User login
Dermatology Articles in Preprint Servers: A Cross-sectional Study
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).
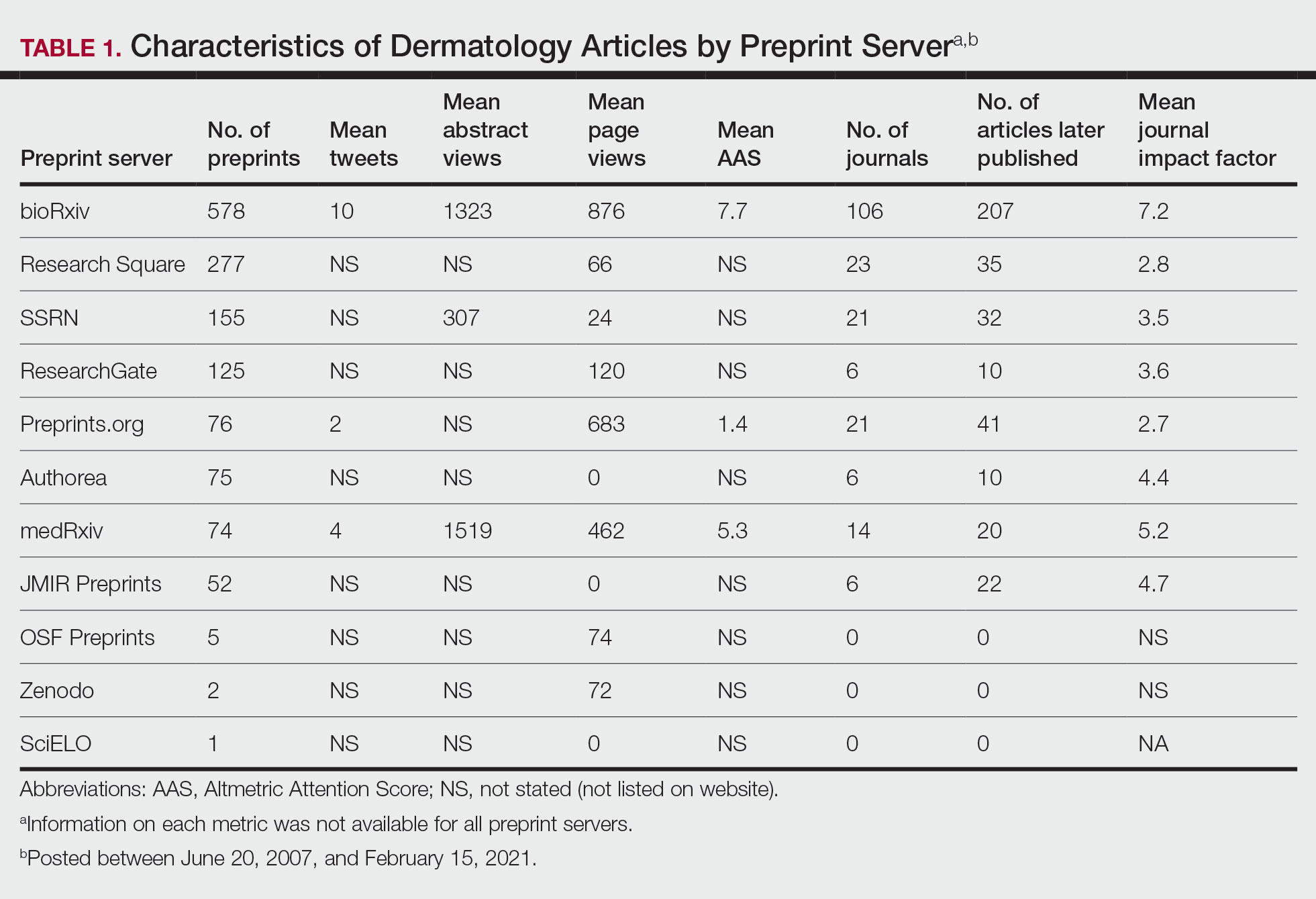
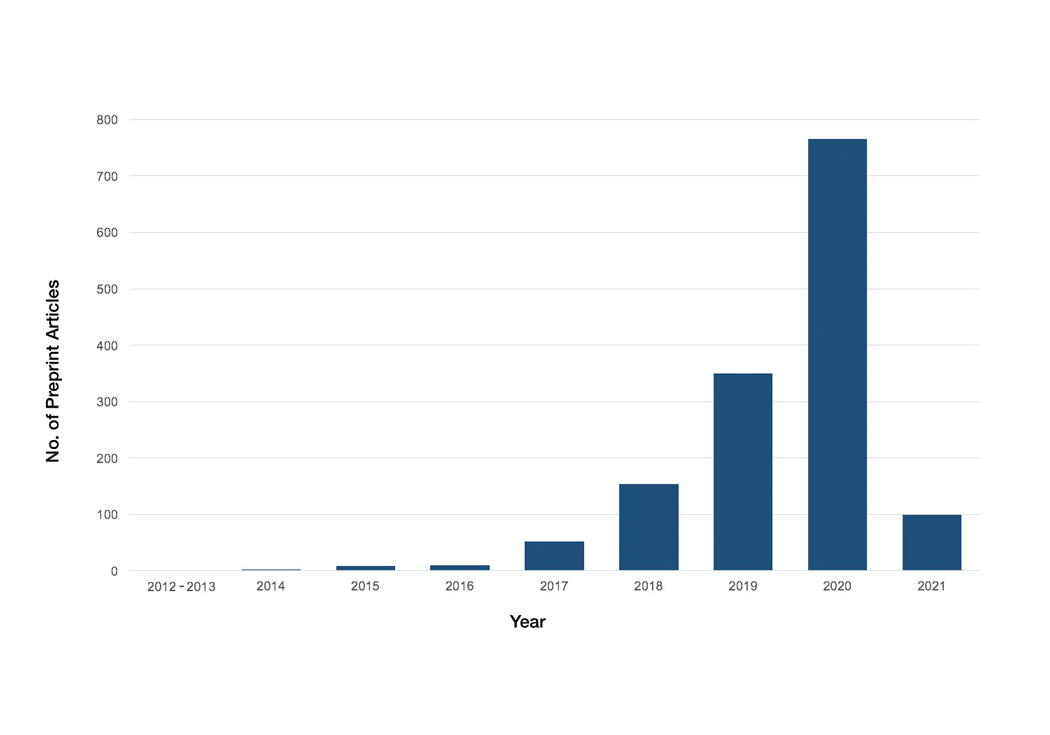
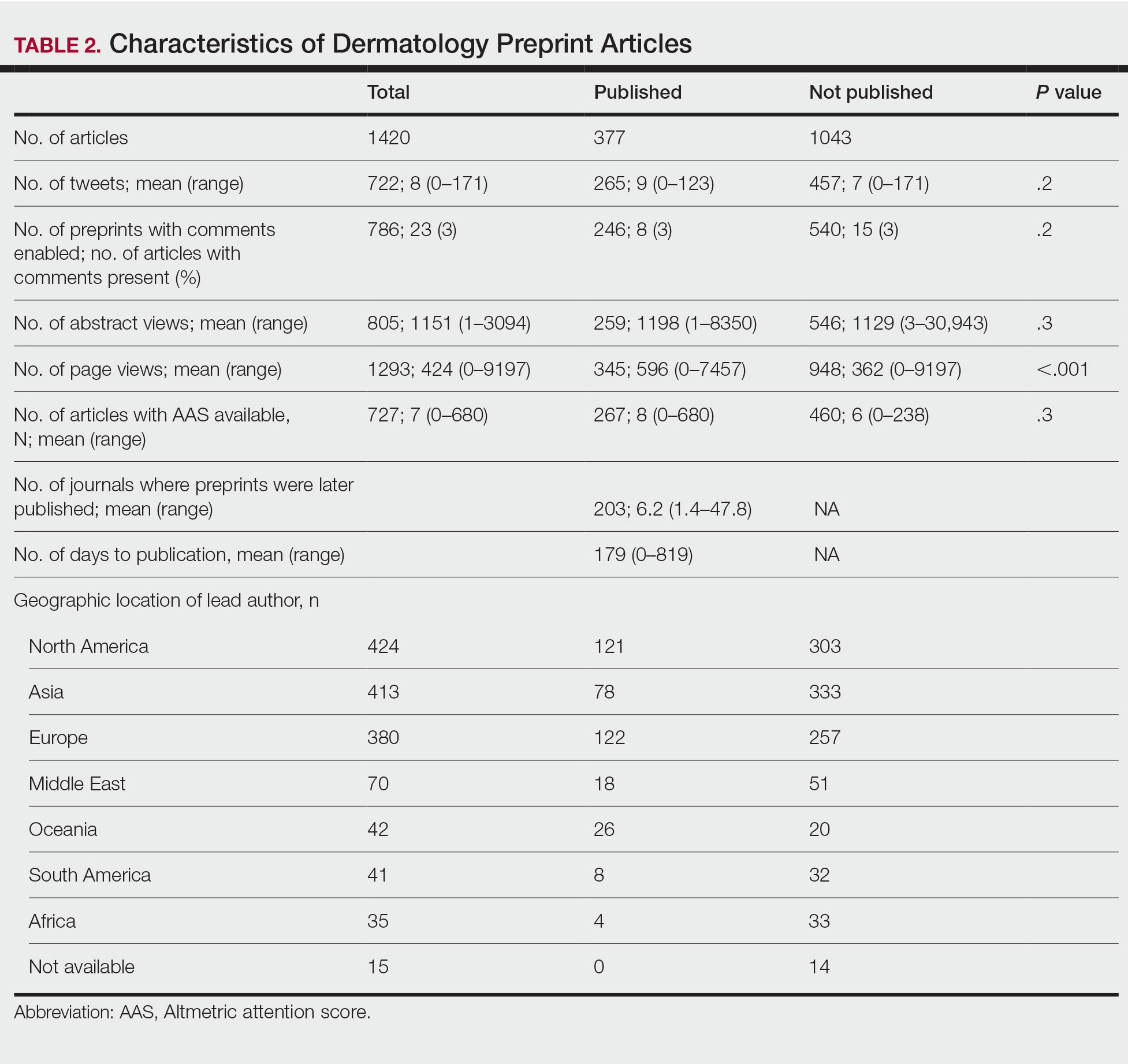
A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.
Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.
Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).

A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.

Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.

Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).

A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.

Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.

Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
PRACTICE POINTS
- Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals.
- The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission; therefore, dermatologists may use these servers to disseminate research quickly and freely but may not receive constructive criticism.
- Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice.
COVID emergency orders ending: What’s next?
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
Developments in wound healing include different treatment options
ORLANDO – , Hadar Lev-Tov, MD, said at the ODAC Dermatology, Aesthetic & Surgery Conference.
At the meeting, Dr. Lev-Tov, associate professor of dermatology at the University of Miami, reviewed some of the latest developments in several conditions involving wound care.
Pyoderma gangrenosum (PG): In this condition, pustules or nodules become large ulcerations, and one-third of patients with PG have pathergy, exaggerated skin injury after a mild trauma such as a bump or a bruise.
“You want to look at the clues in the history because 20% of these patients had histories of PG elsewhere,” Dr. Lev-Tov said. “Ask them about other ulcers, maybe they had some wound dehiscence history.”
Criteria have been developed to help with the diagnosis of ulcerative PG, which includes one major criterion, a biopsy of the ulcer edge showing neutrophilic infiltrate, along with minor criteria, including exclusion of an infection, pathergy, and a history of inflammatory bowel disease or inflammatory arthritis.
“This is no longer a diagnosis of exclusion,” Dr. Lev-Tov said.
Cyclosporine and oral steroids have been found to work well, but it typically takes many months before healing occurs. Tacrolimus or topical steroids can work as well, but healing also takes a fairly long time with those medications, Dr. Lev-Tov said.
The tumor necrosis factor (TNF) blocker infliximab is another option. He had a patient who was referred to him who had been treated with cyclosporine for 3 years for PG on his feet, even though it had not been effective. Dr. Lev-Tov tried infliximab, and the wounds finally cleared, he said.
Apremilast, a phosphodiesterase 4 (PDE4)-inhibitor, is another option for treating PG, he said. “Anecdotally, I used apremilast on three patients with recurrent PG for long-term suppression, with success,” he noted.
Epidermal grafting using suction and heat is an approach that might deserve further exploration for PG, Dr. Lev-Tov suggested. With this procedure, described in an article in 2014, heat and suction are used to induce blistering to separate and remove the epidermis from the dermis at the dermal-epidermal junction, creating an epidermal graft is placed over the wound to promote healing. Patients with PG who are immunosuppressed but demonstrate pathergy do not tend to experience pathergy when epidermal skin grafting is performed, he said.
The heat-suction procedure is simple, painless, and scarless, but better controlled data on this approach are needed, he said.
Corona phlebectatica: This disease involving abnormally dilated veins near the ankle has received formal recognition as a sign of venous insufficiency, in a 2020 update of a classification system for describing patients with chronic venous disorders, Dr. Lev-Tov said.
“We knew about it for years, but now there’s some data that can actually predict the severity of disease,” and, he said, it is now a part of the diagnostic criteria for venous insufficiency .
Venous leg ulcers: These often painful sores on the inside of the leg typically take more than a month to heal. A systematic review of placebo-controlled studies of pentoxifylline as a treatment for venous leg ulcers, published in 2021, supports its use for healing venous leg ulcers, Dr. Lev-Tov said. “It improved the healing rate and increased what [the researchers] called ‘significant improvement,’ ” a category they created to account for the varying methods across the studies, he said.
Topical beta-blockers can improve epithelialization and fibroblast migration in wound healing, he said. A study on topical timolol for various wounds found that a 0.5% formulation of topical timolol, with one drop applied per square centimeter as frequently as possible, was effective in healing. But the healing process was prolonged – a median of 90 days, said Dr. Lev-Tov, one of the study authors.
“When you start this, I don’t want you to expect the wound to heal tomorrow,” he said. “You’ve got to educate your patient.”
Dr. Lev-Tov reports relevant financial relationships with Abbvie, Novartis, Pfizer and other companies.
ORLANDO – , Hadar Lev-Tov, MD, said at the ODAC Dermatology, Aesthetic & Surgery Conference.
At the meeting, Dr. Lev-Tov, associate professor of dermatology at the University of Miami, reviewed some of the latest developments in several conditions involving wound care.
Pyoderma gangrenosum (PG): In this condition, pustules or nodules become large ulcerations, and one-third of patients with PG have pathergy, exaggerated skin injury after a mild trauma such as a bump or a bruise.
“You want to look at the clues in the history because 20% of these patients had histories of PG elsewhere,” Dr. Lev-Tov said. “Ask them about other ulcers, maybe they had some wound dehiscence history.”
Criteria have been developed to help with the diagnosis of ulcerative PG, which includes one major criterion, a biopsy of the ulcer edge showing neutrophilic infiltrate, along with minor criteria, including exclusion of an infection, pathergy, and a history of inflammatory bowel disease or inflammatory arthritis.
“This is no longer a diagnosis of exclusion,” Dr. Lev-Tov said.
Cyclosporine and oral steroids have been found to work well, but it typically takes many months before healing occurs. Tacrolimus or topical steroids can work as well, but healing also takes a fairly long time with those medications, Dr. Lev-Tov said.
The tumor necrosis factor (TNF) blocker infliximab is another option. He had a patient who was referred to him who had been treated with cyclosporine for 3 years for PG on his feet, even though it had not been effective. Dr. Lev-Tov tried infliximab, and the wounds finally cleared, he said.
Apremilast, a phosphodiesterase 4 (PDE4)-inhibitor, is another option for treating PG, he said. “Anecdotally, I used apremilast on three patients with recurrent PG for long-term suppression, with success,” he noted.
Epidermal grafting using suction and heat is an approach that might deserve further exploration for PG, Dr. Lev-Tov suggested. With this procedure, described in an article in 2014, heat and suction are used to induce blistering to separate and remove the epidermis from the dermis at the dermal-epidermal junction, creating an epidermal graft is placed over the wound to promote healing. Patients with PG who are immunosuppressed but demonstrate pathergy do not tend to experience pathergy when epidermal skin grafting is performed, he said.
The heat-suction procedure is simple, painless, and scarless, but better controlled data on this approach are needed, he said.
Corona phlebectatica: This disease involving abnormally dilated veins near the ankle has received formal recognition as a sign of venous insufficiency, in a 2020 update of a classification system for describing patients with chronic venous disorders, Dr. Lev-Tov said.
“We knew about it for years, but now there’s some data that can actually predict the severity of disease,” and, he said, it is now a part of the diagnostic criteria for venous insufficiency .
Venous leg ulcers: These often painful sores on the inside of the leg typically take more than a month to heal. A systematic review of placebo-controlled studies of pentoxifylline as a treatment for venous leg ulcers, published in 2021, supports its use for healing venous leg ulcers, Dr. Lev-Tov said. “It improved the healing rate and increased what [the researchers] called ‘significant improvement,’ ” a category they created to account for the varying methods across the studies, he said.
Topical beta-blockers can improve epithelialization and fibroblast migration in wound healing, he said. A study on topical timolol for various wounds found that a 0.5% formulation of topical timolol, with one drop applied per square centimeter as frequently as possible, was effective in healing. But the healing process was prolonged – a median of 90 days, said Dr. Lev-Tov, one of the study authors.
“When you start this, I don’t want you to expect the wound to heal tomorrow,” he said. “You’ve got to educate your patient.”
Dr. Lev-Tov reports relevant financial relationships with Abbvie, Novartis, Pfizer and other companies.
ORLANDO – , Hadar Lev-Tov, MD, said at the ODAC Dermatology, Aesthetic & Surgery Conference.
At the meeting, Dr. Lev-Tov, associate professor of dermatology at the University of Miami, reviewed some of the latest developments in several conditions involving wound care.
Pyoderma gangrenosum (PG): In this condition, pustules or nodules become large ulcerations, and one-third of patients with PG have pathergy, exaggerated skin injury after a mild trauma such as a bump or a bruise.
“You want to look at the clues in the history because 20% of these patients had histories of PG elsewhere,” Dr. Lev-Tov said. “Ask them about other ulcers, maybe they had some wound dehiscence history.”
Criteria have been developed to help with the diagnosis of ulcerative PG, which includes one major criterion, a biopsy of the ulcer edge showing neutrophilic infiltrate, along with minor criteria, including exclusion of an infection, pathergy, and a history of inflammatory bowel disease or inflammatory arthritis.
“This is no longer a diagnosis of exclusion,” Dr. Lev-Tov said.
Cyclosporine and oral steroids have been found to work well, but it typically takes many months before healing occurs. Tacrolimus or topical steroids can work as well, but healing also takes a fairly long time with those medications, Dr. Lev-Tov said.
The tumor necrosis factor (TNF) blocker infliximab is another option. He had a patient who was referred to him who had been treated with cyclosporine for 3 years for PG on his feet, even though it had not been effective. Dr. Lev-Tov tried infliximab, and the wounds finally cleared, he said.
Apremilast, a phosphodiesterase 4 (PDE4)-inhibitor, is another option for treating PG, he said. “Anecdotally, I used apremilast on three patients with recurrent PG for long-term suppression, with success,” he noted.
Epidermal grafting using suction and heat is an approach that might deserve further exploration for PG, Dr. Lev-Tov suggested. With this procedure, described in an article in 2014, heat and suction are used to induce blistering to separate and remove the epidermis from the dermis at the dermal-epidermal junction, creating an epidermal graft is placed over the wound to promote healing. Patients with PG who are immunosuppressed but demonstrate pathergy do not tend to experience pathergy when epidermal skin grafting is performed, he said.
The heat-suction procedure is simple, painless, and scarless, but better controlled data on this approach are needed, he said.
Corona phlebectatica: This disease involving abnormally dilated veins near the ankle has received formal recognition as a sign of venous insufficiency, in a 2020 update of a classification system for describing patients with chronic venous disorders, Dr. Lev-Tov said.
“We knew about it for years, but now there’s some data that can actually predict the severity of disease,” and, he said, it is now a part of the diagnostic criteria for venous insufficiency .
Venous leg ulcers: These often painful sores on the inside of the leg typically take more than a month to heal. A systematic review of placebo-controlled studies of pentoxifylline as a treatment for venous leg ulcers, published in 2021, supports its use for healing venous leg ulcers, Dr. Lev-Tov said. “It improved the healing rate and increased what [the researchers] called ‘significant improvement,’ ” a category they created to account for the varying methods across the studies, he said.
Topical beta-blockers can improve epithelialization and fibroblast migration in wound healing, he said. A study on topical timolol for various wounds found that a 0.5% formulation of topical timolol, with one drop applied per square centimeter as frequently as possible, was effective in healing. But the healing process was prolonged – a median of 90 days, said Dr. Lev-Tov, one of the study authors.
“When you start this, I don’t want you to expect the wound to heal tomorrow,” he said. “You’ve got to educate your patient.”
Dr. Lev-Tov reports relevant financial relationships with Abbvie, Novartis, Pfizer and other companies.
AT ODAC 2023
Cognitive testing for older drivers: Is there a benefit?
, according to results from a large population-based study using data from Japan.
But the same study, published in the Journal of the American Geriatrics Society, also reported a concurrent increase in pedestrian and cycling injuries, possibly because more older former drivers were getting around by alternative means. That finding echoed a 2012 study from Denmark, which also looked at the effects of an age-based cognitive screening policy for older drivers, and saw more fatal road injuries among older people who were not driving.
While some governments, including those of Denmark, Taiwan, and Japan, have implemented age-based cognitive screening for older drivers, there has been little evidence to date that such policies improve road safety. Guidelines issued in 2010 by the American Academy of Neurology discourage age-based screening, advising instead that people diagnosed with cognitive disorders be carefully evaluated for driving fitness and recommending one widely used scale, the Clinical Dementia Rating, as useful in identifying potentially unsafe drivers.
Japan’s national screening policy: Did it work?
The new study, led by Haruhiko Inada, MD, PhD, an epidemiologist at Johns Hopkins University in Baltimore, used national crash data from Japan, where since 2017 all drivers 75 and older not only must take cognitive tests measuring temporal orientation and memory at license renewal, but are also referred for medical evaluation if they fail them. People receiving a subsequent dementia diagnosis can have their licenses suspended or revoked.
Dr. Inada and his colleagues looked at national data from nearly 603,000 police-reported vehicle collisions and nearly 197,000 pedestrian or cyclist road injuries between March 2012 and December 2019, all involving people aged 70 and older. To assess the screening policy’s impact, the researchers calculated estimated monthly collision or injury incidence rates per 100,000 person-years. This way, they could “control for secular trends that were unaffected by the policy, such as the decreasing incidence of motor vehicle collisions year by year,” the researchers explained.
After the screening was implemented, cumulative estimated collisions among drivers 75 or older decreased by 3,670 (95% confidence interval, 5,125-2,104), while reported pedestrian or cyclist injuries increased by an estimated 959 (95% CI, 24-1,834). Dr. Inada and colleagues found that crashes declined among men but not women, noting also that more older men than women are licensed to drive in Japan. Pedestrian and cyclist injuries were highest among men aged 80-84, and women aged 80 and older.
“Cognitively screening older drivers at license renewal and promoting voluntary surrender of licenses may prevent motor vehicle collisions,” Dr. Inada and his colleagues concluded. “However, they are associated with an increase in road injuries for older pedestrians and cyclists. Future studies should examine the effectiveness of mitigation measures, such as alternative, safe transportation, and accommodations for pedestrians and cyclists.”
No definitive answers
Two investigators who have studied cognitive screening related to road safety were contacted for commentary on the study findings.
Anu Siren, PhD, professor of gerontology at Tampere (Finland) University, who in 2012 reported higher injuries after implementation of older-driver cognitive screening in Denmark, commented that the new study, while benefiting from a much larger data set than earlier studies, still “fails to show that decrease in collisions is because ‘unfit’ drivers were removed from the road. But it does confirm previous findings about how strict screening policies make people shift from cars to unprotected modes of transportation,” which are riskier.
In studies measuring driving safety, the usual definition of risk is incidents per exposure, Dr. Siren noted. In Dr. Inada and colleagues’ study, “the incident measure, or numerator, is the number of collisions. The exposure measure or denominator is population. Because the study uses population and not driver licenses (or distance traveled) as an exposure measure, the observed decrease in collisions does not say much about how the collision risk develops after the implementation of screening.”
Older driver screening “is likely to cause some older persons to cease from driving and probably continue to travel as unprotected road users,” Dr. Siren continued. “Similar to what we found [in 2012], the injury rates for pedestrians and cyclists went up after the introduction of screening, which suggests that screening indirectly causes increasing number of injuries among older unprotected road users.”
Matthew Rizzo, MD, professor and chair of the department of neurological sciences at the University of Nebraska Medical Center and codirector of the Nebraska Neuroscience Alliance in Omaha, Neb., and the lead author of the 2010 AAN guidelines on cognitive impairment and driving risk, cautioned against ageism in designing policies meant to protect motorists.
“We find some erratic/weak effects of age here and there, but the big effects we consistently find are from cognitive and visual decline – which is somewhat correlated with age, but with huge variance,” Dr. Rizzo said. “It is hard to say what an optimal age threshold for risk would be, and if 75 is it.”
U.S. crash data from the last decade points to drivers 80 and older as significantly more accident-prone than those in their 70s, or even late 70s, Dr. Rizzo noted. Moreover, “willingness to get on the road, number of miles driven, type of road (urban, rural, highway, commercial, residential), type of vehicle driven, traffic, and environment (day, night, weather), et cetera, are all factors to consider in driving risk and restriction,” he said.
Dr. Rizzo added that the 2010 AAN guidelines might need to be revisited in light of newer vehicle safety systems and automation.
Dr. Inada and colleagues’ study was funded by Japanese government grants, and Dr. Inada and his coauthors reported no financial conflicts of interest. Dr. Siren and Dr. Rizzo reported no financial conflicts of interest.
, according to results from a large population-based study using data from Japan.
But the same study, published in the Journal of the American Geriatrics Society, also reported a concurrent increase in pedestrian and cycling injuries, possibly because more older former drivers were getting around by alternative means. That finding echoed a 2012 study from Denmark, which also looked at the effects of an age-based cognitive screening policy for older drivers, and saw more fatal road injuries among older people who were not driving.
While some governments, including those of Denmark, Taiwan, and Japan, have implemented age-based cognitive screening for older drivers, there has been little evidence to date that such policies improve road safety. Guidelines issued in 2010 by the American Academy of Neurology discourage age-based screening, advising instead that people diagnosed with cognitive disorders be carefully evaluated for driving fitness and recommending one widely used scale, the Clinical Dementia Rating, as useful in identifying potentially unsafe drivers.
Japan’s national screening policy: Did it work?
The new study, led by Haruhiko Inada, MD, PhD, an epidemiologist at Johns Hopkins University in Baltimore, used national crash data from Japan, where since 2017 all drivers 75 and older not only must take cognitive tests measuring temporal orientation and memory at license renewal, but are also referred for medical evaluation if they fail them. People receiving a subsequent dementia diagnosis can have their licenses suspended or revoked.
Dr. Inada and his colleagues looked at national data from nearly 603,000 police-reported vehicle collisions and nearly 197,000 pedestrian or cyclist road injuries between March 2012 and December 2019, all involving people aged 70 and older. To assess the screening policy’s impact, the researchers calculated estimated monthly collision or injury incidence rates per 100,000 person-years. This way, they could “control for secular trends that were unaffected by the policy, such as the decreasing incidence of motor vehicle collisions year by year,” the researchers explained.
After the screening was implemented, cumulative estimated collisions among drivers 75 or older decreased by 3,670 (95% confidence interval, 5,125-2,104), while reported pedestrian or cyclist injuries increased by an estimated 959 (95% CI, 24-1,834). Dr. Inada and colleagues found that crashes declined among men but not women, noting also that more older men than women are licensed to drive in Japan. Pedestrian and cyclist injuries were highest among men aged 80-84, and women aged 80 and older.
“Cognitively screening older drivers at license renewal and promoting voluntary surrender of licenses may prevent motor vehicle collisions,” Dr. Inada and his colleagues concluded. “However, they are associated with an increase in road injuries for older pedestrians and cyclists. Future studies should examine the effectiveness of mitigation measures, such as alternative, safe transportation, and accommodations for pedestrians and cyclists.”
No definitive answers
Two investigators who have studied cognitive screening related to road safety were contacted for commentary on the study findings.
Anu Siren, PhD, professor of gerontology at Tampere (Finland) University, who in 2012 reported higher injuries after implementation of older-driver cognitive screening in Denmark, commented that the new study, while benefiting from a much larger data set than earlier studies, still “fails to show that decrease in collisions is because ‘unfit’ drivers were removed from the road. But it does confirm previous findings about how strict screening policies make people shift from cars to unprotected modes of transportation,” which are riskier.
In studies measuring driving safety, the usual definition of risk is incidents per exposure, Dr. Siren noted. In Dr. Inada and colleagues’ study, “the incident measure, or numerator, is the number of collisions. The exposure measure or denominator is population. Because the study uses population and not driver licenses (or distance traveled) as an exposure measure, the observed decrease in collisions does not say much about how the collision risk develops after the implementation of screening.”
Older driver screening “is likely to cause some older persons to cease from driving and probably continue to travel as unprotected road users,” Dr. Siren continued. “Similar to what we found [in 2012], the injury rates for pedestrians and cyclists went up after the introduction of screening, which suggests that screening indirectly causes increasing number of injuries among older unprotected road users.”
Matthew Rizzo, MD, professor and chair of the department of neurological sciences at the University of Nebraska Medical Center and codirector of the Nebraska Neuroscience Alliance in Omaha, Neb., and the lead author of the 2010 AAN guidelines on cognitive impairment and driving risk, cautioned against ageism in designing policies meant to protect motorists.
“We find some erratic/weak effects of age here and there, but the big effects we consistently find are from cognitive and visual decline – which is somewhat correlated with age, but with huge variance,” Dr. Rizzo said. “It is hard to say what an optimal age threshold for risk would be, and if 75 is it.”
U.S. crash data from the last decade points to drivers 80 and older as significantly more accident-prone than those in their 70s, or even late 70s, Dr. Rizzo noted. Moreover, “willingness to get on the road, number of miles driven, type of road (urban, rural, highway, commercial, residential), type of vehicle driven, traffic, and environment (day, night, weather), et cetera, are all factors to consider in driving risk and restriction,” he said.
Dr. Rizzo added that the 2010 AAN guidelines might need to be revisited in light of newer vehicle safety systems and automation.
Dr. Inada and colleagues’ study was funded by Japanese government grants, and Dr. Inada and his coauthors reported no financial conflicts of interest. Dr. Siren and Dr. Rizzo reported no financial conflicts of interest.
, according to results from a large population-based study using data from Japan.
But the same study, published in the Journal of the American Geriatrics Society, also reported a concurrent increase in pedestrian and cycling injuries, possibly because more older former drivers were getting around by alternative means. That finding echoed a 2012 study from Denmark, which also looked at the effects of an age-based cognitive screening policy for older drivers, and saw more fatal road injuries among older people who were not driving.
While some governments, including those of Denmark, Taiwan, and Japan, have implemented age-based cognitive screening for older drivers, there has been little evidence to date that such policies improve road safety. Guidelines issued in 2010 by the American Academy of Neurology discourage age-based screening, advising instead that people diagnosed with cognitive disorders be carefully evaluated for driving fitness and recommending one widely used scale, the Clinical Dementia Rating, as useful in identifying potentially unsafe drivers.
Japan’s national screening policy: Did it work?
The new study, led by Haruhiko Inada, MD, PhD, an epidemiologist at Johns Hopkins University in Baltimore, used national crash data from Japan, where since 2017 all drivers 75 and older not only must take cognitive tests measuring temporal orientation and memory at license renewal, but are also referred for medical evaluation if they fail them. People receiving a subsequent dementia diagnosis can have their licenses suspended or revoked.
Dr. Inada and his colleagues looked at national data from nearly 603,000 police-reported vehicle collisions and nearly 197,000 pedestrian or cyclist road injuries between March 2012 and December 2019, all involving people aged 70 and older. To assess the screening policy’s impact, the researchers calculated estimated monthly collision or injury incidence rates per 100,000 person-years. This way, they could “control for secular trends that were unaffected by the policy, such as the decreasing incidence of motor vehicle collisions year by year,” the researchers explained.
After the screening was implemented, cumulative estimated collisions among drivers 75 or older decreased by 3,670 (95% confidence interval, 5,125-2,104), while reported pedestrian or cyclist injuries increased by an estimated 959 (95% CI, 24-1,834). Dr. Inada and colleagues found that crashes declined among men but not women, noting also that more older men than women are licensed to drive in Japan. Pedestrian and cyclist injuries were highest among men aged 80-84, and women aged 80 and older.
“Cognitively screening older drivers at license renewal and promoting voluntary surrender of licenses may prevent motor vehicle collisions,” Dr. Inada and his colleagues concluded. “However, they are associated with an increase in road injuries for older pedestrians and cyclists. Future studies should examine the effectiveness of mitigation measures, such as alternative, safe transportation, and accommodations for pedestrians and cyclists.”
No definitive answers
Two investigators who have studied cognitive screening related to road safety were contacted for commentary on the study findings.
Anu Siren, PhD, professor of gerontology at Tampere (Finland) University, who in 2012 reported higher injuries after implementation of older-driver cognitive screening in Denmark, commented that the new study, while benefiting from a much larger data set than earlier studies, still “fails to show that decrease in collisions is because ‘unfit’ drivers were removed from the road. But it does confirm previous findings about how strict screening policies make people shift from cars to unprotected modes of transportation,” which are riskier.
In studies measuring driving safety, the usual definition of risk is incidents per exposure, Dr. Siren noted. In Dr. Inada and colleagues’ study, “the incident measure, or numerator, is the number of collisions. The exposure measure or denominator is population. Because the study uses population and not driver licenses (or distance traveled) as an exposure measure, the observed decrease in collisions does not say much about how the collision risk develops after the implementation of screening.”
Older driver screening “is likely to cause some older persons to cease from driving and probably continue to travel as unprotected road users,” Dr. Siren continued. “Similar to what we found [in 2012], the injury rates for pedestrians and cyclists went up after the introduction of screening, which suggests that screening indirectly causes increasing number of injuries among older unprotected road users.”
Matthew Rizzo, MD, professor and chair of the department of neurological sciences at the University of Nebraska Medical Center and codirector of the Nebraska Neuroscience Alliance in Omaha, Neb., and the lead author of the 2010 AAN guidelines on cognitive impairment and driving risk, cautioned against ageism in designing policies meant to protect motorists.
“We find some erratic/weak effects of age here and there, but the big effects we consistently find are from cognitive and visual decline – which is somewhat correlated with age, but with huge variance,” Dr. Rizzo said. “It is hard to say what an optimal age threshold for risk would be, and if 75 is it.”
U.S. crash data from the last decade points to drivers 80 and older as significantly more accident-prone than those in their 70s, or even late 70s, Dr. Rizzo noted. Moreover, “willingness to get on the road, number of miles driven, type of road (urban, rural, highway, commercial, residential), type of vehicle driven, traffic, and environment (day, night, weather), et cetera, are all factors to consider in driving risk and restriction,” he said.
Dr. Rizzo added that the 2010 AAN guidelines might need to be revisited in light of newer vehicle safety systems and automation.
Dr. Inada and colleagues’ study was funded by Japanese government grants, and Dr. Inada and his coauthors reported no financial conflicts of interest. Dr. Siren and Dr. Rizzo reported no financial conflicts of interest.
FROM THE JOURNAL OF THE AMERICAN GERIATRICS SOCIETY
CV deaths jumped in 2020, reflecting pandemic toll
Cardiovascular-related deaths increased dramatically in 2020, marking the largest single-year increase since 2015 and surpassing the previous record from 2003, according to the American Heart Association’s 2023 Statistical Update.
During the first year of the COVID-19 pandemic, the largest increases in cardiovascular disease (CVD) deaths were seen among Asian, Black, and Hispanic people.
“We thought we had been improving as a country with respect to CVD deaths over the past few decades,” Connie Tsao, MD, chair of the AHA Statistical Update writing committee, told this news organization.
Since 2020, however, those trends have changed. Dr. Tsao, a staff cardiologist at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, both in Boston, noted the firsthand experience that many clinicians had in seeing the shift.
“We observed this sharp rise in age-adjusted CVD deaths, which corresponds to the COVID-19 pandemic,” she said. “Those of us health care providers knew from the overfull hospitals and ICUs that clearly COVID took a toll, particularly in those with cardiovascular risk factors.”
The AHA Statistical Update was published online in the journal Circulation.
Data on deaths
Each year, the American Heart Association and National Institutes of Health report the latest statistics related to heart disease, stroke, and cardiovascular risk factors. The 2023 update includes additional information about pandemic-related data.
Overall, the number of people who died from cardiovascular disease increased during the first year of the pandemic, rising from 876,613 in 2019 to 928,741 in 2020. This topped the previous high of 910,000 in 2003.
In addition, the age-adjusted mortality rate increased for the first time in several years, Dr. Tsao said, by a “fairly substantial” 4.6%. The age-adjusted mortality rate incorporates the variability in the aging population from year to year, accounting for higher death rates among older people.
“Even though our total number of deaths has been slowly increasing over the past decade, we have seen a decline each year in our age-adjusted rates – until 2020,” she said. “I think that is very indicative of what has been going on within our country – and the world – in light of people of all ages being impacted by the COVID-19 pandemic, especially before vaccines were available to slow the spread.”
The largest increases in CVD-related deaths occurred among Asian, Black, and Hispanic people, who were most heavily affected during the first year of the pandemic.
“People from communities of color were among those most highly impacted, especially early on, often due to a disproportionate burden of cardiovascular risk factors, such as hypertension and obesity,” Michelle Albert, MD, MPH, president of AHA and a professor of medicine at the University of California, San Francisco, said in a statement.
Dr. Albert, who is also the director of UCSF’s Center for the Study of Adversity and Cardiovascular Disease, does research on health equity and noted the disparities seen in the 2020 numbers. “Additionally, there are socioeconomic considerations, as well as the ongoing impact of structural racism on multiple factors, including limiting the ability to access quality health care,” she said.
Additional considerations
In a special commentary, the Statistical Update writing committee pointed to the need to track data for other underrepresented communities, including LGBTQ people and those living in rural or urban areas. The authors outlined several ways to better understand the effects of identity and social determinants of health, as well as strategies to reduce cardiovascular-related disparities.
“This year’s writing group made a concerted effort to gather information on specific social factors related to health risk and outcomes, including sexual orientation, gender identity, urbanization, and socioeconomic position,” Dr. Tsao said. “However, the data are lacking because these communities are grossly underrepresented in clinical and epidemiological research.”
For the next several years, the AHA Statistical Update will likely include more insights about the effects of the COVID-19 pandemic, as well as ongoing disparities.
“For sure, we will be continuing to see the effects of the pandemic for years to come,” Dr. Tsao said. “Recognition of the disparities in outcomes among vulnerable groups should be a call to action among health care providers and researchers, administration, and policy leaders to investigate the reasons and make changes to reverse these trends.”
The statistical update was prepared by a volunteer writing group on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee.
A version of this article first appeared on Medscape.com.
Cardiovascular-related deaths increased dramatically in 2020, marking the largest single-year increase since 2015 and surpassing the previous record from 2003, according to the American Heart Association’s 2023 Statistical Update.
During the first year of the COVID-19 pandemic, the largest increases in cardiovascular disease (CVD) deaths were seen among Asian, Black, and Hispanic people.
“We thought we had been improving as a country with respect to CVD deaths over the past few decades,” Connie Tsao, MD, chair of the AHA Statistical Update writing committee, told this news organization.
Since 2020, however, those trends have changed. Dr. Tsao, a staff cardiologist at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, both in Boston, noted the firsthand experience that many clinicians had in seeing the shift.
“We observed this sharp rise in age-adjusted CVD deaths, which corresponds to the COVID-19 pandemic,” she said. “Those of us health care providers knew from the overfull hospitals and ICUs that clearly COVID took a toll, particularly in those with cardiovascular risk factors.”
The AHA Statistical Update was published online in the journal Circulation.
Data on deaths
Each year, the American Heart Association and National Institutes of Health report the latest statistics related to heart disease, stroke, and cardiovascular risk factors. The 2023 update includes additional information about pandemic-related data.
Overall, the number of people who died from cardiovascular disease increased during the first year of the pandemic, rising from 876,613 in 2019 to 928,741 in 2020. This topped the previous high of 910,000 in 2003.
In addition, the age-adjusted mortality rate increased for the first time in several years, Dr. Tsao said, by a “fairly substantial” 4.6%. The age-adjusted mortality rate incorporates the variability in the aging population from year to year, accounting for higher death rates among older people.
“Even though our total number of deaths has been slowly increasing over the past decade, we have seen a decline each year in our age-adjusted rates – until 2020,” she said. “I think that is very indicative of what has been going on within our country – and the world – in light of people of all ages being impacted by the COVID-19 pandemic, especially before vaccines were available to slow the spread.”
The largest increases in CVD-related deaths occurred among Asian, Black, and Hispanic people, who were most heavily affected during the first year of the pandemic.
“People from communities of color were among those most highly impacted, especially early on, often due to a disproportionate burden of cardiovascular risk factors, such as hypertension and obesity,” Michelle Albert, MD, MPH, president of AHA and a professor of medicine at the University of California, San Francisco, said in a statement.
Dr. Albert, who is also the director of UCSF’s Center for the Study of Adversity and Cardiovascular Disease, does research on health equity and noted the disparities seen in the 2020 numbers. “Additionally, there are socioeconomic considerations, as well as the ongoing impact of structural racism on multiple factors, including limiting the ability to access quality health care,” she said.
Additional considerations
In a special commentary, the Statistical Update writing committee pointed to the need to track data for other underrepresented communities, including LGBTQ people and those living in rural or urban areas. The authors outlined several ways to better understand the effects of identity and social determinants of health, as well as strategies to reduce cardiovascular-related disparities.
“This year’s writing group made a concerted effort to gather information on specific social factors related to health risk and outcomes, including sexual orientation, gender identity, urbanization, and socioeconomic position,” Dr. Tsao said. “However, the data are lacking because these communities are grossly underrepresented in clinical and epidemiological research.”
For the next several years, the AHA Statistical Update will likely include more insights about the effects of the COVID-19 pandemic, as well as ongoing disparities.
“For sure, we will be continuing to see the effects of the pandemic for years to come,” Dr. Tsao said. “Recognition of the disparities in outcomes among vulnerable groups should be a call to action among health care providers and researchers, administration, and policy leaders to investigate the reasons and make changes to reverse these trends.”
The statistical update was prepared by a volunteer writing group on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee.
A version of this article first appeared on Medscape.com.
Cardiovascular-related deaths increased dramatically in 2020, marking the largest single-year increase since 2015 and surpassing the previous record from 2003, according to the American Heart Association’s 2023 Statistical Update.
During the first year of the COVID-19 pandemic, the largest increases in cardiovascular disease (CVD) deaths were seen among Asian, Black, and Hispanic people.
“We thought we had been improving as a country with respect to CVD deaths over the past few decades,” Connie Tsao, MD, chair of the AHA Statistical Update writing committee, told this news organization.
Since 2020, however, those trends have changed. Dr. Tsao, a staff cardiologist at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, both in Boston, noted the firsthand experience that many clinicians had in seeing the shift.
“We observed this sharp rise in age-adjusted CVD deaths, which corresponds to the COVID-19 pandemic,” she said. “Those of us health care providers knew from the overfull hospitals and ICUs that clearly COVID took a toll, particularly in those with cardiovascular risk factors.”
The AHA Statistical Update was published online in the journal Circulation.
Data on deaths
Each year, the American Heart Association and National Institutes of Health report the latest statistics related to heart disease, stroke, and cardiovascular risk factors. The 2023 update includes additional information about pandemic-related data.
Overall, the number of people who died from cardiovascular disease increased during the first year of the pandemic, rising from 876,613 in 2019 to 928,741 in 2020. This topped the previous high of 910,000 in 2003.
In addition, the age-adjusted mortality rate increased for the first time in several years, Dr. Tsao said, by a “fairly substantial” 4.6%. The age-adjusted mortality rate incorporates the variability in the aging population from year to year, accounting for higher death rates among older people.
“Even though our total number of deaths has been slowly increasing over the past decade, we have seen a decline each year in our age-adjusted rates – until 2020,” she said. “I think that is very indicative of what has been going on within our country – and the world – in light of people of all ages being impacted by the COVID-19 pandemic, especially before vaccines were available to slow the spread.”
The largest increases in CVD-related deaths occurred among Asian, Black, and Hispanic people, who were most heavily affected during the first year of the pandemic.
“People from communities of color were among those most highly impacted, especially early on, often due to a disproportionate burden of cardiovascular risk factors, such as hypertension and obesity,” Michelle Albert, MD, MPH, president of AHA and a professor of medicine at the University of California, San Francisco, said in a statement.
Dr. Albert, who is also the director of UCSF’s Center for the Study of Adversity and Cardiovascular Disease, does research on health equity and noted the disparities seen in the 2020 numbers. “Additionally, there are socioeconomic considerations, as well as the ongoing impact of structural racism on multiple factors, including limiting the ability to access quality health care,” she said.
Additional considerations
In a special commentary, the Statistical Update writing committee pointed to the need to track data for other underrepresented communities, including LGBTQ people and those living in rural or urban areas. The authors outlined several ways to better understand the effects of identity and social determinants of health, as well as strategies to reduce cardiovascular-related disparities.
“This year’s writing group made a concerted effort to gather information on specific social factors related to health risk and outcomes, including sexual orientation, gender identity, urbanization, and socioeconomic position,” Dr. Tsao said. “However, the data are lacking because these communities are grossly underrepresented in clinical and epidemiological research.”
For the next several years, the AHA Statistical Update will likely include more insights about the effects of the COVID-19 pandemic, as well as ongoing disparities.
“For sure, we will be continuing to see the effects of the pandemic for years to come,” Dr. Tsao said. “Recognition of the disparities in outcomes among vulnerable groups should be a call to action among health care providers and researchers, administration, and policy leaders to investigate the reasons and make changes to reverse these trends.”
The statistical update was prepared by a volunteer writing group on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Positive top-line results for novel psychedelic in major depression
Top-line results from a phase 2a study of SPL026 (intravenous N,N-Dimethyltryptamine [DMT]) showed a 57% remission rate 3 months after participants received a single dose of the drug, the developer reports.
Small Pharma noted in a press release that this is the first placebo-controlled efficacy trial of a short-duration psychedelic for depression completed to date.
Investigators reported significant improvement in depression symptoms 2 weeks after dosing, which was the primary endpoint, and the improvement persisted at week 12.
“We now have the first evidence that SPL026 DMT, combined with supportive therapy, may be effective for people suffering from MDD,” chief investigator David Erritzoe, MD, PhD, clinical psychiatrist at Imperial College London, said in a statement.
“For patients who are unfortunate to experience little benefit from existing antidepressants, the potential for rapid and durable relief from a single treatment, as shown in this trial, is very promising,” Dr. Erritzoe added.
Randomized trial results
The blinded, randomized, placebo-controlled, two-staged phase 2a study included 34 patients with moderate to severe MDD. Those who were taking pharmacological antidepressant medication at baseline stopped taking the medication prior to dosing with SPL026.
Patients received a placebo (n = 17) or active treatment (n = 17). The latter consisted of a short IV infusion of 21.5 mg of SPL026, resulting in a 20- to 30-minute psychedelic experience, and supportive therapy.
The dose was selected based on data analysis from the company’s phase 1 study in healthy volunteers.
Efficacy was assessed using the Montgomery-Asberg Depression Rating Scale (MADRS) to measure changes in MDD symptoms.
Two weeks after dosing, those receiving the novel therapy showed a significant reduction in depressive symptoms, demonstrating a –7.4-point difference versus the placebo group in MADRS score (P = .02).
Analysis of key secondary endpoints showed a rapid onset of antidepressant effect 1 week post-dose, with a statistically significant difference in MADRS score between the active and placebo groups of –10.8 points (P = .002).
Next steps?
All participants were subsequently enrolled into an open-label phase of the trial where they received a single dose of SPL026 with supportive therapy. They were then followed for a further 12 weeks.
In the open-label phase, patients who received at least one active dose of SPL026 with supportive therapy reported a durable improvement in depression symptoms.
No apparent difference in antidepressant effect was observed between a one- or two-dose regimen of SPL026.
“SPL026 with supportive therapy was shown to have a significant antidepressant effect that was rapid and durable,” Carol Routledge, PhD, chief medical and scientific officer at Small Pharma, said in the statement.
“The results are clinically meaningful and enable us to progress into an international multisite phase 2b study where we seek to further explore the efficacy and safety profile of SPL026 in a larger MDD patient population,” Dr. Routledge added.
A version of this article first appeared on Medscape.com.
Top-line results from a phase 2a study of SPL026 (intravenous N,N-Dimethyltryptamine [DMT]) showed a 57% remission rate 3 months after participants received a single dose of the drug, the developer reports.
Small Pharma noted in a press release that this is the first placebo-controlled efficacy trial of a short-duration psychedelic for depression completed to date.
Investigators reported significant improvement in depression symptoms 2 weeks after dosing, which was the primary endpoint, and the improvement persisted at week 12.
“We now have the first evidence that SPL026 DMT, combined with supportive therapy, may be effective for people suffering from MDD,” chief investigator David Erritzoe, MD, PhD, clinical psychiatrist at Imperial College London, said in a statement.
“For patients who are unfortunate to experience little benefit from existing antidepressants, the potential for rapid and durable relief from a single treatment, as shown in this trial, is very promising,” Dr. Erritzoe added.
Randomized trial results
The blinded, randomized, placebo-controlled, two-staged phase 2a study included 34 patients with moderate to severe MDD. Those who were taking pharmacological antidepressant medication at baseline stopped taking the medication prior to dosing with SPL026.
Patients received a placebo (n = 17) or active treatment (n = 17). The latter consisted of a short IV infusion of 21.5 mg of SPL026, resulting in a 20- to 30-minute psychedelic experience, and supportive therapy.
The dose was selected based on data analysis from the company’s phase 1 study in healthy volunteers.
Efficacy was assessed using the Montgomery-Asberg Depression Rating Scale (MADRS) to measure changes in MDD symptoms.
Two weeks after dosing, those receiving the novel therapy showed a significant reduction in depressive symptoms, demonstrating a –7.4-point difference versus the placebo group in MADRS score (P = .02).
Analysis of key secondary endpoints showed a rapid onset of antidepressant effect 1 week post-dose, with a statistically significant difference in MADRS score between the active and placebo groups of –10.8 points (P = .002).
Next steps?
All participants were subsequently enrolled into an open-label phase of the trial where they received a single dose of SPL026 with supportive therapy. They were then followed for a further 12 weeks.
In the open-label phase, patients who received at least one active dose of SPL026 with supportive therapy reported a durable improvement in depression symptoms.
No apparent difference in antidepressant effect was observed between a one- or two-dose regimen of SPL026.
“SPL026 with supportive therapy was shown to have a significant antidepressant effect that was rapid and durable,” Carol Routledge, PhD, chief medical and scientific officer at Small Pharma, said in the statement.
“The results are clinically meaningful and enable us to progress into an international multisite phase 2b study where we seek to further explore the efficacy and safety profile of SPL026 in a larger MDD patient population,” Dr. Routledge added.
A version of this article first appeared on Medscape.com.
Top-line results from a phase 2a study of SPL026 (intravenous N,N-Dimethyltryptamine [DMT]) showed a 57% remission rate 3 months after participants received a single dose of the drug, the developer reports.
Small Pharma noted in a press release that this is the first placebo-controlled efficacy trial of a short-duration psychedelic for depression completed to date.
Investigators reported significant improvement in depression symptoms 2 weeks after dosing, which was the primary endpoint, and the improvement persisted at week 12.
“We now have the first evidence that SPL026 DMT, combined with supportive therapy, may be effective for people suffering from MDD,” chief investigator David Erritzoe, MD, PhD, clinical psychiatrist at Imperial College London, said in a statement.
“For patients who are unfortunate to experience little benefit from existing antidepressants, the potential for rapid and durable relief from a single treatment, as shown in this trial, is very promising,” Dr. Erritzoe added.
Randomized trial results
The blinded, randomized, placebo-controlled, two-staged phase 2a study included 34 patients with moderate to severe MDD. Those who were taking pharmacological antidepressant medication at baseline stopped taking the medication prior to dosing with SPL026.
Patients received a placebo (n = 17) or active treatment (n = 17). The latter consisted of a short IV infusion of 21.5 mg of SPL026, resulting in a 20- to 30-minute psychedelic experience, and supportive therapy.
The dose was selected based on data analysis from the company’s phase 1 study in healthy volunteers.
Efficacy was assessed using the Montgomery-Asberg Depression Rating Scale (MADRS) to measure changes in MDD symptoms.
Two weeks after dosing, those receiving the novel therapy showed a significant reduction in depressive symptoms, demonstrating a –7.4-point difference versus the placebo group in MADRS score (P = .02).
Analysis of key secondary endpoints showed a rapid onset of antidepressant effect 1 week post-dose, with a statistically significant difference in MADRS score between the active and placebo groups of –10.8 points (P = .002).
Next steps?
All participants were subsequently enrolled into an open-label phase of the trial where they received a single dose of SPL026 with supportive therapy. They were then followed for a further 12 weeks.
In the open-label phase, patients who received at least one active dose of SPL026 with supportive therapy reported a durable improvement in depression symptoms.
No apparent difference in antidepressant effect was observed between a one- or two-dose regimen of SPL026.
“SPL026 with supportive therapy was shown to have a significant antidepressant effect that was rapid and durable,” Carol Routledge, PhD, chief medical and scientific officer at Small Pharma, said in the statement.
“The results are clinically meaningful and enable us to progress into an international multisite phase 2b study where we seek to further explore the efficacy and safety profile of SPL026 in a larger MDD patient population,” Dr. Routledge added.
A version of this article first appeared on Medscape.com.
Decoding endometriosis: Recent research fosters hope
Roughly 4 decades after she first started menstruating, Elizabeth Flanagan finally underwent surgery to repair damage wreaked on her body by endometriosis. She’d spent years struggling with a variety of seemingly random symptoms, from migraines to excruciatingly painful periods to fatigue and irritable bowel syndrome. She’d worried about abnormal labs, including “extremely high” ANA, creatinine, and BUN blood test results that had been out of normal range for more than 10 years.
She was diagnosed with endometriosis in 2016, at age 47, after surgery to remove an ovarian cyst. Still, it took 5 more years before she landed in the office of a surgeon with the proper training to excise the lesions that continued to cause her so much anguish. That physician, Matthew Siedhoff, MD, at Cedars-Sinai Medical Center in Los Angeles, explained why her creatinine and BUN results were so far out of range: The endometriosis was impinging on her ureters.
The appointment left Ms. Flanagan with a range of emotions. “I was shocked that no doctor had identified this before, relieved knowing that I was finally in the hands of an expert who understood my condition, and saddened by the dearth of knowledge and proper treatment of endometriosis,” she wrote in an email.
Although the disease afflicts at least 1 out of every 10 women, endometriosis remains a conundrum for patients and their physicians. It often masquerades as other problems, from mental health issues such as anxiety and depression to physical issues such as irritable bowel syndrome. It often coexists with autoimmune conditions. Short of performing surgery, it can be a diagnosis of exclusion. And the existing, state-of-the-art treatment – hormone therapy that shuts down the reproductive system – doesn’t work for every woman every time.
“It is no wonder that it takes 10 years on average, from the time someone has symptoms of endometriosis, until they get a definitive diagnosis,” said Hugh Taylor, MD, chair of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn. “It’s a combination of [physicians] not taking painful menses seriously and getting distracted by all these other manifestations of the disease throughout the whole body.”
Endometriosis, he said, “is a whole-body disease.”
But recent genetic research offers the tantalizing prospect of new diagnostic tools and treatments. In 5-10 years, scientists say, physicians may be able to diagnose the disease with a simple blood test, and treat it, for example, by preventing a gene receptor from initiating a cascade of inflammatory effects, or crafting treatments tailored to the molecular makeup of a patient’s disease.
“Tomorrow’s therapies will target specifically the molecular defects of endometriosis and be nonhormonal,” Dr. Taylor said.
Guidelines published last year by the European Society of Human Reproduction and Embryology detail the latest standards for diagnosis and treatment of endometriosis.
According to the guidelines, physicians should consider the diagnosis of endometriosis in individuals presenting with the following cyclical and noncyclical signs and symptoms: dysmenorrhea, deep dyspareunia, dysuria, dyschezia, painful rectal bleeding or hematuria, shoulder tip pain, catamenial pneumothorax, cyclical cough/hemoptysis/chest pain, cyclical scar swelling, and pain, fatigue, and infertility.
A clinical exam should be considered, as well as imaging such as ultrasound and/or MRI, the guidelines state, although negative findings should not rule out a diagnosis. Laparoscopy is also an option, particularly for patients who desire a definitive diagnosis or cannot be diagnosed any other way, “although negative histology [of endometriotic lesions] does not entirely rule out the disease,” the guidelines state.
To treat the pain associated with endometriosis, the guidelines advise, as a first-line therapy, beginning with NSAIDs and combined hormonal contraceptives (in oral, vaginal, or transdermal form). Another option is progesterone, including progesterone-only contraceptives, with a recommendation to prescribe a levonorgestrel-releasing intrauterine system or an etonogestrel-releasing subdermal implant to reduce endometriosis-associated pain.
However, progestins and low-dose oral contraceptives are “unsuccessful in a third of women,” Dr. Taylor and his coauthors wrote in a paper published in 2021 in The Lancet.
Until recently, the gold standard for second-line treatment of endometriosis was oral gonadotropin-releasing hormone (GnRH) agonists. These manage the disease by inducing medical menopause – they downregulate pituitary GnRH receptors to create a hypoestrogenic state characterized by low serum levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). GnRH agonists may be administered nasally, or through daily, monthly, or trimonthly injections. But the Food and Drug Administration advises that, when used for longer than 6 months, GnRH agonists be paired with add-back hormone replacement therapy to reduce the risk of bone loss associated with the plunge in hormone levels. Also, treatment may not be appropriate for patients who, when suddenly forced into menopause, suffer from bothersome symptoms.
The latest treatment, GnRH antagonists, are new options for patients who either do not respond adequately to progestins and low-dose contraceptives or develop progesterone resistance, and want to avoid some of the risks and/or symptoms associated with GnRH agonists. Two advantages of GnRH antagonists for patients, Dr. Taylor said, are that they have a fast onset of action and are oral rather than injectable.
“These drugs [GnRH antagonists] cause competitive blockage of the GnRH receptor and hence dose-dependently suppress production of FSH and LH and inhibit secretion of ovarian steroid hormones without inducing a flare-up effect,” Belgian physicians and researchers Jacques Donnez, MD, and Marie-Madeleine Dolmans, MD, PhD, wrote in a paper published last year in the Journal of Clinical Medicine. “The mechanism is different from that of the GnRH agonist which, after a first phase of stimulation, desensitizes GnRH receptors, leading to full suppression of LH and FSH production and subsequently to complete suppression of [estrogen] to levels similar to those observed after bilateral oophorectomy.”
Patients who took Elagolix, the first oral nonpeptide GnRH antagonist available for the treatment of moderate to severe endometriosis-associated pain, had fewer vasomotor side effects and less bone density loss than those on the GnRH agonist leuprorelin, according to a 2018 study in Obstetrics and Gynecology. However, without add-back hormone-replacement therapy, GnRH antagonist use may need to be limited to 24 months, because of loss of bone density, a study in Cell Reports Medicine reported in 2022.
Attempting to explain the pathogenesis of endometriosis, and frustrated by the shortcomings of currently available therapies, researchers have turned to genetics for insight. A team of scientists led by Thomas Tapmeier, PhD, now a senior research fellow at Monash University in Australia, and Prof. Krina Zondervan at the University of Oxford, ran genetic analyses of families with a history of endometriosis, as well as rhesus macaques that spontaneously developed endometriosis. The research, published in Science Translational Medicine, identified NPSR1, the gene encoding neuropeptide S receptor 1, as one commonly associated with endometriosis. In trials with mouse models, they found that the NPSR1 inhibitor SHA 68R was able to reduce endometriosis-related inflammation and pain.
“It’s important to stress that there is no single gene that is responsible for endometriosis,” Dr. Tapmeier said in an interview. “This gene just has a higher frequency in people with endometriosis.”
The next step, then, would be to try to find a compound that would inhibit NPSR1 at some point, or a competitor to the ligand that binds to the receptor and blocks it, he said.
“We’re currently looking at compounds that might be able to inhibit the receptor signaling,” he said.
Such a therapy could potentially reduce the symptoms of endometriosis without interfering with the menstrual cycle and without introducing hormones that cause undesirable side effects in some patients.
“This might be a way to treat the pain and inflammation that goes with endometriosis, as well as leaving the possibility of pregnancy open,” he said.
Other researchers are searching for biomarkers of the disease, both to provide a definitive, nonsurgical diagnostic tool, and for potential, individualized treatment.
In a study published in Nature Genetics, researchers at Cedars-Sinai created a “cellular atlas” of endometriosis by analyzing nearly 400,000 individual cells from 21 patients, some of whom had the disease and some of whom did not. A new technology, single-cell genomics, allowed the scientists to profile the multiple cell types contributing to the disease.
“So the initial question we wanted to ask was about understanding how the cells look in endometriosis, compared to endometrium,” said Kate Lawrenson, PhD, an associate professor in the department of obstetrics and gynecology at Cedars-Sinai, and co–senior author of the study. “We know that they resemble the cells of the womb, but we really don’t understand if they behave the same. We had a good inkling that they would behave differently.”
It turned out they did: Cells of endometriosis interacted atypically with female hormones, compared with cells in the uterus, Dr. Lawrenson said.
“That helps us understand how, even when patients take contraceptive pills, which is a commonly prescribed therapy, it doesn’t always work, or sometimes it stops working after a while,” she said. The next step for researchers, she said, will be to pinpoint the specific causes of these altered interactions.
Meanwhile, the current research also points to diagnostic possibilities. “We were quite excited to see that multiple cell types and endometriosis are upregulating the same sets of genes,” she said. “That makes us optimistic that hopefully there are some protein gene products that are being made in abundance, and hopefully we can detect them in the blood stream. It might be that we could use that information to develop new biomarkers, or even risk stratification tools.”
In the future, a simple blood test could identify signs of endometriosis in at-risk patients and get them “fast-tracked to a specialist for evaluation,” she said. “Whereas now, they might go from PCP to gynecologist to a different gynecologist over the course of 5-10 years before they get that referral.”
This discovery, that endometrial cells use genes differently and cross-talk with nearby cells differently, presents new treatment possibilities. Maybe we can physically block how cells interact with nearby cells, Dr. Lawrenson said. One model for doing that, she said, would be antibody-based therapy, similar to the therapies now changing the treatment of cancer.
What’s most exciting, looking ahead 5-10 years, is that treatment for endometriosis in the future may be significantly more individualized, and less hormone-based, than it is today.
“What we need for endometriosis is more options for patients and something that is tailored to the molecular makeup of their disease rather than a process of trial and error,” she said.
Roughly 4 decades after she first started menstruating, Elizabeth Flanagan finally underwent surgery to repair damage wreaked on her body by endometriosis. She’d spent years struggling with a variety of seemingly random symptoms, from migraines to excruciatingly painful periods to fatigue and irritable bowel syndrome. She’d worried about abnormal labs, including “extremely high” ANA, creatinine, and BUN blood test results that had been out of normal range for more than 10 years.
She was diagnosed with endometriosis in 2016, at age 47, after surgery to remove an ovarian cyst. Still, it took 5 more years before she landed in the office of a surgeon with the proper training to excise the lesions that continued to cause her so much anguish. That physician, Matthew Siedhoff, MD, at Cedars-Sinai Medical Center in Los Angeles, explained why her creatinine and BUN results were so far out of range: The endometriosis was impinging on her ureters.
The appointment left Ms. Flanagan with a range of emotions. “I was shocked that no doctor had identified this before, relieved knowing that I was finally in the hands of an expert who understood my condition, and saddened by the dearth of knowledge and proper treatment of endometriosis,” she wrote in an email.
Although the disease afflicts at least 1 out of every 10 women, endometriosis remains a conundrum for patients and their physicians. It often masquerades as other problems, from mental health issues such as anxiety and depression to physical issues such as irritable bowel syndrome. It often coexists with autoimmune conditions. Short of performing surgery, it can be a diagnosis of exclusion. And the existing, state-of-the-art treatment – hormone therapy that shuts down the reproductive system – doesn’t work for every woman every time.
“It is no wonder that it takes 10 years on average, from the time someone has symptoms of endometriosis, until they get a definitive diagnosis,” said Hugh Taylor, MD, chair of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn. “It’s a combination of [physicians] not taking painful menses seriously and getting distracted by all these other manifestations of the disease throughout the whole body.”
Endometriosis, he said, “is a whole-body disease.”
But recent genetic research offers the tantalizing prospect of new diagnostic tools and treatments. In 5-10 years, scientists say, physicians may be able to diagnose the disease with a simple blood test, and treat it, for example, by preventing a gene receptor from initiating a cascade of inflammatory effects, or crafting treatments tailored to the molecular makeup of a patient’s disease.
“Tomorrow’s therapies will target specifically the molecular defects of endometriosis and be nonhormonal,” Dr. Taylor said.
Guidelines published last year by the European Society of Human Reproduction and Embryology detail the latest standards for diagnosis and treatment of endometriosis.
According to the guidelines, physicians should consider the diagnosis of endometriosis in individuals presenting with the following cyclical and noncyclical signs and symptoms: dysmenorrhea, deep dyspareunia, dysuria, dyschezia, painful rectal bleeding or hematuria, shoulder tip pain, catamenial pneumothorax, cyclical cough/hemoptysis/chest pain, cyclical scar swelling, and pain, fatigue, and infertility.
A clinical exam should be considered, as well as imaging such as ultrasound and/or MRI, the guidelines state, although negative findings should not rule out a diagnosis. Laparoscopy is also an option, particularly for patients who desire a definitive diagnosis or cannot be diagnosed any other way, “although negative histology [of endometriotic lesions] does not entirely rule out the disease,” the guidelines state.
To treat the pain associated with endometriosis, the guidelines advise, as a first-line therapy, beginning with NSAIDs and combined hormonal contraceptives (in oral, vaginal, or transdermal form). Another option is progesterone, including progesterone-only contraceptives, with a recommendation to prescribe a levonorgestrel-releasing intrauterine system or an etonogestrel-releasing subdermal implant to reduce endometriosis-associated pain.
However, progestins and low-dose oral contraceptives are “unsuccessful in a third of women,” Dr. Taylor and his coauthors wrote in a paper published in 2021 in The Lancet.
Until recently, the gold standard for second-line treatment of endometriosis was oral gonadotropin-releasing hormone (GnRH) agonists. These manage the disease by inducing medical menopause – they downregulate pituitary GnRH receptors to create a hypoestrogenic state characterized by low serum levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). GnRH agonists may be administered nasally, or through daily, monthly, or trimonthly injections. But the Food and Drug Administration advises that, when used for longer than 6 months, GnRH agonists be paired with add-back hormone replacement therapy to reduce the risk of bone loss associated with the plunge in hormone levels. Also, treatment may not be appropriate for patients who, when suddenly forced into menopause, suffer from bothersome symptoms.
The latest treatment, GnRH antagonists, are new options for patients who either do not respond adequately to progestins and low-dose contraceptives or develop progesterone resistance, and want to avoid some of the risks and/or symptoms associated with GnRH agonists. Two advantages of GnRH antagonists for patients, Dr. Taylor said, are that they have a fast onset of action and are oral rather than injectable.
“These drugs [GnRH antagonists] cause competitive blockage of the GnRH receptor and hence dose-dependently suppress production of FSH and LH and inhibit secretion of ovarian steroid hormones without inducing a flare-up effect,” Belgian physicians and researchers Jacques Donnez, MD, and Marie-Madeleine Dolmans, MD, PhD, wrote in a paper published last year in the Journal of Clinical Medicine. “The mechanism is different from that of the GnRH agonist which, after a first phase of stimulation, desensitizes GnRH receptors, leading to full suppression of LH and FSH production and subsequently to complete suppression of [estrogen] to levels similar to those observed after bilateral oophorectomy.”
Patients who took Elagolix, the first oral nonpeptide GnRH antagonist available for the treatment of moderate to severe endometriosis-associated pain, had fewer vasomotor side effects and less bone density loss than those on the GnRH agonist leuprorelin, according to a 2018 study in Obstetrics and Gynecology. However, without add-back hormone-replacement therapy, GnRH antagonist use may need to be limited to 24 months, because of loss of bone density, a study in Cell Reports Medicine reported in 2022.
Attempting to explain the pathogenesis of endometriosis, and frustrated by the shortcomings of currently available therapies, researchers have turned to genetics for insight. A team of scientists led by Thomas Tapmeier, PhD, now a senior research fellow at Monash University in Australia, and Prof. Krina Zondervan at the University of Oxford, ran genetic analyses of families with a history of endometriosis, as well as rhesus macaques that spontaneously developed endometriosis. The research, published in Science Translational Medicine, identified NPSR1, the gene encoding neuropeptide S receptor 1, as one commonly associated with endometriosis. In trials with mouse models, they found that the NPSR1 inhibitor SHA 68R was able to reduce endometriosis-related inflammation and pain.
“It’s important to stress that there is no single gene that is responsible for endometriosis,” Dr. Tapmeier said in an interview. “This gene just has a higher frequency in people with endometriosis.”
The next step, then, would be to try to find a compound that would inhibit NPSR1 at some point, or a competitor to the ligand that binds to the receptor and blocks it, he said.
“We’re currently looking at compounds that might be able to inhibit the receptor signaling,” he said.
Such a therapy could potentially reduce the symptoms of endometriosis without interfering with the menstrual cycle and without introducing hormones that cause undesirable side effects in some patients.
“This might be a way to treat the pain and inflammation that goes with endometriosis, as well as leaving the possibility of pregnancy open,” he said.
Other researchers are searching for biomarkers of the disease, both to provide a definitive, nonsurgical diagnostic tool, and for potential, individualized treatment.
In a study published in Nature Genetics, researchers at Cedars-Sinai created a “cellular atlas” of endometriosis by analyzing nearly 400,000 individual cells from 21 patients, some of whom had the disease and some of whom did not. A new technology, single-cell genomics, allowed the scientists to profile the multiple cell types contributing to the disease.
“So the initial question we wanted to ask was about understanding how the cells look in endometriosis, compared to endometrium,” said Kate Lawrenson, PhD, an associate professor in the department of obstetrics and gynecology at Cedars-Sinai, and co–senior author of the study. “We know that they resemble the cells of the womb, but we really don’t understand if they behave the same. We had a good inkling that they would behave differently.”
It turned out they did: Cells of endometriosis interacted atypically with female hormones, compared with cells in the uterus, Dr. Lawrenson said.
“That helps us understand how, even when patients take contraceptive pills, which is a commonly prescribed therapy, it doesn’t always work, or sometimes it stops working after a while,” she said. The next step for researchers, she said, will be to pinpoint the specific causes of these altered interactions.
Meanwhile, the current research also points to diagnostic possibilities. “We were quite excited to see that multiple cell types and endometriosis are upregulating the same sets of genes,” she said. “That makes us optimistic that hopefully there are some protein gene products that are being made in abundance, and hopefully we can detect them in the blood stream. It might be that we could use that information to develop new biomarkers, or even risk stratification tools.”
In the future, a simple blood test could identify signs of endometriosis in at-risk patients and get them “fast-tracked to a specialist for evaluation,” she said. “Whereas now, they might go from PCP to gynecologist to a different gynecologist over the course of 5-10 years before they get that referral.”
This discovery, that endometrial cells use genes differently and cross-talk with nearby cells differently, presents new treatment possibilities. Maybe we can physically block how cells interact with nearby cells, Dr. Lawrenson said. One model for doing that, she said, would be antibody-based therapy, similar to the therapies now changing the treatment of cancer.
What’s most exciting, looking ahead 5-10 years, is that treatment for endometriosis in the future may be significantly more individualized, and less hormone-based, than it is today.
“What we need for endometriosis is more options for patients and something that is tailored to the molecular makeup of their disease rather than a process of trial and error,” she said.
Roughly 4 decades after she first started menstruating, Elizabeth Flanagan finally underwent surgery to repair damage wreaked on her body by endometriosis. She’d spent years struggling with a variety of seemingly random symptoms, from migraines to excruciatingly painful periods to fatigue and irritable bowel syndrome. She’d worried about abnormal labs, including “extremely high” ANA, creatinine, and BUN blood test results that had been out of normal range for more than 10 years.
She was diagnosed with endometriosis in 2016, at age 47, after surgery to remove an ovarian cyst. Still, it took 5 more years before she landed in the office of a surgeon with the proper training to excise the lesions that continued to cause her so much anguish. That physician, Matthew Siedhoff, MD, at Cedars-Sinai Medical Center in Los Angeles, explained why her creatinine and BUN results were so far out of range: The endometriosis was impinging on her ureters.
The appointment left Ms. Flanagan with a range of emotions. “I was shocked that no doctor had identified this before, relieved knowing that I was finally in the hands of an expert who understood my condition, and saddened by the dearth of knowledge and proper treatment of endometriosis,” she wrote in an email.
Although the disease afflicts at least 1 out of every 10 women, endometriosis remains a conundrum for patients and their physicians. It often masquerades as other problems, from mental health issues such as anxiety and depression to physical issues such as irritable bowel syndrome. It often coexists with autoimmune conditions. Short of performing surgery, it can be a diagnosis of exclusion. And the existing, state-of-the-art treatment – hormone therapy that shuts down the reproductive system – doesn’t work for every woman every time.
“It is no wonder that it takes 10 years on average, from the time someone has symptoms of endometriosis, until they get a definitive diagnosis,” said Hugh Taylor, MD, chair of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn. “It’s a combination of [physicians] not taking painful menses seriously and getting distracted by all these other manifestations of the disease throughout the whole body.”
Endometriosis, he said, “is a whole-body disease.”
But recent genetic research offers the tantalizing prospect of new diagnostic tools and treatments. In 5-10 years, scientists say, physicians may be able to diagnose the disease with a simple blood test, and treat it, for example, by preventing a gene receptor from initiating a cascade of inflammatory effects, or crafting treatments tailored to the molecular makeup of a patient’s disease.
“Tomorrow’s therapies will target specifically the molecular defects of endometriosis and be nonhormonal,” Dr. Taylor said.
Guidelines published last year by the European Society of Human Reproduction and Embryology detail the latest standards for diagnosis and treatment of endometriosis.
According to the guidelines, physicians should consider the diagnosis of endometriosis in individuals presenting with the following cyclical and noncyclical signs and symptoms: dysmenorrhea, deep dyspareunia, dysuria, dyschezia, painful rectal bleeding or hematuria, shoulder tip pain, catamenial pneumothorax, cyclical cough/hemoptysis/chest pain, cyclical scar swelling, and pain, fatigue, and infertility.
A clinical exam should be considered, as well as imaging such as ultrasound and/or MRI, the guidelines state, although negative findings should not rule out a diagnosis. Laparoscopy is also an option, particularly for patients who desire a definitive diagnosis or cannot be diagnosed any other way, “although negative histology [of endometriotic lesions] does not entirely rule out the disease,” the guidelines state.
To treat the pain associated with endometriosis, the guidelines advise, as a first-line therapy, beginning with NSAIDs and combined hormonal contraceptives (in oral, vaginal, or transdermal form). Another option is progesterone, including progesterone-only contraceptives, with a recommendation to prescribe a levonorgestrel-releasing intrauterine system or an etonogestrel-releasing subdermal implant to reduce endometriosis-associated pain.
However, progestins and low-dose oral contraceptives are “unsuccessful in a third of women,” Dr. Taylor and his coauthors wrote in a paper published in 2021 in The Lancet.
Until recently, the gold standard for second-line treatment of endometriosis was oral gonadotropin-releasing hormone (GnRH) agonists. These manage the disease by inducing medical menopause – they downregulate pituitary GnRH receptors to create a hypoestrogenic state characterized by low serum levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). GnRH agonists may be administered nasally, or through daily, monthly, or trimonthly injections. But the Food and Drug Administration advises that, when used for longer than 6 months, GnRH agonists be paired with add-back hormone replacement therapy to reduce the risk of bone loss associated with the plunge in hormone levels. Also, treatment may not be appropriate for patients who, when suddenly forced into menopause, suffer from bothersome symptoms.
The latest treatment, GnRH antagonists, are new options for patients who either do not respond adequately to progestins and low-dose contraceptives or develop progesterone resistance, and want to avoid some of the risks and/or symptoms associated with GnRH agonists. Two advantages of GnRH antagonists for patients, Dr. Taylor said, are that they have a fast onset of action and are oral rather than injectable.
“These drugs [GnRH antagonists] cause competitive blockage of the GnRH receptor and hence dose-dependently suppress production of FSH and LH and inhibit secretion of ovarian steroid hormones without inducing a flare-up effect,” Belgian physicians and researchers Jacques Donnez, MD, and Marie-Madeleine Dolmans, MD, PhD, wrote in a paper published last year in the Journal of Clinical Medicine. “The mechanism is different from that of the GnRH agonist which, after a first phase of stimulation, desensitizes GnRH receptors, leading to full suppression of LH and FSH production and subsequently to complete suppression of [estrogen] to levels similar to those observed after bilateral oophorectomy.”
Patients who took Elagolix, the first oral nonpeptide GnRH antagonist available for the treatment of moderate to severe endometriosis-associated pain, had fewer vasomotor side effects and less bone density loss than those on the GnRH agonist leuprorelin, according to a 2018 study in Obstetrics and Gynecology. However, without add-back hormone-replacement therapy, GnRH antagonist use may need to be limited to 24 months, because of loss of bone density, a study in Cell Reports Medicine reported in 2022.
Attempting to explain the pathogenesis of endometriosis, and frustrated by the shortcomings of currently available therapies, researchers have turned to genetics for insight. A team of scientists led by Thomas Tapmeier, PhD, now a senior research fellow at Monash University in Australia, and Prof. Krina Zondervan at the University of Oxford, ran genetic analyses of families with a history of endometriosis, as well as rhesus macaques that spontaneously developed endometriosis. The research, published in Science Translational Medicine, identified NPSR1, the gene encoding neuropeptide S receptor 1, as one commonly associated with endometriosis. In trials with mouse models, they found that the NPSR1 inhibitor SHA 68R was able to reduce endometriosis-related inflammation and pain.
“It’s important to stress that there is no single gene that is responsible for endometriosis,” Dr. Tapmeier said in an interview. “This gene just has a higher frequency in people with endometriosis.”
The next step, then, would be to try to find a compound that would inhibit NPSR1 at some point, or a competitor to the ligand that binds to the receptor and blocks it, he said.
“We’re currently looking at compounds that might be able to inhibit the receptor signaling,” he said.
Such a therapy could potentially reduce the symptoms of endometriosis without interfering with the menstrual cycle and without introducing hormones that cause undesirable side effects in some patients.
“This might be a way to treat the pain and inflammation that goes with endometriosis, as well as leaving the possibility of pregnancy open,” he said.
Other researchers are searching for biomarkers of the disease, both to provide a definitive, nonsurgical diagnostic tool, and for potential, individualized treatment.
In a study published in Nature Genetics, researchers at Cedars-Sinai created a “cellular atlas” of endometriosis by analyzing nearly 400,000 individual cells from 21 patients, some of whom had the disease and some of whom did not. A new technology, single-cell genomics, allowed the scientists to profile the multiple cell types contributing to the disease.
“So the initial question we wanted to ask was about understanding how the cells look in endometriosis, compared to endometrium,” said Kate Lawrenson, PhD, an associate professor in the department of obstetrics and gynecology at Cedars-Sinai, and co–senior author of the study. “We know that they resemble the cells of the womb, but we really don’t understand if they behave the same. We had a good inkling that they would behave differently.”
It turned out they did: Cells of endometriosis interacted atypically with female hormones, compared with cells in the uterus, Dr. Lawrenson said.
“That helps us understand how, even when patients take contraceptive pills, which is a commonly prescribed therapy, it doesn’t always work, or sometimes it stops working after a while,” she said. The next step for researchers, she said, will be to pinpoint the specific causes of these altered interactions.
Meanwhile, the current research also points to diagnostic possibilities. “We were quite excited to see that multiple cell types and endometriosis are upregulating the same sets of genes,” she said. “That makes us optimistic that hopefully there are some protein gene products that are being made in abundance, and hopefully we can detect them in the blood stream. It might be that we could use that information to develop new biomarkers, or even risk stratification tools.”
In the future, a simple blood test could identify signs of endometriosis in at-risk patients and get them “fast-tracked to a specialist for evaluation,” she said. “Whereas now, they might go from PCP to gynecologist to a different gynecologist over the course of 5-10 years before they get that referral.”
This discovery, that endometrial cells use genes differently and cross-talk with nearby cells differently, presents new treatment possibilities. Maybe we can physically block how cells interact with nearby cells, Dr. Lawrenson said. One model for doing that, she said, would be antibody-based therapy, similar to the therapies now changing the treatment of cancer.
What’s most exciting, looking ahead 5-10 years, is that treatment for endometriosis in the future may be significantly more individualized, and less hormone-based, than it is today.
“What we need for endometriosis is more options for patients and something that is tailored to the molecular makeup of their disease rather than a process of trial and error,” she said.
A special tribute: Memorial and honorary gifts
Did you know you can honor a family member, friend, or colleague through a gift to the AGA Research Foundation?
- Giving now or later. Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help fund the AGA Research Awards Program. Your gift will assist in furthering basic digestive disease research, which can ultimately advance research into all digestive diseases. The financial benefits include an income tax deduction and possible elimination of capital gains tax.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly a percentage of your estate will pass to the AGA Research Foundation at your death in honor of your loved one.
- Named opportunities. Individuals interested in receiving name recognition for a listed AGA Institute program can do so by contributing a new, unrestricted gift to the AGA Research Foundation. The gift can be payable over five years. Endowed opportunities are also available.
Your next step
An honorary gift is a wonderful way to acknowledge someone’s vision for the future. To learn more about ways to recognize your honoree, visit our website.
Did you know you can honor a family member, friend, or colleague through a gift to the AGA Research Foundation?
- Giving now or later. Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help fund the AGA Research Awards Program. Your gift will assist in furthering basic digestive disease research, which can ultimately advance research into all digestive diseases. The financial benefits include an income tax deduction and possible elimination of capital gains tax.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly a percentage of your estate will pass to the AGA Research Foundation at your death in honor of your loved one.
- Named opportunities. Individuals interested in receiving name recognition for a listed AGA Institute program can do so by contributing a new, unrestricted gift to the AGA Research Foundation. The gift can be payable over five years. Endowed opportunities are also available.
Your next step
An honorary gift is a wonderful way to acknowledge someone’s vision for the future. To learn more about ways to recognize your honoree, visit our website.
Did you know you can honor a family member, friend, or colleague through a gift to the AGA Research Foundation?
- Giving now or later. Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help fund the AGA Research Awards Program. Your gift will assist in furthering basic digestive disease research, which can ultimately advance research into all digestive diseases. The financial benefits include an income tax deduction and possible elimination of capital gains tax.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly a percentage of your estate will pass to the AGA Research Foundation at your death in honor of your loved one.
- Named opportunities. Individuals interested in receiving name recognition for a listed AGA Institute program can do so by contributing a new, unrestricted gift to the AGA Research Foundation. The gift can be payable over five years. Endowed opportunities are also available.
Your next step
An honorary gift is a wonderful way to acknowledge someone’s vision for the future. To learn more about ways to recognize your honoree, visit our website.
The long-range thrombolysis forecast calls for tiny ultrasonic tornadoes
Sticks and stones may break my bones, but clots will never hurt me
You’ve probably seen “Ghostbusters” or at least heard the theme song. Maybe you even know about the Discovery Channel’s “Mythbusters.” But now there’s a new buster in town, and it eats platitudes for breakfast: Meet Cliche-busters, LOTME’s new recurring feature.
This week, Cliche-busters takes on “Two wrongs don’t make a right.” Yum.
We start with blood clots, which are bad. Doctors go to a lot of trouble to get rid of the things because they are dangerous. A blood clot, then, is a bodily function gone wrong.
Tornadoes are also bad. Out there in the world, these violently rotating columns of air can destroy buildings, toss large objects long distances, and inspire mediocre action movies. They are examples of nature gone wrong.
Seemingly, these two wrongs – blood clots and tornadoes – are not about to make a right. Has Cliche-busters bitten off more than it can chew?
Not according to Xiaoning Jiang of North Carolina State University, Raleigh, and his team of researchers. They’ve figured out a way to use a tiny ultrasonic tornado to break down clots in the brain. “Our new work uses vortex ultrasound, where the ultrasound waves have a helical wavefront. In other words, the ultrasound is swirling as it moves forward,” he said in a statement from the university.
Their new tool’s single transducer is small enough to fit in a catheter, and its “vortex ultrasound-induced shear force has the potential to break down clots safely and improve the efficacy of thrombolysis,” they explained in the open-access journal Research.
The investigators used cow blood in a 3D-printed model of the cerebral venous sinus for the proof-of-concept study and were able to dissolve an acute blood clot in less than 30 minutes, compared with the 15-30 hours needed with a pharmaceutical intervention, according to the written statement.
Can you hear the sound of two wrongs making a right? We can, and that closes the curtain on this cliche.
With age does not come wisdom
We’ve all met this person before. The sort of person who takes a 10-minute IQ test on a shifty-looking website and then proceeds to brag about a 180 IQ until the heat death of the universe. The one who worships at the altar of Mensa. Yeah, that guy. They’re never as smart as they think they are, but they’ll never, ever admit it.
It’s not exactly a secret that IQ as a measurement of intelligence is highly overrated. A lot of scientists doubt we should bother measuring it at all. That said, a higher IQ is associated with greater success in academic and financial endeavors, so it’s not absolutely worthless. And if we’re stuck with it, we may as well study it.
That brings us neatly to new research published in Brain and Behavior. Most studies into IQ and self-estimated intelligence have focused on younger adults, and the author of this study was curious if the stereotype of young men inflating their IQ, a stereotype backed up by research, persisted into older adulthood. So she conducted a survey of 159 younger adults and 152 older adults to find out.
The results in younger adults were not surprising: Younger men overestimated their actual IQ by 5-15 points, which tracks with previous research. We’re in for a bit of a surprise with the older adults, though, because the older men were more humble about their intelligence, with their estimation falling in line with their actual IQ. Older women, however, not so much. In fact, they overestimated their intelligence just as much as the younger men.
In addition, older women who perceived themselves as more attractive reported the highest self-estimated intelligence of all. That isn’t how intelligence works, but honestly, if Grandma’s out and about thinking she looks good and has the brains to go and win “Jeopardy!” do you really have the heart to tell her otherwise?
Fight temptation with empathy … and shoes
Relationships are tough. They all go through their respective ups and downs, but what happens when one person is feeling so down in the partnership that cheating comes to mind? Is there any way to stop it from happening?
Well, a recent study suggests that there is, and it’s as simple as putting yourself in the other person’s shoes. By observing 408 heterosexual, monogamous participants in a series of experiments, psychologists in Israel and New York found that practicing empathy and “perspective taking” doesn’t necessarily stop people from cheating but it does reduces the desire.
People cheat on their significant others for many different reasons – men for a lack of sexual needs being met and women for shortfalls regarding emotional needs – but prioritizing the other person’s perspective gives the idea of being unfaithful a different view and could make one act differently, the investigators said.
Perspective taking also promotes other positive attributes to the relationship, such as the promotion of compassion and the feeling of being understood, lead author Gurit Birnbaum of Reichman University in Herzliya, Israel, said in a written statement. These things ultimately help couples navigate the rough patches and strengthen bonds, making them even less likely to cheat.
The researchers noted that even people in satisfying relationships do cheat, but this approach does encourage people to stop and think before they act. It could ultimately prevent what might be a huge mistake.
Think before they act. Hmm, that’s kind of like look before they leap, right? Sounds like a job for the Cliche-busters.
Sticks and stones may break my bones, but clots will never hurt me
You’ve probably seen “Ghostbusters” or at least heard the theme song. Maybe you even know about the Discovery Channel’s “Mythbusters.” But now there’s a new buster in town, and it eats platitudes for breakfast: Meet Cliche-busters, LOTME’s new recurring feature.
This week, Cliche-busters takes on “Two wrongs don’t make a right.” Yum.
We start with blood clots, which are bad. Doctors go to a lot of trouble to get rid of the things because they are dangerous. A blood clot, then, is a bodily function gone wrong.
Tornadoes are also bad. Out there in the world, these violently rotating columns of air can destroy buildings, toss large objects long distances, and inspire mediocre action movies. They are examples of nature gone wrong.
Seemingly, these two wrongs – blood clots and tornadoes – are not about to make a right. Has Cliche-busters bitten off more than it can chew?
Not according to Xiaoning Jiang of North Carolina State University, Raleigh, and his team of researchers. They’ve figured out a way to use a tiny ultrasonic tornado to break down clots in the brain. “Our new work uses vortex ultrasound, where the ultrasound waves have a helical wavefront. In other words, the ultrasound is swirling as it moves forward,” he said in a statement from the university.
Their new tool’s single transducer is small enough to fit in a catheter, and its “vortex ultrasound-induced shear force has the potential to break down clots safely and improve the efficacy of thrombolysis,” they explained in the open-access journal Research.
The investigators used cow blood in a 3D-printed model of the cerebral venous sinus for the proof-of-concept study and were able to dissolve an acute blood clot in less than 30 minutes, compared with the 15-30 hours needed with a pharmaceutical intervention, according to the written statement.
Can you hear the sound of two wrongs making a right? We can, and that closes the curtain on this cliche.
With age does not come wisdom
We’ve all met this person before. The sort of person who takes a 10-minute IQ test on a shifty-looking website and then proceeds to brag about a 180 IQ until the heat death of the universe. The one who worships at the altar of Mensa. Yeah, that guy. They’re never as smart as they think they are, but they’ll never, ever admit it.
It’s not exactly a secret that IQ as a measurement of intelligence is highly overrated. A lot of scientists doubt we should bother measuring it at all. That said, a higher IQ is associated with greater success in academic and financial endeavors, so it’s not absolutely worthless. And if we’re stuck with it, we may as well study it.
That brings us neatly to new research published in Brain and Behavior. Most studies into IQ and self-estimated intelligence have focused on younger adults, and the author of this study was curious if the stereotype of young men inflating their IQ, a stereotype backed up by research, persisted into older adulthood. So she conducted a survey of 159 younger adults and 152 older adults to find out.
The results in younger adults were not surprising: Younger men overestimated their actual IQ by 5-15 points, which tracks with previous research. We’re in for a bit of a surprise with the older adults, though, because the older men were more humble about their intelligence, with their estimation falling in line with their actual IQ. Older women, however, not so much. In fact, they overestimated their intelligence just as much as the younger men.
In addition, older women who perceived themselves as more attractive reported the highest self-estimated intelligence of all. That isn’t how intelligence works, but honestly, if Grandma’s out and about thinking she looks good and has the brains to go and win “Jeopardy!” do you really have the heart to tell her otherwise?
Fight temptation with empathy … and shoes
Relationships are tough. They all go through their respective ups and downs, but what happens when one person is feeling so down in the partnership that cheating comes to mind? Is there any way to stop it from happening?
Well, a recent study suggests that there is, and it’s as simple as putting yourself in the other person’s shoes. By observing 408 heterosexual, monogamous participants in a series of experiments, psychologists in Israel and New York found that practicing empathy and “perspective taking” doesn’t necessarily stop people from cheating but it does reduces the desire.
People cheat on their significant others for many different reasons – men for a lack of sexual needs being met and women for shortfalls regarding emotional needs – but prioritizing the other person’s perspective gives the idea of being unfaithful a different view and could make one act differently, the investigators said.
Perspective taking also promotes other positive attributes to the relationship, such as the promotion of compassion and the feeling of being understood, lead author Gurit Birnbaum of Reichman University in Herzliya, Israel, said in a written statement. These things ultimately help couples navigate the rough patches and strengthen bonds, making them even less likely to cheat.
The researchers noted that even people in satisfying relationships do cheat, but this approach does encourage people to stop and think before they act. It could ultimately prevent what might be a huge mistake.
Think before they act. Hmm, that’s kind of like look before they leap, right? Sounds like a job for the Cliche-busters.
Sticks and stones may break my bones, but clots will never hurt me
You’ve probably seen “Ghostbusters” or at least heard the theme song. Maybe you even know about the Discovery Channel’s “Mythbusters.” But now there’s a new buster in town, and it eats platitudes for breakfast: Meet Cliche-busters, LOTME’s new recurring feature.
This week, Cliche-busters takes on “Two wrongs don’t make a right.” Yum.
We start with blood clots, which are bad. Doctors go to a lot of trouble to get rid of the things because they are dangerous. A blood clot, then, is a bodily function gone wrong.
Tornadoes are also bad. Out there in the world, these violently rotating columns of air can destroy buildings, toss large objects long distances, and inspire mediocre action movies. They are examples of nature gone wrong.
Seemingly, these two wrongs – blood clots and tornadoes – are not about to make a right. Has Cliche-busters bitten off more than it can chew?
Not according to Xiaoning Jiang of North Carolina State University, Raleigh, and his team of researchers. They’ve figured out a way to use a tiny ultrasonic tornado to break down clots in the brain. “Our new work uses vortex ultrasound, where the ultrasound waves have a helical wavefront. In other words, the ultrasound is swirling as it moves forward,” he said in a statement from the university.
Their new tool’s single transducer is small enough to fit in a catheter, and its “vortex ultrasound-induced shear force has the potential to break down clots safely and improve the efficacy of thrombolysis,” they explained in the open-access journal Research.
The investigators used cow blood in a 3D-printed model of the cerebral venous sinus for the proof-of-concept study and were able to dissolve an acute blood clot in less than 30 minutes, compared with the 15-30 hours needed with a pharmaceutical intervention, according to the written statement.
Can you hear the sound of two wrongs making a right? We can, and that closes the curtain on this cliche.
With age does not come wisdom
We’ve all met this person before. The sort of person who takes a 10-minute IQ test on a shifty-looking website and then proceeds to brag about a 180 IQ until the heat death of the universe. The one who worships at the altar of Mensa. Yeah, that guy. They’re never as smart as they think they are, but they’ll never, ever admit it.
It’s not exactly a secret that IQ as a measurement of intelligence is highly overrated. A lot of scientists doubt we should bother measuring it at all. That said, a higher IQ is associated with greater success in academic and financial endeavors, so it’s not absolutely worthless. And if we’re stuck with it, we may as well study it.
That brings us neatly to new research published in Brain and Behavior. Most studies into IQ and self-estimated intelligence have focused on younger adults, and the author of this study was curious if the stereotype of young men inflating their IQ, a stereotype backed up by research, persisted into older adulthood. So she conducted a survey of 159 younger adults and 152 older adults to find out.
The results in younger adults were not surprising: Younger men overestimated their actual IQ by 5-15 points, which tracks with previous research. We’re in for a bit of a surprise with the older adults, though, because the older men were more humble about their intelligence, with their estimation falling in line with their actual IQ. Older women, however, not so much. In fact, they overestimated their intelligence just as much as the younger men.
In addition, older women who perceived themselves as more attractive reported the highest self-estimated intelligence of all. That isn’t how intelligence works, but honestly, if Grandma’s out and about thinking she looks good and has the brains to go and win “Jeopardy!” do you really have the heart to tell her otherwise?
Fight temptation with empathy … and shoes
Relationships are tough. They all go through their respective ups and downs, but what happens when one person is feeling so down in the partnership that cheating comes to mind? Is there any way to stop it from happening?
Well, a recent study suggests that there is, and it’s as simple as putting yourself in the other person’s shoes. By observing 408 heterosexual, monogamous participants in a series of experiments, psychologists in Israel and New York found that practicing empathy and “perspective taking” doesn’t necessarily stop people from cheating but it does reduces the desire.
People cheat on their significant others for many different reasons – men for a lack of sexual needs being met and women for shortfalls regarding emotional needs – but prioritizing the other person’s perspective gives the idea of being unfaithful a different view and could make one act differently, the investigators said.
Perspective taking also promotes other positive attributes to the relationship, such as the promotion of compassion and the feeling of being understood, lead author Gurit Birnbaum of Reichman University in Herzliya, Israel, said in a written statement. These things ultimately help couples navigate the rough patches and strengthen bonds, making them even less likely to cheat.
The researchers noted that even people in satisfying relationships do cheat, but this approach does encourage people to stop and think before they act. It could ultimately prevent what might be a huge mistake.
Think before they act. Hmm, that’s kind of like look before they leap, right? Sounds like a job for the Cliche-busters.
Herbal combination tames active UC in small study
AURORA, COLO. –
Among 42 patients randomized on a 2:1 basis to receive either an enteric-coated pill containing 3 g of curcumin and qing-dai (CurQD) or placebo for 8 weeks, 43% of those assigned to receive the combination met the co-primary endpoint of a significant reduction in disease activity and objective evidence of response, compared with 8% of those assigned to placebo, reported Shomron Ben-Horin, MD, of Sheba Medical Center in Tel Aviv, Israel, and colleagues.
“In this randomized multicenter placebo-controlled trial, combination CurQD was found effective for inducing remission in active UC patients, including biologic-experienced patients,” they wrote in a scientific poster presented at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Nice spice
Curcumin is a polyphenolic compound derived from the spice turmeric that has been shown to have antioxidative and anti-inflammatory properties. Qing-dai (QD), also known as indigo naturalis, has been used in traditional Chinese medicine as an anti-inflammatory. Both agents are available over the counter in the United States, and have been on the market in Israel as a combination since 2016, said coauthor Nir Salomon, a certified herbalist at Sheba Medical Center.
“What we have here is a combination of these two compounds that are specifically sourced – the gut-directed curcumin, which we developed, and the specifically-sourced QD, and we use them in a specific protocol with a formulation suitable for moderate to severe disease,” he said in an interview.
Mr. Salomon and colleagues in Israel and in Athens, Greece, tested CurQD in a two-part trial. The first part was a 4-week open-label study of CurQD in 10 patients with active UC defined by a Simple Clinical Colitis Activity Index (SCCAI) score of 5 or greater and a modified Mayo endoscopic subscore of 2 or greater.
Part 2 was the placebo-controlled trial described before, with 42 patients with active UC. For 49% of these patients immunomodulatory and/or biologic therapies had failed to induce or maintain remissions.
A total of 43% of patients assigned to CurQD met the primary combined endpoint of a reduction in SCCAI of at least 3 points and objective evidence of response, consisting of either a Mayo endoscopic subscore improvement of 1 or greater, or at least 50% reduction in calprotectin.
In all, 85.7% of patients assigned to CurQD had a clinical response, compared with 30.7% of those assigned to placebo (P < .001).
In addition, 75% of patients on CurQD had endoscopic improvement, compared with 20% on placebo (P = .036), and more patients on the combined supplement had at least 50% reductions in calprotectin levels (46.4% vs. 15.4%, respectively), although the difference did not reach statistical significance.
Patients randomized to CurQD had significantly better resolution of rectal bleeding by day 12 (P value not shown).
Eight additional weeks of maintenance on curcumin alone resulted in 93% retention at week 16 of clinical response, 80% retention of remissions, and 40% maintenance of clinical biomarker responses.
CurQD, but not placebo, was associated with activation of the aryl-hydrocarbon receptor (AhR) pathway. AhR is a nuclear receptor that has been implicated as a mediator of inflammatory bowel disease.
“Induction of AhR merits further study as [a] potential treatment target in active UC,” the investigators wrote.
Small molecule
“This is a very promising and nicely conducted trial. Previously there are separate trials both determining potential mechanisms of action as well as efficacy of curcumin and Qing Dai separately in this population. This is a nice study that uses the combination in patients with mild to moderate UC,” said Ashwin N. Ananthakrishnan, MBBS, MPH, a gastroenterology physician and researcher at Massachusetts General Hospital in Boston.
“Immunosuppressive treatments are very effective in our patients with IBD but there remains concern (particularly for patients) about the consequences of immunosuppression including risk of treatment associated cancer. Thus, there is a lot of interest in rigorous studies of nonimmunosuppressive treatments that may still be effective in relieving objective inflammation (apart from just symptomatic improvement). This study provides a nice evidence base for that. There remain multiple limitations including small sample size, potential generalizability to other populations, and importantly whether the efficacy is driven by curcumin or Qing Dai,” he said in reply to a request for independent commentary.
Dr. Ananthakrishnan was not involved in the study.
“This is great work! We are also studying Qing Dai/indigo naturalis and have developed a single small molecule that works similarly to this therapy,” Matt Davidson, PhD, of Azora Therapeutics in Encino, Calif., said in an online chat section of the meeting website.
In a separate scientific poster presented at the meeting, Dr. Davidson and Julie Saiki, PhD, also from Azora, reported that their company is developing a novel synthetic small molecule prodrug of indirubin, an AhR agonist derived from indigo that is purported to maximize colonic exposure while minimizing systemic exposure.
In mouse models of colitis, oral administration of the prodrug significantly reduced Disease Activity Index and weight loss similar in magnitude to the active compound indirubin, they reported.
The study was supported by Sheba Medical Center. Mr. Salomon disclosed speaking fees from various companies and has received consulting fees and has an equity position in EvNature, the manufacturer of CurQD. Dr. Ananthakrishnan reported having no disclosures relative to the study. Dr. Davidson is CEO and cofounder of Avora Therapeutics.
AURORA, COLO. –
Among 42 patients randomized on a 2:1 basis to receive either an enteric-coated pill containing 3 g of curcumin and qing-dai (CurQD) or placebo for 8 weeks, 43% of those assigned to receive the combination met the co-primary endpoint of a significant reduction in disease activity and objective evidence of response, compared with 8% of those assigned to placebo, reported Shomron Ben-Horin, MD, of Sheba Medical Center in Tel Aviv, Israel, and colleagues.
“In this randomized multicenter placebo-controlled trial, combination CurQD was found effective for inducing remission in active UC patients, including biologic-experienced patients,” they wrote in a scientific poster presented at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Nice spice
Curcumin is a polyphenolic compound derived from the spice turmeric that has been shown to have antioxidative and anti-inflammatory properties. Qing-dai (QD), also known as indigo naturalis, has been used in traditional Chinese medicine as an anti-inflammatory. Both agents are available over the counter in the United States, and have been on the market in Israel as a combination since 2016, said coauthor Nir Salomon, a certified herbalist at Sheba Medical Center.

“What we have here is a combination of these two compounds that are specifically sourced – the gut-directed curcumin, which we developed, and the specifically-sourced QD, and we use them in a specific protocol with a formulation suitable for moderate to severe disease,” he said in an interview.
Mr. Salomon and colleagues in Israel and in Athens, Greece, tested CurQD in a two-part trial. The first part was a 4-week open-label study of CurQD in 10 patients with active UC defined by a Simple Clinical Colitis Activity Index (SCCAI) score of 5 or greater and a modified Mayo endoscopic subscore of 2 or greater.
Part 2 was the placebo-controlled trial described before, with 42 patients with active UC. For 49% of these patients immunomodulatory and/or biologic therapies had failed to induce or maintain remissions.
A total of 43% of patients assigned to CurQD met the primary combined endpoint of a reduction in SCCAI of at least 3 points and objective evidence of response, consisting of either a Mayo endoscopic subscore improvement of 1 or greater, or at least 50% reduction in calprotectin.
In all, 85.7% of patients assigned to CurQD had a clinical response, compared with 30.7% of those assigned to placebo (P < .001).
In addition, 75% of patients on CurQD had endoscopic improvement, compared with 20% on placebo (P = .036), and more patients on the combined supplement had at least 50% reductions in calprotectin levels (46.4% vs. 15.4%, respectively), although the difference did not reach statistical significance.
Patients randomized to CurQD had significantly better resolution of rectal bleeding by day 12 (P value not shown).
Eight additional weeks of maintenance on curcumin alone resulted in 93% retention at week 16 of clinical response, 80% retention of remissions, and 40% maintenance of clinical biomarker responses.
CurQD, but not placebo, was associated with activation of the aryl-hydrocarbon receptor (AhR) pathway. AhR is a nuclear receptor that has been implicated as a mediator of inflammatory bowel disease.
“Induction of AhR merits further study as [a] potential treatment target in active UC,” the investigators wrote.
Small molecule
“This is a very promising and nicely conducted trial. Previously there are separate trials both determining potential mechanisms of action as well as efficacy of curcumin and Qing Dai separately in this population. This is a nice study that uses the combination in patients with mild to moderate UC,” said Ashwin N. Ananthakrishnan, MBBS, MPH, a gastroenterology physician and researcher at Massachusetts General Hospital in Boston.
“Immunosuppressive treatments are very effective in our patients with IBD but there remains concern (particularly for patients) about the consequences of immunosuppression including risk of treatment associated cancer. Thus, there is a lot of interest in rigorous studies of nonimmunosuppressive treatments that may still be effective in relieving objective inflammation (apart from just symptomatic improvement). This study provides a nice evidence base for that. There remain multiple limitations including small sample size, potential generalizability to other populations, and importantly whether the efficacy is driven by curcumin or Qing Dai,” he said in reply to a request for independent commentary.
Dr. Ananthakrishnan was not involved in the study.
“This is great work! We are also studying Qing Dai/indigo naturalis and have developed a single small molecule that works similarly to this therapy,” Matt Davidson, PhD, of Azora Therapeutics in Encino, Calif., said in an online chat section of the meeting website.
In a separate scientific poster presented at the meeting, Dr. Davidson and Julie Saiki, PhD, also from Azora, reported that their company is developing a novel synthetic small molecule prodrug of indirubin, an AhR agonist derived from indigo that is purported to maximize colonic exposure while minimizing systemic exposure.
In mouse models of colitis, oral administration of the prodrug significantly reduced Disease Activity Index and weight loss similar in magnitude to the active compound indirubin, they reported.
The study was supported by Sheba Medical Center. Mr. Salomon disclosed speaking fees from various companies and has received consulting fees and has an equity position in EvNature, the manufacturer of CurQD. Dr. Ananthakrishnan reported having no disclosures relative to the study. Dr. Davidson is CEO and cofounder of Avora Therapeutics.
AURORA, COLO. –
Among 42 patients randomized on a 2:1 basis to receive either an enteric-coated pill containing 3 g of curcumin and qing-dai (CurQD) or placebo for 8 weeks, 43% of those assigned to receive the combination met the co-primary endpoint of a significant reduction in disease activity and objective evidence of response, compared with 8% of those assigned to placebo, reported Shomron Ben-Horin, MD, of Sheba Medical Center in Tel Aviv, Israel, and colleagues.
“In this randomized multicenter placebo-controlled trial, combination CurQD was found effective for inducing remission in active UC patients, including biologic-experienced patients,” they wrote in a scientific poster presented at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Nice spice
Curcumin is a polyphenolic compound derived from the spice turmeric that has been shown to have antioxidative and anti-inflammatory properties. Qing-dai (QD), also known as indigo naturalis, has been used in traditional Chinese medicine as an anti-inflammatory. Both agents are available over the counter in the United States, and have been on the market in Israel as a combination since 2016, said coauthor Nir Salomon, a certified herbalist at Sheba Medical Center.

“What we have here is a combination of these two compounds that are specifically sourced – the gut-directed curcumin, which we developed, and the specifically-sourced QD, and we use them in a specific protocol with a formulation suitable for moderate to severe disease,” he said in an interview.
Mr. Salomon and colleagues in Israel and in Athens, Greece, tested CurQD in a two-part trial. The first part was a 4-week open-label study of CurQD in 10 patients with active UC defined by a Simple Clinical Colitis Activity Index (SCCAI) score of 5 or greater and a modified Mayo endoscopic subscore of 2 or greater.
Part 2 was the placebo-controlled trial described before, with 42 patients with active UC. For 49% of these patients immunomodulatory and/or biologic therapies had failed to induce or maintain remissions.
A total of 43% of patients assigned to CurQD met the primary combined endpoint of a reduction in SCCAI of at least 3 points and objective evidence of response, consisting of either a Mayo endoscopic subscore improvement of 1 or greater, or at least 50% reduction in calprotectin.
In all, 85.7% of patients assigned to CurQD had a clinical response, compared with 30.7% of those assigned to placebo (P < .001).
In addition, 75% of patients on CurQD had endoscopic improvement, compared with 20% on placebo (P = .036), and more patients on the combined supplement had at least 50% reductions in calprotectin levels (46.4% vs. 15.4%, respectively), although the difference did not reach statistical significance.
Patients randomized to CurQD had significantly better resolution of rectal bleeding by day 12 (P value not shown).
Eight additional weeks of maintenance on curcumin alone resulted in 93% retention at week 16 of clinical response, 80% retention of remissions, and 40% maintenance of clinical biomarker responses.
CurQD, but not placebo, was associated with activation of the aryl-hydrocarbon receptor (AhR) pathway. AhR is a nuclear receptor that has been implicated as a mediator of inflammatory bowel disease.
“Induction of AhR merits further study as [a] potential treatment target in active UC,” the investigators wrote.
Small molecule
“This is a very promising and nicely conducted trial. Previously there are separate trials both determining potential mechanisms of action as well as efficacy of curcumin and Qing Dai separately in this population. This is a nice study that uses the combination in patients with mild to moderate UC,” said Ashwin N. Ananthakrishnan, MBBS, MPH, a gastroenterology physician and researcher at Massachusetts General Hospital in Boston.
“Immunosuppressive treatments are very effective in our patients with IBD but there remains concern (particularly for patients) about the consequences of immunosuppression including risk of treatment associated cancer. Thus, there is a lot of interest in rigorous studies of nonimmunosuppressive treatments that may still be effective in relieving objective inflammation (apart from just symptomatic improvement). This study provides a nice evidence base for that. There remain multiple limitations including small sample size, potential generalizability to other populations, and importantly whether the efficacy is driven by curcumin or Qing Dai,” he said in reply to a request for independent commentary.
Dr. Ananthakrishnan was not involved in the study.
“This is great work! We are also studying Qing Dai/indigo naturalis and have developed a single small molecule that works similarly to this therapy,” Matt Davidson, PhD, of Azora Therapeutics in Encino, Calif., said in an online chat section of the meeting website.
In a separate scientific poster presented at the meeting, Dr. Davidson and Julie Saiki, PhD, also from Azora, reported that their company is developing a novel synthetic small molecule prodrug of indirubin, an AhR agonist derived from indigo that is purported to maximize colonic exposure while minimizing systemic exposure.
In mouse models of colitis, oral administration of the prodrug significantly reduced Disease Activity Index and weight loss similar in magnitude to the active compound indirubin, they reported.
The study was supported by Sheba Medical Center. Mr. Salomon disclosed speaking fees from various companies and has received consulting fees and has an equity position in EvNature, the manufacturer of CurQD. Dr. Ananthakrishnan reported having no disclosures relative to the study. Dr. Davidson is CEO and cofounder of Avora Therapeutics.
AT THE CROHN’S & COLITIS CONGRESS