User login
Hospitalists are natural leaders in the COVID-19 battle
Christopher Pribula, MD, a hospitalist at Sanford Broadway Medical Center in Fargo, N.D., didn’t anticipate becoming his hospital’s resident expert on COVID-19. Having just returned from vacation in March, he agreed to cover for a colleague on what would become the special care unit. “When our hospital medicine group decided that it would be the COVID unit, I just ran with it,” he said. Dr. Pribula spent the next 18 days doing 8- to 14-hour shifts and learning as much as he could as the hospital – and the nation – wrestled with the pandemic.
“Because I was the first hospitalist, along with our infectious disease specialist, Dr. Avish Nagpal, to really engage with the virus, people came to me with their questions,” Dr. Pribula said. Working to establish protocols for the care of COVID-19 patients involved a lot of planning, from nursing protocols to discharge planning.
Dr. Pribula was part of the hospital’s incident command structure, thought about how the system could scale up for a potential surge, and worked with the North Dakota Medical Association to reach out to outlying medical centers on safety and infection control. He even drew on his prior work experience as a medical technologist doing negative-pressure containment in a cell-processing facility to help create the hospital’s negative-pressure unit in an old ICU.
“We did a lot of communication from the start. To a certain extent we were making it up as we went along, but we sat down and huddled as a team every day at 9 and 4,” he explained. “We started out with observation and retrospective research, and learned piece by piece. But that’s how science works.”
Hospitalists across the country have played leading roles in their hospitals’ and health systems’ response to the pandemic, and not just because they are on the front lines providing patient care. Their job as doctors who work full-time in the hospital makes them natural leaders in improving clinical quality and hospital administrative protocols as well as studying the latest information and educating their colleagues. Responding to the pandemic has required lots of planning, careful attention to schedules and assignments and staff stress, and working with other departments in the hospital and groups in the community, including public health authorities.
Where is hospital treatment for COVID-19 at today?
As knowledge has grown, Dr. Pribula said, COVID-19 treatment in the hospital has come to incorporate remdesivir, a broad-spectrum antiviral; dexamethasone, a common steroid medication; and convalescent plasma, blood products from people who have recovered from the illness. “We went from no steroids to giving steroids. We went from putting patients on ventilators to avoid acute respiratory distress syndrome (ARDS) initially to now working to avoid intubation at all costs,” he said.
“What we found is that we need to pressure-support these patients. We do proning and CPAP while we let the lungs heal. By the time they arrive at the hospital, more often than not they’re on the backside of the viral load. But now we’re dealing with the body’s inflammatory response.”
Navneet Attri, MD, a hospitalist at Sutter Santa Rosa Regional Hospital in Santa Rosa, Calif., 50 miles north of San Francisco, experienced fears and uncertainties working at a hospital that treated early COVID patients from the Grand Princess cruise ship. Early on, she wrote a post describing her experience for The Hospitalist Leader, the Society of Hospital Medicine’s blog page.
Dr. Attri said she has gone through the gamut of emotions while caring for COVID-19 patients, addressing their fears and trying to support family members who aren’t allowed to enter the hospital to be at their loved one’s side. Sometimes, patient after patient with COVID-19 becomes almost too much. But seeing a lot of them in the intervening 6 months has increased her confidence level.
Understanding of how the disease is spread has continued to evolve, with a recent return to focusing on airborne transmission, she said. Frontline workers need N95 masks and eye shields, even if all of that PPE feels like a burden. Dr. Attri said she hardly notices the PPE anymore. “Putting it on is just a habit.”
She sits on Sonoma County’s COVID-19 surge planning group, which has representatives from the three local hospitals, the public health department, and other community agencies. “I report back to my hospitalist group about the situation in the community. Because our facilities were well prepared, our hospitals have not been overwhelmed,” she said.
The importance of teamwork
Sunil Shah, MD, a hospitalist with Northwell Health’s Southside Hospital in Bay Shore, N.Y., is part of the massive hospital medicine team, including reassigned specialists and volunteers from across the country, deployed at Northwell hospitals in Greater New York City and Long Island during the COVID-19 surge. Northwell probably has cared for more COVID-19 patients than any other health system in the country, and at the height of the surge the intensity of hospital care was like nothing he’s ever seen. But he also expressed gratitude that doctors from other parts of the country were willing to come and help out.
Southside Hospital went almost overnight from a 200-bed acute facility to a full, 350-bed, regional COVID-19–only hospital. “On busy days, our entire hospital was like a floating ICU,” he said. “You’d hear ‘rapid response’ or ‘code blue’ over the intercom every few seconds. Normally we’d have a designated rapid response person for the day, but with COVID, everybody stepped in to help – whoever was closest,” he said.
Majid Sheikh, MD, a hospitalist at Emory University Hospital in Atlanta, also became a go-to COVID-19 expert for his group. “I didn’t specifically volunteer, but my partner and I had the first cases, and the leadership group was happy to have us there,” he explained.
“One interesting thing I learned was the concept of the ‘happy’ hypoxemic patient, who is having a significant drop in oxygen saturation without developing any obvious signs of respiratory distress,” he said. “We’d be checking the accuracy of the reading and trying to figure out if it was real.” Emory was also one of the leaders in studying anticoagulant treatments for COVID-19 patients.
“Six months later I would say we’re definitely getting better outcomes on the floor, and our COVID patients aren’t landing in the ICU as easily,” Dr. Sheikh said. “It was scary at first, and doubly scary when doctors sometimes don’t feel they can say, ‘Hey, I’m scared too,’ or ‘By the way, I really don’t know what I’m doing.’ So, we’d be trying to reassure the patients when the information was coming to us in fragments.”
But he also believes that the pandemic has afforded hospitalists the opportunity to be the clinical detectives they were trained to be, sifting through clues. “I had to think more and really pay attention clinically in a much different way. You could say it was exciting and scary at the same time,” he said.
A human fix in the hospital
Dr. Pribula agreed that the pandemic has been both a difficult experience and a rewarding one. “I think of the people I first admitted. If they had shown up even a month later, would they still be with us?” He believes that his group and his field are going to get to a place where they have solid treatment plans for how to provide optimal care and how to protect providers from exposure.
One of the first COVID-19 patients in Fargo had dementia and was very distressed. “She had no idea why nobody was visiting or why we wouldn’t let her out of her room,” Dr. Pribula said. “Instead of reaching for sedatives, one of our nurses went into the room and talked with her, prayed a rosary, and played two hands of cards with her and didn’t have to sedate her. That’s what people need when they’re alone and scared. It wasn’t a medical fix but a human fix.”
A version of this article originally appeared on Medscape.com.
Christopher Pribula, MD, a hospitalist at Sanford Broadway Medical Center in Fargo, N.D., didn’t anticipate becoming his hospital’s resident expert on COVID-19. Having just returned from vacation in March, he agreed to cover for a colleague on what would become the special care unit. “When our hospital medicine group decided that it would be the COVID unit, I just ran with it,” he said. Dr. Pribula spent the next 18 days doing 8- to 14-hour shifts and learning as much as he could as the hospital – and the nation – wrestled with the pandemic.
“Because I was the first hospitalist, along with our infectious disease specialist, Dr. Avish Nagpal, to really engage with the virus, people came to me with their questions,” Dr. Pribula said. Working to establish protocols for the care of COVID-19 patients involved a lot of planning, from nursing protocols to discharge planning.
Dr. Pribula was part of the hospital’s incident command structure, thought about how the system could scale up for a potential surge, and worked with the North Dakota Medical Association to reach out to outlying medical centers on safety and infection control. He even drew on his prior work experience as a medical technologist doing negative-pressure containment in a cell-processing facility to help create the hospital’s negative-pressure unit in an old ICU.
“We did a lot of communication from the start. To a certain extent we were making it up as we went along, but we sat down and huddled as a team every day at 9 and 4,” he explained. “We started out with observation and retrospective research, and learned piece by piece. But that’s how science works.”
Hospitalists across the country have played leading roles in their hospitals’ and health systems’ response to the pandemic, and not just because they are on the front lines providing patient care. Their job as doctors who work full-time in the hospital makes them natural leaders in improving clinical quality and hospital administrative protocols as well as studying the latest information and educating their colleagues. Responding to the pandemic has required lots of planning, careful attention to schedules and assignments and staff stress, and working with other departments in the hospital and groups in the community, including public health authorities.
Where is hospital treatment for COVID-19 at today?
As knowledge has grown, Dr. Pribula said, COVID-19 treatment in the hospital has come to incorporate remdesivir, a broad-spectrum antiviral; dexamethasone, a common steroid medication; and convalescent plasma, blood products from people who have recovered from the illness. “We went from no steroids to giving steroids. We went from putting patients on ventilators to avoid acute respiratory distress syndrome (ARDS) initially to now working to avoid intubation at all costs,” he said.
“What we found is that we need to pressure-support these patients. We do proning and CPAP while we let the lungs heal. By the time they arrive at the hospital, more often than not they’re on the backside of the viral load. But now we’re dealing with the body’s inflammatory response.”
Navneet Attri, MD, a hospitalist at Sutter Santa Rosa Regional Hospital in Santa Rosa, Calif., 50 miles north of San Francisco, experienced fears and uncertainties working at a hospital that treated early COVID patients from the Grand Princess cruise ship. Early on, she wrote a post describing her experience for The Hospitalist Leader, the Society of Hospital Medicine’s blog page.
Dr. Attri said she has gone through the gamut of emotions while caring for COVID-19 patients, addressing their fears and trying to support family members who aren’t allowed to enter the hospital to be at their loved one’s side. Sometimes, patient after patient with COVID-19 becomes almost too much. But seeing a lot of them in the intervening 6 months has increased her confidence level.
Understanding of how the disease is spread has continued to evolve, with a recent return to focusing on airborne transmission, she said. Frontline workers need N95 masks and eye shields, even if all of that PPE feels like a burden. Dr. Attri said she hardly notices the PPE anymore. “Putting it on is just a habit.”
She sits on Sonoma County’s COVID-19 surge planning group, which has representatives from the three local hospitals, the public health department, and other community agencies. “I report back to my hospitalist group about the situation in the community. Because our facilities were well prepared, our hospitals have not been overwhelmed,” she said.
The importance of teamwork
Sunil Shah, MD, a hospitalist with Northwell Health’s Southside Hospital in Bay Shore, N.Y., is part of the massive hospital medicine team, including reassigned specialists and volunteers from across the country, deployed at Northwell hospitals in Greater New York City and Long Island during the COVID-19 surge. Northwell probably has cared for more COVID-19 patients than any other health system in the country, and at the height of the surge the intensity of hospital care was like nothing he’s ever seen. But he also expressed gratitude that doctors from other parts of the country were willing to come and help out.
Southside Hospital went almost overnight from a 200-bed acute facility to a full, 350-bed, regional COVID-19–only hospital. “On busy days, our entire hospital was like a floating ICU,” he said. “You’d hear ‘rapid response’ or ‘code blue’ over the intercom every few seconds. Normally we’d have a designated rapid response person for the day, but with COVID, everybody stepped in to help – whoever was closest,” he said.
Majid Sheikh, MD, a hospitalist at Emory University Hospital in Atlanta, also became a go-to COVID-19 expert for his group. “I didn’t specifically volunteer, but my partner and I had the first cases, and the leadership group was happy to have us there,” he explained.
“One interesting thing I learned was the concept of the ‘happy’ hypoxemic patient, who is having a significant drop in oxygen saturation without developing any obvious signs of respiratory distress,” he said. “We’d be checking the accuracy of the reading and trying to figure out if it was real.” Emory was also one of the leaders in studying anticoagulant treatments for COVID-19 patients.
“Six months later I would say we’re definitely getting better outcomes on the floor, and our COVID patients aren’t landing in the ICU as easily,” Dr. Sheikh said. “It was scary at first, and doubly scary when doctors sometimes don’t feel they can say, ‘Hey, I’m scared too,’ or ‘By the way, I really don’t know what I’m doing.’ So, we’d be trying to reassure the patients when the information was coming to us in fragments.”
But he also believes that the pandemic has afforded hospitalists the opportunity to be the clinical detectives they were trained to be, sifting through clues. “I had to think more and really pay attention clinically in a much different way. You could say it was exciting and scary at the same time,” he said.
A human fix in the hospital
Dr. Pribula agreed that the pandemic has been both a difficult experience and a rewarding one. “I think of the people I first admitted. If they had shown up even a month later, would they still be with us?” He believes that his group and his field are going to get to a place where they have solid treatment plans for how to provide optimal care and how to protect providers from exposure.
One of the first COVID-19 patients in Fargo had dementia and was very distressed. “She had no idea why nobody was visiting or why we wouldn’t let her out of her room,” Dr. Pribula said. “Instead of reaching for sedatives, one of our nurses went into the room and talked with her, prayed a rosary, and played two hands of cards with her and didn’t have to sedate her. That’s what people need when they’re alone and scared. It wasn’t a medical fix but a human fix.”
A version of this article originally appeared on Medscape.com.
Christopher Pribula, MD, a hospitalist at Sanford Broadway Medical Center in Fargo, N.D., didn’t anticipate becoming his hospital’s resident expert on COVID-19. Having just returned from vacation in March, he agreed to cover for a colleague on what would become the special care unit. “When our hospital medicine group decided that it would be the COVID unit, I just ran with it,” he said. Dr. Pribula spent the next 18 days doing 8- to 14-hour shifts and learning as much as he could as the hospital – and the nation – wrestled with the pandemic.
“Because I was the first hospitalist, along with our infectious disease specialist, Dr. Avish Nagpal, to really engage with the virus, people came to me with their questions,” Dr. Pribula said. Working to establish protocols for the care of COVID-19 patients involved a lot of planning, from nursing protocols to discharge planning.
Dr. Pribula was part of the hospital’s incident command structure, thought about how the system could scale up for a potential surge, and worked with the North Dakota Medical Association to reach out to outlying medical centers on safety and infection control. He even drew on his prior work experience as a medical technologist doing negative-pressure containment in a cell-processing facility to help create the hospital’s negative-pressure unit in an old ICU.
“We did a lot of communication from the start. To a certain extent we were making it up as we went along, but we sat down and huddled as a team every day at 9 and 4,” he explained. “We started out with observation and retrospective research, and learned piece by piece. But that’s how science works.”
Hospitalists across the country have played leading roles in their hospitals’ and health systems’ response to the pandemic, and not just because they are on the front lines providing patient care. Their job as doctors who work full-time in the hospital makes them natural leaders in improving clinical quality and hospital administrative protocols as well as studying the latest information and educating their colleagues. Responding to the pandemic has required lots of planning, careful attention to schedules and assignments and staff stress, and working with other departments in the hospital and groups in the community, including public health authorities.
Where is hospital treatment for COVID-19 at today?
As knowledge has grown, Dr. Pribula said, COVID-19 treatment in the hospital has come to incorporate remdesivir, a broad-spectrum antiviral; dexamethasone, a common steroid medication; and convalescent plasma, blood products from people who have recovered from the illness. “We went from no steroids to giving steroids. We went from putting patients on ventilators to avoid acute respiratory distress syndrome (ARDS) initially to now working to avoid intubation at all costs,” he said.
“What we found is that we need to pressure-support these patients. We do proning and CPAP while we let the lungs heal. By the time they arrive at the hospital, more often than not they’re on the backside of the viral load. But now we’re dealing with the body’s inflammatory response.”
Navneet Attri, MD, a hospitalist at Sutter Santa Rosa Regional Hospital in Santa Rosa, Calif., 50 miles north of San Francisco, experienced fears and uncertainties working at a hospital that treated early COVID patients from the Grand Princess cruise ship. Early on, she wrote a post describing her experience for The Hospitalist Leader, the Society of Hospital Medicine’s blog page.
Dr. Attri said she has gone through the gamut of emotions while caring for COVID-19 patients, addressing their fears and trying to support family members who aren’t allowed to enter the hospital to be at their loved one’s side. Sometimes, patient after patient with COVID-19 becomes almost too much. But seeing a lot of them in the intervening 6 months has increased her confidence level.
Understanding of how the disease is spread has continued to evolve, with a recent return to focusing on airborne transmission, she said. Frontline workers need N95 masks and eye shields, even if all of that PPE feels like a burden. Dr. Attri said she hardly notices the PPE anymore. “Putting it on is just a habit.”
She sits on Sonoma County’s COVID-19 surge planning group, which has representatives from the three local hospitals, the public health department, and other community agencies. “I report back to my hospitalist group about the situation in the community. Because our facilities were well prepared, our hospitals have not been overwhelmed,” she said.
The importance of teamwork
Sunil Shah, MD, a hospitalist with Northwell Health’s Southside Hospital in Bay Shore, N.Y., is part of the massive hospital medicine team, including reassigned specialists and volunteers from across the country, deployed at Northwell hospitals in Greater New York City and Long Island during the COVID-19 surge. Northwell probably has cared for more COVID-19 patients than any other health system in the country, and at the height of the surge the intensity of hospital care was like nothing he’s ever seen. But he also expressed gratitude that doctors from other parts of the country were willing to come and help out.
Southside Hospital went almost overnight from a 200-bed acute facility to a full, 350-bed, regional COVID-19–only hospital. “On busy days, our entire hospital was like a floating ICU,” he said. “You’d hear ‘rapid response’ or ‘code blue’ over the intercom every few seconds. Normally we’d have a designated rapid response person for the day, but with COVID, everybody stepped in to help – whoever was closest,” he said.
Majid Sheikh, MD, a hospitalist at Emory University Hospital in Atlanta, also became a go-to COVID-19 expert for his group. “I didn’t specifically volunteer, but my partner and I had the first cases, and the leadership group was happy to have us there,” he explained.
“One interesting thing I learned was the concept of the ‘happy’ hypoxemic patient, who is having a significant drop in oxygen saturation without developing any obvious signs of respiratory distress,” he said. “We’d be checking the accuracy of the reading and trying to figure out if it was real.” Emory was also one of the leaders in studying anticoagulant treatments for COVID-19 patients.
“Six months later I would say we’re definitely getting better outcomes on the floor, and our COVID patients aren’t landing in the ICU as easily,” Dr. Sheikh said. “It was scary at first, and doubly scary when doctors sometimes don’t feel they can say, ‘Hey, I’m scared too,’ or ‘By the way, I really don’t know what I’m doing.’ So, we’d be trying to reassure the patients when the information was coming to us in fragments.”
But he also believes that the pandemic has afforded hospitalists the opportunity to be the clinical detectives they were trained to be, sifting through clues. “I had to think more and really pay attention clinically in a much different way. You could say it was exciting and scary at the same time,” he said.
A human fix in the hospital
Dr. Pribula agreed that the pandemic has been both a difficult experience and a rewarding one. “I think of the people I first admitted. If they had shown up even a month later, would they still be with us?” He believes that his group and his field are going to get to a place where they have solid treatment plans for how to provide optimal care and how to protect providers from exposure.
One of the first COVID-19 patients in Fargo had dementia and was very distressed. “She had no idea why nobody was visiting or why we wouldn’t let her out of her room,” Dr. Pribula said. “Instead of reaching for sedatives, one of our nurses went into the room and talked with her, prayed a rosary, and played two hands of cards with her and didn’t have to sedate her. That’s what people need when they’re alone and scared. It wasn’t a medical fix but a human fix.”
A version of this article originally appeared on Medscape.com.
COVID frontline physicians afraid to seek mental health care
A new poll of emergency physicians on the front lines of the COVID-19 pandemic shows many are fearful of seeking mental health care for fear of stigma and the potential career impact.
The results of the nationally representative poll, conducted Oct. 7-13 by the American College of Emergency Physicians, showed almost half (45%) of 862 emergency physician respondents reported being uncomfortable seeking available psychiatric care. The poll had a margin of error of plus or minus 3 percentage points.
The findings provide new insight into both the challenges of serving in emergency medicine during the pandemic and the persistent barriers to mental health care in terms of stigma and concerns about potential career setbacks.
In the poll, with another 45% report they were feeling somewhat more stressed.
When asked about causes of stress related directly to COVID-19, 83% cited concerns about family and friends contracting COVID-19. Also factoring into emergency physicians’ stress and burnout were concerns about their own safety (80%) and lack of personal protective equipment or other needed resources (60%).
In the poll, 29% of respondents reported having excellent access to mental health treatment and 42% reported having good access. Despite this, 30% of respondents still reported feeling there was a lot of stigma in their workplace about seeking mental health treatment, with another 43% reporting they felt there was some stigma.
Poll results also showed that 24% of respondents were very concerned about what might happen with their employment if they were to seek mental health treatment, with another 33% saying they were somewhat concerned.
In recent years there have been efforts to break down cultural roadblocks in medicine that deter many physicians from seeking mental health treatment, but more needs to be done, said Mark Rosenberg, DO, MBA, who was elected president of ACEP at last weekend’s annual meeting, ACEP20.
“The pandemic emphatically underscores our need to change the status quo when it comes to physicians’ mental health,” Dr. Rosenberg said.
As previously reported by Medscape Medical News, current efforts to remove such barriers include initiatives to limit inquiries into clinicians’ past or present mental health treatment.
In May, the influential Joint Commission issued a statement urging organizations to refrain from asking about any history of mental health conditions or treatment. The Joint Commission said it supports recommendations already made by the Federation of State Medical Boards and the American Medical Association to limit inquiries on licensing applications to conditions that currently impair a clinician’s ability to perform their job.
Also supporting these efforts is the Dr. Lorna Breen Heroes’ Foundation, created in honor of an emergency physician who died by suicide in April amid the pandemic.
Lorna Breen, MD, had been working intensely in the response to the pandemic. During one shift, she covered two EDs in Manhattan at locations 5 miles apart, according to a backgrounder on the foundation’s web site.
At an ACEP press conference this week, Dr. Breen’s brother-in-law, J. Corey Feist, JD, MBA, cofounder of the foundation, noted that some states’ licensing applications for physicians include questions that fall outside of the boundaries of the Americans With Disabilities Act. He cited an analysis of state medical boards’ initial licensing questions published in 2018 in the Journal of the American Academy of Psychiatry and the Law.
In many cases, states have posed questions that extend beyond an assessment of a physician’s current ability to care for patients, creating a needless hurdle to seeking care, wrote the paper’s lead author, Carol North, MD, of the University of Texas Southwestern Medical Center, Dallas.
“Over the years, many medical licensure boards have asked applicants intrusive questions about whether they have any psychiatric history. This has created a major problem for applicants, and unfortunately this has discouraged many of those who need psychiatric treatment from seeking it because of fear of the questions,” Dr. North and colleagues noted. They cited Ohio as an example of a state that had overhauled its approach to questioning to bring it in compliance with the ADA.
Ohio previously required applicants to answer lengthy questions about their mental health, including:
- Within the last 10 years, have you been diagnosed with or have you been treated for bipolar disorder, schizophrenia, paranoia, or any other psychotic disorder?
- Have you, since attaining the age of eighteen or within the last 10 years, whichever period is shorter, been admitted to a hospital or other facility for the treatment of bipolar disorder, schizophrenia, paranoia, or any other psychotic disorder?
- Do you have, or have you been diagnosed as having, a medical condition which in any way impairs or limits your ability to practice medicine with reasonable skill and safety?
In the new version, the single question reads: “In the past 5 years, have you been diagnosed as having, or been hospitalized for, a medical condition which in any way impairs or limits your ability to practice medicine with reasonable skill and safety?”
Other states such as New York pose no mental health questions on applications for licensure.
Still, even when states have nondiscriminatory laws, physicians may not be aware of them, said Mr. Feist at an ACEP press conference. In addition to his work with the foundation, Mr. Feist is the CEO of the University of Virginia Physicians Group.
He said his sister-in-law Dr. Breen may have worried without cause about potential consequences of seeking psychiatric treatment during the pandemic. In addition, physicians in need of psychiatric care may worry about encountering hitches with medical organizations and insurers.
“This stigma and this fear of professional action on your license or your credentialing or privileging is pervasive throughout the industry,” he said.
A version of this article originally appeared on Medscape.com.
A new poll of emergency physicians on the front lines of the COVID-19 pandemic shows many are fearful of seeking mental health care for fear of stigma and the potential career impact.
The results of the nationally representative poll, conducted Oct. 7-13 by the American College of Emergency Physicians, showed almost half (45%) of 862 emergency physician respondents reported being uncomfortable seeking available psychiatric care. The poll had a margin of error of plus or minus 3 percentage points.
The findings provide new insight into both the challenges of serving in emergency medicine during the pandemic and the persistent barriers to mental health care in terms of stigma and concerns about potential career setbacks.
In the poll, with another 45% report they were feeling somewhat more stressed.
When asked about causes of stress related directly to COVID-19, 83% cited concerns about family and friends contracting COVID-19. Also factoring into emergency physicians’ stress and burnout were concerns about their own safety (80%) and lack of personal protective equipment or other needed resources (60%).
In the poll, 29% of respondents reported having excellent access to mental health treatment and 42% reported having good access. Despite this, 30% of respondents still reported feeling there was a lot of stigma in their workplace about seeking mental health treatment, with another 43% reporting they felt there was some stigma.
Poll results also showed that 24% of respondents were very concerned about what might happen with their employment if they were to seek mental health treatment, with another 33% saying they were somewhat concerned.
In recent years there have been efforts to break down cultural roadblocks in medicine that deter many physicians from seeking mental health treatment, but more needs to be done, said Mark Rosenberg, DO, MBA, who was elected president of ACEP at last weekend’s annual meeting, ACEP20.
“The pandemic emphatically underscores our need to change the status quo when it comes to physicians’ mental health,” Dr. Rosenberg said.
As previously reported by Medscape Medical News, current efforts to remove such barriers include initiatives to limit inquiries into clinicians’ past or present mental health treatment.
In May, the influential Joint Commission issued a statement urging organizations to refrain from asking about any history of mental health conditions or treatment. The Joint Commission said it supports recommendations already made by the Federation of State Medical Boards and the American Medical Association to limit inquiries on licensing applications to conditions that currently impair a clinician’s ability to perform their job.
Also supporting these efforts is the Dr. Lorna Breen Heroes’ Foundation, created in honor of an emergency physician who died by suicide in April amid the pandemic.
Lorna Breen, MD, had been working intensely in the response to the pandemic. During one shift, she covered two EDs in Manhattan at locations 5 miles apart, according to a backgrounder on the foundation’s web site.
At an ACEP press conference this week, Dr. Breen’s brother-in-law, J. Corey Feist, JD, MBA, cofounder of the foundation, noted that some states’ licensing applications for physicians include questions that fall outside of the boundaries of the Americans With Disabilities Act. He cited an analysis of state medical boards’ initial licensing questions published in 2018 in the Journal of the American Academy of Psychiatry and the Law.
In many cases, states have posed questions that extend beyond an assessment of a physician’s current ability to care for patients, creating a needless hurdle to seeking care, wrote the paper’s lead author, Carol North, MD, of the University of Texas Southwestern Medical Center, Dallas.
“Over the years, many medical licensure boards have asked applicants intrusive questions about whether they have any psychiatric history. This has created a major problem for applicants, and unfortunately this has discouraged many of those who need psychiatric treatment from seeking it because of fear of the questions,” Dr. North and colleagues noted. They cited Ohio as an example of a state that had overhauled its approach to questioning to bring it in compliance with the ADA.
Ohio previously required applicants to answer lengthy questions about their mental health, including:
- Within the last 10 years, have you been diagnosed with or have you been treated for bipolar disorder, schizophrenia, paranoia, or any other psychotic disorder?
- Have you, since attaining the age of eighteen or within the last 10 years, whichever period is shorter, been admitted to a hospital or other facility for the treatment of bipolar disorder, schizophrenia, paranoia, or any other psychotic disorder?
- Do you have, or have you been diagnosed as having, a medical condition which in any way impairs or limits your ability to practice medicine with reasonable skill and safety?
In the new version, the single question reads: “In the past 5 years, have you been diagnosed as having, or been hospitalized for, a medical condition which in any way impairs or limits your ability to practice medicine with reasonable skill and safety?”
Other states such as New York pose no mental health questions on applications for licensure.
Still, even when states have nondiscriminatory laws, physicians may not be aware of them, said Mr. Feist at an ACEP press conference. In addition to his work with the foundation, Mr. Feist is the CEO of the University of Virginia Physicians Group.
He said his sister-in-law Dr. Breen may have worried without cause about potential consequences of seeking psychiatric treatment during the pandemic. In addition, physicians in need of psychiatric care may worry about encountering hitches with medical organizations and insurers.
“This stigma and this fear of professional action on your license or your credentialing or privileging is pervasive throughout the industry,” he said.
A version of this article originally appeared on Medscape.com.
A new poll of emergency physicians on the front lines of the COVID-19 pandemic shows many are fearful of seeking mental health care for fear of stigma and the potential career impact.
The results of the nationally representative poll, conducted Oct. 7-13 by the American College of Emergency Physicians, showed almost half (45%) of 862 emergency physician respondents reported being uncomfortable seeking available psychiatric care. The poll had a margin of error of plus or minus 3 percentage points.
The findings provide new insight into both the challenges of serving in emergency medicine during the pandemic and the persistent barriers to mental health care in terms of stigma and concerns about potential career setbacks.
In the poll, with another 45% report they were feeling somewhat more stressed.
When asked about causes of stress related directly to COVID-19, 83% cited concerns about family and friends contracting COVID-19. Also factoring into emergency physicians’ stress and burnout were concerns about their own safety (80%) and lack of personal protective equipment or other needed resources (60%).
In the poll, 29% of respondents reported having excellent access to mental health treatment and 42% reported having good access. Despite this, 30% of respondents still reported feeling there was a lot of stigma in their workplace about seeking mental health treatment, with another 43% reporting they felt there was some stigma.
Poll results also showed that 24% of respondents were very concerned about what might happen with their employment if they were to seek mental health treatment, with another 33% saying they were somewhat concerned.
In recent years there have been efforts to break down cultural roadblocks in medicine that deter many physicians from seeking mental health treatment, but more needs to be done, said Mark Rosenberg, DO, MBA, who was elected president of ACEP at last weekend’s annual meeting, ACEP20.
“The pandemic emphatically underscores our need to change the status quo when it comes to physicians’ mental health,” Dr. Rosenberg said.
As previously reported by Medscape Medical News, current efforts to remove such barriers include initiatives to limit inquiries into clinicians’ past or present mental health treatment.
In May, the influential Joint Commission issued a statement urging organizations to refrain from asking about any history of mental health conditions or treatment. The Joint Commission said it supports recommendations already made by the Federation of State Medical Boards and the American Medical Association to limit inquiries on licensing applications to conditions that currently impair a clinician’s ability to perform their job.
Also supporting these efforts is the Dr. Lorna Breen Heroes’ Foundation, created in honor of an emergency physician who died by suicide in April amid the pandemic.
Lorna Breen, MD, had been working intensely in the response to the pandemic. During one shift, she covered two EDs in Manhattan at locations 5 miles apart, according to a backgrounder on the foundation’s web site.
At an ACEP press conference this week, Dr. Breen’s brother-in-law, J. Corey Feist, JD, MBA, cofounder of the foundation, noted that some states’ licensing applications for physicians include questions that fall outside of the boundaries of the Americans With Disabilities Act. He cited an analysis of state medical boards’ initial licensing questions published in 2018 in the Journal of the American Academy of Psychiatry and the Law.
In many cases, states have posed questions that extend beyond an assessment of a physician’s current ability to care for patients, creating a needless hurdle to seeking care, wrote the paper’s lead author, Carol North, MD, of the University of Texas Southwestern Medical Center, Dallas.
“Over the years, many medical licensure boards have asked applicants intrusive questions about whether they have any psychiatric history. This has created a major problem for applicants, and unfortunately this has discouraged many of those who need psychiatric treatment from seeking it because of fear of the questions,” Dr. North and colleagues noted. They cited Ohio as an example of a state that had overhauled its approach to questioning to bring it in compliance with the ADA.
Ohio previously required applicants to answer lengthy questions about their mental health, including:
- Within the last 10 years, have you been diagnosed with or have you been treated for bipolar disorder, schizophrenia, paranoia, or any other psychotic disorder?
- Have you, since attaining the age of eighteen or within the last 10 years, whichever period is shorter, been admitted to a hospital or other facility for the treatment of bipolar disorder, schizophrenia, paranoia, or any other psychotic disorder?
- Do you have, or have you been diagnosed as having, a medical condition which in any way impairs or limits your ability to practice medicine with reasonable skill and safety?
In the new version, the single question reads: “In the past 5 years, have you been diagnosed as having, or been hospitalized for, a medical condition which in any way impairs or limits your ability to practice medicine with reasonable skill and safety?”
Other states such as New York pose no mental health questions on applications for licensure.
Still, even when states have nondiscriminatory laws, physicians may not be aware of them, said Mr. Feist at an ACEP press conference. In addition to his work with the foundation, Mr. Feist is the CEO of the University of Virginia Physicians Group.
He said his sister-in-law Dr. Breen may have worried without cause about potential consequences of seeking psychiatric treatment during the pandemic. In addition, physicians in need of psychiatric care may worry about encountering hitches with medical organizations and insurers.
“This stigma and this fear of professional action on your license or your credentialing or privileging is pervasive throughout the industry,” he said.
A version of this article originally appeared on Medscape.com.
Fulminant C. diff debate: Fecal transplants or antibiotics?
Two experts at IDWeek 2020 debated the best treatment for patients with the most severe type of Clostridioides difficile infection – fulminant C. diff. The discussion pitted fecal microbiota transplants (FMT) from the stool of healthy donors against traditional antibiotics.
Fulminant C. diff infection (CDI) represents about 8% of all CDI cases and is often fatal. Patients frequently don’t respond to maximum antibiotic therapy.
Should these patients be treated with FMT before surgery is considered?
“Unequivocally, yes,” said Jessica R. Allegretti, MD, MPH, associate director of the Crohn’s and Colitis Center at Brigham and Women’s Hospital in Boston.
Patients face full colectomy
Fulminant infection, she says, typically requires a total abdominal colectomy with end ileostomy.
“Patients have a quite high perioperative and intraoperative mortality because this is typically an older population with significant comorbidities,” she said.
Often the patients are poor candidates for surgery, she added.
She pointed to the efficacy of FMT in studies such as one published in Gut Microbes in 2017. The study, by Monika Fischer, MD, of Indiana University, Indianapolis, and colleagues showed a 91% cure rate at 1 month in severe patients with an average of 1.5 fecal transplants, noting that was “quite remarkable” in this very sick population.
Though FMT is not approved by the US Food and Drug Administration for fulminant CDI, Dr. Allegretti said, the FDA does allow treatment under “enforcement discretion,” which means no investigational new drug license is needed specifically if treating CDI patients who haven’t responded to standard therapy, as long as proper consent has been obtained.
“This is a patient population that is likely going to die,” she said. “If you were the one in the ICU with fulminant C. diff and you’ve been on maximum therapy for 3-5 days and you’re not getting better, wouldn’t you want somebody to offer you a fecal transplant and give you the chance to recover and leave the hospital with your colon intact? The data suggest that is possible, with a high likelihood and a good safety profile.”
She said the most recent guidelines have supported FMT, and emerging guidelines coming within months “will support this as well.”
Unknowns with FMT
Taking the other side of the debate, Kevin Garey, PharmD, chair of the department of pharmacy practice and translational research at University of Houston College of Pharmacy, warned against trading traditional antibiotics, such as vancomycin and fidaxomicin, for the novelty of FMT.
“With the science of the microbiome and the novelty of fecal microbiota transplantation in expanding use, I think people have somewhat forgotten pharmacotherapy,” he said.
He pointed out safety concerns with FMT reported in June 2019, after which the FDA issued an alert. Two immunocompromised patients who received FMT, both from the same donor, developed invasive infections caused by extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli. One died.
The FDA explained that the donated FMT samples the patients received were not tested for ESBL-producing gram-negative organisms before use.
Dr. Allegretti agreed antibiotics play a role in treatment with FMT, but she argued that the safety profile of FMT remains strong and that the safety issues came from isolated incidents at a single center.
Dr. Garey countered that there are just too many unknowns with FMT.
“We will never know what the next superbug that’s going to land in an FMT is until we’ve identified that superbug in somebody – the next Candida auris, the next CRE [carbapenem-resistant Enterobacteriaceae], the next thing that’s going to show up in FMT – until we get rid of the ‘F,’ “ Dr. Garey said.
“[Until] we get microbial therapy that’s generated without the need for healthy donors, I think we’re always going to be in this problem.”
He said although FMT “has an amazing ability to alter a microbiome” it “pales in comparison” to vancomycin’s ability to do so.
Disruption of the microbiome is, without a doubt, a hallmark of C. diff, but we don’t have to run to FMT,” Dr. Garey said. “We can think about prophylaxis strategies, we can think about new drug development that spares the microbiota. The need for FMT might be a consequence of poor pharmacotherapy management, not a part of pharmacotherapy management.”
Moderator Sam Aitken, PharmD, MPH, a clinical pharmacy specialist in infectious disease at MD Anderson Cancer Center in Houston, said in an interview the speakers found some common ground.
“I think there was a general consensus between both Dr. Allegretti and Dr. Garey that both traditional therapeutics and fecal microbiota transplantation have a role to play in these patients, although there is still quite a bit of discussion around where those might be best positioned,” Dr. Aitken said.
He added, “There’s also a general consensus that there is not likely to be one right answer for all patients with multiple recurrent CDI.”
Dr. Allegretti, Dr. Garey, and Dr. Aitken have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Two experts at IDWeek 2020 debated the best treatment for patients with the most severe type of Clostridioides difficile infection – fulminant C. diff. The discussion pitted fecal microbiota transplants (FMT) from the stool of healthy donors against traditional antibiotics.
Fulminant C. diff infection (CDI) represents about 8% of all CDI cases and is often fatal. Patients frequently don’t respond to maximum antibiotic therapy.
Should these patients be treated with FMT before surgery is considered?
“Unequivocally, yes,” said Jessica R. Allegretti, MD, MPH, associate director of the Crohn’s and Colitis Center at Brigham and Women’s Hospital in Boston.
Patients face full colectomy
Fulminant infection, she says, typically requires a total abdominal colectomy with end ileostomy.
“Patients have a quite high perioperative and intraoperative mortality because this is typically an older population with significant comorbidities,” she said.
Often the patients are poor candidates for surgery, she added.
She pointed to the efficacy of FMT in studies such as one published in Gut Microbes in 2017. The study, by Monika Fischer, MD, of Indiana University, Indianapolis, and colleagues showed a 91% cure rate at 1 month in severe patients with an average of 1.5 fecal transplants, noting that was “quite remarkable” in this very sick population.
Though FMT is not approved by the US Food and Drug Administration for fulminant CDI, Dr. Allegretti said, the FDA does allow treatment under “enforcement discretion,” which means no investigational new drug license is needed specifically if treating CDI patients who haven’t responded to standard therapy, as long as proper consent has been obtained.
“This is a patient population that is likely going to die,” she said. “If you were the one in the ICU with fulminant C. diff and you’ve been on maximum therapy for 3-5 days and you’re not getting better, wouldn’t you want somebody to offer you a fecal transplant and give you the chance to recover and leave the hospital with your colon intact? The data suggest that is possible, with a high likelihood and a good safety profile.”
She said the most recent guidelines have supported FMT, and emerging guidelines coming within months “will support this as well.”
Unknowns with FMT
Taking the other side of the debate, Kevin Garey, PharmD, chair of the department of pharmacy practice and translational research at University of Houston College of Pharmacy, warned against trading traditional antibiotics, such as vancomycin and fidaxomicin, for the novelty of FMT.
“With the science of the microbiome and the novelty of fecal microbiota transplantation in expanding use, I think people have somewhat forgotten pharmacotherapy,” he said.
He pointed out safety concerns with FMT reported in June 2019, after which the FDA issued an alert. Two immunocompromised patients who received FMT, both from the same donor, developed invasive infections caused by extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli. One died.
The FDA explained that the donated FMT samples the patients received were not tested for ESBL-producing gram-negative organisms before use.
Dr. Allegretti agreed antibiotics play a role in treatment with FMT, but she argued that the safety profile of FMT remains strong and that the safety issues came from isolated incidents at a single center.
Dr. Garey countered that there are just too many unknowns with FMT.
“We will never know what the next superbug that’s going to land in an FMT is until we’ve identified that superbug in somebody – the next Candida auris, the next CRE [carbapenem-resistant Enterobacteriaceae], the next thing that’s going to show up in FMT – until we get rid of the ‘F,’ “ Dr. Garey said.
“[Until] we get microbial therapy that’s generated without the need for healthy donors, I think we’re always going to be in this problem.”
He said although FMT “has an amazing ability to alter a microbiome” it “pales in comparison” to vancomycin’s ability to do so.
Disruption of the microbiome is, without a doubt, a hallmark of C. diff, but we don’t have to run to FMT,” Dr. Garey said. “We can think about prophylaxis strategies, we can think about new drug development that spares the microbiota. The need for FMT might be a consequence of poor pharmacotherapy management, not a part of pharmacotherapy management.”
Moderator Sam Aitken, PharmD, MPH, a clinical pharmacy specialist in infectious disease at MD Anderson Cancer Center in Houston, said in an interview the speakers found some common ground.
“I think there was a general consensus between both Dr. Allegretti and Dr. Garey that both traditional therapeutics and fecal microbiota transplantation have a role to play in these patients, although there is still quite a bit of discussion around where those might be best positioned,” Dr. Aitken said.
He added, “There’s also a general consensus that there is not likely to be one right answer for all patients with multiple recurrent CDI.”
Dr. Allegretti, Dr. Garey, and Dr. Aitken have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Two experts at IDWeek 2020 debated the best treatment for patients with the most severe type of Clostridioides difficile infection – fulminant C. diff. The discussion pitted fecal microbiota transplants (FMT) from the stool of healthy donors against traditional antibiotics.
Fulminant C. diff infection (CDI) represents about 8% of all CDI cases and is often fatal. Patients frequently don’t respond to maximum antibiotic therapy.
Should these patients be treated with FMT before surgery is considered?
“Unequivocally, yes,” said Jessica R. Allegretti, MD, MPH, associate director of the Crohn’s and Colitis Center at Brigham and Women’s Hospital in Boston.
Patients face full colectomy
Fulminant infection, she says, typically requires a total abdominal colectomy with end ileostomy.
“Patients have a quite high perioperative and intraoperative mortality because this is typically an older population with significant comorbidities,” she said.
Often the patients are poor candidates for surgery, she added.
She pointed to the efficacy of FMT in studies such as one published in Gut Microbes in 2017. The study, by Monika Fischer, MD, of Indiana University, Indianapolis, and colleagues showed a 91% cure rate at 1 month in severe patients with an average of 1.5 fecal transplants, noting that was “quite remarkable” in this very sick population.
Though FMT is not approved by the US Food and Drug Administration for fulminant CDI, Dr. Allegretti said, the FDA does allow treatment under “enforcement discretion,” which means no investigational new drug license is needed specifically if treating CDI patients who haven’t responded to standard therapy, as long as proper consent has been obtained.
“This is a patient population that is likely going to die,” she said. “If you were the one in the ICU with fulminant C. diff and you’ve been on maximum therapy for 3-5 days and you’re not getting better, wouldn’t you want somebody to offer you a fecal transplant and give you the chance to recover and leave the hospital with your colon intact? The data suggest that is possible, with a high likelihood and a good safety profile.”
She said the most recent guidelines have supported FMT, and emerging guidelines coming within months “will support this as well.”
Unknowns with FMT
Taking the other side of the debate, Kevin Garey, PharmD, chair of the department of pharmacy practice and translational research at University of Houston College of Pharmacy, warned against trading traditional antibiotics, such as vancomycin and fidaxomicin, for the novelty of FMT.
“With the science of the microbiome and the novelty of fecal microbiota transplantation in expanding use, I think people have somewhat forgotten pharmacotherapy,” he said.
He pointed out safety concerns with FMT reported in June 2019, after which the FDA issued an alert. Two immunocompromised patients who received FMT, both from the same donor, developed invasive infections caused by extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli. One died.
The FDA explained that the donated FMT samples the patients received were not tested for ESBL-producing gram-negative organisms before use.
Dr. Allegretti agreed antibiotics play a role in treatment with FMT, but she argued that the safety profile of FMT remains strong and that the safety issues came from isolated incidents at a single center.
Dr. Garey countered that there are just too many unknowns with FMT.
“We will never know what the next superbug that’s going to land in an FMT is until we’ve identified that superbug in somebody – the next Candida auris, the next CRE [carbapenem-resistant Enterobacteriaceae], the next thing that’s going to show up in FMT – until we get rid of the ‘F,’ “ Dr. Garey said.
“[Until] we get microbial therapy that’s generated without the need for healthy donors, I think we’re always going to be in this problem.”
He said although FMT “has an amazing ability to alter a microbiome” it “pales in comparison” to vancomycin’s ability to do so.
Disruption of the microbiome is, without a doubt, a hallmark of C. diff, but we don’t have to run to FMT,” Dr. Garey said. “We can think about prophylaxis strategies, we can think about new drug development that spares the microbiota. The need for FMT might be a consequence of poor pharmacotherapy management, not a part of pharmacotherapy management.”
Moderator Sam Aitken, PharmD, MPH, a clinical pharmacy specialist in infectious disease at MD Anderson Cancer Center in Houston, said in an interview the speakers found some common ground.
“I think there was a general consensus between both Dr. Allegretti and Dr. Garey that both traditional therapeutics and fecal microbiota transplantation have a role to play in these patients, although there is still quite a bit of discussion around where those might be best positioned,” Dr. Aitken said.
He added, “There’s also a general consensus that there is not likely to be one right answer for all patients with multiple recurrent CDI.”
Dr. Allegretti, Dr. Garey, and Dr. Aitken have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Potentially practice-changing bacterial therapy trials analyzed
A new formulation of an existing antibacterial agent and a potential therapeutic approach to a challenging clinical problem were the focus of a session on potentially practice-changing clinical trials in antimicrobial therapy presented during IDWeek 2020, an annual scientific meeting on infectious diseases.
“I know it has been a big year for viral disease of course, with COVID, but there has been some really good work that has gone on in the bacterial space, and of course as those of you who are on service know, you may have your fair share of COVID patients, but these are infections that we still deal with on a daily basis,” said Michael Satlin, MD, an infectious disease specialist at Weill Cornell Medicine in New York.
He combed through studies published during the previous 12 months in leading medical journals, including the New England Journal of Medicine, JAMA network publications, Lancet Infectious Diseases, Lancet Respiratory Medicine, Clinical Infectious Diseases, and Clinical Microbiology and Infection, looking for randomized trials of interventions to treat bacterial infections, and selecting those most likely to change practice of U.S. infectious diseases practitioners.
He excluded meta-analyses, post hoc analyses, evaluations of diagnostic tests, stewardship, or any studies presented previously at IDWeek.
Two of the trials he highlighted are described here.
Fosfomycin for injection
In the United States, fosfomycin, the only antibiotic in its class, is currently available only in an oral sachet formulation (Monurol), “and typically we’ve only given this for patients with cystitis because we know that we don’t achieve significant levels [of drug] in the kidney or in the bloodstream for other types of infections,” Dr. Satlin said.
In Europe, however fosfomycin for injection (ZTI-01) has been available for several years.
“There’s been a lot of interest in fosfomycin because it has a different mechanism of action from other agents. It’s an epoxide antibiotic that inhibits early peptidoglycan synthesis by binding to MurA,” he explained.
The phase 2/3 randomized ZEUS trial compared ZTI-01 with piperacillin/tazobactam (pip/taz) for treatment of complicated urinary tract infection (UTI) including acute pyelonephritis.
A total of 465 hospitalized adults with suspected or microbiologically confirmed complicated UTI or acute pyelonephritis were randomized to 6 g of ZTI-01 every 8 hours or 4.5 g of intravenous pip/taz every 8 hours for a fixed 7-day course with no oral switch; patients with concomitant bacteremia (about 9% of the study population) could receive the assigned therapy for up to 14 days.
The primary endpoint of noninferiority of ZTI-01 was met and clinical cure rates were high and similar between the treatments, at approximately 91% each. Treatment-emergent adverse events, including hypokalemia and elevated serum aminotransferases, were mostly mild and transient.
The hypokalemia seen in the trial may be attributable to the high salt load of fosfomycin relative to pip/taz, Dr. Satlin said.
“How might this change your practice? Well, if IV fosfomycin is ever FDA [Food and Drug Administration] approved – and my understanding is that the delays have been more related to manufacturing than scientific quality of data – it could potentially be an alternative to beta-lactams and fluoroquinolones” and has activity against most extend spectrum beta-lactamase (ESBL)–producing Enterobacteriaceae, he said.
Fosfomycin susceptibility testing is challenging, however, with no Clinical & Laboratory Standards Institute (CLSI) or FDA breakpoints for Enterobacterales other than Escherichia coli, and there are questions about the step-down therapy.
“Do you just give a 3-gram sachet chaser when they walk out the door? Do you switch to another agent? I think that needs to be worked out,” he said.
Inhaled amikacin
“We know that some IV antibiotics, particularly for resistant organisms, may not achieve sufficient concentrations in the lung to treat pneumonia. We know that inhaled antibiotics can give a lot of concentration of that drug right at the at the site of infection, but we don’t really have [randomized controlled trial] data to see whether it really helps,” Dr. Satlin said.
The INHALE trial was a double-blind, placebo-controlled superiority trial to see whether adding inhaled amikacin to IV standard-of-care antibiotics could improve outcomes for mechanically ventilated patients with gram-negative pneumonia.
The investigators enrolled 725 adults who were receiving mechanical ventilation for pneumonia, 45% of who had ventilator-associated pneumonia (VAP). Of the total cohort, 508 patients analyzed for efficacy had gram-negative pathogens, including 32% with Pseudomonas aeurginosa, 29% with Acinetobacter baumannii, 30% with E. coli, and the remainder with Klebsiella pneumoniae.
Patients were randomized to standard-of-care intravenous antibiotics plus either inhaled amikacin 400 mg twice daily for 10 days or inhaled saline placebo.
“Of note, the median standard-of-care antibiotics in this study was 18 days, which is certainly longer than what our guidelines recommend.”
There was no significant difference between study arms in the primary endpoint of survival at days 28-32 for all patients who had received at least one dose of study drug, were infected with a gram-negative pathogen, and an Acute Physiology and Chronic Health Evaluation (APACHE) II score of at 10 or higher at diagnosis. The respective survival rates for the inhaled amikacin and placebo groups were 75% and 77%. The incidence of treatment-emergent adverse events or serious treatment-emergent adverse events were similar between the two treatment arms.
“No matter how you sliced and diced it – days of mechanical ventilation, duration of ICU stay – essentially they looked the same. Even for [extensively drug resistant] pathogens where you might expect that you’d see the benefit of inhaled amikacin, they didn’t really see a mortality benefit in this study,” Dr. Satlin said.
The study is practice changing, he said “because I think inhaled aminoglycosides should not be routinely added to the standard of care IV antibiotics for pneumonia in ventilated patients,” he said.
It’s still unclear whether inhaled aminoglycosides might play a role in the treatment of select patients infected with organisms resistant to all beta-lactams and fluoroquinolones, he added.
Tempting strategy
“Adding inhaled antibiotics is a tempting strategy for treatment of ventilated pneumonia, which often has poor outcomes,” commented Thomas Holland, MD, a hospitalist and infectious disease specialist at Duke University Hospital in Durham, N.C. “This is valuable and practical information as clinicians choose antibiotics regimens for this difficult-to-treat syndrome,” he said in an interview.
Dr. Holland comoderated the session in which Dr. Satlin presented the study findings and opinions.
No funding source for the presentation was reported. Dr. Satlin reported consulting for Shionogi and Achaogen and research grants from Allergan, Merck, and BioFire Diagnostics. Dr. Holland disclosed consulting fees and other material support from Basilea Pharmaceutica, Genetech, Karius and Theravance.
A new formulation of an existing antibacterial agent and a potential therapeutic approach to a challenging clinical problem were the focus of a session on potentially practice-changing clinical trials in antimicrobial therapy presented during IDWeek 2020, an annual scientific meeting on infectious diseases.
“I know it has been a big year for viral disease of course, with COVID, but there has been some really good work that has gone on in the bacterial space, and of course as those of you who are on service know, you may have your fair share of COVID patients, but these are infections that we still deal with on a daily basis,” said Michael Satlin, MD, an infectious disease specialist at Weill Cornell Medicine in New York.
He combed through studies published during the previous 12 months in leading medical journals, including the New England Journal of Medicine, JAMA network publications, Lancet Infectious Diseases, Lancet Respiratory Medicine, Clinical Infectious Diseases, and Clinical Microbiology and Infection, looking for randomized trials of interventions to treat bacterial infections, and selecting those most likely to change practice of U.S. infectious diseases practitioners.
He excluded meta-analyses, post hoc analyses, evaluations of diagnostic tests, stewardship, or any studies presented previously at IDWeek.
Two of the trials he highlighted are described here.
Fosfomycin for injection
In the United States, fosfomycin, the only antibiotic in its class, is currently available only in an oral sachet formulation (Monurol), “and typically we’ve only given this for patients with cystitis because we know that we don’t achieve significant levels [of drug] in the kidney or in the bloodstream for other types of infections,” Dr. Satlin said.
In Europe, however fosfomycin for injection (ZTI-01) has been available for several years.
“There’s been a lot of interest in fosfomycin because it has a different mechanism of action from other agents. It’s an epoxide antibiotic that inhibits early peptidoglycan synthesis by binding to MurA,” he explained.
The phase 2/3 randomized ZEUS trial compared ZTI-01 with piperacillin/tazobactam (pip/taz) for treatment of complicated urinary tract infection (UTI) including acute pyelonephritis.
A total of 465 hospitalized adults with suspected or microbiologically confirmed complicated UTI or acute pyelonephritis were randomized to 6 g of ZTI-01 every 8 hours or 4.5 g of intravenous pip/taz every 8 hours for a fixed 7-day course with no oral switch; patients with concomitant bacteremia (about 9% of the study population) could receive the assigned therapy for up to 14 days.
The primary endpoint of noninferiority of ZTI-01 was met and clinical cure rates were high and similar between the treatments, at approximately 91% each. Treatment-emergent adverse events, including hypokalemia and elevated serum aminotransferases, were mostly mild and transient.
The hypokalemia seen in the trial may be attributable to the high salt load of fosfomycin relative to pip/taz, Dr. Satlin said.
“How might this change your practice? Well, if IV fosfomycin is ever FDA [Food and Drug Administration] approved – and my understanding is that the delays have been more related to manufacturing than scientific quality of data – it could potentially be an alternative to beta-lactams and fluoroquinolones” and has activity against most extend spectrum beta-lactamase (ESBL)–producing Enterobacteriaceae, he said.
Fosfomycin susceptibility testing is challenging, however, with no Clinical & Laboratory Standards Institute (CLSI) or FDA breakpoints for Enterobacterales other than Escherichia coli, and there are questions about the step-down therapy.
“Do you just give a 3-gram sachet chaser when they walk out the door? Do you switch to another agent? I think that needs to be worked out,” he said.
Inhaled amikacin
“We know that some IV antibiotics, particularly for resistant organisms, may not achieve sufficient concentrations in the lung to treat pneumonia. We know that inhaled antibiotics can give a lot of concentration of that drug right at the at the site of infection, but we don’t really have [randomized controlled trial] data to see whether it really helps,” Dr. Satlin said.
The INHALE trial was a double-blind, placebo-controlled superiority trial to see whether adding inhaled amikacin to IV standard-of-care antibiotics could improve outcomes for mechanically ventilated patients with gram-negative pneumonia.
The investigators enrolled 725 adults who were receiving mechanical ventilation for pneumonia, 45% of who had ventilator-associated pneumonia (VAP). Of the total cohort, 508 patients analyzed for efficacy had gram-negative pathogens, including 32% with Pseudomonas aeurginosa, 29% with Acinetobacter baumannii, 30% with E. coli, and the remainder with Klebsiella pneumoniae.
Patients were randomized to standard-of-care intravenous antibiotics plus either inhaled amikacin 400 mg twice daily for 10 days or inhaled saline placebo.
“Of note, the median standard-of-care antibiotics in this study was 18 days, which is certainly longer than what our guidelines recommend.”
There was no significant difference between study arms in the primary endpoint of survival at days 28-32 for all patients who had received at least one dose of study drug, were infected with a gram-negative pathogen, and an Acute Physiology and Chronic Health Evaluation (APACHE) II score of at 10 or higher at diagnosis. The respective survival rates for the inhaled amikacin and placebo groups were 75% and 77%. The incidence of treatment-emergent adverse events or serious treatment-emergent adverse events were similar between the two treatment arms.
“No matter how you sliced and diced it – days of mechanical ventilation, duration of ICU stay – essentially they looked the same. Even for [extensively drug resistant] pathogens where you might expect that you’d see the benefit of inhaled amikacin, they didn’t really see a mortality benefit in this study,” Dr. Satlin said.
The study is practice changing, he said “because I think inhaled aminoglycosides should not be routinely added to the standard of care IV antibiotics for pneumonia in ventilated patients,” he said.
It’s still unclear whether inhaled aminoglycosides might play a role in the treatment of select patients infected with organisms resistant to all beta-lactams and fluoroquinolones, he added.
Tempting strategy
“Adding inhaled antibiotics is a tempting strategy for treatment of ventilated pneumonia, which often has poor outcomes,” commented Thomas Holland, MD, a hospitalist and infectious disease specialist at Duke University Hospital in Durham, N.C. “This is valuable and practical information as clinicians choose antibiotics regimens for this difficult-to-treat syndrome,” he said in an interview.
Dr. Holland comoderated the session in which Dr. Satlin presented the study findings and opinions.
No funding source for the presentation was reported. Dr. Satlin reported consulting for Shionogi and Achaogen and research grants from Allergan, Merck, and BioFire Diagnostics. Dr. Holland disclosed consulting fees and other material support from Basilea Pharmaceutica, Genetech, Karius and Theravance.
A new formulation of an existing antibacterial agent and a potential therapeutic approach to a challenging clinical problem were the focus of a session on potentially practice-changing clinical trials in antimicrobial therapy presented during IDWeek 2020, an annual scientific meeting on infectious diseases.
“I know it has been a big year for viral disease of course, with COVID, but there has been some really good work that has gone on in the bacterial space, and of course as those of you who are on service know, you may have your fair share of COVID patients, but these are infections that we still deal with on a daily basis,” said Michael Satlin, MD, an infectious disease specialist at Weill Cornell Medicine in New York.
He combed through studies published during the previous 12 months in leading medical journals, including the New England Journal of Medicine, JAMA network publications, Lancet Infectious Diseases, Lancet Respiratory Medicine, Clinical Infectious Diseases, and Clinical Microbiology and Infection, looking for randomized trials of interventions to treat bacterial infections, and selecting those most likely to change practice of U.S. infectious diseases practitioners.
He excluded meta-analyses, post hoc analyses, evaluations of diagnostic tests, stewardship, or any studies presented previously at IDWeek.
Two of the trials he highlighted are described here.
Fosfomycin for injection
In the United States, fosfomycin, the only antibiotic in its class, is currently available only in an oral sachet formulation (Monurol), “and typically we’ve only given this for patients with cystitis because we know that we don’t achieve significant levels [of drug] in the kidney or in the bloodstream for other types of infections,” Dr. Satlin said.
In Europe, however fosfomycin for injection (ZTI-01) has been available for several years.
“There’s been a lot of interest in fosfomycin because it has a different mechanism of action from other agents. It’s an epoxide antibiotic that inhibits early peptidoglycan synthesis by binding to MurA,” he explained.
The phase 2/3 randomized ZEUS trial compared ZTI-01 with piperacillin/tazobactam (pip/taz) for treatment of complicated urinary tract infection (UTI) including acute pyelonephritis.
A total of 465 hospitalized adults with suspected or microbiologically confirmed complicated UTI or acute pyelonephritis were randomized to 6 g of ZTI-01 every 8 hours or 4.5 g of intravenous pip/taz every 8 hours for a fixed 7-day course with no oral switch; patients with concomitant bacteremia (about 9% of the study population) could receive the assigned therapy for up to 14 days.
The primary endpoint of noninferiority of ZTI-01 was met and clinical cure rates were high and similar between the treatments, at approximately 91% each. Treatment-emergent adverse events, including hypokalemia and elevated serum aminotransferases, were mostly mild and transient.
The hypokalemia seen in the trial may be attributable to the high salt load of fosfomycin relative to pip/taz, Dr. Satlin said.
“How might this change your practice? Well, if IV fosfomycin is ever FDA [Food and Drug Administration] approved – and my understanding is that the delays have been more related to manufacturing than scientific quality of data – it could potentially be an alternative to beta-lactams and fluoroquinolones” and has activity against most extend spectrum beta-lactamase (ESBL)–producing Enterobacteriaceae, he said.
Fosfomycin susceptibility testing is challenging, however, with no Clinical & Laboratory Standards Institute (CLSI) or FDA breakpoints for Enterobacterales other than Escherichia coli, and there are questions about the step-down therapy.
“Do you just give a 3-gram sachet chaser when they walk out the door? Do you switch to another agent? I think that needs to be worked out,” he said.
Inhaled amikacin
“We know that some IV antibiotics, particularly for resistant organisms, may not achieve sufficient concentrations in the lung to treat pneumonia. We know that inhaled antibiotics can give a lot of concentration of that drug right at the at the site of infection, but we don’t really have [randomized controlled trial] data to see whether it really helps,” Dr. Satlin said.
The INHALE trial was a double-blind, placebo-controlled superiority trial to see whether adding inhaled amikacin to IV standard-of-care antibiotics could improve outcomes for mechanically ventilated patients with gram-negative pneumonia.
The investigators enrolled 725 adults who were receiving mechanical ventilation for pneumonia, 45% of who had ventilator-associated pneumonia (VAP). Of the total cohort, 508 patients analyzed for efficacy had gram-negative pathogens, including 32% with Pseudomonas aeurginosa, 29% with Acinetobacter baumannii, 30% with E. coli, and the remainder with Klebsiella pneumoniae.
Patients were randomized to standard-of-care intravenous antibiotics plus either inhaled amikacin 400 mg twice daily for 10 days or inhaled saline placebo.
“Of note, the median standard-of-care antibiotics in this study was 18 days, which is certainly longer than what our guidelines recommend.”
There was no significant difference between study arms in the primary endpoint of survival at days 28-32 for all patients who had received at least one dose of study drug, were infected with a gram-negative pathogen, and an Acute Physiology and Chronic Health Evaluation (APACHE) II score of at 10 or higher at diagnosis. The respective survival rates for the inhaled amikacin and placebo groups were 75% and 77%. The incidence of treatment-emergent adverse events or serious treatment-emergent adverse events were similar between the two treatment arms.
“No matter how you sliced and diced it – days of mechanical ventilation, duration of ICU stay – essentially they looked the same. Even for [extensively drug resistant] pathogens where you might expect that you’d see the benefit of inhaled amikacin, they didn’t really see a mortality benefit in this study,” Dr. Satlin said.
The study is practice changing, he said “because I think inhaled aminoglycosides should not be routinely added to the standard of care IV antibiotics for pneumonia in ventilated patients,” he said.
It’s still unclear whether inhaled aminoglycosides might play a role in the treatment of select patients infected with organisms resistant to all beta-lactams and fluoroquinolones, he added.
Tempting strategy
“Adding inhaled antibiotics is a tempting strategy for treatment of ventilated pneumonia, which often has poor outcomes,” commented Thomas Holland, MD, a hospitalist and infectious disease specialist at Duke University Hospital in Durham, N.C. “This is valuable and practical information as clinicians choose antibiotics regimens for this difficult-to-treat syndrome,” he said in an interview.
Dr. Holland comoderated the session in which Dr. Satlin presented the study findings and opinions.
No funding source for the presentation was reported. Dr. Satlin reported consulting for Shionogi and Achaogen and research grants from Allergan, Merck, and BioFire Diagnostics. Dr. Holland disclosed consulting fees and other material support from Basilea Pharmaceutica, Genetech, Karius and Theravance.
FROM IDWEEK 2020
Vertebral fractures in COVID-19 linked to mortality
Vertebral fractures appear to be common in people with severe COVID-19, and also raise the mortality risk, findings from a retrospective cohort suggest.
Among 114 patients with COVID-19 who underwent lateral chest x-rays at the San Raffaele Hospital ED in Milan, more than a third were found to have thoracic vertebral fractures. And, those individuals were more than twice as likely to die as were those without vertebral fractures.
“Morphometric vertebral fractures are one of the most common comorbidities among adults hospitalized with COVID-19, and the presence of such fractures may predict the severity of disease outcomes,” lead investigator Andrea Giustina, MD, said in an interview.
This is the first study to examine vertebral fracture prevalence in any coronavirus disease, but such fractures have been linked to an increased risk of pneumonia and impaired respiratory function, including restrictive pulmonary dysfunction. One possible mechanism may be that they cause anatomical changes, such as kyphosis, which negatively impact respiratory function by decreasing vital capacity, forced expiratory volume in 1 second, and inspiratory time, explained Dr. Giustina, professor of endocrinology, San Raffaele Vita Salute University, Milan, and president of the European Society of Endocrinology. The results were published in the Journal of Clinical Endocrinology and Metabolism.
Clinically, the findings suggest that all patients with COVID-19 who are undergoing chest x-rays should have morphometric vertebral x-ray evaluation, said Dr. Giustina.
“One interesting aspect of the study is that without morphometry, approximatively two thirds of vertebral fractures [would have been] missed. Therefore, they are largely underestimated in clinical practice,” he noted.
Thoracic vertebral fractures assessed via lateral chest x-rays
The 114 study subjects included were those whose lateral chest x-rays allowed for a high-quality assessment and in which all the thoracic tract of T4-T12 were viewable and assessable. None had been using glucocorticoids and only 3% had a prior diagnosis of osteoporosis.
The majority (75%) were male, and median age was 57 years. Most (79%) were hospitalized after evaluation in the ED. Of those, 12% (13) were admitted to the ICU and 15% (16) died.
Thoracic vertebral fractures were detected on the lateral chest x-rays in 36% (41) of the patients. In contrast, in studies of women aged 50 years and older from the general European population, morphometric vertebral fracture prevalence ranged from 18% to 26%, the investigators noted.
Of the total 65 vertebral fractures detected, 60% were classified as mild (height ratio decrease <25%), 33.3% as moderate (25%-40% decrease) and 7.7% as severe (>40%). Patients with more than one vertebral fracture were classified by their most severe one.
Those with versus without vertebral fractures didn’t differ by sex, body mass index, or clinical or biological parameters evaluated in the ED. But, compared with those without vertebral fractures, those with them were significantly older (68 vs. 54 years) and were more likely to have arterial hypertension (56% vs. 30%) and coronary artery disease (22% vs. 7%).
In multivariate analysis, age was the only statistically significant predictor of vertebral fractures (odds ratio, 1.04; P < .001).
Mortality doubled, though not significantly
Those with vertebral fractures were more likely to be hospitalized, although not significantly (88% vs. 74%). There was no significant difference in ICU admission (11% vs. 12.5%).
However, those with vertebral fractures required noninvasive mechanical ventilation significantly more often (48.8% vs. 27.4%; P = .02), and were more than twice as likely to die (22% vs. 10%; P = .07). While the difference in overall mortality wasn’t quite statistically significant, those with severe vertebral fractures were significantly more likely to die, compared with those with mild or moderate fractures (60%, 7%, 24%, respectively, for severe, moderate, and mild; P = .04), despite no significant differences in clinical or laboratory parameters.
“Our data from the field reinforce the need of implementing previously published recommendations concerning the importance of bone fragility care during the COVID pandemic with at least those patients already treated with antiosteoporotic drugs maintaining their adherence to treatments including vitamin D, which have also been suggested very recently to have no relevant predisposing effect on COVID-19,” Dr. Giustina and colleagues wrote.
Moreover, they added, “continuity of care should also include bone density monitoring despite very restricted access to clinical facilities, during the COVID-19 pandemic. Finally, all patients with fractures should start antiresorptive treatment right away, even during hospital stay.”
The authors reported having no disclosures.
SOURCE: Giustina A et al. J Clin Endocrinol Metab. 2020 Oct 21. doi: 10.1210/clinem/dgaa738.
Vertebral fractures appear to be common in people with severe COVID-19, and also raise the mortality risk, findings from a retrospective cohort suggest.
Among 114 patients with COVID-19 who underwent lateral chest x-rays at the San Raffaele Hospital ED in Milan, more than a third were found to have thoracic vertebral fractures. And, those individuals were more than twice as likely to die as were those without vertebral fractures.
“Morphometric vertebral fractures are one of the most common comorbidities among adults hospitalized with COVID-19, and the presence of such fractures may predict the severity of disease outcomes,” lead investigator Andrea Giustina, MD, said in an interview.
This is the first study to examine vertebral fracture prevalence in any coronavirus disease, but such fractures have been linked to an increased risk of pneumonia and impaired respiratory function, including restrictive pulmonary dysfunction. One possible mechanism may be that they cause anatomical changes, such as kyphosis, which negatively impact respiratory function by decreasing vital capacity, forced expiratory volume in 1 second, and inspiratory time, explained Dr. Giustina, professor of endocrinology, San Raffaele Vita Salute University, Milan, and president of the European Society of Endocrinology. The results were published in the Journal of Clinical Endocrinology and Metabolism.
Clinically, the findings suggest that all patients with COVID-19 who are undergoing chest x-rays should have morphometric vertebral x-ray evaluation, said Dr. Giustina.
“One interesting aspect of the study is that without morphometry, approximatively two thirds of vertebral fractures [would have been] missed. Therefore, they are largely underestimated in clinical practice,” he noted.
Thoracic vertebral fractures assessed via lateral chest x-rays
The 114 study subjects included were those whose lateral chest x-rays allowed for a high-quality assessment and in which all the thoracic tract of T4-T12 were viewable and assessable. None had been using glucocorticoids and only 3% had a prior diagnosis of osteoporosis.
The majority (75%) were male, and median age was 57 years. Most (79%) were hospitalized after evaluation in the ED. Of those, 12% (13) were admitted to the ICU and 15% (16) died.
Thoracic vertebral fractures were detected on the lateral chest x-rays in 36% (41) of the patients. In contrast, in studies of women aged 50 years and older from the general European population, morphometric vertebral fracture prevalence ranged from 18% to 26%, the investigators noted.
Of the total 65 vertebral fractures detected, 60% were classified as mild (height ratio decrease <25%), 33.3% as moderate (25%-40% decrease) and 7.7% as severe (>40%). Patients with more than one vertebral fracture were classified by their most severe one.
Those with versus without vertebral fractures didn’t differ by sex, body mass index, or clinical or biological parameters evaluated in the ED. But, compared with those without vertebral fractures, those with them were significantly older (68 vs. 54 years) and were more likely to have arterial hypertension (56% vs. 30%) and coronary artery disease (22% vs. 7%).
In multivariate analysis, age was the only statistically significant predictor of vertebral fractures (odds ratio, 1.04; P < .001).
Mortality doubled, though not significantly
Those with vertebral fractures were more likely to be hospitalized, although not significantly (88% vs. 74%). There was no significant difference in ICU admission (11% vs. 12.5%).
However, those with vertebral fractures required noninvasive mechanical ventilation significantly more often (48.8% vs. 27.4%; P = .02), and were more than twice as likely to die (22% vs. 10%; P = .07). While the difference in overall mortality wasn’t quite statistically significant, those with severe vertebral fractures were significantly more likely to die, compared with those with mild or moderate fractures (60%, 7%, 24%, respectively, for severe, moderate, and mild; P = .04), despite no significant differences in clinical or laboratory parameters.
“Our data from the field reinforce the need of implementing previously published recommendations concerning the importance of bone fragility care during the COVID pandemic with at least those patients already treated with antiosteoporotic drugs maintaining their adherence to treatments including vitamin D, which have also been suggested very recently to have no relevant predisposing effect on COVID-19,” Dr. Giustina and colleagues wrote.
Moreover, they added, “continuity of care should also include bone density monitoring despite very restricted access to clinical facilities, during the COVID-19 pandemic. Finally, all patients with fractures should start antiresorptive treatment right away, even during hospital stay.”
The authors reported having no disclosures.
SOURCE: Giustina A et al. J Clin Endocrinol Metab. 2020 Oct 21. doi: 10.1210/clinem/dgaa738.
Vertebral fractures appear to be common in people with severe COVID-19, and also raise the mortality risk, findings from a retrospective cohort suggest.
Among 114 patients with COVID-19 who underwent lateral chest x-rays at the San Raffaele Hospital ED in Milan, more than a third were found to have thoracic vertebral fractures. And, those individuals were more than twice as likely to die as were those without vertebral fractures.
“Morphometric vertebral fractures are one of the most common comorbidities among adults hospitalized with COVID-19, and the presence of such fractures may predict the severity of disease outcomes,” lead investigator Andrea Giustina, MD, said in an interview.
This is the first study to examine vertebral fracture prevalence in any coronavirus disease, but such fractures have been linked to an increased risk of pneumonia and impaired respiratory function, including restrictive pulmonary dysfunction. One possible mechanism may be that they cause anatomical changes, such as kyphosis, which negatively impact respiratory function by decreasing vital capacity, forced expiratory volume in 1 second, and inspiratory time, explained Dr. Giustina, professor of endocrinology, San Raffaele Vita Salute University, Milan, and president of the European Society of Endocrinology. The results were published in the Journal of Clinical Endocrinology and Metabolism.
Clinically, the findings suggest that all patients with COVID-19 who are undergoing chest x-rays should have morphometric vertebral x-ray evaluation, said Dr. Giustina.
“One interesting aspect of the study is that without morphometry, approximatively two thirds of vertebral fractures [would have been] missed. Therefore, they are largely underestimated in clinical practice,” he noted.
Thoracic vertebral fractures assessed via lateral chest x-rays
The 114 study subjects included were those whose lateral chest x-rays allowed for a high-quality assessment and in which all the thoracic tract of T4-T12 were viewable and assessable. None had been using glucocorticoids and only 3% had a prior diagnosis of osteoporosis.
The majority (75%) were male, and median age was 57 years. Most (79%) were hospitalized after evaluation in the ED. Of those, 12% (13) were admitted to the ICU and 15% (16) died.
Thoracic vertebral fractures were detected on the lateral chest x-rays in 36% (41) of the patients. In contrast, in studies of women aged 50 years and older from the general European population, morphometric vertebral fracture prevalence ranged from 18% to 26%, the investigators noted.
Of the total 65 vertebral fractures detected, 60% were classified as mild (height ratio decrease <25%), 33.3% as moderate (25%-40% decrease) and 7.7% as severe (>40%). Patients with more than one vertebral fracture were classified by their most severe one.
Those with versus without vertebral fractures didn’t differ by sex, body mass index, or clinical or biological parameters evaluated in the ED. But, compared with those without vertebral fractures, those with them were significantly older (68 vs. 54 years) and were more likely to have arterial hypertension (56% vs. 30%) and coronary artery disease (22% vs. 7%).
In multivariate analysis, age was the only statistically significant predictor of vertebral fractures (odds ratio, 1.04; P < .001).
Mortality doubled, though not significantly
Those with vertebral fractures were more likely to be hospitalized, although not significantly (88% vs. 74%). There was no significant difference in ICU admission (11% vs. 12.5%).
However, those with vertebral fractures required noninvasive mechanical ventilation significantly more often (48.8% vs. 27.4%; P = .02), and were more than twice as likely to die (22% vs. 10%; P = .07). While the difference in overall mortality wasn’t quite statistically significant, those with severe vertebral fractures were significantly more likely to die, compared with those with mild or moderate fractures (60%, 7%, 24%, respectively, for severe, moderate, and mild; P = .04), despite no significant differences in clinical or laboratory parameters.
“Our data from the field reinforce the need of implementing previously published recommendations concerning the importance of bone fragility care during the COVID pandemic with at least those patients already treated with antiosteoporotic drugs maintaining their adherence to treatments including vitamin D, which have also been suggested very recently to have no relevant predisposing effect on COVID-19,” Dr. Giustina and colleagues wrote.
Moreover, they added, “continuity of care should also include bone density monitoring despite very restricted access to clinical facilities, during the COVID-19 pandemic. Finally, all patients with fractures should start antiresorptive treatment right away, even during hospital stay.”
The authors reported having no disclosures.
SOURCE: Giustina A et al. J Clin Endocrinol Metab. 2020 Oct 21. doi: 10.1210/clinem/dgaa738.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
The new one-percenters: Children with COVID-19
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
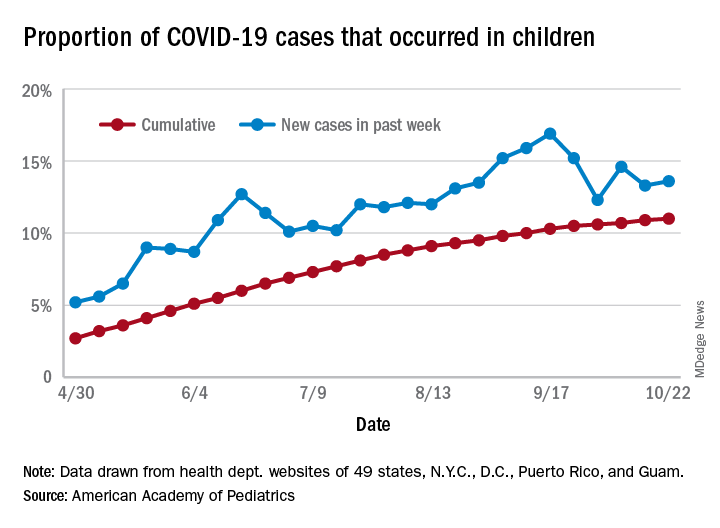
There have been 1,052 cases of COVID-19 per 100,000 children as of Oct. 22, and that works out to 1.05% of all children in the country. The cumulative number of pediatric cases is 792,188, and children now represent 11% of all COVID-19 cases, the AAP and the CHA reported Oct. 26.
There were just over 50,000 new child cases reported in the week ending Oct. 22, which was 13.6% of the national total of almost 370,000. That’s up slightly from the 13.3% the previous week but still down from the spike seen in mid-September, based on the data collected from the websites of 49 state health departments (New York does not report ages), along with the District of Columbia, New York City, Puerto Rico, and Guam.
The state-level data show that California has had more COVID-19 cases in children (92,864) than any other state, although Texas has reported ages for only 7% of its confirmed cases. Illinois is next with 46,006 cases, followed by Florida at 45,575, although Florida is using an age range of 0-14 years to define a child case, the AAP and CHA noted.
Other measures largely put small states at the extremes:
- North Dakota has the highest cumulative rate: 2,954 cases per 100,000 children.
- Vermont has the lowest cumulative rate: 190.5 per 100,000.
- Wyoming has the highest proportion of cases in children: 27.7%.
- New Jersey has the lowest proportion of child cases: 4.6%.
There were no COVID-19–related deaths in children reported the week ending Oct. 22, so the total number remains at 120, which is just 0.06% of the total for all ages, based on data from 42 states and New York City. Hospitalization figures put admissions at almost 5,600 in children, or 1.7% of all hospitalizations, although those data come from just 24 states and New York City, the AAP and CHA said.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There have been 1,052 cases of COVID-19 per 100,000 children as of Oct. 22, and that works out to 1.05% of all children in the country. The cumulative number of pediatric cases is 792,188, and children now represent 11% of all COVID-19 cases, the AAP and the CHA reported Oct. 26.
There were just over 50,000 new child cases reported in the week ending Oct. 22, which was 13.6% of the national total of almost 370,000. That’s up slightly from the 13.3% the previous week but still down from the spike seen in mid-September, based on the data collected from the websites of 49 state health departments (New York does not report ages), along with the District of Columbia, New York City, Puerto Rico, and Guam.
The state-level data show that California has had more COVID-19 cases in children (92,864) than any other state, although Texas has reported ages for only 7% of its confirmed cases. Illinois is next with 46,006 cases, followed by Florida at 45,575, although Florida is using an age range of 0-14 years to define a child case, the AAP and CHA noted.
Other measures largely put small states at the extremes:
- North Dakota has the highest cumulative rate: 2,954 cases per 100,000 children.
- Vermont has the lowest cumulative rate: 190.5 per 100,000.
- Wyoming has the highest proportion of cases in children: 27.7%.
- New Jersey has the lowest proportion of child cases: 4.6%.
There were no COVID-19–related deaths in children reported the week ending Oct. 22, so the total number remains at 120, which is just 0.06% of the total for all ages, based on data from 42 states and New York City. Hospitalization figures put admissions at almost 5,600 in children, or 1.7% of all hospitalizations, although those data come from just 24 states and New York City, the AAP and CHA said.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There have been 1,052 cases of COVID-19 per 100,000 children as of Oct. 22, and that works out to 1.05% of all children in the country. The cumulative number of pediatric cases is 792,188, and children now represent 11% of all COVID-19 cases, the AAP and the CHA reported Oct. 26.
There were just over 50,000 new child cases reported in the week ending Oct. 22, which was 13.6% of the national total of almost 370,000. That’s up slightly from the 13.3% the previous week but still down from the spike seen in mid-September, based on the data collected from the websites of 49 state health departments (New York does not report ages), along with the District of Columbia, New York City, Puerto Rico, and Guam.
The state-level data show that California has had more COVID-19 cases in children (92,864) than any other state, although Texas has reported ages for only 7% of its confirmed cases. Illinois is next with 46,006 cases, followed by Florida at 45,575, although Florida is using an age range of 0-14 years to define a child case, the AAP and CHA noted.
Other measures largely put small states at the extremes:
- North Dakota has the highest cumulative rate: 2,954 cases per 100,000 children.
- Vermont has the lowest cumulative rate: 190.5 per 100,000.
- Wyoming has the highest proportion of cases in children: 27.7%.
- New Jersey has the lowest proportion of child cases: 4.6%.
There were no COVID-19–related deaths in children reported the week ending Oct. 22, so the total number remains at 120, which is just 0.06% of the total for all ages, based on data from 42 states and New York City. Hospitalization figures put admissions at almost 5,600 in children, or 1.7% of all hospitalizations, although those data come from just 24 states and New York City, the AAP and CHA said.
Few women hospitalized for influenza have been vaccinated
Researchers analyzed data from 9,652 women ages 15-44 who were hospitalized with laboratory-confirmed influenza from October through April during the 2010-2019 influenza seasons. Data were pulled from the U.S. Influenza Hospitalization Surveillance Network (FluSurv-NET).
Of those women, 2,697 (28%) were pregnant. Median age was 28 and median gestational age was 32 weeks. Those studied included 36% who were non-Hispanic White; 29% non-Hispanic Black; and 20% Hispanic women.
Some 89% of the women, pregnant and nonpregnant, received antivirals while in the hospital but only 31% reported they had received the flu vaccine in the current season, despite guideline recommendations citing clear evidence that vaccination is safe for mother and baby.
Rachel Holstein, MPH, an epidemiology and information science fellow at the Centers for Disease Control and Prevention, who presented her team’s work as part of IDWeek 2020, explained that the mother’s vaccination can help protect the baby from flu infection for several months after birth, before the baby can be vaccinated.
She noted that pregnant women are at high risk for influenza-associated hospitalization.
“Changes in the immune system, heart, and lungs during pregnancy make pregnant women, and women up to 2 weeks post partum, more prone to severe illness from flu, including illness resulting in hospitalization,” she said in an interview
“Vaccination has been shown to reduce the risk of flu-associated acute respiratory infection in pregnant women by up to one-half,” she said. “A 2018 study showed that getting a flu shot reduced a pregnant woman’s risk of being hospitalized with flu by an average of 40%.»
FluSurv-NET data show hospitalizations were more common in the third trimester of pregnancy compared with the first and second, Holstein said. The most common underlying conditions among these women were asthma (23%) and obesity (10%), and 12% were current tobacco smokers. Overall, 5% of pregnant women with flu required ICU admission, 2% needed mechanical ventilation, and 6% developed pneumonia.
Vaccine uptake lowest in first two trimesters
Holstein said vaccine coverage was lowest among women in their first or second trimesters for all 9 seasons, and overall vaccination coverage increased significantly over time.
Uptake also differed by age. The data showed coverage was lower among women aged 15-34 years, compared with women 35 years and older (34% vs. 50%).
“It was as low as 15% among pregnant women aged 15-34 years in the 2011-12 season,” she added.
Jeanne Sheffield, MD, director of the division of maternal-fetal medicine at Johns Hopkins Medicine, Baltimore, said in an interview the low uptake of vaccine shown in this study is both familiar and frustrating.
She said education from health care providers has improved, but women are nonetheless frequently fearful. She pointed out the widespread phenomenon of vaccine hesitancy in the general population.
Coverage was 45.3% among adults in the 2018-2019 flu season, 8.2 percentage points higher than coverage during the 2017-18 season (37.1%) according to CDC estimates.
Added to that, she said, is further hesitancy when women believe vaccination could harm the unborn baby, despite “very good data that flu vaccine is safe in pregnancy, acceptable in pregnancy in all trimesters, and is optimal standard of care.”
Holstein added, “We know from past research that a range of factors – including negative attitudes and beliefs about vaccines, less knowledge about and access to vaccines, and a lack of trust in healthcare providers and vaccines – can contribute to lower vaccination rates.”
Healthcare providers play a key role in increasing flu vaccinations among pregnant women, she said.
“A provider recommendation, combined with an offer to administer a flu vaccine at the time of visit, remains one of the best ways to accomplish this,” Holstein said.
Holstein and Sheffield have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Researchers analyzed data from 9,652 women ages 15-44 who were hospitalized with laboratory-confirmed influenza from October through April during the 2010-2019 influenza seasons. Data were pulled from the U.S. Influenza Hospitalization Surveillance Network (FluSurv-NET).
Of those women, 2,697 (28%) were pregnant. Median age was 28 and median gestational age was 32 weeks. Those studied included 36% who were non-Hispanic White; 29% non-Hispanic Black; and 20% Hispanic women.
Some 89% of the women, pregnant and nonpregnant, received antivirals while in the hospital but only 31% reported they had received the flu vaccine in the current season, despite guideline recommendations citing clear evidence that vaccination is safe for mother and baby.
Rachel Holstein, MPH, an epidemiology and information science fellow at the Centers for Disease Control and Prevention, who presented her team’s work as part of IDWeek 2020, explained that the mother’s vaccination can help protect the baby from flu infection for several months after birth, before the baby can be vaccinated.
She noted that pregnant women are at high risk for influenza-associated hospitalization.
“Changes in the immune system, heart, and lungs during pregnancy make pregnant women, and women up to 2 weeks post partum, more prone to severe illness from flu, including illness resulting in hospitalization,” she said in an interview
“Vaccination has been shown to reduce the risk of flu-associated acute respiratory infection in pregnant women by up to one-half,” she said. “A 2018 study showed that getting a flu shot reduced a pregnant woman’s risk of being hospitalized with flu by an average of 40%.»
FluSurv-NET data show hospitalizations were more common in the third trimester of pregnancy compared with the first and second, Holstein said. The most common underlying conditions among these women were asthma (23%) and obesity (10%), and 12% were current tobacco smokers. Overall, 5% of pregnant women with flu required ICU admission, 2% needed mechanical ventilation, and 6% developed pneumonia.
Vaccine uptake lowest in first two trimesters
Holstein said vaccine coverage was lowest among women in their first or second trimesters for all 9 seasons, and overall vaccination coverage increased significantly over time.
Uptake also differed by age. The data showed coverage was lower among women aged 15-34 years, compared with women 35 years and older (34% vs. 50%).
“It was as low as 15% among pregnant women aged 15-34 years in the 2011-12 season,” she added.
Jeanne Sheffield, MD, director of the division of maternal-fetal medicine at Johns Hopkins Medicine, Baltimore, said in an interview the low uptake of vaccine shown in this study is both familiar and frustrating.
She said education from health care providers has improved, but women are nonetheless frequently fearful. She pointed out the widespread phenomenon of vaccine hesitancy in the general population.
Coverage was 45.3% among adults in the 2018-2019 flu season, 8.2 percentage points higher than coverage during the 2017-18 season (37.1%) according to CDC estimates.
Added to that, she said, is further hesitancy when women believe vaccination could harm the unborn baby, despite “very good data that flu vaccine is safe in pregnancy, acceptable in pregnancy in all trimesters, and is optimal standard of care.”
Holstein added, “We know from past research that a range of factors – including negative attitudes and beliefs about vaccines, less knowledge about and access to vaccines, and a lack of trust in healthcare providers and vaccines – can contribute to lower vaccination rates.”
Healthcare providers play a key role in increasing flu vaccinations among pregnant women, she said.
“A provider recommendation, combined with an offer to administer a flu vaccine at the time of visit, remains one of the best ways to accomplish this,” Holstein said.
Holstein and Sheffield have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Researchers analyzed data from 9,652 women ages 15-44 who were hospitalized with laboratory-confirmed influenza from October through April during the 2010-2019 influenza seasons. Data were pulled from the U.S. Influenza Hospitalization Surveillance Network (FluSurv-NET).
Of those women, 2,697 (28%) were pregnant. Median age was 28 and median gestational age was 32 weeks. Those studied included 36% who were non-Hispanic White; 29% non-Hispanic Black; and 20% Hispanic women.
Some 89% of the women, pregnant and nonpregnant, received antivirals while in the hospital but only 31% reported they had received the flu vaccine in the current season, despite guideline recommendations citing clear evidence that vaccination is safe for mother and baby.
Rachel Holstein, MPH, an epidemiology and information science fellow at the Centers for Disease Control and Prevention, who presented her team’s work as part of IDWeek 2020, explained that the mother’s vaccination can help protect the baby from flu infection for several months after birth, before the baby can be vaccinated.
She noted that pregnant women are at high risk for influenza-associated hospitalization.
“Changes in the immune system, heart, and lungs during pregnancy make pregnant women, and women up to 2 weeks post partum, more prone to severe illness from flu, including illness resulting in hospitalization,” she said in an interview
“Vaccination has been shown to reduce the risk of flu-associated acute respiratory infection in pregnant women by up to one-half,” she said. “A 2018 study showed that getting a flu shot reduced a pregnant woman’s risk of being hospitalized with flu by an average of 40%.»
FluSurv-NET data show hospitalizations were more common in the third trimester of pregnancy compared with the first and second, Holstein said. The most common underlying conditions among these women were asthma (23%) and obesity (10%), and 12% were current tobacco smokers. Overall, 5% of pregnant women with flu required ICU admission, 2% needed mechanical ventilation, and 6% developed pneumonia.
Vaccine uptake lowest in first two trimesters
Holstein said vaccine coverage was lowest among women in their first or second trimesters for all 9 seasons, and overall vaccination coverage increased significantly over time.
Uptake also differed by age. The data showed coverage was lower among women aged 15-34 years, compared with women 35 years and older (34% vs. 50%).
“It was as low as 15% among pregnant women aged 15-34 years in the 2011-12 season,” she added.
Jeanne Sheffield, MD, director of the division of maternal-fetal medicine at Johns Hopkins Medicine, Baltimore, said in an interview the low uptake of vaccine shown in this study is both familiar and frustrating.
She said education from health care providers has improved, but women are nonetheless frequently fearful. She pointed out the widespread phenomenon of vaccine hesitancy in the general population.
Coverage was 45.3% among adults in the 2018-2019 flu season, 8.2 percentage points higher than coverage during the 2017-18 season (37.1%) according to CDC estimates.
Added to that, she said, is further hesitancy when women believe vaccination could harm the unborn baby, despite “very good data that flu vaccine is safe in pregnancy, acceptable in pregnancy in all trimesters, and is optimal standard of care.”
Holstein added, “We know from past research that a range of factors – including negative attitudes and beliefs about vaccines, less knowledge about and access to vaccines, and a lack of trust in healthcare providers and vaccines – can contribute to lower vaccination rates.”
Healthcare providers play a key role in increasing flu vaccinations among pregnant women, she said.
“A provider recommendation, combined with an offer to administer a flu vaccine at the time of visit, remains one of the best ways to accomplish this,” Holstein said.
Holstein and Sheffield have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Acute HIV cases double in ED. Is COVID-19 responsible?
David Pitrak, MD, an infectious diseases specialist at the University of Chicago Medicine, and colleagues found that the incidence ratio of acute HIV infection (AHI) jumped to 14.4 this year, compared with the 6.8 average for the previous 4 years (IR, 2.14; 95% confidence interval, 1.01-4.54; P < .05).
At a press conference at IDWeek 2020, he said that this year, acute patients made up one quarter of all new diagnoses (9 of 35), “the highest percentage we have ever seen.
“Patients with acute infection, especially those with symptoms, have extremely high viral loads and progress more rapidly. Because of those high viral loads, there’s risk of transmission to others, so rapid linkage to care and ART [antiretroviral treatment] is really important,” he said.
After the IDWeek abstract was submitted in September, Dr. Pitrak said, three additional AHI cases were diagnosed in the ED, bringing the IR of AHI during the pandemic to 2.57 (95% CI, 1.29-5.11).
Should all EDs link HIV screening to COVID-19 testing?
The ED at UCM incorporated blood draws for HIV screening as part of COVID-19 evaluations early on during the pandemic, and they recommend that practice for EDs across the nation.
After a positive test result, the ID team was able to quickly link the HIV patients to care and initiation of antiretroviral treatment without adding staff or resources, Dr. Pitrak said in an interview.
Dr. Pitrak and colleagues reviewed data from 13 health care centers on the south and west sides of Chicago. At most of the centers, fourth- and fifth-generation antibody tests were available. The investigators found that the number of HIV screens that were conducted dropped significantly during the COVID-19 pandemic.
At the height of the pandemic, HIV screening at the sites decreased an average of 58%, the researchers found. As of the end of June, the number was decreased by 32%.
“This is a global problem,” he said. “HIV services have been severely impacted worldwide, with the greatest impact on the LGBTQ community.”
UCM performed 19,111 HIV screens (11,133 in the ED) between Jan. 1 and Aug. 17 this year. It performed 14,754 COVID polymerase chain reaction tests in the ED between March 17 and Aug. 17. All of the acute cases were identified in the ED.
Dr. Pitrak mentioned some possible causes of an increase in the number of patients with acute cases who present in the ED. People who do not suspect they have AHI may be coming to the ED because they think they have COVID-19, inasmuch as many of the symptoms overlap. One of the AHI patients actually did have a coinfection, Dr. Pitrak noted.
“There is also the possibility that this could be bad news,” Dr. Pitrak said in an interview. “It could be that there are more acute cases presenting because there are more community transmissions.”
He noted that follow-up visits have been canceled or converted to telehealth visits during the pandemic, and the number of patients who are initiating pre-exposure prophylaxis has declined significantly.
“I hope we’re not seeing an increase in new transmissions after so much work has been done to decrease transmissions over the past few years,” he said.
Partnership with emergency physicians
Critical to screening these patients is building a solid partnership between ID and ED physicians.
Coauthor Kimberly Stanford, MD, MPH, an assistant professor in emergency medicine at UCM, said, “You need a champion within the emergency department who can help make sure that the work flow is not disrupted, that however you implement your screening program, you’re not putting extra work on the staff.
“We can feel extremely confident that if I send a test and it comes back positive, I know someone is going to call that patient and make sure they get into care.”
Although the testing is performed in the ED at UCM, the follow-up, linkage to care, and initiation of treatment are conducted by the ID specialists.
Beverly E. Sha, MD, professor in the division of infectious diseases, department of internal medicine, Rush Medical College, Chicago, said in an interview that although she agrees that HIV screening programs in EDs “make absolute sense,” there are different ways to conduct such programs. Dr. Sha was not involved in Dr. Pitrak’s study.
At Rush’s ED, she says, HIV testing is linked with a complete blood count.
“If someone presents with fever, we would often be doing that test as well,” she said. “I think just globally increasing screening [in the ED] is what makes the most sense.”
Dr. Sha said they have not seen a similar surge in acute cases in the ED at Rush during the pandemic.
She noted, however, that UCM tested more than 11,000 people for HIV in the ED this year, whereas “we probably only did about 3500.
“The reason testing is so important, whether for HIV or COVID, is the more you test, the more you’re going to find,” she said, “especially in cities like Chicago.”
Dr. Pitrak received grant support from Gilead Sciences. His coauthors and Dr. Sha reported no relevant financial relationships.
This article first appeared on Medscape.com.
David Pitrak, MD, an infectious diseases specialist at the University of Chicago Medicine, and colleagues found that the incidence ratio of acute HIV infection (AHI) jumped to 14.4 this year, compared with the 6.8 average for the previous 4 years (IR, 2.14; 95% confidence interval, 1.01-4.54; P < .05).
At a press conference at IDWeek 2020, he said that this year, acute patients made up one quarter of all new diagnoses (9 of 35), “the highest percentage we have ever seen.
“Patients with acute infection, especially those with symptoms, have extremely high viral loads and progress more rapidly. Because of those high viral loads, there’s risk of transmission to others, so rapid linkage to care and ART [antiretroviral treatment] is really important,” he said.
After the IDWeek abstract was submitted in September, Dr. Pitrak said, three additional AHI cases were diagnosed in the ED, bringing the IR of AHI during the pandemic to 2.57 (95% CI, 1.29-5.11).
Should all EDs link HIV screening to COVID-19 testing?
The ED at UCM incorporated blood draws for HIV screening as part of COVID-19 evaluations early on during the pandemic, and they recommend that practice for EDs across the nation.
After a positive test result, the ID team was able to quickly link the HIV patients to care and initiation of antiretroviral treatment without adding staff or resources, Dr. Pitrak said in an interview.
Dr. Pitrak and colleagues reviewed data from 13 health care centers on the south and west sides of Chicago. At most of the centers, fourth- and fifth-generation antibody tests were available. The investigators found that the number of HIV screens that were conducted dropped significantly during the COVID-19 pandemic.
At the height of the pandemic, HIV screening at the sites decreased an average of 58%, the researchers found. As of the end of June, the number was decreased by 32%.
“This is a global problem,” he said. “HIV services have been severely impacted worldwide, with the greatest impact on the LGBTQ community.”
UCM performed 19,111 HIV screens (11,133 in the ED) between Jan. 1 and Aug. 17 this year. It performed 14,754 COVID polymerase chain reaction tests in the ED between March 17 and Aug. 17. All of the acute cases were identified in the ED.
Dr. Pitrak mentioned some possible causes of an increase in the number of patients with acute cases who present in the ED. People who do not suspect they have AHI may be coming to the ED because they think they have COVID-19, inasmuch as many of the symptoms overlap. One of the AHI patients actually did have a coinfection, Dr. Pitrak noted.
“There is also the possibility that this could be bad news,” Dr. Pitrak said in an interview. “It could be that there are more acute cases presenting because there are more community transmissions.”
He noted that follow-up visits have been canceled or converted to telehealth visits during the pandemic, and the number of patients who are initiating pre-exposure prophylaxis has declined significantly.
“I hope we’re not seeing an increase in new transmissions after so much work has been done to decrease transmissions over the past few years,” he said.
Partnership with emergency physicians
Critical to screening these patients is building a solid partnership between ID and ED physicians.
Coauthor Kimberly Stanford, MD, MPH, an assistant professor in emergency medicine at UCM, said, “You need a champion within the emergency department who can help make sure that the work flow is not disrupted, that however you implement your screening program, you’re not putting extra work on the staff.
“We can feel extremely confident that if I send a test and it comes back positive, I know someone is going to call that patient and make sure they get into care.”
Although the testing is performed in the ED at UCM, the follow-up, linkage to care, and initiation of treatment are conducted by the ID specialists.
Beverly E. Sha, MD, professor in the division of infectious diseases, department of internal medicine, Rush Medical College, Chicago, said in an interview that although she agrees that HIV screening programs in EDs “make absolute sense,” there are different ways to conduct such programs. Dr. Sha was not involved in Dr. Pitrak’s study.
At Rush’s ED, she says, HIV testing is linked with a complete blood count.
“If someone presents with fever, we would often be doing that test as well,” she said. “I think just globally increasing screening [in the ED] is what makes the most sense.”
Dr. Sha said they have not seen a similar surge in acute cases in the ED at Rush during the pandemic.
She noted, however, that UCM tested more than 11,000 people for HIV in the ED this year, whereas “we probably only did about 3500.
“The reason testing is so important, whether for HIV or COVID, is the more you test, the more you’re going to find,” she said, “especially in cities like Chicago.”
Dr. Pitrak received grant support from Gilead Sciences. His coauthors and Dr. Sha reported no relevant financial relationships.
This article first appeared on Medscape.com.
David Pitrak, MD, an infectious diseases specialist at the University of Chicago Medicine, and colleagues found that the incidence ratio of acute HIV infection (AHI) jumped to 14.4 this year, compared with the 6.8 average for the previous 4 years (IR, 2.14; 95% confidence interval, 1.01-4.54; P < .05).
At a press conference at IDWeek 2020, he said that this year, acute patients made up one quarter of all new diagnoses (9 of 35), “the highest percentage we have ever seen.
“Patients with acute infection, especially those with symptoms, have extremely high viral loads and progress more rapidly. Because of those high viral loads, there’s risk of transmission to others, so rapid linkage to care and ART [antiretroviral treatment] is really important,” he said.
After the IDWeek abstract was submitted in September, Dr. Pitrak said, three additional AHI cases were diagnosed in the ED, bringing the IR of AHI during the pandemic to 2.57 (95% CI, 1.29-5.11).
Should all EDs link HIV screening to COVID-19 testing?
The ED at UCM incorporated blood draws for HIV screening as part of COVID-19 evaluations early on during the pandemic, and they recommend that practice for EDs across the nation.
After a positive test result, the ID team was able to quickly link the HIV patients to care and initiation of antiretroviral treatment without adding staff or resources, Dr. Pitrak said in an interview.
Dr. Pitrak and colleagues reviewed data from 13 health care centers on the south and west sides of Chicago. At most of the centers, fourth- and fifth-generation antibody tests were available. The investigators found that the number of HIV screens that were conducted dropped significantly during the COVID-19 pandemic.
At the height of the pandemic, HIV screening at the sites decreased an average of 58%, the researchers found. As of the end of June, the number was decreased by 32%.
“This is a global problem,” he said. “HIV services have been severely impacted worldwide, with the greatest impact on the LGBTQ community.”
UCM performed 19,111 HIV screens (11,133 in the ED) between Jan. 1 and Aug. 17 this year. It performed 14,754 COVID polymerase chain reaction tests in the ED between March 17 and Aug. 17. All of the acute cases were identified in the ED.
Dr. Pitrak mentioned some possible causes of an increase in the number of patients with acute cases who present in the ED. People who do not suspect they have AHI may be coming to the ED because they think they have COVID-19, inasmuch as many of the symptoms overlap. One of the AHI patients actually did have a coinfection, Dr. Pitrak noted.
“There is also the possibility that this could be bad news,” Dr. Pitrak said in an interview. “It could be that there are more acute cases presenting because there are more community transmissions.”
He noted that follow-up visits have been canceled or converted to telehealth visits during the pandemic, and the number of patients who are initiating pre-exposure prophylaxis has declined significantly.
“I hope we’re not seeing an increase in new transmissions after so much work has been done to decrease transmissions over the past few years,” he said.
Partnership with emergency physicians
Critical to screening these patients is building a solid partnership between ID and ED physicians.
Coauthor Kimberly Stanford, MD, MPH, an assistant professor in emergency medicine at UCM, said, “You need a champion within the emergency department who can help make sure that the work flow is not disrupted, that however you implement your screening program, you’re not putting extra work on the staff.
“We can feel extremely confident that if I send a test and it comes back positive, I know someone is going to call that patient and make sure they get into care.”
Although the testing is performed in the ED at UCM, the follow-up, linkage to care, and initiation of treatment are conducted by the ID specialists.
Beverly E. Sha, MD, professor in the division of infectious diseases, department of internal medicine, Rush Medical College, Chicago, said in an interview that although she agrees that HIV screening programs in EDs “make absolute sense,” there are different ways to conduct such programs. Dr. Sha was not involved in Dr. Pitrak’s study.
At Rush’s ED, she says, HIV testing is linked with a complete blood count.
“If someone presents with fever, we would often be doing that test as well,” she said. “I think just globally increasing screening [in the ED] is what makes the most sense.”
Dr. Sha said they have not seen a similar surge in acute cases in the ED at Rush during the pandemic.
She noted, however, that UCM tested more than 11,000 people for HIV in the ED this year, whereas “we probably only did about 3500.
“The reason testing is so important, whether for HIV or COVID, is the more you test, the more you’re going to find,” she said, “especially in cities like Chicago.”
Dr. Pitrak received grant support from Gilead Sciences. His coauthors and Dr. Sha reported no relevant financial relationships.
This article first appeared on Medscape.com.
COVID-19: Thromboembolic events high despite prophylaxis
in a new large observational U.S. study.
“Despite very high rate of antithrombotic prophylaxis there were a high rate of thromboembolic events suggesting that we are probably not providing enough thromboprophylaxis,” lead author Gregory Piazza, MD, Brigham and Women’s Hospital, Boston, said in an interview.
“Standard prophylaxis as recommended in the guidelines is a low dose of low-molecular-weight heparin once daily, but these results suggest [patients] probably need higher doses,” he added.
However, Dr. Piazza cautioned that this is an observational study and randomized trials are needed to make changes in treatment strategies. Several such trials are currently underway.
The current study was published online ahead of print in the Nov. 3 issue of the Journal of the American College of Cardiology.
Rates similar to other very sick patients
The study showed that while thromboembolic complications were high, they were not as high as seen in some of the earlier studies from Asia and Europe, Dr. Piazza noted.
“The numbers we were seeing in early reports were so high we couldn’t figure out how that was possible,” he said. “Our study suggests that, in a U.S. population receiving thromboprophylaxis, the rate of thromboembolic complications [are] more in line with what we would expect to see in other very sick patients who end up in ICU.”
He suggested that the very high rates of thromboembolic complications in the early studies from Asia may have been because of the lack of thromboprophylaxis, which is not routine in hospitalized patients there. “Some of the earlier studies also used routine ultrasound and so picked up asymptomatic thrombotic events, which was not the case in our study. So our results are more representative of the U.S. population.”
Dr. Piazza attributed the high rate of thromboembolic complications being reported with COVID-19 to the sheer number of very sick patients being admitted to the hospital.
“We are accustomed to seeing a rare case of thrombosis despite prophylaxis in hospitalized patients, but we are seeing more in COVID patients. This is probably just because we have more critically ill patients,” he said.
“We are seeing an incredible influx of patients to the ICU that we have never experienced before, so the increase in thromboembolic complications is more obvious. In prior years we probably haven’t had enough critically ill patients at any one time to raise the flag about thromboprophylaxis,” he commented.
The study also found a high rate of cardiovascular complications. They are seeing an increase in the risk of MI, which is to be expected in such sick patients, but they also see quite a bit of new atrial fibrillation, myocarditis, and heart failure in patients who don’t always have underlying cardiovascular disease, he said.
“So this virus does appear to have a predilection to causing cardiovascular complications, but this is probably because it is making patients so sick,” Dr. Piazza said. “If flu was this virulent and resulted in such high rates of acute respiratory distress syndrome (ARDS), we would probably see similar cardiovascular complication rates.”
For the current report, the researchers analyzed a retrospective cohort of 1,114 patients with COVID-19 diagnosed through the Mass General Brigham integrated health network. Of these, 170 had been admitted to the ICU, 229 had been hospitalized but not treated in ICU, and 715 were outpatients. In terms of ethnicity, 22% were Hispanic/Latino and 44% were non-White.
Cardiovascular risk factors were common, with 36% of patients having hypertension, 29% hyperlipidemia, and 18% diabetes. Prophylactic anticoagulation was prescribed in 89% of patients with COVID-19 in the intensive care cohort and 85% of those in the hospitalized non–intensive care setting.
Results showed that major arterial or venous thromboembolism (VTE) occurred in 35% of the intensive care cohort, 2.6% of those hospitalized but not treated in ICU, and 0% of outpatients.
Major adverse cardiovascular events occurred in 46% of the intensive care cohort, 6.1% of those hospitalized but non-ICU, and 0% of outpatients.
Symptomatic VTE occurred in 27% of those admitted to ICU, 2.2% of those hospitalized but non-ICU, and 0% of outpatients.
“We found that outpatients had a very low rate of thromboembolic complications, with the vast majority of the risk being in hospitalized patients, especially those in ICU,” Dr. Piazza said.
“These results suggest that we don’t need routine thromboprophylaxis for all outpatients with COVID-19, but there will probably be some patients who need it – those with risk factors for thromboembolism.”
Catheter- and device-associated deep vein thrombosis accounted for 76.9% of the DVTs observed in the study.
“Our finding of high frequency of catheter-associated DVT supports the judicious use of central venous catheters that have been widely implemented, especially in the ICU, to minimize recurrent health care team exposure and facilitate monitoring,” the researchers wrote.
ARDS biggest risk factor
Of all the markers of disease severity, the presence of ARDS had the strongest association with adverse outcomes, including major arterial or VTE, major adverse cardiovascular events, symptomatic VTE, and death.
“The severe inflammatory state associated with ARDS and other complications of COVID-19 and its resultant hypercoagulability may explain, at least in part, the high frequency of thromboembolic events. Improved risk stratification, utilizing biochemical markers of inflammation and activated coagulation as well as clinical indicators, such as ARDS, may play an important role in the early identification of patients with an increased likelihood of developing symptomatic VTE or arterial thrombosis,” the researchers wrote. “They may benefit from full- or intermediate-intensity antithrombotic therapy rather than prophylactic anticoagulation.”
They point out that this study provides a cross-sectional view of the cardiovascular complications of COVID-19 in a large health care network, consisting of two academic medical centers serving the greater Boston area, several community hospitals, and numerous outpatient care sites.
“The study incorporates a wide scope of clinically meaningful cardiovascular endpoints and utilizes a rigorous process of event adjudication. Although data on patients with COVID-19 in the ICU have been the subject of most reports, our study provides insights into the broad spectrum of all hospitalized and outpatient populations,” the authors noted.
“The high frequency of arterial or venous thromboembolism in hospitalized patients despite routine thromboprophylaxis suggests the need for improved risk stratification and enhanced preventive efforts,” they concluded.
The study is continuing, and the researchers expect to have data on 10,000 patients by the end of winter.
Wait for randomized trials
In an accompanying editorial, Robert McBane, MD, Mayo Clinic, Rochester, Minn., said that these data provide important real-world arterial and venous thrombotic event rates across a large, integrated health care network and an experienced roster of clinician-scientists devoted to thrombosis research.
Noting that whether to interpret these results as alarming or reassuring requires a comparison of expected thromboembolic event rates separate from the pandemic, he pointed out that, while the overall VTE rate among ICU patients was high, the vast majority of these events were attributable to central venous lines, and apart from these, the event rates do not appear inflated relative to prior published incidence rates from the pre–COVID-19 era.
“It is therefore important to resist the urge to overprevent or overtreat patients and expose them to the serious risks of major bleeding,” Dr. McBane wrote, adding that “the systematized approach to delivery of guideline-driven VTE prophylaxis across this large, integrated health network likely contributed to the relatively low rates of serious thrombotic outcomes reported.”
He further noted that, as the majority of VTE events were related to central venous lines in ICU patients, “this underscores the importance of a bundled care approach to central venous line management with daily assessment of the continued necessity of central access.
“A number of important clinical trials aimed at optimizing thromboprophylaxis during hospitalization, following hospital dismissal, and in ambulatory settings are underway. Until available, the lessons of thoughtful anticoagulant prophylaxis and treatment guidelines harvested from years of clinical research appear to apply,” he concluded.
This study was funded, in part, by a research grant from Janssen Pharmaceuticals. Dr. Piazza has received research grant support from EKOS Corporation, Bayer, Bristol-Myers Squibb/Pfizer, Portola Pharmaceuticals, and Janssen Pharmaceuticals; and has received consulting fees from Amgen, Pfizer, Boston Scientific, Agile, and Thrombolex. Dr. McBane reported no relevant disclosures.
A version of this article originally appeared on Medscape.com.
in a new large observational U.S. study.
“Despite very high rate of antithrombotic prophylaxis there were a high rate of thromboembolic events suggesting that we are probably not providing enough thromboprophylaxis,” lead author Gregory Piazza, MD, Brigham and Women’s Hospital, Boston, said in an interview.
“Standard prophylaxis as recommended in the guidelines is a low dose of low-molecular-weight heparin once daily, but these results suggest [patients] probably need higher doses,” he added.
However, Dr. Piazza cautioned that this is an observational study and randomized trials are needed to make changes in treatment strategies. Several such trials are currently underway.
The current study was published online ahead of print in the Nov. 3 issue of the Journal of the American College of Cardiology.
Rates similar to other very sick patients
The study showed that while thromboembolic complications were high, they were not as high as seen in some of the earlier studies from Asia and Europe, Dr. Piazza noted.
“The numbers we were seeing in early reports were so high we couldn’t figure out how that was possible,” he said. “Our study suggests that, in a U.S. population receiving thromboprophylaxis, the rate of thromboembolic complications [are] more in line with what we would expect to see in other very sick patients who end up in ICU.”
He suggested that the very high rates of thromboembolic complications in the early studies from Asia may have been because of the lack of thromboprophylaxis, which is not routine in hospitalized patients there. “Some of the earlier studies also used routine ultrasound and so picked up asymptomatic thrombotic events, which was not the case in our study. So our results are more representative of the U.S. population.”
Dr. Piazza attributed the high rate of thromboembolic complications being reported with COVID-19 to the sheer number of very sick patients being admitted to the hospital.
“We are accustomed to seeing a rare case of thrombosis despite prophylaxis in hospitalized patients, but we are seeing more in COVID patients. This is probably just because we have more critically ill patients,” he said.
“We are seeing an incredible influx of patients to the ICU that we have never experienced before, so the increase in thromboembolic complications is more obvious. In prior years we probably haven’t had enough critically ill patients at any one time to raise the flag about thromboprophylaxis,” he commented.
The study also found a high rate of cardiovascular complications. They are seeing an increase in the risk of MI, which is to be expected in such sick patients, but they also see quite a bit of new atrial fibrillation, myocarditis, and heart failure in patients who don’t always have underlying cardiovascular disease, he said.
“So this virus does appear to have a predilection to causing cardiovascular complications, but this is probably because it is making patients so sick,” Dr. Piazza said. “If flu was this virulent and resulted in such high rates of acute respiratory distress syndrome (ARDS), we would probably see similar cardiovascular complication rates.”
For the current report, the researchers analyzed a retrospective cohort of 1,114 patients with COVID-19 diagnosed through the Mass General Brigham integrated health network. Of these, 170 had been admitted to the ICU, 229 had been hospitalized but not treated in ICU, and 715 were outpatients. In terms of ethnicity, 22% were Hispanic/Latino and 44% were non-White.
Cardiovascular risk factors were common, with 36% of patients having hypertension, 29% hyperlipidemia, and 18% diabetes. Prophylactic anticoagulation was prescribed in 89% of patients with COVID-19 in the intensive care cohort and 85% of those in the hospitalized non–intensive care setting.
Results showed that major arterial or venous thromboembolism (VTE) occurred in 35% of the intensive care cohort, 2.6% of those hospitalized but not treated in ICU, and 0% of outpatients.
Major adverse cardiovascular events occurred in 46% of the intensive care cohort, 6.1% of those hospitalized but non-ICU, and 0% of outpatients.
Symptomatic VTE occurred in 27% of those admitted to ICU, 2.2% of those hospitalized but non-ICU, and 0% of outpatients.
“We found that outpatients had a very low rate of thromboembolic complications, with the vast majority of the risk being in hospitalized patients, especially those in ICU,” Dr. Piazza said.
“These results suggest that we don’t need routine thromboprophylaxis for all outpatients with COVID-19, but there will probably be some patients who need it – those with risk factors for thromboembolism.”
Catheter- and device-associated deep vein thrombosis accounted for 76.9% of the DVTs observed in the study.
“Our finding of high frequency of catheter-associated DVT supports the judicious use of central venous catheters that have been widely implemented, especially in the ICU, to minimize recurrent health care team exposure and facilitate monitoring,” the researchers wrote.
ARDS biggest risk factor
Of all the markers of disease severity, the presence of ARDS had the strongest association with adverse outcomes, including major arterial or VTE, major adverse cardiovascular events, symptomatic VTE, and death.
“The severe inflammatory state associated with ARDS and other complications of COVID-19 and its resultant hypercoagulability may explain, at least in part, the high frequency of thromboembolic events. Improved risk stratification, utilizing biochemical markers of inflammation and activated coagulation as well as clinical indicators, such as ARDS, may play an important role in the early identification of patients with an increased likelihood of developing symptomatic VTE or arterial thrombosis,” the researchers wrote. “They may benefit from full- or intermediate-intensity antithrombotic therapy rather than prophylactic anticoagulation.”
They point out that this study provides a cross-sectional view of the cardiovascular complications of COVID-19 in a large health care network, consisting of two academic medical centers serving the greater Boston area, several community hospitals, and numerous outpatient care sites.
“The study incorporates a wide scope of clinically meaningful cardiovascular endpoints and utilizes a rigorous process of event adjudication. Although data on patients with COVID-19 in the ICU have been the subject of most reports, our study provides insights into the broad spectrum of all hospitalized and outpatient populations,” the authors noted.
“The high frequency of arterial or venous thromboembolism in hospitalized patients despite routine thromboprophylaxis suggests the need for improved risk stratification and enhanced preventive efforts,” they concluded.
The study is continuing, and the researchers expect to have data on 10,000 patients by the end of winter.
Wait for randomized trials
In an accompanying editorial, Robert McBane, MD, Mayo Clinic, Rochester, Minn., said that these data provide important real-world arterial and venous thrombotic event rates across a large, integrated health care network and an experienced roster of clinician-scientists devoted to thrombosis research.
Noting that whether to interpret these results as alarming or reassuring requires a comparison of expected thromboembolic event rates separate from the pandemic, he pointed out that, while the overall VTE rate among ICU patients was high, the vast majority of these events were attributable to central venous lines, and apart from these, the event rates do not appear inflated relative to prior published incidence rates from the pre–COVID-19 era.
“It is therefore important to resist the urge to overprevent or overtreat patients and expose them to the serious risks of major bleeding,” Dr. McBane wrote, adding that “the systematized approach to delivery of guideline-driven VTE prophylaxis across this large, integrated health network likely contributed to the relatively low rates of serious thrombotic outcomes reported.”
He further noted that, as the majority of VTE events were related to central venous lines in ICU patients, “this underscores the importance of a bundled care approach to central venous line management with daily assessment of the continued necessity of central access.
“A number of important clinical trials aimed at optimizing thromboprophylaxis during hospitalization, following hospital dismissal, and in ambulatory settings are underway. Until available, the lessons of thoughtful anticoagulant prophylaxis and treatment guidelines harvested from years of clinical research appear to apply,” he concluded.
This study was funded, in part, by a research grant from Janssen Pharmaceuticals. Dr. Piazza has received research grant support from EKOS Corporation, Bayer, Bristol-Myers Squibb/Pfizer, Portola Pharmaceuticals, and Janssen Pharmaceuticals; and has received consulting fees from Amgen, Pfizer, Boston Scientific, Agile, and Thrombolex. Dr. McBane reported no relevant disclosures.
A version of this article originally appeared on Medscape.com.
in a new large observational U.S. study.
“Despite very high rate of antithrombotic prophylaxis there were a high rate of thromboembolic events suggesting that we are probably not providing enough thromboprophylaxis,” lead author Gregory Piazza, MD, Brigham and Women’s Hospital, Boston, said in an interview.
“Standard prophylaxis as recommended in the guidelines is a low dose of low-molecular-weight heparin once daily, but these results suggest [patients] probably need higher doses,” he added.
However, Dr. Piazza cautioned that this is an observational study and randomized trials are needed to make changes in treatment strategies. Several such trials are currently underway.
The current study was published online ahead of print in the Nov. 3 issue of the Journal of the American College of Cardiology.
Rates similar to other very sick patients
The study showed that while thromboembolic complications were high, they were not as high as seen in some of the earlier studies from Asia and Europe, Dr. Piazza noted.
“The numbers we were seeing in early reports were so high we couldn’t figure out how that was possible,” he said. “Our study suggests that, in a U.S. population receiving thromboprophylaxis, the rate of thromboembolic complications [are] more in line with what we would expect to see in other very sick patients who end up in ICU.”
He suggested that the very high rates of thromboembolic complications in the early studies from Asia may have been because of the lack of thromboprophylaxis, which is not routine in hospitalized patients there. “Some of the earlier studies also used routine ultrasound and so picked up asymptomatic thrombotic events, which was not the case in our study. So our results are more representative of the U.S. population.”
Dr. Piazza attributed the high rate of thromboembolic complications being reported with COVID-19 to the sheer number of very sick patients being admitted to the hospital.
“We are accustomed to seeing a rare case of thrombosis despite prophylaxis in hospitalized patients, but we are seeing more in COVID patients. This is probably just because we have more critically ill patients,” he said.
“We are seeing an incredible influx of patients to the ICU that we have never experienced before, so the increase in thromboembolic complications is more obvious. In prior years we probably haven’t had enough critically ill patients at any one time to raise the flag about thromboprophylaxis,” he commented.
The study also found a high rate of cardiovascular complications. They are seeing an increase in the risk of MI, which is to be expected in such sick patients, but they also see quite a bit of new atrial fibrillation, myocarditis, and heart failure in patients who don’t always have underlying cardiovascular disease, he said.
“So this virus does appear to have a predilection to causing cardiovascular complications, but this is probably because it is making patients so sick,” Dr. Piazza said. “If flu was this virulent and resulted in such high rates of acute respiratory distress syndrome (ARDS), we would probably see similar cardiovascular complication rates.”
For the current report, the researchers analyzed a retrospective cohort of 1,114 patients with COVID-19 diagnosed through the Mass General Brigham integrated health network. Of these, 170 had been admitted to the ICU, 229 had been hospitalized but not treated in ICU, and 715 were outpatients. In terms of ethnicity, 22% were Hispanic/Latino and 44% were non-White.
Cardiovascular risk factors were common, with 36% of patients having hypertension, 29% hyperlipidemia, and 18% diabetes. Prophylactic anticoagulation was prescribed in 89% of patients with COVID-19 in the intensive care cohort and 85% of those in the hospitalized non–intensive care setting.
Results showed that major arterial or venous thromboembolism (VTE) occurred in 35% of the intensive care cohort, 2.6% of those hospitalized but not treated in ICU, and 0% of outpatients.
Major adverse cardiovascular events occurred in 46% of the intensive care cohort, 6.1% of those hospitalized but non-ICU, and 0% of outpatients.
Symptomatic VTE occurred in 27% of those admitted to ICU, 2.2% of those hospitalized but non-ICU, and 0% of outpatients.
“We found that outpatients had a very low rate of thromboembolic complications, with the vast majority of the risk being in hospitalized patients, especially those in ICU,” Dr. Piazza said.
“These results suggest that we don’t need routine thromboprophylaxis for all outpatients with COVID-19, but there will probably be some patients who need it – those with risk factors for thromboembolism.”
Catheter- and device-associated deep vein thrombosis accounted for 76.9% of the DVTs observed in the study.
“Our finding of high frequency of catheter-associated DVT supports the judicious use of central venous catheters that have been widely implemented, especially in the ICU, to minimize recurrent health care team exposure and facilitate monitoring,” the researchers wrote.
ARDS biggest risk factor
Of all the markers of disease severity, the presence of ARDS had the strongest association with adverse outcomes, including major arterial or VTE, major adverse cardiovascular events, symptomatic VTE, and death.
“The severe inflammatory state associated with ARDS and other complications of COVID-19 and its resultant hypercoagulability may explain, at least in part, the high frequency of thromboembolic events. Improved risk stratification, utilizing biochemical markers of inflammation and activated coagulation as well as clinical indicators, such as ARDS, may play an important role in the early identification of patients with an increased likelihood of developing symptomatic VTE or arterial thrombosis,” the researchers wrote. “They may benefit from full- or intermediate-intensity antithrombotic therapy rather than prophylactic anticoagulation.”
They point out that this study provides a cross-sectional view of the cardiovascular complications of COVID-19 in a large health care network, consisting of two academic medical centers serving the greater Boston area, several community hospitals, and numerous outpatient care sites.
“The study incorporates a wide scope of clinically meaningful cardiovascular endpoints and utilizes a rigorous process of event adjudication. Although data on patients with COVID-19 in the ICU have been the subject of most reports, our study provides insights into the broad spectrum of all hospitalized and outpatient populations,” the authors noted.
“The high frequency of arterial or venous thromboembolism in hospitalized patients despite routine thromboprophylaxis suggests the need for improved risk stratification and enhanced preventive efforts,” they concluded.
The study is continuing, and the researchers expect to have data on 10,000 patients by the end of winter.
Wait for randomized trials
In an accompanying editorial, Robert McBane, MD, Mayo Clinic, Rochester, Minn., said that these data provide important real-world arterial and venous thrombotic event rates across a large, integrated health care network and an experienced roster of clinician-scientists devoted to thrombosis research.
Noting that whether to interpret these results as alarming or reassuring requires a comparison of expected thromboembolic event rates separate from the pandemic, he pointed out that, while the overall VTE rate among ICU patients was high, the vast majority of these events were attributable to central venous lines, and apart from these, the event rates do not appear inflated relative to prior published incidence rates from the pre–COVID-19 era.
“It is therefore important to resist the urge to overprevent or overtreat patients and expose them to the serious risks of major bleeding,” Dr. McBane wrote, adding that “the systematized approach to delivery of guideline-driven VTE prophylaxis across this large, integrated health network likely contributed to the relatively low rates of serious thrombotic outcomes reported.”
He further noted that, as the majority of VTE events were related to central venous lines in ICU patients, “this underscores the importance of a bundled care approach to central venous line management with daily assessment of the continued necessity of central access.
“A number of important clinical trials aimed at optimizing thromboprophylaxis during hospitalization, following hospital dismissal, and in ambulatory settings are underway. Until available, the lessons of thoughtful anticoagulant prophylaxis and treatment guidelines harvested from years of clinical research appear to apply,” he concluded.
This study was funded, in part, by a research grant from Janssen Pharmaceuticals. Dr. Piazza has received research grant support from EKOS Corporation, Bayer, Bristol-Myers Squibb/Pfizer, Portola Pharmaceuticals, and Janssen Pharmaceuticals; and has received consulting fees from Amgen, Pfizer, Boston Scientific, Agile, and Thrombolex. Dr. McBane reported no relevant disclosures.
A version of this article originally appeared on Medscape.com.
COVID spikes exacerbate health worker shortages in Rocky Mountains, Great Plains
In Montana, pandemic-induced staffing shortages have shuttered a clinic in the state’s capital, led a northwestern regional hospital to ask employees exposed to COVID-19 to continue to work and emptied a health department 400 miles to the east.
“Just one more person out and we wouldn’t be able to keep the surgeries going,” said Dr. Shelly Harkins, MD, chief medical officer of St. Peter’s Health in Helena, a city of roughly 32,000 where cases continue to spread. “When the virus is just all around you, it’s almost impossible to not be deemed a contact at some point. One case can take out a whole team of people in a blink of an eye.”
In North Dakota, where cases per resident are growing faster than any other state, hospitals may once again curtail elective surgeries and possibly seek government aid to hire more nurses if the situation gets worse, North Dakota Hospital Association President Tim Blasl said.
“How long can we run at this rate with the workforce that we have?” Blasl said. “You can have all the licensed beds you want, but if you don’t have anybody to staff those beds, it doesn’t do you any good.”
The northern Rocky Mountains, Great Plains and Upper Midwest are seeing the highest surge of COVID-19 cases in the nation, as some residents have ignored recommendations for curtailing the virus, such as wearing masks and avoiding large gatherings. Montana, Idaho, Utah, Wyoming, North Dakota, South Dakota, Nebraska, Iowa, and Wisconsin have recently ranked among the top 10 U.S. states in confirmed cases per 100,000 residents over a 7-day period, according to an analysis by the New York Times.
Such coronavirus infections – and the quarantines that occur because of them – are exacerbating the health care worker shortage that existed in these states well before the pandemic. Unlike in the nation’s metropolitan hubs, these outbreaks are scattered across hundreds of miles. And even in these states’ biggest cities, the ranks of medical professionals are in short supply. Specialists and registered nurses are sometimes harder to track down than ventilators, N95 masks or hospital beds. Without enough care providers, patients may not be able to get the medical attention they need.
Hospitals have asked staffers to cover extra shifts and learn new skills. They have brought in temporary workers from other parts of the country and transferred some patients to less-crowded hospitals. But, at St. Peter’s Health, if the hospital’s one kidney doctor gets sick or is told to quarantine, Dr. Harkins doesn’t expect to find a backup.
“We make a point to not have excessive staff because we have an obligation to keep the cost of health care down for a community – we just don’t have a lot of slack in our rope,” Dr. Harkins said. “What we don’t account for is a mass exodus of staff for 14 days.”
Some hospitals are already at patient capacity or are nearly there. That’s not just because of the growing number of COVID-19 patients. Elective surgeries have resumed, and medical emergencies don’t pause for a pandemic.
Some Montana hospitals formed agreements with local affiliates early in the pandemic to share staff if one came up short. But now that the disease is spreading fast – and widely – the hope is that their needs don’t peak all at once.
Montana state officials keep a list of primarily in-state volunteer workers ready to travel to towns with shortages of contact tracers, nurses and more. But during a press conference on Oct. 15, Democratic Gov. Steve Bullock said the state had exhausted that database, and its nationwide request for National Guard medical staffing hadn’t brought in new workers.
“If you are a registered nurse, licensed practical nurse, paramedic, EMT, CNA or contact tracer, and are able to join our workforce, please do consider joining our team,” Gov. Bullock said.
This month, Kalispell Regional Medical Center in northwestern Montana even stopped quarantining COVID-exposed staff who remain asymptomatic, a change allowed by Centers for Disease Control and Prevention guidelines for health facilities facing staffing shortages.
“That’s very telling for what staffing is going through right now,” said Andrea Lueck, a registered nurse at the center. “We’re so tight that employees are called off of quarantine.”
Financial pressure early in the pandemic led the hospital to furlough staff, but it had to bring most of them back to work because it needs those bodies more than ever. The regional hub is based in Flathead County, which has recorded the state’s second-highest number of active COVID-19 cases.
Mellody Sharpton, a hospital spokesperson, said hospital workers who are exposed to someone infected with the virus are tested within three to five days and monitored for symptoms. The hospital is also pulling in new workers, with 25 traveling health professionals on hand and another 25 temporary ones on the way.
But Ms. Sharpton said the best way to conserve the hospital’s workforce is to stop the disease surge in the community.
Earlier in the pandemic, Central Montana Medical Center in Lewistown, a town of fewer than 6,000, experienced an exodus of part-time workers or those close to retirement who decided their jobs weren’t worth the risk. The facility recently secured two traveling workers, but both backed out because they couldn’t find housing. And, so far, roughly 40 of the hospital’s 322 employees have missed work for reasons connected to COVID-19.
“We’re at a critical staffing shortage and have been since the beginning of COVID,” said Joanie Slaybaugh, Central Montana Medical Center’s director of human resources. “We’re small enough, everybody feels an obligation to protect themselves and to protect each other. But it doesn’t take much to take out our staff.”
Roosevelt County, where roughly 11,000 live on the northeastern edge of Montana, had one of the nation’s highest rates of new cases as of Oct. 15. But by the end of the month, the county health department will lose half of its registered nurses as one person is about to retire and another was hired through a grant that’s ending. That leaves only one registered nurse aside from its director, Patty Presser. The health department already had to close earlier during the pandemic because of COVID exposure and not enough staffers to cover the gap. Now, if Ms. Presser can’t find nurse replacements in time, she hopes volunteers will step in, though she added they typically stay for only a few weeks.
“I need someone to do immunizations for my community, and you don’t become an immunization nurse in 14 days,” she said. “We don’t have the workforce here to deal with this virus, not even right now, and then I’m going to have my best two people go.”
Back in Helena, Dr. Harkins said St. Peter’s Health had to close a specialty outpatient clinic that treats chronic diseases for two weeks at the end of September because the entire staff had to quarantine.
Now the hospital is considering having doctors take turns spending a week working from home, so that if another wave of quarantines hits in the hospital, at least one untainted person can be brought back to work. But that won’t help for some specialties, like the hospital’s sole kidney doctor.
Every time Dr. Harkins’ phone rings, she said, she takes a breath and hopes it’s not another case that will force a whole division to close.
“Because I think immediately of the hundreds of people that need that service and won’t have it for 14 days,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
In Montana, pandemic-induced staffing shortages have shuttered a clinic in the state’s capital, led a northwestern regional hospital to ask employees exposed to COVID-19 to continue to work and emptied a health department 400 miles to the east.
“Just one more person out and we wouldn’t be able to keep the surgeries going,” said Dr. Shelly Harkins, MD, chief medical officer of St. Peter’s Health in Helena, a city of roughly 32,000 where cases continue to spread. “When the virus is just all around you, it’s almost impossible to not be deemed a contact at some point. One case can take out a whole team of people in a blink of an eye.”
In North Dakota, where cases per resident are growing faster than any other state, hospitals may once again curtail elective surgeries and possibly seek government aid to hire more nurses if the situation gets worse, North Dakota Hospital Association President Tim Blasl said.
“How long can we run at this rate with the workforce that we have?” Blasl said. “You can have all the licensed beds you want, but if you don’t have anybody to staff those beds, it doesn’t do you any good.”
The northern Rocky Mountains, Great Plains and Upper Midwest are seeing the highest surge of COVID-19 cases in the nation, as some residents have ignored recommendations for curtailing the virus, such as wearing masks and avoiding large gatherings. Montana, Idaho, Utah, Wyoming, North Dakota, South Dakota, Nebraska, Iowa, and Wisconsin have recently ranked among the top 10 U.S. states in confirmed cases per 100,000 residents over a 7-day period, according to an analysis by the New York Times.
Such coronavirus infections – and the quarantines that occur because of them – are exacerbating the health care worker shortage that existed in these states well before the pandemic. Unlike in the nation’s metropolitan hubs, these outbreaks are scattered across hundreds of miles. And even in these states’ biggest cities, the ranks of medical professionals are in short supply. Specialists and registered nurses are sometimes harder to track down than ventilators, N95 masks or hospital beds. Without enough care providers, patients may not be able to get the medical attention they need.
Hospitals have asked staffers to cover extra shifts and learn new skills. They have brought in temporary workers from other parts of the country and transferred some patients to less-crowded hospitals. But, at St. Peter’s Health, if the hospital’s one kidney doctor gets sick or is told to quarantine, Dr. Harkins doesn’t expect to find a backup.
“We make a point to not have excessive staff because we have an obligation to keep the cost of health care down for a community – we just don’t have a lot of slack in our rope,” Dr. Harkins said. “What we don’t account for is a mass exodus of staff for 14 days.”
Some hospitals are already at patient capacity or are nearly there. That’s not just because of the growing number of COVID-19 patients. Elective surgeries have resumed, and medical emergencies don’t pause for a pandemic.
Some Montana hospitals formed agreements with local affiliates early in the pandemic to share staff if one came up short. But now that the disease is spreading fast – and widely – the hope is that their needs don’t peak all at once.
Montana state officials keep a list of primarily in-state volunteer workers ready to travel to towns with shortages of contact tracers, nurses and more. But during a press conference on Oct. 15, Democratic Gov. Steve Bullock said the state had exhausted that database, and its nationwide request for National Guard medical staffing hadn’t brought in new workers.
“If you are a registered nurse, licensed practical nurse, paramedic, EMT, CNA or contact tracer, and are able to join our workforce, please do consider joining our team,” Gov. Bullock said.
This month, Kalispell Regional Medical Center in northwestern Montana even stopped quarantining COVID-exposed staff who remain asymptomatic, a change allowed by Centers for Disease Control and Prevention guidelines for health facilities facing staffing shortages.
“That’s very telling for what staffing is going through right now,” said Andrea Lueck, a registered nurse at the center. “We’re so tight that employees are called off of quarantine.”
Financial pressure early in the pandemic led the hospital to furlough staff, but it had to bring most of them back to work because it needs those bodies more than ever. The regional hub is based in Flathead County, which has recorded the state’s second-highest number of active COVID-19 cases.
Mellody Sharpton, a hospital spokesperson, said hospital workers who are exposed to someone infected with the virus are tested within three to five days and monitored for symptoms. The hospital is also pulling in new workers, with 25 traveling health professionals on hand and another 25 temporary ones on the way.
But Ms. Sharpton said the best way to conserve the hospital’s workforce is to stop the disease surge in the community.
Earlier in the pandemic, Central Montana Medical Center in Lewistown, a town of fewer than 6,000, experienced an exodus of part-time workers or those close to retirement who decided their jobs weren’t worth the risk. The facility recently secured two traveling workers, but both backed out because they couldn’t find housing. And, so far, roughly 40 of the hospital’s 322 employees have missed work for reasons connected to COVID-19.
“We’re at a critical staffing shortage and have been since the beginning of COVID,” said Joanie Slaybaugh, Central Montana Medical Center’s director of human resources. “We’re small enough, everybody feels an obligation to protect themselves and to protect each other. But it doesn’t take much to take out our staff.”
Roosevelt County, where roughly 11,000 live on the northeastern edge of Montana, had one of the nation’s highest rates of new cases as of Oct. 15. But by the end of the month, the county health department will lose half of its registered nurses as one person is about to retire and another was hired through a grant that’s ending. That leaves only one registered nurse aside from its director, Patty Presser. The health department already had to close earlier during the pandemic because of COVID exposure and not enough staffers to cover the gap. Now, if Ms. Presser can’t find nurse replacements in time, she hopes volunteers will step in, though she added they typically stay for only a few weeks.
“I need someone to do immunizations for my community, and you don’t become an immunization nurse in 14 days,” she said. “We don’t have the workforce here to deal with this virus, not even right now, and then I’m going to have my best two people go.”
Back in Helena, Dr. Harkins said St. Peter’s Health had to close a specialty outpatient clinic that treats chronic diseases for two weeks at the end of September because the entire staff had to quarantine.
Now the hospital is considering having doctors take turns spending a week working from home, so that if another wave of quarantines hits in the hospital, at least one untainted person can be brought back to work. But that won’t help for some specialties, like the hospital’s sole kidney doctor.
Every time Dr. Harkins’ phone rings, she said, she takes a breath and hopes it’s not another case that will force a whole division to close.
“Because I think immediately of the hundreds of people that need that service and won’t have it for 14 days,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
In Montana, pandemic-induced staffing shortages have shuttered a clinic in the state’s capital, led a northwestern regional hospital to ask employees exposed to COVID-19 to continue to work and emptied a health department 400 miles to the east.
“Just one more person out and we wouldn’t be able to keep the surgeries going,” said Dr. Shelly Harkins, MD, chief medical officer of St. Peter’s Health in Helena, a city of roughly 32,000 where cases continue to spread. “When the virus is just all around you, it’s almost impossible to not be deemed a contact at some point. One case can take out a whole team of people in a blink of an eye.”
In North Dakota, where cases per resident are growing faster than any other state, hospitals may once again curtail elective surgeries and possibly seek government aid to hire more nurses if the situation gets worse, North Dakota Hospital Association President Tim Blasl said.
“How long can we run at this rate with the workforce that we have?” Blasl said. “You can have all the licensed beds you want, but if you don’t have anybody to staff those beds, it doesn’t do you any good.”
The northern Rocky Mountains, Great Plains and Upper Midwest are seeing the highest surge of COVID-19 cases in the nation, as some residents have ignored recommendations for curtailing the virus, such as wearing masks and avoiding large gatherings. Montana, Idaho, Utah, Wyoming, North Dakota, South Dakota, Nebraska, Iowa, and Wisconsin have recently ranked among the top 10 U.S. states in confirmed cases per 100,000 residents over a 7-day period, according to an analysis by the New York Times.
Such coronavirus infections – and the quarantines that occur because of them – are exacerbating the health care worker shortage that existed in these states well before the pandemic. Unlike in the nation’s metropolitan hubs, these outbreaks are scattered across hundreds of miles. And even in these states’ biggest cities, the ranks of medical professionals are in short supply. Specialists and registered nurses are sometimes harder to track down than ventilators, N95 masks or hospital beds. Without enough care providers, patients may not be able to get the medical attention they need.
Hospitals have asked staffers to cover extra shifts and learn new skills. They have brought in temporary workers from other parts of the country and transferred some patients to less-crowded hospitals. But, at St. Peter’s Health, if the hospital’s one kidney doctor gets sick or is told to quarantine, Dr. Harkins doesn’t expect to find a backup.
“We make a point to not have excessive staff because we have an obligation to keep the cost of health care down for a community – we just don’t have a lot of slack in our rope,” Dr. Harkins said. “What we don’t account for is a mass exodus of staff for 14 days.”
Some hospitals are already at patient capacity or are nearly there. That’s not just because of the growing number of COVID-19 patients. Elective surgeries have resumed, and medical emergencies don’t pause for a pandemic.
Some Montana hospitals formed agreements with local affiliates early in the pandemic to share staff if one came up short. But now that the disease is spreading fast – and widely – the hope is that their needs don’t peak all at once.
Montana state officials keep a list of primarily in-state volunteer workers ready to travel to towns with shortages of contact tracers, nurses and more. But during a press conference on Oct. 15, Democratic Gov. Steve Bullock said the state had exhausted that database, and its nationwide request for National Guard medical staffing hadn’t brought in new workers.
“If you are a registered nurse, licensed practical nurse, paramedic, EMT, CNA or contact tracer, and are able to join our workforce, please do consider joining our team,” Gov. Bullock said.
This month, Kalispell Regional Medical Center in northwestern Montana even stopped quarantining COVID-exposed staff who remain asymptomatic, a change allowed by Centers for Disease Control and Prevention guidelines for health facilities facing staffing shortages.
“That’s very telling for what staffing is going through right now,” said Andrea Lueck, a registered nurse at the center. “We’re so tight that employees are called off of quarantine.”
Financial pressure early in the pandemic led the hospital to furlough staff, but it had to bring most of them back to work because it needs those bodies more than ever. The regional hub is based in Flathead County, which has recorded the state’s second-highest number of active COVID-19 cases.
Mellody Sharpton, a hospital spokesperson, said hospital workers who are exposed to someone infected with the virus are tested within three to five days and monitored for symptoms. The hospital is also pulling in new workers, with 25 traveling health professionals on hand and another 25 temporary ones on the way.
But Ms. Sharpton said the best way to conserve the hospital’s workforce is to stop the disease surge in the community.
Earlier in the pandemic, Central Montana Medical Center in Lewistown, a town of fewer than 6,000, experienced an exodus of part-time workers or those close to retirement who decided their jobs weren’t worth the risk. The facility recently secured two traveling workers, but both backed out because they couldn’t find housing. And, so far, roughly 40 of the hospital’s 322 employees have missed work for reasons connected to COVID-19.
“We’re at a critical staffing shortage and have been since the beginning of COVID,” said Joanie Slaybaugh, Central Montana Medical Center’s director of human resources. “We’re small enough, everybody feels an obligation to protect themselves and to protect each other. But it doesn’t take much to take out our staff.”
Roosevelt County, where roughly 11,000 live on the northeastern edge of Montana, had one of the nation’s highest rates of new cases as of Oct. 15. But by the end of the month, the county health department will lose half of its registered nurses as one person is about to retire and another was hired through a grant that’s ending. That leaves only one registered nurse aside from its director, Patty Presser. The health department already had to close earlier during the pandemic because of COVID exposure and not enough staffers to cover the gap. Now, if Ms. Presser can’t find nurse replacements in time, she hopes volunteers will step in, though she added they typically stay for only a few weeks.
“I need someone to do immunizations for my community, and you don’t become an immunization nurse in 14 days,” she said. “We don’t have the workforce here to deal with this virus, not even right now, and then I’m going to have my best two people go.”
Back in Helena, Dr. Harkins said St. Peter’s Health had to close a specialty outpatient clinic that treats chronic diseases for two weeks at the end of September because the entire staff had to quarantine.
Now the hospital is considering having doctors take turns spending a week working from home, so that if another wave of quarantines hits in the hospital, at least one untainted person can be brought back to work. But that won’t help for some specialties, like the hospital’s sole kidney doctor.
Every time Dr. Harkins’ phone rings, she said, she takes a breath and hopes it’s not another case that will force a whole division to close.
“Because I think immediately of the hundreds of people that need that service and won’t have it for 14 days,” she said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.

