User login
Long-acting injectables noninferior to tablet integrase for HIV
SEATTLE – A long-acting, injectable combination of the novel integrase inhibitor cabotegravir (CAB) and the second-generation nonnucleoside reverse transcriptase inhibitor rilpivirine (RPV) was noninferior to dolutegravir/abacavir/lamivudine (DTG/ABC/3TC) in one phase 3 study (FLAIR) and to three-drug oral antiretroviral therapy (ART) more broadly in the companion ATLAS phase 3 study. Patient acceptance of the injectable formulation was surprisingly high, although researchers admitted there was likely some selection bias because patients already interested in receiving an injection would have been predisposed to entering the trials.
Still, the numbers were impressive: 99% of patients who received the intramuscular injection expressed more satisfaction with it than with their previous oral regimen in the FLAIR study, and 98% expressed a similar opinion in the ATLAS study. Circumstantial evidence also suggests there may be some demand, according to Joseph Eron, MD, professor of medicine at the University of North Carolina at Chapel Hill, who commented during a press conference at the Conference on Retroviruses & Opportunistic Infections. “These studies didn’t take a long time to accrue. People were very interested, so it wasn’t as if people had to go around and beat the bushes to try to find people [to participate],” said Dr. Eron, who was not an author on either report.
“My patients tell me that they like not having to worry about taking their pills every day. There may be some relief from the stigma of HIV. You don’t have to think about it,” Susan Swindells, MBBS, medical director of the Specialty Care Clinic at the University of Nebraska Medical Center in Omaha and first author of the ATLAS trial, said during the press conference.
There were some injection site reactions, but they were generally mild and most resolved within 7 days. In the ATLAS study, 75% of participants reported injection site pain, and 1% discontinued as a result. In the FLAIR study, 82% in the CAB/RPV arm experienced an injection site reaction, with a median duration of 3 days; 99% of reactions were grade 1 or 2.
Should the combination achieve regulatory approval, it remains to be seen how challenging it will be to manage patients with monthly injections and ensure they stick to the schedule. The injections must be administered by a health care provider.
“In terms of generalizability outside of the study, it would be a paradigm shift in our therapy,” Chloe Orkin, MBBCh, said at a press conference. Dr. Orkin is the first author on the FLAIR trial report and a consultant in HIV Medicine at Barts Health National Health Service Trust. She pointed to the example of injectable contraception. “It can be done. It’s just that we haven’t done it. It will require some thought,” Dr. Orkin added.
In the ATLAS study, 616 participants taking two nucleoside reverse transcriptase inhibitor (NRTIs) and an integrase inhibitor, a non-NRTI, or a protease inhibitor, were randomized 1:1 to continue their regimen (CART arm) or switch to CAB/RPV, following a 4-week safety monitoring period of oral CAB/RPV. After 48 weeks, 1.6% in the CAB/RPV arm and 1.0% in the CART arm had HIV-1 RNA greater than or equal to 50 copies/mL, which met the prespecified noninferiority margin. Of patients in the CAB/RPV arm, 93% had HIV-1 RNA less than 50 copies/mL at week 48 versus 95% in the CART arm, and the difference was not statistically significant. Grade 3 or 4 events were seen in 11% of CAB/RPV and 7% of CART patients.
The FLAIR study randomized 566 ART-naive patients to receive either CAB/RPV or DTG/ABC/3TC after a 20-week induction phase, followed by a 4-week safety monitoring period for those going into the CAB/RPV arm. At week 48, 2.1% in the CAB/RPV arm and 2.5% in the DTG/ABC/3TC arm had HIV-1 RNA greater than or equal to 50 copies/mL, which met the prespecified noninferiority margin, while 94% in the CAB/RPV arm and 93% in the DTG/ABC/3TC arm had HIV-1 RNA less than 50 copies/mL. Confirmed virologic failure occurred in four patients (1.4%) in the CAB/RPV arm, and three of those patients had mutations in the NNRTI+INSTI domains, while the fourth patient was not tested. Three failures occurred in the DTG/ABC/3TC arm, and none of those patients had INSTI resistance mutations. A total of 82% of CAB/RPV patients had injection site reactions, 99% of which were grade 1 or 2, and the median duration was 3 days.
The ATLAS and FLAIR studies were sponsored by ViiV. Janssen and GlaxoSmithKline were collaborators.
SOURCES: Swindells S et al. CROI 2019, Abstract 139 LB. Orkin C et al. CROI 2019, Abstract 140.
SEATTLE – A long-acting, injectable combination of the novel integrase inhibitor cabotegravir (CAB) and the second-generation nonnucleoside reverse transcriptase inhibitor rilpivirine (RPV) was noninferior to dolutegravir/abacavir/lamivudine (DTG/ABC/3TC) in one phase 3 study (FLAIR) and to three-drug oral antiretroviral therapy (ART) more broadly in the companion ATLAS phase 3 study. Patient acceptance of the injectable formulation was surprisingly high, although researchers admitted there was likely some selection bias because patients already interested in receiving an injection would have been predisposed to entering the trials.
Still, the numbers were impressive: 99% of patients who received the intramuscular injection expressed more satisfaction with it than with their previous oral regimen in the FLAIR study, and 98% expressed a similar opinion in the ATLAS study. Circumstantial evidence also suggests there may be some demand, according to Joseph Eron, MD, professor of medicine at the University of North Carolina at Chapel Hill, who commented during a press conference at the Conference on Retroviruses & Opportunistic Infections. “These studies didn’t take a long time to accrue. People were very interested, so it wasn’t as if people had to go around and beat the bushes to try to find people [to participate],” said Dr. Eron, who was not an author on either report.
“My patients tell me that they like not having to worry about taking their pills every day. There may be some relief from the stigma of HIV. You don’t have to think about it,” Susan Swindells, MBBS, medical director of the Specialty Care Clinic at the University of Nebraska Medical Center in Omaha and first author of the ATLAS trial, said during the press conference.
There were some injection site reactions, but they were generally mild and most resolved within 7 days. In the ATLAS study, 75% of participants reported injection site pain, and 1% discontinued as a result. In the FLAIR study, 82% in the CAB/RPV arm experienced an injection site reaction, with a median duration of 3 days; 99% of reactions were grade 1 or 2.
Should the combination achieve regulatory approval, it remains to be seen how challenging it will be to manage patients with monthly injections and ensure they stick to the schedule. The injections must be administered by a health care provider.
“In terms of generalizability outside of the study, it would be a paradigm shift in our therapy,” Chloe Orkin, MBBCh, said at a press conference. Dr. Orkin is the first author on the FLAIR trial report and a consultant in HIV Medicine at Barts Health National Health Service Trust. She pointed to the example of injectable contraception. “It can be done. It’s just that we haven’t done it. It will require some thought,” Dr. Orkin added.
In the ATLAS study, 616 participants taking two nucleoside reverse transcriptase inhibitor (NRTIs) and an integrase inhibitor, a non-NRTI, or a protease inhibitor, were randomized 1:1 to continue their regimen (CART arm) or switch to CAB/RPV, following a 4-week safety monitoring period of oral CAB/RPV. After 48 weeks, 1.6% in the CAB/RPV arm and 1.0% in the CART arm had HIV-1 RNA greater than or equal to 50 copies/mL, which met the prespecified noninferiority margin. Of patients in the CAB/RPV arm, 93% had HIV-1 RNA less than 50 copies/mL at week 48 versus 95% in the CART arm, and the difference was not statistically significant. Grade 3 or 4 events were seen in 11% of CAB/RPV and 7% of CART patients.
The FLAIR study randomized 566 ART-naive patients to receive either CAB/RPV or DTG/ABC/3TC after a 20-week induction phase, followed by a 4-week safety monitoring period for those going into the CAB/RPV arm. At week 48, 2.1% in the CAB/RPV arm and 2.5% in the DTG/ABC/3TC arm had HIV-1 RNA greater than or equal to 50 copies/mL, which met the prespecified noninferiority margin, while 94% in the CAB/RPV arm and 93% in the DTG/ABC/3TC arm had HIV-1 RNA less than 50 copies/mL. Confirmed virologic failure occurred in four patients (1.4%) in the CAB/RPV arm, and three of those patients had mutations in the NNRTI+INSTI domains, while the fourth patient was not tested. Three failures occurred in the DTG/ABC/3TC arm, and none of those patients had INSTI resistance mutations. A total of 82% of CAB/RPV patients had injection site reactions, 99% of which were grade 1 or 2, and the median duration was 3 days.
The ATLAS and FLAIR studies were sponsored by ViiV. Janssen and GlaxoSmithKline were collaborators.
SOURCES: Swindells S et al. CROI 2019, Abstract 139 LB. Orkin C et al. CROI 2019, Abstract 140.
SEATTLE – A long-acting, injectable combination of the novel integrase inhibitor cabotegravir (CAB) and the second-generation nonnucleoside reverse transcriptase inhibitor rilpivirine (RPV) was noninferior to dolutegravir/abacavir/lamivudine (DTG/ABC/3TC) in one phase 3 study (FLAIR) and to three-drug oral antiretroviral therapy (ART) more broadly in the companion ATLAS phase 3 study. Patient acceptance of the injectable formulation was surprisingly high, although researchers admitted there was likely some selection bias because patients already interested in receiving an injection would have been predisposed to entering the trials.
Still, the numbers were impressive: 99% of patients who received the intramuscular injection expressed more satisfaction with it than with their previous oral regimen in the FLAIR study, and 98% expressed a similar opinion in the ATLAS study. Circumstantial evidence also suggests there may be some demand, according to Joseph Eron, MD, professor of medicine at the University of North Carolina at Chapel Hill, who commented during a press conference at the Conference on Retroviruses & Opportunistic Infections. “These studies didn’t take a long time to accrue. People were very interested, so it wasn’t as if people had to go around and beat the bushes to try to find people [to participate],” said Dr. Eron, who was not an author on either report.
“My patients tell me that they like not having to worry about taking their pills every day. There may be some relief from the stigma of HIV. You don’t have to think about it,” Susan Swindells, MBBS, medical director of the Specialty Care Clinic at the University of Nebraska Medical Center in Omaha and first author of the ATLAS trial, said during the press conference.
There were some injection site reactions, but they were generally mild and most resolved within 7 days. In the ATLAS study, 75% of participants reported injection site pain, and 1% discontinued as a result. In the FLAIR study, 82% in the CAB/RPV arm experienced an injection site reaction, with a median duration of 3 days; 99% of reactions were grade 1 or 2.
Should the combination achieve regulatory approval, it remains to be seen how challenging it will be to manage patients with monthly injections and ensure they stick to the schedule. The injections must be administered by a health care provider.
“In terms of generalizability outside of the study, it would be a paradigm shift in our therapy,” Chloe Orkin, MBBCh, said at a press conference. Dr. Orkin is the first author on the FLAIR trial report and a consultant in HIV Medicine at Barts Health National Health Service Trust. She pointed to the example of injectable contraception. “It can be done. It’s just that we haven’t done it. It will require some thought,” Dr. Orkin added.
In the ATLAS study, 616 participants taking two nucleoside reverse transcriptase inhibitor (NRTIs) and an integrase inhibitor, a non-NRTI, or a protease inhibitor, were randomized 1:1 to continue their regimen (CART arm) or switch to CAB/RPV, following a 4-week safety monitoring period of oral CAB/RPV. After 48 weeks, 1.6% in the CAB/RPV arm and 1.0% in the CART arm had HIV-1 RNA greater than or equal to 50 copies/mL, which met the prespecified noninferiority margin. Of patients in the CAB/RPV arm, 93% had HIV-1 RNA less than 50 copies/mL at week 48 versus 95% in the CART arm, and the difference was not statistically significant. Grade 3 or 4 events were seen in 11% of CAB/RPV and 7% of CART patients.
The FLAIR study randomized 566 ART-naive patients to receive either CAB/RPV or DTG/ABC/3TC after a 20-week induction phase, followed by a 4-week safety monitoring period for those going into the CAB/RPV arm. At week 48, 2.1% in the CAB/RPV arm and 2.5% in the DTG/ABC/3TC arm had HIV-1 RNA greater than or equal to 50 copies/mL, which met the prespecified noninferiority margin, while 94% in the CAB/RPV arm and 93% in the DTG/ABC/3TC arm had HIV-1 RNA less than 50 copies/mL. Confirmed virologic failure occurred in four patients (1.4%) in the CAB/RPV arm, and three of those patients had mutations in the NNRTI+INSTI domains, while the fourth patient was not tested. Three failures occurred in the DTG/ABC/3TC arm, and none of those patients had INSTI resistance mutations. A total of 82% of CAB/RPV patients had injection site reactions, 99% of which were grade 1 or 2, and the median duration was 3 days.
The ATLAS and FLAIR studies were sponsored by ViiV. Janssen and GlaxoSmithKline were collaborators.
SOURCES: Swindells S et al. CROI 2019, Abstract 139 LB. Orkin C et al. CROI 2019, Abstract 140.
REPORTING FROM CROI 2019
Increased sudden death risk in HIV linked to cardiac fibrosis
SEATTLE – A marked increase in the risk of sudden cardiac death among people with HIV correlates with a significantly higher burden of myocardial fibrosis, according to an autopsy study presented at the Conference on Retroviruses and Opportunistic Infections.
Fibrosis is a known trigger for fatal arrhythmias, so the take home is that fibrosis should be considered as a criteria for defibrillator implantation in HIV patients, said lead investigator Zian Tseng, MD, a cardiologist, cardiac electrophysiologist, and professor of medicine at the University of California, San Francisco.
The finding also speaks to a larger issue. The main criterion right now for implantation is an ejection fraction below 35%, but “there are a lot of people who die suddenly with normal ejection fractions,” and not just people with HIV, he said.
Many of those deaths might be prevented if fibrosis is added to implantation criteria. All that’s needed for assessment is a cardiac MRI, Dr. Tseng said.
The approach would be particularly fruitful for HIV patients, but cardiac fibrosis “isn’t just an” HIV problem, he said.
The conclusions have their roots in an investigation to determine the true incidence of sudden cardiac death (SCD) in the general public. SCD is commonly listed on death certificates, but it’s a presumed diagnosis, based on the best guesses of paramedics and clinicians. Autopsy is the only way to know for sure if a death was truly due to a sudden cardiac arrhythmia, or even related to the heart,
To clear the wheat from the chaff, Dr. Tseng and his colleagues performed autopsies on 525 out-of-hospital SCD cases among adults in San Francisco from 2011-2016; to qualify, the cases had to meet World Health Organization SCD criteria, meaning unexpected death within 1 hour of symptom onset, or, in unwitnessed cases, within 24 hours of when the person was last seen alive and well.
Cases were considered sudden arrhythmic death – and, therefore, true SCD – if no extracardiac causes of death or acute heart failure were found on autopsy. Overall, 40% of deaths attributed to SCD “were not sudden or unexpected, and nearly half of presumed SCDs were not arrhythmic.” The findings had “implications for ... mortality data, clinical trials, and cohort studies,” Dr. Tseng and his team concluded (Circulation. 2018 Jun 19;137[25]:2689-2700).
They next turned their attention to HIV. It’s known that the virus increases the risk of strokes, heart attacks, and heart failure; the researchers wanted to see if it did the same for SCD. The HIV results were presented at CROI.
Forty-seven presumed SCD cases with HIV met inclusion criteria during the study period. Based on the earlier findings and epidemiological data, people with HIV had more than an 80% higher risk of SCD and an almost 60% higher risk of confirmed arrhythmic death than did the general public. Similar to the general population, only about half of presumed SCD cases were confirmed on autopsy. About one-third of what turned out to be non-cardiac HIV deaths were due to occult overdose, versus 13.5% in the general population, which points to the increased need for drug screening and treatment in HIV.
Beyond that, though, the team found that the burden of myocardial fibrosis in HIV “was profound,” far surpassing what was found in SCD deaths in the general population. After adjustment for age, gender, and heart disease, “sudden cardiac deaths with HIV had 60% higher interstitial fibrosis by myocardial trichrome staining. Cardiac fibrosis, a known substrate for fatal arrhythmias in the general population, may underlie the mechanism by which HIV increases the risk” of sudden death in HIV, Dr. Tseng said.
It could be that the virus enters heart cells and sets off an inflammatory cardiomyopathy, or perhaps it’s related to chronic inflammation caused by the virus. Whatever the case, infection seems to have an “independent effect” on increasing fibrosis among people with HIV, he said.
Intriguingly, a large epidemiologic study in United States veterans, also presented at CROI, found a higher risk of SCD among HIV patients, but only if their infections were active over an extended period of time, as indicated by sustained high viral loads and low CD4 cell counts. Dr. Tseng was involved in that work, as well, but noted that the number of HIV SCD cases in the San Francisco study was too small to draw meaningful conclusions regarding the relationship between disease control and cardiac fibrosis.
Cardiac defibrillators can prevent arrhythmic death, so, at least for now, he said that the autopsy study findings mean that criteria for implantation should be broadened to include extensive cardiac fibrosis.
The work was funded by the National Institutes of Health. Dr. Tseng didn’t have any disclosures.
SOURCE: Tseng ZH et al. CROI 2019 abstract 32
SEATTLE – A marked increase in the risk of sudden cardiac death among people with HIV correlates with a significantly higher burden of myocardial fibrosis, according to an autopsy study presented at the Conference on Retroviruses and Opportunistic Infections.
Fibrosis is a known trigger for fatal arrhythmias, so the take home is that fibrosis should be considered as a criteria for defibrillator implantation in HIV patients, said lead investigator Zian Tseng, MD, a cardiologist, cardiac electrophysiologist, and professor of medicine at the University of California, San Francisco.
The finding also speaks to a larger issue. The main criterion right now for implantation is an ejection fraction below 35%, but “there are a lot of people who die suddenly with normal ejection fractions,” and not just people with HIV, he said.
Many of those deaths might be prevented if fibrosis is added to implantation criteria. All that’s needed for assessment is a cardiac MRI, Dr. Tseng said.
The approach would be particularly fruitful for HIV patients, but cardiac fibrosis “isn’t just an” HIV problem, he said.
The conclusions have their roots in an investigation to determine the true incidence of sudden cardiac death (SCD) in the general public. SCD is commonly listed on death certificates, but it’s a presumed diagnosis, based on the best guesses of paramedics and clinicians. Autopsy is the only way to know for sure if a death was truly due to a sudden cardiac arrhythmia, or even related to the heart,
To clear the wheat from the chaff, Dr. Tseng and his colleagues performed autopsies on 525 out-of-hospital SCD cases among adults in San Francisco from 2011-2016; to qualify, the cases had to meet World Health Organization SCD criteria, meaning unexpected death within 1 hour of symptom onset, or, in unwitnessed cases, within 24 hours of when the person was last seen alive and well.
Cases were considered sudden arrhythmic death – and, therefore, true SCD – if no extracardiac causes of death or acute heart failure were found on autopsy. Overall, 40% of deaths attributed to SCD “were not sudden or unexpected, and nearly half of presumed SCDs were not arrhythmic.” The findings had “implications for ... mortality data, clinical trials, and cohort studies,” Dr. Tseng and his team concluded (Circulation. 2018 Jun 19;137[25]:2689-2700).
They next turned their attention to HIV. It’s known that the virus increases the risk of strokes, heart attacks, and heart failure; the researchers wanted to see if it did the same for SCD. The HIV results were presented at CROI.
Forty-seven presumed SCD cases with HIV met inclusion criteria during the study period. Based on the earlier findings and epidemiological data, people with HIV had more than an 80% higher risk of SCD and an almost 60% higher risk of confirmed arrhythmic death than did the general public. Similar to the general population, only about half of presumed SCD cases were confirmed on autopsy. About one-third of what turned out to be non-cardiac HIV deaths were due to occult overdose, versus 13.5% in the general population, which points to the increased need for drug screening and treatment in HIV.
Beyond that, though, the team found that the burden of myocardial fibrosis in HIV “was profound,” far surpassing what was found in SCD deaths in the general population. After adjustment for age, gender, and heart disease, “sudden cardiac deaths with HIV had 60% higher interstitial fibrosis by myocardial trichrome staining. Cardiac fibrosis, a known substrate for fatal arrhythmias in the general population, may underlie the mechanism by which HIV increases the risk” of sudden death in HIV, Dr. Tseng said.
It could be that the virus enters heart cells and sets off an inflammatory cardiomyopathy, or perhaps it’s related to chronic inflammation caused by the virus. Whatever the case, infection seems to have an “independent effect” on increasing fibrosis among people with HIV, he said.
Intriguingly, a large epidemiologic study in United States veterans, also presented at CROI, found a higher risk of SCD among HIV patients, but only if their infections were active over an extended period of time, as indicated by sustained high viral loads and low CD4 cell counts. Dr. Tseng was involved in that work, as well, but noted that the number of HIV SCD cases in the San Francisco study was too small to draw meaningful conclusions regarding the relationship between disease control and cardiac fibrosis.
Cardiac defibrillators can prevent arrhythmic death, so, at least for now, he said that the autopsy study findings mean that criteria for implantation should be broadened to include extensive cardiac fibrosis.
The work was funded by the National Institutes of Health. Dr. Tseng didn’t have any disclosures.
SOURCE: Tseng ZH et al. CROI 2019 abstract 32
SEATTLE – A marked increase in the risk of sudden cardiac death among people with HIV correlates with a significantly higher burden of myocardial fibrosis, according to an autopsy study presented at the Conference on Retroviruses and Opportunistic Infections.
Fibrosis is a known trigger for fatal arrhythmias, so the take home is that fibrosis should be considered as a criteria for defibrillator implantation in HIV patients, said lead investigator Zian Tseng, MD, a cardiologist, cardiac electrophysiologist, and professor of medicine at the University of California, San Francisco.
The finding also speaks to a larger issue. The main criterion right now for implantation is an ejection fraction below 35%, but “there are a lot of people who die suddenly with normal ejection fractions,” and not just people with HIV, he said.
Many of those deaths might be prevented if fibrosis is added to implantation criteria. All that’s needed for assessment is a cardiac MRI, Dr. Tseng said.
The approach would be particularly fruitful for HIV patients, but cardiac fibrosis “isn’t just an” HIV problem, he said.
The conclusions have their roots in an investigation to determine the true incidence of sudden cardiac death (SCD) in the general public. SCD is commonly listed on death certificates, but it’s a presumed diagnosis, based on the best guesses of paramedics and clinicians. Autopsy is the only way to know for sure if a death was truly due to a sudden cardiac arrhythmia, or even related to the heart,
To clear the wheat from the chaff, Dr. Tseng and his colleagues performed autopsies on 525 out-of-hospital SCD cases among adults in San Francisco from 2011-2016; to qualify, the cases had to meet World Health Organization SCD criteria, meaning unexpected death within 1 hour of symptom onset, or, in unwitnessed cases, within 24 hours of when the person was last seen alive and well.
Cases were considered sudden arrhythmic death – and, therefore, true SCD – if no extracardiac causes of death or acute heart failure were found on autopsy. Overall, 40% of deaths attributed to SCD “were not sudden or unexpected, and nearly half of presumed SCDs were not arrhythmic.” The findings had “implications for ... mortality data, clinical trials, and cohort studies,” Dr. Tseng and his team concluded (Circulation. 2018 Jun 19;137[25]:2689-2700).
They next turned their attention to HIV. It’s known that the virus increases the risk of strokes, heart attacks, and heart failure; the researchers wanted to see if it did the same for SCD. The HIV results were presented at CROI.
Forty-seven presumed SCD cases with HIV met inclusion criteria during the study period. Based on the earlier findings and epidemiological data, people with HIV had more than an 80% higher risk of SCD and an almost 60% higher risk of confirmed arrhythmic death than did the general public. Similar to the general population, only about half of presumed SCD cases were confirmed on autopsy. About one-third of what turned out to be non-cardiac HIV deaths were due to occult overdose, versus 13.5% in the general population, which points to the increased need for drug screening and treatment in HIV.
Beyond that, though, the team found that the burden of myocardial fibrosis in HIV “was profound,” far surpassing what was found in SCD deaths in the general population. After adjustment for age, gender, and heart disease, “sudden cardiac deaths with HIV had 60% higher interstitial fibrosis by myocardial trichrome staining. Cardiac fibrosis, a known substrate for fatal arrhythmias in the general population, may underlie the mechanism by which HIV increases the risk” of sudden death in HIV, Dr. Tseng said.
It could be that the virus enters heart cells and sets off an inflammatory cardiomyopathy, or perhaps it’s related to chronic inflammation caused by the virus. Whatever the case, infection seems to have an “independent effect” on increasing fibrosis among people with HIV, he said.
Intriguingly, a large epidemiologic study in United States veterans, also presented at CROI, found a higher risk of SCD among HIV patients, but only if their infections were active over an extended period of time, as indicated by sustained high viral loads and low CD4 cell counts. Dr. Tseng was involved in that work, as well, but noted that the number of HIV SCD cases in the San Francisco study was too small to draw meaningful conclusions regarding the relationship between disease control and cardiac fibrosis.
Cardiac defibrillators can prevent arrhythmic death, so, at least for now, he said that the autopsy study findings mean that criteria for implantation should be broadened to include extensive cardiac fibrosis.
The work was funded by the National Institutes of Health. Dr. Tseng didn’t have any disclosures.
SOURCE: Tseng ZH et al. CROI 2019 abstract 32
REPORTING FROM CROI 2019
H3N2 putting a damper on flu season’s departure
The decline of influenza activity remains slow, largely “driven by a wave of H3N2 virus activity” in recent weeks, according to the Centers for Disease Control and Prevention.
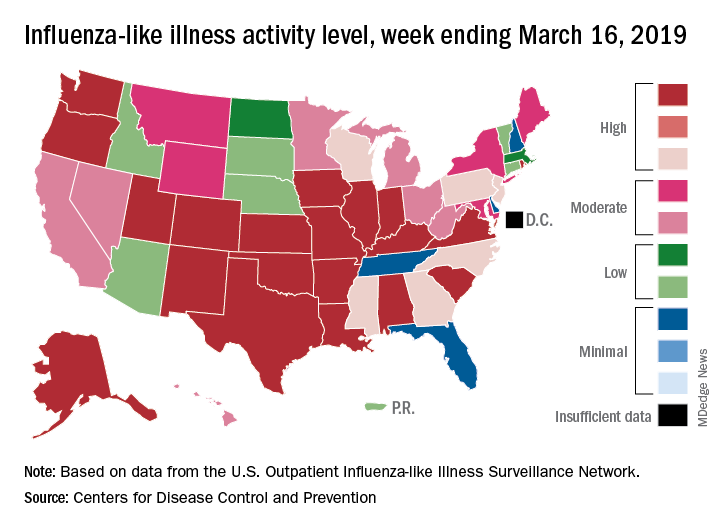
Fewer states reported the highest level of influenza-like illness (ILI) activity on the CDC’s 1-10 scale for the week ending March 16, but the national proportion of outpatient visits for ILI was 4.4% for the second consecutive week, the CDC’s influenza division reported March 22. The outpatient-visit figure for the week ending March 9 was originally reported as 4.5% last week, but it has been revised down to 4.4% this week.
Another measure of activity – the percentage of respiratory specimens testing positive for influenza viruses in clinical laboratories – actually increased slightly during the week ending March 16, the CDC noted.
For the current week, there were 26 states in the high (8-10) range of activity – 20 states were at level 10 and another 6 states were at level 8 – compared with the previous week, when 21 states were at level 10 and 30 states were in the high range, the CDC’s Outpatient ILI Surveillance Network reported.
There were eight ILI-related deaths in children reported during the week ending March 16, seven of which occurred in previous weeks. The total for the 2018-2019 season so far is 76, the CDC said.
New preliminary estimates on influenza’s burden nationally put the total number of deaths at 25,000-41,500 since the beginning of the season on Oct. 1, 2018. There also have been 375,000-454,000 flu-related hospitalizations, 13.2 million to 15.4 million medical visits, and 28.5 to 32.8 million individual illnesses, the CDC said.
Since the CDC “expects flu activity to remain elevated for a number of weeks,” it continues to recommend flu vaccination and the use of influenza antiviral drugs as “an important second line of defense that can be used to treat flu illness. H3N2 viruses are typically associated with more severe illness in older adults, and flu vaccine may protect less well against H3N2 illness in older adults, making prompt treatment with flu antivirals in this age group especially important during the current period of H3N2 predominance.”
The decline of influenza activity remains slow, largely “driven by a wave of H3N2 virus activity” in recent weeks, according to the Centers for Disease Control and Prevention.

Fewer states reported the highest level of influenza-like illness (ILI) activity on the CDC’s 1-10 scale for the week ending March 16, but the national proportion of outpatient visits for ILI was 4.4% for the second consecutive week, the CDC’s influenza division reported March 22. The outpatient-visit figure for the week ending March 9 was originally reported as 4.5% last week, but it has been revised down to 4.4% this week.
Another measure of activity – the percentage of respiratory specimens testing positive for influenza viruses in clinical laboratories – actually increased slightly during the week ending March 16, the CDC noted.
For the current week, there were 26 states in the high (8-10) range of activity – 20 states were at level 10 and another 6 states were at level 8 – compared with the previous week, when 21 states were at level 10 and 30 states were in the high range, the CDC’s Outpatient ILI Surveillance Network reported.
There were eight ILI-related deaths in children reported during the week ending March 16, seven of which occurred in previous weeks. The total for the 2018-2019 season so far is 76, the CDC said.
New preliminary estimates on influenza’s burden nationally put the total number of deaths at 25,000-41,500 since the beginning of the season on Oct. 1, 2018. There also have been 375,000-454,000 flu-related hospitalizations, 13.2 million to 15.4 million medical visits, and 28.5 to 32.8 million individual illnesses, the CDC said.
Since the CDC “expects flu activity to remain elevated for a number of weeks,” it continues to recommend flu vaccination and the use of influenza antiviral drugs as “an important second line of defense that can be used to treat flu illness. H3N2 viruses are typically associated with more severe illness in older adults, and flu vaccine may protect less well against H3N2 illness in older adults, making prompt treatment with flu antivirals in this age group especially important during the current period of H3N2 predominance.”
The decline of influenza activity remains slow, largely “driven by a wave of H3N2 virus activity” in recent weeks, according to the Centers for Disease Control and Prevention.

Fewer states reported the highest level of influenza-like illness (ILI) activity on the CDC’s 1-10 scale for the week ending March 16, but the national proportion of outpatient visits for ILI was 4.4% for the second consecutive week, the CDC’s influenza division reported March 22. The outpatient-visit figure for the week ending March 9 was originally reported as 4.5% last week, but it has been revised down to 4.4% this week.
Another measure of activity – the percentage of respiratory specimens testing positive for influenza viruses in clinical laboratories – actually increased slightly during the week ending March 16, the CDC noted.
For the current week, there were 26 states in the high (8-10) range of activity – 20 states were at level 10 and another 6 states were at level 8 – compared with the previous week, when 21 states were at level 10 and 30 states were in the high range, the CDC’s Outpatient ILI Surveillance Network reported.
There were eight ILI-related deaths in children reported during the week ending March 16, seven of which occurred in previous weeks. The total for the 2018-2019 season so far is 76, the CDC said.
New preliminary estimates on influenza’s burden nationally put the total number of deaths at 25,000-41,500 since the beginning of the season on Oct. 1, 2018. There also have been 375,000-454,000 flu-related hospitalizations, 13.2 million to 15.4 million medical visits, and 28.5 to 32.8 million individual illnesses, the CDC said.
Since the CDC “expects flu activity to remain elevated for a number of weeks,” it continues to recommend flu vaccination and the use of influenza antiviral drugs as “an important second line of defense that can be used to treat flu illness. H3N2 viruses are typically associated with more severe illness in older adults, and flu vaccine may protect less well against H3N2 illness in older adults, making prompt treatment with flu antivirals in this age group especially important during the current period of H3N2 predominance.”
Don’t miss baby scabies
WAIKOLOA, HAWAII –
“It’s really important to think of scabies in any widespread rash that a baby presents with,” said Andrea Zaenglein, MD, professor of dermatology and pediatric dermatology at Penn State University, Hershey. It’s often missed in the ED because it’s not recognized.
While lesions might be limited to the webbing of the hands in older patients, infants generally have a widespread rash with many different lesion types involving the armpits, trunk, and even the scalp. “In older kids, we always think of itch as our primary criteria, but for infants with scabies, that’s not always the case. The younger the kid, the less able they’re to manifest the itch in a way that we recognize,” she said in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Standard treatment for infants with scabies is permethrin cream, which, Dr. Zaenglein advises, should be applied from head to toe. “And make sure to treat all family members, even if they’re not demonstrating any symptoms. It’s really important, because that baby had to get scabies from somebody,” she said. Although permethrin isn’t approved for use under 2 months old, she said she has no problem with it in younger, otherwise healthy infants, but cases below 2 months are uncommon. Even if infants are exposed at birth, it takes several weeks for scabies to manifest.
Topical corticosteroids are useful as well to speed healing and help with itch. Ivermectin is held in reserve for older patients, especially in institutional settings where many people have to be treated at a time, or when permethrin cream is not effective.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII –
“It’s really important to think of scabies in any widespread rash that a baby presents with,” said Andrea Zaenglein, MD, professor of dermatology and pediatric dermatology at Penn State University, Hershey. It’s often missed in the ED because it’s not recognized.
While lesions might be limited to the webbing of the hands in older patients, infants generally have a widespread rash with many different lesion types involving the armpits, trunk, and even the scalp. “In older kids, we always think of itch as our primary criteria, but for infants with scabies, that’s not always the case. The younger the kid, the less able they’re to manifest the itch in a way that we recognize,” she said in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Standard treatment for infants with scabies is permethrin cream, which, Dr. Zaenglein advises, should be applied from head to toe. “And make sure to treat all family members, even if they’re not demonstrating any symptoms. It’s really important, because that baby had to get scabies from somebody,” she said. Although permethrin isn’t approved for use under 2 months old, she said she has no problem with it in younger, otherwise healthy infants, but cases below 2 months are uncommon. Even if infants are exposed at birth, it takes several weeks for scabies to manifest.
Topical corticosteroids are useful as well to speed healing and help with itch. Ivermectin is held in reserve for older patients, especially in institutional settings where many people have to be treated at a time, or when permethrin cream is not effective.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII –
“It’s really important to think of scabies in any widespread rash that a baby presents with,” said Andrea Zaenglein, MD, professor of dermatology and pediatric dermatology at Penn State University, Hershey. It’s often missed in the ED because it’s not recognized.
While lesions might be limited to the webbing of the hands in older patients, infants generally have a widespread rash with many different lesion types involving the armpits, trunk, and even the scalp. “In older kids, we always think of itch as our primary criteria, but for infants with scabies, that’s not always the case. The younger the kid, the less able they’re to manifest the itch in a way that we recognize,” she said in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Standard treatment for infants with scabies is permethrin cream, which, Dr. Zaenglein advises, should be applied from head to toe. “And make sure to treat all family members, even if they’re not demonstrating any symptoms. It’s really important, because that baby had to get scabies from somebody,” she said. Although permethrin isn’t approved for use under 2 months old, she said she has no problem with it in younger, otherwise healthy infants, but cases below 2 months are uncommon. Even if infants are exposed at birth, it takes several weeks for scabies to manifest.
Topical corticosteroids are useful as well to speed healing and help with itch. Ivermectin is held in reserve for older patients, especially in institutional settings where many people have to be treated at a time, or when permethrin cream is not effective.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
FDA examines changing donation policies for men who have sex with men
The
At a meeting of the FDA’s Blood Products Advisory Committee, the agency shared the content of the 5-item questionnaire and reviewed the proposed study design with committee members, who were asked to comment – but not vote – on the best path forward for MSM donation policies.
The FDA is “committed to ongoing evaluation of the MSM deferral policy” and remains open to adjusting the policy based on the best available scientific evidence, said Barbee Whitaker, PhD, a lead scientist in the agency’s Office of Emerging and Transfusion Transmitted Disease
After recruiting 2,000 men who have had sex with men at least once during the past 3 months, the study will aim to identify individuals who have very recently become HIV infected, in order to assess the discriminant function of the set of behavioral questions that are proposed in the questionnaire.
The crux of the problem currently, noted Dr. Whitaker, is identifying those individuals who are very recently infected with HIV. Nucleic acid testing has tightened the window of undetectability considerably, but the current 12-month deferral window after men have had sexual contact with other men is designed to ensure safety of the blood supply.
Social justice concerns have been raised about the blanket deferral, said Dr. Whitaker; the behavioral questions in the pilot study will ask about the number of different sexual partners men have had within the past 1, 3, and 12 months and ask about the type of sexual contact (oral sex, or anal penetrative or receptive intercourse). The questionnaire also asks about sex with a partner known to be HIV positive, condom use, and use of pre-exposure prophylaxis (PrEP).
The FDA will ask for proposals to conduct the study with an eye to having sites in such cities as Washington, Atlanta, and Miami, which have high incidences of HIV, to improve chances of early detection.
The behavioral questionnaire is not seen as an immediate replacement for the 12-month deferral policy, the FDA made clear in its briefing documents and in discussion with the committee. Instead, its utility will be in the information gleaned from the pilot study and a follow-on that may include several hundred thousand individuals. These data should provide “population-based evidence upon which to base regulatory decisions to ensure blood safety,” she said.
Donation policies outside the United States
Whether a change in blood donation deferral policies for MSM would be a shortened window or a move toward a behavioral questionnaire is currently not known. Globally, a variety of practices are used for blood screening, said Mindy Goldman, MD, medical director of Canadian Blood Services, who reviewed international perspectives on blood donation for MSM.
“There’s no general consensus on donation deferrals internationally,” she said. Factors influencing policy can include epidemiology, risk analysis, modeling, and history of response to threats in the past.
However, “there’s basically a couple of main approaches” to handling deferrals for MSM, Dr. Goldman said. One is time-based deferral – the strategy used in the United States, as well as Canada, the United Kingdom, Japan, and Australia.
Japan and the U.K. have recently moved to 3-month deferral periods, a figure arrived at by doubling the window period for nucleic acid testing for HIV, roughly, Dr. Goldman said. Early data from the U.K. experience has not shown an increase in HIV rates among donors, or an increase in NAT-only positive donors, she said. An application to move from a 12-month to a 3-month deferral period is pending in Canada.
A strong advantage of time-based deferral as a risk management strategy, Dr. Goldman said, is standardization. “For us, standardization is close to godliness.”
However, she added, “another major limitation is that you’re still deferring all sexually active MSM, including those who are in a stable monogamous relationship from donating. From a justice perspective for the lowest risk population of MSM – they are still being deferred using this type of approach.”
Some nations, such as Spain and Italy, use individual risk assessment via physician-led interviews. These approaches are often not standardized. “There’s no national uniform questionnaire, so there’s less standardization, and more variability between blood centers,” Dr. Goldman said. “So you wind up trying to compare apples with oranges.”
This means the results are harder to evaluate on a national level. However, there appears to be higher residual risk, with HIV rates among first-time donors approaching those of the general population, Dr. Goldman said.
Another strategy, used in France, is a test-retest model, where blood from first-time MSM that initially tests negative for HIV is held until the individual returns for re-testing or an additional donation, with a second negative test. This approach increases operational complexity and cost, noted Dr. Goldman, and because of the short shelf life of platelets, it’s not practical for this blood component.
In general questioning and discussion after this and other background presentations, the committee could agree on one point: this isn’t an easy question.
“I’m increasingly struck by how difficult this problem is,” said committee member Roger Lewis, MD, PhD, professor at the University of California, Los Angeles, and chair of the department of emergency medicine at Harbor-UCLA Medical Center. Regarding just the problem of completing the pilot study, Dr. Lewis commented, “It sounds like it’s going to be impossible to get the data that directly answers the questions.”
Peter Marx, MD, PhD, who directs the FDA’s Center for Biologics Evaluation and Research (CBER), which oversees blood products safety, joined the discussion to acknowledge the difficulty, but underscore the social importance of a careful examination of the current MSM donation policy.
“We understand the issues here…. With all due respect to our European colleagues, there’s not enough data. That’s the point of this study; we also know that the U.S. has a very different epidemiology of HIV than the U.K. and a lot of other places,” Dr. Marx said. “The pilot study is a way to get some data where we might be able to get away from a time-based deferral. The LGBT community finds any time-based deferral discriminatory.”
Pathogen reduction technology
The committee heard a proposal for a completely different strategy during its afternoon session: pathogen reduction technology (PRT) holds promise to achieve virtual elimination of HIV and other pathogens from donated blood products.
The FDA is reviewing a variance request from the nonprofit blood donation organization Bloodworks Northwest organization to use PRT for apheresis platelet donations from MSM who would otherwise be deferred because of sexual activity within the 12-month deferral window.
James AuBuchon, MD, president of Bloodworks Northwest, explained that his organization takes in about 225,000 donations annually. The variance sought would use the FDA-approved INTERCEPT device to achieve pathogen reduction for donations that meet all requirements except the MSM deferral, and that would still undergo all relevant transfusion transmitted infection testing.
The INTERCEPT device uses amotosalen, which intercalates with DNA and RNA, inactivating it after exposure to ultraviolet A light. Amotosalen is then removed from the blood product before administration. The pathogen reduction activity doesn’t interfere with platelets or plasma, and is active against a wide range of viruses, bacteria, and fungal pathogens, explained Dr. AuBuchon, who is also a professor of hematology at the University of Washington, Seattle.
Dr. AuBuchon walked the committee through procedures designed to flag donors for PRT platelet apheresis, and to ensure these donations receive the intended PRT treatment. Platelets were chosen for this variance request, he explained, because demand outstrips supply. “We are all spending additional time and resources in recruiting a new framework and demographic, and it is exceedingly difficult to keep enough donors coming through the door,” he said. “Our platelet utilization climbs continually – it’s up 15% in the last 4 years.”
Committee members circled around the idea that all risk can’t be eliminated, even with the highly effective PRT technology. But the risk is exceedingly low, said committee chair Richard Kaufman, MD, medical director of the adult transfusion service at Brigham and Women’s Hospital, Boston. “It’s not possible to get rid of the window. We can kind of hammer down the risk by shrinking down the window by using incredibly sensitive tests. But that risk continues to exist. Pathogen reduction can take care of that residual risk…. So what’s left is really quite a low risk,” Dr. Kaufman said.
Susan Stramer, PhD, vice president of scientific affairs for the American Red Cross, concurred, noting that pathogen reduction techniques are already in use for many other blood products, particularly within the plasma industry.
Wrapping up, Dr. Kaufman asked individual committee members to summarize their position on the variance request, though the FDA had not placed a voting question before the committee. Consensus in the room was that this real-world examination of PRT could point to a path to expanding the donor pool while maintaining patient safety – a concern all agreed was paramount.
The FDA usually follows the recommendations of its committees.
The
At a meeting of the FDA’s Blood Products Advisory Committee, the agency shared the content of the 5-item questionnaire and reviewed the proposed study design with committee members, who were asked to comment – but not vote – on the best path forward for MSM donation policies.
The FDA is “committed to ongoing evaluation of the MSM deferral policy” and remains open to adjusting the policy based on the best available scientific evidence, said Barbee Whitaker, PhD, a lead scientist in the agency’s Office of Emerging and Transfusion Transmitted Disease
After recruiting 2,000 men who have had sex with men at least once during the past 3 months, the study will aim to identify individuals who have very recently become HIV infected, in order to assess the discriminant function of the set of behavioral questions that are proposed in the questionnaire.
The crux of the problem currently, noted Dr. Whitaker, is identifying those individuals who are very recently infected with HIV. Nucleic acid testing has tightened the window of undetectability considerably, but the current 12-month deferral window after men have had sexual contact with other men is designed to ensure safety of the blood supply.
Social justice concerns have been raised about the blanket deferral, said Dr. Whitaker; the behavioral questions in the pilot study will ask about the number of different sexual partners men have had within the past 1, 3, and 12 months and ask about the type of sexual contact (oral sex, or anal penetrative or receptive intercourse). The questionnaire also asks about sex with a partner known to be HIV positive, condom use, and use of pre-exposure prophylaxis (PrEP).
The FDA will ask for proposals to conduct the study with an eye to having sites in such cities as Washington, Atlanta, and Miami, which have high incidences of HIV, to improve chances of early detection.
The behavioral questionnaire is not seen as an immediate replacement for the 12-month deferral policy, the FDA made clear in its briefing documents and in discussion with the committee. Instead, its utility will be in the information gleaned from the pilot study and a follow-on that may include several hundred thousand individuals. These data should provide “population-based evidence upon which to base regulatory decisions to ensure blood safety,” she said.
Donation policies outside the United States
Whether a change in blood donation deferral policies for MSM would be a shortened window or a move toward a behavioral questionnaire is currently not known. Globally, a variety of practices are used for blood screening, said Mindy Goldman, MD, medical director of Canadian Blood Services, who reviewed international perspectives on blood donation for MSM.
“There’s no general consensus on donation deferrals internationally,” she said. Factors influencing policy can include epidemiology, risk analysis, modeling, and history of response to threats in the past.
However, “there’s basically a couple of main approaches” to handling deferrals for MSM, Dr. Goldman said. One is time-based deferral – the strategy used in the United States, as well as Canada, the United Kingdom, Japan, and Australia.
Japan and the U.K. have recently moved to 3-month deferral periods, a figure arrived at by doubling the window period for nucleic acid testing for HIV, roughly, Dr. Goldman said. Early data from the U.K. experience has not shown an increase in HIV rates among donors, or an increase in NAT-only positive donors, she said. An application to move from a 12-month to a 3-month deferral period is pending in Canada.
A strong advantage of time-based deferral as a risk management strategy, Dr. Goldman said, is standardization. “For us, standardization is close to godliness.”
However, she added, “another major limitation is that you’re still deferring all sexually active MSM, including those who are in a stable monogamous relationship from donating. From a justice perspective for the lowest risk population of MSM – they are still being deferred using this type of approach.”
Some nations, such as Spain and Italy, use individual risk assessment via physician-led interviews. These approaches are often not standardized. “There’s no national uniform questionnaire, so there’s less standardization, and more variability between blood centers,” Dr. Goldman said. “So you wind up trying to compare apples with oranges.”
This means the results are harder to evaluate on a national level. However, there appears to be higher residual risk, with HIV rates among first-time donors approaching those of the general population, Dr. Goldman said.
Another strategy, used in France, is a test-retest model, where blood from first-time MSM that initially tests negative for HIV is held until the individual returns for re-testing or an additional donation, with a second negative test. This approach increases operational complexity and cost, noted Dr. Goldman, and because of the short shelf life of platelets, it’s not practical for this blood component.
In general questioning and discussion after this and other background presentations, the committee could agree on one point: this isn’t an easy question.
“I’m increasingly struck by how difficult this problem is,” said committee member Roger Lewis, MD, PhD, professor at the University of California, Los Angeles, and chair of the department of emergency medicine at Harbor-UCLA Medical Center. Regarding just the problem of completing the pilot study, Dr. Lewis commented, “It sounds like it’s going to be impossible to get the data that directly answers the questions.”
Peter Marx, MD, PhD, who directs the FDA’s Center for Biologics Evaluation and Research (CBER), which oversees blood products safety, joined the discussion to acknowledge the difficulty, but underscore the social importance of a careful examination of the current MSM donation policy.
“We understand the issues here…. With all due respect to our European colleagues, there’s not enough data. That’s the point of this study; we also know that the U.S. has a very different epidemiology of HIV than the U.K. and a lot of other places,” Dr. Marx said. “The pilot study is a way to get some data where we might be able to get away from a time-based deferral. The LGBT community finds any time-based deferral discriminatory.”
Pathogen reduction technology
The committee heard a proposal for a completely different strategy during its afternoon session: pathogen reduction technology (PRT) holds promise to achieve virtual elimination of HIV and other pathogens from donated blood products.
The FDA is reviewing a variance request from the nonprofit blood donation organization Bloodworks Northwest organization to use PRT for apheresis platelet donations from MSM who would otherwise be deferred because of sexual activity within the 12-month deferral window.
James AuBuchon, MD, president of Bloodworks Northwest, explained that his organization takes in about 225,000 donations annually. The variance sought would use the FDA-approved INTERCEPT device to achieve pathogen reduction for donations that meet all requirements except the MSM deferral, and that would still undergo all relevant transfusion transmitted infection testing.
The INTERCEPT device uses amotosalen, which intercalates with DNA and RNA, inactivating it after exposure to ultraviolet A light. Amotosalen is then removed from the blood product before administration. The pathogen reduction activity doesn’t interfere with platelets or plasma, and is active against a wide range of viruses, bacteria, and fungal pathogens, explained Dr. AuBuchon, who is also a professor of hematology at the University of Washington, Seattle.
Dr. AuBuchon walked the committee through procedures designed to flag donors for PRT platelet apheresis, and to ensure these donations receive the intended PRT treatment. Platelets were chosen for this variance request, he explained, because demand outstrips supply. “We are all spending additional time and resources in recruiting a new framework and demographic, and it is exceedingly difficult to keep enough donors coming through the door,” he said. “Our platelet utilization climbs continually – it’s up 15% in the last 4 years.”
Committee members circled around the idea that all risk can’t be eliminated, even with the highly effective PRT technology. But the risk is exceedingly low, said committee chair Richard Kaufman, MD, medical director of the adult transfusion service at Brigham and Women’s Hospital, Boston. “It’s not possible to get rid of the window. We can kind of hammer down the risk by shrinking down the window by using incredibly sensitive tests. But that risk continues to exist. Pathogen reduction can take care of that residual risk…. So what’s left is really quite a low risk,” Dr. Kaufman said.
Susan Stramer, PhD, vice president of scientific affairs for the American Red Cross, concurred, noting that pathogen reduction techniques are already in use for many other blood products, particularly within the plasma industry.
Wrapping up, Dr. Kaufman asked individual committee members to summarize their position on the variance request, though the FDA had not placed a voting question before the committee. Consensus in the room was that this real-world examination of PRT could point to a path to expanding the donor pool while maintaining patient safety – a concern all agreed was paramount.
The FDA usually follows the recommendations of its committees.
The
At a meeting of the FDA’s Blood Products Advisory Committee, the agency shared the content of the 5-item questionnaire and reviewed the proposed study design with committee members, who were asked to comment – but not vote – on the best path forward for MSM donation policies.
The FDA is “committed to ongoing evaluation of the MSM deferral policy” and remains open to adjusting the policy based on the best available scientific evidence, said Barbee Whitaker, PhD, a lead scientist in the agency’s Office of Emerging and Transfusion Transmitted Disease
After recruiting 2,000 men who have had sex with men at least once during the past 3 months, the study will aim to identify individuals who have very recently become HIV infected, in order to assess the discriminant function of the set of behavioral questions that are proposed in the questionnaire.
The crux of the problem currently, noted Dr. Whitaker, is identifying those individuals who are very recently infected with HIV. Nucleic acid testing has tightened the window of undetectability considerably, but the current 12-month deferral window after men have had sexual contact with other men is designed to ensure safety of the blood supply.
Social justice concerns have been raised about the blanket deferral, said Dr. Whitaker; the behavioral questions in the pilot study will ask about the number of different sexual partners men have had within the past 1, 3, and 12 months and ask about the type of sexual contact (oral sex, or anal penetrative or receptive intercourse). The questionnaire also asks about sex with a partner known to be HIV positive, condom use, and use of pre-exposure prophylaxis (PrEP).
The FDA will ask for proposals to conduct the study with an eye to having sites in such cities as Washington, Atlanta, and Miami, which have high incidences of HIV, to improve chances of early detection.
The behavioral questionnaire is not seen as an immediate replacement for the 12-month deferral policy, the FDA made clear in its briefing documents and in discussion with the committee. Instead, its utility will be in the information gleaned from the pilot study and a follow-on that may include several hundred thousand individuals. These data should provide “population-based evidence upon which to base regulatory decisions to ensure blood safety,” she said.
Donation policies outside the United States
Whether a change in blood donation deferral policies for MSM would be a shortened window or a move toward a behavioral questionnaire is currently not known. Globally, a variety of practices are used for blood screening, said Mindy Goldman, MD, medical director of Canadian Blood Services, who reviewed international perspectives on blood donation for MSM.
“There’s no general consensus on donation deferrals internationally,” she said. Factors influencing policy can include epidemiology, risk analysis, modeling, and history of response to threats in the past.
However, “there’s basically a couple of main approaches” to handling deferrals for MSM, Dr. Goldman said. One is time-based deferral – the strategy used in the United States, as well as Canada, the United Kingdom, Japan, and Australia.
Japan and the U.K. have recently moved to 3-month deferral periods, a figure arrived at by doubling the window period for nucleic acid testing for HIV, roughly, Dr. Goldman said. Early data from the U.K. experience has not shown an increase in HIV rates among donors, or an increase in NAT-only positive donors, she said. An application to move from a 12-month to a 3-month deferral period is pending in Canada.
A strong advantage of time-based deferral as a risk management strategy, Dr. Goldman said, is standardization. “For us, standardization is close to godliness.”
However, she added, “another major limitation is that you’re still deferring all sexually active MSM, including those who are in a stable monogamous relationship from donating. From a justice perspective for the lowest risk population of MSM – they are still being deferred using this type of approach.”
Some nations, such as Spain and Italy, use individual risk assessment via physician-led interviews. These approaches are often not standardized. “There’s no national uniform questionnaire, so there’s less standardization, and more variability between blood centers,” Dr. Goldman said. “So you wind up trying to compare apples with oranges.”
This means the results are harder to evaluate on a national level. However, there appears to be higher residual risk, with HIV rates among first-time donors approaching those of the general population, Dr. Goldman said.
Another strategy, used in France, is a test-retest model, where blood from first-time MSM that initially tests negative for HIV is held until the individual returns for re-testing or an additional donation, with a second negative test. This approach increases operational complexity and cost, noted Dr. Goldman, and because of the short shelf life of platelets, it’s not practical for this blood component.
In general questioning and discussion after this and other background presentations, the committee could agree on one point: this isn’t an easy question.
“I’m increasingly struck by how difficult this problem is,” said committee member Roger Lewis, MD, PhD, professor at the University of California, Los Angeles, and chair of the department of emergency medicine at Harbor-UCLA Medical Center. Regarding just the problem of completing the pilot study, Dr. Lewis commented, “It sounds like it’s going to be impossible to get the data that directly answers the questions.”
Peter Marx, MD, PhD, who directs the FDA’s Center for Biologics Evaluation and Research (CBER), which oversees blood products safety, joined the discussion to acknowledge the difficulty, but underscore the social importance of a careful examination of the current MSM donation policy.
“We understand the issues here…. With all due respect to our European colleagues, there’s not enough data. That’s the point of this study; we also know that the U.S. has a very different epidemiology of HIV than the U.K. and a lot of other places,” Dr. Marx said. “The pilot study is a way to get some data where we might be able to get away from a time-based deferral. The LGBT community finds any time-based deferral discriminatory.”
Pathogen reduction technology
The committee heard a proposal for a completely different strategy during its afternoon session: pathogen reduction technology (PRT) holds promise to achieve virtual elimination of HIV and other pathogens from donated blood products.
The FDA is reviewing a variance request from the nonprofit blood donation organization Bloodworks Northwest organization to use PRT for apheresis platelet donations from MSM who would otherwise be deferred because of sexual activity within the 12-month deferral window.
James AuBuchon, MD, president of Bloodworks Northwest, explained that his organization takes in about 225,000 donations annually. The variance sought would use the FDA-approved INTERCEPT device to achieve pathogen reduction for donations that meet all requirements except the MSM deferral, and that would still undergo all relevant transfusion transmitted infection testing.
The INTERCEPT device uses amotosalen, which intercalates with DNA and RNA, inactivating it after exposure to ultraviolet A light. Amotosalen is then removed from the blood product before administration. The pathogen reduction activity doesn’t interfere with platelets or plasma, and is active against a wide range of viruses, bacteria, and fungal pathogens, explained Dr. AuBuchon, who is also a professor of hematology at the University of Washington, Seattle.
Dr. AuBuchon walked the committee through procedures designed to flag donors for PRT platelet apheresis, and to ensure these donations receive the intended PRT treatment. Platelets were chosen for this variance request, he explained, because demand outstrips supply. “We are all spending additional time and resources in recruiting a new framework and demographic, and it is exceedingly difficult to keep enough donors coming through the door,” he said. “Our platelet utilization climbs continually – it’s up 15% in the last 4 years.”
Committee members circled around the idea that all risk can’t be eliminated, even with the highly effective PRT technology. But the risk is exceedingly low, said committee chair Richard Kaufman, MD, medical director of the adult transfusion service at Brigham and Women’s Hospital, Boston. “It’s not possible to get rid of the window. We can kind of hammer down the risk by shrinking down the window by using incredibly sensitive tests. But that risk continues to exist. Pathogen reduction can take care of that residual risk…. So what’s left is really quite a low risk,” Dr. Kaufman said.
Susan Stramer, PhD, vice president of scientific affairs for the American Red Cross, concurred, noting that pathogen reduction techniques are already in use for many other blood products, particularly within the plasma industry.
Wrapping up, Dr. Kaufman asked individual committee members to summarize their position on the variance request, though the FDA had not placed a voting question before the committee. Consensus in the room was that this real-world examination of PRT could point to a path to expanding the donor pool while maintaining patient safety – a concern all agreed was paramount.
The FDA usually follows the recommendations of its committees.
FROM AN FDA ADVISORY COMMITTEE MEETING
FDA committee advises status quo for blood supply Zika testing
Most members of a Food and Drug Administration advisory committee considered that data support maintaining current testing protocols for Zika virus in the blood donor pool. However, committee discussion entertained the idea of revisiting testing strategies after another year or 2 of Zika virus epidemiological data are available.
In its last guidance regarding Zika virus testing, issued in July 2018, the FDA recommended that either minipool nucleic acid testing (MP NAT) or individual donor (ID) NAT be used to screen for Zika virus. Current guidance still requires conversion to all-ID NAT “when certain threshold conditions are met that indicate an increased risk of suspected mosquito-borne transmission in a defined geographic collection area.”
In the first of three separate votes, 11 of 15 voting members of the FDA’s Blood Products Advisory Committee (BPAC) answered in the affirmative to the question of whether available data support continuing the status quo for Zika testing. Committee members then were asked to weigh whether current data support scaling back to a regional testing strategy targeting at-risk areas. Here, six committee members answered in the affirmative, and nine in the negative.
Just one committee member, F. Blaine Hollinger, MD, voted in favor of the third option, elimination of all Zika virus testing without reintroducing donor screening for risk factors in risk-free areas pending another outbreak in the United States. Dr. Hollinger is a professor of virology and microbiology at Baylor College of Medicine, Houston.
The committee as whole wasn’t swayed by a line of questioning put forward by chairman Richard Kaufman, MD. “I will be the devil’s advocate a little bit: We learned that there have been zero confirmed positives from blood donors for the past year. Would anyone be comfortable with just stopping screening of donors?” asked Dr. Kaufman, medical director of the adult transfusion service at Brigham and Women’s Hospital, Boston.
A wide-ranging morning of presentations put data regarding historical trends and current global Zika hot spots in front of the committee. Current upticks in infection rates in northwest Mexico and in some states in India were areas of concern, given North American travel patterns, noted speaker Marc Fisher, MD, of the Center for Disease Control and Prevention’s Arboviral Disease Branch (Fort Collins, Colo.) “We’re going to see sporadic outbreaks; it’s hard to predict the future,” he said. “The new outbreak in India raises concerns.”
Briefing information from the FDA explained that Zika virus local transmission peaked in the United States in late summer of 2016. More than 5,000 cases were reported in the United States and over 36,000 in Puerto Rico. This has plummeted to 220 in 2018, with about two-thirds of these cases occurring in the territories, mostly (97%) from Puerto Rico across all 3 years.
Zika viremic blood donors dropped by an order of magnitude yearly, totaling 363 in 2016, 38 in 2017, and just 3 in 2018. Of the 363 detected in 2016, 96% came from Puerto Rico or Florida, noted Dr. Fisher.
The number of suspected and confirmed cases in the Americas overall has also dropped from over 650,000 in 2016 to under 30,000 in 2018, with most cases in 2018 being suspected rather than laboratory confirmed. In contrast to testing conducted in North America, few cases in much of Central and South America were laboratory confirmed.
Asymptomatic infections have occurred in blood donors, said the FDA, with 1.8% of blood donations in Puerto Rico testing positive for Zika virus during the peak of the outbreak. Transmission by transfusion is thought to have occurred in Brazil.
Although Zika virus infections have plummeted in the United States and worldwide, prevalence and rates of local transmission are unpredictable, said the FDA, which pointed to sporadic increases in autochthonous transmission of viruses such as dengue and chikungunya that are carried by the same mosquito vector as Zika.
Some of the committee’s discussion centered around finding a way to carve out protection for those most harmed by Zika virus – pregnant women and their fetuses. Martin Schreiber, MD, professor of surgery at Oregon Health and Sciences University, Portland, proposed a point-of-care testing strategy in which only blood destined for pregnant women would be tested for Zika virus. Dr. Schreiber, a trauma surgeon, put forward the rationale that Zika virus causes harm almost exclusively to fetuses, except for rare cases of Guillain-Barré syndrome.
In response, Dr. Kaufman pointed out that with rare exceptions for some bacterial testing, all testing is done from samples taken at the point of donation. The supply chain for donor blood is not set up to accommodate point-of-care testing, he said.
Answering questions about another targeted strategy – maintaining a separate, Zika-tested supply of blood for pregnant women – Susan Stramer, PhD, vice president of scientific affairs for the American Red Cross, said, “Most hospitals do not want, and are very adamant against, carrying a dual inventory.”
Ultimately, the committee’s discussion swung toward the realization that it may be too soon after the recent spike in U.S. Zika cases to plot the best course for ongoing testing strategies. “We are at the tail end of a waning epidemic. ... I think it would probably be a pretty easy question for the committee and for the agency if we actually had some way of having a crystal ball and knowing that the current trend was likely to continue,” said Roger Lewis, MD, PhD, professor at the University of California, Los Angeles, and chair of the department of emergency medicine at Harbor-UCLA Medical Center.
“I think that is not the question,” he went on. “I think the question is, What is the optimal strategy if we have no idea if that tail is going to continue in this current trend. ... And that maybe the committee ought to be thinking about what is the right strategy for the next 2 years – with an underlying assumption that this is a question that can be brought back as we learn more about how this disease behaves.”
The FDA usually follows the recommendations of its advisory committees.
Most members of a Food and Drug Administration advisory committee considered that data support maintaining current testing protocols for Zika virus in the blood donor pool. However, committee discussion entertained the idea of revisiting testing strategies after another year or 2 of Zika virus epidemiological data are available.
In its last guidance regarding Zika virus testing, issued in July 2018, the FDA recommended that either minipool nucleic acid testing (MP NAT) or individual donor (ID) NAT be used to screen for Zika virus. Current guidance still requires conversion to all-ID NAT “when certain threshold conditions are met that indicate an increased risk of suspected mosquito-borne transmission in a defined geographic collection area.”
In the first of three separate votes, 11 of 15 voting members of the FDA’s Blood Products Advisory Committee (BPAC) answered in the affirmative to the question of whether available data support continuing the status quo for Zika testing. Committee members then were asked to weigh whether current data support scaling back to a regional testing strategy targeting at-risk areas. Here, six committee members answered in the affirmative, and nine in the negative.
Just one committee member, F. Blaine Hollinger, MD, voted in favor of the third option, elimination of all Zika virus testing without reintroducing donor screening for risk factors in risk-free areas pending another outbreak in the United States. Dr. Hollinger is a professor of virology and microbiology at Baylor College of Medicine, Houston.
The committee as whole wasn’t swayed by a line of questioning put forward by chairman Richard Kaufman, MD. “I will be the devil’s advocate a little bit: We learned that there have been zero confirmed positives from blood donors for the past year. Would anyone be comfortable with just stopping screening of donors?” asked Dr. Kaufman, medical director of the adult transfusion service at Brigham and Women’s Hospital, Boston.
A wide-ranging morning of presentations put data regarding historical trends and current global Zika hot spots in front of the committee. Current upticks in infection rates in northwest Mexico and in some states in India were areas of concern, given North American travel patterns, noted speaker Marc Fisher, MD, of the Center for Disease Control and Prevention’s Arboviral Disease Branch (Fort Collins, Colo.) “We’re going to see sporadic outbreaks; it’s hard to predict the future,” he said. “The new outbreak in India raises concerns.”
Briefing information from the FDA explained that Zika virus local transmission peaked in the United States in late summer of 2016. More than 5,000 cases were reported in the United States and over 36,000 in Puerto Rico. This has plummeted to 220 in 2018, with about two-thirds of these cases occurring in the territories, mostly (97%) from Puerto Rico across all 3 years.
Zika viremic blood donors dropped by an order of magnitude yearly, totaling 363 in 2016, 38 in 2017, and just 3 in 2018. Of the 363 detected in 2016, 96% came from Puerto Rico or Florida, noted Dr. Fisher.
The number of suspected and confirmed cases in the Americas overall has also dropped from over 650,000 in 2016 to under 30,000 in 2018, with most cases in 2018 being suspected rather than laboratory confirmed. In contrast to testing conducted in North America, few cases in much of Central and South America were laboratory confirmed.
Asymptomatic infections have occurred in blood donors, said the FDA, with 1.8% of blood donations in Puerto Rico testing positive for Zika virus during the peak of the outbreak. Transmission by transfusion is thought to have occurred in Brazil.
Although Zika virus infections have plummeted in the United States and worldwide, prevalence and rates of local transmission are unpredictable, said the FDA, which pointed to sporadic increases in autochthonous transmission of viruses such as dengue and chikungunya that are carried by the same mosquito vector as Zika.
Some of the committee’s discussion centered around finding a way to carve out protection for those most harmed by Zika virus – pregnant women and their fetuses. Martin Schreiber, MD, professor of surgery at Oregon Health and Sciences University, Portland, proposed a point-of-care testing strategy in which only blood destined for pregnant women would be tested for Zika virus. Dr. Schreiber, a trauma surgeon, put forward the rationale that Zika virus causes harm almost exclusively to fetuses, except for rare cases of Guillain-Barré syndrome.
In response, Dr. Kaufman pointed out that with rare exceptions for some bacterial testing, all testing is done from samples taken at the point of donation. The supply chain for donor blood is not set up to accommodate point-of-care testing, he said.
Answering questions about another targeted strategy – maintaining a separate, Zika-tested supply of blood for pregnant women – Susan Stramer, PhD, vice president of scientific affairs for the American Red Cross, said, “Most hospitals do not want, and are very adamant against, carrying a dual inventory.”
Ultimately, the committee’s discussion swung toward the realization that it may be too soon after the recent spike in U.S. Zika cases to plot the best course for ongoing testing strategies. “We are at the tail end of a waning epidemic. ... I think it would probably be a pretty easy question for the committee and for the agency if we actually had some way of having a crystal ball and knowing that the current trend was likely to continue,” said Roger Lewis, MD, PhD, professor at the University of California, Los Angeles, and chair of the department of emergency medicine at Harbor-UCLA Medical Center.
“I think that is not the question,” he went on. “I think the question is, What is the optimal strategy if we have no idea if that tail is going to continue in this current trend. ... And that maybe the committee ought to be thinking about what is the right strategy for the next 2 years – with an underlying assumption that this is a question that can be brought back as we learn more about how this disease behaves.”
The FDA usually follows the recommendations of its advisory committees.
Most members of a Food and Drug Administration advisory committee considered that data support maintaining current testing protocols for Zika virus in the blood donor pool. However, committee discussion entertained the idea of revisiting testing strategies after another year or 2 of Zika virus epidemiological data are available.
In its last guidance regarding Zika virus testing, issued in July 2018, the FDA recommended that either minipool nucleic acid testing (MP NAT) or individual donor (ID) NAT be used to screen for Zika virus. Current guidance still requires conversion to all-ID NAT “when certain threshold conditions are met that indicate an increased risk of suspected mosquito-borne transmission in a defined geographic collection area.”
In the first of three separate votes, 11 of 15 voting members of the FDA’s Blood Products Advisory Committee (BPAC) answered in the affirmative to the question of whether available data support continuing the status quo for Zika testing. Committee members then were asked to weigh whether current data support scaling back to a regional testing strategy targeting at-risk areas. Here, six committee members answered in the affirmative, and nine in the negative.
Just one committee member, F. Blaine Hollinger, MD, voted in favor of the third option, elimination of all Zika virus testing without reintroducing donor screening for risk factors in risk-free areas pending another outbreak in the United States. Dr. Hollinger is a professor of virology and microbiology at Baylor College of Medicine, Houston.
The committee as whole wasn’t swayed by a line of questioning put forward by chairman Richard Kaufman, MD. “I will be the devil’s advocate a little bit: We learned that there have been zero confirmed positives from blood donors for the past year. Would anyone be comfortable with just stopping screening of donors?” asked Dr. Kaufman, medical director of the adult transfusion service at Brigham and Women’s Hospital, Boston.
A wide-ranging morning of presentations put data regarding historical trends and current global Zika hot spots in front of the committee. Current upticks in infection rates in northwest Mexico and in some states in India were areas of concern, given North American travel patterns, noted speaker Marc Fisher, MD, of the Center for Disease Control and Prevention’s Arboviral Disease Branch (Fort Collins, Colo.) “We’re going to see sporadic outbreaks; it’s hard to predict the future,” he said. “The new outbreak in India raises concerns.”
Briefing information from the FDA explained that Zika virus local transmission peaked in the United States in late summer of 2016. More than 5,000 cases were reported in the United States and over 36,000 in Puerto Rico. This has plummeted to 220 in 2018, with about two-thirds of these cases occurring in the territories, mostly (97%) from Puerto Rico across all 3 years.
Zika viremic blood donors dropped by an order of magnitude yearly, totaling 363 in 2016, 38 in 2017, and just 3 in 2018. Of the 363 detected in 2016, 96% came from Puerto Rico or Florida, noted Dr. Fisher.
The number of suspected and confirmed cases in the Americas overall has also dropped from over 650,000 in 2016 to under 30,000 in 2018, with most cases in 2018 being suspected rather than laboratory confirmed. In contrast to testing conducted in North America, few cases in much of Central and South America were laboratory confirmed.
Asymptomatic infections have occurred in blood donors, said the FDA, with 1.8% of blood donations in Puerto Rico testing positive for Zika virus during the peak of the outbreak. Transmission by transfusion is thought to have occurred in Brazil.
Although Zika virus infections have plummeted in the United States and worldwide, prevalence and rates of local transmission are unpredictable, said the FDA, which pointed to sporadic increases in autochthonous transmission of viruses such as dengue and chikungunya that are carried by the same mosquito vector as Zika.
Some of the committee’s discussion centered around finding a way to carve out protection for those most harmed by Zika virus – pregnant women and their fetuses. Martin Schreiber, MD, professor of surgery at Oregon Health and Sciences University, Portland, proposed a point-of-care testing strategy in which only blood destined for pregnant women would be tested for Zika virus. Dr. Schreiber, a trauma surgeon, put forward the rationale that Zika virus causes harm almost exclusively to fetuses, except for rare cases of Guillain-Barré syndrome.
In response, Dr. Kaufman pointed out that with rare exceptions for some bacterial testing, all testing is done from samples taken at the point of donation. The supply chain for donor blood is not set up to accommodate point-of-care testing, he said.
Answering questions about another targeted strategy – maintaining a separate, Zika-tested supply of blood for pregnant women – Susan Stramer, PhD, vice president of scientific affairs for the American Red Cross, said, “Most hospitals do not want, and are very adamant against, carrying a dual inventory.”
Ultimately, the committee’s discussion swung toward the realization that it may be too soon after the recent spike in U.S. Zika cases to plot the best course for ongoing testing strategies. “We are at the tail end of a waning epidemic. ... I think it would probably be a pretty easy question for the committee and for the agency if we actually had some way of having a crystal ball and knowing that the current trend was likely to continue,” said Roger Lewis, MD, PhD, professor at the University of California, Los Angeles, and chair of the department of emergency medicine at Harbor-UCLA Medical Center.
“I think that is not the question,” he went on. “I think the question is, What is the optimal strategy if we have no idea if that tail is going to continue in this current trend. ... And that maybe the committee ought to be thinking about what is the right strategy for the next 2 years – with an underlying assumption that this is a question that can be brought back as we learn more about how this disease behaves.”
The FDA usually follows the recommendations of its advisory committees.
CDC exhorts more testing and treatment of HIV
Approximately 80% of HIV infections in the United States in 2016 were spread by almost 40% of infected individuals who did not know their status or were not receiving care, according to data from the Centers for Disease Control and Prevention.
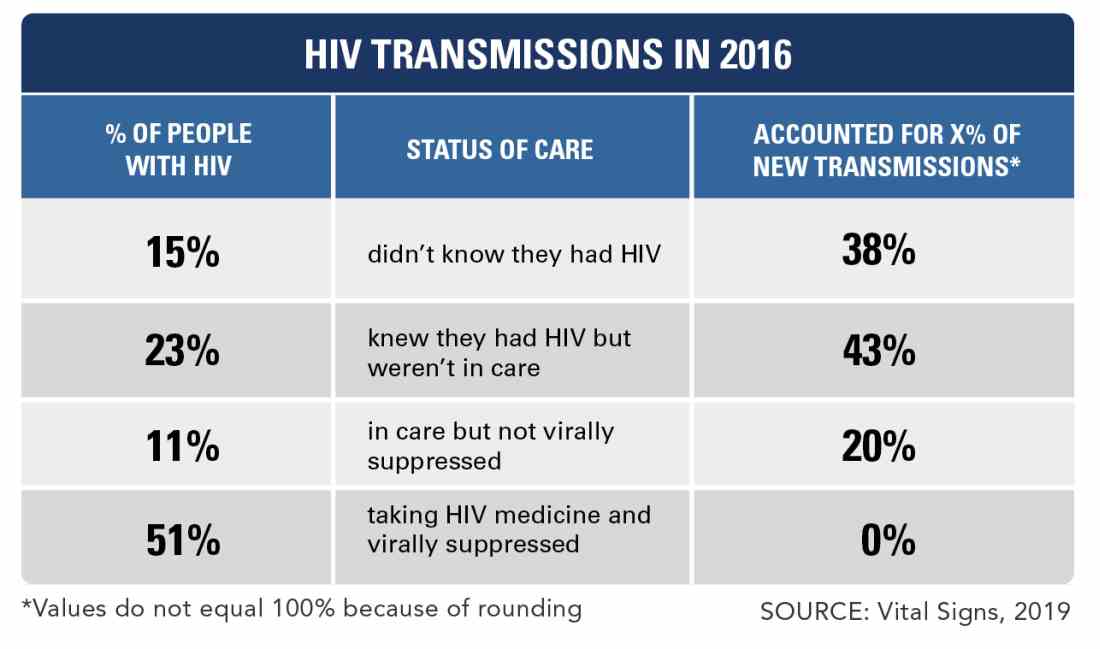
However, leadership at the Department of Health & Human Services has developed a “bold but completely achievable” plan to reduce HIV infections within the next decade, Vice Adm. Jerome M. Adams, MD, the U.S. surgeon general, said in a teleconference to announce the results of a new Vital Signs report on the impact of undiagnosed and untreated HIV.
In the early release Vital Signs from Morbidity and Mortality Weekly Report, Li Zihao, PhD, and his colleagues at the CDC used a model to estimate rates of HIV transmission in 2016 based on data from the National HIV Behavioral Surveillance on needle-sharing behavior and sexual behaviors. The overall transmission rate was 3.5/100,000 person-years. Of these transmissions, 73.0% were from men who have sex with men, 9.7% from intravenous drug users, and 12% from heterosexuals.
The percentages of transmissions for those who were acutely ill with HIV but unaware, not acutely ill but unaware, aware of HIV infection but not treated, receiving care but not virally suppressed, and receiving care and virally suppressed were 4.0%, 33.6%, 42.6%, 19.8%, and 0%, respectively, the researchers said.
The study “emphasizes the impact that HIV resources could have,” by showing the importance of identifying infected individuals early and using the tools now available to treat them before they can transmit the disease, Jonathan Mermin, MD, director of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said during the conference.
“Today’s treatment regimens are simpler than those prescribed in the past, sometimes requiring only single-tablet formulations, with fewer side effects; most persons with HIV infection can achieve viral suppression within 6 months of initiating treatment,” the researchers wrote.
In the wake of the findings and at the start of the CDC’s 2019 National HIV Prevention Conference, the CDC proposed a federal initiative, “Ending the HIV Epidemic: A Plan for America.”
The goal is to reduce the incidence of new HIV infections by at least 90% over the next decade, starting with a focus on parts of the country with the highest disease burden, according to the CDC.
“Today’s Vital Signs report illustrates how a goal that once seemed impossible is now within our reach.” Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention, said during the conference. “If we increase access to testing and treatment for people with HIV, we could prevent a lion’s share of infections,” he said.
The plan involves working to identify individuals at risk, treating those who test positive as quickly as possible, and keeping them in care. Updated information on the CDC website provides more details for clinicians on how to have conversations about HIV with patients, the latest information about antiretroviral therapy, and details about prevention for partners including post- and pre-exposure prophylaxis (PEP and PreP), and condoms.
Eugene McCray, MD, director of the CDC’s Division of HIV/AIDS Prevention, emphasized that the CDC recommends HIV testing for all individuals aged 13-64 years at least once in their lives. He added that everyone who tests positive should seek medical care, that everyone with HIV deserves support to combat stigma, and that those at risk should be empowered to take advantage of proven effective prevention strategies.
Clinicians can access the updated CDC page on caring for HIV patients here.
SOURCE: Li Z et al. MMWR Morb Mortal Wkly Rep. 2019 March 18. doi: org/10.15585/mmwr.mm6811e1.
Approximately 80% of HIV infections in the United States in 2016 were spread by almost 40% of infected individuals who did not know their status or were not receiving care, according to data from the Centers for Disease Control and Prevention.

However, leadership at the Department of Health & Human Services has developed a “bold but completely achievable” plan to reduce HIV infections within the next decade, Vice Adm. Jerome M. Adams, MD, the U.S. surgeon general, said in a teleconference to announce the results of a new Vital Signs report on the impact of undiagnosed and untreated HIV.
In the early release Vital Signs from Morbidity and Mortality Weekly Report, Li Zihao, PhD, and his colleagues at the CDC used a model to estimate rates of HIV transmission in 2016 based on data from the National HIV Behavioral Surveillance on needle-sharing behavior and sexual behaviors. The overall transmission rate was 3.5/100,000 person-years. Of these transmissions, 73.0% were from men who have sex with men, 9.7% from intravenous drug users, and 12% from heterosexuals.
The percentages of transmissions for those who were acutely ill with HIV but unaware, not acutely ill but unaware, aware of HIV infection but not treated, receiving care but not virally suppressed, and receiving care and virally suppressed were 4.0%, 33.6%, 42.6%, 19.8%, and 0%, respectively, the researchers said.
The study “emphasizes the impact that HIV resources could have,” by showing the importance of identifying infected individuals early and using the tools now available to treat them before they can transmit the disease, Jonathan Mermin, MD, director of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said during the conference.
“Today’s treatment regimens are simpler than those prescribed in the past, sometimes requiring only single-tablet formulations, with fewer side effects; most persons with HIV infection can achieve viral suppression within 6 months of initiating treatment,” the researchers wrote.
In the wake of the findings and at the start of the CDC’s 2019 National HIV Prevention Conference, the CDC proposed a federal initiative, “Ending the HIV Epidemic: A Plan for America.”
The goal is to reduce the incidence of new HIV infections by at least 90% over the next decade, starting with a focus on parts of the country with the highest disease burden, according to the CDC.
“Today’s Vital Signs report illustrates how a goal that once seemed impossible is now within our reach.” Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention, said during the conference. “If we increase access to testing and treatment for people with HIV, we could prevent a lion’s share of infections,” he said.
The plan involves working to identify individuals at risk, treating those who test positive as quickly as possible, and keeping them in care. Updated information on the CDC website provides more details for clinicians on how to have conversations about HIV with patients, the latest information about antiretroviral therapy, and details about prevention for partners including post- and pre-exposure prophylaxis (PEP and PreP), and condoms.
Eugene McCray, MD, director of the CDC’s Division of HIV/AIDS Prevention, emphasized that the CDC recommends HIV testing for all individuals aged 13-64 years at least once in their lives. He added that everyone who tests positive should seek medical care, that everyone with HIV deserves support to combat stigma, and that those at risk should be empowered to take advantage of proven effective prevention strategies.
Clinicians can access the updated CDC page on caring for HIV patients here.
SOURCE: Li Z et al. MMWR Morb Mortal Wkly Rep. 2019 March 18. doi: org/10.15585/mmwr.mm6811e1.
Approximately 80% of HIV infections in the United States in 2016 were spread by almost 40% of infected individuals who did not know their status or were not receiving care, according to data from the Centers for Disease Control and Prevention.

However, leadership at the Department of Health & Human Services has developed a “bold but completely achievable” plan to reduce HIV infections within the next decade, Vice Adm. Jerome M. Adams, MD, the U.S. surgeon general, said in a teleconference to announce the results of a new Vital Signs report on the impact of undiagnosed and untreated HIV.
In the early release Vital Signs from Morbidity and Mortality Weekly Report, Li Zihao, PhD, and his colleagues at the CDC used a model to estimate rates of HIV transmission in 2016 based on data from the National HIV Behavioral Surveillance on needle-sharing behavior and sexual behaviors. The overall transmission rate was 3.5/100,000 person-years. Of these transmissions, 73.0% were from men who have sex with men, 9.7% from intravenous drug users, and 12% from heterosexuals.
The percentages of transmissions for those who were acutely ill with HIV but unaware, not acutely ill but unaware, aware of HIV infection but not treated, receiving care but not virally suppressed, and receiving care and virally suppressed were 4.0%, 33.6%, 42.6%, 19.8%, and 0%, respectively, the researchers said.
The study “emphasizes the impact that HIV resources could have,” by showing the importance of identifying infected individuals early and using the tools now available to treat them before they can transmit the disease, Jonathan Mermin, MD, director of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said during the conference.
“Today’s treatment regimens are simpler than those prescribed in the past, sometimes requiring only single-tablet formulations, with fewer side effects; most persons with HIV infection can achieve viral suppression within 6 months of initiating treatment,” the researchers wrote.
In the wake of the findings and at the start of the CDC’s 2019 National HIV Prevention Conference, the CDC proposed a federal initiative, “Ending the HIV Epidemic: A Plan for America.”
The goal is to reduce the incidence of new HIV infections by at least 90% over the next decade, starting with a focus on parts of the country with the highest disease burden, according to the CDC.
“Today’s Vital Signs report illustrates how a goal that once seemed impossible is now within our reach.” Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention, said during the conference. “If we increase access to testing and treatment for people with HIV, we could prevent a lion’s share of infections,” he said.
The plan involves working to identify individuals at risk, treating those who test positive as quickly as possible, and keeping them in care. Updated information on the CDC website provides more details for clinicians on how to have conversations about HIV with patients, the latest information about antiretroviral therapy, and details about prevention for partners including post- and pre-exposure prophylaxis (PEP and PreP), and condoms.
Eugene McCray, MD, director of the CDC’s Division of HIV/AIDS Prevention, emphasized that the CDC recommends HIV testing for all individuals aged 13-64 years at least once in their lives. He added that everyone who tests positive should seek medical care, that everyone with HIV deserves support to combat stigma, and that those at risk should be empowered to take advantage of proven effective prevention strategies.
Clinicians can access the updated CDC page on caring for HIV patients here.
SOURCE: Li Z et al. MMWR Morb Mortal Wkly Rep. 2019 March 18. doi: org/10.15585/mmwr.mm6811e1.
FROM THE CDC
Measles cases confirmed in three more states
Forty more cases and three more states were added to the measles count in the last week, bringing the U.S. total to 268 cases in 15 states so far in 2019, according to the Centers for Disease Control and Prevention.
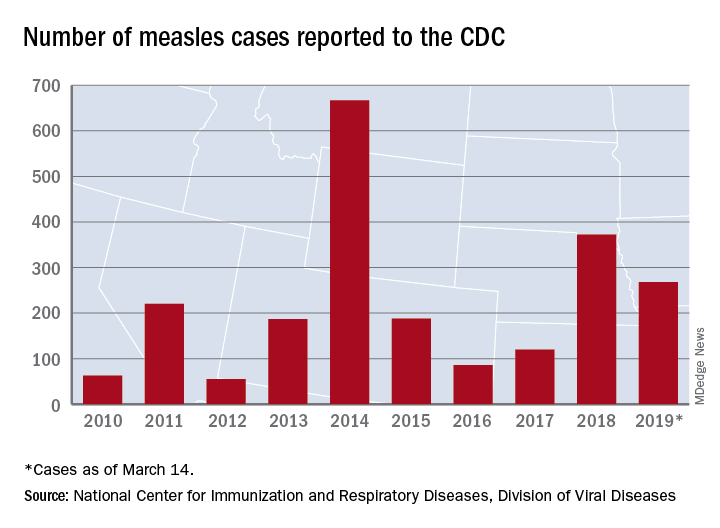
Arizona, Michigan, and Missouri reported their first confirmed cases of the year, joining California (one outbreak), Colorado, Connecticut, Georgia, Illinois (one outbreak), Kentucky, New Hampshire, New Jersey, New York (two outbreaks), Oregon, Texas (one outbreak), and Washington (one outbreak), the CDC reported March 18.
Brooklyn, N.Y., has become the epicenter of measles activity since mid-February, and with 25 of the 40 new cases occurring there, the borough has now led the nation for four consecutive weeks. There have been 157 confirmed cases in Brooklyn and one in Queens since the outbreak began in October of 2018, the New York City Department of Health and Mental Hygiene reported.
Michigan’s first case of measles is travel related and involved an individual visiting from Israel following a stay in New York, the Michigan Department of Health and Human Services and Oakland County Health Division announced March 13.
In Arizona, the state department of health services and the Pima County Public Health Department announced that a 12-month-old infant from Pima County has been diagnosed with measles after traveling to Asia.
A single case of measles, contracted while the person was traveling out of state, has been reported in Jefferson County, Mo., and is being managed by the county health department, according to St. Louis Public Radio.
Forty more cases and three more states were added to the measles count in the last week, bringing the U.S. total to 268 cases in 15 states so far in 2019, according to the Centers for Disease Control and Prevention.

Arizona, Michigan, and Missouri reported their first confirmed cases of the year, joining California (one outbreak), Colorado, Connecticut, Georgia, Illinois (one outbreak), Kentucky, New Hampshire, New Jersey, New York (two outbreaks), Oregon, Texas (one outbreak), and Washington (one outbreak), the CDC reported March 18.
Brooklyn, N.Y., has become the epicenter of measles activity since mid-February, and with 25 of the 40 new cases occurring there, the borough has now led the nation for four consecutive weeks. There have been 157 confirmed cases in Brooklyn and one in Queens since the outbreak began in October of 2018, the New York City Department of Health and Mental Hygiene reported.
Michigan’s first case of measles is travel related and involved an individual visiting from Israel following a stay in New York, the Michigan Department of Health and Human Services and Oakland County Health Division announced March 13.
In Arizona, the state department of health services and the Pima County Public Health Department announced that a 12-month-old infant from Pima County has been diagnosed with measles after traveling to Asia.
A single case of measles, contracted while the person was traveling out of state, has been reported in Jefferson County, Mo., and is being managed by the county health department, according to St. Louis Public Radio.
Forty more cases and three more states were added to the measles count in the last week, bringing the U.S. total to 268 cases in 15 states so far in 2019, according to the Centers for Disease Control and Prevention.

Arizona, Michigan, and Missouri reported their first confirmed cases of the year, joining California (one outbreak), Colorado, Connecticut, Georgia, Illinois (one outbreak), Kentucky, New Hampshire, New Jersey, New York (two outbreaks), Oregon, Texas (one outbreak), and Washington (one outbreak), the CDC reported March 18.
Brooklyn, N.Y., has become the epicenter of measles activity since mid-February, and with 25 of the 40 new cases occurring there, the borough has now led the nation for four consecutive weeks. There have been 157 confirmed cases in Brooklyn and one in Queens since the outbreak began in October of 2018, the New York City Department of Health and Mental Hygiene reported.
Michigan’s first case of measles is travel related and involved an individual visiting from Israel following a stay in New York, the Michigan Department of Health and Human Services and Oakland County Health Division announced March 13.
In Arizona, the state department of health services and the Pima County Public Health Department announced that a 12-month-old infant from Pima County has been diagnosed with measles after traveling to Asia.
A single case of measles, contracted while the person was traveling out of state, has been reported in Jefferson County, Mo., and is being managed by the county health department, according to St. Louis Public Radio.
Repeat VTE risk heightened in HIV patients
SEATTLE – HIV infection is associated with increased risk of recurrent venous thromboembolism, especially within 1 year of the initial episode. The finding, presented during a poster session at the Conference on Retroviruses & Opportunistic Infections, follows up on an earlier study that found that first-time VTE risk also is higher among HIV-positive individuals than in the general population.
The conclusion about first-time VTE risk, published earlier this year in Lancet HIV, came from a comparison between the ATHENA (AIDS Therapy Evaluation in the Netherlands) cohort and European population-level of studies of VTE. It found a crude incidence of 2.33 VTE events per 1,000 person-years In HIV patients, with heightened odds when CD4 cell counts were below 200 cells/mcL (adjusted hazard ratio, 3.40).
The new work represents a follow-up and compared results from ATHENA (153 patients with HIV and first VTE) and the Dutch MEGA cohort (4,005 patients without HIV, with first VTE), which includes the general population. Overall, 26% of patients in the ATHENA cohort experienced a second VTE event, compared with 16% of the general population. At 1 year after anticoagulation withdrawal, HIV-positive individuals were at 67% increased risk (HR, 1.67). At 6-years after withdrawal, the relationship was not statistically significant (HR, 1.22).
Researchers also found that CD4 cell-count recovery was associated with lowered risk, with every 100 cell-count increase between initial VTE diagnosis and anticoagulant withdrawal linked to a 20% reduction in risk (HR, 0.80).
“The clinical question is: If it’s true you have an increased risk of recurrence, should you be continuing anticoagulant therapy longer in people with HIV? This poster doesn’t answer that question and you probably need a randomized, controlled trial to look at that,” Peter Reiss, MD, professor of medicine at Amsterdam University Medical Center, said in an interview during the conference.
In the absence of a clear answer, it’s sensible for clinicians to be aware of the potential increased risk, much as clinicians have internalized the increased risk of atherosclerotic vascular disease in HIV patients. “I think the publication [in Lancet HIV] as well as this poster suggest that on the venous side of things there may also be an accentuated risk,” said Dr. Reiss.
Heidi Crane, MD, a professor of medicine at the University of Washington, Seattle, presented a poster examining the underlying factors that may predispose HIV patients to first-time VTE events. Her team performed an adjudicated review of VTE cases among HIV patients at six institutions and found that the risk factors appeared to be distinct from those seen in the general population.
The traditional long plane ride was less common in this population, while factors such as injected drug use and pneumonia were more common. The VTE events occurred at a median age of 49 years; 30% of the patients had a detectable viral load. “We’re seeing a little more (VTE) than you might expect, and in a younger population than you might have guessed,” said Dr. Crane in an interview.
The most frequent predisposing risk factors were recent hospitalization (40%), infection (40%), or immobilization/bed rest (24%) within the past 90 days, and injectable drug use (22%). “It’s not just the traditional risk factors. Some HIV-specific risk factors are driving this,” said Dr. Crane.
She also aims to learn more about the specifics of risk factors, such as catheter-associated thromboses. The team is working to increase the sample size in order to parse out the relationships with specific outcomes.
In the meantime, the data further characterize the health challenges facing people living with HIV. “This is another example demonstrating that comorbid conditions among patients with HIV that are often considered age related occur at much younger ages in our population,” said Dr. Crane.
SOURCE: Rokx C et al. CROI 2019, Abstract 636; and Tenforde MW et al. CROI 2019, Abstract 637.
.
SEATTLE – HIV infection is associated with increased risk of recurrent venous thromboembolism, especially within 1 year of the initial episode. The finding, presented during a poster session at the Conference on Retroviruses & Opportunistic Infections, follows up on an earlier study that found that first-time VTE risk also is higher among HIV-positive individuals than in the general population.
The conclusion about first-time VTE risk, published earlier this year in Lancet HIV, came from a comparison between the ATHENA (AIDS Therapy Evaluation in the Netherlands) cohort and European population-level of studies of VTE. It found a crude incidence of 2.33 VTE events per 1,000 person-years In HIV patients, with heightened odds when CD4 cell counts were below 200 cells/mcL (adjusted hazard ratio, 3.40).
The new work represents a follow-up and compared results from ATHENA (153 patients with HIV and first VTE) and the Dutch MEGA cohort (4,005 patients without HIV, with first VTE), which includes the general population. Overall, 26% of patients in the ATHENA cohort experienced a second VTE event, compared with 16% of the general population. At 1 year after anticoagulation withdrawal, HIV-positive individuals were at 67% increased risk (HR, 1.67). At 6-years after withdrawal, the relationship was not statistically significant (HR, 1.22).
Researchers also found that CD4 cell-count recovery was associated with lowered risk, with every 100 cell-count increase between initial VTE diagnosis and anticoagulant withdrawal linked to a 20% reduction in risk (HR, 0.80).
“The clinical question is: If it’s true you have an increased risk of recurrence, should you be continuing anticoagulant therapy longer in people with HIV? This poster doesn’t answer that question and you probably need a randomized, controlled trial to look at that,” Peter Reiss, MD, professor of medicine at Amsterdam University Medical Center, said in an interview during the conference.
In the absence of a clear answer, it’s sensible for clinicians to be aware of the potential increased risk, much as clinicians have internalized the increased risk of atherosclerotic vascular disease in HIV patients. “I think the publication [in Lancet HIV] as well as this poster suggest that on the venous side of things there may also be an accentuated risk,” said Dr. Reiss.
Heidi Crane, MD, a professor of medicine at the University of Washington, Seattle, presented a poster examining the underlying factors that may predispose HIV patients to first-time VTE events. Her team performed an adjudicated review of VTE cases among HIV patients at six institutions and found that the risk factors appeared to be distinct from those seen in the general population.
The traditional long plane ride was less common in this population, while factors such as injected drug use and pneumonia were more common. The VTE events occurred at a median age of 49 years; 30% of the patients had a detectable viral load. “We’re seeing a little more (VTE) than you might expect, and in a younger population than you might have guessed,” said Dr. Crane in an interview.
The most frequent predisposing risk factors were recent hospitalization (40%), infection (40%), or immobilization/bed rest (24%) within the past 90 days, and injectable drug use (22%). “It’s not just the traditional risk factors. Some HIV-specific risk factors are driving this,” said Dr. Crane.
She also aims to learn more about the specifics of risk factors, such as catheter-associated thromboses. The team is working to increase the sample size in order to parse out the relationships with specific outcomes.
In the meantime, the data further characterize the health challenges facing people living with HIV. “This is another example demonstrating that comorbid conditions among patients with HIV that are often considered age related occur at much younger ages in our population,” said Dr. Crane.
SOURCE: Rokx C et al. CROI 2019, Abstract 636; and Tenforde MW et al. CROI 2019, Abstract 637.
.
SEATTLE – HIV infection is associated with increased risk of recurrent venous thromboembolism, especially within 1 year of the initial episode. The finding, presented during a poster session at the Conference on Retroviruses & Opportunistic Infections, follows up on an earlier study that found that first-time VTE risk also is higher among HIV-positive individuals than in the general population.
The conclusion about first-time VTE risk, published earlier this year in Lancet HIV, came from a comparison between the ATHENA (AIDS Therapy Evaluation in the Netherlands) cohort and European population-level of studies of VTE. It found a crude incidence of 2.33 VTE events per 1,000 person-years In HIV patients, with heightened odds when CD4 cell counts were below 200 cells/mcL (adjusted hazard ratio, 3.40).
The new work represents a follow-up and compared results from ATHENA (153 patients with HIV and first VTE) and the Dutch MEGA cohort (4,005 patients without HIV, with first VTE), which includes the general population. Overall, 26% of patients in the ATHENA cohort experienced a second VTE event, compared with 16% of the general population. At 1 year after anticoagulation withdrawal, HIV-positive individuals were at 67% increased risk (HR, 1.67). At 6-years after withdrawal, the relationship was not statistically significant (HR, 1.22).
Researchers also found that CD4 cell-count recovery was associated with lowered risk, with every 100 cell-count increase between initial VTE diagnosis and anticoagulant withdrawal linked to a 20% reduction in risk (HR, 0.80).
“The clinical question is: If it’s true you have an increased risk of recurrence, should you be continuing anticoagulant therapy longer in people with HIV? This poster doesn’t answer that question and you probably need a randomized, controlled trial to look at that,” Peter Reiss, MD, professor of medicine at Amsterdam University Medical Center, said in an interview during the conference.
In the absence of a clear answer, it’s sensible for clinicians to be aware of the potential increased risk, much as clinicians have internalized the increased risk of atherosclerotic vascular disease in HIV patients. “I think the publication [in Lancet HIV] as well as this poster suggest that on the venous side of things there may also be an accentuated risk,” said Dr. Reiss.
Heidi Crane, MD, a professor of medicine at the University of Washington, Seattle, presented a poster examining the underlying factors that may predispose HIV patients to first-time VTE events. Her team performed an adjudicated review of VTE cases among HIV patients at six institutions and found that the risk factors appeared to be distinct from those seen in the general population.
The traditional long plane ride was less common in this population, while factors such as injected drug use and pneumonia were more common. The VTE events occurred at a median age of 49 years; 30% of the patients had a detectable viral load. “We’re seeing a little more (VTE) than you might expect, and in a younger population than you might have guessed,” said Dr. Crane in an interview.
The most frequent predisposing risk factors were recent hospitalization (40%), infection (40%), or immobilization/bed rest (24%) within the past 90 days, and injectable drug use (22%). “It’s not just the traditional risk factors. Some HIV-specific risk factors are driving this,” said Dr. Crane.
She also aims to learn more about the specifics of risk factors, such as catheter-associated thromboses. The team is working to increase the sample size in order to parse out the relationships with specific outcomes.
In the meantime, the data further characterize the health challenges facing people living with HIV. “This is another example demonstrating that comorbid conditions among patients with HIV that are often considered age related occur at much younger ages in our population,” said Dr. Crane.
SOURCE: Rokx C et al. CROI 2019, Abstract 636; and Tenforde MW et al. CROI 2019, Abstract 637.
.
REPORTING FROM CROI 2019
Flu activity levels down, but outpatient visits highest since 1998-99
Influenza activity measures declined for a third consecutive week, but levels are higher than usual at this point in the flu season, according to the Centers for Disease Control and Prevention.
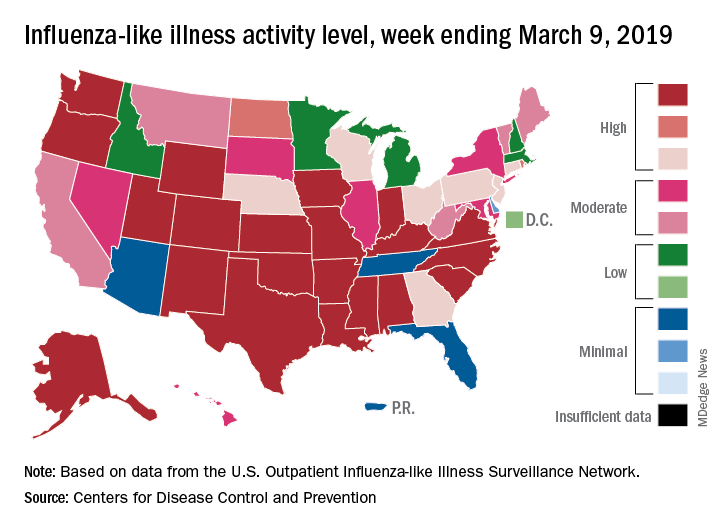
For the week ending March 9, an estimated 4.5% of outpatient visits were for influenza-like illness (ILI), which was down from 4.6% the previous week, the CDC’s influenza division reported March 15, but that is higher than the comparable week for any year since 1998-1999. During last year’s very severe flu season, the outpatient visit rate was just under 3.2% for the week ending March 10.
Although the number of states at level 10 on the CDC’s 1-10 scale remained at 21, the activity map actually looks more red than last week since Rhode Island and West Virgina were replaced by the much larger states of Iowa and Washington. The number of states in the high range (8-10), did go down from 32 to 30, data from the CDC’s Outpatient ILI Surveillance Network show.
Of those four deaths, only one occurred during the most recent reporting week, the CDC said.
Influenza activity measures declined for a third consecutive week, but levels are higher than usual at this point in the flu season, according to the Centers for Disease Control and Prevention.

For the week ending March 9, an estimated 4.5% of outpatient visits were for influenza-like illness (ILI), which was down from 4.6% the previous week, the CDC’s influenza division reported March 15, but that is higher than the comparable week for any year since 1998-1999. During last year’s very severe flu season, the outpatient visit rate was just under 3.2% for the week ending March 10.
Although the number of states at level 10 on the CDC’s 1-10 scale remained at 21, the activity map actually looks more red than last week since Rhode Island and West Virgina were replaced by the much larger states of Iowa and Washington. The number of states in the high range (8-10), did go down from 32 to 30, data from the CDC’s Outpatient ILI Surveillance Network show.
Of those four deaths, only one occurred during the most recent reporting week, the CDC said.
Influenza activity measures declined for a third consecutive week, but levels are higher than usual at this point in the flu season, according to the Centers for Disease Control and Prevention.

For the week ending March 9, an estimated 4.5% of outpatient visits were for influenza-like illness (ILI), which was down from 4.6% the previous week, the CDC’s influenza division reported March 15, but that is higher than the comparable week for any year since 1998-1999. During last year’s very severe flu season, the outpatient visit rate was just under 3.2% for the week ending March 10.
Although the number of states at level 10 on the CDC’s 1-10 scale remained at 21, the activity map actually looks more red than last week since Rhode Island and West Virgina were replaced by the much larger states of Iowa and Washington. The number of states in the high range (8-10), did go down from 32 to 30, data from the CDC’s Outpatient ILI Surveillance Network show.
Of those four deaths, only one occurred during the most recent reporting week, the CDC said.