User login
Tips on Better Patients Communication
SAN DIEGO—Don’t stand when you talk at bedside. Ditch the white gowns, turn away from your computers and pagers, and stop yourself from interrupting all the time.
These tips—and more—can help clinicians provide better and more effective care, said a colorectal surgeon who spoke about communication skills at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Research has suggested that nearly half of Americans don’t think their health care practitioners (HCPs) are compassionate, “and that’s really sad,” said Lorene Valdez-Boyle, MD, MS, surgery chief at the New Mexico VA Health Care Service.
To combat this perception, she said, HCPs can adopt multiple strategies as they work with veterans and their families. The goal, she said, is “to try to get them to trust you and want to be part of their treatment. This is how we're going to have better outcomes.”
Some strategies are simple. Dr. Valdez-Boyle, for example, doesn’t wear a white gown when she sees patients. “Obviously, they’re really gross,” she said. “But also, I want them to be comfortable with me. I sit down at their level, and we have a conversation. We talk about our dogs and we bond, because that’s going to help them trust me and want to work with me. I do that with families too. We joke, and we laugh.”
Sitting bedside instead of standing is important, she said, and a 2016 study backs up this idea. “It’s difficult when you’re running around or you want to get to the next one, and the patient just keeps talking,” she said. But research showed that “when the clinician sat, the patient felt like they listened more carefully, and they explained things in a better way that was much easier for them to understand. They definitely had an improved perception of their [clinician’s] communication skills.”
She highlighted another 2016 study that examined a Commit to Sit initiative in which nurses were urged to sit with patients during each shift. Nurse communication scores and overall patient experience scores went up.
The VA now has a Commit to Sit initiative, which urges clinicians to put away computers, smart phones, and pagers. “The patient feels that we’ve listened more intently to their concerns and care more about them as a patient,” Dr. Valdez-Boyle said. “We have an improved understanding of their health as a result of this. It allows the site employee to continue to be efficient while still delivering compassionate care and fosters trusted relationships in an empathetic and respectful manner.”
For more about the initiative, visit the VA PX SharePoint.
The VA, she said, also has a Take a Moment initiative that emphasizes eye contact, face-to-face interaction without electronics for at least the first 5 minutes of each visit, and seated conversations.
Dr. Valdez-Boyle also urged colleagues to pay attention to how often they interrupt. She pointed to a 2019 study that reported that patients had a median of 11 seconds—yes, seconds—to explain their problem in two-thirds of clinician encounters. “I think some of it is because we think we know what they're going to say.”
In the age of COVID-19, she suggested turning to fist or elbow bumps instead of handshakes. And she said, let patients wear street clothes when appropriate so they’re more comfortable.
In the big picture, she said, good communication and a commitment to shared decision making “really create a shared responsibility. They give your patients ownership over their disease and the ability to make the decisions with their team.
Dr. Valdez-Boyle reported no disclosures.
SAN DIEGO—Don’t stand when you talk at bedside. Ditch the white gowns, turn away from your computers and pagers, and stop yourself from interrupting all the time.
These tips—and more—can help clinicians provide better and more effective care, said a colorectal surgeon who spoke about communication skills at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Research has suggested that nearly half of Americans don’t think their health care practitioners (HCPs) are compassionate, “and that’s really sad,” said Lorene Valdez-Boyle, MD, MS, surgery chief at the New Mexico VA Health Care Service.
To combat this perception, she said, HCPs can adopt multiple strategies as they work with veterans and their families. The goal, she said, is “to try to get them to trust you and want to be part of their treatment. This is how we're going to have better outcomes.”
Some strategies are simple. Dr. Valdez-Boyle, for example, doesn’t wear a white gown when she sees patients. “Obviously, they’re really gross,” she said. “But also, I want them to be comfortable with me. I sit down at their level, and we have a conversation. We talk about our dogs and we bond, because that’s going to help them trust me and want to work with me. I do that with families too. We joke, and we laugh.”
Sitting bedside instead of standing is important, she said, and a 2016 study backs up this idea. “It’s difficult when you’re running around or you want to get to the next one, and the patient just keeps talking,” she said. But research showed that “when the clinician sat, the patient felt like they listened more carefully, and they explained things in a better way that was much easier for them to understand. They definitely had an improved perception of their [clinician’s] communication skills.”
She highlighted another 2016 study that examined a Commit to Sit initiative in which nurses were urged to sit with patients during each shift. Nurse communication scores and overall patient experience scores went up.
The VA now has a Commit to Sit initiative, which urges clinicians to put away computers, smart phones, and pagers. “The patient feels that we’ve listened more intently to their concerns and care more about them as a patient,” Dr. Valdez-Boyle said. “We have an improved understanding of their health as a result of this. It allows the site employee to continue to be efficient while still delivering compassionate care and fosters trusted relationships in an empathetic and respectful manner.”
For more about the initiative, visit the VA PX SharePoint.
The VA, she said, also has a Take a Moment initiative that emphasizes eye contact, face-to-face interaction without electronics for at least the first 5 minutes of each visit, and seated conversations.
Dr. Valdez-Boyle also urged colleagues to pay attention to how often they interrupt. She pointed to a 2019 study that reported that patients had a median of 11 seconds—yes, seconds—to explain their problem in two-thirds of clinician encounters. “I think some of it is because we think we know what they're going to say.”
In the age of COVID-19, she suggested turning to fist or elbow bumps instead of handshakes. And she said, let patients wear street clothes when appropriate so they’re more comfortable.
In the big picture, she said, good communication and a commitment to shared decision making “really create a shared responsibility. They give your patients ownership over their disease and the ability to make the decisions with their team.
Dr. Valdez-Boyle reported no disclosures.
SAN DIEGO—Don’t stand when you talk at bedside. Ditch the white gowns, turn away from your computers and pagers, and stop yourself from interrupting all the time.
These tips—and more—can help clinicians provide better and more effective care, said a colorectal surgeon who spoke about communication skills at the annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Research has suggested that nearly half of Americans don’t think their health care practitioners (HCPs) are compassionate, “and that’s really sad,” said Lorene Valdez-Boyle, MD, MS, surgery chief at the New Mexico VA Health Care Service.
To combat this perception, she said, HCPs can adopt multiple strategies as they work with veterans and their families. The goal, she said, is “to try to get them to trust you and want to be part of their treatment. This is how we're going to have better outcomes.”
Some strategies are simple. Dr. Valdez-Boyle, for example, doesn’t wear a white gown when she sees patients. “Obviously, they’re really gross,” she said. “But also, I want them to be comfortable with me. I sit down at their level, and we have a conversation. We talk about our dogs and we bond, because that’s going to help them trust me and want to work with me. I do that with families too. We joke, and we laugh.”
Sitting bedside instead of standing is important, she said, and a 2016 study backs up this idea. “It’s difficult when you’re running around or you want to get to the next one, and the patient just keeps talking,” she said. But research showed that “when the clinician sat, the patient felt like they listened more carefully, and they explained things in a better way that was much easier for them to understand. They definitely had an improved perception of their [clinician’s] communication skills.”
She highlighted another 2016 study that examined a Commit to Sit initiative in which nurses were urged to sit with patients during each shift. Nurse communication scores and overall patient experience scores went up.
The VA now has a Commit to Sit initiative, which urges clinicians to put away computers, smart phones, and pagers. “The patient feels that we’ve listened more intently to their concerns and care more about them as a patient,” Dr. Valdez-Boyle said. “We have an improved understanding of their health as a result of this. It allows the site employee to continue to be efficient while still delivering compassionate care and fosters trusted relationships in an empathetic and respectful manner.”
For more about the initiative, visit the VA PX SharePoint.
The VA, she said, also has a Take a Moment initiative that emphasizes eye contact, face-to-face interaction without electronics for at least the first 5 minutes of each visit, and seated conversations.
Dr. Valdez-Boyle also urged colleagues to pay attention to how often they interrupt. She pointed to a 2019 study that reported that patients had a median of 11 seconds—yes, seconds—to explain their problem in two-thirds of clinician encounters. “I think some of it is because we think we know what they're going to say.”
In the age of COVID-19, she suggested turning to fist or elbow bumps instead of handshakes. And she said, let patients wear street clothes when appropriate so they’re more comfortable.
In the big picture, she said, good communication and a commitment to shared decision making “really create a shared responsibility. They give your patients ownership over their disease and the ability to make the decisions with their team.
Dr. Valdez-Boyle reported no disclosures.
Health care workers face unimaginable decisions in Ukraine
The effects of the Russian invasion of Ukraine have been devastating, with the loss of tens of thousands of civilian lives and about one-third of the population having been displaced. The war has put a great strain on health care in the country.
“The Russian army is targeting civilian infrastructure, trying to plunge Ukrainians into cold and darkness. (It) is also deliberately targeting hospitals and clinics in Ukraine,” said Nataliya Kovalchuk, PhD, during a session on Ukraine health care at the annual meeting of the American Society for Radiation Oncology held this week in San Antonio. She is a clinical associate professor of radiation oncology at Stanford (Calif.) University.
Analysis of previous wars have shown an increase in cancer incidence and mortality, and the same should be expected in the future in Ukraine, according to Ruslan Zelinskyi, MS, who is president of the Ukrainian Association of Medical Physicists and practices at Spizhenko Clinic in Ukraine. “We must prepare for it now, and we do it. During the full-scale war in Ukraine, three new linear accelerators were installed and put into clinical use,” he said during his talk.
He also gave a personal perspective of the terrible conditions and choices facing Ukrainian health care workers. “I have often heard that science is outside of politics. Perhaps in peacetime, but when fragments of missiles pass through the wall” – here, Mr. Zelinskyi paused to swing the camera around to show bomb damage on the walls of the room where he was conducting his virtual talk – “it is impossible to be outside of politics when 900 medical facilities were destroyed or damaged.” Health care workers have been killed, and patients have lost months or years because they could not receive medical care.
That reality is forcing Ukrainians to make choices most health care workers would struggle to imagine. “For many years, I put on medical clothes and fight against cancer. But (for) me comes the moment when I will change my medical clothes to military (clothes), like many, many other Ukrainians of various professions, because we must preserve the freedom of Ukraine and the security of Europe to do science in a peaceful world,” Mr. Zelinskyi said.
Following Mr. Zelinskyi’s talk, Asya Agulnik, MD, MPH, director of the St. Jude’s Global Critical Care Program and a pediatric intensivist at St. Jude’s Children’s Research Hospital, Memphis, described St. Jude’s efforts with pediatric cancer patients. St. Jude’s Global Critical Care Program created a Eurasian Regional Program in 2018 to serve Central Asia and Eastern Europe, which now includes 48 institutions and more than 250 clinicians and members that help care for children with cancer.
In the aftermath of the invasion, the Eurasian Regional Program launched the Safer Ukraine network to ensure continued care for children with cancer and blood disorders. Initially, the patient’s family or physician identifies a child has having a medical need that cannot be met locally and requiring evacuation. The nongovernmental organization Tabletochki, which has worked with children with cancer in Ukraine since 2011, then arranges transport of the child and his or her medical records to the Ukrainian Specialized Children’s Center in Lviv, Ukraine, for evaluation and stabilization. From there, patients along with their mother and sometimes siblings are transferred to Poland. Transportation methods include ambulance, helicopter, bus, and medical trains that have a full intensive care unit aboard.
In Poland, hospitals estimated they could take on another 200 patients without compromising care for their existing patients, but evacuations reached that number within the first few weeks of the war. It was clear patients would have to be transferred to other countries. To do that, Safer Ukraine set up the Unicorn Marian Wilemski Clinic, which is a converted hotel operated as a partnership between many foundations and governments. It sends unstable patients to nearby emergency rooms, and stable patients stay at the clinic until they can be evaluated and matched with an international care provider from a registry of more than 200 hospitals in 29 countries that have agreed to take patients. A 24/7 Zoom call began on the first day of the war, initially staffed by St. Jude volunteers and now by a contractor. It serves as a command center.
Over the first 12 weeks of the war, the program evacuated over 1,000 children, and over 200 medical records per week were being translated at peak activity. Nearly 300 patients went to Poland, and the rest to 18 countries in Europe and North America.
The success of the initiative stems in part from the fact that it leveraged preexisting collaborations that focused on childhood cancer, according to Dr. Agulnik. “Many stakeholders that came together had previously worked together, but then quickly came together around this unified initiative with the goal of helping these patients,” she said during her talk.
Roman Kowalchuk, MD, who is a radiation oncology resident at Mayo Clinic, spoke about telemedicine efforts to assist physicians and patients in Ukraine.
“I’ve been especially interested in trying to help through telemedicine. We have so much expertise, so many things that we can offer, even if we can’t be there physically, through some of these avenues that really, especially through COVID, have been further developed in terms of virtual support, virtual expertise, and consultations. That presents the opportunity to be able to share some of that knowledge, some of that expertise, to help clinical care in Ukraine given the current circumstance,” said Dr. Kowalchuk during his presentation.
Efforts have used technology like the telemedicine platform Viveo, based in Estonia. One app, called HealUA, takes descriptions from physicians needing advice, and volunteers can scroll through inquiries and provide input. This is especially useful since most physicians in Ukraine are generalists, according to Dr. Kowalchuk, and so may need assistance with diagnosis and management of rare conditions.
Other telehealth approaches include TeleHelp Ukraine. There are WhatsApp groups with hundreds of volunteers who translate Ukrainian medical records to other languages to help non-Ukrainian physicians understand a patient’s history. Expertise is also needed in engineering, emergency medicine, surgery, various fields of oncology, and other specialties. Dr. Kowalchuk noted that Good Samaritan laws generally shield volunteers in these types of programs from legal liability.
International hospital networks taking part in these efforts include St. Jude, European Cancer Organization, American Cancer Society, American Society for Clinical Oncology, and ASTRO.
None of the presenters have relevant financial disclosures.
The effects of the Russian invasion of Ukraine have been devastating, with the loss of tens of thousands of civilian lives and about one-third of the population having been displaced. The war has put a great strain on health care in the country.
“The Russian army is targeting civilian infrastructure, trying to plunge Ukrainians into cold and darkness. (It) is also deliberately targeting hospitals and clinics in Ukraine,” said Nataliya Kovalchuk, PhD, during a session on Ukraine health care at the annual meeting of the American Society for Radiation Oncology held this week in San Antonio. She is a clinical associate professor of radiation oncology at Stanford (Calif.) University.
Analysis of previous wars have shown an increase in cancer incidence and mortality, and the same should be expected in the future in Ukraine, according to Ruslan Zelinskyi, MS, who is president of the Ukrainian Association of Medical Physicists and practices at Spizhenko Clinic in Ukraine. “We must prepare for it now, and we do it. During the full-scale war in Ukraine, three new linear accelerators were installed and put into clinical use,” he said during his talk.
He also gave a personal perspective of the terrible conditions and choices facing Ukrainian health care workers. “I have often heard that science is outside of politics. Perhaps in peacetime, but when fragments of missiles pass through the wall” – here, Mr. Zelinskyi paused to swing the camera around to show bomb damage on the walls of the room where he was conducting his virtual talk – “it is impossible to be outside of politics when 900 medical facilities were destroyed or damaged.” Health care workers have been killed, and patients have lost months or years because they could not receive medical care.
That reality is forcing Ukrainians to make choices most health care workers would struggle to imagine. “For many years, I put on medical clothes and fight against cancer. But (for) me comes the moment when I will change my medical clothes to military (clothes), like many, many other Ukrainians of various professions, because we must preserve the freedom of Ukraine and the security of Europe to do science in a peaceful world,” Mr. Zelinskyi said.
Following Mr. Zelinskyi’s talk, Asya Agulnik, MD, MPH, director of the St. Jude’s Global Critical Care Program and a pediatric intensivist at St. Jude’s Children’s Research Hospital, Memphis, described St. Jude’s efforts with pediatric cancer patients. St. Jude’s Global Critical Care Program created a Eurasian Regional Program in 2018 to serve Central Asia and Eastern Europe, which now includes 48 institutions and more than 250 clinicians and members that help care for children with cancer.
In the aftermath of the invasion, the Eurasian Regional Program launched the Safer Ukraine network to ensure continued care for children with cancer and blood disorders. Initially, the patient’s family or physician identifies a child has having a medical need that cannot be met locally and requiring evacuation. The nongovernmental organization Tabletochki, which has worked with children with cancer in Ukraine since 2011, then arranges transport of the child and his or her medical records to the Ukrainian Specialized Children’s Center in Lviv, Ukraine, for evaluation and stabilization. From there, patients along with their mother and sometimes siblings are transferred to Poland. Transportation methods include ambulance, helicopter, bus, and medical trains that have a full intensive care unit aboard.
In Poland, hospitals estimated they could take on another 200 patients without compromising care for their existing patients, but evacuations reached that number within the first few weeks of the war. It was clear patients would have to be transferred to other countries. To do that, Safer Ukraine set up the Unicorn Marian Wilemski Clinic, which is a converted hotel operated as a partnership between many foundations and governments. It sends unstable patients to nearby emergency rooms, and stable patients stay at the clinic until they can be evaluated and matched with an international care provider from a registry of more than 200 hospitals in 29 countries that have agreed to take patients. A 24/7 Zoom call began on the first day of the war, initially staffed by St. Jude volunteers and now by a contractor. It serves as a command center.
Over the first 12 weeks of the war, the program evacuated over 1,000 children, and over 200 medical records per week were being translated at peak activity. Nearly 300 patients went to Poland, and the rest to 18 countries in Europe and North America.
The success of the initiative stems in part from the fact that it leveraged preexisting collaborations that focused on childhood cancer, according to Dr. Agulnik. “Many stakeholders that came together had previously worked together, but then quickly came together around this unified initiative with the goal of helping these patients,” she said during her talk.
Roman Kowalchuk, MD, who is a radiation oncology resident at Mayo Clinic, spoke about telemedicine efforts to assist physicians and patients in Ukraine.
“I’ve been especially interested in trying to help through telemedicine. We have so much expertise, so many things that we can offer, even if we can’t be there physically, through some of these avenues that really, especially through COVID, have been further developed in terms of virtual support, virtual expertise, and consultations. That presents the opportunity to be able to share some of that knowledge, some of that expertise, to help clinical care in Ukraine given the current circumstance,” said Dr. Kowalchuk during his presentation.
Efforts have used technology like the telemedicine platform Viveo, based in Estonia. One app, called HealUA, takes descriptions from physicians needing advice, and volunteers can scroll through inquiries and provide input. This is especially useful since most physicians in Ukraine are generalists, according to Dr. Kowalchuk, and so may need assistance with diagnosis and management of rare conditions.
Other telehealth approaches include TeleHelp Ukraine. There are WhatsApp groups with hundreds of volunteers who translate Ukrainian medical records to other languages to help non-Ukrainian physicians understand a patient’s history. Expertise is also needed in engineering, emergency medicine, surgery, various fields of oncology, and other specialties. Dr. Kowalchuk noted that Good Samaritan laws generally shield volunteers in these types of programs from legal liability.
International hospital networks taking part in these efforts include St. Jude, European Cancer Organization, American Cancer Society, American Society for Clinical Oncology, and ASTRO.
None of the presenters have relevant financial disclosures.
The effects of the Russian invasion of Ukraine have been devastating, with the loss of tens of thousands of civilian lives and about one-third of the population having been displaced. The war has put a great strain on health care in the country.
“The Russian army is targeting civilian infrastructure, trying to plunge Ukrainians into cold and darkness. (It) is also deliberately targeting hospitals and clinics in Ukraine,” said Nataliya Kovalchuk, PhD, during a session on Ukraine health care at the annual meeting of the American Society for Radiation Oncology held this week in San Antonio. She is a clinical associate professor of radiation oncology at Stanford (Calif.) University.
Analysis of previous wars have shown an increase in cancer incidence and mortality, and the same should be expected in the future in Ukraine, according to Ruslan Zelinskyi, MS, who is president of the Ukrainian Association of Medical Physicists and practices at Spizhenko Clinic in Ukraine. “We must prepare for it now, and we do it. During the full-scale war in Ukraine, three new linear accelerators were installed and put into clinical use,” he said during his talk.
He also gave a personal perspective of the terrible conditions and choices facing Ukrainian health care workers. “I have often heard that science is outside of politics. Perhaps in peacetime, but when fragments of missiles pass through the wall” – here, Mr. Zelinskyi paused to swing the camera around to show bomb damage on the walls of the room where he was conducting his virtual talk – “it is impossible to be outside of politics when 900 medical facilities were destroyed or damaged.” Health care workers have been killed, and patients have lost months or years because they could not receive medical care.
That reality is forcing Ukrainians to make choices most health care workers would struggle to imagine. “For many years, I put on medical clothes and fight against cancer. But (for) me comes the moment when I will change my medical clothes to military (clothes), like many, many other Ukrainians of various professions, because we must preserve the freedom of Ukraine and the security of Europe to do science in a peaceful world,” Mr. Zelinskyi said.
Following Mr. Zelinskyi’s talk, Asya Agulnik, MD, MPH, director of the St. Jude’s Global Critical Care Program and a pediatric intensivist at St. Jude’s Children’s Research Hospital, Memphis, described St. Jude’s efforts with pediatric cancer patients. St. Jude’s Global Critical Care Program created a Eurasian Regional Program in 2018 to serve Central Asia and Eastern Europe, which now includes 48 institutions and more than 250 clinicians and members that help care for children with cancer.
In the aftermath of the invasion, the Eurasian Regional Program launched the Safer Ukraine network to ensure continued care for children with cancer and blood disorders. Initially, the patient’s family or physician identifies a child has having a medical need that cannot be met locally and requiring evacuation. The nongovernmental organization Tabletochki, which has worked with children with cancer in Ukraine since 2011, then arranges transport of the child and his or her medical records to the Ukrainian Specialized Children’s Center in Lviv, Ukraine, for evaluation and stabilization. From there, patients along with their mother and sometimes siblings are transferred to Poland. Transportation methods include ambulance, helicopter, bus, and medical trains that have a full intensive care unit aboard.
In Poland, hospitals estimated they could take on another 200 patients without compromising care for their existing patients, but evacuations reached that number within the first few weeks of the war. It was clear patients would have to be transferred to other countries. To do that, Safer Ukraine set up the Unicorn Marian Wilemski Clinic, which is a converted hotel operated as a partnership between many foundations and governments. It sends unstable patients to nearby emergency rooms, and stable patients stay at the clinic until they can be evaluated and matched with an international care provider from a registry of more than 200 hospitals in 29 countries that have agreed to take patients. A 24/7 Zoom call began on the first day of the war, initially staffed by St. Jude volunteers and now by a contractor. It serves as a command center.
Over the first 12 weeks of the war, the program evacuated over 1,000 children, and over 200 medical records per week were being translated at peak activity. Nearly 300 patients went to Poland, and the rest to 18 countries in Europe and North America.
The success of the initiative stems in part from the fact that it leveraged preexisting collaborations that focused on childhood cancer, according to Dr. Agulnik. “Many stakeholders that came together had previously worked together, but then quickly came together around this unified initiative with the goal of helping these patients,” she said during her talk.
Roman Kowalchuk, MD, who is a radiation oncology resident at Mayo Clinic, spoke about telemedicine efforts to assist physicians and patients in Ukraine.
“I’ve been especially interested in trying to help through telemedicine. We have so much expertise, so many things that we can offer, even if we can’t be there physically, through some of these avenues that really, especially through COVID, have been further developed in terms of virtual support, virtual expertise, and consultations. That presents the opportunity to be able to share some of that knowledge, some of that expertise, to help clinical care in Ukraine given the current circumstance,” said Dr. Kowalchuk during his presentation.
Efforts have used technology like the telemedicine platform Viveo, based in Estonia. One app, called HealUA, takes descriptions from physicians needing advice, and volunteers can scroll through inquiries and provide input. This is especially useful since most physicians in Ukraine are generalists, according to Dr. Kowalchuk, and so may need assistance with diagnosis and management of rare conditions.
Other telehealth approaches include TeleHelp Ukraine. There are WhatsApp groups with hundreds of volunteers who translate Ukrainian medical records to other languages to help non-Ukrainian physicians understand a patient’s history. Expertise is also needed in engineering, emergency medicine, surgery, various fields of oncology, and other specialties. Dr. Kowalchuk noted that Good Samaritan laws generally shield volunteers in these types of programs from legal liability.
International hospital networks taking part in these efforts include St. Jude, European Cancer Organization, American Cancer Society, American Society for Clinical Oncology, and ASTRO.
None of the presenters have relevant financial disclosures.
FROM ASTRO 2022
Antibiotic may enhance noninvasive brain stimulation for depression
“The take-home message is that this proof-of-concept study opens up a new avenue of treatment research so that in the future, we may be able to provide our patients with safe and well-tolerated medications and enhance noninvasive brain stimulation treatments for depression,” senior author Alexander McGirr, MD, PhD, assistant professor of psychiatry, University of Calgary (Alta.), told this news organization.
“Once the safety and efficacy of this strategy have been confirmed with larger multisite studies, this could be deployed within existing health care infrastructure,” he said.
The study was published online in JAMA Psychiatry.
Synaptic plasticity
Repetitive transmagnetic stimulation (rTMS) and the more recently developed intermittent theta-burst stimulation (iTBS) are noninvasive brain stimulation modalities that have the largest evidence base in improving MDD. Although efficacious, an “unacceptable proportion of patients do not significantly improve” with these approaches, the authors write.
“We believe that iTBS improves depression through a process called synaptic plasticity, or how neurons adapt to stimulation, but we know that synaptic plasticity is impacted by the illness,” Dr. McGirr explained. This “could be the reason that only some patients benefit.”
One potential strategy to enhance neuroplasticity is to administer an adjunctive N-methyl D-aspartate (NMDA) receptor agonist during stimulation, since the NMDA receptor is a “key regulator of synaptic plasticity,” the authors state. In fact, synaptic plasticity with continuous and intermittent TBS is NMDA-receptor–dependent.
“DCS is an NMDA receptor partial agonist, and so at the low dose we used in our trial (100 mg), it can facilitate NMDA receptor signaling. The hypothesis was that pairing it with iTBS would enhance synaptic plasticity and clinical outcomes,” Dr. McGirr said.
The group’s previous research demonstrated that targeting the NMDA receptor with low-dose DCS “normalizes long-term motor cortex plasticity in individuals with MDD.” It also led to greater persistence of iTBS-induced changes compared to placebo.
However, “a demonstration that these physiological effects have an impact on treatment outcomes is lacking,” the authors note.
To address this gap, the researchers conducted a 4-week double-blind, placebo-controlled trial in which 50 participants (mean [standard deviation] age, 40.8 [13.4] years; 62% women) were randomly assigned on a 1:1 basis to receive either iTBS plus DCS or iTBS plus placebo (n = 25 per group) for the first 2 weeks of the trial, followed by iTBS without an adjunct for the third and fourth weeks.
Participants were required to be experiencing a major depressive episode and to have failed to respond to at least one adequate antidepressant trial or psychotherapy (but not more than four adequate antidepressant trials during the current episode).
Patients with acute suicidality, psychosis, recent substance use disorder, benzodiazepine use, seizures, unstable medical conditions, history of nonresponse to rTMS or electroconvulsive therapy, or comorbid psychiatric conditions, as well as those for whom psychotherapy was initiated within 3 months of enrollment or during the trial, were excluded.
Depression was measured by the Montgomery-Åsberg Depression Rating Scale (MADRS) (changes in score constituted the primary outcome) and the 17-item Hamilton Depression Rating Scale (17-HDRS).
“Secondary outcomes included clinical response, clinical remission, and Clinical Global Impression (CGI) scores,” the authors state.
“Promising” findings
Most participants in the iTBS plus placebo group were White (80%); 12% were Asian, and 8% were classified as “other.” A smaller proportion of participants in the iTBS plus DCS group were White (68%); the next smallest group was Asian (16%), followed by Hispanic (12%), and “other” (4%).
Participants presented with moderate-severe depressive symptoms, as measured by both the HRDS-17 and the MADRS. The placebo and intervention groups had similar scores at baseline. Resting motor threshold did not differ significantly between the groups, either at baseline or between the weeks with and without adjunctive treatment.
Greater improvements in MADRS scores were found in the intervention group than in the placebo groups (mean difference, –6.15 [95% confidence interval, –2.43 to –9.88]; Hedges g, 0.99 [0.34-1.62]).
A larger treatment effect was found after 4 weeks of treatment than after 2 weeks, although the adjuvant was present for the first 2 weeks. “We speculate that, despite ongoing iTBS, this reflects an erosion of the placebo effect, as 15 of 25 participants (60%) in the iTBS plus placebo group plateaued or had a worsening MADRS score, compared with 9 of 25 participants (36%) in the iTBS plus DCS group,” the authors write.
The intervention group showed higher rates of clinical response compared to the placebo group (73.9% vs. 29.3%, respectively), as well as higher rates of clinical remission (39.1% vs. 4.2%, respectively), as reflected in lower CGI-severity ratings and greater CGI-improvement ratings.
There were no serious adverse events during the trial.
The authors note several limitations, including the small sample size and the fact that participants received the adjunctive treatment for only 2 weeks. Longer treatment courses “require dedicated study.” And the short length of the trial (only 4 weeks) meant the difference between “treatment acceleration” and “treatment enhancement” could not be determined.
Nevertheless, the results are “promising” and suggest additional investigation into “intersectional approaches with other dosing regimens and precision medicine targeting approaches,” the authors state.
Synergistic approach
Commenting on the study, Scott Aaronson, MD, chief science officer, Institute for Advanced Diagnostics and Therapeutics, Sheppard Pratt, Towson, Md., called the findings “heartening.” He noted that the study “demonstrates a creative approach of combining an FDA-approved antibiotic with NMDA partial agonist activity – D-cycloserine – with a brief course of iTBS with the aim of enhancing the neuronal plasticity iTBS creates.”
Dr. Aaronson, who is also an adjunct professor at the University of Maryland, Baltimore, and was not involved with the study, added, “This is an early demonstration of the ability to further exploit neuronal changes from neurostimulation by synergistic use of a pharmacologic intervention.”
The study was supported in part by a Young Investigator Award from the Brain and Behavior Research Foundation and the Campus Alberta Innovates Program Chair in Neurostimulation. Dr. McGirr has a patent for PCT/CA2022/050839 pending with MCGRx Corp and is a shareholder of MCGRx Corp. The other authors’ disclosures are listed on the original article. Dr. Aaronson is a consultant for Neuronetics.
A version of this article first appeared on Medscape.com.
“The take-home message is that this proof-of-concept study opens up a new avenue of treatment research so that in the future, we may be able to provide our patients with safe and well-tolerated medications and enhance noninvasive brain stimulation treatments for depression,” senior author Alexander McGirr, MD, PhD, assistant professor of psychiatry, University of Calgary (Alta.), told this news organization.
“Once the safety and efficacy of this strategy have been confirmed with larger multisite studies, this could be deployed within existing health care infrastructure,” he said.
The study was published online in JAMA Psychiatry.
Synaptic plasticity
Repetitive transmagnetic stimulation (rTMS) and the more recently developed intermittent theta-burst stimulation (iTBS) are noninvasive brain stimulation modalities that have the largest evidence base in improving MDD. Although efficacious, an “unacceptable proportion of patients do not significantly improve” with these approaches, the authors write.
“We believe that iTBS improves depression through a process called synaptic plasticity, or how neurons adapt to stimulation, but we know that synaptic plasticity is impacted by the illness,” Dr. McGirr explained. This “could be the reason that only some patients benefit.”
One potential strategy to enhance neuroplasticity is to administer an adjunctive N-methyl D-aspartate (NMDA) receptor agonist during stimulation, since the NMDA receptor is a “key regulator of synaptic plasticity,” the authors state. In fact, synaptic plasticity with continuous and intermittent TBS is NMDA-receptor–dependent.
“DCS is an NMDA receptor partial agonist, and so at the low dose we used in our trial (100 mg), it can facilitate NMDA receptor signaling. The hypothesis was that pairing it with iTBS would enhance synaptic plasticity and clinical outcomes,” Dr. McGirr said.
The group’s previous research demonstrated that targeting the NMDA receptor with low-dose DCS “normalizes long-term motor cortex plasticity in individuals with MDD.” It also led to greater persistence of iTBS-induced changes compared to placebo.
However, “a demonstration that these physiological effects have an impact on treatment outcomes is lacking,” the authors note.
To address this gap, the researchers conducted a 4-week double-blind, placebo-controlled trial in which 50 participants (mean [standard deviation] age, 40.8 [13.4] years; 62% women) were randomly assigned on a 1:1 basis to receive either iTBS plus DCS or iTBS plus placebo (n = 25 per group) for the first 2 weeks of the trial, followed by iTBS without an adjunct for the third and fourth weeks.
Participants were required to be experiencing a major depressive episode and to have failed to respond to at least one adequate antidepressant trial or psychotherapy (but not more than four adequate antidepressant trials during the current episode).
Patients with acute suicidality, psychosis, recent substance use disorder, benzodiazepine use, seizures, unstable medical conditions, history of nonresponse to rTMS or electroconvulsive therapy, or comorbid psychiatric conditions, as well as those for whom psychotherapy was initiated within 3 months of enrollment or during the trial, were excluded.
Depression was measured by the Montgomery-Åsberg Depression Rating Scale (MADRS) (changes in score constituted the primary outcome) and the 17-item Hamilton Depression Rating Scale (17-HDRS).
“Secondary outcomes included clinical response, clinical remission, and Clinical Global Impression (CGI) scores,” the authors state.
“Promising” findings
Most participants in the iTBS plus placebo group were White (80%); 12% were Asian, and 8% were classified as “other.” A smaller proportion of participants in the iTBS plus DCS group were White (68%); the next smallest group was Asian (16%), followed by Hispanic (12%), and “other” (4%).
Participants presented with moderate-severe depressive symptoms, as measured by both the HRDS-17 and the MADRS. The placebo and intervention groups had similar scores at baseline. Resting motor threshold did not differ significantly between the groups, either at baseline or between the weeks with and without adjunctive treatment.
Greater improvements in MADRS scores were found in the intervention group than in the placebo groups (mean difference, –6.15 [95% confidence interval, –2.43 to –9.88]; Hedges g, 0.99 [0.34-1.62]).
A larger treatment effect was found after 4 weeks of treatment than after 2 weeks, although the adjuvant was present for the first 2 weeks. “We speculate that, despite ongoing iTBS, this reflects an erosion of the placebo effect, as 15 of 25 participants (60%) in the iTBS plus placebo group plateaued or had a worsening MADRS score, compared with 9 of 25 participants (36%) in the iTBS plus DCS group,” the authors write.
The intervention group showed higher rates of clinical response compared to the placebo group (73.9% vs. 29.3%, respectively), as well as higher rates of clinical remission (39.1% vs. 4.2%, respectively), as reflected in lower CGI-severity ratings and greater CGI-improvement ratings.
There were no serious adverse events during the trial.
The authors note several limitations, including the small sample size and the fact that participants received the adjunctive treatment for only 2 weeks. Longer treatment courses “require dedicated study.” And the short length of the trial (only 4 weeks) meant the difference between “treatment acceleration” and “treatment enhancement” could not be determined.
Nevertheless, the results are “promising” and suggest additional investigation into “intersectional approaches with other dosing regimens and precision medicine targeting approaches,” the authors state.
Synergistic approach
Commenting on the study, Scott Aaronson, MD, chief science officer, Institute for Advanced Diagnostics and Therapeutics, Sheppard Pratt, Towson, Md., called the findings “heartening.” He noted that the study “demonstrates a creative approach of combining an FDA-approved antibiotic with NMDA partial agonist activity – D-cycloserine – with a brief course of iTBS with the aim of enhancing the neuronal plasticity iTBS creates.”
Dr. Aaronson, who is also an adjunct professor at the University of Maryland, Baltimore, and was not involved with the study, added, “This is an early demonstration of the ability to further exploit neuronal changes from neurostimulation by synergistic use of a pharmacologic intervention.”
The study was supported in part by a Young Investigator Award from the Brain and Behavior Research Foundation and the Campus Alberta Innovates Program Chair in Neurostimulation. Dr. McGirr has a patent for PCT/CA2022/050839 pending with MCGRx Corp and is a shareholder of MCGRx Corp. The other authors’ disclosures are listed on the original article. Dr. Aaronson is a consultant for Neuronetics.
A version of this article first appeared on Medscape.com.
“The take-home message is that this proof-of-concept study opens up a new avenue of treatment research so that in the future, we may be able to provide our patients with safe and well-tolerated medications and enhance noninvasive brain stimulation treatments for depression,” senior author Alexander McGirr, MD, PhD, assistant professor of psychiatry, University of Calgary (Alta.), told this news organization.
“Once the safety and efficacy of this strategy have been confirmed with larger multisite studies, this could be deployed within existing health care infrastructure,” he said.
The study was published online in JAMA Psychiatry.
Synaptic plasticity
Repetitive transmagnetic stimulation (rTMS) and the more recently developed intermittent theta-burst stimulation (iTBS) are noninvasive brain stimulation modalities that have the largest evidence base in improving MDD. Although efficacious, an “unacceptable proportion of patients do not significantly improve” with these approaches, the authors write.
“We believe that iTBS improves depression through a process called synaptic plasticity, or how neurons adapt to stimulation, but we know that synaptic plasticity is impacted by the illness,” Dr. McGirr explained. This “could be the reason that only some patients benefit.”
One potential strategy to enhance neuroplasticity is to administer an adjunctive N-methyl D-aspartate (NMDA) receptor agonist during stimulation, since the NMDA receptor is a “key regulator of synaptic plasticity,” the authors state. In fact, synaptic plasticity with continuous and intermittent TBS is NMDA-receptor–dependent.
“DCS is an NMDA receptor partial agonist, and so at the low dose we used in our trial (100 mg), it can facilitate NMDA receptor signaling. The hypothesis was that pairing it with iTBS would enhance synaptic plasticity and clinical outcomes,” Dr. McGirr said.
The group’s previous research demonstrated that targeting the NMDA receptor with low-dose DCS “normalizes long-term motor cortex plasticity in individuals with MDD.” It also led to greater persistence of iTBS-induced changes compared to placebo.
However, “a demonstration that these physiological effects have an impact on treatment outcomes is lacking,” the authors note.
To address this gap, the researchers conducted a 4-week double-blind, placebo-controlled trial in which 50 participants (mean [standard deviation] age, 40.8 [13.4] years; 62% women) were randomly assigned on a 1:1 basis to receive either iTBS plus DCS or iTBS plus placebo (n = 25 per group) for the first 2 weeks of the trial, followed by iTBS without an adjunct for the third and fourth weeks.
Participants were required to be experiencing a major depressive episode and to have failed to respond to at least one adequate antidepressant trial or psychotherapy (but not more than four adequate antidepressant trials during the current episode).
Patients with acute suicidality, psychosis, recent substance use disorder, benzodiazepine use, seizures, unstable medical conditions, history of nonresponse to rTMS or electroconvulsive therapy, or comorbid psychiatric conditions, as well as those for whom psychotherapy was initiated within 3 months of enrollment or during the trial, were excluded.
Depression was measured by the Montgomery-Åsberg Depression Rating Scale (MADRS) (changes in score constituted the primary outcome) and the 17-item Hamilton Depression Rating Scale (17-HDRS).
“Secondary outcomes included clinical response, clinical remission, and Clinical Global Impression (CGI) scores,” the authors state.
“Promising” findings
Most participants in the iTBS plus placebo group were White (80%); 12% were Asian, and 8% were classified as “other.” A smaller proportion of participants in the iTBS plus DCS group were White (68%); the next smallest group was Asian (16%), followed by Hispanic (12%), and “other” (4%).
Participants presented with moderate-severe depressive symptoms, as measured by both the HRDS-17 and the MADRS. The placebo and intervention groups had similar scores at baseline. Resting motor threshold did not differ significantly between the groups, either at baseline or between the weeks with and without adjunctive treatment.
Greater improvements in MADRS scores were found in the intervention group than in the placebo groups (mean difference, –6.15 [95% confidence interval, –2.43 to –9.88]; Hedges g, 0.99 [0.34-1.62]).
A larger treatment effect was found after 4 weeks of treatment than after 2 weeks, although the adjuvant was present for the first 2 weeks. “We speculate that, despite ongoing iTBS, this reflects an erosion of the placebo effect, as 15 of 25 participants (60%) in the iTBS plus placebo group plateaued or had a worsening MADRS score, compared with 9 of 25 participants (36%) in the iTBS plus DCS group,” the authors write.
The intervention group showed higher rates of clinical response compared to the placebo group (73.9% vs. 29.3%, respectively), as well as higher rates of clinical remission (39.1% vs. 4.2%, respectively), as reflected in lower CGI-severity ratings and greater CGI-improvement ratings.
There were no serious adverse events during the trial.
The authors note several limitations, including the small sample size and the fact that participants received the adjunctive treatment for only 2 weeks. Longer treatment courses “require dedicated study.” And the short length of the trial (only 4 weeks) meant the difference between “treatment acceleration” and “treatment enhancement” could not be determined.
Nevertheless, the results are “promising” and suggest additional investigation into “intersectional approaches with other dosing regimens and precision medicine targeting approaches,” the authors state.
Synergistic approach
Commenting on the study, Scott Aaronson, MD, chief science officer, Institute for Advanced Diagnostics and Therapeutics, Sheppard Pratt, Towson, Md., called the findings “heartening.” He noted that the study “demonstrates a creative approach of combining an FDA-approved antibiotic with NMDA partial agonist activity – D-cycloserine – with a brief course of iTBS with the aim of enhancing the neuronal plasticity iTBS creates.”
Dr. Aaronson, who is also an adjunct professor at the University of Maryland, Baltimore, and was not involved with the study, added, “This is an early demonstration of the ability to further exploit neuronal changes from neurostimulation by synergistic use of a pharmacologic intervention.”
The study was supported in part by a Young Investigator Award from the Brain and Behavior Research Foundation and the Campus Alberta Innovates Program Chair in Neurostimulation. Dr. McGirr has a patent for PCT/CA2022/050839 pending with MCGRx Corp and is a shareholder of MCGRx Corp. The other authors’ disclosures are listed on the original article. Dr. Aaronson is a consultant for Neuronetics.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
IgA Vasculitis in the Setting of Biologic Therapy for Psoriasis and Recurrent Cutaneous Methicillin-Resistant Staphylococcus aureus Colonization
Case Report
A 47-year-old man presented with a sudden-onset rash consisting of red bumps on the abdomen and legs that had been ongoing for several days. He had known psoriasis and psoriatic arthritis that had been well controlled with adalimumab for the last 18 months. He reported concurrent onset of nausea but denied fevers, chills, night sweats, unintentional weight loss, abdominal pain, and pruritus. He endorsed prior cutaneous infections of methicillin-resistant Staphylococcus aureus (MRSA). His medical history also included diabetes mellitus, hypertension, and obesity. His other medications included oral losartan-hydrochlorothiazide, amlodipine, naproxen, and atorvastatin.
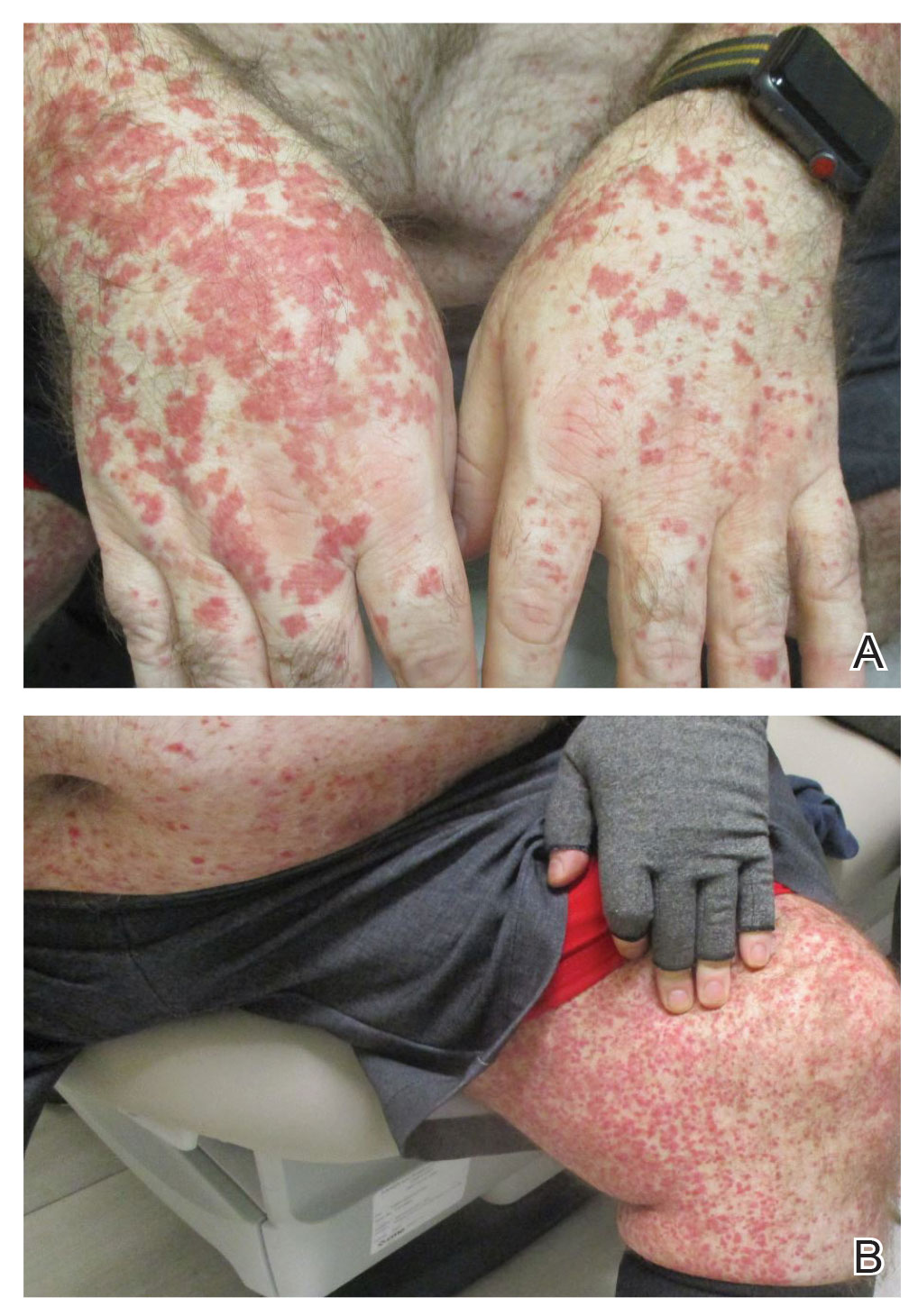
Physical examination revealed numerous thin purpuric papules—some with adherent scale—distributed on the lower legs, extensor forearms, and abdomen. Abdominal lesions were confined to weight-related striae (Figure 1). The palms, soles, oral mucosa, and face were spared. Three punch biopsies were performed, including 1 for direct immunofluorescence (DIF), and the patient was instructed to apply clobetasol to the affected areas twice daily until further notice.
Pathology showed perivascular extravasation of erythrocytes, neutrophils, eosinophils, and leukocytoclasis surrounding blood vessels associated with fibrin (Figure 2). Direct immunofluorescence showed granular deposition of IgA, complement component 3, and fibrinogen in a superficial dermal vascular pattern (Figure 3). These results were consistent with IgA small-vessel vasculitis. One specimen was consistent with the patient’s known psoriasis.
Urinalysis revealed moderate hemoglobinuria, and urine microscopy showed 174 red blood cells per high-power field. Creatinine was high at 1.87 mg/dL (reference range, <1.34 mg/dL; patient’s baseline, 0.81 mg/dL) and glomerular filtration rate was low (42 mL/min, patient’s baseline, >60 mL/min [reference range, 90–120 mL/min]). Erythrocyte sedimentation rate (21 mm/h [reference range, 0–22 mm/h]) and C-reactive protein were elevated (2.2 mg/dL [reference range, 0.3–1.0 mg/dL]). Given his history of cutaneous MRSA infections, a bacterial culture swab was collected from the skin surface to check for colonization, which showed moderate growth of MRSA. Naproxen was discontinued over concern of worsening the patient’s renal status. The patient was instructed to rest at home with his legs elevated, wear compression socks when ambulatory, use chlorhexidine antiseptic daily as a body wash when showering, and apply mupirocin three times daily to the biopsy sites. He was referred to urology for his microhematuria, where cystoscopy revealed no abnormalities.A month passed with no improvement of the patient’s cutaneous vasculitis, and his psoriatic arthritis worsened without his usual use of naproxen. He developed abdominal pain and loss of appetite. A prednisone taper was ordered starting at 40 mg/d (28.8 mg/kg), which provided relief of the skin and joint symptoms only until the course was completed 12 days later.
Five weeks after the initial presentation, the patient returned with a more severe eruption consisting of innumerable purpuric papules that coalesced in plaques on the abdomen, arms, and legs. He also had erythematous facial pustules and mild palmar petechiae (Figure 4). Three biopsies were performed, including 1 for DIF and 1 from a pustule on the forehead. Histology and DIF were again consistent with IgA small-vessel vasculitis. The forehead biopsy was compatible with steroid acne (attributed to recent prednisone use) and psoriasis.
Rheumatology was consulted, and adalimumab was discontinued 6 weeks after the initial presentation out of concern for drug-induced cutaneous vasculitis. Vasculitis work-up was unremarkable, including antineutrophil cytoplasmic antibodies, rheumatoid factor, cyclic citrullinated peptide, and serum protein electrophoresis. Oral dapsone was started at 100 mg/d, with the tentative plan of starting secukinumab if cutaneous symptoms improved. For 3 weeks, the patient’s cutaneous symptoms steadily improved.
Nine weeks after initial presentation to dermatology (3 weeks after discontinuing adalimumab) the patient self-administered his first dose of secukinumab at home. Several hours later, he reported sudden reappearance of vasculitis. He denied diarrhea, abdominal pain, bowel movement urgency, fevers, fatigue, and unintentional weight loss. Antistreptolysin O and hepatitis A antibodies were negative. He was instructed to hold secukinumab indefinitely.
Four weeks after his only secukinumab injection, the patient reported another episode of acute worsening cutaneous symptoms. A 4-week prednisone taper starting at 40 mg/d was ordered. Computed tomography of the chest, abdomen, and pelvis to rule out internal malignancy was unremarkable. Around this time, the patient reported major emotional distress related to an unexpected death in his family, which added to a gradual increase in his stress level related to the COVID-19 pandemic.
Three weeks later, dapsone was increased to 100 mg twice daily on account of the patient’s adiposity and lack of cutaneous improvement on the lower dose. Subsequently, the vasculitis rapidly improved for 2 weeks. The patient then reported symptoms of headache, dizziness, and chills. He was tested for COVID-19 and was negative. Six weeks after increasing the dapsone dose (5 months after initial presentation), the skin was normalizing, showing only faintly hyperpigmented macules confined to areas of resolved vasculitis (forearms, abdomen, legs).
The patient had been on dapsone 100 mg twice daily for 3 months when he was started on ustekinumab (90 mg at weeks 0 and 4, with planned doses every 12 weeks) for psoriatic arthritis in hopes of withdrawing dapsone. His cutaneous symptoms have remained well controlled on this regimen for 18 months. Lowering of dapsone below 100 mg daily has resulted in recurrent mild vasculitis symptoms; he now maintains the once-daily dosing without negative side effects.
Comment
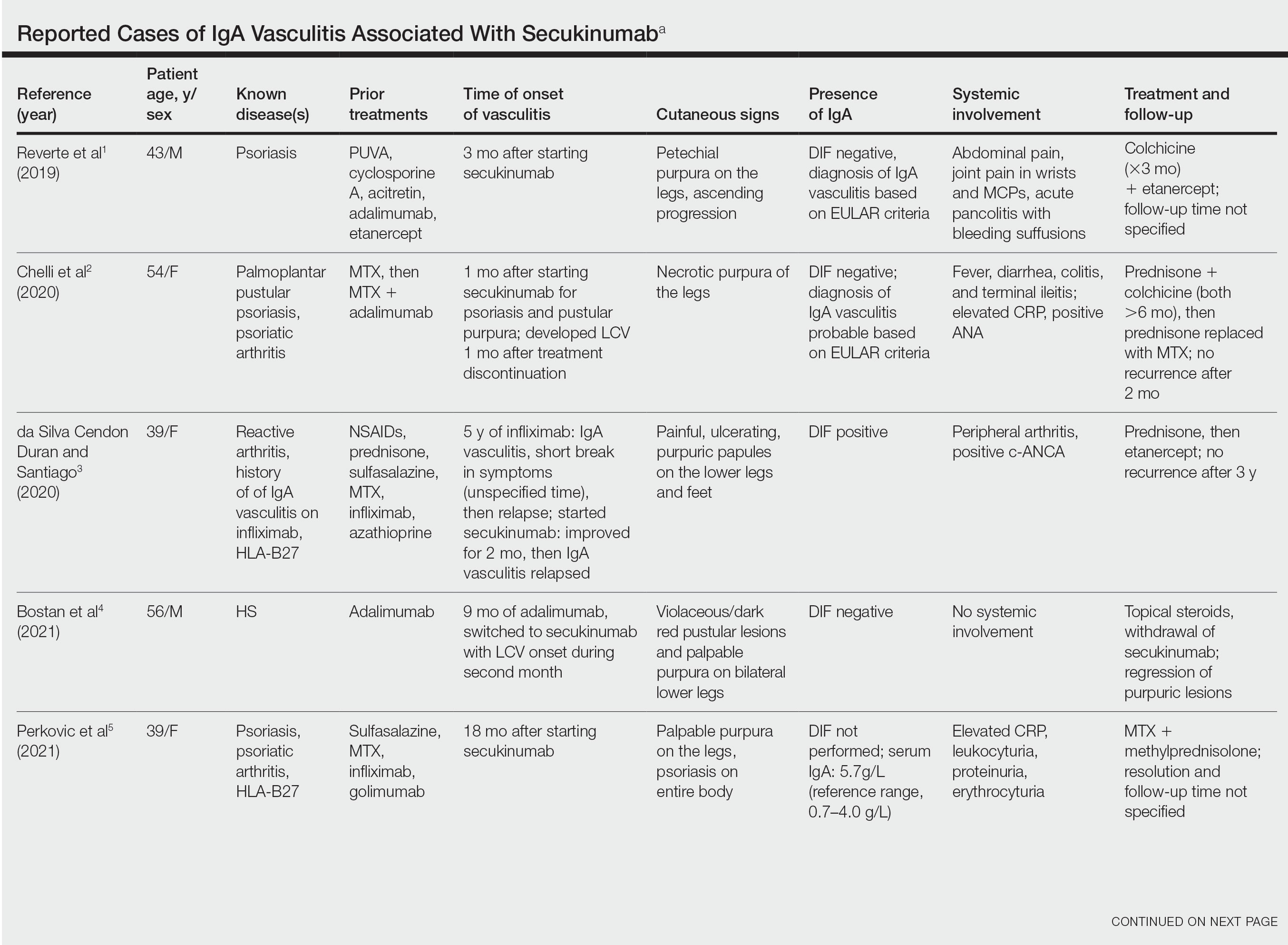
IgA vasculitis is a form of cutaneous small-vessel leukocytoclastic vasculitis (LCV) characterized by episodes of palpable purpura on the extensor surfaces of the arms and legs that may be associated with arthritis, abdominal pain, and/or hematuria. Although vasculitis is a known potential adverse effect of anti–tumor necrosis factor (TNF) α therapy, cases of adalimumab-induced IgA vasculitis are uncommon. As use of more targeted therapies for psoriasis and psoriatic arthritis, such as the IL-17 inhibitor secukinumab, increases so do reports of associated adverse events. Of 6 previously reported cases of secukinumab-associated vasculitis, at least 4 were IgA vasculitis (Table).1-6 Another case described one patient with rheumatoid arthritis undergoing secukinumab treatment who experienced necrotizing glomerulonephritis; however, the authors concluded secukinumab likely was not causative in that case, as serologies and urinalyses suggested gradual onset of the process prior to initiating the medication.7
The exact pathogenesis of IgA vasculitis is unclear, but a prevailing theory involves the dysregulation of IgA synthesis and metabolism. Other than increased serum levels of transforming growth factor β, which is a major stimulating factor for IgA production, it also has been hypothesized that the presence of aberrantly hypoglycosylated IgA exposes an autoepitope for recognition by other pathogenic IgG and IgA, leading to the formation of large immune complexes that can readily deposit in postcapillary venules. The deposition of IgA immune complexes in postcapillary venules and the subsequent activation of the complement system causes direct damage to the endothelial cells of vessel walls. This complement activation is evidenced by vascular complement component 3 deposition on DIF (a nonspecific feature of LCV). Chemotaxis of neutrophils ensues, followed by their firm adherence and transendothelial migration (mediated by monocyte chemoattractant protein 1 [MCP-1]). Neutrophil degranulation releases reactive oxygen species and cytokines, which in turn recruit additional leukocytes to the area of inflammation, subsequently undergoing degeneration (leukocytoclasis). Microvascular permeability also is enhanced by MCP-1, allowing exudation of serum, erythrocytes, and fibrin. In the setting of elevated circulating TNF and IL-1, endothelium is stimulated to activate the intrinsic and extrinsic coagulation pathways. This decreases endothelial fibrinolytic activity, leading to thrombosis. The high venous pressure and low fibrinolytic activity in the lower legs explains why vasculitic lesions often are confined to or begin in this distribution.1,8-10
There also are noteworthy roles for cytokines in LCV. Circulating transforming growth factor β and IL-6—which are necessary for development of T helper 17 (TH17) cells and production of IL-17—are higher in patients with LCV compared to controls. Peripheral blood monocytes in patients with LCV demonstrate higher production of IL-17. Once TH17 cells develop, their survival and phenotype are maintained by IL-23 (considered the master regulator of TH17 differentiation). IL-17 is a potent chemoattractant of IL-8 (CXCL8) and MCP-1, both of which promote neutrophil-mediated perivascular inflammation. The IL-23 and IL-17 pathways implicated in the pathogenesis of psoriasis also cause neutrophil activation and upregulate transcription of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α), which overlap with those implicated in LCV. Autoimmune disease generally entails some positive feedback loop of progressively severe self-recognition and tissue destruction by the immune system. These shared cytokinetic processes may explain how the internal environment of psoriasis could perpetuate IgA vasculitis.1,2,8,10-12
The mechanisms underlying vasculitis associated with adalimumab are unclear, but hypotheses involve direct toxicity on vessels, capillary deposition of anti-TNF/TNF immune complexes, or an inflammatory process resulting in autoantibodies. Similar hypotheses are posited for secukinumab-associated vasculitis, including deposition of secukinumab–IL-17 complexes. Anti–TNF-α medications may increase TH17 cell numbers, leading to increased production of IL-22 and a resultant immunologic microenvironment conducive to vasculitis. All 6 published cases of secukinumab-associated vasculitis that we found had received prior treatment with a TNF-α blocker, but only 1 had occurrence of vasculitis during that treatment.1-6,10
In the 6 cases we reviewed, the time from starting secukinumab to onset of vasculitis ranged from 1 to 18 months. Our patient’s same-day re-emergence of vasculitis after his first secukinumab dose was so acute that we were skeptical of secukinumab as a potential trigger; this may simply have been coincident to the natural waxing and waning of the vasculitis (although onset of IgA vasculitis within 1 day of starting anti–TNF-α therapy has been reported).1-6,13
Specific associations of IgA vasculitis are many and can include bacterial organisms such as Helicobacter pylori, streptococci, and staphylococci. Although internal mucous membrane infections are considered more linked because of the surveillance role of IgA predominantly in mucosal tissues, it is possible that our patient with cutaneous MRSA harbored the same within the nasal mucosa. Our patient also received multiple vaccinations outside our department throughout his clinical course (2 hepatitis B and 1 pneumococcal conjugate), which are known potential triggers for vasculitis. Psychological stress is a known trigger for psoriasis, and given the cytokinetic relationship of psoriasis to vasculitis described previously, it may have indirectly contributed to vasculitis in our case. The anxiety associated with being immunosuppressed during the COVID-19 pandemic and bereavement of losing a family member may have contributed to the refractory nature of our patient’s condition. Renal involvement is relatively common in adults with IgA vasculitis and so should be ruled out, as should occult internal malignancy.8,10,14
It is unclear which of the above factors was causative in our case, but a multifactorial process is likely. Treatment of monoclonal antibody–associated vasculitis entails investigating for triggers and systemic involvement, removing the most likely culprit, quelling the vasculitis acutely, avoiding known potential exacerbators, and introducing an alternative long-term immunomodulant. In all 6 reported similar cases, discontinuation of secukinumab and initiation of prednisone or colchicine led to resolution.1-6 Dapsone also is acceptable for acute control of IgA vasculitis, although this medication is highly lipid soluble and penetrates well into various tissues.15 Thus, lower doses may prove ineffective for obese patients, as was demonstrated in our case. Given the known potential of vaccinations, infections, and other factors (eg, alcohol, penicillin) to trigger IgA vasculitis, these should be avoided.10
Blockade of IL-23 with ustekinumab has been suggested by other authors encountering secukinumab-associated vasculitis, as IL-23 is the main driver and sustainer of TH17 cell differentiation.8 Although 6 previously reported cases of secukinumab-associated vasculitis achieved resolution without long-term recurrence, none did so using an IL-23 inhibitor (nor had any of the described patients received IL-23 inhibitors previously).1-6 Given the established safety of IL-23 inhibitors and that they theoretically are well suited for this unique circumstance (by ceasing the main causative cytokine cascades “upstream”) and were efficacious in quickly resolving our patient’s vasculitis, we suggest that ustekinumab may represent
- Reverte M, Etienne M, Fouchard M, et al. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. J Eur Acad Dermatol Venereol. 2019;33:E455-E457.
- Chelli C, Loget J, Vanhaecke C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. Acta Derm Venereol. 2020;100:adv00077.
- da Silva Cendon Duran C, Santiago MB. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815.
- Bostan E, Gulseren D, Yalici-Armagan B, et al. Vasculitis during certolizumab pegol and secukinumab treatment: report of two cases. Dermatol Ther. 2021;34:E15007.
- Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071-2073.
- Villani A, DE Fata Salvatores G, Nappa P, et al. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. Ital J Dermatol Venerol. 2021;156(suppl 1 to no. 6):9-10.
- Góis M, Messias A, Carvalho D, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58-62.
- Jen HY, Chuang YH, Lin SC, et al. Increased serum interleukin-17 and peripheral Th17 cells in children with acute Henoch-Schönlein purpura. Pediatr Allergy Immunol. 2011;22:862-868.
- Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017;97:1160-1166.
- Weedon D. The vasculopathic reaction pattern. In: Houston M, Davie B, eds. Weedon’s Skin Pathology. 3rd ed. Elsevier Limited; 2010:207-211.
- Puig L. Paradoxical reactions: anti-TNFα ants, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol. 2018;53:49-63.
- Nestle F, Kaplan D, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNFα therapy: a fortuitous event or an indication to stop therapy? Eur J Dermatol. 2017;27:304-305.
- Hello CL, Cohen P, Bousser MG, et al. Suspected hepatitis B vaccination related vasculitis. J Rheumatol. 1999;26:191-194.
- Wolverton SE. Dapsone. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier, Inc; 2021:222-231.
Case Report
A 47-year-old man presented with a sudden-onset rash consisting of red bumps on the abdomen and legs that had been ongoing for several days. He had known psoriasis and psoriatic arthritis that had been well controlled with adalimumab for the last 18 months. He reported concurrent onset of nausea but denied fevers, chills, night sweats, unintentional weight loss, abdominal pain, and pruritus. He endorsed prior cutaneous infections of methicillin-resistant Staphylococcus aureus (MRSA). His medical history also included diabetes mellitus, hypertension, and obesity. His other medications included oral losartan-hydrochlorothiazide, amlodipine, naproxen, and atorvastatin.
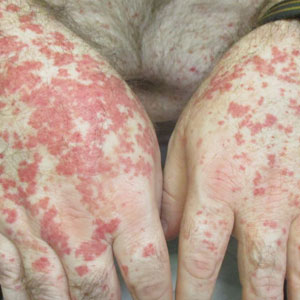
Physical examination revealed numerous thin purpuric papules—some with adherent scale—distributed on the lower legs, extensor forearms, and abdomen. Abdominal lesions were confined to weight-related striae (Figure 1). The palms, soles, oral mucosa, and face were spared. Three punch biopsies were performed, including 1 for direct immunofluorescence (DIF), and the patient was instructed to apply clobetasol to the affected areas twice daily until further notice.

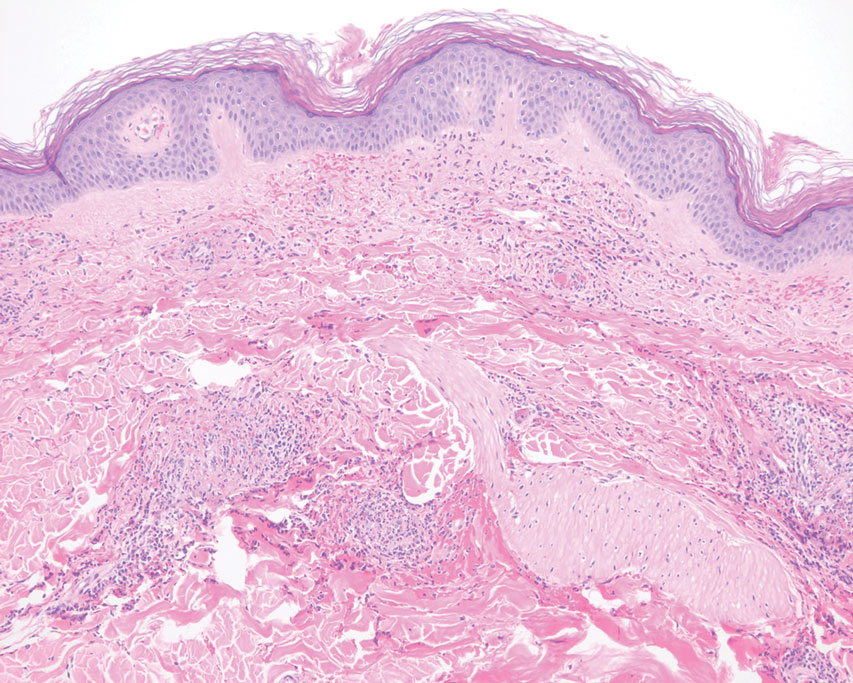
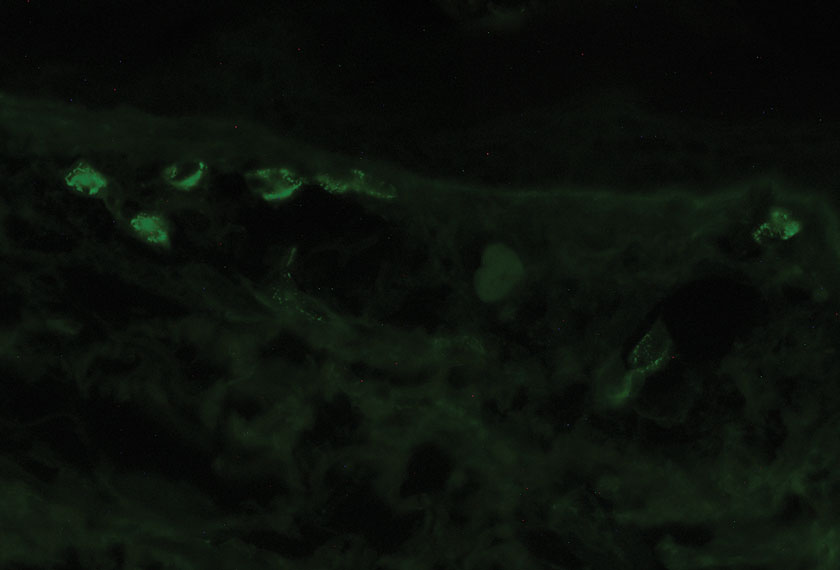
Pathology showed perivascular extravasation of erythrocytes, neutrophils, eosinophils, and leukocytoclasis surrounding blood vessels associated with fibrin (Figure 2). Direct immunofluorescence showed granular deposition of IgA, complement component 3, and fibrinogen in a superficial dermal vascular pattern (Figure 3). These results were consistent with IgA small-vessel vasculitis. One specimen was consistent with the patient’s known psoriasis.

Urinalysis revealed moderate hemoglobinuria, and urine microscopy showed 174 red blood cells per high-power field. Creatinine was high at 1.87 mg/dL (reference range, <1.34 mg/dL; patient’s baseline, 0.81 mg/dL) and glomerular filtration rate was low (42 mL/min, patient’s baseline, >60 mL/min [reference range, 90–120 mL/min]). Erythrocyte sedimentation rate (21 mm/h [reference range, 0–22 mm/h]) and C-reactive protein were elevated (2.2 mg/dL [reference range, 0.3–1.0 mg/dL]). Given his history of cutaneous MRSA infections, a bacterial culture swab was collected from the skin surface to check for colonization, which showed moderate growth of MRSA. Naproxen was discontinued over concern of worsening the patient’s renal status. The patient was instructed to rest at home with his legs elevated, wear compression socks when ambulatory, use chlorhexidine antiseptic daily as a body wash when showering, and apply mupirocin three times daily to the biopsy sites. He was referred to urology for his microhematuria, where cystoscopy revealed no abnormalities.A month passed with no improvement of the patient’s cutaneous vasculitis, and his psoriatic arthritis worsened without his usual use of naproxen. He developed abdominal pain and loss of appetite. A prednisone taper was ordered starting at 40 mg/d (28.8 mg/kg), which provided relief of the skin and joint symptoms only until the course was completed 12 days later.

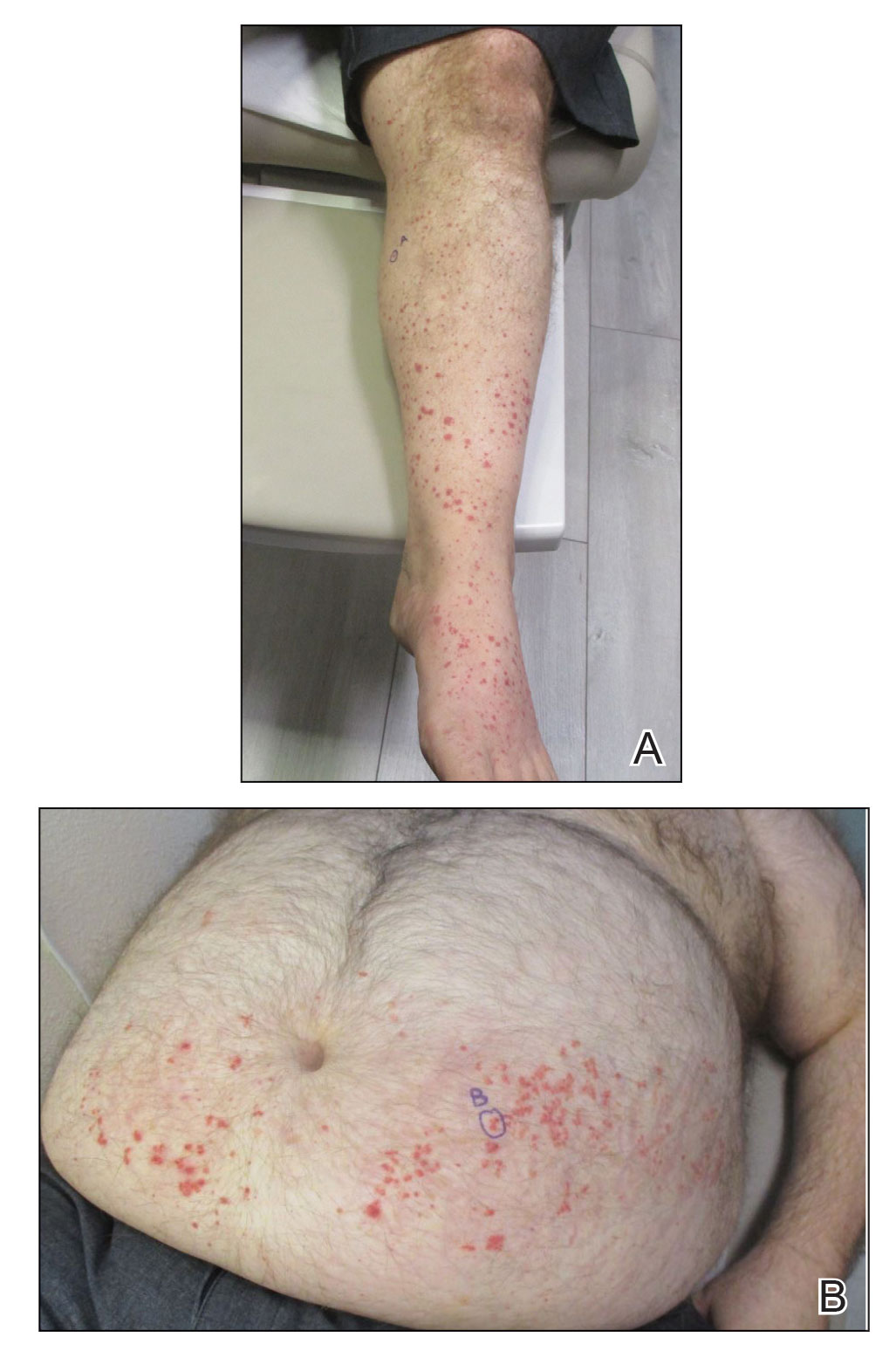
Five weeks after the initial presentation, the patient returned with a more severe eruption consisting of innumerable purpuric papules that coalesced in plaques on the abdomen, arms, and legs. He also had erythematous facial pustules and mild palmar petechiae (Figure 4). Three biopsies were performed, including 1 for DIF and 1 from a pustule on the forehead. Histology and DIF were again consistent with IgA small-vessel vasculitis. The forehead biopsy was compatible with steroid acne (attributed to recent prednisone use) and psoriasis.

Rheumatology was consulted, and adalimumab was discontinued 6 weeks after the initial presentation out of concern for drug-induced cutaneous vasculitis. Vasculitis work-up was unremarkable, including antineutrophil cytoplasmic antibodies, rheumatoid factor, cyclic citrullinated peptide, and serum protein electrophoresis. Oral dapsone was started at 100 mg/d, with the tentative plan of starting secukinumab if cutaneous symptoms improved. For 3 weeks, the patient’s cutaneous symptoms steadily improved.
Nine weeks after initial presentation to dermatology (3 weeks after discontinuing adalimumab) the patient self-administered his first dose of secukinumab at home. Several hours later, he reported sudden reappearance of vasculitis. He denied diarrhea, abdominal pain, bowel movement urgency, fevers, fatigue, and unintentional weight loss. Antistreptolysin O and hepatitis A antibodies were negative. He was instructed to hold secukinumab indefinitely.
Four weeks after his only secukinumab injection, the patient reported another episode of acute worsening cutaneous symptoms. A 4-week prednisone taper starting at 40 mg/d was ordered. Computed tomography of the chest, abdomen, and pelvis to rule out internal malignancy was unremarkable. Around this time, the patient reported major emotional distress related to an unexpected death in his family, which added to a gradual increase in his stress level related to the COVID-19 pandemic.
Three weeks later, dapsone was increased to 100 mg twice daily on account of the patient’s adiposity and lack of cutaneous improvement on the lower dose. Subsequently, the vasculitis rapidly improved for 2 weeks. The patient then reported symptoms of headache, dizziness, and chills. He was tested for COVID-19 and was negative. Six weeks after increasing the dapsone dose (5 months after initial presentation), the skin was normalizing, showing only faintly hyperpigmented macules confined to areas of resolved vasculitis (forearms, abdomen, legs).
The patient had been on dapsone 100 mg twice daily for 3 months when he was started on ustekinumab (90 mg at weeks 0 and 4, with planned doses every 12 weeks) for psoriatic arthritis in hopes of withdrawing dapsone. His cutaneous symptoms have remained well controlled on this regimen for 18 months. Lowering of dapsone below 100 mg daily has resulted in recurrent mild vasculitis symptoms; he now maintains the once-daily dosing without negative side effects.
Comment
IgA vasculitis is a form of cutaneous small-vessel leukocytoclastic vasculitis (LCV) characterized by episodes of palpable purpura on the extensor surfaces of the arms and legs that may be associated with arthritis, abdominal pain, and/or hematuria. Although vasculitis is a known potential adverse effect of anti–tumor necrosis factor (TNF) α therapy, cases of adalimumab-induced IgA vasculitis are uncommon. As use of more targeted therapies for psoriasis and psoriatic arthritis, such as the IL-17 inhibitor secukinumab, increases so do reports of associated adverse events. Of 6 previously reported cases of secukinumab-associated vasculitis, at least 4 were IgA vasculitis (Table).1-6 Another case described one patient with rheumatoid arthritis undergoing secukinumab treatment who experienced necrotizing glomerulonephritis; however, the authors concluded secukinumab likely was not causative in that case, as serologies and urinalyses suggested gradual onset of the process prior to initiating the medication.7

The exact pathogenesis of IgA vasculitis is unclear, but a prevailing theory involves the dysregulation of IgA synthesis and metabolism. Other than increased serum levels of transforming growth factor β, which is a major stimulating factor for IgA production, it also has been hypothesized that the presence of aberrantly hypoglycosylated IgA exposes an autoepitope for recognition by other pathogenic IgG and IgA, leading to the formation of large immune complexes that can readily deposit in postcapillary venules. The deposition of IgA immune complexes in postcapillary venules and the subsequent activation of the complement system causes direct damage to the endothelial cells of vessel walls. This complement activation is evidenced by vascular complement component 3 deposition on DIF (a nonspecific feature of LCV). Chemotaxis of neutrophils ensues, followed by their firm adherence and transendothelial migration (mediated by monocyte chemoattractant protein 1 [MCP-1]). Neutrophil degranulation releases reactive oxygen species and cytokines, which in turn recruit additional leukocytes to the area of inflammation, subsequently undergoing degeneration (leukocytoclasis). Microvascular permeability also is enhanced by MCP-1, allowing exudation of serum, erythrocytes, and fibrin. In the setting of elevated circulating TNF and IL-1, endothelium is stimulated to activate the intrinsic and extrinsic coagulation pathways. This decreases endothelial fibrinolytic activity, leading to thrombosis. The high venous pressure and low fibrinolytic activity in the lower legs explains why vasculitic lesions often are confined to or begin in this distribution.1,8-10

There also are noteworthy roles for cytokines in LCV. Circulating transforming growth factor β and IL-6—which are necessary for development of T helper 17 (TH17) cells and production of IL-17—are higher in patients with LCV compared to controls. Peripheral blood monocytes in patients with LCV demonstrate higher production of IL-17. Once TH17 cells develop, their survival and phenotype are maintained by IL-23 (considered the master regulator of TH17 differentiation). IL-17 is a potent chemoattractant of IL-8 (CXCL8) and MCP-1, both of which promote neutrophil-mediated perivascular inflammation. The IL-23 and IL-17 pathways implicated in the pathogenesis of psoriasis also cause neutrophil activation and upregulate transcription of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α), which overlap with those implicated in LCV. Autoimmune disease generally entails some positive feedback loop of progressively severe self-recognition and tissue destruction by the immune system. These shared cytokinetic processes may explain how the internal environment of psoriasis could perpetuate IgA vasculitis.1,2,8,10-12
The mechanisms underlying vasculitis associated with adalimumab are unclear, but hypotheses involve direct toxicity on vessels, capillary deposition of anti-TNF/TNF immune complexes, or an inflammatory process resulting in autoantibodies. Similar hypotheses are posited for secukinumab-associated vasculitis, including deposition of secukinumab–IL-17 complexes. Anti–TNF-α medications may increase TH17 cell numbers, leading to increased production of IL-22 and a resultant immunologic microenvironment conducive to vasculitis. All 6 published cases of secukinumab-associated vasculitis that we found had received prior treatment with a TNF-α blocker, but only 1 had occurrence of vasculitis during that treatment.1-6,10
In the 6 cases we reviewed, the time from starting secukinumab to onset of vasculitis ranged from 1 to 18 months. Our patient’s same-day re-emergence of vasculitis after his first secukinumab dose was so acute that we were skeptical of secukinumab as a potential trigger; this may simply have been coincident to the natural waxing and waning of the vasculitis (although onset of IgA vasculitis within 1 day of starting anti–TNF-α therapy has been reported).1-6,13
Specific associations of IgA vasculitis are many and can include bacterial organisms such as Helicobacter pylori, streptococci, and staphylococci. Although internal mucous membrane infections are considered more linked because of the surveillance role of IgA predominantly in mucosal tissues, it is possible that our patient with cutaneous MRSA harbored the same within the nasal mucosa. Our patient also received multiple vaccinations outside our department throughout his clinical course (2 hepatitis B and 1 pneumococcal conjugate), which are known potential triggers for vasculitis. Psychological stress is a known trigger for psoriasis, and given the cytokinetic relationship of psoriasis to vasculitis described previously, it may have indirectly contributed to vasculitis in our case. The anxiety associated with being immunosuppressed during the COVID-19 pandemic and bereavement of losing a family member may have contributed to the refractory nature of our patient’s condition. Renal involvement is relatively common in adults with IgA vasculitis and so should be ruled out, as should occult internal malignancy.8,10,14
It is unclear which of the above factors was causative in our case, but a multifactorial process is likely. Treatment of monoclonal antibody–associated vasculitis entails investigating for triggers and systemic involvement, removing the most likely culprit, quelling the vasculitis acutely, avoiding known potential exacerbators, and introducing an alternative long-term immunomodulant. In all 6 reported similar cases, discontinuation of secukinumab and initiation of prednisone or colchicine led to resolution.1-6 Dapsone also is acceptable for acute control of IgA vasculitis, although this medication is highly lipid soluble and penetrates well into various tissues.15 Thus, lower doses may prove ineffective for obese patients, as was demonstrated in our case. Given the known potential of vaccinations, infections, and other factors (eg, alcohol, penicillin) to trigger IgA vasculitis, these should be avoided.10
Blockade of IL-23 with ustekinumab has been suggested by other authors encountering secukinumab-associated vasculitis, as IL-23 is the main driver and sustainer of TH17 cell differentiation.8 Although 6 previously reported cases of secukinumab-associated vasculitis achieved resolution without long-term recurrence, none did so using an IL-23 inhibitor (nor had any of the described patients received IL-23 inhibitors previously).1-6 Given the established safety of IL-23 inhibitors and that they theoretically are well suited for this unique circumstance (by ceasing the main causative cytokine cascades “upstream”) and were efficacious in quickly resolving our patient’s vasculitis, we suggest that ustekinumab may represent
Case Report
A 47-year-old man presented with a sudden-onset rash consisting of red bumps on the abdomen and legs that had been ongoing for several days. He had known psoriasis and psoriatic arthritis that had been well controlled with adalimumab for the last 18 months. He reported concurrent onset of nausea but denied fevers, chills, night sweats, unintentional weight loss, abdominal pain, and pruritus. He endorsed prior cutaneous infections of methicillin-resistant Staphylococcus aureus (MRSA). His medical history also included diabetes mellitus, hypertension, and obesity. His other medications included oral losartan-hydrochlorothiazide, amlodipine, naproxen, and atorvastatin.
Physical examination revealed numerous thin purpuric papules—some with adherent scale—distributed on the lower legs, extensor forearms, and abdomen. Abdominal lesions were confined to weight-related striae (Figure 1). The palms, soles, oral mucosa, and face were spared. Three punch biopsies were performed, including 1 for direct immunofluorescence (DIF), and the patient was instructed to apply clobetasol to the affected areas twice daily until further notice.

Pathology showed perivascular extravasation of erythrocytes, neutrophils, eosinophils, and leukocytoclasis surrounding blood vessels associated with fibrin (Figure 2). Direct immunofluorescence showed granular deposition of IgA, complement component 3, and fibrinogen in a superficial dermal vascular pattern (Figure 3). These results were consistent with IgA small-vessel vasculitis. One specimen was consistent with the patient’s known psoriasis.

Urinalysis revealed moderate hemoglobinuria, and urine microscopy showed 174 red blood cells per high-power field. Creatinine was high at 1.87 mg/dL (reference range, <1.34 mg/dL; patient’s baseline, 0.81 mg/dL) and glomerular filtration rate was low (42 mL/min, patient’s baseline, >60 mL/min [reference range, 90–120 mL/min]). Erythrocyte sedimentation rate (21 mm/h [reference range, 0–22 mm/h]) and C-reactive protein were elevated (2.2 mg/dL [reference range, 0.3–1.0 mg/dL]). Given his history of cutaneous MRSA infections, a bacterial culture swab was collected from the skin surface to check for colonization, which showed moderate growth of MRSA. Naproxen was discontinued over concern of worsening the patient’s renal status. The patient was instructed to rest at home with his legs elevated, wear compression socks when ambulatory, use chlorhexidine antiseptic daily as a body wash when showering, and apply mupirocin three times daily to the biopsy sites. He was referred to urology for his microhematuria, where cystoscopy revealed no abnormalities.A month passed with no improvement of the patient’s cutaneous vasculitis, and his psoriatic arthritis worsened without his usual use of naproxen. He developed abdominal pain and loss of appetite. A prednisone taper was ordered starting at 40 mg/d (28.8 mg/kg), which provided relief of the skin and joint symptoms only until the course was completed 12 days later.

Five weeks after the initial presentation, the patient returned with a more severe eruption consisting of innumerable purpuric papules that coalesced in plaques on the abdomen, arms, and legs. He also had erythematous facial pustules and mild palmar petechiae (Figure 4). Three biopsies were performed, including 1 for DIF and 1 from a pustule on the forehead. Histology and DIF were again consistent with IgA small-vessel vasculitis. The forehead biopsy was compatible with steroid acne (attributed to recent prednisone use) and psoriasis.

Rheumatology was consulted, and adalimumab was discontinued 6 weeks after the initial presentation out of concern for drug-induced cutaneous vasculitis. Vasculitis work-up was unremarkable, including antineutrophil cytoplasmic antibodies, rheumatoid factor, cyclic citrullinated peptide, and serum protein electrophoresis. Oral dapsone was started at 100 mg/d, with the tentative plan of starting secukinumab if cutaneous symptoms improved. For 3 weeks, the patient’s cutaneous symptoms steadily improved.
Nine weeks after initial presentation to dermatology (3 weeks after discontinuing adalimumab) the patient self-administered his first dose of secukinumab at home. Several hours later, he reported sudden reappearance of vasculitis. He denied diarrhea, abdominal pain, bowel movement urgency, fevers, fatigue, and unintentional weight loss. Antistreptolysin O and hepatitis A antibodies were negative. He was instructed to hold secukinumab indefinitely.
Four weeks after his only secukinumab injection, the patient reported another episode of acute worsening cutaneous symptoms. A 4-week prednisone taper starting at 40 mg/d was ordered. Computed tomography of the chest, abdomen, and pelvis to rule out internal malignancy was unremarkable. Around this time, the patient reported major emotional distress related to an unexpected death in his family, which added to a gradual increase in his stress level related to the COVID-19 pandemic.
Three weeks later, dapsone was increased to 100 mg twice daily on account of the patient’s adiposity and lack of cutaneous improvement on the lower dose. Subsequently, the vasculitis rapidly improved for 2 weeks. The patient then reported symptoms of headache, dizziness, and chills. He was tested for COVID-19 and was negative. Six weeks after increasing the dapsone dose (5 months after initial presentation), the skin was normalizing, showing only faintly hyperpigmented macules confined to areas of resolved vasculitis (forearms, abdomen, legs).
The patient had been on dapsone 100 mg twice daily for 3 months when he was started on ustekinumab (90 mg at weeks 0 and 4, with planned doses every 12 weeks) for psoriatic arthritis in hopes of withdrawing dapsone. His cutaneous symptoms have remained well controlled on this regimen for 18 months. Lowering of dapsone below 100 mg daily has resulted in recurrent mild vasculitis symptoms; he now maintains the once-daily dosing without negative side effects.
Comment
IgA vasculitis is a form of cutaneous small-vessel leukocytoclastic vasculitis (LCV) characterized by episodes of palpable purpura on the extensor surfaces of the arms and legs that may be associated with arthritis, abdominal pain, and/or hematuria. Although vasculitis is a known potential adverse effect of anti–tumor necrosis factor (TNF) α therapy, cases of adalimumab-induced IgA vasculitis are uncommon. As use of more targeted therapies for psoriasis and psoriatic arthritis, such as the IL-17 inhibitor secukinumab, increases so do reports of associated adverse events. Of 6 previously reported cases of secukinumab-associated vasculitis, at least 4 were IgA vasculitis (Table).1-6 Another case described one patient with rheumatoid arthritis undergoing secukinumab treatment who experienced necrotizing glomerulonephritis; however, the authors concluded secukinumab likely was not causative in that case, as serologies and urinalyses suggested gradual onset of the process prior to initiating the medication.7

The exact pathogenesis of IgA vasculitis is unclear, but a prevailing theory involves the dysregulation of IgA synthesis and metabolism. Other than increased serum levels of transforming growth factor β, which is a major stimulating factor for IgA production, it also has been hypothesized that the presence of aberrantly hypoglycosylated IgA exposes an autoepitope for recognition by other pathogenic IgG and IgA, leading to the formation of large immune complexes that can readily deposit in postcapillary venules. The deposition of IgA immune complexes in postcapillary venules and the subsequent activation of the complement system causes direct damage to the endothelial cells of vessel walls. This complement activation is evidenced by vascular complement component 3 deposition on DIF (a nonspecific feature of LCV). Chemotaxis of neutrophils ensues, followed by their firm adherence and transendothelial migration (mediated by monocyte chemoattractant protein 1 [MCP-1]). Neutrophil degranulation releases reactive oxygen species and cytokines, which in turn recruit additional leukocytes to the area of inflammation, subsequently undergoing degeneration (leukocytoclasis). Microvascular permeability also is enhanced by MCP-1, allowing exudation of serum, erythrocytes, and fibrin. In the setting of elevated circulating TNF and IL-1, endothelium is stimulated to activate the intrinsic and extrinsic coagulation pathways. This decreases endothelial fibrinolytic activity, leading to thrombosis. The high venous pressure and low fibrinolytic activity in the lower legs explains why vasculitic lesions often are confined to or begin in this distribution.1,8-10

There also are noteworthy roles for cytokines in LCV. Circulating transforming growth factor β and IL-6—which are necessary for development of T helper 17 (TH17) cells and production of IL-17—are higher in patients with LCV compared to controls. Peripheral blood monocytes in patients with LCV demonstrate higher production of IL-17. Once TH17 cells develop, their survival and phenotype are maintained by IL-23 (considered the master regulator of TH17 differentiation). IL-17 is a potent chemoattractant of IL-8 (CXCL8) and MCP-1, both of which promote neutrophil-mediated perivascular inflammation. The IL-23 and IL-17 pathways implicated in the pathogenesis of psoriasis also cause neutrophil activation and upregulate transcription of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α), which overlap with those implicated in LCV. Autoimmune disease generally entails some positive feedback loop of progressively severe self-recognition and tissue destruction by the immune system. These shared cytokinetic processes may explain how the internal environment of psoriasis could perpetuate IgA vasculitis.1,2,8,10-12
The mechanisms underlying vasculitis associated with adalimumab are unclear, but hypotheses involve direct toxicity on vessels, capillary deposition of anti-TNF/TNF immune complexes, or an inflammatory process resulting in autoantibodies. Similar hypotheses are posited for secukinumab-associated vasculitis, including deposition of secukinumab–IL-17 complexes. Anti–TNF-α medications may increase TH17 cell numbers, leading to increased production of IL-22 and a resultant immunologic microenvironment conducive to vasculitis. All 6 published cases of secukinumab-associated vasculitis that we found had received prior treatment with a TNF-α blocker, but only 1 had occurrence of vasculitis during that treatment.1-6,10
In the 6 cases we reviewed, the time from starting secukinumab to onset of vasculitis ranged from 1 to 18 months. Our patient’s same-day re-emergence of vasculitis after his first secukinumab dose was so acute that we were skeptical of secukinumab as a potential trigger; this may simply have been coincident to the natural waxing and waning of the vasculitis (although onset of IgA vasculitis within 1 day of starting anti–TNF-α therapy has been reported).1-6,13
Specific associations of IgA vasculitis are many and can include bacterial organisms such as Helicobacter pylori, streptococci, and staphylococci. Although internal mucous membrane infections are considered more linked because of the surveillance role of IgA predominantly in mucosal tissues, it is possible that our patient with cutaneous MRSA harbored the same within the nasal mucosa. Our patient also received multiple vaccinations outside our department throughout his clinical course (2 hepatitis B and 1 pneumococcal conjugate), which are known potential triggers for vasculitis. Psychological stress is a known trigger for psoriasis, and given the cytokinetic relationship of psoriasis to vasculitis described previously, it may have indirectly contributed to vasculitis in our case. The anxiety associated with being immunosuppressed during the COVID-19 pandemic and bereavement of losing a family member may have contributed to the refractory nature of our patient’s condition. Renal involvement is relatively common in adults with IgA vasculitis and so should be ruled out, as should occult internal malignancy.8,10,14
It is unclear which of the above factors was causative in our case, but a multifactorial process is likely. Treatment of monoclonal antibody–associated vasculitis entails investigating for triggers and systemic involvement, removing the most likely culprit, quelling the vasculitis acutely, avoiding known potential exacerbators, and introducing an alternative long-term immunomodulant. In all 6 reported similar cases, discontinuation of secukinumab and initiation of prednisone or colchicine led to resolution.1-6 Dapsone also is acceptable for acute control of IgA vasculitis, although this medication is highly lipid soluble and penetrates well into various tissues.15 Thus, lower doses may prove ineffective for obese patients, as was demonstrated in our case. Given the known potential of vaccinations, infections, and other factors (eg, alcohol, penicillin) to trigger IgA vasculitis, these should be avoided.10
Blockade of IL-23 with ustekinumab has been suggested by other authors encountering secukinumab-associated vasculitis, as IL-23 is the main driver and sustainer of TH17 cell differentiation.8 Although 6 previously reported cases of secukinumab-associated vasculitis achieved resolution without long-term recurrence, none did so using an IL-23 inhibitor (nor had any of the described patients received IL-23 inhibitors previously).1-6 Given the established safety of IL-23 inhibitors and that they theoretically are well suited for this unique circumstance (by ceasing the main causative cytokine cascades “upstream”) and were efficacious in quickly resolving our patient’s vasculitis, we suggest that ustekinumab may represent
- Reverte M, Etienne M, Fouchard M, et al. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. J Eur Acad Dermatol Venereol. 2019;33:E455-E457.
- Chelli C, Loget J, Vanhaecke C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. Acta Derm Venereol. 2020;100:adv00077.
- da Silva Cendon Duran C, Santiago MB. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815.
- Bostan E, Gulseren D, Yalici-Armagan B, et al. Vasculitis during certolizumab pegol and secukinumab treatment: report of two cases. Dermatol Ther. 2021;34:E15007.
- Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071-2073.
- Villani A, DE Fata Salvatores G, Nappa P, et al. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. Ital J Dermatol Venerol. 2021;156(suppl 1 to no. 6):9-10.
- Góis M, Messias A, Carvalho D, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58-62.
- Jen HY, Chuang YH, Lin SC, et al. Increased serum interleukin-17 and peripheral Th17 cells in children with acute Henoch-Schönlein purpura. Pediatr Allergy Immunol. 2011;22:862-868.
- Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017;97:1160-1166.
- Weedon D. The vasculopathic reaction pattern. In: Houston M, Davie B, eds. Weedon’s Skin Pathology. 3rd ed. Elsevier Limited; 2010:207-211.
- Puig L. Paradoxical reactions: anti-TNFα ants, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol. 2018;53:49-63.
- Nestle F, Kaplan D, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNFα therapy: a fortuitous event or an indication to stop therapy? Eur J Dermatol. 2017;27:304-305.
- Hello CL, Cohen P, Bousser MG, et al. Suspected hepatitis B vaccination related vasculitis. J Rheumatol. 1999;26:191-194.
- Wolverton SE. Dapsone. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier, Inc; 2021:222-231.
- Reverte M, Etienne M, Fouchard M, et al. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. J Eur Acad Dermatol Venereol. 2019;33:E455-E457.
- Chelli C, Loget J, Vanhaecke C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. Acta Derm Venereol. 2020;100:adv00077.
- da Silva Cendon Duran C, Santiago MB. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815.
- Bostan E, Gulseren D, Yalici-Armagan B, et al. Vasculitis during certolizumab pegol and secukinumab treatment: report of two cases. Dermatol Ther. 2021;34:E15007.
- Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071-2073.
- Villani A, DE Fata Salvatores G, Nappa P, et al. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. Ital J Dermatol Venerol. 2021;156(suppl 1 to no. 6):9-10.
- Góis M, Messias A, Carvalho D, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58-62.
- Jen HY, Chuang YH, Lin SC, et al. Increased serum interleukin-17 and peripheral Th17 cells in children with acute Henoch-Schönlein purpura. Pediatr Allergy Immunol. 2011;22:862-868.
- Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017;97:1160-1166.
- Weedon D. The vasculopathic reaction pattern. In: Houston M, Davie B, eds. Weedon’s Skin Pathology. 3rd ed. Elsevier Limited; 2010:207-211.
- Puig L. Paradoxical reactions: anti-TNFα ants, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol. 2018;53:49-63.
- Nestle F, Kaplan D, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNFα therapy: a fortuitous event or an indication to stop therapy? Eur J Dermatol. 2017;27:304-305.
- Hello CL, Cohen P, Bousser MG, et al. Suspected hepatitis B vaccination related vasculitis. J Rheumatol. 1999;26:191-194.
- Wolverton SE. Dapsone. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier, Inc; 2021:222-231.
Practice Points
- Biologic medications including adalimumab and more rarely secukinumab may be associated with leukocytoclastic vasculitis; a smaller subset of patients may experience IgA vasculitis.
- The IL-23 blocker ustekinumab may represent an ideal therapeutic agent when secukinumabassociated vasculitis is suspected. Because IL-23 is the main driver and sustainer of TH17 cell differentiation, it may cease the main causative cytokine cascades “upstream.”
Brussels terror attack victim euthanized in Belgium at age 23
This article was originally published on MediQuality.com, an online service for health care professionals in the Benelux and a member of the Medscape Professional Network.
Performing euthanasia for “mental suffering that cannot be alleviated” is still considered an extraordinary measure in Belgium. Indeed, fewer than 2% of the requests for euthanasia fall within that category, and few such requests are made by young patients.
to be applicable. It’s something that Belgian broadcaster RTBF brought up during a recent episode of #Investigation, which reported on the aftermath of the 2016 Brussels attacks.
On May 7, surrounded by her family, Ms. De Corte was euthanized. She was 23 years old. Six years earlier, on March 22, 2016, Ms. De Corte had been at Brussels Airport when terrorists set off bombs. She was in the departures area with 90 other students from Sint-Rita Campus College, located in the northern town of Kontich. Ms. De Corte was only a few meters away from the blast. Although she was not physically injured, the Flemish teen was traumatized by the attack. This was confirmed by the school psychologist who treated the students. “There were some students who reacted worse than others to these traumatic events. And having had two discussions with Shanti, I can tell you that she was one of these students who were more sensitive to the effects. To me, it’s quite clear. Even before the attacks, she’d experienced serious psychological issues. Therefore, I referred her for psychiatric care.”
Eleven antidepressants daily
A few weeks after that March day, Ms. De Corte was admitted to a psychiatric hospital in Antwerp. It was a place she knew well, having been an inpatient there several times before the attacks. Ms. De Corte was treated with antidepressants. She shared her thoughts about them on numerous occasions. “I get several drugs at breakfast and up to 11 antidepressants a day. I couldn’t do without them. With all the drugs I take, I feel like a ghost who doesn’t feel anything anymore. Perhaps there were solutions other than the drugs.”
It was a brief respite. In 2020, Ms. De Corte attempted suicide. Her spirits were at their lowest. She was heavily medicated, and her medication had been increased over time. She turned down therapeutic help that was offered by a therapist who specializes in treating the victims of the Brussels attacks. The student got in touch with the Life End Information Forum, an association that supports the right to die with dignity. In April 2022, Ms. De Corte submitted a new euthanasia request, stating that she was in a medically futile condition of mental suffering. Two psychiatrists granted their approval.
A small proportion
Last March, Belgium’s Federal Commission for the Control and Evaluation of Euthanasia reported on data from 2021. “There continues to be a very small number of euthanasia requests that cite mental and behavioral disorders (psychiatric conditions, such as personality disorders, and cognitive issues, like Alzheimer’s disease, are included in this group): 1.9% of all cases of euthanasia. Like all euthanasia files, these requests meet the legal conditions (the patient is legally competent, the request is in writing, the condition is medically futile, and the suffering – which is constant, unbearable, and cannot be alleviated – results from a serious and incurable disorder; the request is well-considered and repeated),” the report states.
This article was translated from MediQuality and appeared on Medscape.com.
This article was originally published on MediQuality.com, an online service for health care professionals in the Benelux and a member of the Medscape Professional Network.
Performing euthanasia for “mental suffering that cannot be alleviated” is still considered an extraordinary measure in Belgium. Indeed, fewer than 2% of the requests for euthanasia fall within that category, and few such requests are made by young patients.
to be applicable. It’s something that Belgian broadcaster RTBF brought up during a recent episode of #Investigation, which reported on the aftermath of the 2016 Brussels attacks.
On May 7, surrounded by her family, Ms. De Corte was euthanized. She was 23 years old. Six years earlier, on March 22, 2016, Ms. De Corte had been at Brussels Airport when terrorists set off bombs. She was in the departures area with 90 other students from Sint-Rita Campus College, located in the northern town of Kontich. Ms. De Corte was only a few meters away from the blast. Although she was not physically injured, the Flemish teen was traumatized by the attack. This was confirmed by the school psychologist who treated the students. “There were some students who reacted worse than others to these traumatic events. And having had two discussions with Shanti, I can tell you that she was one of these students who were more sensitive to the effects. To me, it’s quite clear. Even before the attacks, she’d experienced serious psychological issues. Therefore, I referred her for psychiatric care.”
Eleven antidepressants daily
A few weeks after that March day, Ms. De Corte was admitted to a psychiatric hospital in Antwerp. It was a place she knew well, having been an inpatient there several times before the attacks. Ms. De Corte was treated with antidepressants. She shared her thoughts about them on numerous occasions. “I get several drugs at breakfast and up to 11 antidepressants a day. I couldn’t do without them. With all the drugs I take, I feel like a ghost who doesn’t feel anything anymore. Perhaps there were solutions other than the drugs.”
It was a brief respite. In 2020, Ms. De Corte attempted suicide. Her spirits were at their lowest. She was heavily medicated, and her medication had been increased over time. She turned down therapeutic help that was offered by a therapist who specializes in treating the victims of the Brussels attacks. The student got in touch with the Life End Information Forum, an association that supports the right to die with dignity. In April 2022, Ms. De Corte submitted a new euthanasia request, stating that she was in a medically futile condition of mental suffering. Two psychiatrists granted their approval.
A small proportion
Last March, Belgium’s Federal Commission for the Control and Evaluation of Euthanasia reported on data from 2021. “There continues to be a very small number of euthanasia requests that cite mental and behavioral disorders (psychiatric conditions, such as personality disorders, and cognitive issues, like Alzheimer’s disease, are included in this group): 1.9% of all cases of euthanasia. Like all euthanasia files, these requests meet the legal conditions (the patient is legally competent, the request is in writing, the condition is medically futile, and the suffering – which is constant, unbearable, and cannot be alleviated – results from a serious and incurable disorder; the request is well-considered and repeated),” the report states.
This article was translated from MediQuality and appeared on Medscape.com.
This article was originally published on MediQuality.com, an online service for health care professionals in the Benelux and a member of the Medscape Professional Network.
Performing euthanasia for “mental suffering that cannot be alleviated” is still considered an extraordinary measure in Belgium. Indeed, fewer than 2% of the requests for euthanasia fall within that category, and few such requests are made by young patients.
to be applicable. It’s something that Belgian broadcaster RTBF brought up during a recent episode of #Investigation, which reported on the aftermath of the 2016 Brussels attacks.
On May 7, surrounded by her family, Ms. De Corte was euthanized. She was 23 years old. Six years earlier, on March 22, 2016, Ms. De Corte had been at Brussels Airport when terrorists set off bombs. She was in the departures area with 90 other students from Sint-Rita Campus College, located in the northern town of Kontich. Ms. De Corte was only a few meters away from the blast. Although she was not physically injured, the Flemish teen was traumatized by the attack. This was confirmed by the school psychologist who treated the students. “There were some students who reacted worse than others to these traumatic events. And having had two discussions with Shanti, I can tell you that she was one of these students who were more sensitive to the effects. To me, it’s quite clear. Even before the attacks, she’d experienced serious psychological issues. Therefore, I referred her for psychiatric care.”
Eleven antidepressants daily
A few weeks after that March day, Ms. De Corte was admitted to a psychiatric hospital in Antwerp. It was a place she knew well, having been an inpatient there several times before the attacks. Ms. De Corte was treated with antidepressants. She shared her thoughts about them on numerous occasions. “I get several drugs at breakfast and up to 11 antidepressants a day. I couldn’t do without them. With all the drugs I take, I feel like a ghost who doesn’t feel anything anymore. Perhaps there were solutions other than the drugs.”
It was a brief respite. In 2020, Ms. De Corte attempted suicide. Her spirits were at their lowest. She was heavily medicated, and her medication had been increased over time. She turned down therapeutic help that was offered by a therapist who specializes in treating the victims of the Brussels attacks. The student got in touch with the Life End Information Forum, an association that supports the right to die with dignity. In April 2022, Ms. De Corte submitted a new euthanasia request, stating that she was in a medically futile condition of mental suffering. Two psychiatrists granted their approval.
A small proportion
Last March, Belgium’s Federal Commission for the Control and Evaluation of Euthanasia reported on data from 2021. “There continues to be a very small number of euthanasia requests that cite mental and behavioral disorders (psychiatric conditions, such as personality disorders, and cognitive issues, like Alzheimer’s disease, are included in this group): 1.9% of all cases of euthanasia. Like all euthanasia files, these requests meet the legal conditions (the patient is legally competent, the request is in writing, the condition is medically futile, and the suffering – which is constant, unbearable, and cannot be alleviated – results from a serious and incurable disorder; the request is well-considered and repeated),” the report states.
This article was translated from MediQuality and appeared on Medscape.com.
Risk factors ID’d for acute pancreatitis from weight-loss drugs
CHARLOTTE, N.C. – a new study has found.
Type 2 diabetes, advanced chronic kidney disease, and tobacco use were associated with greater risk for acute pancreatitis, researchers report.
On the other hand, a higher body mass index (BMI) – 36 kg/m2 or higher – appeared to protect people against developing the condition.
“As this class of medications becomes increasingly popular in the United States, it is important for providers to know which patients are at a higher or lower risk of developing acute pancreatitis after being started on them,” said lead study author Robert Postlethwaite, MD, a gastroenterology resident at the University of Texas Southwestern Medical Center, Dallas.
The findings were presented at the annual meeting of the American College of Gastroenterology in Charlotte, N.C., being held in person and virtually.
Popularity comes at a price
The U.S. Food and Drug Administration has approved two GLP-1s for weight management – liraglutide (Victoza) in 2014 and semaglutide (Wegovy) in 2021. They work by targeting areas of the brain that control food intake and appetite. Other GLP-1s approved to treat type 2 diabetes include dulaglutide (Trulicity) and two other formulations of semaglutide (Rybelsus and Ozempic).
The demand for Wegovy has been so great that there is an ongoing shortage of the medication in the United States.
Although GLP-1s demonstrate a favorable side-effect profile, compared with other types of antiobesity medications, acute pancreatitis remains a serious and sometimes life-threatening complication, the researchers note. Some patients require hospitalization.
Dr. Postlethwaite and colleagues performed a retrospective, single-center study of 2,245 patients who attended an academic medical center’s Weight Wellness program from 2015 to 2019. The average age was about 50 years, and 81% were female. The average BMI of all patients was 39.7 kg/m2.
The study only included patients starting GLP-1s for treating obesity, not for diabetes.
Of the 2,245 patients, 49 (2.2%) developed acute pancreatitis after starting a GLP-1.
A history of type 2 diabetes mellitus made acute pancreatitis twice as likely (95% confidence interval, 1.04-3.96; P = .04).
Stage 3 or higher chronic kidney disease increased risk 2.3 times (95% CI, 1.18-4.55; P = .01), while tobacco use upped it 3.3 times (95% CI, 1.70-6.50; P < .001).
In contrast, researchers found those with a BMI of 36-40 kg/m2 were 88% less likely to develop acute pancreatitis (95% CI, 0.07-0.67; P = .007), compared with patients with a BMI of less than or equal to 30 kg/m2. Patients with a BMI of greater than 40 kg/m2 had a 73% lower risk (95% CI, 0.10-0.73; P = .01).
Dr. Postlethwaite and colleagues found no association with age, sex, or history of bariatric surgery or acute pancreatitis.
Because a history of acute pancreatitis was not a risk factor, he advised that clinicians not withhold these medications for this reason, “especially given the significant glycemic, cardiovascular, and weight-loss effects.”
“We hope that we can arm clinicians with evidence in order to risk stratify their patients and determine who is at high risk of developing pancreatitis,” Dr. Postlethwaite said.
“Hopefully, we can prevent the development of pancreatitis in some patients, especially high-risk individuals, or at least allow clinicians to be aware of it in higher-risk patients to identify it early enough to prevent complications of acute pancreatitis,” he added.
Larger studies needed
The study is “promising,” said session comoderator Baharak Moshiree, MD, a gastroenterologist at Atrium Health, Charlotte, N.C., who was not affiliated with the research.
However, because the study was retrospective and relatively small, it needs to be validated in larger, prospective studies, she added.
“With obesity being such a global issue, there are many patients on these GLP-1 agonists,” Dr. Moshiree said.
Generally, these medications are prescribed by endocrinologists, not gastroenterologists, she noted, and she said that gastroenterologists should be aware of the risks associated with them, including minor gastrointestinal side effects, like nausea and vomiting, that can occur because of delayed gastric emptying.
Dr. Postlethwaite noted that being unable to assess how much alcohol or tobacco individuals used was a limitation. The relatively low proportion of people who developed acute pancreatitis in the study also means larger studies are warranted, he added.
Going forward, Dr. Postlethwaite and colleagues want to study the risks for each individual GLP-1 and other therapies used to control high blood sugar in people with type 2 diabetes, such as DPP4 (dipeptidyl-peptidase 4) inhibitors.
The study was independently supported. Dr. Postlethwaite and Dr. Moshiree report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHARLOTTE, N.C. – a new study has found.
Type 2 diabetes, advanced chronic kidney disease, and tobacco use were associated with greater risk for acute pancreatitis, researchers report.
On the other hand, a higher body mass index (BMI) – 36 kg/m2 or higher – appeared to protect people against developing the condition.
“As this class of medications becomes increasingly popular in the United States, it is important for providers to know which patients are at a higher or lower risk of developing acute pancreatitis after being started on them,” said lead study author Robert Postlethwaite, MD, a gastroenterology resident at the University of Texas Southwestern Medical Center, Dallas.
The findings were presented at the annual meeting of the American College of Gastroenterology in Charlotte, N.C., being held in person and virtually.
Popularity comes at a price
The U.S. Food and Drug Administration has approved two GLP-1s for weight management – liraglutide (Victoza) in 2014 and semaglutide (Wegovy) in 2021. They work by targeting areas of the brain that control food intake and appetite. Other GLP-1s approved to treat type 2 diabetes include dulaglutide (Trulicity) and two other formulations of semaglutide (Rybelsus and Ozempic).
The demand for Wegovy has been so great that there is an ongoing shortage of the medication in the United States.
Although GLP-1s demonstrate a favorable side-effect profile, compared with other types of antiobesity medications, acute pancreatitis remains a serious and sometimes life-threatening complication, the researchers note. Some patients require hospitalization.
Dr. Postlethwaite and colleagues performed a retrospective, single-center study of 2,245 patients who attended an academic medical center’s Weight Wellness program from 2015 to 2019. The average age was about 50 years, and 81% were female. The average BMI of all patients was 39.7 kg/m2.
The study only included patients starting GLP-1s for treating obesity, not for diabetes.
Of the 2,245 patients, 49 (2.2%) developed acute pancreatitis after starting a GLP-1.
A history of type 2 diabetes mellitus made acute pancreatitis twice as likely (95% confidence interval, 1.04-3.96; P = .04).
Stage 3 or higher chronic kidney disease increased risk 2.3 times (95% CI, 1.18-4.55; P = .01), while tobacco use upped it 3.3 times (95% CI, 1.70-6.50; P < .001).
In contrast, researchers found those with a BMI of 36-40 kg/m2 were 88% less likely to develop acute pancreatitis (95% CI, 0.07-0.67; P = .007), compared with patients with a BMI of less than or equal to 30 kg/m2. Patients with a BMI of greater than 40 kg/m2 had a 73% lower risk (95% CI, 0.10-0.73; P = .01).
Dr. Postlethwaite and colleagues found no association with age, sex, or history of bariatric surgery or acute pancreatitis.
Because a history of acute pancreatitis was not a risk factor, he advised that clinicians not withhold these medications for this reason, “especially given the significant glycemic, cardiovascular, and weight-loss effects.”
“We hope that we can arm clinicians with evidence in order to risk stratify their patients and determine who is at high risk of developing pancreatitis,” Dr. Postlethwaite said.
“Hopefully, we can prevent the development of pancreatitis in some patients, especially high-risk individuals, or at least allow clinicians to be aware of it in higher-risk patients to identify it early enough to prevent complications of acute pancreatitis,” he added.
Larger studies needed
The study is “promising,” said session comoderator Baharak Moshiree, MD, a gastroenterologist at Atrium Health, Charlotte, N.C., who was not affiliated with the research.
However, because the study was retrospective and relatively small, it needs to be validated in larger, prospective studies, she added.
“With obesity being such a global issue, there are many patients on these GLP-1 agonists,” Dr. Moshiree said.
Generally, these medications are prescribed by endocrinologists, not gastroenterologists, she noted, and she said that gastroenterologists should be aware of the risks associated with them, including minor gastrointestinal side effects, like nausea and vomiting, that can occur because of delayed gastric emptying.
Dr. Postlethwaite noted that being unable to assess how much alcohol or tobacco individuals used was a limitation. The relatively low proportion of people who developed acute pancreatitis in the study also means larger studies are warranted, he added.
Going forward, Dr. Postlethwaite and colleagues want to study the risks for each individual GLP-1 and other therapies used to control high blood sugar in people with type 2 diabetes, such as DPP4 (dipeptidyl-peptidase 4) inhibitors.
The study was independently supported. Dr. Postlethwaite and Dr. Moshiree report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHARLOTTE, N.C. – a new study has found.
Type 2 diabetes, advanced chronic kidney disease, and tobacco use were associated with greater risk for acute pancreatitis, researchers report.
On the other hand, a higher body mass index (BMI) – 36 kg/m2 or higher – appeared to protect people against developing the condition.
“As this class of medications becomes increasingly popular in the United States, it is important for providers to know which patients are at a higher or lower risk of developing acute pancreatitis after being started on them,” said lead study author Robert Postlethwaite, MD, a gastroenterology resident at the University of Texas Southwestern Medical Center, Dallas.
The findings were presented at the annual meeting of the American College of Gastroenterology in Charlotte, N.C., being held in person and virtually.
Popularity comes at a price
The U.S. Food and Drug Administration has approved two GLP-1s for weight management – liraglutide (Victoza) in 2014 and semaglutide (Wegovy) in 2021. They work by targeting areas of the brain that control food intake and appetite. Other GLP-1s approved to treat type 2 diabetes include dulaglutide (Trulicity) and two other formulations of semaglutide (Rybelsus and Ozempic).
The demand for Wegovy has been so great that there is an ongoing shortage of the medication in the United States.
Although GLP-1s demonstrate a favorable side-effect profile, compared with other types of antiobesity medications, acute pancreatitis remains a serious and sometimes life-threatening complication, the researchers note. Some patients require hospitalization.
Dr. Postlethwaite and colleagues performed a retrospective, single-center study of 2,245 patients who attended an academic medical center’s Weight Wellness program from 2015 to 2019. The average age was about 50 years, and 81% were female. The average BMI of all patients was 39.7 kg/m2.
The study only included patients starting GLP-1s for treating obesity, not for diabetes.
Of the 2,245 patients, 49 (2.2%) developed acute pancreatitis after starting a GLP-1.
A history of type 2 diabetes mellitus made acute pancreatitis twice as likely (95% confidence interval, 1.04-3.96; P = .04).
Stage 3 or higher chronic kidney disease increased risk 2.3 times (95% CI, 1.18-4.55; P = .01), while tobacco use upped it 3.3 times (95% CI, 1.70-6.50; P < .001).
In contrast, researchers found those with a BMI of 36-40 kg/m2 were 88% less likely to develop acute pancreatitis (95% CI, 0.07-0.67; P = .007), compared with patients with a BMI of less than or equal to 30 kg/m2. Patients with a BMI of greater than 40 kg/m2 had a 73% lower risk (95% CI, 0.10-0.73; P = .01).
Dr. Postlethwaite and colleagues found no association with age, sex, or history of bariatric surgery or acute pancreatitis.
Because a history of acute pancreatitis was not a risk factor, he advised that clinicians not withhold these medications for this reason, “especially given the significant glycemic, cardiovascular, and weight-loss effects.”
“We hope that we can arm clinicians with evidence in order to risk stratify their patients and determine who is at high risk of developing pancreatitis,” Dr. Postlethwaite said.
“Hopefully, we can prevent the development of pancreatitis in some patients, especially high-risk individuals, or at least allow clinicians to be aware of it in higher-risk patients to identify it early enough to prevent complications of acute pancreatitis,” he added.
Larger studies needed
The study is “promising,” said session comoderator Baharak Moshiree, MD, a gastroenterologist at Atrium Health, Charlotte, N.C., who was not affiliated with the research.
However, because the study was retrospective and relatively small, it needs to be validated in larger, prospective studies, she added.
“With obesity being such a global issue, there are many patients on these GLP-1 agonists,” Dr. Moshiree said.
Generally, these medications are prescribed by endocrinologists, not gastroenterologists, she noted, and she said that gastroenterologists should be aware of the risks associated with them, including minor gastrointestinal side effects, like nausea and vomiting, that can occur because of delayed gastric emptying.
Dr. Postlethwaite noted that being unable to assess how much alcohol or tobacco individuals used was a limitation. The relatively low proportion of people who developed acute pancreatitis in the study also means larger studies are warranted, he added.
Going forward, Dr. Postlethwaite and colleagues want to study the risks for each individual GLP-1 and other therapies used to control high blood sugar in people with type 2 diabetes, such as DPP4 (dipeptidyl-peptidase 4) inhibitors.
The study was independently supported. Dr. Postlethwaite and Dr. Moshiree report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACG 2022
Ivermectin for COVID-19: Final nail in the coffin
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.
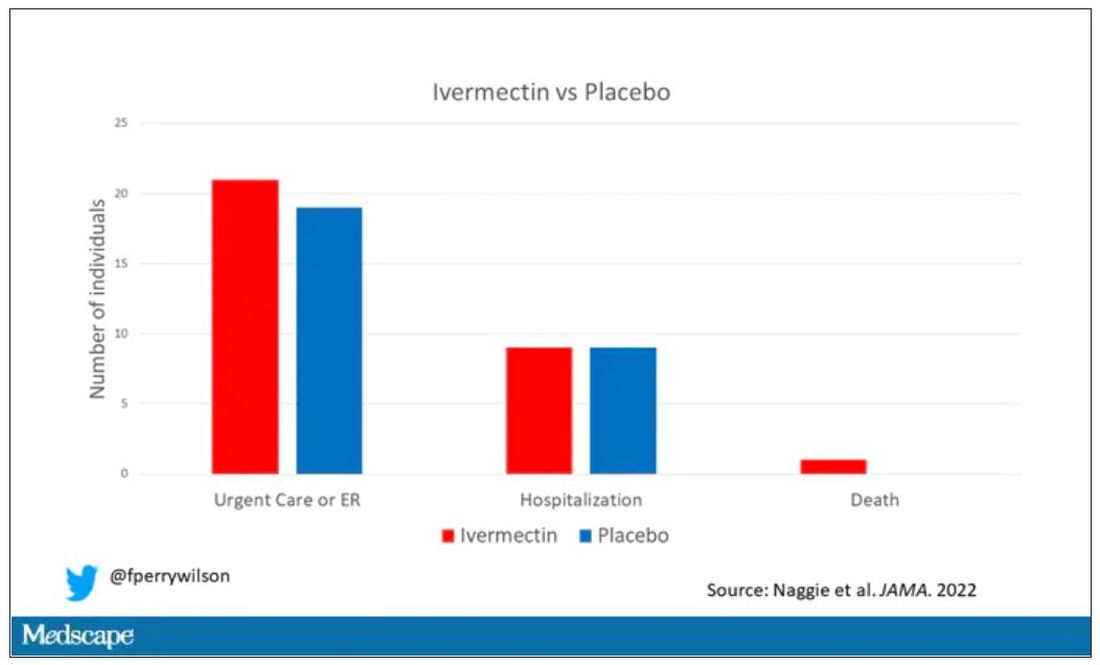
But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.

But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.

But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Study affirms better breast cancer outcomes when chemo comes first
New efficacy and safety data from the monarchE study show that chemotherapy administered before treatment with abemaciclib and estrogen therapy, led to a clinically meaningful improvement in invasive disease-free survival and distant relapse-free survival for women with HR-positive, ERBB2-negative, node-positive, early breast cancer at high risk of recurrence.
The study was published earlier this year in JAMA Oncology.
Neoadjuvant chemotherapy is often provided to such patients in hopes of achieving breast-conserving surgery. Although pathologic complete response rates can be higher than 50% after chemotherapy treatment in triple-negative and ERBB2-positive breast cancer, most patients with HR-positive and ERBB2-negative breast cancer have residual tumor at surgery after neoadjuvant chemotherapy, which is associated with an increased risk of recurrence.
“To our knowledge, abemaciclib is the first agent added to standard adjuvant estrogen therapy that has been shown to reduce the risk of recurrence in patients with HR-positive, ERBB2-negative early breast cancer with residual disease after neoadjuvant chemotherapy,” wrote the authors, who were led by Miguel Martin, MD, PhD, Hospital General Universitario Gregorio Marañon, Spain.
In 2021, Food and Drug Administration approved abemaciclib (Verzenio, Lilly) with endocrine therapy for the treatment of HR-positive/ERBB2-negative, node-positive, high-risk early breast cancer. Their decision was based on data from the monarchE study.
The study is at odds with the previously published Penelope-B study, which found no benefit from treatment with the CDK4/6 inhibitor palbociclib (Ibrance, Pfizer) after 42.8 months of follow-up. The authors suggest that the disparate outcomes may be due to pharmacological differences between the two drugs as well as different dosing schedules: In monarchE, patients received abemaciclib on a continuous basis, while patients in Penelope-B received palbociclib for 21 days, followed by 7 days off. The treatment duration was 2 years in monarchE and 1 year in Penelope-B. Abemaciclib can be dosed continuously because it is a stronger inhibitor of CDK4 versus CDK6 compared to abemaciclib, and in vitro studies suggest that continuous dosing could be a key factor in creating profound inhibition of DNA synthesis.
The monarchE study included 5,637 patients who were randomized to receive standard of care estrogen therapy for 5 years with or without abemaciclib (150 mg, twice per day) for 2 years; 36.5% received abemaciclib. The mean age was 49.9 years; 70.8% were White, 22.8% Asian, and 2.7% Black.
The abemaciclib group had a clinically and statistically significant benefit in invasive disease-free survival (IDFS) (hazard ratio, 0.61; nominal P < .001) and distant relapse-free survival (DRFS) (HR, 0.61; nominal P < .001). At 2 years, DRFS was 89.5% in the abemaciclib group and 82.8% in the estrogen therapy–only group. IDFS was 87.2% and 80.6%, respectively. Patients who underwent neoadjuvant chemotherapy had a similar safety profile to the estrogen therapy–only group, although there was a higher incidence of treatment-emergent adverse events. The most common were diarrhea, infections, neutropenia, and fatigue. The most frequent grade treatment-emergent adverse events (of at least 3) were neutropenia and leucopenia.
The researchers noted that patients who underwent neoadjuvant chemotherapy had a worse prognosis than the intent-to-treat arm, as evidenced by a higher risk of 2-year recurrence (19% versus 11%). Exploratory subgroup analyses revealed that treatment with abemaciclib and estrogen therapy conferred IDFS and DRFS benefits regardless of the pathological tumor size and number of positive axillary lymph nodes.
The study was limited by the fact that it was open label, and the subgroup analyses were not powered to find statistically significant associations.
Dr. Martin has received grants from Eli Lilly, which funded monarchE.
New efficacy and safety data from the monarchE study show that chemotherapy administered before treatment with abemaciclib and estrogen therapy, led to a clinically meaningful improvement in invasive disease-free survival and distant relapse-free survival for women with HR-positive, ERBB2-negative, node-positive, early breast cancer at high risk of recurrence.
The study was published earlier this year in JAMA Oncology.
Neoadjuvant chemotherapy is often provided to such patients in hopes of achieving breast-conserving surgery. Although pathologic complete response rates can be higher than 50% after chemotherapy treatment in triple-negative and ERBB2-positive breast cancer, most patients with HR-positive and ERBB2-negative breast cancer have residual tumor at surgery after neoadjuvant chemotherapy, which is associated with an increased risk of recurrence.
“To our knowledge, abemaciclib is the first agent added to standard adjuvant estrogen therapy that has been shown to reduce the risk of recurrence in patients with HR-positive, ERBB2-negative early breast cancer with residual disease after neoadjuvant chemotherapy,” wrote the authors, who were led by Miguel Martin, MD, PhD, Hospital General Universitario Gregorio Marañon, Spain.
In 2021, Food and Drug Administration approved abemaciclib (Verzenio, Lilly) with endocrine therapy for the treatment of HR-positive/ERBB2-negative, node-positive, high-risk early breast cancer. Their decision was based on data from the monarchE study.
The study is at odds with the previously published Penelope-B study, which found no benefit from treatment with the CDK4/6 inhibitor palbociclib (Ibrance, Pfizer) after 42.8 months of follow-up. The authors suggest that the disparate outcomes may be due to pharmacological differences between the two drugs as well as different dosing schedules: In monarchE, patients received abemaciclib on a continuous basis, while patients in Penelope-B received palbociclib for 21 days, followed by 7 days off. The treatment duration was 2 years in monarchE and 1 year in Penelope-B. Abemaciclib can be dosed continuously because it is a stronger inhibitor of CDK4 versus CDK6 compared to abemaciclib, and in vitro studies suggest that continuous dosing could be a key factor in creating profound inhibition of DNA synthesis.
The monarchE study included 5,637 patients who were randomized to receive standard of care estrogen therapy for 5 years with or without abemaciclib (150 mg, twice per day) for 2 years; 36.5% received abemaciclib. The mean age was 49.9 years; 70.8% were White, 22.8% Asian, and 2.7% Black.
The abemaciclib group had a clinically and statistically significant benefit in invasive disease-free survival (IDFS) (hazard ratio, 0.61; nominal P < .001) and distant relapse-free survival (DRFS) (HR, 0.61; nominal P < .001). At 2 years, DRFS was 89.5% in the abemaciclib group and 82.8% in the estrogen therapy–only group. IDFS was 87.2% and 80.6%, respectively. Patients who underwent neoadjuvant chemotherapy had a similar safety profile to the estrogen therapy–only group, although there was a higher incidence of treatment-emergent adverse events. The most common were diarrhea, infections, neutropenia, and fatigue. The most frequent grade treatment-emergent adverse events (of at least 3) were neutropenia and leucopenia.
The researchers noted that patients who underwent neoadjuvant chemotherapy had a worse prognosis than the intent-to-treat arm, as evidenced by a higher risk of 2-year recurrence (19% versus 11%). Exploratory subgroup analyses revealed that treatment with abemaciclib and estrogen therapy conferred IDFS and DRFS benefits regardless of the pathological tumor size and number of positive axillary lymph nodes.
The study was limited by the fact that it was open label, and the subgroup analyses were not powered to find statistically significant associations.
Dr. Martin has received grants from Eli Lilly, which funded monarchE.
New efficacy and safety data from the monarchE study show that chemotherapy administered before treatment with abemaciclib and estrogen therapy, led to a clinically meaningful improvement in invasive disease-free survival and distant relapse-free survival for women with HR-positive, ERBB2-negative, node-positive, early breast cancer at high risk of recurrence.
The study was published earlier this year in JAMA Oncology.
Neoadjuvant chemotherapy is often provided to such patients in hopes of achieving breast-conserving surgery. Although pathologic complete response rates can be higher than 50% after chemotherapy treatment in triple-negative and ERBB2-positive breast cancer, most patients with HR-positive and ERBB2-negative breast cancer have residual tumor at surgery after neoadjuvant chemotherapy, which is associated with an increased risk of recurrence.
“To our knowledge, abemaciclib is the first agent added to standard adjuvant estrogen therapy that has been shown to reduce the risk of recurrence in patients with HR-positive, ERBB2-negative early breast cancer with residual disease after neoadjuvant chemotherapy,” wrote the authors, who were led by Miguel Martin, MD, PhD, Hospital General Universitario Gregorio Marañon, Spain.
In 2021, Food and Drug Administration approved abemaciclib (Verzenio, Lilly) with endocrine therapy for the treatment of HR-positive/ERBB2-negative, node-positive, high-risk early breast cancer. Their decision was based on data from the monarchE study.
The study is at odds with the previously published Penelope-B study, which found no benefit from treatment with the CDK4/6 inhibitor palbociclib (Ibrance, Pfizer) after 42.8 months of follow-up. The authors suggest that the disparate outcomes may be due to pharmacological differences between the two drugs as well as different dosing schedules: In monarchE, patients received abemaciclib on a continuous basis, while patients in Penelope-B received palbociclib for 21 days, followed by 7 days off. The treatment duration was 2 years in monarchE and 1 year in Penelope-B. Abemaciclib can be dosed continuously because it is a stronger inhibitor of CDK4 versus CDK6 compared to abemaciclib, and in vitro studies suggest that continuous dosing could be a key factor in creating profound inhibition of DNA synthesis.
The monarchE study included 5,637 patients who were randomized to receive standard of care estrogen therapy for 5 years with or without abemaciclib (150 mg, twice per day) for 2 years; 36.5% received abemaciclib. The mean age was 49.9 years; 70.8% were White, 22.8% Asian, and 2.7% Black.
The abemaciclib group had a clinically and statistically significant benefit in invasive disease-free survival (IDFS) (hazard ratio, 0.61; nominal P < .001) and distant relapse-free survival (DRFS) (HR, 0.61; nominal P < .001). At 2 years, DRFS was 89.5% in the abemaciclib group and 82.8% in the estrogen therapy–only group. IDFS was 87.2% and 80.6%, respectively. Patients who underwent neoadjuvant chemotherapy had a similar safety profile to the estrogen therapy–only group, although there was a higher incidence of treatment-emergent adverse events. The most common were diarrhea, infections, neutropenia, and fatigue. The most frequent grade treatment-emergent adverse events (of at least 3) were neutropenia and leucopenia.
The researchers noted that patients who underwent neoadjuvant chemotherapy had a worse prognosis than the intent-to-treat arm, as evidenced by a higher risk of 2-year recurrence (19% versus 11%). Exploratory subgroup analyses revealed that treatment with abemaciclib and estrogen therapy conferred IDFS and DRFS benefits regardless of the pathological tumor size and number of positive axillary lymph nodes.
The study was limited by the fact that it was open label, and the subgroup analyses were not powered to find statistically significant associations.
Dr. Martin has received grants from Eli Lilly, which funded monarchE.
FROM JAMA ONCOLOGY
Side effects from COVID vaccine show its effectiveness
It means your body had a greater antibody response than people who had just a little pain or rash at the injection site, or no reaction at all.
That’s according to new research published in the journal JAMA Network Open .
“These findings support reframing postvaccination symptoms as signals of vaccine effectiveness and reinforce guidelines for vaccine boosters in older adults,” researchers from Columbia University in New York, the University of Vermont, and Boston University wrote.
The vaccines provided strong protection regardless of the level of reaction, researchers said. Almost all the study’s 928 adult participants had a positive antibody response after receiving two doses of vaccine.
“I don’t want a patient to tell me that, ‘Golly, I didn’t get any reaction, my arm wasn’t sore, I didn’t have fever. The vaccine didn’t work.’ I don’t want that conclusion to be out there,” William Schaffner, MD, a professor in the division of infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn., told CNN.
“This is more to reassure people who have had a reaction that that’s their immune system responding, actually in a rather good way, to the vaccine, even though it has caused them some discomfort,” said Dr. Schaffner, who was not involved in the study.
A version of this article first appeared on WebMD.com.
It means your body had a greater antibody response than people who had just a little pain or rash at the injection site, or no reaction at all.
That’s according to new research published in the journal JAMA Network Open .
“These findings support reframing postvaccination symptoms as signals of vaccine effectiveness and reinforce guidelines for vaccine boosters in older adults,” researchers from Columbia University in New York, the University of Vermont, and Boston University wrote.
The vaccines provided strong protection regardless of the level of reaction, researchers said. Almost all the study’s 928 adult participants had a positive antibody response after receiving two doses of vaccine.
“I don’t want a patient to tell me that, ‘Golly, I didn’t get any reaction, my arm wasn’t sore, I didn’t have fever. The vaccine didn’t work.’ I don’t want that conclusion to be out there,” William Schaffner, MD, a professor in the division of infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn., told CNN.
“This is more to reassure people who have had a reaction that that’s their immune system responding, actually in a rather good way, to the vaccine, even though it has caused them some discomfort,” said Dr. Schaffner, who was not involved in the study.
A version of this article first appeared on WebMD.com.
It means your body had a greater antibody response than people who had just a little pain or rash at the injection site, or no reaction at all.
That’s according to new research published in the journal JAMA Network Open .
“These findings support reframing postvaccination symptoms as signals of vaccine effectiveness and reinforce guidelines for vaccine boosters in older adults,” researchers from Columbia University in New York, the University of Vermont, and Boston University wrote.
The vaccines provided strong protection regardless of the level of reaction, researchers said. Almost all the study’s 928 adult participants had a positive antibody response after receiving two doses of vaccine.
“I don’t want a patient to tell me that, ‘Golly, I didn’t get any reaction, my arm wasn’t sore, I didn’t have fever. The vaccine didn’t work.’ I don’t want that conclusion to be out there,” William Schaffner, MD, a professor in the division of infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn., told CNN.
“This is more to reassure people who have had a reaction that that’s their immune system responding, actually in a rather good way, to the vaccine, even though it has caused them some discomfort,” said Dr. Schaffner, who was not involved in the study.
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN
Vitamin D deficiency linked to death, new study finds
Vitamin D deficiency increases mortality risk and raising levels even slightly could decrease the risk, researchers examining data from the UK Biobank have found.
They used a Mendelian randomization approach, which uses genetic variants as “proxy indicators” for external factors that affect vitamin D levels, such as sun exposure or dietary intake. It allows for analysis of the relationship between deficiency and outcomes including mortality, which can’t be done in randomized clinical trials for ethical reasons.
Using this method, nutritionist Joshua P. Sutherland, PhD, of the Australian Centre for Precision Health, Adelaide, and colleagues found an association between genetically predicted vitamin D levels [25-(OH)D] and mortality from several major causes, with evidence of causality among people with measured concentrations below, but not above, 50 nmol/L. The findings were published online in Annals of Internal Medicine.
“Unlike other types of observational studies, we have overcome some of the methodological obstacles. What is special about this new study is we were able to look at people with very low vitamin D concentrations and what would happen if their concentrations were a little bit higher. Most randomized controlled trials don’t show much of an effect. That’s because most people have sufficient concentrations. Ethically you can’t do a trial of people with very low levels without treating them,” senior author Elina Hypp
The data support the 50 nmol/L cut-off endorsed by the United States National Academy of Medicine and align with previous data suggesting the benefit of vitamin D supplementation is largely seen in people with deficiency.
“Everybody with vitamin D levels less than 50 nmol/L is recommended to increase their levels. Our results suggest there’s no need to go very high. The positive message is that if we are able to raise levels to just the current U.S. recommendations, that’s fine. There’s no need to use large supplement doses,” Dr. Hyppönen explained.
Thus, she advised, “Supplementation will clearly help, especially during wintertime or if a person isn’t getting enough vitamin D from the sun or in places where food isn’t fortified with vitamin D.”
But the data don’t support the approach of using large intermittent doses, she added.
“Sometimes doctors want to fix the deficiency quickly with a large ‘bolus’ dose, then continue with a maintenance dose. Increasing evidence suggests that’s not beneficial and might disturb the body’s metabolism so that it can’t get the amount it needs. It’s safe overall but might not work the way we want it to work.”
Rather, Dr. Hyppönen said, “My sense is that daily modest vitamin D dose supplementation when it’s needed is the best way forward.”
Genetic approach reveals causal relationship
The investigators analyzed data from 307,601 individuals in the UK Biobank, a prospective cohort of people recruited from England, Scotland, and Wales during March 2006 and July 2010. Most were of White European ancestry and were aged 37-73 years at baseline.
Genetically predicted vitamin D levels were estimated using 35 confirmed 25-(OH)D variants. Participants were followed for outcomes up to June 2020.
The average baseline measured 25-(OH)D concentration was 45.2 nmol/L, and 11.7% (n = 36,009) of participants had levels between 10.0 and 24.9 nmol/L. Higher levels were seen in people living in southern areas and nonsmokers as well as those with a higher level of physical activity, less socioeconomic deprivation, and lower body mass index.
During follow-up, 6.1% of participants died (n = 18,700). After adjustment for variables, odds ratios for all causes of mortality were highest among people with 25-(OH)D levels below 25 nmol/L and appeared to plateau between 50 and 75 nmol/L, with no further reduction in mortality at values of 75-125 nmol/L.
Mortality 36% higher in those deficient in vitamin D
The risk for mortality was a significant 36% higher for participants with 25-(OH)D 25 nmol/L compared with 50 nmol/L.
With the Mendelian randomization, there was an L-shaped association between genetically predicted 25-(OH)D level and all-cause mortality (P for nonlinearity < .001) and for mortality because of cancer and cardiovascular disease (P for nonlinearity ≤ .033).
Again, the strongest association with those outcomes and genetically predicted 25-(OH)D was found at levels below 25 nmol/L and a plateau was seen by 50 nmol/L.
Compared with a measured 25-(OH)D concentration of 50 nmol/L, investigators estimated that the genetically predicted odds of all-cause mortality would increase sixfold (odds ratio, 6.00) for participants at 10 nmol/L and by 25% (OR, 1.25) for those at 25 nmol/L.
And, compared with a measured 25-(OH)D concentration of 50 nmol/L, those with 10 nmol/L had genetically predicted odds ratios of 5.98 for cardiovascular mortality, 3.37 for cancer mortality, and 12.44 for respiratory mortality.
Comparing measured 25-(OH)D concentrations of 25 nmol/L versus 50 nmol/L, odds ratios for those outcomes were 1.25, 1.16, and 1.96 (95% confidence interval, 1.88-4.67), respectively. All were statistically significant.
Consistent results supportive of a causal effect of genetically predicted 25-(OH)D on all-cause mortality in those with low measured vitamin D concentrations were also found in a sensitivity analysis of 20,837 people of non-White ethnic origin.
The study was funded by the Australian National Health and Medical Research Council. Dr. Sutherland’s studentship is funded by an Australian Research Training Program Scholarship.
A version of this article first appeared on Medscape.com.
Vitamin D deficiency increases mortality risk and raising levels even slightly could decrease the risk, researchers examining data from the UK Biobank have found.
They used a Mendelian randomization approach, which uses genetic variants as “proxy indicators” for external factors that affect vitamin D levels, such as sun exposure or dietary intake. It allows for analysis of the relationship between deficiency and outcomes including mortality, which can’t be done in randomized clinical trials for ethical reasons.
Using this method, nutritionist Joshua P. Sutherland, PhD, of the Australian Centre for Precision Health, Adelaide, and colleagues found an association between genetically predicted vitamin D levels [25-(OH)D] and mortality from several major causes, with evidence of causality among people with measured concentrations below, but not above, 50 nmol/L. The findings were published online in Annals of Internal Medicine.
“Unlike other types of observational studies, we have overcome some of the methodological obstacles. What is special about this new study is we were able to look at people with very low vitamin D concentrations and what would happen if their concentrations were a little bit higher. Most randomized controlled trials don’t show much of an effect. That’s because most people have sufficient concentrations. Ethically you can’t do a trial of people with very low levels without treating them,” senior author Elina Hypp
The data support the 50 nmol/L cut-off endorsed by the United States National Academy of Medicine and align with previous data suggesting the benefit of vitamin D supplementation is largely seen in people with deficiency.
“Everybody with vitamin D levels less than 50 nmol/L is recommended to increase their levels. Our results suggest there’s no need to go very high. The positive message is that if we are able to raise levels to just the current U.S. recommendations, that’s fine. There’s no need to use large supplement doses,” Dr. Hyppönen explained.
Thus, she advised, “Supplementation will clearly help, especially during wintertime or if a person isn’t getting enough vitamin D from the sun or in places where food isn’t fortified with vitamin D.”
But the data don’t support the approach of using large intermittent doses, she added.
“Sometimes doctors want to fix the deficiency quickly with a large ‘bolus’ dose, then continue with a maintenance dose. Increasing evidence suggests that’s not beneficial and might disturb the body’s metabolism so that it can’t get the amount it needs. It’s safe overall but might not work the way we want it to work.”
Rather, Dr. Hyppönen said, “My sense is that daily modest vitamin D dose supplementation when it’s needed is the best way forward.”
Genetic approach reveals causal relationship
The investigators analyzed data from 307,601 individuals in the UK Biobank, a prospective cohort of people recruited from England, Scotland, and Wales during March 2006 and July 2010. Most were of White European ancestry and were aged 37-73 years at baseline.
Genetically predicted vitamin D levels were estimated using 35 confirmed 25-(OH)D variants. Participants were followed for outcomes up to June 2020.
The average baseline measured 25-(OH)D concentration was 45.2 nmol/L, and 11.7% (n = 36,009) of participants had levels between 10.0 and 24.9 nmol/L. Higher levels were seen in people living in southern areas and nonsmokers as well as those with a higher level of physical activity, less socioeconomic deprivation, and lower body mass index.
During follow-up, 6.1% of participants died (n = 18,700). After adjustment for variables, odds ratios for all causes of mortality were highest among people with 25-(OH)D levels below 25 nmol/L and appeared to plateau between 50 and 75 nmol/L, with no further reduction in mortality at values of 75-125 nmol/L.
Mortality 36% higher in those deficient in vitamin D
The risk for mortality was a significant 36% higher for participants with 25-(OH)D 25 nmol/L compared with 50 nmol/L.
With the Mendelian randomization, there was an L-shaped association between genetically predicted 25-(OH)D level and all-cause mortality (P for nonlinearity < .001) and for mortality because of cancer and cardiovascular disease (P for nonlinearity ≤ .033).
Again, the strongest association with those outcomes and genetically predicted 25-(OH)D was found at levels below 25 nmol/L and a plateau was seen by 50 nmol/L.
Compared with a measured 25-(OH)D concentration of 50 nmol/L, investigators estimated that the genetically predicted odds of all-cause mortality would increase sixfold (odds ratio, 6.00) for participants at 10 nmol/L and by 25% (OR, 1.25) for those at 25 nmol/L.
And, compared with a measured 25-(OH)D concentration of 50 nmol/L, those with 10 nmol/L had genetically predicted odds ratios of 5.98 for cardiovascular mortality, 3.37 for cancer mortality, and 12.44 for respiratory mortality.
Comparing measured 25-(OH)D concentrations of 25 nmol/L versus 50 nmol/L, odds ratios for those outcomes were 1.25, 1.16, and 1.96 (95% confidence interval, 1.88-4.67), respectively. All were statistically significant.
Consistent results supportive of a causal effect of genetically predicted 25-(OH)D on all-cause mortality in those with low measured vitamin D concentrations were also found in a sensitivity analysis of 20,837 people of non-White ethnic origin.
The study was funded by the Australian National Health and Medical Research Council. Dr. Sutherland’s studentship is funded by an Australian Research Training Program Scholarship.
A version of this article first appeared on Medscape.com.
Vitamin D deficiency increases mortality risk and raising levels even slightly could decrease the risk, researchers examining data from the UK Biobank have found.
They used a Mendelian randomization approach, which uses genetic variants as “proxy indicators” for external factors that affect vitamin D levels, such as sun exposure or dietary intake. It allows for analysis of the relationship between deficiency and outcomes including mortality, which can’t be done in randomized clinical trials for ethical reasons.
Using this method, nutritionist Joshua P. Sutherland, PhD, of the Australian Centre for Precision Health, Adelaide, and colleagues found an association between genetically predicted vitamin D levels [25-(OH)D] and mortality from several major causes, with evidence of causality among people with measured concentrations below, but not above, 50 nmol/L. The findings were published online in Annals of Internal Medicine.
“Unlike other types of observational studies, we have overcome some of the methodological obstacles. What is special about this new study is we were able to look at people with very low vitamin D concentrations and what would happen if their concentrations were a little bit higher. Most randomized controlled trials don’t show much of an effect. That’s because most people have sufficient concentrations. Ethically you can’t do a trial of people with very low levels without treating them,” senior author Elina Hypp
The data support the 50 nmol/L cut-off endorsed by the United States National Academy of Medicine and align with previous data suggesting the benefit of vitamin D supplementation is largely seen in people with deficiency.
“Everybody with vitamin D levels less than 50 nmol/L is recommended to increase their levels. Our results suggest there’s no need to go very high. The positive message is that if we are able to raise levels to just the current U.S. recommendations, that’s fine. There’s no need to use large supplement doses,” Dr. Hyppönen explained.
Thus, she advised, “Supplementation will clearly help, especially during wintertime or if a person isn’t getting enough vitamin D from the sun or in places where food isn’t fortified with vitamin D.”
But the data don’t support the approach of using large intermittent doses, she added.
“Sometimes doctors want to fix the deficiency quickly with a large ‘bolus’ dose, then continue with a maintenance dose. Increasing evidence suggests that’s not beneficial and might disturb the body’s metabolism so that it can’t get the amount it needs. It’s safe overall but might not work the way we want it to work.”
Rather, Dr. Hyppönen said, “My sense is that daily modest vitamin D dose supplementation when it’s needed is the best way forward.”
Genetic approach reveals causal relationship
The investigators analyzed data from 307,601 individuals in the UK Biobank, a prospective cohort of people recruited from England, Scotland, and Wales during March 2006 and July 2010. Most were of White European ancestry and were aged 37-73 years at baseline.
Genetically predicted vitamin D levels were estimated using 35 confirmed 25-(OH)D variants. Participants were followed for outcomes up to June 2020.
The average baseline measured 25-(OH)D concentration was 45.2 nmol/L, and 11.7% (n = 36,009) of participants had levels between 10.0 and 24.9 nmol/L. Higher levels were seen in people living in southern areas and nonsmokers as well as those with a higher level of physical activity, less socioeconomic deprivation, and lower body mass index.
During follow-up, 6.1% of participants died (n = 18,700). After adjustment for variables, odds ratios for all causes of mortality were highest among people with 25-(OH)D levels below 25 nmol/L and appeared to plateau between 50 and 75 nmol/L, with no further reduction in mortality at values of 75-125 nmol/L.
Mortality 36% higher in those deficient in vitamin D
The risk for mortality was a significant 36% higher for participants with 25-(OH)D 25 nmol/L compared with 50 nmol/L.
With the Mendelian randomization, there was an L-shaped association between genetically predicted 25-(OH)D level and all-cause mortality (P for nonlinearity < .001) and for mortality because of cancer and cardiovascular disease (P for nonlinearity ≤ .033).
Again, the strongest association with those outcomes and genetically predicted 25-(OH)D was found at levels below 25 nmol/L and a plateau was seen by 50 nmol/L.
Compared with a measured 25-(OH)D concentration of 50 nmol/L, investigators estimated that the genetically predicted odds of all-cause mortality would increase sixfold (odds ratio, 6.00) for participants at 10 nmol/L and by 25% (OR, 1.25) for those at 25 nmol/L.
And, compared with a measured 25-(OH)D concentration of 50 nmol/L, those with 10 nmol/L had genetically predicted odds ratios of 5.98 for cardiovascular mortality, 3.37 for cancer mortality, and 12.44 for respiratory mortality.
Comparing measured 25-(OH)D concentrations of 25 nmol/L versus 50 nmol/L, odds ratios for those outcomes were 1.25, 1.16, and 1.96 (95% confidence interval, 1.88-4.67), respectively. All were statistically significant.
Consistent results supportive of a causal effect of genetically predicted 25-(OH)D on all-cause mortality in those with low measured vitamin D concentrations were also found in a sensitivity analysis of 20,837 people of non-White ethnic origin.
The study was funded by the Australian National Health and Medical Research Council. Dr. Sutherland’s studentship is funded by an Australian Research Training Program Scholarship.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE