User login
Impact of Expanded Eligibility for Veterans With Other Than Honorable Discharges on Treatment Courts and VA Mental Health Care
In April 2022, the US Department of Veterans Affairs (VA) revised its behavioral health care eligibility policies to provide comprehensive mental and behavioral health care to former service members who received an Other Than Honorable (OTH) discharge characterization upon separation from military service.1 This policy shift represents a marked expansion in eligibility practices (Table 1 includes amended eligibility criteria).
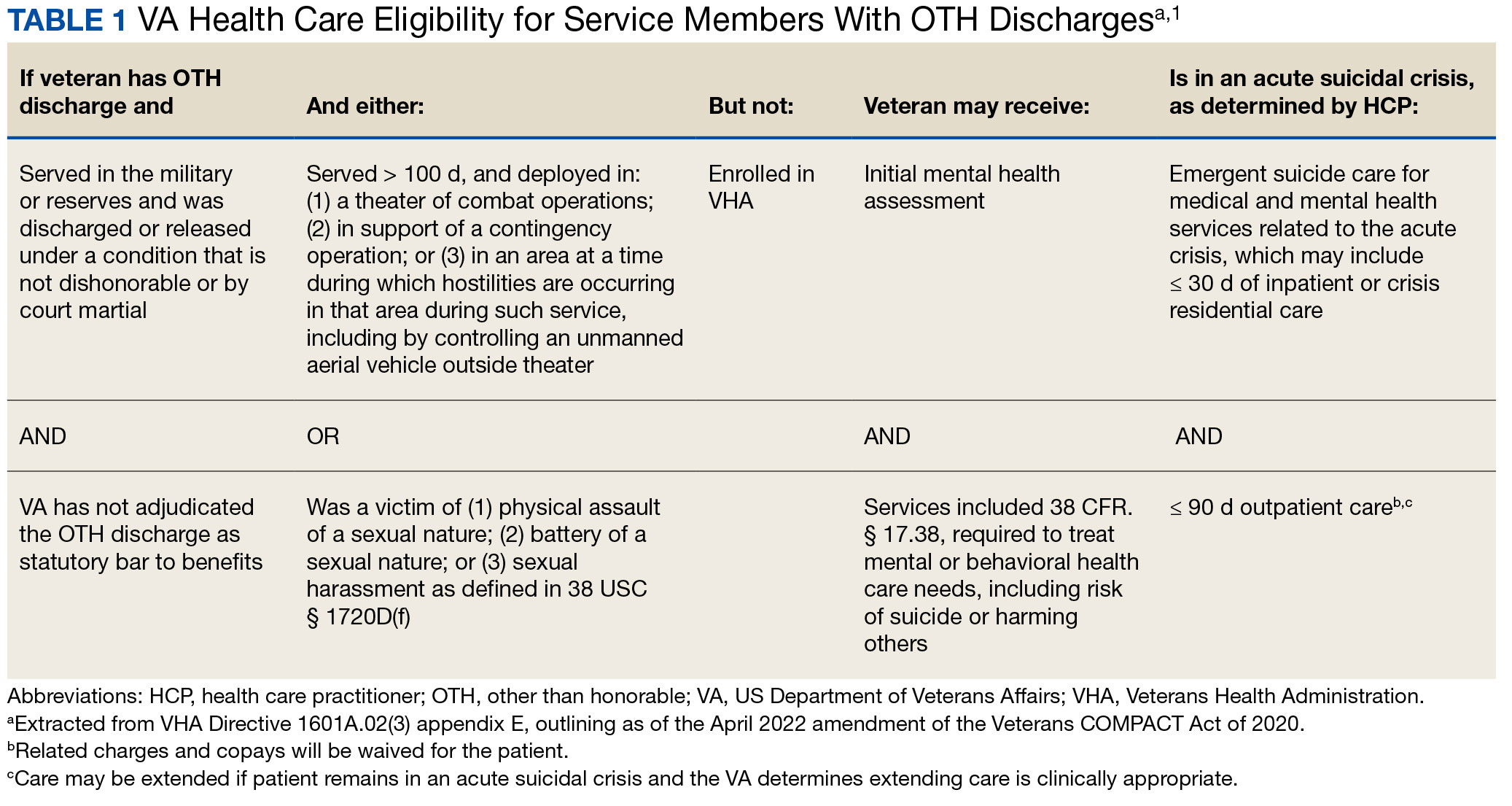
Since June 2017, eligibility policies allowed veterans with OTH discharges to receive “emergent mental health services” needed to stabilize acute mental health crises related to military service (eg, acute escalations in suicide risk).2,3 Previously, veterans with OTH discharges were largely ineligible for VA-based health care; these individuals were only able to access Veterans Health Administration (VHA) mental and behavioral health care through limited channels of eligibility (eg, for treatment of military sexual trauma or psychosis or other mental illness within 2 years of discharge).4,5 The impetus for expansions in eligibility stemmed from VA efforts to reduce the suicide rate among veterans.6-8 Implications of such expansion extend beyond suicide prevention efforts, with notable promised effects on the care of veterans with criminal-legal involvement. This article highlights potential effects of recent eligibility expansions on veterans with criminal-legal involvement and makes specific recommendations for agencies and organizations serving these veterans.
OTHER THAN HONORABLE DISCHARGE
The US Department of Defense delineates 6 discharge characterizations provided to service members upon separation from military service: honorable, general under honorable conditions, OTH, bad conduct, dishonorable, and uncharacterized. Honorable discharge characterizations are considered to reflect general concordance between service member behavior and military standards; general discharge characterizations reflect some disparity between the service member’s behavior and military standards; OTH, bad conduct, and dishonorable discharge characterizations reflect serious disparities between the service member’s behavior and military standards; and uncharacterized discharge characterizations are given when other discharge characterizations are deemed inappropriate.9,10 OTH discharge characterizations are typically issued under instances of misconduct, fraudulent entry, security reasons, or in lieu of trial by court martial.9,10
Recent research suggests that about 85% of service members receive an honorable discharge characterization upon separation from military service, 8% receive general, 6% receive OTH, and 1% receive bad conduct or dishonorable discharges.11 In 2017, the VA estimated there were > 500,000 prior service members with OTH discharge characterizations, which has grown over time (1.9% during the Korean Conflict, 2.5% during the Vietnam War Era, 3.9% during the Cold War, 4.8% in the Persian Gulf War, and 5.8% in the post-9/11 era).7,11
The OTH discharge characterization is 1 of 3 less than honorable discharges informally referred to as bad papers (ie, OTH, bad conduct, or dishonorable). Former service members receiving these discharge characterizations face significant social stigma and structural discrimination upon military discharge, including significant hurdles to employment and educational pursuits as well as notable social alienation.12 Due to their discharge characterization, some are viewed as less deserving of the veteran title, and until recently, many did not qualify for the complex legal definition of veteran as established by the Congress.11,13 Veterans with OTH discharge characterizations have also historically been excluded from services (eg, VHA care),3 benefits (eg, disability compensation),14 and protections (eg, Uniformed Services Employment and Reemployment Rights Act)15 offered to veterans with honorable or general discharge characterizations. However, eligibility policies have gradually expanded, providing veterans with OTH discharges with access to VHA-based mental and behavioral health services and VA supportive housing assistance.1,3,16
Perhaps due to their historical exclusion from VA services, there is limited research available on the behavioral health and associated needs of veterans with OTH discharges. Some scholars argue that historical exclusions have exacerbated underlying difficulties faced by this population, thereby contributing to stark health and social disparities across discharge types.14,15,17 Studies with large veteran samples, for example, reflect notable demographic and behavioral health differences across discharge types. Compared to routinely discharged veterans, veterans with OTH discharges are significantly more likely to be younger, have lower income, use substances, have a history of criminal-legal involvement, and have mental and physical health difficulties.18,19
Substantial evidence also suggests a historical racial bias, with service members of color being disproportionately more likely to receive an OTH discharge.12 Similarly, across all branches of military service, Black service members are significantly more likely to face general or special court martial in military justice proceedings when compared with White service members.20 Service members from gender and sexual minorities are also disproportionately impacted by the OTH designation. Historically, many have been discharged with bad papers due to discriminatory policies, such as Don’t Ask Don’t Tell, which discriminated on the basis of sexual orientation between December 1993 and September 2011, and Directive-type Memorandum-19-004, which banned transgender persons from military service between April 2019 and January 2021.21,22
There is also significant mental health bias in the provision of OTH discharges, such that OTH characterizations are disproportionately represented among individuals with mental health disorders.18-20 Veterans discharged from military service due to behavioral misconduct are significantly more likely to meet diagnostic criteria for various behavioral health conditions and to experience homelessness, criminal-legal involvement, and suicidal ideation and behavior compared with routinely-discharged veterans.23-28
Consistent with their comparatively higher rates of criminal-legal involvement relative to routinely discharged veterans, veterans with OTH discharges are disproportionately represented in criminal justice settings. While veterans with OTH discharges represent only 6% of discharging service members and 2.5% of community-based veterans, they represent 10% of incarcerated veterans.11,18,23,29 Preliminary research suggests veterans with OTH discharges may be at higher risk for lifetime incarceration, though the association between OTH discharge and frequency of lifetime arrests remains unclear.18,30
VETERANS TREATMENT COURTS
Given the overrepresentation of veterans with OTH discharges in criminal-legal settings, consideration for this subset of the veteran population and its unique needs is commonplace among problem-solving courts that service veterans. First conceptualized in 2004, Veterans Treatment Courts (VTCs) are specialized problem-solving courts that divert veterans away from traditional judicial court and penal systems and into community-based supervision and treatment (most commonly behavioral health services).31-34 Although each VTC program is unique in structure, policies, and procedures, most VTCs can be characterized by certain key elements, including voluntary participation, plea requirements, delayed sentencing (often including reduced or dismissed charges), integration of military culture into court proceedings, a rehabilitative vs adversarial approach to decreasing risk of future criminal behavior, mandated treatment and supervision during participation, and use of veteran mentors to provide peer support.32-35 Eligibility requirements vary; however, many restrict participation to veterans with honorable discharge types and charges for nonviolent offenses.32,33,35-37
VTCs connect veterans within the criminal-legal system to needed behavioral health, community, and social services.31-33,37 VTC participants are commonly connected to case management, behavioral health care, therapeutic journaling programs, and vocational rehabilitation.38,39 Accordingly, the most common difficulties faced by veterans participating in these courts include substance use, mental health, family issues, anger management and/or aggressive behavior, and homelessness.36,39 There is limited research on the effectiveness of VTCs. Evidence on their overall effectiveness is largely mixed, though some studies suggest VTC graduates tend to have lower recidivism rates than offenders more broadly or persons who terminate VTC programs prior to completion.40,41 Other studies suggest that VTC participants are more likely to have jail sanctions, new arrests, and new incarcerations relative to nontreatment court participants.42 Notably, experimental designs (considered the gold standard in assessing effectiveness) to date have not been applied to evaluate the effectiveness of VTCs; as such, the effectiveness of these programs remains an area in need of continued empirical investigation.
Like all problem-solving courts, VTCs occasionally struggle to connect participating defendants with appropriate care, particularly when encountering structural barriers (eg, insurance, transportation) and/or complex behavioral health needs (eg, personality disorders).34,43 As suicide rates among veterans experiencing criminal-legal involvement surge (about 150 per 100,000 in 2021, a 10% increase from 2020 to 2021 compared to about 40 per 100,000 and a 1.8% increase among other veterans), efficiency of adequate care coordination is vital.44 Many VTCs rely on VTC-VA partnerships and collaborations to navigate these difficulties and facilitate connection of participating veterans to needed services.32-34,45 For example, within the VHA, Veterans Justice Outreach (VJO) and Health Care for Re-Entry Veterans (HCRV) specialists assist and bridge the gap between the criminal-legal system (including, but not limited to VTCs) and VA services by engaging veterans involved in the criminal-legal system and connecting them to needed VA-based services (Table 2). Generally, VJO specialists support veterans involved with the front end of the criminallegal system (eg, arrest, pretrial incarceration, or participation in VTCs), while HCRV specialists tend to support veterans transitioning back into the community after a period of incarceration.46,47 Specific to VTCs, VJO specialists typically serve as liaisons between the courts and VA, coordinating VA services for defendants to fulfill their terms of VTC participation.46
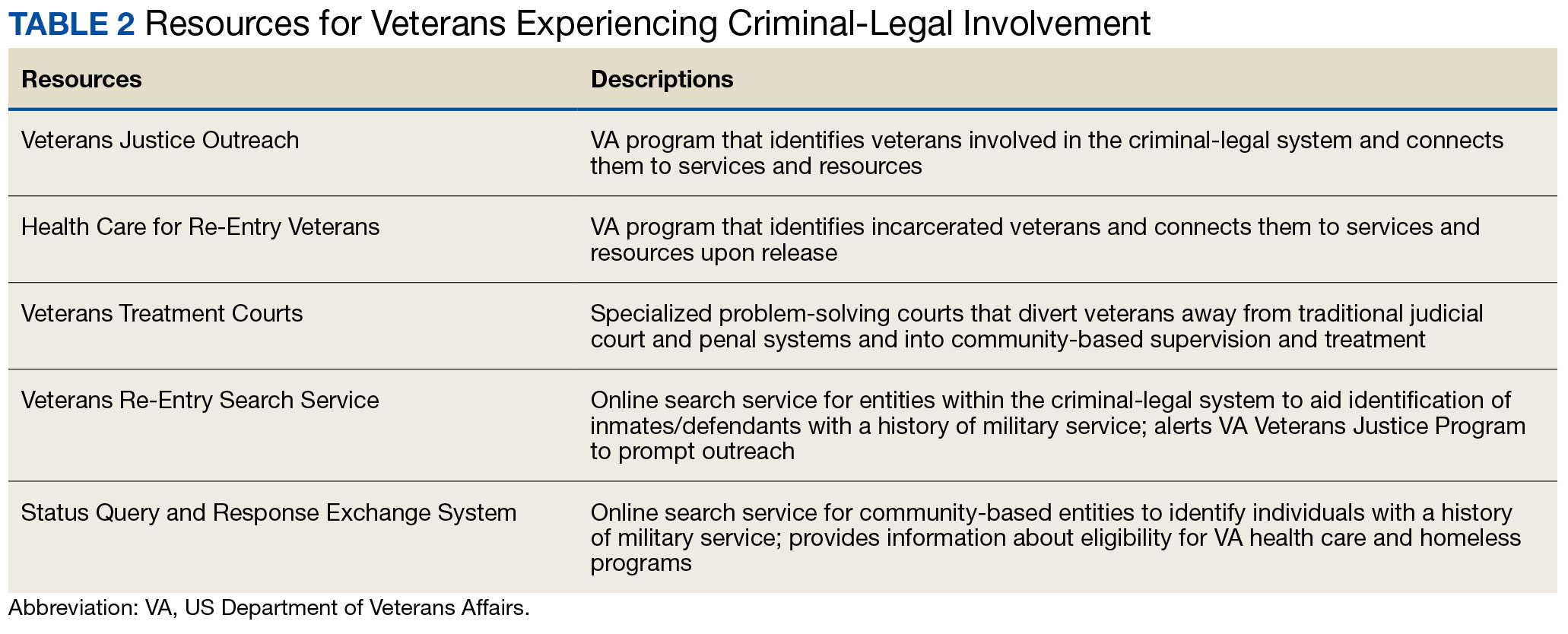
The historical exclusion of veterans with OTH discharge characterizations from VA-based services has restricted many from accessing VTC programs.32 Of 17 VTC programs active in Pennsylvania in 2014, only 5 accepted veterans with OTstayH discharges, and 3 required application to and eligibility for VA benefits.33 Similarly, in national surveys of VTC programs, about 1 in 3 report excluding veterans deemed ineligible for VA services.35,36 When veterans with OTH discharges have accessed VTC programs, they have historically relied on non-VA, community-based programming to fulfill treatment mandates, which may be less suited to addressing the unique needs of veterans.48
Veterans who utilize VTCs receive several benefits, namely peer support and mentorship, acceptance into a veteran-centric space, and connection with specially trained staff capable of supporting the veteran through applications for a range of VA benefits (eg, service connection, housing support).31-33,37 Given the disparate prevalence of OTH discharge characterizations among service members from racial, sexual, and gender minorities and among service members with mental health disorders, exclusion of veterans with OTH discharges from VTCs solely based on the type of discharge likely contributes to structural inequities among these already underserved groups by restricting access to these potential benefits. Such structural inequity stands in direct conflict with VTC best practice standards, which admonish programs to adjust eligibility requirements to facilitate access to treatment court programs for historically marginalized groups.49
ELIGIBILITY EXPANSIONS
Given the overrepresentation of veterans with OTH discharge characterizations within the criminal-legal system and historical barriers of these veterans to access needed mental and behavioral health care, expansions in VA eligibility policies could have immense implications for VTCs. First, these expansions could mitigate common barriers to connecting VTC-participating veterans with OTH discharges with needed behavioral health care by allowing these veterans to access established, VA-based services and programming. Expansion may also allow VTCs to serve as a key intercept point for identifying and engaging veterans with OTH discharges who may be unaware of their eligibility for VA-based behavioral health care.
Access to VA health care services is a major resource for VTC participants and a common requirement.32 Eligibility expansion should ease access barriers veterans with OTH discharges commonly face. By providing a potential source of treatment, expansions may also support OTH eligibility practices within VTCs, particularly practices that require participants to be eligible for VA health care.33,35,36 Some VTCs may continue to determine eligibility on the basis of discharge status and remain inaccessible to veterans with OTH discharge characterizations without program-level policy changes.32,36,37
Communicating changes in eligibility policies relevant to veterans with OTH discharges may be a challenge, because many of these individuals have no established channels of communication with the VA. Because veterans with OTH discharges are at increased risk for legal system involvement, VTCs may serve as a unique point of contact to help facilitate communication.18 For example, upon referral to a VTC, veterans with OTH discharges can be identified, VA health care eligibility can be verified, and veterans can connect to VA staff to facilitate enrollment in VA services and care.
VJO specialists are in a favorable position to serve a critical role in utilizing VTCs as a potential intercept point for engaging veterans with OTH discharge characterizations. As outlined in the STRONG Veterans Act of 2022, VJOs are mandated to “spread awareness and understanding of veteran eligibility for the [VJO] Program, including the eligibility of veterans who were discharged from service in the Armed Forces under conditions other than honorable.”50 The Act further requires VJOs to be annually trained in communicating eligibility changes as they arise. Accordingly, VJOs receive ongoing training in a wide variety of critical outreach topics, including changes in eligibility; while VJOs cannot make eligibility determinations, they are tasked with enrolling all veterans involved in the criminal-legal system with whom they interact into VHA services, whether through typical or special eligibility criteria (M. Stimmel, PhD, National Training Director for Veteran Justice Programming, oral communication, July 14, 2023). VJOs therefore routinely serve in this capacity of facilitating VA enrollment of veterans with OTH discharge characterizations.
Recommendations to Veteran-Servicing Judicial Programs
Considering these potential implications, professionals routinely interacting with veterans involved in the criminal-legal system should become familiarized with recent changes in VA eligibility policies. Such familiarization would support the identification of veterans previously considered ineligible for care; provision of education to these veterans regarding their new eligibility; and referral to appropriate VA-based behavioral health care options. Although conceptually simple, executing such an educational campaign may prove logistically difficult. Given their historical exclusion from VA services, veterans with OTH discharge characterizations are unlikely to seek VA-based services in times of need, instead relying on a broad swath of civilian community-based organizations and resources. Usual approaches to advertising VHA health care policy changes (eg, by notifying VA employees and/or departments providing corresponding services or by circulating information to veteran-focused mailing lists and organizations) likely would prove insufficient. Educational campaigns to disseminate information about recent OTH eligibility changes should instead consider partnering with traditionally civilian, communitybased organizations and institutions, such as state bar associations, legal aid networks, case management services, nonveteran treatment court programs (eg, drug courts, or domestic violence courts), or probation/ parole programs. Because national surveys suggest generally low military cultural competence among civilian populations, providing concurrent support in developing foundational veteran cultural competencies (eg, how to phrase questions about military service history, or understanding discharge characterizations) may be necessary to ensure effective identification and engagement of veteran clients.48
Programs that serve veterans with criminal-legal involvement should also consider potential relevance of recent OTH eligibility changes to program operations. VTC program staff and key partners (eg, judges, case managers, district attorneys, or defense attorneys), should revisit policies and procedures surrounding the engagement of veterans with OTH discharges within VTC programs and strategies for connecting these veterans with needed services. VTC programs that have historically excluded veterans with OTH discharges due to associated difficulties in locating and connecting with needed services should consider expanding eligibility policies considering recent shifts in VA behavioral health care eligibility.33,35,36 Within the VHA, VJO specialists can play a critical role in supporting these VTC eligibility and cultural shifts. Some evidence suggests a large proportion of VTC referrals are facilitated by VJO specialists and that many such referrals are identified when veterans involved with the criminal-legal system seek VA support and/or services.33 Given the historical exclusion of veterans with OTH discharges from VA care, strategies used by VJO specialists to identify, connect, and engage veterans with OTH discharges with VTCs and other services may be beneficial.
Even with knowledge of VA eligibility changes and considerations of these changes on local operations, many forensic settings and programs struggle to identify veterans. These difficulties are likely amplified among veterans with OTH discharge characterizations, who may be hesitant to self-disclose their military service history due to fear of stigma and/or views of OTH discharge characterizations as undeserving of the veteran title.12 The VA offers 2 tools to aid in identification of veterans for these settings: the Veterans Re-Entry Search Service (VRSS) and Status Query and Response Exchange System (SQUARES). For VRSS, correctional facilities, courts, and other criminal justice entities upload a simple spreadsheet that contains basic identifying information of inmates or defendants in their system. VRSS returns information about which inmates or defendants have a history of military service and alerts VA Veterans Justice Programs staff so they can conduct outreach. A pilot study conducted by the California Department of Corrections and Rehabilitation found that 2.7% of its inmate population self-identified as veterans, while VRSS identified 7.7% of inmates with a history of military service. This difference represented about 5000 previously unidentified veterans.51 Similarly, community entities that partner with the VA, such as law enforcement or homeless service programs, can be approved to become a SQUARES user and submit identifying information of individuals with whom they interact directly into the SQUARES search engine. SQUARES then directly returns information about the individual’s veteran status and eligibility for VA health care and homeless programs.
Other Eligibility Limitations
VTCs and other professionals looking to refer veterans with OTH discharge characterizations to VA-based behavioral health care should be aware of potential limitations in eligibility and access. Specifically, although veterans with OTH discharges are now broadly eligible for VA-based behavioral health care and homeless programs, they remain ineligible for other forms of health care, including primary care and nonbehavioral specialty care.1 Research has found a strong comorbidity between behavioral and nonbehavioral health concerns, particularly within historically marginalized demographic groups.52-55 Because these historically marginalized groups are often overrepresented among persons with criminal-legal involvement, veterans with OTH discharges, and VTC participants, such comorbidities require consideration by services or programming designed to support veterans with criminal-legal involvement.12,56-58 Connection with VA-based health care will therefore continue to fall short of addressing all health care needs of veterans with OTH discharges and effective case management will require considerable treatment coordination between VA behavioral health care practitioners (HCPs) and community-based HCPs (eg, primary care professionals or nonbehavioral HCPs).
Implications for VA Mental Health Care
Recent eligibility expansions will also have inevitable consequences for VA mental health care systems. For many years, these systems have been overburdened by high caseloads and clinician burnout.59,60 Given the generally elevated rates of mental health and substance use concerns among veterans with OTH discharge characterizations, expansions hold the potential to further burden caseloads with clinically complex, high-risk, high-need clients. Nevertheless, these expansions are also structured in a way that forces existing systems to absorb the responsibilities of providing necessary care to these veterans. To mitigate detrimental effects of eligibility expansions on the broader VA mental health system, clinicians should be explicitly trained in identifying veterans with OTH discharge characterizations and the implications of discharge status on broader health care eligibility. Treatment of veterans with OTH discharges may also benefit from close coordination between mental health professionals and behavioral health care coordinators to ensure appropriate coordination of care between VA- and non–VA-based HCPs.
CONCLUSIONS
Recent changes to VA eligibility policies now allow comprehensive mental and behavioral health care services to be provided to veterans with OTH discharges.1 Compared to routinely discharged veterans, veterans with OTH discharges are more likely to be persons of color, sexual or gender minorities, and experiencing mental health-related difficulties. Given the disproportionate mental health burden often faced by veterans with OTH discharges and relative overrepresentation of these veterans in judicial and correctional systems, these changes have considerable implications for programs and services designed to support veterans with criminallegal involvement. Professionals within these systems, particularly VTC programs, are therefore encouraged to familiarize themselves with recent changes in VA eligibility policies and to revisit strategies, policies, and procedures surrounding the engagement and enrollment of veterans with OTH discharge characterizations. Doing so may ensure veterans with OTH discharges are effectively connected to needed behavioral health care services.
- US Department of Veterans, Veterans Health Administration. VHA Directive 1601A.02(6): Eligibility Determination. Updated March 6, 2024. Accessed August 8, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8908
- Mental and behavioral health care for certain former members of the Armed Forces. 38 USC §1720I (2018). Accessed August 5, 2024. https://uscode.house.gov/view.xhtml?req=(title:38%20section:1720I%20edition:prelim
- US Department of Veterans, Veterans Health Administration. VHA Directive 1601A.02, Eligibility Determination. June 7, 2017.
- US Department of Veterans, Veterans Health Administration. VHA Directive 1115(1), Military Sexual Trauma (MST) Program. May 8, 2018. Accessed August 5, 2024. https:// www.va.gov/vhapublications/viewpublication.asp?pub_ID=6402
- US Department of Veterans Affairs. Tentative Eligibility Determinations; Presumptive Eligibility for Psychosis and Other Mental Illness. 38 CFR §17.109. May 14, 2013. Accessed August 5, 2024. https://www.federalregister.gov/documents/2013/05/14/2013-11410/tentative-eligibility-determinations-presumptive-eligibility-for-psychosis-and-other-mental-illness
- US Department of Veterans Affairs, VA Office of Mental Health and Suicide Prevention. National strategy for preventing veteran suicide 2018-2028. Published September 2018. Accessed August 5, 2024. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf
- US Department of Veterans Affairs. VA secretary announces intention to expand mental health care to former service members with other-than-honorable discharges and in crisis. Press Release. March 8, 2017. Accessed August 5, 2024. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=2867
- Smith C. Dramatic increase in mental health services to other-than-honorable discharge veterans. VA News. February 23, 2022. Accessed August 5, 2024. https://news.va.gov/100460/dramatic-increase-in-mental-health-services-to-other-than-honorable-discharge-veterans/
- US Department of Defense. DoD Instruction 1332.14. Enlisted administrative separations. Updated August 1, 2024. Accessed August 5, 2024. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/133214p.pdf
- US Department of Defense. DoD Instruction 1332.30. Commissioned officer administrative separations. Updated September 9, 2021. Accessed August 5, 2024. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/133230p.pdf
- OUTVETS, Legal Services Center of Harvard Law School, Veterans Legal Services. Turned away: how the VA unlawfully denies healthcare to veterans with bad paper discharges. 2020. Accessed August 5, 2024. https://legalservicescenter.org/wp-content/uploads/Turn-Away-Report.pdf
- McClean H. Discharged and discarded: the collateral consequences of a less-than-honorable military discharge. Columbia Law Rev. 2021;121(7):2203-2268.
- Veterans Benefits, General Provisions, Definitions. 38 USC §101(2) (1958). Accessed August 5, 2024. https://uscode.house.gov/view.xhtml?req=granuleid:USC-prelim-title38-section101&num=0&edition=prelim
- Bedford JR. Other than honorable discharges: unfair and unjust life sentences of decreased earning capacity. U Penn J Law Pub Affairs. 2021;6(4):687.
- Karin ML. Other than honorable discrimination. Case Western Reserve Law Rev. 2016;67(1):135-191. https://scholarlycommons.law.case.edu/caselrev/vol67/iss1/9
- Veteran HOUSE Act of 2020, HR 2398, 116th Cong, (2020). Accessed August 5, 2024. https://www.congress.gov/bill/116th-congress/house-bill/2398
- Scapardine D. Leaving other than honorable soldiers behind: how the Departments of Defense and Veterans Affairs inadvertently created a health and social crisis. Md Law Rev. 2017;76(4):1133-1165.
- Elbogen EB, Wagner HR, Brancu M, et al. Psychosocial risk factors and other than honorable military discharge: providing healthcare to previously ineligible veterans. Mil Med. 2018;183(9-10):e532-e538. doi:10.1093/milmed/usx128
- Tsai J, Rosenheck RA. Characteristics and health needs of veterans with other-than-honorable discharges: expanding eligibility in the Veterans Health Administration. Mil Med. 2018;183(5-6):e153-e157. doi:10.1093/milmed/usx110
- Christensen DM, Tsilker Y. Racial disparities in military justice: findings of substantial and persistent racial disparities within the United States military justice system. Accessed August 5, 2024. https://www.protectourdefenders.com/wp-content/uploads/2017/05/Report_20.pdf
- Don’t Ask Don’t Tell, 10 USC §654 (1993) (Repealed 2010). Accessed August 5, 2024. http://www.gpo.gov/fdsys/pkg/USCODE-2010-title10/pdf/USCODE-2010-title10-subtitleA-partII-chap37-sec654.pdf
- Palm Center. The making of a ban: how DTM-19-004 works to push transgender people out of military service. 2019. March 20, 2019. Accessed August 5, 2024. https://www.palmcenter.org/wp-content/uploads/2019/04/The-Making-of-a-Ban.pdf
- Edwards ER, Greene AL, Epshteyn G, Gromatsky M, Kinney AR, Holliday R. Mental health of incarcerated veterans and civilians: latent class analysis of the 2016 Survey of Prison Inmates. Crim Justice Behav. 2022;49(12):1800- 1821. doi:10.1177/00938548221121142
- Brignone E, Fargo JD, Blais RK, Carter ME, Samore MH, Gundlapalli AV. Non-routine discharge from military service: mental illness, substance use disorders, and suicidality. Am J Prev Med. 2017;52(5):557-565. doi:10.1016/j.amepre.2016.11.015
- Gamache G, Rosenheck R, Tessler R. Military discharge status of homeless veterans with mental illness. Mil Med. 2000;165(11):803-808. doi:10.1093/milmed/165.11.803
- Gundlapalli AV, Fargo JD, Metraux S, et al. Military Misconduct and Homelessness Among US Veterans Separated From Active Duty, 2001-2012. JAMA. 2015;314(8):832-834. doi:10.1001/jama.2015.8207
- Brooks Holliday S, Pedersen ER. The association between discharge status, mental health, and substance misuse among young adult veterans. Psychiatry Res. 2017;256:428-434. doi:10.1016/j.psychres.2017.07.011
- Williamson RB. DOD Health: Actions Needed to Ensure Post-Traumatic Stress Disorder and Traumatic Brain Injury are Considered in Misconduct Separations. US Government Accountability Office; 2017. Accessed August 5, 2024. https://apps.dtic.mil/sti/pdfs/AD1168610.pdf
- Maruschak LM, Bronson J, Alper M. Indicators of mental health problems reported by prisoners: survey of prison inmates. US Department of Justice Bureau of Justice Statistics. June 2021. Accessed August 5, 2024. https://bjs.ojp.gov/sites/g/files/xyckuh236/files/media/document/imhprpspi16st.pdf
- Brooke E, Gau J. Military service and lifetime arrests: examining the effects of the total military experience on arrests in a sample of prison inmates. Crim Justice Policy Rev. 2018;29(1):24-44. doi:10.1177/0887403415619007
- Russell RT. Veterans treatment court: a proactive approach. N Engl J Crim Civ Confin. 2009;35:357-372.
- Cartwright T. To care for him who shall have borne the battle: the recent development of Veterans Treatment Courts in America. Stanford Law Pol Rev. 2011;22:295-316.
- Douds AS, Ahlin EM, Howard D, Stigerwalt S. Varieties of veterans’ courts: a statewide assessment of veterans’ treatment court components. Crim Justice Policy Rev. 2017;28:740-769. doi:10.1177/0887403415620633
- Rowen J. Worthy of justice: a veterans treatment court in practice. Law Policy. 2020;42(1):78-100. doi:10.1111/lapo.12142
- Timko C, Flatley B, Tjemsland A, et al. A longitudinal examination of veterans treatment courts’ characteristics and eligibility criteria. Justice Res Policy. 2016;17(2):123-136.
- Baldwin JM. Executive summary: national survey of veterans treatment courts. SSRN. Preprint posted online June 5, 2013. Accessed August 5, 2024. doi:10.2139/ssrn.2274138
- Renz T. Veterans treatment court: a hand up rather than lock up. Richmond Public Interest Law Rev. 2013;17(3):697-705. https://scholarship.richmond.edu/pilr/vol17/iss3/6
- Knudsen KJ, Wingenfeld S. A specialized treatment court for veterans with trauma exposure: implications for the field. Community Ment Health J. 2016;52(2):127-135. doi:10.1007/s10597-015-9845-9
- McCall JD, Tsai J, Gordon AJ. Veterans treatment court research: participant characteristics, outcomes, and gaps in the literature. J Offender Rehabil. 2018;57:384-401. doi:10.1080/10509674.2018.1510864
- Smith JS. The Anchorage, Alaska veterans court and recidivism: July 6, 2004 – December 31, 2010. Alsk Law Rev. 2012;29(1):93-111.
- Hartley RD, Baldwin JM. Waging war on recidivism among justice-involved veterans: an impact evaluation of a large urban veterans treatment court. Crim Justice Policy Rev. 2019;30(1):52-78. doi:10.1177/0887403416650490
- Tsai J, Flatley B, Kasprow WJ, Clark S, Finlay A. Diversion of veterans with criminal justice involvement to treatment courts: participant characteristics and outcomes. Psychiatr Serv. 2017;68(4):375-383. doi:10.1176/appi.ps.201600233
- Edwards ER, Sissoko DR, Abrams D, Samost D, La Gamma S, Geraci J. Connecting mental health court participants with services: process, challenges, and recommendations. Psychol Public Policy Law. 2020;26(4):463-475. doi:10.1037/law0000236
- US Department of Veterans Affairs, VA Office of Mental Health and Suicide Prevention. 2023 National Veteran Suicide Prevention Annual Report. US Department of Veterans Affairs; November 2023. Accessed August 5, 2024. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- Finlay AK, Clark S, Blue-Howells J, et al. Logic model of the Department of Veterans Affairs’ role in veterans treatment courts. Drug Court Rev. 2019;2:45-62.
- Finlay AK, Smelson D, Sawh L, et al. U.S. Department of Veterans Affairs veterans justice outreach program: connecting justice-involved veterans with mental health and substance use disorder treatment. Crim Justice Policy Rev. 2016;27(2):10.1177/0887403414562601. doi:10.1177/0887403414562601
- Finlay AK, Stimmel M, Blue-Howells J, et al. Use of Veterans Health Administration mental health and substance use disorder treatment after exiting prison: the health care for reentry veterans program. Adm Policy Ment Health. 2017;44(2):177-187. doi:10.1007/s10488-015-0708-z
- Meyer EG, Writer BW, Brim W. The Importance of Military Cultural Competence. Curr Psychiatry Rep. 2016;18(3):26. doi:10.1007/s11920-016-0662-9
- National Association of Drug Court Professionals. Adult Drug Court Best Practice Standards Volume I. National Association of Drug Court Professionals; 2013. Accessed August 5, 2024. https://allrise.org/publications/standards/
- STRONG Veterans Act of 2022, HR 6411, 117th Cong (2022). https://www.congress.gov/bill/117th-congress/house-bill/6411/text
- Pelletier D, Clark S, Davis L. Veterans reentry search service (VRSS) and the SQUARES application. Presented at: National Association of Drug Court Professionals Conference; August 15-18, 2021; National Harbor, Maryland.
- Scott KM, Lim C, Al-Hamzawi A, et al. Association of Mental Disorders With Subsequent Chronic Physical Conditions: World Mental Health Surveys From 17 Countries. JAMA Psychiatry. 2016;73(2):150-158. doi:10.1001/jamapsychiatry.2015.2688
- Ahmed N, Conway CA. Medical and mental health comorbidities among minority racial/ethnic groups in the United States. J Soc Beh Health Sci. 2020;14(1):153-168. doi:10.5590/JSBHS.2020.14.1.11
- Hanna B, Desai R, Parekh T, Guirguis E, Kumar G, Sachdeva R. Psychiatric disorders in the U.S. transgender population. Ann Epidemiol. 2019;39:1-7.e1. doi:10.1016/j.annepidem.2019.09.009
- Watkins DC, Assari S, Johnson-Lawrence V. Race and ethnic group differences in comorbid major depressive disorder, generalized anxiety disorder, and chronic medical conditions. J Racial Ethn Health Disparities. 2015;2(3):385- 394. doi:10.1007/s40615-015-0085-z
- Baldwin J. Whom do they serve? National examination of veterans treatment court participants and their challenges. Crim Justice Policy Rev. 2017;28(6):515-554. doi:10.1177/0887403415606184
- Beatty LG, Snell TL. Profile of prison inmates, 2016. US Department of Justice Bureau of Justice Statistics. December 2021. Accessed August 5, 2024. https://bjs.ojp.gov/content/pub/pdf/ppi16.pdf
- Al-Rousan T, Rubenstein L, Sieleni B, Deol H, Wallace RB. Inside the nation’s largest mental health institution: a prevalence study in a state prison system. BMC Public Health. 2017;17(1):342. doi:10.1186/s12889-017-4257-0
- Rosen CS, Kaplan AN, Nelson DB, et al. Implementation context and burnout among Department of Veterans Affairs psychotherapists prior to and during the COVID-19 pandemic. J Affect Disord. 2023;320:517-524. doi:10.1016/j.jad.2022.09.141
- Tsai J, Jones N, Klee A, Deegan D. Job burnout among mental health staff at a veterans affairs psychosocial rehabilitation center. Community Ment Health J. 2020;56(2):294- 297. doi:10.1007/s10597-019-00487-5
In April 2022, the US Department of Veterans Affairs (VA) revised its behavioral health care eligibility policies to provide comprehensive mental and behavioral health care to former service members who received an Other Than Honorable (OTH) discharge characterization upon separation from military service.1 This policy shift represents a marked expansion in eligibility practices (Table 1 includes amended eligibility criteria).

Since June 2017, eligibility policies allowed veterans with OTH discharges to receive “emergent mental health services” needed to stabilize acute mental health crises related to military service (eg, acute escalations in suicide risk).2,3 Previously, veterans with OTH discharges were largely ineligible for VA-based health care; these individuals were only able to access Veterans Health Administration (VHA) mental and behavioral health care through limited channels of eligibility (eg, for treatment of military sexual trauma or psychosis or other mental illness within 2 years of discharge).4,5 The impetus for expansions in eligibility stemmed from VA efforts to reduce the suicide rate among veterans.6-8 Implications of such expansion extend beyond suicide prevention efforts, with notable promised effects on the care of veterans with criminal-legal involvement. This article highlights potential effects of recent eligibility expansions on veterans with criminal-legal involvement and makes specific recommendations for agencies and organizations serving these veterans.
OTHER THAN HONORABLE DISCHARGE
The US Department of Defense delineates 6 discharge characterizations provided to service members upon separation from military service: honorable, general under honorable conditions, OTH, bad conduct, dishonorable, and uncharacterized. Honorable discharge characterizations are considered to reflect general concordance between service member behavior and military standards; general discharge characterizations reflect some disparity between the service member’s behavior and military standards; OTH, bad conduct, and dishonorable discharge characterizations reflect serious disparities between the service member’s behavior and military standards; and uncharacterized discharge characterizations are given when other discharge characterizations are deemed inappropriate.9,10 OTH discharge characterizations are typically issued under instances of misconduct, fraudulent entry, security reasons, or in lieu of trial by court martial.9,10
Recent research suggests that about 85% of service members receive an honorable discharge characterization upon separation from military service, 8% receive general, 6% receive OTH, and 1% receive bad conduct or dishonorable discharges.11 In 2017, the VA estimated there were > 500,000 prior service members with OTH discharge characterizations, which has grown over time (1.9% during the Korean Conflict, 2.5% during the Vietnam War Era, 3.9% during the Cold War, 4.8% in the Persian Gulf War, and 5.8% in the post-9/11 era).7,11
The OTH discharge characterization is 1 of 3 less than honorable discharges informally referred to as bad papers (ie, OTH, bad conduct, or dishonorable). Former service members receiving these discharge characterizations face significant social stigma and structural discrimination upon military discharge, including significant hurdles to employment and educational pursuits as well as notable social alienation.12 Due to their discharge characterization, some are viewed as less deserving of the veteran title, and until recently, many did not qualify for the complex legal definition of veteran as established by the Congress.11,13 Veterans with OTH discharge characterizations have also historically been excluded from services (eg, VHA care),3 benefits (eg, disability compensation),14 and protections (eg, Uniformed Services Employment and Reemployment Rights Act)15 offered to veterans with honorable or general discharge characterizations. However, eligibility policies have gradually expanded, providing veterans with OTH discharges with access to VHA-based mental and behavioral health services and VA supportive housing assistance.1,3,16
Perhaps due to their historical exclusion from VA services, there is limited research available on the behavioral health and associated needs of veterans with OTH discharges. Some scholars argue that historical exclusions have exacerbated underlying difficulties faced by this population, thereby contributing to stark health and social disparities across discharge types.14,15,17 Studies with large veteran samples, for example, reflect notable demographic and behavioral health differences across discharge types. Compared to routinely discharged veterans, veterans with OTH discharges are significantly more likely to be younger, have lower income, use substances, have a history of criminal-legal involvement, and have mental and physical health difficulties.18,19
Substantial evidence also suggests a historical racial bias, with service members of color being disproportionately more likely to receive an OTH discharge.12 Similarly, across all branches of military service, Black service members are significantly more likely to face general or special court martial in military justice proceedings when compared with White service members.20 Service members from gender and sexual minorities are also disproportionately impacted by the OTH designation. Historically, many have been discharged with bad papers due to discriminatory policies, such as Don’t Ask Don’t Tell, which discriminated on the basis of sexual orientation between December 1993 and September 2011, and Directive-type Memorandum-19-004, which banned transgender persons from military service between April 2019 and January 2021.21,22
There is also significant mental health bias in the provision of OTH discharges, such that OTH characterizations are disproportionately represented among individuals with mental health disorders.18-20 Veterans discharged from military service due to behavioral misconduct are significantly more likely to meet diagnostic criteria for various behavioral health conditions and to experience homelessness, criminal-legal involvement, and suicidal ideation and behavior compared with routinely-discharged veterans.23-28
Consistent with their comparatively higher rates of criminal-legal involvement relative to routinely discharged veterans, veterans with OTH discharges are disproportionately represented in criminal justice settings. While veterans with OTH discharges represent only 6% of discharging service members and 2.5% of community-based veterans, they represent 10% of incarcerated veterans.11,18,23,29 Preliminary research suggests veterans with OTH discharges may be at higher risk for lifetime incarceration, though the association between OTH discharge and frequency of lifetime arrests remains unclear.18,30
VETERANS TREATMENT COURTS
Given the overrepresentation of veterans with OTH discharges in criminal-legal settings, consideration for this subset of the veteran population and its unique needs is commonplace among problem-solving courts that service veterans. First conceptualized in 2004, Veterans Treatment Courts (VTCs) are specialized problem-solving courts that divert veterans away from traditional judicial court and penal systems and into community-based supervision and treatment (most commonly behavioral health services).31-34 Although each VTC program is unique in structure, policies, and procedures, most VTCs can be characterized by certain key elements, including voluntary participation, plea requirements, delayed sentencing (often including reduced or dismissed charges), integration of military culture into court proceedings, a rehabilitative vs adversarial approach to decreasing risk of future criminal behavior, mandated treatment and supervision during participation, and use of veteran mentors to provide peer support.32-35 Eligibility requirements vary; however, many restrict participation to veterans with honorable discharge types and charges for nonviolent offenses.32,33,35-37
VTCs connect veterans within the criminal-legal system to needed behavioral health, community, and social services.31-33,37 VTC participants are commonly connected to case management, behavioral health care, therapeutic journaling programs, and vocational rehabilitation.38,39 Accordingly, the most common difficulties faced by veterans participating in these courts include substance use, mental health, family issues, anger management and/or aggressive behavior, and homelessness.36,39 There is limited research on the effectiveness of VTCs. Evidence on their overall effectiveness is largely mixed, though some studies suggest VTC graduates tend to have lower recidivism rates than offenders more broadly or persons who terminate VTC programs prior to completion.40,41 Other studies suggest that VTC participants are more likely to have jail sanctions, new arrests, and new incarcerations relative to nontreatment court participants.42 Notably, experimental designs (considered the gold standard in assessing effectiveness) to date have not been applied to evaluate the effectiveness of VTCs; as such, the effectiveness of these programs remains an area in need of continued empirical investigation.
Like all problem-solving courts, VTCs occasionally struggle to connect participating defendants with appropriate care, particularly when encountering structural barriers (eg, insurance, transportation) and/or complex behavioral health needs (eg, personality disorders).34,43 As suicide rates among veterans experiencing criminal-legal involvement surge (about 150 per 100,000 in 2021, a 10% increase from 2020 to 2021 compared to about 40 per 100,000 and a 1.8% increase among other veterans), efficiency of adequate care coordination is vital.44 Many VTCs rely on VTC-VA partnerships and collaborations to navigate these difficulties and facilitate connection of participating veterans to needed services.32-34,45 For example, within the VHA, Veterans Justice Outreach (VJO) and Health Care for Re-Entry Veterans (HCRV) specialists assist and bridge the gap between the criminal-legal system (including, but not limited to VTCs) and VA services by engaging veterans involved in the criminal-legal system and connecting them to needed VA-based services (Table 2). Generally, VJO specialists support veterans involved with the front end of the criminallegal system (eg, arrest, pretrial incarceration, or participation in VTCs), while HCRV specialists tend to support veterans transitioning back into the community after a period of incarceration.46,47 Specific to VTCs, VJO specialists typically serve as liaisons between the courts and VA, coordinating VA services for defendants to fulfill their terms of VTC participation.46

The historical exclusion of veterans with OTH discharge characterizations from VA-based services has restricted many from accessing VTC programs.32 Of 17 VTC programs active in Pennsylvania in 2014, only 5 accepted veterans with OTstayH discharges, and 3 required application to and eligibility for VA benefits.33 Similarly, in national surveys of VTC programs, about 1 in 3 report excluding veterans deemed ineligible for VA services.35,36 When veterans with OTH discharges have accessed VTC programs, they have historically relied on non-VA, community-based programming to fulfill treatment mandates, which may be less suited to addressing the unique needs of veterans.48
Veterans who utilize VTCs receive several benefits, namely peer support and mentorship, acceptance into a veteran-centric space, and connection with specially trained staff capable of supporting the veteran through applications for a range of VA benefits (eg, service connection, housing support).31-33,37 Given the disparate prevalence of OTH discharge characterizations among service members from racial, sexual, and gender minorities and among service members with mental health disorders, exclusion of veterans with OTH discharges from VTCs solely based on the type of discharge likely contributes to structural inequities among these already underserved groups by restricting access to these potential benefits. Such structural inequity stands in direct conflict with VTC best practice standards, which admonish programs to adjust eligibility requirements to facilitate access to treatment court programs for historically marginalized groups.49
ELIGIBILITY EXPANSIONS
Given the overrepresentation of veterans with OTH discharge characterizations within the criminal-legal system and historical barriers of these veterans to access needed mental and behavioral health care, expansions in VA eligibility policies could have immense implications for VTCs. First, these expansions could mitigate common barriers to connecting VTC-participating veterans with OTH discharges with needed behavioral health care by allowing these veterans to access established, VA-based services and programming. Expansion may also allow VTCs to serve as a key intercept point for identifying and engaging veterans with OTH discharges who may be unaware of their eligibility for VA-based behavioral health care.
Access to VA health care services is a major resource for VTC participants and a common requirement.32 Eligibility expansion should ease access barriers veterans with OTH discharges commonly face. By providing a potential source of treatment, expansions may also support OTH eligibility practices within VTCs, particularly practices that require participants to be eligible for VA health care.33,35,36 Some VTCs may continue to determine eligibility on the basis of discharge status and remain inaccessible to veterans with OTH discharge characterizations without program-level policy changes.32,36,37
Communicating changes in eligibility policies relevant to veterans with OTH discharges may be a challenge, because many of these individuals have no established channels of communication with the VA. Because veterans with OTH discharges are at increased risk for legal system involvement, VTCs may serve as a unique point of contact to help facilitate communication.18 For example, upon referral to a VTC, veterans with OTH discharges can be identified, VA health care eligibility can be verified, and veterans can connect to VA staff to facilitate enrollment in VA services and care.
VJO specialists are in a favorable position to serve a critical role in utilizing VTCs as a potential intercept point for engaging veterans with OTH discharge characterizations. As outlined in the STRONG Veterans Act of 2022, VJOs are mandated to “spread awareness and understanding of veteran eligibility for the [VJO] Program, including the eligibility of veterans who were discharged from service in the Armed Forces under conditions other than honorable.”50 The Act further requires VJOs to be annually trained in communicating eligibility changes as they arise. Accordingly, VJOs receive ongoing training in a wide variety of critical outreach topics, including changes in eligibility; while VJOs cannot make eligibility determinations, they are tasked with enrolling all veterans involved in the criminal-legal system with whom they interact into VHA services, whether through typical or special eligibility criteria (M. Stimmel, PhD, National Training Director for Veteran Justice Programming, oral communication, July 14, 2023). VJOs therefore routinely serve in this capacity of facilitating VA enrollment of veterans with OTH discharge characterizations.
Recommendations to Veteran-Servicing Judicial Programs
Considering these potential implications, professionals routinely interacting with veterans involved in the criminal-legal system should become familiarized with recent changes in VA eligibility policies. Such familiarization would support the identification of veterans previously considered ineligible for care; provision of education to these veterans regarding their new eligibility; and referral to appropriate VA-based behavioral health care options. Although conceptually simple, executing such an educational campaign may prove logistically difficult. Given their historical exclusion from VA services, veterans with OTH discharge characterizations are unlikely to seek VA-based services in times of need, instead relying on a broad swath of civilian community-based organizations and resources. Usual approaches to advertising VHA health care policy changes (eg, by notifying VA employees and/or departments providing corresponding services or by circulating information to veteran-focused mailing lists and organizations) likely would prove insufficient. Educational campaigns to disseminate information about recent OTH eligibility changes should instead consider partnering with traditionally civilian, communitybased organizations and institutions, such as state bar associations, legal aid networks, case management services, nonveteran treatment court programs (eg, drug courts, or domestic violence courts), or probation/ parole programs. Because national surveys suggest generally low military cultural competence among civilian populations, providing concurrent support in developing foundational veteran cultural competencies (eg, how to phrase questions about military service history, or understanding discharge characterizations) may be necessary to ensure effective identification and engagement of veteran clients.48
Programs that serve veterans with criminal-legal involvement should also consider potential relevance of recent OTH eligibility changes to program operations. VTC program staff and key partners (eg, judges, case managers, district attorneys, or defense attorneys), should revisit policies and procedures surrounding the engagement of veterans with OTH discharges within VTC programs and strategies for connecting these veterans with needed services. VTC programs that have historically excluded veterans with OTH discharges due to associated difficulties in locating and connecting with needed services should consider expanding eligibility policies considering recent shifts in VA behavioral health care eligibility.33,35,36 Within the VHA, VJO specialists can play a critical role in supporting these VTC eligibility and cultural shifts. Some evidence suggests a large proportion of VTC referrals are facilitated by VJO specialists and that many such referrals are identified when veterans involved with the criminal-legal system seek VA support and/or services.33 Given the historical exclusion of veterans with OTH discharges from VA care, strategies used by VJO specialists to identify, connect, and engage veterans with OTH discharges with VTCs and other services may be beneficial.
Even with knowledge of VA eligibility changes and considerations of these changes on local operations, many forensic settings and programs struggle to identify veterans. These difficulties are likely amplified among veterans with OTH discharge characterizations, who may be hesitant to self-disclose their military service history due to fear of stigma and/or views of OTH discharge characterizations as undeserving of the veteran title.12 The VA offers 2 tools to aid in identification of veterans for these settings: the Veterans Re-Entry Search Service (VRSS) and Status Query and Response Exchange System (SQUARES). For VRSS, correctional facilities, courts, and other criminal justice entities upload a simple spreadsheet that contains basic identifying information of inmates or defendants in their system. VRSS returns information about which inmates or defendants have a history of military service and alerts VA Veterans Justice Programs staff so they can conduct outreach. A pilot study conducted by the California Department of Corrections and Rehabilitation found that 2.7% of its inmate population self-identified as veterans, while VRSS identified 7.7% of inmates with a history of military service. This difference represented about 5000 previously unidentified veterans.51 Similarly, community entities that partner with the VA, such as law enforcement or homeless service programs, can be approved to become a SQUARES user and submit identifying information of individuals with whom they interact directly into the SQUARES search engine. SQUARES then directly returns information about the individual’s veteran status and eligibility for VA health care and homeless programs.
Other Eligibility Limitations
VTCs and other professionals looking to refer veterans with OTH discharge characterizations to VA-based behavioral health care should be aware of potential limitations in eligibility and access. Specifically, although veterans with OTH discharges are now broadly eligible for VA-based behavioral health care and homeless programs, they remain ineligible for other forms of health care, including primary care and nonbehavioral specialty care.1 Research has found a strong comorbidity between behavioral and nonbehavioral health concerns, particularly within historically marginalized demographic groups.52-55 Because these historically marginalized groups are often overrepresented among persons with criminal-legal involvement, veterans with OTH discharges, and VTC participants, such comorbidities require consideration by services or programming designed to support veterans with criminal-legal involvement.12,56-58 Connection with VA-based health care will therefore continue to fall short of addressing all health care needs of veterans with OTH discharges and effective case management will require considerable treatment coordination between VA behavioral health care practitioners (HCPs) and community-based HCPs (eg, primary care professionals or nonbehavioral HCPs).
Implications for VA Mental Health Care
Recent eligibility expansions will also have inevitable consequences for VA mental health care systems. For many years, these systems have been overburdened by high caseloads and clinician burnout.59,60 Given the generally elevated rates of mental health and substance use concerns among veterans with OTH discharge characterizations, expansions hold the potential to further burden caseloads with clinically complex, high-risk, high-need clients. Nevertheless, these expansions are also structured in a way that forces existing systems to absorb the responsibilities of providing necessary care to these veterans. To mitigate detrimental effects of eligibility expansions on the broader VA mental health system, clinicians should be explicitly trained in identifying veterans with OTH discharge characterizations and the implications of discharge status on broader health care eligibility. Treatment of veterans with OTH discharges may also benefit from close coordination between mental health professionals and behavioral health care coordinators to ensure appropriate coordination of care between VA- and non–VA-based HCPs.
CONCLUSIONS
Recent changes to VA eligibility policies now allow comprehensive mental and behavioral health care services to be provided to veterans with OTH discharges.1 Compared to routinely discharged veterans, veterans with OTH discharges are more likely to be persons of color, sexual or gender minorities, and experiencing mental health-related difficulties. Given the disproportionate mental health burden often faced by veterans with OTH discharges and relative overrepresentation of these veterans in judicial and correctional systems, these changes have considerable implications for programs and services designed to support veterans with criminallegal involvement. Professionals within these systems, particularly VTC programs, are therefore encouraged to familiarize themselves with recent changes in VA eligibility policies and to revisit strategies, policies, and procedures surrounding the engagement and enrollment of veterans with OTH discharge characterizations. Doing so may ensure veterans with OTH discharges are effectively connected to needed behavioral health care services.
In April 2022, the US Department of Veterans Affairs (VA) revised its behavioral health care eligibility policies to provide comprehensive mental and behavioral health care to former service members who received an Other Than Honorable (OTH) discharge characterization upon separation from military service.1 This policy shift represents a marked expansion in eligibility practices (Table 1 includes amended eligibility criteria).

Since June 2017, eligibility policies allowed veterans with OTH discharges to receive “emergent mental health services” needed to stabilize acute mental health crises related to military service (eg, acute escalations in suicide risk).2,3 Previously, veterans with OTH discharges were largely ineligible for VA-based health care; these individuals were only able to access Veterans Health Administration (VHA) mental and behavioral health care through limited channels of eligibility (eg, for treatment of military sexual trauma or psychosis or other mental illness within 2 years of discharge).4,5 The impetus for expansions in eligibility stemmed from VA efforts to reduce the suicide rate among veterans.6-8 Implications of such expansion extend beyond suicide prevention efforts, with notable promised effects on the care of veterans with criminal-legal involvement. This article highlights potential effects of recent eligibility expansions on veterans with criminal-legal involvement and makes specific recommendations for agencies and organizations serving these veterans.
OTHER THAN HONORABLE DISCHARGE
The US Department of Defense delineates 6 discharge characterizations provided to service members upon separation from military service: honorable, general under honorable conditions, OTH, bad conduct, dishonorable, and uncharacterized. Honorable discharge characterizations are considered to reflect general concordance between service member behavior and military standards; general discharge characterizations reflect some disparity between the service member’s behavior and military standards; OTH, bad conduct, and dishonorable discharge characterizations reflect serious disparities between the service member’s behavior and military standards; and uncharacterized discharge characterizations are given when other discharge characterizations are deemed inappropriate.9,10 OTH discharge characterizations are typically issued under instances of misconduct, fraudulent entry, security reasons, or in lieu of trial by court martial.9,10
Recent research suggests that about 85% of service members receive an honorable discharge characterization upon separation from military service, 8% receive general, 6% receive OTH, and 1% receive bad conduct or dishonorable discharges.11 In 2017, the VA estimated there were > 500,000 prior service members with OTH discharge characterizations, which has grown over time (1.9% during the Korean Conflict, 2.5% during the Vietnam War Era, 3.9% during the Cold War, 4.8% in the Persian Gulf War, and 5.8% in the post-9/11 era).7,11
The OTH discharge characterization is 1 of 3 less than honorable discharges informally referred to as bad papers (ie, OTH, bad conduct, or dishonorable). Former service members receiving these discharge characterizations face significant social stigma and structural discrimination upon military discharge, including significant hurdles to employment and educational pursuits as well as notable social alienation.12 Due to their discharge characterization, some are viewed as less deserving of the veteran title, and until recently, many did not qualify for the complex legal definition of veteran as established by the Congress.11,13 Veterans with OTH discharge characterizations have also historically been excluded from services (eg, VHA care),3 benefits (eg, disability compensation),14 and protections (eg, Uniformed Services Employment and Reemployment Rights Act)15 offered to veterans with honorable or general discharge characterizations. However, eligibility policies have gradually expanded, providing veterans with OTH discharges with access to VHA-based mental and behavioral health services and VA supportive housing assistance.1,3,16
Perhaps due to their historical exclusion from VA services, there is limited research available on the behavioral health and associated needs of veterans with OTH discharges. Some scholars argue that historical exclusions have exacerbated underlying difficulties faced by this population, thereby contributing to stark health and social disparities across discharge types.14,15,17 Studies with large veteran samples, for example, reflect notable demographic and behavioral health differences across discharge types. Compared to routinely discharged veterans, veterans with OTH discharges are significantly more likely to be younger, have lower income, use substances, have a history of criminal-legal involvement, and have mental and physical health difficulties.18,19
Substantial evidence also suggests a historical racial bias, with service members of color being disproportionately more likely to receive an OTH discharge.12 Similarly, across all branches of military service, Black service members are significantly more likely to face general or special court martial in military justice proceedings when compared with White service members.20 Service members from gender and sexual minorities are also disproportionately impacted by the OTH designation. Historically, many have been discharged with bad papers due to discriminatory policies, such as Don’t Ask Don’t Tell, which discriminated on the basis of sexual orientation between December 1993 and September 2011, and Directive-type Memorandum-19-004, which banned transgender persons from military service between April 2019 and January 2021.21,22
There is also significant mental health bias in the provision of OTH discharges, such that OTH characterizations are disproportionately represented among individuals with mental health disorders.18-20 Veterans discharged from military service due to behavioral misconduct are significantly more likely to meet diagnostic criteria for various behavioral health conditions and to experience homelessness, criminal-legal involvement, and suicidal ideation and behavior compared with routinely-discharged veterans.23-28
Consistent with their comparatively higher rates of criminal-legal involvement relative to routinely discharged veterans, veterans with OTH discharges are disproportionately represented in criminal justice settings. While veterans with OTH discharges represent only 6% of discharging service members and 2.5% of community-based veterans, they represent 10% of incarcerated veterans.11,18,23,29 Preliminary research suggests veterans with OTH discharges may be at higher risk for lifetime incarceration, though the association between OTH discharge and frequency of lifetime arrests remains unclear.18,30
VETERANS TREATMENT COURTS
Given the overrepresentation of veterans with OTH discharges in criminal-legal settings, consideration for this subset of the veteran population and its unique needs is commonplace among problem-solving courts that service veterans. First conceptualized in 2004, Veterans Treatment Courts (VTCs) are specialized problem-solving courts that divert veterans away from traditional judicial court and penal systems and into community-based supervision and treatment (most commonly behavioral health services).31-34 Although each VTC program is unique in structure, policies, and procedures, most VTCs can be characterized by certain key elements, including voluntary participation, plea requirements, delayed sentencing (often including reduced or dismissed charges), integration of military culture into court proceedings, a rehabilitative vs adversarial approach to decreasing risk of future criminal behavior, mandated treatment and supervision during participation, and use of veteran mentors to provide peer support.32-35 Eligibility requirements vary; however, many restrict participation to veterans with honorable discharge types and charges for nonviolent offenses.32,33,35-37
VTCs connect veterans within the criminal-legal system to needed behavioral health, community, and social services.31-33,37 VTC participants are commonly connected to case management, behavioral health care, therapeutic journaling programs, and vocational rehabilitation.38,39 Accordingly, the most common difficulties faced by veterans participating in these courts include substance use, mental health, family issues, anger management and/or aggressive behavior, and homelessness.36,39 There is limited research on the effectiveness of VTCs. Evidence on their overall effectiveness is largely mixed, though some studies suggest VTC graduates tend to have lower recidivism rates than offenders more broadly or persons who terminate VTC programs prior to completion.40,41 Other studies suggest that VTC participants are more likely to have jail sanctions, new arrests, and new incarcerations relative to nontreatment court participants.42 Notably, experimental designs (considered the gold standard in assessing effectiveness) to date have not been applied to evaluate the effectiveness of VTCs; as such, the effectiveness of these programs remains an area in need of continued empirical investigation.
Like all problem-solving courts, VTCs occasionally struggle to connect participating defendants with appropriate care, particularly when encountering structural barriers (eg, insurance, transportation) and/or complex behavioral health needs (eg, personality disorders).34,43 As suicide rates among veterans experiencing criminal-legal involvement surge (about 150 per 100,000 in 2021, a 10% increase from 2020 to 2021 compared to about 40 per 100,000 and a 1.8% increase among other veterans), efficiency of adequate care coordination is vital.44 Many VTCs rely on VTC-VA partnerships and collaborations to navigate these difficulties and facilitate connection of participating veterans to needed services.32-34,45 For example, within the VHA, Veterans Justice Outreach (VJO) and Health Care for Re-Entry Veterans (HCRV) specialists assist and bridge the gap between the criminal-legal system (including, but not limited to VTCs) and VA services by engaging veterans involved in the criminal-legal system and connecting them to needed VA-based services (Table 2). Generally, VJO specialists support veterans involved with the front end of the criminallegal system (eg, arrest, pretrial incarceration, or participation in VTCs), while HCRV specialists tend to support veterans transitioning back into the community after a period of incarceration.46,47 Specific to VTCs, VJO specialists typically serve as liaisons between the courts and VA, coordinating VA services for defendants to fulfill their terms of VTC participation.46

The historical exclusion of veterans with OTH discharge characterizations from VA-based services has restricted many from accessing VTC programs.32 Of 17 VTC programs active in Pennsylvania in 2014, only 5 accepted veterans with OTstayH discharges, and 3 required application to and eligibility for VA benefits.33 Similarly, in national surveys of VTC programs, about 1 in 3 report excluding veterans deemed ineligible for VA services.35,36 When veterans with OTH discharges have accessed VTC programs, they have historically relied on non-VA, community-based programming to fulfill treatment mandates, which may be less suited to addressing the unique needs of veterans.48
Veterans who utilize VTCs receive several benefits, namely peer support and mentorship, acceptance into a veteran-centric space, and connection with specially trained staff capable of supporting the veteran through applications for a range of VA benefits (eg, service connection, housing support).31-33,37 Given the disparate prevalence of OTH discharge characterizations among service members from racial, sexual, and gender minorities and among service members with mental health disorders, exclusion of veterans with OTH discharges from VTCs solely based on the type of discharge likely contributes to structural inequities among these already underserved groups by restricting access to these potential benefits. Such structural inequity stands in direct conflict with VTC best practice standards, which admonish programs to adjust eligibility requirements to facilitate access to treatment court programs for historically marginalized groups.49
ELIGIBILITY EXPANSIONS
Given the overrepresentation of veterans with OTH discharge characterizations within the criminal-legal system and historical barriers of these veterans to access needed mental and behavioral health care, expansions in VA eligibility policies could have immense implications for VTCs. First, these expansions could mitigate common barriers to connecting VTC-participating veterans with OTH discharges with needed behavioral health care by allowing these veterans to access established, VA-based services and programming. Expansion may also allow VTCs to serve as a key intercept point for identifying and engaging veterans with OTH discharges who may be unaware of their eligibility for VA-based behavioral health care.
Access to VA health care services is a major resource for VTC participants and a common requirement.32 Eligibility expansion should ease access barriers veterans with OTH discharges commonly face. By providing a potential source of treatment, expansions may also support OTH eligibility practices within VTCs, particularly practices that require participants to be eligible for VA health care.33,35,36 Some VTCs may continue to determine eligibility on the basis of discharge status and remain inaccessible to veterans with OTH discharge characterizations without program-level policy changes.32,36,37
Communicating changes in eligibility policies relevant to veterans with OTH discharges may be a challenge, because many of these individuals have no established channels of communication with the VA. Because veterans with OTH discharges are at increased risk for legal system involvement, VTCs may serve as a unique point of contact to help facilitate communication.18 For example, upon referral to a VTC, veterans with OTH discharges can be identified, VA health care eligibility can be verified, and veterans can connect to VA staff to facilitate enrollment in VA services and care.
VJO specialists are in a favorable position to serve a critical role in utilizing VTCs as a potential intercept point for engaging veterans with OTH discharge characterizations. As outlined in the STRONG Veterans Act of 2022, VJOs are mandated to “spread awareness and understanding of veteran eligibility for the [VJO] Program, including the eligibility of veterans who were discharged from service in the Armed Forces under conditions other than honorable.”50 The Act further requires VJOs to be annually trained in communicating eligibility changes as they arise. Accordingly, VJOs receive ongoing training in a wide variety of critical outreach topics, including changes in eligibility; while VJOs cannot make eligibility determinations, they are tasked with enrolling all veterans involved in the criminal-legal system with whom they interact into VHA services, whether through typical or special eligibility criteria (M. Stimmel, PhD, National Training Director for Veteran Justice Programming, oral communication, July 14, 2023). VJOs therefore routinely serve in this capacity of facilitating VA enrollment of veterans with OTH discharge characterizations.
Recommendations to Veteran-Servicing Judicial Programs
Considering these potential implications, professionals routinely interacting with veterans involved in the criminal-legal system should become familiarized with recent changes in VA eligibility policies. Such familiarization would support the identification of veterans previously considered ineligible for care; provision of education to these veterans regarding their new eligibility; and referral to appropriate VA-based behavioral health care options. Although conceptually simple, executing such an educational campaign may prove logistically difficult. Given their historical exclusion from VA services, veterans with OTH discharge characterizations are unlikely to seek VA-based services in times of need, instead relying on a broad swath of civilian community-based organizations and resources. Usual approaches to advertising VHA health care policy changes (eg, by notifying VA employees and/or departments providing corresponding services or by circulating information to veteran-focused mailing lists and organizations) likely would prove insufficient. Educational campaigns to disseminate information about recent OTH eligibility changes should instead consider partnering with traditionally civilian, communitybased organizations and institutions, such as state bar associations, legal aid networks, case management services, nonveteran treatment court programs (eg, drug courts, or domestic violence courts), or probation/ parole programs. Because national surveys suggest generally low military cultural competence among civilian populations, providing concurrent support in developing foundational veteran cultural competencies (eg, how to phrase questions about military service history, or understanding discharge characterizations) may be necessary to ensure effective identification and engagement of veteran clients.48
Programs that serve veterans with criminal-legal involvement should also consider potential relevance of recent OTH eligibility changes to program operations. VTC program staff and key partners (eg, judges, case managers, district attorneys, or defense attorneys), should revisit policies and procedures surrounding the engagement of veterans with OTH discharges within VTC programs and strategies for connecting these veterans with needed services. VTC programs that have historically excluded veterans with OTH discharges due to associated difficulties in locating and connecting with needed services should consider expanding eligibility policies considering recent shifts in VA behavioral health care eligibility.33,35,36 Within the VHA, VJO specialists can play a critical role in supporting these VTC eligibility and cultural shifts. Some evidence suggests a large proportion of VTC referrals are facilitated by VJO specialists and that many such referrals are identified when veterans involved with the criminal-legal system seek VA support and/or services.33 Given the historical exclusion of veterans with OTH discharges from VA care, strategies used by VJO specialists to identify, connect, and engage veterans with OTH discharges with VTCs and other services may be beneficial.
Even with knowledge of VA eligibility changes and considerations of these changes on local operations, many forensic settings and programs struggle to identify veterans. These difficulties are likely amplified among veterans with OTH discharge characterizations, who may be hesitant to self-disclose their military service history due to fear of stigma and/or views of OTH discharge characterizations as undeserving of the veteran title.12 The VA offers 2 tools to aid in identification of veterans for these settings: the Veterans Re-Entry Search Service (VRSS) and Status Query and Response Exchange System (SQUARES). For VRSS, correctional facilities, courts, and other criminal justice entities upload a simple spreadsheet that contains basic identifying information of inmates or defendants in their system. VRSS returns information about which inmates or defendants have a history of military service and alerts VA Veterans Justice Programs staff so they can conduct outreach. A pilot study conducted by the California Department of Corrections and Rehabilitation found that 2.7% of its inmate population self-identified as veterans, while VRSS identified 7.7% of inmates with a history of military service. This difference represented about 5000 previously unidentified veterans.51 Similarly, community entities that partner with the VA, such as law enforcement or homeless service programs, can be approved to become a SQUARES user and submit identifying information of individuals with whom they interact directly into the SQUARES search engine. SQUARES then directly returns information about the individual’s veteran status and eligibility for VA health care and homeless programs.
Other Eligibility Limitations
VTCs and other professionals looking to refer veterans with OTH discharge characterizations to VA-based behavioral health care should be aware of potential limitations in eligibility and access. Specifically, although veterans with OTH discharges are now broadly eligible for VA-based behavioral health care and homeless programs, they remain ineligible for other forms of health care, including primary care and nonbehavioral specialty care.1 Research has found a strong comorbidity between behavioral and nonbehavioral health concerns, particularly within historically marginalized demographic groups.52-55 Because these historically marginalized groups are often overrepresented among persons with criminal-legal involvement, veterans with OTH discharges, and VTC participants, such comorbidities require consideration by services or programming designed to support veterans with criminal-legal involvement.12,56-58 Connection with VA-based health care will therefore continue to fall short of addressing all health care needs of veterans with OTH discharges and effective case management will require considerable treatment coordination between VA behavioral health care practitioners (HCPs) and community-based HCPs (eg, primary care professionals or nonbehavioral HCPs).
Implications for VA Mental Health Care
Recent eligibility expansions will also have inevitable consequences for VA mental health care systems. For many years, these systems have been overburdened by high caseloads and clinician burnout.59,60 Given the generally elevated rates of mental health and substance use concerns among veterans with OTH discharge characterizations, expansions hold the potential to further burden caseloads with clinically complex, high-risk, high-need clients. Nevertheless, these expansions are also structured in a way that forces existing systems to absorb the responsibilities of providing necessary care to these veterans. To mitigate detrimental effects of eligibility expansions on the broader VA mental health system, clinicians should be explicitly trained in identifying veterans with OTH discharge characterizations and the implications of discharge status on broader health care eligibility. Treatment of veterans with OTH discharges may also benefit from close coordination between mental health professionals and behavioral health care coordinators to ensure appropriate coordination of care between VA- and non–VA-based HCPs.
CONCLUSIONS
Recent changes to VA eligibility policies now allow comprehensive mental and behavioral health care services to be provided to veterans with OTH discharges.1 Compared to routinely discharged veterans, veterans with OTH discharges are more likely to be persons of color, sexual or gender minorities, and experiencing mental health-related difficulties. Given the disproportionate mental health burden often faced by veterans with OTH discharges and relative overrepresentation of these veterans in judicial and correctional systems, these changes have considerable implications for programs and services designed to support veterans with criminallegal involvement. Professionals within these systems, particularly VTC programs, are therefore encouraged to familiarize themselves with recent changes in VA eligibility policies and to revisit strategies, policies, and procedures surrounding the engagement and enrollment of veterans with OTH discharge characterizations. Doing so may ensure veterans with OTH discharges are effectively connected to needed behavioral health care services.
- US Department of Veterans, Veterans Health Administration. VHA Directive 1601A.02(6): Eligibility Determination. Updated March 6, 2024. Accessed August 8, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8908
- Mental and behavioral health care for certain former members of the Armed Forces. 38 USC §1720I (2018). Accessed August 5, 2024. https://uscode.house.gov/view.xhtml?req=(title:38%20section:1720I%20edition:prelim
- US Department of Veterans, Veterans Health Administration. VHA Directive 1601A.02, Eligibility Determination. June 7, 2017.
- US Department of Veterans, Veterans Health Administration. VHA Directive 1115(1), Military Sexual Trauma (MST) Program. May 8, 2018. Accessed August 5, 2024. https:// www.va.gov/vhapublications/viewpublication.asp?pub_ID=6402
- US Department of Veterans Affairs. Tentative Eligibility Determinations; Presumptive Eligibility for Psychosis and Other Mental Illness. 38 CFR §17.109. May 14, 2013. Accessed August 5, 2024. https://www.federalregister.gov/documents/2013/05/14/2013-11410/tentative-eligibility-determinations-presumptive-eligibility-for-psychosis-and-other-mental-illness
- US Department of Veterans Affairs, VA Office of Mental Health and Suicide Prevention. National strategy for preventing veteran suicide 2018-2028. Published September 2018. Accessed August 5, 2024. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf
- US Department of Veterans Affairs. VA secretary announces intention to expand mental health care to former service members with other-than-honorable discharges and in crisis. Press Release. March 8, 2017. Accessed August 5, 2024. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=2867
- Smith C. Dramatic increase in mental health services to other-than-honorable discharge veterans. VA News. February 23, 2022. Accessed August 5, 2024. https://news.va.gov/100460/dramatic-increase-in-mental-health-services-to-other-than-honorable-discharge-veterans/
- US Department of Defense. DoD Instruction 1332.14. Enlisted administrative separations. Updated August 1, 2024. Accessed August 5, 2024. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/133214p.pdf
- US Department of Defense. DoD Instruction 1332.30. Commissioned officer administrative separations. Updated September 9, 2021. Accessed August 5, 2024. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/133230p.pdf
- OUTVETS, Legal Services Center of Harvard Law School, Veterans Legal Services. Turned away: how the VA unlawfully denies healthcare to veterans with bad paper discharges. 2020. Accessed August 5, 2024. https://legalservicescenter.org/wp-content/uploads/Turn-Away-Report.pdf
- McClean H. Discharged and discarded: the collateral consequences of a less-than-honorable military discharge. Columbia Law Rev. 2021;121(7):2203-2268.
- Veterans Benefits, General Provisions, Definitions. 38 USC §101(2) (1958). Accessed August 5, 2024. https://uscode.house.gov/view.xhtml?req=granuleid:USC-prelim-title38-section101&num=0&edition=prelim
- Bedford JR. Other than honorable discharges: unfair and unjust life sentences of decreased earning capacity. U Penn J Law Pub Affairs. 2021;6(4):687.
- Karin ML. Other than honorable discrimination. Case Western Reserve Law Rev. 2016;67(1):135-191. https://scholarlycommons.law.case.edu/caselrev/vol67/iss1/9
- Veteran HOUSE Act of 2020, HR 2398, 116th Cong, (2020). Accessed August 5, 2024. https://www.congress.gov/bill/116th-congress/house-bill/2398
- Scapardine D. Leaving other than honorable soldiers behind: how the Departments of Defense and Veterans Affairs inadvertently created a health and social crisis. Md Law Rev. 2017;76(4):1133-1165.
- Elbogen EB, Wagner HR, Brancu M, et al. Psychosocial risk factors and other than honorable military discharge: providing healthcare to previously ineligible veterans. Mil Med. 2018;183(9-10):e532-e538. doi:10.1093/milmed/usx128
- Tsai J, Rosenheck RA. Characteristics and health needs of veterans with other-than-honorable discharges: expanding eligibility in the Veterans Health Administration. Mil Med. 2018;183(5-6):e153-e157. doi:10.1093/milmed/usx110
- Christensen DM, Tsilker Y. Racial disparities in military justice: findings of substantial and persistent racial disparities within the United States military justice system. Accessed August 5, 2024. https://www.protectourdefenders.com/wp-content/uploads/2017/05/Report_20.pdf
- Don’t Ask Don’t Tell, 10 USC §654 (1993) (Repealed 2010). Accessed August 5, 2024. http://www.gpo.gov/fdsys/pkg/USCODE-2010-title10/pdf/USCODE-2010-title10-subtitleA-partII-chap37-sec654.pdf
- Palm Center. The making of a ban: how DTM-19-004 works to push transgender people out of military service. 2019. March 20, 2019. Accessed August 5, 2024. https://www.palmcenter.org/wp-content/uploads/2019/04/The-Making-of-a-Ban.pdf
- Edwards ER, Greene AL, Epshteyn G, Gromatsky M, Kinney AR, Holliday R. Mental health of incarcerated veterans and civilians: latent class analysis of the 2016 Survey of Prison Inmates. Crim Justice Behav. 2022;49(12):1800- 1821. doi:10.1177/00938548221121142
- Brignone E, Fargo JD, Blais RK, Carter ME, Samore MH, Gundlapalli AV. Non-routine discharge from military service: mental illness, substance use disorders, and suicidality. Am J Prev Med. 2017;52(5):557-565. doi:10.1016/j.amepre.2016.11.015
- Gamache G, Rosenheck R, Tessler R. Military discharge status of homeless veterans with mental illness. Mil Med. 2000;165(11):803-808. doi:10.1093/milmed/165.11.803
- Gundlapalli AV, Fargo JD, Metraux S, et al. Military Misconduct and Homelessness Among US Veterans Separated From Active Duty, 2001-2012. JAMA. 2015;314(8):832-834. doi:10.1001/jama.2015.8207
- Brooks Holliday S, Pedersen ER. The association between discharge status, mental health, and substance misuse among young adult veterans. Psychiatry Res. 2017;256:428-434. doi:10.1016/j.psychres.2017.07.011
- Williamson RB. DOD Health: Actions Needed to Ensure Post-Traumatic Stress Disorder and Traumatic Brain Injury are Considered in Misconduct Separations. US Government Accountability Office; 2017. Accessed August 5, 2024. https://apps.dtic.mil/sti/pdfs/AD1168610.pdf
- Maruschak LM, Bronson J, Alper M. Indicators of mental health problems reported by prisoners: survey of prison inmates. US Department of Justice Bureau of Justice Statistics. June 2021. Accessed August 5, 2024. https://bjs.ojp.gov/sites/g/files/xyckuh236/files/media/document/imhprpspi16st.pdf
- Brooke E, Gau J. Military service and lifetime arrests: examining the effects of the total military experience on arrests in a sample of prison inmates. Crim Justice Policy Rev. 2018;29(1):24-44. doi:10.1177/0887403415619007
- Russell RT. Veterans treatment court: a proactive approach. N Engl J Crim Civ Confin. 2009;35:357-372.
- Cartwright T. To care for him who shall have borne the battle: the recent development of Veterans Treatment Courts in America. Stanford Law Pol Rev. 2011;22:295-316.
- Douds AS, Ahlin EM, Howard D, Stigerwalt S. Varieties of veterans’ courts: a statewide assessment of veterans’ treatment court components. Crim Justice Policy Rev. 2017;28:740-769. doi:10.1177/0887403415620633
- Rowen J. Worthy of justice: a veterans treatment court in practice. Law Policy. 2020;42(1):78-100. doi:10.1111/lapo.12142
- Timko C, Flatley B, Tjemsland A, et al. A longitudinal examination of veterans treatment courts’ characteristics and eligibility criteria. Justice Res Policy. 2016;17(2):123-136.
- Baldwin JM. Executive summary: national survey of veterans treatment courts. SSRN. Preprint posted online June 5, 2013. Accessed August 5, 2024. doi:10.2139/ssrn.2274138
- Renz T. Veterans treatment court: a hand up rather than lock up. Richmond Public Interest Law Rev. 2013;17(3):697-705. https://scholarship.richmond.edu/pilr/vol17/iss3/6
- Knudsen KJ, Wingenfeld S. A specialized treatment court for veterans with trauma exposure: implications for the field. Community Ment Health J. 2016;52(2):127-135. doi:10.1007/s10597-015-9845-9
- McCall JD, Tsai J, Gordon AJ. Veterans treatment court research: participant characteristics, outcomes, and gaps in the literature. J Offender Rehabil. 2018;57:384-401. doi:10.1080/10509674.2018.1510864
- Smith JS. The Anchorage, Alaska veterans court and recidivism: July 6, 2004 – December 31, 2010. Alsk Law Rev. 2012;29(1):93-111.
- Hartley RD, Baldwin JM. Waging war on recidivism among justice-involved veterans: an impact evaluation of a large urban veterans treatment court. Crim Justice Policy Rev. 2019;30(1):52-78. doi:10.1177/0887403416650490
- Tsai J, Flatley B, Kasprow WJ, Clark S, Finlay A. Diversion of veterans with criminal justice involvement to treatment courts: participant characteristics and outcomes. Psychiatr Serv. 2017;68(4):375-383. doi:10.1176/appi.ps.201600233
- Edwards ER, Sissoko DR, Abrams D, Samost D, La Gamma S, Geraci J. Connecting mental health court participants with services: process, challenges, and recommendations. Psychol Public Policy Law. 2020;26(4):463-475. doi:10.1037/law0000236
- US Department of Veterans Affairs, VA Office of Mental Health and Suicide Prevention. 2023 National Veteran Suicide Prevention Annual Report. US Department of Veterans Affairs; November 2023. Accessed August 5, 2024. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- Finlay AK, Clark S, Blue-Howells J, et al. Logic model of the Department of Veterans Affairs’ role in veterans treatment courts. Drug Court Rev. 2019;2:45-62.
- Finlay AK, Smelson D, Sawh L, et al. U.S. Department of Veterans Affairs veterans justice outreach program: connecting justice-involved veterans with mental health and substance use disorder treatment. Crim Justice Policy Rev. 2016;27(2):10.1177/0887403414562601. doi:10.1177/0887403414562601
- Finlay AK, Stimmel M, Blue-Howells J, et al. Use of Veterans Health Administration mental health and substance use disorder treatment after exiting prison: the health care for reentry veterans program. Adm Policy Ment Health. 2017;44(2):177-187. doi:10.1007/s10488-015-0708-z
- Meyer EG, Writer BW, Brim W. The Importance of Military Cultural Competence. Curr Psychiatry Rep. 2016;18(3):26. doi:10.1007/s11920-016-0662-9
- National Association of Drug Court Professionals. Adult Drug Court Best Practice Standards Volume I. National Association of Drug Court Professionals; 2013. Accessed August 5, 2024. https://allrise.org/publications/standards/
- STRONG Veterans Act of 2022, HR 6411, 117th Cong (2022). https://www.congress.gov/bill/117th-congress/house-bill/6411/text
- Pelletier D, Clark S, Davis L. Veterans reentry search service (VRSS) and the SQUARES application. Presented at: National Association of Drug Court Professionals Conference; August 15-18, 2021; National Harbor, Maryland.
- Scott KM, Lim C, Al-Hamzawi A, et al. Association of Mental Disorders With Subsequent Chronic Physical Conditions: World Mental Health Surveys From 17 Countries. JAMA Psychiatry. 2016;73(2):150-158. doi:10.1001/jamapsychiatry.2015.2688
- Ahmed N, Conway CA. Medical and mental health comorbidities among minority racial/ethnic groups in the United States. J Soc Beh Health Sci. 2020;14(1):153-168. doi:10.5590/JSBHS.2020.14.1.11
- Hanna B, Desai R, Parekh T, Guirguis E, Kumar G, Sachdeva R. Psychiatric disorders in the U.S. transgender population. Ann Epidemiol. 2019;39:1-7.e1. doi:10.1016/j.annepidem.2019.09.009
- Watkins DC, Assari S, Johnson-Lawrence V. Race and ethnic group differences in comorbid major depressive disorder, generalized anxiety disorder, and chronic medical conditions. J Racial Ethn Health Disparities. 2015;2(3):385- 394. doi:10.1007/s40615-015-0085-z
- Baldwin J. Whom do they serve? National examination of veterans treatment court participants and their challenges. Crim Justice Policy Rev. 2017;28(6):515-554. doi:10.1177/0887403415606184
- Beatty LG, Snell TL. Profile of prison inmates, 2016. US Department of Justice Bureau of Justice Statistics. December 2021. Accessed August 5, 2024. https://bjs.ojp.gov/content/pub/pdf/ppi16.pdf
- Al-Rousan T, Rubenstein L, Sieleni B, Deol H, Wallace RB. Inside the nation’s largest mental health institution: a prevalence study in a state prison system. BMC Public Health. 2017;17(1):342. doi:10.1186/s12889-017-4257-0
- Rosen CS, Kaplan AN, Nelson DB, et al. Implementation context and burnout among Department of Veterans Affairs psychotherapists prior to and during the COVID-19 pandemic. J Affect Disord. 2023;320:517-524. doi:10.1016/j.jad.2022.09.141
- Tsai J, Jones N, Klee A, Deegan D. Job burnout among mental health staff at a veterans affairs psychosocial rehabilitation center. Community Ment Health J. 2020;56(2):294- 297. doi:10.1007/s10597-019-00487-5
- US Department of Veterans, Veterans Health Administration. VHA Directive 1601A.02(6): Eligibility Determination. Updated March 6, 2024. Accessed August 8, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8908
- Mental and behavioral health care for certain former members of the Armed Forces. 38 USC §1720I (2018). Accessed August 5, 2024. https://uscode.house.gov/view.xhtml?req=(title:38%20section:1720I%20edition:prelim
- US Department of Veterans, Veterans Health Administration. VHA Directive 1601A.02, Eligibility Determination. June 7, 2017.
- US Department of Veterans, Veterans Health Administration. VHA Directive 1115(1), Military Sexual Trauma (MST) Program. May 8, 2018. Accessed August 5, 2024. https:// www.va.gov/vhapublications/viewpublication.asp?pub_ID=6402
- US Department of Veterans Affairs. Tentative Eligibility Determinations; Presumptive Eligibility for Psychosis and Other Mental Illness. 38 CFR §17.109. May 14, 2013. Accessed August 5, 2024. https://www.federalregister.gov/documents/2013/05/14/2013-11410/tentative-eligibility-determinations-presumptive-eligibility-for-psychosis-and-other-mental-illness
- US Department of Veterans Affairs, VA Office of Mental Health and Suicide Prevention. National strategy for preventing veteran suicide 2018-2028. Published September 2018. Accessed August 5, 2024. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf
- US Department of Veterans Affairs. VA secretary announces intention to expand mental health care to former service members with other-than-honorable discharges and in crisis. Press Release. March 8, 2017. Accessed August 5, 2024. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=2867
- Smith C. Dramatic increase in mental health services to other-than-honorable discharge veterans. VA News. February 23, 2022. Accessed August 5, 2024. https://news.va.gov/100460/dramatic-increase-in-mental-health-services-to-other-than-honorable-discharge-veterans/
- US Department of Defense. DoD Instruction 1332.14. Enlisted administrative separations. Updated August 1, 2024. Accessed August 5, 2024. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/133214p.pdf
- US Department of Defense. DoD Instruction 1332.30. Commissioned officer administrative separations. Updated September 9, 2021. Accessed August 5, 2024. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/133230p.pdf
- OUTVETS, Legal Services Center of Harvard Law School, Veterans Legal Services. Turned away: how the VA unlawfully denies healthcare to veterans with bad paper discharges. 2020. Accessed August 5, 2024. https://legalservicescenter.org/wp-content/uploads/Turn-Away-Report.pdf
- McClean H. Discharged and discarded: the collateral consequences of a less-than-honorable military discharge. Columbia Law Rev. 2021;121(7):2203-2268.
- Veterans Benefits, General Provisions, Definitions. 38 USC §101(2) (1958). Accessed August 5, 2024. https://uscode.house.gov/view.xhtml?req=granuleid:USC-prelim-title38-section101&num=0&edition=prelim
- Bedford JR. Other than honorable discharges: unfair and unjust life sentences of decreased earning capacity. U Penn J Law Pub Affairs. 2021;6(4):687.
- Karin ML. Other than honorable discrimination. Case Western Reserve Law Rev. 2016;67(1):135-191. https://scholarlycommons.law.case.edu/caselrev/vol67/iss1/9
- Veteran HOUSE Act of 2020, HR 2398, 116th Cong, (2020). Accessed August 5, 2024. https://www.congress.gov/bill/116th-congress/house-bill/2398
- Scapardine D. Leaving other than honorable soldiers behind: how the Departments of Defense and Veterans Affairs inadvertently created a health and social crisis. Md Law Rev. 2017;76(4):1133-1165.
- Elbogen EB, Wagner HR, Brancu M, et al. Psychosocial risk factors and other than honorable military discharge: providing healthcare to previously ineligible veterans. Mil Med. 2018;183(9-10):e532-e538. doi:10.1093/milmed/usx128
- Tsai J, Rosenheck RA. Characteristics and health needs of veterans with other-than-honorable discharges: expanding eligibility in the Veterans Health Administration. Mil Med. 2018;183(5-6):e153-e157. doi:10.1093/milmed/usx110
- Christensen DM, Tsilker Y. Racial disparities in military justice: findings of substantial and persistent racial disparities within the United States military justice system. Accessed August 5, 2024. https://www.protectourdefenders.com/wp-content/uploads/2017/05/Report_20.pdf
- Don’t Ask Don’t Tell, 10 USC §654 (1993) (Repealed 2010). Accessed August 5, 2024. http://www.gpo.gov/fdsys/pkg/USCODE-2010-title10/pdf/USCODE-2010-title10-subtitleA-partII-chap37-sec654.pdf
- Palm Center. The making of a ban: how DTM-19-004 works to push transgender people out of military service. 2019. March 20, 2019. Accessed August 5, 2024. https://www.palmcenter.org/wp-content/uploads/2019/04/The-Making-of-a-Ban.pdf
- Edwards ER, Greene AL, Epshteyn G, Gromatsky M, Kinney AR, Holliday R. Mental health of incarcerated veterans and civilians: latent class analysis of the 2016 Survey of Prison Inmates. Crim Justice Behav. 2022;49(12):1800- 1821. doi:10.1177/00938548221121142
- Brignone E, Fargo JD, Blais RK, Carter ME, Samore MH, Gundlapalli AV. Non-routine discharge from military service: mental illness, substance use disorders, and suicidality. Am J Prev Med. 2017;52(5):557-565. doi:10.1016/j.amepre.2016.11.015
- Gamache G, Rosenheck R, Tessler R. Military discharge status of homeless veterans with mental illness. Mil Med. 2000;165(11):803-808. doi:10.1093/milmed/165.11.803
- Gundlapalli AV, Fargo JD, Metraux S, et al. Military Misconduct and Homelessness Among US Veterans Separated From Active Duty, 2001-2012. JAMA. 2015;314(8):832-834. doi:10.1001/jama.2015.8207
- Brooks Holliday S, Pedersen ER. The association between discharge status, mental health, and substance misuse among young adult veterans. Psychiatry Res. 2017;256:428-434. doi:10.1016/j.psychres.2017.07.011
- Williamson RB. DOD Health: Actions Needed to Ensure Post-Traumatic Stress Disorder and Traumatic Brain Injury are Considered in Misconduct Separations. US Government Accountability Office; 2017. Accessed August 5, 2024. https://apps.dtic.mil/sti/pdfs/AD1168610.pdf
- Maruschak LM, Bronson J, Alper M. Indicators of mental health problems reported by prisoners: survey of prison inmates. US Department of Justice Bureau of Justice Statistics. June 2021. Accessed August 5, 2024. https://bjs.ojp.gov/sites/g/files/xyckuh236/files/media/document/imhprpspi16st.pdf
- Brooke E, Gau J. Military service and lifetime arrests: examining the effects of the total military experience on arrests in a sample of prison inmates. Crim Justice Policy Rev. 2018;29(1):24-44. doi:10.1177/0887403415619007
- Russell RT. Veterans treatment court: a proactive approach. N Engl J Crim Civ Confin. 2009;35:357-372.
- Cartwright T. To care for him who shall have borne the battle: the recent development of Veterans Treatment Courts in America. Stanford Law Pol Rev. 2011;22:295-316.
- Douds AS, Ahlin EM, Howard D, Stigerwalt S. Varieties of veterans’ courts: a statewide assessment of veterans’ treatment court components. Crim Justice Policy Rev. 2017;28:740-769. doi:10.1177/0887403415620633
- Rowen J. Worthy of justice: a veterans treatment court in practice. Law Policy. 2020;42(1):78-100. doi:10.1111/lapo.12142
- Timko C, Flatley B, Tjemsland A, et al. A longitudinal examination of veterans treatment courts’ characteristics and eligibility criteria. Justice Res Policy. 2016;17(2):123-136.
- Baldwin JM. Executive summary: national survey of veterans treatment courts. SSRN. Preprint posted online June 5, 2013. Accessed August 5, 2024. doi:10.2139/ssrn.2274138
- Renz T. Veterans treatment court: a hand up rather than lock up. Richmond Public Interest Law Rev. 2013;17(3):697-705. https://scholarship.richmond.edu/pilr/vol17/iss3/6
- Knudsen KJ, Wingenfeld S. A specialized treatment court for veterans with trauma exposure: implications for the field. Community Ment Health J. 2016;52(2):127-135. doi:10.1007/s10597-015-9845-9
- McCall JD, Tsai J, Gordon AJ. Veterans treatment court research: participant characteristics, outcomes, and gaps in the literature. J Offender Rehabil. 2018;57:384-401. doi:10.1080/10509674.2018.1510864
- Smith JS. The Anchorage, Alaska veterans court and recidivism: July 6, 2004 – December 31, 2010. Alsk Law Rev. 2012;29(1):93-111.
- Hartley RD, Baldwin JM. Waging war on recidivism among justice-involved veterans: an impact evaluation of a large urban veterans treatment court. Crim Justice Policy Rev. 2019;30(1):52-78. doi:10.1177/0887403416650490
- Tsai J, Flatley B, Kasprow WJ, Clark S, Finlay A. Diversion of veterans with criminal justice involvement to treatment courts: participant characteristics and outcomes. Psychiatr Serv. 2017;68(4):375-383. doi:10.1176/appi.ps.201600233
- Edwards ER, Sissoko DR, Abrams D, Samost D, La Gamma S, Geraci J. Connecting mental health court participants with services: process, challenges, and recommendations. Psychol Public Policy Law. 2020;26(4):463-475. doi:10.1037/law0000236
- US Department of Veterans Affairs, VA Office of Mental Health and Suicide Prevention. 2023 National Veteran Suicide Prevention Annual Report. US Department of Veterans Affairs; November 2023. Accessed August 5, 2024. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- Finlay AK, Clark S, Blue-Howells J, et al. Logic model of the Department of Veterans Affairs’ role in veterans treatment courts. Drug Court Rev. 2019;2:45-62.
- Finlay AK, Smelson D, Sawh L, et al. U.S. Department of Veterans Affairs veterans justice outreach program: connecting justice-involved veterans with mental health and substance use disorder treatment. Crim Justice Policy Rev. 2016;27(2):10.1177/0887403414562601. doi:10.1177/0887403414562601
- Finlay AK, Stimmel M, Blue-Howells J, et al. Use of Veterans Health Administration mental health and substance use disorder treatment after exiting prison: the health care for reentry veterans program. Adm Policy Ment Health. 2017;44(2):177-187. doi:10.1007/s10488-015-0708-z
- Meyer EG, Writer BW, Brim W. The Importance of Military Cultural Competence. Curr Psychiatry Rep. 2016;18(3):26. doi:10.1007/s11920-016-0662-9
- National Association of Drug Court Professionals. Adult Drug Court Best Practice Standards Volume I. National Association of Drug Court Professionals; 2013. Accessed August 5, 2024. https://allrise.org/publications/standards/
- STRONG Veterans Act of 2022, HR 6411, 117th Cong (2022). https://www.congress.gov/bill/117th-congress/house-bill/6411/text
- Pelletier D, Clark S, Davis L. Veterans reentry search service (VRSS) and the SQUARES application. Presented at: National Association of Drug Court Professionals Conference; August 15-18, 2021; National Harbor, Maryland.
- Scott KM, Lim C, Al-Hamzawi A, et al. Association of Mental Disorders With Subsequent Chronic Physical Conditions: World Mental Health Surveys From 17 Countries. JAMA Psychiatry. 2016;73(2):150-158. doi:10.1001/jamapsychiatry.2015.2688
- Ahmed N, Conway CA. Medical and mental health comorbidities among minority racial/ethnic groups in the United States. J Soc Beh Health Sci. 2020;14(1):153-168. doi:10.5590/JSBHS.2020.14.1.11
- Hanna B, Desai R, Parekh T, Guirguis E, Kumar G, Sachdeva R. Psychiatric disorders in the U.S. transgender population. Ann Epidemiol. 2019;39:1-7.e1. doi:10.1016/j.annepidem.2019.09.009
- Watkins DC, Assari S, Johnson-Lawrence V. Race and ethnic group differences in comorbid major depressive disorder, generalized anxiety disorder, and chronic medical conditions. J Racial Ethn Health Disparities. 2015;2(3):385- 394. doi:10.1007/s40615-015-0085-z
- Baldwin J. Whom do they serve? National examination of veterans treatment court participants and their challenges. Crim Justice Policy Rev. 2017;28(6):515-554. doi:10.1177/0887403415606184
- Beatty LG, Snell TL. Profile of prison inmates, 2016. US Department of Justice Bureau of Justice Statistics. December 2021. Accessed August 5, 2024. https://bjs.ojp.gov/content/pub/pdf/ppi16.pdf
- Al-Rousan T, Rubenstein L, Sieleni B, Deol H, Wallace RB. Inside the nation’s largest mental health institution: a prevalence study in a state prison system. BMC Public Health. 2017;17(1):342. doi:10.1186/s12889-017-4257-0
- Rosen CS, Kaplan AN, Nelson DB, et al. Implementation context and burnout among Department of Veterans Affairs psychotherapists prior to and during the COVID-19 pandemic. J Affect Disord. 2023;320:517-524. doi:10.1016/j.jad.2022.09.141
- Tsai J, Jones N, Klee A, Deegan D. Job burnout among mental health staff at a veterans affairs psychosocial rehabilitation center. Community Ment Health J. 2020;56(2):294- 297. doi:10.1007/s10597-019-00487-5
Will New Obesity Drugs Make Bariatric Surgery Obsolete?
MADRID — In spirited presentations at the annual meeting of the European Association for the Study of Diabetes, Louis J. Aronne, MD, of Weill Cornell Medicine in New York City, made a compelling case that the next generation of obesity medications will make bariatric surgery obsolete, and Francesco Rubino, MD, of King’s College London in England, made an equally compelling case that they will not.
In fact, Dr. Rubino predicted that “metabolic” surgery — new nomenclature reflecting the power of surgery to reduce not only obesity, but also other metabolic conditions, over the long term — will continue and could even increase in years to come.
‘Medical Treatment Will Dominate’
“Obesity treatment is the superhero of treating metabolic disease because it can defeat all of the bad guys at once, not just one, like the other treatments,” Dr. Aronne told meeting attendees. “If you treat somebody’s cholesterol, you’re just treating their cholesterol, and you may actually increase their risk of developing type 2 diabetes (T2D). You treat their blood pressure, you don’t treat their glucose and you don’t treat their lipids — the list goes on and on and on. But by treating obesity, if you can get enough weight loss, you can get all those things at once.”
He pointed to the SELECT trial, which showed that treating obesity with a glucagon-like peptide 1 receptor agonist reduced major adverse cardiovascular events as well as death from any cause, results in line with those from other modes of treatment for cardiovascular disease (CVD) or lipid lowering, he said. “But we get much more with these drugs, including positive effects on heart failure, chronic kidney disease, and a 73% reduction in T2D. So, we’re now on the verge of a major change in the way we manage metabolic disease.”
Dr. Aronne drew a parallel between treating obesity and the historic way of treating hypertension. Years ago, he said, “we waited too long to treat people. We waited until they had severe hypertension that in many cases was irreversible. What would you prefer to do now for obesity — have the patient lose weight with a medicine that is proven to reduce complications or wait until they develop diabetes, high blood pressure, heart disease and then have them undergo surgery to treat that?”
Looking ahead, “the trend could be to treat obesity before it gets out of hand,” he suggested. Treatment might start in people with a body mass index (BMI) of 27 kg/m2, who would be treated to a target BMI of 25. “That’s only a 10% or so change, but our goal would be to keep them in the normal range so they never go above that target. In fact, I think we’re going to be looking at people with severe obesity in a few years and saying, ‘I can’t believe someone didn’t treat that guy earlier.’ What’s going to happen to bariatric surgery if no one gets to a higher weight?”
The plethora of current weight-loss drugs and the large group on the horizon mean that if someone doesn’t respond to one drug, there will be plenty of other choices, Dr. Aronne continued. People will be referred for surgery, but possibly only after they’ve not responded to medical treatment — or just the opposite. “In the United States, it’s much cheaper to have surgery, and I bet the insurance companies are going to make people have surgery before they can get the medicines,” he acknowledged.
A recent report from Morgan Stanley suggests that the global market for the newer weight-loss drugs could increase by 15-fold over the next 5 years as their benefits expand beyond weight loss and that as much as 9% of the US population will be taking the drugs by 2035, Dr. Aronne said, adding that he thinks 9% is an underestimate. By contrast, the number of patients treated by his team’s surgical program is down about 20%.
“I think it’s very clear that medical treatment is going to dominate,” he concluded. “But, it’s also possible that surgery could go up because so many people are going to be coming for medical therapy that we may wind up referring more for surgical therapy.”
‘Surgery Is Saving Lives’
Dr. Rubino is convinced that anti-obesity drugs will not make surgery obsolete, “but it will not be business as usual,” he told meeting attendees. “In fact, I think these drugs will expedite a process that is already ongoing — a transformation of bariatric into metabolic surgery.”
“Bariatric surgery will go down in history as one of the biggest missed opportunities that we, as medical professionals, have seen over the past many years,” he said. “It has been shown beyond any doubt to reduce all-cause mortality — in other words, it saves lives,” and it’s also cost effective and improves quality of life. Yet, fewer than 1% of people globally who meet the criteria actually get the surgery.
Many clinicians don’t inform patients about the treatment and don’t refer them for it, he said. “That would be equivalent to having surgery for CVD [cardiovascular disease], cancer, or other important diseases available but not being accessed as they should be.”
A big reason for the dearth of procedures is that people have unrealistic expectations about diet and exercise interventions for weight loss, he said. His team’s survey, presented at the 26th World Congress of the International Federation for the Surgery of Obesity and Metabolic Disorders, showed that 43% of respondents believed diet and exercise was the best treatment for severe obesity (BMI > 35). A more recent survey asked which among several choices was the most effective weight-loss intervention, and again a large proportion “believed wrongly that diet and exercise is most effective — more so than drugs or surgery — despite plenty of evidence that this is not the case.”
In this context, he said, “any surgery, no matter how safe or effective, would never be very popular.” If obesity is viewed as a modifiable risk factor, patients may say they’ll think about it for 6 months. In contrast, “nobody will tell you ‘I will think about it’ if you tell them they need gallbladder surgery to get rid of gallstone pain.”
Although drugs are available to treat obesity, none of them are curative, and if they’re stopped, the weight comes back, Dr. Rubino pointed out. “Efficacy of drugs is measured in weeks or months, whereas efficacy of surgery is measured in decades of durability — in the case of bariatric surgery, 10-20 years. That’s why bariatric surgery will remain an option,” he said. “It’s not just preventing disease, it’s resolving ongoing disease.”
Furthermore, bariatric surgery is showing value for people with established T2D, whereas in the past, it was mainly considered to be a weight-loss intervention for younger, healthier patients, he said. “In my practice, we’re operating more often in people with T2D, even those at higher risk for anesthesia and surgery — eg, patients with heart failure, chronic kidney disease, on dialysis — and we’re still able to maintain the same safety with minimally invasive laparoscopic surgery that we had with healthier patients.”
A vote held at the end of the session revealed that the audience was split about half and half in favor of drugs making bariatric surgery obsolete or not.
“I think we may have to duke it out now,” Dr. Aronne quipped.
Dr. Aronne disclosed being a consultant, speaker, and adviser for and receiving research support from Altimmune, Amgen, AstraZeneca, Eli Lilly, Intellihealth, Janssen, Novo Nordisk, Pfizer, Senda, UnitedHealth Group, Versanis, and others; he has ownership interests in ERX, Intellihealth, Jamieson, Kallyope, Skye Bioscience, Veru, and others; and he is on the board of directors of ERX, Jamieson Wellness, and Intellihealth/FlyteHealth. Dr. Rubino disclosed receiving research and educational grants from Novo Nordisk, Ethicon, and Medtronic; he is on the scientific advisory board/data safety advisory board for Keyron, Morphic Medical, and GT Metabolic Solutions; he receives speaking honoraria from Medtronic, Ethicon, Novo Nordisk, and Eli Lilly; and he is president of the nonprofit Metabolic Health Institute.
A version of this article first appeared on Medscape.com.
MADRID — In spirited presentations at the annual meeting of the European Association for the Study of Diabetes, Louis J. Aronne, MD, of Weill Cornell Medicine in New York City, made a compelling case that the next generation of obesity medications will make bariatric surgery obsolete, and Francesco Rubino, MD, of King’s College London in England, made an equally compelling case that they will not.
In fact, Dr. Rubino predicted that “metabolic” surgery — new nomenclature reflecting the power of surgery to reduce not only obesity, but also other metabolic conditions, over the long term — will continue and could even increase in years to come.
‘Medical Treatment Will Dominate’
“Obesity treatment is the superhero of treating metabolic disease because it can defeat all of the bad guys at once, not just one, like the other treatments,” Dr. Aronne told meeting attendees. “If you treat somebody’s cholesterol, you’re just treating their cholesterol, and you may actually increase their risk of developing type 2 diabetes (T2D). You treat their blood pressure, you don’t treat their glucose and you don’t treat their lipids — the list goes on and on and on. But by treating obesity, if you can get enough weight loss, you can get all those things at once.”
He pointed to the SELECT trial, which showed that treating obesity with a glucagon-like peptide 1 receptor agonist reduced major adverse cardiovascular events as well as death from any cause, results in line with those from other modes of treatment for cardiovascular disease (CVD) or lipid lowering, he said. “But we get much more with these drugs, including positive effects on heart failure, chronic kidney disease, and a 73% reduction in T2D. So, we’re now on the verge of a major change in the way we manage metabolic disease.”
Dr. Aronne drew a parallel between treating obesity and the historic way of treating hypertension. Years ago, he said, “we waited too long to treat people. We waited until they had severe hypertension that in many cases was irreversible. What would you prefer to do now for obesity — have the patient lose weight with a medicine that is proven to reduce complications or wait until they develop diabetes, high blood pressure, heart disease and then have them undergo surgery to treat that?”
Looking ahead, “the trend could be to treat obesity before it gets out of hand,” he suggested. Treatment might start in people with a body mass index (BMI) of 27 kg/m2, who would be treated to a target BMI of 25. “That’s only a 10% or so change, but our goal would be to keep them in the normal range so they never go above that target. In fact, I think we’re going to be looking at people with severe obesity in a few years and saying, ‘I can’t believe someone didn’t treat that guy earlier.’ What’s going to happen to bariatric surgery if no one gets to a higher weight?”
The plethora of current weight-loss drugs and the large group on the horizon mean that if someone doesn’t respond to one drug, there will be plenty of other choices, Dr. Aronne continued. People will be referred for surgery, but possibly only after they’ve not responded to medical treatment — or just the opposite. “In the United States, it’s much cheaper to have surgery, and I bet the insurance companies are going to make people have surgery before they can get the medicines,” he acknowledged.
A recent report from Morgan Stanley suggests that the global market for the newer weight-loss drugs could increase by 15-fold over the next 5 years as their benefits expand beyond weight loss and that as much as 9% of the US population will be taking the drugs by 2035, Dr. Aronne said, adding that he thinks 9% is an underestimate. By contrast, the number of patients treated by his team’s surgical program is down about 20%.
“I think it’s very clear that medical treatment is going to dominate,” he concluded. “But, it’s also possible that surgery could go up because so many people are going to be coming for medical therapy that we may wind up referring more for surgical therapy.”
‘Surgery Is Saving Lives’
Dr. Rubino is convinced that anti-obesity drugs will not make surgery obsolete, “but it will not be business as usual,” he told meeting attendees. “In fact, I think these drugs will expedite a process that is already ongoing — a transformation of bariatric into metabolic surgery.”
“Bariatric surgery will go down in history as one of the biggest missed opportunities that we, as medical professionals, have seen over the past many years,” he said. “It has been shown beyond any doubt to reduce all-cause mortality — in other words, it saves lives,” and it’s also cost effective and improves quality of life. Yet, fewer than 1% of people globally who meet the criteria actually get the surgery.
Many clinicians don’t inform patients about the treatment and don’t refer them for it, he said. “That would be equivalent to having surgery for CVD [cardiovascular disease], cancer, or other important diseases available but not being accessed as they should be.”
A big reason for the dearth of procedures is that people have unrealistic expectations about diet and exercise interventions for weight loss, he said. His team’s survey, presented at the 26th World Congress of the International Federation for the Surgery of Obesity and Metabolic Disorders, showed that 43% of respondents believed diet and exercise was the best treatment for severe obesity (BMI > 35). A more recent survey asked which among several choices was the most effective weight-loss intervention, and again a large proportion “believed wrongly that diet and exercise is most effective — more so than drugs or surgery — despite plenty of evidence that this is not the case.”
In this context, he said, “any surgery, no matter how safe or effective, would never be very popular.” If obesity is viewed as a modifiable risk factor, patients may say they’ll think about it for 6 months. In contrast, “nobody will tell you ‘I will think about it’ if you tell them they need gallbladder surgery to get rid of gallstone pain.”
Although drugs are available to treat obesity, none of them are curative, and if they’re stopped, the weight comes back, Dr. Rubino pointed out. “Efficacy of drugs is measured in weeks or months, whereas efficacy of surgery is measured in decades of durability — in the case of bariatric surgery, 10-20 years. That’s why bariatric surgery will remain an option,” he said. “It’s not just preventing disease, it’s resolving ongoing disease.”
Furthermore, bariatric surgery is showing value for people with established T2D, whereas in the past, it was mainly considered to be a weight-loss intervention for younger, healthier patients, he said. “In my practice, we’re operating more often in people with T2D, even those at higher risk for anesthesia and surgery — eg, patients with heart failure, chronic kidney disease, on dialysis — and we’re still able to maintain the same safety with minimally invasive laparoscopic surgery that we had with healthier patients.”
A vote held at the end of the session revealed that the audience was split about half and half in favor of drugs making bariatric surgery obsolete or not.
“I think we may have to duke it out now,” Dr. Aronne quipped.
Dr. Aronne disclosed being a consultant, speaker, and adviser for and receiving research support from Altimmune, Amgen, AstraZeneca, Eli Lilly, Intellihealth, Janssen, Novo Nordisk, Pfizer, Senda, UnitedHealth Group, Versanis, and others; he has ownership interests in ERX, Intellihealth, Jamieson, Kallyope, Skye Bioscience, Veru, and others; and he is on the board of directors of ERX, Jamieson Wellness, and Intellihealth/FlyteHealth. Dr. Rubino disclosed receiving research and educational grants from Novo Nordisk, Ethicon, and Medtronic; he is on the scientific advisory board/data safety advisory board for Keyron, Morphic Medical, and GT Metabolic Solutions; he receives speaking honoraria from Medtronic, Ethicon, Novo Nordisk, and Eli Lilly; and he is president of the nonprofit Metabolic Health Institute.
A version of this article first appeared on Medscape.com.
MADRID — In spirited presentations at the annual meeting of the European Association for the Study of Diabetes, Louis J. Aronne, MD, of Weill Cornell Medicine in New York City, made a compelling case that the next generation of obesity medications will make bariatric surgery obsolete, and Francesco Rubino, MD, of King’s College London in England, made an equally compelling case that they will not.
In fact, Dr. Rubino predicted that “metabolic” surgery — new nomenclature reflecting the power of surgery to reduce not only obesity, but also other metabolic conditions, over the long term — will continue and could even increase in years to come.
‘Medical Treatment Will Dominate’
“Obesity treatment is the superhero of treating metabolic disease because it can defeat all of the bad guys at once, not just one, like the other treatments,” Dr. Aronne told meeting attendees. “If you treat somebody’s cholesterol, you’re just treating their cholesterol, and you may actually increase their risk of developing type 2 diabetes (T2D). You treat their blood pressure, you don’t treat their glucose and you don’t treat their lipids — the list goes on and on and on. But by treating obesity, if you can get enough weight loss, you can get all those things at once.”
He pointed to the SELECT trial, which showed that treating obesity with a glucagon-like peptide 1 receptor agonist reduced major adverse cardiovascular events as well as death from any cause, results in line with those from other modes of treatment for cardiovascular disease (CVD) or lipid lowering, he said. “But we get much more with these drugs, including positive effects on heart failure, chronic kidney disease, and a 73% reduction in T2D. So, we’re now on the verge of a major change in the way we manage metabolic disease.”
Dr. Aronne drew a parallel between treating obesity and the historic way of treating hypertension. Years ago, he said, “we waited too long to treat people. We waited until they had severe hypertension that in many cases was irreversible. What would you prefer to do now for obesity — have the patient lose weight with a medicine that is proven to reduce complications or wait until they develop diabetes, high blood pressure, heart disease and then have them undergo surgery to treat that?”
Looking ahead, “the trend could be to treat obesity before it gets out of hand,” he suggested. Treatment might start in people with a body mass index (BMI) of 27 kg/m2, who would be treated to a target BMI of 25. “That’s only a 10% or so change, but our goal would be to keep them in the normal range so they never go above that target. In fact, I think we’re going to be looking at people with severe obesity in a few years and saying, ‘I can’t believe someone didn’t treat that guy earlier.’ What’s going to happen to bariatric surgery if no one gets to a higher weight?”
The plethora of current weight-loss drugs and the large group on the horizon mean that if someone doesn’t respond to one drug, there will be plenty of other choices, Dr. Aronne continued. People will be referred for surgery, but possibly only after they’ve not responded to medical treatment — or just the opposite. “In the United States, it’s much cheaper to have surgery, and I bet the insurance companies are going to make people have surgery before they can get the medicines,” he acknowledged.
A recent report from Morgan Stanley suggests that the global market for the newer weight-loss drugs could increase by 15-fold over the next 5 years as their benefits expand beyond weight loss and that as much as 9% of the US population will be taking the drugs by 2035, Dr. Aronne said, adding that he thinks 9% is an underestimate. By contrast, the number of patients treated by his team’s surgical program is down about 20%.
“I think it’s very clear that medical treatment is going to dominate,” he concluded. “But, it’s also possible that surgery could go up because so many people are going to be coming for medical therapy that we may wind up referring more for surgical therapy.”
‘Surgery Is Saving Lives’
Dr. Rubino is convinced that anti-obesity drugs will not make surgery obsolete, “but it will not be business as usual,” he told meeting attendees. “In fact, I think these drugs will expedite a process that is already ongoing — a transformation of bariatric into metabolic surgery.”
“Bariatric surgery will go down in history as one of the biggest missed opportunities that we, as medical professionals, have seen over the past many years,” he said. “It has been shown beyond any doubt to reduce all-cause mortality — in other words, it saves lives,” and it’s also cost effective and improves quality of life. Yet, fewer than 1% of people globally who meet the criteria actually get the surgery.
Many clinicians don’t inform patients about the treatment and don’t refer them for it, he said. “That would be equivalent to having surgery for CVD [cardiovascular disease], cancer, or other important diseases available but not being accessed as they should be.”
A big reason for the dearth of procedures is that people have unrealistic expectations about diet and exercise interventions for weight loss, he said. His team’s survey, presented at the 26th World Congress of the International Federation for the Surgery of Obesity and Metabolic Disorders, showed that 43% of respondents believed diet and exercise was the best treatment for severe obesity (BMI > 35). A more recent survey asked which among several choices was the most effective weight-loss intervention, and again a large proportion “believed wrongly that diet and exercise is most effective — more so than drugs or surgery — despite plenty of evidence that this is not the case.”
In this context, he said, “any surgery, no matter how safe or effective, would never be very popular.” If obesity is viewed as a modifiable risk factor, patients may say they’ll think about it for 6 months. In contrast, “nobody will tell you ‘I will think about it’ if you tell them they need gallbladder surgery to get rid of gallstone pain.”
Although drugs are available to treat obesity, none of them are curative, and if they’re stopped, the weight comes back, Dr. Rubino pointed out. “Efficacy of drugs is measured in weeks or months, whereas efficacy of surgery is measured in decades of durability — in the case of bariatric surgery, 10-20 years. That’s why bariatric surgery will remain an option,” he said. “It’s not just preventing disease, it’s resolving ongoing disease.”
Furthermore, bariatric surgery is showing value for people with established T2D, whereas in the past, it was mainly considered to be a weight-loss intervention for younger, healthier patients, he said. “In my practice, we’re operating more often in people with T2D, even those at higher risk for anesthesia and surgery — eg, patients with heart failure, chronic kidney disease, on dialysis — and we’re still able to maintain the same safety with minimally invasive laparoscopic surgery that we had with healthier patients.”
A vote held at the end of the session revealed that the audience was split about half and half in favor of drugs making bariatric surgery obsolete or not.
“I think we may have to duke it out now,” Dr. Aronne quipped.
Dr. Aronne disclosed being a consultant, speaker, and adviser for and receiving research support from Altimmune, Amgen, AstraZeneca, Eli Lilly, Intellihealth, Janssen, Novo Nordisk, Pfizer, Senda, UnitedHealth Group, Versanis, and others; he has ownership interests in ERX, Intellihealth, Jamieson, Kallyope, Skye Bioscience, Veru, and others; and he is on the board of directors of ERX, Jamieson Wellness, and Intellihealth/FlyteHealth. Dr. Rubino disclosed receiving research and educational grants from Novo Nordisk, Ethicon, and Medtronic; he is on the scientific advisory board/data safety advisory board for Keyron, Morphic Medical, and GT Metabolic Solutions; he receives speaking honoraria from Medtronic, Ethicon, Novo Nordisk, and Eli Lilly; and he is president of the nonprofit Metabolic Health Institute.
A version of this article first appeared on Medscape.com.
FROM EASD 2024
Hot Flashes: Do They Predict CVD and Dementia?
This transcript has been edited for clarity.
I’d like to talk about a recent report in the journal Menopause linking menopausal symptoms to increased risk for cognitive impairment. I’d also like to discuss some of the recent studies that have addressed whether hot flashes are linked to increased risk for heart disease and other forms of cardiovascular disease (CVD).
Given that 75%-80% of perimenopausal and postmenopausal women have hot flashes and vasomotor symptoms, it’s undoubtedly a more complex relationship between hot flashes and these outcomes than a simple one-size-fits-all, yes-or-no question.
Increasing evidence shows that several additional factors are important, including the age at which the symptoms are occurring, the time since menopause, the severity of the symptoms, whether they co-occur with night sweats and sleep disruption, and the cardiovascular status of the woman.
Several studies suggest that women who have more severe hot flashes and vasomotor symptoms are more likely to have prevalent cardiovascular risk factors — hypertension, dyslipidemia, high body mass index, endothelial dysfunction — as measured by flow-mediated vasodilation and other measures.
It is quite plausible that hot flashes could be a marker for increased risk for cognitive impairment. But the question remains, are hot flashes associated with cognitive impairment independent of these other risk factors? It appears that the associations between hot flashes, vasomotor symptoms, and CVD, and other adverse outcomes, may be more likely when hot flashes persist after age 60 or are newly occurring in later menopause. In the Women’s Health Initiative observational study, the presence of hot flashes and vasomotor symptoms in early menopause was not linked to any increased risk for heart attack, stroke, total CVD, or all-cause mortality.
However, the onset of these symptoms, especially new onset of these symptoms after age 60 or in later menopause, was in fact linked to increased risk for CVD and all-cause mortality. With respect to cognitive impairment, if a woman is having hot flashes and night sweats with regular sleep disruption, performance on cognitive testing would not be as favorable as it would be in the absence of these symptoms.
This brings us to the new study in Menopause that included approximately 1300 Latino women in nine Latin American countries, with an average age of 55 years. Looking at the association between severe menopausal symptoms and cognitive impairment, researchers found that women with severe symptoms were more likely to have cognitive impairment.
Conversely, they found that the women who had a favorable CVD risk factor status (physically active, lower BMI, healthier) and were ever users of estrogen were less likely to have cognitive impairment.
Clearly, for estrogen therapy, we need randomized clinical trials of the presence or absence of vasomotor symptoms and cognitive and CVD outcomes. Such analyses are ongoing, and new randomized trials focused specifically on women in early menopause would be very beneficial.
At the present time, it’s important that we not alarm women about the associations seen in some of these studies because often they are not independent associations; they aren’t independent of other risk factors that are commonly linked to hot flashes and night sweats. There are many other complexities in the relationship between hot flashes and cognitive impairment.
We need to appreciate that women who have moderate to severe hot flashes (especially when associated with disrupted sleep) do have impaired quality of life. It’s important to treat these symptoms, especially in early menopause, and very effective hormonal and nonhormonal treatments are available.
For women with symptoms that persist into later menopause or who have new onset of symptoms in later menopause, it’s important to prioritize cardiovascular health. For example, be more vigilant about behavioral lifestyle counseling to lower risk, and be even more aggressive in treating dyslipidemia and diabetes.
JoAnn E. Manson, Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School; Chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts; and Past President, North American Menopause Society, 2011-2012, has disclosed the following relevant financial relationships: Received study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to talk about a recent report in the journal Menopause linking menopausal symptoms to increased risk for cognitive impairment. I’d also like to discuss some of the recent studies that have addressed whether hot flashes are linked to increased risk for heart disease and other forms of cardiovascular disease (CVD).
Given that 75%-80% of perimenopausal and postmenopausal women have hot flashes and vasomotor symptoms, it’s undoubtedly a more complex relationship between hot flashes and these outcomes than a simple one-size-fits-all, yes-or-no question.
Increasing evidence shows that several additional factors are important, including the age at which the symptoms are occurring, the time since menopause, the severity of the symptoms, whether they co-occur with night sweats and sleep disruption, and the cardiovascular status of the woman.
Several studies suggest that women who have more severe hot flashes and vasomotor symptoms are more likely to have prevalent cardiovascular risk factors — hypertension, dyslipidemia, high body mass index, endothelial dysfunction — as measured by flow-mediated vasodilation and other measures.
It is quite plausible that hot flashes could be a marker for increased risk for cognitive impairment. But the question remains, are hot flashes associated with cognitive impairment independent of these other risk factors? It appears that the associations between hot flashes, vasomotor symptoms, and CVD, and other adverse outcomes, may be more likely when hot flashes persist after age 60 or are newly occurring in later menopause. In the Women’s Health Initiative observational study, the presence of hot flashes and vasomotor symptoms in early menopause was not linked to any increased risk for heart attack, stroke, total CVD, or all-cause mortality.
However, the onset of these symptoms, especially new onset of these symptoms after age 60 or in later menopause, was in fact linked to increased risk for CVD and all-cause mortality. With respect to cognitive impairment, if a woman is having hot flashes and night sweats with regular sleep disruption, performance on cognitive testing would not be as favorable as it would be in the absence of these symptoms.
This brings us to the new study in Menopause that included approximately 1300 Latino women in nine Latin American countries, with an average age of 55 years. Looking at the association between severe menopausal symptoms and cognitive impairment, researchers found that women with severe symptoms were more likely to have cognitive impairment.
Conversely, they found that the women who had a favorable CVD risk factor status (physically active, lower BMI, healthier) and were ever users of estrogen were less likely to have cognitive impairment.
Clearly, for estrogen therapy, we need randomized clinical trials of the presence or absence of vasomotor symptoms and cognitive and CVD outcomes. Such analyses are ongoing, and new randomized trials focused specifically on women in early menopause would be very beneficial.
At the present time, it’s important that we not alarm women about the associations seen in some of these studies because often they are not independent associations; they aren’t independent of other risk factors that are commonly linked to hot flashes and night sweats. There are many other complexities in the relationship between hot flashes and cognitive impairment.
We need to appreciate that women who have moderate to severe hot flashes (especially when associated with disrupted sleep) do have impaired quality of life. It’s important to treat these symptoms, especially in early menopause, and very effective hormonal and nonhormonal treatments are available.
For women with symptoms that persist into later menopause or who have new onset of symptoms in later menopause, it’s important to prioritize cardiovascular health. For example, be more vigilant about behavioral lifestyle counseling to lower risk, and be even more aggressive in treating dyslipidemia and diabetes.
JoAnn E. Manson, Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School; Chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts; and Past President, North American Menopause Society, 2011-2012, has disclosed the following relevant financial relationships: Received study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to talk about a recent report in the journal Menopause linking menopausal symptoms to increased risk for cognitive impairment. I’d also like to discuss some of the recent studies that have addressed whether hot flashes are linked to increased risk for heart disease and other forms of cardiovascular disease (CVD).
Given that 75%-80% of perimenopausal and postmenopausal women have hot flashes and vasomotor symptoms, it’s undoubtedly a more complex relationship between hot flashes and these outcomes than a simple one-size-fits-all, yes-or-no question.
Increasing evidence shows that several additional factors are important, including the age at which the symptoms are occurring, the time since menopause, the severity of the symptoms, whether they co-occur with night sweats and sleep disruption, and the cardiovascular status of the woman.
Several studies suggest that women who have more severe hot flashes and vasomotor symptoms are more likely to have prevalent cardiovascular risk factors — hypertension, dyslipidemia, high body mass index, endothelial dysfunction — as measured by flow-mediated vasodilation and other measures.
It is quite plausible that hot flashes could be a marker for increased risk for cognitive impairment. But the question remains, are hot flashes associated with cognitive impairment independent of these other risk factors? It appears that the associations between hot flashes, vasomotor symptoms, and CVD, and other adverse outcomes, may be more likely when hot flashes persist after age 60 or are newly occurring in later menopause. In the Women’s Health Initiative observational study, the presence of hot flashes and vasomotor symptoms in early menopause was not linked to any increased risk for heart attack, stroke, total CVD, or all-cause mortality.
However, the onset of these symptoms, especially new onset of these symptoms after age 60 or in later menopause, was in fact linked to increased risk for CVD and all-cause mortality. With respect to cognitive impairment, if a woman is having hot flashes and night sweats with regular sleep disruption, performance on cognitive testing would not be as favorable as it would be in the absence of these symptoms.
This brings us to the new study in Menopause that included approximately 1300 Latino women in nine Latin American countries, with an average age of 55 years. Looking at the association between severe menopausal symptoms and cognitive impairment, researchers found that women with severe symptoms were more likely to have cognitive impairment.
Conversely, they found that the women who had a favorable CVD risk factor status (physically active, lower BMI, healthier) and were ever users of estrogen were less likely to have cognitive impairment.
Clearly, for estrogen therapy, we need randomized clinical trials of the presence or absence of vasomotor symptoms and cognitive and CVD outcomes. Such analyses are ongoing, and new randomized trials focused specifically on women in early menopause would be very beneficial.
At the present time, it’s important that we not alarm women about the associations seen in some of these studies because often they are not independent associations; they aren’t independent of other risk factors that are commonly linked to hot flashes and night sweats. There are many other complexities in the relationship between hot flashes and cognitive impairment.
We need to appreciate that women who have moderate to severe hot flashes (especially when associated with disrupted sleep) do have impaired quality of life. It’s important to treat these symptoms, especially in early menopause, and very effective hormonal and nonhormonal treatments are available.
For women with symptoms that persist into later menopause or who have new onset of symptoms in later menopause, it’s important to prioritize cardiovascular health. For example, be more vigilant about behavioral lifestyle counseling to lower risk, and be even more aggressive in treating dyslipidemia and diabetes.
JoAnn E. Manson, Professor of Medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School; Chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts; and Past President, North American Menopause Society, 2011-2012, has disclosed the following relevant financial relationships: Received study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article first appeared on Medscape.com.
Locally Acquired Dengue Case Confirmed in California
A case of locally acquired dengue fever has been confirmed in a resident of Baldwin Park, California, according to a press release from the Los Angeles County Department of Public Health.
“Dengue is the most common insect-borne viral infection in the world, with a wide geographic spread; we know that we have mosquitoes capable of carrying and transmitting the virus in the United States already, and Los Angeles county is a major epicenter for international travel and trade,” James Lawler, MD, associate director for International Programs and Innovation at the Global Center for Health Security and professor in the Infectious Diseases Division at the University of Nebraska Medical Center, Omaha, Nebraska, said in an interview.
Although the patient had no known history of travel to a dengue-endemic area, the potential risk for widespread transmission of the virus in the Los Angeles County area remains low, and no additional suspected cases of locally acquired dengue have been identified, according to the release. However, the recent cases highlight the need for vigilance on the part of the public to reduce transmission of mosquito-borne infections, the public health department noted.
Most cases of dengue occur in people who have traveled to areas where the disease is more common, mainly tropical and subtropical areas, according to the press release. However, the types of mosquitoes that spread dengue exist in parts of the United States, so locally acquired infections can occur.
The Centers for Disease Control and Prevention (CDC) issued an official health advisory in June 2024 about an increased risk for dengue infections in the United States. According to the advisory, 745 cases of dengue were identified in US travelers to endemic areas between January 1, 2024, and June 24, 2024.
The CDC advises clinicians to maintain a high level of suspicion for dengue among individuals with fever and recent travel to areas with frequent dengue transmission, but also to consider locally acquired disease in areas of mosquito vectors.
In clinical practice, dengue may be difficult to differentiate from other febrile systemic infections, Dr. Lawler noted. “Joint pain, low back pain, and headache (often retro-orbital) are common and can be severe, and a rash often appears several days into illness,” he noted.
Do not delay treatment in suspected cases while waiting for test results, the CDC emphasized in the advisory. Food and Drug Administration–approved tests for dengue include RT-PCR and IgM antibody tests or NS1 and IgM antibody tests.
“Severe dengue can be life-threatening and progress to a hemorrhagic fever-like syndrome, and patients with severe dengue should be cared for on a high-acuity or intensive care setting, with close monitoring of labs and fluid status,” Dr. Lawler told this news organization.
The World Health Organization has published guidelines for the management of dengue, which Dr. Lawler strongly recommends to clinicians in the rare event that they are facing a severe case. The treatment for dengue is supportive care, according to the CDC; a vaccine that was deemed safe and effective is no longer being manufactured because of low demand.
Most symptoms last for 2-7 days, and most patients recover within a week, but approximately 1 in 20 may develop severe disease, according to the Los Angeles County Department of Public Health.
Approximately one quarter of dengue infections are symptomatic, and clinicians should know the signs of progression to severe disease, which include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, and liver enlargement, according to the CDC.
Local Dengue Not Unexpected
“Sadly, I am not surprised at another locally acquired case of dengue fever in the United States,” said Dr. Lawler. “We also have seen a trend of more historically tropical, insect-borne diseases popping up with locally acquired cases in the United States,” he noted.
Dr. Lawler suggested that “the erosion of state and local public health” is a major contributor to the increase in dengue cases. For more than 100 years, activities of state and local public health officials had significantly curtailed mosquito-borne diseases through aggressive control programs, “but we seem to be losing ground over the last several years,” he said.
“Locally acquired dengue cases are still rare in the United States,” he added. “However, people can protect themselves against dengue and more common arthropod-borne infections by taking precautions to cover up and wear insect repellent while outdoors.”
In addition, the Los Angeles County Department of Public Health emphasized in its press release that local residents reduce their risk for contact with mosquitoes by removing areas of standing water on their property and ensuring well-fitted screens on doors and windows.
Dr. Lawler had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
A case of locally acquired dengue fever has been confirmed in a resident of Baldwin Park, California, according to a press release from the Los Angeles County Department of Public Health.
“Dengue is the most common insect-borne viral infection in the world, with a wide geographic spread; we know that we have mosquitoes capable of carrying and transmitting the virus in the United States already, and Los Angeles county is a major epicenter for international travel and trade,” James Lawler, MD, associate director for International Programs and Innovation at the Global Center for Health Security and professor in the Infectious Diseases Division at the University of Nebraska Medical Center, Omaha, Nebraska, said in an interview.
Although the patient had no known history of travel to a dengue-endemic area, the potential risk for widespread transmission of the virus in the Los Angeles County area remains low, and no additional suspected cases of locally acquired dengue have been identified, according to the release. However, the recent cases highlight the need for vigilance on the part of the public to reduce transmission of mosquito-borne infections, the public health department noted.
Most cases of dengue occur in people who have traveled to areas where the disease is more common, mainly tropical and subtropical areas, according to the press release. However, the types of mosquitoes that spread dengue exist in parts of the United States, so locally acquired infections can occur.
The Centers for Disease Control and Prevention (CDC) issued an official health advisory in June 2024 about an increased risk for dengue infections in the United States. According to the advisory, 745 cases of dengue were identified in US travelers to endemic areas between January 1, 2024, and June 24, 2024.
The CDC advises clinicians to maintain a high level of suspicion for dengue among individuals with fever and recent travel to areas with frequent dengue transmission, but also to consider locally acquired disease in areas of mosquito vectors.
In clinical practice, dengue may be difficult to differentiate from other febrile systemic infections, Dr. Lawler noted. “Joint pain, low back pain, and headache (often retro-orbital) are common and can be severe, and a rash often appears several days into illness,” he noted.
Do not delay treatment in suspected cases while waiting for test results, the CDC emphasized in the advisory. Food and Drug Administration–approved tests for dengue include RT-PCR and IgM antibody tests or NS1 and IgM antibody tests.
“Severe dengue can be life-threatening and progress to a hemorrhagic fever-like syndrome, and patients with severe dengue should be cared for on a high-acuity or intensive care setting, with close monitoring of labs and fluid status,” Dr. Lawler told this news organization.
The World Health Organization has published guidelines for the management of dengue, which Dr. Lawler strongly recommends to clinicians in the rare event that they are facing a severe case. The treatment for dengue is supportive care, according to the CDC; a vaccine that was deemed safe and effective is no longer being manufactured because of low demand.
Most symptoms last for 2-7 days, and most patients recover within a week, but approximately 1 in 20 may develop severe disease, according to the Los Angeles County Department of Public Health.
Approximately one quarter of dengue infections are symptomatic, and clinicians should know the signs of progression to severe disease, which include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, and liver enlargement, according to the CDC.
Local Dengue Not Unexpected
“Sadly, I am not surprised at another locally acquired case of dengue fever in the United States,” said Dr. Lawler. “We also have seen a trend of more historically tropical, insect-borne diseases popping up with locally acquired cases in the United States,” he noted.
Dr. Lawler suggested that “the erosion of state and local public health” is a major contributor to the increase in dengue cases. For more than 100 years, activities of state and local public health officials had significantly curtailed mosquito-borne diseases through aggressive control programs, “but we seem to be losing ground over the last several years,” he said.
“Locally acquired dengue cases are still rare in the United States,” he added. “However, people can protect themselves against dengue and more common arthropod-borne infections by taking precautions to cover up and wear insect repellent while outdoors.”
In addition, the Los Angeles County Department of Public Health emphasized in its press release that local residents reduce their risk for contact with mosquitoes by removing areas of standing water on their property and ensuring well-fitted screens on doors and windows.
Dr. Lawler had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
A case of locally acquired dengue fever has been confirmed in a resident of Baldwin Park, California, according to a press release from the Los Angeles County Department of Public Health.
“Dengue is the most common insect-borne viral infection in the world, with a wide geographic spread; we know that we have mosquitoes capable of carrying and transmitting the virus in the United States already, and Los Angeles county is a major epicenter for international travel and trade,” James Lawler, MD, associate director for International Programs and Innovation at the Global Center for Health Security and professor in the Infectious Diseases Division at the University of Nebraska Medical Center, Omaha, Nebraska, said in an interview.
Although the patient had no known history of travel to a dengue-endemic area, the potential risk for widespread transmission of the virus in the Los Angeles County area remains low, and no additional suspected cases of locally acquired dengue have been identified, according to the release. However, the recent cases highlight the need for vigilance on the part of the public to reduce transmission of mosquito-borne infections, the public health department noted.
Most cases of dengue occur in people who have traveled to areas where the disease is more common, mainly tropical and subtropical areas, according to the press release. However, the types of mosquitoes that spread dengue exist in parts of the United States, so locally acquired infections can occur.
The Centers for Disease Control and Prevention (CDC) issued an official health advisory in June 2024 about an increased risk for dengue infections in the United States. According to the advisory, 745 cases of dengue were identified in US travelers to endemic areas between January 1, 2024, and June 24, 2024.
The CDC advises clinicians to maintain a high level of suspicion for dengue among individuals with fever and recent travel to areas with frequent dengue transmission, but also to consider locally acquired disease in areas of mosquito vectors.
In clinical practice, dengue may be difficult to differentiate from other febrile systemic infections, Dr. Lawler noted. “Joint pain, low back pain, and headache (often retro-orbital) are common and can be severe, and a rash often appears several days into illness,” he noted.
Do not delay treatment in suspected cases while waiting for test results, the CDC emphasized in the advisory. Food and Drug Administration–approved tests for dengue include RT-PCR and IgM antibody tests or NS1 and IgM antibody tests.
“Severe dengue can be life-threatening and progress to a hemorrhagic fever-like syndrome, and patients with severe dengue should be cared for on a high-acuity or intensive care setting, with close monitoring of labs and fluid status,” Dr. Lawler told this news organization.
The World Health Organization has published guidelines for the management of dengue, which Dr. Lawler strongly recommends to clinicians in the rare event that they are facing a severe case. The treatment for dengue is supportive care, according to the CDC; a vaccine that was deemed safe and effective is no longer being manufactured because of low demand.
Most symptoms last for 2-7 days, and most patients recover within a week, but approximately 1 in 20 may develop severe disease, according to the Los Angeles County Department of Public Health.
Approximately one quarter of dengue infections are symptomatic, and clinicians should know the signs of progression to severe disease, which include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, and liver enlargement, according to the CDC.
Local Dengue Not Unexpected
“Sadly, I am not surprised at another locally acquired case of dengue fever in the United States,” said Dr. Lawler. “We also have seen a trend of more historically tropical, insect-borne diseases popping up with locally acquired cases in the United States,” he noted.
Dr. Lawler suggested that “the erosion of state and local public health” is a major contributor to the increase in dengue cases. For more than 100 years, activities of state and local public health officials had significantly curtailed mosquito-borne diseases through aggressive control programs, “but we seem to be losing ground over the last several years,” he said.
“Locally acquired dengue cases are still rare in the United States,” he added. “However, people can protect themselves against dengue and more common arthropod-borne infections by taking precautions to cover up and wear insect repellent while outdoors.”
In addition, the Los Angeles County Department of Public Health emphasized in its press release that local residents reduce their risk for contact with mosquitoes by removing areas of standing water on their property and ensuring well-fitted screens on doors and windows.
Dr. Lawler had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Is Minimal Access Nipple-Sparing Mastectomy a Safer Option?
TOPLINE:
Given that both procedures appear safe overall, the choice may be guided by patients’ preference.
METHODOLOGY:
- Compared with a conventional mastectomy, a nipple-sparing mastectomy offers superior esthetic outcomes in patients with breast cancer. However, even the typical nipple-sparing approach often results in visible scarring and a high risk for nipple or areola necrosis. A minimal access approach, using endoscopic or robotic techniques, offers the potential to minimize scarring and better outcomes, but the complication risks are not well understood.
- Researchers performed a retrospective study that included 1583 patients with breast cancer who underwent conventional nipple-sparing mastectomy (n = 1356) or minimal access nipple-sparing mastectomy (n = 227) between 2018 and 2020 across 21 institutions in the Republic of Korea.
- Postoperative complications, categorized as short term (< 30 days) and long term (< 90 days), were compared between the two groups.
- The minimal access group had a higher percentage of premenopausal patients (73.57% vs 66.67%) and women with firm breasts (66.08% vs 31.27%).
TAKEAWAY:
- In total, 72 individuals (5.31%) in the conventional nipple-sparing mastectomy group and 7 (3.08%) in the minimal access nipple-sparing mastectomy group developed postoperative complications of grade IIIb or higher.
- The rate of complications between the conventional and minimal access nipple-sparing mastectomy groups in the short term (34.29% for conventional vs 32.16% for minimal access; P = .53) and long term (38.72% vs 32.16%, respectively; P = .06) was not significantly different.
- The conventional group experienced significantly fewer wound infections — both in the short term (1.62% vs 7.49%) and long term (4.28% vs 7.93%) — but a significantly higher rate of seroma (14.23% vs 9.25%), likely because of the variations in surgical instruments used during the procedures.
- Necrosis of the nipple or areola occurred more often in the minimal access group in the short term (8.81% vs 3.91%) but occurred more frequently in the conventional group in the long term (6.71% vs 2.20%).
IN PRACTICE:
“The similar complication rates suggest that both C-NSM [conventional nipple-sparing mastectomy] and M-NSM [minimal access nipple-sparing mastectomy] may be equally safe options,” the authors wrote. “Therefore, the choice of surgical approach should be tailored to patient preferences and individual needs.”
SOURCE:
The study, led by Joo Heung Kim, MD, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, South Korea, was published online on August 14, 2024, in JAMA Surgery.
LIMITATIONS:
The retrospective design comes with inherent biases. The nonrandom assignment of the participants to the two groups and the relatively small number of cases of minimal access nipple-sparing mastectomy may have affected the findings. The involvement of different surgeons and use of early robotic surgery techniques may have introduced bias as well.
DISCLOSURES:
This study was supported by Yonsei University College of Medicine and the National Research Foundation of Korea. Two authors reported receiving grants and consulting fees outside of this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Given that both procedures appear safe overall, the choice may be guided by patients’ preference.
METHODOLOGY:
- Compared with a conventional mastectomy, a nipple-sparing mastectomy offers superior esthetic outcomes in patients with breast cancer. However, even the typical nipple-sparing approach often results in visible scarring and a high risk for nipple or areola necrosis. A minimal access approach, using endoscopic or robotic techniques, offers the potential to minimize scarring and better outcomes, but the complication risks are not well understood.
- Researchers performed a retrospective study that included 1583 patients with breast cancer who underwent conventional nipple-sparing mastectomy (n = 1356) or minimal access nipple-sparing mastectomy (n = 227) between 2018 and 2020 across 21 institutions in the Republic of Korea.
- Postoperative complications, categorized as short term (< 30 days) and long term (< 90 days), were compared between the two groups.
- The minimal access group had a higher percentage of premenopausal patients (73.57% vs 66.67%) and women with firm breasts (66.08% vs 31.27%).
TAKEAWAY:
- In total, 72 individuals (5.31%) in the conventional nipple-sparing mastectomy group and 7 (3.08%) in the minimal access nipple-sparing mastectomy group developed postoperative complications of grade IIIb or higher.
- The rate of complications between the conventional and minimal access nipple-sparing mastectomy groups in the short term (34.29% for conventional vs 32.16% for minimal access; P = .53) and long term (38.72% vs 32.16%, respectively; P = .06) was not significantly different.
- The conventional group experienced significantly fewer wound infections — both in the short term (1.62% vs 7.49%) and long term (4.28% vs 7.93%) — but a significantly higher rate of seroma (14.23% vs 9.25%), likely because of the variations in surgical instruments used during the procedures.
- Necrosis of the nipple or areola occurred more often in the minimal access group in the short term (8.81% vs 3.91%) but occurred more frequently in the conventional group in the long term (6.71% vs 2.20%).
IN PRACTICE:
“The similar complication rates suggest that both C-NSM [conventional nipple-sparing mastectomy] and M-NSM [minimal access nipple-sparing mastectomy] may be equally safe options,” the authors wrote. “Therefore, the choice of surgical approach should be tailored to patient preferences and individual needs.”
SOURCE:
The study, led by Joo Heung Kim, MD, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, South Korea, was published online on August 14, 2024, in JAMA Surgery.
LIMITATIONS:
The retrospective design comes with inherent biases. The nonrandom assignment of the participants to the two groups and the relatively small number of cases of minimal access nipple-sparing mastectomy may have affected the findings. The involvement of different surgeons and use of early robotic surgery techniques may have introduced bias as well.
DISCLOSURES:
This study was supported by Yonsei University College of Medicine and the National Research Foundation of Korea. Two authors reported receiving grants and consulting fees outside of this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Given that both procedures appear safe overall, the choice may be guided by patients’ preference.
METHODOLOGY:
- Compared with a conventional mastectomy, a nipple-sparing mastectomy offers superior esthetic outcomes in patients with breast cancer. However, even the typical nipple-sparing approach often results in visible scarring and a high risk for nipple or areola necrosis. A minimal access approach, using endoscopic or robotic techniques, offers the potential to minimize scarring and better outcomes, but the complication risks are not well understood.
- Researchers performed a retrospective study that included 1583 patients with breast cancer who underwent conventional nipple-sparing mastectomy (n = 1356) or minimal access nipple-sparing mastectomy (n = 227) between 2018 and 2020 across 21 institutions in the Republic of Korea.
- Postoperative complications, categorized as short term (< 30 days) and long term (< 90 days), were compared between the two groups.
- The minimal access group had a higher percentage of premenopausal patients (73.57% vs 66.67%) and women with firm breasts (66.08% vs 31.27%).
TAKEAWAY:
- In total, 72 individuals (5.31%) in the conventional nipple-sparing mastectomy group and 7 (3.08%) in the minimal access nipple-sparing mastectomy group developed postoperative complications of grade IIIb or higher.
- The rate of complications between the conventional and minimal access nipple-sparing mastectomy groups in the short term (34.29% for conventional vs 32.16% for minimal access; P = .53) and long term (38.72% vs 32.16%, respectively; P = .06) was not significantly different.
- The conventional group experienced significantly fewer wound infections — both in the short term (1.62% vs 7.49%) and long term (4.28% vs 7.93%) — but a significantly higher rate of seroma (14.23% vs 9.25%), likely because of the variations in surgical instruments used during the procedures.
- Necrosis of the nipple or areola occurred more often in the minimal access group in the short term (8.81% vs 3.91%) but occurred more frequently in the conventional group in the long term (6.71% vs 2.20%).
IN PRACTICE:
“The similar complication rates suggest that both C-NSM [conventional nipple-sparing mastectomy] and M-NSM [minimal access nipple-sparing mastectomy] may be equally safe options,” the authors wrote. “Therefore, the choice of surgical approach should be tailored to patient preferences and individual needs.”
SOURCE:
The study, led by Joo Heung Kim, MD, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, South Korea, was published online on August 14, 2024, in JAMA Surgery.
LIMITATIONS:
The retrospective design comes with inherent biases. The nonrandom assignment of the participants to the two groups and the relatively small number of cases of minimal access nipple-sparing mastectomy may have affected the findings. The involvement of different surgeons and use of early robotic surgery techniques may have introduced bias as well.
DISCLOSURES:
This study was supported by Yonsei University College of Medicine and the National Research Foundation of Korea. Two authors reported receiving grants and consulting fees outside of this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Should Genetic Testing Be Routine for Breast Cancer?
TOPLINE:
METHODOLOGY:
- Traditional risk-based criteria, including family history and ancestry, are used to guide genetic testing decisions in women with breast cancer. However, these criteria may overlook patients with actionable genetic variants, particularly those outside the typical risk profile.
- To assess the efficacy of universal genetic testing, researchers conducted a cross-sectional study that included 729 women (median age at diagnosis, 53 years; 65.4% White women) newly diagnosed with invasive breast cancer between September 2019 and April 2022 at three Canadian institutions.
- All patients received genetic counseling followed by testing for the presence of germline pathogenic variants in 17 breast cancer susceptibility genes. The primary gene panel included screening for BRCA1, BRCA2, and PALB2, and the optional secondary panel included 14 additional breast cancer susceptibility genes.
- Of the participants, 659 (90.4%) were tested for both primary and secondary gene panels, whereas 70 (9.6%) underwent testing for only the primary panel. The majority of the cohort (66.8) were diagnosed with estrogen receptor–positive breast cancer, while 15.4% had triple-negative breast cancer.
TAKEAWAY:
- The prevalence of germline pathogenic variants was 7.3% (53 patients) — 5.3% for the primary gene panel and 2.1% for the secondary panel.
- Younger age (< 40 years; odds ratio [OR], 6.83), family history of ovarian cancer (OR, 9.75), high-grade disease (OR, 1.68), and triple-negative breast cancer (OR, 3.19) were independently associated with the presence of pathogenic genetic variants in BRCA1, BRCA2, or PALB2.
- Overall, 34.3% of patients with germline pathogenic variants in BRCA1, BRCA2, or PALB2, and 85.7% of carriers of secondary panel variants would not have qualified for traditional genetic testing according to the current risk factors.
- A total of 13 patients with BRCA1, BRCA2, or PALB2 variants had confirmed pathogenic mutations and were eligible for poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors.
IN PRACTICE:
These findings have “informed our clinical practice, and we now offer mainstream, oncology-led genetic testing to all women diagnosed with incident invasive breast cancer younger than 50 years of age, those with triple-negative breast cancer and/or bilateral breast cancer, those potentially eligible for PARP inhibitors,” as well as to men with breast cancer, the authors wrote.
SOURCE:
The study was led by Zoulikha Rezoug, MSc, Lady Davis Institute of the Jewish General Hospital, McGill University in Montreal, Québec, Canada. It was published online on September 3, 2024, in JAMA Network Open.
LIMITATIONS:
The COVID-19 pandemic resulted in a 6-month recruitment pause. Adjustments in recruitment criteria, focus on younger patients and those with triple-negative breast cancer could have overestimated prevalence of genetic pathogenic variants among women aged ≥ 70 years.
DISCLOSURES:
The study was supported by grants from the Jewish General Hospital Foundation and the Québec Breast Cancer Foundation, as well as an award from the Fonds de Recherche du Québec - Santé. Two authors reported receiving grants or personal fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Traditional risk-based criteria, including family history and ancestry, are used to guide genetic testing decisions in women with breast cancer. However, these criteria may overlook patients with actionable genetic variants, particularly those outside the typical risk profile.
- To assess the efficacy of universal genetic testing, researchers conducted a cross-sectional study that included 729 women (median age at diagnosis, 53 years; 65.4% White women) newly diagnosed with invasive breast cancer between September 2019 and April 2022 at three Canadian institutions.
- All patients received genetic counseling followed by testing for the presence of germline pathogenic variants in 17 breast cancer susceptibility genes. The primary gene panel included screening for BRCA1, BRCA2, and PALB2, and the optional secondary panel included 14 additional breast cancer susceptibility genes.
- Of the participants, 659 (90.4%) were tested for both primary and secondary gene panels, whereas 70 (9.6%) underwent testing for only the primary panel. The majority of the cohort (66.8) were diagnosed with estrogen receptor–positive breast cancer, while 15.4% had triple-negative breast cancer.
TAKEAWAY:
- The prevalence of germline pathogenic variants was 7.3% (53 patients) — 5.3% for the primary gene panel and 2.1% for the secondary panel.
- Younger age (< 40 years; odds ratio [OR], 6.83), family history of ovarian cancer (OR, 9.75), high-grade disease (OR, 1.68), and triple-negative breast cancer (OR, 3.19) were independently associated with the presence of pathogenic genetic variants in BRCA1, BRCA2, or PALB2.
- Overall, 34.3% of patients with germline pathogenic variants in BRCA1, BRCA2, or PALB2, and 85.7% of carriers of secondary panel variants would not have qualified for traditional genetic testing according to the current risk factors.
- A total of 13 patients with BRCA1, BRCA2, or PALB2 variants had confirmed pathogenic mutations and were eligible for poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors.
IN PRACTICE:
These findings have “informed our clinical practice, and we now offer mainstream, oncology-led genetic testing to all women diagnosed with incident invasive breast cancer younger than 50 years of age, those with triple-negative breast cancer and/or bilateral breast cancer, those potentially eligible for PARP inhibitors,” as well as to men with breast cancer, the authors wrote.
SOURCE:
The study was led by Zoulikha Rezoug, MSc, Lady Davis Institute of the Jewish General Hospital, McGill University in Montreal, Québec, Canada. It was published online on September 3, 2024, in JAMA Network Open.
LIMITATIONS:
The COVID-19 pandemic resulted in a 6-month recruitment pause. Adjustments in recruitment criteria, focus on younger patients and those with triple-negative breast cancer could have overestimated prevalence of genetic pathogenic variants among women aged ≥ 70 years.
DISCLOSURES:
The study was supported by grants from the Jewish General Hospital Foundation and the Québec Breast Cancer Foundation, as well as an award from the Fonds de Recherche du Québec - Santé. Two authors reported receiving grants or personal fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Traditional risk-based criteria, including family history and ancestry, are used to guide genetic testing decisions in women with breast cancer. However, these criteria may overlook patients with actionable genetic variants, particularly those outside the typical risk profile.
- To assess the efficacy of universal genetic testing, researchers conducted a cross-sectional study that included 729 women (median age at diagnosis, 53 years; 65.4% White women) newly diagnosed with invasive breast cancer between September 2019 and April 2022 at three Canadian institutions.
- All patients received genetic counseling followed by testing for the presence of germline pathogenic variants in 17 breast cancer susceptibility genes. The primary gene panel included screening for BRCA1, BRCA2, and PALB2, and the optional secondary panel included 14 additional breast cancer susceptibility genes.
- Of the participants, 659 (90.4%) were tested for both primary and secondary gene panels, whereas 70 (9.6%) underwent testing for only the primary panel. The majority of the cohort (66.8) were diagnosed with estrogen receptor–positive breast cancer, while 15.4% had triple-negative breast cancer.
TAKEAWAY:
- The prevalence of germline pathogenic variants was 7.3% (53 patients) — 5.3% for the primary gene panel and 2.1% for the secondary panel.
- Younger age (< 40 years; odds ratio [OR], 6.83), family history of ovarian cancer (OR, 9.75), high-grade disease (OR, 1.68), and triple-negative breast cancer (OR, 3.19) were independently associated with the presence of pathogenic genetic variants in BRCA1, BRCA2, or PALB2.
- Overall, 34.3% of patients with germline pathogenic variants in BRCA1, BRCA2, or PALB2, and 85.7% of carriers of secondary panel variants would not have qualified for traditional genetic testing according to the current risk factors.
- A total of 13 patients with BRCA1, BRCA2, or PALB2 variants had confirmed pathogenic mutations and were eligible for poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors.
IN PRACTICE:
These findings have “informed our clinical practice, and we now offer mainstream, oncology-led genetic testing to all women diagnosed with incident invasive breast cancer younger than 50 years of age, those with triple-negative breast cancer and/or bilateral breast cancer, those potentially eligible for PARP inhibitors,” as well as to men with breast cancer, the authors wrote.
SOURCE:
The study was led by Zoulikha Rezoug, MSc, Lady Davis Institute of the Jewish General Hospital, McGill University in Montreal, Québec, Canada. It was published online on September 3, 2024, in JAMA Network Open.
LIMITATIONS:
The COVID-19 pandemic resulted in a 6-month recruitment pause. Adjustments in recruitment criteria, focus on younger patients and those with triple-negative breast cancer could have overestimated prevalence of genetic pathogenic variants among women aged ≥ 70 years.
DISCLOSURES:
The study was supported by grants from the Jewish General Hospital Foundation and the Québec Breast Cancer Foundation, as well as an award from the Fonds de Recherche du Québec - Santé. Two authors reported receiving grants or personal fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
ANCA-Associated Vasculitis Has Five Unique Patient Clusters
TOPLINE:
A data-driven subclassification of antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis has identified five distinct clusters with varying degrees of kidney involvement and systemic inflammation, offering insights into improved patient stratification and treatment approaches.
METHODOLOGY:
- ANCA-associated vasculitis is a rare and complex autoimmune disease that is traditionally classified into granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA).
- Researchers employed advanced artificial intelligence and big data techniques to identify phenotypically distinct subgroups of ANCA-associated vasculitis and developed a classification system using real-world patient data from the Federated Vasculitis Registry consortium.
- They included 3868 patients diagnosed with ANCA-associated vasculitis between November 1, 1966, and March 1, 2023 (mean age at diagnosis, 57.2 years; 51.9% men), across six European vasculitis registries; while a majority of patients (62.9%) were diagnosed with GPA, the remaining 37.1% were diagnosed with MPA.
- Overall, 17 clinical and demographic variables such as the age at diagnosis, gender, serum creatinine and C-reactive protein levels, the type of ANCA, and the involvement of various organ systems were used to create a model for categorizing patients into different clusters.
- The median follow-up duration was 4.2 years.
TAKEAWAY:
- Five distinct clusters were identified in ANCA-associated vasculitis; three had significant kidney involvement (the severe kidney cluster, myeloperoxidase-ANCA-positive kidney cluster, and proteinase 3-ANCA-positive kidney cluster) and two had minimal kidney involvement (young respiratory cluster and inflammatory multisystem cluster).
- The clusters with significant kidney involvement were associated with poorer outcomes, including a higher risk for kidney failure and death. The severe kidney cluster had the poorest prognosis, with mortality and the rate of end-stage kidney failure being 30.5% and 41.6%, respectively.
- The young respiratory cluster, characterized by predominant ear-nose-throat involvement and low systemic inflammation, showed the best prognostic outcomes.
- This cluster membership model showed a greater predictive accuracy for patient and kidney survival than traditional methods based on clinical diagnosis or ANCA specificity.
IN PRACTICE:
“These findings highlight the necessity of recognizing severe kidney disease at the time of diagnosis as an indicator of poor outcome, thereby necessitating intensified treatment approaches,” experts from the Department of Internal Medicine IV (Nephrology and Hypertension), Medical University of Innsbruck, Austria, wrote in an accompanying editorial published online on August 22, 2024, in The Lancet Rheumatology.
SOURCE:
This study was led by Karl Gisslander, Department of Clinical Sciences, Lund University, Lund, Sweden, and was published online on August 22, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Data on estimated glomerular filtration rate recovery in clusters with kidney disease were lacking. Populations from East Asia, where myeloperoxidase-ANCA positivity is more prevalent, were not included.
DISCLOSURES:
This study received funding from the European Union’s Horizon 2020 research and innovation program under the European Joint Programme on Rare Diseases. Some authors declared serving on advisory boards or receiving grants, contracts, travel support, consulting fees, payments, or honoraria from various pharmaceutical companies and other institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A data-driven subclassification of antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis has identified five distinct clusters with varying degrees of kidney involvement and systemic inflammation, offering insights into improved patient stratification and treatment approaches.
METHODOLOGY:
- ANCA-associated vasculitis is a rare and complex autoimmune disease that is traditionally classified into granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA).
- Researchers employed advanced artificial intelligence and big data techniques to identify phenotypically distinct subgroups of ANCA-associated vasculitis and developed a classification system using real-world patient data from the Federated Vasculitis Registry consortium.
- They included 3868 patients diagnosed with ANCA-associated vasculitis between November 1, 1966, and March 1, 2023 (mean age at diagnosis, 57.2 years; 51.9% men), across six European vasculitis registries; while a majority of patients (62.9%) were diagnosed with GPA, the remaining 37.1% were diagnosed with MPA.
- Overall, 17 clinical and demographic variables such as the age at diagnosis, gender, serum creatinine and C-reactive protein levels, the type of ANCA, and the involvement of various organ systems were used to create a model for categorizing patients into different clusters.
- The median follow-up duration was 4.2 years.
TAKEAWAY:
- Five distinct clusters were identified in ANCA-associated vasculitis; three had significant kidney involvement (the severe kidney cluster, myeloperoxidase-ANCA-positive kidney cluster, and proteinase 3-ANCA-positive kidney cluster) and two had minimal kidney involvement (young respiratory cluster and inflammatory multisystem cluster).
- The clusters with significant kidney involvement were associated with poorer outcomes, including a higher risk for kidney failure and death. The severe kidney cluster had the poorest prognosis, with mortality and the rate of end-stage kidney failure being 30.5% and 41.6%, respectively.
- The young respiratory cluster, characterized by predominant ear-nose-throat involvement and low systemic inflammation, showed the best prognostic outcomes.
- This cluster membership model showed a greater predictive accuracy for patient and kidney survival than traditional methods based on clinical diagnosis or ANCA specificity.
IN PRACTICE:
“These findings highlight the necessity of recognizing severe kidney disease at the time of diagnosis as an indicator of poor outcome, thereby necessitating intensified treatment approaches,” experts from the Department of Internal Medicine IV (Nephrology and Hypertension), Medical University of Innsbruck, Austria, wrote in an accompanying editorial published online on August 22, 2024, in The Lancet Rheumatology.
SOURCE:
This study was led by Karl Gisslander, Department of Clinical Sciences, Lund University, Lund, Sweden, and was published online on August 22, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Data on estimated glomerular filtration rate recovery in clusters with kidney disease were lacking. Populations from East Asia, where myeloperoxidase-ANCA positivity is more prevalent, were not included.
DISCLOSURES:
This study received funding from the European Union’s Horizon 2020 research and innovation program under the European Joint Programme on Rare Diseases. Some authors declared serving on advisory boards or receiving grants, contracts, travel support, consulting fees, payments, or honoraria from various pharmaceutical companies and other institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A data-driven subclassification of antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis has identified five distinct clusters with varying degrees of kidney involvement and systemic inflammation, offering insights into improved patient stratification and treatment approaches.
METHODOLOGY:
- ANCA-associated vasculitis is a rare and complex autoimmune disease that is traditionally classified into granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA).
- Researchers employed advanced artificial intelligence and big data techniques to identify phenotypically distinct subgroups of ANCA-associated vasculitis and developed a classification system using real-world patient data from the Federated Vasculitis Registry consortium.
- They included 3868 patients diagnosed with ANCA-associated vasculitis between November 1, 1966, and March 1, 2023 (mean age at diagnosis, 57.2 years; 51.9% men), across six European vasculitis registries; while a majority of patients (62.9%) were diagnosed with GPA, the remaining 37.1% were diagnosed with MPA.
- Overall, 17 clinical and demographic variables such as the age at diagnosis, gender, serum creatinine and C-reactive protein levels, the type of ANCA, and the involvement of various organ systems were used to create a model for categorizing patients into different clusters.
- The median follow-up duration was 4.2 years.
TAKEAWAY:
- Five distinct clusters were identified in ANCA-associated vasculitis; three had significant kidney involvement (the severe kidney cluster, myeloperoxidase-ANCA-positive kidney cluster, and proteinase 3-ANCA-positive kidney cluster) and two had minimal kidney involvement (young respiratory cluster and inflammatory multisystem cluster).
- The clusters with significant kidney involvement were associated with poorer outcomes, including a higher risk for kidney failure and death. The severe kidney cluster had the poorest prognosis, with mortality and the rate of end-stage kidney failure being 30.5% and 41.6%, respectively.
- The young respiratory cluster, characterized by predominant ear-nose-throat involvement and low systemic inflammation, showed the best prognostic outcomes.
- This cluster membership model showed a greater predictive accuracy for patient and kidney survival than traditional methods based on clinical diagnosis or ANCA specificity.
IN PRACTICE:
“These findings highlight the necessity of recognizing severe kidney disease at the time of diagnosis as an indicator of poor outcome, thereby necessitating intensified treatment approaches,” experts from the Department of Internal Medicine IV (Nephrology and Hypertension), Medical University of Innsbruck, Austria, wrote in an accompanying editorial published online on August 22, 2024, in The Lancet Rheumatology.
SOURCE:
This study was led by Karl Gisslander, Department of Clinical Sciences, Lund University, Lund, Sweden, and was published online on August 22, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Data on estimated glomerular filtration rate recovery in clusters with kidney disease were lacking. Populations from East Asia, where myeloperoxidase-ANCA positivity is more prevalent, were not included.
DISCLOSURES:
This study received funding from the European Union’s Horizon 2020 research and innovation program under the European Joint Programme on Rare Diseases. Some authors declared serving on advisory boards or receiving grants, contracts, travel support, consulting fees, payments, or honoraria from various pharmaceutical companies and other institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
‘Reform School’ for Pharmacy Benefit Managers: How Might Legislation Help Patients?
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at [email protected].
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at [email protected].
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at [email protected].
Belimumab Hits Newer Remission, Low Disease Activity Metrics
TOPLINE:
A greater proportion of patients with active systemic lupus erythematosus (SLE) treated with belimumab plus standard therapy achieved the newest definitions for remission and low disease activity compared with those treated with placebo plus standard therapy, with benefits observed as early as week 28 for remission and week 8 for disease activity, according to pooled results from five clinical trials.
METHODOLOGY:
- Researchers conducted an integrated post hoc analysis of five randomized phase 3 clinical trials to evaluate the attainment of remission and low disease activity in adult patients with active, autoantibody-positive SLE.
- A total of 3086 patients (median age, 36 years; 94% women) were randomly assigned to receive standard therapy with intravenous belimumab 10 mg/kg monthly or subcutaneous belimumab 200 mg weekly (n = 1869) or placebo (n = 1217).
- The proportion of patients who achieved definitions of remission in SLE (DORIS) remission and lupus low disease activity state (LLDAS) by visit up to week 52 was assessed.
- The analysis also evaluated the time taken to achieve sustained (at least two consecutive visits) and maintained (up to week 52) DORIS remission and LLDAS.
TAKEAWAY:
- At week 52, a higher proportion of patients receiving belimumab vs placebo achieved DORIS remission (8% vs 6%; risk ratio [RR], 1.51; P = .0055) and LLDAS (17% vs 10%; RR, 1.74; P < .0001).
- The earliest observed significant benefit of belimumab over placebo in patients with a higher baseline disease activity was at week 20 for DORIS remission (RR, 2.09; P = .043) and at week 16 for LLDAS (RR, 1.46; P = .034), with both maintained through week 52.
- The proportion of patients who attained DORIS remission and LLDAS as early as week 28 and week 8, respectively, was higher in the belimumab group than in the placebo group, with both maintained through week 52.
- Patients on belimumab were more likely to have a sustained and maintained DORIS remission (hazard ratio [HR], 1.53; P = .013) and LLDAS (HR, 1.79; P < .0001) at any timepoint.
IN PRACTICE:
“The data clearly support that belimumab is a valuable addition toward accomplishing and maintaining remission or LLDAS,” George Bertsias, MD, PhD, University of Crete Medical School, Heraklion, Greece, and Jinoos Yazdany, MD, University of California San Francisco, wrote in a related comment.
SOURCE:
This study, led by Ioannis Parodis, MD, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden, was published online on August 26, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Due to the post hoc nature of the analysis, the trials were not specifically designed to have adequate statistical power to demonstrate the difference between patients who did or did not achieve DORIS remission or LLDAS. The analysis was limited to patients who met the eligibility criteria, and the outcomes are not generalizable to populations outside a clinical trial setting. The study population had high disease activity, which made it challenging to attain the treatment targets.
DISCLOSURES:
The five trials included in this analysis were funded by GSK. The study was supported by the Swedish Rheumatism Association, King Gustaf V’s 80-year Foundation, the Swedish Society of Medicine, Nyckelfonden, Professor Nanna Svartz Foundation, Ulla and Roland Gustafsson Foundation, Region Stockholm, and Karolinska Institutet. Some authors reported receiving grants, speaker honoraria, or consulting fees from various pharmaceutical companies. Some authors reported being employees and owning stocks and shares of GSK.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A greater proportion of patients with active systemic lupus erythematosus (SLE) treated with belimumab plus standard therapy achieved the newest definitions for remission and low disease activity compared with those treated with placebo plus standard therapy, with benefits observed as early as week 28 for remission and week 8 for disease activity, according to pooled results from five clinical trials.
METHODOLOGY:
- Researchers conducted an integrated post hoc analysis of five randomized phase 3 clinical trials to evaluate the attainment of remission and low disease activity in adult patients with active, autoantibody-positive SLE.
- A total of 3086 patients (median age, 36 years; 94% women) were randomly assigned to receive standard therapy with intravenous belimumab 10 mg/kg monthly or subcutaneous belimumab 200 mg weekly (n = 1869) or placebo (n = 1217).
- The proportion of patients who achieved definitions of remission in SLE (DORIS) remission and lupus low disease activity state (LLDAS) by visit up to week 52 was assessed.
- The analysis also evaluated the time taken to achieve sustained (at least two consecutive visits) and maintained (up to week 52) DORIS remission and LLDAS.
TAKEAWAY:
- At week 52, a higher proportion of patients receiving belimumab vs placebo achieved DORIS remission (8% vs 6%; risk ratio [RR], 1.51; P = .0055) and LLDAS (17% vs 10%; RR, 1.74; P < .0001).
- The earliest observed significant benefit of belimumab over placebo in patients with a higher baseline disease activity was at week 20 for DORIS remission (RR, 2.09; P = .043) and at week 16 for LLDAS (RR, 1.46; P = .034), with both maintained through week 52.
- The proportion of patients who attained DORIS remission and LLDAS as early as week 28 and week 8, respectively, was higher in the belimumab group than in the placebo group, with both maintained through week 52.
- Patients on belimumab were more likely to have a sustained and maintained DORIS remission (hazard ratio [HR], 1.53; P = .013) and LLDAS (HR, 1.79; P < .0001) at any timepoint.
IN PRACTICE:
“The data clearly support that belimumab is a valuable addition toward accomplishing and maintaining remission or LLDAS,” George Bertsias, MD, PhD, University of Crete Medical School, Heraklion, Greece, and Jinoos Yazdany, MD, University of California San Francisco, wrote in a related comment.
SOURCE:
This study, led by Ioannis Parodis, MD, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden, was published online on August 26, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Due to the post hoc nature of the analysis, the trials were not specifically designed to have adequate statistical power to demonstrate the difference between patients who did or did not achieve DORIS remission or LLDAS. The analysis was limited to patients who met the eligibility criteria, and the outcomes are not generalizable to populations outside a clinical trial setting. The study population had high disease activity, which made it challenging to attain the treatment targets.
DISCLOSURES:
The five trials included in this analysis were funded by GSK. The study was supported by the Swedish Rheumatism Association, King Gustaf V’s 80-year Foundation, the Swedish Society of Medicine, Nyckelfonden, Professor Nanna Svartz Foundation, Ulla and Roland Gustafsson Foundation, Region Stockholm, and Karolinska Institutet. Some authors reported receiving grants, speaker honoraria, or consulting fees from various pharmaceutical companies. Some authors reported being employees and owning stocks and shares of GSK.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A greater proportion of patients with active systemic lupus erythematosus (SLE) treated with belimumab plus standard therapy achieved the newest definitions for remission and low disease activity compared with those treated with placebo plus standard therapy, with benefits observed as early as week 28 for remission and week 8 for disease activity, according to pooled results from five clinical trials.
METHODOLOGY:
- Researchers conducted an integrated post hoc analysis of five randomized phase 3 clinical trials to evaluate the attainment of remission and low disease activity in adult patients with active, autoantibody-positive SLE.
- A total of 3086 patients (median age, 36 years; 94% women) were randomly assigned to receive standard therapy with intravenous belimumab 10 mg/kg monthly or subcutaneous belimumab 200 mg weekly (n = 1869) or placebo (n = 1217).
- The proportion of patients who achieved definitions of remission in SLE (DORIS) remission and lupus low disease activity state (LLDAS) by visit up to week 52 was assessed.
- The analysis also evaluated the time taken to achieve sustained (at least two consecutive visits) and maintained (up to week 52) DORIS remission and LLDAS.
TAKEAWAY:
- At week 52, a higher proportion of patients receiving belimumab vs placebo achieved DORIS remission (8% vs 6%; risk ratio [RR], 1.51; P = .0055) and LLDAS (17% vs 10%; RR, 1.74; P < .0001).
- The earliest observed significant benefit of belimumab over placebo in patients with a higher baseline disease activity was at week 20 for DORIS remission (RR, 2.09; P = .043) and at week 16 for LLDAS (RR, 1.46; P = .034), with both maintained through week 52.
- The proportion of patients who attained DORIS remission and LLDAS as early as week 28 and week 8, respectively, was higher in the belimumab group than in the placebo group, with both maintained through week 52.
- Patients on belimumab were more likely to have a sustained and maintained DORIS remission (hazard ratio [HR], 1.53; P = .013) and LLDAS (HR, 1.79; P < .0001) at any timepoint.
IN PRACTICE:
“The data clearly support that belimumab is a valuable addition toward accomplishing and maintaining remission or LLDAS,” George Bertsias, MD, PhD, University of Crete Medical School, Heraklion, Greece, and Jinoos Yazdany, MD, University of California San Francisco, wrote in a related comment.
SOURCE:
This study, led by Ioannis Parodis, MD, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden, was published online on August 26, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Due to the post hoc nature of the analysis, the trials were not specifically designed to have adequate statistical power to demonstrate the difference between patients who did or did not achieve DORIS remission or LLDAS. The analysis was limited to patients who met the eligibility criteria, and the outcomes are not generalizable to populations outside a clinical trial setting. The study population had high disease activity, which made it challenging to attain the treatment targets.
DISCLOSURES:
The five trials included in this analysis were funded by GSK. The study was supported by the Swedish Rheumatism Association, King Gustaf V’s 80-year Foundation, the Swedish Society of Medicine, Nyckelfonden, Professor Nanna Svartz Foundation, Ulla and Roland Gustafsson Foundation, Region Stockholm, and Karolinska Institutet. Some authors reported receiving grants, speaker honoraria, or consulting fees from various pharmaceutical companies. Some authors reported being employees and owning stocks and shares of GSK.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Stones, Bones, Groans, and Moans: Could This Be Primary Hyperparathyroidism?
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams.
Paul, we’re going to talk about our primary hyperparathyroidism podcast with Dr. Lindsay Kuo. It’s a topic that I feel much more clear on now.
Now, Paul, in primary care, you see a lot of calcium that is just slightly high. Can we just blame that on thiazide diuretics?
Paul N. Williams, MD: It’s a place to start. As you’re starting to think about the possible etiologies, primary hyperparathyroidism and malignancy are the two that roll right off the tongue, but it is worth going back to the patient’s medication list and making sure you’re not missing something.
Thiazides famously cause hypercalcemia, but in some of the reading I did for this episode, they may just uncover it a little bit early. Patients who are on thiazides who become hypercalcemic seem to go on to develop primary hyperthyroidism anyway. So I don’t think you can solely blame the thiazide.
Another medication that can be causative is lithium. So a good place to look first after you’ve repeated the labs and confirmed hypercalcemia is the patient’s medication list.
Dr. Watto: We’ve talked before about the basic workup for hypercalcemia, and determining whether it’s PTH dependent or PTH independent. On the podcast, we talk more about the full workup, but I wanted to talk about the classic symptoms. Our expert made the point that we don’t see them as much anymore, although we do see kidney stones. People used to present very late in the disease because they weren’t having labs done routinely.
The classic symptoms include osteoporosis and bone tumors. People can get nephrocalcinosis and kidney stones. I hadn’t really thought of it this way because we’re used to diagnosing it early now. Do you feel the same?
Dr. Williams: As labs have started routinely reporting calcium levels, this is more and more often how it’s picked up. The other aspect is that as we are screening for and finding osteoporosis, part of the workup almost always involves getting a parathyroid hormone and a calcium level. We’re seeing these lab abnormalities before we’re seeing symptoms, which is good.
But it also makes things more diagnostically thorny.
Dr. Watto: Dr. Lindsay Kuo made the point that when she sees patients before and after surgery, she’s aware of these nonclassic symptoms — the stones, bones, groans, and the psychiatric overtones that can be anything from fatigue or irritability to dysphoria.
Some people have a generalized weakness that’s very nonspecific. Dr. Kuo said that sometimes these symptoms will disappear after surgery. The patients may just have gotten used to them, or they thought these symptoms were caused by something else, but after surgery they went away.
There are these nonclassic symptoms that are harder to pin down. I was surprised by that.
Dr. Williams: She mentioned polydipsia and polyuria, which have been reported in other studies. It seems like it can be anything. You have to take a good history, but none of those things in and of themselves is an indication for operating unless the patient has the classic renal or bone manifestations.
Dr. Watto: The other thing we talked about is a normal calcium level in a patient with primary hyperparathyroidism, or the finding of a PTH level in the normal range but with a high calcium level that is inappropriate. Can you talk a little bit about those two situations?
Dr. Williams: They’re hard to say but kind of easy to manage because you treat them the same way as someone who has elevated calcium and PTH levels.
The normocalcemic patient is something we might stumble across with osteoporosis screening. Initially the calcium level is elevated, so you repeat it and it’s normal but with an elevated PTH level. You’re like, shoot. Now what?
It turns out that most endocrine surgeons say that the indications for surgery for the classic form of primary hyperparathyroidism apply to these patients as well, and it probably helps with the bone outcomes, which is one of the things they follow most closely. If you have hypercalcemia, you should have a suppressed PTH level, the so-called normohormonal hyperparathyroidism, which is not normal at all. So even if the PTH is in the normal range, it’s still relatively elevated compared with what it should be. That situation is treated in the same way as the classic elevated PTH and elevated calcium levels.
Dr. Watto: If the calcium is abnormal and the PTH is not quite what you’d expect it to be, you can always ask your friendly neighborhood endocrinologist to help you figure out whether the patient really has one of these conditions. You have to make sure that they don’t have a simple secondary cause like a low vitamin D level. In that case, you fix the vitamin D and then recheck the numbers to see if they’ve normalized. But I have found a bunch of these edge cases in which it has been helpful to confer with an endocrinologist, especially before you send someone to a surgeon to take out their parathyroid gland.
This was a really fantastic conversation. If you want to hear the full podcast episode, click here.
Dr. Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr. Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, served as a director, officer, partner, employee, adviser, consultant, or trustee for The Curbsiders, and has received income in an amount equal to or greater than $250 from The Curbsiders.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams.
Paul, we’re going to talk about our primary hyperparathyroidism podcast with Dr. Lindsay Kuo. It’s a topic that I feel much more clear on now.
Now, Paul, in primary care, you see a lot of calcium that is just slightly high. Can we just blame that on thiazide diuretics?
Paul N. Williams, MD: It’s a place to start. As you’re starting to think about the possible etiologies, primary hyperparathyroidism and malignancy are the two that roll right off the tongue, but it is worth going back to the patient’s medication list and making sure you’re not missing something.
Thiazides famously cause hypercalcemia, but in some of the reading I did for this episode, they may just uncover it a little bit early. Patients who are on thiazides who become hypercalcemic seem to go on to develop primary hyperthyroidism anyway. So I don’t think you can solely blame the thiazide.
Another medication that can be causative is lithium. So a good place to look first after you’ve repeated the labs and confirmed hypercalcemia is the patient’s medication list.
Dr. Watto: We’ve talked before about the basic workup for hypercalcemia, and determining whether it’s PTH dependent or PTH independent. On the podcast, we talk more about the full workup, but I wanted to talk about the classic symptoms. Our expert made the point that we don’t see them as much anymore, although we do see kidney stones. People used to present very late in the disease because they weren’t having labs done routinely.
The classic symptoms include osteoporosis and bone tumors. People can get nephrocalcinosis and kidney stones. I hadn’t really thought of it this way because we’re used to diagnosing it early now. Do you feel the same?
Dr. Williams: As labs have started routinely reporting calcium levels, this is more and more often how it’s picked up. The other aspect is that as we are screening for and finding osteoporosis, part of the workup almost always involves getting a parathyroid hormone and a calcium level. We’re seeing these lab abnormalities before we’re seeing symptoms, which is good.
But it also makes things more diagnostically thorny.
Dr. Watto: Dr. Lindsay Kuo made the point that when she sees patients before and after surgery, she’s aware of these nonclassic symptoms — the stones, bones, groans, and the psychiatric overtones that can be anything from fatigue or irritability to dysphoria.
Some people have a generalized weakness that’s very nonspecific. Dr. Kuo said that sometimes these symptoms will disappear after surgery. The patients may just have gotten used to them, or they thought these symptoms were caused by something else, but after surgery they went away.
There are these nonclassic symptoms that are harder to pin down. I was surprised by that.
Dr. Williams: She mentioned polydipsia and polyuria, which have been reported in other studies. It seems like it can be anything. You have to take a good history, but none of those things in and of themselves is an indication for operating unless the patient has the classic renal or bone manifestations.
Dr. Watto: The other thing we talked about is a normal calcium level in a patient with primary hyperparathyroidism, or the finding of a PTH level in the normal range but with a high calcium level that is inappropriate. Can you talk a little bit about those two situations?
Dr. Williams: They’re hard to say but kind of easy to manage because you treat them the same way as someone who has elevated calcium and PTH levels.
The normocalcemic patient is something we might stumble across with osteoporosis screening. Initially the calcium level is elevated, so you repeat it and it’s normal but with an elevated PTH level. You’re like, shoot. Now what?
It turns out that most endocrine surgeons say that the indications for surgery for the classic form of primary hyperparathyroidism apply to these patients as well, and it probably helps with the bone outcomes, which is one of the things they follow most closely. If you have hypercalcemia, you should have a suppressed PTH level, the so-called normohormonal hyperparathyroidism, which is not normal at all. So even if the PTH is in the normal range, it’s still relatively elevated compared with what it should be. That situation is treated in the same way as the classic elevated PTH and elevated calcium levels.
Dr. Watto: If the calcium is abnormal and the PTH is not quite what you’d expect it to be, you can always ask your friendly neighborhood endocrinologist to help you figure out whether the patient really has one of these conditions. You have to make sure that they don’t have a simple secondary cause like a low vitamin D level. In that case, you fix the vitamin D and then recheck the numbers to see if they’ve normalized. But I have found a bunch of these edge cases in which it has been helpful to confer with an endocrinologist, especially before you send someone to a surgeon to take out their parathyroid gland.
This was a really fantastic conversation. If you want to hear the full podcast episode, click here.
Dr. Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr. Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, served as a director, officer, partner, employee, adviser, consultant, or trustee for The Curbsiders, and has received income in an amount equal to or greater than $250 from The Curbsiders.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams.
Paul, we’re going to talk about our primary hyperparathyroidism podcast with Dr. Lindsay Kuo. It’s a topic that I feel much more clear on now.
Now, Paul, in primary care, you see a lot of calcium that is just slightly high. Can we just blame that on thiazide diuretics?
Paul N. Williams, MD: It’s a place to start. As you’re starting to think about the possible etiologies, primary hyperparathyroidism and malignancy are the two that roll right off the tongue, but it is worth going back to the patient’s medication list and making sure you’re not missing something.
Thiazides famously cause hypercalcemia, but in some of the reading I did for this episode, they may just uncover it a little bit early. Patients who are on thiazides who become hypercalcemic seem to go on to develop primary hyperthyroidism anyway. So I don’t think you can solely blame the thiazide.
Another medication that can be causative is lithium. So a good place to look first after you’ve repeated the labs and confirmed hypercalcemia is the patient’s medication list.
Dr. Watto: We’ve talked before about the basic workup for hypercalcemia, and determining whether it’s PTH dependent or PTH independent. On the podcast, we talk more about the full workup, but I wanted to talk about the classic symptoms. Our expert made the point that we don’t see them as much anymore, although we do see kidney stones. People used to present very late in the disease because they weren’t having labs done routinely.
The classic symptoms include osteoporosis and bone tumors. People can get nephrocalcinosis and kidney stones. I hadn’t really thought of it this way because we’re used to diagnosing it early now. Do you feel the same?
Dr. Williams: As labs have started routinely reporting calcium levels, this is more and more often how it’s picked up. The other aspect is that as we are screening for and finding osteoporosis, part of the workup almost always involves getting a parathyroid hormone and a calcium level. We’re seeing these lab abnormalities before we’re seeing symptoms, which is good.
But it also makes things more diagnostically thorny.
Dr. Watto: Dr. Lindsay Kuo made the point that when she sees patients before and after surgery, she’s aware of these nonclassic symptoms — the stones, bones, groans, and the psychiatric overtones that can be anything from fatigue or irritability to dysphoria.
Some people have a generalized weakness that’s very nonspecific. Dr. Kuo said that sometimes these symptoms will disappear after surgery. The patients may just have gotten used to them, or they thought these symptoms were caused by something else, but after surgery they went away.
There are these nonclassic symptoms that are harder to pin down. I was surprised by that.
Dr. Williams: She mentioned polydipsia and polyuria, which have been reported in other studies. It seems like it can be anything. You have to take a good history, but none of those things in and of themselves is an indication for operating unless the patient has the classic renal or bone manifestations.
Dr. Watto: The other thing we talked about is a normal calcium level in a patient with primary hyperparathyroidism, or the finding of a PTH level in the normal range but with a high calcium level that is inappropriate. Can you talk a little bit about those two situations?
Dr. Williams: They’re hard to say but kind of easy to manage because you treat them the same way as someone who has elevated calcium and PTH levels.
The normocalcemic patient is something we might stumble across with osteoporosis screening. Initially the calcium level is elevated, so you repeat it and it’s normal but with an elevated PTH level. You’re like, shoot. Now what?
It turns out that most endocrine surgeons say that the indications for surgery for the classic form of primary hyperparathyroidism apply to these patients as well, and it probably helps with the bone outcomes, which is one of the things they follow most closely. If you have hypercalcemia, you should have a suppressed PTH level, the so-called normohormonal hyperparathyroidism, which is not normal at all. So even if the PTH is in the normal range, it’s still relatively elevated compared with what it should be. That situation is treated in the same way as the classic elevated PTH and elevated calcium levels.
Dr. Watto: If the calcium is abnormal and the PTH is not quite what you’d expect it to be, you can always ask your friendly neighborhood endocrinologist to help you figure out whether the patient really has one of these conditions. You have to make sure that they don’t have a simple secondary cause like a low vitamin D level. In that case, you fix the vitamin D and then recheck the numbers to see if they’ve normalized. But I have found a bunch of these edge cases in which it has been helpful to confer with an endocrinologist, especially before you send someone to a surgeon to take out their parathyroid gland.
This was a really fantastic conversation. If you want to hear the full podcast episode, click here.
Dr. Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr. Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, served as a director, officer, partner, employee, adviser, consultant, or trustee for The Curbsiders, and has received income in an amount equal to or greater than $250 from The Curbsiders.
A version of this article first appeared on Medscape.com.

