User login
Difficulty fitting family into career: Female oncologists
In a survey of just over 1,000 female oncologists, 95% said their career plans were at least somewhat associated with the timing of when to start a family.
The most striking finding was that one third of respondents had miscarried and another one third reported difficulty with infertility that required fertility counseling and/or treatment.
One third reported experiencing discrimination during pregnancy, and another third said they experienced discrimination for taking maternity leave, and having more than one child increased the likelihood of this.
The most common negative factor associated with family planning was long work hours and heavy workload (66.6%),
These findings suggest there are systemic changes needed not only in the healthcare setting but in society as a whole around women in the workplace and their choices of childbearing, say the authors.
The study was published online in JAMA Network Open and led by Anna Lee MD, MPH, from the department of radiation oncology, University of Texas MD Anderson Cancer Center, Houston.
In an invited commentary, Mona Saleh, MD, and Stephanie Blank, MD, from the department of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai in New York, suggest that cultural changes are needed that go beyond women in medicine.
“These cultural values are so deeply pervasive (one could also say invasive) that they affect even these most educated and wealthy professional women, such as those who participated in this survey,” the editorialists write.
“[The researchers] advocate for early education on assisted reproductive technology (ART) risks, benefits, and success rates, but this is not getting at the underlying issue: Pregnancy discrimination and unfair distribution of childbearing responsibilities are a reflection of a larger problematic culture rather than an issue specific to women in medicine,” they add.
Survey details
The survey comprised a novel 39-item questionnaire distributed to 1,004 U.S. female oncologists from May 7 to June 30, 2020, via email and social media channels.
Most respondents (84.4%) were married, and 71% were currently working full-time.
About one-third (35%) worked in radiation oncology, another third (34.3%) in medical oncology, 18.4% in surgical oncology, and 9.1% in pediatric oncology.
A total of 768 respondents (76.5%) had children, and of these, 415 (41.3%) first gave birth during postgraduate training and 275 (27.4%) gave birth in years 1-5 as an attending physician.
Of all respondents who had been pregnant, approximately two-thirds (65.7%) had some type of pregnancy complication. About one-third of respondents (31.7%) reported having experienced a miscarriage after a confirmed pregnancy; of those, 61.6% reported one miscarriage, while the remainder had two or more miscarriages (38.4%).
Approximately one-third (31.4%) of respondents reported difficulty with infertility that required fertility counseling and/or treatment.
The questionnaire also asked about assisted reproductive technology, and 164 participants (16.3%) reported the use of fertility medications, and 53 (5.3%) reported cryopreservation of eggs. Nearly 13% reported the use of intrauterine insemination and 13.2% reported the use of in vivo fertilization. Among those who experienced fertility concerns, 36.6% (232 of 634) reported facing financial burdens because of fertility or pregnancy that was in some way associated with their career choice.
When asked on the survey if fertility preservation should be discussed with women during medical school and/or residency, 65.7% of respondents stated that it should.
However, the editorialists suggest that “encouraging formal and directed education regarding the infertility risks specifically toward female physicians (which Lee et al. recommend) could be perceived as a blanket recommendation that it is best for women in medicine to delay childbearing and pursue ART.”
“Medical schools and residency and fellowship training programs should instead focus their energy on creating a framework and culture that normalizes conception during these points in training while also subsidizing and supporting trainees and physicians who prefer to use ART and delay fertility until after training,” they suggest.
The editorialists also emphasized that women may choose to become pregnant at any point during the years that it takes to go from being a medical student to resident/fellow to attending physician, and they should be supported by their workplace on their decisions.
The study was funded by grants from National Institutes of Health/National Cancer Institute Cancer Center.
Dr. Lee and coauthors reported no relevant financial relationships. Dr. Blank reported receiving grants from AstraZeneca, Aravive, Akesobio, GlaxoSmithKline, Merck, and Seattle Genetics outside the submitted work. Dr. Saleh reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a survey of just over 1,000 female oncologists, 95% said their career plans were at least somewhat associated with the timing of when to start a family.
The most striking finding was that one third of respondents had miscarried and another one third reported difficulty with infertility that required fertility counseling and/or treatment.
One third reported experiencing discrimination during pregnancy, and another third said they experienced discrimination for taking maternity leave, and having more than one child increased the likelihood of this.
The most common negative factor associated with family planning was long work hours and heavy workload (66.6%),
These findings suggest there are systemic changes needed not only in the healthcare setting but in society as a whole around women in the workplace and their choices of childbearing, say the authors.
The study was published online in JAMA Network Open and led by Anna Lee MD, MPH, from the department of radiation oncology, University of Texas MD Anderson Cancer Center, Houston.
In an invited commentary, Mona Saleh, MD, and Stephanie Blank, MD, from the department of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai in New York, suggest that cultural changes are needed that go beyond women in medicine.
“These cultural values are so deeply pervasive (one could also say invasive) that they affect even these most educated and wealthy professional women, such as those who participated in this survey,” the editorialists write.
“[The researchers] advocate for early education on assisted reproductive technology (ART) risks, benefits, and success rates, but this is not getting at the underlying issue: Pregnancy discrimination and unfair distribution of childbearing responsibilities are a reflection of a larger problematic culture rather than an issue specific to women in medicine,” they add.
Survey details
The survey comprised a novel 39-item questionnaire distributed to 1,004 U.S. female oncologists from May 7 to June 30, 2020, via email and social media channels.
Most respondents (84.4%) were married, and 71% were currently working full-time.
About one-third (35%) worked in radiation oncology, another third (34.3%) in medical oncology, 18.4% in surgical oncology, and 9.1% in pediatric oncology.
A total of 768 respondents (76.5%) had children, and of these, 415 (41.3%) first gave birth during postgraduate training and 275 (27.4%) gave birth in years 1-5 as an attending physician.
Of all respondents who had been pregnant, approximately two-thirds (65.7%) had some type of pregnancy complication. About one-third of respondents (31.7%) reported having experienced a miscarriage after a confirmed pregnancy; of those, 61.6% reported one miscarriage, while the remainder had two or more miscarriages (38.4%).
Approximately one-third (31.4%) of respondents reported difficulty with infertility that required fertility counseling and/or treatment.
The questionnaire also asked about assisted reproductive technology, and 164 participants (16.3%) reported the use of fertility medications, and 53 (5.3%) reported cryopreservation of eggs. Nearly 13% reported the use of intrauterine insemination and 13.2% reported the use of in vivo fertilization. Among those who experienced fertility concerns, 36.6% (232 of 634) reported facing financial burdens because of fertility or pregnancy that was in some way associated with their career choice.
When asked on the survey if fertility preservation should be discussed with women during medical school and/or residency, 65.7% of respondents stated that it should.
However, the editorialists suggest that “encouraging formal and directed education regarding the infertility risks specifically toward female physicians (which Lee et al. recommend) could be perceived as a blanket recommendation that it is best for women in medicine to delay childbearing and pursue ART.”
“Medical schools and residency and fellowship training programs should instead focus their energy on creating a framework and culture that normalizes conception during these points in training while also subsidizing and supporting trainees and physicians who prefer to use ART and delay fertility until after training,” they suggest.
The editorialists also emphasized that women may choose to become pregnant at any point during the years that it takes to go from being a medical student to resident/fellow to attending physician, and they should be supported by their workplace on their decisions.
The study was funded by grants from National Institutes of Health/National Cancer Institute Cancer Center.
Dr. Lee and coauthors reported no relevant financial relationships. Dr. Blank reported receiving grants from AstraZeneca, Aravive, Akesobio, GlaxoSmithKline, Merck, and Seattle Genetics outside the submitted work. Dr. Saleh reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a survey of just over 1,000 female oncologists, 95% said their career plans were at least somewhat associated with the timing of when to start a family.
The most striking finding was that one third of respondents had miscarried and another one third reported difficulty with infertility that required fertility counseling and/or treatment.
One third reported experiencing discrimination during pregnancy, and another third said they experienced discrimination for taking maternity leave, and having more than one child increased the likelihood of this.
The most common negative factor associated with family planning was long work hours and heavy workload (66.6%),
These findings suggest there are systemic changes needed not only in the healthcare setting but in society as a whole around women in the workplace and their choices of childbearing, say the authors.
The study was published online in JAMA Network Open and led by Anna Lee MD, MPH, from the department of radiation oncology, University of Texas MD Anderson Cancer Center, Houston.
In an invited commentary, Mona Saleh, MD, and Stephanie Blank, MD, from the department of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai in New York, suggest that cultural changes are needed that go beyond women in medicine.
“These cultural values are so deeply pervasive (one could also say invasive) that they affect even these most educated and wealthy professional women, such as those who participated in this survey,” the editorialists write.
“[The researchers] advocate for early education on assisted reproductive technology (ART) risks, benefits, and success rates, but this is not getting at the underlying issue: Pregnancy discrimination and unfair distribution of childbearing responsibilities are a reflection of a larger problematic culture rather than an issue specific to women in medicine,” they add.
Survey details
The survey comprised a novel 39-item questionnaire distributed to 1,004 U.S. female oncologists from May 7 to June 30, 2020, via email and social media channels.
Most respondents (84.4%) were married, and 71% were currently working full-time.
About one-third (35%) worked in radiation oncology, another third (34.3%) in medical oncology, 18.4% in surgical oncology, and 9.1% in pediatric oncology.
A total of 768 respondents (76.5%) had children, and of these, 415 (41.3%) first gave birth during postgraduate training and 275 (27.4%) gave birth in years 1-5 as an attending physician.
Of all respondents who had been pregnant, approximately two-thirds (65.7%) had some type of pregnancy complication. About one-third of respondents (31.7%) reported having experienced a miscarriage after a confirmed pregnancy; of those, 61.6% reported one miscarriage, while the remainder had two or more miscarriages (38.4%).
Approximately one-third (31.4%) of respondents reported difficulty with infertility that required fertility counseling and/or treatment.
The questionnaire also asked about assisted reproductive technology, and 164 participants (16.3%) reported the use of fertility medications, and 53 (5.3%) reported cryopreservation of eggs. Nearly 13% reported the use of intrauterine insemination and 13.2% reported the use of in vivo fertilization. Among those who experienced fertility concerns, 36.6% (232 of 634) reported facing financial burdens because of fertility or pregnancy that was in some way associated with their career choice.
When asked on the survey if fertility preservation should be discussed with women during medical school and/or residency, 65.7% of respondents stated that it should.
However, the editorialists suggest that “encouraging formal and directed education regarding the infertility risks specifically toward female physicians (which Lee et al. recommend) could be perceived as a blanket recommendation that it is best for women in medicine to delay childbearing and pursue ART.”
“Medical schools and residency and fellowship training programs should instead focus their energy on creating a framework and culture that normalizes conception during these points in training while also subsidizing and supporting trainees and physicians who prefer to use ART and delay fertility until after training,” they suggest.
The editorialists also emphasized that women may choose to become pregnant at any point during the years that it takes to go from being a medical student to resident/fellow to attending physician, and they should be supported by their workplace on their decisions.
The study was funded by grants from National Institutes of Health/National Cancer Institute Cancer Center.
Dr. Lee and coauthors reported no relevant financial relationships. Dr. Blank reported receiving grants from AstraZeneca, Aravive, Akesobio, GlaxoSmithKline, Merck, and Seattle Genetics outside the submitted work. Dr. Saleh reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Poor sleep quality as a teen may up MS risk in adulthood
Too little sleep or poor sleep quality during the teen years can significantly increase the risk for multiple sclerosis (MS) during adulthood, new research suggests.
In a large case-control study, individuals who slept less than 7 hours a night on average during adolescence were 40% more likely to develop MS later on. The risk was even higher for those who rated their sleep quality as bad.
On the other hand, MS was significantly less common among individuals who slept longer as teens – indicating a possible protective benefit.
While sleep duration has been associated with mortality or disease risk for other conditions, sleep quality usually has little to no effect on risk, lead investigator Torbjörn Åkerstedt, PhD, sleep researcher and professor of psychology, department of neuroscience, Karolinska Institutet, Stockholm, told this news organization.
“I hadn’t really expected that, but those results were quite strong, even stronger than sleep duration,” Dr. Åkerstedt said.
“We don’t really know why this is happening in young age, but the most suitable explanation is that the brain in still developing quite a bit, and you’re interfering with it,” he added.
The findings were published online in the Journal of Neurology, Neurosurgery and Psychiatry.
Strong association
Other studies have tied sleep deprivation to increased risk for serious illness, but the link between sleep and MS risk isn’t as well studied.
Previous research by Dr. Åkerstedt showed that the risk for MS was higher among individuals who took part in shift work before the age of 20. However, the impact of sleep duration or quality among teens was unknown.
The current Swedish population-based case-control study included 2,075 patients with MS and 3,164 without the disorder. All participants were asked to recall how many hours on average they slept per night between the ages of 15 and 19 years and to rate their sleep quality during that time.
Results showed that individuals who slept fewer than 7 hours a night during their teen years were 40% more likely to have MS as adults (odds ratio [OR], 1.4; 95% confidence interval [CI], 1.1-1.7).
Poor sleep quality increased MS risk even more (OR, 1.5; 95% CI, 1.3-1.9).
The association remained strong even after adjustment for additional sleep on weekends and breaks and excluding shift workers.
Long sleep ‘apparently good’
The researchers also conducted several sensitivity studies to rule out confounders that might bias the association, such as excluding participants who reported currently experiencing less sleep or poor sleep.
“You would expect that people who are suffering from sleep problems today would be the people who reported sleep problems during their youth,” but that didn’t happen, Dr. Åkerstedt noted.
The investigators also entered data on sleep duration and sleep quality at the same time, thinking the data would cancel each other out. However, the association remained the same.
“Quite often you see that sleep duration would eliminate the effect of sleep complaints in the prediction of disease, but here both remain significant when they are entered at the same time,” Dr. Åkerstedt said. “You get the feeling that this might mean they act together to produce results,” he added.
“One other thing that surprised me is that long sleep was apparently good,” said Dr. Åkerstedt.
The investigators have conducted several studies on sleep duration and mortality. In recent research, they found that both short sleep and long sleep predicted mortality – “and often, long sleep is a stronger predictor than short sleep,” he said.
Underestimated problem?
Commenting on the findings, Kathleen Zackowski, PhD, associate vice president of research for the National Multiple Sclerosis Society in Baltimore, noted that participants were asked to rate their own sleep quality during adolescence, a subjective report that may mean sleep quality has an even larger association with MS risk.
“That they found a result with sleep quality says to me that there probably is a bigger problem, because I don’t know if people over- or underestimate their sleep quality,” said Dr. Zackowski, who was not involved with the research.
“If we could get to that sleep quality question a little more objectively, I bet that we’d find there’s a lot more to the story,” she said.
That’s a story the researchers would like to explore, Dr. Åkerstedt reported. Designing a prospective study that more closely tracks sleeping habits during adolescence and follows individuals through adulthood could provide valuable information about how sleep quality and duration affect immune system development and MS risk, he said.
Dr. Zackowski said clinicians know that MS is not caused just by a genetic abnormality and that other environmental lifestyle factors seem to play a part.
“If we find out that sleep is one of those lifestyle factors, this is very changeable,” she added.
The study was funded by the Swedish Research Council, the Swedish Research Council for Health, Working Life and Welfare, the Swedish Brain Foundation, AFA Insurance, the European Aviation Safety Authority, the Tercentenary Fund of the Bank of Sweden, the Margaretha af Ugglas Foundation, the Swedish Foundation for MS Research, and NEURO Sweden. Dr. Åkerstadt has been supported by Tercentenary Fund of Bank of Sweden, AFA Insurance, and the European Aviation Safety Authority. Dr. Zackowski reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Too little sleep or poor sleep quality during the teen years can significantly increase the risk for multiple sclerosis (MS) during adulthood, new research suggests.
In a large case-control study, individuals who slept less than 7 hours a night on average during adolescence were 40% more likely to develop MS later on. The risk was even higher for those who rated their sleep quality as bad.
On the other hand, MS was significantly less common among individuals who slept longer as teens – indicating a possible protective benefit.
While sleep duration has been associated with mortality or disease risk for other conditions, sleep quality usually has little to no effect on risk, lead investigator Torbjörn Åkerstedt, PhD, sleep researcher and professor of psychology, department of neuroscience, Karolinska Institutet, Stockholm, told this news organization.
“I hadn’t really expected that, but those results were quite strong, even stronger than sleep duration,” Dr. Åkerstedt said.
“We don’t really know why this is happening in young age, but the most suitable explanation is that the brain in still developing quite a bit, and you’re interfering with it,” he added.
The findings were published online in the Journal of Neurology, Neurosurgery and Psychiatry.
Strong association
Other studies have tied sleep deprivation to increased risk for serious illness, but the link between sleep and MS risk isn’t as well studied.
Previous research by Dr. Åkerstedt showed that the risk for MS was higher among individuals who took part in shift work before the age of 20. However, the impact of sleep duration or quality among teens was unknown.
The current Swedish population-based case-control study included 2,075 patients with MS and 3,164 without the disorder. All participants were asked to recall how many hours on average they slept per night between the ages of 15 and 19 years and to rate their sleep quality during that time.
Results showed that individuals who slept fewer than 7 hours a night during their teen years were 40% more likely to have MS as adults (odds ratio [OR], 1.4; 95% confidence interval [CI], 1.1-1.7).
Poor sleep quality increased MS risk even more (OR, 1.5; 95% CI, 1.3-1.9).
The association remained strong even after adjustment for additional sleep on weekends and breaks and excluding shift workers.
Long sleep ‘apparently good’
The researchers also conducted several sensitivity studies to rule out confounders that might bias the association, such as excluding participants who reported currently experiencing less sleep or poor sleep.
“You would expect that people who are suffering from sleep problems today would be the people who reported sleep problems during their youth,” but that didn’t happen, Dr. Åkerstedt noted.
The investigators also entered data on sleep duration and sleep quality at the same time, thinking the data would cancel each other out. However, the association remained the same.
“Quite often you see that sleep duration would eliminate the effect of sleep complaints in the prediction of disease, but here both remain significant when they are entered at the same time,” Dr. Åkerstedt said. “You get the feeling that this might mean they act together to produce results,” he added.
“One other thing that surprised me is that long sleep was apparently good,” said Dr. Åkerstedt.
The investigators have conducted several studies on sleep duration and mortality. In recent research, they found that both short sleep and long sleep predicted mortality – “and often, long sleep is a stronger predictor than short sleep,” he said.
Underestimated problem?
Commenting on the findings, Kathleen Zackowski, PhD, associate vice president of research for the National Multiple Sclerosis Society in Baltimore, noted that participants were asked to rate their own sleep quality during adolescence, a subjective report that may mean sleep quality has an even larger association with MS risk.
“That they found a result with sleep quality says to me that there probably is a bigger problem, because I don’t know if people over- or underestimate their sleep quality,” said Dr. Zackowski, who was not involved with the research.
“If we could get to that sleep quality question a little more objectively, I bet that we’d find there’s a lot more to the story,” she said.
That’s a story the researchers would like to explore, Dr. Åkerstedt reported. Designing a prospective study that more closely tracks sleeping habits during adolescence and follows individuals through adulthood could provide valuable information about how sleep quality and duration affect immune system development and MS risk, he said.
Dr. Zackowski said clinicians know that MS is not caused just by a genetic abnormality and that other environmental lifestyle factors seem to play a part.
“If we find out that sleep is one of those lifestyle factors, this is very changeable,” she added.
The study was funded by the Swedish Research Council, the Swedish Research Council for Health, Working Life and Welfare, the Swedish Brain Foundation, AFA Insurance, the European Aviation Safety Authority, the Tercentenary Fund of the Bank of Sweden, the Margaretha af Ugglas Foundation, the Swedish Foundation for MS Research, and NEURO Sweden. Dr. Åkerstadt has been supported by Tercentenary Fund of Bank of Sweden, AFA Insurance, and the European Aviation Safety Authority. Dr. Zackowski reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Too little sleep or poor sleep quality during the teen years can significantly increase the risk for multiple sclerosis (MS) during adulthood, new research suggests.
In a large case-control study, individuals who slept less than 7 hours a night on average during adolescence were 40% more likely to develop MS later on. The risk was even higher for those who rated their sleep quality as bad.
On the other hand, MS was significantly less common among individuals who slept longer as teens – indicating a possible protective benefit.
While sleep duration has been associated with mortality or disease risk for other conditions, sleep quality usually has little to no effect on risk, lead investigator Torbjörn Åkerstedt, PhD, sleep researcher and professor of psychology, department of neuroscience, Karolinska Institutet, Stockholm, told this news organization.
“I hadn’t really expected that, but those results were quite strong, even stronger than sleep duration,” Dr. Åkerstedt said.
“We don’t really know why this is happening in young age, but the most suitable explanation is that the brain in still developing quite a bit, and you’re interfering with it,” he added.
The findings were published online in the Journal of Neurology, Neurosurgery and Psychiatry.
Strong association
Other studies have tied sleep deprivation to increased risk for serious illness, but the link between sleep and MS risk isn’t as well studied.
Previous research by Dr. Åkerstedt showed that the risk for MS was higher among individuals who took part in shift work before the age of 20. However, the impact of sleep duration or quality among teens was unknown.
The current Swedish population-based case-control study included 2,075 patients with MS and 3,164 without the disorder. All participants were asked to recall how many hours on average they slept per night between the ages of 15 and 19 years and to rate their sleep quality during that time.
Results showed that individuals who slept fewer than 7 hours a night during their teen years were 40% more likely to have MS as adults (odds ratio [OR], 1.4; 95% confidence interval [CI], 1.1-1.7).
Poor sleep quality increased MS risk even more (OR, 1.5; 95% CI, 1.3-1.9).
The association remained strong even after adjustment for additional sleep on weekends and breaks and excluding shift workers.
Long sleep ‘apparently good’
The researchers also conducted several sensitivity studies to rule out confounders that might bias the association, such as excluding participants who reported currently experiencing less sleep or poor sleep.
“You would expect that people who are suffering from sleep problems today would be the people who reported sleep problems during their youth,” but that didn’t happen, Dr. Åkerstedt noted.
The investigators also entered data on sleep duration and sleep quality at the same time, thinking the data would cancel each other out. However, the association remained the same.
“Quite often you see that sleep duration would eliminate the effect of sleep complaints in the prediction of disease, but here both remain significant when they are entered at the same time,” Dr. Åkerstedt said. “You get the feeling that this might mean they act together to produce results,” he added.
“One other thing that surprised me is that long sleep was apparently good,” said Dr. Åkerstedt.
The investigators have conducted several studies on sleep duration and mortality. In recent research, they found that both short sleep and long sleep predicted mortality – “and often, long sleep is a stronger predictor than short sleep,” he said.
Underestimated problem?
Commenting on the findings, Kathleen Zackowski, PhD, associate vice president of research for the National Multiple Sclerosis Society in Baltimore, noted that participants were asked to rate their own sleep quality during adolescence, a subjective report that may mean sleep quality has an even larger association with MS risk.
“That they found a result with sleep quality says to me that there probably is a bigger problem, because I don’t know if people over- or underestimate their sleep quality,” said Dr. Zackowski, who was not involved with the research.
“If we could get to that sleep quality question a little more objectively, I bet that we’d find there’s a lot more to the story,” she said.
That’s a story the researchers would like to explore, Dr. Åkerstedt reported. Designing a prospective study that more closely tracks sleeping habits during adolescence and follows individuals through adulthood could provide valuable information about how sleep quality and duration affect immune system development and MS risk, he said.
Dr. Zackowski said clinicians know that MS is not caused just by a genetic abnormality and that other environmental lifestyle factors seem to play a part.
“If we find out that sleep is one of those lifestyle factors, this is very changeable,” she added.
The study was funded by the Swedish Research Council, the Swedish Research Council for Health, Working Life and Welfare, the Swedish Brain Foundation, AFA Insurance, the European Aviation Safety Authority, the Tercentenary Fund of the Bank of Sweden, the Margaretha af Ugglas Foundation, the Swedish Foundation for MS Research, and NEURO Sweden. Dr. Åkerstadt has been supported by Tercentenary Fund of Bank of Sweden, AFA Insurance, and the European Aviation Safety Authority. Dr. Zackowski reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Can a nationwide liver paired donation program work?
For a patient who needs a liver, living donation offers an alternative to staying on a list of more than 10,000 people waiting for a transplant. But what happens when your donor is not a match?
“It’s an exciting time to be caring for patients who need liver transplants,” Benjamin Samstein, MD, chief of liver transplantation at New York–Presbyterian/Weill Cornell Medical Center, New York, said in an interview. He is the principal investigator for the UNOS pilot program. “I do believe it is within our grasp to make sure that nobody dies while waiting for an organ,” he said.
The initiative involves 15 U.S. transplant centers. So far, one recipient-donor pair has enrolled in the program. The pilot program has three main goals: Increase access to living donor transplants; increase access to transplants earlier, when recipients are in better health; and work out how to create and sustain a national program.
What is paired donation?
In 2020, 1,095 people died while waiting for a liver transplant, according to a report from the Organ Procurement and Transplant Network (OPTN) – a public-private partnership that includes more than 250 transplant centers and 50 organ procurement organizations across the country.
Most liver transplants involve deceased donors. One way to improve access to lifesaving transplants is through living donation, by which a healthy individual donates part of his or her liver. Someone can participate in nondirected or “altruistic” donation, in which someone donates a liver to someone they don’t know, or they can donate to a specific individual (usually a blood relative or a spouse).
With living liver donation, someone may receive a liver earlier, before getting sick enough to be given priority on the wait-list for deceased donation. Because the recipients are in better health, they may have an easier time recovering from the surgery, Ruthanne Leishman, who manages paired donation programs at UNOS, said in an interview.
In some cases, an individual will want to donate an organ to a specific person, but testing reveals that the two would not be a good match. Paired donation allows incompatible donors and recipients to find matches with other incompatible pairs. Each donor matches with the other pairs’ recipient, so the organs are essentially swapped or exchanged between the two pairs.
“People who want to donate get excited about the fact that they are not just helping their loved one but they’re also helping somebody else,” Ms. Leishman said.
Paired kidney donation programs have been running since 2002, but paired liver donation is relatively new. Since the first U.S. living-donor liver transplant in 1989, the procedure has become safer and is a viable alternative to deceased liver donation. A growing number of living donor programs are popping up at transplant centers across the country.
Still, living-donor liver donation makes up a small percentage of the liver transplants that are performed every year. In 2022, 603 living-donor liver transplants were performed in the United States, compared to 8,925 liver transplants from deceased donors, according to OPTN data. Dr. Samstein estimates a couple dozen paired liver exchanges may have been performed in the United States over the past few years within individual hospital systems. A goal of this pilot program, along with increasing access to liver transplants, is to see whether paired liver donation works on a national level, Ms. Leishman said.
Challenges to building a national program
There are several notable differences between living donor kidney transplants and living donor liver transplants. For example, living donor liver transplant is a more complicated surgery and poses greater risk to the donor. According to the OPTN 2020 Annual Report, from 2015 to 2019, the rehospitalization rate for living liver donors was twice that of living kidney donors up to 6 weeks after transplant (4.7% vs. 2.4%). One year post transplant, the cumulative rehospitalization rate was 11.0% for living liver donors and 4.8% for living kidney donors.
The risk of dying because of living donation is also higher for liver donors compared to kidney donors. The National Kidney Association states that the odds of dying during kidney donation are about 3 in 100,000, while estimates for risk of death for living liver donors range from 1 in 500 to 1 in 1,000. But some of these estimates are from 10 or more years ago, and outcomes have likely improved, said Whitney Jackson, MD, medical director of living donor liver transplant at UCHealth University of Colorado Hospital, Aurora. Her program is participating in the UNOS pilot.
More recent data from OPTN provides some idea of risk: Of 3,967 liver donors who donated between March 1, 2008, to Sept. 30, 2022, three deaths were reported within 30 days of transplant. However, the causes of death were not specified and therefore may be unrelated to the surgery. By comparison, of 74,555 kidney donors during that date range, 10 deaths were reported at 30 days post surgery.
In addition to a more complex surgery, surgeons also have a smaller time window in which to transplant a liver than than they do to transplant a kidney. A kidney can remain viable in cold storage for 24-36 hours, and it can be transported via commercial airlines cross country. Livers have to be transplanted within 8-12 hours, according to the OPTN website. For living donation, the graft needs to be transplanted within about 4 hours, Dr. Samstein noted; this poses a logistical challenge for a national organ paired donation program.
“We worked around that with the idea that we would move the donor rather than the organ,” he said. The program will require a donor (and a support person) to travel to the recipient’s transplant center where the surgery will be performed. While 3 of the 15 pilot paired donation transplant centers are in New York City, the other programs are scattered across the country, meaning a donor may have to fly to a different city to undergo surgery.
Including the preoperative evaluation, meeting the surgical team, the surgery itself, and follow-up, the donor could stay for about a month. The program offers up to $10,000 of financial assistance for travel expenses (for both the donor and support person), as well as lost wages and dependent care (for the donor only). Health insurance coverage will also be provided by the pilot program, in partnership with the American Foundation for Donation and Transplant.
The program requires that transplant candidates (the recipients) be at least 12 years old, be on the waiting list for deceased liver donation at one of the pilot’s transplant centers, and have a Model for End-Stage Liver Disease (MELD) score of 25 or less. All potential donors must be 18 years or older and must undergo a medical and psychosocial evaluation. Nondirected donors can register with the program, and they will be paired with a candidate on the liver transplant waiting list at the same transplant center.
The 1-year pilot program is set to begin when the program conducts its first match run – an algorithm will help match pairs who are enrolled in the program. About five to seven enrolled pairs would be ideal for the first match run, a UNOS spokesperson said. It is possible that the 1-year pilot program could run without performing any paired transplants, but that’s unlikely if multiple pairs are enrolled in the system, the spokesperson said. At the time of this story’s publication, the one enrolled pair are a mother and daughter who are registered at the UCHealth Transplant Center in Colorado.
Is a national liver paired donor program feasible?
While the UNOS pilot program offers financial assistance for expenses related to liver donation, some transplant surgeons are skeptical about the potential travel component of the pilot program.
The pilot program requires that the donor bring one support person if there is a need to travel for the surgery, but undergoing major abdominal surgery from a transplant team they are not familiar with may be stressful, said Peter Abt, MD, a transplant surgeon at the Hospital of the University of Pennsylvania and the Children’s Hospital of Philadelphia. “That’s a big ask,” he said, “and I’m not sure many potential donors would be up to that.”
John Roberts, MD, a transplant surgeon at the University of California, San Francisco, agreed that the travel component may put additional stress on the donor, but “if it’s the only way for the recipient to get a transplant, then the donor might be motivated,” he added.
Dr. Jackson remains optimistic. “Our experience so far has been that, yes, some people have been hesitant for things like traveling, but a lot of people who seem to be genuinely dedicated to the idea of living donation have been very enthusiastic,” she noted.
Dr. Leishman agreed that the travel aspect appears to one of the greatest barriers to participants entering the program but noted that a goal of the pilot program is to understand better what works - and what doesn’t – when considering a liver paired donation program on a national scale. “[Our] steering committee has put together a really nice framework that they think will work, but they know it’s not perfect. We’re going to have to tweak it along the way,” she said.
More information on the paired liver donation pilot program can be found on the UNOS website.
The sources interviewed for this article reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
This article was updated 2/15/23.
For a patient who needs a liver, living donation offers an alternative to staying on a list of more than 10,000 people waiting for a transplant. But what happens when your donor is not a match?
“It’s an exciting time to be caring for patients who need liver transplants,” Benjamin Samstein, MD, chief of liver transplantation at New York–Presbyterian/Weill Cornell Medical Center, New York, said in an interview. He is the principal investigator for the UNOS pilot program. “I do believe it is within our grasp to make sure that nobody dies while waiting for an organ,” he said.
The initiative involves 15 U.S. transplant centers. So far, one recipient-donor pair has enrolled in the program. The pilot program has three main goals: Increase access to living donor transplants; increase access to transplants earlier, when recipients are in better health; and work out how to create and sustain a national program.
What is paired donation?
In 2020, 1,095 people died while waiting for a liver transplant, according to a report from the Organ Procurement and Transplant Network (OPTN) – a public-private partnership that includes more than 250 transplant centers and 50 organ procurement organizations across the country.
Most liver transplants involve deceased donors. One way to improve access to lifesaving transplants is through living donation, by which a healthy individual donates part of his or her liver. Someone can participate in nondirected or “altruistic” donation, in which someone donates a liver to someone they don’t know, or they can donate to a specific individual (usually a blood relative or a spouse).
With living liver donation, someone may receive a liver earlier, before getting sick enough to be given priority on the wait-list for deceased donation. Because the recipients are in better health, they may have an easier time recovering from the surgery, Ruthanne Leishman, who manages paired donation programs at UNOS, said in an interview.
In some cases, an individual will want to donate an organ to a specific person, but testing reveals that the two would not be a good match. Paired donation allows incompatible donors and recipients to find matches with other incompatible pairs. Each donor matches with the other pairs’ recipient, so the organs are essentially swapped or exchanged between the two pairs.
“People who want to donate get excited about the fact that they are not just helping their loved one but they’re also helping somebody else,” Ms. Leishman said.
Paired kidney donation programs have been running since 2002, but paired liver donation is relatively new. Since the first U.S. living-donor liver transplant in 1989, the procedure has become safer and is a viable alternative to deceased liver donation. A growing number of living donor programs are popping up at transplant centers across the country.
Still, living-donor liver donation makes up a small percentage of the liver transplants that are performed every year. In 2022, 603 living-donor liver transplants were performed in the United States, compared to 8,925 liver transplants from deceased donors, according to OPTN data. Dr. Samstein estimates a couple dozen paired liver exchanges may have been performed in the United States over the past few years within individual hospital systems. A goal of this pilot program, along with increasing access to liver transplants, is to see whether paired liver donation works on a national level, Ms. Leishman said.
Challenges to building a national program
There are several notable differences between living donor kidney transplants and living donor liver transplants. For example, living donor liver transplant is a more complicated surgery and poses greater risk to the donor. According to the OPTN 2020 Annual Report, from 2015 to 2019, the rehospitalization rate for living liver donors was twice that of living kidney donors up to 6 weeks after transplant (4.7% vs. 2.4%). One year post transplant, the cumulative rehospitalization rate was 11.0% for living liver donors and 4.8% for living kidney donors.
The risk of dying because of living donation is also higher for liver donors compared to kidney donors. The National Kidney Association states that the odds of dying during kidney donation are about 3 in 100,000, while estimates for risk of death for living liver donors range from 1 in 500 to 1 in 1,000. But some of these estimates are from 10 or more years ago, and outcomes have likely improved, said Whitney Jackson, MD, medical director of living donor liver transplant at UCHealth University of Colorado Hospital, Aurora. Her program is participating in the UNOS pilot.
More recent data from OPTN provides some idea of risk: Of 3,967 liver donors who donated between March 1, 2008, to Sept. 30, 2022, three deaths were reported within 30 days of transplant. However, the causes of death were not specified and therefore may be unrelated to the surgery. By comparison, of 74,555 kidney donors during that date range, 10 deaths were reported at 30 days post surgery.
In addition to a more complex surgery, surgeons also have a smaller time window in which to transplant a liver than than they do to transplant a kidney. A kidney can remain viable in cold storage for 24-36 hours, and it can be transported via commercial airlines cross country. Livers have to be transplanted within 8-12 hours, according to the OPTN website. For living donation, the graft needs to be transplanted within about 4 hours, Dr. Samstein noted; this poses a logistical challenge for a national organ paired donation program.
“We worked around that with the idea that we would move the donor rather than the organ,” he said. The program will require a donor (and a support person) to travel to the recipient’s transplant center where the surgery will be performed. While 3 of the 15 pilot paired donation transplant centers are in New York City, the other programs are scattered across the country, meaning a donor may have to fly to a different city to undergo surgery.
Including the preoperative evaluation, meeting the surgical team, the surgery itself, and follow-up, the donor could stay for about a month. The program offers up to $10,000 of financial assistance for travel expenses (for both the donor and support person), as well as lost wages and dependent care (for the donor only). Health insurance coverage will also be provided by the pilot program, in partnership with the American Foundation for Donation and Transplant.
The program requires that transplant candidates (the recipients) be at least 12 years old, be on the waiting list for deceased liver donation at one of the pilot’s transplant centers, and have a Model for End-Stage Liver Disease (MELD) score of 25 or less. All potential donors must be 18 years or older and must undergo a medical and psychosocial evaluation. Nondirected donors can register with the program, and they will be paired with a candidate on the liver transplant waiting list at the same transplant center.
The 1-year pilot program is set to begin when the program conducts its first match run – an algorithm will help match pairs who are enrolled in the program. About five to seven enrolled pairs would be ideal for the first match run, a UNOS spokesperson said. It is possible that the 1-year pilot program could run without performing any paired transplants, but that’s unlikely if multiple pairs are enrolled in the system, the spokesperson said. At the time of this story’s publication, the one enrolled pair are a mother and daughter who are registered at the UCHealth Transplant Center in Colorado.
Is a national liver paired donor program feasible?
While the UNOS pilot program offers financial assistance for expenses related to liver donation, some transplant surgeons are skeptical about the potential travel component of the pilot program.
The pilot program requires that the donor bring one support person if there is a need to travel for the surgery, but undergoing major abdominal surgery from a transplant team they are not familiar with may be stressful, said Peter Abt, MD, a transplant surgeon at the Hospital of the University of Pennsylvania and the Children’s Hospital of Philadelphia. “That’s a big ask,” he said, “and I’m not sure many potential donors would be up to that.”
John Roberts, MD, a transplant surgeon at the University of California, San Francisco, agreed that the travel component may put additional stress on the donor, but “if it’s the only way for the recipient to get a transplant, then the donor might be motivated,” he added.
Dr. Jackson remains optimistic. “Our experience so far has been that, yes, some people have been hesitant for things like traveling, but a lot of people who seem to be genuinely dedicated to the idea of living donation have been very enthusiastic,” she noted.
Dr. Leishman agreed that the travel aspect appears to one of the greatest barriers to participants entering the program but noted that a goal of the pilot program is to understand better what works - and what doesn’t – when considering a liver paired donation program on a national scale. “[Our] steering committee has put together a really nice framework that they think will work, but they know it’s not perfect. We’re going to have to tweak it along the way,” she said.
More information on the paired liver donation pilot program can be found on the UNOS website.
The sources interviewed for this article reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
This article was updated 2/15/23.
For a patient who needs a liver, living donation offers an alternative to staying on a list of more than 10,000 people waiting for a transplant. But what happens when your donor is not a match?
“It’s an exciting time to be caring for patients who need liver transplants,” Benjamin Samstein, MD, chief of liver transplantation at New York–Presbyterian/Weill Cornell Medical Center, New York, said in an interview. He is the principal investigator for the UNOS pilot program. “I do believe it is within our grasp to make sure that nobody dies while waiting for an organ,” he said.
The initiative involves 15 U.S. transplant centers. So far, one recipient-donor pair has enrolled in the program. The pilot program has three main goals: Increase access to living donor transplants; increase access to transplants earlier, when recipients are in better health; and work out how to create and sustain a national program.
What is paired donation?
In 2020, 1,095 people died while waiting for a liver transplant, according to a report from the Organ Procurement and Transplant Network (OPTN) – a public-private partnership that includes more than 250 transplant centers and 50 organ procurement organizations across the country.
Most liver transplants involve deceased donors. One way to improve access to lifesaving transplants is through living donation, by which a healthy individual donates part of his or her liver. Someone can participate in nondirected or “altruistic” donation, in which someone donates a liver to someone they don’t know, or they can donate to a specific individual (usually a blood relative or a spouse).
With living liver donation, someone may receive a liver earlier, before getting sick enough to be given priority on the wait-list for deceased donation. Because the recipients are in better health, they may have an easier time recovering from the surgery, Ruthanne Leishman, who manages paired donation programs at UNOS, said in an interview.
In some cases, an individual will want to donate an organ to a specific person, but testing reveals that the two would not be a good match. Paired donation allows incompatible donors and recipients to find matches with other incompatible pairs. Each donor matches with the other pairs’ recipient, so the organs are essentially swapped or exchanged between the two pairs.
“People who want to donate get excited about the fact that they are not just helping their loved one but they’re also helping somebody else,” Ms. Leishman said.
Paired kidney donation programs have been running since 2002, but paired liver donation is relatively new. Since the first U.S. living-donor liver transplant in 1989, the procedure has become safer and is a viable alternative to deceased liver donation. A growing number of living donor programs are popping up at transplant centers across the country.
Still, living-donor liver donation makes up a small percentage of the liver transplants that are performed every year. In 2022, 603 living-donor liver transplants were performed in the United States, compared to 8,925 liver transplants from deceased donors, according to OPTN data. Dr. Samstein estimates a couple dozen paired liver exchanges may have been performed in the United States over the past few years within individual hospital systems. A goal of this pilot program, along with increasing access to liver transplants, is to see whether paired liver donation works on a national level, Ms. Leishman said.
Challenges to building a national program
There are several notable differences between living donor kidney transplants and living donor liver transplants. For example, living donor liver transplant is a more complicated surgery and poses greater risk to the donor. According to the OPTN 2020 Annual Report, from 2015 to 2019, the rehospitalization rate for living liver donors was twice that of living kidney donors up to 6 weeks after transplant (4.7% vs. 2.4%). One year post transplant, the cumulative rehospitalization rate was 11.0% for living liver donors and 4.8% for living kidney donors.
The risk of dying because of living donation is also higher for liver donors compared to kidney donors. The National Kidney Association states that the odds of dying during kidney donation are about 3 in 100,000, while estimates for risk of death for living liver donors range from 1 in 500 to 1 in 1,000. But some of these estimates are from 10 or more years ago, and outcomes have likely improved, said Whitney Jackson, MD, medical director of living donor liver transplant at UCHealth University of Colorado Hospital, Aurora. Her program is participating in the UNOS pilot.
More recent data from OPTN provides some idea of risk: Of 3,967 liver donors who donated between March 1, 2008, to Sept. 30, 2022, three deaths were reported within 30 days of transplant. However, the causes of death were not specified and therefore may be unrelated to the surgery. By comparison, of 74,555 kidney donors during that date range, 10 deaths were reported at 30 days post surgery.
In addition to a more complex surgery, surgeons also have a smaller time window in which to transplant a liver than than they do to transplant a kidney. A kidney can remain viable in cold storage for 24-36 hours, and it can be transported via commercial airlines cross country. Livers have to be transplanted within 8-12 hours, according to the OPTN website. For living donation, the graft needs to be transplanted within about 4 hours, Dr. Samstein noted; this poses a logistical challenge for a national organ paired donation program.
“We worked around that with the idea that we would move the donor rather than the organ,” he said. The program will require a donor (and a support person) to travel to the recipient’s transplant center where the surgery will be performed. While 3 of the 15 pilot paired donation transplant centers are in New York City, the other programs are scattered across the country, meaning a donor may have to fly to a different city to undergo surgery.
Including the preoperative evaluation, meeting the surgical team, the surgery itself, and follow-up, the donor could stay for about a month. The program offers up to $10,000 of financial assistance for travel expenses (for both the donor and support person), as well as lost wages and dependent care (for the donor only). Health insurance coverage will also be provided by the pilot program, in partnership with the American Foundation for Donation and Transplant.
The program requires that transplant candidates (the recipients) be at least 12 years old, be on the waiting list for deceased liver donation at one of the pilot’s transplant centers, and have a Model for End-Stage Liver Disease (MELD) score of 25 or less. All potential donors must be 18 years or older and must undergo a medical and psychosocial evaluation. Nondirected donors can register with the program, and they will be paired with a candidate on the liver transplant waiting list at the same transplant center.
The 1-year pilot program is set to begin when the program conducts its first match run – an algorithm will help match pairs who are enrolled in the program. About five to seven enrolled pairs would be ideal for the first match run, a UNOS spokesperson said. It is possible that the 1-year pilot program could run without performing any paired transplants, but that’s unlikely if multiple pairs are enrolled in the system, the spokesperson said. At the time of this story’s publication, the one enrolled pair are a mother and daughter who are registered at the UCHealth Transplant Center in Colorado.
Is a national liver paired donor program feasible?
While the UNOS pilot program offers financial assistance for expenses related to liver donation, some transplant surgeons are skeptical about the potential travel component of the pilot program.
The pilot program requires that the donor bring one support person if there is a need to travel for the surgery, but undergoing major abdominal surgery from a transplant team they are not familiar with may be stressful, said Peter Abt, MD, a transplant surgeon at the Hospital of the University of Pennsylvania and the Children’s Hospital of Philadelphia. “That’s a big ask,” he said, “and I’m not sure many potential donors would be up to that.”
John Roberts, MD, a transplant surgeon at the University of California, San Francisco, agreed that the travel component may put additional stress on the donor, but “if it’s the only way for the recipient to get a transplant, then the donor might be motivated,” he added.
Dr. Jackson remains optimistic. “Our experience so far has been that, yes, some people have been hesitant for things like traveling, but a lot of people who seem to be genuinely dedicated to the idea of living donation have been very enthusiastic,” she noted.
Dr. Leishman agreed that the travel aspect appears to one of the greatest barriers to participants entering the program but noted that a goal of the pilot program is to understand better what works - and what doesn’t – when considering a liver paired donation program on a national scale. “[Our] steering committee has put together a really nice framework that they think will work, but they know it’s not perfect. We’re going to have to tweak it along the way,” she said.
More information on the paired liver donation pilot program can be found on the UNOS website.
The sources interviewed for this article reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
This article was updated 2/15/23.
Muscle weakness predicts poor outcomes in asthma patients
, based on data from 114 individuals.
Previous studies have shown reduced muscle mass in asthma patients, but the impact on clinical and functional outcomes has not been well studied, wrote Edith Visser, MSc, of Medical Centre Leeuwarden (the Netherlands) and colleagues.
“Many asthma patients, especially those with severe disease, report exercise intolerance and limitations in daily activities, severely affecting their quality of life,” they said. Research into the clinical consequences of low muscle mass and low muscle strength for patients with asthma and the role of inflammation could make muscle function a potential treatment target for those with asthma, they said.
In a study published in the Journal of Allergy and Clinical Immunology: In Practice, the researchers recruited 114 consecutive adults aged 18 years and older with a diagnosis of moderate to severe asthma who were seen at a single center between Jun. 2019 and Oct. 2022. The mean age of the patients was 51.9 years, 36% were men, 70% were overweight or obese, and 34 were diagnosed with severe asthma.
Participants underwent clinical, functional, and laboratory assessments at one or two visits within a 2-week period. Assessment tools included the Asthma Quality of Life Questionnaire (AQLQ), the Asthma Control Questionnaire (ACQ-6), a questionnaire on health care use (HCU), and the ‘short questionnaire to assess health-enhancing physical activity’ (SQUASH).
Functional activity was based on the 6-minute walking distance (6MWD), and lung function tests included spirometry and fractional inhaled nitric oxide (FeNO). Muscle mass was based on fat-free mass index (FFMI) and urinary creatinine excretion rate (CER). Muscle strength was measured using hand-grip strength (HGS).
The researchers examined levels of muscle mass and strength and their relation to functional and clinical outcomes.
Overall, the mean measures of muscle mass and strength were higher in males, who had average FFMI, CER, and HGS measures of 20.1 kg/m2, 15.3 mmol/day, and 48.8 kg, respectively. These measures in women were 17.3 kg/m2, 10.8 mmol/day, and 29.3 kg, respectively.
After adjusting for confounding factors, patients in the lowest tertile for muscle mass based on FFMI had significantly more severe asthma based on postbronchodilator forced expiratory volume in 1 second and FEV1/forced vital capacity, as well as lower functional exercise capacity based on the 6MWD compared to those in the highest tertile. A similar association appeared between CER and FEV1, but not FEV1/FVC.
However, no significant associations appeared between the muscle mass measures of FFMI or CER and scores on the ACQ, AQLQ, emergency department visits, or asthma exacerbations, the researchers noted.
No relationship appeared between muscle strength and functional outcomes. However, patients in the lowest tertile of HGS had worse asthma control, worse quality of life, and a higher probability of at least one visit to the emergency department compared to patients in the highest HGS tertile.
Higher leukocyte levels were significantly associated with lower muscle mass after adjusting for age, sex, weight, and physical activity, but no other inflammatory markers were significantly associated with FFMI.
The association between lower muscle strength and poorer asthma control, lower quality of life, and greater odds of emergency department visits reflect findings from previous studies, the researchers said. The mechanisms behind the loss of muscle strength in asthma remain unclear, but physical inactivity and daily oral corticosteroid use may play a role, they added.
The study findings were limited by the cross-sectional design and the possibility that muscle weakness may instead stem from reduced physical activity associated with poor lung function and asthma control, the researchers noted. Other limitations included the potential overestimation of FFMI and the lack of statistical power to show a relationship between FFMI and emergency department visits and asthma exacerbations, they said.
However, the current study is the first known to explore the relationship between lower muscle mass and strength and a range of both functional and clinical outcomes in patients with moderate to severe asthma, they said.
“Our findings encourage longitudinal studies into muscle function as a potential target for treatment to improve asthma outcomes,” they concluded.
The study was supported by unrestricted grants from Medical Centre Leeuwarden research fund. Ms. Visser had no financial conflicts to disclose.
, based on data from 114 individuals.
Previous studies have shown reduced muscle mass in asthma patients, but the impact on clinical and functional outcomes has not been well studied, wrote Edith Visser, MSc, of Medical Centre Leeuwarden (the Netherlands) and colleagues.
“Many asthma patients, especially those with severe disease, report exercise intolerance and limitations in daily activities, severely affecting their quality of life,” they said. Research into the clinical consequences of low muscle mass and low muscle strength for patients with asthma and the role of inflammation could make muscle function a potential treatment target for those with asthma, they said.
In a study published in the Journal of Allergy and Clinical Immunology: In Practice, the researchers recruited 114 consecutive adults aged 18 years and older with a diagnosis of moderate to severe asthma who were seen at a single center between Jun. 2019 and Oct. 2022. The mean age of the patients was 51.9 years, 36% were men, 70% were overweight or obese, and 34 were diagnosed with severe asthma.
Participants underwent clinical, functional, and laboratory assessments at one or two visits within a 2-week period. Assessment tools included the Asthma Quality of Life Questionnaire (AQLQ), the Asthma Control Questionnaire (ACQ-6), a questionnaire on health care use (HCU), and the ‘short questionnaire to assess health-enhancing physical activity’ (SQUASH).
Functional activity was based on the 6-minute walking distance (6MWD), and lung function tests included spirometry and fractional inhaled nitric oxide (FeNO). Muscle mass was based on fat-free mass index (FFMI) and urinary creatinine excretion rate (CER). Muscle strength was measured using hand-grip strength (HGS).
The researchers examined levels of muscle mass and strength and their relation to functional and clinical outcomes.
Overall, the mean measures of muscle mass and strength were higher in males, who had average FFMI, CER, and HGS measures of 20.1 kg/m2, 15.3 mmol/day, and 48.8 kg, respectively. These measures in women were 17.3 kg/m2, 10.8 mmol/day, and 29.3 kg, respectively.
After adjusting for confounding factors, patients in the lowest tertile for muscle mass based on FFMI had significantly more severe asthma based on postbronchodilator forced expiratory volume in 1 second and FEV1/forced vital capacity, as well as lower functional exercise capacity based on the 6MWD compared to those in the highest tertile. A similar association appeared between CER and FEV1, but not FEV1/FVC.
However, no significant associations appeared between the muscle mass measures of FFMI or CER and scores on the ACQ, AQLQ, emergency department visits, or asthma exacerbations, the researchers noted.
No relationship appeared between muscle strength and functional outcomes. However, patients in the lowest tertile of HGS had worse asthma control, worse quality of life, and a higher probability of at least one visit to the emergency department compared to patients in the highest HGS tertile.
Higher leukocyte levels were significantly associated with lower muscle mass after adjusting for age, sex, weight, and physical activity, but no other inflammatory markers were significantly associated with FFMI.
The association between lower muscle strength and poorer asthma control, lower quality of life, and greater odds of emergency department visits reflect findings from previous studies, the researchers said. The mechanisms behind the loss of muscle strength in asthma remain unclear, but physical inactivity and daily oral corticosteroid use may play a role, they added.
The study findings were limited by the cross-sectional design and the possibility that muscle weakness may instead stem from reduced physical activity associated with poor lung function and asthma control, the researchers noted. Other limitations included the potential overestimation of FFMI and the lack of statistical power to show a relationship between FFMI and emergency department visits and asthma exacerbations, they said.
However, the current study is the first known to explore the relationship between lower muscle mass and strength and a range of both functional and clinical outcomes in patients with moderate to severe asthma, they said.
“Our findings encourage longitudinal studies into muscle function as a potential target for treatment to improve asthma outcomes,” they concluded.
The study was supported by unrestricted grants from Medical Centre Leeuwarden research fund. Ms. Visser had no financial conflicts to disclose.
, based on data from 114 individuals.
Previous studies have shown reduced muscle mass in asthma patients, but the impact on clinical and functional outcomes has not been well studied, wrote Edith Visser, MSc, of Medical Centre Leeuwarden (the Netherlands) and colleagues.
“Many asthma patients, especially those with severe disease, report exercise intolerance and limitations in daily activities, severely affecting their quality of life,” they said. Research into the clinical consequences of low muscle mass and low muscle strength for patients with asthma and the role of inflammation could make muscle function a potential treatment target for those with asthma, they said.
In a study published in the Journal of Allergy and Clinical Immunology: In Practice, the researchers recruited 114 consecutive adults aged 18 years and older with a diagnosis of moderate to severe asthma who were seen at a single center between Jun. 2019 and Oct. 2022. The mean age of the patients was 51.9 years, 36% were men, 70% were overweight or obese, and 34 were diagnosed with severe asthma.
Participants underwent clinical, functional, and laboratory assessments at one or two visits within a 2-week period. Assessment tools included the Asthma Quality of Life Questionnaire (AQLQ), the Asthma Control Questionnaire (ACQ-6), a questionnaire on health care use (HCU), and the ‘short questionnaire to assess health-enhancing physical activity’ (SQUASH).
Functional activity was based on the 6-minute walking distance (6MWD), and lung function tests included spirometry and fractional inhaled nitric oxide (FeNO). Muscle mass was based on fat-free mass index (FFMI) and urinary creatinine excretion rate (CER). Muscle strength was measured using hand-grip strength (HGS).
The researchers examined levels of muscle mass and strength and their relation to functional and clinical outcomes.
Overall, the mean measures of muscle mass and strength were higher in males, who had average FFMI, CER, and HGS measures of 20.1 kg/m2, 15.3 mmol/day, and 48.8 kg, respectively. These measures in women were 17.3 kg/m2, 10.8 mmol/day, and 29.3 kg, respectively.
After adjusting for confounding factors, patients in the lowest tertile for muscle mass based on FFMI had significantly more severe asthma based on postbronchodilator forced expiratory volume in 1 second and FEV1/forced vital capacity, as well as lower functional exercise capacity based on the 6MWD compared to those in the highest tertile. A similar association appeared between CER and FEV1, but not FEV1/FVC.
However, no significant associations appeared between the muscle mass measures of FFMI or CER and scores on the ACQ, AQLQ, emergency department visits, or asthma exacerbations, the researchers noted.
No relationship appeared between muscle strength and functional outcomes. However, patients in the lowest tertile of HGS had worse asthma control, worse quality of life, and a higher probability of at least one visit to the emergency department compared to patients in the highest HGS tertile.
Higher leukocyte levels were significantly associated with lower muscle mass after adjusting for age, sex, weight, and physical activity, but no other inflammatory markers were significantly associated with FFMI.
The association between lower muscle strength and poorer asthma control, lower quality of life, and greater odds of emergency department visits reflect findings from previous studies, the researchers said. The mechanisms behind the loss of muscle strength in asthma remain unclear, but physical inactivity and daily oral corticosteroid use may play a role, they added.
The study findings were limited by the cross-sectional design and the possibility that muscle weakness may instead stem from reduced physical activity associated with poor lung function and asthma control, the researchers noted. Other limitations included the potential overestimation of FFMI and the lack of statistical power to show a relationship between FFMI and emergency department visits and asthma exacerbations, they said.
However, the current study is the first known to explore the relationship between lower muscle mass and strength and a range of both functional and clinical outcomes in patients with moderate to severe asthma, they said.
“Our findings encourage longitudinal studies into muscle function as a potential target for treatment to improve asthma outcomes,” they concluded.
The study was supported by unrestricted grants from Medical Centre Leeuwarden research fund. Ms. Visser had no financial conflicts to disclose.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Children and COVID: Weekly cases may have doubled in early January
Although new COVID-19 cases in children, as measured by the American Academy of Pediatrics and the Children’s Hospital Association, have remained fairly steady in recent months, data from the Centers for Diseases Control and Prevention suggest that weekly cases took a big jump in early January.
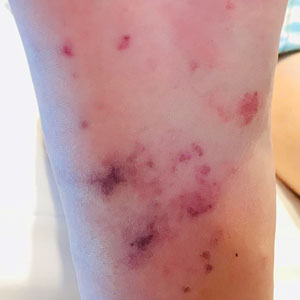
For the most recent week covered . New cases for the first 2 weeks of the year – 31,000 for the week of Dec. 30 to Jan. 5 and 26,000 during Jan. 6-12 – were consistent with the AAP/CHA assertion that “weekly reported child cases have plateaued at an average of about 32,000 cases ... over the past 4 months.”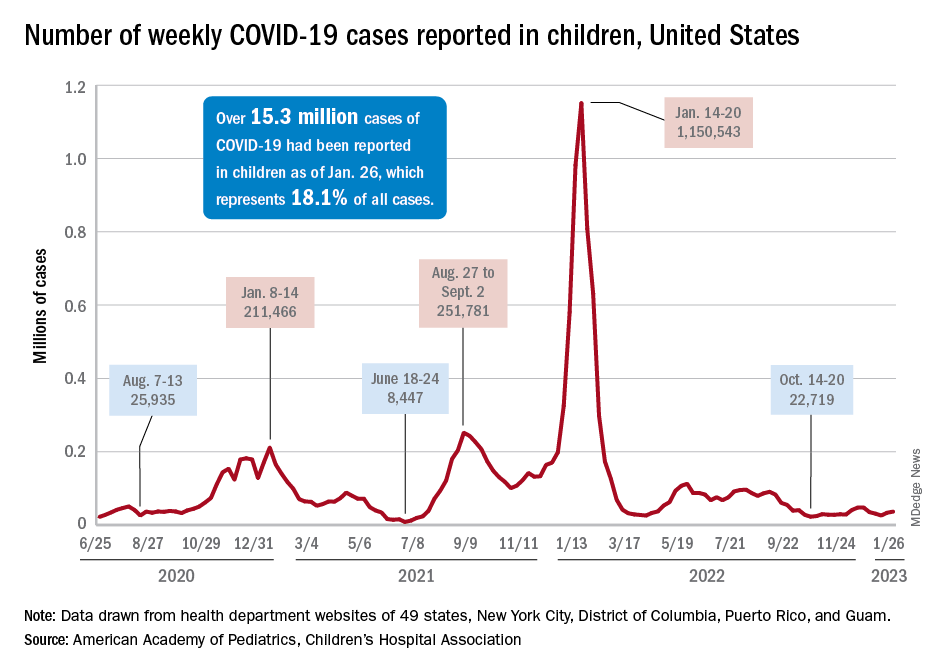
The CDC data, however, show that new cases doubled during the week of Jan. 1-7 to over 65,000, compared with the end of December, and stayed at that level for Jan. 8-14, and since CDC figures are subject to a 6-week reporting delay, the final numbers are likely to be even higher. The composition by age changed somewhat between the 2 weeks, though, as those aged 0-4 years went from almost half of all cases in the first week down to 40% in the second, while cases rose for children aged 5-11 and 12-15, based on data from the COVID-19 response team.
Emergency department visits for January do not show a corresponding increase. ED visits among children aged 0-11 years with COVID-19, measured as a percentage of all ED visits, declined over the course of the month, as did visits for 16- and 17-year-olds, while those aged 12-15 started the month at 1.4% and were at 1.4% on Jan. 27, with a slight dip down to 1.2% in between, the CDC said on its COVID Data Tracker. Daily hospitalizations for children aged 0-17 also declined through mid-January and did not reflect the jump in new cases.
Meanwhile, vaccinated children are still in the minority: 57% of those under age 18 have received no COVID vaccine yet, the AAP said in a separate report. Just 7.4% of children under age 2 years had received at least one dose as of Jan. 25, as had 10.1% of those aged 2-4 years, 39.6% of 5- to 11-year-olds and 71.8% of those 12-17 years old, according to the CDC, with corresponding figures for completion of the primary series at 3.5%, 5.3%, 32.5%, and 61.5%.
Although new COVID-19 cases in children, as measured by the American Academy of Pediatrics and the Children’s Hospital Association, have remained fairly steady in recent months, data from the Centers for Diseases Control and Prevention suggest that weekly cases took a big jump in early January.
For the most recent week covered . New cases for the first 2 weeks of the year – 31,000 for the week of Dec. 30 to Jan. 5 and 26,000 during Jan. 6-12 – were consistent with the AAP/CHA assertion that “weekly reported child cases have plateaued at an average of about 32,000 cases ... over the past 4 months.”
The CDC data, however, show that new cases doubled during the week of Jan. 1-7 to over 65,000, compared with the end of December, and stayed at that level for Jan. 8-14, and since CDC figures are subject to a 6-week reporting delay, the final numbers are likely to be even higher. The composition by age changed somewhat between the 2 weeks, though, as those aged 0-4 years went from almost half of all cases in the first week down to 40% in the second, while cases rose for children aged 5-11 and 12-15, based on data from the COVID-19 response team.
Emergency department visits for January do not show a corresponding increase. ED visits among children aged 0-11 years with COVID-19, measured as a percentage of all ED visits, declined over the course of the month, as did visits for 16- and 17-year-olds, while those aged 12-15 started the month at 1.4% and were at 1.4% on Jan. 27, with a slight dip down to 1.2% in between, the CDC said on its COVID Data Tracker. Daily hospitalizations for children aged 0-17 also declined through mid-January and did not reflect the jump in new cases.
Meanwhile, vaccinated children are still in the minority: 57% of those under age 18 have received no COVID vaccine yet, the AAP said in a separate report. Just 7.4% of children under age 2 years had received at least one dose as of Jan. 25, as had 10.1% of those aged 2-4 years, 39.6% of 5- to 11-year-olds and 71.8% of those 12-17 years old, according to the CDC, with corresponding figures for completion of the primary series at 3.5%, 5.3%, 32.5%, and 61.5%.
Although new COVID-19 cases in children, as measured by the American Academy of Pediatrics and the Children’s Hospital Association, have remained fairly steady in recent months, data from the Centers for Diseases Control and Prevention suggest that weekly cases took a big jump in early January.
For the most recent week covered . New cases for the first 2 weeks of the year – 31,000 for the week of Dec. 30 to Jan. 5 and 26,000 during Jan. 6-12 – were consistent with the AAP/CHA assertion that “weekly reported child cases have plateaued at an average of about 32,000 cases ... over the past 4 months.”
The CDC data, however, show that new cases doubled during the week of Jan. 1-7 to over 65,000, compared with the end of December, and stayed at that level for Jan. 8-14, and since CDC figures are subject to a 6-week reporting delay, the final numbers are likely to be even higher. The composition by age changed somewhat between the 2 weeks, though, as those aged 0-4 years went from almost half of all cases in the first week down to 40% in the second, while cases rose for children aged 5-11 and 12-15, based on data from the COVID-19 response team.
Emergency department visits for January do not show a corresponding increase. ED visits among children aged 0-11 years with COVID-19, measured as a percentage of all ED visits, declined over the course of the month, as did visits for 16- and 17-year-olds, while those aged 12-15 started the month at 1.4% and were at 1.4% on Jan. 27, with a slight dip down to 1.2% in between, the CDC said on its COVID Data Tracker. Daily hospitalizations for children aged 0-17 also declined through mid-January and did not reflect the jump in new cases.
Meanwhile, vaccinated children are still in the minority: 57% of those under age 18 have received no COVID vaccine yet, the AAP said in a separate report. Just 7.4% of children under age 2 years had received at least one dose as of Jan. 25, as had 10.1% of those aged 2-4 years, 39.6% of 5- to 11-year-olds and 71.8% of those 12-17 years old, according to the CDC, with corresponding figures for completion of the primary series at 3.5%, 5.3%, 32.5%, and 61.5%.
Skin of Color Society Scientific Symposium Winners: 2022
The 18th Annual Skin of Color Society Scientific Symposium was held in March 2022 in Boston, Massachusetts. With a theme of Diversity in Action: Science, Healthcare & Society, researchers gathered to present new findings, share key insights, and discuss the continuing evolution of the field. Three awards were presented from the scientific posters at the symposium.
The Best Poster Presentation Award was presented to Brandyn M. White, BS, for “A Preliminary Analysis of the DDB1 Gene: Genome-Wide Association Studies in African and Admixed African American Populations—Is Our Skin Different?” authored by Brandyn M. White, BS; Chidubem A.V. Okeke, BS; Raveena Khanna, MD; Ginette A. Okoye, MD; Michael C. Campbell, PhD; and Angel S. Byrd, MD, PhD. Their research evaluated the association of variant DNA damage binding protein 1, DDB1, with African populations and highlighted the possible phenotypic variations between African and admixed African American populations. Further, it discussed the advantages of conducting future genome-wide association studies in the Washington metropolitan area to better understand dermatological diseases that disproportionately affect skin of color patients.
The Best Oral Presentation Award was presented to Erica Ogwumike, BA, for “Matching into Dermatology Residency: The Impact of Research Fellowships” authored by Erica Ogwumike, BA; Chine Chime, MS, MPH; and Rebecca Vasquez, MD. The aim of this study was to explore what variables were important for 2 events: taking a research fellowship and matching into dermatology. The authors analyzed Electronic Residency Application Service (ERAS) applications for all medical students applying to the UT Southwestern Dermatology Residency Program in the 2014-2015 cycle. They found that 1 of 5 students participated in a research fellowship prior to applying to dermatology residency, and it was not associated with increased odds of matching. They also discovered that students more likely to take a research fellowship were Latinx, attended a medical school ranked in the Top 25, and were not Alpha Omega Alpha members. Nevertheless, total publications did increase the odds of matching; therefore, the authors concluded that when looking for a research fellowship, applicants should look for one that allows productivity so that this measure can be achieved. Further investigation is needed to substantiate these results, but this study was a starting point to examine the characteristics involved in taking a research fellowship in dermatology.
Finally, the Crowd Favorite Award was presented to Jennifer Cucalon, BS, for “Non-invasive, In-Vivo RCM Monitoring of Lentigines Treated With Cryotherapy to Establish Minimum Freeze Time in Seconds (Dose) in Skin of Color” authored by Jennifer Cucalon, BS, and Babar K. Rao, MD. This pilot study showed a minimum freezing time of 3 seconds to be effective in removing lentigines in darker skin; increasing the dose to 6 and 9 seconds had no added benefit. The authors also demonstrated reflectance confocal microscopy to be an appropriate, noninvasive, in vivo tool to visualize pigmentary changes and monitor the effectiveness of treatments for various skin conditions.
The 19th Annual Scientific Symposium will take place on March 16, 2023, in New Orleans, Louisiana. The theme will be Where Science, Innovation & Inclusion Meet. For more information, visit https://skinofcolorsociety.org/19th-annual-skin-of-color-society-scientific-symposium/.
The 18th Annual Skin of Color Society Scientific Symposium was held in March 2022 in Boston, Massachusetts. With a theme of Diversity in Action: Science, Healthcare & Society, researchers gathered to present new findings, share key insights, and discuss the continuing evolution of the field. Three awards were presented from the scientific posters at the symposium.
The Best Poster Presentation Award was presented to Brandyn M. White, BS, for “A Preliminary Analysis of the DDB1 Gene: Genome-Wide Association Studies in African and Admixed African American Populations—Is Our Skin Different?” authored by Brandyn M. White, BS; Chidubem A.V. Okeke, BS; Raveena Khanna, MD; Ginette A. Okoye, MD; Michael C. Campbell, PhD; and Angel S. Byrd, MD, PhD. Their research evaluated the association of variant DNA damage binding protein 1, DDB1, with African populations and highlighted the possible phenotypic variations between African and admixed African American populations. Further, it discussed the advantages of conducting future genome-wide association studies in the Washington metropolitan area to better understand dermatological diseases that disproportionately affect skin of color patients.
The Best Oral Presentation Award was presented to Erica Ogwumike, BA, for “Matching into Dermatology Residency: The Impact of Research Fellowships” authored by Erica Ogwumike, BA; Chine Chime, MS, MPH; and Rebecca Vasquez, MD. The aim of this study was to explore what variables were important for 2 events: taking a research fellowship and matching into dermatology. The authors analyzed Electronic Residency Application Service (ERAS) applications for all medical students applying to the UT Southwestern Dermatology Residency Program in the 2014-2015 cycle. They found that 1 of 5 students participated in a research fellowship prior to applying to dermatology residency, and it was not associated with increased odds of matching. They also discovered that students more likely to take a research fellowship were Latinx, attended a medical school ranked in the Top 25, and were not Alpha Omega Alpha members. Nevertheless, total publications did increase the odds of matching; therefore, the authors concluded that when looking for a research fellowship, applicants should look for one that allows productivity so that this measure can be achieved. Further investigation is needed to substantiate these results, but this study was a starting point to examine the characteristics involved in taking a research fellowship in dermatology.
Finally, the Crowd Favorite Award was presented to Jennifer Cucalon, BS, for “Non-invasive, In-Vivo RCM Monitoring of Lentigines Treated With Cryotherapy to Establish Minimum Freeze Time in Seconds (Dose) in Skin of Color” authored by Jennifer Cucalon, BS, and Babar K. Rao, MD. This pilot study showed a minimum freezing time of 3 seconds to be effective in removing lentigines in darker skin; increasing the dose to 6 and 9 seconds had no added benefit. The authors also demonstrated reflectance confocal microscopy to be an appropriate, noninvasive, in vivo tool to visualize pigmentary changes and monitor the effectiveness of treatments for various skin conditions.
The 19th Annual Scientific Symposium will take place on March 16, 2023, in New Orleans, Louisiana. The theme will be Where Science, Innovation & Inclusion Meet. For more information, visit https://skinofcolorsociety.org/19th-annual-skin-of-color-society-scientific-symposium/.
The 18th Annual Skin of Color Society Scientific Symposium was held in March 2022 in Boston, Massachusetts. With a theme of Diversity in Action: Science, Healthcare & Society, researchers gathered to present new findings, share key insights, and discuss the continuing evolution of the field. Three awards were presented from the scientific posters at the symposium.
The Best Poster Presentation Award was presented to Brandyn M. White, BS, for “A Preliminary Analysis of the DDB1 Gene: Genome-Wide Association Studies in African and Admixed African American Populations—Is Our Skin Different?” authored by Brandyn M. White, BS; Chidubem A.V. Okeke, BS; Raveena Khanna, MD; Ginette A. Okoye, MD; Michael C. Campbell, PhD; and Angel S. Byrd, MD, PhD. Their research evaluated the association of variant DNA damage binding protein 1, DDB1, with African populations and highlighted the possible phenotypic variations between African and admixed African American populations. Further, it discussed the advantages of conducting future genome-wide association studies in the Washington metropolitan area to better understand dermatological diseases that disproportionately affect skin of color patients.
The Best Oral Presentation Award was presented to Erica Ogwumike, BA, for “Matching into Dermatology Residency: The Impact of Research Fellowships” authored by Erica Ogwumike, BA; Chine Chime, MS, MPH; and Rebecca Vasquez, MD. The aim of this study was to explore what variables were important for 2 events: taking a research fellowship and matching into dermatology. The authors analyzed Electronic Residency Application Service (ERAS) applications for all medical students applying to the UT Southwestern Dermatology Residency Program in the 2014-2015 cycle. They found that 1 of 5 students participated in a research fellowship prior to applying to dermatology residency, and it was not associated with increased odds of matching. They also discovered that students more likely to take a research fellowship were Latinx, attended a medical school ranked in the Top 25, and were not Alpha Omega Alpha members. Nevertheless, total publications did increase the odds of matching; therefore, the authors concluded that when looking for a research fellowship, applicants should look for one that allows productivity so that this measure can be achieved. Further investigation is needed to substantiate these results, but this study was a starting point to examine the characteristics involved in taking a research fellowship in dermatology.
Finally, the Crowd Favorite Award was presented to Jennifer Cucalon, BS, for “Non-invasive, In-Vivo RCM Monitoring of Lentigines Treated With Cryotherapy to Establish Minimum Freeze Time in Seconds (Dose) in Skin of Color” authored by Jennifer Cucalon, BS, and Babar K. Rao, MD. This pilot study showed a minimum freezing time of 3 seconds to be effective in removing lentigines in darker skin; increasing the dose to 6 and 9 seconds had no added benefit. The authors also demonstrated reflectance confocal microscopy to be an appropriate, noninvasive, in vivo tool to visualize pigmentary changes and monitor the effectiveness of treatments for various skin conditions.
The 19th Annual Scientific Symposium will take place on March 16, 2023, in New Orleans, Louisiana. The theme will be Where Science, Innovation & Inclusion Meet. For more information, visit https://skinofcolorsociety.org/19th-annual-skin-of-color-society-scientific-symposium/.
Fungal Osler Nodes Indicate Candidal Infective Endocarditis
To the Editor:
A 44-year-old woman presented with a low-grade fever (temperature, 38.0 °C) and painful acral lesions of 1 week’s duration. She had a history of hepatitis C viral infection and intravenous (IV) drug use, as well as polymicrobial infective endocarditis that involved the tricuspid and aortic valves; pathogenic organisms were identified via blood culture as Enterococcus faecalis, Serratia species, Streptococcus viridans, and Candida albicans. The patient had received a mechanical aortic valve and bioprosthetic tricuspid valve replacement 5 months prior with warfarin therapy and had completed a postsurgical 6-week course of high-dose micafungin. She reported that she had developed painful, violaceous, thin papules on the plantar surface of the left foot 2 weeks prior to presentation. Her symptoms improved with a short systemic steroid taper; however, within a week she developed new tender, erythematous, thin papules on the plantar surface of the right foot and the palmar surface of the left hand with associated lower extremity swelling. She denied other symptoms, including fever, chills, neurologic symptoms, shortness of breath, chest pain, nausea, vomiting, hematuria, and hematochezia. Due to worsening cutaneous findings, the patient presented to the emergency department, prompting hospital admission for empiric antibacterial therapy with vancomycin and piperacillin-tazobactam for suspected infectious endocarditis. Dermatology was consulted after 1 day of antibacterial therapy without improvement to determine the etiology of the patient’s skin findings.
Physical examination revealed the patient was afebrile with partially blanching violaceous to purpuric, tender, edematous papules on the left fourth and fifth finger pads, as well as scattered, painful, purpuric patches with stellate borders on the right plantar foot (Figure 1). Laboratory test results revealed mild anemia (hemoglobin, 11.9 g/dL [reference range, 12.0–15.0 g/dL], mild neutrophilia (neutrophils, 8.4×109/L [reference range, 1.9–7.9×109/L], elevated acute-phase reactants (erythrocyte sedimentation rate, 71 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 5.7 mg/dL [reference range, 0.0–0.5 mg/dL]), and positive hepatitis C virus antibody with an undetectable viral load. At the time of dermatologic evaluation, admission blood cultures and transthoracic echocardiogram were negative. Additionally, a transesophageal echocardiogram, limited by artifact from the mechanical aortic valve, was equivocal for valvular pathology. Subsequent ophthalmologic evaluation was negative for lesions associated with endocarditis, such as retinal hemorrhages.
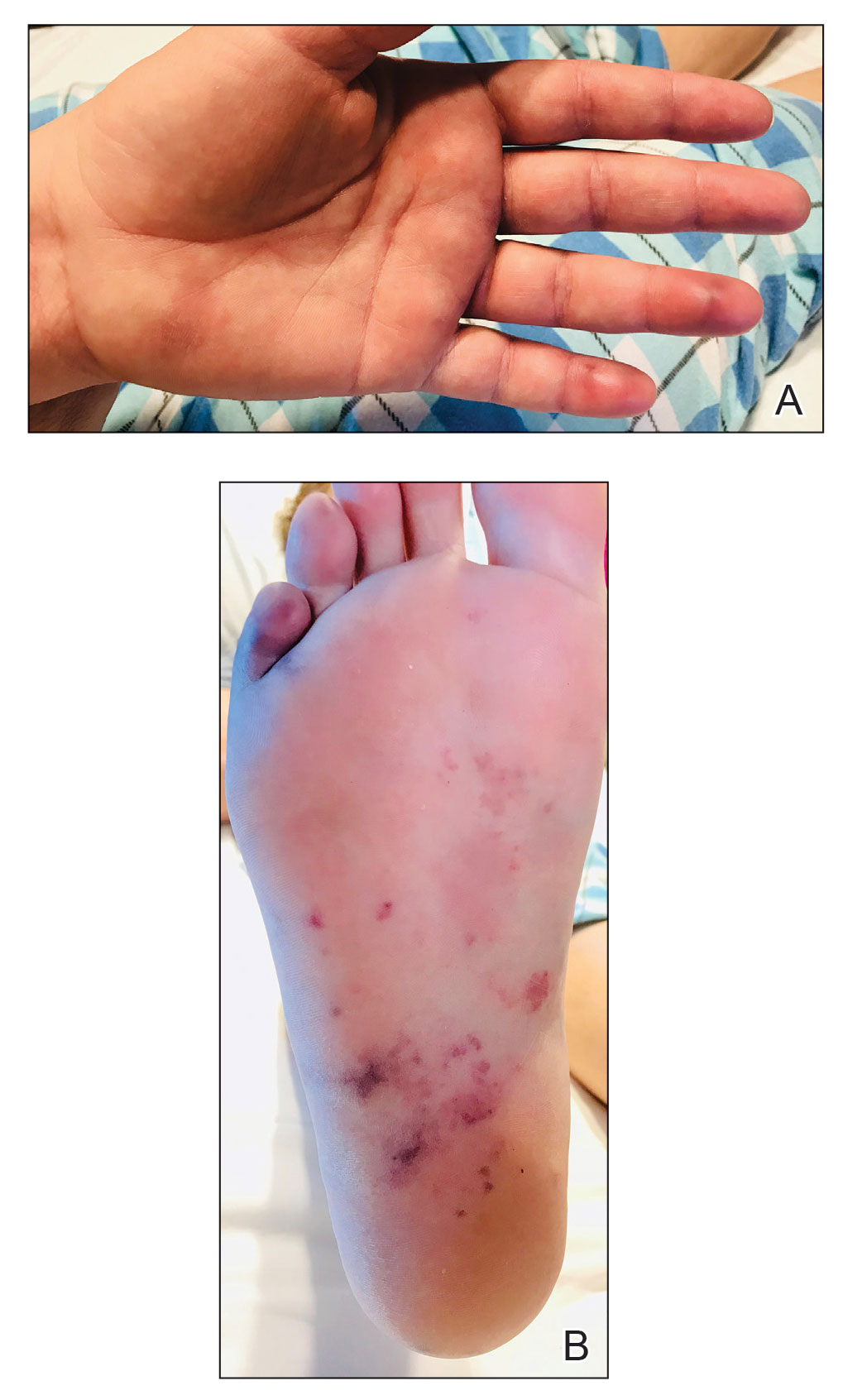
Punch biopsies of the left fourth finger pad were submitted for histopathologic analysis and tissue cultures. Histopathology demonstrated deep dermal perivascular neutrophilic inflammation with multiple intravascular thrombi, perivascular fibrin, and karyorrhectic debris (Figure 2). Periodic acid–Schiff and Grocott-Gomori methenamine-silver stains revealed fungal spores with rare pseudohyphae within the thrombosed vascular spaces and the perivascular dermis, consistent with fungal septic emboli (Figure 3).
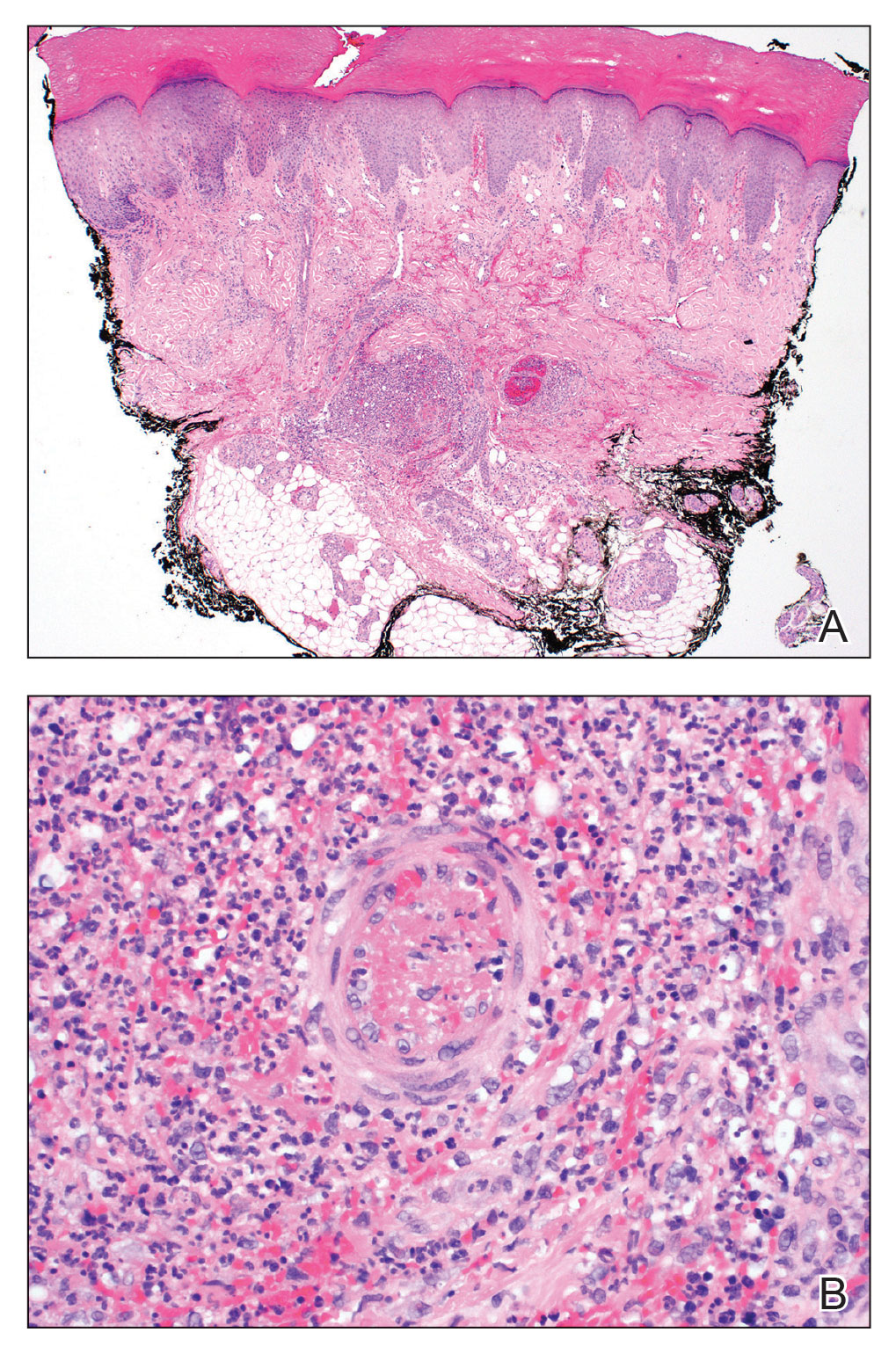
Empiric systemic antifungal coverage composed of IV liposomal amphotericin B and oral flucytosine was initiated, and the patient’s tender acral papules rapidly improved. Within 48 hours of biopsy, skin tissue culture confirmed the presence of C albicans. Four days after the preliminary dermatopathology report, confirmatory blood cultures resulted with pansensitive C albicans. Final tissue and blood cultures were negative for bacteria including mycobacteria. In addition to a 6-week course of IV amphotericin B and flucytosine, repeat surgical intervention was considered, and lifelong suppressive antifungal oral therapy was recommended. Unfortunately, the patient did not present for follow-up. Three months later, she presented to the emergency department with peritonitis; in the operating room, she was found to have ischemia of the entirety of the small and large intestines and died shortly thereafter.
Fungal endocarditis is rare, tending to develop in patient populations with particular risk factors such as immune compromise, structural heart defects or prosthetic valves, and IV drug use. Candida infective endocarditis (CIE) represents less than 2% of infective endocarditis cases and carries a high mortality rate (30%–80%).1-3 Diagnosis may be challenging, as the clinical presentation varies widely. Although some patients may present with classic features of infective endocarditis, including fever, cardiac murmurs, and positive blood cultures, many cases of infective endocarditis present with nonspecific symptoms, raising a broad clinical differential diagnosis. Delay in diagnosis, which is seen in 82% of patients with fungal endocarditis, may be attributed to the slow progression of symptoms, inconclusive cardiac imaging, or negative blood cultures seen in almost one-third of cases.2,3 The feared complication of systemic embolization via infective endocarditis may occur in up to one-half of cases, with the highest rates associated with staphylococcal or fungal pathogens.2 The risk for embolization in fungal endocarditis is independent of the size of the cardiac valve vegetations; accordingly, sequelae of embolic complications may arise despite negative cardiac imaging.4 Embolic complications, which typically are seen within the first 2 to 4 weeks of treatment, may serve as the presenting feature of endocarditis and may even occur after completion of antimicrobial therapy.
Detection of cutaneous manifestations of infective endocarditis, including Janeway lesions, Osler nodes, and splinter hemorrhages, may allow for earlier diagnosis. Despite eponymous recognition, Janeway lesions and Osler nodes are relatively uncommon manifestations of infective endocarditis and may be found in only 5% to 15% of cases.5 Biopsies of suspected Janeway lesions and Osler nodes may allow for recognition of relevant vascular pathology, identification of the causative pathogen, and strong support for the diagnosis of infective endocarditis.4-7
The initial photomicrograph of corresponding Janeway lesion histopathology was published by Kerr in 1955 and revealed dermal microabscesses posited to be secondary to bacterial emboli.8,9 Additional cases through the years have reported overlapping histopathologic features of Janeway lesions and Osler nodes, with the latter often defined by the presence of vasculitis.4 Although there appears to be ongoing debate and overlap between the 2 integumentary findings, a general consensus on differentiation takes into account both the clinical signs and symptoms as well as the histopathologic findings.10,11
Osler nodes present as tender, violaceous, subcutaneous nodules on the acral surfaces, usually on the pads of the fingers and toes.5 The pathogenesis involves the deposition of immune complexes as a sequela of vascular occlusion by microthrombi classically seen in the late phase of subacute endocarditis. Janeway lesions present as nontender erythematous macules on the acral surfaces and are thought to represent microthrombi with dermal microabscesses, more common in acute endocarditis. Our patient demonstrated features of both Osler nodes and Janeway lesions. Despite the presence of fungal thrombi—a pathophysiology closer to that of Janeway lesions—the clinical presentation of painful acral nodules affecting finger pads and histologic features of vasculitis may be better characterized as Osler nodes. Regardless of pathogenesis, these cutaneous findings serve as a minor clinical criterion in the Duke criteria for the diagnosis of infective endocarditis when present.12
Candida infective endocarditis should be suspected in a patient with a history of valvular disease or prior infective endocarditis with fungemia, unexplained neurologic signs, or manifestations of peripheral embolization despite negative blood cultures.3 Particularly in the setting of negative cardiac imaging, recognition of CIE requires heightened diagnostic acumen and clinicopathologic correlation. Although culture and pathologic examination of valvular vegetations represents the gold standard for diagnosis of CIE, aspiration and culture of easily accessible septic emboli may provide rapid identification of the etiologic pathogen. In 1976, Alpert et al13 identified C albicans from an aspirated Osler node. Postmortem examination revealed extensive involvement of the homograft valve and aortic root with C albicans.13 Many other examples exist in the literature demonstrating matching pathogenic isolates from microbiologic cultures of skin and blood.4,9,14,15 Thadepalli and Francis7 investigated 26 cases of endocarditis in heroin users in which the admitting diagnosis was endocarditis in only 4 cases. The etiologic pathogen was aspirated from secondary sites of localized infections secondary to emboli, including cutaneous lesions in 10 of the cases. Gram stain and culture revealed the causative organism leading to the ultimate diagnosis and management in 17 of 26 patients with endocarditis.7
The incidence of fungal endocarditis is increasing, with a reported 67% of cases caused by nosocomial infection.1 Given the rising incidence of fungal endocarditis and its accompanying diagnostic difficulties, including frequently negative blood cultures and cardiac imaging, clinicians must perform careful skin examinations, employ judicious use of skin biopsy, and carefully correlate clinical and pathologic findings to improve recognition of this disease and guide patient care.
- Arnold CJ, Johnson M, Bayer AS, et al. Infective endocarditis: an observational cohort study with a focus on therapy. Antimicrob Agents Chemother. 2015;59:2365. doi:10.1128/AAC.04867-14
- Chaudhary SC, Sawlani KK, Arora R, et al. Native aortic valve fungal endocarditis. BMJ Case Rep. 2013;2013:bcr2012007144. doi:10.1136/bcr-2012-007144
- Ellis ME, Al-Abdely H, Sandridge A, et al. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis. 2001;32:50-62. doi:10.1086/317550
- Gil MP, Velasco M, Botella R, et al. Janeway lesions: differential diagnosis with Osler’s nodes. Int J Dermatol. 1993;32:673-674. doi:10.1111/j.1365-4362.1993.tb04025.x
- Gomes RT, Tiberto LR, Bello VNM, et al. Dermatologic manifestations of infective endocarditis. An Bras Dermatol. 2016;91:92-94.
- Yee JM. Osler’s nodes and the recognition of infective endocarditis: a lesion of diagnostic importance. South Med J. 1987;80:753-757.
- Thadepalli H, Francis C. Diagnostic clues in metastatic lesions of endocarditia in addicts. West J Med. 1978;128:1-5.
- Kerr A Jr. Subacute Bacterial Endocarditis. Charles C. Thomas; 1955.
- Kerr A Jr, Tan JS. Biopsies of the Janeway lesion of infective endocarditis. J Cutan Pathol. 1979;6:124-129. doi:10.1111/j.1600-0560.1979.tb01113.x
- Marrie TJ. Osler’s nodes and Janeway lesions. Am J Med. 2008;121:105-106. doi:10.1016/j.amjmed.2007.07.035
- Gunson TH, Oliver GF. Osler’s nodes and Janeway lesions. Australas J Dermatol. 2007;48:251-255. doi:10.1111/j.1440-0960.2007.00397.x
- Durack DT, Lukes AS, Bright DK, et al. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200-209.
- Alpert JS, Krous HF, Dalen JE, et al. Pathogenesis of Osler’s nodes. Ann Intern Med. 1976;85:471-473. doi:10.7326/0003-4819-85-4-471
- Cardullo AC, Silvers DN, Grossman ME. Janeway lesions and Osler’s nodes: a review of histopathologic findings. J Am Acad Dermatol. 1990;22:1088-1090. doi:10.1016/0190-9622(90)70157-D
- Vinson RP, Chung A, Elston DM, et al. Septic microemboli in a Janeway lesion of bacterial endocarditis. J Am Acad Dermatol. 1996;35:984-985. doi:10.1016/S0190-9622(96)90125-5
To the Editor:
A 44-year-old woman presented with a low-grade fever (temperature, 38.0 °C) and painful acral lesions of 1 week’s duration. She had a history of hepatitis C viral infection and intravenous (IV) drug use, as well as polymicrobial infective endocarditis that involved the tricuspid and aortic valves; pathogenic organisms were identified via blood culture as Enterococcus faecalis, Serratia species, Streptococcus viridans, and Candida albicans. The patient had received a mechanical aortic valve and bioprosthetic tricuspid valve replacement 5 months prior with warfarin therapy and had completed a postsurgical 6-week course of high-dose micafungin. She reported that she had developed painful, violaceous, thin papules on the plantar surface of the left foot 2 weeks prior to presentation. Her symptoms improved with a short systemic steroid taper; however, within a week she developed new tender, erythematous, thin papules on the plantar surface of the right foot and the palmar surface of the left hand with associated lower extremity swelling. She denied other symptoms, including fever, chills, neurologic symptoms, shortness of breath, chest pain, nausea, vomiting, hematuria, and hematochezia. Due to worsening cutaneous findings, the patient presented to the emergency department, prompting hospital admission for empiric antibacterial therapy with vancomycin and piperacillin-tazobactam for suspected infectious endocarditis. Dermatology was consulted after 1 day of antibacterial therapy without improvement to determine the etiology of the patient’s skin findings.
Physical examination revealed the patient was afebrile with partially blanching violaceous to purpuric, tender, edematous papules on the left fourth and fifth finger pads, as well as scattered, painful, purpuric patches with stellate borders on the right plantar foot (Figure 1). Laboratory test results revealed mild anemia (hemoglobin, 11.9 g/dL [reference range, 12.0–15.0 g/dL], mild neutrophilia (neutrophils, 8.4×109/L [reference range, 1.9–7.9×109/L], elevated acute-phase reactants (erythrocyte sedimentation rate, 71 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 5.7 mg/dL [reference range, 0.0–0.5 mg/dL]), and positive hepatitis C virus antibody with an undetectable viral load. At the time of dermatologic evaluation, admission blood cultures and transthoracic echocardiogram were negative. Additionally, a transesophageal echocardiogram, limited by artifact from the mechanical aortic valve, was equivocal for valvular pathology. Subsequent ophthalmologic evaluation was negative for lesions associated with endocarditis, such as retinal hemorrhages.

Punch biopsies of the left fourth finger pad were submitted for histopathologic analysis and tissue cultures. Histopathology demonstrated deep dermal perivascular neutrophilic inflammation with multiple intravascular thrombi, perivascular fibrin, and karyorrhectic debris (Figure 2). Periodic acid–Schiff and Grocott-Gomori methenamine-silver stains revealed fungal spores with rare pseudohyphae within the thrombosed vascular spaces and the perivascular dermis, consistent with fungal septic emboli (Figure 3).

Empiric systemic antifungal coverage composed of IV liposomal amphotericin B and oral flucytosine was initiated, and the patient’s tender acral papules rapidly improved. Within 48 hours of biopsy, skin tissue culture confirmed the presence of C albicans. Four days after the preliminary dermatopathology report, confirmatory blood cultures resulted with pansensitive C albicans. Final tissue and blood cultures were negative for bacteria including mycobacteria. In addition to a 6-week course of IV amphotericin B and flucytosine, repeat surgical intervention was considered, and lifelong suppressive antifungal oral therapy was recommended. Unfortunately, the patient did not present for follow-up. Three months later, she presented to the emergency department with peritonitis; in the operating room, she was found to have ischemia of the entirety of the small and large intestines and died shortly thereafter.

Fungal endocarditis is rare, tending to develop in patient populations with particular risk factors such as immune compromise, structural heart defects or prosthetic valves, and IV drug use. Candida infective endocarditis (CIE) represents less than 2% of infective endocarditis cases and carries a high mortality rate (30%–80%).1-3 Diagnosis may be challenging, as the clinical presentation varies widely. Although some patients may present with classic features of infective endocarditis, including fever, cardiac murmurs, and positive blood cultures, many cases of infective endocarditis present with nonspecific symptoms, raising a broad clinical differential diagnosis. Delay in diagnosis, which is seen in 82% of patients with fungal endocarditis, may be attributed to the slow progression of symptoms, inconclusive cardiac imaging, or negative blood cultures seen in almost one-third of cases.2,3 The feared complication of systemic embolization via infective endocarditis may occur in up to one-half of cases, with the highest rates associated with staphylococcal or fungal pathogens.2 The risk for embolization in fungal endocarditis is independent of the size of the cardiac valve vegetations; accordingly, sequelae of embolic complications may arise despite negative cardiac imaging.4 Embolic complications, which typically are seen within the first 2 to 4 weeks of treatment, may serve as the presenting feature of endocarditis and may even occur after completion of antimicrobial therapy.
Detection of cutaneous manifestations of infective endocarditis, including Janeway lesions, Osler nodes, and splinter hemorrhages, may allow for earlier diagnosis. Despite eponymous recognition, Janeway lesions and Osler nodes are relatively uncommon manifestations of infective endocarditis and may be found in only 5% to 15% of cases.5 Biopsies of suspected Janeway lesions and Osler nodes may allow for recognition of relevant vascular pathology, identification of the causative pathogen, and strong support for the diagnosis of infective endocarditis.4-7
The initial photomicrograph of corresponding Janeway lesion histopathology was published by Kerr in 1955 and revealed dermal microabscesses posited to be secondary to bacterial emboli.8,9 Additional cases through the years have reported overlapping histopathologic features of Janeway lesions and Osler nodes, with the latter often defined by the presence of vasculitis.4 Although there appears to be ongoing debate and overlap between the 2 integumentary findings, a general consensus on differentiation takes into account both the clinical signs and symptoms as well as the histopathologic findings.10,11
Osler nodes present as tender, violaceous, subcutaneous nodules on the acral surfaces, usually on the pads of the fingers and toes.5 The pathogenesis involves the deposition of immune complexes as a sequela of vascular occlusion by microthrombi classically seen in the late phase of subacute endocarditis. Janeway lesions present as nontender erythematous macules on the acral surfaces and are thought to represent microthrombi with dermal microabscesses, more common in acute endocarditis. Our patient demonstrated features of both Osler nodes and Janeway lesions. Despite the presence of fungal thrombi—a pathophysiology closer to that of Janeway lesions—the clinical presentation of painful acral nodules affecting finger pads and histologic features of vasculitis may be better characterized as Osler nodes. Regardless of pathogenesis, these cutaneous findings serve as a minor clinical criterion in the Duke criteria for the diagnosis of infective endocarditis when present.12
Candida infective endocarditis should be suspected in a patient with a history of valvular disease or prior infective endocarditis with fungemia, unexplained neurologic signs, or manifestations of peripheral embolization despite negative blood cultures.3 Particularly in the setting of negative cardiac imaging, recognition of CIE requires heightened diagnostic acumen and clinicopathologic correlation. Although culture and pathologic examination of valvular vegetations represents the gold standard for diagnosis of CIE, aspiration and culture of easily accessible septic emboli may provide rapid identification of the etiologic pathogen. In 1976, Alpert et al13 identified C albicans from an aspirated Osler node. Postmortem examination revealed extensive involvement of the homograft valve and aortic root with C albicans.13 Many other examples exist in the literature demonstrating matching pathogenic isolates from microbiologic cultures of skin and blood.4,9,14,15 Thadepalli and Francis7 investigated 26 cases of endocarditis in heroin users in which the admitting diagnosis was endocarditis in only 4 cases. The etiologic pathogen was aspirated from secondary sites of localized infections secondary to emboli, including cutaneous lesions in 10 of the cases. Gram stain and culture revealed the causative organism leading to the ultimate diagnosis and management in 17 of 26 patients with endocarditis.7
The incidence of fungal endocarditis is increasing, with a reported 67% of cases caused by nosocomial infection.1 Given the rising incidence of fungal endocarditis and its accompanying diagnostic difficulties, including frequently negative blood cultures and cardiac imaging, clinicians must perform careful skin examinations, employ judicious use of skin biopsy, and carefully correlate clinical and pathologic findings to improve recognition of this disease and guide patient care.
To the Editor:
A 44-year-old woman presented with a low-grade fever (temperature, 38.0 °C) and painful acral lesions of 1 week’s duration. She had a history of hepatitis C viral infection and intravenous (IV) drug use, as well as polymicrobial infective endocarditis that involved the tricuspid and aortic valves; pathogenic organisms were identified via blood culture as Enterococcus faecalis, Serratia species, Streptococcus viridans, and Candida albicans. The patient had received a mechanical aortic valve and bioprosthetic tricuspid valve replacement 5 months prior with warfarin therapy and had completed a postsurgical 6-week course of high-dose micafungin. She reported that she had developed painful, violaceous, thin papules on the plantar surface of the left foot 2 weeks prior to presentation. Her symptoms improved with a short systemic steroid taper; however, within a week she developed new tender, erythematous, thin papules on the plantar surface of the right foot and the palmar surface of the left hand with associated lower extremity swelling. She denied other symptoms, including fever, chills, neurologic symptoms, shortness of breath, chest pain, nausea, vomiting, hematuria, and hematochezia. Due to worsening cutaneous findings, the patient presented to the emergency department, prompting hospital admission for empiric antibacterial therapy with vancomycin and piperacillin-tazobactam for suspected infectious endocarditis. Dermatology was consulted after 1 day of antibacterial therapy without improvement to determine the etiology of the patient’s skin findings.
Physical examination revealed the patient was afebrile with partially blanching violaceous to purpuric, tender, edematous papules on the left fourth and fifth finger pads, as well as scattered, painful, purpuric patches with stellate borders on the right plantar foot (Figure 1). Laboratory test results revealed mild anemia (hemoglobin, 11.9 g/dL [reference range, 12.0–15.0 g/dL], mild neutrophilia (neutrophils, 8.4×109/L [reference range, 1.9–7.9×109/L], elevated acute-phase reactants (erythrocyte sedimentation rate, 71 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 5.7 mg/dL [reference range, 0.0–0.5 mg/dL]), and positive hepatitis C virus antibody with an undetectable viral load. At the time of dermatologic evaluation, admission blood cultures and transthoracic echocardiogram were negative. Additionally, a transesophageal echocardiogram, limited by artifact from the mechanical aortic valve, was equivocal for valvular pathology. Subsequent ophthalmologic evaluation was negative for lesions associated with endocarditis, such as retinal hemorrhages.

Punch biopsies of the left fourth finger pad were submitted for histopathologic analysis and tissue cultures. Histopathology demonstrated deep dermal perivascular neutrophilic inflammation with multiple intravascular thrombi, perivascular fibrin, and karyorrhectic debris (Figure 2). Periodic acid–Schiff and Grocott-Gomori methenamine-silver stains revealed fungal spores with rare pseudohyphae within the thrombosed vascular spaces and the perivascular dermis, consistent with fungal septic emboli (Figure 3).

Empiric systemic antifungal coverage composed of IV liposomal amphotericin B and oral flucytosine was initiated, and the patient’s tender acral papules rapidly improved. Within 48 hours of biopsy, skin tissue culture confirmed the presence of C albicans. Four days after the preliminary dermatopathology report, confirmatory blood cultures resulted with pansensitive C albicans. Final tissue and blood cultures were negative for bacteria including mycobacteria. In addition to a 6-week course of IV amphotericin B and flucytosine, repeat surgical intervention was considered, and lifelong suppressive antifungal oral therapy was recommended. Unfortunately, the patient did not present for follow-up. Three months later, she presented to the emergency department with peritonitis; in the operating room, she was found to have ischemia of the entirety of the small and large intestines and died shortly thereafter.

Fungal endocarditis is rare, tending to develop in patient populations with particular risk factors such as immune compromise, structural heart defects or prosthetic valves, and IV drug use. Candida infective endocarditis (CIE) represents less than 2% of infective endocarditis cases and carries a high mortality rate (30%–80%).1-3 Diagnosis may be challenging, as the clinical presentation varies widely. Although some patients may present with classic features of infective endocarditis, including fever, cardiac murmurs, and positive blood cultures, many cases of infective endocarditis present with nonspecific symptoms, raising a broad clinical differential diagnosis. Delay in diagnosis, which is seen in 82% of patients with fungal endocarditis, may be attributed to the slow progression of symptoms, inconclusive cardiac imaging, or negative blood cultures seen in almost one-third of cases.2,3 The feared complication of systemic embolization via infective endocarditis may occur in up to one-half of cases, with the highest rates associated with staphylococcal or fungal pathogens.2 The risk for embolization in fungal endocarditis is independent of the size of the cardiac valve vegetations; accordingly, sequelae of embolic complications may arise despite negative cardiac imaging.4 Embolic complications, which typically are seen within the first 2 to 4 weeks of treatment, may serve as the presenting feature of endocarditis and may even occur after completion of antimicrobial therapy.
Detection of cutaneous manifestations of infective endocarditis, including Janeway lesions, Osler nodes, and splinter hemorrhages, may allow for earlier diagnosis. Despite eponymous recognition, Janeway lesions and Osler nodes are relatively uncommon manifestations of infective endocarditis and may be found in only 5% to 15% of cases.5 Biopsies of suspected Janeway lesions and Osler nodes may allow for recognition of relevant vascular pathology, identification of the causative pathogen, and strong support for the diagnosis of infective endocarditis.4-7
The initial photomicrograph of corresponding Janeway lesion histopathology was published by Kerr in 1955 and revealed dermal microabscesses posited to be secondary to bacterial emboli.8,9 Additional cases through the years have reported overlapping histopathologic features of Janeway lesions and Osler nodes, with the latter often defined by the presence of vasculitis.4 Although there appears to be ongoing debate and overlap between the 2 integumentary findings, a general consensus on differentiation takes into account both the clinical signs and symptoms as well as the histopathologic findings.10,11
Osler nodes present as tender, violaceous, subcutaneous nodules on the acral surfaces, usually on the pads of the fingers and toes.5 The pathogenesis involves the deposition of immune complexes as a sequela of vascular occlusion by microthrombi classically seen in the late phase of subacute endocarditis. Janeway lesions present as nontender erythematous macules on the acral surfaces and are thought to represent microthrombi with dermal microabscesses, more common in acute endocarditis. Our patient demonstrated features of both Osler nodes and Janeway lesions. Despite the presence of fungal thrombi—a pathophysiology closer to that of Janeway lesions—the clinical presentation of painful acral nodules affecting finger pads and histologic features of vasculitis may be better characterized as Osler nodes. Regardless of pathogenesis, these cutaneous findings serve as a minor clinical criterion in the Duke criteria for the diagnosis of infective endocarditis when present.12
Candida infective endocarditis should be suspected in a patient with a history of valvular disease or prior infective endocarditis with fungemia, unexplained neurologic signs, or manifestations of peripheral embolization despite negative blood cultures.3 Particularly in the setting of negative cardiac imaging, recognition of CIE requires heightened diagnostic acumen and clinicopathologic correlation. Although culture and pathologic examination of valvular vegetations represents the gold standard for diagnosis of CIE, aspiration and culture of easily accessible septic emboli may provide rapid identification of the etiologic pathogen. In 1976, Alpert et al13 identified C albicans from an aspirated Osler node. Postmortem examination revealed extensive involvement of the homograft valve and aortic root with C albicans.13 Many other examples exist in the literature demonstrating matching pathogenic isolates from microbiologic cultures of skin and blood.4,9,14,15 Thadepalli and Francis7 investigated 26 cases of endocarditis in heroin users in which the admitting diagnosis was endocarditis in only 4 cases. The etiologic pathogen was aspirated from secondary sites of localized infections secondary to emboli, including cutaneous lesions in 10 of the cases. Gram stain and culture revealed the causative organism leading to the ultimate diagnosis and management in 17 of 26 patients with endocarditis.7
The incidence of fungal endocarditis is increasing, with a reported 67% of cases caused by nosocomial infection.1 Given the rising incidence of fungal endocarditis and its accompanying diagnostic difficulties, including frequently negative blood cultures and cardiac imaging, clinicians must perform careful skin examinations, employ judicious use of skin biopsy, and carefully correlate clinical and pathologic findings to improve recognition of this disease and guide patient care.
- Arnold CJ, Johnson M, Bayer AS, et al. Infective endocarditis: an observational cohort study with a focus on therapy. Antimicrob Agents Chemother. 2015;59:2365. doi:10.1128/AAC.04867-14
- Chaudhary SC, Sawlani KK, Arora R, et al. Native aortic valve fungal endocarditis. BMJ Case Rep. 2013;2013:bcr2012007144. doi:10.1136/bcr-2012-007144
- Ellis ME, Al-Abdely H, Sandridge A, et al. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis. 2001;32:50-62. doi:10.1086/317550
- Gil MP, Velasco M, Botella R, et al. Janeway lesions: differential diagnosis with Osler’s nodes. Int J Dermatol. 1993;32:673-674. doi:10.1111/j.1365-4362.1993.tb04025.x
- Gomes RT, Tiberto LR, Bello VNM, et al. Dermatologic manifestations of infective endocarditis. An Bras Dermatol. 2016;91:92-94.
- Yee JM. Osler’s nodes and the recognition of infective endocarditis: a lesion of diagnostic importance. South Med J. 1987;80:753-757.
- Thadepalli H, Francis C. Diagnostic clues in metastatic lesions of endocarditia in addicts. West J Med. 1978;128:1-5.
- Kerr A Jr. Subacute Bacterial Endocarditis. Charles C. Thomas; 1955.
- Kerr A Jr, Tan JS. Biopsies of the Janeway lesion of infective endocarditis. J Cutan Pathol. 1979;6:124-129. doi:10.1111/j.1600-0560.1979.tb01113.x
- Marrie TJ. Osler’s nodes and Janeway lesions. Am J Med. 2008;121:105-106. doi:10.1016/j.amjmed.2007.07.035
- Gunson TH, Oliver GF. Osler’s nodes and Janeway lesions. Australas J Dermatol. 2007;48:251-255. doi:10.1111/j.1440-0960.2007.00397.x
- Durack DT, Lukes AS, Bright DK, et al. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200-209.
- Alpert JS, Krous HF, Dalen JE, et al. Pathogenesis of Osler’s nodes. Ann Intern Med. 1976;85:471-473. doi:10.7326/0003-4819-85-4-471
- Cardullo AC, Silvers DN, Grossman ME. Janeway lesions and Osler’s nodes: a review of histopathologic findings. J Am Acad Dermatol. 1990;22:1088-1090. doi:10.1016/0190-9622(90)70157-D
- Vinson RP, Chung A, Elston DM, et al. Septic microemboli in a Janeway lesion of bacterial endocarditis. J Am Acad Dermatol. 1996;35:984-985. doi:10.1016/S0190-9622(96)90125-5
- Arnold CJ, Johnson M, Bayer AS, et al. Infective endocarditis: an observational cohort study with a focus on therapy. Antimicrob Agents Chemother. 2015;59:2365. doi:10.1128/AAC.04867-14
- Chaudhary SC, Sawlani KK, Arora R, et al. Native aortic valve fungal endocarditis. BMJ Case Rep. 2013;2013:bcr2012007144. doi:10.1136/bcr-2012-007144
- Ellis ME, Al-Abdely H, Sandridge A, et al. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis. 2001;32:50-62. doi:10.1086/317550
- Gil MP, Velasco M, Botella R, et al. Janeway lesions: differential diagnosis with Osler’s nodes. Int J Dermatol. 1993;32:673-674. doi:10.1111/j.1365-4362.1993.tb04025.x
- Gomes RT, Tiberto LR, Bello VNM, et al. Dermatologic manifestations of infective endocarditis. An Bras Dermatol. 2016;91:92-94.
- Yee JM. Osler’s nodes and the recognition of infective endocarditis: a lesion of diagnostic importance. South Med J. 1987;80:753-757.
- Thadepalli H, Francis C. Diagnostic clues in metastatic lesions of endocarditia in addicts. West J Med. 1978;128:1-5.
- Kerr A Jr. Subacute Bacterial Endocarditis. Charles C. Thomas; 1955.
- Kerr A Jr, Tan JS. Biopsies of the Janeway lesion of infective endocarditis. J Cutan Pathol. 1979;6:124-129. doi:10.1111/j.1600-0560.1979.tb01113.x
- Marrie TJ. Osler’s nodes and Janeway lesions. Am J Med. 2008;121:105-106. doi:10.1016/j.amjmed.2007.07.035
- Gunson TH, Oliver GF. Osler’s nodes and Janeway lesions. Australas J Dermatol. 2007;48:251-255. doi:10.1111/j.1440-0960.2007.00397.x
- Durack DT, Lukes AS, Bright DK, et al. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200-209.
- Alpert JS, Krous HF, Dalen JE, et al. Pathogenesis of Osler’s nodes. Ann Intern Med. 1976;85:471-473. doi:10.7326/0003-4819-85-4-471
- Cardullo AC, Silvers DN, Grossman ME. Janeway lesions and Osler’s nodes: a review of histopathologic findings. J Am Acad Dermatol. 1990;22:1088-1090. doi:10.1016/0190-9622(90)70157-D
- Vinson RP, Chung A, Elston DM, et al. Septic microemboli in a Janeway lesion of bacterial endocarditis. J Am Acad Dermatol. 1996;35:984-985. doi:10.1016/S0190-9622(96)90125-5
PRACTICE POINTS
- Fungal infective endocarditis is rare, and diagnostic tests such as blood cultures and echocardiography may not detect the disease.
- The mortality rate of fungal endocarditis is high, with improved clinical outcomes if diagnosed and treated early.
- Clinicopathologic correlation between integumentary examination and skin biopsy findings may provide timely diagnosis, thereby guiding appropriate therapy.
Adult-onset asthma subtypes associated with both eosinophil, neutrophil levels
The clinical features and inflammatory mediators of adult-onset asthma were associated with distinct endotype groups defined by eosinophil and neutrophil levels, based on data from a real-life long term study of 203 patients.
Asthma is a chronic condition from lower respiratory tract inflammation composed of complex, heterogeneous endotypes with T2 helper cells being one way to distinguish between them. Endotypes have previously been suggested to have differing risks for asthma exacerbations and severity. However, clinical and biomarker information used for recognizing and targeting treatment is largely lacking in those subgroups other than eosinophilic asthma, according to Ella Flinkman, faculty of medicine and health technology, of Tampere University (Finland), and colleagues.
In a study published in The Journal of Allergy and Clinical Immunology: In Practice the researchers reported on their single-center 12-year follow-up phase II Seinäjoki Adult Asthma Study (SAAS). The included cohort of 203 patients had a median age of 58 years and 58% were women; all participants were originally diagnosed by a respiratory specialist physician as having new adult-onset asthma during the years 1999-2000 using asthma symptoms and objective lung function measurements.
To evaluate the association between clinical features and inflammation mediators to venous blood granulocytes this cohort was divided into paucigranulocytic (n = 108), neutrophilic (n = 60), eosinophilic (n = 21), and mixed granulocytic (n = 14) endotype subgroups based on eosinophil and neutrophil levels. Objective comparisons between groups were made using measurements from forced expiratory volume in 1 second (FEV1), fraction of exhaled nitric oxide (FeNO), immunoglobin E (IgE), high-sensitivity C-reactive protein (hsCRP), IL-6, resistin, MMP-9, plasma soluble urokinase plasminogen activator receptor (suPAR), leptin, HMW adiponectin, and periostin tests. Asthma-related medications and disease exacerbation data were collected from medical records accumulated over the 12-year study period.
The neutrophilic group was defined by high (≥ 4.4×109/L) neutrophil but low (< 0.30×109/L) eosinophil counts and conversely the eosinophilic group had low (< 4.4×109/L) neutrophil but high (≥ 0.30×109/L) eosinophil counts. The paucigranulocytic was low and the mixed granulocytic group was high for both eosinophil and neutrophil levels, respectively. Each group was associated with a unique profile of features related to asthma prognosis and treatment. The paucigranulocytic endotype was used as the base comparison group in regression analysis as it was the least likely to meet the definition of severe asthma. This was indicated by the lowest use of inhaled corticosteroid (ICS), antibiotics, and occurrence of unplanned respiratory visits. The other three groups were more likely to fulfill a severe asthma classification.
Negative binomial regression analysis showed significant association of increased incidence rate ratio (IRR) of unplanned respiratory visits, highest body mass index (BMI), and highest dispensed doses of ICS with neutrophilic asthma. Additional significantly associated factors included smoking history and gender. Adjustment for dispensed ICS 2 years prior to the 12-year follow-up visit resulted in a change from borderline to significant association of increased IRR for the eosinophilic group. Both the eosinophilic and neutrophilic groups were associated with the most antibiotic use over the 12-year follow-up period. The authors suggested their data may indicate that antibiotics are overprescribed for asthma and further investigation is required.
Multiple linear regression analysis showed a decline in lung function associated with the eosinophilic but not the neutrophilic group. Connections between specific blood endotypes and molecular features were also identified. Highest periostin and FeNO levels found in the eosinophilic group were consistent with other studies on patients specifically diagnosed with eosinophilic asthma.
The neutrophilic group was distinguished by the highest hsCRP, MMP-9, IL-6, leptin, and suPAR levels. Highest resistin levels were found in both the mixed granulocyte and neutrophilic groups.
This study was strengthened by its real life long-term nature and method for cohort selection, according to the authors, though the value of a larger population to raise numbers particularly in the smaller sized groups was noted.
The authors concluded: “Our study indicates that assays of blood eosinophil and neutrophil counts provide useful information for assessing and treating patients with adult-onset asthma. These granulocyte counts reflect the underlying inflammatory pattern and reveal important differences in asthma clinical features and outcomes.” Additional research “regarding biomarkers used to identify different endotypes of asthma” is needed.
The study was sponsored by a number of research foundations in Finland as well as hospital research center funds. Several of the authors disclosed associations with pharmaceutical companies, including Astra Zeneca, Boehringer-Ingelheim, GSK, Novartis, and Sanofi.
The clinical features and inflammatory mediators of adult-onset asthma were associated with distinct endotype groups defined by eosinophil and neutrophil levels, based on data from a real-life long term study of 203 patients.
Asthma is a chronic condition from lower respiratory tract inflammation composed of complex, heterogeneous endotypes with T2 helper cells being one way to distinguish between them. Endotypes have previously been suggested to have differing risks for asthma exacerbations and severity. However, clinical and biomarker information used for recognizing and targeting treatment is largely lacking in those subgroups other than eosinophilic asthma, according to Ella Flinkman, faculty of medicine and health technology, of Tampere University (Finland), and colleagues.
In a study published in The Journal of Allergy and Clinical Immunology: In Practice the researchers reported on their single-center 12-year follow-up phase II Seinäjoki Adult Asthma Study (SAAS). The included cohort of 203 patients had a median age of 58 years and 58% were women; all participants were originally diagnosed by a respiratory specialist physician as having new adult-onset asthma during the years 1999-2000 using asthma symptoms and objective lung function measurements.
To evaluate the association between clinical features and inflammation mediators to venous blood granulocytes this cohort was divided into paucigranulocytic (n = 108), neutrophilic (n = 60), eosinophilic (n = 21), and mixed granulocytic (n = 14) endotype subgroups based on eosinophil and neutrophil levels. Objective comparisons between groups were made using measurements from forced expiratory volume in 1 second (FEV1), fraction of exhaled nitric oxide (FeNO), immunoglobin E (IgE), high-sensitivity C-reactive protein (hsCRP), IL-6, resistin, MMP-9, plasma soluble urokinase plasminogen activator receptor (suPAR), leptin, HMW adiponectin, and periostin tests. Asthma-related medications and disease exacerbation data were collected from medical records accumulated over the 12-year study period.
The neutrophilic group was defined by high (≥ 4.4×109/L) neutrophil but low (< 0.30×109/L) eosinophil counts and conversely the eosinophilic group had low (< 4.4×109/L) neutrophil but high (≥ 0.30×109/L) eosinophil counts. The paucigranulocytic was low and the mixed granulocytic group was high for both eosinophil and neutrophil levels, respectively. Each group was associated with a unique profile of features related to asthma prognosis and treatment. The paucigranulocytic endotype was used as the base comparison group in regression analysis as it was the least likely to meet the definition of severe asthma. This was indicated by the lowest use of inhaled corticosteroid (ICS), antibiotics, and occurrence of unplanned respiratory visits. The other three groups were more likely to fulfill a severe asthma classification.
Negative binomial regression analysis showed significant association of increased incidence rate ratio (IRR) of unplanned respiratory visits, highest body mass index (BMI), and highest dispensed doses of ICS with neutrophilic asthma. Additional significantly associated factors included smoking history and gender. Adjustment for dispensed ICS 2 years prior to the 12-year follow-up visit resulted in a change from borderline to significant association of increased IRR for the eosinophilic group. Both the eosinophilic and neutrophilic groups were associated with the most antibiotic use over the 12-year follow-up period. The authors suggested their data may indicate that antibiotics are overprescribed for asthma and further investigation is required.
Multiple linear regression analysis showed a decline in lung function associated with the eosinophilic but not the neutrophilic group. Connections between specific blood endotypes and molecular features were also identified. Highest periostin and FeNO levels found in the eosinophilic group were consistent with other studies on patients specifically diagnosed with eosinophilic asthma.
The neutrophilic group was distinguished by the highest hsCRP, MMP-9, IL-6, leptin, and suPAR levels. Highest resistin levels were found in both the mixed granulocyte and neutrophilic groups.
This study was strengthened by its real life long-term nature and method for cohort selection, according to the authors, though the value of a larger population to raise numbers particularly in the smaller sized groups was noted.
The authors concluded: “Our study indicates that assays of blood eosinophil and neutrophil counts provide useful information for assessing and treating patients with adult-onset asthma. These granulocyte counts reflect the underlying inflammatory pattern and reveal important differences in asthma clinical features and outcomes.” Additional research “regarding biomarkers used to identify different endotypes of asthma” is needed.
The study was sponsored by a number of research foundations in Finland as well as hospital research center funds. Several of the authors disclosed associations with pharmaceutical companies, including Astra Zeneca, Boehringer-Ingelheim, GSK, Novartis, and Sanofi.
The clinical features and inflammatory mediators of adult-onset asthma were associated with distinct endotype groups defined by eosinophil and neutrophil levels, based on data from a real-life long term study of 203 patients.
Asthma is a chronic condition from lower respiratory tract inflammation composed of complex, heterogeneous endotypes with T2 helper cells being one way to distinguish between them. Endotypes have previously been suggested to have differing risks for asthma exacerbations and severity. However, clinical and biomarker information used for recognizing and targeting treatment is largely lacking in those subgroups other than eosinophilic asthma, according to Ella Flinkman, faculty of medicine and health technology, of Tampere University (Finland), and colleagues.
In a study published in The Journal of Allergy and Clinical Immunology: In Practice the researchers reported on their single-center 12-year follow-up phase II Seinäjoki Adult Asthma Study (SAAS). The included cohort of 203 patients had a median age of 58 years and 58% were women; all participants were originally diagnosed by a respiratory specialist physician as having new adult-onset asthma during the years 1999-2000 using asthma symptoms and objective lung function measurements.
To evaluate the association between clinical features and inflammation mediators to venous blood granulocytes this cohort was divided into paucigranulocytic (n = 108), neutrophilic (n = 60), eosinophilic (n = 21), and mixed granulocytic (n = 14) endotype subgroups based on eosinophil and neutrophil levels. Objective comparisons between groups were made using measurements from forced expiratory volume in 1 second (FEV1), fraction of exhaled nitric oxide (FeNO), immunoglobin E (IgE), high-sensitivity C-reactive protein (hsCRP), IL-6, resistin, MMP-9, plasma soluble urokinase plasminogen activator receptor (suPAR), leptin, HMW adiponectin, and periostin tests. Asthma-related medications and disease exacerbation data were collected from medical records accumulated over the 12-year study period.
The neutrophilic group was defined by high (≥ 4.4×109/L) neutrophil but low (< 0.30×109/L) eosinophil counts and conversely the eosinophilic group had low (< 4.4×109/L) neutrophil but high (≥ 0.30×109/L) eosinophil counts. The paucigranulocytic was low and the mixed granulocytic group was high for both eosinophil and neutrophil levels, respectively. Each group was associated with a unique profile of features related to asthma prognosis and treatment. The paucigranulocytic endotype was used as the base comparison group in regression analysis as it was the least likely to meet the definition of severe asthma. This was indicated by the lowest use of inhaled corticosteroid (ICS), antibiotics, and occurrence of unplanned respiratory visits. The other three groups were more likely to fulfill a severe asthma classification.
Negative binomial regression analysis showed significant association of increased incidence rate ratio (IRR) of unplanned respiratory visits, highest body mass index (BMI), and highest dispensed doses of ICS with neutrophilic asthma. Additional significantly associated factors included smoking history and gender. Adjustment for dispensed ICS 2 years prior to the 12-year follow-up visit resulted in a change from borderline to significant association of increased IRR for the eosinophilic group. Both the eosinophilic and neutrophilic groups were associated with the most antibiotic use over the 12-year follow-up period. The authors suggested their data may indicate that antibiotics are overprescribed for asthma and further investigation is required.
Multiple linear regression analysis showed a decline in lung function associated with the eosinophilic but not the neutrophilic group. Connections between specific blood endotypes and molecular features were also identified. Highest periostin and FeNO levels found in the eosinophilic group were consistent with other studies on patients specifically diagnosed with eosinophilic asthma.
The neutrophilic group was distinguished by the highest hsCRP, MMP-9, IL-6, leptin, and suPAR levels. Highest resistin levels were found in both the mixed granulocyte and neutrophilic groups.
This study was strengthened by its real life long-term nature and method for cohort selection, according to the authors, though the value of a larger population to raise numbers particularly in the smaller sized groups was noted.
The authors concluded: “Our study indicates that assays of blood eosinophil and neutrophil counts provide useful information for assessing and treating patients with adult-onset asthma. These granulocyte counts reflect the underlying inflammatory pattern and reveal important differences in asthma clinical features and outcomes.” Additional research “regarding biomarkers used to identify different endotypes of asthma” is needed.
The study was sponsored by a number of research foundations in Finland as well as hospital research center funds. Several of the authors disclosed associations with pharmaceutical companies, including Astra Zeneca, Boehringer-Ingelheim, GSK, Novartis, and Sanofi.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Managing respiratory symptoms in the ‘tripledemic’ era
Is it COVID-19, flu, or even RSV? I recently described just such a patient, an obese woman with type 2 diabetes, presenting with fever, cough, myalgia, and fatigue. I asked readers whether they agreed with my management of this patient.
Thank you for your comments as we continue to react to high rates of URIs. Your comments highlight the importance of local resources and practice habits when managing patients with URI.
It was clear that readers value testing to distinguish between infections. However, access to testing is highly variable around the world and is likely to be routinely used only in high-income countries. The Kaiser Family Foundation performed a cost analysis of testing for SARS-CoV-2 in 2020 and found, not surprisingly, wide variability in the cost of testing. Medicare covers tests at rates of $36-$143 per test; a study of list prices for SARS-CoV-2 tests at 93 hospitals found a median cost of $148 per test. And this does not include collection or facility fees. About 20% of tests cost more than $300.
These costs are prohibitive for many health systems. However, more devices have been introduced since that analysis, and competition and evolving technology should drive down prices. Generally, multiplex polymerase chain reaction (PCR) testing for multiple pathogens is less expensive than ordering two or three separate molecular tests and is more convenient for patients and practices alike.
Other reader comments focused on the challenges of getting accurate data on viral epidemiology, and there is certainly a time lag between infection trends and public health reports. This is exacerbated by underreporting of symptoms and more testing at home using antigen tests.
But please do not give up on epidemiology! If a test such as PCR is 90% sensitive for identifying infection, the yield in terms of the number of individuals infected with a particular virus should be high, and that is true when infection is in broad circulation. If 20% of a population of 1,000 has an infection and the test sensitivity is 90%, the yield of testing is 180 true cases versus 20 false positives.
However, if just 2% of the population of 1,000 has the infection in this same scenario, then only 18 true cases are identified. The effect on public health is certainly less, and a lower prevalence rate means that confounding variables, such as how long an individual might shed viral particles and the method of sample collection, have an outsized effect on results. This reduces the validity of diagnostic tests.
Even trends on a national level can provide some insight regarding whom to test. Traditionally, our practice has been to not routinely test patients for influenza or RSV from late spring to early fall unless there was a compelling reason, such as recent travel to an area where these infections were more prevalent. The loss of temporality for these infections since 2020 has altered this approach and made us pay more attention to reports from public health organizations.
I also appreciate the discussion of how to treat Agnes’s symptoms as she waits to improve, and anyone who suffers with or treats a viral URI knows that there are few interventions effective for such symptoms as cough and congestion. A systematic review of 29 randomized controlled trials of over-the-counter medications for cough yielded mixed and largely negative results.
Antihistamines alone do not seem to work, and guaifenesin was successful in only one of three trials. Combinations of different drug classes appeared to be slightly more effective.
My personal favorite for the management of acute cough is something that kids generally love: honey. In a review of 14 studies, 9 of which were limited to pediatric patients, honey was associated with significant reductions in cough frequency, cough severity, and total symptom score. However, there was a moderate risk of bias in the included research, and evidence of honey’s benefit in placebo-controlled trials was limited. Honey used in this research came in a variety of forms, so the best dosage is uncertain.
Clearly, advancements are needed. Better symptom management in viral URI will almost certainly improve productivity across the population and will probably reduce the inappropriate use of antibiotics as well. I have said for years that the scientists who can solve the Gordian knot of pediatric mucus deserve three Nobel prizes. I look forward to that golden day.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported a conflict of interest with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Is it COVID-19, flu, or even RSV? I recently described just such a patient, an obese woman with type 2 diabetes, presenting with fever, cough, myalgia, and fatigue. I asked readers whether they agreed with my management of this patient.
Thank you for your comments as we continue to react to high rates of URIs. Your comments highlight the importance of local resources and practice habits when managing patients with URI.
It was clear that readers value testing to distinguish between infections. However, access to testing is highly variable around the world and is likely to be routinely used only in high-income countries. The Kaiser Family Foundation performed a cost analysis of testing for SARS-CoV-2 in 2020 and found, not surprisingly, wide variability in the cost of testing. Medicare covers tests at rates of $36-$143 per test; a study of list prices for SARS-CoV-2 tests at 93 hospitals found a median cost of $148 per test. And this does not include collection or facility fees. About 20% of tests cost more than $300.
These costs are prohibitive for many health systems. However, more devices have been introduced since that analysis, and competition and evolving technology should drive down prices. Generally, multiplex polymerase chain reaction (PCR) testing for multiple pathogens is less expensive than ordering two or three separate molecular tests and is more convenient for patients and practices alike.
Other reader comments focused on the challenges of getting accurate data on viral epidemiology, and there is certainly a time lag between infection trends and public health reports. This is exacerbated by underreporting of symptoms and more testing at home using antigen tests.
But please do not give up on epidemiology! If a test such as PCR is 90% sensitive for identifying infection, the yield in terms of the number of individuals infected with a particular virus should be high, and that is true when infection is in broad circulation. If 20% of a population of 1,000 has an infection and the test sensitivity is 90%, the yield of testing is 180 true cases versus 20 false positives.
However, if just 2% of the population of 1,000 has the infection in this same scenario, then only 18 true cases are identified. The effect on public health is certainly less, and a lower prevalence rate means that confounding variables, such as how long an individual might shed viral particles and the method of sample collection, have an outsized effect on results. This reduces the validity of diagnostic tests.
Even trends on a national level can provide some insight regarding whom to test. Traditionally, our practice has been to not routinely test patients for influenza or RSV from late spring to early fall unless there was a compelling reason, such as recent travel to an area where these infections were more prevalent. The loss of temporality for these infections since 2020 has altered this approach and made us pay more attention to reports from public health organizations.
I also appreciate the discussion of how to treat Agnes’s symptoms as she waits to improve, and anyone who suffers with or treats a viral URI knows that there are few interventions effective for such symptoms as cough and congestion. A systematic review of 29 randomized controlled trials of over-the-counter medications for cough yielded mixed and largely negative results.
Antihistamines alone do not seem to work, and guaifenesin was successful in only one of three trials. Combinations of different drug classes appeared to be slightly more effective.
My personal favorite for the management of acute cough is something that kids generally love: honey. In a review of 14 studies, 9 of which were limited to pediatric patients, honey was associated with significant reductions in cough frequency, cough severity, and total symptom score. However, there was a moderate risk of bias in the included research, and evidence of honey’s benefit in placebo-controlled trials was limited. Honey used in this research came in a variety of forms, so the best dosage is uncertain.
Clearly, advancements are needed. Better symptom management in viral URI will almost certainly improve productivity across the population and will probably reduce the inappropriate use of antibiotics as well. I have said for years that the scientists who can solve the Gordian knot of pediatric mucus deserve three Nobel prizes. I look forward to that golden day.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported a conflict of interest with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Is it COVID-19, flu, or even RSV? I recently described just such a patient, an obese woman with type 2 diabetes, presenting with fever, cough, myalgia, and fatigue. I asked readers whether they agreed with my management of this patient.
Thank you for your comments as we continue to react to high rates of URIs. Your comments highlight the importance of local resources and practice habits when managing patients with URI.
It was clear that readers value testing to distinguish between infections. However, access to testing is highly variable around the world and is likely to be routinely used only in high-income countries. The Kaiser Family Foundation performed a cost analysis of testing for SARS-CoV-2 in 2020 and found, not surprisingly, wide variability in the cost of testing. Medicare covers tests at rates of $36-$143 per test; a study of list prices for SARS-CoV-2 tests at 93 hospitals found a median cost of $148 per test. And this does not include collection or facility fees. About 20% of tests cost more than $300.
These costs are prohibitive for many health systems. However, more devices have been introduced since that analysis, and competition and evolving technology should drive down prices. Generally, multiplex polymerase chain reaction (PCR) testing for multiple pathogens is less expensive than ordering two or three separate molecular tests and is more convenient for patients and practices alike.
Other reader comments focused on the challenges of getting accurate data on viral epidemiology, and there is certainly a time lag between infection trends and public health reports. This is exacerbated by underreporting of symptoms and more testing at home using antigen tests.
But please do not give up on epidemiology! If a test such as PCR is 90% sensitive for identifying infection, the yield in terms of the number of individuals infected with a particular virus should be high, and that is true when infection is in broad circulation. If 20% of a population of 1,000 has an infection and the test sensitivity is 90%, the yield of testing is 180 true cases versus 20 false positives.
However, if just 2% of the population of 1,000 has the infection in this same scenario, then only 18 true cases are identified. The effect on public health is certainly less, and a lower prevalence rate means that confounding variables, such as how long an individual might shed viral particles and the method of sample collection, have an outsized effect on results. This reduces the validity of diagnostic tests.
Even trends on a national level can provide some insight regarding whom to test. Traditionally, our practice has been to not routinely test patients for influenza or RSV from late spring to early fall unless there was a compelling reason, such as recent travel to an area where these infections were more prevalent. The loss of temporality for these infections since 2020 has altered this approach and made us pay more attention to reports from public health organizations.
I also appreciate the discussion of how to treat Agnes’s symptoms as she waits to improve, and anyone who suffers with or treats a viral URI knows that there are few interventions effective for such symptoms as cough and congestion. A systematic review of 29 randomized controlled trials of over-the-counter medications for cough yielded mixed and largely negative results.
Antihistamines alone do not seem to work, and guaifenesin was successful in only one of three trials. Combinations of different drug classes appeared to be slightly more effective.
My personal favorite for the management of acute cough is something that kids generally love: honey. In a review of 14 studies, 9 of which were limited to pediatric patients, honey was associated with significant reductions in cough frequency, cough severity, and total symptom score. However, there was a moderate risk of bias in the included research, and evidence of honey’s benefit in placebo-controlled trials was limited. Honey used in this research came in a variety of forms, so the best dosage is uncertain.
Clearly, advancements are needed. Better symptom management in viral URI will almost certainly improve productivity across the population and will probably reduce the inappropriate use of antibiotics as well. I have said for years that the scientists who can solve the Gordian knot of pediatric mucus deserve three Nobel prizes. I look forward to that golden day.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported a conflict of interest with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Citing workplace violence, one-fourth of critical care workers are ready to quit
A surgeon in Tulsa shot by a disgruntled patient. A doctor in India beaten by a group of bereaved family members. A general practitioner in the United Kingdom threatened with stabbing. A new study identifies this trend and finds that 25% of health care workers polled were willing to quit because of such violence.
“That was pretty appalling,” Rahul Kashyap, MD, MBA, MBBS, recalls. Dr. Kashyap is one of the leaders of the Violence Study of Healthcare Workers and Systems (ViSHWaS), which polled an international sample of physicians, nurses, and hospital staff. This study has worrying implications, Dr. Kashyap says. In a time when hospital staff are reporting burnout in record numbers, further deterrents may be the last thing our health care system needs. But Dr. Kashyap hopes that bringing awareness to these trends may allow physicians, policymakers, and the public to mobilize and intervene before it’s too late.
Previous studies have revealed similar trends. The rate of workplace violence directed at U.S. health care workers is five times that of workers in any other industry, according to the Bureau of Labor Statistics. The same study found that attacks had increased 63% from 2011 to 2018. Other polls that focus on the pandemic show that nearly half of U.S. nurses believe that violence increased since the world shut down. Well before the pandemic, however, a study from the Indian Medical Association found that 75% of doctors experienced workplace violence.
With this history in mind, perhaps it’s not surprising that the idea for the study came from the authors’ personal experiences. They had seen coworkers go through attacks, or they had endured attacks themselves, Dr. Kashyap says. But they couldn’t find any global data to back up these experiences. So Dr. Kashyap and his colleagues formed a web of volunteers dedicated to creating a cross-sectional study.
They got in touch with researchers from countries across Asia, the Middle East, South America, North America, and Africa. The initial group agreed to reach out to their contacts, casting a wide net. Researchers used WhatsApp, LinkedIn, and text messages to distribute the survey. Health care workers in each country completed the brief questionnaire, recalling their prepandemic world and evaluating their current one.
Within 2 months, they had reached health care workers in more than 100 countries. They concluded the study when they received about 5,000 results, according to Dr. Kashyap, and then began the process of stratifying the data. For this report, they focused on critical care, emergency medicine, and anesthesiology, which resulted in 598 responses from 69 countries. Of these, India and the United States had the highest number of participants.
In all, 73% of participants reported facing physical or verbal violence while in the hospital; 48% said they felt less motivated to work because of that violence; 39% of respondents believed that the amount of violence they experienced was the same as before the COVID-19 pandemic; and 36% of respondents believed that violence had increased. Even though they were trained on guidelines from the Occupational Safety and Health Administration, 20% of participants felt unprepared to face violence.
Although the study didn’t analyze the reasons workers felt this way, Dr. Kashyap speculates that it could be related to the medical distrust that grew during the pandemic or the stress patients and health care professionals experienced during its peak.
Regardless, the researchers say their study is a starting point. Now that the trend has been highlighted, it may be acted on.
Moving forward, Dr. Kashyap believes that controlling for different variables could determine whether factors like gender or shift time put a worker at higher risk for violence. He hopes it’s possible to interrupt these patterns and reestablish trust in the hospital environment. “It’s aspirational, but you’re hoping that through studies like ViSHWaS, which means trust in Hindi ... [we could restore] the trust and confidence among health care providers for the patients and family members.”
A version of this article first appeared on Medscape.com.
A surgeon in Tulsa shot by a disgruntled patient. A doctor in India beaten by a group of bereaved family members. A general practitioner in the United Kingdom threatened with stabbing. A new study identifies this trend and finds that 25% of health care workers polled were willing to quit because of such violence.
“That was pretty appalling,” Rahul Kashyap, MD, MBA, MBBS, recalls. Dr. Kashyap is one of the leaders of the Violence Study of Healthcare Workers and Systems (ViSHWaS), which polled an international sample of physicians, nurses, and hospital staff. This study has worrying implications, Dr. Kashyap says. In a time when hospital staff are reporting burnout in record numbers, further deterrents may be the last thing our health care system needs. But Dr. Kashyap hopes that bringing awareness to these trends may allow physicians, policymakers, and the public to mobilize and intervene before it’s too late.
Previous studies have revealed similar trends. The rate of workplace violence directed at U.S. health care workers is five times that of workers in any other industry, according to the Bureau of Labor Statistics. The same study found that attacks had increased 63% from 2011 to 2018. Other polls that focus on the pandemic show that nearly half of U.S. nurses believe that violence increased since the world shut down. Well before the pandemic, however, a study from the Indian Medical Association found that 75% of doctors experienced workplace violence.
With this history in mind, perhaps it’s not surprising that the idea for the study came from the authors’ personal experiences. They had seen coworkers go through attacks, or they had endured attacks themselves, Dr. Kashyap says. But they couldn’t find any global data to back up these experiences. So Dr. Kashyap and his colleagues formed a web of volunteers dedicated to creating a cross-sectional study.
They got in touch with researchers from countries across Asia, the Middle East, South America, North America, and Africa. The initial group agreed to reach out to their contacts, casting a wide net. Researchers used WhatsApp, LinkedIn, and text messages to distribute the survey. Health care workers in each country completed the brief questionnaire, recalling their prepandemic world and evaluating their current one.
Within 2 months, they had reached health care workers in more than 100 countries. They concluded the study when they received about 5,000 results, according to Dr. Kashyap, and then began the process of stratifying the data. For this report, they focused on critical care, emergency medicine, and anesthesiology, which resulted in 598 responses from 69 countries. Of these, India and the United States had the highest number of participants.
In all, 73% of participants reported facing physical or verbal violence while in the hospital; 48% said they felt less motivated to work because of that violence; 39% of respondents believed that the amount of violence they experienced was the same as before the COVID-19 pandemic; and 36% of respondents believed that violence had increased. Even though they were trained on guidelines from the Occupational Safety and Health Administration, 20% of participants felt unprepared to face violence.
Although the study didn’t analyze the reasons workers felt this way, Dr. Kashyap speculates that it could be related to the medical distrust that grew during the pandemic or the stress patients and health care professionals experienced during its peak.
Regardless, the researchers say their study is a starting point. Now that the trend has been highlighted, it may be acted on.
Moving forward, Dr. Kashyap believes that controlling for different variables could determine whether factors like gender or shift time put a worker at higher risk for violence. He hopes it’s possible to interrupt these patterns and reestablish trust in the hospital environment. “It’s aspirational, but you’re hoping that through studies like ViSHWaS, which means trust in Hindi ... [we could restore] the trust and confidence among health care providers for the patients and family members.”
A version of this article first appeared on Medscape.com.
A surgeon in Tulsa shot by a disgruntled patient. A doctor in India beaten by a group of bereaved family members. A general practitioner in the United Kingdom threatened with stabbing. A new study identifies this trend and finds that 25% of health care workers polled were willing to quit because of such violence.
“That was pretty appalling,” Rahul Kashyap, MD, MBA, MBBS, recalls. Dr. Kashyap is one of the leaders of the Violence Study of Healthcare Workers and Systems (ViSHWaS), which polled an international sample of physicians, nurses, and hospital staff. This study has worrying implications, Dr. Kashyap says. In a time when hospital staff are reporting burnout in record numbers, further deterrents may be the last thing our health care system needs. But Dr. Kashyap hopes that bringing awareness to these trends may allow physicians, policymakers, and the public to mobilize and intervene before it’s too late.
Previous studies have revealed similar trends. The rate of workplace violence directed at U.S. health care workers is five times that of workers in any other industry, according to the Bureau of Labor Statistics. The same study found that attacks had increased 63% from 2011 to 2018. Other polls that focus on the pandemic show that nearly half of U.S. nurses believe that violence increased since the world shut down. Well before the pandemic, however, a study from the Indian Medical Association found that 75% of doctors experienced workplace violence.
With this history in mind, perhaps it’s not surprising that the idea for the study came from the authors’ personal experiences. They had seen coworkers go through attacks, or they had endured attacks themselves, Dr. Kashyap says. But they couldn’t find any global data to back up these experiences. So Dr. Kashyap and his colleagues formed a web of volunteers dedicated to creating a cross-sectional study.
They got in touch with researchers from countries across Asia, the Middle East, South America, North America, and Africa. The initial group agreed to reach out to their contacts, casting a wide net. Researchers used WhatsApp, LinkedIn, and text messages to distribute the survey. Health care workers in each country completed the brief questionnaire, recalling their prepandemic world and evaluating their current one.
Within 2 months, they had reached health care workers in more than 100 countries. They concluded the study when they received about 5,000 results, according to Dr. Kashyap, and then began the process of stratifying the data. For this report, they focused on critical care, emergency medicine, and anesthesiology, which resulted in 598 responses from 69 countries. Of these, India and the United States had the highest number of participants.
In all, 73% of participants reported facing physical or verbal violence while in the hospital; 48% said they felt less motivated to work because of that violence; 39% of respondents believed that the amount of violence they experienced was the same as before the COVID-19 pandemic; and 36% of respondents believed that violence had increased. Even though they were trained on guidelines from the Occupational Safety and Health Administration, 20% of participants felt unprepared to face violence.
Although the study didn’t analyze the reasons workers felt this way, Dr. Kashyap speculates that it could be related to the medical distrust that grew during the pandemic or the stress patients and health care professionals experienced during its peak.
Regardless, the researchers say their study is a starting point. Now that the trend has been highlighted, it may be acted on.
Moving forward, Dr. Kashyap believes that controlling for different variables could determine whether factors like gender or shift time put a worker at higher risk for violence. He hopes it’s possible to interrupt these patterns and reestablish trust in the hospital environment. “It’s aspirational, but you’re hoping that through studies like ViSHWaS, which means trust in Hindi ... [we could restore] the trust and confidence among health care providers for the patients and family members.”
A version of this article first appeared on Medscape.com.