User login
A high proportion of SARS-CoV-2–infected university students are asymptomatic
Many individuals infected with SARS-CoV-2 never become symptomatic. In a South Korean study, these infected individuals remained asymptomatic for a prolonged period while maintaining the same viral load as symptomatic patients, suggesting that they are just as infectious.1 A narrative review found high rates of asymptomatic disease in several younger populations, including women in an obstetric ward (88%), the crew of an aircraft carrier (58%), and prisoners (96%).2 However, there is no published research on the percentage of university students who are asymptomatic.
Methods
The University of Georgia (UGA) began classes on August 20, 2020. Shortly before the beginning of classes, UGA implemented a surveillance program for asymptomatic students, faculty, and staff, testing 300 to 450 people per day. Initially, during Weeks 1 and 2 of data collection, anyone could choose to be tested. In Weeks 3 and 4, students, faculty, and staff were randomly invited to participate.
Over the 4-week period beginning on August 17, we calculated the percent of positive cases in surveillance testing and applied this percentage to the entire UGA student population (n = 38,920) to estimate the total number of asymptomatic COVID-19 students each week.3 Data for symptomatic cases were also reported by the university on a weekly basis. This included positive tests from the University Health Center, as well as voluntary reporting using a smartphone app from other sites.
Positive tests in symptomatic individuals were not stratified by student vs nonstudent until Week 3; students comprised 95% of positive symptomatic reports in Week 3 and 99% in Week 4, so we conservatively estimated that 95% of symptomatic cases in Weeks 1 and 2 were students. These data were used to estimate the percentage of SARS-CoV-2–positive students who were asymptomatic.
Results
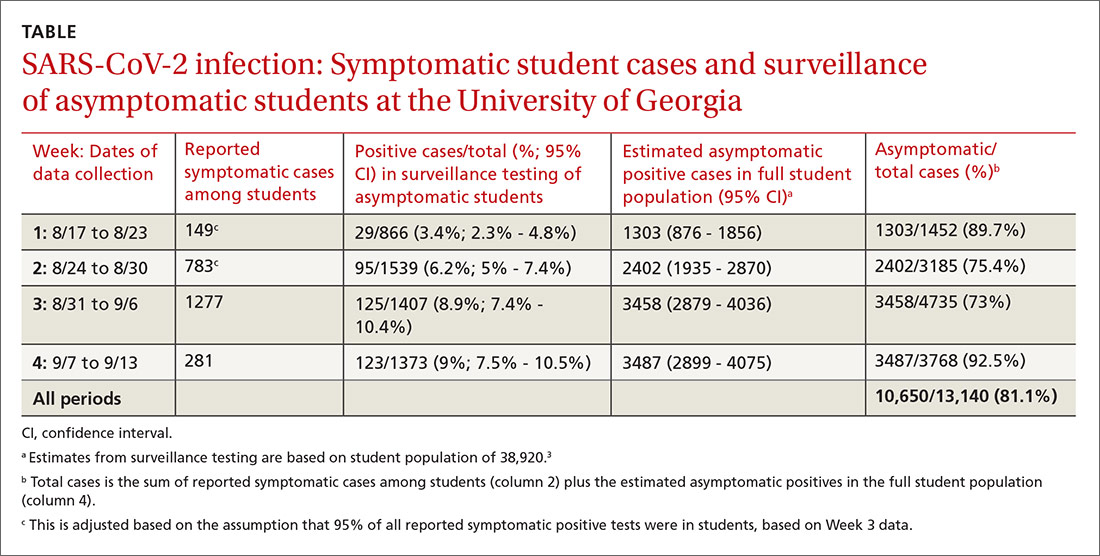
Our results are summarized in the table. The percentage of asymptomatic students testing positive in surveillance testing was 3.4% in Week 1 and rose steadily to 9% by Week 4. We estimated that there were 1303 asymptomatic cases among students in Week 1, increasing to 3487 asymptomatic positive students on campus by Week 4. The estimated percentage of asymptomatic students infected with SARS-CoV-2 ranged from 73% to 92.5% by week and was 81.1% overall.
Discussion
During the reporting period from August 17 to September 13, the 7-day moving average of new cases in Clarke County (home of UGA) increased from 30 to 83 per 100,000 persons/day (https://dph.georgia.gov/covid-19-daily-status-report). During this period, there were large increases in the number of infected students, more than 80% of whom were asymptomatic. With the assumption that anyone could be infected even if asymptomatic, these numbers highlight the importance for infection control to prevent potential spread within a community by taking universal precautions such as wearing a mask, following physical distancing guidelines, and handwashing.
Limitations. First, reporting of positive tests in symptomatic individuals is highly encouraged but not required. The large drop in symptomatic positive test reports between Weeks 3 and 4, with no change in test positivity in surveillance of asymptomatic students (8.9% vs 9%), suggests that students may have chosen to be tested elsewhere in conjunction with evaluation of their symptoms and/or not reported positive tests, possibly to avoid mandatory isolation and other restrictions on their activities. Further evidence to support no change in actual infection rates comes from testing for virus in wastewater, which also remained unchanged.4
Continue to: Second, each week's surveillance...
Second, each week’s surveillance population is not a true random sample, so extrapolating this estimate to the full student population could over- or undercount asymptomatic cases depending on the direction of bias (ie, healthy volunteer bias vs test avoidance by those with high-risk behaviors).
Finally, some students who were positive in surveillance testing may have been presymptomatic, rather than asymptomatic.
In conclusion, we estimate that approximately 80% of students infected with SARS-CoV-2 are asymptomatic. This is consistent with other studies in young adult populations.2
Mark H. Ebell, MD, MS
Cassie Chupp, MPH
Michelle Bentivegna, MPH
Department of Epidemiology and Biostatistics, College of Public Health, University of Georgia, Athens
[email protected]
The authors reported no potential conflict of interest relevant to this article.
1. Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea [published online ahead of print August 6, 2020]. JAMA Intern Med. doi:10.1001/jamainternmed.2020.3862
2. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020;173:362-367.
3. UGA by the Numbers. University of Georgia Web site. www.uga.edu/facts.php. Updated August 2020. Accessed October 20, 2020.
4. Lott M, Norfolk W, Robertson M, et al. Wastewater surveillance for SARS-CoV-2 in Athens, GA. COVID-19 Portal: Center for the Ecology of Infectious Diseases, University of Georgia Web site. www.covid19.uga.edu/wastewater-athens.html. Updated October 15, 2020. Accessed October 20, 2020.
Many individuals infected with SARS-CoV-2 never become symptomatic. In a South Korean study, these infected individuals remained asymptomatic for a prolonged period while maintaining the same viral load as symptomatic patients, suggesting that they are just as infectious.1 A narrative review found high rates of asymptomatic disease in several younger populations, including women in an obstetric ward (88%), the crew of an aircraft carrier (58%), and prisoners (96%).2 However, there is no published research on the percentage of university students who are asymptomatic.
Methods
The University of Georgia (UGA) began classes on August 20, 2020. Shortly before the beginning of classes, UGA implemented a surveillance program for asymptomatic students, faculty, and staff, testing 300 to 450 people per day. Initially, during Weeks 1 and 2 of data collection, anyone could choose to be tested. In Weeks 3 and 4, students, faculty, and staff were randomly invited to participate.
Over the 4-week period beginning on August 17, we calculated the percent of positive cases in surveillance testing and applied this percentage to the entire UGA student population (n = 38,920) to estimate the total number of asymptomatic COVID-19 students each week.3 Data for symptomatic cases were also reported by the university on a weekly basis. This included positive tests from the University Health Center, as well as voluntary reporting using a smartphone app from other sites.
Positive tests in symptomatic individuals were not stratified by student vs nonstudent until Week 3; students comprised 95% of positive symptomatic reports in Week 3 and 99% in Week 4, so we conservatively estimated that 95% of symptomatic cases in Weeks 1 and 2 were students. These data were used to estimate the percentage of SARS-CoV-2–positive students who were asymptomatic.
Results
Our results are summarized in the table. The percentage of asymptomatic students testing positive in surveillance testing was 3.4% in Week 1 and rose steadily to 9% by Week 4. We estimated that there were 1303 asymptomatic cases among students in Week 1, increasing to 3487 asymptomatic positive students on campus by Week 4. The estimated percentage of asymptomatic students infected with SARS-CoV-2 ranged from 73% to 92.5% by week and was 81.1% overall.

Discussion
During the reporting period from August 17 to September 13, the 7-day moving average of new cases in Clarke County (home of UGA) increased from 30 to 83 per 100,000 persons/day (https://dph.georgia.gov/covid-19-daily-status-report). During this period, there were large increases in the number of infected students, more than 80% of whom were asymptomatic. With the assumption that anyone could be infected even if asymptomatic, these numbers highlight the importance for infection control to prevent potential spread within a community by taking universal precautions such as wearing a mask, following physical distancing guidelines, and handwashing.
Limitations. First, reporting of positive tests in symptomatic individuals is highly encouraged but not required. The large drop in symptomatic positive test reports between Weeks 3 and 4, with no change in test positivity in surveillance of asymptomatic students (8.9% vs 9%), suggests that students may have chosen to be tested elsewhere in conjunction with evaluation of their symptoms and/or not reported positive tests, possibly to avoid mandatory isolation and other restrictions on their activities. Further evidence to support no change in actual infection rates comes from testing for virus in wastewater, which also remained unchanged.4
Continue to: Second, each week's surveillance...
Second, each week’s surveillance population is not a true random sample, so extrapolating this estimate to the full student population could over- or undercount asymptomatic cases depending on the direction of bias (ie, healthy volunteer bias vs test avoidance by those with high-risk behaviors).
Finally, some students who were positive in surveillance testing may have been presymptomatic, rather than asymptomatic.
In conclusion, we estimate that approximately 80% of students infected with SARS-CoV-2 are asymptomatic. This is consistent with other studies in young adult populations.2
Mark H. Ebell, MD, MS
Cassie Chupp, MPH
Michelle Bentivegna, MPH
Department of Epidemiology and Biostatistics, College of Public Health, University of Georgia, Athens
[email protected]
The authors reported no potential conflict of interest relevant to this article.
Many individuals infected with SARS-CoV-2 never become symptomatic. In a South Korean study, these infected individuals remained asymptomatic for a prolonged period while maintaining the same viral load as symptomatic patients, suggesting that they are just as infectious.1 A narrative review found high rates of asymptomatic disease in several younger populations, including women in an obstetric ward (88%), the crew of an aircraft carrier (58%), and prisoners (96%).2 However, there is no published research on the percentage of university students who are asymptomatic.
Methods
The University of Georgia (UGA) began classes on August 20, 2020. Shortly before the beginning of classes, UGA implemented a surveillance program for asymptomatic students, faculty, and staff, testing 300 to 450 people per day. Initially, during Weeks 1 and 2 of data collection, anyone could choose to be tested. In Weeks 3 and 4, students, faculty, and staff were randomly invited to participate.
Over the 4-week period beginning on August 17, we calculated the percent of positive cases in surveillance testing and applied this percentage to the entire UGA student population (n = 38,920) to estimate the total number of asymptomatic COVID-19 students each week.3 Data for symptomatic cases were also reported by the university on a weekly basis. This included positive tests from the University Health Center, as well as voluntary reporting using a smartphone app from other sites.
Positive tests in symptomatic individuals were not stratified by student vs nonstudent until Week 3; students comprised 95% of positive symptomatic reports in Week 3 and 99% in Week 4, so we conservatively estimated that 95% of symptomatic cases in Weeks 1 and 2 were students. These data were used to estimate the percentage of SARS-CoV-2–positive students who were asymptomatic.
Results
Our results are summarized in the table. The percentage of asymptomatic students testing positive in surveillance testing was 3.4% in Week 1 and rose steadily to 9% by Week 4. We estimated that there were 1303 asymptomatic cases among students in Week 1, increasing to 3487 asymptomatic positive students on campus by Week 4. The estimated percentage of asymptomatic students infected with SARS-CoV-2 ranged from 73% to 92.5% by week and was 81.1% overall.

Discussion
During the reporting period from August 17 to September 13, the 7-day moving average of new cases in Clarke County (home of UGA) increased from 30 to 83 per 100,000 persons/day (https://dph.georgia.gov/covid-19-daily-status-report). During this period, there were large increases in the number of infected students, more than 80% of whom were asymptomatic. With the assumption that anyone could be infected even if asymptomatic, these numbers highlight the importance for infection control to prevent potential spread within a community by taking universal precautions such as wearing a mask, following physical distancing guidelines, and handwashing.
Limitations. First, reporting of positive tests in symptomatic individuals is highly encouraged but not required. The large drop in symptomatic positive test reports between Weeks 3 and 4, with no change in test positivity in surveillance of asymptomatic students (8.9% vs 9%), suggests that students may have chosen to be tested elsewhere in conjunction with evaluation of their symptoms and/or not reported positive tests, possibly to avoid mandatory isolation and other restrictions on their activities. Further evidence to support no change in actual infection rates comes from testing for virus in wastewater, which also remained unchanged.4
Continue to: Second, each week's surveillance...
Second, each week’s surveillance population is not a true random sample, so extrapolating this estimate to the full student population could over- or undercount asymptomatic cases depending on the direction of bias (ie, healthy volunteer bias vs test avoidance by those with high-risk behaviors).
Finally, some students who were positive in surveillance testing may have been presymptomatic, rather than asymptomatic.
In conclusion, we estimate that approximately 80% of students infected with SARS-CoV-2 are asymptomatic. This is consistent with other studies in young adult populations.2
Mark H. Ebell, MD, MS
Cassie Chupp, MPH
Michelle Bentivegna, MPH
Department of Epidemiology and Biostatistics, College of Public Health, University of Georgia, Athens
[email protected]
The authors reported no potential conflict of interest relevant to this article.
1. Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea [published online ahead of print August 6, 2020]. JAMA Intern Med. doi:10.1001/jamainternmed.2020.3862
2. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020;173:362-367.
3. UGA by the Numbers. University of Georgia Web site. www.uga.edu/facts.php. Updated August 2020. Accessed October 20, 2020.
4. Lott M, Norfolk W, Robertson M, et al. Wastewater surveillance for SARS-CoV-2 in Athens, GA. COVID-19 Portal: Center for the Ecology of Infectious Diseases, University of Georgia Web site. www.covid19.uga.edu/wastewater-athens.html. Updated October 15, 2020. Accessed October 20, 2020.
1. Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea [published online ahead of print August 6, 2020]. JAMA Intern Med. doi:10.1001/jamainternmed.2020.3862
2. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020;173:362-367.
3. UGA by the Numbers. University of Georgia Web site. www.uga.edu/facts.php. Updated August 2020. Accessed October 20, 2020.
4. Lott M, Norfolk W, Robertson M, et al. Wastewater surveillance for SARS-CoV-2 in Athens, GA. COVID-19 Portal: Center for the Ecology of Infectious Diseases, University of Georgia Web site. www.covid19.uga.edu/wastewater-athens.html. Updated October 15, 2020. Accessed October 20, 2020.
Pfizer vaccine data show 90% efficacy in early results
A vaccine candidate against SARS-CoV-2 has been found to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus, results from an interim analysis of a phase 3 study demonstrated.
BTN162b2, a messenger RNA–based vaccine candidate that requires two doses, is being developed by Pfizer and BioNTech SE independently of the Trump administration’s Operation Warp Speed. A global phase 3 clinical trial of BTN162b2 began on July 27 and has enrolled 43,538 participants to date; 42% of enrollees have racially and ethnically diverse backgrounds.
According to a press release issued by the two companies, 38,955 trial volunteers had received a second dose of either vaccine or placebo as of Nov. 8. An interim analysis of 94 individuals conducted by an independent data monitoring committee (DMC) found that the vaccine efficacy rate was above 90% 7 days after the second dose. This means that protection was achieved 28 days after the first vaccine dose.
“It’s promising in that it validates the genetic strategy – whether it’s mRNA vaccines or DNA vaccines,” Paul A. Offit, MD, told Medscape Medical News. Offit is a member of the US Food and Drug Administraiton’s COVID-19 Vaccine Advisory Committee. “All of them have the same approach, which is that they introduce the gene that codes for the coronavirus spike protein into the cell. Your cell makes the spike protein, and your immune system makes antibodies to the spike protein. At least in these preliminary data, which involved 94 people getting sick, it looks like it’s effective. That’s good. We knew that it seemed to work in experimental animals, but you never know until you put it into people.”
According to Pfizer and BioNTech SE, a final data analysis is planned once 164 confirmed COVID-19 cases have accrued. So far, the DMC has not reported any serious safety concerns. It recommends that the study continue to collect safety and efficacy data as planned. The companies plan to apply to the FDA for emergency use authorization soon after the required safety milestone is achieved.
Pfizer CEO Albert Bourla, DVM, PhD, added in a separate press release, “It’s important to note that we cannot apply for FDA Emergency Use Authorization based on these efficacy results alone. More data on safety is also needed, and we are continuing to accumulate that safety data as part of our ongoing clinical study.
“We estimate that a median of two months of safety data following the second and final dose of the vaccine candidate – required by FDA’s guidance for potential Emergency Use Authorization – will be available by the third week of November.”
Offit, professor of pediatrics in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, said that, if BTN162b2 is approved, administering it will be tricky. “This particular vaccine has to be shipped and stored at –70° C or –80° C, which we’ve never done before in this country,” he said. “That means maintaining the product on dry ice. That’s going to be a challenge for distribution, I think.”
Good news, but…
In the press release, BioNTech SE’s cofounder and CEO, Ugur Sahin, MD, characterized the findings as “a victory for innovation, science and a global collaborative effort. When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality.”
President-elect Joe Biden also weighed in, calling the results “excellent news” in a news release.
“At the same time, it is also important to understand that the end of the battle against COVID-19 is still months away,” he said. “This news follows a previously announced timeline by industry officials that forecast vaccine approval by late November. Even if that is achieved, and some Americans are vaccinated later this year, it will be many more months before there is widespread vaccination in this country.
“Today’s news does not change this urgent reality. Americans will have to rely on masking, distancing, contact tracing, hand washing, and other measures to keep themselves safe well into next year,” Biden added.
This article first appeared on Medscape.com.
A vaccine candidate against SARS-CoV-2 has been found to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus, results from an interim analysis of a phase 3 study demonstrated.
BTN162b2, a messenger RNA–based vaccine candidate that requires two doses, is being developed by Pfizer and BioNTech SE independently of the Trump administration’s Operation Warp Speed. A global phase 3 clinical trial of BTN162b2 began on July 27 and has enrolled 43,538 participants to date; 42% of enrollees have racially and ethnically diverse backgrounds.
According to a press release issued by the two companies, 38,955 trial volunteers had received a second dose of either vaccine or placebo as of Nov. 8. An interim analysis of 94 individuals conducted by an independent data monitoring committee (DMC) found that the vaccine efficacy rate was above 90% 7 days after the second dose. This means that protection was achieved 28 days after the first vaccine dose.
“It’s promising in that it validates the genetic strategy – whether it’s mRNA vaccines or DNA vaccines,” Paul A. Offit, MD, told Medscape Medical News. Offit is a member of the US Food and Drug Administraiton’s COVID-19 Vaccine Advisory Committee. “All of them have the same approach, which is that they introduce the gene that codes for the coronavirus spike protein into the cell. Your cell makes the spike protein, and your immune system makes antibodies to the spike protein. At least in these preliminary data, which involved 94 people getting sick, it looks like it’s effective. That’s good. We knew that it seemed to work in experimental animals, but you never know until you put it into people.”
According to Pfizer and BioNTech SE, a final data analysis is planned once 164 confirmed COVID-19 cases have accrued. So far, the DMC has not reported any serious safety concerns. It recommends that the study continue to collect safety and efficacy data as planned. The companies plan to apply to the FDA for emergency use authorization soon after the required safety milestone is achieved.
Pfizer CEO Albert Bourla, DVM, PhD, added in a separate press release, “It’s important to note that we cannot apply for FDA Emergency Use Authorization based on these efficacy results alone. More data on safety is also needed, and we are continuing to accumulate that safety data as part of our ongoing clinical study.
“We estimate that a median of two months of safety data following the second and final dose of the vaccine candidate – required by FDA’s guidance for potential Emergency Use Authorization – will be available by the third week of November.”
Offit, professor of pediatrics in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, said that, if BTN162b2 is approved, administering it will be tricky. “This particular vaccine has to be shipped and stored at –70° C or –80° C, which we’ve never done before in this country,” he said. “That means maintaining the product on dry ice. That’s going to be a challenge for distribution, I think.”
Good news, but…
In the press release, BioNTech SE’s cofounder and CEO, Ugur Sahin, MD, characterized the findings as “a victory for innovation, science and a global collaborative effort. When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality.”
President-elect Joe Biden also weighed in, calling the results “excellent news” in a news release.
“At the same time, it is also important to understand that the end of the battle against COVID-19 is still months away,” he said. “This news follows a previously announced timeline by industry officials that forecast vaccine approval by late November. Even if that is achieved, and some Americans are vaccinated later this year, it will be many more months before there is widespread vaccination in this country.
“Today’s news does not change this urgent reality. Americans will have to rely on masking, distancing, contact tracing, hand washing, and other measures to keep themselves safe well into next year,” Biden added.
This article first appeared on Medscape.com.
A vaccine candidate against SARS-CoV-2 has been found to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus, results from an interim analysis of a phase 3 study demonstrated.
BTN162b2, a messenger RNA–based vaccine candidate that requires two doses, is being developed by Pfizer and BioNTech SE independently of the Trump administration’s Operation Warp Speed. A global phase 3 clinical trial of BTN162b2 began on July 27 and has enrolled 43,538 participants to date; 42% of enrollees have racially and ethnically diverse backgrounds.
According to a press release issued by the two companies, 38,955 trial volunteers had received a second dose of either vaccine or placebo as of Nov. 8. An interim analysis of 94 individuals conducted by an independent data monitoring committee (DMC) found that the vaccine efficacy rate was above 90% 7 days after the second dose. This means that protection was achieved 28 days after the first vaccine dose.
“It’s promising in that it validates the genetic strategy – whether it’s mRNA vaccines or DNA vaccines,” Paul A. Offit, MD, told Medscape Medical News. Offit is a member of the US Food and Drug Administraiton’s COVID-19 Vaccine Advisory Committee. “All of them have the same approach, which is that they introduce the gene that codes for the coronavirus spike protein into the cell. Your cell makes the spike protein, and your immune system makes antibodies to the spike protein. At least in these preliminary data, which involved 94 people getting sick, it looks like it’s effective. That’s good. We knew that it seemed to work in experimental animals, but you never know until you put it into people.”
According to Pfizer and BioNTech SE, a final data analysis is planned once 164 confirmed COVID-19 cases have accrued. So far, the DMC has not reported any serious safety concerns. It recommends that the study continue to collect safety and efficacy data as planned. The companies plan to apply to the FDA for emergency use authorization soon after the required safety milestone is achieved.
Pfizer CEO Albert Bourla, DVM, PhD, added in a separate press release, “It’s important to note that we cannot apply for FDA Emergency Use Authorization based on these efficacy results alone. More data on safety is also needed, and we are continuing to accumulate that safety data as part of our ongoing clinical study.
“We estimate that a median of two months of safety data following the second and final dose of the vaccine candidate – required by FDA’s guidance for potential Emergency Use Authorization – will be available by the third week of November.”
Offit, professor of pediatrics in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, said that, if BTN162b2 is approved, administering it will be tricky. “This particular vaccine has to be shipped and stored at –70° C or –80° C, which we’ve never done before in this country,” he said. “That means maintaining the product on dry ice. That’s going to be a challenge for distribution, I think.”
Good news, but…
In the press release, BioNTech SE’s cofounder and CEO, Ugur Sahin, MD, characterized the findings as “a victory for innovation, science and a global collaborative effort. When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality.”
President-elect Joe Biden also weighed in, calling the results “excellent news” in a news release.
“At the same time, it is also important to understand that the end of the battle against COVID-19 is still months away,” he said. “This news follows a previously announced timeline by industry officials that forecast vaccine approval by late November. Even if that is achieved, and some Americans are vaccinated later this year, it will be many more months before there is widespread vaccination in this country.
“Today’s news does not change this urgent reality. Americans will have to rely on masking, distancing, contact tracing, hand washing, and other measures to keep themselves safe well into next year,” Biden added.
This article first appeared on Medscape.com.
Methotrexate users need tuberculosis tests in high-TB areas
People taking even low-dose methotrexate need tuberculosis screening and ongoing clinical care if they live in areas where TB is common, results of a study presented at the virtual annual meeting of the American College of Rheumatology suggest.
Coauthor Carol Hitchon, MD, MSc, a rheumatologist with the University of Manitoba in Winnipeg, who presented the findings, warned that methotrexate (MTX) users who also take corticosteroids or other immunosuppressants are at particular risk and need TB screening.
Current management guidelines for rheumatic disease address TB in relation to biologics, but not in relation to methotrexate, Dr. Hitchon said.
“We know that methotrexate is the foundational DMARD [disease-modifying antirheumatic drug] for many rheumatic diseases, especially rheumatoid arthritis,” Dr. Hitchon noted at a press conference. “It’s safe and effective when dosed properly. However, methotrexate does have the potential for significant liver toxicity as well as infection, particularly for infectious organisms that are targeted by cell-mediated immunity, and TB is one of those agents.”
Using multiple databases, researchers conducted a systematic review of the literature published from 1990 to 2018 on TB rates among people who take less than 30 mg of methotrexate a week. Of the 4,700 studies they examined, 31 fit the criteria for this analysis.
They collected data on tuberculosis incidence or new TB diagnoses vs. reactivation of latent TB infection as well as TB outcomes, such as pulmonary symptoms, dissemination, and mortality.
They found a modest increase in the risk of TB infections in the setting of low-dose methotrexate. In addition, rates of TB in people with rheumatic disease who are treated with either methotrexate or biologics are generally higher than in the general population.
They also found that methotrexate users had higher rates of the type of TB that spreads beyond a patient’s lungs, compared with the general population.
Safety of INH with methotrexate
Researchers also looked at the safety of isoniazid (INH), the antibiotic used to treat TB, and found that isoniazid-related liver toxicity and neutropenia were more common when people took the antibiotic along with methotrexate, but those effects were usually reversible.
TB is endemic in various regions around the world. Historically there hasn’t been much rheumatology capacity in many of these areas, but as that capacity increases more people who are at high risk for developing or reactivating TB will be receiving methotrexate for rheumatic diseases, Dr. Hitchon said.
“It’s prudent for people managing patients who may be at higher risk for TB either from where they live or from where they travel that we should have a high suspicion for TB and consider screening as part of our workup in the course of initiating treatment like methotrexate,” she said.
Narender Annapureddy, MD, a rheumatologist at Vanderbilt University, Nashville, Tenn., who was not involved in the research, pointed out that a limitation of the work is that only 27% of the studies are from developing countries, which are more likely to have endemic TB, and those studies had very few cases.
“This finding needs to be studied in larger populations in TB-endemic areas and in high-risk populations,” he said in an interview.
As for practice implications in the United States, Dr. Annapureddy noted that TB is rare in the United States and most of the cases occur in people born in other countries.
“This population may be at risk for TB and should probably be screened for TB before initiating methotrexate,” he said. “Since biologics are usually the next step, especially in RA after patients fail methotrexate, having information on TB status may also help guide management options after MTX failure.
“Since high-dose steroids are another important risk factor for TB activation,” Dr. Annapureddy continued, “rheumatologists should likely consider screening patients who are going to be on moderate to high doses of steroids with MTX.”
A version of this article originally appeared on Medscape.com.
People taking even low-dose methotrexate need tuberculosis screening and ongoing clinical care if they live in areas where TB is common, results of a study presented at the virtual annual meeting of the American College of Rheumatology suggest.
Coauthor Carol Hitchon, MD, MSc, a rheumatologist with the University of Manitoba in Winnipeg, who presented the findings, warned that methotrexate (MTX) users who also take corticosteroids or other immunosuppressants are at particular risk and need TB screening.
Current management guidelines for rheumatic disease address TB in relation to biologics, but not in relation to methotrexate, Dr. Hitchon said.
“We know that methotrexate is the foundational DMARD [disease-modifying antirheumatic drug] for many rheumatic diseases, especially rheumatoid arthritis,” Dr. Hitchon noted at a press conference. “It’s safe and effective when dosed properly. However, methotrexate does have the potential for significant liver toxicity as well as infection, particularly for infectious organisms that are targeted by cell-mediated immunity, and TB is one of those agents.”
Using multiple databases, researchers conducted a systematic review of the literature published from 1990 to 2018 on TB rates among people who take less than 30 mg of methotrexate a week. Of the 4,700 studies they examined, 31 fit the criteria for this analysis.
They collected data on tuberculosis incidence or new TB diagnoses vs. reactivation of latent TB infection as well as TB outcomes, such as pulmonary symptoms, dissemination, and mortality.
They found a modest increase in the risk of TB infections in the setting of low-dose methotrexate. In addition, rates of TB in people with rheumatic disease who are treated with either methotrexate or biologics are generally higher than in the general population.
They also found that methotrexate users had higher rates of the type of TB that spreads beyond a patient’s lungs, compared with the general population.
Safety of INH with methotrexate
Researchers also looked at the safety of isoniazid (INH), the antibiotic used to treat TB, and found that isoniazid-related liver toxicity and neutropenia were more common when people took the antibiotic along with methotrexate, but those effects were usually reversible.
TB is endemic in various regions around the world. Historically there hasn’t been much rheumatology capacity in many of these areas, but as that capacity increases more people who are at high risk for developing or reactivating TB will be receiving methotrexate for rheumatic diseases, Dr. Hitchon said.
“It’s prudent for people managing patients who may be at higher risk for TB either from where they live or from where they travel that we should have a high suspicion for TB and consider screening as part of our workup in the course of initiating treatment like methotrexate,” she said.
Narender Annapureddy, MD, a rheumatologist at Vanderbilt University, Nashville, Tenn., who was not involved in the research, pointed out that a limitation of the work is that only 27% of the studies are from developing countries, which are more likely to have endemic TB, and those studies had very few cases.
“This finding needs to be studied in larger populations in TB-endemic areas and in high-risk populations,” he said in an interview.
As for practice implications in the United States, Dr. Annapureddy noted that TB is rare in the United States and most of the cases occur in people born in other countries.
“This population may be at risk for TB and should probably be screened for TB before initiating methotrexate,” he said. “Since biologics are usually the next step, especially in RA after patients fail methotrexate, having information on TB status may also help guide management options after MTX failure.
“Since high-dose steroids are another important risk factor for TB activation,” Dr. Annapureddy continued, “rheumatologists should likely consider screening patients who are going to be on moderate to high doses of steroids with MTX.”
A version of this article originally appeared on Medscape.com.
People taking even low-dose methotrexate need tuberculosis screening and ongoing clinical care if they live in areas where TB is common, results of a study presented at the virtual annual meeting of the American College of Rheumatology suggest.
Coauthor Carol Hitchon, MD, MSc, a rheumatologist with the University of Manitoba in Winnipeg, who presented the findings, warned that methotrexate (MTX) users who also take corticosteroids or other immunosuppressants are at particular risk and need TB screening.
Current management guidelines for rheumatic disease address TB in relation to biologics, but not in relation to methotrexate, Dr. Hitchon said.
“We know that methotrexate is the foundational DMARD [disease-modifying antirheumatic drug] for many rheumatic diseases, especially rheumatoid arthritis,” Dr. Hitchon noted at a press conference. “It’s safe and effective when dosed properly. However, methotrexate does have the potential for significant liver toxicity as well as infection, particularly for infectious organisms that are targeted by cell-mediated immunity, and TB is one of those agents.”
Using multiple databases, researchers conducted a systematic review of the literature published from 1990 to 2018 on TB rates among people who take less than 30 mg of methotrexate a week. Of the 4,700 studies they examined, 31 fit the criteria for this analysis.
They collected data on tuberculosis incidence or new TB diagnoses vs. reactivation of latent TB infection as well as TB outcomes, such as pulmonary symptoms, dissemination, and mortality.
They found a modest increase in the risk of TB infections in the setting of low-dose methotrexate. In addition, rates of TB in people with rheumatic disease who are treated with either methotrexate or biologics are generally higher than in the general population.
They also found that methotrexate users had higher rates of the type of TB that spreads beyond a patient’s lungs, compared with the general population.
Safety of INH with methotrexate
Researchers also looked at the safety of isoniazid (INH), the antibiotic used to treat TB, and found that isoniazid-related liver toxicity and neutropenia were more common when people took the antibiotic along with methotrexate, but those effects were usually reversible.
TB is endemic in various regions around the world. Historically there hasn’t been much rheumatology capacity in many of these areas, but as that capacity increases more people who are at high risk for developing or reactivating TB will be receiving methotrexate for rheumatic diseases, Dr. Hitchon said.
“It’s prudent for people managing patients who may be at higher risk for TB either from where they live or from where they travel that we should have a high suspicion for TB and consider screening as part of our workup in the course of initiating treatment like methotrexate,” she said.
Narender Annapureddy, MD, a rheumatologist at Vanderbilt University, Nashville, Tenn., who was not involved in the research, pointed out that a limitation of the work is that only 27% of the studies are from developing countries, which are more likely to have endemic TB, and those studies had very few cases.
“This finding needs to be studied in larger populations in TB-endemic areas and in high-risk populations,” he said in an interview.
As for practice implications in the United States, Dr. Annapureddy noted that TB is rare in the United States and most of the cases occur in people born in other countries.
“This population may be at risk for TB and should probably be screened for TB before initiating methotrexate,” he said. “Since biologics are usually the next step, especially in RA after patients fail methotrexate, having information on TB status may also help guide management options after MTX failure.
“Since high-dose steroids are another important risk factor for TB activation,” Dr. Annapureddy continued, “rheumatologists should likely consider screening patients who are going to be on moderate to high doses of steroids with MTX.”
A version of this article originally appeared on Medscape.com.
What’s Eating You? Human Flea (Pulex irritans)
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.

Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.
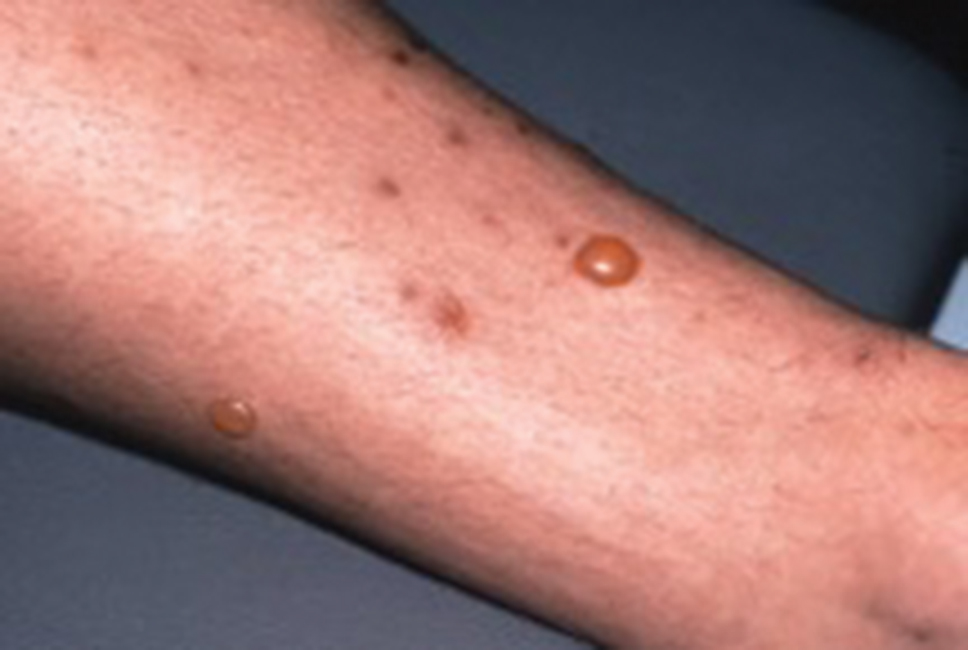
Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.

Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.

Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.

Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.

Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
Practice Points
- The human flea, Pulex irritans, is a vector for various human diseases including the bubonic plague, bartonellosis, and rickettsioses.
- Presenting symptoms of flea bites include intensely pruritic, urticarial to vesicular papules on exposed areas of skin.
- The primary method of flea control includes a combination of insecticidal products and insect growth regulators.
Aging with HIV adds to comorbidity burden
The age of antiretroviral therapy (ART) for HIV is in its third decade, and many of the patients who live in areas of the world fortunate enough to have had early access to therapy have now lived for several decades with complications of HIV and viral suppressive therapy.
But while the life-expectancy of persons with HIV has approached that of noninfected persons over the last 20 years, the higher burden of comorbidities for aging patients with HIV has remained largely the same, according to an epidemiologist who specializes in HIV/AIDS research and aging.
“The pathways from HIV and its treatments to comorbidities are very long and winding, spanning a life course. Social determinants of health and individual risk factors also play an important role, and must be considered,” said Keri N. Althoff, PhD, MPH, of Johns Hopkins University, Baltimore.
Dr. Althoff discussed long-term complications of HIV and its treatment in a virtual symposium during an annual scientific meeting on infectious diseases.
“Many urban HIV providers have an increased proportion of patients who are older long-term survivors of the epidemic. Many, but not all of the comorbidities (including cardiovascular, neurocognitive, renal, and malignancies) have been associated with age, long-term HIV infection, especially uncontrolled HIV infection, and low CD4 nadirs,” commented Harry Lampiris, MD, professor of clinical medicine at the University of California, San Francisco.
“An increasing number of patients are experiencing geriatric syndromes (especially problems with mobility, cognitive decline, food insecurity, polypharmacy, and social isolation) at younger ages than HIV-negative populations,” he added.
Dr. Lampiris, who moderated the session where Dr. Althoff presented her findings, commented on it in an interview, but was not involved in her research.
Pathways to comorbidity
The three primary pathways to comorbidities in people with HIV infections are as follows, according to Dr. Athloff:
- The virus itself, with its associated inflammation, immunosuppression, immune activation, and AIDS.
- HIV therapies, beginning with the notoriously toxic dideoxynucleoside analogues or “d-drugs,” and following with subsequent generations of newer, less toxic agents.
- Individual risk factors, including smoking, stress, diet, exercise, and environment.
Cardiovascular and renal complications
Persons with HIV have an approximately twofold higher risk for major adverse cardiovascular events (myocardial infarction, stroke) compared with persons without HIV. Conditions contributing to cardiovascular disease including hypertension, diabetes, and hyperlipidemia are also significantly higher among persons with HIV, Dr. Althoff said.
Hypertension among persons with HIV from the ages 60-69 years is especially high for Black men and to a lesser degree non-Black men, compared with either White or Black women, she noted.
Pathways to renal disease in persons with HIV include diabetes and hypertension, as well as therapies to treat them, hepatitis B and C coinfection, HIV-associated nephropathy, and immune complex kidney disease, as well as chronic kidney disease resulting from acute kidney injury related to therapy.
“Cardiovascular disease and kidney disease are excellent examples of why the life-course perspective is essential when caring for people with HIV. For those diagnosed with HIV at younger ages, there are points of intervention along the decades-long path, and the timing and implementation of the most effective intervention may preserve comorbidity-free years,” Dr. Althoff said.
Prevention and screening interventions to lower risk for future heart- and kidney-related comorbidities include smoking cessation and lifestyle optimization (diet, exercise, mental health), as well as lipid-lowering medications to lower risk for cardiovascular events.
Liver comorbidities
“Primary drivers of liver disease are social determinants of health and individual lifestyle risk factors that share the same pathways as HIV, resulting in this increased burden of liver disease in people with HIV,” she said.
Risk factors include alcoholic liver disease, nonalcoholic fatty liver disease, hepatitis B and C coinfection, drug use, autoimmune disease, and aging. These risk factors contribute to oxidative stress, mitochondrial injury, lipotoxicity, cytotoxicity, and other mechanisms that can lead to fibrosis, cirrhosis, hepatocellular carcinoma, and end-stage liver disease.
“I want to be sure to acknowledge the importance of liver disease as a comorbidity among people with HIV. Liver disease accounts for nearly 20% of mortality in persons with HIV,” she said.
Neurocognitive problems
HIV has been linked to neurocognitive decline since the beginning of the epidemic, Dr. Althoff noted. The term HIV-associated neurocognitive disorders encompasses the broad spectrum of cognitive effects of HIV, from asymptomatic illness to AIDS-related dementia. Estimates of cognitive impairment in people with HIV range from 14% to 64% across various study populations, but diagnosing and treating it in the community can be challenging.
“Routine monitoring of cognition is often just out of reach in the clinical setting, due to the time it takes to use validated tools. We need a deeper toolbox of quick and validated tools calibrated to people with HIV in order to accurately monitor cognition,” she said.
She noted that the average age of onset of Alzheimer’s disease in the general population is 80 years, and that relatively few people with HIV infection have reached that age.
“But before the population age distribution shifts to the older ages, we can do more to monitor cognition in people with HIV,” she added.
In addition to HIV, factors that can contribute to worse neurocognitive outcomes include major depressive disorder, occurring in and estimated 20%-40% of adults with HIV versus 8% of the U.S. population, generalized anxiety disorder (10%-25% vs. 3%), bipolar disorder (3%-9% vs. 3%), schizophrenia (4%-15% vs. 1%), and posttraumatic stress disorder (10%-30% vs. 8%).
Substance use and polypharmacy, common among adults with HIV, can also contribute to cognitive decline, she said.
Decreased mobility
The Multicenter AIDS Cohort Study (MACS) showed that decreased mobility, defined as a gait speed less than 1 m/sec, occurred earlier in life among HIV-positive men than in HIV-negative men.
In the general aging population, slow gait speed is a predictor for lower extremity limitations, hospitalization, and death, and in more recent MACS studies was associated with increased hemoglobin A1C levels, as well as neurocognitive impairment.
“Hemoglobin A1C is an intervenable target, and perhaps it will help to slow the decline in gait speed,” Dr. Althoff said.
Reduce ‘healthspan’ disparities
The goal for treating aging adults with HIV “is to reduce the disparity in healthspan between people with HIV compared to people without HIV by delaying or eliminating the onset of comorbidities among people with HIV,” she said.
The gerontological concept of extending “healthspan” – the duration of life without significant comorbidities – is to target common mechanisms of aging, thereby delaying the onset of more than one age-related disease at the same time.
“Crude translation of this concept to the population of aging with HIV includes reducing that gap in comorbidity-free survival in people with versus without HIV,” she said.
Modification of care models from geriatrics may help infectious disease specialists manage adults with HIV who have increasingly complex needs.
For example, the geriatric “5 M” model emphasizes focusing on issues of mind (mentation, dementia, delirium, depression), mobility (impaired gait and balance, as well as fall prevention), medications (reducing polypharmacy, optimal prescribing), multicomplexity (multiple morbidities and complex bio-psycho-social situations), and “matters most” (each patient’s individual meaningful health outcome goals and care preferences).
Changing exposures that may influence the pattern of comorbidities for patients with HIV in the future include earlier start on ART, shorter duration of uncontrolled viremia, compared with older populations, newer and less toxic ARTs, long-term viral suppression, and risk factor interventions, Dr. Althoff concluded.
Dr. Lampiris noted that “patients who have initiated therapy in the last 5-10 years are more likely to initiate antiretroviral therapy at higher CD4 counts, and less likely to experience long-term toxicities of antiretroviral therapy. However, African Americans, Hispanics and HIV-positive women continue to lag behind others with regard to timely initiation of treatment.
“In addition there are toxicities associated with the newer agents, particularly weight gain, which disproportionately affect African Americans and women and which may be made worse by poverty, food insecurity, and other health-related behaviors.”
Dr. Athloff’s work is supported by grants from the National Institutes for Health. She disclosed serving as a consultant to the NIH-funded All of US study and to MediQ, and as an adviser to TrioHealth. Dr. Lampiris reported having no disclosures.
The age of antiretroviral therapy (ART) for HIV is in its third decade, and many of the patients who live in areas of the world fortunate enough to have had early access to therapy have now lived for several decades with complications of HIV and viral suppressive therapy.
But while the life-expectancy of persons with HIV has approached that of noninfected persons over the last 20 years, the higher burden of comorbidities for aging patients with HIV has remained largely the same, according to an epidemiologist who specializes in HIV/AIDS research and aging.
“The pathways from HIV and its treatments to comorbidities are very long and winding, spanning a life course. Social determinants of health and individual risk factors also play an important role, and must be considered,” said Keri N. Althoff, PhD, MPH, of Johns Hopkins University, Baltimore.
Dr. Althoff discussed long-term complications of HIV and its treatment in a virtual symposium during an annual scientific meeting on infectious diseases.
“Many urban HIV providers have an increased proportion of patients who are older long-term survivors of the epidemic. Many, but not all of the comorbidities (including cardiovascular, neurocognitive, renal, and malignancies) have been associated with age, long-term HIV infection, especially uncontrolled HIV infection, and low CD4 nadirs,” commented Harry Lampiris, MD, professor of clinical medicine at the University of California, San Francisco.
“An increasing number of patients are experiencing geriatric syndromes (especially problems with mobility, cognitive decline, food insecurity, polypharmacy, and social isolation) at younger ages than HIV-negative populations,” he added.
Dr. Lampiris, who moderated the session where Dr. Althoff presented her findings, commented on it in an interview, but was not involved in her research.
Pathways to comorbidity
The three primary pathways to comorbidities in people with HIV infections are as follows, according to Dr. Athloff:
- The virus itself, with its associated inflammation, immunosuppression, immune activation, and AIDS.
- HIV therapies, beginning with the notoriously toxic dideoxynucleoside analogues or “d-drugs,” and following with subsequent generations of newer, less toxic agents.
- Individual risk factors, including smoking, stress, diet, exercise, and environment.
Cardiovascular and renal complications
Persons with HIV have an approximately twofold higher risk for major adverse cardiovascular events (myocardial infarction, stroke) compared with persons without HIV. Conditions contributing to cardiovascular disease including hypertension, diabetes, and hyperlipidemia are also significantly higher among persons with HIV, Dr. Althoff said.
Hypertension among persons with HIV from the ages 60-69 years is especially high for Black men and to a lesser degree non-Black men, compared with either White or Black women, she noted.
Pathways to renal disease in persons with HIV include diabetes and hypertension, as well as therapies to treat them, hepatitis B and C coinfection, HIV-associated nephropathy, and immune complex kidney disease, as well as chronic kidney disease resulting from acute kidney injury related to therapy.
“Cardiovascular disease and kidney disease are excellent examples of why the life-course perspective is essential when caring for people with HIV. For those diagnosed with HIV at younger ages, there are points of intervention along the decades-long path, and the timing and implementation of the most effective intervention may preserve comorbidity-free years,” Dr. Althoff said.
Prevention and screening interventions to lower risk for future heart- and kidney-related comorbidities include smoking cessation and lifestyle optimization (diet, exercise, mental health), as well as lipid-lowering medications to lower risk for cardiovascular events.
Liver comorbidities
“Primary drivers of liver disease are social determinants of health and individual lifestyle risk factors that share the same pathways as HIV, resulting in this increased burden of liver disease in people with HIV,” she said.
Risk factors include alcoholic liver disease, nonalcoholic fatty liver disease, hepatitis B and C coinfection, drug use, autoimmune disease, and aging. These risk factors contribute to oxidative stress, mitochondrial injury, lipotoxicity, cytotoxicity, and other mechanisms that can lead to fibrosis, cirrhosis, hepatocellular carcinoma, and end-stage liver disease.
“I want to be sure to acknowledge the importance of liver disease as a comorbidity among people with HIV. Liver disease accounts for nearly 20% of mortality in persons with HIV,” she said.
Neurocognitive problems
HIV has been linked to neurocognitive decline since the beginning of the epidemic, Dr. Althoff noted. The term HIV-associated neurocognitive disorders encompasses the broad spectrum of cognitive effects of HIV, from asymptomatic illness to AIDS-related dementia. Estimates of cognitive impairment in people with HIV range from 14% to 64% across various study populations, but diagnosing and treating it in the community can be challenging.
“Routine monitoring of cognition is often just out of reach in the clinical setting, due to the time it takes to use validated tools. We need a deeper toolbox of quick and validated tools calibrated to people with HIV in order to accurately monitor cognition,” she said.
She noted that the average age of onset of Alzheimer’s disease in the general population is 80 years, and that relatively few people with HIV infection have reached that age.
“But before the population age distribution shifts to the older ages, we can do more to monitor cognition in people with HIV,” she added.
In addition to HIV, factors that can contribute to worse neurocognitive outcomes include major depressive disorder, occurring in and estimated 20%-40% of adults with HIV versus 8% of the U.S. population, generalized anxiety disorder (10%-25% vs. 3%), bipolar disorder (3%-9% vs. 3%), schizophrenia (4%-15% vs. 1%), and posttraumatic stress disorder (10%-30% vs. 8%).
Substance use and polypharmacy, common among adults with HIV, can also contribute to cognitive decline, she said.
Decreased mobility
The Multicenter AIDS Cohort Study (MACS) showed that decreased mobility, defined as a gait speed less than 1 m/sec, occurred earlier in life among HIV-positive men than in HIV-negative men.
In the general aging population, slow gait speed is a predictor for lower extremity limitations, hospitalization, and death, and in more recent MACS studies was associated with increased hemoglobin A1C levels, as well as neurocognitive impairment.
“Hemoglobin A1C is an intervenable target, and perhaps it will help to slow the decline in gait speed,” Dr. Althoff said.
Reduce ‘healthspan’ disparities
The goal for treating aging adults with HIV “is to reduce the disparity in healthspan between people with HIV compared to people without HIV by delaying or eliminating the onset of comorbidities among people with HIV,” she said.
The gerontological concept of extending “healthspan” – the duration of life without significant comorbidities – is to target common mechanisms of aging, thereby delaying the onset of more than one age-related disease at the same time.
“Crude translation of this concept to the population of aging with HIV includes reducing that gap in comorbidity-free survival in people with versus without HIV,” she said.
Modification of care models from geriatrics may help infectious disease specialists manage adults with HIV who have increasingly complex needs.
For example, the geriatric “5 M” model emphasizes focusing on issues of mind (mentation, dementia, delirium, depression), mobility (impaired gait and balance, as well as fall prevention), medications (reducing polypharmacy, optimal prescribing), multicomplexity (multiple morbidities and complex bio-psycho-social situations), and “matters most” (each patient’s individual meaningful health outcome goals and care preferences).
Changing exposures that may influence the pattern of comorbidities for patients with HIV in the future include earlier start on ART, shorter duration of uncontrolled viremia, compared with older populations, newer and less toxic ARTs, long-term viral suppression, and risk factor interventions, Dr. Althoff concluded.
Dr. Lampiris noted that “patients who have initiated therapy in the last 5-10 years are more likely to initiate antiretroviral therapy at higher CD4 counts, and less likely to experience long-term toxicities of antiretroviral therapy. However, African Americans, Hispanics and HIV-positive women continue to lag behind others with regard to timely initiation of treatment.
“In addition there are toxicities associated with the newer agents, particularly weight gain, which disproportionately affect African Americans and women and which may be made worse by poverty, food insecurity, and other health-related behaviors.”
Dr. Athloff’s work is supported by grants from the National Institutes for Health. She disclosed serving as a consultant to the NIH-funded All of US study and to MediQ, and as an adviser to TrioHealth. Dr. Lampiris reported having no disclosures.
The age of antiretroviral therapy (ART) for HIV is in its third decade, and many of the patients who live in areas of the world fortunate enough to have had early access to therapy have now lived for several decades with complications of HIV and viral suppressive therapy.
But while the life-expectancy of persons with HIV has approached that of noninfected persons over the last 20 years, the higher burden of comorbidities for aging patients with HIV has remained largely the same, according to an epidemiologist who specializes in HIV/AIDS research and aging.
“The pathways from HIV and its treatments to comorbidities are very long and winding, spanning a life course. Social determinants of health and individual risk factors also play an important role, and must be considered,” said Keri N. Althoff, PhD, MPH, of Johns Hopkins University, Baltimore.
Dr. Althoff discussed long-term complications of HIV and its treatment in a virtual symposium during an annual scientific meeting on infectious diseases.
“Many urban HIV providers have an increased proportion of patients who are older long-term survivors of the epidemic. Many, but not all of the comorbidities (including cardiovascular, neurocognitive, renal, and malignancies) have been associated with age, long-term HIV infection, especially uncontrolled HIV infection, and low CD4 nadirs,” commented Harry Lampiris, MD, professor of clinical medicine at the University of California, San Francisco.
“An increasing number of patients are experiencing geriatric syndromes (especially problems with mobility, cognitive decline, food insecurity, polypharmacy, and social isolation) at younger ages than HIV-negative populations,” he added.
Dr. Lampiris, who moderated the session where Dr. Althoff presented her findings, commented on it in an interview, but was not involved in her research.
Pathways to comorbidity
The three primary pathways to comorbidities in people with HIV infections are as follows, according to Dr. Athloff:
- The virus itself, with its associated inflammation, immunosuppression, immune activation, and AIDS.
- HIV therapies, beginning with the notoriously toxic dideoxynucleoside analogues or “d-drugs,” and following with subsequent generations of newer, less toxic agents.
- Individual risk factors, including smoking, stress, diet, exercise, and environment.
Cardiovascular and renal complications
Persons with HIV have an approximately twofold higher risk for major adverse cardiovascular events (myocardial infarction, stroke) compared with persons without HIV. Conditions contributing to cardiovascular disease including hypertension, diabetes, and hyperlipidemia are also significantly higher among persons with HIV, Dr. Althoff said.
Hypertension among persons with HIV from the ages 60-69 years is especially high for Black men and to a lesser degree non-Black men, compared with either White or Black women, she noted.
Pathways to renal disease in persons with HIV include diabetes and hypertension, as well as therapies to treat them, hepatitis B and C coinfection, HIV-associated nephropathy, and immune complex kidney disease, as well as chronic kidney disease resulting from acute kidney injury related to therapy.
“Cardiovascular disease and kidney disease are excellent examples of why the life-course perspective is essential when caring for people with HIV. For those diagnosed with HIV at younger ages, there are points of intervention along the decades-long path, and the timing and implementation of the most effective intervention may preserve comorbidity-free years,” Dr. Althoff said.
Prevention and screening interventions to lower risk for future heart- and kidney-related comorbidities include smoking cessation and lifestyle optimization (diet, exercise, mental health), as well as lipid-lowering medications to lower risk for cardiovascular events.
Liver comorbidities
“Primary drivers of liver disease are social determinants of health and individual lifestyle risk factors that share the same pathways as HIV, resulting in this increased burden of liver disease in people with HIV,” she said.
Risk factors include alcoholic liver disease, nonalcoholic fatty liver disease, hepatitis B and C coinfection, drug use, autoimmune disease, and aging. These risk factors contribute to oxidative stress, mitochondrial injury, lipotoxicity, cytotoxicity, and other mechanisms that can lead to fibrosis, cirrhosis, hepatocellular carcinoma, and end-stage liver disease.
“I want to be sure to acknowledge the importance of liver disease as a comorbidity among people with HIV. Liver disease accounts for nearly 20% of mortality in persons with HIV,” she said.
Neurocognitive problems
HIV has been linked to neurocognitive decline since the beginning of the epidemic, Dr. Althoff noted. The term HIV-associated neurocognitive disorders encompasses the broad spectrum of cognitive effects of HIV, from asymptomatic illness to AIDS-related dementia. Estimates of cognitive impairment in people with HIV range from 14% to 64% across various study populations, but diagnosing and treating it in the community can be challenging.
“Routine monitoring of cognition is often just out of reach in the clinical setting, due to the time it takes to use validated tools. We need a deeper toolbox of quick and validated tools calibrated to people with HIV in order to accurately monitor cognition,” she said.
She noted that the average age of onset of Alzheimer’s disease in the general population is 80 years, and that relatively few people with HIV infection have reached that age.
“But before the population age distribution shifts to the older ages, we can do more to monitor cognition in people with HIV,” she added.
In addition to HIV, factors that can contribute to worse neurocognitive outcomes include major depressive disorder, occurring in and estimated 20%-40% of adults with HIV versus 8% of the U.S. population, generalized anxiety disorder (10%-25% vs. 3%), bipolar disorder (3%-9% vs. 3%), schizophrenia (4%-15% vs. 1%), and posttraumatic stress disorder (10%-30% vs. 8%).
Substance use and polypharmacy, common among adults with HIV, can also contribute to cognitive decline, she said.
Decreased mobility
The Multicenter AIDS Cohort Study (MACS) showed that decreased mobility, defined as a gait speed less than 1 m/sec, occurred earlier in life among HIV-positive men than in HIV-negative men.
In the general aging population, slow gait speed is a predictor for lower extremity limitations, hospitalization, and death, and in more recent MACS studies was associated with increased hemoglobin A1C levels, as well as neurocognitive impairment.
“Hemoglobin A1C is an intervenable target, and perhaps it will help to slow the decline in gait speed,” Dr. Althoff said.
Reduce ‘healthspan’ disparities
The goal for treating aging adults with HIV “is to reduce the disparity in healthspan between people with HIV compared to people without HIV by delaying or eliminating the onset of comorbidities among people with HIV,” she said.
The gerontological concept of extending “healthspan” – the duration of life without significant comorbidities – is to target common mechanisms of aging, thereby delaying the onset of more than one age-related disease at the same time.
“Crude translation of this concept to the population of aging with HIV includes reducing that gap in comorbidity-free survival in people with versus without HIV,” she said.
Modification of care models from geriatrics may help infectious disease specialists manage adults with HIV who have increasingly complex needs.
For example, the geriatric “5 M” model emphasizes focusing on issues of mind (mentation, dementia, delirium, depression), mobility (impaired gait and balance, as well as fall prevention), medications (reducing polypharmacy, optimal prescribing), multicomplexity (multiple morbidities and complex bio-psycho-social situations), and “matters most” (each patient’s individual meaningful health outcome goals and care preferences).
Changing exposures that may influence the pattern of comorbidities for patients with HIV in the future include earlier start on ART, shorter duration of uncontrolled viremia, compared with older populations, newer and less toxic ARTs, long-term viral suppression, and risk factor interventions, Dr. Althoff concluded.
Dr. Lampiris noted that “patients who have initiated therapy in the last 5-10 years are more likely to initiate antiretroviral therapy at higher CD4 counts, and less likely to experience long-term toxicities of antiretroviral therapy. However, African Americans, Hispanics and HIV-positive women continue to lag behind others with regard to timely initiation of treatment.
“In addition there are toxicities associated with the newer agents, particularly weight gain, which disproportionately affect African Americans and women and which may be made worse by poverty, food insecurity, and other health-related behaviors.”
Dr. Athloff’s work is supported by grants from the National Institutes for Health. She disclosed serving as a consultant to the NIH-funded All of US study and to MediQ, and as an adviser to TrioHealth. Dr. Lampiris reported having no disclosures.
FROM IDWEEK 2020
Biometric changes on fitness trackers, smartwatches detect COVID-19
A smartphone app that combines passively collected physiologic data from wearable devices, such as fitness trackers, and self-reported symptoms can discriminate between COVID-19–positive and –negative individuals among those who report symptoms, new data suggest.
After analyzing data from more than 30,000 participants, researchers from the Digital Engagement and Tracking for Early Control and Treatment (DETECT) study concluded that adding individual changes in sensor data improves models based on symptoms alone for differentiating symptomatic persons who are COVID-19 positive and symptomatic persons who are COVID-19 negative.
The combination can potentially identify infection clusters before wider community spread occurs, Giorgio Quer, PhD, and colleagues report in an article published online Oct. 29 in Nature Medicine. DETECT investigators note that marrying participant-reported symptoms with personal sensor data, such as deviation from normal sleep duration and resting heart rate, resulted in an area under the curve (AUC) of 0.80 (interquartile range [IQR], 0.73-0.86) for differentiating between symptomatic individuals who were positive and those who were negative for COVID-19.
“By better characterizing each individual’s unique baseline, you can then identify changes that may indicate that someone has a viral illness,” said Dr. Quer, director of artificial intelligence at Scripps Research Translational Institute in La Jolla, Calif. “In previous research, we found that the proportion of individuals with elevated resting heart rate and sleep duration compared with their normal could significantly improve real-time detection of influenza-like illness rates at the state level,” he said in an interview.
Thus, continuous passively captured data may be a useful adjunct to bricks-and-mortar site testing, which is generally a one-off or infrequent sampling assay and is not always easily accessible, he added. Furthermore, traditional screening with temperature and symptom reporting is inadequate. An elevation in temperature is not as common as frequently believed for people who test positive for COVID-19, Dr. Quer continued. “Early identification via sensor variables of those who are presymptomatic or even asymptomatic would be especially valuable, as people may potentially be infectious during this period, and early detection is the ultimate goal,” Dr. Quer said.
According to his group, adding these physiologic changes from baseline values significantly outperformed detection (P < .01) using a British model described in an earlier study by by Cristina Menni, PhD, and associates. That method, in which symptoms were considered alone, yielded an AUC of 0.71 (IQR, 0.63-0.79).
According to Dr. Quer, one in five Americans currently wear an electronic device. “If we could enroll even a small percentage of these individuals, we’d be able to potentially identify clusters before they have the opportunity to spread,” he said.
DETECT study details
During the period March 15 to June 7, 2020, the study enrolled 30,529 participants from all 50 states. They ranged in age from younger than 35 years (23.1%) to older than 65 years (12.8%); the majority (63.5%) were aged 35-65 years, and 62% were women. Sensor devices in use by the cohort included Fitbit activity trackers (78.4%) and Apple HealthKit (31.2%).
Participants downloaded an app called MyDataHelps, which collects smartwatch and activity tracker information, including self-reported symptoms and diagnostic testing results. The app also monitors changes from baseline in resting heart rate, sleep duration, and physical activity, as measured by steps.
Overall, 3,811 participants reported having at least one symptom of some kind (e.g., fatigue, cough, dyspnea, loss of taste or smell). Of these, 54 reported testing positive for COVID-19, and 279 reported testing negative.
Sleep and activity were significantly different for the positive and negative groups, with an AUC of 0.68 (IQR, 0.57-0.79) for the sleep metric and 0.69 (IQR, 0.61-0.77) for the activity metric, suggesting that these parameters were more affected in COVID-19–positive participants.
When the investigators combined resting heart rate, sleep, and activity into a single metric, predictive performance improved to an AUC of 0.72 (IQR, 0.64-0.80).
The next step, Dr. Quer said, is to include an alert to notify users of possible infection.
Alerting users to possible COVID-19 infection
In a similar study, an alert feature was already incorporated. The study, led by Michael P. Snyder, PhD, director of the Center for Genomics and Personalized Medicine at Stanford (Calif.) University, will soon be published online in Nature Biomedical Engineering. In that study, presymptomatic detection of COVID-19 was achieved in more than 80% of participants using resting heart rate.
“The median is 4 days prior to symptom formation,” Dr. Snyder said in an interview. “We have an alarm system to notify people when their heart rate is elevated. So a positive signal from a smartwatch can be used to follow up by polymerase chain reaction [testing].”
Dr. Snyder said these approaches offer a roadmap to containing widespread infections. “Public health authorities need to be open to these technologies and begin incorporating them into their tracking,” he said. “Right now, people do temperature checks, which are of limited value. Resting heart rate is much better information.”
Although the DETECT researchers have not yet received feedback on their results, they believe public health authorities could recommend the use of such apps. “These are devices that people routinely wear for tracking their fitness and sleep, so it would be relatively easy to use the data for viral illness tracking,” said co–lead author Jennifer Radin, PhD, an epidemiologist at Scripps. “Testing resources are still limited and don’t allow for routine serial testing of individuals who may be asymptomatic or presymptomatic. Wearables can offer a different way to routinely monitor and screen people for changes in their data that may indicate COVID-19.”
The marshaling of data through consumer digital platforms to fight the coronavirus is gaining ground. New York State and New Jersey are already embracing smartphone apps to alert individuals to possible exposure to the virus.
More than 710,000 New Yorkers have downloaded the COVID NY Alert app, launched in October to help protect individuals and communities from COVID-19 by sending alerts without compromising privacy or personal information. “Upon receiving a notification about a potential exposure, users are then able to self-quarantine, get tested, and reduce the potential exposure risk to family, friends, coworkers, and others,” Jonah Bruno, a spokesperson for the New York State Department of Health, said in an interview.
And recently the Mayo Clinic and Safe Health Systems launched a platform to store COVID-19 testing and vaccination data.
Both the Scripps and Stanford platforms are part of a global technologic response to the COVID-19 pandemic. Prospective studies, led by device manufacturers and academic institutions, allow individuals to voluntarily share sensor and clinical data to address the crisis. Similar approaches have been used to track COVID-19 in large populations in Germany via the Corona Data Donation app.
The study by Dr. Quer and colleagues was funded by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. One coauthor reported grants from Janssen and personal fees from Otsuka and Livongo outside of the submitted work. The other authors have disclosed no relevant financial relationships. Dr. Snyder has ties to Personalis, Qbio, January, SensOmics, Protos, Mirvie, and Oralome.
A version of this article originally appeared on Medscape.com.
A smartphone app that combines passively collected physiologic data from wearable devices, such as fitness trackers, and self-reported symptoms can discriminate between COVID-19–positive and –negative individuals among those who report symptoms, new data suggest.
After analyzing data from more than 30,000 participants, researchers from the Digital Engagement and Tracking for Early Control and Treatment (DETECT) study concluded that adding individual changes in sensor data improves models based on symptoms alone for differentiating symptomatic persons who are COVID-19 positive and symptomatic persons who are COVID-19 negative.
The combination can potentially identify infection clusters before wider community spread occurs, Giorgio Quer, PhD, and colleagues report in an article published online Oct. 29 in Nature Medicine. DETECT investigators note that marrying participant-reported symptoms with personal sensor data, such as deviation from normal sleep duration and resting heart rate, resulted in an area under the curve (AUC) of 0.80 (interquartile range [IQR], 0.73-0.86) for differentiating between symptomatic individuals who were positive and those who were negative for COVID-19.
“By better characterizing each individual’s unique baseline, you can then identify changes that may indicate that someone has a viral illness,” said Dr. Quer, director of artificial intelligence at Scripps Research Translational Institute in La Jolla, Calif. “In previous research, we found that the proportion of individuals with elevated resting heart rate and sleep duration compared with their normal could significantly improve real-time detection of influenza-like illness rates at the state level,” he said in an interview.
Thus, continuous passively captured data may be a useful adjunct to bricks-and-mortar site testing, which is generally a one-off or infrequent sampling assay and is not always easily accessible, he added. Furthermore, traditional screening with temperature and symptom reporting is inadequate. An elevation in temperature is not as common as frequently believed for people who test positive for COVID-19, Dr. Quer continued. “Early identification via sensor variables of those who are presymptomatic or even asymptomatic would be especially valuable, as people may potentially be infectious during this period, and early detection is the ultimate goal,” Dr. Quer said.
According to his group, adding these physiologic changes from baseline values significantly outperformed detection (P < .01) using a British model described in an earlier study by by Cristina Menni, PhD, and associates. That method, in which symptoms were considered alone, yielded an AUC of 0.71 (IQR, 0.63-0.79).
According to Dr. Quer, one in five Americans currently wear an electronic device. “If we could enroll even a small percentage of these individuals, we’d be able to potentially identify clusters before they have the opportunity to spread,” he said.
DETECT study details
During the period March 15 to June 7, 2020, the study enrolled 30,529 participants from all 50 states. They ranged in age from younger than 35 years (23.1%) to older than 65 years (12.8%); the majority (63.5%) were aged 35-65 years, and 62% were women. Sensor devices in use by the cohort included Fitbit activity trackers (78.4%) and Apple HealthKit (31.2%).
Participants downloaded an app called MyDataHelps, which collects smartwatch and activity tracker information, including self-reported symptoms and diagnostic testing results. The app also monitors changes from baseline in resting heart rate, sleep duration, and physical activity, as measured by steps.
Overall, 3,811 participants reported having at least one symptom of some kind (e.g., fatigue, cough, dyspnea, loss of taste or smell). Of these, 54 reported testing positive for COVID-19, and 279 reported testing negative.
Sleep and activity were significantly different for the positive and negative groups, with an AUC of 0.68 (IQR, 0.57-0.79) for the sleep metric and 0.69 (IQR, 0.61-0.77) for the activity metric, suggesting that these parameters were more affected in COVID-19–positive participants.
When the investigators combined resting heart rate, sleep, and activity into a single metric, predictive performance improved to an AUC of 0.72 (IQR, 0.64-0.80).
The next step, Dr. Quer said, is to include an alert to notify users of possible infection.
Alerting users to possible COVID-19 infection
In a similar study, an alert feature was already incorporated. The study, led by Michael P. Snyder, PhD, director of the Center for Genomics and Personalized Medicine at Stanford (Calif.) University, will soon be published online in Nature Biomedical Engineering. In that study, presymptomatic detection of COVID-19 was achieved in more than 80% of participants using resting heart rate.
“The median is 4 days prior to symptom formation,” Dr. Snyder said in an interview. “We have an alarm system to notify people when their heart rate is elevated. So a positive signal from a smartwatch can be used to follow up by polymerase chain reaction [testing].”
Dr. Snyder said these approaches offer a roadmap to containing widespread infections. “Public health authorities need to be open to these technologies and begin incorporating them into their tracking,” he said. “Right now, people do temperature checks, which are of limited value. Resting heart rate is much better information.”
Although the DETECT researchers have not yet received feedback on their results, they believe public health authorities could recommend the use of such apps. “These are devices that people routinely wear for tracking their fitness and sleep, so it would be relatively easy to use the data for viral illness tracking,” said co–lead author Jennifer Radin, PhD, an epidemiologist at Scripps. “Testing resources are still limited and don’t allow for routine serial testing of individuals who may be asymptomatic or presymptomatic. Wearables can offer a different way to routinely monitor and screen people for changes in their data that may indicate COVID-19.”
The marshaling of data through consumer digital platforms to fight the coronavirus is gaining ground. New York State and New Jersey are already embracing smartphone apps to alert individuals to possible exposure to the virus.
More than 710,000 New Yorkers have downloaded the COVID NY Alert app, launched in October to help protect individuals and communities from COVID-19 by sending alerts without compromising privacy or personal information. “Upon receiving a notification about a potential exposure, users are then able to self-quarantine, get tested, and reduce the potential exposure risk to family, friends, coworkers, and others,” Jonah Bruno, a spokesperson for the New York State Department of Health, said in an interview.
And recently the Mayo Clinic and Safe Health Systems launched a platform to store COVID-19 testing and vaccination data.
Both the Scripps and Stanford platforms are part of a global technologic response to the COVID-19 pandemic. Prospective studies, led by device manufacturers and academic institutions, allow individuals to voluntarily share sensor and clinical data to address the crisis. Similar approaches have been used to track COVID-19 in large populations in Germany via the Corona Data Donation app.
The study by Dr. Quer and colleagues was funded by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. One coauthor reported grants from Janssen and personal fees from Otsuka and Livongo outside of the submitted work. The other authors have disclosed no relevant financial relationships. Dr. Snyder has ties to Personalis, Qbio, January, SensOmics, Protos, Mirvie, and Oralome.
A version of this article originally appeared on Medscape.com.
A smartphone app that combines passively collected physiologic data from wearable devices, such as fitness trackers, and self-reported symptoms can discriminate between COVID-19–positive and –negative individuals among those who report symptoms, new data suggest.
After analyzing data from more than 30,000 participants, researchers from the Digital Engagement and Tracking for Early Control and Treatment (DETECT) study concluded that adding individual changes in sensor data improves models based on symptoms alone for differentiating symptomatic persons who are COVID-19 positive and symptomatic persons who are COVID-19 negative.
The combination can potentially identify infection clusters before wider community spread occurs, Giorgio Quer, PhD, and colleagues report in an article published online Oct. 29 in Nature Medicine. DETECT investigators note that marrying participant-reported symptoms with personal sensor data, such as deviation from normal sleep duration and resting heart rate, resulted in an area under the curve (AUC) of 0.80 (interquartile range [IQR], 0.73-0.86) for differentiating between symptomatic individuals who were positive and those who were negative for COVID-19.
“By better characterizing each individual’s unique baseline, you can then identify changes that may indicate that someone has a viral illness,” said Dr. Quer, director of artificial intelligence at Scripps Research Translational Institute in La Jolla, Calif. “In previous research, we found that the proportion of individuals with elevated resting heart rate and sleep duration compared with their normal could significantly improve real-time detection of influenza-like illness rates at the state level,” he said in an interview.
Thus, continuous passively captured data may be a useful adjunct to bricks-and-mortar site testing, which is generally a one-off or infrequent sampling assay and is not always easily accessible, he added. Furthermore, traditional screening with temperature and symptom reporting is inadequate. An elevation in temperature is not as common as frequently believed for people who test positive for COVID-19, Dr. Quer continued. “Early identification via sensor variables of those who are presymptomatic or even asymptomatic would be especially valuable, as people may potentially be infectious during this period, and early detection is the ultimate goal,” Dr. Quer said.
According to his group, adding these physiologic changes from baseline values significantly outperformed detection (P < .01) using a British model described in an earlier study by by Cristina Menni, PhD, and associates. That method, in which symptoms were considered alone, yielded an AUC of 0.71 (IQR, 0.63-0.79).
According to Dr. Quer, one in five Americans currently wear an electronic device. “If we could enroll even a small percentage of these individuals, we’d be able to potentially identify clusters before they have the opportunity to spread,” he said.
DETECT study details
During the period March 15 to June 7, 2020, the study enrolled 30,529 participants from all 50 states. They ranged in age from younger than 35 years (23.1%) to older than 65 years (12.8%); the majority (63.5%) were aged 35-65 years, and 62% were women. Sensor devices in use by the cohort included Fitbit activity trackers (78.4%) and Apple HealthKit (31.2%).
Participants downloaded an app called MyDataHelps, which collects smartwatch and activity tracker information, including self-reported symptoms and diagnostic testing results. The app also monitors changes from baseline in resting heart rate, sleep duration, and physical activity, as measured by steps.
Overall, 3,811 participants reported having at least one symptom of some kind (e.g., fatigue, cough, dyspnea, loss of taste or smell). Of these, 54 reported testing positive for COVID-19, and 279 reported testing negative.
Sleep and activity were significantly different for the positive and negative groups, with an AUC of 0.68 (IQR, 0.57-0.79) for the sleep metric and 0.69 (IQR, 0.61-0.77) for the activity metric, suggesting that these parameters were more affected in COVID-19–positive participants.
When the investigators combined resting heart rate, sleep, and activity into a single metric, predictive performance improved to an AUC of 0.72 (IQR, 0.64-0.80).
The next step, Dr. Quer said, is to include an alert to notify users of possible infection.
Alerting users to possible COVID-19 infection
In a similar study, an alert feature was already incorporated. The study, led by Michael P. Snyder, PhD, director of the Center for Genomics and Personalized Medicine at Stanford (Calif.) University, will soon be published online in Nature Biomedical Engineering. In that study, presymptomatic detection of COVID-19 was achieved in more than 80% of participants using resting heart rate.
“The median is 4 days prior to symptom formation,” Dr. Snyder said in an interview. “We have an alarm system to notify people when their heart rate is elevated. So a positive signal from a smartwatch can be used to follow up by polymerase chain reaction [testing].”
Dr. Snyder said these approaches offer a roadmap to containing widespread infections. “Public health authorities need to be open to these technologies and begin incorporating them into their tracking,” he said. “Right now, people do temperature checks, which are of limited value. Resting heart rate is much better information.”
Although the DETECT researchers have not yet received feedback on their results, they believe public health authorities could recommend the use of such apps. “These are devices that people routinely wear for tracking their fitness and sleep, so it would be relatively easy to use the data for viral illness tracking,” said co–lead author Jennifer Radin, PhD, an epidemiologist at Scripps. “Testing resources are still limited and don’t allow for routine serial testing of individuals who may be asymptomatic or presymptomatic. Wearables can offer a different way to routinely monitor and screen people for changes in their data that may indicate COVID-19.”
The marshaling of data through consumer digital platforms to fight the coronavirus is gaining ground. New York State and New Jersey are already embracing smartphone apps to alert individuals to possible exposure to the virus.
More than 710,000 New Yorkers have downloaded the COVID NY Alert app, launched in October to help protect individuals and communities from COVID-19 by sending alerts without compromising privacy or personal information. “Upon receiving a notification about a potential exposure, users are then able to self-quarantine, get tested, and reduce the potential exposure risk to family, friends, coworkers, and others,” Jonah Bruno, a spokesperson for the New York State Department of Health, said in an interview.
And recently the Mayo Clinic and Safe Health Systems launched a platform to store COVID-19 testing and vaccination data.
Both the Scripps and Stanford platforms are part of a global technologic response to the COVID-19 pandemic. Prospective studies, led by device manufacturers and academic institutions, allow individuals to voluntarily share sensor and clinical data to address the crisis. Similar approaches have been used to track COVID-19 in large populations in Germany via the Corona Data Donation app.
The study by Dr. Quer and colleagues was funded by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. One coauthor reported grants from Janssen and personal fees from Otsuka and Livongo outside of the submitted work. The other authors have disclosed no relevant financial relationships. Dr. Snyder has ties to Personalis, Qbio, January, SensOmics, Protos, Mirvie, and Oralome.
A version of this article originally appeared on Medscape.com.
Black patients less likely to receive H. pylori eradication testing
Black patients may be significantly less likely to receive eradication testing after treatment for Helicobacter pylori infection than patients of other races/ethnic groups, based on a retrospective analysis of more than 1,700 individuals.
This disparity may exacerbate the already increased burden of H. pylori infection and gastric cancer among Black individuals, according to principal author David A. Leiman, MD, MSHP, of Duke University Medical Center in Durham, N.C.
“H. pylori infection disproportionately affects racial/ethnic minorities and those of lower socioeconomic status,” Dr. Leiman, coauthor Julius Wilder, MD, PhD, of Duke University in Durham, and colleagues wrote in their abstract presented at the annual meeting of the American College of Gastroenterology. “ACG guidelines recommend treatment for H. pylori infection followed by confirmation of cure. Adherence to these recommendations varies and its impact on practice patterns is unclear. This study characterizes the management of H. pylori infection and predictors of guideline adherence.”
The investigators analyzed electronic medical records from 1,711 patients diagnosed with H. pylori infection through the Duke University Health System between June 2016 and June 2018, most often (71%) via serum antibody test. Approximately two-thirds of those diagnosed were non-White (66%) and female (63%). Out of 1,711 patients, 622 (36%) underwent eradication testing, of whom 559 (90%) were cured.
Despite publication of the ACG H. pylori guideline midway through the study (February 2017), testing rates dropped significantly from 43.1% in 2016 to 35.9% in 2017, and finally 25.5% in 2018 (P < .0001).
“These findings are consistent with other work that has shown low rates of testing to confirm cure in patients treated for H. pylori,” Dr. Leiman said. “There remains a disappointingly low number of patients who are tested for cure.”
Across the entire study period, patients were significantly more likely to undergo eradication testing if they were treated in the gastroenterology department (52.4%), compared with rates ranging from 33% to 34.6% for internal medicine, family medicine, and other departments (P < .001).
Across all departments, Black patients underwent eradication testing significantly less often than patients of other races/ethnicities, at a rate of 30.5% versus 32.2% for White patients, 35.1% for Asian patients, and 36.7% for patients who were of other backgrounds (P < .001). Compared with White patients, Black patients were 38% less likely to undergo eradication testing (odds ratio, 0.62; 95% confidence interval, 0.48-0.79).
Dr. Leiman noted that these findings contrast with a study by Dr. Shria Kumar and colleagues from earlier this year, which found no racial disparity in eradication testing within a Veterans Health Affairs cohort.
“Black patients are significantly less likely to undergo testing for eradication than [patients of other races/ethnicities],” Dr. Leiman said. “More work is needed to understand the mechanisms driving this disparity.” He suggested a number of possible contributing factors, including provider knowledge gaps, fragmented care, and social determinants of health.
“It is clear that a greater emphasis on characterizing and addressing the social determinants of health, including poverty, education, and location, are needed,” Dr. Leiman said. “Although health systems are not solely responsible for the known and ongoing observations of disparities in care, interventions must be identified and implemented to mitigate these issues.” Such interventions would likely require broad participation, he said, including policy makers, health systems, and individual practitioners.
“We plan to perform a prospective mixed methods study to contextualize which social determinants are associated with a decreased likelihood of receiving appropriate eradication testing by exploring barriers at patient, practitioner, and health-system levels,” Dr. Leiman said. “Ultimately, we aim to leverage these findings to develop an evidence-based intervention to circumnavigate those identified barriers, thereby eliminating the observed disparities in H. pylori care.”
According to Gregory L. Hall, MD, of Northeast Ohio Medical University, Rootstown, and Case Western Reserve University, Cleveland, and codirector of the Partnership for Urban Health Research, Atlanta, the higher rate of H. pylori infection in Black individuals may stem partly from genetic factors.
“Studies have shown that African Americans with a higher proportion of African ancestry have higher rates of H. pylori, suggesting a genetic component to this increased risk,” he said.
Still, Dr. Hall, who is the author of the book Patient-Centered Clinical Care for African Americans, went on to emphasize appropriate H. pylori management and recognition of racial disparities in medicine.
“The ability to test for, treat, and confirm eradication of H. pylori infections represents a great opportunity to improve quality of life through decreased gastritis, gastric ulcers, and gastric cancer,” he said. “[The present findings] show yet another disparity in our clinical care of African Americans that needs increased awareness among providers to these communities.”
Rotonya Carr, MD, of the Hospital of the University of Pennsylvania, Philadelphia, and lead author of a recent publication addressing racism and health disparities in gastroenterology, said the findings of the present study add weight to a known equity gap.
“These data are concerning in view of the twofold higher prevalence of H. pylori seropositivity and twofold higher incidence of gastric cancer in Black patients, compared with White patients,” Dr. Carr said. “These and other data support a comprehensive approach to reduce GI disparities that includes targeted education of both GI specialists and referring providers.”
According to Dr. Leiman, individual practitioners may work toward more equitable outcomes through a comprehensive clinical approach, regardless of patient race or ethnicity.
“Clinicians should consider H. pylori therapy an episode of care that spans diagnosis, treatment, and confirmation of cure,” he said. “Closing the loop in that episode by ensuring eradication is vital to conforming with best practices, and to reduce patients’ long-term risks.”The investigators disclosed relationships with Exact Sciences, Guardant Health, and Phathom Pharmaceuticals. Dr. Hall and Dr. Carr reported no relevant conflicts of interest.
SOURCE: Reichstein J et al. ACG 2020. Abstract S1332.
Black patients may be significantly less likely to receive eradication testing after treatment for Helicobacter pylori infection than patients of other races/ethnic groups, based on a retrospective analysis of more than 1,700 individuals.
This disparity may exacerbate the already increased burden of H. pylori infection and gastric cancer among Black individuals, according to principal author David A. Leiman, MD, MSHP, of Duke University Medical Center in Durham, N.C.
“H. pylori infection disproportionately affects racial/ethnic minorities and those of lower socioeconomic status,” Dr. Leiman, coauthor Julius Wilder, MD, PhD, of Duke University in Durham, and colleagues wrote in their abstract presented at the annual meeting of the American College of Gastroenterology. “ACG guidelines recommend treatment for H. pylori infection followed by confirmation of cure. Adherence to these recommendations varies and its impact on practice patterns is unclear. This study characterizes the management of H. pylori infection and predictors of guideline adherence.”
The investigators analyzed electronic medical records from 1,711 patients diagnosed with H. pylori infection through the Duke University Health System between June 2016 and June 2018, most often (71%) via serum antibody test. Approximately two-thirds of those diagnosed were non-White (66%) and female (63%). Out of 1,711 patients, 622 (36%) underwent eradication testing, of whom 559 (90%) were cured.
Despite publication of the ACG H. pylori guideline midway through the study (February 2017), testing rates dropped significantly from 43.1% in 2016 to 35.9% in 2017, and finally 25.5% in 2018 (P < .0001).
“These findings are consistent with other work that has shown low rates of testing to confirm cure in patients treated for H. pylori,” Dr. Leiman said. “There remains a disappointingly low number of patients who are tested for cure.”
Across the entire study period, patients were significantly more likely to undergo eradication testing if they were treated in the gastroenterology department (52.4%), compared with rates ranging from 33% to 34.6% for internal medicine, family medicine, and other departments (P < .001).
Across all departments, Black patients underwent eradication testing significantly less often than patients of other races/ethnicities, at a rate of 30.5% versus 32.2% for White patients, 35.1% for Asian patients, and 36.7% for patients who were of other backgrounds (P < .001). Compared with White patients, Black patients were 38% less likely to undergo eradication testing (odds ratio, 0.62; 95% confidence interval, 0.48-0.79).
Dr. Leiman noted that these findings contrast with a study by Dr. Shria Kumar and colleagues from earlier this year, which found no racial disparity in eradication testing within a Veterans Health Affairs cohort.
“Black patients are significantly less likely to undergo testing for eradication than [patients of other races/ethnicities],” Dr. Leiman said. “More work is needed to understand the mechanisms driving this disparity.” He suggested a number of possible contributing factors, including provider knowledge gaps, fragmented care, and social determinants of health.
“It is clear that a greater emphasis on characterizing and addressing the social determinants of health, including poverty, education, and location, are needed,” Dr. Leiman said. “Although health systems are not solely responsible for the known and ongoing observations of disparities in care, interventions must be identified and implemented to mitigate these issues.” Such interventions would likely require broad participation, he said, including policy makers, health systems, and individual practitioners.
“We plan to perform a prospective mixed methods study to contextualize which social determinants are associated with a decreased likelihood of receiving appropriate eradication testing by exploring barriers at patient, practitioner, and health-system levels,” Dr. Leiman said. “Ultimately, we aim to leverage these findings to develop an evidence-based intervention to circumnavigate those identified barriers, thereby eliminating the observed disparities in H. pylori care.”
According to Gregory L. Hall, MD, of Northeast Ohio Medical University, Rootstown, and Case Western Reserve University, Cleveland, and codirector of the Partnership for Urban Health Research, Atlanta, the higher rate of H. pylori infection in Black individuals may stem partly from genetic factors.
“Studies have shown that African Americans with a higher proportion of African ancestry have higher rates of H. pylori, suggesting a genetic component to this increased risk,” he said.
Still, Dr. Hall, who is the author of the book Patient-Centered Clinical Care for African Americans, went on to emphasize appropriate H. pylori management and recognition of racial disparities in medicine.
“The ability to test for, treat, and confirm eradication of H. pylori infections represents a great opportunity to improve quality of life through decreased gastritis, gastric ulcers, and gastric cancer,” he said. “[The present findings] show yet another disparity in our clinical care of African Americans that needs increased awareness among providers to these communities.”
Rotonya Carr, MD, of the Hospital of the University of Pennsylvania, Philadelphia, and lead author of a recent publication addressing racism and health disparities in gastroenterology, said the findings of the present study add weight to a known equity gap.
“These data are concerning in view of the twofold higher prevalence of H. pylori seropositivity and twofold higher incidence of gastric cancer in Black patients, compared with White patients,” Dr. Carr said. “These and other data support a comprehensive approach to reduce GI disparities that includes targeted education of both GI specialists and referring providers.”
According to Dr. Leiman, individual practitioners may work toward more equitable outcomes through a comprehensive clinical approach, regardless of patient race or ethnicity.
“Clinicians should consider H. pylori therapy an episode of care that spans diagnosis, treatment, and confirmation of cure,” he said. “Closing the loop in that episode by ensuring eradication is vital to conforming with best practices, and to reduce patients’ long-term risks.”The investigators disclosed relationships with Exact Sciences, Guardant Health, and Phathom Pharmaceuticals. Dr. Hall and Dr. Carr reported no relevant conflicts of interest.
SOURCE: Reichstein J et al. ACG 2020. Abstract S1332.
Black patients may be significantly less likely to receive eradication testing after treatment for Helicobacter pylori infection than patients of other races/ethnic groups, based on a retrospective analysis of more than 1,700 individuals.
This disparity may exacerbate the already increased burden of H. pylori infection and gastric cancer among Black individuals, according to principal author David A. Leiman, MD, MSHP, of Duke University Medical Center in Durham, N.C.
“H. pylori infection disproportionately affects racial/ethnic minorities and those of lower socioeconomic status,” Dr. Leiman, coauthor Julius Wilder, MD, PhD, of Duke University in Durham, and colleagues wrote in their abstract presented at the annual meeting of the American College of Gastroenterology. “ACG guidelines recommend treatment for H. pylori infection followed by confirmation of cure. Adherence to these recommendations varies and its impact on practice patterns is unclear. This study characterizes the management of H. pylori infection and predictors of guideline adherence.”
The investigators analyzed electronic medical records from 1,711 patients diagnosed with H. pylori infection through the Duke University Health System between June 2016 and June 2018, most often (71%) via serum antibody test. Approximately two-thirds of those diagnosed were non-White (66%) and female (63%). Out of 1,711 patients, 622 (36%) underwent eradication testing, of whom 559 (90%) were cured.
Despite publication of the ACG H. pylori guideline midway through the study (February 2017), testing rates dropped significantly from 43.1% in 2016 to 35.9% in 2017, and finally 25.5% in 2018 (P < .0001).
“These findings are consistent with other work that has shown low rates of testing to confirm cure in patients treated for H. pylori,” Dr. Leiman said. “There remains a disappointingly low number of patients who are tested for cure.”
Across the entire study period, patients were significantly more likely to undergo eradication testing if they were treated in the gastroenterology department (52.4%), compared with rates ranging from 33% to 34.6% for internal medicine, family medicine, and other departments (P < .001).
Across all departments, Black patients underwent eradication testing significantly less often than patients of other races/ethnicities, at a rate of 30.5% versus 32.2% for White patients, 35.1% for Asian patients, and 36.7% for patients who were of other backgrounds (P < .001). Compared with White patients, Black patients were 38% less likely to undergo eradication testing (odds ratio, 0.62; 95% confidence interval, 0.48-0.79).
Dr. Leiman noted that these findings contrast with a study by Dr. Shria Kumar and colleagues from earlier this year, which found no racial disparity in eradication testing within a Veterans Health Affairs cohort.
“Black patients are significantly less likely to undergo testing for eradication than [patients of other races/ethnicities],” Dr. Leiman said. “More work is needed to understand the mechanisms driving this disparity.” He suggested a number of possible contributing factors, including provider knowledge gaps, fragmented care, and social determinants of health.
“It is clear that a greater emphasis on characterizing and addressing the social determinants of health, including poverty, education, and location, are needed,” Dr. Leiman said. “Although health systems are not solely responsible for the known and ongoing observations of disparities in care, interventions must be identified and implemented to mitigate these issues.” Such interventions would likely require broad participation, he said, including policy makers, health systems, and individual practitioners.
“We plan to perform a prospective mixed methods study to contextualize which social determinants are associated with a decreased likelihood of receiving appropriate eradication testing by exploring barriers at patient, practitioner, and health-system levels,” Dr. Leiman said. “Ultimately, we aim to leverage these findings to develop an evidence-based intervention to circumnavigate those identified barriers, thereby eliminating the observed disparities in H. pylori care.”
According to Gregory L. Hall, MD, of Northeast Ohio Medical University, Rootstown, and Case Western Reserve University, Cleveland, and codirector of the Partnership for Urban Health Research, Atlanta, the higher rate of H. pylori infection in Black individuals may stem partly from genetic factors.
“Studies have shown that African Americans with a higher proportion of African ancestry have higher rates of H. pylori, suggesting a genetic component to this increased risk,” he said.
Still, Dr. Hall, who is the author of the book Patient-Centered Clinical Care for African Americans, went on to emphasize appropriate H. pylori management and recognition of racial disparities in medicine.
“The ability to test for, treat, and confirm eradication of H. pylori infections represents a great opportunity to improve quality of life through decreased gastritis, gastric ulcers, and gastric cancer,” he said. “[The present findings] show yet another disparity in our clinical care of African Americans that needs increased awareness among providers to these communities.”
Rotonya Carr, MD, of the Hospital of the University of Pennsylvania, Philadelphia, and lead author of a recent publication addressing racism and health disparities in gastroenterology, said the findings of the present study add weight to a known equity gap.
“These data are concerning in view of the twofold higher prevalence of H. pylori seropositivity and twofold higher incidence of gastric cancer in Black patients, compared with White patients,” Dr. Carr said. “These and other data support a comprehensive approach to reduce GI disparities that includes targeted education of both GI specialists and referring providers.”
According to Dr. Leiman, individual practitioners may work toward more equitable outcomes through a comprehensive clinical approach, regardless of patient race or ethnicity.
“Clinicians should consider H. pylori therapy an episode of care that spans diagnosis, treatment, and confirmation of cure,” he said. “Closing the loop in that episode by ensuring eradication is vital to conforming with best practices, and to reduce patients’ long-term risks.”The investigators disclosed relationships with Exact Sciences, Guardant Health, and Phathom Pharmaceuticals. Dr. Hall and Dr. Carr reported no relevant conflicts of interest.
SOURCE: Reichstein J et al. ACG 2020. Abstract S1332.
FROM ACG 2020
Dermatologists as Social Media Contributors During the COVID-19 Pandemic
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
Practice Points
- With the coronavirus disease 2019 (COVID-19) pandemic, strict physical distancing measures have made patients and providers alike reliant on global digital social networks such as Instagram, Twitter, and Facebook to facilitate information sharing about COVID-19.
- Dermatologists should utilize social media as a platform to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during the global pandemic.
COVID-19: U.S. sets new weekly high in children
the American Academy of Pediatrics announced Nov. 2.
For the week, over 61,000 cases were reported in children, bringing the number of COVID-19 cases for the month of October to nearly 200,000 and the total since the start of the pandemic to over 853,000, the AAP and the Children’s Hospital Association said in their weekly report.
“These numbers reflect a disturbing increase in cases throughout most of the United States in all populations, especially among young adults,” Yvonne Maldonado, MD, chair of the AAP Committee on Infectious Diseases, said in a separate statement. “We are entering a heightened wave of infections around the country. We would encourage family holiday gatherings to be avoided if possible, especially if there are high-risk individuals in the household.”
For the week ending Oct. 29, children represented 13.3% of all cases, possibly constituting a minitrend of stability over the past 3 weeks. For the full length of the pandemic, 11.1% of all COVID-19 cases have occurred in children, although severe illness is much less common: 1.7% of all hospitalizations (data from 24 states and New York City) and 0.06% of all deaths (data from 42 states and New York City), the AAP and CHA report said.
Other data show that 1,134 per 100,000 children in the United States have been infected by the coronavirus, up from 1,053 the previous week, with state rates ranging from 221 per 100,000 in Vermont to 3,321 in North Dakota. In Wyoming, 25.5% of all COVID-19 cases have occurred in children, the highest of any state, while New Jersey has the lowest rate at 4.9%, the AAP/CHA report showed.
In the 10 states making testing data available, children represent the lowest percentage of tests in Iowa (5.0%) and the highest in Indiana (16.9%). Iowa, however, has the highest positivity rate for children at 14.6%, along with Nevada, while West Virginia has the lowest at 3.6%, the AAP and CHA said in the report.
These numbers, however, may not be telling the whole story. “The number of reported COVID-19 cases in children is likely an undercount because children’s symptoms are often mild and they may not be tested for every illness,” the AAP said in its statement.
“We urge policy makers to listen to doctors and public health experts rather than level baseless accusations against them. Physicians, nurses and other health care professionals have put their lives on the line to protect our communities. We can all do our part to protect them, and our communities, by wearing masks, practicing physical distancing, and getting our flu immunizations,” AAP President Sally Goza, MD, said in the AAP statement.
the American Academy of Pediatrics announced Nov. 2.

For the week, over 61,000 cases were reported in children, bringing the number of COVID-19 cases for the month of October to nearly 200,000 and the total since the start of the pandemic to over 853,000, the AAP and the Children’s Hospital Association said in their weekly report.
“These numbers reflect a disturbing increase in cases throughout most of the United States in all populations, especially among young adults,” Yvonne Maldonado, MD, chair of the AAP Committee on Infectious Diseases, said in a separate statement. “We are entering a heightened wave of infections around the country. We would encourage family holiday gatherings to be avoided if possible, especially if there are high-risk individuals in the household.”
For the week ending Oct. 29, children represented 13.3% of all cases, possibly constituting a minitrend of stability over the past 3 weeks. For the full length of the pandemic, 11.1% of all COVID-19 cases have occurred in children, although severe illness is much less common: 1.7% of all hospitalizations (data from 24 states and New York City) and 0.06% of all deaths (data from 42 states and New York City), the AAP and CHA report said.
Other data show that 1,134 per 100,000 children in the United States have been infected by the coronavirus, up from 1,053 the previous week, with state rates ranging from 221 per 100,000 in Vermont to 3,321 in North Dakota. In Wyoming, 25.5% of all COVID-19 cases have occurred in children, the highest of any state, while New Jersey has the lowest rate at 4.9%, the AAP/CHA report showed.
In the 10 states making testing data available, children represent the lowest percentage of tests in Iowa (5.0%) and the highest in Indiana (16.9%). Iowa, however, has the highest positivity rate for children at 14.6%, along with Nevada, while West Virginia has the lowest at 3.6%, the AAP and CHA said in the report.
These numbers, however, may not be telling the whole story. “The number of reported COVID-19 cases in children is likely an undercount because children’s symptoms are often mild and they may not be tested for every illness,” the AAP said in its statement.
“We urge policy makers to listen to doctors and public health experts rather than level baseless accusations against them. Physicians, nurses and other health care professionals have put their lives on the line to protect our communities. We can all do our part to protect them, and our communities, by wearing masks, practicing physical distancing, and getting our flu immunizations,” AAP President Sally Goza, MD, said in the AAP statement.
the American Academy of Pediatrics announced Nov. 2.

For the week, over 61,000 cases were reported in children, bringing the number of COVID-19 cases for the month of October to nearly 200,000 and the total since the start of the pandemic to over 853,000, the AAP and the Children’s Hospital Association said in their weekly report.
“These numbers reflect a disturbing increase in cases throughout most of the United States in all populations, especially among young adults,” Yvonne Maldonado, MD, chair of the AAP Committee on Infectious Diseases, said in a separate statement. “We are entering a heightened wave of infections around the country. We would encourage family holiday gatherings to be avoided if possible, especially if there are high-risk individuals in the household.”
For the week ending Oct. 29, children represented 13.3% of all cases, possibly constituting a minitrend of stability over the past 3 weeks. For the full length of the pandemic, 11.1% of all COVID-19 cases have occurred in children, although severe illness is much less common: 1.7% of all hospitalizations (data from 24 states and New York City) and 0.06% of all deaths (data from 42 states and New York City), the AAP and CHA report said.
Other data show that 1,134 per 100,000 children in the United States have been infected by the coronavirus, up from 1,053 the previous week, with state rates ranging from 221 per 100,000 in Vermont to 3,321 in North Dakota. In Wyoming, 25.5% of all COVID-19 cases have occurred in children, the highest of any state, while New Jersey has the lowest rate at 4.9%, the AAP/CHA report showed.
In the 10 states making testing data available, children represent the lowest percentage of tests in Iowa (5.0%) and the highest in Indiana (16.9%). Iowa, however, has the highest positivity rate for children at 14.6%, along with Nevada, while West Virginia has the lowest at 3.6%, the AAP and CHA said in the report.
These numbers, however, may not be telling the whole story. “The number of reported COVID-19 cases in children is likely an undercount because children’s symptoms are often mild and they may not be tested for every illness,” the AAP said in its statement.
“We urge policy makers to listen to doctors and public health experts rather than level baseless accusations against them. Physicians, nurses and other health care professionals have put their lives on the line to protect our communities. We can all do our part to protect them, and our communities, by wearing masks, practicing physical distancing, and getting our flu immunizations,” AAP President Sally Goza, MD, said in the AAP statement.
OTC topical ivermectin lotion earns FDA approval for head lice
in patients aged 6 months and older.
Ivermectin was approved as a prescription treatment for head lice in February 2012, according to an FDA press release, and is now approved as an over-the-counter treatment through an “Rx-to-OTC” switch process. The approval was granted to Arbor Pharmaceuticals.
The expanded approval for ivermectin increases access to effective care for head lice, which is estimated to affect between 6 million and 12 million children each year in the United States, according to the Centers for Disease Control and Prevention.
“The Rx-to-OTC switch process aims to promote public health by increasing consumer access to drugs that would otherwise only be available by prescription,” Theresa Michele, MD, acting director of the Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research, said in the press release.
The FDA also noted in the press release that “Sklice, and its active ingredient ivermectin, have not been shown to be safe or effective for the treatment or prevention of COVID-19 and they are not FDA-approved for this use.”
The drug is approved only for treating head lice, and should be used on the scalp and dry hair, according to the labeling. In the wake of the approval, ivermectin will no longer be available as a prescription drug, according to the FDA, and patients currently using prescription versions should contact their health care providers.
An Rx-to-OTC switch is contingent on the manufacturer’s data showing that the drug is safe and effective when used as directed. In addition, “the manufacturer must show that consumers can understand how to use the drug safely and effectively without the supervision of a health care professional,” according to the FDA.
in patients aged 6 months and older.
Ivermectin was approved as a prescription treatment for head lice in February 2012, according to an FDA press release, and is now approved as an over-the-counter treatment through an “Rx-to-OTC” switch process. The approval was granted to Arbor Pharmaceuticals.
The expanded approval for ivermectin increases access to effective care for head lice, which is estimated to affect between 6 million and 12 million children each year in the United States, according to the Centers for Disease Control and Prevention.
“The Rx-to-OTC switch process aims to promote public health by increasing consumer access to drugs that would otherwise only be available by prescription,” Theresa Michele, MD, acting director of the Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research, said in the press release.
The FDA also noted in the press release that “Sklice, and its active ingredient ivermectin, have not been shown to be safe or effective for the treatment or prevention of COVID-19 and they are not FDA-approved for this use.”
The drug is approved only for treating head lice, and should be used on the scalp and dry hair, according to the labeling. In the wake of the approval, ivermectin will no longer be available as a prescription drug, according to the FDA, and patients currently using prescription versions should contact their health care providers.
An Rx-to-OTC switch is contingent on the manufacturer’s data showing that the drug is safe and effective when used as directed. In addition, “the manufacturer must show that consumers can understand how to use the drug safely and effectively without the supervision of a health care professional,” according to the FDA.
in patients aged 6 months and older.
Ivermectin was approved as a prescription treatment for head lice in February 2012, according to an FDA press release, and is now approved as an over-the-counter treatment through an “Rx-to-OTC” switch process. The approval was granted to Arbor Pharmaceuticals.
The expanded approval for ivermectin increases access to effective care for head lice, which is estimated to affect between 6 million and 12 million children each year in the United States, according to the Centers for Disease Control and Prevention.
“The Rx-to-OTC switch process aims to promote public health by increasing consumer access to drugs that would otherwise only be available by prescription,” Theresa Michele, MD, acting director of the Office of Nonprescription Drugs in the FDA’s Center for Drug Evaluation and Research, said in the press release.
The FDA also noted in the press release that “Sklice, and its active ingredient ivermectin, have not been shown to be safe or effective for the treatment or prevention of COVID-19 and they are not FDA-approved for this use.”
The drug is approved only for treating head lice, and should be used on the scalp and dry hair, according to the labeling. In the wake of the approval, ivermectin will no longer be available as a prescription drug, according to the FDA, and patients currently using prescription versions should contact their health care providers.
An Rx-to-OTC switch is contingent on the manufacturer’s data showing that the drug is safe and effective when used as directed. In addition, “the manufacturer must show that consumers can understand how to use the drug safely and effectively without the supervision of a health care professional,” according to the FDA.