User login
Exercise halves T2D risk in adults with obesity
“Physical exercise combined with diet restriction has been proven to be effective in prevention of diabetes. However, the long-term effect of exercise on prevention of diabetes, and the difference of exercise intensity in prevention of diabetes have not been well studied,” said corresponding author Xiaoying Li, MD, of Zhongshan Hospital, Fudan University, Shanghai, in an interview.
In the research letter published in JAMA Internal Medicine, Dr. Li and colleagues analyzed the results of a study of 220 adults with central obesity and nonalcoholic fatty liver disease, but no incident diabetes, randomized to a 12-month program of vigorous exercise (73 patients), moderate aerobic exercise (73 patients) or no exercise (74 patients).
A total of 208 participants completed the 1-year intervention; of these, 195 and 178 remained to provide data at 2 years and 10 years, respectively. The mean age of the participants was 53.9 years, 32.3% were male, and the mean waist circumference was 96.1 cm at baseline.
The cumulative incidence of type 2 diabetes in the vigorous exercise, moderate exercise, and nonexercise groups was 2.1 per 100 person-years 1.9 per 100 person-years, and 4.1 per 100 person-years, respectively, over the 10-year follow-up period. This translated to a reduction in type 2 diabetes risk of 49% in the vigorous exercise group and 53% in the moderate exercise group compared with the nonexercise group.
In addition, individuals in the vigorous and moderate exercise groups significantly reduced their HbA1c and waist circumference compared with the nonexercisers. Levels of plasma fasting glucose and weight regain were lower in both exercise groups compared with nonexercisers, but these differences were not significant.
The exercise intervention was described in a 2016 study, which was also published in JAMA Internal Medicine. That study’s purpose was to compare the effects of exercise on patients with nonalcoholic fatty liver disease. Participants were coached and supervised for their exercise programs. The program for the vigorous group involved jogging for 150 minutes per week at 65%-80% of maximum heart rate for 6 months and brisk walking 150 minutes per week at 45%-55% of maximum heart rate for another 6 months. The program for the moderate exercise group involved brisk walking 150 minutes per week for 12 months.
Both exercise groups showed a trend towards higher levels of leisure time physical activity after 10 years compared with the nonexercise groups, although the difference was not significant.
The main limitation of the study was that incident prediabetes was not prespecified, which may have led to some confounding, the researchers noted. In addition, the participants were highly supervised for a 12-month program only. However, the results support the long-term value of physical exercise as a method of obesity management and to delay progression to type 2 diabetes in obese individuals, they said. Vigorous and moderate aerobic exercise programs could be implemented for this patient population, they concluded.
“Surprisingly, our findings demonstrated that a 12-month vigorous aerobic exercise or moderate aerobic exercise could significantly reduce the risk of incident diabetes by 50% over the 10-year follow-up,” Dr. Li said in an interview. The results suggest that physical exercise for some period of time can produce a long-term beneficial effect in prevention of type 2 diabetes, he said.
Potential barriers to the routine use of an exercise intervention in patients with obesity include the unwillingness of this population to engage in vigorous exercise, and the potential for musculoskeletal injury, said Dr. Li. In these cases, obese patients should be encouraged to pursue moderate exercise, Dr. Li said.
Looking ahead, more research is needed to examine the potential mechanism behind the effect of exercise on diabetes prevention, said Dr. Li.
Findings fill gap in long-term outcome data
The current study is important because of the long-term follow-up data, said Jill Kanaley, PhD, professor and interim chair of nutrition and exercise physiology at the University of Missouri, in an interview. “We seldom follow up on our training studies, thus it is important to see if there is any long-term impact of these interventions,” she said.
Dr. Kanaley said she was surprised to see the residual benefits of the exercise intervention 10 years later.
“We often wonder how long the impact of the exercise training will stay with someone so that they continue to exercise and watch their weight; this study seems to indicate that there is an educational component that stays with them,” she said.
The main clinical takeaway from the current study was the minimal weight gain over time, Dr. Kanaley said.
Although time may be a barrier to the routine use of an exercise intervention, patients have to realize that they can usually find the time, especially given the multiple benefits, said Dr. Kanaley. “The exercise interventions provide more benefits than just weight control and glucose levels,” she said.
“The 30-60 minutes of exercise does not have to come all at the same time,” Dr. Kanaley noted. “It could be three 15-minute bouts of exercise/physical activity to get their 45 minutes in,” she noted. Exercise does not have to be heavy vigorous exercise, even walking is beneficial, she said. For people who complain of boredom with an exercise routine, Dr. Kanaley encourages mixing it up, with activities such as different exercise classes, running, or walking on a different day of any given week.
Although the current study was conducted in China, the findings may translate to a U.S. population, Dr. Kanaley said in an interview. However, “frequently our Western diet is less healthy than the traditional Chinese diet. This may have provided an immeasurable benefit to these subjects,” although study participants did not make specific adjustments to their diets, she said.
Additional research is needed to confirm the findings, said Dr. Kanaley. “Ideally, the study should be repeated in a population with a Western diet,” she noted.
Next steps for research include maintenance of activity
Evidence on the long-term benefits of exercise programs is limited, said Amanda Paluch, PhD, a physical activity epidemiologist at the University of Massachusetts, Amherst, in an interview.
“Chronic diseases such as diabetes can take years to develop, so understanding these important health outcomes requires years of follow-up. This study followed their study participants for 10 years, which gives us a nice glimpse of the long-term benefits of exercise training on diabetes prevention,” she said.
Data from previous observational studies of individuals’ current activity levels (without an intervention) suggest that adults who are more physically active have a lower risk of diabetes over time, said Dr. Paluch. However, the current study is one of the few with rigorous exercise interventions with extensive follow-up on diabetes risk, and it provides important evidence that a 12-month structured exercise program in inactive adults with obesity can result in meaningful long-term health benefits by lowering the risk of diabetes, she said.
“The individuals in the current study participated in a structured exercise program where their exercise sessions were supervised and coached,” Dr. Paluch noted. “Having a personalized coach may not be within the budget or time constraints for many people,” she said. Her message to clinicians for their patients: “When looking to start an exercise routine, identify an activity you enjoy and find feasible to fit into your existing life and schedule,” she said.
“Although this study was conducted in China, the results are meaningful for the U.S. population, as we would expect the physiological benefit of exercise to be consistent across various populations,” Dr. Paluch said. “However, there are certainly differences across countries at the individual level to the larger community-wide level that may influence a person’s ability to maintain physical activity and prevent diabetes, so replicating similar studies in other countries, including the U.S., would be of value.”
“Additionally, we need more research on how to encourage maintenance of physical activity in the long-term, after the initial exercise program is over,” she said.
“From this current study, we cannot tease out whether diabetes risk is reduced because of the 12-month exercise intervention or the benefit is from maintaining physical activity regularly over the 10 years of follow-up, or a combination of the two,” said Dr. Paluch. Future studies should consider teasing out participants who were only active during the exercise intervention, then ceased being active vs. participants who continued with vigorous activity long-term, she said.
The study was supported by the National Nature Science Foundation, the National Key Research and Development Program of China, and the Shanghai Municipal Science and Technology Major Project. The researchers, Dr. Kanaley, and Dr. Paluch had no financial conflicts to disclose.
“Physical exercise combined with diet restriction has been proven to be effective in prevention of diabetes. However, the long-term effect of exercise on prevention of diabetes, and the difference of exercise intensity in prevention of diabetes have not been well studied,” said corresponding author Xiaoying Li, MD, of Zhongshan Hospital, Fudan University, Shanghai, in an interview.
In the research letter published in JAMA Internal Medicine, Dr. Li and colleagues analyzed the results of a study of 220 adults with central obesity and nonalcoholic fatty liver disease, but no incident diabetes, randomized to a 12-month program of vigorous exercise (73 patients), moderate aerobic exercise (73 patients) or no exercise (74 patients).
A total of 208 participants completed the 1-year intervention; of these, 195 and 178 remained to provide data at 2 years and 10 years, respectively. The mean age of the participants was 53.9 years, 32.3% were male, and the mean waist circumference was 96.1 cm at baseline.
The cumulative incidence of type 2 diabetes in the vigorous exercise, moderate exercise, and nonexercise groups was 2.1 per 100 person-years 1.9 per 100 person-years, and 4.1 per 100 person-years, respectively, over the 10-year follow-up period. This translated to a reduction in type 2 diabetes risk of 49% in the vigorous exercise group and 53% in the moderate exercise group compared with the nonexercise group.
In addition, individuals in the vigorous and moderate exercise groups significantly reduced their HbA1c and waist circumference compared with the nonexercisers. Levels of plasma fasting glucose and weight regain were lower in both exercise groups compared with nonexercisers, but these differences were not significant.
The exercise intervention was described in a 2016 study, which was also published in JAMA Internal Medicine. That study’s purpose was to compare the effects of exercise on patients with nonalcoholic fatty liver disease. Participants were coached and supervised for their exercise programs. The program for the vigorous group involved jogging for 150 minutes per week at 65%-80% of maximum heart rate for 6 months and brisk walking 150 minutes per week at 45%-55% of maximum heart rate for another 6 months. The program for the moderate exercise group involved brisk walking 150 minutes per week for 12 months.
Both exercise groups showed a trend towards higher levels of leisure time physical activity after 10 years compared with the nonexercise groups, although the difference was not significant.
The main limitation of the study was that incident prediabetes was not prespecified, which may have led to some confounding, the researchers noted. In addition, the participants were highly supervised for a 12-month program only. However, the results support the long-term value of physical exercise as a method of obesity management and to delay progression to type 2 diabetes in obese individuals, they said. Vigorous and moderate aerobic exercise programs could be implemented for this patient population, they concluded.
“Surprisingly, our findings demonstrated that a 12-month vigorous aerobic exercise or moderate aerobic exercise could significantly reduce the risk of incident diabetes by 50% over the 10-year follow-up,” Dr. Li said in an interview. The results suggest that physical exercise for some period of time can produce a long-term beneficial effect in prevention of type 2 diabetes, he said.
Potential barriers to the routine use of an exercise intervention in patients with obesity include the unwillingness of this population to engage in vigorous exercise, and the potential for musculoskeletal injury, said Dr. Li. In these cases, obese patients should be encouraged to pursue moderate exercise, Dr. Li said.
Looking ahead, more research is needed to examine the potential mechanism behind the effect of exercise on diabetes prevention, said Dr. Li.
Findings fill gap in long-term outcome data
The current study is important because of the long-term follow-up data, said Jill Kanaley, PhD, professor and interim chair of nutrition and exercise physiology at the University of Missouri, in an interview. “We seldom follow up on our training studies, thus it is important to see if there is any long-term impact of these interventions,” she said.
Dr. Kanaley said she was surprised to see the residual benefits of the exercise intervention 10 years later.
“We often wonder how long the impact of the exercise training will stay with someone so that they continue to exercise and watch their weight; this study seems to indicate that there is an educational component that stays with them,” she said.
The main clinical takeaway from the current study was the minimal weight gain over time, Dr. Kanaley said.
Although time may be a barrier to the routine use of an exercise intervention, patients have to realize that they can usually find the time, especially given the multiple benefits, said Dr. Kanaley. “The exercise interventions provide more benefits than just weight control and glucose levels,” she said.
“The 30-60 minutes of exercise does not have to come all at the same time,” Dr. Kanaley noted. “It could be three 15-minute bouts of exercise/physical activity to get their 45 minutes in,” she noted. Exercise does not have to be heavy vigorous exercise, even walking is beneficial, she said. For people who complain of boredom with an exercise routine, Dr. Kanaley encourages mixing it up, with activities such as different exercise classes, running, or walking on a different day of any given week.
Although the current study was conducted in China, the findings may translate to a U.S. population, Dr. Kanaley said in an interview. However, “frequently our Western diet is less healthy than the traditional Chinese diet. This may have provided an immeasurable benefit to these subjects,” although study participants did not make specific adjustments to their diets, she said.
Additional research is needed to confirm the findings, said Dr. Kanaley. “Ideally, the study should be repeated in a population with a Western diet,” she noted.
Next steps for research include maintenance of activity
Evidence on the long-term benefits of exercise programs is limited, said Amanda Paluch, PhD, a physical activity epidemiologist at the University of Massachusetts, Amherst, in an interview.
“Chronic diseases such as diabetes can take years to develop, so understanding these important health outcomes requires years of follow-up. This study followed their study participants for 10 years, which gives us a nice glimpse of the long-term benefits of exercise training on diabetes prevention,” she said.
Data from previous observational studies of individuals’ current activity levels (without an intervention) suggest that adults who are more physically active have a lower risk of diabetes over time, said Dr. Paluch. However, the current study is one of the few with rigorous exercise interventions with extensive follow-up on diabetes risk, and it provides important evidence that a 12-month structured exercise program in inactive adults with obesity can result in meaningful long-term health benefits by lowering the risk of diabetes, she said.
“The individuals in the current study participated in a structured exercise program where their exercise sessions were supervised and coached,” Dr. Paluch noted. “Having a personalized coach may not be within the budget or time constraints for many people,” she said. Her message to clinicians for their patients: “When looking to start an exercise routine, identify an activity you enjoy and find feasible to fit into your existing life and schedule,” she said.
“Although this study was conducted in China, the results are meaningful for the U.S. population, as we would expect the physiological benefit of exercise to be consistent across various populations,” Dr. Paluch said. “However, there are certainly differences across countries at the individual level to the larger community-wide level that may influence a person’s ability to maintain physical activity and prevent diabetes, so replicating similar studies in other countries, including the U.S., would be of value.”
“Additionally, we need more research on how to encourage maintenance of physical activity in the long-term, after the initial exercise program is over,” she said.
“From this current study, we cannot tease out whether diabetes risk is reduced because of the 12-month exercise intervention or the benefit is from maintaining physical activity regularly over the 10 years of follow-up, or a combination of the two,” said Dr. Paluch. Future studies should consider teasing out participants who were only active during the exercise intervention, then ceased being active vs. participants who continued with vigorous activity long-term, she said.
The study was supported by the National Nature Science Foundation, the National Key Research and Development Program of China, and the Shanghai Municipal Science and Technology Major Project. The researchers, Dr. Kanaley, and Dr. Paluch had no financial conflicts to disclose.
“Physical exercise combined with diet restriction has been proven to be effective in prevention of diabetes. However, the long-term effect of exercise on prevention of diabetes, and the difference of exercise intensity in prevention of diabetes have not been well studied,” said corresponding author Xiaoying Li, MD, of Zhongshan Hospital, Fudan University, Shanghai, in an interview.
In the research letter published in JAMA Internal Medicine, Dr. Li and colleagues analyzed the results of a study of 220 adults with central obesity and nonalcoholic fatty liver disease, but no incident diabetes, randomized to a 12-month program of vigorous exercise (73 patients), moderate aerobic exercise (73 patients) or no exercise (74 patients).
A total of 208 participants completed the 1-year intervention; of these, 195 and 178 remained to provide data at 2 years and 10 years, respectively. The mean age of the participants was 53.9 years, 32.3% were male, and the mean waist circumference was 96.1 cm at baseline.
The cumulative incidence of type 2 diabetes in the vigorous exercise, moderate exercise, and nonexercise groups was 2.1 per 100 person-years 1.9 per 100 person-years, and 4.1 per 100 person-years, respectively, over the 10-year follow-up period. This translated to a reduction in type 2 diabetes risk of 49% in the vigorous exercise group and 53% in the moderate exercise group compared with the nonexercise group.
In addition, individuals in the vigorous and moderate exercise groups significantly reduced their HbA1c and waist circumference compared with the nonexercisers. Levels of plasma fasting glucose and weight regain were lower in both exercise groups compared with nonexercisers, but these differences were not significant.
The exercise intervention was described in a 2016 study, which was also published in JAMA Internal Medicine. That study’s purpose was to compare the effects of exercise on patients with nonalcoholic fatty liver disease. Participants were coached and supervised for their exercise programs. The program for the vigorous group involved jogging for 150 minutes per week at 65%-80% of maximum heart rate for 6 months and brisk walking 150 minutes per week at 45%-55% of maximum heart rate for another 6 months. The program for the moderate exercise group involved brisk walking 150 minutes per week for 12 months.
Both exercise groups showed a trend towards higher levels of leisure time physical activity after 10 years compared with the nonexercise groups, although the difference was not significant.
The main limitation of the study was that incident prediabetes was not prespecified, which may have led to some confounding, the researchers noted. In addition, the participants were highly supervised for a 12-month program only. However, the results support the long-term value of physical exercise as a method of obesity management and to delay progression to type 2 diabetes in obese individuals, they said. Vigorous and moderate aerobic exercise programs could be implemented for this patient population, they concluded.
“Surprisingly, our findings demonstrated that a 12-month vigorous aerobic exercise or moderate aerobic exercise could significantly reduce the risk of incident diabetes by 50% over the 10-year follow-up,” Dr. Li said in an interview. The results suggest that physical exercise for some period of time can produce a long-term beneficial effect in prevention of type 2 diabetes, he said.
Potential barriers to the routine use of an exercise intervention in patients with obesity include the unwillingness of this population to engage in vigorous exercise, and the potential for musculoskeletal injury, said Dr. Li. In these cases, obese patients should be encouraged to pursue moderate exercise, Dr. Li said.
Looking ahead, more research is needed to examine the potential mechanism behind the effect of exercise on diabetes prevention, said Dr. Li.
Findings fill gap in long-term outcome data
The current study is important because of the long-term follow-up data, said Jill Kanaley, PhD, professor and interim chair of nutrition and exercise physiology at the University of Missouri, in an interview. “We seldom follow up on our training studies, thus it is important to see if there is any long-term impact of these interventions,” she said.
Dr. Kanaley said she was surprised to see the residual benefits of the exercise intervention 10 years later.
“We often wonder how long the impact of the exercise training will stay with someone so that they continue to exercise and watch their weight; this study seems to indicate that there is an educational component that stays with them,” she said.
The main clinical takeaway from the current study was the minimal weight gain over time, Dr. Kanaley said.
Although time may be a barrier to the routine use of an exercise intervention, patients have to realize that they can usually find the time, especially given the multiple benefits, said Dr. Kanaley. “The exercise interventions provide more benefits than just weight control and glucose levels,” she said.
“The 30-60 minutes of exercise does not have to come all at the same time,” Dr. Kanaley noted. “It could be three 15-minute bouts of exercise/physical activity to get their 45 minutes in,” she noted. Exercise does not have to be heavy vigorous exercise, even walking is beneficial, she said. For people who complain of boredom with an exercise routine, Dr. Kanaley encourages mixing it up, with activities such as different exercise classes, running, or walking on a different day of any given week.
Although the current study was conducted in China, the findings may translate to a U.S. population, Dr. Kanaley said in an interview. However, “frequently our Western diet is less healthy than the traditional Chinese diet. This may have provided an immeasurable benefit to these subjects,” although study participants did not make specific adjustments to their diets, she said.
Additional research is needed to confirm the findings, said Dr. Kanaley. “Ideally, the study should be repeated in a population with a Western diet,” she noted.
Next steps for research include maintenance of activity
Evidence on the long-term benefits of exercise programs is limited, said Amanda Paluch, PhD, a physical activity epidemiologist at the University of Massachusetts, Amherst, in an interview.
“Chronic diseases such as diabetes can take years to develop, so understanding these important health outcomes requires years of follow-up. This study followed their study participants for 10 years, which gives us a nice glimpse of the long-term benefits of exercise training on diabetes prevention,” she said.
Data from previous observational studies of individuals’ current activity levels (without an intervention) suggest that adults who are more physically active have a lower risk of diabetes over time, said Dr. Paluch. However, the current study is one of the few with rigorous exercise interventions with extensive follow-up on diabetes risk, and it provides important evidence that a 12-month structured exercise program in inactive adults with obesity can result in meaningful long-term health benefits by lowering the risk of diabetes, she said.
“The individuals in the current study participated in a structured exercise program where their exercise sessions were supervised and coached,” Dr. Paluch noted. “Having a personalized coach may not be within the budget or time constraints for many people,” she said. Her message to clinicians for their patients: “When looking to start an exercise routine, identify an activity you enjoy and find feasible to fit into your existing life and schedule,” she said.
“Although this study was conducted in China, the results are meaningful for the U.S. population, as we would expect the physiological benefit of exercise to be consistent across various populations,” Dr. Paluch said. “However, there are certainly differences across countries at the individual level to the larger community-wide level that may influence a person’s ability to maintain physical activity and prevent diabetes, so replicating similar studies in other countries, including the U.S., would be of value.”
“Additionally, we need more research on how to encourage maintenance of physical activity in the long-term, after the initial exercise program is over,” she said.
“From this current study, we cannot tease out whether diabetes risk is reduced because of the 12-month exercise intervention or the benefit is from maintaining physical activity regularly over the 10 years of follow-up, or a combination of the two,” said Dr. Paluch. Future studies should consider teasing out participants who were only active during the exercise intervention, then ceased being active vs. participants who continued with vigorous activity long-term, she said.
The study was supported by the National Nature Science Foundation, the National Key Research and Development Program of China, and the Shanghai Municipal Science and Technology Major Project. The researchers, Dr. Kanaley, and Dr. Paluch had no financial conflicts to disclose.
FROM JAMA INTERNAL MEDICINE
Running does not cause lasting cartilage damage
Running does not appear to cause sustained wear and tear of healthy knee cartilage, with research suggesting that the small, short-term changes to cartilage after a run reverse within hours.
A systematic review and meta-analysis published in the most recent issue of Osteoarthritis and Cartilage presents the findings involving 396 adults, which compared the “before” and “after” state of healthy knee cartilage in runners.
Running is often thought to be detrimental to joint health, wrote Sally Coburn, PhD candidate at the La Trobe Sport & Exercise Medicine Research Centre at La Trobe University in Melbourne and coauthors, but this perception is not supported by evidence.
For the analysis, the researchers included studies that looked at either knee or hip cartilage using MRI to assess its size, shape, structure, and/or composition both in the 48 hours before a single bout of running and in the 48 hours after. The analysis aimed to include adults with or at risk of osteoarthritis, but only 57 of the 446 knees in the analysis fit these criteria.
In studies where participants underwent MRI within 20 minutes of running, there was an immediate postrun decrease in the volume of cartilage, ranging from –3.3% for weight-bearing femoral cartilage to –4.1% for tibial cartilage volume. This also revealed a decrease in T1 and T2 relaxation times, which are specialized MRI measures that reflect the composition of cartilage and which can indicate a breakdown of cartilage structure in the case of diseases such as arthritis.
Reversal of short-term cartilage changes
However, within 48 hours of the run, data from studies that repeated the MRIs more than once after the initial prerun scan suggested these changes reversed back to prerun levels.
“We were able to pool delayed T2 relaxation time measures from studies that repeated scans of the same participants 60 minutes and 91 minutes post-run and found no effect of running on tibiofemoral joint cartilage composition,” the authors write.
For example, one study in marathon runners found no difference in cartilage thickness in the tibiofemoral joint between baseline and at 2-10 hours and 12 hours after the marathon. Another showed the immediate post-run decrease in patellofemoral joint cartilage thickness had reverted back to prerun levels when the scan was repeated 24 hours after the run.
“The changes are very minimal and not inconsistent with what’s expected for your cartilage which is functioning normally,” Ms. Coburn told this news organization.
Sparse data in people with osteoarthritis
The authors said there were not enough data from individuals with osteoarthritis to be able to pool and quantify their cartilage changes. However, one study in the analysis found that cartilage lesions in people considered at risk of osteoarthritis because of prior anterior cruciate ligament reconstruction were unchanged after running.
Another suggested that the decrease in femoral cartilage volume recorded at 15 minutes persisted at 45 minutes, while a separate study found significantly increased T2 relaxation times at 45 minutes after a run in those with knee osteoarthritis but not in those without osteoarthritis.
Senior author Adam Culvenor, PhD, senior research fellow at the La Trobe Centre, said their analysis suggested running was healthy, with small changes in cartilage that resolve quickly, but “we really don’t know yet if running is safe for people with osteoarthritis,” he said. “We need much more work in that space.”
Overall, the study evidence was rated as being of low certainty, which Dr. Coburn said was related to the small numbers in each study, which in turn relates to the cost and logistical challenges of the specialized MRI scan used.
“Study of a repeated exposure over a long duration of time on a disease that has a long natural history, like osteoarthritis, is challenging in that most funding agencies will not fund studies longer than 5 years,” Grace Hsiao-Wei Lo, MD, of the department of immunology, allergy, and rheumatology at the Baylor College of Medicine in Houston, said in an email.
Dr. Lo, who was not involved with this review and meta-analysis, said there are still concerns about the effect of running on knee osteoarthritis among those with the disease, although there are some data to suggest that among those who self-select to run, there are no negative outcomes for the knee.
An accompanying editorial noted that research into the effect of running on those with osteoarthritis was still in its infancy. “This would help to guide clinical practice on how to support people with osteoarthritis, with regard to accessing the health benefits of running participation,” write Jean-Francois Esculier, PT, PhD, from the University of British Columbia, Vancouver, and Christian Barton, PhD, with the La Trobe Centre, pointing out there were a lack of evidence-based clinical recommendations for people with osteoarthritis who want to start or continue running.
It’s a question that PhD candidate Michaela Khan, MSc, is trying to answer at the University of British Columbia. “Our lab did a pilot study for my current study now, and they found that osteoarthritic cartilage took a little bit longer to recover than their healthy counterparts,” Ms. Khan said. Her research is suggesting that people with osteoarthritis not only can run, but even those with severe disease, who might be candidates for knee replacement, can run long distances.
Commenting on the analysis, Ms. Khan said the main take-home message was that healthy cartilage seems to recover after running, and that there is not an ongoing effect of ‘wear and tear.’
“That’s changing the narrative that if you keep running, it will wear away your cartilage, it’ll hurt your knees,” she said. “Now, we have a good synthesis of scientific evidence to prove maybe otherwise.”
Ms. Coburn and Dr. Culvenor report grant support from the National Health & Medical Research Council of Australia, and another author reports grant support from the U.S. National Institute of Arthritis and Musculoskeletal and Skin Diseases. The authors, as well as Dr. Lo and Ms. Khan, report relevant financial relationships.
Running does not appear to cause sustained wear and tear of healthy knee cartilage, with research suggesting that the small, short-term changes to cartilage after a run reverse within hours.
A systematic review and meta-analysis published in the most recent issue of Osteoarthritis and Cartilage presents the findings involving 396 adults, which compared the “before” and “after” state of healthy knee cartilage in runners.
Running is often thought to be detrimental to joint health, wrote Sally Coburn, PhD candidate at the La Trobe Sport & Exercise Medicine Research Centre at La Trobe University in Melbourne and coauthors, but this perception is not supported by evidence.
For the analysis, the researchers included studies that looked at either knee or hip cartilage using MRI to assess its size, shape, structure, and/or composition both in the 48 hours before a single bout of running and in the 48 hours after. The analysis aimed to include adults with or at risk of osteoarthritis, but only 57 of the 446 knees in the analysis fit these criteria.
In studies where participants underwent MRI within 20 minutes of running, there was an immediate postrun decrease in the volume of cartilage, ranging from –3.3% for weight-bearing femoral cartilage to –4.1% for tibial cartilage volume. This also revealed a decrease in T1 and T2 relaxation times, which are specialized MRI measures that reflect the composition of cartilage and which can indicate a breakdown of cartilage structure in the case of diseases such as arthritis.
Reversal of short-term cartilage changes
However, within 48 hours of the run, data from studies that repeated the MRIs more than once after the initial prerun scan suggested these changes reversed back to prerun levels.
“We were able to pool delayed T2 relaxation time measures from studies that repeated scans of the same participants 60 minutes and 91 minutes post-run and found no effect of running on tibiofemoral joint cartilage composition,” the authors write.
For example, one study in marathon runners found no difference in cartilage thickness in the tibiofemoral joint between baseline and at 2-10 hours and 12 hours after the marathon. Another showed the immediate post-run decrease in patellofemoral joint cartilage thickness had reverted back to prerun levels when the scan was repeated 24 hours after the run.
“The changes are very minimal and not inconsistent with what’s expected for your cartilage which is functioning normally,” Ms. Coburn told this news organization.
Sparse data in people with osteoarthritis
The authors said there were not enough data from individuals with osteoarthritis to be able to pool and quantify their cartilage changes. However, one study in the analysis found that cartilage lesions in people considered at risk of osteoarthritis because of prior anterior cruciate ligament reconstruction were unchanged after running.
Another suggested that the decrease in femoral cartilage volume recorded at 15 minutes persisted at 45 minutes, while a separate study found significantly increased T2 relaxation times at 45 minutes after a run in those with knee osteoarthritis but not in those without osteoarthritis.
Senior author Adam Culvenor, PhD, senior research fellow at the La Trobe Centre, said their analysis suggested running was healthy, with small changes in cartilage that resolve quickly, but “we really don’t know yet if running is safe for people with osteoarthritis,” he said. “We need much more work in that space.”
Overall, the study evidence was rated as being of low certainty, which Dr. Coburn said was related to the small numbers in each study, which in turn relates to the cost and logistical challenges of the specialized MRI scan used.
“Study of a repeated exposure over a long duration of time on a disease that has a long natural history, like osteoarthritis, is challenging in that most funding agencies will not fund studies longer than 5 years,” Grace Hsiao-Wei Lo, MD, of the department of immunology, allergy, and rheumatology at the Baylor College of Medicine in Houston, said in an email.
Dr. Lo, who was not involved with this review and meta-analysis, said there are still concerns about the effect of running on knee osteoarthritis among those with the disease, although there are some data to suggest that among those who self-select to run, there are no negative outcomes for the knee.
An accompanying editorial noted that research into the effect of running on those with osteoarthritis was still in its infancy. “This would help to guide clinical practice on how to support people with osteoarthritis, with regard to accessing the health benefits of running participation,” write Jean-Francois Esculier, PT, PhD, from the University of British Columbia, Vancouver, and Christian Barton, PhD, with the La Trobe Centre, pointing out there were a lack of evidence-based clinical recommendations for people with osteoarthritis who want to start or continue running.
It’s a question that PhD candidate Michaela Khan, MSc, is trying to answer at the University of British Columbia. “Our lab did a pilot study for my current study now, and they found that osteoarthritic cartilage took a little bit longer to recover than their healthy counterparts,” Ms. Khan said. Her research is suggesting that people with osteoarthritis not only can run, but even those with severe disease, who might be candidates for knee replacement, can run long distances.
Commenting on the analysis, Ms. Khan said the main take-home message was that healthy cartilage seems to recover after running, and that there is not an ongoing effect of ‘wear and tear.’
“That’s changing the narrative that if you keep running, it will wear away your cartilage, it’ll hurt your knees,” she said. “Now, we have a good synthesis of scientific evidence to prove maybe otherwise.”
Ms. Coburn and Dr. Culvenor report grant support from the National Health & Medical Research Council of Australia, and another author reports grant support from the U.S. National Institute of Arthritis and Musculoskeletal and Skin Diseases. The authors, as well as Dr. Lo and Ms. Khan, report relevant financial relationships.
Running does not appear to cause sustained wear and tear of healthy knee cartilage, with research suggesting that the small, short-term changes to cartilage after a run reverse within hours.
A systematic review and meta-analysis published in the most recent issue of Osteoarthritis and Cartilage presents the findings involving 396 adults, which compared the “before” and “after” state of healthy knee cartilage in runners.
Running is often thought to be detrimental to joint health, wrote Sally Coburn, PhD candidate at the La Trobe Sport & Exercise Medicine Research Centre at La Trobe University in Melbourne and coauthors, but this perception is not supported by evidence.
For the analysis, the researchers included studies that looked at either knee or hip cartilage using MRI to assess its size, shape, structure, and/or composition both in the 48 hours before a single bout of running and in the 48 hours after. The analysis aimed to include adults with or at risk of osteoarthritis, but only 57 of the 446 knees in the analysis fit these criteria.
In studies where participants underwent MRI within 20 minutes of running, there was an immediate postrun decrease in the volume of cartilage, ranging from –3.3% for weight-bearing femoral cartilage to –4.1% for tibial cartilage volume. This also revealed a decrease in T1 and T2 relaxation times, which are specialized MRI measures that reflect the composition of cartilage and which can indicate a breakdown of cartilage structure in the case of diseases such as arthritis.
Reversal of short-term cartilage changes
However, within 48 hours of the run, data from studies that repeated the MRIs more than once after the initial prerun scan suggested these changes reversed back to prerun levels.
“We were able to pool delayed T2 relaxation time measures from studies that repeated scans of the same participants 60 minutes and 91 minutes post-run and found no effect of running on tibiofemoral joint cartilage composition,” the authors write.
For example, one study in marathon runners found no difference in cartilage thickness in the tibiofemoral joint between baseline and at 2-10 hours and 12 hours after the marathon. Another showed the immediate post-run decrease in patellofemoral joint cartilage thickness had reverted back to prerun levels when the scan was repeated 24 hours after the run.
“The changes are very minimal and not inconsistent with what’s expected for your cartilage which is functioning normally,” Ms. Coburn told this news organization.
Sparse data in people with osteoarthritis
The authors said there were not enough data from individuals with osteoarthritis to be able to pool and quantify their cartilage changes. However, one study in the analysis found that cartilage lesions in people considered at risk of osteoarthritis because of prior anterior cruciate ligament reconstruction were unchanged after running.
Another suggested that the decrease in femoral cartilage volume recorded at 15 minutes persisted at 45 minutes, while a separate study found significantly increased T2 relaxation times at 45 minutes after a run in those with knee osteoarthritis but not in those without osteoarthritis.
Senior author Adam Culvenor, PhD, senior research fellow at the La Trobe Centre, said their analysis suggested running was healthy, with small changes in cartilage that resolve quickly, but “we really don’t know yet if running is safe for people with osteoarthritis,” he said. “We need much more work in that space.”
Overall, the study evidence was rated as being of low certainty, which Dr. Coburn said was related to the small numbers in each study, which in turn relates to the cost and logistical challenges of the specialized MRI scan used.
“Study of a repeated exposure over a long duration of time on a disease that has a long natural history, like osteoarthritis, is challenging in that most funding agencies will not fund studies longer than 5 years,” Grace Hsiao-Wei Lo, MD, of the department of immunology, allergy, and rheumatology at the Baylor College of Medicine in Houston, said in an email.
Dr. Lo, who was not involved with this review and meta-analysis, said there are still concerns about the effect of running on knee osteoarthritis among those with the disease, although there are some data to suggest that among those who self-select to run, there are no negative outcomes for the knee.
An accompanying editorial noted that research into the effect of running on those with osteoarthritis was still in its infancy. “This would help to guide clinical practice on how to support people with osteoarthritis, with regard to accessing the health benefits of running participation,” write Jean-Francois Esculier, PT, PhD, from the University of British Columbia, Vancouver, and Christian Barton, PhD, with the La Trobe Centre, pointing out there were a lack of evidence-based clinical recommendations for people with osteoarthritis who want to start or continue running.
It’s a question that PhD candidate Michaela Khan, MSc, is trying to answer at the University of British Columbia. “Our lab did a pilot study for my current study now, and they found that osteoarthritic cartilage took a little bit longer to recover than their healthy counterparts,” Ms. Khan said. Her research is suggesting that people with osteoarthritis not only can run, but even those with severe disease, who might be candidates for knee replacement, can run long distances.
Commenting on the analysis, Ms. Khan said the main take-home message was that healthy cartilage seems to recover after running, and that there is not an ongoing effect of ‘wear and tear.’
“That’s changing the narrative that if you keep running, it will wear away your cartilage, it’ll hurt your knees,” she said. “Now, we have a good synthesis of scientific evidence to prove maybe otherwise.”
Ms. Coburn and Dr. Culvenor report grant support from the National Health & Medical Research Council of Australia, and another author reports grant support from the U.S. National Institute of Arthritis and Musculoskeletal and Skin Diseases. The authors, as well as Dr. Lo and Ms. Khan, report relevant financial relationships.
Adult stem cells can heal intractable perianal Crohn’s fistulae
AURORA, COLO. – Perianal Crohn’s disease with fistula is notoriously difficult to treat and can make patients’ lives miserable, but a new, minimally invasive approach involving local injection of mesenchymal stem cells is both safe and, in a significant proportion of patients, highly effective, according to a colorectal surgeon.
“It’s a really debilitating phenotype, a spectrum of phenotypes,” Amy Lightner, MD, of the Cleveland Clinic said at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Although some patients have minimal symptoms, others may require multiple setons to aid in drainage and healing, while others may require fistulotomy, endorectal advancement flap, intersphincteric fistula tract (LIFT) procedure, diversion, or proctectomy.
“Why is it so difficult to treat? Well, part of it is that this is an anatomic defect, and this is why 90% of patients will come to the operating room and will see their surgeons on a frequent basis. The other part of that is that we have medical therapies to treat these fistulas but they’re really largely ineffective, because there is that anatomical defect, the hole there that needs to be closed,” Dr. Lightner said.
Up to 20% of patients may require a permanent stoma, and an additional 20% may require temporary fecal diversion.
Mesenchymal stem cells (MSC) are derived from bone marrow, fat stores, or umbilical cord tissues. Unlike embryonic stem cells, which have the ability to metamorphose into a multitude of other cell types, mesenchymal stem cells are differentiated “adult” cells.
They work by secreting anti-inflammatory cytokines and recruiting immune cells to stimulate tissue repair and healing. The cells are delivered in a minimally invasive outpatient setting, and there is no risk of incontinence compared with more invasive procedures such as fistulotomy or advancement flaps.
Effective and safe
MSCs were first used in Spain in 2003 to successfully treat a young women with a complex fistula with five perianal tracts converging into a rectovaginal fistula. The investigators injected a single dose of 9 x 106 MSCs into the site, and the fistula healed within 3 months.
Since then in multiple clinical trials involving more than 400 patients, injection of MSCs has resulted in fistula closure and complete healing by 8-12 weeks in 50%-85% of patients, Dr. Lightner said.
The treatment effect is also durable, she said, pointing to data from the ADMIRE-CD study, in which 51.5% of Crohn’s disease patients with treatment-refractory complex perianal fistula were healed at 24 weeks following injection of adipose-derived stem cells, compared with 35.6% of controls. At 1 year of follow-up, respective rates of healing were 56.3% vs. 38.6%.
Dr. Lightner also cited a case report of a patient whose fistula remained healed 4 years after receiving MSCs for refractory perianal Crohn’s fistulas.
Although MSCs are derived from healthy donors, they do not bear cellular surface antigens that would instigate a destructive host immune response, and to date, there have been no reports from clinical trials of systemic infections or complications. The most frequently reported adverse events have been injection-site pain in about 12%-15% of patients, and perianal abscess in 5%-13%, with similar frequencies in treatment and control groups.
Dr. Lightner and colleagues are currently exploring additional indications for stem cell therapy with MSCs, including other complex fistula phenotypes, intestinal Crohn’s disease, and ulcerative colitis.
Other approaches
In a separate presentation, James D. Lewis, MD, MSCE, of the University of Pennsylvania in Philadelphia talked about what would be needed to achieve a “medical moonshot” with the goal of curing inflammatory bowel disease (IBD), and touched on hematopoietic stem cell transplants as a potential option for patients with chronic, severe, and intractable disease.
One of his patients was a woman in her 60s who was diagnosed with stricturing and penetrating Crohn’s disease in her 30s, with the disease involving the ileum and entire colon. She had previously undergone three small bowel resections and a partial colon resection, and had never experienced remission despite taking steroids, azathioprine, methotrexate, four anti-TNF drugs, ustekinumab (Stelara), and vedolizumab (Entyvio).
Following an autologous hematopoietic stem cell transplant, she had a Simple Endoscopic Score for Crohn’s Disease (SES-CD) of 0. Her course was complicated by demand ischemia and acute kidney injury.
An IBD specialist who was not involved in either study commented in an interview that both MSCs and stem cell transplants show promise for treatment-refractory IBD,
“Both approaches are very promising, but stem cell transplants for IBD haven’t been formally studied yet so the data aren’t as strong, but there is promise for the future,” said Berkeley N. Limketkai, MD, PhD, from the University of California, Los Angeles.
“The challenges, however, are also the morbidity associated with actually undergoing such procedures,” he continued. Short- and long-term morbidities associated with hematopoietic stem cell transplants may include mucositis; hemorrhagic cystitis; prolonged, severe pancytopenia; infection; graft-versus-host disease; graft failure; pulmonary complications, veno-occlusive disease of the liver; and thrombotic microangiopathy.
Dr. Limketkai said that over time as the protocols for stem cell transplants in IBD improve, the benefits for select patients may more clearly outweigh the risks.
Dr. Lightner’s work is supported by the Leona M. and Harry B. Helmsley Charitable Trust and the American Society of Colon and Rectal Surgery. She disclosed consulting fees from Boomerang Medical, Mesoblast Limited, Ossium Health, and Takeda Pharmaceuticals USA. Dr. Lewis’ work is supported by grants from the National Institutes of Health, and from AbbVie, Takeda, Janssen, and Nestlé Health Science. He has also served as a consultant to and data safety monitoring board member for several entities. Dr. Limketkai disclosed consulting for Azora Therapeutics.
AURORA, COLO. – Perianal Crohn’s disease with fistula is notoriously difficult to treat and can make patients’ lives miserable, but a new, minimally invasive approach involving local injection of mesenchymal stem cells is both safe and, in a significant proportion of patients, highly effective, according to a colorectal surgeon.
“It’s a really debilitating phenotype, a spectrum of phenotypes,” Amy Lightner, MD, of the Cleveland Clinic said at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Although some patients have minimal symptoms, others may require multiple setons to aid in drainage and healing, while others may require fistulotomy, endorectal advancement flap, intersphincteric fistula tract (LIFT) procedure, diversion, or proctectomy.
“Why is it so difficult to treat? Well, part of it is that this is an anatomic defect, and this is why 90% of patients will come to the operating room and will see their surgeons on a frequent basis. The other part of that is that we have medical therapies to treat these fistulas but they’re really largely ineffective, because there is that anatomical defect, the hole there that needs to be closed,” Dr. Lightner said.
Up to 20% of patients may require a permanent stoma, and an additional 20% may require temporary fecal diversion.
Mesenchymal stem cells (MSC) are derived from bone marrow, fat stores, or umbilical cord tissues. Unlike embryonic stem cells, which have the ability to metamorphose into a multitude of other cell types, mesenchymal stem cells are differentiated “adult” cells.
They work by secreting anti-inflammatory cytokines and recruiting immune cells to stimulate tissue repair and healing. The cells are delivered in a minimally invasive outpatient setting, and there is no risk of incontinence compared with more invasive procedures such as fistulotomy or advancement flaps.
Effective and safe
MSCs were first used in Spain in 2003 to successfully treat a young women with a complex fistula with five perianal tracts converging into a rectovaginal fistula. The investigators injected a single dose of 9 x 106 MSCs into the site, and the fistula healed within 3 months.
Since then in multiple clinical trials involving more than 400 patients, injection of MSCs has resulted in fistula closure and complete healing by 8-12 weeks in 50%-85% of patients, Dr. Lightner said.
The treatment effect is also durable, she said, pointing to data from the ADMIRE-CD study, in which 51.5% of Crohn’s disease patients with treatment-refractory complex perianal fistula were healed at 24 weeks following injection of adipose-derived stem cells, compared with 35.6% of controls. At 1 year of follow-up, respective rates of healing were 56.3% vs. 38.6%.
Dr. Lightner also cited a case report of a patient whose fistula remained healed 4 years after receiving MSCs for refractory perianal Crohn’s fistulas.
Although MSCs are derived from healthy donors, they do not bear cellular surface antigens that would instigate a destructive host immune response, and to date, there have been no reports from clinical trials of systemic infections or complications. The most frequently reported adverse events have been injection-site pain in about 12%-15% of patients, and perianal abscess in 5%-13%, with similar frequencies in treatment and control groups.
Dr. Lightner and colleagues are currently exploring additional indications for stem cell therapy with MSCs, including other complex fistula phenotypes, intestinal Crohn’s disease, and ulcerative colitis.
Other approaches
In a separate presentation, James D. Lewis, MD, MSCE, of the University of Pennsylvania in Philadelphia talked about what would be needed to achieve a “medical moonshot” with the goal of curing inflammatory bowel disease (IBD), and touched on hematopoietic stem cell transplants as a potential option for patients with chronic, severe, and intractable disease.
One of his patients was a woman in her 60s who was diagnosed with stricturing and penetrating Crohn’s disease in her 30s, with the disease involving the ileum and entire colon. She had previously undergone three small bowel resections and a partial colon resection, and had never experienced remission despite taking steroids, azathioprine, methotrexate, four anti-TNF drugs, ustekinumab (Stelara), and vedolizumab (Entyvio).
Following an autologous hematopoietic stem cell transplant, she had a Simple Endoscopic Score for Crohn’s Disease (SES-CD) of 0. Her course was complicated by demand ischemia and acute kidney injury.
An IBD specialist who was not involved in either study commented in an interview that both MSCs and stem cell transplants show promise for treatment-refractory IBD,
“Both approaches are very promising, but stem cell transplants for IBD haven’t been formally studied yet so the data aren’t as strong, but there is promise for the future,” said Berkeley N. Limketkai, MD, PhD, from the University of California, Los Angeles.
“The challenges, however, are also the morbidity associated with actually undergoing such procedures,” he continued. Short- and long-term morbidities associated with hematopoietic stem cell transplants may include mucositis; hemorrhagic cystitis; prolonged, severe pancytopenia; infection; graft-versus-host disease; graft failure; pulmonary complications, veno-occlusive disease of the liver; and thrombotic microangiopathy.
Dr. Limketkai said that over time as the protocols for stem cell transplants in IBD improve, the benefits for select patients may more clearly outweigh the risks.
Dr. Lightner’s work is supported by the Leona M. and Harry B. Helmsley Charitable Trust and the American Society of Colon and Rectal Surgery. She disclosed consulting fees from Boomerang Medical, Mesoblast Limited, Ossium Health, and Takeda Pharmaceuticals USA. Dr. Lewis’ work is supported by grants from the National Institutes of Health, and from AbbVie, Takeda, Janssen, and Nestlé Health Science. He has also served as a consultant to and data safety monitoring board member for several entities. Dr. Limketkai disclosed consulting for Azora Therapeutics.
AURORA, COLO. – Perianal Crohn’s disease with fistula is notoriously difficult to treat and can make patients’ lives miserable, but a new, minimally invasive approach involving local injection of mesenchymal stem cells is both safe and, in a significant proportion of patients, highly effective, according to a colorectal surgeon.
“It’s a really debilitating phenotype, a spectrum of phenotypes,” Amy Lightner, MD, of the Cleveland Clinic said at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Although some patients have minimal symptoms, others may require multiple setons to aid in drainage and healing, while others may require fistulotomy, endorectal advancement flap, intersphincteric fistula tract (LIFT) procedure, diversion, or proctectomy.
“Why is it so difficult to treat? Well, part of it is that this is an anatomic defect, and this is why 90% of patients will come to the operating room and will see their surgeons on a frequent basis. The other part of that is that we have medical therapies to treat these fistulas but they’re really largely ineffective, because there is that anatomical defect, the hole there that needs to be closed,” Dr. Lightner said.
Up to 20% of patients may require a permanent stoma, and an additional 20% may require temporary fecal diversion.
Mesenchymal stem cells (MSC) are derived from bone marrow, fat stores, or umbilical cord tissues. Unlike embryonic stem cells, which have the ability to metamorphose into a multitude of other cell types, mesenchymal stem cells are differentiated “adult” cells.
They work by secreting anti-inflammatory cytokines and recruiting immune cells to stimulate tissue repair and healing. The cells are delivered in a minimally invasive outpatient setting, and there is no risk of incontinence compared with more invasive procedures such as fistulotomy or advancement flaps.
Effective and safe
MSCs were first used in Spain in 2003 to successfully treat a young women with a complex fistula with five perianal tracts converging into a rectovaginal fistula. The investigators injected a single dose of 9 x 106 MSCs into the site, and the fistula healed within 3 months.
Since then in multiple clinical trials involving more than 400 patients, injection of MSCs has resulted in fistula closure and complete healing by 8-12 weeks in 50%-85% of patients, Dr. Lightner said.
The treatment effect is also durable, she said, pointing to data from the ADMIRE-CD study, in which 51.5% of Crohn’s disease patients with treatment-refractory complex perianal fistula were healed at 24 weeks following injection of adipose-derived stem cells, compared with 35.6% of controls. At 1 year of follow-up, respective rates of healing were 56.3% vs. 38.6%.
Dr. Lightner also cited a case report of a patient whose fistula remained healed 4 years after receiving MSCs for refractory perianal Crohn’s fistulas.
Although MSCs are derived from healthy donors, they do not bear cellular surface antigens that would instigate a destructive host immune response, and to date, there have been no reports from clinical trials of systemic infections or complications. The most frequently reported adverse events have been injection-site pain in about 12%-15% of patients, and perianal abscess in 5%-13%, with similar frequencies in treatment and control groups.
Dr. Lightner and colleagues are currently exploring additional indications for stem cell therapy with MSCs, including other complex fistula phenotypes, intestinal Crohn’s disease, and ulcerative colitis.
Other approaches
In a separate presentation, James D. Lewis, MD, MSCE, of the University of Pennsylvania in Philadelphia talked about what would be needed to achieve a “medical moonshot” with the goal of curing inflammatory bowel disease (IBD), and touched on hematopoietic stem cell transplants as a potential option for patients with chronic, severe, and intractable disease.
One of his patients was a woman in her 60s who was diagnosed with stricturing and penetrating Crohn’s disease in her 30s, with the disease involving the ileum and entire colon. She had previously undergone three small bowel resections and a partial colon resection, and had never experienced remission despite taking steroids, azathioprine, methotrexate, four anti-TNF drugs, ustekinumab (Stelara), and vedolizumab (Entyvio).
Following an autologous hematopoietic stem cell transplant, she had a Simple Endoscopic Score for Crohn’s Disease (SES-CD) of 0. Her course was complicated by demand ischemia and acute kidney injury.
An IBD specialist who was not involved in either study commented in an interview that both MSCs and stem cell transplants show promise for treatment-refractory IBD,
“Both approaches are very promising, but stem cell transplants for IBD haven’t been formally studied yet so the data aren’t as strong, but there is promise for the future,” said Berkeley N. Limketkai, MD, PhD, from the University of California, Los Angeles.
“The challenges, however, are also the morbidity associated with actually undergoing such procedures,” he continued. Short- and long-term morbidities associated with hematopoietic stem cell transplants may include mucositis; hemorrhagic cystitis; prolonged, severe pancytopenia; infection; graft-versus-host disease; graft failure; pulmonary complications, veno-occlusive disease of the liver; and thrombotic microangiopathy.
Dr. Limketkai said that over time as the protocols for stem cell transplants in IBD improve, the benefits for select patients may more clearly outweigh the risks.
Dr. Lightner’s work is supported by the Leona M. and Harry B. Helmsley Charitable Trust and the American Society of Colon and Rectal Surgery. She disclosed consulting fees from Boomerang Medical, Mesoblast Limited, Ossium Health, and Takeda Pharmaceuticals USA. Dr. Lewis’ work is supported by grants from the National Institutes of Health, and from AbbVie, Takeda, Janssen, and Nestlé Health Science. He has also served as a consultant to and data safety monitoring board member for several entities. Dr. Limketkai disclosed consulting for Azora Therapeutics.
AT CROHN’S & COLITIS CONGRESS
Massive rise in drug overdose deaths driven by opioids
The 376% represents the change in age-adjusted overdose deaths per 100,000 population, which went from 6.9 in 2001 to 32.4 in 2021, as the total number of deaths rose from 19,394 to 106,699 (450%) over that time period, the NCHS said in a recent data brief. That total made 2021 the first year ever with more than 100,000 overdose deaths.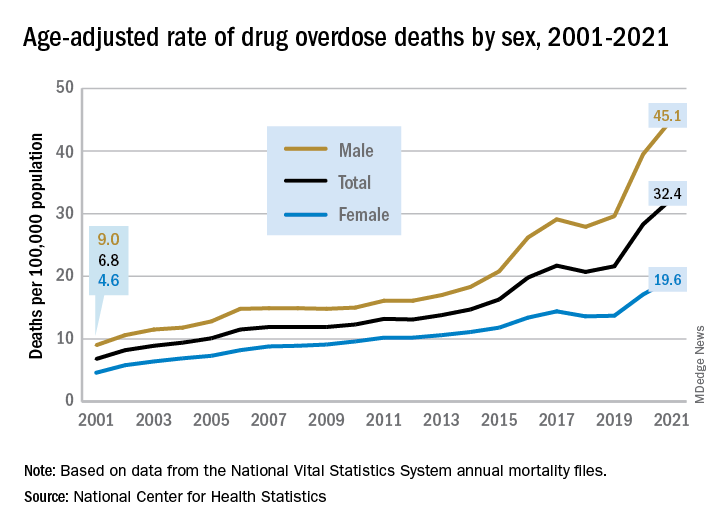
Since the age-adjusted rate stood at 21.6 per 100,000 in 2019, that means 42% of the total increase over 20 years actually occurred in 2020 and 2021. The number of deaths increased by about 36,000 over those 2 years, accounting for 41% of the total annual increase from 2001 to 2021, based on data from the National Vital Statistics System mortality files.
The overdose death rate was significantly higher for males than females for all of the years from 2001 to 2021, with males seeing an increase from 9.0 to 45.1 per 100,000 and females going from 4.6 to 19.6 deaths per 100,000. In the single year from 2020 to 2021, the age-adjusted rate was up by 14% for males and 15% for females, the mortality-file data show.
Analysis by age showed an even larger effect in some groups from 2020 to 2021. Drug overdose deaths jumped 28% among adults aged 65 years and older, more than any other group, and by 21% in those aged 55-64 years, according to the NCHS.
The only age group for which deaths didn’t increase significantly from 2020 to 2021 was 15- to 24-year-olds, whose rate rose by just 3%. The age group with the highest rate in both 2020 and 2021, however, was the 35- to 44-year-olds: 53.9 and 62.0 overdose deaths per 100,000, respectively, for an increase of 15%, the NCHS said in the report.
The drugs now involved in overdose deaths are most often opioids, a change from 2001. That year, opioids were involved in 49% of all overdose deaths, but by 2021 that share had increased to 75%. The trend for opioid-related deaths almost matches that of overall deaths over the 20-year span, and the significantly increasing trend that began for all overdose deaths in 2013 closely follows that of synthetic opioids such as fentanyl and tramadol, the report shows.
Overdose deaths involving cocaine and psychostimulants such as methamphetamine, amphetamine, and methylphenidate also show similar increases. The cocaine-related death rate rose 22% from 2020 to 2021 and is up by 421% since 2012, while the corresponding increases for psychostimulant deaths were 33% and 2,400%, the NCHS said.
The 376% represents the change in age-adjusted overdose deaths per 100,000 population, which went from 6.9 in 2001 to 32.4 in 2021, as the total number of deaths rose from 19,394 to 106,699 (450%) over that time period, the NCHS said in a recent data brief. That total made 2021 the first year ever with more than 100,000 overdose deaths.
Since the age-adjusted rate stood at 21.6 per 100,000 in 2019, that means 42% of the total increase over 20 years actually occurred in 2020 and 2021. The number of deaths increased by about 36,000 over those 2 years, accounting for 41% of the total annual increase from 2001 to 2021, based on data from the National Vital Statistics System mortality files.
The overdose death rate was significantly higher for males than females for all of the years from 2001 to 2021, with males seeing an increase from 9.0 to 45.1 per 100,000 and females going from 4.6 to 19.6 deaths per 100,000. In the single year from 2020 to 2021, the age-adjusted rate was up by 14% for males and 15% for females, the mortality-file data show.
Analysis by age showed an even larger effect in some groups from 2020 to 2021. Drug overdose deaths jumped 28% among adults aged 65 years and older, more than any other group, and by 21% in those aged 55-64 years, according to the NCHS.
The only age group for which deaths didn’t increase significantly from 2020 to 2021 was 15- to 24-year-olds, whose rate rose by just 3%. The age group with the highest rate in both 2020 and 2021, however, was the 35- to 44-year-olds: 53.9 and 62.0 overdose deaths per 100,000, respectively, for an increase of 15%, the NCHS said in the report.
The drugs now involved in overdose deaths are most often opioids, a change from 2001. That year, opioids were involved in 49% of all overdose deaths, but by 2021 that share had increased to 75%. The trend for opioid-related deaths almost matches that of overall deaths over the 20-year span, and the significantly increasing trend that began for all overdose deaths in 2013 closely follows that of synthetic opioids such as fentanyl and tramadol, the report shows.
Overdose deaths involving cocaine and psychostimulants such as methamphetamine, amphetamine, and methylphenidate also show similar increases. The cocaine-related death rate rose 22% from 2020 to 2021 and is up by 421% since 2012, while the corresponding increases for psychostimulant deaths were 33% and 2,400%, the NCHS said.
The 376% represents the change in age-adjusted overdose deaths per 100,000 population, which went from 6.9 in 2001 to 32.4 in 2021, as the total number of deaths rose from 19,394 to 106,699 (450%) over that time period, the NCHS said in a recent data brief. That total made 2021 the first year ever with more than 100,000 overdose deaths.
Since the age-adjusted rate stood at 21.6 per 100,000 in 2019, that means 42% of the total increase over 20 years actually occurred in 2020 and 2021. The number of deaths increased by about 36,000 over those 2 years, accounting for 41% of the total annual increase from 2001 to 2021, based on data from the National Vital Statistics System mortality files.
The overdose death rate was significantly higher for males than females for all of the years from 2001 to 2021, with males seeing an increase from 9.0 to 45.1 per 100,000 and females going from 4.6 to 19.6 deaths per 100,000. In the single year from 2020 to 2021, the age-adjusted rate was up by 14% for males and 15% for females, the mortality-file data show.
Analysis by age showed an even larger effect in some groups from 2020 to 2021. Drug overdose deaths jumped 28% among adults aged 65 years and older, more than any other group, and by 21% in those aged 55-64 years, according to the NCHS.
The only age group for which deaths didn’t increase significantly from 2020 to 2021 was 15- to 24-year-olds, whose rate rose by just 3%. The age group with the highest rate in both 2020 and 2021, however, was the 35- to 44-year-olds: 53.9 and 62.0 overdose deaths per 100,000, respectively, for an increase of 15%, the NCHS said in the report.
The drugs now involved in overdose deaths are most often opioids, a change from 2001. That year, opioids were involved in 49% of all overdose deaths, but by 2021 that share had increased to 75%. The trend for opioid-related deaths almost matches that of overall deaths over the 20-year span, and the significantly increasing trend that began for all overdose deaths in 2013 closely follows that of synthetic opioids such as fentanyl and tramadol, the report shows.
Overdose deaths involving cocaine and psychostimulants such as methamphetamine, amphetamine, and methylphenidate also show similar increases. The cocaine-related death rate rose 22% from 2020 to 2021 and is up by 421% since 2012, while the corresponding increases for psychostimulant deaths were 33% and 2,400%, the NCHS said.
Lipid signature may flag schizophrenia
Although such a test remains a long way off, investigators said, the identification of the unique lipid signature is a critical first step. However, one expert noted that the lipid signature not accurately differentiating patients with schizophrenia from those with bipolar disorder (BD) and major depressive disorder (MDD) limits the findings’ applicability.
The profile includes 77 lipids identified from a large analysis of many different classes of lipid species. Lipids such as cholesterol and triglycerides made up only a small fraction of the classes assessed.
The investigators noted that some of the lipids in the profile associated with schizophrenia are involved in determining cell membrane structure and fluidity or cell-to-cell messaging, which could be important to synaptic function.
“These 77 lipids jointly constitute a lipidomic profile that discriminated between individuals with schizophrenia and individuals without a mental health diagnosis with very high accuracy,” investigator Eva C. Schulte, MD, PhD, of the Institute of Psychiatric Phenomics and Genomics (IPPG) and the department of psychiatry and psychotherapy at University Hospital of Ludwig-Maximilians-University, Munich, told this news organization.
“Of note, we did not see large profile differences between patients with a first psychotic episode who had only been treated for a few days and individuals on long-term antipsychotic therapy,” Dr. Schulte said.
The findings were published online in JAMA Psychiatry.
Detailed analysis
Lipid profiles in patients with psychiatric diagnoses have been reported previously, but those studies were small and did not identify a reliable signature independent of demographic and environmental factors.
For the current study, researchers analyzed blood plasma lipid levels from 980 individuals with severe psychiatric illness and 572 people without mental illness from three cohorts in China, Germany, Austria, and Russia.
The study sample included patients with schizophrenia (n = 478), BD (n = 184), and MDD (n = 256), as well as 104 patients with a first psychotic episode who had no long-term psychopharmacology use.
Results showed 77 lipids in 14 classes were significantly altered between participants with schizophrenia and the healthy control in all three cohorts.
The most prominent alterations at the lipid class level included increases in ceramide, triacylglyceride, and phosphatidylcholine and decreases in acylcarnitine and phosphatidylcholine plasmalogen (P < .05 for each cohort).
Schizophrenia-associated lipid differences were similar between patients with high and low symptom severity (P < .001), suggesting that the lipid alterations might represent a trait of the psychiatric disorder.
No medication effect
Most patients in the study received long-term antipsychotic medication, which has been shown previously to affect some plasma lipid compounds.
So, to assess a possible effect of medication, the investigators evaluated 13 patients with schizophrenia who were not medicated for at least 6 months prior to blood sample collection and the cohort of patients with a first psychotic episode who had been medicated for less than 1 week.
Comparison of the lipid intensity differences between the healthy controls group and either participants receiving medication or those who were not medicated revealed highly correlated alterations in both patient groups (P < .001).
“Taken together, these results indicate that the identified schizophrenia-associated alterations cannot be attributed to medication effects,” the investigators wrote.
Lipidome alterations in BPD and MDD, assessed in 184 and 256 individuals, respectively, were similar to those of schizophrenia but not identical.
Researchers isolated 97 lipids altered in the MDD cohorts and 47 in the BPD cohorts – with 30 and 28, respectively, overlapping with the schizophrenia-associated features and seven of the lipids found among all three disorders.
Although this was significantly more than expected by chance (P < .001), it was not strong enough to demonstrate a clear association, the investigators wrote.
“The profiles were very successful at differentiating individuals with severe mental health conditions from individuals without a diagnosed mental health condition, but much less so at differentiating between the different diagnostic entities,” coinvestigator Thomas G. Schulze, MD, director of IPPG, said in an interview.
“An important caveat, however, is that the available sample sizes for bipolar disorder and major depressive disorder were smaller than those for schizophrenia, which makes a direct comparison between these difficult,” added Dr. Schulze, clinical professor in psychiatry and behavioral sciences at State University of New York, Syracuse.
More work remains
Although the study is thought to be the largest to date to examine lipid profiles associated with serious psychiatric illness, much work remains, Dr. Schulze noted.
“At this time, based on these first results, no clinical diagnostic test can be derived from these results,” he said.
He added that the development of reliable biomarkers based on lipidomic profiles would require large prospective randomized trials, complemented by observational studies assessing full lipidomic profiles across the lifespan.
Researchers also need to better understand the exact mechanism by which lipid alterations are associated with schizophrenia and other illnesses.
Physiologically, the investigated lipids have many additional functions, such as determining cell membrane structure and fluidity or cell-to-cell messaging.
Dr. Schulte noted that several lipid species may be involved in determining mechanisms important to synaptic function, such as cell membrane fluidity and vesicle release.
“As is commonly known, alterations in synaptic function underly many severe psychiatric disorders,” she said. “Changes in lipid species could theoretically be related to these synaptic alterations.”
A better marker needed
In a comment, Stephen Strakowski, MD, professor and vice chair of research in the department of psychiatry, Indiana University, Indianapolis and Evansville, noted that while the findings are interesting, they don’t really offer the kind of information clinicians who treat patients with serious mental illness need most.
“Do we need a marker to tell us if someone’s got a major mental illness compared to a healthy person?” asked Dr. Strakowski, who was not part of the study. “The answer to that is no. We already know how to do that.”
A truly useful marker would help clinicians differentiate between schizophrenia, bipolar disorder, major depression, or another serious mental illness, he said.
“That’s the marker that would be most helpful,” he added. “This can’t address that, but perhaps it could be a step to start designing a test for that.”
Dr. Strakowksi noted that the findings do not clarify whether the lipid profile found in patients with schizophrenia predates diagnosis or whether it is a result of the mental illness, an unrelated illness, or another factor that could be critical in treating patients.
However, he was quick to point out the limitations don’t diminish the importance of the study.
“It’s a large dataset that’s cross-national, cross-diagnostic that says there appears to be a signal here that there’s something about lipid profiles that may be independent of treatment that could be worth understanding,” Dr. Strakowksi said.
“It allows us to think about developing different models based on lipid profiles, and that’s important,” he added.
The study was funded by the National Key R&D Program of China, National One Thousand Foreign Experts Plan, Moscow Center for Innovative Technologies in Healthcare, European Union’s Horizon 2020 Research and Innovation Programme, NARSAD Young Investigator Grant, German Research Foundation, German Ministry for Education and Research, the Dr. Lisa Oehler Foundation, and the Munich Clinician Scientist Program. Dr. Schulze and Dr. Schulte reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although such a test remains a long way off, investigators said, the identification of the unique lipid signature is a critical first step. However, one expert noted that the lipid signature not accurately differentiating patients with schizophrenia from those with bipolar disorder (BD) and major depressive disorder (MDD) limits the findings’ applicability.
The profile includes 77 lipids identified from a large analysis of many different classes of lipid species. Lipids such as cholesterol and triglycerides made up only a small fraction of the classes assessed.
The investigators noted that some of the lipids in the profile associated with schizophrenia are involved in determining cell membrane structure and fluidity or cell-to-cell messaging, which could be important to synaptic function.
“These 77 lipids jointly constitute a lipidomic profile that discriminated between individuals with schizophrenia and individuals without a mental health diagnosis with very high accuracy,” investigator Eva C. Schulte, MD, PhD, of the Institute of Psychiatric Phenomics and Genomics (IPPG) and the department of psychiatry and psychotherapy at University Hospital of Ludwig-Maximilians-University, Munich, told this news organization.
“Of note, we did not see large profile differences between patients with a first psychotic episode who had only been treated for a few days and individuals on long-term antipsychotic therapy,” Dr. Schulte said.
The findings were published online in JAMA Psychiatry.
Detailed analysis
Lipid profiles in patients with psychiatric diagnoses have been reported previously, but those studies were small and did not identify a reliable signature independent of demographic and environmental factors.
For the current study, researchers analyzed blood plasma lipid levels from 980 individuals with severe psychiatric illness and 572 people without mental illness from three cohorts in China, Germany, Austria, and Russia.
The study sample included patients with schizophrenia (n = 478), BD (n = 184), and MDD (n = 256), as well as 104 patients with a first psychotic episode who had no long-term psychopharmacology use.
Results showed 77 lipids in 14 classes were significantly altered between participants with schizophrenia and the healthy control in all three cohorts.
The most prominent alterations at the lipid class level included increases in ceramide, triacylglyceride, and phosphatidylcholine and decreases in acylcarnitine and phosphatidylcholine plasmalogen (P < .05 for each cohort).
Schizophrenia-associated lipid differences were similar between patients with high and low symptom severity (P < .001), suggesting that the lipid alterations might represent a trait of the psychiatric disorder.
No medication effect
Most patients in the study received long-term antipsychotic medication, which has been shown previously to affect some plasma lipid compounds.
So, to assess a possible effect of medication, the investigators evaluated 13 patients with schizophrenia who were not medicated for at least 6 months prior to blood sample collection and the cohort of patients with a first psychotic episode who had been medicated for less than 1 week.
Comparison of the lipid intensity differences between the healthy controls group and either participants receiving medication or those who were not medicated revealed highly correlated alterations in both patient groups (P < .001).
“Taken together, these results indicate that the identified schizophrenia-associated alterations cannot be attributed to medication effects,” the investigators wrote.
Lipidome alterations in BPD and MDD, assessed in 184 and 256 individuals, respectively, were similar to those of schizophrenia but not identical.
Researchers isolated 97 lipids altered in the MDD cohorts and 47 in the BPD cohorts – with 30 and 28, respectively, overlapping with the schizophrenia-associated features and seven of the lipids found among all three disorders.
Although this was significantly more than expected by chance (P < .001), it was not strong enough to demonstrate a clear association, the investigators wrote.
“The profiles were very successful at differentiating individuals with severe mental health conditions from individuals without a diagnosed mental health condition, but much less so at differentiating between the different diagnostic entities,” coinvestigator Thomas G. Schulze, MD, director of IPPG, said in an interview.
“An important caveat, however, is that the available sample sizes for bipolar disorder and major depressive disorder were smaller than those for schizophrenia, which makes a direct comparison between these difficult,” added Dr. Schulze, clinical professor in psychiatry and behavioral sciences at State University of New York, Syracuse.
More work remains
Although the study is thought to be the largest to date to examine lipid profiles associated with serious psychiatric illness, much work remains, Dr. Schulze noted.
“At this time, based on these first results, no clinical diagnostic test can be derived from these results,” he said.
He added that the development of reliable biomarkers based on lipidomic profiles would require large prospective randomized trials, complemented by observational studies assessing full lipidomic profiles across the lifespan.
Researchers also need to better understand the exact mechanism by which lipid alterations are associated with schizophrenia and other illnesses.
Physiologically, the investigated lipids have many additional functions, such as determining cell membrane structure and fluidity or cell-to-cell messaging.
Dr. Schulte noted that several lipid species may be involved in determining mechanisms important to synaptic function, such as cell membrane fluidity and vesicle release.
“As is commonly known, alterations in synaptic function underly many severe psychiatric disorders,” she said. “Changes in lipid species could theoretically be related to these synaptic alterations.”
A better marker needed
In a comment, Stephen Strakowski, MD, professor and vice chair of research in the department of psychiatry, Indiana University, Indianapolis and Evansville, noted that while the findings are interesting, they don’t really offer the kind of information clinicians who treat patients with serious mental illness need most.
“Do we need a marker to tell us if someone’s got a major mental illness compared to a healthy person?” asked Dr. Strakowski, who was not part of the study. “The answer to that is no. We already know how to do that.”
A truly useful marker would help clinicians differentiate between schizophrenia, bipolar disorder, major depression, or another serious mental illness, he said.
“That’s the marker that would be most helpful,” he added. “This can’t address that, but perhaps it could be a step to start designing a test for that.”
Dr. Strakowksi noted that the findings do not clarify whether the lipid profile found in patients with schizophrenia predates diagnosis or whether it is a result of the mental illness, an unrelated illness, or another factor that could be critical in treating patients.
However, he was quick to point out the limitations don’t diminish the importance of the study.
“It’s a large dataset that’s cross-national, cross-diagnostic that says there appears to be a signal here that there’s something about lipid profiles that may be independent of treatment that could be worth understanding,” Dr. Strakowksi said.
“It allows us to think about developing different models based on lipid profiles, and that’s important,” he added.
The study was funded by the National Key R&D Program of China, National One Thousand Foreign Experts Plan, Moscow Center for Innovative Technologies in Healthcare, European Union’s Horizon 2020 Research and Innovation Programme, NARSAD Young Investigator Grant, German Research Foundation, German Ministry for Education and Research, the Dr. Lisa Oehler Foundation, and the Munich Clinician Scientist Program. Dr. Schulze and Dr. Schulte reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although such a test remains a long way off, investigators said, the identification of the unique lipid signature is a critical first step. However, one expert noted that the lipid signature not accurately differentiating patients with schizophrenia from those with bipolar disorder (BD) and major depressive disorder (MDD) limits the findings’ applicability.
The profile includes 77 lipids identified from a large analysis of many different classes of lipid species. Lipids such as cholesterol and triglycerides made up only a small fraction of the classes assessed.
The investigators noted that some of the lipids in the profile associated with schizophrenia are involved in determining cell membrane structure and fluidity or cell-to-cell messaging, which could be important to synaptic function.
“These 77 lipids jointly constitute a lipidomic profile that discriminated between individuals with schizophrenia and individuals without a mental health diagnosis with very high accuracy,” investigator Eva C. Schulte, MD, PhD, of the Institute of Psychiatric Phenomics and Genomics (IPPG) and the department of psychiatry and psychotherapy at University Hospital of Ludwig-Maximilians-University, Munich, told this news organization.
“Of note, we did not see large profile differences between patients with a first psychotic episode who had only been treated for a few days and individuals on long-term antipsychotic therapy,” Dr. Schulte said.
The findings were published online in JAMA Psychiatry.
Detailed analysis
Lipid profiles in patients with psychiatric diagnoses have been reported previously, but those studies were small and did not identify a reliable signature independent of demographic and environmental factors.
For the current study, researchers analyzed blood plasma lipid levels from 980 individuals with severe psychiatric illness and 572 people without mental illness from three cohorts in China, Germany, Austria, and Russia.
The study sample included patients with schizophrenia (n = 478), BD (n = 184), and MDD (n = 256), as well as 104 patients with a first psychotic episode who had no long-term psychopharmacology use.
Results showed 77 lipids in 14 classes were significantly altered between participants with schizophrenia and the healthy control in all three cohorts.
The most prominent alterations at the lipid class level included increases in ceramide, triacylglyceride, and phosphatidylcholine and decreases in acylcarnitine and phosphatidylcholine plasmalogen (P < .05 for each cohort).
Schizophrenia-associated lipid differences were similar between patients with high and low symptom severity (P < .001), suggesting that the lipid alterations might represent a trait of the psychiatric disorder.
No medication effect
Most patients in the study received long-term antipsychotic medication, which has been shown previously to affect some plasma lipid compounds.
So, to assess a possible effect of medication, the investigators evaluated 13 patients with schizophrenia who were not medicated for at least 6 months prior to blood sample collection and the cohort of patients with a first psychotic episode who had been medicated for less than 1 week.
Comparison of the lipid intensity differences between the healthy controls group and either participants receiving medication or those who were not medicated revealed highly correlated alterations in both patient groups (P < .001).
“Taken together, these results indicate that the identified schizophrenia-associated alterations cannot be attributed to medication effects,” the investigators wrote.
Lipidome alterations in BPD and MDD, assessed in 184 and 256 individuals, respectively, were similar to those of schizophrenia but not identical.
Researchers isolated 97 lipids altered in the MDD cohorts and 47 in the BPD cohorts – with 30 and 28, respectively, overlapping with the schizophrenia-associated features and seven of the lipids found among all three disorders.
Although this was significantly more than expected by chance (P < .001), it was not strong enough to demonstrate a clear association, the investigators wrote.
“The profiles were very successful at differentiating individuals with severe mental health conditions from individuals without a diagnosed mental health condition, but much less so at differentiating between the different diagnostic entities,” coinvestigator Thomas G. Schulze, MD, director of IPPG, said in an interview.
“An important caveat, however, is that the available sample sizes for bipolar disorder and major depressive disorder were smaller than those for schizophrenia, which makes a direct comparison between these difficult,” added Dr. Schulze, clinical professor in psychiatry and behavioral sciences at State University of New York, Syracuse.
More work remains
Although the study is thought to be the largest to date to examine lipid profiles associated with serious psychiatric illness, much work remains, Dr. Schulze noted.
“At this time, based on these first results, no clinical diagnostic test can be derived from these results,” he said.
He added that the development of reliable biomarkers based on lipidomic profiles would require large prospective randomized trials, complemented by observational studies assessing full lipidomic profiles across the lifespan.
Researchers also need to better understand the exact mechanism by which lipid alterations are associated with schizophrenia and other illnesses.
Physiologically, the investigated lipids have many additional functions, such as determining cell membrane structure and fluidity or cell-to-cell messaging.
Dr. Schulte noted that several lipid species may be involved in determining mechanisms important to synaptic function, such as cell membrane fluidity and vesicle release.
“As is commonly known, alterations in synaptic function underly many severe psychiatric disorders,” she said. “Changes in lipid species could theoretically be related to these synaptic alterations.”
A better marker needed
In a comment, Stephen Strakowski, MD, professor and vice chair of research in the department of psychiatry, Indiana University, Indianapolis and Evansville, noted that while the findings are interesting, they don’t really offer the kind of information clinicians who treat patients with serious mental illness need most.
“Do we need a marker to tell us if someone’s got a major mental illness compared to a healthy person?” asked Dr. Strakowski, who was not part of the study. “The answer to that is no. We already know how to do that.”
A truly useful marker would help clinicians differentiate between schizophrenia, bipolar disorder, major depression, or another serious mental illness, he said.
“That’s the marker that would be most helpful,” he added. “This can’t address that, but perhaps it could be a step to start designing a test for that.”
Dr. Strakowksi noted that the findings do not clarify whether the lipid profile found in patients with schizophrenia predates diagnosis or whether it is a result of the mental illness, an unrelated illness, or another factor that could be critical in treating patients.
However, he was quick to point out the limitations don’t diminish the importance of the study.
“It’s a large dataset that’s cross-national, cross-diagnostic that says there appears to be a signal here that there’s something about lipid profiles that may be independent of treatment that could be worth understanding,” Dr. Strakowksi said.
“It allows us to think about developing different models based on lipid profiles, and that’s important,” he added.
The study was funded by the National Key R&D Program of China, National One Thousand Foreign Experts Plan, Moscow Center for Innovative Technologies in Healthcare, European Union’s Horizon 2020 Research and Innovation Programme, NARSAD Young Investigator Grant, German Research Foundation, German Ministry for Education and Research, the Dr. Lisa Oehler Foundation, and the Munich Clinician Scientist Program. Dr. Schulze and Dr. Schulte reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Maintenance therapies boost MM survival rates
These results, published in The Lancet Oncology, are not yet practice-changing, but they add to a growing body of evidence supporting this approach.
“Bearing in mind that ATLAS trial data come from unplanned interim analysis, we found that the benefit of combination therapy (KRd) is clearly seen, especially for standard risk patients. We think that, since there are no vast differences in toxicities, there is quite convincing data for considering KRd maintenance for the post autologous stem cell transplantation treatment of MM patients,” ATLAS study coauthor Andrzej Jakubowiak, MD, professor of medicine at the University of Chicago, said in an interview.
Among patients receiving KRd (n = 93), median PFS was 59 months (95% confidence interval) compared with 41 months in the R group (hazard ratio, 0.51, P = .012). These results come from data collected between 2016 and 2020, from 12 academic and clinical centers in the United States and Poland. The cohort included 180 adults randomly assigned to each study arm, (47% were female, median age 59 years) The most common adverse events were neutropenia 48% vs. 60%, thrombocytopenia 13% vs. 7%, and lower respiratory tract infections 8% vs. 1% in the KRd and R groups, respectively. Serious adverse events were reported in 30% of patients in the KRd arm (including one death due to severe pneumonia) and 22% in the R group.
“As expected, there is a slightly higher toxicity in the KRd arm but mostly low-grade events occurred. We believe in KRd maintenance because it provides a longer remission for a relatively small price. The longer a patient is in remission, the better chance that, if relapse does occur, there will be more therapeutic options available to return to remission,” said Dr. Jakubowiak.
Although all patients in the trial received stem cell therapy, the induction therapies were varied.
“We designed the study similarly to the CALGB maintenance trial, one of the key phase 3 trials leading to the approval of lenalidomide as post transplantation maintenance. With so many changes currently occurring MM therapies, having a fixed induction can be its own limitation. Importantly, with our study design we found that all comers may benefit from KRd maintenance,” he said.
It is important to note that KRd treatment occurred for 36 cycles unless a patient withdrew, died, or achieved minimal residual disease (MRD) status.
“Per study design, the duration of KRd treatment was shortened to eight cycles for patients without high-risk cytogenetics who achieved MRD-negativity after cycle six. For these patients, treatment was converted from KRd to R maintenance as of cycle nine. Despite treatment deescalation, this subset of patients (44% of all KRd patients) contributed strongly to KRd PFS. While this approach will need to be validated in a randomized setting, the results provide a proof of concept that if you achieve MRD-negative status and have standard risk cytogenetics, you don’t have to go on a long KRd treatment,” said Dr. Jakubowiak.
Urvi Shah, MD, of Memorial Sloan Kettering Cancer Center in New York, noted the importance of the study as “the first randomized phase-3 trial suggesting progression free survival superiority of an alternative maintenance therapy to lenalidomide alone.”
Dr. Shah did have concerns about the applicability of the study, especially regarding the use of varied induction regimens, saying, “Only 11%-13% of patients in this study got lenalidomide during induction and 4%-6% got carfilzomib induction. Therefore, most patients were getting new drugs during maintenance different from their induction agents, which has the downside of leaving fewer options for patients when they relapse.”
Asked about these concerns, Dr. Jakubowiak responded, “Our team’s perspective is to ‘buy’ the longest remission possible using the most effective available treatment strategy. If we do this, there is a good chance that when the patient relapses, we will have availability of new drugs.”
He added the caveat: “There’s a small proportion of patients who will be progressing while on treatment and will be potentially more challenging to treat if already given KRd. It has been proposed that KRd maintenance might be primarily considered for patients with high-risk cytogenetics. However, at this interim analysis, there were not enough events in high-risk patients to comment on KRd’s usefulness as a maintenance therapy in this population.”
The KRd arm of the ATLAS trial had a high dropout-rate for reasons other than toxicity and death, but “because consent for collecting data was discontinued, we can’t really dig deeper into explaining why they dropped out, but half of the patients who discontinued in the KRd arm did so after already completing KRd treatment and were already on R maintenance. Once taking this into consideration, the dropout rates in both arms are similar, and the data does not appear skewed,” said Dr. Jakubowiak.
In conclusion, Dr. Jakubowiak noted that the phase-2 FORTE trial, also published recently in The Lancet Oncology, provides supportive data for maintenance therapy with KR versus K.
“It is quite important that the results of both trials be presented to public so that people know that KR or KRd could be reasonably considered for posttransplant maintenance,” he said.
Dr. Shah opined that as yet, the results of neither study were practice changing for maintenance therapy in the United States. She said she would consider using KR maintenance for select patients with high-risk myeloma who received these drugs as induction therapy and where there was concern about early progression, given that this aggressive treatment allows for a longer time in remission.
Dr. Shah noted that in these trials, overall survival data on dual maintenance is still being awaited, and that those results would be important when considering whether to switch patients to a more expensive, toxic, and involved therapy that required regular infusion visits for maintenance.
What Dr. Shah found the most groundbreaking and applicable aspect of the study was that “in the ATLAS trial, they’re adapting treatments to disease response, and if patients achieve MRD negativity, which we know is the best kind of response, therapy was deescalated to lenalidomide single agent. I think this is something that we will see more of in newer study designs and this will help individualize patient treatments to their response.”
Dr. Jakubowiak disclosed consulting fees and honoraria for lectures and presentations from AbbVie, Amgen, Celgene (Bristol Myers Squibb), Gracell, GlaxoSmithKline, Janssen, and Sanofi-Aventis. Dr. Shah disclosed ties with Sanofi, Bristol Myers Squibb and Janssen.
These results, published in The Lancet Oncology, are not yet practice-changing, but they add to a growing body of evidence supporting this approach.
“Bearing in mind that ATLAS trial data come from unplanned interim analysis, we found that the benefit of combination therapy (KRd) is clearly seen, especially for standard risk patients. We think that, since there are no vast differences in toxicities, there is quite convincing data for considering KRd maintenance for the post autologous stem cell transplantation treatment of MM patients,” ATLAS study coauthor Andrzej Jakubowiak, MD, professor of medicine at the University of Chicago, said in an interview.
Among patients receiving KRd (n = 93), median PFS was 59 months (95% confidence interval) compared with 41 months in the R group (hazard ratio, 0.51, P = .012). These results come from data collected between 2016 and 2020, from 12 academic and clinical centers in the United States and Poland. The cohort included 180 adults randomly assigned to each study arm, (47% were female, median age 59 years) The most common adverse events were neutropenia 48% vs. 60%, thrombocytopenia 13% vs. 7%, and lower respiratory tract infections 8% vs. 1% in the KRd and R groups, respectively. Serious adverse events were reported in 30% of patients in the KRd arm (including one death due to severe pneumonia) and 22% in the R group.
“As expected, there is a slightly higher toxicity in the KRd arm but mostly low-grade events occurred. We believe in KRd maintenance because it provides a longer remission for a relatively small price. The longer a patient is in remission, the better chance that, if relapse does occur, there will be more therapeutic options available to return to remission,” said Dr. Jakubowiak.
Although all patients in the trial received stem cell therapy, the induction therapies were varied.
“We designed the study similarly to the CALGB maintenance trial, one of the key phase 3 trials leading to the approval of lenalidomide as post transplantation maintenance. With so many changes currently occurring MM therapies, having a fixed induction can be its own limitation. Importantly, with our study design we found that all comers may benefit from KRd maintenance,” he said.
It is important to note that KRd treatment occurred for 36 cycles unless a patient withdrew, died, or achieved minimal residual disease (MRD) status.
“Per study design, the duration of KRd treatment was shortened to eight cycles for patients without high-risk cytogenetics who achieved MRD-negativity after cycle six. For these patients, treatment was converted from KRd to R maintenance as of cycle nine. Despite treatment deescalation, this subset of patients (44% of all KRd patients) contributed strongly to KRd PFS. While this approach will need to be validated in a randomized setting, the results provide a proof of concept that if you achieve MRD-negative status and have standard risk cytogenetics, you don’t have to go on a long KRd treatment,” said Dr. Jakubowiak.
Urvi Shah, MD, of Memorial Sloan Kettering Cancer Center in New York, noted the importance of the study as “the first randomized phase-3 trial suggesting progression free survival superiority of an alternative maintenance therapy to lenalidomide alone.”
Dr. Shah did have concerns about the applicability of the study, especially regarding the use of varied induction regimens, saying, “Only 11%-13% of patients in this study got lenalidomide during induction and 4%-6% got carfilzomib induction. Therefore, most patients were getting new drugs during maintenance different from their induction agents, which has the downside of leaving fewer options for patients when they relapse.”
Asked about these concerns, Dr. Jakubowiak responded, “Our team’s perspective is to ‘buy’ the longest remission possible using the most effective available treatment strategy. If we do this, there is a good chance that when the patient relapses, we will have availability of new drugs.”
He added the caveat: “There’s a small proportion of patients who will be progressing while on treatment and will be potentially more challenging to treat if already given KRd. It has been proposed that KRd maintenance might be primarily considered for patients with high-risk cytogenetics. However, at this interim analysis, there were not enough events in high-risk patients to comment on KRd’s usefulness as a maintenance therapy in this population.”
The KRd arm of the ATLAS trial had a high dropout-rate for reasons other than toxicity and death, but “because consent for collecting data was discontinued, we can’t really dig deeper into explaining why they dropped out, but half of the patients who discontinued in the KRd arm did so after already completing KRd treatment and were already on R maintenance. Once taking this into consideration, the dropout rates in both arms are similar, and the data does not appear skewed,” said Dr. Jakubowiak.
In conclusion, Dr. Jakubowiak noted that the phase-2 FORTE trial, also published recently in The Lancet Oncology, provides supportive data for maintenance therapy with KR versus K.
“It is quite important that the results of both trials be presented to public so that people know that KR or KRd could be reasonably considered for posttransplant maintenance,” he said.
Dr. Shah opined that as yet, the results of neither study were practice changing for maintenance therapy in the United States. She said she would consider using KR maintenance for select patients with high-risk myeloma who received these drugs as induction therapy and where there was concern about early progression, given that this aggressive treatment allows for a longer time in remission.
Dr. Shah noted that in these trials, overall survival data on dual maintenance is still being awaited, and that those results would be important when considering whether to switch patients to a more expensive, toxic, and involved therapy that required regular infusion visits for maintenance.
What Dr. Shah found the most groundbreaking and applicable aspect of the study was that “in the ATLAS trial, they’re adapting treatments to disease response, and if patients achieve MRD negativity, which we know is the best kind of response, therapy was deescalated to lenalidomide single agent. I think this is something that we will see more of in newer study designs and this will help individualize patient treatments to their response.”
Dr. Jakubowiak disclosed consulting fees and honoraria for lectures and presentations from AbbVie, Amgen, Celgene (Bristol Myers Squibb), Gracell, GlaxoSmithKline, Janssen, and Sanofi-Aventis. Dr. Shah disclosed ties with Sanofi, Bristol Myers Squibb and Janssen.
These results, published in The Lancet Oncology, are not yet practice-changing, but they add to a growing body of evidence supporting this approach.
“Bearing in mind that ATLAS trial data come from unplanned interim analysis, we found that the benefit of combination therapy (KRd) is clearly seen, especially for standard risk patients. We think that, since there are no vast differences in toxicities, there is quite convincing data for considering KRd maintenance for the post autologous stem cell transplantation treatment of MM patients,” ATLAS study coauthor Andrzej Jakubowiak, MD, professor of medicine at the University of Chicago, said in an interview.
Among patients receiving KRd (n = 93), median PFS was 59 months (95% confidence interval) compared with 41 months in the R group (hazard ratio, 0.51, P = .012). These results come from data collected between 2016 and 2020, from 12 academic and clinical centers in the United States and Poland. The cohort included 180 adults randomly assigned to each study arm, (47% were female, median age 59 years) The most common adverse events were neutropenia 48% vs. 60%, thrombocytopenia 13% vs. 7%, and lower respiratory tract infections 8% vs. 1% in the KRd and R groups, respectively. Serious adverse events were reported in 30% of patients in the KRd arm (including one death due to severe pneumonia) and 22% in the R group.
“As expected, there is a slightly higher toxicity in the KRd arm but mostly low-grade events occurred. We believe in KRd maintenance because it provides a longer remission for a relatively small price. The longer a patient is in remission, the better chance that, if relapse does occur, there will be more therapeutic options available to return to remission,” said Dr. Jakubowiak.
Although all patients in the trial received stem cell therapy, the induction therapies were varied.
“We designed the study similarly to the CALGB maintenance trial, one of the key phase 3 trials leading to the approval of lenalidomide as post transplantation maintenance. With so many changes currently occurring MM therapies, having a fixed induction can be its own limitation. Importantly, with our study design we found that all comers may benefit from KRd maintenance,” he said.
It is important to note that KRd treatment occurred for 36 cycles unless a patient withdrew, died, or achieved minimal residual disease (MRD) status.
“Per study design, the duration of KRd treatment was shortened to eight cycles for patients without high-risk cytogenetics who achieved MRD-negativity after cycle six. For these patients, treatment was converted from KRd to R maintenance as of cycle nine. Despite treatment deescalation, this subset of patients (44% of all KRd patients) contributed strongly to KRd PFS. While this approach will need to be validated in a randomized setting, the results provide a proof of concept that if you achieve MRD-negative status and have standard risk cytogenetics, you don’t have to go on a long KRd treatment,” said Dr. Jakubowiak.
Urvi Shah, MD, of Memorial Sloan Kettering Cancer Center in New York, noted the importance of the study as “the first randomized phase-3 trial suggesting progression free survival superiority of an alternative maintenance therapy to lenalidomide alone.”
Dr. Shah did have concerns about the applicability of the study, especially regarding the use of varied induction regimens, saying, “Only 11%-13% of patients in this study got lenalidomide during induction and 4%-6% got carfilzomib induction. Therefore, most patients were getting new drugs during maintenance different from their induction agents, which has the downside of leaving fewer options for patients when they relapse.”
Asked about these concerns, Dr. Jakubowiak responded, “Our team’s perspective is to ‘buy’ the longest remission possible using the most effective available treatment strategy. If we do this, there is a good chance that when the patient relapses, we will have availability of new drugs.”
He added the caveat: “There’s a small proportion of patients who will be progressing while on treatment and will be potentially more challenging to treat if already given KRd. It has been proposed that KRd maintenance might be primarily considered for patients with high-risk cytogenetics. However, at this interim analysis, there were not enough events in high-risk patients to comment on KRd’s usefulness as a maintenance therapy in this population.”
The KRd arm of the ATLAS trial had a high dropout-rate for reasons other than toxicity and death, but “because consent for collecting data was discontinued, we can’t really dig deeper into explaining why they dropped out, but half of the patients who discontinued in the KRd arm did so after already completing KRd treatment and were already on R maintenance. Once taking this into consideration, the dropout rates in both arms are similar, and the data does not appear skewed,” said Dr. Jakubowiak.
In conclusion, Dr. Jakubowiak noted that the phase-2 FORTE trial, also published recently in The Lancet Oncology, provides supportive data for maintenance therapy with KR versus K.
“It is quite important that the results of both trials be presented to public so that people know that KR or KRd could be reasonably considered for posttransplant maintenance,” he said.
Dr. Shah opined that as yet, the results of neither study were practice changing for maintenance therapy in the United States. She said she would consider using KR maintenance for select patients with high-risk myeloma who received these drugs as induction therapy and where there was concern about early progression, given that this aggressive treatment allows for a longer time in remission.
Dr. Shah noted that in these trials, overall survival data on dual maintenance is still being awaited, and that those results would be important when considering whether to switch patients to a more expensive, toxic, and involved therapy that required regular infusion visits for maintenance.
What Dr. Shah found the most groundbreaking and applicable aspect of the study was that “in the ATLAS trial, they’re adapting treatments to disease response, and if patients achieve MRD negativity, which we know is the best kind of response, therapy was deescalated to lenalidomide single agent. I think this is something that we will see more of in newer study designs and this will help individualize patient treatments to their response.”
Dr. Jakubowiak disclosed consulting fees and honoraria for lectures and presentations from AbbVie, Amgen, Celgene (Bristol Myers Squibb), Gracell, GlaxoSmithKline, Janssen, and Sanofi-Aventis. Dr. Shah disclosed ties with Sanofi, Bristol Myers Squibb and Janssen.
Autism linked to problems with cardiovascular health
People with autism are more likely to face diabetes, high cholesterol, and heart disease than those without the neurologic condition, according to a study published in JAMA Pediatrics. Researchers also found that children with autism are especially likely to develop diabetes compared with their peers, and are at greater risk of hypertension, too.
While the link between autism and risk for obesity and gastrointestinal ailments is well-established, the new findings suggest that clinicians who care for these patients – particularly children – should focus on cardiometabolic health more broadly.
“Clinicians who are treating kids with autism need to pay more attention to this,” said Chanaka N. Kahathuduwa, MD, PhD, MPhil, of the department of neurology at Texas Tech University Health Sciences Center, in Lubbock, and a coauthor of the new study.
A pediatrician may prescribe an atypical antipsychotic medication such as risperidone to regulate the behavior of an autistic child, Dr. Kahathuduwa said, which may increase their cholesterol levels. Although this or similar drugs may be necessary in some cases, Dr. Kahathuduwa advised that clinicians explore other treatment options first.
Mining data from previously published studies
For the new analysis, Dr. Kahathuduwa and his colleagues pooled the results of 34 previously published studies, which included medical records of more than 276,000 people with autism and close to 8 million people without the condition.
Study participants were an average age of 31 years, and 47% were female. Some studies reported age ranges that enabled the researchers to differentiate between children and adults.
People with autism were 64% more likely to develop type 1 diabetes, 146% more likely to experience type 2 diabetes, and 46% more likely to have heart disease, overall, the study found. Children with autism were almost twice as likely as their peers to develop diabetes (184%) and high blood pressure (154%).
The study found associations, not causation, and does not include detailed data about medication prescribing patterns. While it would be ideal to understand why autism is linked to cardiometabolic risk, to address the link most effectively, Dr. Kahathuduwa said the causes likely are multifactorial. Medication history and genetics each play a role in a way that is hard to untangle. Even so, Dr. Kahathuduwa said he hoped the findings prompt clinicians to reevaluate how they treat their patients with autism.
“This may be an eye opener,” he said.
An editorial accompanying the study noted that people with autism may die up to 30 years earlier than people without autism, in part because of the physical health problems surfaced in the new research. They also are more likely than others to attempt suicide.
Elizabeth M. Weir, PhD, of the Autism Research Centre at the University of Cambridge (England) and author of the editorial, argued that current health delivery models often fail autistic people by not taking their needs into account.
Dr. Weir told this news organization that making adjustments such as dimming the lights for a light-sensitive patient or allowing people with autism to bring an advocate to appointments could build rapport.
“I diagnose autism pretty much every day and I know families get so overwhelmed with all the recommendations that we give,” said Sonia Monteiro, MD, a developmental and behavioral pediatrician at Texas Children’s Hospital in Houston. Still, Dr. Monteiro said clinicians should help parents of children with autism address the potential long-term cardiovascular risks – but to do so by layering in the information rather than merely adding more bullet points to an already long presentation.
“We know this information now, but finding a way to share that with families without overwhelming them even more, I think is challenging,” Dr. Monteiro said. “But it’s not something we can ignore.”
Dr. Kahathuduwa, Dr. Weir, and Dr. Monteiro report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People with autism are more likely to face diabetes, high cholesterol, and heart disease than those without the neurologic condition, according to a study published in JAMA Pediatrics. Researchers also found that children with autism are especially likely to develop diabetes compared with their peers, and are at greater risk of hypertension, too.
While the link between autism and risk for obesity and gastrointestinal ailments is well-established, the new findings suggest that clinicians who care for these patients – particularly children – should focus on cardiometabolic health more broadly.
“Clinicians who are treating kids with autism need to pay more attention to this,” said Chanaka N. Kahathuduwa, MD, PhD, MPhil, of the department of neurology at Texas Tech University Health Sciences Center, in Lubbock, and a coauthor of the new study.
A pediatrician may prescribe an atypical antipsychotic medication such as risperidone to regulate the behavior of an autistic child, Dr. Kahathuduwa said, which may increase their cholesterol levels. Although this or similar drugs may be necessary in some cases, Dr. Kahathuduwa advised that clinicians explore other treatment options first.
Mining data from previously published studies
For the new analysis, Dr. Kahathuduwa and his colleagues pooled the results of 34 previously published studies, which included medical records of more than 276,000 people with autism and close to 8 million people without the condition.
Study participants were an average age of 31 years, and 47% were female. Some studies reported age ranges that enabled the researchers to differentiate between children and adults.
People with autism were 64% more likely to develop type 1 diabetes, 146% more likely to experience type 2 diabetes, and 46% more likely to have heart disease, overall, the study found. Children with autism were almost twice as likely as their peers to develop diabetes (184%) and high blood pressure (154%).
The study found associations, not causation, and does not include detailed data about medication prescribing patterns. While it would be ideal to understand why autism is linked to cardiometabolic risk, to address the link most effectively, Dr. Kahathuduwa said the causes likely are multifactorial. Medication history and genetics each play a role in a way that is hard to untangle. Even so, Dr. Kahathuduwa said he hoped the findings prompt clinicians to reevaluate how they treat their patients with autism.
“This may be an eye opener,” he said.
An editorial accompanying the study noted that people with autism may die up to 30 years earlier than people without autism, in part because of the physical health problems surfaced in the new research. They also are more likely than others to attempt suicide.
Elizabeth M. Weir, PhD, of the Autism Research Centre at the University of Cambridge (England) and author of the editorial, argued that current health delivery models often fail autistic people by not taking their needs into account.
Dr. Weir told this news organization that making adjustments such as dimming the lights for a light-sensitive patient or allowing people with autism to bring an advocate to appointments could build rapport.
“I diagnose autism pretty much every day and I know families get so overwhelmed with all the recommendations that we give,” said Sonia Monteiro, MD, a developmental and behavioral pediatrician at Texas Children’s Hospital in Houston. Still, Dr. Monteiro said clinicians should help parents of children with autism address the potential long-term cardiovascular risks – but to do so by layering in the information rather than merely adding more bullet points to an already long presentation.
“We know this information now, but finding a way to share that with families without overwhelming them even more, I think is challenging,” Dr. Monteiro said. “But it’s not something we can ignore.”
Dr. Kahathuduwa, Dr. Weir, and Dr. Monteiro report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People with autism are more likely to face diabetes, high cholesterol, and heart disease than those without the neurologic condition, according to a study published in JAMA Pediatrics. Researchers also found that children with autism are especially likely to develop diabetes compared with their peers, and are at greater risk of hypertension, too.
While the link between autism and risk for obesity and gastrointestinal ailments is well-established, the new findings suggest that clinicians who care for these patients – particularly children – should focus on cardiometabolic health more broadly.
“Clinicians who are treating kids with autism need to pay more attention to this,” said Chanaka N. Kahathuduwa, MD, PhD, MPhil, of the department of neurology at Texas Tech University Health Sciences Center, in Lubbock, and a coauthor of the new study.
A pediatrician may prescribe an atypical antipsychotic medication such as risperidone to regulate the behavior of an autistic child, Dr. Kahathuduwa said, which may increase their cholesterol levels. Although this or similar drugs may be necessary in some cases, Dr. Kahathuduwa advised that clinicians explore other treatment options first.
Mining data from previously published studies
For the new analysis, Dr. Kahathuduwa and his colleagues pooled the results of 34 previously published studies, which included medical records of more than 276,000 people with autism and close to 8 million people without the condition.
Study participants were an average age of 31 years, and 47% were female. Some studies reported age ranges that enabled the researchers to differentiate between children and adults.
People with autism were 64% more likely to develop type 1 diabetes, 146% more likely to experience type 2 diabetes, and 46% more likely to have heart disease, overall, the study found. Children with autism were almost twice as likely as their peers to develop diabetes (184%) and high blood pressure (154%).
The study found associations, not causation, and does not include detailed data about medication prescribing patterns. While it would be ideal to understand why autism is linked to cardiometabolic risk, to address the link most effectively, Dr. Kahathuduwa said the causes likely are multifactorial. Medication history and genetics each play a role in a way that is hard to untangle. Even so, Dr. Kahathuduwa said he hoped the findings prompt clinicians to reevaluate how they treat their patients with autism.
“This may be an eye opener,” he said.
An editorial accompanying the study noted that people with autism may die up to 30 years earlier than people without autism, in part because of the physical health problems surfaced in the new research. They also are more likely than others to attempt suicide.
Elizabeth M. Weir, PhD, of the Autism Research Centre at the University of Cambridge (England) and author of the editorial, argued that current health delivery models often fail autistic people by not taking their needs into account.
Dr. Weir told this news organization that making adjustments such as dimming the lights for a light-sensitive patient or allowing people with autism to bring an advocate to appointments could build rapport.
“I diagnose autism pretty much every day and I know families get so overwhelmed with all the recommendations that we give,” said Sonia Monteiro, MD, a developmental and behavioral pediatrician at Texas Children’s Hospital in Houston. Still, Dr. Monteiro said clinicians should help parents of children with autism address the potential long-term cardiovascular risks – but to do so by layering in the information rather than merely adding more bullet points to an already long presentation.
“We know this information now, but finding a way to share that with families without overwhelming them even more, I think is challenging,” Dr. Monteiro said. “But it’s not something we can ignore.”
Dr. Kahathuduwa, Dr. Weir, and Dr. Monteiro report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Washington medical board charges doctor with spreading COVID misinformation
Doctors and professional organizations are standing guard, hoping to protect patients from any harm that results from mistruths spread by colleagues.
Case in point: Several physicians and the American Board of Pathology filed complaints with Washington and Idaho medical boards alleging that Ryan Cole, MD, a board-certified pathologist who practices in Boise, Idaho, but who also holds a license in Washington, has spread antivaccine and pro-ivermectin statements on social media. Dr. Cole is one of the founders of America’s Frontline Doctors, a right-wing political organization. Dr. Cole did not respond to a request for comment.
Gary W. Procop, MD, CEO, American Board of Pathology, told this news organization that “as physicians and board-certified pathologists, we have a public trust, and we must be accountable to patients, society, and the profession. Misinformation can cause real harm to patients, which may include death. Misinformation diverts patients away from lifesaving vaccination and other preventive measures, promotes viral transmission, and recommends ineffective therapies that may be toxic instead of evidence-based medical care.”
Cavalcade of complaints
Several doctors also chimed in with formal complaints alleging that Cole is spreading unreliable information, according to a report from KTVB News. For example, a Boise doctor wrote in his complaint that Dr. Cole is “a major purveyor of misinformation” and called it “amazing” that the physician was continuing to publicly support debunked information about COVID-19 more than a year into the pandemic. The doctor also stated, “Cole is a health menace, abusing his status as a physician to mislead the public.”
As a result of such complaints, the Washington medical board has charged Cole with COVID-19–related violations. It is unclear whether or not the Idaho medical board will sanction the doctor. At least 12 medical boards have sanctioned doctors for similar violations since the start of the pandemic.
The statement of charges from the Washington medical board contends that since March 2021, Dr. Cole has made numerous misleading statements regarding the COVID-19 pandemic, vaccines, the use of ivermectin to treat COVID-19, and the effectiveness of masks.
In addition, the statement alleges that Dr. Cole treated several COVID-19 patients via telemedicine. During these sessions, he prescribed ivermectin, an antiparasite drug that has not been found to have any effectiveness in treating, curing, or preventing COVID-19. One of the patients died after receiving this treatment, according to the complaint.
Citing a study published in the New England Journal of Medicine, Dr. Procop pointed out that use of ivermectin, which is not approved by the U.S. Food and Drug Administration to treat COVID-19, is particularly troubling.
“There is a concern whenever an ineffective treatment is prescribed when more effective and scientifically proven therapies are available. Therapeutics have potential side effects, and toxicities have been associated with the use of ivermectin,” Dr. Procop said. “The benefits of therapy should always outweigh the risks of treatment.”
If the Washington medical board finds that Dr. Cole has engaged in unprofessional conduct, possible sanctions include revocation or suspension of his license. Washington state law also provides for a range of other possible sanctions, including restriction or limitation of his practice, requiring that he complete a specific program of remedial education or treatment, monitoring of his practice, censure or reprimand, probation, a fine of up to $5,000 for each violation, or refunding fees that his practice has billed to and collected from patients. Dr. Cole had until January 30 to respond to the medical board’s statement.
“The American Board of Pathology supports the actions of the Washington State Medical Board regarding their inquiries into any physician that holds license in their state who makes false and misleading medical claims, or provides medical care beyond their scope of practice, as indicated by their training,” Dr. Procop said.
Law in limbo
While medical boards are seeking to sanction professionals who spread falsehoods, the pause button has been hit on the California law that allows regulators to punish doctors for spreading false information about COVID-19 vaccinations and treatments.
The law went into effect Jan. 1 but was temporarily halted when U.S. District Judge William B. Shubb of the Eastern District of California granted a preliminary injunction against the law on Jan. 25, according to a report in the Sacramento Bee.
Mr. Shubb said the measure’s definition of “misinformation” was “unconstitutionally vague” under the due process clause of the 14th Amendment. He also criticized the law’s definition of “misinformation” as being “grammatically incoherent.”
A version of this article first appeared on Medscape.com.
Doctors and professional organizations are standing guard, hoping to protect patients from any harm that results from mistruths spread by colleagues.
Case in point: Several physicians and the American Board of Pathology filed complaints with Washington and Idaho medical boards alleging that Ryan Cole, MD, a board-certified pathologist who practices in Boise, Idaho, but who also holds a license in Washington, has spread antivaccine and pro-ivermectin statements on social media. Dr. Cole is one of the founders of America’s Frontline Doctors, a right-wing political organization. Dr. Cole did not respond to a request for comment.
Gary W. Procop, MD, CEO, American Board of Pathology, told this news organization that “as physicians and board-certified pathologists, we have a public trust, and we must be accountable to patients, society, and the profession. Misinformation can cause real harm to patients, which may include death. Misinformation diverts patients away from lifesaving vaccination and other preventive measures, promotes viral transmission, and recommends ineffective therapies that may be toxic instead of evidence-based medical care.”
Cavalcade of complaints
Several doctors also chimed in with formal complaints alleging that Cole is spreading unreliable information, according to a report from KTVB News. For example, a Boise doctor wrote in his complaint that Dr. Cole is “a major purveyor of misinformation” and called it “amazing” that the physician was continuing to publicly support debunked information about COVID-19 more than a year into the pandemic. The doctor also stated, “Cole is a health menace, abusing his status as a physician to mislead the public.”
As a result of such complaints, the Washington medical board has charged Cole with COVID-19–related violations. It is unclear whether or not the Idaho medical board will sanction the doctor. At least 12 medical boards have sanctioned doctors for similar violations since the start of the pandemic.
The statement of charges from the Washington medical board contends that since March 2021, Dr. Cole has made numerous misleading statements regarding the COVID-19 pandemic, vaccines, the use of ivermectin to treat COVID-19, and the effectiveness of masks.
In addition, the statement alleges that Dr. Cole treated several COVID-19 patients via telemedicine. During these sessions, he prescribed ivermectin, an antiparasite drug that has not been found to have any effectiveness in treating, curing, or preventing COVID-19. One of the patients died after receiving this treatment, according to the complaint.
Citing a study published in the New England Journal of Medicine, Dr. Procop pointed out that use of ivermectin, which is not approved by the U.S. Food and Drug Administration to treat COVID-19, is particularly troubling.
“There is a concern whenever an ineffective treatment is prescribed when more effective and scientifically proven therapies are available. Therapeutics have potential side effects, and toxicities have been associated with the use of ivermectin,” Dr. Procop said. “The benefits of therapy should always outweigh the risks of treatment.”
If the Washington medical board finds that Dr. Cole has engaged in unprofessional conduct, possible sanctions include revocation or suspension of his license. Washington state law also provides for a range of other possible sanctions, including restriction or limitation of his practice, requiring that he complete a specific program of remedial education or treatment, monitoring of his practice, censure or reprimand, probation, a fine of up to $5,000 for each violation, or refunding fees that his practice has billed to and collected from patients. Dr. Cole had until January 30 to respond to the medical board’s statement.
“The American Board of Pathology supports the actions of the Washington State Medical Board regarding their inquiries into any physician that holds license in their state who makes false and misleading medical claims, or provides medical care beyond their scope of practice, as indicated by their training,” Dr. Procop said.
Law in limbo
While medical boards are seeking to sanction professionals who spread falsehoods, the pause button has been hit on the California law that allows regulators to punish doctors for spreading false information about COVID-19 vaccinations and treatments.
The law went into effect Jan. 1 but was temporarily halted when U.S. District Judge William B. Shubb of the Eastern District of California granted a preliminary injunction against the law on Jan. 25, according to a report in the Sacramento Bee.
Mr. Shubb said the measure’s definition of “misinformation” was “unconstitutionally vague” under the due process clause of the 14th Amendment. He also criticized the law’s definition of “misinformation” as being “grammatically incoherent.”
A version of this article first appeared on Medscape.com.
Doctors and professional organizations are standing guard, hoping to protect patients from any harm that results from mistruths spread by colleagues.
Case in point: Several physicians and the American Board of Pathology filed complaints with Washington and Idaho medical boards alleging that Ryan Cole, MD, a board-certified pathologist who practices in Boise, Idaho, but who also holds a license in Washington, has spread antivaccine and pro-ivermectin statements on social media. Dr. Cole is one of the founders of America’s Frontline Doctors, a right-wing political organization. Dr. Cole did not respond to a request for comment.
Gary W. Procop, MD, CEO, American Board of Pathology, told this news organization that “as physicians and board-certified pathologists, we have a public trust, and we must be accountable to patients, society, and the profession. Misinformation can cause real harm to patients, which may include death. Misinformation diverts patients away from lifesaving vaccination and other preventive measures, promotes viral transmission, and recommends ineffective therapies that may be toxic instead of evidence-based medical care.”
Cavalcade of complaints
Several doctors also chimed in with formal complaints alleging that Cole is spreading unreliable information, according to a report from KTVB News. For example, a Boise doctor wrote in his complaint that Dr. Cole is “a major purveyor of misinformation” and called it “amazing” that the physician was continuing to publicly support debunked information about COVID-19 more than a year into the pandemic. The doctor also stated, “Cole is a health menace, abusing his status as a physician to mislead the public.”
As a result of such complaints, the Washington medical board has charged Cole with COVID-19–related violations. It is unclear whether or not the Idaho medical board will sanction the doctor. At least 12 medical boards have sanctioned doctors for similar violations since the start of the pandemic.
The statement of charges from the Washington medical board contends that since March 2021, Dr. Cole has made numerous misleading statements regarding the COVID-19 pandemic, vaccines, the use of ivermectin to treat COVID-19, and the effectiveness of masks.
In addition, the statement alleges that Dr. Cole treated several COVID-19 patients via telemedicine. During these sessions, he prescribed ivermectin, an antiparasite drug that has not been found to have any effectiveness in treating, curing, or preventing COVID-19. One of the patients died after receiving this treatment, according to the complaint.
Citing a study published in the New England Journal of Medicine, Dr. Procop pointed out that use of ivermectin, which is not approved by the U.S. Food and Drug Administration to treat COVID-19, is particularly troubling.
“There is a concern whenever an ineffective treatment is prescribed when more effective and scientifically proven therapies are available. Therapeutics have potential side effects, and toxicities have been associated with the use of ivermectin,” Dr. Procop said. “The benefits of therapy should always outweigh the risks of treatment.”
If the Washington medical board finds that Dr. Cole has engaged in unprofessional conduct, possible sanctions include revocation or suspension of his license. Washington state law also provides for a range of other possible sanctions, including restriction or limitation of his practice, requiring that he complete a specific program of remedial education or treatment, monitoring of his practice, censure or reprimand, probation, a fine of up to $5,000 for each violation, or refunding fees that his practice has billed to and collected from patients. Dr. Cole had until January 30 to respond to the medical board’s statement.
“The American Board of Pathology supports the actions of the Washington State Medical Board regarding their inquiries into any physician that holds license in their state who makes false and misleading medical claims, or provides medical care beyond their scope of practice, as indicated by their training,” Dr. Procop said.
Law in limbo
While medical boards are seeking to sanction professionals who spread falsehoods, the pause button has been hit on the California law that allows regulators to punish doctors for spreading false information about COVID-19 vaccinations and treatments.
The law went into effect Jan. 1 but was temporarily halted when U.S. District Judge William B. Shubb of the Eastern District of California granted a preliminary injunction against the law on Jan. 25, according to a report in the Sacramento Bee.
Mr. Shubb said the measure’s definition of “misinformation” was “unconstitutionally vague” under the due process clause of the 14th Amendment. He also criticized the law’s definition of “misinformation” as being “grammatically incoherent.”
A version of this article first appeared on Medscape.com.
Can AI conquer the late-shift dip in colonoscopy quality?
AI systems “may be a potential tool for minimizing time-related degradation of colonoscopy quality and further maintaining high quality and homogeneity of colonoscopies in high-workload centers,” Honggang Yu, MD, with the department of gastroenterology, Renmin Hospital of Wuhan (China) University, said in an interview.
The study was published online in JAMA Network Open.
Fatigue a factor?
Adenoma detection rate (ADR) is a critical quality measure of screening colonoscopy. Time of day is a well-known factor related to suboptimal ADR – with morning colonoscopies associated with improved ADR and afternoon colonoscopies with reduced ADR, Dr. Yu and colleagues write.
“However, an objective approach to solve this problem is still lacking,” Dr. Yu said. AI systems have been shown to improve the ADR, but the performance of AI during different times of the day remains unknown.
This cohort study is a secondary analysis of two prospective randomized controlled trials, in which a total of 1,780 consecutive patients were randomly allocated to either conventional colonoscopy or AI-assisted colonoscopy. The ADR for early and late colonoscopy sessions per half day were then compared.
Colonoscopy procedures were divided into two groups according to the end time of the procedure. The early group included procedures started in the early session per half day (8:00 a.m.–10:59 a.m. or 1:00 p.m.–2:59 p.m.). The late group included procedures started in the later session per half day (11:00 a.m.–12:59 p.m. or 3:00 p.m.–4:59 p.m.).
A total of 1,041 procedures were performed in the early sessions (357 conventional and 684 AI assisted). A total of 739 procedures were performed in the late sessions (263 conventional and 476 AI assisted).
In the unassisted colonoscopy group, later sessions per half day were associated with a decline in ADR (early vs. late, 13.73% vs. 5.7%; P = .005; odds ratio, 2.42; 95% confidence interval, 1.31-4.47).
With AI assistance, however, no such association was found in the ADR (early vs. late, 22.95% vs. 22.06%; P = .78; OR, 0.96; 95% CI, 0.71-1.29). AI provided the highest assistance capability in the last hour per half day.
The decline in ADR in late sessions (vs. early sessions) was evident in different colonoscopy settings. The investigators say accrual of endoscopist fatigue may be an independent factor of time-related degradation of colonoscopy quality.
More exploration required
“We’re excited about the great potential of using the power of AI to assist endoscopists in quality control or disease diagnosis in colonoscopy practice, but it’s too early to see AI as the standard,” Dr. Yu told this news organization.
“Despite recent achievements in the design and validation of AI systems, much more exploration is required in the clinical application of AI,” Dr. Yu said.
Dr. Yu further explained that, in addition to regulatory approval, the results of AI output must be trusted by the endoscopist, which remains a challenge for current AI systems that lack interpretability.
“Therefore, at the current stage of AI development, AI models can only serve as an extra reminder to assist endoscopists in colonoscopy,” Dr. Yu said.
This study was supported by the Innovation Team Project of Health Commission of Hubei Province. The authors have indicated no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
This article was updated 2/1/23.
AI systems “may be a potential tool for minimizing time-related degradation of colonoscopy quality and further maintaining high quality and homogeneity of colonoscopies in high-workload centers,” Honggang Yu, MD, with the department of gastroenterology, Renmin Hospital of Wuhan (China) University, said in an interview.
The study was published online in JAMA Network Open.
Fatigue a factor?
Adenoma detection rate (ADR) is a critical quality measure of screening colonoscopy. Time of day is a well-known factor related to suboptimal ADR – with morning colonoscopies associated with improved ADR and afternoon colonoscopies with reduced ADR, Dr. Yu and colleagues write.
“However, an objective approach to solve this problem is still lacking,” Dr. Yu said. AI systems have been shown to improve the ADR, but the performance of AI during different times of the day remains unknown.
This cohort study is a secondary analysis of two prospective randomized controlled trials, in which a total of 1,780 consecutive patients were randomly allocated to either conventional colonoscopy or AI-assisted colonoscopy. The ADR for early and late colonoscopy sessions per half day were then compared.
Colonoscopy procedures were divided into two groups according to the end time of the procedure. The early group included procedures started in the early session per half day (8:00 a.m.–10:59 a.m. or 1:00 p.m.–2:59 p.m.). The late group included procedures started in the later session per half day (11:00 a.m.–12:59 p.m. or 3:00 p.m.–4:59 p.m.).
A total of 1,041 procedures were performed in the early sessions (357 conventional and 684 AI assisted). A total of 739 procedures were performed in the late sessions (263 conventional and 476 AI assisted).
In the unassisted colonoscopy group, later sessions per half day were associated with a decline in ADR (early vs. late, 13.73% vs. 5.7%; P = .005; odds ratio, 2.42; 95% confidence interval, 1.31-4.47).
With AI assistance, however, no such association was found in the ADR (early vs. late, 22.95% vs. 22.06%; P = .78; OR, 0.96; 95% CI, 0.71-1.29). AI provided the highest assistance capability in the last hour per half day.
The decline in ADR in late sessions (vs. early sessions) was evident in different colonoscopy settings. The investigators say accrual of endoscopist fatigue may be an independent factor of time-related degradation of colonoscopy quality.
More exploration required
“We’re excited about the great potential of using the power of AI to assist endoscopists in quality control or disease diagnosis in colonoscopy practice, but it’s too early to see AI as the standard,” Dr. Yu told this news organization.
“Despite recent achievements in the design and validation of AI systems, much more exploration is required in the clinical application of AI,” Dr. Yu said.
Dr. Yu further explained that, in addition to regulatory approval, the results of AI output must be trusted by the endoscopist, which remains a challenge for current AI systems that lack interpretability.
“Therefore, at the current stage of AI development, AI models can only serve as an extra reminder to assist endoscopists in colonoscopy,” Dr. Yu said.
This study was supported by the Innovation Team Project of Health Commission of Hubei Province. The authors have indicated no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
This article was updated 2/1/23.
AI systems “may be a potential tool for minimizing time-related degradation of colonoscopy quality and further maintaining high quality and homogeneity of colonoscopies in high-workload centers,” Honggang Yu, MD, with the department of gastroenterology, Renmin Hospital of Wuhan (China) University, said in an interview.
The study was published online in JAMA Network Open.
Fatigue a factor?
Adenoma detection rate (ADR) is a critical quality measure of screening colonoscopy. Time of day is a well-known factor related to suboptimal ADR – with morning colonoscopies associated with improved ADR and afternoon colonoscopies with reduced ADR, Dr. Yu and colleagues write.
“However, an objective approach to solve this problem is still lacking,” Dr. Yu said. AI systems have been shown to improve the ADR, but the performance of AI during different times of the day remains unknown.
This cohort study is a secondary analysis of two prospective randomized controlled trials, in which a total of 1,780 consecutive patients were randomly allocated to either conventional colonoscopy or AI-assisted colonoscopy. The ADR for early and late colonoscopy sessions per half day were then compared.
Colonoscopy procedures were divided into two groups according to the end time of the procedure. The early group included procedures started in the early session per half day (8:00 a.m.–10:59 a.m. or 1:00 p.m.–2:59 p.m.). The late group included procedures started in the later session per half day (11:00 a.m.–12:59 p.m. or 3:00 p.m.–4:59 p.m.).
A total of 1,041 procedures were performed in the early sessions (357 conventional and 684 AI assisted). A total of 739 procedures were performed in the late sessions (263 conventional and 476 AI assisted).
In the unassisted colonoscopy group, later sessions per half day were associated with a decline in ADR (early vs. late, 13.73% vs. 5.7%; P = .005; odds ratio, 2.42; 95% confidence interval, 1.31-4.47).
With AI assistance, however, no such association was found in the ADR (early vs. late, 22.95% vs. 22.06%; P = .78; OR, 0.96; 95% CI, 0.71-1.29). AI provided the highest assistance capability in the last hour per half day.
The decline in ADR in late sessions (vs. early sessions) was evident in different colonoscopy settings. The investigators say accrual of endoscopist fatigue may be an independent factor of time-related degradation of colonoscopy quality.
More exploration required
“We’re excited about the great potential of using the power of AI to assist endoscopists in quality control or disease diagnosis in colonoscopy practice, but it’s too early to see AI as the standard,” Dr. Yu told this news organization.
“Despite recent achievements in the design and validation of AI systems, much more exploration is required in the clinical application of AI,” Dr. Yu said.
Dr. Yu further explained that, in addition to regulatory approval, the results of AI output must be trusted by the endoscopist, which remains a challenge for current AI systems that lack interpretability.
“Therefore, at the current stage of AI development, AI models can only serve as an extra reminder to assist endoscopists in colonoscopy,” Dr. Yu said.
This study was supported by the Innovation Team Project of Health Commission of Hubei Province. The authors have indicated no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
This article was updated 2/1/23.
FROM JAMA NETWORK OPEN
Pandemic pregnancy-linked deaths up 35% from 2019
Pregnancy-associated deaths, including drug-related deaths and homicide, were up 35% in 2020, compared with prepandemic 2019, new research indicates.
The data also show a 7.1% decrease in pregnancy-related suicides in 2020 from 2019.
The study, led by Claire E. Margerison, PhD, with the department of epidemiology and biostatistics at Michigan State University, East Lansing, included 4,528 pregnancy-associated deaths. The rate of deaths per 100,000 live births from April to December 2020 was 66.9 (95% confidence interval, 63.9-70.1). The comparative rate from April to December 2019 was 49.6. Researchers looked at that time period because the pandemic started in March 2020.
The findings were published online in JAMA Open Network.
Drug-related deaths up 55.3%
During the study period, drug deaths increased 55.3% and deaths from homicide increased 41.2%. Deaths from obstetric and other causes (mainly vehicle crashes) increased 28.4% and 56.7%, respectively, according to Dr. Margerison's group.
“Although pregnancy-associated deaths increased over time, increases from 2019 to 2020 were substantially larger than increases from 2018 to 2019,” the authors wrote.
The findings align with deaths in the general population in that time frame, they added.
Another study – this one looking at all-cause and cause-specific mortality from 2019 to 2020 in recently pregnant women, also published in JAMA Network Open, found significant racial and ethnic disparities in rates and cause of death.
According to the study, “Compared with non-Hispanic White women, mortality rates were three- to fivefold higher among American Indian or Alaska Native women for every cause, including suicide. Likewise, these findings suggest that non-Hispanic Black women experienced significantly higher mortality rates across causes, with the highest rates for homicide.”
Dr. Margerison and colleagues did not try to answer what caused the increases but pointed to the fentanyl epidemic, the murder of George Floyd, and COVID-19–related economic strain as potential stressors. They also suggest fewer screenings during the pandemic may have played a role.
Prevention opportunities missed
“Although pregnancy is considered an opportunity for screening and prevention related to physical, mental, and behavioral health, our data suggest that such opportunities were missed for hundreds of pregnant people during the pandemic,” the authors wrote.
Researchers analyzed cross-sectional U.S. death certificates from Jan. 1, 2018, to Dec. 31, 2020, for female U.S. residents ages 15-44 years. They then obtained the count for live births for the same population and time frame from the Centers for Disease Control and Prevention WONDER database.
They were able to identify pregnancy-associated deaths as the 2003 Revised Death Certificate contains a standardized pregnancy checkbox that asks whether the person was pregnant at the time of death, within 42 days of death, or within 43 days to 1 year of death.
Researchers also included deaths with ICD-10 codes linked with death from obstetric causes.
Deaths from overdose, suicide, and homicide are making up large and growing proportions of all deaths during pregnancy and in the first year postpartum, the authors report.
Dr. Margerison and coauthors, in research published in 2022, reported that these causes account for more than one-fifth of all pregnancy-related deaths. They also reported that drug-related deaths and homicides in this population have increased over the past 10 years.
“Substantial racial and ethnic inequities in these deaths exist,” they wrote in that paper.
The authors concluded in the current research: “Our study findings suggest that there is a need for prevention and intervention efforts, including harm-reduction strategies, tailored to pregnant and postpartum women, particularly during times of population stress and decreased utilization of preventive care, such as a pandemic.”
Dr. Margerison and coauthors reported receiving grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor received personal fees from the World Health Organization and Population Reference Bureau outside the submitted work. One coauthor reported receiving grant support from the National Institutes of Mental Health during the study.
*This story was updated on 2/1.
Pregnancy-associated deaths, including drug-related deaths and homicide, were up 35% in 2020, compared with prepandemic 2019, new research indicates.
The data also show a 7.1% decrease in pregnancy-related suicides in 2020 from 2019.
The study, led by Claire E. Margerison, PhD, with the department of epidemiology and biostatistics at Michigan State University, East Lansing, included 4,528 pregnancy-associated deaths. The rate of deaths per 100,000 live births from April to December 2020 was 66.9 (95% confidence interval, 63.9-70.1). The comparative rate from April to December 2019 was 49.6. Researchers looked at that time period because the pandemic started in March 2020.
The findings were published online in JAMA Open Network.
Drug-related deaths up 55.3%
During the study period, drug deaths increased 55.3% and deaths from homicide increased 41.2%. Deaths from obstetric and other causes (mainly vehicle crashes) increased 28.4% and 56.7%, respectively, according to Dr. Margerison's group.
“Although pregnancy-associated deaths increased over time, increases from 2019 to 2020 were substantially larger than increases from 2018 to 2019,” the authors wrote.
The findings align with deaths in the general population in that time frame, they added.
Another study – this one looking at all-cause and cause-specific mortality from 2019 to 2020 in recently pregnant women, also published in JAMA Network Open, found significant racial and ethnic disparities in rates and cause of death.
According to the study, “Compared with non-Hispanic White women, mortality rates were three- to fivefold higher among American Indian or Alaska Native women for every cause, including suicide. Likewise, these findings suggest that non-Hispanic Black women experienced significantly higher mortality rates across causes, with the highest rates for homicide.”
Dr. Margerison and colleagues did not try to answer what caused the increases but pointed to the fentanyl epidemic, the murder of George Floyd, and COVID-19–related economic strain as potential stressors. They also suggest fewer screenings during the pandemic may have played a role.
Prevention opportunities missed
“Although pregnancy is considered an opportunity for screening and prevention related to physical, mental, and behavioral health, our data suggest that such opportunities were missed for hundreds of pregnant people during the pandemic,” the authors wrote.
Researchers analyzed cross-sectional U.S. death certificates from Jan. 1, 2018, to Dec. 31, 2020, for female U.S. residents ages 15-44 years. They then obtained the count for live births for the same population and time frame from the Centers for Disease Control and Prevention WONDER database.
They were able to identify pregnancy-associated deaths as the 2003 Revised Death Certificate contains a standardized pregnancy checkbox that asks whether the person was pregnant at the time of death, within 42 days of death, or within 43 days to 1 year of death.
Researchers also included deaths with ICD-10 codes linked with death from obstetric causes.
Deaths from overdose, suicide, and homicide are making up large and growing proportions of all deaths during pregnancy and in the first year postpartum, the authors report.
Dr. Margerison and coauthors, in research published in 2022, reported that these causes account for more than one-fifth of all pregnancy-related deaths. They also reported that drug-related deaths and homicides in this population have increased over the past 10 years.
“Substantial racial and ethnic inequities in these deaths exist,” they wrote in that paper.
The authors concluded in the current research: “Our study findings suggest that there is a need for prevention and intervention efforts, including harm-reduction strategies, tailored to pregnant and postpartum women, particularly during times of population stress and decreased utilization of preventive care, such as a pandemic.”
Dr. Margerison and coauthors reported receiving grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor received personal fees from the World Health Organization and Population Reference Bureau outside the submitted work. One coauthor reported receiving grant support from the National Institutes of Mental Health during the study.
*This story was updated on 2/1.
Pregnancy-associated deaths, including drug-related deaths and homicide, were up 35% in 2020, compared with prepandemic 2019, new research indicates.
The data also show a 7.1% decrease in pregnancy-related suicides in 2020 from 2019.
The study, led by Claire E. Margerison, PhD, with the department of epidemiology and biostatistics at Michigan State University, East Lansing, included 4,528 pregnancy-associated deaths. The rate of deaths per 100,000 live births from April to December 2020 was 66.9 (95% confidence interval, 63.9-70.1). The comparative rate from April to December 2019 was 49.6. Researchers looked at that time period because the pandemic started in March 2020.
The findings were published online in JAMA Open Network.
Drug-related deaths up 55.3%
During the study period, drug deaths increased 55.3% and deaths from homicide increased 41.2%. Deaths from obstetric and other causes (mainly vehicle crashes) increased 28.4% and 56.7%, respectively, according to Dr. Margerison's group.
“Although pregnancy-associated deaths increased over time, increases from 2019 to 2020 were substantially larger than increases from 2018 to 2019,” the authors wrote.
The findings align with deaths in the general population in that time frame, they added.
Another study – this one looking at all-cause and cause-specific mortality from 2019 to 2020 in recently pregnant women, also published in JAMA Network Open, found significant racial and ethnic disparities in rates and cause of death.
According to the study, “Compared with non-Hispanic White women, mortality rates were three- to fivefold higher among American Indian or Alaska Native women for every cause, including suicide. Likewise, these findings suggest that non-Hispanic Black women experienced significantly higher mortality rates across causes, with the highest rates for homicide.”
Dr. Margerison and colleagues did not try to answer what caused the increases but pointed to the fentanyl epidemic, the murder of George Floyd, and COVID-19–related economic strain as potential stressors. They also suggest fewer screenings during the pandemic may have played a role.
Prevention opportunities missed
“Although pregnancy is considered an opportunity for screening and prevention related to physical, mental, and behavioral health, our data suggest that such opportunities were missed for hundreds of pregnant people during the pandemic,” the authors wrote.
Researchers analyzed cross-sectional U.S. death certificates from Jan. 1, 2018, to Dec. 31, 2020, for female U.S. residents ages 15-44 years. They then obtained the count for live births for the same population and time frame from the Centers for Disease Control and Prevention WONDER database.
They were able to identify pregnancy-associated deaths as the 2003 Revised Death Certificate contains a standardized pregnancy checkbox that asks whether the person was pregnant at the time of death, within 42 days of death, or within 43 days to 1 year of death.
Researchers also included deaths with ICD-10 codes linked with death from obstetric causes.
Deaths from overdose, suicide, and homicide are making up large and growing proportions of all deaths during pregnancy and in the first year postpartum, the authors report.
Dr. Margerison and coauthors, in research published in 2022, reported that these causes account for more than one-fifth of all pregnancy-related deaths. They also reported that drug-related deaths and homicides in this population have increased over the past 10 years.
“Substantial racial and ethnic inequities in these deaths exist,” they wrote in that paper.
The authors concluded in the current research: “Our study findings suggest that there is a need for prevention and intervention efforts, including harm-reduction strategies, tailored to pregnant and postpartum women, particularly during times of population stress and decreased utilization of preventive care, such as a pandemic.”
Dr. Margerison and coauthors reported receiving grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor received personal fees from the World Health Organization and Population Reference Bureau outside the submitted work. One coauthor reported receiving grant support from the National Institutes of Mental Health during the study.
*This story was updated on 2/1.
FROM JAMA NETWORK OPEN











