User login
NIH to undertake first in-human trial of universal influenza vaccine
The National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, is launching the first in-human trial of a universal influenza vaccine candidate.
The experimental vaccine, H1ssF_3928, is derived from the stem of an H1N1 virus and has a surface made from hemagglutinin and ferritin. By including only the stem of the virus, which changes less than the head, the vaccine should require fewer updates. A similar vaccine made from the same materials was shown to be safe and well tolerated in humans.
The clinical trial (NCT03814720) will be conducted at the NIH Clinical Center in Bethesda, Md., and will gradually enroll at least 53 healthy adults aged 18-70 years. The first 5 participants will receive one 20-mcg intramuscular injection of the vaccine; the other 48 participants will receive two 60-mcg vaccinations 16 weeks apart. Patients will return for 9-11 follow-ups over a 12- to 15-month period, and will provide blood samples for analysis of anti-influenza antibodies.
“Seasonal influenza is a perpetual public health challenge, and we continually face the possibility of an influenza pandemic resulting from the emergence and spread of novel influenza viruses. This phase 1 clinical trial is a step forward in our efforts to develop a durable and broadly protective universal influenza vaccine,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said in the press release.
Find the full press release on the NIH website.
The National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, is launching the first in-human trial of a universal influenza vaccine candidate.
The experimental vaccine, H1ssF_3928, is derived from the stem of an H1N1 virus and has a surface made from hemagglutinin and ferritin. By including only the stem of the virus, which changes less than the head, the vaccine should require fewer updates. A similar vaccine made from the same materials was shown to be safe and well tolerated in humans.
The clinical trial (NCT03814720) will be conducted at the NIH Clinical Center in Bethesda, Md., and will gradually enroll at least 53 healthy adults aged 18-70 years. The first 5 participants will receive one 20-mcg intramuscular injection of the vaccine; the other 48 participants will receive two 60-mcg vaccinations 16 weeks apart. Patients will return for 9-11 follow-ups over a 12- to 15-month period, and will provide blood samples for analysis of anti-influenza antibodies.
“Seasonal influenza is a perpetual public health challenge, and we continually face the possibility of an influenza pandemic resulting from the emergence and spread of novel influenza viruses. This phase 1 clinical trial is a step forward in our efforts to develop a durable and broadly protective universal influenza vaccine,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said in the press release.
Find the full press release on the NIH website.
The National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, is launching the first in-human trial of a universal influenza vaccine candidate.
The experimental vaccine, H1ssF_3928, is derived from the stem of an H1N1 virus and has a surface made from hemagglutinin and ferritin. By including only the stem of the virus, which changes less than the head, the vaccine should require fewer updates. A similar vaccine made from the same materials was shown to be safe and well tolerated in humans.
The clinical trial (NCT03814720) will be conducted at the NIH Clinical Center in Bethesda, Md., and will gradually enroll at least 53 healthy adults aged 18-70 years. The first 5 participants will receive one 20-mcg intramuscular injection of the vaccine; the other 48 participants will receive two 60-mcg vaccinations 16 weeks apart. Patients will return for 9-11 follow-ups over a 12- to 15-month period, and will provide blood samples for analysis of anti-influenza antibodies.
“Seasonal influenza is a perpetual public health challenge, and we continually face the possibility of an influenza pandemic resulting from the emergence and spread of novel influenza viruses. This phase 1 clinical trial is a step forward in our efforts to develop a durable and broadly protective universal influenza vaccine,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said in the press release.
Find the full press release on the NIH website.
Genetic data boost HIV surveillance efforts
SEATTLE – Advances in genetic sequencing are boosting efforts to identify new clusters of HIV infections and guiding public health interventions to address them. The method relies on resistance testing at diagnosis and virologic failure and allows public health researchers to determine the genetic relatedness of viruses responsible for new infections. If the viruses are genetically, geographically, and temporally associated, it indicates a previously unknown transmission cluster.
“The presence of a cluster indicates gaps in our preventative services, which we must address to improve service delivery and stop transmission,” Alexandra M. Oster, MD, Division of HIV/AIDS Prevention, Surveillance, and Epidemiology at the Centers for Disease Control and Prevention, Atlanta, said during a talk at the Conference on Retroviruses and Opportunistic Infections.
She noted that HIV brings special challenges to outbreak detection. The median delay between infection and diagnosis is 3 years. Individuals are highly mobile, and signals of new outbreaks can be quickly drowned out in high-burden areas. But these challenges aren’t unique. Tuberculosis has a similarly lengthy latency period, yet more than 75% of new TB outbreaks are now identified through the use of genetic data. Sequencing also is used to track food-borne illness. The CDC’s PulseNet is a network of laboratories that examines DNA sequences from bacterial infections in search of previously unrecognized outbreaks.
In the HIV setting, molecular surveillance has great potential in identifying and intervening in evolving networks of HIV transmission, but also carries ethical and other challenges.
Nevertheless, “I hope to make the case that cluster detection and response [using molecular surveillance] can help bring the nation closer to ending the HIV epidemic,” said Dr. Oster.
Molecular surveillance obtains most of its data from drug resistance testing, both at entry to care and after virologic failure, which then gets passed to the U.S. National HIV Surveillance System. The data are then stripped of patient identifying information and submitted to the CDC.
With data from multiple individuals in hand, researchers create a phylogenetic tree, in which closely-related viruses appear as close neighbors on a branch. “By tracing back along the tree, you can see the inferred ancestor of [individual strains], and also the inferred ancestor of all strains on the tree,” said Dr. Oster. Together with geographical data, that information allows researchers to identify clusters of patients connected in a transmission network, and that information can be passed along to federal, state, and local agencies to prevent infections and improve care.
From 1997 through 2012, the CDC’s molecular surveillance program focused on drug resistance patterns, but in 2013 the agency decided to expand to include transmission clusters. It now uses a tool called HIV Trace, which helps public health workers with no background in bioinformatics to visualize the DNA sequences and potential clusters, though Dr. Oster cautioned against overinterpretation of the results. “The links shown can easily be misinterpreted as actual social connections,” she said.
As proof of the approach’s potential, an analysis of the clusters identified showed their potential for HIV spread. On average in the United States, four new HIV infections occur per 100 people living with HIV. In the first 13 clusters that CDC identified, the number of infections was 33 per 100 person-years. The first 60 clusters had an average of 44 transmissions per 100 person-years. “None of these clusters had been found by [standard] epidemiologic methods, demonstrating that rapid transmission can be hard to detect without molecular data,” said Dr. Oster.
In 2018, all health departments began collecting sequencing data, and almost 40% of newly diagnosed patients have had sequencing data reported, more than 340,000 patients in total. Researchers have identified 145 priority clusters.
But use of molecular data is not the only method available. The CDC monitors increases in diagnoses in specific areas and conducts time-space analyses. These more traditional methods are particularly useful in areas with small populations or low HIV burden.
With a cluster identified, public health officials can attempt to identify all of the members of the network and help them to access services, such as testing, preexposure prophylaxis (PrEP), syringe service programs, and linkage to care.
In San Antonio, Tex., an analysis identified a cluster of 24 gay and bisexual men, and further analysis revealed an extended network of 87 sexual or needle-sharing partners. Researchers also identified missed opportunities for diagnosis of acute infection as well as low access to PrEP, so the health department sent out an alert clarifying diagnosis testing guidelines, highlighting the concern over acute infection, and containing PrEP educational material.
Analysis of another network in Michigan found that all identified individuals were virally suppressed, even though the network continued to grow. That suggested that there were unidentified individuals who were contributing to transmission, which prompted efforts by providers to encourage testing, linkage to care, and prevention.
All of these developments are good news for efforts to eradicate HIV, but they come with pitfalls. Local communities have expressed concerned that molecular data could be used to identify direction of transmission and for prosecution, since there are HIV laws that criminalize lack of disclosure and potential exposure to the virus, even when transmission doesn’t occur. “These laws are not aligned with current science and have not been found to help curb HIV,” said Dr. Oster.
She noted that current molecular methods are incapable of identifying direction of transmission. Still, the CDC is reemphasizing efforts to protect public health data from nonpublic health use. “CDC and health departments implement unprecedented policies and procedures to ensure confidentiality and security of the data,” Dr. Oster said.
She reported having no relevant disclosures.
SEATTLE – Advances in genetic sequencing are boosting efforts to identify new clusters of HIV infections and guiding public health interventions to address them. The method relies on resistance testing at diagnosis and virologic failure and allows public health researchers to determine the genetic relatedness of viruses responsible for new infections. If the viruses are genetically, geographically, and temporally associated, it indicates a previously unknown transmission cluster.
“The presence of a cluster indicates gaps in our preventative services, which we must address to improve service delivery and stop transmission,” Alexandra M. Oster, MD, Division of HIV/AIDS Prevention, Surveillance, and Epidemiology at the Centers for Disease Control and Prevention, Atlanta, said during a talk at the Conference on Retroviruses and Opportunistic Infections.
She noted that HIV brings special challenges to outbreak detection. The median delay between infection and diagnosis is 3 years. Individuals are highly mobile, and signals of new outbreaks can be quickly drowned out in high-burden areas. But these challenges aren’t unique. Tuberculosis has a similarly lengthy latency period, yet more than 75% of new TB outbreaks are now identified through the use of genetic data. Sequencing also is used to track food-borne illness. The CDC’s PulseNet is a network of laboratories that examines DNA sequences from bacterial infections in search of previously unrecognized outbreaks.
In the HIV setting, molecular surveillance has great potential in identifying and intervening in evolving networks of HIV transmission, but also carries ethical and other challenges.
Nevertheless, “I hope to make the case that cluster detection and response [using molecular surveillance] can help bring the nation closer to ending the HIV epidemic,” said Dr. Oster.
Molecular surveillance obtains most of its data from drug resistance testing, both at entry to care and after virologic failure, which then gets passed to the U.S. National HIV Surveillance System. The data are then stripped of patient identifying information and submitted to the CDC.
With data from multiple individuals in hand, researchers create a phylogenetic tree, in which closely-related viruses appear as close neighbors on a branch. “By tracing back along the tree, you can see the inferred ancestor of [individual strains], and also the inferred ancestor of all strains on the tree,” said Dr. Oster. Together with geographical data, that information allows researchers to identify clusters of patients connected in a transmission network, and that information can be passed along to federal, state, and local agencies to prevent infections and improve care.
From 1997 through 2012, the CDC’s molecular surveillance program focused on drug resistance patterns, but in 2013 the agency decided to expand to include transmission clusters. It now uses a tool called HIV Trace, which helps public health workers with no background in bioinformatics to visualize the DNA sequences and potential clusters, though Dr. Oster cautioned against overinterpretation of the results. “The links shown can easily be misinterpreted as actual social connections,” she said.
As proof of the approach’s potential, an analysis of the clusters identified showed their potential for HIV spread. On average in the United States, four new HIV infections occur per 100 people living with HIV. In the first 13 clusters that CDC identified, the number of infections was 33 per 100 person-years. The first 60 clusters had an average of 44 transmissions per 100 person-years. “None of these clusters had been found by [standard] epidemiologic methods, demonstrating that rapid transmission can be hard to detect without molecular data,” said Dr. Oster.
In 2018, all health departments began collecting sequencing data, and almost 40% of newly diagnosed patients have had sequencing data reported, more than 340,000 patients in total. Researchers have identified 145 priority clusters.
But use of molecular data is not the only method available. The CDC monitors increases in diagnoses in specific areas and conducts time-space analyses. These more traditional methods are particularly useful in areas with small populations or low HIV burden.
With a cluster identified, public health officials can attempt to identify all of the members of the network and help them to access services, such as testing, preexposure prophylaxis (PrEP), syringe service programs, and linkage to care.
In San Antonio, Tex., an analysis identified a cluster of 24 gay and bisexual men, and further analysis revealed an extended network of 87 sexual or needle-sharing partners. Researchers also identified missed opportunities for diagnosis of acute infection as well as low access to PrEP, so the health department sent out an alert clarifying diagnosis testing guidelines, highlighting the concern over acute infection, and containing PrEP educational material.
Analysis of another network in Michigan found that all identified individuals were virally suppressed, even though the network continued to grow. That suggested that there were unidentified individuals who were contributing to transmission, which prompted efforts by providers to encourage testing, linkage to care, and prevention.
All of these developments are good news for efforts to eradicate HIV, but they come with pitfalls. Local communities have expressed concerned that molecular data could be used to identify direction of transmission and for prosecution, since there are HIV laws that criminalize lack of disclosure and potential exposure to the virus, even when transmission doesn’t occur. “These laws are not aligned with current science and have not been found to help curb HIV,” said Dr. Oster.
She noted that current molecular methods are incapable of identifying direction of transmission. Still, the CDC is reemphasizing efforts to protect public health data from nonpublic health use. “CDC and health departments implement unprecedented policies and procedures to ensure confidentiality and security of the data,” Dr. Oster said.
She reported having no relevant disclosures.
SEATTLE – Advances in genetic sequencing are boosting efforts to identify new clusters of HIV infections and guiding public health interventions to address them. The method relies on resistance testing at diagnosis and virologic failure and allows public health researchers to determine the genetic relatedness of viruses responsible for new infections. If the viruses are genetically, geographically, and temporally associated, it indicates a previously unknown transmission cluster.
“The presence of a cluster indicates gaps in our preventative services, which we must address to improve service delivery and stop transmission,” Alexandra M. Oster, MD, Division of HIV/AIDS Prevention, Surveillance, and Epidemiology at the Centers for Disease Control and Prevention, Atlanta, said during a talk at the Conference on Retroviruses and Opportunistic Infections.
She noted that HIV brings special challenges to outbreak detection. The median delay between infection and diagnosis is 3 years. Individuals are highly mobile, and signals of new outbreaks can be quickly drowned out in high-burden areas. But these challenges aren’t unique. Tuberculosis has a similarly lengthy latency period, yet more than 75% of new TB outbreaks are now identified through the use of genetic data. Sequencing also is used to track food-borne illness. The CDC’s PulseNet is a network of laboratories that examines DNA sequences from bacterial infections in search of previously unrecognized outbreaks.
In the HIV setting, molecular surveillance has great potential in identifying and intervening in evolving networks of HIV transmission, but also carries ethical and other challenges.
Nevertheless, “I hope to make the case that cluster detection and response [using molecular surveillance] can help bring the nation closer to ending the HIV epidemic,” said Dr. Oster.
Molecular surveillance obtains most of its data from drug resistance testing, both at entry to care and after virologic failure, which then gets passed to the U.S. National HIV Surveillance System. The data are then stripped of patient identifying information and submitted to the CDC.
With data from multiple individuals in hand, researchers create a phylogenetic tree, in which closely-related viruses appear as close neighbors on a branch. “By tracing back along the tree, you can see the inferred ancestor of [individual strains], and also the inferred ancestor of all strains on the tree,” said Dr. Oster. Together with geographical data, that information allows researchers to identify clusters of patients connected in a transmission network, and that information can be passed along to federal, state, and local agencies to prevent infections and improve care.
From 1997 through 2012, the CDC’s molecular surveillance program focused on drug resistance patterns, but in 2013 the agency decided to expand to include transmission clusters. It now uses a tool called HIV Trace, which helps public health workers with no background in bioinformatics to visualize the DNA sequences and potential clusters, though Dr. Oster cautioned against overinterpretation of the results. “The links shown can easily be misinterpreted as actual social connections,” she said.
As proof of the approach’s potential, an analysis of the clusters identified showed their potential for HIV spread. On average in the United States, four new HIV infections occur per 100 people living with HIV. In the first 13 clusters that CDC identified, the number of infections was 33 per 100 person-years. The first 60 clusters had an average of 44 transmissions per 100 person-years. “None of these clusters had been found by [standard] epidemiologic methods, demonstrating that rapid transmission can be hard to detect without molecular data,” said Dr. Oster.
In 2018, all health departments began collecting sequencing data, and almost 40% of newly diagnosed patients have had sequencing data reported, more than 340,000 patients in total. Researchers have identified 145 priority clusters.
But use of molecular data is not the only method available. The CDC monitors increases in diagnoses in specific areas and conducts time-space analyses. These more traditional methods are particularly useful in areas with small populations or low HIV burden.
With a cluster identified, public health officials can attempt to identify all of the members of the network and help them to access services, such as testing, preexposure prophylaxis (PrEP), syringe service programs, and linkage to care.
In San Antonio, Tex., an analysis identified a cluster of 24 gay and bisexual men, and further analysis revealed an extended network of 87 sexual or needle-sharing partners. Researchers also identified missed opportunities for diagnosis of acute infection as well as low access to PrEP, so the health department sent out an alert clarifying diagnosis testing guidelines, highlighting the concern over acute infection, and containing PrEP educational material.
Analysis of another network in Michigan found that all identified individuals were virally suppressed, even though the network continued to grow. That suggested that there were unidentified individuals who were contributing to transmission, which prompted efforts by providers to encourage testing, linkage to care, and prevention.
All of these developments are good news for efforts to eradicate HIV, but they come with pitfalls. Local communities have expressed concerned that molecular data could be used to identify direction of transmission and for prosecution, since there are HIV laws that criminalize lack of disclosure and potential exposure to the virus, even when transmission doesn’t occur. “These laws are not aligned with current science and have not been found to help curb HIV,” said Dr. Oster.
She noted that current molecular methods are incapable of identifying direction of transmission. Still, the CDC is reemphasizing efforts to protect public health data from nonpublic health use. “CDC and health departments implement unprecedented policies and procedures to ensure confidentiality and security of the data,” Dr. Oster said.
She reported having no relevant disclosures.
EXPERT ANALYSIS FROM CROI 2019
STIs pose complex challenge to HIV efforts
SEATTLE – Sexually-transmitted infections (STIs) such as gonorrhea, chlamydia, and syphilis are on the rise among HIV-infected individuals, and emerging antimicrobial resistance in these organisms is presenting serious challenges to physicians. The issue may be traceable to the introduction of preexposure prophylaxis (PrEP) in 2011, which previous studies have shown to be associated with less condom use.
In the United States, a 2017 report by the Centers for Disease Control and Prevention showed rising incidences of chlamydia (+5% from 2015 to 2017), gonorrhea (+19%), and syphilis (+18%). “We have an incidence among men who have sex with men [MSM] that is above the pre-AIDS era estimates, and we have evidence of spread into heterosexual networks, and a very scary collision with the methamphetamine and heroine using networks,” said Jeanne Marrazzo, MD, professor of infectious diseases at the University of Alabama at Birmingham.
But the numbers alone don’t tell the whole story. “It’s not just the burden of these infections. What’s characterizing these trends is that we have continuing evolution of microbial resistance, which is really a crisis,” Dr. Marrazzo added during a plenary she delivered at the Conference on Retroviruses & Opportunistic Infections.
These infections also remain intricately linked with HIV. An analysis of syphilis cases found that 88% occurred in men. Of those, 80% were MSM. Of the cases in MSM, 46% were coinfected with HIV. “Those are incredible rates,” said Dr. Marrazzo. Among women, the trends are even more alarming. There has been a greater than 150% increase in primary/secondary and congenital syphilis between 2013 and 2017.
Resistance to ceftriaxone and azithromycin remains on the rise in gonorrhea, with 24% of countries reporting at least a 5% incidence of strains that are less susceptible or resistant to ceftriaxone, and 81% of countries reporting similar trends with azithromycin.
In the absence of new drugs to overcome that resistance, or vaccines that can prevent gonorrhea and other infections, what are clinicians to do?
One option may be postexposure doxycycline. One trial in MSM showed that a 200-mg dose taken 24-72 hours after sex was associated with about a 70% increase in both time to first chlamydia and time to first syphilis infection, though no effect was seen on gonorrhea infections. “We shouldn’t be surprised. We know that gonorrhea is classically resistant to tetracyclines, and the MSM population has the highest prevalence of antimicrobial resistance in gonorrhea,” said Dr. Marrazzo.
There are pros and cons to this strategy, of course. On the one hand, doxycycline works for chlamydia and syphilis, it’s safe, and it’s easy to administer. “We’re up a tree when it comes to syphilis, so why not?” opined Dr. Marrazzo. In fact, some MSM have read the literature and are already using it prophylactically. But there are downsides, including adverse effects such as esophagitis/ulceration and photosensitivity, and it is contraindicated in pregnant women. And then there’s the potential for evolving greater resistance. “The horse is out of the barn with respect to gonorrhea, but I think it’s worth thinking about resistance to other pathogens, where we still rely on doxycycline [to treat] in rare cases,” said Dr. Marrazzo.
Finally, Dr. Marrazzo discussed the role of STI treatment in the effort to eradicate HIV. Should the Getting to 0 strategies include aggressive prevention and treatment of STIs? Despite the potentiating role of some STIs in the spread HIV, some urban areas are approaching zero new infections even as other STIs remain a problem. It could be that undetectable = untransmittable, regardless of the presence an STI. Some view targeting STIs as a regressive practice in a setting where the U=U mantra has opened up an era of sexual freedom living with or at risk of HIV.
On the other hand, there are also good arguments to target STIs while trying to eliminate HIV. Results from high-resource locales such as San Francisco and New York City are unlikely to be replicated in places like Sub-Saharan Africa. The public health burden of STIs is extensive, and antibiotic resistance and antibiotic shortages can make treatment difficult. The situation is also different for women, who may experience impacts on fertility or pregnancies, and do not have the same freedom as men in many countries. “Stigma is highly operative and I would wager that sexual pleasure and freedom remain a very elusive goal for women across the globe,” said Dr. Marrazzo.
Dr. Marrazzo has a research grant/grant pending from Cepheid, and is on the advisory panels of BioFire and Gilead.
SEATTLE – Sexually-transmitted infections (STIs) such as gonorrhea, chlamydia, and syphilis are on the rise among HIV-infected individuals, and emerging antimicrobial resistance in these organisms is presenting serious challenges to physicians. The issue may be traceable to the introduction of preexposure prophylaxis (PrEP) in 2011, which previous studies have shown to be associated with less condom use.
In the United States, a 2017 report by the Centers for Disease Control and Prevention showed rising incidences of chlamydia (+5% from 2015 to 2017), gonorrhea (+19%), and syphilis (+18%). “We have an incidence among men who have sex with men [MSM] that is above the pre-AIDS era estimates, and we have evidence of spread into heterosexual networks, and a very scary collision with the methamphetamine and heroine using networks,” said Jeanne Marrazzo, MD, professor of infectious diseases at the University of Alabama at Birmingham.
But the numbers alone don’t tell the whole story. “It’s not just the burden of these infections. What’s characterizing these trends is that we have continuing evolution of microbial resistance, which is really a crisis,” Dr. Marrazzo added during a plenary she delivered at the Conference on Retroviruses & Opportunistic Infections.
These infections also remain intricately linked with HIV. An analysis of syphilis cases found that 88% occurred in men. Of those, 80% were MSM. Of the cases in MSM, 46% were coinfected with HIV. “Those are incredible rates,” said Dr. Marrazzo. Among women, the trends are even more alarming. There has been a greater than 150% increase in primary/secondary and congenital syphilis between 2013 and 2017.
Resistance to ceftriaxone and azithromycin remains on the rise in gonorrhea, with 24% of countries reporting at least a 5% incidence of strains that are less susceptible or resistant to ceftriaxone, and 81% of countries reporting similar trends with azithromycin.
In the absence of new drugs to overcome that resistance, or vaccines that can prevent gonorrhea and other infections, what are clinicians to do?
One option may be postexposure doxycycline. One trial in MSM showed that a 200-mg dose taken 24-72 hours after sex was associated with about a 70% increase in both time to first chlamydia and time to first syphilis infection, though no effect was seen on gonorrhea infections. “We shouldn’t be surprised. We know that gonorrhea is classically resistant to tetracyclines, and the MSM population has the highest prevalence of antimicrobial resistance in gonorrhea,” said Dr. Marrazzo.
There are pros and cons to this strategy, of course. On the one hand, doxycycline works for chlamydia and syphilis, it’s safe, and it’s easy to administer. “We’re up a tree when it comes to syphilis, so why not?” opined Dr. Marrazzo. In fact, some MSM have read the literature and are already using it prophylactically. But there are downsides, including adverse effects such as esophagitis/ulceration and photosensitivity, and it is contraindicated in pregnant women. And then there’s the potential for evolving greater resistance. “The horse is out of the barn with respect to gonorrhea, but I think it’s worth thinking about resistance to other pathogens, where we still rely on doxycycline [to treat] in rare cases,” said Dr. Marrazzo.
Finally, Dr. Marrazzo discussed the role of STI treatment in the effort to eradicate HIV. Should the Getting to 0 strategies include aggressive prevention and treatment of STIs? Despite the potentiating role of some STIs in the spread HIV, some urban areas are approaching zero new infections even as other STIs remain a problem. It could be that undetectable = untransmittable, regardless of the presence an STI. Some view targeting STIs as a regressive practice in a setting where the U=U mantra has opened up an era of sexual freedom living with or at risk of HIV.
On the other hand, there are also good arguments to target STIs while trying to eliminate HIV. Results from high-resource locales such as San Francisco and New York City are unlikely to be replicated in places like Sub-Saharan Africa. The public health burden of STIs is extensive, and antibiotic resistance and antibiotic shortages can make treatment difficult. The situation is also different for women, who may experience impacts on fertility or pregnancies, and do not have the same freedom as men in many countries. “Stigma is highly operative and I would wager that sexual pleasure and freedom remain a very elusive goal for women across the globe,” said Dr. Marrazzo.
Dr. Marrazzo has a research grant/grant pending from Cepheid, and is on the advisory panels of BioFire and Gilead.
SEATTLE – Sexually-transmitted infections (STIs) such as gonorrhea, chlamydia, and syphilis are on the rise among HIV-infected individuals, and emerging antimicrobial resistance in these organisms is presenting serious challenges to physicians. The issue may be traceable to the introduction of preexposure prophylaxis (PrEP) in 2011, which previous studies have shown to be associated with less condom use.
In the United States, a 2017 report by the Centers for Disease Control and Prevention showed rising incidences of chlamydia (+5% from 2015 to 2017), gonorrhea (+19%), and syphilis (+18%). “We have an incidence among men who have sex with men [MSM] that is above the pre-AIDS era estimates, and we have evidence of spread into heterosexual networks, and a very scary collision with the methamphetamine and heroine using networks,” said Jeanne Marrazzo, MD, professor of infectious diseases at the University of Alabama at Birmingham.
But the numbers alone don’t tell the whole story. “It’s not just the burden of these infections. What’s characterizing these trends is that we have continuing evolution of microbial resistance, which is really a crisis,” Dr. Marrazzo added during a plenary she delivered at the Conference on Retroviruses & Opportunistic Infections.
These infections also remain intricately linked with HIV. An analysis of syphilis cases found that 88% occurred in men. Of those, 80% were MSM. Of the cases in MSM, 46% were coinfected with HIV. “Those are incredible rates,” said Dr. Marrazzo. Among women, the trends are even more alarming. There has been a greater than 150% increase in primary/secondary and congenital syphilis between 2013 and 2017.
Resistance to ceftriaxone and azithromycin remains on the rise in gonorrhea, with 24% of countries reporting at least a 5% incidence of strains that are less susceptible or resistant to ceftriaxone, and 81% of countries reporting similar trends with azithromycin.
In the absence of new drugs to overcome that resistance, or vaccines that can prevent gonorrhea and other infections, what are clinicians to do?
One option may be postexposure doxycycline. One trial in MSM showed that a 200-mg dose taken 24-72 hours after sex was associated with about a 70% increase in both time to first chlamydia and time to first syphilis infection, though no effect was seen on gonorrhea infections. “We shouldn’t be surprised. We know that gonorrhea is classically resistant to tetracyclines, and the MSM population has the highest prevalence of antimicrobial resistance in gonorrhea,” said Dr. Marrazzo.
There are pros and cons to this strategy, of course. On the one hand, doxycycline works for chlamydia and syphilis, it’s safe, and it’s easy to administer. “We’re up a tree when it comes to syphilis, so why not?” opined Dr. Marrazzo. In fact, some MSM have read the literature and are already using it prophylactically. But there are downsides, including adverse effects such as esophagitis/ulceration and photosensitivity, and it is contraindicated in pregnant women. And then there’s the potential for evolving greater resistance. “The horse is out of the barn with respect to gonorrhea, but I think it’s worth thinking about resistance to other pathogens, where we still rely on doxycycline [to treat] in rare cases,” said Dr. Marrazzo.
Finally, Dr. Marrazzo discussed the role of STI treatment in the effort to eradicate HIV. Should the Getting to 0 strategies include aggressive prevention and treatment of STIs? Despite the potentiating role of some STIs in the spread HIV, some urban areas are approaching zero new infections even as other STIs remain a problem. It could be that undetectable = untransmittable, regardless of the presence an STI. Some view targeting STIs as a regressive practice in a setting where the U=U mantra has opened up an era of sexual freedom living with or at risk of HIV.
On the other hand, there are also good arguments to target STIs while trying to eliminate HIV. Results from high-resource locales such as San Francisco and New York City are unlikely to be replicated in places like Sub-Saharan Africa. The public health burden of STIs is extensive, and antibiotic resistance and antibiotic shortages can make treatment difficult. The situation is also different for women, who may experience impacts on fertility or pregnancies, and do not have the same freedom as men in many countries. “Stigma is highly operative and I would wager that sexual pleasure and freedom remain a very elusive goal for women across the globe,” said Dr. Marrazzo.
Dr. Marrazzo has a research grant/grant pending from Cepheid, and is on the advisory panels of BioFire and Gilead.
EXPERT ANALYSIS FROM CROI 2019
Noninfected children of HIV-positive mothers have high rates of obesity
NEW ORLEANS – than are those with no such exposure, according to research that provides a compelling link between inflammatory activity in utero and subsequent risk of metabolic disorders.
Most supportive of that link was a near-linear inverse relationship between CD4 counts during the time of pregnancy and risk of both obesity and reactive respiratory disease more than a decade later, according to research presented by Lindsay Fourman, MD, an instructor in medicine at Massachusetts General Hospital, Boston, during the annual meeting of the Endocrine Society.
In this video interview, Dr. Fourman discusses the effort to understand the long-term health consequences of being exposed to HIV and antiretroviral therapies while in utero, a group known by the acronym HIV-exposed uninfected (HEU). With effective therapies now routinely preventing mother-to-child transmission, this population of children is growing quickly.
For this study, 50 HEU individuals were identified from a patient database. They were matched in a 3:1 ratio to a control group for a variety of demographic and socioeconomic variables. At a median age of 18 years, the HEU population was found to have a “strikingly” higher rate of obesity, compared with controls (42% vs. 25%, respectively; P = .04). The rate of reactive airway disease was similarly increased in the HEU group (40% vs. 24%; P = .04).
These data are important for considering health risks in an HEU population, but Dr. Fourman explained that it provides support for looking at metabolic risks from other in utero exposures linked to upregulated inflammation, such as gestational diabetes or obesity.
Dr Fourman and her colleagues reported no disclosures or financial conflicts of interest.
SOURCE: Fourman L et al. ENDO 2019, Session P10 (SAT-256).
NEW ORLEANS – than are those with no such exposure, according to research that provides a compelling link between inflammatory activity in utero and subsequent risk of metabolic disorders.
Most supportive of that link was a near-linear inverse relationship between CD4 counts during the time of pregnancy and risk of both obesity and reactive respiratory disease more than a decade later, according to research presented by Lindsay Fourman, MD, an instructor in medicine at Massachusetts General Hospital, Boston, during the annual meeting of the Endocrine Society.
In this video interview, Dr. Fourman discusses the effort to understand the long-term health consequences of being exposed to HIV and antiretroviral therapies while in utero, a group known by the acronym HIV-exposed uninfected (HEU). With effective therapies now routinely preventing mother-to-child transmission, this population of children is growing quickly.
For this study, 50 HEU individuals were identified from a patient database. They were matched in a 3:1 ratio to a control group for a variety of demographic and socioeconomic variables. At a median age of 18 years, the HEU population was found to have a “strikingly” higher rate of obesity, compared with controls (42% vs. 25%, respectively; P = .04). The rate of reactive airway disease was similarly increased in the HEU group (40% vs. 24%; P = .04).
These data are important for considering health risks in an HEU population, but Dr. Fourman explained that it provides support for looking at metabolic risks from other in utero exposures linked to upregulated inflammation, such as gestational diabetes or obesity.
Dr Fourman and her colleagues reported no disclosures or financial conflicts of interest.
SOURCE: Fourman L et al. ENDO 2019, Session P10 (SAT-256).
NEW ORLEANS – than are those with no such exposure, according to research that provides a compelling link between inflammatory activity in utero and subsequent risk of metabolic disorders.
Most supportive of that link was a near-linear inverse relationship between CD4 counts during the time of pregnancy and risk of both obesity and reactive respiratory disease more than a decade later, according to research presented by Lindsay Fourman, MD, an instructor in medicine at Massachusetts General Hospital, Boston, during the annual meeting of the Endocrine Society.
In this video interview, Dr. Fourman discusses the effort to understand the long-term health consequences of being exposed to HIV and antiretroviral therapies while in utero, a group known by the acronym HIV-exposed uninfected (HEU). With effective therapies now routinely preventing mother-to-child transmission, this population of children is growing quickly.
For this study, 50 HEU individuals were identified from a patient database. They were matched in a 3:1 ratio to a control group for a variety of demographic and socioeconomic variables. At a median age of 18 years, the HEU population was found to have a “strikingly” higher rate of obesity, compared with controls (42% vs. 25%, respectively; P = .04). The rate of reactive airway disease was similarly increased in the HEU group (40% vs. 24%; P = .04).
These data are important for considering health risks in an HEU population, but Dr. Fourman explained that it provides support for looking at metabolic risks from other in utero exposures linked to upregulated inflammation, such as gestational diabetes or obesity.
Dr Fourman and her colleagues reported no disclosures or financial conflicts of interest.
SOURCE: Fourman L et al. ENDO 2019, Session P10 (SAT-256).
REPORTING FROM ENDO 2019
Measles: Latest weekly count is the highest of the year
according to the Centers for Disease Control and Prevention.
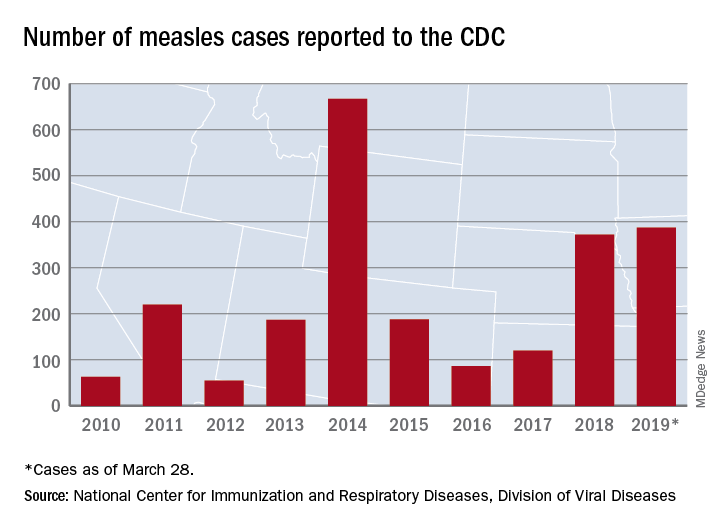
The 73 new cases of measles reported to the CDC during the week ending March 28 – more than any other single week so far in 2019 – brings the total number of cases for the year to 387, the CDC reported April 1. That surpasses the 372 reported in 2018 and is now the highest annual count since 667 cases were reported in 2014.
The ongoing outbreak in Rockland County, N.Y., which resulted in 6 new cases there last week and 52 for the year, prompted County Executive Ed Day to declare a state of emergency effective March 27 that bars unvaccinated individuals under age 18 years from public places for the next 30 days unless they receive an MMR vaccination.
“As this outbreak has continued our inspectors have begun to meet resistance from those they are trying to protect. They have been hung up on or told not to call again. They’ve been told ‘we’re not discussing this, do not come back,’ when visiting the homes of infected individuals as part of their investigations. This type of response is unacceptable and irresponsible. It endangers the health and well-being of others and displays a shocking lack of responsibility and concern for others in our community,” Mr. Day said in a written statement.
In addition to Rockland County, the CDC is currently tracking five other outbreaks: New York City, mainly Brooklyn (33 new cases last week); Washington state (74 cases for the year, but no new cases in the last week); New Jersey (10 total cases, with 8 related to an outbreak in Ocean and Monmouth Counties); and two in California (16 total cases, with 11 related to the outbreaks). One of the California outbreaks and the New Jersey outbreak are new, but the CDC is no longer reporting outbreaks in Texas and Illinois, so the total stays at six nationwide.
In related news from California, state Sen. Richard Pan (D), a pediatrician, and Assemblywoman Lorena Gonzalez (D) introduced a bill to monitor vaccine exemptions “by requiring the state health department to vet each medical exemption form written by physicians [and to] maintain a database of exemptions that would allow officials to monitor which doctors are granting the exemptions,” the Los Angeles Times reported.
according to the Centers for Disease Control and Prevention.

The 73 new cases of measles reported to the CDC during the week ending March 28 – more than any other single week so far in 2019 – brings the total number of cases for the year to 387, the CDC reported April 1. That surpasses the 372 reported in 2018 and is now the highest annual count since 667 cases were reported in 2014.
The ongoing outbreak in Rockland County, N.Y., which resulted in 6 new cases there last week and 52 for the year, prompted County Executive Ed Day to declare a state of emergency effective March 27 that bars unvaccinated individuals under age 18 years from public places for the next 30 days unless they receive an MMR vaccination.
“As this outbreak has continued our inspectors have begun to meet resistance from those they are trying to protect. They have been hung up on or told not to call again. They’ve been told ‘we’re not discussing this, do not come back,’ when visiting the homes of infected individuals as part of their investigations. This type of response is unacceptable and irresponsible. It endangers the health and well-being of others and displays a shocking lack of responsibility and concern for others in our community,” Mr. Day said in a written statement.
In addition to Rockland County, the CDC is currently tracking five other outbreaks: New York City, mainly Brooklyn (33 new cases last week); Washington state (74 cases for the year, but no new cases in the last week); New Jersey (10 total cases, with 8 related to an outbreak in Ocean and Monmouth Counties); and two in California (16 total cases, with 11 related to the outbreaks). One of the California outbreaks and the New Jersey outbreak are new, but the CDC is no longer reporting outbreaks in Texas and Illinois, so the total stays at six nationwide.
In related news from California, state Sen. Richard Pan (D), a pediatrician, and Assemblywoman Lorena Gonzalez (D) introduced a bill to monitor vaccine exemptions “by requiring the state health department to vet each medical exemption form written by physicians [and to] maintain a database of exemptions that would allow officials to monitor which doctors are granting the exemptions,” the Los Angeles Times reported.
according to the Centers for Disease Control and Prevention.

The 73 new cases of measles reported to the CDC during the week ending March 28 – more than any other single week so far in 2019 – brings the total number of cases for the year to 387, the CDC reported April 1. That surpasses the 372 reported in 2018 and is now the highest annual count since 667 cases were reported in 2014.
The ongoing outbreak in Rockland County, N.Y., which resulted in 6 new cases there last week and 52 for the year, prompted County Executive Ed Day to declare a state of emergency effective March 27 that bars unvaccinated individuals under age 18 years from public places for the next 30 days unless they receive an MMR vaccination.
“As this outbreak has continued our inspectors have begun to meet resistance from those they are trying to protect. They have been hung up on or told not to call again. They’ve been told ‘we’re not discussing this, do not come back,’ when visiting the homes of infected individuals as part of their investigations. This type of response is unacceptable and irresponsible. It endangers the health and well-being of others and displays a shocking lack of responsibility and concern for others in our community,” Mr. Day said in a written statement.
In addition to Rockland County, the CDC is currently tracking five other outbreaks: New York City, mainly Brooklyn (33 new cases last week); Washington state (74 cases for the year, but no new cases in the last week); New Jersey (10 total cases, with 8 related to an outbreak in Ocean and Monmouth Counties); and two in California (16 total cases, with 11 related to the outbreaks). One of the California outbreaks and the New Jersey outbreak are new, but the CDC is no longer reporting outbreaks in Texas and Illinois, so the total stays at six nationwide.
In related news from California, state Sen. Richard Pan (D), a pediatrician, and Assemblywoman Lorena Gonzalez (D) introduced a bill to monitor vaccine exemptions “by requiring the state health department to vet each medical exemption form written by physicians [and to] maintain a database of exemptions that would allow officials to monitor which doctors are granting the exemptions,” the Los Angeles Times reported.
Acute kidney injury after hip or knee replacement: Can we lower the risk?
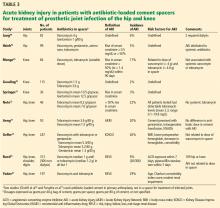
Total hip or knee replacement (also called total joint arthroplasty) is highly successful at relieving pain and restoring function, but at the risk of acute kidney injury, which is a sudden loss of renal function. Various factors have been associated with this risk, some of which are potentially modifiable, notably, the use of nephrotoxic antibiotics and other drugs.
This review examines the incidence of acute kidney injury using current criteria in total joint arthroplasty of the hip or knee in general, and in the setting of revision surgery for prosthetic joint infection in particular, in which the risk is higher. We identify risk factors for acute kidney injury and propose ways to lower the risk.
MILLIONS OF PROCEDURES ANNUALLY
Total replacement of the hip1,2 or knee3 is being done more and more. Kurtz et al4 estimate that by the year 2030, we will see approximately 3.5 million primary total knee and 500,000 primary total hip replacements every year. In addition, revision total knee procedures are expected to exceed 250,000 per year, and revision total hip procedures are expected to exceed 90,000 per year.4
Chronic infection may complicate up to 2% of these procedures and is associated with significant morbidity, death, and financial costs. Currently, it may be the reason for 25% of total joint arthroplasty revisions,5 but by the year 2030, it is projected to account for 66% of revision total knee arthroplasties and 48% of revision total hip arthroplasties.6
PRIMARY TOTAL JOINT ARTHROPLASTY AND ACUTE KIDNEY INJURY
Study designs, findings varied widely
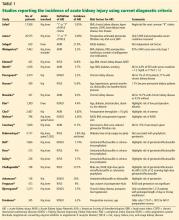
The incidence of acute kidney injury varied markedly among the studies of primary total joint arthroplasty or revision for aseptic reasons. Numerous factors explain this heterogeneity.
Designs ranged from single-center studies with relatively small numbers of patients to large regional and national samples based on administrative data.
Almost all of the studies were retrospective. We are not aware of any randomized controlled trials.
Discharge diagnosis may miss many cases
Several studies based the diagnosis of acute kidney injury on International Classification of Diseases, Ninth Revision (ICD-9) coding from hospital discharge summaries.
Nadkarni et al,29 in the largest study published to date, used the nationwide inpatient sample database of more than 7 million total joint arthroplasties and found an incidence of acute kidney injury based on ICD-9 coding of 1.3% over the years 2002 to 2012, although this increased to 1.8% to 1.9% from 2010 to 2012.
Lopez-de-Andres et al,30 in a similar study using the Spanish national hospital discharge database, evaluated 20,188 patients who underwent revision total hip or knee arthroplasty and found an overall incidence of acute kidney injury of 0.94%, also using ICD-9 coding.
Gharaibeh et al31 used similar methods to diagnose acute kidney injury in a single-center study of 8,949 patients and found an incidence of 1.1%.
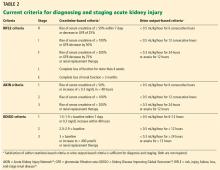
Although these 3 studies suggest that the incidence of acute kidney injury is relatively low, Grams et al35 found the sensitivity of ICD-9 coding from hospital records for the diagnosis of acute kidney injury to be only 11.7% compared with KDIGO serum creatinine and urine output criteria. This suggests that the true incidence in these studies may be many times higher, possibly near 10%.
Do all stages of kidney injury count?
Jafari et al,7 in a large series from a single medical center, used only the “I” (injury) and “F” (failure) levels of the RIFLE criteria (corresponding to stages 2 and 3 of the KDIGO criteria) and found an incidence of 0.55% in more than 17,000 total joint arthroplasties.
Jamsa et al8 used the same criteria for acute kidney injury (only “I” and “F”) and found 58 cases in 5,609 patients in whom postoperative serum creatinine was measured, for an incidence of 1%; the remaining 14,966 patients in their cohort did not have serum creatinine measured, and it was assumed they did not have acute kidney injury. Neither of these studies included the most common “R” (risk) stage of acute kidney injury.
Parr et al36 recently studied a nationwide sample of 657,840 hospitalized veterans and found that of 90,614 who developed acute kidney injury based on KDIGO creatinine criteria, 84% reached only stage R. This suggests that if all stages were considered, the true incidence of acute kidney injury would have been higher—possibly 4% in the Jafari series and possibly 7% in the Jamsa series.
Smaller studies had higher rates
Smaller, single-center series reported much higher incidences of acute kidney injury.
Kimmel et al11 found an incidence of 14.8% in 425 total joint arthroplasties using RIFLE creatinine criteria.
Johansson et al25 found an incidence of 19.9% in 136 total joint arthroplasties using KDIGO creatinine criteria.
Sehgal et al9 found an incidence of 21.9% in 659 total joint arthroplasties using AKIN creatinine criteria.
Challagundla et al24 found an incidence of 23.7% in 198 procedures using RIFLE creatinine criteria.
Weingarten et al,10 in a single-center series of 7,463 total joint arthroplasties, found an incidence of acute kidney injury of only 2.2% using AKIN criteria, although 12% of the patients with acute kidney injury did not return to their baseline serum creatinine levels by 3 months.
Our estimate: Nearly 10%
In total, in the 20 studies in Table 1 that included all stages of acute kidney injury, there were 1,909 cases of acute kidney injury in 34,337 patients, for an incidence of 5.6%. Considering that all studies but one were retrospective and none considered urine output criteria for acute kidney injury, we believe that using current KDIGO criteria, the true incidence of acute kidney injury complicating primary lower-extremity total joint arthroplasties is really closer to 10%.
RISK FACTORS FOR ACUTE KIDNEY INJURY
Various factors have been associated with development of acute kidney injury by multivariate analysis in these studies. Some are modifiable, while others are not, at least in the short term.
Nonmodifiable risk factors
Older age is often significant in studies assessing primary total joint arthroplasty or revision total joint arthroplasty not specifically for infection.11,12,16,17,26,28
Obesity is also a major factor in the development of acute kidney injury,7,10–12,17,18 and, along with age, is a major factor contributing to the need for joint replacement in the first place.
Male sex may increase risk.29
Diabetes mellitus was identified as a risk factor in several studies,10,12,17,20 and hypertension in a few.7,10,24
Other comorbidities and factors such as cardiovascular disease,7,10 liver disease,7 pulmonary disease,7 high American Society of Anesthesiology score,8,19 and benign heart murmurs preoperatively by routine physical examination have also been linked to acute kidney injury after joint arthroplasty.28
Chronic kidney disease as a risk factor
Chronic kidney disease at baseline was associated with acute kidney injury in several of these series.7,11–13,15,19,29
Warth et al12 studied 1,038 patients and found an incidence of acute kidney injury of 11% in the 135 with chronic kidney disease (defined as serum creatinine > 1.2 mg/dL) and who received acetaminophen or narcotics for pain control, compared with 4.8% in the remaining 903 patients without chronic kidney disease, who received ketorolac or celecoxib.
Perregaard et al13 studied 3,410 patients who underwent total hip arthroplasty and found an incidence of acute kidney injury (per KDIGO creatinine criteria) of 2.2% overall, but 7% in the 134 patients with chronic kidney disease based on KDIGO creatinine criteria.
Nowicka et al15 found an incidence of acute kidney injury of 16.7% in the 48 patients with chronic kidney disease (defined as a glomerular filtration rate estimated by the Cockroft-Gault formula of less than 60 mL/min/1.73 m2), compared with 4.5% in the remaining 289.
Modifiable risk factors
Modifiable risk factors that should be considered in high-risk cases include anemia, perioperative blood transfusion, perioperative use of renin-angiotensin-aldosterone system inhibitors such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), particular antibiotics used for prophylaxis, and nonsteroidal anti-inflammatory drugs used postoperatively.
Anemia and blood transfusion
Preoperative anemia has been associated with postoperative acute kidney injury in various surgical settings such as cardiac surgery.37,38 Perioperative red blood cell transfusions have also been associated with acute kidney injury in cardiac surgery; similar results may apply to total joint arthroplasty.
Choi et al,17 in 2,467 patients undergoing hip replacement, found a significant risk for acute kidney injury if postoperative hemoglobin was consistently below 10 g/dL compared with consistently above this level, with an inverse probability-of-treatment weighted odds ratio of 1.817 (P = .011).
Others have found a significant association of perioperative blood transfusion with acute kidney injury in total joint arthroplasty.10,29
Nadkarni et al,29 for example, used the nationwide inpatient sample database and found by multivariate analysis that perioperative blood transfusion was strongly associated with acute kidney injury, with an adjusted odds ratio of 2.28 (95% confidence interval [CI] 2.15–2.42, P < .0001).
Comment. A higher incidence of acute kidney injury may represent confounding by indication bias, as sicker patients or complicated surgeries may require transfusion, and this risk may not be completely accounted for by multivariate analysis. It is also possible, however, that transfusions per se may contribute to acute kidney injury. Possible direct or indirect mechanisms mediating acute kidney injury include hemolytic reactions, circulatory overload, acute lung injury, and immunomodulatory effects.39
Preoperative transfusion in anemic patients undergoing cardiac surgery may also reduce the incidence of postoperative acute kidney injury both by correcting the anemia and by limiting the need for perioperative transfusions.40 It remains to be determined whether elective preoperative transfusion to correct anemia would reduce postoperative development of acute kidney injury in total joint arthroplasty. As an aside, perioperative transfusion has also been linked to development of periprosthetic joint infection.41
Renin-angiotensin-aldosterone system inhibitors
Several studies found perioperative use of renin-angiotensin-aldosterone system inhibitors to be a risk factor for acute kidney injury.
Kimmel et al11 reported adjusted odds ratios of 2.70 (95% CI 1.12–6.48) for ACE inhibitor use and 2.64 (95% CI 1.18–5.93) for ARB use in a study of 425 primary total joint arthroplasties.
Challagundla et al24 found an odds ratio of 3.07 (95% CI 1.40–6.74) with ACE inhibitor or ARB use by multivariate analysis in 198 total joint arthroplasties.
Nielson et al18 studied 798 patients who underwent total joint arthroplasty and found that preoperative use of renin-angiotensin system inhibitors was associated with a significantly higher rate of postoperative acute kidney injury (8.3% vs 1.7% without inhibition), which was statistically significant by multivariate analysis (odds ratio 2.6, 95% CI 1.04–6.51).
We recommend holding renin-angiotensin-aldosterone system inhibitors 7 days before surgery through the postoperative period in high-risk cases.
Aminoglycoside use as a risk factor
Prophylactic administration of systemic antibiotics is the standard of care. In a systematic review of 26 studies and meta-analysis of 7 studies (3,065 patients), prophylactic antibiotics reduced the relative risk of wound infection by 81% with an absolute risk reduction of 8%.42
A modifiable risk factor for acute kidney injury is the specific antibiotic used for prophylaxis. Multiple studies assessed the risk of acute kidney injury comparing regimens containing an aminoglycoside (typically gentamicin) with regimens lacking these agents.20–26 In general, these studies found a significantly higher risk of acute kidney injury when gentamicin was used.
Challagundla et al24 found an incidence of acute kidney injury of 52% using RIFLE creatinine criteria in 52 patients receiving 8 g total of flucloxacillin plus 160 mg of gentamicin (120 mg if they weighed less than 60 kg) compared with 8% in 48 patients given cefuroxime (3 g total) and 14% in an additional 52 patients also given cefuroxime.
Johansson et al25 found an incidence of KDIGO creatinine-based acute kidney injury of 13% in 70 patients given dicloxacillin alone prophylactically compared with 27% given dicloxacillin and gentamicin, with a relative risk of 3.
Bell et al,21 in a large registry-based analysis from Scotland involving 7,666 elective orthopedic procedures, found that use of flucloxacillin 2 g plus a single dose of gentamicin 4 mg/kg was significantly associated with a 94% higher risk of acute kidney injury (KDIGO creatinine criteria) compared with a cefuroxime-based regimen, with absolute rates increasing from 6.2% to 10.8%.
Dubrovskaya et al20 and Ferguson et al,26 in contrast, found no increased risk with addition of gentamicin.
We recommend avoiding aminoglycosides for prophylaxis in primary lower-extremity total joint arthroplasty in patients at higher risk unless required for specific microbiologic reasons.
Vancomycin may also increase risk
Courtney et al19 assessed the risk of adding vancomycin to cefazolin for routine prophylaxis in a retrospective series of 1,828 total hip or knee arthroplasties and found a significantly higher rate of acute kidney injury, using AKIN criteria (13% vs 8%, odds ratio by multivariate analysis 1.82, P = .002).19
Other agents shown to be effective in treating periprosthetic joint infections or complicated skin and soft-tissue infections with resistant organisms include daptomycin43 and linezolid.44 These nonnephrotoxic alternatives to vancomycin may be a consideration if prophylaxis for methicillin-resistant Staphylococcus aureus is deemed necessary in patients at risk for acute kidney injury.
PROSTHETIC JOINT INFECTIONS AND ANTIBIOTIC-LOADED CEMENT
Deep infection may complicate nearly 1% of total hip45 and 2% of total knee arthroplasties.46 Kurtz et al4,6 have projected that by 2030, infection will be the cause of two-thirds of the estimated 268,000 revision total knee arthroplasties and about half of the estimated 96,700 revision total hip arthroplasties.
The most common method of treating a chronically infected replacement joint is a 2-stage procedure.5 First, the prosthesis is removed, all infected bone and soft tissue is debrided, and an antibiotic-loaded cement spacer is implanted. Systemic antibiotics are given concurrently, typically for about 6 weeks. After the infection is brought under control, perhaps 2 to 3 months later, the spacer is removed and a new joint is implanted with antibiotic-loaded cement. A 1-stage procedure may be an option in selected cases and would obviate the need for an antibiotic-loaded cement spacer.47,48
Of obvious relevance to development of acute kidney injury is the choice and amount of antibiotics embedded in the cement used for spacers and in implantation. Very high antibiotic levels are achieved within the joint space, usually with little systemic absorption, although significant systemic exposure has been documented in some cases.
The polymethylmethacrylate cement used for these purposes comes in 40-g bags. Multiple bags are typically required per joint, perhaps 2 to 4.49
The rate of elution of antibiotics is determined by several factors, including surface area, porosity, and the number of antibiotics. In general, elution is greatest early on, with exponential decline lasting perhaps 1 week, followed by slow, sustained release over weeks to months.50 However, several in vitro studies have indicated that only about 5%50,51 of the total antibiotic actually elutes over time.
Initially, multiple antibiotic-laden cement beads were used to fill the joint space, but this significantly limited function and mobility.52 Now, cement spacers are used, and they can be nonarticulating or articulating for maximal joint mobility.53 Although much greater antibiotic elution occurs from beads due to their high surface area-to-volume ratio, spacers still provide an adequate dose.
ANTIBIOTIC-LOADED CEMENT: DOSAGE AND ELUTION CHARACTERISTICS
Antibiotic-loaded cement can be either low-dose or high-dose.
Low-dose cement
Low-dose cement typically consists of 0.5 to 1.0 g of antibiotic per 40-g bag of cement, usually an aminoglycoside (gentamicin or tobramycin) or vancomycin, and can be purchased premixed by the manufacturer. Such cement is only used prophylactically with primary total joint arthroplasty or revision for aseptic reasons, a practice common in Europe but less so in the United States. Some American authors propose antibiotic-loaded cement prophylaxis for patients at high risk, eg, those with immunosuppression, inflammatory cause of arthritis, or diabetes.54
Vrabec et al,55 in a study of low-dose tobramycin-loaded cement used for primary total knee arthroplasty, found a peak median intra-articular tobramycin concentration of 32 mg/L at 6 hours, declining to 6 mg/L at 48 hours with all serum levels 0.3 mg/L or less (unmeasureable) at similar time points.
Sterling et al,56 studying primary total hip arthroplasties with low-dose tobramycin-loaded cement, found mean levels in drainage fluid of 103 mg/L at 6 hours, declining to 15 mg/L at 48 hours. Serum levels peaked at 0.94 mg/L at 3 hours, declining to 0.2 mg/L by 48 hours.
Although most of the antibiotic elution occurs early (within the first week), antibiotic can be found in joint aspirates up to 20 years later.57 We are unaware of any well-documented cases of acute kidney injury ascribable to low-dose antibiotic-loaded cement used prophylactically. One case report making this assertion did not determine serum levels of aminoglycoside.58
High-dose cement
High-dose antibiotic-loaded cement typically contains about 4 to 8 g of antibiotic per 40-g bag of cement and is used in the treatment of prosthetic joint infection to form the spacers. The antibiotic must be mixed into the cement powder by the surgeon in the operating room.
There is no standard combination or dosage. The choice of antibiotic can be tailored to the infecting organism if known. Otherwise, gram-positive organisms are most common, and vancomycin and aminoglycosides are often used together. This particular combination will enhance the elution of both antibiotics when studied in vitro, a process termed “passive opportunism.”59 Other antibiotics in use include aztreonam, piperacillin, teicoplanin, fluoroquinolones, cephalosporins, and daptomycin, among others.
About 8 g of antibiotic total per 40-g bag is the maximum to allow easy molding.52 As an example, this may include 4 g of vancomycin and 3.6 g of tobramycin per 40 g. Given that 3 to 4 such bags are often used per joint, there is significant risk of systemic exposure.
Kalil et al60 studied 8 patients who received high-dose tobramycin-loaded cement to treat periprosthetic joint infections of the hip or knee and found that 7 had detectable serum levels (mean 0.84 mg/L, highest 2.0 mg/L), including 1 with a level of 0.9 mg/L on day 38; 4 of these 8 developed acute kidney injury by AKIN criteria, although other risk factors for acute kidney injury existed. Nearly all had concomitant vancomycin (3 to 8 g) added to the cement as well.
Hsieh et al61 studied 46 patients with infected total hip arthroplasties treated with high-dose antibiotic-loaded cement spacers (vancomycin 4 g and aztreonam 4 g per 40-g bag) and found vancomycin levels in joint drainage higher than 1,500 mg/L on day 1, decreasing to 571 mg/L on day 7; serum levels were low (range 0.1–1.6 mg/L at 24 hours), falling to undetectable by 72 hours.
ANTIBIOTIC-LOADED CEMENT SPACERS AND ACUTE KIDNEY INJURY
Case reports have associated high-dose antibiotic-loaded cement spacers with acute kidney injury.
Curtis et al62 described an 85-year-old patient with stage 3 chronic kidney disease who was treated for an infected total knee arthroplasty with an antibiotic-loaded cement spacer (containing 3.6 g of tobramycin and 3 g of cefazolin per 40-g bag, 3 bags total) and developed stage 3 acute kidney injury. After 16 days and 3 hemodialysis sessions, the patient’s serum tobramycin level was still 2 mg/L despite receiving no systemic tobramycin.
Wu et al63 reported a case of acute kidney injury that required dialysis after implantation of a tobramycin- and vancomycin-loaded spacer, with persistent serum tobramycin levels despite repeated hemodialysis sessions until the spacer was removed.
Chalmers et al64 described 2 patients with acute kidney injury and persistently elevated serum tobramycin levels (3.9 mg/L on day 39 in 1 patient and 2.0 mg/L on day 24 in the other patient) despite no systemic administration.
In these and other case reports,65–67 dialysis and spacer explantation were usually required.
Comment. It is intuitive that acute kidney injury would more likely complicate revision total joint arthroplasties for infection than for primary total joint arthroplasties or revisions for aseptic reasons, given the systemic effects of infection and exposure to nephrotoxic or allergenic antibiotics. And the available data suggest that the risk of acute kidney injury is higher with revision for prosthetic joint infection than with revision for aseptic reasons. However, many of the studies were retrospective, relatively small, single-center series and used different definitions of acute kidney injury.
Luu et al83 performed a systematic review of studies published between January 1989 and June 2012 reporting systemic complications (including acute kidney injury) of 2-stage revision arthroplasties including placement of an antibiotic-loaded cement spacer for treatment of periprosthetic joint infection. Overall, 10 studies were identified with 544 total patients. Five of these studies, with 409 patients, reported at least 1 case of acute kidney injury for a total of 27 patients, giving an incidence of 6.6% in these studies.68–71 The remaining 5 studies, totaling 135 patients, did not report any cases of acute kidney injury,50,61,76–78 although that was not the primary focus of any of those trials.
Most notable from this systematic review, the study of Menge et al69 retrospectively determined the incidence of acute kidney injury (defined as a 50% rise in serum creatinine to > 1.4 mg/dL within 90 days of surgery) to be 17% in 84 patients with infected total knee arthroplasties treated with antibiotic-loaded cement spacers. A mean of 3.5 bags of cement per spacer were used in the 35 articulating spacers, compared with 2.9 per nonarticulating spacer. These spacers contained vancomycin in 82% (median 4.0 g, range 1–16 g) and tobramycin in 94% (median 4.8 g, range 1–12 g), among others in small percentages. The dose of tobramycin in the spacer considered either as a dichotomous variable (> 4.8 g, OR 5.87) or linearly (OR 1.24 per 1-g increase) was significantly associated with acute kidney injury, although systemic administration of aminoglycosides or vancomycin was not.
Additional single-center series that were published subsequent to this review have generally used more current diagnostic criteria.
Noto et al72 found that 10 of 46 patients treated with antibiotic-loaded cement spacers had a greater than 50% rise in serum creatinine (average increase 260%). All spacers contained tobramycin (mean dose 8.2 g), and 9 of 10 also contained vancomycin (mean 7.6 g). All of the 9 patients with acute kidney injury with follow-up data recovered renal function.
Reed et al75 found 26 cases of acute kidney injury (based on RIFLE creatinine criteria) in 306 patients with antibiotic-loaded cement spacers treating various periprosthetic joint infections (including hips, knees, shoulders, and digits) and compared them with 74 controls who did not develop acute kidney injury. By multivariable analysis, receipt of an ACE inhibitor within 7 days of surgery and receipt of piperacillin-tazobactam within 7 days after surgery were both significantly more common in cases with acute kidney injury than in controls without acute kidney injury.
Aeng et al73 prospectively studied 50 consecutive patients receiving antibiotic-loaded spacers containing tobramycin (with or without vancomycin) for treatment of infected hip or knee replacements. Using RIFLE creatinine criteria, they found an incidence of acute kidney injury of 20% (10 of 50). Factors significantly associated with acute kidney injury included cement premixed by the manufacturer with gentamicin (0.5 g per 40-g bag) in addition to the tobramycin they added, intraoperative blood transfusions, and postoperative use of nonsteroidal anti-inflammatory drugs.
Geller et al,74 in a multicenter retrospective study of 247 patients with prosthetic joint infections (156 knees and 91 hips) undergoing antibiotic-loaded cement spacer placement, found an incidence of acute kidney injury of 26% based on KDIGO creatinine criteria. Significant risk factors included higher body mass index, lower preoperative hemoglobin level, drop in hemoglobin after surgery, and comorbidity (hypertension, diabetes, chronic kidney disease, or cardiovascular disease). Most of the spacers contained a combination of vancomycin and either tobramycin (81%) or gentamicin (13%). The spacers contained an average of 5.3 g (range 0.6–18 g) of vancomycin (average 2.65 g per 40-g bag) and an average of 5.2 g (range 0.5–16.4 g) of tobramycin (average 2.6 g per bag).
As in Menge et al,69 this study illustrates the wide range of antibiotic dosages in use and the lack of standardization. In contrast to the study by Menge et al, however, development of acute kidney injury was not related to the amount of vancomycin or tobramycin contained in the spacers. Eventual clearance of infection (at 1 and 2 years) was significantly related to increasing amounts of vancomycin. Multiple different systemic antibiotics were used, most commonly vancomycin (44%), and systemic vancomycin was not associated with acute kidney injury.
Yadav et al,81 in a study of 3,129 consecutive revision procedures of the knee or hip, found an incidence of acute kidney injury by RIFLE creatinine criteria of 29% in the 197 patients who received antibiotic-loaded cement spacers for periprosthetic joint infection compared with 3.4% in the 2,848 who underwent revision for aseptic reasons. In 84 patients with prosthetic joint infection having various surgeries not including placement of a spacer, the acute kidney injury rate at some point in their course was an alarmingly high 82%. In the group that received spacers, only age and comorbidity as assessed by Charlson comorbidity index were independently associated with acute kidney injury by multivariate analysis. Surprisingly, modest renal impairment was protective, possibly because physicians of patients with chronic kidney disease were more vigilant and took appropriate measures to prevent acute kidney injury.
Overall, the risk of acute kidney injury appears to be much higher during treatment of prosthetic joint infection with a 2-stage procedure using an antibiotic-loaded cement spacer than after primary total joint arthroplasty or revision for aseptic reasons, and may complicate up to one-third of cases.
REDUCING RISK DURING TREATMENT OF INFECTED REPLACEMENT JOINTS
As in primary total joint arthroplasty in general, higher-risk cases should be identified based on age, body mass index, chronic kidney disease, comorbidities (hypertension, diabetes, established cardiovascular disease), and anemia.
Preoperative transfusion can be considered case by case depending on degree of anemia and associated risk factors.
All renin-angiotensin-aldosterone system inhibitors should be withheld starting 1 week before surgery.
Both nonselective and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs should be avoided, if possible.
Strict attention should be paid to adequate intraoperative and postoperative fluid resuscitation.
Kidney function should be monitored closely in the early postoperative period, including urine output and daily creatinine for at least 72 hours.
Systemic administration of potentially nephrotoxic antibiotics should be minimized, especially the combination of vancomycin with piperacillin-tazobactam.84 Daptomycin is a consideration.43
If acute kidney injury should develop, serum levels of vancomycin or aminoglycosides should be measured if the spacer contains these antibiotics. The spacer may need to be removed if toxic serum levels persist.
TAKE-HOME POINTS
Acute kidney injury may complicate up to 10% of primary lower-extremity total joint arthroplasties and up to 25% of periprosthetic joint infections treated with a 2-stage procedure including placement of an antibiotic-loaded cement spacer in the first stage.
Risk factors for acute kidney injury include older age, obesity, chronic kidney disease, and overall comorbidity. Potentially modifiable risk factors include anemia, perioperative transfusions, aminoglycoside prophylaxis, perioperative renin-angiotensin system blockade, and postoperative nonsteroidal anti-inflammatory drugs. These should be mitigated when possible.
In patients with periprosthetic joint infection who receive antibiotic-loaded cement spacers, especially patients with additional risk factors for acute kidney injury, strict attention should be paid to the dose of antibiotic in the spacer, with levels checked postoperatively if necessary. Nonnephrotoxic antibiotics should be chosen for systemic administration when possible.
Prospective randomized controlled trials are needed to guide therapy after total joint arthroplasty, and to verify the adverse long-term outcomes of acute kidney injury in this setting.
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007; 370(9597):1508–1519. doi:10.1016/S0140-6736(07)60457-7
- Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet 2012; 380(9855):1768–1777. doi:10.1016/S0140-6736(12)60607-2
- Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012; 379(9823):1331–1340. doi:10.1016/S0140-6736(11)60752-6
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89(4):780–785. doi:10.2106/JBJS.F.00222
- Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet 2016; 387(10016):386–394. doi:10.1016/S0140-6736(14)61798-0
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am 2007; 89(suppl 3):144–151. doi:10.2106/JBJS.G.00587
- Jafari SM, Huang R, Joshi A, Parvizi J, Hozack WJ. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty 2010; 25(6 suppl):49–53, 53.e1–2. doi:10.1016/j.arth.2010.04.008
- Jamsa P, Jamsen E, Lyytikainen LP, Kalliovalkama J, Eskelinen A, Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop 2017; 88(4):370–376. doi:10.1080/17453674.2017.1301743
- Sehgal V, Bajwa SJ, Sehgal R, Eagan J, Reddy P, Lesko SM. Predictors of acute kidney injury in geriatric patients undergoing total knee replacement surgery. Int J Endocrinol Metab 2014; 12(3):e16713. doi:10.5812/ijem.16713
- Weingarten TN, Gurrieri C, Jarett PD, et al. Acute kidney injury following total joint arthroplasty: retrospective analysis. Can J Anaesth 2012; 59(12):1111–1118. doi:10.1007/s12630-012-9797-2
- Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J 2014; 7(6):546–551. doi:10.1093/ckj/sfu108
- Warth LC, Noiseux NO, Hogue MH, Klaassen AL, Liu SS, Callaghan JJ. Risk of acute kidney injury after primary and revision total hip arthroplasty and total knee arthroplasty using a multimodal approach to perioperative pain control including ketorolac and celecoxib. J Arthroplasty 2016; 31(1):253–255. doi:10.1016/j.arth.2015.08.012
- Perregaard H, Damholt MB, Solgaard S, Petersen MB. Renal function after elective total hip replacement. Acta Orthop 2016; 87(3):235–238. doi:10.3109/17453674.2016.1155130
- Hassan BK, Sahlström A, Dessau RB. Risk factors for renal dysfunction after total hip joint replacement; a retrospective cohort study. J Orthop Surg Res 2015; 10:158. doi:10.1186/s13018-015-0299-0
- Nowicka A, Selvaraj T. Incidence of acute kidney injury after elective lower limb arthroplasty. J Clin Anesth 2016; 34:520–523. doi:10.1016/j.jclinane.2016.06.010
- Kim HJ, Koh WU, Kim SG, et al. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: a retrospective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine (Baltimore) 2016; 95(31):e4489. doi:10.1097/MD.0000000000004489
- Choi YJ, Kim S, Sim JH, Hahm K. Postoperative anemia is associated with acute kidney injury in patients undergoing total hip replacement arthroplasty: a retrospective study. Anesth Analg 2016; 122(6):1923–1928. doi:10.1213/ANE.0000000000001003
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9(5):283–288. doi:10.1002/jhm.2155
- Courtney PM, Melnic CM, Zimmer Z, Anari J, Lee GC. Addition of vancomycin to cefazolin prophylaxis is associated with acute kidney injury after primary joint arthroplasty. Clin Orthop Relat Res 2015; 473(7):2197–2203. doi:10.1007/s11999-014-4062-3
- Dubrovskaya Y, Tejada R, Bosco J 3rd, et al. Single high dose gentamicin for perioperative prophylaxis in orthopedic surgery: evaluation of nephrotoxicity. SAGE Open Med 2015; 3:2050312115612803. doi:10.1177/2050312115612803
- Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol 2014; 25(11):2625–2632. doi:10.1681/ASN.2014010035
- Ross AD, Boscainos PJ, Malhas A, Wigderowitz C. Peri-operative renal morbidity secondary to gentamicin and flucloxacillin chemoprophylaxis for hip and knee arthroplasty. Scott Med J 2013; 58(4):209–212. doi:10.1177/0036933013507850
- Bailey O, Torkington MS, Anthony I, Wells J, Blyth M, Jones B. Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J 2014; 96-B(3):395–398. doi:10.1302/0301-620X.96B3.32745
- Challagundla SR, Knox D, Hawkins A, et al. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant 2013; 28(3):612–619. doi:10.1093/ndt/gfs458
- Johansson S, Christensen OM, Thorsmark AH. A retrospective study of acute kidney injury in hip arthroplasty patients receiving gentamicin and dicloxacillin. Acta Orthop 2016; 87(6):589–591. doi:10.1080/17453674.2016.1231008
- Ferguson KB, Winter A, Russo L, et al. Acute kidney injury following primary hip and knee arthroplasty surgery. Ann R Coll Surg Eng 2017; 99(4):307–312. doi:10.1308/rcsann.2016.0324
- Bjerregaard LS, Jorgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-Track Hip and Knee Replacement Collaborative Group. Serious renal and urological complications in fast-track primary total hip and knee arthroplasty; a detailed observational cohort study. Minerva Anestesiol 2016; 82(7):767–776. pmid:27028450
- Friedman JM, Couso R, Kitchens M, et al. Benign heart murmurs as a predictor for complications following total joint arthroplasty. J Orthop 2017; 14(4):470–474. doi:10.1016/j.jor.2017.07.009
- Nadkarni GN, Patel AA, Ahuja Y, et al. Incidence, risk factors, and outcome trends of acute kidney injury in elective total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016; 45(1):E12–E19. pmid:26761921
- Lopez-de-Andres A, Hernandez-Barrera V, Martinez-Huedo MA, Villanueva-Martinez M, Jimenez-Trujillo I, Jimenez-Garcia R. Type 2 diabetes and in-hospital complications after revision of total hip and knee arthroplasty. PLoS One 2017; 12(8):e0183796. doi:10.1371/journal.pone.0183796
- Gharaibeh KA, Hamadah AM, Sierra RJ, Leung N, Kremers WK, El-Zoghby ZM. The rate of acute kidney injury after total hip arthroplasty is low but increases significantly in patients with specific comorbidities. J Bone Joint Surg Am 2017; 99(21):1819–1826. doi:10.2106/JBJS.16.01027
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8(4):R204–R212. doi:10.1186/cc2872
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11(2):R31. doi:10.1186/cc5713
- Section 2: AKI Definition. Kidney Int Suppl (2011) 2012; 2(1):19–36. doi:10.1038/kisup.2011.32
- Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 2014; 9(4):682–689. doi:10.2215/CJN.07650713
- Parr SK, Matheny ME, Abdel-Kader K, et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 2018; 93(2):460–469. doi:10.1016/j.kint.2017.07.007
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Karkouti K, Grocott HP, Hall R, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth 2015; 62(4):377–384. doi:10.1007/s12630-014-0302-y
- Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med 2017; 377(13):1261–1272. doi:10.1056/NEJMra1612789
- Karkouti K, Wijeysundera DN, Yau TM, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology 2012; 116(3):613–621. doi:10.1097/ALN.0b013e3182475e39
- Newman ET, Watters TS, Lewis JS, et al. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J Bone Joint Surg Am 2014; 96(4):279–284. doi:10.2106/JBJS.L.01041
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90(7):915–919. doi:10.1302/0301-620X.90B7.20498
- Corona Pérez-Cardona PS, Barro Ojeda V, Rodriguez Pardo D, et al. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother 2012; 67(7):1749–1754. doi:10.1093/jac/dks119
- Itani KM, Biswas P, Reisman A, Bhattacharyya H, Baruch AM. Clinical efficacy of oral linezolid compared with intravenous vancomycin for the treatment of methicillin-resistant Staphylococcus aureus-complicated skin and soft tissue infections: a retrospective, propensity score-matched, case-control analysis. Clin Ther 2012; 34(8):1667–1673.e1. doi:10.1016/j.clinthera.2012.06.018
- Dale H, Hallan G, Hallan G, Espehaug B, Havelin LI, Engesaeter LB. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop 2009; 80(6):639–645. doi:10.3109/17453670903506658
- Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010; 468(1):52–56. doi:10.1007/s11999-009-1013-5
- Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD; INFORM Team. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One 2016; 11(3):e0151537. doi:10.1371/journal.pone.0151537
- Negus JJ, Gifford PB, Haddad FS. Single-stage revision arthroplasty for infection—an underutilized treatment strategy. J Arthroplasty 2017; 32(7):2051–2055. doi:10.1016/j.arth.2017.02.059
- Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res 2005; 23(1):27–33. doi:10.1016/j.orthres.2004.03.003
- Chohfi M, Langlais F, Fourastier J, Minet J, Thomazeau H, Cormier M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int Orthop 1998; 22(3):171–177. pmid:9728311
- Amin TJ, Lamping JW, Hendricks KJ, McIff TE. Increasing the elution of vancomycin from high-dose antibiotic-loaded bone cement: a novel preparation technique. J Bone Joint Surg Am 2012; 94(21):1946–1951. doi:10.2106/JBJS.L.00014
- Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma 2004; 56(6):1247–1252. pmid:15211133
- Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am 2007; 89(4):871–882. doi:10.2106/JBJS.E.01070
- Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am 2006; 88(11):2487–2500. doi:10.2106/JBJS.E.01126
- Vrabec G, Stevenson W, Elguizaoui S, Kirsch M, Pinkowski J. What is the intraarticular concentration of tobramycin using low-dose tobramycin bone cement in TKA: an in vivo analysis? Clin Orthop Relat Res 2016; 474(11):2441–2447. doi:10.1007/s11999-016-5006-x
- Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg Br 2003; 85(5):646–649. pmid:12892183
- Fletcher MD, Spencer RF, Langkamer VG, Lovering AM. Gentamicin concentrations in diagnostic aspirates from 25 patients with hip and knee arthroplasties. Acta Orthop Scand 2004; 75(2):173–176. doi:10.1080/00016470412331294425
- Lau BP, Kumar VP. Acute kidney injury (AKI) with the use of antibiotic-impregnated bone cement in primary total knee arthroplasty. Ann Acad Med Singapore 2013; 42(12):692–695. pmid:24463833
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty 1996; 11(8):939–944. pmid:8986572
- Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother 2012; 46(7–8):929–934. doi:10.1345/aph.1R049
- Hsieh PH, Chang YH, Chen SH, Ueng SW, Shih CH. High concentration and bioactivity of vancomycin and aztreonam eluted from simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J Orthop Res 2006; 24(8):1615–1621. doi:10.1002/jor.20214
- Curtis JM, Sternhagen V, Batts D. Acute renal failure after placement of tobramycin-impregnated bone cement in an infected total knee arthroplasty. Pharmacotherapy 2005; 25(6):876–880. pmid:15927906
- Wu IM, Marin EP, Kashgarian M, Brewster UC. A case of an acute kidney injury secondary to an implanted aminoglycoside. Kidney Int 2009; 75(10):1109–1112. doi:10.1038/ki.2008.386
- Chalmers PN, Frank J, Sporer SM. Acute postoperative renal failure following insertion of an antibiotic-impregnated cement spacer in revision total joint arthroplasty: two case reports. JBJS Case Connect 2012; 2(1):e12. doi:10.2106/JBJS.CC.K.00094
- Patrick BN, Rivey MP, Allington DR. Acute renal failure associated with vancomycin- and tobramycin-laden cement in total hip arthroplasty. Ann Pharmacother 2006; 40(11):2037–2042. doi:10.1345/aph.1H173
- Dovas S, Liakopoulos V, Papatheodorou L, et al. Acute renal failure after antibiotic-impregnated bone cement treatment of an infected total knee arthroplasty. Clin Nephrol 2008; 69(3):207–212. pmid:18397720
- McGlothan KR, Gosmanova EO. A case report of acute interstitial nephritis associated with antibiotic-impregnated orthopedic bone-cement spacer. Tenn Med 2012; 105(9):37–40, 42. pmid:23097958
- Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci 2009; 6(5):265–273. pmid:19834592
- Menge TJ, Koethe JR, Jenkins CA, et al. Acute kidney injury after placement of an antibiotic-impregnated cement spacer during revision total knee arthroplasty. J Arthroplasty 2012; 27(6):1221–1227.e1–2. doi:10.1016/j.arth.2011.12.005
- Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin Orthop Relat Res 2011; 469(4):985–993. doi:10.1007/s11999-010-1579-y
- Springer BD, Lee GC, Osmon D, Haidukewych GJ, Hanssen AD, Jacofsky DJ. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res 2004; 427:47–51. pmid:15552135
- Noto MJ, Koethe JR, Miller G, Wright PW. Detectable serum tobramycin levels in patients with renal dysfunction and recent placement of antibiotic-impregnated cement knee or hip spacers. Clin Infect Dis 2014; 58(12):1783–1784. doi:10.1093/cid/ciu159
- Aeng ES, Shalansky KF, Lau TT, et al. Acute kidney injury with tobramycin-impregnated bone cement spacers in prosthetic joint infections. Ann Pharmacother 2015; 49(11):1207–1213. doi:10.1177/1060028015600176
- Geller JA, Cunn G, Herschmiller T, Murtaugh T, Chen A. Acute kidney injury after first-stage joint revision for infection: Risk factors and the impact of antibiotic dosing. J Arthroplasty 2017; 32(10):3120–3125. doi:10.1016/j.arth.2017.04.054
- Reed EE, Johnston J, Severing J, Stevenson KB, Deutscher M. Nephrotoxicity risk factors and intravenous vancomycin dosing in the immediate postoperative period following antibiotic-impregnated cement spacer placement. Ann Pharmacother 2014; 48(8):962–969. doi:10.1177/1060028014535360
- Koo KH, Yang JW, Cho SH, et al. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty 2001; 16(7):882–892. doi:10.1054/arth.2001.24444
- Forsythe ME, Crawford S, Sterling GJ, Whitehouse SL, Crawford R. Safeness of simplex-tobramycin bone cement in patients with renal dysfunction undergoing total hip replacement. J Orthop Surg (Hong Kong) 2006; 14(1):38–42. doi:10.1177/230949900601400109
- Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: in vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. J Trauma 2009; 66(3):804–808. doi:10.1097/TA.0b013e31818896cc
- Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res 2005; 430:125–131. pmid:15662313
- Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res 2004; 427:37–46. pmid:15552134
- Yadav A, Alijanipour P, Ackerman CT, Karanth S, Hozack WJ, Filippone EJ. Acute kidney injury following failed total hip and knee arthroplasty. J Arthroplasty 2018; 33(10):3297–3303. doi:10.1016/j.arth.2018.06.019
- Hsieh PH, Huang KC, Lee PC, Lee MS. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: retrospective comparison between short-term and prolonged antibiotic therapy. J Antimicrob Chemother 2009; 64(2):392–397. doi:10.1093/jac/dkp177
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty 2013; 28(9):1490–1498.e1. doi:10.1016/j.arth.2013.02.035
- Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017; 102(3):459–469. doi:10.1002/cpt.726
Total hip or knee replacement (also called total joint arthroplasty) is highly successful at relieving pain and restoring function, but at the risk of acute kidney injury, which is a sudden loss of renal function. Various factors have been associated with this risk, some of which are potentially modifiable, notably, the use of nephrotoxic antibiotics and other drugs.
This review examines the incidence of acute kidney injury using current criteria in total joint arthroplasty of the hip or knee in general, and in the setting of revision surgery for prosthetic joint infection in particular, in which the risk is higher. We identify risk factors for acute kidney injury and propose ways to lower the risk.
MILLIONS OF PROCEDURES ANNUALLY
Total replacement of the hip1,2 or knee3 is being done more and more. Kurtz et al4 estimate that by the year 2030, we will see approximately 3.5 million primary total knee and 500,000 primary total hip replacements every year. In addition, revision total knee procedures are expected to exceed 250,000 per year, and revision total hip procedures are expected to exceed 90,000 per year.4
Chronic infection may complicate up to 2% of these procedures and is associated with significant morbidity, death, and financial costs. Currently, it may be the reason for 25% of total joint arthroplasty revisions,5 but by the year 2030, it is projected to account for 66% of revision total knee arthroplasties and 48% of revision total hip arthroplasties.6
PRIMARY TOTAL JOINT ARTHROPLASTY AND ACUTE KIDNEY INJURY
Study designs, findings varied widely
The incidence of acute kidney injury varied markedly among the studies of primary total joint arthroplasty or revision for aseptic reasons. Numerous factors explain this heterogeneity.
Designs ranged from single-center studies with relatively small numbers of patients to large regional and national samples based on administrative data.
Almost all of the studies were retrospective. We are not aware of any randomized controlled trials.
Discharge diagnosis may miss many cases
Several studies based the diagnosis of acute kidney injury on International Classification of Diseases, Ninth Revision (ICD-9) coding from hospital discharge summaries.
Nadkarni et al,29 in the largest study published to date, used the nationwide inpatient sample database of more than 7 million total joint arthroplasties and found an incidence of acute kidney injury based on ICD-9 coding of 1.3% over the years 2002 to 2012, although this increased to 1.8% to 1.9% from 2010 to 2012.
Lopez-de-Andres et al,30 in a similar study using the Spanish national hospital discharge database, evaluated 20,188 patients who underwent revision total hip or knee arthroplasty and found an overall incidence of acute kidney injury of 0.94%, also using ICD-9 coding.
Gharaibeh et al31 used similar methods to diagnose acute kidney injury in a single-center study of 8,949 patients and found an incidence of 1.1%.
Although these 3 studies suggest that the incidence of acute kidney injury is relatively low, Grams et al35 found the sensitivity of ICD-9 coding from hospital records for the diagnosis of acute kidney injury to be only 11.7% compared with KDIGO serum creatinine and urine output criteria. This suggests that the true incidence in these studies may be many times higher, possibly near 10%.
Do all stages of kidney injury count?
Jafari et al,7 in a large series from a single medical center, used only the “I” (injury) and “F” (failure) levels of the RIFLE criteria (corresponding to stages 2 and 3 of the KDIGO criteria) and found an incidence of 0.55% in more than 17,000 total joint arthroplasties.
Jamsa et al8 used the same criteria for acute kidney injury (only “I” and “F”) and found 58 cases in 5,609 patients in whom postoperative serum creatinine was measured, for an incidence of 1%; the remaining 14,966 patients in their cohort did not have serum creatinine measured, and it was assumed they did not have acute kidney injury. Neither of these studies included the most common “R” (risk) stage of acute kidney injury.
Parr et al36 recently studied a nationwide sample of 657,840 hospitalized veterans and found that of 90,614 who developed acute kidney injury based on KDIGO creatinine criteria, 84% reached only stage R. This suggests that if all stages were considered, the true incidence of acute kidney injury would have been higher—possibly 4% in the Jafari series and possibly 7% in the Jamsa series.
Smaller studies had higher rates
Smaller, single-center series reported much higher incidences of acute kidney injury.
Kimmel et al11 found an incidence of 14.8% in 425 total joint arthroplasties using RIFLE creatinine criteria.
Johansson et al25 found an incidence of 19.9% in 136 total joint arthroplasties using KDIGO creatinine criteria.
Sehgal et al9 found an incidence of 21.9% in 659 total joint arthroplasties using AKIN creatinine criteria.
Challagundla et al24 found an incidence of 23.7% in 198 procedures using RIFLE creatinine criteria.
Weingarten et al,10 in a single-center series of 7,463 total joint arthroplasties, found an incidence of acute kidney injury of only 2.2% using AKIN criteria, although 12% of the patients with acute kidney injury did not return to their baseline serum creatinine levels by 3 months.
Our estimate: Nearly 10%
In total, in the 20 studies in Table 1 that included all stages of acute kidney injury, there were 1,909 cases of acute kidney injury in 34,337 patients, for an incidence of 5.6%. Considering that all studies but one were retrospective and none considered urine output criteria for acute kidney injury, we believe that using current KDIGO criteria, the true incidence of acute kidney injury complicating primary lower-extremity total joint arthroplasties is really closer to 10%.
RISK FACTORS FOR ACUTE KIDNEY INJURY
Various factors have been associated with development of acute kidney injury by multivariate analysis in these studies. Some are modifiable, while others are not, at least in the short term.
Nonmodifiable risk factors
Older age is often significant in studies assessing primary total joint arthroplasty or revision total joint arthroplasty not specifically for infection.11,12,16,17,26,28
Obesity is also a major factor in the development of acute kidney injury,7,10–12,17,18 and, along with age, is a major factor contributing to the need for joint replacement in the first place.
Male sex may increase risk.29
Diabetes mellitus was identified as a risk factor in several studies,10,12,17,20 and hypertension in a few.7,10,24
Other comorbidities and factors such as cardiovascular disease,7,10 liver disease,7 pulmonary disease,7 high American Society of Anesthesiology score,8,19 and benign heart murmurs preoperatively by routine physical examination have also been linked to acute kidney injury after joint arthroplasty.28
Chronic kidney disease as a risk factor
Chronic kidney disease at baseline was associated with acute kidney injury in several of these series.7,11–13,15,19,29
Warth et al12 studied 1,038 patients and found an incidence of acute kidney injury of 11% in the 135 with chronic kidney disease (defined as serum creatinine > 1.2 mg/dL) and who received acetaminophen or narcotics for pain control, compared with 4.8% in the remaining 903 patients without chronic kidney disease, who received ketorolac or celecoxib.
Perregaard et al13 studied 3,410 patients who underwent total hip arthroplasty and found an incidence of acute kidney injury (per KDIGO creatinine criteria) of 2.2% overall, but 7% in the 134 patients with chronic kidney disease based on KDIGO creatinine criteria.
Nowicka et al15 found an incidence of acute kidney injury of 16.7% in the 48 patients with chronic kidney disease (defined as a glomerular filtration rate estimated by the Cockroft-Gault formula of less than 60 mL/min/1.73 m2), compared with 4.5% in the remaining 289.
Modifiable risk factors
Modifiable risk factors that should be considered in high-risk cases include anemia, perioperative blood transfusion, perioperative use of renin-angiotensin-aldosterone system inhibitors such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), particular antibiotics used for prophylaxis, and nonsteroidal anti-inflammatory drugs used postoperatively.
Anemia and blood transfusion
Preoperative anemia has been associated with postoperative acute kidney injury in various surgical settings such as cardiac surgery.37,38 Perioperative red blood cell transfusions have also been associated with acute kidney injury in cardiac surgery; similar results may apply to total joint arthroplasty.
Choi et al,17 in 2,467 patients undergoing hip replacement, found a significant risk for acute kidney injury if postoperative hemoglobin was consistently below 10 g/dL compared with consistently above this level, with an inverse probability-of-treatment weighted odds ratio of 1.817 (P = .011).
Others have found a significant association of perioperative blood transfusion with acute kidney injury in total joint arthroplasty.10,29
Nadkarni et al,29 for example, used the nationwide inpatient sample database and found by multivariate analysis that perioperative blood transfusion was strongly associated with acute kidney injury, with an adjusted odds ratio of 2.28 (95% confidence interval [CI] 2.15–2.42, P < .0001).
Comment. A higher incidence of acute kidney injury may represent confounding by indication bias, as sicker patients or complicated surgeries may require transfusion, and this risk may not be completely accounted for by multivariate analysis. It is also possible, however, that transfusions per se may contribute to acute kidney injury. Possible direct or indirect mechanisms mediating acute kidney injury include hemolytic reactions, circulatory overload, acute lung injury, and immunomodulatory effects.39
Preoperative transfusion in anemic patients undergoing cardiac surgery may also reduce the incidence of postoperative acute kidney injury both by correcting the anemia and by limiting the need for perioperative transfusions.40 It remains to be determined whether elective preoperative transfusion to correct anemia would reduce postoperative development of acute kidney injury in total joint arthroplasty. As an aside, perioperative transfusion has also been linked to development of periprosthetic joint infection.41
Renin-angiotensin-aldosterone system inhibitors
Several studies found perioperative use of renin-angiotensin-aldosterone system inhibitors to be a risk factor for acute kidney injury.
Kimmel et al11 reported adjusted odds ratios of 2.70 (95% CI 1.12–6.48) for ACE inhibitor use and 2.64 (95% CI 1.18–5.93) for ARB use in a study of 425 primary total joint arthroplasties.
Challagundla et al24 found an odds ratio of 3.07 (95% CI 1.40–6.74) with ACE inhibitor or ARB use by multivariate analysis in 198 total joint arthroplasties.
Nielson et al18 studied 798 patients who underwent total joint arthroplasty and found that preoperative use of renin-angiotensin system inhibitors was associated with a significantly higher rate of postoperative acute kidney injury (8.3% vs 1.7% without inhibition), which was statistically significant by multivariate analysis (odds ratio 2.6, 95% CI 1.04–6.51).
We recommend holding renin-angiotensin-aldosterone system inhibitors 7 days before surgery through the postoperative period in high-risk cases.
Aminoglycoside use as a risk factor
Prophylactic administration of systemic antibiotics is the standard of care. In a systematic review of 26 studies and meta-analysis of 7 studies (3,065 patients), prophylactic antibiotics reduced the relative risk of wound infection by 81% with an absolute risk reduction of 8%.42
A modifiable risk factor for acute kidney injury is the specific antibiotic used for prophylaxis. Multiple studies assessed the risk of acute kidney injury comparing regimens containing an aminoglycoside (typically gentamicin) with regimens lacking these agents.20–26 In general, these studies found a significantly higher risk of acute kidney injury when gentamicin was used.
Challagundla et al24 found an incidence of acute kidney injury of 52% using RIFLE creatinine criteria in 52 patients receiving 8 g total of flucloxacillin plus 160 mg of gentamicin (120 mg if they weighed less than 60 kg) compared with 8% in 48 patients given cefuroxime (3 g total) and 14% in an additional 52 patients also given cefuroxime.
Johansson et al25 found an incidence of KDIGO creatinine-based acute kidney injury of 13% in 70 patients given dicloxacillin alone prophylactically compared with 27% given dicloxacillin and gentamicin, with a relative risk of 3.
Bell et al,21 in a large registry-based analysis from Scotland involving 7,666 elective orthopedic procedures, found that use of flucloxacillin 2 g plus a single dose of gentamicin 4 mg/kg was significantly associated with a 94% higher risk of acute kidney injury (KDIGO creatinine criteria) compared with a cefuroxime-based regimen, with absolute rates increasing from 6.2% to 10.8%.
Dubrovskaya et al20 and Ferguson et al,26 in contrast, found no increased risk with addition of gentamicin.
We recommend avoiding aminoglycosides for prophylaxis in primary lower-extremity total joint arthroplasty in patients at higher risk unless required for specific microbiologic reasons.
Vancomycin may also increase risk
Courtney et al19 assessed the risk of adding vancomycin to cefazolin for routine prophylaxis in a retrospective series of 1,828 total hip or knee arthroplasties and found a significantly higher rate of acute kidney injury, using AKIN criteria (13% vs 8%, odds ratio by multivariate analysis 1.82, P = .002).19
Other agents shown to be effective in treating periprosthetic joint infections or complicated skin and soft-tissue infections with resistant organisms include daptomycin43 and linezolid.44 These nonnephrotoxic alternatives to vancomycin may be a consideration if prophylaxis for methicillin-resistant Staphylococcus aureus is deemed necessary in patients at risk for acute kidney injury.
PROSTHETIC JOINT INFECTIONS AND ANTIBIOTIC-LOADED CEMENT
Deep infection may complicate nearly 1% of total hip45 and 2% of total knee arthroplasties.46 Kurtz et al4,6 have projected that by 2030, infection will be the cause of two-thirds of the estimated 268,000 revision total knee arthroplasties and about half of the estimated 96,700 revision total hip arthroplasties.
The most common method of treating a chronically infected replacement joint is a 2-stage procedure.5 First, the prosthesis is removed, all infected bone and soft tissue is debrided, and an antibiotic-loaded cement spacer is implanted. Systemic antibiotics are given concurrently, typically for about 6 weeks. After the infection is brought under control, perhaps 2 to 3 months later, the spacer is removed and a new joint is implanted with antibiotic-loaded cement. A 1-stage procedure may be an option in selected cases and would obviate the need for an antibiotic-loaded cement spacer.47,48
Of obvious relevance to development of acute kidney injury is the choice and amount of antibiotics embedded in the cement used for spacers and in implantation. Very high antibiotic levels are achieved within the joint space, usually with little systemic absorption, although significant systemic exposure has been documented in some cases.
The polymethylmethacrylate cement used for these purposes comes in 40-g bags. Multiple bags are typically required per joint, perhaps 2 to 4.49
The rate of elution of antibiotics is determined by several factors, including surface area, porosity, and the number of antibiotics. In general, elution is greatest early on, with exponential decline lasting perhaps 1 week, followed by slow, sustained release over weeks to months.50 However, several in vitro studies have indicated that only about 5%50,51 of the total antibiotic actually elutes over time.
Initially, multiple antibiotic-laden cement beads were used to fill the joint space, but this significantly limited function and mobility.52 Now, cement spacers are used, and they can be nonarticulating or articulating for maximal joint mobility.53 Although much greater antibiotic elution occurs from beads due to their high surface area-to-volume ratio, spacers still provide an adequate dose.
ANTIBIOTIC-LOADED CEMENT: DOSAGE AND ELUTION CHARACTERISTICS
Antibiotic-loaded cement can be either low-dose or high-dose.
Low-dose cement
Low-dose cement typically consists of 0.5 to 1.0 g of antibiotic per 40-g bag of cement, usually an aminoglycoside (gentamicin or tobramycin) or vancomycin, and can be purchased premixed by the manufacturer. Such cement is only used prophylactically with primary total joint arthroplasty or revision for aseptic reasons, a practice common in Europe but less so in the United States. Some American authors propose antibiotic-loaded cement prophylaxis for patients at high risk, eg, those with immunosuppression, inflammatory cause of arthritis, or diabetes.54
Vrabec et al,55 in a study of low-dose tobramycin-loaded cement used for primary total knee arthroplasty, found a peak median intra-articular tobramycin concentration of 32 mg/L at 6 hours, declining to 6 mg/L at 48 hours with all serum levels 0.3 mg/L or less (unmeasureable) at similar time points.
Sterling et al,56 studying primary total hip arthroplasties with low-dose tobramycin-loaded cement, found mean levels in drainage fluid of 103 mg/L at 6 hours, declining to 15 mg/L at 48 hours. Serum levels peaked at 0.94 mg/L at 3 hours, declining to 0.2 mg/L by 48 hours.
Although most of the antibiotic elution occurs early (within the first week), antibiotic can be found in joint aspirates up to 20 years later.57 We are unaware of any well-documented cases of acute kidney injury ascribable to low-dose antibiotic-loaded cement used prophylactically. One case report making this assertion did not determine serum levels of aminoglycoside.58
High-dose cement
High-dose antibiotic-loaded cement typically contains about 4 to 8 g of antibiotic per 40-g bag of cement and is used in the treatment of prosthetic joint infection to form the spacers. The antibiotic must be mixed into the cement powder by the surgeon in the operating room.
There is no standard combination or dosage. The choice of antibiotic can be tailored to the infecting organism if known. Otherwise, gram-positive organisms are most common, and vancomycin and aminoglycosides are often used together. This particular combination will enhance the elution of both antibiotics when studied in vitro, a process termed “passive opportunism.”59 Other antibiotics in use include aztreonam, piperacillin, teicoplanin, fluoroquinolones, cephalosporins, and daptomycin, among others.
About 8 g of antibiotic total per 40-g bag is the maximum to allow easy molding.52 As an example, this may include 4 g of vancomycin and 3.6 g of tobramycin per 40 g. Given that 3 to 4 such bags are often used per joint, there is significant risk of systemic exposure.
Kalil et al60 studied 8 patients who received high-dose tobramycin-loaded cement to treat periprosthetic joint infections of the hip or knee and found that 7 had detectable serum levels (mean 0.84 mg/L, highest 2.0 mg/L), including 1 with a level of 0.9 mg/L on day 38; 4 of these 8 developed acute kidney injury by AKIN criteria, although other risk factors for acute kidney injury existed. Nearly all had concomitant vancomycin (3 to 8 g) added to the cement as well.
Hsieh et al61 studied 46 patients with infected total hip arthroplasties treated with high-dose antibiotic-loaded cement spacers (vancomycin 4 g and aztreonam 4 g per 40-g bag) and found vancomycin levels in joint drainage higher than 1,500 mg/L on day 1, decreasing to 571 mg/L on day 7; serum levels were low (range 0.1–1.6 mg/L at 24 hours), falling to undetectable by 72 hours.
ANTIBIOTIC-LOADED CEMENT SPACERS AND ACUTE KIDNEY INJURY
Case reports have associated high-dose antibiotic-loaded cement spacers with acute kidney injury.
Curtis et al62 described an 85-year-old patient with stage 3 chronic kidney disease who was treated for an infected total knee arthroplasty with an antibiotic-loaded cement spacer (containing 3.6 g of tobramycin and 3 g of cefazolin per 40-g bag, 3 bags total) and developed stage 3 acute kidney injury. After 16 days and 3 hemodialysis sessions, the patient’s serum tobramycin level was still 2 mg/L despite receiving no systemic tobramycin.
Wu et al63 reported a case of acute kidney injury that required dialysis after implantation of a tobramycin- and vancomycin-loaded spacer, with persistent serum tobramycin levels despite repeated hemodialysis sessions until the spacer was removed.
Chalmers et al64 described 2 patients with acute kidney injury and persistently elevated serum tobramycin levels (3.9 mg/L on day 39 in 1 patient and 2.0 mg/L on day 24 in the other patient) despite no systemic administration.
In these and other case reports,65–67 dialysis and spacer explantation were usually required.
Comment. It is intuitive that acute kidney injury would more likely complicate revision total joint arthroplasties for infection than for primary total joint arthroplasties or revisions for aseptic reasons, given the systemic effects of infection and exposure to nephrotoxic or allergenic antibiotics. And the available data suggest that the risk of acute kidney injury is higher with revision for prosthetic joint infection than with revision for aseptic reasons. However, many of the studies were retrospective, relatively small, single-center series and used different definitions of acute kidney injury.
Luu et al83 performed a systematic review of studies published between January 1989 and June 2012 reporting systemic complications (including acute kidney injury) of 2-stage revision arthroplasties including placement of an antibiotic-loaded cement spacer for treatment of periprosthetic joint infection. Overall, 10 studies were identified with 544 total patients. Five of these studies, with 409 patients, reported at least 1 case of acute kidney injury for a total of 27 patients, giving an incidence of 6.6% in these studies.68–71 The remaining 5 studies, totaling 135 patients, did not report any cases of acute kidney injury,50,61,76–78 although that was not the primary focus of any of those trials.
Most notable from this systematic review, the study of Menge et al69 retrospectively determined the incidence of acute kidney injury (defined as a 50% rise in serum creatinine to > 1.4 mg/dL within 90 days of surgery) to be 17% in 84 patients with infected total knee arthroplasties treated with antibiotic-loaded cement spacers. A mean of 3.5 bags of cement per spacer were used in the 35 articulating spacers, compared with 2.9 per nonarticulating spacer. These spacers contained vancomycin in 82% (median 4.0 g, range 1–16 g) and tobramycin in 94% (median 4.8 g, range 1–12 g), among others in small percentages. The dose of tobramycin in the spacer considered either as a dichotomous variable (> 4.8 g, OR 5.87) or linearly (OR 1.24 per 1-g increase) was significantly associated with acute kidney injury, although systemic administration of aminoglycosides or vancomycin was not.
Additional single-center series that were published subsequent to this review have generally used more current diagnostic criteria.
Noto et al72 found that 10 of 46 patients treated with antibiotic-loaded cement spacers had a greater than 50% rise in serum creatinine (average increase 260%). All spacers contained tobramycin (mean dose 8.2 g), and 9 of 10 also contained vancomycin (mean 7.6 g). All of the 9 patients with acute kidney injury with follow-up data recovered renal function.
Reed et al75 found 26 cases of acute kidney injury (based on RIFLE creatinine criteria) in 306 patients with antibiotic-loaded cement spacers treating various periprosthetic joint infections (including hips, knees, shoulders, and digits) and compared them with 74 controls who did not develop acute kidney injury. By multivariable analysis, receipt of an ACE inhibitor within 7 days of surgery and receipt of piperacillin-tazobactam within 7 days after surgery were both significantly more common in cases with acute kidney injury than in controls without acute kidney injury.
Aeng et al73 prospectively studied 50 consecutive patients receiving antibiotic-loaded spacers containing tobramycin (with or without vancomycin) for treatment of infected hip or knee replacements. Using RIFLE creatinine criteria, they found an incidence of acute kidney injury of 20% (10 of 50). Factors significantly associated with acute kidney injury included cement premixed by the manufacturer with gentamicin (0.5 g per 40-g bag) in addition to the tobramycin they added, intraoperative blood transfusions, and postoperative use of nonsteroidal anti-inflammatory drugs.
Geller et al,74 in a multicenter retrospective study of 247 patients with prosthetic joint infections (156 knees and 91 hips) undergoing antibiotic-loaded cement spacer placement, found an incidence of acute kidney injury of 26% based on KDIGO creatinine criteria. Significant risk factors included higher body mass index, lower preoperative hemoglobin level, drop in hemoglobin after surgery, and comorbidity (hypertension, diabetes, chronic kidney disease, or cardiovascular disease). Most of the spacers contained a combination of vancomycin and either tobramycin (81%) or gentamicin (13%). The spacers contained an average of 5.3 g (range 0.6–18 g) of vancomycin (average 2.65 g per 40-g bag) and an average of 5.2 g (range 0.5–16.4 g) of tobramycin (average 2.6 g per bag).
As in Menge et al,69 this study illustrates the wide range of antibiotic dosages in use and the lack of standardization. In contrast to the study by Menge et al, however, development of acute kidney injury was not related to the amount of vancomycin or tobramycin contained in the spacers. Eventual clearance of infection (at 1 and 2 years) was significantly related to increasing amounts of vancomycin. Multiple different systemic antibiotics were used, most commonly vancomycin (44%), and systemic vancomycin was not associated with acute kidney injury.
Yadav et al,81 in a study of 3,129 consecutive revision procedures of the knee or hip, found an incidence of acute kidney injury by RIFLE creatinine criteria of 29% in the 197 patients who received antibiotic-loaded cement spacers for periprosthetic joint infection compared with 3.4% in the 2,848 who underwent revision for aseptic reasons. In 84 patients with prosthetic joint infection having various surgeries not including placement of a spacer, the acute kidney injury rate at some point in their course was an alarmingly high 82%. In the group that received spacers, only age and comorbidity as assessed by Charlson comorbidity index were independently associated with acute kidney injury by multivariate analysis. Surprisingly, modest renal impairment was protective, possibly because physicians of patients with chronic kidney disease were more vigilant and took appropriate measures to prevent acute kidney injury.
Overall, the risk of acute kidney injury appears to be much higher during treatment of prosthetic joint infection with a 2-stage procedure using an antibiotic-loaded cement spacer than after primary total joint arthroplasty or revision for aseptic reasons, and may complicate up to one-third of cases.
REDUCING RISK DURING TREATMENT OF INFECTED REPLACEMENT JOINTS
As in primary total joint arthroplasty in general, higher-risk cases should be identified based on age, body mass index, chronic kidney disease, comorbidities (hypertension, diabetes, established cardiovascular disease), and anemia.
Preoperative transfusion can be considered case by case depending on degree of anemia and associated risk factors.
All renin-angiotensin-aldosterone system inhibitors should be withheld starting 1 week before surgery.
Both nonselective and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs should be avoided, if possible.
Strict attention should be paid to adequate intraoperative and postoperative fluid resuscitation.
Kidney function should be monitored closely in the early postoperative period, including urine output and daily creatinine for at least 72 hours.
Systemic administration of potentially nephrotoxic antibiotics should be minimized, especially the combination of vancomycin with piperacillin-tazobactam.84 Daptomycin is a consideration.43
If acute kidney injury should develop, serum levels of vancomycin or aminoglycosides should be measured if the spacer contains these antibiotics. The spacer may need to be removed if toxic serum levels persist.
TAKE-HOME POINTS
Acute kidney injury may complicate up to 10% of primary lower-extremity total joint arthroplasties and up to 25% of periprosthetic joint infections treated with a 2-stage procedure including placement of an antibiotic-loaded cement spacer in the first stage.
Risk factors for acute kidney injury include older age, obesity, chronic kidney disease, and overall comorbidity. Potentially modifiable risk factors include anemia, perioperative transfusions, aminoglycoside prophylaxis, perioperative renin-angiotensin system blockade, and postoperative nonsteroidal anti-inflammatory drugs. These should be mitigated when possible.
In patients with periprosthetic joint infection who receive antibiotic-loaded cement spacers, especially patients with additional risk factors for acute kidney injury, strict attention should be paid to the dose of antibiotic in the spacer, with levels checked postoperatively if necessary. Nonnephrotoxic antibiotics should be chosen for systemic administration when possible.
Prospective randomized controlled trials are needed to guide therapy after total joint arthroplasty, and to verify the adverse long-term outcomes of acute kidney injury in this setting.
Total hip or knee replacement (also called total joint arthroplasty) is highly successful at relieving pain and restoring function, but at the risk of acute kidney injury, which is a sudden loss of renal function. Various factors have been associated with this risk, some of which are potentially modifiable, notably, the use of nephrotoxic antibiotics and other drugs.
This review examines the incidence of acute kidney injury using current criteria in total joint arthroplasty of the hip or knee in general, and in the setting of revision surgery for prosthetic joint infection in particular, in which the risk is higher. We identify risk factors for acute kidney injury and propose ways to lower the risk.
MILLIONS OF PROCEDURES ANNUALLY
Total replacement of the hip1,2 or knee3 is being done more and more. Kurtz et al4 estimate that by the year 2030, we will see approximately 3.5 million primary total knee and 500,000 primary total hip replacements every year. In addition, revision total knee procedures are expected to exceed 250,000 per year, and revision total hip procedures are expected to exceed 90,000 per year.4
Chronic infection may complicate up to 2% of these procedures and is associated with significant morbidity, death, and financial costs. Currently, it may be the reason for 25% of total joint arthroplasty revisions,5 but by the year 2030, it is projected to account for 66% of revision total knee arthroplasties and 48% of revision total hip arthroplasties.6
PRIMARY TOTAL JOINT ARTHROPLASTY AND ACUTE KIDNEY INJURY
Study designs, findings varied widely
The incidence of acute kidney injury varied markedly among the studies of primary total joint arthroplasty or revision for aseptic reasons. Numerous factors explain this heterogeneity.
Designs ranged from single-center studies with relatively small numbers of patients to large regional and national samples based on administrative data.
Almost all of the studies were retrospective. We are not aware of any randomized controlled trials.
Discharge diagnosis may miss many cases
Several studies based the diagnosis of acute kidney injury on International Classification of Diseases, Ninth Revision (ICD-9) coding from hospital discharge summaries.
Nadkarni et al,29 in the largest study published to date, used the nationwide inpatient sample database of more than 7 million total joint arthroplasties and found an incidence of acute kidney injury based on ICD-9 coding of 1.3% over the years 2002 to 2012, although this increased to 1.8% to 1.9% from 2010 to 2012.
Lopez-de-Andres et al,30 in a similar study using the Spanish national hospital discharge database, evaluated 20,188 patients who underwent revision total hip or knee arthroplasty and found an overall incidence of acute kidney injury of 0.94%, also using ICD-9 coding.
Gharaibeh et al31 used similar methods to diagnose acute kidney injury in a single-center study of 8,949 patients and found an incidence of 1.1%.
Although these 3 studies suggest that the incidence of acute kidney injury is relatively low, Grams et al35 found the sensitivity of ICD-9 coding from hospital records for the diagnosis of acute kidney injury to be only 11.7% compared with KDIGO serum creatinine and urine output criteria. This suggests that the true incidence in these studies may be many times higher, possibly near 10%.
Do all stages of kidney injury count?
Jafari et al,7 in a large series from a single medical center, used only the “I” (injury) and “F” (failure) levels of the RIFLE criteria (corresponding to stages 2 and 3 of the KDIGO criteria) and found an incidence of 0.55% in more than 17,000 total joint arthroplasties.
Jamsa et al8 used the same criteria for acute kidney injury (only “I” and “F”) and found 58 cases in 5,609 patients in whom postoperative serum creatinine was measured, for an incidence of 1%; the remaining 14,966 patients in their cohort did not have serum creatinine measured, and it was assumed they did not have acute kidney injury. Neither of these studies included the most common “R” (risk) stage of acute kidney injury.
Parr et al36 recently studied a nationwide sample of 657,840 hospitalized veterans and found that of 90,614 who developed acute kidney injury based on KDIGO creatinine criteria, 84% reached only stage R. This suggests that if all stages were considered, the true incidence of acute kidney injury would have been higher—possibly 4% in the Jafari series and possibly 7% in the Jamsa series.
Smaller studies had higher rates
Smaller, single-center series reported much higher incidences of acute kidney injury.
Kimmel et al11 found an incidence of 14.8% in 425 total joint arthroplasties using RIFLE creatinine criteria.
Johansson et al25 found an incidence of 19.9% in 136 total joint arthroplasties using KDIGO creatinine criteria.
Sehgal et al9 found an incidence of 21.9% in 659 total joint arthroplasties using AKIN creatinine criteria.
Challagundla et al24 found an incidence of 23.7% in 198 procedures using RIFLE creatinine criteria.
Weingarten et al,10 in a single-center series of 7,463 total joint arthroplasties, found an incidence of acute kidney injury of only 2.2% using AKIN criteria, although 12% of the patients with acute kidney injury did not return to their baseline serum creatinine levels by 3 months.
Our estimate: Nearly 10%
In total, in the 20 studies in Table 1 that included all stages of acute kidney injury, there were 1,909 cases of acute kidney injury in 34,337 patients, for an incidence of 5.6%. Considering that all studies but one were retrospective and none considered urine output criteria for acute kidney injury, we believe that using current KDIGO criteria, the true incidence of acute kidney injury complicating primary lower-extremity total joint arthroplasties is really closer to 10%.
RISK FACTORS FOR ACUTE KIDNEY INJURY
Various factors have been associated with development of acute kidney injury by multivariate analysis in these studies. Some are modifiable, while others are not, at least in the short term.
Nonmodifiable risk factors
Older age is often significant in studies assessing primary total joint arthroplasty or revision total joint arthroplasty not specifically for infection.11,12,16,17,26,28
Obesity is also a major factor in the development of acute kidney injury,7,10–12,17,18 and, along with age, is a major factor contributing to the need for joint replacement in the first place.
Male sex may increase risk.29
Diabetes mellitus was identified as a risk factor in several studies,10,12,17,20 and hypertension in a few.7,10,24
Other comorbidities and factors such as cardiovascular disease,7,10 liver disease,7 pulmonary disease,7 high American Society of Anesthesiology score,8,19 and benign heart murmurs preoperatively by routine physical examination have also been linked to acute kidney injury after joint arthroplasty.28
Chronic kidney disease as a risk factor
Chronic kidney disease at baseline was associated with acute kidney injury in several of these series.7,11–13,15,19,29
Warth et al12 studied 1,038 patients and found an incidence of acute kidney injury of 11% in the 135 with chronic kidney disease (defined as serum creatinine > 1.2 mg/dL) and who received acetaminophen or narcotics for pain control, compared with 4.8% in the remaining 903 patients without chronic kidney disease, who received ketorolac or celecoxib.
Perregaard et al13 studied 3,410 patients who underwent total hip arthroplasty and found an incidence of acute kidney injury (per KDIGO creatinine criteria) of 2.2% overall, but 7% in the 134 patients with chronic kidney disease based on KDIGO creatinine criteria.
Nowicka et al15 found an incidence of acute kidney injury of 16.7% in the 48 patients with chronic kidney disease (defined as a glomerular filtration rate estimated by the Cockroft-Gault formula of less than 60 mL/min/1.73 m2), compared with 4.5% in the remaining 289.
Modifiable risk factors
Modifiable risk factors that should be considered in high-risk cases include anemia, perioperative blood transfusion, perioperative use of renin-angiotensin-aldosterone system inhibitors such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), particular antibiotics used for prophylaxis, and nonsteroidal anti-inflammatory drugs used postoperatively.
Anemia and blood transfusion
Preoperative anemia has been associated with postoperative acute kidney injury in various surgical settings such as cardiac surgery.37,38 Perioperative red blood cell transfusions have also been associated with acute kidney injury in cardiac surgery; similar results may apply to total joint arthroplasty.
Choi et al,17 in 2,467 patients undergoing hip replacement, found a significant risk for acute kidney injury if postoperative hemoglobin was consistently below 10 g/dL compared with consistently above this level, with an inverse probability-of-treatment weighted odds ratio of 1.817 (P = .011).
Others have found a significant association of perioperative blood transfusion with acute kidney injury in total joint arthroplasty.10,29
Nadkarni et al,29 for example, used the nationwide inpatient sample database and found by multivariate analysis that perioperative blood transfusion was strongly associated with acute kidney injury, with an adjusted odds ratio of 2.28 (95% confidence interval [CI] 2.15–2.42, P < .0001).
Comment. A higher incidence of acute kidney injury may represent confounding by indication bias, as sicker patients or complicated surgeries may require transfusion, and this risk may not be completely accounted for by multivariate analysis. It is also possible, however, that transfusions per se may contribute to acute kidney injury. Possible direct or indirect mechanisms mediating acute kidney injury include hemolytic reactions, circulatory overload, acute lung injury, and immunomodulatory effects.39
Preoperative transfusion in anemic patients undergoing cardiac surgery may also reduce the incidence of postoperative acute kidney injury both by correcting the anemia and by limiting the need for perioperative transfusions.40 It remains to be determined whether elective preoperative transfusion to correct anemia would reduce postoperative development of acute kidney injury in total joint arthroplasty. As an aside, perioperative transfusion has also been linked to development of periprosthetic joint infection.41
Renin-angiotensin-aldosterone system inhibitors
Several studies found perioperative use of renin-angiotensin-aldosterone system inhibitors to be a risk factor for acute kidney injury.
Kimmel et al11 reported adjusted odds ratios of 2.70 (95% CI 1.12–6.48) for ACE inhibitor use and 2.64 (95% CI 1.18–5.93) for ARB use in a study of 425 primary total joint arthroplasties.
Challagundla et al24 found an odds ratio of 3.07 (95% CI 1.40–6.74) with ACE inhibitor or ARB use by multivariate analysis in 198 total joint arthroplasties.
Nielson et al18 studied 798 patients who underwent total joint arthroplasty and found that preoperative use of renin-angiotensin system inhibitors was associated with a significantly higher rate of postoperative acute kidney injury (8.3% vs 1.7% without inhibition), which was statistically significant by multivariate analysis (odds ratio 2.6, 95% CI 1.04–6.51).
We recommend holding renin-angiotensin-aldosterone system inhibitors 7 days before surgery through the postoperative period in high-risk cases.
Aminoglycoside use as a risk factor
Prophylactic administration of systemic antibiotics is the standard of care. In a systematic review of 26 studies and meta-analysis of 7 studies (3,065 patients), prophylactic antibiotics reduced the relative risk of wound infection by 81% with an absolute risk reduction of 8%.42
A modifiable risk factor for acute kidney injury is the specific antibiotic used for prophylaxis. Multiple studies assessed the risk of acute kidney injury comparing regimens containing an aminoglycoside (typically gentamicin) with regimens lacking these agents.20–26 In general, these studies found a significantly higher risk of acute kidney injury when gentamicin was used.
Challagundla et al24 found an incidence of acute kidney injury of 52% using RIFLE creatinine criteria in 52 patients receiving 8 g total of flucloxacillin plus 160 mg of gentamicin (120 mg if they weighed less than 60 kg) compared with 8% in 48 patients given cefuroxime (3 g total) and 14% in an additional 52 patients also given cefuroxime.
Johansson et al25 found an incidence of KDIGO creatinine-based acute kidney injury of 13% in 70 patients given dicloxacillin alone prophylactically compared with 27% given dicloxacillin and gentamicin, with a relative risk of 3.
Bell et al,21 in a large registry-based analysis from Scotland involving 7,666 elective orthopedic procedures, found that use of flucloxacillin 2 g plus a single dose of gentamicin 4 mg/kg was significantly associated with a 94% higher risk of acute kidney injury (KDIGO creatinine criteria) compared with a cefuroxime-based regimen, with absolute rates increasing from 6.2% to 10.8%.
Dubrovskaya et al20 and Ferguson et al,26 in contrast, found no increased risk with addition of gentamicin.
We recommend avoiding aminoglycosides for prophylaxis in primary lower-extremity total joint arthroplasty in patients at higher risk unless required for specific microbiologic reasons.
Vancomycin may also increase risk
Courtney et al19 assessed the risk of adding vancomycin to cefazolin for routine prophylaxis in a retrospective series of 1,828 total hip or knee arthroplasties and found a significantly higher rate of acute kidney injury, using AKIN criteria (13% vs 8%, odds ratio by multivariate analysis 1.82, P = .002).19
Other agents shown to be effective in treating periprosthetic joint infections or complicated skin and soft-tissue infections with resistant organisms include daptomycin43 and linezolid.44 These nonnephrotoxic alternatives to vancomycin may be a consideration if prophylaxis for methicillin-resistant Staphylococcus aureus is deemed necessary in patients at risk for acute kidney injury.
PROSTHETIC JOINT INFECTIONS AND ANTIBIOTIC-LOADED CEMENT
Deep infection may complicate nearly 1% of total hip45 and 2% of total knee arthroplasties.46 Kurtz et al4,6 have projected that by 2030, infection will be the cause of two-thirds of the estimated 268,000 revision total knee arthroplasties and about half of the estimated 96,700 revision total hip arthroplasties.
The most common method of treating a chronically infected replacement joint is a 2-stage procedure.5 First, the prosthesis is removed, all infected bone and soft tissue is debrided, and an antibiotic-loaded cement spacer is implanted. Systemic antibiotics are given concurrently, typically for about 6 weeks. After the infection is brought under control, perhaps 2 to 3 months later, the spacer is removed and a new joint is implanted with antibiotic-loaded cement. A 1-stage procedure may be an option in selected cases and would obviate the need for an antibiotic-loaded cement spacer.47,48
Of obvious relevance to development of acute kidney injury is the choice and amount of antibiotics embedded in the cement used for spacers and in implantation. Very high antibiotic levels are achieved within the joint space, usually with little systemic absorption, although significant systemic exposure has been documented in some cases.
The polymethylmethacrylate cement used for these purposes comes in 40-g bags. Multiple bags are typically required per joint, perhaps 2 to 4.49
The rate of elution of antibiotics is determined by several factors, including surface area, porosity, and the number of antibiotics. In general, elution is greatest early on, with exponential decline lasting perhaps 1 week, followed by slow, sustained release over weeks to months.50 However, several in vitro studies have indicated that only about 5%50,51 of the total antibiotic actually elutes over time.
Initially, multiple antibiotic-laden cement beads were used to fill the joint space, but this significantly limited function and mobility.52 Now, cement spacers are used, and they can be nonarticulating or articulating for maximal joint mobility.53 Although much greater antibiotic elution occurs from beads due to their high surface area-to-volume ratio, spacers still provide an adequate dose.
ANTIBIOTIC-LOADED CEMENT: DOSAGE AND ELUTION CHARACTERISTICS
Antibiotic-loaded cement can be either low-dose or high-dose.
Low-dose cement
Low-dose cement typically consists of 0.5 to 1.0 g of antibiotic per 40-g bag of cement, usually an aminoglycoside (gentamicin or tobramycin) or vancomycin, and can be purchased premixed by the manufacturer. Such cement is only used prophylactically with primary total joint arthroplasty or revision for aseptic reasons, a practice common in Europe but less so in the United States. Some American authors propose antibiotic-loaded cement prophylaxis for patients at high risk, eg, those with immunosuppression, inflammatory cause of arthritis, or diabetes.54
Vrabec et al,55 in a study of low-dose tobramycin-loaded cement used for primary total knee arthroplasty, found a peak median intra-articular tobramycin concentration of 32 mg/L at 6 hours, declining to 6 mg/L at 48 hours with all serum levels 0.3 mg/L or less (unmeasureable) at similar time points.
Sterling et al,56 studying primary total hip arthroplasties with low-dose tobramycin-loaded cement, found mean levels in drainage fluid of 103 mg/L at 6 hours, declining to 15 mg/L at 48 hours. Serum levels peaked at 0.94 mg/L at 3 hours, declining to 0.2 mg/L by 48 hours.
Although most of the antibiotic elution occurs early (within the first week), antibiotic can be found in joint aspirates up to 20 years later.57 We are unaware of any well-documented cases of acute kidney injury ascribable to low-dose antibiotic-loaded cement used prophylactically. One case report making this assertion did not determine serum levels of aminoglycoside.58
High-dose cement
High-dose antibiotic-loaded cement typically contains about 4 to 8 g of antibiotic per 40-g bag of cement and is used in the treatment of prosthetic joint infection to form the spacers. The antibiotic must be mixed into the cement powder by the surgeon in the operating room.
There is no standard combination or dosage. The choice of antibiotic can be tailored to the infecting organism if known. Otherwise, gram-positive organisms are most common, and vancomycin and aminoglycosides are often used together. This particular combination will enhance the elution of both antibiotics when studied in vitro, a process termed “passive opportunism.”59 Other antibiotics in use include aztreonam, piperacillin, teicoplanin, fluoroquinolones, cephalosporins, and daptomycin, among others.
About 8 g of antibiotic total per 40-g bag is the maximum to allow easy molding.52 As an example, this may include 4 g of vancomycin and 3.6 g of tobramycin per 40 g. Given that 3 to 4 such bags are often used per joint, there is significant risk of systemic exposure.
Kalil et al60 studied 8 patients who received high-dose tobramycin-loaded cement to treat periprosthetic joint infections of the hip or knee and found that 7 had detectable serum levels (mean 0.84 mg/L, highest 2.0 mg/L), including 1 with a level of 0.9 mg/L on day 38; 4 of these 8 developed acute kidney injury by AKIN criteria, although other risk factors for acute kidney injury existed. Nearly all had concomitant vancomycin (3 to 8 g) added to the cement as well.
Hsieh et al61 studied 46 patients with infected total hip arthroplasties treated with high-dose antibiotic-loaded cement spacers (vancomycin 4 g and aztreonam 4 g per 40-g bag) and found vancomycin levels in joint drainage higher than 1,500 mg/L on day 1, decreasing to 571 mg/L on day 7; serum levels were low (range 0.1–1.6 mg/L at 24 hours), falling to undetectable by 72 hours.
ANTIBIOTIC-LOADED CEMENT SPACERS AND ACUTE KIDNEY INJURY
Case reports have associated high-dose antibiotic-loaded cement spacers with acute kidney injury.
Curtis et al62 described an 85-year-old patient with stage 3 chronic kidney disease who was treated for an infected total knee arthroplasty with an antibiotic-loaded cement spacer (containing 3.6 g of tobramycin and 3 g of cefazolin per 40-g bag, 3 bags total) and developed stage 3 acute kidney injury. After 16 days and 3 hemodialysis sessions, the patient’s serum tobramycin level was still 2 mg/L despite receiving no systemic tobramycin.
Wu et al63 reported a case of acute kidney injury that required dialysis after implantation of a tobramycin- and vancomycin-loaded spacer, with persistent serum tobramycin levels despite repeated hemodialysis sessions until the spacer was removed.
Chalmers et al64 described 2 patients with acute kidney injury and persistently elevated serum tobramycin levels (3.9 mg/L on day 39 in 1 patient and 2.0 mg/L on day 24 in the other patient) despite no systemic administration.
In these and other case reports,65–67 dialysis and spacer explantation were usually required.
Comment. It is intuitive that acute kidney injury would more likely complicate revision total joint arthroplasties for infection than for primary total joint arthroplasties or revisions for aseptic reasons, given the systemic effects of infection and exposure to nephrotoxic or allergenic antibiotics. And the available data suggest that the risk of acute kidney injury is higher with revision for prosthetic joint infection than with revision for aseptic reasons. However, many of the studies were retrospective, relatively small, single-center series and used different definitions of acute kidney injury.
Luu et al83 performed a systematic review of studies published between January 1989 and June 2012 reporting systemic complications (including acute kidney injury) of 2-stage revision arthroplasties including placement of an antibiotic-loaded cement spacer for treatment of periprosthetic joint infection. Overall, 10 studies were identified with 544 total patients. Five of these studies, with 409 patients, reported at least 1 case of acute kidney injury for a total of 27 patients, giving an incidence of 6.6% in these studies.68–71 The remaining 5 studies, totaling 135 patients, did not report any cases of acute kidney injury,50,61,76–78 although that was not the primary focus of any of those trials.
Most notable from this systematic review, the study of Menge et al69 retrospectively determined the incidence of acute kidney injury (defined as a 50% rise in serum creatinine to > 1.4 mg/dL within 90 days of surgery) to be 17% in 84 patients with infected total knee arthroplasties treated with antibiotic-loaded cement spacers. A mean of 3.5 bags of cement per spacer were used in the 35 articulating spacers, compared with 2.9 per nonarticulating spacer. These spacers contained vancomycin in 82% (median 4.0 g, range 1–16 g) and tobramycin in 94% (median 4.8 g, range 1–12 g), among others in small percentages. The dose of tobramycin in the spacer considered either as a dichotomous variable (> 4.8 g, OR 5.87) or linearly (OR 1.24 per 1-g increase) was significantly associated with acute kidney injury, although systemic administration of aminoglycosides or vancomycin was not.
Additional single-center series that were published subsequent to this review have generally used more current diagnostic criteria.
Noto et al72 found that 10 of 46 patients treated with antibiotic-loaded cement spacers had a greater than 50% rise in serum creatinine (average increase 260%). All spacers contained tobramycin (mean dose 8.2 g), and 9 of 10 also contained vancomycin (mean 7.6 g). All of the 9 patients with acute kidney injury with follow-up data recovered renal function.
Reed et al75 found 26 cases of acute kidney injury (based on RIFLE creatinine criteria) in 306 patients with antibiotic-loaded cement spacers treating various periprosthetic joint infections (including hips, knees, shoulders, and digits) and compared them with 74 controls who did not develop acute kidney injury. By multivariable analysis, receipt of an ACE inhibitor within 7 days of surgery and receipt of piperacillin-tazobactam within 7 days after surgery were both significantly more common in cases with acute kidney injury than in controls without acute kidney injury.
Aeng et al73 prospectively studied 50 consecutive patients receiving antibiotic-loaded spacers containing tobramycin (with or without vancomycin) for treatment of infected hip or knee replacements. Using RIFLE creatinine criteria, they found an incidence of acute kidney injury of 20% (10 of 50). Factors significantly associated with acute kidney injury included cement premixed by the manufacturer with gentamicin (0.5 g per 40-g bag) in addition to the tobramycin they added, intraoperative blood transfusions, and postoperative use of nonsteroidal anti-inflammatory drugs.
Geller et al,74 in a multicenter retrospective study of 247 patients with prosthetic joint infections (156 knees and 91 hips) undergoing antibiotic-loaded cement spacer placement, found an incidence of acute kidney injury of 26% based on KDIGO creatinine criteria. Significant risk factors included higher body mass index, lower preoperative hemoglobin level, drop in hemoglobin after surgery, and comorbidity (hypertension, diabetes, chronic kidney disease, or cardiovascular disease). Most of the spacers contained a combination of vancomycin and either tobramycin (81%) or gentamicin (13%). The spacers contained an average of 5.3 g (range 0.6–18 g) of vancomycin (average 2.65 g per 40-g bag) and an average of 5.2 g (range 0.5–16.4 g) of tobramycin (average 2.6 g per bag).
As in Menge et al,69 this study illustrates the wide range of antibiotic dosages in use and the lack of standardization. In contrast to the study by Menge et al, however, development of acute kidney injury was not related to the amount of vancomycin or tobramycin contained in the spacers. Eventual clearance of infection (at 1 and 2 years) was significantly related to increasing amounts of vancomycin. Multiple different systemic antibiotics were used, most commonly vancomycin (44%), and systemic vancomycin was not associated with acute kidney injury.
Yadav et al,81 in a study of 3,129 consecutive revision procedures of the knee or hip, found an incidence of acute kidney injury by RIFLE creatinine criteria of 29% in the 197 patients who received antibiotic-loaded cement spacers for periprosthetic joint infection compared with 3.4% in the 2,848 who underwent revision for aseptic reasons. In 84 patients with prosthetic joint infection having various surgeries not including placement of a spacer, the acute kidney injury rate at some point in their course was an alarmingly high 82%. In the group that received spacers, only age and comorbidity as assessed by Charlson comorbidity index were independently associated with acute kidney injury by multivariate analysis. Surprisingly, modest renal impairment was protective, possibly because physicians of patients with chronic kidney disease were more vigilant and took appropriate measures to prevent acute kidney injury.
Overall, the risk of acute kidney injury appears to be much higher during treatment of prosthetic joint infection with a 2-stage procedure using an antibiotic-loaded cement spacer than after primary total joint arthroplasty or revision for aseptic reasons, and may complicate up to one-third of cases.
REDUCING RISK DURING TREATMENT OF INFECTED REPLACEMENT JOINTS
As in primary total joint arthroplasty in general, higher-risk cases should be identified based on age, body mass index, chronic kidney disease, comorbidities (hypertension, diabetes, established cardiovascular disease), and anemia.
Preoperative transfusion can be considered case by case depending on degree of anemia and associated risk factors.
All renin-angiotensin-aldosterone system inhibitors should be withheld starting 1 week before surgery.
Both nonselective and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs should be avoided, if possible.
Strict attention should be paid to adequate intraoperative and postoperative fluid resuscitation.
Kidney function should be monitored closely in the early postoperative period, including urine output and daily creatinine for at least 72 hours.
Systemic administration of potentially nephrotoxic antibiotics should be minimized, especially the combination of vancomycin with piperacillin-tazobactam.84 Daptomycin is a consideration.43
If acute kidney injury should develop, serum levels of vancomycin or aminoglycosides should be measured if the spacer contains these antibiotics. The spacer may need to be removed if toxic serum levels persist.
TAKE-HOME POINTS
Acute kidney injury may complicate up to 10% of primary lower-extremity total joint arthroplasties and up to 25% of periprosthetic joint infections treated with a 2-stage procedure including placement of an antibiotic-loaded cement spacer in the first stage.
Risk factors for acute kidney injury include older age, obesity, chronic kidney disease, and overall comorbidity. Potentially modifiable risk factors include anemia, perioperative transfusions, aminoglycoside prophylaxis, perioperative renin-angiotensin system blockade, and postoperative nonsteroidal anti-inflammatory drugs. These should be mitigated when possible.
In patients with periprosthetic joint infection who receive antibiotic-loaded cement spacers, especially patients with additional risk factors for acute kidney injury, strict attention should be paid to the dose of antibiotic in the spacer, with levels checked postoperatively if necessary. Nonnephrotoxic antibiotics should be chosen for systemic administration when possible.
Prospective randomized controlled trials are needed to guide therapy after total joint arthroplasty, and to verify the adverse long-term outcomes of acute kidney injury in this setting.
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007; 370(9597):1508–1519. doi:10.1016/S0140-6736(07)60457-7
- Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet 2012; 380(9855):1768–1777. doi:10.1016/S0140-6736(12)60607-2
- Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012; 379(9823):1331–1340. doi:10.1016/S0140-6736(11)60752-6
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89(4):780–785. doi:10.2106/JBJS.F.00222
- Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet 2016; 387(10016):386–394. doi:10.1016/S0140-6736(14)61798-0
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am 2007; 89(suppl 3):144–151. doi:10.2106/JBJS.G.00587
- Jafari SM, Huang R, Joshi A, Parvizi J, Hozack WJ. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty 2010; 25(6 suppl):49–53, 53.e1–2. doi:10.1016/j.arth.2010.04.008
- Jamsa P, Jamsen E, Lyytikainen LP, Kalliovalkama J, Eskelinen A, Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop 2017; 88(4):370–376. doi:10.1080/17453674.2017.1301743
- Sehgal V, Bajwa SJ, Sehgal R, Eagan J, Reddy P, Lesko SM. Predictors of acute kidney injury in geriatric patients undergoing total knee replacement surgery. Int J Endocrinol Metab 2014; 12(3):e16713. doi:10.5812/ijem.16713
- Weingarten TN, Gurrieri C, Jarett PD, et al. Acute kidney injury following total joint arthroplasty: retrospective analysis. Can J Anaesth 2012; 59(12):1111–1118. doi:10.1007/s12630-012-9797-2
- Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J 2014; 7(6):546–551. doi:10.1093/ckj/sfu108
- Warth LC, Noiseux NO, Hogue MH, Klaassen AL, Liu SS, Callaghan JJ. Risk of acute kidney injury after primary and revision total hip arthroplasty and total knee arthroplasty using a multimodal approach to perioperative pain control including ketorolac and celecoxib. J Arthroplasty 2016; 31(1):253–255. doi:10.1016/j.arth.2015.08.012
- Perregaard H, Damholt MB, Solgaard S, Petersen MB. Renal function after elective total hip replacement. Acta Orthop 2016; 87(3):235–238. doi:10.3109/17453674.2016.1155130
- Hassan BK, Sahlström A, Dessau RB. Risk factors for renal dysfunction after total hip joint replacement; a retrospective cohort study. J Orthop Surg Res 2015; 10:158. doi:10.1186/s13018-015-0299-0
- Nowicka A, Selvaraj T. Incidence of acute kidney injury after elective lower limb arthroplasty. J Clin Anesth 2016; 34:520–523. doi:10.1016/j.jclinane.2016.06.010
- Kim HJ, Koh WU, Kim SG, et al. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: a retrospective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine (Baltimore) 2016; 95(31):e4489. doi:10.1097/MD.0000000000004489
- Choi YJ, Kim S, Sim JH, Hahm K. Postoperative anemia is associated with acute kidney injury in patients undergoing total hip replacement arthroplasty: a retrospective study. Anesth Analg 2016; 122(6):1923–1928. doi:10.1213/ANE.0000000000001003
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9(5):283–288. doi:10.1002/jhm.2155
- Courtney PM, Melnic CM, Zimmer Z, Anari J, Lee GC. Addition of vancomycin to cefazolin prophylaxis is associated with acute kidney injury after primary joint arthroplasty. Clin Orthop Relat Res 2015; 473(7):2197–2203. doi:10.1007/s11999-014-4062-3
- Dubrovskaya Y, Tejada R, Bosco J 3rd, et al. Single high dose gentamicin for perioperative prophylaxis in orthopedic surgery: evaluation of nephrotoxicity. SAGE Open Med 2015; 3:2050312115612803. doi:10.1177/2050312115612803
- Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol 2014; 25(11):2625–2632. doi:10.1681/ASN.2014010035
- Ross AD, Boscainos PJ, Malhas A, Wigderowitz C. Peri-operative renal morbidity secondary to gentamicin and flucloxacillin chemoprophylaxis for hip and knee arthroplasty. Scott Med J 2013; 58(4):209–212. doi:10.1177/0036933013507850
- Bailey O, Torkington MS, Anthony I, Wells J, Blyth M, Jones B. Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J 2014; 96-B(3):395–398. doi:10.1302/0301-620X.96B3.32745
- Challagundla SR, Knox D, Hawkins A, et al. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant 2013; 28(3):612–619. doi:10.1093/ndt/gfs458
- Johansson S, Christensen OM, Thorsmark AH. A retrospective study of acute kidney injury in hip arthroplasty patients receiving gentamicin and dicloxacillin. Acta Orthop 2016; 87(6):589–591. doi:10.1080/17453674.2016.1231008
- Ferguson KB, Winter A, Russo L, et al. Acute kidney injury following primary hip and knee arthroplasty surgery. Ann R Coll Surg Eng 2017; 99(4):307–312. doi:10.1308/rcsann.2016.0324
- Bjerregaard LS, Jorgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-Track Hip and Knee Replacement Collaborative Group. Serious renal and urological complications in fast-track primary total hip and knee arthroplasty; a detailed observational cohort study. Minerva Anestesiol 2016; 82(7):767–776. pmid:27028450
- Friedman JM, Couso R, Kitchens M, et al. Benign heart murmurs as a predictor for complications following total joint arthroplasty. J Orthop 2017; 14(4):470–474. doi:10.1016/j.jor.2017.07.009
- Nadkarni GN, Patel AA, Ahuja Y, et al. Incidence, risk factors, and outcome trends of acute kidney injury in elective total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016; 45(1):E12–E19. pmid:26761921
- Lopez-de-Andres A, Hernandez-Barrera V, Martinez-Huedo MA, Villanueva-Martinez M, Jimenez-Trujillo I, Jimenez-Garcia R. Type 2 diabetes and in-hospital complications after revision of total hip and knee arthroplasty. PLoS One 2017; 12(8):e0183796. doi:10.1371/journal.pone.0183796
- Gharaibeh KA, Hamadah AM, Sierra RJ, Leung N, Kremers WK, El-Zoghby ZM. The rate of acute kidney injury after total hip arthroplasty is low but increases significantly in patients with specific comorbidities. J Bone Joint Surg Am 2017; 99(21):1819–1826. doi:10.2106/JBJS.16.01027
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8(4):R204–R212. doi:10.1186/cc2872
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11(2):R31. doi:10.1186/cc5713
- Section 2: AKI Definition. Kidney Int Suppl (2011) 2012; 2(1):19–36. doi:10.1038/kisup.2011.32
- Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 2014; 9(4):682–689. doi:10.2215/CJN.07650713
- Parr SK, Matheny ME, Abdel-Kader K, et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 2018; 93(2):460–469. doi:10.1016/j.kint.2017.07.007
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Karkouti K, Grocott HP, Hall R, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth 2015; 62(4):377–384. doi:10.1007/s12630-014-0302-y
- Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med 2017; 377(13):1261–1272. doi:10.1056/NEJMra1612789
- Karkouti K, Wijeysundera DN, Yau TM, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology 2012; 116(3):613–621. doi:10.1097/ALN.0b013e3182475e39
- Newman ET, Watters TS, Lewis JS, et al. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J Bone Joint Surg Am 2014; 96(4):279–284. doi:10.2106/JBJS.L.01041
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90(7):915–919. doi:10.1302/0301-620X.90B7.20498
- Corona Pérez-Cardona PS, Barro Ojeda V, Rodriguez Pardo D, et al. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother 2012; 67(7):1749–1754. doi:10.1093/jac/dks119
- Itani KM, Biswas P, Reisman A, Bhattacharyya H, Baruch AM. Clinical efficacy of oral linezolid compared with intravenous vancomycin for the treatment of methicillin-resistant Staphylococcus aureus-complicated skin and soft tissue infections: a retrospective, propensity score-matched, case-control analysis. Clin Ther 2012; 34(8):1667–1673.e1. doi:10.1016/j.clinthera.2012.06.018
- Dale H, Hallan G, Hallan G, Espehaug B, Havelin LI, Engesaeter LB. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop 2009; 80(6):639–645. doi:10.3109/17453670903506658
- Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010; 468(1):52–56. doi:10.1007/s11999-009-1013-5
- Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD; INFORM Team. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One 2016; 11(3):e0151537. doi:10.1371/journal.pone.0151537
- Negus JJ, Gifford PB, Haddad FS. Single-stage revision arthroplasty for infection—an underutilized treatment strategy. J Arthroplasty 2017; 32(7):2051–2055. doi:10.1016/j.arth.2017.02.059
- Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res 2005; 23(1):27–33. doi:10.1016/j.orthres.2004.03.003
- Chohfi M, Langlais F, Fourastier J, Minet J, Thomazeau H, Cormier M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int Orthop 1998; 22(3):171–177. pmid:9728311
- Amin TJ, Lamping JW, Hendricks KJ, McIff TE. Increasing the elution of vancomycin from high-dose antibiotic-loaded bone cement: a novel preparation technique. J Bone Joint Surg Am 2012; 94(21):1946–1951. doi:10.2106/JBJS.L.00014
- Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma 2004; 56(6):1247–1252. pmid:15211133
- Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am 2007; 89(4):871–882. doi:10.2106/JBJS.E.01070
- Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am 2006; 88(11):2487–2500. doi:10.2106/JBJS.E.01126
- Vrabec G, Stevenson W, Elguizaoui S, Kirsch M, Pinkowski J. What is the intraarticular concentration of tobramycin using low-dose tobramycin bone cement in TKA: an in vivo analysis? Clin Orthop Relat Res 2016; 474(11):2441–2447. doi:10.1007/s11999-016-5006-x
- Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg Br 2003; 85(5):646–649. pmid:12892183
- Fletcher MD, Spencer RF, Langkamer VG, Lovering AM. Gentamicin concentrations in diagnostic aspirates from 25 patients with hip and knee arthroplasties. Acta Orthop Scand 2004; 75(2):173–176. doi:10.1080/00016470412331294425
- Lau BP, Kumar VP. Acute kidney injury (AKI) with the use of antibiotic-impregnated bone cement in primary total knee arthroplasty. Ann Acad Med Singapore 2013; 42(12):692–695. pmid:24463833
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty 1996; 11(8):939–944. pmid:8986572
- Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother 2012; 46(7–8):929–934. doi:10.1345/aph.1R049
- Hsieh PH, Chang YH, Chen SH, Ueng SW, Shih CH. High concentration and bioactivity of vancomycin and aztreonam eluted from simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J Orthop Res 2006; 24(8):1615–1621. doi:10.1002/jor.20214
- Curtis JM, Sternhagen V, Batts D. Acute renal failure after placement of tobramycin-impregnated bone cement in an infected total knee arthroplasty. Pharmacotherapy 2005; 25(6):876–880. pmid:15927906
- Wu IM, Marin EP, Kashgarian M, Brewster UC. A case of an acute kidney injury secondary to an implanted aminoglycoside. Kidney Int 2009; 75(10):1109–1112. doi:10.1038/ki.2008.386
- Chalmers PN, Frank J, Sporer SM. Acute postoperative renal failure following insertion of an antibiotic-impregnated cement spacer in revision total joint arthroplasty: two case reports. JBJS Case Connect 2012; 2(1):e12. doi:10.2106/JBJS.CC.K.00094
- Patrick BN, Rivey MP, Allington DR. Acute renal failure associated with vancomycin- and tobramycin-laden cement in total hip arthroplasty. Ann Pharmacother 2006; 40(11):2037–2042. doi:10.1345/aph.1H173
- Dovas S, Liakopoulos V, Papatheodorou L, et al. Acute renal failure after antibiotic-impregnated bone cement treatment of an infected total knee arthroplasty. Clin Nephrol 2008; 69(3):207–212. pmid:18397720
- McGlothan KR, Gosmanova EO. A case report of acute interstitial nephritis associated with antibiotic-impregnated orthopedic bone-cement spacer. Tenn Med 2012; 105(9):37–40, 42. pmid:23097958
- Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci 2009; 6(5):265–273. pmid:19834592
- Menge TJ, Koethe JR, Jenkins CA, et al. Acute kidney injury after placement of an antibiotic-impregnated cement spacer during revision total knee arthroplasty. J Arthroplasty 2012; 27(6):1221–1227.e1–2. doi:10.1016/j.arth.2011.12.005
- Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin Orthop Relat Res 2011; 469(4):985–993. doi:10.1007/s11999-010-1579-y
- Springer BD, Lee GC, Osmon D, Haidukewych GJ, Hanssen AD, Jacofsky DJ. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res 2004; 427:47–51. pmid:15552135
- Noto MJ, Koethe JR, Miller G, Wright PW. Detectable serum tobramycin levels in patients with renal dysfunction and recent placement of antibiotic-impregnated cement knee or hip spacers. Clin Infect Dis 2014; 58(12):1783–1784. doi:10.1093/cid/ciu159
- Aeng ES, Shalansky KF, Lau TT, et al. Acute kidney injury with tobramycin-impregnated bone cement spacers in prosthetic joint infections. Ann Pharmacother 2015; 49(11):1207–1213. doi:10.1177/1060028015600176
- Geller JA, Cunn G, Herschmiller T, Murtaugh T, Chen A. Acute kidney injury after first-stage joint revision for infection: Risk factors and the impact of antibiotic dosing. J Arthroplasty 2017; 32(10):3120–3125. doi:10.1016/j.arth.2017.04.054
- Reed EE, Johnston J, Severing J, Stevenson KB, Deutscher M. Nephrotoxicity risk factors and intravenous vancomycin dosing in the immediate postoperative period following antibiotic-impregnated cement spacer placement. Ann Pharmacother 2014; 48(8):962–969. doi:10.1177/1060028014535360
- Koo KH, Yang JW, Cho SH, et al. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty 2001; 16(7):882–892. doi:10.1054/arth.2001.24444
- Forsythe ME, Crawford S, Sterling GJ, Whitehouse SL, Crawford R. Safeness of simplex-tobramycin bone cement in patients with renal dysfunction undergoing total hip replacement. J Orthop Surg (Hong Kong) 2006; 14(1):38–42. doi:10.1177/230949900601400109
- Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: in vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. J Trauma 2009; 66(3):804–808. doi:10.1097/TA.0b013e31818896cc
- Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res 2005; 430:125–131. pmid:15662313
- Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res 2004; 427:37–46. pmid:15552134
- Yadav A, Alijanipour P, Ackerman CT, Karanth S, Hozack WJ, Filippone EJ. Acute kidney injury following failed total hip and knee arthroplasty. J Arthroplasty 2018; 33(10):3297–3303. doi:10.1016/j.arth.2018.06.019
- Hsieh PH, Huang KC, Lee PC, Lee MS. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: retrospective comparison between short-term and prolonged antibiotic therapy. J Antimicrob Chemother 2009; 64(2):392–397. doi:10.1093/jac/dkp177
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty 2013; 28(9):1490–1498.e1. doi:10.1016/j.arth.2013.02.035
- Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017; 102(3):459–469. doi:10.1002/cpt.726
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007; 370(9597):1508–1519. doi:10.1016/S0140-6736(07)60457-7
- Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet 2012; 380(9855):1768–1777. doi:10.1016/S0140-6736(12)60607-2
- Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012; 379(9823):1331–1340. doi:10.1016/S0140-6736(11)60752-6
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89(4):780–785. doi:10.2106/JBJS.F.00222
- Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet 2016; 387(10016):386–394. doi:10.1016/S0140-6736(14)61798-0
- Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am 2007; 89(suppl 3):144–151. doi:10.2106/JBJS.G.00587
- Jafari SM, Huang R, Joshi A, Parvizi J, Hozack WJ. Renal impairment following total joint arthroplasty: who is at risk? J Arthroplasty 2010; 25(6 suppl):49–53, 53.e1–2. doi:10.1016/j.arth.2010.04.008
- Jamsa P, Jamsen E, Lyytikainen LP, Kalliovalkama J, Eskelinen A, Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop 2017; 88(4):370–376. doi:10.1080/17453674.2017.1301743
- Sehgal V, Bajwa SJ, Sehgal R, Eagan J, Reddy P, Lesko SM. Predictors of acute kidney injury in geriatric patients undergoing total knee replacement surgery. Int J Endocrinol Metab 2014; 12(3):e16713. doi:10.5812/ijem.16713
- Weingarten TN, Gurrieri C, Jarett PD, et al. Acute kidney injury following total joint arthroplasty: retrospective analysis. Can J Anaesth 2012; 59(12):1111–1118. doi:10.1007/s12630-012-9797-2
- Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J 2014; 7(6):546–551. doi:10.1093/ckj/sfu108
- Warth LC, Noiseux NO, Hogue MH, Klaassen AL, Liu SS, Callaghan JJ. Risk of acute kidney injury after primary and revision total hip arthroplasty and total knee arthroplasty using a multimodal approach to perioperative pain control including ketorolac and celecoxib. J Arthroplasty 2016; 31(1):253–255. doi:10.1016/j.arth.2015.08.012
- Perregaard H, Damholt MB, Solgaard S, Petersen MB. Renal function after elective total hip replacement. Acta Orthop 2016; 87(3):235–238. doi:10.3109/17453674.2016.1155130
- Hassan BK, Sahlström A, Dessau RB. Risk factors for renal dysfunction after total hip joint replacement; a retrospective cohort study. J Orthop Surg Res 2015; 10:158. doi:10.1186/s13018-015-0299-0
- Nowicka A, Selvaraj T. Incidence of acute kidney injury after elective lower limb arthroplasty. J Clin Anesth 2016; 34:520–523. doi:10.1016/j.jclinane.2016.06.010
- Kim HJ, Koh WU, Kim SG, et al. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: a retrospective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine (Baltimore) 2016; 95(31):e4489. doi:10.1097/MD.0000000000004489
- Choi YJ, Kim S, Sim JH, Hahm K. Postoperative anemia is associated with acute kidney injury in patients undergoing total hip replacement arthroplasty: a retrospective study. Anesth Analg 2016; 122(6):1923–1928. doi:10.1213/ANE.0000000000001003
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9(5):283–288. doi:10.1002/jhm.2155
- Courtney PM, Melnic CM, Zimmer Z, Anari J, Lee GC. Addition of vancomycin to cefazolin prophylaxis is associated with acute kidney injury after primary joint arthroplasty. Clin Orthop Relat Res 2015; 473(7):2197–2203. doi:10.1007/s11999-014-4062-3
- Dubrovskaya Y, Tejada R, Bosco J 3rd, et al. Single high dose gentamicin for perioperative prophylaxis in orthopedic surgery: evaluation of nephrotoxicity. SAGE Open Med 2015; 3:2050312115612803. doi:10.1177/2050312115612803
- Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol 2014; 25(11):2625–2632. doi:10.1681/ASN.2014010035
- Ross AD, Boscainos PJ, Malhas A, Wigderowitz C. Peri-operative renal morbidity secondary to gentamicin and flucloxacillin chemoprophylaxis for hip and knee arthroplasty. Scott Med J 2013; 58(4):209–212. doi:10.1177/0036933013507850
- Bailey O, Torkington MS, Anthony I, Wells J, Blyth M, Jones B. Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J 2014; 96-B(3):395–398. doi:10.1302/0301-620X.96B3.32745
- Challagundla SR, Knox D, Hawkins A, et al. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol Dial Transplant 2013; 28(3):612–619. doi:10.1093/ndt/gfs458
- Johansson S, Christensen OM, Thorsmark AH. A retrospective study of acute kidney injury in hip arthroplasty patients receiving gentamicin and dicloxacillin. Acta Orthop 2016; 87(6):589–591. doi:10.1080/17453674.2016.1231008
- Ferguson KB, Winter A, Russo L, et al. Acute kidney injury following primary hip and knee arthroplasty surgery. Ann R Coll Surg Eng 2017; 99(4):307–312. doi:10.1308/rcsann.2016.0324
- Bjerregaard LS, Jorgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-Track Hip and Knee Replacement Collaborative Group. Serious renal and urological complications in fast-track primary total hip and knee arthroplasty; a detailed observational cohort study. Minerva Anestesiol 2016; 82(7):767–776. pmid:27028450
- Friedman JM, Couso R, Kitchens M, et al. Benign heart murmurs as a predictor for complications following total joint arthroplasty. J Orthop 2017; 14(4):470–474. doi:10.1016/j.jor.2017.07.009
- Nadkarni GN, Patel AA, Ahuja Y, et al. Incidence, risk factors, and outcome trends of acute kidney injury in elective total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016; 45(1):E12–E19. pmid:26761921
- Lopez-de-Andres A, Hernandez-Barrera V, Martinez-Huedo MA, Villanueva-Martinez M, Jimenez-Trujillo I, Jimenez-Garcia R. Type 2 diabetes and in-hospital complications after revision of total hip and knee arthroplasty. PLoS One 2017; 12(8):e0183796. doi:10.1371/journal.pone.0183796
- Gharaibeh KA, Hamadah AM, Sierra RJ, Leung N, Kremers WK, El-Zoghby ZM. The rate of acute kidney injury after total hip arthroplasty is low but increases significantly in patients with specific comorbidities. J Bone Joint Surg Am 2017; 99(21):1819–1826. doi:10.2106/JBJS.16.01027
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8(4):R204–R212. doi:10.1186/cc2872
- Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11(2):R31. doi:10.1186/cc5713
- Section 2: AKI Definition. Kidney Int Suppl (2011) 2012; 2(1):19–36. doi:10.1038/kisup.2011.32
- Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 2014; 9(4):682–689. doi:10.2215/CJN.07650713
- Parr SK, Matheny ME, Abdel-Kader K, et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int 2018; 93(2):460–469. doi:10.1016/j.kint.2017.07.007
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Karkouti K, Grocott HP, Hall R, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth 2015; 62(4):377–384. doi:10.1007/s12630-014-0302-y
- Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med 2017; 377(13):1261–1272. doi:10.1056/NEJMra1612789
- Karkouti K, Wijeysundera DN, Yau TM, et al. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology 2012; 116(3):613–621. doi:10.1097/ALN.0b013e3182475e39
- Newman ET, Watters TS, Lewis JS, et al. Impact of perioperative allogeneic and autologous blood transfusion on acute wound infection following total knee and total hip arthroplasty. J Bone Joint Surg Am 2014; 96(4):279–284. doi:10.2106/JBJS.L.01041
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90(7):915–919. doi:10.1302/0301-620X.90B7.20498
- Corona Pérez-Cardona PS, Barro Ojeda V, Rodriguez Pardo D, et al. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother 2012; 67(7):1749–1754. doi:10.1093/jac/dks119
- Itani KM, Biswas P, Reisman A, Bhattacharyya H, Baruch AM. Clinical efficacy of oral linezolid compared with intravenous vancomycin for the treatment of methicillin-resistant Staphylococcus aureus-complicated skin and soft tissue infections: a retrospective, propensity score-matched, case-control analysis. Clin Ther 2012; 34(8):1667–1673.e1. doi:10.1016/j.clinthera.2012.06.018
- Dale H, Hallan G, Hallan G, Espehaug B, Havelin LI, Engesaeter LB. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop 2009; 80(6):639–645. doi:10.3109/17453670903506658
- Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010; 468(1):52–56. doi:10.1007/s11999-009-1013-5
- Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD; INFORM Team. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One 2016; 11(3):e0151537. doi:10.1371/journal.pone.0151537
- Negus JJ, Gifford PB, Haddad FS. Single-stage revision arthroplasty for infection—an underutilized treatment strategy. J Arthroplasty 2017; 32(7):2051–2055. doi:10.1016/j.arth.2017.02.059
- Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res 2005; 23(1):27–33. doi:10.1016/j.orthres.2004.03.003
- Chohfi M, Langlais F, Fourastier J, Minet J, Thomazeau H, Cormier M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int Orthop 1998; 22(3):171–177. pmid:9728311
- Amin TJ, Lamping JW, Hendricks KJ, McIff TE. Increasing the elution of vancomycin from high-dose antibiotic-loaded bone cement: a novel preparation technique. J Bone Joint Surg Am 2012; 94(21):1946–1951. doi:10.2106/JBJS.L.00014
- Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma 2004; 56(6):1247–1252. pmid:15211133
- Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am 2007; 89(4):871–882. doi:10.2106/JBJS.E.01070
- Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am 2006; 88(11):2487–2500. doi:10.2106/JBJS.E.01126
- Vrabec G, Stevenson W, Elguizaoui S, Kirsch M, Pinkowski J. What is the intraarticular concentration of tobramycin using low-dose tobramycin bone cement in TKA: an in vivo analysis? Clin Orthop Relat Res 2016; 474(11):2441–2447. doi:10.1007/s11999-016-5006-x
- Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg Br 2003; 85(5):646–649. pmid:12892183
- Fletcher MD, Spencer RF, Langkamer VG, Lovering AM. Gentamicin concentrations in diagnostic aspirates from 25 patients with hip and knee arthroplasties. Acta Orthop Scand 2004; 75(2):173–176. doi:10.1080/00016470412331294425
- Lau BP, Kumar VP. Acute kidney injury (AKI) with the use of antibiotic-impregnated bone cement in primary total knee arthroplasty. Ann Acad Med Singapore 2013; 42(12):692–695. pmid:24463833
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty 1996; 11(8):939–944. pmid:8986572
- Kalil GZ, Ernst EJ, Johnson SJ, et al. Systemic exposure to aminoglycosides following knee and hip arthroplasty with aminoglycoside-loaded bone cement implants. Ann Pharmacother 2012; 46(7–8):929–934. doi:10.1345/aph.1R049
- Hsieh PH, Chang YH, Chen SH, Ueng SW, Shih CH. High concentration and bioactivity of vancomycin and aztreonam eluted from simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J Orthop Res 2006; 24(8):1615–1621. doi:10.1002/jor.20214
- Curtis JM, Sternhagen V, Batts D. Acute renal failure after placement of tobramycin-impregnated bone cement in an infected total knee arthroplasty. Pharmacotherapy 2005; 25(6):876–880. pmid:15927906
- Wu IM, Marin EP, Kashgarian M, Brewster UC. A case of an acute kidney injury secondary to an implanted aminoglycoside. Kidney Int 2009; 75(10):1109–1112. doi:10.1038/ki.2008.386
- Chalmers PN, Frank J, Sporer SM. Acute postoperative renal failure following insertion of an antibiotic-impregnated cement spacer in revision total joint arthroplasty: two case reports. JBJS Case Connect 2012; 2(1):e12. doi:10.2106/JBJS.CC.K.00094
- Patrick BN, Rivey MP, Allington DR. Acute renal failure associated with vancomycin- and tobramycin-laden cement in total hip arthroplasty. Ann Pharmacother 2006; 40(11):2037–2042. doi:10.1345/aph.1H173
- Dovas S, Liakopoulos V, Papatheodorou L, et al. Acute renal failure after antibiotic-impregnated bone cement treatment of an infected total knee arthroplasty. Clin Nephrol 2008; 69(3):207–212. pmid:18397720
- McGlothan KR, Gosmanova EO. A case report of acute interstitial nephritis associated with antibiotic-impregnated orthopedic bone-cement spacer. Tenn Med 2012; 105(9):37–40, 42. pmid:23097958
- Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci 2009; 6(5):265–273. pmid:19834592
- Menge TJ, Koethe JR, Jenkins CA, et al. Acute kidney injury after placement of an antibiotic-impregnated cement spacer during revision total knee arthroplasty. J Arthroplasty 2012; 27(6):1221–1227.e1–2. doi:10.1016/j.arth.2011.12.005
- Gooding CR, Masri BA, Duncan CP, Greidanus NV, Garbuz DS. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin Orthop Relat Res 2011; 469(4):985–993. doi:10.1007/s11999-010-1579-y
- Springer BD, Lee GC, Osmon D, Haidukewych GJ, Hanssen AD, Jacofsky DJ. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res 2004; 427:47–51. pmid:15552135
- Noto MJ, Koethe JR, Miller G, Wright PW. Detectable serum tobramycin levels in patients with renal dysfunction and recent placement of antibiotic-impregnated cement knee or hip spacers. Clin Infect Dis 2014; 58(12):1783–1784. doi:10.1093/cid/ciu159
- Aeng ES, Shalansky KF, Lau TT, et al. Acute kidney injury with tobramycin-impregnated bone cement spacers in prosthetic joint infections. Ann Pharmacother 2015; 49(11):1207–1213. doi:10.1177/1060028015600176
- Geller JA, Cunn G, Herschmiller T, Murtaugh T, Chen A. Acute kidney injury after first-stage joint revision for infection: Risk factors and the impact of antibiotic dosing. J Arthroplasty 2017; 32(10):3120–3125. doi:10.1016/j.arth.2017.04.054
- Reed EE, Johnston J, Severing J, Stevenson KB, Deutscher M. Nephrotoxicity risk factors and intravenous vancomycin dosing in the immediate postoperative period following antibiotic-impregnated cement spacer placement. Ann Pharmacother 2014; 48(8):962–969. doi:10.1177/1060028014535360
- Koo KH, Yang JW, Cho SH, et al. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty 2001; 16(7):882–892. doi:10.1054/arth.2001.24444
- Forsythe ME, Crawford S, Sterling GJ, Whitehouse SL, Crawford R. Safeness of simplex-tobramycin bone cement in patients with renal dysfunction undergoing total hip replacement. J Orthop Surg (Hong Kong) 2006; 14(1):38–42. doi:10.1177/230949900601400109
- Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: in vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. J Trauma 2009; 66(3):804–808. doi:10.1097/TA.0b013e31818896cc
- Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res 2005; 430:125–131. pmid:15662313
- Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res 2004; 427:37–46. pmid:15552134
- Yadav A, Alijanipour P, Ackerman CT, Karanth S, Hozack WJ, Filippone EJ. Acute kidney injury following failed total hip and knee arthroplasty. J Arthroplasty 2018; 33(10):3297–3303. doi:10.1016/j.arth.2018.06.019
- Hsieh PH, Huang KC, Lee PC, Lee MS. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: retrospective comparison between short-term and prolonged antibiotic therapy. J Antimicrob Chemother 2009; 64(2):392–397. doi:10.1093/jac/dkp177
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty 2013; 28(9):1490–1498.e1. doi:10.1016/j.arth.2013.02.035
- Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017; 102(3):459–469. doi:10.1002/cpt.726
KEY POINTS
- Using current diagnostic criteria, the incidence of acute kidney injury complicating primary total joint arthroplasty may be nearly 10%, and 25% after placement of an antibiotic-loaded cement spacer to treat infection.
- In primary total joint arthroplasty, significant risk factors include older age, higher body mass index, chronic kidney disease, comorbidity, anemia, perioperative transfusion, aminoglycoside prophylaxis and treatment, preoperative heart murmur, and renin-angiotensin-aldosterone system blockade.
- Acute kidney injury may arise from infection, systemic administration of nephrotoxic antibiotics, and elution of antibiotics from antibiotic-loaded cement.
- No randomized controlled trial aimed at reducing acute kidney injury in these settings has been published; however, suggestions for practice modification are made based on the available data.
Unusual effects of common antibiotics
A 60-year-old man is admitted for respiratory failure following a massive myocardial infarction. He develops ventilator-associated pneumonia and is treated with cefepime and vancomycin. Three days later, he develops prolonged atypical absence seizures.
What caused these seizures? The neurologist thinks it might be the cefepime. Do you agree?
Antibiotics are widely used in the United States, with 269 million courses of oral therapy prescribed in 2011.1 Adverse effects such as rash are well known, but rare effects such as seizure, hypoglycemia, and hypoxemia may not be immediately attributed to these drugs.
In this article, we review less-recognized but potentially serious adverse effects of antibiotics commonly prescribed in the United States. We have structured our discussion by organ system for ease of reference.
NERVOUS SYSTEM
The potential adverse effects of antibiotics on the nervous system range from encephalopathy and seizure to nonconvulsive status epilepticus.
Encephalopathy and seizure
Encephalopathy has been reported with penicillins, cephalosporins, sulfamethoxazole-trimethoprim, quinolones, and oxazolidinones such as linezolid.2,3
Seizures are known to occur with penicillins, cephalosporins, carbapenems, and quinolones.2–4 For cephalosporins, these effects are more common at higher doses, in elderly patients, and in patients with renal impairment. Carbapenems are associated with seizure activity in elderly patients.2–4
Encephalopathy and seizure can also occur on a continuum, as is the case with piperacillin-induced encephalopathy, with progressive dysarthria, tremor, and progressive confusion culminating in tonic-clonic seizures.2
Nonconvulsive status epilepticus
Nonconvulsive status epilepticus, marked by prolonged atypical absence seizures, has complicated the use of penicillins, quinolones, clarithromycin, and cephalosporins, specifically cefepime.2,3,5 Diagnosis can be difficult and requires clinical awareness and confirmation with electroencephalography.
Class-specific neurologic effects
Certain antibiotics have class-specific effects:
Tetracyclines: cranial nerve toxicity, neuromuscular blockade, and intracranial hypertension.2
Sulfamethoxazole-trimethoprim: tremors and psychosis, with visual and auditory hallucinations.6
Macrolides: dysequilibrium and potentially irreversible hearing loss.2
Quinolones: orofacial dyskinesia and a Tourette-like syndrome, with a higher incidence reported with newer quinolones.7
Linezolid: optic and peripheral neuropathy2; neuropathy can be persistent and can lead to loss of vision. The package insert recommends monitoring visual function in patients taking linezolid for more than 3 months and in any patient reporting visual symptoms.8
Linezolid is also associated with serotonin syndrome when combined with a drug that potentiates serotonergic activity, most commonly selective serotonin reuptake inhibitors. The syndrome is characterized by a triad of cognitive or behavioral changes, autonomic instability, and neuromuscular excitability such as spontaneous clonus.9
Metronidazole: optic and peripheral neuropathy, in addition to cerebellar toxicity and central nervous system lesions on magnetic resonance imaging of the brain. In a series of 11 cases of cerebellar toxicity, most patients presented with ataxia and dysarthria associated with high total doses of metronidazole, and in most cases, magnetic resonance imaging showed resolution of the lesions upon discontinuation of metronidazole.10
HEMATOLOGIC AND RHEUMATOLOGIC EFFECTS
Agranulocytosis has been associated with beta-lactams, in most cases with prolonged exposure. In one report, the average exposure before onset of agranulocytosis was 22 days for nafcillin and 25 days for penicillin. For penicillins, more than 50% of cases involved high daily doses.11
Likewise, most episodes of vancomycin-induced neutropenia were reported to occur after 20 days of therapy.12
In another study, most cases of drug-induced anemia were due to ceftriaxone and piperacillin.13
Drug-induced thrombocytopenia has been described with penicillins, cephalosporins, sulfonamides, and vancomycin14 and is a well-recognized effect of linezolid. The syndrome of drug reaction with eosinophilia and systemic symptoms, a severe and rare adverse reaction, has been reported with minocycline, sulfamethoxazole, and vancomycin.15
The tetracycline minocycline has been reported to cause drug-induced lupus and polyarteritis nodosa-like vasculitis.16 Drug-induced lupus presents as myalgias and arthralgias, serositis, constitutional symptoms, and positive antinuclear antibody titers. The effect is not dose-dependent. Penicillin, cefuroxime, and nitrofurantoin have also been implicated.16
Kermani et al17 described 9 cases of polyarteritis nodosa, in which 5 patients (56%) had systemic involvement including renal artery microaneurysm, mononeuritis multiplex, and mesenteric vasculitis, and some of these patients also had cutaneous involvement. All patients had positive antineutrophil cytoplasmic antibody in a perinuclear pattern. The median time from start of the minocycline to symptom onset was 9 months, and the median duration of use was 2 years.
Quinolones have also been reported to cause fatal hypersensitivity vasculitis.18,19
CARDIOVASCULAR SYSTEM
Macrolides and quinolones have been reported to cause QT-interval prolongation and torsades de pointes. The risk is greatest when a macrolide is co-administered with a CYP3A4 inhibitor.
Of the macrolides, azithromycin is the safest, as clarithromycin and erythromycin are more likely to cause QT prolongation.
While QT prolongation is a class effect of quinolones, there is variability within the class. Ciprofloxacin is thought to be the safest in terms of cardiovascular adverse effects.20 In addition, Owens and Nolin20 reported that quinolone-associated QT prolongation was more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, and bradycardia, and especially in women. Other risk factors for QT prolongation with quinolone use include underlying cardiac disease and advanced age.21
Quinolones have also been associated with an increased risk of aortic dissection. The US Food and Drug Administration has issued a warning advising clinicians to avoid quinolones in patients who have aneurysms or are at risk for aneurysms, such as patients with advanced age, peripheral atherosclerotic vascular disease, hypertension and conditions such as Marfan and Ehlers-Danlos syndrome.22
DIGESTIVE SYSTEM
Tetracyclines are known to cause esophagitis from direct contact with and disruption of the mucosal lining. Doxycycline is the most frequent offender.23
Amoxicillin-clavulanate is the antibiotic most commonly associated with drug-induced liver injury, mainly attributable to the clavulanate component.24 It is more common in men over age 50 and with prolonged and repeated dosing and is sometimes fatal. Other adverse effects include Stevens-Johnson syndrome, interstitial nephritis, and thrombotic thrombocytopenic purpura.25
Cholestatic hepatitis has been reported with penicillins, particularly dicloxacillin, oxacillin, and amoxicillin-clavulanate; cephalosporins; doxycycline; sulfamethoxazole-trimethoprim; macrolides; and ciprofloxacin.24–26 Hepatocellular injury is linked to amoxicillin-clavulanate and doxycycline. Drug-induced mixed liver injury has been observed with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim and, rarely, cephalosporins.
Liver injury is classified as cholestatic if the alkaline phosphatase level is more than 2 times higher than normal, or if the ratio of alanine aminotransferase to alkaline phosphatase is less than 2; if the ratio is greater than 5, the injury is considered hepatocellular.24 Mixed liver injury, the most common, is defined as a ratio from 2 to 5.
Nitrofurantoin has also been linked to hepatotoxicity, cirrhosis, and end-stage liver disease, and to death if the drug is continued after the onset of jaundice.26 Death from liver injury has been reported with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, and erythromycin, and jaundice indicates a poor prognosis, associated with a 10% mortality rate or need for liver transplant in all patients.24
ENDOCRINE SYSTEM
Clarithromycin, sulfonamides, and quinolones are known to precipitate hypoglycemia by interacting with sulfonylureas. A study of Medicare patients age 66 or older who were taking glipizide or glyburide reported that female sex, older age, and a history of hypoglycemic episodes were associated with antibiotic-related hypoglycemia.27 The odds ratio for hypoglycemia was highest for clarithromycin (3.96), sulfamethoxazole-trimethoprim (2.56), metronidazole (2.11), and ciprofloxacin (1.62) when compared with antibiotics that do not cause hypoglycemia. There was no signal for levofloxacin-mediated hypoglycemia in this series.27
RESPIRATORY SYSTEM
Hypersensitivity lung disease has been reported with penicillin, ampicillin, cephalosporins, ciprofloxacin, and sulfonamides including sulfamethoxazole-trimethoprim.28 The lipopeptide daptomycin has been reported to cause acute eosinophilic pneumonia defined as fever for less than 5 days, pulmonary infiltrates, hypoxemia, and a bronchoalveolar lavage or biopsy study with eosinophils. Daptomycin should be stopped early in these cases, and the patient should not be rechallenged, as the reaction can be deadly.29
Nitrofurantoin has a long history of hypersensitivity pneumonitis in its acute form and a chronic allergic response. While more widely recognized, nitrofurantoin pulmonary toxicity is rare, occurring in 1 in 5,000 patients.30
RENAL SYSTEM
Acute interstitial nephritis has been reported with penicillins, cephalosporins, macrolides, quinolones, sulfonamides, and vancomycin.31–33 Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been seen with quinolones and sulfonamides.
Advanced age is an important risk factor for renal dysfunction from quinolones,18 and penicillin G has been reported to cause glomerulonephritis.31
MUSCULOSKELETAL SYSTEM
Quinolones have been associated with arthropathy or tendinitis at a rate of 1%, including cases of Achilles tendon rupture.18 The US Food and Drug Administration announced in 2016 that the serious adverse events with fluoroquinolones outweigh the benefits in patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infection, and that they should be used only if there are no other options.34
Daptomycin is known to cause elevations of creatine kinase.34 Weekly monitoring is recommended based on postmarketing data reports of elevations in 2.5% of patients; myopathy is a rarer effect, occurring in 0.2% of patients.35
REPRODUCTIVE SYSTEM
Antibiotics have long been reported to interact with oral contraceptives, but the data are not compelling for commonly used antibiotics. The strongest association is with rifampicin, which reduces oral contraceptive efficacy and warrants an alternative mode of contraception.36
BACK TO OUR PATIENT
Antibiotics can have serious adverse effects, and it is important for clinicians to be cognizant of this. Our 60-year-old patient who was taking cefepime and vancomycin for pneumonia developed prolonged atypical absence seizures. When the cefepime was discontinued, his mental status improved, and no other seizures were observed.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315(17):1864–1873. doi:10.1001/jama.2016.4151
- Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 2011; 72(3):381–393. doi:10.1111/j.1365-2125.2011.03991.x
- Dakdouki GK, Al-Awar GN. Cefepime-induced encephalopathy. Int J Infect Dis 2004; 8(1):59–61. pmid:14690782
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antiobiotics: doripenem, ceftobiprole, and cefepime. Infect Dis Clin North Am 2009; 23(4):983–999. doi:10.1016/j.idc.2009.06.007
- Bandettini di Poggio M, Anfosso S, Audenino D, Primavera A. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 2011; 18(3):313–318. doi:10.1016/j.jocn.2010.08.014
- Saidinejad M, Ewald MB, Shannon MW. Transient psychosis in an immune-competent patient after oral trimethoprim-sulfamethoxazole administration. Pediatrics 2005; 115(6):e739–e741. doi:10.1542/peds.2004-1352
- Thomas RJ, Reagan DR. Association of a Tourette-like syndrome with ofloxacin. Ann Pharmacother 1996; 30(2):138–141. doi:10.1177/106002809603000205
- Pharmacia and Upjohn Company LLC. Zyvox® Package Insert. http://labeling.pfizer.com/showlabeling.aspx?id=649. Accessed March 5, 2019.
- Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis 2006; 42(11):1578–1583. doi:10.1086/503839
- Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis 2008; 12(6):e111–e114. doi:10.1016/j.ijid.2008.03.006
- Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 2007; 146(9):657–665. pmid:17470834
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose- or duration-related? Ann Pharmacother 2011; 45(5):629–638. doi:10.1345/aph.1P583
- Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program 2009: 73–79. doi:10.1182/asheducation-2009.1.73
- Chong Bh, Choi PY, Khachigian L, Perdomo J. Drug-induced immune thrombocytopenia. Hematol Oncol Clin North Am 2013; 27(3):521–540. doi:10.1016/j.hoc.2013.02.003
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med 2011; 124(7):588–597. doi:10.1016/j.amjmed.2011.01.017
- Chang C, Gershwin ME. Drugs and autoimmunity—a contemporary review and mechanistic approach. J Autoimmun 2010; 34(3):J266–J275. doi:10.1016/j.jaut.2009.11.012
- Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum 2012; 42(2):213–221. doi:10.1016/j.semarthrit.2012.03.006
- Mandell LA, Ball P, Tillotson G. Antimicrobial safety and tolerability: differences and dilemmas. Clin Infect Dis 2001; 32(suppl 1):S72–S79. doi:10.1086/319379
- Christ W, Esch B. Session III: safety. Adverse reactions to fluoroquinolones in adults and children. Infect Dis Clin Pract 1994; 3(3 suppl 3):S168–S176.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43(12):1603–1611. doi:10.1086/508873
- Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother 2002; 49(4):593–596. pmid:11909831
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolones antibiotics in certain patients. https://www.fda.gov/Drugs/DrugSafety/ucm628753.htm. Accessed March 15, 2019.
- Seminerio J, McGrath K, Arnold CA, Voltaggio L, Singhi AD. Medication-associated lesions of the GI tract. Gastrointest Endosc 2014; 79(1):140–150. doi:10.1016/j.gie.2013.08.027
- Bjornsson ES, Jonasson JG. Drug-induced cholestasis. Clin Liver Dis 2013; 17(2):191–209. doi:10.1016/j.cld.2012.11.002
- Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci 2005; 50(10):1785–1790. doi:10.1007/s10620-005-2938-5
- Sakaan SA, Twilla JD, Usery JB, Winton JC, Self TH. Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication. South Med J 2014; 107(2):107–113. doi:10.1097/SMJ.0000000000000059
- Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med 2014; 174(10):1605–1612. doi:10.1001/jamainternmed.2014.3293
- Prasad R, Gupta P, Singh A, Goel N. Drug induced pulmonary parenchymal disease. Drug Discov Ther 2014; 8(6):232–237. doi:10.5582/ddt.2014.01046
- Miller BA, Gray A, Leblanc TW, Sexton DJ, Martin AR, Slama TG. Acute eosinophilic pneumonia secondary to daptomycin: a report of three cases. Clin Infect Dis 2010; 50(11):e63–e68. doi:10.1086/652656
- Kabbara WK, Kordahi MC. Nitrofurantoin-induced pulmonary toxicity: a case report and review of the literature. J Infect Public Health 2015; 8(4):309–313. doi:10.1016/j.jiph.2015.01.007
- Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. J Renal Inj Prev 2015; 4(3):57–60. doi:10.12861/jrip.2015.12
- Mac K, Chavada R, Paull S, Howlin K, Wong J. Cefepime induced acute interstitial nephritis—a case report. BMC Nephrol 2015; 16:15. doi:10.1186/s12882-015-0004-x
- Woodruff AE, Meaney CJ, Hansen EA, Prescott GM. Azithromycin-induced, biopsy-proven cute interstitial nephritis in an adult successfully treated with low-dose corticosteroids. Pharmacotherapy 2015; 35(11):e169–e174. doi:10.1002/phar.1660
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed March 7, 2019.
- Hawkey PM. Pre-clinical experience with daptomycin. J Antimicrob Chemother 2008; 62(suppl 3):iii7–iii14. doi:10.1093/jac/dkn367
- ACOG Committee on Practice Bulletins–Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2006; 107(6):1453–1472. pmid:16738183
A 60-year-old man is admitted for respiratory failure following a massive myocardial infarction. He develops ventilator-associated pneumonia and is treated with cefepime and vancomycin. Three days later, he develops prolonged atypical absence seizures.
What caused these seizures? The neurologist thinks it might be the cefepime. Do you agree?
Antibiotics are widely used in the United States, with 269 million courses of oral therapy prescribed in 2011.1 Adverse effects such as rash are well known, but rare effects such as seizure, hypoglycemia, and hypoxemia may not be immediately attributed to these drugs.
In this article, we review less-recognized but potentially serious adverse effects of antibiotics commonly prescribed in the United States. We have structured our discussion by organ system for ease of reference.
NERVOUS SYSTEM
The potential adverse effects of antibiotics on the nervous system range from encephalopathy and seizure to nonconvulsive status epilepticus.
Encephalopathy and seizure
Encephalopathy has been reported with penicillins, cephalosporins, sulfamethoxazole-trimethoprim, quinolones, and oxazolidinones such as linezolid.2,3
Seizures are known to occur with penicillins, cephalosporins, carbapenems, and quinolones.2–4 For cephalosporins, these effects are more common at higher doses, in elderly patients, and in patients with renal impairment. Carbapenems are associated with seizure activity in elderly patients.2–4
Encephalopathy and seizure can also occur on a continuum, as is the case with piperacillin-induced encephalopathy, with progressive dysarthria, tremor, and progressive confusion culminating in tonic-clonic seizures.2
Nonconvulsive status epilepticus
Nonconvulsive status epilepticus, marked by prolonged atypical absence seizures, has complicated the use of penicillins, quinolones, clarithromycin, and cephalosporins, specifically cefepime.2,3,5 Diagnosis can be difficult and requires clinical awareness and confirmation with electroencephalography.
Class-specific neurologic effects
Certain antibiotics have class-specific effects:
Tetracyclines: cranial nerve toxicity, neuromuscular blockade, and intracranial hypertension.2
Sulfamethoxazole-trimethoprim: tremors and psychosis, with visual and auditory hallucinations.6
Macrolides: dysequilibrium and potentially irreversible hearing loss.2
Quinolones: orofacial dyskinesia and a Tourette-like syndrome, with a higher incidence reported with newer quinolones.7
Linezolid: optic and peripheral neuropathy2; neuropathy can be persistent and can lead to loss of vision. The package insert recommends monitoring visual function in patients taking linezolid for more than 3 months and in any patient reporting visual symptoms.8
Linezolid is also associated with serotonin syndrome when combined with a drug that potentiates serotonergic activity, most commonly selective serotonin reuptake inhibitors. The syndrome is characterized by a triad of cognitive or behavioral changes, autonomic instability, and neuromuscular excitability such as spontaneous clonus.9
Metronidazole: optic and peripheral neuropathy, in addition to cerebellar toxicity and central nervous system lesions on magnetic resonance imaging of the brain. In a series of 11 cases of cerebellar toxicity, most patients presented with ataxia and dysarthria associated with high total doses of metronidazole, and in most cases, magnetic resonance imaging showed resolution of the lesions upon discontinuation of metronidazole.10
HEMATOLOGIC AND RHEUMATOLOGIC EFFECTS
Agranulocytosis has been associated with beta-lactams, in most cases with prolonged exposure. In one report, the average exposure before onset of agranulocytosis was 22 days for nafcillin and 25 days for penicillin. For penicillins, more than 50% of cases involved high daily doses.11
Likewise, most episodes of vancomycin-induced neutropenia were reported to occur after 20 days of therapy.12
In another study, most cases of drug-induced anemia were due to ceftriaxone and piperacillin.13
Drug-induced thrombocytopenia has been described with penicillins, cephalosporins, sulfonamides, and vancomycin14 and is a well-recognized effect of linezolid. The syndrome of drug reaction with eosinophilia and systemic symptoms, a severe and rare adverse reaction, has been reported with minocycline, sulfamethoxazole, and vancomycin.15
The tetracycline minocycline has been reported to cause drug-induced lupus and polyarteritis nodosa-like vasculitis.16 Drug-induced lupus presents as myalgias and arthralgias, serositis, constitutional symptoms, and positive antinuclear antibody titers. The effect is not dose-dependent. Penicillin, cefuroxime, and nitrofurantoin have also been implicated.16
Kermani et al17 described 9 cases of polyarteritis nodosa, in which 5 patients (56%) had systemic involvement including renal artery microaneurysm, mononeuritis multiplex, and mesenteric vasculitis, and some of these patients also had cutaneous involvement. All patients had positive antineutrophil cytoplasmic antibody in a perinuclear pattern. The median time from start of the minocycline to symptom onset was 9 months, and the median duration of use was 2 years.
Quinolones have also been reported to cause fatal hypersensitivity vasculitis.18,19
CARDIOVASCULAR SYSTEM
Macrolides and quinolones have been reported to cause QT-interval prolongation and torsades de pointes. The risk is greatest when a macrolide is co-administered with a CYP3A4 inhibitor.
Of the macrolides, azithromycin is the safest, as clarithromycin and erythromycin are more likely to cause QT prolongation.
While QT prolongation is a class effect of quinolones, there is variability within the class. Ciprofloxacin is thought to be the safest in terms of cardiovascular adverse effects.20 In addition, Owens and Nolin20 reported that quinolone-associated QT prolongation was more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, and bradycardia, and especially in women. Other risk factors for QT prolongation with quinolone use include underlying cardiac disease and advanced age.21
Quinolones have also been associated with an increased risk of aortic dissection. The US Food and Drug Administration has issued a warning advising clinicians to avoid quinolones in patients who have aneurysms or are at risk for aneurysms, such as patients with advanced age, peripheral atherosclerotic vascular disease, hypertension and conditions such as Marfan and Ehlers-Danlos syndrome.22
DIGESTIVE SYSTEM
Tetracyclines are known to cause esophagitis from direct contact with and disruption of the mucosal lining. Doxycycline is the most frequent offender.23
Amoxicillin-clavulanate is the antibiotic most commonly associated with drug-induced liver injury, mainly attributable to the clavulanate component.24 It is more common in men over age 50 and with prolonged and repeated dosing and is sometimes fatal. Other adverse effects include Stevens-Johnson syndrome, interstitial nephritis, and thrombotic thrombocytopenic purpura.25
Cholestatic hepatitis has been reported with penicillins, particularly dicloxacillin, oxacillin, and amoxicillin-clavulanate; cephalosporins; doxycycline; sulfamethoxazole-trimethoprim; macrolides; and ciprofloxacin.24–26 Hepatocellular injury is linked to amoxicillin-clavulanate and doxycycline. Drug-induced mixed liver injury has been observed with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim and, rarely, cephalosporins.
Liver injury is classified as cholestatic if the alkaline phosphatase level is more than 2 times higher than normal, or if the ratio of alanine aminotransferase to alkaline phosphatase is less than 2; if the ratio is greater than 5, the injury is considered hepatocellular.24 Mixed liver injury, the most common, is defined as a ratio from 2 to 5.
Nitrofurantoin has also been linked to hepatotoxicity, cirrhosis, and end-stage liver disease, and to death if the drug is continued after the onset of jaundice.26 Death from liver injury has been reported with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, and erythromycin, and jaundice indicates a poor prognosis, associated with a 10% mortality rate or need for liver transplant in all patients.24
ENDOCRINE SYSTEM
Clarithromycin, sulfonamides, and quinolones are known to precipitate hypoglycemia by interacting with sulfonylureas. A study of Medicare patients age 66 or older who were taking glipizide or glyburide reported that female sex, older age, and a history of hypoglycemic episodes were associated with antibiotic-related hypoglycemia.27 The odds ratio for hypoglycemia was highest for clarithromycin (3.96), sulfamethoxazole-trimethoprim (2.56), metronidazole (2.11), and ciprofloxacin (1.62) when compared with antibiotics that do not cause hypoglycemia. There was no signal for levofloxacin-mediated hypoglycemia in this series.27
RESPIRATORY SYSTEM
Hypersensitivity lung disease has been reported with penicillin, ampicillin, cephalosporins, ciprofloxacin, and sulfonamides including sulfamethoxazole-trimethoprim.28 The lipopeptide daptomycin has been reported to cause acute eosinophilic pneumonia defined as fever for less than 5 days, pulmonary infiltrates, hypoxemia, and a bronchoalveolar lavage or biopsy study with eosinophils. Daptomycin should be stopped early in these cases, and the patient should not be rechallenged, as the reaction can be deadly.29
Nitrofurantoin has a long history of hypersensitivity pneumonitis in its acute form and a chronic allergic response. While more widely recognized, nitrofurantoin pulmonary toxicity is rare, occurring in 1 in 5,000 patients.30
RENAL SYSTEM
Acute interstitial nephritis has been reported with penicillins, cephalosporins, macrolides, quinolones, sulfonamides, and vancomycin.31–33 Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been seen with quinolones and sulfonamides.
Advanced age is an important risk factor for renal dysfunction from quinolones,18 and penicillin G has been reported to cause glomerulonephritis.31
MUSCULOSKELETAL SYSTEM
Quinolones have been associated with arthropathy or tendinitis at a rate of 1%, including cases of Achilles tendon rupture.18 The US Food and Drug Administration announced in 2016 that the serious adverse events with fluoroquinolones outweigh the benefits in patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infection, and that they should be used only if there are no other options.34
Daptomycin is known to cause elevations of creatine kinase.34 Weekly monitoring is recommended based on postmarketing data reports of elevations in 2.5% of patients; myopathy is a rarer effect, occurring in 0.2% of patients.35
REPRODUCTIVE SYSTEM
Antibiotics have long been reported to interact with oral contraceptives, but the data are not compelling for commonly used antibiotics. The strongest association is with rifampicin, which reduces oral contraceptive efficacy and warrants an alternative mode of contraception.36
BACK TO OUR PATIENT
Antibiotics can have serious adverse effects, and it is important for clinicians to be cognizant of this. Our 60-year-old patient who was taking cefepime and vancomycin for pneumonia developed prolonged atypical absence seizures. When the cefepime was discontinued, his mental status improved, and no other seizures were observed.
A 60-year-old man is admitted for respiratory failure following a massive myocardial infarction. He develops ventilator-associated pneumonia and is treated with cefepime and vancomycin. Three days later, he develops prolonged atypical absence seizures.
What caused these seizures? The neurologist thinks it might be the cefepime. Do you agree?
Antibiotics are widely used in the United States, with 269 million courses of oral therapy prescribed in 2011.1 Adverse effects such as rash are well known, but rare effects such as seizure, hypoglycemia, and hypoxemia may not be immediately attributed to these drugs.
In this article, we review less-recognized but potentially serious adverse effects of antibiotics commonly prescribed in the United States. We have structured our discussion by organ system for ease of reference.
NERVOUS SYSTEM
The potential adverse effects of antibiotics on the nervous system range from encephalopathy and seizure to nonconvulsive status epilepticus.
Encephalopathy and seizure
Encephalopathy has been reported with penicillins, cephalosporins, sulfamethoxazole-trimethoprim, quinolones, and oxazolidinones such as linezolid.2,3
Seizures are known to occur with penicillins, cephalosporins, carbapenems, and quinolones.2–4 For cephalosporins, these effects are more common at higher doses, in elderly patients, and in patients with renal impairment. Carbapenems are associated with seizure activity in elderly patients.2–4
Encephalopathy and seizure can also occur on a continuum, as is the case with piperacillin-induced encephalopathy, with progressive dysarthria, tremor, and progressive confusion culminating in tonic-clonic seizures.2
Nonconvulsive status epilepticus
Nonconvulsive status epilepticus, marked by prolonged atypical absence seizures, has complicated the use of penicillins, quinolones, clarithromycin, and cephalosporins, specifically cefepime.2,3,5 Diagnosis can be difficult and requires clinical awareness and confirmation with electroencephalography.
Class-specific neurologic effects
Certain antibiotics have class-specific effects:
Tetracyclines: cranial nerve toxicity, neuromuscular blockade, and intracranial hypertension.2
Sulfamethoxazole-trimethoprim: tremors and psychosis, with visual and auditory hallucinations.6
Macrolides: dysequilibrium and potentially irreversible hearing loss.2
Quinolones: orofacial dyskinesia and a Tourette-like syndrome, with a higher incidence reported with newer quinolones.7
Linezolid: optic and peripheral neuropathy2; neuropathy can be persistent and can lead to loss of vision. The package insert recommends monitoring visual function in patients taking linezolid for more than 3 months and in any patient reporting visual symptoms.8
Linezolid is also associated with serotonin syndrome when combined with a drug that potentiates serotonergic activity, most commonly selective serotonin reuptake inhibitors. The syndrome is characterized by a triad of cognitive or behavioral changes, autonomic instability, and neuromuscular excitability such as spontaneous clonus.9
Metronidazole: optic and peripheral neuropathy, in addition to cerebellar toxicity and central nervous system lesions on magnetic resonance imaging of the brain. In a series of 11 cases of cerebellar toxicity, most patients presented with ataxia and dysarthria associated with high total doses of metronidazole, and in most cases, magnetic resonance imaging showed resolution of the lesions upon discontinuation of metronidazole.10
HEMATOLOGIC AND RHEUMATOLOGIC EFFECTS
Agranulocytosis has been associated with beta-lactams, in most cases with prolonged exposure. In one report, the average exposure before onset of agranulocytosis was 22 days for nafcillin and 25 days for penicillin. For penicillins, more than 50% of cases involved high daily doses.11
Likewise, most episodes of vancomycin-induced neutropenia were reported to occur after 20 days of therapy.12
In another study, most cases of drug-induced anemia were due to ceftriaxone and piperacillin.13
Drug-induced thrombocytopenia has been described with penicillins, cephalosporins, sulfonamides, and vancomycin14 and is a well-recognized effect of linezolid. The syndrome of drug reaction with eosinophilia and systemic symptoms, a severe and rare adverse reaction, has been reported with minocycline, sulfamethoxazole, and vancomycin.15
The tetracycline minocycline has been reported to cause drug-induced lupus and polyarteritis nodosa-like vasculitis.16 Drug-induced lupus presents as myalgias and arthralgias, serositis, constitutional symptoms, and positive antinuclear antibody titers. The effect is not dose-dependent. Penicillin, cefuroxime, and nitrofurantoin have also been implicated.16
Kermani et al17 described 9 cases of polyarteritis nodosa, in which 5 patients (56%) had systemic involvement including renal artery microaneurysm, mononeuritis multiplex, and mesenteric vasculitis, and some of these patients also had cutaneous involvement. All patients had positive antineutrophil cytoplasmic antibody in a perinuclear pattern. The median time from start of the minocycline to symptom onset was 9 months, and the median duration of use was 2 years.
Quinolones have also been reported to cause fatal hypersensitivity vasculitis.18,19
CARDIOVASCULAR SYSTEM
Macrolides and quinolones have been reported to cause QT-interval prolongation and torsades de pointes. The risk is greatest when a macrolide is co-administered with a CYP3A4 inhibitor.
Of the macrolides, azithromycin is the safest, as clarithromycin and erythromycin are more likely to cause QT prolongation.
While QT prolongation is a class effect of quinolones, there is variability within the class. Ciprofloxacin is thought to be the safest in terms of cardiovascular adverse effects.20 In addition, Owens and Nolin20 reported that quinolone-associated QT prolongation was more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, and bradycardia, and especially in women. Other risk factors for QT prolongation with quinolone use include underlying cardiac disease and advanced age.21
Quinolones have also been associated with an increased risk of aortic dissection. The US Food and Drug Administration has issued a warning advising clinicians to avoid quinolones in patients who have aneurysms or are at risk for aneurysms, such as patients with advanced age, peripheral atherosclerotic vascular disease, hypertension and conditions such as Marfan and Ehlers-Danlos syndrome.22
DIGESTIVE SYSTEM
Tetracyclines are known to cause esophagitis from direct contact with and disruption of the mucosal lining. Doxycycline is the most frequent offender.23
Amoxicillin-clavulanate is the antibiotic most commonly associated with drug-induced liver injury, mainly attributable to the clavulanate component.24 It is more common in men over age 50 and with prolonged and repeated dosing and is sometimes fatal. Other adverse effects include Stevens-Johnson syndrome, interstitial nephritis, and thrombotic thrombocytopenic purpura.25
Cholestatic hepatitis has been reported with penicillins, particularly dicloxacillin, oxacillin, and amoxicillin-clavulanate; cephalosporins; doxycycline; sulfamethoxazole-trimethoprim; macrolides; and ciprofloxacin.24–26 Hepatocellular injury is linked to amoxicillin-clavulanate and doxycycline. Drug-induced mixed liver injury has been observed with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim and, rarely, cephalosporins.
Liver injury is classified as cholestatic if the alkaline phosphatase level is more than 2 times higher than normal, or if the ratio of alanine aminotransferase to alkaline phosphatase is less than 2; if the ratio is greater than 5, the injury is considered hepatocellular.24 Mixed liver injury, the most common, is defined as a ratio from 2 to 5.
Nitrofurantoin has also been linked to hepatotoxicity, cirrhosis, and end-stage liver disease, and to death if the drug is continued after the onset of jaundice.26 Death from liver injury has been reported with amoxicillin-clavulanate, sulfamethoxazole-trimethoprim, and erythromycin, and jaundice indicates a poor prognosis, associated with a 10% mortality rate or need for liver transplant in all patients.24
ENDOCRINE SYSTEM
Clarithromycin, sulfonamides, and quinolones are known to precipitate hypoglycemia by interacting with sulfonylureas. A study of Medicare patients age 66 or older who were taking glipizide or glyburide reported that female sex, older age, and a history of hypoglycemic episodes were associated with antibiotic-related hypoglycemia.27 The odds ratio for hypoglycemia was highest for clarithromycin (3.96), sulfamethoxazole-trimethoprim (2.56), metronidazole (2.11), and ciprofloxacin (1.62) when compared with antibiotics that do not cause hypoglycemia. There was no signal for levofloxacin-mediated hypoglycemia in this series.27
RESPIRATORY SYSTEM
Hypersensitivity lung disease has been reported with penicillin, ampicillin, cephalosporins, ciprofloxacin, and sulfonamides including sulfamethoxazole-trimethoprim.28 The lipopeptide daptomycin has been reported to cause acute eosinophilic pneumonia defined as fever for less than 5 days, pulmonary infiltrates, hypoxemia, and a bronchoalveolar lavage or biopsy study with eosinophils. Daptomycin should be stopped early in these cases, and the patient should not be rechallenged, as the reaction can be deadly.29
Nitrofurantoin has a long history of hypersensitivity pneumonitis in its acute form and a chronic allergic response. While more widely recognized, nitrofurantoin pulmonary toxicity is rare, occurring in 1 in 5,000 patients.30
RENAL SYSTEM
Acute interstitial nephritis has been reported with penicillins, cephalosporins, macrolides, quinolones, sulfonamides, and vancomycin.31–33 Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been seen with quinolones and sulfonamides.
Advanced age is an important risk factor for renal dysfunction from quinolones,18 and penicillin G has been reported to cause glomerulonephritis.31
MUSCULOSKELETAL SYSTEM
Quinolones have been associated with arthropathy or tendinitis at a rate of 1%, including cases of Achilles tendon rupture.18 The US Food and Drug Administration announced in 2016 that the serious adverse events with fluoroquinolones outweigh the benefits in patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infection, and that they should be used only if there are no other options.34
Daptomycin is known to cause elevations of creatine kinase.34 Weekly monitoring is recommended based on postmarketing data reports of elevations in 2.5% of patients; myopathy is a rarer effect, occurring in 0.2% of patients.35
REPRODUCTIVE SYSTEM
Antibiotics have long been reported to interact with oral contraceptives, but the data are not compelling for commonly used antibiotics. The strongest association is with rifampicin, which reduces oral contraceptive efficacy and warrants an alternative mode of contraception.36
BACK TO OUR PATIENT
Antibiotics can have serious adverse effects, and it is important for clinicians to be cognizant of this. Our 60-year-old patient who was taking cefepime and vancomycin for pneumonia developed prolonged atypical absence seizures. When the cefepime was discontinued, his mental status improved, and no other seizures were observed.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315(17):1864–1873. doi:10.1001/jama.2016.4151
- Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 2011; 72(3):381–393. doi:10.1111/j.1365-2125.2011.03991.x
- Dakdouki GK, Al-Awar GN. Cefepime-induced encephalopathy. Int J Infect Dis 2004; 8(1):59–61. pmid:14690782
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antiobiotics: doripenem, ceftobiprole, and cefepime. Infect Dis Clin North Am 2009; 23(4):983–999. doi:10.1016/j.idc.2009.06.007
- Bandettini di Poggio M, Anfosso S, Audenino D, Primavera A. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 2011; 18(3):313–318. doi:10.1016/j.jocn.2010.08.014
- Saidinejad M, Ewald MB, Shannon MW. Transient psychosis in an immune-competent patient after oral trimethoprim-sulfamethoxazole administration. Pediatrics 2005; 115(6):e739–e741. doi:10.1542/peds.2004-1352
- Thomas RJ, Reagan DR. Association of a Tourette-like syndrome with ofloxacin. Ann Pharmacother 1996; 30(2):138–141. doi:10.1177/106002809603000205
- Pharmacia and Upjohn Company LLC. Zyvox® Package Insert. http://labeling.pfizer.com/showlabeling.aspx?id=649. Accessed March 5, 2019.
- Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis 2006; 42(11):1578–1583. doi:10.1086/503839
- Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis 2008; 12(6):e111–e114. doi:10.1016/j.ijid.2008.03.006
- Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 2007; 146(9):657–665. pmid:17470834
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose- or duration-related? Ann Pharmacother 2011; 45(5):629–638. doi:10.1345/aph.1P583
- Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program 2009: 73–79. doi:10.1182/asheducation-2009.1.73
- Chong Bh, Choi PY, Khachigian L, Perdomo J. Drug-induced immune thrombocytopenia. Hematol Oncol Clin North Am 2013; 27(3):521–540. doi:10.1016/j.hoc.2013.02.003
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med 2011; 124(7):588–597. doi:10.1016/j.amjmed.2011.01.017
- Chang C, Gershwin ME. Drugs and autoimmunity—a contemporary review and mechanistic approach. J Autoimmun 2010; 34(3):J266–J275. doi:10.1016/j.jaut.2009.11.012
- Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum 2012; 42(2):213–221. doi:10.1016/j.semarthrit.2012.03.006
- Mandell LA, Ball P, Tillotson G. Antimicrobial safety and tolerability: differences and dilemmas. Clin Infect Dis 2001; 32(suppl 1):S72–S79. doi:10.1086/319379
- Christ W, Esch B. Session III: safety. Adverse reactions to fluoroquinolones in adults and children. Infect Dis Clin Pract 1994; 3(3 suppl 3):S168–S176.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43(12):1603–1611. doi:10.1086/508873
- Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother 2002; 49(4):593–596. pmid:11909831
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolones antibiotics in certain patients. https://www.fda.gov/Drugs/DrugSafety/ucm628753.htm. Accessed March 15, 2019.
- Seminerio J, McGrath K, Arnold CA, Voltaggio L, Singhi AD. Medication-associated lesions of the GI tract. Gastrointest Endosc 2014; 79(1):140–150. doi:10.1016/j.gie.2013.08.027
- Bjornsson ES, Jonasson JG. Drug-induced cholestasis. Clin Liver Dis 2013; 17(2):191–209. doi:10.1016/j.cld.2012.11.002
- Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci 2005; 50(10):1785–1790. doi:10.1007/s10620-005-2938-5
- Sakaan SA, Twilla JD, Usery JB, Winton JC, Self TH. Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication. South Med J 2014; 107(2):107–113. doi:10.1097/SMJ.0000000000000059
- Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med 2014; 174(10):1605–1612. doi:10.1001/jamainternmed.2014.3293
- Prasad R, Gupta P, Singh A, Goel N. Drug induced pulmonary parenchymal disease. Drug Discov Ther 2014; 8(6):232–237. doi:10.5582/ddt.2014.01046
- Miller BA, Gray A, Leblanc TW, Sexton DJ, Martin AR, Slama TG. Acute eosinophilic pneumonia secondary to daptomycin: a report of three cases. Clin Infect Dis 2010; 50(11):e63–e68. doi:10.1086/652656
- Kabbara WK, Kordahi MC. Nitrofurantoin-induced pulmonary toxicity: a case report and review of the literature. J Infect Public Health 2015; 8(4):309–313. doi:10.1016/j.jiph.2015.01.007
- Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. J Renal Inj Prev 2015; 4(3):57–60. doi:10.12861/jrip.2015.12
- Mac K, Chavada R, Paull S, Howlin K, Wong J. Cefepime induced acute interstitial nephritis—a case report. BMC Nephrol 2015; 16:15. doi:10.1186/s12882-015-0004-x
- Woodruff AE, Meaney CJ, Hansen EA, Prescott GM. Azithromycin-induced, biopsy-proven cute interstitial nephritis in an adult successfully treated with low-dose corticosteroids. Pharmacotherapy 2015; 35(11):e169–e174. doi:10.1002/phar.1660
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed March 7, 2019.
- Hawkey PM. Pre-clinical experience with daptomycin. J Antimicrob Chemother 2008; 62(suppl 3):iii7–iii14. doi:10.1093/jac/dkn367
- ACOG Committee on Practice Bulletins–Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2006; 107(6):1453–1472. pmid:16738183
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315(17):1864–1873. doi:10.1001/jama.2016.4151
- Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 2011; 72(3):381–393. doi:10.1111/j.1365-2125.2011.03991.x
- Dakdouki GK, Al-Awar GN. Cefepime-induced encephalopathy. Int J Infect Dis 2004; 8(1):59–61. pmid:14690782
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antiobiotics: doripenem, ceftobiprole, and cefepime. Infect Dis Clin North Am 2009; 23(4):983–999. doi:10.1016/j.idc.2009.06.007
- Bandettini di Poggio M, Anfosso S, Audenino D, Primavera A. Clarithromycin-induced neurotoxicity in adults. J Clin Neurosci 2011; 18(3):313–318. doi:10.1016/j.jocn.2010.08.014
- Saidinejad M, Ewald MB, Shannon MW. Transient psychosis in an immune-competent patient after oral trimethoprim-sulfamethoxazole administration. Pediatrics 2005; 115(6):e739–e741. doi:10.1542/peds.2004-1352
- Thomas RJ, Reagan DR. Association of a Tourette-like syndrome with ofloxacin. Ann Pharmacother 1996; 30(2):138–141. doi:10.1177/106002809603000205
- Pharmacia and Upjohn Company LLC. Zyvox® Package Insert. http://labeling.pfizer.com/showlabeling.aspx?id=649. Accessed March 5, 2019.
- Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis 2006; 42(11):1578–1583. doi:10.1086/503839
- Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis 2008; 12(6):e111–e114. doi:10.1016/j.ijid.2008.03.006
- Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 2007; 146(9):657–665. pmid:17470834
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose- or duration-related? Ann Pharmacother 2011; 45(5):629–638. doi:10.1345/aph.1P583
- Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program 2009: 73–79. doi:10.1182/asheducation-2009.1.73
- Chong Bh, Choi PY, Khachigian L, Perdomo J. Drug-induced immune thrombocytopenia. Hematol Oncol Clin North Am 2013; 27(3):521–540. doi:10.1016/j.hoc.2013.02.003
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med 2011; 124(7):588–597. doi:10.1016/j.amjmed.2011.01.017
- Chang C, Gershwin ME. Drugs and autoimmunity—a contemporary review and mechanistic approach. J Autoimmun 2010; 34(3):J266–J275. doi:10.1016/j.jaut.2009.11.012
- Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum 2012; 42(2):213–221. doi:10.1016/j.semarthrit.2012.03.006
- Mandell LA, Ball P, Tillotson G. Antimicrobial safety and tolerability: differences and dilemmas. Clin Infect Dis 2001; 32(suppl 1):S72–S79. doi:10.1086/319379
- Christ W, Esch B. Session III: safety. Adverse reactions to fluoroquinolones in adults and children. Infect Dis Clin Pract 1994; 3(3 suppl 3):S168–S176.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 2006; 43(12):1603–1611. doi:10.1086/508873
- Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother 2002; 49(4):593–596. pmid:11909831
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolones antibiotics in certain patients. https://www.fda.gov/Drugs/DrugSafety/ucm628753.htm. Accessed March 15, 2019.
- Seminerio J, McGrath K, Arnold CA, Voltaggio L, Singhi AD. Medication-associated lesions of the GI tract. Gastrointest Endosc 2014; 79(1):140–150. doi:10.1016/j.gie.2013.08.027
- Bjornsson ES, Jonasson JG. Drug-induced cholestasis. Clin Liver Dis 2013; 17(2):191–209. doi:10.1016/j.cld.2012.11.002
- Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci 2005; 50(10):1785–1790. doi:10.1007/s10620-005-2938-5
- Sakaan SA, Twilla JD, Usery JB, Winton JC, Self TH. Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication. South Med J 2014; 107(2):107–113. doi:10.1097/SMJ.0000000000000059
- Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med 2014; 174(10):1605–1612. doi:10.1001/jamainternmed.2014.3293
- Prasad R, Gupta P, Singh A, Goel N. Drug induced pulmonary parenchymal disease. Drug Discov Ther 2014; 8(6):232–237. doi:10.5582/ddt.2014.01046
- Miller BA, Gray A, Leblanc TW, Sexton DJ, Martin AR, Slama TG. Acute eosinophilic pneumonia secondary to daptomycin: a report of three cases. Clin Infect Dis 2010; 50(11):e63–e68. doi:10.1086/652656
- Kabbara WK, Kordahi MC. Nitrofurantoin-induced pulmonary toxicity: a case report and review of the literature. J Infect Public Health 2015; 8(4):309–313. doi:10.1016/j.jiph.2015.01.007
- Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. J Renal Inj Prev 2015; 4(3):57–60. doi:10.12861/jrip.2015.12
- Mac K, Chavada R, Paull S, Howlin K, Wong J. Cefepime induced acute interstitial nephritis—a case report. BMC Nephrol 2015; 16:15. doi:10.1186/s12882-015-0004-x
- Woodruff AE, Meaney CJ, Hansen EA, Prescott GM. Azithromycin-induced, biopsy-proven cute interstitial nephritis in an adult successfully treated with low-dose corticosteroids. Pharmacotherapy 2015; 35(11):e169–e174. doi:10.1002/phar.1660
- US Food and Drug Administration (FDA). FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed March 7, 2019.
- Hawkey PM. Pre-clinical experience with daptomycin. J Antimicrob Chemother 2008; 62(suppl 3):iii7–iii14. doi:10.1093/jac/dkn367
- ACOG Committee on Practice Bulletins–Gynecology. ACOG practice bulletin. No. 73: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 2006; 107(6):1453–1472. pmid:16738183
KEY POINTS
- Piperacillin-induced encephalopathy and seizure can occur on a continuum, with progressive dysarthria, tremor, and confusion culminating in tonic-clonic seizures.
- Monocycline-induced lupus can present as myalgia, arthralgia, serositis, constitutional symptoms, and a positive antinuclear antibody titer. The effect is not dose-dependent.
- Acute tubular necrosis has been linked to cephalosporins and tetracyclines. Crystal nephropathy has been reported with quinolones and sulfonamides.
- QT-interval prolongation is a class effect of quinolones and is more likely to occur in patients with pre-existing QT prolongation, electrolyte abnormalities, organic heart disease, or bradycardia, or in women.
Flu shot can be given irrespective of the time of last methotrexate dose
Immune response to influenza vaccination in rheumatoid arthritis patients taking methotrexate appears to depend most on stopping the next two weekly doses of the drug rather than any effect from the timing of the last dose, new research concludes.
The new finding, reported in Annals of the Rheumatic Diseases, stems from a post hoc analysis of a randomized, controlled trial that Jin Kyun Park, MD, of Seoul (Korea) National University, and his colleagues had conducted earlier on immune response when patients stopped methotrexate for either 2 or 4 weeks after vaccination. While the main endpoint of that study showed no difference in the improvement in vaccine response with either stopping methotrexate for 2 or 4 weeks and no increase in disease activity with stopping for 2 weeks, it was unclear whether the timing of the last dose mattered when stopping for 2 weeks.
In a bid to identify the optimal time between the last dose of methotrexate and administration of a flu vaccine, Dr. Park and his colleagues conducted a post hoc analysis of the trial, which involved 316 patients with RA receiving methotrexate for 6 weeks or longer to continue (n = 156) or to hold methotrexate (n = 160) for 2 weeks after receiving a quadrivalent influenza vaccine containing H1N1, H3N2, B-Yamagata, and B-Victoria.
The study authors defined a positive vaccine response as a fourfold or greater increase in hemagglutination inhibition (HI) antibody titer. A satisfactory vaccine response was a positive response to two or more of four vaccine antigens.
Patients who stopped taking methotrexate were divided into eight subgroups according to the number of days between their last dose and their vaccination.
The research team reported that response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
However, they conceded that “the absence of impact of the number of days between the last methotrexate dose and vaccination could be due to the small patient numbers in eight subgroups.”
Vaccine response also did not differ between patients who received the influenza vaccination within 3 days of the last methotrexate dose (n = 65) and those who received it between 4-7 days of the last methotrexate dose (n = 95).
Furthermore, RA disease activity, seropositivity, or use of conventional or biologic disease-modifying antirheumatic drugs did not have an impact on methotrexate discontinuation.
The authors concluded that vaccinations could be given irrespective of the time of the last methotrexate dose, and patients should be advised to skip two weekly doses following vaccination.
“This supports the notion that the effects of methotrexate on humeral immunity occur rapidly, despite the delayed effects on arthritis; therefore, the absence of methotrexate during the first 2 weeks postvaccination is critical for humoral immunity,” they wrote.
The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
SOURCE: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187.
Immune response to influenza vaccination in rheumatoid arthritis patients taking methotrexate appears to depend most on stopping the next two weekly doses of the drug rather than any effect from the timing of the last dose, new research concludes.
The new finding, reported in Annals of the Rheumatic Diseases, stems from a post hoc analysis of a randomized, controlled trial that Jin Kyun Park, MD, of Seoul (Korea) National University, and his colleagues had conducted earlier on immune response when patients stopped methotrexate for either 2 or 4 weeks after vaccination. While the main endpoint of that study showed no difference in the improvement in vaccine response with either stopping methotrexate for 2 or 4 weeks and no increase in disease activity with stopping for 2 weeks, it was unclear whether the timing of the last dose mattered when stopping for 2 weeks.
In a bid to identify the optimal time between the last dose of methotrexate and administration of a flu vaccine, Dr. Park and his colleagues conducted a post hoc analysis of the trial, which involved 316 patients with RA receiving methotrexate for 6 weeks or longer to continue (n = 156) or to hold methotrexate (n = 160) for 2 weeks after receiving a quadrivalent influenza vaccine containing H1N1, H3N2, B-Yamagata, and B-Victoria.
The study authors defined a positive vaccine response as a fourfold or greater increase in hemagglutination inhibition (HI) antibody titer. A satisfactory vaccine response was a positive response to two or more of four vaccine antigens.
Patients who stopped taking methotrexate were divided into eight subgroups according to the number of days between their last dose and their vaccination.
The research team reported that response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
However, they conceded that “the absence of impact of the number of days between the last methotrexate dose and vaccination could be due to the small patient numbers in eight subgroups.”
Vaccine response also did not differ between patients who received the influenza vaccination within 3 days of the last methotrexate dose (n = 65) and those who received it between 4-7 days of the last methotrexate dose (n = 95).
Furthermore, RA disease activity, seropositivity, or use of conventional or biologic disease-modifying antirheumatic drugs did not have an impact on methotrexate discontinuation.
The authors concluded that vaccinations could be given irrespective of the time of the last methotrexate dose, and patients should be advised to skip two weekly doses following vaccination.
“This supports the notion that the effects of methotrexate on humeral immunity occur rapidly, despite the delayed effects on arthritis; therefore, the absence of methotrexate during the first 2 weeks postvaccination is critical for humoral immunity,” they wrote.
The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
SOURCE: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187.
Immune response to influenza vaccination in rheumatoid arthritis patients taking methotrexate appears to depend most on stopping the next two weekly doses of the drug rather than any effect from the timing of the last dose, new research concludes.
The new finding, reported in Annals of the Rheumatic Diseases, stems from a post hoc analysis of a randomized, controlled trial that Jin Kyun Park, MD, of Seoul (Korea) National University, and his colleagues had conducted earlier on immune response when patients stopped methotrexate for either 2 or 4 weeks after vaccination. While the main endpoint of that study showed no difference in the improvement in vaccine response with either stopping methotrexate for 2 or 4 weeks and no increase in disease activity with stopping for 2 weeks, it was unclear whether the timing of the last dose mattered when stopping for 2 weeks.
In a bid to identify the optimal time between the last dose of methotrexate and administration of a flu vaccine, Dr. Park and his colleagues conducted a post hoc analysis of the trial, which involved 316 patients with RA receiving methotrexate for 6 weeks or longer to continue (n = 156) or to hold methotrexate (n = 160) for 2 weeks after receiving a quadrivalent influenza vaccine containing H1N1, H3N2, B-Yamagata, and B-Victoria.
The study authors defined a positive vaccine response as a fourfold or greater increase in hemagglutination inhibition (HI) antibody titer. A satisfactory vaccine response was a positive response to two or more of four vaccine antigens.
Patients who stopped taking methotrexate were divided into eight subgroups according to the number of days between their last dose and their vaccination.
The research team reported that response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
However, they conceded that “the absence of impact of the number of days between the last methotrexate dose and vaccination could be due to the small patient numbers in eight subgroups.”
Vaccine response also did not differ between patients who received the influenza vaccination within 3 days of the last methotrexate dose (n = 65) and those who received it between 4-7 days of the last methotrexate dose (n = 95).
Furthermore, RA disease activity, seropositivity, or use of conventional or biologic disease-modifying antirheumatic drugs did not have an impact on methotrexate discontinuation.
The authors concluded that vaccinations could be given irrespective of the time of the last methotrexate dose, and patients should be advised to skip two weekly doses following vaccination.
“This supports the notion that the effects of methotrexate on humeral immunity occur rapidly, despite the delayed effects on arthritis; therefore, the absence of methotrexate during the first 2 weeks postvaccination is critical for humoral immunity,” they wrote.
The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
SOURCE: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Response to vaccine, fold increase in HI antibody titers, and postvaccination seroprotection rates were not associated with the time between the last methotrexate dose and the time of vaccination.
Study details: A post hoc analysis of a randomized, controlled trial involving 316 patients with rheumatoid arthritis who continued or stopped methotrexate for 2 weeks following influenza vaccination.
Disclosures: The study was sponsored by GC Pharma. One author disclosed serving as a consultant to Pfizer and receiving research grants from GC Pharma and Hanmi Pharma.
Source: Park JK et al. Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2019-215187
2018-2019 flu season: Going but not gone yet
The 2018-2019 flu season again showed real signs of ending as influenza activity levels dropped during the week ending March 23, according to the Centers for Disease Control and Prevention.
Despite those declines, however, current levels of influenza-like illness (ILI) activity are still elevated enough that the CDC issued a health advisory on March 28 to inform clinicians about the “increasing proportion of activity due to influenza A(H3N2) viruses, continued circulation of influenza A(H1N1) viruses, and low levels of influenza B viruses.”
The CDC’s weekly flu report, released March 29, does show that the overall burden is improving. The national proportion of outpatient visits for ILI dropped from 4.3% for the week ending March 16 to 3.8% for the latest reporting week, the CDC’s influenza division reported. The figure for March 16 was originally reported to be 4.4% but was revised in the new report.
The length of this years’ flu season, when measured as the number of weeks at or above the baseline level of 2.2%, is now 18 weeks. By this measure, the last five seasons have averaged 16 weeks, the CDC noted.
Influenza was considered widespread in 34 states and Puerto Rico for the week ending March 23, down from 44 states the previous week. The number of states at the highest level of ILI activity on the CDC’s 1-10 scale dropped from 20 to 11, and those in the high range (8-10) dropped from 26 to 20, data from the CDC’s Outpatient ILI Surveillance Network show.
There was one flu-related pediatric death during the week of March 23 but none reported from earlier weeks, which brings the total to 77 for the 2018-2019 season, the CDC said.
The 2018-2019 flu season again showed real signs of ending as influenza activity levels dropped during the week ending March 23, according to the Centers for Disease Control and Prevention.

Despite those declines, however, current levels of influenza-like illness (ILI) activity are still elevated enough that the CDC issued a health advisory on March 28 to inform clinicians about the “increasing proportion of activity due to influenza A(H3N2) viruses, continued circulation of influenza A(H1N1) viruses, and low levels of influenza B viruses.”
The CDC’s weekly flu report, released March 29, does show that the overall burden is improving. The national proportion of outpatient visits for ILI dropped from 4.3% for the week ending March 16 to 3.8% for the latest reporting week, the CDC’s influenza division reported. The figure for March 16 was originally reported to be 4.4% but was revised in the new report.
The length of this years’ flu season, when measured as the number of weeks at or above the baseline level of 2.2%, is now 18 weeks. By this measure, the last five seasons have averaged 16 weeks, the CDC noted.
Influenza was considered widespread in 34 states and Puerto Rico for the week ending March 23, down from 44 states the previous week. The number of states at the highest level of ILI activity on the CDC’s 1-10 scale dropped from 20 to 11, and those in the high range (8-10) dropped from 26 to 20, data from the CDC’s Outpatient ILI Surveillance Network show.
There was one flu-related pediatric death during the week of March 23 but none reported from earlier weeks, which brings the total to 77 for the 2018-2019 season, the CDC said.
The 2018-2019 flu season again showed real signs of ending as influenza activity levels dropped during the week ending March 23, according to the Centers for Disease Control and Prevention.

Despite those declines, however, current levels of influenza-like illness (ILI) activity are still elevated enough that the CDC issued a health advisory on March 28 to inform clinicians about the “increasing proportion of activity due to influenza A(H3N2) viruses, continued circulation of influenza A(H1N1) viruses, and low levels of influenza B viruses.”
The CDC’s weekly flu report, released March 29, does show that the overall burden is improving. The national proportion of outpatient visits for ILI dropped from 4.3% for the week ending March 16 to 3.8% for the latest reporting week, the CDC’s influenza division reported. The figure for March 16 was originally reported to be 4.4% but was revised in the new report.
The length of this years’ flu season, when measured as the number of weeks at or above the baseline level of 2.2%, is now 18 weeks. By this measure, the last five seasons have averaged 16 weeks, the CDC noted.
Influenza was considered widespread in 34 states and Puerto Rico for the week ending March 23, down from 44 states the previous week. The number of states at the highest level of ILI activity on the CDC’s 1-10 scale dropped from 20 to 11, and those in the high range (8-10) dropped from 26 to 20, data from the CDC’s Outpatient ILI Surveillance Network show.
There was one flu-related pediatric death during the week of March 23 but none reported from earlier weeks, which brings the total to 77 for the 2018-2019 season, the CDC said.
Anti-infective update addresses SSSI choices
ORLANDO – What’s new in infectious disease therapeutics for dermatologists? He ran through an array of updates at the Orlando Dermatology Aesthetic and Clinical Conference.
While naturally occurring smallpox was globally eradicated in 1980, small research stores are held in the United States and Russia, and effective antivirals are part of a strategy to combat bioweapons. Tecovirimat (TPOXX) is an antiviral that inhibits a major envelope protein that poxviruses need to produce extracellular virus. Approved by the Food and Drug Administration in mid-2018, it is currently the only antiviral for treating variola virus infection approved in the United States, noted Dr. Finch of the University of Connecticut, Farmington. He added that 2 million doses are currently held in the U.S. Strategic National Stockpile.
Another anti-infective agent that won’t be used by those practicing in the United States, but which promises to alleviate a significant source of suffering in the developing world, is moxidectin. The anthelmintic had previously been approved for veterinary uses, but in June 2018, the FDA approved moxidectin to treat onchocerciasis, also known as river blindness. The drug defeats the parasitic worm by binding to glutamate-gated chloride ion channels; it is licensed by the nonprofit Medicines Development for Global Health.
Another antiparasitic drug, benznidazole, was approved to treat children aged 2-12 years with Chagas disease in 2017, Dr. Finch said.
Also in 2017, a topical quinolone, ozenoxacin (Xepi) was approved to treat impetigo in adults and children aged at least 2 months. Formulated as a 1% cream, ozenoxacin is applied twice daily for 5 days. In clinical trials, ozenoxacin was shown to be noninferior to retapamulin, he said.
A new topical choice is important as mupirocin resistance climbs, Dr. Finch added. A recent Greek study showed that 20% (437) of 2,137 staph infections studied were mupirocin resistant. Of the 20%, all but one were skin and skin structure infections (SSSIs), with 88% of these being impetigo.
In the United States, mupirocin resistance has been seen in one in three outpatients in a Florida study and in 31% of patients in a New York City sample. Other studies have shown mupirocin resistance in Staphylococcus aureus isolates with resistance in the 10%-15% range among children with SSSIs, Dr. Finch said.
Two other new antibiotics to fight SSSIs can each be administered orally or intravenously. One, omadacycline (Nuzyra), is a novel tetracycline that maintains efficacy against bacteria that express tetracycline resistance through efflux and ribosomal protection. Approved in late 2018 for acute bacterial SSSIs, omadacycline treats not just methicillin-sensitive and methicillin-resistant S. aureus, but also Streptococcus species and gram-negative rods such as Enterobacter and Klebsiella pneumoniae, Dr. Finch noted.
Another new fluorinated quinolone, approved in 2017, delafloxacin (Baxdela) has broad spectrum activity against gram-negative and gram-positive bacteria.
Dr. Finch reported that he has no relevant conflicts of interest.
ORLANDO – What’s new in infectious disease therapeutics for dermatologists? He ran through an array of updates at the Orlando Dermatology Aesthetic and Clinical Conference.
While naturally occurring smallpox was globally eradicated in 1980, small research stores are held in the United States and Russia, and effective antivirals are part of a strategy to combat bioweapons. Tecovirimat (TPOXX) is an antiviral that inhibits a major envelope protein that poxviruses need to produce extracellular virus. Approved by the Food and Drug Administration in mid-2018, it is currently the only antiviral for treating variola virus infection approved in the United States, noted Dr. Finch of the University of Connecticut, Farmington. He added that 2 million doses are currently held in the U.S. Strategic National Stockpile.
Another anti-infective agent that won’t be used by those practicing in the United States, but which promises to alleviate a significant source of suffering in the developing world, is moxidectin. The anthelmintic had previously been approved for veterinary uses, but in June 2018, the FDA approved moxidectin to treat onchocerciasis, also known as river blindness. The drug defeats the parasitic worm by binding to glutamate-gated chloride ion channels; it is licensed by the nonprofit Medicines Development for Global Health.
Another antiparasitic drug, benznidazole, was approved to treat children aged 2-12 years with Chagas disease in 2017, Dr. Finch said.
Also in 2017, a topical quinolone, ozenoxacin (Xepi) was approved to treat impetigo in adults and children aged at least 2 months. Formulated as a 1% cream, ozenoxacin is applied twice daily for 5 days. In clinical trials, ozenoxacin was shown to be noninferior to retapamulin, he said.
A new topical choice is important as mupirocin resistance climbs, Dr. Finch added. A recent Greek study showed that 20% (437) of 2,137 staph infections studied were mupirocin resistant. Of the 20%, all but one were skin and skin structure infections (SSSIs), with 88% of these being impetigo.
In the United States, mupirocin resistance has been seen in one in three outpatients in a Florida study and in 31% of patients in a New York City sample. Other studies have shown mupirocin resistance in Staphylococcus aureus isolates with resistance in the 10%-15% range among children with SSSIs, Dr. Finch said.
Two other new antibiotics to fight SSSIs can each be administered orally or intravenously. One, omadacycline (Nuzyra), is a novel tetracycline that maintains efficacy against bacteria that express tetracycline resistance through efflux and ribosomal protection. Approved in late 2018 for acute bacterial SSSIs, omadacycline treats not just methicillin-sensitive and methicillin-resistant S. aureus, but also Streptococcus species and gram-negative rods such as Enterobacter and Klebsiella pneumoniae, Dr. Finch noted.
Another new fluorinated quinolone, approved in 2017, delafloxacin (Baxdela) has broad spectrum activity against gram-negative and gram-positive bacteria.
Dr. Finch reported that he has no relevant conflicts of interest.
ORLANDO – What’s new in infectious disease therapeutics for dermatologists? He ran through an array of updates at the Orlando Dermatology Aesthetic and Clinical Conference.
While naturally occurring smallpox was globally eradicated in 1980, small research stores are held in the United States and Russia, and effective antivirals are part of a strategy to combat bioweapons. Tecovirimat (TPOXX) is an antiviral that inhibits a major envelope protein that poxviruses need to produce extracellular virus. Approved by the Food and Drug Administration in mid-2018, it is currently the only antiviral for treating variola virus infection approved in the United States, noted Dr. Finch of the University of Connecticut, Farmington. He added that 2 million doses are currently held in the U.S. Strategic National Stockpile.
Another anti-infective agent that won’t be used by those practicing in the United States, but which promises to alleviate a significant source of suffering in the developing world, is moxidectin. The anthelmintic had previously been approved for veterinary uses, but in June 2018, the FDA approved moxidectin to treat onchocerciasis, also known as river blindness. The drug defeats the parasitic worm by binding to glutamate-gated chloride ion channels; it is licensed by the nonprofit Medicines Development for Global Health.
Another antiparasitic drug, benznidazole, was approved to treat children aged 2-12 years with Chagas disease in 2017, Dr. Finch said.
Also in 2017, a topical quinolone, ozenoxacin (Xepi) was approved to treat impetigo in adults and children aged at least 2 months. Formulated as a 1% cream, ozenoxacin is applied twice daily for 5 days. In clinical trials, ozenoxacin was shown to be noninferior to retapamulin, he said.
A new topical choice is important as mupirocin resistance climbs, Dr. Finch added. A recent Greek study showed that 20% (437) of 2,137 staph infections studied were mupirocin resistant. Of the 20%, all but one were skin and skin structure infections (SSSIs), with 88% of these being impetigo.
In the United States, mupirocin resistance has been seen in one in three outpatients in a Florida study and in 31% of patients in a New York City sample. Other studies have shown mupirocin resistance in Staphylococcus aureus isolates with resistance in the 10%-15% range among children with SSSIs, Dr. Finch said.
Two other new antibiotics to fight SSSIs can each be administered orally or intravenously. One, omadacycline (Nuzyra), is a novel tetracycline that maintains efficacy against bacteria that express tetracycline resistance through efflux and ribosomal protection. Approved in late 2018 for acute bacterial SSSIs, omadacycline treats not just methicillin-sensitive and methicillin-resistant S. aureus, but also Streptococcus species and gram-negative rods such as Enterobacter and Klebsiella pneumoniae, Dr. Finch noted.
Another new fluorinated quinolone, approved in 2017, delafloxacin (Baxdela) has broad spectrum activity against gram-negative and gram-positive bacteria.
Dr. Finch reported that he has no relevant conflicts of interest.
EXPERT ANALYSIS FROM ODAC 2019