User login
For MD-IQ use only
Screening Tool to Reduce Anticoagulant Clinic Encounters
Metrics from 2017 at the Fayetteville Veterans Affairs Heath Care Center (FVAHCC) Anticoagulation Clinic indicate that 43% of patients with atrial fibrillation (AF) who are prescribed warfarin have difficulty maintaining a therapeutic international normalized ratio (INR). These patients require frequent clinic appointments to adjust their regimens to ensure anticoagulation efficacy. FVAHCC policy requires a patient to return to the clinic for repeat INR evaluation within 5 to 14 days of the visit where INR was outside of the established therapeutic range.1 These frequent INR monitoring appointments increase patient and health care provider burden.
Direct oral anticoagulants (DOACs) are an alternative to warfarin for patients with AF who require anticoagulation. DOACs, which do not require regular efficacy monitoring, can be beneficial to patients who struggle to maintain a therapeutic INR when taking warfarin. FVAHCC policy regarding warfarin therapy monitoring allows for a maximum of 6 weeks between appointments. This period is often extended to 3 to 6 months for patients on DOACs.1
At FVAHCC, patients prescribed warfarin are managed in a centralized Anticoagulation Clinic led by a clinical pharmacy specialist (CPS). When a patient reports for an appointment, a clinical pharmacy technician performs point-of-care INR testing and asks standardized questions regarding therapy, including an assessment of adherence. The CPS then evaluates the patient’s INR test results, adjusts the dosage of warfarin as indicated, and determines appropriate follow-up.
A patient who is prescribed a DOAC is monitored by a CPS who works within a patient aligned care team (PACT). The PACT, a multidisciplinary team providing health care to veterans, includes physicians, nurses, pharmacists, dieticians, and mental health providers. Each CPS covers 3 or 4 PACTs. These pharmacists monitor all aspects of DOAC therapy at regular intervals, including renal and hepatic function, complete blood counts, medication adherence, and adverse effects.
Clinic and patient INR data are tracked using a time in therapeutic range (TTR) report generated by the US Department of Veterans Affairs (VA). The TTR report provides clinical information to enhance patient anticoagulation care.2 The TTR report identifies patients with an active order for warfarin and a diagnosis of AF or venous thromboembolism (VTE) whose INR is within therapeutic range (between 2 and 3) < 60% of the time over the previous 160 days.2 The patient must have had at least 3 INR levels drawn within that time frame for a TTR report calculation.2 The report excludes patients who were first prescribed warfarin within the previous 42 days and those with mechanical heart valves. The TTR report is used by the VA to see concrete facility-level results for quality improvement efforts.2
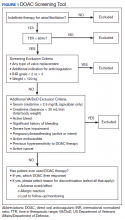
A quality improvement screening tool was developed to identify patients with AF being treated with warfarin who may appropriately transition to DOAC therapy. Anticoagulation Clinic patients were eligible for further evaluation if they had a TTR report level of < 60% and were prescribed indefinite warfarin therapy for AF.
The national VA goal is to have patient TTR report levels read > 60%. Therefore, the primary objective of this project was to improve Anticoagulation Clinic TTR metrics by targeting patients with TTR levels below the national goal.2
Patients who were successfully converted from warfarin to a DOAC were no longer included in Anticoagulation Clinic metrics and instead were followed by a PACT CPS. Thus, it was hypothesized that the average number of monthly Anticoagulation Clinic encounters would decrease on successful implementation of the screening tool. A secondary endpoint of the study evaluated the change in the total number of encounters of those who converted from warfarin to a DOAC.
Fewer clinic encounters could increase time available for the CPS to incorporate other initiatives into workflow and could increase clinic availability for newly referred veterans.
Methods
As this undertaking was considered to be a quality improvement project, institutional review board approval was not required. During an 8-week screening period (August to September 2018), the DOAC screening tool was implemented into the Anticoagulation Clinic workflow. This screening tool (Figure 1) was established based on VA Pharmacy Benefit Management (PBM) Service’s Criteria for Use for Stroke Prevention in Nonvalvular Atrial Fibrillation, a national set of standards used to determine appropriate candidates for DOAC therapy.3
Exclusion criteria included patients with INR goals < 2 or > 3, patients with a diagnosis of VTE, and patients with weight > 120 kg. Patients with a diagnosis of VTE were excluded due to the variability in therapy duration. Weight cutoffs were based on recommendations by the International Society on Thrombosis and Haemostasis. Due to a lack of available data, it was suggested that clinical judgment be used in patients whose weight was > 120 kg.4
During the screening period, weekly TTR reports identified patients in the clinic who had TTR < 60%. When a patient with a TTR report results of < 60% also had a scheduled appointment within a week, a CPS then further reviewed patient eligibility using the DOAC screening tool. On arrival for an appointment, the eligible patient was counseled on DOAC medications and the differences between warfarin and DOACs, including monitoring requirements. Patients had the option to switch to DOAC therapy or remain on warfarin.
The change in the average number of monthly Anticoagulation Clinic encounters for 3 months prior to the screening period (May to July 2018) and 2 months following screening (October to November 2018) was evaluated to measure the impact of the DOAC screening tool. The total number of encounters in the clinic was assessed using the monthly VA reports and were averaged for each period. Then data from the 2 periods were compared.
The monthly encounter reports, a data tool that monitors the number of unique visits per veteran each calendar month, also were used to generate a secondary endpoint showing the number of encounters in the Anticoagulation Clinic associated with patients who switched to a DOAC, including visits prior to changing therapy, and before and after the screening period.
Student’s t test was used to compare the change in encounter frequency before and after screening tool implementation for both primary and secondary endpoints. α was defined as .05 a priori. Continuous data were presented as means and standard deviations. Data were calculated with Microsoft Excel 2016.
Results
For the 3 months before the 8-week screening period, an average of 476 Anticoagulation Clinic encounters per month were documented. Two months of data following the screening period averaged 546 encounters per month. There were an average of 70 additional encounters per month after screening tool implementation (P = .15), reflecting the study’s primary objective.
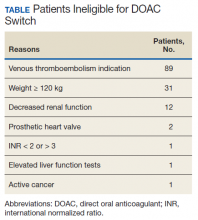
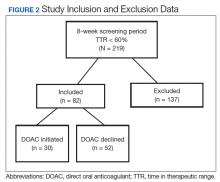
A total of 219 patients in the Anticoagulation Clinic were identified as having a TTR report results of < 60% during the 8-week screening period (Figure 2). Eighty-two of those patients (37.4%) were considered eligible to switch from warfarin to DOAC therapy. Thirty of those eligible patients (13.7%) switched to a DOAC. A total of 107 clinic encounters (22.5%) was associated with these 30 patients prior to screening and 32 associated encounters (5.9%) following screening (P = .01). Of the remaining 137 patients (62.6%) who were ineligible for DOAC therapy, the most common reason for disqualification was a diagnosis of VTE (Table).
Discussion
The general results of this quality improvement project showed that implementation of a screening tool designed to identify patients eligible for DOAC therapy did not decrease the average number of Anticoagulation Clinic encounters. Thirty of 82 eligible patients (36.6%) decided to switch to DOAC therapy during the study period. For those 30 patients, there was a statistically significant decrease in the number of individual clinic encounters. This suggests that the screening tool may positively impact Anticoagulation Clinic metrics when evaluating individual patients, potentially increasing clinic appointment availability.
Confounding Factors
Multiple confounding factors may have affected this project’s results. First, Class I recall for point-of-care test strips used by the clinic was mandated by the US Food and Drug Administration on November 1, 2018.5 Before the recall, investigators found that many nontherapeutic INRs using point-of-care testing later showed results that were within the therapeutic INR range using same-day venous blood collection. This may have led to increases in falsely recorded nontherapeutic INRs and lowered TTR report results. Initially, the project was designed to collect monthly clinic encounter data for 3 months following the 8-week screening period; however, data collection was stopped after 2 months because of the test strip recall.
In addition, in early December 2018, all patients were moved from the Anticoagulation Clinic to the Anticoagulation Telephone Clinic that uses venous blood draws and telephone appointments. Data from venous blood draw results had previously been excluded from this project because results were not available on the same day. Patients in this program are contacted by telephone rather than being offered a face-to-face appointment, thus reducing in-clinic encounters.
Another confounding factor was a FVAHCC policy change in August 2018 requiring that any patient initiated on a DOAC make a onetime visit to the Anticoagulation Clinic prior to establishing care with a PACT CPS. Investigators were unable to exclude these patients from monthly encounter data. Some patients transitioning from warfarin to DOAC therapy were required to continue receiving anticoagulation monitoring from the clinic because of limited PACT CPS clinic availability, thus further increasing postscreening encounters.
Health care providers outside of the Anticoagulation Clinic and uninvolved with the quality improvement project also were switching patients from warfarin to DOAC therapies. Although this may have affected encounter data positively, investigators cannot guarantee these patients would have met criteria outlined by the screening tool.
In September 2018 Hurricane Florence disrupted health care delivery during the 8-week screening period. This event disrupted numerous clinic appointments. Although screening of patients was completed during the 8-week screening period, some patients did not switch to DOAC therapies until November 2018.
Secondary Endpoint Results
Promising results can be seen by specifically looking at the secondary endpoint: the number of encounters associated with patients who chose DOAC therapy. There were 107 encounters associated with the 30 patients who switched to a DOAC prior to screening and only 32 associated encounters after screening, a reduction of 70.1%. This suggests that multiple appointment slots were freed when the screening tool led to successful conversion from warfarin to a DOAC. Further assessment is warranted.
Future Project Development
Future areas for quality improvement project development include expanding project criteria to include patients taking warfarin for VTE. Eighty-nine of 137 patients (65%) who were deemed ineligible to switch to DOAC therapy were excluded due to a diagnosis of VTE. There are existing VA/Department of Defense Criteria for Use for DOAC use in VTE recommendations. Straightforward modification of the screening tool could include this patient group and may be especially useful for patients on indefinite warfarin therapy for recurrent VTE who have poor TTR report results.6
Given the number of confounding factors caused by unforeseen changes to the Anticoagulation Clinic workflow, use of the DOAC screening tool was placed on hold at the conclusion of data collection. This limited the ability to analyze encounter data in the months following project conclusion. Future plans include reimplementation of the screening tool with minor adjustments to include patients on warfarin for VTE and patients with a TTR report results above 60%.
Conclusion
This quality improvement project sought to determine the impact of a screening tool on effecting Anticoagulation Clinic encounter metrics. Results of this project show that the screening tool was unsuccessful in reducing the number of overall clinic encounters. Some promise was shown when evaluating clinic encounters for patients who switched anticoagulation therapies. Numerous confounding factors may have contributed to these results.
1. US Department of Veterans Affairs, Fayetteville Veterans Affairs Health Care Center. MCM 11-188 Anticoagulation Management Program. Revised July 11, 2017. [Source not verified.]
2. US Department of Veterans Affairs, Pharmacy Benefits Management Clinical Pharmacy Practice Office. Anticoagulation percent time in therapeutic range reports. https://spsites.cdw.va.gov/sites/PBM_CPPO/Pages/AnticoagulationTTR.aspx. Revised May 24, 2017. Accessed April 20, 2020. [Source not verified.]
3. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs). Dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis) and edoxaban (SAVAYSA) criteria for use for stroke prevention in nonvalvular atrial fibrillation (AF). https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313.
5. US Food and Drug Administration. Roche Diagnostics recalls CoaguChek XS PT Test Strips due to naccurate INR test results. https://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm624822.htm. Published November 1, 2018. Accessed April 16, 2019.
6. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs) (formerly called TSOACs) dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban(Savaysa) criteria for use for *treatment of venous thromboembolism (VTE)* https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
Metrics from 2017 at the Fayetteville Veterans Affairs Heath Care Center (FVAHCC) Anticoagulation Clinic indicate that 43% of patients with atrial fibrillation (AF) who are prescribed warfarin have difficulty maintaining a therapeutic international normalized ratio (INR). These patients require frequent clinic appointments to adjust their regimens to ensure anticoagulation efficacy. FVAHCC policy requires a patient to return to the clinic for repeat INR evaluation within 5 to 14 days of the visit where INR was outside of the established therapeutic range.1 These frequent INR monitoring appointments increase patient and health care provider burden.
Direct oral anticoagulants (DOACs) are an alternative to warfarin for patients with AF who require anticoagulation. DOACs, which do not require regular efficacy monitoring, can be beneficial to patients who struggle to maintain a therapeutic INR when taking warfarin. FVAHCC policy regarding warfarin therapy monitoring allows for a maximum of 6 weeks between appointments. This period is often extended to 3 to 6 months for patients on DOACs.1
At FVAHCC, patients prescribed warfarin are managed in a centralized Anticoagulation Clinic led by a clinical pharmacy specialist (CPS). When a patient reports for an appointment, a clinical pharmacy technician performs point-of-care INR testing and asks standardized questions regarding therapy, including an assessment of adherence. The CPS then evaluates the patient’s INR test results, adjusts the dosage of warfarin as indicated, and determines appropriate follow-up.
A patient who is prescribed a DOAC is monitored by a CPS who works within a patient aligned care team (PACT). The PACT, a multidisciplinary team providing health care to veterans, includes physicians, nurses, pharmacists, dieticians, and mental health providers. Each CPS covers 3 or 4 PACTs. These pharmacists monitor all aspects of DOAC therapy at regular intervals, including renal and hepatic function, complete blood counts, medication adherence, and adverse effects.
Clinic and patient INR data are tracked using a time in therapeutic range (TTR) report generated by the US Department of Veterans Affairs (VA). The TTR report provides clinical information to enhance patient anticoagulation care.2 The TTR report identifies patients with an active order for warfarin and a diagnosis of AF or venous thromboembolism (VTE) whose INR is within therapeutic range (between 2 and 3) < 60% of the time over the previous 160 days.2 The patient must have had at least 3 INR levels drawn within that time frame for a TTR report calculation.2 The report excludes patients who were first prescribed warfarin within the previous 42 days and those with mechanical heart valves. The TTR report is used by the VA to see concrete facility-level results for quality improvement efforts.2
A quality improvement screening tool was developed to identify patients with AF being treated with warfarin who may appropriately transition to DOAC therapy. Anticoagulation Clinic patients were eligible for further evaluation if they had a TTR report level of < 60% and were prescribed indefinite warfarin therapy for AF.
The national VA goal is to have patient TTR report levels read > 60%. Therefore, the primary objective of this project was to improve Anticoagulation Clinic TTR metrics by targeting patients with TTR levels below the national goal.2
Patients who were successfully converted from warfarin to a DOAC were no longer included in Anticoagulation Clinic metrics and instead were followed by a PACT CPS. Thus, it was hypothesized that the average number of monthly Anticoagulation Clinic encounters would decrease on successful implementation of the screening tool. A secondary endpoint of the study evaluated the change in the total number of encounters of those who converted from warfarin to a DOAC.
Fewer clinic encounters could increase time available for the CPS to incorporate other initiatives into workflow and could increase clinic availability for newly referred veterans.
Methods
As this undertaking was considered to be a quality improvement project, institutional review board approval was not required. During an 8-week screening period (August to September 2018), the DOAC screening tool was implemented into the Anticoagulation Clinic workflow. This screening tool (Figure 1) was established based on VA Pharmacy Benefit Management (PBM) Service’s Criteria for Use for Stroke Prevention in Nonvalvular Atrial Fibrillation, a national set of standards used to determine appropriate candidates for DOAC therapy.3
Exclusion criteria included patients with INR goals < 2 or > 3, patients with a diagnosis of VTE, and patients with weight > 120 kg. Patients with a diagnosis of VTE were excluded due to the variability in therapy duration. Weight cutoffs were based on recommendations by the International Society on Thrombosis and Haemostasis. Due to a lack of available data, it was suggested that clinical judgment be used in patients whose weight was > 120 kg.4
During the screening period, weekly TTR reports identified patients in the clinic who had TTR < 60%. When a patient with a TTR report results of < 60% also had a scheduled appointment within a week, a CPS then further reviewed patient eligibility using the DOAC screening tool. On arrival for an appointment, the eligible patient was counseled on DOAC medications and the differences between warfarin and DOACs, including monitoring requirements. Patients had the option to switch to DOAC therapy or remain on warfarin.
The change in the average number of monthly Anticoagulation Clinic encounters for 3 months prior to the screening period (May to July 2018) and 2 months following screening (October to November 2018) was evaluated to measure the impact of the DOAC screening tool. The total number of encounters in the clinic was assessed using the monthly VA reports and were averaged for each period. Then data from the 2 periods were compared.
The monthly encounter reports, a data tool that monitors the number of unique visits per veteran each calendar month, also were used to generate a secondary endpoint showing the number of encounters in the Anticoagulation Clinic associated with patients who switched to a DOAC, including visits prior to changing therapy, and before and after the screening period.
Student’s t test was used to compare the change in encounter frequency before and after screening tool implementation for both primary and secondary endpoints. α was defined as .05 a priori. Continuous data were presented as means and standard deviations. Data were calculated with Microsoft Excel 2016.
Results
For the 3 months before the 8-week screening period, an average of 476 Anticoagulation Clinic encounters per month were documented. Two months of data following the screening period averaged 546 encounters per month. There were an average of 70 additional encounters per month after screening tool implementation (P = .15), reflecting the study’s primary objective.
A total of 219 patients in the Anticoagulation Clinic were identified as having a TTR report results of < 60% during the 8-week screening period (Figure 2). Eighty-two of those patients (37.4%) were considered eligible to switch from warfarin to DOAC therapy. Thirty of those eligible patients (13.7%) switched to a DOAC. A total of 107 clinic encounters (22.5%) was associated with these 30 patients prior to screening and 32 associated encounters (5.9%) following screening (P = .01). Of the remaining 137 patients (62.6%) who were ineligible for DOAC therapy, the most common reason for disqualification was a diagnosis of VTE (Table).
Discussion
The general results of this quality improvement project showed that implementation of a screening tool designed to identify patients eligible for DOAC therapy did not decrease the average number of Anticoagulation Clinic encounters. Thirty of 82 eligible patients (36.6%) decided to switch to DOAC therapy during the study period. For those 30 patients, there was a statistically significant decrease in the number of individual clinic encounters. This suggests that the screening tool may positively impact Anticoagulation Clinic metrics when evaluating individual patients, potentially increasing clinic appointment availability.
Confounding Factors
Multiple confounding factors may have affected this project’s results. First, Class I recall for point-of-care test strips used by the clinic was mandated by the US Food and Drug Administration on November 1, 2018.5 Before the recall, investigators found that many nontherapeutic INRs using point-of-care testing later showed results that were within the therapeutic INR range using same-day venous blood collection. This may have led to increases in falsely recorded nontherapeutic INRs and lowered TTR report results. Initially, the project was designed to collect monthly clinic encounter data for 3 months following the 8-week screening period; however, data collection was stopped after 2 months because of the test strip recall.
In addition, in early December 2018, all patients were moved from the Anticoagulation Clinic to the Anticoagulation Telephone Clinic that uses venous blood draws and telephone appointments. Data from venous blood draw results had previously been excluded from this project because results were not available on the same day. Patients in this program are contacted by telephone rather than being offered a face-to-face appointment, thus reducing in-clinic encounters.
Another confounding factor was a FVAHCC policy change in August 2018 requiring that any patient initiated on a DOAC make a onetime visit to the Anticoagulation Clinic prior to establishing care with a PACT CPS. Investigators were unable to exclude these patients from monthly encounter data. Some patients transitioning from warfarin to DOAC therapy were required to continue receiving anticoagulation monitoring from the clinic because of limited PACT CPS clinic availability, thus further increasing postscreening encounters.
Health care providers outside of the Anticoagulation Clinic and uninvolved with the quality improvement project also were switching patients from warfarin to DOAC therapies. Although this may have affected encounter data positively, investigators cannot guarantee these patients would have met criteria outlined by the screening tool.
In September 2018 Hurricane Florence disrupted health care delivery during the 8-week screening period. This event disrupted numerous clinic appointments. Although screening of patients was completed during the 8-week screening period, some patients did not switch to DOAC therapies until November 2018.
Secondary Endpoint Results
Promising results can be seen by specifically looking at the secondary endpoint: the number of encounters associated with patients who chose DOAC therapy. There were 107 encounters associated with the 30 patients who switched to a DOAC prior to screening and only 32 associated encounters after screening, a reduction of 70.1%. This suggests that multiple appointment slots were freed when the screening tool led to successful conversion from warfarin to a DOAC. Further assessment is warranted.
Future Project Development
Future areas for quality improvement project development include expanding project criteria to include patients taking warfarin for VTE. Eighty-nine of 137 patients (65%) who were deemed ineligible to switch to DOAC therapy were excluded due to a diagnosis of VTE. There are existing VA/Department of Defense Criteria for Use for DOAC use in VTE recommendations. Straightforward modification of the screening tool could include this patient group and may be especially useful for patients on indefinite warfarin therapy for recurrent VTE who have poor TTR report results.6
Given the number of confounding factors caused by unforeseen changes to the Anticoagulation Clinic workflow, use of the DOAC screening tool was placed on hold at the conclusion of data collection. This limited the ability to analyze encounter data in the months following project conclusion. Future plans include reimplementation of the screening tool with minor adjustments to include patients on warfarin for VTE and patients with a TTR report results above 60%.
Conclusion
This quality improvement project sought to determine the impact of a screening tool on effecting Anticoagulation Clinic encounter metrics. Results of this project show that the screening tool was unsuccessful in reducing the number of overall clinic encounters. Some promise was shown when evaluating clinic encounters for patients who switched anticoagulation therapies. Numerous confounding factors may have contributed to these results.
Metrics from 2017 at the Fayetteville Veterans Affairs Heath Care Center (FVAHCC) Anticoagulation Clinic indicate that 43% of patients with atrial fibrillation (AF) who are prescribed warfarin have difficulty maintaining a therapeutic international normalized ratio (INR). These patients require frequent clinic appointments to adjust their regimens to ensure anticoagulation efficacy. FVAHCC policy requires a patient to return to the clinic for repeat INR evaluation within 5 to 14 days of the visit where INR was outside of the established therapeutic range.1 These frequent INR monitoring appointments increase patient and health care provider burden.
Direct oral anticoagulants (DOACs) are an alternative to warfarin for patients with AF who require anticoagulation. DOACs, which do not require regular efficacy monitoring, can be beneficial to patients who struggle to maintain a therapeutic INR when taking warfarin. FVAHCC policy regarding warfarin therapy monitoring allows for a maximum of 6 weeks between appointments. This period is often extended to 3 to 6 months for patients on DOACs.1
At FVAHCC, patients prescribed warfarin are managed in a centralized Anticoagulation Clinic led by a clinical pharmacy specialist (CPS). When a patient reports for an appointment, a clinical pharmacy technician performs point-of-care INR testing and asks standardized questions regarding therapy, including an assessment of adherence. The CPS then evaluates the patient’s INR test results, adjusts the dosage of warfarin as indicated, and determines appropriate follow-up.
A patient who is prescribed a DOAC is monitored by a CPS who works within a patient aligned care team (PACT). The PACT, a multidisciplinary team providing health care to veterans, includes physicians, nurses, pharmacists, dieticians, and mental health providers. Each CPS covers 3 or 4 PACTs. These pharmacists monitor all aspects of DOAC therapy at regular intervals, including renal and hepatic function, complete blood counts, medication adherence, and adverse effects.
Clinic and patient INR data are tracked using a time in therapeutic range (TTR) report generated by the US Department of Veterans Affairs (VA). The TTR report provides clinical information to enhance patient anticoagulation care.2 The TTR report identifies patients with an active order for warfarin and a diagnosis of AF or venous thromboembolism (VTE) whose INR is within therapeutic range (between 2 and 3) < 60% of the time over the previous 160 days.2 The patient must have had at least 3 INR levels drawn within that time frame for a TTR report calculation.2 The report excludes patients who were first prescribed warfarin within the previous 42 days and those with mechanical heart valves. The TTR report is used by the VA to see concrete facility-level results for quality improvement efforts.2
A quality improvement screening tool was developed to identify patients with AF being treated with warfarin who may appropriately transition to DOAC therapy. Anticoagulation Clinic patients were eligible for further evaluation if they had a TTR report level of < 60% and were prescribed indefinite warfarin therapy for AF.
The national VA goal is to have patient TTR report levels read > 60%. Therefore, the primary objective of this project was to improve Anticoagulation Clinic TTR metrics by targeting patients with TTR levels below the national goal.2
Patients who were successfully converted from warfarin to a DOAC were no longer included in Anticoagulation Clinic metrics and instead were followed by a PACT CPS. Thus, it was hypothesized that the average number of monthly Anticoagulation Clinic encounters would decrease on successful implementation of the screening tool. A secondary endpoint of the study evaluated the change in the total number of encounters of those who converted from warfarin to a DOAC.
Fewer clinic encounters could increase time available for the CPS to incorporate other initiatives into workflow and could increase clinic availability for newly referred veterans.
Methods
As this undertaking was considered to be a quality improvement project, institutional review board approval was not required. During an 8-week screening period (August to September 2018), the DOAC screening tool was implemented into the Anticoagulation Clinic workflow. This screening tool (Figure 1) was established based on VA Pharmacy Benefit Management (PBM) Service’s Criteria for Use for Stroke Prevention in Nonvalvular Atrial Fibrillation, a national set of standards used to determine appropriate candidates for DOAC therapy.3
Exclusion criteria included patients with INR goals < 2 or > 3, patients with a diagnosis of VTE, and patients with weight > 120 kg. Patients with a diagnosis of VTE were excluded due to the variability in therapy duration. Weight cutoffs were based on recommendations by the International Society on Thrombosis and Haemostasis. Due to a lack of available data, it was suggested that clinical judgment be used in patients whose weight was > 120 kg.4
During the screening period, weekly TTR reports identified patients in the clinic who had TTR < 60%. When a patient with a TTR report results of < 60% also had a scheduled appointment within a week, a CPS then further reviewed patient eligibility using the DOAC screening tool. On arrival for an appointment, the eligible patient was counseled on DOAC medications and the differences between warfarin and DOACs, including monitoring requirements. Patients had the option to switch to DOAC therapy or remain on warfarin.
The change in the average number of monthly Anticoagulation Clinic encounters for 3 months prior to the screening period (May to July 2018) and 2 months following screening (October to November 2018) was evaluated to measure the impact of the DOAC screening tool. The total number of encounters in the clinic was assessed using the monthly VA reports and were averaged for each period. Then data from the 2 periods were compared.
The monthly encounter reports, a data tool that monitors the number of unique visits per veteran each calendar month, also were used to generate a secondary endpoint showing the number of encounters in the Anticoagulation Clinic associated with patients who switched to a DOAC, including visits prior to changing therapy, and before and after the screening period.
Student’s t test was used to compare the change in encounter frequency before and after screening tool implementation for both primary and secondary endpoints. α was defined as .05 a priori. Continuous data were presented as means and standard deviations. Data were calculated with Microsoft Excel 2016.
Results
For the 3 months before the 8-week screening period, an average of 476 Anticoagulation Clinic encounters per month were documented. Two months of data following the screening period averaged 546 encounters per month. There were an average of 70 additional encounters per month after screening tool implementation (P = .15), reflecting the study’s primary objective.
A total of 219 patients in the Anticoagulation Clinic were identified as having a TTR report results of < 60% during the 8-week screening period (Figure 2). Eighty-two of those patients (37.4%) were considered eligible to switch from warfarin to DOAC therapy. Thirty of those eligible patients (13.7%) switched to a DOAC. A total of 107 clinic encounters (22.5%) was associated with these 30 patients prior to screening and 32 associated encounters (5.9%) following screening (P = .01). Of the remaining 137 patients (62.6%) who were ineligible for DOAC therapy, the most common reason for disqualification was a diagnosis of VTE (Table).
Discussion
The general results of this quality improvement project showed that implementation of a screening tool designed to identify patients eligible for DOAC therapy did not decrease the average number of Anticoagulation Clinic encounters. Thirty of 82 eligible patients (36.6%) decided to switch to DOAC therapy during the study period. For those 30 patients, there was a statistically significant decrease in the number of individual clinic encounters. This suggests that the screening tool may positively impact Anticoagulation Clinic metrics when evaluating individual patients, potentially increasing clinic appointment availability.
Confounding Factors
Multiple confounding factors may have affected this project’s results. First, Class I recall for point-of-care test strips used by the clinic was mandated by the US Food and Drug Administration on November 1, 2018.5 Before the recall, investigators found that many nontherapeutic INRs using point-of-care testing later showed results that were within the therapeutic INR range using same-day venous blood collection. This may have led to increases in falsely recorded nontherapeutic INRs and lowered TTR report results. Initially, the project was designed to collect monthly clinic encounter data for 3 months following the 8-week screening period; however, data collection was stopped after 2 months because of the test strip recall.
In addition, in early December 2018, all patients were moved from the Anticoagulation Clinic to the Anticoagulation Telephone Clinic that uses venous blood draws and telephone appointments. Data from venous blood draw results had previously been excluded from this project because results were not available on the same day. Patients in this program are contacted by telephone rather than being offered a face-to-face appointment, thus reducing in-clinic encounters.
Another confounding factor was a FVAHCC policy change in August 2018 requiring that any patient initiated on a DOAC make a onetime visit to the Anticoagulation Clinic prior to establishing care with a PACT CPS. Investigators were unable to exclude these patients from monthly encounter data. Some patients transitioning from warfarin to DOAC therapy were required to continue receiving anticoagulation monitoring from the clinic because of limited PACT CPS clinic availability, thus further increasing postscreening encounters.
Health care providers outside of the Anticoagulation Clinic and uninvolved with the quality improvement project also were switching patients from warfarin to DOAC therapies. Although this may have affected encounter data positively, investigators cannot guarantee these patients would have met criteria outlined by the screening tool.
In September 2018 Hurricane Florence disrupted health care delivery during the 8-week screening period. This event disrupted numerous clinic appointments. Although screening of patients was completed during the 8-week screening period, some patients did not switch to DOAC therapies until November 2018.
Secondary Endpoint Results
Promising results can be seen by specifically looking at the secondary endpoint: the number of encounters associated with patients who chose DOAC therapy. There were 107 encounters associated with the 30 patients who switched to a DOAC prior to screening and only 32 associated encounters after screening, a reduction of 70.1%. This suggests that multiple appointment slots were freed when the screening tool led to successful conversion from warfarin to a DOAC. Further assessment is warranted.
Future Project Development
Future areas for quality improvement project development include expanding project criteria to include patients taking warfarin for VTE. Eighty-nine of 137 patients (65%) who were deemed ineligible to switch to DOAC therapy were excluded due to a diagnosis of VTE. There are existing VA/Department of Defense Criteria for Use for DOAC use in VTE recommendations. Straightforward modification of the screening tool could include this patient group and may be especially useful for patients on indefinite warfarin therapy for recurrent VTE who have poor TTR report results.6
Given the number of confounding factors caused by unforeseen changes to the Anticoagulation Clinic workflow, use of the DOAC screening tool was placed on hold at the conclusion of data collection. This limited the ability to analyze encounter data in the months following project conclusion. Future plans include reimplementation of the screening tool with minor adjustments to include patients on warfarin for VTE and patients with a TTR report results above 60%.
Conclusion
This quality improvement project sought to determine the impact of a screening tool on effecting Anticoagulation Clinic encounter metrics. Results of this project show that the screening tool was unsuccessful in reducing the number of overall clinic encounters. Some promise was shown when evaluating clinic encounters for patients who switched anticoagulation therapies. Numerous confounding factors may have contributed to these results.
1. US Department of Veterans Affairs, Fayetteville Veterans Affairs Health Care Center. MCM 11-188 Anticoagulation Management Program. Revised July 11, 2017. [Source not verified.]
2. US Department of Veterans Affairs, Pharmacy Benefits Management Clinical Pharmacy Practice Office. Anticoagulation percent time in therapeutic range reports. https://spsites.cdw.va.gov/sites/PBM_CPPO/Pages/AnticoagulationTTR.aspx. Revised May 24, 2017. Accessed April 20, 2020. [Source not verified.]
3. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs). Dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis) and edoxaban (SAVAYSA) criteria for use for stroke prevention in nonvalvular atrial fibrillation (AF). https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313.
5. US Food and Drug Administration. Roche Diagnostics recalls CoaguChek XS PT Test Strips due to naccurate INR test results. https://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm624822.htm. Published November 1, 2018. Accessed April 16, 2019.
6. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs) (formerly called TSOACs) dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban(Savaysa) criteria for use for *treatment of venous thromboembolism (VTE)* https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
1. US Department of Veterans Affairs, Fayetteville Veterans Affairs Health Care Center. MCM 11-188 Anticoagulation Management Program. Revised July 11, 2017. [Source not verified.]
2. US Department of Veterans Affairs, Pharmacy Benefits Management Clinical Pharmacy Practice Office. Anticoagulation percent time in therapeutic range reports. https://spsites.cdw.va.gov/sites/PBM_CPPO/Pages/AnticoagulationTTR.aspx. Revised May 24, 2017. Accessed April 20, 2020. [Source not verified.]
3. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs). Dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis) and edoxaban (SAVAYSA) criteria for use for stroke prevention in nonvalvular atrial fibrillation (AF). https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313.
5. US Food and Drug Administration. Roche Diagnostics recalls CoaguChek XS PT Test Strips due to naccurate INR test results. https://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm624822.htm. Published November 1, 2018. Accessed April 16, 2019.
6. US Department of Veterans Affairs, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs) (formerly called TSOACs) dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban(Savaysa) criteria for use for *treatment of venous thromboembolism (VTE)* https://www.pbm.va.gov/apps/VANationalFormulary/. Updated December 2017. Accessed April 30, 2020.
Urgent and Emergent Eye Care Strategies to Protect Against COVID-19
COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and its symptoms range from mild to severe respiratory illness, fever, cough, fatigue, and shortness of breath.1 Diarrhea is common early on with infection and loss of taste and smell have also been reported.1 Follicular conjunctivitis has also been reported, either as an early sign of infection or during hospitalization for severe COVID-19 disease.2-4 The incubation period of COVID-19 falls within 2 to 14 days according to the CDC.5
It has been confirmed that COVID-19 is transmitted through both respiratory droplets and direct contact. Another possible route of viral transmission is entry through aerosolized droplets into the tears, which then pass through the nasolacrimal ducts and into the respiratory tract.6
Preparations Prior to Office Visit
It is essential for the eye care provider to prioritize patient care in order of absolute necessity, such as sudden vision loss, sudden onset flashes and floaters, and eye trauma. In cases of potentially sight threatening pathology, it is in the best interest of the patient to conduct a face-to-face appointment. Therefore, it is important to implement new guidelines and protocols as we continue to see these patients (Figure 1).
Prior to the patient entering the medical facility, measures should be implemented to minimize exposure risk. This can be done over the telephone or at vehicle entrance screening stations. The triage technician answering the telephone should have a script of questions to ask. The patient should be instructed to come into the office alone unless, for physical or mental reasons, a caregiver is required.
SARS-CoV-2 Screening Questions
Preparedness through risk mitigation strategies are recommended with a targeted questionnaire and noncontact temperature check at the clinic or hospital entrance. Below are some general questions to further triage patients exposed to SARS-CoV-2.
- Do you have fever or any respiratory symptoms?
- Do you have new or worsening cough or shortness of breath?
- Do you have flulike symptoms?
- Have you been in close contact with someone, including health care workers, confirmed to have the COVID-19?
If the patient answers yes to any of the above questions, the CDC urges health care providers to immediately notify both infection control personnel at your health care facility and your local or state health department.1,2 In regions currently managing significant outbreaks of COVID-19, the AAO recommends that eye care providers assume that any patient could be infected with SARS-CoV-2 and to proceed accordingly.2 If urgent eye care is needed, a referral call should be made to a hospital or center equipped to deal with COVID-19 and urgent eye conditions. When calling the referral center, ensure adequate staffing and space and relay all pertinent information along with receiving approval from the treating physician.
Face-to-Face Office Visits
Once it has been determined that it is in the best interest of the patient to be seen in a face-to-face visit, the patient should be instructed to call the office when they arrive in the parking lot. The CDC recommends limiting points of entry upon arrival and during the visit.1 As soon as an examination lane is ready, the patient can then be messaged to come into the office and escorted into the examination room.
An urgent or emergent ophthalmic examination for a patient with no respiratory symptoms, no fever, and no COVID-19 risk factors should include proper hand hygiene, use of personal protective equipment (PPE), and proper disinfection. Several studies have documented SARS-CoV-2 infection in asymptomatic and presymptomatic patients, making PPE of the up most importance.2,7,8 PPE should include mask, face shield, and gloves. Currently, there are national and international shortages on PPE and a heightened topic of discussion concerning mask use, effectiveness with extended wear, and reuse. Please refer to the CDC and AAO websites for up-to-date guidelines (Table).1,2 According to the CDC, N95 respirators are restricted to those performing or present for an aerosol-generating procedure.9
It is recommended that the eye care provider should only perform necessary tests and procedures. Noncontact tonometry should be avoided, as this might cause aerosolization of virus particles. The close proximity between eye care providers and their patients during slit-lamp examination may require further precautions to lower the risk of transmission via droplets or through hand to eye contact. The patient should be advised not to speak during the examination portion and the AAO also recommends a surgical mask or cloth face covering for the patient.2 An additional protective device that may be used during the slit-lamp exam is a breath shield or a barrier shield (Figures 2 and 3).2 Some manufacturers are offering clinicians free slit-lamp breath shields online.
Infection Prevention and Control Measures
Last, once the patient leaves the examination room, it should be properly disinfected. A disinfection checklist may be made to ensure uniform systematic cleaning. Alcohol and bleach-based disinfectants commonly used in health care settings are likely very effective against virus particles that cause COVID-19.10 During the disinfection process, gloves should be worn and careful attention paid to the contact time. Contact time is the amount of time the surface should appear visibly wet for proper disinfection. For example, Metrex CaviWipes have a recommended contact time of 3 minutes; however, this varies depending on type of virus and formulation, check labels or manufacturers’ websites for further directions.10 Also, the US Environmental Protection Agency has a database search available for disinfectants that meet their criteria for use against SARS-CoV-2.11
In an ever-changing environment, we offer this article to help equip providers to deliver the best possible patient care when face-to-face encounters are necessary. Currently nonurgent eye care follow-up visits are being conducted by telephone or video clinics. It is our goal to inform fellow practitioners on options and strategies to elevate the safety of staff and patients while minimizing the risk of exposure.
1. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): for healthcare professionals. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Updated April 7, 2020. Accessed April 13, 2020.
2. American Academy of Ophthalmology. Important coronavirus context for ophthalmologists. https://www.aao.org/headline/alert-important-coronavirus-context. Updated April 12, 2020. Accessed April 13, 2020.
3. Zhou Y, Zeng Y, Tong Y, Chen CZ. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva [preprint]. https://doi.org/10.1101/2020.02.11.20021956. Published February 12, 2020. Accessed April 13, 2020.
4. Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020; 395(10224):e39.
5. Centers for Disease Control and Prevention. Symptoms of coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Updated March 20, 2020. Accessed April 13, 2020.
6. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;NEJMc2004973. [Published online ahead of print, March 17, 2020].
7. Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and Presymptomatic SARS-CoV-Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377-381.
8. Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) [published online ahead of print, 2020 Mar 16]. Science. 2020; eabb3221.
9. Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Updated April 9, 2020. Accessed April 13, 2020.
10. Centers for Disease Control and Prevention. Cleaning and disinfection for households interim recommendations for U.S. households with suspected or confirmed coronavirus disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cleaning-disinfection.html. Updated March 28, 2020. Accessed April 13, 2020.
11. US Environmental Protection Agency. Pesticide registration: List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 10, 2020. Accessed April 13, 2020.
COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and its symptoms range from mild to severe respiratory illness, fever, cough, fatigue, and shortness of breath.1 Diarrhea is common early on with infection and loss of taste and smell have also been reported.1 Follicular conjunctivitis has also been reported, either as an early sign of infection or during hospitalization for severe COVID-19 disease.2-4 The incubation period of COVID-19 falls within 2 to 14 days according to the CDC.5
It has been confirmed that COVID-19 is transmitted through both respiratory droplets and direct contact. Another possible route of viral transmission is entry through aerosolized droplets into the tears, which then pass through the nasolacrimal ducts and into the respiratory tract.6
Preparations Prior to Office Visit
It is essential for the eye care provider to prioritize patient care in order of absolute necessity, such as sudden vision loss, sudden onset flashes and floaters, and eye trauma. In cases of potentially sight threatening pathology, it is in the best interest of the patient to conduct a face-to-face appointment. Therefore, it is important to implement new guidelines and protocols as we continue to see these patients (Figure 1).
Prior to the patient entering the medical facility, measures should be implemented to minimize exposure risk. This can be done over the telephone or at vehicle entrance screening stations. The triage technician answering the telephone should have a script of questions to ask. The patient should be instructed to come into the office alone unless, for physical or mental reasons, a caregiver is required.
SARS-CoV-2 Screening Questions
Preparedness through risk mitigation strategies are recommended with a targeted questionnaire and noncontact temperature check at the clinic or hospital entrance. Below are some general questions to further triage patients exposed to SARS-CoV-2.
- Do you have fever or any respiratory symptoms?
- Do you have new or worsening cough or shortness of breath?
- Do you have flulike symptoms?
- Have you been in close contact with someone, including health care workers, confirmed to have the COVID-19?
If the patient answers yes to any of the above questions, the CDC urges health care providers to immediately notify both infection control personnel at your health care facility and your local or state health department.1,2 In regions currently managing significant outbreaks of COVID-19, the AAO recommends that eye care providers assume that any patient could be infected with SARS-CoV-2 and to proceed accordingly.2 If urgent eye care is needed, a referral call should be made to a hospital or center equipped to deal with COVID-19 and urgent eye conditions. When calling the referral center, ensure adequate staffing and space and relay all pertinent information along with receiving approval from the treating physician.
Face-to-Face Office Visits
Once it has been determined that it is in the best interest of the patient to be seen in a face-to-face visit, the patient should be instructed to call the office when they arrive in the parking lot. The CDC recommends limiting points of entry upon arrival and during the visit.1 As soon as an examination lane is ready, the patient can then be messaged to come into the office and escorted into the examination room.
An urgent or emergent ophthalmic examination for a patient with no respiratory symptoms, no fever, and no COVID-19 risk factors should include proper hand hygiene, use of personal protective equipment (PPE), and proper disinfection. Several studies have documented SARS-CoV-2 infection in asymptomatic and presymptomatic patients, making PPE of the up most importance.2,7,8 PPE should include mask, face shield, and gloves. Currently, there are national and international shortages on PPE and a heightened topic of discussion concerning mask use, effectiveness with extended wear, and reuse. Please refer to the CDC and AAO websites for up-to-date guidelines (Table).1,2 According to the CDC, N95 respirators are restricted to those performing or present for an aerosol-generating procedure.9
It is recommended that the eye care provider should only perform necessary tests and procedures. Noncontact tonometry should be avoided, as this might cause aerosolization of virus particles. The close proximity between eye care providers and their patients during slit-lamp examination may require further precautions to lower the risk of transmission via droplets or through hand to eye contact. The patient should be advised not to speak during the examination portion and the AAO also recommends a surgical mask or cloth face covering for the patient.2 An additional protective device that may be used during the slit-lamp exam is a breath shield or a barrier shield (Figures 2 and 3).2 Some manufacturers are offering clinicians free slit-lamp breath shields online.
Infection Prevention and Control Measures
Last, once the patient leaves the examination room, it should be properly disinfected. A disinfection checklist may be made to ensure uniform systematic cleaning. Alcohol and bleach-based disinfectants commonly used in health care settings are likely very effective against virus particles that cause COVID-19.10 During the disinfection process, gloves should be worn and careful attention paid to the contact time. Contact time is the amount of time the surface should appear visibly wet for proper disinfection. For example, Metrex CaviWipes have a recommended contact time of 3 minutes; however, this varies depending on type of virus and formulation, check labels or manufacturers’ websites for further directions.10 Also, the US Environmental Protection Agency has a database search available for disinfectants that meet their criteria for use against SARS-CoV-2.11
In an ever-changing environment, we offer this article to help equip providers to deliver the best possible patient care when face-to-face encounters are necessary. Currently nonurgent eye care follow-up visits are being conducted by telephone or video clinics. It is our goal to inform fellow practitioners on options and strategies to elevate the safety of staff and patients while minimizing the risk of exposure.
COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and its symptoms range from mild to severe respiratory illness, fever, cough, fatigue, and shortness of breath.1 Diarrhea is common early on with infection and loss of taste and smell have also been reported.1 Follicular conjunctivitis has also been reported, either as an early sign of infection or during hospitalization for severe COVID-19 disease.2-4 The incubation period of COVID-19 falls within 2 to 14 days according to the CDC.5
It has been confirmed that COVID-19 is transmitted through both respiratory droplets and direct contact. Another possible route of viral transmission is entry through aerosolized droplets into the tears, which then pass through the nasolacrimal ducts and into the respiratory tract.6
Preparations Prior to Office Visit
It is essential for the eye care provider to prioritize patient care in order of absolute necessity, such as sudden vision loss, sudden onset flashes and floaters, and eye trauma. In cases of potentially sight threatening pathology, it is in the best interest of the patient to conduct a face-to-face appointment. Therefore, it is important to implement new guidelines and protocols as we continue to see these patients (Figure 1).
Prior to the patient entering the medical facility, measures should be implemented to minimize exposure risk. This can be done over the telephone or at vehicle entrance screening stations. The triage technician answering the telephone should have a script of questions to ask. The patient should be instructed to come into the office alone unless, for physical or mental reasons, a caregiver is required.
SARS-CoV-2 Screening Questions
Preparedness through risk mitigation strategies are recommended with a targeted questionnaire and noncontact temperature check at the clinic or hospital entrance. Below are some general questions to further triage patients exposed to SARS-CoV-2.
- Do you have fever or any respiratory symptoms?
- Do you have new or worsening cough or shortness of breath?
- Do you have flulike symptoms?
- Have you been in close contact with someone, including health care workers, confirmed to have the COVID-19?
If the patient answers yes to any of the above questions, the CDC urges health care providers to immediately notify both infection control personnel at your health care facility and your local or state health department.1,2 In regions currently managing significant outbreaks of COVID-19, the AAO recommends that eye care providers assume that any patient could be infected with SARS-CoV-2 and to proceed accordingly.2 If urgent eye care is needed, a referral call should be made to a hospital or center equipped to deal with COVID-19 and urgent eye conditions. When calling the referral center, ensure adequate staffing and space and relay all pertinent information along with receiving approval from the treating physician.
Face-to-Face Office Visits
Once it has been determined that it is in the best interest of the patient to be seen in a face-to-face visit, the patient should be instructed to call the office when they arrive in the parking lot. The CDC recommends limiting points of entry upon arrival and during the visit.1 As soon as an examination lane is ready, the patient can then be messaged to come into the office and escorted into the examination room.
An urgent or emergent ophthalmic examination for a patient with no respiratory symptoms, no fever, and no COVID-19 risk factors should include proper hand hygiene, use of personal protective equipment (PPE), and proper disinfection. Several studies have documented SARS-CoV-2 infection in asymptomatic and presymptomatic patients, making PPE of the up most importance.2,7,8 PPE should include mask, face shield, and gloves. Currently, there are national and international shortages on PPE and a heightened topic of discussion concerning mask use, effectiveness with extended wear, and reuse. Please refer to the CDC and AAO websites for up-to-date guidelines (Table).1,2 According to the CDC, N95 respirators are restricted to those performing or present for an aerosol-generating procedure.9
It is recommended that the eye care provider should only perform necessary tests and procedures. Noncontact tonometry should be avoided, as this might cause aerosolization of virus particles. The close proximity between eye care providers and their patients during slit-lamp examination may require further precautions to lower the risk of transmission via droplets or through hand to eye contact. The patient should be advised not to speak during the examination portion and the AAO also recommends a surgical mask or cloth face covering for the patient.2 An additional protective device that may be used during the slit-lamp exam is a breath shield or a barrier shield (Figures 2 and 3).2 Some manufacturers are offering clinicians free slit-lamp breath shields online.
Infection Prevention and Control Measures
Last, once the patient leaves the examination room, it should be properly disinfected. A disinfection checklist may be made to ensure uniform systematic cleaning. Alcohol and bleach-based disinfectants commonly used in health care settings are likely very effective against virus particles that cause COVID-19.10 During the disinfection process, gloves should be worn and careful attention paid to the contact time. Contact time is the amount of time the surface should appear visibly wet for proper disinfection. For example, Metrex CaviWipes have a recommended contact time of 3 minutes; however, this varies depending on type of virus and formulation, check labels or manufacturers’ websites for further directions.10 Also, the US Environmental Protection Agency has a database search available for disinfectants that meet their criteria for use against SARS-CoV-2.11
In an ever-changing environment, we offer this article to help equip providers to deliver the best possible patient care when face-to-face encounters are necessary. Currently nonurgent eye care follow-up visits are being conducted by telephone or video clinics. It is our goal to inform fellow practitioners on options and strategies to elevate the safety of staff and patients while minimizing the risk of exposure.
1. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): for healthcare professionals. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Updated April 7, 2020. Accessed April 13, 2020.
2. American Academy of Ophthalmology. Important coronavirus context for ophthalmologists. https://www.aao.org/headline/alert-important-coronavirus-context. Updated April 12, 2020. Accessed April 13, 2020.
3. Zhou Y, Zeng Y, Tong Y, Chen CZ. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva [preprint]. https://doi.org/10.1101/2020.02.11.20021956. Published February 12, 2020. Accessed April 13, 2020.
4. Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020; 395(10224):e39.
5. Centers for Disease Control and Prevention. Symptoms of coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Updated March 20, 2020. Accessed April 13, 2020.
6. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;NEJMc2004973. [Published online ahead of print, March 17, 2020].
7. Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and Presymptomatic SARS-CoV-Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377-381.
8. Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) [published online ahead of print, 2020 Mar 16]. Science. 2020; eabb3221.
9. Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Updated April 9, 2020. Accessed April 13, 2020.
10. Centers for Disease Control and Prevention. Cleaning and disinfection for households interim recommendations for U.S. households with suspected or confirmed coronavirus disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cleaning-disinfection.html. Updated March 28, 2020. Accessed April 13, 2020.
11. US Environmental Protection Agency. Pesticide registration: List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 10, 2020. Accessed April 13, 2020.
1. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): for healthcare professionals. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Updated April 7, 2020. Accessed April 13, 2020.
2. American Academy of Ophthalmology. Important coronavirus context for ophthalmologists. https://www.aao.org/headline/alert-important-coronavirus-context. Updated April 12, 2020. Accessed April 13, 2020.
3. Zhou Y, Zeng Y, Tong Y, Chen CZ. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva [preprint]. https://doi.org/10.1101/2020.02.11.20021956. Published February 12, 2020. Accessed April 13, 2020.
4. Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020; 395(10224):e39.
5. Centers for Disease Control and Prevention. Symptoms of coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Updated March 20, 2020. Accessed April 13, 2020.
6. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;NEJMc2004973. [Published online ahead of print, March 17, 2020].
7. Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and Presymptomatic SARS-CoV-Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377-381.
8. Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) [published online ahead of print, 2020 Mar 16]. Science. 2020; eabb3221.
9. Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Updated April 9, 2020. Accessed April 13, 2020.
10. Centers for Disease Control and Prevention. Cleaning and disinfection for households interim recommendations for U.S. households with suspected or confirmed coronavirus disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cleaning-disinfection.html. Updated March 28, 2020. Accessed April 13, 2020.
11. US Environmental Protection Agency. Pesticide registration: List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Updated April 10, 2020. Accessed April 13, 2020.
The Duty to Care and Its Exceptions in a Pandemic
As of April 9, 2020, the Centers for Disease Control and Prevention (CDC) reported that 9,282 health care providers in the US had contracted COVID-19, and 27 had died of the virus.2 Medscape reports the toll as much higher. Thousands more nurses, doctors, epidemiologists, social workers, physician assistants, dentists, pharmacists, and other health care workers from Italy, China, and dozens of other countries have died fighting this plague.3
The truth is no one knows how many health care workers are actually sick or even have died. State and federal governments have not been routinely and specifically tracking that data, making these already grim statistics likely a gross underestimation.4 While not all of these health care providers were exposed to COVID-19 in the line of duty, many were, and many more will be as the pandemic subsides in one epicenter only to erupt in another, and smolders for months until a vaccine quenches it.
Each of those lost lives of promise had a story of hard work and sacrifice to become a health care professional, of friends and family who loved and cared for them when ill, who need and grieve for them, now gone far too soon. Nor should we forget to mourn all of the administrative professionals, the line and support staff of health care facilities, who also perished fighting the pestilence. It is fitting then, that this second editorial in my pledge to write each month about COVID-19 until the pandemic ends, be about the duty to care and its limits.
The duty to care is among the most fundamental and ancient ethical obligations of health care providers. It is included even in modern codes of ethics like that of the American Medical Association and American Nurses Association. The obligation to not abandon patients is even more compelling for the Military Health System, Veterans Health Administration (VHA), and the US Public Health Service whose health care mission also is a public trust. The duty is rooted in the fiduciary nature of the health professions in which the interests of the patient should take priority over other considerations, including a risk to their own health and life. Prioritization though has exceptions. Physician and attorney David Orentlicher points out the unconditional obligation that bound physicians in the 14th century Black Death, or the 1918 Spanish influenza, now admits exceptions and qualifications.5
The exception that has become the object of greatest concern to health care workers is personal protective equipment (PPE). In modern public health ethics, health care systems and state and federal governments have a corresponding ethical obligation of reciprocity toward their employees whose work places them at elevated risk of harm—in this case, COVID-19 exposure. The principle of reciprocity encompasses the measures and materials that health care institutions need to provide to health care workers to reasonably minimize the risk of viral transmission. The reasonableness standard does not demand that there be zero risk. It does require that health care workers have adequate and appropriate PPE so that in fulfilling their duty to care they are not exposed to a disproportionate risk.
This last assertion has been the subject of controversy in the media and consternation on the part of health care professionals for several disconcerting reasons. First and foremost, a cascade failure on the part of government and industry has resulted in PPE being the scarcest health care resource in this pandemic.6 The shortage is as serious as that of the life-saving ventilators that are rightly at the center of most crisis standards resource allocation plans.7 Second, the guidance from the CDC and other authoritative sources continues to change. This is, in part, to adjust to the even more rapid pace of knowledge about the virus and its behavior and to adapt to the reality of insufficient PPE.8
Understandably, health care providers, especially those on the frontlines, may lose trust in the scientific experts and the leadership of their institutions, compounding the climate of moral distress in a public health crisis. Health care workers in the community, and even in federal service, have launched socially distanced protests and taken to social media to voice their concern and rally assistance.9,10 In response, VHA Executive-in-Charge Richard Stone, MD, admitted that VHA does have a shortage of PPE in a Washington Post interview.11 He outlined how the organization plans to address staff concerns. The article also reported only a 4% absentee rate of VHA staff as opposed to the 40% that plans predicted was possible. This demonstrates once more the dedication of VHA health care professionals and workers to fulfill their duty to care for veterans even amid fears about inadequate PPE.
In the epigraph, Albert Camus captures the uncertainty and fear that as humans all health care providers experience as they face the unpredictable but very real threat of COVID-19.1 Camus expresses even more strongly the devotion to duty of health care providers to care for vulnerable ill patients in need despite the inherent threat in a highly transmissible and potentially deadly infection that is inextricably linked to that caring. Orentlicher wisely opines that the integrity of the health professions and their respected role in society benefit from a strong duty to care.5 The best way to promote that duty is to do all in our power to protect those who willingly brave the pestilence to treat, and hope and pray someday to cure COVID-19.
1. Camus A. The Plague. Vintage Books: New York; 1948:120.
2. CDC COVID-19 Response Team. Characteristics of Health Care Personnel with COVID-19— United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):477-481.
3. In memoriam: healthcare workers who have died of COVID-19. https://www.medscape.com/viewarticle/927976. Updated April 21, 2020. Accessed April 22, 2020.
4. Galvin G. The great unknown: how many health care workers have coronavirus? https://www.usnews.com/news/national-news/articles/2020-04-03/how-many-health-care-workers-have-coronavirus. Published April 3, 2020. Accessed April 22, 2020.
5. Orentlicher D. The physician’s duty to treat during pandemics. Am J Public Health. 2018;108(11):1459-1461.
6. Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. [Published online ahead of print, 2020 Mar 25.] N Engl J Med. 2020;10.1056/NEJMp2006141.
7. New York State Task Force on Life and the Law, New York State Department of Health. Ventilator allocation guidelines. https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf. Published November 2015. Accessed April 22, 2020.
8. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-2019): Strategies to optimize PPE and equipment. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html. Updated April 3, 2020. Accessed April 22, 2020.
9. Wentling N. ‘It’s out of control’: VA nurses demand more protection against coronavirus. https://www.stripes.com/news/veterans/va-nurses-demand-more-protection-against-coronavirus-1.626910. Updated April 21, 2020. Accessed April 22, 2020.
10. Padilla M. ‘It feels like a war zone’: doctors and nurses plead for masks on social media. https://www.nytimes.com/2020/03/19/us/hospitals-coronavirus-ppe-shortage.html. Updated March 22, 2020. Accessed April 22, 2020.
11. Rein L. VA health chief acknowledges a shortage of protective gear for its hospital workers. https://www.washingtonpost.com/politics/va-health-chief-acknowledges-a-shortage-of-protective-gear-for-its-hospital-workers/2020/04/24/4c1bcd5e-84bf-11ea-ae26-989cfce1c7c7_story.html. Published April 25, 2020. Accessed April 27, 2020.
As of April 9, 2020, the Centers for Disease Control and Prevention (CDC) reported that 9,282 health care providers in the US had contracted COVID-19, and 27 had died of the virus.2 Medscape reports the toll as much higher. Thousands more nurses, doctors, epidemiologists, social workers, physician assistants, dentists, pharmacists, and other health care workers from Italy, China, and dozens of other countries have died fighting this plague.3
The truth is no one knows how many health care workers are actually sick or even have died. State and federal governments have not been routinely and specifically tracking that data, making these already grim statistics likely a gross underestimation.4 While not all of these health care providers were exposed to COVID-19 in the line of duty, many were, and many more will be as the pandemic subsides in one epicenter only to erupt in another, and smolders for months until a vaccine quenches it.
Each of those lost lives of promise had a story of hard work and sacrifice to become a health care professional, of friends and family who loved and cared for them when ill, who need and grieve for them, now gone far too soon. Nor should we forget to mourn all of the administrative professionals, the line and support staff of health care facilities, who also perished fighting the pestilence. It is fitting then, that this second editorial in my pledge to write each month about COVID-19 until the pandemic ends, be about the duty to care and its limits.
The duty to care is among the most fundamental and ancient ethical obligations of health care providers. It is included even in modern codes of ethics like that of the American Medical Association and American Nurses Association. The obligation to not abandon patients is even more compelling for the Military Health System, Veterans Health Administration (VHA), and the US Public Health Service whose health care mission also is a public trust. The duty is rooted in the fiduciary nature of the health professions in which the interests of the patient should take priority over other considerations, including a risk to their own health and life. Prioritization though has exceptions. Physician and attorney David Orentlicher points out the unconditional obligation that bound physicians in the 14th century Black Death, or the 1918 Spanish influenza, now admits exceptions and qualifications.5
The exception that has become the object of greatest concern to health care workers is personal protective equipment (PPE). In modern public health ethics, health care systems and state and federal governments have a corresponding ethical obligation of reciprocity toward their employees whose work places them at elevated risk of harm—in this case, COVID-19 exposure. The principle of reciprocity encompasses the measures and materials that health care institutions need to provide to health care workers to reasonably minimize the risk of viral transmission. The reasonableness standard does not demand that there be zero risk. It does require that health care workers have adequate and appropriate PPE so that in fulfilling their duty to care they are not exposed to a disproportionate risk.
This last assertion has been the subject of controversy in the media and consternation on the part of health care professionals for several disconcerting reasons. First and foremost, a cascade failure on the part of government and industry has resulted in PPE being the scarcest health care resource in this pandemic.6 The shortage is as serious as that of the life-saving ventilators that are rightly at the center of most crisis standards resource allocation plans.7 Second, the guidance from the CDC and other authoritative sources continues to change. This is, in part, to adjust to the even more rapid pace of knowledge about the virus and its behavior and to adapt to the reality of insufficient PPE.8
Understandably, health care providers, especially those on the frontlines, may lose trust in the scientific experts and the leadership of their institutions, compounding the climate of moral distress in a public health crisis. Health care workers in the community, and even in federal service, have launched socially distanced protests and taken to social media to voice their concern and rally assistance.9,10 In response, VHA Executive-in-Charge Richard Stone, MD, admitted that VHA does have a shortage of PPE in a Washington Post interview.11 He outlined how the organization plans to address staff concerns. The article also reported only a 4% absentee rate of VHA staff as opposed to the 40% that plans predicted was possible. This demonstrates once more the dedication of VHA health care professionals and workers to fulfill their duty to care for veterans even amid fears about inadequate PPE.
In the epigraph, Albert Camus captures the uncertainty and fear that as humans all health care providers experience as they face the unpredictable but very real threat of COVID-19.1 Camus expresses even more strongly the devotion to duty of health care providers to care for vulnerable ill patients in need despite the inherent threat in a highly transmissible and potentially deadly infection that is inextricably linked to that caring. Orentlicher wisely opines that the integrity of the health professions and their respected role in society benefit from a strong duty to care.5 The best way to promote that duty is to do all in our power to protect those who willingly brave the pestilence to treat, and hope and pray someday to cure COVID-19.
As of April 9, 2020, the Centers for Disease Control and Prevention (CDC) reported that 9,282 health care providers in the US had contracted COVID-19, and 27 had died of the virus.2 Medscape reports the toll as much higher. Thousands more nurses, doctors, epidemiologists, social workers, physician assistants, dentists, pharmacists, and other health care workers from Italy, China, and dozens of other countries have died fighting this plague.3
The truth is no one knows how many health care workers are actually sick or even have died. State and federal governments have not been routinely and specifically tracking that data, making these already grim statistics likely a gross underestimation.4 While not all of these health care providers were exposed to COVID-19 in the line of duty, many were, and many more will be as the pandemic subsides in one epicenter only to erupt in another, and smolders for months until a vaccine quenches it.
Each of those lost lives of promise had a story of hard work and sacrifice to become a health care professional, of friends and family who loved and cared for them when ill, who need and grieve for them, now gone far too soon. Nor should we forget to mourn all of the administrative professionals, the line and support staff of health care facilities, who also perished fighting the pestilence. It is fitting then, that this second editorial in my pledge to write each month about COVID-19 until the pandemic ends, be about the duty to care and its limits.
The duty to care is among the most fundamental and ancient ethical obligations of health care providers. It is included even in modern codes of ethics like that of the American Medical Association and American Nurses Association. The obligation to not abandon patients is even more compelling for the Military Health System, Veterans Health Administration (VHA), and the US Public Health Service whose health care mission also is a public trust. The duty is rooted in the fiduciary nature of the health professions in which the interests of the patient should take priority over other considerations, including a risk to their own health and life. Prioritization though has exceptions. Physician and attorney David Orentlicher points out the unconditional obligation that bound physicians in the 14th century Black Death, or the 1918 Spanish influenza, now admits exceptions and qualifications.5
The exception that has become the object of greatest concern to health care workers is personal protective equipment (PPE). In modern public health ethics, health care systems and state and federal governments have a corresponding ethical obligation of reciprocity toward their employees whose work places them at elevated risk of harm—in this case, COVID-19 exposure. The principle of reciprocity encompasses the measures and materials that health care institutions need to provide to health care workers to reasonably minimize the risk of viral transmission. The reasonableness standard does not demand that there be zero risk. It does require that health care workers have adequate and appropriate PPE so that in fulfilling their duty to care they are not exposed to a disproportionate risk.
This last assertion has been the subject of controversy in the media and consternation on the part of health care professionals for several disconcerting reasons. First and foremost, a cascade failure on the part of government and industry has resulted in PPE being the scarcest health care resource in this pandemic.6 The shortage is as serious as that of the life-saving ventilators that are rightly at the center of most crisis standards resource allocation plans.7 Second, the guidance from the CDC and other authoritative sources continues to change. This is, in part, to adjust to the even more rapid pace of knowledge about the virus and its behavior and to adapt to the reality of insufficient PPE.8
Understandably, health care providers, especially those on the frontlines, may lose trust in the scientific experts and the leadership of their institutions, compounding the climate of moral distress in a public health crisis. Health care workers in the community, and even in federal service, have launched socially distanced protests and taken to social media to voice their concern and rally assistance.9,10 In response, VHA Executive-in-Charge Richard Stone, MD, admitted that VHA does have a shortage of PPE in a Washington Post interview.11 He outlined how the organization plans to address staff concerns. The article also reported only a 4% absentee rate of VHA staff as opposed to the 40% that plans predicted was possible. This demonstrates once more the dedication of VHA health care professionals and workers to fulfill their duty to care for veterans even amid fears about inadequate PPE.
In the epigraph, Albert Camus captures the uncertainty and fear that as humans all health care providers experience as they face the unpredictable but very real threat of COVID-19.1 Camus expresses even more strongly the devotion to duty of health care providers to care for vulnerable ill patients in need despite the inherent threat in a highly transmissible and potentially deadly infection that is inextricably linked to that caring. Orentlicher wisely opines that the integrity of the health professions and their respected role in society benefit from a strong duty to care.5 The best way to promote that duty is to do all in our power to protect those who willingly brave the pestilence to treat, and hope and pray someday to cure COVID-19.
1. Camus A. The Plague. Vintage Books: New York; 1948:120.
2. CDC COVID-19 Response Team. Characteristics of Health Care Personnel with COVID-19— United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):477-481.
3. In memoriam: healthcare workers who have died of COVID-19. https://www.medscape.com/viewarticle/927976. Updated April 21, 2020. Accessed April 22, 2020.
4. Galvin G. The great unknown: how many health care workers have coronavirus? https://www.usnews.com/news/national-news/articles/2020-04-03/how-many-health-care-workers-have-coronavirus. Published April 3, 2020. Accessed April 22, 2020.
5. Orentlicher D. The physician’s duty to treat during pandemics. Am J Public Health. 2018;108(11):1459-1461.
6. Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. [Published online ahead of print, 2020 Mar 25.] N Engl J Med. 2020;10.1056/NEJMp2006141.
7. New York State Task Force on Life and the Law, New York State Department of Health. Ventilator allocation guidelines. https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf. Published November 2015. Accessed April 22, 2020.
8. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-2019): Strategies to optimize PPE and equipment. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html. Updated April 3, 2020. Accessed April 22, 2020.
9. Wentling N. ‘It’s out of control’: VA nurses demand more protection against coronavirus. https://www.stripes.com/news/veterans/va-nurses-demand-more-protection-against-coronavirus-1.626910. Updated April 21, 2020. Accessed April 22, 2020.
10. Padilla M. ‘It feels like a war zone’: doctors and nurses plead for masks on social media. https://www.nytimes.com/2020/03/19/us/hospitals-coronavirus-ppe-shortage.html. Updated March 22, 2020. Accessed April 22, 2020.
11. Rein L. VA health chief acknowledges a shortage of protective gear for its hospital workers. https://www.washingtonpost.com/politics/va-health-chief-acknowledges-a-shortage-of-protective-gear-for-its-hospital-workers/2020/04/24/4c1bcd5e-84bf-11ea-ae26-989cfce1c7c7_story.html. Published April 25, 2020. Accessed April 27, 2020.
1. Camus A. The Plague. Vintage Books: New York; 1948:120.
2. CDC COVID-19 Response Team. Characteristics of Health Care Personnel with COVID-19— United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):477-481.
3. In memoriam: healthcare workers who have died of COVID-19. https://www.medscape.com/viewarticle/927976. Updated April 21, 2020. Accessed April 22, 2020.
4. Galvin G. The great unknown: how many health care workers have coronavirus? https://www.usnews.com/news/national-news/articles/2020-04-03/how-many-health-care-workers-have-coronavirus. Published April 3, 2020. Accessed April 22, 2020.
5. Orentlicher D. The physician’s duty to treat during pandemics. Am J Public Health. 2018;108(11):1459-1461.
6. Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. [Published online ahead of print, 2020 Mar 25.] N Engl J Med. 2020;10.1056/NEJMp2006141.
7. New York State Task Force on Life and the Law, New York State Department of Health. Ventilator allocation guidelines. https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf. Published November 2015. Accessed April 22, 2020.
8. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-2019): Strategies to optimize PPE and equipment. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html. Updated April 3, 2020. Accessed April 22, 2020.
9. Wentling N. ‘It’s out of control’: VA nurses demand more protection against coronavirus. https://www.stripes.com/news/veterans/va-nurses-demand-more-protection-against-coronavirus-1.626910. Updated April 21, 2020. Accessed April 22, 2020.
10. Padilla M. ‘It feels like a war zone’: doctors and nurses plead for masks on social media. https://www.nytimes.com/2020/03/19/us/hospitals-coronavirus-ppe-shortage.html. Updated March 22, 2020. Accessed April 22, 2020.
11. Rein L. VA health chief acknowledges a shortage of protective gear for its hospital workers. https://www.washingtonpost.com/politics/va-health-chief-acknowledges-a-shortage-of-protective-gear-for-its-hospital-workers/2020/04/24/4c1bcd5e-84bf-11ea-ae26-989cfce1c7c7_story.html. Published April 25, 2020. Accessed April 27, 2020.
Societies offer advice on treating osteoporosis patients during pandemic
Five leading bone health organizations have gotten together to provide new recommendations for managing patients with osteoporosis during the COVID-19 pandemic.
The joint guidance – released by the American Society for Bone and Mineral Research (ASBMR), the American Association of Clinical Endocrinologists, the Endocrine Society, the European Calcified Tissue Society, and the National Osteoporosis Foundation – offered both general and specific recommendations for patients whose osteoporosis treatment plan is either continuing or has been disrupted during the COVID-19 pandemic.
Among the general recommendations are to initiate oral bisphosphonate therapy over either the telephone or through a video visit, with no delays for patients at high risk of fracture. They also noted that, as elective procedures, bone mineral density examinations may need to be postponed.
For patients already on osteoporosis medications – such as oral and IV bisphosphonates, denosumab, estrogen, raloxifene, teriparatide, abaloparatide, and romosozumab – they recommend continuing treatment whenever possible. “There is no evidence that any osteoporosis therapy increases the risk or severity of COVID-19 infection or alters the disease course,” they wrote. They did add, however, that COVID-19 may increase the risk of hypercoagulable complications and so caution should be exercised when treating patients with estrogen or raloxifene.
Separately, in a letter to the editor published in the Journal of Clinical Endocrinology and Metabolism (doi: 10.1210/clinem/dgaa254), Ruban Dhaliwal, MD, MPH, of the State University of New York, Syracuse, and coauthors concur in regard to raloxifene. They wrote that, because of the increased risk of thromboembolic events related to COVID-19, “it is best to discontinue raloxifene, which is also associated with such risk.”
The joint statement recognizes current social distancing policies and therefore recommends avoiding standard pretreatment labs prior to IV bisphosphonate and/or denosumab administration if previous labs were normal and the patient’s recent health has been deemed “stable.” Lab evaluation is recommended, however, for patients with fluctuating renal function and for those at higher risk of developing hypocalcemia.
The statement also provides potential alternative methods for delivering parenteral osteoporosis treatments, including off-site clinics, home delivery and administration, self-injection of denosumab and/or romosozumab, and drive-through administration of denosumab and/or romosozumab. They acknowledged the complications surrounding each alternative, including residents of “socioeconomically challenged communities” being unable to reach clinics if public transportation is not available and the “important medicolegal issues” to consider around self-injection.
For all patients whose treatments have been disrupted, the authors recommend frequent reevaluation “with the goal to resume the original osteoporosis treatment plan once circumstances allow.” As for specific recommendations, patients on denosumab who will not be treatable within 7 months of their previous injection should be transitioned to oral bisphosphonate if at all possible. For patients with underlying gastrointestinal disorders, they recommend monthly ibandronate or weekly/monthly risedronate; for patients with chronic renal insufficiency, they recommend an off-label regimen of lower dose oral bisphosphonate.
For patients on teriparatide or abaloparatide who will be unable to receive continued treatment, they recommend a delay in treatment. If that delay goes beyond several months, they recommend a temporary transition to oral bisphosphonate. For patients on romosozumab who will be unable to receive continued treatment, they also recommend a delay in treatment and a temporary transition to oral bisphosphonate. Finally, they expressed confidence that patients on IV bisphosphonates will not be harmed by treatment delays, even those of several months.
“I think we could fall into a trap during this era of the pandemic and fail to address patients’ underlying chronic conditions, even though those comorbidities will end up greatly affecting their overall health,” said incoming ASBMR president Suzanne Jan de Beur, MD, of the Johns Hopkins University, Baltimore. “As we continue to care for our patients, we need to keep chronic conditions like osteoporosis on the radar screen and not stop diagnosing people at risk or those who present with fractures. Even when we can’t perform full screening tests due to distancing policies, we need to be vigilant for those patients who need treatment and administer the treatments we have available as needed.”
The statement’s authors acknowledged the limitations of their recommendations, noting that “there is a paucity of data to provide clear guidance” and as such they were “based primarily on expert opinion.”
The authors from the five organizations did not disclose any conflicts of interest.
Five leading bone health organizations have gotten together to provide new recommendations for managing patients with osteoporosis during the COVID-19 pandemic.
The joint guidance – released by the American Society for Bone and Mineral Research (ASBMR), the American Association of Clinical Endocrinologists, the Endocrine Society, the European Calcified Tissue Society, and the National Osteoporosis Foundation – offered both general and specific recommendations for patients whose osteoporosis treatment plan is either continuing or has been disrupted during the COVID-19 pandemic.
Among the general recommendations are to initiate oral bisphosphonate therapy over either the telephone or through a video visit, with no delays for patients at high risk of fracture. They also noted that, as elective procedures, bone mineral density examinations may need to be postponed.
For patients already on osteoporosis medications – such as oral and IV bisphosphonates, denosumab, estrogen, raloxifene, teriparatide, abaloparatide, and romosozumab – they recommend continuing treatment whenever possible. “There is no evidence that any osteoporosis therapy increases the risk or severity of COVID-19 infection or alters the disease course,” they wrote. They did add, however, that COVID-19 may increase the risk of hypercoagulable complications and so caution should be exercised when treating patients with estrogen or raloxifene.
Separately, in a letter to the editor published in the Journal of Clinical Endocrinology and Metabolism (doi: 10.1210/clinem/dgaa254), Ruban Dhaliwal, MD, MPH, of the State University of New York, Syracuse, and coauthors concur in regard to raloxifene. They wrote that, because of the increased risk of thromboembolic events related to COVID-19, “it is best to discontinue raloxifene, which is also associated with such risk.”
The joint statement recognizes current social distancing policies and therefore recommends avoiding standard pretreatment labs prior to IV bisphosphonate and/or denosumab administration if previous labs were normal and the patient’s recent health has been deemed “stable.” Lab evaluation is recommended, however, for patients with fluctuating renal function and for those at higher risk of developing hypocalcemia.
The statement also provides potential alternative methods for delivering parenteral osteoporosis treatments, including off-site clinics, home delivery and administration, self-injection of denosumab and/or romosozumab, and drive-through administration of denosumab and/or romosozumab. They acknowledged the complications surrounding each alternative, including residents of “socioeconomically challenged communities” being unable to reach clinics if public transportation is not available and the “important medicolegal issues” to consider around self-injection.
For all patients whose treatments have been disrupted, the authors recommend frequent reevaluation “with the goal to resume the original osteoporosis treatment plan once circumstances allow.” As for specific recommendations, patients on denosumab who will not be treatable within 7 months of their previous injection should be transitioned to oral bisphosphonate if at all possible. For patients with underlying gastrointestinal disorders, they recommend monthly ibandronate or weekly/monthly risedronate; for patients with chronic renal insufficiency, they recommend an off-label regimen of lower dose oral bisphosphonate.
For patients on teriparatide or abaloparatide who will be unable to receive continued treatment, they recommend a delay in treatment. If that delay goes beyond several months, they recommend a temporary transition to oral bisphosphonate. For patients on romosozumab who will be unable to receive continued treatment, they also recommend a delay in treatment and a temporary transition to oral bisphosphonate. Finally, they expressed confidence that patients on IV bisphosphonates will not be harmed by treatment delays, even those of several months.
“I think we could fall into a trap during this era of the pandemic and fail to address patients’ underlying chronic conditions, even though those comorbidities will end up greatly affecting their overall health,” said incoming ASBMR president Suzanne Jan de Beur, MD, of the Johns Hopkins University, Baltimore. “As we continue to care for our patients, we need to keep chronic conditions like osteoporosis on the radar screen and not stop diagnosing people at risk or those who present with fractures. Even when we can’t perform full screening tests due to distancing policies, we need to be vigilant for those patients who need treatment and administer the treatments we have available as needed.”
The statement’s authors acknowledged the limitations of their recommendations, noting that “there is a paucity of data to provide clear guidance” and as such they were “based primarily on expert opinion.”
The authors from the five organizations did not disclose any conflicts of interest.
Five leading bone health organizations have gotten together to provide new recommendations for managing patients with osteoporosis during the COVID-19 pandemic.
The joint guidance – released by the American Society for Bone and Mineral Research (ASBMR), the American Association of Clinical Endocrinologists, the Endocrine Society, the European Calcified Tissue Society, and the National Osteoporosis Foundation – offered both general and specific recommendations for patients whose osteoporosis treatment plan is either continuing or has been disrupted during the COVID-19 pandemic.
Among the general recommendations are to initiate oral bisphosphonate therapy over either the telephone or through a video visit, with no delays for patients at high risk of fracture. They also noted that, as elective procedures, bone mineral density examinations may need to be postponed.
For patients already on osteoporosis medications – such as oral and IV bisphosphonates, denosumab, estrogen, raloxifene, teriparatide, abaloparatide, and romosozumab – they recommend continuing treatment whenever possible. “There is no evidence that any osteoporosis therapy increases the risk or severity of COVID-19 infection or alters the disease course,” they wrote. They did add, however, that COVID-19 may increase the risk of hypercoagulable complications and so caution should be exercised when treating patients with estrogen or raloxifene.
Separately, in a letter to the editor published in the Journal of Clinical Endocrinology and Metabolism (doi: 10.1210/clinem/dgaa254), Ruban Dhaliwal, MD, MPH, of the State University of New York, Syracuse, and coauthors concur in regard to raloxifene. They wrote that, because of the increased risk of thromboembolic events related to COVID-19, “it is best to discontinue raloxifene, which is also associated with such risk.”
The joint statement recognizes current social distancing policies and therefore recommends avoiding standard pretreatment labs prior to IV bisphosphonate and/or denosumab administration if previous labs were normal and the patient’s recent health has been deemed “stable.” Lab evaluation is recommended, however, for patients with fluctuating renal function and for those at higher risk of developing hypocalcemia.
The statement also provides potential alternative methods for delivering parenteral osteoporosis treatments, including off-site clinics, home delivery and administration, self-injection of denosumab and/or romosozumab, and drive-through administration of denosumab and/or romosozumab. They acknowledged the complications surrounding each alternative, including residents of “socioeconomically challenged communities” being unable to reach clinics if public transportation is not available and the “important medicolegal issues” to consider around self-injection.
For all patients whose treatments have been disrupted, the authors recommend frequent reevaluation “with the goal to resume the original osteoporosis treatment plan once circumstances allow.” As for specific recommendations, patients on denosumab who will not be treatable within 7 months of their previous injection should be transitioned to oral bisphosphonate if at all possible. For patients with underlying gastrointestinal disorders, they recommend monthly ibandronate or weekly/monthly risedronate; for patients with chronic renal insufficiency, they recommend an off-label regimen of lower dose oral bisphosphonate.
For patients on teriparatide or abaloparatide who will be unable to receive continued treatment, they recommend a delay in treatment. If that delay goes beyond several months, they recommend a temporary transition to oral bisphosphonate. For patients on romosozumab who will be unable to receive continued treatment, they also recommend a delay in treatment and a temporary transition to oral bisphosphonate. Finally, they expressed confidence that patients on IV bisphosphonates will not be harmed by treatment delays, even those of several months.
“I think we could fall into a trap during this era of the pandemic and fail to address patients’ underlying chronic conditions, even though those comorbidities will end up greatly affecting their overall health,” said incoming ASBMR president Suzanne Jan de Beur, MD, of the Johns Hopkins University, Baltimore. “As we continue to care for our patients, we need to keep chronic conditions like osteoporosis on the radar screen and not stop diagnosing people at risk or those who present with fractures. Even when we can’t perform full screening tests due to distancing policies, we need to be vigilant for those patients who need treatment and administer the treatments we have available as needed.”
The statement’s authors acknowledged the limitations of their recommendations, noting that “there is a paucity of data to provide clear guidance” and as such they were “based primarily on expert opinion.”
The authors from the five organizations did not disclose any conflicts of interest.
Evidence builds linking anticoagulation to COVID-19 survival
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
Petechiae and Ecchymoses on the Arm
The Diagnosis: Rumpel-Leede Phenomenon
Rumpel-Leede (R-L) phenomenon describes the rare benign occurrence of dermal capillaries acutely rupturing following the administration of a tourniquetlike force on an extremity,1 which manifests as asymptomatic petechiae and ecchymoses on a distal extremity, usually following noninvasive measurement of blood pressure.2 Rumpel-Leede phenomenon represents an underrecognized entity that either is excluded or minimally referenced in most dermatology textbooks. The R-L sign initially was described in the early 1900s after it was observed that tourniquets applied to the arms of patients with scarlet fever would lead to the development of petechiae in that extremity.3 It later was elucidated that underlying vascular disease predisposes to dermal capillary fragility, a risk factor for R-L phenomenon to occur upon application of a tourniquet. Rumpel-Leede phenomenon has been noted in patients with diabetes mellitus, acute or chronic hypertension, and thrombocytopenia.4 In addition to being hypertensive and diabetic, our patient had been taking amlodipine and diltiazem. Calcium channel blockers have been linked to R-L phenomenon in case reports as well as in a study of calcium channel blockers inducing capillary fragility in vivo.5 Rumpel-Leede phenomenon also has been noted in patients with tightly fitting garments and infants carried in baby carriers.1
The differential diagnosis for R-L phenomenon includes actinic purpura, small vessel vasculitis, disseminated intravascular coagulation (DIC), and deep vein thrombosis (DVT). Actinic purpura, also called solar purpura or senile purpura, represents the petechiae and ecchymoses that are associated with aging skin. It is thought to occur when DNA damage, UV-induced solar elastosis, and decreased lipids in the stratum corneum cause a weakened ability to contain red blood cell extravasation from capillaries.6 Due to the lack of history of trauma and clear association with the blood pressure cuff placement in our patient, a diagnosis of actinic purpura was unlikely. In small vessel vasculitis, patients classically present with nonblanching palpable purpura frequently distributed over the lower extremities. The isolation of the lesions to only the left arm lowered the suspicion for vasculitis. Cutaneous manifestations of DIC and other hypercoagulable states may include purpura, livedo reticularis, atrophie blanche, and in extreme cases purpura fulminans. Routine laboratory examination reveals thrombocytopenia, prolonged prothrombin time/partial thromboplastin time, and hemolytic anemia.7 Although our patient had the risk factors of recent infection and surgery, a hemoglobin level of 10.9 g/dL (reference range, 14.0-17.5 g/dL) and platelet count of 279,000/µL (reference range, 150,000-350,000/µL) excluded DIC as the probable diagnosis. Our patient was at an overall increased risk for DVT due to his prolonged hospital stay, increased age, and other factors. Despite these risk factors, the lack of pain or swelling made this diagnosis unlikely. Furthermore, our patient was heparinized throughout his hospital stay, and upper extremity DVT accounts for only 4% to 10% of the total DVT incidence.5
Although R-L phenomenon is a benign, self-limited condition, it may be necessary in some cases to rule out more serious underlying etiologies with investigative workup comprised of a complete blood cell count, coagulation profile, and basic metabolic panel. However, recognition of the R-L phenomenon in the right clinical context of localized petechiae or ecchymoses with a history of a tourniquetlike force may prevent an unnecessary and costly workup. Patients should be encouraged that R-L phenomenon will resolve over time with identification and correction of the tourniquetlike force. In this case, we recommended loosening of the sphygmomanometer cuff and alternating extremities to which the cuff was to be placed, which resulted in complete clearance of the petechiae and ecchymoses within 5 days.
- Nguyen T, Garcia D, Wang A, et al. Rumpel-Leede phenomenon associated with tourniquet-like forces of baby carriers in otherwise healthy infants. JAMA Dermatol. 2016;152:728-730.
- Chester M, Barwise J, Holzman M, et al. Acute dermal capillary rupture associated with noninvasive blood pressure monitoring. J Clin Anesth. 2007;19:473-475.
- Hartley A, Lim PB, Hayat SA. Rumpel-Leede phenomenon in a hypertensive patient due to mechanical trauma: a case report. J Med Case Rep. 2016;10:150.
- Varela D, Tran D, Ngamdu K, et al. Rumpel-Leede phenomenon presenting as a hypertensive urgency. Proc (Bayl Univ Med Cent). 2016;29:200-201.
- Cox NH, Walsh ML, Robson RH. Purpura and bleeding due to calcium-channel blockers: an underestimated problem? case reports and a pilot study. Clin Exp Dermatol. 2009;34:487-491.
- Ceilley RI. Treatment of actinic purpura. J Clin Aesthet Dermatol. 2017;10:44-50.
- Rajagopal R, Thachil J, Monagle P. Disseminated intravascular coagulation in paediatrics. Arch Dis Child. 2017;102:187-193.
- Kraaijpoel N, van Es N, Porreca E, et al. The diagnostic management of upper extremity deep venous thrombosis: a review of the literature. Thromb Res. 2017;156:54-59.
The Diagnosis: Rumpel-Leede Phenomenon
Rumpel-Leede (R-L) phenomenon describes the rare benign occurrence of dermal capillaries acutely rupturing following the administration of a tourniquetlike force on an extremity,1 which manifests as asymptomatic petechiae and ecchymoses on a distal extremity, usually following noninvasive measurement of blood pressure.2 Rumpel-Leede phenomenon represents an underrecognized entity that either is excluded or minimally referenced in most dermatology textbooks. The R-L sign initially was described in the early 1900s after it was observed that tourniquets applied to the arms of patients with scarlet fever would lead to the development of petechiae in that extremity.3 It later was elucidated that underlying vascular disease predisposes to dermal capillary fragility, a risk factor for R-L phenomenon to occur upon application of a tourniquet. Rumpel-Leede phenomenon has been noted in patients with diabetes mellitus, acute or chronic hypertension, and thrombocytopenia.4 In addition to being hypertensive and diabetic, our patient had been taking amlodipine and diltiazem. Calcium channel blockers have been linked to R-L phenomenon in case reports as well as in a study of calcium channel blockers inducing capillary fragility in vivo.5 Rumpel-Leede phenomenon also has been noted in patients with tightly fitting garments and infants carried in baby carriers.1
The differential diagnosis for R-L phenomenon includes actinic purpura, small vessel vasculitis, disseminated intravascular coagulation (DIC), and deep vein thrombosis (DVT). Actinic purpura, also called solar purpura or senile purpura, represents the petechiae and ecchymoses that are associated with aging skin. It is thought to occur when DNA damage, UV-induced solar elastosis, and decreased lipids in the stratum corneum cause a weakened ability to contain red blood cell extravasation from capillaries.6 Due to the lack of history of trauma and clear association with the blood pressure cuff placement in our patient, a diagnosis of actinic purpura was unlikely. In small vessel vasculitis, patients classically present with nonblanching palpable purpura frequently distributed over the lower extremities. The isolation of the lesions to only the left arm lowered the suspicion for vasculitis. Cutaneous manifestations of DIC and other hypercoagulable states may include purpura, livedo reticularis, atrophie blanche, and in extreme cases purpura fulminans. Routine laboratory examination reveals thrombocytopenia, prolonged prothrombin time/partial thromboplastin time, and hemolytic anemia.7 Although our patient had the risk factors of recent infection and surgery, a hemoglobin level of 10.9 g/dL (reference range, 14.0-17.5 g/dL) and platelet count of 279,000/µL (reference range, 150,000-350,000/µL) excluded DIC as the probable diagnosis. Our patient was at an overall increased risk for DVT due to his prolonged hospital stay, increased age, and other factors. Despite these risk factors, the lack of pain or swelling made this diagnosis unlikely. Furthermore, our patient was heparinized throughout his hospital stay, and upper extremity DVT accounts for only 4% to 10% of the total DVT incidence.5
Although R-L phenomenon is a benign, self-limited condition, it may be necessary in some cases to rule out more serious underlying etiologies with investigative workup comprised of a complete blood cell count, coagulation profile, and basic metabolic panel. However, recognition of the R-L phenomenon in the right clinical context of localized petechiae or ecchymoses with a history of a tourniquetlike force may prevent an unnecessary and costly workup. Patients should be encouraged that R-L phenomenon will resolve over time with identification and correction of the tourniquetlike force. In this case, we recommended loosening of the sphygmomanometer cuff and alternating extremities to which the cuff was to be placed, which resulted in complete clearance of the petechiae and ecchymoses within 5 days.
The Diagnosis: Rumpel-Leede Phenomenon
Rumpel-Leede (R-L) phenomenon describes the rare benign occurrence of dermal capillaries acutely rupturing following the administration of a tourniquetlike force on an extremity,1 which manifests as asymptomatic petechiae and ecchymoses on a distal extremity, usually following noninvasive measurement of blood pressure.2 Rumpel-Leede phenomenon represents an underrecognized entity that either is excluded or minimally referenced in most dermatology textbooks. The R-L sign initially was described in the early 1900s after it was observed that tourniquets applied to the arms of patients with scarlet fever would lead to the development of petechiae in that extremity.3 It later was elucidated that underlying vascular disease predisposes to dermal capillary fragility, a risk factor for R-L phenomenon to occur upon application of a tourniquet. Rumpel-Leede phenomenon has been noted in patients with diabetes mellitus, acute or chronic hypertension, and thrombocytopenia.4 In addition to being hypertensive and diabetic, our patient had been taking amlodipine and diltiazem. Calcium channel blockers have been linked to R-L phenomenon in case reports as well as in a study of calcium channel blockers inducing capillary fragility in vivo.5 Rumpel-Leede phenomenon also has been noted in patients with tightly fitting garments and infants carried in baby carriers.1
The differential diagnosis for R-L phenomenon includes actinic purpura, small vessel vasculitis, disseminated intravascular coagulation (DIC), and deep vein thrombosis (DVT). Actinic purpura, also called solar purpura or senile purpura, represents the petechiae and ecchymoses that are associated with aging skin. It is thought to occur when DNA damage, UV-induced solar elastosis, and decreased lipids in the stratum corneum cause a weakened ability to contain red blood cell extravasation from capillaries.6 Due to the lack of history of trauma and clear association with the blood pressure cuff placement in our patient, a diagnosis of actinic purpura was unlikely. In small vessel vasculitis, patients classically present with nonblanching palpable purpura frequently distributed over the lower extremities. The isolation of the lesions to only the left arm lowered the suspicion for vasculitis. Cutaneous manifestations of DIC and other hypercoagulable states may include purpura, livedo reticularis, atrophie blanche, and in extreme cases purpura fulminans. Routine laboratory examination reveals thrombocytopenia, prolonged prothrombin time/partial thromboplastin time, and hemolytic anemia.7 Although our patient had the risk factors of recent infection and surgery, a hemoglobin level of 10.9 g/dL (reference range, 14.0-17.5 g/dL) and platelet count of 279,000/µL (reference range, 150,000-350,000/µL) excluded DIC as the probable diagnosis. Our patient was at an overall increased risk for DVT due to his prolonged hospital stay, increased age, and other factors. Despite these risk factors, the lack of pain or swelling made this diagnosis unlikely. Furthermore, our patient was heparinized throughout his hospital stay, and upper extremity DVT accounts for only 4% to 10% of the total DVT incidence.5
Although R-L phenomenon is a benign, self-limited condition, it may be necessary in some cases to rule out more serious underlying etiologies with investigative workup comprised of a complete blood cell count, coagulation profile, and basic metabolic panel. However, recognition of the R-L phenomenon in the right clinical context of localized petechiae or ecchymoses with a history of a tourniquetlike force may prevent an unnecessary and costly workup. Patients should be encouraged that R-L phenomenon will resolve over time with identification and correction of the tourniquetlike force. In this case, we recommended loosening of the sphygmomanometer cuff and alternating extremities to which the cuff was to be placed, which resulted in complete clearance of the petechiae and ecchymoses within 5 days.
- Nguyen T, Garcia D, Wang A, et al. Rumpel-Leede phenomenon associated with tourniquet-like forces of baby carriers in otherwise healthy infants. JAMA Dermatol. 2016;152:728-730.
- Chester M, Barwise J, Holzman M, et al. Acute dermal capillary rupture associated with noninvasive blood pressure monitoring. J Clin Anesth. 2007;19:473-475.
- Hartley A, Lim PB, Hayat SA. Rumpel-Leede phenomenon in a hypertensive patient due to mechanical trauma: a case report. J Med Case Rep. 2016;10:150.
- Varela D, Tran D, Ngamdu K, et al. Rumpel-Leede phenomenon presenting as a hypertensive urgency. Proc (Bayl Univ Med Cent). 2016;29:200-201.
- Cox NH, Walsh ML, Robson RH. Purpura and bleeding due to calcium-channel blockers: an underestimated problem? case reports and a pilot study. Clin Exp Dermatol. 2009;34:487-491.
- Ceilley RI. Treatment of actinic purpura. J Clin Aesthet Dermatol. 2017;10:44-50.
- Rajagopal R, Thachil J, Monagle P. Disseminated intravascular coagulation in paediatrics. Arch Dis Child. 2017;102:187-193.
- Kraaijpoel N, van Es N, Porreca E, et al. The diagnostic management of upper extremity deep venous thrombosis: a review of the literature. Thromb Res. 2017;156:54-59.
- Nguyen T, Garcia D, Wang A, et al. Rumpel-Leede phenomenon associated with tourniquet-like forces of baby carriers in otherwise healthy infants. JAMA Dermatol. 2016;152:728-730.
- Chester M, Barwise J, Holzman M, et al. Acute dermal capillary rupture associated with noninvasive blood pressure monitoring. J Clin Anesth. 2007;19:473-475.
- Hartley A, Lim PB, Hayat SA. Rumpel-Leede phenomenon in a hypertensive patient due to mechanical trauma: a case report. J Med Case Rep. 2016;10:150.
- Varela D, Tran D, Ngamdu K, et al. Rumpel-Leede phenomenon presenting as a hypertensive urgency. Proc (Bayl Univ Med Cent). 2016;29:200-201.
- Cox NH, Walsh ML, Robson RH. Purpura and bleeding due to calcium-channel blockers: an underestimated problem? case reports and a pilot study. Clin Exp Dermatol. 2009;34:487-491.
- Ceilley RI. Treatment of actinic purpura. J Clin Aesthet Dermatol. 2017;10:44-50.
- Rajagopal R, Thachil J, Monagle P. Disseminated intravascular coagulation in paediatrics. Arch Dis Child. 2017;102:187-193.
- Kraaijpoel N, van Es N, Porreca E, et al. The diagnostic management of upper extremity deep venous thrombosis: a review of the literature. Thromb Res. 2017;156:54-59.
A 70-year-old man who had been admitted to the hospital a week prior for a right groin abscess overlying the site of a femoral graft developed a purpuric rash isolated to the left arm of 1 day's duration. The dermatology service was consulted by vascular surgery. The patient denied prior episodes of a similar rash, and there was no associated pruritus or pain. There was no history of trauma to the area. His medical history was remarkable for type 2 diabetes mellitus, hypertension, and peripheral vascular disease. His surgical history was notable for bilateral popliteal aneurysm repair and right femoral aneurysm repair. No pertinent family history was elicited. Cefepime, metronidazole, and vancomycin were administered for the groin abscess. Additional medications included amlodipine, atorvastatin, diltiazem, gabapentin, heparin, insulin, oxycodone, and acetaminophen.
Physical examination revealed broad ecchymoses on the left forearm with scattered petechiae as well as linear ecchymoses on the left upper arm distributed in the area where a sphygmomanometer cuff was applied. Full-body skin examination confirmed that the distribution of the petechiae and ecchymoses was limited to the left arm. The patient was normotensive at the time of examination. Laboratory evaluation revealed a hemoglobin level of 10.9 g/dL (reference range, 14.0-17.5 g/dL), a platelet count of 279,000/µL (reference range, 150,000-350,000/µL), and a glucose level of 121 mg/dL (reference range, 70-110 mg/dL).
New angiotensin studies in COVID-19 give more reassurance
Four more studies of the relationship of angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) with COVID-19 have been published in the past few days in top-tier peer-reviewed journals, and on the whole, the data are reassuring.
Three of the new studies were published in the New England Journal of Medicine on May 1, and one study was published in JAMA Cardiology on May 5.
Although all the studies are observational in design and have some confounding factors, overall, However, there are some contradictory findings in secondary analyses regarding possible differences in the effects of the two drug classes.
Providing commentary, John McMurray, MD, professor of medical cardiology at the University of Glasgow, said: “The overall picture seems to suggest no increase in risk of adverse outcomes in patients taking renin-angiotensin system [RAS] blockers ― but with lots of caveats: These are all observational rather than randomized studies, and there may be residual or unmeasured confounding.”
Was it ‘Much ado about nothing’?
Franz Messerli, MD, professor of medicine at the University of Bern (Switzerland), added: “Given this state of the art, I am inclined to consider RAS blockade and COVID-19 – despite all the hype in the news media – as much ado about nothing.”
But both Dr. McMurray and Dr. Messerli said they were intrigued about possible differences in the effects of ACE inhibitors and ARBs that some of the new results suggest.
In one study, a team led by Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center, Boston, analyzed data from 8,910 patients with COVID-19 admitted to 169 hospitals in Asia, Europe, and North America who had either died in the hospital (5.8%) or survived to hospital discharge (94.2%).
In multivariate logistic-regression analysis, age greater than 65 years, coronary artery disease, congestive heart failure, history of cardiac arrhythmia, chronic obstructive pulmonary disease, and current smoking were associated with an increased risk for in-hospital death. Female sex was associated with a decreased risk. Neither ACE inhibitors nor ARBs were associated with an increased risk for in-hospital death.
In fact, ACE inhibitors were associated with a significant reduction in mortality (odds ratio, 0.33), as were statins (OR, 0.35).
The authors, however, stressed that these observations about reduced mortality with ACE inhibitors and statins “should be considered with extreme caution.”
“Because our study was not a randomized, controlled trial, we cannot exclude the possibility of confounding. In addition, we examined relationships between many variables and in-hospital death, and no primary hypothesis was prespecified; these factors increased the probability of chance associations being found. Therefore, a cause-and-effect relationship between drug therapy and survival should not be inferred,” they wrote.
A secondary analysis that was restricted to patients with hypertension (those for whom an ACE inhibitor or an ARB would be indicated) also did not show harm.
A second study published in the New England Journal of Medicine had a case-control design. The authors, led by Giuseppe Mancia, MD, of the University of Milano-Bicocca (Italy), compared 6,272 patients with confirmed COVID-19 (case patients) with 30,759 control persons who were matched according to age, sex, and municipality of residence.
In a conditional logistic-regression multivariate analysis, neither ACE inhibitors nor ARBs were associated with the likelihood of SARS-CoV-2 infection.
“Thus, our results do not provide evidence of an independent relationship between renin angiotensin aldosterone blockers and the susceptibility to COVID-19 in humans,” the authors concluded.
In addition, a second analysis that compared patients who had severe or fatal infections with matched control persons did not show an association between ACE inhibitors or ARBs and severe disease.
In the third study published in the New England Journal of Medicine, a group led by Harmony R. Reynolds, MD, of New York University, analyzed data from the health records of 12,594 patients in the NYU Langone Health system who had been tested for COVID-19. They found 5,894 patients whose test results were positive. Of these patients, 1,002 had severe illness, which was defined as illness requiring admission to the ICU, need for mechanical ventilation, or death.
Using Bayesian analysis and propensity score matching, the researchers assessed the relation between previous treatment with five different classes of antihypertensive drugs (ACE inhibitors, ARBs, beta blockers, calcium blockers, and thiazide diuretics) and the likelihood of a positive or negative result on COVID-19 testing, as well as the likelihood of severe illness among patients who tested positive.
Results showed no positive association between any of the analyzed drug classes and either a positive test result or severe illness.
In an accompanying editorial, a group led by John A. Jarcho, MD, of Harvard Medical School, Boston, and deputy editor of the New England Journal of Medicine, wrote: “Taken together, these three studies do not provide evidence to support the hypothesis that ACE inhibitor or ARB use is associated with the risk of SARS-CoV-2 infection, the risk of severe COVID-19 among those infected, or the risk of in-hospital death among those with a positive test.
“Each of these studies has weaknesses inherent in observational data, but we find it reassuring that three studies in different populations and with different designs arrive at the consistent message that the continued use of ACE inhibitors and ARBs is unlikely to be harmful in patients with COVID-19. Several other smaller studies from China and the United Kingdom have come to the same conclusion,” the authors of the editorial stated.
In the study published in JAMA Cardiology, a group led by Neil Mehta, MBBS, of the Cleveland Clinic, Ohio, analyzed data on 18,472 patients who had been tested for COVID-19 between March 8 and April 12 in the Cleveland Clinic Health System in Ohio and Florida. Of these patients, 9.4% tested positive.
After overlap propensity score weighting for both ACE inhibitors and ARBs to take into account relevant comorbidities, there was no difference in risk for testing positive among patients taking an ACE inhibitor or an ARB in comparison with those not taking such medication.
Are there different effects between ACE inhibitors and ARBs?
A secondary exploratory analysis showed a higher likelihood of hospital admission among patients who tested positive and who were taking either ACE inhibitors (OR, 1.84) or ARBs (OR, 1.61), and there was a higher likelihood of ICU admission among patients who tested positive and who were taking an ACE inhibitor (OR 1.77), but no such difference was observed among those taking ARBs.
Coauthor Ankur Kalra, MD, of the Cleveland Clinic, said in an interview that results of the exploratory analysis fit with the hypothesis that the two drugs classes may have different effects in patients with COVID-19.
“Angiotensin II promotes vasoconstriction, inflammation, and fibrosis in the lungs, and ARBs block the effects of angiotensin II more effectively than ACE inhibitors. In addition, ACE inhibitors (but not ARBs) increase levels of bradykinin, which may be one factor leading to acute respiratory distress syndrome,” he noted.
“However, these results should only be considered exploratory, as there is inherent bias in observational data,” Dr. Kalra stressed.
In an accompanying editorial in JAMA Cardiology, a group led by Laine E. Thomas, PhD, of Duke Clinical Research Institute, Durham, North Carolina, said that the results of this secondary exploratory analysis are limited by a small number of patients and “are likely explained by confounding and should not be inferred as causal.”
The New England Journal of Medicine editorialists reached a similar conclusion regarding the lower mortality in COVID-19 patients who took ACE inhibitors in the study by Dr. Mehra and colleagues. They say this unexpected result “may be due to unmeasured confounding and, in the absence of a randomized trial, should not be regarded as evidence to prescribe these drugs in patients with COVID-19.”
Providing further comment, Dr. McMurray said: “Normally, I would not read too much into the different effects of ACE inhibitors and ARBs suggested in the Cleveland study because of the small numbers (about 28 ACE inhibitor–treated patients admitted to ICU) and the limited information about matching and/or adjustment for potential differences between groups.
“I could also argue that the comparison that would best answer the question about risk related to type of RAS blocker would be the direct comparison of people taking an ACE inhibitor with those taking an ARB (and that doesn’t look very different). The only thing that makes me a little cautious about completely dismissing the possibility of a difference between ACE inhibitor and ARB here is the suggestion of a similar trend in another large study from the VA [Veterans Affairs] system,” he added.
He also noted that speculation about there being mechanisms that involve different effects of the two drug classes on bradykinin and angiotensin II was “plausible but unproven.”
Dr. Messerli added: “Before turning the page, I would like to see an analysis comparing ACE inhibitors and ARBs, since experimentally, their effect on ACE2 (the receptor to which the virus binds) seems to differ. The study of Mehta et al in JAMA Cardiology may be the first clinical hint indicating that ARBs are more protective than ACEIs. However even here, the looming possibility of confounding cannot be excluded.”
Dr. Messerli also pointed to a hypothesis that suggests that direct viral infection of endothelial cells expressing ACE2 receptors may explain worse outcomes in patients with cardiovascular comorbidities, which provides a rationale for therapies to stabilize the endothelium, particularly with anti-inflammatory anticytokine drugs, ACE inhibitors, and statins.
A version of this article originally appeared on Medscape.com.
Four more studies of the relationship of angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) with COVID-19 have been published in the past few days in top-tier peer-reviewed journals, and on the whole, the data are reassuring.
Three of the new studies were published in the New England Journal of Medicine on May 1, and one study was published in JAMA Cardiology on May 5.
Although all the studies are observational in design and have some confounding factors, overall, However, there are some contradictory findings in secondary analyses regarding possible differences in the effects of the two drug classes.
Providing commentary, John McMurray, MD, professor of medical cardiology at the University of Glasgow, said: “The overall picture seems to suggest no increase in risk of adverse outcomes in patients taking renin-angiotensin system [RAS] blockers ― but with lots of caveats: These are all observational rather than randomized studies, and there may be residual or unmeasured confounding.”
Was it ‘Much ado about nothing’?
Franz Messerli, MD, professor of medicine at the University of Bern (Switzerland), added: “Given this state of the art, I am inclined to consider RAS blockade and COVID-19 – despite all the hype in the news media – as much ado about nothing.”
But both Dr. McMurray and Dr. Messerli said they were intrigued about possible differences in the effects of ACE inhibitors and ARBs that some of the new results suggest.
In one study, a team led by Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center, Boston, analyzed data from 8,910 patients with COVID-19 admitted to 169 hospitals in Asia, Europe, and North America who had either died in the hospital (5.8%) or survived to hospital discharge (94.2%).
In multivariate logistic-regression analysis, age greater than 65 years, coronary artery disease, congestive heart failure, history of cardiac arrhythmia, chronic obstructive pulmonary disease, and current smoking were associated with an increased risk for in-hospital death. Female sex was associated with a decreased risk. Neither ACE inhibitors nor ARBs were associated with an increased risk for in-hospital death.
In fact, ACE inhibitors were associated with a significant reduction in mortality (odds ratio, 0.33), as were statins (OR, 0.35).
The authors, however, stressed that these observations about reduced mortality with ACE inhibitors and statins “should be considered with extreme caution.”
“Because our study was not a randomized, controlled trial, we cannot exclude the possibility of confounding. In addition, we examined relationships between many variables and in-hospital death, and no primary hypothesis was prespecified; these factors increased the probability of chance associations being found. Therefore, a cause-and-effect relationship between drug therapy and survival should not be inferred,” they wrote.
A secondary analysis that was restricted to patients with hypertension (those for whom an ACE inhibitor or an ARB would be indicated) also did not show harm.
A second study published in the New England Journal of Medicine had a case-control design. The authors, led by Giuseppe Mancia, MD, of the University of Milano-Bicocca (Italy), compared 6,272 patients with confirmed COVID-19 (case patients) with 30,759 control persons who were matched according to age, sex, and municipality of residence.
In a conditional logistic-regression multivariate analysis, neither ACE inhibitors nor ARBs were associated with the likelihood of SARS-CoV-2 infection.
“Thus, our results do not provide evidence of an independent relationship between renin angiotensin aldosterone blockers and the susceptibility to COVID-19 in humans,” the authors concluded.
In addition, a second analysis that compared patients who had severe or fatal infections with matched control persons did not show an association between ACE inhibitors or ARBs and severe disease.
In the third study published in the New England Journal of Medicine, a group led by Harmony R. Reynolds, MD, of New York University, analyzed data from the health records of 12,594 patients in the NYU Langone Health system who had been tested for COVID-19. They found 5,894 patients whose test results were positive. Of these patients, 1,002 had severe illness, which was defined as illness requiring admission to the ICU, need for mechanical ventilation, or death.
Using Bayesian analysis and propensity score matching, the researchers assessed the relation between previous treatment with five different classes of antihypertensive drugs (ACE inhibitors, ARBs, beta blockers, calcium blockers, and thiazide diuretics) and the likelihood of a positive or negative result on COVID-19 testing, as well as the likelihood of severe illness among patients who tested positive.
Results showed no positive association between any of the analyzed drug classes and either a positive test result or severe illness.
In an accompanying editorial, a group led by John A. Jarcho, MD, of Harvard Medical School, Boston, and deputy editor of the New England Journal of Medicine, wrote: “Taken together, these three studies do not provide evidence to support the hypothesis that ACE inhibitor or ARB use is associated with the risk of SARS-CoV-2 infection, the risk of severe COVID-19 among those infected, or the risk of in-hospital death among those with a positive test.
“Each of these studies has weaknesses inherent in observational data, but we find it reassuring that three studies in different populations and with different designs arrive at the consistent message that the continued use of ACE inhibitors and ARBs is unlikely to be harmful in patients with COVID-19. Several other smaller studies from China and the United Kingdom have come to the same conclusion,” the authors of the editorial stated.
In the study published in JAMA Cardiology, a group led by Neil Mehta, MBBS, of the Cleveland Clinic, Ohio, analyzed data on 18,472 patients who had been tested for COVID-19 between March 8 and April 12 in the Cleveland Clinic Health System in Ohio and Florida. Of these patients, 9.4% tested positive.
After overlap propensity score weighting for both ACE inhibitors and ARBs to take into account relevant comorbidities, there was no difference in risk for testing positive among patients taking an ACE inhibitor or an ARB in comparison with those not taking such medication.
Are there different effects between ACE inhibitors and ARBs?
A secondary exploratory analysis showed a higher likelihood of hospital admission among patients who tested positive and who were taking either ACE inhibitors (OR, 1.84) or ARBs (OR, 1.61), and there was a higher likelihood of ICU admission among patients who tested positive and who were taking an ACE inhibitor (OR 1.77), but no such difference was observed among those taking ARBs.
Coauthor Ankur Kalra, MD, of the Cleveland Clinic, said in an interview that results of the exploratory analysis fit with the hypothesis that the two drugs classes may have different effects in patients with COVID-19.
“Angiotensin II promotes vasoconstriction, inflammation, and fibrosis in the lungs, and ARBs block the effects of angiotensin II more effectively than ACE inhibitors. In addition, ACE inhibitors (but not ARBs) increase levels of bradykinin, which may be one factor leading to acute respiratory distress syndrome,” he noted.
“However, these results should only be considered exploratory, as there is inherent bias in observational data,” Dr. Kalra stressed.
In an accompanying editorial in JAMA Cardiology, a group led by Laine E. Thomas, PhD, of Duke Clinical Research Institute, Durham, North Carolina, said that the results of this secondary exploratory analysis are limited by a small number of patients and “are likely explained by confounding and should not be inferred as causal.”
The New England Journal of Medicine editorialists reached a similar conclusion regarding the lower mortality in COVID-19 patients who took ACE inhibitors in the study by Dr. Mehra and colleagues. They say this unexpected result “may be due to unmeasured confounding and, in the absence of a randomized trial, should not be regarded as evidence to prescribe these drugs in patients with COVID-19.”
Providing further comment, Dr. McMurray said: “Normally, I would not read too much into the different effects of ACE inhibitors and ARBs suggested in the Cleveland study because of the small numbers (about 28 ACE inhibitor–treated patients admitted to ICU) and the limited information about matching and/or adjustment for potential differences between groups.
“I could also argue that the comparison that would best answer the question about risk related to type of RAS blocker would be the direct comparison of people taking an ACE inhibitor with those taking an ARB (and that doesn’t look very different). The only thing that makes me a little cautious about completely dismissing the possibility of a difference between ACE inhibitor and ARB here is the suggestion of a similar trend in another large study from the VA [Veterans Affairs] system,” he added.
He also noted that speculation about there being mechanisms that involve different effects of the two drug classes on bradykinin and angiotensin II was “plausible but unproven.”
Dr. Messerli added: “Before turning the page, I would like to see an analysis comparing ACE inhibitors and ARBs, since experimentally, their effect on ACE2 (the receptor to which the virus binds) seems to differ. The study of Mehta et al in JAMA Cardiology may be the first clinical hint indicating that ARBs are more protective than ACEIs. However even here, the looming possibility of confounding cannot be excluded.”
Dr. Messerli also pointed to a hypothesis that suggests that direct viral infection of endothelial cells expressing ACE2 receptors may explain worse outcomes in patients with cardiovascular comorbidities, which provides a rationale for therapies to stabilize the endothelium, particularly with anti-inflammatory anticytokine drugs, ACE inhibitors, and statins.
A version of this article originally appeared on Medscape.com.
Four more studies of the relationship of angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) with COVID-19 have been published in the past few days in top-tier peer-reviewed journals, and on the whole, the data are reassuring.
Three of the new studies were published in the New England Journal of Medicine on May 1, and one study was published in JAMA Cardiology on May 5.
Although all the studies are observational in design and have some confounding factors, overall, However, there are some contradictory findings in secondary analyses regarding possible differences in the effects of the two drug classes.
Providing commentary, John McMurray, MD, professor of medical cardiology at the University of Glasgow, said: “The overall picture seems to suggest no increase in risk of adverse outcomes in patients taking renin-angiotensin system [RAS] blockers ― but with lots of caveats: These are all observational rather than randomized studies, and there may be residual or unmeasured confounding.”
Was it ‘Much ado about nothing’?
Franz Messerli, MD, professor of medicine at the University of Bern (Switzerland), added: “Given this state of the art, I am inclined to consider RAS blockade and COVID-19 – despite all the hype in the news media – as much ado about nothing.”
But both Dr. McMurray and Dr. Messerli said they were intrigued about possible differences in the effects of ACE inhibitors and ARBs that some of the new results suggest.
In one study, a team led by Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center, Boston, analyzed data from 8,910 patients with COVID-19 admitted to 169 hospitals in Asia, Europe, and North America who had either died in the hospital (5.8%) or survived to hospital discharge (94.2%).
In multivariate logistic-regression analysis, age greater than 65 years, coronary artery disease, congestive heart failure, history of cardiac arrhythmia, chronic obstructive pulmonary disease, and current smoking were associated with an increased risk for in-hospital death. Female sex was associated with a decreased risk. Neither ACE inhibitors nor ARBs were associated with an increased risk for in-hospital death.
In fact, ACE inhibitors were associated with a significant reduction in mortality (odds ratio, 0.33), as were statins (OR, 0.35).
The authors, however, stressed that these observations about reduced mortality with ACE inhibitors and statins “should be considered with extreme caution.”
“Because our study was not a randomized, controlled trial, we cannot exclude the possibility of confounding. In addition, we examined relationships between many variables and in-hospital death, and no primary hypothesis was prespecified; these factors increased the probability of chance associations being found. Therefore, a cause-and-effect relationship between drug therapy and survival should not be inferred,” they wrote.
A secondary analysis that was restricted to patients with hypertension (those for whom an ACE inhibitor or an ARB would be indicated) also did not show harm.
A second study published in the New England Journal of Medicine had a case-control design. The authors, led by Giuseppe Mancia, MD, of the University of Milano-Bicocca (Italy), compared 6,272 patients with confirmed COVID-19 (case patients) with 30,759 control persons who were matched according to age, sex, and municipality of residence.
In a conditional logistic-regression multivariate analysis, neither ACE inhibitors nor ARBs were associated with the likelihood of SARS-CoV-2 infection.
“Thus, our results do not provide evidence of an independent relationship between renin angiotensin aldosterone blockers and the susceptibility to COVID-19 in humans,” the authors concluded.
In addition, a second analysis that compared patients who had severe or fatal infections with matched control persons did not show an association between ACE inhibitors or ARBs and severe disease.
In the third study published in the New England Journal of Medicine, a group led by Harmony R. Reynolds, MD, of New York University, analyzed data from the health records of 12,594 patients in the NYU Langone Health system who had been tested for COVID-19. They found 5,894 patients whose test results were positive. Of these patients, 1,002 had severe illness, which was defined as illness requiring admission to the ICU, need for mechanical ventilation, or death.
Using Bayesian analysis and propensity score matching, the researchers assessed the relation between previous treatment with five different classes of antihypertensive drugs (ACE inhibitors, ARBs, beta blockers, calcium blockers, and thiazide diuretics) and the likelihood of a positive or negative result on COVID-19 testing, as well as the likelihood of severe illness among patients who tested positive.
Results showed no positive association between any of the analyzed drug classes and either a positive test result or severe illness.
In an accompanying editorial, a group led by John A. Jarcho, MD, of Harvard Medical School, Boston, and deputy editor of the New England Journal of Medicine, wrote: “Taken together, these three studies do not provide evidence to support the hypothesis that ACE inhibitor or ARB use is associated with the risk of SARS-CoV-2 infection, the risk of severe COVID-19 among those infected, or the risk of in-hospital death among those with a positive test.
“Each of these studies has weaknesses inherent in observational data, but we find it reassuring that three studies in different populations and with different designs arrive at the consistent message that the continued use of ACE inhibitors and ARBs is unlikely to be harmful in patients with COVID-19. Several other smaller studies from China and the United Kingdom have come to the same conclusion,” the authors of the editorial stated.
In the study published in JAMA Cardiology, a group led by Neil Mehta, MBBS, of the Cleveland Clinic, Ohio, analyzed data on 18,472 patients who had been tested for COVID-19 between March 8 and April 12 in the Cleveland Clinic Health System in Ohio and Florida. Of these patients, 9.4% tested positive.
After overlap propensity score weighting for both ACE inhibitors and ARBs to take into account relevant comorbidities, there was no difference in risk for testing positive among patients taking an ACE inhibitor or an ARB in comparison with those not taking such medication.
Are there different effects between ACE inhibitors and ARBs?
A secondary exploratory analysis showed a higher likelihood of hospital admission among patients who tested positive and who were taking either ACE inhibitors (OR, 1.84) or ARBs (OR, 1.61), and there was a higher likelihood of ICU admission among patients who tested positive and who were taking an ACE inhibitor (OR 1.77), but no such difference was observed among those taking ARBs.
Coauthor Ankur Kalra, MD, of the Cleveland Clinic, said in an interview that results of the exploratory analysis fit with the hypothesis that the two drugs classes may have different effects in patients with COVID-19.
“Angiotensin II promotes vasoconstriction, inflammation, and fibrosis in the lungs, and ARBs block the effects of angiotensin II more effectively than ACE inhibitors. In addition, ACE inhibitors (but not ARBs) increase levels of bradykinin, which may be one factor leading to acute respiratory distress syndrome,” he noted.
“However, these results should only be considered exploratory, as there is inherent bias in observational data,” Dr. Kalra stressed.
In an accompanying editorial in JAMA Cardiology, a group led by Laine E. Thomas, PhD, of Duke Clinical Research Institute, Durham, North Carolina, said that the results of this secondary exploratory analysis are limited by a small number of patients and “are likely explained by confounding and should not be inferred as causal.”
The New England Journal of Medicine editorialists reached a similar conclusion regarding the lower mortality in COVID-19 patients who took ACE inhibitors in the study by Dr. Mehra and colleagues. They say this unexpected result “may be due to unmeasured confounding and, in the absence of a randomized trial, should not be regarded as evidence to prescribe these drugs in patients with COVID-19.”
Providing further comment, Dr. McMurray said: “Normally, I would not read too much into the different effects of ACE inhibitors and ARBs suggested in the Cleveland study because of the small numbers (about 28 ACE inhibitor–treated patients admitted to ICU) and the limited information about matching and/or adjustment for potential differences between groups.
“I could also argue that the comparison that would best answer the question about risk related to type of RAS blocker would be the direct comparison of people taking an ACE inhibitor with those taking an ARB (and that doesn’t look very different). The only thing that makes me a little cautious about completely dismissing the possibility of a difference between ACE inhibitor and ARB here is the suggestion of a similar trend in another large study from the VA [Veterans Affairs] system,” he added.
He also noted that speculation about there being mechanisms that involve different effects of the two drug classes on bradykinin and angiotensin II was “plausible but unproven.”
Dr. Messerli added: “Before turning the page, I would like to see an analysis comparing ACE inhibitors and ARBs, since experimentally, their effect on ACE2 (the receptor to which the virus binds) seems to differ. The study of Mehta et al in JAMA Cardiology may be the first clinical hint indicating that ARBs are more protective than ACEIs. However even here, the looming possibility of confounding cannot be excluded.”
Dr. Messerli also pointed to a hypothesis that suggests that direct viral infection of endothelial cells expressing ACE2 receptors may explain worse outcomes in patients with cardiovascular comorbidities, which provides a rationale for therapies to stabilize the endothelium, particularly with anti-inflammatory anticytokine drugs, ACE inhibitors, and statins.
A version of this article originally appeared on Medscape.com.
Moving beyond the hospital ward
SHM is entering an exciting new chapter in its history because we will soon see Dr. Eric Howell take the reins from Dr. Larry Wellikson as CEO, as we watch Dr. Danielle Scheurer assume the role of president from Dr. Chris Frost, and as a side note, I will try to fill Dr. Scheurer’s shoes as physician editor of The Hospitalist.
This changing of the guard of SHM’s leadership will take place amid the backdrop of an acrimonious presidential election and the emergence of a novel coronavirus that threatens to upend the typical routines of our social and professional lives.
Without a doubt, our leaders, whether national, regional, or local, will be at the helm during one of the most uncertain times in the history of modern health care. Will we see a U.S. President who is a proponent of supporting the Affordable Care Act? Will we see further erosion of Obamacare under a second term of President Trump? Will we see rural hospitals continue to close or shrink1 as their margins get squeezed by skyrocketing denials for inpatient status in favor of observation or outpatient status?2
Forces that seem beyond our control threaten to drastically alter our professions and even our livelihoods. In the space of the few weeks during which I began and finished this piece, every day brought a whole new world of changes in my hospital, town, state, and country. No leader can predict the future with any semblance of certitude.
In the face of these swirling winds of uncertainty, what is clear is that maintaining our commitment as hospitalists to providing evidence-based, high-quality care to our patients while providing support to our colleagues in the health care industry will greatly benefit from collaborating effectively under the “big tent” philosophy of SHM. Over my career, I have benefited from great role models and colleagues as my career took me from primary care med-peds to the “new” field of hospital medicine as a med-peds hospitalist, to a leadership role in pediatric hospital medicine. I have also benefited from “learning opportunities,” as I have made my fair share of mistakes in efforts to improve systems of care. Nearly all of these mistakes share a common thread – not collaborating effectively with critical stakeholders, both within and outside of my institution.3 As this pandemic progresses, I am (and likely you are) witnessing your leaders succeed or fail based on their ability to collaborate across the institution.
As a field, we risk making similar errors by being too narrowly focused as we strive to improve the care of our patients. Recently, Dr. Russell Buhr and his colleagues at the University of California, Los Angeles, demonstrated that a majority of 30-day readmissions for chronic obstructive pulmonary disease (COPD) are due to non-COPD diagnoses.4 As we discharge our COPD patients, we may be satisfied that we’ve “tuned up” our patient’s COPD, but have we adequately arranged for appropriate ongoing care of their other medical problems? This requires an activity undertaken less and less these days in medicine – a conversation between hospitalists and outpatient medical providers. The coronavirus disease 2019 (COVID-19) pandemic has made this more challenging, but I can assure you that you can neither transmit nor catch the coronavirus from a phone call.
Perhaps we can learn from our hospitalist colleagues trained in family medicine. A recent study found that hospitalists in a team made up of family medicine–trained physicians in an academic health center achieved a 33% shorter length of stay for patients from the family medicine clinic, after adjustment for disease, demographics, and disease severity.5 The conclusion of the authors was that this was likely caused by greater familiarity with outpatient resources. I would conjecture that family medicine hospitalists were also more likely to have a conversation with a patient’s outpatient primary care provider (PCP).
Of course, I am the first to admit that chatting with a PCP is not as easy as it used to be – when we could bump into each other in the doctor’s lounge drinking coffee or in radiology while pulling x-ray films (remember those?) – and in the age of COVID-19, these interactions are even less likely. It can take considerable time and effort to get PCP colleagues on the phone unless you’re chummy enough to have their cell phone numbers. And time is a resource in short supply because most hospital medicine groups are understaffed – in the 2018 SHM State of Hospital Medicine (SoHM) Report, 66.4% of responding groups had open positions, with a median of 12% understaffing reported. The 2020 SoHM report is being compiled as we speak, but I suspect this situation will not have improved, and as the pandemic strikes, staffing models have been completely blown up.
To dig ourselves out of this staffing hole and still stay under (or not too over) budget, bringing more advanced practice providers (APP) into our groups/divisions will be needed. We must recognize, however, that APPs can’t just be hired rapidly and thrown into the schedule. As Tracy Cardin, ACNP-BC, SFHM, stated in her December 2019 blog post on the Hospital Leader website, leaders need to implement consistent onboarding, training, and support of APPs, just as they would for any other hospitalist in their group.6 Physician hospitalists need to develop and maintain proven competency in effectively interacting with APPs practicing at the top of their skills and productivity. No time has ever proven the need to allow APPs to practice at the top of their skills than the age of COVID-19.7
But if your “field” doesn’t even recognize you at all? That is the fate of many providers left behind by the field of pediatric hospital medicine. Over the past year, we have seen PHM attain a great achievement in its recognition as a board-certified subspecialty established by the American Board of Pediatrics (ABP), only to have the process beset by allegations of gender and maternal bias. While a groundswell of opposition from pediatric hospitalists triggered by the exclusion of applicants to the Practice Pathway to board certification led the ABP to remove the practice interruption criteria, other potential sources of gender and maternal bias remain.8
This does not even address pediatric hospitalists trained in family medicine who cannot be eligible for PHM board certification through experience or fellowship, med-peds trained pediatric hospitalists who cannot quality because of insufficient time spent on pediatric inpatient care, newborn hospitalists (who do not qualify), and APPs specialized in pediatric inpatient care. While it is completely understandable that the ABP cannot provide a certification pathway for all of these groups, this still leaves a gap for these providers when it comes to being in a professional community that supports their professional development, ongoing education, and training. Fortunately, leaders of the three societies that have significant numbers of pediatric hospitalists – SHM, American Academy of Pediatrics, and Academic Pediatric Association – are working to develop a PHM designation outside of the ABP board certification pathway that will extend the professional community to those left out of board certification.
As we move bravely into this new era of SHM, our clarion call is to collaborate whenever and wherever we can, with our practice administrators, APPs, outpatient providers, subspecialist providers, and patient/family advocates – pandemic or no pandemic. In fact, what this pandemic has shown us is that rapid cycle, fully 360-degree collaboration is the only way hospitalists and hospital leaders will weather the storms of changing reimbursement, pandemics, or politics. This will be our challenge for the next decade, to ensure that SHM collaboratively moves beyond the confines of the hospital ward.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
References
1. Frakt A. A Sense of Alarm as Rural Hospitals Keep Closing. The New York Times. 2018. https://www.nytimes.com/2018/10/29/upshot/a-sense-of-alarm-as-rural-hospitals-keep-closing.html. Accessed February 28, 2020.
2. Poonacha TK, Chamoun F. The burden of prior authorizations and denials in health care. Medpage Today’s KevinMD. 2019. https://www.kevinmd.com/blog/2019/12/the-burden-of-prior-authorizations-and-denials-in-health-care.html. Accessed February 28, 2020.
3. 10 reasons healthcare leaders fail and how to prevent them. Becker’s Hospital Review. 2015. https://www.beckershospitalreview.com/hospital-management-administration/10-reasons-healthcare-leaders-fail-and-how-to-prevent-them.html. Accessed March 15, 2020
4. Buhr RG et al. Comorbidity and thirty-day hospital readmission odds in chronic obstructive pulmonary disease: a comparison of the Charlson and Elixhauser comorbidity indices. BMC Health Serv Res. 2019;19:701.
5. Garrison GM et al. Family medicine patients have shorter length of stay when cared for on a family medicine inpatient service. J Prim Care Community Health. 2019. doi: 10.1177/2150132719840517.
6. Cardin T. Work the Program for NP/PAs, and the Program Will Work. The Hospital Leader: Official Blog of SHM. 2019. https://thehospitalleader.org/work-the-program-for-np-pas-and-the-program-will-work/
7. Mittman DE. More physician assistants are ready to help with COVID-19 – now governors must empower them. The Hill. 2020. https://thehill.com/opinion/healthcare/489985-more-physician-assistants-are-ready-to-help-with-covid-19-now-governors. Accessed March 31, 2020.
8. Gold JM et al. Collective action and effective dialogue to address gender bias in medicine. J Hosp Med. 2019;14:630-2.
SHM is entering an exciting new chapter in its history because we will soon see Dr. Eric Howell take the reins from Dr. Larry Wellikson as CEO, as we watch Dr. Danielle Scheurer assume the role of president from Dr. Chris Frost, and as a side note, I will try to fill Dr. Scheurer’s shoes as physician editor of The Hospitalist.
This changing of the guard of SHM’s leadership will take place amid the backdrop of an acrimonious presidential election and the emergence of a novel coronavirus that threatens to upend the typical routines of our social and professional lives.
Without a doubt, our leaders, whether national, regional, or local, will be at the helm during one of the most uncertain times in the history of modern health care. Will we see a U.S. President who is a proponent of supporting the Affordable Care Act? Will we see further erosion of Obamacare under a second term of President Trump? Will we see rural hospitals continue to close or shrink1 as their margins get squeezed by skyrocketing denials for inpatient status in favor of observation or outpatient status?2
Forces that seem beyond our control threaten to drastically alter our professions and even our livelihoods. In the space of the few weeks during which I began and finished this piece, every day brought a whole new world of changes in my hospital, town, state, and country. No leader can predict the future with any semblance of certitude.
In the face of these swirling winds of uncertainty, what is clear is that maintaining our commitment as hospitalists to providing evidence-based, high-quality care to our patients while providing support to our colleagues in the health care industry will greatly benefit from collaborating effectively under the “big tent” philosophy of SHM. Over my career, I have benefited from great role models and colleagues as my career took me from primary care med-peds to the “new” field of hospital medicine as a med-peds hospitalist, to a leadership role in pediatric hospital medicine. I have also benefited from “learning opportunities,” as I have made my fair share of mistakes in efforts to improve systems of care. Nearly all of these mistakes share a common thread – not collaborating effectively with critical stakeholders, both within and outside of my institution.3 As this pandemic progresses, I am (and likely you are) witnessing your leaders succeed or fail based on their ability to collaborate across the institution.
As a field, we risk making similar errors by being too narrowly focused as we strive to improve the care of our patients. Recently, Dr. Russell Buhr and his colleagues at the University of California, Los Angeles, demonstrated that a majority of 30-day readmissions for chronic obstructive pulmonary disease (COPD) are due to non-COPD diagnoses.4 As we discharge our COPD patients, we may be satisfied that we’ve “tuned up” our patient’s COPD, but have we adequately arranged for appropriate ongoing care of their other medical problems? This requires an activity undertaken less and less these days in medicine – a conversation between hospitalists and outpatient medical providers. The coronavirus disease 2019 (COVID-19) pandemic has made this more challenging, but I can assure you that you can neither transmit nor catch the coronavirus from a phone call.
Perhaps we can learn from our hospitalist colleagues trained in family medicine. A recent study found that hospitalists in a team made up of family medicine–trained physicians in an academic health center achieved a 33% shorter length of stay for patients from the family medicine clinic, after adjustment for disease, demographics, and disease severity.5 The conclusion of the authors was that this was likely caused by greater familiarity with outpatient resources. I would conjecture that family medicine hospitalists were also more likely to have a conversation with a patient’s outpatient primary care provider (PCP).
Of course, I am the first to admit that chatting with a PCP is not as easy as it used to be – when we could bump into each other in the doctor’s lounge drinking coffee or in radiology while pulling x-ray films (remember those?) – and in the age of COVID-19, these interactions are even less likely. It can take considerable time and effort to get PCP colleagues on the phone unless you’re chummy enough to have their cell phone numbers. And time is a resource in short supply because most hospital medicine groups are understaffed – in the 2018 SHM State of Hospital Medicine (SoHM) Report, 66.4% of responding groups had open positions, with a median of 12% understaffing reported. The 2020 SoHM report is being compiled as we speak, but I suspect this situation will not have improved, and as the pandemic strikes, staffing models have been completely blown up.
To dig ourselves out of this staffing hole and still stay under (or not too over) budget, bringing more advanced practice providers (APP) into our groups/divisions will be needed. We must recognize, however, that APPs can’t just be hired rapidly and thrown into the schedule. As Tracy Cardin, ACNP-BC, SFHM, stated in her December 2019 blog post on the Hospital Leader website, leaders need to implement consistent onboarding, training, and support of APPs, just as they would for any other hospitalist in their group.6 Physician hospitalists need to develop and maintain proven competency in effectively interacting with APPs practicing at the top of their skills and productivity. No time has ever proven the need to allow APPs to practice at the top of their skills than the age of COVID-19.7
But if your “field” doesn’t even recognize you at all? That is the fate of many providers left behind by the field of pediatric hospital medicine. Over the past year, we have seen PHM attain a great achievement in its recognition as a board-certified subspecialty established by the American Board of Pediatrics (ABP), only to have the process beset by allegations of gender and maternal bias. While a groundswell of opposition from pediatric hospitalists triggered by the exclusion of applicants to the Practice Pathway to board certification led the ABP to remove the practice interruption criteria, other potential sources of gender and maternal bias remain.8
This does not even address pediatric hospitalists trained in family medicine who cannot be eligible for PHM board certification through experience or fellowship, med-peds trained pediatric hospitalists who cannot quality because of insufficient time spent on pediatric inpatient care, newborn hospitalists (who do not qualify), and APPs specialized in pediatric inpatient care. While it is completely understandable that the ABP cannot provide a certification pathway for all of these groups, this still leaves a gap for these providers when it comes to being in a professional community that supports their professional development, ongoing education, and training. Fortunately, leaders of the three societies that have significant numbers of pediatric hospitalists – SHM, American Academy of Pediatrics, and Academic Pediatric Association – are working to develop a PHM designation outside of the ABP board certification pathway that will extend the professional community to those left out of board certification.
As we move bravely into this new era of SHM, our clarion call is to collaborate whenever and wherever we can, with our practice administrators, APPs, outpatient providers, subspecialist providers, and patient/family advocates – pandemic or no pandemic. In fact, what this pandemic has shown us is that rapid cycle, fully 360-degree collaboration is the only way hospitalists and hospital leaders will weather the storms of changing reimbursement, pandemics, or politics. This will be our challenge for the next decade, to ensure that SHM collaboratively moves beyond the confines of the hospital ward.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
References
1. Frakt A. A Sense of Alarm as Rural Hospitals Keep Closing. The New York Times. 2018. https://www.nytimes.com/2018/10/29/upshot/a-sense-of-alarm-as-rural-hospitals-keep-closing.html. Accessed February 28, 2020.
2. Poonacha TK, Chamoun F. The burden of prior authorizations and denials in health care. Medpage Today’s KevinMD. 2019. https://www.kevinmd.com/blog/2019/12/the-burden-of-prior-authorizations-and-denials-in-health-care.html. Accessed February 28, 2020.
3. 10 reasons healthcare leaders fail and how to prevent them. Becker’s Hospital Review. 2015. https://www.beckershospitalreview.com/hospital-management-administration/10-reasons-healthcare-leaders-fail-and-how-to-prevent-them.html. Accessed March 15, 2020
4. Buhr RG et al. Comorbidity and thirty-day hospital readmission odds in chronic obstructive pulmonary disease: a comparison of the Charlson and Elixhauser comorbidity indices. BMC Health Serv Res. 2019;19:701.
5. Garrison GM et al. Family medicine patients have shorter length of stay when cared for on a family medicine inpatient service. J Prim Care Community Health. 2019. doi: 10.1177/2150132719840517.
6. Cardin T. Work the Program for NP/PAs, and the Program Will Work. The Hospital Leader: Official Blog of SHM. 2019. https://thehospitalleader.org/work-the-program-for-np-pas-and-the-program-will-work/
7. Mittman DE. More physician assistants are ready to help with COVID-19 – now governors must empower them. The Hill. 2020. https://thehill.com/opinion/healthcare/489985-more-physician-assistants-are-ready-to-help-with-covid-19-now-governors. Accessed March 31, 2020.
8. Gold JM et al. Collective action and effective dialogue to address gender bias in medicine. J Hosp Med. 2019;14:630-2.
SHM is entering an exciting new chapter in its history because we will soon see Dr. Eric Howell take the reins from Dr. Larry Wellikson as CEO, as we watch Dr. Danielle Scheurer assume the role of president from Dr. Chris Frost, and as a side note, I will try to fill Dr. Scheurer’s shoes as physician editor of The Hospitalist.
This changing of the guard of SHM’s leadership will take place amid the backdrop of an acrimonious presidential election and the emergence of a novel coronavirus that threatens to upend the typical routines of our social and professional lives.
Without a doubt, our leaders, whether national, regional, or local, will be at the helm during one of the most uncertain times in the history of modern health care. Will we see a U.S. President who is a proponent of supporting the Affordable Care Act? Will we see further erosion of Obamacare under a second term of President Trump? Will we see rural hospitals continue to close or shrink1 as their margins get squeezed by skyrocketing denials for inpatient status in favor of observation or outpatient status?2
Forces that seem beyond our control threaten to drastically alter our professions and even our livelihoods. In the space of the few weeks during which I began and finished this piece, every day brought a whole new world of changes in my hospital, town, state, and country. No leader can predict the future with any semblance of certitude.
In the face of these swirling winds of uncertainty, what is clear is that maintaining our commitment as hospitalists to providing evidence-based, high-quality care to our patients while providing support to our colleagues in the health care industry will greatly benefit from collaborating effectively under the “big tent” philosophy of SHM. Over my career, I have benefited from great role models and colleagues as my career took me from primary care med-peds to the “new” field of hospital medicine as a med-peds hospitalist, to a leadership role in pediatric hospital medicine. I have also benefited from “learning opportunities,” as I have made my fair share of mistakes in efforts to improve systems of care. Nearly all of these mistakes share a common thread – not collaborating effectively with critical stakeholders, both within and outside of my institution.3 As this pandemic progresses, I am (and likely you are) witnessing your leaders succeed or fail based on their ability to collaborate across the institution.
As a field, we risk making similar errors by being too narrowly focused as we strive to improve the care of our patients. Recently, Dr. Russell Buhr and his colleagues at the University of California, Los Angeles, demonstrated that a majority of 30-day readmissions for chronic obstructive pulmonary disease (COPD) are due to non-COPD diagnoses.4 As we discharge our COPD patients, we may be satisfied that we’ve “tuned up” our patient’s COPD, but have we adequately arranged for appropriate ongoing care of their other medical problems? This requires an activity undertaken less and less these days in medicine – a conversation between hospitalists and outpatient medical providers. The coronavirus disease 2019 (COVID-19) pandemic has made this more challenging, but I can assure you that you can neither transmit nor catch the coronavirus from a phone call.
Perhaps we can learn from our hospitalist colleagues trained in family medicine. A recent study found that hospitalists in a team made up of family medicine–trained physicians in an academic health center achieved a 33% shorter length of stay for patients from the family medicine clinic, after adjustment for disease, demographics, and disease severity.5 The conclusion of the authors was that this was likely caused by greater familiarity with outpatient resources. I would conjecture that family medicine hospitalists were also more likely to have a conversation with a patient’s outpatient primary care provider (PCP).
Of course, I am the first to admit that chatting with a PCP is not as easy as it used to be – when we could bump into each other in the doctor’s lounge drinking coffee or in radiology while pulling x-ray films (remember those?) – and in the age of COVID-19, these interactions are even less likely. It can take considerable time and effort to get PCP colleagues on the phone unless you’re chummy enough to have their cell phone numbers. And time is a resource in short supply because most hospital medicine groups are understaffed – in the 2018 SHM State of Hospital Medicine (SoHM) Report, 66.4% of responding groups had open positions, with a median of 12% understaffing reported. The 2020 SoHM report is being compiled as we speak, but I suspect this situation will not have improved, and as the pandemic strikes, staffing models have been completely blown up.
To dig ourselves out of this staffing hole and still stay under (or not too over) budget, bringing more advanced practice providers (APP) into our groups/divisions will be needed. We must recognize, however, that APPs can’t just be hired rapidly and thrown into the schedule. As Tracy Cardin, ACNP-BC, SFHM, stated in her December 2019 blog post on the Hospital Leader website, leaders need to implement consistent onboarding, training, and support of APPs, just as they would for any other hospitalist in their group.6 Physician hospitalists need to develop and maintain proven competency in effectively interacting with APPs practicing at the top of their skills and productivity. No time has ever proven the need to allow APPs to practice at the top of their skills than the age of COVID-19.7
But if your “field” doesn’t even recognize you at all? That is the fate of many providers left behind by the field of pediatric hospital medicine. Over the past year, we have seen PHM attain a great achievement in its recognition as a board-certified subspecialty established by the American Board of Pediatrics (ABP), only to have the process beset by allegations of gender and maternal bias. While a groundswell of opposition from pediatric hospitalists triggered by the exclusion of applicants to the Practice Pathway to board certification led the ABP to remove the practice interruption criteria, other potential sources of gender and maternal bias remain.8
This does not even address pediatric hospitalists trained in family medicine who cannot be eligible for PHM board certification through experience or fellowship, med-peds trained pediatric hospitalists who cannot quality because of insufficient time spent on pediatric inpatient care, newborn hospitalists (who do not qualify), and APPs specialized in pediatric inpatient care. While it is completely understandable that the ABP cannot provide a certification pathway for all of these groups, this still leaves a gap for these providers when it comes to being in a professional community that supports their professional development, ongoing education, and training. Fortunately, leaders of the three societies that have significant numbers of pediatric hospitalists – SHM, American Academy of Pediatrics, and Academic Pediatric Association – are working to develop a PHM designation outside of the ABP board certification pathway that will extend the professional community to those left out of board certification.
As we move bravely into this new era of SHM, our clarion call is to collaborate whenever and wherever we can, with our practice administrators, APPs, outpatient providers, subspecialist providers, and patient/family advocates – pandemic or no pandemic. In fact, what this pandemic has shown us is that rapid cycle, fully 360-degree collaboration is the only way hospitalists and hospital leaders will weather the storms of changing reimbursement, pandemics, or politics. This will be our challenge for the next decade, to ensure that SHM collaboratively moves beyond the confines of the hospital ward.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
References
1. Frakt A. A Sense of Alarm as Rural Hospitals Keep Closing. The New York Times. 2018. https://www.nytimes.com/2018/10/29/upshot/a-sense-of-alarm-as-rural-hospitals-keep-closing.html. Accessed February 28, 2020.
2. Poonacha TK, Chamoun F. The burden of prior authorizations and denials in health care. Medpage Today’s KevinMD. 2019. https://www.kevinmd.com/blog/2019/12/the-burden-of-prior-authorizations-and-denials-in-health-care.html. Accessed February 28, 2020.
3. 10 reasons healthcare leaders fail and how to prevent them. Becker’s Hospital Review. 2015. https://www.beckershospitalreview.com/hospital-management-administration/10-reasons-healthcare-leaders-fail-and-how-to-prevent-them.html. Accessed March 15, 2020
4. Buhr RG et al. Comorbidity and thirty-day hospital readmission odds in chronic obstructive pulmonary disease: a comparison of the Charlson and Elixhauser comorbidity indices. BMC Health Serv Res. 2019;19:701.
5. Garrison GM et al. Family medicine patients have shorter length of stay when cared for on a family medicine inpatient service. J Prim Care Community Health. 2019. doi: 10.1177/2150132719840517.
6. Cardin T. Work the Program for NP/PAs, and the Program Will Work. The Hospital Leader: Official Blog of SHM. 2019. https://thehospitalleader.org/work-the-program-for-np-pas-and-the-program-will-work/
7. Mittman DE. More physician assistants are ready to help with COVID-19 – now governors must empower them. The Hill. 2020. https://thehill.com/opinion/healthcare/489985-more-physician-assistants-are-ready-to-help-with-covid-19-now-governors. Accessed March 31, 2020.
8. Gold JM et al. Collective action and effective dialogue to address gender bias in medicine. J Hosp Med. 2019;14:630-2.
More on How to Decrease Dermatology Interview Costs
To the Editor:
Ongoing concern about the high costs of dermatology residency interviews has led to several cost-saving proposals, as presented by Hussain1 in the Cutis article, “Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective.” Additional strategies to reduce applicant costs include eliminating travel costs through video or telephone interviews, interviewing students who are visiting during their away rotation, and developing and implementing a mechanism to exempt students from participating in the Electronic Residency Application Service (
First, because applicants would be limited to 1 application to participate in the early decision program, they must realistically consider the strength of their application and weigh their chances for acceptance to that program. Programs could facilitate the process by becoming more transparent about the type of applicants that have previously matched in their program.2 If an early-decision applicant successfully matches, that applicant would be prohibited from applying to additional dermatology residency programs through
Second, early-decision actions by programs—probably by August 1, a time when most third-year medical students have completed their academic year—would be determined before ERAS releases applications to residency programs. This timeline would remove successful applicants in the early decision program from going to additional interviews and incurring the associated travel costs.
Third, early decision could be potentially beneficial to applicants who are tied to a specific geographic region for training and to programs with specific program needs, such as expertise in specific areas of dermatology research or areas of clinical need (eg, adding a dermatopathologist, plastic surgeon, internist, or a pediatrician to the residency program who now wants dermatology training) or other program needs.
Fourth, application costs could potentially be lower for early-decision applicants than through the present application process if participating institutions waived application fees. Applicants would still be responsible for submitting requested academic transcripts, letters of recommendation, and travel expenses if an on-site interview is requested by the program.
Finally, highly desirable applicants who are offered a position through early decision would result in more opportunities for other applicants to interview for the remaining available residency positions through ERAS/NRMP.
Downsides to early decision for dermatology residency include the inability of applicants to compare programs to one another through their personal experiences, such as prior rotations or interviews, and for programs to compare applicants though the interview process and away rotations. In addition, US Medical Licensing Examination Step 2 scores and Alpha Omega Alpha honor medical society status and other academic honors may not be available to programs to consider at the time of early decision. Cooperation would be needed with ERAS and NRMP to create an early decision program for dermatology residency.
One other potential consequence of the early match could involve instances of strained relationships between research fellows and their sponsoring institution or dermatology program. Research fellows often match at their research institution, and failing to early match could potentially sour the relationship between the applicant and the program, thus leading to a less productive year. However, many programs participating in an early match will probably have additional residency positions remaining in the traditional match that would be still available to the fellows.
The concept of an early-binding residency match process has the potential to save both time and money for programs and applicants. Although an early-match process would have many positive effects, there also would be inherent downsides that accompany such a system. Nonetheless, an early-match process in dermatology has the prospect of efficiently pairing applicants and programs that feel strongly about each other while simplifying the match process and reducing costs for all parties involved.
References
1. Hussain AN.
2. Weisert E, Phan M. Thoughts on reducing the cost for dermatology residency applications. DIG@UTMB blog. http://digutmb.blogspot.com/2019/12/thoughts-on-reducing-cost-for.html. Published December 23, 2019. Accessed April 17, 2020.
3. Early decision program. Association of American Medical Colleges website. https://students-residents.aamc.org/applying-medical-school/article/early-decision-program/. Accessed April 8, 2020.
Author’s Response
The early decision option for dermatology residency applications would be a welcomed addition to the process but may be complicated by 2 recent events: the coronavirus disease 2019 (COVID-19) pandemic and the change of US Medical Licensing Examination (USMLE) Step 1 score reporting to a pass/fail system.
The COVID-19 pandemic has caused remarkable economic distress and likely affects medical students more acutely given their high levels of debt. As Ryan and Wagner observed, one advantage of the early-decision option would be financial relief for certain students. If applicants successfully match during the early-decision phase, they will not need to apply to any additional dermatology programs and also can target their preliminary-year applications to the geographic region where they have already matched.
In addition, the COVID-19 pandemic may further reduce early applicants’ ability to visit programs in person. Various medical schools have curtailed away rotations, and programs may opt for virtual interviews in accordance with social distancing guidelines.1 Thus, early applicants will have even fewer opportunities to compare programs before they must make a binding decision about their residency placement. Although away rotations and interview travel are some of the largest drivers of application cost,2 reducing costs in this way might shortchange both students and programs.
Arguably, the change in USMLE Step 1 score reporting beginning in 2022 may impact residency selection for a longer period of time than the COVID-19 pandemic. Program directors cited USMLE Step 1 scores as one of the main factors determining which applicants may be invited to interview.3 The lack of numerical USMLE Step 1 scores may encourage programs to place more weight on other metrics such as USMLE Step 2 CK scores or Alpha Omega Alpha membership.4 However, as Ryan and Wagner point out, such metrics may not be available in time for early-decision applicants.
As such, future program directors will have precious little information to screen early-decision applicants and may need to conduct holistic application review. This would require increased time and manpower compared to screening based on traditional metrics but may lead to a better “fit” for an applicant with a residency.
In general, implementation of any early decision program would benefit dermatology applicants as a group by removing elite candidates from the applicant pool. According to National Resident Matching Program data, just 3% of dermatology applicants account for more than 12% of overall interviews.5 In other words, a small group of the strongest applicants receives a lion’s share of interviews, crowding out many other candidates. Removing these top-tier applicants likely would provide remaining applicants with a higher return on investment per application, and students may choose to save money by applying to fewer programs.
Adopting early-decision options within the dermatology match may be complicated given the COVID-19 pandemic and USMLE score changes but may spur positive changes in the process while also reducing the financial burden on applicants.
Aamir N. Hussain, MD, MAPP
From Northwell Health, Manhasset, New York.
The author reports no conflict of interest.
Correspondence: Aamir N. Hussain, MD, MAPP ([email protected]).
References
1. Coronavirus (COVID-19) and the VSLO program. Association of American Medical Colleges website. https://students-residents.aamc.org/attending-medical-school/article/coronavirus-covid-19-and-vslo-program/. Accessed April 17, 2020.
2. Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
3. National Resident Matching Program, Data Release and Research Committee. Results of the 2018 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; 2018. https://www.nrmp.org/wp-content/uploads/2018/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf. Published June 2018. Accessed April 17, 2020.
4. Crane MA, Chang HA, Azamfirei R. Medical education takes a step in the right direction: where does that leave students? [published online March 6, 2020]. JAMA. doi:10.1001/jama.2020.2950.
5. Lee AH, Young P, Liao R, et al. I dream of Gini: quantifying inequality in otolaryngology residency interviews. Laryngoscope. 2019;129:627-633.
To the Editor:
Ongoing concern about the high costs of dermatology residency interviews has led to several cost-saving proposals, as presented by Hussain1 in the Cutis article, “Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective.” Additional strategies to reduce applicant costs include eliminating travel costs through video or telephone interviews, interviewing students who are visiting during their away rotation, and developing and implementing a mechanism to exempt students from participating in the Electronic Residency Application Service (
First, because applicants would be limited to 1 application to participate in the early decision program, they must realistically consider the strength of their application and weigh their chances for acceptance to that program. Programs could facilitate the process by becoming more transparent about the type of applicants that have previously matched in their program.2 If an early-decision applicant successfully matches, that applicant would be prohibited from applying to additional dermatology residency programs through
Second, early-decision actions by programs—probably by August 1, a time when most third-year medical students have completed their academic year—would be determined before ERAS releases applications to residency programs. This timeline would remove successful applicants in the early decision program from going to additional interviews and incurring the associated travel costs.
Third, early decision could be potentially beneficial to applicants who are tied to a specific geographic region for training and to programs with specific program needs, such as expertise in specific areas of dermatology research or areas of clinical need (eg, adding a dermatopathologist, plastic surgeon, internist, or a pediatrician to the residency program who now wants dermatology training) or other program needs.
Fourth, application costs could potentially be lower for early-decision applicants than through the present application process if participating institutions waived application fees. Applicants would still be responsible for submitting requested academic transcripts, letters of recommendation, and travel expenses if an on-site interview is requested by the program.
Finally, highly desirable applicants who are offered a position through early decision would result in more opportunities for other applicants to interview for the remaining available residency positions through ERAS/NRMP.
Downsides to early decision for dermatology residency include the inability of applicants to compare programs to one another through their personal experiences, such as prior rotations or interviews, and for programs to compare applicants though the interview process and away rotations. In addition, US Medical Licensing Examination Step 2 scores and Alpha Omega Alpha honor medical society status and other academic honors may not be available to programs to consider at the time of early decision. Cooperation would be needed with ERAS and NRMP to create an early decision program for dermatology residency.
One other potential consequence of the early match could involve instances of strained relationships between research fellows and their sponsoring institution or dermatology program. Research fellows often match at their research institution, and failing to early match could potentially sour the relationship between the applicant and the program, thus leading to a less productive year. However, many programs participating in an early match will probably have additional residency positions remaining in the traditional match that would be still available to the fellows.
The concept of an early-binding residency match process has the potential to save both time and money for programs and applicants. Although an early-match process would have many positive effects, there also would be inherent downsides that accompany such a system. Nonetheless, an early-match process in dermatology has the prospect of efficiently pairing applicants and programs that feel strongly about each other while simplifying the match process and reducing costs for all parties involved.
References
1. Hussain AN.
2. Weisert E, Phan M. Thoughts on reducing the cost for dermatology residency applications. DIG@UTMB blog. http://digutmb.blogspot.com/2019/12/thoughts-on-reducing-cost-for.html. Published December 23, 2019. Accessed April 17, 2020.
3. Early decision program. Association of American Medical Colleges website. https://students-residents.aamc.org/applying-medical-school/article/early-decision-program/. Accessed April 8, 2020.
Author’s Response
The early decision option for dermatology residency applications would be a welcomed addition to the process but may be complicated by 2 recent events: the coronavirus disease 2019 (COVID-19) pandemic and the change of US Medical Licensing Examination (USMLE) Step 1 score reporting to a pass/fail system.
The COVID-19 pandemic has caused remarkable economic distress and likely affects medical students more acutely given their high levels of debt. As Ryan and Wagner observed, one advantage of the early-decision option would be financial relief for certain students. If applicants successfully match during the early-decision phase, they will not need to apply to any additional dermatology programs and also can target their preliminary-year applications to the geographic region where they have already matched.
In addition, the COVID-19 pandemic may further reduce early applicants’ ability to visit programs in person. Various medical schools have curtailed away rotations, and programs may opt for virtual interviews in accordance with social distancing guidelines.1 Thus, early applicants will have even fewer opportunities to compare programs before they must make a binding decision about their residency placement. Although away rotations and interview travel are some of the largest drivers of application cost,2 reducing costs in this way might shortchange both students and programs.
Arguably, the change in USMLE Step 1 score reporting beginning in 2022 may impact residency selection for a longer period of time than the COVID-19 pandemic. Program directors cited USMLE Step 1 scores as one of the main factors determining which applicants may be invited to interview.3 The lack of numerical USMLE Step 1 scores may encourage programs to place more weight on other metrics such as USMLE Step 2 CK scores or Alpha Omega Alpha membership.4 However, as Ryan and Wagner point out, such metrics may not be available in time for early-decision applicants.
As such, future program directors will have precious little information to screen early-decision applicants and may need to conduct holistic application review. This would require increased time and manpower compared to screening based on traditional metrics but may lead to a better “fit” for an applicant with a residency.
In general, implementation of any early decision program would benefit dermatology applicants as a group by removing elite candidates from the applicant pool. According to National Resident Matching Program data, just 3% of dermatology applicants account for more than 12% of overall interviews.5 In other words, a small group of the strongest applicants receives a lion’s share of interviews, crowding out many other candidates. Removing these top-tier applicants likely would provide remaining applicants with a higher return on investment per application, and students may choose to save money by applying to fewer programs.
Adopting early-decision options within the dermatology match may be complicated given the COVID-19 pandemic and USMLE score changes but may spur positive changes in the process while also reducing the financial burden on applicants.
Aamir N. Hussain, MD, MAPP
From Northwell Health, Manhasset, New York.
The author reports no conflict of interest.
Correspondence: Aamir N. Hussain, MD, MAPP ([email protected]).
References
1. Coronavirus (COVID-19) and the VSLO program. Association of American Medical Colleges website. https://students-residents.aamc.org/attending-medical-school/article/coronavirus-covid-19-and-vslo-program/. Accessed April 17, 2020.
2. Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
3. National Resident Matching Program, Data Release and Research Committee. Results of the 2018 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; 2018. https://www.nrmp.org/wp-content/uploads/2018/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf. Published June 2018. Accessed April 17, 2020.
4. Crane MA, Chang HA, Azamfirei R. Medical education takes a step in the right direction: where does that leave students? [published online March 6, 2020]. JAMA. doi:10.1001/jama.2020.2950.
5. Lee AH, Young P, Liao R, et al. I dream of Gini: quantifying inequality in otolaryngology residency interviews. Laryngoscope. 2019;129:627-633.
To the Editor:
Ongoing concern about the high costs of dermatology residency interviews has led to several cost-saving proposals, as presented by Hussain1 in the Cutis article, “Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective.” Additional strategies to reduce applicant costs include eliminating travel costs through video or telephone interviews, interviewing students who are visiting during their away rotation, and developing and implementing a mechanism to exempt students from participating in the Electronic Residency Application Service (
First, because applicants would be limited to 1 application to participate in the early decision program, they must realistically consider the strength of their application and weigh their chances for acceptance to that program. Programs could facilitate the process by becoming more transparent about the type of applicants that have previously matched in their program.2 If an early-decision applicant successfully matches, that applicant would be prohibited from applying to additional dermatology residency programs through
Second, early-decision actions by programs—probably by August 1, a time when most third-year medical students have completed their academic year—would be determined before ERAS releases applications to residency programs. This timeline would remove successful applicants in the early decision program from going to additional interviews and incurring the associated travel costs.
Third, early decision could be potentially beneficial to applicants who are tied to a specific geographic region for training and to programs with specific program needs, such as expertise in specific areas of dermatology research or areas of clinical need (eg, adding a dermatopathologist, plastic surgeon, internist, or a pediatrician to the residency program who now wants dermatology training) or other program needs.
Fourth, application costs could potentially be lower for early-decision applicants than through the present application process if participating institutions waived application fees. Applicants would still be responsible for submitting requested academic transcripts, letters of recommendation, and travel expenses if an on-site interview is requested by the program.
Finally, highly desirable applicants who are offered a position through early decision would result in more opportunities for other applicants to interview for the remaining available residency positions through ERAS/NRMP.
Downsides to early decision for dermatology residency include the inability of applicants to compare programs to one another through their personal experiences, such as prior rotations or interviews, and for programs to compare applicants though the interview process and away rotations. In addition, US Medical Licensing Examination Step 2 scores and Alpha Omega Alpha honor medical society status and other academic honors may not be available to programs to consider at the time of early decision. Cooperation would be needed with ERAS and NRMP to create an early decision program for dermatology residency.
One other potential consequence of the early match could involve instances of strained relationships between research fellows and their sponsoring institution or dermatology program. Research fellows often match at their research institution, and failing to early match could potentially sour the relationship between the applicant and the program, thus leading to a less productive year. However, many programs participating in an early match will probably have additional residency positions remaining in the traditional match that would be still available to the fellows.
The concept of an early-binding residency match process has the potential to save both time and money for programs and applicants. Although an early-match process would have many positive effects, there also would be inherent downsides that accompany such a system. Nonetheless, an early-match process in dermatology has the prospect of efficiently pairing applicants and programs that feel strongly about each other while simplifying the match process and reducing costs for all parties involved.
References
1. Hussain AN.
2. Weisert E, Phan M. Thoughts on reducing the cost for dermatology residency applications. DIG@UTMB blog. http://digutmb.blogspot.com/2019/12/thoughts-on-reducing-cost-for.html. Published December 23, 2019. Accessed April 17, 2020.
3. Early decision program. Association of American Medical Colleges website. https://students-residents.aamc.org/applying-medical-school/article/early-decision-program/. Accessed April 8, 2020.
Author’s Response
The early decision option for dermatology residency applications would be a welcomed addition to the process but may be complicated by 2 recent events: the coronavirus disease 2019 (COVID-19) pandemic and the change of US Medical Licensing Examination (USMLE) Step 1 score reporting to a pass/fail system.
The COVID-19 pandemic has caused remarkable economic distress and likely affects medical students more acutely given their high levels of debt. As Ryan and Wagner observed, one advantage of the early-decision option would be financial relief for certain students. If applicants successfully match during the early-decision phase, they will not need to apply to any additional dermatology programs and also can target their preliminary-year applications to the geographic region where they have already matched.
In addition, the COVID-19 pandemic may further reduce early applicants’ ability to visit programs in person. Various medical schools have curtailed away rotations, and programs may opt for virtual interviews in accordance with social distancing guidelines.1 Thus, early applicants will have even fewer opportunities to compare programs before they must make a binding decision about their residency placement. Although away rotations and interview travel are some of the largest drivers of application cost,2 reducing costs in this way might shortchange both students and programs.
Arguably, the change in USMLE Step 1 score reporting beginning in 2022 may impact residency selection for a longer period of time than the COVID-19 pandemic. Program directors cited USMLE Step 1 scores as one of the main factors determining which applicants may be invited to interview.3 The lack of numerical USMLE Step 1 scores may encourage programs to place more weight on other metrics such as USMLE Step 2 CK scores or Alpha Omega Alpha membership.4 However, as Ryan and Wagner point out, such metrics may not be available in time for early-decision applicants.
As such, future program directors will have precious little information to screen early-decision applicants and may need to conduct holistic application review. This would require increased time and manpower compared to screening based on traditional metrics but may lead to a better “fit” for an applicant with a residency.
In general, implementation of any early decision program would benefit dermatology applicants as a group by removing elite candidates from the applicant pool. According to National Resident Matching Program data, just 3% of dermatology applicants account for more than 12% of overall interviews.5 In other words, a small group of the strongest applicants receives a lion’s share of interviews, crowding out many other candidates. Removing these top-tier applicants likely would provide remaining applicants with a higher return on investment per application, and students may choose to save money by applying to fewer programs.
Adopting early-decision options within the dermatology match may be complicated given the COVID-19 pandemic and USMLE score changes but may spur positive changes in the process while also reducing the financial burden on applicants.
Aamir N. Hussain, MD, MAPP
From Northwell Health, Manhasset, New York.
The author reports no conflict of interest.
Correspondence: Aamir N. Hussain, MD, MAPP ([email protected]).
References
1. Coronavirus (COVID-19) and the VSLO program. Association of American Medical Colleges website. https://students-residents.aamc.org/attending-medical-school/article/coronavirus-covid-19-and-vslo-program/. Accessed April 17, 2020.
2. Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
3. National Resident Matching Program, Data Release and Research Committee. Results of the 2018 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; 2018. https://www.nrmp.org/wp-content/uploads/2018/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf. Published June 2018. Accessed April 17, 2020.
4. Crane MA, Chang HA, Azamfirei R. Medical education takes a step in the right direction: where does that leave students? [published online March 6, 2020]. JAMA. doi:10.1001/jama.2020.2950.
5. Lee AH, Young P, Liao R, et al. I dream of Gini: quantifying inequality in otolaryngology residency interviews. Laryngoscope. 2019;129:627-633.
Multisociety roadmap eyes restarting elective cardiac cases
As COVID-19 case levels plateau in some regions, 16 North American cardiovascular societies have released a framework for reintroducing cardiovascular services disrupted by the pandemic.
The consensus document outlines a phased approach to restarting invasive cardiovascular (CV) procedures and diagnostic tests that aims to reduce patient and health care provider exposure to the coronavirus and still provide essential care. It also emphasizes some of the ethical considerations in patient selection and the need for a collaborative approach.
“The key message in our document is we need a new unprecedented collaboration with public health officials so that we can carefully monitor the situation and we’re aware of what’s happening with the penetrance of the pandemic in the community, but they’re aware of the morbidity and mortality that’s occurring on our ever-growing waiting list,” lead author David A. Wood, MD, told theheart.org | Medscape Cardiology.
The recommendations were jointly published May 4 in the Canadian Journal of Cardiology , the Journal of the American College of Cardiology, and The Annals of Thoracic Surgery, and are endorsed by, among others, the American Heart Association, American College of Cardiology (ACC), and Canadian Cardiovascular Society.
The guidance comes as hospitals are facing revenue shortfalls because of canceled elective procedures and resource-intensive COVID-19 cases, prompting some healthcare systems to furlough, lay off, or even fire staff.
“It’s obvious that volumes are down between 40% and 60%,” said Wood, director of the cardiac catheterization laboratory at Vancouver General Hospital and professor of medicine at the University of British Columbia, Canada. “Part of that is that some areas have restricted case volumes totally appropriately and it’s partly because patients are very afraid of coming to the hospital and, unfortunately, are having bad events at home. And some are dying.”
The new report features a detailed table outlining three different response levels: reintroduction of some services (level 2); reintroduction of most services (level 1); and regular services (level 0). It covers a range of services from transthoracic echocardiography and exercise testing with imaging to care for acute coronary syndrome and ST-segment elevation myocardial infarction.
“We’ve learned that we can very quickly turn off the tap and go to doing only 10% of our normal volumes, whether that’s surgery, cath lab, EP, diagnostic tests,” Wood said. “It’s much more difficult to thoughtfully turn the tap part way back on or restart the engine … you don’t just go from 0 to 100 [mph]. You go from 0 to 30 to 60 then maybe to 80 [mph].”
The document also includes eight guiding principles such as:
- The expectation that response levels will be different between regions, and even within a given region.
- A “transparent collaborative plan” for COVID-19 testing and personal protective equipment (PPE) must be in place before restarting cases.
- A less invasive test or alternate imaging modality should be considered, if both tests have similar efficacy.
- In general, a minimally invasive procedure with a shorter length of stay is preferable, if both strategies have similar efficacy and safety.
Although previous reports on cath lab considerations during the pandemic or restarting elective surgeries peg various actions to specific thresholds or time intervals, the language here is noticeably and intentionally broad.
Instead of stating when cardiovascular services should resume, for example, the experts say it’s appropriate to put the guidance document into place if there’s a “sustained reduction” in the rate of new COVID-19 admissions and deaths in the relevant geographic region for a “prespecified time interval.”
As for when or how frequently patients and healthcare providers should be tested for COVID-19, the document encourages “routine screening of all patients prior to any cardiovascular procedure or test.”
Overly prescriptive language in previous documents wasn’t felt to be that helpful, whereas language like “selective” cases and “some” or “most” cardiovascular procedures gives clinicians, health systems, and policy makers flexibility when moving between response levels, Wood explained.
“Different regions might be at different levels based on principles of public health as far as the penetrance of the pandemic in that community, as well as how can you actually do the physical distancing in your hospital or ambulatory clinic. Because, I tell you, that is the Achilles heel,” he said. “Our run rates are going to be determined by testing, the availability of PPE, but also how we’re going to use our existing infrastructure and maintain physical distancing.”
That may mean using telehealth for initial visits, having clinics open earlier in the morning or on weekends, or doing partial volumes for surgery or in the cath lab so patients can be staggered and recover at different times and in different areas of the hospital. “These are very granular, specific infrastructure things that we’ve never really had to consider before,” Wood observed.
The document also had to be flexible and nimble enough to respond to a potential rebound of COVID-19 cases, which in newly released models are projected to rise sharply to 200,000 cases a day and be accompanied by some 3,000 deaths each day by June 1.
“This is my own personal opinion but I think it’s foolish to think that we are going to be able to come back to 100% of the cases we were doing before, even with testing, PPE, and all of that until we have a vaccine,” he said.
Similar to decisions made in preparation for the initial COVID-19 surge, the consensus document outlines the need for ethical considerations when turning the tap back on. This means prioritizing procedures and tests that are likely to benefit more people and to a greater degree, and ensuring that patients are treated fairly and consistently, regardless of their ethnicity, perceived social worth, or ability to pay, said coauthor and ACC President Athena Poppas, MD, Brown University School of Medicine, Providence, Rhode Island.
“It’s an ethical tenet that exists in a lot of places but it’s usually not overtly called out,” Poppas told theheart.org | Medscape Cardiology. “It’s not rationing care; I think people jump to that but it’s actually the opposite of rationing care. It’s about being thoughtful about prioritizing patients.”
“There’s a variety of data that should help in the prioritization, not only how much hospital resources are utilized, that’s on one side, but there’s also the patient risk of delaying or doing a procedure, and then the societal risk,” she said.
Susheel Kodali, MD, of New York–Presbyterian Hospital/Columbia University Irving Medical Center, who recently published recommendations on restructuring structural heart disease practice during the pandemic, said the document is timely as centers, including his own, are trying to restart some outpatient visits, as early as next week.
“They made a point about talking about cohesive partnerships with regional public health officials and I think that’s great. The question is how does that happen,” he told theheart.org | Medscape Cardiology. “In New York, we’re not allowed to do elective cases but what’s considered elective is not so clearly defined. An AS [aortic stenosis] patient that had a syncopal episode 2 weeks ago, is that considered elective or is that semi-urgent? I think that’s one of the challenges and that’s where these partnerships would be useful.”
Other challenges include the need for regional partnerships to better align hospitals, which in the New York area means half a dozen large healthcare systems, and to coordinate care between hospital departments – all of which will be scheduling imaging and OR time for their own backlog of hernia, knee, or hip surgeries.
Finally, there’s the need for a lot of conversation with the patient and their family about returning to a hospital amid a deadly pandemic.
“I had a patient today and the daughter was very concerned about bringing her in,” Kodali said. “She’s in class IV heart failure but her [daughter’s] big concern was: who is she going to be exposed to when she gets the echo? What kind of protection is there for her? Is the tech wearing a mask?
“It’s not just the health care providers that have to have the comfort, but it’s the patients and their families who have to feel comfortable bringing their loved ones here for treatment,” he said. “Because everyone is concerned about the environment.”
Wood reports receiving unrestricted grant support from Edwards Lifesciences and Abbott Vascular and serving as a consultant for Edwards Lifesciences, Medtronic, Abbott Vascular, and Boston Scientific. Poppas reports no relevant conflicts of interest. Kodali reports consultant (honoraria) from Admedus, Meril Life Sciences, JenaValve, and Abbott Vascular; SAB (equity) from Dura Biotech, MicroInterventional Devices, Thubrikar Aortic Valve, Supira, and Admedus; and institutional funding from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve.
This article first appeared on Medscape.com.
As COVID-19 case levels plateau in some regions, 16 North American cardiovascular societies have released a framework for reintroducing cardiovascular services disrupted by the pandemic.
The consensus document outlines a phased approach to restarting invasive cardiovascular (CV) procedures and diagnostic tests that aims to reduce patient and health care provider exposure to the coronavirus and still provide essential care. It also emphasizes some of the ethical considerations in patient selection and the need for a collaborative approach.
“The key message in our document is we need a new unprecedented collaboration with public health officials so that we can carefully monitor the situation and we’re aware of what’s happening with the penetrance of the pandemic in the community, but they’re aware of the morbidity and mortality that’s occurring on our ever-growing waiting list,” lead author David A. Wood, MD, told theheart.org | Medscape Cardiology.
The recommendations were jointly published May 4 in the Canadian Journal of Cardiology , the Journal of the American College of Cardiology, and The Annals of Thoracic Surgery, and are endorsed by, among others, the American Heart Association, American College of Cardiology (ACC), and Canadian Cardiovascular Society.
The guidance comes as hospitals are facing revenue shortfalls because of canceled elective procedures and resource-intensive COVID-19 cases, prompting some healthcare systems to furlough, lay off, or even fire staff.
“It’s obvious that volumes are down between 40% and 60%,” said Wood, director of the cardiac catheterization laboratory at Vancouver General Hospital and professor of medicine at the University of British Columbia, Canada. “Part of that is that some areas have restricted case volumes totally appropriately and it’s partly because patients are very afraid of coming to the hospital and, unfortunately, are having bad events at home. And some are dying.”
The new report features a detailed table outlining three different response levels: reintroduction of some services (level 2); reintroduction of most services (level 1); and regular services (level 0). It covers a range of services from transthoracic echocardiography and exercise testing with imaging to care for acute coronary syndrome and ST-segment elevation myocardial infarction.
“We’ve learned that we can very quickly turn off the tap and go to doing only 10% of our normal volumes, whether that’s surgery, cath lab, EP, diagnostic tests,” Wood said. “It’s much more difficult to thoughtfully turn the tap part way back on or restart the engine … you don’t just go from 0 to 100 [mph]. You go from 0 to 30 to 60 then maybe to 80 [mph].”
The document also includes eight guiding principles such as:
- The expectation that response levels will be different between regions, and even within a given region.
- A “transparent collaborative plan” for COVID-19 testing and personal protective equipment (PPE) must be in place before restarting cases.
- A less invasive test or alternate imaging modality should be considered, if both tests have similar efficacy.
- In general, a minimally invasive procedure with a shorter length of stay is preferable, if both strategies have similar efficacy and safety.
Although previous reports on cath lab considerations during the pandemic or restarting elective surgeries peg various actions to specific thresholds or time intervals, the language here is noticeably and intentionally broad.
Instead of stating when cardiovascular services should resume, for example, the experts say it’s appropriate to put the guidance document into place if there’s a “sustained reduction” in the rate of new COVID-19 admissions and deaths in the relevant geographic region for a “prespecified time interval.”
As for when or how frequently patients and healthcare providers should be tested for COVID-19, the document encourages “routine screening of all patients prior to any cardiovascular procedure or test.”
Overly prescriptive language in previous documents wasn’t felt to be that helpful, whereas language like “selective” cases and “some” or “most” cardiovascular procedures gives clinicians, health systems, and policy makers flexibility when moving between response levels, Wood explained.
“Different regions might be at different levels based on principles of public health as far as the penetrance of the pandemic in that community, as well as how can you actually do the physical distancing in your hospital or ambulatory clinic. Because, I tell you, that is the Achilles heel,” he said. “Our run rates are going to be determined by testing, the availability of PPE, but also how we’re going to use our existing infrastructure and maintain physical distancing.”
That may mean using telehealth for initial visits, having clinics open earlier in the morning or on weekends, or doing partial volumes for surgery or in the cath lab so patients can be staggered and recover at different times and in different areas of the hospital. “These are very granular, specific infrastructure things that we’ve never really had to consider before,” Wood observed.
The document also had to be flexible and nimble enough to respond to a potential rebound of COVID-19 cases, which in newly released models are projected to rise sharply to 200,000 cases a day and be accompanied by some 3,000 deaths each day by June 1.
“This is my own personal opinion but I think it’s foolish to think that we are going to be able to come back to 100% of the cases we were doing before, even with testing, PPE, and all of that until we have a vaccine,” he said.
Similar to decisions made in preparation for the initial COVID-19 surge, the consensus document outlines the need for ethical considerations when turning the tap back on. This means prioritizing procedures and tests that are likely to benefit more people and to a greater degree, and ensuring that patients are treated fairly and consistently, regardless of their ethnicity, perceived social worth, or ability to pay, said coauthor and ACC President Athena Poppas, MD, Brown University School of Medicine, Providence, Rhode Island.
“It’s an ethical tenet that exists in a lot of places but it’s usually not overtly called out,” Poppas told theheart.org | Medscape Cardiology. “It’s not rationing care; I think people jump to that but it’s actually the opposite of rationing care. It’s about being thoughtful about prioritizing patients.”
“There’s a variety of data that should help in the prioritization, not only how much hospital resources are utilized, that’s on one side, but there’s also the patient risk of delaying or doing a procedure, and then the societal risk,” she said.
Susheel Kodali, MD, of New York–Presbyterian Hospital/Columbia University Irving Medical Center, who recently published recommendations on restructuring structural heart disease practice during the pandemic, said the document is timely as centers, including his own, are trying to restart some outpatient visits, as early as next week.
“They made a point about talking about cohesive partnerships with regional public health officials and I think that’s great. The question is how does that happen,” he told theheart.org | Medscape Cardiology. “In New York, we’re not allowed to do elective cases but what’s considered elective is not so clearly defined. An AS [aortic stenosis] patient that had a syncopal episode 2 weeks ago, is that considered elective or is that semi-urgent? I think that’s one of the challenges and that’s where these partnerships would be useful.”
Other challenges include the need for regional partnerships to better align hospitals, which in the New York area means half a dozen large healthcare systems, and to coordinate care between hospital departments – all of which will be scheduling imaging and OR time for their own backlog of hernia, knee, or hip surgeries.
Finally, there’s the need for a lot of conversation with the patient and their family about returning to a hospital amid a deadly pandemic.
“I had a patient today and the daughter was very concerned about bringing her in,” Kodali said. “She’s in class IV heart failure but her [daughter’s] big concern was: who is she going to be exposed to when she gets the echo? What kind of protection is there for her? Is the tech wearing a mask?
“It’s not just the health care providers that have to have the comfort, but it’s the patients and their families who have to feel comfortable bringing their loved ones here for treatment,” he said. “Because everyone is concerned about the environment.”
Wood reports receiving unrestricted grant support from Edwards Lifesciences and Abbott Vascular and serving as a consultant for Edwards Lifesciences, Medtronic, Abbott Vascular, and Boston Scientific. Poppas reports no relevant conflicts of interest. Kodali reports consultant (honoraria) from Admedus, Meril Life Sciences, JenaValve, and Abbott Vascular; SAB (equity) from Dura Biotech, MicroInterventional Devices, Thubrikar Aortic Valve, Supira, and Admedus; and institutional funding from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve.
This article first appeared on Medscape.com.
As COVID-19 case levels plateau in some regions, 16 North American cardiovascular societies have released a framework for reintroducing cardiovascular services disrupted by the pandemic.
The consensus document outlines a phased approach to restarting invasive cardiovascular (CV) procedures and diagnostic tests that aims to reduce patient and health care provider exposure to the coronavirus and still provide essential care. It also emphasizes some of the ethical considerations in patient selection and the need for a collaborative approach.
“The key message in our document is we need a new unprecedented collaboration with public health officials so that we can carefully monitor the situation and we’re aware of what’s happening with the penetrance of the pandemic in the community, but they’re aware of the morbidity and mortality that’s occurring on our ever-growing waiting list,” lead author David A. Wood, MD, told theheart.org | Medscape Cardiology.
The recommendations were jointly published May 4 in the Canadian Journal of Cardiology , the Journal of the American College of Cardiology, and The Annals of Thoracic Surgery, and are endorsed by, among others, the American Heart Association, American College of Cardiology (ACC), and Canadian Cardiovascular Society.
The guidance comes as hospitals are facing revenue shortfalls because of canceled elective procedures and resource-intensive COVID-19 cases, prompting some healthcare systems to furlough, lay off, or even fire staff.
“It’s obvious that volumes are down between 40% and 60%,” said Wood, director of the cardiac catheterization laboratory at Vancouver General Hospital and professor of medicine at the University of British Columbia, Canada. “Part of that is that some areas have restricted case volumes totally appropriately and it’s partly because patients are very afraid of coming to the hospital and, unfortunately, are having bad events at home. And some are dying.”
The new report features a detailed table outlining three different response levels: reintroduction of some services (level 2); reintroduction of most services (level 1); and regular services (level 0). It covers a range of services from transthoracic echocardiography and exercise testing with imaging to care for acute coronary syndrome and ST-segment elevation myocardial infarction.
“We’ve learned that we can very quickly turn off the tap and go to doing only 10% of our normal volumes, whether that’s surgery, cath lab, EP, diagnostic tests,” Wood said. “It’s much more difficult to thoughtfully turn the tap part way back on or restart the engine … you don’t just go from 0 to 100 [mph]. You go from 0 to 30 to 60 then maybe to 80 [mph].”
The document also includes eight guiding principles such as:
- The expectation that response levels will be different between regions, and even within a given region.
- A “transparent collaborative plan” for COVID-19 testing and personal protective equipment (PPE) must be in place before restarting cases.
- A less invasive test or alternate imaging modality should be considered, if both tests have similar efficacy.
- In general, a minimally invasive procedure with a shorter length of stay is preferable, if both strategies have similar efficacy and safety.
Although previous reports on cath lab considerations during the pandemic or restarting elective surgeries peg various actions to specific thresholds or time intervals, the language here is noticeably and intentionally broad.
Instead of stating when cardiovascular services should resume, for example, the experts say it’s appropriate to put the guidance document into place if there’s a “sustained reduction” in the rate of new COVID-19 admissions and deaths in the relevant geographic region for a “prespecified time interval.”
As for when or how frequently patients and healthcare providers should be tested for COVID-19, the document encourages “routine screening of all patients prior to any cardiovascular procedure or test.”
Overly prescriptive language in previous documents wasn’t felt to be that helpful, whereas language like “selective” cases and “some” or “most” cardiovascular procedures gives clinicians, health systems, and policy makers flexibility when moving between response levels, Wood explained.
“Different regions might be at different levels based on principles of public health as far as the penetrance of the pandemic in that community, as well as how can you actually do the physical distancing in your hospital or ambulatory clinic. Because, I tell you, that is the Achilles heel,” he said. “Our run rates are going to be determined by testing, the availability of PPE, but also how we’re going to use our existing infrastructure and maintain physical distancing.”
That may mean using telehealth for initial visits, having clinics open earlier in the morning or on weekends, or doing partial volumes for surgery or in the cath lab so patients can be staggered and recover at different times and in different areas of the hospital. “These are very granular, specific infrastructure things that we’ve never really had to consider before,” Wood observed.
The document also had to be flexible and nimble enough to respond to a potential rebound of COVID-19 cases, which in newly released models are projected to rise sharply to 200,000 cases a day and be accompanied by some 3,000 deaths each day by June 1.
“This is my own personal opinion but I think it’s foolish to think that we are going to be able to come back to 100% of the cases we were doing before, even with testing, PPE, and all of that until we have a vaccine,” he said.
Similar to decisions made in preparation for the initial COVID-19 surge, the consensus document outlines the need for ethical considerations when turning the tap back on. This means prioritizing procedures and tests that are likely to benefit more people and to a greater degree, and ensuring that patients are treated fairly and consistently, regardless of their ethnicity, perceived social worth, or ability to pay, said coauthor and ACC President Athena Poppas, MD, Brown University School of Medicine, Providence, Rhode Island.
“It’s an ethical tenet that exists in a lot of places but it’s usually not overtly called out,” Poppas told theheart.org | Medscape Cardiology. “It’s not rationing care; I think people jump to that but it’s actually the opposite of rationing care. It’s about being thoughtful about prioritizing patients.”
“There’s a variety of data that should help in the prioritization, not only how much hospital resources are utilized, that’s on one side, but there’s also the patient risk of delaying or doing a procedure, and then the societal risk,” she said.
Susheel Kodali, MD, of New York–Presbyterian Hospital/Columbia University Irving Medical Center, who recently published recommendations on restructuring structural heart disease practice during the pandemic, said the document is timely as centers, including his own, are trying to restart some outpatient visits, as early as next week.
“They made a point about talking about cohesive partnerships with regional public health officials and I think that’s great. The question is how does that happen,” he told theheart.org | Medscape Cardiology. “In New York, we’re not allowed to do elective cases but what’s considered elective is not so clearly defined. An AS [aortic stenosis] patient that had a syncopal episode 2 weeks ago, is that considered elective or is that semi-urgent? I think that’s one of the challenges and that’s where these partnerships would be useful.”
Other challenges include the need for regional partnerships to better align hospitals, which in the New York area means half a dozen large healthcare systems, and to coordinate care between hospital departments – all of which will be scheduling imaging and OR time for their own backlog of hernia, knee, or hip surgeries.
Finally, there’s the need for a lot of conversation with the patient and their family about returning to a hospital amid a deadly pandemic.
“I had a patient today and the daughter was very concerned about bringing her in,” Kodali said. “She’s in class IV heart failure but her [daughter’s] big concern was: who is she going to be exposed to when she gets the echo? What kind of protection is there for her? Is the tech wearing a mask?
“It’s not just the health care providers that have to have the comfort, but it’s the patients and their families who have to feel comfortable bringing their loved ones here for treatment,” he said. “Because everyone is concerned about the environment.”
Wood reports receiving unrestricted grant support from Edwards Lifesciences and Abbott Vascular and serving as a consultant for Edwards Lifesciences, Medtronic, Abbott Vascular, and Boston Scientific. Poppas reports no relevant conflicts of interest. Kodali reports consultant (honoraria) from Admedus, Meril Life Sciences, JenaValve, and Abbott Vascular; SAB (equity) from Dura Biotech, MicroInterventional Devices, Thubrikar Aortic Valve, Supira, and Admedus; and institutional funding from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve.
This article first appeared on Medscape.com.