User login
Earlier diagnosis, treatment needed to curb dramatic rise in neonatal HSV
A 56% increase in neonatal herpes simplex virus (HSV) infection over 7 years was determined as part of a retrospective, multistate, longitudinal cohort study using information collected from the MarketScan Medicaid Database, reported Sanjay Mahant, MD, of the University of Toronto, and his associates.
Comprehensive coordinated care – as well as public health strategies targeting disease prevention, early diagnosis, and treatment – are needed to manage the growing number of neonates diagnosed with HSV, Dr. Mahant and his colleagues said.
A total of 900 newborn Medicaid enrollees aged 0-28 days were chosen from 2,107,124 births for inclusion in the study. All patients, who were diagnosed with HSV infection during hospital admission, were born during Jan. 1, 2009–Dec. 31, 2015.
Susceptibility to primary HSV-1 infection among younger women has been attributed to an increase in oral sex practices over the past 2 decades, which is putting adolescents and young adults at greater risk of genital HSV-1 infection (J Infect Dis. 2007;196[12]:1852-9). As a result, more “primary or nonprimary genital HSV-1 infections among childbearing women” are believed to be the likely cause for the increasing numbers of neonatal HSV cases, the authors speculated, citing a recent study (J Infect Dis. 2014 Feb 1;209[3]:315-7).
HSV, a rare infection typically contracted immediately before or after birth, has both high morbidity and mortality rates; transmission rates “after exposure and during delivery increase from 2% in recurrent infection to 25% and 60% in nonprimary and primary infections, respectively,” Dr. Mahant and his colleagues noted.
Over the study period, disease incidence grew from 3.4/10,000 births in 2009 (1/2,941 births) to 5.3/10,000 births in 2015 (1/1,886 births).
Dr. Mahant and his associates noted several limitations in the study that might explain the increase in incidence.
ICD diagnosis codes, which they characterized as imperfect in their ability to correctly identify neonatal HSV infections, may have led researchers to include infants who were not actually infected or (less likely) to have excluded infants who were infected. States participating in the MarketScan Medicaid Database also may have changed over the study period. Incomplete follow-up after hospitalization made it impossible to track infants who had changed insurers, moved to other states, or died during the study. They also cautioned that outcomes may not be transferable to the general population because outcomes were specific to Medicaid enrollees.
The total cost for initial hospitalization and treatments provided during 6 months of follow-up was $60,620,431 ($87,602 median cost per patient) for the cohort of 900 infants. This is significant given that the authors reported a median length of stay of 18 days for initial hospitalization. Of the 846 patients discharged (54, or 6%, died during initial hospitalization), follow-up data was available for 692 (81%). A total of 316 (46%) infants required at least one subsequent visit to the emergency room, and another 112 (16%) experienced at least one hospital readmission.
That Dr. Mahant and his colleagues “observed high health care use and associated payments over the first 6 months, including and after hospitalization for neonatal HSV” suggests that there is a need for comprehensive, coordinated care once neonatal patients receive a diagnosis of HSV.
“Public health strategies that are targeted on disease prevention and early diagnosis and treatment are needed,” they advised.
The authors had no relevant financial disclosures. The study was funded by the National Institutes of Health.
SOURCE: Mahant S et al. Pediatrics. 2019 Mar. doi: 10.1542/peds.2018-3233.
The rise in herpes simplex virus cases among neonates reported by Mahant et al. is significant, but there are other possible explanations that warrant additional research, James Gaensbauer, MD, and Joseph A. Grubenhoff, MD, wrote in an accompanying editorial.
Among those explanations, Dr. Gaensbauer and Dr. Grubenhoff cite recommendations made nationally in 2013 to screen asymptomatic infants who had been exposed to HSV at the time of delivery as one possible factor elevating the number of cases being reported. More widespread use of polymerase chain reaction (PCR)–based diagnostic testing, which is reported to be more sensitive, also could play a role in increasing the number of cases being identified.
As part of a larger diagnostic “conundrum” challenging clinicians, the editorialists noted that, at present, there is no uniform consensus for performing HSV testing and providing empirical treatment. “Current recommendations from the American Academy of Pediatrics identify and emphasize the importance of recognition of the factors associated with increased likelihood of HSV infection but do not specify a more comprehensive (e.g., all febrile infants) strategy.” Stakeholders should build flexibility into their recommended treatment approaches for the benefit of practitioners operating on the front lines, they advised.
Ultimately, if the increase in incidence of neonatal HSV cases proves largely attributable to the changing behaviors of young women, who have been engaging more frequently in oral sex, as Dr. Mahant and his colleagues suggest, further research will be warranted, cautioned Dr. Gaensbauer and Dr. Grubenhoff.
“With their work, the authors contribute further nuance to a complicated and ongoing question: How do we correctly identify all infants with neonatal HSV in a timely manner while avoiding subjecting large numbers of children to unnecessary tests and empirical treatments?” This debate “is likely to be transformed by increasing availability of rapid PCR testing for HSV,” they said.
The “pathway to better clarity will depend on researchers and clinicians such as Mahant et al., who continue to provide important data and ask critical questions,” Dr. Gaensbauer and Dr. Grubenhoff concluded.
Dr. Gaensbauer and Dr. Grubenhoff are affiliated with the Denver Health Medical Center; the Children’s Hospital Colorado, Aurora; and the department of pediatrics at University of Colorado at Denver, Aurora. This is a summarization of their editorial, which accompanied the article by Mahant et al. (Pediatrics. 2019 Mar. doi: 10.1542/peds.2019-0159). They received no external funding and had no relevant financial disclosures.
The rise in herpes simplex virus cases among neonates reported by Mahant et al. is significant, but there are other possible explanations that warrant additional research, James Gaensbauer, MD, and Joseph A. Grubenhoff, MD, wrote in an accompanying editorial.
Among those explanations, Dr. Gaensbauer and Dr. Grubenhoff cite recommendations made nationally in 2013 to screen asymptomatic infants who had been exposed to HSV at the time of delivery as one possible factor elevating the number of cases being reported. More widespread use of polymerase chain reaction (PCR)–based diagnostic testing, which is reported to be more sensitive, also could play a role in increasing the number of cases being identified.
As part of a larger diagnostic “conundrum” challenging clinicians, the editorialists noted that, at present, there is no uniform consensus for performing HSV testing and providing empirical treatment. “Current recommendations from the American Academy of Pediatrics identify and emphasize the importance of recognition of the factors associated with increased likelihood of HSV infection but do not specify a more comprehensive (e.g., all febrile infants) strategy.” Stakeholders should build flexibility into their recommended treatment approaches for the benefit of practitioners operating on the front lines, they advised.
Ultimately, if the increase in incidence of neonatal HSV cases proves largely attributable to the changing behaviors of young women, who have been engaging more frequently in oral sex, as Dr. Mahant and his colleagues suggest, further research will be warranted, cautioned Dr. Gaensbauer and Dr. Grubenhoff.
“With their work, the authors contribute further nuance to a complicated and ongoing question: How do we correctly identify all infants with neonatal HSV in a timely manner while avoiding subjecting large numbers of children to unnecessary tests and empirical treatments?” This debate “is likely to be transformed by increasing availability of rapid PCR testing for HSV,” they said.
The “pathway to better clarity will depend on researchers and clinicians such as Mahant et al., who continue to provide important data and ask critical questions,” Dr. Gaensbauer and Dr. Grubenhoff concluded.
Dr. Gaensbauer and Dr. Grubenhoff are affiliated with the Denver Health Medical Center; the Children’s Hospital Colorado, Aurora; and the department of pediatrics at University of Colorado at Denver, Aurora. This is a summarization of their editorial, which accompanied the article by Mahant et al. (Pediatrics. 2019 Mar. doi: 10.1542/peds.2019-0159). They received no external funding and had no relevant financial disclosures.
The rise in herpes simplex virus cases among neonates reported by Mahant et al. is significant, but there are other possible explanations that warrant additional research, James Gaensbauer, MD, and Joseph A. Grubenhoff, MD, wrote in an accompanying editorial.
Among those explanations, Dr. Gaensbauer and Dr. Grubenhoff cite recommendations made nationally in 2013 to screen asymptomatic infants who had been exposed to HSV at the time of delivery as one possible factor elevating the number of cases being reported. More widespread use of polymerase chain reaction (PCR)–based diagnostic testing, which is reported to be more sensitive, also could play a role in increasing the number of cases being identified.
As part of a larger diagnostic “conundrum” challenging clinicians, the editorialists noted that, at present, there is no uniform consensus for performing HSV testing and providing empirical treatment. “Current recommendations from the American Academy of Pediatrics identify and emphasize the importance of recognition of the factors associated with increased likelihood of HSV infection but do not specify a more comprehensive (e.g., all febrile infants) strategy.” Stakeholders should build flexibility into their recommended treatment approaches for the benefit of practitioners operating on the front lines, they advised.
Ultimately, if the increase in incidence of neonatal HSV cases proves largely attributable to the changing behaviors of young women, who have been engaging more frequently in oral sex, as Dr. Mahant and his colleagues suggest, further research will be warranted, cautioned Dr. Gaensbauer and Dr. Grubenhoff.
“With their work, the authors contribute further nuance to a complicated and ongoing question: How do we correctly identify all infants with neonatal HSV in a timely manner while avoiding subjecting large numbers of children to unnecessary tests and empirical treatments?” This debate “is likely to be transformed by increasing availability of rapid PCR testing for HSV,” they said.
The “pathway to better clarity will depend on researchers and clinicians such as Mahant et al., who continue to provide important data and ask critical questions,” Dr. Gaensbauer and Dr. Grubenhoff concluded.
Dr. Gaensbauer and Dr. Grubenhoff are affiliated with the Denver Health Medical Center; the Children’s Hospital Colorado, Aurora; and the department of pediatrics at University of Colorado at Denver, Aurora. This is a summarization of their editorial, which accompanied the article by Mahant et al. (Pediatrics. 2019 Mar. doi: 10.1542/peds.2019-0159). They received no external funding and had no relevant financial disclosures.
A 56% increase in neonatal herpes simplex virus (HSV) infection over 7 years was determined as part of a retrospective, multistate, longitudinal cohort study using information collected from the MarketScan Medicaid Database, reported Sanjay Mahant, MD, of the University of Toronto, and his associates.
Comprehensive coordinated care – as well as public health strategies targeting disease prevention, early diagnosis, and treatment – are needed to manage the growing number of neonates diagnosed with HSV, Dr. Mahant and his colleagues said.
A total of 900 newborn Medicaid enrollees aged 0-28 days were chosen from 2,107,124 births for inclusion in the study. All patients, who were diagnosed with HSV infection during hospital admission, were born during Jan. 1, 2009–Dec. 31, 2015.
Susceptibility to primary HSV-1 infection among younger women has been attributed to an increase in oral sex practices over the past 2 decades, which is putting adolescents and young adults at greater risk of genital HSV-1 infection (J Infect Dis. 2007;196[12]:1852-9). As a result, more “primary or nonprimary genital HSV-1 infections among childbearing women” are believed to be the likely cause for the increasing numbers of neonatal HSV cases, the authors speculated, citing a recent study (J Infect Dis. 2014 Feb 1;209[3]:315-7).
HSV, a rare infection typically contracted immediately before or after birth, has both high morbidity and mortality rates; transmission rates “after exposure and during delivery increase from 2% in recurrent infection to 25% and 60% in nonprimary and primary infections, respectively,” Dr. Mahant and his colleagues noted.
Over the study period, disease incidence grew from 3.4/10,000 births in 2009 (1/2,941 births) to 5.3/10,000 births in 2015 (1/1,886 births).
Dr. Mahant and his associates noted several limitations in the study that might explain the increase in incidence.
ICD diagnosis codes, which they characterized as imperfect in their ability to correctly identify neonatal HSV infections, may have led researchers to include infants who were not actually infected or (less likely) to have excluded infants who were infected. States participating in the MarketScan Medicaid Database also may have changed over the study period. Incomplete follow-up after hospitalization made it impossible to track infants who had changed insurers, moved to other states, or died during the study. They also cautioned that outcomes may not be transferable to the general population because outcomes were specific to Medicaid enrollees.
The total cost for initial hospitalization and treatments provided during 6 months of follow-up was $60,620,431 ($87,602 median cost per patient) for the cohort of 900 infants. This is significant given that the authors reported a median length of stay of 18 days for initial hospitalization. Of the 846 patients discharged (54, or 6%, died during initial hospitalization), follow-up data was available for 692 (81%). A total of 316 (46%) infants required at least one subsequent visit to the emergency room, and another 112 (16%) experienced at least one hospital readmission.
That Dr. Mahant and his colleagues “observed high health care use and associated payments over the first 6 months, including and after hospitalization for neonatal HSV” suggests that there is a need for comprehensive, coordinated care once neonatal patients receive a diagnosis of HSV.
“Public health strategies that are targeted on disease prevention and early diagnosis and treatment are needed,” they advised.
The authors had no relevant financial disclosures. The study was funded by the National Institutes of Health.
SOURCE: Mahant S et al. Pediatrics. 2019 Mar. doi: 10.1542/peds.2018-3233.
A 56% increase in neonatal herpes simplex virus (HSV) infection over 7 years was determined as part of a retrospective, multistate, longitudinal cohort study using information collected from the MarketScan Medicaid Database, reported Sanjay Mahant, MD, of the University of Toronto, and his associates.
Comprehensive coordinated care – as well as public health strategies targeting disease prevention, early diagnosis, and treatment – are needed to manage the growing number of neonates diagnosed with HSV, Dr. Mahant and his colleagues said.
A total of 900 newborn Medicaid enrollees aged 0-28 days were chosen from 2,107,124 births for inclusion in the study. All patients, who were diagnosed with HSV infection during hospital admission, were born during Jan. 1, 2009–Dec. 31, 2015.
Susceptibility to primary HSV-1 infection among younger women has been attributed to an increase in oral sex practices over the past 2 decades, which is putting adolescents and young adults at greater risk of genital HSV-1 infection (J Infect Dis. 2007;196[12]:1852-9). As a result, more “primary or nonprimary genital HSV-1 infections among childbearing women” are believed to be the likely cause for the increasing numbers of neonatal HSV cases, the authors speculated, citing a recent study (J Infect Dis. 2014 Feb 1;209[3]:315-7).
HSV, a rare infection typically contracted immediately before or after birth, has both high morbidity and mortality rates; transmission rates “after exposure and during delivery increase from 2% in recurrent infection to 25% and 60% in nonprimary and primary infections, respectively,” Dr. Mahant and his colleagues noted.
Over the study period, disease incidence grew from 3.4/10,000 births in 2009 (1/2,941 births) to 5.3/10,000 births in 2015 (1/1,886 births).
Dr. Mahant and his associates noted several limitations in the study that might explain the increase in incidence.
ICD diagnosis codes, which they characterized as imperfect in their ability to correctly identify neonatal HSV infections, may have led researchers to include infants who were not actually infected or (less likely) to have excluded infants who were infected. States participating in the MarketScan Medicaid Database also may have changed over the study period. Incomplete follow-up after hospitalization made it impossible to track infants who had changed insurers, moved to other states, or died during the study. They also cautioned that outcomes may not be transferable to the general population because outcomes were specific to Medicaid enrollees.
The total cost for initial hospitalization and treatments provided during 6 months of follow-up was $60,620,431 ($87,602 median cost per patient) for the cohort of 900 infants. This is significant given that the authors reported a median length of stay of 18 days for initial hospitalization. Of the 846 patients discharged (54, or 6%, died during initial hospitalization), follow-up data was available for 692 (81%). A total of 316 (46%) infants required at least one subsequent visit to the emergency room, and another 112 (16%) experienced at least one hospital readmission.
That Dr. Mahant and his colleagues “observed high health care use and associated payments over the first 6 months, including and after hospitalization for neonatal HSV” suggests that there is a need for comprehensive, coordinated care once neonatal patients receive a diagnosis of HSV.
“Public health strategies that are targeted on disease prevention and early diagnosis and treatment are needed,” they advised.
The authors had no relevant financial disclosures. The study was funded by the National Institutes of Health.
SOURCE: Mahant S et al. Pediatrics. 2019 Mar. doi: 10.1542/peds.2018-3233.
FROM PEDIATRICS
Cellulitis pearls
A 38-year-old man is admitted to the hospital with a painful, swollen left leg. This was not the first instance of this kind for him. He had been admitted for the same problem 3 months earlier. During the earlier admission, he was diagnosed with cellulitis and treated with intravenous cefazolin for 4 days, then discharged on cephalexin with resolution of his swelling and pain. Today, his blood pressure is 120/70, pulse is 90, temperature is 38.2°C, his left leg is edematous from the mid-calf to the ankle, and he has erythema and warmth over the calf. His white blood cell count is 13,000, and a diagnosis of cellulitis is made. Which of the following treatments is most likely to shorten his hospital stay?
A. Vancomycin therapy instead of cefazolin.
B. Piperacillin/tazobactam therapy instead of cefazolin.
C. Prednisolone therapy in addition to antibiotics.
D. Furosemide therapy in addition to antibiotics.
The correct answer is C, prednisolone therapy in addition to antibiotics. Corticosteroids have been used as therapy for a number of infectious diseases, and steroid use has been shown to improve survival in patients with bacterial meningitis, tuberculous meningitis, tuberculous pericarditis, severe typhoid fever, tetanus, or pneumocystis pneumonia with moderate to severe hypoxemia.1 Corticosteroid use in many other infections has been studied, and for many infections, symptomatic benefit has been shown. Berkvist and Sjobeck studied 112 patients admitted to the hospital with lower-extremity erysipelas/cellulitis and randomized the patients to receive prednisolone or placebo in addition to antibiotic treatment.2 The prednisolone-treated patients had a shorter hospital stay (5 days vs. 6 days; P less than .01), and had a shorter length of intravenous antibiotic treatment ( 3 days vs. 4 days; P less than .05). The same researchers followed up the study cohort a year later to see if there was any difference in relapse between the steroid- and placebo-treated patients.3 There was no statistically significant difference in relapse (six patients treated with prednisolone relapsed, compared with 13 who received placebo). Solomon et al. did a retrospective study of patients admitted with erysipelas/cellulitis over a 7-year period.4 The control group was defined as patients who received antibiotics but did not receive prednisone, while the other patients in the study received both antibiotics and prednisone. The patients who received antibiotics and prednisone had more severe cellulitis (most had bullous cellulitis) than the patients in the control group. Long-term follow-up showed a higher incidence of erythema and recurrence of cellulitis in the control group. The return to full function was faster in the prednisone-treated patients than in the control group.
Back to the case. Which of the following is most important to do for this patient to help prevent future episodes of cellulitis?
A. Daily penicillin.
B. Treatment of tinea pedis.
C. Hydrochlorothiazide treatment for leg edema.
D. Topical triamcinolone treatment of dry skin on legs.
The correct answer here is treatment of concurrent tinea pedis infection. Antibiotic prophylaxis is considered in patients who have multiple recurrent episodes. This patient’s unilateral edema is most likely attributable to the cellulitis and should resolve with therapy, so diuretics would not be indicated. Risk factors for recurrent cellulitis are tinea pedis, obesity, venous insufficiency, and lymphedema.5
Concheiro and colleagues did a retrospective study of 122 cases of cellulitis and found tinea pedis in 33% of the cases.6 Muller et al. studied the importance of toe web microorganisms and erysipelas and found that the presence of interdigital tinea pedis was correlated with recurrent infection.7 Treatment of tinea pedis is an easily modifiable risk factor in patients with recurrent cellulitis.
Pearls: Consider adding a short course of steroids in patients with more severe erysipelas/cellulitis, as it can decrease hospital stay and IV antibiotics.
Look for tinea pedis and treat if present in patients who have erysipelas/cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Arch Intern Med. 2008 May 26;168(10):1034-46.
2. Scand J Infect Dis 1997;29(4):377-82.
3. Scand J Infect Dis. 1998;30(2):206-7.
4. Isr Med Assoc J. 2018 Mar;20(3):137-40.
5. J Dtsch Dermatol Ges. 2004 Feb;2(2):89-95.
6. Actas Dermosifiliogr. 2009 Dec;100(10):888-94.
7. J Dtsch Dermatol Ges. 2014 Aug;12(8):691-5.
A 38-year-old man is admitted to the hospital with a painful, swollen left leg. This was not the first instance of this kind for him. He had been admitted for the same problem 3 months earlier. During the earlier admission, he was diagnosed with cellulitis and treated with intravenous cefazolin for 4 days, then discharged on cephalexin with resolution of his swelling and pain. Today, his blood pressure is 120/70, pulse is 90, temperature is 38.2°C, his left leg is edematous from the mid-calf to the ankle, and he has erythema and warmth over the calf. His white blood cell count is 13,000, and a diagnosis of cellulitis is made. Which of the following treatments is most likely to shorten his hospital stay?
A. Vancomycin therapy instead of cefazolin.
B. Piperacillin/tazobactam therapy instead of cefazolin.
C. Prednisolone therapy in addition to antibiotics.
D. Furosemide therapy in addition to antibiotics.
The correct answer is C, prednisolone therapy in addition to antibiotics. Corticosteroids have been used as therapy for a number of infectious diseases, and steroid use has been shown to improve survival in patients with bacterial meningitis, tuberculous meningitis, tuberculous pericarditis, severe typhoid fever, tetanus, or pneumocystis pneumonia with moderate to severe hypoxemia.1 Corticosteroid use in many other infections has been studied, and for many infections, symptomatic benefit has been shown. Berkvist and Sjobeck studied 112 patients admitted to the hospital with lower-extremity erysipelas/cellulitis and randomized the patients to receive prednisolone or placebo in addition to antibiotic treatment.2 The prednisolone-treated patients had a shorter hospital stay (5 days vs. 6 days; P less than .01), and had a shorter length of intravenous antibiotic treatment ( 3 days vs. 4 days; P less than .05). The same researchers followed up the study cohort a year later to see if there was any difference in relapse between the steroid- and placebo-treated patients.3 There was no statistically significant difference in relapse (six patients treated with prednisolone relapsed, compared with 13 who received placebo). Solomon et al. did a retrospective study of patients admitted with erysipelas/cellulitis over a 7-year period.4 The control group was defined as patients who received antibiotics but did not receive prednisone, while the other patients in the study received both antibiotics and prednisone. The patients who received antibiotics and prednisone had more severe cellulitis (most had bullous cellulitis) than the patients in the control group. Long-term follow-up showed a higher incidence of erythema and recurrence of cellulitis in the control group. The return to full function was faster in the prednisone-treated patients than in the control group.
Back to the case. Which of the following is most important to do for this patient to help prevent future episodes of cellulitis?
A. Daily penicillin.
B. Treatment of tinea pedis.
C. Hydrochlorothiazide treatment for leg edema.
D. Topical triamcinolone treatment of dry skin on legs.
The correct answer here is treatment of concurrent tinea pedis infection. Antibiotic prophylaxis is considered in patients who have multiple recurrent episodes. This patient’s unilateral edema is most likely attributable to the cellulitis and should resolve with therapy, so diuretics would not be indicated. Risk factors for recurrent cellulitis are tinea pedis, obesity, venous insufficiency, and lymphedema.5
Concheiro and colleagues did a retrospective study of 122 cases of cellulitis and found tinea pedis in 33% of the cases.6 Muller et al. studied the importance of toe web microorganisms and erysipelas and found that the presence of interdigital tinea pedis was correlated with recurrent infection.7 Treatment of tinea pedis is an easily modifiable risk factor in patients with recurrent cellulitis.
Pearls: Consider adding a short course of steroids in patients with more severe erysipelas/cellulitis, as it can decrease hospital stay and IV antibiotics.
Look for tinea pedis and treat if present in patients who have erysipelas/cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Arch Intern Med. 2008 May 26;168(10):1034-46.
2. Scand J Infect Dis 1997;29(4):377-82.
3. Scand J Infect Dis. 1998;30(2):206-7.
4. Isr Med Assoc J. 2018 Mar;20(3):137-40.
5. J Dtsch Dermatol Ges. 2004 Feb;2(2):89-95.
6. Actas Dermosifiliogr. 2009 Dec;100(10):888-94.
7. J Dtsch Dermatol Ges. 2014 Aug;12(8):691-5.
A 38-year-old man is admitted to the hospital with a painful, swollen left leg. This was not the first instance of this kind for him. He had been admitted for the same problem 3 months earlier. During the earlier admission, he was diagnosed with cellulitis and treated with intravenous cefazolin for 4 days, then discharged on cephalexin with resolution of his swelling and pain. Today, his blood pressure is 120/70, pulse is 90, temperature is 38.2°C, his left leg is edematous from the mid-calf to the ankle, and he has erythema and warmth over the calf. His white blood cell count is 13,000, and a diagnosis of cellulitis is made. Which of the following treatments is most likely to shorten his hospital stay?
A. Vancomycin therapy instead of cefazolin.
B. Piperacillin/tazobactam therapy instead of cefazolin.
C. Prednisolone therapy in addition to antibiotics.
D. Furosemide therapy in addition to antibiotics.
The correct answer is C, prednisolone therapy in addition to antibiotics. Corticosteroids have been used as therapy for a number of infectious diseases, and steroid use has been shown to improve survival in patients with bacterial meningitis, tuberculous meningitis, tuberculous pericarditis, severe typhoid fever, tetanus, or pneumocystis pneumonia with moderate to severe hypoxemia.1 Corticosteroid use in many other infections has been studied, and for many infections, symptomatic benefit has been shown. Berkvist and Sjobeck studied 112 patients admitted to the hospital with lower-extremity erysipelas/cellulitis and randomized the patients to receive prednisolone or placebo in addition to antibiotic treatment.2 The prednisolone-treated patients had a shorter hospital stay (5 days vs. 6 days; P less than .01), and had a shorter length of intravenous antibiotic treatment ( 3 days vs. 4 days; P less than .05). The same researchers followed up the study cohort a year later to see if there was any difference in relapse between the steroid- and placebo-treated patients.3 There was no statistically significant difference in relapse (six patients treated with prednisolone relapsed, compared with 13 who received placebo). Solomon et al. did a retrospective study of patients admitted with erysipelas/cellulitis over a 7-year period.4 The control group was defined as patients who received antibiotics but did not receive prednisone, while the other patients in the study received both antibiotics and prednisone. The patients who received antibiotics and prednisone had more severe cellulitis (most had bullous cellulitis) than the patients in the control group. Long-term follow-up showed a higher incidence of erythema and recurrence of cellulitis in the control group. The return to full function was faster in the prednisone-treated patients than in the control group.
Back to the case. Which of the following is most important to do for this patient to help prevent future episodes of cellulitis?
A. Daily penicillin.
B. Treatment of tinea pedis.
C. Hydrochlorothiazide treatment for leg edema.
D. Topical triamcinolone treatment of dry skin on legs.
The correct answer here is treatment of concurrent tinea pedis infection. Antibiotic prophylaxis is considered in patients who have multiple recurrent episodes. This patient’s unilateral edema is most likely attributable to the cellulitis and should resolve with therapy, so diuretics would not be indicated. Risk factors for recurrent cellulitis are tinea pedis, obesity, venous insufficiency, and lymphedema.5
Concheiro and colleagues did a retrospective study of 122 cases of cellulitis and found tinea pedis in 33% of the cases.6 Muller et al. studied the importance of toe web microorganisms and erysipelas and found that the presence of interdigital tinea pedis was correlated with recurrent infection.7 Treatment of tinea pedis is an easily modifiable risk factor in patients with recurrent cellulitis.
Pearls: Consider adding a short course of steroids in patients with more severe erysipelas/cellulitis, as it can decrease hospital stay and IV antibiotics.
Look for tinea pedis and treat if present in patients who have erysipelas/cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Arch Intern Med. 2008 May 26;168(10):1034-46.
2. Scand J Infect Dis 1997;29(4):377-82.
3. Scand J Infect Dis. 1998;30(2):206-7.
4. Isr Med Assoc J. 2018 Mar;20(3):137-40.
5. J Dtsch Dermatol Ges. 2004 Feb;2(2):89-95.
6. Actas Dermosifiliogr. 2009 Dec;100(10):888-94.
7. J Dtsch Dermatol Ges. 2014 Aug;12(8):691-5.
Artesunate to become first-line malaria treatment in U.S.
Starting April 1, 2019, intravenous artesunate will become the first-line treatment for malaria in the United States, following the discontinuation of quinidine, the only Food and Drug Administration–approved intravenous drug for severe malaria treatment.
Although artesunate is not approved or commercially available in the United States, it is recommended by the World Health Organization. The Centers for Disease Control and Prevention have made the drug available through an expanded use investigational new drug protocol, an FDA regulatory mechanism. Clinicians can obtain the medication through the CDC’s Malaria Hotline (770-488-7788); artesunate will be stocked at 10 quarantine stations and will be released to hospitals free of charge, according to a CDC announcement.
Clinical trials have illustrated that intravenous artesunate is safe, well tolerated, and can be administered even to infants, children, and pregnant women in the second and third trimester.
About 1,700 cases of malaria are reported in the United States per year, 300 of which are classified as severe. The CDC believes the supply of artesunate obtained will be sufficient to treat all cases of severe malaria in the country, according to a CDC press release.
Starting April 1, 2019, intravenous artesunate will become the first-line treatment for malaria in the United States, following the discontinuation of quinidine, the only Food and Drug Administration–approved intravenous drug for severe malaria treatment.
Although artesunate is not approved or commercially available in the United States, it is recommended by the World Health Organization. The Centers for Disease Control and Prevention have made the drug available through an expanded use investigational new drug protocol, an FDA regulatory mechanism. Clinicians can obtain the medication through the CDC’s Malaria Hotline (770-488-7788); artesunate will be stocked at 10 quarantine stations and will be released to hospitals free of charge, according to a CDC announcement.
Clinical trials have illustrated that intravenous artesunate is safe, well tolerated, and can be administered even to infants, children, and pregnant women in the second and third trimester.
About 1,700 cases of malaria are reported in the United States per year, 300 of which are classified as severe. The CDC believes the supply of artesunate obtained will be sufficient to treat all cases of severe malaria in the country, according to a CDC press release.
Starting April 1, 2019, intravenous artesunate will become the first-line treatment for malaria in the United States, following the discontinuation of quinidine, the only Food and Drug Administration–approved intravenous drug for severe malaria treatment.
Although artesunate is not approved or commercially available in the United States, it is recommended by the World Health Organization. The Centers for Disease Control and Prevention have made the drug available through an expanded use investigational new drug protocol, an FDA regulatory mechanism. Clinicians can obtain the medication through the CDC’s Malaria Hotline (770-488-7788); artesunate will be stocked at 10 quarantine stations and will be released to hospitals free of charge, according to a CDC announcement.
Clinical trials have illustrated that intravenous artesunate is safe, well tolerated, and can be administered even to infants, children, and pregnant women in the second and third trimester.
About 1,700 cases of malaria are reported in the United States per year, 300 of which are classified as severe. The CDC believes the supply of artesunate obtained will be sufficient to treat all cases of severe malaria in the country, according to a CDC press release.
One HCV infection leads to another in HIV+ MSM
SEATTLE – Once HIV positive men who have sex with men contract the hepatitis C virus, they are more likely to get it again, according a study of 305 men in New York.
Overall, 38 men (12%) picked up another HCV infection a median of 1.9 years after clearance of their first, yielding a reinfection rate was 4.4/100 person-years, “a solid seven times higher than the primary infection rate” among HIV-positive men who have sex with men (MSM), said senior investigator Daniel Fierer, MD, an associate professor of infectious diseases at Mount Sinai Hospital, New York.
Thirty-three men cleared their second infection. Of those, six picked up a third infection at a median of 1.1 years, yielding an overall third infection incidence of 8.7/100 person-years.
The results held no matter how the men cleared HCV, whether spontaneously, as in about 10%, or by interferon before 2013, and direct-acting antivirals (DAAs) after.
Most reinfections occurred within 2 years of initial clearance, but some occurred more than a decade later.
The results suggest that there’s a particular need for HCV prevention efforts among men who have previously cleared the infection. For those patients, testing for HCV at an annual HIV checkup might not be frequent enough, Dr. Fierer said at the Conference on Retroviruses and Opportunistic Infections.
“Long-term surveillance is warranted for all HIV-infected MSM after clearance of HCV infection. Further, strategies to reduce HCV reinfections are needed to meet the goal of eliminating HCV in these men,” he said.
Also, “the large difference between primary” and secondary infection “rates suggests HCV risk is not distributed evenly between HIV-infected MSM, but concentrated among a small subpopulation. By definition, this subpopulation would have a higher prevalence” of risky behavior, such as condomless receptive anal sex and sexualized injection methamphetamine use, he said.
The high reinfection rate “tells us basically that we have not done a good job of” preventing infection and reinfection among at risk, HIV-positive men. There’s an “inadequate level of HCV treatment ... we need to eliminate restrictions on DAA” access, Dr. Fierer said.
As far as prevention goes, “I believe we just don’t know what to do. I tell all of my patients about the body fluids that have HCV in them,” which is a good start, he said.
The median age at first clearance was about 45 years, 82% of the men were white, and there was about a 50-50 split between people with private and public insurance.
The work was funded by Gilead. Dr. Fierer did not mention any disclosures.
SOURCE: Carollo JR et al. CROI 2019, Abstract 86
SEATTLE – Once HIV positive men who have sex with men contract the hepatitis C virus, they are more likely to get it again, according a study of 305 men in New York.
Overall, 38 men (12%) picked up another HCV infection a median of 1.9 years after clearance of their first, yielding a reinfection rate was 4.4/100 person-years, “a solid seven times higher than the primary infection rate” among HIV-positive men who have sex with men (MSM), said senior investigator Daniel Fierer, MD, an associate professor of infectious diseases at Mount Sinai Hospital, New York.
Thirty-three men cleared their second infection. Of those, six picked up a third infection at a median of 1.1 years, yielding an overall third infection incidence of 8.7/100 person-years.
The results held no matter how the men cleared HCV, whether spontaneously, as in about 10%, or by interferon before 2013, and direct-acting antivirals (DAAs) after.
Most reinfections occurred within 2 years of initial clearance, but some occurred more than a decade later.
The results suggest that there’s a particular need for HCV prevention efforts among men who have previously cleared the infection. For those patients, testing for HCV at an annual HIV checkup might not be frequent enough, Dr. Fierer said at the Conference on Retroviruses and Opportunistic Infections.
“Long-term surveillance is warranted for all HIV-infected MSM after clearance of HCV infection. Further, strategies to reduce HCV reinfections are needed to meet the goal of eliminating HCV in these men,” he said.
Also, “the large difference between primary” and secondary infection “rates suggests HCV risk is not distributed evenly between HIV-infected MSM, but concentrated among a small subpopulation. By definition, this subpopulation would have a higher prevalence” of risky behavior, such as condomless receptive anal sex and sexualized injection methamphetamine use, he said.
The high reinfection rate “tells us basically that we have not done a good job of” preventing infection and reinfection among at risk, HIV-positive men. There’s an “inadequate level of HCV treatment ... we need to eliminate restrictions on DAA” access, Dr. Fierer said.
As far as prevention goes, “I believe we just don’t know what to do. I tell all of my patients about the body fluids that have HCV in them,” which is a good start, he said.
The median age at first clearance was about 45 years, 82% of the men were white, and there was about a 50-50 split between people with private and public insurance.
The work was funded by Gilead. Dr. Fierer did not mention any disclosures.
SOURCE: Carollo JR et al. CROI 2019, Abstract 86
SEATTLE – Once HIV positive men who have sex with men contract the hepatitis C virus, they are more likely to get it again, according a study of 305 men in New York.
Overall, 38 men (12%) picked up another HCV infection a median of 1.9 years after clearance of their first, yielding a reinfection rate was 4.4/100 person-years, “a solid seven times higher than the primary infection rate” among HIV-positive men who have sex with men (MSM), said senior investigator Daniel Fierer, MD, an associate professor of infectious diseases at Mount Sinai Hospital, New York.
Thirty-three men cleared their second infection. Of those, six picked up a third infection at a median of 1.1 years, yielding an overall third infection incidence of 8.7/100 person-years.
The results held no matter how the men cleared HCV, whether spontaneously, as in about 10%, or by interferon before 2013, and direct-acting antivirals (DAAs) after.
Most reinfections occurred within 2 years of initial clearance, but some occurred more than a decade later.
The results suggest that there’s a particular need for HCV prevention efforts among men who have previously cleared the infection. For those patients, testing for HCV at an annual HIV checkup might not be frequent enough, Dr. Fierer said at the Conference on Retroviruses and Opportunistic Infections.
“Long-term surveillance is warranted for all HIV-infected MSM after clearance of HCV infection. Further, strategies to reduce HCV reinfections are needed to meet the goal of eliminating HCV in these men,” he said.
Also, “the large difference between primary” and secondary infection “rates suggests HCV risk is not distributed evenly between HIV-infected MSM, but concentrated among a small subpopulation. By definition, this subpopulation would have a higher prevalence” of risky behavior, such as condomless receptive anal sex and sexualized injection methamphetamine use, he said.
The high reinfection rate “tells us basically that we have not done a good job of” preventing infection and reinfection among at risk, HIV-positive men. There’s an “inadequate level of HCV treatment ... we need to eliminate restrictions on DAA” access, Dr. Fierer said.
As far as prevention goes, “I believe we just don’t know what to do. I tell all of my patients about the body fluids that have HCV in them,” which is a good start, he said.
The median age at first clearance was about 45 years, 82% of the men were white, and there was about a 50-50 split between people with private and public insurance.
The work was funded by Gilead. Dr. Fierer did not mention any disclosures.
SOURCE: Carollo JR et al. CROI 2019, Abstract 86
REPORTING FROM CROI 2019
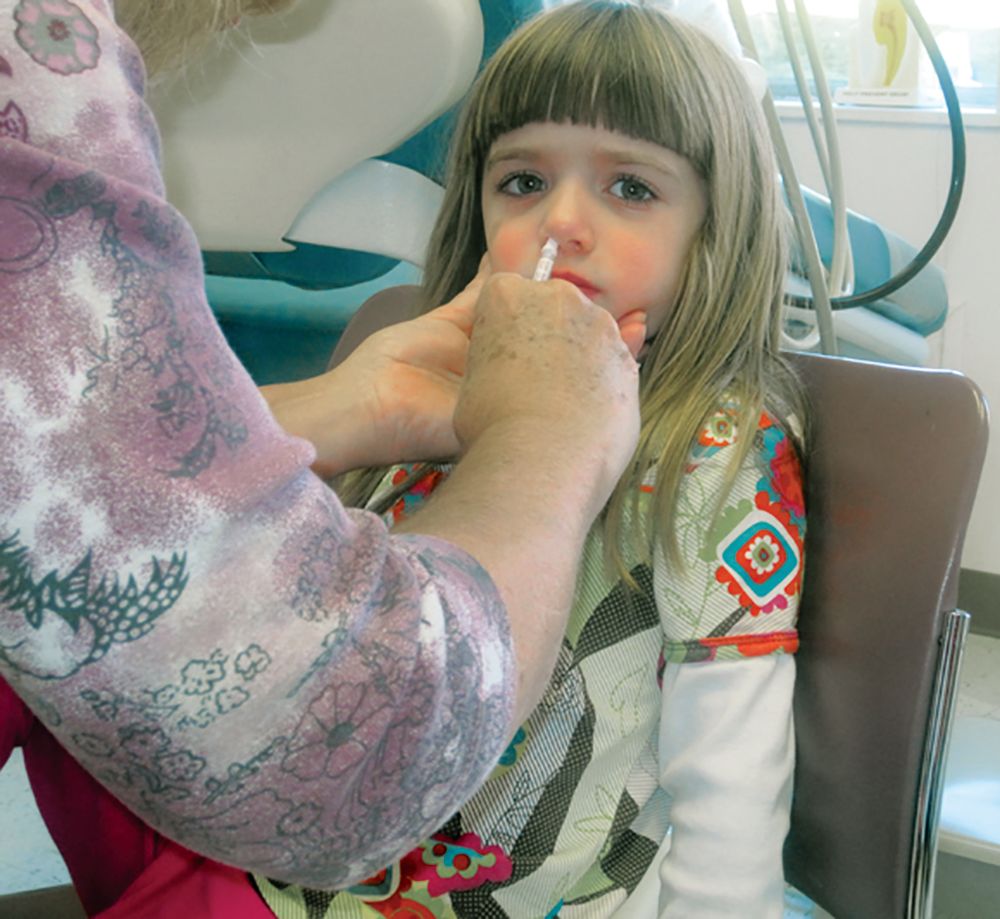
AAP updates 2019-2020 flu vaccine recommendations to include nasal spray
Although the American Academy of Pediatrics had cited a preference for injected flu vaccines for children during the 2018-2019 flu season, this year’s recommendations say either that or the nasal spray formulation are acceptable, according to a press release. The Centers for Disease Control and Prevention has given similar guidance.
Because the spray did not work as well against A/H1N1 as the injected vaccine had during the 2013-2014 and 2014-2015 seasons, the AAP did not recommend the spray during the 2015-2016 and 2016-2017 seasons. However, in 2017 the spray’s manufacturer included a new strain of A/H1N1, and new data has supported the spray’s effectiveness against some strains.
according to the CDC. That said, the spray is especially appropriate for patients who refuse to receive the injected form, so the choice of formulation is at the pediatrician’s discretion, according to the AAP release.
Although the American Academy of Pediatrics had cited a preference for injected flu vaccines for children during the 2018-2019 flu season, this year’s recommendations say either that or the nasal spray formulation are acceptable, according to a press release. The Centers for Disease Control and Prevention has given similar guidance.
Because the spray did not work as well against A/H1N1 as the injected vaccine had during the 2013-2014 and 2014-2015 seasons, the AAP did not recommend the spray during the 2015-2016 and 2016-2017 seasons. However, in 2017 the spray’s manufacturer included a new strain of A/H1N1, and new data has supported the spray’s effectiveness against some strains.
according to the CDC. That said, the spray is especially appropriate for patients who refuse to receive the injected form, so the choice of formulation is at the pediatrician’s discretion, according to the AAP release.
Although the American Academy of Pediatrics had cited a preference for injected flu vaccines for children during the 2018-2019 flu season, this year’s recommendations say either that or the nasal spray formulation are acceptable, according to a press release. The Centers for Disease Control and Prevention has given similar guidance.
Because the spray did not work as well against A/H1N1 as the injected vaccine had during the 2013-2014 and 2014-2015 seasons, the AAP did not recommend the spray during the 2015-2016 and 2016-2017 seasons. However, in 2017 the spray’s manufacturer included a new strain of A/H1N1, and new data has supported the spray’s effectiveness against some strains.
according to the CDC. That said, the spray is especially appropriate for patients who refuse to receive the injected form, so the choice of formulation is at the pediatrician’s discretion, according to the AAP release.
Antibiotic Overprescribing
Erythematous and Necrotic Papules in an Immunosuppressed Woman
The Diagnosis: Disseminated Fusariosis
Histologic evaluation of the punch biopsy demonstrated thrombosed vessels in the deep dermis and along fibrous septae of subcutaneous tissue, as well as delicate, thin-walled, branching hyphae with vesicular swellings (Figure). The hyphae were present within the vascular thrombi and extended into surrounding tissue. The fungal tissue culture eventually grew scant Fusarium. At the time of biopsy, there was a high index of suspicion for fungal infection, which supported the decision to empirically treat with anidulafungin and voriconazole.
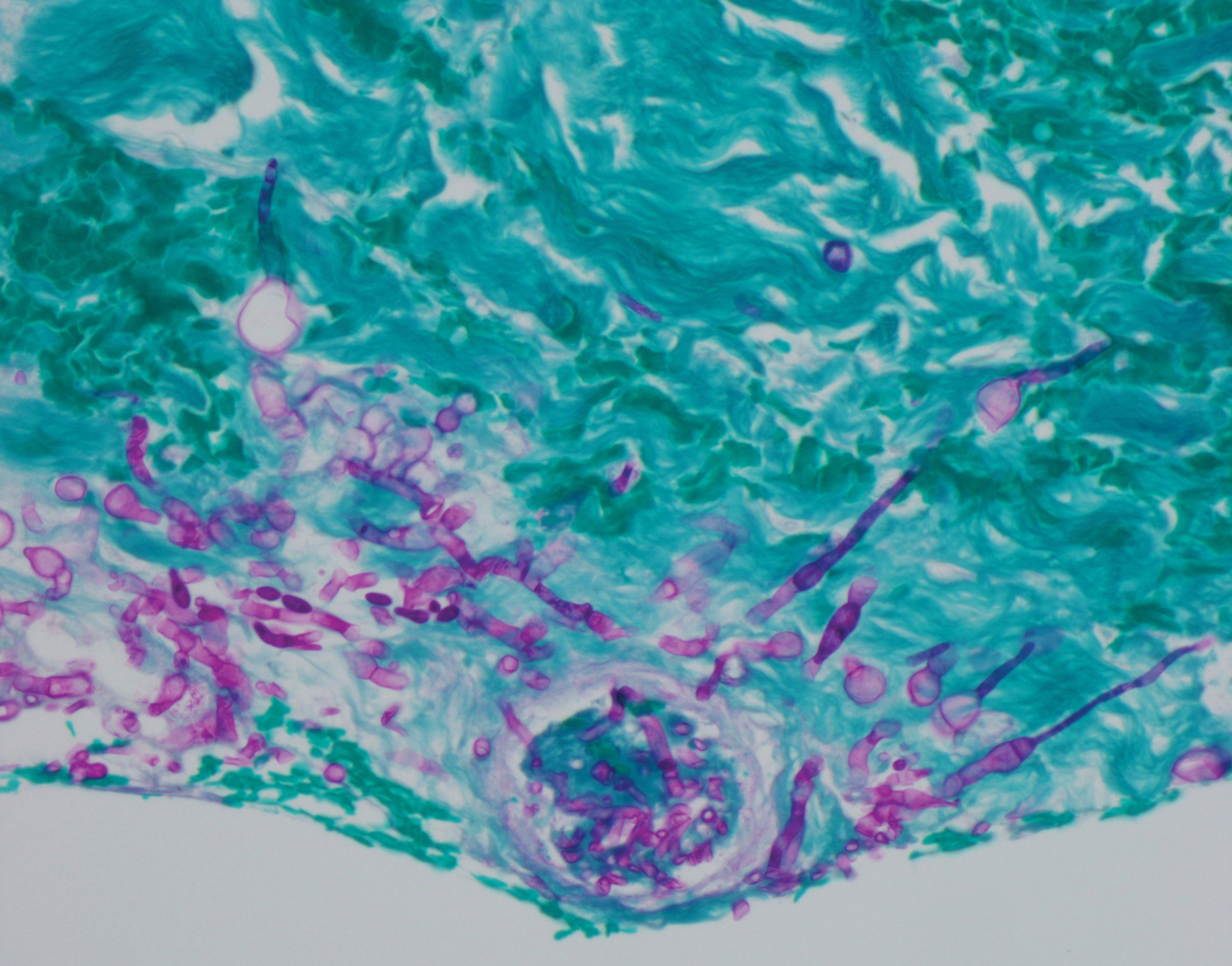
Differentiating the diagnosis in this case was done primarily with histopathology. Although Aspergillus also has slender hyphae, it lacks the vesicular swellings characteristic of fusariosis. Disseminated candidiasis would demonstrate budding yeast and pseudohyphae in the dermis. Ecthyma gangrenosum histologically presents as necrotizing hemorrhagic vasculitis with gram-negative rods in the walls of deeper vessels, characteristically sparing the intima. Leukemia cutis histologically varies but would display a neoplastic infiltrate of atypical monocytoid cells with nuclear pleomorphism.
Our patient had been treated with palliative chemotherapy as a salvage regimen with idarubicin and cytarabine. She had persistent pancytopenia despite granulocyte-macrophage colony-stimulating factor therapy. The mortality rate for disseminated Fusarium infection approaches 100% when risk factors such as angiotropism and prolonged neutropenia are present.1,2 Additionally, our patient's susceptibility profile subsequently demonstrated an elevated minimum inhibitory concentration to amphotericin B, itraconazole, voriconazole, and posaconazole. The neutropenia and Fusarium infection were not responsive to treatment. She was discharged on palliative voriconazole with home hospice care.
Fusarium species are soil-dwelling saprophytes and important plant pathogens that have increasingly emerged as rare but notable causes of morbidity and mortality in immunocompromised patients.1-3 More specifically, Fusarium infection is most commonly observed in patients with hematologic malignancy complicated by persistent neutropenia. The 3 most frequently encountered Fusarium species in human disease are Fusarium solani, Fusarium oxysporum, and Fusarium moniliforme, with F solani being the most virulent.1,2 Infection with Fusarium may manifest as a broad range of presentations depending on the route of entry, such as endophthalmitis, sinusitis, pneumonia, and cutaneous lesions.1 Disseminated infection is marked by skin lesions or positive blood cultures for Fusarium.3 This fungus is notorious for its limited susceptibility profile.1 It requires systemic antifungal medications such as triazoles and amphotericin B. Fusarium is most susceptible in vitro to amphotericin B but often requires toxic dosages to be effective in decreasing fungal load.2,3 The high mortality rate of disseminated fusariosis further emphasizes that prevention is an important component to protecting high-risk patients. Keeping patients in rooms with high-efficiency particulate arresting filters and limiting exposure to unsanitized tap water faucets can help decrease exposure; however, reducing immunosuppression and improving neutropenia are the most effective ways to prevent fusariosis.1 Although skin breakdown can facilitate the spread of infection, it has been observed that immunosuppressed individuals do not necessarily have this finding.4
This case emphasizes the importance of considering disseminated fusariosis in patients with hematologic malignancy or other immunosuppressed conditions. The most important factors that should raise clinical suspicion are persistent neutropenia and recent corticosteroid therapy.1 A clinical picture that suggests fungal infection should warrant consideration of prophylactic treatment as well as tissue and blood cultures to determine species and susceptibility.
- Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007;20:695-704.
- Jossi M, Ambrosioni J, Macedo-Vinas M, et al. Invasive fusariosis with prolonged fungemia in a patient with acute lymphoblastic leukemia: case report and review of the literature. Int J Infect Dis. 2010;14:E354-E356.
- Tan R, Ng KP, Gan GG, et al. Fusarium sp. infection in a patient with Acute Lymphoblastic Leukaemia. Med J Malaysia. 2013;68:479-480.
- Nucci M, Anaissie E. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin Infect Dis. 2002;35:909-920.
The Diagnosis: Disseminated Fusariosis
Histologic evaluation of the punch biopsy demonstrated thrombosed vessels in the deep dermis and along fibrous septae of subcutaneous tissue, as well as delicate, thin-walled, branching hyphae with vesicular swellings (Figure). The hyphae were present within the vascular thrombi and extended into surrounding tissue. The fungal tissue culture eventually grew scant Fusarium. At the time of biopsy, there was a high index of suspicion for fungal infection, which supported the decision to empirically treat with anidulafungin and voriconazole.

Differentiating the diagnosis in this case was done primarily with histopathology. Although Aspergillus also has slender hyphae, it lacks the vesicular swellings characteristic of fusariosis. Disseminated candidiasis would demonstrate budding yeast and pseudohyphae in the dermis. Ecthyma gangrenosum histologically presents as necrotizing hemorrhagic vasculitis with gram-negative rods in the walls of deeper vessels, characteristically sparing the intima. Leukemia cutis histologically varies but would display a neoplastic infiltrate of atypical monocytoid cells with nuclear pleomorphism.
Our patient had been treated with palliative chemotherapy as a salvage regimen with idarubicin and cytarabine. She had persistent pancytopenia despite granulocyte-macrophage colony-stimulating factor therapy. The mortality rate for disseminated Fusarium infection approaches 100% when risk factors such as angiotropism and prolonged neutropenia are present.1,2 Additionally, our patient's susceptibility profile subsequently demonstrated an elevated minimum inhibitory concentration to amphotericin B, itraconazole, voriconazole, and posaconazole. The neutropenia and Fusarium infection were not responsive to treatment. She was discharged on palliative voriconazole with home hospice care.
Fusarium species are soil-dwelling saprophytes and important plant pathogens that have increasingly emerged as rare but notable causes of morbidity and mortality in immunocompromised patients.1-3 More specifically, Fusarium infection is most commonly observed in patients with hematologic malignancy complicated by persistent neutropenia. The 3 most frequently encountered Fusarium species in human disease are Fusarium solani, Fusarium oxysporum, and Fusarium moniliforme, with F solani being the most virulent.1,2 Infection with Fusarium may manifest as a broad range of presentations depending on the route of entry, such as endophthalmitis, sinusitis, pneumonia, and cutaneous lesions.1 Disseminated infection is marked by skin lesions or positive blood cultures for Fusarium.3 This fungus is notorious for its limited susceptibility profile.1 It requires systemic antifungal medications such as triazoles and amphotericin B. Fusarium is most susceptible in vitro to amphotericin B but often requires toxic dosages to be effective in decreasing fungal load.2,3 The high mortality rate of disseminated fusariosis further emphasizes that prevention is an important component to protecting high-risk patients. Keeping patients in rooms with high-efficiency particulate arresting filters and limiting exposure to unsanitized tap water faucets can help decrease exposure; however, reducing immunosuppression and improving neutropenia are the most effective ways to prevent fusariosis.1 Although skin breakdown can facilitate the spread of infection, it has been observed that immunosuppressed individuals do not necessarily have this finding.4
This case emphasizes the importance of considering disseminated fusariosis in patients with hematologic malignancy or other immunosuppressed conditions. The most important factors that should raise clinical suspicion are persistent neutropenia and recent corticosteroid therapy.1 A clinical picture that suggests fungal infection should warrant consideration of prophylactic treatment as well as tissue and blood cultures to determine species and susceptibility.
The Diagnosis: Disseminated Fusariosis
Histologic evaluation of the punch biopsy demonstrated thrombosed vessels in the deep dermis and along fibrous septae of subcutaneous tissue, as well as delicate, thin-walled, branching hyphae with vesicular swellings (Figure). The hyphae were present within the vascular thrombi and extended into surrounding tissue. The fungal tissue culture eventually grew scant Fusarium. At the time of biopsy, there was a high index of suspicion for fungal infection, which supported the decision to empirically treat with anidulafungin and voriconazole.

Differentiating the diagnosis in this case was done primarily with histopathology. Although Aspergillus also has slender hyphae, it lacks the vesicular swellings characteristic of fusariosis. Disseminated candidiasis would demonstrate budding yeast and pseudohyphae in the dermis. Ecthyma gangrenosum histologically presents as necrotizing hemorrhagic vasculitis with gram-negative rods in the walls of deeper vessels, characteristically sparing the intima. Leukemia cutis histologically varies but would display a neoplastic infiltrate of atypical monocytoid cells with nuclear pleomorphism.
Our patient had been treated with palliative chemotherapy as a salvage regimen with idarubicin and cytarabine. She had persistent pancytopenia despite granulocyte-macrophage colony-stimulating factor therapy. The mortality rate for disseminated Fusarium infection approaches 100% when risk factors such as angiotropism and prolonged neutropenia are present.1,2 Additionally, our patient's susceptibility profile subsequently demonstrated an elevated minimum inhibitory concentration to amphotericin B, itraconazole, voriconazole, and posaconazole. The neutropenia and Fusarium infection were not responsive to treatment. She was discharged on palliative voriconazole with home hospice care.
Fusarium species are soil-dwelling saprophytes and important plant pathogens that have increasingly emerged as rare but notable causes of morbidity and mortality in immunocompromised patients.1-3 More specifically, Fusarium infection is most commonly observed in patients with hematologic malignancy complicated by persistent neutropenia. The 3 most frequently encountered Fusarium species in human disease are Fusarium solani, Fusarium oxysporum, and Fusarium moniliforme, with F solani being the most virulent.1,2 Infection with Fusarium may manifest as a broad range of presentations depending on the route of entry, such as endophthalmitis, sinusitis, pneumonia, and cutaneous lesions.1 Disseminated infection is marked by skin lesions or positive blood cultures for Fusarium.3 This fungus is notorious for its limited susceptibility profile.1 It requires systemic antifungal medications such as triazoles and amphotericin B. Fusarium is most susceptible in vitro to amphotericin B but often requires toxic dosages to be effective in decreasing fungal load.2,3 The high mortality rate of disseminated fusariosis further emphasizes that prevention is an important component to protecting high-risk patients. Keeping patients in rooms with high-efficiency particulate arresting filters and limiting exposure to unsanitized tap water faucets can help decrease exposure; however, reducing immunosuppression and improving neutropenia are the most effective ways to prevent fusariosis.1 Although skin breakdown can facilitate the spread of infection, it has been observed that immunosuppressed individuals do not necessarily have this finding.4
This case emphasizes the importance of considering disseminated fusariosis in patients with hematologic malignancy or other immunosuppressed conditions. The most important factors that should raise clinical suspicion are persistent neutropenia and recent corticosteroid therapy.1 A clinical picture that suggests fungal infection should warrant consideration of prophylactic treatment as well as tissue and blood cultures to determine species and susceptibility.
- Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007;20:695-704.
- Jossi M, Ambrosioni J, Macedo-Vinas M, et al. Invasive fusariosis with prolonged fungemia in a patient with acute lymphoblastic leukemia: case report and review of the literature. Int J Infect Dis. 2010;14:E354-E356.
- Tan R, Ng KP, Gan GG, et al. Fusarium sp. infection in a patient with Acute Lymphoblastic Leukaemia. Med J Malaysia. 2013;68:479-480.
- Nucci M, Anaissie E. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin Infect Dis. 2002;35:909-920.
- Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007;20:695-704.
- Jossi M, Ambrosioni J, Macedo-Vinas M, et al. Invasive fusariosis with prolonged fungemia in a patient with acute lymphoblastic leukemia: case report and review of the literature. Int J Infect Dis. 2010;14:E354-E356.
- Tan R, Ng KP, Gan GG, et al. Fusarium sp. infection in a patient with Acute Lymphoblastic Leukaemia. Med J Malaysia. 2013;68:479-480.
- Nucci M, Anaissie E. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin Infect Dis. 2002;35:909-920.
Highlights from the "Updates in Sepsis" session (VIDEO)

HM19 attendees discuss key take-home points from Monday’s Update in Sepsis session.

HM19 attendees discuss key take-home points from Monday’s Update in Sepsis session.

HM19 attendees discuss key take-home points from Monday’s Update in Sepsis session.
For patients with HBV, daily aspirin may reduce risk of liver cancer
Sixteen years of data showed that daily aspirin therapy reduced the risk of HBV-related HCC by 29%, reported lead author Teng-Yu Lee, MD, PhD, of Taichung (Taiwan) Veterans General Hospital and his colleagues. Analysis also showed that antiviral nucleos(t)ide analogue therapy and statin use were independently associated with reduced risk of HCC, whereas older age, cirrhosis, and male sex increased risk.
“Therapy with [nucleos(t)ide analogues] is associated with reductions in HCC risk, but the risk is not erased,” the investigators wrote in JAMA Internal Medicine. “Therefore, using only [nucleos(t)ide analogue] therapy may not be enough for HCC prevention. Antiviral therapy is not indicated in most HBV carriers, so another effective way of reducing HCC risk needs to be developed.”
Previous studies have shown that aspirin can reduce the risk of colorectal cancer; however, data supporting aspirin for HCC prevention are limited to a few animal models and human studies, the latter of which are statistically unreliable.
“Therefore, we conducted a nationwide cohort study to evaluate the association of daily aspirin therapy with HBV-related HCC,” the investigators wrote.
They screened 204,507 patients with HBV included in the Taiwanese National Health Insurance Research Database (NHIRD) between 1997 and 2012, first excluding any with confounding conditions, such as hepatitis C infection or alcoholic liver disease. Next, 2,123 patients were identified who had taken aspirin for 90 days or longer. Finally, these cases were randomly matched with 8,492 control patients with HBV who had never received antiplatelet therapy. The main measured outcome was diagnosis with HCC. Patients were followed until this diagnosis was made, death occurred, or the end of the study period.
Analysis showed that most patients were male (72.4%) and took aspirin for about 4 years, usually prescribed for cardiovascular disease risk factors. Almost all patients in the treatment group (98%) received an aspirin dose of 100 mg or less.
After 5 years, the cumulative incidence of HCC in the aspirin group was 5.20% versus 7.87% in the control group (P less than .001). Multivariable analysis revealed that daily aspirin was associated with a significant risk reduction of 29% (HR 0.71; P less than .001), as were nucleos(t)ide analogues and statins, which lowered risk by 46% and 38%, respectively. In contrast, risk increased with older age at the rate of 1% per year, male sex carried an additional risk of 75%, and liver cirrhosis was associated with a 2.89-fold risk increase.
“In the present study, we report that daily aspirin therapy was associated with a reduced incidence of HCC in patients with [chronic hepatitis B],” the investigators wrote. “Our findings may be of help in future efforts to further improve the chemoprevention of HBV-related HCC, and a proof-of-concept study is thus warranted.”
The investigators described several mechanisms that may have contribute to the possible risk reduction provided by aspirin. For one, aspirin inhibits platelet activation, which is associated with development of HBV-related liver disease. Additional benefit may come from induction of HCC cell apoptosis, control of tumor growth, reduced liver fibrosis, and increased liver regeneration, all of which have been associated with aspirin in rodent models.
“Hepatitis B virus–related HCC is generally a consequence of chronic inflammation due to hepatitis, fibrosis, dysplasia, and tumor growth,” the investigators wrote, suggesting that aspirin-related reductions in inflammation could also explain reduced neoplastic activity.
To assess for increased risk of peptic ulcers secondary to aspirin, the investigators performed a subanalysis of peptic ulcer bleeding. These results showed that rates of peptic ulcer bleeding, at around 5%-6%, were similar between the aspirin group and the control group. Among other variables, cirrhosis didn’t significantly affect rates of peptic ulcer bleeding, and aspirin users had similar rates of peptic ulcer bleeding regardless of HBV status. Because of the study design, however, the investigators cautioned that these analyses could underestimate ulcer risk because patients who could not tolerate aspirin for at least 90 days were excluded from the study.
Although statins stood out as another possible risk reducer, the investigators noted that “randomized clinical trials are required to confirm the chemopreventive effect of statins.”
Similarly, the investigators suggested that a prospective trial is needed before aspirin can be adopted as an HCC preventive.
The study was funded by the Ministry of Science and Technology, National Health Research Institutes, and Taichung (Taiwan) Veterans General Hospital, Taiwan. One author reported financial compensation from Gilead and Bristol-Myers Squibb.
SOURCE: Lee T-Y et al. JAMA Intern Med. 2019 Mar 18. doi:10.1001/jamainternmed.2018.8342.
Sixteen years of data showed that daily aspirin therapy reduced the risk of HBV-related HCC by 29%, reported lead author Teng-Yu Lee, MD, PhD, of Taichung (Taiwan) Veterans General Hospital and his colleagues. Analysis also showed that antiviral nucleos(t)ide analogue therapy and statin use were independently associated with reduced risk of HCC, whereas older age, cirrhosis, and male sex increased risk.
“Therapy with [nucleos(t)ide analogues] is associated with reductions in HCC risk, but the risk is not erased,” the investigators wrote in JAMA Internal Medicine. “Therefore, using only [nucleos(t)ide analogue] therapy may not be enough for HCC prevention. Antiviral therapy is not indicated in most HBV carriers, so another effective way of reducing HCC risk needs to be developed.”
Previous studies have shown that aspirin can reduce the risk of colorectal cancer; however, data supporting aspirin for HCC prevention are limited to a few animal models and human studies, the latter of which are statistically unreliable.
“Therefore, we conducted a nationwide cohort study to evaluate the association of daily aspirin therapy with HBV-related HCC,” the investigators wrote.
They screened 204,507 patients with HBV included in the Taiwanese National Health Insurance Research Database (NHIRD) between 1997 and 2012, first excluding any with confounding conditions, such as hepatitis C infection or alcoholic liver disease. Next, 2,123 patients were identified who had taken aspirin for 90 days or longer. Finally, these cases were randomly matched with 8,492 control patients with HBV who had never received antiplatelet therapy. The main measured outcome was diagnosis with HCC. Patients were followed until this diagnosis was made, death occurred, or the end of the study period.
Analysis showed that most patients were male (72.4%) and took aspirin for about 4 years, usually prescribed for cardiovascular disease risk factors. Almost all patients in the treatment group (98%) received an aspirin dose of 100 mg or less.
After 5 years, the cumulative incidence of HCC in the aspirin group was 5.20% versus 7.87% in the control group (P less than .001). Multivariable analysis revealed that daily aspirin was associated with a significant risk reduction of 29% (HR 0.71; P less than .001), as were nucleos(t)ide analogues and statins, which lowered risk by 46% and 38%, respectively. In contrast, risk increased with older age at the rate of 1% per year, male sex carried an additional risk of 75%, and liver cirrhosis was associated with a 2.89-fold risk increase.
“In the present study, we report that daily aspirin therapy was associated with a reduced incidence of HCC in patients with [chronic hepatitis B],” the investigators wrote. “Our findings may be of help in future efforts to further improve the chemoprevention of HBV-related HCC, and a proof-of-concept study is thus warranted.”
The investigators described several mechanisms that may have contribute to the possible risk reduction provided by aspirin. For one, aspirin inhibits platelet activation, which is associated with development of HBV-related liver disease. Additional benefit may come from induction of HCC cell apoptosis, control of tumor growth, reduced liver fibrosis, and increased liver regeneration, all of which have been associated with aspirin in rodent models.
“Hepatitis B virus–related HCC is generally a consequence of chronic inflammation due to hepatitis, fibrosis, dysplasia, and tumor growth,” the investigators wrote, suggesting that aspirin-related reductions in inflammation could also explain reduced neoplastic activity.
To assess for increased risk of peptic ulcers secondary to aspirin, the investigators performed a subanalysis of peptic ulcer bleeding. These results showed that rates of peptic ulcer bleeding, at around 5%-6%, were similar between the aspirin group and the control group. Among other variables, cirrhosis didn’t significantly affect rates of peptic ulcer bleeding, and aspirin users had similar rates of peptic ulcer bleeding regardless of HBV status. Because of the study design, however, the investigators cautioned that these analyses could underestimate ulcer risk because patients who could not tolerate aspirin for at least 90 days were excluded from the study.
Although statins stood out as another possible risk reducer, the investigators noted that “randomized clinical trials are required to confirm the chemopreventive effect of statins.”
Similarly, the investigators suggested that a prospective trial is needed before aspirin can be adopted as an HCC preventive.
The study was funded by the Ministry of Science and Technology, National Health Research Institutes, and Taichung (Taiwan) Veterans General Hospital, Taiwan. One author reported financial compensation from Gilead and Bristol-Myers Squibb.
SOURCE: Lee T-Y et al. JAMA Intern Med. 2019 Mar 18. doi:10.1001/jamainternmed.2018.8342.
Sixteen years of data showed that daily aspirin therapy reduced the risk of HBV-related HCC by 29%, reported lead author Teng-Yu Lee, MD, PhD, of Taichung (Taiwan) Veterans General Hospital and his colleagues. Analysis also showed that antiviral nucleos(t)ide analogue therapy and statin use were independently associated with reduced risk of HCC, whereas older age, cirrhosis, and male sex increased risk.
“Therapy with [nucleos(t)ide analogues] is associated with reductions in HCC risk, but the risk is not erased,” the investigators wrote in JAMA Internal Medicine. “Therefore, using only [nucleos(t)ide analogue] therapy may not be enough for HCC prevention. Antiviral therapy is not indicated in most HBV carriers, so another effective way of reducing HCC risk needs to be developed.”
Previous studies have shown that aspirin can reduce the risk of colorectal cancer; however, data supporting aspirin for HCC prevention are limited to a few animal models and human studies, the latter of which are statistically unreliable.
“Therefore, we conducted a nationwide cohort study to evaluate the association of daily aspirin therapy with HBV-related HCC,” the investigators wrote.
They screened 204,507 patients with HBV included in the Taiwanese National Health Insurance Research Database (NHIRD) between 1997 and 2012, first excluding any with confounding conditions, such as hepatitis C infection or alcoholic liver disease. Next, 2,123 patients were identified who had taken aspirin for 90 days or longer. Finally, these cases were randomly matched with 8,492 control patients with HBV who had never received antiplatelet therapy. The main measured outcome was diagnosis with HCC. Patients were followed until this diagnosis was made, death occurred, or the end of the study period.
Analysis showed that most patients were male (72.4%) and took aspirin for about 4 years, usually prescribed for cardiovascular disease risk factors. Almost all patients in the treatment group (98%) received an aspirin dose of 100 mg or less.
After 5 years, the cumulative incidence of HCC in the aspirin group was 5.20% versus 7.87% in the control group (P less than .001). Multivariable analysis revealed that daily aspirin was associated with a significant risk reduction of 29% (HR 0.71; P less than .001), as were nucleos(t)ide analogues and statins, which lowered risk by 46% and 38%, respectively. In contrast, risk increased with older age at the rate of 1% per year, male sex carried an additional risk of 75%, and liver cirrhosis was associated with a 2.89-fold risk increase.
“In the present study, we report that daily aspirin therapy was associated with a reduced incidence of HCC in patients with [chronic hepatitis B],” the investigators wrote. “Our findings may be of help in future efforts to further improve the chemoprevention of HBV-related HCC, and a proof-of-concept study is thus warranted.”
The investigators described several mechanisms that may have contribute to the possible risk reduction provided by aspirin. For one, aspirin inhibits platelet activation, which is associated with development of HBV-related liver disease. Additional benefit may come from induction of HCC cell apoptosis, control of tumor growth, reduced liver fibrosis, and increased liver regeneration, all of which have been associated with aspirin in rodent models.
“Hepatitis B virus–related HCC is generally a consequence of chronic inflammation due to hepatitis, fibrosis, dysplasia, and tumor growth,” the investigators wrote, suggesting that aspirin-related reductions in inflammation could also explain reduced neoplastic activity.
To assess for increased risk of peptic ulcers secondary to aspirin, the investigators performed a subanalysis of peptic ulcer bleeding. These results showed that rates of peptic ulcer bleeding, at around 5%-6%, were similar between the aspirin group and the control group. Among other variables, cirrhosis didn’t significantly affect rates of peptic ulcer bleeding, and aspirin users had similar rates of peptic ulcer bleeding regardless of HBV status. Because of the study design, however, the investigators cautioned that these analyses could underestimate ulcer risk because patients who could not tolerate aspirin for at least 90 days were excluded from the study.
Although statins stood out as another possible risk reducer, the investigators noted that “randomized clinical trials are required to confirm the chemopreventive effect of statins.”
Similarly, the investigators suggested that a prospective trial is needed before aspirin can be adopted as an HCC preventive.
The study was funded by the Ministry of Science and Technology, National Health Research Institutes, and Taichung (Taiwan) Veterans General Hospital, Taiwan. One author reported financial compensation from Gilead and Bristol-Myers Squibb.
SOURCE: Lee T-Y et al. JAMA Intern Med. 2019 Mar 18. doi:10.1001/jamainternmed.2018.8342.
FROM JAMA INTERNAL MEDICINE
United States now over 300 measles cases for the year
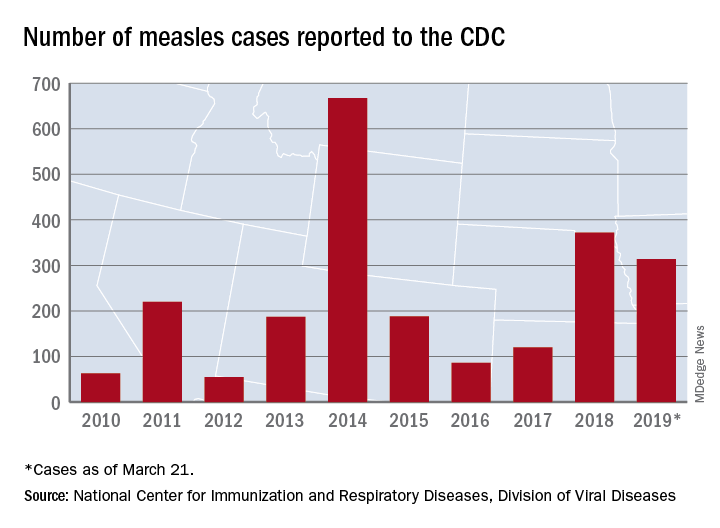
Despite those 46 new cases, the number of states with reported cases remains at 15, the CDC reported March 25.
For the fifth consecutive week the busiest outbreak was in Brooklyn, N.Y., which added 23 new cases. New York’s Rockland County, which is just north of New York City and has 46 confirmed cases for the year, is home to another of the six current outbreaks in the country, with the other four located in Washington (74 total cases for the state), Texas (14 cases), California (7 cases), and Illinois (6 cases). Other states with cases are Arizona, Colorado, Connecticut, Georgia, Kentucky, Missouri, New Hampshire, New Jersey, and Oregon, reported the CDC.
This year’s case total through less than 3 months is nearing the 372 that occurred in 2018, which was the second-worst year for measles in the last decade, but is still well off the 10-year high of 667 reported in 2014, the CDC said.

Despite those 46 new cases, the number of states with reported cases remains at 15, the CDC reported March 25.
For the fifth consecutive week the busiest outbreak was in Brooklyn, N.Y., which added 23 new cases. New York’s Rockland County, which is just north of New York City and has 46 confirmed cases for the year, is home to another of the six current outbreaks in the country, with the other four located in Washington (74 total cases for the state), Texas (14 cases), California (7 cases), and Illinois (6 cases). Other states with cases are Arizona, Colorado, Connecticut, Georgia, Kentucky, Missouri, New Hampshire, New Jersey, and Oregon, reported the CDC.
This year’s case total through less than 3 months is nearing the 372 that occurred in 2018, which was the second-worst year for measles in the last decade, but is still well off the 10-year high of 667 reported in 2014, the CDC said.

Despite those 46 new cases, the number of states with reported cases remains at 15, the CDC reported March 25.
For the fifth consecutive week the busiest outbreak was in Brooklyn, N.Y., which added 23 new cases. New York’s Rockland County, which is just north of New York City and has 46 confirmed cases for the year, is home to another of the six current outbreaks in the country, with the other four located in Washington (74 total cases for the state), Texas (14 cases), California (7 cases), and Illinois (6 cases). Other states with cases are Arizona, Colorado, Connecticut, Georgia, Kentucky, Missouri, New Hampshire, New Jersey, and Oregon, reported the CDC.
This year’s case total through less than 3 months is nearing the 372 that occurred in 2018, which was the second-worst year for measles in the last decade, but is still well off the 10-year high of 667 reported in 2014, the CDC said.