User login
ICYMI: Anti-CD4 antibody maintains viral suppression in HIV patients post ART
according to results from a small, nonrandomized, open-label, phase 2 trial published in the New England Journal of Medicine (2019 Apr 17. doi: 10.1056/NEJMoa1802264).
We reported on this story at the 2017 Conference on Retroviruses & Opportunistic Infections before it was published in the journal. Find our coverage at the link below.
according to results from a small, nonrandomized, open-label, phase 2 trial published in the New England Journal of Medicine (2019 Apr 17. doi: 10.1056/NEJMoa1802264).
We reported on this story at the 2017 Conference on Retroviruses & Opportunistic Infections before it was published in the journal. Find our coverage at the link below.
according to results from a small, nonrandomized, open-label, phase 2 trial published in the New England Journal of Medicine (2019 Apr 17. doi: 10.1056/NEJMoa1802264).
We reported on this story at the 2017 Conference on Retroviruses & Opportunistic Infections before it was published in the journal. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Can immune checkpoint inhibitors treat PML?
investigators reported in the New England Journal of Medicine.
Three research teams described 10 cases in which patients with PML received pembrolizumab or nivolumab.
In one study, researchers administered pembrolizumab to eight adults with PML. Five patients had clinical improvement or stabilization, whereas 3 patients did not. Among the patients with clinical improvement, treatment led to reduced JC viral load in cerebrospinal fluid (CSF) and increased CD4+ and CD8+ anti–JC virus activity in vitro. Among patients without clinical improvement, treatment did not meaningfully change viral load or antiviral cellular immune response.
In a separate letter, researchers in Germany described an additional patient with PML who had clinical stabilization and no disease progression on MRI after treatment with pembrolizumab.
In another letter, researchers in France described a patient with PML whose condition improved after treatment with nivolumab.
“Do pembrolizumab and nivolumab fit the bill for treatment of PML? The current reports are encouraging but suggest that the presence of JC virus–specific T cells in the blood is a prerequisite for their use,” said Igor J. Koralnik, MD, of the department of neurological sciences at Rush University Medical Center in Chicago, in an accompanying editorial. “A controlled trial may be needed to determine whether immune checkpoint inhibitors are indeed able to keep JC virus in check in patients with PML.”
Reinvigorating T cells
Both monoclonal antibodies target programmed cell death protein 1 (PD-1), which inhibits T-cell proliferation and cytokine production when it binds its associated ligand, Dr. Koralnik said. Pembrolizumab and nivolumab block this inhibition and have been used to spur T-cell activity against tumors in patients with cancer.
PML, an often fatal brain infection caused by the JC virus in patients with immunosuppression, has no specific treatment. Management hinges on “recovery of the immune system, either by treating the underlying cause of immunosuppression or by discontinuing the use of immunosuppressive medications,” said Dr. Koralnik.
Pembrolizumab
Prior studies have found that PD-1 expression is elevated on T lymphocytes of patients with PML. To determine whether PD-1 blockade with pembrolizumab reinvigorates anti–JC virus immune activity in patients with PML, Irene Cortese, MD, of the National Institutes of Health’s Neuroimmunology Clinic and her research colleagues administered pembrolizumab at a dose of 2 mg/kg of body weight every 4-6 weeks to eight adults with PML. The patients received 1-3 doses, and each patient had a different underlying condition.
In all patients, treatment induced down-regulation of PD-1 expression on lymphocytes in CSF and peripheral blood, and five of the eight patients had clinical stabilization or improvement. Of the other three patients who did not improve, one had stabilized prior to treatment and remained stable. The other two patients died from PML.
Additional reports
Separately, Sebastian Rauer, MD, of Albert Ludwigs University in Freiburg, Germany, and his colleagues reported that a patient with PML whose symptoms culminated in mutism in February 2018 began speaking again after receiving five infusions of pembrolizumab over 10 weeks. “In addition, the size and number of lesions on MRI decreased, and JCV was no longer detectable in CSF,” Dr. Rauer and his colleagues wrote. “The patient has remained stable as of the end of March 2019, with persistent but abating psychomotor slowing, aphasia, and disorientation.”
Finally, Ondine Walter, of Toulouse (France) University Hospital and colleagues described the case of a 60-year-old woman with PML who received nivolumab on a compassionate-use basis. Two weeks after treatment, JC viral load in CSF and blood had decreased. “Starting 8 weeks after the initiation of nivolumab therapy, the patient’s neurologic symptoms and signs stabilized, and subsequently she showed improved alertness, and the ptosis and hemiplegia abated.”
Reason for caution
Prior studies, however, give reasons for caution when considering the potential use of immune checkpoint inhibitors to treat PML, Dr. Koralnik noted. In one case, a patient developed an inflammatory form of PML known as immune reconstitution inflammatory syndrome after receiving nivolumab (J Neurovirol. 2019 March 12. doi: 10.1007/s13365-019-00738-x). In addition, researchers have reported a case of PML that occurred after 1 year of nivolumab treatment, and four cases of PML related to nivolumab have been reported in pharmacovigilance databases (Emerg Infect Dis. 2018;24:1594-6). The cost and safety profiles of the medications also may be considerations, Dr. Koralnik said.
The study by Dr. Cortese and colleagues was funded by the National Institutes of Health, and the authors had no relevant disclosures. Some of the research letter authors disclosed grants and personal fees from pharmaceutical companies.
SOURCES: Cortese I et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1815039; Rauer S et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1817193; Walter O et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1816198; Koralnik IJ. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1904140.
investigators reported in the New England Journal of Medicine.
Three research teams described 10 cases in which patients with PML received pembrolizumab or nivolumab.
In one study, researchers administered pembrolizumab to eight adults with PML. Five patients had clinical improvement or stabilization, whereas 3 patients did not. Among the patients with clinical improvement, treatment led to reduced JC viral load in cerebrospinal fluid (CSF) and increased CD4+ and CD8+ anti–JC virus activity in vitro. Among patients without clinical improvement, treatment did not meaningfully change viral load or antiviral cellular immune response.
In a separate letter, researchers in Germany described an additional patient with PML who had clinical stabilization and no disease progression on MRI after treatment with pembrolizumab.
In another letter, researchers in France described a patient with PML whose condition improved after treatment with nivolumab.
“Do pembrolizumab and nivolumab fit the bill for treatment of PML? The current reports are encouraging but suggest that the presence of JC virus–specific T cells in the blood is a prerequisite for their use,” said Igor J. Koralnik, MD, of the department of neurological sciences at Rush University Medical Center in Chicago, in an accompanying editorial. “A controlled trial may be needed to determine whether immune checkpoint inhibitors are indeed able to keep JC virus in check in patients with PML.”
Reinvigorating T cells
Both monoclonal antibodies target programmed cell death protein 1 (PD-1), which inhibits T-cell proliferation and cytokine production when it binds its associated ligand, Dr. Koralnik said. Pembrolizumab and nivolumab block this inhibition and have been used to spur T-cell activity against tumors in patients with cancer.
PML, an often fatal brain infection caused by the JC virus in patients with immunosuppression, has no specific treatment. Management hinges on “recovery of the immune system, either by treating the underlying cause of immunosuppression or by discontinuing the use of immunosuppressive medications,” said Dr. Koralnik.
Pembrolizumab
Prior studies have found that PD-1 expression is elevated on T lymphocytes of patients with PML. To determine whether PD-1 blockade with pembrolizumab reinvigorates anti–JC virus immune activity in patients with PML, Irene Cortese, MD, of the National Institutes of Health’s Neuroimmunology Clinic and her research colleagues administered pembrolizumab at a dose of 2 mg/kg of body weight every 4-6 weeks to eight adults with PML. The patients received 1-3 doses, and each patient had a different underlying condition.
In all patients, treatment induced down-regulation of PD-1 expression on lymphocytes in CSF and peripheral blood, and five of the eight patients had clinical stabilization or improvement. Of the other three patients who did not improve, one had stabilized prior to treatment and remained stable. The other two patients died from PML.
Additional reports
Separately, Sebastian Rauer, MD, of Albert Ludwigs University in Freiburg, Germany, and his colleagues reported that a patient with PML whose symptoms culminated in mutism in February 2018 began speaking again after receiving five infusions of pembrolizumab over 10 weeks. “In addition, the size and number of lesions on MRI decreased, and JCV was no longer detectable in CSF,” Dr. Rauer and his colleagues wrote. “The patient has remained stable as of the end of March 2019, with persistent but abating psychomotor slowing, aphasia, and disorientation.”
Finally, Ondine Walter, of Toulouse (France) University Hospital and colleagues described the case of a 60-year-old woman with PML who received nivolumab on a compassionate-use basis. Two weeks after treatment, JC viral load in CSF and blood had decreased. “Starting 8 weeks after the initiation of nivolumab therapy, the patient’s neurologic symptoms and signs stabilized, and subsequently she showed improved alertness, and the ptosis and hemiplegia abated.”
Reason for caution
Prior studies, however, give reasons for caution when considering the potential use of immune checkpoint inhibitors to treat PML, Dr. Koralnik noted. In one case, a patient developed an inflammatory form of PML known as immune reconstitution inflammatory syndrome after receiving nivolumab (J Neurovirol. 2019 March 12. doi: 10.1007/s13365-019-00738-x). In addition, researchers have reported a case of PML that occurred after 1 year of nivolumab treatment, and four cases of PML related to nivolumab have been reported in pharmacovigilance databases (Emerg Infect Dis. 2018;24:1594-6). The cost and safety profiles of the medications also may be considerations, Dr. Koralnik said.
The study by Dr. Cortese and colleagues was funded by the National Institutes of Health, and the authors had no relevant disclosures. Some of the research letter authors disclosed grants and personal fees from pharmaceutical companies.
SOURCES: Cortese I et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1815039; Rauer S et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1817193; Walter O et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1816198; Koralnik IJ. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1904140.
investigators reported in the New England Journal of Medicine.
Three research teams described 10 cases in which patients with PML received pembrolizumab or nivolumab.
In one study, researchers administered pembrolizumab to eight adults with PML. Five patients had clinical improvement or stabilization, whereas 3 patients did not. Among the patients with clinical improvement, treatment led to reduced JC viral load in cerebrospinal fluid (CSF) and increased CD4+ and CD8+ anti–JC virus activity in vitro. Among patients without clinical improvement, treatment did not meaningfully change viral load or antiviral cellular immune response.
In a separate letter, researchers in Germany described an additional patient with PML who had clinical stabilization and no disease progression on MRI after treatment with pembrolizumab.
In another letter, researchers in France described a patient with PML whose condition improved after treatment with nivolumab.
“Do pembrolizumab and nivolumab fit the bill for treatment of PML? The current reports are encouraging but suggest that the presence of JC virus–specific T cells in the blood is a prerequisite for their use,” said Igor J. Koralnik, MD, of the department of neurological sciences at Rush University Medical Center in Chicago, in an accompanying editorial. “A controlled trial may be needed to determine whether immune checkpoint inhibitors are indeed able to keep JC virus in check in patients with PML.”
Reinvigorating T cells
Both monoclonal antibodies target programmed cell death protein 1 (PD-1), which inhibits T-cell proliferation and cytokine production when it binds its associated ligand, Dr. Koralnik said. Pembrolizumab and nivolumab block this inhibition and have been used to spur T-cell activity against tumors in patients with cancer.
PML, an often fatal brain infection caused by the JC virus in patients with immunosuppression, has no specific treatment. Management hinges on “recovery of the immune system, either by treating the underlying cause of immunosuppression or by discontinuing the use of immunosuppressive medications,” said Dr. Koralnik.
Pembrolizumab
Prior studies have found that PD-1 expression is elevated on T lymphocytes of patients with PML. To determine whether PD-1 blockade with pembrolizumab reinvigorates anti–JC virus immune activity in patients with PML, Irene Cortese, MD, of the National Institutes of Health’s Neuroimmunology Clinic and her research colleagues administered pembrolizumab at a dose of 2 mg/kg of body weight every 4-6 weeks to eight adults with PML. The patients received 1-3 doses, and each patient had a different underlying condition.
In all patients, treatment induced down-regulation of PD-1 expression on lymphocytes in CSF and peripheral blood, and five of the eight patients had clinical stabilization or improvement. Of the other three patients who did not improve, one had stabilized prior to treatment and remained stable. The other two patients died from PML.
Additional reports
Separately, Sebastian Rauer, MD, of Albert Ludwigs University in Freiburg, Germany, and his colleagues reported that a patient with PML whose symptoms culminated in mutism in February 2018 began speaking again after receiving five infusions of pembrolizumab over 10 weeks. “In addition, the size and number of lesions on MRI decreased, and JCV was no longer detectable in CSF,” Dr. Rauer and his colleagues wrote. “The patient has remained stable as of the end of March 2019, with persistent but abating psychomotor slowing, aphasia, and disorientation.”
Finally, Ondine Walter, of Toulouse (France) University Hospital and colleagues described the case of a 60-year-old woman with PML who received nivolumab on a compassionate-use basis. Two weeks after treatment, JC viral load in CSF and blood had decreased. “Starting 8 weeks after the initiation of nivolumab therapy, the patient’s neurologic symptoms and signs stabilized, and subsequently she showed improved alertness, and the ptosis and hemiplegia abated.”
Reason for caution
Prior studies, however, give reasons for caution when considering the potential use of immune checkpoint inhibitors to treat PML, Dr. Koralnik noted. In one case, a patient developed an inflammatory form of PML known as immune reconstitution inflammatory syndrome after receiving nivolumab (J Neurovirol. 2019 March 12. doi: 10.1007/s13365-019-00738-x). In addition, researchers have reported a case of PML that occurred after 1 year of nivolumab treatment, and four cases of PML related to nivolumab have been reported in pharmacovigilance databases (Emerg Infect Dis. 2018;24:1594-6). The cost and safety profiles of the medications also may be considerations, Dr. Koralnik said.
The study by Dr. Cortese and colleagues was funded by the National Institutes of Health, and the authors had no relevant disclosures. Some of the research letter authors disclosed grants and personal fees from pharmaceutical companies.
SOURCES: Cortese I et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1815039; Rauer S et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1817193; Walter O et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1816198; Koralnik IJ. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1904140.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Diagnostic test helps clinicians identify IPF with nonsurgical biopsy
, according to recent research published in the Lancet Respiratory Medicine.
The results of the molecular test, called the Envisia Genomic Classifier (Veracyte; San Francisco), had a high positive predictive value of proven usual interstitial pneumonia, and could be used in place of surgical lung biopsy to confirm a diagnosis of idiopathic pulmonary fibrosis (IPF), wrote Ganesh Raghu, MD, director at the Center for Interstitial Lung Diseases and professor of medicine at the University of Washington, Seattle, and his colleagues.* The Envisia Genomic Classifier recently received final Medicare local coverage determination for IPF diagnosis, according to a recent press release by Veracyte.
“IPF is often challenging to distinguish from other [interstitial lung disease], but timely and accurate diagnosis is critical so that patients with IPF can access therapies that may slow progression of the disease, while avoiding potentially harmful treatments,” Dr. Raghu stated in a press release. “Our results with molecular classification through machine learning [the Envisia classifier] are promising and, along with clinical information and radiological features in high-resolution CT imaging, physicians through multidisciplinary discussions, may be able to utilize the molecular classification as a diagnostic tool to make a more informed and confident diagnoses.”
The researchers prospectively recruited 237 patients from 29 centers in the United States and Europe who were evaluated with the Bronchial Sample Collection for a Novel Genomic Test for suspected interstitial lung disease and who underwent surgical biopsy, transbronchial biopsy, or cryobiopsy for sample collection. They used histopathology and RNA sequence data from 90 patients to create a training data set of an unusual interstitial pneumonia pattern for the machine learning algorithm.
The classifier found usual interstitial pneumonia diagnoses in 49 patients; the test had a specificity of 88% (95% confidence interval, 70%-98%) and a sensitivity of 70% (95% CI, 47%-87%). Of 42 patients with inconsistent or possible usual interstitial pneumonia identified from high-resolution CT imaging, there was a positive predictive value of 81% (95% CI, 54%-96%). When multidisciplinary teams made diagnoses with the molecular classifier data, there was a clinical agreement of 86% (95% CI, 78%-92%) with diagnoses made using histopathology data. In 18 cases of IPF, there was an improvement in diagnostic confidence using the molecular classifier data, with 89% of diagnoses designated as high confidence, compared with 56% of cases based on histopathologic data (P = .0339). In 48 patients with nondiagnostic pathology or nonclassifiable fibrosis histopathology, 63% of diagnoses with the molecular classifier data were high confidence, compared with 42% using histopathologic data (P = .0412).
This study was funded by Veracyte, creator of the Envisia Genomic Classifier. Some authors reported relationships with Veracyte and other companies.
SOURCE: Raghu G et al. Lancet Respir Med. 2019 Apr 1. doi: 10.1016/S2213-8587(19)300.
Correction, 4/25/19: An earlier version of this article misstated how the Envisia Genomic Classifier could be used. The Envisia test is not intended to replace high-resolution chest CT (HRCT). It is used when HRCT is inconclusive to help prevent patients from having to undergo invasive diagnostic procedures.
Use of a molecular classifier could be most helpful in situations where patients have atypical radiology results or in cases where multidisciplinary teams disagree on the diagnosis, Simon Hart, PhD, wrote in a related editorial.
According to the 2018 international guidelines for idiopathic pulmonary fibrosis, usual interstitial pneumonia certainty is defined as honeycombing seen on high-resolution CT (HRCT), probable if there is presence of traction bronchiectasis but not honeycombing, and indeterminate if there is no presence of usual interstitial pneumonia or another diagnosis. As radiologists “often disagree on HRCT patterns,” IPF sometimes becomes a working diagnosis based on progression of disease, Dr. Hart wrote. In these cases, molecular classifier samples could help identify IPF in patients who have undergone less invasive transbronchial lung biopsy.
Among patients for whom diagnoses using identical clinical features have different results, HRCT and pathology data, particularly in cases of nonspecific interstitial pneumonia and chronic hypersensitivity pneumonitis that follow a similar disease course to idiopathic pulmonary fibrosis, molecular classifier testing could help identify patients with these diseases so treatments such as to avoid treating these patients with anti-inflammatory or immunosuppressive therapy.
“It seems conceivable that in future interstitial lung diseases could be classified by a simple dichotomy: primarily scarring diseases characterized by molecular usual interstitial pneumonia to be treated with antifibrotics versus immune-driven conditions without usual interstitial pneumonia that need an anti-inflammatory approach,” he wrote.
Dr. Hart is from the respiratory research group at Castle Hill Hospital in Cottingham, England. These comments summarize his editorial in response to Raghu et al. (Lancet Respir Med. 2019 Apr 1. doi 10.1016/S2213-2600[19]30058-X). He reported receiving grants and support to attend conferences, and consultancy fees from Boehringer Ingelheim.
Use of a molecular classifier could be most helpful in situations where patients have atypical radiology results or in cases where multidisciplinary teams disagree on the diagnosis, Simon Hart, PhD, wrote in a related editorial.
According to the 2018 international guidelines for idiopathic pulmonary fibrosis, usual interstitial pneumonia certainty is defined as honeycombing seen on high-resolution CT (HRCT), probable if there is presence of traction bronchiectasis but not honeycombing, and indeterminate if there is no presence of usual interstitial pneumonia or another diagnosis. As radiologists “often disagree on HRCT patterns,” IPF sometimes becomes a working diagnosis based on progression of disease, Dr. Hart wrote. In these cases, molecular classifier samples could help identify IPF in patients who have undergone less invasive transbronchial lung biopsy.
Among patients for whom diagnoses using identical clinical features have different results, HRCT and pathology data, particularly in cases of nonspecific interstitial pneumonia and chronic hypersensitivity pneumonitis that follow a similar disease course to idiopathic pulmonary fibrosis, molecular classifier testing could help identify patients with these diseases so treatments such as to avoid treating these patients with anti-inflammatory or immunosuppressive therapy.
“It seems conceivable that in future interstitial lung diseases could be classified by a simple dichotomy: primarily scarring diseases characterized by molecular usual interstitial pneumonia to be treated with antifibrotics versus immune-driven conditions without usual interstitial pneumonia that need an anti-inflammatory approach,” he wrote.
Dr. Hart is from the respiratory research group at Castle Hill Hospital in Cottingham, England. These comments summarize his editorial in response to Raghu et al. (Lancet Respir Med. 2019 Apr 1. doi 10.1016/S2213-2600[19]30058-X). He reported receiving grants and support to attend conferences, and consultancy fees from Boehringer Ingelheim.
Use of a molecular classifier could be most helpful in situations where patients have atypical radiology results or in cases where multidisciplinary teams disagree on the diagnosis, Simon Hart, PhD, wrote in a related editorial.
According to the 2018 international guidelines for idiopathic pulmonary fibrosis, usual interstitial pneumonia certainty is defined as honeycombing seen on high-resolution CT (HRCT), probable if there is presence of traction bronchiectasis but not honeycombing, and indeterminate if there is no presence of usual interstitial pneumonia or another diagnosis. As radiologists “often disagree on HRCT patterns,” IPF sometimes becomes a working diagnosis based on progression of disease, Dr. Hart wrote. In these cases, molecular classifier samples could help identify IPF in patients who have undergone less invasive transbronchial lung biopsy.
Among patients for whom diagnoses using identical clinical features have different results, HRCT and pathology data, particularly in cases of nonspecific interstitial pneumonia and chronic hypersensitivity pneumonitis that follow a similar disease course to idiopathic pulmonary fibrosis, molecular classifier testing could help identify patients with these diseases so treatments such as to avoid treating these patients with anti-inflammatory or immunosuppressive therapy.
“It seems conceivable that in future interstitial lung diseases could be classified by a simple dichotomy: primarily scarring diseases characterized by molecular usual interstitial pneumonia to be treated with antifibrotics versus immune-driven conditions without usual interstitial pneumonia that need an anti-inflammatory approach,” he wrote.
Dr. Hart is from the respiratory research group at Castle Hill Hospital in Cottingham, England. These comments summarize his editorial in response to Raghu et al. (Lancet Respir Med. 2019 Apr 1. doi 10.1016/S2213-2600[19]30058-X). He reported receiving grants and support to attend conferences, and consultancy fees from Boehringer Ingelheim.
, according to recent research published in the Lancet Respiratory Medicine.
The results of the molecular test, called the Envisia Genomic Classifier (Veracyte; San Francisco), had a high positive predictive value of proven usual interstitial pneumonia, and could be used in place of surgical lung biopsy to confirm a diagnosis of idiopathic pulmonary fibrosis (IPF), wrote Ganesh Raghu, MD, director at the Center for Interstitial Lung Diseases and professor of medicine at the University of Washington, Seattle, and his colleagues.* The Envisia Genomic Classifier recently received final Medicare local coverage determination for IPF diagnosis, according to a recent press release by Veracyte.
“IPF is often challenging to distinguish from other [interstitial lung disease], but timely and accurate diagnosis is critical so that patients with IPF can access therapies that may slow progression of the disease, while avoiding potentially harmful treatments,” Dr. Raghu stated in a press release. “Our results with molecular classification through machine learning [the Envisia classifier] are promising and, along with clinical information and radiological features in high-resolution CT imaging, physicians through multidisciplinary discussions, may be able to utilize the molecular classification as a diagnostic tool to make a more informed and confident diagnoses.”
The researchers prospectively recruited 237 patients from 29 centers in the United States and Europe who were evaluated with the Bronchial Sample Collection for a Novel Genomic Test for suspected interstitial lung disease and who underwent surgical biopsy, transbronchial biopsy, or cryobiopsy for sample collection. They used histopathology and RNA sequence data from 90 patients to create a training data set of an unusual interstitial pneumonia pattern for the machine learning algorithm.
The classifier found usual interstitial pneumonia diagnoses in 49 patients; the test had a specificity of 88% (95% confidence interval, 70%-98%) and a sensitivity of 70% (95% CI, 47%-87%). Of 42 patients with inconsistent or possible usual interstitial pneumonia identified from high-resolution CT imaging, there was a positive predictive value of 81% (95% CI, 54%-96%). When multidisciplinary teams made diagnoses with the molecular classifier data, there was a clinical agreement of 86% (95% CI, 78%-92%) with diagnoses made using histopathology data. In 18 cases of IPF, there was an improvement in diagnostic confidence using the molecular classifier data, with 89% of diagnoses designated as high confidence, compared with 56% of cases based on histopathologic data (P = .0339). In 48 patients with nondiagnostic pathology or nonclassifiable fibrosis histopathology, 63% of diagnoses with the molecular classifier data were high confidence, compared with 42% using histopathologic data (P = .0412).
This study was funded by Veracyte, creator of the Envisia Genomic Classifier. Some authors reported relationships with Veracyte and other companies.
SOURCE: Raghu G et al. Lancet Respir Med. 2019 Apr 1. doi: 10.1016/S2213-8587(19)300.
Correction, 4/25/19: An earlier version of this article misstated how the Envisia Genomic Classifier could be used. The Envisia test is not intended to replace high-resolution chest CT (HRCT). It is used when HRCT is inconclusive to help prevent patients from having to undergo invasive diagnostic procedures.
, according to recent research published in the Lancet Respiratory Medicine.
The results of the molecular test, called the Envisia Genomic Classifier (Veracyte; San Francisco), had a high positive predictive value of proven usual interstitial pneumonia, and could be used in place of surgical lung biopsy to confirm a diagnosis of idiopathic pulmonary fibrosis (IPF), wrote Ganesh Raghu, MD, director at the Center for Interstitial Lung Diseases and professor of medicine at the University of Washington, Seattle, and his colleagues.* The Envisia Genomic Classifier recently received final Medicare local coverage determination for IPF diagnosis, according to a recent press release by Veracyte.
“IPF is often challenging to distinguish from other [interstitial lung disease], but timely and accurate diagnosis is critical so that patients with IPF can access therapies that may slow progression of the disease, while avoiding potentially harmful treatments,” Dr. Raghu stated in a press release. “Our results with molecular classification through machine learning [the Envisia classifier] are promising and, along with clinical information and radiological features in high-resolution CT imaging, physicians through multidisciplinary discussions, may be able to utilize the molecular classification as a diagnostic tool to make a more informed and confident diagnoses.”
The researchers prospectively recruited 237 patients from 29 centers in the United States and Europe who were evaluated with the Bronchial Sample Collection for a Novel Genomic Test for suspected interstitial lung disease and who underwent surgical biopsy, transbronchial biopsy, or cryobiopsy for sample collection. They used histopathology and RNA sequence data from 90 patients to create a training data set of an unusual interstitial pneumonia pattern for the machine learning algorithm.
The classifier found usual interstitial pneumonia diagnoses in 49 patients; the test had a specificity of 88% (95% confidence interval, 70%-98%) and a sensitivity of 70% (95% CI, 47%-87%). Of 42 patients with inconsistent or possible usual interstitial pneumonia identified from high-resolution CT imaging, there was a positive predictive value of 81% (95% CI, 54%-96%). When multidisciplinary teams made diagnoses with the molecular classifier data, there was a clinical agreement of 86% (95% CI, 78%-92%) with diagnoses made using histopathology data. In 18 cases of IPF, there was an improvement in diagnostic confidence using the molecular classifier data, with 89% of diagnoses designated as high confidence, compared with 56% of cases based on histopathologic data (P = .0339). In 48 patients with nondiagnostic pathology or nonclassifiable fibrosis histopathology, 63% of diagnoses with the molecular classifier data were high confidence, compared with 42% using histopathologic data (P = .0412).
This study was funded by Veracyte, creator of the Envisia Genomic Classifier. Some authors reported relationships with Veracyte and other companies.
SOURCE: Raghu G et al. Lancet Respir Med. 2019 Apr 1. doi: 10.1016/S2213-8587(19)300.
Correction, 4/25/19: An earlier version of this article misstated how the Envisia Genomic Classifier could be used. The Envisia test is not intended to replace high-resolution chest CT (HRCT). It is used when HRCT is inconclusive to help prevent patients from having to undergo invasive diagnostic procedures.
FROM THE LANCET RESPIRATORY MEDICINE
Identifying CMV infection in asymptomatic newborns – one step closer?
Cytomegalovirus (CMV) infection is the most common congenital viral infection in U.S. children, with a frequency between 0.5% and 1% of newborn infants resulting in approximately 30,000 infected children annually. A small minority (approximately 10%) can be identified in the neonatal period as symptomatic with jaundice (from direct hyperbilirubinemia), petechiae (from thrombocytopenia), hepatosplenomegaly, microcephaly, or other manifestations. The vast majority are asymptomatic at birth, yet 15% will have or develop sensorineural hearing loss (SNHL) during the first few years of life; others (1%-2%) will develop vision loss associated with retinal scars. Congenital CMV accounts for 20% of those with SNHL detected at birth and 25% of children with SNHL at 4 years of age.
Screening for congenital CMV has been an ongoing subject of debate. The challenges of implementing screening programs are related both to the diagnostics (collecting urine samples on newborns) as well as with the question of whether we have treatment and interventions to offer babies diagnosed with congenital CMV across the complete spectrum of clinical presentations.
Current screening programs implemented in some hospitals, called “targeted screening,” in which babies who fail newborn screening programs are tested for CMV, are not sufficient to achieve the goal of identifying babies who will need follow-up for early detection of SNHL or vision abnormalities, or possibly early antiviral therapy (Valcyte; valganciclovir), because only a small portion of those who eventually develop SNHL are currently identified by the targeted screening programs.1
However, its availability only has added to the debate as to whether the time has arrived for universal screening.
Vertical transmission of CMV occurs in utero (during any of the trimesters), at birth by passage through the birth canal, or postnatally by ingestion of breast milk. Neonatal infection (in utero and postnatal) occurs in both mothers with primary CMV infection during gestation and in those with recurrent infection (from a different viral strain) or reactivation of infection. Severe clinically symptomatic disease and sequelae is associated with primary maternal infection and early transmission to the fetus. However, it is estimated that nonprimary maternal infection accounts for 75% of neonatal infections. Transmission by breast milk to full-term, healthy infants does not appear to be associated with clinical illness or sequelae; however, preterm infants or those with birth weights less than 1,500 g have a small risk of developing clinical disease.
The polymerase chain reaction–based saliva CMV test (Alethia CMV Assay Test System) was licensed by the Food and Drug Administration in November 2018 after studies demonstrated high sensitivity and specificity, compared with viral culture (the gold standard). In one study, 17,327 infants were screened with the liquid-saliva PCR assay, and 0.5% tested positive for CMV on both the saliva test and culture. Sensitivity and specificity of the liquid-saliva PCR assay were 100% and 99.9%, respectively.2 The availability of an approved saliva-based assay that is both highly sensitive and specific overcomes the challenge of collecting urine, which has been a limiting factor in development of pragmatic universal screening programs. To date, most of the focus in identification of congenital CMV infection has been linking newborn hearing testing programs with CMV testing. For some, these have been labeled “targeted screening programs for CMV.” To us, these appear to be best practice for medical evaluations of an infant with identified SNHL. The availability of saliva-based CMV testing should enable virtually all children who fail newborn screening to be tested for CMV. In multiple studies,3,4 6% of infants with confirmed hearing screen failure tested positive for CMV. A recent study5 identified only 1 infant among the 171 infants who failed newborn screening, however only approximately 15% of the infants were eventually confirmed as hearing impaired at audiology follow-up, suggesting that programmatically testing for CMV might be limited to those with confirmed hearing loss if such can be accomplished within a narrow window of time.
The major challenge with linking CMV testing with newborn hearing screening is whether treatment with valganciclovir would be of value in congenital CMV infection and isolated hearing loss. Studies of children with symptomatic central nervous system congenital CMV disease provide evidence of improvement (or lack of progression) in hearing loss in those treated with valganciclovir. Few, if any of these children had isolated hearing loss in this pivotal study.6 An observational study reported improved outcomes in 55 of 59 (93%) children with congenital CMV and isolated SNHL treated with valganciclovir between birth to 12 weeks of life.7 Hearing improved in nearly 70% of ears, 27% showed no change, and only 3% demonstrated progression of hearing loss; most of the improved ears returned to normal hearing. Currently, a National Institutes of Health study (ValEAR) is recruiting CMV-infected infants with isolated SNHL and randomizing them to treatment with valganciclovir or placebo. The goal is to determine if infants treated with valganciclovir will have better hearing and language outcomes.
Linking CMV testing to those who fail newborn hearing screening programs is an important step, as it appears such children are at least five times more likely to be infected with CMV than is the overall birth cohort. However, such strategies fall short of identifying the majority of newborns with congenital CMV infection, who are completely asymptomatic yet are at risk for development of complications that potentially have substantial impact on their quality of life. Although the availability of sensitive and specific PCR testing in saliva provides a pragmatic approach to identify infected children, many questions remain. First, would a confirmatory test be necessary, such as urine PCR (now considered the gold standard by many CMV experts)? Second, once identified, what regimen for follow-up testing would be indicated to identify those with early SNHL or retinopathy, and until what age? Third, is there a role for treatment in asymptomatic infection? Would that treatment be prophylactic, prior to the development of clinical signs, or implemented once early evidence of SNHL or retinopathy is present?
The Valgan Toddler study – sponsored by NIH and the University of Alabama as part of the Collaborative Antiviral Study Group – will enroll children who are aged 1 month through 3 years and who had a recent diagnosis of hearing loss (within the prior 12 weeks) and evidence of congenital CMV infection. The purpose of this study is to compare the effect on hearing and neurologic outcomes in infants aged 1 month through 4 years with recent onset SNHL who receive 6 weeks of valganciclovir versus children who do not receive this drug. The results of such studies will be critical for the development of best practices.
In summary, the licensure of a rapid PCR-based tool for diagnosis of CMV infection from saliva adds to our ability to develop screening programs to detect asymptomatic infants with congenital CMV infection. The ability to link newborns who fail hearing screening programs with CMV testing will lead to more detection of CMV-infected neonates, both with isolated hearing loss, and subsequently with no signs or symptoms of infection. There is an urgent need for evidence from randomized clinical trials to enable the development of best practices for such infants.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Dr. Lapidot is a senior fellow in pediatric infectious diseases, Boston Medical Center. Neither Dr. Pelton nor Dr. Lapidot have any relevant financial disclosures. Email them at [email protected].
References
1. J Pediatric Infect Dis Soc. 2019 Mar 28;8(1):55-9.
2. N Engl J Med 2011 Jun 2; 364:2111-8.
3. Pediatrics. 2008 May;121(5):970-5
4. J Clin Virol. 2018 May;102:110-5.
5. J Pediatric Infect Dis Soc. 2019 Mar;8(1):55-9.
6. J Pediatr. 2003 Jul;143(1):16-25.
7. J Pediatr. 2018 Aug;199:166-70.
Cytomegalovirus (CMV) infection is the most common congenital viral infection in U.S. children, with a frequency between 0.5% and 1% of newborn infants resulting in approximately 30,000 infected children annually. A small minority (approximately 10%) can be identified in the neonatal period as symptomatic with jaundice (from direct hyperbilirubinemia), petechiae (from thrombocytopenia), hepatosplenomegaly, microcephaly, or other manifestations. The vast majority are asymptomatic at birth, yet 15% will have or develop sensorineural hearing loss (SNHL) during the first few years of life; others (1%-2%) will develop vision loss associated with retinal scars. Congenital CMV accounts for 20% of those with SNHL detected at birth and 25% of children with SNHL at 4 years of age.
Screening for congenital CMV has been an ongoing subject of debate. The challenges of implementing screening programs are related both to the diagnostics (collecting urine samples on newborns) as well as with the question of whether we have treatment and interventions to offer babies diagnosed with congenital CMV across the complete spectrum of clinical presentations.
Current screening programs implemented in some hospitals, called “targeted screening,” in which babies who fail newborn screening programs are tested for CMV, are not sufficient to achieve the goal of identifying babies who will need follow-up for early detection of SNHL or vision abnormalities, or possibly early antiviral therapy (Valcyte; valganciclovir), because only a small portion of those who eventually develop SNHL are currently identified by the targeted screening programs.1
However, its availability only has added to the debate as to whether the time has arrived for universal screening.
Vertical transmission of CMV occurs in utero (during any of the trimesters), at birth by passage through the birth canal, or postnatally by ingestion of breast milk. Neonatal infection (in utero and postnatal) occurs in both mothers with primary CMV infection during gestation and in those with recurrent infection (from a different viral strain) or reactivation of infection. Severe clinically symptomatic disease and sequelae is associated with primary maternal infection and early transmission to the fetus. However, it is estimated that nonprimary maternal infection accounts for 75% of neonatal infections. Transmission by breast milk to full-term, healthy infants does not appear to be associated with clinical illness or sequelae; however, preterm infants or those with birth weights less than 1,500 g have a small risk of developing clinical disease.
The polymerase chain reaction–based saliva CMV test (Alethia CMV Assay Test System) was licensed by the Food and Drug Administration in November 2018 after studies demonstrated high sensitivity and specificity, compared with viral culture (the gold standard). In one study, 17,327 infants were screened with the liquid-saliva PCR assay, and 0.5% tested positive for CMV on both the saliva test and culture. Sensitivity and specificity of the liquid-saliva PCR assay were 100% and 99.9%, respectively.2 The availability of an approved saliva-based assay that is both highly sensitive and specific overcomes the challenge of collecting urine, which has been a limiting factor in development of pragmatic universal screening programs. To date, most of the focus in identification of congenital CMV infection has been linking newborn hearing testing programs with CMV testing. For some, these have been labeled “targeted screening programs for CMV.” To us, these appear to be best practice for medical evaluations of an infant with identified SNHL. The availability of saliva-based CMV testing should enable virtually all children who fail newborn screening to be tested for CMV. In multiple studies,3,4 6% of infants with confirmed hearing screen failure tested positive for CMV. A recent study5 identified only 1 infant among the 171 infants who failed newborn screening, however only approximately 15% of the infants were eventually confirmed as hearing impaired at audiology follow-up, suggesting that programmatically testing for CMV might be limited to those with confirmed hearing loss if such can be accomplished within a narrow window of time.
The major challenge with linking CMV testing with newborn hearing screening is whether treatment with valganciclovir would be of value in congenital CMV infection and isolated hearing loss. Studies of children with symptomatic central nervous system congenital CMV disease provide evidence of improvement (or lack of progression) in hearing loss in those treated with valganciclovir. Few, if any of these children had isolated hearing loss in this pivotal study.6 An observational study reported improved outcomes in 55 of 59 (93%) children with congenital CMV and isolated SNHL treated with valganciclovir between birth to 12 weeks of life.7 Hearing improved in nearly 70% of ears, 27% showed no change, and only 3% demonstrated progression of hearing loss; most of the improved ears returned to normal hearing. Currently, a National Institutes of Health study (ValEAR) is recruiting CMV-infected infants with isolated SNHL and randomizing them to treatment with valganciclovir or placebo. The goal is to determine if infants treated with valganciclovir will have better hearing and language outcomes.
Linking CMV testing to those who fail newborn hearing screening programs is an important step, as it appears such children are at least five times more likely to be infected with CMV than is the overall birth cohort. However, such strategies fall short of identifying the majority of newborns with congenital CMV infection, who are completely asymptomatic yet are at risk for development of complications that potentially have substantial impact on their quality of life. Although the availability of sensitive and specific PCR testing in saliva provides a pragmatic approach to identify infected children, many questions remain. First, would a confirmatory test be necessary, such as urine PCR (now considered the gold standard by many CMV experts)? Second, once identified, what regimen for follow-up testing would be indicated to identify those with early SNHL or retinopathy, and until what age? Third, is there a role for treatment in asymptomatic infection? Would that treatment be prophylactic, prior to the development of clinical signs, or implemented once early evidence of SNHL or retinopathy is present?
The Valgan Toddler study – sponsored by NIH and the University of Alabama as part of the Collaborative Antiviral Study Group – will enroll children who are aged 1 month through 3 years and who had a recent diagnosis of hearing loss (within the prior 12 weeks) and evidence of congenital CMV infection. The purpose of this study is to compare the effect on hearing and neurologic outcomes in infants aged 1 month through 4 years with recent onset SNHL who receive 6 weeks of valganciclovir versus children who do not receive this drug. The results of such studies will be critical for the development of best practices.
In summary, the licensure of a rapid PCR-based tool for diagnosis of CMV infection from saliva adds to our ability to develop screening programs to detect asymptomatic infants with congenital CMV infection. The ability to link newborns who fail hearing screening programs with CMV testing will lead to more detection of CMV-infected neonates, both with isolated hearing loss, and subsequently with no signs or symptoms of infection. There is an urgent need for evidence from randomized clinical trials to enable the development of best practices for such infants.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Dr. Lapidot is a senior fellow in pediatric infectious diseases, Boston Medical Center. Neither Dr. Pelton nor Dr. Lapidot have any relevant financial disclosures. Email them at [email protected].
References
1. J Pediatric Infect Dis Soc. 2019 Mar 28;8(1):55-9.
2. N Engl J Med 2011 Jun 2; 364:2111-8.
3. Pediatrics. 2008 May;121(5):970-5
4. J Clin Virol. 2018 May;102:110-5.
5. J Pediatric Infect Dis Soc. 2019 Mar;8(1):55-9.
6. J Pediatr. 2003 Jul;143(1):16-25.
7. J Pediatr. 2018 Aug;199:166-70.
Cytomegalovirus (CMV) infection is the most common congenital viral infection in U.S. children, with a frequency between 0.5% and 1% of newborn infants resulting in approximately 30,000 infected children annually. A small minority (approximately 10%) can be identified in the neonatal period as symptomatic with jaundice (from direct hyperbilirubinemia), petechiae (from thrombocytopenia), hepatosplenomegaly, microcephaly, or other manifestations. The vast majority are asymptomatic at birth, yet 15% will have or develop sensorineural hearing loss (SNHL) during the first few years of life; others (1%-2%) will develop vision loss associated with retinal scars. Congenital CMV accounts for 20% of those with SNHL detected at birth and 25% of children with SNHL at 4 years of age.
Screening for congenital CMV has been an ongoing subject of debate. The challenges of implementing screening programs are related both to the diagnostics (collecting urine samples on newborns) as well as with the question of whether we have treatment and interventions to offer babies diagnosed with congenital CMV across the complete spectrum of clinical presentations.
Current screening programs implemented in some hospitals, called “targeted screening,” in which babies who fail newborn screening programs are tested for CMV, are not sufficient to achieve the goal of identifying babies who will need follow-up for early detection of SNHL or vision abnormalities, or possibly early antiviral therapy (Valcyte; valganciclovir), because only a small portion of those who eventually develop SNHL are currently identified by the targeted screening programs.1
However, its availability only has added to the debate as to whether the time has arrived for universal screening.
Vertical transmission of CMV occurs in utero (during any of the trimesters), at birth by passage through the birth canal, or postnatally by ingestion of breast milk. Neonatal infection (in utero and postnatal) occurs in both mothers with primary CMV infection during gestation and in those with recurrent infection (from a different viral strain) or reactivation of infection. Severe clinically symptomatic disease and sequelae is associated with primary maternal infection and early transmission to the fetus. However, it is estimated that nonprimary maternal infection accounts for 75% of neonatal infections. Transmission by breast milk to full-term, healthy infants does not appear to be associated with clinical illness or sequelae; however, preterm infants or those with birth weights less than 1,500 g have a small risk of developing clinical disease.
The polymerase chain reaction–based saliva CMV test (Alethia CMV Assay Test System) was licensed by the Food and Drug Administration in November 2018 after studies demonstrated high sensitivity and specificity, compared with viral culture (the gold standard). In one study, 17,327 infants were screened with the liquid-saliva PCR assay, and 0.5% tested positive for CMV on both the saliva test and culture. Sensitivity and specificity of the liquid-saliva PCR assay were 100% and 99.9%, respectively.2 The availability of an approved saliva-based assay that is both highly sensitive and specific overcomes the challenge of collecting urine, which has been a limiting factor in development of pragmatic universal screening programs. To date, most of the focus in identification of congenital CMV infection has been linking newborn hearing testing programs with CMV testing. For some, these have been labeled “targeted screening programs for CMV.” To us, these appear to be best practice for medical evaluations of an infant with identified SNHL. The availability of saliva-based CMV testing should enable virtually all children who fail newborn screening to be tested for CMV. In multiple studies,3,4 6% of infants with confirmed hearing screen failure tested positive for CMV. A recent study5 identified only 1 infant among the 171 infants who failed newborn screening, however only approximately 15% of the infants were eventually confirmed as hearing impaired at audiology follow-up, suggesting that programmatically testing for CMV might be limited to those with confirmed hearing loss if such can be accomplished within a narrow window of time.
The major challenge with linking CMV testing with newborn hearing screening is whether treatment with valganciclovir would be of value in congenital CMV infection and isolated hearing loss. Studies of children with symptomatic central nervous system congenital CMV disease provide evidence of improvement (or lack of progression) in hearing loss in those treated with valganciclovir. Few, if any of these children had isolated hearing loss in this pivotal study.6 An observational study reported improved outcomes in 55 of 59 (93%) children with congenital CMV and isolated SNHL treated with valganciclovir between birth to 12 weeks of life.7 Hearing improved in nearly 70% of ears, 27% showed no change, and only 3% demonstrated progression of hearing loss; most of the improved ears returned to normal hearing. Currently, a National Institutes of Health study (ValEAR) is recruiting CMV-infected infants with isolated SNHL and randomizing them to treatment with valganciclovir or placebo. The goal is to determine if infants treated with valganciclovir will have better hearing and language outcomes.
Linking CMV testing to those who fail newborn hearing screening programs is an important step, as it appears such children are at least five times more likely to be infected with CMV than is the overall birth cohort. However, such strategies fall short of identifying the majority of newborns with congenital CMV infection, who are completely asymptomatic yet are at risk for development of complications that potentially have substantial impact on their quality of life. Although the availability of sensitive and specific PCR testing in saliva provides a pragmatic approach to identify infected children, many questions remain. First, would a confirmatory test be necessary, such as urine PCR (now considered the gold standard by many CMV experts)? Second, once identified, what regimen for follow-up testing would be indicated to identify those with early SNHL or retinopathy, and until what age? Third, is there a role for treatment in asymptomatic infection? Would that treatment be prophylactic, prior to the development of clinical signs, or implemented once early evidence of SNHL or retinopathy is present?
The Valgan Toddler study – sponsored by NIH and the University of Alabama as part of the Collaborative Antiviral Study Group – will enroll children who are aged 1 month through 3 years and who had a recent diagnosis of hearing loss (within the prior 12 weeks) and evidence of congenital CMV infection. The purpose of this study is to compare the effect on hearing and neurologic outcomes in infants aged 1 month through 4 years with recent onset SNHL who receive 6 weeks of valganciclovir versus children who do not receive this drug. The results of such studies will be critical for the development of best practices.
In summary, the licensure of a rapid PCR-based tool for diagnosis of CMV infection from saliva adds to our ability to develop screening programs to detect asymptomatic infants with congenital CMV infection. The ability to link newborns who fail hearing screening programs with CMV testing will lead to more detection of CMV-infected neonates, both with isolated hearing loss, and subsequently with no signs or symptoms of infection. There is an urgent need for evidence from randomized clinical trials to enable the development of best practices for such infants.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Dr. Lapidot is a senior fellow in pediatric infectious diseases, Boston Medical Center. Neither Dr. Pelton nor Dr. Lapidot have any relevant financial disclosures. Email them at [email protected].
References
1. J Pediatric Infect Dis Soc. 2019 Mar 28;8(1):55-9.
2. N Engl J Med 2011 Jun 2; 364:2111-8.
3. Pediatrics. 2008 May;121(5):970-5
4. J Clin Virol. 2018 May;102:110-5.
5. J Pediatric Infect Dis Soc. 2019 Mar;8(1):55-9.
6. J Pediatr. 2003 Jul;143(1):16-25.
7. J Pediatr. 2018 Aug;199:166-70.
Busiest week yet brings 2019 measles total to 555 cases
according to the Centers for Disease Control and Prevention.
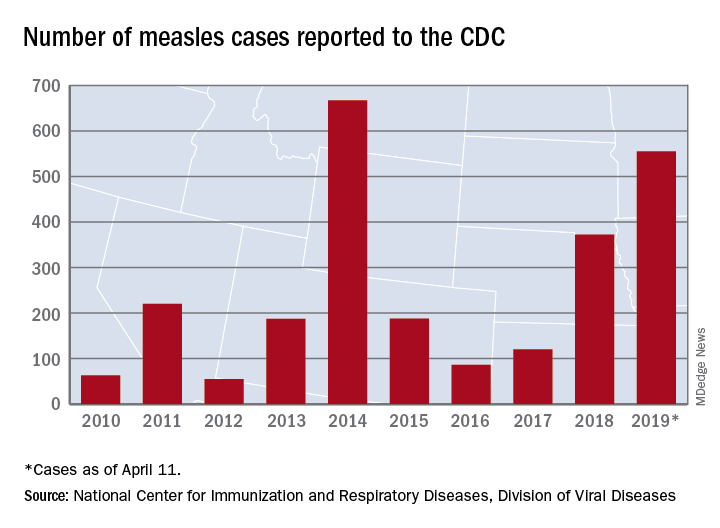
The 90 measles cases reported during the week ending April 11 mark the third consecutive weekly high for 2019, topping the 78 recorded during the week of April 4 and the 73 reported during the week of March 28. Meanwhile, this year’s total trails only the 667 cases reported in 2014 for the highest in the postelimination era, the CDC said April 15.
New York reported 26 new cases in Brooklyn’s Williamsburg neighborhood last week, which puts the borough at 227 for the year, with another two occurring in the Flushing section of Queens. A public health emergency declared on April 9 covers several zip codes in Williamsburg and requires unvaccinated individuals who may have been exposed to measles to receive “the measles-mumps-rubella vaccine in order to protect others in the community and help curtail the ongoing outbreak,” the city’s health department said in a written statement.
Maryland became the 20th state to report a measles case this year, and the state’s department of health said it was notifying those in the vicinity of a medical office building in Pikesville about possible exposure on April 2.
The recent outbreak in Michigan’s Oakland County did not result in any new patients over the last week and remains at 38 cases, with the state reporting one additional case in Wayne County. More recent reports of a case in Washtenaw County and another in Oakland County were reversed after additional testing, the state health department reported.
according to the Centers for Disease Control and Prevention.

The 90 measles cases reported during the week ending April 11 mark the third consecutive weekly high for 2019, topping the 78 recorded during the week of April 4 and the 73 reported during the week of March 28. Meanwhile, this year’s total trails only the 667 cases reported in 2014 for the highest in the postelimination era, the CDC said April 15.
New York reported 26 new cases in Brooklyn’s Williamsburg neighborhood last week, which puts the borough at 227 for the year, with another two occurring in the Flushing section of Queens. A public health emergency declared on April 9 covers several zip codes in Williamsburg and requires unvaccinated individuals who may have been exposed to measles to receive “the measles-mumps-rubella vaccine in order to protect others in the community and help curtail the ongoing outbreak,” the city’s health department said in a written statement.
Maryland became the 20th state to report a measles case this year, and the state’s department of health said it was notifying those in the vicinity of a medical office building in Pikesville about possible exposure on April 2.
The recent outbreak in Michigan’s Oakland County did not result in any new patients over the last week and remains at 38 cases, with the state reporting one additional case in Wayne County. More recent reports of a case in Washtenaw County and another in Oakland County were reversed after additional testing, the state health department reported.
according to the Centers for Disease Control and Prevention.

The 90 measles cases reported during the week ending April 11 mark the third consecutive weekly high for 2019, topping the 78 recorded during the week of April 4 and the 73 reported during the week of March 28. Meanwhile, this year’s total trails only the 667 cases reported in 2014 for the highest in the postelimination era, the CDC said April 15.
New York reported 26 new cases in Brooklyn’s Williamsburg neighborhood last week, which puts the borough at 227 for the year, with another two occurring in the Flushing section of Queens. A public health emergency declared on April 9 covers several zip codes in Williamsburg and requires unvaccinated individuals who may have been exposed to measles to receive “the measles-mumps-rubella vaccine in order to protect others in the community and help curtail the ongoing outbreak,” the city’s health department said in a written statement.
Maryland became the 20th state to report a measles case this year, and the state’s department of health said it was notifying those in the vicinity of a medical office building in Pikesville about possible exposure on April 2.
The recent outbreak in Michigan’s Oakland County did not result in any new patients over the last week and remains at 38 cases, with the state reporting one additional case in Wayne County. More recent reports of a case in Washtenaw County and another in Oakland County were reversed after additional testing, the state health department reported.
A chance to unite
Is America coming apart at the seams? According to the press, there are more things that divide us than bind us together. It’s red state versus blue state, it’s the privileged versus the disadvantaged, people of color versus the white majority. Could the great melting pot have cooled and its contents settled out into a dozen stratified layers?
Despite the image of a divided America that we see portrayed in the newspapers and on television, I continue to believe that there is more that we share in common than separate us, but it’s a struggle. The media operate on the assumption that conflict draws more readers than good news about cooperation and compromise. The situation is compounded by the apparent absence of a leader from either party who wants to unite us.
However, when one scratches the surface, there is surprising amount of agreement among Americans. For example, according to John Gramlich (“7 facts about guns in the U.S.,” Pew Research Center, Dec. 27, 2018), 89% of both Republicans and Democrats feel that people with mental illness should not be allowed to purchase a gun. And 79% of Republicans and 91% of Democrats favor background checks at gun shows and for private sales for purchase of a gun. As of 2018, 58% of Americans feel that abortion should be legal in all or most cases, and only 37% feel it should be illegal in all or most cases. (“Public Opinion on Abortion,” Pew Research Center, Oct. 15, 2018).
At the core of many of our struggles to unite is a question that has bedeviled democracies for millennia: How does one balance a citizen’s freedom of choice with the health and safety of the society in which that person lives? While resolutions on gun control and abortion seem unlikely in the foreseeable future, the current outbreaks of measles offer America a rare opportunity to unite on an issue that pits personal freedom against societal safety.
According to Virginia Villa (“5 facts about vaccines in the U.S.,” Pew Research Center, Mar. 19, 2019), 82% of adults in the United States believe that the MMR vaccine should be required for public school attendance, while only 17% believe that parents should be allowed to leave their child unvaccinated even if their decision creates a health risk for other children and adults.
Why should we expect the government to respond to protect the population from the risk posed by the unvaccinated minority when it has done very little to further gun control? Obviously a key difference is that the antivaccination minority lacks the financial resources and political muscle of a large organization such as the National Rifle Association. While we must never underestimate the power of social media, the publicity surfacing from the mainstream media as the measles outbreaks in the United States have continued has prompted several states to rethink their policies regarding vaccination requirements and school attendance. Here in Maine, there has been strong support among the legislature for eliminating exemptions for philosophic or religious exemptions.
It is probably unrealistic to expect the federal government to act on the health threat caused by the antivaccine movement. However, it is encouraging that, at least at the local level, there is hope for closing one of the wounds that divide us. As providers who care for children, we should seize this opportunity created by the measles outbreaks to promote legislation and policies that strike a sensible balance between the right of the individual and the safety of the society at large.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Is America coming apart at the seams? According to the press, there are more things that divide us than bind us together. It’s red state versus blue state, it’s the privileged versus the disadvantaged, people of color versus the white majority. Could the great melting pot have cooled and its contents settled out into a dozen stratified layers?
Despite the image of a divided America that we see portrayed in the newspapers and on television, I continue to believe that there is more that we share in common than separate us, but it’s a struggle. The media operate on the assumption that conflict draws more readers than good news about cooperation and compromise. The situation is compounded by the apparent absence of a leader from either party who wants to unite us.
However, when one scratches the surface, there is surprising amount of agreement among Americans. For example, according to John Gramlich (“7 facts about guns in the U.S.,” Pew Research Center, Dec. 27, 2018), 89% of both Republicans and Democrats feel that people with mental illness should not be allowed to purchase a gun. And 79% of Republicans and 91% of Democrats favor background checks at gun shows and for private sales for purchase of a gun. As of 2018, 58% of Americans feel that abortion should be legal in all or most cases, and only 37% feel it should be illegal in all or most cases. (“Public Opinion on Abortion,” Pew Research Center, Oct. 15, 2018).
At the core of many of our struggles to unite is a question that has bedeviled democracies for millennia: How does one balance a citizen’s freedom of choice with the health and safety of the society in which that person lives? While resolutions on gun control and abortion seem unlikely in the foreseeable future, the current outbreaks of measles offer America a rare opportunity to unite on an issue that pits personal freedom against societal safety.
According to Virginia Villa (“5 facts about vaccines in the U.S.,” Pew Research Center, Mar. 19, 2019), 82% of adults in the United States believe that the MMR vaccine should be required for public school attendance, while only 17% believe that parents should be allowed to leave their child unvaccinated even if their decision creates a health risk for other children and adults.
Why should we expect the government to respond to protect the population from the risk posed by the unvaccinated minority when it has done very little to further gun control? Obviously a key difference is that the antivaccination minority lacks the financial resources and political muscle of a large organization such as the National Rifle Association. While we must never underestimate the power of social media, the publicity surfacing from the mainstream media as the measles outbreaks in the United States have continued has prompted several states to rethink their policies regarding vaccination requirements and school attendance. Here in Maine, there has been strong support among the legislature for eliminating exemptions for philosophic or religious exemptions.
It is probably unrealistic to expect the federal government to act on the health threat caused by the antivaccine movement. However, it is encouraging that, at least at the local level, there is hope for closing one of the wounds that divide us. As providers who care for children, we should seize this opportunity created by the measles outbreaks to promote legislation and policies that strike a sensible balance between the right of the individual and the safety of the society at large.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Is America coming apart at the seams? According to the press, there are more things that divide us than bind us together. It’s red state versus blue state, it’s the privileged versus the disadvantaged, people of color versus the white majority. Could the great melting pot have cooled and its contents settled out into a dozen stratified layers?
Despite the image of a divided America that we see portrayed in the newspapers and on television, I continue to believe that there is more that we share in common than separate us, but it’s a struggle. The media operate on the assumption that conflict draws more readers than good news about cooperation and compromise. The situation is compounded by the apparent absence of a leader from either party who wants to unite us.
However, when one scratches the surface, there is surprising amount of agreement among Americans. For example, according to John Gramlich (“7 facts about guns in the U.S.,” Pew Research Center, Dec. 27, 2018), 89% of both Republicans and Democrats feel that people with mental illness should not be allowed to purchase a gun. And 79% of Republicans and 91% of Democrats favor background checks at gun shows and for private sales for purchase of a gun. As of 2018, 58% of Americans feel that abortion should be legal in all or most cases, and only 37% feel it should be illegal in all or most cases. (“Public Opinion on Abortion,” Pew Research Center, Oct. 15, 2018).
At the core of many of our struggles to unite is a question that has bedeviled democracies for millennia: How does one balance a citizen’s freedom of choice with the health and safety of the society in which that person lives? While resolutions on gun control and abortion seem unlikely in the foreseeable future, the current outbreaks of measles offer America a rare opportunity to unite on an issue that pits personal freedom against societal safety.
According to Virginia Villa (“5 facts about vaccines in the U.S.,” Pew Research Center, Mar. 19, 2019), 82% of adults in the United States believe that the MMR vaccine should be required for public school attendance, while only 17% believe that parents should be allowed to leave their child unvaccinated even if their decision creates a health risk for other children and adults.
Why should we expect the government to respond to protect the population from the risk posed by the unvaccinated minority when it has done very little to further gun control? Obviously a key difference is that the antivaccination minority lacks the financial resources and political muscle of a large organization such as the National Rifle Association. While we must never underestimate the power of social media, the publicity surfacing from the mainstream media as the measles outbreaks in the United States have continued has prompted several states to rethink their policies regarding vaccination requirements and school attendance. Here in Maine, there has been strong support among the legislature for eliminating exemptions for philosophic or religious exemptions.
It is probably unrealistic to expect the federal government to act on the health threat caused by the antivaccine movement. However, it is encouraging that, at least at the local level, there is hope for closing one of the wounds that divide us. As providers who care for children, we should seize this opportunity created by the measles outbreaks to promote legislation and policies that strike a sensible balance between the right of the individual and the safety of the society at large.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Dr. Douglas Paauw: Consider rechallenging patients with penicillin allergy
PHILADELPHIA – As fluoroquinolone warnings stack up, internists seeking treatment alternatives should consider rechallenging patients with penicillin allergy or referring those patients for testing, said Douglas S. Paauw, MD, during a presentation.
This was one of the pieces of advice provided by Dr. Paauw, professor of medicine in the division of general internal medicine at the University of Washington, Seattle, at the annual meeting of the American College of Physicians.
“The FDA [Food and Drug Administration] has been just killing trees, sending us letters over the last 5-10 years, with fluoroquinolone warnings,” said Dr. Paauw, referencing previous warnings describing risks of tendon rupture, peripheral neuropathy, hypoglycemia, mental health side effects, and more.
“I think the buzz in 2019 is that we should not overreact to a history of penicillin allergy,” he said.
As many as 98% of patients who have reported penicillin allergy don’t have true allergy and can safely receive penicillin, he explained.
“If they don’t have an allergy, make sure you get it out of the electronic record,” Dr. Paauw also advised.
The latest warning on fluoroquinolones from the FDA, issued in Dec. 20, 2018, said that clinicians should avoid prescribing these antibiotics in patients who have, or are at risk of, aortic aneurysm. This comprises a very large proportion of patients in an internist’s practice, Dr. Paauw noted. The warning specifically singled out elderly patients as being in the at-risk population, along with patients who have peripheral atherosclerotic vascular diseases, hypertension, or genetic conditions such as Marfan syndrome or Ehlers-Danlos syndrome, he added.
Dr. Paauw further supported his suggestions by describing two relevant studies.
In one of those studies, which was published this year in an allergy and asthma journal, 20 subjects with a history of penicillin allergy agreed to direct oral amoxicillin rechallenge by an allergist, he said. None of those 20 patients were observed to have developed immediate or delayed hypersensitivity reactions, investigators reported. That study included a total of 50 adults with a penicillin allergy label, of whom 24 (48%) had the label removed from their medical records.
In another recent and reassuring study, penicillin allergy testing was conducted in 100 children with parent-reported penicillin allergy that was considered low risk based on reported symptoms, Dr. Paauw said. Of that group, all 100 children were found to be negative for true penicillin allergy.
Dr. Paauw had no relevant disclosures.
SOURCE: Paauw DS. Annual Meeting of the American College of Physicians, Presentation MTP 013.
PHILADELPHIA – As fluoroquinolone warnings stack up, internists seeking treatment alternatives should consider rechallenging patients with penicillin allergy or referring those patients for testing, said Douglas S. Paauw, MD, during a presentation.
This was one of the pieces of advice provided by Dr. Paauw, professor of medicine in the division of general internal medicine at the University of Washington, Seattle, at the annual meeting of the American College of Physicians.
“The FDA [Food and Drug Administration] has been just killing trees, sending us letters over the last 5-10 years, with fluoroquinolone warnings,” said Dr. Paauw, referencing previous warnings describing risks of tendon rupture, peripheral neuropathy, hypoglycemia, mental health side effects, and more.
“I think the buzz in 2019 is that we should not overreact to a history of penicillin allergy,” he said.
As many as 98% of patients who have reported penicillin allergy don’t have true allergy and can safely receive penicillin, he explained.
“If they don’t have an allergy, make sure you get it out of the electronic record,” Dr. Paauw also advised.
The latest warning on fluoroquinolones from the FDA, issued in Dec. 20, 2018, said that clinicians should avoid prescribing these antibiotics in patients who have, or are at risk of, aortic aneurysm. This comprises a very large proportion of patients in an internist’s practice, Dr. Paauw noted. The warning specifically singled out elderly patients as being in the at-risk population, along with patients who have peripheral atherosclerotic vascular diseases, hypertension, or genetic conditions such as Marfan syndrome or Ehlers-Danlos syndrome, he added.
Dr. Paauw further supported his suggestions by describing two relevant studies.
In one of those studies, which was published this year in an allergy and asthma journal, 20 subjects with a history of penicillin allergy agreed to direct oral amoxicillin rechallenge by an allergist, he said. None of those 20 patients were observed to have developed immediate or delayed hypersensitivity reactions, investigators reported. That study included a total of 50 adults with a penicillin allergy label, of whom 24 (48%) had the label removed from their medical records.
In another recent and reassuring study, penicillin allergy testing was conducted in 100 children with parent-reported penicillin allergy that was considered low risk based on reported symptoms, Dr. Paauw said. Of that group, all 100 children were found to be negative for true penicillin allergy.
Dr. Paauw had no relevant disclosures.
SOURCE: Paauw DS. Annual Meeting of the American College of Physicians, Presentation MTP 013.
PHILADELPHIA – As fluoroquinolone warnings stack up, internists seeking treatment alternatives should consider rechallenging patients with penicillin allergy or referring those patients for testing, said Douglas S. Paauw, MD, during a presentation.
This was one of the pieces of advice provided by Dr. Paauw, professor of medicine in the division of general internal medicine at the University of Washington, Seattle, at the annual meeting of the American College of Physicians.
“The FDA [Food and Drug Administration] has been just killing trees, sending us letters over the last 5-10 years, with fluoroquinolone warnings,” said Dr. Paauw, referencing previous warnings describing risks of tendon rupture, peripheral neuropathy, hypoglycemia, mental health side effects, and more.
“I think the buzz in 2019 is that we should not overreact to a history of penicillin allergy,” he said.
As many as 98% of patients who have reported penicillin allergy don’t have true allergy and can safely receive penicillin, he explained.
“If they don’t have an allergy, make sure you get it out of the electronic record,” Dr. Paauw also advised.
The latest warning on fluoroquinolones from the FDA, issued in Dec. 20, 2018, said that clinicians should avoid prescribing these antibiotics in patients who have, or are at risk of, aortic aneurysm. This comprises a very large proportion of patients in an internist’s practice, Dr. Paauw noted. The warning specifically singled out elderly patients as being in the at-risk population, along with patients who have peripheral atherosclerotic vascular diseases, hypertension, or genetic conditions such as Marfan syndrome or Ehlers-Danlos syndrome, he added.
Dr. Paauw further supported his suggestions by describing two relevant studies.
In one of those studies, which was published this year in an allergy and asthma journal, 20 subjects with a history of penicillin allergy agreed to direct oral amoxicillin rechallenge by an allergist, he said. None of those 20 patients were observed to have developed immediate or delayed hypersensitivity reactions, investigators reported. That study included a total of 50 adults with a penicillin allergy label, of whom 24 (48%) had the label removed from their medical records.
In another recent and reassuring study, penicillin allergy testing was conducted in 100 children with parent-reported penicillin allergy that was considered low risk based on reported symptoms, Dr. Paauw said. Of that group, all 100 children were found to be negative for true penicillin allergy.
Dr. Paauw had no relevant disclosures.
SOURCE: Paauw DS. Annual Meeting of the American College of Physicians, Presentation MTP 013.
REPORTING FROM INTERNAL MEDICINE 2019
Research coalition issues plan for curing hepatitis B virus
VIENNA – They hope either to have a cure or to have made substantial progress toward this goal over the next 10 years.
Treatments already are on the market that effectively inhibit hepatitis B replication in infected patients (and an effective preventive vaccine also exists). Still, these treatments are not curative, and for the vast majority of patients treatment must continue indefinitely, while their risk for liver cancer and their virally induced immune system abnormalities remain, Peter A. Revill, PhD, said during a press briefing that introduced a strategy for hepatitis B virus (HBV) cure development from the International Coalition to Eliminate HBV. Concurrently with the briefing session, the strategy appeared in an article published online (Lancet Gastroenterol Hepatol. 2019 Apr 10. doi: 10.1016/s2468-1253(19)30119-0).
The way forward will likely be a “two-pronged approach or restoring immune responses and targeting the virus,” Dr. Revill, head of molecular virology at the Doherty Institute in Melbourne, said in a video interview.
The new strategy recognizes the huge challenge of devising a treatment that produces a total cure that includes elimination of all traces of viral DNA from patients and for the immediate future focuses on the goal of functional cure. The term functional cure means a sustained period without detectable HBV surface antigen or HBV DNA in a patient’s serum, as well as suppressed virus release. Another feature of a functional cure would be a halt to progression of liver disease, replaced by liver regeneration, said Anna S. Lok, MD, professor of medicine and director of clinical hepatology at the University of Michigan, Ann Arbor, and a member of the strategy-writing group. She and her colleagues who wrote the strategy foresee the need for drug combinations with agents that can hit multiple viral targets as well as agents that restore normal immune function.
Several novel drug classes aimed at new viral targets, such as capsid inhibitors, are in various stages of clinical development, said Fabien Zoulim, MD, head of the gastroenterology and hepatology service at the Red Cross Hospital in Lyon, France, and another member of the writing panel. “We have many drug candidates” that use novel approaches to further restrict viral growth, roughly 50 agents in phase 1 and 2 studies, he said during the press briefing, held during the meeting sponsored by the European Association for the Study of the Liver. The other, immunologic aspect of the two-part cure strategy – restoring the “exhausted” HBV-specific T-cell population and stimulating production of neutralizing antibody to HBV – remains hypothetical right now, however. “It’s a concept that needs development,” Dr. Zoulim said.
A reason members of the coalition are optimistic about eventual prospects for a cure is that currently about 1% of patients on HBV antiviral treatments have a functional cure after relatively brief treatment, and the percentage of cured patients plateaus at about 10% among those who remain on current HBV antiviral drugs for several years. In addition, a substantial fraction of patients spontaneously resolve their HBV infection without any treatment. Experts estimate that more than 1 billion people worldwide have been infected by HBV and then later had their infection clear “naturally,” said Dr. Revill. But the mechanism by which this happens is currently a mystery. “We don’t know how or why” so many infected people are “cured” naturally, Dr. Revill admitted, but it gives him and his colleagues hope that the numbers can expand once more and better treatments for HBV infection are available.
VIENNA – They hope either to have a cure or to have made substantial progress toward this goal over the next 10 years.
Treatments already are on the market that effectively inhibit hepatitis B replication in infected patients (and an effective preventive vaccine also exists). Still, these treatments are not curative, and for the vast majority of patients treatment must continue indefinitely, while their risk for liver cancer and their virally induced immune system abnormalities remain, Peter A. Revill, PhD, said during a press briefing that introduced a strategy for hepatitis B virus (HBV) cure development from the International Coalition to Eliminate HBV. Concurrently with the briefing session, the strategy appeared in an article published online (Lancet Gastroenterol Hepatol. 2019 Apr 10. doi: 10.1016/s2468-1253(19)30119-0).
The way forward will likely be a “two-pronged approach or restoring immune responses and targeting the virus,” Dr. Revill, head of molecular virology at the Doherty Institute in Melbourne, said in a video interview.
The new strategy recognizes the huge challenge of devising a treatment that produces a total cure that includes elimination of all traces of viral DNA from patients and for the immediate future focuses on the goal of functional cure. The term functional cure means a sustained period without detectable HBV surface antigen or HBV DNA in a patient’s serum, as well as suppressed virus release. Another feature of a functional cure would be a halt to progression of liver disease, replaced by liver regeneration, said Anna S. Lok, MD, professor of medicine and director of clinical hepatology at the University of Michigan, Ann Arbor, and a member of the strategy-writing group. She and her colleagues who wrote the strategy foresee the need for drug combinations with agents that can hit multiple viral targets as well as agents that restore normal immune function.
Several novel drug classes aimed at new viral targets, such as capsid inhibitors, are in various stages of clinical development, said Fabien Zoulim, MD, head of the gastroenterology and hepatology service at the Red Cross Hospital in Lyon, France, and another member of the writing panel. “We have many drug candidates” that use novel approaches to further restrict viral growth, roughly 50 agents in phase 1 and 2 studies, he said during the press briefing, held during the meeting sponsored by the European Association for the Study of the Liver. The other, immunologic aspect of the two-part cure strategy – restoring the “exhausted” HBV-specific T-cell population and stimulating production of neutralizing antibody to HBV – remains hypothetical right now, however. “It’s a concept that needs development,” Dr. Zoulim said.
A reason members of the coalition are optimistic about eventual prospects for a cure is that currently about 1% of patients on HBV antiviral treatments have a functional cure after relatively brief treatment, and the percentage of cured patients plateaus at about 10% among those who remain on current HBV antiviral drugs for several years. In addition, a substantial fraction of patients spontaneously resolve their HBV infection without any treatment. Experts estimate that more than 1 billion people worldwide have been infected by HBV and then later had their infection clear “naturally,” said Dr. Revill. But the mechanism by which this happens is currently a mystery. “We don’t know how or why” so many infected people are “cured” naturally, Dr. Revill admitted, but it gives him and his colleagues hope that the numbers can expand once more and better treatments for HBV infection are available.
VIENNA – They hope either to have a cure or to have made substantial progress toward this goal over the next 10 years.
Treatments already are on the market that effectively inhibit hepatitis B replication in infected patients (and an effective preventive vaccine also exists). Still, these treatments are not curative, and for the vast majority of patients treatment must continue indefinitely, while their risk for liver cancer and their virally induced immune system abnormalities remain, Peter A. Revill, PhD, said during a press briefing that introduced a strategy for hepatitis B virus (HBV) cure development from the International Coalition to Eliminate HBV. Concurrently with the briefing session, the strategy appeared in an article published online (Lancet Gastroenterol Hepatol. 2019 Apr 10. doi: 10.1016/s2468-1253(19)30119-0).
The way forward will likely be a “two-pronged approach or restoring immune responses and targeting the virus,” Dr. Revill, head of molecular virology at the Doherty Institute in Melbourne, said in a video interview.
The new strategy recognizes the huge challenge of devising a treatment that produces a total cure that includes elimination of all traces of viral DNA from patients and for the immediate future focuses on the goal of functional cure. The term functional cure means a sustained period without detectable HBV surface antigen or HBV DNA in a patient’s serum, as well as suppressed virus release. Another feature of a functional cure would be a halt to progression of liver disease, replaced by liver regeneration, said Anna S. Lok, MD, professor of medicine and director of clinical hepatology at the University of Michigan, Ann Arbor, and a member of the strategy-writing group. She and her colleagues who wrote the strategy foresee the need for drug combinations with agents that can hit multiple viral targets as well as agents that restore normal immune function.
Several novel drug classes aimed at new viral targets, such as capsid inhibitors, are in various stages of clinical development, said Fabien Zoulim, MD, head of the gastroenterology and hepatology service at the Red Cross Hospital in Lyon, France, and another member of the writing panel. “We have many drug candidates” that use novel approaches to further restrict viral growth, roughly 50 agents in phase 1 and 2 studies, he said during the press briefing, held during the meeting sponsored by the European Association for the Study of the Liver. The other, immunologic aspect of the two-part cure strategy – restoring the “exhausted” HBV-specific T-cell population and stimulating production of neutralizing antibody to HBV – remains hypothetical right now, however. “It’s a concept that needs development,” Dr. Zoulim said.
A reason members of the coalition are optimistic about eventual prospects for a cure is that currently about 1% of patients on HBV antiviral treatments have a functional cure after relatively brief treatment, and the percentage of cured patients plateaus at about 10% among those who remain on current HBV antiviral drugs for several years. In addition, a substantial fraction of patients spontaneously resolve their HBV infection without any treatment. Experts estimate that more than 1 billion people worldwide have been infected by HBV and then later had their infection clear “naturally,” said Dr. Revill. But the mechanism by which this happens is currently a mystery. “We don’t know how or why” so many infected people are “cured” naturally, Dr. Revill admitted, but it gives him and his colleagues hope that the numbers can expand once more and better treatments for HBV infection are available.
REPORTING FROM ILC 2019
Weight gain highest with integrase inhibitors for HIV
SEATTLE – Weight gain in HIV treatment is highest with integrase inhibitors, especially dolutegravir (Tivicay), followed by raltegravir (Isentress), according to a review of thousands of North American patients during their first 5 years of therapy.
People “starting integrase inhibitors are gaining significantly more weight than ART [antiretroviral therapy] naive patients starting NNRTIs [nonnucleoside reverse transcriptase inhibitors]. They are also gaining more weight than ART patients starting PIs [protease inhibitors],” however, the differences do not always reach statistical significance, said lead investigator Kassem Bourgi, MD, an infectious disease fellow at Vanderbilt University, Nashville, Tenn.
“This is clinically important as” integrase inhibitor-based “regimens are now recommended first line,” and, as people with HIV live normal lifespans, they “are at increasing risk for obesity, metabolic comorbidities, and cardiovascular disease,” just like the rest of the population, he said.
Dr. Bourgi’s presentation was one of several at the Conference on Retroviruses and Opportunistic Infections tackling the issue of ART-induced obesity. There was great interest in the topic, and his study wasn’t the only one to find an increased risk of excess weight gain with integrase inhibitors.
“This is a real-world issue, and this is what we are seeing in our patients; they are dealing with this every day,” explained moderator Jane O’Halloran, MD, when asked about the impressive audience turnout for the ART obesity session.
Dr. Bourgi’s team turned to the North American AIDS Cohort Collaboration on Research and Design, which includes data on HIV patients throughout Canada and the United States, to compare weight gain outcomes among 24,001 people who started HIV treatment from 2007-2016; 49% started on NNRTI-based regimens, 31% on PIs, and 20% on integrase inhibitors. Elvitegravir (Vitekta) was most commonly used in the integrase group (45%), followed by raltegravir (35%), and dolutegravir (20%).
At 2 and 5 years, patients started on integrase inhibitors gained 4.9 and 6 kg, respectively, over their predicted weight, compared with 3.3 and 4.3 kg for NNRTIs, and 4.4 and 5.1 kg for PIs, adjusted for age, sex, race, cohort site, HIV acquisition mode, ART initiation year, and baseline weight, HIV-1 RNA, and CD4 cell count.
At 2 years among the integrase group, weight gain was 6 kg for dolutegravir, 4.9 kg for raltegravir, and 3.8 kg for elvitegravir; the weight gain for those on elvitegravir was comparable to that for those on PIs.
“Patients who started dolutegravir or raltegravir gained significantly more weight by year 2 compared with those who started elvitegravir,” Dr. Bourgi noted.
The mechanisms for the differences are unknown.
Integrase inhibitors are very well tolerated, so it could be that once on them, people feel so much better that, as one audience member put it, they just do “what everyone else does”: overeat. Dolutegravir also is associated with psychiatric symptoms, so maybe people on it are more likely to be taking psychiatric drugs that pack on the weight. Perhaps it’s something peculiar to the drugs. More work is needed on the issue, Dr. O’Halloran said.
Most of the subjects were men, and 38% were men who have sex with men. The median age at ART initiation was 42 years, and median weight body mass index 25 kg/m2. The median CD4 cell count was 303 cells/mm3.
The work was funded by Gilead, through an investigator sponsored grant. Dr. Bourgi didn’t say he had any disclosures.
SOURCE: Bourgi K et al. CROI 2019, Abstract 670.
SEATTLE – Weight gain in HIV treatment is highest with integrase inhibitors, especially dolutegravir (Tivicay), followed by raltegravir (Isentress), according to a review of thousands of North American patients during their first 5 years of therapy.
People “starting integrase inhibitors are gaining significantly more weight than ART [antiretroviral therapy] naive patients starting NNRTIs [nonnucleoside reverse transcriptase inhibitors]. They are also gaining more weight than ART patients starting PIs [protease inhibitors],” however, the differences do not always reach statistical significance, said lead investigator Kassem Bourgi, MD, an infectious disease fellow at Vanderbilt University, Nashville, Tenn.
“This is clinically important as” integrase inhibitor-based “regimens are now recommended first line,” and, as people with HIV live normal lifespans, they “are at increasing risk for obesity, metabolic comorbidities, and cardiovascular disease,” just like the rest of the population, he said.
Dr. Bourgi’s presentation was one of several at the Conference on Retroviruses and Opportunistic Infections tackling the issue of ART-induced obesity. There was great interest in the topic, and his study wasn’t the only one to find an increased risk of excess weight gain with integrase inhibitors.
“This is a real-world issue, and this is what we are seeing in our patients; they are dealing with this every day,” explained moderator Jane O’Halloran, MD, when asked about the impressive audience turnout for the ART obesity session.
Dr. Bourgi’s team turned to the North American AIDS Cohort Collaboration on Research and Design, which includes data on HIV patients throughout Canada and the United States, to compare weight gain outcomes among 24,001 people who started HIV treatment from 2007-2016; 49% started on NNRTI-based regimens, 31% on PIs, and 20% on integrase inhibitors. Elvitegravir (Vitekta) was most commonly used in the integrase group (45%), followed by raltegravir (35%), and dolutegravir (20%).
At 2 and 5 years, patients started on integrase inhibitors gained 4.9 and 6 kg, respectively, over their predicted weight, compared with 3.3 and 4.3 kg for NNRTIs, and 4.4 and 5.1 kg for PIs, adjusted for age, sex, race, cohort site, HIV acquisition mode, ART initiation year, and baseline weight, HIV-1 RNA, and CD4 cell count.
At 2 years among the integrase group, weight gain was 6 kg for dolutegravir, 4.9 kg for raltegravir, and 3.8 kg for elvitegravir; the weight gain for those on elvitegravir was comparable to that for those on PIs.
“Patients who started dolutegravir or raltegravir gained significantly more weight by year 2 compared with those who started elvitegravir,” Dr. Bourgi noted.
The mechanisms for the differences are unknown.
Integrase inhibitors are very well tolerated, so it could be that once on them, people feel so much better that, as one audience member put it, they just do “what everyone else does”: overeat. Dolutegravir also is associated with psychiatric symptoms, so maybe people on it are more likely to be taking psychiatric drugs that pack on the weight. Perhaps it’s something peculiar to the drugs. More work is needed on the issue, Dr. O’Halloran said.
Most of the subjects were men, and 38% were men who have sex with men. The median age at ART initiation was 42 years, and median weight body mass index 25 kg/m2. The median CD4 cell count was 303 cells/mm3.
The work was funded by Gilead, through an investigator sponsored grant. Dr. Bourgi didn’t say he had any disclosures.
SOURCE: Bourgi K et al. CROI 2019, Abstract 670.
SEATTLE – Weight gain in HIV treatment is highest with integrase inhibitors, especially dolutegravir (Tivicay), followed by raltegravir (Isentress), according to a review of thousands of North American patients during their first 5 years of therapy.
People “starting integrase inhibitors are gaining significantly more weight than ART [antiretroviral therapy] naive patients starting NNRTIs [nonnucleoside reverse transcriptase inhibitors]. They are also gaining more weight than ART patients starting PIs [protease inhibitors],” however, the differences do not always reach statistical significance, said lead investigator Kassem Bourgi, MD, an infectious disease fellow at Vanderbilt University, Nashville, Tenn.
“This is clinically important as” integrase inhibitor-based “regimens are now recommended first line,” and, as people with HIV live normal lifespans, they “are at increasing risk for obesity, metabolic comorbidities, and cardiovascular disease,” just like the rest of the population, he said.
Dr. Bourgi’s presentation was one of several at the Conference on Retroviruses and Opportunistic Infections tackling the issue of ART-induced obesity. There was great interest in the topic, and his study wasn’t the only one to find an increased risk of excess weight gain with integrase inhibitors.
“This is a real-world issue, and this is what we are seeing in our patients; they are dealing with this every day,” explained moderator Jane O’Halloran, MD, when asked about the impressive audience turnout for the ART obesity session.
Dr. Bourgi’s team turned to the North American AIDS Cohort Collaboration on Research and Design, which includes data on HIV patients throughout Canada and the United States, to compare weight gain outcomes among 24,001 people who started HIV treatment from 2007-2016; 49% started on NNRTI-based regimens, 31% on PIs, and 20% on integrase inhibitors. Elvitegravir (Vitekta) was most commonly used in the integrase group (45%), followed by raltegravir (35%), and dolutegravir (20%).
At 2 and 5 years, patients started on integrase inhibitors gained 4.9 and 6 kg, respectively, over their predicted weight, compared with 3.3 and 4.3 kg for NNRTIs, and 4.4 and 5.1 kg for PIs, adjusted for age, sex, race, cohort site, HIV acquisition mode, ART initiation year, and baseline weight, HIV-1 RNA, and CD4 cell count.
At 2 years among the integrase group, weight gain was 6 kg for dolutegravir, 4.9 kg for raltegravir, and 3.8 kg for elvitegravir; the weight gain for those on elvitegravir was comparable to that for those on PIs.
“Patients who started dolutegravir or raltegravir gained significantly more weight by year 2 compared with those who started elvitegravir,” Dr. Bourgi noted.
The mechanisms for the differences are unknown.
Integrase inhibitors are very well tolerated, so it could be that once on them, people feel so much better that, as one audience member put it, they just do “what everyone else does”: overeat. Dolutegravir also is associated with psychiatric symptoms, so maybe people on it are more likely to be taking psychiatric drugs that pack on the weight. Perhaps it’s something peculiar to the drugs. More work is needed on the issue, Dr. O’Halloran said.
Most of the subjects were men, and 38% were men who have sex with men. The median age at ART initiation was 42 years, and median weight body mass index 25 kg/m2. The median CD4 cell count was 303 cells/mm3.
The work was funded by Gilead, through an investigator sponsored grant. Dr. Bourgi didn’t say he had any disclosures.
SOURCE: Bourgi K et al. CROI 2019, Abstract 670.
REPORTING FROM CROI 2019
An HCV-infected population showed gaps in HBV testing, vaccination, and care
Assessment of a large cohort of hepatitis C virus (HCV)–infected patients revealed a high prevalence of current or past hepatitis B virus. However, within this cohort, there were notable gaps in HBV testing, directed care, and vaccination, according to Aaron M. Harris, MD, of the Centers for Disease Control and Prevention.
Dr. Harris and his colleagues abstracted patient-level data from the Grady Health System EHR in August 2016 to create an HCV patient registry. They found that, among 4,224 HCV-infected patients, 3,629 (86%) had test results for the hepatitis B surface antigen (HBsAg), with 43 (1.2%) being HBsAg positive.
“Our results identified a gap in care as a minority of HBsAg-positive patients with HCV coinfection received HBV DNA and/or e-antigen [HBeAg] testing,” the researchers stated.
Overall, only 2,342 (55.4%) patients had test results for all three HBV serologic markers. Among these, 789 (33.7%) were anti-HBc positive only, 678 (28.9%) were anti-HBc/anti-HBs positive, 190 (8.1%) were anti-HBs positive only, and 642 (27.4%) were HBV susceptible. In addition, only 50% of the HBV-susceptible patients received at least one dose of hepatitis B vaccine, according to the report published in Vaccine.
“Strategies are needed to increase hepatitis B testing, linkage to hepatitis B–directed care of HBV/HCV-coinfected patients, and to increase uptake in hepatitis B vaccination for HCV-infected patients within the Grady Health System,” the researchers concluded.
The study was funded by the CDC and the authors reported that they had no conflicts.
SOURCE: Harris AM et al. Vaccine. 2019;37:2188-93.
Assessment of a large cohort of hepatitis C virus (HCV)–infected patients revealed a high prevalence of current or past hepatitis B virus. However, within this cohort, there were notable gaps in HBV testing, directed care, and vaccination, according to Aaron M. Harris, MD, of the Centers for Disease Control and Prevention.
Dr. Harris and his colleagues abstracted patient-level data from the Grady Health System EHR in August 2016 to create an HCV patient registry. They found that, among 4,224 HCV-infected patients, 3,629 (86%) had test results for the hepatitis B surface antigen (HBsAg), with 43 (1.2%) being HBsAg positive.
“Our results identified a gap in care as a minority of HBsAg-positive patients with HCV coinfection received HBV DNA and/or e-antigen [HBeAg] testing,” the researchers stated.
Overall, only 2,342 (55.4%) patients had test results for all three HBV serologic markers. Among these, 789 (33.7%) were anti-HBc positive only, 678 (28.9%) were anti-HBc/anti-HBs positive, 190 (8.1%) were anti-HBs positive only, and 642 (27.4%) were HBV susceptible. In addition, only 50% of the HBV-susceptible patients received at least one dose of hepatitis B vaccine, according to the report published in Vaccine.
“Strategies are needed to increase hepatitis B testing, linkage to hepatitis B–directed care of HBV/HCV-coinfected patients, and to increase uptake in hepatitis B vaccination for HCV-infected patients within the Grady Health System,” the researchers concluded.
The study was funded by the CDC and the authors reported that they had no conflicts.
SOURCE: Harris AM et al. Vaccine. 2019;37:2188-93.
Assessment of a large cohort of hepatitis C virus (HCV)–infected patients revealed a high prevalence of current or past hepatitis B virus. However, within this cohort, there were notable gaps in HBV testing, directed care, and vaccination, according to Aaron M. Harris, MD, of the Centers for Disease Control and Prevention.
Dr. Harris and his colleagues abstracted patient-level data from the Grady Health System EHR in August 2016 to create an HCV patient registry. They found that, among 4,224 HCV-infected patients, 3,629 (86%) had test results for the hepatitis B surface antigen (HBsAg), with 43 (1.2%) being HBsAg positive.
“Our results identified a gap in care as a minority of HBsAg-positive patients with HCV coinfection received HBV DNA and/or e-antigen [HBeAg] testing,” the researchers stated.
Overall, only 2,342 (55.4%) patients had test results for all three HBV serologic markers. Among these, 789 (33.7%) were anti-HBc positive only, 678 (28.9%) were anti-HBc/anti-HBs positive, 190 (8.1%) were anti-HBs positive only, and 642 (27.4%) were HBV susceptible. In addition, only 50% of the HBV-susceptible patients received at least one dose of hepatitis B vaccine, according to the report published in Vaccine.
“Strategies are needed to increase hepatitis B testing, linkage to hepatitis B–directed care of HBV/HCV-coinfected patients, and to increase uptake in hepatitis B vaccination for HCV-infected patients within the Grady Health System,” the researchers concluded.
The study was funded by the CDC and the authors reported that they had no conflicts.
SOURCE: Harris AM et al. Vaccine. 2019;37:2188-93.
FROM VACCINE