User login
Brain scans show effect of poverty, stress on Black children
Childhood stress can change the brain negatively, according to a new study that says Black children are affected more because they experience more poverty and adversity.
“The researchers analyzed MRI scans to identify small differences in the volume of certain brain structures, and said these could accumulate as children age and play a role in the later development of mental health problems,” STAT News reported. “The finding, part of an emerging research field looking at how racism and other social factors may affect the physical architecture of the brain, may help explain longstanding racial disparities in the prevalence of psychiatric disorders such as PTSD.”
The study was published in The American Journal of Psychiatry.
Brain development is affected by “disparities faced by certain groups of people,” even among children as young as 9 years old, said Nathaniel Harnett, an assistant professor of psychiatry at Harvard Medical School, Boston, and the study’s senior author. “If we’re going to treat the world as colorblind, we’re not going to create mental health solutions that are effective for all people.”
The study used evidence from the Adolescent Brain Cognitive Development Study, which the National Institutes of Health established in 2015 to study the brains and experiences of thousands of American children through early adulthood.
Brain scans revealed that Black children had less gray matter in 11 of 14 brain areas that were examined. Disparities in 8 of the 14 brain areas were affected by childhood adversity, particularly poverty.
A version of this article first appeared on WebMD.com.
Childhood stress can change the brain negatively, according to a new study that says Black children are affected more because they experience more poverty and adversity.
“The researchers analyzed MRI scans to identify small differences in the volume of certain brain structures, and said these could accumulate as children age and play a role in the later development of mental health problems,” STAT News reported. “The finding, part of an emerging research field looking at how racism and other social factors may affect the physical architecture of the brain, may help explain longstanding racial disparities in the prevalence of psychiatric disorders such as PTSD.”
The study was published in The American Journal of Psychiatry.
Brain development is affected by “disparities faced by certain groups of people,” even among children as young as 9 years old, said Nathaniel Harnett, an assistant professor of psychiatry at Harvard Medical School, Boston, and the study’s senior author. “If we’re going to treat the world as colorblind, we’re not going to create mental health solutions that are effective for all people.”
The study used evidence from the Adolescent Brain Cognitive Development Study, which the National Institutes of Health established in 2015 to study the brains and experiences of thousands of American children through early adulthood.
Brain scans revealed that Black children had less gray matter in 11 of 14 brain areas that were examined. Disparities in 8 of the 14 brain areas were affected by childhood adversity, particularly poverty.
A version of this article first appeared on WebMD.com.
Childhood stress can change the brain negatively, according to a new study that says Black children are affected more because they experience more poverty and adversity.
“The researchers analyzed MRI scans to identify small differences in the volume of certain brain structures, and said these could accumulate as children age and play a role in the later development of mental health problems,” STAT News reported. “The finding, part of an emerging research field looking at how racism and other social factors may affect the physical architecture of the brain, may help explain longstanding racial disparities in the prevalence of psychiatric disorders such as PTSD.”
The study was published in The American Journal of Psychiatry.
Brain development is affected by “disparities faced by certain groups of people,” even among children as young as 9 years old, said Nathaniel Harnett, an assistant professor of psychiatry at Harvard Medical School, Boston, and the study’s senior author. “If we’re going to treat the world as colorblind, we’re not going to create mental health solutions that are effective for all people.”
The study used evidence from the Adolescent Brain Cognitive Development Study, which the National Institutes of Health established in 2015 to study the brains and experiences of thousands of American children through early adulthood.
Brain scans revealed that Black children had less gray matter in 11 of 14 brain areas that were examined. Disparities in 8 of the 14 brain areas were affected by childhood adversity, particularly poverty.
A version of this article first appeared on WebMD.com.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY
Topical gene therapy heals dystrophic epidermolysis bullosa wounds
.
In a phase 3 study of patients with DEB, “we found that repeated topical application of B-VEC [beremagene geperpavec], an HSV-1–based gene therapy, resulted in a greater likelihood of complete wound healing than the topical application of placebo at up to 6 months,” the authors wrote. The study was published in The New England Journal of Medicine. “Longer and larger trials are warranted to determine the durability of effect and risks of this approach,” the authors noted.
“The results prove that B-VEC, the first topical in vivo gene therapy to reach late-stage development, can heal DEB,” senior author M. Peter Marinkovich, MD, associate professor of dermatology at Stanford University, Redwood City, Calif., said in an interview.
“In the past, DEB was a very specialized disease that only a handful of dermatologists would see but could not do much to treat,” he said. “With gene therapy, many more dermatologists who may not be familiar with DEB will be able to treat these patients in their offices.” It is expected that nurses will be able to administer the treatment to patients at home, he added.
Rare, life-threatening, genetic blistering disease
DEB, a rare disease that affects one to three persons per million in the United States, is caused by mutations in the COL7A1 gene that encodes the alpha-1 chain of collagen type VII (C7) protein. C7 forms the anchoring fibrils that attach the epidermis to the underlying dermal connective tissue.
COL71A mutations that lead to defective, decreased, or absent C7 can make the skin so fragile it tears with the slightest touch. This has led to patients being called “butterfly children.” Epithelial tissues blister and scar, causing esophageal and genitourinary strictures, adhesion of digits, malnutrition, anemia, infection, and bothersome itch and pain. Morbidity and mortality are high. The leading cause of death in adults is chronic wounds leading to aggressive squamous cell cancers.
The first therapy for DEB, under FDA review
B-VEC restores C7 protein by using an engineered replication-defective herpes simplex virus type 1 (HSV-1) vector to deliver the COL7A1 gene directly to skin cells to restore functional C7 protein fibrils that stabilize the skin structure.
On the basis of manufacturing information submitted to the FDA in December 2022, the agency extended the date for a decision on approval by 3 months, to May 19, 2023, according to a statement from Krystal Biotech, the developer of B-VEC and the sponsor of the NEJM study.
Dr. Marinkovich and his colleagues conducted the double-blind, randomized, controlled GEM-3 trial of B-VEC at three sites in the United States. The 31 study participants ranged in age from 1 to 44 years (median age, 16 years) and had genetically confirmed DEB (30 with the recessive form and 1 with the dominant form).
For each participant, a pair of wounds was chosen that were matched in size, region, and appearance. The wounds within each pair were randomly allocated to receive weekly applications of either B-VEC or placebo gel for 26 weeks.
The results of the study included the following:
- Complete healing at 6 months occurred in 67% of the wounds treated with B-VEC (including a wound in the patient with dominant DEB), vs. 22% of those who received placebo (95% confidence interval [CI], 24-68; P = .002).
- Complete healing at 3 months occurred in 71% of the wounds treated with B-VEC, vs. 20% of those who received placebo (95% CI, 29-73; P < .001).
- The mean change from baseline to week 22 in pain severity during wound-dressing changes for patients aged 6 years and older, as determined on the basis of a visual analogue scale, was –0.88 with B-VEC, vs. –0.71 with placebo (adjusted least-squares mean difference, –0.61; 95% CI, –1.10 to –0.13); similar mean changes were seen at weeks 24 and 26.
- Among all patients, 58% had at least one adverse event. Most adverse events were mild or moderate. The most common were pruritus, chills, and squamous cell carcinoma (SCC), which were reported in three patients each (SCC cases occurred at wound sites that had not been exposed to B-VEC or placebo). Serious adverse events, which were unrelated to the treatment, occurred in three patients: diarrhea, anemia, cellulitis, and a positive blood culture related to a hemodialysis catheter.
“With the ability to treat patients with topical gene therapy, dermatology is entering a new age of treatment possibilities,” Dr. Marinkovich said in the interview.
The researchers were surprised that the redosable in vivo gene therapy worked so well, he added. In vivo gene therapy has been plagued by the occurrence of immune reactions against the viral vectors used, Dr. Marinkovich explained. But because the herpes simplex virus has evolved to evade the immune system, his team could use the viral vector every week for 6 months without inflammatory reactions.
“The immune system’s inability to fight off or get rid of the herpes simplex vector makes it bad as a disease, but as a gene therapy vector, it provides a huge advantage,” he added.
Asked to comment on the results, Christen Ebens, MD, MPH, assistant professor in the department of pediatrics at the University of Minnesota, Minneapolis, whose clinical and research interests include EB, called the results exciting for patients, families, and doctors.
“Side effects were minimal, and importantly, use of the replication-incompetent HSV vector means that the payload gene does not integrate into the patient’s DNA,” Dr. Ebens, who was not involved in the study, said in an interview. “B-VEC is not a lifelong cure but potentially an effective maintenance therapy requiring repeated doses,” she added.
Although the researchers found no clinically important immune reactions to B-VEC, Dr. Ebens said she would like to see results from longer studies of the treatment. “We will want to see that patients do not produce neutralizing antibodies against B-VEC or its components, as such antibodies may yield the treatment ineffective or cause significant side effects.”
In an interview, Vanessa R. Holland, MD, associate clinical professor in the division of dermatology at UCLA Health, Burbank, Calif., who was not involved in the study, said that “topical replication-defective HSV-1 is a brilliant vector to deliver the depleted collagen.” She added that “such a vehicle may significantly alter management of these disorders and improve or extend lives by minimizing potentially fatal complications.”
Paras P. Vakharia, MD, PharmD, assistant professor of dermatology at Northwestern University, Chicago, who was not involved in the study, was surprised by the high percentage of healed wounds and wounds that remained healed over time.
In an interview, Dr. Vakharia said that he’d like to know whether patients develop antibodies against HSV and C7 with long-term treatment and whether problems will arise related to drug availability.
B-VEC for treating other conditions
Dr. Marinkovich noted that an ongoing phase 1 clinical trial, also sponsored by Krystal Biotech, is using the HSV-1 vector to deliver a different biologic (KB105) to establish dose and safety in the treatment of ichthyosis. He added that he would like to explore the use of B-VEC to treat DEB at mucosal surfaces, including inside the mouth, the eye, and the esophagus.
Authors of two editorials that accompanied the study also referred to other conditions B-VEC might treat.
This study “highlights potential future investigations,” David V. Schaffer, PhD, professor of chemical and biomolecular engineering, bioengineering, and molecular and cell biology at the University of California, Berkeley, wrotes in one of the editorials.
Important considerations he mentioned include the likelihood of the treatment becoming lifelong; the inability of HSV to penetrate intact skin, making B-VEC unsuitable for preventing the development of new wounds; and the inability of this treatment to treat EB lesions along the digestive tract. “This important trial builds on and extends gene-therapy successes to new targets and vectors, an advance for patients,” he added.
In the second editorial, Aimee S. Payne, MD, PhD, professor of dermatology at the University of Pennsylvania, Philadelphia, raised the question of whether B-VEC’s clinical success for treating DEB can translate to other genetic diseases.
“Formulations for ophthalmic, oral-gastrointestinal, or respiratory delivery would be of great value to address the extracutaneous manifestations of epidermolysis bullosa and other genetic diseases,” she wrote.
Referring to an ongoing trial of a topical gene therapy for cystic fibrosis that is delivered by a nebulizer, Dr. Payne noted, “Ultimately, the completion of clinical trials such as this one will be required to determine whether HSV-1–mediated gene delivery can go more than skin deep.”
Earlier data and more details of the study were presented in a poster at the annual meeting of the Society for Pediatric Dermatology in July 2022.
Dr. Marinkovich has disclosed no relevant financial relationships. Several coauthors are employees of or have other financial relationships with Krystal Biotech, the study’s sponsor and the developer of beremagene geperpavec. Dr. Schaffer and Dr. Payne have financial relationships with the pharmaceutical industry. Dr. Ebens, Dr. Holland, and Dr. Vakharia have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
.
In a phase 3 study of patients with DEB, “we found that repeated topical application of B-VEC [beremagene geperpavec], an HSV-1–based gene therapy, resulted in a greater likelihood of complete wound healing than the topical application of placebo at up to 6 months,” the authors wrote. The study was published in The New England Journal of Medicine. “Longer and larger trials are warranted to determine the durability of effect and risks of this approach,” the authors noted.
“The results prove that B-VEC, the first topical in vivo gene therapy to reach late-stage development, can heal DEB,” senior author M. Peter Marinkovich, MD, associate professor of dermatology at Stanford University, Redwood City, Calif., said in an interview.
“In the past, DEB was a very specialized disease that only a handful of dermatologists would see but could not do much to treat,” he said. “With gene therapy, many more dermatologists who may not be familiar with DEB will be able to treat these patients in their offices.” It is expected that nurses will be able to administer the treatment to patients at home, he added.
Rare, life-threatening, genetic blistering disease
DEB, a rare disease that affects one to three persons per million in the United States, is caused by mutations in the COL7A1 gene that encodes the alpha-1 chain of collagen type VII (C7) protein. C7 forms the anchoring fibrils that attach the epidermis to the underlying dermal connective tissue.
COL71A mutations that lead to defective, decreased, or absent C7 can make the skin so fragile it tears with the slightest touch. This has led to patients being called “butterfly children.” Epithelial tissues blister and scar, causing esophageal and genitourinary strictures, adhesion of digits, malnutrition, anemia, infection, and bothersome itch and pain. Morbidity and mortality are high. The leading cause of death in adults is chronic wounds leading to aggressive squamous cell cancers.
The first therapy for DEB, under FDA review
B-VEC restores C7 protein by using an engineered replication-defective herpes simplex virus type 1 (HSV-1) vector to deliver the COL7A1 gene directly to skin cells to restore functional C7 protein fibrils that stabilize the skin structure.
On the basis of manufacturing information submitted to the FDA in December 2022, the agency extended the date for a decision on approval by 3 months, to May 19, 2023, according to a statement from Krystal Biotech, the developer of B-VEC and the sponsor of the NEJM study.
Dr. Marinkovich and his colleagues conducted the double-blind, randomized, controlled GEM-3 trial of B-VEC at three sites in the United States. The 31 study participants ranged in age from 1 to 44 years (median age, 16 years) and had genetically confirmed DEB (30 with the recessive form and 1 with the dominant form).
For each participant, a pair of wounds was chosen that were matched in size, region, and appearance. The wounds within each pair were randomly allocated to receive weekly applications of either B-VEC or placebo gel for 26 weeks.
The results of the study included the following:
- Complete healing at 6 months occurred in 67% of the wounds treated with B-VEC (including a wound in the patient with dominant DEB), vs. 22% of those who received placebo (95% confidence interval [CI], 24-68; P = .002).
- Complete healing at 3 months occurred in 71% of the wounds treated with B-VEC, vs. 20% of those who received placebo (95% CI, 29-73; P < .001).
- The mean change from baseline to week 22 in pain severity during wound-dressing changes for patients aged 6 years and older, as determined on the basis of a visual analogue scale, was –0.88 with B-VEC, vs. –0.71 with placebo (adjusted least-squares mean difference, –0.61; 95% CI, –1.10 to –0.13); similar mean changes were seen at weeks 24 and 26.
- Among all patients, 58% had at least one adverse event. Most adverse events were mild or moderate. The most common were pruritus, chills, and squamous cell carcinoma (SCC), which were reported in three patients each (SCC cases occurred at wound sites that had not been exposed to B-VEC or placebo). Serious adverse events, which were unrelated to the treatment, occurred in three patients: diarrhea, anemia, cellulitis, and a positive blood culture related to a hemodialysis catheter.
“With the ability to treat patients with topical gene therapy, dermatology is entering a new age of treatment possibilities,” Dr. Marinkovich said in the interview.
The researchers were surprised that the redosable in vivo gene therapy worked so well, he added. In vivo gene therapy has been plagued by the occurrence of immune reactions against the viral vectors used, Dr. Marinkovich explained. But because the herpes simplex virus has evolved to evade the immune system, his team could use the viral vector every week for 6 months without inflammatory reactions.
“The immune system’s inability to fight off or get rid of the herpes simplex vector makes it bad as a disease, but as a gene therapy vector, it provides a huge advantage,” he added.
Asked to comment on the results, Christen Ebens, MD, MPH, assistant professor in the department of pediatrics at the University of Minnesota, Minneapolis, whose clinical and research interests include EB, called the results exciting for patients, families, and doctors.
“Side effects were minimal, and importantly, use of the replication-incompetent HSV vector means that the payload gene does not integrate into the patient’s DNA,” Dr. Ebens, who was not involved in the study, said in an interview. “B-VEC is not a lifelong cure but potentially an effective maintenance therapy requiring repeated doses,” she added.
Although the researchers found no clinically important immune reactions to B-VEC, Dr. Ebens said she would like to see results from longer studies of the treatment. “We will want to see that patients do not produce neutralizing antibodies against B-VEC or its components, as such antibodies may yield the treatment ineffective or cause significant side effects.”
In an interview, Vanessa R. Holland, MD, associate clinical professor in the division of dermatology at UCLA Health, Burbank, Calif., who was not involved in the study, said that “topical replication-defective HSV-1 is a brilliant vector to deliver the depleted collagen.” She added that “such a vehicle may significantly alter management of these disorders and improve or extend lives by minimizing potentially fatal complications.”
Paras P. Vakharia, MD, PharmD, assistant professor of dermatology at Northwestern University, Chicago, who was not involved in the study, was surprised by the high percentage of healed wounds and wounds that remained healed over time.
In an interview, Dr. Vakharia said that he’d like to know whether patients develop antibodies against HSV and C7 with long-term treatment and whether problems will arise related to drug availability.
B-VEC for treating other conditions
Dr. Marinkovich noted that an ongoing phase 1 clinical trial, also sponsored by Krystal Biotech, is using the HSV-1 vector to deliver a different biologic (KB105) to establish dose and safety in the treatment of ichthyosis. He added that he would like to explore the use of B-VEC to treat DEB at mucosal surfaces, including inside the mouth, the eye, and the esophagus.
Authors of two editorials that accompanied the study also referred to other conditions B-VEC might treat.
This study “highlights potential future investigations,” David V. Schaffer, PhD, professor of chemical and biomolecular engineering, bioengineering, and molecular and cell biology at the University of California, Berkeley, wrotes in one of the editorials.
Important considerations he mentioned include the likelihood of the treatment becoming lifelong; the inability of HSV to penetrate intact skin, making B-VEC unsuitable for preventing the development of new wounds; and the inability of this treatment to treat EB lesions along the digestive tract. “This important trial builds on and extends gene-therapy successes to new targets and vectors, an advance for patients,” he added.
In the second editorial, Aimee S. Payne, MD, PhD, professor of dermatology at the University of Pennsylvania, Philadelphia, raised the question of whether B-VEC’s clinical success for treating DEB can translate to other genetic diseases.
“Formulations for ophthalmic, oral-gastrointestinal, or respiratory delivery would be of great value to address the extracutaneous manifestations of epidermolysis bullosa and other genetic diseases,” she wrote.
Referring to an ongoing trial of a topical gene therapy for cystic fibrosis that is delivered by a nebulizer, Dr. Payne noted, “Ultimately, the completion of clinical trials such as this one will be required to determine whether HSV-1–mediated gene delivery can go more than skin deep.”
Earlier data and more details of the study were presented in a poster at the annual meeting of the Society for Pediatric Dermatology in July 2022.
Dr. Marinkovich has disclosed no relevant financial relationships. Several coauthors are employees of or have other financial relationships with Krystal Biotech, the study’s sponsor and the developer of beremagene geperpavec. Dr. Schaffer and Dr. Payne have financial relationships with the pharmaceutical industry. Dr. Ebens, Dr. Holland, and Dr. Vakharia have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
.
In a phase 3 study of patients with DEB, “we found that repeated topical application of B-VEC [beremagene geperpavec], an HSV-1–based gene therapy, resulted in a greater likelihood of complete wound healing than the topical application of placebo at up to 6 months,” the authors wrote. The study was published in The New England Journal of Medicine. “Longer and larger trials are warranted to determine the durability of effect and risks of this approach,” the authors noted.
“The results prove that B-VEC, the first topical in vivo gene therapy to reach late-stage development, can heal DEB,” senior author M. Peter Marinkovich, MD, associate professor of dermatology at Stanford University, Redwood City, Calif., said in an interview.
“In the past, DEB was a very specialized disease that only a handful of dermatologists would see but could not do much to treat,” he said. “With gene therapy, many more dermatologists who may not be familiar with DEB will be able to treat these patients in their offices.” It is expected that nurses will be able to administer the treatment to patients at home, he added.
Rare, life-threatening, genetic blistering disease
DEB, a rare disease that affects one to three persons per million in the United States, is caused by mutations in the COL7A1 gene that encodes the alpha-1 chain of collagen type VII (C7) protein. C7 forms the anchoring fibrils that attach the epidermis to the underlying dermal connective tissue.
COL71A mutations that lead to defective, decreased, or absent C7 can make the skin so fragile it tears with the slightest touch. This has led to patients being called “butterfly children.” Epithelial tissues blister and scar, causing esophageal and genitourinary strictures, adhesion of digits, malnutrition, anemia, infection, and bothersome itch and pain. Morbidity and mortality are high. The leading cause of death in adults is chronic wounds leading to aggressive squamous cell cancers.
The first therapy for DEB, under FDA review
B-VEC restores C7 protein by using an engineered replication-defective herpes simplex virus type 1 (HSV-1) vector to deliver the COL7A1 gene directly to skin cells to restore functional C7 protein fibrils that stabilize the skin structure.
On the basis of manufacturing information submitted to the FDA in December 2022, the agency extended the date for a decision on approval by 3 months, to May 19, 2023, according to a statement from Krystal Biotech, the developer of B-VEC and the sponsor of the NEJM study.
Dr. Marinkovich and his colleagues conducted the double-blind, randomized, controlled GEM-3 trial of B-VEC at three sites in the United States. The 31 study participants ranged in age from 1 to 44 years (median age, 16 years) and had genetically confirmed DEB (30 with the recessive form and 1 with the dominant form).
For each participant, a pair of wounds was chosen that were matched in size, region, and appearance. The wounds within each pair were randomly allocated to receive weekly applications of either B-VEC or placebo gel for 26 weeks.
The results of the study included the following:
- Complete healing at 6 months occurred in 67% of the wounds treated with B-VEC (including a wound in the patient with dominant DEB), vs. 22% of those who received placebo (95% confidence interval [CI], 24-68; P = .002).
- Complete healing at 3 months occurred in 71% of the wounds treated with B-VEC, vs. 20% of those who received placebo (95% CI, 29-73; P < .001).
- The mean change from baseline to week 22 in pain severity during wound-dressing changes for patients aged 6 years and older, as determined on the basis of a visual analogue scale, was –0.88 with B-VEC, vs. –0.71 with placebo (adjusted least-squares mean difference, –0.61; 95% CI, –1.10 to –0.13); similar mean changes were seen at weeks 24 and 26.
- Among all patients, 58% had at least one adverse event. Most adverse events were mild or moderate. The most common were pruritus, chills, and squamous cell carcinoma (SCC), which were reported in three patients each (SCC cases occurred at wound sites that had not been exposed to B-VEC or placebo). Serious adverse events, which were unrelated to the treatment, occurred in three patients: diarrhea, anemia, cellulitis, and a positive blood culture related to a hemodialysis catheter.
“With the ability to treat patients with topical gene therapy, dermatology is entering a new age of treatment possibilities,” Dr. Marinkovich said in the interview.
The researchers were surprised that the redosable in vivo gene therapy worked so well, he added. In vivo gene therapy has been plagued by the occurrence of immune reactions against the viral vectors used, Dr. Marinkovich explained. But because the herpes simplex virus has evolved to evade the immune system, his team could use the viral vector every week for 6 months without inflammatory reactions.
“The immune system’s inability to fight off or get rid of the herpes simplex vector makes it bad as a disease, but as a gene therapy vector, it provides a huge advantage,” he added.
Asked to comment on the results, Christen Ebens, MD, MPH, assistant professor in the department of pediatrics at the University of Minnesota, Minneapolis, whose clinical and research interests include EB, called the results exciting for patients, families, and doctors.
“Side effects were minimal, and importantly, use of the replication-incompetent HSV vector means that the payload gene does not integrate into the patient’s DNA,” Dr. Ebens, who was not involved in the study, said in an interview. “B-VEC is not a lifelong cure but potentially an effective maintenance therapy requiring repeated doses,” she added.
Although the researchers found no clinically important immune reactions to B-VEC, Dr. Ebens said she would like to see results from longer studies of the treatment. “We will want to see that patients do not produce neutralizing antibodies against B-VEC or its components, as such antibodies may yield the treatment ineffective or cause significant side effects.”
In an interview, Vanessa R. Holland, MD, associate clinical professor in the division of dermatology at UCLA Health, Burbank, Calif., who was not involved in the study, said that “topical replication-defective HSV-1 is a brilliant vector to deliver the depleted collagen.” She added that “such a vehicle may significantly alter management of these disorders and improve or extend lives by minimizing potentially fatal complications.”
Paras P. Vakharia, MD, PharmD, assistant professor of dermatology at Northwestern University, Chicago, who was not involved in the study, was surprised by the high percentage of healed wounds and wounds that remained healed over time.
In an interview, Dr. Vakharia said that he’d like to know whether patients develop antibodies against HSV and C7 with long-term treatment and whether problems will arise related to drug availability.
B-VEC for treating other conditions
Dr. Marinkovich noted that an ongoing phase 1 clinical trial, also sponsored by Krystal Biotech, is using the HSV-1 vector to deliver a different biologic (KB105) to establish dose and safety in the treatment of ichthyosis. He added that he would like to explore the use of B-VEC to treat DEB at mucosal surfaces, including inside the mouth, the eye, and the esophagus.
Authors of two editorials that accompanied the study also referred to other conditions B-VEC might treat.
This study “highlights potential future investigations,” David V. Schaffer, PhD, professor of chemical and biomolecular engineering, bioengineering, and molecular and cell biology at the University of California, Berkeley, wrotes in one of the editorials.
Important considerations he mentioned include the likelihood of the treatment becoming lifelong; the inability of HSV to penetrate intact skin, making B-VEC unsuitable for preventing the development of new wounds; and the inability of this treatment to treat EB lesions along the digestive tract. “This important trial builds on and extends gene-therapy successes to new targets and vectors, an advance for patients,” he added.
In the second editorial, Aimee S. Payne, MD, PhD, professor of dermatology at the University of Pennsylvania, Philadelphia, raised the question of whether B-VEC’s clinical success for treating DEB can translate to other genetic diseases.
“Formulations for ophthalmic, oral-gastrointestinal, or respiratory delivery would be of great value to address the extracutaneous manifestations of epidermolysis bullosa and other genetic diseases,” she wrote.
Referring to an ongoing trial of a topical gene therapy for cystic fibrosis that is delivered by a nebulizer, Dr. Payne noted, “Ultimately, the completion of clinical trials such as this one will be required to determine whether HSV-1–mediated gene delivery can go more than skin deep.”
Earlier data and more details of the study were presented in a poster at the annual meeting of the Society for Pediatric Dermatology in July 2022.
Dr. Marinkovich has disclosed no relevant financial relationships. Several coauthors are employees of or have other financial relationships with Krystal Biotech, the study’s sponsor and the developer of beremagene geperpavec. Dr. Schaffer and Dr. Payne have financial relationships with the pharmaceutical industry. Dr. Ebens, Dr. Holland, and Dr. Vakharia have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Restricted fluid failed to reduce mortality in sepsis-induced hypotension
A restrictive fluid strategy had no significant impact on mortality in patients with sepsis-induced hypotension compared to the typical liberal fluid strategy, based on data from 1,563 individuals.
Intravenous fluids are standard in the early resuscitation of sepsis patients, as are vasopressor agents, but data comparing restrictive or liberal use in these patients are limited, wrote Nathan I. Shapiro, MD, of Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, and colleagues.
In a study published in the New England Journal of Medicine the researchers randomized 782 patients to the restrictive fluid group and 781 to the liberal fluid group. Patients aged 18 years and older were enrolled between March 7, 2018, and Jan. 31, 2022, at 60 centers in the United States. Participants were randomized within 4 hours of meeting the criteria for sepsis-induced hypotension that was refractory to initial treatment with 1-3 L of intravenous fluid. Baseline characteristics were similar between the groups. At randomization, 21% of patients in the restrictive fluid group and 18% in the liberal fluid group received vasopressors.
The primary outcome was 90-day all-cause mortality, which occurred in 109 and 116 patients in the liberal and restricted groups, respectively (approximately 14% of each group). No significant differences were noted among subgroups based on factors including systolic blood pressure and the use of vasopressors at randomization, chronic heart failure, end-stage renal disease, and pneumonia.
The restrictive fluid protocol called for vasopressors as the primary treatment for sepsis-induced hypotension, with “rescue fluids” to be used for prespecified situations of severe intravascular volume depletion. The liberal fluid protocol was a recommended initial intravenous infusion of 2,000 mL of isotonic crystalloid, followed by fluid boluses given based on clinical triggers such as tachycardia, along with “rescue vasopressors,” the researchers wrote.
The median volume of fluid administered in the first 24-hour period after randomization was 1,267 mL in the restrictive group and 3,400 mL in the liberal group. Adherence to the treatment protocols was greater than 90% for both groups.
The current study is distinct in its enrollment of patients with primary presentations of sepsis to a hospital emergency department, the researchers wrote in their discussion. we expect our findings to be generalizable to these types of patients,” they said.
Reported serious adverse events were similar between the groups, though fewer episodes of fluid overload and pulmonary edema occurred in the restricted group.
The findings were limited by several factors including some cases in which patients in the restrictive group received more fluid than called for by the protocol, the researchers noted. Other limitations included the lack of subgroups with different coexisting conditions, the lack of blinding, and the lack of a control with no instructions for treatment protocol, they said. However, the results suggest that a restrictive fluid strategy had no significant advantage over a liberal strategy in terms of mortality for patients with sepsis-induced hypotension, they concluded.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Shapiro disclosed serving as a consultant for and having stock options in Diagnostic Robotics, as well as grant support from Inflammatrix and Rapid Pathogen Screening, and serving as a consultant for Prenosis.
A restrictive fluid strategy had no significant impact on mortality in patients with sepsis-induced hypotension compared to the typical liberal fluid strategy, based on data from 1,563 individuals.
Intravenous fluids are standard in the early resuscitation of sepsis patients, as are vasopressor agents, but data comparing restrictive or liberal use in these patients are limited, wrote Nathan I. Shapiro, MD, of Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, and colleagues.
In a study published in the New England Journal of Medicine the researchers randomized 782 patients to the restrictive fluid group and 781 to the liberal fluid group. Patients aged 18 years and older were enrolled between March 7, 2018, and Jan. 31, 2022, at 60 centers in the United States. Participants were randomized within 4 hours of meeting the criteria for sepsis-induced hypotension that was refractory to initial treatment with 1-3 L of intravenous fluid. Baseline characteristics were similar between the groups. At randomization, 21% of patients in the restrictive fluid group and 18% in the liberal fluid group received vasopressors.
The primary outcome was 90-day all-cause mortality, which occurred in 109 and 116 patients in the liberal and restricted groups, respectively (approximately 14% of each group). No significant differences were noted among subgroups based on factors including systolic blood pressure and the use of vasopressors at randomization, chronic heart failure, end-stage renal disease, and pneumonia.
The restrictive fluid protocol called for vasopressors as the primary treatment for sepsis-induced hypotension, with “rescue fluids” to be used for prespecified situations of severe intravascular volume depletion. The liberal fluid protocol was a recommended initial intravenous infusion of 2,000 mL of isotonic crystalloid, followed by fluid boluses given based on clinical triggers such as tachycardia, along with “rescue vasopressors,” the researchers wrote.
The median volume of fluid administered in the first 24-hour period after randomization was 1,267 mL in the restrictive group and 3,400 mL in the liberal group. Adherence to the treatment protocols was greater than 90% for both groups.
The current study is distinct in its enrollment of patients with primary presentations of sepsis to a hospital emergency department, the researchers wrote in their discussion. we expect our findings to be generalizable to these types of patients,” they said.
Reported serious adverse events were similar between the groups, though fewer episodes of fluid overload and pulmonary edema occurred in the restricted group.
The findings were limited by several factors including some cases in which patients in the restrictive group received more fluid than called for by the protocol, the researchers noted. Other limitations included the lack of subgroups with different coexisting conditions, the lack of blinding, and the lack of a control with no instructions for treatment protocol, they said. However, the results suggest that a restrictive fluid strategy had no significant advantage over a liberal strategy in terms of mortality for patients with sepsis-induced hypotension, they concluded.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Shapiro disclosed serving as a consultant for and having stock options in Diagnostic Robotics, as well as grant support from Inflammatrix and Rapid Pathogen Screening, and serving as a consultant for Prenosis.
A restrictive fluid strategy had no significant impact on mortality in patients with sepsis-induced hypotension compared to the typical liberal fluid strategy, based on data from 1,563 individuals.
Intravenous fluids are standard in the early resuscitation of sepsis patients, as are vasopressor agents, but data comparing restrictive or liberal use in these patients are limited, wrote Nathan I. Shapiro, MD, of Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, and colleagues.
In a study published in the New England Journal of Medicine the researchers randomized 782 patients to the restrictive fluid group and 781 to the liberal fluid group. Patients aged 18 years and older were enrolled between March 7, 2018, and Jan. 31, 2022, at 60 centers in the United States. Participants were randomized within 4 hours of meeting the criteria for sepsis-induced hypotension that was refractory to initial treatment with 1-3 L of intravenous fluid. Baseline characteristics were similar between the groups. At randomization, 21% of patients in the restrictive fluid group and 18% in the liberal fluid group received vasopressors.
The primary outcome was 90-day all-cause mortality, which occurred in 109 and 116 patients in the liberal and restricted groups, respectively (approximately 14% of each group). No significant differences were noted among subgroups based on factors including systolic blood pressure and the use of vasopressors at randomization, chronic heart failure, end-stage renal disease, and pneumonia.
The restrictive fluid protocol called for vasopressors as the primary treatment for sepsis-induced hypotension, with “rescue fluids” to be used for prespecified situations of severe intravascular volume depletion. The liberal fluid protocol was a recommended initial intravenous infusion of 2,000 mL of isotonic crystalloid, followed by fluid boluses given based on clinical triggers such as tachycardia, along with “rescue vasopressors,” the researchers wrote.
The median volume of fluid administered in the first 24-hour period after randomization was 1,267 mL in the restrictive group and 3,400 mL in the liberal group. Adherence to the treatment protocols was greater than 90% for both groups.
The current study is distinct in its enrollment of patients with primary presentations of sepsis to a hospital emergency department, the researchers wrote in their discussion. we expect our findings to be generalizable to these types of patients,” they said.
Reported serious adverse events were similar between the groups, though fewer episodes of fluid overload and pulmonary edema occurred in the restricted group.
The findings were limited by several factors including some cases in which patients in the restrictive group received more fluid than called for by the protocol, the researchers noted. Other limitations included the lack of subgroups with different coexisting conditions, the lack of blinding, and the lack of a control with no instructions for treatment protocol, they said. However, the results suggest that a restrictive fluid strategy had no significant advantage over a liberal strategy in terms of mortality for patients with sepsis-induced hypotension, they concluded.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Shapiro disclosed serving as a consultant for and having stock options in Diagnostic Robotics, as well as grant support from Inflammatrix and Rapid Pathogen Screening, and serving as a consultant for Prenosis.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Sleep abnormalities common in all stages of psychosis
For example, compared with their healthy peers, participants in a chronic psychosis stage had reduced density, amplitude, and duration of spindles – or bursts of brainwave activity during sleep identified by electroencephalography.
“The results suggest sleep could be an important target [and] an area of research and clinical intervention that could make a difference” in the lives of patients at risk for psychosis, study investigator Fabio Ferrarelli, MD, PhD, associate professor of psychiatry and director of the Sleep and Schizophrenia Program, University of Pittsburgh School of Medicine, told this news organization.
The findings were published online in JAMA Psychiatry.
‘Window of opportunity’
Researchers separate psychosis into stages. During the “clinically high-risk for psychosis” (CHR-P) stage, patients have milder symptoms but do not have a diagnosable psychotic disorder. Those in the early psychosis (EP) stage have had a first episode of psychosis. When they reach a cut-off, often at 5 years, they are considered to have chronic psychosis (CP).
Previous studies have shown that altered sleep often precedes a psychotic episode in early psychosis, and disrupted sleep contributes to predicting transition to psychosis in youth at risk for the condition. Individuals with CP commonly report sleep disturbances, such as insomnia.
Following a literature search, the investigators for this current meta-analysis selected 21 studies assessing sleep disturbance prevalence in 5,135 patients. They also selected 39 studies measuring sleep alterations subjectively (for example, sleep quality) and/or objectively (for example, sleep architecture and sleep oscillation) in 1,575 patients and 977 healthy controls.
The included studies measured the prevalence of sleep disturbances and/or sleep characteristics at different psychosis stages using polysomnography, EEG, actigraphy, or self-reports.
The pooled prevalence of sleep disturbances was 50% across clinical stages (95% confidence interval, 40%-61%). The prevalence was 54% in CHR-P, 68% in EP, and 44% in CP.
The prevalence of insomnia as the primary sleep disturbance was 34% of pooled cases, 48% of the EP group, and 27% of the CP group.
“What’s interesting is the rate of sleep disturbances is relatively stable across stages,” said Dr. Ferrarelli. “This is important because you have a window of opportunity to do some early intervention in people who are at risk that can prevent things from getting worse.”
He suggests clinicians screen for insomnia in early-course patients and perhaps recommend cognitive behavioral therapy (CBT) for insomnia. As well, they should promote sleep hygiene measures for at-risk patients, including such things as avoiding caffeine, alcohol, and screen time before bedtime and adopting a regular sleep pattern.
“These are people at risk, which means they have a 20%-30% chance of eventually developing a psychotic disorder,” said Dr. Ferrarelli. “Maybe disrupted sleep is one of the factors that can make a difference.”
Altered sleep architecture
To compare sleep quality between clinical and control groups, studies used total scores on the Pittsburgh Sleep Quality Index (PSQI), where a score over 5 indicates a sleep problem.
There was a significant standardized mean difference in pooled cases versus controls (SMD, 1.0; 95% CI, 0.7-1.3; P < .001). Each clinical group showed poorer sleep quality, compared with controls.
When assessing sleep architecture abnormalities, stage-specific case-control comparisons showed these were driven by EP and CP stages.
Altered sleep characteristics in both these stages included increased sleep onset latency, increased wake after sleep onset, and reduced sleep efficiency.
Compared with controls, CP was the only clinical group with more arousals. Patients with CP also had more arousals than the CHR-P group, and the number of arousals was significantly affected by medication.
The findings indicate the effects of antipsychotic medications on sleep should be closely monitored, especially in CP, the investigators write.
They add that clinicians should consider medication adjustments, such as decreased doses or switches to another compound.
‘Robust’ spindle results
As for spindle parameters, pooled cases showed significantly decreased spindle density (SMD, –1.06), spindle amplitude (SMD, –1.08), and spindle duration (SMD, −1.21), compared with controls. Stage-specific comparisons revealed these deficits were present in both EP and CP relative to controls.
Dr. Ferrarelli noted the results for spindle abnormalities were among “the most robust” and show that these abnormalities “tend to get worse over the course of the illness.”
The spindle data are “a lot more informative” than that provided by other sleep parameters “in the sense they can yield what could be wrong, where it could be, and potentially what you can do about it,” said Dr. Ferrarelli.
“This might be an objective measure that could be used to identify individuals who have a psychosis disorder, monitor progression of illness, and for prognostic reasons,” he added.
He noted that spindles may also represent a promising target for treatment interventions and added that non-invasive transcranial magnetic stimulation has shown promise in restoring sleep oscillations, including spindles.
Another way to evoke target-brain activity may be through auditory tones – with a patient listening to a particular sound through headphones while asleep, Dr. Ferrarelli said.
Reaffirms previous data
Commenting on the study, Jeffrey A. Lieberman, MD, professor and chair in psychiatry at Columbia University, New York, and a past president of the American Psychiatric Association, noted that the review “just reaffirms what has been reported by individual studies for decades.”
That so many at-risk study subjects had a sleep abnormality is not surprising, said Dr. Lieberman, who was not involved with the current research.
“How many individuals in late adolescence or early adulthood have sleep problems?” he asked. “I would venture to say it’s probably a lot. So the question is: How distinctive is this from what occurs in people who don’t develop the illness?”
The aim of sleep research in the area of schizophrenia has long been to disentangle the effects of medication and environmental factors from the disease and to be able to treat patients to normalize their sleep, said Dr. Lieberman.
“But it’s not clear from these results how one would do that,” he added.
The authors “don’t fundamentally tell us anything about the underlying cause of the illness or the pathophysiology, and they don’t really offer any kind of clear direction for clinical intervention,” he said.
The study was supported by the National Institute of Mental Health. Dr. Ferrarelli reported grants from the National Institute of Mental Health during the conduct of the study. Dr. Lieberman has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For example, compared with their healthy peers, participants in a chronic psychosis stage had reduced density, amplitude, and duration of spindles – or bursts of brainwave activity during sleep identified by electroencephalography.
“The results suggest sleep could be an important target [and] an area of research and clinical intervention that could make a difference” in the lives of patients at risk for psychosis, study investigator Fabio Ferrarelli, MD, PhD, associate professor of psychiatry and director of the Sleep and Schizophrenia Program, University of Pittsburgh School of Medicine, told this news organization.
The findings were published online in JAMA Psychiatry.
‘Window of opportunity’
Researchers separate psychosis into stages. During the “clinically high-risk for psychosis” (CHR-P) stage, patients have milder symptoms but do not have a diagnosable psychotic disorder. Those in the early psychosis (EP) stage have had a first episode of psychosis. When they reach a cut-off, often at 5 years, they are considered to have chronic psychosis (CP).
Previous studies have shown that altered sleep often precedes a psychotic episode in early psychosis, and disrupted sleep contributes to predicting transition to psychosis in youth at risk for the condition. Individuals with CP commonly report sleep disturbances, such as insomnia.
Following a literature search, the investigators for this current meta-analysis selected 21 studies assessing sleep disturbance prevalence in 5,135 patients. They also selected 39 studies measuring sleep alterations subjectively (for example, sleep quality) and/or objectively (for example, sleep architecture and sleep oscillation) in 1,575 patients and 977 healthy controls.
The included studies measured the prevalence of sleep disturbances and/or sleep characteristics at different psychosis stages using polysomnography, EEG, actigraphy, or self-reports.
The pooled prevalence of sleep disturbances was 50% across clinical stages (95% confidence interval, 40%-61%). The prevalence was 54% in CHR-P, 68% in EP, and 44% in CP.
The prevalence of insomnia as the primary sleep disturbance was 34% of pooled cases, 48% of the EP group, and 27% of the CP group.
“What’s interesting is the rate of sleep disturbances is relatively stable across stages,” said Dr. Ferrarelli. “This is important because you have a window of opportunity to do some early intervention in people who are at risk that can prevent things from getting worse.”
He suggests clinicians screen for insomnia in early-course patients and perhaps recommend cognitive behavioral therapy (CBT) for insomnia. As well, they should promote sleep hygiene measures for at-risk patients, including such things as avoiding caffeine, alcohol, and screen time before bedtime and adopting a regular sleep pattern.
“These are people at risk, which means they have a 20%-30% chance of eventually developing a psychotic disorder,” said Dr. Ferrarelli. “Maybe disrupted sleep is one of the factors that can make a difference.”
Altered sleep architecture
To compare sleep quality between clinical and control groups, studies used total scores on the Pittsburgh Sleep Quality Index (PSQI), where a score over 5 indicates a sleep problem.
There was a significant standardized mean difference in pooled cases versus controls (SMD, 1.0; 95% CI, 0.7-1.3; P < .001). Each clinical group showed poorer sleep quality, compared with controls.
When assessing sleep architecture abnormalities, stage-specific case-control comparisons showed these were driven by EP and CP stages.
Altered sleep characteristics in both these stages included increased sleep onset latency, increased wake after sleep onset, and reduced sleep efficiency.
Compared with controls, CP was the only clinical group with more arousals. Patients with CP also had more arousals than the CHR-P group, and the number of arousals was significantly affected by medication.
The findings indicate the effects of antipsychotic medications on sleep should be closely monitored, especially in CP, the investigators write.
They add that clinicians should consider medication adjustments, such as decreased doses or switches to another compound.
‘Robust’ spindle results
As for spindle parameters, pooled cases showed significantly decreased spindle density (SMD, –1.06), spindle amplitude (SMD, –1.08), and spindle duration (SMD, −1.21), compared with controls. Stage-specific comparisons revealed these deficits were present in both EP and CP relative to controls.
Dr. Ferrarelli noted the results for spindle abnormalities were among “the most robust” and show that these abnormalities “tend to get worse over the course of the illness.”
The spindle data are “a lot more informative” than that provided by other sleep parameters “in the sense they can yield what could be wrong, where it could be, and potentially what you can do about it,” said Dr. Ferrarelli.
“This might be an objective measure that could be used to identify individuals who have a psychosis disorder, monitor progression of illness, and for prognostic reasons,” he added.
He noted that spindles may also represent a promising target for treatment interventions and added that non-invasive transcranial magnetic stimulation has shown promise in restoring sleep oscillations, including spindles.
Another way to evoke target-brain activity may be through auditory tones – with a patient listening to a particular sound through headphones while asleep, Dr. Ferrarelli said.
Reaffirms previous data
Commenting on the study, Jeffrey A. Lieberman, MD, professor and chair in psychiatry at Columbia University, New York, and a past president of the American Psychiatric Association, noted that the review “just reaffirms what has been reported by individual studies for decades.”
That so many at-risk study subjects had a sleep abnormality is not surprising, said Dr. Lieberman, who was not involved with the current research.
“How many individuals in late adolescence or early adulthood have sleep problems?” he asked. “I would venture to say it’s probably a lot. So the question is: How distinctive is this from what occurs in people who don’t develop the illness?”
The aim of sleep research in the area of schizophrenia has long been to disentangle the effects of medication and environmental factors from the disease and to be able to treat patients to normalize their sleep, said Dr. Lieberman.
“But it’s not clear from these results how one would do that,” he added.
The authors “don’t fundamentally tell us anything about the underlying cause of the illness or the pathophysiology, and they don’t really offer any kind of clear direction for clinical intervention,” he said.
The study was supported by the National Institute of Mental Health. Dr. Ferrarelli reported grants from the National Institute of Mental Health during the conduct of the study. Dr. Lieberman has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For example, compared with their healthy peers, participants in a chronic psychosis stage had reduced density, amplitude, and duration of spindles – or bursts of brainwave activity during sleep identified by electroencephalography.
“The results suggest sleep could be an important target [and] an area of research and clinical intervention that could make a difference” in the lives of patients at risk for psychosis, study investigator Fabio Ferrarelli, MD, PhD, associate professor of psychiatry and director of the Sleep and Schizophrenia Program, University of Pittsburgh School of Medicine, told this news organization.
The findings were published online in JAMA Psychiatry.
‘Window of opportunity’
Researchers separate psychosis into stages. During the “clinically high-risk for psychosis” (CHR-P) stage, patients have milder symptoms but do not have a diagnosable psychotic disorder. Those in the early psychosis (EP) stage have had a first episode of psychosis. When they reach a cut-off, often at 5 years, they are considered to have chronic psychosis (CP).
Previous studies have shown that altered sleep often precedes a psychotic episode in early psychosis, and disrupted sleep contributes to predicting transition to psychosis in youth at risk for the condition. Individuals with CP commonly report sleep disturbances, such as insomnia.
Following a literature search, the investigators for this current meta-analysis selected 21 studies assessing sleep disturbance prevalence in 5,135 patients. They also selected 39 studies measuring sleep alterations subjectively (for example, sleep quality) and/or objectively (for example, sleep architecture and sleep oscillation) in 1,575 patients and 977 healthy controls.
The included studies measured the prevalence of sleep disturbances and/or sleep characteristics at different psychosis stages using polysomnography, EEG, actigraphy, or self-reports.
The pooled prevalence of sleep disturbances was 50% across clinical stages (95% confidence interval, 40%-61%). The prevalence was 54% in CHR-P, 68% in EP, and 44% in CP.
The prevalence of insomnia as the primary sleep disturbance was 34% of pooled cases, 48% of the EP group, and 27% of the CP group.
“What’s interesting is the rate of sleep disturbances is relatively stable across stages,” said Dr. Ferrarelli. “This is important because you have a window of opportunity to do some early intervention in people who are at risk that can prevent things from getting worse.”
He suggests clinicians screen for insomnia in early-course patients and perhaps recommend cognitive behavioral therapy (CBT) for insomnia. As well, they should promote sleep hygiene measures for at-risk patients, including such things as avoiding caffeine, alcohol, and screen time before bedtime and adopting a regular sleep pattern.
“These are people at risk, which means they have a 20%-30% chance of eventually developing a psychotic disorder,” said Dr. Ferrarelli. “Maybe disrupted sleep is one of the factors that can make a difference.”
Altered sleep architecture
To compare sleep quality between clinical and control groups, studies used total scores on the Pittsburgh Sleep Quality Index (PSQI), where a score over 5 indicates a sleep problem.
There was a significant standardized mean difference in pooled cases versus controls (SMD, 1.0; 95% CI, 0.7-1.3; P < .001). Each clinical group showed poorer sleep quality, compared with controls.
When assessing sleep architecture abnormalities, stage-specific case-control comparisons showed these were driven by EP and CP stages.
Altered sleep characteristics in both these stages included increased sleep onset latency, increased wake after sleep onset, and reduced sleep efficiency.
Compared with controls, CP was the only clinical group with more arousals. Patients with CP also had more arousals than the CHR-P group, and the number of arousals was significantly affected by medication.
The findings indicate the effects of antipsychotic medications on sleep should be closely monitored, especially in CP, the investigators write.
They add that clinicians should consider medication adjustments, such as decreased doses or switches to another compound.
‘Robust’ spindle results
As for spindle parameters, pooled cases showed significantly decreased spindle density (SMD, –1.06), spindle amplitude (SMD, –1.08), and spindle duration (SMD, −1.21), compared with controls. Stage-specific comparisons revealed these deficits were present in both EP and CP relative to controls.
Dr. Ferrarelli noted the results for spindle abnormalities were among “the most robust” and show that these abnormalities “tend to get worse over the course of the illness.”
The spindle data are “a lot more informative” than that provided by other sleep parameters “in the sense they can yield what could be wrong, where it could be, and potentially what you can do about it,” said Dr. Ferrarelli.
“This might be an objective measure that could be used to identify individuals who have a psychosis disorder, monitor progression of illness, and for prognostic reasons,” he added.
He noted that spindles may also represent a promising target for treatment interventions and added that non-invasive transcranial magnetic stimulation has shown promise in restoring sleep oscillations, including spindles.
Another way to evoke target-brain activity may be through auditory tones – with a patient listening to a particular sound through headphones while asleep, Dr. Ferrarelli said.
Reaffirms previous data
Commenting on the study, Jeffrey A. Lieberman, MD, professor and chair in psychiatry at Columbia University, New York, and a past president of the American Psychiatric Association, noted that the review “just reaffirms what has been reported by individual studies for decades.”
That so many at-risk study subjects had a sleep abnormality is not surprising, said Dr. Lieberman, who was not involved with the current research.
“How many individuals in late adolescence or early adulthood have sleep problems?” he asked. “I would venture to say it’s probably a lot. So the question is: How distinctive is this from what occurs in people who don’t develop the illness?”
The aim of sleep research in the area of schizophrenia has long been to disentangle the effects of medication and environmental factors from the disease and to be able to treat patients to normalize their sleep, said Dr. Lieberman.
“But it’s not clear from these results how one would do that,” he added.
The authors “don’t fundamentally tell us anything about the underlying cause of the illness or the pathophysiology, and they don’t really offer any kind of clear direction for clinical intervention,” he said.
The study was supported by the National Institute of Mental Health. Dr. Ferrarelli reported grants from the National Institute of Mental Health during the conduct of the study. Dr. Lieberman has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
FDA OKs elacestrant for ESR1+ advanced, metastatic breast cancer
that progressed on at least one line of endocrine therapy.
The agency also approved the Guardant360 CDx assay as a companion diagnostic to identify breast cancer patients who meet the treatment requirements, according to the agency’s press release announcing the approval.
The novel oral selective estrogen receptor degrader was approved based on the phase 3 EMERALD trial, which included 478 postmenopausal women and men with ER-positive, HER2-negative advanced or metastatic breast cancer, about half of whom had ESR1 mutations. Patients had progressed on one or two prior lines of endocrine therapy, including one containing a CDK4/6 inhibitor. Participants could also have had one prior line of chemotherapy in the advanced or metastatic setting.
Participants were randomized 1:1 to either elacestrant 345 mg orally once daily or investigator’s choice of endocrine therapy, which included fulvestrant or an aromatase inhibitor.
In the 228 patients (48%) with ESR1 mutations, median progression-free survival (PFS) was 3.8 months with elacestrant versus 1.9 months in the fulvestrant or aromatase inhibitor arm (hazard ratio, 0.55; P = .0005). Investigators observed no statistically significant PFS difference between the treatment arms in patients who didn’t have the mutation.
Fair comparison?
In June, experts raised concerns about the adequacy of the “standard of care” control arm in EMERALD, particularly that single agents were used at a time when combination therapy is becoming more common.
“The expression ‘standard of care’ is applied generously, as the control arm is restricted” to single agents and no combinations, which “may have led to a substandard” comparison group, Timothée Olivier, MD, Geneva University Hospital, and Vinay Prasad, MD, MPH, University of California, San Francisco, said in an editorial quoted in the piece.
EMERALD investigators acknowledged that there were issues with the control group, noting that in the “United States and Europe, combination therapy with fulvestrant” – instead of single agents – “is increasingly being used as the second-line [standard of care] treatment.”
However, the goal of the study “was to compare a novel endocrine therapy vs. currently available endocrine therapies,” not combination regimens, the investigators said.
Also, “the benefit of elacestrant over fulvestrant and AIs [aromatase inhibitors] in our monotherapy trial ... suggests that incorporating elacestrant as the preferred endocrine therapy backbone in future earlier-line combination studies is a promising strategy.”
Lipid monitoring necessary
The most common adverse events with elacestrant, occurring in 10% or more of patients, are musculoskeletal pain, nausea, increased cholesterol, increased AST, increased triglycerides, fatigue, decreased hemoglobin, vomiting, increased ALT, decreased sodium, increased creatinine, decreased appetite, diarrhea, headache, constipation, abdominal pain, hot flush, and dyspepsia, according to labeling.
Labeling warns that elacestrant “may cause hypercholesterolemia and hypertriglyceridemia. Monitor lipid profile prior to starting treatment and periodically thereafter.”
The recommended elacestrant dose is the trial dose, 345 mg orally with food once daily until disease progression or unacceptable toxicity.
A version of this article first appeared on Medscape.com.
that progressed on at least one line of endocrine therapy.
The agency also approved the Guardant360 CDx assay as a companion diagnostic to identify breast cancer patients who meet the treatment requirements, according to the agency’s press release announcing the approval.
The novel oral selective estrogen receptor degrader was approved based on the phase 3 EMERALD trial, which included 478 postmenopausal women and men with ER-positive, HER2-negative advanced or metastatic breast cancer, about half of whom had ESR1 mutations. Patients had progressed on one or two prior lines of endocrine therapy, including one containing a CDK4/6 inhibitor. Participants could also have had one prior line of chemotherapy in the advanced or metastatic setting.
Participants were randomized 1:1 to either elacestrant 345 mg orally once daily or investigator’s choice of endocrine therapy, which included fulvestrant or an aromatase inhibitor.
In the 228 patients (48%) with ESR1 mutations, median progression-free survival (PFS) was 3.8 months with elacestrant versus 1.9 months in the fulvestrant or aromatase inhibitor arm (hazard ratio, 0.55; P = .0005). Investigators observed no statistically significant PFS difference between the treatment arms in patients who didn’t have the mutation.
Fair comparison?
In June, experts raised concerns about the adequacy of the “standard of care” control arm in EMERALD, particularly that single agents were used at a time when combination therapy is becoming more common.
“The expression ‘standard of care’ is applied generously, as the control arm is restricted” to single agents and no combinations, which “may have led to a substandard” comparison group, Timothée Olivier, MD, Geneva University Hospital, and Vinay Prasad, MD, MPH, University of California, San Francisco, said in an editorial quoted in the piece.
EMERALD investigators acknowledged that there were issues with the control group, noting that in the “United States and Europe, combination therapy with fulvestrant” – instead of single agents – “is increasingly being used as the second-line [standard of care] treatment.”
However, the goal of the study “was to compare a novel endocrine therapy vs. currently available endocrine therapies,” not combination regimens, the investigators said.
Also, “the benefit of elacestrant over fulvestrant and AIs [aromatase inhibitors] in our monotherapy trial ... suggests that incorporating elacestrant as the preferred endocrine therapy backbone in future earlier-line combination studies is a promising strategy.”
Lipid monitoring necessary
The most common adverse events with elacestrant, occurring in 10% or more of patients, are musculoskeletal pain, nausea, increased cholesterol, increased AST, increased triglycerides, fatigue, decreased hemoglobin, vomiting, increased ALT, decreased sodium, increased creatinine, decreased appetite, diarrhea, headache, constipation, abdominal pain, hot flush, and dyspepsia, according to labeling.
Labeling warns that elacestrant “may cause hypercholesterolemia and hypertriglyceridemia. Monitor lipid profile prior to starting treatment and periodically thereafter.”
The recommended elacestrant dose is the trial dose, 345 mg orally with food once daily until disease progression or unacceptable toxicity.
A version of this article first appeared on Medscape.com.
that progressed on at least one line of endocrine therapy.
The agency also approved the Guardant360 CDx assay as a companion diagnostic to identify breast cancer patients who meet the treatment requirements, according to the agency’s press release announcing the approval.
The novel oral selective estrogen receptor degrader was approved based on the phase 3 EMERALD trial, which included 478 postmenopausal women and men with ER-positive, HER2-negative advanced or metastatic breast cancer, about half of whom had ESR1 mutations. Patients had progressed on one or two prior lines of endocrine therapy, including one containing a CDK4/6 inhibitor. Participants could also have had one prior line of chemotherapy in the advanced or metastatic setting.
Participants were randomized 1:1 to either elacestrant 345 mg orally once daily or investigator’s choice of endocrine therapy, which included fulvestrant or an aromatase inhibitor.
In the 228 patients (48%) with ESR1 mutations, median progression-free survival (PFS) was 3.8 months with elacestrant versus 1.9 months in the fulvestrant or aromatase inhibitor arm (hazard ratio, 0.55; P = .0005). Investigators observed no statistically significant PFS difference between the treatment arms in patients who didn’t have the mutation.
Fair comparison?
In June, experts raised concerns about the adequacy of the “standard of care” control arm in EMERALD, particularly that single agents were used at a time when combination therapy is becoming more common.
“The expression ‘standard of care’ is applied generously, as the control arm is restricted” to single agents and no combinations, which “may have led to a substandard” comparison group, Timothée Olivier, MD, Geneva University Hospital, and Vinay Prasad, MD, MPH, University of California, San Francisco, said in an editorial quoted in the piece.
EMERALD investigators acknowledged that there were issues with the control group, noting that in the “United States and Europe, combination therapy with fulvestrant” – instead of single agents – “is increasingly being used as the second-line [standard of care] treatment.”
However, the goal of the study “was to compare a novel endocrine therapy vs. currently available endocrine therapies,” not combination regimens, the investigators said.
Also, “the benefit of elacestrant over fulvestrant and AIs [aromatase inhibitors] in our monotherapy trial ... suggests that incorporating elacestrant as the preferred endocrine therapy backbone in future earlier-line combination studies is a promising strategy.”
Lipid monitoring necessary
The most common adverse events with elacestrant, occurring in 10% or more of patients, are musculoskeletal pain, nausea, increased cholesterol, increased AST, increased triglycerides, fatigue, decreased hemoglobin, vomiting, increased ALT, decreased sodium, increased creatinine, decreased appetite, diarrhea, headache, constipation, abdominal pain, hot flush, and dyspepsia, according to labeling.
Labeling warns that elacestrant “may cause hypercholesterolemia and hypertriglyceridemia. Monitor lipid profile prior to starting treatment and periodically thereafter.”
The recommended elacestrant dose is the trial dose, 345 mg orally with food once daily until disease progression or unacceptable toxicity.
A version of this article first appeared on Medscape.com.
Three wishes: The changes health professionals want
As physicians well know, magic wands don’t exist. If they did, every patient would recover in the exam room, prior authorization wouldn’t exist, and continuing medical education credits would be printed on bearer bonds.
But Because, hey – we all need to dream.
Suzanne C. Boulter, MD, adjunct professor of pediatrics and community and family medicine, Geisel School of Medicine at Dartmouth, Hanover, N.H.
Patients: An end to gun violence.
Practice/hospital: Adequate staffing and pediatric bed availability.
Health system: Universal access to health insurance.
Sarah G. Candler, MD, MPH, care team medical director and director of academic relations, Iora Primary Care, Northside Clinic, Houston
Patients: Systems of health that start with communities of safety, including access to affordable housing, food, transportation, and health care.
Practice/hospital: I.N.T.E.R.O.P.E.R.A.B.I.L.I.T.Y.
Health system: Clinician leadership that has the power (often aka funding) to do what’s right, not just what’s right in front of us.
Arthur L. Caplan, PhD, bioethicist, New York University Langone Health
Patients: I wish for patients in the United States greater access to affordable primary care. There are still too many people without insurance or a reasonably accessible quality provider. And I especially wish for the rapid expansion of affordable training programs to meet staffing needs, including more scholarships, 3-year programs, and more new primary care–oriented schools.
Hospital: Increased staffing, especially nursing. There are too many retirements, too much burnout, and too much privatization into boutique practices to ensure the ability to provide high-quality, safe, patient-oriented care.
Health system: I wish for health systems to seriously move into electronic medicine. While billing has become electronic, there is still much to be done to supplement diagnosis, training, and standardized data collection on key metrics. Systems are not yet behaving in a manner consistent with the hype in this regard.
Stephen Devries, MD, executive director, Gaples Institute (nonprofit) and adjunct associate professor of nutrition, Harvard School of Public Health, Boston
Patients: Patients continue to demand more from their health care professionals and insist that they are offered evidence-based counseling on nutrition and lifestyle strategies.
Practice: Quality-based reimbursement for medical services will take hold that will incentivize much-needed preventive care.
Hospital: Hospitals will more fully embrace the role of serving as true centers of health and focus as much on preventive medicine as on the more lucrative areas of high-tech treatment.
Peter D. Friedmann, MD, MPH, chief research officer, Baystate Health, Springfield, Mass.
Seconded by: Elisabeth Poorman, MD, general internist, University of Washington Clinic, Kent
Patients: Don’t forget the ongoing epidemic of substance use disorder, a major cause of premature mortality. Descheduling of cannabis and expungement of cannabis-related convictions.
Practice/hospital: Commitment of hospitals and practices to address stigma and ensure delivery of medications for opioid use disorder in primary care, the emergency department, and inpatient settings.
Health system: Reform of antiquated methadone regulations to permit office-based prescription and pharmacy dispensing to treat opioid use disorder, as is the case in most of the world.
Robert Glatter, MD, emergency physician, New York
Patients: I want all patients to understand the enormous strain the health care system has been under – not just with the pandemic, the tripledemic, and mpox [previously called monkeypox], but well before the onset of these public health crises.
Hospital: The medical profession has endured not only burnout but a growing mental health crisis, staffing shortages, a physician addiction crisis, and increased attrition in the decade leading up to the pandemic. The pandemic was like a punch in the gut, occurring at the most inopportune time one could imagine.
Health system: The intersection of health and the state of our public health deserves important mention. Unless we take action to bolster our public health infrastructure, our health care system will be in jeopardy, unable to handle the next pandemic, which could be just around the corner.
William E. Golden, MD, medical director of Arkansas Medicaid, professor of medicine and public health, University of Arkansas for Medical Sciences, Little Rock
Patients: Affordable options for diabetes and obesity management.
Health system: Greater investment by health systems and third-party payers in primary care infrastructure.
Gregory A. Hood, MD, Baptist Health, Lexington, Ky.
Patients: To embrace the gift of getting out in the world, being active, and connecting with others – having put down the screens.
Health system: To be freed from the financial gamesmanship of the insurers as they continue to serve their goals of promoting their hedge fund investing over meaningful and productive partnering with primary care physicians, and that they gain insight that they are one of the main reasons they can’t find PCPs to connect with to render care in disadvantaged environments – because they made it economically impossible to do so.
Robert H. Hopkins Jr., MD, associate professor of internal medicine and pediatrics and director of the division of general internal medicine, University of Arkansas for Medical Sciences, Little Rock
Patients/Health system: I would wish for staged implementation of universal basic health coverage for all, perhaps closest to the French or Canadian model. This would need to be coupled with expanded funding for nursing education, graduate medical education, and tracing of other health-related professionals.
Harvey Hsu, MD, Banner Health, Phoenix
Patients: More clear guidelines that are simple to understand. This can apply to colonoscopy (now age 45), immunizations, blood pressure goals. I wish medications were not as expensive so patients can take the best medicine for them and not stop taking them when they hit their donut hole in coverage.
Practice: We have been functioning on a leaner basis to cut down costs. When the pandemic hit, turnover was high and we lost PAs, nurses, front-office staff, and physicians. Having adequate staffing is probably number one on many lists. One way we dealt with lack of staffing was converting in-person visits to telehealth. Video visits are paid the same as in-person visits, but if the patient could not get their video to work, then it would be a telephone visit. Now many insurances do not even pay for telephone visits. So I would wish that we could still be reimbursed for telehealth visits.
Health system: I would wish for our health system to recognize the extra work required to take care of patients while improving quality and meeting quality measures. Allowing more time for patient visits could be one way to meet those goals or having more support staff to make sure patients get their colonoscopy/mammograms done, improve their sugars, and take their medications.
Jan L. Shifren, MD, Vincent Trustees Professor, obstetrics, gynecology, and reproductive biology, Harvard Medical School, and director of the Midlife Women’s Health Center at Massachusetts General Hospital, Boston
Patients: I wish for patients to be actively involved in all aspects of their care, well informed with shared decision-making.
Practice: I wish for the enormous time demands of electronic medical records and documentation to not distract from the pleasure of caring for patients.
Health system: Patient care remains at the center of decisions and programs.
Timothy J. Joos, MD, MPH, internal medicine/pediatrics, Seattle
Health system: I wish someone could figure out how we could be reimbursed for the quality of care we provide instead of the volume of patients we see. I wish EMRs could become less complicated and more user-friendly rather than needing advanced training to use.
Peter Kovacs, MD, medical director, Kaali Institute IVF Center, Budapest
Patients: I work as an infertility specialist, so when we talk about infectious diseases and associated risks, we talk about a minimum of two (female and male partner) and ideally three (plus the pregnancy) individuals. We have learned that SARS-CoV-2 affects reproductive health. It may compromise sperm production, could delay fertility treatment, could be associated with lower success rates; and if the treatment is successful, it may harm the pregnant woman/fetus/newborn. The best preventive measure that we can offer is vaccination. One cannot overemphasize the importance of preventive measures, paying attention to personal hygiene and social distancing. Therefore, I wish those planning to become pregnant to listen to their health care provider and accept the recommended vaccines to minimize the risk of getting infected and to minimize the risk for severe disease, especially if one undergoes successful fertility treatment and achieves a long-desired pregnancy.
Practice: During the 2022 calendar year we had many days when one or more employees were out of work on sick leave. This puts extra stress on the others to allow uncompromised work in the clinic. In addition, we all have to work in a less-comfortable environment if we consider mask use every day, all day. For health care workers, vaccination is mandated but many still are affected by milder forms of coronavirus infection and other respiratory diseases. Therefore, I wish my colleagues patience toward the preventive measures to lower the individual risk for infections. As a result, hopefully we will have a less stressful 2023.
Health system: Many resources had to be delegated to dealing with acute and chronic COVID, and this was at the expense of routine daily elective and preventive medical services. I wish the health care system to return to normal daily operations, to have the personnel and financial resources to carry on with the required preventive and elective medical services to avoid long-term consequences of not being able to provide such services. It would be sad if we had to treat otherwise preventable illnesses in the upcoming years that went undiagnosed and/or were not properly managed due to limited resources as the result of the pandemic.
Alan R. Nelson, MD, internist-endocrinologist, retired
Patients: Expansion of the FDA’s authority into over-the-counter drugs, including the veracity of their advertising claims.
Practice: Make diabetes drugs available at a reasonable cost.
Health system: With the expansion of Medicaid eligibility during COVID-19 coming to a close, federal government actions are necessary for those who once again have been dropped from coverage to have their legitimate needs met.
Kevin Powell, MD, PhD, St. Louis
Patients: To be cared for and about, and not just medically, even when illness strikes and health fails.
Hospitals: To hear the thankfulness of a grateful public for the care you provide, and to hear that above the angry noise of outraged individuals who spout vitriol and focus on how they believe others have harmed them.
Health system: A truer understanding of mercy and justice.
Margaret Thew, DNP, FNP-BC, director, department of adolescent medicine, Children’s Hospital of Wisconsin, Milwaukee
Seconded by: M. Susan Jay, MD, professor of pediatrics, chief of adolescent medicine, Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee
My wish for patients, hospital, and system: health, calm, and grace.
Mark P. Trolice, MD, director of Fertility CARE, the IVF Center, Winter Park, Fla.
Patients: To be proactive in their health care and be their own advocates. Question when unclear and only consult credible resources.
Practice/hospital: Improve support of physicians and all health care providers to allow more input in their practice operations and growth.
Health system: Reduce interference of the “business of medicine” and ensure that the patient experience is the priority.
Charles P. Vega, MD, University of California, Irvine
Three minutes on a routine basis for everyone in health care to reflect on our blessings and the honor and gravity – as well as joy – that are integral to health care. Three minutes that will also help us to recognize our challenges and put them in the proper context. I know 3 minutes is not meeting any standard for reflective practice. But it’s 3 minutes more than I have right now.
Karen Breach Washington, MD, medical director of WellCare of North Carolina/Centene, Charlotte
Seconded by: Lillian M. Beard, MD, physician director, Children’s Pediatricians and Associates, Silver Spring, Md.
Patients: Access to affordable health care.
Hospital: Resources to care for patients (sufficient number of beds and a healthy staff).
Health system: Equity for all.
Andrew Wilner, MD, host of the podcast “The Art of Medicine with Dr. Andrew Wilner,” www.andrewwilner.com
Let’s put patients first! Too many extraneous considerations other than the patient’s best interest obstruct optimal patient care.
Here are just a few examples of patients coming last instead of first.
- If a patient needs to start a new medication in hospital, we shouldn’t have to wait until the patient is an outpatient because “that’s when insurance will pay.”
- If there’s a new medication that’s better than the old medication, we shouldn’t be forced to choose the old medication and provide inferior care because “that’s when insurance will pay.”
- If patients need to stay in hospital, we shouldn’t be pressured to discharge them because the hospital has decided that decreasing “length of stay” is its highest priority.
Dr. Francis Peabody said it best in 1927: “The secret of the care of the patient is in caring for the patient.” How hard is that?
In 2023, why don’t we follow Dr. Peabody’s sage advice from nearly 100 years ago and see what happens?
James M. Wooten, PharmD, University of Missouri–Kansas City, University Health, Kansas City, Mo.
Patients: I want patients to understand and properly realize the advantage of vaccinations – not only for COVID-19 but also for influenza. There is so much misinformation that I spend a lot of time trying to convince patients to get vaccinated. Most patients don’t realize that through their lives, most of them have already been vaccinated for something just to be able to attend school. How the COVID-19 vaccine created so much stigma makes little sense to me. I also want patients to understand that COVID-19 vaccination and boosters do not always prevent infection but will many times prevent severe infection. I believe that better patient communication and education is the key and will always be the key to improving vaccination numbers. Not only communicating and educating patients on vaccination itself but also making patients realize that personal vaccination decisions may affect what happens to your neighbor. Allowing infection means that you may be more likely to infect someone else. As a society, we must take care of each other.
Health system: It will be interesting to see what happens when vaccines are no longer reimbursed by the federal government. Understanding which vaccines work best and are better tolerated will be key to choosing appropriate vaccine brands. Health care providers will need to be very selective regarding which vaccines are selected for formulary inclusion. Thorough meta-analysis studies must be done to provide more evaluable information to allow for appropriate selection. “Knowledge is power!” Appropriate knowledge will help distinguish which vaccines work best for various patient populations.
A version of this article first appeared on Medscape.com.
As physicians well know, magic wands don’t exist. If they did, every patient would recover in the exam room, prior authorization wouldn’t exist, and continuing medical education credits would be printed on bearer bonds.
But Because, hey – we all need to dream.
Suzanne C. Boulter, MD, adjunct professor of pediatrics and community and family medicine, Geisel School of Medicine at Dartmouth, Hanover, N.H.
Patients: An end to gun violence.
Practice/hospital: Adequate staffing and pediatric bed availability.
Health system: Universal access to health insurance.
Sarah G. Candler, MD, MPH, care team medical director and director of academic relations, Iora Primary Care, Northside Clinic, Houston
Patients: Systems of health that start with communities of safety, including access to affordable housing, food, transportation, and health care.
Practice/hospital: I.N.T.E.R.O.P.E.R.A.B.I.L.I.T.Y.
Health system: Clinician leadership that has the power (often aka funding) to do what’s right, not just what’s right in front of us.
Arthur L. Caplan, PhD, bioethicist, New York University Langone Health
Patients: I wish for patients in the United States greater access to affordable primary care. There are still too many people without insurance or a reasonably accessible quality provider. And I especially wish for the rapid expansion of affordable training programs to meet staffing needs, including more scholarships, 3-year programs, and more new primary care–oriented schools.
Hospital: Increased staffing, especially nursing. There are too many retirements, too much burnout, and too much privatization into boutique practices to ensure the ability to provide high-quality, safe, patient-oriented care.
Health system: I wish for health systems to seriously move into electronic medicine. While billing has become electronic, there is still much to be done to supplement diagnosis, training, and standardized data collection on key metrics. Systems are not yet behaving in a manner consistent with the hype in this regard.
Stephen Devries, MD, executive director, Gaples Institute (nonprofit) and adjunct associate professor of nutrition, Harvard School of Public Health, Boston
Patients: Patients continue to demand more from their health care professionals and insist that they are offered evidence-based counseling on nutrition and lifestyle strategies.
Practice: Quality-based reimbursement for medical services will take hold that will incentivize much-needed preventive care.
Hospital: Hospitals will more fully embrace the role of serving as true centers of health and focus as much on preventive medicine as on the more lucrative areas of high-tech treatment.
Peter D. Friedmann, MD, MPH, chief research officer, Baystate Health, Springfield, Mass.
Seconded by: Elisabeth Poorman, MD, general internist, University of Washington Clinic, Kent
Patients: Don’t forget the ongoing epidemic of substance use disorder, a major cause of premature mortality. Descheduling of cannabis and expungement of cannabis-related convictions.
Practice/hospital: Commitment of hospitals and practices to address stigma and ensure delivery of medications for opioid use disorder in primary care, the emergency department, and inpatient settings.
Health system: Reform of antiquated methadone regulations to permit office-based prescription and pharmacy dispensing to treat opioid use disorder, as is the case in most of the world.
Robert Glatter, MD, emergency physician, New York
Patients: I want all patients to understand the enormous strain the health care system has been under – not just with the pandemic, the tripledemic, and mpox [previously called monkeypox], but well before the onset of these public health crises.
Hospital: The medical profession has endured not only burnout but a growing mental health crisis, staffing shortages, a physician addiction crisis, and increased attrition in the decade leading up to the pandemic. The pandemic was like a punch in the gut, occurring at the most inopportune time one could imagine.
Health system: The intersection of health and the state of our public health deserves important mention. Unless we take action to bolster our public health infrastructure, our health care system will be in jeopardy, unable to handle the next pandemic, which could be just around the corner.
William E. Golden, MD, medical director of Arkansas Medicaid, professor of medicine and public health, University of Arkansas for Medical Sciences, Little Rock
Patients: Affordable options for diabetes and obesity management.
Health system: Greater investment by health systems and third-party payers in primary care infrastructure.
Gregory A. Hood, MD, Baptist Health, Lexington, Ky.
Patients: To embrace the gift of getting out in the world, being active, and connecting with others – having put down the screens.
Health system: To be freed from the financial gamesmanship of the insurers as they continue to serve their goals of promoting their hedge fund investing over meaningful and productive partnering with primary care physicians, and that they gain insight that they are one of the main reasons they can’t find PCPs to connect with to render care in disadvantaged environments – because they made it economically impossible to do so.
Robert H. Hopkins Jr., MD, associate professor of internal medicine and pediatrics and director of the division of general internal medicine, University of Arkansas for Medical Sciences, Little Rock
Patients/Health system: I would wish for staged implementation of universal basic health coverage for all, perhaps closest to the French or Canadian model. This would need to be coupled with expanded funding for nursing education, graduate medical education, and tracing of other health-related professionals.
Harvey Hsu, MD, Banner Health, Phoenix
Patients: More clear guidelines that are simple to understand. This can apply to colonoscopy (now age 45), immunizations, blood pressure goals. I wish medications were not as expensive so patients can take the best medicine for them and not stop taking them when they hit their donut hole in coverage.
Practice: We have been functioning on a leaner basis to cut down costs. When the pandemic hit, turnover was high and we lost PAs, nurses, front-office staff, and physicians. Having adequate staffing is probably number one on many lists. One way we dealt with lack of staffing was converting in-person visits to telehealth. Video visits are paid the same as in-person visits, but if the patient could not get their video to work, then it would be a telephone visit. Now many insurances do not even pay for telephone visits. So I would wish that we could still be reimbursed for telehealth visits.
Health system: I would wish for our health system to recognize the extra work required to take care of patients while improving quality and meeting quality measures. Allowing more time for patient visits could be one way to meet those goals or having more support staff to make sure patients get their colonoscopy/mammograms done, improve their sugars, and take their medications.
Jan L. Shifren, MD, Vincent Trustees Professor, obstetrics, gynecology, and reproductive biology, Harvard Medical School, and director of the Midlife Women’s Health Center at Massachusetts General Hospital, Boston
Patients: I wish for patients to be actively involved in all aspects of their care, well informed with shared decision-making.
Practice: I wish for the enormous time demands of electronic medical records and documentation to not distract from the pleasure of caring for patients.
Health system: Patient care remains at the center of decisions and programs.
Timothy J. Joos, MD, MPH, internal medicine/pediatrics, Seattle
Health system: I wish someone could figure out how we could be reimbursed for the quality of care we provide instead of the volume of patients we see. I wish EMRs could become less complicated and more user-friendly rather than needing advanced training to use.
Peter Kovacs, MD, medical director, Kaali Institute IVF Center, Budapest
Patients: I work as an infertility specialist, so when we talk about infectious diseases and associated risks, we talk about a minimum of two (female and male partner) and ideally three (plus the pregnancy) individuals. We have learned that SARS-CoV-2 affects reproductive health. It may compromise sperm production, could delay fertility treatment, could be associated with lower success rates; and if the treatment is successful, it may harm the pregnant woman/fetus/newborn. The best preventive measure that we can offer is vaccination. One cannot overemphasize the importance of preventive measures, paying attention to personal hygiene and social distancing. Therefore, I wish those planning to become pregnant to listen to their health care provider and accept the recommended vaccines to minimize the risk of getting infected and to minimize the risk for severe disease, especially if one undergoes successful fertility treatment and achieves a long-desired pregnancy.
Practice: During the 2022 calendar year we had many days when one or more employees were out of work on sick leave. This puts extra stress on the others to allow uncompromised work in the clinic. In addition, we all have to work in a less-comfortable environment if we consider mask use every day, all day. For health care workers, vaccination is mandated but many still are affected by milder forms of coronavirus infection and other respiratory diseases. Therefore, I wish my colleagues patience toward the preventive measures to lower the individual risk for infections. As a result, hopefully we will have a less stressful 2023.
Health system: Many resources had to be delegated to dealing with acute and chronic COVID, and this was at the expense of routine daily elective and preventive medical services. I wish the health care system to return to normal daily operations, to have the personnel and financial resources to carry on with the required preventive and elective medical services to avoid long-term consequences of not being able to provide such services. It would be sad if we had to treat otherwise preventable illnesses in the upcoming years that went undiagnosed and/or were not properly managed due to limited resources as the result of the pandemic.
Alan R. Nelson, MD, internist-endocrinologist, retired
Patients: Expansion of the FDA’s authority into over-the-counter drugs, including the veracity of their advertising claims.
Practice: Make diabetes drugs available at a reasonable cost.
Health system: With the expansion of Medicaid eligibility during COVID-19 coming to a close, federal government actions are necessary for those who once again have been dropped from coverage to have their legitimate needs met.
Kevin Powell, MD, PhD, St. Louis
Patients: To be cared for and about, and not just medically, even when illness strikes and health fails.
Hospitals: To hear the thankfulness of a grateful public for the care you provide, and to hear that above the angry noise of outraged individuals who spout vitriol and focus on how they believe others have harmed them.
Health system: A truer understanding of mercy and justice.
Margaret Thew, DNP, FNP-BC, director, department of adolescent medicine, Children’s Hospital of Wisconsin, Milwaukee
Seconded by: M. Susan Jay, MD, professor of pediatrics, chief of adolescent medicine, Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee
My wish for patients, hospital, and system: health, calm, and grace.
Mark P. Trolice, MD, director of Fertility CARE, the IVF Center, Winter Park, Fla.
Patients: To be proactive in their health care and be their own advocates. Question when unclear and only consult credible resources.
Practice/hospital: Improve support of physicians and all health care providers to allow more input in their practice operations and growth.
Health system: Reduce interference of the “business of medicine” and ensure that the patient experience is the priority.
Charles P. Vega, MD, University of California, Irvine
Three minutes on a routine basis for everyone in health care to reflect on our blessings and the honor and gravity – as well as joy – that are integral to health care. Three minutes that will also help us to recognize our challenges and put them in the proper context. I know 3 minutes is not meeting any standard for reflective practice. But it’s 3 minutes more than I have right now.
Karen Breach Washington, MD, medical director of WellCare of North Carolina/Centene, Charlotte
Seconded by: Lillian M. Beard, MD, physician director, Children’s Pediatricians and Associates, Silver Spring, Md.
Patients: Access to affordable health care.
Hospital: Resources to care for patients (sufficient number of beds and a healthy staff).
Health system: Equity for all.
Andrew Wilner, MD, host of the podcast “The Art of Medicine with Dr. Andrew Wilner,” www.andrewwilner.com
Let’s put patients first! Too many extraneous considerations other than the patient’s best interest obstruct optimal patient care.
Here are just a few examples of patients coming last instead of first.
- If a patient needs to start a new medication in hospital, we shouldn’t have to wait until the patient is an outpatient because “that’s when insurance will pay.”
- If there’s a new medication that’s better than the old medication, we shouldn’t be forced to choose the old medication and provide inferior care because “that’s when insurance will pay.”
- If patients need to stay in hospital, we shouldn’t be pressured to discharge them because the hospital has decided that decreasing “length of stay” is its highest priority.
Dr. Francis Peabody said it best in 1927: “The secret of the care of the patient is in caring for the patient.” How hard is that?
In 2023, why don’t we follow Dr. Peabody’s sage advice from nearly 100 years ago and see what happens?
James M. Wooten, PharmD, University of Missouri–Kansas City, University Health, Kansas City, Mo.
Patients: I want patients to understand and properly realize the advantage of vaccinations – not only for COVID-19 but also for influenza. There is so much misinformation that I spend a lot of time trying to convince patients to get vaccinated. Most patients don’t realize that through their lives, most of them have already been vaccinated for something just to be able to attend school. How the COVID-19 vaccine created so much stigma makes little sense to me. I also want patients to understand that COVID-19 vaccination and boosters do not always prevent infection but will many times prevent severe infection. I believe that better patient communication and education is the key and will always be the key to improving vaccination numbers. Not only communicating and educating patients on vaccination itself but also making patients realize that personal vaccination decisions may affect what happens to your neighbor. Allowing infection means that you may be more likely to infect someone else. As a society, we must take care of each other.
Health system: It will be interesting to see what happens when vaccines are no longer reimbursed by the federal government. Understanding which vaccines work best and are better tolerated will be key to choosing appropriate vaccine brands. Health care providers will need to be very selective regarding which vaccines are selected for formulary inclusion. Thorough meta-analysis studies must be done to provide more evaluable information to allow for appropriate selection. “Knowledge is power!” Appropriate knowledge will help distinguish which vaccines work best for various patient populations.
A version of this article first appeared on Medscape.com.
As physicians well know, magic wands don’t exist. If they did, every patient would recover in the exam room, prior authorization wouldn’t exist, and continuing medical education credits would be printed on bearer bonds.
But Because, hey – we all need to dream.
Suzanne C. Boulter, MD, adjunct professor of pediatrics and community and family medicine, Geisel School of Medicine at Dartmouth, Hanover, N.H.
Patients: An end to gun violence.
Practice/hospital: Adequate staffing and pediatric bed availability.
Health system: Universal access to health insurance.
Sarah G. Candler, MD, MPH, care team medical director and director of academic relations, Iora Primary Care, Northside Clinic, Houston
Patients: Systems of health that start with communities of safety, including access to affordable housing, food, transportation, and health care.
Practice/hospital: I.N.T.E.R.O.P.E.R.A.B.I.L.I.T.Y.
Health system: Clinician leadership that has the power (often aka funding) to do what’s right, not just what’s right in front of us.
Arthur L. Caplan, PhD, bioethicist, New York University Langone Health
Patients: I wish for patients in the United States greater access to affordable primary care. There are still too many people without insurance or a reasonably accessible quality provider. And I especially wish for the rapid expansion of affordable training programs to meet staffing needs, including more scholarships, 3-year programs, and more new primary care–oriented schools.
Hospital: Increased staffing, especially nursing. There are too many retirements, too much burnout, and too much privatization into boutique practices to ensure the ability to provide high-quality, safe, patient-oriented care.
Health system: I wish for health systems to seriously move into electronic medicine. While billing has become electronic, there is still much to be done to supplement diagnosis, training, and standardized data collection on key metrics. Systems are not yet behaving in a manner consistent with the hype in this regard.
Stephen Devries, MD, executive director, Gaples Institute (nonprofit) and adjunct associate professor of nutrition, Harvard School of Public Health, Boston
Patients: Patients continue to demand more from their health care professionals and insist that they are offered evidence-based counseling on nutrition and lifestyle strategies.
Practice: Quality-based reimbursement for medical services will take hold that will incentivize much-needed preventive care.
Hospital: Hospitals will more fully embrace the role of serving as true centers of health and focus as much on preventive medicine as on the more lucrative areas of high-tech treatment.
Peter D. Friedmann, MD, MPH, chief research officer, Baystate Health, Springfield, Mass.
Seconded by: Elisabeth Poorman, MD, general internist, University of Washington Clinic, Kent
Patients: Don’t forget the ongoing epidemic of substance use disorder, a major cause of premature mortality. Descheduling of cannabis and expungement of cannabis-related convictions.
Practice/hospital: Commitment of hospitals and practices to address stigma and ensure delivery of medications for opioid use disorder in primary care, the emergency department, and inpatient settings.
Health system: Reform of antiquated methadone regulations to permit office-based prescription and pharmacy dispensing to treat opioid use disorder, as is the case in most of the world.
Robert Glatter, MD, emergency physician, New York
Patients: I want all patients to understand the enormous strain the health care system has been under – not just with the pandemic, the tripledemic, and mpox [previously called monkeypox], but well before the onset of these public health crises.
Hospital: The medical profession has endured not only burnout but a growing mental health crisis, staffing shortages, a physician addiction crisis, and increased attrition in the decade leading up to the pandemic. The pandemic was like a punch in the gut, occurring at the most inopportune time one could imagine.
Health system: The intersection of health and the state of our public health deserves important mention. Unless we take action to bolster our public health infrastructure, our health care system will be in jeopardy, unable to handle the next pandemic, which could be just around the corner.
William E. Golden, MD, medical director of Arkansas Medicaid, professor of medicine and public health, University of Arkansas for Medical Sciences, Little Rock
Patients: Affordable options for diabetes and obesity management.
Health system: Greater investment by health systems and third-party payers in primary care infrastructure.
Gregory A. Hood, MD, Baptist Health, Lexington, Ky.
Patients: To embrace the gift of getting out in the world, being active, and connecting with others – having put down the screens.
Health system: To be freed from the financial gamesmanship of the insurers as they continue to serve their goals of promoting their hedge fund investing over meaningful and productive partnering with primary care physicians, and that they gain insight that they are one of the main reasons they can’t find PCPs to connect with to render care in disadvantaged environments – because they made it economically impossible to do so.
Robert H. Hopkins Jr., MD, associate professor of internal medicine and pediatrics and director of the division of general internal medicine, University of Arkansas for Medical Sciences, Little Rock
Patients/Health system: I would wish for staged implementation of universal basic health coverage for all, perhaps closest to the French or Canadian model. This would need to be coupled with expanded funding for nursing education, graduate medical education, and tracing of other health-related professionals.
Harvey Hsu, MD, Banner Health, Phoenix
Patients: More clear guidelines that are simple to understand. This can apply to colonoscopy (now age 45), immunizations, blood pressure goals. I wish medications were not as expensive so patients can take the best medicine for them and not stop taking them when they hit their donut hole in coverage.
Practice: We have been functioning on a leaner basis to cut down costs. When the pandemic hit, turnover was high and we lost PAs, nurses, front-office staff, and physicians. Having adequate staffing is probably number one on many lists. One way we dealt with lack of staffing was converting in-person visits to telehealth. Video visits are paid the same as in-person visits, but if the patient could not get their video to work, then it would be a telephone visit. Now many insurances do not even pay for telephone visits. So I would wish that we could still be reimbursed for telehealth visits.
Health system: I would wish for our health system to recognize the extra work required to take care of patients while improving quality and meeting quality measures. Allowing more time for patient visits could be one way to meet those goals or having more support staff to make sure patients get their colonoscopy/mammograms done, improve their sugars, and take their medications.
Jan L. Shifren, MD, Vincent Trustees Professor, obstetrics, gynecology, and reproductive biology, Harvard Medical School, and director of the Midlife Women’s Health Center at Massachusetts General Hospital, Boston
Patients: I wish for patients to be actively involved in all aspects of their care, well informed with shared decision-making.
Practice: I wish for the enormous time demands of electronic medical records and documentation to not distract from the pleasure of caring for patients.
Health system: Patient care remains at the center of decisions and programs.
Timothy J. Joos, MD, MPH, internal medicine/pediatrics, Seattle
Health system: I wish someone could figure out how we could be reimbursed for the quality of care we provide instead of the volume of patients we see. I wish EMRs could become less complicated and more user-friendly rather than needing advanced training to use.
Peter Kovacs, MD, medical director, Kaali Institute IVF Center, Budapest
Patients: I work as an infertility specialist, so when we talk about infectious diseases and associated risks, we talk about a minimum of two (female and male partner) and ideally three (plus the pregnancy) individuals. We have learned that SARS-CoV-2 affects reproductive health. It may compromise sperm production, could delay fertility treatment, could be associated with lower success rates; and if the treatment is successful, it may harm the pregnant woman/fetus/newborn. The best preventive measure that we can offer is vaccination. One cannot overemphasize the importance of preventive measures, paying attention to personal hygiene and social distancing. Therefore, I wish those planning to become pregnant to listen to their health care provider and accept the recommended vaccines to minimize the risk of getting infected and to minimize the risk for severe disease, especially if one undergoes successful fertility treatment and achieves a long-desired pregnancy.
Practice: During the 2022 calendar year we had many days when one or more employees were out of work on sick leave. This puts extra stress on the others to allow uncompromised work in the clinic. In addition, we all have to work in a less-comfortable environment if we consider mask use every day, all day. For health care workers, vaccination is mandated but many still are affected by milder forms of coronavirus infection and other respiratory diseases. Therefore, I wish my colleagues patience toward the preventive measures to lower the individual risk for infections. As a result, hopefully we will have a less stressful 2023.
Health system: Many resources had to be delegated to dealing with acute and chronic COVID, and this was at the expense of routine daily elective and preventive medical services. I wish the health care system to return to normal daily operations, to have the personnel and financial resources to carry on with the required preventive and elective medical services to avoid long-term consequences of not being able to provide such services. It would be sad if we had to treat otherwise preventable illnesses in the upcoming years that went undiagnosed and/or were not properly managed due to limited resources as the result of the pandemic.
Alan R. Nelson, MD, internist-endocrinologist, retired
Patients: Expansion of the FDA’s authority into over-the-counter drugs, including the veracity of their advertising claims.
Practice: Make diabetes drugs available at a reasonable cost.
Health system: With the expansion of Medicaid eligibility during COVID-19 coming to a close, federal government actions are necessary for those who once again have been dropped from coverage to have their legitimate needs met.
Kevin Powell, MD, PhD, St. Louis
Patients: To be cared for and about, and not just medically, even when illness strikes and health fails.
Hospitals: To hear the thankfulness of a grateful public for the care you provide, and to hear that above the angry noise of outraged individuals who spout vitriol and focus on how they believe others have harmed them.
Health system: A truer understanding of mercy and justice.
Margaret Thew, DNP, FNP-BC, director, department of adolescent medicine, Children’s Hospital of Wisconsin, Milwaukee
Seconded by: M. Susan Jay, MD, professor of pediatrics, chief of adolescent medicine, Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee
My wish for patients, hospital, and system: health, calm, and grace.
Mark P. Trolice, MD, director of Fertility CARE, the IVF Center, Winter Park, Fla.
Patients: To be proactive in their health care and be their own advocates. Question when unclear and only consult credible resources.
Practice/hospital: Improve support of physicians and all health care providers to allow more input in their practice operations and growth.
Health system: Reduce interference of the “business of medicine” and ensure that the patient experience is the priority.
Charles P. Vega, MD, University of California, Irvine
Three minutes on a routine basis for everyone in health care to reflect on our blessings and the honor and gravity – as well as joy – that are integral to health care. Three minutes that will also help us to recognize our challenges and put them in the proper context. I know 3 minutes is not meeting any standard for reflective practice. But it’s 3 minutes more than I have right now.
Karen Breach Washington, MD, medical director of WellCare of North Carolina/Centene, Charlotte
Seconded by: Lillian M. Beard, MD, physician director, Children’s Pediatricians and Associates, Silver Spring, Md.
Patients: Access to affordable health care.
Hospital: Resources to care for patients (sufficient number of beds and a healthy staff).
Health system: Equity for all.
Andrew Wilner, MD, host of the podcast “The Art of Medicine with Dr. Andrew Wilner,” www.andrewwilner.com
Let’s put patients first! Too many extraneous considerations other than the patient’s best interest obstruct optimal patient care.
Here are just a few examples of patients coming last instead of first.
- If a patient needs to start a new medication in hospital, we shouldn’t have to wait until the patient is an outpatient because “that’s when insurance will pay.”
- If there’s a new medication that’s better than the old medication, we shouldn’t be forced to choose the old medication and provide inferior care because “that’s when insurance will pay.”
- If patients need to stay in hospital, we shouldn’t be pressured to discharge them because the hospital has decided that decreasing “length of stay” is its highest priority.
Dr. Francis Peabody said it best in 1927: “The secret of the care of the patient is in caring for the patient.” How hard is that?
In 2023, why don’t we follow Dr. Peabody’s sage advice from nearly 100 years ago and see what happens?
James M. Wooten, PharmD, University of Missouri–Kansas City, University Health, Kansas City, Mo.
Patients: I want patients to understand and properly realize the advantage of vaccinations – not only for COVID-19 but also for influenza. There is so much misinformation that I spend a lot of time trying to convince patients to get vaccinated. Most patients don’t realize that through their lives, most of them have already been vaccinated for something just to be able to attend school. How the COVID-19 vaccine created so much stigma makes little sense to me. I also want patients to understand that COVID-19 vaccination and boosters do not always prevent infection but will many times prevent severe infection. I believe that better patient communication and education is the key and will always be the key to improving vaccination numbers. Not only communicating and educating patients on vaccination itself but also making patients realize that personal vaccination decisions may affect what happens to your neighbor. Allowing infection means that you may be more likely to infect someone else. As a society, we must take care of each other.
Health system: It will be interesting to see what happens when vaccines are no longer reimbursed by the federal government. Understanding which vaccines work best and are better tolerated will be key to choosing appropriate vaccine brands. Health care providers will need to be very selective regarding which vaccines are selected for formulary inclusion. Thorough meta-analysis studies must be done to provide more evaluable information to allow for appropriate selection. “Knowledge is power!” Appropriate knowledge will help distinguish which vaccines work best for various patient populations.
A version of this article first appeared on Medscape.com.
Universal testing for Lp(a): What are we waiting for?
It soon became clear that Lp(a) was associated with atherosclerotic cardiovascular disease (ASCVD), but whether an elevated blood level was a biomarker or a causal factor proved difficult to determine. Studies of inheritance patterns confirmed that blood levels were primarily genetically determined and largely resistant to lifestyle and pharmacologic intervention. It seemed senseless to test for something that was deemed “unmodifiable,” so untreatable. That label stuck for decades.
Fortunately, a resurgent interest in molecular pathophysiology this past decade has clarified Lp(a)’s unique contribution to atherothrombotic disease and calcific aortic stenosis. While there remains much to be learned about this complex, highly atherogenic molecule and its role in cardiac disease, it seems shortsighted not to take the simple step of identifying who carries this risk. Why are we not testing everyone for an extremely common and potent risk factor for the most lethal disease on the planet?
Epidemiologic studies project a stunning number of people in the United States to be at increased risk for Lp(a)-mediated coronary and cerebrovascular events. Because the LPA gene which codes for the apo(a) component of the Lp(a) molecule is fully expressed at age 2, this is a truly lifelong risk factor for a projected 64 million individuals with blood levels (> 60 mg/dL) high enough to double their risk for ASCVD. Because risk increases linearly, this includes 16 million, like me, with levels > 116 mg/dL, who are at four times the risk for ASCVD as those with normal levels (< 30 mg/dL).
Because Lp(a) level remains relatively constant throughout life, a single blood test would help stratify the risk it confers on millions of people who, under current U.S. guidelines, would never be tested. Until Lp(a) is integrated into its algorithms, the commonly used ASCVD Risk Calculator will substantially underestimate risk in 20% of the population.
A potential barrier to universal testing is that the ideal method to measure Lp(a) has yet to be determined. Lp(a) comprises an apoB particle bonded to an apo(a) particle. Apo(a) is complex and has a number of isoforms that can result in large heterogeneity in apo(a) size between, as well as within, individuals. This contributes to controversy about the ideal assay and whether Lp(a) levels should be expressed as mass (mg/dL) or number of particles (nmols/L). This should not, however, deter universal testing.
One-time cost, lifetime benefit?
Absent universal testing, it’s impossible to estimate the economic toll that Lp(a) exacts, but it’s surely an extraordinary number, particularly because the highest-risk individuals are prone to recurrent, nonfatal vascular events. The substantial price tag for my personal decade of Lp(a)-induced vascular havoc included four percutaneous coronary interventions with rapid stent restenosis, an eventual bypass surgery, and an aborted left hemispheric stroke, requiring an urgent carotid endarterectomy.
As a frame of reference, U.S. expenditures related to ASCVD are estimated to be $351 billion annually. If everyone in the United States over the age of 18 were tested for Lp(a) at a cost of $100 per person, this would be a $21 billion expenditure. This nonrecurring expense would identify the 20% – or almost 42 million individuals – at high risk for ASCVD, a number of whom would have already had vascular events. This one-time cost would be a foundational step in securing year-after-year savings from enhanced ASCVD prevention and reduction in recurrent vascular events.
Such savings would be significantly enhanced if and when targeted, effective Lp(a) treatments become available, but it seems shortsighted to make this the linchpin for universal testing. It’s noteworthy that Canadian and European guidelines already endorse one-time testing for all.
The confirmation of Lp(a)’s causal role in ASCVD remains underappreciated by medical providers across all specialties. Much of the elegant Lp(a)-related science of the past decade has yet to translate to the clinical world. What better way to rectify this than by identifying those with high Lp(a)? Since the advent of the statin era, “good” and “bad” cholesterol values are common conversational fare, in part because virtually every adult has had not one, but many lipid panels. Universal Lp(a) testing would spotlight this pervasive and important risk factor that was referred to as the “horrible” cholesterol in a recent review.
U.S. guidelines need updating
To foster this, U.S. guidelines, which influence every aspect of care, including testing, prevention, treatment, reimbursement, and medical legal issues, need to be simplified. The discussion of Lp(a) testing in the 2018 U.S. guidelines on cholesterol management is already obsolete. The contingencies on when testing is “reasonable” or “may be reasonable” are dated and cumbersome. In contrast, a recommendation to test everyone once, perhaps in adolescence, would be a useful, forward-looking strategy.
To date, trials of an antisense oligonucleotide and a small interfering RNA molecule targeting hepatic LPA messenger RNA have confirmed that plasma Lp(a) levels can be significantly and safely lowered. If the ongoing Lp(a) HORIZON and OCEAN(a) phase 3 trials have positive outcomes in patients with known ASCVD, this would spawn a host of clinical trials to explore the possibilities of these therapies in primary prevention as well. These will require tens of thousands of enrollees, and universal testing would expand the pool of potential participants.
The majority of at-risk individuals identified through universal testing would be candidates for primary prevention. This large, currently unidentified cohort should have all coexisting risk factors assessed and managed; lowering elevated LDL cholesterol early and aggressively is paramount. Recent data from the United Kingdom suggest that attainment of specific LDL cholesterol levels may offset the risk for vascular events in those with high Lp(a) levels.
Of note, this was the advice given to the small fraction of high-risk individuals like me, who had their Lp(a) level tested long before its ominous implications were understood. This recommendation was informed mostly by common sense. For any number of reasons, the same might be said for universal testing.
Dr. Leahy, a retired cardiologist in San Diego, has an abiding professional and personal interest in Lp(a), which has been responsible for a number of cardiovascular events in his own life over the past 2 decades. He was a participant in the phase 2 clinical trial of the Lp(a)-lowering antisense oligonucleotide being studied in the Lp(a) HORIZON trial, funded by Novartis, and is currently undergoing apheresis treatment. A version of this article originally appeared on Medscape.com.
It soon became clear that Lp(a) was associated with atherosclerotic cardiovascular disease (ASCVD), but whether an elevated blood level was a biomarker or a causal factor proved difficult to determine. Studies of inheritance patterns confirmed that blood levels were primarily genetically determined and largely resistant to lifestyle and pharmacologic intervention. It seemed senseless to test for something that was deemed “unmodifiable,” so untreatable. That label stuck for decades.
Fortunately, a resurgent interest in molecular pathophysiology this past decade has clarified Lp(a)’s unique contribution to atherothrombotic disease and calcific aortic stenosis. While there remains much to be learned about this complex, highly atherogenic molecule and its role in cardiac disease, it seems shortsighted not to take the simple step of identifying who carries this risk. Why are we not testing everyone for an extremely common and potent risk factor for the most lethal disease on the planet?
Epidemiologic studies project a stunning number of people in the United States to be at increased risk for Lp(a)-mediated coronary and cerebrovascular events. Because the LPA gene which codes for the apo(a) component of the Lp(a) molecule is fully expressed at age 2, this is a truly lifelong risk factor for a projected 64 million individuals with blood levels (> 60 mg/dL) high enough to double their risk for ASCVD. Because risk increases linearly, this includes 16 million, like me, with levels > 116 mg/dL, who are at four times the risk for ASCVD as those with normal levels (< 30 mg/dL).
Because Lp(a) level remains relatively constant throughout life, a single blood test would help stratify the risk it confers on millions of people who, under current U.S. guidelines, would never be tested. Until Lp(a) is integrated into its algorithms, the commonly used ASCVD Risk Calculator will substantially underestimate risk in 20% of the population.
A potential barrier to universal testing is that the ideal method to measure Lp(a) has yet to be determined. Lp(a) comprises an apoB particle bonded to an apo(a) particle. Apo(a) is complex and has a number of isoforms that can result in large heterogeneity in apo(a) size between, as well as within, individuals. This contributes to controversy about the ideal assay and whether Lp(a) levels should be expressed as mass (mg/dL) or number of particles (nmols/L). This should not, however, deter universal testing.
One-time cost, lifetime benefit?
Absent universal testing, it’s impossible to estimate the economic toll that Lp(a) exacts, but it’s surely an extraordinary number, particularly because the highest-risk individuals are prone to recurrent, nonfatal vascular events. The substantial price tag for my personal decade of Lp(a)-induced vascular havoc included four percutaneous coronary interventions with rapid stent restenosis, an eventual bypass surgery, and an aborted left hemispheric stroke, requiring an urgent carotid endarterectomy.
As a frame of reference, U.S. expenditures related to ASCVD are estimated to be $351 billion annually. If everyone in the United States over the age of 18 were tested for Lp(a) at a cost of $100 per person, this would be a $21 billion expenditure. This nonrecurring expense would identify the 20% – or almost 42 million individuals – at high risk for ASCVD, a number of whom would have already had vascular events. This one-time cost would be a foundational step in securing year-after-year savings from enhanced ASCVD prevention and reduction in recurrent vascular events.
Such savings would be significantly enhanced if and when targeted, effective Lp(a) treatments become available, but it seems shortsighted to make this the linchpin for universal testing. It’s noteworthy that Canadian and European guidelines already endorse one-time testing for all.
The confirmation of Lp(a)’s causal role in ASCVD remains underappreciated by medical providers across all specialties. Much of the elegant Lp(a)-related science of the past decade has yet to translate to the clinical world. What better way to rectify this than by identifying those with high Lp(a)? Since the advent of the statin era, “good” and “bad” cholesterol values are common conversational fare, in part because virtually every adult has had not one, but many lipid panels. Universal Lp(a) testing would spotlight this pervasive and important risk factor that was referred to as the “horrible” cholesterol in a recent review.
U.S. guidelines need updating
To foster this, U.S. guidelines, which influence every aspect of care, including testing, prevention, treatment, reimbursement, and medical legal issues, need to be simplified. The discussion of Lp(a) testing in the 2018 U.S. guidelines on cholesterol management is already obsolete. The contingencies on when testing is “reasonable” or “may be reasonable” are dated and cumbersome. In contrast, a recommendation to test everyone once, perhaps in adolescence, would be a useful, forward-looking strategy.
To date, trials of an antisense oligonucleotide and a small interfering RNA molecule targeting hepatic LPA messenger RNA have confirmed that plasma Lp(a) levels can be significantly and safely lowered. If the ongoing Lp(a) HORIZON and OCEAN(a) phase 3 trials have positive outcomes in patients with known ASCVD, this would spawn a host of clinical trials to explore the possibilities of these therapies in primary prevention as well. These will require tens of thousands of enrollees, and universal testing would expand the pool of potential participants.
The majority of at-risk individuals identified through universal testing would be candidates for primary prevention. This large, currently unidentified cohort should have all coexisting risk factors assessed and managed; lowering elevated LDL cholesterol early and aggressively is paramount. Recent data from the United Kingdom suggest that attainment of specific LDL cholesterol levels may offset the risk for vascular events in those with high Lp(a) levels.
Of note, this was the advice given to the small fraction of high-risk individuals like me, who had their Lp(a) level tested long before its ominous implications were understood. This recommendation was informed mostly by common sense. For any number of reasons, the same might be said for universal testing.
Dr. Leahy, a retired cardiologist in San Diego, has an abiding professional and personal interest in Lp(a), which has been responsible for a number of cardiovascular events in his own life over the past 2 decades. He was a participant in the phase 2 clinical trial of the Lp(a)-lowering antisense oligonucleotide being studied in the Lp(a) HORIZON trial, funded by Novartis, and is currently undergoing apheresis treatment. A version of this article originally appeared on Medscape.com.
It soon became clear that Lp(a) was associated with atherosclerotic cardiovascular disease (ASCVD), but whether an elevated blood level was a biomarker or a causal factor proved difficult to determine. Studies of inheritance patterns confirmed that blood levels were primarily genetically determined and largely resistant to lifestyle and pharmacologic intervention. It seemed senseless to test for something that was deemed “unmodifiable,” so untreatable. That label stuck for decades.
Fortunately, a resurgent interest in molecular pathophysiology this past decade has clarified Lp(a)’s unique contribution to atherothrombotic disease and calcific aortic stenosis. While there remains much to be learned about this complex, highly atherogenic molecule and its role in cardiac disease, it seems shortsighted not to take the simple step of identifying who carries this risk. Why are we not testing everyone for an extremely common and potent risk factor for the most lethal disease on the planet?
Epidemiologic studies project a stunning number of people in the United States to be at increased risk for Lp(a)-mediated coronary and cerebrovascular events. Because the LPA gene which codes for the apo(a) component of the Lp(a) molecule is fully expressed at age 2, this is a truly lifelong risk factor for a projected 64 million individuals with blood levels (> 60 mg/dL) high enough to double their risk for ASCVD. Because risk increases linearly, this includes 16 million, like me, with levels > 116 mg/dL, who are at four times the risk for ASCVD as those with normal levels (< 30 mg/dL).
Because Lp(a) level remains relatively constant throughout life, a single blood test would help stratify the risk it confers on millions of people who, under current U.S. guidelines, would never be tested. Until Lp(a) is integrated into its algorithms, the commonly used ASCVD Risk Calculator will substantially underestimate risk in 20% of the population.
A potential barrier to universal testing is that the ideal method to measure Lp(a) has yet to be determined. Lp(a) comprises an apoB particle bonded to an apo(a) particle. Apo(a) is complex and has a number of isoforms that can result in large heterogeneity in apo(a) size between, as well as within, individuals. This contributes to controversy about the ideal assay and whether Lp(a) levels should be expressed as mass (mg/dL) or number of particles (nmols/L). This should not, however, deter universal testing.
One-time cost, lifetime benefit?
Absent universal testing, it’s impossible to estimate the economic toll that Lp(a) exacts, but it’s surely an extraordinary number, particularly because the highest-risk individuals are prone to recurrent, nonfatal vascular events. The substantial price tag for my personal decade of Lp(a)-induced vascular havoc included four percutaneous coronary interventions with rapid stent restenosis, an eventual bypass surgery, and an aborted left hemispheric stroke, requiring an urgent carotid endarterectomy.
As a frame of reference, U.S. expenditures related to ASCVD are estimated to be $351 billion annually. If everyone in the United States over the age of 18 were tested for Lp(a) at a cost of $100 per person, this would be a $21 billion expenditure. This nonrecurring expense would identify the 20% – or almost 42 million individuals – at high risk for ASCVD, a number of whom would have already had vascular events. This one-time cost would be a foundational step in securing year-after-year savings from enhanced ASCVD prevention and reduction in recurrent vascular events.
Such savings would be significantly enhanced if and when targeted, effective Lp(a) treatments become available, but it seems shortsighted to make this the linchpin for universal testing. It’s noteworthy that Canadian and European guidelines already endorse one-time testing for all.
The confirmation of Lp(a)’s causal role in ASCVD remains underappreciated by medical providers across all specialties. Much of the elegant Lp(a)-related science of the past decade has yet to translate to the clinical world. What better way to rectify this than by identifying those with high Lp(a)? Since the advent of the statin era, “good” and “bad” cholesterol values are common conversational fare, in part because virtually every adult has had not one, but many lipid panels. Universal Lp(a) testing would spotlight this pervasive and important risk factor that was referred to as the “horrible” cholesterol in a recent review.
U.S. guidelines need updating
To foster this, U.S. guidelines, which influence every aspect of care, including testing, prevention, treatment, reimbursement, and medical legal issues, need to be simplified. The discussion of Lp(a) testing in the 2018 U.S. guidelines on cholesterol management is already obsolete. The contingencies on when testing is “reasonable” or “may be reasonable” are dated and cumbersome. In contrast, a recommendation to test everyone once, perhaps in adolescence, would be a useful, forward-looking strategy.
To date, trials of an antisense oligonucleotide and a small interfering RNA molecule targeting hepatic LPA messenger RNA have confirmed that plasma Lp(a) levels can be significantly and safely lowered. If the ongoing Lp(a) HORIZON and OCEAN(a) phase 3 trials have positive outcomes in patients with known ASCVD, this would spawn a host of clinical trials to explore the possibilities of these therapies in primary prevention as well. These will require tens of thousands of enrollees, and universal testing would expand the pool of potential participants.
The majority of at-risk individuals identified through universal testing would be candidates for primary prevention. This large, currently unidentified cohort should have all coexisting risk factors assessed and managed; lowering elevated LDL cholesterol early and aggressively is paramount. Recent data from the United Kingdom suggest that attainment of specific LDL cholesterol levels may offset the risk for vascular events in those with high Lp(a) levels.
Of note, this was the advice given to the small fraction of high-risk individuals like me, who had their Lp(a) level tested long before its ominous implications were understood. This recommendation was informed mostly by common sense. For any number of reasons, the same might be said for universal testing.
Dr. Leahy, a retired cardiologist in San Diego, has an abiding professional and personal interest in Lp(a), which has been responsible for a number of cardiovascular events in his own life over the past 2 decades. He was a participant in the phase 2 clinical trial of the Lp(a)-lowering antisense oligonucleotide being studied in the Lp(a) HORIZON trial, funded by Novartis, and is currently undergoing apheresis treatment. A version of this article originally appeared on Medscape.com.
Bone Wax as a Physical Hemostatic Agent
Practice Gap
Hemostasis after cutaneous surgery typically can be aided by mechanical occlusion with petrolatum and gauze known as a pressure bandage. However, in certain scenarios such as bone bleeding or irregularly shaped areas (eg, conchal bowl), difficulty applying a pressure bandage necessitates alternative hemostatic measures.1 In those instances, physical hemostatic agents, such as gelatin, oxidized cellulose, microporous polysaccharide spheres, hydrophilic polymers with potassium salts, microfibrillar collagen, and chitin, also can be used.2 However, those agents are expensive and often adhere to wound edges, inducing repeat trauma with removal. To avoid such concerns, we propose the use of bone wax as an effective hemostatic technique.
The Technique
When secondary intention healing is chosen or a temporary bandage needs to be placed, we offer the use of bone wax as an alternative to help achieve hemostasis. Bone wax—a combination of beeswax, isopropyl palmitate, and a stabilizing agent such as almond oils or sterilized salicylic acid3—helps achieve hemostasis by purely mechanical means. It is malleable and can be easily adapted to the architecture of the surgical site (Figure 1). The bone wax can be applied immediately following surgery and removed during bandage change.

Practice Implications
Use of bone wax as a physical hemostatic agent provides a practical alternative to other options commonly used in dermatologic surgery for deep wounds or irregular surfaces. It offers several advantages.
Bone wax is not absorbed and does not adhere to wound surfaces, which makes removal easy and painless. Furthermore, bone wax allows for excellent growth of granulation tissue2 (Figure 2), most likely due to the healing and emollient properties of the beeswax and the moist occlusive environment created by the bone wax.
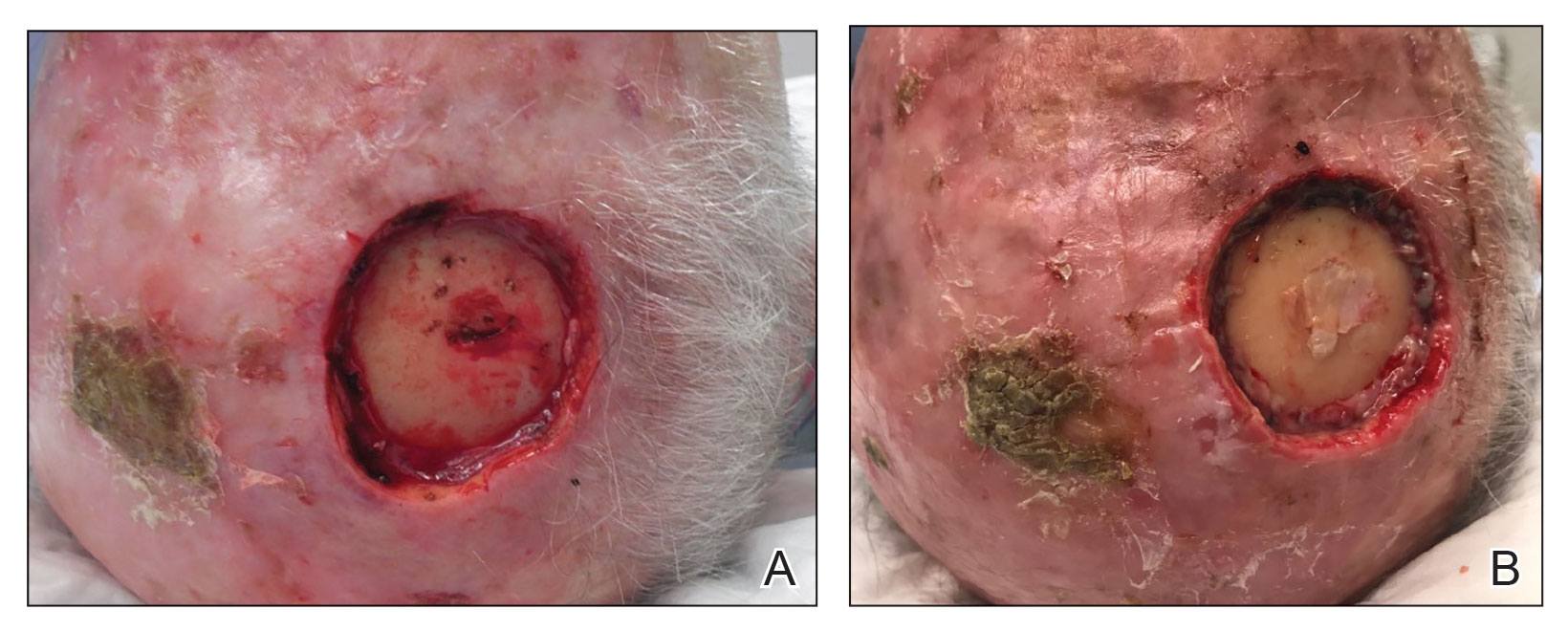
Additional advantages are its low cost, especially compared to other hemostatic agents, and long shelf-life (approximately 5 years).2 Furthermore, in scenarios when cutaneous tumors extend into the calvarium, bone wax can prevent air emboli from entering noncollapsible emissary veins.4
When bone wax is used as a temporary measure in a dermatologic setting, complications inherent to its use in bone healing (eg, granulomatous reaction, infection)—for which it is left in place indefinitely—are avoided.
- Perandones-González H, Fernández-Canga P, Rodríguez-Prieto MA. Bone wax as an ideal dressing for auricle concha. J Am Acad Dermatol. 2021;84:e75-e76. doi:10.1016/j.jaad.2019.08.002
- Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg. 2008;34:431-445. doi:10.1111/j.1524-4725.2007.34090.x
- Alegre M, Garcés JR, Puig L. Bone wax in dermatologic surgery. Actas Dermosifiliogr. 2013;104:299-303. doi:10.1016/j.adengl.2013.03.001
- Goldman G, Altmayer S, Sambandan P, et al. Development of cerebral air emboli during Mohs micrographic surgery. Dermatol Surg. 2009;35:1414-1421. doi:10.1111/j.1524-4725.2009.01250.x
Practice Gap
Hemostasis after cutaneous surgery typically can be aided by mechanical occlusion with petrolatum and gauze known as a pressure bandage. However, in certain scenarios such as bone bleeding or irregularly shaped areas (eg, conchal bowl), difficulty applying a pressure bandage necessitates alternative hemostatic measures.1 In those instances, physical hemostatic agents, such as gelatin, oxidized cellulose, microporous polysaccharide spheres, hydrophilic polymers with potassium salts, microfibrillar collagen, and chitin, also can be used.2 However, those agents are expensive and often adhere to wound edges, inducing repeat trauma with removal. To avoid such concerns, we propose the use of bone wax as an effective hemostatic technique.
The Technique
When secondary intention healing is chosen or a temporary bandage needs to be placed, we offer the use of bone wax as an alternative to help achieve hemostasis. Bone wax—a combination of beeswax, isopropyl palmitate, and a stabilizing agent such as almond oils or sterilized salicylic acid3—helps achieve hemostasis by purely mechanical means. It is malleable and can be easily adapted to the architecture of the surgical site (Figure 1). The bone wax can be applied immediately following surgery and removed during bandage change.

Practice Implications
Use of bone wax as a physical hemostatic agent provides a practical alternative to other options commonly used in dermatologic surgery for deep wounds or irregular surfaces. It offers several advantages.
Bone wax is not absorbed and does not adhere to wound surfaces, which makes removal easy and painless. Furthermore, bone wax allows for excellent growth of granulation tissue2 (Figure 2), most likely due to the healing and emollient properties of the beeswax and the moist occlusive environment created by the bone wax.

Additional advantages are its low cost, especially compared to other hemostatic agents, and long shelf-life (approximately 5 years).2 Furthermore, in scenarios when cutaneous tumors extend into the calvarium, bone wax can prevent air emboli from entering noncollapsible emissary veins.4
When bone wax is used as a temporary measure in a dermatologic setting, complications inherent to its use in bone healing (eg, granulomatous reaction, infection)—for which it is left in place indefinitely—are avoided.
Practice Gap
Hemostasis after cutaneous surgery typically can be aided by mechanical occlusion with petrolatum and gauze known as a pressure bandage. However, in certain scenarios such as bone bleeding or irregularly shaped areas (eg, conchal bowl), difficulty applying a pressure bandage necessitates alternative hemostatic measures.1 In those instances, physical hemostatic agents, such as gelatin, oxidized cellulose, microporous polysaccharide spheres, hydrophilic polymers with potassium salts, microfibrillar collagen, and chitin, also can be used.2 However, those agents are expensive and often adhere to wound edges, inducing repeat trauma with removal. To avoid such concerns, we propose the use of bone wax as an effective hemostatic technique.
The Technique
When secondary intention healing is chosen or a temporary bandage needs to be placed, we offer the use of bone wax as an alternative to help achieve hemostasis. Bone wax—a combination of beeswax, isopropyl palmitate, and a stabilizing agent such as almond oils or sterilized salicylic acid3—helps achieve hemostasis by purely mechanical means. It is malleable and can be easily adapted to the architecture of the surgical site (Figure 1). The bone wax can be applied immediately following surgery and removed during bandage change.

Practice Implications
Use of bone wax as a physical hemostatic agent provides a practical alternative to other options commonly used in dermatologic surgery for deep wounds or irregular surfaces. It offers several advantages.
Bone wax is not absorbed and does not adhere to wound surfaces, which makes removal easy and painless. Furthermore, bone wax allows for excellent growth of granulation tissue2 (Figure 2), most likely due to the healing and emollient properties of the beeswax and the moist occlusive environment created by the bone wax.

Additional advantages are its low cost, especially compared to other hemostatic agents, and long shelf-life (approximately 5 years).2 Furthermore, in scenarios when cutaneous tumors extend into the calvarium, bone wax can prevent air emboli from entering noncollapsible emissary veins.4
When bone wax is used as a temporary measure in a dermatologic setting, complications inherent to its use in bone healing (eg, granulomatous reaction, infection)—for which it is left in place indefinitely—are avoided.
- Perandones-González H, Fernández-Canga P, Rodríguez-Prieto MA. Bone wax as an ideal dressing for auricle concha. J Am Acad Dermatol. 2021;84:e75-e76. doi:10.1016/j.jaad.2019.08.002
- Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg. 2008;34:431-445. doi:10.1111/j.1524-4725.2007.34090.x
- Alegre M, Garcés JR, Puig L. Bone wax in dermatologic surgery. Actas Dermosifiliogr. 2013;104:299-303. doi:10.1016/j.adengl.2013.03.001
- Goldman G, Altmayer S, Sambandan P, et al. Development of cerebral air emboli during Mohs micrographic surgery. Dermatol Surg. 2009;35:1414-1421. doi:10.1111/j.1524-4725.2009.01250.x
- Perandones-González H, Fernández-Canga P, Rodríguez-Prieto MA. Bone wax as an ideal dressing for auricle concha. J Am Acad Dermatol. 2021;84:e75-e76. doi:10.1016/j.jaad.2019.08.002
- Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg. 2008;34:431-445. doi:10.1111/j.1524-4725.2007.34090.x
- Alegre M, Garcés JR, Puig L. Bone wax in dermatologic surgery. Actas Dermosifiliogr. 2013;104:299-303. doi:10.1016/j.adengl.2013.03.001
- Goldman G, Altmayer S, Sambandan P, et al. Development of cerebral air emboli during Mohs micrographic surgery. Dermatol Surg. 2009;35:1414-1421. doi:10.1111/j.1524-4725.2009.01250.x
Inflammation and immunity troubles top long-COVID suspect list
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
Using live pigs in residency training sparks heated debate
Pigs have been long used in medical schools to teach surgical techniques and, more recently, in research trials and experimental xenotransplantation procedures. But
Just last month, the Physicians Committee for Responsible Medicine, a nonprofit group with a decades-long stance against the use of animals in medical education and research, placed billboards around the Portland, Ore., area demanding that Oregon Health and Science University stop using pigs to teach surgical residents.
Undergraduate medical programs no longer use live animals. But a small number of graduate medical education programs still use animals, predominantly pigs, to train physicians in subspecialties like internal medicine, emergency medicine, surgery, and anesthesiology, John Pippin, MD, FACC, director of academic affairs at PCRM, told this news organization.
Dr. Pippin says residents practice establishing emergency airways, inserting chest tubes, and accessing blood vessels on anesthetized pigs before euthanizing them.
Swine lab advocates say pigs make ideal training subjects because of their similarities to humans, including comparably sized organs like the heart, lungs, and kidneys. Pigs share about 85% of their DNA with people. Where pig skin alternatives may suffice for less invasive procedures, supporters say residents’ experiences with live tissue are irreplaceable.
In a statement, Sara Hottman, associate director of media relations at Oregon Health and Science University, told this news organization the school “only uses animal models in its surgical training program when nonanimal methods are inadequate or too dangerous for human participants.”
“We believe that the education and experience surgical trainees gain through the use of relevant animal models are essential to ensuring future surgeons have the knowledge and skills necessary to provide safe, high-quality care.”
Ms. Hottman also noted that the university continues to evaluate alternatives and looks forward to when nonanimal “surgical training methods are capable of faithfully modeling the complexity of a living system,” such as in the management of critical internal complications.
But Dr. Pippin argues that residents can gain sufficient expertise through simulators and hands-on training in the operating room, and that the differences between humans and pigs are too vast to provide meaningful clinical data or skills.
“Pigs have different genetic influences and very thick, tough skin,” he said. If you use the same pressure on a human that you learned on a pig, he added, “you’d slice right through the trachea. Whatever you think you find out in animals, you have to learn all over again with humans.”
Undergraduate medical education programs in the United States and Canada abandoned the practice of using live animals, including pigs, by 2016, with Johns Hopkins University, Baltimore, and the University of Tennessee, Chattanooga, last to announce their shift away from the controversial teaching model following campaigns by PCRM.
Today, most residency training programs have followed suit. Pippin said that pediatric residencies no longer use animals, and all trauma and anesthesiology programs have ceased such practices except two. Just 3% of emergency medicine programs continue to use animals, as do about 21% of surgical residencies, he said, based on PCRM’s latest surveys.
A public debate
Occasionally, PCRM goes public with a campaign against a residency program “if that’s the only way to win,” Dr. Pippin said.
In addition to billboards, the group has held protests, circulated petitions, and filed complaints with the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service, the entity responsible for overseeing the health and welfare of animals used in medical training and research.
In 2021, spurred by a complaint from PCRM, APHIS launched an investigation into the University of Cincinnati’s surgical residency program. At the time, a university spokesperson acknowledged the school’s limited use of pigs to train “highly-skilled, well-prepared surgeons in the most advanced, complex, real-world needs, procedures, and techniques,” adding that the training methods were endorsed by the American College of Surgeons and in compliance with federal guidelines.
Residency programs have caught the attention of state lawmakers, too. In 2020, bills introduced in both the Rhode Island House and Senate sought to ban the use of live animals in medical training when “there is an alternate teaching method that teaches the medical procedure or lesson without the use of an animal.” Violators would incur misdemeanor charges and monetary fines of up to $1,000 per animal.
The bills – backed by PCRM – targeted Brown University’s emergency medicine residency program, Providence, R.I., which sponsoring legislators said was the last program in New England still using the “outdated” and “unnecessary” method.
In testimony before lawmakers, the school said fewer than 15 pigs participate in the annual training, and faculty spoke about the benefits of the experience.
“If it was your brother or sister, or your mother or father who had to come in and get this procedure done, would you want the physician who’s doing it to be the one who does it for the very first time on a human being, on live tissue? Or do you want that provider to have only practiced on plastic and rubber?” said Nicholas Musisca, MD, an assistant program director with Brown University’s emergency medicine residency, NBC affiliate WJAR reported.
The bills have since stalled, and PCRM held a protest at Brown University in October 2022. In response, a university spokesperson told the Brown Daily Herald, “effective synthetic model alternatives simply do not exist for every complex medical procedure that an emergency physician must be prepared to perform,” including establishing an airway in adults and pediatric patients with severe facial trauma.
By the numbers
Annual reports from APHIS do not show the number of pigs dedicated solely to residency training. Instead, reporting indicates the number of animals “upon which experiments, teaching, research, surgery, or tests were conducted involving accompanying pain or distress to the animals and for which appropriate anesthetic, analgesic, or tranquilizing drugs were used.”
For fiscal year 2021 – the most recent data available – Oregon Health and Science University had 154 pigs under its control, while the University of Cincinnati and Brown University had 118 and 71 pigs, respectively, according to APHIS. Primates were more commonly used at Oregon Health and Science University and guinea pigs at the University of Cincinnati.
Similarly, the Association of American Medical Colleges supports the “use of animals to meet essential educational objectives [across] the medical education continuum. ... Further restrictions on the use of animals in biomedical and behavioral research and education threatens progress in health care and disease prevention.”
The debate will likely rage on. “The one thing we don’t do is give up,” Dr. Pippin said.
A version of this article originally appeared on Medscape.com.
Pigs have been long used in medical schools to teach surgical techniques and, more recently, in research trials and experimental xenotransplantation procedures. But
Just last month, the Physicians Committee for Responsible Medicine, a nonprofit group with a decades-long stance against the use of animals in medical education and research, placed billboards around the Portland, Ore., area demanding that Oregon Health and Science University stop using pigs to teach surgical residents.
Undergraduate medical programs no longer use live animals. But a small number of graduate medical education programs still use animals, predominantly pigs, to train physicians in subspecialties like internal medicine, emergency medicine, surgery, and anesthesiology, John Pippin, MD, FACC, director of academic affairs at PCRM, told this news organization.
Dr. Pippin says residents practice establishing emergency airways, inserting chest tubes, and accessing blood vessels on anesthetized pigs before euthanizing them.
Swine lab advocates say pigs make ideal training subjects because of their similarities to humans, including comparably sized organs like the heart, lungs, and kidneys. Pigs share about 85% of their DNA with people. Where pig skin alternatives may suffice for less invasive procedures, supporters say residents’ experiences with live tissue are irreplaceable.
In a statement, Sara Hottman, associate director of media relations at Oregon Health and Science University, told this news organization the school “only uses animal models in its surgical training program when nonanimal methods are inadequate or too dangerous for human participants.”
“We believe that the education and experience surgical trainees gain through the use of relevant animal models are essential to ensuring future surgeons have the knowledge and skills necessary to provide safe, high-quality care.”
Ms. Hottman also noted that the university continues to evaluate alternatives and looks forward to when nonanimal “surgical training methods are capable of faithfully modeling the complexity of a living system,” such as in the management of critical internal complications.
But Dr. Pippin argues that residents can gain sufficient expertise through simulators and hands-on training in the operating room, and that the differences between humans and pigs are too vast to provide meaningful clinical data or skills.
“Pigs have different genetic influences and very thick, tough skin,” he said. If you use the same pressure on a human that you learned on a pig, he added, “you’d slice right through the trachea. Whatever you think you find out in animals, you have to learn all over again with humans.”
Undergraduate medical education programs in the United States and Canada abandoned the practice of using live animals, including pigs, by 2016, with Johns Hopkins University, Baltimore, and the University of Tennessee, Chattanooga, last to announce their shift away from the controversial teaching model following campaigns by PCRM.
Today, most residency training programs have followed suit. Pippin said that pediatric residencies no longer use animals, and all trauma and anesthesiology programs have ceased such practices except two. Just 3% of emergency medicine programs continue to use animals, as do about 21% of surgical residencies, he said, based on PCRM’s latest surveys.
A public debate
Occasionally, PCRM goes public with a campaign against a residency program “if that’s the only way to win,” Dr. Pippin said.
In addition to billboards, the group has held protests, circulated petitions, and filed complaints with the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service, the entity responsible for overseeing the health and welfare of animals used in medical training and research.
In 2021, spurred by a complaint from PCRM, APHIS launched an investigation into the University of Cincinnati’s surgical residency program. At the time, a university spokesperson acknowledged the school’s limited use of pigs to train “highly-skilled, well-prepared surgeons in the most advanced, complex, real-world needs, procedures, and techniques,” adding that the training methods were endorsed by the American College of Surgeons and in compliance with federal guidelines.
Residency programs have caught the attention of state lawmakers, too. In 2020, bills introduced in both the Rhode Island House and Senate sought to ban the use of live animals in medical training when “there is an alternate teaching method that teaches the medical procedure or lesson without the use of an animal.” Violators would incur misdemeanor charges and monetary fines of up to $1,000 per animal.
The bills – backed by PCRM – targeted Brown University’s emergency medicine residency program, Providence, R.I., which sponsoring legislators said was the last program in New England still using the “outdated” and “unnecessary” method.
In testimony before lawmakers, the school said fewer than 15 pigs participate in the annual training, and faculty spoke about the benefits of the experience.
“If it was your brother or sister, or your mother or father who had to come in and get this procedure done, would you want the physician who’s doing it to be the one who does it for the very first time on a human being, on live tissue? Or do you want that provider to have only practiced on plastic and rubber?” said Nicholas Musisca, MD, an assistant program director with Brown University’s emergency medicine residency, NBC affiliate WJAR reported.
The bills have since stalled, and PCRM held a protest at Brown University in October 2022. In response, a university spokesperson told the Brown Daily Herald, “effective synthetic model alternatives simply do not exist for every complex medical procedure that an emergency physician must be prepared to perform,” including establishing an airway in adults and pediatric patients with severe facial trauma.
By the numbers
Annual reports from APHIS do not show the number of pigs dedicated solely to residency training. Instead, reporting indicates the number of animals “upon which experiments, teaching, research, surgery, or tests were conducted involving accompanying pain or distress to the animals and for which appropriate anesthetic, analgesic, or tranquilizing drugs were used.”
For fiscal year 2021 – the most recent data available – Oregon Health and Science University had 154 pigs under its control, while the University of Cincinnati and Brown University had 118 and 71 pigs, respectively, according to APHIS. Primates were more commonly used at Oregon Health and Science University and guinea pigs at the University of Cincinnati.
Similarly, the Association of American Medical Colleges supports the “use of animals to meet essential educational objectives [across] the medical education continuum. ... Further restrictions on the use of animals in biomedical and behavioral research and education threatens progress in health care and disease prevention.”
The debate will likely rage on. “The one thing we don’t do is give up,” Dr. Pippin said.
A version of this article originally appeared on Medscape.com.
Pigs have been long used in medical schools to teach surgical techniques and, more recently, in research trials and experimental xenotransplantation procedures. But
Just last month, the Physicians Committee for Responsible Medicine, a nonprofit group with a decades-long stance against the use of animals in medical education and research, placed billboards around the Portland, Ore., area demanding that Oregon Health and Science University stop using pigs to teach surgical residents.
Undergraduate medical programs no longer use live animals. But a small number of graduate medical education programs still use animals, predominantly pigs, to train physicians in subspecialties like internal medicine, emergency medicine, surgery, and anesthesiology, John Pippin, MD, FACC, director of academic affairs at PCRM, told this news organization.
Dr. Pippin says residents practice establishing emergency airways, inserting chest tubes, and accessing blood vessels on anesthetized pigs before euthanizing them.
Swine lab advocates say pigs make ideal training subjects because of their similarities to humans, including comparably sized organs like the heart, lungs, and kidneys. Pigs share about 85% of their DNA with people. Where pig skin alternatives may suffice for less invasive procedures, supporters say residents’ experiences with live tissue are irreplaceable.
In a statement, Sara Hottman, associate director of media relations at Oregon Health and Science University, told this news organization the school “only uses animal models in its surgical training program when nonanimal methods are inadequate or too dangerous for human participants.”
“We believe that the education and experience surgical trainees gain through the use of relevant animal models are essential to ensuring future surgeons have the knowledge and skills necessary to provide safe, high-quality care.”
Ms. Hottman also noted that the university continues to evaluate alternatives and looks forward to when nonanimal “surgical training methods are capable of faithfully modeling the complexity of a living system,” such as in the management of critical internal complications.
But Dr. Pippin argues that residents can gain sufficient expertise through simulators and hands-on training in the operating room, and that the differences between humans and pigs are too vast to provide meaningful clinical data or skills.
“Pigs have different genetic influences and very thick, tough skin,” he said. If you use the same pressure on a human that you learned on a pig, he added, “you’d slice right through the trachea. Whatever you think you find out in animals, you have to learn all over again with humans.”
Undergraduate medical education programs in the United States and Canada abandoned the practice of using live animals, including pigs, by 2016, with Johns Hopkins University, Baltimore, and the University of Tennessee, Chattanooga, last to announce their shift away from the controversial teaching model following campaigns by PCRM.
Today, most residency training programs have followed suit. Pippin said that pediatric residencies no longer use animals, and all trauma and anesthesiology programs have ceased such practices except two. Just 3% of emergency medicine programs continue to use animals, as do about 21% of surgical residencies, he said, based on PCRM’s latest surveys.
A public debate
Occasionally, PCRM goes public with a campaign against a residency program “if that’s the only way to win,” Dr. Pippin said.
In addition to billboards, the group has held protests, circulated petitions, and filed complaints with the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service, the entity responsible for overseeing the health and welfare of animals used in medical training and research.
In 2021, spurred by a complaint from PCRM, APHIS launched an investigation into the University of Cincinnati’s surgical residency program. At the time, a university spokesperson acknowledged the school’s limited use of pigs to train “highly-skilled, well-prepared surgeons in the most advanced, complex, real-world needs, procedures, and techniques,” adding that the training methods were endorsed by the American College of Surgeons and in compliance with federal guidelines.
Residency programs have caught the attention of state lawmakers, too. In 2020, bills introduced in both the Rhode Island House and Senate sought to ban the use of live animals in medical training when “there is an alternate teaching method that teaches the medical procedure or lesson without the use of an animal.” Violators would incur misdemeanor charges and monetary fines of up to $1,000 per animal.
The bills – backed by PCRM – targeted Brown University’s emergency medicine residency program, Providence, R.I., which sponsoring legislators said was the last program in New England still using the “outdated” and “unnecessary” method.
In testimony before lawmakers, the school said fewer than 15 pigs participate in the annual training, and faculty spoke about the benefits of the experience.
“If it was your brother or sister, or your mother or father who had to come in and get this procedure done, would you want the physician who’s doing it to be the one who does it for the very first time on a human being, on live tissue? Or do you want that provider to have only practiced on plastic and rubber?” said Nicholas Musisca, MD, an assistant program director with Brown University’s emergency medicine residency, NBC affiliate WJAR reported.
The bills have since stalled, and PCRM held a protest at Brown University in October 2022. In response, a university spokesperson told the Brown Daily Herald, “effective synthetic model alternatives simply do not exist for every complex medical procedure that an emergency physician must be prepared to perform,” including establishing an airway in adults and pediatric patients with severe facial trauma.
By the numbers
Annual reports from APHIS do not show the number of pigs dedicated solely to residency training. Instead, reporting indicates the number of animals “upon which experiments, teaching, research, surgery, or tests were conducted involving accompanying pain or distress to the animals and for which appropriate anesthetic, analgesic, or tranquilizing drugs were used.”
For fiscal year 2021 – the most recent data available – Oregon Health and Science University had 154 pigs under its control, while the University of Cincinnati and Brown University had 118 and 71 pigs, respectively, according to APHIS. Primates were more commonly used at Oregon Health and Science University and guinea pigs at the University of Cincinnati.
Similarly, the Association of American Medical Colleges supports the “use of animals to meet essential educational objectives [across] the medical education continuum. ... Further restrictions on the use of animals in biomedical and behavioral research and education threatens progress in health care and disease prevention.”
The debate will likely rage on. “The one thing we don’t do is give up,” Dr. Pippin said.
A version of this article originally appeared on Medscape.com.







