User login
The Ins and Outs of Transferring Residency Programs
Transferring from one residency program to another is rare but not unheard of. According to the most recent Accreditation Council for Graduate Medical Education Data Resource Book, there were 1020 residents who transferred residency programs in the 2020-2021 academic year.1 With a total of 126,759 active residents in specialty programs, the percentage of transferring residents was less than 1%. The specialties with the highest number of transferring residents included psychiatry, general surgery, internal medicine, and family medicine. In dermatology programs, there were only 2 resident transfers during the 2019-2020 academic year and 6 transfers in the 2020-2021 academic year.1,2 A resident contemplating transferring training programs must carefully consider the advantages and disadvantages before undertaking the uncertain transfer process, but transferring residency programs can be achieved successfully with planning and luck.
Deciding to Transfer
The decision to transfer residency programs may be a difficult one that is wrought with anxiety. There are many reasons why a trainee may wish to pursue transferring training programs. A transfer to another geographic area may be necessary for personal or family reasons, such as to reunite with a spouse and children or to care for a sick family member. A resident may find their program to be a poor fit and may wish to train in a different educational environment. Occasionally, a program can lose its accreditation, and its residents will be tasked with finding a new position elsewhere. A trainee also may realize that the specialty they matched into initially does not align with their true passions. It is important for the potential transfer applicant to be levelheaded about their decision. Residency is a demanding period for every trainee; switching programs may not be the best solution for every problem and should only be considered if essential.
Transfer Timing
A trainee may have thoughts of leaving a program soon after starting residency or perhaps even before starting if their National Resident Matching Program (NRMP) Match result was a disappointment; however, there are certain rules related to transfer timing. The NRMP Match represents a binding commitment for both the applicant and program. If for any reason an applicant will not honor the binding commitment, the NRMP requires the applicant to initiate a waiver review, which can be requested for unanticipated serious and extreme hardship, change of specialty, or ineligibility. According to the NRMP rules and regulations, applicants cannot apply for, discuss, interview for, or accept a position in another program until a waiver has been granted.3 Waivers based on change of specialty must be requested by mid-January prior to the start of training, which means most applicants who match to positions that begin in the same year of the Match do not qualify for change of specialty waivers. However, those who matched to an advanced position and are doing a preliminary year position may consider this option if they have a change of heart during their internship. The NRMP may consider a 1-year deferral to delay training if mutually agreed upon by both the matched applicant and the program.3 The binding commitment is in place for the first 45 days of training, and applicants who resign within 45 days or a program that tries to solicit the transfer of a resident prior to that date could be in violation of the Match and can face consequences such as being barred from entering the matching process in future cycles. Of the 1020 transfers that occurred among residents in specialty programs during the 2020-2021 academic year, 354 (34.7%) occurred during the first year of the training program; 228 (22.4%) occurred during the second year; 389 (38.1%) occurred during the third year; and 49 (4.8%) occurred in the fourth, fifth, or sixth year of the program.1 Unlike other jobs/occupations in which one can simply give notice, in medical training even if a transfer position is accepted, the transition date between programs must be mutually agreed upon. Often, this may coincide with the start of the new academic year.
The Transfer Process
Transferring residency programs is a substantial undertaking. Unlike the Match, a trainee seeking to transfer programs does so without a standardized application system or structured support through the process; the transfer applicant must be prepared to navigate the transfer process on their own. The first step after making the decision to transfer is for the resident to meet with the program leadership (ie, program director[s], coordinator, designated official) at their home program to discuss the decision—a nerve-wracking but imperative first step. A receiving program may not favor an applicant secretly applying to a new program without the knowledge of their home program and often will require the home program’s blessing to proceed. The receiving program also would want to ensure the applicant is in good standing and not leaving due to misconduct. Once given the go-ahead, the process is largely in the hands of the applicant. The transfer applicant should identify locations or programs of interest and then take initiative to reach out to potential programs. FREIDA (Fellowship and Residency Electronic Interactive Database Access) is the American Medical Association’s residency and fellowship database that allows vacant position listings to be posted online.4 Additionally, the Association of American Medical Colleges’ FindAResident website is a year-round search tool designed to help find open residency and fellowship positions.5 Various specialties also may have program director listserves that communicate vacant positions. On occasion, there are spots in the main NRMP Match that are reserved positions (“R”). These are postgraduate year 2 positions in specialty programs that begin in the year of the Match and are reserved for physicians with prior graduate medical education; these also are known as “Physician Positions.”6 Ultimately, advertisements for vacancies may be few and far between, requiring the resident to send unsolicited emails with curriculum vitae attached to the program directors at programs of interest to inquire about any vacancies and hope for a favorable response. Even if the transfer applicant is qualified, luck that the right spot will be available at the right time may be the deciding factor in transferring programs.
The next step is interviewing for the position. There likely will be fewer candidates interviewing for an open spot but that does not make the process less competitive. The candidate should highlight their strengths and achievements and discuss why the new program would be a great fit both personally and professionally. Even if an applicant is seeking a transfer due to discontent with a prior program, it is best to act graciously and not speak poorly about another training program.
Prior to selection, the candidate may be asked to provide information such as diplomas, US Medical Licensing Examination Step and residency in-service training examination scores, and academic reviews from their current residency program. The interview process may take several weeks as the graduate medical education office often will need to officially approve of an applicant before a formal offer to transfer is extended.
Finally, once an offer is made and accepted, there still is a great amount of paperwork to complete before the transition. The applicant should stay on track with all off-boarding and on-boarding requirements, such as signing a contract, obtaining background checks, and applying for a new license to ensure the switch is not delayed.
Disadvantages of Transferring Programs
The transfer process is not easy to navigate and can be a source of stress for the applicant. It is natural to fear resentment from colleagues and co-residents. Although transferring programs might be in the best interest of the trainee, it may leave a large gap in the program that they are leaving, which can place a burden on the remaining residents.
There are many adjustments to be made after transferring programs. The transferring resident will again start from scratch, needing to learn the ropes and adapt to the growing pains of being at a new institution. This may require learning a completely new electronic medical record, adapting to a new culture, and in many cases stepping in as a senior resident without fully knowing the ins and outs of the program.
Advantages of Transferring Programs
Successfully transferring programs is something to celebrate. There may be great benefits to transferring to a program that is better suited to the trainee—either personally or professionally. Ameliorating the adversity that led to the decision to transfer such as reuniting a long-distance family or realizing one’s true passion can allow the resident to thrive as a trainee and maximize their potential. Transferring programs can give a resident a more well-rounded training experience, as different programs may have different strengths, patient populations, and practice settings. Working with different faculty members with varied niches and practice styles can create a more comprehensive residency experience.
Final Thoughts
Ultimately, transferring residency programs is not easy but also is not impossible. Successfully switching residency programs can be a rewarding experience providing greater well-being and fulfillment.
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2021-2022. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2021-2022_acgme__databook_document.pdf
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2020-2021. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2020-2021_acgme_databook_document.pdf
- After the Match. National Resident Matching Program website. Accessed January 23, 2023. https://www.nrmp.org/fellowship-applicants/after-the-match/
- FREIDA vacant position listings. American Medical Association website. Accessed January 23, 2023. https://freida.ama-assn.org/vacant-position
- FindAResident. Association of American Medical Colleges website. Accessed January 23, 2023. https://students-residents.aamc.org/findaresident/findaresident
- What are the types of program positions in the main residency match? National Resident Matching Program website. Published August 5, 2021. Accessed January 23, 2023. https://www.nrmp.org/help/item/what-types-of-programs-participate-in-the-main-residency-match/
Transferring from one residency program to another is rare but not unheard of. According to the most recent Accreditation Council for Graduate Medical Education Data Resource Book, there were 1020 residents who transferred residency programs in the 2020-2021 academic year.1 With a total of 126,759 active residents in specialty programs, the percentage of transferring residents was less than 1%. The specialties with the highest number of transferring residents included psychiatry, general surgery, internal medicine, and family medicine. In dermatology programs, there were only 2 resident transfers during the 2019-2020 academic year and 6 transfers in the 2020-2021 academic year.1,2 A resident contemplating transferring training programs must carefully consider the advantages and disadvantages before undertaking the uncertain transfer process, but transferring residency programs can be achieved successfully with planning and luck.
Deciding to Transfer
The decision to transfer residency programs may be a difficult one that is wrought with anxiety. There are many reasons why a trainee may wish to pursue transferring training programs. A transfer to another geographic area may be necessary for personal or family reasons, such as to reunite with a spouse and children or to care for a sick family member. A resident may find their program to be a poor fit and may wish to train in a different educational environment. Occasionally, a program can lose its accreditation, and its residents will be tasked with finding a new position elsewhere. A trainee also may realize that the specialty they matched into initially does not align with their true passions. It is important for the potential transfer applicant to be levelheaded about their decision. Residency is a demanding period for every trainee; switching programs may not be the best solution for every problem and should only be considered if essential.
Transfer Timing
A trainee may have thoughts of leaving a program soon after starting residency or perhaps even before starting if their National Resident Matching Program (NRMP) Match result was a disappointment; however, there are certain rules related to transfer timing. The NRMP Match represents a binding commitment for both the applicant and program. If for any reason an applicant will not honor the binding commitment, the NRMP requires the applicant to initiate a waiver review, which can be requested for unanticipated serious and extreme hardship, change of specialty, or ineligibility. According to the NRMP rules and regulations, applicants cannot apply for, discuss, interview for, or accept a position in another program until a waiver has been granted.3 Waivers based on change of specialty must be requested by mid-January prior to the start of training, which means most applicants who match to positions that begin in the same year of the Match do not qualify for change of specialty waivers. However, those who matched to an advanced position and are doing a preliminary year position may consider this option if they have a change of heart during their internship. The NRMP may consider a 1-year deferral to delay training if mutually agreed upon by both the matched applicant and the program.3 The binding commitment is in place for the first 45 days of training, and applicants who resign within 45 days or a program that tries to solicit the transfer of a resident prior to that date could be in violation of the Match and can face consequences such as being barred from entering the matching process in future cycles. Of the 1020 transfers that occurred among residents in specialty programs during the 2020-2021 academic year, 354 (34.7%) occurred during the first year of the training program; 228 (22.4%) occurred during the second year; 389 (38.1%) occurred during the third year; and 49 (4.8%) occurred in the fourth, fifth, or sixth year of the program.1 Unlike other jobs/occupations in which one can simply give notice, in medical training even if a transfer position is accepted, the transition date between programs must be mutually agreed upon. Often, this may coincide with the start of the new academic year.
The Transfer Process
Transferring residency programs is a substantial undertaking. Unlike the Match, a trainee seeking to transfer programs does so without a standardized application system or structured support through the process; the transfer applicant must be prepared to navigate the transfer process on their own. The first step after making the decision to transfer is for the resident to meet with the program leadership (ie, program director[s], coordinator, designated official) at their home program to discuss the decision—a nerve-wracking but imperative first step. A receiving program may not favor an applicant secretly applying to a new program without the knowledge of their home program and often will require the home program’s blessing to proceed. The receiving program also would want to ensure the applicant is in good standing and not leaving due to misconduct. Once given the go-ahead, the process is largely in the hands of the applicant. The transfer applicant should identify locations or programs of interest and then take initiative to reach out to potential programs. FREIDA (Fellowship and Residency Electronic Interactive Database Access) is the American Medical Association’s residency and fellowship database that allows vacant position listings to be posted online.4 Additionally, the Association of American Medical Colleges’ FindAResident website is a year-round search tool designed to help find open residency and fellowship positions.5 Various specialties also may have program director listserves that communicate vacant positions. On occasion, there are spots in the main NRMP Match that are reserved positions (“R”). These are postgraduate year 2 positions in specialty programs that begin in the year of the Match and are reserved for physicians with prior graduate medical education; these also are known as “Physician Positions.”6 Ultimately, advertisements for vacancies may be few and far between, requiring the resident to send unsolicited emails with curriculum vitae attached to the program directors at programs of interest to inquire about any vacancies and hope for a favorable response. Even if the transfer applicant is qualified, luck that the right spot will be available at the right time may be the deciding factor in transferring programs.
The next step is interviewing for the position. There likely will be fewer candidates interviewing for an open spot but that does not make the process less competitive. The candidate should highlight their strengths and achievements and discuss why the new program would be a great fit both personally and professionally. Even if an applicant is seeking a transfer due to discontent with a prior program, it is best to act graciously and not speak poorly about another training program.
Prior to selection, the candidate may be asked to provide information such as diplomas, US Medical Licensing Examination Step and residency in-service training examination scores, and academic reviews from their current residency program. The interview process may take several weeks as the graduate medical education office often will need to officially approve of an applicant before a formal offer to transfer is extended.
Finally, once an offer is made and accepted, there still is a great amount of paperwork to complete before the transition. The applicant should stay on track with all off-boarding and on-boarding requirements, such as signing a contract, obtaining background checks, and applying for a new license to ensure the switch is not delayed.
Disadvantages of Transferring Programs
The transfer process is not easy to navigate and can be a source of stress for the applicant. It is natural to fear resentment from colleagues and co-residents. Although transferring programs might be in the best interest of the trainee, it may leave a large gap in the program that they are leaving, which can place a burden on the remaining residents.
There are many adjustments to be made after transferring programs. The transferring resident will again start from scratch, needing to learn the ropes and adapt to the growing pains of being at a new institution. This may require learning a completely new electronic medical record, adapting to a new culture, and in many cases stepping in as a senior resident without fully knowing the ins and outs of the program.
Advantages of Transferring Programs
Successfully transferring programs is something to celebrate. There may be great benefits to transferring to a program that is better suited to the trainee—either personally or professionally. Ameliorating the adversity that led to the decision to transfer such as reuniting a long-distance family or realizing one’s true passion can allow the resident to thrive as a trainee and maximize their potential. Transferring programs can give a resident a more well-rounded training experience, as different programs may have different strengths, patient populations, and practice settings. Working with different faculty members with varied niches and practice styles can create a more comprehensive residency experience.
Final Thoughts
Ultimately, transferring residency programs is not easy but also is not impossible. Successfully switching residency programs can be a rewarding experience providing greater well-being and fulfillment.
Transferring from one residency program to another is rare but not unheard of. According to the most recent Accreditation Council for Graduate Medical Education Data Resource Book, there were 1020 residents who transferred residency programs in the 2020-2021 academic year.1 With a total of 126,759 active residents in specialty programs, the percentage of transferring residents was less than 1%. The specialties with the highest number of transferring residents included psychiatry, general surgery, internal medicine, and family medicine. In dermatology programs, there were only 2 resident transfers during the 2019-2020 academic year and 6 transfers in the 2020-2021 academic year.1,2 A resident contemplating transferring training programs must carefully consider the advantages and disadvantages before undertaking the uncertain transfer process, but transferring residency programs can be achieved successfully with planning and luck.
Deciding to Transfer
The decision to transfer residency programs may be a difficult one that is wrought with anxiety. There are many reasons why a trainee may wish to pursue transferring training programs. A transfer to another geographic area may be necessary for personal or family reasons, such as to reunite with a spouse and children or to care for a sick family member. A resident may find their program to be a poor fit and may wish to train in a different educational environment. Occasionally, a program can lose its accreditation, and its residents will be tasked with finding a new position elsewhere. A trainee also may realize that the specialty they matched into initially does not align with their true passions. It is important for the potential transfer applicant to be levelheaded about their decision. Residency is a demanding period for every trainee; switching programs may not be the best solution for every problem and should only be considered if essential.
Transfer Timing
A trainee may have thoughts of leaving a program soon after starting residency or perhaps even before starting if their National Resident Matching Program (NRMP) Match result was a disappointment; however, there are certain rules related to transfer timing. The NRMP Match represents a binding commitment for both the applicant and program. If for any reason an applicant will not honor the binding commitment, the NRMP requires the applicant to initiate a waiver review, which can be requested for unanticipated serious and extreme hardship, change of specialty, or ineligibility. According to the NRMP rules and regulations, applicants cannot apply for, discuss, interview for, or accept a position in another program until a waiver has been granted.3 Waivers based on change of specialty must be requested by mid-January prior to the start of training, which means most applicants who match to positions that begin in the same year of the Match do not qualify for change of specialty waivers. However, those who matched to an advanced position and are doing a preliminary year position may consider this option if they have a change of heart during their internship. The NRMP may consider a 1-year deferral to delay training if mutually agreed upon by both the matched applicant and the program.3 The binding commitment is in place for the first 45 days of training, and applicants who resign within 45 days or a program that tries to solicit the transfer of a resident prior to that date could be in violation of the Match and can face consequences such as being barred from entering the matching process in future cycles. Of the 1020 transfers that occurred among residents in specialty programs during the 2020-2021 academic year, 354 (34.7%) occurred during the first year of the training program; 228 (22.4%) occurred during the second year; 389 (38.1%) occurred during the third year; and 49 (4.8%) occurred in the fourth, fifth, or sixth year of the program.1 Unlike other jobs/occupations in which one can simply give notice, in medical training even if a transfer position is accepted, the transition date between programs must be mutually agreed upon. Often, this may coincide with the start of the new academic year.
The Transfer Process
Transferring residency programs is a substantial undertaking. Unlike the Match, a trainee seeking to transfer programs does so without a standardized application system or structured support through the process; the transfer applicant must be prepared to navigate the transfer process on their own. The first step after making the decision to transfer is for the resident to meet with the program leadership (ie, program director[s], coordinator, designated official) at their home program to discuss the decision—a nerve-wracking but imperative first step. A receiving program may not favor an applicant secretly applying to a new program without the knowledge of their home program and often will require the home program’s blessing to proceed. The receiving program also would want to ensure the applicant is in good standing and not leaving due to misconduct. Once given the go-ahead, the process is largely in the hands of the applicant. The transfer applicant should identify locations or programs of interest and then take initiative to reach out to potential programs. FREIDA (Fellowship and Residency Electronic Interactive Database Access) is the American Medical Association’s residency and fellowship database that allows vacant position listings to be posted online.4 Additionally, the Association of American Medical Colleges’ FindAResident website is a year-round search tool designed to help find open residency and fellowship positions.5 Various specialties also may have program director listserves that communicate vacant positions. On occasion, there are spots in the main NRMP Match that are reserved positions (“R”). These are postgraduate year 2 positions in specialty programs that begin in the year of the Match and are reserved for physicians with prior graduate medical education; these also are known as “Physician Positions.”6 Ultimately, advertisements for vacancies may be few and far between, requiring the resident to send unsolicited emails with curriculum vitae attached to the program directors at programs of interest to inquire about any vacancies and hope for a favorable response. Even if the transfer applicant is qualified, luck that the right spot will be available at the right time may be the deciding factor in transferring programs.
The next step is interviewing for the position. There likely will be fewer candidates interviewing for an open spot but that does not make the process less competitive. The candidate should highlight their strengths and achievements and discuss why the new program would be a great fit both personally and professionally. Even if an applicant is seeking a transfer due to discontent with a prior program, it is best to act graciously and not speak poorly about another training program.
Prior to selection, the candidate may be asked to provide information such as diplomas, US Medical Licensing Examination Step and residency in-service training examination scores, and academic reviews from their current residency program. The interview process may take several weeks as the graduate medical education office often will need to officially approve of an applicant before a formal offer to transfer is extended.
Finally, once an offer is made and accepted, there still is a great amount of paperwork to complete before the transition. The applicant should stay on track with all off-boarding and on-boarding requirements, such as signing a contract, obtaining background checks, and applying for a new license to ensure the switch is not delayed.
Disadvantages of Transferring Programs
The transfer process is not easy to navigate and can be a source of stress for the applicant. It is natural to fear resentment from colleagues and co-residents. Although transferring programs might be in the best interest of the trainee, it may leave a large gap in the program that they are leaving, which can place a burden on the remaining residents.
There are many adjustments to be made after transferring programs. The transferring resident will again start from scratch, needing to learn the ropes and adapt to the growing pains of being at a new institution. This may require learning a completely new electronic medical record, adapting to a new culture, and in many cases stepping in as a senior resident without fully knowing the ins and outs of the program.
Advantages of Transferring Programs
Successfully transferring programs is something to celebrate. There may be great benefits to transferring to a program that is better suited to the trainee—either personally or professionally. Ameliorating the adversity that led to the decision to transfer such as reuniting a long-distance family or realizing one’s true passion can allow the resident to thrive as a trainee and maximize their potential. Transferring programs can give a resident a more well-rounded training experience, as different programs may have different strengths, patient populations, and practice settings. Working with different faculty members with varied niches and practice styles can create a more comprehensive residency experience.
Final Thoughts
Ultimately, transferring residency programs is not easy but also is not impossible. Successfully switching residency programs can be a rewarding experience providing greater well-being and fulfillment.
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2021-2022. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2021-2022_acgme__databook_document.pdf
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2020-2021. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2020-2021_acgme_databook_document.pdf
- After the Match. National Resident Matching Program website. Accessed January 23, 2023. https://www.nrmp.org/fellowship-applicants/after-the-match/
- FREIDA vacant position listings. American Medical Association website. Accessed January 23, 2023. https://freida.ama-assn.org/vacant-position
- FindAResident. Association of American Medical Colleges website. Accessed January 23, 2023. https://students-residents.aamc.org/findaresident/findaresident
- What are the types of program positions in the main residency match? National Resident Matching Program website. Published August 5, 2021. Accessed January 23, 2023. https://www.nrmp.org/help/item/what-types-of-programs-participate-in-the-main-residency-match/
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2021-2022. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2021-2022_acgme__databook_document.pdf
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2020-2021. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2020-2021_acgme_databook_document.pdf
- After the Match. National Resident Matching Program website. Accessed January 23, 2023. https://www.nrmp.org/fellowship-applicants/after-the-match/
- FREIDA vacant position listings. American Medical Association website. Accessed January 23, 2023. https://freida.ama-assn.org/vacant-position
- FindAResident. Association of American Medical Colleges website. Accessed January 23, 2023. https://students-residents.aamc.org/findaresident/findaresident
- What are the types of program positions in the main residency match? National Resident Matching Program website. Published August 5, 2021. Accessed January 23, 2023. https://www.nrmp.org/help/item/what-types-of-programs-participate-in-the-main-residency-match/
RESIDENT PEARL
- Transferring residency programs is difficult but possible. The decision to transfer residencies may be anxiety producing, but with substantial motives, the rewards of transferring can be worthwhile.
Severe Asthma Guidelines
Hemorrhagic Lacrimation and Epistaxis: Rare Findings in Acute Hemorrhagic Edema of Infancy
To the Editor:
Hemorrhagic lacrimation and epistaxis are dramatic presentations with a narrow differential diagnosis. It rarely has been reported to present alongside the more typical features of acute hemorrhagic edema of infancy (AHEI), which is a benign self-limited leukocytoclastic vasculitis most often seen in children aged 4 months to 2 years. Extracutaneous involvement rarely is seen in AHEI, though joint, gastrointestinal tract, and renal involvement have been reported.1 Most patients present with edematous, annular, or cockade purpuric vasculitic lesions classically involving the face and distal extremities with relative sparing of the trunk. We present a case of a well-appearing, 10-month-old infant boy with hemorrhagic vasculitic lesions, acral edema, and an associated episode of hemorrhagic lacrimation and epistaxis.
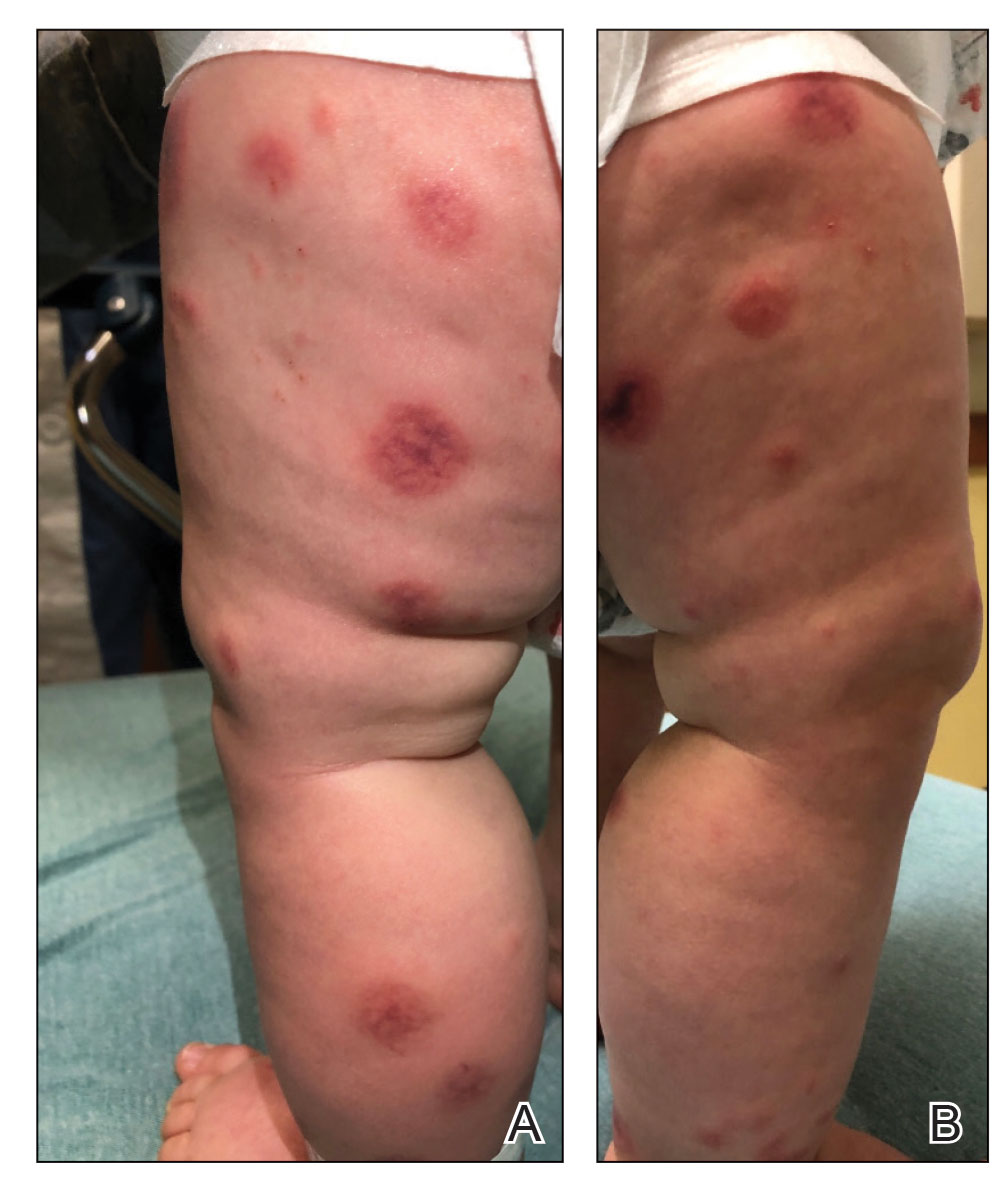
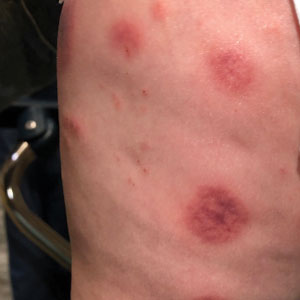
A 10-month-old infant boy who was otherwise healthy presented to the emergency department (ED) with an acute-onset, progressively worsening cutaneous eruption of 2 days’ duration. A thorough history revealed that the eruption initially had presented as several small, bright-red papules on the thighs. The eruption subsequently spread to involve the buttocks, legs, and arms (Figures 1 and 2). The parents also noted that the patient had experienced an episode of bloody tears and epistaxis that lasted a few minutes at the pediatrician’s office earlier that morning, a finding that prompted the urgent referral to the ED.
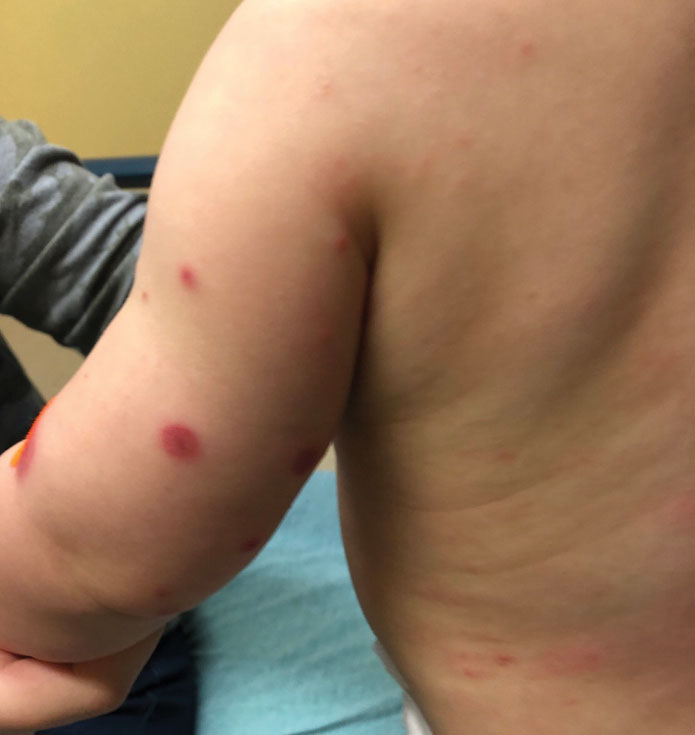
Dermatology was then consulted. A review of systems was notable for rhinorrhea and diarrhea during the week leading to the eruption. The patient’s parents denied fevers, decreased oral intake, or a recent course of antibiotics. The patient’s medical history was notable only for atopic dermatitis treated with emollients and occasional topical steroids. The parents denied recent travel or vaccinations. Physical examination showed an afebrile, well-appearing infant with multiple nontender, slightly edematous, circular, purpuric papules and plaques scattered on the buttocks and extremities with edema on the dorsal feet. The remainder of the patient’s workup in the ED was notable for mild elevations in C-reactive protein levels (1.4 mg/dL [reference range, 0–1.2 mg/dL]) and an elevated erythrocyte sedimentation rate (22 mm/h [reference range, 2–12 mm/h]). A complete blood cell count; liver function tests; urinalysis; and coagulation studies, including prothrombin, partial thromboplastin time, and international normalized ratio, were unremarkable. Acute hemorrhagic edema of infancy was diagnosed based on the clinical manifestations.
Acute hemorrhagic edema of infancy (also known as Finkelstein disease, medallionlike purpura, Seidemayer syndrome, infantile postinfectious irislike purpura and edema, and purpura en cocarde avec oedeme) is believed to result from an immune complex–related reaction, often in the setting of an upper respiratory tract infection; medications, especially antibiotics; or vaccinations. The condition previously was considered a benign form of Henoch-Schönlein purpura; however, it is now recognized as its own clinical entity. Acute hemorrhagic edema of infancy commonly affects children between the ages of 4 months and 2 years. The incidence peaks in the winter months, and males tend to be more affected than females.1
Acute hemorrhagic edema of infancy is clinically characterized by a triad of large purpuric lesions, low-grade fever, and peripheral acral edema. Edema can develop on the hands, feet, and genitalia. Importantly, facial edema has been noted to precede skin lesions.2 Coin-shaped or targetoid hemorrhagic and purpuric lesions in a cockade or rosette pattern with scalloped margins typically begin on the distal extremities and tend to spread proximally. The lesions are variable in size but have been reported to be as large as 5 cm in diameter. Although joint pain, bloody diarrhea, hematuria, and proteinuria can accompany AHEI, most cases are devoid of systemic symptoms.3 Hemorrhagic lacrimation and epistaxis—both present in our patient—are rare findings with AHEI. It is likely that most providers, including dermatologists, may be unfamiliar with these striking clinical findings. Although the pathophysiology of hemorrhagic lacrimation and epistaxis has not been formally investigated, we postulate that it likely is related to the formation of immune complexes that lead to small vessel vasculitis, underpinning the characteristic findings in AHEI.4,5 This reasoning is supported by the complete resolution of symptoms corresponding with clinical clearance of the cutaneous vasculitis in 2 prior cases4,5 as well as in our patient who did not have a relapse of symptoms following cessation of the cutaneous eruption at a pediatric follow-up appointment 2 weeks later.
Acute hemorrhagic edema of infancy is a clinical diagnosis; however, a skin biopsy can be performed to confirm the clinical suspicion and rule out more serious conditions. Histopathologic examination reveals a leukocytoclastic vasculitis involving the capillaries and postcapillary venules of the upper and mid dermis. Laboratory test results usually are nonspecific but can help distinguish AHEI from more serious diseases. The erythrocyte sedimentation rate and C-reactive protein level may be slightly elevated in infants with AHEI. Urinalysis and stool guaiac tests also can be performed to evaluate for any renal or gastrointestinal involvement.6
The differential diagnosis includes IgA vasculitis, erythema multiforme, acute meningococcemia, urticarial vasculitis, Kawasaki disease, and child abuse. IgA vasculitis often presents with more systemic involvement, with abdominal pain, vomiting, hematemesis, diarrhea, and hematochezia occurring in up to 50% of patients. The cutaneous findings of erythema multiforme classically are confined to the limbs and face, and edema of the extremities typically is not seen. Patients with acute meningococcemia appear toxic with high fevers, malaise, and possible septic shock.5
Acute hemorrhagic edema of infancy is a self-limited condition typically lasting 1 to 3 weeks and requires only supportive care.7 Antibiotics should be given to treat concurrent bacterial infections, and antihistamines and steroids may be useful for symptomatic relief. Importantly, however, systemic corticosteroids do not appear to conclusively alter the disease course.8
Acute hemorrhagic edema of infancy is a rare benign leukocytoclastic vasculitis with a striking presentation often seen following an upper respiratory tract infection or course of antibiotics. Our case demonstrates that on rare occasions, AHEI may be accompanied by hemorrhagic lacrimation and epistaxis—findings that can be quite alarming to both parents and medical providers. Nonetheless, patients and their caretakers should be assured that the condition is self-limited and resolves without permanent sequalae.
- Emerich PS, Prebianchi PA, Motta LL, et al. Acute hemorrhagic edema of infancy: report of three cases. An Bras Dermatol. 2011;86:1181-1184.
- Avhad G, Ghuge P, Jerajani H. Acute hemorrhagic edema of infancy. Indian Dermatol Online J. 2014;5:356-357.
- Krause I, Lazarov A, Rachmel A, et al. Acute haemorrhagic oedema of infancy, a benign variant of leucocytoclastic vasculitis. Acta Paediatr. 1996;85:114-117.
- Sneller H, Vega C, Zemel L, et al. Acute hemorrhagic edema of infancy with associated hemorrhagic lacrimation. Pediatr Emerg Care. 2021;37:E70-E72. doi:10.1097/PEC.0000000000001542
- Mreish S, Al-Tatari H. Hemorrhagic lacrimation and epistaxis in acute hemorrhagic edema of infancy. Case Rep Pediatr. 2016;2016:9762185. doi:10.1155/2016/9762185
- Savino F, Lupica MM, Tarasco V, et al. Acute hemorrhagic edema of infancy: a troubling cutaneous presentation with a self-limiting course. Pediatr Dermatol. 2013;30:E149-E152.
- Fiore E, Rizzi M, Ragazzi M, et al. Acute hemorrhagic edema of young children (cockade purpura and edema): a case series and systematic review. J Am Acad Dermatol. 2008;59:684-695.
- Acute hemorrhagic edema of young children: a concise narrative review. Eur J Pediatr. 2011;170:1507-1511.
To the Editor:
Hemorrhagic lacrimation and epistaxis are dramatic presentations with a narrow differential diagnosis. It rarely has been reported to present alongside the more typical features of acute hemorrhagic edema of infancy (AHEI), which is a benign self-limited leukocytoclastic vasculitis most often seen in children aged 4 months to 2 years. Extracutaneous involvement rarely is seen in AHEI, though joint, gastrointestinal tract, and renal involvement have been reported.1 Most patients present with edematous, annular, or cockade purpuric vasculitic lesions classically involving the face and distal extremities with relative sparing of the trunk. We present a case of a well-appearing, 10-month-old infant boy with hemorrhagic vasculitic lesions, acral edema, and an associated episode of hemorrhagic lacrimation and epistaxis.

A 10-month-old infant boy who was otherwise healthy presented to the emergency department (ED) with an acute-onset, progressively worsening cutaneous eruption of 2 days’ duration. A thorough history revealed that the eruption initially had presented as several small, bright-red papules on the thighs. The eruption subsequently spread to involve the buttocks, legs, and arms (Figures 1 and 2). The parents also noted that the patient had experienced an episode of bloody tears and epistaxis that lasted a few minutes at the pediatrician’s office earlier that morning, a finding that prompted the urgent referral to the ED.

Dermatology was then consulted. A review of systems was notable for rhinorrhea and diarrhea during the week leading to the eruption. The patient’s parents denied fevers, decreased oral intake, or a recent course of antibiotics. The patient’s medical history was notable only for atopic dermatitis treated with emollients and occasional topical steroids. The parents denied recent travel or vaccinations. Physical examination showed an afebrile, well-appearing infant with multiple nontender, slightly edematous, circular, purpuric papules and plaques scattered on the buttocks and extremities with edema on the dorsal feet. The remainder of the patient’s workup in the ED was notable for mild elevations in C-reactive protein levels (1.4 mg/dL [reference range, 0–1.2 mg/dL]) and an elevated erythrocyte sedimentation rate (22 mm/h [reference range, 2–12 mm/h]). A complete blood cell count; liver function tests; urinalysis; and coagulation studies, including prothrombin, partial thromboplastin time, and international normalized ratio, were unremarkable. Acute hemorrhagic edema of infancy was diagnosed based on the clinical manifestations.
Acute hemorrhagic edema of infancy (also known as Finkelstein disease, medallionlike purpura, Seidemayer syndrome, infantile postinfectious irislike purpura and edema, and purpura en cocarde avec oedeme) is believed to result from an immune complex–related reaction, often in the setting of an upper respiratory tract infection; medications, especially antibiotics; or vaccinations. The condition previously was considered a benign form of Henoch-Schönlein purpura; however, it is now recognized as its own clinical entity. Acute hemorrhagic edema of infancy commonly affects children between the ages of 4 months and 2 years. The incidence peaks in the winter months, and males tend to be more affected than females.1
Acute hemorrhagic edema of infancy is clinically characterized by a triad of large purpuric lesions, low-grade fever, and peripheral acral edema. Edema can develop on the hands, feet, and genitalia. Importantly, facial edema has been noted to precede skin lesions.2 Coin-shaped or targetoid hemorrhagic and purpuric lesions in a cockade or rosette pattern with scalloped margins typically begin on the distal extremities and tend to spread proximally. The lesions are variable in size but have been reported to be as large as 5 cm in diameter. Although joint pain, bloody diarrhea, hematuria, and proteinuria can accompany AHEI, most cases are devoid of systemic symptoms.3 Hemorrhagic lacrimation and epistaxis—both present in our patient—are rare findings with AHEI. It is likely that most providers, including dermatologists, may be unfamiliar with these striking clinical findings. Although the pathophysiology of hemorrhagic lacrimation and epistaxis has not been formally investigated, we postulate that it likely is related to the formation of immune complexes that lead to small vessel vasculitis, underpinning the characteristic findings in AHEI.4,5 This reasoning is supported by the complete resolution of symptoms corresponding with clinical clearance of the cutaneous vasculitis in 2 prior cases4,5 as well as in our patient who did not have a relapse of symptoms following cessation of the cutaneous eruption at a pediatric follow-up appointment 2 weeks later.
Acute hemorrhagic edema of infancy is a clinical diagnosis; however, a skin biopsy can be performed to confirm the clinical suspicion and rule out more serious conditions. Histopathologic examination reveals a leukocytoclastic vasculitis involving the capillaries and postcapillary venules of the upper and mid dermis. Laboratory test results usually are nonspecific but can help distinguish AHEI from more serious diseases. The erythrocyte sedimentation rate and C-reactive protein level may be slightly elevated in infants with AHEI. Urinalysis and stool guaiac tests also can be performed to evaluate for any renal or gastrointestinal involvement.6
The differential diagnosis includes IgA vasculitis, erythema multiforme, acute meningococcemia, urticarial vasculitis, Kawasaki disease, and child abuse. IgA vasculitis often presents with more systemic involvement, with abdominal pain, vomiting, hematemesis, diarrhea, and hematochezia occurring in up to 50% of patients. The cutaneous findings of erythema multiforme classically are confined to the limbs and face, and edema of the extremities typically is not seen. Patients with acute meningococcemia appear toxic with high fevers, malaise, and possible septic shock.5
Acute hemorrhagic edema of infancy is a self-limited condition typically lasting 1 to 3 weeks and requires only supportive care.7 Antibiotics should be given to treat concurrent bacterial infections, and antihistamines and steroids may be useful for symptomatic relief. Importantly, however, systemic corticosteroids do not appear to conclusively alter the disease course.8
Acute hemorrhagic edema of infancy is a rare benign leukocytoclastic vasculitis with a striking presentation often seen following an upper respiratory tract infection or course of antibiotics. Our case demonstrates that on rare occasions, AHEI may be accompanied by hemorrhagic lacrimation and epistaxis—findings that can be quite alarming to both parents and medical providers. Nonetheless, patients and their caretakers should be assured that the condition is self-limited and resolves without permanent sequalae.
To the Editor:
Hemorrhagic lacrimation and epistaxis are dramatic presentations with a narrow differential diagnosis. It rarely has been reported to present alongside the more typical features of acute hemorrhagic edema of infancy (AHEI), which is a benign self-limited leukocytoclastic vasculitis most often seen in children aged 4 months to 2 years. Extracutaneous involvement rarely is seen in AHEI, though joint, gastrointestinal tract, and renal involvement have been reported.1 Most patients present with edematous, annular, or cockade purpuric vasculitic lesions classically involving the face and distal extremities with relative sparing of the trunk. We present a case of a well-appearing, 10-month-old infant boy with hemorrhagic vasculitic lesions, acral edema, and an associated episode of hemorrhagic lacrimation and epistaxis.

A 10-month-old infant boy who was otherwise healthy presented to the emergency department (ED) with an acute-onset, progressively worsening cutaneous eruption of 2 days’ duration. A thorough history revealed that the eruption initially had presented as several small, bright-red papules on the thighs. The eruption subsequently spread to involve the buttocks, legs, and arms (Figures 1 and 2). The parents also noted that the patient had experienced an episode of bloody tears and epistaxis that lasted a few minutes at the pediatrician’s office earlier that morning, a finding that prompted the urgent referral to the ED.

Dermatology was then consulted. A review of systems was notable for rhinorrhea and diarrhea during the week leading to the eruption. The patient’s parents denied fevers, decreased oral intake, or a recent course of antibiotics. The patient’s medical history was notable only for atopic dermatitis treated with emollients and occasional topical steroids. The parents denied recent travel or vaccinations. Physical examination showed an afebrile, well-appearing infant with multiple nontender, slightly edematous, circular, purpuric papules and plaques scattered on the buttocks and extremities with edema on the dorsal feet. The remainder of the patient’s workup in the ED was notable for mild elevations in C-reactive protein levels (1.4 mg/dL [reference range, 0–1.2 mg/dL]) and an elevated erythrocyte sedimentation rate (22 mm/h [reference range, 2–12 mm/h]). A complete blood cell count; liver function tests; urinalysis; and coagulation studies, including prothrombin, partial thromboplastin time, and international normalized ratio, were unremarkable. Acute hemorrhagic edema of infancy was diagnosed based on the clinical manifestations.
Acute hemorrhagic edema of infancy (also known as Finkelstein disease, medallionlike purpura, Seidemayer syndrome, infantile postinfectious irislike purpura and edema, and purpura en cocarde avec oedeme) is believed to result from an immune complex–related reaction, often in the setting of an upper respiratory tract infection; medications, especially antibiotics; or vaccinations. The condition previously was considered a benign form of Henoch-Schönlein purpura; however, it is now recognized as its own clinical entity. Acute hemorrhagic edema of infancy commonly affects children between the ages of 4 months and 2 years. The incidence peaks in the winter months, and males tend to be more affected than females.1
Acute hemorrhagic edema of infancy is clinically characterized by a triad of large purpuric lesions, low-grade fever, and peripheral acral edema. Edema can develop on the hands, feet, and genitalia. Importantly, facial edema has been noted to precede skin lesions.2 Coin-shaped or targetoid hemorrhagic and purpuric lesions in a cockade or rosette pattern with scalloped margins typically begin on the distal extremities and tend to spread proximally. The lesions are variable in size but have been reported to be as large as 5 cm in diameter. Although joint pain, bloody diarrhea, hematuria, and proteinuria can accompany AHEI, most cases are devoid of systemic symptoms.3 Hemorrhagic lacrimation and epistaxis—both present in our patient—are rare findings with AHEI. It is likely that most providers, including dermatologists, may be unfamiliar with these striking clinical findings. Although the pathophysiology of hemorrhagic lacrimation and epistaxis has not been formally investigated, we postulate that it likely is related to the formation of immune complexes that lead to small vessel vasculitis, underpinning the characteristic findings in AHEI.4,5 This reasoning is supported by the complete resolution of symptoms corresponding with clinical clearance of the cutaneous vasculitis in 2 prior cases4,5 as well as in our patient who did not have a relapse of symptoms following cessation of the cutaneous eruption at a pediatric follow-up appointment 2 weeks later.
Acute hemorrhagic edema of infancy is a clinical diagnosis; however, a skin biopsy can be performed to confirm the clinical suspicion and rule out more serious conditions. Histopathologic examination reveals a leukocytoclastic vasculitis involving the capillaries and postcapillary venules of the upper and mid dermis. Laboratory test results usually are nonspecific but can help distinguish AHEI from more serious diseases. The erythrocyte sedimentation rate and C-reactive protein level may be slightly elevated in infants with AHEI. Urinalysis and stool guaiac tests also can be performed to evaluate for any renal or gastrointestinal involvement.6
The differential diagnosis includes IgA vasculitis, erythema multiforme, acute meningococcemia, urticarial vasculitis, Kawasaki disease, and child abuse. IgA vasculitis often presents with more systemic involvement, with abdominal pain, vomiting, hematemesis, diarrhea, and hematochezia occurring in up to 50% of patients. The cutaneous findings of erythema multiforme classically are confined to the limbs and face, and edema of the extremities typically is not seen. Patients with acute meningococcemia appear toxic with high fevers, malaise, and possible septic shock.5
Acute hemorrhagic edema of infancy is a self-limited condition typically lasting 1 to 3 weeks and requires only supportive care.7 Antibiotics should be given to treat concurrent bacterial infections, and antihistamines and steroids may be useful for symptomatic relief. Importantly, however, systemic corticosteroids do not appear to conclusively alter the disease course.8
Acute hemorrhagic edema of infancy is a rare benign leukocytoclastic vasculitis with a striking presentation often seen following an upper respiratory tract infection or course of antibiotics. Our case demonstrates that on rare occasions, AHEI may be accompanied by hemorrhagic lacrimation and epistaxis—findings that can be quite alarming to both parents and medical providers. Nonetheless, patients and their caretakers should be assured that the condition is self-limited and resolves without permanent sequalae.
- Emerich PS, Prebianchi PA, Motta LL, et al. Acute hemorrhagic edema of infancy: report of three cases. An Bras Dermatol. 2011;86:1181-1184.
- Avhad G, Ghuge P, Jerajani H. Acute hemorrhagic edema of infancy. Indian Dermatol Online J. 2014;5:356-357.
- Krause I, Lazarov A, Rachmel A, et al. Acute haemorrhagic oedema of infancy, a benign variant of leucocytoclastic vasculitis. Acta Paediatr. 1996;85:114-117.
- Sneller H, Vega C, Zemel L, et al. Acute hemorrhagic edema of infancy with associated hemorrhagic lacrimation. Pediatr Emerg Care. 2021;37:E70-E72. doi:10.1097/PEC.0000000000001542
- Mreish S, Al-Tatari H. Hemorrhagic lacrimation and epistaxis in acute hemorrhagic edema of infancy. Case Rep Pediatr. 2016;2016:9762185. doi:10.1155/2016/9762185
- Savino F, Lupica MM, Tarasco V, et al. Acute hemorrhagic edema of infancy: a troubling cutaneous presentation with a self-limiting course. Pediatr Dermatol. 2013;30:E149-E152.
- Fiore E, Rizzi M, Ragazzi M, et al. Acute hemorrhagic edema of young children (cockade purpura and edema): a case series and systematic review. J Am Acad Dermatol. 2008;59:684-695.
- Acute hemorrhagic edema of young children: a concise narrative review. Eur J Pediatr. 2011;170:1507-1511.
- Emerich PS, Prebianchi PA, Motta LL, et al. Acute hemorrhagic edema of infancy: report of three cases. An Bras Dermatol. 2011;86:1181-1184.
- Avhad G, Ghuge P, Jerajani H. Acute hemorrhagic edema of infancy. Indian Dermatol Online J. 2014;5:356-357.
- Krause I, Lazarov A, Rachmel A, et al. Acute haemorrhagic oedema of infancy, a benign variant of leucocytoclastic vasculitis. Acta Paediatr. 1996;85:114-117.
- Sneller H, Vega C, Zemel L, et al. Acute hemorrhagic edema of infancy with associated hemorrhagic lacrimation. Pediatr Emerg Care. 2021;37:E70-E72. doi:10.1097/PEC.0000000000001542
- Mreish S, Al-Tatari H. Hemorrhagic lacrimation and epistaxis in acute hemorrhagic edema of infancy. Case Rep Pediatr. 2016;2016:9762185. doi:10.1155/2016/9762185
- Savino F, Lupica MM, Tarasco V, et al. Acute hemorrhagic edema of infancy: a troubling cutaneous presentation with a self-limiting course. Pediatr Dermatol. 2013;30:E149-E152.
- Fiore E, Rizzi M, Ragazzi M, et al. Acute hemorrhagic edema of young children (cockade purpura and edema): a case series and systematic review. J Am Acad Dermatol. 2008;59:684-695.
- Acute hemorrhagic edema of young children: a concise narrative review. Eur J Pediatr. 2011;170:1507-1511.
PRACTICE POINTS
- Acute hemorrhagic edema of infancy (AHEI) is clinically characterized by a triad of large purpuric lesions, low-grade fever, and peripheral acral edema. Although joint pain, bloody diarrhea, hematuria, and proteinuria can accompany AHEI, most cases are devoid of systemic symptoms.
- It is a self-limited condition typically lasting 1 to 3 weeks and requires only supportive care.
- On rare occasions, AHEI may be accompanied by hemorrhagic lacrimation and epistaxis. Patients should be assured that the condition is self-limited and resolves without permanent sequalae.
Can a ‘smart’ skin patch detect early neurodegenerative diseases?
A new “smart patch” composed of microneedles that can detect proinflammatory markers via simulated skin interstitial fluid (ISF) may help diagnose neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease very early on.
Originally developed to deliver medications and vaccines via the skin in a minimally invasive manner, the microneedle arrays were fitted with molecular sensors that, when placed on the skin, detect neuroinflammatory biomarkers such as interleukin-6 in as little as 6 minutes.
The literature suggests that these biomarkers of neurodegenerative disease are present years before patients become symptomatic, said study investigator Sanjiv Sharma, PhD.
“Neurodegenerative disorders such as Parkinson’s disease and Alzheimer’s disease are [characterized by] progressive loss in nerve cell and brain cells, which leads to memory problems and a loss of mental ability. That is why early diagnosis is key to preventing the loss of brain tissue in dementia, which can go undetected for years,” added Dr. Sharma, who is a lecturer in medical engineering at Swansea (Wales) University.
Dr. Sharma developed the patch with scientists at the Polytechnic of Porto (Portugal) School of Engineering in Portugal. In 2022, they designed, and are currently testing, a microneedle patch that will deliver the COVID vaccine.
The investigators describe their research on the patch’s ability to detect IL-6 in an article published in ACS Omega.
At-home diagnosis?
“The skin is the largest organ in the body – it contains more skin interstitial fluid than the total blood volume,” Dr. Sharma noted. “This fluid is an ultrafiltrate of blood and holds biomarkers that complement other biofluids, such as sweat, saliva, and urine. It can be sampled in a minimally invasive manner and used either for point-of-care testing or real-time using microneedle devices.”
Dr. Sharma and associates tested the microneedle patch in artificial ISF that contained the inflammatory cytokine IL-6. They found that the patch accurately detected IL-6 concentrations as low as 1 pg/mL in the fabricated ISF solution.
“In general, the transdermal sensor presented here showed simplicity in designing, short measuring time, high accuracy, and low detection limit. This approach seems a successful tool for the screening of inflammatory biomarkers in point of care testing wherein the skin acts as a window to the body,” the investigators reported.
Dr. Sharma noted that early detection of neurodegenerative diseases is crucial, as once symptoms appear, the disease may have already progressed significantly, and meaningful intervention is challenging.
The device has yet to be tested in humans, which is the next step, said Dr. Sharma.
“We will have to test the hypothesis through extensive preclinical and clinical studies to determine if bloodless, transdermal (skin) diagnostics can offer a cost-effective device that could allow testing in simpler settings such as a clinician’s practice or even home settings,” he noted.
Early days
Commenting on the research, David K. Simon, MD, PhD, professor of neurology at Harvard Medical School, Boston, said it is “a promising step regarding validation of a potentially beneficial method for rapidly and accurately measuring IL-6.”
However, he added, “many additional steps are needed to validate the method in actual human skin and to determine whether or not measuring these biomarkers in skin will be useful in studies of neurodegenerative diseases.”
He noted that one study limitation is that inflammatory cytokines such as IL-6 are highly nonspecific, and levels are elevated in various diseases associated with inflammation.
“It is highly unlikely that measuring IL-6 will be useful as a diagnostic tool. However, it does have potential as a biomarker for measuring the impact of treatments aimed at reducing inflammation. As the authors point out, it’s more likely that clinicians will require a panel of biomarkers rather than only measuring IL-6,” he said.
The study was funded by Fundação para a Ciência e Tecnologia. The investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new “smart patch” composed of microneedles that can detect proinflammatory markers via simulated skin interstitial fluid (ISF) may help diagnose neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease very early on.
Originally developed to deliver medications and vaccines via the skin in a minimally invasive manner, the microneedle arrays were fitted with molecular sensors that, when placed on the skin, detect neuroinflammatory biomarkers such as interleukin-6 in as little as 6 minutes.
The literature suggests that these biomarkers of neurodegenerative disease are present years before patients become symptomatic, said study investigator Sanjiv Sharma, PhD.
“Neurodegenerative disorders such as Parkinson’s disease and Alzheimer’s disease are [characterized by] progressive loss in nerve cell and brain cells, which leads to memory problems and a loss of mental ability. That is why early diagnosis is key to preventing the loss of brain tissue in dementia, which can go undetected for years,” added Dr. Sharma, who is a lecturer in medical engineering at Swansea (Wales) University.
Dr. Sharma developed the patch with scientists at the Polytechnic of Porto (Portugal) School of Engineering in Portugal. In 2022, they designed, and are currently testing, a microneedle patch that will deliver the COVID vaccine.
The investigators describe their research on the patch’s ability to detect IL-6 in an article published in ACS Omega.
At-home diagnosis?
“The skin is the largest organ in the body – it contains more skin interstitial fluid than the total blood volume,” Dr. Sharma noted. “This fluid is an ultrafiltrate of blood and holds biomarkers that complement other biofluids, such as sweat, saliva, and urine. It can be sampled in a minimally invasive manner and used either for point-of-care testing or real-time using microneedle devices.”
Dr. Sharma and associates tested the microneedle patch in artificial ISF that contained the inflammatory cytokine IL-6. They found that the patch accurately detected IL-6 concentrations as low as 1 pg/mL in the fabricated ISF solution.
“In general, the transdermal sensor presented here showed simplicity in designing, short measuring time, high accuracy, and low detection limit. This approach seems a successful tool for the screening of inflammatory biomarkers in point of care testing wherein the skin acts as a window to the body,” the investigators reported.
Dr. Sharma noted that early detection of neurodegenerative diseases is crucial, as once symptoms appear, the disease may have already progressed significantly, and meaningful intervention is challenging.
The device has yet to be tested in humans, which is the next step, said Dr. Sharma.
“We will have to test the hypothesis through extensive preclinical and clinical studies to determine if bloodless, transdermal (skin) diagnostics can offer a cost-effective device that could allow testing in simpler settings such as a clinician’s practice or even home settings,” he noted.
Early days
Commenting on the research, David K. Simon, MD, PhD, professor of neurology at Harvard Medical School, Boston, said it is “a promising step regarding validation of a potentially beneficial method for rapidly and accurately measuring IL-6.”
However, he added, “many additional steps are needed to validate the method in actual human skin and to determine whether or not measuring these biomarkers in skin will be useful in studies of neurodegenerative diseases.”
He noted that one study limitation is that inflammatory cytokines such as IL-6 are highly nonspecific, and levels are elevated in various diseases associated with inflammation.
“It is highly unlikely that measuring IL-6 will be useful as a diagnostic tool. However, it does have potential as a biomarker for measuring the impact of treatments aimed at reducing inflammation. As the authors point out, it’s more likely that clinicians will require a panel of biomarkers rather than only measuring IL-6,” he said.
The study was funded by Fundação para a Ciência e Tecnologia. The investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new “smart patch” composed of microneedles that can detect proinflammatory markers via simulated skin interstitial fluid (ISF) may help diagnose neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease very early on.
Originally developed to deliver medications and vaccines via the skin in a minimally invasive manner, the microneedle arrays were fitted with molecular sensors that, when placed on the skin, detect neuroinflammatory biomarkers such as interleukin-6 in as little as 6 minutes.
The literature suggests that these biomarkers of neurodegenerative disease are present years before patients become symptomatic, said study investigator Sanjiv Sharma, PhD.
“Neurodegenerative disorders such as Parkinson’s disease and Alzheimer’s disease are [characterized by] progressive loss in nerve cell and brain cells, which leads to memory problems and a loss of mental ability. That is why early diagnosis is key to preventing the loss of brain tissue in dementia, which can go undetected for years,” added Dr. Sharma, who is a lecturer in medical engineering at Swansea (Wales) University.
Dr. Sharma developed the patch with scientists at the Polytechnic of Porto (Portugal) School of Engineering in Portugal. In 2022, they designed, and are currently testing, a microneedle patch that will deliver the COVID vaccine.
The investigators describe their research on the patch’s ability to detect IL-6 in an article published in ACS Omega.
At-home diagnosis?
“The skin is the largest organ in the body – it contains more skin interstitial fluid than the total blood volume,” Dr. Sharma noted. “This fluid is an ultrafiltrate of blood and holds biomarkers that complement other biofluids, such as sweat, saliva, and urine. It can be sampled in a minimally invasive manner and used either for point-of-care testing or real-time using microneedle devices.”
Dr. Sharma and associates tested the microneedle patch in artificial ISF that contained the inflammatory cytokine IL-6. They found that the patch accurately detected IL-6 concentrations as low as 1 pg/mL in the fabricated ISF solution.
“In general, the transdermal sensor presented here showed simplicity in designing, short measuring time, high accuracy, and low detection limit. This approach seems a successful tool for the screening of inflammatory biomarkers in point of care testing wherein the skin acts as a window to the body,” the investigators reported.
Dr. Sharma noted that early detection of neurodegenerative diseases is crucial, as once symptoms appear, the disease may have already progressed significantly, and meaningful intervention is challenging.
The device has yet to be tested in humans, which is the next step, said Dr. Sharma.
“We will have to test the hypothesis through extensive preclinical and clinical studies to determine if bloodless, transdermal (skin) diagnostics can offer a cost-effective device that could allow testing in simpler settings such as a clinician’s practice or even home settings,” he noted.
Early days
Commenting on the research, David K. Simon, MD, PhD, professor of neurology at Harvard Medical School, Boston, said it is “a promising step regarding validation of a potentially beneficial method for rapidly and accurately measuring IL-6.”
However, he added, “many additional steps are needed to validate the method in actual human skin and to determine whether or not measuring these biomarkers in skin will be useful in studies of neurodegenerative diseases.”
He noted that one study limitation is that inflammatory cytokines such as IL-6 are highly nonspecific, and levels are elevated in various diseases associated with inflammation.
“It is highly unlikely that measuring IL-6 will be useful as a diagnostic tool. However, it does have potential as a biomarker for measuring the impact of treatments aimed at reducing inflammation. As the authors point out, it’s more likely that clinicians will require a panel of biomarkers rather than only measuring IL-6,” he said.
The study was funded by Fundação para a Ciência e Tecnologia. The investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACS OMEGA
Biosimilar equal to natalizumab for relapsing remitting MS
An agent biologically similar to the humanized monoclonal antibody natalizumab is as effective and safe as the original reference drug for relapsing remitting multiple sclerosis (RRMS) – and has a similar level of immunogenicity, new research shows.
The investigators noted that these phase 3 trial findings are the final stage in the regulatory approval process.
“There will be a biosimilar that with respect to all parameters – efficacy, side effects, immunogenicity – doesn’t differ from the original drug and will probably be an option to consider to reduce treatment costs in MS,” said lead investigator Bernhard Hemmer, MD, a professor in the department of neurology, Technical University of Munich (Germany).
The findings were published online in JAMA Neurology.
Potential cost savings
Disease-modifying therapies (DMTs), particularly targeted biologics, have revolutionized the treatment of MS, including RRMS. Natalizumab, which was the first targeted biologic therapy approved for RRMS, is very effective and widely used, Dr. Hemmer said.
However, this and other DMTs are costly. Biosimilars, which are medicines clinically similar to an already marketed reference biologic medicine, can address this issue. In the areas of rheumatology and oncology, biosimilars have already demonstrated significant cost savings and improved treatment access.
The biosimilar natalizumab (biosim-NTZ), developed by Polpharma Biologics, is the first biosimilar monoclonal antibody therapy to be developed for MS.
Health authorities such as the Food and Drug Administration require comparative phase 3 studies to confirm there are no clinically relevant differences between a proposed biosimilar and its reference medicine.
The new multicenter, phase 3, double-blind, randomized trial – known as Antelope – included 264 adult patients with RRMS at 48 centers in seven Eastern European countries. Most study participants were women (61.4%), and their mean age was 36.7 years.
All study participants were randomly assigned to receive intravenous infusions every 4 weeks of 300 mg of biosim-NTZ or reference natalizumab (ref-NTZ) for a total of 12 infusions.
At week 24, 30 patients were switched from ref-NTZ to biosim-NTZ for the remainder of their infusions. Including such a population is required by regulatory agencies to ensure switching patients from a drug they’ve been taking to a new biosimilar does not introduce any concerns, said Dr. Hemmer.
Comparable efficacy, safety profile
The primary efficacy endpoint was the cumulative number of new active brain lesions on MRI.
At baseline, 48.1% of the biosimilar group and 45.9% of the reference drug group had at least one gadolinium-enhancing lesion. In addition, 96.9% of the biosimilar group had more than 15 T2 lesions, compared with 96.2% of the reference group.
At week 24, the mean difference between biosim-NTZ and ref-NTZ in the cumulative number of new active lesions was 0.17 (least square means, 0.34 vs. 0.45), with a 95% confidence interval of –0.61 to 0.94 and a point estimate within the prespecified margins of ± 2.1.
The annualized relapse rate for biosim-NTZ and ref-NTZ was similar at 24 weeks (0.21 vs. 0.15), as well as at 48 weeks (0.17 vs. 0.13). For Expanded Disability Status Scale scores, which were similar between treatment groups at baseline (mean, 3.4 vs. 3.2), change at 24 and 48 weeks was minimal and similar in both groups.
The safety profile was as expected for patients with RRMS receiving natalizumab. There were few adverse events of special interest, with similar proportions across all treatment groups.
The overall adverse-event profile for patients who switched from ref-NTZ to biosim-NTZ was similar to patients continuing ref-NTZ treatment and did not indicate any new or increased risks associated with switching.
Rates of treatment-emergent adverse events (TEAEs) were similar, at 64.9% for biosim-NTZ, 68.9% for ref-NTZ, and 73.3% for the switch group. The most-reported TEAEs among all treatment groups were nervous system disorders and infections and infestations.
Progressive multifocal leukoencephalopathy (PML), a rare and potentially fatal demyelinating disease of the central nervous system, is associated with some DMTs – notably ref-NTZ. It is caused by infection with the John Cunningham virus (JCV) (also referred to as human polyomavirus), the researchers noted.
As per the study protocol, no participant had a JCV-positive index of more than 1.5 at baseline. Proportions of patients positive for anti-JCV antibodies were similarly distributed between treatment groups throughout the study.
Similar immunogenicity
There was strong concordance regarding positivity for treatment-emergent antidrug antibodies between the biosim-NTZ and ref-NTZ groups (79.4% and 74.0%). This was also the case for antinatalizumab-neutralizing antibodies (69.0% and 66.2%).
“There was nothing that indicated immunogenicity is different” between the two agents, said Dr. Hemmer.
While this might change “when you look at longer time periods,” antibodies to natalizumab usually develop “very early on,” he added.
Dr. Hemmer noted that this comparison of the proposed biosimilar with the reference drug had no real surprises.
“If the immunogenicity is the same, the mode of action is the same, and the dose is the same, you would expect to have a similar clinical effect and also a similar side-effect profile, which is indeed the case,” he said.
Dr. Hemmer added that he has no insight as to when the drug might be approved but believes developers expect that to occur sometime this year.
Welcome results
Commenting on the study results, Torge Rempe, MD, assistant professor in the department of neurology, University of Florida, Gainesville, and the William T. And Janice M. Neely professor for research in MS, said he welcomes these new results showing the biosimilar matched the reference medication.
“The authors report no significant difference in their primary endpoint of cumulative number of active lesions as well as their secondary clinical endpoints of annualized relapse rate and changes from baseline Expanded Disability Status Scale scores,” said Dr. Rempe, who was not involved with the research.
The study also showed the reported adverse events were similar between the biosimilar and reference natalizumab, he noted.
However, although no cases of PML were uncovered during the study period, further research is needed to determine long-term safety in this area, Dr. Rempe said.
Finally, he agreed that the development of biosimilars such as this one addresses the issue of high annual costs for DMTs, an area of concern in the field of MS.
The study was funded by Polpharma Biologics. Dr. Hemmer has reported receiving personal fees from Polpharma and Sandoz during the conduct of the study and personal fees from Novartis, Biocom, and TG Therapeutics outside the submitted work. He has also received a patent for genetic determinants of antibodies against interferon-beta and a patent for KIR4.1 antibodies in MS; served on scientific advisory boards for Novartis; served as a data monitoring and safety committee member for AllergyCare, Polpharma Biologics, Sandoz, and TG Therapeutics; and received speaker honoraria from Desitin, grants from Regeneron for MS research, and funding from the Multiple MS EU consortium, the CLINSPECT-M consortium, and the German Research Foundation. Dr. Rempe has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An agent biologically similar to the humanized monoclonal antibody natalizumab is as effective and safe as the original reference drug for relapsing remitting multiple sclerosis (RRMS) – and has a similar level of immunogenicity, new research shows.
The investigators noted that these phase 3 trial findings are the final stage in the regulatory approval process.
“There will be a biosimilar that with respect to all parameters – efficacy, side effects, immunogenicity – doesn’t differ from the original drug and will probably be an option to consider to reduce treatment costs in MS,” said lead investigator Bernhard Hemmer, MD, a professor in the department of neurology, Technical University of Munich (Germany).
The findings were published online in JAMA Neurology.
Potential cost savings
Disease-modifying therapies (DMTs), particularly targeted biologics, have revolutionized the treatment of MS, including RRMS. Natalizumab, which was the first targeted biologic therapy approved for RRMS, is very effective and widely used, Dr. Hemmer said.
However, this and other DMTs are costly. Biosimilars, which are medicines clinically similar to an already marketed reference biologic medicine, can address this issue. In the areas of rheumatology and oncology, biosimilars have already demonstrated significant cost savings and improved treatment access.
The biosimilar natalizumab (biosim-NTZ), developed by Polpharma Biologics, is the first biosimilar monoclonal antibody therapy to be developed for MS.
Health authorities such as the Food and Drug Administration require comparative phase 3 studies to confirm there are no clinically relevant differences between a proposed biosimilar and its reference medicine.
The new multicenter, phase 3, double-blind, randomized trial – known as Antelope – included 264 adult patients with RRMS at 48 centers in seven Eastern European countries. Most study participants were women (61.4%), and their mean age was 36.7 years.
All study participants were randomly assigned to receive intravenous infusions every 4 weeks of 300 mg of biosim-NTZ or reference natalizumab (ref-NTZ) for a total of 12 infusions.
At week 24, 30 patients were switched from ref-NTZ to biosim-NTZ for the remainder of their infusions. Including such a population is required by regulatory agencies to ensure switching patients from a drug they’ve been taking to a new biosimilar does not introduce any concerns, said Dr. Hemmer.
Comparable efficacy, safety profile
The primary efficacy endpoint was the cumulative number of new active brain lesions on MRI.
At baseline, 48.1% of the biosimilar group and 45.9% of the reference drug group had at least one gadolinium-enhancing lesion. In addition, 96.9% of the biosimilar group had more than 15 T2 lesions, compared with 96.2% of the reference group.
At week 24, the mean difference between biosim-NTZ and ref-NTZ in the cumulative number of new active lesions was 0.17 (least square means, 0.34 vs. 0.45), with a 95% confidence interval of –0.61 to 0.94 and a point estimate within the prespecified margins of ± 2.1.
The annualized relapse rate for biosim-NTZ and ref-NTZ was similar at 24 weeks (0.21 vs. 0.15), as well as at 48 weeks (0.17 vs. 0.13). For Expanded Disability Status Scale scores, which were similar between treatment groups at baseline (mean, 3.4 vs. 3.2), change at 24 and 48 weeks was minimal and similar in both groups.
The safety profile was as expected for patients with RRMS receiving natalizumab. There were few adverse events of special interest, with similar proportions across all treatment groups.
The overall adverse-event profile for patients who switched from ref-NTZ to biosim-NTZ was similar to patients continuing ref-NTZ treatment and did not indicate any new or increased risks associated with switching.
Rates of treatment-emergent adverse events (TEAEs) were similar, at 64.9% for biosim-NTZ, 68.9% for ref-NTZ, and 73.3% for the switch group. The most-reported TEAEs among all treatment groups were nervous system disorders and infections and infestations.
Progressive multifocal leukoencephalopathy (PML), a rare and potentially fatal demyelinating disease of the central nervous system, is associated with some DMTs – notably ref-NTZ. It is caused by infection with the John Cunningham virus (JCV) (also referred to as human polyomavirus), the researchers noted.
As per the study protocol, no participant had a JCV-positive index of more than 1.5 at baseline. Proportions of patients positive for anti-JCV antibodies were similarly distributed between treatment groups throughout the study.
Similar immunogenicity
There was strong concordance regarding positivity for treatment-emergent antidrug antibodies between the biosim-NTZ and ref-NTZ groups (79.4% and 74.0%). This was also the case for antinatalizumab-neutralizing antibodies (69.0% and 66.2%).
“There was nothing that indicated immunogenicity is different” between the two agents, said Dr. Hemmer.
While this might change “when you look at longer time periods,” antibodies to natalizumab usually develop “very early on,” he added.
Dr. Hemmer noted that this comparison of the proposed biosimilar with the reference drug had no real surprises.
“If the immunogenicity is the same, the mode of action is the same, and the dose is the same, you would expect to have a similar clinical effect and also a similar side-effect profile, which is indeed the case,” he said.
Dr. Hemmer added that he has no insight as to when the drug might be approved but believes developers expect that to occur sometime this year.
Welcome results
Commenting on the study results, Torge Rempe, MD, assistant professor in the department of neurology, University of Florida, Gainesville, and the William T. And Janice M. Neely professor for research in MS, said he welcomes these new results showing the biosimilar matched the reference medication.
“The authors report no significant difference in their primary endpoint of cumulative number of active lesions as well as their secondary clinical endpoints of annualized relapse rate and changes from baseline Expanded Disability Status Scale scores,” said Dr. Rempe, who was not involved with the research.
The study also showed the reported adverse events were similar between the biosimilar and reference natalizumab, he noted.
However, although no cases of PML were uncovered during the study period, further research is needed to determine long-term safety in this area, Dr. Rempe said.
Finally, he agreed that the development of biosimilars such as this one addresses the issue of high annual costs for DMTs, an area of concern in the field of MS.
The study was funded by Polpharma Biologics. Dr. Hemmer has reported receiving personal fees from Polpharma and Sandoz during the conduct of the study and personal fees from Novartis, Biocom, and TG Therapeutics outside the submitted work. He has also received a patent for genetic determinants of antibodies against interferon-beta and a patent for KIR4.1 antibodies in MS; served on scientific advisory boards for Novartis; served as a data monitoring and safety committee member for AllergyCare, Polpharma Biologics, Sandoz, and TG Therapeutics; and received speaker honoraria from Desitin, grants from Regeneron for MS research, and funding from the Multiple MS EU consortium, the CLINSPECT-M consortium, and the German Research Foundation. Dr. Rempe has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An agent biologically similar to the humanized monoclonal antibody natalizumab is as effective and safe as the original reference drug for relapsing remitting multiple sclerosis (RRMS) – and has a similar level of immunogenicity, new research shows.
The investigators noted that these phase 3 trial findings are the final stage in the regulatory approval process.
“There will be a biosimilar that with respect to all parameters – efficacy, side effects, immunogenicity – doesn’t differ from the original drug and will probably be an option to consider to reduce treatment costs in MS,” said lead investigator Bernhard Hemmer, MD, a professor in the department of neurology, Technical University of Munich (Germany).
The findings were published online in JAMA Neurology.
Potential cost savings
Disease-modifying therapies (DMTs), particularly targeted biologics, have revolutionized the treatment of MS, including RRMS. Natalizumab, which was the first targeted biologic therapy approved for RRMS, is very effective and widely used, Dr. Hemmer said.
However, this and other DMTs are costly. Biosimilars, which are medicines clinically similar to an already marketed reference biologic medicine, can address this issue. In the areas of rheumatology and oncology, biosimilars have already demonstrated significant cost savings and improved treatment access.
The biosimilar natalizumab (biosim-NTZ), developed by Polpharma Biologics, is the first biosimilar monoclonal antibody therapy to be developed for MS.
Health authorities such as the Food and Drug Administration require comparative phase 3 studies to confirm there are no clinically relevant differences between a proposed biosimilar and its reference medicine.
The new multicenter, phase 3, double-blind, randomized trial – known as Antelope – included 264 adult patients with RRMS at 48 centers in seven Eastern European countries. Most study participants were women (61.4%), and their mean age was 36.7 years.
All study participants were randomly assigned to receive intravenous infusions every 4 weeks of 300 mg of biosim-NTZ or reference natalizumab (ref-NTZ) for a total of 12 infusions.
At week 24, 30 patients were switched from ref-NTZ to biosim-NTZ for the remainder of their infusions. Including such a population is required by regulatory agencies to ensure switching patients from a drug they’ve been taking to a new biosimilar does not introduce any concerns, said Dr. Hemmer.
Comparable efficacy, safety profile
The primary efficacy endpoint was the cumulative number of new active brain lesions on MRI.
At baseline, 48.1% of the biosimilar group and 45.9% of the reference drug group had at least one gadolinium-enhancing lesion. In addition, 96.9% of the biosimilar group had more than 15 T2 lesions, compared with 96.2% of the reference group.
At week 24, the mean difference between biosim-NTZ and ref-NTZ in the cumulative number of new active lesions was 0.17 (least square means, 0.34 vs. 0.45), with a 95% confidence interval of –0.61 to 0.94 and a point estimate within the prespecified margins of ± 2.1.
The annualized relapse rate for biosim-NTZ and ref-NTZ was similar at 24 weeks (0.21 vs. 0.15), as well as at 48 weeks (0.17 vs. 0.13). For Expanded Disability Status Scale scores, which were similar between treatment groups at baseline (mean, 3.4 vs. 3.2), change at 24 and 48 weeks was minimal and similar in both groups.
The safety profile was as expected for patients with RRMS receiving natalizumab. There were few adverse events of special interest, with similar proportions across all treatment groups.
The overall adverse-event profile for patients who switched from ref-NTZ to biosim-NTZ was similar to patients continuing ref-NTZ treatment and did not indicate any new or increased risks associated with switching.
Rates of treatment-emergent adverse events (TEAEs) were similar, at 64.9% for biosim-NTZ, 68.9% for ref-NTZ, and 73.3% for the switch group. The most-reported TEAEs among all treatment groups were nervous system disorders and infections and infestations.
Progressive multifocal leukoencephalopathy (PML), a rare and potentially fatal demyelinating disease of the central nervous system, is associated with some DMTs – notably ref-NTZ. It is caused by infection with the John Cunningham virus (JCV) (also referred to as human polyomavirus), the researchers noted.
As per the study protocol, no participant had a JCV-positive index of more than 1.5 at baseline. Proportions of patients positive for anti-JCV antibodies were similarly distributed between treatment groups throughout the study.
Similar immunogenicity
There was strong concordance regarding positivity for treatment-emergent antidrug antibodies between the biosim-NTZ and ref-NTZ groups (79.4% and 74.0%). This was also the case for antinatalizumab-neutralizing antibodies (69.0% and 66.2%).
“There was nothing that indicated immunogenicity is different” between the two agents, said Dr. Hemmer.
While this might change “when you look at longer time periods,” antibodies to natalizumab usually develop “very early on,” he added.
Dr. Hemmer noted that this comparison of the proposed biosimilar with the reference drug had no real surprises.
“If the immunogenicity is the same, the mode of action is the same, and the dose is the same, you would expect to have a similar clinical effect and also a similar side-effect profile, which is indeed the case,” he said.
Dr. Hemmer added that he has no insight as to when the drug might be approved but believes developers expect that to occur sometime this year.
Welcome results
Commenting on the study results, Torge Rempe, MD, assistant professor in the department of neurology, University of Florida, Gainesville, and the William T. And Janice M. Neely professor for research in MS, said he welcomes these new results showing the biosimilar matched the reference medication.
“The authors report no significant difference in their primary endpoint of cumulative number of active lesions as well as their secondary clinical endpoints of annualized relapse rate and changes from baseline Expanded Disability Status Scale scores,” said Dr. Rempe, who was not involved with the research.
The study also showed the reported adverse events were similar between the biosimilar and reference natalizumab, he noted.
However, although no cases of PML were uncovered during the study period, further research is needed to determine long-term safety in this area, Dr. Rempe said.
Finally, he agreed that the development of biosimilars such as this one addresses the issue of high annual costs for DMTs, an area of concern in the field of MS.
The study was funded by Polpharma Biologics. Dr. Hemmer has reported receiving personal fees from Polpharma and Sandoz during the conduct of the study and personal fees from Novartis, Biocom, and TG Therapeutics outside the submitted work. He has also received a patent for genetic determinants of antibodies against interferon-beta and a patent for KIR4.1 antibodies in MS; served on scientific advisory boards for Novartis; served as a data monitoring and safety committee member for AllergyCare, Polpharma Biologics, Sandoz, and TG Therapeutics; and received speaker honoraria from Desitin, grants from Regeneron for MS research, and funding from the Multiple MS EU consortium, the CLINSPECT-M consortium, and the German Research Foundation. Dr. Rempe has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Atopic Dermatitis Medication
Metastatic Breast Cancer Workup
The future of GI
Dear friends,
Since the last issue of The New Gastroenterologist, the GI Fellowship Match has occurred and CONGRATULATIONS to the Class of 2026! You’ve all been on an arduous journey to get here, and it’s really time to slow down and soak up as much as you can. For those who did not match, do not give up, because you are still the future of GI!
This issue of TNG is particularly special to me, because it marks my first official selection of articles as I embark on my own TNG journey, and the theme is the future of GI. In the “In Focus” article this quarter, Dr. Eugenia N. Uche-Anya and Dr. Tyler M. Berzin review the vast and emerging advances of artificial intelligence (AI) in colonoscopy, its role in augmenting patient care, obstacles in incorporating AI into current practice, and the future of AI in gastroenterology and hepatology. One important aspect of developing our future in these technologies includes getting involved with industry. Dr. Raman Muthusamy gives practical tips on developing and navigating relationships with industry, with highlights on understanding intellectual property and conflicts of interest.
Continuing our trek into the future of GI, telemedicine came into the fold with the COVID-19 pandemic, and it is clearly here to stay. Dr. Russ R. Arjal repositions telemedicine as a way to increase access to care and optimize practice revenue, with the aim of improving patient outcomes in the future.
Last, to ground this issue clinically, Dr. Jason Kwon and Dr. Paul T. Kroner review the gastrointestinal, hepatic, and pancreaticobiliary adverse manifestations and management of immune checkpoint inhibitors, especially now that immunotherapies have revolutionized the treatment of cancer. As gastroenterologists, we are and will be seeing more and more of these adverse events.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]). You may also contact Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact: Philipp Bozzini is credited with having developed the first endoscope in 1805, called the Lichtleiter (German for “light conductor”), using a candle as its light source. Adolf Kussmaul, however, developed the first rigid gastroscope in 1868, recruiting a sword-swallower in his first demonstration.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Advanced Endoscopy Fellow
Division of Gastroenterology & Hepatology
University of North Carolina at Chapel Hill
Dear friends,
Since the last issue of The New Gastroenterologist, the GI Fellowship Match has occurred and CONGRATULATIONS to the Class of 2026! You’ve all been on an arduous journey to get here, and it’s really time to slow down and soak up as much as you can. For those who did not match, do not give up, because you are still the future of GI!
This issue of TNG is particularly special to me, because it marks my first official selection of articles as I embark on my own TNG journey, and the theme is the future of GI. In the “In Focus” article this quarter, Dr. Eugenia N. Uche-Anya and Dr. Tyler M. Berzin review the vast and emerging advances of artificial intelligence (AI) in colonoscopy, its role in augmenting patient care, obstacles in incorporating AI into current practice, and the future of AI in gastroenterology and hepatology. One important aspect of developing our future in these technologies includes getting involved with industry. Dr. Raman Muthusamy gives practical tips on developing and navigating relationships with industry, with highlights on understanding intellectual property and conflicts of interest.
Continuing our trek into the future of GI, telemedicine came into the fold with the COVID-19 pandemic, and it is clearly here to stay. Dr. Russ R. Arjal repositions telemedicine as a way to increase access to care and optimize practice revenue, with the aim of improving patient outcomes in the future.
Last, to ground this issue clinically, Dr. Jason Kwon and Dr. Paul T. Kroner review the gastrointestinal, hepatic, and pancreaticobiliary adverse manifestations and management of immune checkpoint inhibitors, especially now that immunotherapies have revolutionized the treatment of cancer. As gastroenterologists, we are and will be seeing more and more of these adverse events.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]). You may also contact Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact: Philipp Bozzini is credited with having developed the first endoscope in 1805, called the Lichtleiter (German for “light conductor”), using a candle as its light source. Adolf Kussmaul, however, developed the first rigid gastroscope in 1868, recruiting a sword-swallower in his first demonstration.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Advanced Endoscopy Fellow
Division of Gastroenterology & Hepatology
University of North Carolina at Chapel Hill
Dear friends,
Since the last issue of The New Gastroenterologist, the GI Fellowship Match has occurred and CONGRATULATIONS to the Class of 2026! You’ve all been on an arduous journey to get here, and it’s really time to slow down and soak up as much as you can. For those who did not match, do not give up, because you are still the future of GI!
This issue of TNG is particularly special to me, because it marks my first official selection of articles as I embark on my own TNG journey, and the theme is the future of GI. In the “In Focus” article this quarter, Dr. Eugenia N. Uche-Anya and Dr. Tyler M. Berzin review the vast and emerging advances of artificial intelligence (AI) in colonoscopy, its role in augmenting patient care, obstacles in incorporating AI into current practice, and the future of AI in gastroenterology and hepatology. One important aspect of developing our future in these technologies includes getting involved with industry. Dr. Raman Muthusamy gives practical tips on developing and navigating relationships with industry, with highlights on understanding intellectual property and conflicts of interest.
Continuing our trek into the future of GI, telemedicine came into the fold with the COVID-19 pandemic, and it is clearly here to stay. Dr. Russ R. Arjal repositions telemedicine as a way to increase access to care and optimize practice revenue, with the aim of improving patient outcomes in the future.
Last, to ground this issue clinically, Dr. Jason Kwon and Dr. Paul T. Kroner review the gastrointestinal, hepatic, and pancreaticobiliary adverse manifestations and management of immune checkpoint inhibitors, especially now that immunotherapies have revolutionized the treatment of cancer. As gastroenterologists, we are and will be seeing more and more of these adverse events.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]). You may also contact Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact: Philipp Bozzini is credited with having developed the first endoscope in 1805, called the Lichtleiter (German for “light conductor”), using a candle as its light source. Adolf Kussmaul, however, developed the first rigid gastroscope in 1868, recruiting a sword-swallower in his first demonstration.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Advanced Endoscopy Fellow
Division of Gastroenterology & Hepatology
University of North Carolina at Chapel Hill
Investing in GI innovation
Innovations in biomedical technology – from modern endoscopic devices and techniques to harnessing the microbiome to prevent and treat disease – have fundamentally changed the way in which we practice medicine and significantly improved the lives of our patients. In our February issue, we are pleased to highlight the launch of AGA’s GI Opportunity Fund, a new investment vehicle that provides AGA members and others a direct pathway to support development of promising, early-stage innovations by funding carefully vetted, cutting-edge start-up companies. We hope you will enjoy learning more about this exciting new initiative, which recently made its first major investment.
I want to thank GIHN Associate Editor Dr. Janice Jou for agreeing to spearhead this new column as its section editor – again, we invite you to nominate your colleagues, mentees, and others to be featured in future Member Spotlight columns.
We also highlight several recent papers published in AGA’s flagship journals, including a study assessing clinical outcomes and adverse events in patients receiving oral vs. colonic fecal microbiota transplant (FMT) for recurrent C. difficile infection, and another evaluating the cost-effectiveness of earlier colorectal cancer screening in patients with obesity. On the policy front, we summarize GI-relevant portions of the $1.7 trillion FY 2023 Omnibus Appropriations bill, signed into law on Dec. 30, 2022, by President Biden, and assess its impact on Medicare payments, continuation of support for telehealth/virtual care, and NIH-funding. We hope you enjoy reading these and other articles presented in our February issue.
Don’t forget to register for DDW 2023, May 6-9, 2023, in Chicago – general registration is now open!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Innovations in biomedical technology – from modern endoscopic devices and techniques to harnessing the microbiome to prevent and treat disease – have fundamentally changed the way in which we practice medicine and significantly improved the lives of our patients. In our February issue, we are pleased to highlight the launch of AGA’s GI Opportunity Fund, a new investment vehicle that provides AGA members and others a direct pathway to support development of promising, early-stage innovations by funding carefully vetted, cutting-edge start-up companies. We hope you will enjoy learning more about this exciting new initiative, which recently made its first major investment.
I want to thank GIHN Associate Editor Dr. Janice Jou for agreeing to spearhead this new column as its section editor – again, we invite you to nominate your colleagues, mentees, and others to be featured in future Member Spotlight columns.
We also highlight several recent papers published in AGA’s flagship journals, including a study assessing clinical outcomes and adverse events in patients receiving oral vs. colonic fecal microbiota transplant (FMT) for recurrent C. difficile infection, and another evaluating the cost-effectiveness of earlier colorectal cancer screening in patients with obesity. On the policy front, we summarize GI-relevant portions of the $1.7 trillion FY 2023 Omnibus Appropriations bill, signed into law on Dec. 30, 2022, by President Biden, and assess its impact on Medicare payments, continuation of support for telehealth/virtual care, and NIH-funding. We hope you enjoy reading these and other articles presented in our February issue.
Don’t forget to register for DDW 2023, May 6-9, 2023, in Chicago – general registration is now open!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Innovations in biomedical technology – from modern endoscopic devices and techniques to harnessing the microbiome to prevent and treat disease – have fundamentally changed the way in which we practice medicine and significantly improved the lives of our patients. In our February issue, we are pleased to highlight the launch of AGA’s GI Opportunity Fund, a new investment vehicle that provides AGA members and others a direct pathway to support development of promising, early-stage innovations by funding carefully vetted, cutting-edge start-up companies. We hope you will enjoy learning more about this exciting new initiative, which recently made its first major investment.
I want to thank GIHN Associate Editor Dr. Janice Jou for agreeing to spearhead this new column as its section editor – again, we invite you to nominate your colleagues, mentees, and others to be featured in future Member Spotlight columns.
We also highlight several recent papers published in AGA’s flagship journals, including a study assessing clinical outcomes and adverse events in patients receiving oral vs. colonic fecal microbiota transplant (FMT) for recurrent C. difficile infection, and another evaluating the cost-effectiveness of earlier colorectal cancer screening in patients with obesity. On the policy front, we summarize GI-relevant portions of the $1.7 trillion FY 2023 Omnibus Appropriations bill, signed into law on Dec. 30, 2022, by President Biden, and assess its impact on Medicare payments, continuation of support for telehealth/virtual care, and NIH-funding. We hope you enjoy reading these and other articles presented in our February issue.
Don’t forget to register for DDW 2023, May 6-9, 2023, in Chicago – general registration is now open!
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Setting higher standards for digital health technologies
“It’s influenced the way I see medicine and the work that I do around identifying quality, not in the conventional context in a hospital or a clinic, but applying that lens to the world of technology,” said Dr. Mathews, assistant professor of medicine at Johns Hopkins Medicine in Baltimore.
Bringing greater visibility to digital health technologies is part of his life’s work.
“There is now an expectation that high quality must be part of the development process of these new technologies,” said Dr. Mathews.
In particular, he’d like to see noninvasive diagnostic technologies in the gastroenterology world become more patient-centric.
Bringing somebody into the hospital is often inconvenient and disruptive. The field is heading toward technologies that can be used in the home or in an outpatient setting. “I have some research in that area, and I’d love to see it ultimately reach the patient at the bedside, if possible.”
Dr. Mathews is a member of the AGA Center for GI Innovation and Technology and a previous mentee in the Future Leaders Program.
In an interview, Dr. Mathews discussed his push to validate health technologies in the GI field and to make them more transparent to physicians and patients.
Question: Why did you choose GI?
Answer: I think the world of gastroenterology offers a tremendous amount of diversity in the way we manage and treat patients. There’s a huge spectrum of disease. There’s also the procedural aspect, which is very different from a lot of other medical specialties. For me particularly, there’s the opportunity to work on technology as it relates to GI, as well as research in that space.
Q: It seems like gastroenterology involves a lot of detective work. Would you say that’s true?
A: When you think of something like abdominal pain or GI symptoms, any place in the body can cause those symptoms to be present. You have to think broadly about all of the contributing factors, the whole patient as it relates to travel, pets, exposures, food, diet. You really can’t be myopic when you think about all the potential causes.
The name of the game is to provide answers whenever possible, but I will settle for getting someone feeling better, even if we don’t have the answer etched in stone.
Q: What gives you the most joy in your day-to-day practice?
A: I work in an academic institution at Johns Hopkins. I really enjoy the direct connection with patients. I’ve switched mostly to a hospital-based practice, which means I’m getting patients at their sickest. It’s really a privilege to provide an opportunity for improvement or support in that context. I also enjoy the teaching and training of the next generation of folks that are going into this field. There’s so much to learn, and I think trying to set that example and teach by doing is a great opportunity, and I really enjoy that as well.
Q: Describe your biggest practice-related challenge and what you’re doing to address it.A: One of my focus areas on the research front is about providing greater transparency and validation around health technologies. How do patients know which health technologies to use? How do doctors know which ones to recommend or advocate for?
Q: Can you give an example of a technology of concern?
A: Looking at oncology and mobile apps, one study I coauthored in 2021 found that well over half did not meet physician or patient expectations. These were the most popular and highest rated apps available at the time. It shows that there’s a real disconnect between what the end users – the doctors and the patients – want from these solutions and what’s actually being provided.
There’s a flood of different solutions that are out there, and there really isn’t a streamlined way to know, as a clinician or as a patient, which ones really make a difference clinically and which ones are going to be helpful for you. And that’s been the focus of my research – understanding ways to evaluate technologies that are not so burdensome as to be purely in the realm of academics, but to be pragmatic.
Q: Who has had the strongest influence on your life?
A: I would say my spouse. She’s an academic physician at Hopkins. One of the things she has shown me is the importance of finding alignment in what you do professionally with the sort of goals that you have or the values that you hold as an individual. That’s why I’ve done some nontraditional things in my academic career. It’s really been in search of finding that alignment that matches my interests and goals, as opposed to just doing something because it’s a popular thing to do.
Lightning Round
Favorite sport: Soccer
What song do you have to sing along with when you hear it? 80s pop music
Introvert or extrovert? Introvert
Favorite holiday: Christmas
Optimist or pessimist? Realist
Dr. Mathews is on LinkedIn . His health tech blog is Digital Differential.
“It’s influenced the way I see medicine and the work that I do around identifying quality, not in the conventional context in a hospital or a clinic, but applying that lens to the world of technology,” said Dr. Mathews, assistant professor of medicine at Johns Hopkins Medicine in Baltimore.
Bringing greater visibility to digital health technologies is part of his life’s work.
“There is now an expectation that high quality must be part of the development process of these new technologies,” said Dr. Mathews.
In particular, he’d like to see noninvasive diagnostic technologies in the gastroenterology world become more patient-centric.
Bringing somebody into the hospital is often inconvenient and disruptive. The field is heading toward technologies that can be used in the home or in an outpatient setting. “I have some research in that area, and I’d love to see it ultimately reach the patient at the bedside, if possible.”
Dr. Mathews is a member of the AGA Center for GI Innovation and Technology and a previous mentee in the Future Leaders Program.
In an interview, Dr. Mathews discussed his push to validate health technologies in the GI field and to make them more transparent to physicians and patients.
Question: Why did you choose GI?
Answer: I think the world of gastroenterology offers a tremendous amount of diversity in the way we manage and treat patients. There’s a huge spectrum of disease. There’s also the procedural aspect, which is very different from a lot of other medical specialties. For me particularly, there’s the opportunity to work on technology as it relates to GI, as well as research in that space.
Q: It seems like gastroenterology involves a lot of detective work. Would you say that’s true?
A: When you think of something like abdominal pain or GI symptoms, any place in the body can cause those symptoms to be present. You have to think broadly about all of the contributing factors, the whole patient as it relates to travel, pets, exposures, food, diet. You really can’t be myopic when you think about all the potential causes.
The name of the game is to provide answers whenever possible, but I will settle for getting someone feeling better, even if we don’t have the answer etched in stone.
Q: What gives you the most joy in your day-to-day practice?
A: I work in an academic institution at Johns Hopkins. I really enjoy the direct connection with patients. I’ve switched mostly to a hospital-based practice, which means I’m getting patients at their sickest. It’s really a privilege to provide an opportunity for improvement or support in that context. I also enjoy the teaching and training of the next generation of folks that are going into this field. There’s so much to learn, and I think trying to set that example and teach by doing is a great opportunity, and I really enjoy that as well.
Q: Describe your biggest practice-related challenge and what you’re doing to address it.A: One of my focus areas on the research front is about providing greater transparency and validation around health technologies. How do patients know which health technologies to use? How do doctors know which ones to recommend or advocate for?
Q: Can you give an example of a technology of concern?
A: Looking at oncology and mobile apps, one study I coauthored in 2021 found that well over half did not meet physician or patient expectations. These were the most popular and highest rated apps available at the time. It shows that there’s a real disconnect between what the end users – the doctors and the patients – want from these solutions and what’s actually being provided.
There’s a flood of different solutions that are out there, and there really isn’t a streamlined way to know, as a clinician or as a patient, which ones really make a difference clinically and which ones are going to be helpful for you. And that’s been the focus of my research – understanding ways to evaluate technologies that are not so burdensome as to be purely in the realm of academics, but to be pragmatic.
Q: Who has had the strongest influence on your life?
A: I would say my spouse. She’s an academic physician at Hopkins. One of the things she has shown me is the importance of finding alignment in what you do professionally with the sort of goals that you have or the values that you hold as an individual. That’s why I’ve done some nontraditional things in my academic career. It’s really been in search of finding that alignment that matches my interests and goals, as opposed to just doing something because it’s a popular thing to do.
Lightning Round
Favorite sport: Soccer
What song do you have to sing along with when you hear it? 80s pop music
Introvert or extrovert? Introvert
Favorite holiday: Christmas
Optimist or pessimist? Realist
Dr. Mathews is on LinkedIn . His health tech blog is Digital Differential.
“It’s influenced the way I see medicine and the work that I do around identifying quality, not in the conventional context in a hospital or a clinic, but applying that lens to the world of technology,” said Dr. Mathews, assistant professor of medicine at Johns Hopkins Medicine in Baltimore.
Bringing greater visibility to digital health technologies is part of his life’s work.
“There is now an expectation that high quality must be part of the development process of these new technologies,” said Dr. Mathews.
In particular, he’d like to see noninvasive diagnostic technologies in the gastroenterology world become more patient-centric.
Bringing somebody into the hospital is often inconvenient and disruptive. The field is heading toward technologies that can be used in the home or in an outpatient setting. “I have some research in that area, and I’d love to see it ultimately reach the patient at the bedside, if possible.”
Dr. Mathews is a member of the AGA Center for GI Innovation and Technology and a previous mentee in the Future Leaders Program.
In an interview, Dr. Mathews discussed his push to validate health technologies in the GI field and to make them more transparent to physicians and patients.
Question: Why did you choose GI?
Answer: I think the world of gastroenterology offers a tremendous amount of diversity in the way we manage and treat patients. There’s a huge spectrum of disease. There’s also the procedural aspect, which is very different from a lot of other medical specialties. For me particularly, there’s the opportunity to work on technology as it relates to GI, as well as research in that space.
Q: It seems like gastroenterology involves a lot of detective work. Would you say that’s true?
A: When you think of something like abdominal pain or GI symptoms, any place in the body can cause those symptoms to be present. You have to think broadly about all of the contributing factors, the whole patient as it relates to travel, pets, exposures, food, diet. You really can’t be myopic when you think about all the potential causes.
The name of the game is to provide answers whenever possible, but I will settle for getting someone feeling better, even if we don’t have the answer etched in stone.
Q: What gives you the most joy in your day-to-day practice?
A: I work in an academic institution at Johns Hopkins. I really enjoy the direct connection with patients. I’ve switched mostly to a hospital-based practice, which means I’m getting patients at their sickest. It’s really a privilege to provide an opportunity for improvement or support in that context. I also enjoy the teaching and training of the next generation of folks that are going into this field. There’s so much to learn, and I think trying to set that example and teach by doing is a great opportunity, and I really enjoy that as well.
Q: Describe your biggest practice-related challenge and what you’re doing to address it.A: One of my focus areas on the research front is about providing greater transparency and validation around health technologies. How do patients know which health technologies to use? How do doctors know which ones to recommend or advocate for?
Q: Can you give an example of a technology of concern?
A: Looking at oncology and mobile apps, one study I coauthored in 2021 found that well over half did not meet physician or patient expectations. These were the most popular and highest rated apps available at the time. It shows that there’s a real disconnect between what the end users – the doctors and the patients – want from these solutions and what’s actually being provided.
There’s a flood of different solutions that are out there, and there really isn’t a streamlined way to know, as a clinician or as a patient, which ones really make a difference clinically and which ones are going to be helpful for you. And that’s been the focus of my research – understanding ways to evaluate technologies that are not so burdensome as to be purely in the realm of academics, but to be pragmatic.
Q: Who has had the strongest influence on your life?
A: I would say my spouse. She’s an academic physician at Hopkins. One of the things she has shown me is the importance of finding alignment in what you do professionally with the sort of goals that you have or the values that you hold as an individual. That’s why I’ve done some nontraditional things in my academic career. It’s really been in search of finding that alignment that matches my interests and goals, as opposed to just doing something because it’s a popular thing to do.
Lightning Round
Favorite sport: Soccer
What song do you have to sing along with when you hear it? 80s pop music
Introvert or extrovert? Introvert
Favorite holiday: Christmas
Optimist or pessimist? Realist
Dr. Mathews is on LinkedIn . His health tech blog is Digital Differential.