User login
FDA approves first two-drug tablet for HIV
The U.S. Food and Drug Administration has approved the first two-drug, fixed-dose, complete regimen for HIV-infected adults, according to an FDA press announcement.
Dovato (dolutegravir and lamivudine), a product of ViiV Healthcare, is intended to serve “as a complete regimen” for the treatment of HIV-1 infection in adults who have had no previous antiretroviral treatment and who have an infection with no known or suspected genetic substitutions associated with resistance to the individual components of Dovato.
“With this approval, patients who have never been treated have the option of taking a two-drug regimen in a single tablet while eliminating additional toxicity and potential drug interactions from a third drug,” said Debra Birnkrant, MD, director of the FDA’s Division of Antiviral Products.
The Dovato labeling includes a Boxed Warning that patients infected with both HIV and hepatitis B should add additional treatment for their HBV or consider a different drug regimen. The most common adverse reactions with Dovato were headache, diarrhea, nausea, insomnia, and fatigue. In addition, the FDA warned that, as there is a known risk for neural tube defects with dolutegravir, patients are advised to avoid use of Dovato at the time of conception through the first trimester of pregnancy.
[email protected]
The U.S. Food and Drug Administration has approved the first two-drug, fixed-dose, complete regimen for HIV-infected adults, according to an FDA press announcement.
Dovato (dolutegravir and lamivudine), a product of ViiV Healthcare, is intended to serve “as a complete regimen” for the treatment of HIV-1 infection in adults who have had no previous antiretroviral treatment and who have an infection with no known or suspected genetic substitutions associated with resistance to the individual components of Dovato.
“With this approval, patients who have never been treated have the option of taking a two-drug regimen in a single tablet while eliminating additional toxicity and potential drug interactions from a third drug,” said Debra Birnkrant, MD, director of the FDA’s Division of Antiviral Products.
The Dovato labeling includes a Boxed Warning that patients infected with both HIV and hepatitis B should add additional treatment for their HBV or consider a different drug regimen. The most common adverse reactions with Dovato were headache, diarrhea, nausea, insomnia, and fatigue. In addition, the FDA warned that, as there is a known risk for neural tube defects with dolutegravir, patients are advised to avoid use of Dovato at the time of conception through the first trimester of pregnancy.
[email protected]
The U.S. Food and Drug Administration has approved the first two-drug, fixed-dose, complete regimen for HIV-infected adults, according to an FDA press announcement.
Dovato (dolutegravir and lamivudine), a product of ViiV Healthcare, is intended to serve “as a complete regimen” for the treatment of HIV-1 infection in adults who have had no previous antiretroviral treatment and who have an infection with no known or suspected genetic substitutions associated with resistance to the individual components of Dovato.
“With this approval, patients who have never been treated have the option of taking a two-drug regimen in a single tablet while eliminating additional toxicity and potential drug interactions from a third drug,” said Debra Birnkrant, MD, director of the FDA’s Division of Antiviral Products.
The Dovato labeling includes a Boxed Warning that patients infected with both HIV and hepatitis B should add additional treatment for their HBV or consider a different drug regimen. The most common adverse reactions with Dovato were headache, diarrhea, nausea, insomnia, and fatigue. In addition, the FDA warned that, as there is a known risk for neural tube defects with dolutegravir, patients are advised to avoid use of Dovato at the time of conception through the first trimester of pregnancy.
[email protected]
Large measles outbreak reported in Michigan
A new measles outbreak in Michigan has already resulted in 39 cases, and four more states reported their first cases of 2019 during the week ending April 4, according to the Centers for Disease Control and Prevention
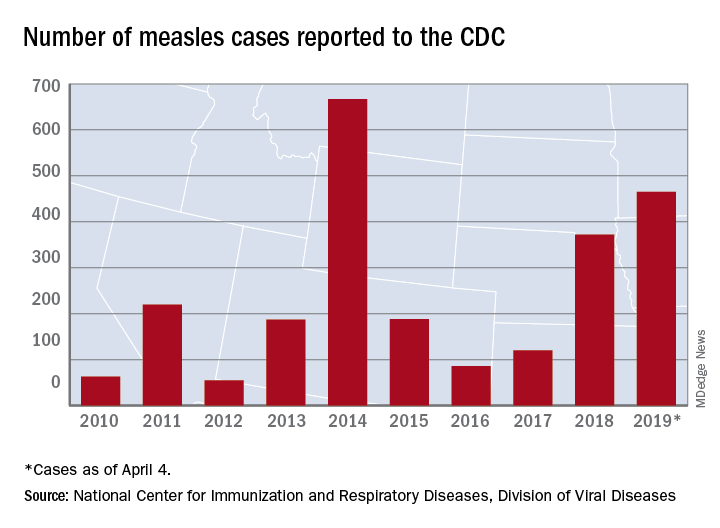
The measles virus has now infected individuals in Florida, Indiana, Massachusetts, and Nevada, which means that 19 states have now reported a total of 465 cases this year, and that is the second-highest total “reported in the U.S. since measles was eliminated in 2000,” the CDC said April 8.
The Michigan outbreak is mostly concentrated in Oakland County, where 38 cases have occurred. The county has posted an up-to-date list of exposure locations.
Not to be outdone, New York reported 45 new cases last week: 44 in Brooklyn and 1 in Queens. There have been 259 confirmed cases in the two boroughs since the outbreak began in October of last year.
Besides Michigan and New York City, there are five other outbreaks ongoing in the United States: Rockland County, N.Y.; Washington State (no new cases since March 22); Butte County, Calif.; Santa Cruz County, Calif.; and New Jersey, the CDC reported.
A judge in New York State temporarily blocked an order banning unimmunized children from public spaces in Rockland County and has set a hearing date of April 19, CNN reported. The ban, ordered by Rockland County Executive Ed Day, went into effect on March 27.
On April 2, the Maine Center for Disease Control & Prevention announced that an out-of-state resident with a confirmed case of measles had visited two health care offices – one in Falmouth and one in Westbrook – on March 27. No cases in Maine residents have been reported yet.
On a vaccine-related note, the Washington State Senate’s Health and Long Term Care Committee approved a proposal on April 1 that would “end the personal exemption for parents who don’t want their children vaccinated against measles,” the Spokane Spokesman-Review said. The bill, which would still allow medical and religious exemptions, has already passed the state’s House of Representatives and goes next to the full senate.
A new measles outbreak in Michigan has already resulted in 39 cases, and four more states reported their first cases of 2019 during the week ending April 4, according to the Centers for Disease Control and Prevention

The measles virus has now infected individuals in Florida, Indiana, Massachusetts, and Nevada, which means that 19 states have now reported a total of 465 cases this year, and that is the second-highest total “reported in the U.S. since measles was eliminated in 2000,” the CDC said April 8.
The Michigan outbreak is mostly concentrated in Oakland County, where 38 cases have occurred. The county has posted an up-to-date list of exposure locations.
Not to be outdone, New York reported 45 new cases last week: 44 in Brooklyn and 1 in Queens. There have been 259 confirmed cases in the two boroughs since the outbreak began in October of last year.
Besides Michigan and New York City, there are five other outbreaks ongoing in the United States: Rockland County, N.Y.; Washington State (no new cases since March 22); Butte County, Calif.; Santa Cruz County, Calif.; and New Jersey, the CDC reported.
A judge in New York State temporarily blocked an order banning unimmunized children from public spaces in Rockland County and has set a hearing date of April 19, CNN reported. The ban, ordered by Rockland County Executive Ed Day, went into effect on March 27.
On April 2, the Maine Center for Disease Control & Prevention announced that an out-of-state resident with a confirmed case of measles had visited two health care offices – one in Falmouth and one in Westbrook – on March 27. No cases in Maine residents have been reported yet.
On a vaccine-related note, the Washington State Senate’s Health and Long Term Care Committee approved a proposal on April 1 that would “end the personal exemption for parents who don’t want their children vaccinated against measles,” the Spokane Spokesman-Review said. The bill, which would still allow medical and religious exemptions, has already passed the state’s House of Representatives and goes next to the full senate.
A new measles outbreak in Michigan has already resulted in 39 cases, and four more states reported their first cases of 2019 during the week ending April 4, according to the Centers for Disease Control and Prevention

The measles virus has now infected individuals in Florida, Indiana, Massachusetts, and Nevada, which means that 19 states have now reported a total of 465 cases this year, and that is the second-highest total “reported in the U.S. since measles was eliminated in 2000,” the CDC said April 8.
The Michigan outbreak is mostly concentrated in Oakland County, where 38 cases have occurred. The county has posted an up-to-date list of exposure locations.
Not to be outdone, New York reported 45 new cases last week: 44 in Brooklyn and 1 in Queens. There have been 259 confirmed cases in the two boroughs since the outbreak began in October of last year.
Besides Michigan and New York City, there are five other outbreaks ongoing in the United States: Rockland County, N.Y.; Washington State (no new cases since March 22); Butte County, Calif.; Santa Cruz County, Calif.; and New Jersey, the CDC reported.
A judge in New York State temporarily blocked an order banning unimmunized children from public spaces in Rockland County and has set a hearing date of April 19, CNN reported. The ban, ordered by Rockland County Executive Ed Day, went into effect on March 27.
On April 2, the Maine Center for Disease Control & Prevention announced that an out-of-state resident with a confirmed case of measles had visited two health care offices – one in Falmouth and one in Westbrook – on March 27. No cases in Maine residents have been reported yet.
On a vaccine-related note, the Washington State Senate’s Health and Long Term Care Committee approved a proposal on April 1 that would “end the personal exemption for parents who don’t want their children vaccinated against measles,” the Spokane Spokesman-Review said. The bill, which would still allow medical and religious exemptions, has already passed the state’s House of Representatives and goes next to the full senate.
How common are noninfectious complications of Foley catheters?
CLINICAL QUESTION: How common are noninfectious complications of Foley catheters?
BACKGROUND: Approximately 20% of hospitalized patients have a Foley catheter inserted at some time during their admission. Infectious complications associated with the use of Foley catheters are widely recognized; however, much less is known about noninfectious complications.
STUDY DESIGN: Prospective cohort study.
SETTING: Four U.S. hospitals in two states.SYNOPSIS: The study included 2,076 hospitalized patients with a Foley catheter. They were followed for 30 days after its insertion, even if catheter removal occurred during this time period. Data about infectious and noninfectious complications were collected through patient interviews.
At least one complication was noted in 1,184 of 2,076 patients (57%) during the 30-day period following Foley catheter insertion. While infectious complications occurred in 219 of 2,076 patients (10.5%), noninfectious complications (such as pain, urinary urgency, hematuria) were reported by 1,150 patients (55.4%; P less than .001). For those with catheters still in place, the most common complication was pain or discomfort (54.5%). Postremoval leaking urine (20.3%) and/or urgency and bladder spasms (24.0%) were the most common complications.
The study only included patients who had a Foley catheter placed during a hospitalization; the results may not apply to patients who receive catheters in other settings.
BOTTOM LINE: Noninfectious complications affect over half of patients with a Foley catheters. These types of complications should be targeted in future harm prevention efforts and should be considered when deciding to place a Foley catheter.
CITATION: Saint S et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018;178(8):1078-85.
Dr. Clarke is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
CLINICAL QUESTION: How common are noninfectious complications of Foley catheters?
BACKGROUND: Approximately 20% of hospitalized patients have a Foley catheter inserted at some time during their admission. Infectious complications associated with the use of Foley catheters are widely recognized; however, much less is known about noninfectious complications.
STUDY DESIGN: Prospective cohort study.
SETTING: Four U.S. hospitals in two states.SYNOPSIS: The study included 2,076 hospitalized patients with a Foley catheter. They were followed for 30 days after its insertion, even if catheter removal occurred during this time period. Data about infectious and noninfectious complications were collected through patient interviews.
At least one complication was noted in 1,184 of 2,076 patients (57%) during the 30-day period following Foley catheter insertion. While infectious complications occurred in 219 of 2,076 patients (10.5%), noninfectious complications (such as pain, urinary urgency, hematuria) were reported by 1,150 patients (55.4%; P less than .001). For those with catheters still in place, the most common complication was pain or discomfort (54.5%). Postremoval leaking urine (20.3%) and/or urgency and bladder spasms (24.0%) were the most common complications.
The study only included patients who had a Foley catheter placed during a hospitalization; the results may not apply to patients who receive catheters in other settings.
BOTTOM LINE: Noninfectious complications affect over half of patients with a Foley catheters. These types of complications should be targeted in future harm prevention efforts and should be considered when deciding to place a Foley catheter.
CITATION: Saint S et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018;178(8):1078-85.
Dr. Clarke is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
CLINICAL QUESTION: How common are noninfectious complications of Foley catheters?
BACKGROUND: Approximately 20% of hospitalized patients have a Foley catheter inserted at some time during their admission. Infectious complications associated with the use of Foley catheters are widely recognized; however, much less is known about noninfectious complications.
STUDY DESIGN: Prospective cohort study.
SETTING: Four U.S. hospitals in two states.SYNOPSIS: The study included 2,076 hospitalized patients with a Foley catheter. They were followed for 30 days after its insertion, even if catheter removal occurred during this time period. Data about infectious and noninfectious complications were collected through patient interviews.
At least one complication was noted in 1,184 of 2,076 patients (57%) during the 30-day period following Foley catheter insertion. While infectious complications occurred in 219 of 2,076 patients (10.5%), noninfectious complications (such as pain, urinary urgency, hematuria) were reported by 1,150 patients (55.4%; P less than .001). For those with catheters still in place, the most common complication was pain or discomfort (54.5%). Postremoval leaking urine (20.3%) and/or urgency and bladder spasms (24.0%) were the most common complications.
The study only included patients who had a Foley catheter placed during a hospitalization; the results may not apply to patients who receive catheters in other settings.
BOTTOM LINE: Noninfectious complications affect over half of patients with a Foley catheters. These types of complications should be targeted in future harm prevention efforts and should be considered when deciding to place a Foley catheter.
CITATION: Saint S et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018;178(8):1078-85.
Dr. Clarke is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Direct-to-consumer telemedicine visits may lead to pediatric antibiotic overprescribing
(ARIs), according to a study of antibiotic prescriptions for ARIs across 3 clinical settings.
“These differences in antibiotic prescribing for children contrast with previous studies of DTC telemedicine quality among adult patients in which quality differences have been smaller or nonexistent,” wrote Kristin N. Ray, MD, of Children’s Hospital of Pittsburgh, and her coauthors. The study was published in Pediatrics.
To determine quality of care during pediatric DTC telemedicine visits, the researchers embarked on a retrospective cohort study using 2015–2016 claims data from a large national commercial health plan. They identified visits for ARIs and matched them across 3 settings: DTC telemedicine, urgent care, and PCP offices. The matched sample included 4,604 DTC telemedicine visits, 38,408 urgent care visits, and 485,201 PCP visits.
Their analysis showed that children were more likely to be prescribed antibiotics at DTC telemedicine visits than in other settings (52% versus 42% for urgent care and 31% for PCP, P less than .001). In addition, they were less likely to receive guideline-concordant antibiotic management (59% versus 67% and 78%, P less than .001). This was primarily attributed to “antibiotic prescribing for visits with viral ARI diagnoses that do not warrant antibiotics,” antibiotics were appropriately not prescribed in only 54% of those DTC telemedicine visits, compared with 66% for urgent care and 80% for PCP (P less than .001).
The authors shared the limitations of their study, including a lack of sociodemographic or clinical data stemming from a reliance on insurance claims. They also noted that their analysis was limited to a specific health plan and its contracted DTC telemedicine vendor, recognizing that “antibiotic prescribing among other DTC telemedicine companies, models, and populations may differ.”
The study was funded by the National Institutes of Health and supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and gifts from Melvin Hall. The authors reported no conflicts of interest.
SOURCE: Ray KN et al. Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2018-2491.
These findings from this study illustrate the issues with direct-to-consumer (DTC) telemedicine, especially when treating children, according to Jeffrey S. Gerber, MD, medical director of the antimicrobial stewardship program at Children’s Hospital of Philadelphia.
The best way to get a 5-star rating after a DTC telemedicine visit is to prescribe an antibiotic, Dr. Gerber wrote, so it shouldn’t be surprising that doctors are handing them out at a higher rate than after an urgent care or a primary care visit. It should also be noted that this study covers a very specific privately insured population and that DTC telemedicine remains a “small piece of the pie,” for now, in terms of patient care.
But, he added, the most problematic element of this study may be that none of the 3 most common pediatric acute respiratory tract infection (ARTI) diagnoses should be followed with an immediate prescription, especially after a virtual visit.
“It could be argued that essentially no ARTI encounters should lead to antibiotic prescriptions solely on the basis of a DTC telemedicine visit,” he wrote, recognizing that – though there may be value for telemedicine in a screening capacity – the DTC version seems to be a “low quality encounter” at best and “a vehicle for antibiotic overuse” at worst.
These comments are adapted from an accompanying editorial (Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2019-0631 ). Dr. Gerber reported receiving personal fees from Medtronic outside the submitted work.
These findings from this study illustrate the issues with direct-to-consumer (DTC) telemedicine, especially when treating children, according to Jeffrey S. Gerber, MD, medical director of the antimicrobial stewardship program at Children’s Hospital of Philadelphia.
The best way to get a 5-star rating after a DTC telemedicine visit is to prescribe an antibiotic, Dr. Gerber wrote, so it shouldn’t be surprising that doctors are handing them out at a higher rate than after an urgent care or a primary care visit. It should also be noted that this study covers a very specific privately insured population and that DTC telemedicine remains a “small piece of the pie,” for now, in terms of patient care.
But, he added, the most problematic element of this study may be that none of the 3 most common pediatric acute respiratory tract infection (ARTI) diagnoses should be followed with an immediate prescription, especially after a virtual visit.
“It could be argued that essentially no ARTI encounters should lead to antibiotic prescriptions solely on the basis of a DTC telemedicine visit,” he wrote, recognizing that – though there may be value for telemedicine in a screening capacity – the DTC version seems to be a “low quality encounter” at best and “a vehicle for antibiotic overuse” at worst.
These comments are adapted from an accompanying editorial (Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2019-0631 ). Dr. Gerber reported receiving personal fees from Medtronic outside the submitted work.
These findings from this study illustrate the issues with direct-to-consumer (DTC) telemedicine, especially when treating children, according to Jeffrey S. Gerber, MD, medical director of the antimicrobial stewardship program at Children’s Hospital of Philadelphia.
The best way to get a 5-star rating after a DTC telemedicine visit is to prescribe an antibiotic, Dr. Gerber wrote, so it shouldn’t be surprising that doctors are handing them out at a higher rate than after an urgent care or a primary care visit. It should also be noted that this study covers a very specific privately insured population and that DTC telemedicine remains a “small piece of the pie,” for now, in terms of patient care.
But, he added, the most problematic element of this study may be that none of the 3 most common pediatric acute respiratory tract infection (ARTI) diagnoses should be followed with an immediate prescription, especially after a virtual visit.
“It could be argued that essentially no ARTI encounters should lead to antibiotic prescriptions solely on the basis of a DTC telemedicine visit,” he wrote, recognizing that – though there may be value for telemedicine in a screening capacity – the DTC version seems to be a “low quality encounter” at best and “a vehicle for antibiotic overuse” at worst.
These comments are adapted from an accompanying editorial (Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2019-0631 ). Dr. Gerber reported receiving personal fees from Medtronic outside the submitted work.
(ARIs), according to a study of antibiotic prescriptions for ARIs across 3 clinical settings.
“These differences in antibiotic prescribing for children contrast with previous studies of DTC telemedicine quality among adult patients in which quality differences have been smaller or nonexistent,” wrote Kristin N. Ray, MD, of Children’s Hospital of Pittsburgh, and her coauthors. The study was published in Pediatrics.
To determine quality of care during pediatric DTC telemedicine visits, the researchers embarked on a retrospective cohort study using 2015–2016 claims data from a large national commercial health plan. They identified visits for ARIs and matched them across 3 settings: DTC telemedicine, urgent care, and PCP offices. The matched sample included 4,604 DTC telemedicine visits, 38,408 urgent care visits, and 485,201 PCP visits.
Their analysis showed that children were more likely to be prescribed antibiotics at DTC telemedicine visits than in other settings (52% versus 42% for urgent care and 31% for PCP, P less than .001). In addition, they were less likely to receive guideline-concordant antibiotic management (59% versus 67% and 78%, P less than .001). This was primarily attributed to “antibiotic prescribing for visits with viral ARI diagnoses that do not warrant antibiotics,” antibiotics were appropriately not prescribed in only 54% of those DTC telemedicine visits, compared with 66% for urgent care and 80% for PCP (P less than .001).
The authors shared the limitations of their study, including a lack of sociodemographic or clinical data stemming from a reliance on insurance claims. They also noted that their analysis was limited to a specific health plan and its contracted DTC telemedicine vendor, recognizing that “antibiotic prescribing among other DTC telemedicine companies, models, and populations may differ.”
The study was funded by the National Institutes of Health and supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and gifts from Melvin Hall. The authors reported no conflicts of interest.
SOURCE: Ray KN et al. Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2018-2491.
(ARIs), according to a study of antibiotic prescriptions for ARIs across 3 clinical settings.
“These differences in antibiotic prescribing for children contrast with previous studies of DTC telemedicine quality among adult patients in which quality differences have been smaller or nonexistent,” wrote Kristin N. Ray, MD, of Children’s Hospital of Pittsburgh, and her coauthors. The study was published in Pediatrics.
To determine quality of care during pediatric DTC telemedicine visits, the researchers embarked on a retrospective cohort study using 2015–2016 claims data from a large national commercial health plan. They identified visits for ARIs and matched them across 3 settings: DTC telemedicine, urgent care, and PCP offices. The matched sample included 4,604 DTC telemedicine visits, 38,408 urgent care visits, and 485,201 PCP visits.
Their analysis showed that children were more likely to be prescribed antibiotics at DTC telemedicine visits than in other settings (52% versus 42% for urgent care and 31% for PCP, P less than .001). In addition, they were less likely to receive guideline-concordant antibiotic management (59% versus 67% and 78%, P less than .001). This was primarily attributed to “antibiotic prescribing for visits with viral ARI diagnoses that do not warrant antibiotics,” antibiotics were appropriately not prescribed in only 54% of those DTC telemedicine visits, compared with 66% for urgent care and 80% for PCP (P less than .001).
The authors shared the limitations of their study, including a lack of sociodemographic or clinical data stemming from a reliance on insurance claims. They also noted that their analysis was limited to a specific health plan and its contracted DTC telemedicine vendor, recognizing that “antibiotic prescribing among other DTC telemedicine companies, models, and populations may differ.”
The study was funded by the National Institutes of Health and supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and gifts from Melvin Hall. The authors reported no conflicts of interest.
SOURCE: Ray KN et al. Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2018-2491.
FROM PEDIATRICS
Key clinical point: For children diagnosed with acute respiratory infections, antibiotic prescribing was higher and guideline-concordant antibiotic management was lower at direct-to-consumer (DTC) telemedicine visits.
Major finding: Children at DTC telemedicine visits were prescribed antibiotics for respiratory infections 52% of the time, compared with 42% at urgent care visits and 31% at primary care provider visits.
Study details: A retrospective cohort study of DTC telemedicine, urgent care, and primary care provider visits for acute respiratory infections and subsequent antibiotic prescriptions.
Disclosures: The study was funded by the National Institutes of Health and supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and gifts from Melvin Hall. The authors reported no conflicts of interest.
Source: Ray KN et al. Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2018-2491.
Flu activity falling but still elevated
Measures of influenza activity fell again as the flu season continues to make its later-than-usual departure this year, according to the Centers for Disease Control and Prevention.
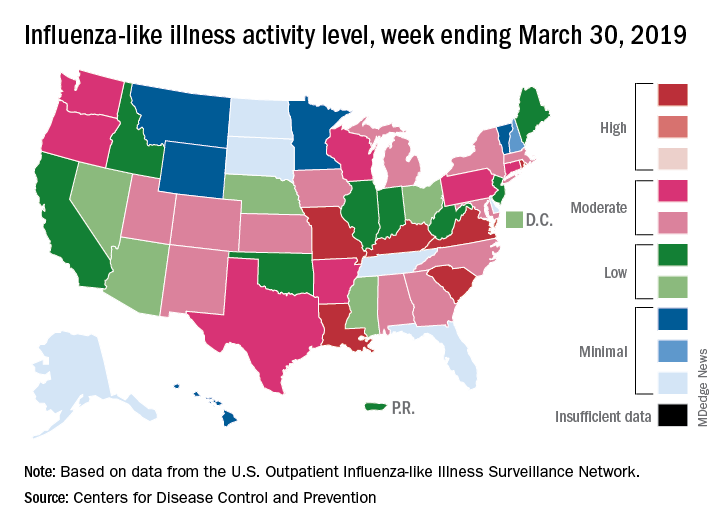
On the geographic front, the map of influenza-like illness (ILI) activity for the week ending March 30 shows that only 6 states are at level 10 on the CDC’s 1-10 scale, compared with 11 for the previous week, and that those same 6 states make up the entire membership of the high range of levels 8-10, which is down from 20 states a week ago, data from the CDC’s Outpatient ILI Surveillance Network show.
The proportion of outpatient visits for ILI, now at 3.2%, dropped for the sixth consecutive week after reaching its season high of 5.1% back in mid-February. The outpatient rate has now been at or above the national baseline of 2.2% for 19 weeks this season, the CDC’s influenza division said April 5, noting that the average for the past five seasons is 16 weeks.
Six flu-related pediatric deaths were reported in the week ending March 30, and the total is now 82 for the 2018-2019 season. Five of the six occurred during previous weeks of this season, and one occurred in the 2017-2018 season, the CDC said.
Measures of influenza activity fell again as the flu season continues to make its later-than-usual departure this year, according to the Centers for Disease Control and Prevention.

On the geographic front, the map of influenza-like illness (ILI) activity for the week ending March 30 shows that only 6 states are at level 10 on the CDC’s 1-10 scale, compared with 11 for the previous week, and that those same 6 states make up the entire membership of the high range of levels 8-10, which is down from 20 states a week ago, data from the CDC’s Outpatient ILI Surveillance Network show.
The proportion of outpatient visits for ILI, now at 3.2%, dropped for the sixth consecutive week after reaching its season high of 5.1% back in mid-February. The outpatient rate has now been at or above the national baseline of 2.2% for 19 weeks this season, the CDC’s influenza division said April 5, noting that the average for the past five seasons is 16 weeks.
Six flu-related pediatric deaths were reported in the week ending March 30, and the total is now 82 for the 2018-2019 season. Five of the six occurred during previous weeks of this season, and one occurred in the 2017-2018 season, the CDC said.
Measures of influenza activity fell again as the flu season continues to make its later-than-usual departure this year, according to the Centers for Disease Control and Prevention.

On the geographic front, the map of influenza-like illness (ILI) activity for the week ending March 30 shows that only 6 states are at level 10 on the CDC’s 1-10 scale, compared with 11 for the previous week, and that those same 6 states make up the entire membership of the high range of levels 8-10, which is down from 20 states a week ago, data from the CDC’s Outpatient ILI Surveillance Network show.
The proportion of outpatient visits for ILI, now at 3.2%, dropped for the sixth consecutive week after reaching its season high of 5.1% back in mid-February. The outpatient rate has now been at or above the national baseline of 2.2% for 19 weeks this season, the CDC’s influenza division said April 5, noting that the average for the past five seasons is 16 weeks.
Six flu-related pediatric deaths were reported in the week ending March 30, and the total is now 82 for the 2018-2019 season. Five of the six occurred during previous weeks of this season, and one occurred in the 2017-2018 season, the CDC said.
Nucleic acid testing before PrEP urged to detect occult HIV
SEATTLE – Almost a third of patients who developed HIV while on pre-exposure prophylaxis (PrEP) had strains that were resistant to emtricitabine – a component of the PrEP medication Truvada along with tenofovir – at time of diagnosis, compared with just 2% of those not on PrEP, in a review of more than 3,500 newly diagnosed HIV cases in New York.
The finding points to a growing concern as use of PrEP becomes more common: the induction of resistance to HIV treatments.
PrEP is highly effective, so it’s likely that the PrEP patients already had HIV when they started treatment. Nucleic acid amplification testing (NAAT) is the only way to rule it out definitively, but only 5% of PrEP users in the study were screened with NAAT within 2 days of initiation.
The usual test – HIV antibody screening – returns a false negative in the window between HIV exposure and active infection, when antibodies turn positive, which can take months. NAAT, on the other hand, looks for evidence of the virus directly.
The findings led the investigators to urge routine NAAT screening before PrEP, something that New York State guidelines currently recommend only if patients present with symptoms of acute HIV infection or report condomless sex in the previous 4 weeks.
To prevent drug resistance, “rigorous screening that includes NAAT is critical.” It reduces “the likelihood of PrEP start during undetected HIV infection,” said lead investigator Kavita Misra, PhD, MPH, a senior epidemiologist at the New York City Department of Health and Mental Hygiene.
Her team reviewed 3,685 people in New York who were diagnosed with HIV from November 2015 to August 2017; 91 had been on PrEP beforehand for a median of 106 days.
Postdiagnosis genotyping was available for 75% of PrEP users and 63% in the no-PrEP group. Resistance mutations to emtricitabine were significantly more prevalent with PrEP than without (29% versus 2%, respectively; P less than .0001). None of the PrEP users, but four in the no-PrEP group, had resistance to the other component of Truvada, tenofovir.
PrEP users were more likely to be diagnosed with HIV in the acute phase of infection than were those not using PrEP (33% versus 9%; P less than .0001), probably because they were also more likely to have regular office visits while on PrEP.
Skeptics at the Conference on Retroviruses and Opportunistic Infections, where the study was presented, wondered whether emtricitabine resistance would have been more common in the no-PrEP group if the infection had been picked up earlier because resistance fades as the infection progresses.
Dr. Misra said it was a good question and that her team will look into it. However, she stood by her conclusions.
PrEP use was most common among white men who have sex with men and among people under 30 years old.
There was no external funding, and the investigators didn’t have any disclosures.
SOURCE: Misra K et al. 2019 CROI, Abstract 107.
SEATTLE – Almost a third of patients who developed HIV while on pre-exposure prophylaxis (PrEP) had strains that were resistant to emtricitabine – a component of the PrEP medication Truvada along with tenofovir – at time of diagnosis, compared with just 2% of those not on PrEP, in a review of more than 3,500 newly diagnosed HIV cases in New York.
The finding points to a growing concern as use of PrEP becomes more common: the induction of resistance to HIV treatments.
PrEP is highly effective, so it’s likely that the PrEP patients already had HIV when they started treatment. Nucleic acid amplification testing (NAAT) is the only way to rule it out definitively, but only 5% of PrEP users in the study were screened with NAAT within 2 days of initiation.
The usual test – HIV antibody screening – returns a false negative in the window between HIV exposure and active infection, when antibodies turn positive, which can take months. NAAT, on the other hand, looks for evidence of the virus directly.
The findings led the investigators to urge routine NAAT screening before PrEP, something that New York State guidelines currently recommend only if patients present with symptoms of acute HIV infection or report condomless sex in the previous 4 weeks.
To prevent drug resistance, “rigorous screening that includes NAAT is critical.” It reduces “the likelihood of PrEP start during undetected HIV infection,” said lead investigator Kavita Misra, PhD, MPH, a senior epidemiologist at the New York City Department of Health and Mental Hygiene.
Her team reviewed 3,685 people in New York who were diagnosed with HIV from November 2015 to August 2017; 91 had been on PrEP beforehand for a median of 106 days.
Postdiagnosis genotyping was available for 75% of PrEP users and 63% in the no-PrEP group. Resistance mutations to emtricitabine were significantly more prevalent with PrEP than without (29% versus 2%, respectively; P less than .0001). None of the PrEP users, but four in the no-PrEP group, had resistance to the other component of Truvada, tenofovir.
PrEP users were more likely to be diagnosed with HIV in the acute phase of infection than were those not using PrEP (33% versus 9%; P less than .0001), probably because they were also more likely to have regular office visits while on PrEP.
Skeptics at the Conference on Retroviruses and Opportunistic Infections, where the study was presented, wondered whether emtricitabine resistance would have been more common in the no-PrEP group if the infection had been picked up earlier because resistance fades as the infection progresses.
Dr. Misra said it was a good question and that her team will look into it. However, she stood by her conclusions.
PrEP use was most common among white men who have sex with men and among people under 30 years old.
There was no external funding, and the investigators didn’t have any disclosures.
SOURCE: Misra K et al. 2019 CROI, Abstract 107.
SEATTLE – Almost a third of patients who developed HIV while on pre-exposure prophylaxis (PrEP) had strains that were resistant to emtricitabine – a component of the PrEP medication Truvada along with tenofovir – at time of diagnosis, compared with just 2% of those not on PrEP, in a review of more than 3,500 newly diagnosed HIV cases in New York.
The finding points to a growing concern as use of PrEP becomes more common: the induction of resistance to HIV treatments.
PrEP is highly effective, so it’s likely that the PrEP patients already had HIV when they started treatment. Nucleic acid amplification testing (NAAT) is the only way to rule it out definitively, but only 5% of PrEP users in the study were screened with NAAT within 2 days of initiation.
The usual test – HIV antibody screening – returns a false negative in the window between HIV exposure and active infection, when antibodies turn positive, which can take months. NAAT, on the other hand, looks for evidence of the virus directly.
The findings led the investigators to urge routine NAAT screening before PrEP, something that New York State guidelines currently recommend only if patients present with symptoms of acute HIV infection or report condomless sex in the previous 4 weeks.
To prevent drug resistance, “rigorous screening that includes NAAT is critical.” It reduces “the likelihood of PrEP start during undetected HIV infection,” said lead investigator Kavita Misra, PhD, MPH, a senior epidemiologist at the New York City Department of Health and Mental Hygiene.
Her team reviewed 3,685 people in New York who were diagnosed with HIV from November 2015 to August 2017; 91 had been on PrEP beforehand for a median of 106 days.
Postdiagnosis genotyping was available for 75% of PrEP users and 63% in the no-PrEP group. Resistance mutations to emtricitabine were significantly more prevalent with PrEP than without (29% versus 2%, respectively; P less than .0001). None of the PrEP users, but four in the no-PrEP group, had resistance to the other component of Truvada, tenofovir.
PrEP users were more likely to be diagnosed with HIV in the acute phase of infection than were those not using PrEP (33% versus 9%; P less than .0001), probably because they were also more likely to have regular office visits while on PrEP.
Skeptics at the Conference on Retroviruses and Opportunistic Infections, where the study was presented, wondered whether emtricitabine resistance would have been more common in the no-PrEP group if the infection had been picked up earlier because resistance fades as the infection progresses.
Dr. Misra said it was a good question and that her team will look into it. However, she stood by her conclusions.
PrEP use was most common among white men who have sex with men and among people under 30 years old.
There was no external funding, and the investigators didn’t have any disclosures.
SOURCE: Misra K et al. 2019 CROI, Abstract 107.
REPORTING FROM CROI 2019
Newborn with desquamating rash
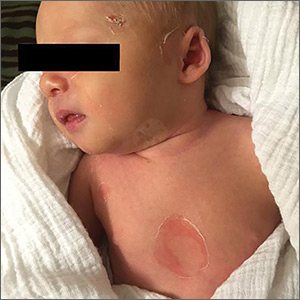
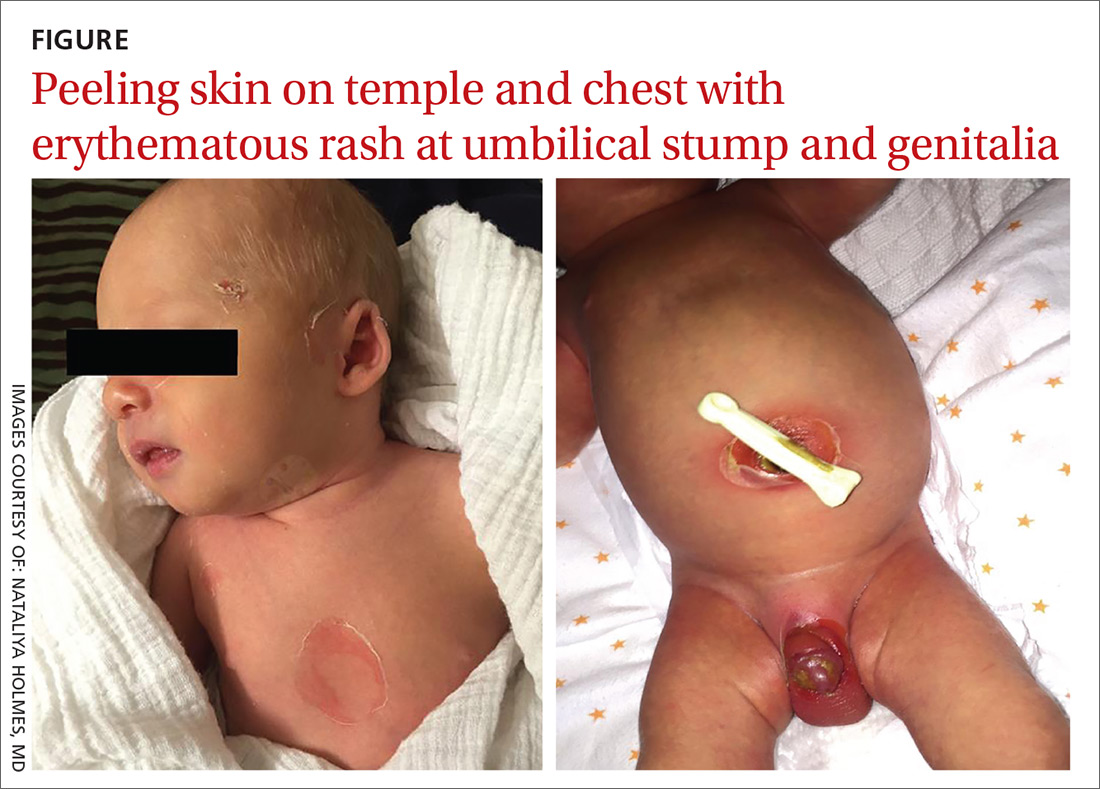
A 9-day-old boy was brought to the emergency department by his mother. The infant had been doing well until his most recent diaper change when his mother noticed a rash around the umbilicus (FIGURE), genitalia, and anus.
The infant was born at term via spontaneous vaginal delivery. The pregnancy was uncomplicated; the infant’s mother was group B strep negative. Following a routine postpartum course, the infant underwent an elective circumcision before hospital discharge on his second day of life. There were no interval reports of irritability, poor feeding, fevers, vomiting, or changes in urine or stool output.
The mother denied any recent unusual exposures, sick contacts, or travel. However, upon further questioning, the mother noted that she herself had several small open wounds on the torso that she attributed to untreated methicillin-resistant Staphylococcus aureus (MRSA).
On physical examination, the infant was overall well-appearing and was breastfeeding vigorously without respiratory distress or cyanosis. He was afebrile with normal vital signs. The majority of the physical examination was normal; however, there was erythematous desquamation around the umbilical stump and genitalia with no vesicles noted. The umbilical stump had a small amount of purulent drainage and necrosis centrally. The infant had a 1-cm round, peeling lesion on the left temple (FIGURE) with a small amount of dried serosanguinous drainage and similar superficial peeling lesions at the left preauricular area and anterior chest. There was no underlying fluctuance and only minimal surrounding erythema.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Staphylococcal scalded skin syndrome
Based on the age of the patient, clinical presentation, and suspected maternal MRSA infection (with possible transmission to the infant), we diagnosed staphylococcal scalded skin syndrome (SSSS) in this patient. SSSS is rare, with annual incidence of 45 cases per million US infants under the age of 2.1 Newborns with a generalized form of SSSS commonly present with fever, poor feeding, irritability, and lethargy. This is followed by a generalized erythematous rash that initially may appear on the head and neck and spread to the rest of the body. Large, fragile blisters subsequently appear. These blisters rupture on gentle pressure, which is known as a positive Nikolsky sign. Ultimately, large sheets of skin easily slough off, leaving raw, denuded skin.2
S aureus is not part of normal skin flora, yet it is found on the skin and mucous membranes of 19% to 55% of healthy adults and children.3S aureus can cause a wide range of infections ranging from abscesses to cellulitis; SSSS is caused by hematogenous spread of S aureus exfoliative toxin. Newborns and immunocompromised patients are particularly susceptible.
Neonatal patients with SSSS most commonly present at 3 to 16 days of age.2 The lack of antitoxin antibody in neonates allows the toxin to reach the epidermis where it acts locally to produce the characteristic fragile skin lesions that often rupture prior to clinical presentation.2,4 During progression of the disease, flaky skin desquamation will occur as the lesions heal.
A retrospective review of 39 cases of SSSS identified pneumonia as the most frequent complication, occurring in 74.4% of the cases.5 The mortality rate of SSSS is up to 5%, and is associated with sepsis, superinfection, electrolyte imbalances, and extensive skin involvement.2,6
If SSSS is suspected, obtain cultures from the blood, urine, eyes, nose, throat, and skin lesions to identify the primary focus of infection.7 However, the retrospective review of 39 cases (noted above) found a positive rate of S aureus isolation of only 23.5%.5 Physicians will often have to make a diagnosis based on clinical presentation and empirically initiate broad-spectrum antibiotics while considering alternative diagnoses.
Continue to: A clinical diagnosis with a large differential
A clinical diagnosis with a large differential
While biopsy rarely is required, it may be helpful to distinguish SSSS from other entities in the differential diagnosis (TABLE2,3,7-13).
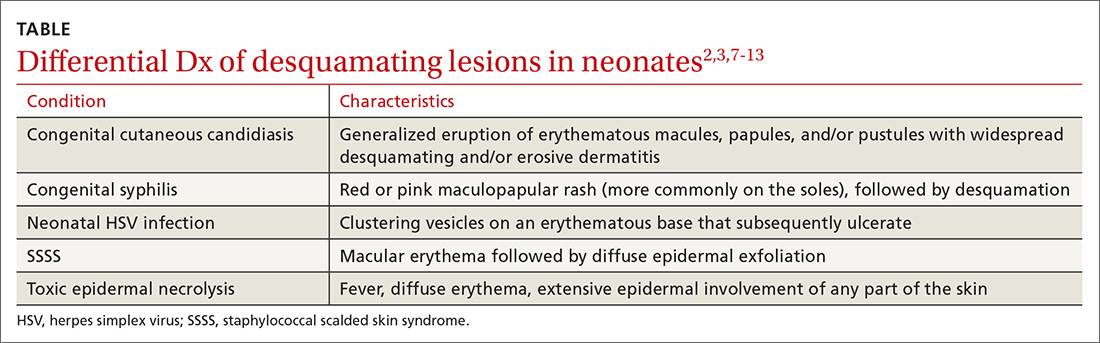
Toxic epidermal necrolysis (TEN) is a rare and life-threatening desquamating disease nearly always caused by a reaction to medications, including antibiotics. TEN can occur at any age. Fever, diffuse erythema, and extensive epidermal involvement (>30% of skin) differentiate TEN from Stevens-Johnson syndrome (SJS), which affects less than 10% of the epidermis. It is worth mentioning that TEN and SJS are now considered to be a spectrum of one disease, and an overlap syndrome has been described with 10% to 30% of skin affected.8 Diagnosis is made clinically, although skin biopsy routinely is performed.7,9
Congenital syphilis features a red or pink maculopapular rash followed by desquamation. Lesions are more common on the soles.10 Desquamation or ulcerative skin lesions should be examined for spirochetes.11 A quantitative, nontreponemal test such as the rapid plasma reagin (RPR) or the Venereal Disease Research Laboratory (VDRL) will be positive in most infants if exposed through the placenta, but antibodies will disappear in uninfected infants by 6 months of age.8
Congenital cutaneous candidiasis presents with a generalized eruption of erythematous macules, papules, and/or pustules with widespread desquamating and/or erosive dermatitis. Premature neonates with extremely low birth weight are at higher risk.13 Diagnosis is confirmed on microscopy by the presence of Candida albicans spores in skin scrapings.13
Neonatal herpes simplex virus (HSV) symptoms typically appear between 1 and 3 weeks of life, with 60% to 70% of cases presenting with classic clustering vesicles on an erythematous base.14 Diagnosis is made with HSV viral culture or polymerase chain reaction (PCR).
Continue to: SSSS should be considered a pediatrics emergency
SSSS should be considered a pediatric emergency
SSSS should be considered a pediatric emergency due to potential complications. Core measures of SSSS treatment include immediate administration of intravenous (IV) antibiotics. US population studies suggest clindamycin and penicillinase-resistant penicillin as empiric therapy.15 However, local strains and resistance patterns, including the prevalence of MRSA, as well as age, comorbidities, and severity of illness should influence antibiotic selection.
IV nafcillin or oxacillin may be used with pediatric dosing of 150 mg/kg daily divided every 6 hours for methicillin-sensitive Staphylococcus aureus (MSSA). For suspected MRSA, IV vancomycin should be considered, with an infant dose of 40 to 60 mg/kg daily divided every 6 hours.16 Fluid, electrolyte, and nutritional management should be addressed immediately. Ongoing fluid losses due to exfoliated skin must be replaced, and skin care to desquamated areas also should be addressed urgently.
Our patient. Phone consultation with an infectious disease specialist at a local children’s hospital resulted in a recommendation to treat for sepsis empirically with IV vancomycin, cefotaxime, and acyclovir. Acyclovir was discontinued once the HSV PCR came back negative. The antibiotic coverage was narrowed to IV ampicillin 50 mg/kg every 8 hours when cerebrospinal fluid and blood cultures returned negative at 48 hours, wound culture sensitivity grew MSSA, and the patient’s clinical condition stabilized. Our patient received 10 days of IV antibiotics and was discharged on oral amoxicillin 50 mg/kg divided twice daily for a total of 14 days of treatment per recommendations by the infectious disease specialist. Our patient fully recovered without any residual skin findings after completion of the antibiotic course.
CORRESPONDENCE
Jennifer J. Walker, MD, MPH, Hawaii Island Family Health Center at Hilo Medical Center, 1190 Waianuenue Ave, Hilo, HI 96720; [email protected]
1. Staiman A, Hsu D, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in US children. Br J Dermatol. 2018;178:704-708.
2. Ladhani S, Joannou CL, Lochrie DP, et al. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224-242.
3. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505-520.
4. Ladhani S. Understanding the mechanism of action of the exfoliative toxins of Staphylococcus aureus. FEMS Immunol Med Microbiol. 2003;39:181-189.
5. Li MY, Hua Y, Wei GH, et al. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol. 2014;31:43-47.
6. Berk DR, Bayliss SJ. MRSA, staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann. 2010;39:627-633.
7. Ely JW, Seabury Stone M. The generalized rash: part I. differential diagnosis. Am Fam Physician. 2010;81:726-734.
8. Bastuji-Garin SB, Stern RS, Shear NH, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92.
9. Elias PM, Fritsch P, Epstein EH. Staphylococcal scalded skin syndrome. clinical features, pathogenesis, and recent microbiological and biochemical developments. Arch Dermatol. 1977;113:207-219.
10. O’Connor NR, McLaughlin M, Ham P. Newborn skin: part I: common rashes. Am Fam Physician. 2008;77:47-52.
11. Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995;8:1-21.
12. Arnold SR, Ford-Jones EL. Congenital syphilis: a guide to diagnosis and management. Paediatr Child Health. 2000;5:463-469.
13. Darmstadt GL, Dinulos JG, Miller Z. Congenital cutaneous candidiasis: clinical presentation, pathogenesis, and management guidelines. Pediatrics. 2000;105:438-444.
14. Kimberlin DW. Neonatal herpes simplex infection. Clin Microbiol Rev. 2004;17:1-13.
15. Braunstein I, Wanat KA, Abuabara K, et al. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31:305-308.
16. Gilbert DN, Chambers HF, Eliopoulos GM, et al. The Sanford Guide to Antimicrobial Therapy. 48th ed. Sperryville, VA: Antimicrobial Therapy, Inc; 2014:56.
A 9-day-old boy was brought to the emergency department by his mother. The infant had been doing well until his most recent diaper change when his mother noticed a rash around the umbilicus (FIGURE), genitalia, and anus.
The infant was born at term via spontaneous vaginal delivery. The pregnancy was uncomplicated; the infant’s mother was group B strep negative. Following a routine postpartum course, the infant underwent an elective circumcision before hospital discharge on his second day of life. There were no interval reports of irritability, poor feeding, fevers, vomiting, or changes in urine or stool output.
The mother denied any recent unusual exposures, sick contacts, or travel. However, upon further questioning, the mother noted that she herself had several small open wounds on the torso that she attributed to untreated methicillin-resistant Staphylococcus aureus (MRSA).
On physical examination, the infant was overall well-appearing and was breastfeeding vigorously without respiratory distress or cyanosis. He was afebrile with normal vital signs. The majority of the physical examination was normal; however, there was erythematous desquamation around the umbilical stump and genitalia with no vesicles noted. The umbilical stump had a small amount of purulent drainage and necrosis centrally. The infant had a 1-cm round, peeling lesion on the left temple (FIGURE) with a small amount of dried serosanguinous drainage and similar superficial peeling lesions at the left preauricular area and anterior chest. There was no underlying fluctuance and only minimal surrounding erythema.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Staphylococcal scalded skin syndrome
Based on the age of the patient, clinical presentation, and suspected maternal MRSA infection (with possible transmission to the infant), we diagnosed staphylococcal scalded skin syndrome (SSSS) in this patient. SSSS is rare, with annual incidence of 45 cases per million US infants under the age of 2.1 Newborns with a generalized form of SSSS commonly present with fever, poor feeding, irritability, and lethargy. This is followed by a generalized erythematous rash that initially may appear on the head and neck and spread to the rest of the body. Large, fragile blisters subsequently appear. These blisters rupture on gentle pressure, which is known as a positive Nikolsky sign. Ultimately, large sheets of skin easily slough off, leaving raw, denuded skin.2
S aureus is not part of normal skin flora, yet it is found on the skin and mucous membranes of 19% to 55% of healthy adults and children.3S aureus can cause a wide range of infections ranging from abscesses to cellulitis; SSSS is caused by hematogenous spread of S aureus exfoliative toxin. Newborns and immunocompromised patients are particularly susceptible.
Neonatal patients with SSSS most commonly present at 3 to 16 days of age.2 The lack of antitoxin antibody in neonates allows the toxin to reach the epidermis where it acts locally to produce the characteristic fragile skin lesions that often rupture prior to clinical presentation.2,4 During progression of the disease, flaky skin desquamation will occur as the lesions heal.
A retrospective review of 39 cases of SSSS identified pneumonia as the most frequent complication, occurring in 74.4% of the cases.5 The mortality rate of SSSS is up to 5%, and is associated with sepsis, superinfection, electrolyte imbalances, and extensive skin involvement.2,6
If SSSS is suspected, obtain cultures from the blood, urine, eyes, nose, throat, and skin lesions to identify the primary focus of infection.7 However, the retrospective review of 39 cases (noted above) found a positive rate of S aureus isolation of only 23.5%.5 Physicians will often have to make a diagnosis based on clinical presentation and empirically initiate broad-spectrum antibiotics while considering alternative diagnoses.
Continue to: A clinical diagnosis with a large differential
A clinical diagnosis with a large differential
While biopsy rarely is required, it may be helpful to distinguish SSSS from other entities in the differential diagnosis (TABLE2,3,7-13).

Toxic epidermal necrolysis (TEN) is a rare and life-threatening desquamating disease nearly always caused by a reaction to medications, including antibiotics. TEN can occur at any age. Fever, diffuse erythema, and extensive epidermal involvement (>30% of skin) differentiate TEN from Stevens-Johnson syndrome (SJS), which affects less than 10% of the epidermis. It is worth mentioning that TEN and SJS are now considered to be a spectrum of one disease, and an overlap syndrome has been described with 10% to 30% of skin affected.8 Diagnosis is made clinically, although skin biopsy routinely is performed.7,9
Congenital syphilis features a red or pink maculopapular rash followed by desquamation. Lesions are more common on the soles.10 Desquamation or ulcerative skin lesions should be examined for spirochetes.11 A quantitative, nontreponemal test such as the rapid plasma reagin (RPR) or the Venereal Disease Research Laboratory (VDRL) will be positive in most infants if exposed through the placenta, but antibodies will disappear in uninfected infants by 6 months of age.8
Congenital cutaneous candidiasis presents with a generalized eruption of erythematous macules, papules, and/or pustules with widespread desquamating and/or erosive dermatitis. Premature neonates with extremely low birth weight are at higher risk.13 Diagnosis is confirmed on microscopy by the presence of Candida albicans spores in skin scrapings.13
Neonatal herpes simplex virus (HSV) symptoms typically appear between 1 and 3 weeks of life, with 60% to 70% of cases presenting with classic clustering vesicles on an erythematous base.14 Diagnosis is made with HSV viral culture or polymerase chain reaction (PCR).
Continue to: SSSS should be considered a pediatrics emergency
SSSS should be considered a pediatric emergency
SSSS should be considered a pediatric emergency due to potential complications. Core measures of SSSS treatment include immediate administration of intravenous (IV) antibiotics. US population studies suggest clindamycin and penicillinase-resistant penicillin as empiric therapy.15 However, local strains and resistance patterns, including the prevalence of MRSA, as well as age, comorbidities, and severity of illness should influence antibiotic selection.
IV nafcillin or oxacillin may be used with pediatric dosing of 150 mg/kg daily divided every 6 hours for methicillin-sensitive Staphylococcus aureus (MSSA). For suspected MRSA, IV vancomycin should be considered, with an infant dose of 40 to 60 mg/kg daily divided every 6 hours.16 Fluid, electrolyte, and nutritional management should be addressed immediately. Ongoing fluid losses due to exfoliated skin must be replaced, and skin care to desquamated areas also should be addressed urgently.
Our patient. Phone consultation with an infectious disease specialist at a local children’s hospital resulted in a recommendation to treat for sepsis empirically with IV vancomycin, cefotaxime, and acyclovir. Acyclovir was discontinued once the HSV PCR came back negative. The antibiotic coverage was narrowed to IV ampicillin 50 mg/kg every 8 hours when cerebrospinal fluid and blood cultures returned negative at 48 hours, wound culture sensitivity grew MSSA, and the patient’s clinical condition stabilized. Our patient received 10 days of IV antibiotics and was discharged on oral amoxicillin 50 mg/kg divided twice daily for a total of 14 days of treatment per recommendations by the infectious disease specialist. Our patient fully recovered without any residual skin findings after completion of the antibiotic course.
CORRESPONDENCE
Jennifer J. Walker, MD, MPH, Hawaii Island Family Health Center at Hilo Medical Center, 1190 Waianuenue Ave, Hilo, HI 96720; [email protected]
A 9-day-old boy was brought to the emergency department by his mother. The infant had been doing well until his most recent diaper change when his mother noticed a rash around the umbilicus (FIGURE), genitalia, and anus.
The infant was born at term via spontaneous vaginal delivery. The pregnancy was uncomplicated; the infant’s mother was group B strep negative. Following a routine postpartum course, the infant underwent an elective circumcision before hospital discharge on his second day of life. There were no interval reports of irritability, poor feeding, fevers, vomiting, or changes in urine or stool output.
The mother denied any recent unusual exposures, sick contacts, or travel. However, upon further questioning, the mother noted that she herself had several small open wounds on the torso that she attributed to untreated methicillin-resistant Staphylococcus aureus (MRSA).
On physical examination, the infant was overall well-appearing and was breastfeeding vigorously without respiratory distress or cyanosis. He was afebrile with normal vital signs. The majority of the physical examination was normal; however, there was erythematous desquamation around the umbilical stump and genitalia with no vesicles noted. The umbilical stump had a small amount of purulent drainage and necrosis centrally. The infant had a 1-cm round, peeling lesion on the left temple (FIGURE) with a small amount of dried serosanguinous drainage and similar superficial peeling lesions at the left preauricular area and anterior chest. There was no underlying fluctuance and only minimal surrounding erythema.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Staphylococcal scalded skin syndrome
Based on the age of the patient, clinical presentation, and suspected maternal MRSA infection (with possible transmission to the infant), we diagnosed staphylococcal scalded skin syndrome (SSSS) in this patient. SSSS is rare, with annual incidence of 45 cases per million US infants under the age of 2.1 Newborns with a generalized form of SSSS commonly present with fever, poor feeding, irritability, and lethargy. This is followed by a generalized erythematous rash that initially may appear on the head and neck and spread to the rest of the body. Large, fragile blisters subsequently appear. These blisters rupture on gentle pressure, which is known as a positive Nikolsky sign. Ultimately, large sheets of skin easily slough off, leaving raw, denuded skin.2
S aureus is not part of normal skin flora, yet it is found on the skin and mucous membranes of 19% to 55% of healthy adults and children.3S aureus can cause a wide range of infections ranging from abscesses to cellulitis; SSSS is caused by hematogenous spread of S aureus exfoliative toxin. Newborns and immunocompromised patients are particularly susceptible.
Neonatal patients with SSSS most commonly present at 3 to 16 days of age.2 The lack of antitoxin antibody in neonates allows the toxin to reach the epidermis where it acts locally to produce the characteristic fragile skin lesions that often rupture prior to clinical presentation.2,4 During progression of the disease, flaky skin desquamation will occur as the lesions heal.
A retrospective review of 39 cases of SSSS identified pneumonia as the most frequent complication, occurring in 74.4% of the cases.5 The mortality rate of SSSS is up to 5%, and is associated with sepsis, superinfection, electrolyte imbalances, and extensive skin involvement.2,6
If SSSS is suspected, obtain cultures from the blood, urine, eyes, nose, throat, and skin lesions to identify the primary focus of infection.7 However, the retrospective review of 39 cases (noted above) found a positive rate of S aureus isolation of only 23.5%.5 Physicians will often have to make a diagnosis based on clinical presentation and empirically initiate broad-spectrum antibiotics while considering alternative diagnoses.
Continue to: A clinical diagnosis with a large differential
A clinical diagnosis with a large differential
While biopsy rarely is required, it may be helpful to distinguish SSSS from other entities in the differential diagnosis (TABLE2,3,7-13).

Toxic epidermal necrolysis (TEN) is a rare and life-threatening desquamating disease nearly always caused by a reaction to medications, including antibiotics. TEN can occur at any age. Fever, diffuse erythema, and extensive epidermal involvement (>30% of skin) differentiate TEN from Stevens-Johnson syndrome (SJS), which affects less than 10% of the epidermis. It is worth mentioning that TEN and SJS are now considered to be a spectrum of one disease, and an overlap syndrome has been described with 10% to 30% of skin affected.8 Diagnosis is made clinically, although skin biopsy routinely is performed.7,9
Congenital syphilis features a red or pink maculopapular rash followed by desquamation. Lesions are more common on the soles.10 Desquamation or ulcerative skin lesions should be examined for spirochetes.11 A quantitative, nontreponemal test such as the rapid plasma reagin (RPR) or the Venereal Disease Research Laboratory (VDRL) will be positive in most infants if exposed through the placenta, but antibodies will disappear in uninfected infants by 6 months of age.8
Congenital cutaneous candidiasis presents with a generalized eruption of erythematous macules, papules, and/or pustules with widespread desquamating and/or erosive dermatitis. Premature neonates with extremely low birth weight are at higher risk.13 Diagnosis is confirmed on microscopy by the presence of Candida albicans spores in skin scrapings.13
Neonatal herpes simplex virus (HSV) symptoms typically appear between 1 and 3 weeks of life, with 60% to 70% of cases presenting with classic clustering vesicles on an erythematous base.14 Diagnosis is made with HSV viral culture or polymerase chain reaction (PCR).
Continue to: SSSS should be considered a pediatrics emergency
SSSS should be considered a pediatric emergency
SSSS should be considered a pediatric emergency due to potential complications. Core measures of SSSS treatment include immediate administration of intravenous (IV) antibiotics. US population studies suggest clindamycin and penicillinase-resistant penicillin as empiric therapy.15 However, local strains and resistance patterns, including the prevalence of MRSA, as well as age, comorbidities, and severity of illness should influence antibiotic selection.
IV nafcillin or oxacillin may be used with pediatric dosing of 150 mg/kg daily divided every 6 hours for methicillin-sensitive Staphylococcus aureus (MSSA). For suspected MRSA, IV vancomycin should be considered, with an infant dose of 40 to 60 mg/kg daily divided every 6 hours.16 Fluid, electrolyte, and nutritional management should be addressed immediately. Ongoing fluid losses due to exfoliated skin must be replaced, and skin care to desquamated areas also should be addressed urgently.
Our patient. Phone consultation with an infectious disease specialist at a local children’s hospital resulted in a recommendation to treat for sepsis empirically with IV vancomycin, cefotaxime, and acyclovir. Acyclovir was discontinued once the HSV PCR came back negative. The antibiotic coverage was narrowed to IV ampicillin 50 mg/kg every 8 hours when cerebrospinal fluid and blood cultures returned negative at 48 hours, wound culture sensitivity grew MSSA, and the patient’s clinical condition stabilized. Our patient received 10 days of IV antibiotics and was discharged on oral amoxicillin 50 mg/kg divided twice daily for a total of 14 days of treatment per recommendations by the infectious disease specialist. Our patient fully recovered without any residual skin findings after completion of the antibiotic course.
CORRESPONDENCE
Jennifer J. Walker, MD, MPH, Hawaii Island Family Health Center at Hilo Medical Center, 1190 Waianuenue Ave, Hilo, HI 96720; [email protected]
1. Staiman A, Hsu D, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in US children. Br J Dermatol. 2018;178:704-708.
2. Ladhani S, Joannou CL, Lochrie DP, et al. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224-242.
3. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505-520.
4. Ladhani S. Understanding the mechanism of action of the exfoliative toxins of Staphylococcus aureus. FEMS Immunol Med Microbiol. 2003;39:181-189.
5. Li MY, Hua Y, Wei GH, et al. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol. 2014;31:43-47.
6. Berk DR, Bayliss SJ. MRSA, staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann. 2010;39:627-633.
7. Ely JW, Seabury Stone M. The generalized rash: part I. differential diagnosis. Am Fam Physician. 2010;81:726-734.
8. Bastuji-Garin SB, Stern RS, Shear NH, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92.
9. Elias PM, Fritsch P, Epstein EH. Staphylococcal scalded skin syndrome. clinical features, pathogenesis, and recent microbiological and biochemical developments. Arch Dermatol. 1977;113:207-219.
10. O’Connor NR, McLaughlin M, Ham P. Newborn skin: part I: common rashes. Am Fam Physician. 2008;77:47-52.
11. Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995;8:1-21.
12. Arnold SR, Ford-Jones EL. Congenital syphilis: a guide to diagnosis and management. Paediatr Child Health. 2000;5:463-469.
13. Darmstadt GL, Dinulos JG, Miller Z. Congenital cutaneous candidiasis: clinical presentation, pathogenesis, and management guidelines. Pediatrics. 2000;105:438-444.
14. Kimberlin DW. Neonatal herpes simplex infection. Clin Microbiol Rev. 2004;17:1-13.
15. Braunstein I, Wanat KA, Abuabara K, et al. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31:305-308.
16. Gilbert DN, Chambers HF, Eliopoulos GM, et al. The Sanford Guide to Antimicrobial Therapy. 48th ed. Sperryville, VA: Antimicrobial Therapy, Inc; 2014:56.
1. Staiman A, Hsu D, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in US children. Br J Dermatol. 2018;178:704-708.
2. Ladhani S, Joannou CL, Lochrie DP, et al. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224-242.
3. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505-520.
4. Ladhani S. Understanding the mechanism of action of the exfoliative toxins of Staphylococcus aureus. FEMS Immunol Med Microbiol. 2003;39:181-189.
5. Li MY, Hua Y, Wei GH, et al. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol. 2014;31:43-47.
6. Berk DR, Bayliss SJ. MRSA, staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann. 2010;39:627-633.
7. Ely JW, Seabury Stone M. The generalized rash: part I. differential diagnosis. Am Fam Physician. 2010;81:726-734.
8. Bastuji-Garin SB, Stern RS, Shear NH, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92.
9. Elias PM, Fritsch P, Epstein EH. Staphylococcal scalded skin syndrome. clinical features, pathogenesis, and recent microbiological and biochemical developments. Arch Dermatol. 1977;113:207-219.
10. O’Connor NR, McLaughlin M, Ham P. Newborn skin: part I: common rashes. Am Fam Physician. 2008;77:47-52.
11. Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995;8:1-21.
12. Arnold SR, Ford-Jones EL. Congenital syphilis: a guide to diagnosis and management. Paediatr Child Health. 2000;5:463-469.
13. Darmstadt GL, Dinulos JG, Miller Z. Congenital cutaneous candidiasis: clinical presentation, pathogenesis, and management guidelines. Pediatrics. 2000;105:438-444.
14. Kimberlin DW. Neonatal herpes simplex infection. Clin Microbiol Rev. 2004;17:1-13.
15. Braunstein I, Wanat KA, Abuabara K, et al. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31:305-308.
16. Gilbert DN, Chambers HF, Eliopoulos GM, et al. The Sanford Guide to Antimicrobial Therapy. 48th ed. Sperryville, VA: Antimicrobial Therapy, Inc; 2014:56.
Subacute polyarticular arthralgias • swelling of the ankles and right knee • recent travel to the Dominican Republic • Dx?
THE CASE
A 78-year-old woman with a history of anxiety and hypertension presented to our family medicine residency practice in Massachusetts with subacute polyarticular arthralgias that had been present for 2 months. She complained of pain and swelling of both ankles and the right knee. She noted that her symptoms had started on a recent trip to the Dominican Republic, where she developed generalized joint pain and a fever that lasted 1 to 2 weeks and subsequently resolved with the lingering polyarthralgias. She denied any rash, constitutional symptoms, photosensitivity, headaches, photophobia, or history of tick bite. Physical examination revealed normal vital signs, notable warmth and swelling of the bilateral ankles that was worse on the right side, and swelling of the right knee with effusion—but no tenderness—to palpation.
THE DIAGNOSIS
The patient’s labwork revealed a white blood cell count of 5900/mcL (reference range, 4500–11,000/mcL), hemoglobin count of 12.5 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 230×103/mcL. Electrolytes and renal function were normal. She had an elevated erythrocyte sedimentation rate of 34 mm/h (reference range, 0–20 mm/h) and a positive antinuclear antibody (ANA) test, but no titer was reported. Anti-chikungunya IgG and IgM antibodies were positive on enzyme-linked immunosorbent assay (ELISA) serologic testing.
DISCUSSION
Chikungunya is an infectious disease that is relatively rare in the United States. Chikungunya was rarely identified in American travelers prior to 2006, but incidence increased over the next decade. In 2014, a total of 2811 cases were reported.1 Chikungunya is an RNA arbovirus that is transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is endemic to West Africa. Within the last 2 decades, there has been an increasing number of outbreaks in India, Asia, Europe, and the Americas, where the highest incidence is in South America, followed by Central America. In the United States, almost all reported cases of chikungunya infection have been in travelers returning from endemic areas.2 The first 2 known cases of local transmission in the United States were reported in Florida in July 2014.3 Local transmission of chikungunya is significant in that it represents the possibility of a local reservoir for sustained transmission.
Disease presentation. Patients will initially complain of a high fever and severe distal polyarthralgias that usually are symmetric. The most common symptoms are polyarthralgias (87%–98% of patients), myalgias (46%–59%), and a maculopapular rash
The term chikungunya is derived from a Kimakonde (central Bantu) word meaning “that which bends up” because of the arthralgia caused by the disease. Fever usually lasts 3 to 7 days; polyarthralgia begins shortly after the onset of fever.4 Frank arthritis also may be present. Infection often exacerbates a previously damaged or diseased joint. Acute symptoms usually persist for 1 to 2 weeks, but arthralgias and arthritis can persist for months to years following resolution of the acute disease.6 In one study of 47 patients with acute chikungunya in Marseilles, France, the number of patients who were symptomatic declined from 88% to 86%, 48%, and 4% at 1, 3, 6, and 15 months, respectively.7
The differential diagnosis includes tropical infectious diseases (dengue, chikungunya, Zika, and leptospirosis) in patients who have recently traveled to the tropics and who complain of subacute polyarticular arthralgias or arthritis; locally acquired infections associated with arthralgia/arthritis such as Lyme disease and other tick-borne diseases and rickettsial infections; parvovirus B19 and other postinfectious arthritides; and rheumatologic conditions such as systemic lupus.
Clinical differentiation among dengue, chikungunya, and Zika may be difficult, although persistent frank arthritis is much more common in chikungunya than in dengue or Zika. Furthermore, conjunctivitis is present in Zika but is absent in chikungunya. Chikungunya also is more likely to cause high fever, severe arthralgia, arthritis, rash, and lymphopenia than Zika or dengue. Dengue is more likely to cause lymphopenia and hemorrhagic consequences than is chikungunya or Zika.8
Continue to: In our patient...
In our patient, dengue titers were not obtained because the duration of symptoms was thought to be more consistent with chikungunya, but testing for dengue also would have been appropriate.
The most common test for diagnosing acute chikungunya is ELISA serologic testing for IgM antibodies, which develop toward the end of the first week of infection; earlier in that first week, serum testing for viral RNA may be performed by polymerase chain reaction.
Treatment is largely supportive
Treatment of acute chikungunya is largely supportive and includes anti-inflammatory agents. To our knowledge, no antiviral agents have been shown to be effective. Postacute or chronic symptoms may require treatment with glucocorticoids or other immunomodulatory medications. A 2017 literature review of treatments for chikungunya-associated rheumatic disorders showed evidence that chloroquine was more effective than placebo for chronic pain relief. Also, adding a disease-modifying antirheumatic agent in combination with chloroquine was more effective for controlling pain and reducing disability than hydroxychloroquine monotherapy.10
Our patient was treated with ibuprofen only and experienced resolution of joint symptoms several months after the initial presentation. A repeat ANA test 12 months later was negative.
A 2009 review of the medical literature revealed a single case report of chikungunya associated with positive ANA.8 Although a positive ANA may be associated with acute viral infections, significantly elevated ANA levels typically are associated with autoimmunity. Resolution of the patient’s serum ANA 1 year later suggested that the positive ANA was not secondary to a pre-existing rheumatologic condition but rather a consequence of her body’s response to the chikungunya infection itself. Our case raises the hypothesis that, at least in some cases, chikungunya somehow stimulates a temporary autoimmune response, which may help explain why immunomodulatory medications can be effective treatment options.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Chikungunya is increasingly common in tropical and subtropical regions. Family physicians practicing in the United States should become familiar with the common patterns of presentation of viruses such as chikungunya, dengue, and Zika. Obtaining a travel history for patients presenting with arthritis improves the differential diagnosis and may even reveal the cause of the condition.
CORRESPONDENCE
Jeremy Golding, MD, 279 Lincoln Street, Worcester, MA 01605; [email protected]
1. Chikungunya virus. Centers for Disease Control and Prevention website. https://www.cdc.gov/chikungunya/geo/united-states.html. Reviewed December 17, 2018. Accessed March 5, 2019.
2. Pan American Health Organization. Preparedness and response for chikungunya virus: introduction into the Americas. https://www.paho.org/hq/dmdocuments/2012/CHIKV-English.pdf. Published 2011. Accessed March 5, 2019.
3. First chikungunya case acquired in the United States reported in Florida [press release]. Atlanta, GA: Centers for Disease Control and Prevention; July 17, 2014. http://www.cdc.gov/media/releases/2014/p0717-chikungunya.html. Accessed March 5, 2019.
4. Taubitz W, Cramer JP, Kapaun A, et al. Chikungunya fever in travelers: clinical presentation and course [published online May 23, 2007]. Clin Infect Dis. 2007;45:e1-e4.
5. Thiberville SD, Moyen N, Dupuis-Maguiraga L, et al. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013;99:345-370.
6. Burt FJ, Rolph MS, Rulli NE, et al. Chikungunya: a re-emerging virus. Lancet. 2012;379:662-671.
7. Simon F, Parola P, Grandadam M, et al. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine (Baltimore). 2007;86:123-137.
8. Chikungunya virus. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/chikungunya/hc/clinicalevaluation.html. Reviewed December 17, 2018. Accessed March 5, 2019.
9. Petersen LR, Jamieson DJ, Powers AM, et al. Zika virus. N Engl J Med. 2016;374:1552-1563.
10. Martí-Carvajal A, Ramon-Pardo P, Javelle E, et al. Interventions for treating patients with chikungunya virus infection-related rheumatic and musculoskeletal disorders: a systematic review. PLoS One. 2017;12:e0179028.
THE CASE
A 78-year-old woman with a history of anxiety and hypertension presented to our family medicine residency practice in Massachusetts with subacute polyarticular arthralgias that had been present for 2 months. She complained of pain and swelling of both ankles and the right knee. She noted that her symptoms had started on a recent trip to the Dominican Republic, where she developed generalized joint pain and a fever that lasted 1 to 2 weeks and subsequently resolved with the lingering polyarthralgias. She denied any rash, constitutional symptoms, photosensitivity, headaches, photophobia, or history of tick bite. Physical examination revealed normal vital signs, notable warmth and swelling of the bilateral ankles that was worse on the right side, and swelling of the right knee with effusion—but no tenderness—to palpation.
THE DIAGNOSIS
The patient’s labwork revealed a white blood cell count of 5900/mcL (reference range, 4500–11,000/mcL), hemoglobin count of 12.5 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 230×103/mcL. Electrolytes and renal function were normal. She had an elevated erythrocyte sedimentation rate of 34 mm/h (reference range, 0–20 mm/h) and a positive antinuclear antibody (ANA) test, but no titer was reported. Anti-chikungunya IgG and IgM antibodies were positive on enzyme-linked immunosorbent assay (ELISA) serologic testing.
DISCUSSION
Chikungunya is an infectious disease that is relatively rare in the United States. Chikungunya was rarely identified in American travelers prior to 2006, but incidence increased over the next decade. In 2014, a total of 2811 cases were reported.1 Chikungunya is an RNA arbovirus that is transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is endemic to West Africa. Within the last 2 decades, there has been an increasing number of outbreaks in India, Asia, Europe, and the Americas, where the highest incidence is in South America, followed by Central America. In the United States, almost all reported cases of chikungunya infection have been in travelers returning from endemic areas.2 The first 2 known cases of local transmission in the United States were reported in Florida in July 2014.3 Local transmission of chikungunya is significant in that it represents the possibility of a local reservoir for sustained transmission.
Disease presentation. Patients will initially complain of a high fever and severe distal polyarthralgias that usually are symmetric. The most common symptoms are polyarthralgias (87%–98% of patients), myalgias (46%–59%), and a maculopapular rash
The term chikungunya is derived from a Kimakonde (central Bantu) word meaning “that which bends up” because of the arthralgia caused by the disease. Fever usually lasts 3 to 7 days; polyarthralgia begins shortly after the onset of fever.4 Frank arthritis also may be present. Infection often exacerbates a previously damaged or diseased joint. Acute symptoms usually persist for 1 to 2 weeks, but arthralgias and arthritis can persist for months to years following resolution of the acute disease.6 In one study of 47 patients with acute chikungunya in Marseilles, France, the number of patients who were symptomatic declined from 88% to 86%, 48%, and 4% at 1, 3, 6, and 15 months, respectively.7
The differential diagnosis includes tropical infectious diseases (dengue, chikungunya, Zika, and leptospirosis) in patients who have recently traveled to the tropics and who complain of subacute polyarticular arthralgias or arthritis; locally acquired infections associated with arthralgia/arthritis such as Lyme disease and other tick-borne diseases and rickettsial infections; parvovirus B19 and other postinfectious arthritides; and rheumatologic conditions such as systemic lupus.
Clinical differentiation among dengue, chikungunya, and Zika may be difficult, although persistent frank arthritis is much more common in chikungunya than in dengue or Zika. Furthermore, conjunctivitis is present in Zika but is absent in chikungunya. Chikungunya also is more likely to cause high fever, severe arthralgia, arthritis, rash, and lymphopenia than Zika or dengue. Dengue is more likely to cause lymphopenia and hemorrhagic consequences than is chikungunya or Zika.8
Continue to: In our patient...
In our patient, dengue titers were not obtained because the duration of symptoms was thought to be more consistent with chikungunya, but testing for dengue also would have been appropriate.
The most common test for diagnosing acute chikungunya is ELISA serologic testing for IgM antibodies, which develop toward the end of the first week of infection; earlier in that first week, serum testing for viral RNA may be performed by polymerase chain reaction.
Treatment is largely supportive
Treatment of acute chikungunya is largely supportive and includes anti-inflammatory agents. To our knowledge, no antiviral agents have been shown to be effective. Postacute or chronic symptoms may require treatment with glucocorticoids or other immunomodulatory medications. A 2017 literature review of treatments for chikungunya-associated rheumatic disorders showed evidence that chloroquine was more effective than placebo for chronic pain relief. Also, adding a disease-modifying antirheumatic agent in combination with chloroquine was more effective for controlling pain and reducing disability than hydroxychloroquine monotherapy.10
Our patient was treated with ibuprofen only and experienced resolution of joint symptoms several months after the initial presentation. A repeat ANA test 12 months later was negative.
A 2009 review of the medical literature revealed a single case report of chikungunya associated with positive ANA.8 Although a positive ANA may be associated with acute viral infections, significantly elevated ANA levels typically are associated with autoimmunity. Resolution of the patient’s serum ANA 1 year later suggested that the positive ANA was not secondary to a pre-existing rheumatologic condition but rather a consequence of her body’s response to the chikungunya infection itself. Our case raises the hypothesis that, at least in some cases, chikungunya somehow stimulates a temporary autoimmune response, which may help explain why immunomodulatory medications can be effective treatment options.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Chikungunya is increasingly common in tropical and subtropical regions. Family physicians practicing in the United States should become familiar with the common patterns of presentation of viruses such as chikungunya, dengue, and Zika. Obtaining a travel history for patients presenting with arthritis improves the differential diagnosis and may even reveal the cause of the condition.
CORRESPONDENCE
Jeremy Golding, MD, 279 Lincoln Street, Worcester, MA 01605; [email protected]
THE CASE
A 78-year-old woman with a history of anxiety and hypertension presented to our family medicine residency practice in Massachusetts with subacute polyarticular arthralgias that had been present for 2 months. She complained of pain and swelling of both ankles and the right knee. She noted that her symptoms had started on a recent trip to the Dominican Republic, where she developed generalized joint pain and a fever that lasted 1 to 2 weeks and subsequently resolved with the lingering polyarthralgias. She denied any rash, constitutional symptoms, photosensitivity, headaches, photophobia, or history of tick bite. Physical examination revealed normal vital signs, notable warmth and swelling of the bilateral ankles that was worse on the right side, and swelling of the right knee with effusion—but no tenderness—to palpation.
THE DIAGNOSIS
The patient’s labwork revealed a white blood cell count of 5900/mcL (reference range, 4500–11,000/mcL), hemoglobin count of 12.5 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 230×103/mcL. Electrolytes and renal function were normal. She had an elevated erythrocyte sedimentation rate of 34 mm/h (reference range, 0–20 mm/h) and a positive antinuclear antibody (ANA) test, but no titer was reported. Anti-chikungunya IgG and IgM antibodies were positive on enzyme-linked immunosorbent assay (ELISA) serologic testing.
DISCUSSION
Chikungunya is an infectious disease that is relatively rare in the United States. Chikungunya was rarely identified in American travelers prior to 2006, but incidence increased over the next decade. In 2014, a total of 2811 cases were reported.1 Chikungunya is an RNA arbovirus that is transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is endemic to West Africa. Within the last 2 decades, there has been an increasing number of outbreaks in India, Asia, Europe, and the Americas, where the highest incidence is in South America, followed by Central America. In the United States, almost all reported cases of chikungunya infection have been in travelers returning from endemic areas.2 The first 2 known cases of local transmission in the United States were reported in Florida in July 2014.3 Local transmission of chikungunya is significant in that it represents the possibility of a local reservoir for sustained transmission.
Disease presentation. Patients will initially complain of a high fever and severe distal polyarthralgias that usually are symmetric. The most common symptoms are polyarthralgias (87%–98% of patients), myalgias (46%–59%), and a maculopapular rash
The term chikungunya is derived from a Kimakonde (central Bantu) word meaning “that which bends up” because of the arthralgia caused by the disease. Fever usually lasts 3 to 7 days; polyarthralgia begins shortly after the onset of fever.4 Frank arthritis also may be present. Infection often exacerbates a previously damaged or diseased joint. Acute symptoms usually persist for 1 to 2 weeks, but arthralgias and arthritis can persist for months to years following resolution of the acute disease.6 In one study of 47 patients with acute chikungunya in Marseilles, France, the number of patients who were symptomatic declined from 88% to 86%, 48%, and 4% at 1, 3, 6, and 15 months, respectively.7
The differential diagnosis includes tropical infectious diseases (dengue, chikungunya, Zika, and leptospirosis) in patients who have recently traveled to the tropics and who complain of subacute polyarticular arthralgias or arthritis; locally acquired infections associated with arthralgia/arthritis such as Lyme disease and other tick-borne diseases and rickettsial infections; parvovirus B19 and other postinfectious arthritides; and rheumatologic conditions such as systemic lupus.
Clinical differentiation among dengue, chikungunya, and Zika may be difficult, although persistent frank arthritis is much more common in chikungunya than in dengue or Zika. Furthermore, conjunctivitis is present in Zika but is absent in chikungunya. Chikungunya also is more likely to cause high fever, severe arthralgia, arthritis, rash, and lymphopenia than Zika or dengue. Dengue is more likely to cause lymphopenia and hemorrhagic consequences than is chikungunya or Zika.8
Continue to: In our patient...
In our patient, dengue titers were not obtained because the duration of symptoms was thought to be more consistent with chikungunya, but testing for dengue also would have been appropriate.
The most common test for diagnosing acute chikungunya is ELISA serologic testing for IgM antibodies, which develop toward the end of the first week of infection; earlier in that first week, serum testing for viral RNA may be performed by polymerase chain reaction.
Treatment is largely supportive
Treatment of acute chikungunya is largely supportive and includes anti-inflammatory agents. To our knowledge, no antiviral agents have been shown to be effective. Postacute or chronic symptoms may require treatment with glucocorticoids or other immunomodulatory medications. A 2017 literature review of treatments for chikungunya-associated rheumatic disorders showed evidence that chloroquine was more effective than placebo for chronic pain relief. Also, adding a disease-modifying antirheumatic agent in combination with chloroquine was more effective for controlling pain and reducing disability than hydroxychloroquine monotherapy.10
Our patient was treated with ibuprofen only and experienced resolution of joint symptoms several months after the initial presentation. A repeat ANA test 12 months later was negative.
A 2009 review of the medical literature revealed a single case report of chikungunya associated with positive ANA.8 Although a positive ANA may be associated with acute viral infections, significantly elevated ANA levels typically are associated with autoimmunity. Resolution of the patient’s serum ANA 1 year later suggested that the positive ANA was not secondary to a pre-existing rheumatologic condition but rather a consequence of her body’s response to the chikungunya infection itself. Our case raises the hypothesis that, at least in some cases, chikungunya somehow stimulates a temporary autoimmune response, which may help explain why immunomodulatory medications can be effective treatment options.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Chikungunya is increasingly common in tropical and subtropical regions. Family physicians practicing in the United States should become familiar with the common patterns of presentation of viruses such as chikungunya, dengue, and Zika. Obtaining a travel history for patients presenting with arthritis improves the differential diagnosis and may even reveal the cause of the condition.
CORRESPONDENCE
Jeremy Golding, MD, 279 Lincoln Street, Worcester, MA 01605; [email protected]
1. Chikungunya virus. Centers for Disease Control and Prevention website. https://www.cdc.gov/chikungunya/geo/united-states.html. Reviewed December 17, 2018. Accessed March 5, 2019.
2. Pan American Health Organization. Preparedness and response for chikungunya virus: introduction into the Americas. https://www.paho.org/hq/dmdocuments/2012/CHIKV-English.pdf. Published 2011. Accessed March 5, 2019.
3. First chikungunya case acquired in the United States reported in Florida [press release]. Atlanta, GA: Centers for Disease Control and Prevention; July 17, 2014. http://www.cdc.gov/media/releases/2014/p0717-chikungunya.html. Accessed March 5, 2019.
4. Taubitz W, Cramer JP, Kapaun A, et al. Chikungunya fever in travelers: clinical presentation and course [published online May 23, 2007]. Clin Infect Dis. 2007;45:e1-e4.
5. Thiberville SD, Moyen N, Dupuis-Maguiraga L, et al. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013;99:345-370.
6. Burt FJ, Rolph MS, Rulli NE, et al. Chikungunya: a re-emerging virus. Lancet. 2012;379:662-671.
7. Simon F, Parola P, Grandadam M, et al. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine (Baltimore). 2007;86:123-137.
8. Chikungunya virus. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/chikungunya/hc/clinicalevaluation.html. Reviewed December 17, 2018. Accessed March 5, 2019.
9. Petersen LR, Jamieson DJ, Powers AM, et al. Zika virus. N Engl J Med. 2016;374:1552-1563.
10. Martí-Carvajal A, Ramon-Pardo P, Javelle E, et al. Interventions for treating patients with chikungunya virus infection-related rheumatic and musculoskeletal disorders: a systematic review. PLoS One. 2017;12:e0179028.
1. Chikungunya virus. Centers for Disease Control and Prevention website. https://www.cdc.gov/chikungunya/geo/united-states.html. Reviewed December 17, 2018. Accessed March 5, 2019.
2. Pan American Health Organization. Preparedness and response for chikungunya virus: introduction into the Americas. https://www.paho.org/hq/dmdocuments/2012/CHIKV-English.pdf. Published 2011. Accessed March 5, 2019.
3. First chikungunya case acquired in the United States reported in Florida [press release]. Atlanta, GA: Centers for Disease Control and Prevention; July 17, 2014. http://www.cdc.gov/media/releases/2014/p0717-chikungunya.html. Accessed March 5, 2019.
4. Taubitz W, Cramer JP, Kapaun A, et al. Chikungunya fever in travelers: clinical presentation and course [published online May 23, 2007]. Clin Infect Dis. 2007;45:e1-e4.
5. Thiberville SD, Moyen N, Dupuis-Maguiraga L, et al. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013;99:345-370.
6. Burt FJ, Rolph MS, Rulli NE, et al. Chikungunya: a re-emerging virus. Lancet. 2012;379:662-671.
7. Simon F, Parola P, Grandadam M, et al. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine (Baltimore). 2007;86:123-137.
8. Chikungunya virus. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/chikungunya/hc/clinicalevaluation.html. Reviewed December 17, 2018. Accessed March 5, 2019.
9. Petersen LR, Jamieson DJ, Powers AM, et al. Zika virus. N Engl J Med. 2016;374:1552-1563.
10. Martí-Carvajal A, Ramon-Pardo P, Javelle E, et al. Interventions for treating patients with chikungunya virus infection-related rheumatic and musculoskeletal disorders: a systematic review. PLoS One. 2017;12:e0179028.
PrEP adherence lowest among Medicaid patients, others
SEATTLE – Female sex, young age, residing in a rural location, black race, and Medicaid insurance were all associated with reduced adherence to HIV pre-exposure prophylaxis in a study from the Centers for Disease Control and Prevention.
Daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir (Truvada) prevents infection, but not everyone sticks with it. The investigators wanted to find out who struggles the most with adherence to help focus future intervention efforts.
The team identified 7,250 commercially insured and 349 Medicaid PrEP users in IBM MarketScan databases during 2011-2016. They tracked them from their initial PrEP prescription until there was a gap of 30 days or more in their PrEP refills, at which point they met the study’s definition of nonpersistence.
Overall, “commercially insured nonpersistent PrEP users were young, female, and rural. Medicaid insured nonpersistent users were young, female, and black,” said lead investigator Ya-Lin Huang, PhD, a health scientist in the CDC Division of HIV/AIDS Prevention.
“It is concerning that some populations with low persistence were among those with the highest rates of HIV diagnosis, such as young, black men. Research is needed to understand reasons for discontinuing PrEP. Interventions tailored for priority populations are needed to improve PrEP persistence,” she said at the Conference on Retroviruses and Opportunistic Infections.
Her team found that commercially insured patients stuck with PrEP longer than did those on Medicaid, a median of 14.5 months, with 56% still filling their prescriptions after a year, versus a median Medicaid persistence of 7.6 months, with only a third of Medicaid patients still on PrEP after 12 months.
Men were more persistent with PrEP than were women in both groups, as were older people versus younger. The median length of adherence among commercially insured patients aged 45-54 years, for instance, was 20.5 months, versus 8.6 months among people aged 18-24 years. Older PrEP users persisted longer among Medicaid patients as well, a median of 10 versus 4 months.
Also in the Medicaid group, white patients stuck with PrEP longer than did black patients, a median of 8.5 months versus 4.1 months. There were no racial differences with commercial insurance.
The findings held even when the team used gaps of 60 and 90 days, instead of 30 days, to define nonpersistence.
The study says nothing about why people stopped PrEP, or why there were such stark differences between the groups.
Perhaps, in some cases, people quit risky behavior or entered new relationships. Maybe PrEP was too expensive for some, or transportation to the clinic was an issue. Side effects might have been a problem, or people could have lost their insurance coverage, or maybe didn’t want to deal with the hassle. It’s impossible to know from the data, Dr. Huang said.
She said her team wants to figure it out, so they can help people overcome barriers to treatment, which likely vary across subgroups.
The study also was limited to patients who were enrolled in coverage at least 6 months before and 6 months after their first PrEP prescription; the investigators want to exam the situation for people with less consistent coverage, and no coverage at all.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no disclosures.
SOURCE: Huang YA et al. CROI 2019, Abstract 106.
SEATTLE – Female sex, young age, residing in a rural location, black race, and Medicaid insurance were all associated with reduced adherence to HIV pre-exposure prophylaxis in a study from the Centers for Disease Control and Prevention.
Daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir (Truvada) prevents infection, but not everyone sticks with it. The investigators wanted to find out who struggles the most with adherence to help focus future intervention efforts.
The team identified 7,250 commercially insured and 349 Medicaid PrEP users in IBM MarketScan databases during 2011-2016. They tracked them from their initial PrEP prescription until there was a gap of 30 days or more in their PrEP refills, at which point they met the study’s definition of nonpersistence.
Overall, “commercially insured nonpersistent PrEP users were young, female, and rural. Medicaid insured nonpersistent users were young, female, and black,” said lead investigator Ya-Lin Huang, PhD, a health scientist in the CDC Division of HIV/AIDS Prevention.
“It is concerning that some populations with low persistence were among those with the highest rates of HIV diagnosis, such as young, black men. Research is needed to understand reasons for discontinuing PrEP. Interventions tailored for priority populations are needed to improve PrEP persistence,” she said at the Conference on Retroviruses and Opportunistic Infections.
Her team found that commercially insured patients stuck with PrEP longer than did those on Medicaid, a median of 14.5 months, with 56% still filling their prescriptions after a year, versus a median Medicaid persistence of 7.6 months, with only a third of Medicaid patients still on PrEP after 12 months.
Men were more persistent with PrEP than were women in both groups, as were older people versus younger. The median length of adherence among commercially insured patients aged 45-54 years, for instance, was 20.5 months, versus 8.6 months among people aged 18-24 years. Older PrEP users persisted longer among Medicaid patients as well, a median of 10 versus 4 months.
Also in the Medicaid group, white patients stuck with PrEP longer than did black patients, a median of 8.5 months versus 4.1 months. There were no racial differences with commercial insurance.
The findings held even when the team used gaps of 60 and 90 days, instead of 30 days, to define nonpersistence.
The study says nothing about why people stopped PrEP, or why there were such stark differences between the groups.
Perhaps, in some cases, people quit risky behavior or entered new relationships. Maybe PrEP was too expensive for some, or transportation to the clinic was an issue. Side effects might have been a problem, or people could have lost their insurance coverage, or maybe didn’t want to deal with the hassle. It’s impossible to know from the data, Dr. Huang said.
She said her team wants to figure it out, so they can help people overcome barriers to treatment, which likely vary across subgroups.
The study also was limited to patients who were enrolled in coverage at least 6 months before and 6 months after their first PrEP prescription; the investigators want to exam the situation for people with less consistent coverage, and no coverage at all.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no disclosures.
SOURCE: Huang YA et al. CROI 2019, Abstract 106.
SEATTLE – Female sex, young age, residing in a rural location, black race, and Medicaid insurance were all associated with reduced adherence to HIV pre-exposure prophylaxis in a study from the Centers for Disease Control and Prevention.
Daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir (Truvada) prevents infection, but not everyone sticks with it. The investigators wanted to find out who struggles the most with adherence to help focus future intervention efforts.
The team identified 7,250 commercially insured and 349 Medicaid PrEP users in IBM MarketScan databases during 2011-2016. They tracked them from their initial PrEP prescription until there was a gap of 30 days or more in their PrEP refills, at which point they met the study’s definition of nonpersistence.
Overall, “commercially insured nonpersistent PrEP users were young, female, and rural. Medicaid insured nonpersistent users were young, female, and black,” said lead investigator Ya-Lin Huang, PhD, a health scientist in the CDC Division of HIV/AIDS Prevention.
“It is concerning that some populations with low persistence were among those with the highest rates of HIV diagnosis, such as young, black men. Research is needed to understand reasons for discontinuing PrEP. Interventions tailored for priority populations are needed to improve PrEP persistence,” she said at the Conference on Retroviruses and Opportunistic Infections.
Her team found that commercially insured patients stuck with PrEP longer than did those on Medicaid, a median of 14.5 months, with 56% still filling their prescriptions after a year, versus a median Medicaid persistence of 7.6 months, with only a third of Medicaid patients still on PrEP after 12 months.
Men were more persistent with PrEP than were women in both groups, as were older people versus younger. The median length of adherence among commercially insured patients aged 45-54 years, for instance, was 20.5 months, versus 8.6 months among people aged 18-24 years. Older PrEP users persisted longer among Medicaid patients as well, a median of 10 versus 4 months.
Also in the Medicaid group, white patients stuck with PrEP longer than did black patients, a median of 8.5 months versus 4.1 months. There were no racial differences with commercial insurance.
The findings held even when the team used gaps of 60 and 90 days, instead of 30 days, to define nonpersistence.
The study says nothing about why people stopped PrEP, or why there were such stark differences between the groups.
Perhaps, in some cases, people quit risky behavior or entered new relationships. Maybe PrEP was too expensive for some, or transportation to the clinic was an issue. Side effects might have been a problem, or people could have lost their insurance coverage, or maybe didn’t want to deal with the hassle. It’s impossible to know from the data, Dr. Huang said.
She said her team wants to figure it out, so they can help people overcome barriers to treatment, which likely vary across subgroups.
The study also was limited to patients who were enrolled in coverage at least 6 months before and 6 months after their first PrEP prescription; the investigators want to exam the situation for people with less consistent coverage, and no coverage at all.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no disclosures.
SOURCE: Huang YA et al. CROI 2019, Abstract 106.
REPORTING FROM CROI 2019
Harness EHRs to identify PrEP candidates
SEATTLE – Kaiser Permanente Northern California has developed an EHR program that automatically flags patients at high risk for HIV.
The idea was to come up with a way to help clinicians focus their pre-exposure prophylaxis (PrEP) outreach on the people who need it most. PrEP prevents HIV, but often “it’s difficult for providers to identify patients who are at risk. Prediction models using EHR data can identify patients who are at high risk but not using PrEP,” said study lead Julia Marcus, PhD, an assistant professor in the department of population medicine at Harvard Medical School, Boston.
She proved that assertion in a presentation at the Conference on Retroviruses & Opportunistic Infections.
The Kaiser program uses 44 variables routinely collected in EHRs from five categories: demographics, social history, lab data, medication use, and diagnoses. Specific variables include living in a zip code with high HIV incidence; men who have sex with men (MSM); black race; urine tests positive for cocaine or methadone; use of erectile dysfunction medications; and diagnoses of depression, anal warts, and other conditions.
The development cohort included 3,143,963 Kaiser members from 2007 to 2014 with 2 or more years of enrollment; at least one outpatient visit; no prior PrEP use; and no HIV diagnosis.
There were 701 incident HIV cases; the model did a good job at predicting them, with a C-statistic of 0.86 (95% confidence interval, 0.85-0.87). A score of 1.0 would be perfect prediction, and 0.5 no predictive value. Previous efforts at HIV prediction – relying generally on just MSM status and STD history – have C-statistics of around 0.6; prediction models commonly used for cardiovascular and other diseases often have C-statistics of around 0.7, Dr. Marcus explained.
The model was validated in 606,701 Kaiser members during 2015-2017. The validation cohort was slightly younger than the development cohort, with a mean age of 37 versus 45 years, and slightly more diverse, with fewer white patients, 44% versus 52%. Both cohorts had slightly more women than men.
There were 83 new HIV diagnoses in the validation cohort. The C-statistic for HIV prediction was 0.84 (95% CI, 0.8-0.89). The model predicted 32 of 69 (46%) incident HIV cases among men tagged as high risk – at least a 0.2% chance of contracting HIV within 3 years – or very high risk, a 1% chance or higher, which is more than 50 times the risk among the general population. Relying on just MSM and STD history predicted 32% of cases.
Overall, “our model identified nearly half of new HIV cases among males by flagging only 2% of the general population. The results suggest our model would perform well if implemented today. You could replicate our approach in any health system with an EHR. Our specific variables may not translate to every setting, but any health care system can develop this model based on the EHR data they do have,” Dr. Marcus said.
“You could embed this in any EHR system and have it update in real time to flag providers to do a sexual history and talk with patients about PrEP,” she said.
The next step is a pilot project at Kaiser Permanente San Francisco to evaluate the impact on PrEP prescribing and HIV incidence. The model failed to predict 14 incident HIV cases among women in the validation cohort, a problem that also needs to be addressed.
The work was funded by the National Institutes of Health and Kaiser Permanente. Dr. Marcus didn’t have any relevant disclosures.
SOURCE: Marcus JL et al. CROI 2019, Abstract 105.
SEATTLE – Kaiser Permanente Northern California has developed an EHR program that automatically flags patients at high risk for HIV.
The idea was to come up with a way to help clinicians focus their pre-exposure prophylaxis (PrEP) outreach on the people who need it most. PrEP prevents HIV, but often “it’s difficult for providers to identify patients who are at risk. Prediction models using EHR data can identify patients who are at high risk but not using PrEP,” said study lead Julia Marcus, PhD, an assistant professor in the department of population medicine at Harvard Medical School, Boston.
She proved that assertion in a presentation at the Conference on Retroviruses & Opportunistic Infections.
The Kaiser program uses 44 variables routinely collected in EHRs from five categories: demographics, social history, lab data, medication use, and diagnoses. Specific variables include living in a zip code with high HIV incidence; men who have sex with men (MSM); black race; urine tests positive for cocaine or methadone; use of erectile dysfunction medications; and diagnoses of depression, anal warts, and other conditions.
The development cohort included 3,143,963 Kaiser members from 2007 to 2014 with 2 or more years of enrollment; at least one outpatient visit; no prior PrEP use; and no HIV diagnosis.
There were 701 incident HIV cases; the model did a good job at predicting them, with a C-statistic of 0.86 (95% confidence interval, 0.85-0.87). A score of 1.0 would be perfect prediction, and 0.5 no predictive value. Previous efforts at HIV prediction – relying generally on just MSM status and STD history – have C-statistics of around 0.6; prediction models commonly used for cardiovascular and other diseases often have C-statistics of around 0.7, Dr. Marcus explained.
The model was validated in 606,701 Kaiser members during 2015-2017. The validation cohort was slightly younger than the development cohort, with a mean age of 37 versus 45 years, and slightly more diverse, with fewer white patients, 44% versus 52%. Both cohorts had slightly more women than men.
There were 83 new HIV diagnoses in the validation cohort. The C-statistic for HIV prediction was 0.84 (95% CI, 0.8-0.89). The model predicted 32 of 69 (46%) incident HIV cases among men tagged as high risk – at least a 0.2% chance of contracting HIV within 3 years – or very high risk, a 1% chance or higher, which is more than 50 times the risk among the general population. Relying on just MSM and STD history predicted 32% of cases.
Overall, “our model identified nearly half of new HIV cases among males by flagging only 2% of the general population. The results suggest our model would perform well if implemented today. You could replicate our approach in any health system with an EHR. Our specific variables may not translate to every setting, but any health care system can develop this model based on the EHR data they do have,” Dr. Marcus said.
“You could embed this in any EHR system and have it update in real time to flag providers to do a sexual history and talk with patients about PrEP,” she said.
The next step is a pilot project at Kaiser Permanente San Francisco to evaluate the impact on PrEP prescribing and HIV incidence. The model failed to predict 14 incident HIV cases among women in the validation cohort, a problem that also needs to be addressed.
The work was funded by the National Institutes of Health and Kaiser Permanente. Dr. Marcus didn’t have any relevant disclosures.
SOURCE: Marcus JL et al. CROI 2019, Abstract 105.
SEATTLE – Kaiser Permanente Northern California has developed an EHR program that automatically flags patients at high risk for HIV.
The idea was to come up with a way to help clinicians focus their pre-exposure prophylaxis (PrEP) outreach on the people who need it most. PrEP prevents HIV, but often “it’s difficult for providers to identify patients who are at risk. Prediction models using EHR data can identify patients who are at high risk but not using PrEP,” said study lead Julia Marcus, PhD, an assistant professor in the department of population medicine at Harvard Medical School, Boston.
She proved that assertion in a presentation at the Conference on Retroviruses & Opportunistic Infections.
The Kaiser program uses 44 variables routinely collected in EHRs from five categories: demographics, social history, lab data, medication use, and diagnoses. Specific variables include living in a zip code with high HIV incidence; men who have sex with men (MSM); black race; urine tests positive for cocaine or methadone; use of erectile dysfunction medications; and diagnoses of depression, anal warts, and other conditions.
The development cohort included 3,143,963 Kaiser members from 2007 to 2014 with 2 or more years of enrollment; at least one outpatient visit; no prior PrEP use; and no HIV diagnosis.
There were 701 incident HIV cases; the model did a good job at predicting them, with a C-statistic of 0.86 (95% confidence interval, 0.85-0.87). A score of 1.0 would be perfect prediction, and 0.5 no predictive value. Previous efforts at HIV prediction – relying generally on just MSM status and STD history – have C-statistics of around 0.6; prediction models commonly used for cardiovascular and other diseases often have C-statistics of around 0.7, Dr. Marcus explained.
The model was validated in 606,701 Kaiser members during 2015-2017. The validation cohort was slightly younger than the development cohort, with a mean age of 37 versus 45 years, and slightly more diverse, with fewer white patients, 44% versus 52%. Both cohorts had slightly more women than men.
There were 83 new HIV diagnoses in the validation cohort. The C-statistic for HIV prediction was 0.84 (95% CI, 0.8-0.89). The model predicted 32 of 69 (46%) incident HIV cases among men tagged as high risk – at least a 0.2% chance of contracting HIV within 3 years – or very high risk, a 1% chance or higher, which is more than 50 times the risk among the general population. Relying on just MSM and STD history predicted 32% of cases.
Overall, “our model identified nearly half of new HIV cases among males by flagging only 2% of the general population. The results suggest our model would perform well if implemented today. You could replicate our approach in any health system with an EHR. Our specific variables may not translate to every setting, but any health care system can develop this model based on the EHR data they do have,” Dr. Marcus said.
“You could embed this in any EHR system and have it update in real time to flag providers to do a sexual history and talk with patients about PrEP,” she said.
The next step is a pilot project at Kaiser Permanente San Francisco to evaluate the impact on PrEP prescribing and HIV incidence. The model failed to predict 14 incident HIV cases among women in the validation cohort, a problem that also needs to be addressed.
The work was funded by the National Institutes of Health and Kaiser Permanente. Dr. Marcus didn’t have any relevant disclosures.
SOURCE: Marcus JL et al. CROI 2019, Abstract 105.
REPORTING FROM CROI 2019