User login
New ACC guidance on cardiovascular consequences of COVID-19
The American College of Cardiology has issued an expert consensus clinical guidance document for the evaluation and management of adults with key cardiovascular consequences of COVID-19.
The document makes recommendations on how to evaluate and manage COVID-associated myocarditis and long COVID and gives advice on resumption of exercise following COVID-19 infection.
The clinical guidance was published online March 16 in the Journal of the American College of Cardiology.
“The best means to diagnose and treat myocarditis and long COVID following SARS-CoV-2 infection continues to evolve,” said Ty Gluckman, MD, MHA, cochair of the expert consensus decision pathway. “This document attempts to provide key recommendations for how to evaluate and manage adults with these conditions, including guidance for safe return to play for both competitive and noncompetitive athletes.”
The authors of the guidance note that COVID-19 can be associated with various abnormalities in cardiac testing and a wide range of cardiovascular complications. For some patients, cardiac symptoms such as chest pain, shortness of breath, fatigue, and palpitations persist, lasting months after the initial illness, and evidence of myocardial injury has also been observed in both symptomatic and asymptomatic individuals, as well as after receipt of the COVID-19 mRNA vaccine.
“For clinicians treating these individuals, a growing number of questions exist related to evaluation and management of these conditions, as well as safe resumption of physical activity,” they say. This report is intended to provide practical guidance on these issues.
Myocarditis
The report states that myocarditis has been recognized as a rare but serious complication of SARS-CoV-2 infection as well as COVID-19 mRNA vaccination.
It defines myocarditis as: 1.cardiac symptoms such as chest pain, dyspnea, palpitations, or syncope; 2. elevated cardiac troponin; and 3. abnormal electrocardiographic, echocardiographic, cardiac MRI, and/or histopathologic findings on biopsy.
The document makes the following recommendations in regard to COVID-related myocarditis:
When there is increased suspicion for cardiac involvement with COVID-19, initial testing should consist of an ECG, measurement of cardiac troponin, and an echocardiogram. Cardiology consultation is recommended for those with a rising cardiac troponin and/or echocardiographic abnormalities. Cardiac MRI is recommended in hemodynamically stable patients with suspected myocarditis.
Hospitalization is recommended for patients with definite myocarditis, ideally at an advanced heart failure center. Patients with fulminant myocarditis should be managed at centers with an expertise in advanced heart failure, mechanical circulatory support, and other advanced therapies.
Patients with myocarditis and COVID-19 pneumonia (with an ongoing need for supplemental oxygen) should be treated with corticosteroids. For patients with suspected pericardial involvement, treatment with NSAIDs, colchicine, and/or prednisone is reasonable. Intravenous corticosteroids may be considered in those with suspected or confirmed COVID-19 myocarditis with hemodynamic compromise or MIS-A (multisystem inflammatory syndrome in adults). Empiric use of corticosteroids may also be considered in those with biopsy evidence of severe myocardial infiltrates or fulminant myocarditis, balanced against infection risk.
As appropriate, guideline-directed medical therapy for heart failure should be initiated and continued after discharge.
The document notes that myocarditis following COVID-19 mRNA vaccination is rare, with highest rates seen in young males after the second vaccine dose. As of May 22, 2021, the U.S. Vaccine Adverse Event Reporting System noted rates of 40.6 cases per million after the second vaccine dose among male individuals aged 12-29 years and 2.4 cases per million among male individuals aged 30 and older. Corresponding rates in female individuals were 4.2 and 1 cases per million, respectively.
But the report says that COVID-19 vaccination is associated with “a very favorable benefit-to-risk ratio” for all age and sex groups evaluated thus far.
In general, vaccine-associated myocarditis should be diagnosed, categorized, and treated in a manner analogous to myocarditis following SARS-CoV-2 infection, the guidance advises.
Long COVID
The document refers to long COVID as postacute sequelae of SARS-CoV-2 infection (PASC), and reports that this condition is experienced by up to 10%-30% of infected individuals. It is defined by a constellation of new, returning, or persistent health problems experienced by individuals 4 or more weeks after COVID-19 infection.
Although individuals with this condition may experience wide-ranging symptoms, the symptoms that draw increased attention to the cardiovascular system include tachycardia, exercise intolerance, chest pain, and shortness of breath.
Nicole Bhave, MD, cochair of the expert consensus decision pathway, says: “There appears to be a ‘downward spiral’ for long-COVID patients. Fatigue and decreased exercise capacity lead to diminished activity and bed rest, in turn leading to worsening symptoms and decreased quality of life.” She adds that “the writing committee recommends a basic cardiopulmonary evaluation performed up front to determine if further specialty care and formalized medical therapy is needed for these patients.”
The authors propose two terms to better understand potential etiologies for those with cardiovascular symptoms:
PASC-CVD, or PASC-cardiovascular disease, refers to a broad group of cardiovascular conditions (including myocarditis) that manifest at least 4 weeks after COVID-19 infection.
PASC-CVS, or PASC-cardiovascular syndrome, includes a wide range of cardiovascular symptoms without objective evidence of cardiovascular disease following standard diagnostic testing.
The document makes the following recommendations for the management of PASC-CVD and PASC-CVS.
For patients with cardiovascular symptoms and suspected PASC, the authors suggest that a reasonable initial testing approach includes basic laboratory testing, including cardiac troponin, an ECG, an echocardiogram, an ambulatory rhythm monitor, chest imaging, and/or pulmonary function tests.
Cardiology consultation is recommended for patients with PASC who have abnormal cardiac test results, known cardiovascular disease with new or worsening symptoms, documented cardiac complications during SARS-CoV-2 infection, and/or persistent cardiopulmonary symptoms that are not otherwise explained.
Recumbent or semirecumbent exercise (for example, rowing, swimming, or cycling) is recommended initially for PASC-CVS patients with tachycardia, exercise/orthostatic intolerance, and/or deconditioning, with transition to upright exercise as orthostatic intolerance improves. Exercise duration should also be short (5-10 minutes/day) initially, with gradual increases as functional capacity improves.
Salt and fluid loading represent nonpharmacologic interventions that may provide symptomatic relief for patients with tachycardia, palpitations, and/or orthostatic hypotension.
Beta-blockers, nondihydropyridine calcium-channel blockers, ivabradine, fludrocortisone, and midodrine may be used empirically as well.
Return to play for athletes
The authors note that concerns about possible cardiac injury after COVID-19 fueled early apprehension regarding the safety of competitive sports for athletes recovering from the infection.
But they say that subsequent data from large registries have demonstrated an overall low prevalence of clinical myocarditis, without a rise in the rate of adverse cardiac events. Based on this, updated guidance is provided with a practical, evidence-based framework to guide resumption of athletics and intense exercise training.
They make the following recommendations:
- For athletes recovering from COVID-19 with ongoing cardiopulmonary symptoms (chest pain, shortness of breath, palpitations, lightheadedness) or those requiring hospitalization with increased suspicion for cardiac involvement, further evaluation with triad testing – an ECG, measurement of cardiac troponin, and an echocardiogram – should be performed.
- For those with abnormal test results, further evaluation with cardiac MRI should be considered. Individuals diagnosed with clinical myocarditis should abstain from exercise for 3-6 months.
- Cardiac testing is not recommended for asymptomatic individuals following COVID-19 infection. Individuals should abstain from training for 3 days to ensure that symptoms do not develop.
- For those with mild or moderate noncardiopulmonary symptoms (fever, lethargy, muscle aches), training may resume after symptom resolution.
- For those with remote infection (≥3 months) without ongoing cardiopulmonary symptoms, a gradual increase in exercise is recommended without the need for cardiac testing.
Based on the low prevalence of myocarditis observed in competitive athletes with COVID-19, the authors note that these recommendations can be reasonably applied to high-school athletes (aged 14 and older) along with adult recreational exercise enthusiasts.
Future study is needed, however, to better understand how long cardiac abnormalities persist following COVID-19 infection and the role of exercise training in long COVID.
The authors conclude that the current guidance is intended to help clinicians understand not only when testing may be warranted, but also when it is not.
“Given that it reflects the current state of knowledge through early 2022, it is anticipated that recommendations will change over time as our understanding evolves,” they say.
The 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19: Myocarditis, Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), and Return to Play will be discussed in a session at the American College of Cardiology’s annual scientific session meeting in Washington in April.
A version of this article first appeared on Medscape.com.
The American College of Cardiology has issued an expert consensus clinical guidance document for the evaluation and management of adults with key cardiovascular consequences of COVID-19.
The document makes recommendations on how to evaluate and manage COVID-associated myocarditis and long COVID and gives advice on resumption of exercise following COVID-19 infection.
The clinical guidance was published online March 16 in the Journal of the American College of Cardiology.
“The best means to diagnose and treat myocarditis and long COVID following SARS-CoV-2 infection continues to evolve,” said Ty Gluckman, MD, MHA, cochair of the expert consensus decision pathway. “This document attempts to provide key recommendations for how to evaluate and manage adults with these conditions, including guidance for safe return to play for both competitive and noncompetitive athletes.”
The authors of the guidance note that COVID-19 can be associated with various abnormalities in cardiac testing and a wide range of cardiovascular complications. For some patients, cardiac symptoms such as chest pain, shortness of breath, fatigue, and palpitations persist, lasting months after the initial illness, and evidence of myocardial injury has also been observed in both symptomatic and asymptomatic individuals, as well as after receipt of the COVID-19 mRNA vaccine.
“For clinicians treating these individuals, a growing number of questions exist related to evaluation and management of these conditions, as well as safe resumption of physical activity,” they say. This report is intended to provide practical guidance on these issues.
Myocarditis
The report states that myocarditis has been recognized as a rare but serious complication of SARS-CoV-2 infection as well as COVID-19 mRNA vaccination.
It defines myocarditis as: 1.cardiac symptoms such as chest pain, dyspnea, palpitations, or syncope; 2. elevated cardiac troponin; and 3. abnormal electrocardiographic, echocardiographic, cardiac MRI, and/or histopathologic findings on biopsy.
The document makes the following recommendations in regard to COVID-related myocarditis:
When there is increased suspicion for cardiac involvement with COVID-19, initial testing should consist of an ECG, measurement of cardiac troponin, and an echocardiogram. Cardiology consultation is recommended for those with a rising cardiac troponin and/or echocardiographic abnormalities. Cardiac MRI is recommended in hemodynamically stable patients with suspected myocarditis.
Hospitalization is recommended for patients with definite myocarditis, ideally at an advanced heart failure center. Patients with fulminant myocarditis should be managed at centers with an expertise in advanced heart failure, mechanical circulatory support, and other advanced therapies.
Patients with myocarditis and COVID-19 pneumonia (with an ongoing need for supplemental oxygen) should be treated with corticosteroids. For patients with suspected pericardial involvement, treatment with NSAIDs, colchicine, and/or prednisone is reasonable. Intravenous corticosteroids may be considered in those with suspected or confirmed COVID-19 myocarditis with hemodynamic compromise or MIS-A (multisystem inflammatory syndrome in adults). Empiric use of corticosteroids may also be considered in those with biopsy evidence of severe myocardial infiltrates or fulminant myocarditis, balanced against infection risk.
As appropriate, guideline-directed medical therapy for heart failure should be initiated and continued after discharge.
The document notes that myocarditis following COVID-19 mRNA vaccination is rare, with highest rates seen in young males after the second vaccine dose. As of May 22, 2021, the U.S. Vaccine Adverse Event Reporting System noted rates of 40.6 cases per million after the second vaccine dose among male individuals aged 12-29 years and 2.4 cases per million among male individuals aged 30 and older. Corresponding rates in female individuals were 4.2 and 1 cases per million, respectively.
But the report says that COVID-19 vaccination is associated with “a very favorable benefit-to-risk ratio” for all age and sex groups evaluated thus far.
In general, vaccine-associated myocarditis should be diagnosed, categorized, and treated in a manner analogous to myocarditis following SARS-CoV-2 infection, the guidance advises.
Long COVID
The document refers to long COVID as postacute sequelae of SARS-CoV-2 infection (PASC), and reports that this condition is experienced by up to 10%-30% of infected individuals. It is defined by a constellation of new, returning, or persistent health problems experienced by individuals 4 or more weeks after COVID-19 infection.
Although individuals with this condition may experience wide-ranging symptoms, the symptoms that draw increased attention to the cardiovascular system include tachycardia, exercise intolerance, chest pain, and shortness of breath.
Nicole Bhave, MD, cochair of the expert consensus decision pathway, says: “There appears to be a ‘downward spiral’ for long-COVID patients. Fatigue and decreased exercise capacity lead to diminished activity and bed rest, in turn leading to worsening symptoms and decreased quality of life.” She adds that “the writing committee recommends a basic cardiopulmonary evaluation performed up front to determine if further specialty care and formalized medical therapy is needed for these patients.”
The authors propose two terms to better understand potential etiologies for those with cardiovascular symptoms:
PASC-CVD, or PASC-cardiovascular disease, refers to a broad group of cardiovascular conditions (including myocarditis) that manifest at least 4 weeks after COVID-19 infection.
PASC-CVS, or PASC-cardiovascular syndrome, includes a wide range of cardiovascular symptoms without objective evidence of cardiovascular disease following standard diagnostic testing.
The document makes the following recommendations for the management of PASC-CVD and PASC-CVS.
For patients with cardiovascular symptoms and suspected PASC, the authors suggest that a reasonable initial testing approach includes basic laboratory testing, including cardiac troponin, an ECG, an echocardiogram, an ambulatory rhythm monitor, chest imaging, and/or pulmonary function tests.
Cardiology consultation is recommended for patients with PASC who have abnormal cardiac test results, known cardiovascular disease with new or worsening symptoms, documented cardiac complications during SARS-CoV-2 infection, and/or persistent cardiopulmonary symptoms that are not otherwise explained.
Recumbent or semirecumbent exercise (for example, rowing, swimming, or cycling) is recommended initially for PASC-CVS patients with tachycardia, exercise/orthostatic intolerance, and/or deconditioning, with transition to upright exercise as orthostatic intolerance improves. Exercise duration should also be short (5-10 minutes/day) initially, with gradual increases as functional capacity improves.
Salt and fluid loading represent nonpharmacologic interventions that may provide symptomatic relief for patients with tachycardia, palpitations, and/or orthostatic hypotension.
Beta-blockers, nondihydropyridine calcium-channel blockers, ivabradine, fludrocortisone, and midodrine may be used empirically as well.
Return to play for athletes
The authors note that concerns about possible cardiac injury after COVID-19 fueled early apprehension regarding the safety of competitive sports for athletes recovering from the infection.
But they say that subsequent data from large registries have demonstrated an overall low prevalence of clinical myocarditis, without a rise in the rate of adverse cardiac events. Based on this, updated guidance is provided with a practical, evidence-based framework to guide resumption of athletics and intense exercise training.
They make the following recommendations:
- For athletes recovering from COVID-19 with ongoing cardiopulmonary symptoms (chest pain, shortness of breath, palpitations, lightheadedness) or those requiring hospitalization with increased suspicion for cardiac involvement, further evaluation with triad testing – an ECG, measurement of cardiac troponin, and an echocardiogram – should be performed.
- For those with abnormal test results, further evaluation with cardiac MRI should be considered. Individuals diagnosed with clinical myocarditis should abstain from exercise for 3-6 months.
- Cardiac testing is not recommended for asymptomatic individuals following COVID-19 infection. Individuals should abstain from training for 3 days to ensure that symptoms do not develop.
- For those with mild or moderate noncardiopulmonary symptoms (fever, lethargy, muscle aches), training may resume after symptom resolution.
- For those with remote infection (≥3 months) without ongoing cardiopulmonary symptoms, a gradual increase in exercise is recommended without the need for cardiac testing.
Based on the low prevalence of myocarditis observed in competitive athletes with COVID-19, the authors note that these recommendations can be reasonably applied to high-school athletes (aged 14 and older) along with adult recreational exercise enthusiasts.
Future study is needed, however, to better understand how long cardiac abnormalities persist following COVID-19 infection and the role of exercise training in long COVID.
The authors conclude that the current guidance is intended to help clinicians understand not only when testing may be warranted, but also when it is not.
“Given that it reflects the current state of knowledge through early 2022, it is anticipated that recommendations will change over time as our understanding evolves,” they say.
The 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19: Myocarditis, Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), and Return to Play will be discussed in a session at the American College of Cardiology’s annual scientific session meeting in Washington in April.
A version of this article first appeared on Medscape.com.
The American College of Cardiology has issued an expert consensus clinical guidance document for the evaluation and management of adults with key cardiovascular consequences of COVID-19.
The document makes recommendations on how to evaluate and manage COVID-associated myocarditis and long COVID and gives advice on resumption of exercise following COVID-19 infection.
The clinical guidance was published online March 16 in the Journal of the American College of Cardiology.
“The best means to diagnose and treat myocarditis and long COVID following SARS-CoV-2 infection continues to evolve,” said Ty Gluckman, MD, MHA, cochair of the expert consensus decision pathway. “This document attempts to provide key recommendations for how to evaluate and manage adults with these conditions, including guidance for safe return to play for both competitive and noncompetitive athletes.”
The authors of the guidance note that COVID-19 can be associated with various abnormalities in cardiac testing and a wide range of cardiovascular complications. For some patients, cardiac symptoms such as chest pain, shortness of breath, fatigue, and palpitations persist, lasting months after the initial illness, and evidence of myocardial injury has also been observed in both symptomatic and asymptomatic individuals, as well as after receipt of the COVID-19 mRNA vaccine.
“For clinicians treating these individuals, a growing number of questions exist related to evaluation and management of these conditions, as well as safe resumption of physical activity,” they say. This report is intended to provide practical guidance on these issues.
Myocarditis
The report states that myocarditis has been recognized as a rare but serious complication of SARS-CoV-2 infection as well as COVID-19 mRNA vaccination.
It defines myocarditis as: 1.cardiac symptoms such as chest pain, dyspnea, palpitations, or syncope; 2. elevated cardiac troponin; and 3. abnormal electrocardiographic, echocardiographic, cardiac MRI, and/or histopathologic findings on biopsy.
The document makes the following recommendations in regard to COVID-related myocarditis:
When there is increased suspicion for cardiac involvement with COVID-19, initial testing should consist of an ECG, measurement of cardiac troponin, and an echocardiogram. Cardiology consultation is recommended for those with a rising cardiac troponin and/or echocardiographic abnormalities. Cardiac MRI is recommended in hemodynamically stable patients with suspected myocarditis.
Hospitalization is recommended for patients with definite myocarditis, ideally at an advanced heart failure center. Patients with fulminant myocarditis should be managed at centers with an expertise in advanced heart failure, mechanical circulatory support, and other advanced therapies.
Patients with myocarditis and COVID-19 pneumonia (with an ongoing need for supplemental oxygen) should be treated with corticosteroids. For patients with suspected pericardial involvement, treatment with NSAIDs, colchicine, and/or prednisone is reasonable. Intravenous corticosteroids may be considered in those with suspected or confirmed COVID-19 myocarditis with hemodynamic compromise or MIS-A (multisystem inflammatory syndrome in adults). Empiric use of corticosteroids may also be considered in those with biopsy evidence of severe myocardial infiltrates or fulminant myocarditis, balanced against infection risk.
As appropriate, guideline-directed medical therapy for heart failure should be initiated and continued after discharge.
The document notes that myocarditis following COVID-19 mRNA vaccination is rare, with highest rates seen in young males after the second vaccine dose. As of May 22, 2021, the U.S. Vaccine Adverse Event Reporting System noted rates of 40.6 cases per million after the second vaccine dose among male individuals aged 12-29 years and 2.4 cases per million among male individuals aged 30 and older. Corresponding rates in female individuals were 4.2 and 1 cases per million, respectively.
But the report says that COVID-19 vaccination is associated with “a very favorable benefit-to-risk ratio” for all age and sex groups evaluated thus far.
In general, vaccine-associated myocarditis should be diagnosed, categorized, and treated in a manner analogous to myocarditis following SARS-CoV-2 infection, the guidance advises.
Long COVID
The document refers to long COVID as postacute sequelae of SARS-CoV-2 infection (PASC), and reports that this condition is experienced by up to 10%-30% of infected individuals. It is defined by a constellation of new, returning, or persistent health problems experienced by individuals 4 or more weeks after COVID-19 infection.
Although individuals with this condition may experience wide-ranging symptoms, the symptoms that draw increased attention to the cardiovascular system include tachycardia, exercise intolerance, chest pain, and shortness of breath.
Nicole Bhave, MD, cochair of the expert consensus decision pathway, says: “There appears to be a ‘downward spiral’ for long-COVID patients. Fatigue and decreased exercise capacity lead to diminished activity and bed rest, in turn leading to worsening symptoms and decreased quality of life.” She adds that “the writing committee recommends a basic cardiopulmonary evaluation performed up front to determine if further specialty care and formalized medical therapy is needed for these patients.”
The authors propose two terms to better understand potential etiologies for those with cardiovascular symptoms:
PASC-CVD, or PASC-cardiovascular disease, refers to a broad group of cardiovascular conditions (including myocarditis) that manifest at least 4 weeks after COVID-19 infection.
PASC-CVS, or PASC-cardiovascular syndrome, includes a wide range of cardiovascular symptoms without objective evidence of cardiovascular disease following standard diagnostic testing.
The document makes the following recommendations for the management of PASC-CVD and PASC-CVS.
For patients with cardiovascular symptoms and suspected PASC, the authors suggest that a reasonable initial testing approach includes basic laboratory testing, including cardiac troponin, an ECG, an echocardiogram, an ambulatory rhythm monitor, chest imaging, and/or pulmonary function tests.
Cardiology consultation is recommended for patients with PASC who have abnormal cardiac test results, known cardiovascular disease with new or worsening symptoms, documented cardiac complications during SARS-CoV-2 infection, and/or persistent cardiopulmonary symptoms that are not otherwise explained.
Recumbent or semirecumbent exercise (for example, rowing, swimming, or cycling) is recommended initially for PASC-CVS patients with tachycardia, exercise/orthostatic intolerance, and/or deconditioning, with transition to upright exercise as orthostatic intolerance improves. Exercise duration should also be short (5-10 minutes/day) initially, with gradual increases as functional capacity improves.
Salt and fluid loading represent nonpharmacologic interventions that may provide symptomatic relief for patients with tachycardia, palpitations, and/or orthostatic hypotension.
Beta-blockers, nondihydropyridine calcium-channel blockers, ivabradine, fludrocortisone, and midodrine may be used empirically as well.
Return to play for athletes
The authors note that concerns about possible cardiac injury after COVID-19 fueled early apprehension regarding the safety of competitive sports for athletes recovering from the infection.
But they say that subsequent data from large registries have demonstrated an overall low prevalence of clinical myocarditis, without a rise in the rate of adverse cardiac events. Based on this, updated guidance is provided with a practical, evidence-based framework to guide resumption of athletics and intense exercise training.
They make the following recommendations:
- For athletes recovering from COVID-19 with ongoing cardiopulmonary symptoms (chest pain, shortness of breath, palpitations, lightheadedness) or those requiring hospitalization with increased suspicion for cardiac involvement, further evaluation with triad testing – an ECG, measurement of cardiac troponin, and an echocardiogram – should be performed.
- For those with abnormal test results, further evaluation with cardiac MRI should be considered. Individuals diagnosed with clinical myocarditis should abstain from exercise for 3-6 months.
- Cardiac testing is not recommended for asymptomatic individuals following COVID-19 infection. Individuals should abstain from training for 3 days to ensure that symptoms do not develop.
- For those with mild or moderate noncardiopulmonary symptoms (fever, lethargy, muscle aches), training may resume after symptom resolution.
- For those with remote infection (≥3 months) without ongoing cardiopulmonary symptoms, a gradual increase in exercise is recommended without the need for cardiac testing.
Based on the low prevalence of myocarditis observed in competitive athletes with COVID-19, the authors note that these recommendations can be reasonably applied to high-school athletes (aged 14 and older) along with adult recreational exercise enthusiasts.
Future study is needed, however, to better understand how long cardiac abnormalities persist following COVID-19 infection and the role of exercise training in long COVID.
The authors conclude that the current guidance is intended to help clinicians understand not only when testing may be warranted, but also when it is not.
“Given that it reflects the current state of knowledge through early 2022, it is anticipated that recommendations will change over time as our understanding evolves,” they say.
The 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19: Myocarditis, Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), and Return to Play will be discussed in a session at the American College of Cardiology’s annual scientific session meeting in Washington in April.
A version of this article first appeared on Medscape.com.
Children and COVID: Decline in new cases reaches 7th week
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.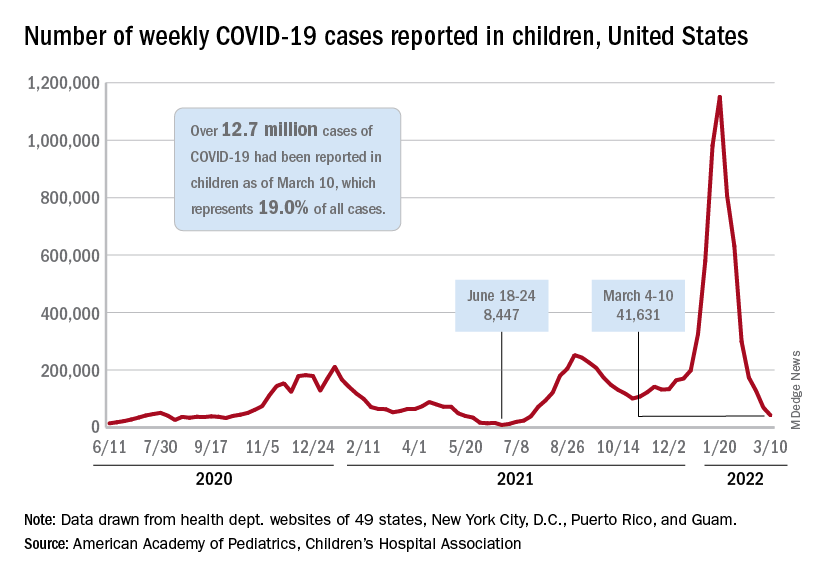
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
Air trapping common in patients with long COVID
, according to a prospective study that compared 100 COVID-19 survivors who had persistent symptoms and 106 healthy control persons.
“Something is going on in the distal airways related to either inflammation or fibrosis that is giving us a signal of air trapping,” noted senior author Alejandro P. Comellas, MD, in a press release. The study was stimulated by reports from University of Iowa clinicians noting that many patients with initial SARS-CoV-2 infection who were either hospitalized or were treated in the ambulatory setting later reported shortness of breath and other respiratory symptoms indicative of chronic lung disease.
Study results
Investigators classified patients (mean age, 48 years; 66 women) with post-acute sequelae of COVID-19 according to whether they were ambulatory (67%), hospitalized (17%), or required treatment in the intensive care unit (16%). They then compared CT findings of patients who had COVID-19 and persistent symptoms with those of a healthy control group.
COVID-19 severity did not affect the percentage of cases of lung with air trapping among these patients. Air trapping occurred at rates of 25.4% among ambulatory patients, 34.6% in hospitalized patients, and in 27.3% of those requiring intensive care (P = .10). The percentage of lungs affected by air trapping in ambulatory participants was sharply and significantly higher than in healthy controls (25.4% vs. 7.2%; P < .001). Also, air trapping persisted; it was still present in 8 of 9 participants who underwent imaging more than 200 days post diagnosis.
Qualitative analysis of chest CT images showed that the most common imaging abnormality was air trapping (58%); ground glass opacities (GGOs) were found in 51% (46/91), note Dr. Comellas and coauthors. This suggests ongoing lung inflammation, edema, or fibrosis. These symptoms are often observed during acute COVID-19, frequently in an organizing pneumonia pattern, and have been shown to persist for months after infection in survivors of severe disease. The mean percentage of total lung classified as having regional GGOs on chest CT scans was 13.2% and 28.7%, respectively, in the hospitalized and ICU groups, both very much higher than in the ambulatory group, at 3.7% (P < .001 for both). Among healthy controls, the GGO rate on chest CT was only 0.06% (P < .001).
In addition, air trapping correlated with the ratio of residual volume to total lung capacity (r = 0.6; P < .001) but not with spirometry results. In fact, the investigators did not observe airflow obstruction by spirometry in any group, suggesting that air trapping in these patients involves only small rather than large airways and that these small airways contribute little to total airway resistance. Only when a large percentage, perhaps 75% or more, of all small airways are obstructed will spirometry pick up small airways disease, the authors observe.
Continuing disease
The findings taken together suggest that functional small airways disease and air trapping are a consequence of SARS-CoV-2 infection, according to Dr. Comellas. “If a portion of patients continues to have small airways disease, then we need to think about the mechanisms behind it,” he said. “It could be something related to inflammation that’s reversible, or it may be something related to a scar that is irreversible, and then we need to look at ways to prevent further progression of the disease.” Furthermore, “studies aimed at determining the natural history of functional small airways disease in patients with post-acute sequelae of COVID-19 and the biological mechanisms that underlie these findings are urgently needed to identify therapeutic and preventative interventions,” Dr. Comellas, professor of internal medicine at Carver College of Medicine, University of Iowa, Iowa City, concluded.
The study limitations, the authors state, include the fact that theirs was a single-center study that enrolled participants infected early during the COVID-19 pandemic and did not include patients with Delta or Omicron variants, thus limiting the generalizability of the findings.
The study was published in Radiology.
The reported findings “indicate a long-term impact on bronchiolar obstruction,” states Brett M. Elicker, MD, professor of clinical radiology, University of California, San Francisco, in an accompanying editorial . Because collagen may be absorbed for months after an acute insult, it is not entirely clear whether the abnormalities seen in the current study will be permanent. He said further, “the presence of ground glass opacity and/or fibrosis on CT were most common in the patients admitted to the ICU and likely correspond to post-organizing pneumonia and/or post-diffuse alveolar damage fibrosis.”
Dr. Elicker also pointed out that organizing pneumonia is especially common among patients with COVID-19 and is usually highly steroid-responsive. The opacities improve or resolve with treatment, but sometimes residual fibrosis occurs. “Longer-term studies assessing the clinical and imaging manifestations 1-2 years after the initial infection are needed to fully ascertain the permanent manifestations of post-COVID fibrosis.”
The study was supported by grants from the National Institutes of Health. The authors and Dr. Elicker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a prospective study that compared 100 COVID-19 survivors who had persistent symptoms and 106 healthy control persons.
“Something is going on in the distal airways related to either inflammation or fibrosis that is giving us a signal of air trapping,” noted senior author Alejandro P. Comellas, MD, in a press release. The study was stimulated by reports from University of Iowa clinicians noting that many patients with initial SARS-CoV-2 infection who were either hospitalized or were treated in the ambulatory setting later reported shortness of breath and other respiratory symptoms indicative of chronic lung disease.
Study results
Investigators classified patients (mean age, 48 years; 66 women) with post-acute sequelae of COVID-19 according to whether they were ambulatory (67%), hospitalized (17%), or required treatment in the intensive care unit (16%). They then compared CT findings of patients who had COVID-19 and persistent symptoms with those of a healthy control group.
COVID-19 severity did not affect the percentage of cases of lung with air trapping among these patients. Air trapping occurred at rates of 25.4% among ambulatory patients, 34.6% in hospitalized patients, and in 27.3% of those requiring intensive care (P = .10). The percentage of lungs affected by air trapping in ambulatory participants was sharply and significantly higher than in healthy controls (25.4% vs. 7.2%; P < .001). Also, air trapping persisted; it was still present in 8 of 9 participants who underwent imaging more than 200 days post diagnosis.
Qualitative analysis of chest CT images showed that the most common imaging abnormality was air trapping (58%); ground glass opacities (GGOs) were found in 51% (46/91), note Dr. Comellas and coauthors. This suggests ongoing lung inflammation, edema, or fibrosis. These symptoms are often observed during acute COVID-19, frequently in an organizing pneumonia pattern, and have been shown to persist for months after infection in survivors of severe disease. The mean percentage of total lung classified as having regional GGOs on chest CT scans was 13.2% and 28.7%, respectively, in the hospitalized and ICU groups, both very much higher than in the ambulatory group, at 3.7% (P < .001 for both). Among healthy controls, the GGO rate on chest CT was only 0.06% (P < .001).
In addition, air trapping correlated with the ratio of residual volume to total lung capacity (r = 0.6; P < .001) but not with spirometry results. In fact, the investigators did not observe airflow obstruction by spirometry in any group, suggesting that air trapping in these patients involves only small rather than large airways and that these small airways contribute little to total airway resistance. Only when a large percentage, perhaps 75% or more, of all small airways are obstructed will spirometry pick up small airways disease, the authors observe.
Continuing disease
The findings taken together suggest that functional small airways disease and air trapping are a consequence of SARS-CoV-2 infection, according to Dr. Comellas. “If a portion of patients continues to have small airways disease, then we need to think about the mechanisms behind it,” he said. “It could be something related to inflammation that’s reversible, or it may be something related to a scar that is irreversible, and then we need to look at ways to prevent further progression of the disease.” Furthermore, “studies aimed at determining the natural history of functional small airways disease in patients with post-acute sequelae of COVID-19 and the biological mechanisms that underlie these findings are urgently needed to identify therapeutic and preventative interventions,” Dr. Comellas, professor of internal medicine at Carver College of Medicine, University of Iowa, Iowa City, concluded.
The study limitations, the authors state, include the fact that theirs was a single-center study that enrolled participants infected early during the COVID-19 pandemic and did not include patients with Delta or Omicron variants, thus limiting the generalizability of the findings.
The study was published in Radiology.
The reported findings “indicate a long-term impact on bronchiolar obstruction,” states Brett M. Elicker, MD, professor of clinical radiology, University of California, San Francisco, in an accompanying editorial . Because collagen may be absorbed for months after an acute insult, it is not entirely clear whether the abnormalities seen in the current study will be permanent. He said further, “the presence of ground glass opacity and/or fibrosis on CT were most common in the patients admitted to the ICU and likely correspond to post-organizing pneumonia and/or post-diffuse alveolar damage fibrosis.”
Dr. Elicker also pointed out that organizing pneumonia is especially common among patients with COVID-19 and is usually highly steroid-responsive. The opacities improve or resolve with treatment, but sometimes residual fibrosis occurs. “Longer-term studies assessing the clinical and imaging manifestations 1-2 years after the initial infection are needed to fully ascertain the permanent manifestations of post-COVID fibrosis.”
The study was supported by grants from the National Institutes of Health. The authors and Dr. Elicker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a prospective study that compared 100 COVID-19 survivors who had persistent symptoms and 106 healthy control persons.
“Something is going on in the distal airways related to either inflammation or fibrosis that is giving us a signal of air trapping,” noted senior author Alejandro P. Comellas, MD, in a press release. The study was stimulated by reports from University of Iowa clinicians noting that many patients with initial SARS-CoV-2 infection who were either hospitalized or were treated in the ambulatory setting later reported shortness of breath and other respiratory symptoms indicative of chronic lung disease.
Study results
Investigators classified patients (mean age, 48 years; 66 women) with post-acute sequelae of COVID-19 according to whether they were ambulatory (67%), hospitalized (17%), or required treatment in the intensive care unit (16%). They then compared CT findings of patients who had COVID-19 and persistent symptoms with those of a healthy control group.
COVID-19 severity did not affect the percentage of cases of lung with air trapping among these patients. Air trapping occurred at rates of 25.4% among ambulatory patients, 34.6% in hospitalized patients, and in 27.3% of those requiring intensive care (P = .10). The percentage of lungs affected by air trapping in ambulatory participants was sharply and significantly higher than in healthy controls (25.4% vs. 7.2%; P < .001). Also, air trapping persisted; it was still present in 8 of 9 participants who underwent imaging more than 200 days post diagnosis.
Qualitative analysis of chest CT images showed that the most common imaging abnormality was air trapping (58%); ground glass opacities (GGOs) were found in 51% (46/91), note Dr. Comellas and coauthors. This suggests ongoing lung inflammation, edema, or fibrosis. These symptoms are often observed during acute COVID-19, frequently in an organizing pneumonia pattern, and have been shown to persist for months after infection in survivors of severe disease. The mean percentage of total lung classified as having regional GGOs on chest CT scans was 13.2% and 28.7%, respectively, in the hospitalized and ICU groups, both very much higher than in the ambulatory group, at 3.7% (P < .001 for both). Among healthy controls, the GGO rate on chest CT was only 0.06% (P < .001).
In addition, air trapping correlated with the ratio of residual volume to total lung capacity (r = 0.6; P < .001) but not with spirometry results. In fact, the investigators did not observe airflow obstruction by spirometry in any group, suggesting that air trapping in these patients involves only small rather than large airways and that these small airways contribute little to total airway resistance. Only when a large percentage, perhaps 75% or more, of all small airways are obstructed will spirometry pick up small airways disease, the authors observe.
Continuing disease
The findings taken together suggest that functional small airways disease and air trapping are a consequence of SARS-CoV-2 infection, according to Dr. Comellas. “If a portion of patients continues to have small airways disease, then we need to think about the mechanisms behind it,” he said. “It could be something related to inflammation that’s reversible, or it may be something related to a scar that is irreversible, and then we need to look at ways to prevent further progression of the disease.” Furthermore, “studies aimed at determining the natural history of functional small airways disease in patients with post-acute sequelae of COVID-19 and the biological mechanisms that underlie these findings are urgently needed to identify therapeutic and preventative interventions,” Dr. Comellas, professor of internal medicine at Carver College of Medicine, University of Iowa, Iowa City, concluded.
The study limitations, the authors state, include the fact that theirs was a single-center study that enrolled participants infected early during the COVID-19 pandemic and did not include patients with Delta or Omicron variants, thus limiting the generalizability of the findings.
The study was published in Radiology.
The reported findings “indicate a long-term impact on bronchiolar obstruction,” states Brett M. Elicker, MD, professor of clinical radiology, University of California, San Francisco, in an accompanying editorial . Because collagen may be absorbed for months after an acute insult, it is not entirely clear whether the abnormalities seen in the current study will be permanent. He said further, “the presence of ground glass opacity and/or fibrosis on CT were most common in the patients admitted to the ICU and likely correspond to post-organizing pneumonia and/or post-diffuse alveolar damage fibrosis.”
Dr. Elicker also pointed out that organizing pneumonia is especially common among patients with COVID-19 and is usually highly steroid-responsive. The opacities improve or resolve with treatment, but sometimes residual fibrosis occurs. “Longer-term studies assessing the clinical and imaging manifestations 1-2 years after the initial infection are needed to fully ascertain the permanent manifestations of post-COVID fibrosis.”
The study was supported by grants from the National Institutes of Health. The authors and Dr. Elicker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM RADIOLOGY
COVID-19 vaccination and pregnancy: What’s the latest?

COVID-19 vaccination is recommended for all reproductive-aged women, regardless of pregnancy status.1 Yet, national vaccination rates in pregnancy remain woefully low—lower than vaccine coverage rates for other recommended vaccines during pregnancy.2,3 COVID-19 infection has clearly documented risks for maternal and fetal health, and data continue to accumulate on the maternal and neonatal benefits of COVID-19 vaccination in pregnancy, as well as the safety of vaccination during pregnancy.
Maternal and neonatal benefits of COVID-19 vaccination
Does vaccination in pregnancy result in decreased rates of severe COVID-19 infection? Results from a study from a Louisiana health system comparing maternal outcomes between fully vaccinated (defined as 2 weeks after the final vaccine dose) and unvaccinated or partially vaccinated pregnant women during the delta variant—predominant COVID-19 surge clearly answer this question. Vaccination in pregnancy resulted in a 90% risk reduction in severe or critical COVID-19 infection and a 70% risk reduction in COVID-19 infection of any severity among fully vaccinated women. The study also provides some useful absolute numbers for patient counseling: Although none of the 1,332 vaccinated pregnant women in the study required supplemental oxygen or intensive care unit (ICU) admission, there was 1 maternal death, 5 ICU admissions, and 6 stillbirths among the 8,760 unvaccinated pregnant women.4
A larger population-based data set from Scotland and Israel demonstrated similar findings.5 Most importantly, the Scotland data, with most patients having had an mRNA-based vaccine, showed that, while 77% of all COVID-19 infections occurred in unvaccinated pregnant women, 91% of all hospital admissions occurred in unvaccinated women, and 98% of all critical care admissions occurred in unvaccinated women. Furthermore, although 13% of all COVID-19 hospitalizations in pregnancy occurred among vaccinated women, only 2% of critical care admissions occurred among vaccinated women. The Israeli experience (which identified nearly 30,000 eligible pregnancies from 1 of 4 state-mandated health funds in the country), demonstrated that the efficacy of the Pfizer/BioNTech vaccine to prevent a SARS-CoV-2 infection of any severity once fully vaccinated is more than 80%.6
Breakthrough infections, which were more prevalent during the omicron surge, have caused some patients to question the utility of COVID-19 vaccination. Recent data from South Africa, where the omicron variant was first identified, noted that efficacy of the Pfizer/ BioNTech vaccine to prevent hospitalization with COVID-19 infection during an omicron-predominant period was 70%—versus 93% efficacy in a delta-predominant period.7 These data, however, were in the absence of a booster dose, and in vitro studies suggest increased vaccine efficacy with a booster dose.8
Continue to: Counseling women on vaccination benefits and risks...
Counseling women on vaccination benefits and risks. No matter the specific numeric rate of efficacy against a COVID-19 infection, it is important to counsel women that the goal of vaccination is to prevent severe or critical COVID-19 infections, and these data all demonstrate that COVID-19 vaccination meets this goal. However, women may have additional questions regarding both fetal/neonatal benefits and safety with immunization in pregnancy.
Let us address the question of benefit first. In a large cohort of more than 1,300 women vaccinated during pregnancy and delivering at >34 weeks’ gestation, a few observations are worth noting.9 The first is that women who were fully vaccinated by the time of delivery had detectable antibodies at birth, even with first trimester vaccination, and these antibodies did cross the placenta to the neonate. Although higher maternal and neonatal antibody levels are achieved with early third trimester vaccination, it is key that women interpret this finding in light of 2 important points:
- women cannot know what gestational age they will deliver, thus waiting until the early third trimester for vaccination to optimize neonatal antibody levels could result in delivery prior to planned vaccination, with benefit for neither the woman nor the baby
- partial vaccination in the early third trimester resulted in lower maternal and neonatal antibody levels than full vaccination in the first trimester.
In addition, while the data were limited, a booster dose in the third trimester results in the highest antibody levels at delivery. Given the recommendation to initiate a booster dose 5 months after the completion of the primary vaccine series,10 many women will be eligible for a booster prior to delivery and thus can achieve the goals of high maternal and neonatal antibody levels simultaneously. One caveat to these data is that, while higher antibody levels seem comforting and may be better, we do not yet know the level of neonatal antibody necessary to decrease risks of COVID-19 infection in early newborn life.9 Recent data from the Centers for Disease Control and Prevention provide real-world evidence that maternal vaccination decreases the risk of hospitalization from COVID-19 for infants aged <6 months, with vaccine efficacy estimated to be 61% during a period of both Delta and Omicron predominance.11
The evidence is clear—the time for COVID-19 vaccination is now. There is no “optimal” time of vaccination in pregnancy for neonatal benefit that would be worth risking any amount of time a woman is susceptible to COVID-19, especially given the promising data regarding maternal and neonatal antibody levels achieved after a booster dose.
Although the COVID-19 vaccine is currently approved by the US Food and Drug Administration for ages 5 and above, Pfizer-BioNTech has plans to submit for approval for their vaccine’s use among kids as young as 6 months.1 Assuming that this approval occurs, this will leave newborns as the only group without possible vaccination against COVID-19. But can vaccination during pregnancy protect these infants against infection, as vaccination with the flu vaccine during pregnancy confers protective benefit to newborns?2
In a recent research letter published in Journal of the American Medical Association, Shook and colleagues present their data on antibody levels against COVID-19 present in newborns of women who were either naturally infected with COVID-19 at 20 to 32 weeks’ gestation (12 women) or who received mRNA vaccination during pregnancy at 20 to 32 weeks’ gestation (77 women).3 (They chose the 20- to 32-week timeframe during pregnancy because it had “demonstrated superior transplacental transfer of antibodies during this window.”)
They found that COVID-19 antibody levels were higher in both maternal and cord blood at birth in the women who were vaccinated versus the women who had infection. At 6 months, 16 of the 28 infants from the vaccinated-mother group had detectable antibodies compared with 1 of 12 infants from the infected-mother group. The researchers pointed out that the “antibody titer known to be protective against COVID-19 in infants is unknown;” however, they say that their findings provide further supportive evidence for COVID-19 vaccination in pregnant women.3
References
- Pfizer-BioNTech coronavirus vaccine for children under 5 could be available by the end of February, people with knowledge say. The Washington Post. https://www.washingtonpost.com /health/2022/01/31/coronavirus-vaccine-children-under-5/. Accessed February 11, 2022.
- Sakala IG, Honda-Okubo Y, Fung J, et al. Influenza immunization during pregnancy: benefits for mother and infant. Hum Vaccin Immunother. 2016;12:3065-3071. doi:10.1080/21645515.2016 .1215392.
- Shook LL, Atyeo CG, Yonker LM, et al. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA. doi:10.1001/jama.2022.1206.
Safety of COVID-19 vaccination: Current data
Risks for pregnancy loss, birth defects, and preterm delivery often are concerns of pregnant women considering a COVID-19 vaccination. Data from more than 2,400 women who submitted their information to the v-SAFE registry demonstrated a 14% risk for pregnancy loss between 6 and 20 weeks’ gestation—well within the expected rate of pregnancy loss in this gestational age range.12
Data from more than 46,000 pregnancies included in the Vaccine Safety Datalink, which includes data from health care organizations in 6 states, demonstrated a preterm birth rate of 6.6% and a small-for-gestational-age rate of 8.2% among fully vaccinated women, rates that were no different among unvaccinated women. There were no differences in the outcomes by trimester of vaccination, and these rates are comparable to the expected rates of these outcomes.13
Women also worry about the risks of vaccine side effects, such as fever or rare adverse events. Although all adverse events (ie, Guillain-Barre syndrome, pericarditis/myocarditis, thrombosis with thrombocytopenia syndrome [TTS]) are very rare, the American College of Obstetricians and Gynecologists does recommend that women get an mRNA COVID-19 vaccine, as the Johnson & Johnson/Janssen vaccine is associated with TTS, which occurred more commonly (although still rare) in women of reproductive age.14
Two large studies of typical side effects experienced after COVID-19 vaccination in pregnancy are incredibly reassuring. In the first, authors of a large study of more than 12,000 pregnant women enrolled in the v-SAFE registry reported that the most common side effect after each mRNA dose was injection site pain (88% after dose 1, 92% after dose 2).15 Self-reported fever occurred in 4% of women after dose 1 and 35% after dose 2. Although this frequency may seem high, a fever of 38.0°C (100.4°F) or higher only occurred among 8% of all participants.
In another study of almost 8,000 women self-reporting side effects (some of whom also may have contributed data to the v-SAFE study), fever occurred in approximately 5% after dose 1 and in about 20% after dose 2.16 In this study, the highest mean temperature was 38.1°C (100.6°F) after dose 1 and 38.2°C (100.7°F) after dose 2. Although it is a reasonable expectation for fever to follow COVID-19 vaccination, particularly after the second dose, the typical fever is a low-grade temperature that will not harm a developing fetus and will be responsive to acetaminophen administration. Moreover, if the fever were the harbinger of harm, then it might stand to reason that an increased signal of preterm delivery may be observed, but data from nearly 10,000 pregnant women vaccinated during the second or third trimesters showed no association with preterm birth (adjusted hazard ratio, 0.91; 95% confidence interval, 0.82–1.01).13
The bottom line
The data are clear. COVID-19 vaccination decreases the risks of severe infection in pregnancy, confers antibodies to neonates with at least some level of protection, and has no demonstrated harmful side effects in pregnancy. ●
- Interim clinical considerations for use of COVID-19 vaccines. CDC website. Published January 24, 2022. Accessed February 22, 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- Cumulative data: percent of pregnant people aged 18-49 years receiving at least one dose of a COVID-19 vaccine during pregnancy overall, by race/ethnicity, and date reported to CDC—Vaccine Safety Datalink, United States. CDC website. Accessed February 22, 2022. https://data.cdc.gov/Vaccinations/Cumulative-Data-Percent-of-Pregnant-People-aged-18/4ht3-nbmd/data
- Razzaghi H, Kahn KE, Black CL, et al. Influenza and Tdap vaccination coverage among pregnant women—United States, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1391-1397.
- Morgan JA, Biggio JRJ, Martin JK, et al. Maternal outcomes after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet Gynecol. 2022;139:107-109.
- Stock SJ, Carruthers J, Calvert C, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland [published online January 13, 2022]. Nat Med. doi:10.1038/s41591-021-01666-2
- Goldshtein I, Nevo D, Steinberg DM, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326:728-735.
- Collie S, Champion J, Moultrie H, et al. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa [published online December 29, 2021]. N Engl J Med. doi:10.1056/NEJMc2119270
- Nemet I, Kliker L, Lustig Y, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection [published online December 29, 2021]. N Engl J Med. doi:10.1056/NEJMc2119358
- Yang YJ, Murphy EA, Singh S, et al. Association of gestational age at coronavirus disease 2019 (COVID-19) vaccination, history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and a vaccine booster dose with maternal and umbilical cord antibody levels at delivery [published online December 28, 2021]. Obstet Gynecol. doi:10.1097/AOG.0000000000004693
- COVID-19 vaccine booster shots. Centers for Disease Control and Prevention web site. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html. Accessed March 2, 2022.
- Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19–associated hospitalization in infants aged <6 months—17 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264–270. doi: http://dx.doi.org/10.15585/mmwr.mm7107e3external icon.
- Zauche LH, Wallace B, Smoots AN, et al. Receipt of mRNA COVID-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385:1533-1535.
- Lipkind HS. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth—eight integrated health care organizations, United States, December 15, 2020–July 22, 2021. MMWR Morb Mortal Wkly Rep. doi:10.15585/mmwr.mm7101e1
- COVID-19 vaccination considerations for obstetric-gynecologic care. ACOG website. Updated February 8, 2022. Accessed February 22, 2022. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282.
- Kachikis A, Englund JA, Singleton M, et al. Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout. JAMA Netw Open. 2021;4:E2121310.

COVID-19 vaccination is recommended for all reproductive-aged women, regardless of pregnancy status.1 Yet, national vaccination rates in pregnancy remain woefully low—lower than vaccine coverage rates for other recommended vaccines during pregnancy.2,3 COVID-19 infection has clearly documented risks for maternal and fetal health, and data continue to accumulate on the maternal and neonatal benefits of COVID-19 vaccination in pregnancy, as well as the safety of vaccination during pregnancy.
Maternal and neonatal benefits of COVID-19 vaccination
Does vaccination in pregnancy result in decreased rates of severe COVID-19 infection? Results from a study from a Louisiana health system comparing maternal outcomes between fully vaccinated (defined as 2 weeks after the final vaccine dose) and unvaccinated or partially vaccinated pregnant women during the delta variant—predominant COVID-19 surge clearly answer this question. Vaccination in pregnancy resulted in a 90% risk reduction in severe or critical COVID-19 infection and a 70% risk reduction in COVID-19 infection of any severity among fully vaccinated women. The study also provides some useful absolute numbers for patient counseling: Although none of the 1,332 vaccinated pregnant women in the study required supplemental oxygen or intensive care unit (ICU) admission, there was 1 maternal death, 5 ICU admissions, and 6 stillbirths among the 8,760 unvaccinated pregnant women.4
A larger population-based data set from Scotland and Israel demonstrated similar findings.5 Most importantly, the Scotland data, with most patients having had an mRNA-based vaccine, showed that, while 77% of all COVID-19 infections occurred in unvaccinated pregnant women, 91% of all hospital admissions occurred in unvaccinated women, and 98% of all critical care admissions occurred in unvaccinated women. Furthermore, although 13% of all COVID-19 hospitalizations in pregnancy occurred among vaccinated women, only 2% of critical care admissions occurred among vaccinated women. The Israeli experience (which identified nearly 30,000 eligible pregnancies from 1 of 4 state-mandated health funds in the country), demonstrated that the efficacy of the Pfizer/BioNTech vaccine to prevent a SARS-CoV-2 infection of any severity once fully vaccinated is more than 80%.6
Breakthrough infections, which were more prevalent during the omicron surge, have caused some patients to question the utility of COVID-19 vaccination. Recent data from South Africa, where the omicron variant was first identified, noted that efficacy of the Pfizer/ BioNTech vaccine to prevent hospitalization with COVID-19 infection during an omicron-predominant period was 70%—versus 93% efficacy in a delta-predominant period.7 These data, however, were in the absence of a booster dose, and in vitro studies suggest increased vaccine efficacy with a booster dose.8
Continue to: Counseling women on vaccination benefits and risks...
Counseling women on vaccination benefits and risks. No matter the specific numeric rate of efficacy against a COVID-19 infection, it is important to counsel women that the goal of vaccination is to prevent severe or critical COVID-19 infections, and these data all demonstrate that COVID-19 vaccination meets this goal. However, women may have additional questions regarding both fetal/neonatal benefits and safety with immunization in pregnancy.
Let us address the question of benefit first. In a large cohort of more than 1,300 women vaccinated during pregnancy and delivering at >34 weeks’ gestation, a few observations are worth noting.9 The first is that women who were fully vaccinated by the time of delivery had detectable antibodies at birth, even with first trimester vaccination, and these antibodies did cross the placenta to the neonate. Although higher maternal and neonatal antibody levels are achieved with early third trimester vaccination, it is key that women interpret this finding in light of 2 important points:
- women cannot know what gestational age they will deliver, thus waiting until the early third trimester for vaccination to optimize neonatal antibody levels could result in delivery prior to planned vaccination, with benefit for neither the woman nor the baby
- partial vaccination in the early third trimester resulted in lower maternal and neonatal antibody levels than full vaccination in the first trimester.
In addition, while the data were limited, a booster dose in the third trimester results in the highest antibody levels at delivery. Given the recommendation to initiate a booster dose 5 months after the completion of the primary vaccine series,10 many women will be eligible for a booster prior to delivery and thus can achieve the goals of high maternal and neonatal antibody levels simultaneously. One caveat to these data is that, while higher antibody levels seem comforting and may be better, we do not yet know the level of neonatal antibody necessary to decrease risks of COVID-19 infection in early newborn life.9 Recent data from the Centers for Disease Control and Prevention provide real-world evidence that maternal vaccination decreases the risk of hospitalization from COVID-19 for infants aged <6 months, with vaccine efficacy estimated to be 61% during a period of both Delta and Omicron predominance.11
The evidence is clear—the time for COVID-19 vaccination is now. There is no “optimal” time of vaccination in pregnancy for neonatal benefit that would be worth risking any amount of time a woman is susceptible to COVID-19, especially given the promising data regarding maternal and neonatal antibody levels achieved after a booster dose.
Although the COVID-19 vaccine is currently approved by the US Food and Drug Administration for ages 5 and above, Pfizer-BioNTech has plans to submit for approval for their vaccine’s use among kids as young as 6 months.1 Assuming that this approval occurs, this will leave newborns as the only group without possible vaccination against COVID-19. But can vaccination during pregnancy protect these infants against infection, as vaccination with the flu vaccine during pregnancy confers protective benefit to newborns?2
In a recent research letter published in Journal of the American Medical Association, Shook and colleagues present their data on antibody levels against COVID-19 present in newborns of women who were either naturally infected with COVID-19 at 20 to 32 weeks’ gestation (12 women) or who received mRNA vaccination during pregnancy at 20 to 32 weeks’ gestation (77 women).3 (They chose the 20- to 32-week timeframe during pregnancy because it had “demonstrated superior transplacental transfer of antibodies during this window.”)
They found that COVID-19 antibody levels were higher in both maternal and cord blood at birth in the women who were vaccinated versus the women who had infection. At 6 months, 16 of the 28 infants from the vaccinated-mother group had detectable antibodies compared with 1 of 12 infants from the infected-mother group. The researchers pointed out that the “antibody titer known to be protective against COVID-19 in infants is unknown;” however, they say that their findings provide further supportive evidence for COVID-19 vaccination in pregnant women.3
References
- Pfizer-BioNTech coronavirus vaccine for children under 5 could be available by the end of February, people with knowledge say. The Washington Post. https://www.washingtonpost.com /health/2022/01/31/coronavirus-vaccine-children-under-5/. Accessed February 11, 2022.
- Sakala IG, Honda-Okubo Y, Fung J, et al. Influenza immunization during pregnancy: benefits for mother and infant. Hum Vaccin Immunother. 2016;12:3065-3071. doi:10.1080/21645515.2016 .1215392.
- Shook LL, Atyeo CG, Yonker LM, et al. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA. doi:10.1001/jama.2022.1206.
Safety of COVID-19 vaccination: Current data
Risks for pregnancy loss, birth defects, and preterm delivery often are concerns of pregnant women considering a COVID-19 vaccination. Data from more than 2,400 women who submitted their information to the v-SAFE registry demonstrated a 14% risk for pregnancy loss between 6 and 20 weeks’ gestation—well within the expected rate of pregnancy loss in this gestational age range.12
Data from more than 46,000 pregnancies included in the Vaccine Safety Datalink, which includes data from health care organizations in 6 states, demonstrated a preterm birth rate of 6.6% and a small-for-gestational-age rate of 8.2% among fully vaccinated women, rates that were no different among unvaccinated women. There were no differences in the outcomes by trimester of vaccination, and these rates are comparable to the expected rates of these outcomes.13
Women also worry about the risks of vaccine side effects, such as fever or rare adverse events. Although all adverse events (ie, Guillain-Barre syndrome, pericarditis/myocarditis, thrombosis with thrombocytopenia syndrome [TTS]) are very rare, the American College of Obstetricians and Gynecologists does recommend that women get an mRNA COVID-19 vaccine, as the Johnson & Johnson/Janssen vaccine is associated with TTS, which occurred more commonly (although still rare) in women of reproductive age.14
Two large studies of typical side effects experienced after COVID-19 vaccination in pregnancy are incredibly reassuring. In the first, authors of a large study of more than 12,000 pregnant women enrolled in the v-SAFE registry reported that the most common side effect after each mRNA dose was injection site pain (88% after dose 1, 92% after dose 2).15 Self-reported fever occurred in 4% of women after dose 1 and 35% after dose 2. Although this frequency may seem high, a fever of 38.0°C (100.4°F) or higher only occurred among 8% of all participants.
In another study of almost 8,000 women self-reporting side effects (some of whom also may have contributed data to the v-SAFE study), fever occurred in approximately 5% after dose 1 and in about 20% after dose 2.16 In this study, the highest mean temperature was 38.1°C (100.6°F) after dose 1 and 38.2°C (100.7°F) after dose 2. Although it is a reasonable expectation for fever to follow COVID-19 vaccination, particularly after the second dose, the typical fever is a low-grade temperature that will not harm a developing fetus and will be responsive to acetaminophen administration. Moreover, if the fever were the harbinger of harm, then it might stand to reason that an increased signal of preterm delivery may be observed, but data from nearly 10,000 pregnant women vaccinated during the second or third trimesters showed no association with preterm birth (adjusted hazard ratio, 0.91; 95% confidence interval, 0.82–1.01).13
The bottom line
The data are clear. COVID-19 vaccination decreases the risks of severe infection in pregnancy, confers antibodies to neonates with at least some level of protection, and has no demonstrated harmful side effects in pregnancy. ●

COVID-19 vaccination is recommended for all reproductive-aged women, regardless of pregnancy status.1 Yet, national vaccination rates in pregnancy remain woefully low—lower than vaccine coverage rates for other recommended vaccines during pregnancy.2,3 COVID-19 infection has clearly documented risks for maternal and fetal health, and data continue to accumulate on the maternal and neonatal benefits of COVID-19 vaccination in pregnancy, as well as the safety of vaccination during pregnancy.
Maternal and neonatal benefits of COVID-19 vaccination
Does vaccination in pregnancy result in decreased rates of severe COVID-19 infection? Results from a study from a Louisiana health system comparing maternal outcomes between fully vaccinated (defined as 2 weeks after the final vaccine dose) and unvaccinated or partially vaccinated pregnant women during the delta variant—predominant COVID-19 surge clearly answer this question. Vaccination in pregnancy resulted in a 90% risk reduction in severe or critical COVID-19 infection and a 70% risk reduction in COVID-19 infection of any severity among fully vaccinated women. The study also provides some useful absolute numbers for patient counseling: Although none of the 1,332 vaccinated pregnant women in the study required supplemental oxygen or intensive care unit (ICU) admission, there was 1 maternal death, 5 ICU admissions, and 6 stillbirths among the 8,760 unvaccinated pregnant women.4
A larger population-based data set from Scotland and Israel demonstrated similar findings.5 Most importantly, the Scotland data, with most patients having had an mRNA-based vaccine, showed that, while 77% of all COVID-19 infections occurred in unvaccinated pregnant women, 91% of all hospital admissions occurred in unvaccinated women, and 98% of all critical care admissions occurred in unvaccinated women. Furthermore, although 13% of all COVID-19 hospitalizations in pregnancy occurred among vaccinated women, only 2% of critical care admissions occurred among vaccinated women. The Israeli experience (which identified nearly 30,000 eligible pregnancies from 1 of 4 state-mandated health funds in the country), demonstrated that the efficacy of the Pfizer/BioNTech vaccine to prevent a SARS-CoV-2 infection of any severity once fully vaccinated is more than 80%.6
Breakthrough infections, which were more prevalent during the omicron surge, have caused some patients to question the utility of COVID-19 vaccination. Recent data from South Africa, where the omicron variant was first identified, noted that efficacy of the Pfizer/ BioNTech vaccine to prevent hospitalization with COVID-19 infection during an omicron-predominant period was 70%—versus 93% efficacy in a delta-predominant period.7 These data, however, were in the absence of a booster dose, and in vitro studies suggest increased vaccine efficacy with a booster dose.8
Continue to: Counseling women on vaccination benefits and risks...
Counseling women on vaccination benefits and risks. No matter the specific numeric rate of efficacy against a COVID-19 infection, it is important to counsel women that the goal of vaccination is to prevent severe or critical COVID-19 infections, and these data all demonstrate that COVID-19 vaccination meets this goal. However, women may have additional questions regarding both fetal/neonatal benefits and safety with immunization in pregnancy.
Let us address the question of benefit first. In a large cohort of more than 1,300 women vaccinated during pregnancy and delivering at >34 weeks’ gestation, a few observations are worth noting.9 The first is that women who were fully vaccinated by the time of delivery had detectable antibodies at birth, even with first trimester vaccination, and these antibodies did cross the placenta to the neonate. Although higher maternal and neonatal antibody levels are achieved with early third trimester vaccination, it is key that women interpret this finding in light of 2 important points:
- women cannot know what gestational age they will deliver, thus waiting until the early third trimester for vaccination to optimize neonatal antibody levels could result in delivery prior to planned vaccination, with benefit for neither the woman nor the baby
- partial vaccination in the early third trimester resulted in lower maternal and neonatal antibody levels than full vaccination in the first trimester.
In addition, while the data were limited, a booster dose in the third trimester results in the highest antibody levels at delivery. Given the recommendation to initiate a booster dose 5 months after the completion of the primary vaccine series,10 many women will be eligible for a booster prior to delivery and thus can achieve the goals of high maternal and neonatal antibody levels simultaneously. One caveat to these data is that, while higher antibody levels seem comforting and may be better, we do not yet know the level of neonatal antibody necessary to decrease risks of COVID-19 infection in early newborn life.9 Recent data from the Centers for Disease Control and Prevention provide real-world evidence that maternal vaccination decreases the risk of hospitalization from COVID-19 for infants aged <6 months, with vaccine efficacy estimated to be 61% during a period of both Delta and Omicron predominance.11
The evidence is clear—the time for COVID-19 vaccination is now. There is no “optimal” time of vaccination in pregnancy for neonatal benefit that would be worth risking any amount of time a woman is susceptible to COVID-19, especially given the promising data regarding maternal and neonatal antibody levels achieved after a booster dose.
Although the COVID-19 vaccine is currently approved by the US Food and Drug Administration for ages 5 and above, Pfizer-BioNTech has plans to submit for approval for their vaccine’s use among kids as young as 6 months.1 Assuming that this approval occurs, this will leave newborns as the only group without possible vaccination against COVID-19. But can vaccination during pregnancy protect these infants against infection, as vaccination with the flu vaccine during pregnancy confers protective benefit to newborns?2
In a recent research letter published in Journal of the American Medical Association, Shook and colleagues present their data on antibody levels against COVID-19 present in newborns of women who were either naturally infected with COVID-19 at 20 to 32 weeks’ gestation (12 women) or who received mRNA vaccination during pregnancy at 20 to 32 weeks’ gestation (77 women).3 (They chose the 20- to 32-week timeframe during pregnancy because it had “demonstrated superior transplacental transfer of antibodies during this window.”)
They found that COVID-19 antibody levels were higher in both maternal and cord blood at birth in the women who were vaccinated versus the women who had infection. At 6 months, 16 of the 28 infants from the vaccinated-mother group had detectable antibodies compared with 1 of 12 infants from the infected-mother group. The researchers pointed out that the “antibody titer known to be protective against COVID-19 in infants is unknown;” however, they say that their findings provide further supportive evidence for COVID-19 vaccination in pregnant women.3
References
- Pfizer-BioNTech coronavirus vaccine for children under 5 could be available by the end of February, people with knowledge say. The Washington Post. https://www.washingtonpost.com /health/2022/01/31/coronavirus-vaccine-children-under-5/. Accessed February 11, 2022.
- Sakala IG, Honda-Okubo Y, Fung J, et al. Influenza immunization during pregnancy: benefits for mother and infant. Hum Vaccin Immunother. 2016;12:3065-3071. doi:10.1080/21645515.2016 .1215392.
- Shook LL, Atyeo CG, Yonker LM, et al. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA. doi:10.1001/jama.2022.1206.
Safety of COVID-19 vaccination: Current data
Risks for pregnancy loss, birth defects, and preterm delivery often are concerns of pregnant women considering a COVID-19 vaccination. Data from more than 2,400 women who submitted their information to the v-SAFE registry demonstrated a 14% risk for pregnancy loss between 6 and 20 weeks’ gestation—well within the expected rate of pregnancy loss in this gestational age range.12
Data from more than 46,000 pregnancies included in the Vaccine Safety Datalink, which includes data from health care organizations in 6 states, demonstrated a preterm birth rate of 6.6% and a small-for-gestational-age rate of 8.2% among fully vaccinated women, rates that were no different among unvaccinated women. There were no differences in the outcomes by trimester of vaccination, and these rates are comparable to the expected rates of these outcomes.13
Women also worry about the risks of vaccine side effects, such as fever or rare adverse events. Although all adverse events (ie, Guillain-Barre syndrome, pericarditis/myocarditis, thrombosis with thrombocytopenia syndrome [TTS]) are very rare, the American College of Obstetricians and Gynecologists does recommend that women get an mRNA COVID-19 vaccine, as the Johnson & Johnson/Janssen vaccine is associated with TTS, which occurred more commonly (although still rare) in women of reproductive age.14
Two large studies of typical side effects experienced after COVID-19 vaccination in pregnancy are incredibly reassuring. In the first, authors of a large study of more than 12,000 pregnant women enrolled in the v-SAFE registry reported that the most common side effect after each mRNA dose was injection site pain (88% after dose 1, 92% after dose 2).15 Self-reported fever occurred in 4% of women after dose 1 and 35% after dose 2. Although this frequency may seem high, a fever of 38.0°C (100.4°F) or higher only occurred among 8% of all participants.
In another study of almost 8,000 women self-reporting side effects (some of whom also may have contributed data to the v-SAFE study), fever occurred in approximately 5% after dose 1 and in about 20% after dose 2.16 In this study, the highest mean temperature was 38.1°C (100.6°F) after dose 1 and 38.2°C (100.7°F) after dose 2. Although it is a reasonable expectation for fever to follow COVID-19 vaccination, particularly after the second dose, the typical fever is a low-grade temperature that will not harm a developing fetus and will be responsive to acetaminophen administration. Moreover, if the fever were the harbinger of harm, then it might stand to reason that an increased signal of preterm delivery may be observed, but data from nearly 10,000 pregnant women vaccinated during the second or third trimesters showed no association with preterm birth (adjusted hazard ratio, 0.91; 95% confidence interval, 0.82–1.01).13
The bottom line
The data are clear. COVID-19 vaccination decreases the risks of severe infection in pregnancy, confers antibodies to neonates with at least some level of protection, and has no demonstrated harmful side effects in pregnancy. ●
- Interim clinical considerations for use of COVID-19 vaccines. CDC website. Published January 24, 2022. Accessed February 22, 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- Cumulative data: percent of pregnant people aged 18-49 years receiving at least one dose of a COVID-19 vaccine during pregnancy overall, by race/ethnicity, and date reported to CDC—Vaccine Safety Datalink, United States. CDC website. Accessed February 22, 2022. https://data.cdc.gov/Vaccinations/Cumulative-Data-Percent-of-Pregnant-People-aged-18/4ht3-nbmd/data
- Razzaghi H, Kahn KE, Black CL, et al. Influenza and Tdap vaccination coverage among pregnant women—United States, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1391-1397.
- Morgan JA, Biggio JRJ, Martin JK, et al. Maternal outcomes after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet Gynecol. 2022;139:107-109.
- Stock SJ, Carruthers J, Calvert C, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland [published online January 13, 2022]. Nat Med. doi:10.1038/s41591-021-01666-2
- Goldshtein I, Nevo D, Steinberg DM, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326:728-735.
- Collie S, Champion J, Moultrie H, et al. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa [published online December 29, 2021]. N Engl J Med. doi:10.1056/NEJMc2119270
- Nemet I, Kliker L, Lustig Y, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection [published online December 29, 2021]. N Engl J Med. doi:10.1056/NEJMc2119358
- Yang YJ, Murphy EA, Singh S, et al. Association of gestational age at coronavirus disease 2019 (COVID-19) vaccination, history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and a vaccine booster dose with maternal and umbilical cord antibody levels at delivery [published online December 28, 2021]. Obstet Gynecol. doi:10.1097/AOG.0000000000004693
- COVID-19 vaccine booster shots. Centers for Disease Control and Prevention web site. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html. Accessed March 2, 2022.
- Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19–associated hospitalization in infants aged <6 months—17 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264–270. doi: http://dx.doi.org/10.15585/mmwr.mm7107e3external icon.
- Zauche LH, Wallace B, Smoots AN, et al. Receipt of mRNA COVID-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385:1533-1535.
- Lipkind HS. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth—eight integrated health care organizations, United States, December 15, 2020–July 22, 2021. MMWR Morb Mortal Wkly Rep. doi:10.15585/mmwr.mm7101e1
- COVID-19 vaccination considerations for obstetric-gynecologic care. ACOG website. Updated February 8, 2022. Accessed February 22, 2022. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282.
- Kachikis A, Englund JA, Singleton M, et al. Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout. JAMA Netw Open. 2021;4:E2121310.
- Interim clinical considerations for use of COVID-19 vaccines. CDC website. Published January 24, 2022. Accessed February 22, 2022. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- Cumulative data: percent of pregnant people aged 18-49 years receiving at least one dose of a COVID-19 vaccine during pregnancy overall, by race/ethnicity, and date reported to CDC—Vaccine Safety Datalink, United States. CDC website. Accessed February 22, 2022. https://data.cdc.gov/Vaccinations/Cumulative-Data-Percent-of-Pregnant-People-aged-18/4ht3-nbmd/data
- Razzaghi H, Kahn KE, Black CL, et al. Influenza and Tdap vaccination coverage among pregnant women—United States, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1391-1397.
- Morgan JA, Biggio JRJ, Martin JK, et al. Maternal outcomes after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet Gynecol. 2022;139:107-109.
- Stock SJ, Carruthers J, Calvert C, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland [published online January 13, 2022]. Nat Med. doi:10.1038/s41591-021-01666-2
- Goldshtein I, Nevo D, Steinberg DM, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326:728-735.
- Collie S, Champion J, Moultrie H, et al. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa [published online December 29, 2021]. N Engl J Med. doi:10.1056/NEJMc2119270
- Nemet I, Kliker L, Lustig Y, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection [published online December 29, 2021]. N Engl J Med. doi:10.1056/NEJMc2119358
- Yang YJ, Murphy EA, Singh S, et al. Association of gestational age at coronavirus disease 2019 (COVID-19) vaccination, history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and a vaccine booster dose with maternal and umbilical cord antibody levels at delivery [published online December 28, 2021]. Obstet Gynecol. doi:10.1097/AOG.0000000000004693
- COVID-19 vaccine booster shots. Centers for Disease Control and Prevention web site. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html. Accessed March 2, 2022.
- Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19–associated hospitalization in infants aged <6 months—17 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264–270. doi: http://dx.doi.org/10.15585/mmwr.mm7107e3external icon.
- Zauche LH, Wallace B, Smoots AN, et al. Receipt of mRNA COVID-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385:1533-1535.
- Lipkind HS. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth—eight integrated health care organizations, United States, December 15, 2020–July 22, 2021. MMWR Morb Mortal Wkly Rep. doi:10.15585/mmwr.mm7101e1
- COVID-19 vaccination considerations for obstetric-gynecologic care. ACOG website. Updated February 8, 2022. Accessed February 22, 2022. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282.
- Kachikis A, Englund JA, Singleton M, et al. Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout. JAMA Netw Open. 2021;4:E2121310.
‘Overwhelming’ need to study COVID vaccine–associated tinnitus
It’s now known that tinnitus may be an unexpected side effect of SARS-CoV-2 vaccination, and there is an urgent need to understand the precise mechanisms and best treatment for vaccine-associated tinnitus, researchers say.
As of mid-September 2021, 12,247 cases of tinnitus, or ringing in the ears, following COVID-19 vaccination had been reported to the Vaccine Adverse Event Reporting System of the U.S. Centers for Disease Control and Prevention.
“Despite several cases of tinnitus being reported following SARS-CoV-2 vaccination, the precise pathophysiology is still not clear,” write Syed Hassan Ahmed, 3rd-year MBBS student, Dow University of Health Sciences, Karachi, Pakistan, and coauthors.
The researchers review what is known and unknown about SARS-CoV-2 vaccine-associated tinnitus in an article published online Feb. 11 in Annals of Medicine and Surgery.
Molecular mimicry?
The researchers say cross-reactivity between anti-spike SARS-CoV-2 antibodies and otologic antigens is one possibility, based on the mechanisms behind other COVID-19 vaccine–induced disorders and the phenomenon of molecular mimicry.
“The heptapeptide resemblance between coronavirus spike glycoprotein and numerous human proteins further supports molecular mimicry as a potential mechanism behind such vaccine-induced disorders,” they write.
Anti-spike antibodies may react with antigens anywhere along the auditory pathway and fuel an inflammatory reaction, they point out.
“Therefore, understanding the phenomenon of cross-reactivity and molecular mimicry may be helpful in postulating potential treatment behind not only tinnitus but also the rare events of vaccination associated hearing loss and other otologic manifestations,” the authors say.
Genetic predispositions and associated conditions may also play a significant role in determining whether an individual develops vaccine-induced tinnitus.
Stress and anxiety following COVID vaccination may also play a role, inasmuch as anxiety-related adverse events following vaccination have been reported. Vaccine-related anxiety as a potential cause of tinnitus developing after vaccination needs to be explored, they write.
Jury out on best management
How best to manage COVID vaccine-associated tinnitus also remains unclear, but it starts with a well-established diagnosis, the authors say.
A well-focused and detailed history and examination are essential, with particular emphasis placed on preexisting health conditions, specifically, autoimmune diseases, such as Hashimoto thyroiditis; otologic conditions, such as sensorineural hearing loss; glaucoma; and psychological well-being. According to the review, patients often present with a history of one or more of these disorders.
“However, any such association has not yet been established and requires further investigation to be concluded as potential risk factors for vaccine-induced tinnitus,” they caution.
Routine cranial nerve examination, otoscopy, Weber test, and Rinne test, which are used for tinnitus diagnosis in general, may be helpful for confirmation of vaccine-associated tinnitus.
Owing to the significant association between tinnitus and hearing impairment, audiology should also performed, the authors say.
Although treatments for non–vaccine-induced tinnitus vary significantly, corticosteroids are the top treatment choice for SARS-CoV-2 vaccine-induced tinnitus reported in the literature.
Trials of other drug and nondrug interventions that may uniquely help with vaccine-associated tinnitus are urgently needed, the authors say.
Summing up, the reviewers say, “Although the incidence of COVID-19 vaccine-associated tinnitus is rare, there is an overwhelming need to discern the precise pathophysiology and clinical management as a better understanding of adverse events may help in encountering vaccine hesitancy and hence fostering the COVID-19 global vaccination program.
“Despite the incidence of adverse events, the benefits of the SARS-CoV-2 vaccine in reducing hospitalization and deaths continue to outweigh the rare ramifications,” they conclude.
The research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s now known that tinnitus may be an unexpected side effect of SARS-CoV-2 vaccination, and there is an urgent need to understand the precise mechanisms and best treatment for vaccine-associated tinnitus, researchers say.
As of mid-September 2021, 12,247 cases of tinnitus, or ringing in the ears, following COVID-19 vaccination had been reported to the Vaccine Adverse Event Reporting System of the U.S. Centers for Disease Control and Prevention.
“Despite several cases of tinnitus being reported following SARS-CoV-2 vaccination, the precise pathophysiology is still not clear,” write Syed Hassan Ahmed, 3rd-year MBBS student, Dow University of Health Sciences, Karachi, Pakistan, and coauthors.
The researchers review what is known and unknown about SARS-CoV-2 vaccine-associated tinnitus in an article published online Feb. 11 in Annals of Medicine and Surgery.
Molecular mimicry?
The researchers say cross-reactivity between anti-spike SARS-CoV-2 antibodies and otologic antigens is one possibility, based on the mechanisms behind other COVID-19 vaccine–induced disorders and the phenomenon of molecular mimicry.
“The heptapeptide resemblance between coronavirus spike glycoprotein and numerous human proteins further supports molecular mimicry as a potential mechanism behind such vaccine-induced disorders,” they write.
Anti-spike antibodies may react with antigens anywhere along the auditory pathway and fuel an inflammatory reaction, they point out.
“Therefore, understanding the phenomenon of cross-reactivity and molecular mimicry may be helpful in postulating potential treatment behind not only tinnitus but also the rare events of vaccination associated hearing loss and other otologic manifestations,” the authors say.
Genetic predispositions and associated conditions may also play a significant role in determining whether an individual develops vaccine-induced tinnitus.
Stress and anxiety following COVID vaccination may also play a role, inasmuch as anxiety-related adverse events following vaccination have been reported. Vaccine-related anxiety as a potential cause of tinnitus developing after vaccination needs to be explored, they write.
Jury out on best management
How best to manage COVID vaccine-associated tinnitus also remains unclear, but it starts with a well-established diagnosis, the authors say.
A well-focused and detailed history and examination are essential, with particular emphasis placed on preexisting health conditions, specifically, autoimmune diseases, such as Hashimoto thyroiditis; otologic conditions, such as sensorineural hearing loss; glaucoma; and psychological well-being. According to the review, patients often present with a history of one or more of these disorders.
“However, any such association has not yet been established and requires further investigation to be concluded as potential risk factors for vaccine-induced tinnitus,” they caution.
Routine cranial nerve examination, otoscopy, Weber test, and Rinne test, which are used for tinnitus diagnosis in general, may be helpful for confirmation of vaccine-associated tinnitus.
Owing to the significant association between tinnitus and hearing impairment, audiology should also performed, the authors say.
Although treatments for non–vaccine-induced tinnitus vary significantly, corticosteroids are the top treatment choice for SARS-CoV-2 vaccine-induced tinnitus reported in the literature.
Trials of other drug and nondrug interventions that may uniquely help with vaccine-associated tinnitus are urgently needed, the authors say.
Summing up, the reviewers say, “Although the incidence of COVID-19 vaccine-associated tinnitus is rare, there is an overwhelming need to discern the precise pathophysiology and clinical management as a better understanding of adverse events may help in encountering vaccine hesitancy and hence fostering the COVID-19 global vaccination program.
“Despite the incidence of adverse events, the benefits of the SARS-CoV-2 vaccine in reducing hospitalization and deaths continue to outweigh the rare ramifications,” they conclude.
The research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s now known that tinnitus may be an unexpected side effect of SARS-CoV-2 vaccination, and there is an urgent need to understand the precise mechanisms and best treatment for vaccine-associated tinnitus, researchers say.
As of mid-September 2021, 12,247 cases of tinnitus, or ringing in the ears, following COVID-19 vaccination had been reported to the Vaccine Adverse Event Reporting System of the U.S. Centers for Disease Control and Prevention.
“Despite several cases of tinnitus being reported following SARS-CoV-2 vaccination, the precise pathophysiology is still not clear,” write Syed Hassan Ahmed, 3rd-year MBBS student, Dow University of Health Sciences, Karachi, Pakistan, and coauthors.
The researchers review what is known and unknown about SARS-CoV-2 vaccine-associated tinnitus in an article published online Feb. 11 in Annals of Medicine and Surgery.
Molecular mimicry?
The researchers say cross-reactivity between anti-spike SARS-CoV-2 antibodies and otologic antigens is one possibility, based on the mechanisms behind other COVID-19 vaccine–induced disorders and the phenomenon of molecular mimicry.
“The heptapeptide resemblance between coronavirus spike glycoprotein and numerous human proteins further supports molecular mimicry as a potential mechanism behind such vaccine-induced disorders,” they write.
Anti-spike antibodies may react with antigens anywhere along the auditory pathway and fuel an inflammatory reaction, they point out.
“Therefore, understanding the phenomenon of cross-reactivity and molecular mimicry may be helpful in postulating potential treatment behind not only tinnitus but also the rare events of vaccination associated hearing loss and other otologic manifestations,” the authors say.
Genetic predispositions and associated conditions may also play a significant role in determining whether an individual develops vaccine-induced tinnitus.
Stress and anxiety following COVID vaccination may also play a role, inasmuch as anxiety-related adverse events following vaccination have been reported. Vaccine-related anxiety as a potential cause of tinnitus developing after vaccination needs to be explored, they write.
Jury out on best management
How best to manage COVID vaccine-associated tinnitus also remains unclear, but it starts with a well-established diagnosis, the authors say.
A well-focused and detailed history and examination are essential, with particular emphasis placed on preexisting health conditions, specifically, autoimmune diseases, such as Hashimoto thyroiditis; otologic conditions, such as sensorineural hearing loss; glaucoma; and psychological well-being. According to the review, patients often present with a history of one or more of these disorders.
“However, any such association has not yet been established and requires further investigation to be concluded as potential risk factors for vaccine-induced tinnitus,” they caution.
Routine cranial nerve examination, otoscopy, Weber test, and Rinne test, which are used for tinnitus diagnosis in general, may be helpful for confirmation of vaccine-associated tinnitus.
Owing to the significant association between tinnitus and hearing impairment, audiology should also performed, the authors say.
Although treatments for non–vaccine-induced tinnitus vary significantly, corticosteroids are the top treatment choice for SARS-CoV-2 vaccine-induced tinnitus reported in the literature.
Trials of other drug and nondrug interventions that may uniquely help with vaccine-associated tinnitus are urgently needed, the authors say.
Summing up, the reviewers say, “Although the incidence of COVID-19 vaccine-associated tinnitus is rare, there is an overwhelming need to discern the precise pathophysiology and clinical management as a better understanding of adverse events may help in encountering vaccine hesitancy and hence fostering the COVID-19 global vaccination program.
“Despite the incidence of adverse events, the benefits of the SARS-CoV-2 vaccine in reducing hospitalization and deaths continue to outweigh the rare ramifications,” they conclude.
The research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF MEDICINE AND SURGERY
Biden administration’s new test-to-treat program pits pharmacists against physicians
The Biden administration’s new test-to-treat program is simple on the surface: if you feel like you may have COVID-19, go to a pharmacy, get tested, and, if positive, get treated with an antiviral medication on the spot.
One large physicians’ group is concerned that the program leaves doctors on the margins, and may put patients at risk if there are adverse effects from the medications. Pharmacists groups, on the other hand, say the program is too restrictive, according to an article by the research group Advisory Board.
Recently, the White House announced that more than 1,000 pharmacy clinics across the United States had registered to participate in the initiative, according to CNN. Ordering of the drugs is underway in many of these clinics, a White House official told the network.
Besides retail clinics in chain pharmacies, the antivirals will also be available in community health centers, long-term-care facilities, and Veterans Health Administration clinics, according to a statement from the U.S. Department of Health and Human Services.
The two antiviral pills authorized by the U.S. Food and Drug Administration include Pfizer’s Paxlovid, for people 12 and older, and Merck’s molnupiravir, for adults. Either drug has to be taken within 5 days after symptoms appear to be effective in preventing serious illness.
The need for speed is a major reason why the government chose to work with retail clinics that are more accessible than most primary care offices. However, the American Medical Association (AMA), the National Community Pharmacists Association (NCPA), and the American Pharmacists Association (APhA) have publicly criticized the administration’s approach.
The pharmacists’ groups are concerned that the program is limited only to pharmacies with clinics on site, thus restricting the number of pharmacies qualified to participate. Fourteen pharmacy groups, including the NCPA and the APhA, have also sent a letter to the Biden administration urging it to remove barriers to pharmacies ordering the medications.
The groups also want permission as “clinically trained medication experts” to prescribe the drugs and ensure their safe use.
The AMA on March 4 took issue with the prescribing component, saying that “the pharmacy-based clinic component of the test-to-treat plan flouts patient safety and risks significant negative health outcomes.”
In the AMA’s view, prescribing Paxlovid without a patient’s physician being present poses a risk for adverse drug interactions, as neither the nurse practitioners in retail clinics nor the pharmacists who dispense the drug have full knowledge of a patient›s medical history.
The next day, the AMA released another statement, saying it was reassured by comments from administration officials “that patients who have access to a regular source of care should contact their physician shortly after testing positive for COVID-19 to assess their treatment options.”
“Traditional doctor-only approach”
Having patients call their doctors after testing positive for COVID in a pharmacy “strikes me as unnecessary in the vast majority of cases, and it will delay treatment,” Robert Wachter, MD, professor and chair of the department of medicine at the University of California San Francisco, said in an interview. “In this case, it seems like the AMA is taking a very traditional doctor-only approach. And the world has changed. It’s much more of a team sport than an individual sport, the way it was years ago.”
Dr. Wachter said he has the utmost respect for pharmacists’ ability to screen prescriptions for adverse drug interactions. “We’re required to do medication reconciliation when patients see us,” he says. “And in many hospitals, we delegate that to pharmacists. They’re at least as good at it if not better than physicians are.”
While it’s essential to know what other medications a patient is taking, he noted, pharmacies have computer records of all the prescriptions they’ve filled for patients. In addition, pharmacies have access to complete medication histories through Surescripts, the company that enables electronic prescribing transactions between prescribers and pharmacies.
Drug interactions “not trivial”
Preeti Malani, MD, the chief health officer and a professor of medicine in the division of infectious diseases at the University of Michigan in Ann Arbor, told this news organization that the potential interactions between Paxlovid and some other medications are “not trivial.”
However, she said, “The really dangerous drugs are the ones for people who have had organ transplants and the like. Those aren’t individuals who are going to shop at a pharmacy.”
Besides the antirejection drugs, Dr. Wachter said, there can be serious interactions with cholesterol-lowering medications. If a person is taking Lipitor, for instance, “Someone would have to make the decision on whether it’s ok for me to stop it for a while, or to lower the dose. But I trust the pharmacist to do that as well as anybody.”
Except for these potential drug interactions with Paxlovid, the antiviral medications are “quite safe,” he said, adding that being able to treat people who test positive for COVID-19 right away is a big advantage of the test-to-treat program, considering how difficult it is for many people to get access to a doctor. That delay could mean that the antivirals are not prescribed and taken until they are no longer effective.
Both Dr. Wachter and Dr. Malani said that the widespread distribution of pharmacies and their extended hours are other big pluses, especially for people who can’t easily leave work or travel far to visit a physician.
Dr. Malani cautioned that there are still kinks to work out in the test-to-treat program. It will be a while before the retail clinics all have the antiviral drugs, and many pharmacies don’t have clinics on site.
Still, she said people can still go to their physicians to be tested, and presumably those doctors can also write antiviral prescriptions. But it’s not clear where the antivirals will be available in the near term.
“Right now, we’re playing catch-up,” Dr. Malani said. “But pharmacies are an important piece of the puzzle.”
Looking at the big picture, she said, “We know that neither vaccination nor natural infection provides long lasting immunity, and so there will be a role for antivirals in order to make this a manageable illness. And when you’re talking about millions of cases, as we were having a few months ago, the health system can’t field all those patients. So we do need a system where I can go to a pharmacy and get a test and treatment.”
A version of this article first appeared on Medscape.com.
The Biden administration’s new test-to-treat program is simple on the surface: if you feel like you may have COVID-19, go to a pharmacy, get tested, and, if positive, get treated with an antiviral medication on the spot.
One large physicians’ group is concerned that the program leaves doctors on the margins, and may put patients at risk if there are adverse effects from the medications. Pharmacists groups, on the other hand, say the program is too restrictive, according to an article by the research group Advisory Board.
Recently, the White House announced that more than 1,000 pharmacy clinics across the United States had registered to participate in the initiative, according to CNN. Ordering of the drugs is underway in many of these clinics, a White House official told the network.
Besides retail clinics in chain pharmacies, the antivirals will also be available in community health centers, long-term-care facilities, and Veterans Health Administration clinics, according to a statement from the U.S. Department of Health and Human Services.
The two antiviral pills authorized by the U.S. Food and Drug Administration include Pfizer’s Paxlovid, for people 12 and older, and Merck’s molnupiravir, for adults. Either drug has to be taken within 5 days after symptoms appear to be effective in preventing serious illness.
The need for speed is a major reason why the government chose to work with retail clinics that are more accessible than most primary care offices. However, the American Medical Association (AMA), the National Community Pharmacists Association (NCPA), and the American Pharmacists Association (APhA) have publicly criticized the administration’s approach.
The pharmacists’ groups are concerned that the program is limited only to pharmacies with clinics on site, thus restricting the number of pharmacies qualified to participate. Fourteen pharmacy groups, including the NCPA and the APhA, have also sent a letter to the Biden administration urging it to remove barriers to pharmacies ordering the medications.
The groups also want permission as “clinically trained medication experts” to prescribe the drugs and ensure their safe use.
The AMA on March 4 took issue with the prescribing component, saying that “the pharmacy-based clinic component of the test-to-treat plan flouts patient safety and risks significant negative health outcomes.”
In the AMA’s view, prescribing Paxlovid without a patient’s physician being present poses a risk for adverse drug interactions, as neither the nurse practitioners in retail clinics nor the pharmacists who dispense the drug have full knowledge of a patient›s medical history.
The next day, the AMA released another statement, saying it was reassured by comments from administration officials “that patients who have access to a regular source of care should contact their physician shortly after testing positive for COVID-19 to assess their treatment options.”
“Traditional doctor-only approach”
Having patients call their doctors after testing positive for COVID in a pharmacy “strikes me as unnecessary in the vast majority of cases, and it will delay treatment,” Robert Wachter, MD, professor and chair of the department of medicine at the University of California San Francisco, said in an interview. “In this case, it seems like the AMA is taking a very traditional doctor-only approach. And the world has changed. It’s much more of a team sport than an individual sport, the way it was years ago.”
Dr. Wachter said he has the utmost respect for pharmacists’ ability to screen prescriptions for adverse drug interactions. “We’re required to do medication reconciliation when patients see us,” he says. “And in many hospitals, we delegate that to pharmacists. They’re at least as good at it if not better than physicians are.”
While it’s essential to know what other medications a patient is taking, he noted, pharmacies have computer records of all the prescriptions they’ve filled for patients. In addition, pharmacies have access to complete medication histories through Surescripts, the company that enables electronic prescribing transactions between prescribers and pharmacies.
Drug interactions “not trivial”
Preeti Malani, MD, the chief health officer and a professor of medicine in the division of infectious diseases at the University of Michigan in Ann Arbor, told this news organization that the potential interactions between Paxlovid and some other medications are “not trivial.”
However, she said, “The really dangerous drugs are the ones for people who have had organ transplants and the like. Those aren’t individuals who are going to shop at a pharmacy.”
Besides the antirejection drugs, Dr. Wachter said, there can be serious interactions with cholesterol-lowering medications. If a person is taking Lipitor, for instance, “Someone would have to make the decision on whether it’s ok for me to stop it for a while, or to lower the dose. But I trust the pharmacist to do that as well as anybody.”
Except for these potential drug interactions with Paxlovid, the antiviral medications are “quite safe,” he said, adding that being able to treat people who test positive for COVID-19 right away is a big advantage of the test-to-treat program, considering how difficult it is for many people to get access to a doctor. That delay could mean that the antivirals are not prescribed and taken until they are no longer effective.
Both Dr. Wachter and Dr. Malani said that the widespread distribution of pharmacies and their extended hours are other big pluses, especially for people who can’t easily leave work or travel far to visit a physician.
Dr. Malani cautioned that there are still kinks to work out in the test-to-treat program. It will be a while before the retail clinics all have the antiviral drugs, and many pharmacies don’t have clinics on site.
Still, she said people can still go to their physicians to be tested, and presumably those doctors can also write antiviral prescriptions. But it’s not clear where the antivirals will be available in the near term.
“Right now, we’re playing catch-up,” Dr. Malani said. “But pharmacies are an important piece of the puzzle.”
Looking at the big picture, she said, “We know that neither vaccination nor natural infection provides long lasting immunity, and so there will be a role for antivirals in order to make this a manageable illness. And when you’re talking about millions of cases, as we were having a few months ago, the health system can’t field all those patients. So we do need a system where I can go to a pharmacy and get a test and treatment.”
A version of this article first appeared on Medscape.com.
The Biden administration’s new test-to-treat program is simple on the surface: if you feel like you may have COVID-19, go to a pharmacy, get tested, and, if positive, get treated with an antiviral medication on the spot.
One large physicians’ group is concerned that the program leaves doctors on the margins, and may put patients at risk if there are adverse effects from the medications. Pharmacists groups, on the other hand, say the program is too restrictive, according to an article by the research group Advisory Board.
Recently, the White House announced that more than 1,000 pharmacy clinics across the United States had registered to participate in the initiative, according to CNN. Ordering of the drugs is underway in many of these clinics, a White House official told the network.
Besides retail clinics in chain pharmacies, the antivirals will also be available in community health centers, long-term-care facilities, and Veterans Health Administration clinics, according to a statement from the U.S. Department of Health and Human Services.
The two antiviral pills authorized by the U.S. Food and Drug Administration include Pfizer’s Paxlovid, for people 12 and older, and Merck’s molnupiravir, for adults. Either drug has to be taken within 5 days after symptoms appear to be effective in preventing serious illness.
The need for speed is a major reason why the government chose to work with retail clinics that are more accessible than most primary care offices. However, the American Medical Association (AMA), the National Community Pharmacists Association (NCPA), and the American Pharmacists Association (APhA) have publicly criticized the administration’s approach.
The pharmacists’ groups are concerned that the program is limited only to pharmacies with clinics on site, thus restricting the number of pharmacies qualified to participate. Fourteen pharmacy groups, including the NCPA and the APhA, have also sent a letter to the Biden administration urging it to remove barriers to pharmacies ordering the medications.
The groups also want permission as “clinically trained medication experts” to prescribe the drugs and ensure their safe use.
The AMA on March 4 took issue with the prescribing component, saying that “the pharmacy-based clinic component of the test-to-treat plan flouts patient safety and risks significant negative health outcomes.”
In the AMA’s view, prescribing Paxlovid without a patient’s physician being present poses a risk for adverse drug interactions, as neither the nurse practitioners in retail clinics nor the pharmacists who dispense the drug have full knowledge of a patient›s medical history.
The next day, the AMA released another statement, saying it was reassured by comments from administration officials “that patients who have access to a regular source of care should contact their physician shortly after testing positive for COVID-19 to assess their treatment options.”
“Traditional doctor-only approach”
Having patients call their doctors after testing positive for COVID in a pharmacy “strikes me as unnecessary in the vast majority of cases, and it will delay treatment,” Robert Wachter, MD, professor and chair of the department of medicine at the University of California San Francisco, said in an interview. “In this case, it seems like the AMA is taking a very traditional doctor-only approach. And the world has changed. It’s much more of a team sport than an individual sport, the way it was years ago.”
Dr. Wachter said he has the utmost respect for pharmacists’ ability to screen prescriptions for adverse drug interactions. “We’re required to do medication reconciliation when patients see us,” he says. “And in many hospitals, we delegate that to pharmacists. They’re at least as good at it if not better than physicians are.”
While it’s essential to know what other medications a patient is taking, he noted, pharmacies have computer records of all the prescriptions they’ve filled for patients. In addition, pharmacies have access to complete medication histories through Surescripts, the company that enables electronic prescribing transactions between prescribers and pharmacies.
Drug interactions “not trivial”
Preeti Malani, MD, the chief health officer and a professor of medicine in the division of infectious diseases at the University of Michigan in Ann Arbor, told this news organization that the potential interactions between Paxlovid and some other medications are “not trivial.”
However, she said, “The really dangerous drugs are the ones for people who have had organ transplants and the like. Those aren’t individuals who are going to shop at a pharmacy.”
Besides the antirejection drugs, Dr. Wachter said, there can be serious interactions with cholesterol-lowering medications. If a person is taking Lipitor, for instance, “Someone would have to make the decision on whether it’s ok for me to stop it for a while, or to lower the dose. But I trust the pharmacist to do that as well as anybody.”
Except for these potential drug interactions with Paxlovid, the antiviral medications are “quite safe,” he said, adding that being able to treat people who test positive for COVID-19 right away is a big advantage of the test-to-treat program, considering how difficult it is for many people to get access to a doctor. That delay could mean that the antivirals are not prescribed and taken until they are no longer effective.
Both Dr. Wachter and Dr. Malani said that the widespread distribution of pharmacies and their extended hours are other big pluses, especially for people who can’t easily leave work or travel far to visit a physician.
Dr. Malani cautioned that there are still kinks to work out in the test-to-treat program. It will be a while before the retail clinics all have the antiviral drugs, and many pharmacies don’t have clinics on site.
Still, she said people can still go to their physicians to be tested, and presumably those doctors can also write antiviral prescriptions. But it’s not clear where the antivirals will be available in the near term.
“Right now, we’re playing catch-up,” Dr. Malani said. “But pharmacies are an important piece of the puzzle.”
Looking at the big picture, she said, “We know that neither vaccination nor natural infection provides long lasting immunity, and so there will be a role for antivirals in order to make this a manageable illness. And when you’re talking about millions of cases, as we were having a few months ago, the health system can’t field all those patients. So we do need a system where I can go to a pharmacy and get a test and treatment.”
A version of this article first appeared on Medscape.com.
COVID-19 often more severe with congenital heart defects
Adults with a congenital heart defect (CHD) are at increased risk for serious illness and death when hospitalized with COVID-19, making vaccination and other preventive measures even important in this population, say researchers with the Centers for Disease Control and Prevention.
“We found that hospitalized patients with heart defects are up to twice as likely to have critical outcomes of COVID-19 illness (admission to the intensive care unit, use of a ventilator to help with breathing, or death) compared to hospitalized COVID-19 patients without heart defects,” Karrie Downing, MPH, epidemiologist, with the CDC’s National Center on Birth Defects and Developmental Disabilities, said in an interview.
“Additionally, we learned that people with hearts defects who were older or who also had other conditions like heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity were the most likely to have critical COVID-19 illness, but children and adults with heart defects without these other conditions were still at increased risk,” Ms. Downing said.
The message for health care providers is clear: “Encourage your patients with heart defects to get vaccinated and discuss with your patients the need for other preventive measures to avoid infection that may progress to severe COVID-19 illness,” Ms. Downing added.
The study was published online March 7, 2022, in Circulation.
The researchers analyzed data on 235,638 patients hospitalized with COVID-19 between March 2020 and January 2021, including 421 (0.2%) with CHD. Most CHD patients were older than 30 years (73%) and 61% were men, with 55% non-Hispanic white, 19% Hispanic and 16% non-Hispanic Black.
Overall, 68% of CHD patients had at least one comorbidity, as did 59% of patients without CHD.
Rates of ICU admission were higher in the CHD group (54% vs. 43%), as were rates of invasive mechanical ventilation (24% vs. 15%) and in-hospital death (11% vs. 7%).
After accounting for patient characteristics, ICU admission, invasive mechanical ventilation and death were more prevalent among COVID-19 patients with rather than without CHD, with adjusted prevalence ratios of 1.4, 1.8 and 2.0, respectively.
When stratified by high-risk characteristics, prevalence estimates for ICU admission, invasive mechanical ventilation and death remained higher among patients with COVID-19 and CHD across nearly all strata, including younger age groups and those without heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity, the researchers reported.
Ms. Downing said more work is needed to identify why the clinical course of COVID-19 disease results in admission to the ICU, the need for a ventilator, or death for some hospitalized patients with CHD and not for others.
“There could be a number of social, environmental, economic, medical, and genetic factors playing a role. But staying up to date with COVID-19 vaccines and following preventive measures for COVID-19 are effective ways to reduce the risk of severe illness from COVID-19,” Ms. Downing said.
The study had no specific funding. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Adults with a congenital heart defect (CHD) are at increased risk for serious illness and death when hospitalized with COVID-19, making vaccination and other preventive measures even important in this population, say researchers with the Centers for Disease Control and Prevention.
“We found that hospitalized patients with heart defects are up to twice as likely to have critical outcomes of COVID-19 illness (admission to the intensive care unit, use of a ventilator to help with breathing, or death) compared to hospitalized COVID-19 patients without heart defects,” Karrie Downing, MPH, epidemiologist, with the CDC’s National Center on Birth Defects and Developmental Disabilities, said in an interview.
“Additionally, we learned that people with hearts defects who were older or who also had other conditions like heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity were the most likely to have critical COVID-19 illness, but children and adults with heart defects without these other conditions were still at increased risk,” Ms. Downing said.
The message for health care providers is clear: “Encourage your patients with heart defects to get vaccinated and discuss with your patients the need for other preventive measures to avoid infection that may progress to severe COVID-19 illness,” Ms. Downing added.
The study was published online March 7, 2022, in Circulation.
The researchers analyzed data on 235,638 patients hospitalized with COVID-19 between March 2020 and January 2021, including 421 (0.2%) with CHD. Most CHD patients were older than 30 years (73%) and 61% were men, with 55% non-Hispanic white, 19% Hispanic and 16% non-Hispanic Black.
Overall, 68% of CHD patients had at least one comorbidity, as did 59% of patients without CHD.
Rates of ICU admission were higher in the CHD group (54% vs. 43%), as were rates of invasive mechanical ventilation (24% vs. 15%) and in-hospital death (11% vs. 7%).
After accounting for patient characteristics, ICU admission, invasive mechanical ventilation and death were more prevalent among COVID-19 patients with rather than without CHD, with adjusted prevalence ratios of 1.4, 1.8 and 2.0, respectively.
When stratified by high-risk characteristics, prevalence estimates for ICU admission, invasive mechanical ventilation and death remained higher among patients with COVID-19 and CHD across nearly all strata, including younger age groups and those without heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity, the researchers reported.
Ms. Downing said more work is needed to identify why the clinical course of COVID-19 disease results in admission to the ICU, the need for a ventilator, or death for some hospitalized patients with CHD and not for others.
“There could be a number of social, environmental, economic, medical, and genetic factors playing a role. But staying up to date with COVID-19 vaccines and following preventive measures for COVID-19 are effective ways to reduce the risk of severe illness from COVID-19,” Ms. Downing said.
The study had no specific funding. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Adults with a congenital heart defect (CHD) are at increased risk for serious illness and death when hospitalized with COVID-19, making vaccination and other preventive measures even important in this population, say researchers with the Centers for Disease Control and Prevention.
“We found that hospitalized patients with heart defects are up to twice as likely to have critical outcomes of COVID-19 illness (admission to the intensive care unit, use of a ventilator to help with breathing, or death) compared to hospitalized COVID-19 patients without heart defects,” Karrie Downing, MPH, epidemiologist, with the CDC’s National Center on Birth Defects and Developmental Disabilities, said in an interview.
“Additionally, we learned that people with hearts defects who were older or who also had other conditions like heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity were the most likely to have critical COVID-19 illness, but children and adults with heart defects without these other conditions were still at increased risk,” Ms. Downing said.
The message for health care providers is clear: “Encourage your patients with heart defects to get vaccinated and discuss with your patients the need for other preventive measures to avoid infection that may progress to severe COVID-19 illness,” Ms. Downing added.
The study was published online March 7, 2022, in Circulation.
The researchers analyzed data on 235,638 patients hospitalized with COVID-19 between March 2020 and January 2021, including 421 (0.2%) with CHD. Most CHD patients were older than 30 years (73%) and 61% were men, with 55% non-Hispanic white, 19% Hispanic and 16% non-Hispanic Black.
Overall, 68% of CHD patients had at least one comorbidity, as did 59% of patients without CHD.
Rates of ICU admission were higher in the CHD group (54% vs. 43%), as were rates of invasive mechanical ventilation (24% vs. 15%) and in-hospital death (11% vs. 7%).
After accounting for patient characteristics, ICU admission, invasive mechanical ventilation and death were more prevalent among COVID-19 patients with rather than without CHD, with adjusted prevalence ratios of 1.4, 1.8 and 2.0, respectively.
When stratified by high-risk characteristics, prevalence estimates for ICU admission, invasive mechanical ventilation and death remained higher among patients with COVID-19 and CHD across nearly all strata, including younger age groups and those without heart failure, pulmonary hypertension, Down syndrome, diabetes, or obesity, the researchers reported.
Ms. Downing said more work is needed to identify why the clinical course of COVID-19 disease results in admission to the ICU, the need for a ventilator, or death for some hospitalized patients with CHD and not for others.
“There could be a number of social, environmental, economic, medical, and genetic factors playing a role. But staying up to date with COVID-19 vaccines and following preventive measures for COVID-19 are effective ways to reduce the risk of severe illness from COVID-19,” Ms. Downing said.
The study had no specific funding. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
COVID-19 vax effectiveness quantified in immunosuppressed patients
People taking immunosuppressive drugs benefit significantly from SARS-CoV-2 vaccines approved in the United States to prevent and reduce the severity of COVID-19, according to the first study to quantify the vaccines’ real-world effectiveness in this population.
Researchers’ analysis of the electronic medical records of more than 150,000 people in the University of Michigan’s health care system showed that even after becoming fully vaccinated, immunosuppressed individuals remain at higher risk for COVID-19 than are vaccinated people in the wider population who aren’t receiving immunosuppressive therapy. However, they still derive benefit from vaccination, particularly when bolstered with a booster dose.
The study, published online in Annals of the Rheumatic Diseases, also claims to be the first to show that the Moderna (mRNA-1273) vaccine is as effective as the Pfizer-BioNTech (BNT162b2) vaccine for people taking immunosuppressants.
“Booster doses are effective and important for individuals on immunosuppressants,” corresponding author Lili Zhao, PhD, a research associate professor in biostatistics at the University of Michigan, Ann Arbor, said in an interview. “Previous studies focused mostly on the Pfizer vaccine, whereas our study is the first that also investigates the Moderna vaccine in a large, immunosuppressed population.”
The epidemiologic study included 154,519 fully vaccinated and unvaccinated adults in the Michigan Medicine electronic health record database. Participants were considered fully vaccinated if they were within 2 weeks of having received a second dose of the Pfizer-BioNTech and Moderna vaccines or the single-dose Johnson & Johnson (Ad26.COV2.S) vaccine. The study population included 5,536 immunosuppressed patients; of those, 4,283 were fully vaccinated, and 1,253 were unvaccinated.
The researchers focused on data collected from Jan. 1 to Dec. 7, 2021, so the study doesn’t cover the Omicron variant. “The conclusions for immunosuppressed individuals are likely to remain the same during the Omicron period,” Dr. Zhao said. “We are currently investigating this.” Johnson & Johnson paused production of its vaccine in February.
The researchers found that, among unvaccinated individuals, the immunosuppressed group had about a 40% higher risk of infection than did the immunocompetent patients (hazard ratio, 1.398; 95% confidence interval, 1.068-1.829; P = .0075) but a similar risk of COVID-19 hospitalization (HR, 0.951; 95% CI, 0.435-2.080; P = .9984). For the fully vaccinated, the gap was significantly wider: Immunosuppressed patients had more than double the risk of infection (HR, 2.173; 95% CI, 1.690-2.794; P < .0001) and almost five times the risk of hospitalization (HR, 4.861; 95% CI, 2.238-10.56; P < .0001), compared with immunocompetent patients.
However, among immunosuppressed individuals, the vaccinations significantly lowered risks, compared with not being vaccinated. There was a statistically significant 45% lower risk of infection (HR, 0.550; 95% CI, 0.387-0.781; P = .001) and similarly lower risk of hospitalization that did not reach statistical significance (HR, 0.534; 95% CI, 0.196-1.452; P = .3724).
When those immunosuppressed patients received a booster dose, their protection against COVID-19 improved, compared with their immunosuppressed counterparts who didn’t get a booster, with a 58% lower risk of infection after adjustment for age, gender, race, and Charlson Comorbidity Index (adjusted HR, 0.42; 95% CI, 0.24-0.76; P = .0037). The study included nearly 4 months of data after the Centers for Disease Control and Prevention recommended a booster dose of the Moderna and Pfizer-BioNTech vaccines for immunocompromised individuals in August 2021. Among the immunosuppressed patients, 38.5% had received a booster dose.
There also was no apparent difference in the effectiveness between the Moderna and Pfizer-BioNTech vaccines, with adjusted hazard ratios showing 41%-48% lower risk of infection. Too few individuals in the study were vaccinated with the Johnson & Johnson vaccine to enable a sufficiently powered calculation of its effectiveness.
Other studies reach similar conclusions
The study findings fall into line with other studies of patient populations on immunosuppressants. A retrospective cohort study of Veterans Affairs patients with inflammatory bowel disease who were taking immunosuppressants, published in Gastroenterology, found that full vaccination with either Moderna and Pfizer-BioNTech vaccines was about 80% effective. Another retrospective cohort study of data from the National COVID Cohort Collaborative, published in JAMA Internal Medicine, reported that full vaccination significantly reduced the risk of COVID-19 breakthrough infection regardless of immune status. Immunosuppressed patients in this study had higher rates of breakthrough infections than immunocompetent patients, but the disparities were in line with what Dr. Zhao and the University of Michigan researchers reported.
A review of 23 studies of COVID-19 vaccinations, published in Lancet Global Health, found that immunocompromised people – 1,722 of whom were included in the studies – had lower rates of producing antibodies after two vaccine doses than did immunocompetent people, ranging from 27% to 92%, depending on the nature of their immunocompromised status, compared with 99% for the immunocompetent.
Strengths and limitations
One strength of the Michigan study is the quality of data, which were drawn from the Michigan Medicine electronic health record, Dr. Zhao said. “So, we know who received the vaccine and who didn’t. We also have access to data on patient health conditions, such as comorbidities, in addition to demographic variables (age, gender, and race), which were controlled in making fair comparisons between immunosuppressants and immunocompetent groups.”
Alfred Kim, MD, PhD, an assistant professor of internal medicine and rheumatology at Washington University in St. Louis, who was not involved with the study, credited Dr. Zhao and associates for delivering the first data that specifically quantified COVID-19 risk reduction in a large study population. Although he noted that the large sample size and the design reduced the chances of confounding and were strengths, he said in an interview that “lumping” the patients taking immunosuppressive drugs into one group was a weakness of the study.
“Clearly, there are certain medications (B-cell depleters, mycophenolate, for example) that carry the greatest risk of poor antibody responses post vaccination,” he said. “One would have to guess that the greatest risk of breakthrough infections continues to be in those patients taking these high-risk medications.”
Another possible problem, which the authors acknowledged, is spotty SARS-CoV-2 testing of study participants – “a systemic issue,” Dr. Kim noted.
“The easiest and most durable way to reduce the risk of getting COVID-19 is through vaccination, period,” he said. “Now we have infection-rates data from a real-world study cohort to prove this. Furthermore, boosting clearly provides additional benefit to this population.”
The National Institute of Allergy and Infectious Diseases provided funding for the study. Dr. Zhao, Dr. Zhao’s coauthors, and Kim disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People taking immunosuppressive drugs benefit significantly from SARS-CoV-2 vaccines approved in the United States to prevent and reduce the severity of COVID-19, according to the first study to quantify the vaccines’ real-world effectiveness in this population.
Researchers’ analysis of the electronic medical records of more than 150,000 people in the University of Michigan’s health care system showed that even after becoming fully vaccinated, immunosuppressed individuals remain at higher risk for COVID-19 than are vaccinated people in the wider population who aren’t receiving immunosuppressive therapy. However, they still derive benefit from vaccination, particularly when bolstered with a booster dose.
The study, published online in Annals of the Rheumatic Diseases, also claims to be the first to show that the Moderna (mRNA-1273) vaccine is as effective as the Pfizer-BioNTech (BNT162b2) vaccine for people taking immunosuppressants.
“Booster doses are effective and important for individuals on immunosuppressants,” corresponding author Lili Zhao, PhD, a research associate professor in biostatistics at the University of Michigan, Ann Arbor, said in an interview. “Previous studies focused mostly on the Pfizer vaccine, whereas our study is the first that also investigates the Moderna vaccine in a large, immunosuppressed population.”
The epidemiologic study included 154,519 fully vaccinated and unvaccinated adults in the Michigan Medicine electronic health record database. Participants were considered fully vaccinated if they were within 2 weeks of having received a second dose of the Pfizer-BioNTech and Moderna vaccines or the single-dose Johnson & Johnson (Ad26.COV2.S) vaccine. The study population included 5,536 immunosuppressed patients; of those, 4,283 were fully vaccinated, and 1,253 were unvaccinated.
The researchers focused on data collected from Jan. 1 to Dec. 7, 2021, so the study doesn’t cover the Omicron variant. “The conclusions for immunosuppressed individuals are likely to remain the same during the Omicron period,” Dr. Zhao said. “We are currently investigating this.” Johnson & Johnson paused production of its vaccine in February.
The researchers found that, among unvaccinated individuals, the immunosuppressed group had about a 40% higher risk of infection than did the immunocompetent patients (hazard ratio, 1.398; 95% confidence interval, 1.068-1.829; P = .0075) but a similar risk of COVID-19 hospitalization (HR, 0.951; 95% CI, 0.435-2.080; P = .9984). For the fully vaccinated, the gap was significantly wider: Immunosuppressed patients had more than double the risk of infection (HR, 2.173; 95% CI, 1.690-2.794; P < .0001) and almost five times the risk of hospitalization (HR, 4.861; 95% CI, 2.238-10.56; P < .0001), compared with immunocompetent patients.
However, among immunosuppressed individuals, the vaccinations significantly lowered risks, compared with not being vaccinated. There was a statistically significant 45% lower risk of infection (HR, 0.550; 95% CI, 0.387-0.781; P = .001) and similarly lower risk of hospitalization that did not reach statistical significance (HR, 0.534; 95% CI, 0.196-1.452; P = .3724).
When those immunosuppressed patients received a booster dose, their protection against COVID-19 improved, compared with their immunosuppressed counterparts who didn’t get a booster, with a 58% lower risk of infection after adjustment for age, gender, race, and Charlson Comorbidity Index (adjusted HR, 0.42; 95% CI, 0.24-0.76; P = .0037). The study included nearly 4 months of data after the Centers for Disease Control and Prevention recommended a booster dose of the Moderna and Pfizer-BioNTech vaccines for immunocompromised individuals in August 2021. Among the immunosuppressed patients, 38.5% had received a booster dose.
There also was no apparent difference in the effectiveness between the Moderna and Pfizer-BioNTech vaccines, with adjusted hazard ratios showing 41%-48% lower risk of infection. Too few individuals in the study were vaccinated with the Johnson & Johnson vaccine to enable a sufficiently powered calculation of its effectiveness.
Other studies reach similar conclusions
The study findings fall into line with other studies of patient populations on immunosuppressants. A retrospective cohort study of Veterans Affairs patients with inflammatory bowel disease who were taking immunosuppressants, published in Gastroenterology, found that full vaccination with either Moderna and Pfizer-BioNTech vaccines was about 80% effective. Another retrospective cohort study of data from the National COVID Cohort Collaborative, published in JAMA Internal Medicine, reported that full vaccination significantly reduced the risk of COVID-19 breakthrough infection regardless of immune status. Immunosuppressed patients in this study had higher rates of breakthrough infections than immunocompetent patients, but the disparities were in line with what Dr. Zhao and the University of Michigan researchers reported.
A review of 23 studies of COVID-19 vaccinations, published in Lancet Global Health, found that immunocompromised people – 1,722 of whom were included in the studies – had lower rates of producing antibodies after two vaccine doses than did immunocompetent people, ranging from 27% to 92%, depending on the nature of their immunocompromised status, compared with 99% for the immunocompetent.
Strengths and limitations
One strength of the Michigan study is the quality of data, which were drawn from the Michigan Medicine electronic health record, Dr. Zhao said. “So, we know who received the vaccine and who didn’t. We also have access to data on patient health conditions, such as comorbidities, in addition to demographic variables (age, gender, and race), which were controlled in making fair comparisons between immunosuppressants and immunocompetent groups.”
Alfred Kim, MD, PhD, an assistant professor of internal medicine and rheumatology at Washington University in St. Louis, who was not involved with the study, credited Dr. Zhao and associates for delivering the first data that specifically quantified COVID-19 risk reduction in a large study population. Although he noted that the large sample size and the design reduced the chances of confounding and were strengths, he said in an interview that “lumping” the patients taking immunosuppressive drugs into one group was a weakness of the study.
“Clearly, there are certain medications (B-cell depleters, mycophenolate, for example) that carry the greatest risk of poor antibody responses post vaccination,” he said. “One would have to guess that the greatest risk of breakthrough infections continues to be in those patients taking these high-risk medications.”
Another possible problem, which the authors acknowledged, is spotty SARS-CoV-2 testing of study participants – “a systemic issue,” Dr. Kim noted.
“The easiest and most durable way to reduce the risk of getting COVID-19 is through vaccination, period,” he said. “Now we have infection-rates data from a real-world study cohort to prove this. Furthermore, boosting clearly provides additional benefit to this population.”
The National Institute of Allergy and Infectious Diseases provided funding for the study. Dr. Zhao, Dr. Zhao’s coauthors, and Kim disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People taking immunosuppressive drugs benefit significantly from SARS-CoV-2 vaccines approved in the United States to prevent and reduce the severity of COVID-19, according to the first study to quantify the vaccines’ real-world effectiveness in this population.
Researchers’ analysis of the electronic medical records of more than 150,000 people in the University of Michigan’s health care system showed that even after becoming fully vaccinated, immunosuppressed individuals remain at higher risk for COVID-19 than are vaccinated people in the wider population who aren’t receiving immunosuppressive therapy. However, they still derive benefit from vaccination, particularly when bolstered with a booster dose.
The study, published online in Annals of the Rheumatic Diseases, also claims to be the first to show that the Moderna (mRNA-1273) vaccine is as effective as the Pfizer-BioNTech (BNT162b2) vaccine for people taking immunosuppressants.
“Booster doses are effective and important for individuals on immunosuppressants,” corresponding author Lili Zhao, PhD, a research associate professor in biostatistics at the University of Michigan, Ann Arbor, said in an interview. “Previous studies focused mostly on the Pfizer vaccine, whereas our study is the first that also investigates the Moderna vaccine in a large, immunosuppressed population.”
The epidemiologic study included 154,519 fully vaccinated and unvaccinated adults in the Michigan Medicine electronic health record database. Participants were considered fully vaccinated if they were within 2 weeks of having received a second dose of the Pfizer-BioNTech and Moderna vaccines or the single-dose Johnson & Johnson (Ad26.COV2.S) vaccine. The study population included 5,536 immunosuppressed patients; of those, 4,283 were fully vaccinated, and 1,253 were unvaccinated.
The researchers focused on data collected from Jan. 1 to Dec. 7, 2021, so the study doesn’t cover the Omicron variant. “The conclusions for immunosuppressed individuals are likely to remain the same during the Omicron period,” Dr. Zhao said. “We are currently investigating this.” Johnson & Johnson paused production of its vaccine in February.
The researchers found that, among unvaccinated individuals, the immunosuppressed group had about a 40% higher risk of infection than did the immunocompetent patients (hazard ratio, 1.398; 95% confidence interval, 1.068-1.829; P = .0075) but a similar risk of COVID-19 hospitalization (HR, 0.951; 95% CI, 0.435-2.080; P = .9984). For the fully vaccinated, the gap was significantly wider: Immunosuppressed patients had more than double the risk of infection (HR, 2.173; 95% CI, 1.690-2.794; P < .0001) and almost five times the risk of hospitalization (HR, 4.861; 95% CI, 2.238-10.56; P < .0001), compared with immunocompetent patients.
However, among immunosuppressed individuals, the vaccinations significantly lowered risks, compared with not being vaccinated. There was a statistically significant 45% lower risk of infection (HR, 0.550; 95% CI, 0.387-0.781; P = .001) and similarly lower risk of hospitalization that did not reach statistical significance (HR, 0.534; 95% CI, 0.196-1.452; P = .3724).
When those immunosuppressed patients received a booster dose, their protection against COVID-19 improved, compared with their immunosuppressed counterparts who didn’t get a booster, with a 58% lower risk of infection after adjustment for age, gender, race, and Charlson Comorbidity Index (adjusted HR, 0.42; 95% CI, 0.24-0.76; P = .0037). The study included nearly 4 months of data after the Centers for Disease Control and Prevention recommended a booster dose of the Moderna and Pfizer-BioNTech vaccines for immunocompromised individuals in August 2021. Among the immunosuppressed patients, 38.5% had received a booster dose.
There also was no apparent difference in the effectiveness between the Moderna and Pfizer-BioNTech vaccines, with adjusted hazard ratios showing 41%-48% lower risk of infection. Too few individuals in the study were vaccinated with the Johnson & Johnson vaccine to enable a sufficiently powered calculation of its effectiveness.
Other studies reach similar conclusions
The study findings fall into line with other studies of patient populations on immunosuppressants. A retrospective cohort study of Veterans Affairs patients with inflammatory bowel disease who were taking immunosuppressants, published in Gastroenterology, found that full vaccination with either Moderna and Pfizer-BioNTech vaccines was about 80% effective. Another retrospective cohort study of data from the National COVID Cohort Collaborative, published in JAMA Internal Medicine, reported that full vaccination significantly reduced the risk of COVID-19 breakthrough infection regardless of immune status. Immunosuppressed patients in this study had higher rates of breakthrough infections than immunocompetent patients, but the disparities were in line with what Dr. Zhao and the University of Michigan researchers reported.
A review of 23 studies of COVID-19 vaccinations, published in Lancet Global Health, found that immunocompromised people – 1,722 of whom were included in the studies – had lower rates of producing antibodies after two vaccine doses than did immunocompetent people, ranging from 27% to 92%, depending on the nature of their immunocompromised status, compared with 99% for the immunocompetent.
Strengths and limitations
One strength of the Michigan study is the quality of data, which were drawn from the Michigan Medicine electronic health record, Dr. Zhao said. “So, we know who received the vaccine and who didn’t. We also have access to data on patient health conditions, such as comorbidities, in addition to demographic variables (age, gender, and race), which were controlled in making fair comparisons between immunosuppressants and immunocompetent groups.”
Alfred Kim, MD, PhD, an assistant professor of internal medicine and rheumatology at Washington University in St. Louis, who was not involved with the study, credited Dr. Zhao and associates for delivering the first data that specifically quantified COVID-19 risk reduction in a large study population. Although he noted that the large sample size and the design reduced the chances of confounding and were strengths, he said in an interview that “lumping” the patients taking immunosuppressive drugs into one group was a weakness of the study.
“Clearly, there are certain medications (B-cell depleters, mycophenolate, for example) that carry the greatest risk of poor antibody responses post vaccination,” he said. “One would have to guess that the greatest risk of breakthrough infections continues to be in those patients taking these high-risk medications.”
Another possible problem, which the authors acknowledged, is spotty SARS-CoV-2 testing of study participants – “a systemic issue,” Dr. Kim noted.
“The easiest and most durable way to reduce the risk of getting COVID-19 is through vaccination, period,” he said. “Now we have infection-rates data from a real-world study cohort to prove this. Furthermore, boosting clearly provides additional benefit to this population.”
The National Institute of Allergy and Infectious Diseases provided funding for the study. Dr. Zhao, Dr. Zhao’s coauthors, and Kim disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES
If you’ve never had COVID, should you relax or worry?
If you’re among those people in the United States who never had COVID-19, how should you think about your risk?
According to the Centers for Disease Control and Prevention (CDC), more than half of people in the United States are in the never-got-COVID category.
The CDC estimated that by the end of January, 43.4% in the United States had developed antibodies to SARS-CoV-2 triggered by infection, not by vaccination — suggesting nearly 60% of people have never been infected.
Now mask mandates are lifting, and daily case and death numbers are plunging. According to the New York Times tracker, new cases are down 51% for the past 2 weeks, and deaths have fallen 30% in that period.
So as those who have so far escaped the virus venture further out into reopened environments, should they worry more or less about risk than their previously infected counterparts?
No “suit of armor”
William Schaffner, MD, an infectious disease expert at Vanderbilt University School of Medicine in Nashville, Tenn., said in an interview that science has not been able to determine why some people have been able to be stay COVID-free when the virus was raging and exposure was ubiquitous.
He said it’s important to remember that though some people think they have never had COVID, they may have been asymptomatic or attributed mild symptoms to something else.
“People may have conceivably — but we can’t define them yet — different capacities to ward off viruses or bacteria,” Dr. Schaffner said.
Could it be that some people have a better immune system or genetic component or environmental reason that they are less susceptible to infectious disease? “We can’t define that in 2022 medicine, but it could be,” he said.
More is known about why people with the same COVID exposure may have different levels of illness severity.
“They’re more likely to get seriously ill if they have a list of predisposing conditions — if they’re older, if they’re frail, if they have underlying illness or are obese. All of those things clearly impair the body’s response to virus,” Dr. Schaffner said.
He warns those who have never been infected, though, not to assume they have “a suit of armor.”
All should continue to follow guidance on getting vaccinated, and those vaccinated should continue to get boosted, Dr. Schaffner said.
“Clearly, the data show that if you are vaccinated and boosted, you’re protected much more securely against severe disease,” he said.
If the never-COVIDs develop a respiratory infection, they should still get tested for COVID, Dr. Schaffner said.
He said while both vaccines and previous natural infection offer protection, the duration of that protection is not yet known.
“We have to stay tuned,” Dr. Schaffner said. “There may be a recommendation in the future to get a booster annually or something like that. We need to be open to those down the road.”
Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security in Baltimore, says it’s unclear why some people have been able to avoid COVID.
“The explanation is likely multifactorial and involves behaviors as well as possible idiosyncrasies with their immune systems that are genetically based,” he said. “It also may be the case that inapparent infections occurred and went undiagnosed.”
Dr. Adalja agrees, though, that this isn’t the time to get overconfident with risk-taking where COVID is concerned.
“People who have not knowingly been infected with COVID should be vaccinated, and after that, be assured that they are protected against serious disease from this virus,” he said.
Genetic protection?
A new study in Nature Genetics explains a potential genetic relationship. Study authors found evidence that levels of expression of angiotensin-converting enzyme 2 (ACE2) – which helps regulate blood pressure, wound healing, and inflammation, but has also been shown to serve as an entry point into cells for some coronaviruses like SARS-CoV-2 — influence COVID-19 risk.
Manuel A. Ferreira, PhD, an executive director for analytic genetics at Regeneron Pharmaceuticals, said in an interview that ACE2 receptors — what he calls the “gateways” for SARS-CoV-2 to enter the body — are different in people who have inherited a particular allele.
The researchers have found that that allele is associated with lower risk of SARS-CoV-2 infection.
“It’s quite substantial -- a 40% risk reduction if you carry the allele that reduces ACE2 expression,” he said. They were not able to discern from this study, however, whether that could predict severity of disease.
The team also looked at a series of six genetic variants elsewhere in the genome and developed a risk score to see if it was possible to predict who might be more susceptible to severe COVID.
Dr. Ferreira said the score only slightly improved predictive abilities beyond factors such as age, sex, weight, and comorbidities. Further information will help hone the ability to predict the likelihood of developing severe disease on the basis of genetics, Ferreira said.
“As we identify more genetic risk factors for COVID — variants like the ACE2 variant that will affect your risk of having COVID — the more informative the risk score will be,” he said.
Several authors of the Nature Genetics article are current and/or former employees of AncestryDNA and may hold equity in AncestryDNA. Several are Regeneron employees and/or hold stock in the company. Dr. Ferreira is an employee of Regeneron and holds stock in the company. Dr. Schaffner and Dr. Adalja report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
If you’re among those people in the United States who never had COVID-19, how should you think about your risk?
According to the Centers for Disease Control and Prevention (CDC), more than half of people in the United States are in the never-got-COVID category.
The CDC estimated that by the end of January, 43.4% in the United States had developed antibodies to SARS-CoV-2 triggered by infection, not by vaccination — suggesting nearly 60% of people have never been infected.
Now mask mandates are lifting, and daily case and death numbers are plunging. According to the New York Times tracker, new cases are down 51% for the past 2 weeks, and deaths have fallen 30% in that period.
So as those who have so far escaped the virus venture further out into reopened environments, should they worry more or less about risk than their previously infected counterparts?
No “suit of armor”
William Schaffner, MD, an infectious disease expert at Vanderbilt University School of Medicine in Nashville, Tenn., said in an interview that science has not been able to determine why some people have been able to be stay COVID-free when the virus was raging and exposure was ubiquitous.
He said it’s important to remember that though some people think they have never had COVID, they may have been asymptomatic or attributed mild symptoms to something else.
“People may have conceivably — but we can’t define them yet — different capacities to ward off viruses or bacteria,” Dr. Schaffner said.
Could it be that some people have a better immune system or genetic component or environmental reason that they are less susceptible to infectious disease? “We can’t define that in 2022 medicine, but it could be,” he said.
More is known about why people with the same COVID exposure may have different levels of illness severity.
“They’re more likely to get seriously ill if they have a list of predisposing conditions — if they’re older, if they’re frail, if they have underlying illness or are obese. All of those things clearly impair the body’s response to virus,” Dr. Schaffner said.
He warns those who have never been infected, though, not to assume they have “a suit of armor.”
All should continue to follow guidance on getting vaccinated, and those vaccinated should continue to get boosted, Dr. Schaffner said.
“Clearly, the data show that if you are vaccinated and boosted, you’re protected much more securely against severe disease,” he said.
If the never-COVIDs develop a respiratory infection, they should still get tested for COVID, Dr. Schaffner said.
He said while both vaccines and previous natural infection offer protection, the duration of that protection is not yet known.
“We have to stay tuned,” Dr. Schaffner said. “There may be a recommendation in the future to get a booster annually or something like that. We need to be open to those down the road.”
Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security in Baltimore, says it’s unclear why some people have been able to avoid COVID.
“The explanation is likely multifactorial and involves behaviors as well as possible idiosyncrasies with their immune systems that are genetically based,” he said. “It also may be the case that inapparent infections occurred and went undiagnosed.”
Dr. Adalja agrees, though, that this isn’t the time to get overconfident with risk-taking where COVID is concerned.
“People who have not knowingly been infected with COVID should be vaccinated, and after that, be assured that they are protected against serious disease from this virus,” he said.
Genetic protection?
A new study in Nature Genetics explains a potential genetic relationship. Study authors found evidence that levels of expression of angiotensin-converting enzyme 2 (ACE2) – which helps regulate blood pressure, wound healing, and inflammation, but has also been shown to serve as an entry point into cells for some coronaviruses like SARS-CoV-2 — influence COVID-19 risk.
Manuel A. Ferreira, PhD, an executive director for analytic genetics at Regeneron Pharmaceuticals, said in an interview that ACE2 receptors — what he calls the “gateways” for SARS-CoV-2 to enter the body — are different in people who have inherited a particular allele.
The researchers have found that that allele is associated with lower risk of SARS-CoV-2 infection.
“It’s quite substantial -- a 40% risk reduction if you carry the allele that reduces ACE2 expression,” he said. They were not able to discern from this study, however, whether that could predict severity of disease.
The team also looked at a series of six genetic variants elsewhere in the genome and developed a risk score to see if it was possible to predict who might be more susceptible to severe COVID.
Dr. Ferreira said the score only slightly improved predictive abilities beyond factors such as age, sex, weight, and comorbidities. Further information will help hone the ability to predict the likelihood of developing severe disease on the basis of genetics, Ferreira said.
“As we identify more genetic risk factors for COVID — variants like the ACE2 variant that will affect your risk of having COVID — the more informative the risk score will be,” he said.
Several authors of the Nature Genetics article are current and/or former employees of AncestryDNA and may hold equity in AncestryDNA. Several are Regeneron employees and/or hold stock in the company. Dr. Ferreira is an employee of Regeneron and holds stock in the company. Dr. Schaffner and Dr. Adalja report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
If you’re among those people in the United States who never had COVID-19, how should you think about your risk?
According to the Centers for Disease Control and Prevention (CDC), more than half of people in the United States are in the never-got-COVID category.
The CDC estimated that by the end of January, 43.4% in the United States had developed antibodies to SARS-CoV-2 triggered by infection, not by vaccination — suggesting nearly 60% of people have never been infected.
Now mask mandates are lifting, and daily case and death numbers are plunging. According to the New York Times tracker, new cases are down 51% for the past 2 weeks, and deaths have fallen 30% in that period.
So as those who have so far escaped the virus venture further out into reopened environments, should they worry more or less about risk than their previously infected counterparts?
No “suit of armor”
William Schaffner, MD, an infectious disease expert at Vanderbilt University School of Medicine in Nashville, Tenn., said in an interview that science has not been able to determine why some people have been able to be stay COVID-free when the virus was raging and exposure was ubiquitous.
He said it’s important to remember that though some people think they have never had COVID, they may have been asymptomatic or attributed mild symptoms to something else.
“People may have conceivably — but we can’t define them yet — different capacities to ward off viruses or bacteria,” Dr. Schaffner said.
Could it be that some people have a better immune system or genetic component or environmental reason that they are less susceptible to infectious disease? “We can’t define that in 2022 medicine, but it could be,” he said.
More is known about why people with the same COVID exposure may have different levels of illness severity.
“They’re more likely to get seriously ill if they have a list of predisposing conditions — if they’re older, if they’re frail, if they have underlying illness or are obese. All of those things clearly impair the body’s response to virus,” Dr. Schaffner said.
He warns those who have never been infected, though, not to assume they have “a suit of armor.”
All should continue to follow guidance on getting vaccinated, and those vaccinated should continue to get boosted, Dr. Schaffner said.
“Clearly, the data show that if you are vaccinated and boosted, you’re protected much more securely against severe disease,” he said.
If the never-COVIDs develop a respiratory infection, they should still get tested for COVID, Dr. Schaffner said.
He said while both vaccines and previous natural infection offer protection, the duration of that protection is not yet known.
“We have to stay tuned,” Dr. Schaffner said. “There may be a recommendation in the future to get a booster annually or something like that. We need to be open to those down the road.”
Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security in Baltimore, says it’s unclear why some people have been able to avoid COVID.
“The explanation is likely multifactorial and involves behaviors as well as possible idiosyncrasies with their immune systems that are genetically based,” he said. “It also may be the case that inapparent infections occurred and went undiagnosed.”
Dr. Adalja agrees, though, that this isn’t the time to get overconfident with risk-taking where COVID is concerned.
“People who have not knowingly been infected with COVID should be vaccinated, and after that, be assured that they are protected against serious disease from this virus,” he said.
Genetic protection?
A new study in Nature Genetics explains a potential genetic relationship. Study authors found evidence that levels of expression of angiotensin-converting enzyme 2 (ACE2) – which helps regulate blood pressure, wound healing, and inflammation, but has also been shown to serve as an entry point into cells for some coronaviruses like SARS-CoV-2 — influence COVID-19 risk.
Manuel A. Ferreira, PhD, an executive director for analytic genetics at Regeneron Pharmaceuticals, said in an interview that ACE2 receptors — what he calls the “gateways” for SARS-CoV-2 to enter the body — are different in people who have inherited a particular allele.
The researchers have found that that allele is associated with lower risk of SARS-CoV-2 infection.
“It’s quite substantial -- a 40% risk reduction if you carry the allele that reduces ACE2 expression,” he said. They were not able to discern from this study, however, whether that could predict severity of disease.
The team also looked at a series of six genetic variants elsewhere in the genome and developed a risk score to see if it was possible to predict who might be more susceptible to severe COVID.
Dr. Ferreira said the score only slightly improved predictive abilities beyond factors such as age, sex, weight, and comorbidities. Further information will help hone the ability to predict the likelihood of developing severe disease on the basis of genetics, Ferreira said.
“As we identify more genetic risk factors for COVID — variants like the ACE2 variant that will affect your risk of having COVID — the more informative the risk score will be,” he said.
Several authors of the Nature Genetics article are current and/or former employees of AncestryDNA and may hold equity in AncestryDNA. Several are Regeneron employees and/or hold stock in the company. Dr. Ferreira is an employee of Regeneron and holds stock in the company. Dr. Schaffner and Dr. Adalja report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
First ‘before-and-after’ COVID-19 brain imaging study shows structural changes
, a new imaging study shows.
In the first study to use magnetic resonance brain imaging, before and after COVID-19, investigators found “greater reduction in grey matter thickness and tissue-contrast in the orbitofrontal cortex and parahippocampal gyrus, greater changes in markers of tissue damage in regions functionally connected to the primary olfactory cortex and greater reduction in global brain size.” However, the researchers urge caution when interpreting the findings.
Gwenaëlle Douaud, PhD, Wellcome Center for Integrative Neuroimaging, Nuffield Department of Clinical Neurosciences, University of Oxford, England, and colleagues describe these brain changes as “modest.”
“Whether these abnormal changes are the hallmark of the spread of the pathogenic effects in the brain, or of the virus itself, and whether these may prefigure a future vulnerability of the limbic system in particular, including memory, for these participants, remains to be investigated,” the researchers wrote.
The findings were published online March 7 in the journal Nature.
Gray matter loss
The investigators analyzed data from the UK Biobank, a large-scale biomedical database with genetic and health information for about 500,000 individuals living in the UK. They identified 785 adults aged 51-81 years who had undergone two brain MRIs about 3 years apart. Of these, 401 tested positive for SARS-CoV-2 before the second scan.
Participants also completed cognitive tests at the time of both scans.
Biobank centers use identical MRI scans and scanning methods, including six types of MRI scans, to image distinct regions of the brain and brain function. Results showed that although some loss of gray matter over time is normal, individuals who were infected with SARS-CoV-2 showed a 0.2% to 2% brain tissue loss in the parahippocampal gyrus, the orbitofrontal cortex, and the insula – all of which are largely involved in the sense of smell.
Participants who had contracted COVID-19 also showed a greater reduction in overall brain volume and a decrease in cognitive function.
Most of those with COVID-19 had only mild or moderate symptoms. However, the findings held even after the researchers excluded patients who had been hospitalized.
More research needed
“These findings might help explain why some people experience brain symptoms long after the acute infection,” Max Taquet, PhD, National Institute for Health Research Oxford Health BRC senior research fellow, University of Oxford, said in a press release.
Dr. Taquet, who was not a part of the study, noted the causes of these brain changes remain to be determined. Questions remain as to “whether they can be prevented or even reverted, as well as whether similar changes are observed in hospitalized patients,” children, younger adults, and minority groups.
“It is possible that these brain changes are not caused by COVID-19 but represent the natural progression of a disease that itself increased the risk of COVID-19,” Dr. Taquet said.
Other experts expressed concern over the findings and emphasized the need for more research.
“I am very concerned by the alarming use of language in the report with terms such as ‘neurodegenerative,’ “ Alan Carson, MD, professor of neuropsychiatry at the Center for Clinical Brain Sciences at the University of Edinburgh, Scotland, said in a press release. “The size and magnitude of brain changes found is very modest and such changes can be caused by a simple change in mental experience,” Dr. Carson said.
“What this study almost certainly shows is the impact, in terms of neural changes, of being disconnected from one’s sense of smell,” he added.
The study was funded by the Wellcome Trust Collaborative. Full financial conflict information for the study authors is included in the original article. Dr. Taquet has collaborated previously with some of the investigators.
A version of this article first appeared on Medscape.com.
, a new imaging study shows.
In the first study to use magnetic resonance brain imaging, before and after COVID-19, investigators found “greater reduction in grey matter thickness and tissue-contrast in the orbitofrontal cortex and parahippocampal gyrus, greater changes in markers of tissue damage in regions functionally connected to the primary olfactory cortex and greater reduction in global brain size.” However, the researchers urge caution when interpreting the findings.
Gwenaëlle Douaud, PhD, Wellcome Center for Integrative Neuroimaging, Nuffield Department of Clinical Neurosciences, University of Oxford, England, and colleagues describe these brain changes as “modest.”
“Whether these abnormal changes are the hallmark of the spread of the pathogenic effects in the brain, or of the virus itself, and whether these may prefigure a future vulnerability of the limbic system in particular, including memory, for these participants, remains to be investigated,” the researchers wrote.
The findings were published online March 7 in the journal Nature.
Gray matter loss
The investigators analyzed data from the UK Biobank, a large-scale biomedical database with genetic and health information for about 500,000 individuals living in the UK. They identified 785 adults aged 51-81 years who had undergone two brain MRIs about 3 years apart. Of these, 401 tested positive for SARS-CoV-2 before the second scan.
Participants also completed cognitive tests at the time of both scans.
Biobank centers use identical MRI scans and scanning methods, including six types of MRI scans, to image distinct regions of the brain and brain function. Results showed that although some loss of gray matter over time is normal, individuals who were infected with SARS-CoV-2 showed a 0.2% to 2% brain tissue loss in the parahippocampal gyrus, the orbitofrontal cortex, and the insula – all of which are largely involved in the sense of smell.
Participants who had contracted COVID-19 also showed a greater reduction in overall brain volume and a decrease in cognitive function.
Most of those with COVID-19 had only mild or moderate symptoms. However, the findings held even after the researchers excluded patients who had been hospitalized.
More research needed
“These findings might help explain why some people experience brain symptoms long after the acute infection,” Max Taquet, PhD, National Institute for Health Research Oxford Health BRC senior research fellow, University of Oxford, said in a press release.
Dr. Taquet, who was not a part of the study, noted the causes of these brain changes remain to be determined. Questions remain as to “whether they can be prevented or even reverted, as well as whether similar changes are observed in hospitalized patients,” children, younger adults, and minority groups.
“It is possible that these brain changes are not caused by COVID-19 but represent the natural progression of a disease that itself increased the risk of COVID-19,” Dr. Taquet said.
Other experts expressed concern over the findings and emphasized the need for more research.
“I am very concerned by the alarming use of language in the report with terms such as ‘neurodegenerative,’ “ Alan Carson, MD, professor of neuropsychiatry at the Center for Clinical Brain Sciences at the University of Edinburgh, Scotland, said in a press release. “The size and magnitude of brain changes found is very modest and such changes can be caused by a simple change in mental experience,” Dr. Carson said.
“What this study almost certainly shows is the impact, in terms of neural changes, of being disconnected from one’s sense of smell,” he added.
The study was funded by the Wellcome Trust Collaborative. Full financial conflict information for the study authors is included in the original article. Dr. Taquet has collaborated previously with some of the investigators.
A version of this article first appeared on Medscape.com.
, a new imaging study shows.
In the first study to use magnetic resonance brain imaging, before and after COVID-19, investigators found “greater reduction in grey matter thickness and tissue-contrast in the orbitofrontal cortex and parahippocampal gyrus, greater changes in markers of tissue damage in regions functionally connected to the primary olfactory cortex and greater reduction in global brain size.” However, the researchers urge caution when interpreting the findings.
Gwenaëlle Douaud, PhD, Wellcome Center for Integrative Neuroimaging, Nuffield Department of Clinical Neurosciences, University of Oxford, England, and colleagues describe these brain changes as “modest.”
“Whether these abnormal changes are the hallmark of the spread of the pathogenic effects in the brain, or of the virus itself, and whether these may prefigure a future vulnerability of the limbic system in particular, including memory, for these participants, remains to be investigated,” the researchers wrote.
The findings were published online March 7 in the journal Nature.
Gray matter loss
The investigators analyzed data from the UK Biobank, a large-scale biomedical database with genetic and health information for about 500,000 individuals living in the UK. They identified 785 adults aged 51-81 years who had undergone two brain MRIs about 3 years apart. Of these, 401 tested positive for SARS-CoV-2 before the second scan.
Participants also completed cognitive tests at the time of both scans.
Biobank centers use identical MRI scans and scanning methods, including six types of MRI scans, to image distinct regions of the brain and brain function. Results showed that although some loss of gray matter over time is normal, individuals who were infected with SARS-CoV-2 showed a 0.2% to 2% brain tissue loss in the parahippocampal gyrus, the orbitofrontal cortex, and the insula – all of which are largely involved in the sense of smell.
Participants who had contracted COVID-19 also showed a greater reduction in overall brain volume and a decrease in cognitive function.
Most of those with COVID-19 had only mild or moderate symptoms. However, the findings held even after the researchers excluded patients who had been hospitalized.
More research needed
“These findings might help explain why some people experience brain symptoms long after the acute infection,” Max Taquet, PhD, National Institute for Health Research Oxford Health BRC senior research fellow, University of Oxford, said in a press release.
Dr. Taquet, who was not a part of the study, noted the causes of these brain changes remain to be determined. Questions remain as to “whether they can be prevented or even reverted, as well as whether similar changes are observed in hospitalized patients,” children, younger adults, and minority groups.
“It is possible that these brain changes are not caused by COVID-19 but represent the natural progression of a disease that itself increased the risk of COVID-19,” Dr. Taquet said.
Other experts expressed concern over the findings and emphasized the need for more research.
“I am very concerned by the alarming use of language in the report with terms such as ‘neurodegenerative,’ “ Alan Carson, MD, professor of neuropsychiatry at the Center for Clinical Brain Sciences at the University of Edinburgh, Scotland, said in a press release. “The size and magnitude of brain changes found is very modest and such changes can be caused by a simple change in mental experience,” Dr. Carson said.
“What this study almost certainly shows is the impact, in terms of neural changes, of being disconnected from one’s sense of smell,” he added.
The study was funded by the Wellcome Trust Collaborative. Full financial conflict information for the study authors is included in the original article. Dr. Taquet has collaborated previously with some of the investigators.
A version of this article first appeared on Medscape.com.
From Nature