User login
Feds charge 25 nursing school execs, staff in fake diploma scheme
The U.S. Department of Justice recently announced charges against 25 owners, operators, and employees of three Florida nursing schools in a fraud scheme in which they sold as many as 7,600 fake nursing degrees.
The purchasers in the diploma scheme paid $10,000 to $15,000 for degrees and transcripts and some 2,800 of the buyers passed the national nursing licensing exam to become registered nurses (RNs) and licensed practice nurses/vocational nurses (LPN/VNs) around the country, according to The New York Times.
Many of the degree recipients went on to work at hospitals, nursing homes, and Veterans Affairs medical centers, according to the U.S. Attorney’s Office for the Southern District of Florida.
Several national nursing organizations cooperated with the investigation, and the Delaware Division of Professional Regulation already annulled 26 licenses, according to the Delaware Nurses Association. Fake licenses were issued in five states, according to federal reports.
“We are deeply unsettled by this egregious act,” DNA President Stephanie McClellan, MSN, RN, CMSRN, said in the group’s press statement. “We want all Delaware nurses to be aware of this active issue and to speak up if there is a concern regarding capacity to practice safely by a colleague/peer,” she said.
The Oregon State Board of Nursing is also investigating at least a dozen nurses who may have paid for their degrees, according to a Portland CBS affiliate.
The National Council of State Boards of Nursing said in a statement that it had helped authorities identify and monitor the individuals who allegedly provided the false degrees.
Nursing community reacts
News of the fraud scheme spread through the nursing community, including social media. “The recent report on falsified nursing school degrees is both heartbreaking and serves as an eye-opener,” tweeted Usha Menon, PhD, RN, FAAN, dean and health professor of the University of South Florida Health College of Nursing. “There was enough of a need that prompted these bad actors to develop a scheme that could’ve endangered dozens of lives.”
Jennifer Mensik Kennedy, PhD, MBA, RN, the new president of the American Nurses Association, also weighed in. “The accusation that personnel at once-accredited nursing schools allegedly participated in this scheme is simply deplorable. These unlawful and unethical acts disparage the reputation of actual nurses everywhere who have rightfully earned [their titles] through their education, hard work, dedication, and time.”
The false degrees and transcripts were issued by three once-accredited and now-shuttered nursing schools in South Florida: Palm Beach School of Nursing, Sacred Heart International Institute, and Sienna College.
The alleged co-conspirators reportedly made $114 million from the scheme, which dates back to 2016, according to several news reports. Each defendant faces up to 20 years in prison.
Most LPN programs charge $10,000 to $15,000 to complete a program, Robert Rosseter, a spokesperson for the American Association of Colleges of Nursing (AACN), told this news organization.
None were AACN members, and none were accredited by the Commission on Collegiate Nursing Education, which is AACN’s autonomous accrediting agency, Mr. Rosseter said. AACN membership is voluntary and is open to schools offering baccalaureate or higher degrees, he explained.
“What is disturbing about this investigation is that there are over 7,600 people around the country with fraudulent nursing credentials who are potentially in critical health care roles treating patients,” Chad Yarbrough, acting special agent in charge for the FBI in Miami, said in the federal justice department release.
‘Operation Nightingale’ based on tip
The federal action, dubbed “Operation Nightingale” after the nursing pioneer Florence Nightingale, began in 2019. It was based on a tip related to a case in Maryland, according to Nurse.org.
That case ensnared Palm Beach School of Nursing owner Johanah Napoleon, who reportedly was selling fake degrees for $6,000 to $18,000 each to two individuals in Maryland and Virginia. Ms. Napoleon was charged in 2021 and eventually pled guilty. The Florida Board of Nursing shut down the Palm Beach school in 2017 owing to its students’ low passing rate on the national licensing exam.
Two participants in the bigger scheme who had also worked with Ms. Napoleon – Geralda Adrien and Woosvelt Predestin – were indicted in 2021. Ms. Adrien owned private education companies for people who at aspired to be nurses, and Mr. Predestin was an employee. They were sentenced to 27 months in prison last year and helped the federal officials build the larger case.
The 25 individuals who were charged Jan. 25 operated in Delaware, New York, New Jersey, Texas, and Florida.
Schemes lured immigrants
In the scheme involving Siena College, some of the individuals acted as recruiters to direct nurses who were looking for employment to the school, where they allegedly would then pay for an RN or LPN/VN degree. The recipients of the false documents then used them to obtain jobs, including at a hospital in Georgia and a Veterans Affairs medical center in Maryland, according to one indictment. The president of Siena and her co-conspirators sold more than 2,000 fake diplomas, according to charging documents.
At the Palm Beach College of Nursing, individuals at various nursing prep and education programs allegedly helped others obtain fake degrees and transcripts, which were then used to pass RN and LPN/VN licensing exams in states that included Massachusetts, New Jersey, New York, and Ohio, according to the indictment.
Some individuals then secured employment with a nursing home in Ohio, a home health agency for pediatric patients in Massachusetts, and skilled nursing facilities in New York and New Jersey.
Prosecutors allege that the president of Sacred Heart International Institute and two other co-conspirators sold 588 fake diplomas.
The FBI said that some of the aspiring nurses who were talked into buying the degrees were LPNs who wanted to become RNs and that most of those lured into the scheme were from South Florida’s Haitian American immigrant community, Nurse.org reported.
A version of this article first appeared on Medscape.com.
The U.S. Department of Justice recently announced charges against 25 owners, operators, and employees of three Florida nursing schools in a fraud scheme in which they sold as many as 7,600 fake nursing degrees.
The purchasers in the diploma scheme paid $10,000 to $15,000 for degrees and transcripts and some 2,800 of the buyers passed the national nursing licensing exam to become registered nurses (RNs) and licensed practice nurses/vocational nurses (LPN/VNs) around the country, according to The New York Times.
Many of the degree recipients went on to work at hospitals, nursing homes, and Veterans Affairs medical centers, according to the U.S. Attorney’s Office for the Southern District of Florida.
Several national nursing organizations cooperated with the investigation, and the Delaware Division of Professional Regulation already annulled 26 licenses, according to the Delaware Nurses Association. Fake licenses were issued in five states, according to federal reports.
“We are deeply unsettled by this egregious act,” DNA President Stephanie McClellan, MSN, RN, CMSRN, said in the group’s press statement. “We want all Delaware nurses to be aware of this active issue and to speak up if there is a concern regarding capacity to practice safely by a colleague/peer,” she said.
The Oregon State Board of Nursing is also investigating at least a dozen nurses who may have paid for their degrees, according to a Portland CBS affiliate.
The National Council of State Boards of Nursing said in a statement that it had helped authorities identify and monitor the individuals who allegedly provided the false degrees.
Nursing community reacts
News of the fraud scheme spread through the nursing community, including social media. “The recent report on falsified nursing school degrees is both heartbreaking and serves as an eye-opener,” tweeted Usha Menon, PhD, RN, FAAN, dean and health professor of the University of South Florida Health College of Nursing. “There was enough of a need that prompted these bad actors to develop a scheme that could’ve endangered dozens of lives.”
Jennifer Mensik Kennedy, PhD, MBA, RN, the new president of the American Nurses Association, also weighed in. “The accusation that personnel at once-accredited nursing schools allegedly participated in this scheme is simply deplorable. These unlawful and unethical acts disparage the reputation of actual nurses everywhere who have rightfully earned [their titles] through their education, hard work, dedication, and time.”
The false degrees and transcripts were issued by three once-accredited and now-shuttered nursing schools in South Florida: Palm Beach School of Nursing, Sacred Heart International Institute, and Sienna College.
The alleged co-conspirators reportedly made $114 million from the scheme, which dates back to 2016, according to several news reports. Each defendant faces up to 20 years in prison.
Most LPN programs charge $10,000 to $15,000 to complete a program, Robert Rosseter, a spokesperson for the American Association of Colleges of Nursing (AACN), told this news organization.
None were AACN members, and none were accredited by the Commission on Collegiate Nursing Education, which is AACN’s autonomous accrediting agency, Mr. Rosseter said. AACN membership is voluntary and is open to schools offering baccalaureate or higher degrees, he explained.
“What is disturbing about this investigation is that there are over 7,600 people around the country with fraudulent nursing credentials who are potentially in critical health care roles treating patients,” Chad Yarbrough, acting special agent in charge for the FBI in Miami, said in the federal justice department release.
‘Operation Nightingale’ based on tip
The federal action, dubbed “Operation Nightingale” after the nursing pioneer Florence Nightingale, began in 2019. It was based on a tip related to a case in Maryland, according to Nurse.org.
That case ensnared Palm Beach School of Nursing owner Johanah Napoleon, who reportedly was selling fake degrees for $6,000 to $18,000 each to two individuals in Maryland and Virginia. Ms. Napoleon was charged in 2021 and eventually pled guilty. The Florida Board of Nursing shut down the Palm Beach school in 2017 owing to its students’ low passing rate on the national licensing exam.
Two participants in the bigger scheme who had also worked with Ms. Napoleon – Geralda Adrien and Woosvelt Predestin – were indicted in 2021. Ms. Adrien owned private education companies for people who at aspired to be nurses, and Mr. Predestin was an employee. They were sentenced to 27 months in prison last year and helped the federal officials build the larger case.
The 25 individuals who were charged Jan. 25 operated in Delaware, New York, New Jersey, Texas, and Florida.
Schemes lured immigrants
In the scheme involving Siena College, some of the individuals acted as recruiters to direct nurses who were looking for employment to the school, where they allegedly would then pay for an RN or LPN/VN degree. The recipients of the false documents then used them to obtain jobs, including at a hospital in Georgia and a Veterans Affairs medical center in Maryland, according to one indictment. The president of Siena and her co-conspirators sold more than 2,000 fake diplomas, according to charging documents.
At the Palm Beach College of Nursing, individuals at various nursing prep and education programs allegedly helped others obtain fake degrees and transcripts, which were then used to pass RN and LPN/VN licensing exams in states that included Massachusetts, New Jersey, New York, and Ohio, according to the indictment.
Some individuals then secured employment with a nursing home in Ohio, a home health agency for pediatric patients in Massachusetts, and skilled nursing facilities in New York and New Jersey.
Prosecutors allege that the president of Sacred Heart International Institute and two other co-conspirators sold 588 fake diplomas.
The FBI said that some of the aspiring nurses who were talked into buying the degrees were LPNs who wanted to become RNs and that most of those lured into the scheme were from South Florida’s Haitian American immigrant community, Nurse.org reported.
A version of this article first appeared on Medscape.com.
The U.S. Department of Justice recently announced charges against 25 owners, operators, and employees of three Florida nursing schools in a fraud scheme in which they sold as many as 7,600 fake nursing degrees.
The purchasers in the diploma scheme paid $10,000 to $15,000 for degrees and transcripts and some 2,800 of the buyers passed the national nursing licensing exam to become registered nurses (RNs) and licensed practice nurses/vocational nurses (LPN/VNs) around the country, according to The New York Times.
Many of the degree recipients went on to work at hospitals, nursing homes, and Veterans Affairs medical centers, according to the U.S. Attorney’s Office for the Southern District of Florida.
Several national nursing organizations cooperated with the investigation, and the Delaware Division of Professional Regulation already annulled 26 licenses, according to the Delaware Nurses Association. Fake licenses were issued in five states, according to federal reports.
“We are deeply unsettled by this egregious act,” DNA President Stephanie McClellan, MSN, RN, CMSRN, said in the group’s press statement. “We want all Delaware nurses to be aware of this active issue and to speak up if there is a concern regarding capacity to practice safely by a colleague/peer,” she said.
The Oregon State Board of Nursing is also investigating at least a dozen nurses who may have paid for their degrees, according to a Portland CBS affiliate.
The National Council of State Boards of Nursing said in a statement that it had helped authorities identify and monitor the individuals who allegedly provided the false degrees.
Nursing community reacts
News of the fraud scheme spread through the nursing community, including social media. “The recent report on falsified nursing school degrees is both heartbreaking and serves as an eye-opener,” tweeted Usha Menon, PhD, RN, FAAN, dean and health professor of the University of South Florida Health College of Nursing. “There was enough of a need that prompted these bad actors to develop a scheme that could’ve endangered dozens of lives.”
Jennifer Mensik Kennedy, PhD, MBA, RN, the new president of the American Nurses Association, also weighed in. “The accusation that personnel at once-accredited nursing schools allegedly participated in this scheme is simply deplorable. These unlawful and unethical acts disparage the reputation of actual nurses everywhere who have rightfully earned [their titles] through their education, hard work, dedication, and time.”
The false degrees and transcripts were issued by three once-accredited and now-shuttered nursing schools in South Florida: Palm Beach School of Nursing, Sacred Heart International Institute, and Sienna College.
The alleged co-conspirators reportedly made $114 million from the scheme, which dates back to 2016, according to several news reports. Each defendant faces up to 20 years in prison.
Most LPN programs charge $10,000 to $15,000 to complete a program, Robert Rosseter, a spokesperson for the American Association of Colleges of Nursing (AACN), told this news organization.
None were AACN members, and none were accredited by the Commission on Collegiate Nursing Education, which is AACN’s autonomous accrediting agency, Mr. Rosseter said. AACN membership is voluntary and is open to schools offering baccalaureate or higher degrees, he explained.
“What is disturbing about this investigation is that there are over 7,600 people around the country with fraudulent nursing credentials who are potentially in critical health care roles treating patients,” Chad Yarbrough, acting special agent in charge for the FBI in Miami, said in the federal justice department release.
‘Operation Nightingale’ based on tip
The federal action, dubbed “Operation Nightingale” after the nursing pioneer Florence Nightingale, began in 2019. It was based on a tip related to a case in Maryland, according to Nurse.org.
That case ensnared Palm Beach School of Nursing owner Johanah Napoleon, who reportedly was selling fake degrees for $6,000 to $18,000 each to two individuals in Maryland and Virginia. Ms. Napoleon was charged in 2021 and eventually pled guilty. The Florida Board of Nursing shut down the Palm Beach school in 2017 owing to its students’ low passing rate on the national licensing exam.
Two participants in the bigger scheme who had also worked with Ms. Napoleon – Geralda Adrien and Woosvelt Predestin – were indicted in 2021. Ms. Adrien owned private education companies for people who at aspired to be nurses, and Mr. Predestin was an employee. They were sentenced to 27 months in prison last year and helped the federal officials build the larger case.
The 25 individuals who were charged Jan. 25 operated in Delaware, New York, New Jersey, Texas, and Florida.
Schemes lured immigrants
In the scheme involving Siena College, some of the individuals acted as recruiters to direct nurses who were looking for employment to the school, where they allegedly would then pay for an RN or LPN/VN degree. The recipients of the false documents then used them to obtain jobs, including at a hospital in Georgia and a Veterans Affairs medical center in Maryland, according to one indictment. The president of Siena and her co-conspirators sold more than 2,000 fake diplomas, according to charging documents.
At the Palm Beach College of Nursing, individuals at various nursing prep and education programs allegedly helped others obtain fake degrees and transcripts, which were then used to pass RN and LPN/VN licensing exams in states that included Massachusetts, New Jersey, New York, and Ohio, according to the indictment.
Some individuals then secured employment with a nursing home in Ohio, a home health agency for pediatric patients in Massachusetts, and skilled nursing facilities in New York and New Jersey.
Prosecutors allege that the president of Sacred Heart International Institute and two other co-conspirators sold 588 fake diplomas.
The FBI said that some of the aspiring nurses who were talked into buying the degrees were LPNs who wanted to become RNs and that most of those lured into the scheme were from South Florida’s Haitian American immigrant community, Nurse.org reported.
A version of this article first appeared on Medscape.com.
Biden to end COVID emergencies in May
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Veteran study helps decode GWI phenotypes
To paraphrase Winston Churchill, Gulf War Illness (GWI) is a mystery wrapped in an enigma—a complex interplay of multiple symptoms, caused by a variety of environmental and chemical hazards. To make things more difficult, there are no diagnostic biomarkers or objective laboratory tests with which to confirm a GWI case. Instead, clinicians rely on patients’ reports of symptoms and the absence of other explanations for the symptoms.
Looking to provide more information on the epidemiology and biology of GWI, US Department of Veterans Affairs (VA) researchers analyzed data from the VA Cooperative Studies Program 2006/Million Veteran Program 029 cohort, the largest sample of GW-era veterans available for research to date: 35,902 veterans, of whom 13,107 deployed to a post 9/11 Persian Gulf conflict.
The researchers used the Kansas (KS) and Centers for Disease Control and Prevention (CDC) definitions of GWI, both of which are based on patient self-reports. The KS GWI criteria for phenotype KS Sym+ require ≥ 2 mild symptoms or ≥ 1 moderate or severe symptoms in at least 3 of 6 domains: fatigue/sleep problems, pain, neurologic/cognitive/mood, gastrointestinal, respiratory, and skin. The criteria for phenotype KS Sym+/Dx- also exclude some diagnosed health conditions, such as cancer, diabetes mellitus, and heart disease. The researchers examined both of these phenotypes.
They also used 2 phenotypes of the CDC definition: CDC GWI is met if the veteran reports ≥ 1 symptoms in 2 of 3 domains (fatigue, musculoskeletal, and mood/cognition). The second, CDC GWI severe, is met if the veteran rates ≥ 1 symptoms as severe in ≥ 2 domains.
Of the veterans studied, 67.1% met the KS Sym+ phenotype; 21.5% met the KS Sym+/Dx– definition. A majority (81.1%) met the CDC GWI phenotype; 18.6% met the severe phenotype. The most prevalent KS GWI domains were neurologic/cognitive/mood (81.9%), fatigue/sleep problems (73.9%), and pain (71.5%).
Although their findings mainly laid a foundation for further research, the researchers pointed to some potential new avenues for exploration. For instance, “Importantly,” the researchers say, “we consistently observed that deployed relative to nondeployed veterans had higher odds of meeting each GWI phenotype.” For both deployed and nondeployed veterans, those who served in the Army or Marine Corps had higher odds of meeting the KS Sym+, CDC GWI, and CDC GWI severe phenotypes. Among the deployed, Reservists had higher odds of CDC GWI and CDC GWI severe than did active-duty veterans.
Their findings also revealed that older age was associated with lower odds of meeting the GWI phenotypes. “[S]omewhat surprisingly,” they note, this finding held in both nondeployed and deployed samples, even after adjusting for military rank during the war. The researchers cite other research that has suggested younger service members are at greater risk for GWI (because they’re more likely, for example, to be exposed to deployment-related toxins). Most studies, the researchers note, have shown GWI and related symptoms to be more common among enlisted personnel than officers. Biomarkers of aging, such as epigenetic age acceleration, they suggest, “may be useful in untangling the relationship between age and GWI case status.”
Because they separately examined the association of demographic characteristics with the GWI phenotypes, the researchers also found that women, regardless of deployment status, had higher odds of meeting the GWI phenotypes compared with men.
Their findings will be used, the researchers say, “to understand how genetic variation is associated with the GWI phenotypes and to identify potential pathophysiologic underpinnings of GWI, pleiotropy with other traits, and gene by environment interactions.” With information from this large dataset of GW-era veterans, they will have a “powerful tool” for in-depth study of exposures and underlying genetic susceptibility to GWI—studies that could not be performed, they say, without the full description of the GWI phenotypes they have documented.
The study had several strengths, the researchers say. For example, unlike previous studies, this one had a sample size large enough to allow more representation of subpopulations, including age, sex, race, ethnicity, education, and military service. The researchers also collected data from surveys, especially data on veterans’ self-reported symptoms and other information “incompletely and infrequently documented in medical records.”
Finally, the data for the study were collected more than 27 years after the GW. It, therefore, gives an “updated, detailed description” of symptoms and conditions affecting GW-era veterans, decades after their return from service.
To paraphrase Winston Churchill, Gulf War Illness (GWI) is a mystery wrapped in an enigma—a complex interplay of multiple symptoms, caused by a variety of environmental and chemical hazards. To make things more difficult, there are no diagnostic biomarkers or objective laboratory tests with which to confirm a GWI case. Instead, clinicians rely on patients’ reports of symptoms and the absence of other explanations for the symptoms.
Looking to provide more information on the epidemiology and biology of GWI, US Department of Veterans Affairs (VA) researchers analyzed data from the VA Cooperative Studies Program 2006/Million Veteran Program 029 cohort, the largest sample of GW-era veterans available for research to date: 35,902 veterans, of whom 13,107 deployed to a post 9/11 Persian Gulf conflict.
The researchers used the Kansas (KS) and Centers for Disease Control and Prevention (CDC) definitions of GWI, both of which are based on patient self-reports. The KS GWI criteria for phenotype KS Sym+ require ≥ 2 mild symptoms or ≥ 1 moderate or severe symptoms in at least 3 of 6 domains: fatigue/sleep problems, pain, neurologic/cognitive/mood, gastrointestinal, respiratory, and skin. The criteria for phenotype KS Sym+/Dx- also exclude some diagnosed health conditions, such as cancer, diabetes mellitus, and heart disease. The researchers examined both of these phenotypes.
They also used 2 phenotypes of the CDC definition: CDC GWI is met if the veteran reports ≥ 1 symptoms in 2 of 3 domains (fatigue, musculoskeletal, and mood/cognition). The second, CDC GWI severe, is met if the veteran rates ≥ 1 symptoms as severe in ≥ 2 domains.
Of the veterans studied, 67.1% met the KS Sym+ phenotype; 21.5% met the KS Sym+/Dx– definition. A majority (81.1%) met the CDC GWI phenotype; 18.6% met the severe phenotype. The most prevalent KS GWI domains were neurologic/cognitive/mood (81.9%), fatigue/sleep problems (73.9%), and pain (71.5%).
Although their findings mainly laid a foundation for further research, the researchers pointed to some potential new avenues for exploration. For instance, “Importantly,” the researchers say, “we consistently observed that deployed relative to nondeployed veterans had higher odds of meeting each GWI phenotype.” For both deployed and nondeployed veterans, those who served in the Army or Marine Corps had higher odds of meeting the KS Sym+, CDC GWI, and CDC GWI severe phenotypes. Among the deployed, Reservists had higher odds of CDC GWI and CDC GWI severe than did active-duty veterans.
Their findings also revealed that older age was associated with lower odds of meeting the GWI phenotypes. “[S]omewhat surprisingly,” they note, this finding held in both nondeployed and deployed samples, even after adjusting for military rank during the war. The researchers cite other research that has suggested younger service members are at greater risk for GWI (because they’re more likely, for example, to be exposed to deployment-related toxins). Most studies, the researchers note, have shown GWI and related symptoms to be more common among enlisted personnel than officers. Biomarkers of aging, such as epigenetic age acceleration, they suggest, “may be useful in untangling the relationship between age and GWI case status.”
Because they separately examined the association of demographic characteristics with the GWI phenotypes, the researchers also found that women, regardless of deployment status, had higher odds of meeting the GWI phenotypes compared with men.
Their findings will be used, the researchers say, “to understand how genetic variation is associated with the GWI phenotypes and to identify potential pathophysiologic underpinnings of GWI, pleiotropy with other traits, and gene by environment interactions.” With information from this large dataset of GW-era veterans, they will have a “powerful tool” for in-depth study of exposures and underlying genetic susceptibility to GWI—studies that could not be performed, they say, without the full description of the GWI phenotypes they have documented.
The study had several strengths, the researchers say. For example, unlike previous studies, this one had a sample size large enough to allow more representation of subpopulations, including age, sex, race, ethnicity, education, and military service. The researchers also collected data from surveys, especially data on veterans’ self-reported symptoms and other information “incompletely and infrequently documented in medical records.”
Finally, the data for the study were collected more than 27 years after the GW. It, therefore, gives an “updated, detailed description” of symptoms and conditions affecting GW-era veterans, decades after their return from service.
To paraphrase Winston Churchill, Gulf War Illness (GWI) is a mystery wrapped in an enigma—a complex interplay of multiple symptoms, caused by a variety of environmental and chemical hazards. To make things more difficult, there are no diagnostic biomarkers or objective laboratory tests with which to confirm a GWI case. Instead, clinicians rely on patients’ reports of symptoms and the absence of other explanations for the symptoms.
Looking to provide more information on the epidemiology and biology of GWI, US Department of Veterans Affairs (VA) researchers analyzed data from the VA Cooperative Studies Program 2006/Million Veteran Program 029 cohort, the largest sample of GW-era veterans available for research to date: 35,902 veterans, of whom 13,107 deployed to a post 9/11 Persian Gulf conflict.
The researchers used the Kansas (KS) and Centers for Disease Control and Prevention (CDC) definitions of GWI, both of which are based on patient self-reports. The KS GWI criteria for phenotype KS Sym+ require ≥ 2 mild symptoms or ≥ 1 moderate or severe symptoms in at least 3 of 6 domains: fatigue/sleep problems, pain, neurologic/cognitive/mood, gastrointestinal, respiratory, and skin. The criteria for phenotype KS Sym+/Dx- also exclude some diagnosed health conditions, such as cancer, diabetes mellitus, and heart disease. The researchers examined both of these phenotypes.
They also used 2 phenotypes of the CDC definition: CDC GWI is met if the veteran reports ≥ 1 symptoms in 2 of 3 domains (fatigue, musculoskeletal, and mood/cognition). The second, CDC GWI severe, is met if the veteran rates ≥ 1 symptoms as severe in ≥ 2 domains.
Of the veterans studied, 67.1% met the KS Sym+ phenotype; 21.5% met the KS Sym+/Dx– definition. A majority (81.1%) met the CDC GWI phenotype; 18.6% met the severe phenotype. The most prevalent KS GWI domains were neurologic/cognitive/mood (81.9%), fatigue/sleep problems (73.9%), and pain (71.5%).
Although their findings mainly laid a foundation for further research, the researchers pointed to some potential new avenues for exploration. For instance, “Importantly,” the researchers say, “we consistently observed that deployed relative to nondeployed veterans had higher odds of meeting each GWI phenotype.” For both deployed and nondeployed veterans, those who served in the Army or Marine Corps had higher odds of meeting the KS Sym+, CDC GWI, and CDC GWI severe phenotypes. Among the deployed, Reservists had higher odds of CDC GWI and CDC GWI severe than did active-duty veterans.
Their findings also revealed that older age was associated with lower odds of meeting the GWI phenotypes. “[S]omewhat surprisingly,” they note, this finding held in both nondeployed and deployed samples, even after adjusting for military rank during the war. The researchers cite other research that has suggested younger service members are at greater risk for GWI (because they’re more likely, for example, to be exposed to deployment-related toxins). Most studies, the researchers note, have shown GWI and related symptoms to be more common among enlisted personnel than officers. Biomarkers of aging, such as epigenetic age acceleration, they suggest, “may be useful in untangling the relationship between age and GWI case status.”
Because they separately examined the association of demographic characteristics with the GWI phenotypes, the researchers also found that women, regardless of deployment status, had higher odds of meeting the GWI phenotypes compared with men.
Their findings will be used, the researchers say, “to understand how genetic variation is associated with the GWI phenotypes and to identify potential pathophysiologic underpinnings of GWI, pleiotropy with other traits, and gene by environment interactions.” With information from this large dataset of GW-era veterans, they will have a “powerful tool” for in-depth study of exposures and underlying genetic susceptibility to GWI—studies that could not be performed, they say, without the full description of the GWI phenotypes they have documented.
The study had several strengths, the researchers say. For example, unlike previous studies, this one had a sample size large enough to allow more representation of subpopulations, including age, sex, race, ethnicity, education, and military service. The researchers also collected data from surveys, especially data on veterans’ self-reported symptoms and other information “incompletely and infrequently documented in medical records.”
Finally, the data for the study were collected more than 27 years after the GW. It, therefore, gives an “updated, detailed description” of symptoms and conditions affecting GW-era veterans, decades after their return from service.
Generalized Pustular Psoriasis Treated With Risankizumab
To the Editor:
Generalized pustular psoriasis (GPP) is a rare but severe subtype of psoriasis that can present with systemic symptoms and organ failure, sometimes leading to hospitalization and even death.1,2 Due to the rarity of this subtype and GPP being excluded from clinical trials for plaque psoriasis, there is limited information on the optimal treatment of this disease.
More than 20 systemic medications have been described in the literature for treating GPP, including systemic steroids, traditional immunosuppressants, retinoids, and biologics, which often are used in combination; none have been consistently effective.3 Among biologic therapies, the use of tumor necrosis factor α as well as IL-12/23 and IL-17 inhibitors has been reported, with the least amount of experience with IL-17 inhibitors.4
A 53-year-old Korean woman presented to the dermatology clinic for evaluation of a widespread painful rash involving the face, neck, torso, arms, and legs that had been treated intermittently with systemic steroids by her primary care physician for several months before presentation. She had no relevant medical or dermatologic history. She denied taking prescription or over-the-counter medications.
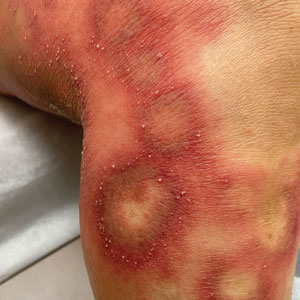
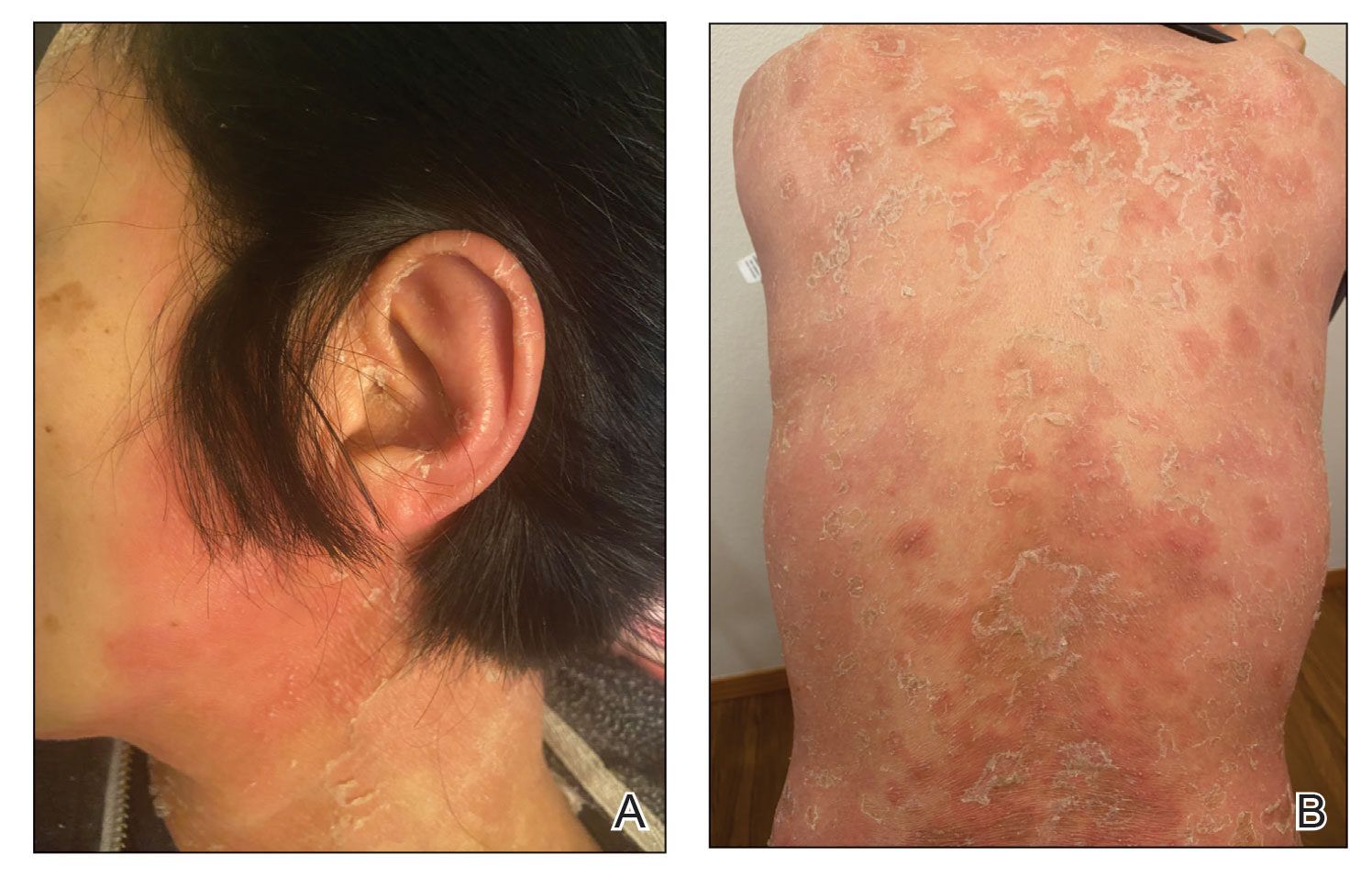
Physical examination revealed the patient was afebrile, but she reported general malaise and chills. She had widespread erythematous, annular, scaly plaques that coalesced into polycyclic plaques studded with nonfollicular-based pustules on the forehead, frontal hairline, neck, chest, abdomen, back, arms, and legs (Figure 1).
![Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively. Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively.](https://cdn.mdedge.com/files/s3fs-public/CT111002096_Fig1_AB.jpg)
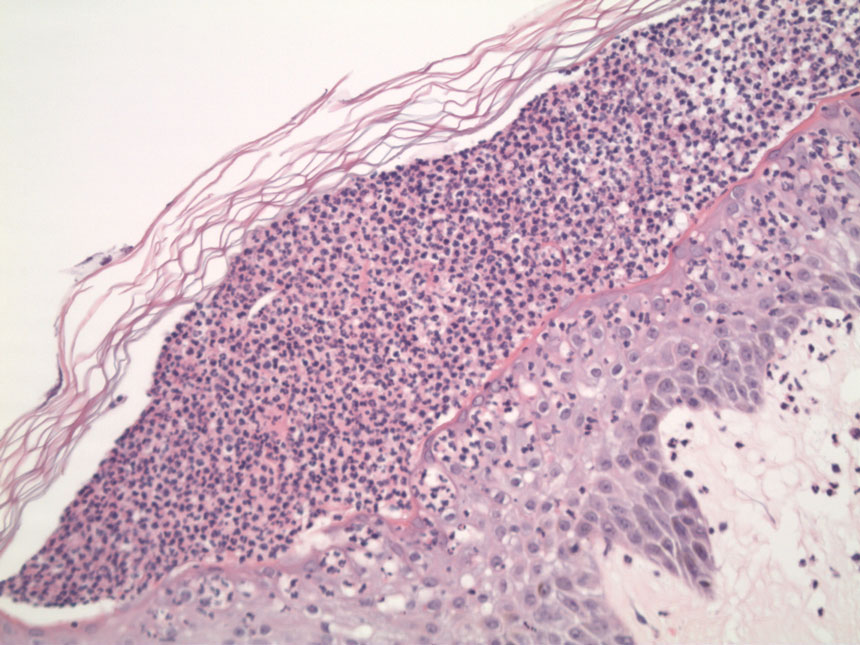
Two 4-mm punch biopsies were performed for hematoxylin and eosin staining and direct immunofluorescence. Histopathologic analysis showed prominent subcorneal neutrophilic pustules and spongiform collections of neutrophils in the spinous layer without notable eosinophils (Figure 2). Direct immunofluorescence was negative.
Based on the clinical history, physical examination, histopathology, and unremarkable drug history, a diagnosis of GPP was made. Initially, acitretin 25 mg/d was prescribed, but the patient was unable to start treatment because the cost of the drug was prohibitive. Her condition worsened, and she returned to the clinic 2 days later. Based on knowledge of an ongoing phase 3, open-label study for risankizumab in GPP, a sample of risankizumab 150 mg was administered subcutaneously in this patient. Three days later, most of the pustules on the upper half of the patient’s body had dried up and she began to desquamate from head to toe (Figure 3).The patient developed notable edema of the lower extremities, which required furosemide 20 mg/d andibuprofen 600 mg every 6 hours for symptom relief.
Ten days after the initial dose of risankizumab, the patient continued to steadily improve. All the pustules had dried up and she was already showing signs of re-epithelialization. Edema and pain also had notably improved. She received 2 additional samples of risankizumab 150 mg at weeks 4 and 16, at which point she was able to receive compassionate care through the drug manufacturer’s program. At follow-up 151 days after the initial dose of risankizumab, the patient’s skin was completely clear.
Generalized pustular psoriasis remains a difficult disease to study, given its rarity and unpredictable course. Spesolimab, a humanized anti–IL-36 receptor monoclonal antibody, was recently approved by the US Food and Drug Administration (FDA) for the treatment of GPP.5 In the pivotal trial (ClinicalTrials.gov Identifier NCT03782792),5 an astonishingly high 54% of patients (19/35) given a single dose of intravenous spesolimab reached the primary end point of no pustules at day 7. However, safety concerns, such as serious infections and severe cutaneous adverse reactions, as well as logistical challenges that come with intravenous administration for an acute disease, may prevent widespread adoption by community dermatologists.
Tumor necrosis factor α, IL-17, and IL-23 inhibitors currently are approved for the treatment of GPP in Japan, Thailand, and Taiwan based on small, nonrandomized, open-label studies.6-10 More recently, results from a phase 3, randomized, open-label study to assess the efficacy and safety of 2 different dosing regimens of risankizumab with 8 Japanese patients with GPP were published.11 However, there currently is only a single approved medication for GPP in Europe and the United States. Therefore, additional therapies, particularly those that have already been established in dermatology, would be welcome in treating this disease.
A number of questions still need to be answered regarding treating GPP with risankizumab:
• What is the optimal dose and schedule of this drug? Our patient received the standard 150-mg dose that is FDA approved for moderate to severe plaque psoriasis; would a higher dose, such as the FDA-approved 600-mg dosing used to treat Crohn disease, have led to a more rapid and durable response?12
• For how long should these patients be treated? Will their disease follow the same course as psoriasis vulgaris, requiring long-term, continuous treatment?
• An ongoing 5-year, open-label extension study of spesolimab might eventually answer that question and currently is recruiting participants (NCT03886246).
• Is there a way to predict a priori which patients will be responders? Biomarkers—especially through the use of tape stripping—are promising, but validation studies are still needed.13
• Because 69% (24/35) of enrolled patients in the treatment group of the spesolimab trial did not harbor a mutation of the IL36RN gene, how reliable is mutation status in predicting treatment response?5
Of note, some of these questions also apply to guttate psoriasis, a far more common subtype of psoriasis that also is worth exploring.
Nevertheless, these are exciting times for patients with GPP. What was once considered an obscure orphan disease is the focus of major recent publications3 and phase 3, randomized, placebo-controlled studies.5 We can be cautiously optimistic that in the next few years we will be in a better position to care for patients with GPP.
- Shah M, Aboud DM Al, Crane JS, et al. Pustular psoriasis. In. Zeichner J, ed. Acneiform Eruptions in Dermatology: A Differential Diagnosis. 2021:295-307. doi:10.1007/978-1-4614-8344-1_42
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509. doi:10.1056/NEJMra0804595
- Noe MH, Wan MT, Mostaghimi A, et al. Evaluation of a case series of patients with generalized pustular psoriasis in the United States. JAMA Dermatol. 2022;158:73-78. doi:10.1001/jamadermatol.2021.4640
- Miyachi H, Konishi T, Kumazawa R, et al. Treatments and outcomes of generalized pustular psoriasis: a cohort of 1516 patients in a nationwide inpatient database in Japan. J Am Acad Dermatol. 2022;86:1266-1274. doi:10.1016/J.JAAD.2021.06.008
- Bachelez H, Choon S-E, Marrakchi S, et al; . Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/J.JAAD.2011.01.032
- Torii H, Nakagawa H; . Long-term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J Dermatol. 2011;38:321-334. doi:10.1111/J.1346-8138.2010.00971.X
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155. doi:10.1111/JDV.12773
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017. doi:10.1111/1346-8138.13306
- Torii H, Terui T, Matsukawa M, et al. Safety profiles and efficacy of infliximab therapy in Japanese patients with plaque psoriasis with or without psoriatic arthritis, pustular psoriasis or psoriatic erythroderma: results from the prospective post-marketing surveillance. J Dermatol. 2016;43:767-778. doi:10.1111/1346-8138.13214
- Yamanaka K, Okubo Y, Yasuda I, et al. Efficacy and safety of risankizumab in Japanese patients with generalized pustular psoriasis or erythrodermic psoriasis: primary analysis and 180-week follow-up results from the phase 3, multicenter IMMspire study [published online December 13, 2022]. J Dermatol. doi:10.1111/1346-8138.16667
- D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399:2015-2030. doi:10.1016/S0140-6736(22)00467-6
- Hughes AJ, Tawfik SS, Baruah KP, et al. Tape strips in dermatology research. Br J Dermatol. 2021;185:26-35. doi:10.1111/BJD.19760
To the Editor:
Generalized pustular psoriasis (GPP) is a rare but severe subtype of psoriasis that can present with systemic symptoms and organ failure, sometimes leading to hospitalization and even death.1,2 Due to the rarity of this subtype and GPP being excluded from clinical trials for plaque psoriasis, there is limited information on the optimal treatment of this disease.
More than 20 systemic medications have been described in the literature for treating GPP, including systemic steroids, traditional immunosuppressants, retinoids, and biologics, which often are used in combination; none have been consistently effective.3 Among biologic therapies, the use of tumor necrosis factor α as well as IL-12/23 and IL-17 inhibitors has been reported, with the least amount of experience with IL-17 inhibitors.4
A 53-year-old Korean woman presented to the dermatology clinic for evaluation of a widespread painful rash involving the face, neck, torso, arms, and legs that had been treated intermittently with systemic steroids by her primary care physician for several months before presentation. She had no relevant medical or dermatologic history. She denied taking prescription or over-the-counter medications.
Physical examination revealed the patient was afebrile, but she reported general malaise and chills. She had widespread erythematous, annular, scaly plaques that coalesced into polycyclic plaques studded with nonfollicular-based pustules on the forehead, frontal hairline, neck, chest, abdomen, back, arms, and legs (Figure 1).
![Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively. Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively.](https://cdn.mdedge.com/files/s3fs-public/CT111002096_Fig1_AB.jpg)
Two 4-mm punch biopsies were performed for hematoxylin and eosin staining and direct immunofluorescence. Histopathologic analysis showed prominent subcorneal neutrophilic pustules and spongiform collections of neutrophils in the spinous layer without notable eosinophils (Figure 2). Direct immunofluorescence was negative.

Based on the clinical history, physical examination, histopathology, and unremarkable drug history, a diagnosis of GPP was made. Initially, acitretin 25 mg/d was prescribed, but the patient was unable to start treatment because the cost of the drug was prohibitive. Her condition worsened, and she returned to the clinic 2 days later. Based on knowledge of an ongoing phase 3, open-label study for risankizumab in GPP, a sample of risankizumab 150 mg was administered subcutaneously in this patient. Three days later, most of the pustules on the upper half of the patient’s body had dried up and she began to desquamate from head to toe (Figure 3).The patient developed notable edema of the lower extremities, which required furosemide 20 mg/d andibuprofen 600 mg every 6 hours for symptom relief.

Ten days after the initial dose of risankizumab, the patient continued to steadily improve. All the pustules had dried up and she was already showing signs of re-epithelialization. Edema and pain also had notably improved. She received 2 additional samples of risankizumab 150 mg at weeks 4 and 16, at which point she was able to receive compassionate care through the drug manufacturer’s program. At follow-up 151 days after the initial dose of risankizumab, the patient’s skin was completely clear.
Generalized pustular psoriasis remains a difficult disease to study, given its rarity and unpredictable course. Spesolimab, a humanized anti–IL-36 receptor monoclonal antibody, was recently approved by the US Food and Drug Administration (FDA) for the treatment of GPP.5 In the pivotal trial (ClinicalTrials.gov Identifier NCT03782792),5 an astonishingly high 54% of patients (19/35) given a single dose of intravenous spesolimab reached the primary end point of no pustules at day 7. However, safety concerns, such as serious infections and severe cutaneous adverse reactions, as well as logistical challenges that come with intravenous administration for an acute disease, may prevent widespread adoption by community dermatologists.
Tumor necrosis factor α, IL-17, and IL-23 inhibitors currently are approved for the treatment of GPP in Japan, Thailand, and Taiwan based on small, nonrandomized, open-label studies.6-10 More recently, results from a phase 3, randomized, open-label study to assess the efficacy and safety of 2 different dosing regimens of risankizumab with 8 Japanese patients with GPP were published.11 However, there currently is only a single approved medication for GPP in Europe and the United States. Therefore, additional therapies, particularly those that have already been established in dermatology, would be welcome in treating this disease.
A number of questions still need to be answered regarding treating GPP with risankizumab:
• What is the optimal dose and schedule of this drug? Our patient received the standard 150-mg dose that is FDA approved for moderate to severe plaque psoriasis; would a higher dose, such as the FDA-approved 600-mg dosing used to treat Crohn disease, have led to a more rapid and durable response?12
• For how long should these patients be treated? Will their disease follow the same course as psoriasis vulgaris, requiring long-term, continuous treatment?
• An ongoing 5-year, open-label extension study of spesolimab might eventually answer that question and currently is recruiting participants (NCT03886246).
• Is there a way to predict a priori which patients will be responders? Biomarkers—especially through the use of tape stripping—are promising, but validation studies are still needed.13
• Because 69% (24/35) of enrolled patients in the treatment group of the spesolimab trial did not harbor a mutation of the IL36RN gene, how reliable is mutation status in predicting treatment response?5
Of note, some of these questions also apply to guttate psoriasis, a far more common subtype of psoriasis that also is worth exploring.
Nevertheless, these are exciting times for patients with GPP. What was once considered an obscure orphan disease is the focus of major recent publications3 and phase 3, randomized, placebo-controlled studies.5 We can be cautiously optimistic that in the next few years we will be in a better position to care for patients with GPP.
To the Editor:
Generalized pustular psoriasis (GPP) is a rare but severe subtype of psoriasis that can present with systemic symptoms and organ failure, sometimes leading to hospitalization and even death.1,2 Due to the rarity of this subtype and GPP being excluded from clinical trials for plaque psoriasis, there is limited information on the optimal treatment of this disease.
More than 20 systemic medications have been described in the literature for treating GPP, including systemic steroids, traditional immunosuppressants, retinoids, and biologics, which often are used in combination; none have been consistently effective.3 Among biologic therapies, the use of tumor necrosis factor α as well as IL-12/23 and IL-17 inhibitors has been reported, with the least amount of experience with IL-17 inhibitors.4
A 53-year-old Korean woman presented to the dermatology clinic for evaluation of a widespread painful rash involving the face, neck, torso, arms, and legs that had been treated intermittently with systemic steroids by her primary care physician for several months before presentation. She had no relevant medical or dermatologic history. She denied taking prescription or over-the-counter medications.
Physical examination revealed the patient was afebrile, but she reported general malaise and chills. She had widespread erythematous, annular, scaly plaques that coalesced into polycyclic plaques studded with nonfollicular-based pustules on the forehead, frontal hairline, neck, chest, abdomen, back, arms, and legs (Figure 1).
![Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively. Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively.](https://cdn.mdedge.com/files/s3fs-public/CT111002096_Fig1_AB.jpg)
Two 4-mm punch biopsies were performed for hematoxylin and eosin staining and direct immunofluorescence. Histopathologic analysis showed prominent subcorneal neutrophilic pustules and spongiform collections of neutrophils in the spinous layer without notable eosinophils (Figure 2). Direct immunofluorescence was negative.

Based on the clinical history, physical examination, histopathology, and unremarkable drug history, a diagnosis of GPP was made. Initially, acitretin 25 mg/d was prescribed, but the patient was unable to start treatment because the cost of the drug was prohibitive. Her condition worsened, and she returned to the clinic 2 days later. Based on knowledge of an ongoing phase 3, open-label study for risankizumab in GPP, a sample of risankizumab 150 mg was administered subcutaneously in this patient. Three days later, most of the pustules on the upper half of the patient’s body had dried up and she began to desquamate from head to toe (Figure 3).The patient developed notable edema of the lower extremities, which required furosemide 20 mg/d andibuprofen 600 mg every 6 hours for symptom relief.

Ten days after the initial dose of risankizumab, the patient continued to steadily improve. All the pustules had dried up and she was already showing signs of re-epithelialization. Edema and pain also had notably improved. She received 2 additional samples of risankizumab 150 mg at weeks 4 and 16, at which point she was able to receive compassionate care through the drug manufacturer’s program. At follow-up 151 days after the initial dose of risankizumab, the patient’s skin was completely clear.
Generalized pustular psoriasis remains a difficult disease to study, given its rarity and unpredictable course. Spesolimab, a humanized anti–IL-36 receptor monoclonal antibody, was recently approved by the US Food and Drug Administration (FDA) for the treatment of GPP.5 In the pivotal trial (ClinicalTrials.gov Identifier NCT03782792),5 an astonishingly high 54% of patients (19/35) given a single dose of intravenous spesolimab reached the primary end point of no pustules at day 7. However, safety concerns, such as serious infections and severe cutaneous adverse reactions, as well as logistical challenges that come with intravenous administration for an acute disease, may prevent widespread adoption by community dermatologists.
Tumor necrosis factor α, IL-17, and IL-23 inhibitors currently are approved for the treatment of GPP in Japan, Thailand, and Taiwan based on small, nonrandomized, open-label studies.6-10 More recently, results from a phase 3, randomized, open-label study to assess the efficacy and safety of 2 different dosing regimens of risankizumab with 8 Japanese patients with GPP were published.11 However, there currently is only a single approved medication for GPP in Europe and the United States. Therefore, additional therapies, particularly those that have already been established in dermatology, would be welcome in treating this disease.
A number of questions still need to be answered regarding treating GPP with risankizumab:
• What is the optimal dose and schedule of this drug? Our patient received the standard 150-mg dose that is FDA approved for moderate to severe plaque psoriasis; would a higher dose, such as the FDA-approved 600-mg dosing used to treat Crohn disease, have led to a more rapid and durable response?12
• For how long should these patients be treated? Will their disease follow the same course as psoriasis vulgaris, requiring long-term, continuous treatment?
• An ongoing 5-year, open-label extension study of spesolimab might eventually answer that question and currently is recruiting participants (NCT03886246).
• Is there a way to predict a priori which patients will be responders? Biomarkers—especially through the use of tape stripping—are promising, but validation studies are still needed.13
• Because 69% (24/35) of enrolled patients in the treatment group of the spesolimab trial did not harbor a mutation of the IL36RN gene, how reliable is mutation status in predicting treatment response?5
Of note, some of these questions also apply to guttate psoriasis, a far more common subtype of psoriasis that also is worth exploring.
Nevertheless, these are exciting times for patients with GPP. What was once considered an obscure orphan disease is the focus of major recent publications3 and phase 3, randomized, placebo-controlled studies.5 We can be cautiously optimistic that in the next few years we will be in a better position to care for patients with GPP.
- Shah M, Aboud DM Al, Crane JS, et al. Pustular psoriasis. In. Zeichner J, ed. Acneiform Eruptions in Dermatology: A Differential Diagnosis. 2021:295-307. doi:10.1007/978-1-4614-8344-1_42
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509. doi:10.1056/NEJMra0804595
- Noe MH, Wan MT, Mostaghimi A, et al. Evaluation of a case series of patients with generalized pustular psoriasis in the United States. JAMA Dermatol. 2022;158:73-78. doi:10.1001/jamadermatol.2021.4640
- Miyachi H, Konishi T, Kumazawa R, et al. Treatments and outcomes of generalized pustular psoriasis: a cohort of 1516 patients in a nationwide inpatient database in Japan. J Am Acad Dermatol. 2022;86:1266-1274. doi:10.1016/J.JAAD.2021.06.008
- Bachelez H, Choon S-E, Marrakchi S, et al; . Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/J.JAAD.2011.01.032
- Torii H, Nakagawa H; . Long-term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J Dermatol. 2011;38:321-334. doi:10.1111/J.1346-8138.2010.00971.X
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155. doi:10.1111/JDV.12773
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017. doi:10.1111/1346-8138.13306
- Torii H, Terui T, Matsukawa M, et al. Safety profiles and efficacy of infliximab therapy in Japanese patients with plaque psoriasis with or without psoriatic arthritis, pustular psoriasis or psoriatic erythroderma: results from the prospective post-marketing surveillance. J Dermatol. 2016;43:767-778. doi:10.1111/1346-8138.13214
- Yamanaka K, Okubo Y, Yasuda I, et al. Efficacy and safety of risankizumab in Japanese patients with generalized pustular psoriasis or erythrodermic psoriasis: primary analysis and 180-week follow-up results from the phase 3, multicenter IMMspire study [published online December 13, 2022]. J Dermatol. doi:10.1111/1346-8138.16667
- D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399:2015-2030. doi:10.1016/S0140-6736(22)00467-6
- Hughes AJ, Tawfik SS, Baruah KP, et al. Tape strips in dermatology research. Br J Dermatol. 2021;185:26-35. doi:10.1111/BJD.19760
- Shah M, Aboud DM Al, Crane JS, et al. Pustular psoriasis. In. Zeichner J, ed. Acneiform Eruptions in Dermatology: A Differential Diagnosis. 2021:295-307. doi:10.1007/978-1-4614-8344-1_42
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509. doi:10.1056/NEJMra0804595
- Noe MH, Wan MT, Mostaghimi A, et al. Evaluation of a case series of patients with generalized pustular psoriasis in the United States. JAMA Dermatol. 2022;158:73-78. doi:10.1001/jamadermatol.2021.4640
- Miyachi H, Konishi T, Kumazawa R, et al. Treatments and outcomes of generalized pustular psoriasis: a cohort of 1516 patients in a nationwide inpatient database in Japan. J Am Acad Dermatol. 2022;86:1266-1274. doi:10.1016/J.JAAD.2021.06.008
- Bachelez H, Choon S-E, Marrakchi S, et al; . Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/J.JAAD.2011.01.032
- Torii H, Nakagawa H; . Long-term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J Dermatol. 2011;38:321-334. doi:10.1111/J.1346-8138.2010.00971.X
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155. doi:10.1111/JDV.12773
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017. doi:10.1111/1346-8138.13306
- Torii H, Terui T, Matsukawa M, et al. Safety profiles and efficacy of infliximab therapy in Japanese patients with plaque psoriasis with or without psoriatic arthritis, pustular psoriasis or psoriatic erythroderma: results from the prospective post-marketing surveillance. J Dermatol. 2016;43:767-778. doi:10.1111/1346-8138.13214
- Yamanaka K, Okubo Y, Yasuda I, et al. Efficacy and safety of risankizumab in Japanese patients with generalized pustular psoriasis or erythrodermic psoriasis: primary analysis and 180-week follow-up results from the phase 3, multicenter IMMspire study [published online December 13, 2022]. J Dermatol. doi:10.1111/1346-8138.16667
- D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399:2015-2030. doi:10.1016/S0140-6736(22)00467-6
- Hughes AJ, Tawfik SS, Baruah KP, et al. Tape strips in dermatology research. Br J Dermatol. 2021;185:26-35. doi:10.1111/BJD.19760
PRACTICE POINTS
- Generalized pustular psoriasis (GPP) is a potentially life-threatening condition that can be precipitated by systemic steroids.
- Although more than 20 systemic medications have been tried with varying success, there has not been a single US Food and Drug Administration–approved medication for GPP until recently with the approval of spesolimab, an IL-36 receptor inhibitor.
- Risankizumab, a high-affinity humanized monoclonal antibody that targets the p19 subunit of the IL-23 cytokine, also has shown promise in a recent phase 3, open-label study for GPP.
Adverse Effects of the COVID-19 Vaccine in Patients With Psoriasis
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.
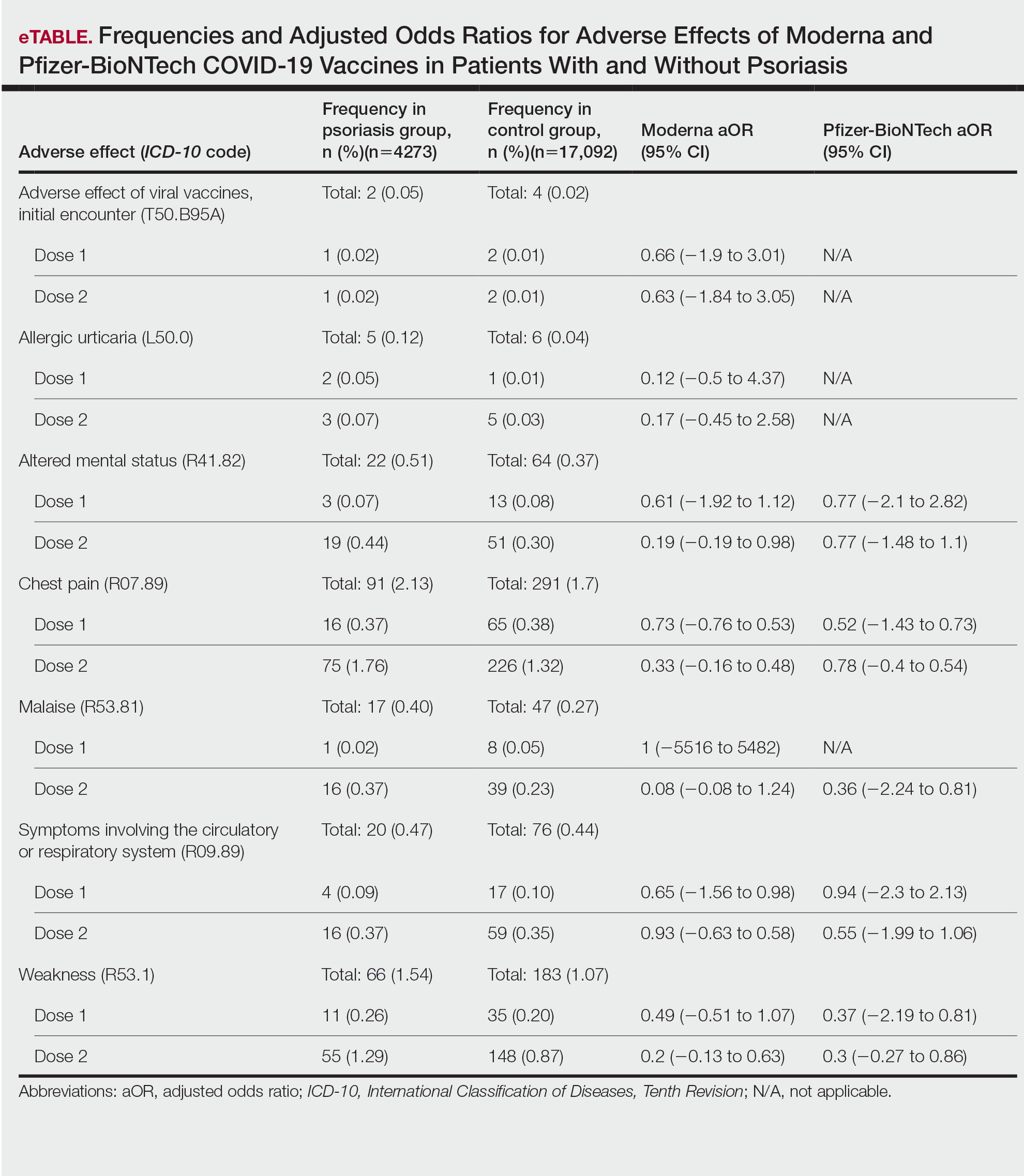
This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
PRACTICE POINTS
- Patients who have psoriasis do not appear to have an increased incidence of adverse effects from messenger RNA COVID-19 vaccines.
- Clinicians can safely recommend COVID-19 vaccines to patients who have psoriasis.
How to Effectively Utilize Consultation Codes: 2023 Updates
Consultations and referrals are an important component of many dermatology practices. There are several families of consultation codes that can be utilized based on the setting and format of the patient encounter. In this article, I describe appropriate use of 3 families of consultation codes and recent updates in these areas.
Consultation Definitions
For all of these code sets, the same definition of consultationapplies—namely that the encounter is provided at the request of another physician, other qualified health care professional, or other appropriate source (eg, nonclinical social worker, educator, lawyer, insurance company) for a specific condition or problem. Importantly, a consultation initiated by a patient or family, or both, and not requested by one of the professionals listed above is not reported using a consultation code.1
The consultant’s opinion and any services that were ordered or performed also must be communicated to the requesting provider. The type of communication required varies based on the consultation code set in question.
Outpatient Consultation Codes
Outpatient consultation CPT (Current Procedural Terminology) codes (99241-99245) are a family of codes that can be utilized for evaluation of a new patient or an existing patient with a new problem in the outpatient setting. These codes are not reimbursed by the Centers for Medicare & Medicaid Services, but some private payers do recognize and reimburse for them.2
The consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1 Modifier -32 should not be used for a second request by a patient or a patient’s family.1
This family of codes has been revised in tandem with other evaluation and management (E/M) code sets; changes went into effect January 1, 2023. These updates are part of the ongoing effort to update code wording and structures to reflect guiding principles of the American Medical Association when redesigning E/M codes. These principles include decreasing administrative burden and the need for audits, decreasing unnecessary documentation that is not needed for patient care, and ensuring that payment for E/M is resource based.3 Updated code language and payment structure is found in Table 1.1,2 The main updates to these codes include:
• Code 99241 was deleted. This was in line with removal of 99201 from the outpatient E/M family set.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 99417 can be utilized.

Inpatient Consultation Codes
Similar to the outpatient consultation codes, the inpatient consultation codes also have been revised as part of E/M updates; revisions went into effect January 1, 2023. Also, as with the outpatient consultation codes, the consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1
When inpatient consultations are performed, 2 code families generally are utilized. For initial consultation, initial inpatient consultation codes (99251-99255) are used; for any follow-up encounters performed while the patient is an inpatient, subsequent inpatient consultation codes (99231-99233) are used. The subsequent code family is the same that is utilized for all subsequent care within the inpatient or observation care setting, regardless of how the care was initiated.1
“Initial service” is when the patient has not received any professional services from either the physician or other qualified health care professional or from another physician or other qualified health care professional ofthe exact same specialty and subspecialty who belongs to the same group practice during the inpatient, observation, or nursing facility admission and stay. “Subsequent service” is when the patient has received professional service(s) from either the physician or other qualified health care professional or from another physician or other qualified health care professional.1 Updated code language and payment structure is found in Table 2.1,2 Major changes include:
• Code 99251 was deleted. This is in line with deletion of a new low-level patient encounter in the outpatient E/M family set and consultation code family set, as noted above.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 993X0 can be utilized.
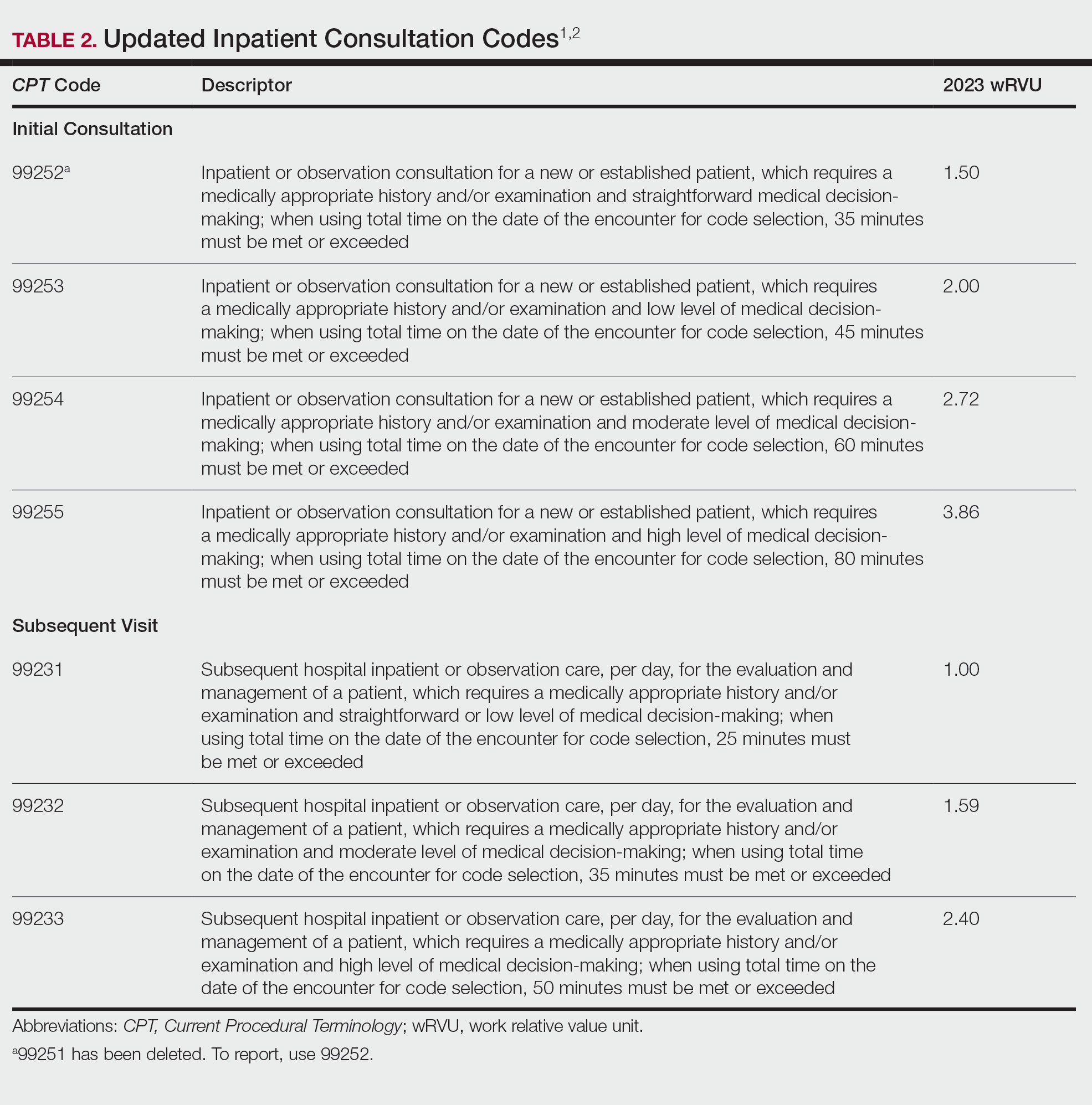
Interprofessional Consultation Codes
An additional code family that can be utilized for consultations is the interprofessional consultation codes. These codes can be utilized when assisting in the diagnosis or management, or both, of a patient without face-to-face contact. These codes are listed in Table 3.2,4 For all of these codes, the consultation is performed by telephone, internet or electronic health record, or a combination of these means. The consultation can be for a new problem or a worsening existing problem. The patient can be a new or established patient to the consultant. Documentation should be performed in the patient’s medical record, including the reason for the request.
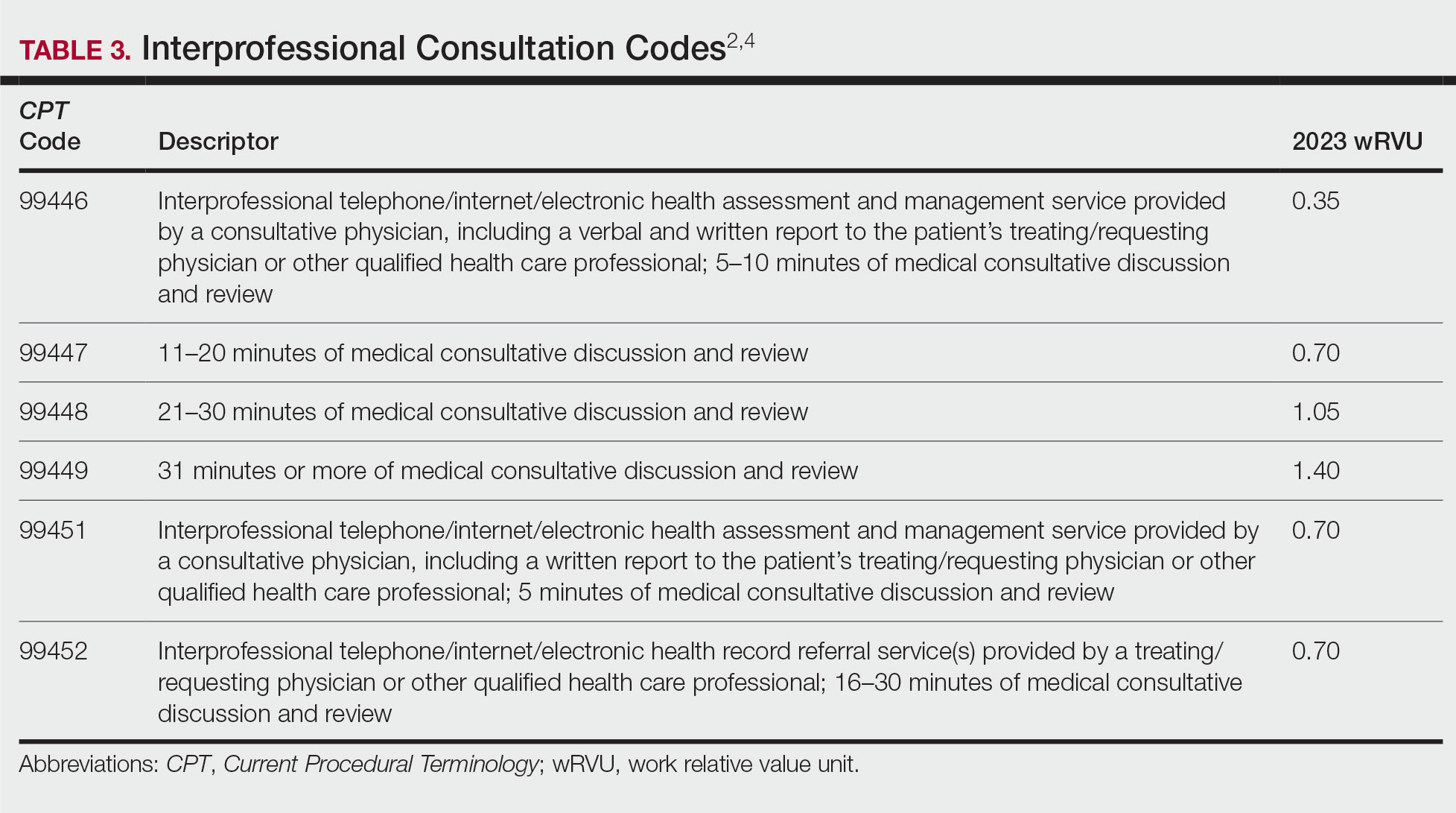
To bill for interprofessional consultation, the consultant should not have seen the patient in a face-to-face encounter within the prior 14 days or see them in the following 14 days. The codes should not be reported more than once in a 7-day period or more than once in a 14-day period in the case of code 99452.4 For codes 99446 to 99449, more than 50% of the time spent by the consulting physician must be devoted to verbal or internet discussion, or both, with the referring physician. For code 99451, service time is based on total review and interprofessional communication time.4 The correct code is chosen based on the following parameters:
• 99446-99449: Describes interprofessional consultation services, which include both a written and a verbal report to the patient’s treating or requesting physician or qualified health care professional. These codes can be utilized by a consulting physician. The correct code is chosen based on time spent by the consulting physician.
• 99451: Describes an interprofessional consultation service, which includes a written report to the patient’s treating or requesting physician or qualified health care professional. This code can be utilized by a consulting physician once 5 minutes of consultative discussion and review has been performed.
• 99452: Describes an interprofessional consultation service provided by the requesting physician. This code can be utilized when a requesting physician spends 16 to 30 minutes in medical consultative discussion and review.
Final Thoughts
Consultation codes can be an important part of a dermatologist’s practice. Differences exist between consultation code sets based on the encounter setting and whether the encounter was performed with or without face-to-face contact. In addition, updates to the E/M inpatient and outpatient consultation codes went into effect January 1, 2023. It is important to understand those changes to correctly bill for these encounters.
- CPT® evaluation and management (E/M) code and guideline changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- RVU23A. US Centers for Medicare and Medicaid Services; January 2023. Accessed January 18, 2023. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu23a
- Understanding the landmark E/M office visit changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/practice-management/cpt/understanding-landmark-em-office-visit-changes
- Synovec MS, Jagmin CL, Hochstetler Z, et al, eds. CPT 2022: Professional Edition. 4th ed. American Medical Association Press; 2021.
Consultations and referrals are an important component of many dermatology practices. There are several families of consultation codes that can be utilized based on the setting and format of the patient encounter. In this article, I describe appropriate use of 3 families of consultation codes and recent updates in these areas.
Consultation Definitions
For all of these code sets, the same definition of consultationapplies—namely that the encounter is provided at the request of another physician, other qualified health care professional, or other appropriate source (eg, nonclinical social worker, educator, lawyer, insurance company) for a specific condition or problem. Importantly, a consultation initiated by a patient or family, or both, and not requested by one of the professionals listed above is not reported using a consultation code.1
The consultant’s opinion and any services that were ordered or performed also must be communicated to the requesting provider. The type of communication required varies based on the consultation code set in question.
Outpatient Consultation Codes
Outpatient consultation CPT (Current Procedural Terminology) codes (99241-99245) are a family of codes that can be utilized for evaluation of a new patient or an existing patient with a new problem in the outpatient setting. These codes are not reimbursed by the Centers for Medicare & Medicaid Services, but some private payers do recognize and reimburse for them.2
The consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1 Modifier -32 should not be used for a second request by a patient or a patient’s family.1
This family of codes has been revised in tandem with other evaluation and management (E/M) code sets; changes went into effect January 1, 2023. These updates are part of the ongoing effort to update code wording and structures to reflect guiding principles of the American Medical Association when redesigning E/M codes. These principles include decreasing administrative burden and the need for audits, decreasing unnecessary documentation that is not needed for patient care, and ensuring that payment for E/M is resource based.3 Updated code language and payment structure is found in Table 1.1,2 The main updates to these codes include:
• Code 99241 was deleted. This was in line with removal of 99201 from the outpatient E/M family set.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 99417 can be utilized.

Inpatient Consultation Codes
Similar to the outpatient consultation codes, the inpatient consultation codes also have been revised as part of E/M updates; revisions went into effect January 1, 2023. Also, as with the outpatient consultation codes, the consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1
When inpatient consultations are performed, 2 code families generally are utilized. For initial consultation, initial inpatient consultation codes (99251-99255) are used; for any follow-up encounters performed while the patient is an inpatient, subsequent inpatient consultation codes (99231-99233) are used. The subsequent code family is the same that is utilized for all subsequent care within the inpatient or observation care setting, regardless of how the care was initiated.1
“Initial service” is when the patient has not received any professional services from either the physician or other qualified health care professional or from another physician or other qualified health care professional ofthe exact same specialty and subspecialty who belongs to the same group practice during the inpatient, observation, or nursing facility admission and stay. “Subsequent service” is when the patient has received professional service(s) from either the physician or other qualified health care professional or from another physician or other qualified health care professional.1 Updated code language and payment structure is found in Table 2.1,2 Major changes include:
• Code 99251 was deleted. This is in line with deletion of a new low-level patient encounter in the outpatient E/M family set and consultation code family set, as noted above.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 993X0 can be utilized.

Interprofessional Consultation Codes
An additional code family that can be utilized for consultations is the interprofessional consultation codes. These codes can be utilized when assisting in the diagnosis or management, or both, of a patient without face-to-face contact. These codes are listed in Table 3.2,4 For all of these codes, the consultation is performed by telephone, internet or electronic health record, or a combination of these means. The consultation can be for a new problem or a worsening existing problem. The patient can be a new or established patient to the consultant. Documentation should be performed in the patient’s medical record, including the reason for the request.

To bill for interprofessional consultation, the consultant should not have seen the patient in a face-to-face encounter within the prior 14 days or see them in the following 14 days. The codes should not be reported more than once in a 7-day period or more than once in a 14-day period in the case of code 99452.4 For codes 99446 to 99449, more than 50% of the time spent by the consulting physician must be devoted to verbal or internet discussion, or both, with the referring physician. For code 99451, service time is based on total review and interprofessional communication time.4 The correct code is chosen based on the following parameters:
• 99446-99449: Describes interprofessional consultation services, which include both a written and a verbal report to the patient’s treating or requesting physician or qualified health care professional. These codes can be utilized by a consulting physician. The correct code is chosen based on time spent by the consulting physician.
• 99451: Describes an interprofessional consultation service, which includes a written report to the patient’s treating or requesting physician or qualified health care professional. This code can be utilized by a consulting physician once 5 minutes of consultative discussion and review has been performed.
• 99452: Describes an interprofessional consultation service provided by the requesting physician. This code can be utilized when a requesting physician spends 16 to 30 minutes in medical consultative discussion and review.
Final Thoughts
Consultation codes can be an important part of a dermatologist’s practice. Differences exist between consultation code sets based on the encounter setting and whether the encounter was performed with or without face-to-face contact. In addition, updates to the E/M inpatient and outpatient consultation codes went into effect January 1, 2023. It is important to understand those changes to correctly bill for these encounters.
Consultations and referrals are an important component of many dermatology practices. There are several families of consultation codes that can be utilized based on the setting and format of the patient encounter. In this article, I describe appropriate use of 3 families of consultation codes and recent updates in these areas.
Consultation Definitions
For all of these code sets, the same definition of consultationapplies—namely that the encounter is provided at the request of another physician, other qualified health care professional, or other appropriate source (eg, nonclinical social worker, educator, lawyer, insurance company) for a specific condition or problem. Importantly, a consultation initiated by a patient or family, or both, and not requested by one of the professionals listed above is not reported using a consultation code.1
The consultant’s opinion and any services that were ordered or performed also must be communicated to the requesting provider. The type of communication required varies based on the consultation code set in question.
Outpatient Consultation Codes
Outpatient consultation CPT (Current Procedural Terminology) codes (99241-99245) are a family of codes that can be utilized for evaluation of a new patient or an existing patient with a new problem in the outpatient setting. These codes are not reimbursed by the Centers for Medicare & Medicaid Services, but some private payers do recognize and reimburse for them.2
The consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1 Modifier -32 should not be used for a second request by a patient or a patient’s family.1
This family of codes has been revised in tandem with other evaluation and management (E/M) code sets; changes went into effect January 1, 2023. These updates are part of the ongoing effort to update code wording and structures to reflect guiding principles of the American Medical Association when redesigning E/M codes. These principles include decreasing administrative burden and the need for audits, decreasing unnecessary documentation that is not needed for patient care, and ensuring that payment for E/M is resource based.3 Updated code language and payment structure is found in Table 1.1,2 The main updates to these codes include:
• Code 99241 was deleted. This was in line with removal of 99201 from the outpatient E/M family set.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 99417 can be utilized.

Inpatient Consultation Codes
Similar to the outpatient consultation codes, the inpatient consultation codes also have been revised as part of E/M updates; revisions went into effect January 1, 2023. Also, as with the outpatient consultation codes, the consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1
When inpatient consultations are performed, 2 code families generally are utilized. For initial consultation, initial inpatient consultation codes (99251-99255) are used; for any follow-up encounters performed while the patient is an inpatient, subsequent inpatient consultation codes (99231-99233) are used. The subsequent code family is the same that is utilized for all subsequent care within the inpatient or observation care setting, regardless of how the care was initiated.1
“Initial service” is when the patient has not received any professional services from either the physician or other qualified health care professional or from another physician or other qualified health care professional ofthe exact same specialty and subspecialty who belongs to the same group practice during the inpatient, observation, or nursing facility admission and stay. “Subsequent service” is when the patient has received professional service(s) from either the physician or other qualified health care professional or from another physician or other qualified health care professional.1 Updated code language and payment structure is found in Table 2.1,2 Major changes include:
• Code 99251 was deleted. This is in line with deletion of a new low-level patient encounter in the outpatient E/M family set and consultation code family set, as noted above.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 993X0 can be utilized.

Interprofessional Consultation Codes
An additional code family that can be utilized for consultations is the interprofessional consultation codes. These codes can be utilized when assisting in the diagnosis or management, or both, of a patient without face-to-face contact. These codes are listed in Table 3.2,4 For all of these codes, the consultation is performed by telephone, internet or electronic health record, or a combination of these means. The consultation can be for a new problem or a worsening existing problem. The patient can be a new or established patient to the consultant. Documentation should be performed in the patient’s medical record, including the reason for the request.

To bill for interprofessional consultation, the consultant should not have seen the patient in a face-to-face encounter within the prior 14 days or see them in the following 14 days. The codes should not be reported more than once in a 7-day period or more than once in a 14-day period in the case of code 99452.4 For codes 99446 to 99449, more than 50% of the time spent by the consulting physician must be devoted to verbal or internet discussion, or both, with the referring physician. For code 99451, service time is based on total review and interprofessional communication time.4 The correct code is chosen based on the following parameters:
• 99446-99449: Describes interprofessional consultation services, which include both a written and a verbal report to the patient’s treating or requesting physician or qualified health care professional. These codes can be utilized by a consulting physician. The correct code is chosen based on time spent by the consulting physician.
• 99451: Describes an interprofessional consultation service, which includes a written report to the patient’s treating or requesting physician or qualified health care professional. This code can be utilized by a consulting physician once 5 minutes of consultative discussion and review has been performed.
• 99452: Describes an interprofessional consultation service provided by the requesting physician. This code can be utilized when a requesting physician spends 16 to 30 minutes in medical consultative discussion and review.
Final Thoughts
Consultation codes can be an important part of a dermatologist’s practice. Differences exist between consultation code sets based on the encounter setting and whether the encounter was performed with or without face-to-face contact. In addition, updates to the E/M inpatient and outpatient consultation codes went into effect January 1, 2023. It is important to understand those changes to correctly bill for these encounters.
- CPT® evaluation and management (E/M) code and guideline changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- RVU23A. US Centers for Medicare and Medicaid Services; January 2023. Accessed January 18, 2023. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu23a
- Understanding the landmark E/M office visit changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/practice-management/cpt/understanding-landmark-em-office-visit-changes
- Synovec MS, Jagmin CL, Hochstetler Z, et al, eds. CPT 2022: Professional Edition. 4th ed. American Medical Association Press; 2021.
- CPT® evaluation and management (E/M) code and guideline changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- RVU23A. US Centers for Medicare and Medicaid Services; January 2023. Accessed January 18, 2023. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu23a
- Understanding the landmark E/M office visit changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/practice-management/cpt/understanding-landmark-em-office-visit-changes
- Synovec MS, Jagmin CL, Hochstetler Z, et al, eds. CPT 2022: Professional Edition. 4th ed. American Medical Association Press; 2021.
PRACTICE POINTS
- Updates to the inpatient and outpatient consultation codes went into effect January 1, 2023.
- For inpatient and outpatient consultation codes, level of service is now solely based on either time on the date of encounter or medical decision-making.
- Interprofessional consultation codes can be utilized when assisting in the diagnosis and/or management of a patient without face-to-face contact.
Commentary: Genetic variants, ovarian suppression, and metabolic syndrome in BC, February 2023
Poly-(ADP-ribose) polymerase (PARP) inhibitors have emerged as essential therapeutic agents in patients with germline BRCA1/2-mutated BC. A BRCA-like phenotype is displayed by a large subset of patients with germline BRCA1/2-wildtype BC who present with homologous recombination deficiency (HRD). The randomized phase 2 S1416 trial (Rodler et al) evaluated the efficacy of cisplatin combined with the PARP inhibitor veliparib in three cohorts of metastatic BC: mutated germline BRCA1/2, BRCA-like, and non-BRCA–like. A total of 335 patients with metastatic or recurrent triple-negative BC (TNBC) or germline BRCA1/2-mutated metastatic BC were randomly assigned (1:1) to receive cisplatin plus either veliparib or a matching placebo. The findings showed that the addition of veliparib to cisplatin significantly improved progression-free survival (PFS) in patients with BRCA-like metastatic TNBC compared with placebo (5.9 vs 4.2 months; HR 0.57; log-rank P = .01), but not in mutated germline BRCA1/2 (6.2 vs 6.4 months; P = .54) and non-BRCA–like (4.0 vs 3.0 months; P = .57) groups. No new toxicity signals were observed. These findings suggest BRCA-like TNBC might show sensitivity to PARP inhibitors and therefore these agents should be explored further in this cohort.
A recent update from the combined analysis of the SOFT and TEXT studies comparing outcomes in 4690 premenopausal women with estrogen/progesterone receptor–positive early BC (Pagani et al) showed that exemestane plus ovarian function suppression (OFS) led to a greater reduction in recurrence risk compared with tamoxifen plus OFS in premenopausal women. After a median follow-up of 13 years, results showed a 4.6% absolute improvement in 12-year disease-free survival (HR 0.79; P < .001) and a 1.8% absolute improvement in disease recurrence-free interval (HR 0.83; P = .03) with exemestane plus OFS compared with tamoxifen plus OFS. These treatment effects on recurrence began to attenuate over time, being strongest in the first 5 years with no further improvement after 10 or more years. No improvement in overall survival (OS) was noted with exemestane vs tamoxifen, although both arms had excellent survival outcomes (90.1% vs 89.1%; HR 0.93; 95% CI 0.78-1.11). It is important to note that there was a 3.3% absolute improvement in 12-year OS with exemestane plus OFS among patients with HER2-negative tumors who received chemotherapy. This OS benefit was also noted amongst patients with high-risk clinicopathologic characteristics (<35 years and those with > 2 cm or grade 3 tumors), ranging from 4.0% to 5.5% absolute improvement. In conclusion, sustained recurrence risk reductions were noted with adjuvant exemestane plus OFS compared with tamoxifen plus OFS, with the most clinically meaningful survival benefit noted for patients with higher risk tumors. Proper selection of patients who are most likely to benefit from exemestane over tamoxifen is vital to maximize the survival benefit while minimizing the burden of treatment intensification.
Findings from a retrospective study including 221 women with BC who received preoperative neoadjuvant chemotherapy (NAC) showed that the presence of metabolic syndrome (MetS) worsened survival outcomes and increased disease recurrence risk (Zhou et al). Patients were divided into MetS and non-MetS groups according to National Cholesterol Education Program Adult Treatment Panel III criteria to investigate the association between MetS and clinicopathologic characteristics, pathologic complete response (pCR), and long-term survival. The MetS group had a significantly lower likelihood of achieving pCR after NAC compared with the non-MetS group (odds ratio [OR] 0.316; P = .028), with the risk for death (OR 2.587; P = .004) and disease recurrence (OR 2.228; P = .007) being significantly higher in patients with vs without MetS. In a multivariate analysis, MetS (P = 0.028) and hormone receptors status were independent predictors of pCR after NAC in BC. These findings emphasize the importance of timely intervention of metabolic syndrome to improve outcomes in patients with BC.
Poly-(ADP-ribose) polymerase (PARP) inhibitors have emerged as essential therapeutic agents in patients with germline BRCA1/2-mutated BC. A BRCA-like phenotype is displayed by a large subset of patients with germline BRCA1/2-wildtype BC who present with homologous recombination deficiency (HRD). The randomized phase 2 S1416 trial (Rodler et al) evaluated the efficacy of cisplatin combined with the PARP inhibitor veliparib in three cohorts of metastatic BC: mutated germline BRCA1/2, BRCA-like, and non-BRCA–like. A total of 335 patients with metastatic or recurrent triple-negative BC (TNBC) or germline BRCA1/2-mutated metastatic BC were randomly assigned (1:1) to receive cisplatin plus either veliparib or a matching placebo. The findings showed that the addition of veliparib to cisplatin significantly improved progression-free survival (PFS) in patients with BRCA-like metastatic TNBC compared with placebo (5.9 vs 4.2 months; HR 0.57; log-rank P = .01), but not in mutated germline BRCA1/2 (6.2 vs 6.4 months; P = .54) and non-BRCA–like (4.0 vs 3.0 months; P = .57) groups. No new toxicity signals were observed. These findings suggest BRCA-like TNBC might show sensitivity to PARP inhibitors and therefore these agents should be explored further in this cohort.
A recent update from the combined analysis of the SOFT and TEXT studies comparing outcomes in 4690 premenopausal women with estrogen/progesterone receptor–positive early BC (Pagani et al) showed that exemestane plus ovarian function suppression (OFS) led to a greater reduction in recurrence risk compared with tamoxifen plus OFS in premenopausal women. After a median follow-up of 13 years, results showed a 4.6% absolute improvement in 12-year disease-free survival (HR 0.79; P < .001) and a 1.8% absolute improvement in disease recurrence-free interval (HR 0.83; P = .03) with exemestane plus OFS compared with tamoxifen plus OFS. These treatment effects on recurrence began to attenuate over time, being strongest in the first 5 years with no further improvement after 10 or more years. No improvement in overall survival (OS) was noted with exemestane vs tamoxifen, although both arms had excellent survival outcomes (90.1% vs 89.1%; HR 0.93; 95% CI 0.78-1.11). It is important to note that there was a 3.3% absolute improvement in 12-year OS with exemestane plus OFS among patients with HER2-negative tumors who received chemotherapy. This OS benefit was also noted amongst patients with high-risk clinicopathologic characteristics (<35 years and those with > 2 cm or grade 3 tumors), ranging from 4.0% to 5.5% absolute improvement. In conclusion, sustained recurrence risk reductions were noted with adjuvant exemestane plus OFS compared with tamoxifen plus OFS, with the most clinically meaningful survival benefit noted for patients with higher risk tumors. Proper selection of patients who are most likely to benefit from exemestane over tamoxifen is vital to maximize the survival benefit while minimizing the burden of treatment intensification.
Findings from a retrospective study including 221 women with BC who received preoperative neoadjuvant chemotherapy (NAC) showed that the presence of metabolic syndrome (MetS) worsened survival outcomes and increased disease recurrence risk (Zhou et al). Patients were divided into MetS and non-MetS groups according to National Cholesterol Education Program Adult Treatment Panel III criteria to investigate the association between MetS and clinicopathologic characteristics, pathologic complete response (pCR), and long-term survival. The MetS group had a significantly lower likelihood of achieving pCR after NAC compared with the non-MetS group (odds ratio [OR] 0.316; P = .028), with the risk for death (OR 2.587; P = .004) and disease recurrence (OR 2.228; P = .007) being significantly higher in patients with vs without MetS. In a multivariate analysis, MetS (P = 0.028) and hormone receptors status were independent predictors of pCR after NAC in BC. These findings emphasize the importance of timely intervention of metabolic syndrome to improve outcomes in patients with BC.
Poly-(ADP-ribose) polymerase (PARP) inhibitors have emerged as essential therapeutic agents in patients with germline BRCA1/2-mutated BC. A BRCA-like phenotype is displayed by a large subset of patients with germline BRCA1/2-wildtype BC who present with homologous recombination deficiency (HRD). The randomized phase 2 S1416 trial (Rodler et al) evaluated the efficacy of cisplatin combined with the PARP inhibitor veliparib in three cohorts of metastatic BC: mutated germline BRCA1/2, BRCA-like, and non-BRCA–like. A total of 335 patients with metastatic or recurrent triple-negative BC (TNBC) or germline BRCA1/2-mutated metastatic BC were randomly assigned (1:1) to receive cisplatin plus either veliparib or a matching placebo. The findings showed that the addition of veliparib to cisplatin significantly improved progression-free survival (PFS) in patients with BRCA-like metastatic TNBC compared with placebo (5.9 vs 4.2 months; HR 0.57; log-rank P = .01), but not in mutated germline BRCA1/2 (6.2 vs 6.4 months; P = .54) and non-BRCA–like (4.0 vs 3.0 months; P = .57) groups. No new toxicity signals were observed. These findings suggest BRCA-like TNBC might show sensitivity to PARP inhibitors and therefore these agents should be explored further in this cohort.
A recent update from the combined analysis of the SOFT and TEXT studies comparing outcomes in 4690 premenopausal women with estrogen/progesterone receptor–positive early BC (Pagani et al) showed that exemestane plus ovarian function suppression (OFS) led to a greater reduction in recurrence risk compared with tamoxifen plus OFS in premenopausal women. After a median follow-up of 13 years, results showed a 4.6% absolute improvement in 12-year disease-free survival (HR 0.79; P < .001) and a 1.8% absolute improvement in disease recurrence-free interval (HR 0.83; P = .03) with exemestane plus OFS compared with tamoxifen plus OFS. These treatment effects on recurrence began to attenuate over time, being strongest in the first 5 years with no further improvement after 10 or more years. No improvement in overall survival (OS) was noted with exemestane vs tamoxifen, although both arms had excellent survival outcomes (90.1% vs 89.1%; HR 0.93; 95% CI 0.78-1.11). It is important to note that there was a 3.3% absolute improvement in 12-year OS with exemestane plus OFS among patients with HER2-negative tumors who received chemotherapy. This OS benefit was also noted amongst patients with high-risk clinicopathologic characteristics (<35 years and those with > 2 cm or grade 3 tumors), ranging from 4.0% to 5.5% absolute improvement. In conclusion, sustained recurrence risk reductions were noted with adjuvant exemestane plus OFS compared with tamoxifen plus OFS, with the most clinically meaningful survival benefit noted for patients with higher risk tumors. Proper selection of patients who are most likely to benefit from exemestane over tamoxifen is vital to maximize the survival benefit while minimizing the burden of treatment intensification.
Findings from a retrospective study including 221 women with BC who received preoperative neoadjuvant chemotherapy (NAC) showed that the presence of metabolic syndrome (MetS) worsened survival outcomes and increased disease recurrence risk (Zhou et al). Patients were divided into MetS and non-MetS groups according to National Cholesterol Education Program Adult Treatment Panel III criteria to investigate the association between MetS and clinicopathologic characteristics, pathologic complete response (pCR), and long-term survival. The MetS group had a significantly lower likelihood of achieving pCR after NAC compared with the non-MetS group (odds ratio [OR] 0.316; P = .028), with the risk for death (OR 2.587; P = .004) and disease recurrence (OR 2.228; P = .007) being significantly higher in patients with vs without MetS. In a multivariate analysis, MetS (P = 0.028) and hormone receptors status were independent predictors of pCR after NAC in BC. These findings emphasize the importance of timely intervention of metabolic syndrome to improve outcomes in patients with BC.
More New Therapeutics for Psoriasis
New treatments for psoriasis constitute an embarrassment of riches compared to any other area of dermatology. Despite the many advances over the last 25 years, additional topical and systemic treatments have recently become available. Gosh, it’s great!
In May 2022, once-daily tapinarof cream 1% was approved for the topical treatment of plaque psoriasis in adults.1 Tapinarof was identified as a metabolite made by bacteria symbiotic to a nematode, allowing the nematode to infect insects.2 Tapinarof’s anti-inflammatory effect extends to mammals. The drug works by activating the aryl hydrocarbon receptor, downregulating proinflammatory cytokines such as IL-17, and normalizing the expression of skin barrier proteins such as filaggrin.2 In two 12-week, phase 3, randomized trials with 510 and 515 patients, respectively, 35% to 40% of tapinarof-treated psoriasis patients were clear or almost clear compared with only 6% of patients in the placebo group. The drug appears safe; common adverse events (AEs) included folliculitis, nasopharyngitis, contact dermatitis, headache, upper respiratory tract infection, and pruritus.3
A second new topical treatment for plaque psoriasis was approved in July 2022—once-daily roflumilast 0.3% cream—for patients 12 years and older.4 Similar to apremilast, roflumilast is a phosphodiesterase 4 inhibitor that blocks the degradation of cAMP and reduces the downstream production of inflammatory molecules implicated in psoriasis.5 In two 8-week, phase 3 clinical trials (ClinicalTrials.gov Identifiers NCT04211363 and NCT04211389)(N=881), approximately 40% of roflumilast-treated patients were clear or almost clear vs approximately 6% in the placebo group. Topical roflumilast was well-tolerated; the most common AEs included diarrhea, headache, insomnia, nausea, application-site pain, upper respiratory tract infection, and urinary tract infection.6
We have so many patients—and many more people with psoriasis who are not yet patients—with limited psoriasis who would be amenable to topical treatment but who are not responding to current treatments. There is considerable enthusiasm for the new topicals, but it is still questionable how much they will help our patients. The main reason the current topicals fail is poor adherence to the treatment. If we give these new treatments to patients who used existing topicals and failed, thereby inadvertently selecting patients with poor adherence to topicals, it will be surprising if the new treatments live up to expectations. Perhaps tapinarof and roflumilast will revolutionize the management of localized psoriasis; perhaps their impact will be similar to topical crisaborole— exciting in trials and less practical in real life. It may be that apremilast, which is now approved for psoriasis of any severity, will make a bigger difference for patients who can access it for limited psoriasis.
Deucravacitinib is a once-daily oral selective tyrosine kinase 2 inhibitor that blocks IL-23 and type I interferon signaling. It was approved for adults with moderate to severe plaque psoriasis in September 2021.7 We know patients want oral treatment; they ask for apremilast even though injections may be much more potent. In a 16-week, phase 3 clinical trial comparing daily deucravacitinib (n=332), apremilast (n=168), and placebo (n=166), rates of clear or almost clear were approximately 55% in the deucravacitinib group, 32% in the apremilast group, and 7% with placebo. The most common AEs included nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and nausea.8 Although deucravacitinib is much more effective than apremilast, deucravacitinib will require monitoring and may have some risk for viral reactivation of herpes simplex and zoster (and hopefully not much else). Whether physicians view it as a replacement for apremilast, which requires no laboratory monitoring, remains to be seen.
Bimekizumab, a humanized monoclonal IgG1 antibody expected to receive US Food and Drug Administration approval in the coming months, inhibits both IL-17A and IL-17F and may become our most effective treatment of psoriasis. Although we are probably not hungering for a more effective psoriasis treatment (given our current embarrassment of riches), bimekizumab’s remarkably high efficacy for psoriatic arthritis may be a quantum leap forward, especially if no new safety signals are identified; bimekizumab treatment is associated with a higher risk of oral candidiasis than other currently available IL-17 antagonists.9 Biosimilars may reduce the cost of psoriasis management to the health system, but it seems unlikely that biosimilars will allow us to help patients who we cannot already help with the existing extensive psoriasis treatment armamentarium.
- Dermavant announces FDA approval for VTAMA® (Tapinarof) cream. International Psoriasis Council. Published May 26, 2022. Accessed January 10, 2023. https://www.psoriasiscouncil.org/treatment/dermavant-vtama/#:~:text=Dermavant%20Sciences%20announced%20that%20VTAMA,and%20Drug%20Administration%20(FDA)
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent [published online November 3, 2020]. J Am Acad Dermatol. 2021;84:1059-1067. doi:10.1016/j.jaad.2020.10.085
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- FDA approves Arcutis’ ZORYVE™ (Roflumilast) cream 0.3% for the treatment of plaque psoriasis in individuals age 12 and older. News release. Arcutis Biotherapeutics; July 29, 2022. Accessed January 10, 2023. https://www.arcutis.com/fda-approves-arcutis-zoryve-roflumilast-cream-0-3-for-the-treatment-of-plaque-psoriasis-in-individuals-age-12-and-older/
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;17:11:21-29. doi:10.2147/PTT.S303634
- Zoryve. Package insert. Arcutis Biotherapeutics; 2022.
- Hoy SM. Deucravacitinib: first approval. Drugs. 2022;82:1671-1679. doi:10.1007/s40265-022-01796-y
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis [published online October 8, 2021]. Drugs. 2021;81:1751-1762. doi:10.1007/s40265-021-01612-z
New treatments for psoriasis constitute an embarrassment of riches compared to any other area of dermatology. Despite the many advances over the last 25 years, additional topical and systemic treatments have recently become available. Gosh, it’s great!
In May 2022, once-daily tapinarof cream 1% was approved for the topical treatment of plaque psoriasis in adults.1 Tapinarof was identified as a metabolite made by bacteria symbiotic to a nematode, allowing the nematode to infect insects.2 Tapinarof’s anti-inflammatory effect extends to mammals. The drug works by activating the aryl hydrocarbon receptor, downregulating proinflammatory cytokines such as IL-17, and normalizing the expression of skin barrier proteins such as filaggrin.2 In two 12-week, phase 3, randomized trials with 510 and 515 patients, respectively, 35% to 40% of tapinarof-treated psoriasis patients were clear or almost clear compared with only 6% of patients in the placebo group. The drug appears safe; common adverse events (AEs) included folliculitis, nasopharyngitis, contact dermatitis, headache, upper respiratory tract infection, and pruritus.3
A second new topical treatment for plaque psoriasis was approved in July 2022—once-daily roflumilast 0.3% cream—for patients 12 years and older.4 Similar to apremilast, roflumilast is a phosphodiesterase 4 inhibitor that blocks the degradation of cAMP and reduces the downstream production of inflammatory molecules implicated in psoriasis.5 In two 8-week, phase 3 clinical trials (ClinicalTrials.gov Identifiers NCT04211363 and NCT04211389)(N=881), approximately 40% of roflumilast-treated patients were clear or almost clear vs approximately 6% in the placebo group. Topical roflumilast was well-tolerated; the most common AEs included diarrhea, headache, insomnia, nausea, application-site pain, upper respiratory tract infection, and urinary tract infection.6
We have so many patients—and many more people with psoriasis who are not yet patients—with limited psoriasis who would be amenable to topical treatment but who are not responding to current treatments. There is considerable enthusiasm for the new topicals, but it is still questionable how much they will help our patients. The main reason the current topicals fail is poor adherence to the treatment. If we give these new treatments to patients who used existing topicals and failed, thereby inadvertently selecting patients with poor adherence to topicals, it will be surprising if the new treatments live up to expectations. Perhaps tapinarof and roflumilast will revolutionize the management of localized psoriasis; perhaps their impact will be similar to topical crisaborole— exciting in trials and less practical in real life. It may be that apremilast, which is now approved for psoriasis of any severity, will make a bigger difference for patients who can access it for limited psoriasis.
Deucravacitinib is a once-daily oral selective tyrosine kinase 2 inhibitor that blocks IL-23 and type I interferon signaling. It was approved for adults with moderate to severe plaque psoriasis in September 2021.7 We know patients want oral treatment; they ask for apremilast even though injections may be much more potent. In a 16-week, phase 3 clinical trial comparing daily deucravacitinib (n=332), apremilast (n=168), and placebo (n=166), rates of clear or almost clear were approximately 55% in the deucravacitinib group, 32% in the apremilast group, and 7% with placebo. The most common AEs included nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and nausea.8 Although deucravacitinib is much more effective than apremilast, deucravacitinib will require monitoring and may have some risk for viral reactivation of herpes simplex and zoster (and hopefully not much else). Whether physicians view it as a replacement for apremilast, which requires no laboratory monitoring, remains to be seen.
Bimekizumab, a humanized monoclonal IgG1 antibody expected to receive US Food and Drug Administration approval in the coming months, inhibits both IL-17A and IL-17F and may become our most effective treatment of psoriasis. Although we are probably not hungering for a more effective psoriasis treatment (given our current embarrassment of riches), bimekizumab’s remarkably high efficacy for psoriatic arthritis may be a quantum leap forward, especially if no new safety signals are identified; bimekizumab treatment is associated with a higher risk of oral candidiasis than other currently available IL-17 antagonists.9 Biosimilars may reduce the cost of psoriasis management to the health system, but it seems unlikely that biosimilars will allow us to help patients who we cannot already help with the existing extensive psoriasis treatment armamentarium.
New treatments for psoriasis constitute an embarrassment of riches compared to any other area of dermatology. Despite the many advances over the last 25 years, additional topical and systemic treatments have recently become available. Gosh, it’s great!
In May 2022, once-daily tapinarof cream 1% was approved for the topical treatment of plaque psoriasis in adults.1 Tapinarof was identified as a metabolite made by bacteria symbiotic to a nematode, allowing the nematode to infect insects.2 Tapinarof’s anti-inflammatory effect extends to mammals. The drug works by activating the aryl hydrocarbon receptor, downregulating proinflammatory cytokines such as IL-17, and normalizing the expression of skin barrier proteins such as filaggrin.2 In two 12-week, phase 3, randomized trials with 510 and 515 patients, respectively, 35% to 40% of tapinarof-treated psoriasis patients were clear or almost clear compared with only 6% of patients in the placebo group. The drug appears safe; common adverse events (AEs) included folliculitis, nasopharyngitis, contact dermatitis, headache, upper respiratory tract infection, and pruritus.3
A second new topical treatment for plaque psoriasis was approved in July 2022—once-daily roflumilast 0.3% cream—for patients 12 years and older.4 Similar to apremilast, roflumilast is a phosphodiesterase 4 inhibitor that blocks the degradation of cAMP and reduces the downstream production of inflammatory molecules implicated in psoriasis.5 In two 8-week, phase 3 clinical trials (ClinicalTrials.gov Identifiers NCT04211363 and NCT04211389)(N=881), approximately 40% of roflumilast-treated patients were clear or almost clear vs approximately 6% in the placebo group. Topical roflumilast was well-tolerated; the most common AEs included diarrhea, headache, insomnia, nausea, application-site pain, upper respiratory tract infection, and urinary tract infection.6
We have so many patients—and many more people with psoriasis who are not yet patients—with limited psoriasis who would be amenable to topical treatment but who are not responding to current treatments. There is considerable enthusiasm for the new topicals, but it is still questionable how much they will help our patients. The main reason the current topicals fail is poor adherence to the treatment. If we give these new treatments to patients who used existing topicals and failed, thereby inadvertently selecting patients with poor adherence to topicals, it will be surprising if the new treatments live up to expectations. Perhaps tapinarof and roflumilast will revolutionize the management of localized psoriasis; perhaps their impact will be similar to topical crisaborole— exciting in trials and less practical in real life. It may be that apremilast, which is now approved for psoriasis of any severity, will make a bigger difference for patients who can access it for limited psoriasis.
Deucravacitinib is a once-daily oral selective tyrosine kinase 2 inhibitor that blocks IL-23 and type I interferon signaling. It was approved for adults with moderate to severe plaque psoriasis in September 2021.7 We know patients want oral treatment; they ask for apremilast even though injections may be much more potent. In a 16-week, phase 3 clinical trial comparing daily deucravacitinib (n=332), apremilast (n=168), and placebo (n=166), rates of clear or almost clear were approximately 55% in the deucravacitinib group, 32% in the apremilast group, and 7% with placebo. The most common AEs included nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and nausea.8 Although deucravacitinib is much more effective than apremilast, deucravacitinib will require monitoring and may have some risk for viral reactivation of herpes simplex and zoster (and hopefully not much else). Whether physicians view it as a replacement for apremilast, which requires no laboratory monitoring, remains to be seen.
Bimekizumab, a humanized monoclonal IgG1 antibody expected to receive US Food and Drug Administration approval in the coming months, inhibits both IL-17A and IL-17F and may become our most effective treatment of psoriasis. Although we are probably not hungering for a more effective psoriasis treatment (given our current embarrassment of riches), bimekizumab’s remarkably high efficacy for psoriatic arthritis may be a quantum leap forward, especially if no new safety signals are identified; bimekizumab treatment is associated with a higher risk of oral candidiasis than other currently available IL-17 antagonists.9 Biosimilars may reduce the cost of psoriasis management to the health system, but it seems unlikely that biosimilars will allow us to help patients who we cannot already help with the existing extensive psoriasis treatment armamentarium.
- Dermavant announces FDA approval for VTAMA® (Tapinarof) cream. International Psoriasis Council. Published May 26, 2022. Accessed January 10, 2023. https://www.psoriasiscouncil.org/treatment/dermavant-vtama/#:~:text=Dermavant%20Sciences%20announced%20that%20VTAMA,and%20Drug%20Administration%20(FDA)
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent [published online November 3, 2020]. J Am Acad Dermatol. 2021;84:1059-1067. doi:10.1016/j.jaad.2020.10.085
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- FDA approves Arcutis’ ZORYVE™ (Roflumilast) cream 0.3% for the treatment of plaque psoriasis in individuals age 12 and older. News release. Arcutis Biotherapeutics; July 29, 2022. Accessed January 10, 2023. https://www.arcutis.com/fda-approves-arcutis-zoryve-roflumilast-cream-0-3-for-the-treatment-of-plaque-psoriasis-in-individuals-age-12-and-older/
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;17:11:21-29. doi:10.2147/PTT.S303634
- Zoryve. Package insert. Arcutis Biotherapeutics; 2022.
- Hoy SM. Deucravacitinib: first approval. Drugs. 2022;82:1671-1679. doi:10.1007/s40265-022-01796-y
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis [published online October 8, 2021]. Drugs. 2021;81:1751-1762. doi:10.1007/s40265-021-01612-z
- Dermavant announces FDA approval for VTAMA® (Tapinarof) cream. International Psoriasis Council. Published May 26, 2022. Accessed January 10, 2023. https://www.psoriasiscouncil.org/treatment/dermavant-vtama/#:~:text=Dermavant%20Sciences%20announced%20that%20VTAMA,and%20Drug%20Administration%20(FDA)
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent [published online November 3, 2020]. J Am Acad Dermatol. 2021;84:1059-1067. doi:10.1016/j.jaad.2020.10.085
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- FDA approves Arcutis’ ZORYVE™ (Roflumilast) cream 0.3% for the treatment of plaque psoriasis in individuals age 12 and older. News release. Arcutis Biotherapeutics; July 29, 2022. Accessed January 10, 2023. https://www.arcutis.com/fda-approves-arcutis-zoryve-roflumilast-cream-0-3-for-the-treatment-of-plaque-psoriasis-in-individuals-age-12-and-older/
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;17:11:21-29. doi:10.2147/PTT.S303634
- Zoryve. Package insert. Arcutis Biotherapeutics; 2022.
- Hoy SM. Deucravacitinib: first approval. Drugs. 2022;82:1671-1679. doi:10.1007/s40265-022-01796-y
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis [published online October 8, 2021]. Drugs. 2021;81:1751-1762. doi:10.1007/s40265-021-01612-z
New Treatments for Psoriasis: An Update on a Therapeutic Frontier
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
PRACTICE POINTS
- Roflumilast, a phosphodiesterase 4 inhibitor, and tapinarof, an aryl hydrocarbon receptor–modulating agent, are 2 novel nonsteroidal topical treatments safe for regular long-term use on all affected areas of the skin in adult patients with plaque psoriasis.
- Deucravacitinib is an oral selective tyrosine kinase 2 allosteric inhibitor that has demonstrated a favorable safety profile and greater levels of efficacy than other available oral medications for plaque psoriasis.
- The dual inhibition of IL-17A and IL-17F with bimekizumab provides faster responses and greater clinical benefits for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone, achieving higher levels of efficacy than has been reported with any other biologic therapy.
- Spesolimab, an IL-36 receptor inhibitor, is an effective, US Food and Drug Administration–approved treatment for patients with generalized pustular psoriasis.
CDC updates guidance on opioid prescribing in adults
The Centers for Disease Control and Prevention (CDC) recently published updated guidelines on prescribing opioids for pain that stress the need for a flexible and individual approach to pain management.1 New recommendations emphasize the use of nonopioid therapies whenever appropriate, support consideration of opioid therapy for patients with acute pain when the benefits are expected to outweigh the risks, and urge clinicians to work with patients receiving opioid therapy to determine whether it should be continued or tapered.
This revision to the agency’s 2016 guidelines is aimed at primary care clinicians who prescribe opioids to adult outpatients for treatment of pain. The recommendations are not meant for patients with sickle-cell disease or cancer-related pain, or those receiving palliative and end-of-life care.
Why an update was needed. In 2021, more than 107,000 Americans died of a drug overdose.2 Although prescription opioids caused only about 16% of these deaths, they account for a population death rate of 4:100,000—which, despite national efforts, has not changed much since 2013.3,4
Following publication of the CDC’s 2016 guidelines on prescribing opioids for chronic pain,5 there was a decline in opioid prescribing but not in related deaths. Furthermore, there appeared to have been some negative effects of reduced prescribing, including untreated and undertreated pain, and rapid tapering or sudden discontinuation of opioids in chronic users, causing withdrawal symptoms and psychological distress in these patients. To address these issues, the CDC published the new guideline in 2022.1
Categories of pain. The guideline panel classified pain into 3 categories: acute pain (duration of < 1 month), subacute pain (duration of 1-3 months), and chronic pain (duration of > 3 months).
When to prescribe opioids. The guidelines recommend a new approach to deciding whether to prescribe opioid therapy. In most cases, nonopioid options—such as nonsteroidal anti-inflammatory drugs (NSAIDs) and exercise—should be tried first, since they are as effective as opioids for many types of acute, subacute, and chronic pain. Opioids should be considered if these options fail and the potential benefits outweigh the risks. In moderate-to-severe acute pain, opioids are an option if NSAIDs are unlikely to be effective or are contraindicated.1
How to prescribe opioids. Before prescribing opioids, clinicians should discuss with the patient the known risks and benefits and offer an accompanying prescription for naloxone. Opioids should be prescribed at the lowest effective dose and for a time period limited to the expected duration of the pain. When starting opioids, immediate-release opioids should be prescribed instead of extended-release or long-acting opioids.1
Precautionary measures. Clinicians should review the patient’s history of controlled substance prescriptions via their state’s prescription drug monitoring program and consider the use of toxicology testing to determine whether the patient is receiving high-risk opioid dosages or combinations. Clinicians should be especially cautious about prescribing opioids and benzodiazepines concurrently.1
Continue or stop opioid treatment? A new recommendation advises clinicians to individually assess the benefits and risks of continuing therapy for patients who have been receiving opioids chronically. Whenever the decision is made to stop or reduce treatment, remember that opioid therapy should not be stopped abruptly or reduced quickly. The guideline panel suggests tapering by 10% per month.1
Finally, patients with opioid use disorder should be offered or referred for treatment with medications. Detoxification alone, without medication, is not recommended.1
1. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
2. CDC. US overdose deaths in 2021 increased half as much as in 2020—but are still up 15%. Published May 11, 2022. Accessed January 25, 2023. www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm
3. CDC. SUDORS Dashboard: fatal overdose data. Updated December 8, 2022. Accessed January 25, 2023. www.cdc.gov/drugoverdose/fatal/dashboard/index.html
4. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-207. doi: 10.15585/mmwr.mm7006a4
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49. doi: 10.15585/mmwr.rr6501e1:26987082
The Centers for Disease Control and Prevention (CDC) recently published updated guidelines on prescribing opioids for pain that stress the need for a flexible and individual approach to pain management.1 New recommendations emphasize the use of nonopioid therapies whenever appropriate, support consideration of opioid therapy for patients with acute pain when the benefits are expected to outweigh the risks, and urge clinicians to work with patients receiving opioid therapy to determine whether it should be continued or tapered.
This revision to the agency’s 2016 guidelines is aimed at primary care clinicians who prescribe opioids to adult outpatients for treatment of pain. The recommendations are not meant for patients with sickle-cell disease or cancer-related pain, or those receiving palliative and end-of-life care.
Why an update was needed. In 2021, more than 107,000 Americans died of a drug overdose.2 Although prescription opioids caused only about 16% of these deaths, they account for a population death rate of 4:100,000—which, despite national efforts, has not changed much since 2013.3,4
Following publication of the CDC’s 2016 guidelines on prescribing opioids for chronic pain,5 there was a decline in opioid prescribing but not in related deaths. Furthermore, there appeared to have been some negative effects of reduced prescribing, including untreated and undertreated pain, and rapid tapering or sudden discontinuation of opioids in chronic users, causing withdrawal symptoms and psychological distress in these patients. To address these issues, the CDC published the new guideline in 2022.1
Categories of pain. The guideline panel classified pain into 3 categories: acute pain (duration of < 1 month), subacute pain (duration of 1-3 months), and chronic pain (duration of > 3 months).
When to prescribe opioids. The guidelines recommend a new approach to deciding whether to prescribe opioid therapy. In most cases, nonopioid options—such as nonsteroidal anti-inflammatory drugs (NSAIDs) and exercise—should be tried first, since they are as effective as opioids for many types of acute, subacute, and chronic pain. Opioids should be considered if these options fail and the potential benefits outweigh the risks. In moderate-to-severe acute pain, opioids are an option if NSAIDs are unlikely to be effective or are contraindicated.1
How to prescribe opioids. Before prescribing opioids, clinicians should discuss with the patient the known risks and benefits and offer an accompanying prescription for naloxone. Opioids should be prescribed at the lowest effective dose and for a time period limited to the expected duration of the pain. When starting opioids, immediate-release opioids should be prescribed instead of extended-release or long-acting opioids.1
Precautionary measures. Clinicians should review the patient’s history of controlled substance prescriptions via their state’s prescription drug monitoring program and consider the use of toxicology testing to determine whether the patient is receiving high-risk opioid dosages or combinations. Clinicians should be especially cautious about prescribing opioids and benzodiazepines concurrently.1
Continue or stop opioid treatment? A new recommendation advises clinicians to individually assess the benefits and risks of continuing therapy for patients who have been receiving opioids chronically. Whenever the decision is made to stop or reduce treatment, remember that opioid therapy should not be stopped abruptly or reduced quickly. The guideline panel suggests tapering by 10% per month.1
Finally, patients with opioid use disorder should be offered or referred for treatment with medications. Detoxification alone, without medication, is not recommended.1
The Centers for Disease Control and Prevention (CDC) recently published updated guidelines on prescribing opioids for pain that stress the need for a flexible and individual approach to pain management.1 New recommendations emphasize the use of nonopioid therapies whenever appropriate, support consideration of opioid therapy for patients with acute pain when the benefits are expected to outweigh the risks, and urge clinicians to work with patients receiving opioid therapy to determine whether it should be continued or tapered.
This revision to the agency’s 2016 guidelines is aimed at primary care clinicians who prescribe opioids to adult outpatients for treatment of pain. The recommendations are not meant for patients with sickle-cell disease or cancer-related pain, or those receiving palliative and end-of-life care.
Why an update was needed. In 2021, more than 107,000 Americans died of a drug overdose.2 Although prescription opioids caused only about 16% of these deaths, they account for a population death rate of 4:100,000—which, despite national efforts, has not changed much since 2013.3,4
Following publication of the CDC’s 2016 guidelines on prescribing opioids for chronic pain,5 there was a decline in opioid prescribing but not in related deaths. Furthermore, there appeared to have been some negative effects of reduced prescribing, including untreated and undertreated pain, and rapid tapering or sudden discontinuation of opioids in chronic users, causing withdrawal symptoms and psychological distress in these patients. To address these issues, the CDC published the new guideline in 2022.1
Categories of pain. The guideline panel classified pain into 3 categories: acute pain (duration of < 1 month), subacute pain (duration of 1-3 months), and chronic pain (duration of > 3 months).
When to prescribe opioids. The guidelines recommend a new approach to deciding whether to prescribe opioid therapy. In most cases, nonopioid options—such as nonsteroidal anti-inflammatory drugs (NSAIDs) and exercise—should be tried first, since they are as effective as opioids for many types of acute, subacute, and chronic pain. Opioids should be considered if these options fail and the potential benefits outweigh the risks. In moderate-to-severe acute pain, opioids are an option if NSAIDs are unlikely to be effective or are contraindicated.1
How to prescribe opioids. Before prescribing opioids, clinicians should discuss with the patient the known risks and benefits and offer an accompanying prescription for naloxone. Opioids should be prescribed at the lowest effective dose and for a time period limited to the expected duration of the pain. When starting opioids, immediate-release opioids should be prescribed instead of extended-release or long-acting opioids.1
Precautionary measures. Clinicians should review the patient’s history of controlled substance prescriptions via their state’s prescription drug monitoring program and consider the use of toxicology testing to determine whether the patient is receiving high-risk opioid dosages or combinations. Clinicians should be especially cautious about prescribing opioids and benzodiazepines concurrently.1
Continue or stop opioid treatment? A new recommendation advises clinicians to individually assess the benefits and risks of continuing therapy for patients who have been receiving opioids chronically. Whenever the decision is made to stop or reduce treatment, remember that opioid therapy should not be stopped abruptly or reduced quickly. The guideline panel suggests tapering by 10% per month.1
Finally, patients with opioid use disorder should be offered or referred for treatment with medications. Detoxification alone, without medication, is not recommended.1
1. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
2. CDC. US overdose deaths in 2021 increased half as much as in 2020—but are still up 15%. Published May 11, 2022. Accessed January 25, 2023. www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm
3. CDC. SUDORS Dashboard: fatal overdose data. Updated December 8, 2022. Accessed January 25, 2023. www.cdc.gov/drugoverdose/fatal/dashboard/index.html
4. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-207. doi: 10.15585/mmwr.mm7006a4
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49. doi: 10.15585/mmwr.rr6501e1:26987082
1. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
2. CDC. US overdose deaths in 2021 increased half as much as in 2020—but are still up 15%. Published May 11, 2022. Accessed January 25, 2023. www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm
3. CDC. SUDORS Dashboard: fatal overdose data. Updated December 8, 2022. Accessed January 25, 2023. www.cdc.gov/drugoverdose/fatal/dashboard/index.html
4. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-207. doi: 10.15585/mmwr.mm7006a4
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49. doi: 10.15585/mmwr.rr6501e1:26987082