User login
Standard measure may underestimate OSA in Black patients
Measurement error may be the culprit in underdiagnosing obstructive sleep apnea in Black patients, compared with White patients, based on data from nearly 2,000 individuals.
, according to Ali Azarbarzin, PhD, of Harvard Medical School, Boston.
“We wanted to examine the implications for obstructive sleep apnea,” which is often caused by a reduction in air flow, Dr. Azarbarzin said in an interview.
In a study presented at the American Thoracic Society’s international conference, Dr. Azarbarzin and colleagues examined data from 1,955 adults who were enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) Exam 5. The study participants underwent unattended 15-channel polysomnography that included a finger pulse oximeter. The mean age of the participants was 68.3 years, and 53.7% were women. A total of 12.1%, 23.7%, 27.7%, and 36.5% of the participants were Asian, Hispanic, Black, and White, respectively.
Apnea hypopnea index (AHI3P) was similar between Black and White patients, at approximately 19 events per hour. Black participants had higher wake SpO2, higher current smoking rates, and higher body mass index, compared with White participants, but these differences were not significant.
Severity of obstructive sleep apnea (OSA) was based on the hypoxic burden, which was defined as the total area under the respiratory curve. The total ventilatory burden was defined as the event-specific area under the ventilation signal and identified by amplitude changes in the nasal pressure signal. The researchers then calculated desaturation sensitivity (the primary outcome) as hypoxic burden divided by ventilatory burden.
In an unadjusted analysis, desaturation sensitivity was significantly lower in Black patients and Asian patients, compared with White patients (P < .001 and P < .02, respectively). After adjusting for age, sex, body mass index, and time spent in a supine position, desaturation sensitivity was lower only in Black patients, compared with White patients, and this difference persisted in both men and women.
The difference in desaturation sensitivity by race could be caused by differences in physiology or in measurement error, Dr. Azarbarzin told this news organization. If measurement error is the culprit, “we may be underestimating OSA severity in [Black people],” especially in Black women, he said.
However, more research is needed to understand the potential impact of both physiology and device accuracy on differences in oxygen saturation across ethnicities and to effectively identify and treat OSA in all patients, Dr. Azarbarzin said.
The MESA Study was supported by the National Institutes of Health and the National Institute on Aging. Data from MESA were obtained through support from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Dr. Azarbarzin disclosed funding from the National Institutes of Health, the American Health Association, and the American Academy of Sleep Medicine.
A version of this article first appeared on Medscape.com.
Measurement error may be the culprit in underdiagnosing obstructive sleep apnea in Black patients, compared with White patients, based on data from nearly 2,000 individuals.
, according to Ali Azarbarzin, PhD, of Harvard Medical School, Boston.
“We wanted to examine the implications for obstructive sleep apnea,” which is often caused by a reduction in air flow, Dr. Azarbarzin said in an interview.
In a study presented at the American Thoracic Society’s international conference, Dr. Azarbarzin and colleagues examined data from 1,955 adults who were enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) Exam 5. The study participants underwent unattended 15-channel polysomnography that included a finger pulse oximeter. The mean age of the participants was 68.3 years, and 53.7% were women. A total of 12.1%, 23.7%, 27.7%, and 36.5% of the participants were Asian, Hispanic, Black, and White, respectively.
Apnea hypopnea index (AHI3P) was similar between Black and White patients, at approximately 19 events per hour. Black participants had higher wake SpO2, higher current smoking rates, and higher body mass index, compared with White participants, but these differences were not significant.
Severity of obstructive sleep apnea (OSA) was based on the hypoxic burden, which was defined as the total area under the respiratory curve. The total ventilatory burden was defined as the event-specific area under the ventilation signal and identified by amplitude changes in the nasal pressure signal. The researchers then calculated desaturation sensitivity (the primary outcome) as hypoxic burden divided by ventilatory burden.
In an unadjusted analysis, desaturation sensitivity was significantly lower in Black patients and Asian patients, compared with White patients (P < .001 and P < .02, respectively). After adjusting for age, sex, body mass index, and time spent in a supine position, desaturation sensitivity was lower only in Black patients, compared with White patients, and this difference persisted in both men and women.
The difference in desaturation sensitivity by race could be caused by differences in physiology or in measurement error, Dr. Azarbarzin told this news organization. If measurement error is the culprit, “we may be underestimating OSA severity in [Black people],” especially in Black women, he said.
However, more research is needed to understand the potential impact of both physiology and device accuracy on differences in oxygen saturation across ethnicities and to effectively identify and treat OSA in all patients, Dr. Azarbarzin said.
The MESA Study was supported by the National Institutes of Health and the National Institute on Aging. Data from MESA were obtained through support from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Dr. Azarbarzin disclosed funding from the National Institutes of Health, the American Health Association, and the American Academy of Sleep Medicine.
A version of this article first appeared on Medscape.com.
Measurement error may be the culprit in underdiagnosing obstructive sleep apnea in Black patients, compared with White patients, based on data from nearly 2,000 individuals.
, according to Ali Azarbarzin, PhD, of Harvard Medical School, Boston.
“We wanted to examine the implications for obstructive sleep apnea,” which is often caused by a reduction in air flow, Dr. Azarbarzin said in an interview.
In a study presented at the American Thoracic Society’s international conference, Dr. Azarbarzin and colleagues examined data from 1,955 adults who were enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) Exam 5. The study participants underwent unattended 15-channel polysomnography that included a finger pulse oximeter. The mean age of the participants was 68.3 years, and 53.7% were women. A total of 12.1%, 23.7%, 27.7%, and 36.5% of the participants were Asian, Hispanic, Black, and White, respectively.
Apnea hypopnea index (AHI3P) was similar between Black and White patients, at approximately 19 events per hour. Black participants had higher wake SpO2, higher current smoking rates, and higher body mass index, compared with White participants, but these differences were not significant.
Severity of obstructive sleep apnea (OSA) was based on the hypoxic burden, which was defined as the total area under the respiratory curve. The total ventilatory burden was defined as the event-specific area under the ventilation signal and identified by amplitude changes in the nasal pressure signal. The researchers then calculated desaturation sensitivity (the primary outcome) as hypoxic burden divided by ventilatory burden.
In an unadjusted analysis, desaturation sensitivity was significantly lower in Black patients and Asian patients, compared with White patients (P < .001 and P < .02, respectively). After adjusting for age, sex, body mass index, and time spent in a supine position, desaturation sensitivity was lower only in Black patients, compared with White patients, and this difference persisted in both men and women.
The difference in desaturation sensitivity by race could be caused by differences in physiology or in measurement error, Dr. Azarbarzin told this news organization. If measurement error is the culprit, “we may be underestimating OSA severity in [Black people],” especially in Black women, he said.
However, more research is needed to understand the potential impact of both physiology and device accuracy on differences in oxygen saturation across ethnicities and to effectively identify and treat OSA in all patients, Dr. Azarbarzin said.
The MESA Study was supported by the National Institutes of Health and the National Institute on Aging. Data from MESA were obtained through support from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Dr. Azarbarzin disclosed funding from the National Institutes of Health, the American Health Association, and the American Academy of Sleep Medicine.
A version of this article first appeared on Medscape.com.
FROM ATS 2023
Maternal health clinic teams with legal services to aid patients
BALTIMORE – A novel partnership between a legal services program and a maternal health clinic is helping pregnant patients with issues such as housing or employment discrimination.
The Perinatal Legal Assistance and Well-being (P-LAW) program at Georgetown University, Washington, launched 2 years ago as a collaboration between GU’s Health Justice Alliance clinic and the Women’s and Infants Services division of nearby MedStar Washington Hospital Center, integrating attorneys into the health care team to offer no-cost legal aid for its diverse, urban population during the perinatal period. Since then, the effort has assisted more than 120 women.
“Our goal was to see how integrating a lawyer can help address some of those issues that, unfortunately, providers are not able to assist with because they go beyond the hospital or clinic walls,” said Roxana Richardson, JD, the project director and managing attorney for P-LAW, during a poster presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “Our initial findings showed that there are issues that patients were facing that needed an intervention from an attorney. We trained the providers and social workers to identify these issues so that we could intervene.”
Improving health by tackling legal barriers
, Ms. Richardson said.
The program is one of few medical-legal partnerships specifically focused on the perinatal population. P-LAW is one component of a larger initiative at MedStar Health called DC Safe Babies Safe Moms. The initiative includes integrated mental health programming, treatment of health conditions that complicate pregnancy, assessments of social determinants of health, expanded support for lactation and nutrition, access to home visiting referrals, and extended postpartum follow-up. The work is supported through the A. James & Alice B. Clark Foundation.
Patients are evaluated for health-harming legal needs as part of a comprehensive social and behavioral health screening at their initial prenatal visit, 28-week appointment, and postpartum visit. Those who screen positive are contacted by a referral specialist on the health care team who confirms the patient has an active legal need and would like to be connected to the P-LAW team. The team then reaches out to conduct a legal intake and determine the appropriate course of action.
From March 2021 through February of this year, Ms. Richardson and others with the program have provided legal representation to 123 patients on 186 legal issues in areas such as public benefits, employment, and housing and family concerns. Services range from advising patients on steps they can take on their own (like reporting a housing condition issue to the Department of Buildings), to sending letters on patients’ behalf, to appearing in court. Most patients served were in their second and third trimesters of pregnancy. The majority were Black or African American, aged 20-34 years, and had incomes below 100% of the federal poverty level.
The most common legal issues were in the areas of public benefits (SNAP/food stamps, cash assistance), employment (parental leave, discrimination), housing (conditions, eviction), and family law (child support, domestic violence). Among the 186 issues, work has been completed on 106 concerns and 33 still have a case open; for 47, the client withdrew or ceased contact, Ms. Richardson reported.
Most times when obstetricians hear concerns like these, they wonder what to do, said Tamika Auguste, MD, chair of obstetrics and gynecology at MedStar Health. Having the P-LAW program as a resource is a huge help, she said. If patients express concerns, or if obstetricians uncover concerns during office visits, doctors can enter a referral directly in the electronic medical record.
Patients are “so relieved,” Dr. Auguste said in an interview, because they often wonder if their doctor can help. “Your doctor is only going to be able to help to a certain point. But to know they’re pregnant and they have this resource, and they’re going to get legal help, has been game-changing for so many patients.”
COVID ... or morning sickness?
In one rewarding case, Ms. Richardson said, a single mother of one child who was pregnant and experiencing hyperemesis explained that her employer would forbid her from working if she had any symptoms similar to COVID-19. The employer mistook her vomiting, nausea, and exhaustion as COVID symptoms and docked her pay. That started a cascade in which earning less meant she was facing eviction and car repossession – and, eventually, overdraft fees and withdrawals from her bank. She was so despondent she was thinking about self-harm, Ms. Richardson said.
With the aid of the P-LAW program, the woman had short-term disability approved within 72 hours, was referred to the hospital for inpatient mental health treatment, and received the care she needed. She ultimately delivered a healthy baby girl and found a new job.
Tiffany Moore Simas, MD, MPH, MEd, chair of the department of obstetrics and gynecology at the University of Massachusetts and UMass Memorial Health in Worcester, said she encounters similar concerns among her patients, with the vast majority having one or more issues with social determinants of health.
“I think it’s incredible, as we’re trying to address equity in perinatal health and maternal mortality and morbidity, to have a more holistic view of what health means, and all of the social determinants of health, and actually helping our patients address that in real time at their visits and connecting them,” said Dr. Simas, who also is professor of ob/gyn, pediatrics, psychiatry, and population and quantitative health sciences at UMass. “It has really opened my mind to the possibilities of things we need to explore and do differently.”
Ms. Richardson, Dr. Auguste, and Dr. Simas reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BALTIMORE – A novel partnership between a legal services program and a maternal health clinic is helping pregnant patients with issues such as housing or employment discrimination.
The Perinatal Legal Assistance and Well-being (P-LAW) program at Georgetown University, Washington, launched 2 years ago as a collaboration between GU’s Health Justice Alliance clinic and the Women’s and Infants Services division of nearby MedStar Washington Hospital Center, integrating attorneys into the health care team to offer no-cost legal aid for its diverse, urban population during the perinatal period. Since then, the effort has assisted more than 120 women.
“Our goal was to see how integrating a lawyer can help address some of those issues that, unfortunately, providers are not able to assist with because they go beyond the hospital or clinic walls,” said Roxana Richardson, JD, the project director and managing attorney for P-LAW, during a poster presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “Our initial findings showed that there are issues that patients were facing that needed an intervention from an attorney. We trained the providers and social workers to identify these issues so that we could intervene.”
Improving health by tackling legal barriers
, Ms. Richardson said.
The program is one of few medical-legal partnerships specifically focused on the perinatal population. P-LAW is one component of a larger initiative at MedStar Health called DC Safe Babies Safe Moms. The initiative includes integrated mental health programming, treatment of health conditions that complicate pregnancy, assessments of social determinants of health, expanded support for lactation and nutrition, access to home visiting referrals, and extended postpartum follow-up. The work is supported through the A. James & Alice B. Clark Foundation.
Patients are evaluated for health-harming legal needs as part of a comprehensive social and behavioral health screening at their initial prenatal visit, 28-week appointment, and postpartum visit. Those who screen positive are contacted by a referral specialist on the health care team who confirms the patient has an active legal need and would like to be connected to the P-LAW team. The team then reaches out to conduct a legal intake and determine the appropriate course of action.
From March 2021 through February of this year, Ms. Richardson and others with the program have provided legal representation to 123 patients on 186 legal issues in areas such as public benefits, employment, and housing and family concerns. Services range from advising patients on steps they can take on their own (like reporting a housing condition issue to the Department of Buildings), to sending letters on patients’ behalf, to appearing in court. Most patients served were in their second and third trimesters of pregnancy. The majority were Black or African American, aged 20-34 years, and had incomes below 100% of the federal poverty level.
The most common legal issues were in the areas of public benefits (SNAP/food stamps, cash assistance), employment (parental leave, discrimination), housing (conditions, eviction), and family law (child support, domestic violence). Among the 186 issues, work has been completed on 106 concerns and 33 still have a case open; for 47, the client withdrew or ceased contact, Ms. Richardson reported.
Most times when obstetricians hear concerns like these, they wonder what to do, said Tamika Auguste, MD, chair of obstetrics and gynecology at MedStar Health. Having the P-LAW program as a resource is a huge help, she said. If patients express concerns, or if obstetricians uncover concerns during office visits, doctors can enter a referral directly in the electronic medical record.
Patients are “so relieved,” Dr. Auguste said in an interview, because they often wonder if their doctor can help. “Your doctor is only going to be able to help to a certain point. But to know they’re pregnant and they have this resource, and they’re going to get legal help, has been game-changing for so many patients.”
COVID ... or morning sickness?
In one rewarding case, Ms. Richardson said, a single mother of one child who was pregnant and experiencing hyperemesis explained that her employer would forbid her from working if she had any symptoms similar to COVID-19. The employer mistook her vomiting, nausea, and exhaustion as COVID symptoms and docked her pay. That started a cascade in which earning less meant she was facing eviction and car repossession – and, eventually, overdraft fees and withdrawals from her bank. She was so despondent she was thinking about self-harm, Ms. Richardson said.
With the aid of the P-LAW program, the woman had short-term disability approved within 72 hours, was referred to the hospital for inpatient mental health treatment, and received the care she needed. She ultimately delivered a healthy baby girl and found a new job.
Tiffany Moore Simas, MD, MPH, MEd, chair of the department of obstetrics and gynecology at the University of Massachusetts and UMass Memorial Health in Worcester, said she encounters similar concerns among her patients, with the vast majority having one or more issues with social determinants of health.
“I think it’s incredible, as we’re trying to address equity in perinatal health and maternal mortality and morbidity, to have a more holistic view of what health means, and all of the social determinants of health, and actually helping our patients address that in real time at their visits and connecting them,” said Dr. Simas, who also is professor of ob/gyn, pediatrics, psychiatry, and population and quantitative health sciences at UMass. “It has really opened my mind to the possibilities of things we need to explore and do differently.”
Ms. Richardson, Dr. Auguste, and Dr. Simas reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
BALTIMORE – A novel partnership between a legal services program and a maternal health clinic is helping pregnant patients with issues such as housing or employment discrimination.
The Perinatal Legal Assistance and Well-being (P-LAW) program at Georgetown University, Washington, launched 2 years ago as a collaboration between GU’s Health Justice Alliance clinic and the Women’s and Infants Services division of nearby MedStar Washington Hospital Center, integrating attorneys into the health care team to offer no-cost legal aid for its diverse, urban population during the perinatal period. Since then, the effort has assisted more than 120 women.
“Our goal was to see how integrating a lawyer can help address some of those issues that, unfortunately, providers are not able to assist with because they go beyond the hospital or clinic walls,” said Roxana Richardson, JD, the project director and managing attorney for P-LAW, during a poster presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “Our initial findings showed that there are issues that patients were facing that needed an intervention from an attorney. We trained the providers and social workers to identify these issues so that we could intervene.”
Improving health by tackling legal barriers
, Ms. Richardson said.
The program is one of few medical-legal partnerships specifically focused on the perinatal population. P-LAW is one component of a larger initiative at MedStar Health called DC Safe Babies Safe Moms. The initiative includes integrated mental health programming, treatment of health conditions that complicate pregnancy, assessments of social determinants of health, expanded support for lactation and nutrition, access to home visiting referrals, and extended postpartum follow-up. The work is supported through the A. James & Alice B. Clark Foundation.
Patients are evaluated for health-harming legal needs as part of a comprehensive social and behavioral health screening at their initial prenatal visit, 28-week appointment, and postpartum visit. Those who screen positive are contacted by a referral specialist on the health care team who confirms the patient has an active legal need and would like to be connected to the P-LAW team. The team then reaches out to conduct a legal intake and determine the appropriate course of action.
From March 2021 through February of this year, Ms. Richardson and others with the program have provided legal representation to 123 patients on 186 legal issues in areas such as public benefits, employment, and housing and family concerns. Services range from advising patients on steps they can take on their own (like reporting a housing condition issue to the Department of Buildings), to sending letters on patients’ behalf, to appearing in court. Most patients served were in their second and third trimesters of pregnancy. The majority were Black or African American, aged 20-34 years, and had incomes below 100% of the federal poverty level.
The most common legal issues were in the areas of public benefits (SNAP/food stamps, cash assistance), employment (parental leave, discrimination), housing (conditions, eviction), and family law (child support, domestic violence). Among the 186 issues, work has been completed on 106 concerns and 33 still have a case open; for 47, the client withdrew or ceased contact, Ms. Richardson reported.
Most times when obstetricians hear concerns like these, they wonder what to do, said Tamika Auguste, MD, chair of obstetrics and gynecology at MedStar Health. Having the P-LAW program as a resource is a huge help, she said. If patients express concerns, or if obstetricians uncover concerns during office visits, doctors can enter a referral directly in the electronic medical record.
Patients are “so relieved,” Dr. Auguste said in an interview, because they often wonder if their doctor can help. “Your doctor is only going to be able to help to a certain point. But to know they’re pregnant and they have this resource, and they’re going to get legal help, has been game-changing for so many patients.”
COVID ... or morning sickness?
In one rewarding case, Ms. Richardson said, a single mother of one child who was pregnant and experiencing hyperemesis explained that her employer would forbid her from working if she had any symptoms similar to COVID-19. The employer mistook her vomiting, nausea, and exhaustion as COVID symptoms and docked her pay. That started a cascade in which earning less meant she was facing eviction and car repossession – and, eventually, overdraft fees and withdrawals from her bank. She was so despondent she was thinking about self-harm, Ms. Richardson said.
With the aid of the P-LAW program, the woman had short-term disability approved within 72 hours, was referred to the hospital for inpatient mental health treatment, and received the care she needed. She ultimately delivered a healthy baby girl and found a new job.
Tiffany Moore Simas, MD, MPH, MEd, chair of the department of obstetrics and gynecology at the University of Massachusetts and UMass Memorial Health in Worcester, said she encounters similar concerns among her patients, with the vast majority having one or more issues with social determinants of health.
“I think it’s incredible, as we’re trying to address equity in perinatal health and maternal mortality and morbidity, to have a more holistic view of what health means, and all of the social determinants of health, and actually helping our patients address that in real time at their visits and connecting them,” said Dr. Simas, who also is professor of ob/gyn, pediatrics, psychiatry, and population and quantitative health sciences at UMass. “It has really opened my mind to the possibilities of things we need to explore and do differently.”
Ms. Richardson, Dr. Auguste, and Dr. Simas reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT ACOG 2023
Breast cancer outcomes are worse for Black men
A new study finds that racial disparities in male breast cancer are persisting in the United States.
From 2000 to 2019, Black men were diagnosed at later ages than White males (median ages, 69 and 63 years, respectively) and were more likely to die from the disease (22.4% vs. 16.8%, respectively). Male breast cancer (MBC) was more likely to kill Black men in rural vs. urban areas (hazard ratio = 1.4; 95% confidence interval, 1.0-2.1; P less than .05). Among White males, in contrast, there was no difference on that front, according to the research, which was presented in a poster (Abstract No. 87P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
It’s not clear why the disparities exist, said lead author Lekha Yadukumar, MBBS, an internal medicine resident at the Wright Center for Graduate Medical Education in Scranton, Penn., in an interview.
“Several potential factors may contribute to the higher rate of breast cancer diagnosis in older [Black] men, including the pathology of the disease, limited awareness about breast cancer, and potential barriers to accessibility,” she said. “The increased mortality among [Black men] may be linked to variations in tumor pathology and molecular biology. Social factors may also potentially impact survival rates, including [having] limited access to health care in rural areas and inadequate social support.”
Male breast cancer is rare, accounting for less than 1% of all breast cancer cases in the United States, according to the Breast Cancer Research Foundation. An estimated 2,700 men are diagnosed each year, and about 530 will die. Previous research has suggested Black men have worse outcomes than White men, but the data covered earlier years than the new study.
Methods and results
Dr. Yadukumar and colleagues retrospectively analyzed statistics from the Surveillance, Epidemiology, and End Results database for patients diagnosed with primary male breast cancer from 2000 to 2019 (n = 8,373; Black men, 1,111 [13.26%]; White men, 6,817 [81.41%]).
Median income didn’t affect mortality, whereas men in both racial groups were less likely to die if they were married vs. single/divorced (hazard ratio = 0.6; P less than .05).
Other studies have shown that “[Black American] men diagnosed with breast cancer experience longer time intervals before receiving treatment, encounter more severe disease manifestations, and exhibit lower rates of survivorship,” Dr. Yadukumar said. “Despite these findings, there remains a scarcity of genetic studies aimed at comprehending the underlying causes of these disparities. Moreover, there is a dearth of research investigating other factors that may influence survival outcomes among men with breast cancer.”
Findings reflect the disparities in female breast cancer
In an interview, Duke University, Durham, N.C., oncologist Arif Kamal, MD, MBA, MHS, the chief patient officer at the American Cancer Society, said the study is impressive since the number of patients is large for a rare cancer and the population is diverse. Plus, the findings reflect the disparities in female breast cancer, he noted.
“We know that Black women’s mortality is worse vs. White women in breast cancer, and we believe that most of that has nothing to do with cancer screening,” said Dr. Kamal, who was not involved in the new study. “When the clock starts from diagnosis onwards, you start to see less introduction to clinical trials and standard care medications and more time to treatment, surgery, and radiation,” he said.
“You see similar disparities as related to mortality in Black vs. White men,” he noted.
The new findings about higher death rates for Black men, especially in rural areas, suggest that “distance matters, and race matters,” he said. In rural areas, it can be hard to access pathologists, radiologists, and surgeons with more experience with breast cancer, he said.
But, he noted, the study finds that income doesn’t appear to be a factor.
In the big picture, he said, the results suggest that when it comes to barriers to better outcomes, “things that are systemic don’t make exceptions because you are a man vs. a woman.”
No study funding was reported. The study authors and Dr. Kamal have no relevant financial disclosures.
A new study finds that racial disparities in male breast cancer are persisting in the United States.
From 2000 to 2019, Black men were diagnosed at later ages than White males (median ages, 69 and 63 years, respectively) and were more likely to die from the disease (22.4% vs. 16.8%, respectively). Male breast cancer (MBC) was more likely to kill Black men in rural vs. urban areas (hazard ratio = 1.4; 95% confidence interval, 1.0-2.1; P less than .05). Among White males, in contrast, there was no difference on that front, according to the research, which was presented in a poster (Abstract No. 87P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
It’s not clear why the disparities exist, said lead author Lekha Yadukumar, MBBS, an internal medicine resident at the Wright Center for Graduate Medical Education in Scranton, Penn., in an interview.
“Several potential factors may contribute to the higher rate of breast cancer diagnosis in older [Black] men, including the pathology of the disease, limited awareness about breast cancer, and potential barriers to accessibility,” she said. “The increased mortality among [Black men] may be linked to variations in tumor pathology and molecular biology. Social factors may also potentially impact survival rates, including [having] limited access to health care in rural areas and inadequate social support.”
Male breast cancer is rare, accounting for less than 1% of all breast cancer cases in the United States, according to the Breast Cancer Research Foundation. An estimated 2,700 men are diagnosed each year, and about 530 will die. Previous research has suggested Black men have worse outcomes than White men, but the data covered earlier years than the new study.
Methods and results
Dr. Yadukumar and colleagues retrospectively analyzed statistics from the Surveillance, Epidemiology, and End Results database for patients diagnosed with primary male breast cancer from 2000 to 2019 (n = 8,373; Black men, 1,111 [13.26%]; White men, 6,817 [81.41%]).
Median income didn’t affect mortality, whereas men in both racial groups were less likely to die if they were married vs. single/divorced (hazard ratio = 0.6; P less than .05).
Other studies have shown that “[Black American] men diagnosed with breast cancer experience longer time intervals before receiving treatment, encounter more severe disease manifestations, and exhibit lower rates of survivorship,” Dr. Yadukumar said. “Despite these findings, there remains a scarcity of genetic studies aimed at comprehending the underlying causes of these disparities. Moreover, there is a dearth of research investigating other factors that may influence survival outcomes among men with breast cancer.”
Findings reflect the disparities in female breast cancer
In an interview, Duke University, Durham, N.C., oncologist Arif Kamal, MD, MBA, MHS, the chief patient officer at the American Cancer Society, said the study is impressive since the number of patients is large for a rare cancer and the population is diverse. Plus, the findings reflect the disparities in female breast cancer, he noted.
“We know that Black women’s mortality is worse vs. White women in breast cancer, and we believe that most of that has nothing to do with cancer screening,” said Dr. Kamal, who was not involved in the new study. “When the clock starts from diagnosis onwards, you start to see less introduction to clinical trials and standard care medications and more time to treatment, surgery, and radiation,” he said.
“You see similar disparities as related to mortality in Black vs. White men,” he noted.
The new findings about higher death rates for Black men, especially in rural areas, suggest that “distance matters, and race matters,” he said. In rural areas, it can be hard to access pathologists, radiologists, and surgeons with more experience with breast cancer, he said.
But, he noted, the study finds that income doesn’t appear to be a factor.
In the big picture, he said, the results suggest that when it comes to barriers to better outcomes, “things that are systemic don’t make exceptions because you are a man vs. a woman.”
No study funding was reported. The study authors and Dr. Kamal have no relevant financial disclosures.
A new study finds that racial disparities in male breast cancer are persisting in the United States.
From 2000 to 2019, Black men were diagnosed at later ages than White males (median ages, 69 and 63 years, respectively) and were more likely to die from the disease (22.4% vs. 16.8%, respectively). Male breast cancer (MBC) was more likely to kill Black men in rural vs. urban areas (hazard ratio = 1.4; 95% confidence interval, 1.0-2.1; P less than .05). Among White males, in contrast, there was no difference on that front, according to the research, which was presented in a poster (Abstract No. 87P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
It’s not clear why the disparities exist, said lead author Lekha Yadukumar, MBBS, an internal medicine resident at the Wright Center for Graduate Medical Education in Scranton, Penn., in an interview.
“Several potential factors may contribute to the higher rate of breast cancer diagnosis in older [Black] men, including the pathology of the disease, limited awareness about breast cancer, and potential barriers to accessibility,” she said. “The increased mortality among [Black men] may be linked to variations in tumor pathology and molecular biology. Social factors may also potentially impact survival rates, including [having] limited access to health care in rural areas and inadequate social support.”
Male breast cancer is rare, accounting for less than 1% of all breast cancer cases in the United States, according to the Breast Cancer Research Foundation. An estimated 2,700 men are diagnosed each year, and about 530 will die. Previous research has suggested Black men have worse outcomes than White men, but the data covered earlier years than the new study.
Methods and results
Dr. Yadukumar and colleagues retrospectively analyzed statistics from the Surveillance, Epidemiology, and End Results database for patients diagnosed with primary male breast cancer from 2000 to 2019 (n = 8,373; Black men, 1,111 [13.26%]; White men, 6,817 [81.41%]).
Median income didn’t affect mortality, whereas men in both racial groups were less likely to die if they were married vs. single/divorced (hazard ratio = 0.6; P less than .05).
Other studies have shown that “[Black American] men diagnosed with breast cancer experience longer time intervals before receiving treatment, encounter more severe disease manifestations, and exhibit lower rates of survivorship,” Dr. Yadukumar said. “Despite these findings, there remains a scarcity of genetic studies aimed at comprehending the underlying causes of these disparities. Moreover, there is a dearth of research investigating other factors that may influence survival outcomes among men with breast cancer.”
Findings reflect the disparities in female breast cancer
In an interview, Duke University, Durham, N.C., oncologist Arif Kamal, MD, MBA, MHS, the chief patient officer at the American Cancer Society, said the study is impressive since the number of patients is large for a rare cancer and the population is diverse. Plus, the findings reflect the disparities in female breast cancer, he noted.
“We know that Black women’s mortality is worse vs. White women in breast cancer, and we believe that most of that has nothing to do with cancer screening,” said Dr. Kamal, who was not involved in the new study. “When the clock starts from diagnosis onwards, you start to see less introduction to clinical trials and standard care medications and more time to treatment, surgery, and radiation,” he said.
“You see similar disparities as related to mortality in Black vs. White men,” he noted.
The new findings about higher death rates for Black men, especially in rural areas, suggest that “distance matters, and race matters,” he said. In rural areas, it can be hard to access pathologists, radiologists, and surgeons with more experience with breast cancer, he said.
But, he noted, the study finds that income doesn’t appear to be a factor.
In the big picture, he said, the results suggest that when it comes to barriers to better outcomes, “things that are systemic don’t make exceptions because you are a man vs. a woman.”
No study funding was reported. The study authors and Dr. Kamal have no relevant financial disclosures.
FROM ESMO BREAST CANCER 2023
Black patients most likely to be restrained in EDs, Latino patients least likely
SAN FRANCISCO – .
In contrast, Hispanic/Latino patients were less likely to be restrained than both Black and White patients, researchers reported in a poster presented at the annual meeting of the American Psychiatric Association. The study authors also found that clinicians rarely turned to restraints, using them in just 2,712 of 882,390 ED visits (0.3%) over a 7-year period.
The study doesn’t examine why the disparities exist. But lead author Erika Chang-Sing, a medical student at Yale University, New Haven, Conn., said in an interview that it’s clear that racial bias is the cause of the differences in restraint rates among White, Black, and Hispanics/Latino patients. “We think that there are multiple contributing factors to the higher rates of restraint for Black patients brought to the hospital by police, and all of them are rooted in systemic racism,” she said, adding that “the lower odds of restraint in the Hispanic or Latino group are also rooted in systemic racism and inequity.”
According to Ms. Chang-Sing, researchers launched the study to gain insight into the use of the restraints in the Southeast and to see what’s happening in light of the recent publicizing of killings of Black people by police. Being taken to the hospital by police “might contribute both to the individual patient’s behavior and the health care provider’s assessment of risk in determining whether or not to apply restraints,” she said.
Other research has linked ethnicity to higher rates of restraint use. For example, a 2021 study of 32,054 cases of patients under mandatory psychiatric hold in 11 Massachusetts emergency rooms found that Black (adjusted odds ratio, 1.22) and Hispanic (aOR, 1.45) patients were more likely to be restrained than White patients.
For the new study, researchers retrospectively tracked 885,102 emergency room visits at three North Carolina emergency departments from 2015 to 2022, including 9,130 who were brought in by police and 2,712 who were physically restrained because of the perceived risk of violence. “Providers use restraints, or straps, to secure the patient’s wrists and ankles to the bed,” Ms. Chang-Sing said.
Among all patients, 52.5% were Black, but 66% of those who were restrained were Black. The numbers for White patients were 35.7% and 23.9%, respectively, and 5.7% and 3.2% for Hispanics/Latino patients. Black patients were less likely than White patients to get a psychiatric primary emergency department diagnosis (aOR, 0.67), but those in that category were more likely than their White counterparts to be restrained (aOR, 1.36).
The higher risk of restraint use in Black patients overall disappeared when researchers adjusted their statistics to account for the effects of sex, age, and type of insurance (aOR, 0.86). Ms. Chang-Sing said the study team is reanalyzing the data since they think insurance may not be a confounder.
Why might Hispanic/Latino ethnicity be protective against restraint use? “This may be due to language barriers, fear of law enforcement, and avoidance of the hospital in the first place,” Ms. Chang-Sing said.
Emergency physician Wendy Macias-Konstantopoulos, MD, MPH, MBA, of Harvard Medical School and Massachusetts General Hospital, both in Boston, coauthored the 2021 study on police restraints. In an interview, she said the new findings add to previous research by providing data about the role played by the police who bring patients to the ED. She added that there is no evidence that certain populations simply need more restraints.
What can be done to reduce disparities in restraint use? Mental health teams can make a difference by responding to mental health emergencies, Ms. Chang-Sing said. “These providers can be instrumental in communicating to patients that the intention is to care for them, not to punish them.”
Another strategy is to increase the number of clinics and crisis response centers, she said. Hospital-based crisis response teams can also be helpful, she said. “Because these teams are focused only on behavioral emergencies, they can be more thoughtful in avoiding the use of restraints.”
No study funding was reported. The study authors and Dr. Macias-Konstantopoulos have no disclosures.
SAN FRANCISCO – .
In contrast, Hispanic/Latino patients were less likely to be restrained than both Black and White patients, researchers reported in a poster presented at the annual meeting of the American Psychiatric Association. The study authors also found that clinicians rarely turned to restraints, using them in just 2,712 of 882,390 ED visits (0.3%) over a 7-year period.
The study doesn’t examine why the disparities exist. But lead author Erika Chang-Sing, a medical student at Yale University, New Haven, Conn., said in an interview that it’s clear that racial bias is the cause of the differences in restraint rates among White, Black, and Hispanics/Latino patients. “We think that there are multiple contributing factors to the higher rates of restraint for Black patients brought to the hospital by police, and all of them are rooted in systemic racism,” she said, adding that “the lower odds of restraint in the Hispanic or Latino group are also rooted in systemic racism and inequity.”
According to Ms. Chang-Sing, researchers launched the study to gain insight into the use of the restraints in the Southeast and to see what’s happening in light of the recent publicizing of killings of Black people by police. Being taken to the hospital by police “might contribute both to the individual patient’s behavior and the health care provider’s assessment of risk in determining whether or not to apply restraints,” she said.
Other research has linked ethnicity to higher rates of restraint use. For example, a 2021 study of 32,054 cases of patients under mandatory psychiatric hold in 11 Massachusetts emergency rooms found that Black (adjusted odds ratio, 1.22) and Hispanic (aOR, 1.45) patients were more likely to be restrained than White patients.
For the new study, researchers retrospectively tracked 885,102 emergency room visits at three North Carolina emergency departments from 2015 to 2022, including 9,130 who were brought in by police and 2,712 who were physically restrained because of the perceived risk of violence. “Providers use restraints, or straps, to secure the patient’s wrists and ankles to the bed,” Ms. Chang-Sing said.
Among all patients, 52.5% were Black, but 66% of those who were restrained were Black. The numbers for White patients were 35.7% and 23.9%, respectively, and 5.7% and 3.2% for Hispanics/Latino patients. Black patients were less likely than White patients to get a psychiatric primary emergency department diagnosis (aOR, 0.67), but those in that category were more likely than their White counterparts to be restrained (aOR, 1.36).
The higher risk of restraint use in Black patients overall disappeared when researchers adjusted their statistics to account for the effects of sex, age, and type of insurance (aOR, 0.86). Ms. Chang-Sing said the study team is reanalyzing the data since they think insurance may not be a confounder.
Why might Hispanic/Latino ethnicity be protective against restraint use? “This may be due to language barriers, fear of law enforcement, and avoidance of the hospital in the first place,” Ms. Chang-Sing said.
Emergency physician Wendy Macias-Konstantopoulos, MD, MPH, MBA, of Harvard Medical School and Massachusetts General Hospital, both in Boston, coauthored the 2021 study on police restraints. In an interview, she said the new findings add to previous research by providing data about the role played by the police who bring patients to the ED. She added that there is no evidence that certain populations simply need more restraints.
What can be done to reduce disparities in restraint use? Mental health teams can make a difference by responding to mental health emergencies, Ms. Chang-Sing said. “These providers can be instrumental in communicating to patients that the intention is to care for them, not to punish them.”
Another strategy is to increase the number of clinics and crisis response centers, she said. Hospital-based crisis response teams can also be helpful, she said. “Because these teams are focused only on behavioral emergencies, they can be more thoughtful in avoiding the use of restraints.”
No study funding was reported. The study authors and Dr. Macias-Konstantopoulos have no disclosures.
SAN FRANCISCO – .
In contrast, Hispanic/Latino patients were less likely to be restrained than both Black and White patients, researchers reported in a poster presented at the annual meeting of the American Psychiatric Association. The study authors also found that clinicians rarely turned to restraints, using them in just 2,712 of 882,390 ED visits (0.3%) over a 7-year period.
The study doesn’t examine why the disparities exist. But lead author Erika Chang-Sing, a medical student at Yale University, New Haven, Conn., said in an interview that it’s clear that racial bias is the cause of the differences in restraint rates among White, Black, and Hispanics/Latino patients. “We think that there are multiple contributing factors to the higher rates of restraint for Black patients brought to the hospital by police, and all of them are rooted in systemic racism,” she said, adding that “the lower odds of restraint in the Hispanic or Latino group are also rooted in systemic racism and inequity.”
According to Ms. Chang-Sing, researchers launched the study to gain insight into the use of the restraints in the Southeast and to see what’s happening in light of the recent publicizing of killings of Black people by police. Being taken to the hospital by police “might contribute both to the individual patient’s behavior and the health care provider’s assessment of risk in determining whether or not to apply restraints,” she said.
Other research has linked ethnicity to higher rates of restraint use. For example, a 2021 study of 32,054 cases of patients under mandatory psychiatric hold in 11 Massachusetts emergency rooms found that Black (adjusted odds ratio, 1.22) and Hispanic (aOR, 1.45) patients were more likely to be restrained than White patients.
For the new study, researchers retrospectively tracked 885,102 emergency room visits at three North Carolina emergency departments from 2015 to 2022, including 9,130 who were brought in by police and 2,712 who were physically restrained because of the perceived risk of violence. “Providers use restraints, or straps, to secure the patient’s wrists and ankles to the bed,” Ms. Chang-Sing said.
Among all patients, 52.5% were Black, but 66% of those who were restrained were Black. The numbers for White patients were 35.7% and 23.9%, respectively, and 5.7% and 3.2% for Hispanics/Latino patients. Black patients were less likely than White patients to get a psychiatric primary emergency department diagnosis (aOR, 0.67), but those in that category were more likely than their White counterparts to be restrained (aOR, 1.36).
The higher risk of restraint use in Black patients overall disappeared when researchers adjusted their statistics to account for the effects of sex, age, and type of insurance (aOR, 0.86). Ms. Chang-Sing said the study team is reanalyzing the data since they think insurance may not be a confounder.
Why might Hispanic/Latino ethnicity be protective against restraint use? “This may be due to language barriers, fear of law enforcement, and avoidance of the hospital in the first place,” Ms. Chang-Sing said.
Emergency physician Wendy Macias-Konstantopoulos, MD, MPH, MBA, of Harvard Medical School and Massachusetts General Hospital, both in Boston, coauthored the 2021 study on police restraints. In an interview, she said the new findings add to previous research by providing data about the role played by the police who bring patients to the ED. She added that there is no evidence that certain populations simply need more restraints.
What can be done to reduce disparities in restraint use? Mental health teams can make a difference by responding to mental health emergencies, Ms. Chang-Sing said. “These providers can be instrumental in communicating to patients that the intention is to care for them, not to punish them.”
Another strategy is to increase the number of clinics and crisis response centers, she said. Hospital-based crisis response teams can also be helpful, she said. “Because these teams are focused only on behavioral emergencies, they can be more thoughtful in avoiding the use of restraints.”
No study funding was reported. The study authors and Dr. Macias-Konstantopoulos have no disclosures.
AT APA 2023
Differences in 30-Day Readmission Rates in Older Adults With Dementia
Study 1 Overview (Park et al)
Objective: To compare rates of adverse events and 30-day readmission among patients with dementia who undergo percutaneous coronary intervention (PCI) with those without dementia.
Design: This cohort study used a national database of hospital readmissions developed by the Agency for Healthcare Research and Quality.
Setting and participants: Data from State Inpatient Databases were used to derive this national readmissions database representing 80% of hospitals from 28 states that contribute data. The study included all individuals aged 18 years and older who were identified to have had a PCI procedure in the years 2017 and 2018. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify PCI procedures, including drug-eluting stent placement, bare-metal stent placement, and balloon angioplasty, performed in patients who presented with myocardial infarction and unstable angina and those with stable ischemic heart disease. Patients were stratified into those with or without dementia, also defined using ICD-10 codes. A total of 755,406 index hospitalizations were included; 2.3% of the patients had dementia.
Main outcome measures: The primary study outcome was 30-day all-cause readmission, with the cause classified as cardiovascular or noncardiovascular. Secondary outcome measures examined were delirium, in-hospital mortality, cardiac arrest, blood transfusion, acute kidney injury, fall in hospital, length of hospital stay, and other adverse outcomes. Location at discharge was also examined. Other covariates included in the analysis were age, sex, comorbidities, hospital characteristics, primary payer, and median income. For analysis, a propensity score matching algorithm was applied to match patients with and without dementia. Kaplan-Meier curves were used to examine 30-day readmission rates, and a Cox proportional hazards model was used to calculate hazard ratios (HR) for those with and without dementia. For secondary outcomes, logistic regression models were used to calculate odds ratios (OR) of outcomes between those with and without dementia.
Main results: The average age of those with dementia was 78.8 years vs 64.9 years in those without dementia. Women made up 42.8% of those with dementia and 31.3% of those without dementia. Those with dementia also had higher rates of comorbidities, such as heart failure, renal failure, and depression. After propensity score matching, 17,309 and 17,187 patients with and without dementia, respectively, were included. Covariates were balanced between the 2 groups after matching. For the primary outcome, patients with dementia were more likely to be readmitted at 30 days (HR, 1.11; 95% CI, 1.05-1.18; P < .01) when compared to those without dementia. For other adverse outcomes, delirium was significantly more likely to occur for those with dementia (OR, 4.37; 95% CI, 3.69-5.16; P < .01). Patients with dementia were also more likely to die in hospital (OR, 1.15; 95% CI, 1.01-1.30; P = .03), have cardiac arrest (OR, 1.19; 95% CI, 1.01-1.39; P = .04), receive a blood transfusion (OR, 1.17; 95% CI, 1.00-1.36; P = .05), experience acute kidney injury (OR, 1.30; 95% CI, 1.21-1.39; P < .01), and fall in hospital (OR, 2.51; 95% CI, 2.06-3.07; P < .01). Hospital length of stay was higher for those with dementia, with a mean difference of 1.43 days. For discharge location, patients with dementia were more likely to be sent to a skilled nursing facility (30.1% vs 12.2%) and less likely to be discharged home.
Conclusion: Patients with dementia are more likely to experience adverse events, including delirium, mortality, kidney injury, and falls after PCI, and are more likely to be readmitted to the hospital in 30 days compared to those without dementia.
Study 2 Overview (Gilmore-Bykovskyi et al)
Objective: To examine the association between race and 30-day readmissions in Black and non-Hispanic White Medicare beneficiaries with dementia.
Design: This was a retrospective cohort study that used 100% Medicare fee-for service claims data from all hospitalizations between January 1, 2014, and November 30, 2014, for all enrollees with a dementia diagnosis. The claims data were linked to the patient, hospital stay, and hospital factors. Patients with dementia were identified using a validated algorithm that requires an inpatient, skilled nursing facility, home health, or Part B institutional or noninstitutional claim with a qualifying diagnostic code during a 3-year period. Persons enrolled in a health maintenance organization plan were excluded.
Main outcome measures: The primary outcome examined in this study was 30-day all-cause readmission. Self-reported race and ethnic identity was a baseline covariate. Persons who self-reported Black or non-Hispanic White race were included in the study; other categories of race and ethnicity were excluded because of prior evidence suggesting low accuracy of these categories in Medicare claims data. Other covariates included neighborhood disadvantage, measured using the Area Deprivation Index (ADI), and rurality; hospital-level and hospital stay–level characteristics such as for-profit status and number of annual discharges; and individual demographic characteristics and comorbidities. The ADI is constructed using variables of poverty, education, housing, and employment and is represented as a percentile ranking of level of disadvantage. Unadjusted and adjusted analyses of 30-day hospital readmission were conducted. Models using various levels of adjustment were constructed to examine the contributions of the identified covariates to the estimated association between 30-day readmission and race.
Main results: A total of 1,523,142 index hospital stays among 945,481 beneficiaries were included; 215,815 episodes were among Black beneficiaries and 1,307,327 episodes were among non-Hispanic White beneficiaries. Mean age was 81.5 years, and approximately 61% of beneficiaries were female. Black beneficiaries were younger but had higher rates of dual Medicare/Medicaid eligibility and disability; they were also more likely to reside in disadvantaged neighborhoods. Black beneficiaries had a 30-day readmission rate of 24.1% compared with 18.5% in non-Hispanic White beneficiaries (unadjusted OR, 1.37; 95% CI, 1.35-1.39). The differences in outcomes persisted after adjusting for geographic factors, social factors, hospital characteristics, hospital stay factors, demographics, and comorbidities, suggesting that unmeasured underlying racial disparities not included in this model accounted for the differences. The effects of certain variables, such as neighborhood, differed by race; for example, the protective effect of living in a less disadvantaged neighborhood was observed among White beneficiaries but not Black beneficiaries.
Conclusion: Racial and geographic disparities in 30-day readmission rates were observed among Medicare beneficiaries with dementia. Protective effects associated with neighborhood advantage may confer different levels of benefit for people of different race.
Commentary
Adults living with dementia are at higher risk of adverse outcomes across settings. In the first study, by Park et al, among adults who underwent a cardiac procedure (PCI), those with dementia were more likely to experience adverse events compared to those without dementia. These outcomes include increased rates of 30-day readmissions, delirium, cardiac arrest, and falls. These findings are consistent with other studies that found a similar association among patients who underwent other cardiac procedures, such as transcatheter aortic valve replacement.1 Because dementia is a strong predisposing factor for delirium, it is not surprising that delirium is observed across patients who underwent different procedures or hospitalization episodes.2 Because of the potential hazards for inpatients with dementia, hospitals have developed risk-reduction programs, such as those that promote recognition of dementia, and management strategies that reduce the risk of delirium.3 Delirium prevention may also impact other adverse outcomes, such as falls, discharge to institutional care, and readmissions.
Racial disparities in care outcomes have been documented across settings, including hospital4 and hospice care settings.5 In study 2, by Gilmore-Bykovskyi et al, the findings of higher rates of hospital readmission among Black patients when compared to non-Hispanic White patients were not surprising. The central finding of this study is that even when accounting for various levels of factors, including hospital-level, hospital stay–level, individual (demographics, comorbidities), and neighborhood characteristics (disadvantage), the observed disparity diminished but persisted, suggesting that while these various levels of factors contributed to the observed disparity, other unmeasured factors also contributed. Another key finding is that the effect of the various factors examined in this study may affect different subgroups in different ways, suggesting underlying factors, and thus potential solutions to reduce disparities in care outcomes, could differ among subgroups.
Applications for Clinical Practice and System Implementation
These 2 studies add to the literature on factors that can affect 30-day hospital readmission rates in patients with dementia. These data could allow for more robust discussions of what to anticipate when adults with dementia undergo specific procedures, and also further build the case that improvements in care, such as delirium prevention programs, could offer benefits. The observation about racial and ethnic disparities in care outcomes among patients with dementia highlights the continued need to better understand the drivers of these disparities so that hospital systems and policy makers can consider and test possible solutions. Future studies should further disentangle the relationships among the various levels of factors and observed disparities in outcomes, especially for this vulnerable population of adults living with dementia.
Practice Points
- Clinicians should be aware of the additional risks for poor outcomes that dementia confers.
- Awareness of this increased risk will inform discussions of risks and benefits for older adults considered for procedures.
–William W. Hung, MD, MPH
1. Park DY, Sana MK, Shoura S, et al. Readmission and in-hospital outcomes after transcatheter aortic valve replacement in patients with dementia. Cardiovasc Revasc Med. 2023;46:70-77. doi:10.1016/j.carrev.2022.08.016
2. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598. doi:10.1034/j.1600-0579.2003.00201.x
3. Weldingh NM, Mellingsæter MR, Hegna BW, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266. doi:10.1186/s12877-022-02949-0
4. Hermosura AH, Noonan CJ, Fyfe-Johnson AL, et al. Hospital disparities between native Hawaiian and other pacific islanders and non-Hispanic whites with Alzheimer’s disease and related dementias. J Aging Health. 2020;32(10):1579-1590. doi:10.1177/0898264320945177
5. Zhang Y, Shao H, Zhang M, Li J. Healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias. J Gen Intern Med. 2023 Jan 17. doi:10.1007/s11606-023-08031-8
Study 1 Overview (Park et al)
Objective: To compare rates of adverse events and 30-day readmission among patients with dementia who undergo percutaneous coronary intervention (PCI) with those without dementia.
Design: This cohort study used a national database of hospital readmissions developed by the Agency for Healthcare Research and Quality.
Setting and participants: Data from State Inpatient Databases were used to derive this national readmissions database representing 80% of hospitals from 28 states that contribute data. The study included all individuals aged 18 years and older who were identified to have had a PCI procedure in the years 2017 and 2018. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify PCI procedures, including drug-eluting stent placement, bare-metal stent placement, and balloon angioplasty, performed in patients who presented with myocardial infarction and unstable angina and those with stable ischemic heart disease. Patients were stratified into those with or without dementia, also defined using ICD-10 codes. A total of 755,406 index hospitalizations were included; 2.3% of the patients had dementia.
Main outcome measures: The primary study outcome was 30-day all-cause readmission, with the cause classified as cardiovascular or noncardiovascular. Secondary outcome measures examined were delirium, in-hospital mortality, cardiac arrest, blood transfusion, acute kidney injury, fall in hospital, length of hospital stay, and other adverse outcomes. Location at discharge was also examined. Other covariates included in the analysis were age, sex, comorbidities, hospital characteristics, primary payer, and median income. For analysis, a propensity score matching algorithm was applied to match patients with and without dementia. Kaplan-Meier curves were used to examine 30-day readmission rates, and a Cox proportional hazards model was used to calculate hazard ratios (HR) for those with and without dementia. For secondary outcomes, logistic regression models were used to calculate odds ratios (OR) of outcomes between those with and without dementia.
Main results: The average age of those with dementia was 78.8 years vs 64.9 years in those without dementia. Women made up 42.8% of those with dementia and 31.3% of those without dementia. Those with dementia also had higher rates of comorbidities, such as heart failure, renal failure, and depression. After propensity score matching, 17,309 and 17,187 patients with and without dementia, respectively, were included. Covariates were balanced between the 2 groups after matching. For the primary outcome, patients with dementia were more likely to be readmitted at 30 days (HR, 1.11; 95% CI, 1.05-1.18; P < .01) when compared to those without dementia. For other adverse outcomes, delirium was significantly more likely to occur for those with dementia (OR, 4.37; 95% CI, 3.69-5.16; P < .01). Patients with dementia were also more likely to die in hospital (OR, 1.15; 95% CI, 1.01-1.30; P = .03), have cardiac arrest (OR, 1.19; 95% CI, 1.01-1.39; P = .04), receive a blood transfusion (OR, 1.17; 95% CI, 1.00-1.36; P = .05), experience acute kidney injury (OR, 1.30; 95% CI, 1.21-1.39; P < .01), and fall in hospital (OR, 2.51; 95% CI, 2.06-3.07; P < .01). Hospital length of stay was higher for those with dementia, with a mean difference of 1.43 days. For discharge location, patients with dementia were more likely to be sent to a skilled nursing facility (30.1% vs 12.2%) and less likely to be discharged home.
Conclusion: Patients with dementia are more likely to experience adverse events, including delirium, mortality, kidney injury, and falls after PCI, and are more likely to be readmitted to the hospital in 30 days compared to those without dementia.
Study 2 Overview (Gilmore-Bykovskyi et al)
Objective: To examine the association between race and 30-day readmissions in Black and non-Hispanic White Medicare beneficiaries with dementia.
Design: This was a retrospective cohort study that used 100% Medicare fee-for service claims data from all hospitalizations between January 1, 2014, and November 30, 2014, for all enrollees with a dementia diagnosis. The claims data were linked to the patient, hospital stay, and hospital factors. Patients with dementia were identified using a validated algorithm that requires an inpatient, skilled nursing facility, home health, or Part B institutional or noninstitutional claim with a qualifying diagnostic code during a 3-year period. Persons enrolled in a health maintenance organization plan were excluded.
Main outcome measures: The primary outcome examined in this study was 30-day all-cause readmission. Self-reported race and ethnic identity was a baseline covariate. Persons who self-reported Black or non-Hispanic White race were included in the study; other categories of race and ethnicity were excluded because of prior evidence suggesting low accuracy of these categories in Medicare claims data. Other covariates included neighborhood disadvantage, measured using the Area Deprivation Index (ADI), and rurality; hospital-level and hospital stay–level characteristics such as for-profit status and number of annual discharges; and individual demographic characteristics and comorbidities. The ADI is constructed using variables of poverty, education, housing, and employment and is represented as a percentile ranking of level of disadvantage. Unadjusted and adjusted analyses of 30-day hospital readmission were conducted. Models using various levels of adjustment were constructed to examine the contributions of the identified covariates to the estimated association between 30-day readmission and race.
Main results: A total of 1,523,142 index hospital stays among 945,481 beneficiaries were included; 215,815 episodes were among Black beneficiaries and 1,307,327 episodes were among non-Hispanic White beneficiaries. Mean age was 81.5 years, and approximately 61% of beneficiaries were female. Black beneficiaries were younger but had higher rates of dual Medicare/Medicaid eligibility and disability; they were also more likely to reside in disadvantaged neighborhoods. Black beneficiaries had a 30-day readmission rate of 24.1% compared with 18.5% in non-Hispanic White beneficiaries (unadjusted OR, 1.37; 95% CI, 1.35-1.39). The differences in outcomes persisted after adjusting for geographic factors, social factors, hospital characteristics, hospital stay factors, demographics, and comorbidities, suggesting that unmeasured underlying racial disparities not included in this model accounted for the differences. The effects of certain variables, such as neighborhood, differed by race; for example, the protective effect of living in a less disadvantaged neighborhood was observed among White beneficiaries but not Black beneficiaries.
Conclusion: Racial and geographic disparities in 30-day readmission rates were observed among Medicare beneficiaries with dementia. Protective effects associated with neighborhood advantage may confer different levels of benefit for people of different race.
Commentary
Adults living with dementia are at higher risk of adverse outcomes across settings. In the first study, by Park et al, among adults who underwent a cardiac procedure (PCI), those with dementia were more likely to experience adverse events compared to those without dementia. These outcomes include increased rates of 30-day readmissions, delirium, cardiac arrest, and falls. These findings are consistent with other studies that found a similar association among patients who underwent other cardiac procedures, such as transcatheter aortic valve replacement.1 Because dementia is a strong predisposing factor for delirium, it is not surprising that delirium is observed across patients who underwent different procedures or hospitalization episodes.2 Because of the potential hazards for inpatients with dementia, hospitals have developed risk-reduction programs, such as those that promote recognition of dementia, and management strategies that reduce the risk of delirium.3 Delirium prevention may also impact other adverse outcomes, such as falls, discharge to institutional care, and readmissions.
Racial disparities in care outcomes have been documented across settings, including hospital4 and hospice care settings.5 In study 2, by Gilmore-Bykovskyi et al, the findings of higher rates of hospital readmission among Black patients when compared to non-Hispanic White patients were not surprising. The central finding of this study is that even when accounting for various levels of factors, including hospital-level, hospital stay–level, individual (demographics, comorbidities), and neighborhood characteristics (disadvantage), the observed disparity diminished but persisted, suggesting that while these various levels of factors contributed to the observed disparity, other unmeasured factors also contributed. Another key finding is that the effect of the various factors examined in this study may affect different subgroups in different ways, suggesting underlying factors, and thus potential solutions to reduce disparities in care outcomes, could differ among subgroups.
Applications for Clinical Practice and System Implementation
These 2 studies add to the literature on factors that can affect 30-day hospital readmission rates in patients with dementia. These data could allow for more robust discussions of what to anticipate when adults with dementia undergo specific procedures, and also further build the case that improvements in care, such as delirium prevention programs, could offer benefits. The observation about racial and ethnic disparities in care outcomes among patients with dementia highlights the continued need to better understand the drivers of these disparities so that hospital systems and policy makers can consider and test possible solutions. Future studies should further disentangle the relationships among the various levels of factors and observed disparities in outcomes, especially for this vulnerable population of adults living with dementia.
Practice Points
- Clinicians should be aware of the additional risks for poor outcomes that dementia confers.
- Awareness of this increased risk will inform discussions of risks and benefits for older adults considered for procedures.
–William W. Hung, MD, MPH
Study 1 Overview (Park et al)
Objective: To compare rates of adverse events and 30-day readmission among patients with dementia who undergo percutaneous coronary intervention (PCI) with those without dementia.
Design: This cohort study used a national database of hospital readmissions developed by the Agency for Healthcare Research and Quality.
Setting and participants: Data from State Inpatient Databases were used to derive this national readmissions database representing 80% of hospitals from 28 states that contribute data. The study included all individuals aged 18 years and older who were identified to have had a PCI procedure in the years 2017 and 2018. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify PCI procedures, including drug-eluting stent placement, bare-metal stent placement, and balloon angioplasty, performed in patients who presented with myocardial infarction and unstable angina and those with stable ischemic heart disease. Patients were stratified into those with or without dementia, also defined using ICD-10 codes. A total of 755,406 index hospitalizations were included; 2.3% of the patients had dementia.
Main outcome measures: The primary study outcome was 30-day all-cause readmission, with the cause classified as cardiovascular or noncardiovascular. Secondary outcome measures examined were delirium, in-hospital mortality, cardiac arrest, blood transfusion, acute kidney injury, fall in hospital, length of hospital stay, and other adverse outcomes. Location at discharge was also examined. Other covariates included in the analysis were age, sex, comorbidities, hospital characteristics, primary payer, and median income. For analysis, a propensity score matching algorithm was applied to match patients with and without dementia. Kaplan-Meier curves were used to examine 30-day readmission rates, and a Cox proportional hazards model was used to calculate hazard ratios (HR) for those with and without dementia. For secondary outcomes, logistic regression models were used to calculate odds ratios (OR) of outcomes between those with and without dementia.
Main results: The average age of those with dementia was 78.8 years vs 64.9 years in those without dementia. Women made up 42.8% of those with dementia and 31.3% of those without dementia. Those with dementia also had higher rates of comorbidities, such as heart failure, renal failure, and depression. After propensity score matching, 17,309 and 17,187 patients with and without dementia, respectively, were included. Covariates were balanced between the 2 groups after matching. For the primary outcome, patients with dementia were more likely to be readmitted at 30 days (HR, 1.11; 95% CI, 1.05-1.18; P < .01) when compared to those without dementia. For other adverse outcomes, delirium was significantly more likely to occur for those with dementia (OR, 4.37; 95% CI, 3.69-5.16; P < .01). Patients with dementia were also more likely to die in hospital (OR, 1.15; 95% CI, 1.01-1.30; P = .03), have cardiac arrest (OR, 1.19; 95% CI, 1.01-1.39; P = .04), receive a blood transfusion (OR, 1.17; 95% CI, 1.00-1.36; P = .05), experience acute kidney injury (OR, 1.30; 95% CI, 1.21-1.39; P < .01), and fall in hospital (OR, 2.51; 95% CI, 2.06-3.07; P < .01). Hospital length of stay was higher for those with dementia, with a mean difference of 1.43 days. For discharge location, patients with dementia were more likely to be sent to a skilled nursing facility (30.1% vs 12.2%) and less likely to be discharged home.
Conclusion: Patients with dementia are more likely to experience adverse events, including delirium, mortality, kidney injury, and falls after PCI, and are more likely to be readmitted to the hospital in 30 days compared to those without dementia.
Study 2 Overview (Gilmore-Bykovskyi et al)
Objective: To examine the association between race and 30-day readmissions in Black and non-Hispanic White Medicare beneficiaries with dementia.
Design: This was a retrospective cohort study that used 100% Medicare fee-for service claims data from all hospitalizations between January 1, 2014, and November 30, 2014, for all enrollees with a dementia diagnosis. The claims data were linked to the patient, hospital stay, and hospital factors. Patients with dementia were identified using a validated algorithm that requires an inpatient, skilled nursing facility, home health, or Part B institutional or noninstitutional claim with a qualifying diagnostic code during a 3-year period. Persons enrolled in a health maintenance organization plan were excluded.
Main outcome measures: The primary outcome examined in this study was 30-day all-cause readmission. Self-reported race and ethnic identity was a baseline covariate. Persons who self-reported Black or non-Hispanic White race were included in the study; other categories of race and ethnicity were excluded because of prior evidence suggesting low accuracy of these categories in Medicare claims data. Other covariates included neighborhood disadvantage, measured using the Area Deprivation Index (ADI), and rurality; hospital-level and hospital stay–level characteristics such as for-profit status and number of annual discharges; and individual demographic characteristics and comorbidities. The ADI is constructed using variables of poverty, education, housing, and employment and is represented as a percentile ranking of level of disadvantage. Unadjusted and adjusted analyses of 30-day hospital readmission were conducted. Models using various levels of adjustment were constructed to examine the contributions of the identified covariates to the estimated association between 30-day readmission and race.
Main results: A total of 1,523,142 index hospital stays among 945,481 beneficiaries were included; 215,815 episodes were among Black beneficiaries and 1,307,327 episodes were among non-Hispanic White beneficiaries. Mean age was 81.5 years, and approximately 61% of beneficiaries were female. Black beneficiaries were younger but had higher rates of dual Medicare/Medicaid eligibility and disability; they were also more likely to reside in disadvantaged neighborhoods. Black beneficiaries had a 30-day readmission rate of 24.1% compared with 18.5% in non-Hispanic White beneficiaries (unadjusted OR, 1.37; 95% CI, 1.35-1.39). The differences in outcomes persisted after adjusting for geographic factors, social factors, hospital characteristics, hospital stay factors, demographics, and comorbidities, suggesting that unmeasured underlying racial disparities not included in this model accounted for the differences. The effects of certain variables, such as neighborhood, differed by race; for example, the protective effect of living in a less disadvantaged neighborhood was observed among White beneficiaries but not Black beneficiaries.
Conclusion: Racial and geographic disparities in 30-day readmission rates were observed among Medicare beneficiaries with dementia. Protective effects associated with neighborhood advantage may confer different levels of benefit for people of different race.
Commentary
Adults living with dementia are at higher risk of adverse outcomes across settings. In the first study, by Park et al, among adults who underwent a cardiac procedure (PCI), those with dementia were more likely to experience adverse events compared to those without dementia. These outcomes include increased rates of 30-day readmissions, delirium, cardiac arrest, and falls. These findings are consistent with other studies that found a similar association among patients who underwent other cardiac procedures, such as transcatheter aortic valve replacement.1 Because dementia is a strong predisposing factor for delirium, it is not surprising that delirium is observed across patients who underwent different procedures or hospitalization episodes.2 Because of the potential hazards for inpatients with dementia, hospitals have developed risk-reduction programs, such as those that promote recognition of dementia, and management strategies that reduce the risk of delirium.3 Delirium prevention may also impact other adverse outcomes, such as falls, discharge to institutional care, and readmissions.
Racial disparities in care outcomes have been documented across settings, including hospital4 and hospice care settings.5 In study 2, by Gilmore-Bykovskyi et al, the findings of higher rates of hospital readmission among Black patients when compared to non-Hispanic White patients were not surprising. The central finding of this study is that even when accounting for various levels of factors, including hospital-level, hospital stay–level, individual (demographics, comorbidities), and neighborhood characteristics (disadvantage), the observed disparity diminished but persisted, suggesting that while these various levels of factors contributed to the observed disparity, other unmeasured factors also contributed. Another key finding is that the effect of the various factors examined in this study may affect different subgroups in different ways, suggesting underlying factors, and thus potential solutions to reduce disparities in care outcomes, could differ among subgroups.
Applications for Clinical Practice and System Implementation
These 2 studies add to the literature on factors that can affect 30-day hospital readmission rates in patients with dementia. These data could allow for more robust discussions of what to anticipate when adults with dementia undergo specific procedures, and also further build the case that improvements in care, such as delirium prevention programs, could offer benefits. The observation about racial and ethnic disparities in care outcomes among patients with dementia highlights the continued need to better understand the drivers of these disparities so that hospital systems and policy makers can consider and test possible solutions. Future studies should further disentangle the relationships among the various levels of factors and observed disparities in outcomes, especially for this vulnerable population of adults living with dementia.
Practice Points
- Clinicians should be aware of the additional risks for poor outcomes that dementia confers.
- Awareness of this increased risk will inform discussions of risks and benefits for older adults considered for procedures.
–William W. Hung, MD, MPH
1. Park DY, Sana MK, Shoura S, et al. Readmission and in-hospital outcomes after transcatheter aortic valve replacement in patients with dementia. Cardiovasc Revasc Med. 2023;46:70-77. doi:10.1016/j.carrev.2022.08.016
2. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598. doi:10.1034/j.1600-0579.2003.00201.x
3. Weldingh NM, Mellingsæter MR, Hegna BW, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266. doi:10.1186/s12877-022-02949-0
4. Hermosura AH, Noonan CJ, Fyfe-Johnson AL, et al. Hospital disparities between native Hawaiian and other pacific islanders and non-Hispanic whites with Alzheimer’s disease and related dementias. J Aging Health. 2020;32(10):1579-1590. doi:10.1177/0898264320945177
5. Zhang Y, Shao H, Zhang M, Li J. Healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias. J Gen Intern Med. 2023 Jan 17. doi:10.1007/s11606-023-08031-8
1. Park DY, Sana MK, Shoura S, et al. Readmission and in-hospital outcomes after transcatheter aortic valve replacement in patients with dementia. Cardiovasc Revasc Med. 2023;46:70-77. doi:10.1016/j.carrev.2022.08.016
2. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598. doi:10.1034/j.1600-0579.2003.00201.x
3. Weldingh NM, Mellingsæter MR, Hegna BW, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266. doi:10.1186/s12877-022-02949-0
4. Hermosura AH, Noonan CJ, Fyfe-Johnson AL, et al. Hospital disparities between native Hawaiian and other pacific islanders and non-Hispanic whites with Alzheimer’s disease and related dementias. J Aging Health. 2020;32(10):1579-1590. doi:10.1177/0898264320945177
5. Zhang Y, Shao H, Zhang M, Li J. Healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias. J Gen Intern Med. 2023 Jan 17. doi:10.1007/s11606-023-08031-8
Persistent Wounds Refractory to Broad-Spectrum Antibiotics
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.
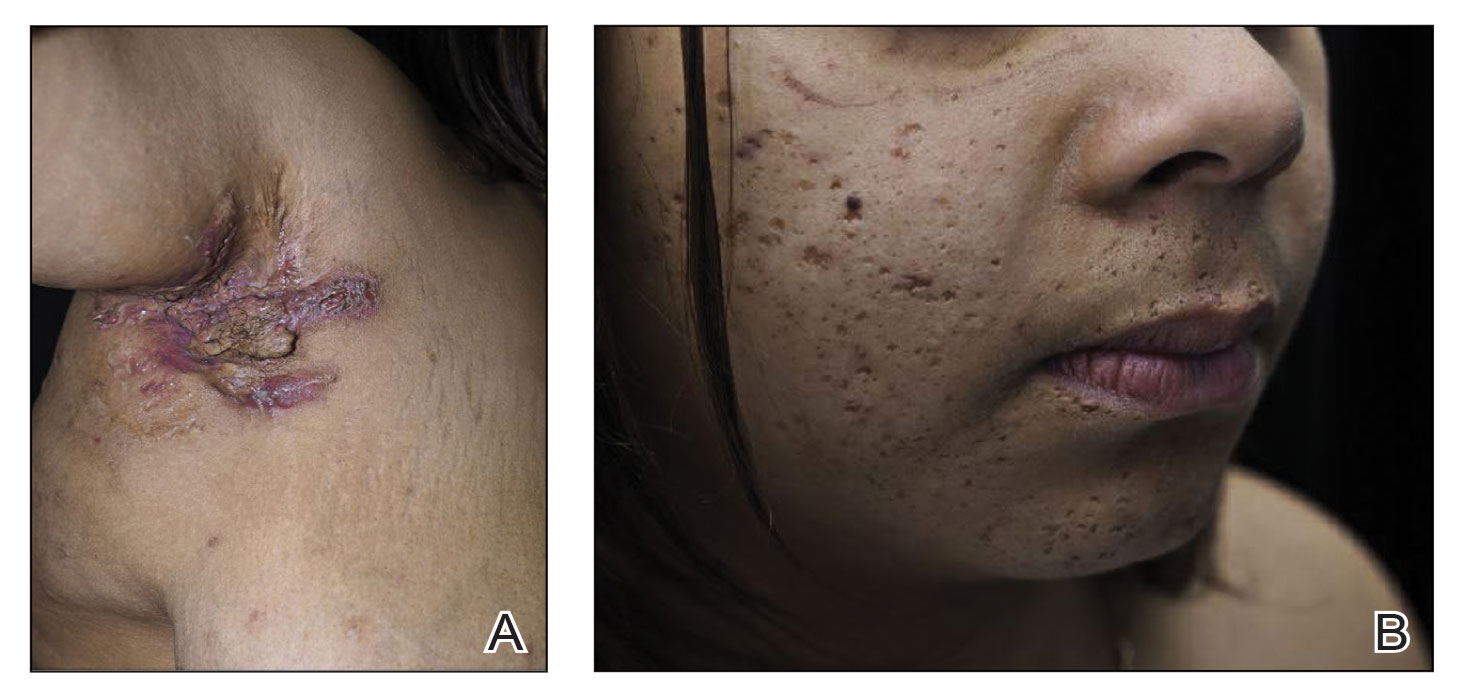
Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.

Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.

Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
A 28-year-old Black woman presented to the hospital for evaluation of worsening leg wounds as well as a similar eroding plaque on the left breast of 1 month’s duration. Broad-spectrum antibiotics prescribed during a prior emergency department visit resulted in no improvement. Her medical history was notable for hidradenitis suppurativa that previously was well controlled on adalimumab prior to discontinuation 1 year prior. A review of systems was negative for fever, chills, shortness of breath, chest pain, night sweats, and arthralgia. The patient had discontinued the antibiotics and was not taking any other medications at the time of presentation. She reported a history of smoking cigarettes (5 pack years). Physical examination revealed hyperkeratotic eroded plaques with violaceous borders circumferentially around the left breast (top) and legs with notable undermining (bottom). Inflammatory nodulocystic acne of the face as well as sinus tract formation with purulent drainage in the axillae also were present. Laboratory workup revealed an elevated erythrocyte sedimentation rate (116 mm/h [reference range, <20 mm/h]). Computed tomography of the leg wound was negative for soft-tissue infection. Aerobic and anaerobic tissue cultures demonstrated no growth.

Number of cancer survivors with functional limitations doubled in 20 years
Vishal Patel, BS, a student at the Dell Medical School at The University of Texas at Austin, and colleagues identified 51,258 cancer survivors from the National Health Interview Survey, representing a weighted population of approximately 178.8 million from 1999 to 2018.
Most survivors were women (60.2%) and were at least 65 years old (55.4%). In 1999, 3.6 million weighted survivors reported functional limitation. In 2018, the number increased to 8.2 million, a 2.25-fold increase.
The number of survivors who reported no limitations also increased, but not by as much. That group grew 1.34-fold during the study period.
For context, “the 70% prevalence of functional limitation among survivors in 2018 is nearly twice that of the general population,” the authors wrote.
Patients surveyed on function
Functional limitation was defined as “self-reported difficulty performing any of 12 routine physical or social activities without assistance.” Examples of the activities included difficulty sitting for more than 2 hours, difficulty participating in social activities or difficulty pushing or pulling an object the size of a living room chair.
Over the 2 decades analyzed, the adjusted prevalence of functional limitation was highest among survivors of pancreatic cancer (80.3%) and lung cancer (76.5%). Prevalence was lowest for survivors of melanoma (62.2%), breast (61.8%) and prostate (59.5%) cancers.
Not just a result of living longer
Mr. Patel told this publication that one assumption people might make when they read these results is that people are just living longer with cancer and losing functional ability accordingly.
“But, in fact, we found that the youngest [– those less than 65 years–] actually contributed to this trend more than the oldest people, which means it’s not just [happening], because people are getting older,” he said.
Hispanic and Black individuals had disproportionately higher increases in functional limitation; percentage point increases over the 2 decades were 19.5 for Black people, 25.1 for Hispanic people and 12.5 for White people. There may be a couple of reasons for that, Mr. Patel noted.
Those who are Black or Hispanic tend to have less access to cancer survivorship care for reasons including insurance status and historic health care inequities, he noted.
“The other potential reason is that they have had less access to cancer care historically. And if, 20 years ago Black and Hispanic individuals didn’t have access to some chemotherapies, and now they do, maybe it’s the increased access to care that’s causing these functional limitations. Because chemotherapy can sometimes be very toxic. It may be sort of a catch-up toxicity,” he said.
Quality of life beyond survivorship
Mr. Patel said the results seem to call for building on improved survival rates by tracking and improving function.
“It’s good to celebrate that there are more survivors. But now that we can keep people alive longer, maybe we can shift gears to improving their quality of life,” he said.
The more-than-doubling of functional limitations over 2 decades “is a very sobering trend,” he noted, while pointing out that the functional limitations applied to 8 million people in the United States – people whose needs are not being met.
There’s no sign of the trend stopping, he continued. “We saw no downward trend, only an upward trend.”
Increasingly, including functionality as an endpoint in cancer trials, in addition to improvements in mortality, is one place to start, he added.
“Our findings suggest an urgent need for care teams to understand and address function, for researchers to evaluate function as a core outcome in trials, and for health systems and policy makers to reimagine survivorship care, recognizing the burden of cancer and its treatment on physical, psychosocial, and cognitive function,” the authors wrote in their paper. Limitations of the study include the potential for recall bias, lack of cancer staging or treatment information, and the subjective perception of function.
A coauthor reported personal fees from Astellas, AstraZeneca, AAA, Blue Earth, Janssen, Lantheus, Myovant, Myriad Genetics, Novartis, Telix, and Sanofi, as well as grants from Pfizer and Bayer during the conduct of the study. No other disclosures were reported.
Vishal Patel, BS, a student at the Dell Medical School at The University of Texas at Austin, and colleagues identified 51,258 cancer survivors from the National Health Interview Survey, representing a weighted population of approximately 178.8 million from 1999 to 2018.
Most survivors were women (60.2%) and were at least 65 years old (55.4%). In 1999, 3.6 million weighted survivors reported functional limitation. In 2018, the number increased to 8.2 million, a 2.25-fold increase.
The number of survivors who reported no limitations also increased, but not by as much. That group grew 1.34-fold during the study period.
For context, “the 70% prevalence of functional limitation among survivors in 2018 is nearly twice that of the general population,” the authors wrote.
Patients surveyed on function
Functional limitation was defined as “self-reported difficulty performing any of 12 routine physical or social activities without assistance.” Examples of the activities included difficulty sitting for more than 2 hours, difficulty participating in social activities or difficulty pushing or pulling an object the size of a living room chair.
Over the 2 decades analyzed, the adjusted prevalence of functional limitation was highest among survivors of pancreatic cancer (80.3%) and lung cancer (76.5%). Prevalence was lowest for survivors of melanoma (62.2%), breast (61.8%) and prostate (59.5%) cancers.
Not just a result of living longer
Mr. Patel told this publication that one assumption people might make when they read these results is that people are just living longer with cancer and losing functional ability accordingly.
“But, in fact, we found that the youngest [– those less than 65 years–] actually contributed to this trend more than the oldest people, which means it’s not just [happening], because people are getting older,” he said.
Hispanic and Black individuals had disproportionately higher increases in functional limitation; percentage point increases over the 2 decades were 19.5 for Black people, 25.1 for Hispanic people and 12.5 for White people. There may be a couple of reasons for that, Mr. Patel noted.
Those who are Black or Hispanic tend to have less access to cancer survivorship care for reasons including insurance status and historic health care inequities, he noted.
“The other potential reason is that they have had less access to cancer care historically. And if, 20 years ago Black and Hispanic individuals didn’t have access to some chemotherapies, and now they do, maybe it’s the increased access to care that’s causing these functional limitations. Because chemotherapy can sometimes be very toxic. It may be sort of a catch-up toxicity,” he said.
Quality of life beyond survivorship
Mr. Patel said the results seem to call for building on improved survival rates by tracking and improving function.
“It’s good to celebrate that there are more survivors. But now that we can keep people alive longer, maybe we can shift gears to improving their quality of life,” he said.
The more-than-doubling of functional limitations over 2 decades “is a very sobering trend,” he noted, while pointing out that the functional limitations applied to 8 million people in the United States – people whose needs are not being met.
There’s no sign of the trend stopping, he continued. “We saw no downward trend, only an upward trend.”
Increasingly, including functionality as an endpoint in cancer trials, in addition to improvements in mortality, is one place to start, he added.
“Our findings suggest an urgent need for care teams to understand and address function, for researchers to evaluate function as a core outcome in trials, and for health systems and policy makers to reimagine survivorship care, recognizing the burden of cancer and its treatment on physical, psychosocial, and cognitive function,” the authors wrote in their paper. Limitations of the study include the potential for recall bias, lack of cancer staging or treatment information, and the subjective perception of function.
A coauthor reported personal fees from Astellas, AstraZeneca, AAA, Blue Earth, Janssen, Lantheus, Myovant, Myriad Genetics, Novartis, Telix, and Sanofi, as well as grants from Pfizer and Bayer during the conduct of the study. No other disclosures were reported.
Vishal Patel, BS, a student at the Dell Medical School at The University of Texas at Austin, and colleagues identified 51,258 cancer survivors from the National Health Interview Survey, representing a weighted population of approximately 178.8 million from 1999 to 2018.
Most survivors were women (60.2%) and were at least 65 years old (55.4%). In 1999, 3.6 million weighted survivors reported functional limitation. In 2018, the number increased to 8.2 million, a 2.25-fold increase.
The number of survivors who reported no limitations also increased, but not by as much. That group grew 1.34-fold during the study period.
For context, “the 70% prevalence of functional limitation among survivors in 2018 is nearly twice that of the general population,” the authors wrote.
Patients surveyed on function
Functional limitation was defined as “self-reported difficulty performing any of 12 routine physical or social activities without assistance.” Examples of the activities included difficulty sitting for more than 2 hours, difficulty participating in social activities or difficulty pushing or pulling an object the size of a living room chair.
Over the 2 decades analyzed, the adjusted prevalence of functional limitation was highest among survivors of pancreatic cancer (80.3%) and lung cancer (76.5%). Prevalence was lowest for survivors of melanoma (62.2%), breast (61.8%) and prostate (59.5%) cancers.
Not just a result of living longer
Mr. Patel told this publication that one assumption people might make when they read these results is that people are just living longer with cancer and losing functional ability accordingly.
“But, in fact, we found that the youngest [– those less than 65 years–] actually contributed to this trend more than the oldest people, which means it’s not just [happening], because people are getting older,” he said.
Hispanic and Black individuals had disproportionately higher increases in functional limitation; percentage point increases over the 2 decades were 19.5 for Black people, 25.1 for Hispanic people and 12.5 for White people. There may be a couple of reasons for that, Mr. Patel noted.
Those who are Black or Hispanic tend to have less access to cancer survivorship care for reasons including insurance status and historic health care inequities, he noted.
“The other potential reason is that they have had less access to cancer care historically. And if, 20 years ago Black and Hispanic individuals didn’t have access to some chemotherapies, and now they do, maybe it’s the increased access to care that’s causing these functional limitations. Because chemotherapy can sometimes be very toxic. It may be sort of a catch-up toxicity,” he said.
Quality of life beyond survivorship
Mr. Patel said the results seem to call for building on improved survival rates by tracking and improving function.
“It’s good to celebrate that there are more survivors. But now that we can keep people alive longer, maybe we can shift gears to improving their quality of life,” he said.
The more-than-doubling of functional limitations over 2 decades “is a very sobering trend,” he noted, while pointing out that the functional limitations applied to 8 million people in the United States – people whose needs are not being met.
There’s no sign of the trend stopping, he continued. “We saw no downward trend, only an upward trend.”
Increasingly, including functionality as an endpoint in cancer trials, in addition to improvements in mortality, is one place to start, he added.
“Our findings suggest an urgent need for care teams to understand and address function, for researchers to evaluate function as a core outcome in trials, and for health systems and policy makers to reimagine survivorship care, recognizing the burden of cancer and its treatment on physical, psychosocial, and cognitive function,” the authors wrote in their paper. Limitations of the study include the potential for recall bias, lack of cancer staging or treatment information, and the subjective perception of function.
A coauthor reported personal fees from Astellas, AstraZeneca, AAA, Blue Earth, Janssen, Lantheus, Myovant, Myriad Genetics, Novartis, Telix, and Sanofi, as well as grants from Pfizer and Bayer during the conduct of the study. No other disclosures were reported.
FROM JAMA ONCOLOGY
Diversity – We’re not one size fits all
The United States has often been described as a “melting pot,” defined as diverse cultures and ethnicities coming together to form the rich fabric of our nation. These days, it seems that our fabric is a bit frayed.
DEIB (diversity, equity, inclusion, and belonging) is dawning as a significant conversation. Each and every one of us is unique by age, gender, culture/ethnicity, religion, socioeconomic status, geographical location, race, and sexual identity – to name just a few aspects of our identity. Keeping these differences in mind, it is evident that none of us fits a “one size fits all” mold.
Some of these differences, such as cross-cultural cuisine and holidays, are enjoyed and celebrated as wonderful opportunities to learn from others, embrace our distinctions, and have them beneficially contribute to our lives. Other differences, however, are not understood or embraced and are, in fact, belittled and stigmatized. Sexual identity falls into this category. It behooves us as a country to become more aware and educated about this category in our identities, in order to understand it, quell our unfounded fear, learn to support one another, and improve our collective mental health.
Recent reports have shown that exposing students and teachers to sexual identity diversity education has sparked some backlash from parents and communities alike. Those opposed are citing concerns over introducing children to LGBTQ+ information, either embedded in the school curriculum or made available in school library reading materials. “Children should remain innocent” seems to be the message. Perhaps parents prefer to discuss this topic privately, at home. Either way, teaching about diversity does not damage one’s innocence or deprive parents of private conversations. In fact, it educates children by improving their awareness, tolerance, and acceptance of others’ differences, and can serve as a catalyst to further parental conversation.
There are kids everywhere who are starting to develop and understand their identities. Wouldn’t it be wonderful for them to know that whichever way they identify is okay, that they are not ‘weird’ or ‘different,’ but that in fact we are all different? Wouldn’t it be great for them to be able to explore and discuss their identities and journeys openly, and not have to hide for fear of retribution or bullying?
It is important for these children to know that they are not alone, that they have options, and that they don’t need to contemplate suicide because they believe that their identity makes them not worthy of being in this world.
Starting the conversation early on in life can empower our youth by planting the seed that people are not “one size fits all,” which is the element responsible for our being unique and human. Diversity can be woven into the rich fabric that defines our nation, rather than be a factor that unravels it.
April was National Diversity Awareness Month and we took time to celebrate our country’s cultural melting pot. By embracing our differences, we can show our children and ourselves how to better navigate diversity, which can help us all fit in.
Dr. Jarkon is a psychiatrist and director of the Center for Behavioral Health at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y.
The United States has often been described as a “melting pot,” defined as diverse cultures and ethnicities coming together to form the rich fabric of our nation. These days, it seems that our fabric is a bit frayed.
DEIB (diversity, equity, inclusion, and belonging) is dawning as a significant conversation. Each and every one of us is unique by age, gender, culture/ethnicity, religion, socioeconomic status, geographical location, race, and sexual identity – to name just a few aspects of our identity. Keeping these differences in mind, it is evident that none of us fits a “one size fits all” mold.
Some of these differences, such as cross-cultural cuisine and holidays, are enjoyed and celebrated as wonderful opportunities to learn from others, embrace our distinctions, and have them beneficially contribute to our lives. Other differences, however, are not understood or embraced and are, in fact, belittled and stigmatized. Sexual identity falls into this category. It behooves us as a country to become more aware and educated about this category in our identities, in order to understand it, quell our unfounded fear, learn to support one another, and improve our collective mental health.
Recent reports have shown that exposing students and teachers to sexual identity diversity education has sparked some backlash from parents and communities alike. Those opposed are citing concerns over introducing children to LGBTQ+ information, either embedded in the school curriculum or made available in school library reading materials. “Children should remain innocent” seems to be the message. Perhaps parents prefer to discuss this topic privately, at home. Either way, teaching about diversity does not damage one’s innocence or deprive parents of private conversations. In fact, it educates children by improving their awareness, tolerance, and acceptance of others’ differences, and can serve as a catalyst to further parental conversation.
There are kids everywhere who are starting to develop and understand their identities. Wouldn’t it be wonderful for them to know that whichever way they identify is okay, that they are not ‘weird’ or ‘different,’ but that in fact we are all different? Wouldn’t it be great for them to be able to explore and discuss their identities and journeys openly, and not have to hide for fear of retribution or bullying?
It is important for these children to know that they are not alone, that they have options, and that they don’t need to contemplate suicide because they believe that their identity makes them not worthy of being in this world.
Starting the conversation early on in life can empower our youth by planting the seed that people are not “one size fits all,” which is the element responsible for our being unique and human. Diversity can be woven into the rich fabric that defines our nation, rather than be a factor that unravels it.
April was National Diversity Awareness Month and we took time to celebrate our country’s cultural melting pot. By embracing our differences, we can show our children and ourselves how to better navigate diversity, which can help us all fit in.
Dr. Jarkon is a psychiatrist and director of the Center for Behavioral Health at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y.
The United States has often been described as a “melting pot,” defined as diverse cultures and ethnicities coming together to form the rich fabric of our nation. These days, it seems that our fabric is a bit frayed.
DEIB (diversity, equity, inclusion, and belonging) is dawning as a significant conversation. Each and every one of us is unique by age, gender, culture/ethnicity, religion, socioeconomic status, geographical location, race, and sexual identity – to name just a few aspects of our identity. Keeping these differences in mind, it is evident that none of us fits a “one size fits all” mold.
Some of these differences, such as cross-cultural cuisine and holidays, are enjoyed and celebrated as wonderful opportunities to learn from others, embrace our distinctions, and have them beneficially contribute to our lives. Other differences, however, are not understood or embraced and are, in fact, belittled and stigmatized. Sexual identity falls into this category. It behooves us as a country to become more aware and educated about this category in our identities, in order to understand it, quell our unfounded fear, learn to support one another, and improve our collective mental health.
Recent reports have shown that exposing students and teachers to sexual identity diversity education has sparked some backlash from parents and communities alike. Those opposed are citing concerns over introducing children to LGBTQ+ information, either embedded in the school curriculum or made available in school library reading materials. “Children should remain innocent” seems to be the message. Perhaps parents prefer to discuss this topic privately, at home. Either way, teaching about diversity does not damage one’s innocence or deprive parents of private conversations. In fact, it educates children by improving their awareness, tolerance, and acceptance of others’ differences, and can serve as a catalyst to further parental conversation.
There are kids everywhere who are starting to develop and understand their identities. Wouldn’t it be wonderful for them to know that whichever way they identify is okay, that they are not ‘weird’ or ‘different,’ but that in fact we are all different? Wouldn’t it be great for them to be able to explore and discuss their identities and journeys openly, and not have to hide for fear of retribution or bullying?
It is important for these children to know that they are not alone, that they have options, and that they don’t need to contemplate suicide because they believe that their identity makes them not worthy of being in this world.
Starting the conversation early on in life can empower our youth by planting the seed that people are not “one size fits all,” which is the element responsible for our being unique and human. Diversity can be woven into the rich fabric that defines our nation, rather than be a factor that unravels it.
April was National Diversity Awareness Month and we took time to celebrate our country’s cultural melting pot. By embracing our differences, we can show our children and ourselves how to better navigate diversity, which can help us all fit in.
Dr. Jarkon is a psychiatrist and director of the Center for Behavioral Health at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y.
CLL: Black patients die sooner than Whites
The findings, published in the American Journal of Hematology, hint that the racial disparity has shrunk over time, especially within the first few years of the targeted-therapy era. Still, “Black patients had a shorter median overall survival of 7 years compared to 9 years for White patients,” study coauthor Deborah Stephens, DO, of the University of Utah Huntsman Cancer Institute, said in an interview. “Clearly, more research is needed to tease out the biologic or economic barriers to achieving prolonged survival.”
As the researchers noted, CLL is far more common among White patients (5.1 cases per 100,000) than other races (Black patients: 3.2 cases per 100,000; Hispanic patients: 2.1 cases per 100,000; Asian American patients: 1.1 per 100,000). In total, non-White patients make up just 11%-13% of CLL cases in the United States.
According to Dr. Stephens, “little is known or published” about Black patients with CLL, “and it is still a mystery why fewer patients that are Black develop CLL and why this group would have shorter survival.”
Dr. Stephens and colleagues launched the new study – the largest of its kind to date – to understand disparities between White and Black patients over most of the past 20 years. The researchers especially wanted to analyze trends during the last decade, when targeted therapies revolutionized treatment of the disease.
The study authors analyzed data in the National Cancer Database for 97,804 patients diagnosed from 2004 to 2018 (90.7% White, 7.6% Black, 0.6% Asian, 1.1% other). Of patients who reported ethnicity (n = 93,555), 2.6% were Hispanic.
Black patients were more likely to have begun CLL therapy at diagnosis (35.9%) than were White patients (23.6%), a sign that Black patients had more advanced disease. Black patients also had shorter overall survival (7.0 years, 95% confidence interval [CI], 6.7–7.3 years) vs. White patients (9.1 years, 95% CI, 9.0–9.3 years, P < .001).
“This finding could be due to underlying biologic differences in the pathology of CLL, when comparing patients across racial groups,” Dr. Stephens said. “Additionally, there could be differences in access to care. Notably, there are fewer racial minorities enrolled in clinical trials, and perhaps we are not individualizing therapy for unique biologic factors seen in CLL affecting racial minorities.”
Other factors also could be at play. Black patients were more likely than were White patients to have one or more comorbidities (27.9% vs. 21.3%, P < .001), lack insurance (6.6% vs. 2.1%, P < .001) and live in lower-income neighborhoods (47.7% vs. 13.1%, P < .001).
What explains the gap in outcomes? In an interview, study lead author Victoria Vardell, MD, of the University of Utah, Salt Lake City, noted that researchers often attribute worse medical outcomes in Black patients to economic and social disparities.
“However, when we adjusted for a number of surrogate markers of health care access, including income, comorbidities, and location, among others, this disparity remained. That indicates that this may be a more complex problem in CLL in particular. Certainly, we cannot adjust for all the socioeconomic strain placed on Black Americans, including those with CLL, but there may be molecular features related to ancestry or environmental exposures that also play a role,” Dr. Vardell said.
She added that “the high cost and difficulty obtaining many novel therapies, particularly in the clinical trial setting, places significantly higher burdens on already disadvantaged populations.”
There is some good news in the new report. “Promisingly, our data suggest that the survival disparity between White and Black patients with CLL may be improving, particularly within the last 5 years, though longer follow-up is needed to confirm significance,” the researchers reported.
Alessandra Ferrajoli, MD, of M.D. Anderson Cancer Center, Houston, who has studied racial disparities in CLL, praised the study in an interview. As she noted, it examines an impressively large population.
The explanations for the disparities are still elusive, she said, although it seems clear there are multiple factors at play. “We don’t know if the disease has the same characteristics in African-Americans as in Whites,” Dr. Ferrajoli said. However, she noted, there’s “no indication that the response to treatment is different according to race.”
Moving forward, she said, the study findings “reinforce the fact that we need to pay attention to this population and be quite attentive to their characteristics.”
No study funding was reported. The authors and Dr. Ferrajoli have no disclosures.
The findings, published in the American Journal of Hematology, hint that the racial disparity has shrunk over time, especially within the first few years of the targeted-therapy era. Still, “Black patients had a shorter median overall survival of 7 years compared to 9 years for White patients,” study coauthor Deborah Stephens, DO, of the University of Utah Huntsman Cancer Institute, said in an interview. “Clearly, more research is needed to tease out the biologic or economic barriers to achieving prolonged survival.”
As the researchers noted, CLL is far more common among White patients (5.1 cases per 100,000) than other races (Black patients: 3.2 cases per 100,000; Hispanic patients: 2.1 cases per 100,000; Asian American patients: 1.1 per 100,000). In total, non-White patients make up just 11%-13% of CLL cases in the United States.
According to Dr. Stephens, “little is known or published” about Black patients with CLL, “and it is still a mystery why fewer patients that are Black develop CLL and why this group would have shorter survival.”
Dr. Stephens and colleagues launched the new study – the largest of its kind to date – to understand disparities between White and Black patients over most of the past 20 years. The researchers especially wanted to analyze trends during the last decade, when targeted therapies revolutionized treatment of the disease.
The study authors analyzed data in the National Cancer Database for 97,804 patients diagnosed from 2004 to 2018 (90.7% White, 7.6% Black, 0.6% Asian, 1.1% other). Of patients who reported ethnicity (n = 93,555), 2.6% were Hispanic.
Black patients were more likely to have begun CLL therapy at diagnosis (35.9%) than were White patients (23.6%), a sign that Black patients had more advanced disease. Black patients also had shorter overall survival (7.0 years, 95% confidence interval [CI], 6.7–7.3 years) vs. White patients (9.1 years, 95% CI, 9.0–9.3 years, P < .001).
“This finding could be due to underlying biologic differences in the pathology of CLL, when comparing patients across racial groups,” Dr. Stephens said. “Additionally, there could be differences in access to care. Notably, there are fewer racial minorities enrolled in clinical trials, and perhaps we are not individualizing therapy for unique biologic factors seen in CLL affecting racial minorities.”
Other factors also could be at play. Black patients were more likely than were White patients to have one or more comorbidities (27.9% vs. 21.3%, P < .001), lack insurance (6.6% vs. 2.1%, P < .001) and live in lower-income neighborhoods (47.7% vs. 13.1%, P < .001).
What explains the gap in outcomes? In an interview, study lead author Victoria Vardell, MD, of the University of Utah, Salt Lake City, noted that researchers often attribute worse medical outcomes in Black patients to economic and social disparities.
“However, when we adjusted for a number of surrogate markers of health care access, including income, comorbidities, and location, among others, this disparity remained. That indicates that this may be a more complex problem in CLL in particular. Certainly, we cannot adjust for all the socioeconomic strain placed on Black Americans, including those with CLL, but there may be molecular features related to ancestry or environmental exposures that also play a role,” Dr. Vardell said.
She added that “the high cost and difficulty obtaining many novel therapies, particularly in the clinical trial setting, places significantly higher burdens on already disadvantaged populations.”
There is some good news in the new report. “Promisingly, our data suggest that the survival disparity between White and Black patients with CLL may be improving, particularly within the last 5 years, though longer follow-up is needed to confirm significance,” the researchers reported.
Alessandra Ferrajoli, MD, of M.D. Anderson Cancer Center, Houston, who has studied racial disparities in CLL, praised the study in an interview. As she noted, it examines an impressively large population.
The explanations for the disparities are still elusive, she said, although it seems clear there are multiple factors at play. “We don’t know if the disease has the same characteristics in African-Americans as in Whites,” Dr. Ferrajoli said. However, she noted, there’s “no indication that the response to treatment is different according to race.”
Moving forward, she said, the study findings “reinforce the fact that we need to pay attention to this population and be quite attentive to their characteristics.”
No study funding was reported. The authors and Dr. Ferrajoli have no disclosures.
The findings, published in the American Journal of Hematology, hint that the racial disparity has shrunk over time, especially within the first few years of the targeted-therapy era. Still, “Black patients had a shorter median overall survival of 7 years compared to 9 years for White patients,” study coauthor Deborah Stephens, DO, of the University of Utah Huntsman Cancer Institute, said in an interview. “Clearly, more research is needed to tease out the biologic or economic barriers to achieving prolonged survival.”
As the researchers noted, CLL is far more common among White patients (5.1 cases per 100,000) than other races (Black patients: 3.2 cases per 100,000; Hispanic patients: 2.1 cases per 100,000; Asian American patients: 1.1 per 100,000). In total, non-White patients make up just 11%-13% of CLL cases in the United States.
According to Dr. Stephens, “little is known or published” about Black patients with CLL, “and it is still a mystery why fewer patients that are Black develop CLL and why this group would have shorter survival.”
Dr. Stephens and colleagues launched the new study – the largest of its kind to date – to understand disparities between White and Black patients over most of the past 20 years. The researchers especially wanted to analyze trends during the last decade, when targeted therapies revolutionized treatment of the disease.
The study authors analyzed data in the National Cancer Database for 97,804 patients diagnosed from 2004 to 2018 (90.7% White, 7.6% Black, 0.6% Asian, 1.1% other). Of patients who reported ethnicity (n = 93,555), 2.6% were Hispanic.
Black patients were more likely to have begun CLL therapy at diagnosis (35.9%) than were White patients (23.6%), a sign that Black patients had more advanced disease. Black patients also had shorter overall survival (7.0 years, 95% confidence interval [CI], 6.7–7.3 years) vs. White patients (9.1 years, 95% CI, 9.0–9.3 years, P < .001).
“This finding could be due to underlying biologic differences in the pathology of CLL, when comparing patients across racial groups,” Dr. Stephens said. “Additionally, there could be differences in access to care. Notably, there are fewer racial minorities enrolled in clinical trials, and perhaps we are not individualizing therapy for unique biologic factors seen in CLL affecting racial minorities.”
Other factors also could be at play. Black patients were more likely than were White patients to have one or more comorbidities (27.9% vs. 21.3%, P < .001), lack insurance (6.6% vs. 2.1%, P < .001) and live in lower-income neighborhoods (47.7% vs. 13.1%, P < .001).
What explains the gap in outcomes? In an interview, study lead author Victoria Vardell, MD, of the University of Utah, Salt Lake City, noted that researchers often attribute worse medical outcomes in Black patients to economic and social disparities.
“However, when we adjusted for a number of surrogate markers of health care access, including income, comorbidities, and location, among others, this disparity remained. That indicates that this may be a more complex problem in CLL in particular. Certainly, we cannot adjust for all the socioeconomic strain placed on Black Americans, including those with CLL, but there may be molecular features related to ancestry or environmental exposures that also play a role,” Dr. Vardell said.
She added that “the high cost and difficulty obtaining many novel therapies, particularly in the clinical trial setting, places significantly higher burdens on already disadvantaged populations.”
There is some good news in the new report. “Promisingly, our data suggest that the survival disparity between White and Black patients with CLL may be improving, particularly within the last 5 years, though longer follow-up is needed to confirm significance,” the researchers reported.
Alessandra Ferrajoli, MD, of M.D. Anderson Cancer Center, Houston, who has studied racial disparities in CLL, praised the study in an interview. As she noted, it examines an impressively large population.
The explanations for the disparities are still elusive, she said, although it seems clear there are multiple factors at play. “We don’t know if the disease has the same characteristics in African-Americans as in Whites,” Dr. Ferrajoli said. However, she noted, there’s “no indication that the response to treatment is different according to race.”
Moving forward, she said, the study findings “reinforce the fact that we need to pay attention to this population and be quite attentive to their characteristics.”
No study funding was reported. The authors and Dr. Ferrajoli have no disclosures.
FROM AMERICAN JOURNAL OF HEMATOLOGY
Disparities in Melanoma Demographics, Tumor Stage, and Metastases in Hispanic and Latino Patients: A Retrospective Study
To the Editor:
Melanoma is an aggressive form of skin cancer with a high rate of metastasis and poor prognosis.1 Historically, Hispanic and/or Latino patients have presented with more advanced-stage melanomas and have lower survival rates compared with non-Hispanic and/or non-Latino White patients.2 In this study, we evaluated recent data from the last decade to investigate if disparities in melanoma tumor stage at diagnosis and risk for metastases continue to exist in the Hispanic and/or Latino population.
We conducted a retrospective review of melanoma patients at 2 major medical centers in Los Angeles, California—Keck Medicine of USC and Los Angeles County-USC Medical Center—from January 2010 to January 2020. The data collected from electronic medical records included age at melanoma diagnosis, sex, race and ethnicity, insurance type, Breslow depth of lesion, presence of ulceration, and presence of lymph node or distant metastases. Melanoma tumor stage was determined using the American Joint Committee on Cancer classification. Patients who self-reported their ethnicity as not Hispanic and/or Latino were designated to this group regardless of their reported race. Those patients who reported their ethnicity as not Hispanic and/or Latino and reported their race as White were designated as non-Hispanic and/or non-Latino White. This study was approved by the institutional review board of the University of Southern California (Los Angeles). Data analysis was performed using the Pearson χ2 test, Fisher exact test, and Wilcoxon rank sum test. Statistical significance was determined at P<.05.
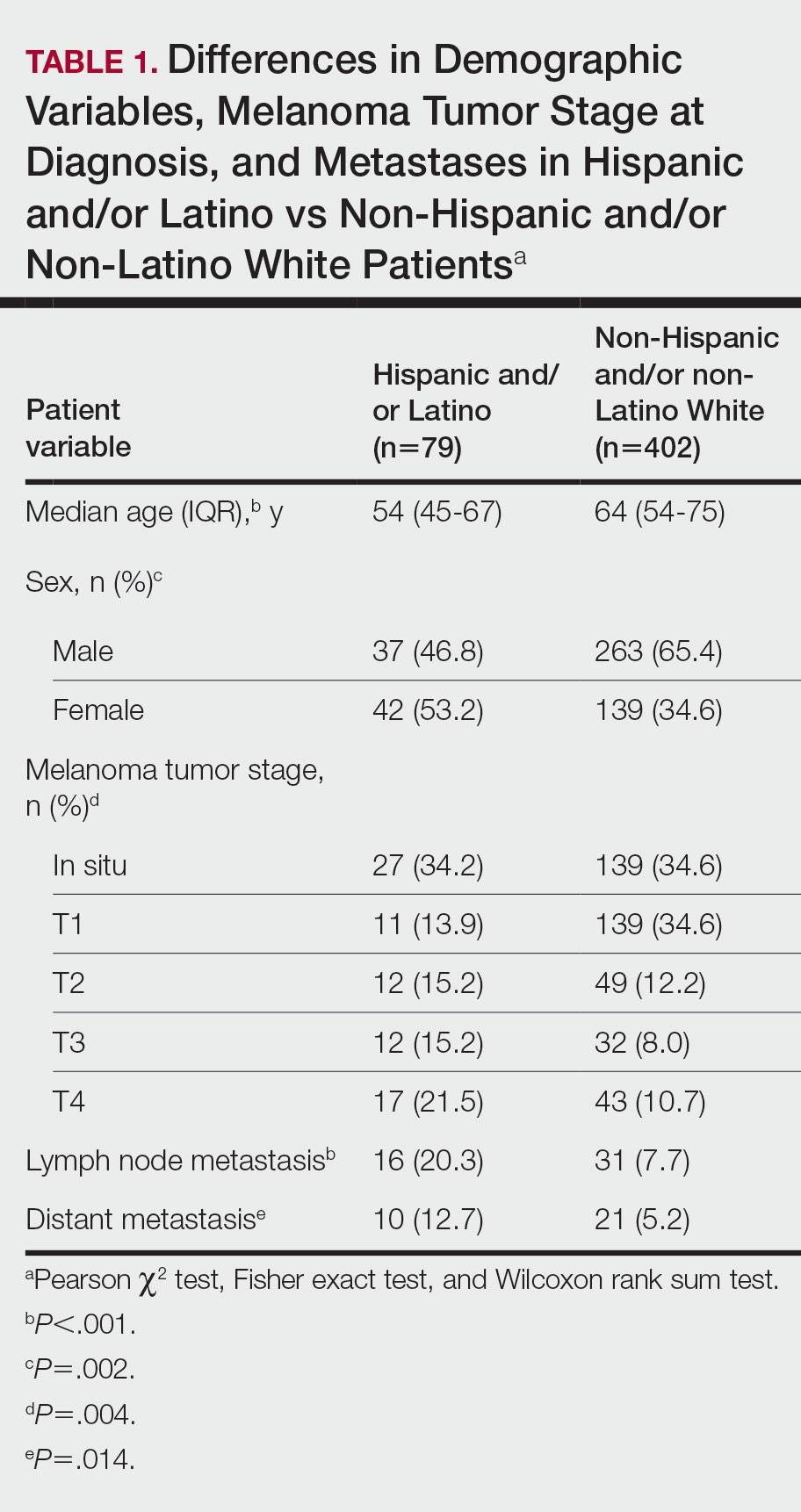
The final cohort of patients included 79 Hispanic and/or Latino patients and 402 non-Hispanic and/or non-Latino White patients. The median age for the Hispanic and/or Latino group was 54 years and 64 years for the non-Hispanic and/or non-Latino White group (P<.001). There was a greater percentage of females in the Hispanic and/or Latino group compared with the non-Hispanic and/or non-Latino White group (53.2% vs 34.6%)(P=.002). Hispanic and/or Latino patients presented with more advanced tumor stage melanomas (T3: 15.2%; T4: 21.5%) compared with non-Hispanic and/or non-Latino White patients (T3: 8.0%; T4: 10.7%)(P=.004). Furthermore, Hispanic and/or Latino patients had higher rates of lymph node metastases compared with non-Hispanic and/or non-Latino White patients (20.3% vs 7.7% [P<.001]) and higher rates of distant metastases (12.7% vs 5.2% [P=.014])(Table 1). The majority of Hispanic and/or Latino patients had Medicaid (39.2%), while most non-Hispanic and/or non-Latino White patients had a preferred provider organization insurance plan (37.3%) or Medicare (34.3%)(P<.001)(Table 2).
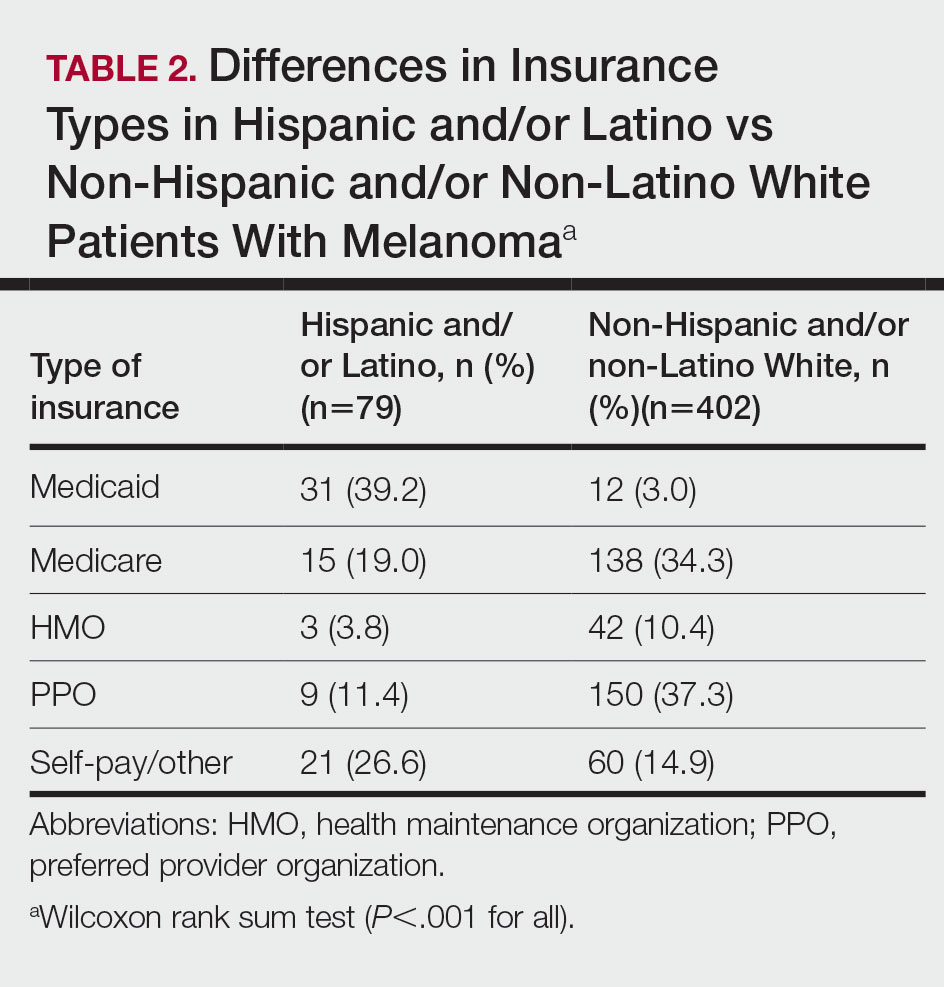
This retrospective study analyzing nearly 10 years of recent melanoma data found that disparities in melanoma diagnosis and treatment continue to exist among Hispanic and/or Latino patients. Compared to non-Hispanic and/or non-Latino White patients, Hispanic and/or Latino patients were diagnosed with melanoma at a younger age and the proportion of females with melanoma was higher. Cormier et al2 also reported that Hispanic patients were younger at melanoma diagnosis, and females represented a larger majority of patients in the Hispanic population compared with the White population. Hispanic and/or Latino patients in our study had more advanced melanoma tumor stage at diagnosis and a higher risk of lymph node and distant metastases, similar to findings reported by Koblinksi et al.3
Our retrospective cohort study demonstrated that the demographics of Hispanic and/or Latino patients with melanoma differ from non-Hispanic and/or non-Latino White patients, specifically with a greater proportion of younger and female patients in the Hispanic and/or Latino population. We also found that Hispanic and/or Latino patients continue to experience worse melanoma outcomes compared with non-Hispanic and/or non-Latino White patients. Further studies are needed to investigate the etiologies behind these health care disparities and potential interventions to address them. In addition, there needs to be increased awareness of the risk for melanoma in Hispanic and/or Latino patients among both health care providers and patients.
Limitations of this study included a smaller sample size of patients from one geographic region. The retrospective design of this study also increased the risk for selection bias, as some of the patients may have had incomplete records or were lost to follow-up. Therefore, the study cohort may not be representative of the general population. Additionally, patients’ skin types could not be determined using standardized tools such as the Fitzpatrick scale, thus we could not assess how patient skin type may have affected melanoma outcomes.
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907. doi:10.1001/archinte.166.17.1907
- Koblinski JE, Maykowski P, Zeitouni NC. Disparities in melanoma stage at diagnosis in Arizona: a 10-year Arizona Cancer Registry study. J Am Acad Dermatol. 2021;84:1776-1779. doi:10.1016/j.jaad.2021.02.045
To the Editor:
Melanoma is an aggressive form of skin cancer with a high rate of metastasis and poor prognosis.1 Historically, Hispanic and/or Latino patients have presented with more advanced-stage melanomas and have lower survival rates compared with non-Hispanic and/or non-Latino White patients.2 In this study, we evaluated recent data from the last decade to investigate if disparities in melanoma tumor stage at diagnosis and risk for metastases continue to exist in the Hispanic and/or Latino population.
We conducted a retrospective review of melanoma patients at 2 major medical centers in Los Angeles, California—Keck Medicine of USC and Los Angeles County-USC Medical Center—from January 2010 to January 2020. The data collected from electronic medical records included age at melanoma diagnosis, sex, race and ethnicity, insurance type, Breslow depth of lesion, presence of ulceration, and presence of lymph node or distant metastases. Melanoma tumor stage was determined using the American Joint Committee on Cancer classification. Patients who self-reported their ethnicity as not Hispanic and/or Latino were designated to this group regardless of their reported race. Those patients who reported their ethnicity as not Hispanic and/or Latino and reported their race as White were designated as non-Hispanic and/or non-Latino White. This study was approved by the institutional review board of the University of Southern California (Los Angeles). Data analysis was performed using the Pearson χ2 test, Fisher exact test, and Wilcoxon rank sum test. Statistical significance was determined at P<.05.

The final cohort of patients included 79 Hispanic and/or Latino patients and 402 non-Hispanic and/or non-Latino White patients. The median age for the Hispanic and/or Latino group was 54 years and 64 years for the non-Hispanic and/or non-Latino White group (P<.001). There was a greater percentage of females in the Hispanic and/or Latino group compared with the non-Hispanic and/or non-Latino White group (53.2% vs 34.6%)(P=.002). Hispanic and/or Latino patients presented with more advanced tumor stage melanomas (T3: 15.2%; T4: 21.5%) compared with non-Hispanic and/or non-Latino White patients (T3: 8.0%; T4: 10.7%)(P=.004). Furthermore, Hispanic and/or Latino patients had higher rates of lymph node metastases compared with non-Hispanic and/or non-Latino White patients (20.3% vs 7.7% [P<.001]) and higher rates of distant metastases (12.7% vs 5.2% [P=.014])(Table 1). The majority of Hispanic and/or Latino patients had Medicaid (39.2%), while most non-Hispanic and/or non-Latino White patients had a preferred provider organization insurance plan (37.3%) or Medicare (34.3%)(P<.001)(Table 2).

This retrospective study analyzing nearly 10 years of recent melanoma data found that disparities in melanoma diagnosis and treatment continue to exist among Hispanic and/or Latino patients. Compared to non-Hispanic and/or non-Latino White patients, Hispanic and/or Latino patients were diagnosed with melanoma at a younger age and the proportion of females with melanoma was higher. Cormier et al2 also reported that Hispanic patients were younger at melanoma diagnosis, and females represented a larger majority of patients in the Hispanic population compared with the White population. Hispanic and/or Latino patients in our study had more advanced melanoma tumor stage at diagnosis and a higher risk of lymph node and distant metastases, similar to findings reported by Koblinksi et al.3
Our retrospective cohort study demonstrated that the demographics of Hispanic and/or Latino patients with melanoma differ from non-Hispanic and/or non-Latino White patients, specifically with a greater proportion of younger and female patients in the Hispanic and/or Latino population. We also found that Hispanic and/or Latino patients continue to experience worse melanoma outcomes compared with non-Hispanic and/or non-Latino White patients. Further studies are needed to investigate the etiologies behind these health care disparities and potential interventions to address them. In addition, there needs to be increased awareness of the risk for melanoma in Hispanic and/or Latino patients among both health care providers and patients.
Limitations of this study included a smaller sample size of patients from one geographic region. The retrospective design of this study also increased the risk for selection bias, as some of the patients may have had incomplete records or were lost to follow-up. Therefore, the study cohort may not be representative of the general population. Additionally, patients’ skin types could not be determined using standardized tools such as the Fitzpatrick scale, thus we could not assess how patient skin type may have affected melanoma outcomes.
To the Editor:
Melanoma is an aggressive form of skin cancer with a high rate of metastasis and poor prognosis.1 Historically, Hispanic and/or Latino patients have presented with more advanced-stage melanomas and have lower survival rates compared with non-Hispanic and/or non-Latino White patients.2 In this study, we evaluated recent data from the last decade to investigate if disparities in melanoma tumor stage at diagnosis and risk for metastases continue to exist in the Hispanic and/or Latino population.
We conducted a retrospective review of melanoma patients at 2 major medical centers in Los Angeles, California—Keck Medicine of USC and Los Angeles County-USC Medical Center—from January 2010 to January 2020. The data collected from electronic medical records included age at melanoma diagnosis, sex, race and ethnicity, insurance type, Breslow depth of lesion, presence of ulceration, and presence of lymph node or distant metastases. Melanoma tumor stage was determined using the American Joint Committee on Cancer classification. Patients who self-reported their ethnicity as not Hispanic and/or Latino were designated to this group regardless of their reported race. Those patients who reported their ethnicity as not Hispanic and/or Latino and reported their race as White were designated as non-Hispanic and/or non-Latino White. This study was approved by the institutional review board of the University of Southern California (Los Angeles). Data analysis was performed using the Pearson χ2 test, Fisher exact test, and Wilcoxon rank sum test. Statistical significance was determined at P<.05.

The final cohort of patients included 79 Hispanic and/or Latino patients and 402 non-Hispanic and/or non-Latino White patients. The median age for the Hispanic and/or Latino group was 54 years and 64 years for the non-Hispanic and/or non-Latino White group (P<.001). There was a greater percentage of females in the Hispanic and/or Latino group compared with the non-Hispanic and/or non-Latino White group (53.2% vs 34.6%)(P=.002). Hispanic and/or Latino patients presented with more advanced tumor stage melanomas (T3: 15.2%; T4: 21.5%) compared with non-Hispanic and/or non-Latino White patients (T3: 8.0%; T4: 10.7%)(P=.004). Furthermore, Hispanic and/or Latino patients had higher rates of lymph node metastases compared with non-Hispanic and/or non-Latino White patients (20.3% vs 7.7% [P<.001]) and higher rates of distant metastases (12.7% vs 5.2% [P=.014])(Table 1). The majority of Hispanic and/or Latino patients had Medicaid (39.2%), while most non-Hispanic and/or non-Latino White patients had a preferred provider organization insurance plan (37.3%) or Medicare (34.3%)(P<.001)(Table 2).

This retrospective study analyzing nearly 10 years of recent melanoma data found that disparities in melanoma diagnosis and treatment continue to exist among Hispanic and/or Latino patients. Compared to non-Hispanic and/or non-Latino White patients, Hispanic and/or Latino patients were diagnosed with melanoma at a younger age and the proportion of females with melanoma was higher. Cormier et al2 also reported that Hispanic patients were younger at melanoma diagnosis, and females represented a larger majority of patients in the Hispanic population compared with the White population. Hispanic and/or Latino patients in our study had more advanced melanoma tumor stage at diagnosis and a higher risk of lymph node and distant metastases, similar to findings reported by Koblinksi et al.3
Our retrospective cohort study demonstrated that the demographics of Hispanic and/or Latino patients with melanoma differ from non-Hispanic and/or non-Latino White patients, specifically with a greater proportion of younger and female patients in the Hispanic and/or Latino population. We also found that Hispanic and/or Latino patients continue to experience worse melanoma outcomes compared with non-Hispanic and/or non-Latino White patients. Further studies are needed to investigate the etiologies behind these health care disparities and potential interventions to address them. In addition, there needs to be increased awareness of the risk for melanoma in Hispanic and/or Latino patients among both health care providers and patients.
Limitations of this study included a smaller sample size of patients from one geographic region. The retrospective design of this study also increased the risk for selection bias, as some of the patients may have had incomplete records or were lost to follow-up. Therefore, the study cohort may not be representative of the general population. Additionally, patients’ skin types could not be determined using standardized tools such as the Fitzpatrick scale, thus we could not assess how patient skin type may have affected melanoma outcomes.
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907. doi:10.1001/archinte.166.17.1907
- Koblinski JE, Maykowski P, Zeitouni NC. Disparities in melanoma stage at diagnosis in Arizona: a 10-year Arizona Cancer Registry study. J Am Acad Dermatol. 2021;84:1776-1779. doi:10.1016/j.jaad.2021.02.045
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85:388-395. doi:10.1016/j.jaad.2021.03.109
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907. doi:10.1001/archinte.166.17.1907
- Koblinski JE, Maykowski P, Zeitouni NC. Disparities in melanoma stage at diagnosis in Arizona: a 10-year Arizona Cancer Registry study. J Am Acad Dermatol. 2021;84:1776-1779. doi:10.1016/j.jaad.2021.02.045
Practice Points
- Hispanic and/or Latino patients often present with more advanced-stage melanomas and have decreased survival rates compared with non-Hispanic and/or non-Latino White patients.
- More education and awareness on the risk for melanoma as well as sun-protective behaviors in the Hispanic and/or Latino population is needed among both health care providers and patients to prevent diagnosis of melanoma in later stages and improve outcomes.


