User login
Rebuttal: Routine Daily Physical Exam
While we agree that a well-honed physical exam is one of the most important diagnostic tools than an internist can use, we have several responses to Drs Kanjee and McNamara’s point that a routine physical exam is essential for hospitalized patients.1 They argue that this exam might be helpful as a deliberate practice to improve skills for effective diagnostic exams. To this, we have two responses: the first is that the typical “routine” exam—a brief auscultation of the chest and abdomen—is performed frequently enough that additional practice should not be necessary for any practicing hospitalist. Performing a true full exam that would hone infrequently used skills, such as a full neurological exam, an orthopedic knee exam, or fundoscopy, comes at the expense of time spent talking to patients, as well as the potential harm of downstream testing cascades leading to adverse events. Second, we would argue that the real skill being developed is not “recognizing normal” but instead learning how to appropriately use physical diagnostic skills. Knowing precisely what exam maneuvers might be beneficial in a given hospitalized patient is incredibly complex, far more so than charts in evidence-based exam textbooks would suggest. It is this skill, not “recognizing normal,” that requires deliberate practice.
We agree that even during routine hospitalizations, daily exams may help detect complications of therapy, such as a patient with cellulitis on intravenous fluids developing volume overload. We are not against performing physical exams for diagnostic or monitoring purposes. In fact, it may be that most hospitalized patients would benefit from some sort of daily exam. However, rarely performed maneuvers, such as walking with patients or performing a validated delirium screen, are likely to have a higher yield than routine lung auscultation. It may also be true that hospitalized patients would benefit from certain screening exam maneuvers, but again, evidence is lacking, and decades of experience in the outpatient world would suggest the contrary.
Finally, and most ardently, we disagree that performing a routine daily physical exam can somehow inoculate against burnout. That is a view wholly unsupported by any evidence. The physical exam was originally developed as a diagnostic tool, not as a method to connect with patients. However, this traditional “routine” exam has been taught in medical schools as normal ever since, with very little serious interrogation of its utility or downstream effects. Increased cynicism about the exam’s usefulness, in our opinion, reflects physician cognizance of actual disutility of routine exams, rather than pining for a halcyon era that never existed. In fact, we believe a more hypothesis-driven diagnostic use of exams enriches physical diagnosis. For instance, listening to the chest of a patient with cellulitis on intravenous fluids is no longer “just listening,” it is an exercise specifically looking for a finding that affects management. Patient-centered care means tailoring all of our care—including the physical exam—to the needs of the patient. Doing a cursory, routine exam day after day for every patient with the goal of “recognizing normal” is not patient-centered, but rather physician-centered.
We do not doubt the importance of ritual, especially in such a stressful situation as a modern hospitalization. But rather than use a diagnostic procedure with downstream effects, we urge hospitalists to consider instead a ritual dating back to the time of Hippocrates—the compassionate physician sitting at the bedside, laying a hand on the shoulder, and listening to the patient’s concerns. That is authentic human connection rather than performance.
Acknowledgment
The authors of this point-counterpoint to thank Chris Smith, MD, and the members of the BIDMC Internal Medicine Residency Clinician Educator Track for thoughtful discussion around these topics.
1. McNamara LC, Kanjee Z. Counterpoint: routine daily physical exams add value for the hospitalist and patient. J Hosp Med. Published online August 18, 2021. https://doi.org/10.12788/jhm.3671
While we agree that a well-honed physical exam is one of the most important diagnostic tools than an internist can use, we have several responses to Drs Kanjee and McNamara’s point that a routine physical exam is essential for hospitalized patients.1 They argue that this exam might be helpful as a deliberate practice to improve skills for effective diagnostic exams. To this, we have two responses: the first is that the typical “routine” exam—a brief auscultation of the chest and abdomen—is performed frequently enough that additional practice should not be necessary for any practicing hospitalist. Performing a true full exam that would hone infrequently used skills, such as a full neurological exam, an orthopedic knee exam, or fundoscopy, comes at the expense of time spent talking to patients, as well as the potential harm of downstream testing cascades leading to adverse events. Second, we would argue that the real skill being developed is not “recognizing normal” but instead learning how to appropriately use physical diagnostic skills. Knowing precisely what exam maneuvers might be beneficial in a given hospitalized patient is incredibly complex, far more so than charts in evidence-based exam textbooks would suggest. It is this skill, not “recognizing normal,” that requires deliberate practice.
We agree that even during routine hospitalizations, daily exams may help detect complications of therapy, such as a patient with cellulitis on intravenous fluids developing volume overload. We are not against performing physical exams for diagnostic or monitoring purposes. In fact, it may be that most hospitalized patients would benefit from some sort of daily exam. However, rarely performed maneuvers, such as walking with patients or performing a validated delirium screen, are likely to have a higher yield than routine lung auscultation. It may also be true that hospitalized patients would benefit from certain screening exam maneuvers, but again, evidence is lacking, and decades of experience in the outpatient world would suggest the contrary.
Finally, and most ardently, we disagree that performing a routine daily physical exam can somehow inoculate against burnout. That is a view wholly unsupported by any evidence. The physical exam was originally developed as a diagnostic tool, not as a method to connect with patients. However, this traditional “routine” exam has been taught in medical schools as normal ever since, with very little serious interrogation of its utility or downstream effects. Increased cynicism about the exam’s usefulness, in our opinion, reflects physician cognizance of actual disutility of routine exams, rather than pining for a halcyon era that never existed. In fact, we believe a more hypothesis-driven diagnostic use of exams enriches physical diagnosis. For instance, listening to the chest of a patient with cellulitis on intravenous fluids is no longer “just listening,” it is an exercise specifically looking for a finding that affects management. Patient-centered care means tailoring all of our care—including the physical exam—to the needs of the patient. Doing a cursory, routine exam day after day for every patient with the goal of “recognizing normal” is not patient-centered, but rather physician-centered.
We do not doubt the importance of ritual, especially in such a stressful situation as a modern hospitalization. But rather than use a diagnostic procedure with downstream effects, we urge hospitalists to consider instead a ritual dating back to the time of Hippocrates—the compassionate physician sitting at the bedside, laying a hand on the shoulder, and listening to the patient’s concerns. That is authentic human connection rather than performance.
Acknowledgment
The authors of this point-counterpoint to thank Chris Smith, MD, and the members of the BIDMC Internal Medicine Residency Clinician Educator Track for thoughtful discussion around these topics.
While we agree that a well-honed physical exam is one of the most important diagnostic tools than an internist can use, we have several responses to Drs Kanjee and McNamara’s point that a routine physical exam is essential for hospitalized patients.1 They argue that this exam might be helpful as a deliberate practice to improve skills for effective diagnostic exams. To this, we have two responses: the first is that the typical “routine” exam—a brief auscultation of the chest and abdomen—is performed frequently enough that additional practice should not be necessary for any practicing hospitalist. Performing a true full exam that would hone infrequently used skills, such as a full neurological exam, an orthopedic knee exam, or fundoscopy, comes at the expense of time spent talking to patients, as well as the potential harm of downstream testing cascades leading to adverse events. Second, we would argue that the real skill being developed is not “recognizing normal” but instead learning how to appropriately use physical diagnostic skills. Knowing precisely what exam maneuvers might be beneficial in a given hospitalized patient is incredibly complex, far more so than charts in evidence-based exam textbooks would suggest. It is this skill, not “recognizing normal,” that requires deliberate practice.
We agree that even during routine hospitalizations, daily exams may help detect complications of therapy, such as a patient with cellulitis on intravenous fluids developing volume overload. We are not against performing physical exams for diagnostic or monitoring purposes. In fact, it may be that most hospitalized patients would benefit from some sort of daily exam. However, rarely performed maneuvers, such as walking with patients or performing a validated delirium screen, are likely to have a higher yield than routine lung auscultation. It may also be true that hospitalized patients would benefit from certain screening exam maneuvers, but again, evidence is lacking, and decades of experience in the outpatient world would suggest the contrary.
Finally, and most ardently, we disagree that performing a routine daily physical exam can somehow inoculate against burnout. That is a view wholly unsupported by any evidence. The physical exam was originally developed as a diagnostic tool, not as a method to connect with patients. However, this traditional “routine” exam has been taught in medical schools as normal ever since, with very little serious interrogation of its utility or downstream effects. Increased cynicism about the exam’s usefulness, in our opinion, reflects physician cognizance of actual disutility of routine exams, rather than pining for a halcyon era that never existed. In fact, we believe a more hypothesis-driven diagnostic use of exams enriches physical diagnosis. For instance, listening to the chest of a patient with cellulitis on intravenous fluids is no longer “just listening,” it is an exercise specifically looking for a finding that affects management. Patient-centered care means tailoring all of our care—including the physical exam—to the needs of the patient. Doing a cursory, routine exam day after day for every patient with the goal of “recognizing normal” is not patient-centered, but rather physician-centered.
We do not doubt the importance of ritual, especially in such a stressful situation as a modern hospitalization. But rather than use a diagnostic procedure with downstream effects, we urge hospitalists to consider instead a ritual dating back to the time of Hippocrates—the compassionate physician sitting at the bedside, laying a hand on the shoulder, and listening to the patient’s concerns. That is authentic human connection rather than performance.
Acknowledgment
The authors of this point-counterpoint to thank Chris Smith, MD, and the members of the BIDMC Internal Medicine Residency Clinician Educator Track for thoughtful discussion around these topics.
1. McNamara LC, Kanjee Z. Counterpoint: routine daily physical exams add value for the hospitalist and patient. J Hosp Med. Published online August 18, 2021. https://doi.org/10.12788/jhm.3671
1. McNamara LC, Kanjee Z. Counterpoint: routine daily physical exams add value for the hospitalist and patient. J Hosp Med. Published online August 18, 2021. https://doi.org/10.12788/jhm.3671
© 2021 Society of Hospital Medicine
Counterpoint: Routine Daily Physical Exams Add Value for the Hospitalist and Patient
We read with interest the perspective of Drs Rodman and Warnock,1 but disagree with the authors on several points. We find that the routine daily physical examination, often neglected or unappreciated amidst technological advances and new diagnostic testing, provides significant value to both hospitalist and patient in terms of diagnosis, treatment, patient-centered care, and maintaining the patient-doctor relationship.
First, the daily physical exam provides the practice that hospitalists need to develop and maintain necessary diagnostic finesse. We are taught fundamental physical exam maneuvers in medical school, but these skills often atrophy during residency and throughout our careers.2 This leaves us, as practicing physicians, potentially worse at these competencies than when we were students. The daily physical exam, on the other hand, provides frequent and effective practice to keep up and build upon these skills. We gain in part from our repeated normal exams, which help us to skillfully recognize the rare abnormal finding; a hospitalist must likely feel hundreds of normal abdomens to reliably discover a furtive abdominal mass. Our exams also benefit from several forms of prompt and relevant feedback, including that which is provided by subspecialist consultants (like a cardiologist agreeing with your assessment of jugular venous pressure) and other diagnostic tests (like the echocardiogram without the valvulopathy thought to be detected at the bedside). The physical examination is best learned at the bedside, and the daily exam offers an unparalleled opportunity to do so. Such continual skill improvement is necessary for hospitalists to accurately apply data from the evidence-based physical diagnosis literature. Many studies of the utility of various physical exam findings3 involve maneuvers performed by experts; to truly apply their results to our generalist practice, we are required to push ourselves to obtain the diagnostic expertise of the specialist. The daily physical examination, being the most concrete way for hospitalists to do so, is therefore essential to practicing better evidence-based physical diagnosis.
Beyond these larger benefits of the daily physical examination on our own practice and skills, patients in our care benefit diagnostically from these exams as well. Time and again, we see an inadequate or incomplete physical exam leading to errors or adverse patient outcomes.4,5 Even after completion of initial laboratory and imaging tests, laying of hands and stethoscope can lead to dramatic changes in inpatient diagnosis and management.6 The subsequent routine daily physical provides fresh opportunities to reexamine the evidence for or against our own working diagnoses and management plans. The adage that “you don’t know what you don’t know” is especially fitting here. We often do not know to look for and rule a disease process in or out if it is not on our initial differential on hospital day one or two; the daily physical exam allows us to be on the lookout for diagnoses we have not yet considered. The two of us have more than once made a serendipitous discovery of a new rash or other physical finding on hospital day three or four that helps suggest another, and ultimately correct, final diagnosis. Particularly in a setting in which so many inpatient diagnoses are wrong and can lead to patient harm,7 the daily physical examination provides an important check on our own diagnostic reasoning.
Even if we are right about the diagnosis, the daily exam also allows for timely recognition of complications from our management. Listening to each patient’s lungs every day, including those of patients with seemingly unrelated lower-extremity cellulitis, means we will more promptly notice when they retain fluid due to as yet unknown underlying heart failure. Those subtle bibasilar crackles not only become diagnostically useful, but also allow us the possibility of intervening and changing course even before the patient reports shortness of breath or the nurse notes hypoxemia on routine vital signs a day or two later. In an era when our treatment regimens are more complex, with frequent off-target results and side effects, the daily exam is a key screening tool for adverse outcomes in an increasingly ill population. Having a frequently updated and accurate baseline exam is also exceptionally important in the event of sudden neurologic deficits; an inpatient with new facial droop and left-arm weakness at 10
Finally, the daily physical examination is important to patient-centered care and potentially preventing physician burnout.8 Patients have more confidence in us when we conduct a thorough exam. The ritual of the physical exam is also an important contributor to the patient-doctor relationship, and a daily exam can help strengthen that bond each morning.9 Such benefits also extend to physicians. Hospitalists are spending less and less time at the bedside,10 a reality at least partially responsible for rising rates of burnout.11 We all went into clinical medicine to take care of and connect with people. The daily physical examination offers valuable time to show our patients we care about them while also giving us the opportunity to spend time with them, rather than with the “iPatient” that can otherwise become our focus.12 In this way, the daily physical examination can be immensely satisfying and may not only inoculate against burnout but also contribute to a stronger patient-doctor relationship.
For so many reasons, the daily physical exam is of great benefit to hospitalists looking to develop and maintain diagnostic skills, to our patients as we stay on the lookout for unexpected diagnoses and complications, and to the relationships we have with those for whom we care. It is a practice worth not only continuing but celebrating.
1. Rodman A, Warnock S. Point: routine daily physical exams in hospitalized patients are a waste of time. J Hosp Med. Published online August 18, 2021. https://doi.org/10.12788/jhm.3670
2. Vukanovic-Criley JM, Criley S, Warde CM, et al. Competency in cardiac examination skills in medical students, trainees, physicians, and faculty: a multicenter study. Arch Intern Med. 2006;166(6):610-616. https://doi.org/10.1001/archinte.166.6.610
3. McGee S. Evidence-Based Physical Diagnosis. 4th ed. Elsevier; 2018.
4. Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JPA. Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med. 2015;128(12):1322-1324.e3. https://doi.org/10.1016/j.amjmed.2015.06.004
5. Singh H, Giardina TD, Meyer AND, Forjuoh SN, Reis MD, Thomas EJ. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med. 2013;173(6):418-425. https://doi.org/10.1001/jamainternmed.2013.2777
6. Reilly BM. Physical examination in the care of medical inpatients: an observational study. Lancet. 2003;362(9390):1100-1105. https://doi.org/10.1016/S0140-6736(03)14464-9
7. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. https://doi.org/10.1136/bmjqs-2019-010822
8. Silverman B, Gertz A. Present role of the precordial examination in patient care. Am J Cardiol. 2015;115(2):253-255. https://doi.org/10.1016/j.amjcard.2014.10.031
9. Costanzo C, Verghese A. The physical examination as ritual: social sciences and embodiment in the context of the physical examination. Med Clin North Am. 2018;102(3):425-431. https://doi.org/10.1016/j.mcna.2017.12.004
10. Malkenson D, Siegal EM, Leff JA, Weber R, Struck R. Comparing academic and community-based hospitalists. J Hosp Med. 2010;5(6):349-352. https://doi.org/10.1002/jhm.793
11. Hipp DM, Rialon KL, Nevel K, Kothari AN, Jardine LDA. “Back to bedside”: Residents’ and fellows’ perspectives on finding meaning in work. J Grad Med Educ. 2017;9(2):269-273. https://doi.org/10.4300/JGME-D-17-00136.1
12. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. https://doi.org/10.1056/NEJMp0807461
We read with interest the perspective of Drs Rodman and Warnock,1 but disagree with the authors on several points. We find that the routine daily physical examination, often neglected or unappreciated amidst technological advances and new diagnostic testing, provides significant value to both hospitalist and patient in terms of diagnosis, treatment, patient-centered care, and maintaining the patient-doctor relationship.
First, the daily physical exam provides the practice that hospitalists need to develop and maintain necessary diagnostic finesse. We are taught fundamental physical exam maneuvers in medical school, but these skills often atrophy during residency and throughout our careers.2 This leaves us, as practicing physicians, potentially worse at these competencies than when we were students. The daily physical exam, on the other hand, provides frequent and effective practice to keep up and build upon these skills. We gain in part from our repeated normal exams, which help us to skillfully recognize the rare abnormal finding; a hospitalist must likely feel hundreds of normal abdomens to reliably discover a furtive abdominal mass. Our exams also benefit from several forms of prompt and relevant feedback, including that which is provided by subspecialist consultants (like a cardiologist agreeing with your assessment of jugular venous pressure) and other diagnostic tests (like the echocardiogram without the valvulopathy thought to be detected at the bedside). The physical examination is best learned at the bedside, and the daily exam offers an unparalleled opportunity to do so. Such continual skill improvement is necessary for hospitalists to accurately apply data from the evidence-based physical diagnosis literature. Many studies of the utility of various physical exam findings3 involve maneuvers performed by experts; to truly apply their results to our generalist practice, we are required to push ourselves to obtain the diagnostic expertise of the specialist. The daily physical examination, being the most concrete way for hospitalists to do so, is therefore essential to practicing better evidence-based physical diagnosis.
Beyond these larger benefits of the daily physical examination on our own practice and skills, patients in our care benefit diagnostically from these exams as well. Time and again, we see an inadequate or incomplete physical exam leading to errors or adverse patient outcomes.4,5 Even after completion of initial laboratory and imaging tests, laying of hands and stethoscope can lead to dramatic changes in inpatient diagnosis and management.6 The subsequent routine daily physical provides fresh opportunities to reexamine the evidence for or against our own working diagnoses and management plans. The adage that “you don’t know what you don’t know” is especially fitting here. We often do not know to look for and rule a disease process in or out if it is not on our initial differential on hospital day one or two; the daily physical exam allows us to be on the lookout for diagnoses we have not yet considered. The two of us have more than once made a serendipitous discovery of a new rash or other physical finding on hospital day three or four that helps suggest another, and ultimately correct, final diagnosis. Particularly in a setting in which so many inpatient diagnoses are wrong and can lead to patient harm,7 the daily physical examination provides an important check on our own diagnostic reasoning.
Even if we are right about the diagnosis, the daily exam also allows for timely recognition of complications from our management. Listening to each patient’s lungs every day, including those of patients with seemingly unrelated lower-extremity cellulitis, means we will more promptly notice when they retain fluid due to as yet unknown underlying heart failure. Those subtle bibasilar crackles not only become diagnostically useful, but also allow us the possibility of intervening and changing course even before the patient reports shortness of breath or the nurse notes hypoxemia on routine vital signs a day or two later. In an era when our treatment regimens are more complex, with frequent off-target results and side effects, the daily exam is a key screening tool for adverse outcomes in an increasingly ill population. Having a frequently updated and accurate baseline exam is also exceptionally important in the event of sudden neurologic deficits; an inpatient with new facial droop and left-arm weakness at 10
Finally, the daily physical examination is important to patient-centered care and potentially preventing physician burnout.8 Patients have more confidence in us when we conduct a thorough exam. The ritual of the physical exam is also an important contributor to the patient-doctor relationship, and a daily exam can help strengthen that bond each morning.9 Such benefits also extend to physicians. Hospitalists are spending less and less time at the bedside,10 a reality at least partially responsible for rising rates of burnout.11 We all went into clinical medicine to take care of and connect with people. The daily physical examination offers valuable time to show our patients we care about them while also giving us the opportunity to spend time with them, rather than with the “iPatient” that can otherwise become our focus.12 In this way, the daily physical examination can be immensely satisfying and may not only inoculate against burnout but also contribute to a stronger patient-doctor relationship.
For so many reasons, the daily physical exam is of great benefit to hospitalists looking to develop and maintain diagnostic skills, to our patients as we stay on the lookout for unexpected diagnoses and complications, and to the relationships we have with those for whom we care. It is a practice worth not only continuing but celebrating.
We read with interest the perspective of Drs Rodman and Warnock,1 but disagree with the authors on several points. We find that the routine daily physical examination, often neglected or unappreciated amidst technological advances and new diagnostic testing, provides significant value to both hospitalist and patient in terms of diagnosis, treatment, patient-centered care, and maintaining the patient-doctor relationship.
First, the daily physical exam provides the practice that hospitalists need to develop and maintain necessary diagnostic finesse. We are taught fundamental physical exam maneuvers in medical school, but these skills often atrophy during residency and throughout our careers.2 This leaves us, as practicing physicians, potentially worse at these competencies than when we were students. The daily physical exam, on the other hand, provides frequent and effective practice to keep up and build upon these skills. We gain in part from our repeated normal exams, which help us to skillfully recognize the rare abnormal finding; a hospitalist must likely feel hundreds of normal abdomens to reliably discover a furtive abdominal mass. Our exams also benefit from several forms of prompt and relevant feedback, including that which is provided by subspecialist consultants (like a cardiologist agreeing with your assessment of jugular venous pressure) and other diagnostic tests (like the echocardiogram without the valvulopathy thought to be detected at the bedside). The physical examination is best learned at the bedside, and the daily exam offers an unparalleled opportunity to do so. Such continual skill improvement is necessary for hospitalists to accurately apply data from the evidence-based physical diagnosis literature. Many studies of the utility of various physical exam findings3 involve maneuvers performed by experts; to truly apply their results to our generalist practice, we are required to push ourselves to obtain the diagnostic expertise of the specialist. The daily physical examination, being the most concrete way for hospitalists to do so, is therefore essential to practicing better evidence-based physical diagnosis.
Beyond these larger benefits of the daily physical examination on our own practice and skills, patients in our care benefit diagnostically from these exams as well. Time and again, we see an inadequate or incomplete physical exam leading to errors or adverse patient outcomes.4,5 Even after completion of initial laboratory and imaging tests, laying of hands and stethoscope can lead to dramatic changes in inpatient diagnosis and management.6 The subsequent routine daily physical provides fresh opportunities to reexamine the evidence for or against our own working diagnoses and management plans. The adage that “you don’t know what you don’t know” is especially fitting here. We often do not know to look for and rule a disease process in or out if it is not on our initial differential on hospital day one or two; the daily physical exam allows us to be on the lookout for diagnoses we have not yet considered. The two of us have more than once made a serendipitous discovery of a new rash or other physical finding on hospital day three or four that helps suggest another, and ultimately correct, final diagnosis. Particularly in a setting in which so many inpatient diagnoses are wrong and can lead to patient harm,7 the daily physical examination provides an important check on our own diagnostic reasoning.
Even if we are right about the diagnosis, the daily exam also allows for timely recognition of complications from our management. Listening to each patient’s lungs every day, including those of patients with seemingly unrelated lower-extremity cellulitis, means we will more promptly notice when they retain fluid due to as yet unknown underlying heart failure. Those subtle bibasilar crackles not only become diagnostically useful, but also allow us the possibility of intervening and changing course even before the patient reports shortness of breath or the nurse notes hypoxemia on routine vital signs a day or two later. In an era when our treatment regimens are more complex, with frequent off-target results and side effects, the daily exam is a key screening tool for adverse outcomes in an increasingly ill population. Having a frequently updated and accurate baseline exam is also exceptionally important in the event of sudden neurologic deficits; an inpatient with new facial droop and left-arm weakness at 10
Finally, the daily physical examination is important to patient-centered care and potentially preventing physician burnout.8 Patients have more confidence in us when we conduct a thorough exam. The ritual of the physical exam is also an important contributor to the patient-doctor relationship, and a daily exam can help strengthen that bond each morning.9 Such benefits also extend to physicians. Hospitalists are spending less and less time at the bedside,10 a reality at least partially responsible for rising rates of burnout.11 We all went into clinical medicine to take care of and connect with people. The daily physical examination offers valuable time to show our patients we care about them while also giving us the opportunity to spend time with them, rather than with the “iPatient” that can otherwise become our focus.12 In this way, the daily physical examination can be immensely satisfying and may not only inoculate against burnout but also contribute to a stronger patient-doctor relationship.
For so many reasons, the daily physical exam is of great benefit to hospitalists looking to develop and maintain diagnostic skills, to our patients as we stay on the lookout for unexpected diagnoses and complications, and to the relationships we have with those for whom we care. It is a practice worth not only continuing but celebrating.
1. Rodman A, Warnock S. Point: routine daily physical exams in hospitalized patients are a waste of time. J Hosp Med. Published online August 18, 2021. https://doi.org/10.12788/jhm.3670
2. Vukanovic-Criley JM, Criley S, Warde CM, et al. Competency in cardiac examination skills in medical students, trainees, physicians, and faculty: a multicenter study. Arch Intern Med. 2006;166(6):610-616. https://doi.org/10.1001/archinte.166.6.610
3. McGee S. Evidence-Based Physical Diagnosis. 4th ed. Elsevier; 2018.
4. Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JPA. Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med. 2015;128(12):1322-1324.e3. https://doi.org/10.1016/j.amjmed.2015.06.004
5. Singh H, Giardina TD, Meyer AND, Forjuoh SN, Reis MD, Thomas EJ. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med. 2013;173(6):418-425. https://doi.org/10.1001/jamainternmed.2013.2777
6. Reilly BM. Physical examination in the care of medical inpatients: an observational study. Lancet. 2003;362(9390):1100-1105. https://doi.org/10.1016/S0140-6736(03)14464-9
7. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. https://doi.org/10.1136/bmjqs-2019-010822
8. Silverman B, Gertz A. Present role of the precordial examination in patient care. Am J Cardiol. 2015;115(2):253-255. https://doi.org/10.1016/j.amjcard.2014.10.031
9. Costanzo C, Verghese A. The physical examination as ritual: social sciences and embodiment in the context of the physical examination. Med Clin North Am. 2018;102(3):425-431. https://doi.org/10.1016/j.mcna.2017.12.004
10. Malkenson D, Siegal EM, Leff JA, Weber R, Struck R. Comparing academic and community-based hospitalists. J Hosp Med. 2010;5(6):349-352. https://doi.org/10.1002/jhm.793
11. Hipp DM, Rialon KL, Nevel K, Kothari AN, Jardine LDA. “Back to bedside”: Residents’ and fellows’ perspectives on finding meaning in work. J Grad Med Educ. 2017;9(2):269-273. https://doi.org/10.4300/JGME-D-17-00136.1
12. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. https://doi.org/10.1056/NEJMp0807461
1. Rodman A, Warnock S. Point: routine daily physical exams in hospitalized patients are a waste of time. J Hosp Med. Published online August 18, 2021. https://doi.org/10.12788/jhm.3670
2. Vukanovic-Criley JM, Criley S, Warde CM, et al. Competency in cardiac examination skills in medical students, trainees, physicians, and faculty: a multicenter study. Arch Intern Med. 2006;166(6):610-616. https://doi.org/10.1001/archinte.166.6.610
3. McGee S. Evidence-Based Physical Diagnosis. 4th ed. Elsevier; 2018.
4. Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JPA. Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med. 2015;128(12):1322-1324.e3. https://doi.org/10.1016/j.amjmed.2015.06.004
5. Singh H, Giardina TD, Meyer AND, Forjuoh SN, Reis MD, Thomas EJ. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med. 2013;173(6):418-425. https://doi.org/10.1001/jamainternmed.2013.2777
6. Reilly BM. Physical examination in the care of medical inpatients: an observational study. Lancet. 2003;362(9390):1100-1105. https://doi.org/10.1016/S0140-6736(03)14464-9
7. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. https://doi.org/10.1136/bmjqs-2019-010822
8. Silverman B, Gertz A. Present role of the precordial examination in patient care. Am J Cardiol. 2015;115(2):253-255. https://doi.org/10.1016/j.amjcard.2014.10.031
9. Costanzo C, Verghese A. The physical examination as ritual: social sciences and embodiment in the context of the physical examination. Med Clin North Am. 2018;102(3):425-431. https://doi.org/10.1016/j.mcna.2017.12.004
10. Malkenson D, Siegal EM, Leff JA, Weber R, Struck R. Comparing academic and community-based hospitalists. J Hosp Med. 2010;5(6):349-352. https://doi.org/10.1002/jhm.793
11. Hipp DM, Rialon KL, Nevel K, Kothari AN, Jardine LDA. “Back to bedside”: Residents’ and fellows’ perspectives on finding meaning in work. J Grad Med Educ. 2017;9(2):269-273. https://doi.org/10.4300/JGME-D-17-00136.1
12. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. https://doi.org/10.1056/NEJMp0807461
© 2021 Society of Hospital Medicine
Point: Routine Daily Physical Exams in Hospitalized Patients Are a Waste of Time
Every day, physicians engage in an elaborate performance with their patients—the routine complete physical exam. We argue that this purportedly time-tested ritual is at best a waste of time, and at worst potentially harmful.
The modern physical exam evolved throughout the 19th century as the first diagnostic tool in a medical field that was rapidly transforming from its traditional roots to a modern scientific discipline.1 Despite the vast increase in diagnostic tools since then, the physical exam remains one of the most predictive. Several decades of investigation into the “evidence-based” physical exam have attempted to calculate the test characteristics of individual exam findings, confirming that the exam remains as useful a diagnostic tool today as it was for Laënnec or Osler.2
Performing a physical exam for the purposes of diagnosis and prognosis—not only on admission, but also on a daily basis to assess treatment response—remains a fundamental part of a hospitalist’s job. For example, a daily volume assessment, including cardiac auscultation for an S3, evaluation of the jugular venous pulse, and measurement of edema, is essential in managing patients with decompensated heart failure. However, when we stray from these diagnostic purposes, we are no longer using the exam as intended.
The physical exam most frequently performed in the hospital today is the so-called routine daily exam. Generally, this involves passing a stethoscope fleetingly across the chest and abdomen, perhaps with some additional palpation of the abdomen. Cranial nerves II through XII may also occasionally be checked. This routine exam—and by extension, the templated physical exams that fill hospitalists’ documentation—not only lack an evidence base, but also are arguably harmful to patients. Such exams should not be part of a hospitalist’s daily practice.
The most concerning aspect of a routine daily exam is that examination of an asymptomatic patient—for example, auscultation of the lungs of a patient admitted with lower extremity cellulitis—is fundamentally a screening rather than a diagnostic test. While little work has been done in the inpatient setting, decades of studies on outpatient screening exams demonstrate that very few of them are effective.3 For example, a review of commonly used exam maneuvers in wellness visits concluded that “for the asymptomatic, nonpregnant adult of any age, no evidence supports the need for a complete physical exam as traditionally defined,” recommending against such popular maneuvers as lung and heart auscultation and peripheral pulse palpation.4 While the inpatient hospital medicine population has different characteristics that may warrant a routine exam, there is no evidence to support such practice.
It is often argued that the routine physical exam is “cheap” and “quick” and, therefore, should be performed regardless of evidence. While this is certainly true for many diagnostic physical exams, the literature suggests that there is no reason to think that a routine physical exam would be cost-effective.5 Even cost-effective screening physical exam tests, such as an outpatient nurse performing a 1-minute pulse palpation starting at age 55, have incremental costs measuring in the thousands of dollars.6 Furthermore, screening tests can have unexpected downstream effects that are both costly and associated with morbidity and mortality.7 For example, abdominal palpation of a “prominent” aorta can lead to imaging, where incidental findings can trigger procedures that may involve complications.
In addition to potentially adding more risk, the routine daily physical exam represents time that can be better allocated. Medical residents spend the vast majority of their day at the computer, while spending less than 10% of their time at the patient’s bedside.8 Anything that takes up that valuable time, including a “routine exam,” is time spent not talking to the patient, learning about their symptoms, their fears, and who they are as human beings.
It is also true that patients expect a physical exam to be performed, and that additional exam maneuvers, including potentially invasive exams, are associated with increasing patient satisfaction.9 However, these arguments miss much of the nuance of why patients have these expectations. Qualitative research suggests that much of a patient’s desire for unnecessary tests or exams is actually their concern about a lack of validation or empathy from the physician, as well as general skepticism about evidence-based medical decision-making.10 Perhaps spending more face time with patients discussing their issues, rather than idle time performing routine maneuvers, would lead to even greater patient satisfaction.
Finally, one of the most popular arguments in defense of a routine physical exam is that the exam is a “sacred ritual” essential to the patient-physician relationship.11 However, this is an argument not supported by historical interpretation. The physical exam was developed as an explicitly diagnostic procedure in the early 19th century, while the primacy of the doctor-physician relationship dates back millennia, long before the development of the modern physical exam. Furthermore, modern historiography has identified the development of the physical exam as part of a movement to minimize the experience of the patient in their own disease, and to situate the physician as the ultimate source of knowledge about a patient’s body rather than an attempt to strengthen a relationship.12
Ritual is indeed important, and the exam as currently practiced may indeed reinforce the physician-patient relationship. But we should also keep in mind what that relationship entails. Having full access to a patient’s unclothed body and having the ability to perform invasive procedures are far beyond regular social norms—these are powerful diagnostic tools, yes, but they also serve to reinforce an imbalance of power in the relationship. Medical rituals have also changed dramatically over time. Modern evidence suggests that pulse palpation alone, the form of the exam that was dominant for millennia, has profound physiological effects even on critically ill patients.13 Rather than a diagnostic exam that has potential downstream cost implications and consumes valuable time from an encounter, we suggest a return to a more traditional ritual of physical touch: sitting at the patient’s bedside, holding their hand, and speaking to them compassionately about their fears and hopes. This would be a far more valuable “routine” encounter to incorporate into the busy hospitalist’s day.
Acknowledgment
The authors of this point-counterpoint thank Chris Smith, MD, and the members of the BIDMC Internal Medicine Residency Clinician Educator Track for thoughtful discussion around these topics.
1. Laënnec RTH. De l’auscultation médiate ou Traité du Diagnostic des Maladies des Poumon et du Coeur fondé principalement sur ce Nouveau Moyen d’Exploration. Brosson & Chaudé; 1819.
2. McGee S. Evidence-Based Physical Diagnosis. 4th ed. Elsevier; 2018.
3. Bloomfield HE, Wilt TJ. Evidence brief: Role of the annual comprehensive physical examination in the asymptomatic adult. VA Evidence Synthesis Program Evidence Briefs. US Department of Veterans Affairs; October 2011.
4. Oboler SK, LaForce FM. The periodic physical examination in asymptomatic adults. Ann Intern Med. 1989;110(3):214-226. https://doi.org/10.7326/0003-4819-110-3-214
5. Angus S. The cost-effective evaluation of syncope. Med Clin North Am. 2016;100(5):1019-1032. https://doi.org/10.1016/j.mcna.2016.04.010
6. Welton NJ, McAleenan A, Thom HH, et al. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2017;21(29):1-236. https://doi.org/10.3310/hta21290
7. Rothberg MB. The $50 000 physical. JAMA. 2020;323(17):1682-1683. https://doi.org/10.1001/jama.2020.2866
8. Mamykina L, Vawdrey DK, Hripcsak G. How do residents spend their shift time? A time and motion study with a particular focus on the use of computers. Acad Med. 2016;91(6):827-832. https://doi.org/10.1097/ACM.0000000000001148
9. Duan L, Mukherjee EM, Federman DG. The physical examination: a survey of patient preferences and expectations during primary care visits. Postgrad Med. 2020;132(1):102-108. https://doi.org/10.1080/00325481.2020.1713618
10. Kravitz RL, Callahan EJ. Patients’ perceptions of omitted examinations and tests: a qualitative analysis. J Gen Intern Med. 2000;15(1):38-45. https://doi.org/10.1046/j.1525-1497.2000.12058.x
11. Costanzo C, Verghese A. The physical examination as ritual: social sciences and embodiment in the context of the physical examination. Med Clin North Am. 2018;102(3):425-431. https://doi.org/10.1016/j.mcna.2017.12.004
12. Jewson ND. The disappearance of the sick-man from medical cosmology, 1770–1870. Int J Epidemiol. 2009;38(3):622-633. https://doi.org/10.1093/ije/dyp180
13. Arnold MH, Komesaroff P, Kerridge I. Understanding the ethical implications of the rituals of medicine. Intern Med J. 2020;50(9):1123-1131. https://doi.org/10.1111/imj.14990
Every day, physicians engage in an elaborate performance with their patients—the routine complete physical exam. We argue that this purportedly time-tested ritual is at best a waste of time, and at worst potentially harmful.
The modern physical exam evolved throughout the 19th century as the first diagnostic tool in a medical field that was rapidly transforming from its traditional roots to a modern scientific discipline.1 Despite the vast increase in diagnostic tools since then, the physical exam remains one of the most predictive. Several decades of investigation into the “evidence-based” physical exam have attempted to calculate the test characteristics of individual exam findings, confirming that the exam remains as useful a diagnostic tool today as it was for Laënnec or Osler.2
Performing a physical exam for the purposes of diagnosis and prognosis—not only on admission, but also on a daily basis to assess treatment response—remains a fundamental part of a hospitalist’s job. For example, a daily volume assessment, including cardiac auscultation for an S3, evaluation of the jugular venous pulse, and measurement of edema, is essential in managing patients with decompensated heart failure. However, when we stray from these diagnostic purposes, we are no longer using the exam as intended.
The physical exam most frequently performed in the hospital today is the so-called routine daily exam. Generally, this involves passing a stethoscope fleetingly across the chest and abdomen, perhaps with some additional palpation of the abdomen. Cranial nerves II through XII may also occasionally be checked. This routine exam—and by extension, the templated physical exams that fill hospitalists’ documentation—not only lack an evidence base, but also are arguably harmful to patients. Such exams should not be part of a hospitalist’s daily practice.
The most concerning aspect of a routine daily exam is that examination of an asymptomatic patient—for example, auscultation of the lungs of a patient admitted with lower extremity cellulitis—is fundamentally a screening rather than a diagnostic test. While little work has been done in the inpatient setting, decades of studies on outpatient screening exams demonstrate that very few of them are effective.3 For example, a review of commonly used exam maneuvers in wellness visits concluded that “for the asymptomatic, nonpregnant adult of any age, no evidence supports the need for a complete physical exam as traditionally defined,” recommending against such popular maneuvers as lung and heart auscultation and peripheral pulse palpation.4 While the inpatient hospital medicine population has different characteristics that may warrant a routine exam, there is no evidence to support such practice.
It is often argued that the routine physical exam is “cheap” and “quick” and, therefore, should be performed regardless of evidence. While this is certainly true for many diagnostic physical exams, the literature suggests that there is no reason to think that a routine physical exam would be cost-effective.5 Even cost-effective screening physical exam tests, such as an outpatient nurse performing a 1-minute pulse palpation starting at age 55, have incremental costs measuring in the thousands of dollars.6 Furthermore, screening tests can have unexpected downstream effects that are both costly and associated with morbidity and mortality.7 For example, abdominal palpation of a “prominent” aorta can lead to imaging, where incidental findings can trigger procedures that may involve complications.
In addition to potentially adding more risk, the routine daily physical exam represents time that can be better allocated. Medical residents spend the vast majority of their day at the computer, while spending less than 10% of their time at the patient’s bedside.8 Anything that takes up that valuable time, including a “routine exam,” is time spent not talking to the patient, learning about their symptoms, their fears, and who they are as human beings.
It is also true that patients expect a physical exam to be performed, and that additional exam maneuvers, including potentially invasive exams, are associated with increasing patient satisfaction.9 However, these arguments miss much of the nuance of why patients have these expectations. Qualitative research suggests that much of a patient’s desire for unnecessary tests or exams is actually their concern about a lack of validation or empathy from the physician, as well as general skepticism about evidence-based medical decision-making.10 Perhaps spending more face time with patients discussing their issues, rather than idle time performing routine maneuvers, would lead to even greater patient satisfaction.
Finally, one of the most popular arguments in defense of a routine physical exam is that the exam is a “sacred ritual” essential to the patient-physician relationship.11 However, this is an argument not supported by historical interpretation. The physical exam was developed as an explicitly diagnostic procedure in the early 19th century, while the primacy of the doctor-physician relationship dates back millennia, long before the development of the modern physical exam. Furthermore, modern historiography has identified the development of the physical exam as part of a movement to minimize the experience of the patient in their own disease, and to situate the physician as the ultimate source of knowledge about a patient’s body rather than an attempt to strengthen a relationship.12
Ritual is indeed important, and the exam as currently practiced may indeed reinforce the physician-patient relationship. But we should also keep in mind what that relationship entails. Having full access to a patient’s unclothed body and having the ability to perform invasive procedures are far beyond regular social norms—these are powerful diagnostic tools, yes, but they also serve to reinforce an imbalance of power in the relationship. Medical rituals have also changed dramatically over time. Modern evidence suggests that pulse palpation alone, the form of the exam that was dominant for millennia, has profound physiological effects even on critically ill patients.13 Rather than a diagnostic exam that has potential downstream cost implications and consumes valuable time from an encounter, we suggest a return to a more traditional ritual of physical touch: sitting at the patient’s bedside, holding their hand, and speaking to them compassionately about their fears and hopes. This would be a far more valuable “routine” encounter to incorporate into the busy hospitalist’s day.
Acknowledgment
The authors of this point-counterpoint thank Chris Smith, MD, and the members of the BIDMC Internal Medicine Residency Clinician Educator Track for thoughtful discussion around these topics.
Every day, physicians engage in an elaborate performance with their patients—the routine complete physical exam. We argue that this purportedly time-tested ritual is at best a waste of time, and at worst potentially harmful.
The modern physical exam evolved throughout the 19th century as the first diagnostic tool in a medical field that was rapidly transforming from its traditional roots to a modern scientific discipline.1 Despite the vast increase in diagnostic tools since then, the physical exam remains one of the most predictive. Several decades of investigation into the “evidence-based” physical exam have attempted to calculate the test characteristics of individual exam findings, confirming that the exam remains as useful a diagnostic tool today as it was for Laënnec or Osler.2
Performing a physical exam for the purposes of diagnosis and prognosis—not only on admission, but also on a daily basis to assess treatment response—remains a fundamental part of a hospitalist’s job. For example, a daily volume assessment, including cardiac auscultation for an S3, evaluation of the jugular venous pulse, and measurement of edema, is essential in managing patients with decompensated heart failure. However, when we stray from these diagnostic purposes, we are no longer using the exam as intended.
The physical exam most frequently performed in the hospital today is the so-called routine daily exam. Generally, this involves passing a stethoscope fleetingly across the chest and abdomen, perhaps with some additional palpation of the abdomen. Cranial nerves II through XII may also occasionally be checked. This routine exam—and by extension, the templated physical exams that fill hospitalists’ documentation—not only lack an evidence base, but also are arguably harmful to patients. Such exams should not be part of a hospitalist’s daily practice.
The most concerning aspect of a routine daily exam is that examination of an asymptomatic patient—for example, auscultation of the lungs of a patient admitted with lower extremity cellulitis—is fundamentally a screening rather than a diagnostic test. While little work has been done in the inpatient setting, decades of studies on outpatient screening exams demonstrate that very few of them are effective.3 For example, a review of commonly used exam maneuvers in wellness visits concluded that “for the asymptomatic, nonpregnant adult of any age, no evidence supports the need for a complete physical exam as traditionally defined,” recommending against such popular maneuvers as lung and heart auscultation and peripheral pulse palpation.4 While the inpatient hospital medicine population has different characteristics that may warrant a routine exam, there is no evidence to support such practice.
It is often argued that the routine physical exam is “cheap” and “quick” and, therefore, should be performed regardless of evidence. While this is certainly true for many diagnostic physical exams, the literature suggests that there is no reason to think that a routine physical exam would be cost-effective.5 Even cost-effective screening physical exam tests, such as an outpatient nurse performing a 1-minute pulse palpation starting at age 55, have incremental costs measuring in the thousands of dollars.6 Furthermore, screening tests can have unexpected downstream effects that are both costly and associated with morbidity and mortality.7 For example, abdominal palpation of a “prominent” aorta can lead to imaging, where incidental findings can trigger procedures that may involve complications.
In addition to potentially adding more risk, the routine daily physical exam represents time that can be better allocated. Medical residents spend the vast majority of their day at the computer, while spending less than 10% of their time at the patient’s bedside.8 Anything that takes up that valuable time, including a “routine exam,” is time spent not talking to the patient, learning about their symptoms, their fears, and who they are as human beings.
It is also true that patients expect a physical exam to be performed, and that additional exam maneuvers, including potentially invasive exams, are associated with increasing patient satisfaction.9 However, these arguments miss much of the nuance of why patients have these expectations. Qualitative research suggests that much of a patient’s desire for unnecessary tests or exams is actually their concern about a lack of validation or empathy from the physician, as well as general skepticism about evidence-based medical decision-making.10 Perhaps spending more face time with patients discussing their issues, rather than idle time performing routine maneuvers, would lead to even greater patient satisfaction.
Finally, one of the most popular arguments in defense of a routine physical exam is that the exam is a “sacred ritual” essential to the patient-physician relationship.11 However, this is an argument not supported by historical interpretation. The physical exam was developed as an explicitly diagnostic procedure in the early 19th century, while the primacy of the doctor-physician relationship dates back millennia, long before the development of the modern physical exam. Furthermore, modern historiography has identified the development of the physical exam as part of a movement to minimize the experience of the patient in their own disease, and to situate the physician as the ultimate source of knowledge about a patient’s body rather than an attempt to strengthen a relationship.12
Ritual is indeed important, and the exam as currently practiced may indeed reinforce the physician-patient relationship. But we should also keep in mind what that relationship entails. Having full access to a patient’s unclothed body and having the ability to perform invasive procedures are far beyond regular social norms—these are powerful diagnostic tools, yes, but they also serve to reinforce an imbalance of power in the relationship. Medical rituals have also changed dramatically over time. Modern evidence suggests that pulse palpation alone, the form of the exam that was dominant for millennia, has profound physiological effects even on critically ill patients.13 Rather than a diagnostic exam that has potential downstream cost implications and consumes valuable time from an encounter, we suggest a return to a more traditional ritual of physical touch: sitting at the patient’s bedside, holding their hand, and speaking to them compassionately about their fears and hopes. This would be a far more valuable “routine” encounter to incorporate into the busy hospitalist’s day.
Acknowledgment
The authors of this point-counterpoint thank Chris Smith, MD, and the members of the BIDMC Internal Medicine Residency Clinician Educator Track for thoughtful discussion around these topics.
1. Laënnec RTH. De l’auscultation médiate ou Traité du Diagnostic des Maladies des Poumon et du Coeur fondé principalement sur ce Nouveau Moyen d’Exploration. Brosson & Chaudé; 1819.
2. McGee S. Evidence-Based Physical Diagnosis. 4th ed. Elsevier; 2018.
3. Bloomfield HE, Wilt TJ. Evidence brief: Role of the annual comprehensive physical examination in the asymptomatic adult. VA Evidence Synthesis Program Evidence Briefs. US Department of Veterans Affairs; October 2011.
4. Oboler SK, LaForce FM. The periodic physical examination in asymptomatic adults. Ann Intern Med. 1989;110(3):214-226. https://doi.org/10.7326/0003-4819-110-3-214
5. Angus S. The cost-effective evaluation of syncope. Med Clin North Am. 2016;100(5):1019-1032. https://doi.org/10.1016/j.mcna.2016.04.010
6. Welton NJ, McAleenan A, Thom HH, et al. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2017;21(29):1-236. https://doi.org/10.3310/hta21290
7. Rothberg MB. The $50 000 physical. JAMA. 2020;323(17):1682-1683. https://doi.org/10.1001/jama.2020.2866
8. Mamykina L, Vawdrey DK, Hripcsak G. How do residents spend their shift time? A time and motion study with a particular focus on the use of computers. Acad Med. 2016;91(6):827-832. https://doi.org/10.1097/ACM.0000000000001148
9. Duan L, Mukherjee EM, Federman DG. The physical examination: a survey of patient preferences and expectations during primary care visits. Postgrad Med. 2020;132(1):102-108. https://doi.org/10.1080/00325481.2020.1713618
10. Kravitz RL, Callahan EJ. Patients’ perceptions of omitted examinations and tests: a qualitative analysis. J Gen Intern Med. 2000;15(1):38-45. https://doi.org/10.1046/j.1525-1497.2000.12058.x
11. Costanzo C, Verghese A. The physical examination as ritual: social sciences and embodiment in the context of the physical examination. Med Clin North Am. 2018;102(3):425-431. https://doi.org/10.1016/j.mcna.2017.12.004
12. Jewson ND. The disappearance of the sick-man from medical cosmology, 1770–1870. Int J Epidemiol. 2009;38(3):622-633. https://doi.org/10.1093/ije/dyp180
13. Arnold MH, Komesaroff P, Kerridge I. Understanding the ethical implications of the rituals of medicine. Intern Med J. 2020;50(9):1123-1131. https://doi.org/10.1111/imj.14990
1. Laënnec RTH. De l’auscultation médiate ou Traité du Diagnostic des Maladies des Poumon et du Coeur fondé principalement sur ce Nouveau Moyen d’Exploration. Brosson & Chaudé; 1819.
2. McGee S. Evidence-Based Physical Diagnosis. 4th ed. Elsevier; 2018.
3. Bloomfield HE, Wilt TJ. Evidence brief: Role of the annual comprehensive physical examination in the asymptomatic adult. VA Evidence Synthesis Program Evidence Briefs. US Department of Veterans Affairs; October 2011.
4. Oboler SK, LaForce FM. The periodic physical examination in asymptomatic adults. Ann Intern Med. 1989;110(3):214-226. https://doi.org/10.7326/0003-4819-110-3-214
5. Angus S. The cost-effective evaluation of syncope. Med Clin North Am. 2016;100(5):1019-1032. https://doi.org/10.1016/j.mcna.2016.04.010
6. Welton NJ, McAleenan A, Thom HH, et al. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2017;21(29):1-236. https://doi.org/10.3310/hta21290
7. Rothberg MB. The $50 000 physical. JAMA. 2020;323(17):1682-1683. https://doi.org/10.1001/jama.2020.2866
8. Mamykina L, Vawdrey DK, Hripcsak G. How do residents spend their shift time? A time and motion study with a particular focus on the use of computers. Acad Med. 2016;91(6):827-832. https://doi.org/10.1097/ACM.0000000000001148
9. Duan L, Mukherjee EM, Federman DG. The physical examination: a survey of patient preferences and expectations during primary care visits. Postgrad Med. 2020;132(1):102-108. https://doi.org/10.1080/00325481.2020.1713618
10. Kravitz RL, Callahan EJ. Patients’ perceptions of omitted examinations and tests: a qualitative analysis. J Gen Intern Med. 2000;15(1):38-45. https://doi.org/10.1046/j.1525-1497.2000.12058.x
11. Costanzo C, Verghese A. The physical examination as ritual: social sciences and embodiment in the context of the physical examination. Med Clin North Am. 2018;102(3):425-431. https://doi.org/10.1016/j.mcna.2017.12.004
12. Jewson ND. The disappearance of the sick-man from medical cosmology, 1770–1870. Int J Epidemiol. 2009;38(3):622-633. https://doi.org/10.1093/ije/dyp180
13. Arnold MH, Komesaroff P, Kerridge I. Understanding the ethical implications of the rituals of medicine. Intern Med J. 2020;50(9):1123-1131. https://doi.org/10.1111/imj.14990
© 2021 Society of Hospital Medicine
Lessons Learned From the Pediatric Overflow Planning Contingency Response Network: A Transdisciplinary Virtual Collaboration Addressing Health System Fragmentation and Disparity During the COVID-19 Pandemic
As the COVID-19 pandemic surged in March 2020 in the United States, it was clear that severe COVID-19 and rates of hospitalization were much higher in adults than in children.1 Pediatric facilities grappled with how to leverage empty beds and other underutilized human, clinical, and material resources to offset the overflowing adult facilities.2,3 Pediatricians agonized about how to identify adult patients for whom they could provide safe and effective care, not only as individual clinicians, but also with adequate support from their local pediatric facility and health system.
Maria* (*name changed) was a young adult whose experience with her local health system highlighted common and addressable issues that arose when pediatric facilities aimed to care for adult populations. Adult hospitals were already above capacity caring for acutely ill patients with COVID-19, and a local freestanding children’s hospital offered to offload young adult patients up to age 30 years. Maria, a 26-year-old, had just been transferred from an adult emergency department (ED) to the children’s hospital ED for management of postoperative pain after a recent appendectomy. There was concern for possible abscess formation, but no evidence of sepsis. During his oral presentation, a pediatric resident in the ED reported, “This patient has a history of drug abuse and should not be admitted to a children’s hospital. She has been demanding pain meds and I feel she would be better served at the adult hospital.”
At the intersection of these seemingly impossible questions, dually trained internal medicine and pediatrics (med-peds) physicians had a unique vantage point, as they were accustomed to bridging the divide between adult and pediatric medicine in their practices.
As POPCoRN members shared their challenges and institutional learnings, common themes were identified, such as management of intubated patients in non–intensive care unit (ICU) spaces; gaps in staffing with redeployment of residents and hospitalists; and dissemination of education, such as Advanced Cardiac Life Support (ACLS) webinars to frontline staff.
IDENTIFY THE “CORRECT” PATIENT POPULATION, BUT DO NOT LET PERFECTION BE A BARRIER TO PROGRESS
Many pediatric facilities reported perseveration over the adult age cutoff accepted to the pediatric facility, only to realize the initial arbitrary age cutoff usually did not encompass enough patients to benefit local adult health systems. Using only strict age cutoffs also created an unnecessary barrier to accepting otherwise appropriate adult patients (eg, adult patient with controlled hypertension and a soft tissue infection).
USE REPETITIVE STAKEHOLDER ANALYSIS TO ADAPT TO A RAPIDLY CHANGING ENVIRONMENT
The pandemic response was rapidly evolving and unpredictable. Planning required all affected parties at the table to effectively identify problems and solutions. Clinical and nonclinical groups were critical to planning operational logistics to provide safe care for adults in pediatric facilities.
COMMUNICATE WITH INTENTION AND TRANSPARENCY: WHEN LESS IS NOT MORE
Across care settings and training levels, the power of timely, honest, and transparent communication with leadership echoed throughout the network and could not be overemphasized. The cadence and modes of communication, while established by facility leaders, was best determined by explicitly asking team members for their needs. Often, leaders attempted to avoid communicating abrupt protocol changes to spare their teams additional stress and excessive correspondence. However, POPCoRN members found this approach often increased the perception among staff of a lack of transparency, which exacerbated feelings of discomfort and stress. While other specific examples of communication strategies are included in the POPCoRN guide, network members consistently noted that virtual open forums with leadership at regular intervals allowed teams to ask questions, raise concerns, and share ideas. In addition to open forums, leaders’ written communications regarding local medicolegal limitations and malpractice protection related to adult care should be distributed to staff. In Maria’s case, would provider discomfort and anxiety have been ameliorated with a proactive open forum to discuss the care of adults at the pediatric facility? Would that forum have called attention to staff educational and preparation needs around taking care of adults with a history substance use disorder? If so, this may have added a downstream benefit of decreasing effects of implicit bias amplified by stress.10
MAKE “JUST-IN-TIME” RESOURCES AVAILABLE FOR PEDIATRICIANS CARING FOR ADULT PATIENTS
DESIGN AN EMERGENCY RESPONSE SYSTEM FOR ADULT PATIENTS IN PEDIATRIC FACILITIES
CONCLUSION
Acknowledgments
Collaborators: All the collaborating authors listed below have contributed to the guide available in the appendix of the online version of this article, “Lessons Learned From COVID-19: A Practical Guide for Pediatric Facility Preparedness and Repurposing.” All the authors have provided consent to be listed.
Francisco Alvarez, MD, Stanford, California; Elizabeth Boggs, MD, MS, Aurora, Colorado; Rachel Boykan, MD, Stony Brook, New York; Alicia Caldwell, MD, Cincinnati, Ohio; Maryanne M. Chumpia, MD, MS, Torrance, California; Katharine N Clouser, MD, Hackensack, New Jersey; Alexandra L Coria, MD, Brooklyn, New York; Clare C Crosh, DO, Cincinnati, Ohio; Magna Dias, MD, Bridgeport, Connecticut; Laura N El-Hage, MD, Philadelphia, Pennsylvania; Jeff Foti, MD, Seattle, Washington; Mirna Giordano, MD, New York, New York; Sheena Gupta, MD, MBA, Evanston, Illinois; Laura Nell Hodo, MD, New York, New York; Ashley Jenkins, MD, MS, Rochester, New York; Anika Kumar, MD, Cleveland, Ohio; Merlin C Lowe, MD, Tuscon, Arizona; Brittany Middleton, MD, Pasadena, California; Sage Myers, MD, Philadelphia, Pennsylvania; Anik Patel, MD, Salt Lake City, Utah; Leah Ratner, MD, MS, Boston, Massachusetts; Shela Sridhar, MD, MPH, Boston, Massachusetts; Nathan Stehouwer, MD, Cleveland, Ohio; Julie Sylvester, DO, Mount Kisco, New York; Dava Szalda, MD, MSHP, Philadelphia, Pennsylvania; Heather Toth, MD, Milwaukee, Wisconsin; Krista Tuomela, MD, Milwaukee, Wisconsin; Ronald Williams, MD, Hershey, Pennsylvania.
1. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. https://doi.org/10.1542/peds.2020-0702
2. Osborn R, Doolittle B, Loyal J. When pediatric hospitalists took care of adults during the COVID-19 pandemic. Hosp Pediatr. 2021;11(1):e15-e18. https://doi.org/10.1542/hpeds.2020-001040
3. Yager PH, Whalen KA, Cummings BM. Repurposing a pediatric ICU for adults. N Engl J Med. 2020;382(22):e80. https://doi.org/10.1056/NEJMc2014819
4. Conway-Habes EE, Herbst BF Jr, Herbst LA, et al. Using quality improvement to introduce and standardize the National Early Warning Score (NEWS) for adult inpatients at a children’s hospital. Hosp Pediatr. 2017;7(3):156-163. https://doi.org/10.1542/hpeds.2016-0117
5. Kinnear B, O’Toole JK. Care of adults in children’s hospitals: acknowledging the aging elephant in the room. JAMA Pediatr. 2015;169(12):1081-1082. https://doi.org/10.1001/jamapediatrics.2015.2215
6. Szalda D, Steinway C, Greenberg A, et al. Developing a hospital-wide transition program for young adults with medical complexity. J Adolesc Health. 2019;65(4):476-482. https://doi.org/10.1016/j.jadohealth.2019.04.008
7. Jenkins A, Ratner L, Caldwell A, Sharma N, Uluer A, White C. Children’s hospitals caring for adults during a pandemic: pragmatic considerations and approaches. J Hosp Med. 2020;15(5):311-313. https://doi.org/10.12788/jhm.3432
8. Botwinick L, Bisognano M, Haraden C. Leadership Guide to Patient Safety. IHI Innovation Series white paper. Cambridge, MA: Institute for Healthcare Improvement; 2006. Accessed January 20, 2021. http://www.ihi.org/resources/Pages/IHIWhitePapers/LeadershipGuidetoPatientSafetyWhitePaper.aspx
9. Essien UR, Eneanya ND, Crews DC. Prioritizing equity in a time of scarcity: the COVID-19 pandemic. J Gen Intern Med. 2020;35(9):2760-2762. https://doi.org/10.1007/s11606-020-05976-y
10. Yu R. Stress potentiates decision biases: a stress induced deliberation-to-intuition (SIDI) model. Neurobiol Stress. 2016;3:83-95. https://doi.org/10.1016/j.ynstr.2015.12.006
As the COVID-19 pandemic surged in March 2020 in the United States, it was clear that severe COVID-19 and rates of hospitalization were much higher in adults than in children.1 Pediatric facilities grappled with how to leverage empty beds and other underutilized human, clinical, and material resources to offset the overflowing adult facilities.2,3 Pediatricians agonized about how to identify adult patients for whom they could provide safe and effective care, not only as individual clinicians, but also with adequate support from their local pediatric facility and health system.
Maria* (*name changed) was a young adult whose experience with her local health system highlighted common and addressable issues that arose when pediatric facilities aimed to care for adult populations. Adult hospitals were already above capacity caring for acutely ill patients with COVID-19, and a local freestanding children’s hospital offered to offload young adult patients up to age 30 years. Maria, a 26-year-old, had just been transferred from an adult emergency department (ED) to the children’s hospital ED for management of postoperative pain after a recent appendectomy. There was concern for possible abscess formation, but no evidence of sepsis. During his oral presentation, a pediatric resident in the ED reported, “This patient has a history of drug abuse and should not be admitted to a children’s hospital. She has been demanding pain meds and I feel she would be better served at the adult hospital.”
At the intersection of these seemingly impossible questions, dually trained internal medicine and pediatrics (med-peds) physicians had a unique vantage point, as they were accustomed to bridging the divide between adult and pediatric medicine in their practices.
As POPCoRN members shared their challenges and institutional learnings, common themes were identified, such as management of intubated patients in non–intensive care unit (ICU) spaces; gaps in staffing with redeployment of residents and hospitalists; and dissemination of education, such as Advanced Cardiac Life Support (ACLS) webinars to frontline staff.
IDENTIFY THE “CORRECT” PATIENT POPULATION, BUT DO NOT LET PERFECTION BE A BARRIER TO PROGRESS
Many pediatric facilities reported perseveration over the adult age cutoff accepted to the pediatric facility, only to realize the initial arbitrary age cutoff usually did not encompass enough patients to benefit local adult health systems. Using only strict age cutoffs also created an unnecessary barrier to accepting otherwise appropriate adult patients (eg, adult patient with controlled hypertension and a soft tissue infection).
USE REPETITIVE STAKEHOLDER ANALYSIS TO ADAPT TO A RAPIDLY CHANGING ENVIRONMENT
The pandemic response was rapidly evolving and unpredictable. Planning required all affected parties at the table to effectively identify problems and solutions. Clinical and nonclinical groups were critical to planning operational logistics to provide safe care for adults in pediatric facilities.
COMMUNICATE WITH INTENTION AND TRANSPARENCY: WHEN LESS IS NOT MORE
Across care settings and training levels, the power of timely, honest, and transparent communication with leadership echoed throughout the network and could not be overemphasized. The cadence and modes of communication, while established by facility leaders, was best determined by explicitly asking team members for their needs. Often, leaders attempted to avoid communicating abrupt protocol changes to spare their teams additional stress and excessive correspondence. However, POPCoRN members found this approach often increased the perception among staff of a lack of transparency, which exacerbated feelings of discomfort and stress. While other specific examples of communication strategies are included in the POPCoRN guide, network members consistently noted that virtual open forums with leadership at regular intervals allowed teams to ask questions, raise concerns, and share ideas. In addition to open forums, leaders’ written communications regarding local medicolegal limitations and malpractice protection related to adult care should be distributed to staff. In Maria’s case, would provider discomfort and anxiety have been ameliorated with a proactive open forum to discuss the care of adults at the pediatric facility? Would that forum have called attention to staff educational and preparation needs around taking care of adults with a history substance use disorder? If so, this may have added a downstream benefit of decreasing effects of implicit bias amplified by stress.10
MAKE “JUST-IN-TIME” RESOURCES AVAILABLE FOR PEDIATRICIANS CARING FOR ADULT PATIENTS
DESIGN AN EMERGENCY RESPONSE SYSTEM FOR ADULT PATIENTS IN PEDIATRIC FACILITIES
CONCLUSION
Acknowledgments
Collaborators: All the collaborating authors listed below have contributed to the guide available in the appendix of the online version of this article, “Lessons Learned From COVID-19: A Practical Guide for Pediatric Facility Preparedness and Repurposing.” All the authors have provided consent to be listed.
Francisco Alvarez, MD, Stanford, California; Elizabeth Boggs, MD, MS, Aurora, Colorado; Rachel Boykan, MD, Stony Brook, New York; Alicia Caldwell, MD, Cincinnati, Ohio; Maryanne M. Chumpia, MD, MS, Torrance, California; Katharine N Clouser, MD, Hackensack, New Jersey; Alexandra L Coria, MD, Brooklyn, New York; Clare C Crosh, DO, Cincinnati, Ohio; Magna Dias, MD, Bridgeport, Connecticut; Laura N El-Hage, MD, Philadelphia, Pennsylvania; Jeff Foti, MD, Seattle, Washington; Mirna Giordano, MD, New York, New York; Sheena Gupta, MD, MBA, Evanston, Illinois; Laura Nell Hodo, MD, New York, New York; Ashley Jenkins, MD, MS, Rochester, New York; Anika Kumar, MD, Cleveland, Ohio; Merlin C Lowe, MD, Tuscon, Arizona; Brittany Middleton, MD, Pasadena, California; Sage Myers, MD, Philadelphia, Pennsylvania; Anik Patel, MD, Salt Lake City, Utah; Leah Ratner, MD, MS, Boston, Massachusetts; Shela Sridhar, MD, MPH, Boston, Massachusetts; Nathan Stehouwer, MD, Cleveland, Ohio; Julie Sylvester, DO, Mount Kisco, New York; Dava Szalda, MD, MSHP, Philadelphia, Pennsylvania; Heather Toth, MD, Milwaukee, Wisconsin; Krista Tuomela, MD, Milwaukee, Wisconsin; Ronald Williams, MD, Hershey, Pennsylvania.
As the COVID-19 pandemic surged in March 2020 in the United States, it was clear that severe COVID-19 and rates of hospitalization were much higher in adults than in children.1 Pediatric facilities grappled with how to leverage empty beds and other underutilized human, clinical, and material resources to offset the overflowing adult facilities.2,3 Pediatricians agonized about how to identify adult patients for whom they could provide safe and effective care, not only as individual clinicians, but also with adequate support from their local pediatric facility and health system.
Maria* (*name changed) was a young adult whose experience with her local health system highlighted common and addressable issues that arose when pediatric facilities aimed to care for adult populations. Adult hospitals were already above capacity caring for acutely ill patients with COVID-19, and a local freestanding children’s hospital offered to offload young adult patients up to age 30 years. Maria, a 26-year-old, had just been transferred from an adult emergency department (ED) to the children’s hospital ED for management of postoperative pain after a recent appendectomy. There was concern for possible abscess formation, but no evidence of sepsis. During his oral presentation, a pediatric resident in the ED reported, “This patient has a history of drug abuse and should not be admitted to a children’s hospital. She has been demanding pain meds and I feel she would be better served at the adult hospital.”
At the intersection of these seemingly impossible questions, dually trained internal medicine and pediatrics (med-peds) physicians had a unique vantage point, as they were accustomed to bridging the divide between adult and pediatric medicine in their practices.
As POPCoRN members shared their challenges and institutional learnings, common themes were identified, such as management of intubated patients in non–intensive care unit (ICU) spaces; gaps in staffing with redeployment of residents and hospitalists; and dissemination of education, such as Advanced Cardiac Life Support (ACLS) webinars to frontline staff.
IDENTIFY THE “CORRECT” PATIENT POPULATION, BUT DO NOT LET PERFECTION BE A BARRIER TO PROGRESS
Many pediatric facilities reported perseveration over the adult age cutoff accepted to the pediatric facility, only to realize the initial arbitrary age cutoff usually did not encompass enough patients to benefit local adult health systems. Using only strict age cutoffs also created an unnecessary barrier to accepting otherwise appropriate adult patients (eg, adult patient with controlled hypertension and a soft tissue infection).
USE REPETITIVE STAKEHOLDER ANALYSIS TO ADAPT TO A RAPIDLY CHANGING ENVIRONMENT
The pandemic response was rapidly evolving and unpredictable. Planning required all affected parties at the table to effectively identify problems and solutions. Clinical and nonclinical groups were critical to planning operational logistics to provide safe care for adults in pediatric facilities.
COMMUNICATE WITH INTENTION AND TRANSPARENCY: WHEN LESS IS NOT MORE
Across care settings and training levels, the power of timely, honest, and transparent communication with leadership echoed throughout the network and could not be overemphasized. The cadence and modes of communication, while established by facility leaders, was best determined by explicitly asking team members for their needs. Often, leaders attempted to avoid communicating abrupt protocol changes to spare their teams additional stress and excessive correspondence. However, POPCoRN members found this approach often increased the perception among staff of a lack of transparency, which exacerbated feelings of discomfort and stress. While other specific examples of communication strategies are included in the POPCoRN guide, network members consistently noted that virtual open forums with leadership at regular intervals allowed teams to ask questions, raise concerns, and share ideas. In addition to open forums, leaders’ written communications regarding local medicolegal limitations and malpractice protection related to adult care should be distributed to staff. In Maria’s case, would provider discomfort and anxiety have been ameliorated with a proactive open forum to discuss the care of adults at the pediatric facility? Would that forum have called attention to staff educational and preparation needs around taking care of adults with a history substance use disorder? If so, this may have added a downstream benefit of decreasing effects of implicit bias amplified by stress.10
MAKE “JUST-IN-TIME” RESOURCES AVAILABLE FOR PEDIATRICIANS CARING FOR ADULT PATIENTS
DESIGN AN EMERGENCY RESPONSE SYSTEM FOR ADULT PATIENTS IN PEDIATRIC FACILITIES
CONCLUSION
Acknowledgments
Collaborators: All the collaborating authors listed below have contributed to the guide available in the appendix of the online version of this article, “Lessons Learned From COVID-19: A Practical Guide for Pediatric Facility Preparedness and Repurposing.” All the authors have provided consent to be listed.
Francisco Alvarez, MD, Stanford, California; Elizabeth Boggs, MD, MS, Aurora, Colorado; Rachel Boykan, MD, Stony Brook, New York; Alicia Caldwell, MD, Cincinnati, Ohio; Maryanne M. Chumpia, MD, MS, Torrance, California; Katharine N Clouser, MD, Hackensack, New Jersey; Alexandra L Coria, MD, Brooklyn, New York; Clare C Crosh, DO, Cincinnati, Ohio; Magna Dias, MD, Bridgeport, Connecticut; Laura N El-Hage, MD, Philadelphia, Pennsylvania; Jeff Foti, MD, Seattle, Washington; Mirna Giordano, MD, New York, New York; Sheena Gupta, MD, MBA, Evanston, Illinois; Laura Nell Hodo, MD, New York, New York; Ashley Jenkins, MD, MS, Rochester, New York; Anika Kumar, MD, Cleveland, Ohio; Merlin C Lowe, MD, Tuscon, Arizona; Brittany Middleton, MD, Pasadena, California; Sage Myers, MD, Philadelphia, Pennsylvania; Anik Patel, MD, Salt Lake City, Utah; Leah Ratner, MD, MS, Boston, Massachusetts; Shela Sridhar, MD, MPH, Boston, Massachusetts; Nathan Stehouwer, MD, Cleveland, Ohio; Julie Sylvester, DO, Mount Kisco, New York; Dava Szalda, MD, MSHP, Philadelphia, Pennsylvania; Heather Toth, MD, Milwaukee, Wisconsin; Krista Tuomela, MD, Milwaukee, Wisconsin; Ronald Williams, MD, Hershey, Pennsylvania.
1. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. https://doi.org/10.1542/peds.2020-0702
2. Osborn R, Doolittle B, Loyal J. When pediatric hospitalists took care of adults during the COVID-19 pandemic. Hosp Pediatr. 2021;11(1):e15-e18. https://doi.org/10.1542/hpeds.2020-001040
3. Yager PH, Whalen KA, Cummings BM. Repurposing a pediatric ICU for adults. N Engl J Med. 2020;382(22):e80. https://doi.org/10.1056/NEJMc2014819
4. Conway-Habes EE, Herbst BF Jr, Herbst LA, et al. Using quality improvement to introduce and standardize the National Early Warning Score (NEWS) for adult inpatients at a children’s hospital. Hosp Pediatr. 2017;7(3):156-163. https://doi.org/10.1542/hpeds.2016-0117
5. Kinnear B, O’Toole JK. Care of adults in children’s hospitals: acknowledging the aging elephant in the room. JAMA Pediatr. 2015;169(12):1081-1082. https://doi.org/10.1001/jamapediatrics.2015.2215
6. Szalda D, Steinway C, Greenberg A, et al. Developing a hospital-wide transition program for young adults with medical complexity. J Adolesc Health. 2019;65(4):476-482. https://doi.org/10.1016/j.jadohealth.2019.04.008
7. Jenkins A, Ratner L, Caldwell A, Sharma N, Uluer A, White C. Children’s hospitals caring for adults during a pandemic: pragmatic considerations and approaches. J Hosp Med. 2020;15(5):311-313. https://doi.org/10.12788/jhm.3432
8. Botwinick L, Bisognano M, Haraden C. Leadership Guide to Patient Safety. IHI Innovation Series white paper. Cambridge, MA: Institute for Healthcare Improvement; 2006. Accessed January 20, 2021. http://www.ihi.org/resources/Pages/IHIWhitePapers/LeadershipGuidetoPatientSafetyWhitePaper.aspx
9. Essien UR, Eneanya ND, Crews DC. Prioritizing equity in a time of scarcity: the COVID-19 pandemic. J Gen Intern Med. 2020;35(9):2760-2762. https://doi.org/10.1007/s11606-020-05976-y
10. Yu R. Stress potentiates decision biases: a stress induced deliberation-to-intuition (SIDI) model. Neurobiol Stress. 2016;3:83-95. https://doi.org/10.1016/j.ynstr.2015.12.006
1. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. https://doi.org/10.1542/peds.2020-0702
2. Osborn R, Doolittle B, Loyal J. When pediatric hospitalists took care of adults during the COVID-19 pandemic. Hosp Pediatr. 2021;11(1):e15-e18. https://doi.org/10.1542/hpeds.2020-001040
3. Yager PH, Whalen KA, Cummings BM. Repurposing a pediatric ICU for adults. N Engl J Med. 2020;382(22):e80. https://doi.org/10.1056/NEJMc2014819
4. Conway-Habes EE, Herbst BF Jr, Herbst LA, et al. Using quality improvement to introduce and standardize the National Early Warning Score (NEWS) for adult inpatients at a children’s hospital. Hosp Pediatr. 2017;7(3):156-163. https://doi.org/10.1542/hpeds.2016-0117
5. Kinnear B, O’Toole JK. Care of adults in children’s hospitals: acknowledging the aging elephant in the room. JAMA Pediatr. 2015;169(12):1081-1082. https://doi.org/10.1001/jamapediatrics.2015.2215
6. Szalda D, Steinway C, Greenberg A, et al. Developing a hospital-wide transition program for young adults with medical complexity. J Adolesc Health. 2019;65(4):476-482. https://doi.org/10.1016/j.jadohealth.2019.04.008
7. Jenkins A, Ratner L, Caldwell A, Sharma N, Uluer A, White C. Children’s hospitals caring for adults during a pandemic: pragmatic considerations and approaches. J Hosp Med. 2020;15(5):311-313. https://doi.org/10.12788/jhm.3432
8. Botwinick L, Bisognano M, Haraden C. Leadership Guide to Patient Safety. IHI Innovation Series white paper. Cambridge, MA: Institute for Healthcare Improvement; 2006. Accessed January 20, 2021. http://www.ihi.org/resources/Pages/IHIWhitePapers/LeadershipGuidetoPatientSafetyWhitePaper.aspx
9. Essien UR, Eneanya ND, Crews DC. Prioritizing equity in a time of scarcity: the COVID-19 pandemic. J Gen Intern Med. 2020;35(9):2760-2762. https://doi.org/10.1007/s11606-020-05976-y
10. Yu R. Stress potentiates decision biases: a stress induced deliberation-to-intuition (SIDI) model. Neurobiol Stress. 2016;3:83-95. https://doi.org/10.1016/j.ynstr.2015.12.006
© 2021 Society of Hospital Medicine
Defining Potential Overutilization of Physical Therapy Consults on Hospital Medicine Services
During hospitalization, patients spend 87% to 100% of their time in bed.1 This prolonged immobilization is a key contributor to the development of hospital-associated disability (HAD), defined as a new loss of ability to complete one or more activities of daily living (ADLs) without assistance after hospital discharge. HAD can lead to readmissions, institutionalization, and death and occurs in approximately one-third of all hospitalized patients.2,3 The most effective way to prevent HAD is by mobilizing patients early and throughout their hospitalization.4 Typically, physical therapists are the primary team members responsible for mobilizing patients, but they are a constrained resource in most inpatient settings.
The Activity Measure-Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a validated tool for measuring physical function.5 The AM-PAC score has been used to predict discharge destination within 48 hours of admission6 and as a guide to allocate inpatient therapy referrals on a medical and a neurosurgical service.7,8 To date, however, no studies have used AM-PAC scores to evaluate overutilization of physical therapy consults on direct care hospital medicine services. In this study, we aimed to assess the potential overutilization of physical therapy consults on direct care hospital medicine services using validated AM-PAC score cutoffs.
METHODS
Study Design and Setting
We analyzed a retrospective cohort of admissions from September 30, 2018, through September 29, 2019, on all direct care hospital medicine services at the University of Chicago Medical Center (UC), Illinois. These services included general medicine, oncology, transplant (renal, lung, and liver), cardiology, and cirrhotic populations at the medical-surgical and telemetry level of care. All patients were hospitalized for longer than 48 hours. Patients who left against medical advice; died; were discharged to hospice, another hospital, or an inpatient psychiatric facility; or received no physical therapy referral during admission were excluded. For the remaining patients, we obtained age, sex, admission and discharge dates, admission and discharge AM-PAC scores, and discharge disposition.
Mobility Measure
At UC, the inpatient mobility protocol requires nursing staff to assess and document AM-PAC mobility scores for each patient at the time of admission and every nursing shift thereafter. They utilize the original version of the AM-PAC “6-Clicks” Basic Mobility score, which includes three questions assessing difficulty with mobility and three questions assessing help needed with mobility activities. It has high interrater reliability, with an intraclass correlation coefficient of 0.85.9
Outcomes and Predictors
The primary outcome was “potential overutilization.” Secondary outcomes were discharge disposition and change in mobility. Our predictors included admission AM-PAC score, age, and sex. Based on previous studies that validated an AM-PAC score of 42.9 (raw score, 17) as a cutoff for predicting discharge to home,6 we defined physical therapy consults as “potentially inappropriate” in patients with admission AM-PAC scores >43.63 (raw score, 18) who were discharged to home. Likewise, in the UC mobility protocol, nursing staff independently mobilize patients with AM-PAC scores >18, another rationale to use this cutoff for defining physical therapy consult inappropriateness. “Discharge to home” was defined as going home with no additional needs or services, going home with outpatient physical therapy, or going home with home health physical therapy services, since none of these require inpatient physical therapy assessment for the order to be placed. Discharge to long-term acute care, skilled nursing facility, subacute rehabilitation facility, or acute rehabilitation facility were considered “discharge to post–acute care.” Loss of mobility was calculated as: discharge AM-PAC − admission AM-PAC, termed delta AM-PAC.
Statistical Analysis
Descriptive statistics were used to summarize age (mean and SD) and age categorized as <65 years or ≥65 years, sex (male or female), admission AM-PAC score (mean and SD) and categorization (≤43.63 or >43.63), discharge AM-PAC score (mean and SD), and discharge destination (home vs post–acute care). Chi-square analysis was used to test for associations between admission AM-PAC score and delta AM-PAC. Two-sample t-test was used to test for difference in mean delta AM-PAC between admission AM-PAC groups. Multivariable logistic regression was used to test for independent associations between age, sex, and admission AM-PAC score and odds of being discharged to home, controlling for length of stay. P values of <.05 were considered statistically significant for all tests. Analyses were performed using Stata statistical software, release 16 (StataCorp LLC).
RESULTS
During the 1-year study period, 3592 admissions with physical therapy consults occurred on the direct care hospital medicine services (58% of all admissions). Mean age was 66.3 years (SD, 15.4 years), and 48% of patients were female. The mean admission AM-PAC score was 43.9 (SD, 11.1), and the mean discharge AM-PAC score was 46.8 (SD, 10.8). In our sample, 38% of physical therapy consults were for patients with an AM-PAC score >43.63 who were discharged to home and were therefore deemed “potential overutilization.” Of those, 40% were for patients who were 65 years or younger (18% of all physical therapy consults) (Table 1).
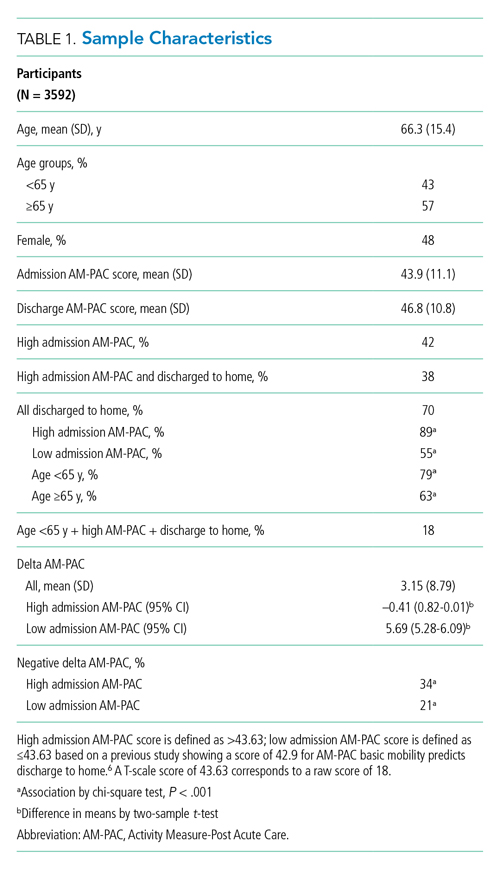
A higher proportion of patients with AM-PAC scores >43.63 were discharged to home compared with those with AM-PAC scores ≤43.63 (89% vs 55%; χ2 [1, N = 3099], 396.5; P < .001). More patients younger than 65 years were discharged to home compared with those 65 years and older (79% vs 63%; χ2 [1, N = 3099], 113.6; P < .001). Additionally, for all patients younger than 65 years, those with AM-PAC score >43.63 were discharged to home more frequently than those with AM-PAC ≤43.63 (92% vs 66%, χ2 [1, N = 1,354], 134.4; P < .001). For 11% (n = 147) of the high-mobility group, the patient was not discharged home but was sent to post–acute care. Reviewing these patient charts showed the reasons for discharge to post–acute care were predominantly personal or social needs (eg, homelessness, need for 24-hour supervision with no family support, patient request) or medical needs (eg, intravenous antibiotics or new tubes, lines, drains, or medications requiring extra nursing support or management). Only 16% of patients in this group (n = 23) experienced deconditioning necessitating physical therapy consult during hospitalization, per their record.
Compared with patients with admission AM-PAC score >43.63, patients with admission AM-PAC ≤43.63 had significantly different changes in mobility as measured by mean delta AM-PAC score (delta AM-PAC, –0.41 for AM-PAC >43.63 vs +5.69 for AM-PAC ≤43.63; t (3097) = –20.3; P < .001) (Table 1).
In multivariate logistic regression, AM-PAC >43.63 (OR, 5.38; 95% CI, 4.36-2.89; P < .001) and age younger than 65 years (OR, 2.40; 95% CI, 1.99-2.90; P < .001) were associated with increased odds of discharge to home (Table 2).
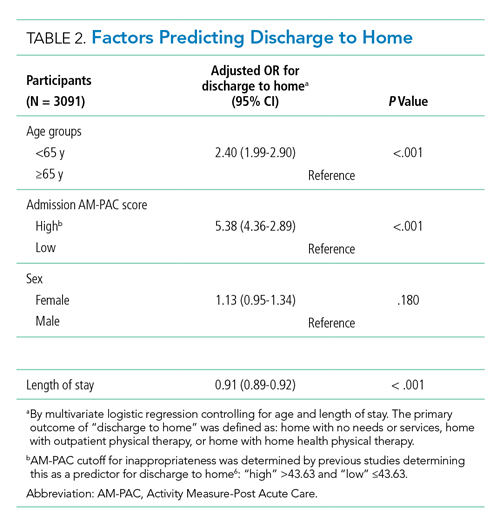
DISCUSSION
In this study, we found that physical therapists may be unnecessarily consulted on direct care hospitalist services as much as 38% of the time based on AM-PAC score. We also demonstrated that patients admitted with high mobility by AM-PAC score are more than five times as likely to be discharged to home. When admitted with high AM-PAC scores, patients had virtually no change in mobility during hospitalization, whereas patients with low AM-PAC scores gained mobility during hospitalization, underscoring the benefit of physical therapy referrals for this group.
Given resource scarcity and cost, achieving optimal physical therapy utilization is an important goal for healthcare systems.10 Appropriate allocation of physical therapy has the potential to improve outcomes from the patient to the payor level. While it may be necessary to consult physical therapy for reasons other than mobility later in the hospitalization, identifying patients who will benefit from skilled physical therapy at the time of admission can help prevent disability and institutionalization and shorten length of stay.5,6 Likewise, decreasing physical therapy referrals for low-risk patients can increase the amount of time spent rehabilitating at-risk patients.
There are limitations of our study worth considering. First, our analyses did not consider whether physical therapy contributed to patients’ ability to return home after discharge. However, in our hospital, patients with AM-PAC >43.63 who cannot safely ambulate independently do progressive mobility with nursing staff. Our physical therapy leadership has also observed that the vast majority of highly mobile patients who are referred for physical therapy ultimately receive no treatment. Second, we did not consider discharge diagnosis, but our patient populations present with a wide variety of conditions, and it is impossible to predict their discharge diagnosis. By not including discharge diagnosis, we assess how AM-PAC performs on admission regardless of the medical condition for which someone is treated. Our hospital treats a high proportion of African American and a low proportion of White, Hispanic, and Asian American patients, limiting the generalizability of our findings. Although the AM-PAC “6-Clicks” score has been shown to have high interrater reliability among physical therapists, our AM-PAC scores are assessed and documented by our nursing staff, which might decrease accuracy. However, one single-center study noted an intraclass correlation coefficient of 0.96 between nurses and physical therapists for the AM-PAC “6-Clicks.”11Despite these limitations, this study underscores the need to be more judicious in the decision to refer a patient for inpatient physical therapy, especially at the time of admission, and demonstrates the utility of using standardized mobility assessment to help in that decision-making process.
1. Fazio S, Stocking J, Kuhn B, et al. How much do hospitalized adults move? A systematic review and meta-analysis. Appl Nurs Res. 2020;51:151189. https://doi.org/10.1016/j.apnr.2019.151189
2. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x
3. Brown C.J, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52:1263-1270. https://doi.org/10.1111/j.1532-5415.2004.52354.x
4. Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63:55-62. https://doi.org/10.1111/jgs.13193
5. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94(3):379-391. https://doi.org/10.2522/ptj.20130199
6. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94(9):1252-1261. https://doi.org/10.2522/ptj.20130359
7. Probasco JC, Lavezza A, Cassell A, et al. Choosing wisely together: physical and occupational therapy consultation for acute neurology inpatients. Neurohospitalist. 2018;8(2):53-59. https://doi.org/10.1177/1941874417729981
8. Young DL, Colantuoni E, Friedman LA, et al. Prediction of disposition within 48 hours of hospital admission using patient mobility scores. J Hosp Med. 2020;15(9);540-543. https://doi.org/10.12788/jhm.3332
9. Jette DU, Stilphen M, Ranganathan VK, Passek S, Frost FS, Jette AM. Interrater reliability of AM-PAC “6-Clicks” basic mobility and daily activity short forms. Phys Ther. 2015;95(5):758-766. https://doi.org/10.2522/ptj.20140174
10. Juneau A, Bolduc A, Nguyen P, et al. Feasibility of implementing an exercise program in a geriatric assessment unit: the SPRINT program. Can Geriatr J. 2018;21(3):284-289. https://doi.org/10.5770/cgj.21.311
11. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110
During hospitalization, patients spend 87% to 100% of their time in bed.1 This prolonged immobilization is a key contributor to the development of hospital-associated disability (HAD), defined as a new loss of ability to complete one or more activities of daily living (ADLs) without assistance after hospital discharge. HAD can lead to readmissions, institutionalization, and death and occurs in approximately one-third of all hospitalized patients.2,3 The most effective way to prevent HAD is by mobilizing patients early and throughout their hospitalization.4 Typically, physical therapists are the primary team members responsible for mobilizing patients, but they are a constrained resource in most inpatient settings.
The Activity Measure-Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a validated tool for measuring physical function.5 The AM-PAC score has been used to predict discharge destination within 48 hours of admission6 and as a guide to allocate inpatient therapy referrals on a medical and a neurosurgical service.7,8 To date, however, no studies have used AM-PAC scores to evaluate overutilization of physical therapy consults on direct care hospital medicine services. In this study, we aimed to assess the potential overutilization of physical therapy consults on direct care hospital medicine services using validated AM-PAC score cutoffs.
METHODS
Study Design and Setting
We analyzed a retrospective cohort of admissions from September 30, 2018, through September 29, 2019, on all direct care hospital medicine services at the University of Chicago Medical Center (UC), Illinois. These services included general medicine, oncology, transplant (renal, lung, and liver), cardiology, and cirrhotic populations at the medical-surgical and telemetry level of care. All patients were hospitalized for longer than 48 hours. Patients who left against medical advice; died; were discharged to hospice, another hospital, or an inpatient psychiatric facility; or received no physical therapy referral during admission were excluded. For the remaining patients, we obtained age, sex, admission and discharge dates, admission and discharge AM-PAC scores, and discharge disposition.
Mobility Measure
At UC, the inpatient mobility protocol requires nursing staff to assess and document AM-PAC mobility scores for each patient at the time of admission and every nursing shift thereafter. They utilize the original version of the AM-PAC “6-Clicks” Basic Mobility score, which includes three questions assessing difficulty with mobility and three questions assessing help needed with mobility activities. It has high interrater reliability, with an intraclass correlation coefficient of 0.85.9
Outcomes and Predictors
The primary outcome was “potential overutilization.” Secondary outcomes were discharge disposition and change in mobility. Our predictors included admission AM-PAC score, age, and sex. Based on previous studies that validated an AM-PAC score of 42.9 (raw score, 17) as a cutoff for predicting discharge to home,6 we defined physical therapy consults as “potentially inappropriate” in patients with admission AM-PAC scores >43.63 (raw score, 18) who were discharged to home. Likewise, in the UC mobility protocol, nursing staff independently mobilize patients with AM-PAC scores >18, another rationale to use this cutoff for defining physical therapy consult inappropriateness. “Discharge to home” was defined as going home with no additional needs or services, going home with outpatient physical therapy, or going home with home health physical therapy services, since none of these require inpatient physical therapy assessment for the order to be placed. Discharge to long-term acute care, skilled nursing facility, subacute rehabilitation facility, or acute rehabilitation facility were considered “discharge to post–acute care.” Loss of mobility was calculated as: discharge AM-PAC − admission AM-PAC, termed delta AM-PAC.
Statistical Analysis
Descriptive statistics were used to summarize age (mean and SD) and age categorized as <65 years or ≥65 years, sex (male or female), admission AM-PAC score (mean and SD) and categorization (≤43.63 or >43.63), discharge AM-PAC score (mean and SD), and discharge destination (home vs post–acute care). Chi-square analysis was used to test for associations between admission AM-PAC score and delta AM-PAC. Two-sample t-test was used to test for difference in mean delta AM-PAC between admission AM-PAC groups. Multivariable logistic regression was used to test for independent associations between age, sex, and admission AM-PAC score and odds of being discharged to home, controlling for length of stay. P values of <.05 were considered statistically significant for all tests. Analyses were performed using Stata statistical software, release 16 (StataCorp LLC).
RESULTS
During the 1-year study period, 3592 admissions with physical therapy consults occurred on the direct care hospital medicine services (58% of all admissions). Mean age was 66.3 years (SD, 15.4 years), and 48% of patients were female. The mean admission AM-PAC score was 43.9 (SD, 11.1), and the mean discharge AM-PAC score was 46.8 (SD, 10.8). In our sample, 38% of physical therapy consults were for patients with an AM-PAC score >43.63 who were discharged to home and were therefore deemed “potential overutilization.” Of those, 40% were for patients who were 65 years or younger (18% of all physical therapy consults) (Table 1).

A higher proportion of patients with AM-PAC scores >43.63 were discharged to home compared with those with AM-PAC scores ≤43.63 (89% vs 55%; χ2 [1, N = 3099], 396.5; P < .001). More patients younger than 65 years were discharged to home compared with those 65 years and older (79% vs 63%; χ2 [1, N = 3099], 113.6; P < .001). Additionally, for all patients younger than 65 years, those with AM-PAC score >43.63 were discharged to home more frequently than those with AM-PAC ≤43.63 (92% vs 66%, χ2 [1, N = 1,354], 134.4; P < .001). For 11% (n = 147) of the high-mobility group, the patient was not discharged home but was sent to post–acute care. Reviewing these patient charts showed the reasons for discharge to post–acute care were predominantly personal or social needs (eg, homelessness, need for 24-hour supervision with no family support, patient request) or medical needs (eg, intravenous antibiotics or new tubes, lines, drains, or medications requiring extra nursing support or management). Only 16% of patients in this group (n = 23) experienced deconditioning necessitating physical therapy consult during hospitalization, per their record.
Compared with patients with admission AM-PAC score >43.63, patients with admission AM-PAC ≤43.63 had significantly different changes in mobility as measured by mean delta AM-PAC score (delta AM-PAC, –0.41 for AM-PAC >43.63 vs +5.69 for AM-PAC ≤43.63; t (3097) = –20.3; P < .001) (Table 1).
In multivariate logistic regression, AM-PAC >43.63 (OR, 5.38; 95% CI, 4.36-2.89; P < .001) and age younger than 65 years (OR, 2.40; 95% CI, 1.99-2.90; P < .001) were associated with increased odds of discharge to home (Table 2).

DISCUSSION
In this study, we found that physical therapists may be unnecessarily consulted on direct care hospitalist services as much as 38% of the time based on AM-PAC score. We also demonstrated that patients admitted with high mobility by AM-PAC score are more than five times as likely to be discharged to home. When admitted with high AM-PAC scores, patients had virtually no change in mobility during hospitalization, whereas patients with low AM-PAC scores gained mobility during hospitalization, underscoring the benefit of physical therapy referrals for this group.
Given resource scarcity and cost, achieving optimal physical therapy utilization is an important goal for healthcare systems.10 Appropriate allocation of physical therapy has the potential to improve outcomes from the patient to the payor level. While it may be necessary to consult physical therapy for reasons other than mobility later in the hospitalization, identifying patients who will benefit from skilled physical therapy at the time of admission can help prevent disability and institutionalization and shorten length of stay.5,6 Likewise, decreasing physical therapy referrals for low-risk patients can increase the amount of time spent rehabilitating at-risk patients.
There are limitations of our study worth considering. First, our analyses did not consider whether physical therapy contributed to patients’ ability to return home after discharge. However, in our hospital, patients with AM-PAC >43.63 who cannot safely ambulate independently do progressive mobility with nursing staff. Our physical therapy leadership has also observed that the vast majority of highly mobile patients who are referred for physical therapy ultimately receive no treatment. Second, we did not consider discharge diagnosis, but our patient populations present with a wide variety of conditions, and it is impossible to predict their discharge diagnosis. By not including discharge diagnosis, we assess how AM-PAC performs on admission regardless of the medical condition for which someone is treated. Our hospital treats a high proportion of African American and a low proportion of White, Hispanic, and Asian American patients, limiting the generalizability of our findings. Although the AM-PAC “6-Clicks” score has been shown to have high interrater reliability among physical therapists, our AM-PAC scores are assessed and documented by our nursing staff, which might decrease accuracy. However, one single-center study noted an intraclass correlation coefficient of 0.96 between nurses and physical therapists for the AM-PAC “6-Clicks.”11Despite these limitations, this study underscores the need to be more judicious in the decision to refer a patient for inpatient physical therapy, especially at the time of admission, and demonstrates the utility of using standardized mobility assessment to help in that decision-making process.
During hospitalization, patients spend 87% to 100% of their time in bed.1 This prolonged immobilization is a key contributor to the development of hospital-associated disability (HAD), defined as a new loss of ability to complete one or more activities of daily living (ADLs) without assistance after hospital discharge. HAD can lead to readmissions, institutionalization, and death and occurs in approximately one-third of all hospitalized patients.2,3 The most effective way to prevent HAD is by mobilizing patients early and throughout their hospitalization.4 Typically, physical therapists are the primary team members responsible for mobilizing patients, but they are a constrained resource in most inpatient settings.
The Activity Measure-Post Acute Care Inpatient Mobility Short Form (AM-PAC IMSF) is a validated tool for measuring physical function.5 The AM-PAC score has been used to predict discharge destination within 48 hours of admission6 and as a guide to allocate inpatient therapy referrals on a medical and a neurosurgical service.7,8 To date, however, no studies have used AM-PAC scores to evaluate overutilization of physical therapy consults on direct care hospital medicine services. In this study, we aimed to assess the potential overutilization of physical therapy consults on direct care hospital medicine services using validated AM-PAC score cutoffs.
METHODS
Study Design and Setting
We analyzed a retrospective cohort of admissions from September 30, 2018, through September 29, 2019, on all direct care hospital medicine services at the University of Chicago Medical Center (UC), Illinois. These services included general medicine, oncology, transplant (renal, lung, and liver), cardiology, and cirrhotic populations at the medical-surgical and telemetry level of care. All patients were hospitalized for longer than 48 hours. Patients who left against medical advice; died; were discharged to hospice, another hospital, or an inpatient psychiatric facility; or received no physical therapy referral during admission were excluded. For the remaining patients, we obtained age, sex, admission and discharge dates, admission and discharge AM-PAC scores, and discharge disposition.
Mobility Measure
At UC, the inpatient mobility protocol requires nursing staff to assess and document AM-PAC mobility scores for each patient at the time of admission and every nursing shift thereafter. They utilize the original version of the AM-PAC “6-Clicks” Basic Mobility score, which includes three questions assessing difficulty with mobility and three questions assessing help needed with mobility activities. It has high interrater reliability, with an intraclass correlation coefficient of 0.85.9
Outcomes and Predictors
The primary outcome was “potential overutilization.” Secondary outcomes were discharge disposition and change in mobility. Our predictors included admission AM-PAC score, age, and sex. Based on previous studies that validated an AM-PAC score of 42.9 (raw score, 17) as a cutoff for predicting discharge to home,6 we defined physical therapy consults as “potentially inappropriate” in patients with admission AM-PAC scores >43.63 (raw score, 18) who were discharged to home. Likewise, in the UC mobility protocol, nursing staff independently mobilize patients with AM-PAC scores >18, another rationale to use this cutoff for defining physical therapy consult inappropriateness. “Discharge to home” was defined as going home with no additional needs or services, going home with outpatient physical therapy, or going home with home health physical therapy services, since none of these require inpatient physical therapy assessment for the order to be placed. Discharge to long-term acute care, skilled nursing facility, subacute rehabilitation facility, or acute rehabilitation facility were considered “discharge to post–acute care.” Loss of mobility was calculated as: discharge AM-PAC − admission AM-PAC, termed delta AM-PAC.
Statistical Analysis
Descriptive statistics were used to summarize age (mean and SD) and age categorized as <65 years or ≥65 years, sex (male or female), admission AM-PAC score (mean and SD) and categorization (≤43.63 or >43.63), discharge AM-PAC score (mean and SD), and discharge destination (home vs post–acute care). Chi-square analysis was used to test for associations between admission AM-PAC score and delta AM-PAC. Two-sample t-test was used to test for difference in mean delta AM-PAC between admission AM-PAC groups. Multivariable logistic regression was used to test for independent associations between age, sex, and admission AM-PAC score and odds of being discharged to home, controlling for length of stay. P values of <.05 were considered statistically significant for all tests. Analyses were performed using Stata statistical software, release 16 (StataCorp LLC).
RESULTS
During the 1-year study period, 3592 admissions with physical therapy consults occurred on the direct care hospital medicine services (58% of all admissions). Mean age was 66.3 years (SD, 15.4 years), and 48% of patients were female. The mean admission AM-PAC score was 43.9 (SD, 11.1), and the mean discharge AM-PAC score was 46.8 (SD, 10.8). In our sample, 38% of physical therapy consults were for patients with an AM-PAC score >43.63 who were discharged to home and were therefore deemed “potential overutilization.” Of those, 40% were for patients who were 65 years or younger (18% of all physical therapy consults) (Table 1).

A higher proportion of patients with AM-PAC scores >43.63 were discharged to home compared with those with AM-PAC scores ≤43.63 (89% vs 55%; χ2 [1, N = 3099], 396.5; P < .001). More patients younger than 65 years were discharged to home compared with those 65 years and older (79% vs 63%; χ2 [1, N = 3099], 113.6; P < .001). Additionally, for all patients younger than 65 years, those with AM-PAC score >43.63 were discharged to home more frequently than those with AM-PAC ≤43.63 (92% vs 66%, χ2 [1, N = 1,354], 134.4; P < .001). For 11% (n = 147) of the high-mobility group, the patient was not discharged home but was sent to post–acute care. Reviewing these patient charts showed the reasons for discharge to post–acute care were predominantly personal or social needs (eg, homelessness, need for 24-hour supervision with no family support, patient request) or medical needs (eg, intravenous antibiotics or new tubes, lines, drains, or medications requiring extra nursing support or management). Only 16% of patients in this group (n = 23) experienced deconditioning necessitating physical therapy consult during hospitalization, per their record.
Compared with patients with admission AM-PAC score >43.63, patients with admission AM-PAC ≤43.63 had significantly different changes in mobility as measured by mean delta AM-PAC score (delta AM-PAC, –0.41 for AM-PAC >43.63 vs +5.69 for AM-PAC ≤43.63; t (3097) = –20.3; P < .001) (Table 1).
In multivariate logistic regression, AM-PAC >43.63 (OR, 5.38; 95% CI, 4.36-2.89; P < .001) and age younger than 65 years (OR, 2.40; 95% CI, 1.99-2.90; P < .001) were associated with increased odds of discharge to home (Table 2).

DISCUSSION
In this study, we found that physical therapists may be unnecessarily consulted on direct care hospitalist services as much as 38% of the time based on AM-PAC score. We also demonstrated that patients admitted with high mobility by AM-PAC score are more than five times as likely to be discharged to home. When admitted with high AM-PAC scores, patients had virtually no change in mobility during hospitalization, whereas patients with low AM-PAC scores gained mobility during hospitalization, underscoring the benefit of physical therapy referrals for this group.
Given resource scarcity and cost, achieving optimal physical therapy utilization is an important goal for healthcare systems.10 Appropriate allocation of physical therapy has the potential to improve outcomes from the patient to the payor level. While it may be necessary to consult physical therapy for reasons other than mobility later in the hospitalization, identifying patients who will benefit from skilled physical therapy at the time of admission can help prevent disability and institutionalization and shorten length of stay.5,6 Likewise, decreasing physical therapy referrals for low-risk patients can increase the amount of time spent rehabilitating at-risk patients.
There are limitations of our study worth considering. First, our analyses did not consider whether physical therapy contributed to patients’ ability to return home after discharge. However, in our hospital, patients with AM-PAC >43.63 who cannot safely ambulate independently do progressive mobility with nursing staff. Our physical therapy leadership has also observed that the vast majority of highly mobile patients who are referred for physical therapy ultimately receive no treatment. Second, we did not consider discharge diagnosis, but our patient populations present with a wide variety of conditions, and it is impossible to predict their discharge diagnosis. By not including discharge diagnosis, we assess how AM-PAC performs on admission regardless of the medical condition for which someone is treated. Our hospital treats a high proportion of African American and a low proportion of White, Hispanic, and Asian American patients, limiting the generalizability of our findings. Although the AM-PAC “6-Clicks” score has been shown to have high interrater reliability among physical therapists, our AM-PAC scores are assessed and documented by our nursing staff, which might decrease accuracy. However, one single-center study noted an intraclass correlation coefficient of 0.96 between nurses and physical therapists for the AM-PAC “6-Clicks.”11Despite these limitations, this study underscores the need to be more judicious in the decision to refer a patient for inpatient physical therapy, especially at the time of admission, and demonstrates the utility of using standardized mobility assessment to help in that decision-making process.
1. Fazio S, Stocking J, Kuhn B, et al. How much do hospitalized adults move? A systematic review and meta-analysis. Appl Nurs Res. 2020;51:151189. https://doi.org/10.1016/j.apnr.2019.151189
2. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x
3. Brown C.J, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52:1263-1270. https://doi.org/10.1111/j.1532-5415.2004.52354.x
4. Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63:55-62. https://doi.org/10.1111/jgs.13193
5. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94(3):379-391. https://doi.org/10.2522/ptj.20130199
6. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94(9):1252-1261. https://doi.org/10.2522/ptj.20130359
7. Probasco JC, Lavezza A, Cassell A, et al. Choosing wisely together: physical and occupational therapy consultation for acute neurology inpatients. Neurohospitalist. 2018;8(2):53-59. https://doi.org/10.1177/1941874417729981
8. Young DL, Colantuoni E, Friedman LA, et al. Prediction of disposition within 48 hours of hospital admission using patient mobility scores. J Hosp Med. 2020;15(9);540-543. https://doi.org/10.12788/jhm.3332
9. Jette DU, Stilphen M, Ranganathan VK, Passek S, Frost FS, Jette AM. Interrater reliability of AM-PAC “6-Clicks” basic mobility and daily activity short forms. Phys Ther. 2015;95(5):758-766. https://doi.org/10.2522/ptj.20140174
10. Juneau A, Bolduc A, Nguyen P, et al. Feasibility of implementing an exercise program in a geriatric assessment unit: the SPRINT program. Can Geriatr J. 2018;21(3):284-289. https://doi.org/10.5770/cgj.21.311
11. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110
1. Fazio S, Stocking J, Kuhn B, et al. How much do hospitalized adults move? A systematic review and meta-analysis. Appl Nurs Res. 2020;51:151189. https://doi.org/10.1016/j.apnr.2019.151189
2. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. https://doi.org/10.1111/j.1532-5415.2009.02393.x
3. Brown C.J, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52:1263-1270. https://doi.org/10.1111/j.1532-5415.2004.52354.x
4. Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63:55-62. https://doi.org/10.1111/jgs.13193
5. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94(3):379-391. https://doi.org/10.2522/ptj.20130199
6. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94(9):1252-1261. https://doi.org/10.2522/ptj.20130359
7. Probasco JC, Lavezza A, Cassell A, et al. Choosing wisely together: physical and occupational therapy consultation for acute neurology inpatients. Neurohospitalist. 2018;8(2):53-59. https://doi.org/10.1177/1941874417729981
8. Young DL, Colantuoni E, Friedman LA, et al. Prediction of disposition within 48 hours of hospital admission using patient mobility scores. J Hosp Med. 2020;15(9);540-543. https://doi.org/10.12788/jhm.3332
9. Jette DU, Stilphen M, Ranganathan VK, Passek S, Frost FS, Jette AM. Interrater reliability of AM-PAC “6-Clicks” basic mobility and daily activity short forms. Phys Ther. 2015;95(5):758-766. https://doi.org/10.2522/ptj.20140174
10. Juneau A, Bolduc A, Nguyen P, et al. Feasibility of implementing an exercise program in a geriatric assessment unit: the SPRINT program. Can Geriatr J. 2018;21(3):284-289. https://doi.org/10.5770/cgj.21.311
11. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142. https://doi.org/10.1093/ptj/pzx110
© 2021 Society of Hospital Medicine
Objective Measures of Physical Distancing in the Hospital During the COVID-19 Pandemic
The COVID-19 pandemic dramatically altered how healthcare providers care for hospitalized patients. Many hospitals provided physical-distancing guidance to minimize viral transmission and preserve personal protective equipment. This guidance informed clinician behavior on rounds and in workspaces.1 One study reported that clinicians maintained distance from patients by grouping medical interventions, utilizing telemedicine for rounding and consultations, and implementing respiratory isolation units (RIUs) to cohort patients with COVID-19.2
Although physical distancing is recommended during inpatient care, no study to date has used objective measures to quantify the degree to which clinical practice was influenced. We aimed to objectively quantify changes in 24-hour patient room–entries before and during the COVID-19 pandemic using data from existing heat sensors to assess differences in physical distancing in RIUs and general medicine units.
METHODS
Study Design
A single-institution study was conducted at the University of Chicago Medicine, Illinois. Room entries were compared between a general medicine unit that transitioned to an RIU (unit A/RIU) and four general medicine units (unit B) using 24-hour patient room–entry data. Unit A was commissioned as an RIU to care exclusively for patients with confirmed COVID-19 on March 25, 2020, and decommissioned on June 23, 2020. Unit B cared for patients under investigation (PUIs) for COVID-19 and patients admitted for other reasons. PUIs were transferred to the RIU if positive for COVID-19. Hospital visitor restrictions were implemented on March 14, 2020, and lifted on June 29, 2020. The University of Chicago Institutional Review Board granted this project an exempt determination.
Data Collection
From January 1, 2020, to August 10, 2020, room-entry data were collected using the PURELL SMARTLINK hand-hygiene system (GOJO Industries, Inc.). This hand-hygiene compliance system tracks unit-level sanitizer dispenses and total room entries and exits via body heat sensors. Similar to our prior studies, this study extracted heat-sensor data to monitor room entries.3,4
Data Analysis
Objective 24-hour room-entry data were analyzed for all units. Rooms with less than two daily entries were assumed to be unoccupied and excluded from the analysis. Hospital-wide physical-distancing guidance published on March 10, 2020, was used to delineate “prepandemic” and “pandemic” periods. Each department adopted these recommendations (eg, physical distancing, conducting prerounds virtually, limiting the number of people seeing patients, using iPads for virtual patient visits) as appropriate.
Interrupted time series analyses were used to examine room-entry changes before and during the pandemic. The segmented function in R 4.0.2 (R Core Team) was used to create a model and estimate final fitting parameters, uncertainties, and data breakpoints using a bootstrap restarting algorithm.5 The Davies test was used to determine statistical significance of breakpoints, which was defined as P < .05.
RESULTS
We examined data from January 1, 2020, to August 10, 2020, from 3283 patients who collectively experienced 655,615 room entries. Unit A/RIU cared for 395 patients during the prepandemic period and 542 patients during the pandemic period. Compared with patients from the prepandemic period, patients during the pandemic period were more likely to be Black (73.7% vs 77.9%) and less likely to be White (17.0% vs 8.7%) (P = .002); were less likely to have respiratory (19.8% vs 8.0%, P < .001) or gastrointestinal (12.4% vs 6.5%, P = .008) primary diagnoses; and had a higher mean case-mix index (1.74 vs 2.38, P < .001) (Table).
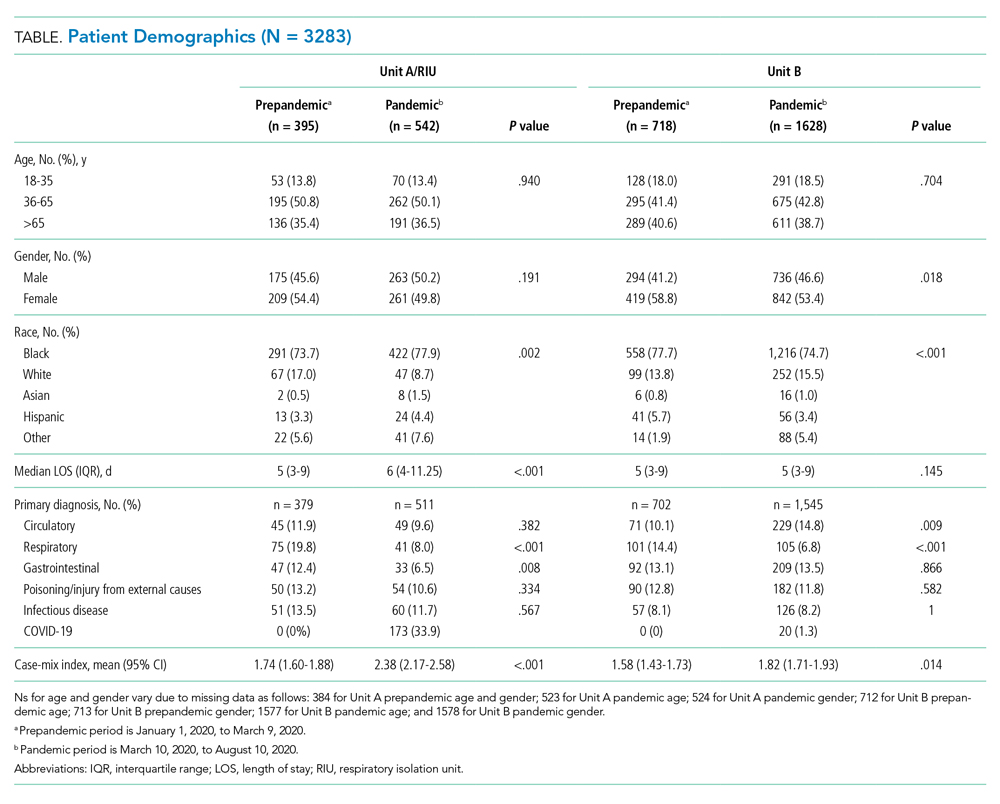
Unit B had 718 patients during the prepandemic period and 1628 patients during the pandemic period. Compared with patients from the prepandemic period, patients during the pandemic period were less likely to be female (58.8% vs 53.4%, P = .018); less likely to be Black (77.7% vs 74.7%) and Hispanic (5.7% vs 3.4%) (P < .001); more likely to have circulatory (10.1% vs 14.8%, P = .009) primary diagnoses; less likely to have respiratory (14.4% vs 6.8%, P < .001) primary diagnoses; and had a higher mean case-mix index (1.58 vs 1.82, P = .014) (Table).
During the prepandemic period, unit A/RIU averaged 27 occupied rooms per day. These rooms averaged 85.0 entries per room per day, with no statistically significant change over time. During the pandemic period, this unit averaged 24 daily occupied rooms, and these rooms averaged 44.4 entries per room per day. At the start of the pandemic, daily entries per room decreased by 51.9 (95% CI, 51.1-52.7). This equated to a 60.6% reduction from baseline (95% CI, 59.6%-61.5%; P < .001), with the lowest average occurring after RIU conversion on March 25, 2020 (letter F in Figure, A). Entries remained constant through the end of statewide stay-at-home orders (letter G in Figure, A) until RIU decommission on June 23, 2020 (letter H in Figure, A). Entries then increased by an average of 0.150 entries per room per day (95% CI, 0.097-0.202; P < .001), reaching 52.5 daily entries on August 10, 2020. This equated to 61.3% of prepandemic levels (95% CI, 61.3%-61.6%; P < .001) (Figure, A).
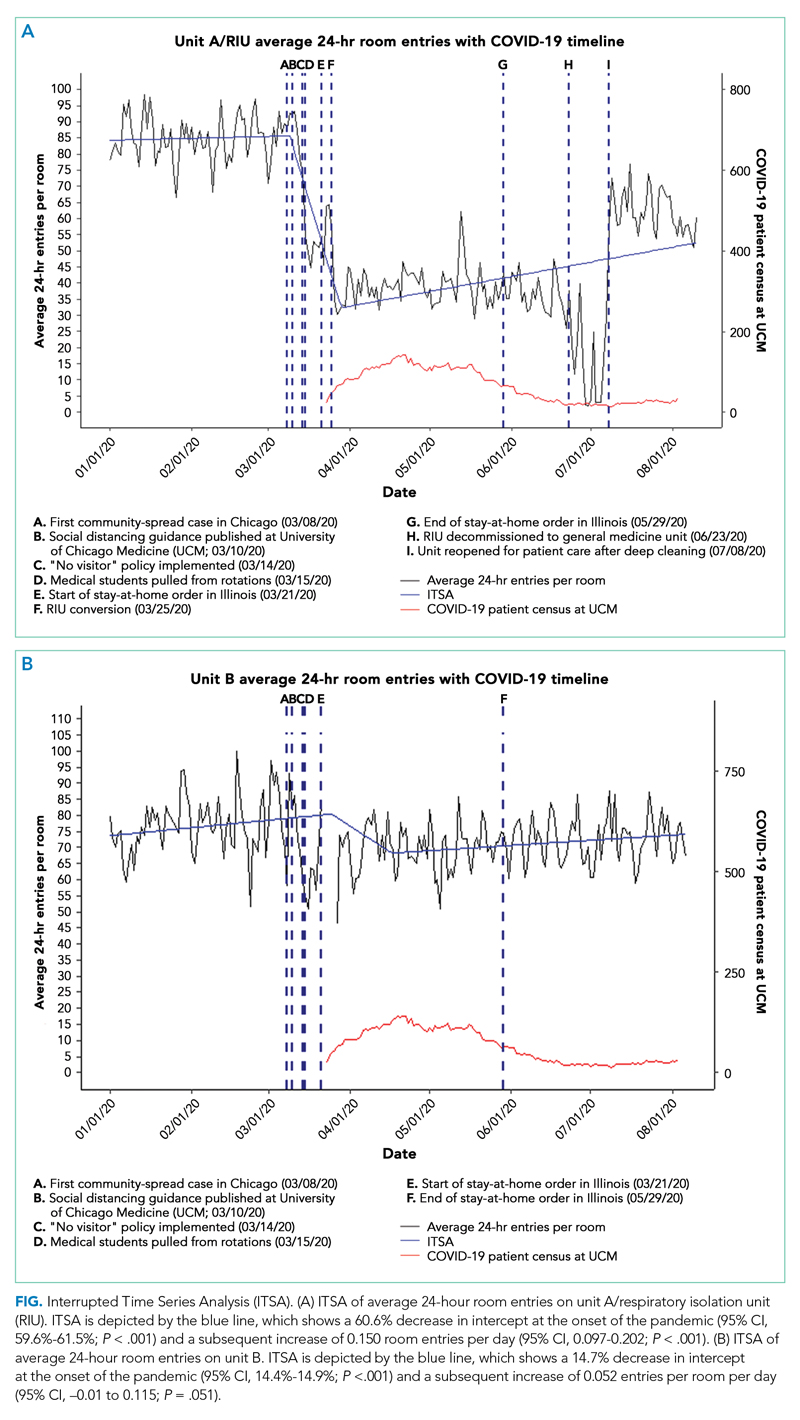
During the prepandemic period, Unit B averaged 63 daily occupied rooms, and these rooms averaged 76.9 entries per room per day, with no statistically significant change over time. During the pandemic period, these units averaged 64 daily occupied rooms, and these rooms averaged 72.4 entries per room per day. Briefly, at the start of the pandemic, daily entries per room decreased by 11.8 (95% CI, 11.6-12), equating to a 14.7% reduction from baseline (95% CI, 14.4%-14.9%; P < .001). Entries then increased by an average of 0.052 entries per room per day (95% CI, –0.01 to 0.115; P = .051), stabilizing in early August 2020 at an average of 74.1 daily entries. This equated to 92.2% of prepandemic levels (95% CI, 92%-92.3%; P < .001) (Figure, B).
Unit A/RIU experienced significantly greater average daily room entries during the prepandemic period (P < .001) and significantly fewer average daily room entries during the pandemic period (P < .001) than unit B. Although unit A and unit B cared for similar patient populations prior to the pandemic, unit B was located in a different building from the resident work room. This likely resulted in batched visits to patients, leading to fewer total room entries per day.
DISCUSSION
This is the first study to measure 24-hour patient room– entries as an objective proxy for physical distancing during the pandemic. Unit A/RIU saw an initial 60.6% decrease in room entries. In contrast, unit B, which cared for PUIs, saw a brief 14.7% decrease in room entries before returning to baseline. In all units, room entries increased over time, although this increase was greater in unit B.
Despite the institutional recommendation of physical distancing, only unit A/RIU saw a large and sustained decrease in room entries. The presence of patients with COVID-19 within this unit likely reminded clinicians of the ongoing need to physically distance. Clinicians may have been fearful of contracting COVID-19 and therefore more stringently followed physical-distancing guidance.
Changes in unit A/RIU room entries tracked with RIU conversion and decommission timeline (letters F and H in Figure, A, respectively) rather than statewide stay-at-home orders (letters E and G in Figure, A). Caring for patients with COVID-19 within the unit might have influenced clinician physical distancing more than state policy. Correspondingly, as the number of hospitalized patients with COVID-19 decreased, room entries trended toward baseline. The difficulty of sustaining behavioral changes has been demonstrated in healthcare settings, including at our own institution.6-8 This gradual extinction in physical distancing could be due to several factors, such as fewer patients with COVID-19 or staff fatigue. Physical distancing may have been more extreme and suboptimal for care at the beginning of the pandemic owing to uncertainty or fear.
This work has implications for how to monitor physical distancing in healthcare facilities. Our study shows that behaviors can change rapidly, but sustaining change is difficult. This suggests the need for regular reinforcement of physical distancing with all staff. Additionally, cohorting patients on RIUs may result in greater physical distancing. It also highlights that PUIs serve as less of a cue to promote physical distancing, possibly due to increased confidence in and availability of COVID-19 tests and/or precautions fatigue.9 Objective room-entry monitoring systems, such as the one used in this study, can provide hospital leaders with crucial real-time feedback to monitor physical distancing practices and determine when and where re-education may be needed.
This study was conducted at a single urban, academic medical center, limiting its generalizability. Many other hospital policies implemented at the beginning of the pandemic may have influenced our results. We are unable to examine the type of clinician entering each room and for how long as well as entries in workrooms and breakrooms. Clinicians were not given real-time or retrospective feedback on room entries during the pandemic period. These data would be important to understand staff responses to physical distancing. Finally, while clinicians responded differently as the pandemic progressed and depending on which unit they were in, the ideal degree of physical distancing remains unknown. Although minimizing patient contact limits nosocomial viral spread, too little contact can also cause harm.
Conclusion
At the onset of the COVID-19 pandemic, 24-hour patient room–entries fell significantly in all units before increasing. This decrease was more pronounced in unit A/RIU. As the pandemic continues, hospitals could consider utilizing novel room-entry monitoring systems to guide physical-distancing implementation and staff education.
Acknowledgment
The authors thank Vera Chu for her support with data requests.
1. Auerbach A, O’Leary KJ, Greysen SR, et al. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
2. Arora VM, Chivu M, Schram A, Meltzer D. Implementing physical distancing in the hospital: a key strategy to prevent nosocomial transmission of COVID-19. J Hosp Med. 2020;15(5):290-291. https://doi.org/10.12788/jhm.3434
3. Erondu AI, Orlov NM, Peirce LB, et al. Characterizing pediatric inpatient sleep duration and disruptions. Sleep Med. 2019;57:87-91. https://doi.org/10.1016/j.sleep.2019.01.030
4. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
5. Muggeo VMR. Segmented: an R package to fit regression models with broken-line relationships. R News. 2008;8:20-25.
6. Cook DJ, Arora VM, Chamberlain M, et al. Improving hospitalized children’s sleep by reducing excessive overnight blood pressure monitoring. Pediatrics. 2020;146(3):e20192217. https://doi.org/10.1542/peds.2019-2217
7. Bernstein M, Hou JK, Weizman AV, et al. Quality improvement primer series: how to sustain a quality improvement effort. Clin Gastroenterol Hepatol. 2016;14(10):1371-1375. https://doi.org/10.1016/j.cgh.2016.05.019
8. Makhni S, Umscheid CA, Soo J, et al. Hand hygiene compliance rate during the COVID-19 pandemic. JAMA Intern Med. 2021;181(7):1006-1008. https://doi.org/10.1001/jamainternmed.2021.1429
9. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
The COVID-19 pandemic dramatically altered how healthcare providers care for hospitalized patients. Many hospitals provided physical-distancing guidance to minimize viral transmission and preserve personal protective equipment. This guidance informed clinician behavior on rounds and in workspaces.1 One study reported that clinicians maintained distance from patients by grouping medical interventions, utilizing telemedicine for rounding and consultations, and implementing respiratory isolation units (RIUs) to cohort patients with COVID-19.2
Although physical distancing is recommended during inpatient care, no study to date has used objective measures to quantify the degree to which clinical practice was influenced. We aimed to objectively quantify changes in 24-hour patient room–entries before and during the COVID-19 pandemic using data from existing heat sensors to assess differences in physical distancing in RIUs and general medicine units.
METHODS
Study Design
A single-institution study was conducted at the University of Chicago Medicine, Illinois. Room entries were compared between a general medicine unit that transitioned to an RIU (unit A/RIU) and four general medicine units (unit B) using 24-hour patient room–entry data. Unit A was commissioned as an RIU to care exclusively for patients with confirmed COVID-19 on March 25, 2020, and decommissioned on June 23, 2020. Unit B cared for patients under investigation (PUIs) for COVID-19 and patients admitted for other reasons. PUIs were transferred to the RIU if positive for COVID-19. Hospital visitor restrictions were implemented on March 14, 2020, and lifted on June 29, 2020. The University of Chicago Institutional Review Board granted this project an exempt determination.
Data Collection
From January 1, 2020, to August 10, 2020, room-entry data were collected using the PURELL SMARTLINK hand-hygiene system (GOJO Industries, Inc.). This hand-hygiene compliance system tracks unit-level sanitizer dispenses and total room entries and exits via body heat sensors. Similar to our prior studies, this study extracted heat-sensor data to monitor room entries.3,4
Data Analysis
Objective 24-hour room-entry data were analyzed for all units. Rooms with less than two daily entries were assumed to be unoccupied and excluded from the analysis. Hospital-wide physical-distancing guidance published on March 10, 2020, was used to delineate “prepandemic” and “pandemic” periods. Each department adopted these recommendations (eg, physical distancing, conducting prerounds virtually, limiting the number of people seeing patients, using iPads for virtual patient visits) as appropriate.
Interrupted time series analyses were used to examine room-entry changes before and during the pandemic. The segmented function in R 4.0.2 (R Core Team) was used to create a model and estimate final fitting parameters, uncertainties, and data breakpoints using a bootstrap restarting algorithm.5 The Davies test was used to determine statistical significance of breakpoints, which was defined as P < .05.
RESULTS
We examined data from January 1, 2020, to August 10, 2020, from 3283 patients who collectively experienced 655,615 room entries. Unit A/RIU cared for 395 patients during the prepandemic period and 542 patients during the pandemic period. Compared with patients from the prepandemic period, patients during the pandemic period were more likely to be Black (73.7% vs 77.9%) and less likely to be White (17.0% vs 8.7%) (P = .002); were less likely to have respiratory (19.8% vs 8.0%, P < .001) or gastrointestinal (12.4% vs 6.5%, P = .008) primary diagnoses; and had a higher mean case-mix index (1.74 vs 2.38, P < .001) (Table).

Unit B had 718 patients during the prepandemic period and 1628 patients during the pandemic period. Compared with patients from the prepandemic period, patients during the pandemic period were less likely to be female (58.8% vs 53.4%, P = .018); less likely to be Black (77.7% vs 74.7%) and Hispanic (5.7% vs 3.4%) (P < .001); more likely to have circulatory (10.1% vs 14.8%, P = .009) primary diagnoses; less likely to have respiratory (14.4% vs 6.8%, P < .001) primary diagnoses; and had a higher mean case-mix index (1.58 vs 1.82, P = .014) (Table).
During the prepandemic period, unit A/RIU averaged 27 occupied rooms per day. These rooms averaged 85.0 entries per room per day, with no statistically significant change over time. During the pandemic period, this unit averaged 24 daily occupied rooms, and these rooms averaged 44.4 entries per room per day. At the start of the pandemic, daily entries per room decreased by 51.9 (95% CI, 51.1-52.7). This equated to a 60.6% reduction from baseline (95% CI, 59.6%-61.5%; P < .001), with the lowest average occurring after RIU conversion on March 25, 2020 (letter F in Figure, A). Entries remained constant through the end of statewide stay-at-home orders (letter G in Figure, A) until RIU decommission on June 23, 2020 (letter H in Figure, A). Entries then increased by an average of 0.150 entries per room per day (95% CI, 0.097-0.202; P < .001), reaching 52.5 daily entries on August 10, 2020. This equated to 61.3% of prepandemic levels (95% CI, 61.3%-61.6%; P < .001) (Figure, A).

During the prepandemic period, Unit B averaged 63 daily occupied rooms, and these rooms averaged 76.9 entries per room per day, with no statistically significant change over time. During the pandemic period, these units averaged 64 daily occupied rooms, and these rooms averaged 72.4 entries per room per day. Briefly, at the start of the pandemic, daily entries per room decreased by 11.8 (95% CI, 11.6-12), equating to a 14.7% reduction from baseline (95% CI, 14.4%-14.9%; P < .001). Entries then increased by an average of 0.052 entries per room per day (95% CI, –0.01 to 0.115; P = .051), stabilizing in early August 2020 at an average of 74.1 daily entries. This equated to 92.2% of prepandemic levels (95% CI, 92%-92.3%; P < .001) (Figure, B).
Unit A/RIU experienced significantly greater average daily room entries during the prepandemic period (P < .001) and significantly fewer average daily room entries during the pandemic period (P < .001) than unit B. Although unit A and unit B cared for similar patient populations prior to the pandemic, unit B was located in a different building from the resident work room. This likely resulted in batched visits to patients, leading to fewer total room entries per day.
DISCUSSION
This is the first study to measure 24-hour patient room– entries as an objective proxy for physical distancing during the pandemic. Unit A/RIU saw an initial 60.6% decrease in room entries. In contrast, unit B, which cared for PUIs, saw a brief 14.7% decrease in room entries before returning to baseline. In all units, room entries increased over time, although this increase was greater in unit B.
Despite the institutional recommendation of physical distancing, only unit A/RIU saw a large and sustained decrease in room entries. The presence of patients with COVID-19 within this unit likely reminded clinicians of the ongoing need to physically distance. Clinicians may have been fearful of contracting COVID-19 and therefore more stringently followed physical-distancing guidance.
Changes in unit A/RIU room entries tracked with RIU conversion and decommission timeline (letters F and H in Figure, A, respectively) rather than statewide stay-at-home orders (letters E and G in Figure, A). Caring for patients with COVID-19 within the unit might have influenced clinician physical distancing more than state policy. Correspondingly, as the number of hospitalized patients with COVID-19 decreased, room entries trended toward baseline. The difficulty of sustaining behavioral changes has been demonstrated in healthcare settings, including at our own institution.6-8 This gradual extinction in physical distancing could be due to several factors, such as fewer patients with COVID-19 or staff fatigue. Physical distancing may have been more extreme and suboptimal for care at the beginning of the pandemic owing to uncertainty or fear.
This work has implications for how to monitor physical distancing in healthcare facilities. Our study shows that behaviors can change rapidly, but sustaining change is difficult. This suggests the need for regular reinforcement of physical distancing with all staff. Additionally, cohorting patients on RIUs may result in greater physical distancing. It also highlights that PUIs serve as less of a cue to promote physical distancing, possibly due to increased confidence in and availability of COVID-19 tests and/or precautions fatigue.9 Objective room-entry monitoring systems, such as the one used in this study, can provide hospital leaders with crucial real-time feedback to monitor physical distancing practices and determine when and where re-education may be needed.
This study was conducted at a single urban, academic medical center, limiting its generalizability. Many other hospital policies implemented at the beginning of the pandemic may have influenced our results. We are unable to examine the type of clinician entering each room and for how long as well as entries in workrooms and breakrooms. Clinicians were not given real-time or retrospective feedback on room entries during the pandemic period. These data would be important to understand staff responses to physical distancing. Finally, while clinicians responded differently as the pandemic progressed and depending on which unit they were in, the ideal degree of physical distancing remains unknown. Although minimizing patient contact limits nosocomial viral spread, too little contact can also cause harm.
Conclusion
At the onset of the COVID-19 pandemic, 24-hour patient room–entries fell significantly in all units before increasing. This decrease was more pronounced in unit A/RIU. As the pandemic continues, hospitals could consider utilizing novel room-entry monitoring systems to guide physical-distancing implementation and staff education.
Acknowledgment
The authors thank Vera Chu for her support with data requests.
The COVID-19 pandemic dramatically altered how healthcare providers care for hospitalized patients. Many hospitals provided physical-distancing guidance to minimize viral transmission and preserve personal protective equipment. This guidance informed clinician behavior on rounds and in workspaces.1 One study reported that clinicians maintained distance from patients by grouping medical interventions, utilizing telemedicine for rounding and consultations, and implementing respiratory isolation units (RIUs) to cohort patients with COVID-19.2
Although physical distancing is recommended during inpatient care, no study to date has used objective measures to quantify the degree to which clinical practice was influenced. We aimed to objectively quantify changes in 24-hour patient room–entries before and during the COVID-19 pandemic using data from existing heat sensors to assess differences in physical distancing in RIUs and general medicine units.
METHODS
Study Design
A single-institution study was conducted at the University of Chicago Medicine, Illinois. Room entries were compared between a general medicine unit that transitioned to an RIU (unit A/RIU) and four general medicine units (unit B) using 24-hour patient room–entry data. Unit A was commissioned as an RIU to care exclusively for patients with confirmed COVID-19 on March 25, 2020, and decommissioned on June 23, 2020. Unit B cared for patients under investigation (PUIs) for COVID-19 and patients admitted for other reasons. PUIs were transferred to the RIU if positive for COVID-19. Hospital visitor restrictions were implemented on March 14, 2020, and lifted on June 29, 2020. The University of Chicago Institutional Review Board granted this project an exempt determination.
Data Collection
From January 1, 2020, to August 10, 2020, room-entry data were collected using the PURELL SMARTLINK hand-hygiene system (GOJO Industries, Inc.). This hand-hygiene compliance system tracks unit-level sanitizer dispenses and total room entries and exits via body heat sensors. Similar to our prior studies, this study extracted heat-sensor data to monitor room entries.3,4
Data Analysis
Objective 24-hour room-entry data were analyzed for all units. Rooms with less than two daily entries were assumed to be unoccupied and excluded from the analysis. Hospital-wide physical-distancing guidance published on March 10, 2020, was used to delineate “prepandemic” and “pandemic” periods. Each department adopted these recommendations (eg, physical distancing, conducting prerounds virtually, limiting the number of people seeing patients, using iPads for virtual patient visits) as appropriate.
Interrupted time series analyses were used to examine room-entry changes before and during the pandemic. The segmented function in R 4.0.2 (R Core Team) was used to create a model and estimate final fitting parameters, uncertainties, and data breakpoints using a bootstrap restarting algorithm.5 The Davies test was used to determine statistical significance of breakpoints, which was defined as P < .05.
RESULTS
We examined data from January 1, 2020, to August 10, 2020, from 3283 patients who collectively experienced 655,615 room entries. Unit A/RIU cared for 395 patients during the prepandemic period and 542 patients during the pandemic period. Compared with patients from the prepandemic period, patients during the pandemic period were more likely to be Black (73.7% vs 77.9%) and less likely to be White (17.0% vs 8.7%) (P = .002); were less likely to have respiratory (19.8% vs 8.0%, P < .001) or gastrointestinal (12.4% vs 6.5%, P = .008) primary diagnoses; and had a higher mean case-mix index (1.74 vs 2.38, P < .001) (Table).

Unit B had 718 patients during the prepandemic period and 1628 patients during the pandemic period. Compared with patients from the prepandemic period, patients during the pandemic period were less likely to be female (58.8% vs 53.4%, P = .018); less likely to be Black (77.7% vs 74.7%) and Hispanic (5.7% vs 3.4%) (P < .001); more likely to have circulatory (10.1% vs 14.8%, P = .009) primary diagnoses; less likely to have respiratory (14.4% vs 6.8%, P < .001) primary diagnoses; and had a higher mean case-mix index (1.58 vs 1.82, P = .014) (Table).
During the prepandemic period, unit A/RIU averaged 27 occupied rooms per day. These rooms averaged 85.0 entries per room per day, with no statistically significant change over time. During the pandemic period, this unit averaged 24 daily occupied rooms, and these rooms averaged 44.4 entries per room per day. At the start of the pandemic, daily entries per room decreased by 51.9 (95% CI, 51.1-52.7). This equated to a 60.6% reduction from baseline (95% CI, 59.6%-61.5%; P < .001), with the lowest average occurring after RIU conversion on March 25, 2020 (letter F in Figure, A). Entries remained constant through the end of statewide stay-at-home orders (letter G in Figure, A) until RIU decommission on June 23, 2020 (letter H in Figure, A). Entries then increased by an average of 0.150 entries per room per day (95% CI, 0.097-0.202; P < .001), reaching 52.5 daily entries on August 10, 2020. This equated to 61.3% of prepandemic levels (95% CI, 61.3%-61.6%; P < .001) (Figure, A).

During the prepandemic period, Unit B averaged 63 daily occupied rooms, and these rooms averaged 76.9 entries per room per day, with no statistically significant change over time. During the pandemic period, these units averaged 64 daily occupied rooms, and these rooms averaged 72.4 entries per room per day. Briefly, at the start of the pandemic, daily entries per room decreased by 11.8 (95% CI, 11.6-12), equating to a 14.7% reduction from baseline (95% CI, 14.4%-14.9%; P < .001). Entries then increased by an average of 0.052 entries per room per day (95% CI, –0.01 to 0.115; P = .051), stabilizing in early August 2020 at an average of 74.1 daily entries. This equated to 92.2% of prepandemic levels (95% CI, 92%-92.3%; P < .001) (Figure, B).
Unit A/RIU experienced significantly greater average daily room entries during the prepandemic period (P < .001) and significantly fewer average daily room entries during the pandemic period (P < .001) than unit B. Although unit A and unit B cared for similar patient populations prior to the pandemic, unit B was located in a different building from the resident work room. This likely resulted in batched visits to patients, leading to fewer total room entries per day.
DISCUSSION
This is the first study to measure 24-hour patient room– entries as an objective proxy for physical distancing during the pandemic. Unit A/RIU saw an initial 60.6% decrease in room entries. In contrast, unit B, which cared for PUIs, saw a brief 14.7% decrease in room entries before returning to baseline. In all units, room entries increased over time, although this increase was greater in unit B.
Despite the institutional recommendation of physical distancing, only unit A/RIU saw a large and sustained decrease in room entries. The presence of patients with COVID-19 within this unit likely reminded clinicians of the ongoing need to physically distance. Clinicians may have been fearful of contracting COVID-19 and therefore more stringently followed physical-distancing guidance.
Changes in unit A/RIU room entries tracked with RIU conversion and decommission timeline (letters F and H in Figure, A, respectively) rather than statewide stay-at-home orders (letters E and G in Figure, A). Caring for patients with COVID-19 within the unit might have influenced clinician physical distancing more than state policy. Correspondingly, as the number of hospitalized patients with COVID-19 decreased, room entries trended toward baseline. The difficulty of sustaining behavioral changes has been demonstrated in healthcare settings, including at our own institution.6-8 This gradual extinction in physical distancing could be due to several factors, such as fewer patients with COVID-19 or staff fatigue. Physical distancing may have been more extreme and suboptimal for care at the beginning of the pandemic owing to uncertainty or fear.
This work has implications for how to monitor physical distancing in healthcare facilities. Our study shows that behaviors can change rapidly, but sustaining change is difficult. This suggests the need for regular reinforcement of physical distancing with all staff. Additionally, cohorting patients on RIUs may result in greater physical distancing. It also highlights that PUIs serve as less of a cue to promote physical distancing, possibly due to increased confidence in and availability of COVID-19 tests and/or precautions fatigue.9 Objective room-entry monitoring systems, such as the one used in this study, can provide hospital leaders with crucial real-time feedback to monitor physical distancing practices and determine when and where re-education may be needed.
This study was conducted at a single urban, academic medical center, limiting its generalizability. Many other hospital policies implemented at the beginning of the pandemic may have influenced our results. We are unable to examine the type of clinician entering each room and for how long as well as entries in workrooms and breakrooms. Clinicians were not given real-time or retrospective feedback on room entries during the pandemic period. These data would be important to understand staff responses to physical distancing. Finally, while clinicians responded differently as the pandemic progressed and depending on which unit they were in, the ideal degree of physical distancing remains unknown. Although minimizing patient contact limits nosocomial viral spread, too little contact can also cause harm.
Conclusion
At the onset of the COVID-19 pandemic, 24-hour patient room–entries fell significantly in all units before increasing. This decrease was more pronounced in unit A/RIU. As the pandemic continues, hospitals could consider utilizing novel room-entry monitoring systems to guide physical-distancing implementation and staff education.
Acknowledgment
The authors thank Vera Chu for her support with data requests.
1. Auerbach A, O’Leary KJ, Greysen SR, et al. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
2. Arora VM, Chivu M, Schram A, Meltzer D. Implementing physical distancing in the hospital: a key strategy to prevent nosocomial transmission of COVID-19. J Hosp Med. 2020;15(5):290-291. https://doi.org/10.12788/jhm.3434
3. Erondu AI, Orlov NM, Peirce LB, et al. Characterizing pediatric inpatient sleep duration and disruptions. Sleep Med. 2019;57:87-91. https://doi.org/10.1016/j.sleep.2019.01.030
4. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
5. Muggeo VMR. Segmented: an R package to fit regression models with broken-line relationships. R News. 2008;8:20-25.
6. Cook DJ, Arora VM, Chamberlain M, et al. Improving hospitalized children’s sleep by reducing excessive overnight blood pressure monitoring. Pediatrics. 2020;146(3):e20192217. https://doi.org/10.1542/peds.2019-2217
7. Bernstein M, Hou JK, Weizman AV, et al. Quality improvement primer series: how to sustain a quality improvement effort. Clin Gastroenterol Hepatol. 2016;14(10):1371-1375. https://doi.org/10.1016/j.cgh.2016.05.019
8. Makhni S, Umscheid CA, Soo J, et al. Hand hygiene compliance rate during the COVID-19 pandemic. JAMA Intern Med. 2021;181(7):1006-1008. https://doi.org/10.1001/jamainternmed.2021.1429
9. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
1. Auerbach A, O’Leary KJ, Greysen SR, et al. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
2. Arora VM, Chivu M, Schram A, Meltzer D. Implementing physical distancing in the hospital: a key strategy to prevent nosocomial transmission of COVID-19. J Hosp Med. 2020;15(5):290-291. https://doi.org/10.12788/jhm.3434
3. Erondu AI, Orlov NM, Peirce LB, et al. Characterizing pediatric inpatient sleep duration and disruptions. Sleep Med. 2019;57:87-91. https://doi.org/10.1016/j.sleep.2019.01.030
4. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091
5. Muggeo VMR. Segmented: an R package to fit regression models with broken-line relationships. R News. 2008;8:20-25.
6. Cook DJ, Arora VM, Chamberlain M, et al. Improving hospitalized children’s sleep by reducing excessive overnight blood pressure monitoring. Pediatrics. 2020;146(3):e20192217. https://doi.org/10.1542/peds.2019-2217
7. Bernstein M, Hou JK, Weizman AV, et al. Quality improvement primer series: how to sustain a quality improvement effort. Clin Gastroenterol Hepatol. 2016;14(10):1371-1375. https://doi.org/10.1016/j.cgh.2016.05.019
8. Makhni S, Umscheid CA, Soo J, et al. Hand hygiene compliance rate during the COVID-19 pandemic. JAMA Intern Med. 2021;181(7):1006-1008. https://doi.org/10.1001/jamainternmed.2021.1429
9. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
© 2021 Society of Hospital Medicine
Socioeconomic and Racial Disparities in Diabetic Ketoacidosis Admissions in Youth With Type 1 Diabetes
Type 1 diabetes mellitus (T1D) is a common chronic condition of childhood. Its incidence has risen steadily over the past two decades.1 Treatment requires complex daily tasks, including blood glucose monitoring and insulin administration. The potential long-term complications of T1D—kidney failure, retinopathy, cardiovascular disease, and death—are grave but can be attenuated with effective glycemic management.2 Still, less than 25% of youths aged 13 to 19 years achieve target hemoglobin A1c (HbA1c) levels.3
Diabetic ketoacidosis (DKA) is an acute, life-threatening complication of T1D associated with suboptimal glycemic management. In the United States, DKA hospitalizations increased during 2009-2014.4 One study found the average standardized cost of a DKA-related pediatric hospitalization exceeded $7000,5 an amount not including missed school days, parent/caregiver workdays, and social and family disruptions. Costs extend to psychological well-being, with patients reporting that they “keep thinking [DKA] may happen again.”6
The burden of T1D-related morbidity is not equitably distributed. Recent evidence suggests that racial/ethnic minorities and those with public insurance experience disproportionately high rates of DKA.7,8 Neighborhood socioeconomic measures, like poverty, add an important dimension illustrative of ecological conditions in which patients reside. These measures have been studied in relation to hospitalization rates for chronic conditions like asthma and heart failure.9,10 For example, one recent study found links between area-level poverty in the United States and the likelihood of readmissions for pediatric patients following an admission for DKA.11 We sought to build on this finding by investigating whether area-level poverty, which we measured as the census tract poverty rate, patient race, and insurance status were associated with the likelihood of initial DKA hospitalization and severity of DKA presentation.
METHODS
Design
We conducted a single-center, retrospective cohort study that examined data on youth with T1D extracted from the Cincinnati Children’s Hospital Medical Center (CCHMC) electronic medical record (EMR). CCHMC is an urban, tertiary-care, freestanding pediatric hospital, with near-complete market penetration in Hamilton County, Ohio, particularly for subspecialty care. The racial and ethnic breakdown of Hamilton County’s general population is 68% White, 27% Black/African American, 3% Asian, and 4% Hispanic or Latino (adds to more than 100% due to rounding).12 The CCHMC Institutional Review Board approved this study.
Study Population
We identified eligible patients through a clinical registry within the EMR, which includes all T1D patients seen at CCHMC within the preceding 24 months. Patients are added to this registry upon initial T1D diagnosis by a research nurse who evaluates autoantibody status and clinical presentation. All patients ≤18 years old who had T1D, were active on the registry, and had an address within Hamilton County as of December 31, 2017, were included. Those with type 2 diabetes mellitus or secondary causes of diabetes were excluded. We captured patients on the registry as of December 31, 2017, and then examined whether they had been hospitalized from January 1, 2011, to December 31, 2017.
Covariates
We extracted demographic and clinical data for patients within the T1D registry as of December 31, 2017, and, for those hospitalized, at the time of their admission. Specifically, we extracted date of birth (to calculate age at encounter), sex, and ethnicity. Age was treated as a continuous variable. We defined ethnicity as Latinx (inclusive of Hispanic) or non-Latinx. We calculated duration of T1D diagnosis for patients within the registry by taking the difference of the diabetes onset date and the end of the study period. We extracted whether registry patients had an insulin pump or a continuous glucose monitor (CGM). Hospitalized patients were identified as new-onset or established T1D by comparing their hospitalization with the T1D diagnosis onset date.
For the entire T1D registry, we also assessed a key clinical characteristic: a patient’s median HbA1c. We enumerated the median HbA1c of all patients’ medians within the calendar year 2017, consistent with the methodology of a large international T1D registry.13 Among the subset of patients who were hospitalized, we obtained the HbA1c value during or prior to hospitalization.
Exposures
We captured exposure variables from EMR documentation of patients’ address, race, and insurance status. Given the small regional population of Latinx patients, we did not include ethnicity as an exposure. We used the address and insurance status at the beginning of the study period for established T1D patients, or at the time of diagnosis for new-onset patients, for analyses related to the likelihood of DKA admission. We used the address at the time of hospitalization for analyses of DKA presentation severity. We geocoded home addresses using ArcGIS software (Version 10.5.1; Esri), successfully geocoding >95% to the street level and assigning patients to a corresponding census tract—a census-defined geography consisting of approximately 4000 people.14 Census tracts, when drawn after the decennial (every 10 years) census, are designed to be sociodemographically homogenous15 and have long been used in medical and public health research.16 We connected geocoded addresses to the census tract poverty rate, defined as the percentage of individuals within a tract living below the federal poverty level. For our analyses, we primarily treated census tract poverty as a continuous variable ranging from 0% to 100%. We visualized the distribution of census tract poverty across Hamilton County using a choropleth map (Figure, part A). We also grouped the registry population into quartiles, ordered by census tract poverty levels, to ease the graphical display for Figure, part B. We defined race as White, Black (inclusive of African American), or other. We categorized insurance as Medicaid/public or Non-Medicaid/private.
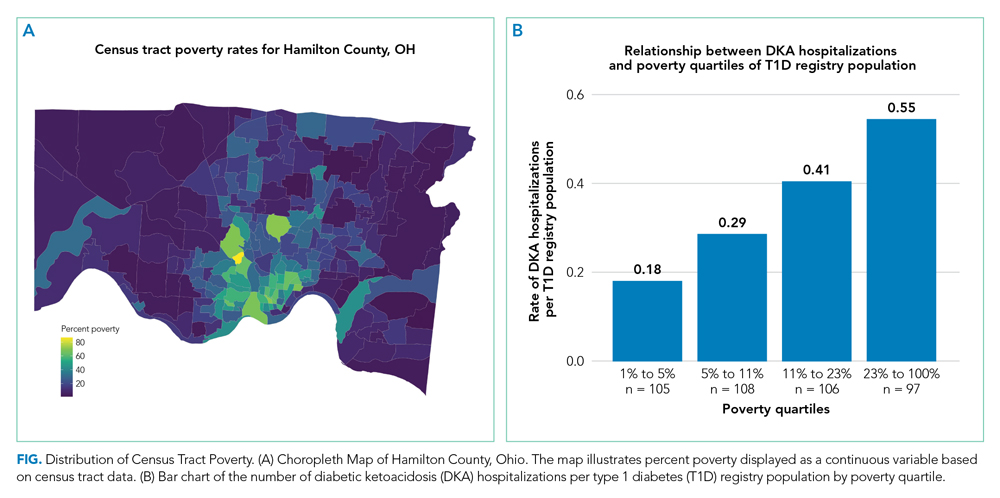
Outcomes
The primary outcome was a hospitalization for DKA during the study period. We identified eligible events by admission diagnosis of DKA (International Classification of Disease, 10th Revision, Clinical Modification, E13.10 or E10.10). Secondarily, we assessed admission severity for each patient’s first DKA hospitalization using initial pH from a venous blood gas and initial bicarbonate from the basic metabolic panel. These measures were chosen in accordance with the American Diabetes Association’s published classification of DKA severity, which uses pH, bicarbonate, and mental status.17 We defined the time for correction as the minutes from admission time until the anion gap was ≤12 or the bicarbonate was ≥18. We calculated total inpatient bed-days and pediatric intensive care unit (PICU) bed-days, and identified whether a patient was readmitted for DKA during the study period.
Statistical Analyses
We used descriptive statistics to characterize demographic and clinical information for patients within the T1D registry and for the hospitalized subset. We examined DKA severity data for all admitted patients by diagnosis status (new-onset T1D vs established). Associations between categorical outcomes were assessed using the Chi-square or Fisher exact test, and continuous outcomes were evaluated using the Mann-Whitney Rank Sum test.
We individually examined the effects of census tract poverty, race, and insurance on the odds of hospitalization through logistic regression. After confirming overlap and variability in census tract poverty rates by race and admission status (Appendix Table 1), we included these exposures in a multivariable model to capture their independent effects. Because the impact of census tract poverty, race, and insurance on hospitalization odds could vary depending on T1D diagnosis status, we conducted a sensitivity analysis by repeating the multivariable model with only established T1D patients.
Given that median HbA1c levels differed for T1D registry patients based on census tract poverty, race, and insurance, we used linear regression in a post-hoc analysis to evaluate associations between those exposures and median HbA1c. Age, gender, ethnicity, and duration of diabetes were not included in the models given that these variables did not differ by outcome variables. Diabetes technology—CGM and insulin pumps—was not included as a confounder because we hypothesized it mediated the effect of area-level poverty. For analyses, SigmaPlot (Version 14.0; SPSS Inc.) and R statistical software were used (The R Project for Statistical Computing).
RESULTS
A total of 439 patients were in the T1D registry and lived in Hamilton County as of December 31, 2017. Their demographic characteristics are listed in Table 1. Their median age was 14 years; 48% were female. The median poverty rate of the census tracts in which these youth lived was 11%. A total of 24.6% of patients identified as Black, 73.1% as White, and 2.3% as Hispanic; 35.8% were publicly insured. Patients had a median duration of T1D of 61 months (5 years, 1 month), with a median HbA1c (of all patients’ medians) of 8.7%. A total of 58.1% had a CGM, and 56% used an insulin pump.
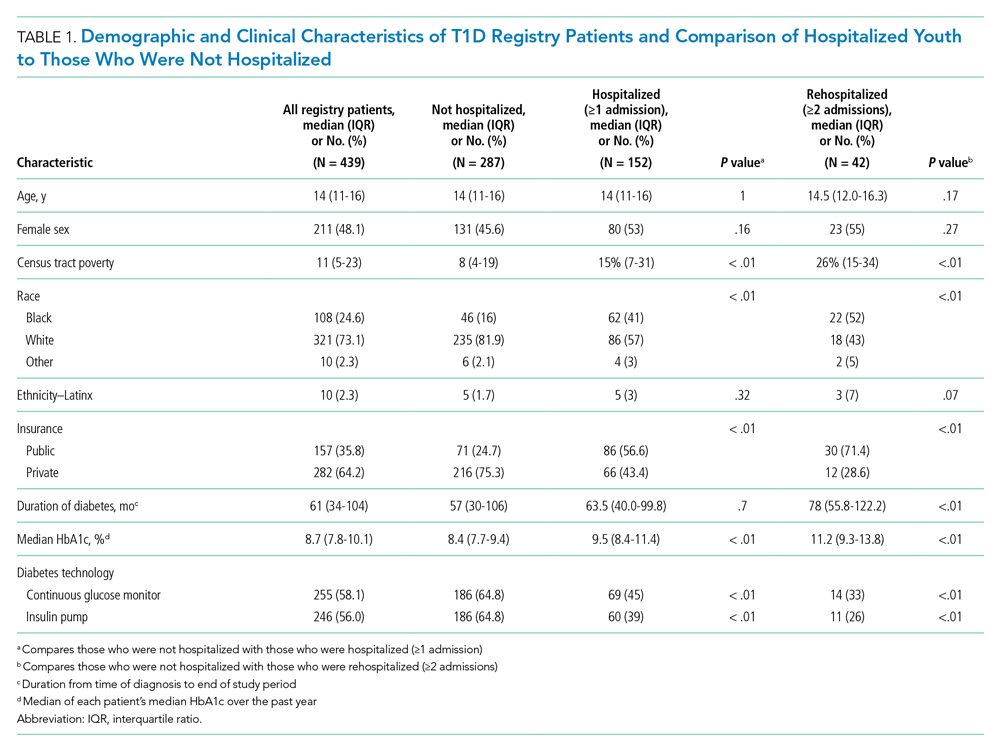
Hospitalization Characteristics of Those in the T1D Registry
Approximately one-third of registry patients (n = 152) experienced ≥1 hospitalization for DKA during the study period (inclusive of new-onset and established diagnosis). Age, gender, ethnicity, and duration of T1D diagnosis were similar between those who experienced a hospitalization and those who did not. The median census tract poverty rate was higher among those who were hospitalized compared to those who were not (15% vs 8%, P < .01). Among hospitalized patients, 41% identified as Black; among all who were not hospitalized, 16% identified as Black (P < .01). Among all hospitalized patients, 56.6% were covered by public insurance, while 24.7% of non-hospitalized patients were covered by public insurance (P < .01). Hospitalized patients were more likely to have a higher median HbA1c (9.5% vs 8.4%, P < .01) and were significantly less likely to have a CGM or insulin pump (both P < .01). These associations were magnified among the roughly 10% of the T1D registry population (n = 42) who experienced ≥2 hospitalizations.
Figure, part A is a choropleth map depicting the distribution of poverty by census tract across Hamilton County. The map is complemented by part B of the Figure, which displays the T1D registry population split into poverty quartiles and each quartile’s DKA admission rate per T1D registry population. As census tract poverty increases, so too does the rate of DKA hospitalizations. There is an almost threefold difference between the hospitalization rate of the highest poverty quartile and that of the lowest.
Likelihood of Hospitalization
We examined the exposures of interest—census tract poverty, race, and insurance—and odds of hospitalization at a patient level using logistic regression models (Table 2). In the multivariable model that included these exposures, we found that for every 10% increase in the census tract poverty rate, the odds of being hospitalized for DKA increased by 22% (95% CI, 1.03-1.47). Race was not significantly associated with odds of DKA hospitalization in the multivariable model. Public insurance was associated with a 2.71-times higher odds of hospitalization compared to those with private insurance (95% CI, 1.62-4.55). Our sensitivity analysis of only those with established T1D diagnoses showed similar results to that of the entire T1D registry population.
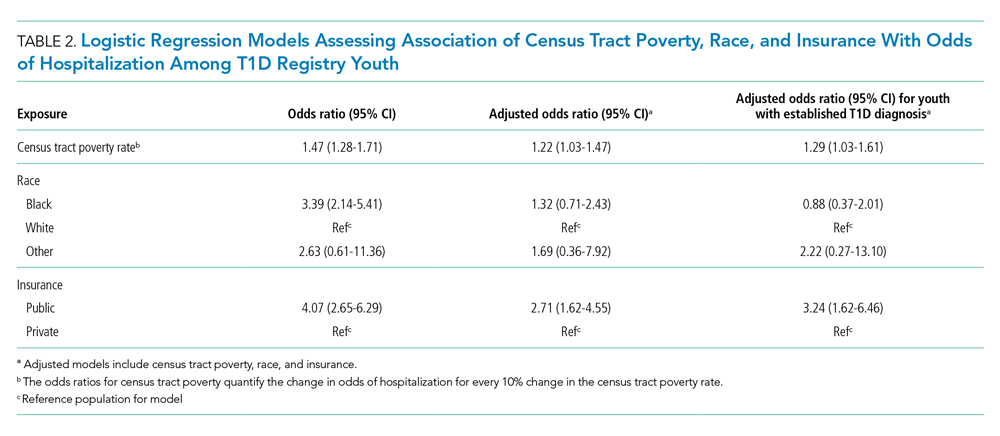
Severity of DKA Presentation
A total of 152 registry patients were admitted; 89 patients had new-onset T1D, and 63 had an established T1D diagnosis. We examined presenting factors (initial pH, initial bicarbonate, HbA1c) and clinical characteristics (time to correction, inpatient bed-days, PICU bed-days) by census tract poverty, race, and insurance for all hospitalized patients. There were no significant differences in presenting factors or outcomes by census tract poverty or insurance. However, we did note certain differences by race. Indeed, though initial pH, bicarbonate, and time to correction did not meaningfully differ by race, Black patients had a longer length of stay than their White peers (Appendix Table 2; 3.06 vs 2.16 days, P < .01). We further examined this by diagnosis status, as newly diagnosed patients often stay longer for diabetes education.18 Black patients had a longer length of stay than White patients, regardless of whether they had new-onset (3.85 vs 2.99 days, P < .01) or established T1D (1.83 vs 0.97 days, P < .01). Additionally, we found that the HbA1c was significantly higher in Black versus White patients (12.4% vs 10.8%, P = .01). HbA1c was significantly different between established T1D patients identifying as Black and those identifying as White (11.7% vs 9.5%, P < .01); however, HbA1c did not differ by race for newly diagnosed patients (12.5% vs 13.0%, P = .63).
Median HbA1c of All T1D Registry Patients
Given the suggestion of HbA1c variability by exposure variables, we performed a post-hoc linear regression analysis. Table 3 displays the linear regression models assessing associations of census tract poverty, race, and insurance on median HbA1c for all T1D registry patients. In the multivariable model, census tract poverty was no longer associated with higher HbA1c levels (0.13, 95% CI, –0.01 to 0.26). However, Black patients continued to have significantly higher HbA1c levels than White patients (1.09% higher; 95% CI, 0.59-1.59). Also, those with public insurance had significantly higher HbA1c levels than those with private insurance (0.93% higher; 95% CI, 0.51-1.35).
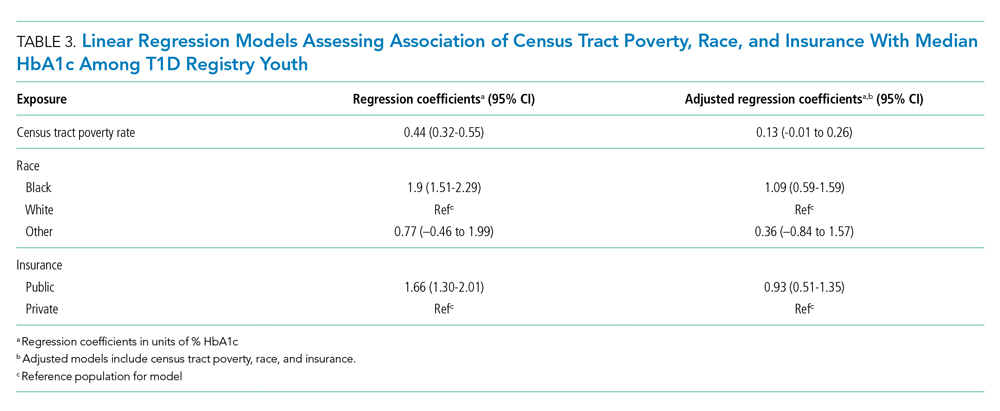
DISCUSSION
Living in high poverty areas, identifying as Black, and having public insurance were each associated with a higher likelihood of hospitalization for DKA in an unadjusted model. When we examined these exposures together in a multivariable model, census tract poverty and insurance remained associated with a higher likelihood of hospitalization for DKA. Race—with a wide confidence interval—was no longer associated with a higher likelihood of hospitalization. The increased likelihood of hospitalization initially seen for Black patients may be because they are more likely to reside in high poverty areas and be on public insurance—inequities associated with structural racism.
These results mirror those of other studies that have found area-level poverty to be associated with higher rates of intensive care admissions and hospitalizations for conditions like asthma.10,19 Our findings are also similar to those of a previous study that found that area-level deprivation was associated with a greater likelihood of DKA readmissions in pediatric and adult patients, as well as an adult study that showed increased neighborhood deprivation was associated with higher odds of DKA hospitalization.11,20 Further examination of the mechanisms underlying DKA hospitalization is clearly warranted and would be informed by a deeper awareness of the social determinants of health—such as community context and the neighborhood/built environment21—and their links to racial inequities.
Neighborhood socioeconomic status, here measured as census tract poverty, is likely linked to hospitalization for DKA through contextual factors like food insecurity, limited access to supportive care (eg, presence of a school nurse who can co-manage T1D with the patient/family and medical team), and adverse environmental exposures.22-24 To improve the health of patients, prevent hospitalizations, and achieve health equity, we suggest that both individual factors and these contextual factors need to be evaluated and addressed through community-connected interventions.25,26 For example, interventions that deploy community health workers to navigate the complexities of health systems have been effective in reducing HbA1c levels in adults with type 2 diabetes mellitus27 and are beginning to be studied in pediatrics.28
Black patients had a higher median HbA1c compared to their White counterparts in the T1D registry. One study has previously shown that hemoglobin glycation biologically differed by around 0.5% in Black patients compared to White patients.29 Despite this purported biological difference, a greater difference in HbA1c—nearly 2 points—was seen in the univariate model, illustrating that contextual factors such as socioeconomic status and treatment patterns, like the use of diabetes technology, exceed this biological difference.30-32 Our multivariable results support the importance of these contextual factors, as the HbA1c difference decreased from 1.9 to 1.09 after census tract poverty and insurance were included.
Such differences by race also emerge from experiences of discrimination and bias. Thus, to improve outcomes and equity, we must understand and address racism and its many ill effects. Different levels of racism, such as structural or personally mediated racism, interact with social determinants of health in various ways.33,34 For example, redlining—racially discriminatory grading of neighborhoods’ creditworthiness in the 1930s—is an example of structural racism that continues to affect the health of patients today.35,36 Black families are more likely to live in areas with higher poverty, even after controlling for household income level.37 The interaction between race and poverty is complex given these historical underpinnings of structural racism, as evidenced by race no longer being associated with an increased likelihood of DKA hospitalization in our multivariable analysis. The longer length of stay for Black patients despite similar presenting severity also exemplifies this complexity. For example, longer stays could result from explicit or implicit biases that lead providers to keep patients longer due to preconceived beliefs that the caregiver does not understand diabetes management.38 However, it also could be that the caregiver takes longer to complete diabetes education due to systemic barriers, such as work-related obligations during business hours when diabetes educators are present, or transportation and childcare needs.39 These barriers are often tied to socioeconomic status, making it more difficult for those in poverty to complete required education. We cannot conclude from our study the reasons for the difference in length of stay, just that differences exist. Moving forward, we suggest that design of interventions aimed at achieving health equity consider the complex interplay between health, healthcare systems, racism, and neighborhood context.
There were limitations to our study. First, given that this was an observational study, unmeasured confounders like patient or parental education and personal income could affect the strength of associations seen in our models. Second, although the CCHMC’s Diabetes Center treats nearly all children with T1D in Hamilton County, some patients, or hospitalizations at other centers, could have been missed. Third, the demographics of Hamilton County may not generalize to other diabetes centers. Fourth, although more granular data—such as census block groups or blocks—are available from the US Census Bureau, we chose census tracts as our unit of analysis to reduce sampling error that emerges from the use of such smaller geographies. Last, we measured census tract poverty rather than other contextual markers of one’s neighborhood, like vacant housing or the proportion of those receiving public benefits. However, these measures are often highly correlated with census tract poverty.40
CONCLUSION
Census tract poverty, race, and insurance were each associated with an increased likelihood of hospitalization for DKA. However, in a multivariable model, the effect of race was reduced so it was no longer significant, indicating that racial differences in DKA hospitalization are at least partially explained by differences in socioeconomic factors. The severity of DKA presentation did not differ by census tract poverty or insurance, although Black patients experienced differences in care despite similar markers of DKA severity. Addressing contextual factors—such as social determinants of health and racism—is warranted for future interventions that aim to eliminate equity gaps for T1D patients.
1. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376(15):1419-1429. https://doi.org/10.1056/NEJMoa1610187
2. Writing Group for the DCCT/EDIC Research Group, Orchard TJ, Nathan DM, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA. 2015;313(1):45-53. https://doi.org/10.1001/jama.2014.16107
3. Wood JR, Miller KM, Maahs DM, et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36(7):2035-2037. https://doi.org/10.2337/dc12-1959
4. Benoit SR. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018;67(12):362-365. https://doi.org/10.15585/mmwr.mm6712a3
5. Tieder JS, McLeod L, Keren R, et al. Variation in resource use and readmission for diabetic ketoacidosis in children’s hospitals. Pediatrics. 2013;132(2):229-236. https://doi.org/10.1542/peds.2013-0359
6. Moffett MA, Buckingham JC, Baker CR, Hawthorne G, Leech NJ. Patients’ experience of admission to hospital with diabetic ketoacidosis and its psychological impact: an exploratory qualitative study. Practical Diabetes. 2013;30(5):203-207. https://doi.org/10.1002/pdi.1777
7. Maahs DM, Hermann JM, Holman N, et al. Rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the U.S., Austria, and Germany. Diabetes Care. 2015;38(10):1876-1882. https://doi.org/10.2337/dc15-0780
8. Malik FS, Hall M, Mangione-Smith R, et al. Patient characteristics associated with differences in admission frequency for diabetic ketoacidosis in United States children’s hospitals. J Pediatr. 2016;171:104-110. https://doi.org/10.1016/j.jpeds.2015.12.015
9. Manickam RN, Mu Y, Kshirsagar AV, Bang H. Area-level poverty and excess hospital readmission ratios. Am J Med. 2017;130(4):e153-e155. https://doi.org/10.1016/j.amjmed.2016.08.047
10. Beck AF, Moncrief T, Huang B, et al. Inequalities in neighborhood child asthma admission rates and underlying community characteristics in one US county. J Pediatr. 2013;163(2):574-580.e1. https://doi.org/10.1016/j.jpeds.2013.01.064
11. Everett E, Mathioudakis N. Association of area deprivation and diabetic ketoacidosis readmissions: comparative risk analysis of adults vs children with type 1 diabetes. J Clin Endocrinol Metab. 2019;104(8):3473-3480. https://doi.org/10.1210/jc.2018-02232
12. U.S. Census Bureau. QuickFacts: Hamilton County, Ohio. Accessed March 2, 2020. https://www.census.gov/quickfacts/hamiltoncountyohio
13. Witsch M, Kosteria I, Kordonouri O, et al. Possibilities and challenges of a large international benchmarking in pediatric diabetology—the SWEET experience. Pediatr Diabetes. 2016;17(S23):7-15. https://doi.org/10.1111/pedi.12432
14. U.S. Census Bureau. Glossary. Accessed April 22, 2021. https://www.census.gov/programs-surveys/geography/about/glossary.html
15. U.S. Census Bureau. Geographic areas reference manual. Chapter 10: Census tracts and block numbering areas. Accessed April 22, 2021. https://www.census.gov/programs-surveys/geography/guidance/geographic-areas-reference-manual.html
16. Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: The Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312-323. https://doi.org/10.2105/AJPH.2003.032482
17. Kitabchi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153. https://doi.org/10.2337/diacare.24.1.131
18. Lawson S, Redel JM, Smego A, et al. Assessment of a day hospital management program for children with type 1 diabetes. JAMA Netw Open. 2020;3(3):e200347. https://doi.org/10.1001/jamanetworkopen.2020.0347
19. Andrist E, Riley CL, Brokamp C, Taylor S, Beck AF. Neighborhood poverty and pediatric intensive care use. Pediatrics. 2019;144(6):e20190748. https://doi.org/10.1542/peds.2019-0748
20. Govan L, Maietti E, Torsney B, et al. The effect of deprivation and HbA1c on admission to hospital for diabetic ketoacidosis in type 1 diabetes. Diabetologia. 2012;55(9):2356-2360. https://doi.org/10.1007/s00125-012-2601-6
21. U.S. Department of Health and Human Services. Healthy People 2030—Social Determinants of Health. Accessed May 13, 2021. https://health.gov/healthypeople/objectives-and-data/social-determinants-health
22. Berkowitz SA, Karter AJ, Corbie-Smith G, et al. Food insecurity, food “deserts,” and glycemic control in patients with diabetes: a longitudinal analysis. Diabetes Care. 2018;41(6):1188-1195. https://doi.org/10.2337/dc17-1981
23. Nguyen TM, Mason KJ, Sanders CG, Yazdani P, Heptulla RA. Targeting blood glucose management in school improves glycemic control in children with poorly controlled type 1 diabetes mellitus. J Pediatr. 2008;153(4):575-578. https://doi.org/10.1016/j.jpeds.2008.04.066
24. Izquierdo R, Morin PC, Bratt K, et al. School-centered telemedicine for children with type 1 diabetes mellitus. J Pediatr. 2009;155(3):374-379. https://doi.org/10.1016/j.jpeds.2009.03.014
25. Council on Community Pediatrics and Committee on Native American Child Health. Policy statement—health equity and children’s rights. Pediatrics. 2010;125(4):838-849. https://doi.org/10.1542/peds.2010-0235
26. Braveman P. What are health disparities and health equity? We need to be clear. Public Health Rep. 2014;129(suppl 2):5-8.
27. Palmas W, March D, Darakjy S, et al. Community health worker interventions to improve glycemic control in people with diabetes: a systematic review and meta-analysis. J Gen Intern Med. 2015;30(7):1004-1012. https://doi.org/10.1007/s11606-015-3247-0
28. Lipman TH, Smith JA, Hawkes CP. Community health workers and the care of children with type 1 diabetes. J Pediatr Nurs. 2019;49:111-112. https://doi.org/10.1016/j.pedn.2019.08.014
29. Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95-102. https://doi.org/10.7326/M16-2596
30. Agarwal S, Kanapka LG, Raymond JK, et al. Racial-ethnic inequity in young adults with type 1 diabetes. J Clin Endocrinol Metab. 2020;105(8):e2960-e2969. https://doi.org/10.1210/clinem/dgaa236
31. Addala A, Auzanneau M, Miller K, et al. A decade of disparities in diabetes technology use and HbA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care. 2021;44(1):133-140. https://doi.org/10.2337/dc20-0257
32. Lipman TH, Smith JA, Patil O, Willi SM, Hawkes CP. Racial disparities in treatment and outcomes of children with type 1 diabetes. Pediatr Diabetes. 2021;22(2):241-248. https://doi.org/10.1111/pedi.13139
33. Boyd RW, Lindo EG, Weeks LD, McLemore MR. On racism: a new standard for publishing on racial health inequities. Health Affairs. Accessed July 9, 2020. https://www.healthaffairs.org/do/10.1377/hblog20200630.939347/full/
34. Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90(8):1212-1215. https://doi.org/10.2105/AJPH.90.8.1212
35. Jacoby SF, Dong B, Beard JH, Wiebe DJ, Morrison CN. The enduring impact of historical and structural racism on urban violence in Philadelphia. Soc Sci Med. 2018;199:87-95. https://doi.org/10.1016/j.socscimed.2017.05.038
36. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453-1463. https://doi.org/10.1016/S0140-6736(17)30569-X
37. Reardon SF, Fox L, Townsend J. Neighborhood income composition by household race and income, 1990–2009. Ann Am Acad Pol Soc Sci. 2015;660(1):78-97. https://doi.org/10.1177/0002716215576104
38. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. https://doi.org/10.2105/AJPH.2011.300558
39. Lawton J, Waugh N, Noyes K, et al. Improving communication and recall of information in paediatric diabetes consultations: a qualitative study of parents’ experiences and views. BMC Pediatr. 2015;15:67. https://doi.org/10.1186/s12887-015-0388-6
40. Brokamp C, Beck AF, Goyal NK, Ryan P, Greenberg JM, Hall ES. Material community deprivation and hospital utilization during the first year of life: an urban population-based cohort study. Ann Epidemiol. 2019;30:37-43. https://doi.org/10.1016/j.annepidem.2018.11.008
Type 1 diabetes mellitus (T1D) is a common chronic condition of childhood. Its incidence has risen steadily over the past two decades.1 Treatment requires complex daily tasks, including blood glucose monitoring and insulin administration. The potential long-term complications of T1D—kidney failure, retinopathy, cardiovascular disease, and death—are grave but can be attenuated with effective glycemic management.2 Still, less than 25% of youths aged 13 to 19 years achieve target hemoglobin A1c (HbA1c) levels.3
Diabetic ketoacidosis (DKA) is an acute, life-threatening complication of T1D associated with suboptimal glycemic management. In the United States, DKA hospitalizations increased during 2009-2014.4 One study found the average standardized cost of a DKA-related pediatric hospitalization exceeded $7000,5 an amount not including missed school days, parent/caregiver workdays, and social and family disruptions. Costs extend to psychological well-being, with patients reporting that they “keep thinking [DKA] may happen again.”6
The burden of T1D-related morbidity is not equitably distributed. Recent evidence suggests that racial/ethnic minorities and those with public insurance experience disproportionately high rates of DKA.7,8 Neighborhood socioeconomic measures, like poverty, add an important dimension illustrative of ecological conditions in which patients reside. These measures have been studied in relation to hospitalization rates for chronic conditions like asthma and heart failure.9,10 For example, one recent study found links between area-level poverty in the United States and the likelihood of readmissions for pediatric patients following an admission for DKA.11 We sought to build on this finding by investigating whether area-level poverty, which we measured as the census tract poverty rate, patient race, and insurance status were associated with the likelihood of initial DKA hospitalization and severity of DKA presentation.
METHODS
Design
We conducted a single-center, retrospective cohort study that examined data on youth with T1D extracted from the Cincinnati Children’s Hospital Medical Center (CCHMC) electronic medical record (EMR). CCHMC is an urban, tertiary-care, freestanding pediatric hospital, with near-complete market penetration in Hamilton County, Ohio, particularly for subspecialty care. The racial and ethnic breakdown of Hamilton County’s general population is 68% White, 27% Black/African American, 3% Asian, and 4% Hispanic or Latino (adds to more than 100% due to rounding).12 The CCHMC Institutional Review Board approved this study.
Study Population
We identified eligible patients through a clinical registry within the EMR, which includes all T1D patients seen at CCHMC within the preceding 24 months. Patients are added to this registry upon initial T1D diagnosis by a research nurse who evaluates autoantibody status and clinical presentation. All patients ≤18 years old who had T1D, were active on the registry, and had an address within Hamilton County as of December 31, 2017, were included. Those with type 2 diabetes mellitus or secondary causes of diabetes were excluded. We captured patients on the registry as of December 31, 2017, and then examined whether they had been hospitalized from January 1, 2011, to December 31, 2017.
Covariates
We extracted demographic and clinical data for patients within the T1D registry as of December 31, 2017, and, for those hospitalized, at the time of their admission. Specifically, we extracted date of birth (to calculate age at encounter), sex, and ethnicity. Age was treated as a continuous variable. We defined ethnicity as Latinx (inclusive of Hispanic) or non-Latinx. We calculated duration of T1D diagnosis for patients within the registry by taking the difference of the diabetes onset date and the end of the study period. We extracted whether registry patients had an insulin pump or a continuous glucose monitor (CGM). Hospitalized patients were identified as new-onset or established T1D by comparing their hospitalization with the T1D diagnosis onset date.
For the entire T1D registry, we also assessed a key clinical characteristic: a patient’s median HbA1c. We enumerated the median HbA1c of all patients’ medians within the calendar year 2017, consistent with the methodology of a large international T1D registry.13 Among the subset of patients who were hospitalized, we obtained the HbA1c value during or prior to hospitalization.
Exposures
We captured exposure variables from EMR documentation of patients’ address, race, and insurance status. Given the small regional population of Latinx patients, we did not include ethnicity as an exposure. We used the address and insurance status at the beginning of the study period for established T1D patients, or at the time of diagnosis for new-onset patients, for analyses related to the likelihood of DKA admission. We used the address at the time of hospitalization for analyses of DKA presentation severity. We geocoded home addresses using ArcGIS software (Version 10.5.1; Esri), successfully geocoding >95% to the street level and assigning patients to a corresponding census tract—a census-defined geography consisting of approximately 4000 people.14 Census tracts, when drawn after the decennial (every 10 years) census, are designed to be sociodemographically homogenous15 and have long been used in medical and public health research.16 We connected geocoded addresses to the census tract poverty rate, defined as the percentage of individuals within a tract living below the federal poverty level. For our analyses, we primarily treated census tract poverty as a continuous variable ranging from 0% to 100%. We visualized the distribution of census tract poverty across Hamilton County using a choropleth map (Figure, part A). We also grouped the registry population into quartiles, ordered by census tract poverty levels, to ease the graphical display for Figure, part B. We defined race as White, Black (inclusive of African American), or other. We categorized insurance as Medicaid/public or Non-Medicaid/private.

Outcomes
The primary outcome was a hospitalization for DKA during the study period. We identified eligible events by admission diagnosis of DKA (International Classification of Disease, 10th Revision, Clinical Modification, E13.10 or E10.10). Secondarily, we assessed admission severity for each patient’s first DKA hospitalization using initial pH from a venous blood gas and initial bicarbonate from the basic metabolic panel. These measures were chosen in accordance with the American Diabetes Association’s published classification of DKA severity, which uses pH, bicarbonate, and mental status.17 We defined the time for correction as the minutes from admission time until the anion gap was ≤12 or the bicarbonate was ≥18. We calculated total inpatient bed-days and pediatric intensive care unit (PICU) bed-days, and identified whether a patient was readmitted for DKA during the study period.
Statistical Analyses
We used descriptive statistics to characterize demographic and clinical information for patients within the T1D registry and for the hospitalized subset. We examined DKA severity data for all admitted patients by diagnosis status (new-onset T1D vs established). Associations between categorical outcomes were assessed using the Chi-square or Fisher exact test, and continuous outcomes were evaluated using the Mann-Whitney Rank Sum test.
We individually examined the effects of census tract poverty, race, and insurance on the odds of hospitalization through logistic regression. After confirming overlap and variability in census tract poverty rates by race and admission status (Appendix Table 1), we included these exposures in a multivariable model to capture their independent effects. Because the impact of census tract poverty, race, and insurance on hospitalization odds could vary depending on T1D diagnosis status, we conducted a sensitivity analysis by repeating the multivariable model with only established T1D patients.
Given that median HbA1c levels differed for T1D registry patients based on census tract poverty, race, and insurance, we used linear regression in a post-hoc analysis to evaluate associations between those exposures and median HbA1c. Age, gender, ethnicity, and duration of diabetes were not included in the models given that these variables did not differ by outcome variables. Diabetes technology—CGM and insulin pumps—was not included as a confounder because we hypothesized it mediated the effect of area-level poverty. For analyses, SigmaPlot (Version 14.0; SPSS Inc.) and R statistical software were used (The R Project for Statistical Computing).
RESULTS
A total of 439 patients were in the T1D registry and lived in Hamilton County as of December 31, 2017. Their demographic characteristics are listed in Table 1. Their median age was 14 years; 48% were female. The median poverty rate of the census tracts in which these youth lived was 11%. A total of 24.6% of patients identified as Black, 73.1% as White, and 2.3% as Hispanic; 35.8% were publicly insured. Patients had a median duration of T1D of 61 months (5 years, 1 month), with a median HbA1c (of all patients’ medians) of 8.7%. A total of 58.1% had a CGM, and 56% used an insulin pump.

Hospitalization Characteristics of Those in the T1D Registry
Approximately one-third of registry patients (n = 152) experienced ≥1 hospitalization for DKA during the study period (inclusive of new-onset and established diagnosis). Age, gender, ethnicity, and duration of T1D diagnosis were similar between those who experienced a hospitalization and those who did not. The median census tract poverty rate was higher among those who were hospitalized compared to those who were not (15% vs 8%, P < .01). Among hospitalized patients, 41% identified as Black; among all who were not hospitalized, 16% identified as Black (P < .01). Among all hospitalized patients, 56.6% were covered by public insurance, while 24.7% of non-hospitalized patients were covered by public insurance (P < .01). Hospitalized patients were more likely to have a higher median HbA1c (9.5% vs 8.4%, P < .01) and were significantly less likely to have a CGM or insulin pump (both P < .01). These associations were magnified among the roughly 10% of the T1D registry population (n = 42) who experienced ≥2 hospitalizations.
Figure, part A is a choropleth map depicting the distribution of poverty by census tract across Hamilton County. The map is complemented by part B of the Figure, which displays the T1D registry population split into poverty quartiles and each quartile’s DKA admission rate per T1D registry population. As census tract poverty increases, so too does the rate of DKA hospitalizations. There is an almost threefold difference between the hospitalization rate of the highest poverty quartile and that of the lowest.
Likelihood of Hospitalization
We examined the exposures of interest—census tract poverty, race, and insurance—and odds of hospitalization at a patient level using logistic regression models (Table 2). In the multivariable model that included these exposures, we found that for every 10% increase in the census tract poverty rate, the odds of being hospitalized for DKA increased by 22% (95% CI, 1.03-1.47). Race was not significantly associated with odds of DKA hospitalization in the multivariable model. Public insurance was associated with a 2.71-times higher odds of hospitalization compared to those with private insurance (95% CI, 1.62-4.55). Our sensitivity analysis of only those with established T1D diagnoses showed similar results to that of the entire T1D registry population.

Severity of DKA Presentation
A total of 152 registry patients were admitted; 89 patients had new-onset T1D, and 63 had an established T1D diagnosis. We examined presenting factors (initial pH, initial bicarbonate, HbA1c) and clinical characteristics (time to correction, inpatient bed-days, PICU bed-days) by census tract poverty, race, and insurance for all hospitalized patients. There were no significant differences in presenting factors or outcomes by census tract poverty or insurance. However, we did note certain differences by race. Indeed, though initial pH, bicarbonate, and time to correction did not meaningfully differ by race, Black patients had a longer length of stay than their White peers (Appendix Table 2; 3.06 vs 2.16 days, P < .01). We further examined this by diagnosis status, as newly diagnosed patients often stay longer for diabetes education.18 Black patients had a longer length of stay than White patients, regardless of whether they had new-onset (3.85 vs 2.99 days, P < .01) or established T1D (1.83 vs 0.97 days, P < .01). Additionally, we found that the HbA1c was significantly higher in Black versus White patients (12.4% vs 10.8%, P = .01). HbA1c was significantly different between established T1D patients identifying as Black and those identifying as White (11.7% vs 9.5%, P < .01); however, HbA1c did not differ by race for newly diagnosed patients (12.5% vs 13.0%, P = .63).
Median HbA1c of All T1D Registry Patients
Given the suggestion of HbA1c variability by exposure variables, we performed a post-hoc linear regression analysis. Table 3 displays the linear regression models assessing associations of census tract poverty, race, and insurance on median HbA1c for all T1D registry patients. In the multivariable model, census tract poverty was no longer associated with higher HbA1c levels (0.13, 95% CI, –0.01 to 0.26). However, Black patients continued to have significantly higher HbA1c levels than White patients (1.09% higher; 95% CI, 0.59-1.59). Also, those with public insurance had significantly higher HbA1c levels than those with private insurance (0.93% higher; 95% CI, 0.51-1.35).

DISCUSSION
Living in high poverty areas, identifying as Black, and having public insurance were each associated with a higher likelihood of hospitalization for DKA in an unadjusted model. When we examined these exposures together in a multivariable model, census tract poverty and insurance remained associated with a higher likelihood of hospitalization for DKA. Race—with a wide confidence interval—was no longer associated with a higher likelihood of hospitalization. The increased likelihood of hospitalization initially seen for Black patients may be because they are more likely to reside in high poverty areas and be on public insurance—inequities associated with structural racism.
These results mirror those of other studies that have found area-level poverty to be associated with higher rates of intensive care admissions and hospitalizations for conditions like asthma.10,19 Our findings are also similar to those of a previous study that found that area-level deprivation was associated with a greater likelihood of DKA readmissions in pediatric and adult patients, as well as an adult study that showed increased neighborhood deprivation was associated with higher odds of DKA hospitalization.11,20 Further examination of the mechanisms underlying DKA hospitalization is clearly warranted and would be informed by a deeper awareness of the social determinants of health—such as community context and the neighborhood/built environment21—and their links to racial inequities.
Neighborhood socioeconomic status, here measured as census tract poverty, is likely linked to hospitalization for DKA through contextual factors like food insecurity, limited access to supportive care (eg, presence of a school nurse who can co-manage T1D with the patient/family and medical team), and adverse environmental exposures.22-24 To improve the health of patients, prevent hospitalizations, and achieve health equity, we suggest that both individual factors and these contextual factors need to be evaluated and addressed through community-connected interventions.25,26 For example, interventions that deploy community health workers to navigate the complexities of health systems have been effective in reducing HbA1c levels in adults with type 2 diabetes mellitus27 and are beginning to be studied in pediatrics.28
Black patients had a higher median HbA1c compared to their White counterparts in the T1D registry. One study has previously shown that hemoglobin glycation biologically differed by around 0.5% in Black patients compared to White patients.29 Despite this purported biological difference, a greater difference in HbA1c—nearly 2 points—was seen in the univariate model, illustrating that contextual factors such as socioeconomic status and treatment patterns, like the use of diabetes technology, exceed this biological difference.30-32 Our multivariable results support the importance of these contextual factors, as the HbA1c difference decreased from 1.9 to 1.09 after census tract poverty and insurance were included.
Such differences by race also emerge from experiences of discrimination and bias. Thus, to improve outcomes and equity, we must understand and address racism and its many ill effects. Different levels of racism, such as structural or personally mediated racism, interact with social determinants of health in various ways.33,34 For example, redlining—racially discriminatory grading of neighborhoods’ creditworthiness in the 1930s—is an example of structural racism that continues to affect the health of patients today.35,36 Black families are more likely to live in areas with higher poverty, even after controlling for household income level.37 The interaction between race and poverty is complex given these historical underpinnings of structural racism, as evidenced by race no longer being associated with an increased likelihood of DKA hospitalization in our multivariable analysis. The longer length of stay for Black patients despite similar presenting severity also exemplifies this complexity. For example, longer stays could result from explicit or implicit biases that lead providers to keep patients longer due to preconceived beliefs that the caregiver does not understand diabetes management.38 However, it also could be that the caregiver takes longer to complete diabetes education due to systemic barriers, such as work-related obligations during business hours when diabetes educators are present, or transportation and childcare needs.39 These barriers are often tied to socioeconomic status, making it more difficult for those in poverty to complete required education. We cannot conclude from our study the reasons for the difference in length of stay, just that differences exist. Moving forward, we suggest that design of interventions aimed at achieving health equity consider the complex interplay between health, healthcare systems, racism, and neighborhood context.
There were limitations to our study. First, given that this was an observational study, unmeasured confounders like patient or parental education and personal income could affect the strength of associations seen in our models. Second, although the CCHMC’s Diabetes Center treats nearly all children with T1D in Hamilton County, some patients, or hospitalizations at other centers, could have been missed. Third, the demographics of Hamilton County may not generalize to other diabetes centers. Fourth, although more granular data—such as census block groups or blocks—are available from the US Census Bureau, we chose census tracts as our unit of analysis to reduce sampling error that emerges from the use of such smaller geographies. Last, we measured census tract poverty rather than other contextual markers of one’s neighborhood, like vacant housing or the proportion of those receiving public benefits. However, these measures are often highly correlated with census tract poverty.40
CONCLUSION
Census tract poverty, race, and insurance were each associated with an increased likelihood of hospitalization for DKA. However, in a multivariable model, the effect of race was reduced so it was no longer significant, indicating that racial differences in DKA hospitalization are at least partially explained by differences in socioeconomic factors. The severity of DKA presentation did not differ by census tract poverty or insurance, although Black patients experienced differences in care despite similar markers of DKA severity. Addressing contextual factors—such as social determinants of health and racism—is warranted for future interventions that aim to eliminate equity gaps for T1D patients.
Type 1 diabetes mellitus (T1D) is a common chronic condition of childhood. Its incidence has risen steadily over the past two decades.1 Treatment requires complex daily tasks, including blood glucose monitoring and insulin administration. The potential long-term complications of T1D—kidney failure, retinopathy, cardiovascular disease, and death—are grave but can be attenuated with effective glycemic management.2 Still, less than 25% of youths aged 13 to 19 years achieve target hemoglobin A1c (HbA1c) levels.3
Diabetic ketoacidosis (DKA) is an acute, life-threatening complication of T1D associated with suboptimal glycemic management. In the United States, DKA hospitalizations increased during 2009-2014.4 One study found the average standardized cost of a DKA-related pediatric hospitalization exceeded $7000,5 an amount not including missed school days, parent/caregiver workdays, and social and family disruptions. Costs extend to psychological well-being, with patients reporting that they “keep thinking [DKA] may happen again.”6
The burden of T1D-related morbidity is not equitably distributed. Recent evidence suggests that racial/ethnic minorities and those with public insurance experience disproportionately high rates of DKA.7,8 Neighborhood socioeconomic measures, like poverty, add an important dimension illustrative of ecological conditions in which patients reside. These measures have been studied in relation to hospitalization rates for chronic conditions like asthma and heart failure.9,10 For example, one recent study found links between area-level poverty in the United States and the likelihood of readmissions for pediatric patients following an admission for DKA.11 We sought to build on this finding by investigating whether area-level poverty, which we measured as the census tract poverty rate, patient race, and insurance status were associated with the likelihood of initial DKA hospitalization and severity of DKA presentation.
METHODS
Design
We conducted a single-center, retrospective cohort study that examined data on youth with T1D extracted from the Cincinnati Children’s Hospital Medical Center (CCHMC) electronic medical record (EMR). CCHMC is an urban, tertiary-care, freestanding pediatric hospital, with near-complete market penetration in Hamilton County, Ohio, particularly for subspecialty care. The racial and ethnic breakdown of Hamilton County’s general population is 68% White, 27% Black/African American, 3% Asian, and 4% Hispanic or Latino (adds to more than 100% due to rounding).12 The CCHMC Institutional Review Board approved this study.
Study Population
We identified eligible patients through a clinical registry within the EMR, which includes all T1D patients seen at CCHMC within the preceding 24 months. Patients are added to this registry upon initial T1D diagnosis by a research nurse who evaluates autoantibody status and clinical presentation. All patients ≤18 years old who had T1D, were active on the registry, and had an address within Hamilton County as of December 31, 2017, were included. Those with type 2 diabetes mellitus or secondary causes of diabetes were excluded. We captured patients on the registry as of December 31, 2017, and then examined whether they had been hospitalized from January 1, 2011, to December 31, 2017.
Covariates
We extracted demographic and clinical data for patients within the T1D registry as of December 31, 2017, and, for those hospitalized, at the time of their admission. Specifically, we extracted date of birth (to calculate age at encounter), sex, and ethnicity. Age was treated as a continuous variable. We defined ethnicity as Latinx (inclusive of Hispanic) or non-Latinx. We calculated duration of T1D diagnosis for patients within the registry by taking the difference of the diabetes onset date and the end of the study period. We extracted whether registry patients had an insulin pump or a continuous glucose monitor (CGM). Hospitalized patients were identified as new-onset or established T1D by comparing their hospitalization with the T1D diagnosis onset date.
For the entire T1D registry, we also assessed a key clinical characteristic: a patient’s median HbA1c. We enumerated the median HbA1c of all patients’ medians within the calendar year 2017, consistent with the methodology of a large international T1D registry.13 Among the subset of patients who were hospitalized, we obtained the HbA1c value during or prior to hospitalization.
Exposures
We captured exposure variables from EMR documentation of patients’ address, race, and insurance status. Given the small regional population of Latinx patients, we did not include ethnicity as an exposure. We used the address and insurance status at the beginning of the study period for established T1D patients, or at the time of diagnosis for new-onset patients, for analyses related to the likelihood of DKA admission. We used the address at the time of hospitalization for analyses of DKA presentation severity. We geocoded home addresses using ArcGIS software (Version 10.5.1; Esri), successfully geocoding >95% to the street level and assigning patients to a corresponding census tract—a census-defined geography consisting of approximately 4000 people.14 Census tracts, when drawn after the decennial (every 10 years) census, are designed to be sociodemographically homogenous15 and have long been used in medical and public health research.16 We connected geocoded addresses to the census tract poverty rate, defined as the percentage of individuals within a tract living below the federal poverty level. For our analyses, we primarily treated census tract poverty as a continuous variable ranging from 0% to 100%. We visualized the distribution of census tract poverty across Hamilton County using a choropleth map (Figure, part A). We also grouped the registry population into quartiles, ordered by census tract poverty levels, to ease the graphical display for Figure, part B. We defined race as White, Black (inclusive of African American), or other. We categorized insurance as Medicaid/public or Non-Medicaid/private.

Outcomes
The primary outcome was a hospitalization for DKA during the study period. We identified eligible events by admission diagnosis of DKA (International Classification of Disease, 10th Revision, Clinical Modification, E13.10 or E10.10). Secondarily, we assessed admission severity for each patient’s first DKA hospitalization using initial pH from a venous blood gas and initial bicarbonate from the basic metabolic panel. These measures were chosen in accordance with the American Diabetes Association’s published classification of DKA severity, which uses pH, bicarbonate, and mental status.17 We defined the time for correction as the minutes from admission time until the anion gap was ≤12 or the bicarbonate was ≥18. We calculated total inpatient bed-days and pediatric intensive care unit (PICU) bed-days, and identified whether a patient was readmitted for DKA during the study period.
Statistical Analyses
We used descriptive statistics to characterize demographic and clinical information for patients within the T1D registry and for the hospitalized subset. We examined DKA severity data for all admitted patients by diagnosis status (new-onset T1D vs established). Associations between categorical outcomes were assessed using the Chi-square or Fisher exact test, and continuous outcomes were evaluated using the Mann-Whitney Rank Sum test.
We individually examined the effects of census tract poverty, race, and insurance on the odds of hospitalization through logistic regression. After confirming overlap and variability in census tract poverty rates by race and admission status (Appendix Table 1), we included these exposures in a multivariable model to capture their independent effects. Because the impact of census tract poverty, race, and insurance on hospitalization odds could vary depending on T1D diagnosis status, we conducted a sensitivity analysis by repeating the multivariable model with only established T1D patients.
Given that median HbA1c levels differed for T1D registry patients based on census tract poverty, race, and insurance, we used linear regression in a post-hoc analysis to evaluate associations between those exposures and median HbA1c. Age, gender, ethnicity, and duration of diabetes were not included in the models given that these variables did not differ by outcome variables. Diabetes technology—CGM and insulin pumps—was not included as a confounder because we hypothesized it mediated the effect of area-level poverty. For analyses, SigmaPlot (Version 14.0; SPSS Inc.) and R statistical software were used (The R Project for Statistical Computing).
RESULTS
A total of 439 patients were in the T1D registry and lived in Hamilton County as of December 31, 2017. Their demographic characteristics are listed in Table 1. Their median age was 14 years; 48% were female. The median poverty rate of the census tracts in which these youth lived was 11%. A total of 24.6% of patients identified as Black, 73.1% as White, and 2.3% as Hispanic; 35.8% were publicly insured. Patients had a median duration of T1D of 61 months (5 years, 1 month), with a median HbA1c (of all patients’ medians) of 8.7%. A total of 58.1% had a CGM, and 56% used an insulin pump.

Hospitalization Characteristics of Those in the T1D Registry
Approximately one-third of registry patients (n = 152) experienced ≥1 hospitalization for DKA during the study period (inclusive of new-onset and established diagnosis). Age, gender, ethnicity, and duration of T1D diagnosis were similar between those who experienced a hospitalization and those who did not. The median census tract poverty rate was higher among those who were hospitalized compared to those who were not (15% vs 8%, P < .01). Among hospitalized patients, 41% identified as Black; among all who were not hospitalized, 16% identified as Black (P < .01). Among all hospitalized patients, 56.6% were covered by public insurance, while 24.7% of non-hospitalized patients were covered by public insurance (P < .01). Hospitalized patients were more likely to have a higher median HbA1c (9.5% vs 8.4%, P < .01) and were significantly less likely to have a CGM or insulin pump (both P < .01). These associations were magnified among the roughly 10% of the T1D registry population (n = 42) who experienced ≥2 hospitalizations.
Figure, part A is a choropleth map depicting the distribution of poverty by census tract across Hamilton County. The map is complemented by part B of the Figure, which displays the T1D registry population split into poverty quartiles and each quartile’s DKA admission rate per T1D registry population. As census tract poverty increases, so too does the rate of DKA hospitalizations. There is an almost threefold difference between the hospitalization rate of the highest poverty quartile and that of the lowest.
Likelihood of Hospitalization
We examined the exposures of interest—census tract poverty, race, and insurance—and odds of hospitalization at a patient level using logistic regression models (Table 2). In the multivariable model that included these exposures, we found that for every 10% increase in the census tract poverty rate, the odds of being hospitalized for DKA increased by 22% (95% CI, 1.03-1.47). Race was not significantly associated with odds of DKA hospitalization in the multivariable model. Public insurance was associated with a 2.71-times higher odds of hospitalization compared to those with private insurance (95% CI, 1.62-4.55). Our sensitivity analysis of only those with established T1D diagnoses showed similar results to that of the entire T1D registry population.

Severity of DKA Presentation
A total of 152 registry patients were admitted; 89 patients had new-onset T1D, and 63 had an established T1D diagnosis. We examined presenting factors (initial pH, initial bicarbonate, HbA1c) and clinical characteristics (time to correction, inpatient bed-days, PICU bed-days) by census tract poverty, race, and insurance for all hospitalized patients. There were no significant differences in presenting factors or outcomes by census tract poverty or insurance. However, we did note certain differences by race. Indeed, though initial pH, bicarbonate, and time to correction did not meaningfully differ by race, Black patients had a longer length of stay than their White peers (Appendix Table 2; 3.06 vs 2.16 days, P < .01). We further examined this by diagnosis status, as newly diagnosed patients often stay longer for diabetes education.18 Black patients had a longer length of stay than White patients, regardless of whether they had new-onset (3.85 vs 2.99 days, P < .01) or established T1D (1.83 vs 0.97 days, P < .01). Additionally, we found that the HbA1c was significantly higher in Black versus White patients (12.4% vs 10.8%, P = .01). HbA1c was significantly different between established T1D patients identifying as Black and those identifying as White (11.7% vs 9.5%, P < .01); however, HbA1c did not differ by race for newly diagnosed patients (12.5% vs 13.0%, P = .63).
Median HbA1c of All T1D Registry Patients
Given the suggestion of HbA1c variability by exposure variables, we performed a post-hoc linear regression analysis. Table 3 displays the linear regression models assessing associations of census tract poverty, race, and insurance on median HbA1c for all T1D registry patients. In the multivariable model, census tract poverty was no longer associated with higher HbA1c levels (0.13, 95% CI, –0.01 to 0.26). However, Black patients continued to have significantly higher HbA1c levels than White patients (1.09% higher; 95% CI, 0.59-1.59). Also, those with public insurance had significantly higher HbA1c levels than those with private insurance (0.93% higher; 95% CI, 0.51-1.35).

DISCUSSION
Living in high poverty areas, identifying as Black, and having public insurance were each associated with a higher likelihood of hospitalization for DKA in an unadjusted model. When we examined these exposures together in a multivariable model, census tract poverty and insurance remained associated with a higher likelihood of hospitalization for DKA. Race—with a wide confidence interval—was no longer associated with a higher likelihood of hospitalization. The increased likelihood of hospitalization initially seen for Black patients may be because they are more likely to reside in high poverty areas and be on public insurance—inequities associated with structural racism.
These results mirror those of other studies that have found area-level poverty to be associated with higher rates of intensive care admissions and hospitalizations for conditions like asthma.10,19 Our findings are also similar to those of a previous study that found that area-level deprivation was associated with a greater likelihood of DKA readmissions in pediatric and adult patients, as well as an adult study that showed increased neighborhood deprivation was associated with higher odds of DKA hospitalization.11,20 Further examination of the mechanisms underlying DKA hospitalization is clearly warranted and would be informed by a deeper awareness of the social determinants of health—such as community context and the neighborhood/built environment21—and their links to racial inequities.
Neighborhood socioeconomic status, here measured as census tract poverty, is likely linked to hospitalization for DKA through contextual factors like food insecurity, limited access to supportive care (eg, presence of a school nurse who can co-manage T1D with the patient/family and medical team), and adverse environmental exposures.22-24 To improve the health of patients, prevent hospitalizations, and achieve health equity, we suggest that both individual factors and these contextual factors need to be evaluated and addressed through community-connected interventions.25,26 For example, interventions that deploy community health workers to navigate the complexities of health systems have been effective in reducing HbA1c levels in adults with type 2 diabetes mellitus27 and are beginning to be studied in pediatrics.28
Black patients had a higher median HbA1c compared to their White counterparts in the T1D registry. One study has previously shown that hemoglobin glycation biologically differed by around 0.5% in Black patients compared to White patients.29 Despite this purported biological difference, a greater difference in HbA1c—nearly 2 points—was seen in the univariate model, illustrating that contextual factors such as socioeconomic status and treatment patterns, like the use of diabetes technology, exceed this biological difference.30-32 Our multivariable results support the importance of these contextual factors, as the HbA1c difference decreased from 1.9 to 1.09 after census tract poverty and insurance were included.
Such differences by race also emerge from experiences of discrimination and bias. Thus, to improve outcomes and equity, we must understand and address racism and its many ill effects. Different levels of racism, such as structural or personally mediated racism, interact with social determinants of health in various ways.33,34 For example, redlining—racially discriminatory grading of neighborhoods’ creditworthiness in the 1930s—is an example of structural racism that continues to affect the health of patients today.35,36 Black families are more likely to live in areas with higher poverty, even after controlling for household income level.37 The interaction between race and poverty is complex given these historical underpinnings of structural racism, as evidenced by race no longer being associated with an increased likelihood of DKA hospitalization in our multivariable analysis. The longer length of stay for Black patients despite similar presenting severity also exemplifies this complexity. For example, longer stays could result from explicit or implicit biases that lead providers to keep patients longer due to preconceived beliefs that the caregiver does not understand diabetes management.38 However, it also could be that the caregiver takes longer to complete diabetes education due to systemic barriers, such as work-related obligations during business hours when diabetes educators are present, or transportation and childcare needs.39 These barriers are often tied to socioeconomic status, making it more difficult for those in poverty to complete required education. We cannot conclude from our study the reasons for the difference in length of stay, just that differences exist. Moving forward, we suggest that design of interventions aimed at achieving health equity consider the complex interplay between health, healthcare systems, racism, and neighborhood context.
There were limitations to our study. First, given that this was an observational study, unmeasured confounders like patient or parental education and personal income could affect the strength of associations seen in our models. Second, although the CCHMC’s Diabetes Center treats nearly all children with T1D in Hamilton County, some patients, or hospitalizations at other centers, could have been missed. Third, the demographics of Hamilton County may not generalize to other diabetes centers. Fourth, although more granular data—such as census block groups or blocks—are available from the US Census Bureau, we chose census tracts as our unit of analysis to reduce sampling error that emerges from the use of such smaller geographies. Last, we measured census tract poverty rather than other contextual markers of one’s neighborhood, like vacant housing or the proportion of those receiving public benefits. However, these measures are often highly correlated with census tract poverty.40
CONCLUSION
Census tract poverty, race, and insurance were each associated with an increased likelihood of hospitalization for DKA. However, in a multivariable model, the effect of race was reduced so it was no longer significant, indicating that racial differences in DKA hospitalization are at least partially explained by differences in socioeconomic factors. The severity of DKA presentation did not differ by census tract poverty or insurance, although Black patients experienced differences in care despite similar markers of DKA severity. Addressing contextual factors—such as social determinants of health and racism—is warranted for future interventions that aim to eliminate equity gaps for T1D patients.
1. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376(15):1419-1429. https://doi.org/10.1056/NEJMoa1610187
2. Writing Group for the DCCT/EDIC Research Group, Orchard TJ, Nathan DM, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA. 2015;313(1):45-53. https://doi.org/10.1001/jama.2014.16107
3. Wood JR, Miller KM, Maahs DM, et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36(7):2035-2037. https://doi.org/10.2337/dc12-1959
4. Benoit SR. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018;67(12):362-365. https://doi.org/10.15585/mmwr.mm6712a3
5. Tieder JS, McLeod L, Keren R, et al. Variation in resource use and readmission for diabetic ketoacidosis in children’s hospitals. Pediatrics. 2013;132(2):229-236. https://doi.org/10.1542/peds.2013-0359
6. Moffett MA, Buckingham JC, Baker CR, Hawthorne G, Leech NJ. Patients’ experience of admission to hospital with diabetic ketoacidosis and its psychological impact: an exploratory qualitative study. Practical Diabetes. 2013;30(5):203-207. https://doi.org/10.1002/pdi.1777
7. Maahs DM, Hermann JM, Holman N, et al. Rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the U.S., Austria, and Germany. Diabetes Care. 2015;38(10):1876-1882. https://doi.org/10.2337/dc15-0780
8. Malik FS, Hall M, Mangione-Smith R, et al. Patient characteristics associated with differences in admission frequency for diabetic ketoacidosis in United States children’s hospitals. J Pediatr. 2016;171:104-110. https://doi.org/10.1016/j.jpeds.2015.12.015
9. Manickam RN, Mu Y, Kshirsagar AV, Bang H. Area-level poverty and excess hospital readmission ratios. Am J Med. 2017;130(4):e153-e155. https://doi.org/10.1016/j.amjmed.2016.08.047
10. Beck AF, Moncrief T, Huang B, et al. Inequalities in neighborhood child asthma admission rates and underlying community characteristics in one US county. J Pediatr. 2013;163(2):574-580.e1. https://doi.org/10.1016/j.jpeds.2013.01.064
11. Everett E, Mathioudakis N. Association of area deprivation and diabetic ketoacidosis readmissions: comparative risk analysis of adults vs children with type 1 diabetes. J Clin Endocrinol Metab. 2019;104(8):3473-3480. https://doi.org/10.1210/jc.2018-02232
12. U.S. Census Bureau. QuickFacts: Hamilton County, Ohio. Accessed March 2, 2020. https://www.census.gov/quickfacts/hamiltoncountyohio
13. Witsch M, Kosteria I, Kordonouri O, et al. Possibilities and challenges of a large international benchmarking in pediatric diabetology—the SWEET experience. Pediatr Diabetes. 2016;17(S23):7-15. https://doi.org/10.1111/pedi.12432
14. U.S. Census Bureau. Glossary. Accessed April 22, 2021. https://www.census.gov/programs-surveys/geography/about/glossary.html
15. U.S. Census Bureau. Geographic areas reference manual. Chapter 10: Census tracts and block numbering areas. Accessed April 22, 2021. https://www.census.gov/programs-surveys/geography/guidance/geographic-areas-reference-manual.html
16. Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: The Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312-323. https://doi.org/10.2105/AJPH.2003.032482
17. Kitabchi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153. https://doi.org/10.2337/diacare.24.1.131
18. Lawson S, Redel JM, Smego A, et al. Assessment of a day hospital management program for children with type 1 diabetes. JAMA Netw Open. 2020;3(3):e200347. https://doi.org/10.1001/jamanetworkopen.2020.0347
19. Andrist E, Riley CL, Brokamp C, Taylor S, Beck AF. Neighborhood poverty and pediatric intensive care use. Pediatrics. 2019;144(6):e20190748. https://doi.org/10.1542/peds.2019-0748
20. Govan L, Maietti E, Torsney B, et al. The effect of deprivation and HbA1c on admission to hospital for diabetic ketoacidosis in type 1 diabetes. Diabetologia. 2012;55(9):2356-2360. https://doi.org/10.1007/s00125-012-2601-6
21. U.S. Department of Health and Human Services. Healthy People 2030—Social Determinants of Health. Accessed May 13, 2021. https://health.gov/healthypeople/objectives-and-data/social-determinants-health
22. Berkowitz SA, Karter AJ, Corbie-Smith G, et al. Food insecurity, food “deserts,” and glycemic control in patients with diabetes: a longitudinal analysis. Diabetes Care. 2018;41(6):1188-1195. https://doi.org/10.2337/dc17-1981
23. Nguyen TM, Mason KJ, Sanders CG, Yazdani P, Heptulla RA. Targeting blood glucose management in school improves glycemic control in children with poorly controlled type 1 diabetes mellitus. J Pediatr. 2008;153(4):575-578. https://doi.org/10.1016/j.jpeds.2008.04.066
24. Izquierdo R, Morin PC, Bratt K, et al. School-centered telemedicine for children with type 1 diabetes mellitus. J Pediatr. 2009;155(3):374-379. https://doi.org/10.1016/j.jpeds.2009.03.014
25. Council on Community Pediatrics and Committee on Native American Child Health. Policy statement—health equity and children’s rights. Pediatrics. 2010;125(4):838-849. https://doi.org/10.1542/peds.2010-0235
26. Braveman P. What are health disparities and health equity? We need to be clear. Public Health Rep. 2014;129(suppl 2):5-8.
27. Palmas W, March D, Darakjy S, et al. Community health worker interventions to improve glycemic control in people with diabetes: a systematic review and meta-analysis. J Gen Intern Med. 2015;30(7):1004-1012. https://doi.org/10.1007/s11606-015-3247-0
28. Lipman TH, Smith JA, Hawkes CP. Community health workers and the care of children with type 1 diabetes. J Pediatr Nurs. 2019;49:111-112. https://doi.org/10.1016/j.pedn.2019.08.014
29. Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95-102. https://doi.org/10.7326/M16-2596
30. Agarwal S, Kanapka LG, Raymond JK, et al. Racial-ethnic inequity in young adults with type 1 diabetes. J Clin Endocrinol Metab. 2020;105(8):e2960-e2969. https://doi.org/10.1210/clinem/dgaa236
31. Addala A, Auzanneau M, Miller K, et al. A decade of disparities in diabetes technology use and HbA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care. 2021;44(1):133-140. https://doi.org/10.2337/dc20-0257
32. Lipman TH, Smith JA, Patil O, Willi SM, Hawkes CP. Racial disparities in treatment and outcomes of children with type 1 diabetes. Pediatr Diabetes. 2021;22(2):241-248. https://doi.org/10.1111/pedi.13139
33. Boyd RW, Lindo EG, Weeks LD, McLemore MR. On racism: a new standard for publishing on racial health inequities. Health Affairs. Accessed July 9, 2020. https://www.healthaffairs.org/do/10.1377/hblog20200630.939347/full/
34. Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90(8):1212-1215. https://doi.org/10.2105/AJPH.90.8.1212
35. Jacoby SF, Dong B, Beard JH, Wiebe DJ, Morrison CN. The enduring impact of historical and structural racism on urban violence in Philadelphia. Soc Sci Med. 2018;199:87-95. https://doi.org/10.1016/j.socscimed.2017.05.038
36. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453-1463. https://doi.org/10.1016/S0140-6736(17)30569-X
37. Reardon SF, Fox L, Townsend J. Neighborhood income composition by household race and income, 1990–2009. Ann Am Acad Pol Soc Sci. 2015;660(1):78-97. https://doi.org/10.1177/0002716215576104
38. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. https://doi.org/10.2105/AJPH.2011.300558
39. Lawton J, Waugh N, Noyes K, et al. Improving communication and recall of information in paediatric diabetes consultations: a qualitative study of parents’ experiences and views. BMC Pediatr. 2015;15:67. https://doi.org/10.1186/s12887-015-0388-6
40. Brokamp C, Beck AF, Goyal NK, Ryan P, Greenberg JM, Hall ES. Material community deprivation and hospital utilization during the first year of life: an urban population-based cohort study. Ann Epidemiol. 2019;30:37-43. https://doi.org/10.1016/j.annepidem.2018.11.008
1. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376(15):1419-1429. https://doi.org/10.1056/NEJMoa1610187
2. Writing Group for the DCCT/EDIC Research Group, Orchard TJ, Nathan DM, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA. 2015;313(1):45-53. https://doi.org/10.1001/jama.2014.16107
3. Wood JR, Miller KM, Maahs DM, et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36(7):2035-2037. https://doi.org/10.2337/dc12-1959
4. Benoit SR. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018;67(12):362-365. https://doi.org/10.15585/mmwr.mm6712a3
5. Tieder JS, McLeod L, Keren R, et al. Variation in resource use and readmission for diabetic ketoacidosis in children’s hospitals. Pediatrics. 2013;132(2):229-236. https://doi.org/10.1542/peds.2013-0359
6. Moffett MA, Buckingham JC, Baker CR, Hawthorne G, Leech NJ. Patients’ experience of admission to hospital with diabetic ketoacidosis and its psychological impact: an exploratory qualitative study. Practical Diabetes. 2013;30(5):203-207. https://doi.org/10.1002/pdi.1777
7. Maahs DM, Hermann JM, Holman N, et al. Rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the U.S., Austria, and Germany. Diabetes Care. 2015;38(10):1876-1882. https://doi.org/10.2337/dc15-0780
8. Malik FS, Hall M, Mangione-Smith R, et al. Patient characteristics associated with differences in admission frequency for diabetic ketoacidosis in United States children’s hospitals. J Pediatr. 2016;171:104-110. https://doi.org/10.1016/j.jpeds.2015.12.015
9. Manickam RN, Mu Y, Kshirsagar AV, Bang H. Area-level poverty and excess hospital readmission ratios. Am J Med. 2017;130(4):e153-e155. https://doi.org/10.1016/j.amjmed.2016.08.047
10. Beck AF, Moncrief T, Huang B, et al. Inequalities in neighborhood child asthma admission rates and underlying community characteristics in one US county. J Pediatr. 2013;163(2):574-580.e1. https://doi.org/10.1016/j.jpeds.2013.01.064
11. Everett E, Mathioudakis N. Association of area deprivation and diabetic ketoacidosis readmissions: comparative risk analysis of adults vs children with type 1 diabetes. J Clin Endocrinol Metab. 2019;104(8):3473-3480. https://doi.org/10.1210/jc.2018-02232
12. U.S. Census Bureau. QuickFacts: Hamilton County, Ohio. Accessed March 2, 2020. https://www.census.gov/quickfacts/hamiltoncountyohio
13. Witsch M, Kosteria I, Kordonouri O, et al. Possibilities and challenges of a large international benchmarking in pediatric diabetology—the SWEET experience. Pediatr Diabetes. 2016;17(S23):7-15. https://doi.org/10.1111/pedi.12432
14. U.S. Census Bureau. Glossary. Accessed April 22, 2021. https://www.census.gov/programs-surveys/geography/about/glossary.html
15. U.S. Census Bureau. Geographic areas reference manual. Chapter 10: Census tracts and block numbering areas. Accessed April 22, 2021. https://www.census.gov/programs-surveys/geography/guidance/geographic-areas-reference-manual.html
16. Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: The Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312-323. https://doi.org/10.2105/AJPH.2003.032482
17. Kitabchi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153. https://doi.org/10.2337/diacare.24.1.131
18. Lawson S, Redel JM, Smego A, et al. Assessment of a day hospital management program for children with type 1 diabetes. JAMA Netw Open. 2020;3(3):e200347. https://doi.org/10.1001/jamanetworkopen.2020.0347
19. Andrist E, Riley CL, Brokamp C, Taylor S, Beck AF. Neighborhood poverty and pediatric intensive care use. Pediatrics. 2019;144(6):e20190748. https://doi.org/10.1542/peds.2019-0748
20. Govan L, Maietti E, Torsney B, et al. The effect of deprivation and HbA1c on admission to hospital for diabetic ketoacidosis in type 1 diabetes. Diabetologia. 2012;55(9):2356-2360. https://doi.org/10.1007/s00125-012-2601-6
21. U.S. Department of Health and Human Services. Healthy People 2030—Social Determinants of Health. Accessed May 13, 2021. https://health.gov/healthypeople/objectives-and-data/social-determinants-health
22. Berkowitz SA, Karter AJ, Corbie-Smith G, et al. Food insecurity, food “deserts,” and glycemic control in patients with diabetes: a longitudinal analysis. Diabetes Care. 2018;41(6):1188-1195. https://doi.org/10.2337/dc17-1981
23. Nguyen TM, Mason KJ, Sanders CG, Yazdani P, Heptulla RA. Targeting blood glucose management in school improves glycemic control in children with poorly controlled type 1 diabetes mellitus. J Pediatr. 2008;153(4):575-578. https://doi.org/10.1016/j.jpeds.2008.04.066
24. Izquierdo R, Morin PC, Bratt K, et al. School-centered telemedicine for children with type 1 diabetes mellitus. J Pediatr. 2009;155(3):374-379. https://doi.org/10.1016/j.jpeds.2009.03.014
25. Council on Community Pediatrics and Committee on Native American Child Health. Policy statement—health equity and children’s rights. Pediatrics. 2010;125(4):838-849. https://doi.org/10.1542/peds.2010-0235
26. Braveman P. What are health disparities and health equity? We need to be clear. Public Health Rep. 2014;129(suppl 2):5-8.
27. Palmas W, March D, Darakjy S, et al. Community health worker interventions to improve glycemic control in people with diabetes: a systematic review and meta-analysis. J Gen Intern Med. 2015;30(7):1004-1012. https://doi.org/10.1007/s11606-015-3247-0
28. Lipman TH, Smith JA, Hawkes CP. Community health workers and the care of children with type 1 diabetes. J Pediatr Nurs. 2019;49:111-112. https://doi.org/10.1016/j.pedn.2019.08.014
29. Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95-102. https://doi.org/10.7326/M16-2596
30. Agarwal S, Kanapka LG, Raymond JK, et al. Racial-ethnic inequity in young adults with type 1 diabetes. J Clin Endocrinol Metab. 2020;105(8):e2960-e2969. https://doi.org/10.1210/clinem/dgaa236
31. Addala A, Auzanneau M, Miller K, et al. A decade of disparities in diabetes technology use and HbA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care. 2021;44(1):133-140. https://doi.org/10.2337/dc20-0257
32. Lipman TH, Smith JA, Patil O, Willi SM, Hawkes CP. Racial disparities in treatment and outcomes of children with type 1 diabetes. Pediatr Diabetes. 2021;22(2):241-248. https://doi.org/10.1111/pedi.13139
33. Boyd RW, Lindo EG, Weeks LD, McLemore MR. On racism: a new standard for publishing on racial health inequities. Health Affairs. Accessed July 9, 2020. https://www.healthaffairs.org/do/10.1377/hblog20200630.939347/full/
34. Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90(8):1212-1215. https://doi.org/10.2105/AJPH.90.8.1212
35. Jacoby SF, Dong B, Beard JH, Wiebe DJ, Morrison CN. The enduring impact of historical and structural racism on urban violence in Philadelphia. Soc Sci Med. 2018;199:87-95. https://doi.org/10.1016/j.socscimed.2017.05.038
36. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453-1463. https://doi.org/10.1016/S0140-6736(17)30569-X
37. Reardon SF, Fox L, Townsend J. Neighborhood income composition by household race and income, 1990–2009. Ann Am Acad Pol Soc Sci. 2015;660(1):78-97. https://doi.org/10.1177/0002716215576104
38. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979-987. https://doi.org/10.2105/AJPH.2011.300558
39. Lawton J, Waugh N, Noyes K, et al. Improving communication and recall of information in paediatric diabetes consultations: a qualitative study of parents’ experiences and views. BMC Pediatr. 2015;15:67. https://doi.org/10.1186/s12887-015-0388-6
40. Brokamp C, Beck AF, Goyal NK, Ryan P, Greenberg JM, Hall ES. Material community deprivation and hospital utilization during the first year of life: an urban population-based cohort study. Ann Epidemiol. 2019;30:37-43. https://doi.org/10.1016/j.annepidem.2018.11.008
© 2021 Society of Hospital Medicine
Continuing Cardiopulmonary Symptoms, Disability, and Financial Toxicity 1 Month After Hospitalization for Third-Wave COVID-19: Early Results From a US Nationwide Cohort
For many patients hospitalized with COVID-19, the impact of the illness continues well beyond hospital discharge.1 Heavy burdens of persistent symptoms have been reported, albeit often from regional and single-hospital samples.2-7 Critically, not all initial reports capture information on pre-COVID-19 symptom burden, so it is unclear whether these highly prevalent problems are truly new; an alternative explanation might be that patients already with symptoms were more likely to be infected with or seek care for SARS-CoV-2.8
Fewer data are available about patients’ abilities to go about the activities of their lives, nor is as much known about the relationships between new symptoms and other impacts. Most of the available information is from health systems during the initial surge of COVID-19 in early 2020—when testing for SARS-CoV-2 was limited even in the inpatient setting; when hospitals’ postdischarge care systems may have been heavily disrupted; and when clinicians were often reasonably focused primarily on reducing mortality in their first cases of COVID-19 rather than promoting recovery from an often-survivable illness. Increasing evidence shows that the inpatient case-fatality rate of COVID-19 is improving over time9,10; this makes unclear the generalizability of outcomes data from early COVID-19 patients to more recent patients.11
Therefore, we report multicenter measurements of incident levels of persistent cardiopulmonary symptoms, disability, return to baseline, and impact on employment among a recent cohort of COVID-19 patients hospitalized around the United States during the “third wave” of COVID-19—fall and winter 2020-2021. We focus on the 1-month time point after hospital discharge, as this time point is still in the early vulnerable period during which hospital transition-of-care programs are understood to have responsibility.
METHODS
The first 253 patients who completed 1-month postdischarge telephone follow-up surveys from the ongoing nationwide BLUE CORAL study were included. BLUE CORAL will enroll up to 1,500 hospitalized COVID-19 patients at 36 US centers (the identities of which are reported in Appendix 1) as a part of the National Heart, Lung, and Blood Institute’s Prevention and Early Treatment of Acute Lung Injury (PETAL) Network. We report here on survey questions that allowed for a clear comparison to be made between 1-month follow-up responses and pre-COVID baseline variables; these comparisons were based on (1) previous in-hospital assessment; (2) explicitly asking patients to compare to pre-COVID-19 levels; or (3) explicitly asking patients for changes in relation to their COVID-19 hospitalization. Items were chosen for inclusion in this report without looking at their association with other variables.
This research was approved by the Vanderbilt Institutional Review Board (IRB), serving as central IRB for the PETAL Network; patients or their surrogates provided informed consent.
Participants
Patients with COVID-19 were identified during hospitalization and within 14 days of a positive molecular test for SARS-CoV-2. Eligible patients presented with fever and/or respiratory signs/symptoms, such as hypoxemia, shortness of breath, or infiltrates on chest imaging. Patients were enrolled within the first 72 hours of hospitalization (in order to avoid oversampling patients with relatively longer stays, and to study the biology of early COVID-19), and excluded if they had comfort-care orders (because of their limited likelihood of surviving to follow-up), or were incarcerated (because of difficulties in obtaining truly open informed consent and likely difficulties in follow-up). Pertinently, patients were not required to be in the intensive care unit.
Surviving patients who spoke English or Spanish, were not homeless on hospital admission, and were neither significantly disabled nor significantly cognitively impaired were eligible for follow-up. “Not significantly disabled” was defined as having limitations due to health on no more than three activities of daily living before their COVID-19 hospitalization, as assessed at BLUE CORAL enrollment; this was chosen because of the potentially limited sensitivity of many of our questionnaires to detect an impact of COVID-19 in patients with greater than this level of disability. We included patients who were able to consent for themselves in the study, or for whom the legally appointed representative consenting on their behalf in the hospital reported no evidence of cognitive impairment, defined as no more than four of the problems on the eight-item Alzheimer’s Dementia (AD8) scale.12-14
Data Collection
One-month surveys were administered to patients or, when necessary, their proxies; the complete English- and Spanish-language instruments are presented in Appendix 2. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Michigan.15,16
Patients were contacted via phone by trained interviewers beginning 21 days after hospital discharge; interviews were completed a median of 47 days after discharge (interquartile range [IQR], 26-61). Efforts prioritized former patients completing surveys themselves by phone, but a well-informed proxy was approached if needed. Proxies, who included spouses, adult children, or other relatives, family friends, or primary caregivers, were in regular contact with the patient and understood the patient’s health status. If necessary, the survey could be completed over multiple phone calls, and a written, mail-back option was available. Other best practices in accurate survey data collection and cohort retention were used.17-19 Participants were given a $10 gift card.
New cardiopulmonary symptoms were queried with symptom-targeted questions informed by the Airways Questionnaire 20,20 the Kansas City Cardiomyopathy Questionnaire,21,22 and the Seattle Angina Questionnaire.23 Whenever a respondent reported a given symptom, they were asked, “Compared to 1 month before your COVID-19 hospitalization, is this better, worse, or about the same?” We counted the number of symptoms which the patient reported as worse.
Using wording from the Health and Retirement Study,24 disability was assessed based on a self-report of any of 14 health-related limitations in activities of daily living or instrumental activities of daily living, as in past studies25: dressing, walking across a room, bathing, eating, getting out of bed, using a toilet, using a map, preparing a hot meal, shopping for groceries, making a phone call, taking medications, paying bills, carrying 10 lb (eg, a heavy bag of groceries), and, as a combined single item, stooping, kneeling, or crouching. Well-chosen proxy reports appear reliable for these items.26 We counted the number of activities for which the patient reported a limitation, comparing those reported at 1 month to those reported during the in-hospital survey assessing pre-illness functioning.
The financial consequences of the COVID-19 hospitalization were assessed in two ways. First, we used a modified version of a World Health Organization Disability Assessment Schedule (WHODAS) 2.0 question27: “Since your COVID-19 hospitalization, how much has your health been a drain on the financial resources of you or your family?” Second, we used the financial toxicity items developed with the Mi-COVID19 study3 based on extensive qualitative interviews with respiratory failure survivors28; these questions were anchored explicitly on “the financial cost of dealing with your COVID-19 hospitalization and related care.”
Data Analysis
There were few missing data, and almost all were on outcome variables. Where present, the degree of missingness is reported and casewise deletion used. Because this was a planned early look at responses to an ongoing survey, with analysis based on the number of accrued responses, the ultimate denominator for response rate calculation is unknown. Therefore, two bounds are presented—the minimum, on the assumption that all remaining uncompleted surveys will be missed; and the maximum, as if the uncompleted surveys were not yet in the eligible denominator.
Variables were summarized with medians and IQRs. Multilevel logistic regression was used to test for differences across demographic characteristics in the rates of development of any new symptom or disability; site-level differences were modeled using a random effect. Gender, race/ethnicity, and age were included in all regressions unless noted otherwise; age was included with both linear and quadratic terms when used as a control variable. For the degree of return to baseline and for the number of new limitations in activities of daily living, we explored associations as dichotomized variables (any/none, using multilevel logistic regression) and as continuous variables (using multilevel linear regression). Percent of variance explained was calculated using the R2 in unadjusted linear regression, and Spearman rank correlations were used to allow nonlinearities in comparisons across outcomes. All adjusted models are presented in Appendix Table 1. Analyses were conducted in Stata 16.1 (StataCorp, 2020); analytic code is presented in Appendix 3, and a log file of all analyses is in Appendix 4.
RESULTS
The 250th 1-month follow-up was completed on February 26, 2021. One month prior, 647 patients had been recruited at 26 centers in the inpatient phase of the study. Patient demographics for the 253 patients surveyed through that date are shown in Appendix Table 2. On the day of the early look at the data, 460 patients had become eligible for 1-month follow-up and 64 patients had been missed for 1-month follow-up (maximum response rate of 79.8%, minimum possible final response rate of 55.0%) (Figure 1). Seven surveys were completed by proxies. Respondents’ median age was 60 years (IQR, 45-68), and 111 (43.4%) were female. Their median hospital length of stay was 5 days(IQR, 3-8) . A total of 236 (93.3%) patients were discharged home, including 197 (77.9%) without home care services and 39 (15.4%) with home care services.
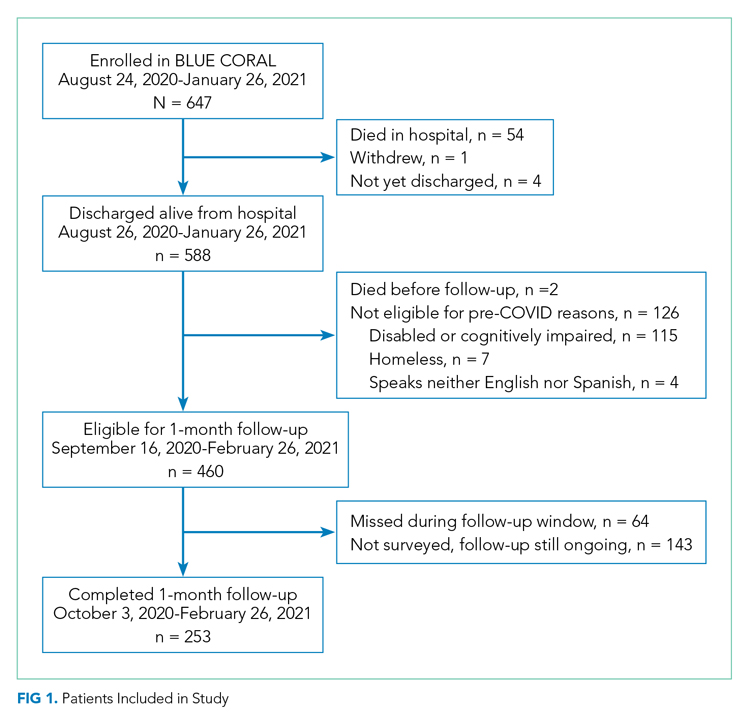
One hundred and thirty-nine patients (56.5%; 95% CI, 50.1%-62.8%) reported at least one new or worsened cardiopulmonary symptom after their COVID-19 hospitalization (Table; seven patients did not respond to these questions). Most patients with new symptoms had one (48 [19.5%]; 95% CI, 14.8%-25.0%) or two (32 [13%]; 95% CI, 9.7%-17.7%) of the new symptoms queried. The most common new cardiopulmonary symptom was cough, reported by 57 (23.2%; 95% CI, 18.0%-29.0%) patients. New oxygen use was reported by 28 (11.4%; 95% CI, 7.7%-16.0%) patients, with another 11 (4.5%; 95% CI, 2.3%-7.9%) reporting increased oxygen requirements. Women were twice as likely as men to report any new cardiopulmonary symptom (adjusted odds ratio [aOR], 2.24; 95% CI, 1.29-3.90) and non-Hispanic Black and Hispanic patients were less likely than White patients to report new symptoms (aOR, 0.31; 95% CI, 0.12-0.83; and aOR, 0.38; 95% CI, 0.21-0.71, respectively). Longer lengths of hospital stay were associated with greater 1-month cardiopulmonary symptoms (aOR, 1.82 per additional week in the hospital; 95% CI, 1.11-2.98), but discharge destination was not (aOR, 0.92; 95% CI, 0.39-1.71).
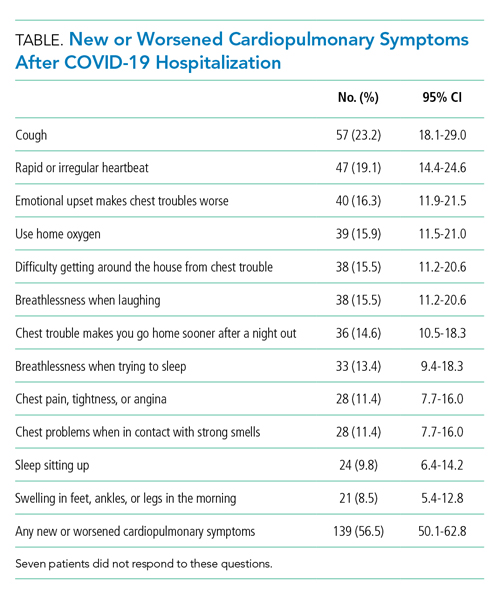
New limitations in activities of daily living or instrumental activities of daily living were present in 130 (52.8%; 95% CI, 46.4%-59.5%) patients (seven not responding), all of whom had 0 to 3 limitations before their COVID-19 hospitalization. Indeed, 62 (25.2%; 95% CI, 19.9%-31.1%) reported 3 or more new health-related limitations in activities of daily living or instrumental activities of daily living compared to their pre-COVID-19 baseline, as assessed separately during their hospitalization (Figure 2; rates of limitations in individual activities are shown in Appendix Table 3). Older patients were more likely to report a new health-related limitation, and Hispanic patients were less likely to have a new limitation. New limitations were common among patients discharged home without home health services. The number of new cardiopulmonary symptoms explained 11.2% of the variance in the number of new limitations in activities of daily living, a Spearman rank correlation of 0.30 (P < .0001; see Appendix Table 4). More than three in four COVID-19 patients reported new or worsened cardiopulmonary symptoms or new health-related limitations in activities of daily living at 1 month—only 62 (24.5%) patients reported neither.
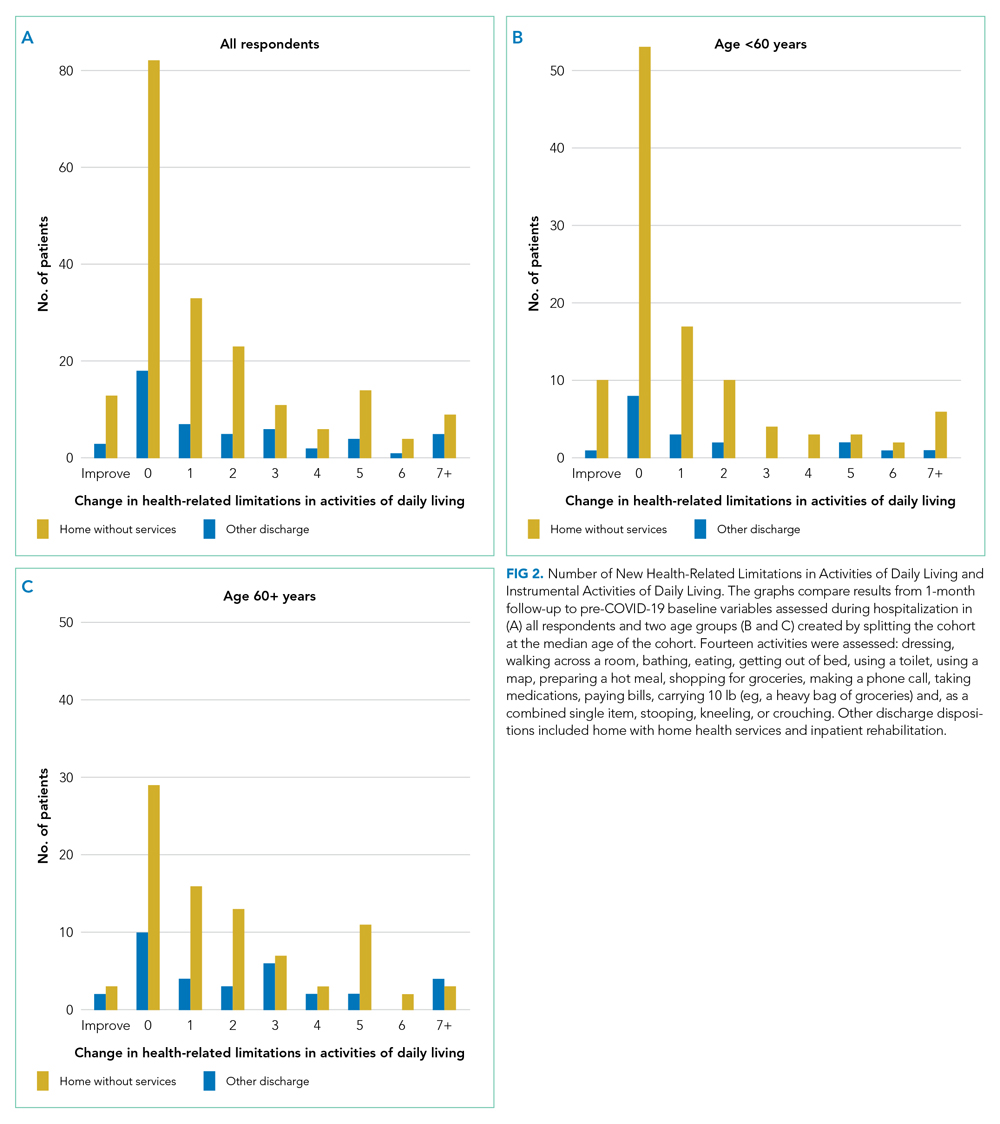
At 1 month after hospital discharge, 213 (84.2%) patients reported that they were not fully back to their pre-COVID-19 level of functioning (3 declined to answer the question). When asked, “On a scale of 1 to 100, with 100 being all the way back to what you could do before COVID, how close to being back are you?” the median response was 80, with an IQR of 64-95 (Figure 3). Forty-two (16.8%; 95% CI, 12.4%-22.0%) patients reported a level of 50 or below. Women and older patients reported lower levels of return of functioning, as did those with longer hospital stays and new or worsened cardiopulmonary symptoms. Each additional week in hospital length of stay was associated with a 7.5-point lower response to the question (95% CI, –11.2 to –3.8), but discharge destination was not associated with the answer after adjusting for demographics. Patients with and without new limitations in activities of daily living and with and without new cardiopulmonary symptoms were found across the range of self-reported degree of recovery, although patients without a new problem in one of those domains were rarer among those reporting recovery of less than 70. The number of new cardiopulmonary symptoms explained 19.7% of the variance in the response to this question, a Spearman rank correlation of 0.47 (P < .0001).
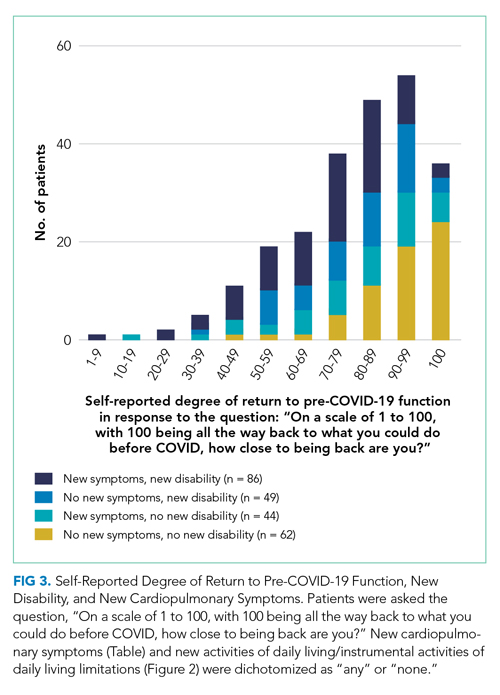
More than half of respondents (115 [55.0%]; 95% CI, 48.0%-61.9%; 44 not responding) stated that their COVID-19 hospitalization had been a drain on the finances of their family; 53 (25.4%; 95% CI, 19.6%-31.8%; 44 not responding) rated that drain as moderate, severe, or extreme within the first month after hospital discharge. Forty-nine patients (19.8%; 95% CI, 15.1%-25.4%; 6 not responding) reported that they had to change their work because of their COVID-19 hospitalization, and 93 patients (37.8%; 95% CI, 31.7%-44.2%; 7 not responding) reported that a loved one had taken time off work to care for them. Altogether, one in five COVID-19 patients reported that, within the first month after hospital discharge, they used all or most of their savings because of their COVID-19 illness or hospitalization (58 [23.2%]; 95% CI, 18.1%-29.9%; 3 not responding). There were no demographic differences in the likelihood of losing a job or having a loved one take time off for caregiving, but non-Hispanic Black and Hispanic patients were much more likely to report having used all or most of their savings (aOR, 2.96; 95% CI, 1.09-8.04; and aOR, 2.68; 95% CI, 1.35-5.31, respectively) than White patients. Hospital length of stay and discharge destination were not consistently associated with these financial toxicities. The development of new or worsened cardiopulmonary symptoms was not associated with job change or having a caregiver take time off but was associated with increased likelihood of having used all or most savings (aOR, 2.30; 95% CI, 1.12-4.37).
DISCUSSION
In a geographically and demographically diverse national US cohort, we found that a decline in perceived health, new or worsened cardiopulmonary symptoms, new limitations in activities of daily living, and new financial stresses were common among patients hospitalized during the US third wave of COVID-19 at 1 month after hospital discharge. The new cardiopulmonary symptoms were significantly associated with the self-report of incomplete recovery and financial stress, but less closely associated with incident disability, inability to work, and caregiving receipt. There were not consistent differences between any demographic groups on these outcomes. Patients with longer lengths of stay generally reported more problems. New problems were very common among patients discharged directly home without home health services.
These data suggest a broad range of new problems among survivors of COVID-19 hospitalization. Moreover, these problems are not well-correlated with each other. This raises the possibility that there may be multiple phenotypes of post-acute sequelae after COVID-19 hospitalization. It is not clear to what extent these differences are mediated by differences in tissue damage from or immunologic response to SARS-CoV-2, distinct from or interacting with other elements of treatment, hospitalization, or the illness experience. The degree of financial stress, savings loss, and job dislocation reported here suggests these patients will face substantial challenges in guiding their own recovery in the absence of a dedicated set of services.28,29The persistent symptoms faced by these COVID-19 patients can be considered in the context of post-acute sequelae among survivors of community-acquired pneumonia in previous studies, as summarized in a recent systematic review.30 For example, only 35% of a large cohort of adults with community-acquired pneumonia who were evaluated in the emergency department were completely free of pneumonia-related symptoms 6 weeks after antibiotic therapy.31,32 Limitations in activities of daily living have been reported at 1 month after community-acquired pneumonia33; rehospitalization and early post-discharge mortality rates may also be similar.34,35 These findings suggest that the persistent problems of both COVID-19 and other pneumonia patients may highlight important opportunities for improvements in healthcare systems,36 and that burdensome postacute sequelae of COVID-19 may not be attributable solely to distinctive features of the SARS-CoV-2 virus.
A majority of patients discharged home without home health services reported new difficulties in their activities of daily living; 77% of patients with new disability at 1 month had been discharged without home services. These data, however, do not show to what extent this lack of home health services resulted from lack of referral for services, home health provider unavailability, or patient refusal of recommended services. Nonetheless, this nonreceipt of home health services may have been consequential. Among hospitalized patients recovering from pneumonia pre-COVID, the use of post-hospital physical and occupational therapy was associated with reduced risk of readmissions and death.37 This association was greater among patients with lower baseline mobility scores and in patients discharged to home directly. Further, the risk of poor outcomes decreased in a dose-response fashion with increased post-hospital therapy delivery. Failure to provide services for postdischarge disability was previously identified as a potential vulnerability of patients during COVID-19.38
This study adds to the literature. The focus on sequelae perceived by the patient to be incident, as distinguished from symptoms and disability existing before COVID-19, increases the likelihood that these data reflect the influence of the COVID-19 hospitalization. These data emphasize that, despite relatively brief hospitalizations, diverse problems are quite common and consequential for patients’ ability to return to their pre-COVID-19 roles. They further add to the literature by demonstrating the relatively loose coupling between various ways in which postacute sequelae of COVID-19 might be defined: the cardiopulmonary symptoms examined here, the patient’s reported completeness of recovery, the financial stresses the hospitalization placed on the patient and their family, or the development of new limitations in activities of daily living.
Our findings highlight a potential second public health crisis from COVID, related to post-COVID recovery, resulting from the incident disability and economic loss among COVID survivors. While much attention is paid to deaths from COVID, there is less (albeit growing) recognition of the long-term consequences in survivors of COVID-19.39 The downstream economic impacts from job loss and financial insolvency for COVID-19 survivors have ramifications for caregivers, family units that include dependents, and the broader US economy—and may do so for generations if uncorrected, as has been suggested after the 1918 influenza pandemic.40 These data may, indeed, look worse at later follow-up given the delay in hospital billing and new expenses in the wake of illness and hospitalization.28,36,41 It is important that the healthcare system and policymakers consider early investments in post-hospital rehabilitation and adaptive services to allow workers to return to the workforce as soon as possible, and prepare for an increased need for financial support for recovering COVID patients.42
Importantly, these data cannot distinguish between the impact of SARS-CoV-2 infection itself from the treatment received for COVID-19 or other non-COVID-19-specific aspects of hospital care. COVID-19 inpatient case fatality rates and management have changed over time, and so generalizability to future cohorts is unknown.9-11 This cohort was recruited in the inpatient setting at largely teaching hospitals; therefore, these patients’ experience may be not be representative of all hospitalized COVID-19 patients during this time period. The generalizability of hospital-based studies to patients not hospitalized for COVID-19 remains a subject of active inquiry. We only interviewed patients who were not homeless (excluding 7 of 588 eligible, 1.2%) and who spoke English or Spanish (excluding 4 of 588 eligible, 0.7%); these and other inclusion/exclusion criteria should be considered when evaluating the generalizability of these findings to other patients. We did not prospectively collect measures of fatigue to examine this important and complex symptom, nor did we evaluate outpatient therapy. Finally, self-report was used, rather than using objective measurements of what the patient did or did not do in their home environment. This is consistent with clinical practice that emphasizes patients as primary reporters of their present state, but may introduce measurement error compared to more invasive strategies if those are considered the gold standard.
Conclusion
Patients who survived hospitalization from COVID-19 during the period of August 2020 to January 2021 continued to face significant burdens of new cardiopulmonary symptoms, incomplete recovery, disability, and financial toxicity, all of which extend to patients discharged directly home without services. The correlations between these potential symptoms are no more than partial, and an exclusive focus on one area may neglect other areas of patient need.
Acknowledgments
The authors thank the patients and families of the Biology and Longitudinal Epidemiology: COVID-19 Observational (BLUE CORAL) study for their generous sharing of their time with us. We acknowledge Hallie C Prescott (University of Michigan and VA Ann Arbor) for her assistance in developing the financial toxicity questions.
1. Rajan S, Khunti K, Alwan N, et al. In the Wake of the Pandemic: Preparing for Long COVID. World Health Organization, Regional Office for Europe; 2021.
2. Bowles KH, McDonald M, Barrón Y, Kennedy E, O’Connor M, Mikkelsen M. Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients. Ann Intern Med. 2021;174(3):316-325. https://doi.org/10.7326/M20-5206
3. Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. https://doi.org/10.7326/M20-5661
4. Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4(1):e2036142. https://doi.org/10.1001/jamanetworkopen.2020.36142
5. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. https://doi.org/10.1016/S0140-6736(20)32656-8
6. Robillard R, Daros AR, Phillips JL, et al. Emerging new psychiatric symptoms and the worsening of pre-existing mental disorders during the COVID-19 pandemic: a Canadian multisite study. Can J Psychiatry. 2021 Jan 19. [Epub ahead of print] https://doi.org/10.1177/0706743720986786
7. Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2):e210830. https://doi.org/10.1001/jamanetworkopen.2021.0830
8. Fan VS, Dominitz JA, Eastment MC, et al. Risk factors for testing positive for SARS-CoV-2 in a national US healthcare system. Clin Infect Dis. 2020 Oct 27. [Epub ahead of print] https://doi.org/10.1093/cid/ciaa1624
9. Prescott HC, Levy MM. Survival from severe coronavirus disease 2019: is it changing? Crit Care Med. 2021;49(2):351-353. https://doi.org/10.1097/CCM.0000000000004753
10. Nguyen NT, Chinn J, Nahmias J, et al. Outcomes and mortality among adults hospitalized with COVID-19 at US medical centers. JAMA Netw Open. 2021;4(3):e210417. https://doi.org/10.1001/jamanetworkopen.2021.0417
11. Iwashyna TJ, Angus DC. Declining case fatality rates for severe sepsis: good data bring good news with ambiguous implications. JAMA. 2014;311(13):1295-1297. https://doi.org/10.1001/jama.2014.2639
12. Galvin JE, Roe CM, Coats MA, Morris JC. Patient’s rating of cognitive ability: using the AD8, a brief informant interview, as a self-rating tool to detect dementia. Arch Neurol. 2007;64(5):725-730. https://doi.org/10.1001/archneur.64.5.725
13. Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942-1948. https://doi.org/10.1212/01.wnl.0000247042.15547.eb
14. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559-564. https://doi.org/10.1212/01.wnl.0000172958.95282.2a
15. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010
16. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. https://doi.org/10.1016/j.jbi.2019.103208
17. Robinson KA, Dinglas VD, Sukrithan V, et al. Updated systematic review identifies substantial number of retention strategies: using more strategies retains more study participants. J Clin Epidemiol. 2015;68(12):1481-1487. https://doi.org/10.1016/j.jclinepi.2015.04.013
18. Groves RM, Fowler FJ, Couper MP, Lepkowski JM, Singer E, Tourangeau R. Survey Methodology. 2nd ed. Wiley; 2009.
19. Lynn P. Methodology of Longitudinal Studies. Wiley; 2009.
20. Quirk F, Jones P. Repeatability of two new short airways questionnaires. Thorax. 1994;49:1075.
21. Pettersen KI, Reikvam A, Rollag A, Stavem K. Reliability and validity of the Kansas City cardiomyopathy questionnaire in patients with previous myocardial infarction. Eur J Heart Fail. 2005;7(2):235-242. https://doi.org/10.1016/j.ejheart.2004.05.012
22. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245-1255. https://doi.org/10.1016/s0735-1097(00)00531-3
23. Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333-341. https://doi.org/10.1016/0735-1097(94)00397-9
24. Fonda S, Herzog AR. Documentation of Physical Functioning Measured in the Health and Retirement Study and the Asset and Health Dynamics Among the Oldest Old Study. Institute for Social Research Survey Research Center; 2004.
25. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Moss M, Huang DT, Brower RG, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997-2008. https://doi.org/10.1056/NEJMoa1901686
26. Ahasic AM, Van Ness PH, Murphy TE, Araujo KL, Pisani MA. Functional status after critical illness: agreement between patient and proxy assessments. Age Ageing. 2015;44(3):506-510. https://doi.org/10.1093/ageing/afu163
27. Üstün T, Kostanjsek N, Chatterji S, Rehm J. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule WHODAS 2.0. World Health Organization; 2010.
28. Hauschildt KE, Seigworth C, Kamphuis LA, et al. Financial toxicity after acute respiratory distress syndrome: a national qualitative cohort study. Crit Care Med. 2020;48(8):1103-1110. https://doi.org/10.1097/CCM.0000000000004378
29. Watkins-Taylor C. Remaking a Life: How Women Living with HIV/AIDS Confront Inequality. University of California Press; 2019.
30. Pick HJ, Bolton CE, Lim WS, McKeever TM. Patient-reported outcome measures in the recovery of adults hospitalised with community-acquired pneumonia: a systematic review. Eur Respir J. 2019;53(3):1802165. https://doi.org/1183/13993003.02165-2018
31. Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Feagan BG. Predictors of symptom resolution in patients with community-acquired pneumonia. Clin Infect Dis. 2000;31(6):1362-1367. https://doi.org/10.1086/317495
32. Wyrwich KW, Yu H, Sato R, Powers JH. Observational longitudinal study of symptom burden and time for recovery from community-acquired pneumonia reported by older adults surveyed nationwide using the CAP Burden of Illness Questionnaire. Patient Relat Outcome Meas. 2015;6:215-223. https://doi.org/10.2147/PROM.S85779
33. Daniel P, Bewick T, McKeever TM, et al. Healthcare reconsultation in working-age adults following hospitalisation for community-acquired pneumonia. Clin Med (Lond). 2018;18(1):41-46. https://doi.org/10.7861/clinmedicine.18-1-41
34. Donnelly JP, Wang XQ, Iwashyna TJ, Prescott HC. Readmission and death after hospitalization for COVID-19 in a large multihospital system. JAMA. 2021;325(3):304-306. https://doi.org/10.1001/jama.2020.21465
35. Viglianti EM, Prescott HC, Liu V, Escobar GJ, Iwashyna TJ. Individual and health system variation in rehospitalizations the year after pneumonia. Medicine (Baltimore). 2017;96(31):e7695. https://doi.org/10.1097/MD.0000000000007695
36. McPeake J, Boehm LM, Hibbert E, et al. Key components of ICU recovery programs: what did patients report provided benefit? Crit Care Explor. 2020;2(4):e0088. https://doi.org/10.1097/CCE.0000000000000088
37. Freburger JK, Chou A, Euloth T, Matcho B. Variation in acute care rehabilitation and 30-day hospital readmission or mortality in adult patients with pneumonia. JAMA Netw Open. 2020;3(9):e2012979. https://doi.org/10.1001/jamanetworkopen.2020.12979
38. Iwashyna TJ, Johnson AB, McPeake JM, McSparron J, Prescott HC, Sevin C. The dirty dozen: common errors on discharging patients recovering from critical illness. Life in the Fastlane. November 3, 2020. Accessed July 1, 2021. https://litfl.com/the-dirty-dozen-common-errors-on-discharging-patients-recovering-from-critical-illness/
39. Lowenstein F, Davis H. Long Covid is not rare. It’s a health crisis. New York Times. March 17, 2021. Accessed July 1, 2021. https://www.nytimes.com/2021/03/17/opinion/long-covid.html
40. Cook CJ, Fletcher JM, Forgues A. Multigenerational effects of early-life health shocks. Demography. 2019;56(5):1855-1874. https://doi.org/10.1007/s13524-019-00804-3
41. McPeake J, Mikkelsen ME, Quasim T, et al. Return to employment after critical illness and its association with psychosocial outcomes. A systematic review and meta-analysis. Ann Am Thorac Soc. 2019;16(10):1304-1311. https://doi.org/10.1513/AnnalsATS.201903-248OC
42. McPeake JM, Henderson P, Darroch G, et al. Social and economic problems of ICU survivors identified by a structured social welfare consultation. Crit Care. 2019;23(1):153. https://doi.org/10.1186/s13054-019-2442-5
For many patients hospitalized with COVID-19, the impact of the illness continues well beyond hospital discharge.1 Heavy burdens of persistent symptoms have been reported, albeit often from regional and single-hospital samples.2-7 Critically, not all initial reports capture information on pre-COVID-19 symptom burden, so it is unclear whether these highly prevalent problems are truly new; an alternative explanation might be that patients already with symptoms were more likely to be infected with or seek care for SARS-CoV-2.8
Fewer data are available about patients’ abilities to go about the activities of their lives, nor is as much known about the relationships between new symptoms and other impacts. Most of the available information is from health systems during the initial surge of COVID-19 in early 2020—when testing for SARS-CoV-2 was limited even in the inpatient setting; when hospitals’ postdischarge care systems may have been heavily disrupted; and when clinicians were often reasonably focused primarily on reducing mortality in their first cases of COVID-19 rather than promoting recovery from an often-survivable illness. Increasing evidence shows that the inpatient case-fatality rate of COVID-19 is improving over time9,10; this makes unclear the generalizability of outcomes data from early COVID-19 patients to more recent patients.11
Therefore, we report multicenter measurements of incident levels of persistent cardiopulmonary symptoms, disability, return to baseline, and impact on employment among a recent cohort of COVID-19 patients hospitalized around the United States during the “third wave” of COVID-19—fall and winter 2020-2021. We focus on the 1-month time point after hospital discharge, as this time point is still in the early vulnerable period during which hospital transition-of-care programs are understood to have responsibility.
METHODS
The first 253 patients who completed 1-month postdischarge telephone follow-up surveys from the ongoing nationwide BLUE CORAL study were included. BLUE CORAL will enroll up to 1,500 hospitalized COVID-19 patients at 36 US centers (the identities of which are reported in Appendix 1) as a part of the National Heart, Lung, and Blood Institute’s Prevention and Early Treatment of Acute Lung Injury (PETAL) Network. We report here on survey questions that allowed for a clear comparison to be made between 1-month follow-up responses and pre-COVID baseline variables; these comparisons were based on (1) previous in-hospital assessment; (2) explicitly asking patients to compare to pre-COVID-19 levels; or (3) explicitly asking patients for changes in relation to their COVID-19 hospitalization. Items were chosen for inclusion in this report without looking at their association with other variables.
This research was approved by the Vanderbilt Institutional Review Board (IRB), serving as central IRB for the PETAL Network; patients or their surrogates provided informed consent.
Participants
Patients with COVID-19 were identified during hospitalization and within 14 days of a positive molecular test for SARS-CoV-2. Eligible patients presented with fever and/or respiratory signs/symptoms, such as hypoxemia, shortness of breath, or infiltrates on chest imaging. Patients were enrolled within the first 72 hours of hospitalization (in order to avoid oversampling patients with relatively longer stays, and to study the biology of early COVID-19), and excluded if they had comfort-care orders (because of their limited likelihood of surviving to follow-up), or were incarcerated (because of difficulties in obtaining truly open informed consent and likely difficulties in follow-up). Pertinently, patients were not required to be in the intensive care unit.
Surviving patients who spoke English or Spanish, were not homeless on hospital admission, and were neither significantly disabled nor significantly cognitively impaired were eligible for follow-up. “Not significantly disabled” was defined as having limitations due to health on no more than three activities of daily living before their COVID-19 hospitalization, as assessed at BLUE CORAL enrollment; this was chosen because of the potentially limited sensitivity of many of our questionnaires to detect an impact of COVID-19 in patients with greater than this level of disability. We included patients who were able to consent for themselves in the study, or for whom the legally appointed representative consenting on their behalf in the hospital reported no evidence of cognitive impairment, defined as no more than four of the problems on the eight-item Alzheimer’s Dementia (AD8) scale.12-14
Data Collection
One-month surveys were administered to patients or, when necessary, their proxies; the complete English- and Spanish-language instruments are presented in Appendix 2. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Michigan.15,16
Patients were contacted via phone by trained interviewers beginning 21 days after hospital discharge; interviews were completed a median of 47 days after discharge (interquartile range [IQR], 26-61). Efforts prioritized former patients completing surveys themselves by phone, but a well-informed proxy was approached if needed. Proxies, who included spouses, adult children, or other relatives, family friends, or primary caregivers, were in regular contact with the patient and understood the patient’s health status. If necessary, the survey could be completed over multiple phone calls, and a written, mail-back option was available. Other best practices in accurate survey data collection and cohort retention were used.17-19 Participants were given a $10 gift card.
New cardiopulmonary symptoms were queried with symptom-targeted questions informed by the Airways Questionnaire 20,20 the Kansas City Cardiomyopathy Questionnaire,21,22 and the Seattle Angina Questionnaire.23 Whenever a respondent reported a given symptom, they were asked, “Compared to 1 month before your COVID-19 hospitalization, is this better, worse, or about the same?” We counted the number of symptoms which the patient reported as worse.
Using wording from the Health and Retirement Study,24 disability was assessed based on a self-report of any of 14 health-related limitations in activities of daily living or instrumental activities of daily living, as in past studies25: dressing, walking across a room, bathing, eating, getting out of bed, using a toilet, using a map, preparing a hot meal, shopping for groceries, making a phone call, taking medications, paying bills, carrying 10 lb (eg, a heavy bag of groceries), and, as a combined single item, stooping, kneeling, or crouching. Well-chosen proxy reports appear reliable for these items.26 We counted the number of activities for which the patient reported a limitation, comparing those reported at 1 month to those reported during the in-hospital survey assessing pre-illness functioning.
The financial consequences of the COVID-19 hospitalization were assessed in two ways. First, we used a modified version of a World Health Organization Disability Assessment Schedule (WHODAS) 2.0 question27: “Since your COVID-19 hospitalization, how much has your health been a drain on the financial resources of you or your family?” Second, we used the financial toxicity items developed with the Mi-COVID19 study3 based on extensive qualitative interviews with respiratory failure survivors28; these questions were anchored explicitly on “the financial cost of dealing with your COVID-19 hospitalization and related care.”
Data Analysis
There were few missing data, and almost all were on outcome variables. Where present, the degree of missingness is reported and casewise deletion used. Because this was a planned early look at responses to an ongoing survey, with analysis based on the number of accrued responses, the ultimate denominator for response rate calculation is unknown. Therefore, two bounds are presented—the minimum, on the assumption that all remaining uncompleted surveys will be missed; and the maximum, as if the uncompleted surveys were not yet in the eligible denominator.
Variables were summarized with medians and IQRs. Multilevel logistic regression was used to test for differences across demographic characteristics in the rates of development of any new symptom or disability; site-level differences were modeled using a random effect. Gender, race/ethnicity, and age were included in all regressions unless noted otherwise; age was included with both linear and quadratic terms when used as a control variable. For the degree of return to baseline and for the number of new limitations in activities of daily living, we explored associations as dichotomized variables (any/none, using multilevel logistic regression) and as continuous variables (using multilevel linear regression). Percent of variance explained was calculated using the R2 in unadjusted linear regression, and Spearman rank correlations were used to allow nonlinearities in comparisons across outcomes. All adjusted models are presented in Appendix Table 1. Analyses were conducted in Stata 16.1 (StataCorp, 2020); analytic code is presented in Appendix 3, and a log file of all analyses is in Appendix 4.
RESULTS
The 250th 1-month follow-up was completed on February 26, 2021. One month prior, 647 patients had been recruited at 26 centers in the inpatient phase of the study. Patient demographics for the 253 patients surveyed through that date are shown in Appendix Table 2. On the day of the early look at the data, 460 patients had become eligible for 1-month follow-up and 64 patients had been missed for 1-month follow-up (maximum response rate of 79.8%, minimum possible final response rate of 55.0%) (Figure 1). Seven surveys were completed by proxies. Respondents’ median age was 60 years (IQR, 45-68), and 111 (43.4%) were female. Their median hospital length of stay was 5 days(IQR, 3-8) . A total of 236 (93.3%) patients were discharged home, including 197 (77.9%) without home care services and 39 (15.4%) with home care services.

One hundred and thirty-nine patients (56.5%; 95% CI, 50.1%-62.8%) reported at least one new or worsened cardiopulmonary symptom after their COVID-19 hospitalization (Table; seven patients did not respond to these questions). Most patients with new symptoms had one (48 [19.5%]; 95% CI, 14.8%-25.0%) or two (32 [13%]; 95% CI, 9.7%-17.7%) of the new symptoms queried. The most common new cardiopulmonary symptom was cough, reported by 57 (23.2%; 95% CI, 18.0%-29.0%) patients. New oxygen use was reported by 28 (11.4%; 95% CI, 7.7%-16.0%) patients, with another 11 (4.5%; 95% CI, 2.3%-7.9%) reporting increased oxygen requirements. Women were twice as likely as men to report any new cardiopulmonary symptom (adjusted odds ratio [aOR], 2.24; 95% CI, 1.29-3.90) and non-Hispanic Black and Hispanic patients were less likely than White patients to report new symptoms (aOR, 0.31; 95% CI, 0.12-0.83; and aOR, 0.38; 95% CI, 0.21-0.71, respectively). Longer lengths of hospital stay were associated with greater 1-month cardiopulmonary symptoms (aOR, 1.82 per additional week in the hospital; 95% CI, 1.11-2.98), but discharge destination was not (aOR, 0.92; 95% CI, 0.39-1.71).

New limitations in activities of daily living or instrumental activities of daily living were present in 130 (52.8%; 95% CI, 46.4%-59.5%) patients (seven not responding), all of whom had 0 to 3 limitations before their COVID-19 hospitalization. Indeed, 62 (25.2%; 95% CI, 19.9%-31.1%) reported 3 or more new health-related limitations in activities of daily living or instrumental activities of daily living compared to their pre-COVID-19 baseline, as assessed separately during their hospitalization (Figure 2; rates of limitations in individual activities are shown in Appendix Table 3). Older patients were more likely to report a new health-related limitation, and Hispanic patients were less likely to have a new limitation. New limitations were common among patients discharged home without home health services. The number of new cardiopulmonary symptoms explained 11.2% of the variance in the number of new limitations in activities of daily living, a Spearman rank correlation of 0.30 (P < .0001; see Appendix Table 4). More than three in four COVID-19 patients reported new or worsened cardiopulmonary symptoms or new health-related limitations in activities of daily living at 1 month—only 62 (24.5%) patients reported neither.

At 1 month after hospital discharge, 213 (84.2%) patients reported that they were not fully back to their pre-COVID-19 level of functioning (3 declined to answer the question). When asked, “On a scale of 1 to 100, with 100 being all the way back to what you could do before COVID, how close to being back are you?” the median response was 80, with an IQR of 64-95 (Figure 3). Forty-two (16.8%; 95% CI, 12.4%-22.0%) patients reported a level of 50 or below. Women and older patients reported lower levels of return of functioning, as did those with longer hospital stays and new or worsened cardiopulmonary symptoms. Each additional week in hospital length of stay was associated with a 7.5-point lower response to the question (95% CI, –11.2 to –3.8), but discharge destination was not associated with the answer after adjusting for demographics. Patients with and without new limitations in activities of daily living and with and without new cardiopulmonary symptoms were found across the range of self-reported degree of recovery, although patients without a new problem in one of those domains were rarer among those reporting recovery of less than 70. The number of new cardiopulmonary symptoms explained 19.7% of the variance in the response to this question, a Spearman rank correlation of 0.47 (P < .0001).

More than half of respondents (115 [55.0%]; 95% CI, 48.0%-61.9%; 44 not responding) stated that their COVID-19 hospitalization had been a drain on the finances of their family; 53 (25.4%; 95% CI, 19.6%-31.8%; 44 not responding) rated that drain as moderate, severe, or extreme within the first month after hospital discharge. Forty-nine patients (19.8%; 95% CI, 15.1%-25.4%; 6 not responding) reported that they had to change their work because of their COVID-19 hospitalization, and 93 patients (37.8%; 95% CI, 31.7%-44.2%; 7 not responding) reported that a loved one had taken time off work to care for them. Altogether, one in five COVID-19 patients reported that, within the first month after hospital discharge, they used all or most of their savings because of their COVID-19 illness or hospitalization (58 [23.2%]; 95% CI, 18.1%-29.9%; 3 not responding). There were no demographic differences in the likelihood of losing a job or having a loved one take time off for caregiving, but non-Hispanic Black and Hispanic patients were much more likely to report having used all or most of their savings (aOR, 2.96; 95% CI, 1.09-8.04; and aOR, 2.68; 95% CI, 1.35-5.31, respectively) than White patients. Hospital length of stay and discharge destination were not consistently associated with these financial toxicities. The development of new or worsened cardiopulmonary symptoms was not associated with job change or having a caregiver take time off but was associated with increased likelihood of having used all or most savings (aOR, 2.30; 95% CI, 1.12-4.37).
DISCUSSION
In a geographically and demographically diverse national US cohort, we found that a decline in perceived health, new or worsened cardiopulmonary symptoms, new limitations in activities of daily living, and new financial stresses were common among patients hospitalized during the US third wave of COVID-19 at 1 month after hospital discharge. The new cardiopulmonary symptoms were significantly associated with the self-report of incomplete recovery and financial stress, but less closely associated with incident disability, inability to work, and caregiving receipt. There were not consistent differences between any demographic groups on these outcomes. Patients with longer lengths of stay generally reported more problems. New problems were very common among patients discharged directly home without home health services.
These data suggest a broad range of new problems among survivors of COVID-19 hospitalization. Moreover, these problems are not well-correlated with each other. This raises the possibility that there may be multiple phenotypes of post-acute sequelae after COVID-19 hospitalization. It is not clear to what extent these differences are mediated by differences in tissue damage from or immunologic response to SARS-CoV-2, distinct from or interacting with other elements of treatment, hospitalization, or the illness experience. The degree of financial stress, savings loss, and job dislocation reported here suggests these patients will face substantial challenges in guiding their own recovery in the absence of a dedicated set of services.28,29The persistent symptoms faced by these COVID-19 patients can be considered in the context of post-acute sequelae among survivors of community-acquired pneumonia in previous studies, as summarized in a recent systematic review.30 For example, only 35% of a large cohort of adults with community-acquired pneumonia who were evaluated in the emergency department were completely free of pneumonia-related symptoms 6 weeks after antibiotic therapy.31,32 Limitations in activities of daily living have been reported at 1 month after community-acquired pneumonia33; rehospitalization and early post-discharge mortality rates may also be similar.34,35 These findings suggest that the persistent problems of both COVID-19 and other pneumonia patients may highlight important opportunities for improvements in healthcare systems,36 and that burdensome postacute sequelae of COVID-19 may not be attributable solely to distinctive features of the SARS-CoV-2 virus.
A majority of patients discharged home without home health services reported new difficulties in their activities of daily living; 77% of patients with new disability at 1 month had been discharged without home services. These data, however, do not show to what extent this lack of home health services resulted from lack of referral for services, home health provider unavailability, or patient refusal of recommended services. Nonetheless, this nonreceipt of home health services may have been consequential. Among hospitalized patients recovering from pneumonia pre-COVID, the use of post-hospital physical and occupational therapy was associated with reduced risk of readmissions and death.37 This association was greater among patients with lower baseline mobility scores and in patients discharged to home directly. Further, the risk of poor outcomes decreased in a dose-response fashion with increased post-hospital therapy delivery. Failure to provide services for postdischarge disability was previously identified as a potential vulnerability of patients during COVID-19.38
This study adds to the literature. The focus on sequelae perceived by the patient to be incident, as distinguished from symptoms and disability existing before COVID-19, increases the likelihood that these data reflect the influence of the COVID-19 hospitalization. These data emphasize that, despite relatively brief hospitalizations, diverse problems are quite common and consequential for patients’ ability to return to their pre-COVID-19 roles. They further add to the literature by demonstrating the relatively loose coupling between various ways in which postacute sequelae of COVID-19 might be defined: the cardiopulmonary symptoms examined here, the patient’s reported completeness of recovery, the financial stresses the hospitalization placed on the patient and their family, or the development of new limitations in activities of daily living.
Our findings highlight a potential second public health crisis from COVID, related to post-COVID recovery, resulting from the incident disability and economic loss among COVID survivors. While much attention is paid to deaths from COVID, there is less (albeit growing) recognition of the long-term consequences in survivors of COVID-19.39 The downstream economic impacts from job loss and financial insolvency for COVID-19 survivors have ramifications for caregivers, family units that include dependents, and the broader US economy—and may do so for generations if uncorrected, as has been suggested after the 1918 influenza pandemic.40 These data may, indeed, look worse at later follow-up given the delay in hospital billing and new expenses in the wake of illness and hospitalization.28,36,41 It is important that the healthcare system and policymakers consider early investments in post-hospital rehabilitation and adaptive services to allow workers to return to the workforce as soon as possible, and prepare for an increased need for financial support for recovering COVID patients.42
Importantly, these data cannot distinguish between the impact of SARS-CoV-2 infection itself from the treatment received for COVID-19 or other non-COVID-19-specific aspects of hospital care. COVID-19 inpatient case fatality rates and management have changed over time, and so generalizability to future cohorts is unknown.9-11 This cohort was recruited in the inpatient setting at largely teaching hospitals; therefore, these patients’ experience may be not be representative of all hospitalized COVID-19 patients during this time period. The generalizability of hospital-based studies to patients not hospitalized for COVID-19 remains a subject of active inquiry. We only interviewed patients who were not homeless (excluding 7 of 588 eligible, 1.2%) and who spoke English or Spanish (excluding 4 of 588 eligible, 0.7%); these and other inclusion/exclusion criteria should be considered when evaluating the generalizability of these findings to other patients. We did not prospectively collect measures of fatigue to examine this important and complex symptom, nor did we evaluate outpatient therapy. Finally, self-report was used, rather than using objective measurements of what the patient did or did not do in their home environment. This is consistent with clinical practice that emphasizes patients as primary reporters of their present state, but may introduce measurement error compared to more invasive strategies if those are considered the gold standard.
Conclusion
Patients who survived hospitalization from COVID-19 during the period of August 2020 to January 2021 continued to face significant burdens of new cardiopulmonary symptoms, incomplete recovery, disability, and financial toxicity, all of which extend to patients discharged directly home without services. The correlations between these potential symptoms are no more than partial, and an exclusive focus on one area may neglect other areas of patient need.
Acknowledgments
The authors thank the patients and families of the Biology and Longitudinal Epidemiology: COVID-19 Observational (BLUE CORAL) study for their generous sharing of their time with us. We acknowledge Hallie C Prescott (University of Michigan and VA Ann Arbor) for her assistance in developing the financial toxicity questions.
For many patients hospitalized with COVID-19, the impact of the illness continues well beyond hospital discharge.1 Heavy burdens of persistent symptoms have been reported, albeit often from regional and single-hospital samples.2-7 Critically, not all initial reports capture information on pre-COVID-19 symptom burden, so it is unclear whether these highly prevalent problems are truly new; an alternative explanation might be that patients already with symptoms were more likely to be infected with or seek care for SARS-CoV-2.8
Fewer data are available about patients’ abilities to go about the activities of their lives, nor is as much known about the relationships between new symptoms and other impacts. Most of the available information is from health systems during the initial surge of COVID-19 in early 2020—when testing for SARS-CoV-2 was limited even in the inpatient setting; when hospitals’ postdischarge care systems may have been heavily disrupted; and when clinicians were often reasonably focused primarily on reducing mortality in their first cases of COVID-19 rather than promoting recovery from an often-survivable illness. Increasing evidence shows that the inpatient case-fatality rate of COVID-19 is improving over time9,10; this makes unclear the generalizability of outcomes data from early COVID-19 patients to more recent patients.11
Therefore, we report multicenter measurements of incident levels of persistent cardiopulmonary symptoms, disability, return to baseline, and impact on employment among a recent cohort of COVID-19 patients hospitalized around the United States during the “third wave” of COVID-19—fall and winter 2020-2021. We focus on the 1-month time point after hospital discharge, as this time point is still in the early vulnerable period during which hospital transition-of-care programs are understood to have responsibility.
METHODS
The first 253 patients who completed 1-month postdischarge telephone follow-up surveys from the ongoing nationwide BLUE CORAL study were included. BLUE CORAL will enroll up to 1,500 hospitalized COVID-19 patients at 36 US centers (the identities of which are reported in Appendix 1) as a part of the National Heart, Lung, and Blood Institute’s Prevention and Early Treatment of Acute Lung Injury (PETAL) Network. We report here on survey questions that allowed for a clear comparison to be made between 1-month follow-up responses and pre-COVID baseline variables; these comparisons were based on (1) previous in-hospital assessment; (2) explicitly asking patients to compare to pre-COVID-19 levels; or (3) explicitly asking patients for changes in relation to their COVID-19 hospitalization. Items were chosen for inclusion in this report without looking at their association with other variables.
This research was approved by the Vanderbilt Institutional Review Board (IRB), serving as central IRB for the PETAL Network; patients or their surrogates provided informed consent.
Participants
Patients with COVID-19 were identified during hospitalization and within 14 days of a positive molecular test for SARS-CoV-2. Eligible patients presented with fever and/or respiratory signs/symptoms, such as hypoxemia, shortness of breath, or infiltrates on chest imaging. Patients were enrolled within the first 72 hours of hospitalization (in order to avoid oversampling patients with relatively longer stays, and to study the biology of early COVID-19), and excluded if they had comfort-care orders (because of their limited likelihood of surviving to follow-up), or were incarcerated (because of difficulties in obtaining truly open informed consent and likely difficulties in follow-up). Pertinently, patients were not required to be in the intensive care unit.
Surviving patients who spoke English or Spanish, were not homeless on hospital admission, and were neither significantly disabled nor significantly cognitively impaired were eligible for follow-up. “Not significantly disabled” was defined as having limitations due to health on no more than three activities of daily living before their COVID-19 hospitalization, as assessed at BLUE CORAL enrollment; this was chosen because of the potentially limited sensitivity of many of our questionnaires to detect an impact of COVID-19 in patients with greater than this level of disability. We included patients who were able to consent for themselves in the study, or for whom the legally appointed representative consenting on their behalf in the hospital reported no evidence of cognitive impairment, defined as no more than four of the problems on the eight-item Alzheimer’s Dementia (AD8) scale.12-14
Data Collection
One-month surveys were administered to patients or, when necessary, their proxies; the complete English- and Spanish-language instruments are presented in Appendix 2. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Michigan.15,16
Patients were contacted via phone by trained interviewers beginning 21 days after hospital discharge; interviews were completed a median of 47 days after discharge (interquartile range [IQR], 26-61). Efforts prioritized former patients completing surveys themselves by phone, but a well-informed proxy was approached if needed. Proxies, who included spouses, adult children, or other relatives, family friends, or primary caregivers, were in regular contact with the patient and understood the patient’s health status. If necessary, the survey could be completed over multiple phone calls, and a written, mail-back option was available. Other best practices in accurate survey data collection and cohort retention were used.17-19 Participants were given a $10 gift card.
New cardiopulmonary symptoms were queried with symptom-targeted questions informed by the Airways Questionnaire 20,20 the Kansas City Cardiomyopathy Questionnaire,21,22 and the Seattle Angina Questionnaire.23 Whenever a respondent reported a given symptom, they were asked, “Compared to 1 month before your COVID-19 hospitalization, is this better, worse, or about the same?” We counted the number of symptoms which the patient reported as worse.
Using wording from the Health and Retirement Study,24 disability was assessed based on a self-report of any of 14 health-related limitations in activities of daily living or instrumental activities of daily living, as in past studies25: dressing, walking across a room, bathing, eating, getting out of bed, using a toilet, using a map, preparing a hot meal, shopping for groceries, making a phone call, taking medications, paying bills, carrying 10 lb (eg, a heavy bag of groceries), and, as a combined single item, stooping, kneeling, or crouching. Well-chosen proxy reports appear reliable for these items.26 We counted the number of activities for which the patient reported a limitation, comparing those reported at 1 month to those reported during the in-hospital survey assessing pre-illness functioning.
The financial consequences of the COVID-19 hospitalization were assessed in two ways. First, we used a modified version of a World Health Organization Disability Assessment Schedule (WHODAS) 2.0 question27: “Since your COVID-19 hospitalization, how much has your health been a drain on the financial resources of you or your family?” Second, we used the financial toxicity items developed with the Mi-COVID19 study3 based on extensive qualitative interviews with respiratory failure survivors28; these questions were anchored explicitly on “the financial cost of dealing with your COVID-19 hospitalization and related care.”
Data Analysis
There were few missing data, and almost all were on outcome variables. Where present, the degree of missingness is reported and casewise deletion used. Because this was a planned early look at responses to an ongoing survey, with analysis based on the number of accrued responses, the ultimate denominator for response rate calculation is unknown. Therefore, two bounds are presented—the minimum, on the assumption that all remaining uncompleted surveys will be missed; and the maximum, as if the uncompleted surveys were not yet in the eligible denominator.
Variables were summarized with medians and IQRs. Multilevel logistic regression was used to test for differences across demographic characteristics in the rates of development of any new symptom or disability; site-level differences were modeled using a random effect. Gender, race/ethnicity, and age were included in all regressions unless noted otherwise; age was included with both linear and quadratic terms when used as a control variable. For the degree of return to baseline and for the number of new limitations in activities of daily living, we explored associations as dichotomized variables (any/none, using multilevel logistic regression) and as continuous variables (using multilevel linear regression). Percent of variance explained was calculated using the R2 in unadjusted linear regression, and Spearman rank correlations were used to allow nonlinearities in comparisons across outcomes. All adjusted models are presented in Appendix Table 1. Analyses were conducted in Stata 16.1 (StataCorp, 2020); analytic code is presented in Appendix 3, and a log file of all analyses is in Appendix 4.
RESULTS
The 250th 1-month follow-up was completed on February 26, 2021. One month prior, 647 patients had been recruited at 26 centers in the inpatient phase of the study. Patient demographics for the 253 patients surveyed through that date are shown in Appendix Table 2. On the day of the early look at the data, 460 patients had become eligible for 1-month follow-up and 64 patients had been missed for 1-month follow-up (maximum response rate of 79.8%, minimum possible final response rate of 55.0%) (Figure 1). Seven surveys were completed by proxies. Respondents’ median age was 60 years (IQR, 45-68), and 111 (43.4%) were female. Their median hospital length of stay was 5 days(IQR, 3-8) . A total of 236 (93.3%) patients were discharged home, including 197 (77.9%) without home care services and 39 (15.4%) with home care services.

One hundred and thirty-nine patients (56.5%; 95% CI, 50.1%-62.8%) reported at least one new or worsened cardiopulmonary symptom after their COVID-19 hospitalization (Table; seven patients did not respond to these questions). Most patients with new symptoms had one (48 [19.5%]; 95% CI, 14.8%-25.0%) or two (32 [13%]; 95% CI, 9.7%-17.7%) of the new symptoms queried. The most common new cardiopulmonary symptom was cough, reported by 57 (23.2%; 95% CI, 18.0%-29.0%) patients. New oxygen use was reported by 28 (11.4%; 95% CI, 7.7%-16.0%) patients, with another 11 (4.5%; 95% CI, 2.3%-7.9%) reporting increased oxygen requirements. Women were twice as likely as men to report any new cardiopulmonary symptom (adjusted odds ratio [aOR], 2.24; 95% CI, 1.29-3.90) and non-Hispanic Black and Hispanic patients were less likely than White patients to report new symptoms (aOR, 0.31; 95% CI, 0.12-0.83; and aOR, 0.38; 95% CI, 0.21-0.71, respectively). Longer lengths of hospital stay were associated with greater 1-month cardiopulmonary symptoms (aOR, 1.82 per additional week in the hospital; 95% CI, 1.11-2.98), but discharge destination was not (aOR, 0.92; 95% CI, 0.39-1.71).

New limitations in activities of daily living or instrumental activities of daily living were present in 130 (52.8%; 95% CI, 46.4%-59.5%) patients (seven not responding), all of whom had 0 to 3 limitations before their COVID-19 hospitalization. Indeed, 62 (25.2%; 95% CI, 19.9%-31.1%) reported 3 or more new health-related limitations in activities of daily living or instrumental activities of daily living compared to their pre-COVID-19 baseline, as assessed separately during their hospitalization (Figure 2; rates of limitations in individual activities are shown in Appendix Table 3). Older patients were more likely to report a new health-related limitation, and Hispanic patients were less likely to have a new limitation. New limitations were common among patients discharged home without home health services. The number of new cardiopulmonary symptoms explained 11.2% of the variance in the number of new limitations in activities of daily living, a Spearman rank correlation of 0.30 (P < .0001; see Appendix Table 4). More than three in four COVID-19 patients reported new or worsened cardiopulmonary symptoms or new health-related limitations in activities of daily living at 1 month—only 62 (24.5%) patients reported neither.

At 1 month after hospital discharge, 213 (84.2%) patients reported that they were not fully back to their pre-COVID-19 level of functioning (3 declined to answer the question). When asked, “On a scale of 1 to 100, with 100 being all the way back to what you could do before COVID, how close to being back are you?” the median response was 80, with an IQR of 64-95 (Figure 3). Forty-two (16.8%; 95% CI, 12.4%-22.0%) patients reported a level of 50 or below. Women and older patients reported lower levels of return of functioning, as did those with longer hospital stays and new or worsened cardiopulmonary symptoms. Each additional week in hospital length of stay was associated with a 7.5-point lower response to the question (95% CI, –11.2 to –3.8), but discharge destination was not associated with the answer after adjusting for demographics. Patients with and without new limitations in activities of daily living and with and without new cardiopulmonary symptoms were found across the range of self-reported degree of recovery, although patients without a new problem in one of those domains were rarer among those reporting recovery of less than 70. The number of new cardiopulmonary symptoms explained 19.7% of the variance in the response to this question, a Spearman rank correlation of 0.47 (P < .0001).

More than half of respondents (115 [55.0%]; 95% CI, 48.0%-61.9%; 44 not responding) stated that their COVID-19 hospitalization had been a drain on the finances of their family; 53 (25.4%; 95% CI, 19.6%-31.8%; 44 not responding) rated that drain as moderate, severe, or extreme within the first month after hospital discharge. Forty-nine patients (19.8%; 95% CI, 15.1%-25.4%; 6 not responding) reported that they had to change their work because of their COVID-19 hospitalization, and 93 patients (37.8%; 95% CI, 31.7%-44.2%; 7 not responding) reported that a loved one had taken time off work to care for them. Altogether, one in five COVID-19 patients reported that, within the first month after hospital discharge, they used all or most of their savings because of their COVID-19 illness or hospitalization (58 [23.2%]; 95% CI, 18.1%-29.9%; 3 not responding). There were no demographic differences in the likelihood of losing a job or having a loved one take time off for caregiving, but non-Hispanic Black and Hispanic patients were much more likely to report having used all or most of their savings (aOR, 2.96; 95% CI, 1.09-8.04; and aOR, 2.68; 95% CI, 1.35-5.31, respectively) than White patients. Hospital length of stay and discharge destination were not consistently associated with these financial toxicities. The development of new or worsened cardiopulmonary symptoms was not associated with job change or having a caregiver take time off but was associated with increased likelihood of having used all or most savings (aOR, 2.30; 95% CI, 1.12-4.37).
DISCUSSION
In a geographically and demographically diverse national US cohort, we found that a decline in perceived health, new or worsened cardiopulmonary symptoms, new limitations in activities of daily living, and new financial stresses were common among patients hospitalized during the US third wave of COVID-19 at 1 month after hospital discharge. The new cardiopulmonary symptoms were significantly associated with the self-report of incomplete recovery and financial stress, but less closely associated with incident disability, inability to work, and caregiving receipt. There were not consistent differences between any demographic groups on these outcomes. Patients with longer lengths of stay generally reported more problems. New problems were very common among patients discharged directly home without home health services.
These data suggest a broad range of new problems among survivors of COVID-19 hospitalization. Moreover, these problems are not well-correlated with each other. This raises the possibility that there may be multiple phenotypes of post-acute sequelae after COVID-19 hospitalization. It is not clear to what extent these differences are mediated by differences in tissue damage from or immunologic response to SARS-CoV-2, distinct from or interacting with other elements of treatment, hospitalization, or the illness experience. The degree of financial stress, savings loss, and job dislocation reported here suggests these patients will face substantial challenges in guiding their own recovery in the absence of a dedicated set of services.28,29The persistent symptoms faced by these COVID-19 patients can be considered in the context of post-acute sequelae among survivors of community-acquired pneumonia in previous studies, as summarized in a recent systematic review.30 For example, only 35% of a large cohort of adults with community-acquired pneumonia who were evaluated in the emergency department were completely free of pneumonia-related symptoms 6 weeks after antibiotic therapy.31,32 Limitations in activities of daily living have been reported at 1 month after community-acquired pneumonia33; rehospitalization and early post-discharge mortality rates may also be similar.34,35 These findings suggest that the persistent problems of both COVID-19 and other pneumonia patients may highlight important opportunities for improvements in healthcare systems,36 and that burdensome postacute sequelae of COVID-19 may not be attributable solely to distinctive features of the SARS-CoV-2 virus.
A majority of patients discharged home without home health services reported new difficulties in their activities of daily living; 77% of patients with new disability at 1 month had been discharged without home services. These data, however, do not show to what extent this lack of home health services resulted from lack of referral for services, home health provider unavailability, or patient refusal of recommended services. Nonetheless, this nonreceipt of home health services may have been consequential. Among hospitalized patients recovering from pneumonia pre-COVID, the use of post-hospital physical and occupational therapy was associated with reduced risk of readmissions and death.37 This association was greater among patients with lower baseline mobility scores and in patients discharged to home directly. Further, the risk of poor outcomes decreased in a dose-response fashion with increased post-hospital therapy delivery. Failure to provide services for postdischarge disability was previously identified as a potential vulnerability of patients during COVID-19.38
This study adds to the literature. The focus on sequelae perceived by the patient to be incident, as distinguished from symptoms and disability existing before COVID-19, increases the likelihood that these data reflect the influence of the COVID-19 hospitalization. These data emphasize that, despite relatively brief hospitalizations, diverse problems are quite common and consequential for patients’ ability to return to their pre-COVID-19 roles. They further add to the literature by demonstrating the relatively loose coupling between various ways in which postacute sequelae of COVID-19 might be defined: the cardiopulmonary symptoms examined here, the patient’s reported completeness of recovery, the financial stresses the hospitalization placed on the patient and their family, or the development of new limitations in activities of daily living.
Our findings highlight a potential second public health crisis from COVID, related to post-COVID recovery, resulting from the incident disability and economic loss among COVID survivors. While much attention is paid to deaths from COVID, there is less (albeit growing) recognition of the long-term consequences in survivors of COVID-19.39 The downstream economic impacts from job loss and financial insolvency for COVID-19 survivors have ramifications for caregivers, family units that include dependents, and the broader US economy—and may do so for generations if uncorrected, as has been suggested after the 1918 influenza pandemic.40 These data may, indeed, look worse at later follow-up given the delay in hospital billing and new expenses in the wake of illness and hospitalization.28,36,41 It is important that the healthcare system and policymakers consider early investments in post-hospital rehabilitation and adaptive services to allow workers to return to the workforce as soon as possible, and prepare for an increased need for financial support for recovering COVID patients.42
Importantly, these data cannot distinguish between the impact of SARS-CoV-2 infection itself from the treatment received for COVID-19 or other non-COVID-19-specific aspects of hospital care. COVID-19 inpatient case fatality rates and management have changed over time, and so generalizability to future cohorts is unknown.9-11 This cohort was recruited in the inpatient setting at largely teaching hospitals; therefore, these patients’ experience may be not be representative of all hospitalized COVID-19 patients during this time period. The generalizability of hospital-based studies to patients not hospitalized for COVID-19 remains a subject of active inquiry. We only interviewed patients who were not homeless (excluding 7 of 588 eligible, 1.2%) and who spoke English or Spanish (excluding 4 of 588 eligible, 0.7%); these and other inclusion/exclusion criteria should be considered when evaluating the generalizability of these findings to other patients. We did not prospectively collect measures of fatigue to examine this important and complex symptom, nor did we evaluate outpatient therapy. Finally, self-report was used, rather than using objective measurements of what the patient did or did not do in their home environment. This is consistent with clinical practice that emphasizes patients as primary reporters of their present state, but may introduce measurement error compared to more invasive strategies if those are considered the gold standard.
Conclusion
Patients who survived hospitalization from COVID-19 during the period of August 2020 to January 2021 continued to face significant burdens of new cardiopulmonary symptoms, incomplete recovery, disability, and financial toxicity, all of which extend to patients discharged directly home without services. The correlations between these potential symptoms are no more than partial, and an exclusive focus on one area may neglect other areas of patient need.
Acknowledgments
The authors thank the patients and families of the Biology and Longitudinal Epidemiology: COVID-19 Observational (BLUE CORAL) study for their generous sharing of their time with us. We acknowledge Hallie C Prescott (University of Michigan and VA Ann Arbor) for her assistance in developing the financial toxicity questions.
1. Rajan S, Khunti K, Alwan N, et al. In the Wake of the Pandemic: Preparing for Long COVID. World Health Organization, Regional Office for Europe; 2021.
2. Bowles KH, McDonald M, Barrón Y, Kennedy E, O’Connor M, Mikkelsen M. Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients. Ann Intern Med. 2021;174(3):316-325. https://doi.org/10.7326/M20-5206
3. Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. https://doi.org/10.7326/M20-5661
4. Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4(1):e2036142. https://doi.org/10.1001/jamanetworkopen.2020.36142
5. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. https://doi.org/10.1016/S0140-6736(20)32656-8
6. Robillard R, Daros AR, Phillips JL, et al. Emerging new psychiatric symptoms and the worsening of pre-existing mental disorders during the COVID-19 pandemic: a Canadian multisite study. Can J Psychiatry. 2021 Jan 19. [Epub ahead of print] https://doi.org/10.1177/0706743720986786
7. Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2):e210830. https://doi.org/10.1001/jamanetworkopen.2021.0830
8. Fan VS, Dominitz JA, Eastment MC, et al. Risk factors for testing positive for SARS-CoV-2 in a national US healthcare system. Clin Infect Dis. 2020 Oct 27. [Epub ahead of print] https://doi.org/10.1093/cid/ciaa1624
9. Prescott HC, Levy MM. Survival from severe coronavirus disease 2019: is it changing? Crit Care Med. 2021;49(2):351-353. https://doi.org/10.1097/CCM.0000000000004753
10. Nguyen NT, Chinn J, Nahmias J, et al. Outcomes and mortality among adults hospitalized with COVID-19 at US medical centers. JAMA Netw Open. 2021;4(3):e210417. https://doi.org/10.1001/jamanetworkopen.2021.0417
11. Iwashyna TJ, Angus DC. Declining case fatality rates for severe sepsis: good data bring good news with ambiguous implications. JAMA. 2014;311(13):1295-1297. https://doi.org/10.1001/jama.2014.2639
12. Galvin JE, Roe CM, Coats MA, Morris JC. Patient’s rating of cognitive ability: using the AD8, a brief informant interview, as a self-rating tool to detect dementia. Arch Neurol. 2007;64(5):725-730. https://doi.org/10.1001/archneur.64.5.725
13. Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942-1948. https://doi.org/10.1212/01.wnl.0000247042.15547.eb
14. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559-564. https://doi.org/10.1212/01.wnl.0000172958.95282.2a
15. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010
16. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. https://doi.org/10.1016/j.jbi.2019.103208
17. Robinson KA, Dinglas VD, Sukrithan V, et al. Updated systematic review identifies substantial number of retention strategies: using more strategies retains more study participants. J Clin Epidemiol. 2015;68(12):1481-1487. https://doi.org/10.1016/j.jclinepi.2015.04.013
18. Groves RM, Fowler FJ, Couper MP, Lepkowski JM, Singer E, Tourangeau R. Survey Methodology. 2nd ed. Wiley; 2009.
19. Lynn P. Methodology of Longitudinal Studies. Wiley; 2009.
20. Quirk F, Jones P. Repeatability of two new short airways questionnaires. Thorax. 1994;49:1075.
21. Pettersen KI, Reikvam A, Rollag A, Stavem K. Reliability and validity of the Kansas City cardiomyopathy questionnaire in patients with previous myocardial infarction. Eur J Heart Fail. 2005;7(2):235-242. https://doi.org/10.1016/j.ejheart.2004.05.012
22. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245-1255. https://doi.org/10.1016/s0735-1097(00)00531-3
23. Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333-341. https://doi.org/10.1016/0735-1097(94)00397-9
24. Fonda S, Herzog AR. Documentation of Physical Functioning Measured in the Health and Retirement Study and the Asset and Health Dynamics Among the Oldest Old Study. Institute for Social Research Survey Research Center; 2004.
25. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Moss M, Huang DT, Brower RG, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997-2008. https://doi.org/10.1056/NEJMoa1901686
26. Ahasic AM, Van Ness PH, Murphy TE, Araujo KL, Pisani MA. Functional status after critical illness: agreement between patient and proxy assessments. Age Ageing. 2015;44(3):506-510. https://doi.org/10.1093/ageing/afu163
27. Üstün T, Kostanjsek N, Chatterji S, Rehm J. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule WHODAS 2.0. World Health Organization; 2010.
28. Hauschildt KE, Seigworth C, Kamphuis LA, et al. Financial toxicity after acute respiratory distress syndrome: a national qualitative cohort study. Crit Care Med. 2020;48(8):1103-1110. https://doi.org/10.1097/CCM.0000000000004378
29. Watkins-Taylor C. Remaking a Life: How Women Living with HIV/AIDS Confront Inequality. University of California Press; 2019.
30. Pick HJ, Bolton CE, Lim WS, McKeever TM. Patient-reported outcome measures in the recovery of adults hospitalised with community-acquired pneumonia: a systematic review. Eur Respir J. 2019;53(3):1802165. https://doi.org/1183/13993003.02165-2018
31. Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Feagan BG. Predictors of symptom resolution in patients with community-acquired pneumonia. Clin Infect Dis. 2000;31(6):1362-1367. https://doi.org/10.1086/317495
32. Wyrwich KW, Yu H, Sato R, Powers JH. Observational longitudinal study of symptom burden and time for recovery from community-acquired pneumonia reported by older adults surveyed nationwide using the CAP Burden of Illness Questionnaire. Patient Relat Outcome Meas. 2015;6:215-223. https://doi.org/10.2147/PROM.S85779
33. Daniel P, Bewick T, McKeever TM, et al. Healthcare reconsultation in working-age adults following hospitalisation for community-acquired pneumonia. Clin Med (Lond). 2018;18(1):41-46. https://doi.org/10.7861/clinmedicine.18-1-41
34. Donnelly JP, Wang XQ, Iwashyna TJ, Prescott HC. Readmission and death after hospitalization for COVID-19 in a large multihospital system. JAMA. 2021;325(3):304-306. https://doi.org/10.1001/jama.2020.21465
35. Viglianti EM, Prescott HC, Liu V, Escobar GJ, Iwashyna TJ. Individual and health system variation in rehospitalizations the year after pneumonia. Medicine (Baltimore). 2017;96(31):e7695. https://doi.org/10.1097/MD.0000000000007695
36. McPeake J, Boehm LM, Hibbert E, et al. Key components of ICU recovery programs: what did patients report provided benefit? Crit Care Explor. 2020;2(4):e0088. https://doi.org/10.1097/CCE.0000000000000088
37. Freburger JK, Chou A, Euloth T, Matcho B. Variation in acute care rehabilitation and 30-day hospital readmission or mortality in adult patients with pneumonia. JAMA Netw Open. 2020;3(9):e2012979. https://doi.org/10.1001/jamanetworkopen.2020.12979
38. Iwashyna TJ, Johnson AB, McPeake JM, McSparron J, Prescott HC, Sevin C. The dirty dozen: common errors on discharging patients recovering from critical illness. Life in the Fastlane. November 3, 2020. Accessed July 1, 2021. https://litfl.com/the-dirty-dozen-common-errors-on-discharging-patients-recovering-from-critical-illness/
39. Lowenstein F, Davis H. Long Covid is not rare. It’s a health crisis. New York Times. March 17, 2021. Accessed July 1, 2021. https://www.nytimes.com/2021/03/17/opinion/long-covid.html
40. Cook CJ, Fletcher JM, Forgues A. Multigenerational effects of early-life health shocks. Demography. 2019;56(5):1855-1874. https://doi.org/10.1007/s13524-019-00804-3
41. McPeake J, Mikkelsen ME, Quasim T, et al. Return to employment after critical illness and its association with psychosocial outcomes. A systematic review and meta-analysis. Ann Am Thorac Soc. 2019;16(10):1304-1311. https://doi.org/10.1513/AnnalsATS.201903-248OC
42. McPeake JM, Henderson P, Darroch G, et al. Social and economic problems of ICU survivors identified by a structured social welfare consultation. Crit Care. 2019;23(1):153. https://doi.org/10.1186/s13054-019-2442-5
1. Rajan S, Khunti K, Alwan N, et al. In the Wake of the Pandemic: Preparing for Long COVID. World Health Organization, Regional Office for Europe; 2021.
2. Bowles KH, McDonald M, Barrón Y, Kennedy E, O’Connor M, Mikkelsen M. Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients. Ann Intern Med. 2021;174(3):316-325. https://doi.org/10.7326/M20-5206
3. Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. https://doi.org/10.7326/M20-5661
4. Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4(1):e2036142. https://doi.org/10.1001/jamanetworkopen.2020.36142
5. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. https://doi.org/10.1016/S0140-6736(20)32656-8
6. Robillard R, Daros AR, Phillips JL, et al. Emerging new psychiatric symptoms and the worsening of pre-existing mental disorders during the COVID-19 pandemic: a Canadian multisite study. Can J Psychiatry. 2021 Jan 19. [Epub ahead of print] https://doi.org/10.1177/0706743720986786
7. Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4(2):e210830. https://doi.org/10.1001/jamanetworkopen.2021.0830
8. Fan VS, Dominitz JA, Eastment MC, et al. Risk factors for testing positive for SARS-CoV-2 in a national US healthcare system. Clin Infect Dis. 2020 Oct 27. [Epub ahead of print] https://doi.org/10.1093/cid/ciaa1624
9. Prescott HC, Levy MM. Survival from severe coronavirus disease 2019: is it changing? Crit Care Med. 2021;49(2):351-353. https://doi.org/10.1097/CCM.0000000000004753
10. Nguyen NT, Chinn J, Nahmias J, et al. Outcomes and mortality among adults hospitalized with COVID-19 at US medical centers. JAMA Netw Open. 2021;4(3):e210417. https://doi.org/10.1001/jamanetworkopen.2021.0417
11. Iwashyna TJ, Angus DC. Declining case fatality rates for severe sepsis: good data bring good news with ambiguous implications. JAMA. 2014;311(13):1295-1297. https://doi.org/10.1001/jama.2014.2639
12. Galvin JE, Roe CM, Coats MA, Morris JC. Patient’s rating of cognitive ability: using the AD8, a brief informant interview, as a self-rating tool to detect dementia. Arch Neurol. 2007;64(5):725-730. https://doi.org/10.1001/archneur.64.5.725
13. Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942-1948. https://doi.org/10.1212/01.wnl.0000247042.15547.eb
14. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559-564. https://doi.org/10.1212/01.wnl.0000172958.95282.2a
15. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010
16. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. https://doi.org/10.1016/j.jbi.2019.103208
17. Robinson KA, Dinglas VD, Sukrithan V, et al. Updated systematic review identifies substantial number of retention strategies: using more strategies retains more study participants. J Clin Epidemiol. 2015;68(12):1481-1487. https://doi.org/10.1016/j.jclinepi.2015.04.013
18. Groves RM, Fowler FJ, Couper MP, Lepkowski JM, Singer E, Tourangeau R. Survey Methodology. 2nd ed. Wiley; 2009.
19. Lynn P. Methodology of Longitudinal Studies. Wiley; 2009.
20. Quirk F, Jones P. Repeatability of two new short airways questionnaires. Thorax. 1994;49:1075.
21. Pettersen KI, Reikvam A, Rollag A, Stavem K. Reliability and validity of the Kansas City cardiomyopathy questionnaire in patients with previous myocardial infarction. Eur J Heart Fail. 2005;7(2):235-242. https://doi.org/10.1016/j.ejheart.2004.05.012
22. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245-1255. https://doi.org/10.1016/s0735-1097(00)00531-3
23. Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333-341. https://doi.org/10.1016/0735-1097(94)00397-9
24. Fonda S, Herzog AR. Documentation of Physical Functioning Measured in the Health and Retirement Study and the Asset and Health Dynamics Among the Oldest Old Study. Institute for Social Research Survey Research Center; 2004.
25. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Moss M, Huang DT, Brower RG, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997-2008. https://doi.org/10.1056/NEJMoa1901686
26. Ahasic AM, Van Ness PH, Murphy TE, Araujo KL, Pisani MA. Functional status after critical illness: agreement between patient and proxy assessments. Age Ageing. 2015;44(3):506-510. https://doi.org/10.1093/ageing/afu163
27. Üstün T, Kostanjsek N, Chatterji S, Rehm J. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule WHODAS 2.0. World Health Organization; 2010.
28. Hauschildt KE, Seigworth C, Kamphuis LA, et al. Financial toxicity after acute respiratory distress syndrome: a national qualitative cohort study. Crit Care Med. 2020;48(8):1103-1110. https://doi.org/10.1097/CCM.0000000000004378
29. Watkins-Taylor C. Remaking a Life: How Women Living with HIV/AIDS Confront Inequality. University of California Press; 2019.
30. Pick HJ, Bolton CE, Lim WS, McKeever TM. Patient-reported outcome measures in the recovery of adults hospitalised with community-acquired pneumonia: a systematic review. Eur Respir J. 2019;53(3):1802165. https://doi.org/1183/13993003.02165-2018
31. Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Feagan BG. Predictors of symptom resolution in patients with community-acquired pneumonia. Clin Infect Dis. 2000;31(6):1362-1367. https://doi.org/10.1086/317495
32. Wyrwich KW, Yu H, Sato R, Powers JH. Observational longitudinal study of symptom burden and time for recovery from community-acquired pneumonia reported by older adults surveyed nationwide using the CAP Burden of Illness Questionnaire. Patient Relat Outcome Meas. 2015;6:215-223. https://doi.org/10.2147/PROM.S85779
33. Daniel P, Bewick T, McKeever TM, et al. Healthcare reconsultation in working-age adults following hospitalisation for community-acquired pneumonia. Clin Med (Lond). 2018;18(1):41-46. https://doi.org/10.7861/clinmedicine.18-1-41
34. Donnelly JP, Wang XQ, Iwashyna TJ, Prescott HC. Readmission and death after hospitalization for COVID-19 in a large multihospital system. JAMA. 2021;325(3):304-306. https://doi.org/10.1001/jama.2020.21465
35. Viglianti EM, Prescott HC, Liu V, Escobar GJ, Iwashyna TJ. Individual and health system variation in rehospitalizations the year after pneumonia. Medicine (Baltimore). 2017;96(31):e7695. https://doi.org/10.1097/MD.0000000000007695
36. McPeake J, Boehm LM, Hibbert E, et al. Key components of ICU recovery programs: what did patients report provided benefit? Crit Care Explor. 2020;2(4):e0088. https://doi.org/10.1097/CCE.0000000000000088
37. Freburger JK, Chou A, Euloth T, Matcho B. Variation in acute care rehabilitation and 30-day hospital readmission or mortality in adult patients with pneumonia. JAMA Netw Open. 2020;3(9):e2012979. https://doi.org/10.1001/jamanetworkopen.2020.12979
38. Iwashyna TJ, Johnson AB, McPeake JM, McSparron J, Prescott HC, Sevin C. The dirty dozen: common errors on discharging patients recovering from critical illness. Life in the Fastlane. November 3, 2020. Accessed July 1, 2021. https://litfl.com/the-dirty-dozen-common-errors-on-discharging-patients-recovering-from-critical-illness/
39. Lowenstein F, Davis H. Long Covid is not rare. It’s a health crisis. New York Times. March 17, 2021. Accessed July 1, 2021. https://www.nytimes.com/2021/03/17/opinion/long-covid.html
40. Cook CJ, Fletcher JM, Forgues A. Multigenerational effects of early-life health shocks. Demography. 2019;56(5):1855-1874. https://doi.org/10.1007/s13524-019-00804-3
41. McPeake J, Mikkelsen ME, Quasim T, et al. Return to employment after critical illness and its association with psychosocial outcomes. A systematic review and meta-analysis. Ann Am Thorac Soc. 2019;16(10):1304-1311. https://doi.org/10.1513/AnnalsATS.201903-248OC
42. McPeake JM, Henderson P, Darroch G, et al. Social and economic problems of ICU survivors identified by a structured social welfare consultation. Crit Care. 2019;23(1):153. https://doi.org/10.1186/s13054-019-2442-5
© 2021 Society of Hospital Medicine
Identifying and Supporting the Needs of Internal Medicine and Pediatrics Residents Interested in Pediatric Hospital Medicine Fellowship
The American Board of Medical Specialties approved subspecialty designation for the field of pediatric hospital medicine (PHM) in 2016.1 For those who started independent practice prior to July 2019, there were two options for board eligibility: the “practice pathway” or completion of a PHM fellowship. The practice pathway allows for pediatric and combined internal medicine–pediatric (med-peds) providers who graduated by July 2019 to sit for the PHM board-certification examination if they meet specific criteria in their pediatric practice.2 For pediatric and med-peds residents who graduated after July 2019, PHM board eligibility is available only through completion of a PHM fellowship.
PHM subspecialty designation with fellowship training requirements may pose unique challenges to med-peds residents interested in practicing both pediatric and adult hospital medicine (HM).3,4 Each year, an estimated 25% of med-peds residency graduates go on to practice HM.5 The majority (62%-83%) of currently practicing med-peds–trained hospitalists care for both adults and children.5,6 Further, med-peds–trained hospitalists comprise at least 10% of the PHM workforce5 and play an important role in caring for adult survivors of childhood diseases.3
Limited existing data suggest that the future practice patterns of med-peds residents may be affected by PHM fellowship requirements. One previous survey study indicated that, although med-peds residents see value in additional training opportunities offered by fellowship, the majority are less likely to pursue PHM as a result of the new requirements.4 Prominent factors dissuading residents from pursuing PHM fellowship included forfeited earnings during fellowship, student loan obligations, family obligations, and the perception that training received during residency was sufficient. Although these data provide important insights into potential changes in practice patterns, they do not explore qualities of PHM fellowship that may make additional training more appealing to med-peds residents and promote retention of med-peds–trained providers in the PHM workforce.
Further, there is no existing literature exploring if and how PHM fellowship programs are equipped to support the needs of med-peds–trained fellows. Other subspecialties have supported med-peds trainees in combined fellowship training programs, including rheumatology, neurology, pediatric emergency medicine, allergy/immunology, physical medicine and rehabilitation, and psychiatry.7,8 However, the extent to which PHM fellowships follow a similar model to accommodate the career goals of med-peds participants is unclear.
Given the large numbers of med-peds residents who go on to practice combined PHM and adult HM, it is crucial to understand the training needs of this group within the context of PHM fellowship and board certification. The primary objectives of this study were to understand (1) the perceived PHM fellowship needs of med-peds residents interested in HM, and (2) how the current PHM fellowship training environment can meet those needs. Understanding that additional training requirements to practice PHM may affect the career trajectory of residents interested in HM, secondary objectives included describing perceptions of med-peds residents on PHM specialty designation and whether designation affected their career plans.
METHODS
Study Design
This cross-sectional study took place over a 3-month period from May to July 2019 and included two surveys of different populations to develop a comprehensive understanding of stakeholder perceptions of PHM fellowship. The first survey (resident survey) invited med-peds residents who were members of the National Med-Peds Residents’ Association (NMPRA)9 in 2019 and who were interested in HM. The second survey (fellowship director [FD] survey) included PHM FDs. The study was determined to be exempt by the University of Pittsburgh Institutional Review Board.
Study Population and Recruitment
Resident Survey
Two attempts were made to elicit participation via the NMPRA electronic mailing list. The NMPRA membership includes med-peds residents and chief residents from US med-peds residency programs. As of May 2019, 77 med-peds residency programs and their residents were members of NMPRA, which encompassed all med-peds programs in the United States and its territories. NMPRA maintains a listserv for all members, and all existing US/territory programs were members at the time of the survey. Med-peds interns, residents, and chief residents interested in HM were invited to participate in this study.
FD Survey
Forty-eight FDs, representing member institutions of the PHM Fellowship Directors’ Council, were surveyed via the PHM Fellowship Directors listserv.
Survey Instruments
We constructed two de novo surveys consisting of multiple-choice and short-answer questions (Appendix 1 and Appendix 2). To enhance the validity of survey responses, questions were designed and tested using an iterative consensus process among authors and additional participants, including current med-peds PHM fellows, PHM fellowship program directors, med-peds residency program directors, and current med-peds residents. These revisions were repeated for a total of four cycles. Items were created to increase knowledge on the following key areas: resident-perceived needs in fellowship training, impact of PHM subspecialty designation on career choices related to HM, health system structure of fellowship programs, and ability to accommodate med-peds clinical training within a PHM fellowship. A combined med-peds fellowship, as defined in the survey and referenced in this study, is a “combined internal medicine–pediatrics hospital medicine fellowship whereby you would remain eligible for PHM board certification.” To ensure a broad and inclusive view of potential needs of med-peds trainees considering fellowship, all respondents were asked to complete questions pertaining to anticipated fellowship needs regardless of their indicated interest in fellowship.
Data Collection
Survey completion was voluntary. Email identifiers were not linked to completed surveys. Study data were collected and managed by using Qualtrics XM. Only completed survey entries were included in analysis.
Statistical Methods and Data Analysis
R software version 4.0.2 (R Foundation for Statistical Computing) was used for statistical analysis. Demographic data were summarized using frequency distributions. The intent of the free-text questions for both surveys was qualitative explanatory thematic analysis. Authors EB, HL, and AJ used a deductive approach to identify common themes that elucidated med-peds resident–anticipated needs in fellowship and PHM program strategies and barriers to accommodate these needs. Preliminary themes and action items were reviewed and discussed among the full authorship team until consensus was reached.
RESULTS
Demographic Data
Resident Survey
A total of 466 med-peds residents completed the resident survey. There are approximately 1300 med-peds residents annually, creating an estimated response rate of 35.8% of all US med-peds residents. The majority (n = 380, 81.5%) of respondents were med-peds postgraduate years 1 through 3 and thus only eligible for PHM board certification via the PHM fellowship pathway (Table 1). Most (n = 446, 95.7%) respondents had considered a career in adult, pediatric, or combined HM at some point. Of those med-peds residents who considered a career in HM (Appendix Table 1), 92.8% (n = 414) would prefer to practice combined adult HM and PHM.
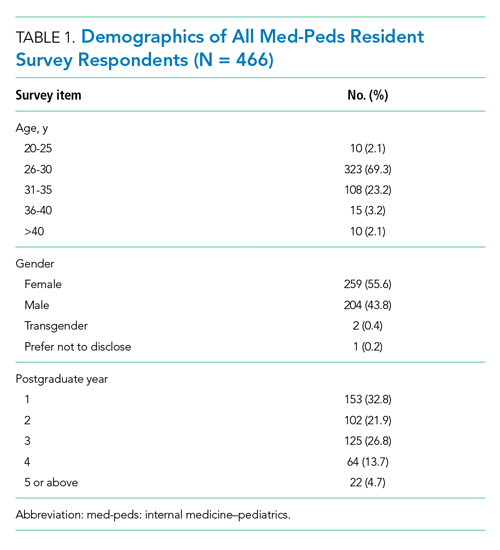
FD Survey
Twenty-eight FDs completed the FD survey, representing 58.3% of 2019 PHM fellowship programs. Of the responding programs, 23 (82.1%) were associated with a freestanding children’s hospital, and 24 (85.7%) were integrated or affiliated with a health system that provides adult inpatient care (Table 2). Sixteen (57.1%) programs had a med-peds residency program at their institution.
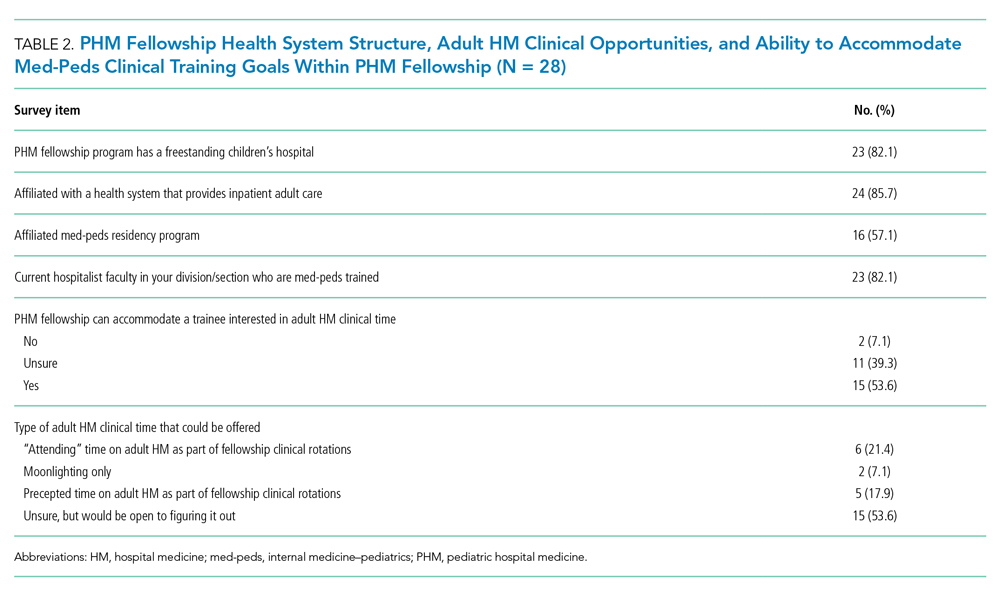
Med-Peds Resident Perceptions of PHM Fellowship
In considering the importance of PHM board certification for physicians practicing PHM, 59.0% (n= 275) of respondents rated board certification as “not at all important” (Appendix Table 2). Most (n = 420, 90.1%) med-peds trainees responded that PHM subspecialty designation “decreased” or “significantly decreased” their desire to pursue a career that includes PHM. Of the respondents who reported no interest in hospital medicine, eight (40%) reported that PHM subspecialty status dissuaded them from a career in HM at least a moderate amount (Appendix Table 3). Roughly one third (n=158, 33.9%) of respondents reported that PHM subspecialty designation increased or significantly increased their desire to pursue a career that includes adult HM (Appenidx Table 2). Finally, although the majority (n = 275, 59%) of respondents said they had no interest in a HM fellowship, 114 (24.5%) indicated interest in a combined med-peds HM fellowship (Appendix Table 1). Short-answer questions revealed that commitment to additional training on top of a 4-year residency program was a possible deterring factor, particularly in light of student loan debt and family obligations. Respondents reported adequate clinical training during residency as another deterring factor.
Med-Peds Resident–Perceived Needs in PHM Fellowship
Regardless of interest in completing a PHM fellowship, all resident survey respondents were asked how their ideal PHM fellowship should be structured. Almost all (n = 456, 97.9%) respondents indicated that they would prefer to complete a combined med-peds HM fellowship (Table 3), and most preferred to complete a fellowship in 2 years. Only 10 (2.1%) respondents preferred to complete a PHM fellowship alone in 2 or 3 years. More than half (n=253, 54.3%) of respondents indicated that it would be ideal to obtain a master’s degree as part of fellowship.
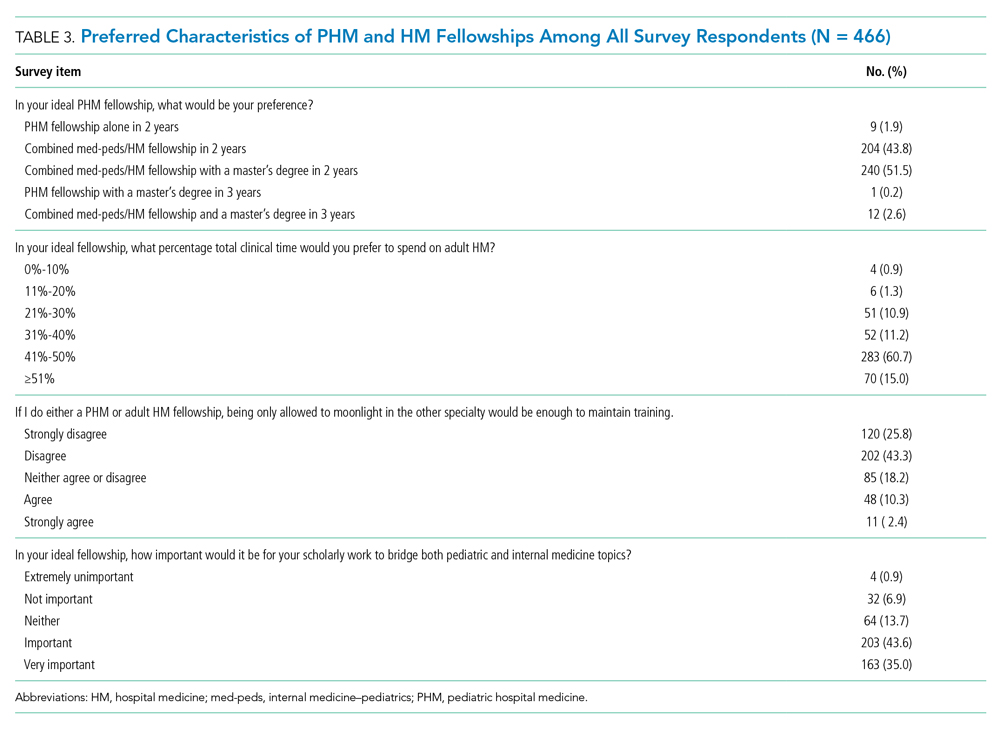
Three quarters (n = 355, 75.8%) of med-peds residents reported that they would want 41% or more of clinical time in an ideal fellowship dedicated to adult HM. Importantly, most (n = 322, 69.1%) of the med-peds residents did not consider moonlighting alone in either PHM or adult HM to be enough to maintain training. In addition, many (n = 366, 78.5%) respondents felt that it was important or very important for scholarly work during fellowship to bridge pediatrics and internal medicine.
Short-answer questions indicated that the ability to practice both internal medicine and pediatrics during fellowship emerged as an important deciding factor, with emphasis on adequate opportunities to maintain internal medicine knowledge base (Figure). Similarly, access to med-peds mentorship was an important component of the decision. Compensation both during fellowship and potential future earnings was also a prominent consideration.
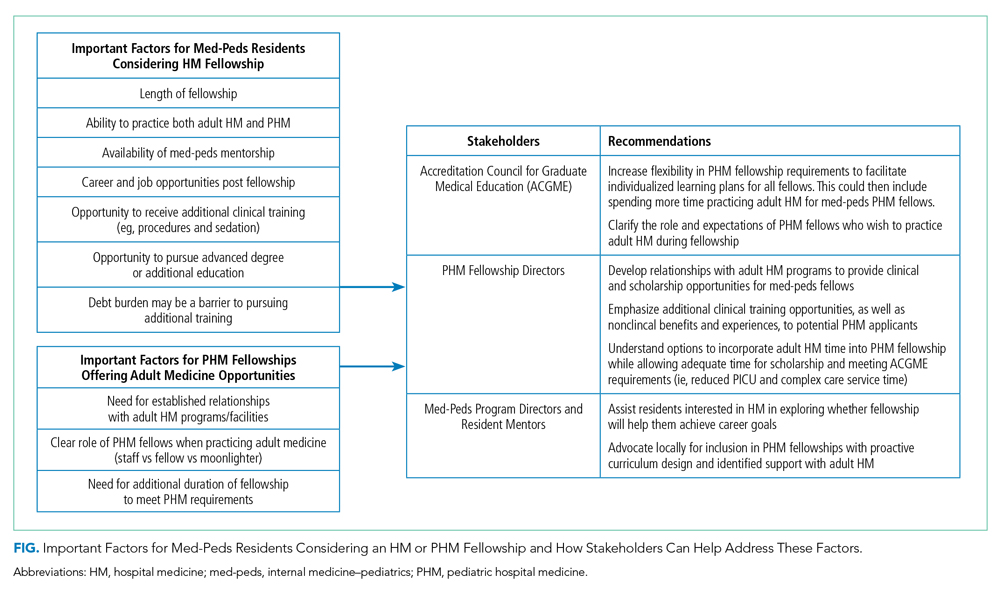
Capacity of PHM Programs to Support Med-Peds Fellows
Fifteen (53.6%) FDs reported that their programs were able to accommodate both PHM and adult HM clinical time during fellowship, 11 (39.3%) were unsure, and 2 (7.1%) were unable to accommodate both (Table 2).
The options for adult HM clinical time varied by institution and included precepted time on adult HM, full attending privileges on adult HM, and adult HM time through moonlighting only. Short-answer responses from FDs with experience training med-peds fellows cited using PHM elective time for adult HM and offering moonlighting in adult HM as ways to address career goals of med-peds trainees. Scholarship time for fellows was preserved by decreasing required time on pediatric intensive care unit and complex care services.
Accessibility of Med-Peds Mentorship
As noted above, med-peds residents identified mentorship as an important factor in consideration of PHM fellowship. A total of 23 (82.1%) FDs reported their programs had med-peds faculty members within their PHM team (Table 2). The majority (n = 21, 91.3%) of those med-peds faculty had both PHM and adult HM clinical time.
DISCUSSION
This study characterized the ideal PHM fellowship structure from the perspective of med-peds residents and described the current ability of PHM fellowships to support med-peds residents. The majority of residents stated that they had no interest in an HM fellowship. However, for med-peds residents who considered a career in HM, 88.8% preferred to complete a combined internal medicine and pediatrics HM fellowship with close to half of clinical time dedicated to adult HM. Just over half (53.6%) of programs reported that they could currently accommodate both PHM and adult clinical time during fellowship, and all but two programs reported that they could accommodate both PHM and HM time in the future.
PHM subspecialty designation with associated fellowship training requirements decreased desire to practice HM among med-peds residents who responded to our survey. This reflects findings from a recently published study that evaluated whether PHM fellowship requirements for board certification influenced pediatric and med-peds residents’ decision to pursue PHM in 2018.4 Additionally, Chandrasekar et al4 found that 87% of respondents indicated that sufficient residency training was an important factor in discouraging them from pursuing PHM fellowship. We noted similar findings in our open-ended survey responses, which indicate that med-peds respondents perceived that the intended purpose of PHM fellowship was to provide additional clinical training, and that served as a deterrent for fellowship. However, the survey by Chandrasekar et al4 assessed only four factors for understanding what was important in encouraging pursuit of a PHM fellowship: opportunity to gain new skills, potential increase in salary, opportunity for a master’s degree, and increased prestige. Our survey expands on med-peds residents’ needs, indicating that med-peds residents want a combined med-peds/HM fellowship that allows them to meet PHM board-eligibility requirements while also continuing to develop their adult HM clinical practice and other nonclinical training objectives in a way that combines both adult HM and PHM. Both surveys demonstrate the role that residency program directors and other resident mentors can have in counseling trainees on the nonclinical training objectives of PHM fellowship, including research, quality improvement, medical education, and leadership and clinical operations. Additional emphasis can be placed on opportunities for an individualized curriculum to address the specific career aims of each resident.
In this study, med-peds trainees viewed distribution of clinical time during fellowship as an important factor in pursuing PHM fellowship. The perceived importance of balancing clinical time is not surprising considering that most survey respondents interested in HM ultimately intend to practice both PHM and adult HM. This finding corresponds with current practice patterns of med-peds hospitalists, the majority of whom care for both children and adults.4,5 Moonlighting in adult medicine was not considered sufficient, suggesting desire for mentorship and training integration on the internal medicine side. Opportunities for trainees to maintain and expand their internal medicine knowledge base and clinical decision-making outside of moonlighting will be key to meeting the needs of med-peds residents in PHM fellowship.
Fortunately, more than half of responding programs reported that they could allow for adult HM practice during PHM fellowship. Twelve programs were unsure if they could accommodate adult HM clinical time, and only two programs reported they could not. We suspect that the ability to support this training with clinical time in both adult HM and PHM is more likely available at programs with established internal medicine relationships, often in the form of med-peds residency programs and med-peds faculty. Further, these established relationships may be more common at pediatric health systems that are integrated or affiliated with an adult health system. Most PHM fellowships surveyed indicated that their pediatric institution had an affiliation with an adult facility, and most had med-peds HM faculty.
Precedent for supporting med-peds fellows is somewhat limited given that only five of the responding PHM fellowship programs reported having fellows with med-peds residency training. However, discrepancies between the expressed needs of med-peds residents and the current Accreditation Council for Graduate Medical Education (ACGME)–accredited PHM fellowship structure highlight opportunities to tailor fellowship training to support the career goals of med-peds residents. The current PHM fellowship structure consists of 26 educational units, with each unit representing 4 calendar weeks. A minimum of eight units are spent on each of the following: core clinical rotations, systems and scholarship, and individualized curriculum.10,11 The Society of Hospital Medicine has published core competencies for both PHM and adult HM, which highlight significant overlap in each field’s skill competency, particularly in areas such as quality improvement, legal issues and risk management, and handoffs and transitions of care.12,13 We contend that competencies addressed within PHM fellowship core clinical rotations may overlap with adult HM. Training in adult HM could be completed as part of the individualized curriculum with the ACGME, allowing adult HM practice to count toward this requirement. This would offer med-peds fellows the option to maintain their adult HM knowledge base without eliminating all elective time. Ultimately, it will be important to be creative in how training is accomplished and skills are acquired during both core clinical and individualized training blocks for med-peds trainees completing PHM fellowship.
In order to meet the expressed needs of med-peds residents interested in incorporating both adult HM and PHM into their future careers through PHM fellowship, we offer key recommendations for consideration by the ACGME, PHM FDs, and med-peds program directors (Figure). We encourage current PHM fellowship programs to establish relationships with adult HM programs to develop structured clinical opportunities that will allow fellows to gain the additional clinical training desired.
There were important limitations in this study. First, our estimated response rate for the resident survey was 35.8% of all med-peds residents in 2019, which may be interpreted as low. However, it is important to note that the survey was targeted to residents interested in HM. More than 25% of med-peds residents pursue a career in HM,5 suggesting our response rate may be attributed to residents who did not complete the survey because they were interested in other fields. The program director survey response rate was higher at 58.3%, though it is possible that response bias resulted in a higher response rate from programs with the ability to support med-peds trainees. Regardless, data from programs with the ability to support med-peds trainees are highly valuable in describing how PHM fellowship can be inclusive of med-peds–trained physicians interested in pursuing HM.
Both surveys were completed in 2019, prior to the ACGME accreditation of PHM fellowship, which likely presents new, unique challenges to fellowship programs trying to support the needs of med-peds fellows. However, insights noted above from programs with experience training med-peds fellows are still applicable within the constraints of ACGME requirements.
CONCLUSION
Many med-peds residents express strong interest in practicing HM and including PHM as part of their future hospitalist practice. With the introduction of PHM subspecialty board certification through the American Board of Pediatrics, med-peds residents face new considerations when choosing a career path after residency. The majority of resident respondents express the desire to spend a substantial portion of their clinical practice and/or fellowship practicing adult HM. A majority of PHM fellowships can or are willing to explore how to provide both pediatric and adult hospitalist training to med-peds residency–trained fellows. Understanding the facilitators and barriers to recruiting med-peds trainees for PHM fellowship ultimately has significant implications for the future of the PHM workforce. Incorporating the recommendations noted in this study may increase retention of med-peds providers in PHM by enabling fellowship training and ultimately board certification. Collaboration among the ACGME, PHM program directors, and med-peds residency program directors could help to develop PHM fellowship training programs that will meet the needs of med-peds residents interested in practicing PHM while still meeting ACGME requirements for PHM board eligibility.
Acknowledgment
The authors thank Dr Anoop Agrawal of National Med-Peds Residents’ Association (NMPRA).
1. Blankenburg B, Bode R, Carlson D, et al. National Pediatric Hospital Medicine Leaders Conference. Published April 4, 2013. https://medpeds.org/wp-content/uploads/2015/02/PediatricHospitalMedicineCertificationMeeting_Update.pdf
2. The American Board of Pediatrics. Pediatric Hospital Medicine Certification. Revised December 18, 2020. Accessed January 26, 2021. https://www.abp.org/content/pediatric-hospital-medicine-certification
3. Feldman LS, Monash B, Eniasivam A, Chang W. Why required pediatric hospital medicine fellowships are unnecessary. Hospitalist. 2016;10. https://www.the-hospitalist.org/hospitalist/article/121461/pediatrics/why-required-pediatric-hospital-medicine-fellowships-are
4. Chandrasekar H, White YN, Ribeiro C, Landrigan CP, Marcus CH. A changing landscape: exploring resident perspectives on pursuing pediatric hospital medicine fellowships. Hosp Pediatr. 2021;11(2):109-115. https://doi.org/10.1542/hpeds.2020-0034
5. O’Toole JK, Friedland AR, Gonzaga AMR, et al. The practice patterns of recently graduated internal medicine-pediatric hospitalists. Hosp Pediatr. 2015;5(6):309-314. https://doi.org/10.1542/hpeds.2014-0135
6. Donnelly MJ, Lubrano L, Radabaugh CL, Lukela MP, Friedland AR, Ruch-Ross HS. The med-peds hospitalist workforce: results from the American Academy of Pediatrics Workforce Survey. Hosp Pediatr. 2015;5(11):574-579. https://doi.org/10.1542/hpeds.2015-0031
7. Patwardhan A, Henrickson M, Laskosz L, Duyenhong S, Spencer CH. Current pediatric rheumatology fellowship training in the United States: what fellows actually do. Pediatr Rheumatol Online J. 2014;12(1):8. https://doi.org/10.1186/1546-0096-12-8
8. Howell E, Kravet S, Kisuule F, Wright SM. An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3(4):314-318. https://doi.org/10.1002/jhm.327
9. The National Med-Peds Residents’ Association. About. Accessed May 11, 2021. https://medpeds.org/about-nmpra/
10. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698.https://doi.org/10.1542/peds.2017-0698
11. ACGME Program Requirements for Graduate Medical Education in Pediatric Hospital Medicine. Pediatr Hosp Med. Published online July 1, 2020:55.
12. Maniscalco J, Gage S, Teferi S, Fisher ES. The Pediatric Hospital Medicine Core Competencies: 2020 Revision. J Hosp Med. 2020;15(7):389-394. https://doi.org/10.12788/jhm.3391
13. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the Core Competencies in Hospital Medicine--2017 Revision: Introduction and Methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715
The American Board of Medical Specialties approved subspecialty designation for the field of pediatric hospital medicine (PHM) in 2016.1 For those who started independent practice prior to July 2019, there were two options for board eligibility: the “practice pathway” or completion of a PHM fellowship. The practice pathway allows for pediatric and combined internal medicine–pediatric (med-peds) providers who graduated by July 2019 to sit for the PHM board-certification examination if they meet specific criteria in their pediatric practice.2 For pediatric and med-peds residents who graduated after July 2019, PHM board eligibility is available only through completion of a PHM fellowship.
PHM subspecialty designation with fellowship training requirements may pose unique challenges to med-peds residents interested in practicing both pediatric and adult hospital medicine (HM).3,4 Each year, an estimated 25% of med-peds residency graduates go on to practice HM.5 The majority (62%-83%) of currently practicing med-peds–trained hospitalists care for both adults and children.5,6 Further, med-peds–trained hospitalists comprise at least 10% of the PHM workforce5 and play an important role in caring for adult survivors of childhood diseases.3
Limited existing data suggest that the future practice patterns of med-peds residents may be affected by PHM fellowship requirements. One previous survey study indicated that, although med-peds residents see value in additional training opportunities offered by fellowship, the majority are less likely to pursue PHM as a result of the new requirements.4 Prominent factors dissuading residents from pursuing PHM fellowship included forfeited earnings during fellowship, student loan obligations, family obligations, and the perception that training received during residency was sufficient. Although these data provide important insights into potential changes in practice patterns, they do not explore qualities of PHM fellowship that may make additional training more appealing to med-peds residents and promote retention of med-peds–trained providers in the PHM workforce.
Further, there is no existing literature exploring if and how PHM fellowship programs are equipped to support the needs of med-peds–trained fellows. Other subspecialties have supported med-peds trainees in combined fellowship training programs, including rheumatology, neurology, pediatric emergency medicine, allergy/immunology, physical medicine and rehabilitation, and psychiatry.7,8 However, the extent to which PHM fellowships follow a similar model to accommodate the career goals of med-peds participants is unclear.
Given the large numbers of med-peds residents who go on to practice combined PHM and adult HM, it is crucial to understand the training needs of this group within the context of PHM fellowship and board certification. The primary objectives of this study were to understand (1) the perceived PHM fellowship needs of med-peds residents interested in HM, and (2) how the current PHM fellowship training environment can meet those needs. Understanding that additional training requirements to practice PHM may affect the career trajectory of residents interested in HM, secondary objectives included describing perceptions of med-peds residents on PHM specialty designation and whether designation affected their career plans.
METHODS
Study Design
This cross-sectional study took place over a 3-month period from May to July 2019 and included two surveys of different populations to develop a comprehensive understanding of stakeholder perceptions of PHM fellowship. The first survey (resident survey) invited med-peds residents who were members of the National Med-Peds Residents’ Association (NMPRA)9 in 2019 and who were interested in HM. The second survey (fellowship director [FD] survey) included PHM FDs. The study was determined to be exempt by the University of Pittsburgh Institutional Review Board.
Study Population and Recruitment
Resident Survey
Two attempts were made to elicit participation via the NMPRA electronic mailing list. The NMPRA membership includes med-peds residents and chief residents from US med-peds residency programs. As of May 2019, 77 med-peds residency programs and their residents were members of NMPRA, which encompassed all med-peds programs in the United States and its territories. NMPRA maintains a listserv for all members, and all existing US/territory programs were members at the time of the survey. Med-peds interns, residents, and chief residents interested in HM were invited to participate in this study.
FD Survey
Forty-eight FDs, representing member institutions of the PHM Fellowship Directors’ Council, were surveyed via the PHM Fellowship Directors listserv.
Survey Instruments
We constructed two de novo surveys consisting of multiple-choice and short-answer questions (Appendix 1 and Appendix 2). To enhance the validity of survey responses, questions were designed and tested using an iterative consensus process among authors and additional participants, including current med-peds PHM fellows, PHM fellowship program directors, med-peds residency program directors, and current med-peds residents. These revisions were repeated for a total of four cycles. Items were created to increase knowledge on the following key areas: resident-perceived needs in fellowship training, impact of PHM subspecialty designation on career choices related to HM, health system structure of fellowship programs, and ability to accommodate med-peds clinical training within a PHM fellowship. A combined med-peds fellowship, as defined in the survey and referenced in this study, is a “combined internal medicine–pediatrics hospital medicine fellowship whereby you would remain eligible for PHM board certification.” To ensure a broad and inclusive view of potential needs of med-peds trainees considering fellowship, all respondents were asked to complete questions pertaining to anticipated fellowship needs regardless of their indicated interest in fellowship.
Data Collection
Survey completion was voluntary. Email identifiers were not linked to completed surveys. Study data were collected and managed by using Qualtrics XM. Only completed survey entries were included in analysis.
Statistical Methods and Data Analysis
R software version 4.0.2 (R Foundation for Statistical Computing) was used for statistical analysis. Demographic data were summarized using frequency distributions. The intent of the free-text questions for both surveys was qualitative explanatory thematic analysis. Authors EB, HL, and AJ used a deductive approach to identify common themes that elucidated med-peds resident–anticipated needs in fellowship and PHM program strategies and barriers to accommodate these needs. Preliminary themes and action items were reviewed and discussed among the full authorship team until consensus was reached.
RESULTS
Demographic Data
Resident Survey
A total of 466 med-peds residents completed the resident survey. There are approximately 1300 med-peds residents annually, creating an estimated response rate of 35.8% of all US med-peds residents. The majority (n = 380, 81.5%) of respondents were med-peds postgraduate years 1 through 3 and thus only eligible for PHM board certification via the PHM fellowship pathway (Table 1). Most (n = 446, 95.7%) respondents had considered a career in adult, pediatric, or combined HM at some point. Of those med-peds residents who considered a career in HM (Appendix Table 1), 92.8% (n = 414) would prefer to practice combined adult HM and PHM.

FD Survey
Twenty-eight FDs completed the FD survey, representing 58.3% of 2019 PHM fellowship programs. Of the responding programs, 23 (82.1%) were associated with a freestanding children’s hospital, and 24 (85.7%) were integrated or affiliated with a health system that provides adult inpatient care (Table 2). Sixteen (57.1%) programs had a med-peds residency program at their institution.

Med-Peds Resident Perceptions of PHM Fellowship
In considering the importance of PHM board certification for physicians practicing PHM, 59.0% (n= 275) of respondents rated board certification as “not at all important” (Appendix Table 2). Most (n = 420, 90.1%) med-peds trainees responded that PHM subspecialty designation “decreased” or “significantly decreased” their desire to pursue a career that includes PHM. Of the respondents who reported no interest in hospital medicine, eight (40%) reported that PHM subspecialty status dissuaded them from a career in HM at least a moderate amount (Appendix Table 3). Roughly one third (n=158, 33.9%) of respondents reported that PHM subspecialty designation increased or significantly increased their desire to pursue a career that includes adult HM (Appenidx Table 2). Finally, although the majority (n = 275, 59%) of respondents said they had no interest in a HM fellowship, 114 (24.5%) indicated interest in a combined med-peds HM fellowship (Appendix Table 1). Short-answer questions revealed that commitment to additional training on top of a 4-year residency program was a possible deterring factor, particularly in light of student loan debt and family obligations. Respondents reported adequate clinical training during residency as another deterring factor.
Med-Peds Resident–Perceived Needs in PHM Fellowship
Regardless of interest in completing a PHM fellowship, all resident survey respondents were asked how their ideal PHM fellowship should be structured. Almost all (n = 456, 97.9%) respondents indicated that they would prefer to complete a combined med-peds HM fellowship (Table 3), and most preferred to complete a fellowship in 2 years. Only 10 (2.1%) respondents preferred to complete a PHM fellowship alone in 2 or 3 years. More than half (n=253, 54.3%) of respondents indicated that it would be ideal to obtain a master’s degree as part of fellowship.

Three quarters (n = 355, 75.8%) of med-peds residents reported that they would want 41% or more of clinical time in an ideal fellowship dedicated to adult HM. Importantly, most (n = 322, 69.1%) of the med-peds residents did not consider moonlighting alone in either PHM or adult HM to be enough to maintain training. In addition, many (n = 366, 78.5%) respondents felt that it was important or very important for scholarly work during fellowship to bridge pediatrics and internal medicine.
Short-answer questions indicated that the ability to practice both internal medicine and pediatrics during fellowship emerged as an important deciding factor, with emphasis on adequate opportunities to maintain internal medicine knowledge base (Figure). Similarly, access to med-peds mentorship was an important component of the decision. Compensation both during fellowship and potential future earnings was also a prominent consideration.

Capacity of PHM Programs to Support Med-Peds Fellows
Fifteen (53.6%) FDs reported that their programs were able to accommodate both PHM and adult HM clinical time during fellowship, 11 (39.3%) were unsure, and 2 (7.1%) were unable to accommodate both (Table 2).
The options for adult HM clinical time varied by institution and included precepted time on adult HM, full attending privileges on adult HM, and adult HM time through moonlighting only. Short-answer responses from FDs with experience training med-peds fellows cited using PHM elective time for adult HM and offering moonlighting in adult HM as ways to address career goals of med-peds trainees. Scholarship time for fellows was preserved by decreasing required time on pediatric intensive care unit and complex care services.
Accessibility of Med-Peds Mentorship
As noted above, med-peds residents identified mentorship as an important factor in consideration of PHM fellowship. A total of 23 (82.1%) FDs reported their programs had med-peds faculty members within their PHM team (Table 2). The majority (n = 21, 91.3%) of those med-peds faculty had both PHM and adult HM clinical time.
DISCUSSION
This study characterized the ideal PHM fellowship structure from the perspective of med-peds residents and described the current ability of PHM fellowships to support med-peds residents. The majority of residents stated that they had no interest in an HM fellowship. However, for med-peds residents who considered a career in HM, 88.8% preferred to complete a combined internal medicine and pediatrics HM fellowship with close to half of clinical time dedicated to adult HM. Just over half (53.6%) of programs reported that they could currently accommodate both PHM and adult clinical time during fellowship, and all but two programs reported that they could accommodate both PHM and HM time in the future.
PHM subspecialty designation with associated fellowship training requirements decreased desire to practice HM among med-peds residents who responded to our survey. This reflects findings from a recently published study that evaluated whether PHM fellowship requirements for board certification influenced pediatric and med-peds residents’ decision to pursue PHM in 2018.4 Additionally, Chandrasekar et al4 found that 87% of respondents indicated that sufficient residency training was an important factor in discouraging them from pursuing PHM fellowship. We noted similar findings in our open-ended survey responses, which indicate that med-peds respondents perceived that the intended purpose of PHM fellowship was to provide additional clinical training, and that served as a deterrent for fellowship. However, the survey by Chandrasekar et al4 assessed only four factors for understanding what was important in encouraging pursuit of a PHM fellowship: opportunity to gain new skills, potential increase in salary, opportunity for a master’s degree, and increased prestige. Our survey expands on med-peds residents’ needs, indicating that med-peds residents want a combined med-peds/HM fellowship that allows them to meet PHM board-eligibility requirements while also continuing to develop their adult HM clinical practice and other nonclinical training objectives in a way that combines both adult HM and PHM. Both surveys demonstrate the role that residency program directors and other resident mentors can have in counseling trainees on the nonclinical training objectives of PHM fellowship, including research, quality improvement, medical education, and leadership and clinical operations. Additional emphasis can be placed on opportunities for an individualized curriculum to address the specific career aims of each resident.
In this study, med-peds trainees viewed distribution of clinical time during fellowship as an important factor in pursuing PHM fellowship. The perceived importance of balancing clinical time is not surprising considering that most survey respondents interested in HM ultimately intend to practice both PHM and adult HM. This finding corresponds with current practice patterns of med-peds hospitalists, the majority of whom care for both children and adults.4,5 Moonlighting in adult medicine was not considered sufficient, suggesting desire for mentorship and training integration on the internal medicine side. Opportunities for trainees to maintain and expand their internal medicine knowledge base and clinical decision-making outside of moonlighting will be key to meeting the needs of med-peds residents in PHM fellowship.
Fortunately, more than half of responding programs reported that they could allow for adult HM practice during PHM fellowship. Twelve programs were unsure if they could accommodate adult HM clinical time, and only two programs reported they could not. We suspect that the ability to support this training with clinical time in both adult HM and PHM is more likely available at programs with established internal medicine relationships, often in the form of med-peds residency programs and med-peds faculty. Further, these established relationships may be more common at pediatric health systems that are integrated or affiliated with an adult health system. Most PHM fellowships surveyed indicated that their pediatric institution had an affiliation with an adult facility, and most had med-peds HM faculty.
Precedent for supporting med-peds fellows is somewhat limited given that only five of the responding PHM fellowship programs reported having fellows with med-peds residency training. However, discrepancies between the expressed needs of med-peds residents and the current Accreditation Council for Graduate Medical Education (ACGME)–accredited PHM fellowship structure highlight opportunities to tailor fellowship training to support the career goals of med-peds residents. The current PHM fellowship structure consists of 26 educational units, with each unit representing 4 calendar weeks. A minimum of eight units are spent on each of the following: core clinical rotations, systems and scholarship, and individualized curriculum.10,11 The Society of Hospital Medicine has published core competencies for both PHM and adult HM, which highlight significant overlap in each field’s skill competency, particularly in areas such as quality improvement, legal issues and risk management, and handoffs and transitions of care.12,13 We contend that competencies addressed within PHM fellowship core clinical rotations may overlap with adult HM. Training in adult HM could be completed as part of the individualized curriculum with the ACGME, allowing adult HM practice to count toward this requirement. This would offer med-peds fellows the option to maintain their adult HM knowledge base without eliminating all elective time. Ultimately, it will be important to be creative in how training is accomplished and skills are acquired during both core clinical and individualized training blocks for med-peds trainees completing PHM fellowship.
In order to meet the expressed needs of med-peds residents interested in incorporating both adult HM and PHM into their future careers through PHM fellowship, we offer key recommendations for consideration by the ACGME, PHM FDs, and med-peds program directors (Figure). We encourage current PHM fellowship programs to establish relationships with adult HM programs to develop structured clinical opportunities that will allow fellows to gain the additional clinical training desired.
There were important limitations in this study. First, our estimated response rate for the resident survey was 35.8% of all med-peds residents in 2019, which may be interpreted as low. However, it is important to note that the survey was targeted to residents interested in HM. More than 25% of med-peds residents pursue a career in HM,5 suggesting our response rate may be attributed to residents who did not complete the survey because they were interested in other fields. The program director survey response rate was higher at 58.3%, though it is possible that response bias resulted in a higher response rate from programs with the ability to support med-peds trainees. Regardless, data from programs with the ability to support med-peds trainees are highly valuable in describing how PHM fellowship can be inclusive of med-peds–trained physicians interested in pursuing HM.
Both surveys were completed in 2019, prior to the ACGME accreditation of PHM fellowship, which likely presents new, unique challenges to fellowship programs trying to support the needs of med-peds fellows. However, insights noted above from programs with experience training med-peds fellows are still applicable within the constraints of ACGME requirements.
CONCLUSION
Many med-peds residents express strong interest in practicing HM and including PHM as part of their future hospitalist practice. With the introduction of PHM subspecialty board certification through the American Board of Pediatrics, med-peds residents face new considerations when choosing a career path after residency. The majority of resident respondents express the desire to spend a substantial portion of their clinical practice and/or fellowship practicing adult HM. A majority of PHM fellowships can or are willing to explore how to provide both pediatric and adult hospitalist training to med-peds residency–trained fellows. Understanding the facilitators and barriers to recruiting med-peds trainees for PHM fellowship ultimately has significant implications for the future of the PHM workforce. Incorporating the recommendations noted in this study may increase retention of med-peds providers in PHM by enabling fellowship training and ultimately board certification. Collaboration among the ACGME, PHM program directors, and med-peds residency program directors could help to develop PHM fellowship training programs that will meet the needs of med-peds residents interested in practicing PHM while still meeting ACGME requirements for PHM board eligibility.
Acknowledgment
The authors thank Dr Anoop Agrawal of National Med-Peds Residents’ Association (NMPRA).
The American Board of Medical Specialties approved subspecialty designation for the field of pediatric hospital medicine (PHM) in 2016.1 For those who started independent practice prior to July 2019, there were two options for board eligibility: the “practice pathway” or completion of a PHM fellowship. The practice pathway allows for pediatric and combined internal medicine–pediatric (med-peds) providers who graduated by July 2019 to sit for the PHM board-certification examination if they meet specific criteria in their pediatric practice.2 For pediatric and med-peds residents who graduated after July 2019, PHM board eligibility is available only through completion of a PHM fellowship.
PHM subspecialty designation with fellowship training requirements may pose unique challenges to med-peds residents interested in practicing both pediatric and adult hospital medicine (HM).3,4 Each year, an estimated 25% of med-peds residency graduates go on to practice HM.5 The majority (62%-83%) of currently practicing med-peds–trained hospitalists care for both adults and children.5,6 Further, med-peds–trained hospitalists comprise at least 10% of the PHM workforce5 and play an important role in caring for adult survivors of childhood diseases.3
Limited existing data suggest that the future practice patterns of med-peds residents may be affected by PHM fellowship requirements. One previous survey study indicated that, although med-peds residents see value in additional training opportunities offered by fellowship, the majority are less likely to pursue PHM as a result of the new requirements.4 Prominent factors dissuading residents from pursuing PHM fellowship included forfeited earnings during fellowship, student loan obligations, family obligations, and the perception that training received during residency was sufficient. Although these data provide important insights into potential changes in practice patterns, they do not explore qualities of PHM fellowship that may make additional training more appealing to med-peds residents and promote retention of med-peds–trained providers in the PHM workforce.
Further, there is no existing literature exploring if and how PHM fellowship programs are equipped to support the needs of med-peds–trained fellows. Other subspecialties have supported med-peds trainees in combined fellowship training programs, including rheumatology, neurology, pediatric emergency medicine, allergy/immunology, physical medicine and rehabilitation, and psychiatry.7,8 However, the extent to which PHM fellowships follow a similar model to accommodate the career goals of med-peds participants is unclear.
Given the large numbers of med-peds residents who go on to practice combined PHM and adult HM, it is crucial to understand the training needs of this group within the context of PHM fellowship and board certification. The primary objectives of this study were to understand (1) the perceived PHM fellowship needs of med-peds residents interested in HM, and (2) how the current PHM fellowship training environment can meet those needs. Understanding that additional training requirements to practice PHM may affect the career trajectory of residents interested in HM, secondary objectives included describing perceptions of med-peds residents on PHM specialty designation and whether designation affected their career plans.
METHODS
Study Design
This cross-sectional study took place over a 3-month period from May to July 2019 and included two surveys of different populations to develop a comprehensive understanding of stakeholder perceptions of PHM fellowship. The first survey (resident survey) invited med-peds residents who were members of the National Med-Peds Residents’ Association (NMPRA)9 in 2019 and who were interested in HM. The second survey (fellowship director [FD] survey) included PHM FDs. The study was determined to be exempt by the University of Pittsburgh Institutional Review Board.
Study Population and Recruitment
Resident Survey
Two attempts were made to elicit participation via the NMPRA electronic mailing list. The NMPRA membership includes med-peds residents and chief residents from US med-peds residency programs. As of May 2019, 77 med-peds residency programs and their residents were members of NMPRA, which encompassed all med-peds programs in the United States and its territories. NMPRA maintains a listserv for all members, and all existing US/territory programs were members at the time of the survey. Med-peds interns, residents, and chief residents interested in HM were invited to participate in this study.
FD Survey
Forty-eight FDs, representing member institutions of the PHM Fellowship Directors’ Council, were surveyed via the PHM Fellowship Directors listserv.
Survey Instruments
We constructed two de novo surveys consisting of multiple-choice and short-answer questions (Appendix 1 and Appendix 2). To enhance the validity of survey responses, questions were designed and tested using an iterative consensus process among authors and additional participants, including current med-peds PHM fellows, PHM fellowship program directors, med-peds residency program directors, and current med-peds residents. These revisions were repeated for a total of four cycles. Items were created to increase knowledge on the following key areas: resident-perceived needs in fellowship training, impact of PHM subspecialty designation on career choices related to HM, health system structure of fellowship programs, and ability to accommodate med-peds clinical training within a PHM fellowship. A combined med-peds fellowship, as defined in the survey and referenced in this study, is a “combined internal medicine–pediatrics hospital medicine fellowship whereby you would remain eligible for PHM board certification.” To ensure a broad and inclusive view of potential needs of med-peds trainees considering fellowship, all respondents were asked to complete questions pertaining to anticipated fellowship needs regardless of their indicated interest in fellowship.
Data Collection
Survey completion was voluntary. Email identifiers were not linked to completed surveys. Study data were collected and managed by using Qualtrics XM. Only completed survey entries were included in analysis.
Statistical Methods and Data Analysis
R software version 4.0.2 (R Foundation for Statistical Computing) was used for statistical analysis. Demographic data were summarized using frequency distributions. The intent of the free-text questions for both surveys was qualitative explanatory thematic analysis. Authors EB, HL, and AJ used a deductive approach to identify common themes that elucidated med-peds resident–anticipated needs in fellowship and PHM program strategies and barriers to accommodate these needs. Preliminary themes and action items were reviewed and discussed among the full authorship team until consensus was reached.
RESULTS
Demographic Data
Resident Survey
A total of 466 med-peds residents completed the resident survey. There are approximately 1300 med-peds residents annually, creating an estimated response rate of 35.8% of all US med-peds residents. The majority (n = 380, 81.5%) of respondents were med-peds postgraduate years 1 through 3 and thus only eligible for PHM board certification via the PHM fellowship pathway (Table 1). Most (n = 446, 95.7%) respondents had considered a career in adult, pediatric, or combined HM at some point. Of those med-peds residents who considered a career in HM (Appendix Table 1), 92.8% (n = 414) would prefer to practice combined adult HM and PHM.

FD Survey
Twenty-eight FDs completed the FD survey, representing 58.3% of 2019 PHM fellowship programs. Of the responding programs, 23 (82.1%) were associated with a freestanding children’s hospital, and 24 (85.7%) were integrated or affiliated with a health system that provides adult inpatient care (Table 2). Sixteen (57.1%) programs had a med-peds residency program at their institution.

Med-Peds Resident Perceptions of PHM Fellowship
In considering the importance of PHM board certification for physicians practicing PHM, 59.0% (n= 275) of respondents rated board certification as “not at all important” (Appendix Table 2). Most (n = 420, 90.1%) med-peds trainees responded that PHM subspecialty designation “decreased” or “significantly decreased” their desire to pursue a career that includes PHM. Of the respondents who reported no interest in hospital medicine, eight (40%) reported that PHM subspecialty status dissuaded them from a career in HM at least a moderate amount (Appendix Table 3). Roughly one third (n=158, 33.9%) of respondents reported that PHM subspecialty designation increased or significantly increased their desire to pursue a career that includes adult HM (Appenidx Table 2). Finally, although the majority (n = 275, 59%) of respondents said they had no interest in a HM fellowship, 114 (24.5%) indicated interest in a combined med-peds HM fellowship (Appendix Table 1). Short-answer questions revealed that commitment to additional training on top of a 4-year residency program was a possible deterring factor, particularly in light of student loan debt and family obligations. Respondents reported adequate clinical training during residency as another deterring factor.
Med-Peds Resident–Perceived Needs in PHM Fellowship
Regardless of interest in completing a PHM fellowship, all resident survey respondents were asked how their ideal PHM fellowship should be structured. Almost all (n = 456, 97.9%) respondents indicated that they would prefer to complete a combined med-peds HM fellowship (Table 3), and most preferred to complete a fellowship in 2 years. Only 10 (2.1%) respondents preferred to complete a PHM fellowship alone in 2 or 3 years. More than half (n=253, 54.3%) of respondents indicated that it would be ideal to obtain a master’s degree as part of fellowship.

Three quarters (n = 355, 75.8%) of med-peds residents reported that they would want 41% or more of clinical time in an ideal fellowship dedicated to adult HM. Importantly, most (n = 322, 69.1%) of the med-peds residents did not consider moonlighting alone in either PHM or adult HM to be enough to maintain training. In addition, many (n = 366, 78.5%) respondents felt that it was important or very important for scholarly work during fellowship to bridge pediatrics and internal medicine.
Short-answer questions indicated that the ability to practice both internal medicine and pediatrics during fellowship emerged as an important deciding factor, with emphasis on adequate opportunities to maintain internal medicine knowledge base (Figure). Similarly, access to med-peds mentorship was an important component of the decision. Compensation both during fellowship and potential future earnings was also a prominent consideration.

Capacity of PHM Programs to Support Med-Peds Fellows
Fifteen (53.6%) FDs reported that their programs were able to accommodate both PHM and adult HM clinical time during fellowship, 11 (39.3%) were unsure, and 2 (7.1%) were unable to accommodate both (Table 2).
The options for adult HM clinical time varied by institution and included precepted time on adult HM, full attending privileges on adult HM, and adult HM time through moonlighting only. Short-answer responses from FDs with experience training med-peds fellows cited using PHM elective time for adult HM and offering moonlighting in adult HM as ways to address career goals of med-peds trainees. Scholarship time for fellows was preserved by decreasing required time on pediatric intensive care unit and complex care services.
Accessibility of Med-Peds Mentorship
As noted above, med-peds residents identified mentorship as an important factor in consideration of PHM fellowship. A total of 23 (82.1%) FDs reported their programs had med-peds faculty members within their PHM team (Table 2). The majority (n = 21, 91.3%) of those med-peds faculty had both PHM and adult HM clinical time.
DISCUSSION
This study characterized the ideal PHM fellowship structure from the perspective of med-peds residents and described the current ability of PHM fellowships to support med-peds residents. The majority of residents stated that they had no interest in an HM fellowship. However, for med-peds residents who considered a career in HM, 88.8% preferred to complete a combined internal medicine and pediatrics HM fellowship with close to half of clinical time dedicated to adult HM. Just over half (53.6%) of programs reported that they could currently accommodate both PHM and adult clinical time during fellowship, and all but two programs reported that they could accommodate both PHM and HM time in the future.
PHM subspecialty designation with associated fellowship training requirements decreased desire to practice HM among med-peds residents who responded to our survey. This reflects findings from a recently published study that evaluated whether PHM fellowship requirements for board certification influenced pediatric and med-peds residents’ decision to pursue PHM in 2018.4 Additionally, Chandrasekar et al4 found that 87% of respondents indicated that sufficient residency training was an important factor in discouraging them from pursuing PHM fellowship. We noted similar findings in our open-ended survey responses, which indicate that med-peds respondents perceived that the intended purpose of PHM fellowship was to provide additional clinical training, and that served as a deterrent for fellowship. However, the survey by Chandrasekar et al4 assessed only four factors for understanding what was important in encouraging pursuit of a PHM fellowship: opportunity to gain new skills, potential increase in salary, opportunity for a master’s degree, and increased prestige. Our survey expands on med-peds residents’ needs, indicating that med-peds residents want a combined med-peds/HM fellowship that allows them to meet PHM board-eligibility requirements while also continuing to develop their adult HM clinical practice and other nonclinical training objectives in a way that combines both adult HM and PHM. Both surveys demonstrate the role that residency program directors and other resident mentors can have in counseling trainees on the nonclinical training objectives of PHM fellowship, including research, quality improvement, medical education, and leadership and clinical operations. Additional emphasis can be placed on opportunities for an individualized curriculum to address the specific career aims of each resident.
In this study, med-peds trainees viewed distribution of clinical time during fellowship as an important factor in pursuing PHM fellowship. The perceived importance of balancing clinical time is not surprising considering that most survey respondents interested in HM ultimately intend to practice both PHM and adult HM. This finding corresponds with current practice patterns of med-peds hospitalists, the majority of whom care for both children and adults.4,5 Moonlighting in adult medicine was not considered sufficient, suggesting desire for mentorship and training integration on the internal medicine side. Opportunities for trainees to maintain and expand their internal medicine knowledge base and clinical decision-making outside of moonlighting will be key to meeting the needs of med-peds residents in PHM fellowship.
Fortunately, more than half of responding programs reported that they could allow for adult HM practice during PHM fellowship. Twelve programs were unsure if they could accommodate adult HM clinical time, and only two programs reported they could not. We suspect that the ability to support this training with clinical time in both adult HM and PHM is more likely available at programs with established internal medicine relationships, often in the form of med-peds residency programs and med-peds faculty. Further, these established relationships may be more common at pediatric health systems that are integrated or affiliated with an adult health system. Most PHM fellowships surveyed indicated that their pediatric institution had an affiliation with an adult facility, and most had med-peds HM faculty.
Precedent for supporting med-peds fellows is somewhat limited given that only five of the responding PHM fellowship programs reported having fellows with med-peds residency training. However, discrepancies between the expressed needs of med-peds residents and the current Accreditation Council for Graduate Medical Education (ACGME)–accredited PHM fellowship structure highlight opportunities to tailor fellowship training to support the career goals of med-peds residents. The current PHM fellowship structure consists of 26 educational units, with each unit representing 4 calendar weeks. A minimum of eight units are spent on each of the following: core clinical rotations, systems and scholarship, and individualized curriculum.10,11 The Society of Hospital Medicine has published core competencies for both PHM and adult HM, which highlight significant overlap in each field’s skill competency, particularly in areas such as quality improvement, legal issues and risk management, and handoffs and transitions of care.12,13 We contend that competencies addressed within PHM fellowship core clinical rotations may overlap with adult HM. Training in adult HM could be completed as part of the individualized curriculum with the ACGME, allowing adult HM practice to count toward this requirement. This would offer med-peds fellows the option to maintain their adult HM knowledge base without eliminating all elective time. Ultimately, it will be important to be creative in how training is accomplished and skills are acquired during both core clinical and individualized training blocks for med-peds trainees completing PHM fellowship.
In order to meet the expressed needs of med-peds residents interested in incorporating both adult HM and PHM into their future careers through PHM fellowship, we offer key recommendations for consideration by the ACGME, PHM FDs, and med-peds program directors (Figure). We encourage current PHM fellowship programs to establish relationships with adult HM programs to develop structured clinical opportunities that will allow fellows to gain the additional clinical training desired.
There were important limitations in this study. First, our estimated response rate for the resident survey was 35.8% of all med-peds residents in 2019, which may be interpreted as low. However, it is important to note that the survey was targeted to residents interested in HM. More than 25% of med-peds residents pursue a career in HM,5 suggesting our response rate may be attributed to residents who did not complete the survey because they were interested in other fields. The program director survey response rate was higher at 58.3%, though it is possible that response bias resulted in a higher response rate from programs with the ability to support med-peds trainees. Regardless, data from programs with the ability to support med-peds trainees are highly valuable in describing how PHM fellowship can be inclusive of med-peds–trained physicians interested in pursuing HM.
Both surveys were completed in 2019, prior to the ACGME accreditation of PHM fellowship, which likely presents new, unique challenges to fellowship programs trying to support the needs of med-peds fellows. However, insights noted above from programs with experience training med-peds fellows are still applicable within the constraints of ACGME requirements.
CONCLUSION
Many med-peds residents express strong interest in practicing HM and including PHM as part of their future hospitalist practice. With the introduction of PHM subspecialty board certification through the American Board of Pediatrics, med-peds residents face new considerations when choosing a career path after residency. The majority of resident respondents express the desire to spend a substantial portion of their clinical practice and/or fellowship practicing adult HM. A majority of PHM fellowships can or are willing to explore how to provide both pediatric and adult hospitalist training to med-peds residency–trained fellows. Understanding the facilitators and barriers to recruiting med-peds trainees for PHM fellowship ultimately has significant implications for the future of the PHM workforce. Incorporating the recommendations noted in this study may increase retention of med-peds providers in PHM by enabling fellowship training and ultimately board certification. Collaboration among the ACGME, PHM program directors, and med-peds residency program directors could help to develop PHM fellowship training programs that will meet the needs of med-peds residents interested in practicing PHM while still meeting ACGME requirements for PHM board eligibility.
Acknowledgment
The authors thank Dr Anoop Agrawal of National Med-Peds Residents’ Association (NMPRA).
1. Blankenburg B, Bode R, Carlson D, et al. National Pediatric Hospital Medicine Leaders Conference. Published April 4, 2013. https://medpeds.org/wp-content/uploads/2015/02/PediatricHospitalMedicineCertificationMeeting_Update.pdf
2. The American Board of Pediatrics. Pediatric Hospital Medicine Certification. Revised December 18, 2020. Accessed January 26, 2021. https://www.abp.org/content/pediatric-hospital-medicine-certification
3. Feldman LS, Monash B, Eniasivam A, Chang W. Why required pediatric hospital medicine fellowships are unnecessary. Hospitalist. 2016;10. https://www.the-hospitalist.org/hospitalist/article/121461/pediatrics/why-required-pediatric-hospital-medicine-fellowships-are
4. Chandrasekar H, White YN, Ribeiro C, Landrigan CP, Marcus CH. A changing landscape: exploring resident perspectives on pursuing pediatric hospital medicine fellowships. Hosp Pediatr. 2021;11(2):109-115. https://doi.org/10.1542/hpeds.2020-0034
5. O’Toole JK, Friedland AR, Gonzaga AMR, et al. The practice patterns of recently graduated internal medicine-pediatric hospitalists. Hosp Pediatr. 2015;5(6):309-314. https://doi.org/10.1542/hpeds.2014-0135
6. Donnelly MJ, Lubrano L, Radabaugh CL, Lukela MP, Friedland AR, Ruch-Ross HS. The med-peds hospitalist workforce: results from the American Academy of Pediatrics Workforce Survey. Hosp Pediatr. 2015;5(11):574-579. https://doi.org/10.1542/hpeds.2015-0031
7. Patwardhan A, Henrickson M, Laskosz L, Duyenhong S, Spencer CH. Current pediatric rheumatology fellowship training in the United States: what fellows actually do. Pediatr Rheumatol Online J. 2014;12(1):8. https://doi.org/10.1186/1546-0096-12-8
8. Howell E, Kravet S, Kisuule F, Wright SM. An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3(4):314-318. https://doi.org/10.1002/jhm.327
9. The National Med-Peds Residents’ Association. About. Accessed May 11, 2021. https://medpeds.org/about-nmpra/
10. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698.https://doi.org/10.1542/peds.2017-0698
11. ACGME Program Requirements for Graduate Medical Education in Pediatric Hospital Medicine. Pediatr Hosp Med. Published online July 1, 2020:55.
12. Maniscalco J, Gage S, Teferi S, Fisher ES. The Pediatric Hospital Medicine Core Competencies: 2020 Revision. J Hosp Med. 2020;15(7):389-394. https://doi.org/10.12788/jhm.3391
13. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the Core Competencies in Hospital Medicine--2017 Revision: Introduction and Methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715
1. Blankenburg B, Bode R, Carlson D, et al. National Pediatric Hospital Medicine Leaders Conference. Published April 4, 2013. https://medpeds.org/wp-content/uploads/2015/02/PediatricHospitalMedicineCertificationMeeting_Update.pdf
2. The American Board of Pediatrics. Pediatric Hospital Medicine Certification. Revised December 18, 2020. Accessed January 26, 2021. https://www.abp.org/content/pediatric-hospital-medicine-certification
3. Feldman LS, Monash B, Eniasivam A, Chang W. Why required pediatric hospital medicine fellowships are unnecessary. Hospitalist. 2016;10. https://www.the-hospitalist.org/hospitalist/article/121461/pediatrics/why-required-pediatric-hospital-medicine-fellowships-are
4. Chandrasekar H, White YN, Ribeiro C, Landrigan CP, Marcus CH. A changing landscape: exploring resident perspectives on pursuing pediatric hospital medicine fellowships. Hosp Pediatr. 2021;11(2):109-115. https://doi.org/10.1542/hpeds.2020-0034
5. O’Toole JK, Friedland AR, Gonzaga AMR, et al. The practice patterns of recently graduated internal medicine-pediatric hospitalists. Hosp Pediatr. 2015;5(6):309-314. https://doi.org/10.1542/hpeds.2014-0135
6. Donnelly MJ, Lubrano L, Radabaugh CL, Lukela MP, Friedland AR, Ruch-Ross HS. The med-peds hospitalist workforce: results from the American Academy of Pediatrics Workforce Survey. Hosp Pediatr. 2015;5(11):574-579. https://doi.org/10.1542/hpeds.2015-0031
7. Patwardhan A, Henrickson M, Laskosz L, Duyenhong S, Spencer CH. Current pediatric rheumatology fellowship training in the United States: what fellows actually do. Pediatr Rheumatol Online J. 2014;12(1):8. https://doi.org/10.1186/1546-0096-12-8
8. Howell E, Kravet S, Kisuule F, Wright SM. An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3(4):314-318. https://doi.org/10.1002/jhm.327
9. The National Med-Peds Residents’ Association. About. Accessed May 11, 2021. https://medpeds.org/about-nmpra/
10. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1):e20170698.https://doi.org/10.1542/peds.2017-0698
11. ACGME Program Requirements for Graduate Medical Education in Pediatric Hospital Medicine. Pediatr Hosp Med. Published online July 1, 2020:55.
12. Maniscalco J, Gage S, Teferi S, Fisher ES. The Pediatric Hospital Medicine Core Competencies: 2020 Revision. J Hosp Med. 2020;15(7):389-394. https://doi.org/10.12788/jhm.3391
13. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the Core Competencies in Hospital Medicine--2017 Revision: Introduction and Methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715
© 2021 Society of Hospital Medicine
‘Reassuring’ findings for second-generation antipsychotics during pregnancy
Second-generation antipsychotics (SGAs) taken by pregnant women are linked to a low rate of adverse effects in their children, new research suggests.
Data from a large registry study of almost 2,000 women showed that 2.5% of the live births in a group that had been exposed to antipsychotics had confirmed major malformations compared with 2% of the live births in a non-exposed group. This translated into an estimated odds ratio of 1.5 for major malformations.
“The 2.5% absolute risk for major malformations is consistent with the estimates of the Centers for Disease Control and Prevention’s national baseline rate of major malformations in the general population,” lead author Adele Viguera, MD, MPH, director of research for women’s mental health, Cleveland Clinic Neurological Institute, told this news organization.
“Our results are reassuring and suggest that second-generation antipsychotics, as a class, do not substantially increase the risk of major malformations,” Dr. Viguera said.
The findings were published online August 3 in the Journal of Clinical Psychiatry.
Safety data scarce
Despite the increasing use of SGAs to treat a “spectrum of psychiatric disorders,” relatively little data are available on the reproductive safety of these agents, Dr. Viguera said.
The National Pregnancy Registry for Atypical Antipsychotics (NPRAA) was established in 2008 to determine risk for major malformation among infants exposed to these medications during the first trimester, relative to a comparison group of unexposed infants of mothers with histories of psychiatric morbidity.
The NPRAA follows pregnant women (aged 18 to 45 years) with psychiatric illness who are exposed or unexposed to SGAs during pregnancy. Participants are recruited through nationwide provider referral, self-referral, and advertisement through the Massachusetts General Hospital Center for Women’s Mental Health website.
Specific data collected are shown in the following table.
Since publication of the first results in 2015, the sample size for the trial has increased – and the absolute and relative risk for major malformations observed in the study population are “more precise,” the investigators note. The current study presented updated previous findings.
Demographic differences
Of the 1,906 women who enrolled as of April 2020, 1,311 (mean age, 32.6 years; 81.3% White) completed the study and were eligible for inclusion in the analysis.
Although the groups had a virtually identical mean age, fewer women in the exposure group were married compared with those in the non-exposure group (77% vs. 90%, respectively) and fewer had a college education (71.2% vs. 87.8%). There was also a higher percentage of first-trimester cigarette smokers in the exposure group (18.4% vs. 5.1%).
On the other hand, more women in the non-exposure group used alcohol than in the exposure group (28.6% vs. 21.4%, respectively).
The most frequent psychiatric disorder in the exposure group was bipolar disorder (63.9%), followed by major depression (12.9%), anxiety (5.8%), and schizophrenia (4.5%). Only 11.4% of women in the non-exposure group were diagnosed with bipolar disorder, whereas 34.1% were diagnosed with major depression, 31.3% with anxiety, and none with schizophrenia.
Notably, a large percentage of women in both groups had a history of postpartum depression and/or psychosis (41.4% and 35.5%, respectively).
The most frequently used SGAs in the exposure group were quetiapine (Seroquel), aripiprazole (Abilify), and lurasidone (Latuda).
Participants in the exposure group had a higher age at initial onset of primary psychiatric diagnosis and a lower proportion of lifetime illness compared with those in the non-exposure group.
Major clinical implication?
Among 640 live births in the exposure group, which included 17 twin pregnancies and 1 triplet pregnancy, 2.5% reported major malformations. Among 704 live births in the control group, which included 14 twin pregnancies, 1.99% reported major malformations.
The estimated OR for major malformations comparing exposed and unexposed infants was 1.48 (95% confidence interval, 0.625-3.517).
The authors note that their findings were consistent with one of the largest studies to date, which included a nationwide sample of more than 1 million women. Its results showed that, among infants exposed to SGAs versus those who were not exposed, the estimated risk ratio after adjusting for psychiatric conditions was 1.05 (95% CI, 0.96-1.16).
Additionally, “a hallmark of a teratogen is that it tends to cause a specific type or pattern of malformations, and we found no preponderance of one single type of major malformation or specific pattern of malformations among the exposed and unexposed groups,” Dr. Viguera said
“A major clinical implication of these findings is that for women with major mood and/or psychotic disorders, treatment with an atypical antipsychotic during pregnancy may be the most prudent clinical decision, much as continued treatment is recommended for pregnant women with other serious and chronic medical conditions, such as epilepsy,” she added.
The concept of ‘satisficing’
Commenting on the study, Vivien Burt, MD, PhD, founder and director/consultant of the Women’s Life Center at the Resnick University of California, Los Angeles (UCLA) Neuropsychiatric Hospital, called the findings “reassuring.”
The results “support the conclusion that in pregnant women with serious psychiatric illnesses, the use of SGAs is often a better option than avoiding these medications and exposing both the women and their offspring to the adverse consequences of maternal mental illness,” she said.
An accompanying editorial co-authored by Dr. Burt and colleague Sonya Rasminsky, MD, introduced the concept of “satisficing” – a term coined by Herbert Simon, a behavioral economist and Nobel Laureate. “Satisficing” is a “decision-making strategy that aims for a satisfactory (‘good enough’) outcome rather than a perfect one.”
The concept applies to decision-making beyond the field of economics “and is critical to how physicians help patients make decisions when they are faced with multiple treatment options,” said Dr. Burt, a professor emeritus of psychiatry at UCLA.
“The goal of ‘satisficing’ is to plan for the most satisfactory outcome, knowing that there are always unknowns, so in an uncertain world, clinicians should carefully help their patients make decisions that will allow them to achieve an outcome they can best live with,” she noted.
The investigators note that their findings may not be generalizable to the larger population of women taking SGAs, given that their participants were “overwhelmingly White, married, and well-educated women.”
They add that enrollment into the NPRAA registry is ongoing and larger sample sizes will “further narrow the confidence interval around the risk estimates and allow for adjustment of likely sources of confounding.”
The NPRAA is supported by Alkermes, Johnson & Johnson/Janssen Pharmaceuticals, Otsuka America Pharmaceutical, Sunovion Pharmaceuticals, SAGE Therapeutics, Teva Pharmaceuticals, and Aurobindo Pharma. Past sponsors of the NPRAA are listed in the original paper. Dr. Viguera receives research support from the NPRAA, Alkermes Biopharmaceuticals, Aurobindo Pharma, Janssen Pharmaceuticals, Otsuka Pharmaceutical, Sunovion Pharmaceuticals, Teva Pharmaceuticals, and SAGE Therapeutics and receives adviser/consulting fees from Up-to-Date. Dr. Burt has been a consultant/speaker for Sage Therapeutics. Dr. Rasminsky has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Second-generation antipsychotics (SGAs) taken by pregnant women are linked to a low rate of adverse effects in their children, new research suggests.
Data from a large registry study of almost 2,000 women showed that 2.5% of the live births in a group that had been exposed to antipsychotics had confirmed major malformations compared with 2% of the live births in a non-exposed group. This translated into an estimated odds ratio of 1.5 for major malformations.
“The 2.5% absolute risk for major malformations is consistent with the estimates of the Centers for Disease Control and Prevention’s national baseline rate of major malformations in the general population,” lead author Adele Viguera, MD, MPH, director of research for women’s mental health, Cleveland Clinic Neurological Institute, told this news organization.
“Our results are reassuring and suggest that second-generation antipsychotics, as a class, do not substantially increase the risk of major malformations,” Dr. Viguera said.
The findings were published online August 3 in the Journal of Clinical Psychiatry.
Safety data scarce
Despite the increasing use of SGAs to treat a “spectrum of psychiatric disorders,” relatively little data are available on the reproductive safety of these agents, Dr. Viguera said.
The National Pregnancy Registry for Atypical Antipsychotics (NPRAA) was established in 2008 to determine risk for major malformation among infants exposed to these medications during the first trimester, relative to a comparison group of unexposed infants of mothers with histories of psychiatric morbidity.
The NPRAA follows pregnant women (aged 18 to 45 years) with psychiatric illness who are exposed or unexposed to SGAs during pregnancy. Participants are recruited through nationwide provider referral, self-referral, and advertisement through the Massachusetts General Hospital Center for Women’s Mental Health website.
Specific data collected are shown in the following table.
Since publication of the first results in 2015, the sample size for the trial has increased – and the absolute and relative risk for major malformations observed in the study population are “more precise,” the investigators note. The current study presented updated previous findings.
Demographic differences
Of the 1,906 women who enrolled as of April 2020, 1,311 (mean age, 32.6 years; 81.3% White) completed the study and were eligible for inclusion in the analysis.
Although the groups had a virtually identical mean age, fewer women in the exposure group were married compared with those in the non-exposure group (77% vs. 90%, respectively) and fewer had a college education (71.2% vs. 87.8%). There was also a higher percentage of first-trimester cigarette smokers in the exposure group (18.4% vs. 5.1%).
On the other hand, more women in the non-exposure group used alcohol than in the exposure group (28.6% vs. 21.4%, respectively).
The most frequent psychiatric disorder in the exposure group was bipolar disorder (63.9%), followed by major depression (12.9%), anxiety (5.8%), and schizophrenia (4.5%). Only 11.4% of women in the non-exposure group were diagnosed with bipolar disorder, whereas 34.1% were diagnosed with major depression, 31.3% with anxiety, and none with schizophrenia.
Notably, a large percentage of women in both groups had a history of postpartum depression and/or psychosis (41.4% and 35.5%, respectively).
The most frequently used SGAs in the exposure group were quetiapine (Seroquel), aripiprazole (Abilify), and lurasidone (Latuda).
Participants in the exposure group had a higher age at initial onset of primary psychiatric diagnosis and a lower proportion of lifetime illness compared with those in the non-exposure group.
Major clinical implication?
Among 640 live births in the exposure group, which included 17 twin pregnancies and 1 triplet pregnancy, 2.5% reported major malformations. Among 704 live births in the control group, which included 14 twin pregnancies, 1.99% reported major malformations.
The estimated OR for major malformations comparing exposed and unexposed infants was 1.48 (95% confidence interval, 0.625-3.517).
The authors note that their findings were consistent with one of the largest studies to date, which included a nationwide sample of more than 1 million women. Its results showed that, among infants exposed to SGAs versus those who were not exposed, the estimated risk ratio after adjusting for psychiatric conditions was 1.05 (95% CI, 0.96-1.16).
Additionally, “a hallmark of a teratogen is that it tends to cause a specific type or pattern of malformations, and we found no preponderance of one single type of major malformation or specific pattern of malformations among the exposed and unexposed groups,” Dr. Viguera said
“A major clinical implication of these findings is that for women with major mood and/or psychotic disorders, treatment with an atypical antipsychotic during pregnancy may be the most prudent clinical decision, much as continued treatment is recommended for pregnant women with other serious and chronic medical conditions, such as epilepsy,” she added.
The concept of ‘satisficing’
Commenting on the study, Vivien Burt, MD, PhD, founder and director/consultant of the Women’s Life Center at the Resnick University of California, Los Angeles (UCLA) Neuropsychiatric Hospital, called the findings “reassuring.”
The results “support the conclusion that in pregnant women with serious psychiatric illnesses, the use of SGAs is often a better option than avoiding these medications and exposing both the women and their offspring to the adverse consequences of maternal mental illness,” she said.
An accompanying editorial co-authored by Dr. Burt and colleague Sonya Rasminsky, MD, introduced the concept of “satisficing” – a term coined by Herbert Simon, a behavioral economist and Nobel Laureate. “Satisficing” is a “decision-making strategy that aims for a satisfactory (‘good enough’) outcome rather than a perfect one.”
The concept applies to decision-making beyond the field of economics “and is critical to how physicians help patients make decisions when they are faced with multiple treatment options,” said Dr. Burt, a professor emeritus of psychiatry at UCLA.
“The goal of ‘satisficing’ is to plan for the most satisfactory outcome, knowing that there are always unknowns, so in an uncertain world, clinicians should carefully help their patients make decisions that will allow them to achieve an outcome they can best live with,” she noted.
The investigators note that their findings may not be generalizable to the larger population of women taking SGAs, given that their participants were “overwhelmingly White, married, and well-educated women.”
They add that enrollment into the NPRAA registry is ongoing and larger sample sizes will “further narrow the confidence interval around the risk estimates and allow for adjustment of likely sources of confounding.”
The NPRAA is supported by Alkermes, Johnson & Johnson/Janssen Pharmaceuticals, Otsuka America Pharmaceutical, Sunovion Pharmaceuticals, SAGE Therapeutics, Teva Pharmaceuticals, and Aurobindo Pharma. Past sponsors of the NPRAA are listed in the original paper. Dr. Viguera receives research support from the NPRAA, Alkermes Biopharmaceuticals, Aurobindo Pharma, Janssen Pharmaceuticals, Otsuka Pharmaceutical, Sunovion Pharmaceuticals, Teva Pharmaceuticals, and SAGE Therapeutics and receives adviser/consulting fees from Up-to-Date. Dr. Burt has been a consultant/speaker for Sage Therapeutics. Dr. Rasminsky has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Second-generation antipsychotics (SGAs) taken by pregnant women are linked to a low rate of adverse effects in their children, new research suggests.
Data from a large registry study of almost 2,000 women showed that 2.5% of the live births in a group that had been exposed to antipsychotics had confirmed major malformations compared with 2% of the live births in a non-exposed group. This translated into an estimated odds ratio of 1.5 for major malformations.
“The 2.5% absolute risk for major malformations is consistent with the estimates of the Centers for Disease Control and Prevention’s national baseline rate of major malformations in the general population,” lead author Adele Viguera, MD, MPH, director of research for women’s mental health, Cleveland Clinic Neurological Institute, told this news organization.
“Our results are reassuring and suggest that second-generation antipsychotics, as a class, do not substantially increase the risk of major malformations,” Dr. Viguera said.
The findings were published online August 3 in the Journal of Clinical Psychiatry.
Safety data scarce
Despite the increasing use of SGAs to treat a “spectrum of psychiatric disorders,” relatively little data are available on the reproductive safety of these agents, Dr. Viguera said.
The National Pregnancy Registry for Atypical Antipsychotics (NPRAA) was established in 2008 to determine risk for major malformation among infants exposed to these medications during the first trimester, relative to a comparison group of unexposed infants of mothers with histories of psychiatric morbidity.
The NPRAA follows pregnant women (aged 18 to 45 years) with psychiatric illness who are exposed or unexposed to SGAs during pregnancy. Participants are recruited through nationwide provider referral, self-referral, and advertisement through the Massachusetts General Hospital Center for Women’s Mental Health website.
Specific data collected are shown in the following table.
Since publication of the first results in 2015, the sample size for the trial has increased – and the absolute and relative risk for major malformations observed in the study population are “more precise,” the investigators note. The current study presented updated previous findings.
Demographic differences
Of the 1,906 women who enrolled as of April 2020, 1,311 (mean age, 32.6 years; 81.3% White) completed the study and were eligible for inclusion in the analysis.
Although the groups had a virtually identical mean age, fewer women in the exposure group were married compared with those in the non-exposure group (77% vs. 90%, respectively) and fewer had a college education (71.2% vs. 87.8%). There was also a higher percentage of first-trimester cigarette smokers in the exposure group (18.4% vs. 5.1%).
On the other hand, more women in the non-exposure group used alcohol than in the exposure group (28.6% vs. 21.4%, respectively).
The most frequent psychiatric disorder in the exposure group was bipolar disorder (63.9%), followed by major depression (12.9%), anxiety (5.8%), and schizophrenia (4.5%). Only 11.4% of women in the non-exposure group were diagnosed with bipolar disorder, whereas 34.1% were diagnosed with major depression, 31.3% with anxiety, and none with schizophrenia.
Notably, a large percentage of women in both groups had a history of postpartum depression and/or psychosis (41.4% and 35.5%, respectively).
The most frequently used SGAs in the exposure group were quetiapine (Seroquel), aripiprazole (Abilify), and lurasidone (Latuda).
Participants in the exposure group had a higher age at initial onset of primary psychiatric diagnosis and a lower proportion of lifetime illness compared with those in the non-exposure group.
Major clinical implication?
Among 640 live births in the exposure group, which included 17 twin pregnancies and 1 triplet pregnancy, 2.5% reported major malformations. Among 704 live births in the control group, which included 14 twin pregnancies, 1.99% reported major malformations.
The estimated OR for major malformations comparing exposed and unexposed infants was 1.48 (95% confidence interval, 0.625-3.517).
The authors note that their findings were consistent with one of the largest studies to date, which included a nationwide sample of more than 1 million women. Its results showed that, among infants exposed to SGAs versus those who were not exposed, the estimated risk ratio after adjusting for psychiatric conditions was 1.05 (95% CI, 0.96-1.16).
Additionally, “a hallmark of a teratogen is that it tends to cause a specific type or pattern of malformations, and we found no preponderance of one single type of major malformation or specific pattern of malformations among the exposed and unexposed groups,” Dr. Viguera said
“A major clinical implication of these findings is that for women with major mood and/or psychotic disorders, treatment with an atypical antipsychotic during pregnancy may be the most prudent clinical decision, much as continued treatment is recommended for pregnant women with other serious and chronic medical conditions, such as epilepsy,” she added.
The concept of ‘satisficing’
Commenting on the study, Vivien Burt, MD, PhD, founder and director/consultant of the Women’s Life Center at the Resnick University of California, Los Angeles (UCLA) Neuropsychiatric Hospital, called the findings “reassuring.”
The results “support the conclusion that in pregnant women with serious psychiatric illnesses, the use of SGAs is often a better option than avoiding these medications and exposing both the women and their offspring to the adverse consequences of maternal mental illness,” she said.
An accompanying editorial co-authored by Dr. Burt and colleague Sonya Rasminsky, MD, introduced the concept of “satisficing” – a term coined by Herbert Simon, a behavioral economist and Nobel Laureate. “Satisficing” is a “decision-making strategy that aims for a satisfactory (‘good enough’) outcome rather than a perfect one.”
The concept applies to decision-making beyond the field of economics “and is critical to how physicians help patients make decisions when they are faced with multiple treatment options,” said Dr. Burt, a professor emeritus of psychiatry at UCLA.
“The goal of ‘satisficing’ is to plan for the most satisfactory outcome, knowing that there are always unknowns, so in an uncertain world, clinicians should carefully help their patients make decisions that will allow them to achieve an outcome they can best live with,” she noted.
The investigators note that their findings may not be generalizable to the larger population of women taking SGAs, given that their participants were “overwhelmingly White, married, and well-educated women.”
They add that enrollment into the NPRAA registry is ongoing and larger sample sizes will “further narrow the confidence interval around the risk estimates and allow for adjustment of likely sources of confounding.”
The NPRAA is supported by Alkermes, Johnson & Johnson/Janssen Pharmaceuticals, Otsuka America Pharmaceutical, Sunovion Pharmaceuticals, SAGE Therapeutics, Teva Pharmaceuticals, and Aurobindo Pharma. Past sponsors of the NPRAA are listed in the original paper. Dr. Viguera receives research support from the NPRAA, Alkermes Biopharmaceuticals, Aurobindo Pharma, Janssen Pharmaceuticals, Otsuka Pharmaceutical, Sunovion Pharmaceuticals, Teva Pharmaceuticals, and SAGE Therapeutics and receives adviser/consulting fees from Up-to-Date. Dr. Burt has been a consultant/speaker for Sage Therapeutics. Dr. Rasminsky has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.

