User login
Smoking worsens disease outcomes in RA
Key clinical point: Smoking worsened disease activity and health-related quality of life at 1 year in patients with rheumatoid arthritis (RA), with effects being persistent at 3 years and early smoking cessation vs. continued smoking being associated with improved disease activity.
Major finding: At 1 year, current smokers vs. non-smokers were at a higher risk for a swollen joint number above the median (odds ratio [OR] 1.7; P = .001) and 36-Item Short-Form Health Survey physical (OR 1.5; P = .006) and mental (OR 1.4; P = .03) scores below the median, with effects being persistent at 3 years. Patients who stopped vs. continued smoking within 1 year reported a lower swollen joint number (P = .002).
Study details: Findings are from a population-based case-control study including 1531 patients with newly diagnosed RA who were followed-up for 3 years, of which 376 patients were current smokers.
Disclosures: This study was supported by grants from the Swedish Medical Research Council and other sources. The authors declared no conflicts of interest.
Source: Alfredsson L et al. Influence of smoking on disease activity and quality of life in patients with rheumatoid arthritis: Results from a Swedish case-control study with longitudinal follow-up. Arthritis Care Res (Hoboken). 2022 (Sep 23). Doi: 10.1002/acr.25026
Key clinical point: Smoking worsened disease activity and health-related quality of life at 1 year in patients with rheumatoid arthritis (RA), with effects being persistent at 3 years and early smoking cessation vs. continued smoking being associated with improved disease activity.
Major finding: At 1 year, current smokers vs. non-smokers were at a higher risk for a swollen joint number above the median (odds ratio [OR] 1.7; P = .001) and 36-Item Short-Form Health Survey physical (OR 1.5; P = .006) and mental (OR 1.4; P = .03) scores below the median, with effects being persistent at 3 years. Patients who stopped vs. continued smoking within 1 year reported a lower swollen joint number (P = .002).
Study details: Findings are from a population-based case-control study including 1531 patients with newly diagnosed RA who were followed-up for 3 years, of which 376 patients were current smokers.
Disclosures: This study was supported by grants from the Swedish Medical Research Council and other sources. The authors declared no conflicts of interest.
Source: Alfredsson L et al. Influence of smoking on disease activity and quality of life in patients with rheumatoid arthritis: Results from a Swedish case-control study with longitudinal follow-up. Arthritis Care Res (Hoboken). 2022 (Sep 23). Doi: 10.1002/acr.25026
Key clinical point: Smoking worsened disease activity and health-related quality of life at 1 year in patients with rheumatoid arthritis (RA), with effects being persistent at 3 years and early smoking cessation vs. continued smoking being associated with improved disease activity.
Major finding: At 1 year, current smokers vs. non-smokers were at a higher risk for a swollen joint number above the median (odds ratio [OR] 1.7; P = .001) and 36-Item Short-Form Health Survey physical (OR 1.5; P = .006) and mental (OR 1.4; P = .03) scores below the median, with effects being persistent at 3 years. Patients who stopped vs. continued smoking within 1 year reported a lower swollen joint number (P = .002).
Study details: Findings are from a population-based case-control study including 1531 patients with newly diagnosed RA who were followed-up for 3 years, of which 376 patients were current smokers.
Disclosures: This study was supported by grants from the Swedish Medical Research Council and other sources. The authors declared no conflicts of interest.
Source: Alfredsson L et al. Influence of smoking on disease activity and quality of life in patients with rheumatoid arthritis: Results from a Swedish case-control study with longitudinal follow-up. Arthritis Care Res (Hoboken). 2022 (Sep 23). Doi: 10.1002/acr.25026
Suboptimal early RA management predicts difficult-to-treat RA
Key clinical point: Failure to initiate methotrexate within 3 months and discontinue glucocorticoids within 6 months during early disease management were associated with difficult-to-treat rheumatoid arthritis (D2T-RA).
Major finding: A significantly lower proportion of patients with D2T-RA had adequate methotrexate treatment duration vs. those with non-D2T-RA (70.8% v. 85.5%; P = .022). Additionally, a significantly higher proportion of patients with D2T-RA vs non-D2T-RA continued glucocorticoids beyond 6 months (70.8% vs 33.8%; P < .001), with a delay of <3 months vs >12 months in methotrexate treatment (odds ratio [OR] 0.3; P = .031) and failure to discontinue glucocorticoids (OR 4.6; P < .001) being significantly associated with D2T-RA.
Study details: Findings are from a retrospective cohort study including 48 patients with D2T-RA and 145 patients with non-D2T-RA.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Giollo A et al. Early characterisation of difficult-to-treat rheumatoid arthritis by suboptimal initial management A multicentre cohort study. Rheumatology (Oxford). 2022 (Oct 3). Doi: 10.1093/rheumatology/keac563
Key clinical point: Failure to initiate methotrexate within 3 months and discontinue glucocorticoids within 6 months during early disease management were associated with difficult-to-treat rheumatoid arthritis (D2T-RA).
Major finding: A significantly lower proportion of patients with D2T-RA had adequate methotrexate treatment duration vs. those with non-D2T-RA (70.8% v. 85.5%; P = .022). Additionally, a significantly higher proportion of patients with D2T-RA vs non-D2T-RA continued glucocorticoids beyond 6 months (70.8% vs 33.8%; P < .001), with a delay of <3 months vs >12 months in methotrexate treatment (odds ratio [OR] 0.3; P = .031) and failure to discontinue glucocorticoids (OR 4.6; P < .001) being significantly associated with D2T-RA.
Study details: Findings are from a retrospective cohort study including 48 patients with D2T-RA and 145 patients with non-D2T-RA.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Giollo A et al. Early characterisation of difficult-to-treat rheumatoid arthritis by suboptimal initial management A multicentre cohort study. Rheumatology (Oxford). 2022 (Oct 3). Doi: 10.1093/rheumatology/keac563
Key clinical point: Failure to initiate methotrexate within 3 months and discontinue glucocorticoids within 6 months during early disease management were associated with difficult-to-treat rheumatoid arthritis (D2T-RA).
Major finding: A significantly lower proportion of patients with D2T-RA had adequate methotrexate treatment duration vs. those with non-D2T-RA (70.8% v. 85.5%; P = .022). Additionally, a significantly higher proportion of patients with D2T-RA vs non-D2T-RA continued glucocorticoids beyond 6 months (70.8% vs 33.8%; P < .001), with a delay of <3 months vs >12 months in methotrexate treatment (odds ratio [OR] 0.3; P = .031) and failure to discontinue glucocorticoids (OR 4.6; P < .001) being significantly associated with D2T-RA.
Study details: Findings are from a retrospective cohort study including 48 patients with D2T-RA and 145 patients with non-D2T-RA.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Giollo A et al. Early characterisation of difficult-to-treat rheumatoid arthritis by suboptimal initial management A multicentre cohort study. Rheumatology (Oxford). 2022 (Oct 3). Doi: 10.1093/rheumatology/keac563
RA: Increased risk for VTE with JAK inhibitors
Key clinical point: Patients with rheumatoid arthritis (RA) treated with Janus kinase (JAK) vs tumor necrosis factor (TNF) inhibitors were at a higher risk for venous thromboembolism (VTE), particularly pulmonary embolism.
Major finding: Patients treated with JAK vs TNF inhibitors were at a 73% higher risk for VTE (adjusted hazard ratio [aHR] 1.73; 95% CI 1.24-2.42), with the higher risk appearing to be confined to pulmonary embolism (aHR 3.21; 95% CI 2.11-4.88) rather than deep vein thrombosis.
Study details: Findings are from a prospective, register-based, active comparator study including 85,722 patients with RA, of which 27,610 patients initiated biologic/targeted synthetic disease-modifying antirheumatic drugs and were matched with 91,207 healthy controls.
Disclosures: This study was funded by Swedish Research Council, the Swedish Heart Lung Foundation, and other sources. Karolinska Institutet has or has had research agreements with various sources for safety monitoring of biologics through ARTIS/Swedish Biologics Register.
Source: Molander V et al. Venous thromboembolism with JAK inhibitors and other immune-modulatory drugs: A Swedish comparative safety study among patients with rheumatoid arthritis. Ann Rheum Dis. 2022 (Sep 23). Doi: 10.1136/ard-2022-223050
Key clinical point: Patients with rheumatoid arthritis (RA) treated with Janus kinase (JAK) vs tumor necrosis factor (TNF) inhibitors were at a higher risk for venous thromboembolism (VTE), particularly pulmonary embolism.
Major finding: Patients treated with JAK vs TNF inhibitors were at a 73% higher risk for VTE (adjusted hazard ratio [aHR] 1.73; 95% CI 1.24-2.42), with the higher risk appearing to be confined to pulmonary embolism (aHR 3.21; 95% CI 2.11-4.88) rather than deep vein thrombosis.
Study details: Findings are from a prospective, register-based, active comparator study including 85,722 patients with RA, of which 27,610 patients initiated biologic/targeted synthetic disease-modifying antirheumatic drugs and were matched with 91,207 healthy controls.
Disclosures: This study was funded by Swedish Research Council, the Swedish Heart Lung Foundation, and other sources. Karolinska Institutet has or has had research agreements with various sources for safety monitoring of biologics through ARTIS/Swedish Biologics Register.
Source: Molander V et al. Venous thromboembolism with JAK inhibitors and other immune-modulatory drugs: A Swedish comparative safety study among patients with rheumatoid arthritis. Ann Rheum Dis. 2022 (Sep 23). Doi: 10.1136/ard-2022-223050
Key clinical point: Patients with rheumatoid arthritis (RA) treated with Janus kinase (JAK) vs tumor necrosis factor (TNF) inhibitors were at a higher risk for venous thromboembolism (VTE), particularly pulmonary embolism.
Major finding: Patients treated with JAK vs TNF inhibitors were at a 73% higher risk for VTE (adjusted hazard ratio [aHR] 1.73; 95% CI 1.24-2.42), with the higher risk appearing to be confined to pulmonary embolism (aHR 3.21; 95% CI 2.11-4.88) rather than deep vein thrombosis.
Study details: Findings are from a prospective, register-based, active comparator study including 85,722 patients with RA, of which 27,610 patients initiated biologic/targeted synthetic disease-modifying antirheumatic drugs and were matched with 91,207 healthy controls.
Disclosures: This study was funded by Swedish Research Council, the Swedish Heart Lung Foundation, and other sources. Karolinska Institutet has or has had research agreements with various sources for safety monitoring of biologics through ARTIS/Swedish Biologics Register.
Source: Molander V et al. Venous thromboembolism with JAK inhibitors and other immune-modulatory drugs: A Swedish comparative safety study among patients with rheumatoid arthritis. Ann Rheum Dis. 2022 (Sep 23). Doi: 10.1136/ard-2022-223050
Withdrawing methotrexate increased disease activity without affecting remission rates in RA patients at target
Key clinical point: Withdrawal of methotrexate slightly worsened disease activity without affecting remission rates in patients with rheumatoid arthritis (RA) at target who were treated with the combination of biologic disease-modifying antirheumatic drugs (bDMARD)/targeted synthetic DMARD (tsDMARD) and methotrexate.
Major finding: Withdrawing methotrexate vs maintaining combination therapy increased the disease activity score of 28-joints by 0.20 (95% CI 0.09-0.32) and decreased the proportion of patients achieving low disease activity (risk ratio [RR] 0.88; 95% CI 0.80-0.97); however, the remission rates remained unaffected (RR 0.90; 95% CI 0.81-1.01).
Study details: Findings are from a systematic review and meta-analysis of six randomized controlled trials including 1430 patients with RA at target who were treated with bDMARD or tsDMARD+methotrexate combination therapy, of which 734 withdrew and 696 continued methotrexate.
Disclosures: This study was supported by West China Hospital, Sichuan University. The authors declared no conflicts of interest.
Source: Wang X et al. Withdrawal of MTX in rheumatoid arthritis patients on bDMARD/tsDMARD plus methotrexate at target: A systematic review and meta-analysis. Rheumatology (Oxford). 2022 (Sep 20). Doi: 10.1093/rheumatology/keac515
Key clinical point: Withdrawal of methotrexate slightly worsened disease activity without affecting remission rates in patients with rheumatoid arthritis (RA) at target who were treated with the combination of biologic disease-modifying antirheumatic drugs (bDMARD)/targeted synthetic DMARD (tsDMARD) and methotrexate.
Major finding: Withdrawing methotrexate vs maintaining combination therapy increased the disease activity score of 28-joints by 0.20 (95% CI 0.09-0.32) and decreased the proportion of patients achieving low disease activity (risk ratio [RR] 0.88; 95% CI 0.80-0.97); however, the remission rates remained unaffected (RR 0.90; 95% CI 0.81-1.01).
Study details: Findings are from a systematic review and meta-analysis of six randomized controlled trials including 1430 patients with RA at target who were treated with bDMARD or tsDMARD+methotrexate combination therapy, of which 734 withdrew and 696 continued methotrexate.
Disclosures: This study was supported by West China Hospital, Sichuan University. The authors declared no conflicts of interest.
Source: Wang X et al. Withdrawal of MTX in rheumatoid arthritis patients on bDMARD/tsDMARD plus methotrexate at target: A systematic review and meta-analysis. Rheumatology (Oxford). 2022 (Sep 20). Doi: 10.1093/rheumatology/keac515
Key clinical point: Withdrawal of methotrexate slightly worsened disease activity without affecting remission rates in patients with rheumatoid arthritis (RA) at target who were treated with the combination of biologic disease-modifying antirheumatic drugs (bDMARD)/targeted synthetic DMARD (tsDMARD) and methotrexate.
Major finding: Withdrawing methotrexate vs maintaining combination therapy increased the disease activity score of 28-joints by 0.20 (95% CI 0.09-0.32) and decreased the proportion of patients achieving low disease activity (risk ratio [RR] 0.88; 95% CI 0.80-0.97); however, the remission rates remained unaffected (RR 0.90; 95% CI 0.81-1.01).
Study details: Findings are from a systematic review and meta-analysis of six randomized controlled trials including 1430 patients with RA at target who were treated with bDMARD or tsDMARD+methotrexate combination therapy, of which 734 withdrew and 696 continued methotrexate.
Disclosures: This study was supported by West China Hospital, Sichuan University. The authors declared no conflicts of interest.
Source: Wang X et al. Withdrawal of MTX in rheumatoid arthritis patients on bDMARD/tsDMARD plus methotrexate at target: A systematic review and meta-analysis. Rheumatology (Oxford). 2022 (Sep 20). Doi: 10.1093/rheumatology/keac515
RA in remission: Subclinical inflammation lower with cs/bDMARDs combination vs. monotherapy
Key clinical point: Ultrasound-detected subclinical inflammation of tendons and joints was better controlled in patients with rheumatoid arthritis (RA) in clinical remission who received the combination therapy of conventional synthetic and biologic disease-modifying antirheumatic drugs (csDMARD+bDMARD) vs csDMARD or bDMARD monotherapy.
Major finding: Grey-scale tenosynovitis (P = .025) and power Doppler (PD) tenosynovitis (P = .047) were better controlled with csDMARD+bDMARD than with csDMARD alone. csDMARD+bDMARD was also associated with better treatment results for PD synovitis vs csDMARD (P = .01) or bDMARD (P = .02) alone.
Study details: Findings are from a longitudinal analysis of the STARTER study including 256 patients with RA in clinical remission who received csDMARD alone, bDMARD alone, or csDMARD+bDMARD.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Parisi S et al. Relationship between the prevalence of subclinical tenosynovitis and treatment in patients with RA in clinical remission: STARTER study. Rheumatology (Oxford). 2022 (Sep 6). Doi: 10.1093/rheumatology/keac518
Key clinical point: Ultrasound-detected subclinical inflammation of tendons and joints was better controlled in patients with rheumatoid arthritis (RA) in clinical remission who received the combination therapy of conventional synthetic and biologic disease-modifying antirheumatic drugs (csDMARD+bDMARD) vs csDMARD or bDMARD monotherapy.
Major finding: Grey-scale tenosynovitis (P = .025) and power Doppler (PD) tenosynovitis (P = .047) were better controlled with csDMARD+bDMARD than with csDMARD alone. csDMARD+bDMARD was also associated with better treatment results for PD synovitis vs csDMARD (P = .01) or bDMARD (P = .02) alone.
Study details: Findings are from a longitudinal analysis of the STARTER study including 256 patients with RA in clinical remission who received csDMARD alone, bDMARD alone, or csDMARD+bDMARD.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Parisi S et al. Relationship between the prevalence of subclinical tenosynovitis and treatment in patients with RA in clinical remission: STARTER study. Rheumatology (Oxford). 2022 (Sep 6). Doi: 10.1093/rheumatology/keac518
Key clinical point: Ultrasound-detected subclinical inflammation of tendons and joints was better controlled in patients with rheumatoid arthritis (RA) in clinical remission who received the combination therapy of conventional synthetic and biologic disease-modifying antirheumatic drugs (csDMARD+bDMARD) vs csDMARD or bDMARD monotherapy.
Major finding: Grey-scale tenosynovitis (P = .025) and power Doppler (PD) tenosynovitis (P = .047) were better controlled with csDMARD+bDMARD than with csDMARD alone. csDMARD+bDMARD was also associated with better treatment results for PD synovitis vs csDMARD (P = .01) or bDMARD (P = .02) alone.
Study details: Findings are from a longitudinal analysis of the STARTER study including 256 patients with RA in clinical remission who received csDMARD alone, bDMARD alone, or csDMARD+bDMARD.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Parisi S et al. Relationship between the prevalence of subclinical tenosynovitis and treatment in patients with RA in clinical remission: STARTER study. Rheumatology (Oxford). 2022 (Sep 6). Doi: 10.1093/rheumatology/keac518
Increased risk for severe COVID-19 in rheumatoid arthritis and its phenotypic subgroups
Key clinical point: Patients with rheumatoid arthritis (RA), including those across different phenotypic subgroups, were at an increased risk for severe COVID-19 compared with patients without RA, with a pronounced association being observed in patients with RA-associated interstitial lung disease (RA-ILD).
Major finding: Risk for severe COVID-19 was significantly higher in patients with RA vs. those without RA (adjusted hazard ratio [aHR], 1.75; P < .0001). Risk was persistent among the sub-group of patients who were seropositive (aHR, 1.97; P < .0001) or had erosive disease (aHR, 1.93; P < .0001) and most prominent among patients with RA-ILD (aHR, 2.50; P < .0001).
Study details: Findings are from a retrospective study of 582 patients with RA and 2,875 matched comparators without RA, all of whom had COVID-19.
Disclosures: This study did not receive any funding. Some authors reported receiving research support, consulting fees, and/or grants unrelated to this study from various sources.
Source: Figueroa-Parra G et al. Risk of severe COVID-19 outcomes associated with rheumatoid arthritis and phenotypic subgroups: A retrospective, comparative, multicentre cohort study. Lancet Rheumatol. 2022 (Sep 13). Doi: 10.1016/S2665-9913(22)00227-2.
Key clinical point: Patients with rheumatoid arthritis (RA), including those across different phenotypic subgroups, were at an increased risk for severe COVID-19 compared with patients without RA, with a pronounced association being observed in patients with RA-associated interstitial lung disease (RA-ILD).
Major finding: Risk for severe COVID-19 was significantly higher in patients with RA vs. those without RA (adjusted hazard ratio [aHR], 1.75; P < .0001). Risk was persistent among the sub-group of patients who were seropositive (aHR, 1.97; P < .0001) or had erosive disease (aHR, 1.93; P < .0001) and most prominent among patients with RA-ILD (aHR, 2.50; P < .0001).
Study details: Findings are from a retrospective study of 582 patients with RA and 2,875 matched comparators without RA, all of whom had COVID-19.
Disclosures: This study did not receive any funding. Some authors reported receiving research support, consulting fees, and/or grants unrelated to this study from various sources.
Source: Figueroa-Parra G et al. Risk of severe COVID-19 outcomes associated with rheumatoid arthritis and phenotypic subgroups: A retrospective, comparative, multicentre cohort study. Lancet Rheumatol. 2022 (Sep 13). Doi: 10.1016/S2665-9913(22)00227-2.
Key clinical point: Patients with rheumatoid arthritis (RA), including those across different phenotypic subgroups, were at an increased risk for severe COVID-19 compared with patients without RA, with a pronounced association being observed in patients with RA-associated interstitial lung disease (RA-ILD).
Major finding: Risk for severe COVID-19 was significantly higher in patients with RA vs. those without RA (adjusted hazard ratio [aHR], 1.75; P < .0001). Risk was persistent among the sub-group of patients who were seropositive (aHR, 1.97; P < .0001) or had erosive disease (aHR, 1.93; P < .0001) and most prominent among patients with RA-ILD (aHR, 2.50; P < .0001).
Study details: Findings are from a retrospective study of 582 patients with RA and 2,875 matched comparators without RA, all of whom had COVID-19.
Disclosures: This study did not receive any funding. Some authors reported receiving research support, consulting fees, and/or grants unrelated to this study from various sources.
Source: Figueroa-Parra G et al. Risk of severe COVID-19 outcomes associated with rheumatoid arthritis and phenotypic subgroups: A retrospective, comparative, multicentre cohort study. Lancet Rheumatol. 2022 (Sep 13). Doi: 10.1016/S2665-9913(22)00227-2.
FDA OKs teclistamab for relapsed/refractory multiple myeloma
The results made the case for “teclistamab as a monotherapy for eligible patients with heavily pretreated multiple myeloma, in need of new treatment options,” investigator Maria-Victoria Mateos, MD, PhD, a hematologist at the University Hospital of Salamanca (Spain) said in a June press release from drug maker Janssen/Johnson & Johnson.
The approval was based on the phase 1-2 single-arm MajesTEC-1 trial. The MajesTEC-1 findings, published in August in the New England Journal of Medicine, included 165 patients with relapsed or refractory multiple myeloma after at least three therapy lines – including an immunomodulatory drug, a proteasome inhibitor, and an anti-CD38 antibody. These patients received a weekly subcutaneous 1.5 mg/kg injection of teclistamab after stepping up from 0.06 mg and 0.3 mg/kg doses.
According to the FDA announcement, the overall response rate was nearly 62%. And the estimated duration of response rate among responders was 90.6% at 6 months and 66.5% at 9 months.
The NEJM results also indicated that almost 40% of patients had a complete response to the therapy, over a median follow-up of 14.1 months. More than a quarter of patients (26.7%) had no minimal residual disease.
The study investigators concluded that “teclistamab resulted in a high rate of deep and durable response in patients with triple-class exposed relapsed or refractory multiple myeloma.”
In a press release, Michael Andreini, president and CEO of the Multiple Myeloma Research Foundation, commented that “teclistamab is an important new treatment option for patients who have faced multiple relapses.”
The recommended dose for teclistamab is 0.06 mg/kg via subcutaneous injection on day 1, 0.3 mg/kg on day 4, and 1.5 mg/kg on day 7, followed by 1.5 mg/kg once weekly until disease progression or unacceptable toxicity.
The FDA noted, however, that the prescribing information for teclistamab comes with a Boxed warning for “life-threatening or fatal cytokine-release syndrome (CRS) and neurologic toxicity, including immune effector cell–associated neurotoxicity (ICANS).”
CRS was reported in 72.1% of patients (grade 3 in one patient but no grade 4 cases), neurologic toxicity in 57%, and ICANS in 6%. Other common adverse events in the NEJM report included neutropenia in 71% of subjects (grade 3 or 4 in 64%); anemia in 52% (grade 3 or 4 in 37%), and thrombocytopenia in 40% (grade 3 or 4 in 21%). Overall, 45% of patients developed grade 3 or 4 infections.
“Because of the risks of CRS and neurologic toxicity, including ICANS, teclistamab-cqyv is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS), called the Tecvayli REMS,” according to the FDA’s press release.
Teclistamab is a T-cell–bispecific antibody that targets both CD3 expressed on the surface of T cells and B-cell maturation antigen (BCMA) expressed on the surface of myeloma cells, activating T-cells to kill cancer cells that express the antigen.
Three BCMA-directed therapies are already on the market in the United States that carry teclistamab’s indication: the antibody-drug conjugate belantamab mafodotin (Blenrep) and two chimeric antigen receptor (CAR) T-cell therapies, idecabtagene vicleucel (Abecma) and ciltacabtagene autoleucel (Carvykti).
The overall response rate is approximately 31% with belantamab mafodotin, 67% for idecabtagene vicleucel, and 83% for ciltacabtagene autoleucel.
Pfizer also has a bispecific BCMA-CD3–targeted antibody in development, elranatamab, for triple-class refractory multiple myeloma that is expected to compete with teclistamab.
MajesTEC-1 was funded by Janssen. Dr. Mateos is a paid speaker and consultant for the company.
A version of this article first appeared on Medscape.com.
The results made the case for “teclistamab as a monotherapy for eligible patients with heavily pretreated multiple myeloma, in need of new treatment options,” investigator Maria-Victoria Mateos, MD, PhD, a hematologist at the University Hospital of Salamanca (Spain) said in a June press release from drug maker Janssen/Johnson & Johnson.
The approval was based on the phase 1-2 single-arm MajesTEC-1 trial. The MajesTEC-1 findings, published in August in the New England Journal of Medicine, included 165 patients with relapsed or refractory multiple myeloma after at least three therapy lines – including an immunomodulatory drug, a proteasome inhibitor, and an anti-CD38 antibody. These patients received a weekly subcutaneous 1.5 mg/kg injection of teclistamab after stepping up from 0.06 mg and 0.3 mg/kg doses.
According to the FDA announcement, the overall response rate was nearly 62%. And the estimated duration of response rate among responders was 90.6% at 6 months and 66.5% at 9 months.
The NEJM results also indicated that almost 40% of patients had a complete response to the therapy, over a median follow-up of 14.1 months. More than a quarter of patients (26.7%) had no minimal residual disease.
The study investigators concluded that “teclistamab resulted in a high rate of deep and durable response in patients with triple-class exposed relapsed or refractory multiple myeloma.”
In a press release, Michael Andreini, president and CEO of the Multiple Myeloma Research Foundation, commented that “teclistamab is an important new treatment option for patients who have faced multiple relapses.”
The recommended dose for teclistamab is 0.06 mg/kg via subcutaneous injection on day 1, 0.3 mg/kg on day 4, and 1.5 mg/kg on day 7, followed by 1.5 mg/kg once weekly until disease progression or unacceptable toxicity.
The FDA noted, however, that the prescribing information for teclistamab comes with a Boxed warning for “life-threatening or fatal cytokine-release syndrome (CRS) and neurologic toxicity, including immune effector cell–associated neurotoxicity (ICANS).”
CRS was reported in 72.1% of patients (grade 3 in one patient but no grade 4 cases), neurologic toxicity in 57%, and ICANS in 6%. Other common adverse events in the NEJM report included neutropenia in 71% of subjects (grade 3 or 4 in 64%); anemia in 52% (grade 3 or 4 in 37%), and thrombocytopenia in 40% (grade 3 or 4 in 21%). Overall, 45% of patients developed grade 3 or 4 infections.
“Because of the risks of CRS and neurologic toxicity, including ICANS, teclistamab-cqyv is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS), called the Tecvayli REMS,” according to the FDA’s press release.
Teclistamab is a T-cell–bispecific antibody that targets both CD3 expressed on the surface of T cells and B-cell maturation antigen (BCMA) expressed on the surface of myeloma cells, activating T-cells to kill cancer cells that express the antigen.
Three BCMA-directed therapies are already on the market in the United States that carry teclistamab’s indication: the antibody-drug conjugate belantamab mafodotin (Blenrep) and two chimeric antigen receptor (CAR) T-cell therapies, idecabtagene vicleucel (Abecma) and ciltacabtagene autoleucel (Carvykti).
The overall response rate is approximately 31% with belantamab mafodotin, 67% for idecabtagene vicleucel, and 83% for ciltacabtagene autoleucel.
Pfizer also has a bispecific BCMA-CD3–targeted antibody in development, elranatamab, for triple-class refractory multiple myeloma that is expected to compete with teclistamab.
MajesTEC-1 was funded by Janssen. Dr. Mateos is a paid speaker and consultant for the company.
A version of this article first appeared on Medscape.com.
The results made the case for “teclistamab as a monotherapy for eligible patients with heavily pretreated multiple myeloma, in need of new treatment options,” investigator Maria-Victoria Mateos, MD, PhD, a hematologist at the University Hospital of Salamanca (Spain) said in a June press release from drug maker Janssen/Johnson & Johnson.
The approval was based on the phase 1-2 single-arm MajesTEC-1 trial. The MajesTEC-1 findings, published in August in the New England Journal of Medicine, included 165 patients with relapsed or refractory multiple myeloma after at least three therapy lines – including an immunomodulatory drug, a proteasome inhibitor, and an anti-CD38 antibody. These patients received a weekly subcutaneous 1.5 mg/kg injection of teclistamab after stepping up from 0.06 mg and 0.3 mg/kg doses.
According to the FDA announcement, the overall response rate was nearly 62%. And the estimated duration of response rate among responders was 90.6% at 6 months and 66.5% at 9 months.
The NEJM results also indicated that almost 40% of patients had a complete response to the therapy, over a median follow-up of 14.1 months. More than a quarter of patients (26.7%) had no minimal residual disease.
The study investigators concluded that “teclistamab resulted in a high rate of deep and durable response in patients with triple-class exposed relapsed or refractory multiple myeloma.”
In a press release, Michael Andreini, president and CEO of the Multiple Myeloma Research Foundation, commented that “teclistamab is an important new treatment option for patients who have faced multiple relapses.”
The recommended dose for teclistamab is 0.06 mg/kg via subcutaneous injection on day 1, 0.3 mg/kg on day 4, and 1.5 mg/kg on day 7, followed by 1.5 mg/kg once weekly until disease progression or unacceptable toxicity.
The FDA noted, however, that the prescribing information for teclistamab comes with a Boxed warning for “life-threatening or fatal cytokine-release syndrome (CRS) and neurologic toxicity, including immune effector cell–associated neurotoxicity (ICANS).”
CRS was reported in 72.1% of patients (grade 3 in one patient but no grade 4 cases), neurologic toxicity in 57%, and ICANS in 6%. Other common adverse events in the NEJM report included neutropenia in 71% of subjects (grade 3 or 4 in 64%); anemia in 52% (grade 3 or 4 in 37%), and thrombocytopenia in 40% (grade 3 or 4 in 21%). Overall, 45% of patients developed grade 3 or 4 infections.
“Because of the risks of CRS and neurologic toxicity, including ICANS, teclistamab-cqyv is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS), called the Tecvayli REMS,” according to the FDA’s press release.
Teclistamab is a T-cell–bispecific antibody that targets both CD3 expressed on the surface of T cells and B-cell maturation antigen (BCMA) expressed on the surface of myeloma cells, activating T-cells to kill cancer cells that express the antigen.
Three BCMA-directed therapies are already on the market in the United States that carry teclistamab’s indication: the antibody-drug conjugate belantamab mafodotin (Blenrep) and two chimeric antigen receptor (CAR) T-cell therapies, idecabtagene vicleucel (Abecma) and ciltacabtagene autoleucel (Carvykti).
The overall response rate is approximately 31% with belantamab mafodotin, 67% for idecabtagene vicleucel, and 83% for ciltacabtagene autoleucel.
Pfizer also has a bispecific BCMA-CD3–targeted antibody in development, elranatamab, for triple-class refractory multiple myeloma that is expected to compete with teclistamab.
MajesTEC-1 was funded by Janssen. Dr. Mateos is a paid speaker and consultant for the company.
A version of this article first appeared on Medscape.com.
Some young CRC patients are missing out on genetic counseling, testing
CHARLOTTE, N.C. – Nearly one-fourth of patients with early-onset colorectal cancer don’t get referrals for genetic counseling or testing, and although acceptance of genetic counseling has improved over the last 10 years, there is still a notable gap between referrals and uptake, investigators have found.
Among 791 patients with young- or early-onset colorectal cancer (YOCRC) seen at a large medical center from 2010 through 2019, 62.1% were referred for genetic counseling, but only 80.1% of this group followed through with the referrals by scheduling an appointment with a counselor or having genetic testing performed, reported Hareem Syed, MD, from the department of internal medicine at the Cleveland Clinic.
“Our findings highlight the need for health systems to implement care pathways to optimize genetic counseling referral and testing in all young-onset colorectal cancer patients,” she said at the annual meeting of the American College of Gastroenterology.
The incidence of CRC diagnosed in persons younger than 50 years is increasing and has been projected to double by 2030, Dr. Syed noted.
In 2009, the Eastern Cooperative Oncology Group recommended that all patients with colorectal cancer be screened for the Lynch syndrome, and earlier this year the National Comprehensive Cancer Network issued a recommendation that patients with YOCRC undergo germline multigene panel testing (MGPT). MGPT has shown that as many as 30% of patients with YOCRC carry a germline pathogenic variant that predisposes them to CRC, regardless of family history, she said.
“We hypothesized that the rate of referral to genetic counseling in this population is low despite the high incidence of pathogenic germline variants, but the uptake of genetic counseling is high [when referred],” Dr. Syed said.
How often, and who needs it?
The investigators sought to determine the frequency of referral to genetic counseling and patient uptake of referrals to assess factors associated with referrals and with uptake, and to evaluate the results of genetic testing.
They reviewed records on all patients younger than 50 years seen at the Cleveland Clinic for CRC from 2010 through 2019, excluding those with appendiceal cancers, a family history of a hereditary cancer syndrome, or irritable bowel syndrome.
The information they extracted from electronic medical records included patient age, sex, family history of CRC, income, tumor stage, and the location and time period of CRC diagnosis.
They considered a genetic counseling referral to be either an order for counseling in the record; clinical documentation of a referral in an office visit with colorectal surgery, oncology, or gastroenterology specialists; or documentation of a completed visit with a genetic counselor.
They considered patient uptake of a counseling referral as either a completed visit to the counselor or documentation of genetic testing results.
The mean patient age at diagnosis was 44 years, with 57.3% of patients male, and 42.7% female. The large majority of patients (86.5%) were White. In all, 40.2% of patients had a family history of CRC.
As noted above, 62.1% of the 791 patients included in the study were referred for counseling, and 80.1% of those referred followed through with uptake. Of this group, nearly all (97.1%) completed genetic testing.
In univariate analysis, factors associated with referral included older patient age at diagnosis, which showed that patients approaching 50 were less likely to receive a referral (odds ratio, 0.904), year of diagnosis with patients diagnosed in the most recent period more likely to receive a referral (OR, 1.247), and family history of CRC (OR, 2.195).
In multivariate analysis, factors significantly associated with referral were age at diagnosis (OR, 0.89), family history of CRC (OR, 2.112), and year of diagnosis (for 2017-19 vs. 2010-13, OR, 5.361).
Among 377 patients who completed genetic testing, 21% were found to have a pathogenic variant, 23% had variants of unknown significance, and 56% had no variants detected. The most commonly detected pathogenic variants were the Lynch syndrome and adenomatous polyposis.
Educate patients and physicians
In an interview, Daniel J. Pambianco, MD, from Charlottesville (Va.) Gastroenterology Associates, who was not involved in the study, commented that patient perceptions about the consequences of genetic testing may be a barrier to either getting a referral for counseling or following through on one.
“Oftentimes patients will perceive anything with ‘genetic’ in it as if their genes are somehow being manipulated, and we need to do a better job at educating patients in that regard,” he said.
Physicians, both primary care practitioners and gastroenterologists, also need to fully appreciate the importance of genetic testing in this population, “because in essence there may be a 4%, 5%, or 6% risk of genetic syndromes that we’re missing and cannot pick up just from getting patients’ histories,” he said.
The investigators did not report a study funding source. Dr. Syed and Dr. Pambianco reported having no relevant financial disclosures.
CHARLOTTE, N.C. – Nearly one-fourth of patients with early-onset colorectal cancer don’t get referrals for genetic counseling or testing, and although acceptance of genetic counseling has improved over the last 10 years, there is still a notable gap between referrals and uptake, investigators have found.
Among 791 patients with young- or early-onset colorectal cancer (YOCRC) seen at a large medical center from 2010 through 2019, 62.1% were referred for genetic counseling, but only 80.1% of this group followed through with the referrals by scheduling an appointment with a counselor or having genetic testing performed, reported Hareem Syed, MD, from the department of internal medicine at the Cleveland Clinic.
“Our findings highlight the need for health systems to implement care pathways to optimize genetic counseling referral and testing in all young-onset colorectal cancer patients,” she said at the annual meeting of the American College of Gastroenterology.
The incidence of CRC diagnosed in persons younger than 50 years is increasing and has been projected to double by 2030, Dr. Syed noted.
In 2009, the Eastern Cooperative Oncology Group recommended that all patients with colorectal cancer be screened for the Lynch syndrome, and earlier this year the National Comprehensive Cancer Network issued a recommendation that patients with YOCRC undergo germline multigene panel testing (MGPT). MGPT has shown that as many as 30% of patients with YOCRC carry a germline pathogenic variant that predisposes them to CRC, regardless of family history, she said.
“We hypothesized that the rate of referral to genetic counseling in this population is low despite the high incidence of pathogenic germline variants, but the uptake of genetic counseling is high [when referred],” Dr. Syed said.
How often, and who needs it?
The investigators sought to determine the frequency of referral to genetic counseling and patient uptake of referrals to assess factors associated with referrals and with uptake, and to evaluate the results of genetic testing.
They reviewed records on all patients younger than 50 years seen at the Cleveland Clinic for CRC from 2010 through 2019, excluding those with appendiceal cancers, a family history of a hereditary cancer syndrome, or irritable bowel syndrome.
The information they extracted from electronic medical records included patient age, sex, family history of CRC, income, tumor stage, and the location and time period of CRC diagnosis.
They considered a genetic counseling referral to be either an order for counseling in the record; clinical documentation of a referral in an office visit with colorectal surgery, oncology, or gastroenterology specialists; or documentation of a completed visit with a genetic counselor.
They considered patient uptake of a counseling referral as either a completed visit to the counselor or documentation of genetic testing results.
The mean patient age at diagnosis was 44 years, with 57.3% of patients male, and 42.7% female. The large majority of patients (86.5%) were White. In all, 40.2% of patients had a family history of CRC.
As noted above, 62.1% of the 791 patients included in the study were referred for counseling, and 80.1% of those referred followed through with uptake. Of this group, nearly all (97.1%) completed genetic testing.
In univariate analysis, factors associated with referral included older patient age at diagnosis, which showed that patients approaching 50 were less likely to receive a referral (odds ratio, 0.904), year of diagnosis with patients diagnosed in the most recent period more likely to receive a referral (OR, 1.247), and family history of CRC (OR, 2.195).
In multivariate analysis, factors significantly associated with referral were age at diagnosis (OR, 0.89), family history of CRC (OR, 2.112), and year of diagnosis (for 2017-19 vs. 2010-13, OR, 5.361).
Among 377 patients who completed genetic testing, 21% were found to have a pathogenic variant, 23% had variants of unknown significance, and 56% had no variants detected. The most commonly detected pathogenic variants were the Lynch syndrome and adenomatous polyposis.
Educate patients and physicians
In an interview, Daniel J. Pambianco, MD, from Charlottesville (Va.) Gastroenterology Associates, who was not involved in the study, commented that patient perceptions about the consequences of genetic testing may be a barrier to either getting a referral for counseling or following through on one.
“Oftentimes patients will perceive anything with ‘genetic’ in it as if their genes are somehow being manipulated, and we need to do a better job at educating patients in that regard,” he said.
Physicians, both primary care practitioners and gastroenterologists, also need to fully appreciate the importance of genetic testing in this population, “because in essence there may be a 4%, 5%, or 6% risk of genetic syndromes that we’re missing and cannot pick up just from getting patients’ histories,” he said.
The investigators did not report a study funding source. Dr. Syed and Dr. Pambianco reported having no relevant financial disclosures.
CHARLOTTE, N.C. – Nearly one-fourth of patients with early-onset colorectal cancer don’t get referrals for genetic counseling or testing, and although acceptance of genetic counseling has improved over the last 10 years, there is still a notable gap between referrals and uptake, investigators have found.
Among 791 patients with young- or early-onset colorectal cancer (YOCRC) seen at a large medical center from 2010 through 2019, 62.1% were referred for genetic counseling, but only 80.1% of this group followed through with the referrals by scheduling an appointment with a counselor or having genetic testing performed, reported Hareem Syed, MD, from the department of internal medicine at the Cleveland Clinic.
“Our findings highlight the need for health systems to implement care pathways to optimize genetic counseling referral and testing in all young-onset colorectal cancer patients,” she said at the annual meeting of the American College of Gastroenterology.
The incidence of CRC diagnosed in persons younger than 50 years is increasing and has been projected to double by 2030, Dr. Syed noted.
In 2009, the Eastern Cooperative Oncology Group recommended that all patients with colorectal cancer be screened for the Lynch syndrome, and earlier this year the National Comprehensive Cancer Network issued a recommendation that patients with YOCRC undergo germline multigene panel testing (MGPT). MGPT has shown that as many as 30% of patients with YOCRC carry a germline pathogenic variant that predisposes them to CRC, regardless of family history, she said.
“We hypothesized that the rate of referral to genetic counseling in this population is low despite the high incidence of pathogenic germline variants, but the uptake of genetic counseling is high [when referred],” Dr. Syed said.
How often, and who needs it?
The investigators sought to determine the frequency of referral to genetic counseling and patient uptake of referrals to assess factors associated with referrals and with uptake, and to evaluate the results of genetic testing.
They reviewed records on all patients younger than 50 years seen at the Cleveland Clinic for CRC from 2010 through 2019, excluding those with appendiceal cancers, a family history of a hereditary cancer syndrome, or irritable bowel syndrome.
The information they extracted from electronic medical records included patient age, sex, family history of CRC, income, tumor stage, and the location and time period of CRC diagnosis.
They considered a genetic counseling referral to be either an order for counseling in the record; clinical documentation of a referral in an office visit with colorectal surgery, oncology, or gastroenterology specialists; or documentation of a completed visit with a genetic counselor.
They considered patient uptake of a counseling referral as either a completed visit to the counselor or documentation of genetic testing results.
The mean patient age at diagnosis was 44 years, with 57.3% of patients male, and 42.7% female. The large majority of patients (86.5%) were White. In all, 40.2% of patients had a family history of CRC.
As noted above, 62.1% of the 791 patients included in the study were referred for counseling, and 80.1% of those referred followed through with uptake. Of this group, nearly all (97.1%) completed genetic testing.
In univariate analysis, factors associated with referral included older patient age at diagnosis, which showed that patients approaching 50 were less likely to receive a referral (odds ratio, 0.904), year of diagnosis with patients diagnosed in the most recent period more likely to receive a referral (OR, 1.247), and family history of CRC (OR, 2.195).
In multivariate analysis, factors significantly associated with referral were age at diagnosis (OR, 0.89), family history of CRC (OR, 2.112), and year of diagnosis (for 2017-19 vs. 2010-13, OR, 5.361).
Among 377 patients who completed genetic testing, 21% were found to have a pathogenic variant, 23% had variants of unknown significance, and 56% had no variants detected. The most commonly detected pathogenic variants were the Lynch syndrome and adenomatous polyposis.
Educate patients and physicians
In an interview, Daniel J. Pambianco, MD, from Charlottesville (Va.) Gastroenterology Associates, who was not involved in the study, commented that patient perceptions about the consequences of genetic testing may be a barrier to either getting a referral for counseling or following through on one.
“Oftentimes patients will perceive anything with ‘genetic’ in it as if their genes are somehow being manipulated, and we need to do a better job at educating patients in that regard,” he said.
Physicians, both primary care practitioners and gastroenterologists, also need to fully appreciate the importance of genetic testing in this population, “because in essence there may be a 4%, 5%, or 6% risk of genetic syndromes that we’re missing and cannot pick up just from getting patients’ histories,” he said.
The investigators did not report a study funding source. Dr. Syed and Dr. Pambianco reported having no relevant financial disclosures.
AT ACG 2022
Children and COVID: Weekly cases fall to lowest level in over a year
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.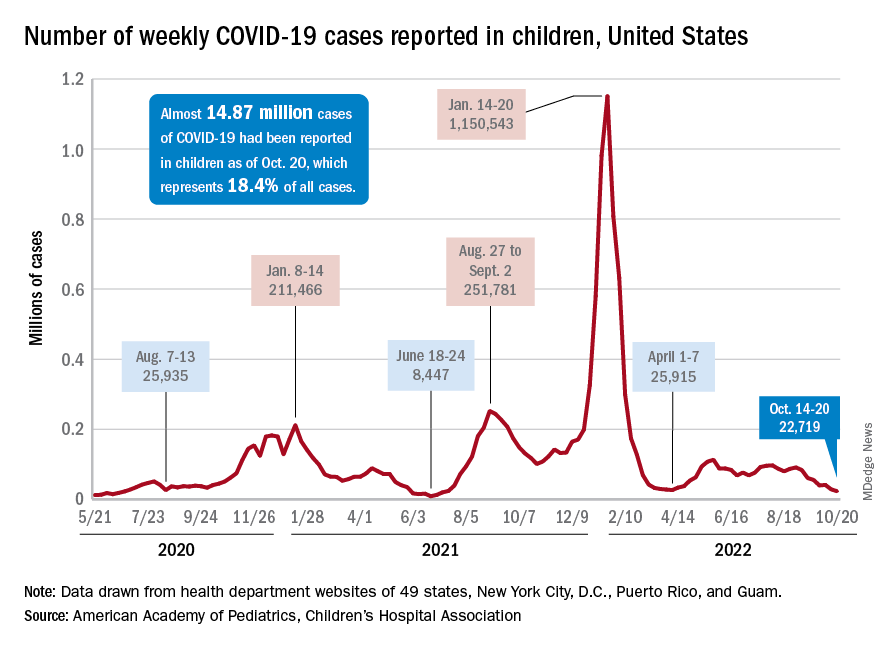
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.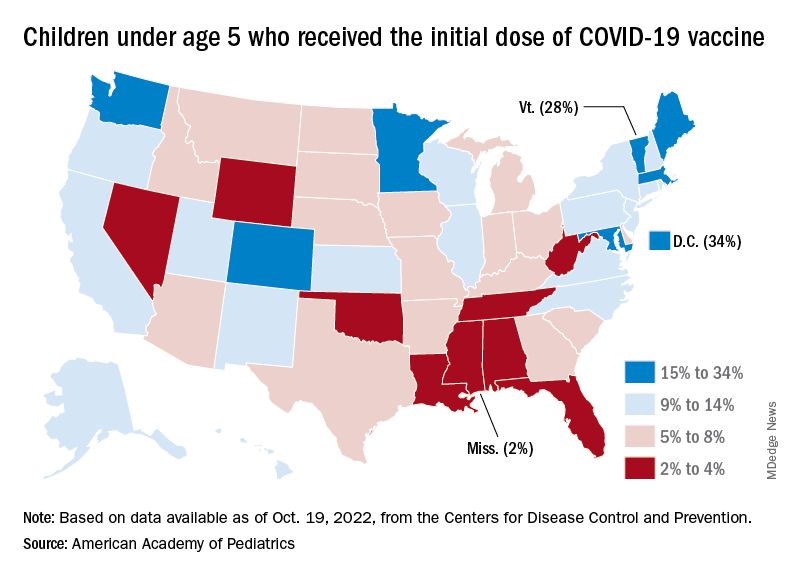
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
EUS-guided RF ablation doubles survival for unresectable pancreatic cancer
CHARLOTTE, N.C. – In a small proof-of-concept study, patients with small unresectable pancreatic cancers treated with endoscopic ultrasound–guided radiofrequency ablation (EUS-RFA) had a more than twofold improvement in overall survival compared with historical controls with a similar disease history, investigators in Thailand found.
In a weighted analysis, median weighted overall survival – the primary outcome – was 14 months among 11 patients who underwent EUS-RFA, compared with 6.1 months for 35 matched controls, translating into a hazard ratio for death with EUS-RFA of 0.38 (P = .016), reported Chawin Lopimpisuth, MD, from King Chulalongkorn Memorial Hospital in Bangkok, Thailand.
Median weighted progression-free survival (PFS) was longer among cases than controls, but did not differ significantly, at 6.1 months and 3.9 months, respectively.
“In patients with unresectable pancreatic ductal adenocarcinomas that are less than 4 cm, EUS-RFA alone or combined with chemotherapy resulted in significantly improved overall survival and tended to improve progression-free survival with minimal adverse events,” Dr. Lopimpisuth reported at the annual meeting of the American College of Gastroenterology.
Small but unresectable tumors
Endoscopically guided radiofrequency ablation of pancreatic ductal tumors has been shown to be both feasible and safe in previous studies, he said, prompting his group to explore whether EUS-RFA could help to control the primary tumor and improve survival outcomes.
They enrolled 11 patients with primary pancreatic ductal adenocarcinoma tumors less than 4 cm in diameter that were unresectable due to blood vessel involvement or distant metastasis, and used propensity-score matching to pair them with a total of 35 controls. Controls were matched by tumor size, staging, age-adjusted Charlson Comorbidity Index, chemotherapy regimen received, and interactions between CCI, regimen, and staging.
The results were weighted to assure that covariate distribution among patients treated with chemotherapy only equaled that of patients who underwent EUS-RFA.
Patients underwent EUS-RFA with a 19-gauge needle, with 50 watts of energy delivered with an impedance of 100 ohms. Those patients deemed able to tolerate chemotherapy received that as well.
After a minimum of 1 year of follow-up, the median weighted survival, as noted before, was 14 months for patients who received EUS-RFA, compared with 6.1 months for controls.
Adjusted survival probabilities at 6 and 12 months were 73% and 64%, respectively, for patients in the EUS-RFA group, compared with 69% and 17% for controls. Adjusted PFS rates at 6 and 12 months were 55% and 36% in the EUS-RFA group, compared with 28% and 4% in the control group.
The only adverse event of significance was mild abdominal pain, reported by 8.3% of total EUS-RFA procedures.
Promising but preliminary
In an interview with this news organization, ACG President Samir A. Shah, MD, from Brown University and Miriam Hospital in Providence, R.I., who was not involved in the study, commented that “we have limited options with these patients, so it’s really exciting to see an initial trend toward efficacy, and their survival improvement was significant by several months.”
Dr. Shah was a moderator of the presidential symposium where the data were presented.
Comoderator Brooks D. Cash, MD, from the University of Texas Health Science Center at Houston, said that the advantage of EUS-RFA is that it’s only minimally invasive and appears to offer a significant survival advantage for patients with few effective treatment options.
He cautioned, however, that “it’s a small study and needs to be replicated in a larger venue and different sites as well, but I think it looks very promising.”
The investigators did not report a funding source for the study. Dr. Lopimpisuth, Dr. Shah, and Dr. Cash all reported having no relevant financial relationships to disclose.
CHARLOTTE, N.C. – In a small proof-of-concept study, patients with small unresectable pancreatic cancers treated with endoscopic ultrasound–guided radiofrequency ablation (EUS-RFA) had a more than twofold improvement in overall survival compared with historical controls with a similar disease history, investigators in Thailand found.
In a weighted analysis, median weighted overall survival – the primary outcome – was 14 months among 11 patients who underwent EUS-RFA, compared with 6.1 months for 35 matched controls, translating into a hazard ratio for death with EUS-RFA of 0.38 (P = .016), reported Chawin Lopimpisuth, MD, from King Chulalongkorn Memorial Hospital in Bangkok, Thailand.
Median weighted progression-free survival (PFS) was longer among cases than controls, but did not differ significantly, at 6.1 months and 3.9 months, respectively.
“In patients with unresectable pancreatic ductal adenocarcinomas that are less than 4 cm, EUS-RFA alone or combined with chemotherapy resulted in significantly improved overall survival and tended to improve progression-free survival with minimal adverse events,” Dr. Lopimpisuth reported at the annual meeting of the American College of Gastroenterology.
Small but unresectable tumors
Endoscopically guided radiofrequency ablation of pancreatic ductal tumors has been shown to be both feasible and safe in previous studies, he said, prompting his group to explore whether EUS-RFA could help to control the primary tumor and improve survival outcomes.
They enrolled 11 patients with primary pancreatic ductal adenocarcinoma tumors less than 4 cm in diameter that were unresectable due to blood vessel involvement or distant metastasis, and used propensity-score matching to pair them with a total of 35 controls. Controls were matched by tumor size, staging, age-adjusted Charlson Comorbidity Index, chemotherapy regimen received, and interactions between CCI, regimen, and staging.
The results were weighted to assure that covariate distribution among patients treated with chemotherapy only equaled that of patients who underwent EUS-RFA.
Patients underwent EUS-RFA with a 19-gauge needle, with 50 watts of energy delivered with an impedance of 100 ohms. Those patients deemed able to tolerate chemotherapy received that as well.
After a minimum of 1 year of follow-up, the median weighted survival, as noted before, was 14 months for patients who received EUS-RFA, compared with 6.1 months for controls.
Adjusted survival probabilities at 6 and 12 months were 73% and 64%, respectively, for patients in the EUS-RFA group, compared with 69% and 17% for controls. Adjusted PFS rates at 6 and 12 months were 55% and 36% in the EUS-RFA group, compared with 28% and 4% in the control group.
The only adverse event of significance was mild abdominal pain, reported by 8.3% of total EUS-RFA procedures.
Promising but preliminary
In an interview with this news organization, ACG President Samir A. Shah, MD, from Brown University and Miriam Hospital in Providence, R.I., who was not involved in the study, commented that “we have limited options with these patients, so it’s really exciting to see an initial trend toward efficacy, and their survival improvement was significant by several months.”
Dr. Shah was a moderator of the presidential symposium where the data were presented.
Comoderator Brooks D. Cash, MD, from the University of Texas Health Science Center at Houston, said that the advantage of EUS-RFA is that it’s only minimally invasive and appears to offer a significant survival advantage for patients with few effective treatment options.
He cautioned, however, that “it’s a small study and needs to be replicated in a larger venue and different sites as well, but I think it looks very promising.”
The investigators did not report a funding source for the study. Dr. Lopimpisuth, Dr. Shah, and Dr. Cash all reported having no relevant financial relationships to disclose.
CHARLOTTE, N.C. – In a small proof-of-concept study, patients with small unresectable pancreatic cancers treated with endoscopic ultrasound–guided radiofrequency ablation (EUS-RFA) had a more than twofold improvement in overall survival compared with historical controls with a similar disease history, investigators in Thailand found.
In a weighted analysis, median weighted overall survival – the primary outcome – was 14 months among 11 patients who underwent EUS-RFA, compared with 6.1 months for 35 matched controls, translating into a hazard ratio for death with EUS-RFA of 0.38 (P = .016), reported Chawin Lopimpisuth, MD, from King Chulalongkorn Memorial Hospital in Bangkok, Thailand.
Median weighted progression-free survival (PFS) was longer among cases than controls, but did not differ significantly, at 6.1 months and 3.9 months, respectively.
“In patients with unresectable pancreatic ductal adenocarcinomas that are less than 4 cm, EUS-RFA alone or combined with chemotherapy resulted in significantly improved overall survival and tended to improve progression-free survival with minimal adverse events,” Dr. Lopimpisuth reported at the annual meeting of the American College of Gastroenterology.
Small but unresectable tumors
Endoscopically guided radiofrequency ablation of pancreatic ductal tumors has been shown to be both feasible and safe in previous studies, he said, prompting his group to explore whether EUS-RFA could help to control the primary tumor and improve survival outcomes.
They enrolled 11 patients with primary pancreatic ductal adenocarcinoma tumors less than 4 cm in diameter that were unresectable due to blood vessel involvement or distant metastasis, and used propensity-score matching to pair them with a total of 35 controls. Controls were matched by tumor size, staging, age-adjusted Charlson Comorbidity Index, chemotherapy regimen received, and interactions between CCI, regimen, and staging.
The results were weighted to assure that covariate distribution among patients treated with chemotherapy only equaled that of patients who underwent EUS-RFA.
Patients underwent EUS-RFA with a 19-gauge needle, with 50 watts of energy delivered with an impedance of 100 ohms. Those patients deemed able to tolerate chemotherapy received that as well.
After a minimum of 1 year of follow-up, the median weighted survival, as noted before, was 14 months for patients who received EUS-RFA, compared with 6.1 months for controls.
Adjusted survival probabilities at 6 and 12 months were 73% and 64%, respectively, for patients in the EUS-RFA group, compared with 69% and 17% for controls. Adjusted PFS rates at 6 and 12 months were 55% and 36% in the EUS-RFA group, compared with 28% and 4% in the control group.
The only adverse event of significance was mild abdominal pain, reported by 8.3% of total EUS-RFA procedures.
Promising but preliminary
In an interview with this news organization, ACG President Samir A. Shah, MD, from Brown University and Miriam Hospital in Providence, R.I., who was not involved in the study, commented that “we have limited options with these patients, so it’s really exciting to see an initial trend toward efficacy, and their survival improvement was significant by several months.”
Dr. Shah was a moderator of the presidential symposium where the data were presented.
Comoderator Brooks D. Cash, MD, from the University of Texas Health Science Center at Houston, said that the advantage of EUS-RFA is that it’s only minimally invasive and appears to offer a significant survival advantage for patients with few effective treatment options.
He cautioned, however, that “it’s a small study and needs to be replicated in a larger venue and different sites as well, but I think it looks very promising.”
The investigators did not report a funding source for the study. Dr. Lopimpisuth, Dr. Shah, and Dr. Cash all reported having no relevant financial relationships to disclose.
AT ACG 2022