User login
Infectious disease pop quiz: Clinical challenge #9 for the ObGyn
For uncomplicated chlamydia infection in a pregnant woman, what is the most appropriate treatment?
Continue to the answer...
Uncomplicated chlamydia infection in a pregnant woman should be treated with a single 1,000-mg oral dose of azithromycin. An acceptable alternative is amoxicillin 500 mg orally 3 times daily for 7 days.
In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days is also an appropriate alternative. However, doxycycline is relatively expensive and may not be well tolerated because of gastrointestinal adverse effects. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
For uncomplicated chlamydia infection in a pregnant woman, what is the most appropriate treatment?
Continue to the answer...
Uncomplicated chlamydia infection in a pregnant woman should be treated with a single 1,000-mg oral dose of azithromycin. An acceptable alternative is amoxicillin 500 mg orally 3 times daily for 7 days.
In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days is also an appropriate alternative. However, doxycycline is relatively expensive and may not be well tolerated because of gastrointestinal adverse effects. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
For uncomplicated chlamydia infection in a pregnant woman, what is the most appropriate treatment?
Continue to the answer...
Uncomplicated chlamydia infection in a pregnant woman should be treated with a single 1,000-mg oral dose of azithromycin. An acceptable alternative is amoxicillin 500 mg orally 3 times daily for 7 days.
In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days is also an appropriate alternative. However, doxycycline is relatively expensive and may not be well tolerated because of gastrointestinal adverse effects. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
AAN updates treatment guidance on painful diabetic neuropathy
Painful diabetic neuropathy is very common and can greatly affect an individual’s quality of life, guideline author Brian Callaghan, MD, University of Michigan, Ann Arbor, noted in a news release.
“This guideline aims to help neurologists and other doctors provide the highest quality patient care based on the latest evidence,” Dr. Callaghan said.
The recommendations update the 2011 AAN guideline on the treatment of painful diabetic neuropathy. The new guidance was published online Dec. 27, 2021, in Neurology and has been endorsed by the American Association of Neuromuscular & Electrodiagnostic Medicine.
Multiple options
To update the guideline, an expert panel reviewed data from more than 100 randomized controlled trials published from January 2008 to April 2020.
The panel noted that more than 16% of individuals with diabetes experience painful diabetic neuropathy, but it often goes unrecognized and untreated. The guideline recommends clinicians assess patients with diabetes for peripheral neuropathic pain and its effect on their function and quality of life.
Before prescribing treatment, health providers should determine if the patient also has mood or sleep problems as both can influence pain perception.
The guideline recommends offering one of four classes of oral medications found to be effective for neuropathic pain: tricyclic antidepressants such as amitriptyline, nortriptyline, or imipramine; serotonin norepinephrine reuptake inhibitors such as duloxetine, venlafaxine, or desvenlafaxine; gabapentinoids such as gabapentin or pregabalin; and/or sodium channel blockers such as carbamazepine, oxcarbazepine, lamotrigine, or lacosamide.
All four classes of medications have “comparable effect sizes just above or just below our cutoff for a medium effect size” (standardized median difference, 0.5), the panel noted.
In addition, “new studies on sodium channel blockers published since the last guideline have resulted in these drugs now being recommended and considered as effective at providing pain relief as the other drug classes recommended in this guideline,” said Dr. Callaghan.
When an initial medication fails to provide meaningful improvement in pain, or produces significant side effects, a trial of another medication from a different class is recommended.
Pain reduction, not elimination
Opioids are not recommended for painful diabetic neuropathy. Not only do they come with risks, there is also no strong evidence they are effective for painful diabetic neuropathy in the long term, the panel wrote. Tramadol and tapentadol are also not recommended for the treatment of painful diabetic neuropathy.
“Current evidence suggests that the risks of the use of opioids for painful diabetic neuropathy therapy outweigh the benefits, so they should not be prescribed,” Dr. Callaghan said.
For patients interested in trying topical, nontraditional, or nondrug interventions to reduce pain, the guideline recommends a number of options including capsaicin, glyceryl trinitrate spray, and Citrullus colocynthis. Ginkgo biloba, exercise, mindfulness, cognitive-behavioral therapy, and tai chi are also suggested.
“It is important to note that the recommended drugs and topical treatments in this guideline may not eliminate pain, but they have been shown to reduce pain,” Dr. Callaghan said. “The good news is there are many treatment options for painful diabetic neuropathy, so a treatment plan can be tailored specifically to each person living with this condition.”
Along with the updated guideline, the AAN has also published a new Polyneuropathy Quality Measurement Set to assist neurologists and other health care providers in treating patients with painful diabetic neuropathy.
The updated guideline was developed with financial support from the AAN.
A version of this article first appeared on Medscape.com.
Painful diabetic neuropathy is very common and can greatly affect an individual’s quality of life, guideline author Brian Callaghan, MD, University of Michigan, Ann Arbor, noted in a news release.
“This guideline aims to help neurologists and other doctors provide the highest quality patient care based on the latest evidence,” Dr. Callaghan said.
The recommendations update the 2011 AAN guideline on the treatment of painful diabetic neuropathy. The new guidance was published online Dec. 27, 2021, in Neurology and has been endorsed by the American Association of Neuromuscular & Electrodiagnostic Medicine.
Multiple options
To update the guideline, an expert panel reviewed data from more than 100 randomized controlled trials published from January 2008 to April 2020.
The panel noted that more than 16% of individuals with diabetes experience painful diabetic neuropathy, but it often goes unrecognized and untreated. The guideline recommends clinicians assess patients with diabetes for peripheral neuropathic pain and its effect on their function and quality of life.
Before prescribing treatment, health providers should determine if the patient also has mood or sleep problems as both can influence pain perception.
The guideline recommends offering one of four classes of oral medications found to be effective for neuropathic pain: tricyclic antidepressants such as amitriptyline, nortriptyline, or imipramine; serotonin norepinephrine reuptake inhibitors such as duloxetine, venlafaxine, or desvenlafaxine; gabapentinoids such as gabapentin or pregabalin; and/or sodium channel blockers such as carbamazepine, oxcarbazepine, lamotrigine, or lacosamide.
All four classes of medications have “comparable effect sizes just above or just below our cutoff for a medium effect size” (standardized median difference, 0.5), the panel noted.
In addition, “new studies on sodium channel blockers published since the last guideline have resulted in these drugs now being recommended and considered as effective at providing pain relief as the other drug classes recommended in this guideline,” said Dr. Callaghan.
When an initial medication fails to provide meaningful improvement in pain, or produces significant side effects, a trial of another medication from a different class is recommended.
Pain reduction, not elimination
Opioids are not recommended for painful diabetic neuropathy. Not only do they come with risks, there is also no strong evidence they are effective for painful diabetic neuropathy in the long term, the panel wrote. Tramadol and tapentadol are also not recommended for the treatment of painful diabetic neuropathy.
“Current evidence suggests that the risks of the use of opioids for painful diabetic neuropathy therapy outweigh the benefits, so they should not be prescribed,” Dr. Callaghan said.
For patients interested in trying topical, nontraditional, or nondrug interventions to reduce pain, the guideline recommends a number of options including capsaicin, glyceryl trinitrate spray, and Citrullus colocynthis. Ginkgo biloba, exercise, mindfulness, cognitive-behavioral therapy, and tai chi are also suggested.
“It is important to note that the recommended drugs and topical treatments in this guideline may not eliminate pain, but they have been shown to reduce pain,” Dr. Callaghan said. “The good news is there are many treatment options for painful diabetic neuropathy, so a treatment plan can be tailored specifically to each person living with this condition.”
Along with the updated guideline, the AAN has also published a new Polyneuropathy Quality Measurement Set to assist neurologists and other health care providers in treating patients with painful diabetic neuropathy.
The updated guideline was developed with financial support from the AAN.
A version of this article first appeared on Medscape.com.
Painful diabetic neuropathy is very common and can greatly affect an individual’s quality of life, guideline author Brian Callaghan, MD, University of Michigan, Ann Arbor, noted in a news release.
“This guideline aims to help neurologists and other doctors provide the highest quality patient care based on the latest evidence,” Dr. Callaghan said.
The recommendations update the 2011 AAN guideline on the treatment of painful diabetic neuropathy. The new guidance was published online Dec. 27, 2021, in Neurology and has been endorsed by the American Association of Neuromuscular & Electrodiagnostic Medicine.
Multiple options
To update the guideline, an expert panel reviewed data from more than 100 randomized controlled trials published from January 2008 to April 2020.
The panel noted that more than 16% of individuals with diabetes experience painful diabetic neuropathy, but it often goes unrecognized and untreated. The guideline recommends clinicians assess patients with diabetes for peripheral neuropathic pain and its effect on their function and quality of life.
Before prescribing treatment, health providers should determine if the patient also has mood or sleep problems as both can influence pain perception.
The guideline recommends offering one of four classes of oral medications found to be effective for neuropathic pain: tricyclic antidepressants such as amitriptyline, nortriptyline, or imipramine; serotonin norepinephrine reuptake inhibitors such as duloxetine, venlafaxine, or desvenlafaxine; gabapentinoids such as gabapentin or pregabalin; and/or sodium channel blockers such as carbamazepine, oxcarbazepine, lamotrigine, or lacosamide.
All four classes of medications have “comparable effect sizes just above or just below our cutoff for a medium effect size” (standardized median difference, 0.5), the panel noted.
In addition, “new studies on sodium channel blockers published since the last guideline have resulted in these drugs now being recommended and considered as effective at providing pain relief as the other drug classes recommended in this guideline,” said Dr. Callaghan.
When an initial medication fails to provide meaningful improvement in pain, or produces significant side effects, a trial of another medication from a different class is recommended.
Pain reduction, not elimination
Opioids are not recommended for painful diabetic neuropathy. Not only do they come with risks, there is also no strong evidence they are effective for painful diabetic neuropathy in the long term, the panel wrote. Tramadol and tapentadol are also not recommended for the treatment of painful diabetic neuropathy.
“Current evidence suggests that the risks of the use of opioids for painful diabetic neuropathy therapy outweigh the benefits, so they should not be prescribed,” Dr. Callaghan said.
For patients interested in trying topical, nontraditional, or nondrug interventions to reduce pain, the guideline recommends a number of options including capsaicin, glyceryl trinitrate spray, and Citrullus colocynthis. Ginkgo biloba, exercise, mindfulness, cognitive-behavioral therapy, and tai chi are also suggested.
“It is important to note that the recommended drugs and topical treatments in this guideline may not eliminate pain, but they have been shown to reduce pain,” Dr. Callaghan said. “The good news is there are many treatment options for painful diabetic neuropathy, so a treatment plan can be tailored specifically to each person living with this condition.”
Along with the updated guideline, the AAN has also published a new Polyneuropathy Quality Measurement Set to assist neurologists and other health care providers in treating patients with painful diabetic neuropathy.
The updated guideline was developed with financial support from the AAN.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Posttraumatic epilepsy is common, even after ‘mild’ TBI
, new research suggests.
Results from a multicenter, prospective cohort study showed 2.7% of nearly 1,500 participants with TBI reported also having posttraumatic epilepsy, and these patients had significantly worse outcomes than those without posttraumatic epilepsy.
“Posttraumatic epilepsy is common even in so-called mild TBI, and we should be on the lookout for patients reporting these kinds of spells,” said coinvestigator Ramon Diaz-Arrastia, MD, PhD, professor of neurology and director of the TBI Clinical Research Center, University of Pennsylvania, Philadelphia.
Dr. Diaz-Arrastia said he dislikes the term “mild TBI” because many of these injuries have “pretty substantial consequences.”
The findings were published online Dec. 29 in JAMA Network Open.
Novel study
Seizures can occur after TBI, most commonly after a severe brain injury, such as those leading to coma or bleeding in the brain or requiring surgical intervention. However, there have been “hints” that some patients with milder brain injuries are also at increased risk for epilepsy, said Dr. Diaz-Arrastia.
To investigate, the researchers assessed data from the large, multicenter Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) database. Participants with TBI, defined as a Glasgow Coma Scale (GCS) score of 3-15, had presented to a level I trauma center within 24 hours of a head trauma needing evaluation with a CT scan.
The study included patients with relatively mild TBI (GCS score, 13-15), which is a “novel feature” of the study, the authors noted. Most prior studies of posttraumatic epilepsy focused on moderate to severe TBI.
The researchers included two sex- and age-matched control groups. The orthopedic trauma control (OTC) group consisted of patients with isolated trauma to the limbs, pelvis, and/or ribs. The “friend” or peer control group had backgrounds and lifestyles similar to those with TBI but had no history of TBI, concussion, or traumatic injury in the previous year.
The analysis included 1,885 participants (mean age, 41.3 years; 65.8% men). Of these, 1,493 had TBI, 182 were in the OTC group, and 210 were in the friends group. At 6- and 12-month follow-ups, investigators administered the Epilepsy Screening Questionnaire (ESQ), developed by the National Institute of Neurological Disorders and Stroke (NINDS).
Confirmatory data
Participants were asked about experiencing uncontrolled movements, unexplained changes in mental state, and repeated unusual attacks or convulsions, and whether they had been told they had epilepsy or seizures. If they answered yes to any of these questions, they received second-level screening, which asked about seizures.
Patients were deemed to have posttraumatic epilepsy if they answered affirmatively to any first-level screening item, experienced seizures 7 days after injury, and were diagnosed with epilepsy.
The primary outcome was rate of positive posttraumatic epilepsy diagnoses. At 12 months, 2.7% of those with TBI reported a posttraumatic epilepsy diagnosis compared with none of either of the control groups (P < .001).
This rate is consistent with prior literature and is “pretty close to what we expected,” said Dr. Diaz-Arrastia.
Among those with TBI and posttraumatic epilepsy, 12.2% had GCS scores of 3-8 (severe), 5.3% had scores of 9-12 (moderate), and 0.9% had scores of 13-15 (mild). That figure for mild TBI is not insignificant, said Dr. Diaz-Arrastia.
“Probably 90% of all those coming to the emergency room with a brain injury are diagnosed with mild TBI not requiring admission,” he noted.
The risk for posttraumatic epilepsy was higher the more severe the head injury, and among those with hemorrhage on head CT imaging. In patients with mild TBI, hemorrhage was associated with a two- to threefold risk of developing posttraumatic epilepsy.
“This prospective observational study confirms the epidemiologic data that even after mild brain injury, there is an increased risk for epilepsy,” said Dr. Diaz-Arrastia.
Universal screening?
The researchers also looked at whether seizures worsen other outcomes. Compared with those who had TBI but not posttraumatic epilepsy, those with posttraumatic epilepsy had significantly lower Glasgow Outcome Scale Extended (GOSE) scores (mean, 4.7 vs. 6.1; P < .001), higher Brief Symptom Inventory (BSI) scores (58.6 vs. 50.2; P = .02), and higher Rivermead Cognitive Metric (RCM) scores (5.3 vs. 3.1; P = .002) at 12 months after adjustment for age, initial GCS score, and imaging findings.
Higher GOSE and RCM scores reflect better outcomes, but a higher score on the BSI, which assesses overall mood, reflects a worse outcome, the investigators noted.
Previous evidence suggests prophylactic use of antiepileptic drugs in patients with TBI does not reduce risks. These drugs “are neither 100% safe nor 100% effective,” said Dr. Diaz-Arrastia. Some studies showed that certain agents actually worsen outcomes, he added.
What the field needs instead are antiepileptogenic drugs – those that interfere with the maladaptive synaptic plasticity that ends up in an epileptic circuit, he noted.
The new results suggest screening for posttraumatic epilepsy using the NINDS-ESQ “should be done pretty much routinely as a follow-up for all brain injuries,” Dr. Diaz-Arrastia said.
The investigators plan to have study participants assessed by an epileptologist later. A significant number of people with TBI, he noted, won’t develop posttraumatic epilepsy until 1-5 years after their injury – and even later in some cases.
A limitation of the study was that some patients reporting posttraumatic epilepsy may have had psychogenic nonepileptiform seizures, which are common in TBI patients, the investigators noted.
The study was supported by grants from One Mind, National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS, and Department of Defence. Dr. Diaz-Arrastia reported receiving grants from the NIH, NINDS, and DOD during the conduct of the study.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a multicenter, prospective cohort study showed 2.7% of nearly 1,500 participants with TBI reported also having posttraumatic epilepsy, and these patients had significantly worse outcomes than those without posttraumatic epilepsy.
“Posttraumatic epilepsy is common even in so-called mild TBI, and we should be on the lookout for patients reporting these kinds of spells,” said coinvestigator Ramon Diaz-Arrastia, MD, PhD, professor of neurology and director of the TBI Clinical Research Center, University of Pennsylvania, Philadelphia.
Dr. Diaz-Arrastia said he dislikes the term “mild TBI” because many of these injuries have “pretty substantial consequences.”
The findings were published online Dec. 29 in JAMA Network Open.
Novel study
Seizures can occur after TBI, most commonly after a severe brain injury, such as those leading to coma or bleeding in the brain or requiring surgical intervention. However, there have been “hints” that some patients with milder brain injuries are also at increased risk for epilepsy, said Dr. Diaz-Arrastia.
To investigate, the researchers assessed data from the large, multicenter Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) database. Participants with TBI, defined as a Glasgow Coma Scale (GCS) score of 3-15, had presented to a level I trauma center within 24 hours of a head trauma needing evaluation with a CT scan.
The study included patients with relatively mild TBI (GCS score, 13-15), which is a “novel feature” of the study, the authors noted. Most prior studies of posttraumatic epilepsy focused on moderate to severe TBI.
The researchers included two sex- and age-matched control groups. The orthopedic trauma control (OTC) group consisted of patients with isolated trauma to the limbs, pelvis, and/or ribs. The “friend” or peer control group had backgrounds and lifestyles similar to those with TBI but had no history of TBI, concussion, or traumatic injury in the previous year.
The analysis included 1,885 participants (mean age, 41.3 years; 65.8% men). Of these, 1,493 had TBI, 182 were in the OTC group, and 210 were in the friends group. At 6- and 12-month follow-ups, investigators administered the Epilepsy Screening Questionnaire (ESQ), developed by the National Institute of Neurological Disorders and Stroke (NINDS).
Confirmatory data
Participants were asked about experiencing uncontrolled movements, unexplained changes in mental state, and repeated unusual attacks or convulsions, and whether they had been told they had epilepsy or seizures. If they answered yes to any of these questions, they received second-level screening, which asked about seizures.
Patients were deemed to have posttraumatic epilepsy if they answered affirmatively to any first-level screening item, experienced seizures 7 days after injury, and were diagnosed with epilepsy.
The primary outcome was rate of positive posttraumatic epilepsy diagnoses. At 12 months, 2.7% of those with TBI reported a posttraumatic epilepsy diagnosis compared with none of either of the control groups (P < .001).
This rate is consistent with prior literature and is “pretty close to what we expected,” said Dr. Diaz-Arrastia.
Among those with TBI and posttraumatic epilepsy, 12.2% had GCS scores of 3-8 (severe), 5.3% had scores of 9-12 (moderate), and 0.9% had scores of 13-15 (mild). That figure for mild TBI is not insignificant, said Dr. Diaz-Arrastia.
“Probably 90% of all those coming to the emergency room with a brain injury are diagnosed with mild TBI not requiring admission,” he noted.
The risk for posttraumatic epilepsy was higher the more severe the head injury, and among those with hemorrhage on head CT imaging. In patients with mild TBI, hemorrhage was associated with a two- to threefold risk of developing posttraumatic epilepsy.
“This prospective observational study confirms the epidemiologic data that even after mild brain injury, there is an increased risk for epilepsy,” said Dr. Diaz-Arrastia.
Universal screening?
The researchers also looked at whether seizures worsen other outcomes. Compared with those who had TBI but not posttraumatic epilepsy, those with posttraumatic epilepsy had significantly lower Glasgow Outcome Scale Extended (GOSE) scores (mean, 4.7 vs. 6.1; P < .001), higher Brief Symptom Inventory (BSI) scores (58.6 vs. 50.2; P = .02), and higher Rivermead Cognitive Metric (RCM) scores (5.3 vs. 3.1; P = .002) at 12 months after adjustment for age, initial GCS score, and imaging findings.
Higher GOSE and RCM scores reflect better outcomes, but a higher score on the BSI, which assesses overall mood, reflects a worse outcome, the investigators noted.
Previous evidence suggests prophylactic use of antiepileptic drugs in patients with TBI does not reduce risks. These drugs “are neither 100% safe nor 100% effective,” said Dr. Diaz-Arrastia. Some studies showed that certain agents actually worsen outcomes, he added.
What the field needs instead are antiepileptogenic drugs – those that interfere with the maladaptive synaptic plasticity that ends up in an epileptic circuit, he noted.
The new results suggest screening for posttraumatic epilepsy using the NINDS-ESQ “should be done pretty much routinely as a follow-up for all brain injuries,” Dr. Diaz-Arrastia said.
The investigators plan to have study participants assessed by an epileptologist later. A significant number of people with TBI, he noted, won’t develop posttraumatic epilepsy until 1-5 years after their injury – and even later in some cases.
A limitation of the study was that some patients reporting posttraumatic epilepsy may have had psychogenic nonepileptiform seizures, which are common in TBI patients, the investigators noted.
The study was supported by grants from One Mind, National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS, and Department of Defence. Dr. Diaz-Arrastia reported receiving grants from the NIH, NINDS, and DOD during the conduct of the study.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a multicenter, prospective cohort study showed 2.7% of nearly 1,500 participants with TBI reported also having posttraumatic epilepsy, and these patients had significantly worse outcomes than those without posttraumatic epilepsy.
“Posttraumatic epilepsy is common even in so-called mild TBI, and we should be on the lookout for patients reporting these kinds of spells,” said coinvestigator Ramon Diaz-Arrastia, MD, PhD, professor of neurology and director of the TBI Clinical Research Center, University of Pennsylvania, Philadelphia.
Dr. Diaz-Arrastia said he dislikes the term “mild TBI” because many of these injuries have “pretty substantial consequences.”
The findings were published online Dec. 29 in JAMA Network Open.
Novel study
Seizures can occur after TBI, most commonly after a severe brain injury, such as those leading to coma or bleeding in the brain or requiring surgical intervention. However, there have been “hints” that some patients with milder brain injuries are also at increased risk for epilepsy, said Dr. Diaz-Arrastia.
To investigate, the researchers assessed data from the large, multicenter Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) database. Participants with TBI, defined as a Glasgow Coma Scale (GCS) score of 3-15, had presented to a level I trauma center within 24 hours of a head trauma needing evaluation with a CT scan.
The study included patients with relatively mild TBI (GCS score, 13-15), which is a “novel feature” of the study, the authors noted. Most prior studies of posttraumatic epilepsy focused on moderate to severe TBI.
The researchers included two sex- and age-matched control groups. The orthopedic trauma control (OTC) group consisted of patients with isolated trauma to the limbs, pelvis, and/or ribs. The “friend” or peer control group had backgrounds and lifestyles similar to those with TBI but had no history of TBI, concussion, or traumatic injury in the previous year.
The analysis included 1,885 participants (mean age, 41.3 years; 65.8% men). Of these, 1,493 had TBI, 182 were in the OTC group, and 210 were in the friends group. At 6- and 12-month follow-ups, investigators administered the Epilepsy Screening Questionnaire (ESQ), developed by the National Institute of Neurological Disorders and Stroke (NINDS).
Confirmatory data
Participants were asked about experiencing uncontrolled movements, unexplained changes in mental state, and repeated unusual attacks or convulsions, and whether they had been told they had epilepsy or seizures. If they answered yes to any of these questions, they received second-level screening, which asked about seizures.
Patients were deemed to have posttraumatic epilepsy if they answered affirmatively to any first-level screening item, experienced seizures 7 days after injury, and were diagnosed with epilepsy.
The primary outcome was rate of positive posttraumatic epilepsy diagnoses. At 12 months, 2.7% of those with TBI reported a posttraumatic epilepsy diagnosis compared with none of either of the control groups (P < .001).
This rate is consistent with prior literature and is “pretty close to what we expected,” said Dr. Diaz-Arrastia.
Among those with TBI and posttraumatic epilepsy, 12.2% had GCS scores of 3-8 (severe), 5.3% had scores of 9-12 (moderate), and 0.9% had scores of 13-15 (mild). That figure for mild TBI is not insignificant, said Dr. Diaz-Arrastia.
“Probably 90% of all those coming to the emergency room with a brain injury are diagnosed with mild TBI not requiring admission,” he noted.
The risk for posttraumatic epilepsy was higher the more severe the head injury, and among those with hemorrhage on head CT imaging. In patients with mild TBI, hemorrhage was associated with a two- to threefold risk of developing posttraumatic epilepsy.
“This prospective observational study confirms the epidemiologic data that even after mild brain injury, there is an increased risk for epilepsy,” said Dr. Diaz-Arrastia.
Universal screening?
The researchers also looked at whether seizures worsen other outcomes. Compared with those who had TBI but not posttraumatic epilepsy, those with posttraumatic epilepsy had significantly lower Glasgow Outcome Scale Extended (GOSE) scores (mean, 4.7 vs. 6.1; P < .001), higher Brief Symptom Inventory (BSI) scores (58.6 vs. 50.2; P = .02), and higher Rivermead Cognitive Metric (RCM) scores (5.3 vs. 3.1; P = .002) at 12 months after adjustment for age, initial GCS score, and imaging findings.
Higher GOSE and RCM scores reflect better outcomes, but a higher score on the BSI, which assesses overall mood, reflects a worse outcome, the investigators noted.
Previous evidence suggests prophylactic use of antiepileptic drugs in patients with TBI does not reduce risks. These drugs “are neither 100% safe nor 100% effective,” said Dr. Diaz-Arrastia. Some studies showed that certain agents actually worsen outcomes, he added.
What the field needs instead are antiepileptogenic drugs – those that interfere with the maladaptive synaptic plasticity that ends up in an epileptic circuit, he noted.
The new results suggest screening for posttraumatic epilepsy using the NINDS-ESQ “should be done pretty much routinely as a follow-up for all brain injuries,” Dr. Diaz-Arrastia said.
The investigators plan to have study participants assessed by an epileptologist later. A significant number of people with TBI, he noted, won’t develop posttraumatic epilepsy until 1-5 years after their injury – and even later in some cases.
A limitation of the study was that some patients reporting posttraumatic epilepsy may have had psychogenic nonepileptiform seizures, which are common in TBI patients, the investigators noted.
The study was supported by grants from One Mind, National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS, and Department of Defence. Dr. Diaz-Arrastia reported receiving grants from the NIH, NINDS, and DOD during the conduct of the study.
A version of this article first appeared on Medscape.com.
Obesity prevention in infants benefits second-born too
According to a statement from the National Institutes of Health, who funded the study, it is the first such infant obesity intervention to show the spillover effect. Findings were published online Dec. 21, 2021, in Obesity.
The program is called Infants Growing on Healthy Trajectories (INSIGHT) responsive parenting (RP) intervention, and it included guidance on feeding, sleep, interactive play, and regulating emotion.
Parents were given guidance by nurses who came to their homes on how to respond when their child is drowsy, sleeping, fussy, and alert. They also learned how to put infants to bed drowsy, but awake, and avoid feeding infants to get them to sleep; how to respond to infants waking up at night; when to start solid foods; how to limit inactive time; and how to use growth charts.
The control group program focused on safety and matched the guidance categories. For example, early visits included information on prevention of sudden infant death syndrome for sleep, breast milk storage and formula for feeding, and safe bathing.
Jennifer S. Savage, Center for Childhood Obesity Research, Penn State University, University Park, led the study that enrolled 117 infants in a randomized controlled trial. Mother and firstborn children were randomized to the RP or home safety intervention (control) group 10-14 days after delivery. Their second-born siblings were enrolled in an observation-only ancillary study.
Second-born children were delivered 2.5 (standard deviation, 0.9) years after firstborns. Anthropometrics were measured in both siblings at ages 3, 16, 28, and 52 weeks.
Firstborn children at 1 year had a body mass index (BMI) that was 0.44 kg/m2 lower than the control group (95% confidence interval, −0.82 to −0.06), and second-born children whose parents received the RP intervention with their first child had BMI that was 0.36 kg/m2 lower.
“What we saw here is that it worked again,” coauthor Ian Paul, MD, MSc, professor of pediatrics and public health sciences at Penn State University, Hershey, said in an interview.
“Once we imprint them with a certain approach to parenting with the first child, they’re doing the same thing with the second child, which is wonderful to see,” he said.
He noted that this happened with second children without any reinforcements or booster information.
Dr. Paul said it’s still not clear which of the interventions – whether related to feeding techniques or sleeping or activity – helps most. And for each family the problematic behaviors may be different.
Responsive parenting programs have shown success previously among firstborns, the authors wrote, but 80% of those children grow up with younger siblings, so an intervention that also benefits them is important.
Weighing the costs of the intervention
The intervention was extensive. It involved four hour-plus nurse visits a year, often by the same nurse who built a relationship with the family.
But Ms. Savage said that it is possible to replicate INSIGHT on a larger scale in the United States with the dozens of home visitation models.
“Currently, 21 home visitation models meet the U.S. Department of Health & Human Services criteria for evidence of effectiveness, such as Nurse Family Partnership, Family Check-up, and Early Head Start Home Based Option. There is an opportunity to use home visitation models at the national scale to potentially interrupt the cycle of poor multigenerational outcomes such as obesity,” she said.
Dr. Paul said the initial investment “can save money in the long term,” given what’s at stake. “We know that 20%-25% of 2- to 5-year-olds are already overweight or obese and if they are already overweight or obese at that age, that;’s likely to persist.”
However, he acknowledged that staff shortages and costs are a challenge.
“Other countries have made that investment in their health care system,” he said. “In the U.S. only a fraction of new mothers and babies get home visitation. The kind of work that we did for obesity prevention has not yet been incorporated into evidence-based models of home visitation, though it certainly could be.”
Dr. Paul said his team is hoping to collaborate with others in the near future on expanding this program to such models of home visitation.
Telehealth, though a less desirable option, compared with in-home visits, could also be utilized, he said.
Short of the comprehensive intervention, he said, many of the concepts can be put into practice by pediatricians and parents.
Dr. Paul noted that the Robert Wood Johnson Foundation has endorsed “responsive feeding” as the preferred approach to feeding infants and toddlers. Responsive feeding – helping parents recognize hunger and satiety cues as opposed to other distress cues – is a big part of the intervention.
“Feeding to soothe is not the preferred approach,” he said. “Food and milk and formula should be used for hunger.” That’s something pediatricians may not be stressing to parents, he said.
Pediatricians can also counsel parents on not using food as a reward. “We shouldn’t be giving kids M&Ms to teach them how to potty train,” he said.
‘Promising’ findings
Charles Wood, MD, a childhood obesity specialist at Duke University, Durham, N.C., who was not part of the study, called the findings “very promising.”
It also makes sense that the “aha moments” of first-time parents learning from the INSIGHT intervention would carry over to the second sibling, he said.
Dr. Wood agreed costs are a big factor. However, he said, the potential downstream costs of not preventing obesity are worse. And this study indicates the benefits may keep spreading with future siblings.
Dr. Wood said accessing obesity interventions outside the pediatrician visit can also help. Connecting patients with support groups or dietitians or with a counselor from Women, Infants, and Children can help. However, consistent messaging among the providers is key, he noted.
Dr. Wood’s research group is investigating text messaging platforms so parents can get answer to real-time questions, such as those about feeding behaviors.
He pointed to a limitation the authors mention, which is that the study was done in mostly White, highly educated, higher-income families.
“There’s a big problem with racial disparities and obesity,” Dr. Wood noted. “We definitely need solutions that address disparities as well.”
Mothers included in the study had given birth for the first time and their infants were enrolled after birth from a single maternity ward between January 2012 and March 2014. Major eligibility criteria were that the babies were full term (at least 37 weeks’ gestation), single births, and delivered to English-speaking mothers at least 20 years of age. Infants who weighed less than 2,500 g at birth were excluded.
The paper’s authors and Dr. Wood declared no relevant financial relationships.
This research was supported by the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases; and the Department of Agriculture.
According to a statement from the National Institutes of Health, who funded the study, it is the first such infant obesity intervention to show the spillover effect. Findings were published online Dec. 21, 2021, in Obesity.
The program is called Infants Growing on Healthy Trajectories (INSIGHT) responsive parenting (RP) intervention, and it included guidance on feeding, sleep, interactive play, and regulating emotion.
Parents were given guidance by nurses who came to their homes on how to respond when their child is drowsy, sleeping, fussy, and alert. They also learned how to put infants to bed drowsy, but awake, and avoid feeding infants to get them to sleep; how to respond to infants waking up at night; when to start solid foods; how to limit inactive time; and how to use growth charts.
The control group program focused on safety and matched the guidance categories. For example, early visits included information on prevention of sudden infant death syndrome for sleep, breast milk storage and formula for feeding, and safe bathing.
Jennifer S. Savage, Center for Childhood Obesity Research, Penn State University, University Park, led the study that enrolled 117 infants in a randomized controlled trial. Mother and firstborn children were randomized to the RP or home safety intervention (control) group 10-14 days after delivery. Their second-born siblings were enrolled in an observation-only ancillary study.
Second-born children were delivered 2.5 (standard deviation, 0.9) years after firstborns. Anthropometrics were measured in both siblings at ages 3, 16, 28, and 52 weeks.
Firstborn children at 1 year had a body mass index (BMI) that was 0.44 kg/m2 lower than the control group (95% confidence interval, −0.82 to −0.06), and second-born children whose parents received the RP intervention with their first child had BMI that was 0.36 kg/m2 lower.
“What we saw here is that it worked again,” coauthor Ian Paul, MD, MSc, professor of pediatrics and public health sciences at Penn State University, Hershey, said in an interview.
“Once we imprint them with a certain approach to parenting with the first child, they’re doing the same thing with the second child, which is wonderful to see,” he said.
He noted that this happened with second children without any reinforcements or booster information.
Dr. Paul said it’s still not clear which of the interventions – whether related to feeding techniques or sleeping or activity – helps most. And for each family the problematic behaviors may be different.
Responsive parenting programs have shown success previously among firstborns, the authors wrote, but 80% of those children grow up with younger siblings, so an intervention that also benefits them is important.
Weighing the costs of the intervention
The intervention was extensive. It involved four hour-plus nurse visits a year, often by the same nurse who built a relationship with the family.
But Ms. Savage said that it is possible to replicate INSIGHT on a larger scale in the United States with the dozens of home visitation models.
“Currently, 21 home visitation models meet the U.S. Department of Health & Human Services criteria for evidence of effectiveness, such as Nurse Family Partnership, Family Check-up, and Early Head Start Home Based Option. There is an opportunity to use home visitation models at the national scale to potentially interrupt the cycle of poor multigenerational outcomes such as obesity,” she said.
Dr. Paul said the initial investment “can save money in the long term,” given what’s at stake. “We know that 20%-25% of 2- to 5-year-olds are already overweight or obese and if they are already overweight or obese at that age, that;’s likely to persist.”
However, he acknowledged that staff shortages and costs are a challenge.
“Other countries have made that investment in their health care system,” he said. “In the U.S. only a fraction of new mothers and babies get home visitation. The kind of work that we did for obesity prevention has not yet been incorporated into evidence-based models of home visitation, though it certainly could be.”
Dr. Paul said his team is hoping to collaborate with others in the near future on expanding this program to such models of home visitation.
Telehealth, though a less desirable option, compared with in-home visits, could also be utilized, he said.
Short of the comprehensive intervention, he said, many of the concepts can be put into practice by pediatricians and parents.
Dr. Paul noted that the Robert Wood Johnson Foundation has endorsed “responsive feeding” as the preferred approach to feeding infants and toddlers. Responsive feeding – helping parents recognize hunger and satiety cues as opposed to other distress cues – is a big part of the intervention.
“Feeding to soothe is not the preferred approach,” he said. “Food and milk and formula should be used for hunger.” That’s something pediatricians may not be stressing to parents, he said.
Pediatricians can also counsel parents on not using food as a reward. “We shouldn’t be giving kids M&Ms to teach them how to potty train,” he said.
‘Promising’ findings
Charles Wood, MD, a childhood obesity specialist at Duke University, Durham, N.C., who was not part of the study, called the findings “very promising.”
It also makes sense that the “aha moments” of first-time parents learning from the INSIGHT intervention would carry over to the second sibling, he said.
Dr. Wood agreed costs are a big factor. However, he said, the potential downstream costs of not preventing obesity are worse. And this study indicates the benefits may keep spreading with future siblings.
Dr. Wood said accessing obesity interventions outside the pediatrician visit can also help. Connecting patients with support groups or dietitians or with a counselor from Women, Infants, and Children can help. However, consistent messaging among the providers is key, he noted.
Dr. Wood’s research group is investigating text messaging platforms so parents can get answer to real-time questions, such as those about feeding behaviors.
He pointed to a limitation the authors mention, which is that the study was done in mostly White, highly educated, higher-income families.
“There’s a big problem with racial disparities and obesity,” Dr. Wood noted. “We definitely need solutions that address disparities as well.”
Mothers included in the study had given birth for the first time and their infants were enrolled after birth from a single maternity ward between January 2012 and March 2014. Major eligibility criteria were that the babies were full term (at least 37 weeks’ gestation), single births, and delivered to English-speaking mothers at least 20 years of age. Infants who weighed less than 2,500 g at birth were excluded.
The paper’s authors and Dr. Wood declared no relevant financial relationships.
This research was supported by the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases; and the Department of Agriculture.
According to a statement from the National Institutes of Health, who funded the study, it is the first such infant obesity intervention to show the spillover effect. Findings were published online Dec. 21, 2021, in Obesity.
The program is called Infants Growing on Healthy Trajectories (INSIGHT) responsive parenting (RP) intervention, and it included guidance on feeding, sleep, interactive play, and regulating emotion.
Parents were given guidance by nurses who came to their homes on how to respond when their child is drowsy, sleeping, fussy, and alert. They also learned how to put infants to bed drowsy, but awake, and avoid feeding infants to get them to sleep; how to respond to infants waking up at night; when to start solid foods; how to limit inactive time; and how to use growth charts.
The control group program focused on safety and matched the guidance categories. For example, early visits included information on prevention of sudden infant death syndrome for sleep, breast milk storage and formula for feeding, and safe bathing.
Jennifer S. Savage, Center for Childhood Obesity Research, Penn State University, University Park, led the study that enrolled 117 infants in a randomized controlled trial. Mother and firstborn children were randomized to the RP or home safety intervention (control) group 10-14 days after delivery. Their second-born siblings were enrolled in an observation-only ancillary study.
Second-born children were delivered 2.5 (standard deviation, 0.9) years after firstborns. Anthropometrics were measured in both siblings at ages 3, 16, 28, and 52 weeks.
Firstborn children at 1 year had a body mass index (BMI) that was 0.44 kg/m2 lower than the control group (95% confidence interval, −0.82 to −0.06), and second-born children whose parents received the RP intervention with their first child had BMI that was 0.36 kg/m2 lower.
“What we saw here is that it worked again,” coauthor Ian Paul, MD, MSc, professor of pediatrics and public health sciences at Penn State University, Hershey, said in an interview.
“Once we imprint them with a certain approach to parenting with the first child, they’re doing the same thing with the second child, which is wonderful to see,” he said.
He noted that this happened with second children without any reinforcements or booster information.
Dr. Paul said it’s still not clear which of the interventions – whether related to feeding techniques or sleeping or activity – helps most. And for each family the problematic behaviors may be different.
Responsive parenting programs have shown success previously among firstborns, the authors wrote, but 80% of those children grow up with younger siblings, so an intervention that also benefits them is important.
Weighing the costs of the intervention
The intervention was extensive. It involved four hour-plus nurse visits a year, often by the same nurse who built a relationship with the family.
But Ms. Savage said that it is possible to replicate INSIGHT on a larger scale in the United States with the dozens of home visitation models.
“Currently, 21 home visitation models meet the U.S. Department of Health & Human Services criteria for evidence of effectiveness, such as Nurse Family Partnership, Family Check-up, and Early Head Start Home Based Option. There is an opportunity to use home visitation models at the national scale to potentially interrupt the cycle of poor multigenerational outcomes such as obesity,” she said.
Dr. Paul said the initial investment “can save money in the long term,” given what’s at stake. “We know that 20%-25% of 2- to 5-year-olds are already overweight or obese and if they are already overweight or obese at that age, that;’s likely to persist.”
However, he acknowledged that staff shortages and costs are a challenge.
“Other countries have made that investment in their health care system,” he said. “In the U.S. only a fraction of new mothers and babies get home visitation. The kind of work that we did for obesity prevention has not yet been incorporated into evidence-based models of home visitation, though it certainly could be.”
Dr. Paul said his team is hoping to collaborate with others in the near future on expanding this program to such models of home visitation.
Telehealth, though a less desirable option, compared with in-home visits, could also be utilized, he said.
Short of the comprehensive intervention, he said, many of the concepts can be put into practice by pediatricians and parents.
Dr. Paul noted that the Robert Wood Johnson Foundation has endorsed “responsive feeding” as the preferred approach to feeding infants and toddlers. Responsive feeding – helping parents recognize hunger and satiety cues as opposed to other distress cues – is a big part of the intervention.
“Feeding to soothe is not the preferred approach,” he said. “Food and milk and formula should be used for hunger.” That’s something pediatricians may not be stressing to parents, he said.
Pediatricians can also counsel parents on not using food as a reward. “We shouldn’t be giving kids M&Ms to teach them how to potty train,” he said.
‘Promising’ findings
Charles Wood, MD, a childhood obesity specialist at Duke University, Durham, N.C., who was not part of the study, called the findings “very promising.”
It also makes sense that the “aha moments” of first-time parents learning from the INSIGHT intervention would carry over to the second sibling, he said.
Dr. Wood agreed costs are a big factor. However, he said, the potential downstream costs of not preventing obesity are worse. And this study indicates the benefits may keep spreading with future siblings.
Dr. Wood said accessing obesity interventions outside the pediatrician visit can also help. Connecting patients with support groups or dietitians or with a counselor from Women, Infants, and Children can help. However, consistent messaging among the providers is key, he noted.
Dr. Wood’s research group is investigating text messaging platforms so parents can get answer to real-time questions, such as those about feeding behaviors.
He pointed to a limitation the authors mention, which is that the study was done in mostly White, highly educated, higher-income families.
“There’s a big problem with racial disparities and obesity,” Dr. Wood noted. “We definitely need solutions that address disparities as well.”
Mothers included in the study had given birth for the first time and their infants were enrolled after birth from a single maternity ward between January 2012 and March 2014. Major eligibility criteria were that the babies were full term (at least 37 weeks’ gestation), single births, and delivered to English-speaking mothers at least 20 years of age. Infants who weighed less than 2,500 g at birth were excluded.
The paper’s authors and Dr. Wood declared no relevant financial relationships.
This research was supported by the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases; and the Department of Agriculture.
FROM OBESITY
Herpes Zoster Following a Nucleoside-Modified Messenger RNA COVID-19 Vaccine
Since the end of 2019, COVID-19 infection caused by SARS-CoV-2 has spread in a worldwide pandemic. The first cutaneous manifestations possibly linked to COVID-19 were reported in spring 2020.1 Herpes zoster (HZ) was suspected as a predictive cutaneous manifestation of COVID-19 with a debated prognostic significance.2 The end of 2020 was marked with the beginning of vaccination against COVID-19, and safety studies reported few side effects after vaccination with nucleoside-modified messenger RNA (mRNA) COVID-19 vaccines.3 Real-life use of vaccines could lead to the occurrence of potential side effects (or fortuitous medical events) that were not observed in these studies. We report a series of 5 cases of HZ occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine extracted from a declarative cohort of cutaneous reactions in our vaccination center.
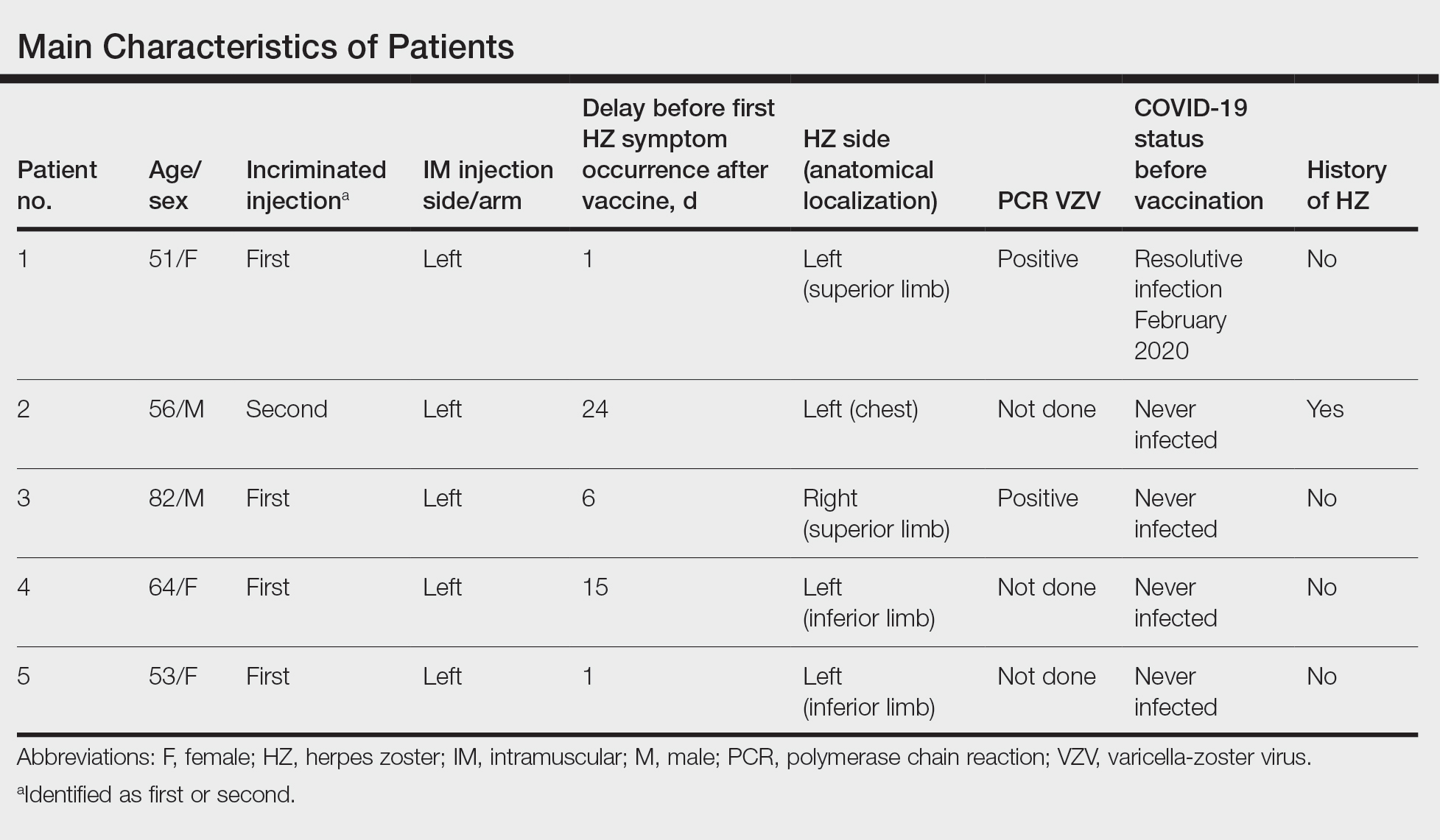
Case Series
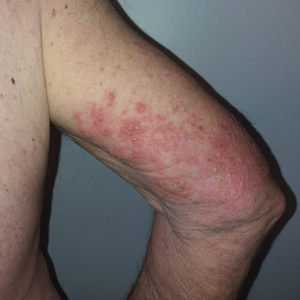
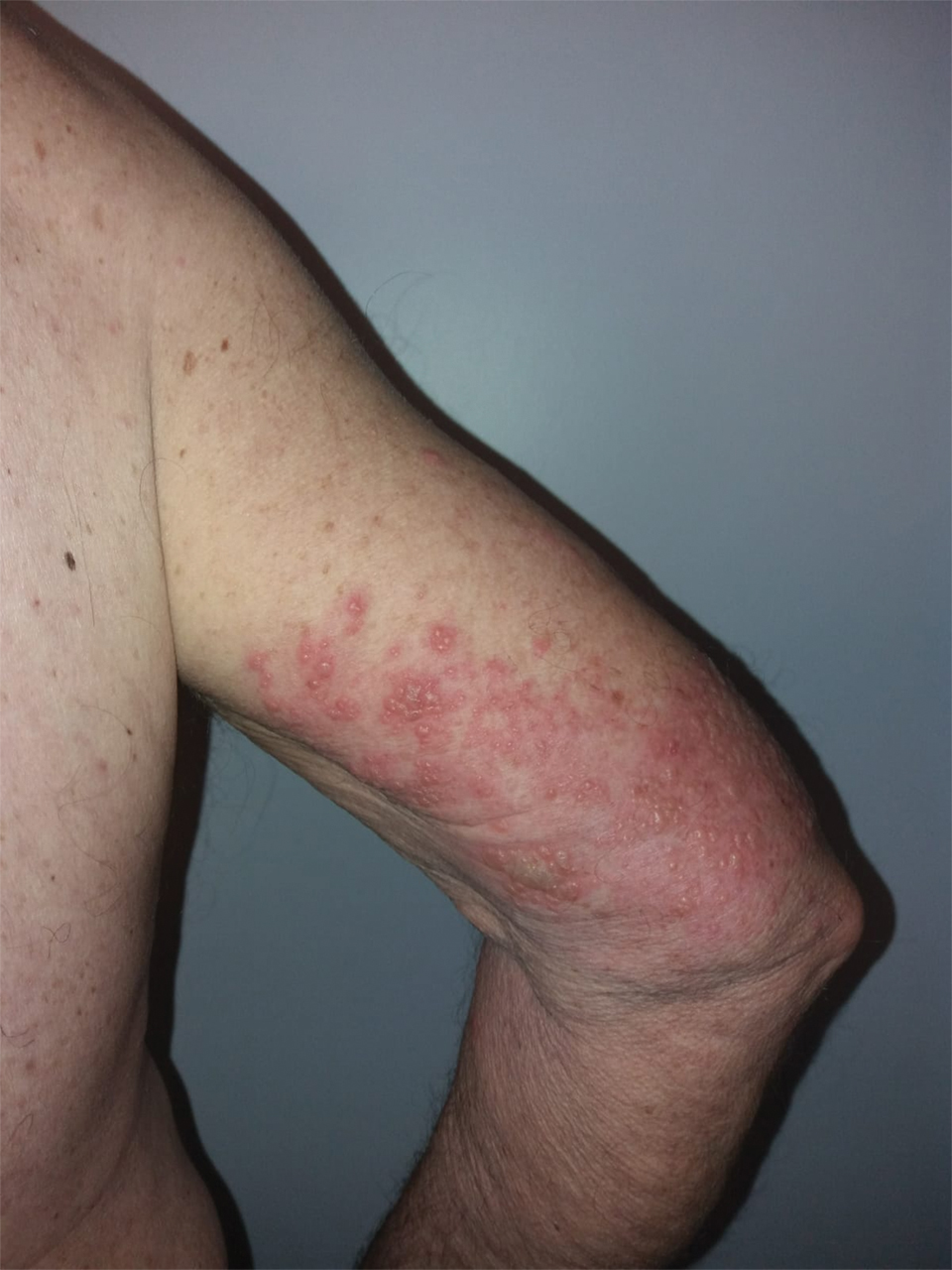
We identified 2 men and 3 women (Table) who experienced HZ after vaccination with a nucleoside-modified mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech). Patients fulfilled French governmental criteria for vaccination at the time of the report—older than 75 years or a health care professional—and they were vaccinated at the vaccination center of a French university hospital. The median age of the patients was 56 years (interquartile range [IQR], 51–82 years). One patient was diagnosed with COVID-19 in February 2020. A medical history of HZ was found in 1 patient. No medical history of immunosuppression was noted. Herpes zoster was observed on the same side of the body as the vaccination site in 4 patients. The median delay before the onset of symptoms was 6 days (IQR, 1–15 days) after injection. The median duration of the symptoms was 13 days (IQR, 11.5–16.5 days). Clinical signs of HZ were mild with few vesicles in 4 patients, and we observed a notably long delay between the onset of pain and the eruption of vesicles in 2 cases (4 and 10 days, respectively). The clinical diagnosis of HZ was confirmed by a dermatologist for all patients (Figures 1 and 2). Polymerase chain reaction assays for the detection of the varicella-zoster virus were performed in 2 cases and were positive. A complete blood cell count was performed in 1 patient, and we observed isolated lymphopenia (500/mm3 [reference range, 1000–4000/mm3]). Herpes zoster occurred after the first dose of vaccine in 4 patients and after the second dose for 1 patient. Three patients were treated with antiviral therapy (acyclovir) for 7 days. Three patients recovered from symptoms within 2 weeks and 2 patients within 1 week.
Comment
We report a series of HZ cases occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine. We did not observe complicated HZ, and most of the time, HZ lesions were located on the same side of the body as the vaccine injection. One case of HZ after COVID-19 vaccination was reported by Bostan and Yalici-Armagan,4 but it followed injection with an inactivated vaccine, which is different from our series. Herpes zoster remains rarely reported, mainly following mRNA COVID-19 vaccination.5
Cases of HZ after vaccination have been reported after the live attenuated zoster or yellow fever vaccines, but HZ should not appear as a concomitant effect after any type of vaccines.6,7 Kawai et al8 reported that the incidence rate of HZ ranged from 3 to 5 cases per 1000 person-years in North America, Europe, and Asia-Pacific. The risk for recurrence of HZ ranged from 1% to 6% depending on the type of study design, age distribution of studied populations, and definition.8 In another retrospective database analysis in Israel, the incidence density rate of HZ was 3.46 cases per 1000 person-years in the total population and 12.8 cases per 1000 person-years in immunocompromised patients, therefore the immunocompromised status is important to consider.9
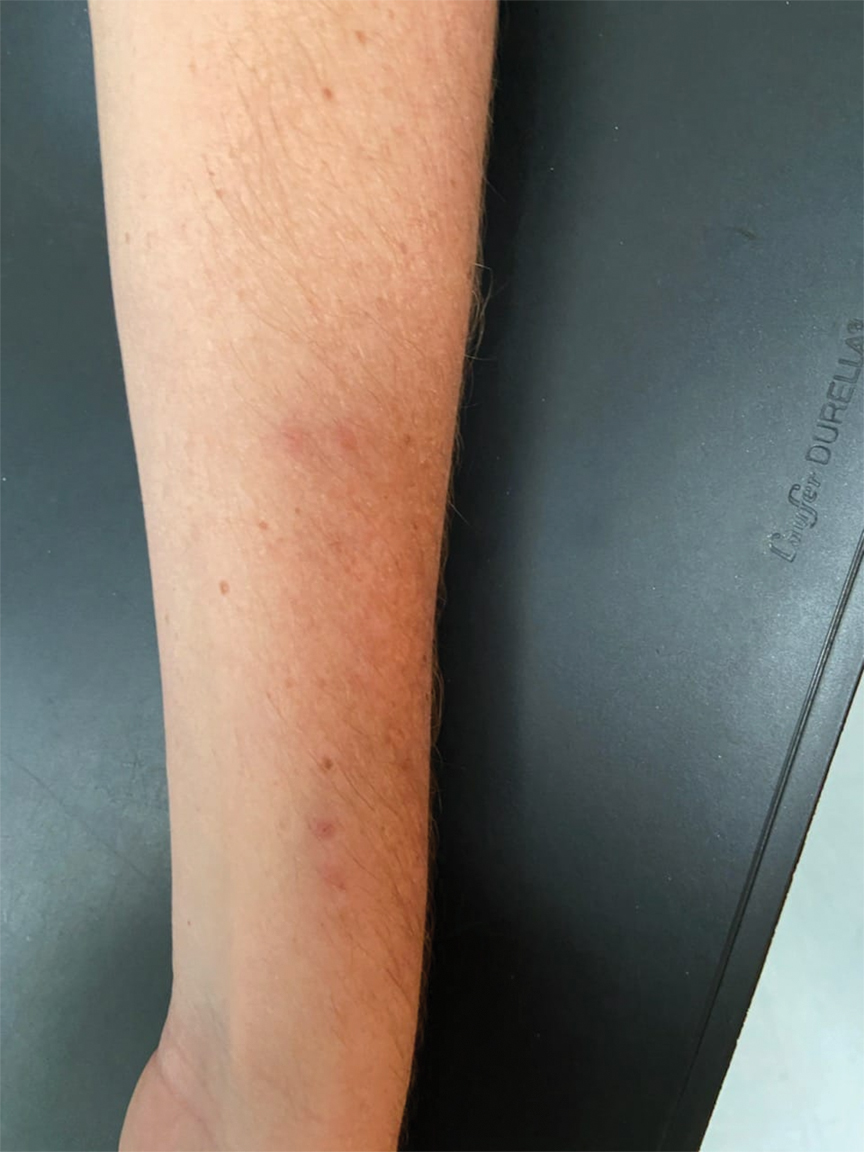
In our declarative cohort of skin eruptions before vaccination, we recorded 11 cases of HZ among 148 skin eruptions (7.43%) at the time of the study, but the design of the study did not allow us to estimate the exact incidence of HZ in the global COVID-19–vaccinated population because our study was not based on a systematic and prospective analysis of all vaccinated patients. The comparison between the prevalence of HZ in the COVID-19–vaccinated population and the nonvaccinated population is difficult owing to the lack of data about HZ in the nonvaccinated population at the time of our analysis. Furthermore, we did not include all vaccinated patients in a prospective follow-up. We highlight the importance of medical history of patients that differed between vaccinated patients (at the time of our analysis) and the global population due to French governmental access criteria to vaccination. The link to prior SARS-CoV-2 infection was uncertain because a medical history of COVID-19 was found in only 1 patient. Only 1 patient had a history of HZ, which is not a contraindication of COVID-19 vaccination.
Postinjection pains are frequent with COVID-19 vaccines, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay between the injection and the symptoms. Indeed, the onset of symptoms could be late, and the clinical presentation initially may be mistaken for an injection-site reaction, which is a frequent known side effect of vaccines. These new cases do not prove causality between COVID-19 vaccination and HZ. Varicella-zoster virus remains latent in dorsal-root or ganglia after primary infection, and HZ caused by reactivation of varicella-zoster virus may occur spontaneously or be triggered. In our series, we did not observe medical history of immunosuppression, and no other known risk factors of HZ (eg, radiation therapy, physical trauma, fever after vaccination) were recorded. The pathophysiologic mechanism remains elusive, but local vaccine-induced immunomodulation or an inflammatory state may be involved.
Conclusion
Our case series highlights that clinicians must remain vigilant to diagnose HZ early to prevent potential complications, such as postherpetic neuralgia. Also, vaccination should not be contraindicated in patients with medical history of HZ; the occurrence of HZ does not justify avoiding the second injection of the vaccine due to the benefit of vaccination.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Desai HD, Sharma K, Shah A, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? a systematic review. J Cosmet Dermatol. 2021;20:3350-3361.
- Fahlbusch M, Wesselmann U, Lehmann P. Herpes zoster after varicella-zoster vaccination [in German]. Hautarzt. 2013;64:107-109.
- Bayas JM, González-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14:65-66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;10;4:E004833.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463-469.
Since the end of 2019, COVID-19 infection caused by SARS-CoV-2 has spread in a worldwide pandemic. The first cutaneous manifestations possibly linked to COVID-19 were reported in spring 2020.1 Herpes zoster (HZ) was suspected as a predictive cutaneous manifestation of COVID-19 with a debated prognostic significance.2 The end of 2020 was marked with the beginning of vaccination against COVID-19, and safety studies reported few side effects after vaccination with nucleoside-modified messenger RNA (mRNA) COVID-19 vaccines.3 Real-life use of vaccines could lead to the occurrence of potential side effects (or fortuitous medical events) that were not observed in these studies. We report a series of 5 cases of HZ occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine extracted from a declarative cohort of cutaneous reactions in our vaccination center.
Case Series
We identified 2 men and 3 women (Table) who experienced HZ after vaccination with a nucleoside-modified mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech). Patients fulfilled French governmental criteria for vaccination at the time of the report—older than 75 years or a health care professional—and they were vaccinated at the vaccination center of a French university hospital. The median age of the patients was 56 years (interquartile range [IQR], 51–82 years). One patient was diagnosed with COVID-19 in February 2020. A medical history of HZ was found in 1 patient. No medical history of immunosuppression was noted. Herpes zoster was observed on the same side of the body as the vaccination site in 4 patients. The median delay before the onset of symptoms was 6 days (IQR, 1–15 days) after injection. The median duration of the symptoms was 13 days (IQR, 11.5–16.5 days). Clinical signs of HZ were mild with few vesicles in 4 patients, and we observed a notably long delay between the onset of pain and the eruption of vesicles in 2 cases (4 and 10 days, respectively). The clinical diagnosis of HZ was confirmed by a dermatologist for all patients (Figures 1 and 2). Polymerase chain reaction assays for the detection of the varicella-zoster virus were performed in 2 cases and were positive. A complete blood cell count was performed in 1 patient, and we observed isolated lymphopenia (500/mm3 [reference range, 1000–4000/mm3]). Herpes zoster occurred after the first dose of vaccine in 4 patients and after the second dose for 1 patient. Three patients were treated with antiviral therapy (acyclovir) for 7 days. Three patients recovered from symptoms within 2 weeks and 2 patients within 1 week.

Comment
We report a series of HZ cases occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine. We did not observe complicated HZ, and most of the time, HZ lesions were located on the same side of the body as the vaccine injection. One case of HZ after COVID-19 vaccination was reported by Bostan and Yalici-Armagan,4 but it followed injection with an inactivated vaccine, which is different from our series. Herpes zoster remains rarely reported, mainly following mRNA COVID-19 vaccination.5

Cases of HZ after vaccination have been reported after the live attenuated zoster or yellow fever vaccines, but HZ should not appear as a concomitant effect after any type of vaccines.6,7 Kawai et al8 reported that the incidence rate of HZ ranged from 3 to 5 cases per 1000 person-years in North America, Europe, and Asia-Pacific. The risk for recurrence of HZ ranged from 1% to 6% depending on the type of study design, age distribution of studied populations, and definition.8 In another retrospective database analysis in Israel, the incidence density rate of HZ was 3.46 cases per 1000 person-years in the total population and 12.8 cases per 1000 person-years in immunocompromised patients, therefore the immunocompromised status is important to consider.9

In our declarative cohort of skin eruptions before vaccination, we recorded 11 cases of HZ among 148 skin eruptions (7.43%) at the time of the study, but the design of the study did not allow us to estimate the exact incidence of HZ in the global COVID-19–vaccinated population because our study was not based on a systematic and prospective analysis of all vaccinated patients. The comparison between the prevalence of HZ in the COVID-19–vaccinated population and the nonvaccinated population is difficult owing to the lack of data about HZ in the nonvaccinated population at the time of our analysis. Furthermore, we did not include all vaccinated patients in a prospective follow-up. We highlight the importance of medical history of patients that differed between vaccinated patients (at the time of our analysis) and the global population due to French governmental access criteria to vaccination. The link to prior SARS-CoV-2 infection was uncertain because a medical history of COVID-19 was found in only 1 patient. Only 1 patient had a history of HZ, which is not a contraindication of COVID-19 vaccination.
Postinjection pains are frequent with COVID-19 vaccines, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay between the injection and the symptoms. Indeed, the onset of symptoms could be late, and the clinical presentation initially may be mistaken for an injection-site reaction, which is a frequent known side effect of vaccines. These new cases do not prove causality between COVID-19 vaccination and HZ. Varicella-zoster virus remains latent in dorsal-root or ganglia after primary infection, and HZ caused by reactivation of varicella-zoster virus may occur spontaneously or be triggered. In our series, we did not observe medical history of immunosuppression, and no other known risk factors of HZ (eg, radiation therapy, physical trauma, fever after vaccination) were recorded. The pathophysiologic mechanism remains elusive, but local vaccine-induced immunomodulation or an inflammatory state may be involved.
Conclusion
Our case series highlights that clinicians must remain vigilant to diagnose HZ early to prevent potential complications, such as postherpetic neuralgia. Also, vaccination should not be contraindicated in patients with medical history of HZ; the occurrence of HZ does not justify avoiding the second injection of the vaccine due to the benefit of vaccination.
Since the end of 2019, COVID-19 infection caused by SARS-CoV-2 has spread in a worldwide pandemic. The first cutaneous manifestations possibly linked to COVID-19 were reported in spring 2020.1 Herpes zoster (HZ) was suspected as a predictive cutaneous manifestation of COVID-19 with a debated prognostic significance.2 The end of 2020 was marked with the beginning of vaccination against COVID-19, and safety studies reported few side effects after vaccination with nucleoside-modified messenger RNA (mRNA) COVID-19 vaccines.3 Real-life use of vaccines could lead to the occurrence of potential side effects (or fortuitous medical events) that were not observed in these studies. We report a series of 5 cases of HZ occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine extracted from a declarative cohort of cutaneous reactions in our vaccination center.
Case Series
We identified 2 men and 3 women (Table) who experienced HZ after vaccination with a nucleoside-modified mRNA COVID-19 vaccine (Comirnaty, Pfizer-BioNTech). Patients fulfilled French governmental criteria for vaccination at the time of the report—older than 75 years or a health care professional—and they were vaccinated at the vaccination center of a French university hospital. The median age of the patients was 56 years (interquartile range [IQR], 51–82 years). One patient was diagnosed with COVID-19 in February 2020. A medical history of HZ was found in 1 patient. No medical history of immunosuppression was noted. Herpes zoster was observed on the same side of the body as the vaccination site in 4 patients. The median delay before the onset of symptoms was 6 days (IQR, 1–15 days) after injection. The median duration of the symptoms was 13 days (IQR, 11.5–16.5 days). Clinical signs of HZ were mild with few vesicles in 4 patients, and we observed a notably long delay between the onset of pain and the eruption of vesicles in 2 cases (4 and 10 days, respectively). The clinical diagnosis of HZ was confirmed by a dermatologist for all patients (Figures 1 and 2). Polymerase chain reaction assays for the detection of the varicella-zoster virus were performed in 2 cases and were positive. A complete blood cell count was performed in 1 patient, and we observed isolated lymphopenia (500/mm3 [reference range, 1000–4000/mm3]). Herpes zoster occurred after the first dose of vaccine in 4 patients and after the second dose for 1 patient. Three patients were treated with antiviral therapy (acyclovir) for 7 days. Three patients recovered from symptoms within 2 weeks and 2 patients within 1 week.

Comment
We report a series of HZ cases occurring after vaccination with a nucleoside-modified mRNA COVID-19 vaccine. We did not observe complicated HZ, and most of the time, HZ lesions were located on the same side of the body as the vaccine injection. One case of HZ after COVID-19 vaccination was reported by Bostan and Yalici-Armagan,4 but it followed injection with an inactivated vaccine, which is different from our series. Herpes zoster remains rarely reported, mainly following mRNA COVID-19 vaccination.5

Cases of HZ after vaccination have been reported after the live attenuated zoster or yellow fever vaccines, but HZ should not appear as a concomitant effect after any type of vaccines.6,7 Kawai et al8 reported that the incidence rate of HZ ranged from 3 to 5 cases per 1000 person-years in North America, Europe, and Asia-Pacific. The risk for recurrence of HZ ranged from 1% to 6% depending on the type of study design, age distribution of studied populations, and definition.8 In another retrospective database analysis in Israel, the incidence density rate of HZ was 3.46 cases per 1000 person-years in the total population and 12.8 cases per 1000 person-years in immunocompromised patients, therefore the immunocompromised status is important to consider.9

In our declarative cohort of skin eruptions before vaccination, we recorded 11 cases of HZ among 148 skin eruptions (7.43%) at the time of the study, but the design of the study did not allow us to estimate the exact incidence of HZ in the global COVID-19–vaccinated population because our study was not based on a systematic and prospective analysis of all vaccinated patients. The comparison between the prevalence of HZ in the COVID-19–vaccinated population and the nonvaccinated population is difficult owing to the lack of data about HZ in the nonvaccinated population at the time of our analysis. Furthermore, we did not include all vaccinated patients in a prospective follow-up. We highlight the importance of medical history of patients that differed between vaccinated patients (at the time of our analysis) and the global population due to French governmental access criteria to vaccination. The link to prior SARS-CoV-2 infection was uncertain because a medical history of COVID-19 was found in only 1 patient. Only 1 patient had a history of HZ, which is not a contraindication of COVID-19 vaccination.
Postinjection pains are frequent with COVID-19 vaccines, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay between the injection and the symptoms. Indeed, the onset of symptoms could be late, and the clinical presentation initially may be mistaken for an injection-site reaction, which is a frequent known side effect of vaccines. These new cases do not prove causality between COVID-19 vaccination and HZ. Varicella-zoster virus remains latent in dorsal-root or ganglia after primary infection, and HZ caused by reactivation of varicella-zoster virus may occur spontaneously or be triggered. In our series, we did not observe medical history of immunosuppression, and no other known risk factors of HZ (eg, radiation therapy, physical trauma, fever after vaccination) were recorded. The pathophysiologic mechanism remains elusive, but local vaccine-induced immunomodulation or an inflammatory state may be involved.
Conclusion
Our case series highlights that clinicians must remain vigilant to diagnose HZ early to prevent potential complications, such as postherpetic neuralgia. Also, vaccination should not be contraindicated in patients with medical history of HZ; the occurrence of HZ does not justify avoiding the second injection of the vaccine due to the benefit of vaccination.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Desai HD, Sharma K, Shah A, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? a systematic review. J Cosmet Dermatol. 2021;20:3350-3361.
- Fahlbusch M, Wesselmann U, Lehmann P. Herpes zoster after varicella-zoster vaccination [in German]. Hautarzt. 2013;64:107-109.
- Bayas JM, González-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14:65-66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;10;4:E004833.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463-469.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Desai HD, Sharma K, Shah A, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of varicella zoster? a systematic review. J Cosmet Dermatol. 2021;20:3350-3361.
- Fahlbusch M, Wesselmann U, Lehmann P. Herpes zoster after varicella-zoster vaccination [in German]. Hautarzt. 2013;64:107-109.
- Bayas JM, González-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14:65-66.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;10;4:E004833.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463-469.
Practice Points
- Herpes zoster (HZ) has been reported following COVID-19 vaccination.
- Postinjection pain is common with COVID-19 vaccination, but clinical signs such as extension of pain, burning sensation, and eruption of vesicles should lead the physician to consider the diagnosis of HZ, regardless of the delay in onset between the injection and the symptoms.
- When indicated, the second vaccine dose should not be avoided in patients who are diagnosed with HZ.
Pursuit of a Research Year or Dual Degree by Dermatology Residency Applicants: A Cross-Sectional Study
To the Editor:
Securing a dermatology residency position is extraordinarily competitive. The match rate for US allopathic seniors for dermatology is 84.7%, among the lowest of all medical specialties. Matched dermatology applicants boast a mean US Medical Licensing Examination (USMLE) Step 1 score of 248, the second highest of all specialties.1 To gain an edge, applicants are faced with decisions regarding pursuit of dedicated research time and additional professional degrees.
We conducted a cross-sectional study to determine how many dermatology residency applicants pursue additional years of training and how this decision relates to USMLE scores and other metrics. This study was approved by the University of Michigan institutional review board. Using Electronic Residency Application Service applicant data, all applicants to the University of Michigan Medical School (Ann Arbor, Michigan) dermatology residency program for the 2018-2019 application cycle were included.
Analysis of variance was performed to determine differences in mean USMLE Step 1 scores, Step 2 Clinical Knowledge scores, and number of research experiences (eg, presentations, publications) between groups. A 2-tailed z test of independent samples was performed for individual pairwise subgroup analyses.
There were 608 (377 female, 231 male; mean age, 27.9 years) applicants from 199 different medical schools; 550 graduated with an MD degree, 40 with a DO degree, and 18 were international medical graduates (IMGs)(eg, MBBS, MBBCh, BAO, MBChB). One hundred eighty-four applicants (30.2%) pursued either a second professional degree or a dedicated research period lasting at least 12 months. Twenty-eight applicants (4.6%) obtained a master’s degree, 21 (3.5%) obtained a doctorate, and 135 (22.2%) pursued dedicated research.
Of the 40 DO applicants, 1 (2.5%) pursued dedicated research time; 0 (zero) completed a dual degree. None (zero) of the 18 IMGs pursued a dual degree or dedicated research time. When the scores of applicants who pursued additional training and the scores of applicants who did not were compared, neither mean USMLE Step 1 scores nor mean USMLE Step 2 Clinical Knowledge scores were statistically different (P=.31 and P=.44, respectively). Applicants who completed medical school in 4 years had fewer research experiences (mean [SD] experiences, 13.9 [13.2]) than students with a master’s degree (18.5 [8.4]), doctorate (24.5 [17.5]), or dedicated research time (23.9 [14.9])(P<.001).
Utilizing US News & World Report rankings (2019 Best Medical Schools: Research), we determined that 146 applicants (24.0%) attended a top 25 medical school in 2019.2 Of those 146 applicants, 77 (52.7%) pursued additional training through dedicated research or a second professional degree. Only 107 of the 462 applicants (23.2%) from medical schools that were not in the top 25 as determined by the US News & World Report pursued additional training (P<.0001)(Figure).
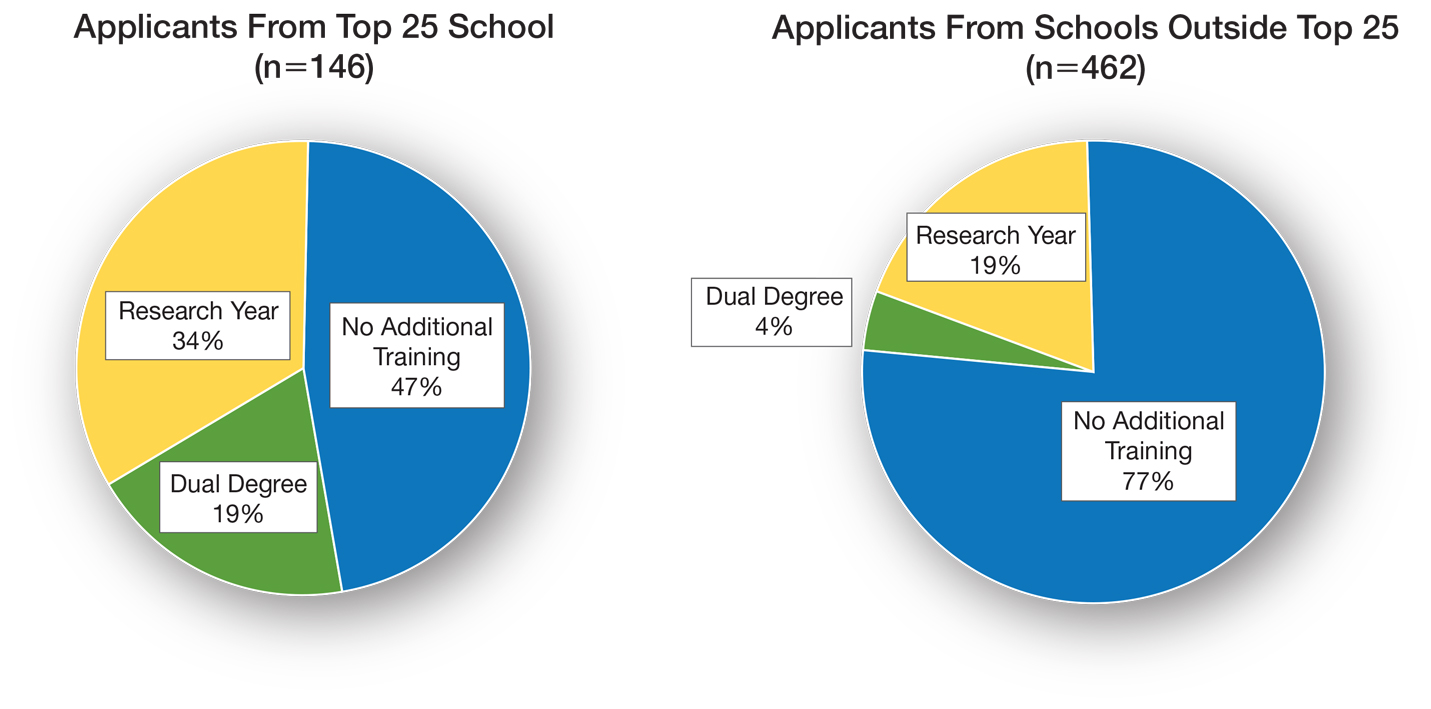
There is sentiment among applicants that a weaker dermatology residency application can be bolstered through a dedicated research year or a second professional degree. Whether this additional training has an impact on an applicant’s chances of matching is unclear and requires further investigation. Our data showed that applicants from the top 25 medical schools were more likely to pursue additional training than graduates at other institutions. These highly ranked academic institutions might encourage students to pursue a dual degree or research fellowship. In addition, year-long research opportunities might be more available through top medical schools; these schools might be more likely to offer dual-degree programs or provide funding to support student research opportunities.
It is important to comment on the potential importance of funding to support research years; the unpaid nature of many research fellowships in dermatology tends to favor applicants from a higher socioeconomic background. In that respect, the pervasive trend of encouraging research years in dermatology might widen already apparent disparities in our field, likely impacting underrepresented minorities disproportionately.3 Importantly, students with an MD degree represent nearly all applicants who completed a dual degree or dedicated research time. This might be due to fewer opportunities available to IMGs and DO students or secondary to incentivization by MD institutions.
Our data also suggest that students who pursue additional training have academic achievement metrics similar to those who do not. Additional training might increase medical students’ debt burden, thus catering to more affluent applicants, which, in turn, might have an impact on the diversity of the dermatology residency applicant pool.
Our data come from a single institution during a single application cycle, comprising 608 applicants. Nationwide, there were 701 dermatology residency applicants for the 2018-2019 application cycle; our pool therefore represents most (86.7%) but not all applicants.
We decided to use the US News & World Report 2019 rankings to identify top medical schools. Although this ranking system is imperfect and inherently subjective, it is widely utilized by prospective applicants and administrative faculty; we deemed it the best ranking that we could utilize to identify top medical schools. Because the University of Michigan Medical School was in the top 25 of Best Medical Schools: Research, according to the US News & World Report 2019 rankings, our applicant pool might be skewed to applicants interested in a more academic, research-focused residency program.
Our study revealed that 30% (n=184) of dermatology residency applicants pursued a second professional degree or dedicated research time. There was no difference in UMLE Step 1 and Step 2 scores for those who pursued additional training compared to those who did not.
- Charting outcomes in the match: U.S. allopathic seniors. 2nd ed. National Residency Matching Program. Published July 2020. Accessed January 3, 2022. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- 2019 Best Medical Schools: Research. US News & World Report; 2019.
- Oussedik E. Important considerations for diversity in the selection of dermatology applicants. JAMA Dermatol. 2017;153:948-949. doi:10.1001/jamadermatol.2017.1814
To the Editor:
Securing a dermatology residency position is extraordinarily competitive. The match rate for US allopathic seniors for dermatology is 84.7%, among the lowest of all medical specialties. Matched dermatology applicants boast a mean US Medical Licensing Examination (USMLE) Step 1 score of 248, the second highest of all specialties.1 To gain an edge, applicants are faced with decisions regarding pursuit of dedicated research time and additional professional degrees.
We conducted a cross-sectional study to determine how many dermatology residency applicants pursue additional years of training and how this decision relates to USMLE scores and other metrics. This study was approved by the University of Michigan institutional review board. Using Electronic Residency Application Service applicant data, all applicants to the University of Michigan Medical School (Ann Arbor, Michigan) dermatology residency program for the 2018-2019 application cycle were included.
Analysis of variance was performed to determine differences in mean USMLE Step 1 scores, Step 2 Clinical Knowledge scores, and number of research experiences (eg, presentations, publications) between groups. A 2-tailed z test of independent samples was performed for individual pairwise subgroup analyses.
There were 608 (377 female, 231 male; mean age, 27.9 years) applicants from 199 different medical schools; 550 graduated with an MD degree, 40 with a DO degree, and 18 were international medical graduates (IMGs)(eg, MBBS, MBBCh, BAO, MBChB). One hundred eighty-four applicants (30.2%) pursued either a second professional degree or a dedicated research period lasting at least 12 months. Twenty-eight applicants (4.6%) obtained a master’s degree, 21 (3.5%) obtained a doctorate, and 135 (22.2%) pursued dedicated research.
Of the 40 DO applicants, 1 (2.5%) pursued dedicated research time; 0 (zero) completed a dual degree. None (zero) of the 18 IMGs pursued a dual degree or dedicated research time. When the scores of applicants who pursued additional training and the scores of applicants who did not were compared, neither mean USMLE Step 1 scores nor mean USMLE Step 2 Clinical Knowledge scores were statistically different (P=.31 and P=.44, respectively). Applicants who completed medical school in 4 years had fewer research experiences (mean [SD] experiences, 13.9 [13.2]) than students with a master’s degree (18.5 [8.4]), doctorate (24.5 [17.5]), or dedicated research time (23.9 [14.9])(P<.001).
Utilizing US News & World Report rankings (2019 Best Medical Schools: Research), we determined that 146 applicants (24.0%) attended a top 25 medical school in 2019.2 Of those 146 applicants, 77 (52.7%) pursued additional training through dedicated research or a second professional degree. Only 107 of the 462 applicants (23.2%) from medical schools that were not in the top 25 as determined by the US News & World Report pursued additional training (P<.0001)(Figure).

There is sentiment among applicants that a weaker dermatology residency application can be bolstered through a dedicated research year or a second professional degree. Whether this additional training has an impact on an applicant’s chances of matching is unclear and requires further investigation. Our data showed that applicants from the top 25 medical schools were more likely to pursue additional training than graduates at other institutions. These highly ranked academic institutions might encourage students to pursue a dual degree or research fellowship. In addition, year-long research opportunities might be more available through top medical schools; these schools might be more likely to offer dual-degree programs or provide funding to support student research opportunities.
It is important to comment on the potential importance of funding to support research years; the unpaid nature of many research fellowships in dermatology tends to favor applicants from a higher socioeconomic background. In that respect, the pervasive trend of encouraging research years in dermatology might widen already apparent disparities in our field, likely impacting underrepresented minorities disproportionately.3 Importantly, students with an MD degree represent nearly all applicants who completed a dual degree or dedicated research time. This might be due to fewer opportunities available to IMGs and DO students or secondary to incentivization by MD institutions.
Our data also suggest that students who pursue additional training have academic achievement metrics similar to those who do not. Additional training might increase medical students’ debt burden, thus catering to more affluent applicants, which, in turn, might have an impact on the diversity of the dermatology residency applicant pool.
Our data come from a single institution during a single application cycle, comprising 608 applicants. Nationwide, there were 701 dermatology residency applicants for the 2018-2019 application cycle; our pool therefore represents most (86.7%) but not all applicants.
We decided to use the US News & World Report 2019 rankings to identify top medical schools. Although this ranking system is imperfect and inherently subjective, it is widely utilized by prospective applicants and administrative faculty; we deemed it the best ranking that we could utilize to identify top medical schools. Because the University of Michigan Medical School was in the top 25 of Best Medical Schools: Research, according to the US News & World Report 2019 rankings, our applicant pool might be skewed to applicants interested in a more academic, research-focused residency program.
Our study revealed that 30% (n=184) of dermatology residency applicants pursued a second professional degree or dedicated research time. There was no difference in UMLE Step 1 and Step 2 scores for those who pursued additional training compared to those who did not.
To the Editor:
Securing a dermatology residency position is extraordinarily competitive. The match rate for US allopathic seniors for dermatology is 84.7%, among the lowest of all medical specialties. Matched dermatology applicants boast a mean US Medical Licensing Examination (USMLE) Step 1 score of 248, the second highest of all specialties.1 To gain an edge, applicants are faced with decisions regarding pursuit of dedicated research time and additional professional degrees.
We conducted a cross-sectional study to determine how many dermatology residency applicants pursue additional years of training and how this decision relates to USMLE scores and other metrics. This study was approved by the University of Michigan institutional review board. Using Electronic Residency Application Service applicant data, all applicants to the University of Michigan Medical School (Ann Arbor, Michigan) dermatology residency program for the 2018-2019 application cycle were included.
Analysis of variance was performed to determine differences in mean USMLE Step 1 scores, Step 2 Clinical Knowledge scores, and number of research experiences (eg, presentations, publications) between groups. A 2-tailed z test of independent samples was performed for individual pairwise subgroup analyses.
There were 608 (377 female, 231 male; mean age, 27.9 years) applicants from 199 different medical schools; 550 graduated with an MD degree, 40 with a DO degree, and 18 were international medical graduates (IMGs)(eg, MBBS, MBBCh, BAO, MBChB). One hundred eighty-four applicants (30.2%) pursued either a second professional degree or a dedicated research period lasting at least 12 months. Twenty-eight applicants (4.6%) obtained a master’s degree, 21 (3.5%) obtained a doctorate, and 135 (22.2%) pursued dedicated research.
Of the 40 DO applicants, 1 (2.5%) pursued dedicated research time; 0 (zero) completed a dual degree. None (zero) of the 18 IMGs pursued a dual degree or dedicated research time. When the scores of applicants who pursued additional training and the scores of applicants who did not were compared, neither mean USMLE Step 1 scores nor mean USMLE Step 2 Clinical Knowledge scores were statistically different (P=.31 and P=.44, respectively). Applicants who completed medical school in 4 years had fewer research experiences (mean [SD] experiences, 13.9 [13.2]) than students with a master’s degree (18.5 [8.4]), doctorate (24.5 [17.5]), or dedicated research time (23.9 [14.9])(P<.001).
Utilizing US News & World Report rankings (2019 Best Medical Schools: Research), we determined that 146 applicants (24.0%) attended a top 25 medical school in 2019.2 Of those 146 applicants, 77 (52.7%) pursued additional training through dedicated research or a second professional degree. Only 107 of the 462 applicants (23.2%) from medical schools that were not in the top 25 as determined by the US News & World Report pursued additional training (P<.0001)(Figure).

There is sentiment among applicants that a weaker dermatology residency application can be bolstered through a dedicated research year or a second professional degree. Whether this additional training has an impact on an applicant’s chances of matching is unclear and requires further investigation. Our data showed that applicants from the top 25 medical schools were more likely to pursue additional training than graduates at other institutions. These highly ranked academic institutions might encourage students to pursue a dual degree or research fellowship. In addition, year-long research opportunities might be more available through top medical schools; these schools might be more likely to offer dual-degree programs or provide funding to support student research opportunities.
It is important to comment on the potential importance of funding to support research years; the unpaid nature of many research fellowships in dermatology tends to favor applicants from a higher socioeconomic background. In that respect, the pervasive trend of encouraging research years in dermatology might widen already apparent disparities in our field, likely impacting underrepresented minorities disproportionately.3 Importantly, students with an MD degree represent nearly all applicants who completed a dual degree or dedicated research time. This might be due to fewer opportunities available to IMGs and DO students or secondary to incentivization by MD institutions.
Our data also suggest that students who pursue additional training have academic achievement metrics similar to those who do not. Additional training might increase medical students’ debt burden, thus catering to more affluent applicants, which, in turn, might have an impact on the diversity of the dermatology residency applicant pool.
Our data come from a single institution during a single application cycle, comprising 608 applicants. Nationwide, there were 701 dermatology residency applicants for the 2018-2019 application cycle; our pool therefore represents most (86.7%) but not all applicants.
We decided to use the US News & World Report 2019 rankings to identify top medical schools. Although this ranking system is imperfect and inherently subjective, it is widely utilized by prospective applicants and administrative faculty; we deemed it the best ranking that we could utilize to identify top medical schools. Because the University of Michigan Medical School was in the top 25 of Best Medical Schools: Research, according to the US News & World Report 2019 rankings, our applicant pool might be skewed to applicants interested in a more academic, research-focused residency program.
Our study revealed that 30% (n=184) of dermatology residency applicants pursued a second professional degree or dedicated research time. There was no difference in UMLE Step 1 and Step 2 scores for those who pursued additional training compared to those who did not.
- Charting outcomes in the match: U.S. allopathic seniors. 2nd ed. National Residency Matching Program. Published July 2020. Accessed January 3, 2022. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- 2019 Best Medical Schools: Research. US News & World Report; 2019.
- Oussedik E. Important considerations for diversity in the selection of dermatology applicants. JAMA Dermatol. 2017;153:948-949. doi:10.1001/jamadermatol.2017.1814
- Charting outcomes in the match: U.S. allopathic seniors. 2nd ed. National Residency Matching Program. Published July 2020. Accessed January 3, 2022. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- 2019 Best Medical Schools: Research. US News & World Report; 2019.
- Oussedik E. Important considerations for diversity in the selection of dermatology applicants. JAMA Dermatol. 2017;153:948-949. doi:10.1001/jamadermatol.2017.1814
PRACTICE POINTS
- In our study of dermatology residency applicants (N11=608), 30% pursued a second professional degree or dedicated research time.
- US Medical Licensing Examination Step 1 and Step 2 scores did not differ among applicants who pursued additional training and those who did not.
- Additional training might increase medical students’ debt burden, thus catering to more affluent applicants and reducing the diversity of applicant and resident pools.
Febrile Ulceronecrotic Mucha-Habermann Disease: A Rare Form of Pityriasis Lichenoides et Varioliformis Acuta
To the Editor:
Pityriasis lichenoides is a papulosquamous dermatologic disorder that is characterized by recurrent papules.1 There is a spectrum of disease in pityriasis lichenoides that includes pityriasis lichenoides et varioliformis acuta (PLEVA) at one end and pityriasis lichenoides chronica at the other. Pityriasis lichenoides et varioliformis acuta is more common in younger individuals and is characterized by erythematous papules that often crust; these lesions resolve over weeks. The lesions of pityriasis lichenoides chronica are characteristically scaly, pink to red-brown papules that tend to resolve over months.1
Histologically, PLEVA exhibits parakeratosis, interface dermatitis, and a wedge-shaped infiltrate.1 Necrotic keratinocytes and extravasated erythrocytes also are common features. Additionally, monoclonal T cells may be present in the infiltrate.1
Febrile ulceronecrotic Mucha-Habermann disease (FUMHD) is a rare and severe variant of PLEVA. Febrile ulceronecrotic Mucha-Habermann disease is characterized by ulceronecrotic lesions, fever, and systemic symptoms.2 Herein, we present a case of FUMHD.
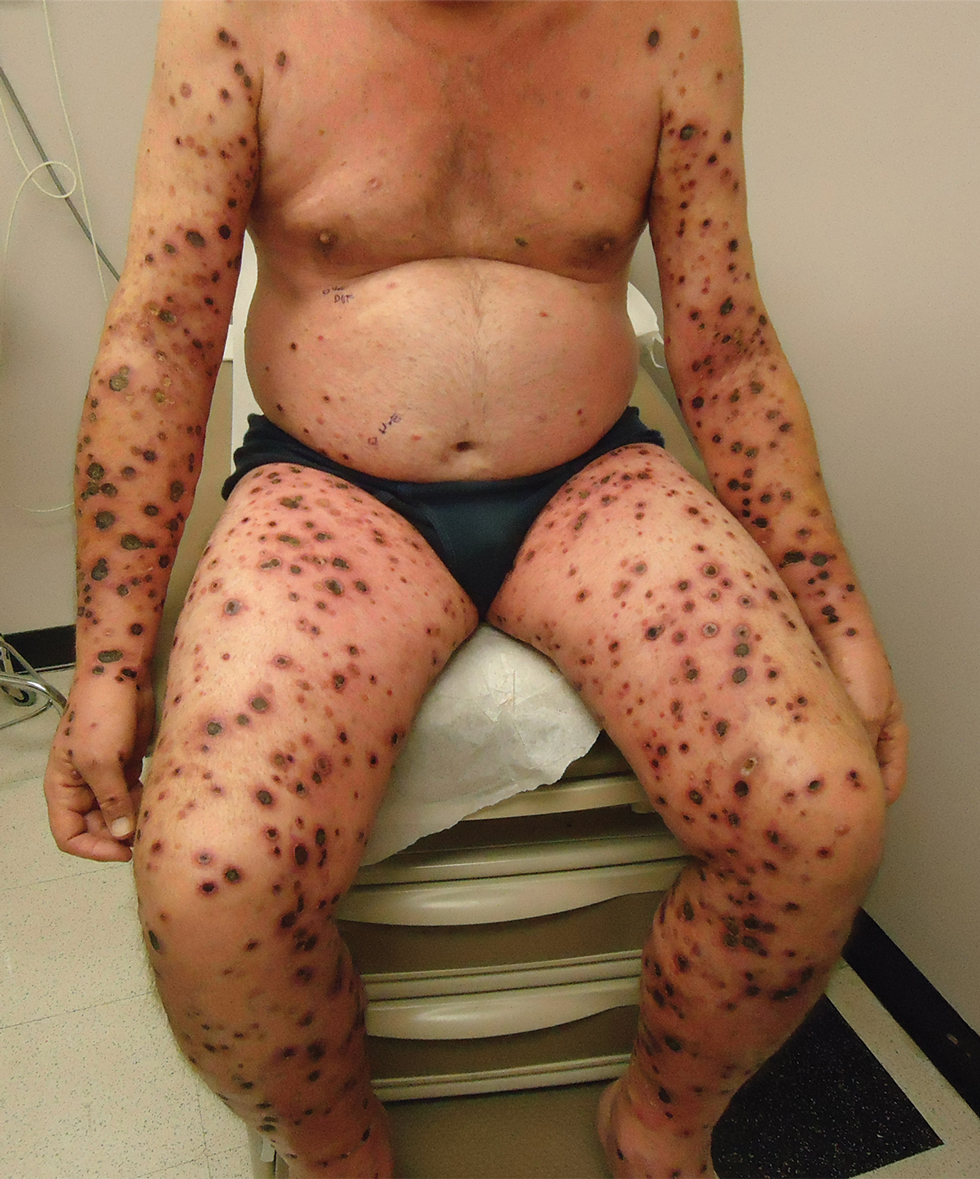
A 57-year-old man presented with an eruption of painful lesions involving the face, trunk, arms, legs, and genitalia of 1 month’s duration. The patient denied oral and ocular involvement. He had soreness and swelling of the arms and legs. A prior 12-day course of prednisone prescribed by a community dermatologist failed to improve the rash. A biopsy performed by a community dermatologist was nondiagnostic. The patient denied fever but did report chills. He had no preceding illness and was not taking new medications. On physical examination, the patient was afebrile and normotensive with innumerable deep-seated pustules and crusted ulcerations on the face, palms, soles, trunk, extremities, and penis (Figures 1 and 2). There was a background morbilliform eruption on the trunk. The ocular and oral mucosae were spared. The upper and lower extremities had pitting edema.
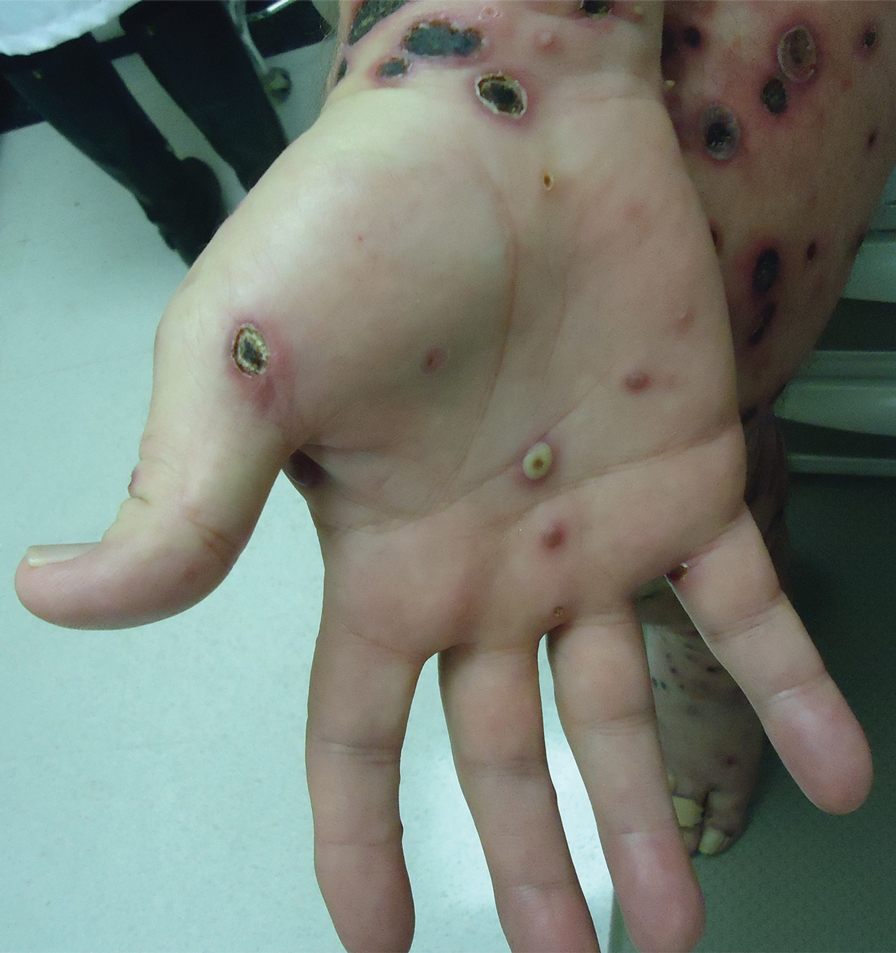
The patient’s alanine aminotransaminase and aspartate aminotransaminase levels were elevated at 55 and 51 U/L, respectively. His white blood cell count was within reference range; however, there was an elevated absolute neutrophil count (8.7×103/μL). No eosinophilia was noted. Laboratory evaluation showed a positive antimitochondrial antibody, and magnetic resonance imaging showed evidence of steatohepatitis. Punch biopsies from both the morbilliform eruption and a deep-seated pustule showed epidermal necrosis, parakeratosis, necrotic keratinocytes, and a lichenoid infiltrate of lymphocytes at the dermoepidermal interface. In the dermis, there was a wedge-shaped superficial and deep, perivascular infiltrate with extravasated erythrocytes (Figures 3 and 4). Tissue Gram stain was negative for bacteria. Varicella-zoster virus and herpes simplex virus immunostains were negative. Direct immunofluorescence showed colloid bodies, as can be seen in lichenoid dermatitis.
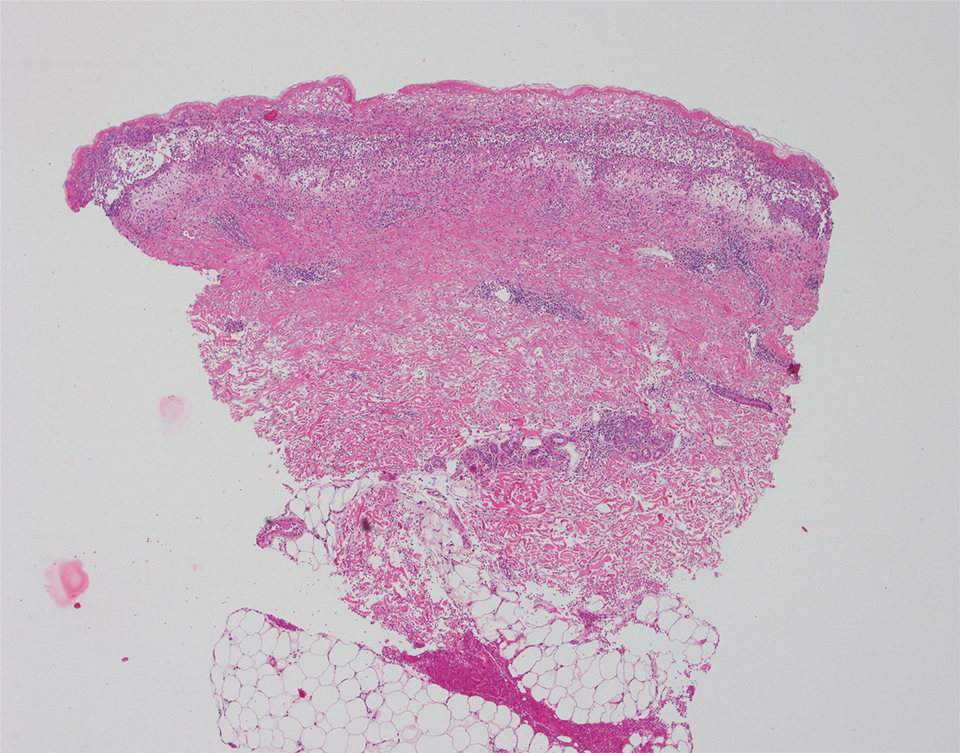
At the next clinic visit, the patient reported a fever of 39.4 °C. After reviewing the patient’s histopathology and clinical picture, along with the presence of fever, a final diagnosis of FUMHD was made. The patient was started on an oral regimen of prednisone 80 mg once daily, minocycline 100 mg twice daily, and methotrexate 15 mg weekly. Unna boots (specialized compression wraps) with triamcinolone acetonide ointment 0.1% were placed weekly until the leg edema and ulcerations healed. He was maintained on methotrexate 15 mg weekly and 5 to 10 mg of prednisone once daily. The patient demonstrated residual scarring, with only rare new papulonodules that did not ulcerate when attempts were made to taper his medications. He was followed for nearly 3 years, with a recurrence of symptoms 2 years and 3 months after initial presentation to the academic dermatology clinic.
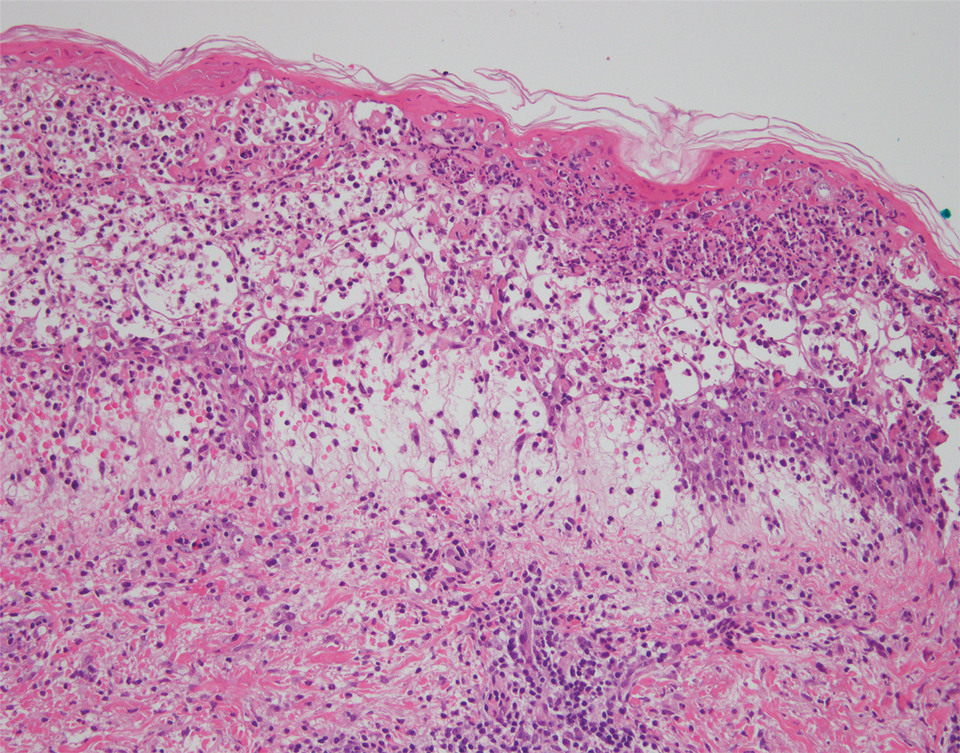
Febrile ulceronecrotic Mucha-Habermann disease is a rare and severe variant of PLEVA that can present with the rapid appearance of necrotic skin lesions, fever, and systemic manifestations, including pulmonary, gastrointestinal, central nervous system, cardiac, hematologic, and rheumatologic symptoms.2-4 The evolution from PLEVA to FUMHD ranges from days to weeks, and patientsrarely can have an initial presentation of FUMHD.2 The duration of illness has been reported to be 1 to 24 months5; however, the length of illness still remains unclear, as many studies of FUMHD are case reports with limited follow-up. Our patient had a disease duration of at least 27 months. The lesions of FUMHD usually are generalized with flexural prominence, and mucosal involvement occurs in approximately one-quarter of cases. Hypertrophic scarring may be seen after the ulcerated lesions heal.2 The incidence of FUMHD is higher in men than in women, and it is more common in younger individuals.2,6 There have been reported fatalities associated with FUMHD, mostly in adults.2,4
The clinical differential diagnosis for PLEVA includes disseminated herpes zoster, varicella-zoster virus or coxsackievirus infections, lymphomatoid papulosis, angiodestructive lymphoma such as extranodal natural killer/T-cell lymphoma, drug eruption, arthropod bite, erythema multiforme, ecthyma, ecthyma gangrenosum, necrotic folliculitis, and cutaneous small vessel vasculitis. To differentiate between these diagnoses and PLEVA or FUMHD, it is important to take a strong clinical history. For example, for varicella-zoster virus and coxsackievirus infections, exposure history to the viruses and vaccination history for varicella-zoster virus can help elucidate the diagnosis.
Skin biopsy can help differentiate between these entities and PLEVA or FUMHD. The histopathology of a nonulcerated lesion of FUMHD shows parakeratosis, spongiosis, and lymphocyte exocytosis, as well as lymphocytic vasculitis—findings commonly seen in PLEVA. With the ulceronecrotic lesions of FUMHD, epidermal necrosis and ulceration can be seen microscopically.2 Although skin biopsy is not absolutely necessary for making the diagnosis of PLEVA, it can be helpful.3 However, given the dramatic and extreme clinical impression with an extensive differential diagnosis that includes disorders ranging from infectious to neoplastic, biopsy of FUMHD with clinicopathologic correlation often is required.
It is important to avoid biopsying ulcerated lesions of FUMHD, as the histopathologic findings are more likely to be nonspecific. Additionally, nonspecific features often are seen with immunohistochemistry; abnormal laboratory testing may be seen in FUMHD, but there is no specific test to diagnose FUMHD.2 Finally, a predominantly CD8+ cell infiltrate was seen in 4 of 6 cases of FUMHD, with 2 cases showing a mixed infiltrate of CD8+ and CD4+ cells.5,7-10
Although no unified diagnostic criterion exists for FUMHD, Nofal et al2 proposed criteria comprised of constant features, which are found in every case of FUMHD and can confirm the diagnosis alone, and variable features to help ensure that cases of FUMHD are not missed. The constant features include fever, acute onset of generalized ulceronecrotic papules and plaques, a course that is rapid and progressive (without a tendency for spontaneous resolution), and histopathology that is consistent with PLEVA. The variable features include history of PLEVA, involvement of mucous membranes, and systemic involvement.2
No single unifying treatment modality for all cases of FUMHD has been described. Immunosuppressive drugs (eg, systemic steroids, methotrexate), antibiotics, antivirals, phototherapy, intravenous immunoglobulin, and dapsone have been tried in patients with FUMHD.2 Combination therapy with an oral medication such as erythromycin or methotrexate and psoralen plus UVA may be effective for FUMHD.3 Additionally, some authors believe that patients with FUMHD should be treated similar to burn victims with intensive supportive care.2
The etiology of PLEVA is unknown, but it is presumed to be associated with an effector cytotoxic T-cell response to either an infectious agent or a drug.11
Four cases of FUMHD with monoclonality have been reported,4,7,8 and some researchers propose that FUMHD may be a subset of cutaneous T-cell lymphoma.7 However, 2 other cases of FUMHD did not show monoclonality of T cells,5,18 suggesting that FUMHD may represent an inflammatory disorder, rather than a lymphoproliferative process of T cells.18 Given the controversy surrounding the clonality of FUMHD, T-cell gene rearrangement studies were not performed in our case.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other papulosquamous disorders. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. Elsevier Saunders; 2014:68-69.
- Nofal A, Assaf M, Alakad R, et al. Febrile ulceronecrotic Mucha-Habermann disease: proposed diagnostic criteria and therapeutic evaluation. Int J Dermatol. 2016;55:729-738.
- Milligan A, Johnston GA. Pityriasis lichenoides et varioliformis acuta. In: Lebwohl MG, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease, Comprehensive Therapeutic Strategies. 4th ed. Saunders; 2013:580-582.
- Miyamoto T, Takayama N, Kitada S, et al. Febrile ulceronecrotic Mucha-Habermann disease: a case report and a review of the literature. J Clin Pathol. 2003;56:795-797.
- Meziane L, Caudron A, Dhaille F, et al. Febrile ulceronecrotic Mucha-Habermann disease: treatment with infliximab and intravenous immunoglobulins and review of the literature. Dermatology. 2012;225:344-348.
- Robinson AB, Stein LD. Miscellaneous conditions associated with arthritis. In: Kliegman RM, Stanton BF, St. Geme JW III, et al, eds. Nelson Textbook of Pediatrics. 19th ed. W.B. Saunders Company; 2011:880.
- Cozzio A, Hafner J, Kempf W, et al. Febrile ulceronecrotic Mucha-Habermann disease with clonality: a cutaneous T-cell lymphoma entity? J Am Acad Dermatol. 2004;51:1014-1017.
- Tsianakas A, Hoeger PH. Transition of pityriasis lichenoides et varioliformis acuta to febrile ulceronecrotic Mucha-Habermann disease is associated with elevated serum tumour necrosis factor-alpha. Br J Dermatol. 2005;152:794-799.
- Yanaba K, Ito M, Sasaki H, et al. A case of febrile ulceronecrotic Mucha-Habermann disease requiring debridement of necrotic skin and epidermal autograft. Br J Dermatol. 2002;147:1249-1253.
- Lode HN, Döring P, Lauenstein P, et al. Febrile ulceronecrotic Mucha-Habermann disease following suspected hemorrhagic chickenpox infection in a 20-month-old boy. Infection. 2015;43:583-588.
- Tomasini D, Tomasini CF, Cerri A, et al. Pityriasis lichenoides: a cytotoxic T-cell-mediated skin disorder: evidence of human parvovirus B19 DNA in nine cases. J Cutan Pathol. 2004;31:531-538.
- Weiss LM, Wood GS, Ellisen LW, et al. Clonal T-cell populations in pityriasis lichenoides et varioliformis acuta (Mucha-Habermann disease). Am J Pathol. 1987;126:417-421.
- Dereure O, Levi E, Kadin ME. T-cell clonality in pityriasis lichenoides et varioliformis acuta: a heteroduplex analysis of 20 cases. Arch Dermatol. 2000;136:1483-1486.
- Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dermatol. 2002;138:1063-1067.
- Fortson JS, Schroeter AL, Esterly NB. Cutaneous T-cell lymphoma (parapsoriasis en plaque): an association with pityriasis lichenoides et varioliformis acuta in young children. Arch Dermatol. 1990;126:1449-1453.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Cutaneous T-cell lymphoma. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. Elsevier Saunders; 2014:958.
- Kim JE, Yun WJ, Mun SK, et al. Pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronica: comparison of lesional T-cell subsets and investigation of viral associations. J Cutan Pathol. 2011;38:649-656.
- López-Estebaran´z JL, Vanaclocha F, Gil R, et al. Febrile ulceronecrotic Mucha-Habermann disease. J Am Acad Dermatol. 1993;29(5, pt 2):903-906.
To the Editor:
Pityriasis lichenoides is a papulosquamous dermatologic disorder that is characterized by recurrent papules.1 There is a spectrum of disease in pityriasis lichenoides that includes pityriasis lichenoides et varioliformis acuta (PLEVA) at one end and pityriasis lichenoides chronica at the other. Pityriasis lichenoides et varioliformis acuta is more common in younger individuals and is characterized by erythematous papules that often crust; these lesions resolve over weeks. The lesions of pityriasis lichenoides chronica are characteristically scaly, pink to red-brown papules that tend to resolve over months.1
Histologically, PLEVA exhibits parakeratosis, interface dermatitis, and a wedge-shaped infiltrate.1 Necrotic keratinocytes and extravasated erythrocytes also are common features. Additionally, monoclonal T cells may be present in the infiltrate.1
Febrile ulceronecrotic Mucha-Habermann disease (FUMHD) is a rare and severe variant of PLEVA. Febrile ulceronecrotic Mucha-Habermann disease is characterized by ulceronecrotic lesions, fever, and systemic symptoms.2 Herein, we present a case of FUMHD.

A 57-year-old man presented with an eruption of painful lesions involving the face, trunk, arms, legs, and genitalia of 1 month’s duration. The patient denied oral and ocular involvement. He had soreness and swelling of the arms and legs. A prior 12-day course of prednisone prescribed by a community dermatologist failed to improve the rash. A biopsy performed by a community dermatologist was nondiagnostic. The patient denied fever but did report chills. He had no preceding illness and was not taking new medications. On physical examination, the patient was afebrile and normotensive with innumerable deep-seated pustules and crusted ulcerations on the face, palms, soles, trunk, extremities, and penis (Figures 1 and 2). There was a background morbilliform eruption on the trunk. The ocular and oral mucosae were spared. The upper and lower extremities had pitting edema.

The patient’s alanine aminotransaminase and aspartate aminotransaminase levels were elevated at 55 and 51 U/L, respectively. His white blood cell count was within reference range; however, there was an elevated absolute neutrophil count (8.7×103/μL). No eosinophilia was noted. Laboratory evaluation showed a positive antimitochondrial antibody, and magnetic resonance imaging showed evidence of steatohepatitis. Punch biopsies from both the morbilliform eruption and a deep-seated pustule showed epidermal necrosis, parakeratosis, necrotic keratinocytes, and a lichenoid infiltrate of lymphocytes at the dermoepidermal interface. In the dermis, there was a wedge-shaped superficial and deep, perivascular infiltrate with extravasated erythrocytes (Figures 3 and 4). Tissue Gram stain was negative for bacteria. Varicella-zoster virus and herpes simplex virus immunostains were negative. Direct immunofluorescence showed colloid bodies, as can be seen in lichenoid dermatitis.

At the next clinic visit, the patient reported a fever of 39.4 °C. After reviewing the patient’s histopathology and clinical picture, along with the presence of fever, a final diagnosis of FUMHD was made. The patient was started on an oral regimen of prednisone 80 mg once daily, minocycline 100 mg twice daily, and methotrexate 15 mg weekly. Unna boots (specialized compression wraps) with triamcinolone acetonide ointment 0.1% were placed weekly until the leg edema and ulcerations healed. He was maintained on methotrexate 15 mg weekly and 5 to 10 mg of prednisone once daily. The patient demonstrated residual scarring, with only rare new papulonodules that did not ulcerate when attempts were made to taper his medications. He was followed for nearly 3 years, with a recurrence of symptoms 2 years and 3 months after initial presentation to the academic dermatology clinic.

Febrile ulceronecrotic Mucha-Habermann disease is a rare and severe variant of PLEVA that can present with the rapid appearance of necrotic skin lesions, fever, and systemic manifestations, including pulmonary, gastrointestinal, central nervous system, cardiac, hematologic, and rheumatologic symptoms.2-4 The evolution from PLEVA to FUMHD ranges from days to weeks, and patientsrarely can have an initial presentation of FUMHD.2 The duration of illness has been reported to be 1 to 24 months5; however, the length of illness still remains unclear, as many studies of FUMHD are case reports with limited follow-up. Our patient had a disease duration of at least 27 months. The lesions of FUMHD usually are generalized with flexural prominence, and mucosal involvement occurs in approximately one-quarter of cases. Hypertrophic scarring may be seen after the ulcerated lesions heal.2 The incidence of FUMHD is higher in men than in women, and it is more common in younger individuals.2,6 There have been reported fatalities associated with FUMHD, mostly in adults.2,4
The clinical differential diagnosis for PLEVA includes disseminated herpes zoster, varicella-zoster virus or coxsackievirus infections, lymphomatoid papulosis, angiodestructive lymphoma such as extranodal natural killer/T-cell lymphoma, drug eruption, arthropod bite, erythema multiforme, ecthyma, ecthyma gangrenosum, necrotic folliculitis, and cutaneous small vessel vasculitis. To differentiate between these diagnoses and PLEVA or FUMHD, it is important to take a strong clinical history. For example, for varicella-zoster virus and coxsackievirus infections, exposure history to the viruses and vaccination history for varicella-zoster virus can help elucidate the diagnosis.
Skin biopsy can help differentiate between these entities and PLEVA or FUMHD. The histopathology of a nonulcerated lesion of FUMHD shows parakeratosis, spongiosis, and lymphocyte exocytosis, as well as lymphocytic vasculitis—findings commonly seen in PLEVA. With the ulceronecrotic lesions of FUMHD, epidermal necrosis and ulceration can be seen microscopically.2 Although skin biopsy is not absolutely necessary for making the diagnosis of PLEVA, it can be helpful.3 However, given the dramatic and extreme clinical impression with an extensive differential diagnosis that includes disorders ranging from infectious to neoplastic, biopsy of FUMHD with clinicopathologic correlation often is required.
It is important to avoid biopsying ulcerated lesions of FUMHD, as the histopathologic findings are more likely to be nonspecific. Additionally, nonspecific features often are seen with immunohistochemistry; abnormal laboratory testing may be seen in FUMHD, but there is no specific test to diagnose FUMHD.2 Finally, a predominantly CD8+ cell infiltrate was seen in 4 of 6 cases of FUMHD, with 2 cases showing a mixed infiltrate of CD8+ and CD4+ cells.5,7-10
Although no unified diagnostic criterion exists for FUMHD, Nofal et al2 proposed criteria comprised of constant features, which are found in every case of FUMHD and can confirm the diagnosis alone, and variable features to help ensure that cases of FUMHD are not missed. The constant features include fever, acute onset of generalized ulceronecrotic papules and plaques, a course that is rapid and progressive (without a tendency for spontaneous resolution), and histopathology that is consistent with PLEVA. The variable features include history of PLEVA, involvement of mucous membranes, and systemic involvement.2
No single unifying treatment modality for all cases of FUMHD has been described. Immunosuppressive drugs (eg, systemic steroids, methotrexate), antibiotics, antivirals, phototherapy, intravenous immunoglobulin, and dapsone have been tried in patients with FUMHD.2 Combination therapy with an oral medication such as erythromycin or methotrexate and psoralen plus UVA may be effective for FUMHD.3 Additionally, some authors believe that patients with FUMHD should be treated similar to burn victims with intensive supportive care.2
The etiology of PLEVA is unknown, but it is presumed to be associated with an effector cytotoxic T-cell response to either an infectious agent or a drug.11
Four cases of FUMHD with monoclonality have been reported,4,7,8 and some researchers propose that FUMHD may be a subset of cutaneous T-cell lymphoma.7 However, 2 other cases of FUMHD did not show monoclonality of T cells,5,18 suggesting that FUMHD may represent an inflammatory disorder, rather than a lymphoproliferative process of T cells.18 Given the controversy surrounding the clonality of FUMHD, T-cell gene rearrangement studies were not performed in our case.
To the Editor:
Pityriasis lichenoides is a papulosquamous dermatologic disorder that is characterized by recurrent papules.1 There is a spectrum of disease in pityriasis lichenoides that includes pityriasis lichenoides et varioliformis acuta (PLEVA) at one end and pityriasis lichenoides chronica at the other. Pityriasis lichenoides et varioliformis acuta is more common in younger individuals and is characterized by erythematous papules that often crust; these lesions resolve over weeks. The lesions of pityriasis lichenoides chronica are characteristically scaly, pink to red-brown papules that tend to resolve over months.1
Histologically, PLEVA exhibits parakeratosis, interface dermatitis, and a wedge-shaped infiltrate.1 Necrotic keratinocytes and extravasated erythrocytes also are common features. Additionally, monoclonal T cells may be present in the infiltrate.1
Febrile ulceronecrotic Mucha-Habermann disease (FUMHD) is a rare and severe variant of PLEVA. Febrile ulceronecrotic Mucha-Habermann disease is characterized by ulceronecrotic lesions, fever, and systemic symptoms.2 Herein, we present a case of FUMHD.

A 57-year-old man presented with an eruption of painful lesions involving the face, trunk, arms, legs, and genitalia of 1 month’s duration. The patient denied oral and ocular involvement. He had soreness and swelling of the arms and legs. A prior 12-day course of prednisone prescribed by a community dermatologist failed to improve the rash. A biopsy performed by a community dermatologist was nondiagnostic. The patient denied fever but did report chills. He had no preceding illness and was not taking new medications. On physical examination, the patient was afebrile and normotensive with innumerable deep-seated pustules and crusted ulcerations on the face, palms, soles, trunk, extremities, and penis (Figures 1 and 2). There was a background morbilliform eruption on the trunk. The ocular and oral mucosae were spared. The upper and lower extremities had pitting edema.

The patient’s alanine aminotransaminase and aspartate aminotransaminase levels were elevated at 55 and 51 U/L, respectively. His white blood cell count was within reference range; however, there was an elevated absolute neutrophil count (8.7×103/μL). No eosinophilia was noted. Laboratory evaluation showed a positive antimitochondrial antibody, and magnetic resonance imaging showed evidence of steatohepatitis. Punch biopsies from both the morbilliform eruption and a deep-seated pustule showed epidermal necrosis, parakeratosis, necrotic keratinocytes, and a lichenoid infiltrate of lymphocytes at the dermoepidermal interface. In the dermis, there was a wedge-shaped superficial and deep, perivascular infiltrate with extravasated erythrocytes (Figures 3 and 4). Tissue Gram stain was negative for bacteria. Varicella-zoster virus and herpes simplex virus immunostains were negative. Direct immunofluorescence showed colloid bodies, as can be seen in lichenoid dermatitis.

At the next clinic visit, the patient reported a fever of 39.4 °C. After reviewing the patient’s histopathology and clinical picture, along with the presence of fever, a final diagnosis of FUMHD was made. The patient was started on an oral regimen of prednisone 80 mg once daily, minocycline 100 mg twice daily, and methotrexate 15 mg weekly. Unna boots (specialized compression wraps) with triamcinolone acetonide ointment 0.1% were placed weekly until the leg edema and ulcerations healed. He was maintained on methotrexate 15 mg weekly and 5 to 10 mg of prednisone once daily. The patient demonstrated residual scarring, with only rare new papulonodules that did not ulcerate when attempts were made to taper his medications. He was followed for nearly 3 years, with a recurrence of symptoms 2 years and 3 months after initial presentation to the academic dermatology clinic.

Febrile ulceronecrotic Mucha-Habermann disease is a rare and severe variant of PLEVA that can present with the rapid appearance of necrotic skin lesions, fever, and systemic manifestations, including pulmonary, gastrointestinal, central nervous system, cardiac, hematologic, and rheumatologic symptoms.2-4 The evolution from PLEVA to FUMHD ranges from days to weeks, and patientsrarely can have an initial presentation of FUMHD.2 The duration of illness has been reported to be 1 to 24 months5; however, the length of illness still remains unclear, as many studies of FUMHD are case reports with limited follow-up. Our patient had a disease duration of at least 27 months. The lesions of FUMHD usually are generalized with flexural prominence, and mucosal involvement occurs in approximately one-quarter of cases. Hypertrophic scarring may be seen after the ulcerated lesions heal.2 The incidence of FUMHD is higher in men than in women, and it is more common in younger individuals.2,6 There have been reported fatalities associated with FUMHD, mostly in adults.2,4
The clinical differential diagnosis for PLEVA includes disseminated herpes zoster, varicella-zoster virus or coxsackievirus infections, lymphomatoid papulosis, angiodestructive lymphoma such as extranodal natural killer/T-cell lymphoma, drug eruption, arthropod bite, erythema multiforme, ecthyma, ecthyma gangrenosum, necrotic folliculitis, and cutaneous small vessel vasculitis. To differentiate between these diagnoses and PLEVA or FUMHD, it is important to take a strong clinical history. For example, for varicella-zoster virus and coxsackievirus infections, exposure history to the viruses and vaccination history for varicella-zoster virus can help elucidate the diagnosis.
Skin biopsy can help differentiate between these entities and PLEVA or FUMHD. The histopathology of a nonulcerated lesion of FUMHD shows parakeratosis, spongiosis, and lymphocyte exocytosis, as well as lymphocytic vasculitis—findings commonly seen in PLEVA. With the ulceronecrotic lesions of FUMHD, epidermal necrosis and ulceration can be seen microscopically.2 Although skin biopsy is not absolutely necessary for making the diagnosis of PLEVA, it can be helpful.3 However, given the dramatic and extreme clinical impression with an extensive differential diagnosis that includes disorders ranging from infectious to neoplastic, biopsy of FUMHD with clinicopathologic correlation often is required.
It is important to avoid biopsying ulcerated lesions of FUMHD, as the histopathologic findings are more likely to be nonspecific. Additionally, nonspecific features often are seen with immunohistochemistry; abnormal laboratory testing may be seen in FUMHD, but there is no specific test to diagnose FUMHD.2 Finally, a predominantly CD8+ cell infiltrate was seen in 4 of 6 cases of FUMHD, with 2 cases showing a mixed infiltrate of CD8+ and CD4+ cells.5,7-10
Although no unified diagnostic criterion exists for FUMHD, Nofal et al2 proposed criteria comprised of constant features, which are found in every case of FUMHD and can confirm the diagnosis alone, and variable features to help ensure that cases of FUMHD are not missed. The constant features include fever, acute onset of generalized ulceronecrotic papules and plaques, a course that is rapid and progressive (without a tendency for spontaneous resolution), and histopathology that is consistent with PLEVA. The variable features include history of PLEVA, involvement of mucous membranes, and systemic involvement.2
No single unifying treatment modality for all cases of FUMHD has been described. Immunosuppressive drugs (eg, systemic steroids, methotrexate), antibiotics, antivirals, phototherapy, intravenous immunoglobulin, and dapsone have been tried in patients with FUMHD.2 Combination therapy with an oral medication such as erythromycin or methotrexate and psoralen plus UVA may be effective for FUMHD.3 Additionally, some authors believe that patients with FUMHD should be treated similar to burn victims with intensive supportive care.2
The etiology of PLEVA is unknown, but it is presumed to be associated with an effector cytotoxic T-cell response to either an infectious agent or a drug.11
Four cases of FUMHD with monoclonality have been reported,4,7,8 and some researchers propose that FUMHD may be a subset of cutaneous T-cell lymphoma.7 However, 2 other cases of FUMHD did not show monoclonality of T cells,5,18 suggesting that FUMHD may represent an inflammatory disorder, rather than a lymphoproliferative process of T cells.18 Given the controversy surrounding the clonality of FUMHD, T-cell gene rearrangement studies were not performed in our case.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other papulosquamous disorders. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. Elsevier Saunders; 2014:68-69.
- Nofal A, Assaf M, Alakad R, et al. Febrile ulceronecrotic Mucha-Habermann disease: proposed diagnostic criteria and therapeutic evaluation. Int J Dermatol. 2016;55:729-738.
- Milligan A, Johnston GA. Pityriasis lichenoides et varioliformis acuta. In: Lebwohl MG, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease, Comprehensive Therapeutic Strategies. 4th ed. Saunders; 2013:580-582.
- Miyamoto T, Takayama N, Kitada S, et al. Febrile ulceronecrotic Mucha-Habermann disease: a case report and a review of the literature. J Clin Pathol. 2003;56:795-797.
- Meziane L, Caudron A, Dhaille F, et al. Febrile ulceronecrotic Mucha-Habermann disease: treatment with infliximab and intravenous immunoglobulins and review of the literature. Dermatology. 2012;225:344-348.
- Robinson AB, Stein LD. Miscellaneous conditions associated with arthritis. In: Kliegman RM, Stanton BF, St. Geme JW III, et al, eds. Nelson Textbook of Pediatrics. 19th ed. W.B. Saunders Company; 2011:880.
- Cozzio A, Hafner J, Kempf W, et al. Febrile ulceronecrotic Mucha-Habermann disease with clonality: a cutaneous T-cell lymphoma entity? J Am Acad Dermatol. 2004;51:1014-1017.
- Tsianakas A, Hoeger PH. Transition of pityriasis lichenoides et varioliformis acuta to febrile ulceronecrotic Mucha-Habermann disease is associated with elevated serum tumour necrosis factor-alpha. Br J Dermatol. 2005;152:794-799.
- Yanaba K, Ito M, Sasaki H, et al. A case of febrile ulceronecrotic Mucha-Habermann disease requiring debridement of necrotic skin and epidermal autograft. Br J Dermatol. 2002;147:1249-1253.
- Lode HN, Döring P, Lauenstein P, et al. Febrile ulceronecrotic Mucha-Habermann disease following suspected hemorrhagic chickenpox infection in a 20-month-old boy. Infection. 2015;43:583-588.
- Tomasini D, Tomasini CF, Cerri A, et al. Pityriasis lichenoides: a cytotoxic T-cell-mediated skin disorder: evidence of human parvovirus B19 DNA in nine cases. J Cutan Pathol. 2004;31:531-538.
- Weiss LM, Wood GS, Ellisen LW, et al. Clonal T-cell populations in pityriasis lichenoides et varioliformis acuta (Mucha-Habermann disease). Am J Pathol. 1987;126:417-421.
- Dereure O, Levi E, Kadin ME. T-cell clonality in pityriasis lichenoides et varioliformis acuta: a heteroduplex analysis of 20 cases. Arch Dermatol. 2000;136:1483-1486.
- Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dermatol. 2002;138:1063-1067.
- Fortson JS, Schroeter AL, Esterly NB. Cutaneous T-cell lymphoma (parapsoriasis en plaque): an association with pityriasis lichenoides et varioliformis acuta in young children. Arch Dermatol. 1990;126:1449-1453.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Cutaneous T-cell lymphoma. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. Elsevier Saunders; 2014:958.
- Kim JE, Yun WJ, Mun SK, et al. Pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronica: comparison of lesional T-cell subsets and investigation of viral associations. J Cutan Pathol. 2011;38:649-656.
- López-Estebaran´z JL, Vanaclocha F, Gil R, et al. Febrile ulceronecrotic Mucha-Habermann disease. J Am Acad Dermatol. 1993;29(5, pt 2):903-906.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other papulosquamous disorders. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. Elsevier Saunders; 2014:68-69.
- Nofal A, Assaf M, Alakad R, et al. Febrile ulceronecrotic Mucha-Habermann disease: proposed diagnostic criteria and therapeutic evaluation. Int J Dermatol. 2016;55:729-738.
- Milligan A, Johnston GA. Pityriasis lichenoides et varioliformis acuta. In: Lebwohl MG, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease, Comprehensive Therapeutic Strategies. 4th ed. Saunders; 2013:580-582.
- Miyamoto T, Takayama N, Kitada S, et al. Febrile ulceronecrotic Mucha-Habermann disease: a case report and a review of the literature. J Clin Pathol. 2003;56:795-797.
- Meziane L, Caudron A, Dhaille F, et al. Febrile ulceronecrotic Mucha-Habermann disease: treatment with infliximab and intravenous immunoglobulins and review of the literature. Dermatology. 2012;225:344-348.
- Robinson AB, Stein LD. Miscellaneous conditions associated with arthritis. In: Kliegman RM, Stanton BF, St. Geme JW III, et al, eds. Nelson Textbook of Pediatrics. 19th ed. W.B. Saunders Company; 2011:880.
- Cozzio A, Hafner J, Kempf W, et al. Febrile ulceronecrotic Mucha-Habermann disease with clonality: a cutaneous T-cell lymphoma entity? J Am Acad Dermatol. 2004;51:1014-1017.
- Tsianakas A, Hoeger PH. Transition of pityriasis lichenoides et varioliformis acuta to febrile ulceronecrotic Mucha-Habermann disease is associated with elevated serum tumour necrosis factor-alpha. Br J Dermatol. 2005;152:794-799.
- Yanaba K, Ito M, Sasaki H, et al. A case of febrile ulceronecrotic Mucha-Habermann disease requiring debridement of necrotic skin and epidermal autograft. Br J Dermatol. 2002;147:1249-1253.
- Lode HN, Döring P, Lauenstein P, et al. Febrile ulceronecrotic Mucha-Habermann disease following suspected hemorrhagic chickenpox infection in a 20-month-old boy. Infection. 2015;43:583-588.
- Tomasini D, Tomasini CF, Cerri A, et al. Pityriasis lichenoides: a cytotoxic T-cell-mediated skin disorder: evidence of human parvovirus B19 DNA in nine cases. J Cutan Pathol. 2004;31:531-538.
- Weiss LM, Wood GS, Ellisen LW, et al. Clonal T-cell populations in pityriasis lichenoides et varioliformis acuta (Mucha-Habermann disease). Am J Pathol. 1987;126:417-421.
- Dereure O, Levi E, Kadin ME. T-cell clonality in pityriasis lichenoides et varioliformis acuta: a heteroduplex analysis of 20 cases. Arch Dermatol. 2000;136:1483-1486.
- Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dermatol. 2002;138:1063-1067.
- Fortson JS, Schroeter AL, Esterly NB. Cutaneous T-cell lymphoma (parapsoriasis en plaque): an association with pityriasis lichenoides et varioliformis acuta in young children. Arch Dermatol. 1990;126:1449-1453.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Cutaneous T-cell lymphoma. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. Elsevier Saunders; 2014:958.
- Kim JE, Yun WJ, Mun SK, et al. Pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronica: comparison of lesional T-cell subsets and investigation of viral associations. J Cutan Pathol. 2011;38:649-656.
- López-Estebaran´z JL, Vanaclocha F, Gil R, et al. Febrile ulceronecrotic Mucha-Habermann disease. J Am Acad Dermatol. 1993;29(5, pt 2):903-906.
Practice Points
- Febrile ulceronecrotic Mucha-Habermann disease (FUMHD) is a rare variant of pityriasis lichenoides et varioliformis acuta, characterized by ulceronecrotic lesions, fever, and systemic symptoms.
- A variety of treatments including immunosuppressive drugs (eg, systemic steroids, methotrexate), antibiotics, antivirals, phototherapy, intravenous immunoglobulin, and dapsone have been used in patients with FUMHD.
‘This makes no sense’: Florida oncologist charged with prescription and insurance fraud
Michael Dattoli, MD, a radiation oncologist and physician-in-chief of the Dattoli Cancer Center in Sarasota, Fla., has been charged with prescription and insurance fraud, according to the Sarasota County Sheriff’s Office.
The charges include three counts of possessing a controlled substance by fraud, three counts of criminal use of personal identification information, and three counts of insurance fraud.
Dr. Dattoli was arrested on December 16.
According to investigators, a former employee of the Dattoli Cancer Center alleged that Dr. Dattoli filled prescriptions for diazepam (Valium) three times in his wife’s name using a different healthcare provider’s information.
Some experts find it bizarre for a physician of his stature to have possibly engaged in such a transgression — a relatively minor fraud that comes with serious consequences.
“This is a very well-respected physician who has done a lot of good in the community, and this makes no sense at all,” said Jay Wolfson, PhD, JD, Distinguished Service Professor of Public Health Medicine and Pharmacy and associate vice president for health law, policy, and safety at the University of South Florida, Tampa. “It’s low-level fraud, and not like he was laundering money or involved in pill mills, which has been problematic in Florida.”
According to recent accounts from local news agencies, in August 2021, the Sarasota police connected with investigators from the Sarasota County Sheriff’s Office’s Pharmaceutical Diversion Unit regarding prescription fraud dating back to 2019 and 2020 involving Dr. Dattoli and a “victim.”
The victim, a former employee of the Dattoli Cancer Center, had left his job at the end of 2020 after 5 years. His name and position at the cancer center were redacted in the arrest warrant, but the warrant mentions that he treated patients while he worked at the center.
The former employee told police that when checking the Florida prescription drug database for controlled substances in September 2021, he noticed that several fraudulent prescriptions for diazepam — a controlled substance — had been entered from 2020. The recipient was Dr. Dattoli’s wife, Rita Beatrice Dattoli, but the former employee stated he had never authorized these prescriptions and that Dr. Dattoli’s wife was never his patient.
In September 2021, the police obtained copies of multiple prescriptions from local pharmacies that were phoned in throughout 2020 by the Dattoli Cancer Center. The prescriptions were filled and picked up the same day by Dr. Dattoli himself, whose identity had been verified by his driver’s license.
Dr. Dattoli’s wife, who was interviewed by the police in October 2021, stated she had never been a patient at the center, that the prescription was not hers, and that she had never used the prescribed drug.
A month later, the Sarasota police subpoenaed bank records that matched accounts belonging to Dr. Dattoli, which showed the same dates, total purchase price, and stores where fraudulent prescriptions were filled, picked up, and purchased.
None of this really makes any sense, Dr. Wolfson told this news organizataion. “Any physician in need of Valium doesn’t have to forge a prescription, he can get it from any of his colleagues,” he noted. “And why put it in his wife’s name? He also submitted it to his insurance, which leaves more of a paper trail. And he didn’t need to have insurance pay for it — Valium is a very inexpensive drug.”
Plus, Dr. Wolfson added, “I know people who have been treated by Dr. Dattoli and they have nothing but good things to say about him — he’s an excellent doctor with a great bedside manner. His record is clean, he’s never been reprimanded, he’s built a successful practice, and then this thing just parachutes out of the sky.”
The investigation is ongoing, and detectives from the Sarasota police department have stated that they “believe there may be additional victims.”
Dr. Dattoli was released the day after his arrest on a $1,500 bond. His arraignment is scheduled for January 22. If convicted, he could face prison time, fines, or even lose his license to practice medicine.
Dr. Wolfson added that the arraignment is the first step in the process. “But even if it can be determined that he forged a signature, I don’t think it will rise to a level where his license will be revoked,” he said.
A version of this article first appeared on Medscape.com.
Michael Dattoli, MD, a radiation oncologist and physician-in-chief of the Dattoli Cancer Center in Sarasota, Fla., has been charged with prescription and insurance fraud, according to the Sarasota County Sheriff’s Office.
The charges include three counts of possessing a controlled substance by fraud, three counts of criminal use of personal identification information, and three counts of insurance fraud.
Dr. Dattoli was arrested on December 16.
According to investigators, a former employee of the Dattoli Cancer Center alleged that Dr. Dattoli filled prescriptions for diazepam (Valium) three times in his wife’s name using a different healthcare provider’s information.
Some experts find it bizarre for a physician of his stature to have possibly engaged in such a transgression — a relatively minor fraud that comes with serious consequences.
“This is a very well-respected physician who has done a lot of good in the community, and this makes no sense at all,” said Jay Wolfson, PhD, JD, Distinguished Service Professor of Public Health Medicine and Pharmacy and associate vice president for health law, policy, and safety at the University of South Florida, Tampa. “It’s low-level fraud, and not like he was laundering money or involved in pill mills, which has been problematic in Florida.”
According to recent accounts from local news agencies, in August 2021, the Sarasota police connected with investigators from the Sarasota County Sheriff’s Office’s Pharmaceutical Diversion Unit regarding prescription fraud dating back to 2019 and 2020 involving Dr. Dattoli and a “victim.”
The victim, a former employee of the Dattoli Cancer Center, had left his job at the end of 2020 after 5 years. His name and position at the cancer center were redacted in the arrest warrant, but the warrant mentions that he treated patients while he worked at the center.
The former employee told police that when checking the Florida prescription drug database for controlled substances in September 2021, he noticed that several fraudulent prescriptions for diazepam — a controlled substance — had been entered from 2020. The recipient was Dr. Dattoli’s wife, Rita Beatrice Dattoli, but the former employee stated he had never authorized these prescriptions and that Dr. Dattoli’s wife was never his patient.
In September 2021, the police obtained copies of multiple prescriptions from local pharmacies that were phoned in throughout 2020 by the Dattoli Cancer Center. The prescriptions were filled and picked up the same day by Dr. Dattoli himself, whose identity had been verified by his driver’s license.
Dr. Dattoli’s wife, who was interviewed by the police in October 2021, stated she had never been a patient at the center, that the prescription was not hers, and that she had never used the prescribed drug.
A month later, the Sarasota police subpoenaed bank records that matched accounts belonging to Dr. Dattoli, which showed the same dates, total purchase price, and stores where fraudulent prescriptions were filled, picked up, and purchased.
None of this really makes any sense, Dr. Wolfson told this news organizataion. “Any physician in need of Valium doesn’t have to forge a prescription, he can get it from any of his colleagues,” he noted. “And why put it in his wife’s name? He also submitted it to his insurance, which leaves more of a paper trail. And he didn’t need to have insurance pay for it — Valium is a very inexpensive drug.”
Plus, Dr. Wolfson added, “I know people who have been treated by Dr. Dattoli and they have nothing but good things to say about him — he’s an excellent doctor with a great bedside manner. His record is clean, he’s never been reprimanded, he’s built a successful practice, and then this thing just parachutes out of the sky.”
The investigation is ongoing, and detectives from the Sarasota police department have stated that they “believe there may be additional victims.”
Dr. Dattoli was released the day after his arrest on a $1,500 bond. His arraignment is scheduled for January 22. If convicted, he could face prison time, fines, or even lose his license to practice medicine.
Dr. Wolfson added that the arraignment is the first step in the process. “But even if it can be determined that he forged a signature, I don’t think it will rise to a level where his license will be revoked,” he said.
A version of this article first appeared on Medscape.com.
Michael Dattoli, MD, a radiation oncologist and physician-in-chief of the Dattoli Cancer Center in Sarasota, Fla., has been charged with prescription and insurance fraud, according to the Sarasota County Sheriff’s Office.
The charges include three counts of possessing a controlled substance by fraud, three counts of criminal use of personal identification information, and three counts of insurance fraud.
Dr. Dattoli was arrested on December 16.
According to investigators, a former employee of the Dattoli Cancer Center alleged that Dr. Dattoli filled prescriptions for diazepam (Valium) three times in his wife’s name using a different healthcare provider’s information.
Some experts find it bizarre for a physician of his stature to have possibly engaged in such a transgression — a relatively minor fraud that comes with serious consequences.
“This is a very well-respected physician who has done a lot of good in the community, and this makes no sense at all,” said Jay Wolfson, PhD, JD, Distinguished Service Professor of Public Health Medicine and Pharmacy and associate vice president for health law, policy, and safety at the University of South Florida, Tampa. “It’s low-level fraud, and not like he was laundering money or involved in pill mills, which has been problematic in Florida.”
According to recent accounts from local news agencies, in August 2021, the Sarasota police connected with investigators from the Sarasota County Sheriff’s Office’s Pharmaceutical Diversion Unit regarding prescription fraud dating back to 2019 and 2020 involving Dr. Dattoli and a “victim.”
The victim, a former employee of the Dattoli Cancer Center, had left his job at the end of 2020 after 5 years. His name and position at the cancer center were redacted in the arrest warrant, but the warrant mentions that he treated patients while he worked at the center.
The former employee told police that when checking the Florida prescription drug database for controlled substances in September 2021, he noticed that several fraudulent prescriptions for diazepam — a controlled substance — had been entered from 2020. The recipient was Dr. Dattoli’s wife, Rita Beatrice Dattoli, but the former employee stated he had never authorized these prescriptions and that Dr. Dattoli’s wife was never his patient.
In September 2021, the police obtained copies of multiple prescriptions from local pharmacies that were phoned in throughout 2020 by the Dattoli Cancer Center. The prescriptions were filled and picked up the same day by Dr. Dattoli himself, whose identity had been verified by his driver’s license.
Dr. Dattoli’s wife, who was interviewed by the police in October 2021, stated she had never been a patient at the center, that the prescription was not hers, and that she had never used the prescribed drug.
A month later, the Sarasota police subpoenaed bank records that matched accounts belonging to Dr. Dattoli, which showed the same dates, total purchase price, and stores where fraudulent prescriptions were filled, picked up, and purchased.
None of this really makes any sense, Dr. Wolfson told this news organizataion. “Any physician in need of Valium doesn’t have to forge a prescription, he can get it from any of his colleagues,” he noted. “And why put it in his wife’s name? He also submitted it to his insurance, which leaves more of a paper trail. And he didn’t need to have insurance pay for it — Valium is a very inexpensive drug.”
Plus, Dr. Wolfson added, “I know people who have been treated by Dr. Dattoli and they have nothing but good things to say about him — he’s an excellent doctor with a great bedside manner. His record is clean, he’s never been reprimanded, he’s built a successful practice, and then this thing just parachutes out of the sky.”
The investigation is ongoing, and detectives from the Sarasota police department have stated that they “believe there may be additional victims.”
Dr. Dattoli was released the day after his arrest on a $1,500 bond. His arraignment is scheduled for January 22. If convicted, he could face prison time, fines, or even lose his license to practice medicine.
Dr. Wolfson added that the arraignment is the first step in the process. “But even if it can be determined that he forged a signature, I don’t think it will rise to a level where his license will be revoked,” he said.
A version of this article first appeared on Medscape.com.
Frail COPD patients at high risk of disability and death
, a prospective cohort study of community-dwelling adults has shown.
“Frailty, a widely recognized geriatric syndrome characterized by multidimensional functional decline in bio-psycho-social factors, is associated with functional disability and mortality,” senior author Tze Pin Ng, MD, National University of Singapore, and colleagues explain.“Our results ... suggest that beyond traditional prognostic markers such as FEV1% (forced expiratory volume in 1 second) and dyspnea, the physical frailty phenotype provides additional useful prognostic information on future risks of disability and mortality,” the authors suggest.
The study was published online Dec. 12 in the journal CHEST®.
SLAS-1 and SLAS-2
Data from the Singapore Longitudinal Ageing Study (SLAS-1) and SLAS-2 were collected and analyzed. SLAS-1 recruited 2,804 participants 55 years of age and older from Sept. 2003 through Dec. 2004, while SLAS-2 recruited 3,270 participants of the same age between March 2009 and June 2013. “Follow-up visits and assessments were conducted approximately 3-5 years apart,” the investigators noted.
Mortality was determined at a mean of 9.5 years of follow-up for SLAS-1 participants and a mean of 6.5 years’ follow-up for SLAS-2 participants. A total of 4,627 participants were eventually included in the analysis, of whom 1,162 patients had COPD and 3,465 patients did not. COPD was classified as mild if FEV1% was greater than or equal to 80%; moderate if FEV1% was greater than or equal to 50% to less than 80%, and severe if FEV1% was less than 50%.
Frailty in turn was based on five clinical criteria, including weakness, slowness, low physical activity, exhaustion, and shrinking. Participants were classified as frail if they met three or more of these criteria and prefrail if they met one or two criteria.
Adverse health outcomes were judged on the basis of instrumental or basic activities of daily living (IADL/ADL), while disability was judged by self-reported difficulties in or requiring assistance with at least one IADL or ADL.
Frail or prefrail
Almost half of the participants were frail or prefrail, as the authors reported, while 25% had COPD. Among the participants with COPD, 30% had moderate to severe COPD, 6.4% had dyspnea, and almost half had prefrailty, while approximately 7% were classified as frail.
This percentage was 86% higher than it was for participants without COPD, among whom just 3.2% were assessed as frail, at an odds ratio of 1.86 (95% CI, 1.35-2.56). Further adjustments for possible confounders reduced the gap between frail COPD and frail non-COPD participants, but frailty remained significantly associated with COPD, at an OR of 1.61 (95% CI, 1.15-2.26), the investigators note.
Furthermore, compared to those without COPD, a diagnosis of COPD without and with dyspnea was associated with a 1.5- and 4.2-fold increase in prevalent frailty (95% CI, 1.04-2.08; 1.84-9.19), respectively, although not with prefrailty. Again, adjusting for multiple confounders, FEV1%, dyspnea, and both prefrailty and frailty were associated with an approximately twofold higher prevalence of IADL/ADL disability, while the prevalence of IADL/ADL disability for participants with COPD was approximately fourfold higher in those with co-occurring FEV1% less than 80% with either prefrailty, frailty, or dyspnea.
Furthermore, the presence of prefrailty or frailty in combination with a lower FEV1% or dyspnea was associated with a 3.7- to 3.8-fold increased risk of having an IADL or ADL disability.
Frailty and mortality
Some 1,116 participants with COPD were followed for a mean of 2,981 days for mortality outcomes. Both FEV1% less than 50% and the presence of prefrailty and frailty almost doubled the risk of mortality, at an adjusted hazard ratio of 1.8 (95% CI, 1.24-2.68) compared to patients with an FEV1% greater than or equal to 80%. In combination with either FEV1% less than 80% or prefrailty/frailty, dyspnea almost more than doubled the risk of mortality, at an HR of 2.4 for both combinations.
“However, the mortality risk of participants with COPD was highest among those with FEV1% less than 80% and prefrailty/frailty,” the authors note, more than tripling mortality risk at an adjusted HR of 3.25 (95% CI, 1.97-5.36). Interestingly, FEV1 less than 80% and prefrailty/frailty – both alone and in combination – were also associated with a twofold to fourfold increased risk of IADL or ADL disability in participants without COPD but were less strongly associated with mortality.
Researchers then went on to create a summary risk score containing all relevant variables with values ranging from 0 to 5. The highest risk category of 3 to 5 was associated with a 7- to 8.5-fold increased risk for IADL and ADL disability and mortality among participants with COPD, and that risk remained high after adjusting for multiple confounders.
Interestingly, frailty did not significantly predict mortality in women, while dyspnea did not significantly predict mortality in men. “Recognition and assessment of physical frailty in addition to FEV1% and dyspnea would allow for more accurate identification and targeted treatment of COPD at risk of future adverse outcomes,” the authors suggest.
Frailty scoring system
Asked to comment on the study, Sachin Gupta, MD, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif., noted that the current study adds to the body of literature that outcomes in patients with COPD depend as much on objectively measured variables as on qualitative measures. “By applying a frailty scoring system, these researchers were able to categorize frailty and study its impact on patient characteristics and outcomes,” he told this news organization in an email.
The summary risk assessment tool developed and assessed is familiar: It carries parallels to the widely utilized BODE Index, replacing body mass index and 6-minute walk distance with the frailty scale, he added. “Findings from this study support the idea that what meets the eye in face-to-face visits – frailty – can be codified and be part of a tool that is predictive of outcomes,” Dr. Gupta underscored.
The authors had no conflicts of interest to declare. Dr. Gupta disclosed that he is also an employee and shareholder at Genentech.
A version of this article first appeared on Medscape.com.
, a prospective cohort study of community-dwelling adults has shown.
“Frailty, a widely recognized geriatric syndrome characterized by multidimensional functional decline in bio-psycho-social factors, is associated with functional disability and mortality,” senior author Tze Pin Ng, MD, National University of Singapore, and colleagues explain.“Our results ... suggest that beyond traditional prognostic markers such as FEV1% (forced expiratory volume in 1 second) and dyspnea, the physical frailty phenotype provides additional useful prognostic information on future risks of disability and mortality,” the authors suggest.
The study was published online Dec. 12 in the journal CHEST®.
SLAS-1 and SLAS-2
Data from the Singapore Longitudinal Ageing Study (SLAS-1) and SLAS-2 were collected and analyzed. SLAS-1 recruited 2,804 participants 55 years of age and older from Sept. 2003 through Dec. 2004, while SLAS-2 recruited 3,270 participants of the same age between March 2009 and June 2013. “Follow-up visits and assessments were conducted approximately 3-5 years apart,” the investigators noted.
Mortality was determined at a mean of 9.5 years of follow-up for SLAS-1 participants and a mean of 6.5 years’ follow-up for SLAS-2 participants. A total of 4,627 participants were eventually included in the analysis, of whom 1,162 patients had COPD and 3,465 patients did not. COPD was classified as mild if FEV1% was greater than or equal to 80%; moderate if FEV1% was greater than or equal to 50% to less than 80%, and severe if FEV1% was less than 50%.
Frailty in turn was based on five clinical criteria, including weakness, slowness, low physical activity, exhaustion, and shrinking. Participants were classified as frail if they met three or more of these criteria and prefrail if they met one or two criteria.
Adverse health outcomes were judged on the basis of instrumental or basic activities of daily living (IADL/ADL), while disability was judged by self-reported difficulties in or requiring assistance with at least one IADL or ADL.
Frail or prefrail
Almost half of the participants were frail or prefrail, as the authors reported, while 25% had COPD. Among the participants with COPD, 30% had moderate to severe COPD, 6.4% had dyspnea, and almost half had prefrailty, while approximately 7% were classified as frail.
This percentage was 86% higher than it was for participants without COPD, among whom just 3.2% were assessed as frail, at an odds ratio of 1.86 (95% CI, 1.35-2.56). Further adjustments for possible confounders reduced the gap between frail COPD and frail non-COPD participants, but frailty remained significantly associated with COPD, at an OR of 1.61 (95% CI, 1.15-2.26), the investigators note.
Furthermore, compared to those without COPD, a diagnosis of COPD without and with dyspnea was associated with a 1.5- and 4.2-fold increase in prevalent frailty (95% CI, 1.04-2.08; 1.84-9.19), respectively, although not with prefrailty. Again, adjusting for multiple confounders, FEV1%, dyspnea, and both prefrailty and frailty were associated with an approximately twofold higher prevalence of IADL/ADL disability, while the prevalence of IADL/ADL disability for participants with COPD was approximately fourfold higher in those with co-occurring FEV1% less than 80% with either prefrailty, frailty, or dyspnea.
Furthermore, the presence of prefrailty or frailty in combination with a lower FEV1% or dyspnea was associated with a 3.7- to 3.8-fold increased risk of having an IADL or ADL disability.
Frailty and mortality
Some 1,116 participants with COPD were followed for a mean of 2,981 days for mortality outcomes. Both FEV1% less than 50% and the presence of prefrailty and frailty almost doubled the risk of mortality, at an adjusted hazard ratio of 1.8 (95% CI, 1.24-2.68) compared to patients with an FEV1% greater than or equal to 80%. In combination with either FEV1% less than 80% or prefrailty/frailty, dyspnea almost more than doubled the risk of mortality, at an HR of 2.4 for both combinations.
“However, the mortality risk of participants with COPD was highest among those with FEV1% less than 80% and prefrailty/frailty,” the authors note, more than tripling mortality risk at an adjusted HR of 3.25 (95% CI, 1.97-5.36). Interestingly, FEV1 less than 80% and prefrailty/frailty – both alone and in combination – were also associated with a twofold to fourfold increased risk of IADL or ADL disability in participants without COPD but were less strongly associated with mortality.
Researchers then went on to create a summary risk score containing all relevant variables with values ranging from 0 to 5. The highest risk category of 3 to 5 was associated with a 7- to 8.5-fold increased risk for IADL and ADL disability and mortality among participants with COPD, and that risk remained high after adjusting for multiple confounders.
Interestingly, frailty did not significantly predict mortality in women, while dyspnea did not significantly predict mortality in men. “Recognition and assessment of physical frailty in addition to FEV1% and dyspnea would allow for more accurate identification and targeted treatment of COPD at risk of future adverse outcomes,” the authors suggest.
Frailty scoring system
Asked to comment on the study, Sachin Gupta, MD, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif., noted that the current study adds to the body of literature that outcomes in patients with COPD depend as much on objectively measured variables as on qualitative measures. “By applying a frailty scoring system, these researchers were able to categorize frailty and study its impact on patient characteristics and outcomes,” he told this news organization in an email.
The summary risk assessment tool developed and assessed is familiar: It carries parallels to the widely utilized BODE Index, replacing body mass index and 6-minute walk distance with the frailty scale, he added. “Findings from this study support the idea that what meets the eye in face-to-face visits – frailty – can be codified and be part of a tool that is predictive of outcomes,” Dr. Gupta underscored.
The authors had no conflicts of interest to declare. Dr. Gupta disclosed that he is also an employee and shareholder at Genentech.
A version of this article first appeared on Medscape.com.
, a prospective cohort study of community-dwelling adults has shown.
“Frailty, a widely recognized geriatric syndrome characterized by multidimensional functional decline in bio-psycho-social factors, is associated with functional disability and mortality,” senior author Tze Pin Ng, MD, National University of Singapore, and colleagues explain.“Our results ... suggest that beyond traditional prognostic markers such as FEV1% (forced expiratory volume in 1 second) and dyspnea, the physical frailty phenotype provides additional useful prognostic information on future risks of disability and mortality,” the authors suggest.
The study was published online Dec. 12 in the journal CHEST®.
SLAS-1 and SLAS-2
Data from the Singapore Longitudinal Ageing Study (SLAS-1) and SLAS-2 were collected and analyzed. SLAS-1 recruited 2,804 participants 55 years of age and older from Sept. 2003 through Dec. 2004, while SLAS-2 recruited 3,270 participants of the same age between March 2009 and June 2013. “Follow-up visits and assessments were conducted approximately 3-5 years apart,” the investigators noted.
Mortality was determined at a mean of 9.5 years of follow-up for SLAS-1 participants and a mean of 6.5 years’ follow-up for SLAS-2 participants. A total of 4,627 participants were eventually included in the analysis, of whom 1,162 patients had COPD and 3,465 patients did not. COPD was classified as mild if FEV1% was greater than or equal to 80%; moderate if FEV1% was greater than or equal to 50% to less than 80%, and severe if FEV1% was less than 50%.
Frailty in turn was based on five clinical criteria, including weakness, slowness, low physical activity, exhaustion, and shrinking. Participants were classified as frail if they met three or more of these criteria and prefrail if they met one or two criteria.
Adverse health outcomes were judged on the basis of instrumental or basic activities of daily living (IADL/ADL), while disability was judged by self-reported difficulties in or requiring assistance with at least one IADL or ADL.
Frail or prefrail
Almost half of the participants were frail or prefrail, as the authors reported, while 25% had COPD. Among the participants with COPD, 30% had moderate to severe COPD, 6.4% had dyspnea, and almost half had prefrailty, while approximately 7% were classified as frail.
This percentage was 86% higher than it was for participants without COPD, among whom just 3.2% were assessed as frail, at an odds ratio of 1.86 (95% CI, 1.35-2.56). Further adjustments for possible confounders reduced the gap between frail COPD and frail non-COPD participants, but frailty remained significantly associated with COPD, at an OR of 1.61 (95% CI, 1.15-2.26), the investigators note.
Furthermore, compared to those without COPD, a diagnosis of COPD without and with dyspnea was associated with a 1.5- and 4.2-fold increase in prevalent frailty (95% CI, 1.04-2.08; 1.84-9.19), respectively, although not with prefrailty. Again, adjusting for multiple confounders, FEV1%, dyspnea, and both prefrailty and frailty were associated with an approximately twofold higher prevalence of IADL/ADL disability, while the prevalence of IADL/ADL disability for participants with COPD was approximately fourfold higher in those with co-occurring FEV1% less than 80% with either prefrailty, frailty, or dyspnea.
Furthermore, the presence of prefrailty or frailty in combination with a lower FEV1% or dyspnea was associated with a 3.7- to 3.8-fold increased risk of having an IADL or ADL disability.
Frailty and mortality
Some 1,116 participants with COPD were followed for a mean of 2,981 days for mortality outcomes. Both FEV1% less than 50% and the presence of prefrailty and frailty almost doubled the risk of mortality, at an adjusted hazard ratio of 1.8 (95% CI, 1.24-2.68) compared to patients with an FEV1% greater than or equal to 80%. In combination with either FEV1% less than 80% or prefrailty/frailty, dyspnea almost more than doubled the risk of mortality, at an HR of 2.4 for both combinations.
“However, the mortality risk of participants with COPD was highest among those with FEV1% less than 80% and prefrailty/frailty,” the authors note, more than tripling mortality risk at an adjusted HR of 3.25 (95% CI, 1.97-5.36). Interestingly, FEV1 less than 80% and prefrailty/frailty – both alone and in combination – were also associated with a twofold to fourfold increased risk of IADL or ADL disability in participants without COPD but were less strongly associated with mortality.
Researchers then went on to create a summary risk score containing all relevant variables with values ranging from 0 to 5. The highest risk category of 3 to 5 was associated with a 7- to 8.5-fold increased risk for IADL and ADL disability and mortality among participants with COPD, and that risk remained high after adjusting for multiple confounders.
Interestingly, frailty did not significantly predict mortality in women, while dyspnea did not significantly predict mortality in men. “Recognition and assessment of physical frailty in addition to FEV1% and dyspnea would allow for more accurate identification and targeted treatment of COPD at risk of future adverse outcomes,” the authors suggest.
Frailty scoring system
Asked to comment on the study, Sachin Gupta, MD, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif., noted that the current study adds to the body of literature that outcomes in patients with COPD depend as much on objectively measured variables as on qualitative measures. “By applying a frailty scoring system, these researchers were able to categorize frailty and study its impact on patient characteristics and outcomes,” he told this news organization in an email.
The summary risk assessment tool developed and assessed is familiar: It carries parallels to the widely utilized BODE Index, replacing body mass index and 6-minute walk distance with the frailty scale, he added. “Findings from this study support the idea that what meets the eye in face-to-face visits – frailty – can be codified and be part of a tool that is predictive of outcomes,” Dr. Gupta underscored.
The authors had no conflicts of interest to declare. Dr. Gupta disclosed that he is also an employee and shareholder at Genentech.
A version of this article first appeared on Medscape.com.
FROM CHEST
COVID affects executive functioning in young to middle-age adults: Study
than people in the general population with no such infection, according to new data published on the preprint server medRxiv.
Researchers, led by Peter A. Hall, PhD, with the University of Waterloo (Ont.), found that COVID infection is associated with executive dysfunction among young and middle-aged adults, including for those not exposed to intubation or hospitalization.
The findings have not been peer reviewed.
The study included a representative cohort of 1,958 community-dwelling young and middle-aged adults. It used a balanced proportion of infected and uninfected people to estimate the link between SARS-CoV-2 infection and cognitive/executive dysfunction.
The authors noted that the survey was conducted from Sept. 28 to Oct. 21, 2021, when the primary variant in Canada was Delta.
The research was a cross-sectional observational study with data from the ongoing Canadian COVID-19 Experiences Survey. It included equal representation of vaccinated and vaccine-hesitant adults aged 18-54 years. COVID-19 symptoms ranged from negligible to life-threatening cases requiring hospitalization.
Half in the cohort (50.2%) received two vaccine shots; 43.3% had received no shots; and 5.5% received one shot, but were not intending to receive a second shot.
Dose-response relationship
According to the paper, those with prior COVID-19 infection, regardless of symptom severity, reported a significantly higher number of symptoms of executive dysfunction than their noninfected counterparts (mechanical adjustment, 1.63, standard error, 0.08; 95% confidence interval, 1.47-1.80; P = .001).
The researchers also found a dose-response relationship between COVID-19 symptom severity and cognitive dysfunction. Those with moderate and very/extremely severe COVID-19 symptoms were linked with significantly greater dysfunction.
“This reinforces what we’re hearing about – that COVID is not ‘one and done.’ It can have lasting and quite subtle and damaging effects on the human body,” William Schaffner, MD, infectious disease specialist with Vanderbilt University, Nashville, Tenn., said in an interview.
Measuring executive functioning – including the ability to make sound decisions – is something other studies haven’t typically addressed, he said.
Men were likely to report more cognitive dysfunction symptoms than women (beta, 0.15; P < .001). Younger adults (25-39 years) were more likely to experience cognitive dysfunction than those age 40-54 (beta, 0.30; P < .001).
Dr. Schaffner said it was troubling that young people are more likely to experience the dysfunction.
“When we think of ‘brain fog’ we think of older persons who are already predisposed to have more memory lapses as they get older,” he said.
The link between cognitive dysfunction and COVID-19 infection has been shown in other studies, but many have not used representative samples and have not compared results with noninfected controls in the general population, the authors wrote.
Executive dysfunction was measured using four questions from the Deficits in Executive Functioning Scale. Respondents were asked how often they have experienced these scenarios in the past 6 months:
- “I am unable to inhibit my reactions or responses to events or to other people.”
- “I make impulsive comments to others.”
- “I am likely to do things without considering the consequences for doing them.”
- “I act without thinking.”
“This makes it even more important that we talk about vaccination,” Dr. Schaffner said, “because clearly the more seriously ill you are, the more likely this sort of thing is likely to happen and vaccines have been shown time and again to avert hospitalizations and more serious illness. It also makes more important the monoclonal antibody treatments we have and the antivirals, which will prevent the evolution of mild disease into something more serious.”
This research was supported by a grant from the Canadian Institutes for Health Research, Institute for Population and Public Health. The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
than people in the general population with no such infection, according to new data published on the preprint server medRxiv.
Researchers, led by Peter A. Hall, PhD, with the University of Waterloo (Ont.), found that COVID infection is associated with executive dysfunction among young and middle-aged adults, including for those not exposed to intubation or hospitalization.
The findings have not been peer reviewed.
The study included a representative cohort of 1,958 community-dwelling young and middle-aged adults. It used a balanced proportion of infected and uninfected people to estimate the link between SARS-CoV-2 infection and cognitive/executive dysfunction.
The authors noted that the survey was conducted from Sept. 28 to Oct. 21, 2021, when the primary variant in Canada was Delta.
The research was a cross-sectional observational study with data from the ongoing Canadian COVID-19 Experiences Survey. It included equal representation of vaccinated and vaccine-hesitant adults aged 18-54 years. COVID-19 symptoms ranged from negligible to life-threatening cases requiring hospitalization.
Half in the cohort (50.2%) received two vaccine shots; 43.3% had received no shots; and 5.5% received one shot, but were not intending to receive a second shot.
Dose-response relationship
According to the paper, those with prior COVID-19 infection, regardless of symptom severity, reported a significantly higher number of symptoms of executive dysfunction than their noninfected counterparts (mechanical adjustment, 1.63, standard error, 0.08; 95% confidence interval, 1.47-1.80; P = .001).
The researchers also found a dose-response relationship between COVID-19 symptom severity and cognitive dysfunction. Those with moderate and very/extremely severe COVID-19 symptoms were linked with significantly greater dysfunction.
“This reinforces what we’re hearing about – that COVID is not ‘one and done.’ It can have lasting and quite subtle and damaging effects on the human body,” William Schaffner, MD, infectious disease specialist with Vanderbilt University, Nashville, Tenn., said in an interview.
Measuring executive functioning – including the ability to make sound decisions – is something other studies haven’t typically addressed, he said.
Men were likely to report more cognitive dysfunction symptoms than women (beta, 0.15; P < .001). Younger adults (25-39 years) were more likely to experience cognitive dysfunction than those age 40-54 (beta, 0.30; P < .001).
Dr. Schaffner said it was troubling that young people are more likely to experience the dysfunction.
“When we think of ‘brain fog’ we think of older persons who are already predisposed to have more memory lapses as they get older,” he said.
The link between cognitive dysfunction and COVID-19 infection has been shown in other studies, but many have not used representative samples and have not compared results with noninfected controls in the general population, the authors wrote.
Executive dysfunction was measured using four questions from the Deficits in Executive Functioning Scale. Respondents were asked how often they have experienced these scenarios in the past 6 months:
- “I am unable to inhibit my reactions or responses to events or to other people.”
- “I make impulsive comments to others.”
- “I am likely to do things without considering the consequences for doing them.”
- “I act without thinking.”
“This makes it even more important that we talk about vaccination,” Dr. Schaffner said, “because clearly the more seriously ill you are, the more likely this sort of thing is likely to happen and vaccines have been shown time and again to avert hospitalizations and more serious illness. It also makes more important the monoclonal antibody treatments we have and the antivirals, which will prevent the evolution of mild disease into something more serious.”
This research was supported by a grant from the Canadian Institutes for Health Research, Institute for Population and Public Health. The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
than people in the general population with no such infection, according to new data published on the preprint server medRxiv.
Researchers, led by Peter A. Hall, PhD, with the University of Waterloo (Ont.), found that COVID infection is associated with executive dysfunction among young and middle-aged adults, including for those not exposed to intubation or hospitalization.
The findings have not been peer reviewed.
The study included a representative cohort of 1,958 community-dwelling young and middle-aged adults. It used a balanced proportion of infected and uninfected people to estimate the link between SARS-CoV-2 infection and cognitive/executive dysfunction.
The authors noted that the survey was conducted from Sept. 28 to Oct. 21, 2021, when the primary variant in Canada was Delta.
The research was a cross-sectional observational study with data from the ongoing Canadian COVID-19 Experiences Survey. It included equal representation of vaccinated and vaccine-hesitant adults aged 18-54 years. COVID-19 symptoms ranged from negligible to life-threatening cases requiring hospitalization.
Half in the cohort (50.2%) received two vaccine shots; 43.3% had received no shots; and 5.5% received one shot, but were not intending to receive a second shot.
Dose-response relationship
According to the paper, those with prior COVID-19 infection, regardless of symptom severity, reported a significantly higher number of symptoms of executive dysfunction than their noninfected counterparts (mechanical adjustment, 1.63, standard error, 0.08; 95% confidence interval, 1.47-1.80; P = .001).
The researchers also found a dose-response relationship between COVID-19 symptom severity and cognitive dysfunction. Those with moderate and very/extremely severe COVID-19 symptoms were linked with significantly greater dysfunction.
“This reinforces what we’re hearing about – that COVID is not ‘one and done.’ It can have lasting and quite subtle and damaging effects on the human body,” William Schaffner, MD, infectious disease specialist with Vanderbilt University, Nashville, Tenn., said in an interview.
Measuring executive functioning – including the ability to make sound decisions – is something other studies haven’t typically addressed, he said.
Men were likely to report more cognitive dysfunction symptoms than women (beta, 0.15; P < .001). Younger adults (25-39 years) were more likely to experience cognitive dysfunction than those age 40-54 (beta, 0.30; P < .001).
Dr. Schaffner said it was troubling that young people are more likely to experience the dysfunction.
“When we think of ‘brain fog’ we think of older persons who are already predisposed to have more memory lapses as they get older,” he said.
The link between cognitive dysfunction and COVID-19 infection has been shown in other studies, but many have not used representative samples and have not compared results with noninfected controls in the general population, the authors wrote.
Executive dysfunction was measured using four questions from the Deficits in Executive Functioning Scale. Respondents were asked how often they have experienced these scenarios in the past 6 months:
- “I am unable to inhibit my reactions or responses to events or to other people.”
- “I make impulsive comments to others.”
- “I am likely to do things without considering the consequences for doing them.”
- “I act without thinking.”
“This makes it even more important that we talk about vaccination,” Dr. Schaffner said, “because clearly the more seriously ill you are, the more likely this sort of thing is likely to happen and vaccines have been shown time and again to avert hospitalizations and more serious illness. It also makes more important the monoclonal antibody treatments we have and the antivirals, which will prevent the evolution of mild disease into something more serious.”
This research was supported by a grant from the Canadian Institutes for Health Research, Institute for Population and Public Health. The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MEDRXIV