User login
Children and COVID: Weekly cases at lowest level since August
New cases of COVID-19 in children continued their descent toward normalcy, falling below 100,000 in a week for the first time since early August 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
and 94% since the Omicron-fueled peak of 1.15 million during the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. The total number of child cases is 12.7 million since the pandemic began, with children representing 19% of all cases.
New admissions also stayed on a downward path, as the rate dropped to 0.24 per 100,000 children aged 0-17 years on March 5, a decline of nearly 81% since hitting 1.25 per 100,000 on Jan. 15. The latest 7-day average for daily admissions, 178 per day from Feb. 27 to March 5, was 29% lower than the previous week and almost 81% lower than the peak of 914 per day for Jan. 10-16, the Centers for Disease Control and Prevention reported.
The story is the same for emergency department visits with diagnosed COVID-19, which are reported as a percentage of all ED visits. On March 4, the 7-day average for children aged 0-11 years was 0.8%, compared with a high of 13.9% in mid-January, while 12- to 15-year-olds had dropped from 12.4% to 0.5% and 16- to 17-year-olds went from 12.6% down to 0.5%, the CDC said on its COVID Data Tracker.
Florida’s surgeon general says no to the vaccine
Vaccination, in the meantime, is struggling to maintain a foothold against the current of declining cases. Florida Surgeon General Joseph Ladapo said that “the Florida Department of Health is going to be the first state to officially recommend against the COVID-19 vaccines for healthy children,” NBC News reported March 7. With such a move, “Florida would become the first state to break from the CDC on vaccines for children,” CNN said in its report.
Vaccinations among children aged 5-11 years, which hit 1.6 million in 1 week shortly after emergency use was authorized in early November, declined quickly shorty thereafter and only rose slightly during the Omicron surge. Since mid-January, the number of children receiving an initial dose has declined for seven consecutive weeks and is now lower than ever, based on CDC data compiled by the AAP.
Just over one-third of children aged 5-11 have gotten at least one dose of COVID-19 vaccine, while 26.4% are fully vaccinated. Among children aged 12-17, just over two-thirds (67.8%) have received at least one dose, 57.8% have completed the vaccine regimen, and 21.9% have gotten a booster, the CDC reported.
As of March 2, “about 8.4 million children 12-17 have yet to receive their initial COVID-19 vaccine dose,” the AAP said. About 64,000 children aged 12-17 had received their first dose in the previous week, the group noted, which was the second-lowest weekly total since the vaccine was approved for children aged 12-15 in May of 2021.
New cases of COVID-19 in children continued their descent toward normalcy, falling below 100,000 in a week for the first time since early August 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
and 94% since the Omicron-fueled peak of 1.15 million during the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. The total number of child cases is 12.7 million since the pandemic began, with children representing 19% of all cases.
New admissions also stayed on a downward path, as the rate dropped to 0.24 per 100,000 children aged 0-17 years on March 5, a decline of nearly 81% since hitting 1.25 per 100,000 on Jan. 15. The latest 7-day average for daily admissions, 178 per day from Feb. 27 to March 5, was 29% lower than the previous week and almost 81% lower than the peak of 914 per day for Jan. 10-16, the Centers for Disease Control and Prevention reported.
The story is the same for emergency department visits with diagnosed COVID-19, which are reported as a percentage of all ED visits. On March 4, the 7-day average for children aged 0-11 years was 0.8%, compared with a high of 13.9% in mid-January, while 12- to 15-year-olds had dropped from 12.4% to 0.5% and 16- to 17-year-olds went from 12.6% down to 0.5%, the CDC said on its COVID Data Tracker.
Florida’s surgeon general says no to the vaccine
Vaccination, in the meantime, is struggling to maintain a foothold against the current of declining cases. Florida Surgeon General Joseph Ladapo said that “the Florida Department of Health is going to be the first state to officially recommend against the COVID-19 vaccines for healthy children,” NBC News reported March 7. With such a move, “Florida would become the first state to break from the CDC on vaccines for children,” CNN said in its report.
Vaccinations among children aged 5-11 years, which hit 1.6 million in 1 week shortly after emergency use was authorized in early November, declined quickly shorty thereafter and only rose slightly during the Omicron surge. Since mid-January, the number of children receiving an initial dose has declined for seven consecutive weeks and is now lower than ever, based on CDC data compiled by the AAP.
Just over one-third of children aged 5-11 have gotten at least one dose of COVID-19 vaccine, while 26.4% are fully vaccinated. Among children aged 12-17, just over two-thirds (67.8%) have received at least one dose, 57.8% have completed the vaccine regimen, and 21.9% have gotten a booster, the CDC reported.
As of March 2, “about 8.4 million children 12-17 have yet to receive their initial COVID-19 vaccine dose,” the AAP said. About 64,000 children aged 12-17 had received their first dose in the previous week, the group noted, which was the second-lowest weekly total since the vaccine was approved for children aged 12-15 in May of 2021.
New cases of COVID-19 in children continued their descent toward normalcy, falling below 100,000 in a week for the first time since early August 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
and 94% since the Omicron-fueled peak of 1.15 million during the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. The total number of child cases is 12.7 million since the pandemic began, with children representing 19% of all cases.
New admissions also stayed on a downward path, as the rate dropped to 0.24 per 100,000 children aged 0-17 years on March 5, a decline of nearly 81% since hitting 1.25 per 100,000 on Jan. 15. The latest 7-day average for daily admissions, 178 per day from Feb. 27 to March 5, was 29% lower than the previous week and almost 81% lower than the peak of 914 per day for Jan. 10-16, the Centers for Disease Control and Prevention reported.
The story is the same for emergency department visits with diagnosed COVID-19, which are reported as a percentage of all ED visits. On March 4, the 7-day average for children aged 0-11 years was 0.8%, compared with a high of 13.9% in mid-January, while 12- to 15-year-olds had dropped from 12.4% to 0.5% and 16- to 17-year-olds went from 12.6% down to 0.5%, the CDC said on its COVID Data Tracker.
Florida’s surgeon general says no to the vaccine
Vaccination, in the meantime, is struggling to maintain a foothold against the current of declining cases. Florida Surgeon General Joseph Ladapo said that “the Florida Department of Health is going to be the first state to officially recommend against the COVID-19 vaccines for healthy children,” NBC News reported March 7. With such a move, “Florida would become the first state to break from the CDC on vaccines for children,” CNN said in its report.
Vaccinations among children aged 5-11 years, which hit 1.6 million in 1 week shortly after emergency use was authorized in early November, declined quickly shorty thereafter and only rose slightly during the Omicron surge. Since mid-January, the number of children receiving an initial dose has declined for seven consecutive weeks and is now lower than ever, based on CDC data compiled by the AAP.
Just over one-third of children aged 5-11 have gotten at least one dose of COVID-19 vaccine, while 26.4% are fully vaccinated. Among children aged 12-17, just over two-thirds (67.8%) have received at least one dose, 57.8% have completed the vaccine regimen, and 21.9% have gotten a booster, the CDC reported.
As of March 2, “about 8.4 million children 12-17 have yet to receive their initial COVID-19 vaccine dose,” the AAP said. About 64,000 children aged 12-17 had received their first dose in the previous week, the group noted, which was the second-lowest weekly total since the vaccine was approved for children aged 12-15 in May of 2021.
Double-dose COVID-19 vaccines showed limited effectiveness against Omicron
, as determined on the basis of data from more than 800,000 Omicron-infected individuals.
Early laboratory data suggested a substantially lower neutralizing antibody response to the Omicron variant, compared with both the original COVID-19 strain and the Delta variant, write Nick Andrews, PhD, of the United Kingdom Health Security Agency, London, and colleagues.
Vaccines have shown high levels of effectiveness against symptomatic disease and severe disease and death resulting from the original COVID-19 virus and the Alpha variant and modest effectiveness against the Beta and Delta variants, they say.
“Neutralizing antibodies correlate with protection against reinfection and vaccine effectiveness against infection; therefore, reduced vaccine effectiveness against the omicron variant is anticipated on the basis of these early laboratory findings,” they explain.
In a study published in the New England Journal of Medicine, the researchers identified 886,774 adults aged 18 years and older who had been infected with the Omicron variant, 204,154 who had been infected with the Delta variant, and 1,572,621 symptomatic control patients who tested negative for COVID-19 between Nov. 27, 2021, and Jan. 12, 2022. The participants had been vaccinated with two doses of BNT162b2 (Pfizer–BioNTech), ChAdOx1 nCoV-19 (AstraZeneca), or mRNA-1273 (Moderna) vaccine, plus a booster given at least 175 days after a second dose, after Sept. 13, 2021.
Vaccine effectiveness was calculated after primary immunization at weeks 2-4, 5-9, 10-14, 15-19, 20-24, and 25 or longer after the second dose, and at 2-4, 5-9, and 10 or more weeks after boosters.
Omicron infections that occurred starting 14 or more days after a booster occurred a median of 39 days after the booster.
“Vaccine effectiveness was lower for the Omicron variant than for the Delta variant at all intervals after vaccination and for all combinations of primary courses and booster doses investigated,” the researchers write.
Individuals who received two doses of ChAdOx1 nCoV-19 had almost no protection against symptomatic disease caused by Omicron from 20-24 weeks after the second dose. For individuals who received two doses of BNT162b2, effectiveness was 65.5% 2-4 weeks after the second dose, but effectiveness declined to 15.4% after 15-19 weeks and to 8.8% after 25 or more weeks. For individuals who received two doses of mRNA-1273, vaccine effectiveness was 75.1% after 2-4 weeks, but effectiveness declined to 14.9% after 25 or more weeks.
Boosters created a short-term improvement in vaccine effectiveness against the Omicron variant, but this effect also declined over time.
Among individuals who received primary doses of ChAdOx1 nCoV-19, vaccine effectiveness increased to 62.4% 2-4 weeks after a BNT162b2 booster, then declined to 39.6% after 10 or more weeks. After an mRNA-1273 booster, vaccine effectiveness increased to 70.1% at 2-4 weeks and decreased to 60.9% at 5-9 weeks.
Among individuals who received primary doses of BNT162b2, vaccine effectiveness increased to 67.2% 2-4 weeks after a BNT162b2 booster, then declined to 45.7% at 10 or more weeks. After an mRNA-1273 booster, vaccine effectiveness increased to 73.9% at 2-4 weeks, then declined to 64.4% at 5-9 weeks.
Among individuals who received primary doses of mRNA-1273, vaccine effectiveness increased to 64.9% 2-4 weeks after a BNT162b2 booster and 66.3% 2-4 weeks after an mRNA-1273 booster.
The study findings were limited by potential confounding from study participants who had traveled and may have had different levels of vaccine coverage and by the inability to break down estimates on the basis of age and clinical risk that might affect vaccine effectiveness, the researchers note. Other limitations include a lack of data on vaccine effectiveness for a longer period after boosters, they say.
However, the results are consistent with neutralization data for the Omicron variant in studies from the United Kingdom, South Africa, and Germany, they write. “Our findings support maximizing coverage with third doses of vaccine in highly vaccinated populations such as in the United Kingdom. Further follow-up will be needed to assess protection against severe disease and the duration of protection after booster vaccination,” they conclude.
Focus on severe disease prevention
Paul Offit, MD, of the University of Pennsylvania, Philadelphia, addressed the topic of vaccine effectiveness in an op-ed published on March 4 in The Philadelphia Inquirer. The following is adapted from the op-ed, with his permission.
“The goal of the COVID vaccine – as is true for all vaccines – is to prevent serious illness,” Dr. Offit wrote.
“For most people with normal immune systems, two doses of mRNA vaccines appear to do exactly that. But not everyone,” wrote Dr. Offit, who serves as director of the Vaccine Education Center at the Children’s Hospital of Philadelphia and also serves on the Food and Drug Administration’s Vaccine Advisory Committee. “Three doses are required to induce high levels of protection against serious illness for people over 65 years of age or for people with other conditions that make them vulnerable, which can be anything from being overweight to having cancer. For people who are immune compromised, four doses might be required,” he noted.
Frequent vaccine boosting, although it may help prevent milder cases of COVID-19, such as those seen with the Omicron variant, is impractical, Dr. Offit emphasized. Instead, a newer, variant-specific vaccine might be needed if a variant emerges that overrides the protection against severe disease currently afforded by the available vaccines, he said. “But we’re not there yet. For now, we are going to have to realize that it is virtually impossible to prevent mild COVID without frequent boosting. So, let’s learn to accept that the goal of COVID vaccines is to prevent severe and not mild illness and stop talking about frequent boosting. Otherwise, we will never be able to live our lives as before,” he wrote.
The study was supported by the U.K. Health Security Agency. The researchers and Dr. Offit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, as determined on the basis of data from more than 800,000 Omicron-infected individuals.
Early laboratory data suggested a substantially lower neutralizing antibody response to the Omicron variant, compared with both the original COVID-19 strain and the Delta variant, write Nick Andrews, PhD, of the United Kingdom Health Security Agency, London, and colleagues.
Vaccines have shown high levels of effectiveness against symptomatic disease and severe disease and death resulting from the original COVID-19 virus and the Alpha variant and modest effectiveness against the Beta and Delta variants, they say.
“Neutralizing antibodies correlate with protection against reinfection and vaccine effectiveness against infection; therefore, reduced vaccine effectiveness against the omicron variant is anticipated on the basis of these early laboratory findings,” they explain.
In a study published in the New England Journal of Medicine, the researchers identified 886,774 adults aged 18 years and older who had been infected with the Omicron variant, 204,154 who had been infected with the Delta variant, and 1,572,621 symptomatic control patients who tested negative for COVID-19 between Nov. 27, 2021, and Jan. 12, 2022. The participants had been vaccinated with two doses of BNT162b2 (Pfizer–BioNTech), ChAdOx1 nCoV-19 (AstraZeneca), or mRNA-1273 (Moderna) vaccine, plus a booster given at least 175 days after a second dose, after Sept. 13, 2021.
Vaccine effectiveness was calculated after primary immunization at weeks 2-4, 5-9, 10-14, 15-19, 20-24, and 25 or longer after the second dose, and at 2-4, 5-9, and 10 or more weeks after boosters.
Omicron infections that occurred starting 14 or more days after a booster occurred a median of 39 days after the booster.
“Vaccine effectiveness was lower for the Omicron variant than for the Delta variant at all intervals after vaccination and for all combinations of primary courses and booster doses investigated,” the researchers write.
Individuals who received two doses of ChAdOx1 nCoV-19 had almost no protection against symptomatic disease caused by Omicron from 20-24 weeks after the second dose. For individuals who received two doses of BNT162b2, effectiveness was 65.5% 2-4 weeks after the second dose, but effectiveness declined to 15.4% after 15-19 weeks and to 8.8% after 25 or more weeks. For individuals who received two doses of mRNA-1273, vaccine effectiveness was 75.1% after 2-4 weeks, but effectiveness declined to 14.9% after 25 or more weeks.
Boosters created a short-term improvement in vaccine effectiveness against the Omicron variant, but this effect also declined over time.
Among individuals who received primary doses of ChAdOx1 nCoV-19, vaccine effectiveness increased to 62.4% 2-4 weeks after a BNT162b2 booster, then declined to 39.6% after 10 or more weeks. After an mRNA-1273 booster, vaccine effectiveness increased to 70.1% at 2-4 weeks and decreased to 60.9% at 5-9 weeks.
Among individuals who received primary doses of BNT162b2, vaccine effectiveness increased to 67.2% 2-4 weeks after a BNT162b2 booster, then declined to 45.7% at 10 or more weeks. After an mRNA-1273 booster, vaccine effectiveness increased to 73.9% at 2-4 weeks, then declined to 64.4% at 5-9 weeks.
Among individuals who received primary doses of mRNA-1273, vaccine effectiveness increased to 64.9% 2-4 weeks after a BNT162b2 booster and 66.3% 2-4 weeks after an mRNA-1273 booster.
The study findings were limited by potential confounding from study participants who had traveled and may have had different levels of vaccine coverage and by the inability to break down estimates on the basis of age and clinical risk that might affect vaccine effectiveness, the researchers note. Other limitations include a lack of data on vaccine effectiveness for a longer period after boosters, they say.
However, the results are consistent with neutralization data for the Omicron variant in studies from the United Kingdom, South Africa, and Germany, they write. “Our findings support maximizing coverage with third doses of vaccine in highly vaccinated populations such as in the United Kingdom. Further follow-up will be needed to assess protection against severe disease and the duration of protection after booster vaccination,” they conclude.
Focus on severe disease prevention
Paul Offit, MD, of the University of Pennsylvania, Philadelphia, addressed the topic of vaccine effectiveness in an op-ed published on March 4 in The Philadelphia Inquirer. The following is adapted from the op-ed, with his permission.
“The goal of the COVID vaccine – as is true for all vaccines – is to prevent serious illness,” Dr. Offit wrote.
“For most people with normal immune systems, two doses of mRNA vaccines appear to do exactly that. But not everyone,” wrote Dr. Offit, who serves as director of the Vaccine Education Center at the Children’s Hospital of Philadelphia and also serves on the Food and Drug Administration’s Vaccine Advisory Committee. “Three doses are required to induce high levels of protection against serious illness for people over 65 years of age or for people with other conditions that make them vulnerable, which can be anything from being overweight to having cancer. For people who are immune compromised, four doses might be required,” he noted.
Frequent vaccine boosting, although it may help prevent milder cases of COVID-19, such as those seen with the Omicron variant, is impractical, Dr. Offit emphasized. Instead, a newer, variant-specific vaccine might be needed if a variant emerges that overrides the protection against severe disease currently afforded by the available vaccines, he said. “But we’re not there yet. For now, we are going to have to realize that it is virtually impossible to prevent mild COVID without frequent boosting. So, let’s learn to accept that the goal of COVID vaccines is to prevent severe and not mild illness and stop talking about frequent boosting. Otherwise, we will never be able to live our lives as before,” he wrote.
The study was supported by the U.K. Health Security Agency. The researchers and Dr. Offit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, as determined on the basis of data from more than 800,000 Omicron-infected individuals.
Early laboratory data suggested a substantially lower neutralizing antibody response to the Omicron variant, compared with both the original COVID-19 strain and the Delta variant, write Nick Andrews, PhD, of the United Kingdom Health Security Agency, London, and colleagues.
Vaccines have shown high levels of effectiveness against symptomatic disease and severe disease and death resulting from the original COVID-19 virus and the Alpha variant and modest effectiveness against the Beta and Delta variants, they say.
“Neutralizing antibodies correlate with protection against reinfection and vaccine effectiveness against infection; therefore, reduced vaccine effectiveness against the omicron variant is anticipated on the basis of these early laboratory findings,” they explain.
In a study published in the New England Journal of Medicine, the researchers identified 886,774 adults aged 18 years and older who had been infected with the Omicron variant, 204,154 who had been infected with the Delta variant, and 1,572,621 symptomatic control patients who tested negative for COVID-19 between Nov. 27, 2021, and Jan. 12, 2022. The participants had been vaccinated with two doses of BNT162b2 (Pfizer–BioNTech), ChAdOx1 nCoV-19 (AstraZeneca), or mRNA-1273 (Moderna) vaccine, plus a booster given at least 175 days after a second dose, after Sept. 13, 2021.
Vaccine effectiveness was calculated after primary immunization at weeks 2-4, 5-9, 10-14, 15-19, 20-24, and 25 or longer after the second dose, and at 2-4, 5-9, and 10 or more weeks after boosters.
Omicron infections that occurred starting 14 or more days after a booster occurred a median of 39 days after the booster.
“Vaccine effectiveness was lower for the Omicron variant than for the Delta variant at all intervals after vaccination and for all combinations of primary courses and booster doses investigated,” the researchers write.
Individuals who received two doses of ChAdOx1 nCoV-19 had almost no protection against symptomatic disease caused by Omicron from 20-24 weeks after the second dose. For individuals who received two doses of BNT162b2, effectiveness was 65.5% 2-4 weeks after the second dose, but effectiveness declined to 15.4% after 15-19 weeks and to 8.8% after 25 or more weeks. For individuals who received two doses of mRNA-1273, vaccine effectiveness was 75.1% after 2-4 weeks, but effectiveness declined to 14.9% after 25 or more weeks.
Boosters created a short-term improvement in vaccine effectiveness against the Omicron variant, but this effect also declined over time.
Among individuals who received primary doses of ChAdOx1 nCoV-19, vaccine effectiveness increased to 62.4% 2-4 weeks after a BNT162b2 booster, then declined to 39.6% after 10 or more weeks. After an mRNA-1273 booster, vaccine effectiveness increased to 70.1% at 2-4 weeks and decreased to 60.9% at 5-9 weeks.
Among individuals who received primary doses of BNT162b2, vaccine effectiveness increased to 67.2% 2-4 weeks after a BNT162b2 booster, then declined to 45.7% at 10 or more weeks. After an mRNA-1273 booster, vaccine effectiveness increased to 73.9% at 2-4 weeks, then declined to 64.4% at 5-9 weeks.
Among individuals who received primary doses of mRNA-1273, vaccine effectiveness increased to 64.9% 2-4 weeks after a BNT162b2 booster and 66.3% 2-4 weeks after an mRNA-1273 booster.
The study findings were limited by potential confounding from study participants who had traveled and may have had different levels of vaccine coverage and by the inability to break down estimates on the basis of age and clinical risk that might affect vaccine effectiveness, the researchers note. Other limitations include a lack of data on vaccine effectiveness for a longer period after boosters, they say.
However, the results are consistent with neutralization data for the Omicron variant in studies from the United Kingdom, South Africa, and Germany, they write. “Our findings support maximizing coverage with third doses of vaccine in highly vaccinated populations such as in the United Kingdom. Further follow-up will be needed to assess protection against severe disease and the duration of protection after booster vaccination,” they conclude.
Focus on severe disease prevention
Paul Offit, MD, of the University of Pennsylvania, Philadelphia, addressed the topic of vaccine effectiveness in an op-ed published on March 4 in The Philadelphia Inquirer. The following is adapted from the op-ed, with his permission.
“The goal of the COVID vaccine – as is true for all vaccines – is to prevent serious illness,” Dr. Offit wrote.
“For most people with normal immune systems, two doses of mRNA vaccines appear to do exactly that. But not everyone,” wrote Dr. Offit, who serves as director of the Vaccine Education Center at the Children’s Hospital of Philadelphia and also serves on the Food and Drug Administration’s Vaccine Advisory Committee. “Three doses are required to induce high levels of protection against serious illness for people over 65 years of age or for people with other conditions that make them vulnerable, which can be anything from being overweight to having cancer. For people who are immune compromised, four doses might be required,” he noted.
Frequent vaccine boosting, although it may help prevent milder cases of COVID-19, such as those seen with the Omicron variant, is impractical, Dr. Offit emphasized. Instead, a newer, variant-specific vaccine might be needed if a variant emerges that overrides the protection against severe disease currently afforded by the available vaccines, he said. “But we’re not there yet. For now, we are going to have to realize that it is virtually impossible to prevent mild COVID without frequent boosting. So, let’s learn to accept that the goal of COVID vaccines is to prevent severe and not mild illness and stop talking about frequent boosting. Otherwise, we will never be able to live our lives as before,” he wrote.
The study was supported by the U.K. Health Security Agency. The researchers and Dr. Offit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEW ENGLAND JOURNAL OF MEDICINE
COVID-19 found in 29 types of animals, scientists say
according to researchers’ latest tally.
In most cases, humans infect animals, and animals don’t transmit the virus back to humans. But scientists have expressed concerns about recent research that shows some animals – such as mink and deer – appear to be able to spread the virus to humans.
In addition, the virus will likely continue to circulate in wild animals, which could lead to new mutations, some of which may make the virus less susceptible to people’s immunity from current vaccines. Researchers are calling for better surveillance of animals, especially in the wild, to track any new variants.
“It could be evolving in hosts we are not aware of,” Eman Anis, PhD, an assistant professor of microbiology at the University of Pennsylvania, Philadelphia, told the Philadelphia Inquirer.
Scientists have identified the virus in a growing list of animals, according to the Centers for Disease Control and Prevention, including cats, dogs, ferrets, gorillas, hamsters, hippos, hyenas, mice, otters, pigs, rabbits, and tigers. In many cases, humans spread the coronavirus to pets at home or to wildlife in zoos and sanctuaries.
In the study, published in bioRxiv, researchers identified a person who tested positive after close contact with infected white-tailed deer. The coronavirus had evolved dozens of mutations not found in other strains.
Even with the changes, the virus they found doesn’t appear different enough to evade current vaccines, the researchers reported. The vaccines target the spike protein on the outside of coronavirus cells, and the mutations that happened in deer occurred elsewhere in the virus.
At the same time, scientists have noted that this points to the need to step up monitoring in wild animals before mutations become a problem.
“This is no need to panic, but this is not something we can ignore,” Suresh Kuchipudi, PhD, a professor of veterinary and biomedical sciences at Pennsylvania State University in University Park, told the Inquirer.
Dr. Kuchipudi, who wasn’t involved with the Canadian study, has done other studies that found COVID-19 in deer. As the coronavirus continues to circulate in deer, more mutations will arise, he noted.
“It’s hard to predict what evolution’s going to come up with,” Frederic Bushman, a microbiology professor at the University of Pennsylvania, told the Inquirer.
“The virus will probably change different ways in different animals. Some of them probably won’t infect humans as well,” he said. “But the fear is that maybe some new one will come along that does infect humans well.”
A version of this article first appeared on WebMD.com.
according to researchers’ latest tally.
In most cases, humans infect animals, and animals don’t transmit the virus back to humans. But scientists have expressed concerns about recent research that shows some animals – such as mink and deer – appear to be able to spread the virus to humans.
In addition, the virus will likely continue to circulate in wild animals, which could lead to new mutations, some of which may make the virus less susceptible to people’s immunity from current vaccines. Researchers are calling for better surveillance of animals, especially in the wild, to track any new variants.
“It could be evolving in hosts we are not aware of,” Eman Anis, PhD, an assistant professor of microbiology at the University of Pennsylvania, Philadelphia, told the Philadelphia Inquirer.
Scientists have identified the virus in a growing list of animals, according to the Centers for Disease Control and Prevention, including cats, dogs, ferrets, gorillas, hamsters, hippos, hyenas, mice, otters, pigs, rabbits, and tigers. In many cases, humans spread the coronavirus to pets at home or to wildlife in zoos and sanctuaries.
In the study, published in bioRxiv, researchers identified a person who tested positive after close contact with infected white-tailed deer. The coronavirus had evolved dozens of mutations not found in other strains.
Even with the changes, the virus they found doesn’t appear different enough to evade current vaccines, the researchers reported. The vaccines target the spike protein on the outside of coronavirus cells, and the mutations that happened in deer occurred elsewhere in the virus.
At the same time, scientists have noted that this points to the need to step up monitoring in wild animals before mutations become a problem.
“This is no need to panic, but this is not something we can ignore,” Suresh Kuchipudi, PhD, a professor of veterinary and biomedical sciences at Pennsylvania State University in University Park, told the Inquirer.
Dr. Kuchipudi, who wasn’t involved with the Canadian study, has done other studies that found COVID-19 in deer. As the coronavirus continues to circulate in deer, more mutations will arise, he noted.
“It’s hard to predict what evolution’s going to come up with,” Frederic Bushman, a microbiology professor at the University of Pennsylvania, told the Inquirer.
“The virus will probably change different ways in different animals. Some of them probably won’t infect humans as well,” he said. “But the fear is that maybe some new one will come along that does infect humans well.”
A version of this article first appeared on WebMD.com.
according to researchers’ latest tally.
In most cases, humans infect animals, and animals don’t transmit the virus back to humans. But scientists have expressed concerns about recent research that shows some animals – such as mink and deer – appear to be able to spread the virus to humans.
In addition, the virus will likely continue to circulate in wild animals, which could lead to new mutations, some of which may make the virus less susceptible to people’s immunity from current vaccines. Researchers are calling for better surveillance of animals, especially in the wild, to track any new variants.
“It could be evolving in hosts we are not aware of,” Eman Anis, PhD, an assistant professor of microbiology at the University of Pennsylvania, Philadelphia, told the Philadelphia Inquirer.
Scientists have identified the virus in a growing list of animals, according to the Centers for Disease Control and Prevention, including cats, dogs, ferrets, gorillas, hamsters, hippos, hyenas, mice, otters, pigs, rabbits, and tigers. In many cases, humans spread the coronavirus to pets at home or to wildlife in zoos and sanctuaries.
In the study, published in bioRxiv, researchers identified a person who tested positive after close contact with infected white-tailed deer. The coronavirus had evolved dozens of mutations not found in other strains.
Even with the changes, the virus they found doesn’t appear different enough to evade current vaccines, the researchers reported. The vaccines target the spike protein on the outside of coronavirus cells, and the mutations that happened in deer occurred elsewhere in the virus.
At the same time, scientists have noted that this points to the need to step up monitoring in wild animals before mutations become a problem.
“This is no need to panic, but this is not something we can ignore,” Suresh Kuchipudi, PhD, a professor of veterinary and biomedical sciences at Pennsylvania State University in University Park, told the Inquirer.
Dr. Kuchipudi, who wasn’t involved with the Canadian study, has done other studies that found COVID-19 in deer. As the coronavirus continues to circulate in deer, more mutations will arise, he noted.
“It’s hard to predict what evolution’s going to come up with,” Frederic Bushman, a microbiology professor at the University of Pennsylvania, told the Inquirer.
“The virus will probably change different ways in different animals. Some of them probably won’t infect humans as well,” he said. “But the fear is that maybe some new one will come along that does infect humans well.”
A version of this article first appeared on WebMD.com.
Pan-coronavirus vaccines may be key to fighting future pandemics
As the COVID-19 pandemic winds down – for the time being at least – efforts are ramping up to develop next-generation vaccines that can protect against future novel coronaviruses and variants. Several projects are presenting clever combinations of viral parts to the immune system that evoke a robust and hopefully lasting response.
The coming generation of “pan” vaccines aims to tamp down SARS-CoV-2, its closest relatives, and whatever may come into tamer respiratory viruses like the common cold. Whatever the eventual components of this new generation of vaccines, experts agree on the goal: preventing severe disease and death. And a broader approach is critical.
“All the vaccines have been amazing. But we’re playing a whack-a-mole game with the variants. We need to take a step back and ask if a pan-variant vaccine is possible. That’s important because Omicron isn’t the last variant,” said Jacob Lemieux, MD, PhD, instructor in medicine and infectious disease specialist at Massachusetts General Hospital, Boston.
A broad spectrum vaccine
The drive to create a vaccine that would deter multiple coronaviruses arose early, among many researchers. An article published in Nature in May 2020 by National Institute of Allergy and Infectious Diseases researcher Luca T. Giurgea, MD, and colleagues said it all in the title: “Universal coronavirus vaccines: the time to start is now.”
Their concerns? The diversity of bat coronaviruses poised to jump into humans; the high mutability of the spike gene that the immune response recognizes; and the persistence of mutations in an RNA virus, which can’t repair errors.
Work on broader vaccines began in several labs as SARS-CoV-2 spawned variant after variant.
On Sept. 28, NIAID announced funding for developing ‘pan-coronavirus’ vaccines – the quotation marks theirs to indicate that a magic bullet against any new coronavirus is unrealistic. “These new awards are designed to look ahead and prepare for the next generation of coronaviruses with pandemic potential,” said NIAID director Anthony S. Fauci, MD. An initial three awards went to groups at the University of Wisconsin, Brigham and Women’s Hospital, and Duke University.
President Biden mentioned the NIAID funding in his State of the Union Address. He also talked about how the Biomedical Advanced Research and Development Authority, founded in 2006 to prepare for public health emergencies, is spearheading development of new vaccine platforms and vaccines that target a broader swath of pathogen parts.
Meanwhile, individual researchers from eclectic fields are finding new ways to prevent future pandemics.
Artem Babaian, PhD, a computational biologist at the University of Cambridge (England), had the idea to probe National Institutes of Health genome databases, going back more than a decade, for overlooked novel coronaviruses. He started the project while he was between jobs as the pandemic was unfurling, using a telltale enzyme unique to the RNA viruses to fish out COVID cousins. The work is published in Nature and the data freely available at serratus.io.
Among the nearly 132,000 novel RNA viruses Dr. Babaian’s team found, 9 were from previously unrecognized coronaviruses. The novel nine came from “ecologically diverse sources”: a seahorse, an axolotl, an eel, and several fishes. Deciphering the topographies of these coronaviruses may provide clues to developing vaccines that stay ahead of future pandemics.
But optics are important in keeping expectations reasonable. “‘Universal vaccine’ is a misnomer. I think about it as ‘broad spectrum vaccines.’ It’s critical to be up front that these vaccines can never guarantee immunity against all coronaviruses. There are no absolutes in biology, but they hopefully will work against the dangers that we do know exist. A vaccine that mimics exposure to many coronaviruses could protect against a currently unknown coronavirus, especially if slower-evolving antigens are included,” Dr. Babaian said in an interview.
Nikolai Petrovsky, MD, PhD, of Flinders University, Adelaide, and the biotechnology company Vaccine Pty, agrees, calling a literal pan-coronavirus vaccine a “pipe dream. What I do think is achievable is a broadly protective, pan–CoV-19 vaccine – I can say that because we have already developed and tested it, combining antigens rather than trying just one that can do everything.”
Immunity lures
The broader vaccines in development display viral antigens, such as spike proteins, to the immune system on diverse frameworks. Here are a few approaches.
Ferritin nanoparticles: A candidate vaccine from the emerging infectious diseases branch of Water Reed National Military Medical Center began phase 1 human trials in April 2021. Called SpFN, the vaccine consists of arrays of ferritin nanoparticles linked to spike proteins from various variants and species. Ferritin is a protein that binds and stores iron in the body.
“The repetitive and ordered display of the coronavirus spike protein on a multifaced nanoparticle may stimulate immunity in such a way as to translate into significantly broader protection,” said Walter Reed’s branch director and vaccine coinventor Kayvon Modjarrad, MD, PhD.
A second vaccine targets only the “bullseye” part of the spike that the virus uses to attach and gain access to human cells, called the receptor-binding domain (RBD), of SARS-CoV-2 variants and of the virus behind the original SARS. The preclinical data appeared in Science Translational Medicine.
Barton Haynes, MD and colleagues at the Duke Human Vaccine Institute are also using ferritin to design and develop a “pan-betacoronavirus vaccine,” referring to the genus to which SARS-CoV-2 belongs. They say their results in macaques, published in Nature, “demonstrate that current mRNA-based vaccines may provide some protection from future outbreaks of zoonotic betacoronaviruses.”
Mosaic nanoparticles: Graduate student Alexander Cohen is leading an effort at CalTech, in the lab of Pamela Bjorkman, PhD, that uses nanoparticles consisting of proteins from a bacterium (Strep pyogenes) to which RBDs from spike proteins of four or eight different betacoronaviruses are attached. The strategy demonstrates that the whole is greater than the sum of the parts.
“Alex’s results show that it is possible to raise diverse neutralizing antibody responses, even against coronavirus strains that were not represented on the injected nanoparticle. We are hopeful that this technology could be used to protect against future animal coronaviruses that cross into humans,” said Dr. Björkman. The work appeared in Science.
Candidate vaccines from Inovio Pharmaceuticals also use a mosaic spike strategy, but with DNA rings (plasmids) rather than nanoparticles. One version works against pre-Omicron variants and is being tested against Omicron, and another with “pan–COVID-19” coverage has tested well in animal models. Inovio’s vaccines are delivered into the skin using a special device that applies an electric pulse that increases the cells’ permeability.
Chimeric spikes: Yet another approach is to fashion vaccines from various parts of the betacoronaviruses that are most closely related to SARS-CoV-2 – the pathogens behind Middle East respiratory syndrome and severe acute respiratory syndrome as well as several bat viruses and a few pangolin ones. The abundance and ubiquity of these viruses provide a toolbox of sorts, with instructions written in the language of RNA, from which to select, dissect, recombine, and customize vaccines.
“SARS-like viruses can recombine and exhibit great genetic diversity in several parts of the genome. We designed chimeric spikes to improve coverage of a multiplexed vaccine,” said David Martinez, PhD.
His team at the University of North Carolina at Chapel Hill has developed mRNA vaccines that deliver “scrambled coronavirus spikes” representing various parts, not just the RBD, as described in Science.
In mice, the chimeric vaccines elicit robust T- and B-cell immune responses, which stimulate antibody production and control other facets of building immunity.
Beyond the spike bullseye
The challenge of developing pan-coronavirus vaccines is dual. “The very best vaccines are highly specific to each strain, and the universal vaccines have to sacrifice effectiveness to get broad coverage. Life is a trade-off.” Dr. Petrovsky told this news organization.
Efforts to broaden vaccine efficacy venture beyond targeting the RBD bullseyes of the spike triplets that festoon the virus. Some projects are focusing on less changeable spike parts that are more alike among less closely related coronaviruses than is the mutation-prone RBD. For example, the peptides that twist into the “stem-helix” portion of the part of the spike that adheres to host cells are the basis of some candidate vaccines now in preclinical studies.
Still other vaccines aren’t spike based at all. French company Osivax, for example, is working on a vaccine that targets the nucleocapsid protein that shields the viral RNA. The hope is that presenting various faces of the pathogen may spark immunity beyond an initial antibody rush and evoke more diverse and lasting T-cell responses.
With the myriad efforts to back up the first generation of COVID-19 vaccines with new ones offering broader protection, it appears that science may have finally learned from history.
“After the SARS outbreak, we lost interest and failed to complete development of a vaccine for use in case of a recurrent outbreak. We must not make the same mistake again,” Dr. Giurgea and colleagues wrote in their Nature article about universal coronavirus vaccines.
A version of this article first appeared on Medscape.com.
As the COVID-19 pandemic winds down – for the time being at least – efforts are ramping up to develop next-generation vaccines that can protect against future novel coronaviruses and variants. Several projects are presenting clever combinations of viral parts to the immune system that evoke a robust and hopefully lasting response.
The coming generation of “pan” vaccines aims to tamp down SARS-CoV-2, its closest relatives, and whatever may come into tamer respiratory viruses like the common cold. Whatever the eventual components of this new generation of vaccines, experts agree on the goal: preventing severe disease and death. And a broader approach is critical.
“All the vaccines have been amazing. But we’re playing a whack-a-mole game with the variants. We need to take a step back and ask if a pan-variant vaccine is possible. That’s important because Omicron isn’t the last variant,” said Jacob Lemieux, MD, PhD, instructor in medicine and infectious disease specialist at Massachusetts General Hospital, Boston.
A broad spectrum vaccine
The drive to create a vaccine that would deter multiple coronaviruses arose early, among many researchers. An article published in Nature in May 2020 by National Institute of Allergy and Infectious Diseases researcher Luca T. Giurgea, MD, and colleagues said it all in the title: “Universal coronavirus vaccines: the time to start is now.”
Their concerns? The diversity of bat coronaviruses poised to jump into humans; the high mutability of the spike gene that the immune response recognizes; and the persistence of mutations in an RNA virus, which can’t repair errors.
Work on broader vaccines began in several labs as SARS-CoV-2 spawned variant after variant.
On Sept. 28, NIAID announced funding for developing ‘pan-coronavirus’ vaccines – the quotation marks theirs to indicate that a magic bullet against any new coronavirus is unrealistic. “These new awards are designed to look ahead and prepare for the next generation of coronaviruses with pandemic potential,” said NIAID director Anthony S. Fauci, MD. An initial three awards went to groups at the University of Wisconsin, Brigham and Women’s Hospital, and Duke University.
President Biden mentioned the NIAID funding in his State of the Union Address. He also talked about how the Biomedical Advanced Research and Development Authority, founded in 2006 to prepare for public health emergencies, is spearheading development of new vaccine platforms and vaccines that target a broader swath of pathogen parts.
Meanwhile, individual researchers from eclectic fields are finding new ways to prevent future pandemics.
Artem Babaian, PhD, a computational biologist at the University of Cambridge (England), had the idea to probe National Institutes of Health genome databases, going back more than a decade, for overlooked novel coronaviruses. He started the project while he was between jobs as the pandemic was unfurling, using a telltale enzyme unique to the RNA viruses to fish out COVID cousins. The work is published in Nature and the data freely available at serratus.io.
Among the nearly 132,000 novel RNA viruses Dr. Babaian’s team found, 9 were from previously unrecognized coronaviruses. The novel nine came from “ecologically diverse sources”: a seahorse, an axolotl, an eel, and several fishes. Deciphering the topographies of these coronaviruses may provide clues to developing vaccines that stay ahead of future pandemics.
But optics are important in keeping expectations reasonable. “‘Universal vaccine’ is a misnomer. I think about it as ‘broad spectrum vaccines.’ It’s critical to be up front that these vaccines can never guarantee immunity against all coronaviruses. There are no absolutes in biology, but they hopefully will work against the dangers that we do know exist. A vaccine that mimics exposure to many coronaviruses could protect against a currently unknown coronavirus, especially if slower-evolving antigens are included,” Dr. Babaian said in an interview.
Nikolai Petrovsky, MD, PhD, of Flinders University, Adelaide, and the biotechnology company Vaccine Pty, agrees, calling a literal pan-coronavirus vaccine a “pipe dream. What I do think is achievable is a broadly protective, pan–CoV-19 vaccine – I can say that because we have already developed and tested it, combining antigens rather than trying just one that can do everything.”
Immunity lures
The broader vaccines in development display viral antigens, such as spike proteins, to the immune system on diverse frameworks. Here are a few approaches.
Ferritin nanoparticles: A candidate vaccine from the emerging infectious diseases branch of Water Reed National Military Medical Center began phase 1 human trials in April 2021. Called SpFN, the vaccine consists of arrays of ferritin nanoparticles linked to spike proteins from various variants and species. Ferritin is a protein that binds and stores iron in the body.
“The repetitive and ordered display of the coronavirus spike protein on a multifaced nanoparticle may stimulate immunity in such a way as to translate into significantly broader protection,” said Walter Reed’s branch director and vaccine coinventor Kayvon Modjarrad, MD, PhD.
A second vaccine targets only the “bullseye” part of the spike that the virus uses to attach and gain access to human cells, called the receptor-binding domain (RBD), of SARS-CoV-2 variants and of the virus behind the original SARS. The preclinical data appeared in Science Translational Medicine.
Barton Haynes, MD and colleagues at the Duke Human Vaccine Institute are also using ferritin to design and develop a “pan-betacoronavirus vaccine,” referring to the genus to which SARS-CoV-2 belongs. They say their results in macaques, published in Nature, “demonstrate that current mRNA-based vaccines may provide some protection from future outbreaks of zoonotic betacoronaviruses.”
Mosaic nanoparticles: Graduate student Alexander Cohen is leading an effort at CalTech, in the lab of Pamela Bjorkman, PhD, that uses nanoparticles consisting of proteins from a bacterium (Strep pyogenes) to which RBDs from spike proteins of four or eight different betacoronaviruses are attached. The strategy demonstrates that the whole is greater than the sum of the parts.
“Alex’s results show that it is possible to raise diverse neutralizing antibody responses, even against coronavirus strains that were not represented on the injected nanoparticle. We are hopeful that this technology could be used to protect against future animal coronaviruses that cross into humans,” said Dr. Björkman. The work appeared in Science.
Candidate vaccines from Inovio Pharmaceuticals also use a mosaic spike strategy, but with DNA rings (plasmids) rather than nanoparticles. One version works against pre-Omicron variants and is being tested against Omicron, and another with “pan–COVID-19” coverage has tested well in animal models. Inovio’s vaccines are delivered into the skin using a special device that applies an electric pulse that increases the cells’ permeability.
Chimeric spikes: Yet another approach is to fashion vaccines from various parts of the betacoronaviruses that are most closely related to SARS-CoV-2 – the pathogens behind Middle East respiratory syndrome and severe acute respiratory syndrome as well as several bat viruses and a few pangolin ones. The abundance and ubiquity of these viruses provide a toolbox of sorts, with instructions written in the language of RNA, from which to select, dissect, recombine, and customize vaccines.
“SARS-like viruses can recombine and exhibit great genetic diversity in several parts of the genome. We designed chimeric spikes to improve coverage of a multiplexed vaccine,” said David Martinez, PhD.
His team at the University of North Carolina at Chapel Hill has developed mRNA vaccines that deliver “scrambled coronavirus spikes” representing various parts, not just the RBD, as described in Science.
In mice, the chimeric vaccines elicit robust T- and B-cell immune responses, which stimulate antibody production and control other facets of building immunity.
Beyond the spike bullseye
The challenge of developing pan-coronavirus vaccines is dual. “The very best vaccines are highly specific to each strain, and the universal vaccines have to sacrifice effectiveness to get broad coverage. Life is a trade-off.” Dr. Petrovsky told this news organization.
Efforts to broaden vaccine efficacy venture beyond targeting the RBD bullseyes of the spike triplets that festoon the virus. Some projects are focusing on less changeable spike parts that are more alike among less closely related coronaviruses than is the mutation-prone RBD. For example, the peptides that twist into the “stem-helix” portion of the part of the spike that adheres to host cells are the basis of some candidate vaccines now in preclinical studies.
Still other vaccines aren’t spike based at all. French company Osivax, for example, is working on a vaccine that targets the nucleocapsid protein that shields the viral RNA. The hope is that presenting various faces of the pathogen may spark immunity beyond an initial antibody rush and evoke more diverse and lasting T-cell responses.
With the myriad efforts to back up the first generation of COVID-19 vaccines with new ones offering broader protection, it appears that science may have finally learned from history.
“After the SARS outbreak, we lost interest and failed to complete development of a vaccine for use in case of a recurrent outbreak. We must not make the same mistake again,” Dr. Giurgea and colleagues wrote in their Nature article about universal coronavirus vaccines.
A version of this article first appeared on Medscape.com.
As the COVID-19 pandemic winds down – for the time being at least – efforts are ramping up to develop next-generation vaccines that can protect against future novel coronaviruses and variants. Several projects are presenting clever combinations of viral parts to the immune system that evoke a robust and hopefully lasting response.
The coming generation of “pan” vaccines aims to tamp down SARS-CoV-2, its closest relatives, and whatever may come into tamer respiratory viruses like the common cold. Whatever the eventual components of this new generation of vaccines, experts agree on the goal: preventing severe disease and death. And a broader approach is critical.
“All the vaccines have been amazing. But we’re playing a whack-a-mole game with the variants. We need to take a step back and ask if a pan-variant vaccine is possible. That’s important because Omicron isn’t the last variant,” said Jacob Lemieux, MD, PhD, instructor in medicine and infectious disease specialist at Massachusetts General Hospital, Boston.
A broad spectrum vaccine
The drive to create a vaccine that would deter multiple coronaviruses arose early, among many researchers. An article published in Nature in May 2020 by National Institute of Allergy and Infectious Diseases researcher Luca T. Giurgea, MD, and colleagues said it all in the title: “Universal coronavirus vaccines: the time to start is now.”
Their concerns? The diversity of bat coronaviruses poised to jump into humans; the high mutability of the spike gene that the immune response recognizes; and the persistence of mutations in an RNA virus, which can’t repair errors.
Work on broader vaccines began in several labs as SARS-CoV-2 spawned variant after variant.
On Sept. 28, NIAID announced funding for developing ‘pan-coronavirus’ vaccines – the quotation marks theirs to indicate that a magic bullet against any new coronavirus is unrealistic. “These new awards are designed to look ahead and prepare for the next generation of coronaviruses with pandemic potential,” said NIAID director Anthony S. Fauci, MD. An initial three awards went to groups at the University of Wisconsin, Brigham and Women’s Hospital, and Duke University.
President Biden mentioned the NIAID funding in his State of the Union Address. He also talked about how the Biomedical Advanced Research and Development Authority, founded in 2006 to prepare for public health emergencies, is spearheading development of new vaccine platforms and vaccines that target a broader swath of pathogen parts.
Meanwhile, individual researchers from eclectic fields are finding new ways to prevent future pandemics.
Artem Babaian, PhD, a computational biologist at the University of Cambridge (England), had the idea to probe National Institutes of Health genome databases, going back more than a decade, for overlooked novel coronaviruses. He started the project while he was between jobs as the pandemic was unfurling, using a telltale enzyme unique to the RNA viruses to fish out COVID cousins. The work is published in Nature and the data freely available at serratus.io.
Among the nearly 132,000 novel RNA viruses Dr. Babaian’s team found, 9 were from previously unrecognized coronaviruses. The novel nine came from “ecologically diverse sources”: a seahorse, an axolotl, an eel, and several fishes. Deciphering the topographies of these coronaviruses may provide clues to developing vaccines that stay ahead of future pandemics.
But optics are important in keeping expectations reasonable. “‘Universal vaccine’ is a misnomer. I think about it as ‘broad spectrum vaccines.’ It’s critical to be up front that these vaccines can never guarantee immunity against all coronaviruses. There are no absolutes in biology, but they hopefully will work against the dangers that we do know exist. A vaccine that mimics exposure to many coronaviruses could protect against a currently unknown coronavirus, especially if slower-evolving antigens are included,” Dr. Babaian said in an interview.
Nikolai Petrovsky, MD, PhD, of Flinders University, Adelaide, and the biotechnology company Vaccine Pty, agrees, calling a literal pan-coronavirus vaccine a “pipe dream. What I do think is achievable is a broadly protective, pan–CoV-19 vaccine – I can say that because we have already developed and tested it, combining antigens rather than trying just one that can do everything.”
Immunity lures
The broader vaccines in development display viral antigens, such as spike proteins, to the immune system on diverse frameworks. Here are a few approaches.
Ferritin nanoparticles: A candidate vaccine from the emerging infectious diseases branch of Water Reed National Military Medical Center began phase 1 human trials in April 2021. Called SpFN, the vaccine consists of arrays of ferritin nanoparticles linked to spike proteins from various variants and species. Ferritin is a protein that binds and stores iron in the body.
“The repetitive and ordered display of the coronavirus spike protein on a multifaced nanoparticle may stimulate immunity in such a way as to translate into significantly broader protection,” said Walter Reed’s branch director and vaccine coinventor Kayvon Modjarrad, MD, PhD.
A second vaccine targets only the “bullseye” part of the spike that the virus uses to attach and gain access to human cells, called the receptor-binding domain (RBD), of SARS-CoV-2 variants and of the virus behind the original SARS. The preclinical data appeared in Science Translational Medicine.
Barton Haynes, MD and colleagues at the Duke Human Vaccine Institute are also using ferritin to design and develop a “pan-betacoronavirus vaccine,” referring to the genus to which SARS-CoV-2 belongs. They say their results in macaques, published in Nature, “demonstrate that current mRNA-based vaccines may provide some protection from future outbreaks of zoonotic betacoronaviruses.”
Mosaic nanoparticles: Graduate student Alexander Cohen is leading an effort at CalTech, in the lab of Pamela Bjorkman, PhD, that uses nanoparticles consisting of proteins from a bacterium (Strep pyogenes) to which RBDs from spike proteins of four or eight different betacoronaviruses are attached. The strategy demonstrates that the whole is greater than the sum of the parts.
“Alex’s results show that it is possible to raise diverse neutralizing antibody responses, even against coronavirus strains that were not represented on the injected nanoparticle. We are hopeful that this technology could be used to protect against future animal coronaviruses that cross into humans,” said Dr. Björkman. The work appeared in Science.
Candidate vaccines from Inovio Pharmaceuticals also use a mosaic spike strategy, but with DNA rings (plasmids) rather than nanoparticles. One version works against pre-Omicron variants and is being tested against Omicron, and another with “pan–COVID-19” coverage has tested well in animal models. Inovio’s vaccines are delivered into the skin using a special device that applies an electric pulse that increases the cells’ permeability.
Chimeric spikes: Yet another approach is to fashion vaccines from various parts of the betacoronaviruses that are most closely related to SARS-CoV-2 – the pathogens behind Middle East respiratory syndrome and severe acute respiratory syndrome as well as several bat viruses and a few pangolin ones. The abundance and ubiquity of these viruses provide a toolbox of sorts, with instructions written in the language of RNA, from which to select, dissect, recombine, and customize vaccines.
“SARS-like viruses can recombine and exhibit great genetic diversity in several parts of the genome. We designed chimeric spikes to improve coverage of a multiplexed vaccine,” said David Martinez, PhD.
His team at the University of North Carolina at Chapel Hill has developed mRNA vaccines that deliver “scrambled coronavirus spikes” representing various parts, not just the RBD, as described in Science.
In mice, the chimeric vaccines elicit robust T- and B-cell immune responses, which stimulate antibody production and control other facets of building immunity.
Beyond the spike bullseye
The challenge of developing pan-coronavirus vaccines is dual. “The very best vaccines are highly specific to each strain, and the universal vaccines have to sacrifice effectiveness to get broad coverage. Life is a trade-off.” Dr. Petrovsky told this news organization.
Efforts to broaden vaccine efficacy venture beyond targeting the RBD bullseyes of the spike triplets that festoon the virus. Some projects are focusing on less changeable spike parts that are more alike among less closely related coronaviruses than is the mutation-prone RBD. For example, the peptides that twist into the “stem-helix” portion of the part of the spike that adheres to host cells are the basis of some candidate vaccines now in preclinical studies.
Still other vaccines aren’t spike based at all. French company Osivax, for example, is working on a vaccine that targets the nucleocapsid protein that shields the viral RNA. The hope is that presenting various faces of the pathogen may spark immunity beyond an initial antibody rush and evoke more diverse and lasting T-cell responses.
With the myriad efforts to back up the first generation of COVID-19 vaccines with new ones offering broader protection, it appears that science may have finally learned from history.
“After the SARS outbreak, we lost interest and failed to complete development of a vaccine for use in case of a recurrent outbreak. We must not make the same mistake again,” Dr. Giurgea and colleagues wrote in their Nature article about universal coronavirus vaccines.
A version of this article first appeared on Medscape.com.
Side effects of COVID mRNA vaccines are mild and short, large study confirms
Data from the first 6 months after the rollout of mRNA COVID-19 vaccines in the United States released today show that adverse effects from shots are typically mild and short-lived.
Findings of the large study, compiled after nearly 300 million doses were administered, were published online March 7 in The Lancet Infectious Diseases.
Researchers, led by Hannah G. Rosenblum, MD, with the Centers for Disease Control and Prevention COVID Response Team, used passive U.S. surveillance data collected through the Vaccine Adverse Event Reporting System (VAERS), and the active system, v-safe, starting in December 2020 through the first 6 months of the U.S. COVID-19 vaccination program. V-safe is a voluntary, smartphone-based system set up in 2020 specifically for monitoring reactions to COVID-19 and health effects after vaccination. The health effects information from v-safe is presented in this study for the first time.
Of the 298.7 million doses of mRNA vaccines administered in the U.S. during the study period, VAERS processed 340,522 reports. Of those, 313,499 (92.1%) were nonserious; 22,527 (6.6%) were serious (nondeath); and 4,496 (1.3%) were deaths.
From v-safe reporting, researchers learned that about 71% of the 7.9 million participants reported local or systemic reactions, more frequently after dose 2 than after dose 1. Of those reporting reactions after dose 1, about two-thirds (68.6%) reported a local reaction and 52.7% reported a systemic reaction.
Among other findings:
- Injection-site pain occurred after dose 1 in 66.2% of participants and 68.6% after dose 2.
- One-third of participants (33.9%) reported fatigue after dose 1 and 55.7% after dose 2.
- Headache was reported among 27% of participants after dose 1 and 46.2% after dose 2.
- When injection site pain, fatigue, or headaches were reported, the reports were usually in the first week after vaccination.
- Reports of being unable to work or do normal daily activities, or instances of seeking medical care, occurred more commonly after dose 2 (32.1%) than after dose 1 (11.9%). Fewer than 1% of participants reported seeking medical care after dose 1 or 2 of the vaccine.
- Reactions and health effects were reported more often in female than in male recipients, and in people younger than 65 years, compared with older people.
- Serious adverse events, including myocarditis, have been identified following mRNA vaccinations, but the events are rare.
The authors wrote that these results are consistent with preauthorization clinical trials and early postauthorization reports.
“On the basis of our findings, mild to moderate transient reactogenicity should be anticipated,” they said, “particularly among younger and female vaccine recipients.”
‘Robust and reassuring data’
“The safety monitoring of the mRNA COVID-19 vaccines stands out as the most comprehensive of any vaccine in U.S. history. The use of these complementary monitoring systems has provided robust and reassuring data,” Matthew S. Krantz, MD, with the division of allergy, pulmonary, and critical care medicine at Vanderbilt University, Nashville, Tenn., and Elizabeth J. Phillips, MD, with the department of pathology, microbiology, and immunology at Vanderbilt, wrote in a related commentary in The Lancet Infectious Diseases.
They point out that the v-safe reports of reactions are consistent with those reported from clinical trials and a large population study in the United Kingdom.
Dr. Phillips said in a press release, “[A]lthough approximately one in 1,000 individuals vaccinated may have an adverse effect, most of these are nonserious. No unusual patterns emerged in the cause of death or serious adverse effects among VAERS reports. For adverse events of special interest, it is reassuring that there were no unexpected signals other than myopericarditis and anaphylaxis, already known to be associated with mRNA vaccines.”
The study authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from the first 6 months after the rollout of mRNA COVID-19 vaccines in the United States released today show that adverse effects from shots are typically mild and short-lived.
Findings of the large study, compiled after nearly 300 million doses were administered, were published online March 7 in The Lancet Infectious Diseases.
Researchers, led by Hannah G. Rosenblum, MD, with the Centers for Disease Control and Prevention COVID Response Team, used passive U.S. surveillance data collected through the Vaccine Adverse Event Reporting System (VAERS), and the active system, v-safe, starting in December 2020 through the first 6 months of the U.S. COVID-19 vaccination program. V-safe is a voluntary, smartphone-based system set up in 2020 specifically for monitoring reactions to COVID-19 and health effects after vaccination. The health effects information from v-safe is presented in this study for the first time.
Of the 298.7 million doses of mRNA vaccines administered in the U.S. during the study period, VAERS processed 340,522 reports. Of those, 313,499 (92.1%) were nonserious; 22,527 (6.6%) were serious (nondeath); and 4,496 (1.3%) were deaths.
From v-safe reporting, researchers learned that about 71% of the 7.9 million participants reported local or systemic reactions, more frequently after dose 2 than after dose 1. Of those reporting reactions after dose 1, about two-thirds (68.6%) reported a local reaction and 52.7% reported a systemic reaction.
Among other findings:
- Injection-site pain occurred after dose 1 in 66.2% of participants and 68.6% after dose 2.
- One-third of participants (33.9%) reported fatigue after dose 1 and 55.7% after dose 2.
- Headache was reported among 27% of participants after dose 1 and 46.2% after dose 2.
- When injection site pain, fatigue, or headaches were reported, the reports were usually in the first week after vaccination.
- Reports of being unable to work or do normal daily activities, or instances of seeking medical care, occurred more commonly after dose 2 (32.1%) than after dose 1 (11.9%). Fewer than 1% of participants reported seeking medical care after dose 1 or 2 of the vaccine.
- Reactions and health effects were reported more often in female than in male recipients, and in people younger than 65 years, compared with older people.
- Serious adverse events, including myocarditis, have been identified following mRNA vaccinations, but the events are rare.
The authors wrote that these results are consistent with preauthorization clinical trials and early postauthorization reports.
“On the basis of our findings, mild to moderate transient reactogenicity should be anticipated,” they said, “particularly among younger and female vaccine recipients.”
‘Robust and reassuring data’
“The safety monitoring of the mRNA COVID-19 vaccines stands out as the most comprehensive of any vaccine in U.S. history. The use of these complementary monitoring systems has provided robust and reassuring data,” Matthew S. Krantz, MD, with the division of allergy, pulmonary, and critical care medicine at Vanderbilt University, Nashville, Tenn., and Elizabeth J. Phillips, MD, with the department of pathology, microbiology, and immunology at Vanderbilt, wrote in a related commentary in The Lancet Infectious Diseases.
They point out that the v-safe reports of reactions are consistent with those reported from clinical trials and a large population study in the United Kingdom.
Dr. Phillips said in a press release, “[A]lthough approximately one in 1,000 individuals vaccinated may have an adverse effect, most of these are nonserious. No unusual patterns emerged in the cause of death or serious adverse effects among VAERS reports. For adverse events of special interest, it is reassuring that there were no unexpected signals other than myopericarditis and anaphylaxis, already known to be associated with mRNA vaccines.”
The study authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from the first 6 months after the rollout of mRNA COVID-19 vaccines in the United States released today show that adverse effects from shots are typically mild and short-lived.
Findings of the large study, compiled after nearly 300 million doses were administered, were published online March 7 in The Lancet Infectious Diseases.
Researchers, led by Hannah G. Rosenblum, MD, with the Centers for Disease Control and Prevention COVID Response Team, used passive U.S. surveillance data collected through the Vaccine Adverse Event Reporting System (VAERS), and the active system, v-safe, starting in December 2020 through the first 6 months of the U.S. COVID-19 vaccination program. V-safe is a voluntary, smartphone-based system set up in 2020 specifically for monitoring reactions to COVID-19 and health effects after vaccination. The health effects information from v-safe is presented in this study for the first time.
Of the 298.7 million doses of mRNA vaccines administered in the U.S. during the study period, VAERS processed 340,522 reports. Of those, 313,499 (92.1%) were nonserious; 22,527 (6.6%) were serious (nondeath); and 4,496 (1.3%) were deaths.
From v-safe reporting, researchers learned that about 71% of the 7.9 million participants reported local or systemic reactions, more frequently after dose 2 than after dose 1. Of those reporting reactions after dose 1, about two-thirds (68.6%) reported a local reaction and 52.7% reported a systemic reaction.
Among other findings:
- Injection-site pain occurred after dose 1 in 66.2% of participants and 68.6% after dose 2.
- One-third of participants (33.9%) reported fatigue after dose 1 and 55.7% after dose 2.
- Headache was reported among 27% of participants after dose 1 and 46.2% after dose 2.
- When injection site pain, fatigue, or headaches were reported, the reports were usually in the first week after vaccination.
- Reports of being unable to work or do normal daily activities, or instances of seeking medical care, occurred more commonly after dose 2 (32.1%) than after dose 1 (11.9%). Fewer than 1% of participants reported seeking medical care after dose 1 or 2 of the vaccine.
- Reactions and health effects were reported more often in female than in male recipients, and in people younger than 65 years, compared with older people.
- Serious adverse events, including myocarditis, have been identified following mRNA vaccinations, but the events are rare.
The authors wrote that these results are consistent with preauthorization clinical trials and early postauthorization reports.
“On the basis of our findings, mild to moderate transient reactogenicity should be anticipated,” they said, “particularly among younger and female vaccine recipients.”
‘Robust and reassuring data’
“The safety monitoring of the mRNA COVID-19 vaccines stands out as the most comprehensive of any vaccine in U.S. history. The use of these complementary monitoring systems has provided robust and reassuring data,” Matthew S. Krantz, MD, with the division of allergy, pulmonary, and critical care medicine at Vanderbilt University, Nashville, Tenn., and Elizabeth J. Phillips, MD, with the department of pathology, microbiology, and immunology at Vanderbilt, wrote in a related commentary in The Lancet Infectious Diseases.
They point out that the v-safe reports of reactions are consistent with those reported from clinical trials and a large population study in the United Kingdom.
Dr. Phillips said in a press release, “[A]lthough approximately one in 1,000 individuals vaccinated may have an adverse effect, most of these are nonserious. No unusual patterns emerged in the cause of death or serious adverse effects among VAERS reports. For adverse events of special interest, it is reassuring that there were no unexpected signals other than myopericarditis and anaphylaxis, already known to be associated with mRNA vaccines.”
The study authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
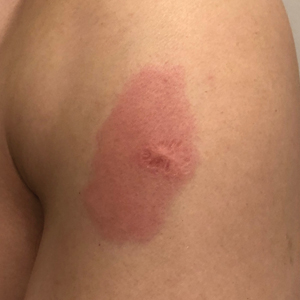
Reactivation of a BCG Vaccination Scar Following the First Dose of the Moderna COVID-19 Vaccine
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).
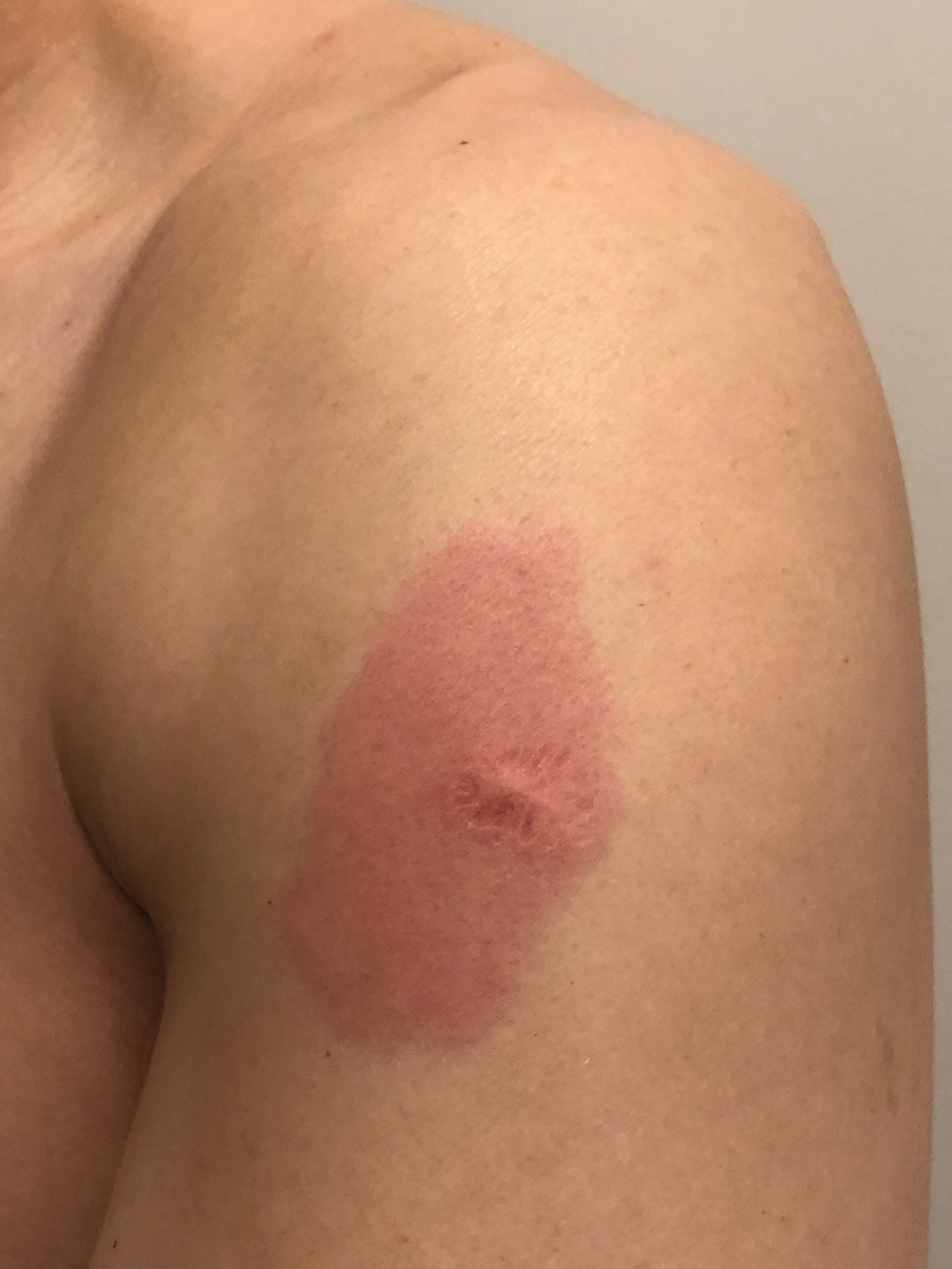
The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).

The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).

The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
Practice Points
- BCG vaccination scar reactivation is a potential benign, self-limited reaction in patients who receive the Moderna COVID-19 Vaccine.
- Symptoms of BCG vaccination scar reactivation, which is seen more commonly in children with Kawasaki disease, include redness, swelling, and ulceration.
Antivaccine physician pleads guilty to role in Capitol riot
California-based emergency physician Simone Melissa Gold, MD, JD, founder of the antivaccine group America’s Frontline Doctors (AFD) and leading voice in the antivaccine movement, has pleaded guilty to one of five charges related to the Jan. 6 Capitol riot.
According to the plea deal, Dr. Gold pleaded guilty to charges that she “did unlawfully and knowingly enter and remain in a restricted building and grounds, that is, any posted, cordoned-off, or otherwise restricted area within the United States Capitol and its grounds, during a time when the vice president was in the building without lawful authority to do so.” As part of the agreement, additional charges against her – obstructing an official proceeding and intent to disrupt the orderly conduct of government business – will be dismissed. She also agreed to cooperate with investigators, including allowing them to review social media postings made during the time surrounding the event.
Shortly after she was indicted, Dr. Gold told The Washington Post that she did not see any violence and that the event was “peaceful.” However, according to news reports, Dr. Gold acknowledged in her plea deal that she and her codefendant, John Herbert Strand, witnessed the assault of a police officer while they were outside the building.
Dr. Gold, 56, based in Beverly Hills, Calif., founded AFD in 2019. The group notes its goal is to “amplify the voices of concerned physicians and patients nationwide to combat those who push political and economic agendas at the expense of science and quality health care solutions.” Mr. Strand is the organization’s communication’s director.
The group has been a leading proponent of the use of ivermectin as a “safe and effective treatment” for COVID-19, according to its website.
In 2021, Dr. Gold spoke at an event called The Stand, representing AFD, where she promised to tell “the truth” about COVID vaccines, including that it was actually giving people the virus, that COVID was renamed from the “Wuhan Virus” as part of a cover-up, and touted treatments, including hydroxycholoroquine and ivermectin.
Dr. Gold has been one of the leading voices in the anti-vaccine movement. She has more than 400,000 Twitter followers; her Twitter profile includes a pinned tweet saying: “We are living in Orwellian times.” In addition to spreading vaccine misinformation, Dr. Gold has promoted the use of unproven treatments such as hydroxychloroquine and ivermectin.
Calls and emails to AFD regarding a statement on Gold’s plea made by this news organization were not returned by press time.
In October, Representative James E. Clyburn (D-S.C.), chairman of the Select Subcommittee on the Coronavirus Crisis, launched an investigation into organizations, including AFD, that spread misinformation and facilitate access to disproven and potentially hazardous treatments for COVID-19. According to news reports, Rep. Clyburn called the AFD and other such groups “predatory actors.”
Hospitals where Dr. Gold previously worked, including Providence St. Joseph Medical Center in Santa Monica, Calif., and Cedars-Sinai in Los Angeles, have disassociated themselves from her. On July 29, 2020, Cedars-Sinai Medical Center, where Gold previously worked, issued a statement that said, in part, “Simone Gold, MD, has not worked with Cedars-Sinai Medical Center or any of its offices or affiliates since 2015. For 3 weeks in late 2015, Dr. Gold was employed on a per diem basis by Cedars-Sinai Medical Network, a component of Cedars-Sinai. She worked during this brief time in a network urgent care clinic. Dr. Gold is not authorized to represent or speak about any information on behalf of Cedars-Sinai.”
Dr. Gold’s medical license in the state of California is current and she has no pending hearings before the state medical board, according to its website. On her own website, Dr. Gold says she “voluntarily refused” to renew her board certification last year, “due to the unethical behavior of the medical boards.”
Dr. Gold is also a licensed attorney, having earned her law degree in health policy analysis at Stanford (Calif.) Law School.
Dr. Gold faces 6 months in prison. Sentencing is scheduled for June 16.
A version of this article first appeared on Medscape.com.
California-based emergency physician Simone Melissa Gold, MD, JD, founder of the antivaccine group America’s Frontline Doctors (AFD) and leading voice in the antivaccine movement, has pleaded guilty to one of five charges related to the Jan. 6 Capitol riot.
According to the plea deal, Dr. Gold pleaded guilty to charges that she “did unlawfully and knowingly enter and remain in a restricted building and grounds, that is, any posted, cordoned-off, or otherwise restricted area within the United States Capitol and its grounds, during a time when the vice president was in the building without lawful authority to do so.” As part of the agreement, additional charges against her – obstructing an official proceeding and intent to disrupt the orderly conduct of government business – will be dismissed. She also agreed to cooperate with investigators, including allowing them to review social media postings made during the time surrounding the event.
Shortly after she was indicted, Dr. Gold told The Washington Post that she did not see any violence and that the event was “peaceful.” However, according to news reports, Dr. Gold acknowledged in her plea deal that she and her codefendant, John Herbert Strand, witnessed the assault of a police officer while they were outside the building.
Dr. Gold, 56, based in Beverly Hills, Calif., founded AFD in 2019. The group notes its goal is to “amplify the voices of concerned physicians and patients nationwide to combat those who push political and economic agendas at the expense of science and quality health care solutions.” Mr. Strand is the organization’s communication’s director.
The group has been a leading proponent of the use of ivermectin as a “safe and effective treatment” for COVID-19, according to its website.
In 2021, Dr. Gold spoke at an event called The Stand, representing AFD, where she promised to tell “the truth” about COVID vaccines, including that it was actually giving people the virus, that COVID was renamed from the “Wuhan Virus” as part of a cover-up, and touted treatments, including hydroxycholoroquine and ivermectin.
Dr. Gold has been one of the leading voices in the anti-vaccine movement. She has more than 400,000 Twitter followers; her Twitter profile includes a pinned tweet saying: “We are living in Orwellian times.” In addition to spreading vaccine misinformation, Dr. Gold has promoted the use of unproven treatments such as hydroxychloroquine and ivermectin.
Calls and emails to AFD regarding a statement on Gold’s plea made by this news organization were not returned by press time.
In October, Representative James E. Clyburn (D-S.C.), chairman of the Select Subcommittee on the Coronavirus Crisis, launched an investigation into organizations, including AFD, that spread misinformation and facilitate access to disproven and potentially hazardous treatments for COVID-19. According to news reports, Rep. Clyburn called the AFD and other such groups “predatory actors.”
Hospitals where Dr. Gold previously worked, including Providence St. Joseph Medical Center in Santa Monica, Calif., and Cedars-Sinai in Los Angeles, have disassociated themselves from her. On July 29, 2020, Cedars-Sinai Medical Center, where Gold previously worked, issued a statement that said, in part, “Simone Gold, MD, has not worked with Cedars-Sinai Medical Center or any of its offices or affiliates since 2015. For 3 weeks in late 2015, Dr. Gold was employed on a per diem basis by Cedars-Sinai Medical Network, a component of Cedars-Sinai. She worked during this brief time in a network urgent care clinic. Dr. Gold is not authorized to represent or speak about any information on behalf of Cedars-Sinai.”
Dr. Gold’s medical license in the state of California is current and she has no pending hearings before the state medical board, according to its website. On her own website, Dr. Gold says she “voluntarily refused” to renew her board certification last year, “due to the unethical behavior of the medical boards.”
Dr. Gold is also a licensed attorney, having earned her law degree in health policy analysis at Stanford (Calif.) Law School.
Dr. Gold faces 6 months in prison. Sentencing is scheduled for June 16.
A version of this article first appeared on Medscape.com.
California-based emergency physician Simone Melissa Gold, MD, JD, founder of the antivaccine group America’s Frontline Doctors (AFD) and leading voice in the antivaccine movement, has pleaded guilty to one of five charges related to the Jan. 6 Capitol riot.
According to the plea deal, Dr. Gold pleaded guilty to charges that she “did unlawfully and knowingly enter and remain in a restricted building and grounds, that is, any posted, cordoned-off, or otherwise restricted area within the United States Capitol and its grounds, during a time when the vice president was in the building without lawful authority to do so.” As part of the agreement, additional charges against her – obstructing an official proceeding and intent to disrupt the orderly conduct of government business – will be dismissed. She also agreed to cooperate with investigators, including allowing them to review social media postings made during the time surrounding the event.
Shortly after she was indicted, Dr. Gold told The Washington Post that she did not see any violence and that the event was “peaceful.” However, according to news reports, Dr. Gold acknowledged in her plea deal that she and her codefendant, John Herbert Strand, witnessed the assault of a police officer while they were outside the building.
Dr. Gold, 56, based in Beverly Hills, Calif., founded AFD in 2019. The group notes its goal is to “amplify the voices of concerned physicians and patients nationwide to combat those who push political and economic agendas at the expense of science and quality health care solutions.” Mr. Strand is the organization’s communication’s director.
The group has been a leading proponent of the use of ivermectin as a “safe and effective treatment” for COVID-19, according to its website.
In 2021, Dr. Gold spoke at an event called The Stand, representing AFD, where she promised to tell “the truth” about COVID vaccines, including that it was actually giving people the virus, that COVID was renamed from the “Wuhan Virus” as part of a cover-up, and touted treatments, including hydroxycholoroquine and ivermectin.
Dr. Gold has been one of the leading voices in the anti-vaccine movement. She has more than 400,000 Twitter followers; her Twitter profile includes a pinned tweet saying: “We are living in Orwellian times.” In addition to spreading vaccine misinformation, Dr. Gold has promoted the use of unproven treatments such as hydroxychloroquine and ivermectin.
Calls and emails to AFD regarding a statement on Gold’s plea made by this news organization were not returned by press time.
In October, Representative James E. Clyburn (D-S.C.), chairman of the Select Subcommittee on the Coronavirus Crisis, launched an investigation into organizations, including AFD, that spread misinformation and facilitate access to disproven and potentially hazardous treatments for COVID-19. According to news reports, Rep. Clyburn called the AFD and other such groups “predatory actors.”
Hospitals where Dr. Gold previously worked, including Providence St. Joseph Medical Center in Santa Monica, Calif., and Cedars-Sinai in Los Angeles, have disassociated themselves from her. On July 29, 2020, Cedars-Sinai Medical Center, where Gold previously worked, issued a statement that said, in part, “Simone Gold, MD, has not worked with Cedars-Sinai Medical Center or any of its offices or affiliates since 2015. For 3 weeks in late 2015, Dr. Gold was employed on a per diem basis by Cedars-Sinai Medical Network, a component of Cedars-Sinai. She worked during this brief time in a network urgent care clinic. Dr. Gold is not authorized to represent or speak about any information on behalf of Cedars-Sinai.”
Dr. Gold’s medical license in the state of California is current and she has no pending hearings before the state medical board, according to its website. On her own website, Dr. Gold says she “voluntarily refused” to renew her board certification last year, “due to the unethical behavior of the medical boards.”
Dr. Gold is also a licensed attorney, having earned her law degree in health policy analysis at Stanford (Calif.) Law School.
Dr. Gold faces 6 months in prison. Sentencing is scheduled for June 16.
A version of this article first appeared on Medscape.com.
COVID-19 vaccine does not affect in vitro fertilization outcomes
Getting a COVID-19 mRNA vaccine did not affect pregnancy rates for women trying to conceive with in vitro fertilization or ovarian response to treatment, findings of a new study indicate.
The study was led by Sarit Avraham, MD, with the IVF unit, department of obstetrics and gynecology, Shamir Medical Center in Tzrifi, Israel. The findings were published online in Fertility and Sterility in a preproof version.
“Women should be vaccinated for COVID-19 prior to attempting to conceive via IVF treatments, given the higher risk of severe illness in pregnant women,” the authors wrote.
Doubts arose from “the theoretical concept of the supposed similarity between the SARS-CoV-2 spike protein and the syncytin protein that is speculated to take part in the fertilization process and the formation of the placenta,” the authors wrote.
Some then assumed that the COVID vaccine might kick off an immune response that could affect implantation and pregnancy. But this study and others before it found otherwise.
Researchers included 200 vaccinated women trying to conceive with IVF treatments in the retrospective study, and compared them with 200 unvaccinated patients of similar age (average age in both groups, 36 years) who were not previously infected with COVID-19. All the women were undergoing IVF from January to April 2021 and all the vaccinated women completed two doses of the BNT162b2 (Pfizer/BioNTech) vaccine at least 2 weeks before ovarian stimulation.
Researchers compared the average number of oocytes retrieved and clinical pregnancy rates between the two groups.
No difference between groups
Two hundred patients underwent oocyte retrieval 14-68 days after receiving a COVID shot; there was no significant difference by vaccination status in the number retrieved per cycle (10.63 in the vaccinated group vs. 10.72 in the unvaccinated group; P = .93).
There was also no difference in the clinical pregnancy rates after fresh embryo transfers. The rate among 128 vaccinated patients was 32.8% versus 33.1% in the 133 unvaccinated patients (P = .96), with 42 and 44 clinical pregnancies, respectively.
A total of 113 patients (66 in the study group and 47 in the controls) underwent freeze-all cycles to preserve fertility and fertilization rates were similar between vaccinated and unvaccinated (55.43% vaccinated vs. 54.29% unvaccinated; P = .73). The average number of cryopreserved embryos was 3.59 (vaccinated) versus 3.28 (unvaccinated) (P = .80).
In a subanalysis of outcomes by age, researchers found vaccination status had no effect on number of oocytes or pregnancy rates in the 39-and-older group. That’s important because it shows the vaccine did not affect outcomes even in a population with reduced ovarian reserves, the authors wrote.
The authors noted one of the study’s limitations is that it didn’t include information about vaccination or past infection status of the male partners.
Question should be put to rest
Sarah Cross, MD, a maternal-fetal medicine specialist at the University of Minnesota, Minneapolis, said the study is the biggest she’s seen that concludes COVID vaccinations are safe and highly encouraged for women before trying to conceive, but other smaller studies have come to the same conclusion.
She pointed to research including a study from 2021 with similar findings that concluded: “Physicians and public health personnel can counsel women of reproductive age that neither previous illness with COVID-19 nor antibodies produced from vaccination to COVID-19 will cause sterility.”
She said she thinks the question of whether COVID shots are safe with IVF has been answered and the results of the latest study add proof to counter misinformation around the issue.
“The COVID-19 vaccine does not affect fertility,” she said. “I don’t know how many more [studies] we need.”
The harm is in not getting vaccinated, she said. Pregnancy significantly increases a woman’s chance of getting severe COVID, the need for hospitalization, mechanical ventilation, and risk of death.
“I personally have never had a hospitalized patient who’s been vaccinated,” Dr. Cross said. “The worst thing for the fetus is to have a critically ill mother.”
Dr. Cross, whose high-risk patients include those seeking counseling before IVF, added: “I would counsel all of them that they should absolutely get vaccinated prior to pregnancy, when they’re pregnant, whenever it is, as soon as they possibly can.”
The study authors and Dr. Cross report no relevant financial relationships.
Getting a COVID-19 mRNA vaccine did not affect pregnancy rates for women trying to conceive with in vitro fertilization or ovarian response to treatment, findings of a new study indicate.
The study was led by Sarit Avraham, MD, with the IVF unit, department of obstetrics and gynecology, Shamir Medical Center in Tzrifi, Israel. The findings were published online in Fertility and Sterility in a preproof version.
“Women should be vaccinated for COVID-19 prior to attempting to conceive via IVF treatments, given the higher risk of severe illness in pregnant women,” the authors wrote.
Doubts arose from “the theoretical concept of the supposed similarity between the SARS-CoV-2 spike protein and the syncytin protein that is speculated to take part in the fertilization process and the formation of the placenta,” the authors wrote.
Some then assumed that the COVID vaccine might kick off an immune response that could affect implantation and pregnancy. But this study and others before it found otherwise.
Researchers included 200 vaccinated women trying to conceive with IVF treatments in the retrospective study, and compared them with 200 unvaccinated patients of similar age (average age in both groups, 36 years) who were not previously infected with COVID-19. All the women were undergoing IVF from January to April 2021 and all the vaccinated women completed two doses of the BNT162b2 (Pfizer/BioNTech) vaccine at least 2 weeks before ovarian stimulation.
Researchers compared the average number of oocytes retrieved and clinical pregnancy rates between the two groups.
No difference between groups
Two hundred patients underwent oocyte retrieval 14-68 days after receiving a COVID shot; there was no significant difference by vaccination status in the number retrieved per cycle (10.63 in the vaccinated group vs. 10.72 in the unvaccinated group; P = .93).
There was also no difference in the clinical pregnancy rates after fresh embryo transfers. The rate among 128 vaccinated patients was 32.8% versus 33.1% in the 133 unvaccinated patients (P = .96), with 42 and 44 clinical pregnancies, respectively.
A total of 113 patients (66 in the study group and 47 in the controls) underwent freeze-all cycles to preserve fertility and fertilization rates were similar between vaccinated and unvaccinated (55.43% vaccinated vs. 54.29% unvaccinated; P = .73). The average number of cryopreserved embryos was 3.59 (vaccinated) versus 3.28 (unvaccinated) (P = .80).
In a subanalysis of outcomes by age, researchers found vaccination status had no effect on number of oocytes or pregnancy rates in the 39-and-older group. That’s important because it shows the vaccine did not affect outcomes even in a population with reduced ovarian reserves, the authors wrote.
The authors noted one of the study’s limitations is that it didn’t include information about vaccination or past infection status of the male partners.
Question should be put to rest
Sarah Cross, MD, a maternal-fetal medicine specialist at the University of Minnesota, Minneapolis, said the study is the biggest she’s seen that concludes COVID vaccinations are safe and highly encouraged for women before trying to conceive, but other smaller studies have come to the same conclusion.
She pointed to research including a study from 2021 with similar findings that concluded: “Physicians and public health personnel can counsel women of reproductive age that neither previous illness with COVID-19 nor antibodies produced from vaccination to COVID-19 will cause sterility.”
She said she thinks the question of whether COVID shots are safe with IVF has been answered and the results of the latest study add proof to counter misinformation around the issue.
“The COVID-19 vaccine does not affect fertility,” she said. “I don’t know how many more [studies] we need.”
The harm is in not getting vaccinated, she said. Pregnancy significantly increases a woman’s chance of getting severe COVID, the need for hospitalization, mechanical ventilation, and risk of death.
“I personally have never had a hospitalized patient who’s been vaccinated,” Dr. Cross said. “The worst thing for the fetus is to have a critically ill mother.”
Dr. Cross, whose high-risk patients include those seeking counseling before IVF, added: “I would counsel all of them that they should absolutely get vaccinated prior to pregnancy, when they’re pregnant, whenever it is, as soon as they possibly can.”
The study authors and Dr. Cross report no relevant financial relationships.
Getting a COVID-19 mRNA vaccine did not affect pregnancy rates for women trying to conceive with in vitro fertilization or ovarian response to treatment, findings of a new study indicate.
The study was led by Sarit Avraham, MD, with the IVF unit, department of obstetrics and gynecology, Shamir Medical Center in Tzrifi, Israel. The findings were published online in Fertility and Sterility in a preproof version.
“Women should be vaccinated for COVID-19 prior to attempting to conceive via IVF treatments, given the higher risk of severe illness in pregnant women,” the authors wrote.
Doubts arose from “the theoretical concept of the supposed similarity between the SARS-CoV-2 spike protein and the syncytin protein that is speculated to take part in the fertilization process and the formation of the placenta,” the authors wrote.
Some then assumed that the COVID vaccine might kick off an immune response that could affect implantation and pregnancy. But this study and others before it found otherwise.
Researchers included 200 vaccinated women trying to conceive with IVF treatments in the retrospective study, and compared them with 200 unvaccinated patients of similar age (average age in both groups, 36 years) who were not previously infected with COVID-19. All the women were undergoing IVF from January to April 2021 and all the vaccinated women completed two doses of the BNT162b2 (Pfizer/BioNTech) vaccine at least 2 weeks before ovarian stimulation.
Researchers compared the average number of oocytes retrieved and clinical pregnancy rates between the two groups.
No difference between groups
Two hundred patients underwent oocyte retrieval 14-68 days after receiving a COVID shot; there was no significant difference by vaccination status in the number retrieved per cycle (10.63 in the vaccinated group vs. 10.72 in the unvaccinated group; P = .93).
There was also no difference in the clinical pregnancy rates after fresh embryo transfers. The rate among 128 vaccinated patients was 32.8% versus 33.1% in the 133 unvaccinated patients (P = .96), with 42 and 44 clinical pregnancies, respectively.
A total of 113 patients (66 in the study group and 47 in the controls) underwent freeze-all cycles to preserve fertility and fertilization rates were similar between vaccinated and unvaccinated (55.43% vaccinated vs. 54.29% unvaccinated; P = .73). The average number of cryopreserved embryos was 3.59 (vaccinated) versus 3.28 (unvaccinated) (P = .80).
In a subanalysis of outcomes by age, researchers found vaccination status had no effect on number of oocytes or pregnancy rates in the 39-and-older group. That’s important because it shows the vaccine did not affect outcomes even in a population with reduced ovarian reserves, the authors wrote.
The authors noted one of the study’s limitations is that it didn’t include information about vaccination or past infection status of the male partners.
Question should be put to rest
Sarah Cross, MD, a maternal-fetal medicine specialist at the University of Minnesota, Minneapolis, said the study is the biggest she’s seen that concludes COVID vaccinations are safe and highly encouraged for women before trying to conceive, but other smaller studies have come to the same conclusion.
She pointed to research including a study from 2021 with similar findings that concluded: “Physicians and public health personnel can counsel women of reproductive age that neither previous illness with COVID-19 nor antibodies produced from vaccination to COVID-19 will cause sterility.”
She said she thinks the question of whether COVID shots are safe with IVF has been answered and the results of the latest study add proof to counter misinformation around the issue.
“The COVID-19 vaccine does not affect fertility,” she said. “I don’t know how many more [studies] we need.”
The harm is in not getting vaccinated, she said. Pregnancy significantly increases a woman’s chance of getting severe COVID, the need for hospitalization, mechanical ventilation, and risk of death.
“I personally have never had a hospitalized patient who’s been vaccinated,” Dr. Cross said. “The worst thing for the fetus is to have a critically ill mother.”
Dr. Cross, whose high-risk patients include those seeking counseling before IVF, added: “I would counsel all of them that they should absolutely get vaccinated prior to pregnancy, when they’re pregnant, whenever it is, as soon as they possibly can.”
The study authors and Dr. Cross report no relevant financial relationships.
FROM FERTILITY AND STERILITY
Long COVID patients may develop nerve damage: Study
according to a new study published in the journal Neurology: Neuroimmunology & Neuroinflammation (doi: 10.1212/NXI.0000000000001146).
The nerve damage, which has been seen even among mild coronavirus cases, appears to be caused by immunity problems triggered by infection.
“This is one of the early papers looking into causes of long COVID, which will steadily increase in importance as acute COVID wanes,” Anne Louise Oaklander, MD, the lead study author and a neurologist at Massachusetts General Hospital, Boston, said in a statement.
“Our findings suggest that some long COVID patients had damage to their peripheral nerve fibers and that damage to the small-fiber type of nerve cell may be prominent,” she said.
The research team analyzed data from 17 COVID-19 survivors with lingering symptoms who had no history or risks of neuropathy, or nerve damage or disease. The patients were from 10 states and territories, and all but one had mild infections.
They found that 10 patients – or 59% – had at least one test that confirmed neuropathy. Two patients had rare neuropathies that affected muscle nerves, and 10 were diagnosed with small-fiber neuropathy, which is a cause of chronic pain. Common symptoms included fatigue, weakness, changes in their senses, and pain in their hands and feet.
For treatment, 11 patients were given immunotherapies such as corticosteroids or intravenous immunoglobulins, and the five patients who received repeated IgG treatments appeared to benefit. Over time, 52% of patients improved, though none had all of their symptoms go away.
“Research from our team and others is clarifying what the different types of post-COVID neuropathy are and how best to diagnose and treat them,” she said. “Most long COVID neuropathies described so far appear to reflect immune responses to the virus that went off course.”
Dr. Oaklander noted that researchers haven’t been able to do clinical trials to evaluate specific post-COVID neuropathy treatments. But some existing treatments may help.
“Some patients seem to improve from standard treatments for other immune-related neuropathies,” she said.
A version of this article first appeared on WebMD.com.
according to a new study published in the journal Neurology: Neuroimmunology & Neuroinflammation (doi: 10.1212/NXI.0000000000001146).
The nerve damage, which has been seen even among mild coronavirus cases, appears to be caused by immunity problems triggered by infection.
“This is one of the early papers looking into causes of long COVID, which will steadily increase in importance as acute COVID wanes,” Anne Louise Oaklander, MD, the lead study author and a neurologist at Massachusetts General Hospital, Boston, said in a statement.
“Our findings suggest that some long COVID patients had damage to their peripheral nerve fibers and that damage to the small-fiber type of nerve cell may be prominent,” she said.
The research team analyzed data from 17 COVID-19 survivors with lingering symptoms who had no history or risks of neuropathy, or nerve damage or disease. The patients were from 10 states and territories, and all but one had mild infections.
They found that 10 patients – or 59% – had at least one test that confirmed neuropathy. Two patients had rare neuropathies that affected muscle nerves, and 10 were diagnosed with small-fiber neuropathy, which is a cause of chronic pain. Common symptoms included fatigue, weakness, changes in their senses, and pain in their hands and feet.
For treatment, 11 patients were given immunotherapies such as corticosteroids or intravenous immunoglobulins, and the five patients who received repeated IgG treatments appeared to benefit. Over time, 52% of patients improved, though none had all of their symptoms go away.
“Research from our team and others is clarifying what the different types of post-COVID neuropathy are and how best to diagnose and treat them,” she said. “Most long COVID neuropathies described so far appear to reflect immune responses to the virus that went off course.”
Dr. Oaklander noted that researchers haven’t been able to do clinical trials to evaluate specific post-COVID neuropathy treatments. But some existing treatments may help.
“Some patients seem to improve from standard treatments for other immune-related neuropathies,” she said.
A version of this article first appeared on WebMD.com.
according to a new study published in the journal Neurology: Neuroimmunology & Neuroinflammation (doi: 10.1212/NXI.0000000000001146).
The nerve damage, which has been seen even among mild coronavirus cases, appears to be caused by immunity problems triggered by infection.
“This is one of the early papers looking into causes of long COVID, which will steadily increase in importance as acute COVID wanes,” Anne Louise Oaklander, MD, the lead study author and a neurologist at Massachusetts General Hospital, Boston, said in a statement.
“Our findings suggest that some long COVID patients had damage to their peripheral nerve fibers and that damage to the small-fiber type of nerve cell may be prominent,” she said.
The research team analyzed data from 17 COVID-19 survivors with lingering symptoms who had no history or risks of neuropathy, or nerve damage or disease. The patients were from 10 states and territories, and all but one had mild infections.
They found that 10 patients – or 59% – had at least one test that confirmed neuropathy. Two patients had rare neuropathies that affected muscle nerves, and 10 were diagnosed with small-fiber neuropathy, which is a cause of chronic pain. Common symptoms included fatigue, weakness, changes in their senses, and pain in their hands and feet.
For treatment, 11 patients were given immunotherapies such as corticosteroids or intravenous immunoglobulins, and the five patients who received repeated IgG treatments appeared to benefit. Over time, 52% of patients improved, though none had all of their symptoms go away.
“Research from our team and others is clarifying what the different types of post-COVID neuropathy are and how best to diagnose and treat them,” she said. “Most long COVID neuropathies described so far appear to reflect immune responses to the virus that went off course.”
Dr. Oaklander noted that researchers haven’t been able to do clinical trials to evaluate specific post-COVID neuropathy treatments. But some existing treatments may help.
“Some patients seem to improve from standard treatments for other immune-related neuropathies,” she said.
A version of this article first appeared on WebMD.com.
FROM NEUROLOGY: NEUROIMMUNOLOGY & NEUROINFLAMMATION
Cardiac arrest survival lower in COVID-19 inpatients
Survival after in-hospital cardiac arrest was roughly one-third lower in patients with COVID-19 infections compared to uninfected patients, based on data from nearly 25,000 individuals.
Survival rates of less than 3% were reported in the United States and China for patients who suffered in-hospital cardiac arrest (IHCA) while infected with COVID-19 early in the pandemic, but the data came from small, single-center studies in overwhelmed hospitals, wrote Saket Girotra, MD, of the University of Iowa, Iowa City, and fellow American Heart Association Get With the Guidelines–Resuscitation Investigators. Whether these early reports reflect the broader experience of patients with COVID-19 in hospitals in the United States remains unknown.
In a study published as a research letter in JAMA Network Open, the researchers reviewed data from the American Heart Association Get With the Guidelines–Resuscitation registry. The registry collects detailed information on patients aged 18 years and older who experience cardiac arrest at participating hospitals in the United States. The study population included 24,915 patients aged 18 years and older from 286 hospitals who experienced IHCA during March–December 2020. The mean age of the patients was 64.7 years; 61.1% were White, 24.8% were Black, 3.8% were of other race or ethnicity, and 10.3% were of unknown race or ethnicity.
The primary outcomes were survival to discharge and return of spontaneous circulation (ROSC) for at least 20 minutes.
A total of 5,916 patients (23.7%) had suspected or confirmed COVID-19 infections, and infected patients were more likely to be younger, male, and Black. Patients with COVID-19 infections also were significantly more likely than noninfected patients to have nonshockable rhythm, pneumonia, respiratory insufficiency, or sepsis, and to be on mechanical ventilation or vasopressors when the IHCA occurred, the researchers noted.
Survival rates to hospital discharge were 11.9% for COVID-19 patients, compared with 23.5% for noninfected patients (adjusted relative risk, 0.65; P < .001). ROSC was 53.7% and 63.6%, for infected and noninfected patients, respectively (aRR, 0.86; P < .001).
COVID-19 patients also were more likely than noninfected patients to receive delayed defibrillation, the researchers said. “Although delays in resuscitation, especially defibrillation, may have contributed to lower survival, the negative association of COVID-19 with survival in this study was consistent across subgroups, including patients who received timely treatment with defibrillation and epinephrine.”
The extremely low survival rate in early pandemic studies likely reflected the overwhelming burden on health systems at the time, the researchers said in their discussion.
The study findings were limited by several factors, including potential confounding from unmeasured variables, the use of a quality improvement registry that may not reflect nonparticipating hospitals, and potential false-positive COVID-19 cases. However, the result support findings from recent studies of multiple centers and extend clinical knowledge by comparing infected and noninfected patients from a larger group of hospitals than previously studied, the researchers said.
“We believe that these data will be relevant to health care providers and hospital administrators as the COVID-19 pandemic continues,” they concluded.
Think beyond COVID-19 for cardiac care
“Early during the pandemic, questions were raised whether COVID-19 patients should be treated with CPR,” Dr. Girotra said in an interview. “This was because initial studies had found a dismal survival of 0%-3% in COVID patients treated with CPR. The potential of transmitting the virus to health care professionals during CPR further heightened these concerns. We wanted to know whether the poor survival reported in these initial studies were broadly representative.”
Dr. Girotra said that some of the study findings were surprising. “We found that of all patients with IHCA in 2020 in our study, one in four were suspected or confirmed to have COVID-19 infection. We were surprised by the magnitude of COVID’s impact on the cardiac arrest incidence.”
The implications for clinical decision-making are to think outside of COVID-19 infection, said Dr. Girotra. In the current study, “Although overall survival of cardiac arrest in COVID-positive patients was 30% lower, compared to non-COVID patients, it was not as poor as previously reported. COVID-19 infection alone should not be considered the sole factor for making decisions regarding CPR.
“Over the past 2 decades, we have experienced large gains in survival for in-hospital cardiac arrest. However, the COVID-19 pandemic has eroded these gains,” said Dr. Girotra. “Future studies are needed to monitor the impact of any new variants on cardiac arrest care,” as well as studies “to see whether we return to the prepandemic levels of IHCA survival once the pandemic recedes.”
Dr. Girotra has no relevant financial disclosures.
Survival after in-hospital cardiac arrest was roughly one-third lower in patients with COVID-19 infections compared to uninfected patients, based on data from nearly 25,000 individuals.
Survival rates of less than 3% were reported in the United States and China for patients who suffered in-hospital cardiac arrest (IHCA) while infected with COVID-19 early in the pandemic, but the data came from small, single-center studies in overwhelmed hospitals, wrote Saket Girotra, MD, of the University of Iowa, Iowa City, and fellow American Heart Association Get With the Guidelines–Resuscitation Investigators. Whether these early reports reflect the broader experience of patients with COVID-19 in hospitals in the United States remains unknown.
In a study published as a research letter in JAMA Network Open, the researchers reviewed data from the American Heart Association Get With the Guidelines–Resuscitation registry. The registry collects detailed information on patients aged 18 years and older who experience cardiac arrest at participating hospitals in the United States. The study population included 24,915 patients aged 18 years and older from 286 hospitals who experienced IHCA during March–December 2020. The mean age of the patients was 64.7 years; 61.1% were White, 24.8% were Black, 3.8% were of other race or ethnicity, and 10.3% were of unknown race or ethnicity.
The primary outcomes were survival to discharge and return of spontaneous circulation (ROSC) for at least 20 minutes.
A total of 5,916 patients (23.7%) had suspected or confirmed COVID-19 infections, and infected patients were more likely to be younger, male, and Black. Patients with COVID-19 infections also were significantly more likely than noninfected patients to have nonshockable rhythm, pneumonia, respiratory insufficiency, or sepsis, and to be on mechanical ventilation or vasopressors when the IHCA occurred, the researchers noted.
Survival rates to hospital discharge were 11.9% for COVID-19 patients, compared with 23.5% for noninfected patients (adjusted relative risk, 0.65; P < .001). ROSC was 53.7% and 63.6%, for infected and noninfected patients, respectively (aRR, 0.86; P < .001).
COVID-19 patients also were more likely than noninfected patients to receive delayed defibrillation, the researchers said. “Although delays in resuscitation, especially defibrillation, may have contributed to lower survival, the negative association of COVID-19 with survival in this study was consistent across subgroups, including patients who received timely treatment with defibrillation and epinephrine.”
The extremely low survival rate in early pandemic studies likely reflected the overwhelming burden on health systems at the time, the researchers said in their discussion.
The study findings were limited by several factors, including potential confounding from unmeasured variables, the use of a quality improvement registry that may not reflect nonparticipating hospitals, and potential false-positive COVID-19 cases. However, the result support findings from recent studies of multiple centers and extend clinical knowledge by comparing infected and noninfected patients from a larger group of hospitals than previously studied, the researchers said.
“We believe that these data will be relevant to health care providers and hospital administrators as the COVID-19 pandemic continues,” they concluded.
Think beyond COVID-19 for cardiac care
“Early during the pandemic, questions were raised whether COVID-19 patients should be treated with CPR,” Dr. Girotra said in an interview. “This was because initial studies had found a dismal survival of 0%-3% in COVID patients treated with CPR. The potential of transmitting the virus to health care professionals during CPR further heightened these concerns. We wanted to know whether the poor survival reported in these initial studies were broadly representative.”
Dr. Girotra said that some of the study findings were surprising. “We found that of all patients with IHCA in 2020 in our study, one in four were suspected or confirmed to have COVID-19 infection. We were surprised by the magnitude of COVID’s impact on the cardiac arrest incidence.”
The implications for clinical decision-making are to think outside of COVID-19 infection, said Dr. Girotra. In the current study, “Although overall survival of cardiac arrest in COVID-positive patients was 30% lower, compared to non-COVID patients, it was not as poor as previously reported. COVID-19 infection alone should not be considered the sole factor for making decisions regarding CPR.
“Over the past 2 decades, we have experienced large gains in survival for in-hospital cardiac arrest. However, the COVID-19 pandemic has eroded these gains,” said Dr. Girotra. “Future studies are needed to monitor the impact of any new variants on cardiac arrest care,” as well as studies “to see whether we return to the prepandemic levels of IHCA survival once the pandemic recedes.”
Dr. Girotra has no relevant financial disclosures.
Survival after in-hospital cardiac arrest was roughly one-third lower in patients with COVID-19 infections compared to uninfected patients, based on data from nearly 25,000 individuals.
Survival rates of less than 3% were reported in the United States and China for patients who suffered in-hospital cardiac arrest (IHCA) while infected with COVID-19 early in the pandemic, but the data came from small, single-center studies in overwhelmed hospitals, wrote Saket Girotra, MD, of the University of Iowa, Iowa City, and fellow American Heart Association Get With the Guidelines–Resuscitation Investigators. Whether these early reports reflect the broader experience of patients with COVID-19 in hospitals in the United States remains unknown.
In a study published as a research letter in JAMA Network Open, the researchers reviewed data from the American Heart Association Get With the Guidelines–Resuscitation registry. The registry collects detailed information on patients aged 18 years and older who experience cardiac arrest at participating hospitals in the United States. The study population included 24,915 patients aged 18 years and older from 286 hospitals who experienced IHCA during March–December 2020. The mean age of the patients was 64.7 years; 61.1% were White, 24.8% were Black, 3.8% were of other race or ethnicity, and 10.3% were of unknown race or ethnicity.
The primary outcomes were survival to discharge and return of spontaneous circulation (ROSC) for at least 20 minutes.
A total of 5,916 patients (23.7%) had suspected or confirmed COVID-19 infections, and infected patients were more likely to be younger, male, and Black. Patients with COVID-19 infections also were significantly more likely than noninfected patients to have nonshockable rhythm, pneumonia, respiratory insufficiency, or sepsis, and to be on mechanical ventilation or vasopressors when the IHCA occurred, the researchers noted.
Survival rates to hospital discharge were 11.9% for COVID-19 patients, compared with 23.5% for noninfected patients (adjusted relative risk, 0.65; P < .001). ROSC was 53.7% and 63.6%, for infected and noninfected patients, respectively (aRR, 0.86; P < .001).
COVID-19 patients also were more likely than noninfected patients to receive delayed defibrillation, the researchers said. “Although delays in resuscitation, especially defibrillation, may have contributed to lower survival, the negative association of COVID-19 with survival in this study was consistent across subgroups, including patients who received timely treatment with defibrillation and epinephrine.”
The extremely low survival rate in early pandemic studies likely reflected the overwhelming burden on health systems at the time, the researchers said in their discussion.
The study findings were limited by several factors, including potential confounding from unmeasured variables, the use of a quality improvement registry that may not reflect nonparticipating hospitals, and potential false-positive COVID-19 cases. However, the result support findings from recent studies of multiple centers and extend clinical knowledge by comparing infected and noninfected patients from a larger group of hospitals than previously studied, the researchers said.
“We believe that these data will be relevant to health care providers and hospital administrators as the COVID-19 pandemic continues,” they concluded.
Think beyond COVID-19 for cardiac care
“Early during the pandemic, questions were raised whether COVID-19 patients should be treated with CPR,” Dr. Girotra said in an interview. “This was because initial studies had found a dismal survival of 0%-3% in COVID patients treated with CPR. The potential of transmitting the virus to health care professionals during CPR further heightened these concerns. We wanted to know whether the poor survival reported in these initial studies were broadly representative.”
Dr. Girotra said that some of the study findings were surprising. “We found that of all patients with IHCA in 2020 in our study, one in four were suspected or confirmed to have COVID-19 infection. We were surprised by the magnitude of COVID’s impact on the cardiac arrest incidence.”
The implications for clinical decision-making are to think outside of COVID-19 infection, said Dr. Girotra. In the current study, “Although overall survival of cardiac arrest in COVID-positive patients was 30% lower, compared to non-COVID patients, it was not as poor as previously reported. COVID-19 infection alone should not be considered the sole factor for making decisions regarding CPR.
“Over the past 2 decades, we have experienced large gains in survival for in-hospital cardiac arrest. However, the COVID-19 pandemic has eroded these gains,” said Dr. Girotra. “Future studies are needed to monitor the impact of any new variants on cardiac arrest care,” as well as studies “to see whether we return to the prepandemic levels of IHCA survival once the pandemic recedes.”
Dr. Girotra has no relevant financial disclosures.
FROM JAMA NETWORK OPEN