User login
COVID-19 doesn’t spike A1c levels
Key takeaways
Results from a retrospective, observational, case-control study of more than 20,000 people from a single U.S. medical center showed a statistically significant but clinically insignificant increase in A1c in people following COVID-19 infection, in both those with and without diabetes.
After people received a diagnosis of COVID-19 infection, they were 40% more likely to also receive a diagnosis of type 2 diabetes, compared with people who tested negative for COVID-19, a difference that was significant and could be explained by the increased medical care received by people who test positive for COVID-19.
The risk of incident diabetic ketoacidosis (DKA) among people who tested positive for COVID-19 was significantly higher among those with pre-existing type 2 diabetes, those using insulin, and among Black individuals.
Why this matters
The authors said that their study is the first report of evidence that infection with COVID-19 affects A1c levels in a large, real-world clinical cohort.
Until now, the impact of COVID-19 infection on A1c remained unclear. Results from previous studies indicated that COVID-19 infection may increase A1c levels, but the studied cohorts were small and lacked uninfected controls.
The current study included 8,755 people infected with COVID-19, had data from both before and after the infection on diabetes status and A1c levels, and also included many matched, uninfected people who served as controls.
Study design
Data came from a Cleveland Clinic registry that included 81,093 people who tested positive for COVID-19 between March 2020 and May 2021 and 153,034 matched individuals who tested negative for COVID-19 during the same period.
The researchers retrospectively selected patients with an A1c recorded within 12 months before their COVID-19 test, as well as a second A1c value recorded within 12 months after COVID-19 testing. This produced a study cohort of 8,755 COVID-positive people and 11,998 matched people who tested negative for COVID-19.
To evaluate the risk of DKA onset after COVID-19 infection, the authors identified two sub-cohorts that excluded those with a history of DKA. The sub-cohorts were 701 people with type 1 diabetes and 21,830 with type 2 diabetes.
Key results
The investigators found a statistically significant but clinically insignificant A1c increase following a positive COVID-19 test, an average A1c increase of 0.06 percentage points. Those who tested negative for COVID-19 had a clinically insignificant change in their average A1c level that was of borderline statistical significance, an average increase of 0.02 percentage points (P = .05).
The statistically significant but clinically insignificant increase in A1c following infection with COVID-19 was similar in people with and without type 2 diabetes prior to infection.
In patients with type 2 diabetes who became infected with COVID-19, the researchers saw significant positive associations between higher A1c levels before infection and time to hospitalization (hazard ratio, 1.07), need for assisted breathing (HR, 1.06), and ICU admission (HR, 1.07).
Following a COVID-19 infection, people were 40% more likely to receive a diagnosis of incident type 2 diabetes, compared with matched uninfected people. The authors said a possible explanation is that after diagnosis of COVID-19, infected people in general received more intensified care that led to better identification of those with underlying type 2 diabetes.
The 701 people included with pre-existing type 1 diabetes showed no significant difference in their rate of developing DKA between those infected and not infected with COVID-19.
Among the 21,830 people with pre-existing type 2 diabetes, the DKA risk was a significant 35% greater for those who were infected with COVID-19, compared with those who were uninfected. The magnitude of this increased relative risk was even higher among the patients with type 2 diabetes who used insulin as part of their treatment.
The difference in DKA risk didn’t differ between Black and White patients who were not infected with COVID-19, but among those infected by COVID-19, Black patients were more than twice as likely to be diagnosed with DKA, compared with White patients, a significant difference.
Black patients with type 2 diabetes who became infected with COVID-19 had a significant (63%) increased rate of DKA compared with Black patients with type 2 diabetes who remained uninfected.
Limitations
The study included patients with A1c measurements made up to 12 months prior to their COVID-19 test, and hence comorbid conditions, medication changes during this period, or other factors may have affected subsequent A1c levels. To address this, the authors also assessed outcomes at 3- and 6-month intervals, which produced results consistent with the 12-month findings.
The researchers did not have A1c values for many of the more than 234,000 people in the entire registry who underwent COVID-19 testing from March 2020-May 2021 at the Cleveland Clinic, omissions that may have biased the study cohort.
This was a single-center study. Some patients may have received care outside of the center, hence records of those episodes could not be included.
Disclosures
The study received no commercial funding. Four authors received consulting and speaker honoraria and research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Corcept Therapeutics, Diasome, Eli Lilly, Merck, Novo Nordisk, and Sanofi. Three authors have intellectual property related to treatment decisionmaking in the context of type 2 diabetes.
This is a summary of a preprint research study “Impacts of COVID-19 on glycemia and risk of diabetic ketoacidosis,” written by researchers at the Cleveland Clinic on medRxiv. The study has not yet been peer reviewed. The full text of the study can be found on medRxiv.org.
A version of this article first appeared on Medscape.com.
Key takeaways
Results from a retrospective, observational, case-control study of more than 20,000 people from a single U.S. medical center showed a statistically significant but clinically insignificant increase in A1c in people following COVID-19 infection, in both those with and without diabetes.
After people received a diagnosis of COVID-19 infection, they were 40% more likely to also receive a diagnosis of type 2 diabetes, compared with people who tested negative for COVID-19, a difference that was significant and could be explained by the increased medical care received by people who test positive for COVID-19.
The risk of incident diabetic ketoacidosis (DKA) among people who tested positive for COVID-19 was significantly higher among those with pre-existing type 2 diabetes, those using insulin, and among Black individuals.
Why this matters
The authors said that their study is the first report of evidence that infection with COVID-19 affects A1c levels in a large, real-world clinical cohort.
Until now, the impact of COVID-19 infection on A1c remained unclear. Results from previous studies indicated that COVID-19 infection may increase A1c levels, but the studied cohorts were small and lacked uninfected controls.
The current study included 8,755 people infected with COVID-19, had data from both before and after the infection on diabetes status and A1c levels, and also included many matched, uninfected people who served as controls.
Study design
Data came from a Cleveland Clinic registry that included 81,093 people who tested positive for COVID-19 between March 2020 and May 2021 and 153,034 matched individuals who tested negative for COVID-19 during the same period.
The researchers retrospectively selected patients with an A1c recorded within 12 months before their COVID-19 test, as well as a second A1c value recorded within 12 months after COVID-19 testing. This produced a study cohort of 8,755 COVID-positive people and 11,998 matched people who tested negative for COVID-19.
To evaluate the risk of DKA onset after COVID-19 infection, the authors identified two sub-cohorts that excluded those with a history of DKA. The sub-cohorts were 701 people with type 1 diabetes and 21,830 with type 2 diabetes.
Key results
The investigators found a statistically significant but clinically insignificant A1c increase following a positive COVID-19 test, an average A1c increase of 0.06 percentage points. Those who tested negative for COVID-19 had a clinically insignificant change in their average A1c level that was of borderline statistical significance, an average increase of 0.02 percentage points (P = .05).
The statistically significant but clinically insignificant increase in A1c following infection with COVID-19 was similar in people with and without type 2 diabetes prior to infection.
In patients with type 2 diabetes who became infected with COVID-19, the researchers saw significant positive associations between higher A1c levels before infection and time to hospitalization (hazard ratio, 1.07), need for assisted breathing (HR, 1.06), and ICU admission (HR, 1.07).
Following a COVID-19 infection, people were 40% more likely to receive a diagnosis of incident type 2 diabetes, compared with matched uninfected people. The authors said a possible explanation is that after diagnosis of COVID-19, infected people in general received more intensified care that led to better identification of those with underlying type 2 diabetes.
The 701 people included with pre-existing type 1 diabetes showed no significant difference in their rate of developing DKA between those infected and not infected with COVID-19.
Among the 21,830 people with pre-existing type 2 diabetes, the DKA risk was a significant 35% greater for those who were infected with COVID-19, compared with those who were uninfected. The magnitude of this increased relative risk was even higher among the patients with type 2 diabetes who used insulin as part of their treatment.
The difference in DKA risk didn’t differ between Black and White patients who were not infected with COVID-19, but among those infected by COVID-19, Black patients were more than twice as likely to be diagnosed with DKA, compared with White patients, a significant difference.
Black patients with type 2 diabetes who became infected with COVID-19 had a significant (63%) increased rate of DKA compared with Black patients with type 2 diabetes who remained uninfected.
Limitations
The study included patients with A1c measurements made up to 12 months prior to their COVID-19 test, and hence comorbid conditions, medication changes during this period, or other factors may have affected subsequent A1c levels. To address this, the authors also assessed outcomes at 3- and 6-month intervals, which produced results consistent with the 12-month findings.
The researchers did not have A1c values for many of the more than 234,000 people in the entire registry who underwent COVID-19 testing from March 2020-May 2021 at the Cleveland Clinic, omissions that may have biased the study cohort.
This was a single-center study. Some patients may have received care outside of the center, hence records of those episodes could not be included.
Disclosures
The study received no commercial funding. Four authors received consulting and speaker honoraria and research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Corcept Therapeutics, Diasome, Eli Lilly, Merck, Novo Nordisk, and Sanofi. Three authors have intellectual property related to treatment decisionmaking in the context of type 2 diabetes.
This is a summary of a preprint research study “Impacts of COVID-19 on glycemia and risk of diabetic ketoacidosis,” written by researchers at the Cleveland Clinic on medRxiv. The study has not yet been peer reviewed. The full text of the study can be found on medRxiv.org.
A version of this article first appeared on Medscape.com.
Key takeaways
Results from a retrospective, observational, case-control study of more than 20,000 people from a single U.S. medical center showed a statistically significant but clinically insignificant increase in A1c in people following COVID-19 infection, in both those with and without diabetes.
After people received a diagnosis of COVID-19 infection, they were 40% more likely to also receive a diagnosis of type 2 diabetes, compared with people who tested negative for COVID-19, a difference that was significant and could be explained by the increased medical care received by people who test positive for COVID-19.
The risk of incident diabetic ketoacidosis (DKA) among people who tested positive for COVID-19 was significantly higher among those with pre-existing type 2 diabetes, those using insulin, and among Black individuals.
Why this matters
The authors said that their study is the first report of evidence that infection with COVID-19 affects A1c levels in a large, real-world clinical cohort.
Until now, the impact of COVID-19 infection on A1c remained unclear. Results from previous studies indicated that COVID-19 infection may increase A1c levels, but the studied cohorts were small and lacked uninfected controls.
The current study included 8,755 people infected with COVID-19, had data from both before and after the infection on diabetes status and A1c levels, and also included many matched, uninfected people who served as controls.
Study design
Data came from a Cleveland Clinic registry that included 81,093 people who tested positive for COVID-19 between March 2020 and May 2021 and 153,034 matched individuals who tested negative for COVID-19 during the same period.
The researchers retrospectively selected patients with an A1c recorded within 12 months before their COVID-19 test, as well as a second A1c value recorded within 12 months after COVID-19 testing. This produced a study cohort of 8,755 COVID-positive people and 11,998 matched people who tested negative for COVID-19.
To evaluate the risk of DKA onset after COVID-19 infection, the authors identified two sub-cohorts that excluded those with a history of DKA. The sub-cohorts were 701 people with type 1 diabetes and 21,830 with type 2 diabetes.
Key results
The investigators found a statistically significant but clinically insignificant A1c increase following a positive COVID-19 test, an average A1c increase of 0.06 percentage points. Those who tested negative for COVID-19 had a clinically insignificant change in their average A1c level that was of borderline statistical significance, an average increase of 0.02 percentage points (P = .05).
The statistically significant but clinically insignificant increase in A1c following infection with COVID-19 was similar in people with and without type 2 diabetes prior to infection.
In patients with type 2 diabetes who became infected with COVID-19, the researchers saw significant positive associations between higher A1c levels before infection and time to hospitalization (hazard ratio, 1.07), need for assisted breathing (HR, 1.06), and ICU admission (HR, 1.07).
Following a COVID-19 infection, people were 40% more likely to receive a diagnosis of incident type 2 diabetes, compared with matched uninfected people. The authors said a possible explanation is that after diagnosis of COVID-19, infected people in general received more intensified care that led to better identification of those with underlying type 2 diabetes.
The 701 people included with pre-existing type 1 diabetes showed no significant difference in their rate of developing DKA between those infected and not infected with COVID-19.
Among the 21,830 people with pre-existing type 2 diabetes, the DKA risk was a significant 35% greater for those who were infected with COVID-19, compared with those who were uninfected. The magnitude of this increased relative risk was even higher among the patients with type 2 diabetes who used insulin as part of their treatment.
The difference in DKA risk didn’t differ between Black and White patients who were not infected with COVID-19, but among those infected by COVID-19, Black patients were more than twice as likely to be diagnosed with DKA, compared with White patients, a significant difference.
Black patients with type 2 diabetes who became infected with COVID-19 had a significant (63%) increased rate of DKA compared with Black patients with type 2 diabetes who remained uninfected.
Limitations
The study included patients with A1c measurements made up to 12 months prior to their COVID-19 test, and hence comorbid conditions, medication changes during this period, or other factors may have affected subsequent A1c levels. To address this, the authors also assessed outcomes at 3- and 6-month intervals, which produced results consistent with the 12-month findings.
The researchers did not have A1c values for many of the more than 234,000 people in the entire registry who underwent COVID-19 testing from March 2020-May 2021 at the Cleveland Clinic, omissions that may have biased the study cohort.
This was a single-center study. Some patients may have received care outside of the center, hence records of those episodes could not be included.
Disclosures
The study received no commercial funding. Four authors received consulting and speaker honoraria and research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Corcept Therapeutics, Diasome, Eli Lilly, Merck, Novo Nordisk, and Sanofi. Three authors have intellectual property related to treatment decisionmaking in the context of type 2 diabetes.
This is a summary of a preprint research study “Impacts of COVID-19 on glycemia and risk of diabetic ketoacidosis,” written by researchers at the Cleveland Clinic on medRxiv. The study has not yet been peer reviewed. The full text of the study can be found on medRxiv.org.
A version of this article first appeared on Medscape.com.
U.S. health officials tracking COVID-19 increase in U.K.
according to NPR.
Daily cases counts have increased 38% in the past week, according to the latest data from the U.K. Health Security Agency. Hospitalizations are up about 25% as well.
“Over the last year or so, what happens in the U.K. usually happens here a few weeks later,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told NPR.
“And right now, the U.K. is seeing somewhat of a rebound in cases,” he said.
Health officials in the United Kingdom have noted the latest increase is likely due to the contagious BA.2 Omicron subvariant, the recent loosening of coronavirus restrictions, and waning immunity from vaccinations and infections.
“All three of those factors we have here in the United States,” Dr. Fauci said. “So I would not be surprised if, in the next few weeks, we see either a plateauing … of cases or even [the curve] rebounds and slightly goes up.”
Right now, COVID-19 cases in the United Stastes have dropped to their lowest levels since July 2021, according to the latest Centers for Disease Control and Prevention data, with fewer than 30,000 daily cases. At the same time, the rate of decline in cases has slowed significantly and is beginning to plateau.
Public health experts are also pointing to wastewater surveillance data that shows an uptick in viral activity across the country. The CDC’s wastewater dashboard indicates that about 35% of sites that monitor wastewater are seeing an increase, with consistent growth in Florida, Rhode Island, and West Virginia.
“The power of wastewater surveillance is that it’s an early warning system,” Amy Kirby, the program lead for the CDC’s National Wastewater Surveillance System, told NPR.
“We are seeing evidence of increases in some communities across the country,” she said. “What looked like noise at the beginning of the week is starting to look like a true signal here at the end of the week.”
The wastewater system doesn’t distinguish between Omicron and subvariants such as BA.2. However, other CDC data has found an increase in BA.2 cases in the United States, making up about a quarter of new COVID-19 cases.
The BA.2 variant has roughly doubled each week for the last month, which means it could become the dominant coronavirus strain in the United States in coming weeks, according to USA Today. Cases appear to be spreading more quickly in the Northeast and West, making up about 39% of cases in New York and New Jersey last week.
BA.2 also accounts for nearly 39% of cases across the Northeast, including Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island and Vermont, USA Today reported. In the West, which includes Arizona, California and Nevada, the subvariant makes up about 28% of new cases. In the upper West, which includes Alaska, Oregon and Washington, about 26% of cases are BA.2.
The good news is that BA.2 “doesn’t seem to evade our vaccines or immunity any more than the prior Omicron [variant]. And it doesn’t seem to lead to any more increased severity of disease,” Rochelle Walensky, MD, the CDC director, told NPR’s Morning Edition on March 18.
The effects of BA.2 will likely depend on the immunity profile in the United States, including how long it’s been since someone was vaccinated, boosted, or recovered from an infection, she said.
Health officials are watching other countries with BA.2 increases, such as Germany, Italy, and the Netherlands. Many European countries have been reporting an uptick but not implementing major restrictions or shutdowns, USA Today reported.
The BA.2 variant likely won’t lead to a major surge in severe disease or strict COVID-19 measures, Dr. Fauci told NPR, but some coronavirus protocols may need to be implemented again if cases grow dramatically.
“We must be ready to pivot and, if necessary, to go back to stricter mitigation with regard to masks,” he said.
A version of this article first appeared on WebMD.com.
according to NPR.
Daily cases counts have increased 38% in the past week, according to the latest data from the U.K. Health Security Agency. Hospitalizations are up about 25% as well.
“Over the last year or so, what happens in the U.K. usually happens here a few weeks later,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told NPR.
“And right now, the U.K. is seeing somewhat of a rebound in cases,” he said.
Health officials in the United Kingdom have noted the latest increase is likely due to the contagious BA.2 Omicron subvariant, the recent loosening of coronavirus restrictions, and waning immunity from vaccinations and infections.
“All three of those factors we have here in the United States,” Dr. Fauci said. “So I would not be surprised if, in the next few weeks, we see either a plateauing … of cases or even [the curve] rebounds and slightly goes up.”
Right now, COVID-19 cases in the United Stastes have dropped to their lowest levels since July 2021, according to the latest Centers for Disease Control and Prevention data, with fewer than 30,000 daily cases. At the same time, the rate of decline in cases has slowed significantly and is beginning to plateau.
Public health experts are also pointing to wastewater surveillance data that shows an uptick in viral activity across the country. The CDC’s wastewater dashboard indicates that about 35% of sites that monitor wastewater are seeing an increase, with consistent growth in Florida, Rhode Island, and West Virginia.
“The power of wastewater surveillance is that it’s an early warning system,” Amy Kirby, the program lead for the CDC’s National Wastewater Surveillance System, told NPR.
“We are seeing evidence of increases in some communities across the country,” she said. “What looked like noise at the beginning of the week is starting to look like a true signal here at the end of the week.”
The wastewater system doesn’t distinguish between Omicron and subvariants such as BA.2. However, other CDC data has found an increase in BA.2 cases in the United States, making up about a quarter of new COVID-19 cases.
The BA.2 variant has roughly doubled each week for the last month, which means it could become the dominant coronavirus strain in the United States in coming weeks, according to USA Today. Cases appear to be spreading more quickly in the Northeast and West, making up about 39% of cases in New York and New Jersey last week.
BA.2 also accounts for nearly 39% of cases across the Northeast, including Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island and Vermont, USA Today reported. In the West, which includes Arizona, California and Nevada, the subvariant makes up about 28% of new cases. In the upper West, which includes Alaska, Oregon and Washington, about 26% of cases are BA.2.
The good news is that BA.2 “doesn’t seem to evade our vaccines or immunity any more than the prior Omicron [variant]. And it doesn’t seem to lead to any more increased severity of disease,” Rochelle Walensky, MD, the CDC director, told NPR’s Morning Edition on March 18.
The effects of BA.2 will likely depend on the immunity profile in the United States, including how long it’s been since someone was vaccinated, boosted, or recovered from an infection, she said.
Health officials are watching other countries with BA.2 increases, such as Germany, Italy, and the Netherlands. Many European countries have been reporting an uptick but not implementing major restrictions or shutdowns, USA Today reported.
The BA.2 variant likely won’t lead to a major surge in severe disease or strict COVID-19 measures, Dr. Fauci told NPR, but some coronavirus protocols may need to be implemented again if cases grow dramatically.
“We must be ready to pivot and, if necessary, to go back to stricter mitigation with regard to masks,” he said.
A version of this article first appeared on WebMD.com.
according to NPR.
Daily cases counts have increased 38% in the past week, according to the latest data from the U.K. Health Security Agency. Hospitalizations are up about 25% as well.
“Over the last year or so, what happens in the U.K. usually happens here a few weeks later,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told NPR.
“And right now, the U.K. is seeing somewhat of a rebound in cases,” he said.
Health officials in the United Kingdom have noted the latest increase is likely due to the contagious BA.2 Omicron subvariant, the recent loosening of coronavirus restrictions, and waning immunity from vaccinations and infections.
“All three of those factors we have here in the United States,” Dr. Fauci said. “So I would not be surprised if, in the next few weeks, we see either a plateauing … of cases or even [the curve] rebounds and slightly goes up.”
Right now, COVID-19 cases in the United Stastes have dropped to their lowest levels since July 2021, according to the latest Centers for Disease Control and Prevention data, with fewer than 30,000 daily cases. At the same time, the rate of decline in cases has slowed significantly and is beginning to plateau.
Public health experts are also pointing to wastewater surveillance data that shows an uptick in viral activity across the country. The CDC’s wastewater dashboard indicates that about 35% of sites that monitor wastewater are seeing an increase, with consistent growth in Florida, Rhode Island, and West Virginia.
“The power of wastewater surveillance is that it’s an early warning system,” Amy Kirby, the program lead for the CDC’s National Wastewater Surveillance System, told NPR.
“We are seeing evidence of increases in some communities across the country,” she said. “What looked like noise at the beginning of the week is starting to look like a true signal here at the end of the week.”
The wastewater system doesn’t distinguish between Omicron and subvariants such as BA.2. However, other CDC data has found an increase in BA.2 cases in the United States, making up about a quarter of new COVID-19 cases.
The BA.2 variant has roughly doubled each week for the last month, which means it could become the dominant coronavirus strain in the United States in coming weeks, according to USA Today. Cases appear to be spreading more quickly in the Northeast and West, making up about 39% of cases in New York and New Jersey last week.
BA.2 also accounts for nearly 39% of cases across the Northeast, including Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island and Vermont, USA Today reported. In the West, which includes Arizona, California and Nevada, the subvariant makes up about 28% of new cases. In the upper West, which includes Alaska, Oregon and Washington, about 26% of cases are BA.2.
The good news is that BA.2 “doesn’t seem to evade our vaccines or immunity any more than the prior Omicron [variant]. And it doesn’t seem to lead to any more increased severity of disease,” Rochelle Walensky, MD, the CDC director, told NPR’s Morning Edition on March 18.
The effects of BA.2 will likely depend on the immunity profile in the United States, including how long it’s been since someone was vaccinated, boosted, or recovered from an infection, she said.
Health officials are watching other countries with BA.2 increases, such as Germany, Italy, and the Netherlands. Many European countries have been reporting an uptick but not implementing major restrictions or shutdowns, USA Today reported.
The BA.2 variant likely won’t lead to a major surge in severe disease or strict COVID-19 measures, Dr. Fauci told NPR, but some coronavirus protocols may need to be implemented again if cases grow dramatically.
“We must be ready to pivot and, if necessary, to go back to stricter mitigation with regard to masks,” he said.
A version of this article first appeared on WebMD.com.
‘Vast majority’ of COVID patients wake up after mechanical ventilation
COVID-19 patients who are successfully weaned off a ventilator may take days, or even weeks, to regain consciousness, especially those who experienced episodes of hypoxemia while intubated, a new study shows.
“As we started to see the first patients waking up after successful COVID-19 ICU treatments, we also encountered many patients who remained comatose for days and weeks and then regained consciousness to become fully oriented,” co-senior investigator Nicholas Schiff, MD, with NewYork-Presbyterian/Weill Cornell Medical Center, says in a news release.
The findings have immediate implications regarding life-sustaining therapies for unresponsive COVID-19 patients, the investigators note.
“In critical care medicine, one of our main tasks is to advise families about planning in the event a patient does not regain consciousness,” said co-senior author Jan Claassen, MD, with New York-Presbyterian/Columbia University Irving Medical Center.
“Our findings suggest that for patients with severe COVID, the decision to withdraw life support shouldn’t be based solely on prolonged periods of unconsciousness, as these patients may eventually recover,” Dr. Claassen adds.
The study was published online March 7 in Annals of Neurology.
Slow road back
The researchers examined 795 intubated patients with severe COVID-19 at three medical centers in New York during the first wave of the pandemic (March-July 2020). All patients had impaired consciousness (Glasgow Coma Scale [GCS] motor score less than 6) on day 7 of intubation.
A total of 571 patients (72%) survived and regained consciousness.
The median time to recovery of consciousness was 30 days. One-quarter of the patients recovered consciousness 10 days or longer after they stopped receiving ventilator support and 10% took 23 days or longer to recover.
Time to recovery of consciousness was associated with hypoxemia. The hazard ratio was 0.56 (95% confidence interval, 0.46-0.68) with arterial partial pressure of oxygen (PaO2) less than or equal to 55 mm Hg and 0.88 (95% CI, 0.85-0.91) with a PaO2 less than or equal to 70 mm Hg.
Each additional day of hypoxemia decreased the odds of recovery of consciousness after accounting for confounding factors including sedation.
These findings were confirmed among patients without any imaging evidence of structural brain injury and in a non-overlapping cohort of 427 patients from the second wave of the pandemic (October-April 2021).
“These findings provide us with more accurate information to guide families who are deciding whether to continue life-sustaining therapy in unconscious COVID-19 patients,” co-senior author Brian Edlow, MD, with Massachusetts General Hospital and Harvard Medical School in Boston, says in the news release.
“Encouragingly,” adds Dr. Claassen, “our study shows that the vast majority of unconscious COVID patients recover consciousness, but it is important to consider that we did not look at the quality of recovery. That’s something that should be the focus of long-term follow-up studies.”
The study was supported by the James S. McDonnell Foundation (JSMF). Dr. Schiff, Dr. Claassen, and Dr. Edlow have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 patients who are successfully weaned off a ventilator may take days, or even weeks, to regain consciousness, especially those who experienced episodes of hypoxemia while intubated, a new study shows.
“As we started to see the first patients waking up after successful COVID-19 ICU treatments, we also encountered many patients who remained comatose for days and weeks and then regained consciousness to become fully oriented,” co-senior investigator Nicholas Schiff, MD, with NewYork-Presbyterian/Weill Cornell Medical Center, says in a news release.
The findings have immediate implications regarding life-sustaining therapies for unresponsive COVID-19 patients, the investigators note.
“In critical care medicine, one of our main tasks is to advise families about planning in the event a patient does not regain consciousness,” said co-senior author Jan Claassen, MD, with New York-Presbyterian/Columbia University Irving Medical Center.
“Our findings suggest that for patients with severe COVID, the decision to withdraw life support shouldn’t be based solely on prolonged periods of unconsciousness, as these patients may eventually recover,” Dr. Claassen adds.
The study was published online March 7 in Annals of Neurology.
Slow road back
The researchers examined 795 intubated patients with severe COVID-19 at three medical centers in New York during the first wave of the pandemic (March-July 2020). All patients had impaired consciousness (Glasgow Coma Scale [GCS] motor score less than 6) on day 7 of intubation.
A total of 571 patients (72%) survived and regained consciousness.
The median time to recovery of consciousness was 30 days. One-quarter of the patients recovered consciousness 10 days or longer after they stopped receiving ventilator support and 10% took 23 days or longer to recover.
Time to recovery of consciousness was associated with hypoxemia. The hazard ratio was 0.56 (95% confidence interval, 0.46-0.68) with arterial partial pressure of oxygen (PaO2) less than or equal to 55 mm Hg and 0.88 (95% CI, 0.85-0.91) with a PaO2 less than or equal to 70 mm Hg.
Each additional day of hypoxemia decreased the odds of recovery of consciousness after accounting for confounding factors including sedation.
These findings were confirmed among patients without any imaging evidence of structural brain injury and in a non-overlapping cohort of 427 patients from the second wave of the pandemic (October-April 2021).
“These findings provide us with more accurate information to guide families who are deciding whether to continue life-sustaining therapy in unconscious COVID-19 patients,” co-senior author Brian Edlow, MD, with Massachusetts General Hospital and Harvard Medical School in Boston, says in the news release.
“Encouragingly,” adds Dr. Claassen, “our study shows that the vast majority of unconscious COVID patients recover consciousness, but it is important to consider that we did not look at the quality of recovery. That’s something that should be the focus of long-term follow-up studies.”
The study was supported by the James S. McDonnell Foundation (JSMF). Dr. Schiff, Dr. Claassen, and Dr. Edlow have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 patients who are successfully weaned off a ventilator may take days, or even weeks, to regain consciousness, especially those who experienced episodes of hypoxemia while intubated, a new study shows.
“As we started to see the first patients waking up after successful COVID-19 ICU treatments, we also encountered many patients who remained comatose for days and weeks and then regained consciousness to become fully oriented,” co-senior investigator Nicholas Schiff, MD, with NewYork-Presbyterian/Weill Cornell Medical Center, says in a news release.
The findings have immediate implications regarding life-sustaining therapies for unresponsive COVID-19 patients, the investigators note.
“In critical care medicine, one of our main tasks is to advise families about planning in the event a patient does not regain consciousness,” said co-senior author Jan Claassen, MD, with New York-Presbyterian/Columbia University Irving Medical Center.
“Our findings suggest that for patients with severe COVID, the decision to withdraw life support shouldn’t be based solely on prolonged periods of unconsciousness, as these patients may eventually recover,” Dr. Claassen adds.
The study was published online March 7 in Annals of Neurology.
Slow road back
The researchers examined 795 intubated patients with severe COVID-19 at three medical centers in New York during the first wave of the pandemic (March-July 2020). All patients had impaired consciousness (Glasgow Coma Scale [GCS] motor score less than 6) on day 7 of intubation.
A total of 571 patients (72%) survived and regained consciousness.
The median time to recovery of consciousness was 30 days. One-quarter of the patients recovered consciousness 10 days or longer after they stopped receiving ventilator support and 10% took 23 days or longer to recover.
Time to recovery of consciousness was associated with hypoxemia. The hazard ratio was 0.56 (95% confidence interval, 0.46-0.68) with arterial partial pressure of oxygen (PaO2) less than or equal to 55 mm Hg and 0.88 (95% CI, 0.85-0.91) with a PaO2 less than or equal to 70 mm Hg.
Each additional day of hypoxemia decreased the odds of recovery of consciousness after accounting for confounding factors including sedation.
These findings were confirmed among patients without any imaging evidence of structural brain injury and in a non-overlapping cohort of 427 patients from the second wave of the pandemic (October-April 2021).
“These findings provide us with more accurate information to guide families who are deciding whether to continue life-sustaining therapy in unconscious COVID-19 patients,” co-senior author Brian Edlow, MD, with Massachusetts General Hospital and Harvard Medical School in Boston, says in the news release.
“Encouragingly,” adds Dr. Claassen, “our study shows that the vast majority of unconscious COVID patients recover consciousness, but it is important to consider that we did not look at the quality of recovery. That’s something that should be the focus of long-term follow-up studies.”
The study was supported by the James S. McDonnell Foundation (JSMF). Dr. Schiff, Dr. Claassen, and Dr. Edlow have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF NEUROLOGY
Aiming for System Improvement While Transitioning to the New Normal
As we transition out of the Omicron surge, the lessons we’ve learned from the prior surges carry forward and add to our knowledge foundation. Medical journals have published numerous research and perspectives manuscripts on all aspects of COVID-19 over the past 2 years, adding much-needed knowledge to our clinical practice during the pandemic. However, the story does not stop there, as the pandemic has impacted the usual, non-COVID-19 clinical care we provide. The value-based health care delivery model accounts for both COVID-19 clinical care and the usual care we provide our patients every day. Clinicians, administrators, and health care workers will need to know how to balance both worlds in the years to come.
In this issue of JCOM, the work of balancing the demands of COVID-19 care with those of system improvement continues. Two original research articles address the former, with Liesching et al1 reporting data on improving clinical outcomes of patients with COVID-19 through acute care oxygen therapies, and Ali et al2 explaining the impact of COVID-19 on STEMI care delivery models. Liesching et al’s study showed that patients admitted for COVID-19 after the first surge were more likely to receive high-flow nasal cannula and had better outcomes, while Ali et al showed that patients with STEMI yet again experienced worse outcomes during the first wave.
On the system improvement front, Cusick et al3 report on a quality improvement (QI) project that addressed acute disease management of heparin-induced thrombocytopenia (HIT) during hospitalization, Sosa et al4 discuss efforts to improve comorbidity capture at their institution, and Uche et al5 present the results of a nonpharmacologic initiative to improve management of chronic pain among veterans. Cusick et al’s QI project showed that a HIT testing strategy could be safely implemented through an evidence-based process to nudge resource utilization using specific management pathways. While capturing and measuring the complexity of diseases and comorbidities can be challenging, accurate capture is essential, as patient acuity has implications for reimbursement and quality comparisons for hospitals and physicians; Sosa et al describe a series of initiatives implemented at their institution that improved comorbidity capture. Furthermore, Uche et al report on a 10-week complementary and integrative health program for veterans with noncancer chronic pain that reduced pain intensity and improved quality of life for its participants. These QI reports show that, though the health care landscape has changed over the past 2 years, the aim remains the same: to provide the best care for patients regardless of the diagnosis, location, or time.
Conducting QI projects during the COVID-19 pandemic has been difficult, especially in terms of implementing consistent processes and management pathways while contending with staff and supply shortages. The pandemic, however, has highlighted the importance of continuing QI efforts, specifically around infectious disease prevention and good clinical practices. Moreover, the recent continuous learning and implementation around COVID-19 patient care has been a significant achievement, as clinicians and administrators worked continuously to understand and improve processes, create a supporting culture, and redesign care delivery on the fly. The management of both COVID-19 care and our usual care QI efforts should incorporate the lessons learned from the pandemic and leverage system redesign for future steps. As we’ve seen, survival in COVID-19 improved dramatically since the beginning of the pandemic, as clinical trials became more adaptive and efficient and system upgrades like telemedicine and digital technologies in the public health response led to major advancements. The work to improve the care provided in the clinic and at the bedside will continue through one collective approach in the new normal.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine Brigham and Women’s Hospital, Boston, MA; [email protected]
1. Liesching TN, Lei Y. Oxygen therapies and clinical outcomes for patients hospitalized with covid-19: first surge vs second surge. J Clin Outcomes Manag. 2022;29(2):58-64. doi:10.12788/jcom.0086
2. Ali SH, Hyer S, Davis K, Murrow JR. Acute STEMI during the COVID-19 pandemic at Piedmont Athens Regional: incidence, clinical characteristics, and outcomes. J Clin Outcomes Manag. 2022;29(2):65-71. doi:10.12788/jcom.0085
3. Cusick A, Hanigan S, Bashaw L, et al. A practical and cost-effective approach to the diagnosis of heparin-induced thrombocytopenia: a single-center quality improvement study. J Clin Outcomes Manag. 2022;29(2):72-77.
4. Sosa MA, Ferreira T, Gershengorn H, et al. Improving hospital metrics through the implementation of a comorbidity capture tool and other quality initiatives. J Clin Outcomes Manag. 2022;29(2):80-87. doi:10.12788/jcom.00885. Uche JU, Jamison M, Waugh S. Evaluation of the Empower Veterans Program for military veterans with chronic pain. J Clin Outcomes Manag. 2022;29(2):88-95. doi:10.12788/jcom.0089
As we transition out of the Omicron surge, the lessons we’ve learned from the prior surges carry forward and add to our knowledge foundation. Medical journals have published numerous research and perspectives manuscripts on all aspects of COVID-19 over the past 2 years, adding much-needed knowledge to our clinical practice during the pandemic. However, the story does not stop there, as the pandemic has impacted the usual, non-COVID-19 clinical care we provide. The value-based health care delivery model accounts for both COVID-19 clinical care and the usual care we provide our patients every day. Clinicians, administrators, and health care workers will need to know how to balance both worlds in the years to come.
In this issue of JCOM, the work of balancing the demands of COVID-19 care with those of system improvement continues. Two original research articles address the former, with Liesching et al1 reporting data on improving clinical outcomes of patients with COVID-19 through acute care oxygen therapies, and Ali et al2 explaining the impact of COVID-19 on STEMI care delivery models. Liesching et al’s study showed that patients admitted for COVID-19 after the first surge were more likely to receive high-flow nasal cannula and had better outcomes, while Ali et al showed that patients with STEMI yet again experienced worse outcomes during the first wave.
On the system improvement front, Cusick et al3 report on a quality improvement (QI) project that addressed acute disease management of heparin-induced thrombocytopenia (HIT) during hospitalization, Sosa et al4 discuss efforts to improve comorbidity capture at their institution, and Uche et al5 present the results of a nonpharmacologic initiative to improve management of chronic pain among veterans. Cusick et al’s QI project showed that a HIT testing strategy could be safely implemented through an evidence-based process to nudge resource utilization using specific management pathways. While capturing and measuring the complexity of diseases and comorbidities can be challenging, accurate capture is essential, as patient acuity has implications for reimbursement and quality comparisons for hospitals and physicians; Sosa et al describe a series of initiatives implemented at their institution that improved comorbidity capture. Furthermore, Uche et al report on a 10-week complementary and integrative health program for veterans with noncancer chronic pain that reduced pain intensity and improved quality of life for its participants. These QI reports show that, though the health care landscape has changed over the past 2 years, the aim remains the same: to provide the best care for patients regardless of the diagnosis, location, or time.
Conducting QI projects during the COVID-19 pandemic has been difficult, especially in terms of implementing consistent processes and management pathways while contending with staff and supply shortages. The pandemic, however, has highlighted the importance of continuing QI efforts, specifically around infectious disease prevention and good clinical practices. Moreover, the recent continuous learning and implementation around COVID-19 patient care has been a significant achievement, as clinicians and administrators worked continuously to understand and improve processes, create a supporting culture, and redesign care delivery on the fly. The management of both COVID-19 care and our usual care QI efforts should incorporate the lessons learned from the pandemic and leverage system redesign for future steps. As we’ve seen, survival in COVID-19 improved dramatically since the beginning of the pandemic, as clinical trials became more adaptive and efficient and system upgrades like telemedicine and digital technologies in the public health response led to major advancements. The work to improve the care provided in the clinic and at the bedside will continue through one collective approach in the new normal.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine Brigham and Women’s Hospital, Boston, MA; [email protected]
As we transition out of the Omicron surge, the lessons we’ve learned from the prior surges carry forward and add to our knowledge foundation. Medical journals have published numerous research and perspectives manuscripts on all aspects of COVID-19 over the past 2 years, adding much-needed knowledge to our clinical practice during the pandemic. However, the story does not stop there, as the pandemic has impacted the usual, non-COVID-19 clinical care we provide. The value-based health care delivery model accounts for both COVID-19 clinical care and the usual care we provide our patients every day. Clinicians, administrators, and health care workers will need to know how to balance both worlds in the years to come.
In this issue of JCOM, the work of balancing the demands of COVID-19 care with those of system improvement continues. Two original research articles address the former, with Liesching et al1 reporting data on improving clinical outcomes of patients with COVID-19 through acute care oxygen therapies, and Ali et al2 explaining the impact of COVID-19 on STEMI care delivery models. Liesching et al’s study showed that patients admitted for COVID-19 after the first surge were more likely to receive high-flow nasal cannula and had better outcomes, while Ali et al showed that patients with STEMI yet again experienced worse outcomes during the first wave.
On the system improvement front, Cusick et al3 report on a quality improvement (QI) project that addressed acute disease management of heparin-induced thrombocytopenia (HIT) during hospitalization, Sosa et al4 discuss efforts to improve comorbidity capture at their institution, and Uche et al5 present the results of a nonpharmacologic initiative to improve management of chronic pain among veterans. Cusick et al’s QI project showed that a HIT testing strategy could be safely implemented through an evidence-based process to nudge resource utilization using specific management pathways. While capturing and measuring the complexity of diseases and comorbidities can be challenging, accurate capture is essential, as patient acuity has implications for reimbursement and quality comparisons for hospitals and physicians; Sosa et al describe a series of initiatives implemented at their institution that improved comorbidity capture. Furthermore, Uche et al report on a 10-week complementary and integrative health program for veterans with noncancer chronic pain that reduced pain intensity and improved quality of life for its participants. These QI reports show that, though the health care landscape has changed over the past 2 years, the aim remains the same: to provide the best care for patients regardless of the diagnosis, location, or time.
Conducting QI projects during the COVID-19 pandemic has been difficult, especially in terms of implementing consistent processes and management pathways while contending with staff and supply shortages. The pandemic, however, has highlighted the importance of continuing QI efforts, specifically around infectious disease prevention and good clinical practices. Moreover, the recent continuous learning and implementation around COVID-19 patient care has been a significant achievement, as clinicians and administrators worked continuously to understand and improve processes, create a supporting culture, and redesign care delivery on the fly. The management of both COVID-19 care and our usual care QI efforts should incorporate the lessons learned from the pandemic and leverage system redesign for future steps. As we’ve seen, survival in COVID-19 improved dramatically since the beginning of the pandemic, as clinical trials became more adaptive and efficient and system upgrades like telemedicine and digital technologies in the public health response led to major advancements. The work to improve the care provided in the clinic and at the bedside will continue through one collective approach in the new normal.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine Brigham and Women’s Hospital, Boston, MA; [email protected]
1. Liesching TN, Lei Y. Oxygen therapies and clinical outcomes for patients hospitalized with covid-19: first surge vs second surge. J Clin Outcomes Manag. 2022;29(2):58-64. doi:10.12788/jcom.0086
2. Ali SH, Hyer S, Davis K, Murrow JR. Acute STEMI during the COVID-19 pandemic at Piedmont Athens Regional: incidence, clinical characteristics, and outcomes. J Clin Outcomes Manag. 2022;29(2):65-71. doi:10.12788/jcom.0085
3. Cusick A, Hanigan S, Bashaw L, et al. A practical and cost-effective approach to the diagnosis of heparin-induced thrombocytopenia: a single-center quality improvement study. J Clin Outcomes Manag. 2022;29(2):72-77.
4. Sosa MA, Ferreira T, Gershengorn H, et al. Improving hospital metrics through the implementation of a comorbidity capture tool and other quality initiatives. J Clin Outcomes Manag. 2022;29(2):80-87. doi:10.12788/jcom.00885. Uche JU, Jamison M, Waugh S. Evaluation of the Empower Veterans Program for military veterans with chronic pain. J Clin Outcomes Manag. 2022;29(2):88-95. doi:10.12788/jcom.0089
1. Liesching TN, Lei Y. Oxygen therapies and clinical outcomes for patients hospitalized with covid-19: first surge vs second surge. J Clin Outcomes Manag. 2022;29(2):58-64. doi:10.12788/jcom.0086
2. Ali SH, Hyer S, Davis K, Murrow JR. Acute STEMI during the COVID-19 pandemic at Piedmont Athens Regional: incidence, clinical characteristics, and outcomes. J Clin Outcomes Manag. 2022;29(2):65-71. doi:10.12788/jcom.0085
3. Cusick A, Hanigan S, Bashaw L, et al. A practical and cost-effective approach to the diagnosis of heparin-induced thrombocytopenia: a single-center quality improvement study. J Clin Outcomes Manag. 2022;29(2):72-77.
4. Sosa MA, Ferreira T, Gershengorn H, et al. Improving hospital metrics through the implementation of a comorbidity capture tool and other quality initiatives. J Clin Outcomes Manag. 2022;29(2):80-87. doi:10.12788/jcom.00885. Uche JU, Jamison M, Waugh S. Evaluation of the Empower Veterans Program for military veterans with chronic pain. J Clin Outcomes Manag. 2022;29(2):88-95. doi:10.12788/jcom.0089
Acute STEMI During the COVID-19 Pandemic at a Regional Hospital: Incidence, Clinical Characteristics, and Outcomes
From the Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, Athens, GA (Syed H. Ali, Syed Hyder, and Dr. Murrow), and the Department of Cardiology, Piedmont Heart Institute, Piedmont Athens Regional, Athens, GA (Dr. Murrow and Mrs. Davis).
Abstract
Objectives: The aim of this study was to describe the characteristics and in-hospital outcomes of patients with acute ST-segment elevation myocardial infarction (STEMI) during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods: A retrospective study was conducted at PAR to evaluate patients with acute STEMI admitted over an 8-week period during the initial COVID-19 outbreak. This study group was compared to patients admitted during the corresponding period in 2019. The primary endpoint of this study was defined as a composite of sustained ventricular arrhythmia, congestive heart failure (CHF) with pulmonary congestion, and/or in-hospital mortality.
Results: This study cohort was composed of 64 patients with acute STEMI; 30 patients (46.9%) were hospitalized during the COVID-19 pandemic. Patients with STEMI in both the COVID-19 and control groups had similar comorbidities, Killip classification score, and clinical presentations. The median (interquartile range) time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (84.8-132) in 2019 to 149 minutes (96.3-231.8; P = .032) in 2020. Hospitalization during the COVID-19 period was associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046).
Conclusion: Patients with STEMI admitted during the first wave of the COVID-19 outbreak experienced longer total ischemic time and increased risk for combined in-hospital outcomes compared to patients admitted during the corresponding period in 2019.
Keywords: myocardial infarction, acute coronary syndrome, hospitalization, outcomes.

The emergence of the SARS-Cov-2 virus in December 2019 caused a worldwide shift in resource allocation and the restructuring of health care systems within the span of a few months. With the rapid spread of infection, the World Health Organization officially declared a pandemic in March 2020. The pandemic led to the deferral and cancellation of in-person patient visits, routine diagnostic studies, and nonessential surgeries and procedures. This response occurred secondary to a joint effort to reduce transmission via stay-at-home mandates and appropriate social distancing.1
Alongside the reduction in elective procedures and health care visits, significant reductions in hospitalization rates due to decreases in acute ST-segment elevation myocardial infarction (STEMI) and catheterization laboratory utilization have been reported in many studies from around the world.2-7 Comprehensive data demonstrating the impact of the COVID-19 pandemic on acute STEMI patient characteristics, clinical presentation, and in-hospital outcomes are lacking. Although patients with previously diagnosed cardiovascular disease are more likely to encounter worse outcomes in the setting of COVID-19, there may also be an indirect impact of the pandemic on high-risk patients, including those without the infection.8 Several theories have been hypothesized to explain this phenomenon. One theory postulates that the fear of contracting the virus during hospitalization is great enough to prevent patients from seeking care.2 Another theory suggests that the increased utilization of telemedicine prevents exacerbation of chronic conditions and the need for hospitalization.9 Contrary to this trend, previous studies have shown an increased incidence of acute STEMI following stressful events such as natural disasters.10
The aim of this study was to describe trends pertaining to clinical characteristics and in-hospital outcomes of patients with acute STEMI during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods
A retrospective cohort study was conducted at PAR to evaluate patients with STEMI admitted to the cardiovascular intensive care unit over an 8-week period (March 5 to May 5, 2020) during the COVID-19 outbreak. COVID-19 was declared a national emergency on March 13, 2020, in the United States. The institutional review board at PAR approved the study; the need for individual consent was waived under the condition that participant data would undergo de-identification and be strictly safeguarded.
Data Collection
Because there are seasonal variations in cardiovascular admissions, patient data from a control period (March 9 to May 9, 2019) were obtained to compare with data from the 2020 period. The number of patients with the diagnosis of acute STEMI during the COVID-19 period was recorded. Demographic data, clinical characteristics, and primary angiographic findings were gathered for all patients. Time from symptom onset to hospital admission and time from hospital admission to reperfusion (defined as door-to-balloon time) were documented for each patient. Killip classification was used to assess patients’ clinical status on admission. Length of stay was determined as days from hospital admission to discharge or death (if occurring during the same hospitalization).
Adverse in-hospital complications were also recorded. These were selected based on inclusion of the following categories of acute STEMI complications: ischemic, mechanical, arrhythmic, embolic, and inflammatory. The following complications occurred in our patient cohort: sustained ventricular arrhythmia, congestive heart failure (CHF) defined as congestion requiring intravenous diuretics, re-infarction, mechanical complications (free-wall rupture, ventricular septal defect, or mitral regurgitation), second- or third-degree atrioventricular block, atrial fibrillation, stroke, mechanical ventilation, major bleeding, pericarditis, cardiogenic shock, cardiac arrest, and in-hospital mortality. The primary outcome of this study was defined as a composite of sustained ventricular arrhythmia, CHF with congestion requiring intravenous diuretics, and/or in-hospital mortality. Ventricular arrythmia and CHF were included in the composite outcome because they are defined as the 2 most common causes of sudden cardiac death following acute STEMI.11,12
Statistical Analysis
Normally distributed continuous variables and categorical variables were compared using the paired t-test. A 2-sided P value <.05 was considered to be statistically significant. Mean admission rates for acute STEMI hospitalizations were determined by dividing the number of admissions by the number of days in each time period. The daily rate of COVID-19 cases per 100,000 individuals was obtained from the Centers for Disease Control and Prevention COVID-19 database. All data analyses were performed using Microsoft Excel.
Results
The study cohort consisted of 64 patients, of whom 30 (46.9%) were hospitalized between March 5 and May 5, 2020, and 34 (53.1%) who were admitted during the analogous time period in 2019. This reflected a 6% decrease in STEMI admissions at PAR in the COVID-19 cohort.
Acute STEMI Hospitalization Rates and COVID-19 Incidence
The mean daily acute STEMI admission rate was 0.50 during the study period compared to 0.57 during the control period. During the study period in 2020 in the state of Georgia, the daily rate of newly confirmed COVID-19 cases ranged from 0.194 per 100,000 on March 5 to 8.778 per 100,000 on May 5. Results of COVID-19 testing were available for 9 STEMI patients, and of these 0 tests were positive.
Baseline Characteristics
Baseline characteristics of the acute STEMI cohorts are presented in Table 1. Approximately 75% were male; median (interquartile range [IQR]) age was 60 (51-72) years. There were no significant differences in age and gender between the study periods. Three-quarters of patients had a history of hypertension, and 87.5% had a history of dyslipidemia. There was no significant difference in baseline comorbidity profiles between the 2 study periods; therefore, our sample populations shared similar characteristics.

Clinical Presentation
Significant differences were observed regarding the time intervals of STEMI patients in the COVID-19 period and the control period (Table 2). Median time from symptom onset to hospital admission (patient delay) was extended from 57.5 minutes (IQR, 40.3-106) in 2019 to 93 minutes (IQR, 48.8-132) in 2020; however, this difference was not statistically significant (P = .697). Median time from hospital admission to reperfusion (system delay) was prolonged from 45 minutes (IQR, 28-61) in 2019 to 78 minutes (IQR, 50-110) in 2020 (P < .001). Overall time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (IQR, 84.8-132) in 2019 to 149 minutes (IQR, 96.3-231.8) in 2020 (P = .032).

Regarding mode of transportation, 23.5% of patients in 2019 were walk-in admissions to the emergency department. During the COVID-19 period, walk-in admissions decreased to 6.7% (P = .065). There were no significant differences between emergency medical service, transfer, or in-patient admissions for STEMI cases between the 2 study periods.
Killip classification scores were calculated for all patients on admission; 90.6% of patients were classified as Killip Class 1. There was no significant difference between hemodynamic presentations during the COVID-19 period compared to the control period.
Angiographic Data
Overall, 53 (82.8%) patients admitted with acute STEMI underwent coronary angiography during their hospital stay. The proportion of patients who underwent primary reperfusion was greater in the control period than in the COVID-19 period (85.3% vs 80%; P = .582). Angiographic characteristics and findings were similar between the 2 study groups (Table 2).
In-Hospital Outcomes
In-hospital outcome data were available for all patients. As shown in Table 3, hospitalization during the COVID-19 period was independently associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046). The rate of in-hospital mortality was greater in the COVID-19 period (P = .013). We found no significant difference when comparing secondary outcomes from admissions during the COVID-19 period and the control period in 2019. For the 5 patients who died during the study period, the primary diagnosis at death was acute STEMI complicated by CHF (3 patients) or cardiogenic shock (2 patients).

Discussion
This single-center retrospective study at PAR looks at the impact of COVID-19 on hospitalizations for acute STEMI during the initial peak of the pandemic. The key findings of this study show a significant increase in ischemic time parameters (symptom onset to reperfusion, hospital admission to reperfusion), in-hospital mortality, and combined in-hospital outcomes.
There was a 49.5-minute increase in total ischemic time noted in this study (P = .032). Though there was a numerical increase in time of symptom onset to hospital admission by 23.5 minutes, this difference was not statistically significant (P = .697). However, this study observed a statistically significant 33-minute increase in ischemic time from hospital admission to reperfusion (P < .001). Multiple studies globally have found a similar increase in total ischemic times, including those conducted in China and Europe.13-15 Every level of potential delay must be considered, including pre-hospital, triage and emergency department, and/or reperfusion team. Pre-hospital sources of delays that have been suggested include “stay-at-home” orders and apprehension to seek medical care due to concern about contracting the virus or overwhelming the health care facilities. There was a clinically significant 4-fold decrease in the number of walk-in acute STEMI cases in the study period. In 2019, there were 8 walk-in cases compared to 2 cases in 2020 (P = .065). However, this change was not statistically significant. In-hospital/systemic sources of delays have been mentioned in other studies; they include increased time taken to rule out COVID-19 (nasopharyngeal swab/chest x-ray) and increased time due to the need for intensive gowning and gloving procedures by staff. It was difficult to objectively determine the sources of system delay by the reperfusion team due to a lack of quantitative data.
In the current study, we found a significant increase in in-hospital mortality during the COVID-19 period compared to a parallel time frame in 2019. This finding is contrary to a multicenter study from Spain that reported no difference in in-hospital outcomes or mortality rates among all acute coronary syndrome cases.16 The worsening outcomes and prognosis may simply be a result of increased ischemic time; however, the virus that causes COVID-19 itself may play a role as well. Studies have found that SARS-Cov-2 infection places patients at greater risk for cardiovascular conditions such as hypercoagulability, myocarditis, and arrhythmias.17 In our study, however, there were no acute STEMI patients who tested positive for COVID-19. Therefore, we cannot discuss the impact of increased thrombus burden in patients with COVID-19. Piedmont Healthcare published a STEMI treatment protocol in May 2020 that advised increased use of tissue plasminogen activator (tPA) in COVID-19-positive cases; during the study period, however, there were no occasions when tPA use was deemed appropriate based on clinical judgment.
Our findings align with previous studies that describe an increase in combined in-hospital adverse outcomes during the COVID-19 era. Previous studies detected a higher rate of complications in the COVID-19 cohort, but in the current study, the adverse in-hospital course is unrelated to underlying infection.18,19 This study reports a higher incidence of major in-hospital outcomes, including a 65% increase in the rate of combined in-hospital outcomes, which is similar to a multicenter study conducted in Israel.19 There was a 2.3-fold numerical increase in sustained ventricular arrhythmias and a 2.5-fold numerical increase in the incidence of cardiac arrest in the study period. This phenomenon was observed despite a similar rate of reperfusion procedures in both groups.
Acute STEMI is a highly fatal condition with an incidence of 8.5 in 10,000 annually in the United States. While studies across the world have shown a 25% to 40% reduction in the rate of hospitalized acute coronary syndrome cases during the COVID-19 pandemic, the decrease from 34 to 30 STEMI admissions at PAR is not statistically significant.20 Possible reasons for the reduction globally include increased out-of-hospital mortality and decreased incidence of acute STEMI across the general population as a result of improved access to telemedicine or decreased levels of life stressors.20
In summary, there was an increase in ischemic time to reperfusion, in-hospital mortality, and combined in-hospital outcomes for acute STEMI patients at PAR during the COVID period.
Limitations
This study has several limitations. This is a single-center study, so the sample size is small and may not be generalizable to a larger population. This is a retrospective observational study, so causation cannot be inferred. This study analyzed ischemic time parameters as average rates over time rather than in an interrupted time series. Post-reperfusion outcomes were limited to hospital stay. Post-hospital follow-up would provide a better picture of the effects of STEMI intervention. There is no account of patients who died out-of-hospital secondary to acute STEMI. COVID-19 testing was not introduced until midway in our study period. Therefore, we cannot rule out the possibility of the SARS-Cov-2 virus inciting acute STEMI and subsequently leading to worse outcomes and poor prognosis.
Conclusions
This study provides an analysis of the incidence, characteristics, and clinical outcomes of patients presenting with acute STEMI during the early period of the COVID-19 pandemic. In-hospital mortality and ischemic time to reperfusion increased while combined in-hospital outcomes worsened.
Acknowledgment: The authors thank Piedmont Athens Regional IRB for approving this project and allowing access to patient data.
Corresponding author: Syed H. Ali; Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, 30606, Athens, GA; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0085
1. Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
2. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJR. Decline of acute coronary syndrome admissions in Austria since the outbreak of Covid-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852-1853. doi:10.1093/eurheartj/ehaa314
3. De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the Covid-19 era. Eur Heart J. 2020;41(22):2083-2088.
4. Wilson SJ, Connolly MJ, Elghamry Z, et al. Effect of the COVID-19 pandemic on ST-segment-elevation myocardial infarction presentations and in-hospital outcomes. Circ Cardiovasc Interv. 2020; 13(7):e009438. doi:10.1161/CIRCINTERVENTIONS.120.009438
5. Mafham MM, Spata E, Goldacre R, et al. Covid-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396 (10248):381-389. doi:10.1016/S0140-6736(20)31356-8
6. Bhatt AS, Moscone A, McElrath EE, et al. Fewer Hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
7. Tam CF, Cheung KS, Lam S, et al. Impact of Coronavirus disease 2019 (Covid-19) outbreak on ST-segment elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13(4):e006631. doi:10.1161/CIRCOUTCOMES.120.006631
8. Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648-1655. doi:10.1161/CIRCULATIONAHA.120.046941
9. Ebinger JE, Shah PK. Declining admissions for acute cardiovascular illness: The Covid-19 paradox. J Am Coll Cardiol. 2020;76(3):289-291. doi:10.1016/j.jacc.2020.05.039
10 Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334(7):413-419. doi:10.1056/NEJM199602153340701
11. Hiramori K. Major causes of death from acute myocardial infarction in a coronary care unit. Jpn Circ J. 1987;51(9):1041-1047. doi:10.1253/jcj.51.1041
12. Bui AH, Waks JW. Risk stratification of sudden cardiac death after acute myocardial infarction. J Innov Card Rhythm Manag. 2018;9(2):3035-3049. doi:10.19102/icrm.2018.090201
13. Xiang D, Xiang X, Zhang W, et al. Management and outcomes of patients with STEMI during the COVID-19 pandemic in China. J Am Coll Cardiol. 2020;76(11):1318-1324. doi:10.1016/j.jacc.2020.06.039
14. Hakim R, Motreff P, Rangé G. COVID-19 and STEMI. [Article in French]. Ann Cardiol Angeiol (Paris). 2020;69(6):355-359. doi:10.1016/j.ancard.2020.09.034
15. Soylu K, Coksevim M, Yanık A, Bugra Cerik I, Aksan G. Effect of Covid-19 pandemic process on STEMI patients timeline. Int J Clin Pract. 2021;75(5):e14005. doi:10.1111/ijcp.14005
16. Salinas P, Travieso A, Vergara-Uzcategui C, et al. Clinical profile and 30-day mortality of invasively managed patients with suspected acute coronary syndrome during the COVID-19 outbreak. Int Heart J. 2021;62(2):274-281. doi:10.1536/ihj.20-574
17. Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (Covid-19): a systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi:10.1016/j.jcv.2020.104371
18. Rodriguez-Leor O, Cid Alvarez AB, Perez de Prado A, et al. In-hospital outcomes of COVID-19 ST-elevation myocardial infarction patients. EuroIntervention. 2021;16(17):1426-1433. doi:10.4244/EIJ-D-20-00935
19. Fardman A, Zahger D, Orvin K, et al. Acute myocardial infarction in the Covid-19 era: incidence, clinical characteristics and in-hospital outcomes—A multicenter registry. PLoS ONE. 2021;16(6): e0253524. doi:10.1371/journal.pone.0253524
20. Pessoa-Amorim G, Camm CF, Gajendragadkar P, et al. Admission of patients with STEMI since the outbreak of the COVID-19 pandemic: a survey by the European Society of Cardiology. Eur Heart J Qual Care Clin Outcomes. 2020;6(3):210-216. doi:10.1093/ehjqcco/qcaa046
From the Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, Athens, GA (Syed H. Ali, Syed Hyder, and Dr. Murrow), and the Department of Cardiology, Piedmont Heart Institute, Piedmont Athens Regional, Athens, GA (Dr. Murrow and Mrs. Davis).
Abstract
Objectives: The aim of this study was to describe the characteristics and in-hospital outcomes of patients with acute ST-segment elevation myocardial infarction (STEMI) during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods: A retrospective study was conducted at PAR to evaluate patients with acute STEMI admitted over an 8-week period during the initial COVID-19 outbreak. This study group was compared to patients admitted during the corresponding period in 2019. The primary endpoint of this study was defined as a composite of sustained ventricular arrhythmia, congestive heart failure (CHF) with pulmonary congestion, and/or in-hospital mortality.
Results: This study cohort was composed of 64 patients with acute STEMI; 30 patients (46.9%) were hospitalized during the COVID-19 pandemic. Patients with STEMI in both the COVID-19 and control groups had similar comorbidities, Killip classification score, and clinical presentations. The median (interquartile range) time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (84.8-132) in 2019 to 149 minutes (96.3-231.8; P = .032) in 2020. Hospitalization during the COVID-19 period was associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046).
Conclusion: Patients with STEMI admitted during the first wave of the COVID-19 outbreak experienced longer total ischemic time and increased risk for combined in-hospital outcomes compared to patients admitted during the corresponding period in 2019.
Keywords: myocardial infarction, acute coronary syndrome, hospitalization, outcomes.

The emergence of the SARS-Cov-2 virus in December 2019 caused a worldwide shift in resource allocation and the restructuring of health care systems within the span of a few months. With the rapid spread of infection, the World Health Organization officially declared a pandemic in March 2020. The pandemic led to the deferral and cancellation of in-person patient visits, routine diagnostic studies, and nonessential surgeries and procedures. This response occurred secondary to a joint effort to reduce transmission via stay-at-home mandates and appropriate social distancing.1
Alongside the reduction in elective procedures and health care visits, significant reductions in hospitalization rates due to decreases in acute ST-segment elevation myocardial infarction (STEMI) and catheterization laboratory utilization have been reported in many studies from around the world.2-7 Comprehensive data demonstrating the impact of the COVID-19 pandemic on acute STEMI patient characteristics, clinical presentation, and in-hospital outcomes are lacking. Although patients with previously diagnosed cardiovascular disease are more likely to encounter worse outcomes in the setting of COVID-19, there may also be an indirect impact of the pandemic on high-risk patients, including those without the infection.8 Several theories have been hypothesized to explain this phenomenon. One theory postulates that the fear of contracting the virus during hospitalization is great enough to prevent patients from seeking care.2 Another theory suggests that the increased utilization of telemedicine prevents exacerbation of chronic conditions and the need for hospitalization.9 Contrary to this trend, previous studies have shown an increased incidence of acute STEMI following stressful events such as natural disasters.10
The aim of this study was to describe trends pertaining to clinical characteristics and in-hospital outcomes of patients with acute STEMI during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods
A retrospective cohort study was conducted at PAR to evaluate patients with STEMI admitted to the cardiovascular intensive care unit over an 8-week period (March 5 to May 5, 2020) during the COVID-19 outbreak. COVID-19 was declared a national emergency on March 13, 2020, in the United States. The institutional review board at PAR approved the study; the need for individual consent was waived under the condition that participant data would undergo de-identification and be strictly safeguarded.
Data Collection
Because there are seasonal variations in cardiovascular admissions, patient data from a control period (March 9 to May 9, 2019) were obtained to compare with data from the 2020 period. The number of patients with the diagnosis of acute STEMI during the COVID-19 period was recorded. Demographic data, clinical characteristics, and primary angiographic findings were gathered for all patients. Time from symptom onset to hospital admission and time from hospital admission to reperfusion (defined as door-to-balloon time) were documented for each patient. Killip classification was used to assess patients’ clinical status on admission. Length of stay was determined as days from hospital admission to discharge or death (if occurring during the same hospitalization).
Adverse in-hospital complications were also recorded. These were selected based on inclusion of the following categories of acute STEMI complications: ischemic, mechanical, arrhythmic, embolic, and inflammatory. The following complications occurred in our patient cohort: sustained ventricular arrhythmia, congestive heart failure (CHF) defined as congestion requiring intravenous diuretics, re-infarction, mechanical complications (free-wall rupture, ventricular septal defect, or mitral regurgitation), second- or third-degree atrioventricular block, atrial fibrillation, stroke, mechanical ventilation, major bleeding, pericarditis, cardiogenic shock, cardiac arrest, and in-hospital mortality. The primary outcome of this study was defined as a composite of sustained ventricular arrhythmia, CHF with congestion requiring intravenous diuretics, and/or in-hospital mortality. Ventricular arrythmia and CHF were included in the composite outcome because they are defined as the 2 most common causes of sudden cardiac death following acute STEMI.11,12
Statistical Analysis
Normally distributed continuous variables and categorical variables were compared using the paired t-test. A 2-sided P value <.05 was considered to be statistically significant. Mean admission rates for acute STEMI hospitalizations were determined by dividing the number of admissions by the number of days in each time period. The daily rate of COVID-19 cases per 100,000 individuals was obtained from the Centers for Disease Control and Prevention COVID-19 database. All data analyses were performed using Microsoft Excel.
Results
The study cohort consisted of 64 patients, of whom 30 (46.9%) were hospitalized between March 5 and May 5, 2020, and 34 (53.1%) who were admitted during the analogous time period in 2019. This reflected a 6% decrease in STEMI admissions at PAR in the COVID-19 cohort.
Acute STEMI Hospitalization Rates and COVID-19 Incidence
The mean daily acute STEMI admission rate was 0.50 during the study period compared to 0.57 during the control period. During the study period in 2020 in the state of Georgia, the daily rate of newly confirmed COVID-19 cases ranged from 0.194 per 100,000 on March 5 to 8.778 per 100,000 on May 5. Results of COVID-19 testing were available for 9 STEMI patients, and of these 0 tests were positive.
Baseline Characteristics
Baseline characteristics of the acute STEMI cohorts are presented in Table 1. Approximately 75% were male; median (interquartile range [IQR]) age was 60 (51-72) years. There were no significant differences in age and gender between the study periods. Three-quarters of patients had a history of hypertension, and 87.5% had a history of dyslipidemia. There was no significant difference in baseline comorbidity profiles between the 2 study periods; therefore, our sample populations shared similar characteristics.

Clinical Presentation
Significant differences were observed regarding the time intervals of STEMI patients in the COVID-19 period and the control period (Table 2). Median time from symptom onset to hospital admission (patient delay) was extended from 57.5 minutes (IQR, 40.3-106) in 2019 to 93 minutes (IQR, 48.8-132) in 2020; however, this difference was not statistically significant (P = .697). Median time from hospital admission to reperfusion (system delay) was prolonged from 45 minutes (IQR, 28-61) in 2019 to 78 minutes (IQR, 50-110) in 2020 (P < .001). Overall time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (IQR, 84.8-132) in 2019 to 149 minutes (IQR, 96.3-231.8) in 2020 (P = .032).

Regarding mode of transportation, 23.5% of patients in 2019 were walk-in admissions to the emergency department. During the COVID-19 period, walk-in admissions decreased to 6.7% (P = .065). There were no significant differences between emergency medical service, transfer, or in-patient admissions for STEMI cases between the 2 study periods.
Killip classification scores were calculated for all patients on admission; 90.6% of patients were classified as Killip Class 1. There was no significant difference between hemodynamic presentations during the COVID-19 period compared to the control period.
Angiographic Data
Overall, 53 (82.8%) patients admitted with acute STEMI underwent coronary angiography during their hospital stay. The proportion of patients who underwent primary reperfusion was greater in the control period than in the COVID-19 period (85.3% vs 80%; P = .582). Angiographic characteristics and findings were similar between the 2 study groups (Table 2).
In-Hospital Outcomes
In-hospital outcome data were available for all patients. As shown in Table 3, hospitalization during the COVID-19 period was independently associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046). The rate of in-hospital mortality was greater in the COVID-19 period (P = .013). We found no significant difference when comparing secondary outcomes from admissions during the COVID-19 period and the control period in 2019. For the 5 patients who died during the study period, the primary diagnosis at death was acute STEMI complicated by CHF (3 patients) or cardiogenic shock (2 patients).

Discussion
This single-center retrospective study at PAR looks at the impact of COVID-19 on hospitalizations for acute STEMI during the initial peak of the pandemic. The key findings of this study show a significant increase in ischemic time parameters (symptom onset to reperfusion, hospital admission to reperfusion), in-hospital mortality, and combined in-hospital outcomes.
There was a 49.5-minute increase in total ischemic time noted in this study (P = .032). Though there was a numerical increase in time of symptom onset to hospital admission by 23.5 minutes, this difference was not statistically significant (P = .697). However, this study observed a statistically significant 33-minute increase in ischemic time from hospital admission to reperfusion (P < .001). Multiple studies globally have found a similar increase in total ischemic times, including those conducted in China and Europe.13-15 Every level of potential delay must be considered, including pre-hospital, triage and emergency department, and/or reperfusion team. Pre-hospital sources of delays that have been suggested include “stay-at-home” orders and apprehension to seek medical care due to concern about contracting the virus or overwhelming the health care facilities. There was a clinically significant 4-fold decrease in the number of walk-in acute STEMI cases in the study period. In 2019, there were 8 walk-in cases compared to 2 cases in 2020 (P = .065). However, this change was not statistically significant. In-hospital/systemic sources of delays have been mentioned in other studies; they include increased time taken to rule out COVID-19 (nasopharyngeal swab/chest x-ray) and increased time due to the need for intensive gowning and gloving procedures by staff. It was difficult to objectively determine the sources of system delay by the reperfusion team due to a lack of quantitative data.
In the current study, we found a significant increase in in-hospital mortality during the COVID-19 period compared to a parallel time frame in 2019. This finding is contrary to a multicenter study from Spain that reported no difference in in-hospital outcomes or mortality rates among all acute coronary syndrome cases.16 The worsening outcomes and prognosis may simply be a result of increased ischemic time; however, the virus that causes COVID-19 itself may play a role as well. Studies have found that SARS-Cov-2 infection places patients at greater risk for cardiovascular conditions such as hypercoagulability, myocarditis, and arrhythmias.17 In our study, however, there were no acute STEMI patients who tested positive for COVID-19. Therefore, we cannot discuss the impact of increased thrombus burden in patients with COVID-19. Piedmont Healthcare published a STEMI treatment protocol in May 2020 that advised increased use of tissue plasminogen activator (tPA) in COVID-19-positive cases; during the study period, however, there were no occasions when tPA use was deemed appropriate based on clinical judgment.
Our findings align with previous studies that describe an increase in combined in-hospital adverse outcomes during the COVID-19 era. Previous studies detected a higher rate of complications in the COVID-19 cohort, but in the current study, the adverse in-hospital course is unrelated to underlying infection.18,19 This study reports a higher incidence of major in-hospital outcomes, including a 65% increase in the rate of combined in-hospital outcomes, which is similar to a multicenter study conducted in Israel.19 There was a 2.3-fold numerical increase in sustained ventricular arrhythmias and a 2.5-fold numerical increase in the incidence of cardiac arrest in the study period. This phenomenon was observed despite a similar rate of reperfusion procedures in both groups.
Acute STEMI is a highly fatal condition with an incidence of 8.5 in 10,000 annually in the United States. While studies across the world have shown a 25% to 40% reduction in the rate of hospitalized acute coronary syndrome cases during the COVID-19 pandemic, the decrease from 34 to 30 STEMI admissions at PAR is not statistically significant.20 Possible reasons for the reduction globally include increased out-of-hospital mortality and decreased incidence of acute STEMI across the general population as a result of improved access to telemedicine or decreased levels of life stressors.20
In summary, there was an increase in ischemic time to reperfusion, in-hospital mortality, and combined in-hospital outcomes for acute STEMI patients at PAR during the COVID period.
Limitations
This study has several limitations. This is a single-center study, so the sample size is small and may not be generalizable to a larger population. This is a retrospective observational study, so causation cannot be inferred. This study analyzed ischemic time parameters as average rates over time rather than in an interrupted time series. Post-reperfusion outcomes were limited to hospital stay. Post-hospital follow-up would provide a better picture of the effects of STEMI intervention. There is no account of patients who died out-of-hospital secondary to acute STEMI. COVID-19 testing was not introduced until midway in our study period. Therefore, we cannot rule out the possibility of the SARS-Cov-2 virus inciting acute STEMI and subsequently leading to worse outcomes and poor prognosis.
Conclusions
This study provides an analysis of the incidence, characteristics, and clinical outcomes of patients presenting with acute STEMI during the early period of the COVID-19 pandemic. In-hospital mortality and ischemic time to reperfusion increased while combined in-hospital outcomes worsened.
Acknowledgment: The authors thank Piedmont Athens Regional IRB for approving this project and allowing access to patient data.
Corresponding author: Syed H. Ali; Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, 30606, Athens, GA; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0085
From the Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, Athens, GA (Syed H. Ali, Syed Hyder, and Dr. Murrow), and the Department of Cardiology, Piedmont Heart Institute, Piedmont Athens Regional, Athens, GA (Dr. Murrow and Mrs. Davis).
Abstract
Objectives: The aim of this study was to describe the characteristics and in-hospital outcomes of patients with acute ST-segment elevation myocardial infarction (STEMI) during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods: A retrospective study was conducted at PAR to evaluate patients with acute STEMI admitted over an 8-week period during the initial COVID-19 outbreak. This study group was compared to patients admitted during the corresponding period in 2019. The primary endpoint of this study was defined as a composite of sustained ventricular arrhythmia, congestive heart failure (CHF) with pulmonary congestion, and/or in-hospital mortality.
Results: This study cohort was composed of 64 patients with acute STEMI; 30 patients (46.9%) were hospitalized during the COVID-19 pandemic. Patients with STEMI in both the COVID-19 and control groups had similar comorbidities, Killip classification score, and clinical presentations. The median (interquartile range) time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (84.8-132) in 2019 to 149 minutes (96.3-231.8; P = .032) in 2020. Hospitalization during the COVID-19 period was associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046).
Conclusion: Patients with STEMI admitted during the first wave of the COVID-19 outbreak experienced longer total ischemic time and increased risk for combined in-hospital outcomes compared to patients admitted during the corresponding period in 2019.
Keywords: myocardial infarction, acute coronary syndrome, hospitalization, outcomes.

The emergence of the SARS-Cov-2 virus in December 2019 caused a worldwide shift in resource allocation and the restructuring of health care systems within the span of a few months. With the rapid spread of infection, the World Health Organization officially declared a pandemic in March 2020. The pandemic led to the deferral and cancellation of in-person patient visits, routine diagnostic studies, and nonessential surgeries and procedures. This response occurred secondary to a joint effort to reduce transmission via stay-at-home mandates and appropriate social distancing.1
Alongside the reduction in elective procedures and health care visits, significant reductions in hospitalization rates due to decreases in acute ST-segment elevation myocardial infarction (STEMI) and catheterization laboratory utilization have been reported in many studies from around the world.2-7 Comprehensive data demonstrating the impact of the COVID-19 pandemic on acute STEMI patient characteristics, clinical presentation, and in-hospital outcomes are lacking. Although patients with previously diagnosed cardiovascular disease are more likely to encounter worse outcomes in the setting of COVID-19, there may also be an indirect impact of the pandemic on high-risk patients, including those without the infection.8 Several theories have been hypothesized to explain this phenomenon. One theory postulates that the fear of contracting the virus during hospitalization is great enough to prevent patients from seeking care.2 Another theory suggests that the increased utilization of telemedicine prevents exacerbation of chronic conditions and the need for hospitalization.9 Contrary to this trend, previous studies have shown an increased incidence of acute STEMI following stressful events such as natural disasters.10
The aim of this study was to describe trends pertaining to clinical characteristics and in-hospital outcomes of patients with acute STEMI during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods
A retrospective cohort study was conducted at PAR to evaluate patients with STEMI admitted to the cardiovascular intensive care unit over an 8-week period (March 5 to May 5, 2020) during the COVID-19 outbreak. COVID-19 was declared a national emergency on March 13, 2020, in the United States. The institutional review board at PAR approved the study; the need for individual consent was waived under the condition that participant data would undergo de-identification and be strictly safeguarded.
Data Collection
Because there are seasonal variations in cardiovascular admissions, patient data from a control period (March 9 to May 9, 2019) were obtained to compare with data from the 2020 period. The number of patients with the diagnosis of acute STEMI during the COVID-19 period was recorded. Demographic data, clinical characteristics, and primary angiographic findings were gathered for all patients. Time from symptom onset to hospital admission and time from hospital admission to reperfusion (defined as door-to-balloon time) were documented for each patient. Killip classification was used to assess patients’ clinical status on admission. Length of stay was determined as days from hospital admission to discharge or death (if occurring during the same hospitalization).
Adverse in-hospital complications were also recorded. These were selected based on inclusion of the following categories of acute STEMI complications: ischemic, mechanical, arrhythmic, embolic, and inflammatory. The following complications occurred in our patient cohort: sustained ventricular arrhythmia, congestive heart failure (CHF) defined as congestion requiring intravenous diuretics, re-infarction, mechanical complications (free-wall rupture, ventricular septal defect, or mitral regurgitation), second- or third-degree atrioventricular block, atrial fibrillation, stroke, mechanical ventilation, major bleeding, pericarditis, cardiogenic shock, cardiac arrest, and in-hospital mortality. The primary outcome of this study was defined as a composite of sustained ventricular arrhythmia, CHF with congestion requiring intravenous diuretics, and/or in-hospital mortality. Ventricular arrythmia and CHF were included in the composite outcome because they are defined as the 2 most common causes of sudden cardiac death following acute STEMI.11,12
Statistical Analysis
Normally distributed continuous variables and categorical variables were compared using the paired t-test. A 2-sided P value <.05 was considered to be statistically significant. Mean admission rates for acute STEMI hospitalizations were determined by dividing the number of admissions by the number of days in each time period. The daily rate of COVID-19 cases per 100,000 individuals was obtained from the Centers for Disease Control and Prevention COVID-19 database. All data analyses were performed using Microsoft Excel.
Results
The study cohort consisted of 64 patients, of whom 30 (46.9%) were hospitalized between March 5 and May 5, 2020, and 34 (53.1%) who were admitted during the analogous time period in 2019. This reflected a 6% decrease in STEMI admissions at PAR in the COVID-19 cohort.
Acute STEMI Hospitalization Rates and COVID-19 Incidence
The mean daily acute STEMI admission rate was 0.50 during the study period compared to 0.57 during the control period. During the study period in 2020 in the state of Georgia, the daily rate of newly confirmed COVID-19 cases ranged from 0.194 per 100,000 on March 5 to 8.778 per 100,000 on May 5. Results of COVID-19 testing were available for 9 STEMI patients, and of these 0 tests were positive.
Baseline Characteristics
Baseline characteristics of the acute STEMI cohorts are presented in Table 1. Approximately 75% were male; median (interquartile range [IQR]) age was 60 (51-72) years. There were no significant differences in age and gender between the study periods. Three-quarters of patients had a history of hypertension, and 87.5% had a history of dyslipidemia. There was no significant difference in baseline comorbidity profiles between the 2 study periods; therefore, our sample populations shared similar characteristics.

Clinical Presentation
Significant differences were observed regarding the time intervals of STEMI patients in the COVID-19 period and the control period (Table 2). Median time from symptom onset to hospital admission (patient delay) was extended from 57.5 minutes (IQR, 40.3-106) in 2019 to 93 minutes (IQR, 48.8-132) in 2020; however, this difference was not statistically significant (P = .697). Median time from hospital admission to reperfusion (system delay) was prolonged from 45 minutes (IQR, 28-61) in 2019 to 78 minutes (IQR, 50-110) in 2020 (P < .001). Overall time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (IQR, 84.8-132) in 2019 to 149 minutes (IQR, 96.3-231.8) in 2020 (P = .032).

Regarding mode of transportation, 23.5% of patients in 2019 were walk-in admissions to the emergency department. During the COVID-19 period, walk-in admissions decreased to 6.7% (P = .065). There were no significant differences between emergency medical service, transfer, or in-patient admissions for STEMI cases between the 2 study periods.
Killip classification scores were calculated for all patients on admission; 90.6% of patients were classified as Killip Class 1. There was no significant difference between hemodynamic presentations during the COVID-19 period compared to the control period.
Angiographic Data
Overall, 53 (82.8%) patients admitted with acute STEMI underwent coronary angiography during their hospital stay. The proportion of patients who underwent primary reperfusion was greater in the control period than in the COVID-19 period (85.3% vs 80%; P = .582). Angiographic characteristics and findings were similar between the 2 study groups (Table 2).
In-Hospital Outcomes
In-hospital outcome data were available for all patients. As shown in Table 3, hospitalization during the COVID-19 period was independently associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046). The rate of in-hospital mortality was greater in the COVID-19 period (P = .013). We found no significant difference when comparing secondary outcomes from admissions during the COVID-19 period and the control period in 2019. For the 5 patients who died during the study period, the primary diagnosis at death was acute STEMI complicated by CHF (3 patients) or cardiogenic shock (2 patients).

Discussion
This single-center retrospective study at PAR looks at the impact of COVID-19 on hospitalizations for acute STEMI during the initial peak of the pandemic. The key findings of this study show a significant increase in ischemic time parameters (symptom onset to reperfusion, hospital admission to reperfusion), in-hospital mortality, and combined in-hospital outcomes.
There was a 49.5-minute increase in total ischemic time noted in this study (P = .032). Though there was a numerical increase in time of symptom onset to hospital admission by 23.5 minutes, this difference was not statistically significant (P = .697). However, this study observed a statistically significant 33-minute increase in ischemic time from hospital admission to reperfusion (P < .001). Multiple studies globally have found a similar increase in total ischemic times, including those conducted in China and Europe.13-15 Every level of potential delay must be considered, including pre-hospital, triage and emergency department, and/or reperfusion team. Pre-hospital sources of delays that have been suggested include “stay-at-home” orders and apprehension to seek medical care due to concern about contracting the virus or overwhelming the health care facilities. There was a clinically significant 4-fold decrease in the number of walk-in acute STEMI cases in the study period. In 2019, there were 8 walk-in cases compared to 2 cases in 2020 (P = .065). However, this change was not statistically significant. In-hospital/systemic sources of delays have been mentioned in other studies; they include increased time taken to rule out COVID-19 (nasopharyngeal swab/chest x-ray) and increased time due to the need for intensive gowning and gloving procedures by staff. It was difficult to objectively determine the sources of system delay by the reperfusion team due to a lack of quantitative data.
In the current study, we found a significant increase in in-hospital mortality during the COVID-19 period compared to a parallel time frame in 2019. This finding is contrary to a multicenter study from Spain that reported no difference in in-hospital outcomes or mortality rates among all acute coronary syndrome cases.16 The worsening outcomes and prognosis may simply be a result of increased ischemic time; however, the virus that causes COVID-19 itself may play a role as well. Studies have found that SARS-Cov-2 infection places patients at greater risk for cardiovascular conditions such as hypercoagulability, myocarditis, and arrhythmias.17 In our study, however, there were no acute STEMI patients who tested positive for COVID-19. Therefore, we cannot discuss the impact of increased thrombus burden in patients with COVID-19. Piedmont Healthcare published a STEMI treatment protocol in May 2020 that advised increased use of tissue plasminogen activator (tPA) in COVID-19-positive cases; during the study period, however, there were no occasions when tPA use was deemed appropriate based on clinical judgment.
Our findings align with previous studies that describe an increase in combined in-hospital adverse outcomes during the COVID-19 era. Previous studies detected a higher rate of complications in the COVID-19 cohort, but in the current study, the adverse in-hospital course is unrelated to underlying infection.18,19 This study reports a higher incidence of major in-hospital outcomes, including a 65% increase in the rate of combined in-hospital outcomes, which is similar to a multicenter study conducted in Israel.19 There was a 2.3-fold numerical increase in sustained ventricular arrhythmias and a 2.5-fold numerical increase in the incidence of cardiac arrest in the study period. This phenomenon was observed despite a similar rate of reperfusion procedures in both groups.
Acute STEMI is a highly fatal condition with an incidence of 8.5 in 10,000 annually in the United States. While studies across the world have shown a 25% to 40% reduction in the rate of hospitalized acute coronary syndrome cases during the COVID-19 pandemic, the decrease from 34 to 30 STEMI admissions at PAR is not statistically significant.20 Possible reasons for the reduction globally include increased out-of-hospital mortality and decreased incidence of acute STEMI across the general population as a result of improved access to telemedicine or decreased levels of life stressors.20
In summary, there was an increase in ischemic time to reperfusion, in-hospital mortality, and combined in-hospital outcomes for acute STEMI patients at PAR during the COVID period.
Limitations
This study has several limitations. This is a single-center study, so the sample size is small and may not be generalizable to a larger population. This is a retrospective observational study, so causation cannot be inferred. This study analyzed ischemic time parameters as average rates over time rather than in an interrupted time series. Post-reperfusion outcomes were limited to hospital stay. Post-hospital follow-up would provide a better picture of the effects of STEMI intervention. There is no account of patients who died out-of-hospital secondary to acute STEMI. COVID-19 testing was not introduced until midway in our study period. Therefore, we cannot rule out the possibility of the SARS-Cov-2 virus inciting acute STEMI and subsequently leading to worse outcomes and poor prognosis.
Conclusions
This study provides an analysis of the incidence, characteristics, and clinical outcomes of patients presenting with acute STEMI during the early period of the COVID-19 pandemic. In-hospital mortality and ischemic time to reperfusion increased while combined in-hospital outcomes worsened.
Acknowledgment: The authors thank Piedmont Athens Regional IRB for approving this project and allowing access to patient data.
Corresponding author: Syed H. Ali; Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, 30606, Athens, GA; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0085
1. Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
2. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJR. Decline of acute coronary syndrome admissions in Austria since the outbreak of Covid-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852-1853. doi:10.1093/eurheartj/ehaa314
3. De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the Covid-19 era. Eur Heart J. 2020;41(22):2083-2088.
4. Wilson SJ, Connolly MJ, Elghamry Z, et al. Effect of the COVID-19 pandemic on ST-segment-elevation myocardial infarction presentations and in-hospital outcomes. Circ Cardiovasc Interv. 2020; 13(7):e009438. doi:10.1161/CIRCINTERVENTIONS.120.009438
5. Mafham MM, Spata E, Goldacre R, et al. Covid-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396 (10248):381-389. doi:10.1016/S0140-6736(20)31356-8
6. Bhatt AS, Moscone A, McElrath EE, et al. Fewer Hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
7. Tam CF, Cheung KS, Lam S, et al. Impact of Coronavirus disease 2019 (Covid-19) outbreak on ST-segment elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13(4):e006631. doi:10.1161/CIRCOUTCOMES.120.006631
8. Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648-1655. doi:10.1161/CIRCULATIONAHA.120.046941
9. Ebinger JE, Shah PK. Declining admissions for acute cardiovascular illness: The Covid-19 paradox. J Am Coll Cardiol. 2020;76(3):289-291. doi:10.1016/j.jacc.2020.05.039
10 Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334(7):413-419. doi:10.1056/NEJM199602153340701
11. Hiramori K. Major causes of death from acute myocardial infarction in a coronary care unit. Jpn Circ J. 1987;51(9):1041-1047. doi:10.1253/jcj.51.1041
12. Bui AH, Waks JW. Risk stratification of sudden cardiac death after acute myocardial infarction. J Innov Card Rhythm Manag. 2018;9(2):3035-3049. doi:10.19102/icrm.2018.090201
13. Xiang D, Xiang X, Zhang W, et al. Management and outcomes of patients with STEMI during the COVID-19 pandemic in China. J Am Coll Cardiol. 2020;76(11):1318-1324. doi:10.1016/j.jacc.2020.06.039
14. Hakim R, Motreff P, Rangé G. COVID-19 and STEMI. [Article in French]. Ann Cardiol Angeiol (Paris). 2020;69(6):355-359. doi:10.1016/j.ancard.2020.09.034
15. Soylu K, Coksevim M, Yanık A, Bugra Cerik I, Aksan G. Effect of Covid-19 pandemic process on STEMI patients timeline. Int J Clin Pract. 2021;75(5):e14005. doi:10.1111/ijcp.14005
16. Salinas P, Travieso A, Vergara-Uzcategui C, et al. Clinical profile and 30-day mortality of invasively managed patients with suspected acute coronary syndrome during the COVID-19 outbreak. Int Heart J. 2021;62(2):274-281. doi:10.1536/ihj.20-574
17. Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (Covid-19): a systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi:10.1016/j.jcv.2020.104371
18. Rodriguez-Leor O, Cid Alvarez AB, Perez de Prado A, et al. In-hospital outcomes of COVID-19 ST-elevation myocardial infarction patients. EuroIntervention. 2021;16(17):1426-1433. doi:10.4244/EIJ-D-20-00935
19. Fardman A, Zahger D, Orvin K, et al. Acute myocardial infarction in the Covid-19 era: incidence, clinical characteristics and in-hospital outcomes—A multicenter registry. PLoS ONE. 2021;16(6): e0253524. doi:10.1371/journal.pone.0253524
20. Pessoa-Amorim G, Camm CF, Gajendragadkar P, et al. Admission of patients with STEMI since the outbreak of the COVID-19 pandemic: a survey by the European Society of Cardiology. Eur Heart J Qual Care Clin Outcomes. 2020;6(3):210-216. doi:10.1093/ehjqcco/qcaa046
1. Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
2. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJR. Decline of acute coronary syndrome admissions in Austria since the outbreak of Covid-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852-1853. doi:10.1093/eurheartj/ehaa314
3. De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the Covid-19 era. Eur Heart J. 2020;41(22):2083-2088.
4. Wilson SJ, Connolly MJ, Elghamry Z, et al. Effect of the COVID-19 pandemic on ST-segment-elevation myocardial infarction presentations and in-hospital outcomes. Circ Cardiovasc Interv. 2020; 13(7):e009438. doi:10.1161/CIRCINTERVENTIONS.120.009438
5. Mafham MM, Spata E, Goldacre R, et al. Covid-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396 (10248):381-389. doi:10.1016/S0140-6736(20)31356-8
6. Bhatt AS, Moscone A, McElrath EE, et al. Fewer Hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
7. Tam CF, Cheung KS, Lam S, et al. Impact of Coronavirus disease 2019 (Covid-19) outbreak on ST-segment elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13(4):e006631. doi:10.1161/CIRCOUTCOMES.120.006631
8. Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648-1655. doi:10.1161/CIRCULATIONAHA.120.046941
9. Ebinger JE, Shah PK. Declining admissions for acute cardiovascular illness: The Covid-19 paradox. J Am Coll Cardiol. 2020;76(3):289-291. doi:10.1016/j.jacc.2020.05.039
10 Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334(7):413-419. doi:10.1056/NEJM199602153340701
11. Hiramori K. Major causes of death from acute myocardial infarction in a coronary care unit. Jpn Circ J. 1987;51(9):1041-1047. doi:10.1253/jcj.51.1041
12. Bui AH, Waks JW. Risk stratification of sudden cardiac death after acute myocardial infarction. J Innov Card Rhythm Manag. 2018;9(2):3035-3049. doi:10.19102/icrm.2018.090201
13. Xiang D, Xiang X, Zhang W, et al. Management and outcomes of patients with STEMI during the COVID-19 pandemic in China. J Am Coll Cardiol. 2020;76(11):1318-1324. doi:10.1016/j.jacc.2020.06.039
14. Hakim R, Motreff P, Rangé G. COVID-19 and STEMI. [Article in French]. Ann Cardiol Angeiol (Paris). 2020;69(6):355-359. doi:10.1016/j.ancard.2020.09.034
15. Soylu K, Coksevim M, Yanık A, Bugra Cerik I, Aksan G. Effect of Covid-19 pandemic process on STEMI patients timeline. Int J Clin Pract. 2021;75(5):e14005. doi:10.1111/ijcp.14005
16. Salinas P, Travieso A, Vergara-Uzcategui C, et al. Clinical profile and 30-day mortality of invasively managed patients with suspected acute coronary syndrome during the COVID-19 outbreak. Int Heart J. 2021;62(2):274-281. doi:10.1536/ihj.20-574
17. Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (Covid-19): a systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi:10.1016/j.jcv.2020.104371
18. Rodriguez-Leor O, Cid Alvarez AB, Perez de Prado A, et al. In-hospital outcomes of COVID-19 ST-elevation myocardial infarction patients. EuroIntervention. 2021;16(17):1426-1433. doi:10.4244/EIJ-D-20-00935
19. Fardman A, Zahger D, Orvin K, et al. Acute myocardial infarction in the Covid-19 era: incidence, clinical characteristics and in-hospital outcomes—A multicenter registry. PLoS ONE. 2021;16(6): e0253524. doi:10.1371/journal.pone.0253524
20. Pessoa-Amorim G, Camm CF, Gajendragadkar P, et al. Admission of patients with STEMI since the outbreak of the COVID-19 pandemic: a survey by the European Society of Cardiology. Eur Heart J Qual Care Clin Outcomes. 2020;6(3):210-216. doi:10.1093/ehjqcco/qcaa046
Oxygen Therapies and Clinical Outcomes for Patients Hospitalized With COVID-19: First Surge vs Second Surge
From Lahey Hospital and Medical Center, Burlington, MA (Drs. Liesching and Lei), and Tufts University School of Medicine, Boston, MA (Dr. Liesching)
ABSTRACT
Objective: To compare the utilization of oxygen therapies and clinical outcomes of patients admitted for COVID-19 during the second surge of the pandemic to that of patients admitted during the first surge.
Design: Observational study using a registry database.
Setting: Three hospitals (791 inpatient beds and 76 intensive care unit [ICU] beds) within the Beth Israel Lahey Health system in Massachusetts.
Participants: We included 3183 patients with COVID-19 admitted to hospitals.
Measurements: Baseline data included demographics and comorbidities. Treatments included low-flow supplemental oxygen (2-6 L/min), high-flow oxygen via nasal cannula, and invasive mechanical ventilation. Outcomes included ICU admission, length of stay, ventilator days, and mortality.
Results: A total of 3183 patients were included: 1586 during the first surge and 1597 during the second surge. Compared to the first surge, patients admitted during the second surge had a similar rate of receiving low-flow supplemental oxygen (65.8% vs 64.1%, P = .3), a higher rate of receiving high-flow nasal cannula (15.4% vs 10.8%, P = .0001), and a lower ventilation rate (5.6% vs 9.7%, P < .0001). The outcomes during the second surge were better than those during the first surge: lower ICU admission rate (8.1% vs 12.7%, P < .0001), shorter length of hospital stay (5 vs 6 days, P < .0001), fewer ventilator days (10 vs 16, P = .01), and lower mortality (8.3% vs 19.2%, P < .0001). Among ventilated patients, those who received high-flow nasal cannula had lower mortality.
Conclusion: Compared to the first surge of the COVID-19 pandemic, patients admitted during the second surge had similar likelihood of receiving low-flow supplemental oxygen, were more likely to receive high-flow nasal cannula, were less likely to be ventilated, and had better outcomes.
Keywords: supplemental oxygen, high-flow nasal cannula, ventilator.
The respiratory system receives the major impact of SARS-CoV-2 virus, and hypoxemia has been the predominant diagnosis for patients hospitalized with COVID-19.1,2 During the initial stage of the pandemic, oxygen therapies and mechanical ventilation were the only choices for these patients.3-6 Standard-of-care treatment for patients with COVID-19 during the initial surge included oxygen therapies and mechanical ventilation for hypoxemia and medications for comorbidities and COVID-19–associated sequelae, such as multi-organ dysfunction and failure. A report from New York during the first surge (May 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received supplemental oxygen and 12.2% received invasive mechanical ventilation.7 High-flow nasal cannula (HFNC) oxygen delivery has been utilized widely throughout the pandemic due to its superiority over other noninvasive respiratory support techniques.8-12 Mechanical ventilation is always necessary for critically ill patients with acute respiratory distress syndrome. However, ventilator scarcity has become a bottleneck in caring for severely ill patients with COVID-19 during the pandemic.13
The clinical outcomes of hospitalized COVID-19 patients include a high intubation rate, long length of hospital and intensive care unit (ICU) stay, and high mortality.14,15 As the pandemic evolved, new medications, including remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a, were used in addition to the standard of care, but these did not result in significantly different mortality from standard of care.16 Steroids are becoming foundational to the treatment of severe COVID-19 pneumonia, but evidence from high-quality randomized controlled clinical trials is lacking.17
During the first surge from March to May 2020, Massachusetts had the third highest number of COVID-19 cases among states in the United States.18 In early 2021, COVID-19 cases were climbing close to the peak of the second surge in Massachusetts. In this study, we compared utilization of low-flow supplemental oxygen, HFNC, and mechanical ventilation and clinical outcomes of patients admitted to 3 hospitals in Massachusetts during the second surge of the pandemic to that of patients admitted during the first surge.
Methods
Setting
Beth Israel Lahey Health is a system of academic and teaching hospitals with primary care and specialty care providers. We included 3 centers within the Beth Israel Lahey Health system in Massachusetts: Lahey Hospital and Medical Center, with 335 inpatient hospital beds and 52 critical care beds; Beverly Hospital, with 227 beds and 14 critical care beds; and Winchester Hospital, with 229 beds and 10 ICU beds.
Participants
We included patients admitted to the 3 hospitals with COVID-19 as a primary or secondary diagnosis during the first surge of the pandemic (March 1, 2020 to June 15, 2020) and the second surge (November 15, 2020 to January 27, 2021). The timeframe of the first surge was defined as the window between the start date and the end date of data collection. During the time window of the first surge, 1586 patients were included. The start time of the second surge was defined as the date when the data collection was restarted; the end date was set when the number of patients (1597) accumulated was close to the number of patients in the first surge (1586), so that the two groups had similar sample size.
Study Design
A data registry of COVID-19 patients was created by our institution, and the data were prospectively collected starting in March 2020. We retrospectively extracted data on the following from the registry database for this observational study: demographics and baseline comorbidities; the use of low-flow supplemental oxygen, HFNC, and invasive mechanical ventilator; and ICU admission, length of hospital stay, length of ICU stay, and hospital discharge disposition. Start and end times for each oxygen therapy were not entered in the registry. Data about other oxygen therapies, such as noninvasive positive-pressure ventilation, were not collected in the registry database, and therefore were not included in the analysis.
Statistical Analysis
Continuous variables (eg, age) were tested for data distribution normality using the Shapiro-Wilk test. Normally distributed data were tested using unpaired t-tests and displayed as mean (SD). The skewed data were tested using the Wilcoxon rank sum test and displayed as median (interquartile range [IQR]). The categorical variables were compared using chi-square test. Comparisons with P ≤ .05 were considered significantly different. Statistical analysis for this study was generated using Statistical Analysis Software (SAS), version 9.4 for Windows (SAS Institute Inc.).
Results
Baseline Characteristics
We included 3183 patients: 1586 admitted during the first surge and 1597 admitted during the second surge. Baseline characteristics of patients with COVID-19 admitted during the first and second surges are shown in Table 1. Patients admitted during the second surge were older (73 years vs 71 years, P = .01) and had higher rates of hypertension (64.8% vs 59.6%, P = .003) and asthma (12.9% vs 10.7%, P = .049) but a lower rate of interstitial lung disease (3.3% vs 7.7%, P < .001). Sequential organ failure assessment scores at admission and the rates of other comorbidities were not significantly different between the 2 surges.

Oxygen Therapies
The number of patients who were hospitalized and received low-flow supplemental oxygen, and/or HFNC, and/or ventilator in the first surge and the second surge is shown in the Figure. Of all patients included, 2067 (64.9%) received low-flow supplemental oxygen; of these, 374 (18.1%) subsequently received HFNC, and 85 (22.7%) of these subsequently received mechanical ventilation. Of all 3183 patients, 417 (13.1%) received HFNC; 43 of these patients received HFNC without receiving low-flow supplemental oxygen, and 98 (23.5%) subsequently received mechanical ventilation. Out of all 3183 patients, 244 (7.7%) received mechanical ventilation; 98 (40.2%) of these received HFNC while the remaining 146 (59.8%) did not. At the beginning of the first surge, the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was close to 1:1 (10/10); the ratio decreased to 6:10 in May and June 2020. At the beginning of the second surge, the ratio was 8:10 and then decreased to 3:10 in December 2020 and January 2021.

As shown in Table 2, the proportion of patients who received low-flow supplemental oxygen during the second surge was similar to that during the first surge (65.8% vs 64.1%, P = .3). Patients admitted during the second surge were more likely to receive HFNC than patients admitted during the first surge (15.4% vs 10.8%, P = .0001). Patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001).

Clinical Outcomes
As shown in Table 3, second surge outcomes were much better than first surge outcomes: the ICU admission rate was lower (8.1% vs 12.7%, P < .0001); patients were more likely to be discharged to home (60.2% vs 47.4%, P < .0001), had a shorter length of hospital stay (5 vs 6 days, P < .0001), and had fewer ventilator days (10 vs 16, P = .01); and mortality was lower (8.3% vs 19.2%, P < .0001). There was a trend that length of ICU stay was shorter during the second surge than during the first surge (7 days vs 9 days, P = .09).

As noted (Figure), the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was decreasing during both the first surge and the second surge. To further analyze the relation between ventilator and HFNC, we performed a subgroup analysis for 244 ventilated patients during both surges to compare outcomes between patients who received HFNC and those who did not receive HFNC (Table 4). Ninety-eight (40%) patients received HFNC. Ventilated patients who received HFNC had lower mortality than those patients who did not receive HFNC (31.6% vs 48%, P = .01), but had a longer length of hospital stay (29 days vs 14 days, P < .0001), longer length of ICU stay (17 days vs 9 days, P < .0001), and a higher number of ventilator days (16 vs 11, P = .001).

Discussion
Our study compared the baseline patient characteristics; utilization of low-flow supplemental oxygen therapy, HFNC, and mechanical ventilation; and clinical outcomes between the first surge (n = 1586) and the second surge (n = 1597) of the COVID-19 pandemic. During both surges, about two-thirds of admitted patients received low-flow supplemental oxygen. A higher proportion of the admitted patients received HFNC during the second surge than during the first surge, while the intubation rate was lower during the second surge than during the first surge.
Reported low-flow supplemental oxygen use ranged from 28% to 63% depending on the cohort characteristics and location during the first surge.6,7,19 A report from New York during the first surge (March 1 to April 4, 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received low-flow supplemental oxygen.7 HFNC is recommended in guidelines on management of patients with acute respiratory failure due to COVID-19.20 In our study, HFNC was utilized in a higher proportion of patients admitted for COVID-19 during the second surge (15.5% vs 10.8%, P = .0001). During the early pandemic period in Wuhan, China, 11% to 21% of admitted COVID-19 patients received HFNC.21,22 Utilization of HFNC in New York during the first surge (March to May 2020) varied from 5% to 14.3% of patients admitted with COVID-19.23,24 Our subgroup analysis of the ventilated patients showed that patients who received HFNC had lower mortality than those who did not (31.6% vs 48.0%, P = .011). Comparably, a report from Paris, France, showed that among patients admitted to ICUs for acute hypoxemic respiratory failure, those who received HFNC had lower mortality at day 60 than those who did not (21% vs 31%, P = .052).25 Our recent analysis showed that patients treated with HFNC prior to mechanical ventilation had lower mortality than those treated with only conventional oxygen (30% vs 52%, P = .05).26 In this subgroup analysis, we could not determine if HFNC treatment was administered before or after ventilation because HFNC was entered as dichotomous data (“Yes” or “No”) in the registry database. We merely showed the beneficial effect of HFNC on reducing mortality for ventilated COVID-19 patients, but did not mean to focus on how and when to apply HFNC.
We observed that the patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001). During the first surge in New York, among 5700 patients admitted with COVID-19, 12.2% received invasive mechanical ventilation.7 In another report, also from New York during the first surge, 26.1% of 2015 hospitalized COVID-19 patients received mechanical ventilation.27 In our study, the ventilation rate of 9.7% during the first surge was lower.
Outcomes during the second surge were better than during the first surge, including ICU admission rate, hospital and ICU length of stay, ventilator days, and mortality. The mortality was 19.2% during the first surge vs 8.3% during the second surge (P < .0001). The mortality of 19.2% was lower than the 30.6% mortality reported for 2015 hospitalized COVID-19 patients in New York during the first surge.27 A retrospective study showed that early administration of remdesivir was associated with reduced ICU admission, ventilation use, and mortality.28 The RECOVERY clinical trial showed that dexamethasone improved mortality for COVID-19 patients who received respiratory support, but not for patients who did not receive any respiratory support.29 Perhaps some, if not all, of the improvement in ICU admission and mortality during the second surge was attributed to the new medications, such as antivirals and steroids.
The length of hospital stay for patients with moderate to severe COVID-19 varied from 4 to 53 days at different locations of the world, as shown in a meta-analysis by Rees and colleagues.30 Our results showing a length of stay of 6 days during the first surge and 5 days during the second surge fell into the shorter end of this range. In a retrospective analysis of 1643 adults with severe COVID-19 admitted to hospitals in New York City between March 9, 2020 and April 23, 2020, median hospital length of stay was 7 (IQR, 3-14) days.31 For the ventilated patients in our study, the length of stay of 14 days (did not receive HFNC) and 29 days (received HFNC) was much longer. This longer length of stay might be attributed to the patients in our study being older and having more severe comorbidities.
The main purpose of this study was to compare the oxygen therapies and outcomes between 2 surges. It is difficult to associate the clinical outcomes with the oxygen therapies because new therapies and medications were available after the first surge. It was not possible to adjust the outcomes with confounders (other therapies and medications) because the registry data did not include the new therapies and medications.
A strength of this study was that we included a large, balanced number of patients in the first surge and the second surge. We did not plan the sample size in both groups as we could not predict the number of admissions. We set the end date of data collection for analysis as the time when the number of patients admitted during the second surge was similar to the number of patients admitted during the first surge. A limitation was that the registry database was created by the institution and was not designed solely for this study. The data for oxygen therapies were limited to low-flow supplemental oxygen, HFNC, and invasive mechanical ventilation; data for noninvasive ventilation were not included.
Conclusion
At our centers, during the second surge of COVID-19 pandemic, patients hospitalized with COVID-19 infection were more likely to receive HFNC but less likely to be ventilated. Compared to the first surge, the hospitalized patients with COVID-19 infection had a lower ICU admission rate, shorter length of hospital stay, fewer ventilator days, and lower mortality. For ventilated patients, those who received HFNC had lower mortality than those who did not.
Corresponding author: Timothy N. Liesching, MD, 41 Mall Road, Burlington, MA 01805; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0086
1. Xie J, Covassin N, Fan Z, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95(6):1138-1147. doi:10.1016/j.mayocp.2020.04.006
2. Asleh R, Asher E, Yagel O, et al. Predictors of hypoxemia and related adverse outcomes in patients hospitalized with COVID-19: a double-center retrospective study. J Clin Med. 2021;10(16):3581. doi:10.3390/jcm10163581
3. Choi KJ, Hong HL, Kim EJ. Association between oxygen saturation/fraction of inhaled oxygen and mortality in patients with COVID-19 associated pneumonia requiring oxygen therapy. Tuberc Respir Dis (Seoul). 2021;84(2):125-133. doi:10.4046/trd.2020.0126
4. Dixit SB. Role of noninvasive oxygen therapy strategies in COVID-19 patients: Where are we going? Indian J Crit Care Med. 2020;24(10):897-898. doi:10.5005/jp-journals-10071-23625
5. Gonzalez-Castro A, Fajardo Campoverde A, Medina A, et al. Non-invasive mechanical ventilation and high-flow oxygen therapy in the COVID-19 pandemic: the value of a draw. Med Intensiva (Engl Ed). 2021;45(5):320-321. doi:10.1016/j.medine.2021.04.001
6. Pan W, Li J, Ou Y, et al. Clinical outcome of standardized oxygen therapy nursing strategy in COVID-19. Ann Palliat Med. 2020;9(4):2171-2177. doi:10.21037/apm-20-1272
7. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
8. He G, Han Y, Fang Q, et al. Clinical experience of high-flow nasal cannula oxygen therapy in severe COVID-19 patients. Article in Chinese. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):232-239. doi:10.3785/j.issn.1008-9292.2020.03.13
9. Lalla U, Allwood BW, Louw EH, et al. The utility of high-flow nasal cannula oxygen therapy in the management of respiratory failure secondary to COVID-19 pneumonia. S Afr Med J. 2020;110(6):12941.
10. Zhang TT, Dai B, Wang W. Should the high-flow nasal oxygen therapy be used or avoided in COVID-19? J Transl Int Med. 2020;8(2):57-58. doi:10.2478/jtim-2020-0018
11. Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020;67(9):1217-1248. doi:10.1007/s12630-020-01740-2
12. Geng S, Mei Q, Zhu C, et al. High flow nasal cannula is a good treatment option for COVID-19. Heart Lung. 2020;49(5):444-445. doi:10.1016/j.hrtlng.2020.03.018
13. Feinstein MM, Niforatos JD, Hyun I, et al. Considerations for ventilator triage during the COVID-19 pandemic. Lancet Respir Med. 2020;8(6):e53. doi:10.1016/S2213-2600(20)30192-2
14. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
15. Rojas-Marte G, Hashmi AT, Khalid M, et al. Outcomes in patients with COVID-19 disease and high oxygen requirements. J Clin Med Res. 2021;13(1):26-37. doi:10.14740/jocmr4405
16. Zhang R, Mylonakis E. In inpatients with COVID-19, none of remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a differed from standard care for in-hospital mortality. Ann Intern Med. 2021;174(2):JC17. doi:10.7326/ACPJ202102160-017
17. Rello J, Waterer GW, Bourdiol A, Roquilly A. COVID-19, steroids and other immunomodulators: The jigsaw is not complete. Anaesth Crit Care Pain Med. 2020;39(6):699-701. doi:10.1016/j.accpm.2020.10.011
18. Dargin J, Stempek S, Lei Y, Gray Jr. A, Liesching T. The effect of a tiered provider staffing model on patient outcomes during the coronavirus disease 2019 pandemic: A single-center observational study. Int J Crit Illn Inj Sci. 2021;11(3). doi:10.4103/ijciis.ijciis_37_21
19. Ni YN, Wang T, Liang BM, Liang ZA. The independent factors associated with oxygen therapy in COVID-19 patients under 65 years old. PLoS One. 2021;16(1):e0245690. doi:10.1371/journal.pone.0245690
20. Alhazzani W, Moller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. doi:10.1097/CCM.0000000000004363
21. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi:10.1001/jama.2020.1585
22. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3
23. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi:10.1136/bmj.m1996
24. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
25. Demoule A, Vieillard Baron A, Darmon M, et al. High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202(7):1039-1042. doi:10.1164/rccm.202005-2007LE
26. Hansen CK, Stempek S, Liesching T, Lei Y, Dargin J. Characteristics and outcomes of patients receiving high flow nasal cannula therapy prior to mechanical ventilation in COVID-19 respiratory failure: a prospective observational study. Int J Crit Illn Inj Sci. 2021;11(2):56-60. doi:10.4103/IJCIIS.IJCIIS_181_20
27. van Gerwen M, Alsen M, Little C, et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93(2):907-915. doi:10.1002/jmv.26337
28. Hussain Alsayed HA, Saheb Sharif-Askari F, Saheb Sharif-Askari N, Hussain AAS, Hamid Q, Halwani R. Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: A retrospective cohort study. PLoS One. 2021;16(10):e0258643. doi:10.1371/journal.pone.0258643
29. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi:10.1056/NEJMoa2021436
30. Rees EM, Nightingale ES, Jafari Y, et al. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18(1):270. doi:10.1186/s12916-020-01726-3
31. Anderson M, Bach P, Baldwin MR. Hospital length of stay for severe COVID-19: implications for Remdesivir’s value. medRxiv. 2020;2020.08.10.20171637. doi:10.1101/2020.08.10.20171637
From Lahey Hospital and Medical Center, Burlington, MA (Drs. Liesching and Lei), and Tufts University School of Medicine, Boston, MA (Dr. Liesching)
ABSTRACT
Objective: To compare the utilization of oxygen therapies and clinical outcomes of patients admitted for COVID-19 during the second surge of the pandemic to that of patients admitted during the first surge.
Design: Observational study using a registry database.
Setting: Three hospitals (791 inpatient beds and 76 intensive care unit [ICU] beds) within the Beth Israel Lahey Health system in Massachusetts.
Participants: We included 3183 patients with COVID-19 admitted to hospitals.
Measurements: Baseline data included demographics and comorbidities. Treatments included low-flow supplemental oxygen (2-6 L/min), high-flow oxygen via nasal cannula, and invasive mechanical ventilation. Outcomes included ICU admission, length of stay, ventilator days, and mortality.
Results: A total of 3183 patients were included: 1586 during the first surge and 1597 during the second surge. Compared to the first surge, patients admitted during the second surge had a similar rate of receiving low-flow supplemental oxygen (65.8% vs 64.1%, P = .3), a higher rate of receiving high-flow nasal cannula (15.4% vs 10.8%, P = .0001), and a lower ventilation rate (5.6% vs 9.7%, P < .0001). The outcomes during the second surge were better than those during the first surge: lower ICU admission rate (8.1% vs 12.7%, P < .0001), shorter length of hospital stay (5 vs 6 days, P < .0001), fewer ventilator days (10 vs 16, P = .01), and lower mortality (8.3% vs 19.2%, P < .0001). Among ventilated patients, those who received high-flow nasal cannula had lower mortality.
Conclusion: Compared to the first surge of the COVID-19 pandemic, patients admitted during the second surge had similar likelihood of receiving low-flow supplemental oxygen, were more likely to receive high-flow nasal cannula, were less likely to be ventilated, and had better outcomes.
Keywords: supplemental oxygen, high-flow nasal cannula, ventilator.
The respiratory system receives the major impact of SARS-CoV-2 virus, and hypoxemia has been the predominant diagnosis for patients hospitalized with COVID-19.1,2 During the initial stage of the pandemic, oxygen therapies and mechanical ventilation were the only choices for these patients.3-6 Standard-of-care treatment for patients with COVID-19 during the initial surge included oxygen therapies and mechanical ventilation for hypoxemia and medications for comorbidities and COVID-19–associated sequelae, such as multi-organ dysfunction and failure. A report from New York during the first surge (May 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received supplemental oxygen and 12.2% received invasive mechanical ventilation.7 High-flow nasal cannula (HFNC) oxygen delivery has been utilized widely throughout the pandemic due to its superiority over other noninvasive respiratory support techniques.8-12 Mechanical ventilation is always necessary for critically ill patients with acute respiratory distress syndrome. However, ventilator scarcity has become a bottleneck in caring for severely ill patients with COVID-19 during the pandemic.13
The clinical outcomes of hospitalized COVID-19 patients include a high intubation rate, long length of hospital and intensive care unit (ICU) stay, and high mortality.14,15 As the pandemic evolved, new medications, including remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a, were used in addition to the standard of care, but these did not result in significantly different mortality from standard of care.16 Steroids are becoming foundational to the treatment of severe COVID-19 pneumonia, but evidence from high-quality randomized controlled clinical trials is lacking.17
During the first surge from March to May 2020, Massachusetts had the third highest number of COVID-19 cases among states in the United States.18 In early 2021, COVID-19 cases were climbing close to the peak of the second surge in Massachusetts. In this study, we compared utilization of low-flow supplemental oxygen, HFNC, and mechanical ventilation and clinical outcomes of patients admitted to 3 hospitals in Massachusetts during the second surge of the pandemic to that of patients admitted during the first surge.
Methods
Setting
Beth Israel Lahey Health is a system of academic and teaching hospitals with primary care and specialty care providers. We included 3 centers within the Beth Israel Lahey Health system in Massachusetts: Lahey Hospital and Medical Center, with 335 inpatient hospital beds and 52 critical care beds; Beverly Hospital, with 227 beds and 14 critical care beds; and Winchester Hospital, with 229 beds and 10 ICU beds.
Participants
We included patients admitted to the 3 hospitals with COVID-19 as a primary or secondary diagnosis during the first surge of the pandemic (March 1, 2020 to June 15, 2020) and the second surge (November 15, 2020 to January 27, 2021). The timeframe of the first surge was defined as the window between the start date and the end date of data collection. During the time window of the first surge, 1586 patients were included. The start time of the second surge was defined as the date when the data collection was restarted; the end date was set when the number of patients (1597) accumulated was close to the number of patients in the first surge (1586), so that the two groups had similar sample size.
Study Design
A data registry of COVID-19 patients was created by our institution, and the data were prospectively collected starting in March 2020. We retrospectively extracted data on the following from the registry database for this observational study: demographics and baseline comorbidities; the use of low-flow supplemental oxygen, HFNC, and invasive mechanical ventilator; and ICU admission, length of hospital stay, length of ICU stay, and hospital discharge disposition. Start and end times for each oxygen therapy were not entered in the registry. Data about other oxygen therapies, such as noninvasive positive-pressure ventilation, were not collected in the registry database, and therefore were not included in the analysis.
Statistical Analysis
Continuous variables (eg, age) were tested for data distribution normality using the Shapiro-Wilk test. Normally distributed data were tested using unpaired t-tests and displayed as mean (SD). The skewed data were tested using the Wilcoxon rank sum test and displayed as median (interquartile range [IQR]). The categorical variables were compared using chi-square test. Comparisons with P ≤ .05 were considered significantly different. Statistical analysis for this study was generated using Statistical Analysis Software (SAS), version 9.4 for Windows (SAS Institute Inc.).
Results
Baseline Characteristics
We included 3183 patients: 1586 admitted during the first surge and 1597 admitted during the second surge. Baseline characteristics of patients with COVID-19 admitted during the first and second surges are shown in Table 1. Patients admitted during the second surge were older (73 years vs 71 years, P = .01) and had higher rates of hypertension (64.8% vs 59.6%, P = .003) and asthma (12.9% vs 10.7%, P = .049) but a lower rate of interstitial lung disease (3.3% vs 7.7%, P < .001). Sequential organ failure assessment scores at admission and the rates of other comorbidities were not significantly different between the 2 surges.

Oxygen Therapies
The number of patients who were hospitalized and received low-flow supplemental oxygen, and/or HFNC, and/or ventilator in the first surge and the second surge is shown in the Figure. Of all patients included, 2067 (64.9%) received low-flow supplemental oxygen; of these, 374 (18.1%) subsequently received HFNC, and 85 (22.7%) of these subsequently received mechanical ventilation. Of all 3183 patients, 417 (13.1%) received HFNC; 43 of these patients received HFNC without receiving low-flow supplemental oxygen, and 98 (23.5%) subsequently received mechanical ventilation. Out of all 3183 patients, 244 (7.7%) received mechanical ventilation; 98 (40.2%) of these received HFNC while the remaining 146 (59.8%) did not. At the beginning of the first surge, the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was close to 1:1 (10/10); the ratio decreased to 6:10 in May and June 2020. At the beginning of the second surge, the ratio was 8:10 and then decreased to 3:10 in December 2020 and January 2021.

As shown in Table 2, the proportion of patients who received low-flow supplemental oxygen during the second surge was similar to that during the first surge (65.8% vs 64.1%, P = .3). Patients admitted during the second surge were more likely to receive HFNC than patients admitted during the first surge (15.4% vs 10.8%, P = .0001). Patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001).

Clinical Outcomes
As shown in Table 3, second surge outcomes were much better than first surge outcomes: the ICU admission rate was lower (8.1% vs 12.7%, P < .0001); patients were more likely to be discharged to home (60.2% vs 47.4%, P < .0001), had a shorter length of hospital stay (5 vs 6 days, P < .0001), and had fewer ventilator days (10 vs 16, P = .01); and mortality was lower (8.3% vs 19.2%, P < .0001). There was a trend that length of ICU stay was shorter during the second surge than during the first surge (7 days vs 9 days, P = .09).

As noted (Figure), the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was decreasing during both the first surge and the second surge. To further analyze the relation between ventilator and HFNC, we performed a subgroup analysis for 244 ventilated patients during both surges to compare outcomes between patients who received HFNC and those who did not receive HFNC (Table 4). Ninety-eight (40%) patients received HFNC. Ventilated patients who received HFNC had lower mortality than those patients who did not receive HFNC (31.6% vs 48%, P = .01), but had a longer length of hospital stay (29 days vs 14 days, P < .0001), longer length of ICU stay (17 days vs 9 days, P < .0001), and a higher number of ventilator days (16 vs 11, P = .001).

Discussion
Our study compared the baseline patient characteristics; utilization of low-flow supplemental oxygen therapy, HFNC, and mechanical ventilation; and clinical outcomes between the first surge (n = 1586) and the second surge (n = 1597) of the COVID-19 pandemic. During both surges, about two-thirds of admitted patients received low-flow supplemental oxygen. A higher proportion of the admitted patients received HFNC during the second surge than during the first surge, while the intubation rate was lower during the second surge than during the first surge.
Reported low-flow supplemental oxygen use ranged from 28% to 63% depending on the cohort characteristics and location during the first surge.6,7,19 A report from New York during the first surge (March 1 to April 4, 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received low-flow supplemental oxygen.7 HFNC is recommended in guidelines on management of patients with acute respiratory failure due to COVID-19.20 In our study, HFNC was utilized in a higher proportion of patients admitted for COVID-19 during the second surge (15.5% vs 10.8%, P = .0001). During the early pandemic period in Wuhan, China, 11% to 21% of admitted COVID-19 patients received HFNC.21,22 Utilization of HFNC in New York during the first surge (March to May 2020) varied from 5% to 14.3% of patients admitted with COVID-19.23,24 Our subgroup analysis of the ventilated patients showed that patients who received HFNC had lower mortality than those who did not (31.6% vs 48.0%, P = .011). Comparably, a report from Paris, France, showed that among patients admitted to ICUs for acute hypoxemic respiratory failure, those who received HFNC had lower mortality at day 60 than those who did not (21% vs 31%, P = .052).25 Our recent analysis showed that patients treated with HFNC prior to mechanical ventilation had lower mortality than those treated with only conventional oxygen (30% vs 52%, P = .05).26 In this subgroup analysis, we could not determine if HFNC treatment was administered before or after ventilation because HFNC was entered as dichotomous data (“Yes” or “No”) in the registry database. We merely showed the beneficial effect of HFNC on reducing mortality for ventilated COVID-19 patients, but did not mean to focus on how and when to apply HFNC.
We observed that the patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001). During the first surge in New York, among 5700 patients admitted with COVID-19, 12.2% received invasive mechanical ventilation.7 In another report, also from New York during the first surge, 26.1% of 2015 hospitalized COVID-19 patients received mechanical ventilation.27 In our study, the ventilation rate of 9.7% during the first surge was lower.
Outcomes during the second surge were better than during the first surge, including ICU admission rate, hospital and ICU length of stay, ventilator days, and mortality. The mortality was 19.2% during the first surge vs 8.3% during the second surge (P < .0001). The mortality of 19.2% was lower than the 30.6% mortality reported for 2015 hospitalized COVID-19 patients in New York during the first surge.27 A retrospective study showed that early administration of remdesivir was associated with reduced ICU admission, ventilation use, and mortality.28 The RECOVERY clinical trial showed that dexamethasone improved mortality for COVID-19 patients who received respiratory support, but not for patients who did not receive any respiratory support.29 Perhaps some, if not all, of the improvement in ICU admission and mortality during the second surge was attributed to the new medications, such as antivirals and steroids.
The length of hospital stay for patients with moderate to severe COVID-19 varied from 4 to 53 days at different locations of the world, as shown in a meta-analysis by Rees and colleagues.30 Our results showing a length of stay of 6 days during the first surge and 5 days during the second surge fell into the shorter end of this range. In a retrospective analysis of 1643 adults with severe COVID-19 admitted to hospitals in New York City between March 9, 2020 and April 23, 2020, median hospital length of stay was 7 (IQR, 3-14) days.31 For the ventilated patients in our study, the length of stay of 14 days (did not receive HFNC) and 29 days (received HFNC) was much longer. This longer length of stay might be attributed to the patients in our study being older and having more severe comorbidities.
The main purpose of this study was to compare the oxygen therapies and outcomes between 2 surges. It is difficult to associate the clinical outcomes with the oxygen therapies because new therapies and medications were available after the first surge. It was not possible to adjust the outcomes with confounders (other therapies and medications) because the registry data did not include the new therapies and medications.
A strength of this study was that we included a large, balanced number of patients in the first surge and the second surge. We did not plan the sample size in both groups as we could not predict the number of admissions. We set the end date of data collection for analysis as the time when the number of patients admitted during the second surge was similar to the number of patients admitted during the first surge. A limitation was that the registry database was created by the institution and was not designed solely for this study. The data for oxygen therapies were limited to low-flow supplemental oxygen, HFNC, and invasive mechanical ventilation; data for noninvasive ventilation were not included.
Conclusion
At our centers, during the second surge of COVID-19 pandemic, patients hospitalized with COVID-19 infection were more likely to receive HFNC but less likely to be ventilated. Compared to the first surge, the hospitalized patients with COVID-19 infection had a lower ICU admission rate, shorter length of hospital stay, fewer ventilator days, and lower mortality. For ventilated patients, those who received HFNC had lower mortality than those who did not.
Corresponding author: Timothy N. Liesching, MD, 41 Mall Road, Burlington, MA 01805; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0086
From Lahey Hospital and Medical Center, Burlington, MA (Drs. Liesching and Lei), and Tufts University School of Medicine, Boston, MA (Dr. Liesching)
ABSTRACT
Objective: To compare the utilization of oxygen therapies and clinical outcomes of patients admitted for COVID-19 during the second surge of the pandemic to that of patients admitted during the first surge.
Design: Observational study using a registry database.
Setting: Three hospitals (791 inpatient beds and 76 intensive care unit [ICU] beds) within the Beth Israel Lahey Health system in Massachusetts.
Participants: We included 3183 patients with COVID-19 admitted to hospitals.
Measurements: Baseline data included demographics and comorbidities. Treatments included low-flow supplemental oxygen (2-6 L/min), high-flow oxygen via nasal cannula, and invasive mechanical ventilation. Outcomes included ICU admission, length of stay, ventilator days, and mortality.
Results: A total of 3183 patients were included: 1586 during the first surge and 1597 during the second surge. Compared to the first surge, patients admitted during the second surge had a similar rate of receiving low-flow supplemental oxygen (65.8% vs 64.1%, P = .3), a higher rate of receiving high-flow nasal cannula (15.4% vs 10.8%, P = .0001), and a lower ventilation rate (5.6% vs 9.7%, P < .0001). The outcomes during the second surge were better than those during the first surge: lower ICU admission rate (8.1% vs 12.7%, P < .0001), shorter length of hospital stay (5 vs 6 days, P < .0001), fewer ventilator days (10 vs 16, P = .01), and lower mortality (8.3% vs 19.2%, P < .0001). Among ventilated patients, those who received high-flow nasal cannula had lower mortality.
Conclusion: Compared to the first surge of the COVID-19 pandemic, patients admitted during the second surge had similar likelihood of receiving low-flow supplemental oxygen, were more likely to receive high-flow nasal cannula, were less likely to be ventilated, and had better outcomes.
Keywords: supplemental oxygen, high-flow nasal cannula, ventilator.
The respiratory system receives the major impact of SARS-CoV-2 virus, and hypoxemia has been the predominant diagnosis for patients hospitalized with COVID-19.1,2 During the initial stage of the pandemic, oxygen therapies and mechanical ventilation were the only choices for these patients.3-6 Standard-of-care treatment for patients with COVID-19 during the initial surge included oxygen therapies and mechanical ventilation for hypoxemia and medications for comorbidities and COVID-19–associated sequelae, such as multi-organ dysfunction and failure. A report from New York during the first surge (May 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received supplemental oxygen and 12.2% received invasive mechanical ventilation.7 High-flow nasal cannula (HFNC) oxygen delivery has been utilized widely throughout the pandemic due to its superiority over other noninvasive respiratory support techniques.8-12 Mechanical ventilation is always necessary for critically ill patients with acute respiratory distress syndrome. However, ventilator scarcity has become a bottleneck in caring for severely ill patients with COVID-19 during the pandemic.13
The clinical outcomes of hospitalized COVID-19 patients include a high intubation rate, long length of hospital and intensive care unit (ICU) stay, and high mortality.14,15 As the pandemic evolved, new medications, including remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a, were used in addition to the standard of care, but these did not result in significantly different mortality from standard of care.16 Steroids are becoming foundational to the treatment of severe COVID-19 pneumonia, but evidence from high-quality randomized controlled clinical trials is lacking.17
During the first surge from March to May 2020, Massachusetts had the third highest number of COVID-19 cases among states in the United States.18 In early 2021, COVID-19 cases were climbing close to the peak of the second surge in Massachusetts. In this study, we compared utilization of low-flow supplemental oxygen, HFNC, and mechanical ventilation and clinical outcomes of patients admitted to 3 hospitals in Massachusetts during the second surge of the pandemic to that of patients admitted during the first surge.
Methods
Setting
Beth Israel Lahey Health is a system of academic and teaching hospitals with primary care and specialty care providers. We included 3 centers within the Beth Israel Lahey Health system in Massachusetts: Lahey Hospital and Medical Center, with 335 inpatient hospital beds and 52 critical care beds; Beverly Hospital, with 227 beds and 14 critical care beds; and Winchester Hospital, with 229 beds and 10 ICU beds.
Participants
We included patients admitted to the 3 hospitals with COVID-19 as a primary or secondary diagnosis during the first surge of the pandemic (March 1, 2020 to June 15, 2020) and the second surge (November 15, 2020 to January 27, 2021). The timeframe of the first surge was defined as the window between the start date and the end date of data collection. During the time window of the first surge, 1586 patients were included. The start time of the second surge was defined as the date when the data collection was restarted; the end date was set when the number of patients (1597) accumulated was close to the number of patients in the first surge (1586), so that the two groups had similar sample size.
Study Design
A data registry of COVID-19 patients was created by our institution, and the data were prospectively collected starting in March 2020. We retrospectively extracted data on the following from the registry database for this observational study: demographics and baseline comorbidities; the use of low-flow supplemental oxygen, HFNC, and invasive mechanical ventilator; and ICU admission, length of hospital stay, length of ICU stay, and hospital discharge disposition. Start and end times for each oxygen therapy were not entered in the registry. Data about other oxygen therapies, such as noninvasive positive-pressure ventilation, were not collected in the registry database, and therefore were not included in the analysis.
Statistical Analysis
Continuous variables (eg, age) were tested for data distribution normality using the Shapiro-Wilk test. Normally distributed data were tested using unpaired t-tests and displayed as mean (SD). The skewed data were tested using the Wilcoxon rank sum test and displayed as median (interquartile range [IQR]). The categorical variables were compared using chi-square test. Comparisons with P ≤ .05 were considered significantly different. Statistical analysis for this study was generated using Statistical Analysis Software (SAS), version 9.4 for Windows (SAS Institute Inc.).
Results
Baseline Characteristics
We included 3183 patients: 1586 admitted during the first surge and 1597 admitted during the second surge. Baseline characteristics of patients with COVID-19 admitted during the first and second surges are shown in Table 1. Patients admitted during the second surge were older (73 years vs 71 years, P = .01) and had higher rates of hypertension (64.8% vs 59.6%, P = .003) and asthma (12.9% vs 10.7%, P = .049) but a lower rate of interstitial lung disease (3.3% vs 7.7%, P < .001). Sequential organ failure assessment scores at admission and the rates of other comorbidities were not significantly different between the 2 surges.

Oxygen Therapies
The number of patients who were hospitalized and received low-flow supplemental oxygen, and/or HFNC, and/or ventilator in the first surge and the second surge is shown in the Figure. Of all patients included, 2067 (64.9%) received low-flow supplemental oxygen; of these, 374 (18.1%) subsequently received HFNC, and 85 (22.7%) of these subsequently received mechanical ventilation. Of all 3183 patients, 417 (13.1%) received HFNC; 43 of these patients received HFNC without receiving low-flow supplemental oxygen, and 98 (23.5%) subsequently received mechanical ventilation. Out of all 3183 patients, 244 (7.7%) received mechanical ventilation; 98 (40.2%) of these received HFNC while the remaining 146 (59.8%) did not. At the beginning of the first surge, the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was close to 1:1 (10/10); the ratio decreased to 6:10 in May and June 2020. At the beginning of the second surge, the ratio was 8:10 and then decreased to 3:10 in December 2020 and January 2021.

As shown in Table 2, the proportion of patients who received low-flow supplemental oxygen during the second surge was similar to that during the first surge (65.8% vs 64.1%, P = .3). Patients admitted during the second surge were more likely to receive HFNC than patients admitted during the first surge (15.4% vs 10.8%, P = .0001). Patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001).

Clinical Outcomes
As shown in Table 3, second surge outcomes were much better than first surge outcomes: the ICU admission rate was lower (8.1% vs 12.7%, P < .0001); patients were more likely to be discharged to home (60.2% vs 47.4%, P < .0001), had a shorter length of hospital stay (5 vs 6 days, P < .0001), and had fewer ventilator days (10 vs 16, P = .01); and mortality was lower (8.3% vs 19.2%, P < .0001). There was a trend that length of ICU stay was shorter during the second surge than during the first surge (7 days vs 9 days, P = .09).

As noted (Figure), the ratio of patients who received invasive mechanical ventilation to patients who received HFNC was decreasing during both the first surge and the second surge. To further analyze the relation between ventilator and HFNC, we performed a subgroup analysis for 244 ventilated patients during both surges to compare outcomes between patients who received HFNC and those who did not receive HFNC (Table 4). Ninety-eight (40%) patients received HFNC. Ventilated patients who received HFNC had lower mortality than those patients who did not receive HFNC (31.6% vs 48%, P = .01), but had a longer length of hospital stay (29 days vs 14 days, P < .0001), longer length of ICU stay (17 days vs 9 days, P < .0001), and a higher number of ventilator days (16 vs 11, P = .001).

Discussion
Our study compared the baseline patient characteristics; utilization of low-flow supplemental oxygen therapy, HFNC, and mechanical ventilation; and clinical outcomes between the first surge (n = 1586) and the second surge (n = 1597) of the COVID-19 pandemic. During both surges, about two-thirds of admitted patients received low-flow supplemental oxygen. A higher proportion of the admitted patients received HFNC during the second surge than during the first surge, while the intubation rate was lower during the second surge than during the first surge.
Reported low-flow supplemental oxygen use ranged from 28% to 63% depending on the cohort characteristics and location during the first surge.6,7,19 A report from New York during the first surge (March 1 to April 4, 2020) showed that among 5700 hospitalized patients with COVID-19, 27.8% received low-flow supplemental oxygen.7 HFNC is recommended in guidelines on management of patients with acute respiratory failure due to COVID-19.20 In our study, HFNC was utilized in a higher proportion of patients admitted for COVID-19 during the second surge (15.5% vs 10.8%, P = .0001). During the early pandemic period in Wuhan, China, 11% to 21% of admitted COVID-19 patients received HFNC.21,22 Utilization of HFNC in New York during the first surge (March to May 2020) varied from 5% to 14.3% of patients admitted with COVID-19.23,24 Our subgroup analysis of the ventilated patients showed that patients who received HFNC had lower mortality than those who did not (31.6% vs 48.0%, P = .011). Comparably, a report from Paris, France, showed that among patients admitted to ICUs for acute hypoxemic respiratory failure, those who received HFNC had lower mortality at day 60 than those who did not (21% vs 31%, P = .052).25 Our recent analysis showed that patients treated with HFNC prior to mechanical ventilation had lower mortality than those treated with only conventional oxygen (30% vs 52%, P = .05).26 In this subgroup analysis, we could not determine if HFNC treatment was administered before or after ventilation because HFNC was entered as dichotomous data (“Yes” or “No”) in the registry database. We merely showed the beneficial effect of HFNC on reducing mortality for ventilated COVID-19 patients, but did not mean to focus on how and when to apply HFNC.
We observed that the patients admitted during the second surge were less likely to be ventilated than the patients admitted during the first surge (5.6% vs 9.7%, P < .0001). During the first surge in New York, among 5700 patients admitted with COVID-19, 12.2% received invasive mechanical ventilation.7 In another report, also from New York during the first surge, 26.1% of 2015 hospitalized COVID-19 patients received mechanical ventilation.27 In our study, the ventilation rate of 9.7% during the first surge was lower.
Outcomes during the second surge were better than during the first surge, including ICU admission rate, hospital and ICU length of stay, ventilator days, and mortality. The mortality was 19.2% during the first surge vs 8.3% during the second surge (P < .0001). The mortality of 19.2% was lower than the 30.6% mortality reported for 2015 hospitalized COVID-19 patients in New York during the first surge.27 A retrospective study showed that early administration of remdesivir was associated with reduced ICU admission, ventilation use, and mortality.28 The RECOVERY clinical trial showed that dexamethasone improved mortality for COVID-19 patients who received respiratory support, but not for patients who did not receive any respiratory support.29 Perhaps some, if not all, of the improvement in ICU admission and mortality during the second surge was attributed to the new medications, such as antivirals and steroids.
The length of hospital stay for patients with moderate to severe COVID-19 varied from 4 to 53 days at different locations of the world, as shown in a meta-analysis by Rees and colleagues.30 Our results showing a length of stay of 6 days during the first surge and 5 days during the second surge fell into the shorter end of this range. In a retrospective analysis of 1643 adults with severe COVID-19 admitted to hospitals in New York City between March 9, 2020 and April 23, 2020, median hospital length of stay was 7 (IQR, 3-14) days.31 For the ventilated patients in our study, the length of stay of 14 days (did not receive HFNC) and 29 days (received HFNC) was much longer. This longer length of stay might be attributed to the patients in our study being older and having more severe comorbidities.
The main purpose of this study was to compare the oxygen therapies and outcomes between 2 surges. It is difficult to associate the clinical outcomes with the oxygen therapies because new therapies and medications were available after the first surge. It was not possible to adjust the outcomes with confounders (other therapies and medications) because the registry data did not include the new therapies and medications.
A strength of this study was that we included a large, balanced number of patients in the first surge and the second surge. We did not plan the sample size in both groups as we could not predict the number of admissions. We set the end date of data collection for analysis as the time when the number of patients admitted during the second surge was similar to the number of patients admitted during the first surge. A limitation was that the registry database was created by the institution and was not designed solely for this study. The data for oxygen therapies were limited to low-flow supplemental oxygen, HFNC, and invasive mechanical ventilation; data for noninvasive ventilation were not included.
Conclusion
At our centers, during the second surge of COVID-19 pandemic, patients hospitalized with COVID-19 infection were more likely to receive HFNC but less likely to be ventilated. Compared to the first surge, the hospitalized patients with COVID-19 infection had a lower ICU admission rate, shorter length of hospital stay, fewer ventilator days, and lower mortality. For ventilated patients, those who received HFNC had lower mortality than those who did not.
Corresponding author: Timothy N. Liesching, MD, 41 Mall Road, Burlington, MA 01805; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0086
1. Xie J, Covassin N, Fan Z, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95(6):1138-1147. doi:10.1016/j.mayocp.2020.04.006
2. Asleh R, Asher E, Yagel O, et al. Predictors of hypoxemia and related adverse outcomes in patients hospitalized with COVID-19: a double-center retrospective study. J Clin Med. 2021;10(16):3581. doi:10.3390/jcm10163581
3. Choi KJ, Hong HL, Kim EJ. Association between oxygen saturation/fraction of inhaled oxygen and mortality in patients with COVID-19 associated pneumonia requiring oxygen therapy. Tuberc Respir Dis (Seoul). 2021;84(2):125-133. doi:10.4046/trd.2020.0126
4. Dixit SB. Role of noninvasive oxygen therapy strategies in COVID-19 patients: Where are we going? Indian J Crit Care Med. 2020;24(10):897-898. doi:10.5005/jp-journals-10071-23625
5. Gonzalez-Castro A, Fajardo Campoverde A, Medina A, et al. Non-invasive mechanical ventilation and high-flow oxygen therapy in the COVID-19 pandemic: the value of a draw. Med Intensiva (Engl Ed). 2021;45(5):320-321. doi:10.1016/j.medine.2021.04.001
6. Pan W, Li J, Ou Y, et al. Clinical outcome of standardized oxygen therapy nursing strategy in COVID-19. Ann Palliat Med. 2020;9(4):2171-2177. doi:10.21037/apm-20-1272
7. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
8. He G, Han Y, Fang Q, et al. Clinical experience of high-flow nasal cannula oxygen therapy in severe COVID-19 patients. Article in Chinese. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):232-239. doi:10.3785/j.issn.1008-9292.2020.03.13
9. Lalla U, Allwood BW, Louw EH, et al. The utility of high-flow nasal cannula oxygen therapy in the management of respiratory failure secondary to COVID-19 pneumonia. S Afr Med J. 2020;110(6):12941.
10. Zhang TT, Dai B, Wang W. Should the high-flow nasal oxygen therapy be used or avoided in COVID-19? J Transl Int Med. 2020;8(2):57-58. doi:10.2478/jtim-2020-0018
11. Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020;67(9):1217-1248. doi:10.1007/s12630-020-01740-2
12. Geng S, Mei Q, Zhu C, et al. High flow nasal cannula is a good treatment option for COVID-19. Heart Lung. 2020;49(5):444-445. doi:10.1016/j.hrtlng.2020.03.018
13. Feinstein MM, Niforatos JD, Hyun I, et al. Considerations for ventilator triage during the COVID-19 pandemic. Lancet Respir Med. 2020;8(6):e53. doi:10.1016/S2213-2600(20)30192-2
14. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
15. Rojas-Marte G, Hashmi AT, Khalid M, et al. Outcomes in patients with COVID-19 disease and high oxygen requirements. J Clin Med Res. 2021;13(1):26-37. doi:10.14740/jocmr4405
16. Zhang R, Mylonakis E. In inpatients with COVID-19, none of remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a differed from standard care for in-hospital mortality. Ann Intern Med. 2021;174(2):JC17. doi:10.7326/ACPJ202102160-017
17. Rello J, Waterer GW, Bourdiol A, Roquilly A. COVID-19, steroids and other immunomodulators: The jigsaw is not complete. Anaesth Crit Care Pain Med. 2020;39(6):699-701. doi:10.1016/j.accpm.2020.10.011
18. Dargin J, Stempek S, Lei Y, Gray Jr. A, Liesching T. The effect of a tiered provider staffing model on patient outcomes during the coronavirus disease 2019 pandemic: A single-center observational study. Int J Crit Illn Inj Sci. 2021;11(3). doi:10.4103/ijciis.ijciis_37_21
19. Ni YN, Wang T, Liang BM, Liang ZA. The independent factors associated with oxygen therapy in COVID-19 patients under 65 years old. PLoS One. 2021;16(1):e0245690. doi:10.1371/journal.pone.0245690
20. Alhazzani W, Moller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. doi:10.1097/CCM.0000000000004363
21. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi:10.1001/jama.2020.1585
22. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3
23. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi:10.1136/bmj.m1996
24. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
25. Demoule A, Vieillard Baron A, Darmon M, et al. High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202(7):1039-1042. doi:10.1164/rccm.202005-2007LE
26. Hansen CK, Stempek S, Liesching T, Lei Y, Dargin J. Characteristics and outcomes of patients receiving high flow nasal cannula therapy prior to mechanical ventilation in COVID-19 respiratory failure: a prospective observational study. Int J Crit Illn Inj Sci. 2021;11(2):56-60. doi:10.4103/IJCIIS.IJCIIS_181_20
27. van Gerwen M, Alsen M, Little C, et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93(2):907-915. doi:10.1002/jmv.26337
28. Hussain Alsayed HA, Saheb Sharif-Askari F, Saheb Sharif-Askari N, Hussain AAS, Hamid Q, Halwani R. Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: A retrospective cohort study. PLoS One. 2021;16(10):e0258643. doi:10.1371/journal.pone.0258643
29. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi:10.1056/NEJMoa2021436
30. Rees EM, Nightingale ES, Jafari Y, et al. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18(1):270. doi:10.1186/s12916-020-01726-3
31. Anderson M, Bach P, Baldwin MR. Hospital length of stay for severe COVID-19: implications for Remdesivir’s value. medRxiv. 2020;2020.08.10.20171637. doi:10.1101/2020.08.10.20171637
1. Xie J, Covassin N, Fan Z, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95(6):1138-1147. doi:10.1016/j.mayocp.2020.04.006
2. Asleh R, Asher E, Yagel O, et al. Predictors of hypoxemia and related adverse outcomes in patients hospitalized with COVID-19: a double-center retrospective study. J Clin Med. 2021;10(16):3581. doi:10.3390/jcm10163581
3. Choi KJ, Hong HL, Kim EJ. Association between oxygen saturation/fraction of inhaled oxygen and mortality in patients with COVID-19 associated pneumonia requiring oxygen therapy. Tuberc Respir Dis (Seoul). 2021;84(2):125-133. doi:10.4046/trd.2020.0126
4. Dixit SB. Role of noninvasive oxygen therapy strategies in COVID-19 patients: Where are we going? Indian J Crit Care Med. 2020;24(10):897-898. doi:10.5005/jp-journals-10071-23625
5. Gonzalez-Castro A, Fajardo Campoverde A, Medina A, et al. Non-invasive mechanical ventilation and high-flow oxygen therapy in the COVID-19 pandemic: the value of a draw. Med Intensiva (Engl Ed). 2021;45(5):320-321. doi:10.1016/j.medine.2021.04.001
6. Pan W, Li J, Ou Y, et al. Clinical outcome of standardized oxygen therapy nursing strategy in COVID-19. Ann Palliat Med. 2020;9(4):2171-2177. doi:10.21037/apm-20-1272
7. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
8. He G, Han Y, Fang Q, et al. Clinical experience of high-flow nasal cannula oxygen therapy in severe COVID-19 patients. Article in Chinese. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):232-239. doi:10.3785/j.issn.1008-9292.2020.03.13
9. Lalla U, Allwood BW, Louw EH, et al. The utility of high-flow nasal cannula oxygen therapy in the management of respiratory failure secondary to COVID-19 pneumonia. S Afr Med J. 2020;110(6):12941.
10. Zhang TT, Dai B, Wang W. Should the high-flow nasal oxygen therapy be used or avoided in COVID-19? J Transl Int Med. 2020;8(2):57-58. doi:10.2478/jtim-2020-0018
11. Agarwal A, Basmaji J, Muttalib F, et al. High-flow nasal cannula for acute hypoxemic respiratory failure in patients with COVID-19: systematic reviews of effectiveness and its risks of aerosolization, dispersion, and infection transmission. Can J Anaesth. 2020;67(9):1217-1248. doi:10.1007/s12630-020-01740-2
12. Geng S, Mei Q, Zhu C, et al. High flow nasal cannula is a good treatment option for COVID-19. Heart Lung. 2020;49(5):444-445. doi:10.1016/j.hrtlng.2020.03.018
13. Feinstein MM, Niforatos JD, Hyun I, et al. Considerations for ventilator triage during the COVID-19 pandemic. Lancet Respir Med. 2020;8(6):e53. doi:10.1016/S2213-2600(20)30192-2
14. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
15. Rojas-Marte G, Hashmi AT, Khalid M, et al. Outcomes in patients with COVID-19 disease and high oxygen requirements. J Clin Med Res. 2021;13(1):26-37. doi:10.14740/jocmr4405
16. Zhang R, Mylonakis E. In inpatients with COVID-19, none of remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a differed from standard care for in-hospital mortality. Ann Intern Med. 2021;174(2):JC17. doi:10.7326/ACPJ202102160-017
17. Rello J, Waterer GW, Bourdiol A, Roquilly A. COVID-19, steroids and other immunomodulators: The jigsaw is not complete. Anaesth Crit Care Pain Med. 2020;39(6):699-701. doi:10.1016/j.accpm.2020.10.011
18. Dargin J, Stempek S, Lei Y, Gray Jr. A, Liesching T. The effect of a tiered provider staffing model on patient outcomes during the coronavirus disease 2019 pandemic: A single-center observational study. Int J Crit Illn Inj Sci. 2021;11(3). doi:10.4103/ijciis.ijciis_37_21
19. Ni YN, Wang T, Liang BM, Liang ZA. The independent factors associated with oxygen therapy in COVID-19 patients under 65 years old. PLoS One. 2021;16(1):e0245690. doi:10.1371/journal.pone.0245690
20. Alhazzani W, Moller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. doi:10.1097/CCM.0000000000004363
21. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi:10.1001/jama.2020.1585
22. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3
23. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi:10.1136/bmj.m1996
24. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
25. Demoule A, Vieillard Baron A, Darmon M, et al. High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202(7):1039-1042. doi:10.1164/rccm.202005-2007LE
26. Hansen CK, Stempek S, Liesching T, Lei Y, Dargin J. Characteristics and outcomes of patients receiving high flow nasal cannula therapy prior to mechanical ventilation in COVID-19 respiratory failure: a prospective observational study. Int J Crit Illn Inj Sci. 2021;11(2):56-60. doi:10.4103/IJCIIS.IJCIIS_181_20
27. van Gerwen M, Alsen M, Little C, et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93(2):907-915. doi:10.1002/jmv.26337
28. Hussain Alsayed HA, Saheb Sharif-Askari F, Saheb Sharif-Askari N, Hussain AAS, Hamid Q, Halwani R. Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: A retrospective cohort study. PLoS One. 2021;16(10):e0258643. doi:10.1371/journal.pone.0258643
29. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi:10.1056/NEJMoa2021436
30. Rees EM, Nightingale ES, Jafari Y, et al. COVID-19 length of hospital stay: a systematic review and data synthesis. BMC Med. 2020;18(1):270. doi:10.1186/s12916-020-01726-3
31. Anderson M, Bach P, Baldwin MR. Hospital length of stay for severe COVID-19: implications for Remdesivir’s value. medRxiv. 2020;2020.08.10.20171637. doi:10.1101/2020.08.10.20171637
COVID-19–alopecia areata link? Review doesn’t find much evidence
A new
If there is a connection, it’s likely not a strong one, said study author Rachel E. Christensen, a graduate student at Rutgers Robert Wood Johnson Medical School, in an interview. “Based on the reported number of cases following COVID-19, alopecia areata appears to be low on the list of common skin manifestations of COVID-19,” she said. Of 402 articles screened from three databases in the review, only 11 were identified as related to alopecia areata (AA) and COVID-19, and only 9 of those met the study inclusion criteria. “This number alone highlights the very low number of published articles investigating this connection.”
The review was published in JAAD International.
While COVID-19 has been linked to a variety of skin conditions, a 2021 South Korean study of 7,958 cases and 218,779 controls found no connection between infection and AA even after covariates such as age, gender, and income level were taken into account. In a letter to the editor published in 2020, dermatologists in Turkey reported that the percentage of patients with AA at the dermatology outpatient clinic jumped from 0.97% in May 2019 to 1.48% in May 2020. The number of patients in each group wasn’t reported.
Systematic review
The investigators launched the systematic review to gain a wider perspective, although there are still limitations. On the one hand, Ms. Christensen said, “we do know that COVID-19, like other viruses, has been linked to various dermatological disorders.”
However, “it is difficult to tease apart whether any worsening of alopecia areata we see following COVID-19 is due to the virus itself or the increased psychological burden related to the infection or to the pandemic in general,” she said. Indeed, the authors of the report in Turkey attributed the rise in cases to stress.
For the review, the researchers analyzed studies from Italy (four), Turkey (two), Brazil (one), the United States (one), and Poland (one).
Six of the studies reported cases of new-onset AA following COVID-19 infection (seven cases; average age, 37 years; females, three). Another study was a retrospective review of 32 patients with preexisting AA who developed COVID-19; none experienced significant worsening of AA within 6 months.
The review also included a study based on a survey of 389 patients with AA. The investigators found that, at a median 2.14 months after infection, 44% of those who had COVID-19 vs. 12% of those who were COVID negative had a relapse. Finally, a case report noted a patient with preexisting AA whose condition worsened following COVID infection.
The findings suggest that AA “could be a dermatological manifestation of COVID-19, with cases most often appearing 1-2 months following infection,” the authors wrote. “However, the heterogeneity of study designs and high proportion of case reports make it challenging to draw any conclusion.”
In an interview, dermatologist Brett King, MD, PhD, of the department of dermatology, Yale University, New Haven, Conn., said the review findings suggest that “there is little concern of alopecia areata following COVID infection.
Does new-onset AA happen, and are there exacerbations of preexisting disease related to COVID infection? Probably yes, but rarely.”
However, he noted that another form of alopecia, telogen effluvium (TE), is more common after COVID-19 infection. According to Dr. King, who was not involved with the systematic review, TE is typically time-limited, compared with AA’s more common chronic waxing-and-waning course.
“Distinguishing TE and AA is usually straightforward because AA typically presents with well-circumscribed patches of hair loss,” such as circular patches, “while TE manifests as diffuse hair loss,” he explained. “Rarely, however, AA does manifest diffuse hair loss without patches, similar to TE. In those cases, it may be difficult to distinguish them. A biopsy may be helpful if there is a question of the diagnosis.”
No study funding is reported. The review authors and Dr. King report no relevant disclosures.
A new
If there is a connection, it’s likely not a strong one, said study author Rachel E. Christensen, a graduate student at Rutgers Robert Wood Johnson Medical School, in an interview. “Based on the reported number of cases following COVID-19, alopecia areata appears to be low on the list of common skin manifestations of COVID-19,” she said. Of 402 articles screened from three databases in the review, only 11 were identified as related to alopecia areata (AA) and COVID-19, and only 9 of those met the study inclusion criteria. “This number alone highlights the very low number of published articles investigating this connection.”
The review was published in JAAD International.
While COVID-19 has been linked to a variety of skin conditions, a 2021 South Korean study of 7,958 cases and 218,779 controls found no connection between infection and AA even after covariates such as age, gender, and income level were taken into account. In a letter to the editor published in 2020, dermatologists in Turkey reported that the percentage of patients with AA at the dermatology outpatient clinic jumped from 0.97% in May 2019 to 1.48% in May 2020. The number of patients in each group wasn’t reported.
Systematic review
The investigators launched the systematic review to gain a wider perspective, although there are still limitations. On the one hand, Ms. Christensen said, “we do know that COVID-19, like other viruses, has been linked to various dermatological disorders.”
However, “it is difficult to tease apart whether any worsening of alopecia areata we see following COVID-19 is due to the virus itself or the increased psychological burden related to the infection or to the pandemic in general,” she said. Indeed, the authors of the report in Turkey attributed the rise in cases to stress.
For the review, the researchers analyzed studies from Italy (four), Turkey (two), Brazil (one), the United States (one), and Poland (one).
Six of the studies reported cases of new-onset AA following COVID-19 infection (seven cases; average age, 37 years; females, three). Another study was a retrospective review of 32 patients with preexisting AA who developed COVID-19; none experienced significant worsening of AA within 6 months.
The review also included a study based on a survey of 389 patients with AA. The investigators found that, at a median 2.14 months after infection, 44% of those who had COVID-19 vs. 12% of those who were COVID negative had a relapse. Finally, a case report noted a patient with preexisting AA whose condition worsened following COVID infection.
The findings suggest that AA “could be a dermatological manifestation of COVID-19, with cases most often appearing 1-2 months following infection,” the authors wrote. “However, the heterogeneity of study designs and high proportion of case reports make it challenging to draw any conclusion.”
In an interview, dermatologist Brett King, MD, PhD, of the department of dermatology, Yale University, New Haven, Conn., said the review findings suggest that “there is little concern of alopecia areata following COVID infection.
Does new-onset AA happen, and are there exacerbations of preexisting disease related to COVID infection? Probably yes, but rarely.”
However, he noted that another form of alopecia, telogen effluvium (TE), is more common after COVID-19 infection. According to Dr. King, who was not involved with the systematic review, TE is typically time-limited, compared with AA’s more common chronic waxing-and-waning course.
“Distinguishing TE and AA is usually straightforward because AA typically presents with well-circumscribed patches of hair loss,” such as circular patches, “while TE manifests as diffuse hair loss,” he explained. “Rarely, however, AA does manifest diffuse hair loss without patches, similar to TE. In those cases, it may be difficult to distinguish them. A biopsy may be helpful if there is a question of the diagnosis.”
No study funding is reported. The review authors and Dr. King report no relevant disclosures.
A new
If there is a connection, it’s likely not a strong one, said study author Rachel E. Christensen, a graduate student at Rutgers Robert Wood Johnson Medical School, in an interview. “Based on the reported number of cases following COVID-19, alopecia areata appears to be low on the list of common skin manifestations of COVID-19,” she said. Of 402 articles screened from three databases in the review, only 11 were identified as related to alopecia areata (AA) and COVID-19, and only 9 of those met the study inclusion criteria. “This number alone highlights the very low number of published articles investigating this connection.”
The review was published in JAAD International.
While COVID-19 has been linked to a variety of skin conditions, a 2021 South Korean study of 7,958 cases and 218,779 controls found no connection between infection and AA even after covariates such as age, gender, and income level were taken into account. In a letter to the editor published in 2020, dermatologists in Turkey reported that the percentage of patients with AA at the dermatology outpatient clinic jumped from 0.97% in May 2019 to 1.48% in May 2020. The number of patients in each group wasn’t reported.
Systematic review
The investigators launched the systematic review to gain a wider perspective, although there are still limitations. On the one hand, Ms. Christensen said, “we do know that COVID-19, like other viruses, has been linked to various dermatological disorders.”
However, “it is difficult to tease apart whether any worsening of alopecia areata we see following COVID-19 is due to the virus itself or the increased psychological burden related to the infection or to the pandemic in general,” she said. Indeed, the authors of the report in Turkey attributed the rise in cases to stress.
For the review, the researchers analyzed studies from Italy (four), Turkey (two), Brazil (one), the United States (one), and Poland (one).
Six of the studies reported cases of new-onset AA following COVID-19 infection (seven cases; average age, 37 years; females, three). Another study was a retrospective review of 32 patients with preexisting AA who developed COVID-19; none experienced significant worsening of AA within 6 months.
The review also included a study based on a survey of 389 patients with AA. The investigators found that, at a median 2.14 months after infection, 44% of those who had COVID-19 vs. 12% of those who were COVID negative had a relapse. Finally, a case report noted a patient with preexisting AA whose condition worsened following COVID infection.
The findings suggest that AA “could be a dermatological manifestation of COVID-19, with cases most often appearing 1-2 months following infection,” the authors wrote. “However, the heterogeneity of study designs and high proportion of case reports make it challenging to draw any conclusion.”
In an interview, dermatologist Brett King, MD, PhD, of the department of dermatology, Yale University, New Haven, Conn., said the review findings suggest that “there is little concern of alopecia areata following COVID infection.
Does new-onset AA happen, and are there exacerbations of preexisting disease related to COVID infection? Probably yes, but rarely.”
However, he noted that another form of alopecia, telogen effluvium (TE), is more common after COVID-19 infection. According to Dr. King, who was not involved with the systematic review, TE is typically time-limited, compared with AA’s more common chronic waxing-and-waning course.
“Distinguishing TE and AA is usually straightforward because AA typically presents with well-circumscribed patches of hair loss,” such as circular patches, “while TE manifests as diffuse hair loss,” he explained. “Rarely, however, AA does manifest diffuse hair loss without patches, similar to TE. In those cases, it may be difficult to distinguish them. A biopsy may be helpful if there is a question of the diagnosis.”
No study funding is reported. The review authors and Dr. King report no relevant disclosures.
FROM JAAD INTERNATIONAL
‘Alarming’ worldwide decline in mental health
The Mental Health Million project of Sapien Labs issued its second report, published online March 15, encompassing 34 countries and over 220,000 Internet-enabled adults. It found a continued decline in mental health in all age groups and genders, with English-speaking countries having the lowest mental well-being.
The decline was significantly correlated with the stringency of COVID-19 lockdown measures in each country and was directionally correlated to the cases and deaths per million.
The youngest age group (18-24 years) reported the poorest mental well-being, with better mental health scores rising in every successively older age group.
“Some of our findings, especially regarding mental health in young adults, are alarming,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, told this news organization.
“Our data, which are continually updated in real time, are freely available for nonprofit, noncommercial use and research, and we hope that researchers will get involved in an interdisciplinary way that spans sociology, economics, psychiatry, and other fields,” she said.
Pioneering research
Dr. Thiagarajan and her team pioneered the Mental Health Million project, an ongoing research initiative utilizing a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability.”
The MHQ consists of 47 “elements of mental well-being,” with scores ranging from –100 to +200. (Negative scores indicate poorer mental well-being.) The MHQ categorizes respondents as “clinical, at-risk, enduring, managing, succeeding, and thriving” and computes scores on the basis of six broad dimensions of mental health: core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
As reported by this news organization, Sapien Lab’s first Mental Health State of the World report (n = 49,000 adults) was conducted in eight English-speaking countries in 2020. Participants were compared to a smaller sample of people from the same countries polled in 2019.
In this year’s report, “we expanded quite substantially,” Dr. Thiagarajan said. The project added Spanish, French, and Arabic and recruited participants from 34 countries on six continents (n = 223,087) via advertising on Google and Facebook.
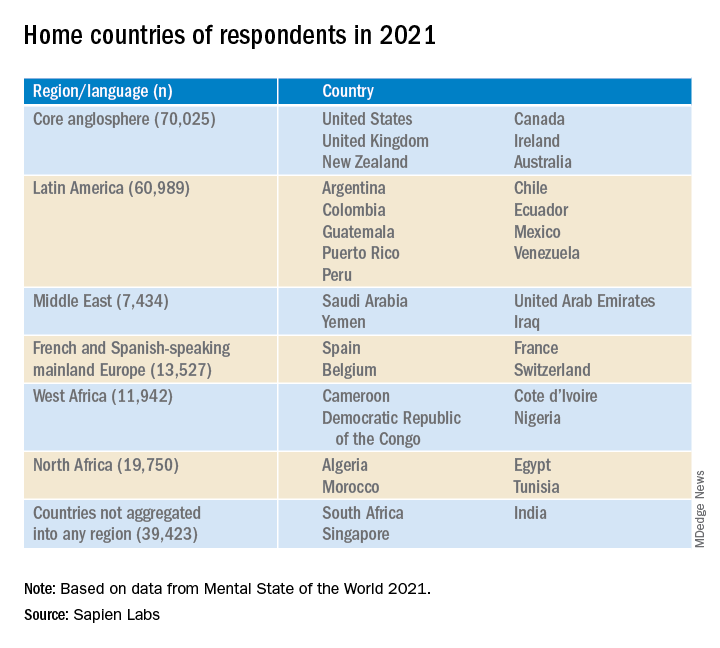
Economic prosperity not protective
Across the eight English-speaking countries, there was a decline in mental well-being of 3% from 2020 to 2021, which was smaller than the 8% decline from 2019 to 2020. The percentage of people who were “distressed or struggling” increased from 26% to 30% in 2021.
“Now that a lot of pandemic issue seems to be easing up, I hope we’ll see mental well-being coming back up, but at least it’s a smaller decline than we saw between 2019 and 2020,” said Dr. Thiagarajan.
The decline across countries from 2019 to 2021 was significantly correlated with the stringency of governmental COVID-19-related measures (based on the Oxford COVID-19 Government Response Tracker, 2022; r = .54) and directionally correlated to the cases and deaths per million.
In total, 30% of respondents in English-speaking countries had mental well-being scores in the “distressed” or “struggling” range – higher than the Middle Eastern countries, North Africa, Latin America, and Europe (23%, 23%, 24%, and 18%, respectively).
Only 36% of participants in the English-speaking countries, the Middle East, and North Africa reported “thriving or succeeding,” vs. 45% and 46% in Latin America and Europe, respectively. Venezuela topped the list with an average MHQ of 91, while the United Kingdom and South Africa had the lowest scores, at 46 each.
Mental well-being was slightly higher in males than in females but was dramatically lower in nonbinary/third-gender respondents. In fact, those identifying as nonbinary/third gender had the lowest mental well-being of any group.
Across all countries and languages, higher education was associated with better mental well-being. Employment was also associated with superior mental well-being, compared with being unemployed – particularly in core English-speaking countries.
However, “country indicators of economic prosperity were negatively correlated with mental well-being, particularly for young adults and males, belying the commonly held belief that national economic prosperity translates into greater mental well-being,” said Dr. Thiagarajan.
‘Stark’ contrast
The most dramatic finding was the difference in mental well-being between younger and older adults, which was two- to threefold larger than differences in other dimensions (for example, age, gender, employment). Even the maximum difference between countries overall (15%) was still smaller than the generational gap within any region.
While only 7% (6%- 9%) of participants aged ≥65 years were “distressed and struggling” with their mental well-being to a “clinical” extent, 44% (38%-50%) of those aged 18-24 years reported mental well-being scores in the “distressed or struggling” range – representing a “growing gap between generations that, while present prior to the COVID-19 pandemic, has since been exacerbated,” the authors state.
With every successive decrement in age group, mental well-being “plummeted,” Dr. Thiagarajan said. She noted that research conducted prior to 2010 in several regions of the world showed that young adults typically had the highest well-being. “Our findings stand in stark contrast to these previous patterns.”
The relationship between lockdown stringency and poorer mental health could play a role. “The impact of social isolation may be most strongly felt in younger people,” she said.
Internet a culprit?
“Within almost every region, scores for cognition and drive and motivation were highest while mood and outlook and social self were the lowest,” the authors report.
The aggregate percentage of respondents who reported being “distressed or struggling” in the various MHQ dimensions is shown in the following table.
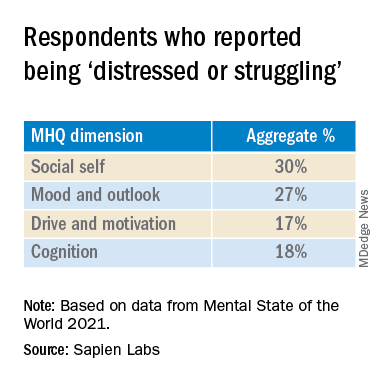
In particular, English-speaking countries scored lowest on the social self scale.
The sense of social self is “how you see yourself with respect to others, how you relate to others and the ability to form strong, stable relationships and maintain them with other people,” said Dr. Thiagarajan.
Internet use might account for the “massive” difference between the youngest and the oldest generations, she suggested. “Following 2010, mobile phone penetration picked up and rose rapidly. ... Mobile phones took over the world.”
Time spent on the Internet – an estimated 7-10 hours per day – “eats into the time people in older generations used in building the social self. Kids who grow up on the Internet are losing thousands of hours in social interactions, which is challenging their ability to form relationships, how they see themselves, and how they fit into the social fabric,” Dr. Thiagarajan added
Sedentary time
Commenting for this news organization, Bernardo Ng, MD, a member of the American Psychiatric Association’s Council on International Psychiatry and Global Health and medical director of Sun Valley Research Center, Imperial, Calif., called the report “interesting, with an impressive sample size” and an “impressive geographic distribution.”
Dr. Ng, who was not involved in the report, said, “I did not think the impact of Internet use on mental health was as dramatic before looking at this report.
“On the other hand, I have personally been interested in the impact of sedentarism in mental health – not only emotionally but also biologically. Sedentarism, which is directly related to screen use time, produces inflammation that worsens brain function.”
Also commenting, Ken Duckworth, MD, chief medical officer of the National Alliance of Mental Illness, called the survey “extremely well timed and creative, although it looked only at Internet-enabled populations, so one cannot make too many overall pronouncements, because a lot of people don’t have access to the Internet.”
The data regarding young people are particularly powerful. “The idea that young people are having a decrease in their experience of mental health across the world is something I haven’t seen before.”
Dr. Duckworth suggested the reason might “have to do with the impact of the COVID lockdown on normal development that young people go through, while older people don’t struggle with these developmental challenges in the same way.”
A version of this article first appeared on Medscape.com.
The Mental Health Million project of Sapien Labs issued its second report, published online March 15, encompassing 34 countries and over 220,000 Internet-enabled adults. It found a continued decline in mental health in all age groups and genders, with English-speaking countries having the lowest mental well-being.
The decline was significantly correlated with the stringency of COVID-19 lockdown measures in each country and was directionally correlated to the cases and deaths per million.
The youngest age group (18-24 years) reported the poorest mental well-being, with better mental health scores rising in every successively older age group.
“Some of our findings, especially regarding mental health in young adults, are alarming,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, told this news organization.
“Our data, which are continually updated in real time, are freely available for nonprofit, noncommercial use and research, and we hope that researchers will get involved in an interdisciplinary way that spans sociology, economics, psychiatry, and other fields,” she said.
Pioneering research
Dr. Thiagarajan and her team pioneered the Mental Health Million project, an ongoing research initiative utilizing a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability.”
The MHQ consists of 47 “elements of mental well-being,” with scores ranging from –100 to +200. (Negative scores indicate poorer mental well-being.) The MHQ categorizes respondents as “clinical, at-risk, enduring, managing, succeeding, and thriving” and computes scores on the basis of six broad dimensions of mental health: core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
As reported by this news organization, Sapien Lab’s first Mental Health State of the World report (n = 49,000 adults) was conducted in eight English-speaking countries in 2020. Participants were compared to a smaller sample of people from the same countries polled in 2019.
In this year’s report, “we expanded quite substantially,” Dr. Thiagarajan said. The project added Spanish, French, and Arabic and recruited participants from 34 countries on six continents (n = 223,087) via advertising on Google and Facebook.

Economic prosperity not protective
Across the eight English-speaking countries, there was a decline in mental well-being of 3% from 2020 to 2021, which was smaller than the 8% decline from 2019 to 2020. The percentage of people who were “distressed or struggling” increased from 26% to 30% in 2021.
“Now that a lot of pandemic issue seems to be easing up, I hope we’ll see mental well-being coming back up, but at least it’s a smaller decline than we saw between 2019 and 2020,” said Dr. Thiagarajan.
The decline across countries from 2019 to 2021 was significantly correlated with the stringency of governmental COVID-19-related measures (based on the Oxford COVID-19 Government Response Tracker, 2022; r = .54) and directionally correlated to the cases and deaths per million.
In total, 30% of respondents in English-speaking countries had mental well-being scores in the “distressed” or “struggling” range – higher than the Middle Eastern countries, North Africa, Latin America, and Europe (23%, 23%, 24%, and 18%, respectively).
Only 36% of participants in the English-speaking countries, the Middle East, and North Africa reported “thriving or succeeding,” vs. 45% and 46% in Latin America and Europe, respectively. Venezuela topped the list with an average MHQ of 91, while the United Kingdom and South Africa had the lowest scores, at 46 each.
Mental well-being was slightly higher in males than in females but was dramatically lower in nonbinary/third-gender respondents. In fact, those identifying as nonbinary/third gender had the lowest mental well-being of any group.
Across all countries and languages, higher education was associated with better mental well-being. Employment was also associated with superior mental well-being, compared with being unemployed – particularly in core English-speaking countries.
However, “country indicators of economic prosperity were negatively correlated with mental well-being, particularly for young adults and males, belying the commonly held belief that national economic prosperity translates into greater mental well-being,” said Dr. Thiagarajan.
‘Stark’ contrast
The most dramatic finding was the difference in mental well-being between younger and older adults, which was two- to threefold larger than differences in other dimensions (for example, age, gender, employment). Even the maximum difference between countries overall (15%) was still smaller than the generational gap within any region.
While only 7% (6%- 9%) of participants aged ≥65 years were “distressed and struggling” with their mental well-being to a “clinical” extent, 44% (38%-50%) of those aged 18-24 years reported mental well-being scores in the “distressed or struggling” range – representing a “growing gap between generations that, while present prior to the COVID-19 pandemic, has since been exacerbated,” the authors state.
With every successive decrement in age group, mental well-being “plummeted,” Dr. Thiagarajan said. She noted that research conducted prior to 2010 in several regions of the world showed that young adults typically had the highest well-being. “Our findings stand in stark contrast to these previous patterns.”
The relationship between lockdown stringency and poorer mental health could play a role. “The impact of social isolation may be most strongly felt in younger people,” she said.
Internet a culprit?
“Within almost every region, scores for cognition and drive and motivation were highest while mood and outlook and social self were the lowest,” the authors report.
The aggregate percentage of respondents who reported being “distressed or struggling” in the various MHQ dimensions is shown in the following table.

In particular, English-speaking countries scored lowest on the social self scale.
The sense of social self is “how you see yourself with respect to others, how you relate to others and the ability to form strong, stable relationships and maintain them with other people,” said Dr. Thiagarajan.
Internet use might account for the “massive” difference between the youngest and the oldest generations, she suggested. “Following 2010, mobile phone penetration picked up and rose rapidly. ... Mobile phones took over the world.”
Time spent on the Internet – an estimated 7-10 hours per day – “eats into the time people in older generations used in building the social self. Kids who grow up on the Internet are losing thousands of hours in social interactions, which is challenging their ability to form relationships, how they see themselves, and how they fit into the social fabric,” Dr. Thiagarajan added
Sedentary time
Commenting for this news organization, Bernardo Ng, MD, a member of the American Psychiatric Association’s Council on International Psychiatry and Global Health and medical director of Sun Valley Research Center, Imperial, Calif., called the report “interesting, with an impressive sample size” and an “impressive geographic distribution.”
Dr. Ng, who was not involved in the report, said, “I did not think the impact of Internet use on mental health was as dramatic before looking at this report.
“On the other hand, I have personally been interested in the impact of sedentarism in mental health – not only emotionally but also biologically. Sedentarism, which is directly related to screen use time, produces inflammation that worsens brain function.”
Also commenting, Ken Duckworth, MD, chief medical officer of the National Alliance of Mental Illness, called the survey “extremely well timed and creative, although it looked only at Internet-enabled populations, so one cannot make too many overall pronouncements, because a lot of people don’t have access to the Internet.”
The data regarding young people are particularly powerful. “The idea that young people are having a decrease in their experience of mental health across the world is something I haven’t seen before.”
Dr. Duckworth suggested the reason might “have to do with the impact of the COVID lockdown on normal development that young people go through, while older people don’t struggle with these developmental challenges in the same way.”
A version of this article first appeared on Medscape.com.
The Mental Health Million project of Sapien Labs issued its second report, published online March 15, encompassing 34 countries and over 220,000 Internet-enabled adults. It found a continued decline in mental health in all age groups and genders, with English-speaking countries having the lowest mental well-being.
The decline was significantly correlated with the stringency of COVID-19 lockdown measures in each country and was directionally correlated to the cases and deaths per million.
The youngest age group (18-24 years) reported the poorest mental well-being, with better mental health scores rising in every successively older age group.
“Some of our findings, especially regarding mental health in young adults, are alarming,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, told this news organization.
“Our data, which are continually updated in real time, are freely available for nonprofit, noncommercial use and research, and we hope that researchers will get involved in an interdisciplinary way that spans sociology, economics, psychiatry, and other fields,” she said.
Pioneering research
Dr. Thiagarajan and her team pioneered the Mental Health Million project, an ongoing research initiative utilizing a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability.”
The MHQ consists of 47 “elements of mental well-being,” with scores ranging from –100 to +200. (Negative scores indicate poorer mental well-being.) The MHQ categorizes respondents as “clinical, at-risk, enduring, managing, succeeding, and thriving” and computes scores on the basis of six broad dimensions of mental health: core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
As reported by this news organization, Sapien Lab’s first Mental Health State of the World report (n = 49,000 adults) was conducted in eight English-speaking countries in 2020. Participants were compared to a smaller sample of people from the same countries polled in 2019.
In this year’s report, “we expanded quite substantially,” Dr. Thiagarajan said. The project added Spanish, French, and Arabic and recruited participants from 34 countries on six continents (n = 223,087) via advertising on Google and Facebook.

Economic prosperity not protective
Across the eight English-speaking countries, there was a decline in mental well-being of 3% from 2020 to 2021, which was smaller than the 8% decline from 2019 to 2020. The percentage of people who were “distressed or struggling” increased from 26% to 30% in 2021.
“Now that a lot of pandemic issue seems to be easing up, I hope we’ll see mental well-being coming back up, but at least it’s a smaller decline than we saw between 2019 and 2020,” said Dr. Thiagarajan.
The decline across countries from 2019 to 2021 was significantly correlated with the stringency of governmental COVID-19-related measures (based on the Oxford COVID-19 Government Response Tracker, 2022; r = .54) and directionally correlated to the cases and deaths per million.
In total, 30% of respondents in English-speaking countries had mental well-being scores in the “distressed” or “struggling” range – higher than the Middle Eastern countries, North Africa, Latin America, and Europe (23%, 23%, 24%, and 18%, respectively).
Only 36% of participants in the English-speaking countries, the Middle East, and North Africa reported “thriving or succeeding,” vs. 45% and 46% in Latin America and Europe, respectively. Venezuela topped the list with an average MHQ of 91, while the United Kingdom and South Africa had the lowest scores, at 46 each.
Mental well-being was slightly higher in males than in females but was dramatically lower in nonbinary/third-gender respondents. In fact, those identifying as nonbinary/third gender had the lowest mental well-being of any group.
Across all countries and languages, higher education was associated with better mental well-being. Employment was also associated with superior mental well-being, compared with being unemployed – particularly in core English-speaking countries.
However, “country indicators of economic prosperity were negatively correlated with mental well-being, particularly for young adults and males, belying the commonly held belief that national economic prosperity translates into greater mental well-being,” said Dr. Thiagarajan.
‘Stark’ contrast
The most dramatic finding was the difference in mental well-being between younger and older adults, which was two- to threefold larger than differences in other dimensions (for example, age, gender, employment). Even the maximum difference between countries overall (15%) was still smaller than the generational gap within any region.
While only 7% (6%- 9%) of participants aged ≥65 years were “distressed and struggling” with their mental well-being to a “clinical” extent, 44% (38%-50%) of those aged 18-24 years reported mental well-being scores in the “distressed or struggling” range – representing a “growing gap between generations that, while present prior to the COVID-19 pandemic, has since been exacerbated,” the authors state.
With every successive decrement in age group, mental well-being “plummeted,” Dr. Thiagarajan said. She noted that research conducted prior to 2010 in several regions of the world showed that young adults typically had the highest well-being. “Our findings stand in stark contrast to these previous patterns.”
The relationship between lockdown stringency and poorer mental health could play a role. “The impact of social isolation may be most strongly felt in younger people,” she said.
Internet a culprit?
“Within almost every region, scores for cognition and drive and motivation were highest while mood and outlook and social self were the lowest,” the authors report.
The aggregate percentage of respondents who reported being “distressed or struggling” in the various MHQ dimensions is shown in the following table.

In particular, English-speaking countries scored lowest on the social self scale.
The sense of social self is “how you see yourself with respect to others, how you relate to others and the ability to form strong, stable relationships and maintain them with other people,” said Dr. Thiagarajan.
Internet use might account for the “massive” difference between the youngest and the oldest generations, she suggested. “Following 2010, mobile phone penetration picked up and rose rapidly. ... Mobile phones took over the world.”
Time spent on the Internet – an estimated 7-10 hours per day – “eats into the time people in older generations used in building the social self. Kids who grow up on the Internet are losing thousands of hours in social interactions, which is challenging their ability to form relationships, how they see themselves, and how they fit into the social fabric,” Dr. Thiagarajan added
Sedentary time
Commenting for this news organization, Bernardo Ng, MD, a member of the American Psychiatric Association’s Council on International Psychiatry and Global Health and medical director of Sun Valley Research Center, Imperial, Calif., called the report “interesting, with an impressive sample size” and an “impressive geographic distribution.”
Dr. Ng, who was not involved in the report, said, “I did not think the impact of Internet use on mental health was as dramatic before looking at this report.
“On the other hand, I have personally been interested in the impact of sedentarism in mental health – not only emotionally but also biologically. Sedentarism, which is directly related to screen use time, produces inflammation that worsens brain function.”
Also commenting, Ken Duckworth, MD, chief medical officer of the National Alliance of Mental Illness, called the survey “extremely well timed and creative, although it looked only at Internet-enabled populations, so one cannot make too many overall pronouncements, because a lot of people don’t have access to the Internet.”
The data regarding young people are particularly powerful. “The idea that young people are having a decrease in their experience of mental health across the world is something I haven’t seen before.”
Dr. Duckworth suggested the reason might “have to do with the impact of the COVID lockdown on normal development that young people go through, while older people don’t struggle with these developmental challenges in the same way.”
A version of this article first appeared on Medscape.com.
COVID surge in Western Europe puts U.S. health experts on alert
, even as states and cities continue to lift restrictions amid low case numbers.
Infectious disease experts are watching BA.2, the Omicron subvariant that appears to be more transmissible than the original strain. BA.2 is fueling outbreaks across Europe and is growing in dominance across the United States.
“It’s picking up steam. It’s across at least 12 countries … from Finland to Greece,” Eric Topol, MD, director of the Scripps Research Translational Institute, told The Washington Post.
He has been following the surge and has posted recent charts of the outbreak on Twitter. Hospitalizations appear to be increasing in some places as well, he noted, despite the higher vaccination rates of many Western European countries.
“There’s no question there’s a significant wave there,” Dr. Topol said.
Germany recorded more than 260,000 new cases on March 15, according to the data tracker from the New York Times, but coronavirus restrictions are still being lifted this week. The U.K. is reporting more than 75,000 daily cases, and the Netherlands is reporting more than 60,000 daily cases, which are considered major numbers, compared to their population sizes. Meanwhile, France, Italy, and Switzerland are also reporting large increases in infections.
During the past 2 years, widespread outbreaks in Europe have been followed by similar surges in the U.S. weeks later. Most experts interviewed by the Post predicted that it’s likely to happen again.
In the United States, the BA.2 subvariant accounted for 23% of new COVID-19 cases for the week ending March 12, according to the latest estimate from the Centers for Disease Control and Prevention, while the original Omicron strain made up about 66% of cases. The BA.2 percentage is up from 13.7% of new cases for the week ending March 5, 7.1% the previous week, and 4.1% the week before that. In parts of the Northeast and New England, BA.2 makes up more than 38% of new cases.
At the same time, the 7 -day average of COVID-19 cases continues to drop in the United States, with about 31,000 daily cases currently, the New York Times data tracker shows. About 25,000 COVID-19 patients are hospitalized across the country, which has fallen 44% in the past 2 weeks, and about 1,200 deaths are being reported daily.
Several variables could affect the course of a future surge, the Post reported. Vaccination rates, coronavirus safety protocols, and access to antiviral medications could dictate how another wave unfolds across the country.
About 82% of the eligible U.S. population has received at least one vaccine dose, and 69% is fully vaccinated, according to the latest CDC data. About half of those who are eligible for booster doses have received one. In Germany, nearly 76% of people are fully vaccinated, the newspaper reported, and in the United Kingdom, about 74% are fully vaccinated.
Health experts are also considering how natural immunity from a previous infection could affect a BA.2 surge. Millions of Americans were infected with the original Omicron strain, BA.1, which could provide protection. That said, researchers aren’t quite sure whether BA.1 infection protects against BA.2.
“It’s like a weather alert. Right now, the skies are sunny and bright, and we hope they stay that way,” Michael Osterholm, PhD, director of the University of Minnesota’s Center for Infectious Disease Research and Policy, told CNN.
“But we could have some bad weather by evening,” he said. “We just don’t know.”
A version of this article first appeared on WebMD.com.
, even as states and cities continue to lift restrictions amid low case numbers.
Infectious disease experts are watching BA.2, the Omicron subvariant that appears to be more transmissible than the original strain. BA.2 is fueling outbreaks across Europe and is growing in dominance across the United States.
“It’s picking up steam. It’s across at least 12 countries … from Finland to Greece,” Eric Topol, MD, director of the Scripps Research Translational Institute, told The Washington Post.
He has been following the surge and has posted recent charts of the outbreak on Twitter. Hospitalizations appear to be increasing in some places as well, he noted, despite the higher vaccination rates of many Western European countries.
“There’s no question there’s a significant wave there,” Dr. Topol said.
Germany recorded more than 260,000 new cases on March 15, according to the data tracker from the New York Times, but coronavirus restrictions are still being lifted this week. The U.K. is reporting more than 75,000 daily cases, and the Netherlands is reporting more than 60,000 daily cases, which are considered major numbers, compared to their population sizes. Meanwhile, France, Italy, and Switzerland are also reporting large increases in infections.
During the past 2 years, widespread outbreaks in Europe have been followed by similar surges in the U.S. weeks later. Most experts interviewed by the Post predicted that it’s likely to happen again.
In the United States, the BA.2 subvariant accounted for 23% of new COVID-19 cases for the week ending March 12, according to the latest estimate from the Centers for Disease Control and Prevention, while the original Omicron strain made up about 66% of cases. The BA.2 percentage is up from 13.7% of new cases for the week ending March 5, 7.1% the previous week, and 4.1% the week before that. In parts of the Northeast and New England, BA.2 makes up more than 38% of new cases.
At the same time, the 7 -day average of COVID-19 cases continues to drop in the United States, with about 31,000 daily cases currently, the New York Times data tracker shows. About 25,000 COVID-19 patients are hospitalized across the country, which has fallen 44% in the past 2 weeks, and about 1,200 deaths are being reported daily.
Several variables could affect the course of a future surge, the Post reported. Vaccination rates, coronavirus safety protocols, and access to antiviral medications could dictate how another wave unfolds across the country.
About 82% of the eligible U.S. population has received at least one vaccine dose, and 69% is fully vaccinated, according to the latest CDC data. About half of those who are eligible for booster doses have received one. In Germany, nearly 76% of people are fully vaccinated, the newspaper reported, and in the United Kingdom, about 74% are fully vaccinated.
Health experts are also considering how natural immunity from a previous infection could affect a BA.2 surge. Millions of Americans were infected with the original Omicron strain, BA.1, which could provide protection. That said, researchers aren’t quite sure whether BA.1 infection protects against BA.2.
“It’s like a weather alert. Right now, the skies are sunny and bright, and we hope they stay that way,” Michael Osterholm, PhD, director of the University of Minnesota’s Center for Infectious Disease Research and Policy, told CNN.
“But we could have some bad weather by evening,” he said. “We just don’t know.”
A version of this article first appeared on WebMD.com.
, even as states and cities continue to lift restrictions amid low case numbers.
Infectious disease experts are watching BA.2, the Omicron subvariant that appears to be more transmissible than the original strain. BA.2 is fueling outbreaks across Europe and is growing in dominance across the United States.
“It’s picking up steam. It’s across at least 12 countries … from Finland to Greece,” Eric Topol, MD, director of the Scripps Research Translational Institute, told The Washington Post.
He has been following the surge and has posted recent charts of the outbreak on Twitter. Hospitalizations appear to be increasing in some places as well, he noted, despite the higher vaccination rates of many Western European countries.
“There’s no question there’s a significant wave there,” Dr. Topol said.
Germany recorded more than 260,000 new cases on March 15, according to the data tracker from the New York Times, but coronavirus restrictions are still being lifted this week. The U.K. is reporting more than 75,000 daily cases, and the Netherlands is reporting more than 60,000 daily cases, which are considered major numbers, compared to their population sizes. Meanwhile, France, Italy, and Switzerland are also reporting large increases in infections.
During the past 2 years, widespread outbreaks in Europe have been followed by similar surges in the U.S. weeks later. Most experts interviewed by the Post predicted that it’s likely to happen again.
In the United States, the BA.2 subvariant accounted for 23% of new COVID-19 cases for the week ending March 12, according to the latest estimate from the Centers for Disease Control and Prevention, while the original Omicron strain made up about 66% of cases. The BA.2 percentage is up from 13.7% of new cases for the week ending March 5, 7.1% the previous week, and 4.1% the week before that. In parts of the Northeast and New England, BA.2 makes up more than 38% of new cases.
At the same time, the 7 -day average of COVID-19 cases continues to drop in the United States, with about 31,000 daily cases currently, the New York Times data tracker shows. About 25,000 COVID-19 patients are hospitalized across the country, which has fallen 44% in the past 2 weeks, and about 1,200 deaths are being reported daily.
Several variables could affect the course of a future surge, the Post reported. Vaccination rates, coronavirus safety protocols, and access to antiviral medications could dictate how another wave unfolds across the country.
About 82% of the eligible U.S. population has received at least one vaccine dose, and 69% is fully vaccinated, according to the latest CDC data. About half of those who are eligible for booster doses have received one. In Germany, nearly 76% of people are fully vaccinated, the newspaper reported, and in the United Kingdom, about 74% are fully vaccinated.
Health experts are also considering how natural immunity from a previous infection could affect a BA.2 surge. Millions of Americans were infected with the original Omicron strain, BA.1, which could provide protection. That said, researchers aren’t quite sure whether BA.1 infection protects against BA.2.
“It’s like a weather alert. Right now, the skies are sunny and bright, and we hope they stay that way,” Michael Osterholm, PhD, director of the University of Minnesota’s Center for Infectious Disease Research and Policy, told CNN.
“But we could have some bad weather by evening,” he said. “We just don’t know.”
A version of this article first appeared on WebMD.com.
Waiting for the under-5 COVID-19 vaccine
In February, citing the need for more data, Pfizer and BioNTech announced that they were delaying the application for their COVID-19 vaccine for children under the age of 5. Earlier evidence suggests that two doses may not provide adequate protection in the 2- to 4-year old age group. With the larger number of infections and illness in the younger age group from the Omicron variant, Pfizer and BioNTech felt they needed more data on the effectiveness of a third dose.
This delay came as a disappointment to parents of children under 5 who have been eager to have them receive the vaccination. However, Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the Food and Drug Administration, told parents that this delay should be reassuring – that the companies were doing important due diligence before releasing a product that is both safe and effective. The American Academy of Pediatrics wisely released a similar statement of reassurance and support.
It is difficult to know how many parents will eventually immunize their young children once the vaccine is approved. Any survey done more than a few weeks ago must be viewed cautiously as “the COVID numbers” around the country continue to improve and parental attitudes are likely to change.
There will always remain subgroups of parents on either extreme of the bell-shaped curve. Some will reject the under-5 vaccine simply because it is a vaccine. Some parents are so anxious to vaccinate that they will want to be first in line even if waiting is the more prudent approach. In a recent opinion piece appearing in the New York Times, a statistician writes that he is so eager to have his young children immunized that he is encouraging the FDA to replace its traditional reliance on “statistical significance” with a less rigid and binary method such as one based on Bayesian theory (Aubrey Carlton, “I’m a parent and a statistician. There’s a smarter way to think about the under-5 vaccine.” The New York Times. 2022 Mar 1.). However, what this statistician misses in his haste to vaccinate his own children is that we are dealing with an entire population with varying levels of scientific sophistication and appetite for risk. While “statistical significance” may no longer be cutting edge to some statisticians, most of the rest of the country finds the term reassuring.
It will be interesting to see what happens if and when the vaccine is approved. Will the American Academy of Pediatrics come out with a strong recommendation? I hope they are careful and provide a sufficient number of caveats, otherwise we in the trenches will again be left to provide more nuanced advice to families who are both anxious and hesitant.
Despite the recent surge in cases among young children, apparently as a result of the Omicron variant, the disease continues to cause less and milder disease among young children than it does in adults. And the degree to which illness in the pediatric population contributes to the health of the general population appears to still be a matter of debate. This may be yet another instance of when the crafty COVID-19 has moved with a pace that will make an under–age-5 vaccine of relatively little value.
First, we must be careful to assure ourselves that any side effects the vaccine might generate are well within an even more restricted acceptable range. Second, we must be careful not to squander our persuasive currency by promoting a vaccine that in retrospect may turn out to be of relatively little value.
Although there is ample evidence that education often fails to convince the committed anti-vaxxers, pediatricians continue to be held in high regard by most parents, many of whom are understandably confused by the tsunami of health information of mixed quality generated by the pandemic. We must be cautious not to cast ourselves as a group whose knee-jerk reaction is to recommend every vaccine with equal vigor. All vaccines are not created equal. We must be patient and prepared to adjust the level of our enthusiasm. We must continue to tailor our advice based on the hard data. Otherwise, parents will stop asking for our advice because they will believe that they already know what we’re going to say.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In February, citing the need for more data, Pfizer and BioNTech announced that they were delaying the application for their COVID-19 vaccine for children under the age of 5. Earlier evidence suggests that two doses may not provide adequate protection in the 2- to 4-year old age group. With the larger number of infections and illness in the younger age group from the Omicron variant, Pfizer and BioNTech felt they needed more data on the effectiveness of a third dose.
This delay came as a disappointment to parents of children under 5 who have been eager to have them receive the vaccination. However, Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the Food and Drug Administration, told parents that this delay should be reassuring – that the companies were doing important due diligence before releasing a product that is both safe and effective. The American Academy of Pediatrics wisely released a similar statement of reassurance and support.
It is difficult to know how many parents will eventually immunize their young children once the vaccine is approved. Any survey done more than a few weeks ago must be viewed cautiously as “the COVID numbers” around the country continue to improve and parental attitudes are likely to change.
There will always remain subgroups of parents on either extreme of the bell-shaped curve. Some will reject the under-5 vaccine simply because it is a vaccine. Some parents are so anxious to vaccinate that they will want to be first in line even if waiting is the more prudent approach. In a recent opinion piece appearing in the New York Times, a statistician writes that he is so eager to have his young children immunized that he is encouraging the FDA to replace its traditional reliance on “statistical significance” with a less rigid and binary method such as one based on Bayesian theory (Aubrey Carlton, “I’m a parent and a statistician. There’s a smarter way to think about the under-5 vaccine.” The New York Times. 2022 Mar 1.). However, what this statistician misses in his haste to vaccinate his own children is that we are dealing with an entire population with varying levels of scientific sophistication and appetite for risk. While “statistical significance” may no longer be cutting edge to some statisticians, most of the rest of the country finds the term reassuring.
It will be interesting to see what happens if and when the vaccine is approved. Will the American Academy of Pediatrics come out with a strong recommendation? I hope they are careful and provide a sufficient number of caveats, otherwise we in the trenches will again be left to provide more nuanced advice to families who are both anxious and hesitant.
Despite the recent surge in cases among young children, apparently as a result of the Omicron variant, the disease continues to cause less and milder disease among young children than it does in adults. And the degree to which illness in the pediatric population contributes to the health of the general population appears to still be a matter of debate. This may be yet another instance of when the crafty COVID-19 has moved with a pace that will make an under–age-5 vaccine of relatively little value.
First, we must be careful to assure ourselves that any side effects the vaccine might generate are well within an even more restricted acceptable range. Second, we must be careful not to squander our persuasive currency by promoting a vaccine that in retrospect may turn out to be of relatively little value.
Although there is ample evidence that education often fails to convince the committed anti-vaxxers, pediatricians continue to be held in high regard by most parents, many of whom are understandably confused by the tsunami of health information of mixed quality generated by the pandemic. We must be cautious not to cast ourselves as a group whose knee-jerk reaction is to recommend every vaccine with equal vigor. All vaccines are not created equal. We must be patient and prepared to adjust the level of our enthusiasm. We must continue to tailor our advice based on the hard data. Otherwise, parents will stop asking for our advice because they will believe that they already know what we’re going to say.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In February, citing the need for more data, Pfizer and BioNTech announced that they were delaying the application for their COVID-19 vaccine for children under the age of 5. Earlier evidence suggests that two doses may not provide adequate protection in the 2- to 4-year old age group. With the larger number of infections and illness in the younger age group from the Omicron variant, Pfizer and BioNTech felt they needed more data on the effectiveness of a third dose.
This delay came as a disappointment to parents of children under 5 who have been eager to have them receive the vaccination. However, Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the Food and Drug Administration, told parents that this delay should be reassuring – that the companies were doing important due diligence before releasing a product that is both safe and effective. The American Academy of Pediatrics wisely released a similar statement of reassurance and support.
It is difficult to know how many parents will eventually immunize their young children once the vaccine is approved. Any survey done more than a few weeks ago must be viewed cautiously as “the COVID numbers” around the country continue to improve and parental attitudes are likely to change.
There will always remain subgroups of parents on either extreme of the bell-shaped curve. Some will reject the under-5 vaccine simply because it is a vaccine. Some parents are so anxious to vaccinate that they will want to be first in line even if waiting is the more prudent approach. In a recent opinion piece appearing in the New York Times, a statistician writes that he is so eager to have his young children immunized that he is encouraging the FDA to replace its traditional reliance on “statistical significance” with a less rigid and binary method such as one based on Bayesian theory (Aubrey Carlton, “I’m a parent and a statistician. There’s a smarter way to think about the under-5 vaccine.” The New York Times. 2022 Mar 1.). However, what this statistician misses in his haste to vaccinate his own children is that we are dealing with an entire population with varying levels of scientific sophistication and appetite for risk. While “statistical significance” may no longer be cutting edge to some statisticians, most of the rest of the country finds the term reassuring.
It will be interesting to see what happens if and when the vaccine is approved. Will the American Academy of Pediatrics come out with a strong recommendation? I hope they are careful and provide a sufficient number of caveats, otherwise we in the trenches will again be left to provide more nuanced advice to families who are both anxious and hesitant.
Despite the recent surge in cases among young children, apparently as a result of the Omicron variant, the disease continues to cause less and milder disease among young children than it does in adults. And the degree to which illness in the pediatric population contributes to the health of the general population appears to still be a matter of debate. This may be yet another instance of when the crafty COVID-19 has moved with a pace that will make an under–age-5 vaccine of relatively little value.
First, we must be careful to assure ourselves that any side effects the vaccine might generate are well within an even more restricted acceptable range. Second, we must be careful not to squander our persuasive currency by promoting a vaccine that in retrospect may turn out to be of relatively little value.
Although there is ample evidence that education often fails to convince the committed anti-vaxxers, pediatricians continue to be held in high regard by most parents, many of whom are understandably confused by the tsunami of health information of mixed quality generated by the pandemic. We must be cautious not to cast ourselves as a group whose knee-jerk reaction is to recommend every vaccine with equal vigor. All vaccines are not created equal. We must be patient and prepared to adjust the level of our enthusiasm. We must continue to tailor our advice based on the hard data. Otherwise, parents will stop asking for our advice because they will believe that they already know what we’re going to say.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].





