User login
For MD-IQ use only
The SHM 2019 Chapter Excellence Awards
The Society of Hospital Medicine is proud to recognize its chapters for their hard work and dedication in 2019 through Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustenance, and innovation within their chapter activities, which are then applauded for their successes throughout the subsequent year. In 2019, a new Bronze category was established, for a total of four Status Awards that chapters can earn.
Please join SHM in congratulating the following chapters on their year of success in 2019!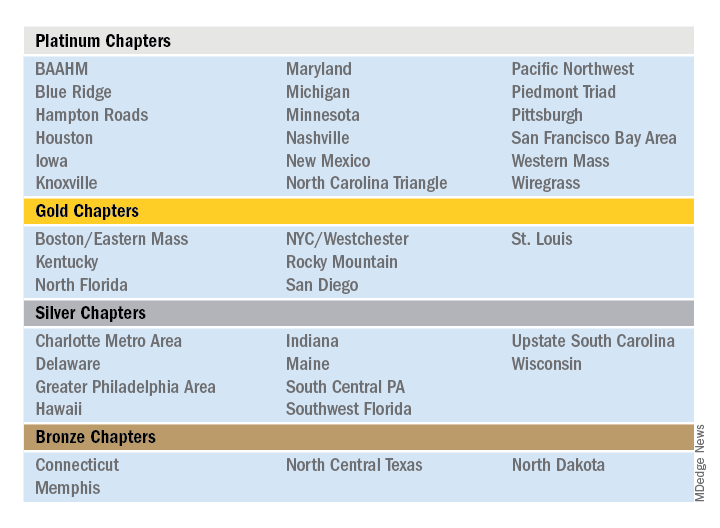
Outstanding Chapter of the Year
The Outstanding Chapter of the Year Award goes to one chapter who exemplifies high performance, going above and beyond the basic chapter requirements. The recipient of the Outstanding Chapter of the Year Award for 2019 is the Wiregrass Chapter of SHM. The chapter has a strong and engaged leadership which includes representation at all levels of the hospital medicine team, including physician hospitalists, advanced care provider hospitalists, practice administrators, nurses, residents, and medical students.
In the last year, the Wiregrass leadership team has organized programs and events to cater to and engage all the chapter’s members. This includes a variety of innovative ideas that catered toward medical education, health care provider well-being, engagement, mentorship, and community involvement.
The SHM Wiregrass Chapter’s biggest accomplishment in 2019 was the creation of an exchange program for physician and advanced practice provider hospitalists between the SHM New Mexico Chapter and the SHM Wiregrass Chapter. This idea first arose at HM19, where the chapter leaders had met during a networking event and debated the role of clinician wellbeing, quality of medical education, and faculty development to individual hospital medicine group (HMG) practice styles.
Clinician well-being is the prerequisite to the triple aim of improving the health of populations, enhancing the patient experience, and reducing the cost of care. Each HMG faces similar challenges but approaches to solving them vary. Professional challenges can affect the well-being of the individual clinicians. Having interinstitutional exchange programs provides a platform to exchange ideas and establish mentors. Also, the quality of medical education is directly linked to the quality of faculty development. Improving the quality of medical education requires a multifaceted approach by highly developed faculty. The complex factors affecting medical education and faculty development are further complicated by geographic location, patient characteristics, and professional growth opportunities. Overcoming these obstacles requires an innovative and collaborative approach. Although faculty exchanges are common in academic medicine, they are not commonly attempted with HMGs.
Hospitalists are responsible for a significant part of inpatient training for residents, medical students, and nurse practitioners/physician assistants (NPs/PAs), but their faculty training can vary based on location. Being a young specialty, only 2 decades old, hospital medicine is still evolving and incorporating NP/PA and physician hospitalists in varied practice models. Each HMG addresses common obstacles differently based on their culture and practice styles. The chapter leaders determined an exchange program would afford the opportunity for visiting faculty members to experience these differences. This emphasized the role and importance of exchanging ideas and contemplated a solution to benefit more practicing hospitalists.
The chapter leaders researched the characteristics of individual academic HMGs and structured a tailored faculty exchange involving physicians and NPs/PAs. During the exchange program planning, the visiting faculty itinerary was tailored to a well-planned agenda for 1 week, with separate tracks for physicians and NPs/PAs giving increased access to their individual peer practice styles. Additionally, the visiting faculty had meetings and discussions with HMG and hospital leadership, to specifically address each visiting faculty institution’s challenges. The overall goal of this exchange program was to promote cross-institutional collaboration, increase engagement, improve medical education through faculty development, and improve the quality of care. The focus of the exchange program was to share ideas and innovation and learn the approaches to unique challenges at each institution. Out of this also came collaboration and mentoring opportunities.
The evaluation process of the exchange involved interviews, a survey, and the establishment of shared QI projects in mutual areas of challenge. The survey provided feedback, lessons learned from the exchange, and areas to be improved. Collaborative QI projects currently underway as a result of the exchange include paging etiquette, quality of sleep for hospitalized patients, and onboarding of NPs/PAs in HMGs.
This innovation addressed faculty development and medical education via clinician well-being. The physician and NP/PA Faculty Exchange was an essential and meaningful innovation that resulted in increased SHM member engagement, cross-institutional collaboration, networking, and mentorship.
Additional projects that the SHM Wiregrass Chapter successfully implemented in 2019 include a “Women in Medicine” event that recognized women physician and advanced practice provider hospitalist leaders, a poster competition that expanded its research, clinical vignettes, and quality categories to include a fourth category of innovation, featuring 75 posters. Additionally, the chapter held a policy meeting with six Alabama state legislators, creating new channels of collaboration between the legislators and the chapter. Lastly, the chapter held a successful community event and launched a mentor program targeting medical students and residents.
Rising Star Chapter
The Rising Star Chapter Award goes to one chapter who has been active for 2 years or less, who in the past 12 months have made improvements to their leadership, stability and growth, and membership. The recipient of the Rising Star Chapter Award for 2019 is the Blue Ridge Chapter of SHM, which has made significant strides to develop since its launch in the fall of 2018. The chapter represents counties in northwest Tennessee, southwest Virginia, and western North Carolina.
The chapter held three meetings in 2019 which were well attended by hospitalists, residents/fellows, administrators, advanced practice providers, and nurses. On average, attendees from five to six different hospitalist groups are represented. The chapter hosted both Dr. Chris Frost, immediate past president of the SHM board of directors, and Dr. Ron Greeno, a past president of the SHM board of directors.
The SHM Blue Ridge Chapter has collaborated with both the ACP Tennessee Chapter and the Healthcare MBA program at Haslam College of Business at the University of Tennessee.
The chapter leadership regularly attends local medical residency programs at noon conferences to attract and recruit young physicians into chapter activities. Overall, the chapter has seen a growth in membership in 2019. The Blue Ridge Chapter is an active, enthusiastic chapter that is rapidly growing and thriving.
Outstanding Membership Recruitment and Retention
The Outstanding Membership Recruitment and Retention Award is a new exemplary award for 2019 that goes to one chapter who has gone above and beyond to implement initiatives to recruit and retain SHM members in their chapter. The recipient of the Outstanding Membership Recruitment and Retention Award for 2019 is the Western Massachusetts Chapter of SHM, which has done outstanding work to recruit and retain the membership. In 2019, the SHM membership in the chapter grew by 24%. The chapter utilized Chapter Development Funds to launch new initiatives to conduct outreach to nonmember hospitalists in the community and invite them to meetings to obtain the SHM experience. Additionally, the chapter encouraged residents to join and get involved by hosting a poster competition.
The Western Massachusetts Chapter focused on being innovative, inclusive, and creative to retain their existing meetings. For example, the chapter hosted a new “Jeopardy Session” event that featured a nontraditional jeopardy game that attracted a large attendance including local residents. Additionally, the chapter insured that all clinical and nonclinical members of the hospital medicine team were included and encouraged to participate in all chapter meetings. Lastly, the chapter launched a local awards program to recognize senior hospitalist and early career hospitalist who contributed to chapter development.
Most Engaged Chapter Leader
The Most Engaged Chapter Leader Award is a new exemplary award for 2019 that goes to one chapter leader or district chair who is either nominated or self-nominated and has demonstrated how they or their nominee has gone above and beyond in the past year to grow and sustain their chapter and/or district and continues to carry out the SHM mission. The recipient of the Most Engaged Chapter Leader Award for 2019 goes to Thérèse Franco, MD, SFHM, president of the Pacific Northwest Chapter.
Dr. Franco has served as the chapter’s president for 2 years and has served on the SHM Chapter Support Committee for 3 years. She has previously participated as a mentor in the glycemic control mentored implementation program, and as chair and cochair of the RIV contest. She continues to review abstracts, volunteer as a judge and offer local education on glycemic control through the Washington State Hospital Association, promoting SHM’s work there. One of Dr. Franco’s core strengths has been effective collaboration with past leaders (such as Rachel Thompson, MD, and Kimberly Bell, MD), future leaders, and other organizations (such as the Washington State Medical Association and the King County Medical Association). Dr. Franco has recruited an outstanding leadership team and new advisory committee for the Pacific Northwest Chapter, resulting a fantastic year of growth, innovation, and development.
The Society of Hospital Medicine is proud to recognize its chapters for their hard work and dedication in 2019 through Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustenance, and innovation within their chapter activities, which are then applauded for their successes throughout the subsequent year. In 2019, a new Bronze category was established, for a total of four Status Awards that chapters can earn.
Please join SHM in congratulating the following chapters on their year of success in 2019!
Outstanding Chapter of the Year
The Outstanding Chapter of the Year Award goes to one chapter who exemplifies high performance, going above and beyond the basic chapter requirements. The recipient of the Outstanding Chapter of the Year Award for 2019 is the Wiregrass Chapter of SHM. The chapter has a strong and engaged leadership which includes representation at all levels of the hospital medicine team, including physician hospitalists, advanced care provider hospitalists, practice administrators, nurses, residents, and medical students.
In the last year, the Wiregrass leadership team has organized programs and events to cater to and engage all the chapter’s members. This includes a variety of innovative ideas that catered toward medical education, health care provider well-being, engagement, mentorship, and community involvement.
The SHM Wiregrass Chapter’s biggest accomplishment in 2019 was the creation of an exchange program for physician and advanced practice provider hospitalists between the SHM New Mexico Chapter and the SHM Wiregrass Chapter. This idea first arose at HM19, where the chapter leaders had met during a networking event and debated the role of clinician wellbeing, quality of medical education, and faculty development to individual hospital medicine group (HMG) practice styles.
Clinician well-being is the prerequisite to the triple aim of improving the health of populations, enhancing the patient experience, and reducing the cost of care. Each HMG faces similar challenges but approaches to solving them vary. Professional challenges can affect the well-being of the individual clinicians. Having interinstitutional exchange programs provides a platform to exchange ideas and establish mentors. Also, the quality of medical education is directly linked to the quality of faculty development. Improving the quality of medical education requires a multifaceted approach by highly developed faculty. The complex factors affecting medical education and faculty development are further complicated by geographic location, patient characteristics, and professional growth opportunities. Overcoming these obstacles requires an innovative and collaborative approach. Although faculty exchanges are common in academic medicine, they are not commonly attempted with HMGs.
Hospitalists are responsible for a significant part of inpatient training for residents, medical students, and nurse practitioners/physician assistants (NPs/PAs), but their faculty training can vary based on location. Being a young specialty, only 2 decades old, hospital medicine is still evolving and incorporating NP/PA and physician hospitalists in varied practice models. Each HMG addresses common obstacles differently based on their culture and practice styles. The chapter leaders determined an exchange program would afford the opportunity for visiting faculty members to experience these differences. This emphasized the role and importance of exchanging ideas and contemplated a solution to benefit more practicing hospitalists.
The chapter leaders researched the characteristics of individual academic HMGs and structured a tailored faculty exchange involving physicians and NPs/PAs. During the exchange program planning, the visiting faculty itinerary was tailored to a well-planned agenda for 1 week, with separate tracks for physicians and NPs/PAs giving increased access to their individual peer practice styles. Additionally, the visiting faculty had meetings and discussions with HMG and hospital leadership, to specifically address each visiting faculty institution’s challenges. The overall goal of this exchange program was to promote cross-institutional collaboration, increase engagement, improve medical education through faculty development, and improve the quality of care. The focus of the exchange program was to share ideas and innovation and learn the approaches to unique challenges at each institution. Out of this also came collaboration and mentoring opportunities.
The evaluation process of the exchange involved interviews, a survey, and the establishment of shared QI projects in mutual areas of challenge. The survey provided feedback, lessons learned from the exchange, and areas to be improved. Collaborative QI projects currently underway as a result of the exchange include paging etiquette, quality of sleep for hospitalized patients, and onboarding of NPs/PAs in HMGs.
This innovation addressed faculty development and medical education via clinician well-being. The physician and NP/PA Faculty Exchange was an essential and meaningful innovation that resulted in increased SHM member engagement, cross-institutional collaboration, networking, and mentorship.
Additional projects that the SHM Wiregrass Chapter successfully implemented in 2019 include a “Women in Medicine” event that recognized women physician and advanced practice provider hospitalist leaders, a poster competition that expanded its research, clinical vignettes, and quality categories to include a fourth category of innovation, featuring 75 posters. Additionally, the chapter held a policy meeting with six Alabama state legislators, creating new channels of collaboration between the legislators and the chapter. Lastly, the chapter held a successful community event and launched a mentor program targeting medical students and residents.
Rising Star Chapter
The Rising Star Chapter Award goes to one chapter who has been active for 2 years or less, who in the past 12 months have made improvements to their leadership, stability and growth, and membership. The recipient of the Rising Star Chapter Award for 2019 is the Blue Ridge Chapter of SHM, which has made significant strides to develop since its launch in the fall of 2018. The chapter represents counties in northwest Tennessee, southwest Virginia, and western North Carolina.
The chapter held three meetings in 2019 which were well attended by hospitalists, residents/fellows, administrators, advanced practice providers, and nurses. On average, attendees from five to six different hospitalist groups are represented. The chapter hosted both Dr. Chris Frost, immediate past president of the SHM board of directors, and Dr. Ron Greeno, a past president of the SHM board of directors.
The SHM Blue Ridge Chapter has collaborated with both the ACP Tennessee Chapter and the Healthcare MBA program at Haslam College of Business at the University of Tennessee.
The chapter leadership regularly attends local medical residency programs at noon conferences to attract and recruit young physicians into chapter activities. Overall, the chapter has seen a growth in membership in 2019. The Blue Ridge Chapter is an active, enthusiastic chapter that is rapidly growing and thriving.
Outstanding Membership Recruitment and Retention
The Outstanding Membership Recruitment and Retention Award is a new exemplary award for 2019 that goes to one chapter who has gone above and beyond to implement initiatives to recruit and retain SHM members in their chapter. The recipient of the Outstanding Membership Recruitment and Retention Award for 2019 is the Western Massachusetts Chapter of SHM, which has done outstanding work to recruit and retain the membership. In 2019, the SHM membership in the chapter grew by 24%. The chapter utilized Chapter Development Funds to launch new initiatives to conduct outreach to nonmember hospitalists in the community and invite them to meetings to obtain the SHM experience. Additionally, the chapter encouraged residents to join and get involved by hosting a poster competition.
The Western Massachusetts Chapter focused on being innovative, inclusive, and creative to retain their existing meetings. For example, the chapter hosted a new “Jeopardy Session” event that featured a nontraditional jeopardy game that attracted a large attendance including local residents. Additionally, the chapter insured that all clinical and nonclinical members of the hospital medicine team were included and encouraged to participate in all chapter meetings. Lastly, the chapter launched a local awards program to recognize senior hospitalist and early career hospitalist who contributed to chapter development.
Most Engaged Chapter Leader
The Most Engaged Chapter Leader Award is a new exemplary award for 2019 that goes to one chapter leader or district chair who is either nominated or self-nominated and has demonstrated how they or their nominee has gone above and beyond in the past year to grow and sustain their chapter and/or district and continues to carry out the SHM mission. The recipient of the Most Engaged Chapter Leader Award for 2019 goes to Thérèse Franco, MD, SFHM, president of the Pacific Northwest Chapter.
Dr. Franco has served as the chapter’s president for 2 years and has served on the SHM Chapter Support Committee for 3 years. She has previously participated as a mentor in the glycemic control mentored implementation program, and as chair and cochair of the RIV contest. She continues to review abstracts, volunteer as a judge and offer local education on glycemic control through the Washington State Hospital Association, promoting SHM’s work there. One of Dr. Franco’s core strengths has been effective collaboration with past leaders (such as Rachel Thompson, MD, and Kimberly Bell, MD), future leaders, and other organizations (such as the Washington State Medical Association and the King County Medical Association). Dr. Franco has recruited an outstanding leadership team and new advisory committee for the Pacific Northwest Chapter, resulting a fantastic year of growth, innovation, and development.
The Society of Hospital Medicine is proud to recognize its chapters for their hard work and dedication in 2019 through Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustenance, and innovation within their chapter activities, which are then applauded for their successes throughout the subsequent year. In 2019, a new Bronze category was established, for a total of four Status Awards that chapters can earn.
Please join SHM in congratulating the following chapters on their year of success in 2019!
Outstanding Chapter of the Year
The Outstanding Chapter of the Year Award goes to one chapter who exemplifies high performance, going above and beyond the basic chapter requirements. The recipient of the Outstanding Chapter of the Year Award for 2019 is the Wiregrass Chapter of SHM. The chapter has a strong and engaged leadership which includes representation at all levels of the hospital medicine team, including physician hospitalists, advanced care provider hospitalists, practice administrators, nurses, residents, and medical students.
In the last year, the Wiregrass leadership team has organized programs and events to cater to and engage all the chapter’s members. This includes a variety of innovative ideas that catered toward medical education, health care provider well-being, engagement, mentorship, and community involvement.
The SHM Wiregrass Chapter’s biggest accomplishment in 2019 was the creation of an exchange program for physician and advanced practice provider hospitalists between the SHM New Mexico Chapter and the SHM Wiregrass Chapter. This idea first arose at HM19, where the chapter leaders had met during a networking event and debated the role of clinician wellbeing, quality of medical education, and faculty development to individual hospital medicine group (HMG) practice styles.
Clinician well-being is the prerequisite to the triple aim of improving the health of populations, enhancing the patient experience, and reducing the cost of care. Each HMG faces similar challenges but approaches to solving them vary. Professional challenges can affect the well-being of the individual clinicians. Having interinstitutional exchange programs provides a platform to exchange ideas and establish mentors. Also, the quality of medical education is directly linked to the quality of faculty development. Improving the quality of medical education requires a multifaceted approach by highly developed faculty. The complex factors affecting medical education and faculty development are further complicated by geographic location, patient characteristics, and professional growth opportunities. Overcoming these obstacles requires an innovative and collaborative approach. Although faculty exchanges are common in academic medicine, they are not commonly attempted with HMGs.
Hospitalists are responsible for a significant part of inpatient training for residents, medical students, and nurse practitioners/physician assistants (NPs/PAs), but their faculty training can vary based on location. Being a young specialty, only 2 decades old, hospital medicine is still evolving and incorporating NP/PA and physician hospitalists in varied practice models. Each HMG addresses common obstacles differently based on their culture and practice styles. The chapter leaders determined an exchange program would afford the opportunity for visiting faculty members to experience these differences. This emphasized the role and importance of exchanging ideas and contemplated a solution to benefit more practicing hospitalists.
The chapter leaders researched the characteristics of individual academic HMGs and structured a tailored faculty exchange involving physicians and NPs/PAs. During the exchange program planning, the visiting faculty itinerary was tailored to a well-planned agenda for 1 week, with separate tracks for physicians and NPs/PAs giving increased access to their individual peer practice styles. Additionally, the visiting faculty had meetings and discussions with HMG and hospital leadership, to specifically address each visiting faculty institution’s challenges. The overall goal of this exchange program was to promote cross-institutional collaboration, increase engagement, improve medical education through faculty development, and improve the quality of care. The focus of the exchange program was to share ideas and innovation and learn the approaches to unique challenges at each institution. Out of this also came collaboration and mentoring opportunities.
The evaluation process of the exchange involved interviews, a survey, and the establishment of shared QI projects in mutual areas of challenge. The survey provided feedback, lessons learned from the exchange, and areas to be improved. Collaborative QI projects currently underway as a result of the exchange include paging etiquette, quality of sleep for hospitalized patients, and onboarding of NPs/PAs in HMGs.
This innovation addressed faculty development and medical education via clinician well-being. The physician and NP/PA Faculty Exchange was an essential and meaningful innovation that resulted in increased SHM member engagement, cross-institutional collaboration, networking, and mentorship.
Additional projects that the SHM Wiregrass Chapter successfully implemented in 2019 include a “Women in Medicine” event that recognized women physician and advanced practice provider hospitalist leaders, a poster competition that expanded its research, clinical vignettes, and quality categories to include a fourth category of innovation, featuring 75 posters. Additionally, the chapter held a policy meeting with six Alabama state legislators, creating new channels of collaboration between the legislators and the chapter. Lastly, the chapter held a successful community event and launched a mentor program targeting medical students and residents.
Rising Star Chapter
The Rising Star Chapter Award goes to one chapter who has been active for 2 years or less, who in the past 12 months have made improvements to their leadership, stability and growth, and membership. The recipient of the Rising Star Chapter Award for 2019 is the Blue Ridge Chapter of SHM, which has made significant strides to develop since its launch in the fall of 2018. The chapter represents counties in northwest Tennessee, southwest Virginia, and western North Carolina.
The chapter held three meetings in 2019 which were well attended by hospitalists, residents/fellows, administrators, advanced practice providers, and nurses. On average, attendees from five to six different hospitalist groups are represented. The chapter hosted both Dr. Chris Frost, immediate past president of the SHM board of directors, and Dr. Ron Greeno, a past president of the SHM board of directors.
The SHM Blue Ridge Chapter has collaborated with both the ACP Tennessee Chapter and the Healthcare MBA program at Haslam College of Business at the University of Tennessee.
The chapter leadership regularly attends local medical residency programs at noon conferences to attract and recruit young physicians into chapter activities. Overall, the chapter has seen a growth in membership in 2019. The Blue Ridge Chapter is an active, enthusiastic chapter that is rapidly growing and thriving.
Outstanding Membership Recruitment and Retention
The Outstanding Membership Recruitment and Retention Award is a new exemplary award for 2019 that goes to one chapter who has gone above and beyond to implement initiatives to recruit and retain SHM members in their chapter. The recipient of the Outstanding Membership Recruitment and Retention Award for 2019 is the Western Massachusetts Chapter of SHM, which has done outstanding work to recruit and retain the membership. In 2019, the SHM membership in the chapter grew by 24%. The chapter utilized Chapter Development Funds to launch new initiatives to conduct outreach to nonmember hospitalists in the community and invite them to meetings to obtain the SHM experience. Additionally, the chapter encouraged residents to join and get involved by hosting a poster competition.
The Western Massachusetts Chapter focused on being innovative, inclusive, and creative to retain their existing meetings. For example, the chapter hosted a new “Jeopardy Session” event that featured a nontraditional jeopardy game that attracted a large attendance including local residents. Additionally, the chapter insured that all clinical and nonclinical members of the hospital medicine team were included and encouraged to participate in all chapter meetings. Lastly, the chapter launched a local awards program to recognize senior hospitalist and early career hospitalist who contributed to chapter development.
Most Engaged Chapter Leader
The Most Engaged Chapter Leader Award is a new exemplary award for 2019 that goes to one chapter leader or district chair who is either nominated or self-nominated and has demonstrated how they or their nominee has gone above and beyond in the past year to grow and sustain their chapter and/or district and continues to carry out the SHM mission. The recipient of the Most Engaged Chapter Leader Award for 2019 goes to Thérèse Franco, MD, SFHM, president of the Pacific Northwest Chapter.
Dr. Franco has served as the chapter’s president for 2 years and has served on the SHM Chapter Support Committee for 3 years. She has previously participated as a mentor in the glycemic control mentored implementation program, and as chair and cochair of the RIV contest. She continues to review abstracts, volunteer as a judge and offer local education on glycemic control through the Washington State Hospital Association, promoting SHM’s work there. One of Dr. Franco’s core strengths has been effective collaboration with past leaders (such as Rachel Thompson, MD, and Kimberly Bell, MD), future leaders, and other organizations (such as the Washington State Medical Association and the King County Medical Association). Dr. Franco has recruited an outstanding leadership team and new advisory committee for the Pacific Northwest Chapter, resulting a fantastic year of growth, innovation, and development.
COVID-19 exacerbating challenges for Latino patients
Disproportionate burden of pandemic complicates mental health care
Pamela Montano, MD, recalls the recent case of a patient with bipolar II disorder who was improving after treatment with medication and therapy when her life was upended by the COVID-19 pandemic.
The patient, who is Puerto Rican, lost two cousins to the virus, two of her brothers fell ill, and her sister became sick with coronavirus, said Dr. Montano, director of the Latino Bicultural Clinic at Gouverneur Health in New York. The patient was then left to care for her sister’s toddlers along with the patient’s own children, one of whom has special needs.
“After this happened, it increased her anxiety,” Dr. Montano said in an interview. “She’s not sleeping, and she started having panic attacks. My main concern was how to help her cope.”
Across the country, clinicians who treat mental illness and behavioral disorders in Latino patients are facing similar experiences and challenges associated with COVID-19 and the ensuing pandemic response. Current data suggest a disproportionate burden of illness and death from the novel coronavirus among racial and ethnic groups, particularly black and Hispanic patients. The disparities are likely attributable to economic and social conditions more common among such populations, compared with non-Hispanic whites, in addition to isolation from resources, according to the Centers for Disease Control and Prevention.
A recent New York City Department of Health study based on data that were available in late April found that deaths from COVID-19 were substantially higher for black and Hispanic/Latino patients than for white and Asian patients. The death rate per 100,000 population was 209.4 for blacks, 195.3 for Hispanics/Latinos, 107.7 for whites, and 90.8 for Asians.
“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and nonimmigrant,” said Dr. Montano, a board member of the American Society of Hispanic Psychiatry and the officer of infrastructure and advocacy for the Hispanic Caucus of the American Psychiatric Association. “This includes income inequality, poor nutrition, history of trauma and discrimination, employment issues, quality education, access to technology, and overall access to appropriate cultural linguistic health care.”
Navigating challenges
For mental health professionals treating Latino patients, COVID-19 and the pandemic response have generated a range of treatment obstacles.
The transition to telehealth for example, has not been easy for some patients, said Jacqueline Posada, MD, consultation-liaison psychiatry fellow at the Inova Fairfax Hospital–George Washington University program in Falls Church, Va., and an APA Substance Abuse and Mental Health Services Administration minority fellow. Some patients lack Internet services, others forget virtual visits, and some do not have working phones, she said.
“I’ve had to be very flexible,” she said in an interview. “Ideally, I’d love to see everybody via video chat, but a lot of people either don’t have a stable Internet connection or Internet, so I meet the patient where they are. Whatever they have available, that’s what I’m going to use. If they don’t answer on the first call, I will call again at least three to five times in the first 15 minutes to make sure I’m giving them an opportunity to pick up the phone.”
In addition, Dr. Posada has encountered disconnected phones when calling patients for appointments. In such cases, Dr. Posada contacts the patient’s primary care physician to relay medication recommendations in case the patient resurfaces at the clinic.
In other instances, patients are not familiar with video technology, or they must travel to a friend or neighbor’s house to access the technology, said Hector Colón-Rivera, MD, an addiction psychiatrist and medical director of the Asociación Puertorriqueños en Marcha Behavioral Health Program, a nonprofit organization based in the Philadelphia area. Telehealth visits frequently include appearances by children, family members, barking dogs, and other distractions, said Dr. Colón-Rivera, president of the APA Hispanic Caucus.
“We’re seeing things that we didn’t used to see when they came to our office – for good or for bad,” said Dr. Colón-Rivera, an attending telemedicine physician at the University of Pittsburgh Medical Center. “It could be a good chance to meet our patient in a different way. Of course, it creates different stressors. If you have five kids on top of you and you’re the only one at home, it’s hard to do therapy.”
Psychiatrists are also seeing prior health conditions in patients exacerbated by COVID-19 fears and new health problems arising from the current pandemic environment. Dr. Posada recalls a patient whom she successfully treated for premenstrual dysphoric disorder who recently descended into severe clinical depression. The patient, from Colombia, was attending school in the United States on a student visa and supporting herself through child care jobs.
“So much of her depression was based on her social circumstance,” Dr. Posada said. “She had lost her job, her sister had lost her job so they were scraping by on her sister’s husband’s income, and the thing that brought her joy, which was going to school and studying so she could make a different life for herself than what her parents had in Colombia, also seemed like it was out of reach.”
Dr. Colón-Rivera recently received a call from a hospital where one of his patients was admitted after becoming delusional and psychotic. The patient was correctly taking medication prescribed by Dr. Colón-Rivera, but her diabetes had become uncontrolled because she was unable to reach her primary care doctor and couldn’t access the pharmacy. Her blood sugar level became elevated, leading to the delusions.
“A patient that was perfectly stable now is unstable,” he said. “Her diet has not been good enough through the pandemic, exacerbating her diabetes. She was admitted to the hospital for delirium.
Compounding of traumas
For many Latino patients, the adverse impacts of the pandemic comes on top of multiple prior traumas, such as violence exposures, discrimination, and economic issues, said Lisa Fortuna, MD, MPH, MDiv, chief of psychiatry and vice chair at Zuckerberg San Francisco General Hospital. A 2017 analysis found that nearly four in five Latino youth face at least one traumatic childhood experience, like poverty or abuse, and that about 29% of Latino youth experience four or more of these traumas.
Immigrants in particular, may have faced trauma in their home country and/or immigration trauma, Dr. Fortuna added. A 2013 study on immigrant Latino adolescents for example, found that 29% of foreign-born adolescents and 34% of foreign-born parents experienced trauma during the migration process (Int Migr Rev. 2013 Dec;47(4):10).
“All of these things are cumulative,” Dr. Fortuna said. “Then when you’re hit with a pandemic, all of the disparities that you already have and all the stress that you already have are compounded. This is for the kids, too, who have been exposed to a lot of stressors and now maybe have family members that have been ill or have died. All of these things definitely put people at risk for increased depression [and] the worsening of any preexisting posttraumatic stress disorder. We’ve seen this in previous disasters, and I expect that’s what we’re going to see more of with the COVID-19 pandemic.”
At the same time, a central cultural value of many Latinos is family unity, Dr. Montano said, a foundation that is now being strained by social distancing and severed connections.
“This has separated many families,” she said. “There has been a lot of loneliness and grief.”
Mistrust and fear toward the government, public agencies, and even the health system itself act as further hurdles for some Latinos in the face of COVID-19. In areas with large immigrant populations such as San Francisco, Dr. Fortuna noted, it’s not uncommon for undocumented patients to avoid accessing medical care and social services, or visiting emergency departments for needed care for fear of drawing attention to themselves or possible detainment.
“The fact that so many people showed up at our hospital so ill and ended up in the ICU – that could be a combination of factors. Because the population has high rates of diabetes and hypertension, that might have put people at increased risk for severe illness,” she said. “But some people may have been holding out for care because they wanted to avoid being in places out of fear of immigration scrutiny.”
Overcoming language barriers
Compounding the challenging pandemic landscape for Latino patients is the fact that many state resources about COVID-19 have not been translated to Spanish, Dr. Colón-Rivera said. He was troubled recently when he went to several state websites and found limited to no information in Spanish about the coronavirus. Some data about COVID-19 from the federal government were not translated to Spanish until officials received pushback, he added. Even now, press releases and other information disseminated by the federal government about the virus appear to be translated by an automated service – and lack sense and context.
The state agencies in Pennsylvania have been alerted to the absence of Spanish information, but change has been slow, he noted.
“In Philadelphia, 23% speaks a language other than English,” he said. “So we missed a lot of critical information that could have helped to avoid spreading the illness and access support.”
Dr. Fortuna said that California has done better with providing COVID-19–related information in Spanish, compared with some other states, but misinformation about the virus and lingering myths have still been a problem among the Latino community. The University of California, San Francisco, recently launched a Latino Task Force resource website for the Latino community that includes information in English, Spanish, and Yucatec Maya about COVID-19, health and wellness tips, and resources for various assistance needs.
The concerning lack of COVID-19 information translated to Spanish led Dr. Montano to start a Facebook page in Spanish about mental health tips and guidance for managing COVID-19–related issues. She and her team of clinicians share information, videos, relaxation exercises, and community resources on the page, among other posts. “There is also general info and recommendations about COVID-19 that I think can be useful for the community,” she said. “The idea is that patients, the general community, and providers can have share information, hope messages, and ask questions in Spanish.”
Feeling ‘helpless’
A central part of caring for Latino patients during the COVID-19 crisis has been referring them to outside agencies and social services, psychiatrists say. But finding the right resources amid a pandemic and ensuring that patients connect with the correct aid has been an uphill battle.
“We sometimes feel like our hands are tied,” Dr. Colón-Rivera said. “Sometimes, we need to call a place to bring food. Some of the state agencies and nonprofits don’t have delivery systems, so the patient has to go pick up for food or medication. Some of our patients don’t want to go outside. Some do not have cars.”
As a clinician, it can be easy to feel helpless when trying to navigate new challenges posed by the pandemic in addition to other longstanding barriers, Dr. Posada said.
“Already, mental health disorders are so influenced by social situations like poverty, job insecurity, or family issues, and now it just seems those obstacles are even more insurmountable,” she said. “At the end of the day, I can feel like: ‘Did I make a difference?’ That’s a big struggle.”
Dr. Montano’s team, which includes psychiatrists, psychologists, and social workers, have come to rely on virtual debriefings to vent, express frustrations, and support one another, she said. She also recently joined a virtual mind-body skills group as a participant.
“I recognize the importance of getting additional support and ways to alleviate burnout,” she said. “We need to take care of ourselves or we won’t be able to help others.”
Focusing on resilience during the current crisis can be beneficial for both patients and providers in coping and drawing strength, Dr. Posada said.
“When it comes to fostering resilience during times of hardship, I think it’s most helpful to reflect on what skills or attributes have helped during past crises and apply those now – whether it’s turning to comfort from close relationships, looking to religion and spirituality, practicing self-care like rest or exercise, or really tapping into one’s purpose and reason for practicing psychiatry and being a physician,” she said. “The same advice goes for clinicians: We’ve all been through hard times in the past, it’s part of the human condition and we’ve also witnessed a lot of suffering in our patients, so now is the time to practice those skills that have gotten us through hard times in the past.”
Learning lessons from COVID-19
Despite the challenges with moving to telehealth, Dr. Fortuna said the tool has proved beneficial overall for mental health care. For Dr. Fortuna’s team for example, telehealth by phone has decreased the no-show rate, compared with clinic visits, and improved care access.
“We need to figure out how to maintain that,” she said. “If we can build ways for equity and access to Internet, especially equipment, I think that’s going to help.”
In addition, more data are needed about the ways in which COVID-19 is affecting Latino patients, Dr. Colón-Rivera said. Mortality statistics have been published, but information is needed about the rates of infection and manifestation of illness.
Most importantly, the COVID-19 crisis has emphasized the critical need to address and improve the underlying inequity issues among Latino patients, psychiatrists say.
“We really need to think about how there can be partnerships, in terms of community-based Latino business and leaders, multisector resources, trying to think about how we can improve conditions both work and safety for Latinos,” Dr. Fortuna said. “How can schools get support in integrating mental health and support for families, especially now after COVID-19? And really looking at some of these underlying inequities that are the underpinnings of why people were at risk for the disproportionate effects of the COVID-19 pandemic.”
Disproportionate burden of pandemic complicates mental health care
Disproportionate burden of pandemic complicates mental health care
Pamela Montano, MD, recalls the recent case of a patient with bipolar II disorder who was improving after treatment with medication and therapy when her life was upended by the COVID-19 pandemic.
The patient, who is Puerto Rican, lost two cousins to the virus, two of her brothers fell ill, and her sister became sick with coronavirus, said Dr. Montano, director of the Latino Bicultural Clinic at Gouverneur Health in New York. The patient was then left to care for her sister’s toddlers along with the patient’s own children, one of whom has special needs.
“After this happened, it increased her anxiety,” Dr. Montano said in an interview. “She’s not sleeping, and she started having panic attacks. My main concern was how to help her cope.”
Across the country, clinicians who treat mental illness and behavioral disorders in Latino patients are facing similar experiences and challenges associated with COVID-19 and the ensuing pandemic response. Current data suggest a disproportionate burden of illness and death from the novel coronavirus among racial and ethnic groups, particularly black and Hispanic patients. The disparities are likely attributable to economic and social conditions more common among such populations, compared with non-Hispanic whites, in addition to isolation from resources, according to the Centers for Disease Control and Prevention.
A recent New York City Department of Health study based on data that were available in late April found that deaths from COVID-19 were substantially higher for black and Hispanic/Latino patients than for white and Asian patients. The death rate per 100,000 population was 209.4 for blacks, 195.3 for Hispanics/Latinos, 107.7 for whites, and 90.8 for Asians.
“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and nonimmigrant,” said Dr. Montano, a board member of the American Society of Hispanic Psychiatry and the officer of infrastructure and advocacy for the Hispanic Caucus of the American Psychiatric Association. “This includes income inequality, poor nutrition, history of trauma and discrimination, employment issues, quality education, access to technology, and overall access to appropriate cultural linguistic health care.”
Navigating challenges
For mental health professionals treating Latino patients, COVID-19 and the pandemic response have generated a range of treatment obstacles.
The transition to telehealth for example, has not been easy for some patients, said Jacqueline Posada, MD, consultation-liaison psychiatry fellow at the Inova Fairfax Hospital–George Washington University program in Falls Church, Va., and an APA Substance Abuse and Mental Health Services Administration minority fellow. Some patients lack Internet services, others forget virtual visits, and some do not have working phones, she said.
“I’ve had to be very flexible,” she said in an interview. “Ideally, I’d love to see everybody via video chat, but a lot of people either don’t have a stable Internet connection or Internet, so I meet the patient where they are. Whatever they have available, that’s what I’m going to use. If they don’t answer on the first call, I will call again at least three to five times in the first 15 minutes to make sure I’m giving them an opportunity to pick up the phone.”
In addition, Dr. Posada has encountered disconnected phones when calling patients for appointments. In such cases, Dr. Posada contacts the patient’s primary care physician to relay medication recommendations in case the patient resurfaces at the clinic.
In other instances, patients are not familiar with video technology, or they must travel to a friend or neighbor’s house to access the technology, said Hector Colón-Rivera, MD, an addiction psychiatrist and medical director of the Asociación Puertorriqueños en Marcha Behavioral Health Program, a nonprofit organization based in the Philadelphia area. Telehealth visits frequently include appearances by children, family members, barking dogs, and other distractions, said Dr. Colón-Rivera, president of the APA Hispanic Caucus.
“We’re seeing things that we didn’t used to see when they came to our office – for good or for bad,” said Dr. Colón-Rivera, an attending telemedicine physician at the University of Pittsburgh Medical Center. “It could be a good chance to meet our patient in a different way. Of course, it creates different stressors. If you have five kids on top of you and you’re the only one at home, it’s hard to do therapy.”
Psychiatrists are also seeing prior health conditions in patients exacerbated by COVID-19 fears and new health problems arising from the current pandemic environment. Dr. Posada recalls a patient whom she successfully treated for premenstrual dysphoric disorder who recently descended into severe clinical depression. The patient, from Colombia, was attending school in the United States on a student visa and supporting herself through child care jobs.
“So much of her depression was based on her social circumstance,” Dr. Posada said. “She had lost her job, her sister had lost her job so they were scraping by on her sister’s husband’s income, and the thing that brought her joy, which was going to school and studying so she could make a different life for herself than what her parents had in Colombia, also seemed like it was out of reach.”
Dr. Colón-Rivera recently received a call from a hospital where one of his patients was admitted after becoming delusional and psychotic. The patient was correctly taking medication prescribed by Dr. Colón-Rivera, but her diabetes had become uncontrolled because she was unable to reach her primary care doctor and couldn’t access the pharmacy. Her blood sugar level became elevated, leading to the delusions.
“A patient that was perfectly stable now is unstable,” he said. “Her diet has not been good enough through the pandemic, exacerbating her diabetes. She was admitted to the hospital for delirium.
Compounding of traumas
For many Latino patients, the adverse impacts of the pandemic comes on top of multiple prior traumas, such as violence exposures, discrimination, and economic issues, said Lisa Fortuna, MD, MPH, MDiv, chief of psychiatry and vice chair at Zuckerberg San Francisco General Hospital. A 2017 analysis found that nearly four in five Latino youth face at least one traumatic childhood experience, like poverty or abuse, and that about 29% of Latino youth experience four or more of these traumas.
Immigrants in particular, may have faced trauma in their home country and/or immigration trauma, Dr. Fortuna added. A 2013 study on immigrant Latino adolescents for example, found that 29% of foreign-born adolescents and 34% of foreign-born parents experienced trauma during the migration process (Int Migr Rev. 2013 Dec;47(4):10).
“All of these things are cumulative,” Dr. Fortuna said. “Then when you’re hit with a pandemic, all of the disparities that you already have and all the stress that you already have are compounded. This is for the kids, too, who have been exposed to a lot of stressors and now maybe have family members that have been ill or have died. All of these things definitely put people at risk for increased depression [and] the worsening of any preexisting posttraumatic stress disorder. We’ve seen this in previous disasters, and I expect that’s what we’re going to see more of with the COVID-19 pandemic.”
At the same time, a central cultural value of many Latinos is family unity, Dr. Montano said, a foundation that is now being strained by social distancing and severed connections.
“This has separated many families,” she said. “There has been a lot of loneliness and grief.”
Mistrust and fear toward the government, public agencies, and even the health system itself act as further hurdles for some Latinos in the face of COVID-19. In areas with large immigrant populations such as San Francisco, Dr. Fortuna noted, it’s not uncommon for undocumented patients to avoid accessing medical care and social services, or visiting emergency departments for needed care for fear of drawing attention to themselves or possible detainment.
“The fact that so many people showed up at our hospital so ill and ended up in the ICU – that could be a combination of factors. Because the population has high rates of diabetes and hypertension, that might have put people at increased risk for severe illness,” she said. “But some people may have been holding out for care because they wanted to avoid being in places out of fear of immigration scrutiny.”
Overcoming language barriers
Compounding the challenging pandemic landscape for Latino patients is the fact that many state resources about COVID-19 have not been translated to Spanish, Dr. Colón-Rivera said. He was troubled recently when he went to several state websites and found limited to no information in Spanish about the coronavirus. Some data about COVID-19 from the federal government were not translated to Spanish until officials received pushback, he added. Even now, press releases and other information disseminated by the federal government about the virus appear to be translated by an automated service – and lack sense and context.
The state agencies in Pennsylvania have been alerted to the absence of Spanish information, but change has been slow, he noted.
“In Philadelphia, 23% speaks a language other than English,” he said. “So we missed a lot of critical information that could have helped to avoid spreading the illness and access support.”
Dr. Fortuna said that California has done better with providing COVID-19–related information in Spanish, compared with some other states, but misinformation about the virus and lingering myths have still been a problem among the Latino community. The University of California, San Francisco, recently launched a Latino Task Force resource website for the Latino community that includes information in English, Spanish, and Yucatec Maya about COVID-19, health and wellness tips, and resources for various assistance needs.
The concerning lack of COVID-19 information translated to Spanish led Dr. Montano to start a Facebook page in Spanish about mental health tips and guidance for managing COVID-19–related issues. She and her team of clinicians share information, videos, relaxation exercises, and community resources on the page, among other posts. “There is also general info and recommendations about COVID-19 that I think can be useful for the community,” she said. “The idea is that patients, the general community, and providers can have share information, hope messages, and ask questions in Spanish.”
Feeling ‘helpless’
A central part of caring for Latino patients during the COVID-19 crisis has been referring them to outside agencies and social services, psychiatrists say. But finding the right resources amid a pandemic and ensuring that patients connect with the correct aid has been an uphill battle.
“We sometimes feel like our hands are tied,” Dr. Colón-Rivera said. “Sometimes, we need to call a place to bring food. Some of the state agencies and nonprofits don’t have delivery systems, so the patient has to go pick up for food or medication. Some of our patients don’t want to go outside. Some do not have cars.”
As a clinician, it can be easy to feel helpless when trying to navigate new challenges posed by the pandemic in addition to other longstanding barriers, Dr. Posada said.
“Already, mental health disorders are so influenced by social situations like poverty, job insecurity, or family issues, and now it just seems those obstacles are even more insurmountable,” she said. “At the end of the day, I can feel like: ‘Did I make a difference?’ That’s a big struggle.”
Dr. Montano’s team, which includes psychiatrists, psychologists, and social workers, have come to rely on virtual debriefings to vent, express frustrations, and support one another, she said. She also recently joined a virtual mind-body skills group as a participant.
“I recognize the importance of getting additional support and ways to alleviate burnout,” she said. “We need to take care of ourselves or we won’t be able to help others.”
Focusing on resilience during the current crisis can be beneficial for both patients and providers in coping and drawing strength, Dr. Posada said.
“When it comes to fostering resilience during times of hardship, I think it’s most helpful to reflect on what skills or attributes have helped during past crises and apply those now – whether it’s turning to comfort from close relationships, looking to religion and spirituality, practicing self-care like rest or exercise, or really tapping into one’s purpose and reason for practicing psychiatry and being a physician,” she said. “The same advice goes for clinicians: We’ve all been through hard times in the past, it’s part of the human condition and we’ve also witnessed a lot of suffering in our patients, so now is the time to practice those skills that have gotten us through hard times in the past.”
Learning lessons from COVID-19
Despite the challenges with moving to telehealth, Dr. Fortuna said the tool has proved beneficial overall for mental health care. For Dr. Fortuna’s team for example, telehealth by phone has decreased the no-show rate, compared with clinic visits, and improved care access.
“We need to figure out how to maintain that,” she said. “If we can build ways for equity and access to Internet, especially equipment, I think that’s going to help.”
In addition, more data are needed about the ways in which COVID-19 is affecting Latino patients, Dr. Colón-Rivera said. Mortality statistics have been published, but information is needed about the rates of infection and manifestation of illness.
Most importantly, the COVID-19 crisis has emphasized the critical need to address and improve the underlying inequity issues among Latino patients, psychiatrists say.
“We really need to think about how there can be partnerships, in terms of community-based Latino business and leaders, multisector resources, trying to think about how we can improve conditions both work and safety for Latinos,” Dr. Fortuna said. “How can schools get support in integrating mental health and support for families, especially now after COVID-19? And really looking at some of these underlying inequities that are the underpinnings of why people were at risk for the disproportionate effects of the COVID-19 pandemic.”
Pamela Montano, MD, recalls the recent case of a patient with bipolar II disorder who was improving after treatment with medication and therapy when her life was upended by the COVID-19 pandemic.
The patient, who is Puerto Rican, lost two cousins to the virus, two of her brothers fell ill, and her sister became sick with coronavirus, said Dr. Montano, director of the Latino Bicultural Clinic at Gouverneur Health in New York. The patient was then left to care for her sister’s toddlers along with the patient’s own children, one of whom has special needs.
“After this happened, it increased her anxiety,” Dr. Montano said in an interview. “She’s not sleeping, and she started having panic attacks. My main concern was how to help her cope.”
Across the country, clinicians who treat mental illness and behavioral disorders in Latino patients are facing similar experiences and challenges associated with COVID-19 and the ensuing pandemic response. Current data suggest a disproportionate burden of illness and death from the novel coronavirus among racial and ethnic groups, particularly black and Hispanic patients. The disparities are likely attributable to economic and social conditions more common among such populations, compared with non-Hispanic whites, in addition to isolation from resources, according to the Centers for Disease Control and Prevention.
A recent New York City Department of Health study based on data that were available in late April found that deaths from COVID-19 were substantially higher for black and Hispanic/Latino patients than for white and Asian patients. The death rate per 100,000 population was 209.4 for blacks, 195.3 for Hispanics/Latinos, 107.7 for whites, and 90.8 for Asians.
“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and nonimmigrant,” said Dr. Montano, a board member of the American Society of Hispanic Psychiatry and the officer of infrastructure and advocacy for the Hispanic Caucus of the American Psychiatric Association. “This includes income inequality, poor nutrition, history of trauma and discrimination, employment issues, quality education, access to technology, and overall access to appropriate cultural linguistic health care.”
Navigating challenges
For mental health professionals treating Latino patients, COVID-19 and the pandemic response have generated a range of treatment obstacles.
The transition to telehealth for example, has not been easy for some patients, said Jacqueline Posada, MD, consultation-liaison psychiatry fellow at the Inova Fairfax Hospital–George Washington University program in Falls Church, Va., and an APA Substance Abuse and Mental Health Services Administration minority fellow. Some patients lack Internet services, others forget virtual visits, and some do not have working phones, she said.
“I’ve had to be very flexible,” she said in an interview. “Ideally, I’d love to see everybody via video chat, but a lot of people either don’t have a stable Internet connection or Internet, so I meet the patient where they are. Whatever they have available, that’s what I’m going to use. If they don’t answer on the first call, I will call again at least three to five times in the first 15 minutes to make sure I’m giving them an opportunity to pick up the phone.”
In addition, Dr. Posada has encountered disconnected phones when calling patients for appointments. In such cases, Dr. Posada contacts the patient’s primary care physician to relay medication recommendations in case the patient resurfaces at the clinic.
In other instances, patients are not familiar with video technology, or they must travel to a friend or neighbor’s house to access the technology, said Hector Colón-Rivera, MD, an addiction psychiatrist and medical director of the Asociación Puertorriqueños en Marcha Behavioral Health Program, a nonprofit organization based in the Philadelphia area. Telehealth visits frequently include appearances by children, family members, barking dogs, and other distractions, said Dr. Colón-Rivera, president of the APA Hispanic Caucus.
“We’re seeing things that we didn’t used to see when they came to our office – for good or for bad,” said Dr. Colón-Rivera, an attending telemedicine physician at the University of Pittsburgh Medical Center. “It could be a good chance to meet our patient in a different way. Of course, it creates different stressors. If you have five kids on top of you and you’re the only one at home, it’s hard to do therapy.”
Psychiatrists are also seeing prior health conditions in patients exacerbated by COVID-19 fears and new health problems arising from the current pandemic environment. Dr. Posada recalls a patient whom she successfully treated for premenstrual dysphoric disorder who recently descended into severe clinical depression. The patient, from Colombia, was attending school in the United States on a student visa and supporting herself through child care jobs.
“So much of her depression was based on her social circumstance,” Dr. Posada said. “She had lost her job, her sister had lost her job so they were scraping by on her sister’s husband’s income, and the thing that brought her joy, which was going to school and studying so she could make a different life for herself than what her parents had in Colombia, also seemed like it was out of reach.”
Dr. Colón-Rivera recently received a call from a hospital where one of his patients was admitted after becoming delusional and psychotic. The patient was correctly taking medication prescribed by Dr. Colón-Rivera, but her diabetes had become uncontrolled because she was unable to reach her primary care doctor and couldn’t access the pharmacy. Her blood sugar level became elevated, leading to the delusions.
“A patient that was perfectly stable now is unstable,” he said. “Her diet has not been good enough through the pandemic, exacerbating her diabetes. She was admitted to the hospital for delirium.
Compounding of traumas
For many Latino patients, the adverse impacts of the pandemic comes on top of multiple prior traumas, such as violence exposures, discrimination, and economic issues, said Lisa Fortuna, MD, MPH, MDiv, chief of psychiatry and vice chair at Zuckerberg San Francisco General Hospital. A 2017 analysis found that nearly four in five Latino youth face at least one traumatic childhood experience, like poverty or abuse, and that about 29% of Latino youth experience four or more of these traumas.
Immigrants in particular, may have faced trauma in their home country and/or immigration trauma, Dr. Fortuna added. A 2013 study on immigrant Latino adolescents for example, found that 29% of foreign-born adolescents and 34% of foreign-born parents experienced trauma during the migration process (Int Migr Rev. 2013 Dec;47(4):10).
“All of these things are cumulative,” Dr. Fortuna said. “Then when you’re hit with a pandemic, all of the disparities that you already have and all the stress that you already have are compounded. This is for the kids, too, who have been exposed to a lot of stressors and now maybe have family members that have been ill or have died. All of these things definitely put people at risk for increased depression [and] the worsening of any preexisting posttraumatic stress disorder. We’ve seen this in previous disasters, and I expect that’s what we’re going to see more of with the COVID-19 pandemic.”
At the same time, a central cultural value of many Latinos is family unity, Dr. Montano said, a foundation that is now being strained by social distancing and severed connections.
“This has separated many families,” she said. “There has been a lot of loneliness and grief.”
Mistrust and fear toward the government, public agencies, and even the health system itself act as further hurdles for some Latinos in the face of COVID-19. In areas with large immigrant populations such as San Francisco, Dr. Fortuna noted, it’s not uncommon for undocumented patients to avoid accessing medical care and social services, or visiting emergency departments for needed care for fear of drawing attention to themselves or possible detainment.
“The fact that so many people showed up at our hospital so ill and ended up in the ICU – that could be a combination of factors. Because the population has high rates of diabetes and hypertension, that might have put people at increased risk for severe illness,” she said. “But some people may have been holding out for care because they wanted to avoid being in places out of fear of immigration scrutiny.”
Overcoming language barriers
Compounding the challenging pandemic landscape for Latino patients is the fact that many state resources about COVID-19 have not been translated to Spanish, Dr. Colón-Rivera said. He was troubled recently when he went to several state websites and found limited to no information in Spanish about the coronavirus. Some data about COVID-19 from the federal government were not translated to Spanish until officials received pushback, he added. Even now, press releases and other information disseminated by the federal government about the virus appear to be translated by an automated service – and lack sense and context.
The state agencies in Pennsylvania have been alerted to the absence of Spanish information, but change has been slow, he noted.
“In Philadelphia, 23% speaks a language other than English,” he said. “So we missed a lot of critical information that could have helped to avoid spreading the illness and access support.”
Dr. Fortuna said that California has done better with providing COVID-19–related information in Spanish, compared with some other states, but misinformation about the virus and lingering myths have still been a problem among the Latino community. The University of California, San Francisco, recently launched a Latino Task Force resource website for the Latino community that includes information in English, Spanish, and Yucatec Maya about COVID-19, health and wellness tips, and resources for various assistance needs.
The concerning lack of COVID-19 information translated to Spanish led Dr. Montano to start a Facebook page in Spanish about mental health tips and guidance for managing COVID-19–related issues. She and her team of clinicians share information, videos, relaxation exercises, and community resources on the page, among other posts. “There is also general info and recommendations about COVID-19 that I think can be useful for the community,” she said. “The idea is that patients, the general community, and providers can have share information, hope messages, and ask questions in Spanish.”
Feeling ‘helpless’
A central part of caring for Latino patients during the COVID-19 crisis has been referring them to outside agencies and social services, psychiatrists say. But finding the right resources amid a pandemic and ensuring that patients connect with the correct aid has been an uphill battle.
“We sometimes feel like our hands are tied,” Dr. Colón-Rivera said. “Sometimes, we need to call a place to bring food. Some of the state agencies and nonprofits don’t have delivery systems, so the patient has to go pick up for food or medication. Some of our patients don’t want to go outside. Some do not have cars.”
As a clinician, it can be easy to feel helpless when trying to navigate new challenges posed by the pandemic in addition to other longstanding barriers, Dr. Posada said.
“Already, mental health disorders are so influenced by social situations like poverty, job insecurity, or family issues, and now it just seems those obstacles are even more insurmountable,” she said. “At the end of the day, I can feel like: ‘Did I make a difference?’ That’s a big struggle.”
Dr. Montano’s team, which includes psychiatrists, psychologists, and social workers, have come to rely on virtual debriefings to vent, express frustrations, and support one another, she said. She also recently joined a virtual mind-body skills group as a participant.
“I recognize the importance of getting additional support and ways to alleviate burnout,” she said. “We need to take care of ourselves or we won’t be able to help others.”
Focusing on resilience during the current crisis can be beneficial for both patients and providers in coping and drawing strength, Dr. Posada said.
“When it comes to fostering resilience during times of hardship, I think it’s most helpful to reflect on what skills or attributes have helped during past crises and apply those now – whether it’s turning to comfort from close relationships, looking to religion and spirituality, practicing self-care like rest or exercise, or really tapping into one’s purpose and reason for practicing psychiatry and being a physician,” she said. “The same advice goes for clinicians: We’ve all been through hard times in the past, it’s part of the human condition and we’ve also witnessed a lot of suffering in our patients, so now is the time to practice those skills that have gotten us through hard times in the past.”
Learning lessons from COVID-19
Despite the challenges with moving to telehealth, Dr. Fortuna said the tool has proved beneficial overall for mental health care. For Dr. Fortuna’s team for example, telehealth by phone has decreased the no-show rate, compared with clinic visits, and improved care access.
“We need to figure out how to maintain that,” she said. “If we can build ways for equity and access to Internet, especially equipment, I think that’s going to help.”
In addition, more data are needed about the ways in which COVID-19 is affecting Latino patients, Dr. Colón-Rivera said. Mortality statistics have been published, but information is needed about the rates of infection and manifestation of illness.
Most importantly, the COVID-19 crisis has emphasized the critical need to address and improve the underlying inequity issues among Latino patients, psychiatrists say.
“We really need to think about how there can be partnerships, in terms of community-based Latino business and leaders, multisector resources, trying to think about how we can improve conditions both work and safety for Latinos,” Dr. Fortuna said. “How can schools get support in integrating mental health and support for families, especially now after COVID-19? And really looking at some of these underlying inequities that are the underpinnings of why people were at risk for the disproportionate effects of the COVID-19 pandemic.”
Psoriasis patients with mental illness report lower satisfaction with physicians
according to a retrospective analysis of survey data.
The findings highlight the importance of clinicians being supportive and adaptable in their communication style when interacting with psoriasis patients with mental illness.
“This study aims to evaluate whether an association exists between a patient’s psychological state and the perception of patient-clinician encounters,” wrote Charlotte Read, MBBS, of Imperial College London, and April W. Armstrong, MD, MPH, of the University of Southern California, Los Angeles, in JAMA Dermatology.
The researchers retrospectively analyzed longitudinal data from over 8.8 million U.S. adults (unweighted, 652) with psoriasis who participated in the Medical Expenditure Panel Survey from 2004 to 2017. The nationally representative database includes various clinical information, such as data on patient demographics, health care use, and mental health comorbidities.
The primary outcome, patient satisfaction with their physician, was assessed using a patient-physician communication composite score. Mental health comorbidities were evaluated using standard questionnaires.
The mean age of study patients was 52.1 years (range, 0.7 years), and most were female (54%). In all, 73% of participants had no or mild psychological distress symptoms, and 27% had moderate or severe symptoms.
After analysis, the researchers found that patients with moderate psychological distress symptoms were 2.8 times more likely to report lower satisfaction with their physician than were those with no or mild symptoms (adjusted odds ratio, 2.8; P = .001). They also reported that patients with severe symptoms were more likely to report lower satisfaction (aOR, 2.3; P = .03).
“Patients with moderate or severe depression symptoms were less satisfied with their clinicians, compared with those with no or mild depression symptoms,” they further explained.
Based on the results, the coinvestigators emphasized the importance of bettering the patient experience for those with mental illness given the potential association with improved health outcomes.
“Because depressed patients can be more sensitive to negative communication, the clinician needs to be more conscious about using a positive and supportive communication style,” they recommended.
The authors acknowledged the inadequacy of evaluating clinician performance using patient satisfaction alone. As a result, the findings may not be generalizable to all clinical settings.
The study was funded by the National Psoriasis Foundation. Dr. Armstrong reported financial affiliations with several pharmaceutical companies.
SOURCE: Read C, Armstrong AW. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.1054.
according to a retrospective analysis of survey data.
The findings highlight the importance of clinicians being supportive and adaptable in their communication style when interacting with psoriasis patients with mental illness.
“This study aims to evaluate whether an association exists between a patient’s psychological state and the perception of patient-clinician encounters,” wrote Charlotte Read, MBBS, of Imperial College London, and April W. Armstrong, MD, MPH, of the University of Southern California, Los Angeles, in JAMA Dermatology.
The researchers retrospectively analyzed longitudinal data from over 8.8 million U.S. adults (unweighted, 652) with psoriasis who participated in the Medical Expenditure Panel Survey from 2004 to 2017. The nationally representative database includes various clinical information, such as data on patient demographics, health care use, and mental health comorbidities.
The primary outcome, patient satisfaction with their physician, was assessed using a patient-physician communication composite score. Mental health comorbidities were evaluated using standard questionnaires.
The mean age of study patients was 52.1 years (range, 0.7 years), and most were female (54%). In all, 73% of participants had no or mild psychological distress symptoms, and 27% had moderate or severe symptoms.
After analysis, the researchers found that patients with moderate psychological distress symptoms were 2.8 times more likely to report lower satisfaction with their physician than were those with no or mild symptoms (adjusted odds ratio, 2.8; P = .001). They also reported that patients with severe symptoms were more likely to report lower satisfaction (aOR, 2.3; P = .03).
“Patients with moderate or severe depression symptoms were less satisfied with their clinicians, compared with those with no or mild depression symptoms,” they further explained.
Based on the results, the coinvestigators emphasized the importance of bettering the patient experience for those with mental illness given the potential association with improved health outcomes.
“Because depressed patients can be more sensitive to negative communication, the clinician needs to be more conscious about using a positive and supportive communication style,” they recommended.
The authors acknowledged the inadequacy of evaluating clinician performance using patient satisfaction alone. As a result, the findings may not be generalizable to all clinical settings.
The study was funded by the National Psoriasis Foundation. Dr. Armstrong reported financial affiliations with several pharmaceutical companies.
SOURCE: Read C, Armstrong AW. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.1054.
according to a retrospective analysis of survey data.
The findings highlight the importance of clinicians being supportive and adaptable in their communication style when interacting with psoriasis patients with mental illness.
“This study aims to evaluate whether an association exists between a patient’s psychological state and the perception of patient-clinician encounters,” wrote Charlotte Read, MBBS, of Imperial College London, and April W. Armstrong, MD, MPH, of the University of Southern California, Los Angeles, in JAMA Dermatology.
The researchers retrospectively analyzed longitudinal data from over 8.8 million U.S. adults (unweighted, 652) with psoriasis who participated in the Medical Expenditure Panel Survey from 2004 to 2017. The nationally representative database includes various clinical information, such as data on patient demographics, health care use, and mental health comorbidities.
The primary outcome, patient satisfaction with their physician, was assessed using a patient-physician communication composite score. Mental health comorbidities were evaluated using standard questionnaires.
The mean age of study patients was 52.1 years (range, 0.7 years), and most were female (54%). In all, 73% of participants had no or mild psychological distress symptoms, and 27% had moderate or severe symptoms.
After analysis, the researchers found that patients with moderate psychological distress symptoms were 2.8 times more likely to report lower satisfaction with their physician than were those with no or mild symptoms (adjusted odds ratio, 2.8; P = .001). They also reported that patients with severe symptoms were more likely to report lower satisfaction (aOR, 2.3; P = .03).
“Patients with moderate or severe depression symptoms were less satisfied with their clinicians, compared with those with no or mild depression symptoms,” they further explained.
Based on the results, the coinvestigators emphasized the importance of bettering the patient experience for those with mental illness given the potential association with improved health outcomes.
“Because depressed patients can be more sensitive to negative communication, the clinician needs to be more conscious about using a positive and supportive communication style,” they recommended.
The authors acknowledged the inadequacy of evaluating clinician performance using patient satisfaction alone. As a result, the findings may not be generalizable to all clinical settings.
The study was funded by the National Psoriasis Foundation. Dr. Armstrong reported financial affiliations with several pharmaceutical companies.
SOURCE: Read C, Armstrong AW. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.1054.
FROM JAMA DERMATOLOGY
Even with mild COVID-19, athletes need cardiac testing before returning to play
Potential risks of cardiac injury posed by coronavirus disease 2019 (COVID-19) infection warrant a cautious return-to-play for highly active people and competitive athletes who test positive, according to leading sports cardiologists.
To prevent cardiac injury, athletes should rest for at least 2 weeks after symptoms resolve, then undergo cardiac testing before returning high-level competitive sports, reported lead author Dermot Phelan, MD, PhD, of Atrium Health in Charlotte, N.C., and colleagues.
These recommendations, which were published in JAMA Cardiology, are part of a clinical algorithm that sorts athletes based on coronavirus test status and symptom severity. The algorithm offers a clear timeline for resumption of activity, with management decisions for symptomatic individuals based on additional diagnostics, such as high-sensitivity troponin testing and electrocardiogram.
Despite a scarcity of relevant clinical data, Dr. Phelan said that he and his colleagues wanted to offer their best recommendations to the athletic community, who had been reaching out for help.
“We were getting calls and messages from amateur and professional sporting organizations from around the country asking for guidance about what to do,” Dr. Phelan said. “So a number of us from the American College of Cardiology Sports and Exercise Council decided that we really should provide some guidance even in the absence of good, strong data, for what we feel is a reasonable approach.”
The recommendations were based on what is known of other viral infections, as well as risks posed by COVID-19 that may be worsened by athletic activity.
“We know that, when people have an active infection, vigorous exercise can lower immunity, and that can make the infection worse,” Dr. Phelan said. “That really applies very strongly in people who have had myocarditis. If you exercise when you have myocarditis, it actually increases viral replication and results in increased necrosis of the heart muscle. We really want to avoid exercising during that active infection phase.”
Myocarditis is one of the top causes of sudden cardiac death among young athletes, Dr. Phelan said, “so that’s a major concern for us.”
According to Dr. Phelan, existing data suggest a wide range of incidence of 7%-33% for cardiac injury among patients hospitalized for COVID-19. Even the low end of this range, at 7%, is significantly higher than the incidence rate of 1% found in patients with non–COVID-19 acute viral infections.
“This particular virus appears to cause more cardiac insults than other viruses,” Dr. Phelan said.
The incidence of cardiac injury among nonhospitalized patients remains unknown, leaving a wide knowledge gap that shaped the conservative nature of the present recommendations.
With more information, however, the guidance may “change dramatically,” Dr. Phelan said.
“If the data come back and show that no nonhospitalized patients got cardiac injury, then we would be much more comfortable allowing return to play without the need for cardiac testing,” he said.
Conversely, if cardiac injury is more common than anticipated, then more extensive testing may be needed, he added.
As the algorithm stands, high-sensitivity troponin testing and/or cardiac studies are recommended for all symptomatic athletes; if troponin levels are greater than the 99th percentile or a cardiac study is abnormal, then clinicians should follow return-to-play guidelines for myocarditis. For athletes with normal tests, slow resumption of activity is recommended, including close monitoring for clinical deterioration.
As Dr. Phelan discussed these recommendations in a broader context, he emphasized the need for caution, both preventively, and for cardiologists working with recovering athletes.
“For the early stage of this reentry into normal life while this is still an active pandemic, we need to be cautious,” Dr. Phelan said. “We need to follow the regular CDC guidelines, in terms of social distancing and handwashing, but we also need to consider that those people who have suffered from COVID-19 may have had cardiac injury. We don’t know that yet. But we need to be cautious with these individuals and test them before they return to high-level competitive sports.”
One author disclosed a relationship with the Atlanta Falcons.
SOURCE: Phelan D et al. JAMA Cardiology. 2020 Apr 13. doi: 10.1001/jamacardio.2020.2136.
Potential risks of cardiac injury posed by coronavirus disease 2019 (COVID-19) infection warrant a cautious return-to-play for highly active people and competitive athletes who test positive, according to leading sports cardiologists.
To prevent cardiac injury, athletes should rest for at least 2 weeks after symptoms resolve, then undergo cardiac testing before returning high-level competitive sports, reported lead author Dermot Phelan, MD, PhD, of Atrium Health in Charlotte, N.C., and colleagues.
These recommendations, which were published in JAMA Cardiology, are part of a clinical algorithm that sorts athletes based on coronavirus test status and symptom severity. The algorithm offers a clear timeline for resumption of activity, with management decisions for symptomatic individuals based on additional diagnostics, such as high-sensitivity troponin testing and electrocardiogram.
Despite a scarcity of relevant clinical data, Dr. Phelan said that he and his colleagues wanted to offer their best recommendations to the athletic community, who had been reaching out for help.
“We were getting calls and messages from amateur and professional sporting organizations from around the country asking for guidance about what to do,” Dr. Phelan said. “So a number of us from the American College of Cardiology Sports and Exercise Council decided that we really should provide some guidance even in the absence of good, strong data, for what we feel is a reasonable approach.”
The recommendations were based on what is known of other viral infections, as well as risks posed by COVID-19 that may be worsened by athletic activity.
“We know that, when people have an active infection, vigorous exercise can lower immunity, and that can make the infection worse,” Dr. Phelan said. “That really applies very strongly in people who have had myocarditis. If you exercise when you have myocarditis, it actually increases viral replication and results in increased necrosis of the heart muscle. We really want to avoid exercising during that active infection phase.”
Myocarditis is one of the top causes of sudden cardiac death among young athletes, Dr. Phelan said, “so that’s a major concern for us.”
According to Dr. Phelan, existing data suggest a wide range of incidence of 7%-33% for cardiac injury among patients hospitalized for COVID-19. Even the low end of this range, at 7%, is significantly higher than the incidence rate of 1% found in patients with non–COVID-19 acute viral infections.
“This particular virus appears to cause more cardiac insults than other viruses,” Dr. Phelan said.
The incidence of cardiac injury among nonhospitalized patients remains unknown, leaving a wide knowledge gap that shaped the conservative nature of the present recommendations.
With more information, however, the guidance may “change dramatically,” Dr. Phelan said.
“If the data come back and show that no nonhospitalized patients got cardiac injury, then we would be much more comfortable allowing return to play without the need for cardiac testing,” he said.
Conversely, if cardiac injury is more common than anticipated, then more extensive testing may be needed, he added.
As the algorithm stands, high-sensitivity troponin testing and/or cardiac studies are recommended for all symptomatic athletes; if troponin levels are greater than the 99th percentile or a cardiac study is abnormal, then clinicians should follow return-to-play guidelines for myocarditis. For athletes with normal tests, slow resumption of activity is recommended, including close monitoring for clinical deterioration.
As Dr. Phelan discussed these recommendations in a broader context, he emphasized the need for caution, both preventively, and for cardiologists working with recovering athletes.
“For the early stage of this reentry into normal life while this is still an active pandemic, we need to be cautious,” Dr. Phelan said. “We need to follow the regular CDC guidelines, in terms of social distancing and handwashing, but we also need to consider that those people who have suffered from COVID-19 may have had cardiac injury. We don’t know that yet. But we need to be cautious with these individuals and test them before they return to high-level competitive sports.”
One author disclosed a relationship with the Atlanta Falcons.
SOURCE: Phelan D et al. JAMA Cardiology. 2020 Apr 13. doi: 10.1001/jamacardio.2020.2136.
Potential risks of cardiac injury posed by coronavirus disease 2019 (COVID-19) infection warrant a cautious return-to-play for highly active people and competitive athletes who test positive, according to leading sports cardiologists.
To prevent cardiac injury, athletes should rest for at least 2 weeks after symptoms resolve, then undergo cardiac testing before returning high-level competitive sports, reported lead author Dermot Phelan, MD, PhD, of Atrium Health in Charlotte, N.C., and colleagues.
These recommendations, which were published in JAMA Cardiology, are part of a clinical algorithm that sorts athletes based on coronavirus test status and symptom severity. The algorithm offers a clear timeline for resumption of activity, with management decisions for symptomatic individuals based on additional diagnostics, such as high-sensitivity troponin testing and electrocardiogram.
Despite a scarcity of relevant clinical data, Dr. Phelan said that he and his colleagues wanted to offer their best recommendations to the athletic community, who had been reaching out for help.
“We were getting calls and messages from amateur and professional sporting organizations from around the country asking for guidance about what to do,” Dr. Phelan said. “So a number of us from the American College of Cardiology Sports and Exercise Council decided that we really should provide some guidance even in the absence of good, strong data, for what we feel is a reasonable approach.”
The recommendations were based on what is known of other viral infections, as well as risks posed by COVID-19 that may be worsened by athletic activity.
“We know that, when people have an active infection, vigorous exercise can lower immunity, and that can make the infection worse,” Dr. Phelan said. “That really applies very strongly in people who have had myocarditis. If you exercise when you have myocarditis, it actually increases viral replication and results in increased necrosis of the heart muscle. We really want to avoid exercising during that active infection phase.”
Myocarditis is one of the top causes of sudden cardiac death among young athletes, Dr. Phelan said, “so that’s a major concern for us.”
According to Dr. Phelan, existing data suggest a wide range of incidence of 7%-33% for cardiac injury among patients hospitalized for COVID-19. Even the low end of this range, at 7%, is significantly higher than the incidence rate of 1% found in patients with non–COVID-19 acute viral infections.
“This particular virus appears to cause more cardiac insults than other viruses,” Dr. Phelan said.
The incidence of cardiac injury among nonhospitalized patients remains unknown, leaving a wide knowledge gap that shaped the conservative nature of the present recommendations.
With more information, however, the guidance may “change dramatically,” Dr. Phelan said.
“If the data come back and show that no nonhospitalized patients got cardiac injury, then we would be much more comfortable allowing return to play without the need for cardiac testing,” he said.
Conversely, if cardiac injury is more common than anticipated, then more extensive testing may be needed, he added.
As the algorithm stands, high-sensitivity troponin testing and/or cardiac studies are recommended for all symptomatic athletes; if troponin levels are greater than the 99th percentile or a cardiac study is abnormal, then clinicians should follow return-to-play guidelines for myocarditis. For athletes with normal tests, slow resumption of activity is recommended, including close monitoring for clinical deterioration.
As Dr. Phelan discussed these recommendations in a broader context, he emphasized the need for caution, both preventively, and for cardiologists working with recovering athletes.
“For the early stage of this reentry into normal life while this is still an active pandemic, we need to be cautious,” Dr. Phelan said. “We need to follow the regular CDC guidelines, in terms of social distancing and handwashing, but we also need to consider that those people who have suffered from COVID-19 may have had cardiac injury. We don’t know that yet. But we need to be cautious with these individuals and test them before they return to high-level competitive sports.”
One author disclosed a relationship with the Atlanta Falcons.
SOURCE: Phelan D et al. JAMA Cardiology. 2020 Apr 13. doi: 10.1001/jamacardio.2020.2136.
FROM JAMA CARDIOLOGY
Letters: Disappointment in the Wrapping
To the Editor: I am very disappointed to continue receiving my copies of the Federal Practitioner in nonrecyclable plastic. As a health care professional, I am on the mailing list for numerous other medical publications, all of which seem to be able to utilize recyclable plastic wrappers. I hope you can rectify this problem, which is an embarrassment to me, a serviceconnected veteran.
C. William Kaiser, MD
To the Editor: I am very disappointed to continue receiving my copies of the Federal Practitioner in nonrecyclable plastic. As a health care professional, I am on the mailing list for numerous other medical publications, all of which seem to be able to utilize recyclable plastic wrappers. I hope you can rectify this problem, which is an embarrassment to me, a serviceconnected veteran.
C. William Kaiser, MD
To the Editor: I am very disappointed to continue receiving my copies of the Federal Practitioner in nonrecyclable plastic. As a health care professional, I am on the mailing list for numerous other medical publications, all of which seem to be able to utilize recyclable plastic wrappers. I hope you can rectify this problem, which is an embarrassment to me, a serviceconnected veteran.
C. William Kaiser, MD
Presenting the 2020 SHM Award of Excellence winners
Outstanding Service in Hospital Medicine
Efren Manjarrez, MD, SFHM, FACP, is an associate professor of clinical medicine at the University of Miami Miller School of Medicine, where he also serves as a hospitalist in the division of hospital medicine. His high-impact work at his home institution and through SHM has been extensive.
He founded the division of hospital medicine at the University of Miami in 2000 and later served as the division chief and patient safety officer. Dr. Manjarrez served in the prestigious role of course director for HM15 and as co-course director for the Adult Hospital Medicine Boot Camp.
One of his most enduring contributions is as an author of the white paper on hospitalist handoffs, published in the Journal of Hospital Medicine in 2009, which continues to be cited and validated. He was an assistant editor for the Journal of Hospital Medicine and continues to review articles for JHM. Dr. Manjarrez is also a senior fellow in hospital medicine.
Excellence in Research
Shoshana J. Herzig, MD, MPH, is the director of hospital medicine research at Beth Israel Deaconess Medical Center in Boston, where she also serves as a hospitalist. She is also an associate professor of medicine at Harvard Medical School, also in Boston.
She has published nearly 50 original peer-reviewed manuscripts in some of medicine’s top journals. Her impressive research, which primarily focuses on patterns of medication utilization and associated outcomes in hospitalized adults, has been cited more than 1,500 times in the medical literature.
In addition to her work on medication safety, she is also a site PI for the Hospital Medicine Research Network (HOMERuN), a nationwide collaborative of hospital medicine researchers.
Dr. Herzig has been a member of SHM since 2008 and has attended the annual conference every year since. She has served as an RIV abstract judge, was instrumental in developing SHM’s consensus statement on safe opioid prescribing, and has served as an editor for the Journal of Hospital Medicine since 2012 and has been a senior deputy editor since 2015.
Clinical Leadership for Physicians
Karen Smith, MD, MEd, SFHM, is the chief of the division of hospitalist medicine and past president of the medical staff at Children’s National Medical Center in Washington. She also serves as associate professor of pediatrics at the George Washington University School of Medicine. She has consistently worked to create a supportive environment in which to promote wellness among her staff and colleagues.
She was one of three founding faculty members of the division of hospital medicine at Children’s National, and under her leadership, the division has seen substantial growth. It has evolved from a single site to a comprehensive model of services, spanning six community hospitals and a specialty hospital for rehabilitation and subacute care.
To increase morale, Dr. Smith spearheaded the development of a virtual physician lounge. She reserved a conference room once a month and provided free lunch to medical staff members of different specialties. Its success led to the construction of a full-time lounge – all because of Dr. Smith’s perseverance and forward thinking.
She is a past member of SHM’s Pediatrics Committee and Hospital Quality and Patient Safety Committee and is a senior fellow in hospital medicine.
Excellence in Teaching
Kathleen M. Finn, MD, M.Phil, SFHM, is the senior associate program director for resident and faculty development in the Massachusetts General Hospital internal medicine residency program at Harvard Medical School, both in Boston, where she also is an assistant professor of medicine. She has excelled at teaching at all levels and in all kinds of settings, from clinical teaching on inpatient rounds, educating faculty through workshops to serving as course director for Hospital Medicine 2018 in Orlando. She constantly strives to think creatively and to teach in new ways and considers her career to be a synergy of all three domains in medical education: clinical teaching, leadership, and research.
Her interest in improving the art of inpatient teaching has also taken Dr. Finn into the medical education research space, where she has conducted and published several significant studies.
She was the codirector of the Boston chapter of SHM for 18 years and is well known for her dedication to SHM’s annual conference. She gained a reputation on the Annual Conference Committee for coming up with creative topics, including the Great Debate series.
Dr. Finn has previously served on the editorial board for the Journal of Hospital Medicine, where she continues to be a reviewer. She is a senior fellow in hospital medicine.
Excellence in Teaching
Juan Nicolás Lessing, MD, is an assistant professor of medicine within the division of hospital medicine at the Medical School at the University of Colorado at Denver, Aurora. He has dedicated himself to the teaching and study of clinical reasoning processes and has cocreated a resident clinical reasoning curriculum, which has been expended to all residency classes.
Dr. Lessing’s dedication to mentorship has been extraordinary. In fewer than 5 years, he has mentored more than 50 learners, resulting in 54 competitive abstracts, posters, and presentations. He has led more than 24 workshops and consistently sponsors junior colleagues to join him. In summary, he teaches learners how to learn rather than what to learn. Additionally, Dr. Lessing created and facilitated several impactful department-wide sessions on how we can learn from our mistakes to openly discuss missed diagnoses. He served as a co-PI on the LOOP study, a multicenter endeavor to provide real-time feedback to admitting residents on a patient’s clinical course, which was published in the Journal of Hospital Medicine.
Dr. Lessing has been actively involved with SHM since medical school, is a graduate of SHM’s Academic Hospitalist Academy, and serves on the executive board for the Rocky Mountain chapter of SHM.
Clinical Leadership for NPs/PAs
Ilaria Gadalla, DMSc, PA-C, is a hospitalist at Treasure Coast Hospitalists in Port St. Lucia, Fla., and also serves as the physician assistant department chair/program director at South University, where she supervises more than 40 PAs, medical directors, and administrative staff.
She continuously drives innovative projects for NPs and PAs to demonstrate excellence in collaboration by working closely with C-suite administration to expand QI (quality improvement) and education efforts. A prime example is the optimal communication system that she developed within her first week as a hospitalist in the Port St. Lucie area. Nursing, ED, and pharmacy staff had difficulty contacting hospitalists since the EMR would not reflect the assigned hospitalist, so she developed a simple contact sheet that included the hospitalist team each day. This method is still in use today.
Ms. Gadalla is the chair of SHM’s NP/PA special interest group who was integral in drafting the recent white paper on NP/PA integration and optimization.
Excellence in Humanitarian Services
Khaalisha Ajala, MD, MBA, is a hospitalist and associate site director for education at Grady Memorial Hospital in Atlanta. She cares for patients of diverse backgrounds directly and also has a deep-seeded passion for public health and patient education, always demonstrating how to bring this passion to trainee education.
Using her knowledge as an MBA, Dr. Ajala has designed, developed, and now maintains her own nonprofit agency, Heart Beats & Hip-Hop. Through this organization, she has hosted public health fairs to conduct health screenings in less-traditional local settings, where community members who may not have access to care can gain exposure to a health care provider.
More broadly, in the last year, she has made two journeys – one to Thailand and another to Ethiopia – to work with Emory trainees in educational and clinical efforts to help them engage the global community in health improvement. In Thailand, she taught students how to care for patients at risk for trafficking and sexual exploitation. While in Ethiopia, she served as an educator and clinical preceptor to Emory residents in the global health pathway, teaching them to care for high-risk patients at a local hospital.
With her active and unrelenting humanitarian efforts in mind, she was also chosen as a member of the executive council for SHM’s Care for Vulnerable Populations special interest group.
Diversity Leadership
Kimberly D. Manning, MD, FACP, FAAP, is a professor of medicine and the associate vice chair of diversity, equity and inclusion at the Emory University School of Medicine in Atlanta, where she also is a hospitalist at Grady Memorial Hospital. She demonstrates a strong passion for building and strengthening diverse clinical learning environments. This inspired her to promote cultural competency via lectures, curriculum development, and more.
Dr. Manning has designed a new educational modality – Bite-Sized Teaching (abbreviated “BST” and read as “BEAST”-Mode Teaching). This engages trainees as the teachers of their peers. As part of those sessions, Dr. Manning intentionally encourages and engages trainees from all backgrounds, including women, minorities, and trainees with varied ethnic and cultural perspectives.
Her leadership on the Emory Task Force on Diversity, Equity and Inclusion led her to be named the department of medicine’s first associate vice chair of diversity, equity and inclusion. Due in large part to her engagement, the medical school just admitted its largest class of underrepresented minorities, nearly doubling numbers from prior years.
She has received the 2018 AGCME Parker J. Palmer Courage to Teach Award and the 2019 Lifetime Achievement Award by the Association of Black Women Physicians.
Leadership for Practice Manager
Douglas G. Philpot, MHA, MBA, MHR, FACHE, currently the hospitalist program director at Intermountain Healthcare in Salt Lake City, epitomizes excellence in practice management.
In mid-2018, Intermountain Healthcare transitioned to a new organizational structure that brought all medical and surgical operations under one leadership team. Prior to this reorganization, hospitalist groups were largely divided by the geographies they served, each operating independently.
After the reorganization, it was apparent that staffing structures among groups varied greatly. Dr. Philpot pored over the workload and billing data and determined the most efficient use of how to staff hospitalist providers. He recently created a program that allows all stakeholders to meet and discuss in an unbiased manner how and when to add resources to a given group. As a result, the team is better able to make smart decisions that translate into improved quality, better patient experience, a more engaged hospitalist group and improved financial decisions. This is a model that Intermountain is now looking to apply to other specialties.
Team Award in Quality Improvement
The Michigan Hospital Medicine Safety Consortium has been in place for a decade and has worked together to improve quality and safety for patients across Michigan and the nation. It has been led since its inception by Scott Flanders, MD, a hospitalist at the University of Michigan, Ann Arbor.
At each participating hospital, teams include hospitalists, infectious disease clinicians, interventional radiologists, nephrologists, nurses, pharmacists, administrators, and more. This integration ensures that the team’s work is highly relevant and generalizable for hospitals around the country.
Their initiatives have informed regulatory and guideline writing authorities in the United States and beyond. For example, findings from their venous thromboembolism project demonstrated that the majority of hospitalized patients do not benefit from VTE prophylaxis, but rather, targeted strategies to define those at high risk. In 2016, their work helped to prevent 852 VTEs in Michigan alone. This led to changes in national guidelines that now emphasize deimplementing pharmacologic VTE prophylaxis and focused risk-assessment in U.S. hospitals.
Their antimicrobial use initiative has led to a robust partnership between hospitalists, hospitals, and national partners, such as the Centers for Disease Control and Prevention. Early work has informed a key gap in stewardship – discharge antibiotic prescribing – which has been a focus for SHM, the CDC, and many others. Efforts have already led to a reduction in thousands of unnecessary antibiotic prescriptions in Michigan.
Junior Investigator Award
SHM’s Research Committee presents the Junior Investigator Award to recognize early-career hospitalist researchers who are leading the way in their field. We are pleased to present the HM20 Junior Investigator Award to Valerie Vaughn, MD, MSc.
Dr. Vaughn is an assistant professor and research scientist in the division of hospital medicine at the University of Michigan and Veterans Affairs Ann Arbor Healthcare System.
Her research is focused on engaging hospitalists in antibiotic prescribing, especially at discharge. She is the hospitalist lead for an initiative to improve antibiotic prescribing in 46 hospitals across Michigan. She has already made a national contribution to the field – two manuscripts that have received high praise and have been cited by the Joint Commission and the CDC in their updated recommendations for antibiotic stewardship. She has a grant from the Gordon and Betty Moore Foundation to study the role of diagnostic error in antibiotic overuse and just received a K08 career development award from the Agency for Healthcare Research and Quality to study methods to improve antibiotic prescribing at hospital discharge.
One of Dr. Vaughn’s career goals is to advance hospital medicine through mentoring the next generation of hospitalists. In 2017, she authored a manuscript titled “Mentee Missteps” in JAMA, which has been viewed nearly 40,000 times since publication. She continues to give talks on this topic and mentors clinical hospitalists on research projects to improve quality and safety.
Dr. Vaughn has worked closely with SHM and represents the society at the CDC’s Healthcare Infection Control Practices Advisory Committee quarterly meetings.
Certificate of Leadership in Hospital Medicine
The Certificate of Leadership in Hospital Medicine (CLHM) cultivates leadership skills in the context of specific hospital medicine challenges. This designation informs employers – or potential employers – with confidence that a candidate is equipped and ready to lead teams and grow an organization.
Charmaine Lewis, MD, MPH, FHM, CLHM, is the quality director for New Hanover Hospitalists in Wilmington, N.C., a role she has held for 7 years. She is also clinical assistant professor, department of medicine, University of North Carolina School of Medicine, Chapel Hill, serving as a mentor for internal medicine, surgery, and obstetrics residents completing projects in quality improvement.
While sitting on the CHF and readmissions committees at her institution, Dr. Lewis was asked why patients with heart failure came back to the hospital. This question launched an in-depth search for real-time and accurate data on heart failure patients in her institution. She worked with the Heart Failure Steering Committee to develop a process to close care gaps and document compliance to the ACC/AHA Get with the Guidelines: Heart Failure recommendations. She facilitated order set revisions, smart-phrase documentation in EPIC, and scripted bedside interdisciplinary rounding to facilitate compliance prior to patient discharge. She also created an end-user friendly dashboard to report compliance with medical leaders, and eventually this project was selected by the department of medicine as their annual quality goal. The project has led to the improvement of CHF GWTG Composite Bundle compliance from 76% to 93%, and compliance with use of aldosterone antagonists from 22% to 85%.
Outstanding Service in Hospital Medicine
Efren Manjarrez, MD, SFHM, FACP, is an associate professor of clinical medicine at the University of Miami Miller School of Medicine, where he also serves as a hospitalist in the division of hospital medicine. His high-impact work at his home institution and through SHM has been extensive.
He founded the division of hospital medicine at the University of Miami in 2000 and later served as the division chief and patient safety officer. Dr. Manjarrez served in the prestigious role of course director for HM15 and as co-course director for the Adult Hospital Medicine Boot Camp.
One of his most enduring contributions is as an author of the white paper on hospitalist handoffs, published in the Journal of Hospital Medicine in 2009, which continues to be cited and validated. He was an assistant editor for the Journal of Hospital Medicine and continues to review articles for JHM. Dr. Manjarrez is also a senior fellow in hospital medicine.
Excellence in Research
Shoshana J. Herzig, MD, MPH, is the director of hospital medicine research at Beth Israel Deaconess Medical Center in Boston, where she also serves as a hospitalist. She is also an associate professor of medicine at Harvard Medical School, also in Boston.
She has published nearly 50 original peer-reviewed manuscripts in some of medicine’s top journals. Her impressive research, which primarily focuses on patterns of medication utilization and associated outcomes in hospitalized adults, has been cited more than 1,500 times in the medical literature.
In addition to her work on medication safety, she is also a site PI for the Hospital Medicine Research Network (HOMERuN), a nationwide collaborative of hospital medicine researchers.
Dr. Herzig has been a member of SHM since 2008 and has attended the annual conference every year since. She has served as an RIV abstract judge, was instrumental in developing SHM’s consensus statement on safe opioid prescribing, and has served as an editor for the Journal of Hospital Medicine since 2012 and has been a senior deputy editor since 2015.
Clinical Leadership for Physicians
Karen Smith, MD, MEd, SFHM, is the chief of the division of hospitalist medicine and past president of the medical staff at Children’s National Medical Center in Washington. She also serves as associate professor of pediatrics at the George Washington University School of Medicine. She has consistently worked to create a supportive environment in which to promote wellness among her staff and colleagues.
She was one of three founding faculty members of the division of hospital medicine at Children’s National, and under her leadership, the division has seen substantial growth. It has evolved from a single site to a comprehensive model of services, spanning six community hospitals and a specialty hospital for rehabilitation and subacute care.
To increase morale, Dr. Smith spearheaded the development of a virtual physician lounge. She reserved a conference room once a month and provided free lunch to medical staff members of different specialties. Its success led to the construction of a full-time lounge – all because of Dr. Smith’s perseverance and forward thinking.
She is a past member of SHM’s Pediatrics Committee and Hospital Quality and Patient Safety Committee and is a senior fellow in hospital medicine.
Excellence in Teaching
Kathleen M. Finn, MD, M.Phil, SFHM, is the senior associate program director for resident and faculty development in the Massachusetts General Hospital internal medicine residency program at Harvard Medical School, both in Boston, where she also is an assistant professor of medicine. She has excelled at teaching at all levels and in all kinds of settings, from clinical teaching on inpatient rounds, educating faculty through workshops to serving as course director for Hospital Medicine 2018 in Orlando. She constantly strives to think creatively and to teach in new ways and considers her career to be a synergy of all three domains in medical education: clinical teaching, leadership, and research.
Her interest in improving the art of inpatient teaching has also taken Dr. Finn into the medical education research space, where she has conducted and published several significant studies.
She was the codirector of the Boston chapter of SHM for 18 years and is well known for her dedication to SHM’s annual conference. She gained a reputation on the Annual Conference Committee for coming up with creative topics, including the Great Debate series.
Dr. Finn has previously served on the editorial board for the Journal of Hospital Medicine, where she continues to be a reviewer. She is a senior fellow in hospital medicine.
Excellence in Teaching
Juan Nicolás Lessing, MD, is an assistant professor of medicine within the division of hospital medicine at the Medical School at the University of Colorado at Denver, Aurora. He has dedicated himself to the teaching and study of clinical reasoning processes and has cocreated a resident clinical reasoning curriculum, which has been expended to all residency classes.
Dr. Lessing’s dedication to mentorship has been extraordinary. In fewer than 5 years, he has mentored more than 50 learners, resulting in 54 competitive abstracts, posters, and presentations. He has led more than 24 workshops and consistently sponsors junior colleagues to join him. In summary, he teaches learners how to learn rather than what to learn. Additionally, Dr. Lessing created and facilitated several impactful department-wide sessions on how we can learn from our mistakes to openly discuss missed diagnoses. He served as a co-PI on the LOOP study, a multicenter endeavor to provide real-time feedback to admitting residents on a patient’s clinical course, which was published in the Journal of Hospital Medicine.
Dr. Lessing has been actively involved with SHM since medical school, is a graduate of SHM’s Academic Hospitalist Academy, and serves on the executive board for the Rocky Mountain chapter of SHM.
Clinical Leadership for NPs/PAs
Ilaria Gadalla, DMSc, PA-C, is a hospitalist at Treasure Coast Hospitalists in Port St. Lucia, Fla., and also serves as the physician assistant department chair/program director at South University, where she supervises more than 40 PAs, medical directors, and administrative staff.
She continuously drives innovative projects for NPs and PAs to demonstrate excellence in collaboration by working closely with C-suite administration to expand QI (quality improvement) and education efforts. A prime example is the optimal communication system that she developed within her first week as a hospitalist in the Port St. Lucie area. Nursing, ED, and pharmacy staff had difficulty contacting hospitalists since the EMR would not reflect the assigned hospitalist, so she developed a simple contact sheet that included the hospitalist team each day. This method is still in use today.
Ms. Gadalla is the chair of SHM’s NP/PA special interest group who was integral in drafting the recent white paper on NP/PA integration and optimization.
Excellence in Humanitarian Services
Khaalisha Ajala, MD, MBA, is a hospitalist and associate site director for education at Grady Memorial Hospital in Atlanta. She cares for patients of diverse backgrounds directly and also has a deep-seeded passion for public health and patient education, always demonstrating how to bring this passion to trainee education.
Using her knowledge as an MBA, Dr. Ajala has designed, developed, and now maintains her own nonprofit agency, Heart Beats & Hip-Hop. Through this organization, she has hosted public health fairs to conduct health screenings in less-traditional local settings, where community members who may not have access to care can gain exposure to a health care provider.
More broadly, in the last year, she has made two journeys – one to Thailand and another to Ethiopia – to work with Emory trainees in educational and clinical efforts to help them engage the global community in health improvement. In Thailand, she taught students how to care for patients at risk for trafficking and sexual exploitation. While in Ethiopia, she served as an educator and clinical preceptor to Emory residents in the global health pathway, teaching them to care for high-risk patients at a local hospital.
With her active and unrelenting humanitarian efforts in mind, she was also chosen as a member of the executive council for SHM’s Care for Vulnerable Populations special interest group.
Diversity Leadership
Kimberly D. Manning, MD, FACP, FAAP, is a professor of medicine and the associate vice chair of diversity, equity and inclusion at the Emory University School of Medicine in Atlanta, where she also is a hospitalist at Grady Memorial Hospital. She demonstrates a strong passion for building and strengthening diverse clinical learning environments. This inspired her to promote cultural competency via lectures, curriculum development, and more.
Dr. Manning has designed a new educational modality – Bite-Sized Teaching (abbreviated “BST” and read as “BEAST”-Mode Teaching). This engages trainees as the teachers of their peers. As part of those sessions, Dr. Manning intentionally encourages and engages trainees from all backgrounds, including women, minorities, and trainees with varied ethnic and cultural perspectives.
Her leadership on the Emory Task Force on Diversity, Equity and Inclusion led her to be named the department of medicine’s first associate vice chair of diversity, equity and inclusion. Due in large part to her engagement, the medical school just admitted its largest class of underrepresented minorities, nearly doubling numbers from prior years.
She has received the 2018 AGCME Parker J. Palmer Courage to Teach Award and the 2019 Lifetime Achievement Award by the Association of Black Women Physicians.
Leadership for Practice Manager
Douglas G. Philpot, MHA, MBA, MHR, FACHE, currently the hospitalist program director at Intermountain Healthcare in Salt Lake City, epitomizes excellence in practice management.
In mid-2018, Intermountain Healthcare transitioned to a new organizational structure that brought all medical and surgical operations under one leadership team. Prior to this reorganization, hospitalist groups were largely divided by the geographies they served, each operating independently.
After the reorganization, it was apparent that staffing structures among groups varied greatly. Dr. Philpot pored over the workload and billing data and determined the most efficient use of how to staff hospitalist providers. He recently created a program that allows all stakeholders to meet and discuss in an unbiased manner how and when to add resources to a given group. As a result, the team is better able to make smart decisions that translate into improved quality, better patient experience, a more engaged hospitalist group and improved financial decisions. This is a model that Intermountain is now looking to apply to other specialties.
Team Award in Quality Improvement
The Michigan Hospital Medicine Safety Consortium has been in place for a decade and has worked together to improve quality and safety for patients across Michigan and the nation. It has been led since its inception by Scott Flanders, MD, a hospitalist at the University of Michigan, Ann Arbor.
At each participating hospital, teams include hospitalists, infectious disease clinicians, interventional radiologists, nephrologists, nurses, pharmacists, administrators, and more. This integration ensures that the team’s work is highly relevant and generalizable for hospitals around the country.
Their initiatives have informed regulatory and guideline writing authorities in the United States and beyond. For example, findings from their venous thromboembolism project demonstrated that the majority of hospitalized patients do not benefit from VTE prophylaxis, but rather, targeted strategies to define those at high risk. In 2016, their work helped to prevent 852 VTEs in Michigan alone. This led to changes in national guidelines that now emphasize deimplementing pharmacologic VTE prophylaxis and focused risk-assessment in U.S. hospitals.
Their antimicrobial use initiative has led to a robust partnership between hospitalists, hospitals, and national partners, such as the Centers for Disease Control and Prevention. Early work has informed a key gap in stewardship – discharge antibiotic prescribing – which has been a focus for SHM, the CDC, and many others. Efforts have already led to a reduction in thousands of unnecessary antibiotic prescriptions in Michigan.
Junior Investigator Award
SHM’s Research Committee presents the Junior Investigator Award to recognize early-career hospitalist researchers who are leading the way in their field. We are pleased to present the HM20 Junior Investigator Award to Valerie Vaughn, MD, MSc.
Dr. Vaughn is an assistant professor and research scientist in the division of hospital medicine at the University of Michigan and Veterans Affairs Ann Arbor Healthcare System.
Her research is focused on engaging hospitalists in antibiotic prescribing, especially at discharge. She is the hospitalist lead for an initiative to improve antibiotic prescribing in 46 hospitals across Michigan. She has already made a national contribution to the field – two manuscripts that have received high praise and have been cited by the Joint Commission and the CDC in their updated recommendations for antibiotic stewardship. She has a grant from the Gordon and Betty Moore Foundation to study the role of diagnostic error in antibiotic overuse and just received a K08 career development award from the Agency for Healthcare Research and Quality to study methods to improve antibiotic prescribing at hospital discharge.
One of Dr. Vaughn’s career goals is to advance hospital medicine through mentoring the next generation of hospitalists. In 2017, she authored a manuscript titled “Mentee Missteps” in JAMA, which has been viewed nearly 40,000 times since publication. She continues to give talks on this topic and mentors clinical hospitalists on research projects to improve quality and safety.
Dr. Vaughn has worked closely with SHM and represents the society at the CDC’s Healthcare Infection Control Practices Advisory Committee quarterly meetings.
Certificate of Leadership in Hospital Medicine
The Certificate of Leadership in Hospital Medicine (CLHM) cultivates leadership skills in the context of specific hospital medicine challenges. This designation informs employers – or potential employers – with confidence that a candidate is equipped and ready to lead teams and grow an organization.
Charmaine Lewis, MD, MPH, FHM, CLHM, is the quality director for New Hanover Hospitalists in Wilmington, N.C., a role she has held for 7 years. She is also clinical assistant professor, department of medicine, University of North Carolina School of Medicine, Chapel Hill, serving as a mentor for internal medicine, surgery, and obstetrics residents completing projects in quality improvement.
While sitting on the CHF and readmissions committees at her institution, Dr. Lewis was asked why patients with heart failure came back to the hospital. This question launched an in-depth search for real-time and accurate data on heart failure patients in her institution. She worked with the Heart Failure Steering Committee to develop a process to close care gaps and document compliance to the ACC/AHA Get with the Guidelines: Heart Failure recommendations. She facilitated order set revisions, smart-phrase documentation in EPIC, and scripted bedside interdisciplinary rounding to facilitate compliance prior to patient discharge. She also created an end-user friendly dashboard to report compliance with medical leaders, and eventually this project was selected by the department of medicine as their annual quality goal. The project has led to the improvement of CHF GWTG Composite Bundle compliance from 76% to 93%, and compliance with use of aldosterone antagonists from 22% to 85%.
Outstanding Service in Hospital Medicine
Efren Manjarrez, MD, SFHM, FACP, is an associate professor of clinical medicine at the University of Miami Miller School of Medicine, where he also serves as a hospitalist in the division of hospital medicine. His high-impact work at his home institution and through SHM has been extensive.
He founded the division of hospital medicine at the University of Miami in 2000 and later served as the division chief and patient safety officer. Dr. Manjarrez served in the prestigious role of course director for HM15 and as co-course director for the Adult Hospital Medicine Boot Camp.
One of his most enduring contributions is as an author of the white paper on hospitalist handoffs, published in the Journal of Hospital Medicine in 2009, which continues to be cited and validated. He was an assistant editor for the Journal of Hospital Medicine and continues to review articles for JHM. Dr. Manjarrez is also a senior fellow in hospital medicine.
Excellence in Research
Shoshana J. Herzig, MD, MPH, is the director of hospital medicine research at Beth Israel Deaconess Medical Center in Boston, where she also serves as a hospitalist. She is also an associate professor of medicine at Harvard Medical School, also in Boston.
She has published nearly 50 original peer-reviewed manuscripts in some of medicine’s top journals. Her impressive research, which primarily focuses on patterns of medication utilization and associated outcomes in hospitalized adults, has been cited more than 1,500 times in the medical literature.
In addition to her work on medication safety, she is also a site PI for the Hospital Medicine Research Network (HOMERuN), a nationwide collaborative of hospital medicine researchers.
Dr. Herzig has been a member of SHM since 2008 and has attended the annual conference every year since. She has served as an RIV abstract judge, was instrumental in developing SHM’s consensus statement on safe opioid prescribing, and has served as an editor for the Journal of Hospital Medicine since 2012 and has been a senior deputy editor since 2015.
Clinical Leadership for Physicians
Karen Smith, MD, MEd, SFHM, is the chief of the division of hospitalist medicine and past president of the medical staff at Children’s National Medical Center in Washington. She also serves as associate professor of pediatrics at the George Washington University School of Medicine. She has consistently worked to create a supportive environment in which to promote wellness among her staff and colleagues.
She was one of three founding faculty members of the division of hospital medicine at Children’s National, and under her leadership, the division has seen substantial growth. It has evolved from a single site to a comprehensive model of services, spanning six community hospitals and a specialty hospital for rehabilitation and subacute care.
To increase morale, Dr. Smith spearheaded the development of a virtual physician lounge. She reserved a conference room once a month and provided free lunch to medical staff members of different specialties. Its success led to the construction of a full-time lounge – all because of Dr. Smith’s perseverance and forward thinking.
She is a past member of SHM’s Pediatrics Committee and Hospital Quality and Patient Safety Committee and is a senior fellow in hospital medicine.
Excellence in Teaching
Kathleen M. Finn, MD, M.Phil, SFHM, is the senior associate program director for resident and faculty development in the Massachusetts General Hospital internal medicine residency program at Harvard Medical School, both in Boston, where she also is an assistant professor of medicine. She has excelled at teaching at all levels and in all kinds of settings, from clinical teaching on inpatient rounds, educating faculty through workshops to serving as course director for Hospital Medicine 2018 in Orlando. She constantly strives to think creatively and to teach in new ways and considers her career to be a synergy of all three domains in medical education: clinical teaching, leadership, and research.
Her interest in improving the art of inpatient teaching has also taken Dr. Finn into the medical education research space, where she has conducted and published several significant studies.
She was the codirector of the Boston chapter of SHM for 18 years and is well known for her dedication to SHM’s annual conference. She gained a reputation on the Annual Conference Committee for coming up with creative topics, including the Great Debate series.
Dr. Finn has previously served on the editorial board for the Journal of Hospital Medicine, where she continues to be a reviewer. She is a senior fellow in hospital medicine.
Excellence in Teaching
Juan Nicolás Lessing, MD, is an assistant professor of medicine within the division of hospital medicine at the Medical School at the University of Colorado at Denver, Aurora. He has dedicated himself to the teaching and study of clinical reasoning processes and has cocreated a resident clinical reasoning curriculum, which has been expended to all residency classes.
Dr. Lessing’s dedication to mentorship has been extraordinary. In fewer than 5 years, he has mentored more than 50 learners, resulting in 54 competitive abstracts, posters, and presentations. He has led more than 24 workshops and consistently sponsors junior colleagues to join him. In summary, he teaches learners how to learn rather than what to learn. Additionally, Dr. Lessing created and facilitated several impactful department-wide sessions on how we can learn from our mistakes to openly discuss missed diagnoses. He served as a co-PI on the LOOP study, a multicenter endeavor to provide real-time feedback to admitting residents on a patient’s clinical course, which was published in the Journal of Hospital Medicine.
Dr. Lessing has been actively involved with SHM since medical school, is a graduate of SHM’s Academic Hospitalist Academy, and serves on the executive board for the Rocky Mountain chapter of SHM.
Clinical Leadership for NPs/PAs
Ilaria Gadalla, DMSc, PA-C, is a hospitalist at Treasure Coast Hospitalists in Port St. Lucia, Fla., and also serves as the physician assistant department chair/program director at South University, where she supervises more than 40 PAs, medical directors, and administrative staff.
She continuously drives innovative projects for NPs and PAs to demonstrate excellence in collaboration by working closely with C-suite administration to expand QI (quality improvement) and education efforts. A prime example is the optimal communication system that she developed within her first week as a hospitalist in the Port St. Lucie area. Nursing, ED, and pharmacy staff had difficulty contacting hospitalists since the EMR would not reflect the assigned hospitalist, so she developed a simple contact sheet that included the hospitalist team each day. This method is still in use today.
Ms. Gadalla is the chair of SHM’s NP/PA special interest group who was integral in drafting the recent white paper on NP/PA integration and optimization.
Excellence in Humanitarian Services
Khaalisha Ajala, MD, MBA, is a hospitalist and associate site director for education at Grady Memorial Hospital in Atlanta. She cares for patients of diverse backgrounds directly and also has a deep-seeded passion for public health and patient education, always demonstrating how to bring this passion to trainee education.
Using her knowledge as an MBA, Dr. Ajala has designed, developed, and now maintains her own nonprofit agency, Heart Beats & Hip-Hop. Through this organization, she has hosted public health fairs to conduct health screenings in less-traditional local settings, where community members who may not have access to care can gain exposure to a health care provider.
More broadly, in the last year, she has made two journeys – one to Thailand and another to Ethiopia – to work with Emory trainees in educational and clinical efforts to help them engage the global community in health improvement. In Thailand, she taught students how to care for patients at risk for trafficking and sexual exploitation. While in Ethiopia, she served as an educator and clinical preceptor to Emory residents in the global health pathway, teaching them to care for high-risk patients at a local hospital.
With her active and unrelenting humanitarian efforts in mind, she was also chosen as a member of the executive council for SHM’s Care for Vulnerable Populations special interest group.
Diversity Leadership
Kimberly D. Manning, MD, FACP, FAAP, is a professor of medicine and the associate vice chair of diversity, equity and inclusion at the Emory University School of Medicine in Atlanta, where she also is a hospitalist at Grady Memorial Hospital. She demonstrates a strong passion for building and strengthening diverse clinical learning environments. This inspired her to promote cultural competency via lectures, curriculum development, and more.
Dr. Manning has designed a new educational modality – Bite-Sized Teaching (abbreviated “BST” and read as “BEAST”-Mode Teaching). This engages trainees as the teachers of their peers. As part of those sessions, Dr. Manning intentionally encourages and engages trainees from all backgrounds, including women, minorities, and trainees with varied ethnic and cultural perspectives.
Her leadership on the Emory Task Force on Diversity, Equity and Inclusion led her to be named the department of medicine’s first associate vice chair of diversity, equity and inclusion. Due in large part to her engagement, the medical school just admitted its largest class of underrepresented minorities, nearly doubling numbers from prior years.
She has received the 2018 AGCME Parker J. Palmer Courage to Teach Award and the 2019 Lifetime Achievement Award by the Association of Black Women Physicians.
Leadership for Practice Manager
Douglas G. Philpot, MHA, MBA, MHR, FACHE, currently the hospitalist program director at Intermountain Healthcare in Salt Lake City, epitomizes excellence in practice management.
In mid-2018, Intermountain Healthcare transitioned to a new organizational structure that brought all medical and surgical operations under one leadership team. Prior to this reorganization, hospitalist groups were largely divided by the geographies they served, each operating independently.
After the reorganization, it was apparent that staffing structures among groups varied greatly. Dr. Philpot pored over the workload and billing data and determined the most efficient use of how to staff hospitalist providers. He recently created a program that allows all stakeholders to meet and discuss in an unbiased manner how and when to add resources to a given group. As a result, the team is better able to make smart decisions that translate into improved quality, better patient experience, a more engaged hospitalist group and improved financial decisions. This is a model that Intermountain is now looking to apply to other specialties.
Team Award in Quality Improvement
The Michigan Hospital Medicine Safety Consortium has been in place for a decade and has worked together to improve quality and safety for patients across Michigan and the nation. It has been led since its inception by Scott Flanders, MD, a hospitalist at the University of Michigan, Ann Arbor.
At each participating hospital, teams include hospitalists, infectious disease clinicians, interventional radiologists, nephrologists, nurses, pharmacists, administrators, and more. This integration ensures that the team’s work is highly relevant and generalizable for hospitals around the country.
Their initiatives have informed regulatory and guideline writing authorities in the United States and beyond. For example, findings from their venous thromboembolism project demonstrated that the majority of hospitalized patients do not benefit from VTE prophylaxis, but rather, targeted strategies to define those at high risk. In 2016, their work helped to prevent 852 VTEs in Michigan alone. This led to changes in national guidelines that now emphasize deimplementing pharmacologic VTE prophylaxis and focused risk-assessment in U.S. hospitals.
Their antimicrobial use initiative has led to a robust partnership between hospitalists, hospitals, and national partners, such as the Centers for Disease Control and Prevention. Early work has informed a key gap in stewardship – discharge antibiotic prescribing – which has been a focus for SHM, the CDC, and many others. Efforts have already led to a reduction in thousands of unnecessary antibiotic prescriptions in Michigan.
Junior Investigator Award
SHM’s Research Committee presents the Junior Investigator Award to recognize early-career hospitalist researchers who are leading the way in their field. We are pleased to present the HM20 Junior Investigator Award to Valerie Vaughn, MD, MSc.
Dr. Vaughn is an assistant professor and research scientist in the division of hospital medicine at the University of Michigan and Veterans Affairs Ann Arbor Healthcare System.
Her research is focused on engaging hospitalists in antibiotic prescribing, especially at discharge. She is the hospitalist lead for an initiative to improve antibiotic prescribing in 46 hospitals across Michigan. She has already made a national contribution to the field – two manuscripts that have received high praise and have been cited by the Joint Commission and the CDC in their updated recommendations for antibiotic stewardship. She has a grant from the Gordon and Betty Moore Foundation to study the role of diagnostic error in antibiotic overuse and just received a K08 career development award from the Agency for Healthcare Research and Quality to study methods to improve antibiotic prescribing at hospital discharge.
One of Dr. Vaughn’s career goals is to advance hospital medicine through mentoring the next generation of hospitalists. In 2017, she authored a manuscript titled “Mentee Missteps” in JAMA, which has been viewed nearly 40,000 times since publication. She continues to give talks on this topic and mentors clinical hospitalists on research projects to improve quality and safety.
Dr. Vaughn has worked closely with SHM and represents the society at the CDC’s Healthcare Infection Control Practices Advisory Committee quarterly meetings.
Certificate of Leadership in Hospital Medicine
The Certificate of Leadership in Hospital Medicine (CLHM) cultivates leadership skills in the context of specific hospital medicine challenges. This designation informs employers – or potential employers – with confidence that a candidate is equipped and ready to lead teams and grow an organization.
Charmaine Lewis, MD, MPH, FHM, CLHM, is the quality director for New Hanover Hospitalists in Wilmington, N.C., a role she has held for 7 years. She is also clinical assistant professor, department of medicine, University of North Carolina School of Medicine, Chapel Hill, serving as a mentor for internal medicine, surgery, and obstetrics residents completing projects in quality improvement.
While sitting on the CHF and readmissions committees at her institution, Dr. Lewis was asked why patients with heart failure came back to the hospital. This question launched an in-depth search for real-time and accurate data on heart failure patients in her institution. She worked with the Heart Failure Steering Committee to develop a process to close care gaps and document compliance to the ACC/AHA Get with the Guidelines: Heart Failure recommendations. She facilitated order set revisions, smart-phrase documentation in EPIC, and scripted bedside interdisciplinary rounding to facilitate compliance prior to patient discharge. She also created an end-user friendly dashboard to report compliance with medical leaders, and eventually this project was selected by the department of medicine as their annual quality goal. The project has led to the improvement of CHF GWTG Composite Bundle compliance from 76% to 93%, and compliance with use of aldosterone antagonists from 22% to 85%.
Many hydroxychloroquine COVID-19 prophylaxis trials lack ECG screening
Many planned randomized trials to test the efficacy of hydroxychloroquine or related drugs for preventing COVID-19 infection have, as of the end of April 2020, failed to include ECG assessment to either exclude people at the highest risk for possibly developing a life-threatening cardiac arrhythmia or to flag people who achieve a dangerous QTc interval on treatment, according to an analysis of the posted designs of several dozen studies.
Hydroxychloroquine, the related agent chloroquine, and azithromycin have all recently received attention as potentially effective but unproven agents for both reducing the severity and duration of established COVID-19 infection as well as possibly preventing or mitigating an incident infection. As of April 30, 155 randomized, control trials listed on a major index for pending and in-progress trials, clinicaltrials.gov, had designs that intended to randomized an overall total of more than 85,000 healthy people to receive hydroxychloroquine or chloroquine, in some cases in combination with azithromycin, to test their efficacy and safety for COVID-19 prophylaxis, Michael H. Gollob, MD, said in an article posted by the Journal of the American College of Cardiology (2020 May 11. doi: 10.1016/j.jacc.2020.05.008).
The problem is that all three agents are documented to potentially produce lengthening of the corrected QT interval (QTc), and if this happens in a person who starts treatment with a QTc on the high end, the incremental prolongation from drug treatment could push their heart rhythm into a range where their risk for a life-threatening arrhythmia becomes substantial, said Dr. Gollob, a cardiac arrhythmia researcher at Toronto General Hospital and the University of Toronto. As a consequence, he recommended excluding from these prophylaxis trials anyone with a resting QTc at baseline assessment of greater than 450 msec, as well as discontinuing treatment from anyone who develops a resting QTc of more than 480 ms while on treatment.
“Though this may seem like a conservative value for subject withdrawal from a study, this is a prudent QTc cut-off, particularly when the severity of the adverse event, sudden death, may be worse than the study endpoint” of reduced incidence of COVID-19 infection, he wrote in his opinion piece.
“We cannot provide an accurate number for elevated risk” faced by people whose QTc climbs above these thresholds, “but we know that events will occur, which is why most trials that involve QT-prolonging drugs typically have an ECG exclusion criterion of QTc greater than 450 msec,” Dr. Gollob said in an interview.
His analysis of the 155 planned randomized prophylaxis trials on clinicaltrials.gov that he examined in detail had enrollment goals that would translate into more than 85,000 uninfected people who would receive hydroxychloroquine or chloroquine plus, in come cases, azithromycin. Only six relatively small studies from among these 155 included a plan for ECG screening and monitoring in its design, he noted. “It is reasonable to estimate that among the 80,000 patients randomized to a QT-prolonging drug [without ECG screening or monitoring] there will certainly be arrhythmic events.” If some of these people were to then die from a drug-induced arrhythmic event that could have been prevented by ECG screening or monitoring, it would be a “tragedy,” Dr. Gollob said.
“It is not only inexplicable, but also inexcusable that clinical investigators would dare to include healthy individuals in a clinical trial involving QT-prolonging medications without bothering to screen their electrocardiogram,” commented Sami Viskin, MD, an electrophysiologist at Tel Aviv Sourasky Medical Center. “The fact that we needed Dr. Gollob to ring this alarm is, itself, shocking,” he said in an interview.
“ECG screening is a good option to minimize the risk. You don’t eliminate the risk, but you can minimize it,” commented Arthur Wilde, MD, a cardiac electrophysiologist and professor of medicine at the Academic Medical Center in Amsterdam. Both Dr. Viskin and Dr. Wilde agreed with the QTc interval thresholds Dr. Gollob recommended using for excluding or discontinuing study participants.
In his commentary, Dr. Gollob estimated that if 85,000 otherwise healthy adults were randomized to received a drug that can increase the QTc interval, as many as about 3,400 people (4%) in the group could statistically be expected to have an especially high vulnerability to QT prolongation because of genetic variants they might carry that collectively have roughly this prevalence. In some people of African heritage, the prevalence of genetic risk for excessive QTc lengthening can be even higher, approaching about 10%, noted Dr. Wilde.
Dr. Gollob hoped the concerns he raised will prompt the organizers of many of these studies to revise their design, and he said he already knew of one study based in Toronto that recently added an ECG-monitoring strategy in response to the concerns he raised. He expressed optimism that more studies will follow.
“It’s a real issue to have these trials designed without ECG exclusions or monitoring. I’m glad that Dr. Gollob sent this warning, because he is right. ECG monitoring during treatment is important so you can stop the treatment in time,” Dr. Wilde said. Dr. Wilde also noted that many, if not most, of the studies listed on clinicaltrials.gov may not actually launch.
In April, representatives from several cardiology societies coauthored a document of considerations when using hydroxychloroquine, chloroquine, or azithromycin to treat patients with a diagnosed COVID-19 infection, and highlighted a QTc interval of 500 msec or greater as flagging patients who should no longer receive these drugs (J Am Coll Cardiol. 2020 Apr 10. doi: 10.1016/j.jacc.2020.04.016). For patients who do not yet have COVID-19 disease and the goal from treatment is prevention the potential efficacy of these drugs is reasonable to explore, but “does not exclude the need to minimize risk to research participants, especially when enrolling healthy subjects,” Dr. Gollob said.
Dr. Gollob, Dr. Viskin, and Dr. Wilde had no relevant financial disclosures.
Many planned randomized trials to test the efficacy of hydroxychloroquine or related drugs for preventing COVID-19 infection have, as of the end of April 2020, failed to include ECG assessment to either exclude people at the highest risk for possibly developing a life-threatening cardiac arrhythmia or to flag people who achieve a dangerous QTc interval on treatment, according to an analysis of the posted designs of several dozen studies.
Hydroxychloroquine, the related agent chloroquine, and azithromycin have all recently received attention as potentially effective but unproven agents for both reducing the severity and duration of established COVID-19 infection as well as possibly preventing or mitigating an incident infection. As of April 30, 155 randomized, control trials listed on a major index for pending and in-progress trials, clinicaltrials.gov, had designs that intended to randomized an overall total of more than 85,000 healthy people to receive hydroxychloroquine or chloroquine, in some cases in combination with azithromycin, to test their efficacy and safety for COVID-19 prophylaxis, Michael H. Gollob, MD, said in an article posted by the Journal of the American College of Cardiology (2020 May 11. doi: 10.1016/j.jacc.2020.05.008).
The problem is that all three agents are documented to potentially produce lengthening of the corrected QT interval (QTc), and if this happens in a person who starts treatment with a QTc on the high end, the incremental prolongation from drug treatment could push their heart rhythm into a range where their risk for a life-threatening arrhythmia becomes substantial, said Dr. Gollob, a cardiac arrhythmia researcher at Toronto General Hospital and the University of Toronto. As a consequence, he recommended excluding from these prophylaxis trials anyone with a resting QTc at baseline assessment of greater than 450 msec, as well as discontinuing treatment from anyone who develops a resting QTc of more than 480 ms while on treatment.
“Though this may seem like a conservative value for subject withdrawal from a study, this is a prudent QTc cut-off, particularly when the severity of the adverse event, sudden death, may be worse than the study endpoint” of reduced incidence of COVID-19 infection, he wrote in his opinion piece.
“We cannot provide an accurate number for elevated risk” faced by people whose QTc climbs above these thresholds, “but we know that events will occur, which is why most trials that involve QT-prolonging drugs typically have an ECG exclusion criterion of QTc greater than 450 msec,” Dr. Gollob said in an interview.
His analysis of the 155 planned randomized prophylaxis trials on clinicaltrials.gov that he examined in detail had enrollment goals that would translate into more than 85,000 uninfected people who would receive hydroxychloroquine or chloroquine plus, in come cases, azithromycin. Only six relatively small studies from among these 155 included a plan for ECG screening and monitoring in its design, he noted. “It is reasonable to estimate that among the 80,000 patients randomized to a QT-prolonging drug [without ECG screening or monitoring] there will certainly be arrhythmic events.” If some of these people were to then die from a drug-induced arrhythmic event that could have been prevented by ECG screening or monitoring, it would be a “tragedy,” Dr. Gollob said.
“It is not only inexplicable, but also inexcusable that clinical investigators would dare to include healthy individuals in a clinical trial involving QT-prolonging medications without bothering to screen their electrocardiogram,” commented Sami Viskin, MD, an electrophysiologist at Tel Aviv Sourasky Medical Center. “The fact that we needed Dr. Gollob to ring this alarm is, itself, shocking,” he said in an interview.
“ECG screening is a good option to minimize the risk. You don’t eliminate the risk, but you can minimize it,” commented Arthur Wilde, MD, a cardiac electrophysiologist and professor of medicine at the Academic Medical Center in Amsterdam. Both Dr. Viskin and Dr. Wilde agreed with the QTc interval thresholds Dr. Gollob recommended using for excluding or discontinuing study participants.
In his commentary, Dr. Gollob estimated that if 85,000 otherwise healthy adults were randomized to received a drug that can increase the QTc interval, as many as about 3,400 people (4%) in the group could statistically be expected to have an especially high vulnerability to QT prolongation because of genetic variants they might carry that collectively have roughly this prevalence. In some people of African heritage, the prevalence of genetic risk for excessive QTc lengthening can be even higher, approaching about 10%, noted Dr. Wilde.
Dr. Gollob hoped the concerns he raised will prompt the organizers of many of these studies to revise their design, and he said he already knew of one study based in Toronto that recently added an ECG-monitoring strategy in response to the concerns he raised. He expressed optimism that more studies will follow.
“It’s a real issue to have these trials designed without ECG exclusions or monitoring. I’m glad that Dr. Gollob sent this warning, because he is right. ECG monitoring during treatment is important so you can stop the treatment in time,” Dr. Wilde said. Dr. Wilde also noted that many, if not most, of the studies listed on clinicaltrials.gov may not actually launch.
In April, representatives from several cardiology societies coauthored a document of considerations when using hydroxychloroquine, chloroquine, or azithromycin to treat patients with a diagnosed COVID-19 infection, and highlighted a QTc interval of 500 msec or greater as flagging patients who should no longer receive these drugs (J Am Coll Cardiol. 2020 Apr 10. doi: 10.1016/j.jacc.2020.04.016). For patients who do not yet have COVID-19 disease and the goal from treatment is prevention the potential efficacy of these drugs is reasonable to explore, but “does not exclude the need to minimize risk to research participants, especially when enrolling healthy subjects,” Dr. Gollob said.
Dr. Gollob, Dr. Viskin, and Dr. Wilde had no relevant financial disclosures.
Many planned randomized trials to test the efficacy of hydroxychloroquine or related drugs for preventing COVID-19 infection have, as of the end of April 2020, failed to include ECG assessment to either exclude people at the highest risk for possibly developing a life-threatening cardiac arrhythmia or to flag people who achieve a dangerous QTc interval on treatment, according to an analysis of the posted designs of several dozen studies.
Hydroxychloroquine, the related agent chloroquine, and azithromycin have all recently received attention as potentially effective but unproven agents for both reducing the severity and duration of established COVID-19 infection as well as possibly preventing or mitigating an incident infection. As of April 30, 155 randomized, control trials listed on a major index for pending and in-progress trials, clinicaltrials.gov, had designs that intended to randomized an overall total of more than 85,000 healthy people to receive hydroxychloroquine or chloroquine, in some cases in combination with azithromycin, to test their efficacy and safety for COVID-19 prophylaxis, Michael H. Gollob, MD, said in an article posted by the Journal of the American College of Cardiology (2020 May 11. doi: 10.1016/j.jacc.2020.05.008).
The problem is that all three agents are documented to potentially produce lengthening of the corrected QT interval (QTc), and if this happens in a person who starts treatment with a QTc on the high end, the incremental prolongation from drug treatment could push their heart rhythm into a range where their risk for a life-threatening arrhythmia becomes substantial, said Dr. Gollob, a cardiac arrhythmia researcher at Toronto General Hospital and the University of Toronto. As a consequence, he recommended excluding from these prophylaxis trials anyone with a resting QTc at baseline assessment of greater than 450 msec, as well as discontinuing treatment from anyone who develops a resting QTc of more than 480 ms while on treatment.
“Though this may seem like a conservative value for subject withdrawal from a study, this is a prudent QTc cut-off, particularly when the severity of the adverse event, sudden death, may be worse than the study endpoint” of reduced incidence of COVID-19 infection, he wrote in his opinion piece.
“We cannot provide an accurate number for elevated risk” faced by people whose QTc climbs above these thresholds, “but we know that events will occur, which is why most trials that involve QT-prolonging drugs typically have an ECG exclusion criterion of QTc greater than 450 msec,” Dr. Gollob said in an interview.
His analysis of the 155 planned randomized prophylaxis trials on clinicaltrials.gov that he examined in detail had enrollment goals that would translate into more than 85,000 uninfected people who would receive hydroxychloroquine or chloroquine plus, in come cases, azithromycin. Only six relatively small studies from among these 155 included a plan for ECG screening and monitoring in its design, he noted. “It is reasonable to estimate that among the 80,000 patients randomized to a QT-prolonging drug [without ECG screening or monitoring] there will certainly be arrhythmic events.” If some of these people were to then die from a drug-induced arrhythmic event that could have been prevented by ECG screening or monitoring, it would be a “tragedy,” Dr. Gollob said.
“It is not only inexplicable, but also inexcusable that clinical investigators would dare to include healthy individuals in a clinical trial involving QT-prolonging medications without bothering to screen their electrocardiogram,” commented Sami Viskin, MD, an electrophysiologist at Tel Aviv Sourasky Medical Center. “The fact that we needed Dr. Gollob to ring this alarm is, itself, shocking,” he said in an interview.
“ECG screening is a good option to minimize the risk. You don’t eliminate the risk, but you can minimize it,” commented Arthur Wilde, MD, a cardiac electrophysiologist and professor of medicine at the Academic Medical Center in Amsterdam. Both Dr. Viskin and Dr. Wilde agreed with the QTc interval thresholds Dr. Gollob recommended using for excluding or discontinuing study participants.
In his commentary, Dr. Gollob estimated that if 85,000 otherwise healthy adults were randomized to received a drug that can increase the QTc interval, as many as about 3,400 people (4%) in the group could statistically be expected to have an especially high vulnerability to QT prolongation because of genetic variants they might carry that collectively have roughly this prevalence. In some people of African heritage, the prevalence of genetic risk for excessive QTc lengthening can be even higher, approaching about 10%, noted Dr. Wilde.
Dr. Gollob hoped the concerns he raised will prompt the organizers of many of these studies to revise their design, and he said he already knew of one study based in Toronto that recently added an ECG-monitoring strategy in response to the concerns he raised. He expressed optimism that more studies will follow.
“It’s a real issue to have these trials designed without ECG exclusions or monitoring. I’m glad that Dr. Gollob sent this warning, because he is right. ECG monitoring during treatment is important so you can stop the treatment in time,” Dr. Wilde said. Dr. Wilde also noted that many, if not most, of the studies listed on clinicaltrials.gov may not actually launch.
In April, representatives from several cardiology societies coauthored a document of considerations when using hydroxychloroquine, chloroquine, or azithromycin to treat patients with a diagnosed COVID-19 infection, and highlighted a QTc interval of 500 msec or greater as flagging patients who should no longer receive these drugs (J Am Coll Cardiol. 2020 Apr 10. doi: 10.1016/j.jacc.2020.04.016). For patients who do not yet have COVID-19 disease and the goal from treatment is prevention the potential efficacy of these drugs is reasonable to explore, but “does not exclude the need to minimize risk to research participants, especially when enrolling healthy subjects,” Dr. Gollob said.
Dr. Gollob, Dr. Viskin, and Dr. Wilde had no relevant financial disclosures.
REPORTING FROM JACC
ASCO goes ahead online, as conference center is used as hospital
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Bacteroides Fragilis Vertebral Osteomyelitis and Discitis: “Back” to Susceptibility Testing
Acute pyogenic vertebral osteomyelitis is often due to hematogenous spread of aerobic bacteria.1-4 Conversely, only 0.5% of anaerobic bacteremias lead to osteomyelitis.5 Anaerobic osteomyelitis typically results from the contiguous spread of polymicrobial infections through breaks in the gut mucosal barrier and involves the vertebral bodies in only 2% to 5% of cases.5,6 Although Bacteroides fragilis (B fragilis) is the most common anaerobic pathogen cultivated from blood, accounting for about half of all anaerobic blood isolates, it seldom leads to osteomyelitis.1,2,7-11 We report an uncommon case of B fragilis bacteremia and vertebral osteomyelitis confounded by uncertainties in anaerobic identification and susceptibilities.
Case Presentation
A healthy-appearing male aged 55 years presented to the Naval Medical Center Portsmouth (NMCP) with subacute low back pain and fevers of 103 °F for > 3 weeks. While traveling 4 weeks prior, he completed a course of oseltamivir for influenza B infection; afterward, he was diagnosed with community-acquired pneumonia and treated with a dose of ceftriaxone and a 7-day course of doxycycline. The patient presented to the same facility a week later for low back pain and nonresolving respiratory symptoms, and his therapy was changed to azithromycin, cefuroxime, prednisone, and inhalers. Additionally, after being treated for influenza, he developed constipation and hematochezia for which he did not seek care. The hematochezia was similar to a previous episode from an anal fissure 1 year prior that resolved with stool softeners. When he was finally seen at NMCP after 3 weeks of worsening back pain and fevers, lumbosacral magnetic resonance imaging (MRI) demonstrated vertebral osteomyelitis and discitis at L4-L5 and admitted to the hospital (Figure 1).
After a fluoroscopy-guided biopsy of the L4 vertebral body on hospital day 1, the patient was started on cefepime and vancomycin. The biopsy sample was inoculated onto solid media (blood agar, chocolate agar, and MacConkey agar) and incubated at 36 °C for 24 hours in a 5% CO2 atmosphere, as well as onto Shaedler agar with vitamin K and chopped meat glucose broth and incubated at 36 °C for 48 hours under anaerobic conditions. Metronidazole was added and vancomycin discontinued after 2 anaerobic blood culture vials obtained on hospital day 1, incubated in a Becton Dickinson BACTEC FX automated system, which demonstrated Gram-negative bacilli after 48 hours. The blood culture isolates demonstrated a > 99% probability of being identified as ß-lactamase positive Prevotella loescheii using Thermo Fischer Scientific RapID ANA II biochemical testing. Nitrocefinase discs were used to detect ß-lactamase activity.
The biopsy demonstrated nongranulomatous focal areas of necrotic bone and neutrophilia in a hematopoietic background consistent with acute osteomyelitis (Figure 2); on hospital day 4, ß-lactamase positive B fragilis grew from the bone culture. Additionally, 1 anaerobic vial from a surveillance blood culture set that was obtained on hospital day 3 grew ß-lactamasepositive B fragilis using the same identification methods. With these results he was thought to have a polymicrobial infection (B fragilis and Prevotella loescheii [P loescheii]) from a suspected bowel source based on his hematochezia and history of anal fissure. No aerobic, Gram-negative enterobacteriaceae were isolated, but he had previously been on cefuroxime, which has potential activity against these organisms, for ≥ 2 weeks prior to hospitalization and cultures. He was discharged on moxifloxacin and metronidazole pending final culture results, including requested anaerobic susceptibility testing.
At 1-week follow-up, both aerobic and anaerobic vials from surveillance blood cultures remained negative for any microbes, so antibiotics were deescalated to moxifloxacin monotherapy. However, after 3 days the patient was readmitted for increasing C-reactive protein (CRP) levels and intractable back pain with worsening bilateral radiculopathy. A repeat MRI demonstrated interval disease progression with near obliteration of the L4-L5 disc space and hyperenhancement of the prevertebral soft tissues and adjacent psoas musculature without focal rim-enhancing fluid collection (Figure 3). After repeat L4 biopsy, metronidazole was restarted and ertapenem added for enterobacteriaceae coverage, given the known B fragilis and potential suppression from previous cephalosporin therapy; moxifloxacin was discontinued. L4 biopsy cultures showed no growth, and CRP levels trended down from 154.2 mg/L (start of first admission) to 42.4 mg/L (start of second admission) to 14.9 mg/L (day of discharge) (reference range, 5-9.9 mg/L). He was discharged on ertapenem and metronidazole. He completed a 6-week course without further complication.
During antibiotic therapy he had an unremarkable colonoscopy, CRP normalized to 2.6 mg/L (reference range, 0-4.9 mg/L), and he underwent successful L4-L5 transforaminal lumbar interbody fusion 2 weeks after finishing antibiotics.
We retroactively sent both P loescheii isolates and the 1 B fragilis isolate that grew from the surveillance blood culture to the Multidrug-resistant Organism Repository and Surveillance Network (MRSN) at the Walter Reed Army Institute of Research for identification confirmation and susceptibility analysis. Whole genome sequencing with single nucleotide polymorphism (SNP)-based analysis revealed all isolates were 100% identical and consistent with B fragilis and not P loescheii, based on clustering around other B fragilis sequences found in the National Center for Biotechnology Information (NCBI) Genbank database (Figure 4). All isolates carried the antibiotic resistance genes— cepA, sul(2), tetQ— encoding for possible resistance to cephalosporins, sulphonamides, and tetracyclines, respectively; as well as a point mutation in the gyrA gene (Ser82Phe). None of the isolates carried the nim gene, and screening for the 3 subtypes of B fragilis enterotoxin gene (bft-1, bft-2, bft-3) was negative. Eventual susceptibility testing at the Mayo Clinic several months after the conclusion of the case indicated that the B fragilis isolate was sensitive to piperacillin-tazobactam, ertapenem, clindamycin, and metronidazole; however, testing was not performed against moxifloxacin.
Discussion
In the era of growing antibiotic resistance patterns, antimicrobial stewardship programs recommend interventions to improve antimicrobial use through targeted narrow- spectrum antibiotics.12 The Clinical and Laboratory Standards Institute (CLSI) maintains guidelines on the major indications for anaerobic antimicrobial susceptibility testing (AST) to help direct narrow-targeted antimicrobial therapy. However, in a 2008 practice survey Goldstein and colleagues reported that less than half of US hospitals performed anaerobic AST, and only 21% of these facilities did it in-house, while the remainder sent out their isolates for testing.11-14 The CLSI major indications for AST include situations in which the selection of agents is important because of the (1) known resistance of a particular species; (2) confirmation of appropriate therapy for severe infections or for those that may require long-term therapy; (3) persistence of infection despite adequate treatment with an appropriate therapeutic regimen; and (4) difficulty in making empirical decisions based on precedent.14 Additionally, isolates from brain abscess, endocarditis, osteomyelitis, joint infection, infection of prosthetic devices or vascular grafts, bacteremia, and normally sterile body sites (unless contamination suspected) should be tested.14
Because of the lack of anaerobic AST, health care providers must base empiric treatment on reported sensitivities from the medical literature. Empiric selection of antimicrobials for anaerobic infections is made even more challenging by the increased rates of resistance reported in the literature, leading to recommendations to increase susceptibility testing to guide therapy.13,15,16 Empiric therapy of deep-seated anaerobic infections may lead to use of inactive agents or overly broad-spectrum antibiotics. Current antimicrobial stewardship initiatives recognize the importance of narrow-spectrum antibiotics to minimize risk of adverse events and selective pressure for antimicrobial resistance.
Although we attempted to confirm the identification of the anaerobic isolates via commercially available methods, it was not until we performed genetic testing that we were able verify the isolates as B fragilis. Furthermore, earlier susceptibility testing would have allowed for more narrow-targeted antimicrobial therapy and could have potentially prevented our patient’s readmission and use of ertapenem, despite its > 98% susceptibility rates against B fragilis.13,17
All of the B fragilis isolates carried the cepA gene, which is a cephalosporinase that encodes for resistance to cephalosporins and aminopenicillins but not to ß-lactam ß-lactamase inhibitor combinations.13 Although not a substitution for susceptibility analysis, genetic testing showed that all of the isolates carried a nonsynonymous mutation from serine to a phenylalanine at amino acid position 82 (S82F) in the gyrA gene. The S82F mutation has been implicated in fluoroquinolone resistance, via inhibition of substrate–target recognition and binding between fluoroquinolones and the target topoisomerase protein,18 and may potentially explain why our patient clinically worsened while on moxifloxacin monotherapy. Although moxifloxacin susceptibility was not performed, susceptibility rates remain highly variable, ranging from 50% to 70% for B fragilis.13,15,16
It is important to note that the metronidazole the patient received during his first hospital admission could have sterilized the vertebral body without completely eradicating the microbe; thus could explain his clinical worsening while on moxifloxacin monotherapy despite no growth from the repeat biopsy culture. Our rationale for initially continuing moxifloxacin was based on its excellent bioavailability and bone penetration properties. Additionally, of the fluoroquinolones it has the most reliable anaerobic activity and is the only one recommended as monotherapy for complicated intraabdominal infections.19 However, guidelines recommend avoiding its use in patients who have received a fluoroquinolone in the past 90 days or at institutions with high rates of resistance. At our institution Escherichia coli has a > 90% susceptibility rate to fluoroquinolones. Given this rate and our concern that the patient had a polymicrobial infection, we felt that moxifloxacin would provide appropriate anaerobic and aerobic coverage, especially since he had no previous fluoroquinolone exposure.
Additionally, none of the isolates carried the nim or bft toxin genes. Although the nim gene is associated with metronidazole resistance,its presence does not invariably result in resistant strains of B fragilis; in fact, metronidazole resistance is relatively uncommon, with the majority of B fragilis showing < 1% resistance, based on CLSI breakpoints (≥ 32 mg/L).13,20,21 However, one recent epidemiologic study on anaerobic wound isolates from Iraq and Afghanistan casualties found that 12% (2/17) of B fragilis isolates were resistant to metronidazole.15 Given the improvement of the patient’s symptoms while on metronidazole, it is likely that the B fragilis was susceptible. Nevertheless, susceptibility testing with minimum inhibitory concentrations is necessary to verify this result. Also, although enterotoxigenic strains of B fragilis have been associated with bloodstream infections, our patient’s isolates lacked the 3 subtypes of B fragilis enterotoxin gene.22
Conclusions
We report a case of B fragilis bacteremia and vertebral osteomyelitis complicated by challenges in anaerobic identification and sensitivities that led to brief use of a possibly inactive antimicrobial and the subsequent use of carbapenem therapy, which may have been avoided if susceptibility testing were more readily available. This case led to changes in our hospital’s processing of anaerobic isolates to include susceptibility testing on request.
Acknowledgments
We thank Keith Thompson, MD (staff pathologist, Naval Medical Center Portsmouth Virginia), for providing the pathology images from the initial vertebral biopsy, and Dr. Kate Hinkle (director, Multidrug-Resistant Organism Repository and Surveillance Network, Silver Spring, Maryland ) for providing the whole genome sequencing results from the B fragilis isolates.
1. Zimmerli W. Vertebral osteomyelitis. N Eng J Med. 2010;362(11):1022-1029.
2. Chazan B, Strahilevitz J, Millgram MA, Kaufmann S, Raz R. Bacteroides fragilis vertebral osteomyelitis secondary to anal dilatation. Spine (Phila PA 1976). 2001;26(16):E377-E378.
3. Kierzkowska M, Pedzisz P, Babiak I, et al. Orthopedic infections caused by obligatory anaerobic Gram-negative rods: report of two cases. Med Microbiol Immunol. 2017;206(5):363-366.
4. McHenry M, Easley K, Locker G. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342-1350.
5. Raff MJ, Melo JC. Anaerobic osteomyelitis. Medicine (Baltimore).1978;57(1):83-103.
6. Lewis R, Sutter V, Finegold S. Bone infections involving anaerobic bacteria. Medicine (Baltimore). 1978;57(1):279-305.
7. Brook I. The role of anaerobic bacteria in bacteremia. Anaerobe. 2010;16(3):183-189.
8. Lassmann B, Gustafson DR, Wood CM, Rosenblatt JE. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007;44(7):895-900.
9. Lazarovitch T, Freimann S, Shapira G, Blank H. Decrease in anaerobe-related bacteraemias and increase in Bacteroides species isolation rate from 1998 to 2007: a retrospective study. Anaerobe. 2010;16(3):201-205.
10. Keukeleire S, Wybo I, Naessens A, et al. Anaerobic bacteraemia: a 10-year retrospective epidemiological survey. Anaerobe. 2016;39:54-59.
11. Goldstein EJC, Citron DM, Goldman PJ, Goldman RJ. National hospital survey of anaerobic culture and susceptibility methods: III. Anaerobe. 2008;14(2):68-72.
12. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77.
13. Schuetz AN. Antimicrobial resistance and susceptibility testing of anaerobic bacteria. Antimicr Resist. 2014;59(5):698-705.
14. Clinical and Laboratory Standards Institute. M11-A8: Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard. 8th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
15. White B, Mende K, Weintrob A, et al; Infectious Disease Clinical Research Program Trauma Infectious Disease Outcome Study Group. Epidemiology and antimicrobial susceptibilities of wound isolates of obligate anaerobes from combat casualties. Diagn Mircrobiol Infect Dis. 2016;84(2):144-150.
16. Hastey CJ, Boyd H, Schuetz AN, et al; Ad Hoc Working Group on Antimicrobial Susceptibility Testing of Anaerobic Bacteria of CLSI. Changes in the antibiotic susceptibility of anaerobic bacteria from 2007-2009 to 2010-2012 based on the CLSI methodology. Anaerobe. 2016;42:27-30.
17. Brook I, Wexler HM, Goldstein EJC. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev. 2013;26(3):526-546.
18. Pumbwe L, Wareham D, Aduse-Opoku J, Brazier JS, Wexler HM. Genetic analysis of mechanisms of multidrug resistance in a clinical isolate of Bacteroides fragilis. Clin Microbiol Infect. 2007;13(2):183-189.
19. Solomkin J, Mazuski J, Bradley J, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164.
20. Breuil J, Dublanchet A, Truffaut N, Sebald M. Transferable 5-nitroimidazole resistance Bacteroides fragilis group. Plasmid. 1989;21(2):151-154.
21. Nagy E, Urbán E, Nord CE; ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin Microbiol Infect. 2011;17(3):371-379.
22. Avila-Campos M, Liu C, Song Y, Rowlinson M-C, Finegold SM. Determination of bft gene subtypes in Bacteroides fragilis clinical isolates. J Clin Microbiol. 2007;45(4):1336-1338.
Acute pyogenic vertebral osteomyelitis is often due to hematogenous spread of aerobic bacteria.1-4 Conversely, only 0.5% of anaerobic bacteremias lead to osteomyelitis.5 Anaerobic osteomyelitis typically results from the contiguous spread of polymicrobial infections through breaks in the gut mucosal barrier and involves the vertebral bodies in only 2% to 5% of cases.5,6 Although Bacteroides fragilis (B fragilis) is the most common anaerobic pathogen cultivated from blood, accounting for about half of all anaerobic blood isolates, it seldom leads to osteomyelitis.1,2,7-11 We report an uncommon case of B fragilis bacteremia and vertebral osteomyelitis confounded by uncertainties in anaerobic identification and susceptibilities.
Case Presentation
A healthy-appearing male aged 55 years presented to the Naval Medical Center Portsmouth (NMCP) with subacute low back pain and fevers of 103 °F for > 3 weeks. While traveling 4 weeks prior, he completed a course of oseltamivir for influenza B infection; afterward, he was diagnosed with community-acquired pneumonia and treated with a dose of ceftriaxone and a 7-day course of doxycycline. The patient presented to the same facility a week later for low back pain and nonresolving respiratory symptoms, and his therapy was changed to azithromycin, cefuroxime, prednisone, and inhalers. Additionally, after being treated for influenza, he developed constipation and hematochezia for which he did not seek care. The hematochezia was similar to a previous episode from an anal fissure 1 year prior that resolved with stool softeners. When he was finally seen at NMCP after 3 weeks of worsening back pain and fevers, lumbosacral magnetic resonance imaging (MRI) demonstrated vertebral osteomyelitis and discitis at L4-L5 and admitted to the hospital (Figure 1).
After a fluoroscopy-guided biopsy of the L4 vertebral body on hospital day 1, the patient was started on cefepime and vancomycin. The biopsy sample was inoculated onto solid media (blood agar, chocolate agar, and MacConkey agar) and incubated at 36 °C for 24 hours in a 5% CO2 atmosphere, as well as onto Shaedler agar with vitamin K and chopped meat glucose broth and incubated at 36 °C for 48 hours under anaerobic conditions. Metronidazole was added and vancomycin discontinued after 2 anaerobic blood culture vials obtained on hospital day 1, incubated in a Becton Dickinson BACTEC FX automated system, which demonstrated Gram-negative bacilli after 48 hours. The blood culture isolates demonstrated a > 99% probability of being identified as ß-lactamase positive Prevotella loescheii using Thermo Fischer Scientific RapID ANA II biochemical testing. Nitrocefinase discs were used to detect ß-lactamase activity.
The biopsy demonstrated nongranulomatous focal areas of necrotic bone and neutrophilia in a hematopoietic background consistent with acute osteomyelitis (Figure 2); on hospital day 4, ß-lactamase positive B fragilis grew from the bone culture. Additionally, 1 anaerobic vial from a surveillance blood culture set that was obtained on hospital day 3 grew ß-lactamasepositive B fragilis using the same identification methods. With these results he was thought to have a polymicrobial infection (B fragilis and Prevotella loescheii [P loescheii]) from a suspected bowel source based on his hematochezia and history of anal fissure. No aerobic, Gram-negative enterobacteriaceae were isolated, but he had previously been on cefuroxime, which has potential activity against these organisms, for ≥ 2 weeks prior to hospitalization and cultures. He was discharged on moxifloxacin and metronidazole pending final culture results, including requested anaerobic susceptibility testing.
At 1-week follow-up, both aerobic and anaerobic vials from surveillance blood cultures remained negative for any microbes, so antibiotics were deescalated to moxifloxacin monotherapy. However, after 3 days the patient was readmitted for increasing C-reactive protein (CRP) levels and intractable back pain with worsening bilateral radiculopathy. A repeat MRI demonstrated interval disease progression with near obliteration of the L4-L5 disc space and hyperenhancement of the prevertebral soft tissues and adjacent psoas musculature without focal rim-enhancing fluid collection (Figure 3). After repeat L4 biopsy, metronidazole was restarted and ertapenem added for enterobacteriaceae coverage, given the known B fragilis and potential suppression from previous cephalosporin therapy; moxifloxacin was discontinued. L4 biopsy cultures showed no growth, and CRP levels trended down from 154.2 mg/L (start of first admission) to 42.4 mg/L (start of second admission) to 14.9 mg/L (day of discharge) (reference range, 5-9.9 mg/L). He was discharged on ertapenem and metronidazole. He completed a 6-week course without further complication.
During antibiotic therapy he had an unremarkable colonoscopy, CRP normalized to 2.6 mg/L (reference range, 0-4.9 mg/L), and he underwent successful L4-L5 transforaminal lumbar interbody fusion 2 weeks after finishing antibiotics.
We retroactively sent both P loescheii isolates and the 1 B fragilis isolate that grew from the surveillance blood culture to the Multidrug-resistant Organism Repository and Surveillance Network (MRSN) at the Walter Reed Army Institute of Research for identification confirmation and susceptibility analysis. Whole genome sequencing with single nucleotide polymorphism (SNP)-based analysis revealed all isolates were 100% identical and consistent with B fragilis and not P loescheii, based on clustering around other B fragilis sequences found in the National Center for Biotechnology Information (NCBI) Genbank database (Figure 4). All isolates carried the antibiotic resistance genes— cepA, sul(2), tetQ— encoding for possible resistance to cephalosporins, sulphonamides, and tetracyclines, respectively; as well as a point mutation in the gyrA gene (Ser82Phe). None of the isolates carried the nim gene, and screening for the 3 subtypes of B fragilis enterotoxin gene (bft-1, bft-2, bft-3) was negative. Eventual susceptibility testing at the Mayo Clinic several months after the conclusion of the case indicated that the B fragilis isolate was sensitive to piperacillin-tazobactam, ertapenem, clindamycin, and metronidazole; however, testing was not performed against moxifloxacin.
Discussion
In the era of growing antibiotic resistance patterns, antimicrobial stewardship programs recommend interventions to improve antimicrobial use through targeted narrow- spectrum antibiotics.12 The Clinical and Laboratory Standards Institute (CLSI) maintains guidelines on the major indications for anaerobic antimicrobial susceptibility testing (AST) to help direct narrow-targeted antimicrobial therapy. However, in a 2008 practice survey Goldstein and colleagues reported that less than half of US hospitals performed anaerobic AST, and only 21% of these facilities did it in-house, while the remainder sent out their isolates for testing.11-14 The CLSI major indications for AST include situations in which the selection of agents is important because of the (1) known resistance of a particular species; (2) confirmation of appropriate therapy for severe infections or for those that may require long-term therapy; (3) persistence of infection despite adequate treatment with an appropriate therapeutic regimen; and (4) difficulty in making empirical decisions based on precedent.14 Additionally, isolates from brain abscess, endocarditis, osteomyelitis, joint infection, infection of prosthetic devices or vascular grafts, bacteremia, and normally sterile body sites (unless contamination suspected) should be tested.14
Because of the lack of anaerobic AST, health care providers must base empiric treatment on reported sensitivities from the medical literature. Empiric selection of antimicrobials for anaerobic infections is made even more challenging by the increased rates of resistance reported in the literature, leading to recommendations to increase susceptibility testing to guide therapy.13,15,16 Empiric therapy of deep-seated anaerobic infections may lead to use of inactive agents or overly broad-spectrum antibiotics. Current antimicrobial stewardship initiatives recognize the importance of narrow-spectrum antibiotics to minimize risk of adverse events and selective pressure for antimicrobial resistance.
Although we attempted to confirm the identification of the anaerobic isolates via commercially available methods, it was not until we performed genetic testing that we were able verify the isolates as B fragilis. Furthermore, earlier susceptibility testing would have allowed for more narrow-targeted antimicrobial therapy and could have potentially prevented our patient’s readmission and use of ertapenem, despite its > 98% susceptibility rates against B fragilis.13,17
All of the B fragilis isolates carried the cepA gene, which is a cephalosporinase that encodes for resistance to cephalosporins and aminopenicillins but not to ß-lactam ß-lactamase inhibitor combinations.13 Although not a substitution for susceptibility analysis, genetic testing showed that all of the isolates carried a nonsynonymous mutation from serine to a phenylalanine at amino acid position 82 (S82F) in the gyrA gene. The S82F mutation has been implicated in fluoroquinolone resistance, via inhibition of substrate–target recognition and binding between fluoroquinolones and the target topoisomerase protein,18 and may potentially explain why our patient clinically worsened while on moxifloxacin monotherapy. Although moxifloxacin susceptibility was not performed, susceptibility rates remain highly variable, ranging from 50% to 70% for B fragilis.13,15,16
It is important to note that the metronidazole the patient received during his first hospital admission could have sterilized the vertebral body without completely eradicating the microbe; thus could explain his clinical worsening while on moxifloxacin monotherapy despite no growth from the repeat biopsy culture. Our rationale for initially continuing moxifloxacin was based on its excellent bioavailability and bone penetration properties. Additionally, of the fluoroquinolones it has the most reliable anaerobic activity and is the only one recommended as monotherapy for complicated intraabdominal infections.19 However, guidelines recommend avoiding its use in patients who have received a fluoroquinolone in the past 90 days or at institutions with high rates of resistance. At our institution Escherichia coli has a > 90% susceptibility rate to fluoroquinolones. Given this rate and our concern that the patient had a polymicrobial infection, we felt that moxifloxacin would provide appropriate anaerobic and aerobic coverage, especially since he had no previous fluoroquinolone exposure.
Additionally, none of the isolates carried the nim or bft toxin genes. Although the nim gene is associated with metronidazole resistance,its presence does not invariably result in resistant strains of B fragilis; in fact, metronidazole resistance is relatively uncommon, with the majority of B fragilis showing < 1% resistance, based on CLSI breakpoints (≥ 32 mg/L).13,20,21 However, one recent epidemiologic study on anaerobic wound isolates from Iraq and Afghanistan casualties found that 12% (2/17) of B fragilis isolates were resistant to metronidazole.15 Given the improvement of the patient’s symptoms while on metronidazole, it is likely that the B fragilis was susceptible. Nevertheless, susceptibility testing with minimum inhibitory concentrations is necessary to verify this result. Also, although enterotoxigenic strains of B fragilis have been associated with bloodstream infections, our patient’s isolates lacked the 3 subtypes of B fragilis enterotoxin gene.22
Conclusions
We report a case of B fragilis bacteremia and vertebral osteomyelitis complicated by challenges in anaerobic identification and sensitivities that led to brief use of a possibly inactive antimicrobial and the subsequent use of carbapenem therapy, which may have been avoided if susceptibility testing were more readily available. This case led to changes in our hospital’s processing of anaerobic isolates to include susceptibility testing on request.
Acknowledgments
We thank Keith Thompson, MD (staff pathologist, Naval Medical Center Portsmouth Virginia), for providing the pathology images from the initial vertebral biopsy, and Dr. Kate Hinkle (director, Multidrug-Resistant Organism Repository and Surveillance Network, Silver Spring, Maryland ) for providing the whole genome sequencing results from the B fragilis isolates.
Acute pyogenic vertebral osteomyelitis is often due to hematogenous spread of aerobic bacteria.1-4 Conversely, only 0.5% of anaerobic bacteremias lead to osteomyelitis.5 Anaerobic osteomyelitis typically results from the contiguous spread of polymicrobial infections through breaks in the gut mucosal barrier and involves the vertebral bodies in only 2% to 5% of cases.5,6 Although Bacteroides fragilis (B fragilis) is the most common anaerobic pathogen cultivated from blood, accounting for about half of all anaerobic blood isolates, it seldom leads to osteomyelitis.1,2,7-11 We report an uncommon case of B fragilis bacteremia and vertebral osteomyelitis confounded by uncertainties in anaerobic identification and susceptibilities.
Case Presentation
A healthy-appearing male aged 55 years presented to the Naval Medical Center Portsmouth (NMCP) with subacute low back pain and fevers of 103 °F for > 3 weeks. While traveling 4 weeks prior, he completed a course of oseltamivir for influenza B infection; afterward, he was diagnosed with community-acquired pneumonia and treated with a dose of ceftriaxone and a 7-day course of doxycycline. The patient presented to the same facility a week later for low back pain and nonresolving respiratory symptoms, and his therapy was changed to azithromycin, cefuroxime, prednisone, and inhalers. Additionally, after being treated for influenza, he developed constipation and hematochezia for which he did not seek care. The hematochezia was similar to a previous episode from an anal fissure 1 year prior that resolved with stool softeners. When he was finally seen at NMCP after 3 weeks of worsening back pain and fevers, lumbosacral magnetic resonance imaging (MRI) demonstrated vertebral osteomyelitis and discitis at L4-L5 and admitted to the hospital (Figure 1).
After a fluoroscopy-guided biopsy of the L4 vertebral body on hospital day 1, the patient was started on cefepime and vancomycin. The biopsy sample was inoculated onto solid media (blood agar, chocolate agar, and MacConkey agar) and incubated at 36 °C for 24 hours in a 5% CO2 atmosphere, as well as onto Shaedler agar with vitamin K and chopped meat glucose broth and incubated at 36 °C for 48 hours under anaerobic conditions. Metronidazole was added and vancomycin discontinued after 2 anaerobic blood culture vials obtained on hospital day 1, incubated in a Becton Dickinson BACTEC FX automated system, which demonstrated Gram-negative bacilli after 48 hours. The blood culture isolates demonstrated a > 99% probability of being identified as ß-lactamase positive Prevotella loescheii using Thermo Fischer Scientific RapID ANA II biochemical testing. Nitrocefinase discs were used to detect ß-lactamase activity.
The biopsy demonstrated nongranulomatous focal areas of necrotic bone and neutrophilia in a hematopoietic background consistent with acute osteomyelitis (Figure 2); on hospital day 4, ß-lactamase positive B fragilis grew from the bone culture. Additionally, 1 anaerobic vial from a surveillance blood culture set that was obtained on hospital day 3 grew ß-lactamasepositive B fragilis using the same identification methods. With these results he was thought to have a polymicrobial infection (B fragilis and Prevotella loescheii [P loescheii]) from a suspected bowel source based on his hematochezia and history of anal fissure. No aerobic, Gram-negative enterobacteriaceae were isolated, but he had previously been on cefuroxime, which has potential activity against these organisms, for ≥ 2 weeks prior to hospitalization and cultures. He was discharged on moxifloxacin and metronidazole pending final culture results, including requested anaerobic susceptibility testing.
At 1-week follow-up, both aerobic and anaerobic vials from surveillance blood cultures remained negative for any microbes, so antibiotics were deescalated to moxifloxacin monotherapy. However, after 3 days the patient was readmitted for increasing C-reactive protein (CRP) levels and intractable back pain with worsening bilateral radiculopathy. A repeat MRI demonstrated interval disease progression with near obliteration of the L4-L5 disc space and hyperenhancement of the prevertebral soft tissues and adjacent psoas musculature without focal rim-enhancing fluid collection (Figure 3). After repeat L4 biopsy, metronidazole was restarted and ertapenem added for enterobacteriaceae coverage, given the known B fragilis and potential suppression from previous cephalosporin therapy; moxifloxacin was discontinued. L4 biopsy cultures showed no growth, and CRP levels trended down from 154.2 mg/L (start of first admission) to 42.4 mg/L (start of second admission) to 14.9 mg/L (day of discharge) (reference range, 5-9.9 mg/L). He was discharged on ertapenem and metronidazole. He completed a 6-week course without further complication.
During antibiotic therapy he had an unremarkable colonoscopy, CRP normalized to 2.6 mg/L (reference range, 0-4.9 mg/L), and he underwent successful L4-L5 transforaminal lumbar interbody fusion 2 weeks after finishing antibiotics.
We retroactively sent both P loescheii isolates and the 1 B fragilis isolate that grew from the surveillance blood culture to the Multidrug-resistant Organism Repository and Surveillance Network (MRSN) at the Walter Reed Army Institute of Research for identification confirmation and susceptibility analysis. Whole genome sequencing with single nucleotide polymorphism (SNP)-based analysis revealed all isolates were 100% identical and consistent with B fragilis and not P loescheii, based on clustering around other B fragilis sequences found in the National Center for Biotechnology Information (NCBI) Genbank database (Figure 4). All isolates carried the antibiotic resistance genes— cepA, sul(2), tetQ— encoding for possible resistance to cephalosporins, sulphonamides, and tetracyclines, respectively; as well as a point mutation in the gyrA gene (Ser82Phe). None of the isolates carried the nim gene, and screening for the 3 subtypes of B fragilis enterotoxin gene (bft-1, bft-2, bft-3) was negative. Eventual susceptibility testing at the Mayo Clinic several months after the conclusion of the case indicated that the B fragilis isolate was sensitive to piperacillin-tazobactam, ertapenem, clindamycin, and metronidazole; however, testing was not performed against moxifloxacin.
Discussion
In the era of growing antibiotic resistance patterns, antimicrobial stewardship programs recommend interventions to improve antimicrobial use through targeted narrow- spectrum antibiotics.12 The Clinical and Laboratory Standards Institute (CLSI) maintains guidelines on the major indications for anaerobic antimicrobial susceptibility testing (AST) to help direct narrow-targeted antimicrobial therapy. However, in a 2008 practice survey Goldstein and colleagues reported that less than half of US hospitals performed anaerobic AST, and only 21% of these facilities did it in-house, while the remainder sent out their isolates for testing.11-14 The CLSI major indications for AST include situations in which the selection of agents is important because of the (1) known resistance of a particular species; (2) confirmation of appropriate therapy for severe infections or for those that may require long-term therapy; (3) persistence of infection despite adequate treatment with an appropriate therapeutic regimen; and (4) difficulty in making empirical decisions based on precedent.14 Additionally, isolates from brain abscess, endocarditis, osteomyelitis, joint infection, infection of prosthetic devices or vascular grafts, bacteremia, and normally sterile body sites (unless contamination suspected) should be tested.14
Because of the lack of anaerobic AST, health care providers must base empiric treatment on reported sensitivities from the medical literature. Empiric selection of antimicrobials for anaerobic infections is made even more challenging by the increased rates of resistance reported in the literature, leading to recommendations to increase susceptibility testing to guide therapy.13,15,16 Empiric therapy of deep-seated anaerobic infections may lead to use of inactive agents or overly broad-spectrum antibiotics. Current antimicrobial stewardship initiatives recognize the importance of narrow-spectrum antibiotics to minimize risk of adverse events and selective pressure for antimicrobial resistance.
Although we attempted to confirm the identification of the anaerobic isolates via commercially available methods, it was not until we performed genetic testing that we were able verify the isolates as B fragilis. Furthermore, earlier susceptibility testing would have allowed for more narrow-targeted antimicrobial therapy and could have potentially prevented our patient’s readmission and use of ertapenem, despite its > 98% susceptibility rates against B fragilis.13,17
All of the B fragilis isolates carried the cepA gene, which is a cephalosporinase that encodes for resistance to cephalosporins and aminopenicillins but not to ß-lactam ß-lactamase inhibitor combinations.13 Although not a substitution for susceptibility analysis, genetic testing showed that all of the isolates carried a nonsynonymous mutation from serine to a phenylalanine at amino acid position 82 (S82F) in the gyrA gene. The S82F mutation has been implicated in fluoroquinolone resistance, via inhibition of substrate–target recognition and binding between fluoroquinolones and the target topoisomerase protein,18 and may potentially explain why our patient clinically worsened while on moxifloxacin monotherapy. Although moxifloxacin susceptibility was not performed, susceptibility rates remain highly variable, ranging from 50% to 70% for B fragilis.13,15,16
It is important to note that the metronidazole the patient received during his first hospital admission could have sterilized the vertebral body without completely eradicating the microbe; thus could explain his clinical worsening while on moxifloxacin monotherapy despite no growth from the repeat biopsy culture. Our rationale for initially continuing moxifloxacin was based on its excellent bioavailability and bone penetration properties. Additionally, of the fluoroquinolones it has the most reliable anaerobic activity and is the only one recommended as monotherapy for complicated intraabdominal infections.19 However, guidelines recommend avoiding its use in patients who have received a fluoroquinolone in the past 90 days or at institutions with high rates of resistance. At our institution Escherichia coli has a > 90% susceptibility rate to fluoroquinolones. Given this rate and our concern that the patient had a polymicrobial infection, we felt that moxifloxacin would provide appropriate anaerobic and aerobic coverage, especially since he had no previous fluoroquinolone exposure.
Additionally, none of the isolates carried the nim or bft toxin genes. Although the nim gene is associated with metronidazole resistance,its presence does not invariably result in resistant strains of B fragilis; in fact, metronidazole resistance is relatively uncommon, with the majority of B fragilis showing < 1% resistance, based on CLSI breakpoints (≥ 32 mg/L).13,20,21 However, one recent epidemiologic study on anaerobic wound isolates from Iraq and Afghanistan casualties found that 12% (2/17) of B fragilis isolates were resistant to metronidazole.15 Given the improvement of the patient’s symptoms while on metronidazole, it is likely that the B fragilis was susceptible. Nevertheless, susceptibility testing with minimum inhibitory concentrations is necessary to verify this result. Also, although enterotoxigenic strains of B fragilis have been associated with bloodstream infections, our patient’s isolates lacked the 3 subtypes of B fragilis enterotoxin gene.22
Conclusions
We report a case of B fragilis bacteremia and vertebral osteomyelitis complicated by challenges in anaerobic identification and sensitivities that led to brief use of a possibly inactive antimicrobial and the subsequent use of carbapenem therapy, which may have been avoided if susceptibility testing were more readily available. This case led to changes in our hospital’s processing of anaerobic isolates to include susceptibility testing on request.
Acknowledgments
We thank Keith Thompson, MD (staff pathologist, Naval Medical Center Portsmouth Virginia), for providing the pathology images from the initial vertebral biopsy, and Dr. Kate Hinkle (director, Multidrug-Resistant Organism Repository and Surveillance Network, Silver Spring, Maryland ) for providing the whole genome sequencing results from the B fragilis isolates.
1. Zimmerli W. Vertebral osteomyelitis. N Eng J Med. 2010;362(11):1022-1029.
2. Chazan B, Strahilevitz J, Millgram MA, Kaufmann S, Raz R. Bacteroides fragilis vertebral osteomyelitis secondary to anal dilatation. Spine (Phila PA 1976). 2001;26(16):E377-E378.
3. Kierzkowska M, Pedzisz P, Babiak I, et al. Orthopedic infections caused by obligatory anaerobic Gram-negative rods: report of two cases. Med Microbiol Immunol. 2017;206(5):363-366.
4. McHenry M, Easley K, Locker G. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342-1350.
5. Raff MJ, Melo JC. Anaerobic osteomyelitis. Medicine (Baltimore).1978;57(1):83-103.
6. Lewis R, Sutter V, Finegold S. Bone infections involving anaerobic bacteria. Medicine (Baltimore). 1978;57(1):279-305.
7. Brook I. The role of anaerobic bacteria in bacteremia. Anaerobe. 2010;16(3):183-189.
8. Lassmann B, Gustafson DR, Wood CM, Rosenblatt JE. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007;44(7):895-900.
9. Lazarovitch T, Freimann S, Shapira G, Blank H. Decrease in anaerobe-related bacteraemias and increase in Bacteroides species isolation rate from 1998 to 2007: a retrospective study. Anaerobe. 2010;16(3):201-205.
10. Keukeleire S, Wybo I, Naessens A, et al. Anaerobic bacteraemia: a 10-year retrospective epidemiological survey. Anaerobe. 2016;39:54-59.
11. Goldstein EJC, Citron DM, Goldman PJ, Goldman RJ. National hospital survey of anaerobic culture and susceptibility methods: III. Anaerobe. 2008;14(2):68-72.
12. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77.
13. Schuetz AN. Antimicrobial resistance and susceptibility testing of anaerobic bacteria. Antimicr Resist. 2014;59(5):698-705.
14. Clinical and Laboratory Standards Institute. M11-A8: Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard. 8th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
15. White B, Mende K, Weintrob A, et al; Infectious Disease Clinical Research Program Trauma Infectious Disease Outcome Study Group. Epidemiology and antimicrobial susceptibilities of wound isolates of obligate anaerobes from combat casualties. Diagn Mircrobiol Infect Dis. 2016;84(2):144-150.
16. Hastey CJ, Boyd H, Schuetz AN, et al; Ad Hoc Working Group on Antimicrobial Susceptibility Testing of Anaerobic Bacteria of CLSI. Changes in the antibiotic susceptibility of anaerobic bacteria from 2007-2009 to 2010-2012 based on the CLSI methodology. Anaerobe. 2016;42:27-30.
17. Brook I, Wexler HM, Goldstein EJC. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev. 2013;26(3):526-546.
18. Pumbwe L, Wareham D, Aduse-Opoku J, Brazier JS, Wexler HM. Genetic analysis of mechanisms of multidrug resistance in a clinical isolate of Bacteroides fragilis. Clin Microbiol Infect. 2007;13(2):183-189.
19. Solomkin J, Mazuski J, Bradley J, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164.
20. Breuil J, Dublanchet A, Truffaut N, Sebald M. Transferable 5-nitroimidazole resistance Bacteroides fragilis group. Plasmid. 1989;21(2):151-154.
21. Nagy E, Urbán E, Nord CE; ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin Microbiol Infect. 2011;17(3):371-379.
22. Avila-Campos M, Liu C, Song Y, Rowlinson M-C, Finegold SM. Determination of bft gene subtypes in Bacteroides fragilis clinical isolates. J Clin Microbiol. 2007;45(4):1336-1338.
1. Zimmerli W. Vertebral osteomyelitis. N Eng J Med. 2010;362(11):1022-1029.
2. Chazan B, Strahilevitz J, Millgram MA, Kaufmann S, Raz R. Bacteroides fragilis vertebral osteomyelitis secondary to anal dilatation. Spine (Phila PA 1976). 2001;26(16):E377-E378.
3. Kierzkowska M, Pedzisz P, Babiak I, et al. Orthopedic infections caused by obligatory anaerobic Gram-negative rods: report of two cases. Med Microbiol Immunol. 2017;206(5):363-366.
4. McHenry M, Easley K, Locker G. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342-1350.
5. Raff MJ, Melo JC. Anaerobic osteomyelitis. Medicine (Baltimore).1978;57(1):83-103.
6. Lewis R, Sutter V, Finegold S. Bone infections involving anaerobic bacteria. Medicine (Baltimore). 1978;57(1):279-305.
7. Brook I. The role of anaerobic bacteria in bacteremia. Anaerobe. 2010;16(3):183-189.
8. Lassmann B, Gustafson DR, Wood CM, Rosenblatt JE. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007;44(7):895-900.
9. Lazarovitch T, Freimann S, Shapira G, Blank H. Decrease in anaerobe-related bacteraemias and increase in Bacteroides species isolation rate from 1998 to 2007: a retrospective study. Anaerobe. 2010;16(3):201-205.
10. Keukeleire S, Wybo I, Naessens A, et al. Anaerobic bacteraemia: a 10-year retrospective epidemiological survey. Anaerobe. 2016;39:54-59.
11. Goldstein EJC, Citron DM, Goldman PJ, Goldman RJ. National hospital survey of anaerobic culture and susceptibility methods: III. Anaerobe. 2008;14(2):68-72.
12. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77.
13. Schuetz AN. Antimicrobial resistance and susceptibility testing of anaerobic bacteria. Antimicr Resist. 2014;59(5):698-705.
14. Clinical and Laboratory Standards Institute. M11-A8: Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard. 8th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
15. White B, Mende K, Weintrob A, et al; Infectious Disease Clinical Research Program Trauma Infectious Disease Outcome Study Group. Epidemiology and antimicrobial susceptibilities of wound isolates of obligate anaerobes from combat casualties. Diagn Mircrobiol Infect Dis. 2016;84(2):144-150.
16. Hastey CJ, Boyd H, Schuetz AN, et al; Ad Hoc Working Group on Antimicrobial Susceptibility Testing of Anaerobic Bacteria of CLSI. Changes in the antibiotic susceptibility of anaerobic bacteria from 2007-2009 to 2010-2012 based on the CLSI methodology. Anaerobe. 2016;42:27-30.
17. Brook I, Wexler HM, Goldstein EJC. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev. 2013;26(3):526-546.
18. Pumbwe L, Wareham D, Aduse-Opoku J, Brazier JS, Wexler HM. Genetic analysis of mechanisms of multidrug resistance in a clinical isolate of Bacteroides fragilis. Clin Microbiol Infect. 2007;13(2):183-189.
19. Solomkin J, Mazuski J, Bradley J, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164.
20. Breuil J, Dublanchet A, Truffaut N, Sebald M. Transferable 5-nitroimidazole resistance Bacteroides fragilis group. Plasmid. 1989;21(2):151-154.
21. Nagy E, Urbán E, Nord CE; ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin Microbiol Infect. 2011;17(3):371-379.
22. Avila-Campos M, Liu C, Song Y, Rowlinson M-C, Finegold SM. Determination of bft gene subtypes in Bacteroides fragilis clinical isolates. J Clin Microbiol. 2007;45(4):1336-1338.
Where Dysphagia Begins: Polypharmacy and Xerostomia
Xerostomia, the subjective sensation of dry mouth, is a common problem developed by geriatric patients. In practice, xerostomia can impair swallowing, speech, and oral hygiene, and if left unchecked, symptoms such as dysphagia and dysarthria can diminish patients’ quality of life (QOL). Salivary gland hypofunction (SGH) is the objective measure of decreased saliva production, determined by sialometry. Although xerostomia and SGH can coexist, the 2 conditions are not necessarily related.1-4 For this discussion, the term xerostomia will denote dry mouth with or without a concomitant diagnosis of SGH.
Xerostomia is seen in a wide variety of patients with varied comorbidities. It is commonly associated with Sjögren syndrome and head and neck irradiation. The diagnosis and treatment of xerostomia often involves rheumatologists, dentists, otolaryngologists, and oncologists. Additionally, most of the scientific literature about this topic exists in dental journals, such as the Journal of the American Dental Association and the British Dental Journal. Rarer still are studies in the veteran population.5
Faced with increasing time pressure to treat the many chronic diseases affecting aging veterans, health care providers (HCPs) tend to deprioritize diagnosing dry mouth. To that point, saliva is often not considered in the same category as other bodily fluids. According to Mandel, “It lacks the drama of blood, the sincerity of sweat…[and] the emotional appeal of tears.”6 In reality, saliva plays a critical role in the oral-digestive tract and in swallowing. It contains the first digestive enzymes in the gastrointestinal tract and is key for maintaining homeostasis in the oral cavity.7 Decreased saliva production results in difficulties with speech and mastication as well as problems of dysphagia, esophageal dysfunction, dysgeusia, nutritional compromises, new and recurrent dental caries, candidiasis, glossitis, impaired use of dentures, halitosis, and susceptibility to mucosal injury.7,8 Problems with the production of saliva may lead to loss of QOL, such as enjoying a meal or conversing with others.4
Although xerostomia is often associated with advanced age, it is more often explained by the diseases that afflict geriatric patients and the arsenal of medications used to treat them.2,9-16 Polypharmacy, the simultaneous use of multiple drugs by a single patient for ≥ 1 conditions, is an independent risk factor for xerostomia regardless of the types of medication taken.16 From 2005 to 2011, older adults in the US significantly increased their prescription medication use and dietary supplements. More than one-third of older adults used ≥ 5 prescription medications concurrently, and two-thirds of older adults used combinations of prescribed medications, over-the-counter medications, and dietary supplements.17 Several drug classes have the capacity to induce xerostomia, such as antihypertensives, antiulcer agents, anticholinergics, and antidepressants.2,5,12 Prevalence of dry mouth also can range from 10% to 46%, and women typically are more medicated and symptomatic.2,3,9,13,14,16 Xerostomia can also lead to depression and even reduce patients’ will to live.18 Despite xerostomia’s prevalence and impact on QOL, few patients report it as their chief symptom, and few physicians attempt to treat it.19
In order to target polypharmacy as a cause of dry mouth, the objectives for this study were to evaluate (1) the prevalence of xerostomia; (2) the relationship between xerostomia and other oral conditions; and (3) the impact of polypharmacy on dry mouth in a veteran population.
Methods
This is a retrospective cross-sectional study of all outpatient visits in fiscal year (FY) 2015 (October 1, 2014 to September 30, 2015) at the VA Palo Alto Health Care System (VAPAHCS), a tertiary care US Department of Veterans Affairs (VA) hospital. This study was approved by the Stanford University Institutional Review Board. All patients diagnosed with xerostomia in the 1-year study period were identified using ICD-9 diagnosis codes for dry mouth or disturbance of salivary gland secretion (527.7, 527.8, R68.2) and Systemized Nomenclature of Medicine Clinical Terms (SNOMED CT) codes covering dry mouth, xerostomia, aptyalism, absent salivary secretion, and disturbance of salivary secretion (87715008, 78948009). Data analysts in the VA Office of Business Analytics assisted in gathering data from the Veterans Information Systems and Technology Architecture (VistA) electronic health record.
The statistical analysis of that data was performed using Microsoft Excel. Age and gender distributions were determined for the patients. The relationship between xerostomia and the number and types of medications taken by patients also was examined. A previous Swedish study examining the link between dry mouth and quantities of medications used a scale ranging from 0 to ≥ 7 medications.16 The scale for this study was made wider to include the following groups: 0-2, 3-5, 6-8, 9-11, and ≥ 12 medications. Items that do not have xerogenic risks, such as medical supplies (eg, gloves, syringes, etc) and topical medications, were excluded from the analysis. Finally, the number of subjects with comorbid problems with speech, dentition, or swallowing (SDS) was recorded. Non-VA medications were included to capture any self- or externally prescribed xerogenic medications.
Results
Of the patients seen at VAPAHCS during FY 2015, 138 had a diagnostic code for xerostomia, including 129 men (93.5%) and 9 women (6.5%). The average (SD) age of this xerostomia cohort was 69.3 (12.6) years, and the 3 most common age groups were 60 to 69 years (37.7%), 70 to 79 years (28.3%), and 80 to 89 years (13.0%) (Table 1). Of those 138 patients with a xerostomia diagnosis, a majority (84; 60.9%) had at least 1 documented SDS problem (Table 2). Conversely, during FY 2015, although 4,971 patients seen at VAPAHCS had documented SDS problems, only 77 (1.5%) had a recorded diagnosis of xerostomia
Of the 138 patients with xerostomia, 55 (39.9%) were taking ≥ 12 medications, more than twice as many patients as in any of the other groups studied (0-2, 3-5, 6-8, and 9-11 medications taken) (Table 3). On average, each patient with xerostomia filled prescriptions for 10.4 (SD, 7.2) different drugs. In this cohort of 138 patients diagnosed with xerostomia, antihypertensive medications or analgesics were taken by > 50% of patients, while statins, psychiatric medications, antibiotics, proton pump inhibitors, or drugs known to have anticholinergic activity were taken by > 40%. Antihistamines, anticonvulsants, diuretics, or inhaled respiratory agents were used by > 20% of the patients in this cohort (Table 4).
Data on each individual medication were split into 2 categories: the percentage of patients that filled ≥ 1 prescription for that drug, and the total number of prescriptions filled and/or refilled for that drug (ie, including all fills and refills made by individual patients). The 5 most widely used medications in this cohort were omeprazole (39.1%), docusate sodium (29.7%), gabapentin (29.7%), aspirin (27.5%), and hydrocodone/acetaminophen (26.1%) (Table 5). The 5 prescriptions that were cumulatively most filled and/or refilled were omeprazole (128), sildenafil citrate (108), gabapentin (101), hydrocodone/acetaminophen (100), and oxycodone (92) (Table 6). Though sildenafil citrate and oxycodone were among the most-filled prescriptions, these were not included in Table 5 as neither was taken by > 15% of the patients studied. These prescriptions were filled multiple times by a small subset of patients.
Regarding treatment for dry mouth, artificial saliva spray was one of the most widely used (23.2%) and the seventh most-filled prescription within this cohort (86). The only other medication taken by > 15% of patients in a formulation other than a tablet or capsule was chlorhexidine, a germicidal mouthwash used to improve oral care.
Also, 30 (21.7%) patients with a documented xerostomia diagnosis had a history of substance misuse involving use of ≥ 1 of tobacco, alcohol, marijuana, or other illicit drugs.
Discussion
Saliva is an essential component for the maintenance of normal oral health.20,21 Decreased saliva production causes problems, including difficulties with speech, mastication, dysphagia, changes in taste, dental caries, impaired use of prostheses, recurrent infections, halitosis, deterioration of soft tissues, and compromised QOL.22,23 Among patients with a diagnosed SDS abnormality who were seen at this facility during FY 2015, the prevalence of xerostomia was only 1.5%. However, the true prevalence and incidence of xerostomia among veterans is not known. Given the role of xerostomia as a common risk factor for SDS problems and the polypharmacy exhibited by those presented here with SDS problems, it is probable that xerostomia was underreported in this veteran cohort.
Additionally, although salivary acinar cells are known to atrophy with age, as is consistent with this xerostomia cohort’s average age (SD) of 69.3 (12.6) years, the development of dry mouth is a multifactorial process. The current scientific literature asserts that most salivary loss is due to local and systemic diseases, immunologic disorders, external radiation, and as was highlighted by this study, multiple prescription and nonprescription medications.24-26
It has also been demonstrated previously that dry mouth complaints and low salivary flow rates are directly proportional to the number of medications taken by patients.2,27-30 Polypharmacy is therefore an area of great interest, and ≥ 40 categories of xerogenic medications have been identified by investigators such as Sreebny and Schwartz.31 Among those, some of the most xerogenic medication classes include antihypertensives, antiulcer agents, anticholinergics, and antidepressants, are all very commonly consumed in this cohort of patients with dry mouth (58.7%, 42.0%, 47.1%, and 38.4%, respectively). The medication regimens within this cohort of veterans with xerostomia were prime examples of polypharmacy as each patient took an average (SD) of 10.4 (7.2) medications, 39.9% took ≥ 12, and 72.5% of patients with xerostomia were taking ≥ 6 prescription drugs during a 1-year period.
Given the dangers of polypharmacy, a more conservative approach to prescribing medications could feasibly help with preventing xerostomia and SGH. In practice, while clinicians try to avoid prescribing anticholinergics, antimuscarinics, and antihistaminergic drugs for geriatric patients, they are tasked with the complex management of medication adverse effects (AEs) when dealing with multiple health conditions. The clinicians’ primary responsibilities are to follow the standard of care and not to introduce unnecessary harm when managing patients, but they also must push for, stay abreast of, and conduct more basic research and clinical trials to inform, adjust, and improve our current standard.
Research into polypharmacy and its role in inducing dry mouth is ongoing. Twenty years ago, Thomson and colleagues identified reduced salivary flow in patients who used antianginals, thyroxine, diuretics, antidepressants, and medications for asthma, while only 5 years earlier Loesche and colleagues reported the role of antiulcer medications, such as proton pump inhibitors, in the development of xerostomia.2,32 Within the past 5 years, Viljakainen and colleagues and Ohara and colleagues have echoed some of those findings by identifying associations between xerostomia and agents that impact digestive organs.33,34 A strong association recently was identified between the use of antipsychotic drugs and xerostomia.35 Additionally, when attributing xerostomia to polypharmacy, the interaction between medications is often overlooked in favor of considering the raw number of prescriptions taken. Whereas 1 medication alone may not have drying properties, combinations of medications might be more likely to induce xerostomia. Thomson and colleagues suggested such a situation regarding the interaction between thyroxine and diuretics.36 Future studies should focus on identifying viable substitutes for existing medications that reduce risk for xerostomia without compromising the management of other serious conditions.
Treatment
Another pressing question for clinicians concerns artificial saliva. Although 23.2% of patients with dry mouth in this xerostomia cohort used artificial saliva, the efficacy of this treatment is still unproven. Saliva substitutes are often used by patients who cannot produce sufficient amounts of natural saliva. In practice, artificial saliva produces, at best, modest temporary improvement in dry mouth symptoms in up to 40% of patients. At worst, as put forth by the Cochrane Review, artificial saliva may be no better than placebo in treating dry mouth.37,38 The volumes needed for symptom relief are large, ranging between 40 mL and 150 mL per day depending on the substitute’s composition. Saliva substitutes also must be reapplied throughout each day. This is particularly bothersome when patients must wake up repeatedly to reapply the treatment at night.37 In short, these substances do not seem wholly effective in managing dry mouth, and other options must be made available to patients with refractory xerostomia when artificial saliva and lifestyle modifications fail.
For now, few alternatives exist. Chewing gums and lozenges help to stimulate salivary flow, as do muscarinic agonists like pilocarpine. Unfortunately, muscarinic agonists are seldom used due to cholinergic AEs. Humidifiers are effective in increasing nighttime moisture but are contraindicated in patients with dust mite allergies.39 Reservoir-based devices with automated pumps funnel water and/or salivary substitutes from a fanny pack into patients’ mouths for lubrication.37 Other more esoteric pharmacologic treatments include D-limonene, yohimbine, and amifostine, which purportedly protect salivary progenitor cells, increase peripheral cholinergic activity, and protect salivary glands from free-radical damage during radiation treatment, respectively. Although these agents have shown some promise, D-limonene is difficult to administer given the high dosage required for treatment, yohimbine hasn’t been seriously investigated for improving salivary secretion since 1997, and amifostine isn’t used widely due to its AE profile despite its US Food and Drug Administration approval for prevention of xerostomia.39
Substance Abuse
The impact of smoking on xerostomia remains controversial. Some studies report an association between active smoking and xerostomia; others suggest that the local irritant effect of tobacco smoke may increase salivary gland output.40,41 The same may be true for chronic alcohol use as there are no epidemiologic studies showing a causal relationship between alcohol use and xerostomia. Studies with rats that are chronically exposed to ethanol have found increased salivary flow rates.42 In the xerostomia cohort presented here, 30 patients (21.7%) had a documented history of substance misuse. That percentage is likely underestimated, as substance misuse is often underreported, and frequent use may not always constitute misuse. Therefore, nicotine exposure, alcohol exposure, illicit drug use, and vaping all should be considered during the workup of a patient with xerostomia.
Limitations
It is common for medications to remain in a patient’s health record long after that patient stops taking them. Developing methods to track when patients discontinue their prescriptions will be essential for clearing up uncertainty in our data and in other similar studies. This study also did not account for patients’ medication adherence and the duration of exposure to medications and illicit substances. Furthermore, the results of this veteran study are not easily generalizable as this cohort is disproportionately male, of advanced age, and especially prone to exhibiting both substance use and psychiatric diagnoses relative to the general population. As described by Viljakainen and colleagues, risk factors for xerostomia include advanced age, female gender, low body mass index (BMI), malnutrition, and depressive symptoms, but because the demographic scope of this veteran population was narrow, it was not possible to discern the impact of, for example, gender.33 Data on variables like BMI, malnutrition, and depressive symptoms were not available. For this study, xerostomia could only be considered as an all-or-nothing phenomenon because the dataset did not describe different levels of dry mouth severity (eg, mild, moderate, severe).
Additionally, past polypharmacy studies have acknowledged an inability to tell whether xerostomia is mainly due to medications or to underlying medical conditions. For example, for emphysema, ß-adrenergic stimulation from bronchodilators could cause dry mouth by thickening saliva and decreasing salivary volume, but the pathophysiology and/or cardinal symptoms of emphysema, including chronic obstructive pulmonary disease-associated tachypnea, might contribute independently to dryness.
Though we can make inferences based on the medications taken by this cohort (eg, those taking antihypertensives have high blood pressure), this dataset did not explicitly detail comorbid conditions and ICD codes for chronic diseases that commonly arise with xerostomia. Those conditions, however, are of great clinical importance. Diabetes mellitus, HIV/AIDS, and, classically, Sjögren syndrome, all are known to cause dry mouth.43 Identifying new conditions that co-occur with xerostomia would allow clinicians to describe the root causes of and risk factors for dry mouth and SDS conditions in greater detail. Patients with dry mouth without SDS problems in this dataset are of particular interest as closer examination of their medications and comorbid conditions could help us understand why some individuals and not others develop SDS problems. The subjects of how comorbidities contribute to dry mouth and how their influences can be judged independently from the effects of medications are of great interest to us and will be investigated rigorously in our future studies.
Conclusions
In this cohort, few patients with SDS problems had documentation of a concomitant xerostomia diagnosis. This could represent a true infrequency of dry mouth or more likely, an underrecognition by clinicians. Heightened physician awareness regarding the signs and symptoms of and risk factors for xerostomia is needed to improve providers’ ability to diagnose this condition.
In particular, polypharmacy should be a major consideration when evaluating patients for xerostomia. This continues to be an important area of research, and some of the latest data on polypharmacy among older patients were compiled in a recent meta-analysis by Tan and colleagues. The authors of that systematic review reiterated the significant association between salivary gland hypofunction and the number of medications taken by patients. They also advocated for the creation of a risk score for medication-induced dry mouth to aid in medication management.44 Per their recommendations, it is now as crucial as ever to consider the numbers and types of medications taken by patients, to discontinue unnecessary prescriptions when possible, and to continue developing new strategies for preventing and treating xerostomia.
1. Thomson WM, Chalmers JM, Spencer AJ, Ketabi M. The occurrence of xerostomia and salivary gland hypofunction in a population-based sample of older South Australians. Spec Care Dentist. 1999;19(1):20-23.
2. Thomson WM, Chalmers JM, Spencer AJ, Slade GD. Medication and dry mouth: findings from a cohort study of older people. J Public Health Dent. 2000;60(1):12-20.
3. Sasportas LS, Hosford DN, Sodini MA, et al. Cost-effectiveness landscape analysis of treatments addressing xerostomia in patients receiving head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):e37-e51.
4. Bivona PL. Xerostomia. A common problem among the elderly. N Y State Dent J. 1998;64(6):46-52.
5. Ness J, Hoth A, Barnett MJ, Shorr RI, Kaboli PJ. Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother. 2006;4(1):42-51.
6. Mandel ID. The diagnostic uses of saliva. J Oral Pathol Med. 1990;19(3):119-125.
7. Friedman PK, Isfeld D. Xerostomia: the “invisible” oral health condition. J Mass Dent Soc. 2008;57(3):42-44.
8. Ship JA, McCutcheon JA, Spivakovsky S, Kerr AR. Safety and effectiveness of topical dry mouth products containing olive oil, betaine, and xylitol in reducing xerostomia for polypharmacy-induced dry mouth. J Oral Rehabil. 2007;34(10):724-732.
9. Field EA, Fear S, Higham SM, et al. Age and medication are significant risk factors for xerostomia in an English population, attending general dental practice. Gerodontology. 2001;18(1):21-24.
10. Villa A, Connell CL, Abati S. Diagnosis and management of xerostomia and hyposalivation. Ther Clin Risk Manag. 2015;11:45-51.
11. Geuiros LA, Soares MS, Leao JC. Impact of ageing and drug consumption on oral health. Gerodontology. 2009;26(4):297-301.
12. Singh ML, Papas A. Oral implications of polypharmacy in the elderly. Dent Clin North Am. 2014;58(4):783-796.
13. Shinkai RS, Hatch JP, Schmidt CB, Sartori EA. Exposure to the oral side effects of medication in a community-based sample. Spec Care Dentist. 2006;26(3):116-120.
14. Hopcraft MS, Tan C. Xerostomia: an update for clinicians. Aust Dent J. 2010;55(3):238-244; quiz 353.
15. Ettinger RL. Review: xerostomia: a symptom which acts like a disease. Age Ageing. 1996;25(5):409-412.
16. Nederfors T, Isaksson R, Mornstad H, Dahlof C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population—relation to age, sex and pharmacotherapy. Community Dent Oral Epidemiol. 1997;25(3):211-216.
17. Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473-482.
18. Dirix P, Nuyts S, Vander Poorten V, Delaere P, Van den Boaert W. The influence of xerostomia after radiotherapy on quality of life: results of a questionnaire in head and neck cancer. Support Care Cancer. 2008;16(2):171-179.
19. Sreebny LM, Valdini A. Xerostomia. A neglected symptom. Arch Intern Med. 1987;147(7):1333-1337.
20. Sreebny LM. Saliva in health and disease: an appraisal and update. Int Dent J. 2000;50(3):140-161.
21. Amerongen AV, Veeran EC. Saliva—the defender of the oral cavity. Oral Dis. 2002;8(1):12-22.
22. Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. 2003;134(1):61-69; quiz 118-119.
23. Atkinson JC, Baum BJ. Salivary enhancement: current status and future therapies. J Dent Educ. 2001;65(10):1096-1101.
24. Narhi TO, Meurman JH, Ainamo A. Xerostomia and hyposalivation: causes, consequences and treatment in the elderly. Drugs Aging. 1999;15(2):103-116.
25. Ship JA, Baum BJ. Is reduced salivary flow normal in old people? Lancet. 1990;336(8729):1507.
26. Ghezzi EM, Wagner-Lange LA, Schork MA, et al. Longitudinal influence of age, menopause, hormone replacement therapy, and other medications on parotid flow rates in healthy women. J Gerontol A Biol Sci Med Sci. 2000;55(1):M34-M42.
27. Fox PC. Acquired salivary dysfunction. Drugs and radiation. Ann N Y Acad Sci. 1998;842:132-137.
28. Bergdahl M, Bergdahl J. Low unstimulated salivary flow and subjective oral dryness: association with medication, anxiety, depression, and stress. J Dent Res. 2000;79(9):1652-1658.
29. Ship JA, Pillemer SR, Baum BJ. Xerostomia and the geriatric patient. J Am Geriatr Soc. 2002;50(3):535-543.
30. Sreebny LM, Valdini A, Yu A. Xerostomia. Part II: Relationship to nonoral symptoms, drugs, and diseases. Oral Surg Oral Med Oral Pathol. 1989;68(4):419-427.
31. Sreebny LM, Schwartz SS. A reference guide to drugs and dry mouth—2nd edition. Gerodontology. 1997;14(1):33-47.
32. Loesche WJ, Bromberg J, Terpenning MS, et al. Xerostomia, xerogenic medications and food avoidances in selected geriatric groups. J Am Geriatr Soc. 1995;43(4):401-407.

33. Viljakainen S, Nykanen I, Ahonen R, et al. Xerostomia among older home care clients. Community Dent Oral Epidemiol. 2016;44(3):232-238.
34. Ohara Y, Hirano H, Yoshida H, et al. Prevalence and factors associated with xerostomia and hyposalivation among community-dwelling older people in Japan. Gerodontology. 2016;33(1):20-27.
35. Okamoto A, Miyachi H, Tanaka K, Chikazu D, Miyaoka H. Relationship between xerostomia and psychotropic drugs in patients with schizophrenia: evaluation using an oral moisture meter. J Clin Pharm Ther. 2016;41(6):684-688.
36. Thomson WM. Dry mouth and older people. Aust Dent J. 2015;60(suppl 1):54-63.
37. Sasportas LS, Hosford AT, Sodini MA, et al. Cost-effectiveness landscape analysis of treatments addressing xerostomia in patients receiving head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):e37-e51.
38. Furness S, Worthington HV, Bryan G, Birchenough S, McMillan R. Interventions for the management of dry mouth: topical therapies. Cochrane Database Syst Rev. 2011;(12):CD008924.
39. Roblegg E, Coughran A, Sirjani D. Saliva: an all-rounder of our body. Eur J Pharm Biopharm. 2019;142:133-141.
40. Billings RJ, Proskin HM, Moss ME. Xerostomia and associated factors in a community-dwelling adult population. Community Dent Oral Epidemiol. 1996;24(5):312-316.
41. Norlen P, Ostberg H, Bjorn AL. Relationship between general health, social factors and oral health in women at the age of retirement. Community Dent Oral Epidemiol. 1991;19(5):296-301.
42. Berry MR, Scott J. Functional and structural adaptation of the parotid gland to medium-term chronic ethanol exposure in the rat. Alcohol Alcoholism. 1990;25(5):523-531.
43. von Bultzingslowen I, Sollecito TP, Fox PC, et al. Salivary dysfunction associated with systemic diseases: systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol. 2007;103:S57.e1-e15.
44. Tan ECK, Lexomboon D, Sandborgh-Englund G, et al. Medications that cause dry mouth as an adverse effect in older people: a systematic review and metaanalysis. J Am Geriatr Soc. 2018;66(1):76-84.
Xerostomia, the subjective sensation of dry mouth, is a common problem developed by geriatric patients. In practice, xerostomia can impair swallowing, speech, and oral hygiene, and if left unchecked, symptoms such as dysphagia and dysarthria can diminish patients’ quality of life (QOL). Salivary gland hypofunction (SGH) is the objective measure of decreased saliva production, determined by sialometry. Although xerostomia and SGH can coexist, the 2 conditions are not necessarily related.1-4 For this discussion, the term xerostomia will denote dry mouth with or without a concomitant diagnosis of SGH.
Xerostomia is seen in a wide variety of patients with varied comorbidities. It is commonly associated with Sjögren syndrome and head and neck irradiation. The diagnosis and treatment of xerostomia often involves rheumatologists, dentists, otolaryngologists, and oncologists. Additionally, most of the scientific literature about this topic exists in dental journals, such as the Journal of the American Dental Association and the British Dental Journal. Rarer still are studies in the veteran population.5
Faced with increasing time pressure to treat the many chronic diseases affecting aging veterans, health care providers (HCPs) tend to deprioritize diagnosing dry mouth. To that point, saliva is often not considered in the same category as other bodily fluids. According to Mandel, “It lacks the drama of blood, the sincerity of sweat…[and] the emotional appeal of tears.”6 In reality, saliva plays a critical role in the oral-digestive tract and in swallowing. It contains the first digestive enzymes in the gastrointestinal tract and is key for maintaining homeostasis in the oral cavity.7 Decreased saliva production results in difficulties with speech and mastication as well as problems of dysphagia, esophageal dysfunction, dysgeusia, nutritional compromises, new and recurrent dental caries, candidiasis, glossitis, impaired use of dentures, halitosis, and susceptibility to mucosal injury.7,8 Problems with the production of saliva may lead to loss of QOL, such as enjoying a meal or conversing with others.4
Although xerostomia is often associated with advanced age, it is more often explained by the diseases that afflict geriatric patients and the arsenal of medications used to treat them.2,9-16 Polypharmacy, the simultaneous use of multiple drugs by a single patient for ≥ 1 conditions, is an independent risk factor for xerostomia regardless of the types of medication taken.16 From 2005 to 2011, older adults in the US significantly increased their prescription medication use and dietary supplements. More than one-third of older adults used ≥ 5 prescription medications concurrently, and two-thirds of older adults used combinations of prescribed medications, over-the-counter medications, and dietary supplements.17 Several drug classes have the capacity to induce xerostomia, such as antihypertensives, antiulcer agents, anticholinergics, and antidepressants.2,5,12 Prevalence of dry mouth also can range from 10% to 46%, and women typically are more medicated and symptomatic.2,3,9,13,14,16 Xerostomia can also lead to depression and even reduce patients’ will to live.18 Despite xerostomia’s prevalence and impact on QOL, few patients report it as their chief symptom, and few physicians attempt to treat it.19
In order to target polypharmacy as a cause of dry mouth, the objectives for this study were to evaluate (1) the prevalence of xerostomia; (2) the relationship between xerostomia and other oral conditions; and (3) the impact of polypharmacy on dry mouth in a veteran population.
Methods
This is a retrospective cross-sectional study of all outpatient visits in fiscal year (FY) 2015 (October 1, 2014 to September 30, 2015) at the VA Palo Alto Health Care System (VAPAHCS), a tertiary care US Department of Veterans Affairs (VA) hospital. This study was approved by the Stanford University Institutional Review Board. All patients diagnosed with xerostomia in the 1-year study period were identified using ICD-9 diagnosis codes for dry mouth or disturbance of salivary gland secretion (527.7, 527.8, R68.2) and Systemized Nomenclature of Medicine Clinical Terms (SNOMED CT) codes covering dry mouth, xerostomia, aptyalism, absent salivary secretion, and disturbance of salivary secretion (87715008, 78948009). Data analysts in the VA Office of Business Analytics assisted in gathering data from the Veterans Information Systems and Technology Architecture (VistA) electronic health record.
The statistical analysis of that data was performed using Microsoft Excel. Age and gender distributions were determined for the patients. The relationship between xerostomia and the number and types of medications taken by patients also was examined. A previous Swedish study examining the link between dry mouth and quantities of medications used a scale ranging from 0 to ≥ 7 medications.16 The scale for this study was made wider to include the following groups: 0-2, 3-5, 6-8, 9-11, and ≥ 12 medications. Items that do not have xerogenic risks, such as medical supplies (eg, gloves, syringes, etc) and topical medications, were excluded from the analysis. Finally, the number of subjects with comorbid problems with speech, dentition, or swallowing (SDS) was recorded. Non-VA medications were included to capture any self- or externally prescribed xerogenic medications.
Results
Of the patients seen at VAPAHCS during FY 2015, 138 had a diagnostic code for xerostomia, including 129 men (93.5%) and 9 women (6.5%). The average (SD) age of this xerostomia cohort was 69.3 (12.6) years, and the 3 most common age groups were 60 to 69 years (37.7%), 70 to 79 years (28.3%), and 80 to 89 years (13.0%) (Table 1). Of those 138 patients with a xerostomia diagnosis, a majority (84; 60.9%) had at least 1 documented SDS problem (Table 2). Conversely, during FY 2015, although 4,971 patients seen at VAPAHCS had documented SDS problems, only 77 (1.5%) had a recorded diagnosis of xerostomia
Of the 138 patients with xerostomia, 55 (39.9%) were taking ≥ 12 medications, more than twice as many patients as in any of the other groups studied (0-2, 3-5, 6-8, and 9-11 medications taken) (Table 3). On average, each patient with xerostomia filled prescriptions for 10.4 (SD, 7.2) different drugs. In this cohort of 138 patients diagnosed with xerostomia, antihypertensive medications or analgesics were taken by > 50% of patients, while statins, psychiatric medications, antibiotics, proton pump inhibitors, or drugs known to have anticholinergic activity were taken by > 40%. Antihistamines, anticonvulsants, diuretics, or inhaled respiratory agents were used by > 20% of the patients in this cohort (Table 4).
Data on each individual medication were split into 2 categories: the percentage of patients that filled ≥ 1 prescription for that drug, and the total number of prescriptions filled and/or refilled for that drug (ie, including all fills and refills made by individual patients). The 5 most widely used medications in this cohort were omeprazole (39.1%), docusate sodium (29.7%), gabapentin (29.7%), aspirin (27.5%), and hydrocodone/acetaminophen (26.1%) (Table 5). The 5 prescriptions that were cumulatively most filled and/or refilled were omeprazole (128), sildenafil citrate (108), gabapentin (101), hydrocodone/acetaminophen (100), and oxycodone (92) (Table 6). Though sildenafil citrate and oxycodone were among the most-filled prescriptions, these were not included in Table 5 as neither was taken by > 15% of the patients studied. These prescriptions were filled multiple times by a small subset of patients.
Regarding treatment for dry mouth, artificial saliva spray was one of the most widely used (23.2%) and the seventh most-filled prescription within this cohort (86). The only other medication taken by > 15% of patients in a formulation other than a tablet or capsule was chlorhexidine, a germicidal mouthwash used to improve oral care.
Also, 30 (21.7%) patients with a documented xerostomia diagnosis had a history of substance misuse involving use of ≥ 1 of tobacco, alcohol, marijuana, or other illicit drugs.
Discussion
Saliva is an essential component for the maintenance of normal oral health.20,21 Decreased saliva production causes problems, including difficulties with speech, mastication, dysphagia, changes in taste, dental caries, impaired use of prostheses, recurrent infections, halitosis, deterioration of soft tissues, and compromised QOL.22,23 Among patients with a diagnosed SDS abnormality who were seen at this facility during FY 2015, the prevalence of xerostomia was only 1.5%. However, the true prevalence and incidence of xerostomia among veterans is not known. Given the role of xerostomia as a common risk factor for SDS problems and the polypharmacy exhibited by those presented here with SDS problems, it is probable that xerostomia was underreported in this veteran cohort.
Additionally, although salivary acinar cells are known to atrophy with age, as is consistent with this xerostomia cohort’s average age (SD) of 69.3 (12.6) years, the development of dry mouth is a multifactorial process. The current scientific literature asserts that most salivary loss is due to local and systemic diseases, immunologic disorders, external radiation, and as was highlighted by this study, multiple prescription and nonprescription medications.24-26
It has also been demonstrated previously that dry mouth complaints and low salivary flow rates are directly proportional to the number of medications taken by patients.2,27-30 Polypharmacy is therefore an area of great interest, and ≥ 40 categories of xerogenic medications have been identified by investigators such as Sreebny and Schwartz.31 Among those, some of the most xerogenic medication classes include antihypertensives, antiulcer agents, anticholinergics, and antidepressants, are all very commonly consumed in this cohort of patients with dry mouth (58.7%, 42.0%, 47.1%, and 38.4%, respectively). The medication regimens within this cohort of veterans with xerostomia were prime examples of polypharmacy as each patient took an average (SD) of 10.4 (7.2) medications, 39.9% took ≥ 12, and 72.5% of patients with xerostomia were taking ≥ 6 prescription drugs during a 1-year period.
Given the dangers of polypharmacy, a more conservative approach to prescribing medications could feasibly help with preventing xerostomia and SGH. In practice, while clinicians try to avoid prescribing anticholinergics, antimuscarinics, and antihistaminergic drugs for geriatric patients, they are tasked with the complex management of medication adverse effects (AEs) when dealing with multiple health conditions. The clinicians’ primary responsibilities are to follow the standard of care and not to introduce unnecessary harm when managing patients, but they also must push for, stay abreast of, and conduct more basic research and clinical trials to inform, adjust, and improve our current standard.
Research into polypharmacy and its role in inducing dry mouth is ongoing. Twenty years ago, Thomson and colleagues identified reduced salivary flow in patients who used antianginals, thyroxine, diuretics, antidepressants, and medications for asthma, while only 5 years earlier Loesche and colleagues reported the role of antiulcer medications, such as proton pump inhibitors, in the development of xerostomia.2,32 Within the past 5 years, Viljakainen and colleagues and Ohara and colleagues have echoed some of those findings by identifying associations between xerostomia and agents that impact digestive organs.33,34 A strong association recently was identified between the use of antipsychotic drugs and xerostomia.35 Additionally, when attributing xerostomia to polypharmacy, the interaction between medications is often overlooked in favor of considering the raw number of prescriptions taken. Whereas 1 medication alone may not have drying properties, combinations of medications might be more likely to induce xerostomia. Thomson and colleagues suggested such a situation regarding the interaction between thyroxine and diuretics.36 Future studies should focus on identifying viable substitutes for existing medications that reduce risk for xerostomia without compromising the management of other serious conditions.
Treatment
Another pressing question for clinicians concerns artificial saliva. Although 23.2% of patients with dry mouth in this xerostomia cohort used artificial saliva, the efficacy of this treatment is still unproven. Saliva substitutes are often used by patients who cannot produce sufficient amounts of natural saliva. In practice, artificial saliva produces, at best, modest temporary improvement in dry mouth symptoms in up to 40% of patients. At worst, as put forth by the Cochrane Review, artificial saliva may be no better than placebo in treating dry mouth.37,38 The volumes needed for symptom relief are large, ranging between 40 mL and 150 mL per day depending on the substitute’s composition. Saliva substitutes also must be reapplied throughout each day. This is particularly bothersome when patients must wake up repeatedly to reapply the treatment at night.37 In short, these substances do not seem wholly effective in managing dry mouth, and other options must be made available to patients with refractory xerostomia when artificial saliva and lifestyle modifications fail.
For now, few alternatives exist. Chewing gums and lozenges help to stimulate salivary flow, as do muscarinic agonists like pilocarpine. Unfortunately, muscarinic agonists are seldom used due to cholinergic AEs. Humidifiers are effective in increasing nighttime moisture but are contraindicated in patients with dust mite allergies.39 Reservoir-based devices with automated pumps funnel water and/or salivary substitutes from a fanny pack into patients’ mouths for lubrication.37 Other more esoteric pharmacologic treatments include D-limonene, yohimbine, and amifostine, which purportedly protect salivary progenitor cells, increase peripheral cholinergic activity, and protect salivary glands from free-radical damage during radiation treatment, respectively. Although these agents have shown some promise, D-limonene is difficult to administer given the high dosage required for treatment, yohimbine hasn’t been seriously investigated for improving salivary secretion since 1997, and amifostine isn’t used widely due to its AE profile despite its US Food and Drug Administration approval for prevention of xerostomia.39
Substance Abuse
The impact of smoking on xerostomia remains controversial. Some studies report an association between active smoking and xerostomia; others suggest that the local irritant effect of tobacco smoke may increase salivary gland output.40,41 The same may be true for chronic alcohol use as there are no epidemiologic studies showing a causal relationship between alcohol use and xerostomia. Studies with rats that are chronically exposed to ethanol have found increased salivary flow rates.42 In the xerostomia cohort presented here, 30 patients (21.7%) had a documented history of substance misuse. That percentage is likely underestimated, as substance misuse is often underreported, and frequent use may not always constitute misuse. Therefore, nicotine exposure, alcohol exposure, illicit drug use, and vaping all should be considered during the workup of a patient with xerostomia.
Limitations
It is common for medications to remain in a patient’s health record long after that patient stops taking them. Developing methods to track when patients discontinue their prescriptions will be essential for clearing up uncertainty in our data and in other similar studies. This study also did not account for patients’ medication adherence and the duration of exposure to medications and illicit substances. Furthermore, the results of this veteran study are not easily generalizable as this cohort is disproportionately male, of advanced age, and especially prone to exhibiting both substance use and psychiatric diagnoses relative to the general population. As described by Viljakainen and colleagues, risk factors for xerostomia include advanced age, female gender, low body mass index (BMI), malnutrition, and depressive symptoms, but because the demographic scope of this veteran population was narrow, it was not possible to discern the impact of, for example, gender.33 Data on variables like BMI, malnutrition, and depressive symptoms were not available. For this study, xerostomia could only be considered as an all-or-nothing phenomenon because the dataset did not describe different levels of dry mouth severity (eg, mild, moderate, severe).
Additionally, past polypharmacy studies have acknowledged an inability to tell whether xerostomia is mainly due to medications or to underlying medical conditions. For example, for emphysema, ß-adrenergic stimulation from bronchodilators could cause dry mouth by thickening saliva and decreasing salivary volume, but the pathophysiology and/or cardinal symptoms of emphysema, including chronic obstructive pulmonary disease-associated tachypnea, might contribute independently to dryness.
Though we can make inferences based on the medications taken by this cohort (eg, those taking antihypertensives have high blood pressure), this dataset did not explicitly detail comorbid conditions and ICD codes for chronic diseases that commonly arise with xerostomia. Those conditions, however, are of great clinical importance. Diabetes mellitus, HIV/AIDS, and, classically, Sjögren syndrome, all are known to cause dry mouth.43 Identifying new conditions that co-occur with xerostomia would allow clinicians to describe the root causes of and risk factors for dry mouth and SDS conditions in greater detail. Patients with dry mouth without SDS problems in this dataset are of particular interest as closer examination of their medications and comorbid conditions could help us understand why some individuals and not others develop SDS problems. The subjects of how comorbidities contribute to dry mouth and how their influences can be judged independently from the effects of medications are of great interest to us and will be investigated rigorously in our future studies.
Conclusions
In this cohort, few patients with SDS problems had documentation of a concomitant xerostomia diagnosis. This could represent a true infrequency of dry mouth or more likely, an underrecognition by clinicians. Heightened physician awareness regarding the signs and symptoms of and risk factors for xerostomia is needed to improve providers’ ability to diagnose this condition.
In particular, polypharmacy should be a major consideration when evaluating patients for xerostomia. This continues to be an important area of research, and some of the latest data on polypharmacy among older patients were compiled in a recent meta-analysis by Tan and colleagues. The authors of that systematic review reiterated the significant association between salivary gland hypofunction and the number of medications taken by patients. They also advocated for the creation of a risk score for medication-induced dry mouth to aid in medication management.44 Per their recommendations, it is now as crucial as ever to consider the numbers and types of medications taken by patients, to discontinue unnecessary prescriptions when possible, and to continue developing new strategies for preventing and treating xerostomia.
Xerostomia, the subjective sensation of dry mouth, is a common problem developed by geriatric patients. In practice, xerostomia can impair swallowing, speech, and oral hygiene, and if left unchecked, symptoms such as dysphagia and dysarthria can diminish patients’ quality of life (QOL). Salivary gland hypofunction (SGH) is the objective measure of decreased saliva production, determined by sialometry. Although xerostomia and SGH can coexist, the 2 conditions are not necessarily related.1-4 For this discussion, the term xerostomia will denote dry mouth with or without a concomitant diagnosis of SGH.
Xerostomia is seen in a wide variety of patients with varied comorbidities. It is commonly associated with Sjögren syndrome and head and neck irradiation. The diagnosis and treatment of xerostomia often involves rheumatologists, dentists, otolaryngologists, and oncologists. Additionally, most of the scientific literature about this topic exists in dental journals, such as the Journal of the American Dental Association and the British Dental Journal. Rarer still are studies in the veteran population.5
Faced with increasing time pressure to treat the many chronic diseases affecting aging veterans, health care providers (HCPs) tend to deprioritize diagnosing dry mouth. To that point, saliva is often not considered in the same category as other bodily fluids. According to Mandel, “It lacks the drama of blood, the sincerity of sweat…[and] the emotional appeal of tears.”6 In reality, saliva plays a critical role in the oral-digestive tract and in swallowing. It contains the first digestive enzymes in the gastrointestinal tract and is key for maintaining homeostasis in the oral cavity.7 Decreased saliva production results in difficulties with speech and mastication as well as problems of dysphagia, esophageal dysfunction, dysgeusia, nutritional compromises, new and recurrent dental caries, candidiasis, glossitis, impaired use of dentures, halitosis, and susceptibility to mucosal injury.7,8 Problems with the production of saliva may lead to loss of QOL, such as enjoying a meal or conversing with others.4
Although xerostomia is often associated with advanced age, it is more often explained by the diseases that afflict geriatric patients and the arsenal of medications used to treat them.2,9-16 Polypharmacy, the simultaneous use of multiple drugs by a single patient for ≥ 1 conditions, is an independent risk factor for xerostomia regardless of the types of medication taken.16 From 2005 to 2011, older adults in the US significantly increased their prescription medication use and dietary supplements. More than one-third of older adults used ≥ 5 prescription medications concurrently, and two-thirds of older adults used combinations of prescribed medications, over-the-counter medications, and dietary supplements.17 Several drug classes have the capacity to induce xerostomia, such as antihypertensives, antiulcer agents, anticholinergics, and antidepressants.2,5,12 Prevalence of dry mouth also can range from 10% to 46%, and women typically are more medicated and symptomatic.2,3,9,13,14,16 Xerostomia can also lead to depression and even reduce patients’ will to live.18 Despite xerostomia’s prevalence and impact on QOL, few patients report it as their chief symptom, and few physicians attempt to treat it.19
In order to target polypharmacy as a cause of dry mouth, the objectives for this study were to evaluate (1) the prevalence of xerostomia; (2) the relationship between xerostomia and other oral conditions; and (3) the impact of polypharmacy on dry mouth in a veteran population.
Methods
This is a retrospective cross-sectional study of all outpatient visits in fiscal year (FY) 2015 (October 1, 2014 to September 30, 2015) at the VA Palo Alto Health Care System (VAPAHCS), a tertiary care US Department of Veterans Affairs (VA) hospital. This study was approved by the Stanford University Institutional Review Board. All patients diagnosed with xerostomia in the 1-year study period were identified using ICD-9 diagnosis codes for dry mouth or disturbance of salivary gland secretion (527.7, 527.8, R68.2) and Systemized Nomenclature of Medicine Clinical Terms (SNOMED CT) codes covering dry mouth, xerostomia, aptyalism, absent salivary secretion, and disturbance of salivary secretion (87715008, 78948009). Data analysts in the VA Office of Business Analytics assisted in gathering data from the Veterans Information Systems and Technology Architecture (VistA) electronic health record.
The statistical analysis of that data was performed using Microsoft Excel. Age and gender distributions were determined for the patients. The relationship between xerostomia and the number and types of medications taken by patients also was examined. A previous Swedish study examining the link between dry mouth and quantities of medications used a scale ranging from 0 to ≥ 7 medications.16 The scale for this study was made wider to include the following groups: 0-2, 3-5, 6-8, 9-11, and ≥ 12 medications. Items that do not have xerogenic risks, such as medical supplies (eg, gloves, syringes, etc) and topical medications, were excluded from the analysis. Finally, the number of subjects with comorbid problems with speech, dentition, or swallowing (SDS) was recorded. Non-VA medications were included to capture any self- or externally prescribed xerogenic medications.
Results
Of the patients seen at VAPAHCS during FY 2015, 138 had a diagnostic code for xerostomia, including 129 men (93.5%) and 9 women (6.5%). The average (SD) age of this xerostomia cohort was 69.3 (12.6) years, and the 3 most common age groups were 60 to 69 years (37.7%), 70 to 79 years (28.3%), and 80 to 89 years (13.0%) (Table 1). Of those 138 patients with a xerostomia diagnosis, a majority (84; 60.9%) had at least 1 documented SDS problem (Table 2). Conversely, during FY 2015, although 4,971 patients seen at VAPAHCS had documented SDS problems, only 77 (1.5%) had a recorded diagnosis of xerostomia
Of the 138 patients with xerostomia, 55 (39.9%) were taking ≥ 12 medications, more than twice as many patients as in any of the other groups studied (0-2, 3-5, 6-8, and 9-11 medications taken) (Table 3). On average, each patient with xerostomia filled prescriptions for 10.4 (SD, 7.2) different drugs. In this cohort of 138 patients diagnosed with xerostomia, antihypertensive medications or analgesics were taken by > 50% of patients, while statins, psychiatric medications, antibiotics, proton pump inhibitors, or drugs known to have anticholinergic activity were taken by > 40%. Antihistamines, anticonvulsants, diuretics, or inhaled respiratory agents were used by > 20% of the patients in this cohort (Table 4).
Data on each individual medication were split into 2 categories: the percentage of patients that filled ≥ 1 prescription for that drug, and the total number of prescriptions filled and/or refilled for that drug (ie, including all fills and refills made by individual patients). The 5 most widely used medications in this cohort were omeprazole (39.1%), docusate sodium (29.7%), gabapentin (29.7%), aspirin (27.5%), and hydrocodone/acetaminophen (26.1%) (Table 5). The 5 prescriptions that were cumulatively most filled and/or refilled were omeprazole (128), sildenafil citrate (108), gabapentin (101), hydrocodone/acetaminophen (100), and oxycodone (92) (Table 6). Though sildenafil citrate and oxycodone were among the most-filled prescriptions, these were not included in Table 5 as neither was taken by > 15% of the patients studied. These prescriptions were filled multiple times by a small subset of patients.
Regarding treatment for dry mouth, artificial saliva spray was one of the most widely used (23.2%) and the seventh most-filled prescription within this cohort (86). The only other medication taken by > 15% of patients in a formulation other than a tablet or capsule was chlorhexidine, a germicidal mouthwash used to improve oral care.
Also, 30 (21.7%) patients with a documented xerostomia diagnosis had a history of substance misuse involving use of ≥ 1 of tobacco, alcohol, marijuana, or other illicit drugs.
Discussion
Saliva is an essential component for the maintenance of normal oral health.20,21 Decreased saliva production causes problems, including difficulties with speech, mastication, dysphagia, changes in taste, dental caries, impaired use of prostheses, recurrent infections, halitosis, deterioration of soft tissues, and compromised QOL.22,23 Among patients with a diagnosed SDS abnormality who were seen at this facility during FY 2015, the prevalence of xerostomia was only 1.5%. However, the true prevalence and incidence of xerostomia among veterans is not known. Given the role of xerostomia as a common risk factor for SDS problems and the polypharmacy exhibited by those presented here with SDS problems, it is probable that xerostomia was underreported in this veteran cohort.
Additionally, although salivary acinar cells are known to atrophy with age, as is consistent with this xerostomia cohort’s average age (SD) of 69.3 (12.6) years, the development of dry mouth is a multifactorial process. The current scientific literature asserts that most salivary loss is due to local and systemic diseases, immunologic disorders, external radiation, and as was highlighted by this study, multiple prescription and nonprescription medications.24-26
It has also been demonstrated previously that dry mouth complaints and low salivary flow rates are directly proportional to the number of medications taken by patients.2,27-30 Polypharmacy is therefore an area of great interest, and ≥ 40 categories of xerogenic medications have been identified by investigators such as Sreebny and Schwartz.31 Among those, some of the most xerogenic medication classes include antihypertensives, antiulcer agents, anticholinergics, and antidepressants, are all very commonly consumed in this cohort of patients with dry mouth (58.7%, 42.0%, 47.1%, and 38.4%, respectively). The medication regimens within this cohort of veterans with xerostomia were prime examples of polypharmacy as each patient took an average (SD) of 10.4 (7.2) medications, 39.9% took ≥ 12, and 72.5% of patients with xerostomia were taking ≥ 6 prescription drugs during a 1-year period.
Given the dangers of polypharmacy, a more conservative approach to prescribing medications could feasibly help with preventing xerostomia and SGH. In practice, while clinicians try to avoid prescribing anticholinergics, antimuscarinics, and antihistaminergic drugs for geriatric patients, they are tasked with the complex management of medication adverse effects (AEs) when dealing with multiple health conditions. The clinicians’ primary responsibilities are to follow the standard of care and not to introduce unnecessary harm when managing patients, but they also must push for, stay abreast of, and conduct more basic research and clinical trials to inform, adjust, and improve our current standard.
Research into polypharmacy and its role in inducing dry mouth is ongoing. Twenty years ago, Thomson and colleagues identified reduced salivary flow in patients who used antianginals, thyroxine, diuretics, antidepressants, and medications for asthma, while only 5 years earlier Loesche and colleagues reported the role of antiulcer medications, such as proton pump inhibitors, in the development of xerostomia.2,32 Within the past 5 years, Viljakainen and colleagues and Ohara and colleagues have echoed some of those findings by identifying associations between xerostomia and agents that impact digestive organs.33,34 A strong association recently was identified between the use of antipsychotic drugs and xerostomia.35 Additionally, when attributing xerostomia to polypharmacy, the interaction between medications is often overlooked in favor of considering the raw number of prescriptions taken. Whereas 1 medication alone may not have drying properties, combinations of medications might be more likely to induce xerostomia. Thomson and colleagues suggested such a situation regarding the interaction between thyroxine and diuretics.36 Future studies should focus on identifying viable substitutes for existing medications that reduce risk for xerostomia without compromising the management of other serious conditions.
Treatment
Another pressing question for clinicians concerns artificial saliva. Although 23.2% of patients with dry mouth in this xerostomia cohort used artificial saliva, the efficacy of this treatment is still unproven. Saliva substitutes are often used by patients who cannot produce sufficient amounts of natural saliva. In practice, artificial saliva produces, at best, modest temporary improvement in dry mouth symptoms in up to 40% of patients. At worst, as put forth by the Cochrane Review, artificial saliva may be no better than placebo in treating dry mouth.37,38 The volumes needed for symptom relief are large, ranging between 40 mL and 150 mL per day depending on the substitute’s composition. Saliva substitutes also must be reapplied throughout each day. This is particularly bothersome when patients must wake up repeatedly to reapply the treatment at night.37 In short, these substances do not seem wholly effective in managing dry mouth, and other options must be made available to patients with refractory xerostomia when artificial saliva and lifestyle modifications fail.
For now, few alternatives exist. Chewing gums and lozenges help to stimulate salivary flow, as do muscarinic agonists like pilocarpine. Unfortunately, muscarinic agonists are seldom used due to cholinergic AEs. Humidifiers are effective in increasing nighttime moisture but are contraindicated in patients with dust mite allergies.39 Reservoir-based devices with automated pumps funnel water and/or salivary substitutes from a fanny pack into patients’ mouths for lubrication.37 Other more esoteric pharmacologic treatments include D-limonene, yohimbine, and amifostine, which purportedly protect salivary progenitor cells, increase peripheral cholinergic activity, and protect salivary glands from free-radical damage during radiation treatment, respectively. Although these agents have shown some promise, D-limonene is difficult to administer given the high dosage required for treatment, yohimbine hasn’t been seriously investigated for improving salivary secretion since 1997, and amifostine isn’t used widely due to its AE profile despite its US Food and Drug Administration approval for prevention of xerostomia.39
Substance Abuse
The impact of smoking on xerostomia remains controversial. Some studies report an association between active smoking and xerostomia; others suggest that the local irritant effect of tobacco smoke may increase salivary gland output.40,41 The same may be true for chronic alcohol use as there are no epidemiologic studies showing a causal relationship between alcohol use and xerostomia. Studies with rats that are chronically exposed to ethanol have found increased salivary flow rates.42 In the xerostomia cohort presented here, 30 patients (21.7%) had a documented history of substance misuse. That percentage is likely underestimated, as substance misuse is often underreported, and frequent use may not always constitute misuse. Therefore, nicotine exposure, alcohol exposure, illicit drug use, and vaping all should be considered during the workup of a patient with xerostomia.
Limitations
It is common for medications to remain in a patient’s health record long after that patient stops taking them. Developing methods to track when patients discontinue their prescriptions will be essential for clearing up uncertainty in our data and in other similar studies. This study also did not account for patients’ medication adherence and the duration of exposure to medications and illicit substances. Furthermore, the results of this veteran study are not easily generalizable as this cohort is disproportionately male, of advanced age, and especially prone to exhibiting both substance use and psychiatric diagnoses relative to the general population. As described by Viljakainen and colleagues, risk factors for xerostomia include advanced age, female gender, low body mass index (BMI), malnutrition, and depressive symptoms, but because the demographic scope of this veteran population was narrow, it was not possible to discern the impact of, for example, gender.33 Data on variables like BMI, malnutrition, and depressive symptoms were not available. For this study, xerostomia could only be considered as an all-or-nothing phenomenon because the dataset did not describe different levels of dry mouth severity (eg, mild, moderate, severe).
Additionally, past polypharmacy studies have acknowledged an inability to tell whether xerostomia is mainly due to medications or to underlying medical conditions. For example, for emphysema, ß-adrenergic stimulation from bronchodilators could cause dry mouth by thickening saliva and decreasing salivary volume, but the pathophysiology and/or cardinal symptoms of emphysema, including chronic obstructive pulmonary disease-associated tachypnea, might contribute independently to dryness.
Though we can make inferences based on the medications taken by this cohort (eg, those taking antihypertensives have high blood pressure), this dataset did not explicitly detail comorbid conditions and ICD codes for chronic diseases that commonly arise with xerostomia. Those conditions, however, are of great clinical importance. Diabetes mellitus, HIV/AIDS, and, classically, Sjögren syndrome, all are known to cause dry mouth.43 Identifying new conditions that co-occur with xerostomia would allow clinicians to describe the root causes of and risk factors for dry mouth and SDS conditions in greater detail. Patients with dry mouth without SDS problems in this dataset are of particular interest as closer examination of their medications and comorbid conditions could help us understand why some individuals and not others develop SDS problems. The subjects of how comorbidities contribute to dry mouth and how their influences can be judged independently from the effects of medications are of great interest to us and will be investigated rigorously in our future studies.
Conclusions
In this cohort, few patients with SDS problems had documentation of a concomitant xerostomia diagnosis. This could represent a true infrequency of dry mouth or more likely, an underrecognition by clinicians. Heightened physician awareness regarding the signs and symptoms of and risk factors for xerostomia is needed to improve providers’ ability to diagnose this condition.
In particular, polypharmacy should be a major consideration when evaluating patients for xerostomia. This continues to be an important area of research, and some of the latest data on polypharmacy among older patients were compiled in a recent meta-analysis by Tan and colleagues. The authors of that systematic review reiterated the significant association between salivary gland hypofunction and the number of medications taken by patients. They also advocated for the creation of a risk score for medication-induced dry mouth to aid in medication management.44 Per their recommendations, it is now as crucial as ever to consider the numbers and types of medications taken by patients, to discontinue unnecessary prescriptions when possible, and to continue developing new strategies for preventing and treating xerostomia.
1. Thomson WM, Chalmers JM, Spencer AJ, Ketabi M. The occurrence of xerostomia and salivary gland hypofunction in a population-based sample of older South Australians. Spec Care Dentist. 1999;19(1):20-23.
2. Thomson WM, Chalmers JM, Spencer AJ, Slade GD. Medication and dry mouth: findings from a cohort study of older people. J Public Health Dent. 2000;60(1):12-20.
3. Sasportas LS, Hosford DN, Sodini MA, et al. Cost-effectiveness landscape analysis of treatments addressing xerostomia in patients receiving head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):e37-e51.
4. Bivona PL. Xerostomia. A common problem among the elderly. N Y State Dent J. 1998;64(6):46-52.
5. Ness J, Hoth A, Barnett MJ, Shorr RI, Kaboli PJ. Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother. 2006;4(1):42-51.
6. Mandel ID. The diagnostic uses of saliva. J Oral Pathol Med. 1990;19(3):119-125.
7. Friedman PK, Isfeld D. Xerostomia: the “invisible” oral health condition. J Mass Dent Soc. 2008;57(3):42-44.
8. Ship JA, McCutcheon JA, Spivakovsky S, Kerr AR. Safety and effectiveness of topical dry mouth products containing olive oil, betaine, and xylitol in reducing xerostomia for polypharmacy-induced dry mouth. J Oral Rehabil. 2007;34(10):724-732.
9. Field EA, Fear S, Higham SM, et al. Age and medication are significant risk factors for xerostomia in an English population, attending general dental practice. Gerodontology. 2001;18(1):21-24.
10. Villa A, Connell CL, Abati S. Diagnosis and management of xerostomia and hyposalivation. Ther Clin Risk Manag. 2015;11:45-51.
11. Geuiros LA, Soares MS, Leao JC. Impact of ageing and drug consumption on oral health. Gerodontology. 2009;26(4):297-301.
12. Singh ML, Papas A. Oral implications of polypharmacy in the elderly. Dent Clin North Am. 2014;58(4):783-796.
13. Shinkai RS, Hatch JP, Schmidt CB, Sartori EA. Exposure to the oral side effects of medication in a community-based sample. Spec Care Dentist. 2006;26(3):116-120.
14. Hopcraft MS, Tan C. Xerostomia: an update for clinicians. Aust Dent J. 2010;55(3):238-244; quiz 353.
15. Ettinger RL. Review: xerostomia: a symptom which acts like a disease. Age Ageing. 1996;25(5):409-412.
16. Nederfors T, Isaksson R, Mornstad H, Dahlof C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population—relation to age, sex and pharmacotherapy. Community Dent Oral Epidemiol. 1997;25(3):211-216.
17. Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473-482.
18. Dirix P, Nuyts S, Vander Poorten V, Delaere P, Van den Boaert W. The influence of xerostomia after radiotherapy on quality of life: results of a questionnaire in head and neck cancer. Support Care Cancer. 2008;16(2):171-179.
19. Sreebny LM, Valdini A. Xerostomia. A neglected symptom. Arch Intern Med. 1987;147(7):1333-1337.
20. Sreebny LM. Saliva in health and disease: an appraisal and update. Int Dent J. 2000;50(3):140-161.
21. Amerongen AV, Veeran EC. Saliva—the defender of the oral cavity. Oral Dis. 2002;8(1):12-22.
22. Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. 2003;134(1):61-69; quiz 118-119.
23. Atkinson JC, Baum BJ. Salivary enhancement: current status and future therapies. J Dent Educ. 2001;65(10):1096-1101.
24. Narhi TO, Meurman JH, Ainamo A. Xerostomia and hyposalivation: causes, consequences and treatment in the elderly. Drugs Aging. 1999;15(2):103-116.
25. Ship JA, Baum BJ. Is reduced salivary flow normal in old people? Lancet. 1990;336(8729):1507.
26. Ghezzi EM, Wagner-Lange LA, Schork MA, et al. Longitudinal influence of age, menopause, hormone replacement therapy, and other medications on parotid flow rates in healthy women. J Gerontol A Biol Sci Med Sci. 2000;55(1):M34-M42.
27. Fox PC. Acquired salivary dysfunction. Drugs and radiation. Ann N Y Acad Sci. 1998;842:132-137.
28. Bergdahl M, Bergdahl J. Low unstimulated salivary flow and subjective oral dryness: association with medication, anxiety, depression, and stress. J Dent Res. 2000;79(9):1652-1658.
29. Ship JA, Pillemer SR, Baum BJ. Xerostomia and the geriatric patient. J Am Geriatr Soc. 2002;50(3):535-543.
30. Sreebny LM, Valdini A, Yu A. Xerostomia. Part II: Relationship to nonoral symptoms, drugs, and diseases. Oral Surg Oral Med Oral Pathol. 1989;68(4):419-427.
31. Sreebny LM, Schwartz SS. A reference guide to drugs and dry mouth—2nd edition. Gerodontology. 1997;14(1):33-47.
32. Loesche WJ, Bromberg J, Terpenning MS, et al. Xerostomia, xerogenic medications and food avoidances in selected geriatric groups. J Am Geriatr Soc. 1995;43(4):401-407.

33. Viljakainen S, Nykanen I, Ahonen R, et al. Xerostomia among older home care clients. Community Dent Oral Epidemiol. 2016;44(3):232-238.
34. Ohara Y, Hirano H, Yoshida H, et al. Prevalence and factors associated with xerostomia and hyposalivation among community-dwelling older people in Japan. Gerodontology. 2016;33(1):20-27.
35. Okamoto A, Miyachi H, Tanaka K, Chikazu D, Miyaoka H. Relationship between xerostomia and psychotropic drugs in patients with schizophrenia: evaluation using an oral moisture meter. J Clin Pharm Ther. 2016;41(6):684-688.
36. Thomson WM. Dry mouth and older people. Aust Dent J. 2015;60(suppl 1):54-63.
37. Sasportas LS, Hosford AT, Sodini MA, et al. Cost-effectiveness landscape analysis of treatments addressing xerostomia in patients receiving head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):e37-e51.
38. Furness S, Worthington HV, Bryan G, Birchenough S, McMillan R. Interventions for the management of dry mouth: topical therapies. Cochrane Database Syst Rev. 2011;(12):CD008924.
39. Roblegg E, Coughran A, Sirjani D. Saliva: an all-rounder of our body. Eur J Pharm Biopharm. 2019;142:133-141.
40. Billings RJ, Proskin HM, Moss ME. Xerostomia and associated factors in a community-dwelling adult population. Community Dent Oral Epidemiol. 1996;24(5):312-316.
41. Norlen P, Ostberg H, Bjorn AL. Relationship between general health, social factors and oral health in women at the age of retirement. Community Dent Oral Epidemiol. 1991;19(5):296-301.
42. Berry MR, Scott J. Functional and structural adaptation of the parotid gland to medium-term chronic ethanol exposure in the rat. Alcohol Alcoholism. 1990;25(5):523-531.
43. von Bultzingslowen I, Sollecito TP, Fox PC, et al. Salivary dysfunction associated with systemic diseases: systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol. 2007;103:S57.e1-e15.
44. Tan ECK, Lexomboon D, Sandborgh-Englund G, et al. Medications that cause dry mouth as an adverse effect in older people: a systematic review and metaanalysis. J Am Geriatr Soc. 2018;66(1):76-84.
1. Thomson WM, Chalmers JM, Spencer AJ, Ketabi M. The occurrence of xerostomia and salivary gland hypofunction in a population-based sample of older South Australians. Spec Care Dentist. 1999;19(1):20-23.
2. Thomson WM, Chalmers JM, Spencer AJ, Slade GD. Medication and dry mouth: findings from a cohort study of older people. J Public Health Dent. 2000;60(1):12-20.
3. Sasportas LS, Hosford DN, Sodini MA, et al. Cost-effectiveness landscape analysis of treatments addressing xerostomia in patients receiving head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):e37-e51.
4. Bivona PL. Xerostomia. A common problem among the elderly. N Y State Dent J. 1998;64(6):46-52.
5. Ness J, Hoth A, Barnett MJ, Shorr RI, Kaboli PJ. Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother. 2006;4(1):42-51.
6. Mandel ID. The diagnostic uses of saliva. J Oral Pathol Med. 1990;19(3):119-125.
7. Friedman PK, Isfeld D. Xerostomia: the “invisible” oral health condition. J Mass Dent Soc. 2008;57(3):42-44.
8. Ship JA, McCutcheon JA, Spivakovsky S, Kerr AR. Safety and effectiveness of topical dry mouth products containing olive oil, betaine, and xylitol in reducing xerostomia for polypharmacy-induced dry mouth. J Oral Rehabil. 2007;34(10):724-732.
9. Field EA, Fear S, Higham SM, et al. Age and medication are significant risk factors for xerostomia in an English population, attending general dental practice. Gerodontology. 2001;18(1):21-24.
10. Villa A, Connell CL, Abati S. Diagnosis and management of xerostomia and hyposalivation. Ther Clin Risk Manag. 2015;11:45-51.
11. Geuiros LA, Soares MS, Leao JC. Impact of ageing and drug consumption on oral health. Gerodontology. 2009;26(4):297-301.
12. Singh ML, Papas A. Oral implications of polypharmacy in the elderly. Dent Clin North Am. 2014;58(4):783-796.
13. Shinkai RS, Hatch JP, Schmidt CB, Sartori EA. Exposure to the oral side effects of medication in a community-based sample. Spec Care Dentist. 2006;26(3):116-120.
14. Hopcraft MS, Tan C. Xerostomia: an update for clinicians. Aust Dent J. 2010;55(3):238-244; quiz 353.
15. Ettinger RL. Review: xerostomia: a symptom which acts like a disease. Age Ageing. 1996;25(5):409-412.
16. Nederfors T, Isaksson R, Mornstad H, Dahlof C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population—relation to age, sex and pharmacotherapy. Community Dent Oral Epidemiol. 1997;25(3):211-216.
17. Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473-482.
18. Dirix P, Nuyts S, Vander Poorten V, Delaere P, Van den Boaert W. The influence of xerostomia after radiotherapy on quality of life: results of a questionnaire in head and neck cancer. Support Care Cancer. 2008;16(2):171-179.
19. Sreebny LM, Valdini A. Xerostomia. A neglected symptom. Arch Intern Med. 1987;147(7):1333-1337.
20. Sreebny LM. Saliva in health and disease: an appraisal and update. Int Dent J. 2000;50(3):140-161.
21. Amerongen AV, Veeran EC. Saliva—the defender of the oral cavity. Oral Dis. 2002;8(1):12-22.
22. Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. 2003;134(1):61-69; quiz 118-119.
23. Atkinson JC, Baum BJ. Salivary enhancement: current status and future therapies. J Dent Educ. 2001;65(10):1096-1101.
24. Narhi TO, Meurman JH, Ainamo A. Xerostomia and hyposalivation: causes, consequences and treatment in the elderly. Drugs Aging. 1999;15(2):103-116.
25. Ship JA, Baum BJ. Is reduced salivary flow normal in old people? Lancet. 1990;336(8729):1507.
26. Ghezzi EM, Wagner-Lange LA, Schork MA, et al. Longitudinal influence of age, menopause, hormone replacement therapy, and other medications on parotid flow rates in healthy women. J Gerontol A Biol Sci Med Sci. 2000;55(1):M34-M42.
27. Fox PC. Acquired salivary dysfunction. Drugs and radiation. Ann N Y Acad Sci. 1998;842:132-137.
28. Bergdahl M, Bergdahl J. Low unstimulated salivary flow and subjective oral dryness: association with medication, anxiety, depression, and stress. J Dent Res. 2000;79(9):1652-1658.
29. Ship JA, Pillemer SR, Baum BJ. Xerostomia and the geriatric patient. J Am Geriatr Soc. 2002;50(3):535-543.
30. Sreebny LM, Valdini A, Yu A. Xerostomia. Part II: Relationship to nonoral symptoms, drugs, and diseases. Oral Surg Oral Med Oral Pathol. 1989;68(4):419-427.
31. Sreebny LM, Schwartz SS. A reference guide to drugs and dry mouth—2nd edition. Gerodontology. 1997;14(1):33-47.
32. Loesche WJ, Bromberg J, Terpenning MS, et al. Xerostomia, xerogenic medications and food avoidances in selected geriatric groups. J Am Geriatr Soc. 1995;43(4):401-407.

33. Viljakainen S, Nykanen I, Ahonen R, et al. Xerostomia among older home care clients. Community Dent Oral Epidemiol. 2016;44(3):232-238.
34. Ohara Y, Hirano H, Yoshida H, et al. Prevalence and factors associated with xerostomia and hyposalivation among community-dwelling older people in Japan. Gerodontology. 2016;33(1):20-27.
35. Okamoto A, Miyachi H, Tanaka K, Chikazu D, Miyaoka H. Relationship between xerostomia and psychotropic drugs in patients with schizophrenia: evaluation using an oral moisture meter. J Clin Pharm Ther. 2016;41(6):684-688.
36. Thomson WM. Dry mouth and older people. Aust Dent J. 2015;60(suppl 1):54-63.
37. Sasportas LS, Hosford AT, Sodini MA, et al. Cost-effectiveness landscape analysis of treatments addressing xerostomia in patients receiving head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):e37-e51.
38. Furness S, Worthington HV, Bryan G, Birchenough S, McMillan R. Interventions for the management of dry mouth: topical therapies. Cochrane Database Syst Rev. 2011;(12):CD008924.
39. Roblegg E, Coughran A, Sirjani D. Saliva: an all-rounder of our body. Eur J Pharm Biopharm. 2019;142:133-141.
40. Billings RJ, Proskin HM, Moss ME. Xerostomia and associated factors in a community-dwelling adult population. Community Dent Oral Epidemiol. 1996;24(5):312-316.
41. Norlen P, Ostberg H, Bjorn AL. Relationship between general health, social factors and oral health in women at the age of retirement. Community Dent Oral Epidemiol. 1991;19(5):296-301.
42. Berry MR, Scott J. Functional and structural adaptation of the parotid gland to medium-term chronic ethanol exposure in the rat. Alcohol Alcoholism. 1990;25(5):523-531.
43. von Bultzingslowen I, Sollecito TP, Fox PC, et al. Salivary dysfunction associated with systemic diseases: systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol. 2007;103:S57.e1-e15.
44. Tan ECK, Lexomboon D, Sandborgh-Englund G, et al. Medications that cause dry mouth as an adverse effect in older people: a systematic review and metaanalysis. J Am Geriatr Soc. 2018;66(1):76-84.