User login
Tips, contraindications for superficial chemical peels reviewed
CHICAGO – Heather Woolery-Lloyd, MD, says she’s generally “risk averse,” but when it comes to superficial chemical peels, she’s in her comfort zone.
Superficial peeling is “one of the most common cosmetic procedures that I do,” Dr. Woolery-Lloyd, director of the skin of color division in the dermatology department at the University of Miami, said at the Pigmentary Disorders Exchange Symposium.
In her practice, .
Contraindications are an active bacterial infection, open wounds, and active herpes simplex virus. “If someone looks like they even have a remnant of a cold sore, I tell them to come back,” she said.
Setting expectations for patients is critical, Dr. Woolery-Lloyd said, as a series of superficial peels is needed before the desired results are evident.
The peel she uses most is salicylic acid, a beta-hydroxy acid, at a strength of 20%-30%. “It’s very effective on our acne patients,” she said at the meeting, provided by MedscapeLIVE! “If you’re just starting with peels, I think this is a very safe one. You don’t have to time it, and you don’t have to neutralize it,” and at lower concentrations, is “very safe.”
Dr. Woolery-Lloyd provided these other tips during her presentation:
- Even superficial peels can be uncomfortable, she noted, so she keeps a fan nearby to use when needed to help with discomfort.
- Find the peel you’re comfortable with, master that peel, and don’t jump from peel to peel. Get familiar with the side effects and how to predict results.
- Stop retinoids up to 7 days before a peel. Consider placing the patient on hydroquinone before the chemical peel to decrease the risk of hyperpigmentation.
- Before the procedure, prep the skin with acetone or alcohol. Applying petrolatum helps protect around the eyes, alar crease, and other sensitive areas, “or anywhere you’re concerned about the depth of the peel.”
- Application with rough gauze helps avoid the waste that comes with makeup sponges soaking up the product. It also helps add exfoliation.
- Have everything ready before starting the procedure, including (depending on the peel), a neutralizer or soapless cleanser. Although peels are generally safe, you want to be able to remove one quickly, if needed, without having to leave the room.
- Start with the lowest concentration (salicylic acid or glycolic acid) then titrate up. Ask patients about any reactions they experienced with the previous peel before making the decision on the next concentration.
- For a peel to treat hyperpigmentation, she recommends one peel about every 4 weeks for a series of 5-6 peels.
- After a peel, the patient should use a mineral sunscreen; chemical sunscreens will sting.
Know your comfort zone
Conference chair Pearl Grimes, MD, director of The Vitiligo & Pigmentation Institute of Southern California in Los Angeles, said superficial peels are best for dermatologists new to peeling until they gain comfort with experience.
Superficial and medium-depth peels work well for mild to moderate photoaging, she said at the meeting.
“We know that in darker skin we have more intrinsic aging rather than photoaging. We have more textural changes, hyperpigmentation,” Dr. Grimes said.
For Fitzpatrick skin types I-III, she said, “you can do superficial, medium, and deep peels.” For darker skin types, “I typically stay in the superficial, medium range.”
She said that she uses retinoids to exfoliate before a superficial peel but added, “you’ve got to stop them early because retinoids can make a superficial peel a medium-depth peel.”
Taking photos is important before any procedure, she said, as is spending time with patients clarifying their outcome expectations.
“I love peeling,” Dr. Grimes said. “And it’s cost effective. If you don’t want to spend a ton of money, it’s amazing what you can achieve with chemical peeling.”
When asked by a member of the audience whether they avoid superficial peels in women who are pregnant or breastfeeding, both Dr. Woolery-Lloyd and Dr. Grimes said they do avoid them in those patients.
Dr. Grimes said she tells her patients, especially in the first trimester, “I am the most conservative woman on the planet. I do nothing during the first trimester.”
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, Loreal and EPI, and has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion. She has been on advisory boards for Loreal, Allergan, Ortho Dermatologics, Pfize,r and Merz. Dr. Grimes reports grant/research Support from Clinuvel Pharmaceuticals, Incyte, Johnson & Johnson, LASEROPTEK, L’Oréal USA, Pfizer, Procter & Gamble, skinbetter science, and Versicolor Technologies, and is on the speakers bureau/receives honoraria for non-CME for Incyte and Procter & Gamble; and is a consultant or is on the advisory board for L’Oréal USA and Procter & Gamble. She has stock options in Versicolor Technologies.
CHICAGO – Heather Woolery-Lloyd, MD, says she’s generally “risk averse,” but when it comes to superficial chemical peels, she’s in her comfort zone.
Superficial peeling is “one of the most common cosmetic procedures that I do,” Dr. Woolery-Lloyd, director of the skin of color division in the dermatology department at the University of Miami, said at the Pigmentary Disorders Exchange Symposium.
In her practice, .
Contraindications are an active bacterial infection, open wounds, and active herpes simplex virus. “If someone looks like they even have a remnant of a cold sore, I tell them to come back,” she said.
Setting expectations for patients is critical, Dr. Woolery-Lloyd said, as a series of superficial peels is needed before the desired results are evident.
The peel she uses most is salicylic acid, a beta-hydroxy acid, at a strength of 20%-30%. “It’s very effective on our acne patients,” she said at the meeting, provided by MedscapeLIVE! “If you’re just starting with peels, I think this is a very safe one. You don’t have to time it, and you don’t have to neutralize it,” and at lower concentrations, is “very safe.”
Dr. Woolery-Lloyd provided these other tips during her presentation:
- Even superficial peels can be uncomfortable, she noted, so she keeps a fan nearby to use when needed to help with discomfort.
- Find the peel you’re comfortable with, master that peel, and don’t jump from peel to peel. Get familiar with the side effects and how to predict results.
- Stop retinoids up to 7 days before a peel. Consider placing the patient on hydroquinone before the chemical peel to decrease the risk of hyperpigmentation.
- Before the procedure, prep the skin with acetone or alcohol. Applying petrolatum helps protect around the eyes, alar crease, and other sensitive areas, “or anywhere you’re concerned about the depth of the peel.”
- Application with rough gauze helps avoid the waste that comes with makeup sponges soaking up the product. It also helps add exfoliation.
- Have everything ready before starting the procedure, including (depending on the peel), a neutralizer or soapless cleanser. Although peels are generally safe, you want to be able to remove one quickly, if needed, without having to leave the room.
- Start with the lowest concentration (salicylic acid or glycolic acid) then titrate up. Ask patients about any reactions they experienced with the previous peel before making the decision on the next concentration.
- For a peel to treat hyperpigmentation, she recommends one peel about every 4 weeks for a series of 5-6 peels.
- After a peel, the patient should use a mineral sunscreen; chemical sunscreens will sting.
Know your comfort zone
Conference chair Pearl Grimes, MD, director of The Vitiligo & Pigmentation Institute of Southern California in Los Angeles, said superficial peels are best for dermatologists new to peeling until they gain comfort with experience.
Superficial and medium-depth peels work well for mild to moderate photoaging, she said at the meeting.
“We know that in darker skin we have more intrinsic aging rather than photoaging. We have more textural changes, hyperpigmentation,” Dr. Grimes said.
For Fitzpatrick skin types I-III, she said, “you can do superficial, medium, and deep peels.” For darker skin types, “I typically stay in the superficial, medium range.”
She said that she uses retinoids to exfoliate before a superficial peel but added, “you’ve got to stop them early because retinoids can make a superficial peel a medium-depth peel.”
Taking photos is important before any procedure, she said, as is spending time with patients clarifying their outcome expectations.
“I love peeling,” Dr. Grimes said. “And it’s cost effective. If you don’t want to spend a ton of money, it’s amazing what you can achieve with chemical peeling.”
When asked by a member of the audience whether they avoid superficial peels in women who are pregnant or breastfeeding, both Dr. Woolery-Lloyd and Dr. Grimes said they do avoid them in those patients.
Dr. Grimes said she tells her patients, especially in the first trimester, “I am the most conservative woman on the planet. I do nothing during the first trimester.”
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, Loreal and EPI, and has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion. She has been on advisory boards for Loreal, Allergan, Ortho Dermatologics, Pfize,r and Merz. Dr. Grimes reports grant/research Support from Clinuvel Pharmaceuticals, Incyte, Johnson & Johnson, LASEROPTEK, L’Oréal USA, Pfizer, Procter & Gamble, skinbetter science, and Versicolor Technologies, and is on the speakers bureau/receives honoraria for non-CME for Incyte and Procter & Gamble; and is a consultant or is on the advisory board for L’Oréal USA and Procter & Gamble. She has stock options in Versicolor Technologies.
CHICAGO – Heather Woolery-Lloyd, MD, says she’s generally “risk averse,” but when it comes to superficial chemical peels, she’s in her comfort zone.
Superficial peeling is “one of the most common cosmetic procedures that I do,” Dr. Woolery-Lloyd, director of the skin of color division in the dermatology department at the University of Miami, said at the Pigmentary Disorders Exchange Symposium.
In her practice, .
Contraindications are an active bacterial infection, open wounds, and active herpes simplex virus. “If someone looks like they even have a remnant of a cold sore, I tell them to come back,” she said.
Setting expectations for patients is critical, Dr. Woolery-Lloyd said, as a series of superficial peels is needed before the desired results are evident.
The peel she uses most is salicylic acid, a beta-hydroxy acid, at a strength of 20%-30%. “It’s very effective on our acne patients,” she said at the meeting, provided by MedscapeLIVE! “If you’re just starting with peels, I think this is a very safe one. You don’t have to time it, and you don’t have to neutralize it,” and at lower concentrations, is “very safe.”
Dr. Woolery-Lloyd provided these other tips during her presentation:
- Even superficial peels can be uncomfortable, she noted, so she keeps a fan nearby to use when needed to help with discomfort.
- Find the peel you’re comfortable with, master that peel, and don’t jump from peel to peel. Get familiar with the side effects and how to predict results.
- Stop retinoids up to 7 days before a peel. Consider placing the patient on hydroquinone before the chemical peel to decrease the risk of hyperpigmentation.
- Before the procedure, prep the skin with acetone or alcohol. Applying petrolatum helps protect around the eyes, alar crease, and other sensitive areas, “or anywhere you’re concerned about the depth of the peel.”
- Application with rough gauze helps avoid the waste that comes with makeup sponges soaking up the product. It also helps add exfoliation.
- Have everything ready before starting the procedure, including (depending on the peel), a neutralizer or soapless cleanser. Although peels are generally safe, you want to be able to remove one quickly, if needed, without having to leave the room.
- Start with the lowest concentration (salicylic acid or glycolic acid) then titrate up. Ask patients about any reactions they experienced with the previous peel before making the decision on the next concentration.
- For a peel to treat hyperpigmentation, she recommends one peel about every 4 weeks for a series of 5-6 peels.
- After a peel, the patient should use a mineral sunscreen; chemical sunscreens will sting.
Know your comfort zone
Conference chair Pearl Grimes, MD, director of The Vitiligo & Pigmentation Institute of Southern California in Los Angeles, said superficial peels are best for dermatologists new to peeling until they gain comfort with experience.
Superficial and medium-depth peels work well for mild to moderate photoaging, she said at the meeting.
“We know that in darker skin we have more intrinsic aging rather than photoaging. We have more textural changes, hyperpigmentation,” Dr. Grimes said.
For Fitzpatrick skin types I-III, she said, “you can do superficial, medium, and deep peels.” For darker skin types, “I typically stay in the superficial, medium range.”
She said that she uses retinoids to exfoliate before a superficial peel but added, “you’ve got to stop them early because retinoids can make a superficial peel a medium-depth peel.”
Taking photos is important before any procedure, she said, as is spending time with patients clarifying their outcome expectations.
“I love peeling,” Dr. Grimes said. “And it’s cost effective. If you don’t want to spend a ton of money, it’s amazing what you can achieve with chemical peeling.”
When asked by a member of the audience whether they avoid superficial peels in women who are pregnant or breastfeeding, both Dr. Woolery-Lloyd and Dr. Grimes said they do avoid them in those patients.
Dr. Grimes said she tells her patients, especially in the first trimester, “I am the most conservative woman on the planet. I do nothing during the first trimester.”
Dr. Woolery-Lloyd has been a speaker for Ortho Dermatologics, Loreal and EPI, and has done research for Pfizer, Galderma, Allergan, Arcutis, Vyne, Merz, and Eirion. She has been on advisory boards for Loreal, Allergan, Ortho Dermatologics, Pfize,r and Merz. Dr. Grimes reports grant/research Support from Clinuvel Pharmaceuticals, Incyte, Johnson & Johnson, LASEROPTEK, L’Oréal USA, Pfizer, Procter & Gamble, skinbetter science, and Versicolor Technologies, and is on the speakers bureau/receives honoraria for non-CME for Incyte and Procter & Gamble; and is a consultant or is on the advisory board for L’Oréal USA and Procter & Gamble. She has stock options in Versicolor Technologies.
AT THE MEDSCAPE LIVE! PIGMENTARY DISORDERS SYMPOSIUM
Medicaid patients with heart failure get poor follow-up after hospital discharge
Nearly 60% of Medicaid-covered adults with concurrent diabetes and heart failure did not receive guideline-concordant postdischarge care within 7-10 days of leaving the hospital, according to a large Alabama study. Moreover, affected Black and Hispanic/other Alabamians were less likely than were their White counterparts to receive recommended postdischarge care.
In comparison with White participants, Black and Hispanic adults were less likely to have any postdischarge ambulatory care visits after HF hospitalization or had a delayed visit, according to researchers led by Yulia Khodneva, MD, PhD, an internist at the University of Alabama at Birmingham. “This is likely a reflection of a structural racism and implicit bias against racial and ethnic minorities that persists in the U.S. health care system,” she and her colleagues wrote.
The findings point to the need for strategies to improve access to postdischarge care for lower-income HF patients.
Among U.S. states, Alabama is the sixth-poorest, the third in diabetes prevalence (14%), and has the highest rates of heart failure hospitalizations and cardiovascular mortality, the authors noted.
Study details
The cohort included 9,857 adults with diabetes and first hospitalizations for heart failure who were covered by Alabama Medicaid during 2010-2019. The investigators analyzed patients’ claims for ambulatory care (any, primary, cardiology, or endocrinology) within 60 days of discharge.
The mean age of participants was 53.7 years; 47.3% were Black; 41.8% non-Hispanic White; and 10.9% Hispanic/other, with other including those identifying as non-White Hispanic, American Indian, Pacific Islander, and Asian. About two-thirds (65.4%) of participants were women.
Analysis revealed low rates of follow-up care after hospital discharge; 26.7% had an ambulatory visit within 0-7 days, 15.2% within 8-14 days, 31.3% within 15-60 days, and 26.8% had no follow-up visit at all. Of those having a follow-up visit, 71% saw a primary care physician and 12% saw a cardiologist.
In contrast, a much higher proportion of heart failure patients in a Swedish registry – 63% – received ambulatory follow-up in cardiology.
Ethnic/gender/age disparities
Black and Hispanic/other adults were less likely to have any postdischarge ambulatory visit (P <.0001) or had the visit delayed by 1.8 days (P = .0006) and 2.8 days (P = .0016), respectively. They were less likely to see a primary care physician than were non-Hispanic White adults: adjusted incidence rate ratio, 0.96 (95% confidence interval [CI], 0.91-1.00) and 0.91 (95% CI, 0.89-0.98), respectively.
Men and those with longer-standing heart failure were less likely to be seen in primary care, while the presence of multiple comorbidities was associated with a higher likelihood of a postdischarge primary care visit. Men were more likely to be seen by a cardiologist, while older discharged patients were less likely to be seen by an endocrinologist within 60 days. There was a U-shaped relationship between the timing of the first postdischarge ambulatory visit and all-cause mortality among adults with diabetes and heart failure. Higher rates of 60-day all-cause mortality were observed both in those who had seen a provider within 0-7 days after discharge and in those who had not seen any provider during the 60-day study period compared with those having an ambulatory care visit within 7-14 or 15-60 days. “The group with early follow-up (0-7 days) likely represents a sicker population of patients with heart failure with more comorbidity burden and higher overall health care use, including readmissions, as was demonstrated in our analysis,” Dr. Khodneva and associates wrote. “Interventions that improve access to postdischarge ambulatory care for low-income patients with diabetes and heart failure and eliminate racial and ethnic disparities may be warranted,” they added.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Alabama at Birmingham Diabetes Research Center. Dr. Khodneva reported funding from the University of Alabama at Birmingham and the Forge Ahead Center as well as from the NIDDK, the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Alabama Medicaid Agency. Coauthor Emily Levitan, ScD, reported research funding from Amgen and has served on Amgen advisory boards. She has also served as a scientific consultant for a research project funded by Novartis.
Nearly 60% of Medicaid-covered adults with concurrent diabetes and heart failure did not receive guideline-concordant postdischarge care within 7-10 days of leaving the hospital, according to a large Alabama study. Moreover, affected Black and Hispanic/other Alabamians were less likely than were their White counterparts to receive recommended postdischarge care.
In comparison with White participants, Black and Hispanic adults were less likely to have any postdischarge ambulatory care visits after HF hospitalization or had a delayed visit, according to researchers led by Yulia Khodneva, MD, PhD, an internist at the University of Alabama at Birmingham. “This is likely a reflection of a structural racism and implicit bias against racial and ethnic minorities that persists in the U.S. health care system,” she and her colleagues wrote.
The findings point to the need for strategies to improve access to postdischarge care for lower-income HF patients.
Among U.S. states, Alabama is the sixth-poorest, the third in diabetes prevalence (14%), and has the highest rates of heart failure hospitalizations and cardiovascular mortality, the authors noted.
Study details
The cohort included 9,857 adults with diabetes and first hospitalizations for heart failure who were covered by Alabama Medicaid during 2010-2019. The investigators analyzed patients’ claims for ambulatory care (any, primary, cardiology, or endocrinology) within 60 days of discharge.
The mean age of participants was 53.7 years; 47.3% were Black; 41.8% non-Hispanic White; and 10.9% Hispanic/other, with other including those identifying as non-White Hispanic, American Indian, Pacific Islander, and Asian. About two-thirds (65.4%) of participants were women.
Analysis revealed low rates of follow-up care after hospital discharge; 26.7% had an ambulatory visit within 0-7 days, 15.2% within 8-14 days, 31.3% within 15-60 days, and 26.8% had no follow-up visit at all. Of those having a follow-up visit, 71% saw a primary care physician and 12% saw a cardiologist.
In contrast, a much higher proportion of heart failure patients in a Swedish registry – 63% – received ambulatory follow-up in cardiology.
Ethnic/gender/age disparities
Black and Hispanic/other adults were less likely to have any postdischarge ambulatory visit (P <.0001) or had the visit delayed by 1.8 days (P = .0006) and 2.8 days (P = .0016), respectively. They were less likely to see a primary care physician than were non-Hispanic White adults: adjusted incidence rate ratio, 0.96 (95% confidence interval [CI], 0.91-1.00) and 0.91 (95% CI, 0.89-0.98), respectively.
Men and those with longer-standing heart failure were less likely to be seen in primary care, while the presence of multiple comorbidities was associated with a higher likelihood of a postdischarge primary care visit. Men were more likely to be seen by a cardiologist, while older discharged patients were less likely to be seen by an endocrinologist within 60 days. There was a U-shaped relationship between the timing of the first postdischarge ambulatory visit and all-cause mortality among adults with diabetes and heart failure. Higher rates of 60-day all-cause mortality were observed both in those who had seen a provider within 0-7 days after discharge and in those who had not seen any provider during the 60-day study period compared with those having an ambulatory care visit within 7-14 or 15-60 days. “The group with early follow-up (0-7 days) likely represents a sicker population of patients with heart failure with more comorbidity burden and higher overall health care use, including readmissions, as was demonstrated in our analysis,” Dr. Khodneva and associates wrote. “Interventions that improve access to postdischarge ambulatory care for low-income patients with diabetes and heart failure and eliminate racial and ethnic disparities may be warranted,” they added.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Alabama at Birmingham Diabetes Research Center. Dr. Khodneva reported funding from the University of Alabama at Birmingham and the Forge Ahead Center as well as from the NIDDK, the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Alabama Medicaid Agency. Coauthor Emily Levitan, ScD, reported research funding from Amgen and has served on Amgen advisory boards. She has also served as a scientific consultant for a research project funded by Novartis.
Nearly 60% of Medicaid-covered adults with concurrent diabetes and heart failure did not receive guideline-concordant postdischarge care within 7-10 days of leaving the hospital, according to a large Alabama study. Moreover, affected Black and Hispanic/other Alabamians were less likely than were their White counterparts to receive recommended postdischarge care.
In comparison with White participants, Black and Hispanic adults were less likely to have any postdischarge ambulatory care visits after HF hospitalization or had a delayed visit, according to researchers led by Yulia Khodneva, MD, PhD, an internist at the University of Alabama at Birmingham. “This is likely a reflection of a structural racism and implicit bias against racial and ethnic minorities that persists in the U.S. health care system,” she and her colleagues wrote.
The findings point to the need for strategies to improve access to postdischarge care for lower-income HF patients.
Among U.S. states, Alabama is the sixth-poorest, the third in diabetes prevalence (14%), and has the highest rates of heart failure hospitalizations and cardiovascular mortality, the authors noted.
Study details
The cohort included 9,857 adults with diabetes and first hospitalizations for heart failure who were covered by Alabama Medicaid during 2010-2019. The investigators analyzed patients’ claims for ambulatory care (any, primary, cardiology, or endocrinology) within 60 days of discharge.
The mean age of participants was 53.7 years; 47.3% were Black; 41.8% non-Hispanic White; and 10.9% Hispanic/other, with other including those identifying as non-White Hispanic, American Indian, Pacific Islander, and Asian. About two-thirds (65.4%) of participants were women.
Analysis revealed low rates of follow-up care after hospital discharge; 26.7% had an ambulatory visit within 0-7 days, 15.2% within 8-14 days, 31.3% within 15-60 days, and 26.8% had no follow-up visit at all. Of those having a follow-up visit, 71% saw a primary care physician and 12% saw a cardiologist.
In contrast, a much higher proportion of heart failure patients in a Swedish registry – 63% – received ambulatory follow-up in cardiology.
Ethnic/gender/age disparities
Black and Hispanic/other adults were less likely to have any postdischarge ambulatory visit (P <.0001) or had the visit delayed by 1.8 days (P = .0006) and 2.8 days (P = .0016), respectively. They were less likely to see a primary care physician than were non-Hispanic White adults: adjusted incidence rate ratio, 0.96 (95% confidence interval [CI], 0.91-1.00) and 0.91 (95% CI, 0.89-0.98), respectively.
Men and those with longer-standing heart failure were less likely to be seen in primary care, while the presence of multiple comorbidities was associated with a higher likelihood of a postdischarge primary care visit. Men were more likely to be seen by a cardiologist, while older discharged patients were less likely to be seen by an endocrinologist within 60 days. There was a U-shaped relationship between the timing of the first postdischarge ambulatory visit and all-cause mortality among adults with diabetes and heart failure. Higher rates of 60-day all-cause mortality were observed both in those who had seen a provider within 0-7 days after discharge and in those who had not seen any provider during the 60-day study period compared with those having an ambulatory care visit within 7-14 or 15-60 days. “The group with early follow-up (0-7 days) likely represents a sicker population of patients with heart failure with more comorbidity burden and higher overall health care use, including readmissions, as was demonstrated in our analysis,” Dr. Khodneva and associates wrote. “Interventions that improve access to postdischarge ambulatory care for low-income patients with diabetes and heart failure and eliminate racial and ethnic disparities may be warranted,” they added.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Alabama at Birmingham Diabetes Research Center. Dr. Khodneva reported funding from the University of Alabama at Birmingham and the Forge Ahead Center as well as from the NIDDK, the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Alabama Medicaid Agency. Coauthor Emily Levitan, ScD, reported research funding from Amgen and has served on Amgen advisory boards. She has also served as a scientific consultant for a research project funded by Novartis.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Racial, ethnic disparities persist in access to MS care
Aurora, Colo. – , according to research on patient-reported health inequities presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
”Equal access to and quality of care are critical for managing a progressive disease such as multiple sclerosis,” said Chris Hardy, of Publicis Health Media, and her associates. “Despite increased awareness of health outcome disparities in the U.S., certain patients still experience inequities in care.”
The researchers sent emails to members of MyMSTeam, an online support network of more than 197,000 members, to request completion of a 34-question online survey. Questions addressed respondents’ ability to access care, resources in their neighborhood, and their interactions with their health care providers. Questions also addressed the burden of MS on individuals’ quality of life, which was considerable across all demographics. The 1,935 patients with MS who responded were overwhelmingly White, though the demographics varied by question.
A ‘widespread and significant problem’
“This study is important in pointing out the unfortunate, obvious [fact] that lack of access and lack of availability to treatment is still a widespread and significant problem in this country,” commented Mark Gudesblatt, MD, a neurologist at South Shore Neurologic Associates who was not involved in the study. “Improving effective treatment of disease requires a more granular understanding of disease impact on a quantitative, multidimensional, objective patient-centric approach,” he added. “Racial and ethnic barriers to effective treatment cannot be allowed nor tolerated. We need to be more acutely aware that outreach, digital health, and remote assessments are tools that we need to incorporate to improve access and do better.”
The pervasive impact of MS
Overall, 85% of respondents reported that MS made it harder to do everyday chores, and 84% said their MS made it harder to exercise and interfered with their everyday life. Similarly high proportions of respondents reported that their MS causes them a lot of stress (80%), makes them feel anxious or depressed (77%), disrupts their work/employment (75%), and interferes with their social life (75%). In addition, more than half said their diagnosis negatively affects their family (59%) and makes them feel judged (53%).
Deanne Power, RN, MSCN, the lead nurse care partner at Octave Bioscience, who spoke as a representative of the study authors, said it’s critical that clinicians be aware of the health inequities that exist among their patient population.
“Some patients have lower income or language issues where English is not their primary language, and they don’t have access and are even afraid to call doctor or reach out [for help],” Ms. Power said. “If providers aren’t actively aware of these situations and talk to their patients, they can’t just say, ‘Oh, well, I just want you to go fill this prescription,’ when they don’t have money to put food on their table. Providers have got to know their patients as [more than] just an MS patient. This is a human being in front of you, and you better know what their life is like, because it’s impacting their MS.”
Access to care varied by race
Among the 1,906 respondents who answered questions about access to care, 9% were Black, 5% were Hispanic, and the rest were White. In these questions, differences between demographics arose when it came to individuals’ ability to conveniently see an MS specialist and their subsequent use of emergency services. For example, only 64% of Hispanic respondents reported convenient access to a health care provider specializing in MS, compared with 76% of White and 78% of Black respondents.
A significantly higher proportion of Hispanics also reported that they could not take time off from work when they were sick (25%) or to attend a doctor appointment (20%), compared with White (15% and 9%, respectively) and Black (18% and 12%) respondents. Meanwhile, a significantly higher proportion of Hispanics (35%) reported visiting the emergency department in the past year for MS-related issues, compared with White (19%) or Black (25%) respondents.
White respondents consistently had greater convenient access to dental offices, healthy foods, outpatient care, gyms, and parks and trails, compared with Black and Hispanic patients’ access. For example, 85% of White patients had convenient access to dental offices and 72% had access to outpatient care, compared with Black (74% and 65%) and Hispanic (78% and 52%) patients. Two-thirds of Hispanic respondents (67%) reported access to healthy foods and to gyms, parks, or trails, compared with more than three-quarters of both White and Black patients.
Other barriers to MS care
Both racial/ethnic and gender disparities emerged in how patients felt treated by their health care providers. Men were significantly more likely (70%) than women (65%) to say their health care provider listens to and understands them. A statistically significant higher proportion of men (71%) also said their clinician explained their MS test results to them, compared with women (62%), and only 28% of women, versus 37% of men, said their provider developed a long-term plan for them.
Anne Foelsch, the vice president of strategic partnerships at MyHealthTeam, who works with the authors, noted the large discrepancy that was seen particularly for Hispanic patients in terms of how they felt treated by their health care provider.
“Doctors might perceive that the relationship is the same with all of their patients when their patients have a very different perception of what that relationship is and whether they’re not being heard,” Ms. Foelsch said. “It’s important that clinicians take a little bit of time and learn a little bit more about a patient’s perspective and what it’s like when they have a chronic condition like MS and how it impacts their life, looking for those nuances that are different based on your ethnicity.”
Just over half of Hispanic patients (54%) said their provider explained their MS test results, compared with nearly two-thirds of White patients (65%) and 61% of Black patients. Hispanic patients were also less likely (55%) to say they felt their provider listens to and understands them than White (67%) or Black (65%) patients. Two-thirds of White respondents (67%) said their doctor recommended regular check-ups, compared with just over half of Black and Hispanic respondents (55%).
Other statistically significant disparities by race/ethnicity, where a higher proportion of White patients responded affirmatively than Black or Hispanic patients, included feeling treated with respect by their health care provider, feeling their provider is nonjudgmental, and saying their provider spends enough time with them, addresses their MS symptoms, and encourages shared decision-making.
“This study nicely documents and points out that despite our best intentions, we need to do much better as a community to help those with chronic and potentially disabling diseases like MS,” Dr. Gudesblatt said. “The racial, ethnic, and gender disparities only result in greater disability and societal costs by those who can least afford it. All therapies fail due to nonadherence, limited access, lack of insurance coverage, limited insurance coverage, high copays, long waits, cultural biases, and more.”
The researchers acknowledged that their survey respondents may not be representative of all patients with MS because the survey relied on those who chose to respond to the online survey.
The study authors were all employees of Publicis Health Media or MyHealthTeam. Dr. Gudesblatt reported no disclosures.
Aurora, Colo. – , according to research on patient-reported health inequities presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
”Equal access to and quality of care are critical for managing a progressive disease such as multiple sclerosis,” said Chris Hardy, of Publicis Health Media, and her associates. “Despite increased awareness of health outcome disparities in the U.S., certain patients still experience inequities in care.”
The researchers sent emails to members of MyMSTeam, an online support network of more than 197,000 members, to request completion of a 34-question online survey. Questions addressed respondents’ ability to access care, resources in their neighborhood, and their interactions with their health care providers. Questions also addressed the burden of MS on individuals’ quality of life, which was considerable across all demographics. The 1,935 patients with MS who responded were overwhelmingly White, though the demographics varied by question.
A ‘widespread and significant problem’
“This study is important in pointing out the unfortunate, obvious [fact] that lack of access and lack of availability to treatment is still a widespread and significant problem in this country,” commented Mark Gudesblatt, MD, a neurologist at South Shore Neurologic Associates who was not involved in the study. “Improving effective treatment of disease requires a more granular understanding of disease impact on a quantitative, multidimensional, objective patient-centric approach,” he added. “Racial and ethnic barriers to effective treatment cannot be allowed nor tolerated. We need to be more acutely aware that outreach, digital health, and remote assessments are tools that we need to incorporate to improve access and do better.”
The pervasive impact of MS
Overall, 85% of respondents reported that MS made it harder to do everyday chores, and 84% said their MS made it harder to exercise and interfered with their everyday life. Similarly high proportions of respondents reported that their MS causes them a lot of stress (80%), makes them feel anxious or depressed (77%), disrupts their work/employment (75%), and interferes with their social life (75%). In addition, more than half said their diagnosis negatively affects their family (59%) and makes them feel judged (53%).
Deanne Power, RN, MSCN, the lead nurse care partner at Octave Bioscience, who spoke as a representative of the study authors, said it’s critical that clinicians be aware of the health inequities that exist among their patient population.
“Some patients have lower income or language issues where English is not their primary language, and they don’t have access and are even afraid to call doctor or reach out [for help],” Ms. Power said. “If providers aren’t actively aware of these situations and talk to their patients, they can’t just say, ‘Oh, well, I just want you to go fill this prescription,’ when they don’t have money to put food on their table. Providers have got to know their patients as [more than] just an MS patient. This is a human being in front of you, and you better know what their life is like, because it’s impacting their MS.”
Access to care varied by race
Among the 1,906 respondents who answered questions about access to care, 9% were Black, 5% were Hispanic, and the rest were White. In these questions, differences between demographics arose when it came to individuals’ ability to conveniently see an MS specialist and their subsequent use of emergency services. For example, only 64% of Hispanic respondents reported convenient access to a health care provider specializing in MS, compared with 76% of White and 78% of Black respondents.
A significantly higher proportion of Hispanics also reported that they could not take time off from work when they were sick (25%) or to attend a doctor appointment (20%), compared with White (15% and 9%, respectively) and Black (18% and 12%) respondents. Meanwhile, a significantly higher proportion of Hispanics (35%) reported visiting the emergency department in the past year for MS-related issues, compared with White (19%) or Black (25%) respondents.
White respondents consistently had greater convenient access to dental offices, healthy foods, outpatient care, gyms, and parks and trails, compared with Black and Hispanic patients’ access. For example, 85% of White patients had convenient access to dental offices and 72% had access to outpatient care, compared with Black (74% and 65%) and Hispanic (78% and 52%) patients. Two-thirds of Hispanic respondents (67%) reported access to healthy foods and to gyms, parks, or trails, compared with more than three-quarters of both White and Black patients.
Other barriers to MS care
Both racial/ethnic and gender disparities emerged in how patients felt treated by their health care providers. Men were significantly more likely (70%) than women (65%) to say their health care provider listens to and understands them. A statistically significant higher proportion of men (71%) also said their clinician explained their MS test results to them, compared with women (62%), and only 28% of women, versus 37% of men, said their provider developed a long-term plan for them.
Anne Foelsch, the vice president of strategic partnerships at MyHealthTeam, who works with the authors, noted the large discrepancy that was seen particularly for Hispanic patients in terms of how they felt treated by their health care provider.
“Doctors might perceive that the relationship is the same with all of their patients when their patients have a very different perception of what that relationship is and whether they’re not being heard,” Ms. Foelsch said. “It’s important that clinicians take a little bit of time and learn a little bit more about a patient’s perspective and what it’s like when they have a chronic condition like MS and how it impacts their life, looking for those nuances that are different based on your ethnicity.”
Just over half of Hispanic patients (54%) said their provider explained their MS test results, compared with nearly two-thirds of White patients (65%) and 61% of Black patients. Hispanic patients were also less likely (55%) to say they felt their provider listens to and understands them than White (67%) or Black (65%) patients. Two-thirds of White respondents (67%) said their doctor recommended regular check-ups, compared with just over half of Black and Hispanic respondents (55%).
Other statistically significant disparities by race/ethnicity, where a higher proportion of White patients responded affirmatively than Black or Hispanic patients, included feeling treated with respect by their health care provider, feeling their provider is nonjudgmental, and saying their provider spends enough time with them, addresses their MS symptoms, and encourages shared decision-making.
“This study nicely documents and points out that despite our best intentions, we need to do much better as a community to help those with chronic and potentially disabling diseases like MS,” Dr. Gudesblatt said. “The racial, ethnic, and gender disparities only result in greater disability and societal costs by those who can least afford it. All therapies fail due to nonadherence, limited access, lack of insurance coverage, limited insurance coverage, high copays, long waits, cultural biases, and more.”
The researchers acknowledged that their survey respondents may not be representative of all patients with MS because the survey relied on those who chose to respond to the online survey.
The study authors were all employees of Publicis Health Media or MyHealthTeam. Dr. Gudesblatt reported no disclosures.
Aurora, Colo. – , according to research on patient-reported health inequities presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
”Equal access to and quality of care are critical for managing a progressive disease such as multiple sclerosis,” said Chris Hardy, of Publicis Health Media, and her associates. “Despite increased awareness of health outcome disparities in the U.S., certain patients still experience inequities in care.”
The researchers sent emails to members of MyMSTeam, an online support network of more than 197,000 members, to request completion of a 34-question online survey. Questions addressed respondents’ ability to access care, resources in their neighborhood, and their interactions with their health care providers. Questions also addressed the burden of MS on individuals’ quality of life, which was considerable across all demographics. The 1,935 patients with MS who responded were overwhelmingly White, though the demographics varied by question.
A ‘widespread and significant problem’
“This study is important in pointing out the unfortunate, obvious [fact] that lack of access and lack of availability to treatment is still a widespread and significant problem in this country,” commented Mark Gudesblatt, MD, a neurologist at South Shore Neurologic Associates who was not involved in the study. “Improving effective treatment of disease requires a more granular understanding of disease impact on a quantitative, multidimensional, objective patient-centric approach,” he added. “Racial and ethnic barriers to effective treatment cannot be allowed nor tolerated. We need to be more acutely aware that outreach, digital health, and remote assessments are tools that we need to incorporate to improve access and do better.”
The pervasive impact of MS
Overall, 85% of respondents reported that MS made it harder to do everyday chores, and 84% said their MS made it harder to exercise and interfered with their everyday life. Similarly high proportions of respondents reported that their MS causes them a lot of stress (80%), makes them feel anxious or depressed (77%), disrupts their work/employment (75%), and interferes with their social life (75%). In addition, more than half said their diagnosis negatively affects their family (59%) and makes them feel judged (53%).
Deanne Power, RN, MSCN, the lead nurse care partner at Octave Bioscience, who spoke as a representative of the study authors, said it’s critical that clinicians be aware of the health inequities that exist among their patient population.
“Some patients have lower income or language issues where English is not their primary language, and they don’t have access and are even afraid to call doctor or reach out [for help],” Ms. Power said. “If providers aren’t actively aware of these situations and talk to their patients, they can’t just say, ‘Oh, well, I just want you to go fill this prescription,’ when they don’t have money to put food on their table. Providers have got to know their patients as [more than] just an MS patient. This is a human being in front of you, and you better know what their life is like, because it’s impacting their MS.”
Access to care varied by race
Among the 1,906 respondents who answered questions about access to care, 9% were Black, 5% were Hispanic, and the rest were White. In these questions, differences between demographics arose when it came to individuals’ ability to conveniently see an MS specialist and their subsequent use of emergency services. For example, only 64% of Hispanic respondents reported convenient access to a health care provider specializing in MS, compared with 76% of White and 78% of Black respondents.
A significantly higher proportion of Hispanics also reported that they could not take time off from work when they were sick (25%) or to attend a doctor appointment (20%), compared with White (15% and 9%, respectively) and Black (18% and 12%) respondents. Meanwhile, a significantly higher proportion of Hispanics (35%) reported visiting the emergency department in the past year for MS-related issues, compared with White (19%) or Black (25%) respondents.
White respondents consistently had greater convenient access to dental offices, healthy foods, outpatient care, gyms, and parks and trails, compared with Black and Hispanic patients’ access. For example, 85% of White patients had convenient access to dental offices and 72% had access to outpatient care, compared with Black (74% and 65%) and Hispanic (78% and 52%) patients. Two-thirds of Hispanic respondents (67%) reported access to healthy foods and to gyms, parks, or trails, compared with more than three-quarters of both White and Black patients.
Other barriers to MS care
Both racial/ethnic and gender disparities emerged in how patients felt treated by their health care providers. Men were significantly more likely (70%) than women (65%) to say their health care provider listens to and understands them. A statistically significant higher proportion of men (71%) also said their clinician explained their MS test results to them, compared with women (62%), and only 28% of women, versus 37% of men, said their provider developed a long-term plan for them.
Anne Foelsch, the vice president of strategic partnerships at MyHealthTeam, who works with the authors, noted the large discrepancy that was seen particularly for Hispanic patients in terms of how they felt treated by their health care provider.
“Doctors might perceive that the relationship is the same with all of their patients when their patients have a very different perception of what that relationship is and whether they’re not being heard,” Ms. Foelsch said. “It’s important that clinicians take a little bit of time and learn a little bit more about a patient’s perspective and what it’s like when they have a chronic condition like MS and how it impacts their life, looking for those nuances that are different based on your ethnicity.”
Just over half of Hispanic patients (54%) said their provider explained their MS test results, compared with nearly two-thirds of White patients (65%) and 61% of Black patients. Hispanic patients were also less likely (55%) to say they felt their provider listens to and understands them than White (67%) or Black (65%) patients. Two-thirds of White respondents (67%) said their doctor recommended regular check-ups, compared with just over half of Black and Hispanic respondents (55%).
Other statistically significant disparities by race/ethnicity, where a higher proportion of White patients responded affirmatively than Black or Hispanic patients, included feeling treated with respect by their health care provider, feeling their provider is nonjudgmental, and saying their provider spends enough time with them, addresses their MS symptoms, and encourages shared decision-making.
“This study nicely documents and points out that despite our best intentions, we need to do much better as a community to help those with chronic and potentially disabling diseases like MS,” Dr. Gudesblatt said. “The racial, ethnic, and gender disparities only result in greater disability and societal costs by those who can least afford it. All therapies fail due to nonadherence, limited access, lack of insurance coverage, limited insurance coverage, high copays, long waits, cultural biases, and more.”
The researchers acknowledged that their survey respondents may not be representative of all patients with MS because the survey relied on those who chose to respond to the online survey.
The study authors were all employees of Publicis Health Media or MyHealthTeam. Dr. Gudesblatt reported no disclosures.
At CMSC 2023
Why Is There a Lack of Representation of Skin of Color in the COVID-19 Literature?
Throughout the COVID-19 pandemic, there has been a striking paucity of representations of patients with skin of color (SOC) in the dermatology literature. Was COVID-19 underdiagnosed in this patient population due to a lack of patient-centered resources and inadequate dermatology training; reduced access to care, resulting from social determinants of health and reduced skin-color concordance; or the absence of population-based prevalence studies?
Tan et al1 reviewed 51 articles describing skin findings secondary to COVID-19. Patients were stratified by country of origin, which yielded an increased prevalence of cutaneous manifestations among Americans and Europeans compared to Asians, but patients were not stratified by race.1 However, in one case series of 318 predominantly American patients, 89% were White and 0.7% were Black.2 This systematic review by Tan et al1 suggested that skin manifestations of COVID-19 were present in patients with SOC but less frequently than in White patients. However, case series are not a strong proxy for population-level prevalence.
More broadly, patients with SOC are underrepresented in Google image search results, as the medical resource websites (eg, DermNet [https://dermnetnz.org], MedicalNewsToday [www.medicalnewstoday.com], and Healthline [www.healthline.com]) are lacking these images.3 As a result, it is difficult for patients with SOC to recognize diseases presenting in darker skin types. This same tendency may exist for COVID-19 skin manifestations. A systematic review found that articles describing cutaneous manifestations of COVID-19 almost exclusively presented images of lighter skin and completely omitted darker skin.4 If images of patients with SOC are absent from online resources, it is increasingly unlikely for these patients to recognize if their skin lesions are associated with COVID-19, which may result in a decrease in the number of patients with SOC presenting with skin lesions secondary to COVID-19, thereby influencing the representation of patients with SOC in case studies.
The lack of representation of SOC in online resources mirrors the paucity of images in dermatology textbooks. According to a search of 7170 images in major dermatology textbooks, most images depicted light or white skin (80.6%), followed by medium or brown skin in 15.5% of images and dark or black skin in only 3.9%.5 Physicians rely on online and print resources for making diagnoses; inadequate resources highlight a component of a larger issue: inadequate training of dermatologists in SOC. In a survey of American dermatologists and dermatology residents (N=262), 47% thought that their medical education had not adequately trained them on skin conditions in Black patients.6
A lack of adequate training for dermatologists may decrease the rate of correct diagnosis of skin lesions secondary to COVID-19 in patients with SOC. A lack of trust in the health care system and social determinants of health may hinder patients with SOC from seeking medical help. Dermatology is the second least diverse of medical specialties; only 3% of dermatologists are Black.7 This is impactful: First, because minority physicians are increasingly likely to provide care for patients of the same race or background, and second, because race-concordant physician visits are associated with greater patient-reported positive affect.7 A lack of availability of race-concordant physicians or physicians with perceived cultural competence may deter patients with SOC from seeking help, which may be further prevalent in dermatologic practice.
Barriers at all levels of social determinants of health hinder access to health care. Patients with SOC experience greater housing insecurity, increased reliance on public transportation, more issues with health literacy, and limited English-language fluency.8 Combined, these factors equate to decreased access to health care resources and subsequently a lack of inclusion in case studies.
COVID-19 infection disproportionately affects patients with SOC,8 but there is a clear lack of representation of SOC in the COVID-19 dermatology literature. It is imperative to investigate factors that may contribute to this inequity. Recognizing skin manifestations can play a role in diagnosing COVID-19; increased awareness of its presentation in darker skin types may help bridge existing racial inequities. It is vital that physicians receive adequate resources and training to be able to recognize cutaneous manifestations of COVID-19 in all skin types. Finally, it is important to recognize that the lack of representation of SOC in the COVID-19 literature represents a larger trend that exists in dermatologic research that warrants further investigation and advocacy for inclusivity.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133. doi:10.1016/j.jdin.2020.12.003
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dematol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Fathy R, Lipoff JB. Lack of skin of color in Google image searches may reflect under-representation in all educational resources. J Am Acad Dermatol. 2022;86:E113-E114. doi:10.1016/j.jaad.2021.04.097
- Lester JC, Jia JL, Zhang L, et al. Absence of images of skin of colour in publications of COVID-19 skin manifestations. Br J Dermatol. 2020;183:593-595. doi:10.1111/bjd.19258
- Kamath P, Sundaram N, Morillo-Hernandez C, et al. Visual racism in internet searches and dermatology textbooks. J Am Acad Dermatol. 2021;85:1348-1349. doi:10.1016/j.jaad.2020.10.072
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Tai DBG, Shah A, Doubeni CA, et al. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72:703-706. doi:10.1093/cid/ciaa815
Throughout the COVID-19 pandemic, there has been a striking paucity of representations of patients with skin of color (SOC) in the dermatology literature. Was COVID-19 underdiagnosed in this patient population due to a lack of patient-centered resources and inadequate dermatology training; reduced access to care, resulting from social determinants of health and reduced skin-color concordance; or the absence of population-based prevalence studies?
Tan et al1 reviewed 51 articles describing skin findings secondary to COVID-19. Patients were stratified by country of origin, which yielded an increased prevalence of cutaneous manifestations among Americans and Europeans compared to Asians, but patients were not stratified by race.1 However, in one case series of 318 predominantly American patients, 89% were White and 0.7% were Black.2 This systematic review by Tan et al1 suggested that skin manifestations of COVID-19 were present in patients with SOC but less frequently than in White patients. However, case series are not a strong proxy for population-level prevalence.
More broadly, patients with SOC are underrepresented in Google image search results, as the medical resource websites (eg, DermNet [https://dermnetnz.org], MedicalNewsToday [www.medicalnewstoday.com], and Healthline [www.healthline.com]) are lacking these images.3 As a result, it is difficult for patients with SOC to recognize diseases presenting in darker skin types. This same tendency may exist for COVID-19 skin manifestations. A systematic review found that articles describing cutaneous manifestations of COVID-19 almost exclusively presented images of lighter skin and completely omitted darker skin.4 If images of patients with SOC are absent from online resources, it is increasingly unlikely for these patients to recognize if their skin lesions are associated with COVID-19, which may result in a decrease in the number of patients with SOC presenting with skin lesions secondary to COVID-19, thereby influencing the representation of patients with SOC in case studies.
The lack of representation of SOC in online resources mirrors the paucity of images in dermatology textbooks. According to a search of 7170 images in major dermatology textbooks, most images depicted light or white skin (80.6%), followed by medium or brown skin in 15.5% of images and dark or black skin in only 3.9%.5 Physicians rely on online and print resources for making diagnoses; inadequate resources highlight a component of a larger issue: inadequate training of dermatologists in SOC. In a survey of American dermatologists and dermatology residents (N=262), 47% thought that their medical education had not adequately trained them on skin conditions in Black patients.6
A lack of adequate training for dermatologists may decrease the rate of correct diagnosis of skin lesions secondary to COVID-19 in patients with SOC. A lack of trust in the health care system and social determinants of health may hinder patients with SOC from seeking medical help. Dermatology is the second least diverse of medical specialties; only 3% of dermatologists are Black.7 This is impactful: First, because minority physicians are increasingly likely to provide care for patients of the same race or background, and second, because race-concordant physician visits are associated with greater patient-reported positive affect.7 A lack of availability of race-concordant physicians or physicians with perceived cultural competence may deter patients with SOC from seeking help, which may be further prevalent in dermatologic practice.
Barriers at all levels of social determinants of health hinder access to health care. Patients with SOC experience greater housing insecurity, increased reliance on public transportation, more issues with health literacy, and limited English-language fluency.8 Combined, these factors equate to decreased access to health care resources and subsequently a lack of inclusion in case studies.
COVID-19 infection disproportionately affects patients with SOC,8 but there is a clear lack of representation of SOC in the COVID-19 dermatology literature. It is imperative to investigate factors that may contribute to this inequity. Recognizing skin manifestations can play a role in diagnosing COVID-19; increased awareness of its presentation in darker skin types may help bridge existing racial inequities. It is vital that physicians receive adequate resources and training to be able to recognize cutaneous manifestations of COVID-19 in all skin types. Finally, it is important to recognize that the lack of representation of SOC in the COVID-19 literature represents a larger trend that exists in dermatologic research that warrants further investigation and advocacy for inclusivity.
Throughout the COVID-19 pandemic, there has been a striking paucity of representations of patients with skin of color (SOC) in the dermatology literature. Was COVID-19 underdiagnosed in this patient population due to a lack of patient-centered resources and inadequate dermatology training; reduced access to care, resulting from social determinants of health and reduced skin-color concordance; or the absence of population-based prevalence studies?
Tan et al1 reviewed 51 articles describing skin findings secondary to COVID-19. Patients were stratified by country of origin, which yielded an increased prevalence of cutaneous manifestations among Americans and Europeans compared to Asians, but patients were not stratified by race.1 However, in one case series of 318 predominantly American patients, 89% were White and 0.7% were Black.2 This systematic review by Tan et al1 suggested that skin manifestations of COVID-19 were present in patients with SOC but less frequently than in White patients. However, case series are not a strong proxy for population-level prevalence.
More broadly, patients with SOC are underrepresented in Google image search results, as the medical resource websites (eg, DermNet [https://dermnetnz.org], MedicalNewsToday [www.medicalnewstoday.com], and Healthline [www.healthline.com]) are lacking these images.3 As a result, it is difficult for patients with SOC to recognize diseases presenting in darker skin types. This same tendency may exist for COVID-19 skin manifestations. A systematic review found that articles describing cutaneous manifestations of COVID-19 almost exclusively presented images of lighter skin and completely omitted darker skin.4 If images of patients with SOC are absent from online resources, it is increasingly unlikely for these patients to recognize if their skin lesions are associated with COVID-19, which may result in a decrease in the number of patients with SOC presenting with skin lesions secondary to COVID-19, thereby influencing the representation of patients with SOC in case studies.
The lack of representation of SOC in online resources mirrors the paucity of images in dermatology textbooks. According to a search of 7170 images in major dermatology textbooks, most images depicted light or white skin (80.6%), followed by medium or brown skin in 15.5% of images and dark or black skin in only 3.9%.5 Physicians rely on online and print resources for making diagnoses; inadequate resources highlight a component of a larger issue: inadequate training of dermatologists in SOC. In a survey of American dermatologists and dermatology residents (N=262), 47% thought that their medical education had not adequately trained them on skin conditions in Black patients.6
A lack of adequate training for dermatologists may decrease the rate of correct diagnosis of skin lesions secondary to COVID-19 in patients with SOC. A lack of trust in the health care system and social determinants of health may hinder patients with SOC from seeking medical help. Dermatology is the second least diverse of medical specialties; only 3% of dermatologists are Black.7 This is impactful: First, because minority physicians are increasingly likely to provide care for patients of the same race or background, and second, because race-concordant physician visits are associated with greater patient-reported positive affect.7 A lack of availability of race-concordant physicians or physicians with perceived cultural competence may deter patients with SOC from seeking help, which may be further prevalent in dermatologic practice.
Barriers at all levels of social determinants of health hinder access to health care. Patients with SOC experience greater housing insecurity, increased reliance on public transportation, more issues with health literacy, and limited English-language fluency.8 Combined, these factors equate to decreased access to health care resources and subsequently a lack of inclusion in case studies.
COVID-19 infection disproportionately affects patients with SOC,8 but there is a clear lack of representation of SOC in the COVID-19 dermatology literature. It is imperative to investigate factors that may contribute to this inequity. Recognizing skin manifestations can play a role in diagnosing COVID-19; increased awareness of its presentation in darker skin types may help bridge existing racial inequities. It is vital that physicians receive adequate resources and training to be able to recognize cutaneous manifestations of COVID-19 in all skin types. Finally, it is important to recognize that the lack of representation of SOC in the COVID-19 literature represents a larger trend that exists in dermatologic research that warrants further investigation and advocacy for inclusivity.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133. doi:10.1016/j.jdin.2020.12.003
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dematol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Fathy R, Lipoff JB. Lack of skin of color in Google image searches may reflect under-representation in all educational resources. J Am Acad Dermatol. 2022;86:E113-E114. doi:10.1016/j.jaad.2021.04.097
- Lester JC, Jia JL, Zhang L, et al. Absence of images of skin of colour in publications of COVID-19 skin manifestations. Br J Dermatol. 2020;183:593-595. doi:10.1111/bjd.19258
- Kamath P, Sundaram N, Morillo-Hernandez C, et al. Visual racism in internet searches and dermatology textbooks. J Am Acad Dermatol. 2021;85:1348-1349. doi:10.1016/j.jaad.2020.10.072
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Tai DBG, Shah A, Doubeni CA, et al. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72:703-706. doi:10.1093/cid/ciaa815
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133. doi:10.1016/j.jdin.2020.12.003
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dematol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Fathy R, Lipoff JB. Lack of skin of color in Google image searches may reflect under-representation in all educational resources. J Am Acad Dermatol. 2022;86:E113-E114. doi:10.1016/j.jaad.2021.04.097
- Lester JC, Jia JL, Zhang L, et al. Absence of images of skin of colour in publications of COVID-19 skin manifestations. Br J Dermatol. 2020;183:593-595. doi:10.1111/bjd.19258
- Kamath P, Sundaram N, Morillo-Hernandez C, et al. Visual racism in internet searches and dermatology textbooks. J Am Acad Dermatol. 2021;85:1348-1349. doi:10.1016/j.jaad.2020.10.072
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Tai DBG, Shah A, Doubeni CA, et al. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72:703-706. doi:10.1093/cid/ciaa815
Systemic Lupus Erythematosus
THE COMPARISON
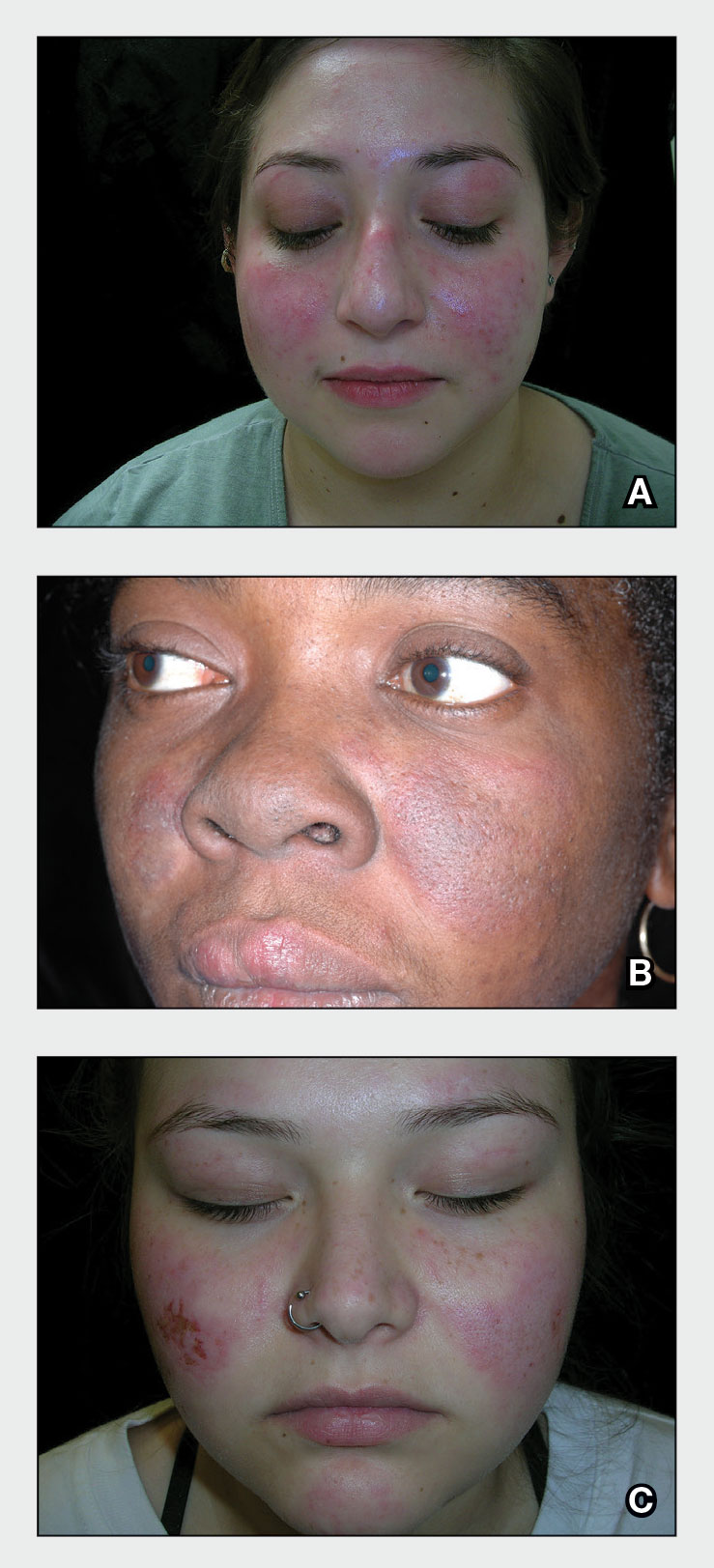
A A 23-year-old White woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose and eyelids but spares the nasolabial folds.
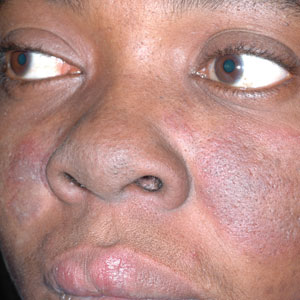
B A Black woman with malar erythema and hyperpigmentation from acute cutaneous lupus erythematosus. The nasolabial folds are spared.
C A 19-year-old Latina woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose, chin, and eyelids but spares the nasolabial folds. Cutaneous erosions are present on the right cheek as part of the lupus flare. Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects the kidneys, lungs, brain, and heart, though it is not limited to these organs. Dermatologists and primary care physicians play a critical role in the early identification of SLE, particularly in those with skin of color, as the standardized mortality rate is 2.6-fold higher in patients with SLE compared to the general population.1 The clinical manifestations of SLE vary.
Epidemiology
A meta-analysis of data from the Centers for Disease Control and Prevention National Lupus Registry network including 5417 patients revealed a prevalence of 72.8 cases per 100,000 person-years.2 The prevalence was higher in females than males and highest among females identifying as Black. White and Asian/Pacific Islander females had the lowest prevalence. The American Indian (indigenous)/Alaska Native–identifying population had the highest race-specific SLE estimates among both females and males compared to other racial/ethnic groups.2
Key clinical features in people with darker skin tones
The diagnosis of SLE is based on clinical and immunologic criteria from the European League Against Rheumatism/American College of Rheumatology.3,4 An antinuclear antibody titer of 1:80 or higher at least once is required for the diagnosis of SLE, as long as there is not another more likely diagnosis. If it is present, 22 additive weighted classification criteria are considered; each criterion is assigned points, ranging from 2 to 10. Patients with at least 1 clinical criterion and 10 or more points are classified as having SLE. If more than 1 of the criteria are met in a domain, then the one with the highest numerical value is counted.3,4 Aringer et al3,4 outline the criteria and numerical points to make the diagnosis of SLE. The mucocutaneous component of the SLE diagnostic criteria3,4 includes nonscarring alopecia, oral ulcers, subacute cutaneous or discoid lupus erythematosus,5 and acute cutaneous lupus erythematosus, with acute cutaneous lupus erythematosus being the highest-weighted criterion in that domain. The other clinical domains are constitutional, hematologic, neuropsychiatric, serosal, musculoskeletal, renal, antiphosopholipid antibodies, complement proteins, and SLE-specific antibodies.3,4
The malar (“butterfly”) rash of SLE characteristically includes erythema that spares the nasolabial folds but affects the nasal bridge and cheeks.6 The rash occasionally may be pruritic and painful, lasting days to weeks. Photosensitivity occurs, resulting in rashes or even an overall worsening of SLE symptoms. In those with darker skin tones, erythema may appear violaceous or may not be as readily appreciated.6
Worth noting
• Patients with skin of color are at an increased risk for postinflammatory hypopigmentation and hyperpigmentation (pigment alteration), hypertrophic scars, and keloids.7,8
• The mortality rate for those with SLE is high despite early recognition and treatment when compared to the general population.1,9
Health disparity highlight
Those at greatest risk for death from SLE in the United States are those of African descent, Hispanic individuals, men, and those with low socioeconomic status,9 which likely is primarily driven by social determinants of health instead of genetic patterns. Income level, educational attainment, insurance status, and environmental factors10 have far-reaching effects, negatively impacting quality of life and even mortality.
- Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727-734.
- Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries [published online April 23, 2021]. Arthritis Rheumatol. 2021;73:991-996. doi:10.1002/art.41632
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-1412. doi:10.1002/art.40930
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159.
- Heath CR, Usatine RP. Discoid lupus. Cutis. 2022;109:172-173.
- Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley’s Textbook of Rheumatology. 8th ed. Saunders Elsevier; 2008.
- Nozile W, Adgerson CH, Cohen GF. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343-349.
- Cardinali F, Kovacs D, Picardo M. Mechanisms underlying postinflammatory hyperpigmentation: lessons for solar. Ann Dermatol Venereol. 2012;139(suppl 4):S148-S152.
- Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14:1043-1053. doi:10.1080/17446 66X.2018.1538789
- Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605-620. doi:10.1038/nrrheum.2016.137
THE COMPARISON
A A 23-year-old White woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose and eyelids but spares the nasolabial folds.
B A Black woman with malar erythema and hyperpigmentation from acute cutaneous lupus erythematosus. The nasolabial folds are spared.
C A 19-year-old Latina woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose, chin, and eyelids but spares the nasolabial folds. Cutaneous erosions are present on the right cheek as part of the lupus flare. Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects the kidneys, lungs, brain, and heart, though it is not limited to these organs. Dermatologists and primary care physicians play a critical role in the early identification of SLE, particularly in those with skin of color, as the standardized mortality rate is 2.6-fold higher in patients with SLE compared to the general population.1 The clinical manifestations of SLE vary.

Epidemiology
A meta-analysis of data from the Centers for Disease Control and Prevention National Lupus Registry network including 5417 patients revealed a prevalence of 72.8 cases per 100,000 person-years.2 The prevalence was higher in females than males and highest among females identifying as Black. White and Asian/Pacific Islander females had the lowest prevalence. The American Indian (indigenous)/Alaska Native–identifying population had the highest race-specific SLE estimates among both females and males compared to other racial/ethnic groups.2
Key clinical features in people with darker skin tones
The diagnosis of SLE is based on clinical and immunologic criteria from the European League Against Rheumatism/American College of Rheumatology.3,4 An antinuclear antibody titer of 1:80 or higher at least once is required for the diagnosis of SLE, as long as there is not another more likely diagnosis. If it is present, 22 additive weighted classification criteria are considered; each criterion is assigned points, ranging from 2 to 10. Patients with at least 1 clinical criterion and 10 or more points are classified as having SLE. If more than 1 of the criteria are met in a domain, then the one with the highest numerical value is counted.3,4 Aringer et al3,4 outline the criteria and numerical points to make the diagnosis of SLE. The mucocutaneous component of the SLE diagnostic criteria3,4 includes nonscarring alopecia, oral ulcers, subacute cutaneous or discoid lupus erythematosus,5 and acute cutaneous lupus erythematosus, with acute cutaneous lupus erythematosus being the highest-weighted criterion in that domain. The other clinical domains are constitutional, hematologic, neuropsychiatric, serosal, musculoskeletal, renal, antiphosopholipid antibodies, complement proteins, and SLE-specific antibodies.3,4
The malar (“butterfly”) rash of SLE characteristically includes erythema that spares the nasolabial folds but affects the nasal bridge and cheeks.6 The rash occasionally may be pruritic and painful, lasting days to weeks. Photosensitivity occurs, resulting in rashes or even an overall worsening of SLE symptoms. In those with darker skin tones, erythema may appear violaceous or may not be as readily appreciated.6
Worth noting
• Patients with skin of color are at an increased risk for postinflammatory hypopigmentation and hyperpigmentation (pigment alteration), hypertrophic scars, and keloids.7,8
• The mortality rate for those with SLE is high despite early recognition and treatment when compared to the general population.1,9
Health disparity highlight
Those at greatest risk for death from SLE in the United States are those of African descent, Hispanic individuals, men, and those with low socioeconomic status,9 which likely is primarily driven by social determinants of health instead of genetic patterns. Income level, educational attainment, insurance status, and environmental factors10 have far-reaching effects, negatively impacting quality of life and even mortality.
THE COMPARISON
A A 23-year-old White woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose and eyelids but spares the nasolabial folds.
B A Black woman with malar erythema and hyperpigmentation from acute cutaneous lupus erythematosus. The nasolabial folds are spared.
C A 19-year-old Latina woman with malar erythema from acute cutaneous lupus erythematosus. The erythema also can be seen on the nose, chin, and eyelids but spares the nasolabial folds. Cutaneous erosions are present on the right cheek as part of the lupus flare. Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects the kidneys, lungs, brain, and heart, though it is not limited to these organs. Dermatologists and primary care physicians play a critical role in the early identification of SLE, particularly in those with skin of color, as the standardized mortality rate is 2.6-fold higher in patients with SLE compared to the general population.1 The clinical manifestations of SLE vary.

Epidemiology
A meta-analysis of data from the Centers for Disease Control and Prevention National Lupus Registry network including 5417 patients revealed a prevalence of 72.8 cases per 100,000 person-years.2 The prevalence was higher in females than males and highest among females identifying as Black. White and Asian/Pacific Islander females had the lowest prevalence. The American Indian (indigenous)/Alaska Native–identifying population had the highest race-specific SLE estimates among both females and males compared to other racial/ethnic groups.2
Key clinical features in people with darker skin tones
The diagnosis of SLE is based on clinical and immunologic criteria from the European League Against Rheumatism/American College of Rheumatology.3,4 An antinuclear antibody titer of 1:80 or higher at least once is required for the diagnosis of SLE, as long as there is not another more likely diagnosis. If it is present, 22 additive weighted classification criteria are considered; each criterion is assigned points, ranging from 2 to 10. Patients with at least 1 clinical criterion and 10 or more points are classified as having SLE. If more than 1 of the criteria are met in a domain, then the one with the highest numerical value is counted.3,4 Aringer et al3,4 outline the criteria and numerical points to make the diagnosis of SLE. The mucocutaneous component of the SLE diagnostic criteria3,4 includes nonscarring alopecia, oral ulcers, subacute cutaneous or discoid lupus erythematosus,5 and acute cutaneous lupus erythematosus, with acute cutaneous lupus erythematosus being the highest-weighted criterion in that domain. The other clinical domains are constitutional, hematologic, neuropsychiatric, serosal, musculoskeletal, renal, antiphosopholipid antibodies, complement proteins, and SLE-specific antibodies.3,4
The malar (“butterfly”) rash of SLE characteristically includes erythema that spares the nasolabial folds but affects the nasal bridge and cheeks.6 The rash occasionally may be pruritic and painful, lasting days to weeks. Photosensitivity occurs, resulting in rashes or even an overall worsening of SLE symptoms. In those with darker skin tones, erythema may appear violaceous or may not be as readily appreciated.6
Worth noting
• Patients with skin of color are at an increased risk for postinflammatory hypopigmentation and hyperpigmentation (pigment alteration), hypertrophic scars, and keloids.7,8
• The mortality rate for those with SLE is high despite early recognition and treatment when compared to the general population.1,9
Health disparity highlight
Those at greatest risk for death from SLE in the United States are those of African descent, Hispanic individuals, men, and those with low socioeconomic status,9 which likely is primarily driven by social determinants of health instead of genetic patterns. Income level, educational attainment, insurance status, and environmental factors10 have far-reaching effects, negatively impacting quality of life and even mortality.
- Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727-734.
- Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries [published online April 23, 2021]. Arthritis Rheumatol. 2021;73:991-996. doi:10.1002/art.41632
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-1412. doi:10.1002/art.40930
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159.
- Heath CR, Usatine RP. Discoid lupus. Cutis. 2022;109:172-173.
- Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley’s Textbook of Rheumatology. 8th ed. Saunders Elsevier; 2008.
- Nozile W, Adgerson CH, Cohen GF. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343-349.
- Cardinali F, Kovacs D, Picardo M. Mechanisms underlying postinflammatory hyperpigmentation: lessons for solar. Ann Dermatol Venereol. 2012;139(suppl 4):S148-S152.
- Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14:1043-1053. doi:10.1080/17446 66X.2018.1538789
- Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605-620. doi:10.1038/nrrheum.2016.137
- Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25:727-734.
- Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries [published online April 23, 2021]. Arthritis Rheumatol. 2021;73:991-996. doi:10.1002/art.41632
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:1400-1412. doi:10.1002/art.40930
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159.
- Heath CR, Usatine RP. Discoid lupus. Cutis. 2022;109:172-173.
- Firestein GS, Budd RC, Harris ED Jr, et al, eds. Kelley’s Textbook of Rheumatology. 8th ed. Saunders Elsevier; 2008.
- Nozile W, Adgerson CH, Cohen GF. Cutaneous lupus erythematosus in skin of color. J Drugs Dermatol. 2015;14:343-349.
- Cardinali F, Kovacs D, Picardo M. Mechanisms underlying postinflammatory hyperpigmentation: lessons for solar. Ann Dermatol Venereol. 2012;139(suppl 4):S148-S152.
- Ocampo-Piraquive V, Nieto-Aristizábal I, Cañas CA, et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14:1043-1053. doi:10.1080/17446 66X.2018.1538789
- Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol. 2016;12:605-620. doi:10.1038/nrrheum.2016.137
Interacting With Dermatology Patients Online: Private Practice vs Academic Institute Website Content
Patients are finding it easier to use online resources to discover health care providers who fit their personalized needs. In the United States, approximately 70% of individuals use the internet to find health care information, and 80% are influenced by the information presented to them on health care websites.1 Patients utilize the internet to better understand treatments offered by providers and their prices as well as how other patients have rated their experience. Providers in private practice also have noticed that many patients are referring themselves vs obtaining a referral from another provider.2 As a result, it is critical for practice websites to have information that is of value to their patients, including the unique qualities and treatments offered. The purpose of this study was to analyze the differences between the content presented on dermatology private practice websites and academic institutional websites.
Methods
Websites Searched —All 140 academic dermatology programs, including both allopathic and osteopathic programs, were queried from the Association of American Medical Colleges (AAMC) database in March 2022. 3 First, the dermatology departmental websites for each program were analyzed to see if they contained information pertinent to patients. Any website that lacked this information or only had information relevant to the dermatology residency program was excluded from the study. After exclusion, a total of 113 websites were used in the academic website cohort. The private practices were found through an incognito Google search with the search term dermatologist and matched to be within 5 miles of each academic institution. The private practices that included at least one board-certified dermatologist and received the highest number of reviews on Google compared to other practices in the same region—a measure of online reputation—were selected to be in the private practice cohort (N = 113). Any duplicate practices, practices belonging to the same conglomerate company, or multispecialty clinics were excluded from the study. Board-certified dermatologists were confirmed using the Find a Dermatologist tool on the American Academy of Dermatology (AAD) website. 4
Website Assessments —Each website was assessed using 23 criteria divided into 4 categories: practice, physician(s), patient, and treatment/procedure (Table). Criteria for social media and publicity were further assessed. Criteria for social media included links on the website to a Facebook page, an Instagram account, a Twitter account, a Pinterest account, a LinkedIn account, a blog, a Yelp page, a YouTube channel, and/or any other social media. Criteria for publicity included links on the website to local television news, national news, newspapers, and/or magazines. 5-8 Ease of site access was determined if the website was the first search result found on Google when searching for each website. Nondermatology professionals included listing of mid-level providers or researchers.
Four individuals (V.S.J., A.C.B., M.E.O., and M.B.B.) independently assessed each of the websites using the established criteria. Each criterion was defined and discussed prior to data collection to maintain consistency. The criteria were determined as being present if the website clearly displayed, stated, explained, or linked to the relevant content. If the website did not directly contain the content, it was determined that the criteria were absent. One other individual (J.P.) independently cross-examined the data for consistency and evaluated for any discrepancies. 8
A raw analysis was done between each cohort. Another analysis was done that controlled for population density and the proportionate population age in each city 9 in which an academic institution/private practice was located. We proposed that more densely populated cities naturally may have more competition between practices, which may result in more optimized websites. 10 We also anticipated similar findings in cities with younger populations, as the younger demographic may be more likely to utilize and value online information when compared to older populations. 11 The websites for each cohort were equally divided into 3 tiers of population density (not shown) and population age (not shown).
Statistical Analysis —Statistical analysis was completed using descriptive statistics, χ 2 testing, and Fisher exact tests where appropriate with a predetermined level of significance of P < .05 in Microsoft Excel.
Results
Demographics —A total of 226 websites from both private practices and academic institutions were evaluated. Of them, only 108 private practices and 108 academic institutions listed practicing dermatologists on their site. Of 108 private practices, 76 (70.4%) had more than one practicing board-certified dermatologist. Of 108 academic institutions, all 108 (100%) institutions had more than one practicing board-certified dermatologist.
Of the dermatologists who practiced at academic institutions (n=2014) and private practices (n=817), 1157 (57.4%) and 419 (51.2%) were females, respectively. The population density of the cities with each of these practices/institutions ranged from 137 individuals per square kilometer to 11,232 individuals per square kilometer (mean [SD] population density, 2579 [2485] individuals per square kilometer). Densely populated, moderately populated, and sparsely populated cities had a median population density of 4618, 1708, and 760 individuals per square kilometer, respectively. The data also were divided into 3 age groups. In the older population tier, the median percentage of individuals older than 64 years was 14.2%, the median percentage of individuals aged 18 to 64 years was 63.8%, and the median percentage of individuals aged 5 to 17 years was 14.9%. In the moderately aged population tier, the median percentage of individuals older than 64 years was 10.2%, the median percentage of individuals aged 18 to 64 years was 70.3%, and the median percentage of individuals aged 5 to 17 years was 13.6%. In the younger population tier, the median percentage of individuals older than 64 years was 12%, the median percentage of individuals aged 18 to 64 years was 66.8%, and the median percentage of individuals aged 5 to 17 years was 15%.
Practice and Physician Content—In the raw analysis (Figure), the most commonly listed types of content (>90% of websites) in both private practice and academic sites was address (range, 95% to 100%), telephone number (range, 97% to 100%), and dermatologist profiles (both 92%). The least commonly listed types of content in both cohorts was publicity (range, 20% to 23%). Private practices were more likely to list profiles of nondermatology professionals (73% vs 56%; P<.02), email (47% vs 17%; P<.0001), and social media (29% vs 8%; P<.0001) compared with academic institution websites. Although Facebook was the most-linked social media account for both groups, 75% of private practice sites included the link compared with 16% of academic institutions. Academic institutions were more likely to list fellowship availability (66% vs 1%; P<.0001). Accessing each website was significantly easier in the private practice cohort (99% vs 61%; P<.0001).
When controlling for population density, private practices were only more likely to list nondermatology professionals’ profiles in densely populated cities when compared with academic institutions (73% vs 41%; P<.01). Academic institutions continued to list fellowship availability more often than private practices regardless of population density. The same trend was observed for private practices with ease of site access and listing of social media.
When controlling for population age, similar trends were seen as when controlling for population density. However, private practices listing nondermatology professionals’ profiles was only more likely in the cities with a proportionately younger population when compared with academic institutions (74% vs 47%; P<.04).
Patient and Treatment/Procedure—The most commonly listed content types on both private practice websites and academic institution websites were available treatments/procedures (range, 89% to 98%). The least commonly listed content included financing for elective procedures (range, 4% to 16%), consultation fees (range, 1% to 2%), FAQs (frequently asked questions)(range, 4% to 20%), and HIPAA (Health Insurance Portability and Accountability Act) policy (range, 12% to 22%). Private practices were more likely to list patient testimonials (52% vs 35%; P<.005), financing (16% vs 4%; P<.005), FAQs (20% vs 4%; P<.001), online appointments (77% vs 56%; P<.001), available treatments/procedures (98% vs 86%; P<.004), product advertisements (66% vs 16%; P<.0001), pictures of dermatology conditions (33% vs 13%; P<.001), and HIPAA policy (22% vs 12%; P<.04). Academic institutions were more likely to list research trials (65% vs 13%; P<.0001).
When controlling for population density, private practices were only more likely to list patient testimonials in densely populated (P=.035) and moderately populated cities (P=.019). The same trend was observed for online appointments in densely populated (P=.0023) and moderately populated cities (P=.037). Private practices continued to list product availability more often than academic institutions regardless of population density or population age. Academic institutions also continued to list research trials more often than private practices regardless of population density or population age.
Comment
Our study uniquely analyzed the differences in website content between private practices and academic institutions in dermatology. Of the 140 academic institutions accredited by the Accreditation Council for Graduate Medical Education (ACGME), only 113 had patient-pertinent websites.
Access to Websites —There was a significant difference in many website content criteria between the 2 groups. Private practice sites were easier to access via a Google search when compared with academic sites, which likely is influenced by the Google search algorithm that ranks websites higher based on several criteria including but not limited to keyword use in the title tag, link popularity of the site, and historic ranking. 12,13 Academic sites often were only accessible through portals found on their main institutional site or institution’s residency site.
Role of Social Media —Social media has been found to assist in educating patients on medical practices as well as selecting a physician. 14,15 Our study found that private practice websites listed links to social media more often than their academic counterparts. Social media consumption is increasing, in part due to the COVID-19 pandemic, and it may be optimal for patients and practices alike to include links on their websites. 16 Facebook and Instagram were listed more often on private practice sites when compared with academic institution sites, which was similar to a recent study analyzing the websites of plastic surgery private practices (N = 310) in which 90% of private practices included some type of social media, with Instagram and Facebook being the most used. 8 Social networking accounts can act as convenient platforms for marketing, providing patient education, and generating referrals, which suggests that the prominence of their usage in private practice poses benefits in patient decision-making when seeking care. 17-19 A study analyzing the impact of Facebook in medicine concluded that a Facebook page can serve as an effective vehicle for medical education, particularly in younger generations that favor technology-oriented teaching methods. 20 A survey on trends in cosmetic facial procedures in plastic surgery found that the most influential online methods patients used for choosing their providers were social media platforms and practice websites. Front-page placement on Google also was commonly associated with the number of social media followers. 21,22 A lack of social media prominence could hinder a website’s potential to reach patients.
Communication With Practices —Our study also found significant differences in other metrics related to a patient’s ability to directly communicate with a practice, such as physical addresses, telephone numbers, products available for direct purchase, and online appointment booking, all of which were listed more often on private practice websites compared with academic institution websites. Online appointment booking also was found more frequently on private practice websites. Although physical addresses and telephone numbers were listed significantly more often on private practice sites, this information was ubiquitous and easily accessible elsewhere. Academic institution websites listed research trials and fellowship training significantly more often than private practices. These differences imply a divergence in focus between private practices and academic institutions, likely because academic institutions are funded in large part from research grants, begetting a cycle of academic contribution. 23 In contrast, private practices may not rely as heavily on academic revenue and may be more likely to prioritize other revenue streams such as product sales. 24
HIPAA Policy —Surprisingly, HIPAA policy rarely was listed on any private (22%) or academic site (12%). Conversely, in the plastic surgery study, HIPAA policy was listed much more often, with more than half of private practices with board-certified plastic surgeons accredited in the year 2015 including it on their website, 8 which may suggest that surgically oriented specialties, particularly cosmetic subspecialties, aim to more noticeably display their privacy policies for patient reassurance.
Study Limitations —There are several limitations of our study. First, it is common for a conglomerate company to own multiple private practices in different specialties. As with academic sites, private practice sites may be limited by the hosting platforms, which often are tedious to navigate. Also noteworthy is the emergence of designated social media management positions—both by practice employees and by third-party firms 25 —but the impact of these positions in private practices and academic institutions has not been fully explored. Finally, inclusion criteria and standardized criteria definitions were chosen based on the precedent established by the authors of similar analyses in plastic surgery and radiology. 5-8 Further investigation into the most valued aspects of care by patients within the context of the type of practice chosen would be valuable in refining inclusion criteria. Additionally, this study did not stratify the data collected based on factors such as gender, race, and geographical location; studies conducted on website traffic analysis patterns that focus on these aspects likely would further explain the significance of these findings. Differences in the length of time to the next available appointment between private practices and academic institutions also may help support our findings. Finally, there is a need for further investigation into the preferences of patients themselves garnered from website traffic alone.
Conclusion
Our study examined a diverse compilation of private practice and academic institution websites and uncovered numerous differences in content. As technology and health care continuously evolve, it is imperative that both private practices and academic institutions are actively adapting to optimize their online presence. In doing so, patients will be better equipped at accessing provider information, gaining familiarity with the practice, and understanding treatment options.
- Gentry ZL, Ananthasekar S, Yeatts M, et al. Can patients find an endocrine surgeon? how hospital websites hide the expertise of these medical professionals. Am J Surg . 2021;221:101-105.
- Pollack CE, Rastegar A, Keating NL, et al. Is self-referral associated with higher quality care? Health Serv Res . 2015;50:1472-1490.
- Association of American Medical Colleges. Residency Explorer TM tool. Accessed May 15, 2023. https://students-residents.aamc.org/apply-smart-residency/residency-explorer-tool
- Find a dermatologist. American Academy of Dermatology website. Accessed May 15, 2023. https://find-a-derm.aad.org/
- Johnson EJ, Doshi AM, Rosenkrantz AB. Strengths and deficiencies in the content of US radiology private practices’ websites. J Am Coll Radiol. 2017;14:431-435.
- Brunk D. Medical website expert shares design tips. Dermatology News . February 9, 2012. Accessed May 15, 2023. https://www.mdedge.com/dermatology/article/47413/health-policy/medical-website-expert-shares-design-tips
- Kuhnigk O, Ramuschkat M, Schreiner J, et al. Internet presence of neurologists, psychiatrists and medical psychotherapists in private practice [in German]. Psychiatr Prax . 2013;41:142-147.
- Ananthasekar S, Patel JJ, Patel NJ, et al. The content of US plastic surgery private practices’ websites. Ann Plast Surg . 2021;86(6S suppl 5):S578-S584.
- US Census Bureau. Age and Sex: 2021. Updated December 2, 2021. Accessed March 15, 2023. https://www.census.gov/topics/population/age-and-sex/data/tables.2021.List_897222059.html#list-tab-List_897222059
- Porter ME. The competitive advantage of the inner city. Harvard Business Review . Published August 1, 2014. https://hbr.org/1995/05/the-competitive-advantage-of-the-inner-city
- Clark PG. The social allocation of health care resources: ethical dilemmas in age-group competition. Gerontologist. 1985;25:119-125.
- Su A-J, Hu YC, Kuzmanovic A, et al. How to improve your Google ranking: myths and reality. ACM Transactions on the Web . 2014;8. https://dl.acm.org/doi/abs/10.1145/2579990
- McCormick K. 39 ways to increase traffic to your website. WordStream website. Published March 28, 2023. Accessed May 22, 2023. https://www.wordstream.com/blog/ws/2014/08/14/increase-traffic-to-my-website
- Montemurro P, Porcnik A, Hedén P, et al. The influence of social media and easily accessible online information on the aesthetic plastic surgery practice: literature review and our own experience. Aesthetic Plast Surg . 2015;39:270-277.
- Steehler KR, Steehler MK, Pierce ML, et al. Social media’s role in otolaryngology–head and neck surgery. Otolaryngol Head Neck Surg . 2013;149:521-524.
- Tsao S-F, Chen H, Tisseverasinghe T, et al. What social media told us in the time of COVID-19: a scoping review. Lancet Digit Health . 2021;3:E175-E194.
- Geist R, Militello M, Albrecht JM, et al. Social media and clinical research in dermatology. Curr Dermatol Rep . 2021;10:105-111.
- McLawhorn AS, De Martino I, Fehring KA, et al. Social media and your practice: navigating the surgeon-patient relationship. Curr Rev Musculoskelet Med . 2016;9:487-495.
- Thomas RB, Johnson PT, Fishman EK. Social media for global education: pearls and pitfalls of using Facebook, Twitter, and Instagram. J Am Coll Radiol . 2018;15:1513-1516.
- Lugo-Fagundo C, Johnson MB, Thomas RB, et al. New frontiers in education: Facebook as a vehicle for medical information delivery. J Am Coll Radiol . 2016;13:316-319.
- Ho T-VT, Dayan SH. How to leverage social media in private practice. Facial Plast Surg Clin North Am . 2020;28:515-522.
- Fan KL, Graziano F, Economides JM, et al. The public’s preferences on plastic surgery social media engagement and professionalism. Plast Reconstr Surg . 2019;143:619-630.
- Jacob BA, Lefgren L. The impact of research grant funding on scientific productivity. J Public Econ. 2011;95:1168-1177.
- Baumann L. Ethics in cosmetic dermatology. Clin Dermatol. 2012;30:522-527.
- Miller AR, Tucker C. Active social media management: the case of health care. Info Sys Res . 2013;24:52-70.
Patients are finding it easier to use online resources to discover health care providers who fit their personalized needs. In the United States, approximately 70% of individuals use the internet to find health care information, and 80% are influenced by the information presented to them on health care websites.1 Patients utilize the internet to better understand treatments offered by providers and their prices as well as how other patients have rated their experience. Providers in private practice also have noticed that many patients are referring themselves vs obtaining a referral from another provider.2 As a result, it is critical for practice websites to have information that is of value to their patients, including the unique qualities and treatments offered. The purpose of this study was to analyze the differences between the content presented on dermatology private practice websites and academic institutional websites.
Methods
Websites Searched —All 140 academic dermatology programs, including both allopathic and osteopathic programs, were queried from the Association of American Medical Colleges (AAMC) database in March 2022. 3 First, the dermatology departmental websites for each program were analyzed to see if they contained information pertinent to patients. Any website that lacked this information or only had information relevant to the dermatology residency program was excluded from the study. After exclusion, a total of 113 websites were used in the academic website cohort. The private practices were found through an incognito Google search with the search term dermatologist and matched to be within 5 miles of each academic institution. The private practices that included at least one board-certified dermatologist and received the highest number of reviews on Google compared to other practices in the same region—a measure of online reputation—were selected to be in the private practice cohort (N = 113). Any duplicate practices, practices belonging to the same conglomerate company, or multispecialty clinics were excluded from the study. Board-certified dermatologists were confirmed using the Find a Dermatologist tool on the American Academy of Dermatology (AAD) website. 4
Website Assessments —Each website was assessed using 23 criteria divided into 4 categories: practice, physician(s), patient, and treatment/procedure (Table). Criteria for social media and publicity were further assessed. Criteria for social media included links on the website to a Facebook page, an Instagram account, a Twitter account, a Pinterest account, a LinkedIn account, a blog, a Yelp page, a YouTube channel, and/or any other social media. Criteria for publicity included links on the website to local television news, national news, newspapers, and/or magazines. 5-8 Ease of site access was determined if the website was the first search result found on Google when searching for each website. Nondermatology professionals included listing of mid-level providers or researchers.

Four individuals (V.S.J., A.C.B., M.E.O., and M.B.B.) independently assessed each of the websites using the established criteria. Each criterion was defined and discussed prior to data collection to maintain consistency. The criteria were determined as being present if the website clearly displayed, stated, explained, or linked to the relevant content. If the website did not directly contain the content, it was determined that the criteria were absent. One other individual (J.P.) independently cross-examined the data for consistency and evaluated for any discrepancies. 8
A raw analysis was done between each cohort. Another analysis was done that controlled for population density and the proportionate population age in each city 9 in which an academic institution/private practice was located. We proposed that more densely populated cities naturally may have more competition between practices, which may result in more optimized websites. 10 We also anticipated similar findings in cities with younger populations, as the younger demographic may be more likely to utilize and value online information when compared to older populations. 11 The websites for each cohort were equally divided into 3 tiers of population density (not shown) and population age (not shown).
Statistical Analysis —Statistical analysis was completed using descriptive statistics, χ 2 testing, and Fisher exact tests where appropriate with a predetermined level of significance of P < .05 in Microsoft Excel.
Results
Demographics —A total of 226 websites from both private practices and academic institutions were evaluated. Of them, only 108 private practices and 108 academic institutions listed practicing dermatologists on their site. Of 108 private practices, 76 (70.4%) had more than one practicing board-certified dermatologist. Of 108 academic institutions, all 108 (100%) institutions had more than one practicing board-certified dermatologist.
Of the dermatologists who practiced at academic institutions (n=2014) and private practices (n=817), 1157 (57.4%) and 419 (51.2%) were females, respectively. The population density of the cities with each of these practices/institutions ranged from 137 individuals per square kilometer to 11,232 individuals per square kilometer (mean [SD] population density, 2579 [2485] individuals per square kilometer). Densely populated, moderately populated, and sparsely populated cities had a median population density of 4618, 1708, and 760 individuals per square kilometer, respectively. The data also were divided into 3 age groups. In the older population tier, the median percentage of individuals older than 64 years was 14.2%, the median percentage of individuals aged 18 to 64 years was 63.8%, and the median percentage of individuals aged 5 to 17 years was 14.9%. In the moderately aged population tier, the median percentage of individuals older than 64 years was 10.2%, the median percentage of individuals aged 18 to 64 years was 70.3%, and the median percentage of individuals aged 5 to 17 years was 13.6%. In the younger population tier, the median percentage of individuals older than 64 years was 12%, the median percentage of individuals aged 18 to 64 years was 66.8%, and the median percentage of individuals aged 5 to 17 years was 15%.
Practice and Physician Content—In the raw analysis (Figure), the most commonly listed types of content (>90% of websites) in both private practice and academic sites was address (range, 95% to 100%), telephone number (range, 97% to 100%), and dermatologist profiles (both 92%). The least commonly listed types of content in both cohorts was publicity (range, 20% to 23%). Private practices were more likely to list profiles of nondermatology professionals (73% vs 56%; P<.02), email (47% vs 17%; P<.0001), and social media (29% vs 8%; P<.0001) compared with academic institution websites. Although Facebook was the most-linked social media account for both groups, 75% of private practice sites included the link compared with 16% of academic institutions. Academic institutions were more likely to list fellowship availability (66% vs 1%; P<.0001). Accessing each website was significantly easier in the private practice cohort (99% vs 61%; P<.0001).

When controlling for population density, private practices were only more likely to list nondermatology professionals’ profiles in densely populated cities when compared with academic institutions (73% vs 41%; P<.01). Academic institutions continued to list fellowship availability more often than private practices regardless of population density. The same trend was observed for private practices with ease of site access and listing of social media.
When controlling for population age, similar trends were seen as when controlling for population density. However, private practices listing nondermatology professionals’ profiles was only more likely in the cities with a proportionately younger population when compared with academic institutions (74% vs 47%; P<.04).
Patient and Treatment/Procedure—The most commonly listed content types on both private practice websites and academic institution websites were available treatments/procedures (range, 89% to 98%). The least commonly listed content included financing for elective procedures (range, 4% to 16%), consultation fees (range, 1% to 2%), FAQs (frequently asked questions)(range, 4% to 20%), and HIPAA (Health Insurance Portability and Accountability Act) policy (range, 12% to 22%). Private practices were more likely to list patient testimonials (52% vs 35%; P<.005), financing (16% vs 4%; P<.005), FAQs (20% vs 4%; P<.001), online appointments (77% vs 56%; P<.001), available treatments/procedures (98% vs 86%; P<.004), product advertisements (66% vs 16%; P<.0001), pictures of dermatology conditions (33% vs 13%; P<.001), and HIPAA policy (22% vs 12%; P<.04). Academic institutions were more likely to list research trials (65% vs 13%; P<.0001).
When controlling for population density, private practices were only more likely to list patient testimonials in densely populated (P=.035) and moderately populated cities (P=.019). The same trend was observed for online appointments in densely populated (P=.0023) and moderately populated cities (P=.037). Private practices continued to list product availability more often than academic institutions regardless of population density or population age. Academic institutions also continued to list research trials more often than private practices regardless of population density or population age.
Comment
Our study uniquely analyzed the differences in website content between private practices and academic institutions in dermatology. Of the 140 academic institutions accredited by the Accreditation Council for Graduate Medical Education (ACGME), only 113 had patient-pertinent websites.
Access to Websites —There was a significant difference in many website content criteria between the 2 groups. Private practice sites were easier to access via a Google search when compared with academic sites, which likely is influenced by the Google search algorithm that ranks websites higher based on several criteria including but not limited to keyword use in the title tag, link popularity of the site, and historic ranking. 12,13 Academic sites often were only accessible through portals found on their main institutional site or institution’s residency site.
Role of Social Media —Social media has been found to assist in educating patients on medical practices as well as selecting a physician. 14,15 Our study found that private practice websites listed links to social media more often than their academic counterparts. Social media consumption is increasing, in part due to the COVID-19 pandemic, and it may be optimal for patients and practices alike to include links on their websites. 16 Facebook and Instagram were listed more often on private practice sites when compared with academic institution sites, which was similar to a recent study analyzing the websites of plastic surgery private practices (N = 310) in which 90% of private practices included some type of social media, with Instagram and Facebook being the most used. 8 Social networking accounts can act as convenient platforms for marketing, providing patient education, and generating referrals, which suggests that the prominence of their usage in private practice poses benefits in patient decision-making when seeking care. 17-19 A study analyzing the impact of Facebook in medicine concluded that a Facebook page can serve as an effective vehicle for medical education, particularly in younger generations that favor technology-oriented teaching methods. 20 A survey on trends in cosmetic facial procedures in plastic surgery found that the most influential online methods patients used for choosing their providers were social media platforms and practice websites. Front-page placement on Google also was commonly associated with the number of social media followers. 21,22 A lack of social media prominence could hinder a website’s potential to reach patients.
Communication With Practices —Our study also found significant differences in other metrics related to a patient’s ability to directly communicate with a practice, such as physical addresses, telephone numbers, products available for direct purchase, and online appointment booking, all of which were listed more often on private practice websites compared with academic institution websites. Online appointment booking also was found more frequently on private practice websites. Although physical addresses and telephone numbers were listed significantly more often on private practice sites, this information was ubiquitous and easily accessible elsewhere. Academic institution websites listed research trials and fellowship training significantly more often than private practices. These differences imply a divergence in focus between private practices and academic institutions, likely because academic institutions are funded in large part from research grants, begetting a cycle of academic contribution. 23 In contrast, private practices may not rely as heavily on academic revenue and may be more likely to prioritize other revenue streams such as product sales. 24
HIPAA Policy —Surprisingly, HIPAA policy rarely was listed on any private (22%) or academic site (12%). Conversely, in the plastic surgery study, HIPAA policy was listed much more often, with more than half of private practices with board-certified plastic surgeons accredited in the year 2015 including it on their website, 8 which may suggest that surgically oriented specialties, particularly cosmetic subspecialties, aim to more noticeably display their privacy policies for patient reassurance.
Study Limitations —There are several limitations of our study. First, it is common for a conglomerate company to own multiple private practices in different specialties. As with academic sites, private practice sites may be limited by the hosting platforms, which often are tedious to navigate. Also noteworthy is the emergence of designated social media management positions—both by practice employees and by third-party firms 25 —but the impact of these positions in private practices and academic institutions has not been fully explored. Finally, inclusion criteria and standardized criteria definitions were chosen based on the precedent established by the authors of similar analyses in plastic surgery and radiology. 5-8 Further investigation into the most valued aspects of care by patients within the context of the type of practice chosen would be valuable in refining inclusion criteria. Additionally, this study did not stratify the data collected based on factors such as gender, race, and geographical location; studies conducted on website traffic analysis patterns that focus on these aspects likely would further explain the significance of these findings. Differences in the length of time to the next available appointment between private practices and academic institutions also may help support our findings. Finally, there is a need for further investigation into the preferences of patients themselves garnered from website traffic alone.
Conclusion
Our study examined a diverse compilation of private practice and academic institution websites and uncovered numerous differences in content. As technology and health care continuously evolve, it is imperative that both private practices and academic institutions are actively adapting to optimize their online presence. In doing so, patients will be better equipped at accessing provider information, gaining familiarity with the practice, and understanding treatment options.
Patients are finding it easier to use online resources to discover health care providers who fit their personalized needs. In the United States, approximately 70% of individuals use the internet to find health care information, and 80% are influenced by the information presented to them on health care websites.1 Patients utilize the internet to better understand treatments offered by providers and their prices as well as how other patients have rated their experience. Providers in private practice also have noticed that many patients are referring themselves vs obtaining a referral from another provider.2 As a result, it is critical for practice websites to have information that is of value to their patients, including the unique qualities and treatments offered. The purpose of this study was to analyze the differences between the content presented on dermatology private practice websites and academic institutional websites.
Methods
Websites Searched —All 140 academic dermatology programs, including both allopathic and osteopathic programs, were queried from the Association of American Medical Colleges (AAMC) database in March 2022. 3 First, the dermatology departmental websites for each program were analyzed to see if they contained information pertinent to patients. Any website that lacked this information or only had information relevant to the dermatology residency program was excluded from the study. After exclusion, a total of 113 websites were used in the academic website cohort. The private practices were found through an incognito Google search with the search term dermatologist and matched to be within 5 miles of each academic institution. The private practices that included at least one board-certified dermatologist and received the highest number of reviews on Google compared to other practices in the same region—a measure of online reputation—were selected to be in the private practice cohort (N = 113). Any duplicate practices, practices belonging to the same conglomerate company, or multispecialty clinics were excluded from the study. Board-certified dermatologists were confirmed using the Find a Dermatologist tool on the American Academy of Dermatology (AAD) website. 4
Website Assessments —Each website was assessed using 23 criteria divided into 4 categories: practice, physician(s), patient, and treatment/procedure (Table). Criteria for social media and publicity were further assessed. Criteria for social media included links on the website to a Facebook page, an Instagram account, a Twitter account, a Pinterest account, a LinkedIn account, a blog, a Yelp page, a YouTube channel, and/or any other social media. Criteria for publicity included links on the website to local television news, national news, newspapers, and/or magazines. 5-8 Ease of site access was determined if the website was the first search result found on Google when searching for each website. Nondermatology professionals included listing of mid-level providers or researchers.

Four individuals (V.S.J., A.C.B., M.E.O., and M.B.B.) independently assessed each of the websites using the established criteria. Each criterion was defined and discussed prior to data collection to maintain consistency. The criteria were determined as being present if the website clearly displayed, stated, explained, or linked to the relevant content. If the website did not directly contain the content, it was determined that the criteria were absent. One other individual (J.P.) independently cross-examined the data for consistency and evaluated for any discrepancies. 8
A raw analysis was done between each cohort. Another analysis was done that controlled for population density and the proportionate population age in each city 9 in which an academic institution/private practice was located. We proposed that more densely populated cities naturally may have more competition between practices, which may result in more optimized websites. 10 We also anticipated similar findings in cities with younger populations, as the younger demographic may be more likely to utilize and value online information when compared to older populations. 11 The websites for each cohort were equally divided into 3 tiers of population density (not shown) and population age (not shown).
Statistical Analysis —Statistical analysis was completed using descriptive statistics, χ 2 testing, and Fisher exact tests where appropriate with a predetermined level of significance of P < .05 in Microsoft Excel.
Results
Demographics —A total of 226 websites from both private practices and academic institutions were evaluated. Of them, only 108 private practices and 108 academic institutions listed practicing dermatologists on their site. Of 108 private practices, 76 (70.4%) had more than one practicing board-certified dermatologist. Of 108 academic institutions, all 108 (100%) institutions had more than one practicing board-certified dermatologist.
Of the dermatologists who practiced at academic institutions (n=2014) and private practices (n=817), 1157 (57.4%) and 419 (51.2%) were females, respectively. The population density of the cities with each of these practices/institutions ranged from 137 individuals per square kilometer to 11,232 individuals per square kilometer (mean [SD] population density, 2579 [2485] individuals per square kilometer). Densely populated, moderately populated, and sparsely populated cities had a median population density of 4618, 1708, and 760 individuals per square kilometer, respectively. The data also were divided into 3 age groups. In the older population tier, the median percentage of individuals older than 64 years was 14.2%, the median percentage of individuals aged 18 to 64 years was 63.8%, and the median percentage of individuals aged 5 to 17 years was 14.9%. In the moderately aged population tier, the median percentage of individuals older than 64 years was 10.2%, the median percentage of individuals aged 18 to 64 years was 70.3%, and the median percentage of individuals aged 5 to 17 years was 13.6%. In the younger population tier, the median percentage of individuals older than 64 years was 12%, the median percentage of individuals aged 18 to 64 years was 66.8%, and the median percentage of individuals aged 5 to 17 years was 15%.
Practice and Physician Content—In the raw analysis (Figure), the most commonly listed types of content (>90% of websites) in both private practice and academic sites was address (range, 95% to 100%), telephone number (range, 97% to 100%), and dermatologist profiles (both 92%). The least commonly listed types of content in both cohorts was publicity (range, 20% to 23%). Private practices were more likely to list profiles of nondermatology professionals (73% vs 56%; P<.02), email (47% vs 17%; P<.0001), and social media (29% vs 8%; P<.0001) compared with academic institution websites. Although Facebook was the most-linked social media account for both groups, 75% of private practice sites included the link compared with 16% of academic institutions. Academic institutions were more likely to list fellowship availability (66% vs 1%; P<.0001). Accessing each website was significantly easier in the private practice cohort (99% vs 61%; P<.0001).

When controlling for population density, private practices were only more likely to list nondermatology professionals’ profiles in densely populated cities when compared with academic institutions (73% vs 41%; P<.01). Academic institutions continued to list fellowship availability more often than private practices regardless of population density. The same trend was observed for private practices with ease of site access and listing of social media.
When controlling for population age, similar trends were seen as when controlling for population density. However, private practices listing nondermatology professionals’ profiles was only more likely in the cities with a proportionately younger population when compared with academic institutions (74% vs 47%; P<.04).
Patient and Treatment/Procedure—The most commonly listed content types on both private practice websites and academic institution websites were available treatments/procedures (range, 89% to 98%). The least commonly listed content included financing for elective procedures (range, 4% to 16%), consultation fees (range, 1% to 2%), FAQs (frequently asked questions)(range, 4% to 20%), and HIPAA (Health Insurance Portability and Accountability Act) policy (range, 12% to 22%). Private practices were more likely to list patient testimonials (52% vs 35%; P<.005), financing (16% vs 4%; P<.005), FAQs (20% vs 4%; P<.001), online appointments (77% vs 56%; P<.001), available treatments/procedures (98% vs 86%; P<.004), product advertisements (66% vs 16%; P<.0001), pictures of dermatology conditions (33% vs 13%; P<.001), and HIPAA policy (22% vs 12%; P<.04). Academic institutions were more likely to list research trials (65% vs 13%; P<.0001).
When controlling for population density, private practices were only more likely to list patient testimonials in densely populated (P=.035) and moderately populated cities (P=.019). The same trend was observed for online appointments in densely populated (P=.0023) and moderately populated cities (P=.037). Private practices continued to list product availability more often than academic institutions regardless of population density or population age. Academic institutions also continued to list research trials more often than private practices regardless of population density or population age.
Comment
Our study uniquely analyzed the differences in website content between private practices and academic institutions in dermatology. Of the 140 academic institutions accredited by the Accreditation Council for Graduate Medical Education (ACGME), only 113 had patient-pertinent websites.
Access to Websites —There was a significant difference in many website content criteria between the 2 groups. Private practice sites were easier to access via a Google search when compared with academic sites, which likely is influenced by the Google search algorithm that ranks websites higher based on several criteria including but not limited to keyword use in the title tag, link popularity of the site, and historic ranking. 12,13 Academic sites often were only accessible through portals found on their main institutional site or institution’s residency site.
Role of Social Media —Social media has been found to assist in educating patients on medical practices as well as selecting a physician. 14,15 Our study found that private practice websites listed links to social media more often than their academic counterparts. Social media consumption is increasing, in part due to the COVID-19 pandemic, and it may be optimal for patients and practices alike to include links on their websites. 16 Facebook and Instagram were listed more often on private practice sites when compared with academic institution sites, which was similar to a recent study analyzing the websites of plastic surgery private practices (N = 310) in which 90% of private practices included some type of social media, with Instagram and Facebook being the most used. 8 Social networking accounts can act as convenient platforms for marketing, providing patient education, and generating referrals, which suggests that the prominence of their usage in private practice poses benefits in patient decision-making when seeking care. 17-19 A study analyzing the impact of Facebook in medicine concluded that a Facebook page can serve as an effective vehicle for medical education, particularly in younger generations that favor technology-oriented teaching methods. 20 A survey on trends in cosmetic facial procedures in plastic surgery found that the most influential online methods patients used for choosing their providers were social media platforms and practice websites. Front-page placement on Google also was commonly associated with the number of social media followers. 21,22 A lack of social media prominence could hinder a website’s potential to reach patients.
Communication With Practices —Our study also found significant differences in other metrics related to a patient’s ability to directly communicate with a practice, such as physical addresses, telephone numbers, products available for direct purchase, and online appointment booking, all of which were listed more often on private practice websites compared with academic institution websites. Online appointment booking also was found more frequently on private practice websites. Although physical addresses and telephone numbers were listed significantly more often on private practice sites, this information was ubiquitous and easily accessible elsewhere. Academic institution websites listed research trials and fellowship training significantly more often than private practices. These differences imply a divergence in focus between private practices and academic institutions, likely because academic institutions are funded in large part from research grants, begetting a cycle of academic contribution. 23 In contrast, private practices may not rely as heavily on academic revenue and may be more likely to prioritize other revenue streams such as product sales. 24
HIPAA Policy —Surprisingly, HIPAA policy rarely was listed on any private (22%) or academic site (12%). Conversely, in the plastic surgery study, HIPAA policy was listed much more often, with more than half of private practices with board-certified plastic surgeons accredited in the year 2015 including it on their website, 8 which may suggest that surgically oriented specialties, particularly cosmetic subspecialties, aim to more noticeably display their privacy policies for patient reassurance.
Study Limitations —There are several limitations of our study. First, it is common for a conglomerate company to own multiple private practices in different specialties. As with academic sites, private practice sites may be limited by the hosting platforms, which often are tedious to navigate. Also noteworthy is the emergence of designated social media management positions—both by practice employees and by third-party firms 25 —but the impact of these positions in private practices and academic institutions has not been fully explored. Finally, inclusion criteria and standardized criteria definitions were chosen based on the precedent established by the authors of similar analyses in plastic surgery and radiology. 5-8 Further investigation into the most valued aspects of care by patients within the context of the type of practice chosen would be valuable in refining inclusion criteria. Additionally, this study did not stratify the data collected based on factors such as gender, race, and geographical location; studies conducted on website traffic analysis patterns that focus on these aspects likely would further explain the significance of these findings. Differences in the length of time to the next available appointment between private practices and academic institutions also may help support our findings. Finally, there is a need for further investigation into the preferences of patients themselves garnered from website traffic alone.
Conclusion
Our study examined a diverse compilation of private practice and academic institution websites and uncovered numerous differences in content. As technology and health care continuously evolve, it is imperative that both private practices and academic institutions are actively adapting to optimize their online presence. In doing so, patients will be better equipped at accessing provider information, gaining familiarity with the practice, and understanding treatment options.
- Gentry ZL, Ananthasekar S, Yeatts M, et al. Can patients find an endocrine surgeon? how hospital websites hide the expertise of these medical professionals. Am J Surg . 2021;221:101-105.
- Pollack CE, Rastegar A, Keating NL, et al. Is self-referral associated with higher quality care? Health Serv Res . 2015;50:1472-1490.
- Association of American Medical Colleges. Residency Explorer TM tool. Accessed May 15, 2023. https://students-residents.aamc.org/apply-smart-residency/residency-explorer-tool
- Find a dermatologist. American Academy of Dermatology website. Accessed May 15, 2023. https://find-a-derm.aad.org/
- Johnson EJ, Doshi AM, Rosenkrantz AB. Strengths and deficiencies in the content of US radiology private practices’ websites. J Am Coll Radiol. 2017;14:431-435.
- Brunk D. Medical website expert shares design tips. Dermatology News . February 9, 2012. Accessed May 15, 2023. https://www.mdedge.com/dermatology/article/47413/health-policy/medical-website-expert-shares-design-tips
- Kuhnigk O, Ramuschkat M, Schreiner J, et al. Internet presence of neurologists, psychiatrists and medical psychotherapists in private practice [in German]. Psychiatr Prax . 2013;41:142-147.
- Ananthasekar S, Patel JJ, Patel NJ, et al. The content of US plastic surgery private practices’ websites. Ann Plast Surg . 2021;86(6S suppl 5):S578-S584.
- US Census Bureau. Age and Sex: 2021. Updated December 2, 2021. Accessed March 15, 2023. https://www.census.gov/topics/population/age-and-sex/data/tables.2021.List_897222059.html#list-tab-List_897222059
- Porter ME. The competitive advantage of the inner city. Harvard Business Review . Published August 1, 2014. https://hbr.org/1995/05/the-competitive-advantage-of-the-inner-city
- Clark PG. The social allocation of health care resources: ethical dilemmas in age-group competition. Gerontologist. 1985;25:119-125.
- Su A-J, Hu YC, Kuzmanovic A, et al. How to improve your Google ranking: myths and reality. ACM Transactions on the Web . 2014;8. https://dl.acm.org/doi/abs/10.1145/2579990
- McCormick K. 39 ways to increase traffic to your website. WordStream website. Published March 28, 2023. Accessed May 22, 2023. https://www.wordstream.com/blog/ws/2014/08/14/increase-traffic-to-my-website
- Montemurro P, Porcnik A, Hedén P, et al. The influence of social media and easily accessible online information on the aesthetic plastic surgery practice: literature review and our own experience. Aesthetic Plast Surg . 2015;39:270-277.
- Steehler KR, Steehler MK, Pierce ML, et al. Social media’s role in otolaryngology–head and neck surgery. Otolaryngol Head Neck Surg . 2013;149:521-524.
- Tsao S-F, Chen H, Tisseverasinghe T, et al. What social media told us in the time of COVID-19: a scoping review. Lancet Digit Health . 2021;3:E175-E194.
- Geist R, Militello M, Albrecht JM, et al. Social media and clinical research in dermatology. Curr Dermatol Rep . 2021;10:105-111.
- McLawhorn AS, De Martino I, Fehring KA, et al. Social media and your practice: navigating the surgeon-patient relationship. Curr Rev Musculoskelet Med . 2016;9:487-495.
- Thomas RB, Johnson PT, Fishman EK. Social media for global education: pearls and pitfalls of using Facebook, Twitter, and Instagram. J Am Coll Radiol . 2018;15:1513-1516.
- Lugo-Fagundo C, Johnson MB, Thomas RB, et al. New frontiers in education: Facebook as a vehicle for medical information delivery. J Am Coll Radiol . 2016;13:316-319.
- Ho T-VT, Dayan SH. How to leverage social media in private practice. Facial Plast Surg Clin North Am . 2020;28:515-522.
- Fan KL, Graziano F, Economides JM, et al. The public’s preferences on plastic surgery social media engagement and professionalism. Plast Reconstr Surg . 2019;143:619-630.
- Jacob BA, Lefgren L. The impact of research grant funding on scientific productivity. J Public Econ. 2011;95:1168-1177.
- Baumann L. Ethics in cosmetic dermatology. Clin Dermatol. 2012;30:522-527.
- Miller AR, Tucker C. Active social media management: the case of health care. Info Sys Res . 2013;24:52-70.
- Gentry ZL, Ananthasekar S, Yeatts M, et al. Can patients find an endocrine surgeon? how hospital websites hide the expertise of these medical professionals. Am J Surg . 2021;221:101-105.
- Pollack CE, Rastegar A, Keating NL, et al. Is self-referral associated with higher quality care? Health Serv Res . 2015;50:1472-1490.
- Association of American Medical Colleges. Residency Explorer TM tool. Accessed May 15, 2023. https://students-residents.aamc.org/apply-smart-residency/residency-explorer-tool
- Find a dermatologist. American Academy of Dermatology website. Accessed May 15, 2023. https://find-a-derm.aad.org/
- Johnson EJ, Doshi AM, Rosenkrantz AB. Strengths and deficiencies in the content of US radiology private practices’ websites. J Am Coll Radiol. 2017;14:431-435.
- Brunk D. Medical website expert shares design tips. Dermatology News . February 9, 2012. Accessed May 15, 2023. https://www.mdedge.com/dermatology/article/47413/health-policy/medical-website-expert-shares-design-tips
- Kuhnigk O, Ramuschkat M, Schreiner J, et al. Internet presence of neurologists, psychiatrists and medical psychotherapists in private practice [in German]. Psychiatr Prax . 2013;41:142-147.
- Ananthasekar S, Patel JJ, Patel NJ, et al. The content of US plastic surgery private practices’ websites. Ann Plast Surg . 2021;86(6S suppl 5):S578-S584.
- US Census Bureau. Age and Sex: 2021. Updated December 2, 2021. Accessed March 15, 2023. https://www.census.gov/topics/population/age-and-sex/data/tables.2021.List_897222059.html#list-tab-List_897222059
- Porter ME. The competitive advantage of the inner city. Harvard Business Review . Published August 1, 2014. https://hbr.org/1995/05/the-competitive-advantage-of-the-inner-city
- Clark PG. The social allocation of health care resources: ethical dilemmas in age-group competition. Gerontologist. 1985;25:119-125.
- Su A-J, Hu YC, Kuzmanovic A, et al. How to improve your Google ranking: myths and reality. ACM Transactions on the Web . 2014;8. https://dl.acm.org/doi/abs/10.1145/2579990
- McCormick K. 39 ways to increase traffic to your website. WordStream website. Published March 28, 2023. Accessed May 22, 2023. https://www.wordstream.com/blog/ws/2014/08/14/increase-traffic-to-my-website
- Montemurro P, Porcnik A, Hedén P, et al. The influence of social media and easily accessible online information on the aesthetic plastic surgery practice: literature review and our own experience. Aesthetic Plast Surg . 2015;39:270-277.
- Steehler KR, Steehler MK, Pierce ML, et al. Social media’s role in otolaryngology–head and neck surgery. Otolaryngol Head Neck Surg . 2013;149:521-524.
- Tsao S-F, Chen H, Tisseverasinghe T, et al. What social media told us in the time of COVID-19: a scoping review. Lancet Digit Health . 2021;3:E175-E194.
- Geist R, Militello M, Albrecht JM, et al. Social media and clinical research in dermatology. Curr Dermatol Rep . 2021;10:105-111.
- McLawhorn AS, De Martino I, Fehring KA, et al. Social media and your practice: navigating the surgeon-patient relationship. Curr Rev Musculoskelet Med . 2016;9:487-495.
- Thomas RB, Johnson PT, Fishman EK. Social media for global education: pearls and pitfalls of using Facebook, Twitter, and Instagram. J Am Coll Radiol . 2018;15:1513-1516.
- Lugo-Fagundo C, Johnson MB, Thomas RB, et al. New frontiers in education: Facebook as a vehicle for medical information delivery. J Am Coll Radiol . 2016;13:316-319.
- Ho T-VT, Dayan SH. How to leverage social media in private practice. Facial Plast Surg Clin North Am . 2020;28:515-522.
- Fan KL, Graziano F, Economides JM, et al. The public’s preferences on plastic surgery social media engagement and professionalism. Plast Reconstr Surg . 2019;143:619-630.
- Jacob BA, Lefgren L. The impact of research grant funding on scientific productivity. J Public Econ. 2011;95:1168-1177.
- Baumann L. Ethics in cosmetic dermatology. Clin Dermatol. 2012;30:522-527.
- Miller AR, Tucker C. Active social media management: the case of health care. Info Sys Res . 2013;24:52-70.
Practice Points
- Dermatologists at both private practices and academic institutions should understand that website content often may be the most accessible source of information about the practice available to patients and should be as specific and detailed as possible.
- When compared to private practices, academic institutions largely fail to have a social media presence, which may limit patient interaction with their websites.
Omitting radiation in rectal cancer: ‘Less is more’
“This study establishes preoperative therapy with FOLFOX and only selective use of chemoradiation for patients with locally advanced rectal cancer,” commented principal investigator Deborah Schrag, MD, MPH, gastrointestinal oncologist at Memorial Sloan Kettering Cancer Center, New York.
“Having this option is important for several reasons,” she said. “First, in many parts of the world, radiation therapy is not readily accessible. An all-chemotherapy approach may make curative intent treatment accessible for patients in these resource-constrained settings. Additionally, given the rising rates of colorectal cancer in young patients, this provides an option for patients who wish to preserve fertility or avoid early menopause.”
Reacting to the findings, Pamela Kunz, MD, leader of the gastrointestinal cancers program at Yale Cancer Center, New Haven, Conn., commented: “What’s important here is that radiation can be safely omitted in many patients with clinically advanced rectal cancer – this is really ‘less is more.’ ”
“We can spare select patients from receiving radiation without compromising efficacy,” she said. “This leads to improved quality of life, and reduced side effects, including things like early menopause and infertility.”
Dr. Kunz spoke at a press briefing where the results were highlighted prior to being presented at a plenary session at the annual meeting of the American Society of Clinical Oncology.
She said the trial was “practice changing, and it aligns incredibly well with the theme at this year’s annual meeting around de-escalation of therapy and partnering with patients.”
Julie R. Gralow, MD, ASCO chief medical officer and executive vice president, added that the theme may be de-escalation, but in this case it is “really more accurately optimization, because you were able to de-escalate in 91%, but you found the 9% who really needed that escalation, if you will.”
The efficacy results were published simultaneously in the New England Journal of Medicine, while the patient-reported outcomes were published in the Journal of Clinical Oncology.
Chemoradiation is standard approach
Presenting the findings, Dr. Schrag began by noting that approximately half of all new rectal cancer diagnoses have locally advanced disease, which in the United States represents 48,000 cases per year.
She explained that the “standard approach” to treatment is 5½ weeks of daily chemoradiation, comprising radiation to the pelvis alongside sensitizing chemotherapy with a fluoropyrimidine, followed by surgery, and then approximately 16 weeks of adjuvant chemotherapy.
“This way, we have achieved very good outcomes,” Dr. Schrag noted, with the inclusion of radiation in the 1980s a “critically important advance” in slowing rates of recurrence, which is a “cause of enormous suffering.”
However, the treatment is “long and hard,” and the pelvic radiation causes “real toxicities,” she said. These can include impaired bowel, bladder, and sexual function, and an increase in late effects, such as increased risk of pelvic fractures and secondary cancers, as well as infertility and early menopause, and impaired bone marrow function.
This depressed bone marrow function can “become a problem for people going into chemotherapy into the future,” while infertility and early menopause are “a big deal because we’re seeing increasing diagnosis of rectal cancer in people before the age of 50 years.”
Dr. Schrag said that, since chemoradiotherapy became the standard of care, there has been “so much progress,” with better chemotherapy, better surgical techniques, and more screening, so we’re finding more tumors when they are smaller and easier to treat.”
The impetus for the PROSPECT trial, Dr. Schrag said, was therefore: “Could we use radiotherapy more selectively, and only give it to people who don’t respond to chemotherapy rather than giving [it] to everybody?”
The trial enrolled 1,128 patients with rectal cancer with clinically staged T2 node–positive, T3 node–negative, or T3 node–positive disease and who were candidates for sphincter-sparing surgery.
They were randomly assigned to receive either a modified chemotherapy regimen (n = 585) or standard chemoradiation (n = 543). The mean age of the patients was 57 years; 34.5% were female. The majority (85%) were White.
All patients then underwent surgery, with low anterior resection with total mesorectal excision.
The standard chemoradiotherapy consisted of pelvic radiotherapy at 50.4 Gy over 28 fractions alongside sensitizing chemotherapy with either fluorouracil or capecitabine.
The modified chemotherapy regimen consisted of mFOLFOX6, which included modified oxaliplatin with l-leucovorin, and bolus/continuous infusion of 5-fluorouracil.
Patients in the mFOLFOX6 group whose primary tumor decreased in size by at least 20% after the six cycles proceeded straight to surgery, while were rest given the chemoradiotherapy before surgery (9% ended up receiving chemoradiotherapy).
Postoperative adjuvant chemotherapy with six cycles in the mFOLFOX6 group, or eight cycles in the chemoradiotherapy group, was suggested but not mandated.
Dr. Schrag said in an interview that the team chose to use mFOLFOX6 because of their long experience in using it.
FOLFOX first became available in 2002, and randomized controlled trials showed that it was more effective than 5-fluorouracil alone in colon cancer trials.
She explained that locally advanced rectal cancer patients were “never included” in those trials because they were already receiving chemoradiotherapy, but that its use in metastatic rectal cancer, coupled with the data in colon cancer, led them to try it in the current setting.
“It’s also quite tolerable, it’s given every 2 weeks, and it’s familiar to oncologists everywhere,” Dr. Schrag added.
She noted that it’s “not risk free,” with an increased risk of neuropathy, “which is not a great side effect, but we’re able to modulate that.”
Trial results
After a median follow-up of 58 months, mFOLFOX6 was found to be noninferior to chemoradiotherapy for disease-free survival, at a hazard ratio for disease recurrence or death of 0.92 (P = .005 for noninferiority).
The 5-year disease-free survival was 80.8% in the mFOLFOX6 group and 78.6% among patients assigned to chemoradiotherapy, while 5-year overall survival was 89.5% versus 90.2%, at a nonsignificant HR for death of 1.04.
Rates of local recurrence at 5 years were low, at 1.8% with mFOLFOX6 and 1.6% with chemoradiotherapy.
Grade 3 or higher adverse effects were twice as common in the mFOLFOX6 group than among patients who received chemoradiotherapy, at 41% versus 22.8%, although the researchers noted that the treatment period was also twice as long in the chemotherapy arm.
The most common grade 3 or higher adverse effects with mFOLFOX6 were neutropenia (20.3%), pain (3.1%), and hypertension (2.9%), while those with chemoradiotherapy were lymphopenia (8.3%), diarrhea (6.4%), and hypertension (1.7%).
In terms of patient reported outcomes, patients receiving mFOLFOX6 reported lower rates of diarrhea and better overall bowel function during the neoadjuvant phase than those given chemoradiotherapy (P < .05 for all).
However, those assigned to chemoradiotherapy experienced lower rates of anxiety, appetite loss, constipation, depression, dysphagia, dyspnea, edema, fatigue, mucositis, nausea, neuropathy, and vomiting during treatment (P < .05 for all).
At 12-month postoperative follow-up, however, those differences had disappeared, and patients originally assigned to mFOLFOX6 had lower rates of fatigue and neuropathy, and better sexual function, than those given chemoradiotherapy (P < .05 for all).
“During the treatment itself, multiple symptoms were worse with chemotherapy, but a year after treatment ended, those symptoms resolved and the pattern flipped so that patients who received radiation exhibited lingering symptoms,” said coinvestigator Ethan Basch, MD, MSc, chief of medical oncology, and director of the cancer outcomes research program at University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill.
“Patients ideally will understand the potential impact of treatments on how they feel and function when making choices, so as oncologists we need to talk with our patients about their options and the consequences of those options,” he said in a statement.
The study was funded by the National Cancer Institute of the National Institutes of Health. Dr. Schrag reported relationships with Merck, JAMA, the American Association for Cancer Research, and Grail. Dr. Basch reported relationships with AstraZeneca, Navigating Cancer, Resilience Care, SIVAN Innovation, ASCO, Centers for Medicare & Medicaid Services, JAMA, the National Cancer Institute, and the Patient-Centered Outcomes Research Institute. Dr. Kunz reported relationships with Novartis, Genentech/Roche, Amgen, Crinetics Pharmaceuticals, Natera, HUTCHMED, and Isotope Technologies Munich. Dr. Gralow reported relationships with Genentech/Roche.
A version of this article first appeared on Medscape.com.
“This study establishes preoperative therapy with FOLFOX and only selective use of chemoradiation for patients with locally advanced rectal cancer,” commented principal investigator Deborah Schrag, MD, MPH, gastrointestinal oncologist at Memorial Sloan Kettering Cancer Center, New York.
“Having this option is important for several reasons,” she said. “First, in many parts of the world, radiation therapy is not readily accessible. An all-chemotherapy approach may make curative intent treatment accessible for patients in these resource-constrained settings. Additionally, given the rising rates of colorectal cancer in young patients, this provides an option for patients who wish to preserve fertility or avoid early menopause.”
Reacting to the findings, Pamela Kunz, MD, leader of the gastrointestinal cancers program at Yale Cancer Center, New Haven, Conn., commented: “What’s important here is that radiation can be safely omitted in many patients with clinically advanced rectal cancer – this is really ‘less is more.’ ”
“We can spare select patients from receiving radiation without compromising efficacy,” she said. “This leads to improved quality of life, and reduced side effects, including things like early menopause and infertility.”
Dr. Kunz spoke at a press briefing where the results were highlighted prior to being presented at a plenary session at the annual meeting of the American Society of Clinical Oncology.
She said the trial was “practice changing, and it aligns incredibly well with the theme at this year’s annual meeting around de-escalation of therapy and partnering with patients.”
Julie R. Gralow, MD, ASCO chief medical officer and executive vice president, added that the theme may be de-escalation, but in this case it is “really more accurately optimization, because you were able to de-escalate in 91%, but you found the 9% who really needed that escalation, if you will.”
The efficacy results were published simultaneously in the New England Journal of Medicine, while the patient-reported outcomes were published in the Journal of Clinical Oncology.
Chemoradiation is standard approach
Presenting the findings, Dr. Schrag began by noting that approximately half of all new rectal cancer diagnoses have locally advanced disease, which in the United States represents 48,000 cases per year.
She explained that the “standard approach” to treatment is 5½ weeks of daily chemoradiation, comprising radiation to the pelvis alongside sensitizing chemotherapy with a fluoropyrimidine, followed by surgery, and then approximately 16 weeks of adjuvant chemotherapy.
“This way, we have achieved very good outcomes,” Dr. Schrag noted, with the inclusion of radiation in the 1980s a “critically important advance” in slowing rates of recurrence, which is a “cause of enormous suffering.”
However, the treatment is “long and hard,” and the pelvic radiation causes “real toxicities,” she said. These can include impaired bowel, bladder, and sexual function, and an increase in late effects, such as increased risk of pelvic fractures and secondary cancers, as well as infertility and early menopause, and impaired bone marrow function.
This depressed bone marrow function can “become a problem for people going into chemotherapy into the future,” while infertility and early menopause are “a big deal because we’re seeing increasing diagnosis of rectal cancer in people before the age of 50 years.”
Dr. Schrag said that, since chemoradiotherapy became the standard of care, there has been “so much progress,” with better chemotherapy, better surgical techniques, and more screening, so we’re finding more tumors when they are smaller and easier to treat.”
The impetus for the PROSPECT trial, Dr. Schrag said, was therefore: “Could we use radiotherapy more selectively, and only give it to people who don’t respond to chemotherapy rather than giving [it] to everybody?”
The trial enrolled 1,128 patients with rectal cancer with clinically staged T2 node–positive, T3 node–negative, or T3 node–positive disease and who were candidates for sphincter-sparing surgery.
They were randomly assigned to receive either a modified chemotherapy regimen (n = 585) or standard chemoradiation (n = 543). The mean age of the patients was 57 years; 34.5% were female. The majority (85%) were White.
All patients then underwent surgery, with low anterior resection with total mesorectal excision.
The standard chemoradiotherapy consisted of pelvic radiotherapy at 50.4 Gy over 28 fractions alongside sensitizing chemotherapy with either fluorouracil or capecitabine.
The modified chemotherapy regimen consisted of mFOLFOX6, which included modified oxaliplatin with l-leucovorin, and bolus/continuous infusion of 5-fluorouracil.
Patients in the mFOLFOX6 group whose primary tumor decreased in size by at least 20% after the six cycles proceeded straight to surgery, while were rest given the chemoradiotherapy before surgery (9% ended up receiving chemoradiotherapy).
Postoperative adjuvant chemotherapy with six cycles in the mFOLFOX6 group, or eight cycles in the chemoradiotherapy group, was suggested but not mandated.
Dr. Schrag said in an interview that the team chose to use mFOLFOX6 because of their long experience in using it.
FOLFOX first became available in 2002, and randomized controlled trials showed that it was more effective than 5-fluorouracil alone in colon cancer trials.
She explained that locally advanced rectal cancer patients were “never included” in those trials because they were already receiving chemoradiotherapy, but that its use in metastatic rectal cancer, coupled with the data in colon cancer, led them to try it in the current setting.
“It’s also quite tolerable, it’s given every 2 weeks, and it’s familiar to oncologists everywhere,” Dr. Schrag added.
She noted that it’s “not risk free,” with an increased risk of neuropathy, “which is not a great side effect, but we’re able to modulate that.”
Trial results
After a median follow-up of 58 months, mFOLFOX6 was found to be noninferior to chemoradiotherapy for disease-free survival, at a hazard ratio for disease recurrence or death of 0.92 (P = .005 for noninferiority).
The 5-year disease-free survival was 80.8% in the mFOLFOX6 group and 78.6% among patients assigned to chemoradiotherapy, while 5-year overall survival was 89.5% versus 90.2%, at a nonsignificant HR for death of 1.04.
Rates of local recurrence at 5 years were low, at 1.8% with mFOLFOX6 and 1.6% with chemoradiotherapy.
Grade 3 or higher adverse effects were twice as common in the mFOLFOX6 group than among patients who received chemoradiotherapy, at 41% versus 22.8%, although the researchers noted that the treatment period was also twice as long in the chemotherapy arm.
The most common grade 3 or higher adverse effects with mFOLFOX6 were neutropenia (20.3%), pain (3.1%), and hypertension (2.9%), while those with chemoradiotherapy were lymphopenia (8.3%), diarrhea (6.4%), and hypertension (1.7%).
In terms of patient reported outcomes, patients receiving mFOLFOX6 reported lower rates of diarrhea and better overall bowel function during the neoadjuvant phase than those given chemoradiotherapy (P < .05 for all).
However, those assigned to chemoradiotherapy experienced lower rates of anxiety, appetite loss, constipation, depression, dysphagia, dyspnea, edema, fatigue, mucositis, nausea, neuropathy, and vomiting during treatment (P < .05 for all).
At 12-month postoperative follow-up, however, those differences had disappeared, and patients originally assigned to mFOLFOX6 had lower rates of fatigue and neuropathy, and better sexual function, than those given chemoradiotherapy (P < .05 for all).
“During the treatment itself, multiple symptoms were worse with chemotherapy, but a year after treatment ended, those symptoms resolved and the pattern flipped so that patients who received radiation exhibited lingering symptoms,” said coinvestigator Ethan Basch, MD, MSc, chief of medical oncology, and director of the cancer outcomes research program at University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill.
“Patients ideally will understand the potential impact of treatments on how they feel and function when making choices, so as oncologists we need to talk with our patients about their options and the consequences of those options,” he said in a statement.
The study was funded by the National Cancer Institute of the National Institutes of Health. Dr. Schrag reported relationships with Merck, JAMA, the American Association for Cancer Research, and Grail. Dr. Basch reported relationships with AstraZeneca, Navigating Cancer, Resilience Care, SIVAN Innovation, ASCO, Centers for Medicare & Medicaid Services, JAMA, the National Cancer Institute, and the Patient-Centered Outcomes Research Institute. Dr. Kunz reported relationships with Novartis, Genentech/Roche, Amgen, Crinetics Pharmaceuticals, Natera, HUTCHMED, and Isotope Technologies Munich. Dr. Gralow reported relationships with Genentech/Roche.
A version of this article first appeared on Medscape.com.
“This study establishes preoperative therapy with FOLFOX and only selective use of chemoradiation for patients with locally advanced rectal cancer,” commented principal investigator Deborah Schrag, MD, MPH, gastrointestinal oncologist at Memorial Sloan Kettering Cancer Center, New York.
“Having this option is important for several reasons,” she said. “First, in many parts of the world, radiation therapy is not readily accessible. An all-chemotherapy approach may make curative intent treatment accessible for patients in these resource-constrained settings. Additionally, given the rising rates of colorectal cancer in young patients, this provides an option for patients who wish to preserve fertility or avoid early menopause.”
Reacting to the findings, Pamela Kunz, MD, leader of the gastrointestinal cancers program at Yale Cancer Center, New Haven, Conn., commented: “What’s important here is that radiation can be safely omitted in many patients with clinically advanced rectal cancer – this is really ‘less is more.’ ”
“We can spare select patients from receiving radiation without compromising efficacy,” she said. “This leads to improved quality of life, and reduced side effects, including things like early menopause and infertility.”
Dr. Kunz spoke at a press briefing where the results were highlighted prior to being presented at a plenary session at the annual meeting of the American Society of Clinical Oncology.
She said the trial was “practice changing, and it aligns incredibly well with the theme at this year’s annual meeting around de-escalation of therapy and partnering with patients.”
Julie R. Gralow, MD, ASCO chief medical officer and executive vice president, added that the theme may be de-escalation, but in this case it is “really more accurately optimization, because you were able to de-escalate in 91%, but you found the 9% who really needed that escalation, if you will.”
The efficacy results were published simultaneously in the New England Journal of Medicine, while the patient-reported outcomes were published in the Journal of Clinical Oncology.
Chemoradiation is standard approach
Presenting the findings, Dr. Schrag began by noting that approximately half of all new rectal cancer diagnoses have locally advanced disease, which in the United States represents 48,000 cases per year.
She explained that the “standard approach” to treatment is 5½ weeks of daily chemoradiation, comprising radiation to the pelvis alongside sensitizing chemotherapy with a fluoropyrimidine, followed by surgery, and then approximately 16 weeks of adjuvant chemotherapy.
“This way, we have achieved very good outcomes,” Dr. Schrag noted, with the inclusion of radiation in the 1980s a “critically important advance” in slowing rates of recurrence, which is a “cause of enormous suffering.”
However, the treatment is “long and hard,” and the pelvic radiation causes “real toxicities,” she said. These can include impaired bowel, bladder, and sexual function, and an increase in late effects, such as increased risk of pelvic fractures and secondary cancers, as well as infertility and early menopause, and impaired bone marrow function.
This depressed bone marrow function can “become a problem for people going into chemotherapy into the future,” while infertility and early menopause are “a big deal because we’re seeing increasing diagnosis of rectal cancer in people before the age of 50 years.”
Dr. Schrag said that, since chemoradiotherapy became the standard of care, there has been “so much progress,” with better chemotherapy, better surgical techniques, and more screening, so we’re finding more tumors when they are smaller and easier to treat.”
The impetus for the PROSPECT trial, Dr. Schrag said, was therefore: “Could we use radiotherapy more selectively, and only give it to people who don’t respond to chemotherapy rather than giving [it] to everybody?”
The trial enrolled 1,128 patients with rectal cancer with clinically staged T2 node–positive, T3 node–negative, or T3 node–positive disease and who were candidates for sphincter-sparing surgery.
They were randomly assigned to receive either a modified chemotherapy regimen (n = 585) or standard chemoradiation (n = 543). The mean age of the patients was 57 years; 34.5% were female. The majority (85%) were White.
All patients then underwent surgery, with low anterior resection with total mesorectal excision.
The standard chemoradiotherapy consisted of pelvic radiotherapy at 50.4 Gy over 28 fractions alongside sensitizing chemotherapy with either fluorouracil or capecitabine.
The modified chemotherapy regimen consisted of mFOLFOX6, which included modified oxaliplatin with l-leucovorin, and bolus/continuous infusion of 5-fluorouracil.
Patients in the mFOLFOX6 group whose primary tumor decreased in size by at least 20% after the six cycles proceeded straight to surgery, while were rest given the chemoradiotherapy before surgery (9% ended up receiving chemoradiotherapy).
Postoperative adjuvant chemotherapy with six cycles in the mFOLFOX6 group, or eight cycles in the chemoradiotherapy group, was suggested but not mandated.
Dr. Schrag said in an interview that the team chose to use mFOLFOX6 because of their long experience in using it.
FOLFOX first became available in 2002, and randomized controlled trials showed that it was more effective than 5-fluorouracil alone in colon cancer trials.
She explained that locally advanced rectal cancer patients were “never included” in those trials because they were already receiving chemoradiotherapy, but that its use in metastatic rectal cancer, coupled with the data in colon cancer, led them to try it in the current setting.
“It’s also quite tolerable, it’s given every 2 weeks, and it’s familiar to oncologists everywhere,” Dr. Schrag added.
She noted that it’s “not risk free,” with an increased risk of neuropathy, “which is not a great side effect, but we’re able to modulate that.”
Trial results
After a median follow-up of 58 months, mFOLFOX6 was found to be noninferior to chemoradiotherapy for disease-free survival, at a hazard ratio for disease recurrence or death of 0.92 (P = .005 for noninferiority).
The 5-year disease-free survival was 80.8% in the mFOLFOX6 group and 78.6% among patients assigned to chemoradiotherapy, while 5-year overall survival was 89.5% versus 90.2%, at a nonsignificant HR for death of 1.04.
Rates of local recurrence at 5 years were low, at 1.8% with mFOLFOX6 and 1.6% with chemoradiotherapy.
Grade 3 or higher adverse effects were twice as common in the mFOLFOX6 group than among patients who received chemoradiotherapy, at 41% versus 22.8%, although the researchers noted that the treatment period was also twice as long in the chemotherapy arm.
The most common grade 3 or higher adverse effects with mFOLFOX6 were neutropenia (20.3%), pain (3.1%), and hypertension (2.9%), while those with chemoradiotherapy were lymphopenia (8.3%), diarrhea (6.4%), and hypertension (1.7%).
In terms of patient reported outcomes, patients receiving mFOLFOX6 reported lower rates of diarrhea and better overall bowel function during the neoadjuvant phase than those given chemoradiotherapy (P < .05 for all).
However, those assigned to chemoradiotherapy experienced lower rates of anxiety, appetite loss, constipation, depression, dysphagia, dyspnea, edema, fatigue, mucositis, nausea, neuropathy, and vomiting during treatment (P < .05 for all).
At 12-month postoperative follow-up, however, those differences had disappeared, and patients originally assigned to mFOLFOX6 had lower rates of fatigue and neuropathy, and better sexual function, than those given chemoradiotherapy (P < .05 for all).
“During the treatment itself, multiple symptoms were worse with chemotherapy, but a year after treatment ended, those symptoms resolved and the pattern flipped so that patients who received radiation exhibited lingering symptoms,” said coinvestigator Ethan Basch, MD, MSc, chief of medical oncology, and director of the cancer outcomes research program at University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill.
“Patients ideally will understand the potential impact of treatments on how they feel and function when making choices, so as oncologists we need to talk with our patients about their options and the consequences of those options,” he said in a statement.
The study was funded by the National Cancer Institute of the National Institutes of Health. Dr. Schrag reported relationships with Merck, JAMA, the American Association for Cancer Research, and Grail. Dr. Basch reported relationships with AstraZeneca, Navigating Cancer, Resilience Care, SIVAN Innovation, ASCO, Centers for Medicare & Medicaid Services, JAMA, the National Cancer Institute, and the Patient-Centered Outcomes Research Institute. Dr. Kunz reported relationships with Novartis, Genentech/Roche, Amgen, Crinetics Pharmaceuticals, Natera, HUTCHMED, and Isotope Technologies Munich. Dr. Gralow reported relationships with Genentech/Roche.
A version of this article first appeared on Medscape.com.
AT ASCO 2023
Medicaid expansion closing racial gap in GI cancer deaths
Across the United States, minority patients with cancer often have worse outcomes than White patients, with Black patients more likely to die sooner.
But new data suggest that these racial disparities are lessening. They come from a cross-sectional cohort study of patients with gastrointestinal cancers and show that the gap in mortality rates was reduced in Medicaid expansion states, compared with nonexpansion states.
The results were particularly notable for Black patients, for whom there was a consistent increase in receiving therapy (chemotherapy or surgery) and a decrease in mortality from stomach, colorectal, and pancreatic cancer, the investigators commented.
The study was highlighted at a press briefing held in advance of the annual meeting of the American Society of Clinical Oncology.
“The findings of this study provide a solid step for closing the gap, showing that the Medicaid expansion opportunity offered by the Affordable Care Act, which allows participating states to improve health care access for disadvantaged populations, results in better cancer outcomes and mitigation of racial disparities in cancer survival,” commented Julie Gralow, MD, chief medical officer and executive vice president of ASCO.
The study included 86,052 patients from the National Cancer Database who, from 2009 to 2019, were diagnosed with pancreatic cancer, colorectal cancer, or stomach cancer. Just over 22,000 patients (25.7%) were Black; the remainder 63,943 (74.3%) were White.
In Medicaid expansion states, there was a greater absolute reduction in 2-year mortality among Black patients with pancreatic cancer of –11.8%, compared with nonexpansion states, at –2.4%, a difference-in-difference (DID) of –9.4%. Additionally, there was an increase in treatment with chemotherapy for patients with stage III-IV pancreatic cancer (4.5% for Black patients and 3.2% for White), compared with patients in nonexpansion states (0.8% for Black patients and 0.4% for White; DID, 3.7% for Black patients and DID, 2.7% for White).
“We found similar results in colorectal cancer, but this effect is primarily observed among the stage IV patients,” commented lead author Naveen Manisundaram, MD, a research fellow at the University of Texas MD Anderson Cancer Center, Houston. “Black patients with advanced stage disease experienced a 12.6% reduction in mortality in expansion states.”
Among Black patients with stage IV colorectal cancer, there was an increase in rates of surgery in expansion states, compared with nonexpansion states (DID, 5.7%). However, there was no increase in treatment with chemotherapy (DID, 1%; P = .66).
Mortality rates for Black patients with stomach cancer also decreased. In expansion states, there was a –13% absolute decrease in mortality, compared with a –5.2% decrease in nonexpansion states.
The investigators noted that Medicaid coverage was a key component in access to care through the Affordable Care Act. About two-thirds (66.7%) of Black patients had Medicaid; 33.3% were uninsured. Coverage was similar among White patients; 64.1% had Medicaid and 35.9% were uninsured.
“Our study provides compelling data that show Medicaid expansion was associated with improvement in survival for both Black and White patients with gastrointestinal cancers. Additionally, it suggests that Medicaid expansion is one potential avenue to mitigate existing racial survival disparities among these patients,” Dr. Manisundaram concluded.
The study was funded by the National Institutes of Health. One coauthor reported an advisory role with Medicaroid. Dr. Gralow has had a consulting or advisory role with Genentech and Roche.
A version of this article first appeared on Medscape.com.
Across the United States, minority patients with cancer often have worse outcomes than White patients, with Black patients more likely to die sooner.
But new data suggest that these racial disparities are lessening. They come from a cross-sectional cohort study of patients with gastrointestinal cancers and show that the gap in mortality rates was reduced in Medicaid expansion states, compared with nonexpansion states.
The results were particularly notable for Black patients, for whom there was a consistent increase in receiving therapy (chemotherapy or surgery) and a decrease in mortality from stomach, colorectal, and pancreatic cancer, the investigators commented.
The study was highlighted at a press briefing held in advance of the annual meeting of the American Society of Clinical Oncology.
“The findings of this study provide a solid step for closing the gap, showing that the Medicaid expansion opportunity offered by the Affordable Care Act, which allows participating states to improve health care access for disadvantaged populations, results in better cancer outcomes and mitigation of racial disparities in cancer survival,” commented Julie Gralow, MD, chief medical officer and executive vice president of ASCO.
The study included 86,052 patients from the National Cancer Database who, from 2009 to 2019, were diagnosed with pancreatic cancer, colorectal cancer, or stomach cancer. Just over 22,000 patients (25.7%) were Black; the remainder 63,943 (74.3%) were White.
In Medicaid expansion states, there was a greater absolute reduction in 2-year mortality among Black patients with pancreatic cancer of –11.8%, compared with nonexpansion states, at –2.4%, a difference-in-difference (DID) of –9.4%. Additionally, there was an increase in treatment with chemotherapy for patients with stage III-IV pancreatic cancer (4.5% for Black patients and 3.2% for White), compared with patients in nonexpansion states (0.8% for Black patients and 0.4% for White; DID, 3.7% for Black patients and DID, 2.7% for White).
“We found similar results in colorectal cancer, but this effect is primarily observed among the stage IV patients,” commented lead author Naveen Manisundaram, MD, a research fellow at the University of Texas MD Anderson Cancer Center, Houston. “Black patients with advanced stage disease experienced a 12.6% reduction in mortality in expansion states.”
Among Black patients with stage IV colorectal cancer, there was an increase in rates of surgery in expansion states, compared with nonexpansion states (DID, 5.7%). However, there was no increase in treatment with chemotherapy (DID, 1%; P = .66).
Mortality rates for Black patients with stomach cancer also decreased. In expansion states, there was a –13% absolute decrease in mortality, compared with a –5.2% decrease in nonexpansion states.
The investigators noted that Medicaid coverage was a key component in access to care through the Affordable Care Act. About two-thirds (66.7%) of Black patients had Medicaid; 33.3% were uninsured. Coverage was similar among White patients; 64.1% had Medicaid and 35.9% were uninsured.
“Our study provides compelling data that show Medicaid expansion was associated with improvement in survival for both Black and White patients with gastrointestinal cancers. Additionally, it suggests that Medicaid expansion is one potential avenue to mitigate existing racial survival disparities among these patients,” Dr. Manisundaram concluded.
The study was funded by the National Institutes of Health. One coauthor reported an advisory role with Medicaroid. Dr. Gralow has had a consulting or advisory role with Genentech and Roche.
A version of this article first appeared on Medscape.com.
Across the United States, minority patients with cancer often have worse outcomes than White patients, with Black patients more likely to die sooner.
But new data suggest that these racial disparities are lessening. They come from a cross-sectional cohort study of patients with gastrointestinal cancers and show that the gap in mortality rates was reduced in Medicaid expansion states, compared with nonexpansion states.
The results were particularly notable for Black patients, for whom there was a consistent increase in receiving therapy (chemotherapy or surgery) and a decrease in mortality from stomach, colorectal, and pancreatic cancer, the investigators commented.
The study was highlighted at a press briefing held in advance of the annual meeting of the American Society of Clinical Oncology.
“The findings of this study provide a solid step for closing the gap, showing that the Medicaid expansion opportunity offered by the Affordable Care Act, which allows participating states to improve health care access for disadvantaged populations, results in better cancer outcomes and mitigation of racial disparities in cancer survival,” commented Julie Gralow, MD, chief medical officer and executive vice president of ASCO.
The study included 86,052 patients from the National Cancer Database who, from 2009 to 2019, were diagnosed with pancreatic cancer, colorectal cancer, or stomach cancer. Just over 22,000 patients (25.7%) were Black; the remainder 63,943 (74.3%) were White.
In Medicaid expansion states, there was a greater absolute reduction in 2-year mortality among Black patients with pancreatic cancer of –11.8%, compared with nonexpansion states, at –2.4%, a difference-in-difference (DID) of –9.4%. Additionally, there was an increase in treatment with chemotherapy for patients with stage III-IV pancreatic cancer (4.5% for Black patients and 3.2% for White), compared with patients in nonexpansion states (0.8% for Black patients and 0.4% for White; DID, 3.7% for Black patients and DID, 2.7% for White).
“We found similar results in colorectal cancer, but this effect is primarily observed among the stage IV patients,” commented lead author Naveen Manisundaram, MD, a research fellow at the University of Texas MD Anderson Cancer Center, Houston. “Black patients with advanced stage disease experienced a 12.6% reduction in mortality in expansion states.”
Among Black patients with stage IV colorectal cancer, there was an increase in rates of surgery in expansion states, compared with nonexpansion states (DID, 5.7%). However, there was no increase in treatment with chemotherapy (DID, 1%; P = .66).
Mortality rates for Black patients with stomach cancer also decreased. In expansion states, there was a –13% absolute decrease in mortality, compared with a –5.2% decrease in nonexpansion states.
The investigators noted that Medicaid coverage was a key component in access to care through the Affordable Care Act. About two-thirds (66.7%) of Black patients had Medicaid; 33.3% were uninsured. Coverage was similar among White patients; 64.1% had Medicaid and 35.9% were uninsured.
“Our study provides compelling data that show Medicaid expansion was associated with improvement in survival for both Black and White patients with gastrointestinal cancers. Additionally, it suggests that Medicaid expansion is one potential avenue to mitigate existing racial survival disparities among these patients,” Dr. Manisundaram concluded.
The study was funded by the National Institutes of Health. One coauthor reported an advisory role with Medicaroid. Dr. Gralow has had a consulting or advisory role with Genentech and Roche.
A version of this article first appeared on Medscape.com.
FROM ASCO 2023
Cross-border U.S.-Mexican collaboration drives up ALL survival
A team from a hospital in San Diego combined a previously established training program from the World Health Organization with a new collaboration, which resulted in improvements in care standards and sustainability of care in a center in Tijuana, Mexico, just 23 miles away.
Implementation of the program in 2013 led to a significant 6% improvement in 5-year overall survival for children with ALL.
For patients at standard risk, 5-year overall survival increased from 73% to 100% after implementation of the program.
“This is really remarkable because this survival is the same as we have here in San Diego,” commented Paula Aristizabal, MD, MAS, a pediatric hematologist/oncologist at Rady Children’s Hospital, San Diego, at a press briefing before the annual meeting of the American Society of Clinical Oncology.
The findings show that “sustained improvements in cancer outcomes in low- and middle-income countries [LMICs] are feasible with innovative cross-border programs, particularly in borders that are shared” between a high- and low-income country, she commented. In other words, “it takes a village in both countries” to drive up standards.
Dr. Aristizabal also noted that the partnership will continue with a particularly focus on improving survival among patients with high-risk disease.
“We like to call it ‘twinning,’ because that means we are twins forever,” she said. “This is not a marriage that can be dissolved.”
‘Huge survival gap’
“The burden of childhood cancer has increased globally, but unfortunately, survival in low- and middle-income countries has not improved at the same level as in high-income countries,” Dr. Aristizabal commented.
This has resulted in a “huge survival gap” between high-income countries and the LMICs. ALL is now a leading cause of death among children in these countries, she commented.
“This study illustrates collaborative strategies that can be put into place today that could greatly improve outcomes for children with cancer globally,” commented Julie R. Gralow, MD, ASCO chief medical officer and executive vice president.
Speaking at the press conference, she added: “As I’ve heard Princess Dina Mired of Jordan say many times: ‘Your ZIP code should not determine if you survive cancer.’ ”
She said the differences in ALL survival between the United States and Mexico are an “example of children being so close in terms of proximity not having the same advantages.”
Also commenting, ASCO President Eric Winer, MD, from the Yale Cancer Center, New Haven, Conn., asked whether the proximity of the hospitals in San Diego and Tijuana “makes a difference, or do you think this is something that done ... at a distance?”
Dr. Aristizabal said that the proximity between the institutions “has been extremely helpful,” as they can go between hospitals in just 30 minutes.
However, “one of the things that we learned with COVID is that we can do a lot of things remotely,” she answered.
“Some of the projects that we started in Tijuana, through our collaboration with St. Jude Children’s Research Hospital, we have been able to implement in many other centers in Mexico,” she said.
Study details
Rady Children’s Hospital partnered with the public sector in Baja California, with the aim of improving outcomes in children’s cancer, she explained.
In 2008, the team collaborated with St. Jude Children’s Research Hospital, Memphis, to establish a training program in the Hospital General Tijuana in Tijuana that shared knowledge, technology, and organizational skills.
The team also consulted on clinical cases and set up education and research programs, all with the aim of building capacity and sustainability in Mexico.
“As the number of leukemia patients increased, we wanted to decrease depending on their international collaborators in the U.S. and ensure long-term sustainability,” Dr. Aristizabal explained.
This led in 2013 to the implementation of the WHO Framework for Action HSS training model, which has several components, including health service delivery.
Combined with the previously established model, the overall goals of the program were to improve health outcomes, systems efficiency, timely access to care, and social and financial risk protection.
Dr. Aristizabal said in an interview that this involved developing highly specific leukemia treatment guidelines, which have now gone through three iterations, as well as guidelines for supportive care.
Working with a local foundation, the team has also “focused on providing psychosocial support, nutritional support, a shelter for families that live 12-14 hours away from the pediatric cancer center, as well as food subsidies, trying to address financial toxicity and food insecurity in these families.”
Impact of the collaboration
To assess the impact of the WHO framework, the researchers conducted a study that involved 109 children with ALL who were treated at Hospital General Tijuana over the preimplementation phase in 2008-2012 and the postimplementation phase in 2013-2017.
The mean age of the patients was 7.04 years, and 50.4% were girls. The majority (67%) were classified as having high-risk disease.
Over the entire study period, the 5-year overall survival rate was 65%. Analysis revealed that between the pre- and postimplementation periods, 5-year overall survival increased from 59% to 65%, which Dr. Aristizabal described as “a significant improvement.”
Among high-risk patients, the improvement in 5-year survival between the pre- and postimplementation period went from 48% to 55%.
“This is an area for improvement,” Dr. Aristizabal said, “and we’re working on additional strategies to help improve survival for high-risk patients.
The study was funded by Rady Children’s Hospital, the Mexican Secretary of Health, and the Patronato Foundation. Dr. Aristizabal and coauthors reported no relevant financial relationships. Dr. Gralow reported relationships with Genentech and Roche. Dr. Winer reported relationships with Leap Therapeutics, Jounce Therapeutics, Carrick Therapeutics, and Genentech.
A version of this article first appeared on Medscape.com.
A team from a hospital in San Diego combined a previously established training program from the World Health Organization with a new collaboration, which resulted in improvements in care standards and sustainability of care in a center in Tijuana, Mexico, just 23 miles away.
Implementation of the program in 2013 led to a significant 6% improvement in 5-year overall survival for children with ALL.
For patients at standard risk, 5-year overall survival increased from 73% to 100% after implementation of the program.
“This is really remarkable because this survival is the same as we have here in San Diego,” commented Paula Aristizabal, MD, MAS, a pediatric hematologist/oncologist at Rady Children’s Hospital, San Diego, at a press briefing before the annual meeting of the American Society of Clinical Oncology.
The findings show that “sustained improvements in cancer outcomes in low- and middle-income countries [LMICs] are feasible with innovative cross-border programs, particularly in borders that are shared” between a high- and low-income country, she commented. In other words, “it takes a village in both countries” to drive up standards.
Dr. Aristizabal also noted that the partnership will continue with a particularly focus on improving survival among patients with high-risk disease.
“We like to call it ‘twinning,’ because that means we are twins forever,” she said. “This is not a marriage that can be dissolved.”
‘Huge survival gap’
“The burden of childhood cancer has increased globally, but unfortunately, survival in low- and middle-income countries has not improved at the same level as in high-income countries,” Dr. Aristizabal commented.
This has resulted in a “huge survival gap” between high-income countries and the LMICs. ALL is now a leading cause of death among children in these countries, she commented.
“This study illustrates collaborative strategies that can be put into place today that could greatly improve outcomes for children with cancer globally,” commented Julie R. Gralow, MD, ASCO chief medical officer and executive vice president.
Speaking at the press conference, she added: “As I’ve heard Princess Dina Mired of Jordan say many times: ‘Your ZIP code should not determine if you survive cancer.’ ”
She said the differences in ALL survival between the United States and Mexico are an “example of children being so close in terms of proximity not having the same advantages.”
Also commenting, ASCO President Eric Winer, MD, from the Yale Cancer Center, New Haven, Conn., asked whether the proximity of the hospitals in San Diego and Tijuana “makes a difference, or do you think this is something that done ... at a distance?”
Dr. Aristizabal said that the proximity between the institutions “has been extremely helpful,” as they can go between hospitals in just 30 minutes.
However, “one of the things that we learned with COVID is that we can do a lot of things remotely,” she answered.
“Some of the projects that we started in Tijuana, through our collaboration with St. Jude Children’s Research Hospital, we have been able to implement in many other centers in Mexico,” she said.
Study details
Rady Children’s Hospital partnered with the public sector in Baja California, with the aim of improving outcomes in children’s cancer, she explained.
In 2008, the team collaborated with St. Jude Children’s Research Hospital, Memphis, to establish a training program in the Hospital General Tijuana in Tijuana that shared knowledge, technology, and organizational skills.
The team also consulted on clinical cases and set up education and research programs, all with the aim of building capacity and sustainability in Mexico.
“As the number of leukemia patients increased, we wanted to decrease depending on their international collaborators in the U.S. and ensure long-term sustainability,” Dr. Aristizabal explained.
This led in 2013 to the implementation of the WHO Framework for Action HSS training model, which has several components, including health service delivery.
Combined with the previously established model, the overall goals of the program were to improve health outcomes, systems efficiency, timely access to care, and social and financial risk protection.
Dr. Aristizabal said in an interview that this involved developing highly specific leukemia treatment guidelines, which have now gone through three iterations, as well as guidelines for supportive care.
Working with a local foundation, the team has also “focused on providing psychosocial support, nutritional support, a shelter for families that live 12-14 hours away from the pediatric cancer center, as well as food subsidies, trying to address financial toxicity and food insecurity in these families.”
Impact of the collaboration
To assess the impact of the WHO framework, the researchers conducted a study that involved 109 children with ALL who were treated at Hospital General Tijuana over the preimplementation phase in 2008-2012 and the postimplementation phase in 2013-2017.
The mean age of the patients was 7.04 years, and 50.4% were girls. The majority (67%) were classified as having high-risk disease.
Over the entire study period, the 5-year overall survival rate was 65%. Analysis revealed that between the pre- and postimplementation periods, 5-year overall survival increased from 59% to 65%, which Dr. Aristizabal described as “a significant improvement.”
Among high-risk patients, the improvement in 5-year survival between the pre- and postimplementation period went from 48% to 55%.
“This is an area for improvement,” Dr. Aristizabal said, “and we’re working on additional strategies to help improve survival for high-risk patients.
The study was funded by Rady Children’s Hospital, the Mexican Secretary of Health, and the Patronato Foundation. Dr. Aristizabal and coauthors reported no relevant financial relationships. Dr. Gralow reported relationships with Genentech and Roche. Dr. Winer reported relationships with Leap Therapeutics, Jounce Therapeutics, Carrick Therapeutics, and Genentech.
A version of this article first appeared on Medscape.com.
A team from a hospital in San Diego combined a previously established training program from the World Health Organization with a new collaboration, which resulted in improvements in care standards and sustainability of care in a center in Tijuana, Mexico, just 23 miles away.
Implementation of the program in 2013 led to a significant 6% improvement in 5-year overall survival for children with ALL.
For patients at standard risk, 5-year overall survival increased from 73% to 100% after implementation of the program.
“This is really remarkable because this survival is the same as we have here in San Diego,” commented Paula Aristizabal, MD, MAS, a pediatric hematologist/oncologist at Rady Children’s Hospital, San Diego, at a press briefing before the annual meeting of the American Society of Clinical Oncology.
The findings show that “sustained improvements in cancer outcomes in low- and middle-income countries [LMICs] are feasible with innovative cross-border programs, particularly in borders that are shared” between a high- and low-income country, she commented. In other words, “it takes a village in both countries” to drive up standards.
Dr. Aristizabal also noted that the partnership will continue with a particularly focus on improving survival among patients with high-risk disease.
“We like to call it ‘twinning,’ because that means we are twins forever,” she said. “This is not a marriage that can be dissolved.”
‘Huge survival gap’
“The burden of childhood cancer has increased globally, but unfortunately, survival in low- and middle-income countries has not improved at the same level as in high-income countries,” Dr. Aristizabal commented.
This has resulted in a “huge survival gap” between high-income countries and the LMICs. ALL is now a leading cause of death among children in these countries, she commented.
“This study illustrates collaborative strategies that can be put into place today that could greatly improve outcomes for children with cancer globally,” commented Julie R. Gralow, MD, ASCO chief medical officer and executive vice president.
Speaking at the press conference, she added: “As I’ve heard Princess Dina Mired of Jordan say many times: ‘Your ZIP code should not determine if you survive cancer.’ ”
She said the differences in ALL survival between the United States and Mexico are an “example of children being so close in terms of proximity not having the same advantages.”
Also commenting, ASCO President Eric Winer, MD, from the Yale Cancer Center, New Haven, Conn., asked whether the proximity of the hospitals in San Diego and Tijuana “makes a difference, or do you think this is something that done ... at a distance?”
Dr. Aristizabal said that the proximity between the institutions “has been extremely helpful,” as they can go between hospitals in just 30 minutes.
However, “one of the things that we learned with COVID is that we can do a lot of things remotely,” she answered.
“Some of the projects that we started in Tijuana, through our collaboration with St. Jude Children’s Research Hospital, we have been able to implement in many other centers in Mexico,” she said.
Study details
Rady Children’s Hospital partnered with the public sector in Baja California, with the aim of improving outcomes in children’s cancer, she explained.
In 2008, the team collaborated with St. Jude Children’s Research Hospital, Memphis, to establish a training program in the Hospital General Tijuana in Tijuana that shared knowledge, technology, and organizational skills.
The team also consulted on clinical cases and set up education and research programs, all with the aim of building capacity and sustainability in Mexico.
“As the number of leukemia patients increased, we wanted to decrease depending on their international collaborators in the U.S. and ensure long-term sustainability,” Dr. Aristizabal explained.
This led in 2013 to the implementation of the WHO Framework for Action HSS training model, which has several components, including health service delivery.
Combined with the previously established model, the overall goals of the program were to improve health outcomes, systems efficiency, timely access to care, and social and financial risk protection.
Dr. Aristizabal said in an interview that this involved developing highly specific leukemia treatment guidelines, which have now gone through three iterations, as well as guidelines for supportive care.
Working with a local foundation, the team has also “focused on providing psychosocial support, nutritional support, a shelter for families that live 12-14 hours away from the pediatric cancer center, as well as food subsidies, trying to address financial toxicity and food insecurity in these families.”
Impact of the collaboration
To assess the impact of the WHO framework, the researchers conducted a study that involved 109 children with ALL who were treated at Hospital General Tijuana over the preimplementation phase in 2008-2012 and the postimplementation phase in 2013-2017.
The mean age of the patients was 7.04 years, and 50.4% were girls. The majority (67%) were classified as having high-risk disease.
Over the entire study period, the 5-year overall survival rate was 65%. Analysis revealed that between the pre- and postimplementation periods, 5-year overall survival increased from 59% to 65%, which Dr. Aristizabal described as “a significant improvement.”
Among high-risk patients, the improvement in 5-year survival between the pre- and postimplementation period went from 48% to 55%.
“This is an area for improvement,” Dr. Aristizabal said, “and we’re working on additional strategies to help improve survival for high-risk patients.
The study was funded by Rady Children’s Hospital, the Mexican Secretary of Health, and the Patronato Foundation. Dr. Aristizabal and coauthors reported no relevant financial relationships. Dr. Gralow reported relationships with Genentech and Roche. Dr. Winer reported relationships with Leap Therapeutics, Jounce Therapeutics, Carrick Therapeutics, and Genentech.
A version of this article first appeared on Medscape.com.
FROM ASCO 2023
Blood cancer patient takes on bias and ‘gaslighting’
Diagnosed with Hodgkin lymphoma in 2021, Ms. Ngon underwent port surgery to allow chemotherapy to be administered. Her right arm lost circulation and went numb, so she sought guidance from her blood cancer specialist. He dismissed her worries, saying that her tumors were pinching a nerve. She’d get better, he predicted, after more chemo.
“I knew in my body that something was wrong,” Ms. Ngon recalled. When the oncologist continued to downplay her concerns, she and a fellow communications specialist sat down together in the hospital lobby to draft an email to her physician. “We were trying to articulate the urgency in an email that expresses that I’m not being dramatic. We had to do it in a way that didn’t insult his intelligence: ‘Respectfully, you’re the doctor, but I know something is wrong.’ ”
In essence, Ms. Ngon was trying to be diplomatic and not trigger her oncologist’s defenses, while still convincing him to take action. Her approach to getting her doctor’s attention worked. He referred Ms. Ngon to a radiologist, who discovered that she had blood clots in her arm. Ms. Ngon then landed in the ICU for a week, as clinicians tried to break up the clots.
“I was the perfect person for this to happen to, because of my job and education. But it makes me sad because I understand I was in a fortunate position, with a background in communication. Most people don’t have that,” Ms. Ngon said.
This and other negative experiences during her medical saga inspired Ms. Ngon to partner with the Lymphoma Research Foundation in order to spread the word about unique challenges facing patients like her: people of color.
Ms. Ngon, who is Black, said her goal as a patient advocate is to “empower communities of color to speak up for themselves and hold oncologists responsible for listening and understanding differences across cultures.” And she wants to take a stand against the “gaslighting” of patients.
African Americans with hematologic disease like Ms. Ngon face a higher risk of poor outcomes than Whites, even as they are less likely than Whites to develop certain blood cancers. The reasons for this disparity aren’t clear, but researchers suspect they’re related to factors such as poverty, lack of insurance, genetics, and limited access to high-quality care.
Some researchers have blamed another factor: racism. A 2022 study sought to explain why Black and Hispanic patients with acute myeloid leukemia in urban areas have higher mortality rates than Whites, “despite more favorable genetics and younger age” (hazard ratio, 1.59, 95% confidence interval, 1.15-2.22 and HR, 1.25; 95% CI, 0.88-1.79). The study authors determined that “structural racism” – which they measured by examining segregation and “disadvantage” in neighborhoods where patients lived – accounted for nearly all of the disparities.
Ms. Ngon said her experiences and her awareness about poorer outcomes in medicine for African Americans – such as higher death rates for Black women during pregnancy – affect how she interacts with clinicians. “I automatically assume a barrier between me and my doctors, and it’s their responsibility to dismantle it.”
Making an connection with a physician can make a huge difference, she said. “I walked into my primary care doctor’s office and saw that she was a Latino woman. My guard went down, and I could feel her care for me as a human being. Whether that was because she was also a woman of color or not, I don’t know. But I did feel more cared for.”
However, Ms. Ngon could not find a Black oncologist to care for her in New York City, and that’s no surprise.
Ethnic and gender diversity remains an immense challenge in the hematology/oncology field. According to the American Society of Clinical Oncology, only about a third of oncologists are women, and the percentages identifying themselves as Black/African American and Hispanic are just 2.3% and 5.8%, respectively.
These numbers don’t seem likely to budge much any time soon. An analysis of medical students in U.S. oncology training programs from 2015-2020 found that just 3.8% identified themselves as Black/African American and 5.1% as Hispanic/Latino versus 52.15% as White and 31% as Asian/Pacific Islander/Native Hawaiian.
Ms. Ngon encountered challenges on other fronts during her cancer care. When she needed a wig during chemotherapy, a list of insurer-approved shops didn’t include any that catered to African Americans. Essentially, she said, she was being told that she couldn’t “purchase a wig from a place that makes you feel comfortable and from a woman who understand your needs as a Black woman. It needs to be from these specific shops that really don’t cater to my community.”
She also found it difficult to find fellow patients who shared her unique challenges. “I remember when I was diagnosed, I was looking through the support groups on Facebook, trying to find someone Black to ask about whether braiding my hair might stop it from falling out.”
Now, Ms. Ngon is in remission. And she’s happy with her oncologist, who’s White. “He listened to me, and he promised me that I would have the most boring recovery process ever, after everything I’d experienced. That explains a lot of why I felt so comfortable with him.”
She hopes to use her partnership with the Lymphoma Research Foundation to be a resource for people of color and alert them to the support that’s available for them. “I would love to let them know how to advocate for themselves as patients, how to trust their bodies, how to push back if they feel like they’re not getting the care that they deserve.”
Ms. Ngon would also like to see more support for medical students of color. “I hope to exist in a world one day where it wouldn’t be so hard to find an oncologist who looks like me in a city as large as this one,” she said.
As for oncologists, she urged them to “go the extra mile and really, really listen to what patients are saying. It’s easier said than done because there are natural biases in this world, and it’s hard to overcome those obstacles. But to not be heard and have to push every time. It was just exhausting to do that on top of trying to beat cancer.”
Diagnosed with Hodgkin lymphoma in 2021, Ms. Ngon underwent port surgery to allow chemotherapy to be administered. Her right arm lost circulation and went numb, so she sought guidance from her blood cancer specialist. He dismissed her worries, saying that her tumors were pinching a nerve. She’d get better, he predicted, after more chemo.

“I knew in my body that something was wrong,” Ms. Ngon recalled. When the oncologist continued to downplay her concerns, she and a fellow communications specialist sat down together in the hospital lobby to draft an email to her physician. “We were trying to articulate the urgency in an email that expresses that I’m not being dramatic. We had to do it in a way that didn’t insult his intelligence: ‘Respectfully, you’re the doctor, but I know something is wrong.’ ”
In essence, Ms. Ngon was trying to be diplomatic and not trigger her oncologist’s defenses, while still convincing him to take action. Her approach to getting her doctor’s attention worked. He referred Ms. Ngon to a radiologist, who discovered that she had blood clots in her arm. Ms. Ngon then landed in the ICU for a week, as clinicians tried to break up the clots.
“I was the perfect person for this to happen to, because of my job and education. But it makes me sad because I understand I was in a fortunate position, with a background in communication. Most people don’t have that,” Ms. Ngon said.
This and other negative experiences during her medical saga inspired Ms. Ngon to partner with the Lymphoma Research Foundation in order to spread the word about unique challenges facing patients like her: people of color.
Ms. Ngon, who is Black, said her goal as a patient advocate is to “empower communities of color to speak up for themselves and hold oncologists responsible for listening and understanding differences across cultures.” And she wants to take a stand against the “gaslighting” of patients.
African Americans with hematologic disease like Ms. Ngon face a higher risk of poor outcomes than Whites, even as they are less likely than Whites to develop certain blood cancers. The reasons for this disparity aren’t clear, but researchers suspect they’re related to factors such as poverty, lack of insurance, genetics, and limited access to high-quality care.
Some researchers have blamed another factor: racism. A 2022 study sought to explain why Black and Hispanic patients with acute myeloid leukemia in urban areas have higher mortality rates than Whites, “despite more favorable genetics and younger age” (hazard ratio, 1.59, 95% confidence interval, 1.15-2.22 and HR, 1.25; 95% CI, 0.88-1.79). The study authors determined that “structural racism” – which they measured by examining segregation and “disadvantage” in neighborhoods where patients lived – accounted for nearly all of the disparities.
Ms. Ngon said her experiences and her awareness about poorer outcomes in medicine for African Americans – such as higher death rates for Black women during pregnancy – affect how she interacts with clinicians. “I automatically assume a barrier between me and my doctors, and it’s their responsibility to dismantle it.”
Making an connection with a physician can make a huge difference, she said. “I walked into my primary care doctor’s office and saw that she was a Latino woman. My guard went down, and I could feel her care for me as a human being. Whether that was because she was also a woman of color or not, I don’t know. But I did feel more cared for.”
However, Ms. Ngon could not find a Black oncologist to care for her in New York City, and that’s no surprise.
Ethnic and gender diversity remains an immense challenge in the hematology/oncology field. According to the American Society of Clinical Oncology, only about a third of oncologists are women, and the percentages identifying themselves as Black/African American and Hispanic are just 2.3% and 5.8%, respectively.
These numbers don’t seem likely to budge much any time soon. An analysis of medical students in U.S. oncology training programs from 2015-2020 found that just 3.8% identified themselves as Black/African American and 5.1% as Hispanic/Latino versus 52.15% as White and 31% as Asian/Pacific Islander/Native Hawaiian.
Ms. Ngon encountered challenges on other fronts during her cancer care. When she needed a wig during chemotherapy, a list of insurer-approved shops didn’t include any that catered to African Americans. Essentially, she said, she was being told that she couldn’t “purchase a wig from a place that makes you feel comfortable and from a woman who understand your needs as a Black woman. It needs to be from these specific shops that really don’t cater to my community.”
She also found it difficult to find fellow patients who shared her unique challenges. “I remember when I was diagnosed, I was looking through the support groups on Facebook, trying to find someone Black to ask about whether braiding my hair might stop it from falling out.”
Now, Ms. Ngon is in remission. And she’s happy with her oncologist, who’s White. “He listened to me, and he promised me that I would have the most boring recovery process ever, after everything I’d experienced. That explains a lot of why I felt so comfortable with him.”
She hopes to use her partnership with the Lymphoma Research Foundation to be a resource for people of color and alert them to the support that’s available for them. “I would love to let them know how to advocate for themselves as patients, how to trust their bodies, how to push back if they feel like they’re not getting the care that they deserve.”
Ms. Ngon would also like to see more support for medical students of color. “I hope to exist in a world one day where it wouldn’t be so hard to find an oncologist who looks like me in a city as large as this one,” she said.
As for oncologists, she urged them to “go the extra mile and really, really listen to what patients are saying. It’s easier said than done because there are natural biases in this world, and it’s hard to overcome those obstacles. But to not be heard and have to push every time. It was just exhausting to do that on top of trying to beat cancer.”
Diagnosed with Hodgkin lymphoma in 2021, Ms. Ngon underwent port surgery to allow chemotherapy to be administered. Her right arm lost circulation and went numb, so she sought guidance from her blood cancer specialist. He dismissed her worries, saying that her tumors were pinching a nerve. She’d get better, he predicted, after more chemo.

“I knew in my body that something was wrong,” Ms. Ngon recalled. When the oncologist continued to downplay her concerns, she and a fellow communications specialist sat down together in the hospital lobby to draft an email to her physician. “We were trying to articulate the urgency in an email that expresses that I’m not being dramatic. We had to do it in a way that didn’t insult his intelligence: ‘Respectfully, you’re the doctor, but I know something is wrong.’ ”
In essence, Ms. Ngon was trying to be diplomatic and not trigger her oncologist’s defenses, while still convincing him to take action. Her approach to getting her doctor’s attention worked. He referred Ms. Ngon to a radiologist, who discovered that she had blood clots in her arm. Ms. Ngon then landed in the ICU for a week, as clinicians tried to break up the clots.
“I was the perfect person for this to happen to, because of my job and education. But it makes me sad because I understand I was in a fortunate position, with a background in communication. Most people don’t have that,” Ms. Ngon said.
This and other negative experiences during her medical saga inspired Ms. Ngon to partner with the Lymphoma Research Foundation in order to spread the word about unique challenges facing patients like her: people of color.
Ms. Ngon, who is Black, said her goal as a patient advocate is to “empower communities of color to speak up for themselves and hold oncologists responsible for listening and understanding differences across cultures.” And she wants to take a stand against the “gaslighting” of patients.
African Americans with hematologic disease like Ms. Ngon face a higher risk of poor outcomes than Whites, even as they are less likely than Whites to develop certain blood cancers. The reasons for this disparity aren’t clear, but researchers suspect they’re related to factors such as poverty, lack of insurance, genetics, and limited access to high-quality care.
Some researchers have blamed another factor: racism. A 2022 study sought to explain why Black and Hispanic patients with acute myeloid leukemia in urban areas have higher mortality rates than Whites, “despite more favorable genetics and younger age” (hazard ratio, 1.59, 95% confidence interval, 1.15-2.22 and HR, 1.25; 95% CI, 0.88-1.79). The study authors determined that “structural racism” – which they measured by examining segregation and “disadvantage” in neighborhoods where patients lived – accounted for nearly all of the disparities.
Ms. Ngon said her experiences and her awareness about poorer outcomes in medicine for African Americans – such as higher death rates for Black women during pregnancy – affect how she interacts with clinicians. “I automatically assume a barrier between me and my doctors, and it’s their responsibility to dismantle it.”
Making an connection with a physician can make a huge difference, she said. “I walked into my primary care doctor’s office and saw that she was a Latino woman. My guard went down, and I could feel her care for me as a human being. Whether that was because she was also a woman of color or not, I don’t know. But I did feel more cared for.”
However, Ms. Ngon could not find a Black oncologist to care for her in New York City, and that’s no surprise.
Ethnic and gender diversity remains an immense challenge in the hematology/oncology field. According to the American Society of Clinical Oncology, only about a third of oncologists are women, and the percentages identifying themselves as Black/African American and Hispanic are just 2.3% and 5.8%, respectively.
These numbers don’t seem likely to budge much any time soon. An analysis of medical students in U.S. oncology training programs from 2015-2020 found that just 3.8% identified themselves as Black/African American and 5.1% as Hispanic/Latino versus 52.15% as White and 31% as Asian/Pacific Islander/Native Hawaiian.
Ms. Ngon encountered challenges on other fronts during her cancer care. When she needed a wig during chemotherapy, a list of insurer-approved shops didn’t include any that catered to African Americans. Essentially, she said, she was being told that she couldn’t “purchase a wig from a place that makes you feel comfortable and from a woman who understand your needs as a Black woman. It needs to be from these specific shops that really don’t cater to my community.”
She also found it difficult to find fellow patients who shared her unique challenges. “I remember when I was diagnosed, I was looking through the support groups on Facebook, trying to find someone Black to ask about whether braiding my hair might stop it from falling out.”
Now, Ms. Ngon is in remission. And she’s happy with her oncologist, who’s White. “He listened to me, and he promised me that I would have the most boring recovery process ever, after everything I’d experienced. That explains a lot of why I felt so comfortable with him.”
She hopes to use her partnership with the Lymphoma Research Foundation to be a resource for people of color and alert them to the support that’s available for them. “I would love to let them know how to advocate for themselves as patients, how to trust their bodies, how to push back if they feel like they’re not getting the care that they deserve.”
Ms. Ngon would also like to see more support for medical students of color. “I hope to exist in a world one day where it wouldn’t be so hard to find an oncologist who looks like me in a city as large as this one,” she said.
As for oncologists, she urged them to “go the extra mile and really, really listen to what patients are saying. It’s easier said than done because there are natural biases in this world, and it’s hard to overcome those obstacles. But to not be heard and have to push every time. It was just exhausting to do that on top of trying to beat cancer.”