User login
Major life stressors ‘strongly predictive’ of long COVID symptoms
new research suggests.
Major life stressors in the year after hospital discharge for COVID-19 are “strongly predictive of a lot of the important outcomes that people may face after COVID,” lead investigator Jennifer A. Frontera, MD, a professor in the department of neurology at New York University Langone Health, said in an interview.
These outcomes include depression, brain fog, fatigue, trouble sleeping, and other long COVID symptoms.
The findings were published online in the Journal of the Neurological Sciences.
Major stressful events common
Dr. Frontera and the NYU Neurology COVID-19 study team evaluated 451 adults who survived a COVID hospital stay. Of these, 383 completed a 6-month follow-up, 242 completed a 12-month follow-up, and 174 completed follow-up at both time points.
Within 1 year of discharge, 77 (17%) patients died and 51% suffered a major stressful life event.
In multivariable analyses, major life stressors – including financial insecurity, food insecurity, death of a close contact, and new disability – were strong independent predictors of disability, trouble with activities of daily living, depression, fatigue, sleep problems, and prolonged post-acute COVID symptoms. The adjusted odds ratios for these outcomes ranged from 2.5 to 20.8.
The research also confirmed the contribution of traditional risk factors for long COVID symptoms, as shown in past studies. These include older age, poor pre-COVID functional status, and more severe initial COVID-19 infection.
Long-term sequelae of COVID are increasingly recognized as major public health issues.
It has been estimated that roughly 16 million U.S. adults aged 18-65 years ave long COVID, with the often debilitating symptoms keeping up to 4 million out of work.
Holistic approach
Dr. Frontera said it’s important to realize that “sleep, fatigue, anxiety, depression, even cognition are so interwoven with each other that anything that impacts any one of them could have repercussions on the other.”
She added that it “certainly makes sense that there is an interplay or even a bidirectional relationship between the stressors that people face and how well they can recover after COVID.”
Therapies that lessen the trauma of the most stress-inducing life events need to be a central part of treatment for long COVID, with more research needed to validate the best approaches, Dr. Frontera said.
She also noted that social services or case management resources may be able to help address at least some of the stressors that individuals are under – and it is important to refer them to these resources. Referral to mental health services is also important.
“I think it’s really important to take a holistic approach and try to deal with whatever the problem may be,” said Dr. Frontera.
“I’m a neurologist, but as part of my evaluation, I really need to address if there are life stressors or mental health issues that may be impacting this person’s function,” she added.
The study had no commercial funding. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Major life stressors in the year after hospital discharge for COVID-19 are “strongly predictive of a lot of the important outcomes that people may face after COVID,” lead investigator Jennifer A. Frontera, MD, a professor in the department of neurology at New York University Langone Health, said in an interview.
These outcomes include depression, brain fog, fatigue, trouble sleeping, and other long COVID symptoms.
The findings were published online in the Journal of the Neurological Sciences.
Major stressful events common
Dr. Frontera and the NYU Neurology COVID-19 study team evaluated 451 adults who survived a COVID hospital stay. Of these, 383 completed a 6-month follow-up, 242 completed a 12-month follow-up, and 174 completed follow-up at both time points.
Within 1 year of discharge, 77 (17%) patients died and 51% suffered a major stressful life event.
In multivariable analyses, major life stressors – including financial insecurity, food insecurity, death of a close contact, and new disability – were strong independent predictors of disability, trouble with activities of daily living, depression, fatigue, sleep problems, and prolonged post-acute COVID symptoms. The adjusted odds ratios for these outcomes ranged from 2.5 to 20.8.
The research also confirmed the contribution of traditional risk factors for long COVID symptoms, as shown in past studies. These include older age, poor pre-COVID functional status, and more severe initial COVID-19 infection.
Long-term sequelae of COVID are increasingly recognized as major public health issues.
It has been estimated that roughly 16 million U.S. adults aged 18-65 years ave long COVID, with the often debilitating symptoms keeping up to 4 million out of work.
Holistic approach
Dr. Frontera said it’s important to realize that “sleep, fatigue, anxiety, depression, even cognition are so interwoven with each other that anything that impacts any one of them could have repercussions on the other.”
She added that it “certainly makes sense that there is an interplay or even a bidirectional relationship between the stressors that people face and how well they can recover after COVID.”
Therapies that lessen the trauma of the most stress-inducing life events need to be a central part of treatment for long COVID, with more research needed to validate the best approaches, Dr. Frontera said.
She also noted that social services or case management resources may be able to help address at least some of the stressors that individuals are under – and it is important to refer them to these resources. Referral to mental health services is also important.
“I think it’s really important to take a holistic approach and try to deal with whatever the problem may be,” said Dr. Frontera.
“I’m a neurologist, but as part of my evaluation, I really need to address if there are life stressors or mental health issues that may be impacting this person’s function,” she added.
The study had no commercial funding. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Major life stressors in the year after hospital discharge for COVID-19 are “strongly predictive of a lot of the important outcomes that people may face after COVID,” lead investigator Jennifer A. Frontera, MD, a professor in the department of neurology at New York University Langone Health, said in an interview.
These outcomes include depression, brain fog, fatigue, trouble sleeping, and other long COVID symptoms.
The findings were published online in the Journal of the Neurological Sciences.
Major stressful events common
Dr. Frontera and the NYU Neurology COVID-19 study team evaluated 451 adults who survived a COVID hospital stay. Of these, 383 completed a 6-month follow-up, 242 completed a 12-month follow-up, and 174 completed follow-up at both time points.
Within 1 year of discharge, 77 (17%) patients died and 51% suffered a major stressful life event.
In multivariable analyses, major life stressors – including financial insecurity, food insecurity, death of a close contact, and new disability – were strong independent predictors of disability, trouble with activities of daily living, depression, fatigue, sleep problems, and prolonged post-acute COVID symptoms. The adjusted odds ratios for these outcomes ranged from 2.5 to 20.8.
The research also confirmed the contribution of traditional risk factors for long COVID symptoms, as shown in past studies. These include older age, poor pre-COVID functional status, and more severe initial COVID-19 infection.
Long-term sequelae of COVID are increasingly recognized as major public health issues.
It has been estimated that roughly 16 million U.S. adults aged 18-65 years ave long COVID, with the often debilitating symptoms keeping up to 4 million out of work.
Holistic approach
Dr. Frontera said it’s important to realize that “sleep, fatigue, anxiety, depression, even cognition are so interwoven with each other that anything that impacts any one of them could have repercussions on the other.”
She added that it “certainly makes sense that there is an interplay or even a bidirectional relationship between the stressors that people face and how well they can recover after COVID.”
Therapies that lessen the trauma of the most stress-inducing life events need to be a central part of treatment for long COVID, with more research needed to validate the best approaches, Dr. Frontera said.
She also noted that social services or case management resources may be able to help address at least some of the stressors that individuals are under – and it is important to refer them to these resources. Referral to mental health services is also important.
“I think it’s really important to take a holistic approach and try to deal with whatever the problem may be,” said Dr. Frontera.
“I’m a neurologist, but as part of my evaluation, I really need to address if there are life stressors or mental health issues that may be impacting this person’s function,” she added.
The study had no commercial funding. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE NEUROLOGICAL SCIENCES
Experts explain the ‘perfect storm’ of rampant RSV and flu
Headlines over the past few weeks are ringing the alarm about earlier and more serious influenza (flu) and respiratory syncytial virus (RSV) outbreaks compared with previous years. Add COVID-19 to the mix and you have a dangerous mash of viruses that have many experts calling for caution and searching for explanations.
RSV and the flu “are certainly getting more attention, and they’re getting more attention for two reasons,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University, Nashville, Tenn.
“The first is that they’re both extraordinarily early. The second is that they’re both out there spreading very, very rapidly,” he told this news organization.
RSV usually follows a seasonal pattern with cases peaking in January and February. Both viruses tend to hit different regions of the country at different times, and that’s not the case in 2022.
“This is particularly striking for RSV, which usually doesn’t affect the entire country simultaneously,” Dr. Schaffner said.
“Yes, RSV is causing many more hospitalizations and earlier than any previously recorded season in the U.S.,” according to figures from the Centers for Disease Control and Prevention on RSV hospitalizations, said Kevin Messacar, MD, PhD, associate professor at the University of Colorado at Denver, Aurora, and a pediatric infectious disease specialist at Children’s Hospital Colorado in Aurora.
Although there could be some increase in diagnoses because of increased awareness, the jump in RSV and flu cases “is a real phenomenon for multiple reasons,” said Peter Chin-Hong, MD, professor in the division of infectious diseases at the University of California, San Francisco.
With fewer COVID-related restrictions, people are moving around more. Also, during fall and winter, people tend to gather indoors. Colder temperatures and lower humidity contribute as well, Dr. Chin-Hong said, because “the droplets are just simply lighter.
“I think those are all factors,” he told this news organization.
Paul Auwaerter, MD, agreed that there are likely multiple causes for the unusual timing and severity of RSV and flu this year.
“Change in behaviors is a leading cause,” said the clinical director for the division of infectious diseases at the Johns Hopkins University, Baltimore. More people returning to the workplace and children going to school without masks are examples, he added.
Less exposure to these three viruses also means there was less immune boosting among existing populations, he said. This can lead to “larger susceptible populations, especially infants and younger children, due to the relative absence of circulating virus in past years.”
A leading theory
Are we paying a price now for people following the edicts from officials to mask up, stand apart, and take other personal and public health precautions during the COVID-19 pandemic?
It’s possible, but that may not be the whole story.
“When it comes to RSV, I think that theory of isolation, social distancing, mask wearing, and not attending schools is a very valid one,” Dr. Schaffner said. “That’s everybody’s favorite [reason].”
He said he is confident that the jump in RSV cases is being driven by previous COVID public health protections. However, he’s “a little more cautious about influenza, in part because influenza is so variable.
“Like people in influenza say, if you’ve seen one influenza season, you’ve seen one influenza season,” Dr. Schaffner said.
“There’s a lot of debate,” he added. “Nobody can say definitively whether the immune deficit or debt is a consequence of not being stimulated and restimulated by the influenza virus over the past two seasons.”
‘A perfect storm’
“Now you kind of have the perfect storm,” Dr. Chin-Hong said. “It’s not a good situation for COVID with the variants that are emerging. For influenza, not having seen a lot of influenza the last 2 years, we’re probably more susceptible to getting infected.”
RSV cases rose during summer 2021, but now the weather is colder, and people are interacting more closely. “And it’s very, very transmissible,” he said.
Dr. Chin-Hong also predicted that “even though we don’t have a lot of COVID now, COVID will probably pick up.”
The rise in RSV was unexpected by some experts. “This early influenza is also a bit of a surprise and may be influenced by the fact that lots of us are going back and seeing each other again close-to-close, face-to-face in many enclosed environments,” Dr. Schaffner said.
He estimated the 2022-2023 flu season started 4-6 weeks early “and it’s taken off like a rocket. It started in the Southeast, quickly went to the Southwest and up the East Coast. Now it’s moving dramatically through the Midwest and will continue. It’s quite sure to hit the West Coast if it isn’t there already.”
A phenomenon by any other name
Some are calling the situation an “immunity debt,” while others dub it an “immunity pause” or an “immunity deficit.” Many physicians and immunologists have taken to social media to push back on the term “immunity debt,” saying it’s a mischaracterization that is being used to vilify COVID precautions, such as masking, social distancing, and other protective measures taken during the pandemic.
“I prefer the term ‘immunity gap’ ... which is more established in the epidemiology literature, especially given the politicization of the term ‘immunity debt’ by folks recently,” Dr. Messacar said.
“To me, the immunity gap is a scientific observation, not a political argument,” he added.
In a July 2022 publication in The Lancet, Dr. Messacar and his colleagues stated that “decreased exposure to endemic viruses created an immunity gap – a group of susceptible individuals who avoided infection and therefore lack pathogen-specific immunity to protect against future infection. Decreases in childhood vaccinations with pandemic disruptions to health care delivery contribute to this immunity gap for vaccine-preventable diseases, such as influenza,measles, and polio.”
The researchers noted that because of isolation during the pandemic, older children and newborns are being exposed to RSV for the first time. Returning to birthday parties, playing with friends, and going to school without masks means “children are being exposed to RSV, and that’s likely the reason that RSV is moving early and very, very substantially through this now expanded pool of susceptible children,” Dr. Schaffner said.
How likely are coinfections?
With peaks in RSV, flu, and COVID-19 cases each predicted in the coming months, how likely is it that someone could get sick with more than one infection at the same time?
Early in the pandemic, coinfection with COVID and the flu was reported in people at some centers on the West Coast, Dr. Auwaerter said. Now, however, “the unpredictable nature of the Omicron subvariants and the potential for further change, along with the never-before-seen significant lessening of influenza over 2 years, leave little for predictability.
“I do think it is less likely, given the extent of immunity now to SARS-CoV-2 in the population,” Dr. Auwaerter said.
“I most worry about viral coinfections ... in people with suppressed immune systems if we have high community rates of the SARS-CoV-2 and influenza circulating this fall and winter,” he added.
Studies during the pandemic suggest that coinfection with the SARS-CoV-2 virus and another respiratory virus were either rare or nonexistent.
Dr. Schaffner said these findings align with his experience at Vanderbilt University, which is part of a CDC-sponsored network that tracks laboratory-confirmed RSV, flu, and COVID cases among people in the hospital. “Coinfections are, at least to date, very unusual.”
There needs to be an asterisk next to that, Dr. Schaffner added. “Looking back over the last 2 years, we’ve had very little influenza, and we’ve had curtailed RSV seasons. So there hasn’t been a whole lot of opportunity for dual infections to occur.
“So this year may be more revelatory as we go forward,” he said.
Future concerns
The future is uncertain, Dr. Messacar and colleagues wrote in The Lancet: “Crucially, the patterns of these returning viral outbreaks have been heterogeneous across locations, populations, and pathogens, making predictions and preparations challenging.”
Dr. Chin-Hong used a horse race analogy to illustrate the situation now and going forward. RSV is the front-running horse, and influenza is running behind but trying to catch up. “And then COVID is the dark horse. It’s trailing the race right now – but all these variants are giving the horse extra supplements.
“And the COVID horse is probably going to be very competitive with the front-runner,” he said.
“We’re just at the beginning of the race right now,” Dr. Chin-Hong said, “so that’s why we’re worried that these three [viruses] will be even more pronounced come later in the year.”
A version of this article first appeared on Medscape.com.
Headlines over the past few weeks are ringing the alarm about earlier and more serious influenza (flu) and respiratory syncytial virus (RSV) outbreaks compared with previous years. Add COVID-19 to the mix and you have a dangerous mash of viruses that have many experts calling for caution and searching for explanations.
RSV and the flu “are certainly getting more attention, and they’re getting more attention for two reasons,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University, Nashville, Tenn.
“The first is that they’re both extraordinarily early. The second is that they’re both out there spreading very, very rapidly,” he told this news organization.
RSV usually follows a seasonal pattern with cases peaking in January and February. Both viruses tend to hit different regions of the country at different times, and that’s not the case in 2022.
“This is particularly striking for RSV, which usually doesn’t affect the entire country simultaneously,” Dr. Schaffner said.
“Yes, RSV is causing many more hospitalizations and earlier than any previously recorded season in the U.S.,” according to figures from the Centers for Disease Control and Prevention on RSV hospitalizations, said Kevin Messacar, MD, PhD, associate professor at the University of Colorado at Denver, Aurora, and a pediatric infectious disease specialist at Children’s Hospital Colorado in Aurora.
Although there could be some increase in diagnoses because of increased awareness, the jump in RSV and flu cases “is a real phenomenon for multiple reasons,” said Peter Chin-Hong, MD, professor in the division of infectious diseases at the University of California, San Francisco.
With fewer COVID-related restrictions, people are moving around more. Also, during fall and winter, people tend to gather indoors. Colder temperatures and lower humidity contribute as well, Dr. Chin-Hong said, because “the droplets are just simply lighter.
“I think those are all factors,” he told this news organization.
Paul Auwaerter, MD, agreed that there are likely multiple causes for the unusual timing and severity of RSV and flu this year.
“Change in behaviors is a leading cause,” said the clinical director for the division of infectious diseases at the Johns Hopkins University, Baltimore. More people returning to the workplace and children going to school without masks are examples, he added.
Less exposure to these three viruses also means there was less immune boosting among existing populations, he said. This can lead to “larger susceptible populations, especially infants and younger children, due to the relative absence of circulating virus in past years.”
A leading theory
Are we paying a price now for people following the edicts from officials to mask up, stand apart, and take other personal and public health precautions during the COVID-19 pandemic?
It’s possible, but that may not be the whole story.
“When it comes to RSV, I think that theory of isolation, social distancing, mask wearing, and not attending schools is a very valid one,” Dr. Schaffner said. “That’s everybody’s favorite [reason].”
He said he is confident that the jump in RSV cases is being driven by previous COVID public health protections. However, he’s “a little more cautious about influenza, in part because influenza is so variable.
“Like people in influenza say, if you’ve seen one influenza season, you’ve seen one influenza season,” Dr. Schaffner said.
“There’s a lot of debate,” he added. “Nobody can say definitively whether the immune deficit or debt is a consequence of not being stimulated and restimulated by the influenza virus over the past two seasons.”
‘A perfect storm’
“Now you kind of have the perfect storm,” Dr. Chin-Hong said. “It’s not a good situation for COVID with the variants that are emerging. For influenza, not having seen a lot of influenza the last 2 years, we’re probably more susceptible to getting infected.”
RSV cases rose during summer 2021, but now the weather is colder, and people are interacting more closely. “And it’s very, very transmissible,” he said.
Dr. Chin-Hong also predicted that “even though we don’t have a lot of COVID now, COVID will probably pick up.”
The rise in RSV was unexpected by some experts. “This early influenza is also a bit of a surprise and may be influenced by the fact that lots of us are going back and seeing each other again close-to-close, face-to-face in many enclosed environments,” Dr. Schaffner said.
He estimated the 2022-2023 flu season started 4-6 weeks early “and it’s taken off like a rocket. It started in the Southeast, quickly went to the Southwest and up the East Coast. Now it’s moving dramatically through the Midwest and will continue. It’s quite sure to hit the West Coast if it isn’t there already.”
A phenomenon by any other name
Some are calling the situation an “immunity debt,” while others dub it an “immunity pause” or an “immunity deficit.” Many physicians and immunologists have taken to social media to push back on the term “immunity debt,” saying it’s a mischaracterization that is being used to vilify COVID precautions, such as masking, social distancing, and other protective measures taken during the pandemic.
“I prefer the term ‘immunity gap’ ... which is more established in the epidemiology literature, especially given the politicization of the term ‘immunity debt’ by folks recently,” Dr. Messacar said.
“To me, the immunity gap is a scientific observation, not a political argument,” he added.
In a July 2022 publication in The Lancet, Dr. Messacar and his colleagues stated that “decreased exposure to endemic viruses created an immunity gap – a group of susceptible individuals who avoided infection and therefore lack pathogen-specific immunity to protect against future infection. Decreases in childhood vaccinations with pandemic disruptions to health care delivery contribute to this immunity gap for vaccine-preventable diseases, such as influenza,measles, and polio.”
The researchers noted that because of isolation during the pandemic, older children and newborns are being exposed to RSV for the first time. Returning to birthday parties, playing with friends, and going to school without masks means “children are being exposed to RSV, and that’s likely the reason that RSV is moving early and very, very substantially through this now expanded pool of susceptible children,” Dr. Schaffner said.
How likely are coinfections?
With peaks in RSV, flu, and COVID-19 cases each predicted in the coming months, how likely is it that someone could get sick with more than one infection at the same time?
Early in the pandemic, coinfection with COVID and the flu was reported in people at some centers on the West Coast, Dr. Auwaerter said. Now, however, “the unpredictable nature of the Omicron subvariants and the potential for further change, along with the never-before-seen significant lessening of influenza over 2 years, leave little for predictability.
“I do think it is less likely, given the extent of immunity now to SARS-CoV-2 in the population,” Dr. Auwaerter said.
“I most worry about viral coinfections ... in people with suppressed immune systems if we have high community rates of the SARS-CoV-2 and influenza circulating this fall and winter,” he added.
Studies during the pandemic suggest that coinfection with the SARS-CoV-2 virus and another respiratory virus were either rare or nonexistent.
Dr. Schaffner said these findings align with his experience at Vanderbilt University, which is part of a CDC-sponsored network that tracks laboratory-confirmed RSV, flu, and COVID cases among people in the hospital. “Coinfections are, at least to date, very unusual.”
There needs to be an asterisk next to that, Dr. Schaffner added. “Looking back over the last 2 years, we’ve had very little influenza, and we’ve had curtailed RSV seasons. So there hasn’t been a whole lot of opportunity for dual infections to occur.
“So this year may be more revelatory as we go forward,” he said.
Future concerns
The future is uncertain, Dr. Messacar and colleagues wrote in The Lancet: “Crucially, the patterns of these returning viral outbreaks have been heterogeneous across locations, populations, and pathogens, making predictions and preparations challenging.”
Dr. Chin-Hong used a horse race analogy to illustrate the situation now and going forward. RSV is the front-running horse, and influenza is running behind but trying to catch up. “And then COVID is the dark horse. It’s trailing the race right now – but all these variants are giving the horse extra supplements.
“And the COVID horse is probably going to be very competitive with the front-runner,” he said.
“We’re just at the beginning of the race right now,” Dr. Chin-Hong said, “so that’s why we’re worried that these three [viruses] will be even more pronounced come later in the year.”
A version of this article first appeared on Medscape.com.
Headlines over the past few weeks are ringing the alarm about earlier and more serious influenza (flu) and respiratory syncytial virus (RSV) outbreaks compared with previous years. Add COVID-19 to the mix and you have a dangerous mash of viruses that have many experts calling for caution and searching for explanations.
RSV and the flu “are certainly getting more attention, and they’re getting more attention for two reasons,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University, Nashville, Tenn.
“The first is that they’re both extraordinarily early. The second is that they’re both out there spreading very, very rapidly,” he told this news organization.
RSV usually follows a seasonal pattern with cases peaking in January and February. Both viruses tend to hit different regions of the country at different times, and that’s not the case in 2022.
“This is particularly striking for RSV, which usually doesn’t affect the entire country simultaneously,” Dr. Schaffner said.
“Yes, RSV is causing many more hospitalizations and earlier than any previously recorded season in the U.S.,” according to figures from the Centers for Disease Control and Prevention on RSV hospitalizations, said Kevin Messacar, MD, PhD, associate professor at the University of Colorado at Denver, Aurora, and a pediatric infectious disease specialist at Children’s Hospital Colorado in Aurora.
Although there could be some increase in diagnoses because of increased awareness, the jump in RSV and flu cases “is a real phenomenon for multiple reasons,” said Peter Chin-Hong, MD, professor in the division of infectious diseases at the University of California, San Francisco.
With fewer COVID-related restrictions, people are moving around more. Also, during fall and winter, people tend to gather indoors. Colder temperatures and lower humidity contribute as well, Dr. Chin-Hong said, because “the droplets are just simply lighter.
“I think those are all factors,” he told this news organization.
Paul Auwaerter, MD, agreed that there are likely multiple causes for the unusual timing and severity of RSV and flu this year.
“Change in behaviors is a leading cause,” said the clinical director for the division of infectious diseases at the Johns Hopkins University, Baltimore. More people returning to the workplace and children going to school without masks are examples, he added.
Less exposure to these three viruses also means there was less immune boosting among existing populations, he said. This can lead to “larger susceptible populations, especially infants and younger children, due to the relative absence of circulating virus in past years.”
A leading theory
Are we paying a price now for people following the edicts from officials to mask up, stand apart, and take other personal and public health precautions during the COVID-19 pandemic?
It’s possible, but that may not be the whole story.
“When it comes to RSV, I think that theory of isolation, social distancing, mask wearing, and not attending schools is a very valid one,” Dr. Schaffner said. “That’s everybody’s favorite [reason].”
He said he is confident that the jump in RSV cases is being driven by previous COVID public health protections. However, he’s “a little more cautious about influenza, in part because influenza is so variable.
“Like people in influenza say, if you’ve seen one influenza season, you’ve seen one influenza season,” Dr. Schaffner said.
“There’s a lot of debate,” he added. “Nobody can say definitively whether the immune deficit or debt is a consequence of not being stimulated and restimulated by the influenza virus over the past two seasons.”
‘A perfect storm’
“Now you kind of have the perfect storm,” Dr. Chin-Hong said. “It’s not a good situation for COVID with the variants that are emerging. For influenza, not having seen a lot of influenza the last 2 years, we’re probably more susceptible to getting infected.”
RSV cases rose during summer 2021, but now the weather is colder, and people are interacting more closely. “And it’s very, very transmissible,” he said.
Dr. Chin-Hong also predicted that “even though we don’t have a lot of COVID now, COVID will probably pick up.”
The rise in RSV was unexpected by some experts. “This early influenza is also a bit of a surprise and may be influenced by the fact that lots of us are going back and seeing each other again close-to-close, face-to-face in many enclosed environments,” Dr. Schaffner said.
He estimated the 2022-2023 flu season started 4-6 weeks early “and it’s taken off like a rocket. It started in the Southeast, quickly went to the Southwest and up the East Coast. Now it’s moving dramatically through the Midwest and will continue. It’s quite sure to hit the West Coast if it isn’t there already.”
A phenomenon by any other name
Some are calling the situation an “immunity debt,” while others dub it an “immunity pause” or an “immunity deficit.” Many physicians and immunologists have taken to social media to push back on the term “immunity debt,” saying it’s a mischaracterization that is being used to vilify COVID precautions, such as masking, social distancing, and other protective measures taken during the pandemic.
“I prefer the term ‘immunity gap’ ... which is more established in the epidemiology literature, especially given the politicization of the term ‘immunity debt’ by folks recently,” Dr. Messacar said.
“To me, the immunity gap is a scientific observation, not a political argument,” he added.
In a July 2022 publication in The Lancet, Dr. Messacar and his colleagues stated that “decreased exposure to endemic viruses created an immunity gap – a group of susceptible individuals who avoided infection and therefore lack pathogen-specific immunity to protect against future infection. Decreases in childhood vaccinations with pandemic disruptions to health care delivery contribute to this immunity gap for vaccine-preventable diseases, such as influenza,measles, and polio.”
The researchers noted that because of isolation during the pandemic, older children and newborns are being exposed to RSV for the first time. Returning to birthday parties, playing with friends, and going to school without masks means “children are being exposed to RSV, and that’s likely the reason that RSV is moving early and very, very substantially through this now expanded pool of susceptible children,” Dr. Schaffner said.
How likely are coinfections?
With peaks in RSV, flu, and COVID-19 cases each predicted in the coming months, how likely is it that someone could get sick with more than one infection at the same time?
Early in the pandemic, coinfection with COVID and the flu was reported in people at some centers on the West Coast, Dr. Auwaerter said. Now, however, “the unpredictable nature of the Omicron subvariants and the potential for further change, along with the never-before-seen significant lessening of influenza over 2 years, leave little for predictability.
“I do think it is less likely, given the extent of immunity now to SARS-CoV-2 in the population,” Dr. Auwaerter said.
“I most worry about viral coinfections ... in people with suppressed immune systems if we have high community rates of the SARS-CoV-2 and influenza circulating this fall and winter,” he added.
Studies during the pandemic suggest that coinfection with the SARS-CoV-2 virus and another respiratory virus were either rare or nonexistent.
Dr. Schaffner said these findings align with his experience at Vanderbilt University, which is part of a CDC-sponsored network that tracks laboratory-confirmed RSV, flu, and COVID cases among people in the hospital. “Coinfections are, at least to date, very unusual.”
There needs to be an asterisk next to that, Dr. Schaffner added. “Looking back over the last 2 years, we’ve had very little influenza, and we’ve had curtailed RSV seasons. So there hasn’t been a whole lot of opportunity for dual infections to occur.
“So this year may be more revelatory as we go forward,” he said.
Future concerns
The future is uncertain, Dr. Messacar and colleagues wrote in The Lancet: “Crucially, the patterns of these returning viral outbreaks have been heterogeneous across locations, populations, and pathogens, making predictions and preparations challenging.”
Dr. Chin-Hong used a horse race analogy to illustrate the situation now and going forward. RSV is the front-running horse, and influenza is running behind but trying to catch up. “And then COVID is the dark horse. It’s trailing the race right now – but all these variants are giving the horse extra supplements.
“And the COVID horse is probably going to be very competitive with the front-runner,” he said.
“We’re just at the beginning of the race right now,” Dr. Chin-Hong said, “so that’s why we’re worried that these three [viruses] will be even more pronounced come later in the year.”
A version of this article first appeared on Medscape.com.
Hypertension linked to risk of severe COVID
U.K. researchers have established that hypertension is associated with a 22% greater risk of severe COVID-19, with the odds of severe COVID-19 unaffected by medication type.
Hypertension “appears to be one of the commonest comorbidities in COVID-19 patients”, explained the authors of a new study, published in PLOS ONE. The authors highlighted that previous research had shown that hypertension was more prevalent in severe and fatal cases compared with all cases of COVID-19.
They pointed out, however, that whether hypertensive individuals have a higher risk of severe COVID-19, compared with nonhypertensives, and whether the absolute level of systolic blood pressure or the type of antihypertensive medication is related to this risk, remained “unclear.”
To try to answer these questions, the research team, led by University of Cambridge researchers, analyzed data from 16,134 individuals who tested positive for COVID-19 (mean age 65.3 years, 47% male, 90% white), 40% were diagnosed with essential hypertension at the analysis baseline – 22% of whom had developed severe COVID-19.
Systolic blood pressure (SBP) was categorized by 10–mm Hg ranges, starting from < 120 mm Hg up to 180+ mm Hg, with the reference category defined as 120-129 mm Hg, based on data from the SPRINT study, which demonstrated that intensive SBP lowering to below 120 mm Hg, as compared with the traditional threshold of 140 mm Hg, was beneficial. Diastolic blood pressure was categorized by 10–mm Hg ranges, starting from < 60 mm Hg up to 100+ mm Hg with 80-90 mm Hg being the reference category.
In their analyses the researchers adjusted for age, sex, body mass index, ethnicity, smoking status, diabetes status, socioeconomic status, and inflammation (C-reactive protein [CRP]), as these were proposed as potential confounders. To assess the direct effect of hypertension on COVID-19, they also adjusted for intermediate variables, including cardiovascular comorbidities and stroke, on the causal pathway between hypertension and severe COVID-19.
Majority of effect of hypertension on severe COVID-19 was direct
The unadjusted odds ratio of the association between hypertension and severe COVID-19 was 2.33 (95% confidence interval, 2.16-2.51), the authors emphasized. They found that, after adjusting for all confounding variables, hypertension was associated with 22% higher odds of severe COVID-19 (OR, 1.22; 95% CI, 1.12-1.33), compared with normotension.
Individuals with severe COVID-19 were marginally older, more likely to be male, and more deprived, the authors said. “They were also more likely to be hypertensive, compared with individuals without severe COVID-19, and a greater proportion of individuals with severe COVID-19 had cardiovascular comorbidities.”
The majority of the effect of hypertension on development of severe COVID-19 was “direct,” they said. However, a modest proportion of the effect was mediated via cardiovascular comorbidities such as peripheral vascular disease, MI, coronary heart disease, arrhythmias, and stroke. Of note, those with a history of stroke had a 47% higher risk of severe COVID-19 and those with a history of other cardiovascular comorbidities had a 30% higher risk of severe COVID-19, the authors commented.
J-shaped relationship
Of the total of 6,517 (40%) individuals who had a diagnosis of essential hypertension at baseline, 67% were treated (41% with monotherapy, 59% with combination therapy), and 33% were untreated.
There were similar numbers of severe COVID-19 in each medication group: ACE inhibitors, 34%; angiotensin receptor blockers (ARBs), 36%; and “other” medications 34%.
In hypertensive individuals receiving antihypertensive medications, there was a “J-shaped relationship” between the level of blood pressure and risk of severe COVID-19 when using a systolic blood pressure level of 120-129 mm Hg as a reference – 150-159 mm Hg versus 120-129 mm Hg (OR 1.91; 95% CI, 1.44-2.53), > 180+ mm Hg versus 120-129 mm Hg (OR 1.93; 95% CI, 1.06-3.51).
The authors commented that there was no evidence of a higher risk of severe COVID-19 until systolic blood pressure “exceeded 150 mm Hg.”
They said it was an interesting finding that “very well-controlled” systolic blood pressure < 120 mm Hg was associated with a 40% (OR, 1.40; 95% CI, 1.11-1.78) greater odds of severe COVID-19. “This may be due to reverse causality, where low systolic blood pressure levels may indicate poorer health, such that the occurrence of severe COVID-19 may be related to underlying disease rather than the level of SBP per se,” they suggested.
The J-shaped association observed remained after multiple adjustments, including presence of known cardiovascular comorbidities, which suggested a possible “real effect” of low SBP on severe COVID-19, “at least in treated hypertensive individuals.”
Their analyses also identified that, compared with a “normal” diastolic blood pressure (80-90 mm Hg), having a diastolic blood pressure higher than 90 mm Hg was associated with higher odds of severe COVID-19.
The association between hypertension and COVID-19 was “amplified” if the individuals were treated and their BP remained uncontrolled, the authors pointed out.
There did not appear to be any difference in the risk of severe COVID-19 between individuals taking ACE inhibitors and those taking ARBs or other antihypertensive medications, the authors said.
Better understanding of underlying mechanisms needed
Individuals with hypertension who tested positive for COVID-19 had “over twice” the risk of developing severe COVID-19, compared with nonhypertensive individuals, the authors said.
They highlighted that their findings also suggest that there are “further effects” influencing the severity of COVID-19 beyond a “dichotomous” diagnosis of hypertension.
“Individuals with a higher-than-target systolic blood pressure may be less healthy, less active, suffering more severe hypertension, or have developed drug-resistant hypertension, all suggesting that the effects of hypertension have already had detrimental physiological effects on the cardiovascular system, which in turn may offer some explanation for the higher risk of severe COVID-19 with uncontrolled SBP,” they explained.
“Hypertension is an important risk factor for COVID-19,” reiterated the authors, who emphasized that a better understanding of the underlying mechanisms driving this increased risk is warranted in case of “more severe strains or other viruses” in the future.
The authors have declared no competing interests.
A version of this article first appeared on Medscape UK.
U.K. researchers have established that hypertension is associated with a 22% greater risk of severe COVID-19, with the odds of severe COVID-19 unaffected by medication type.
Hypertension “appears to be one of the commonest comorbidities in COVID-19 patients”, explained the authors of a new study, published in PLOS ONE. The authors highlighted that previous research had shown that hypertension was more prevalent in severe and fatal cases compared with all cases of COVID-19.
They pointed out, however, that whether hypertensive individuals have a higher risk of severe COVID-19, compared with nonhypertensives, and whether the absolute level of systolic blood pressure or the type of antihypertensive medication is related to this risk, remained “unclear.”
To try to answer these questions, the research team, led by University of Cambridge researchers, analyzed data from 16,134 individuals who tested positive for COVID-19 (mean age 65.3 years, 47% male, 90% white), 40% were diagnosed with essential hypertension at the analysis baseline – 22% of whom had developed severe COVID-19.
Systolic blood pressure (SBP) was categorized by 10–mm Hg ranges, starting from < 120 mm Hg up to 180+ mm Hg, with the reference category defined as 120-129 mm Hg, based on data from the SPRINT study, which demonstrated that intensive SBP lowering to below 120 mm Hg, as compared with the traditional threshold of 140 mm Hg, was beneficial. Diastolic blood pressure was categorized by 10–mm Hg ranges, starting from < 60 mm Hg up to 100+ mm Hg with 80-90 mm Hg being the reference category.
In their analyses the researchers adjusted for age, sex, body mass index, ethnicity, smoking status, diabetes status, socioeconomic status, and inflammation (C-reactive protein [CRP]), as these were proposed as potential confounders. To assess the direct effect of hypertension on COVID-19, they also adjusted for intermediate variables, including cardiovascular comorbidities and stroke, on the causal pathway between hypertension and severe COVID-19.
Majority of effect of hypertension on severe COVID-19 was direct
The unadjusted odds ratio of the association between hypertension and severe COVID-19 was 2.33 (95% confidence interval, 2.16-2.51), the authors emphasized. They found that, after adjusting for all confounding variables, hypertension was associated with 22% higher odds of severe COVID-19 (OR, 1.22; 95% CI, 1.12-1.33), compared with normotension.
Individuals with severe COVID-19 were marginally older, more likely to be male, and more deprived, the authors said. “They were also more likely to be hypertensive, compared with individuals without severe COVID-19, and a greater proportion of individuals with severe COVID-19 had cardiovascular comorbidities.”
The majority of the effect of hypertension on development of severe COVID-19 was “direct,” they said. However, a modest proportion of the effect was mediated via cardiovascular comorbidities such as peripheral vascular disease, MI, coronary heart disease, arrhythmias, and stroke. Of note, those with a history of stroke had a 47% higher risk of severe COVID-19 and those with a history of other cardiovascular comorbidities had a 30% higher risk of severe COVID-19, the authors commented.
J-shaped relationship
Of the total of 6,517 (40%) individuals who had a diagnosis of essential hypertension at baseline, 67% were treated (41% with monotherapy, 59% with combination therapy), and 33% were untreated.
There were similar numbers of severe COVID-19 in each medication group: ACE inhibitors, 34%; angiotensin receptor blockers (ARBs), 36%; and “other” medications 34%.
In hypertensive individuals receiving antihypertensive medications, there was a “J-shaped relationship” between the level of blood pressure and risk of severe COVID-19 when using a systolic blood pressure level of 120-129 mm Hg as a reference – 150-159 mm Hg versus 120-129 mm Hg (OR 1.91; 95% CI, 1.44-2.53), > 180+ mm Hg versus 120-129 mm Hg (OR 1.93; 95% CI, 1.06-3.51).
The authors commented that there was no evidence of a higher risk of severe COVID-19 until systolic blood pressure “exceeded 150 mm Hg.”
They said it was an interesting finding that “very well-controlled” systolic blood pressure < 120 mm Hg was associated with a 40% (OR, 1.40; 95% CI, 1.11-1.78) greater odds of severe COVID-19. “This may be due to reverse causality, where low systolic blood pressure levels may indicate poorer health, such that the occurrence of severe COVID-19 may be related to underlying disease rather than the level of SBP per se,” they suggested.
The J-shaped association observed remained after multiple adjustments, including presence of known cardiovascular comorbidities, which suggested a possible “real effect” of low SBP on severe COVID-19, “at least in treated hypertensive individuals.”
Their analyses also identified that, compared with a “normal” diastolic blood pressure (80-90 mm Hg), having a diastolic blood pressure higher than 90 mm Hg was associated with higher odds of severe COVID-19.
The association between hypertension and COVID-19 was “amplified” if the individuals were treated and their BP remained uncontrolled, the authors pointed out.
There did not appear to be any difference in the risk of severe COVID-19 between individuals taking ACE inhibitors and those taking ARBs or other antihypertensive medications, the authors said.
Better understanding of underlying mechanisms needed
Individuals with hypertension who tested positive for COVID-19 had “over twice” the risk of developing severe COVID-19, compared with nonhypertensive individuals, the authors said.
They highlighted that their findings also suggest that there are “further effects” influencing the severity of COVID-19 beyond a “dichotomous” diagnosis of hypertension.
“Individuals with a higher-than-target systolic blood pressure may be less healthy, less active, suffering more severe hypertension, or have developed drug-resistant hypertension, all suggesting that the effects of hypertension have already had detrimental physiological effects on the cardiovascular system, which in turn may offer some explanation for the higher risk of severe COVID-19 with uncontrolled SBP,” they explained.
“Hypertension is an important risk factor for COVID-19,” reiterated the authors, who emphasized that a better understanding of the underlying mechanisms driving this increased risk is warranted in case of “more severe strains or other viruses” in the future.
The authors have declared no competing interests.
A version of this article first appeared on Medscape UK.
U.K. researchers have established that hypertension is associated with a 22% greater risk of severe COVID-19, with the odds of severe COVID-19 unaffected by medication type.
Hypertension “appears to be one of the commonest comorbidities in COVID-19 patients”, explained the authors of a new study, published in PLOS ONE. The authors highlighted that previous research had shown that hypertension was more prevalent in severe and fatal cases compared with all cases of COVID-19.
They pointed out, however, that whether hypertensive individuals have a higher risk of severe COVID-19, compared with nonhypertensives, and whether the absolute level of systolic blood pressure or the type of antihypertensive medication is related to this risk, remained “unclear.”
To try to answer these questions, the research team, led by University of Cambridge researchers, analyzed data from 16,134 individuals who tested positive for COVID-19 (mean age 65.3 years, 47% male, 90% white), 40% were diagnosed with essential hypertension at the analysis baseline – 22% of whom had developed severe COVID-19.
Systolic blood pressure (SBP) was categorized by 10–mm Hg ranges, starting from < 120 mm Hg up to 180+ mm Hg, with the reference category defined as 120-129 mm Hg, based on data from the SPRINT study, which demonstrated that intensive SBP lowering to below 120 mm Hg, as compared with the traditional threshold of 140 mm Hg, was beneficial. Diastolic blood pressure was categorized by 10–mm Hg ranges, starting from < 60 mm Hg up to 100+ mm Hg with 80-90 mm Hg being the reference category.
In their analyses the researchers adjusted for age, sex, body mass index, ethnicity, smoking status, diabetes status, socioeconomic status, and inflammation (C-reactive protein [CRP]), as these were proposed as potential confounders. To assess the direct effect of hypertension on COVID-19, they also adjusted for intermediate variables, including cardiovascular comorbidities and stroke, on the causal pathway between hypertension and severe COVID-19.
Majority of effect of hypertension on severe COVID-19 was direct
The unadjusted odds ratio of the association between hypertension and severe COVID-19 was 2.33 (95% confidence interval, 2.16-2.51), the authors emphasized. They found that, after adjusting for all confounding variables, hypertension was associated with 22% higher odds of severe COVID-19 (OR, 1.22; 95% CI, 1.12-1.33), compared with normotension.
Individuals with severe COVID-19 were marginally older, more likely to be male, and more deprived, the authors said. “They were also more likely to be hypertensive, compared with individuals without severe COVID-19, and a greater proportion of individuals with severe COVID-19 had cardiovascular comorbidities.”
The majority of the effect of hypertension on development of severe COVID-19 was “direct,” they said. However, a modest proportion of the effect was mediated via cardiovascular comorbidities such as peripheral vascular disease, MI, coronary heart disease, arrhythmias, and stroke. Of note, those with a history of stroke had a 47% higher risk of severe COVID-19 and those with a history of other cardiovascular comorbidities had a 30% higher risk of severe COVID-19, the authors commented.
J-shaped relationship
Of the total of 6,517 (40%) individuals who had a diagnosis of essential hypertension at baseline, 67% were treated (41% with monotherapy, 59% with combination therapy), and 33% were untreated.
There were similar numbers of severe COVID-19 in each medication group: ACE inhibitors, 34%; angiotensin receptor blockers (ARBs), 36%; and “other” medications 34%.
In hypertensive individuals receiving antihypertensive medications, there was a “J-shaped relationship” between the level of blood pressure and risk of severe COVID-19 when using a systolic blood pressure level of 120-129 mm Hg as a reference – 150-159 mm Hg versus 120-129 mm Hg (OR 1.91; 95% CI, 1.44-2.53), > 180+ mm Hg versus 120-129 mm Hg (OR 1.93; 95% CI, 1.06-3.51).
The authors commented that there was no evidence of a higher risk of severe COVID-19 until systolic blood pressure “exceeded 150 mm Hg.”
They said it was an interesting finding that “very well-controlled” systolic blood pressure < 120 mm Hg was associated with a 40% (OR, 1.40; 95% CI, 1.11-1.78) greater odds of severe COVID-19. “This may be due to reverse causality, where low systolic blood pressure levels may indicate poorer health, such that the occurrence of severe COVID-19 may be related to underlying disease rather than the level of SBP per se,” they suggested.
The J-shaped association observed remained after multiple adjustments, including presence of known cardiovascular comorbidities, which suggested a possible “real effect” of low SBP on severe COVID-19, “at least in treated hypertensive individuals.”
Their analyses also identified that, compared with a “normal” diastolic blood pressure (80-90 mm Hg), having a diastolic blood pressure higher than 90 mm Hg was associated with higher odds of severe COVID-19.
The association between hypertension and COVID-19 was “amplified” if the individuals were treated and their BP remained uncontrolled, the authors pointed out.
There did not appear to be any difference in the risk of severe COVID-19 between individuals taking ACE inhibitors and those taking ARBs or other antihypertensive medications, the authors said.
Better understanding of underlying mechanisms needed
Individuals with hypertension who tested positive for COVID-19 had “over twice” the risk of developing severe COVID-19, compared with nonhypertensive individuals, the authors said.
They highlighted that their findings also suggest that there are “further effects” influencing the severity of COVID-19 beyond a “dichotomous” diagnosis of hypertension.
“Individuals with a higher-than-target systolic blood pressure may be less healthy, less active, suffering more severe hypertension, or have developed drug-resistant hypertension, all suggesting that the effects of hypertension have already had detrimental physiological effects on the cardiovascular system, which in turn may offer some explanation for the higher risk of severe COVID-19 with uncontrolled SBP,” they explained.
“Hypertension is an important risk factor for COVID-19,” reiterated the authors, who emphasized that a better understanding of the underlying mechanisms driving this increased risk is warranted in case of “more severe strains or other viruses” in the future.
The authors have declared no competing interests.
A version of this article first appeared on Medscape UK.
FROM PLOS ONE
Neurosurgery Operating Room Efficiency During the COVID-19 Era
From the Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN (Stefan W. Koester, Puja Jagasia, and Drs. Liles, Dambrino IV, Feldman, and Chambless), and the Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN (Drs. Mathews and Tiwari).
ABSTRACT
Background: The COVID-19 pandemic has had broad effects on surgical care, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and newly implemented anti-infective measures. Our aim was to assess neurosurgery OR efficiency before the COVID-19 pandemic, during peak COVID-19, and during current times.
Methods: Institutional perioperative databases at a single, high-volume neurosurgical center were queried for operations performed from December 2019 until October 2021. March 12, 2020, the day that the state of Tennessee declared a state of emergency, was chosen as the onset of the COVID-19 pandemic. The 90-day periods before and after this day were used to define the pre-COVID-19, peak-COVID-19, and post-peak restrictions time periods for comparative analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover). Univariate analysis used Wilcoxon rank-sum test for continuous outcomes, while chi-square test and Fisher’s exact test were used for categorical comparisons. Significance was defined as P < .05.
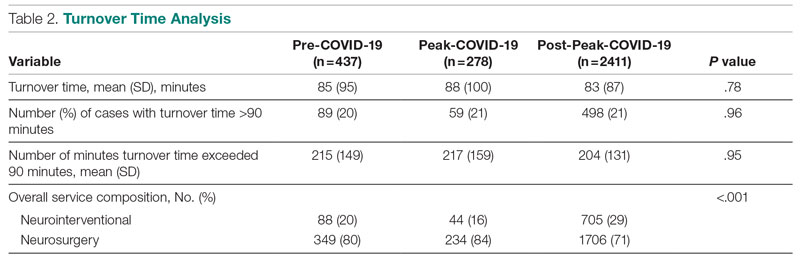
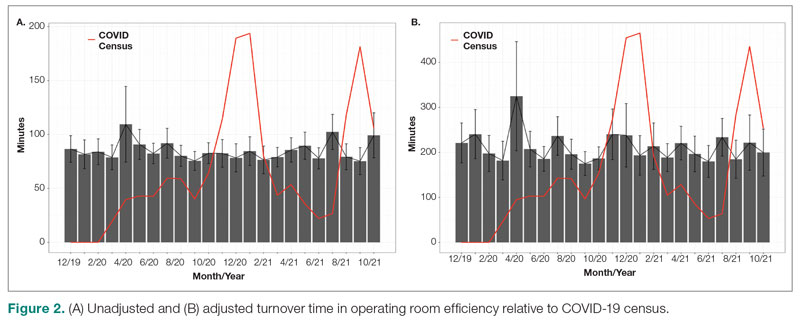
Results: First-start time was analyzed in 426 pre-COVID-19, 357 peak-restrictions, and 2304 post-peak-restrictions cases. The unadjusted mean delay length was found to be significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different. The proportion of cases that started early, as well as significantly early past a 15-minute threshold, have not been impacted. There was no significant change in turnover time during peak restrictions relative to the pre-COVID-19 period (88 [100] minutes vs 85 [95] minutes), and turnover time has since remained unchanged (83 [87] minutes).
Conclusion: Our center was able to maintain OR efficiency before, during, and after peak restrictions even while instituting advanced infection-control strategies. While there were significant changes, delays were relatively small in magnitude.
Keywords: operating room timing, hospital efficiency, socioeconomics, pandemic.
The COVID-19 pandemic has led to major changes in patient care both from a surgical perspective and in regard to inpatient hospital course. Safety protocols nationwide have been implemented to protect both patients and providers. Some elements of surgical care have drastically changed, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and increased sterilization measures. Furloughs, layoffs, and reassignments due to the focus on nonelective and COVID-19–related cases challenged OR staffing and efficiency. Operating room staff with COVID-19 exposures or COVID-19 infections also caused last-minute changes in staffing. All of these scenarios can cause issues due to actual understaffing or due to staff members being pushed into highly specialized areas, such as neurosurgery, in which they have very little experience. A further obstacle to OR efficiency included policy changes involving PPE utilization, sterilization measures, and supply chain shortages of necessary resources such as PPE.
Neurosurgery in particular has been susceptible to COVID-19–related system-wide changes given operator proximity to the patient’s respiratory passages, frequency of emergent cases, and varying anesthetic needs, as well as the high level of specialization needed to perform neurosurgical care. Previous studies have shown a change in the makeup of neurosurgical patients seeking care, as well as in the acuity of neurological consult of these patients.1 A study in orthopedic surgery by Andreata et al demonstrated worsened OR efficiency, with significantly increased first-start and turnover times.2 In the COVID-19 era, OR quality and safety are crucially important to both patients and providers. Providing this safe and effective care in an efficient manner is important for optimal neurosurgical management in the long term.3 Moreover, the financial burden of implementing new protocols and standards can be compounded by additional financial losses due to reduced OR efficiency.
Methods
To analyze the effect of COVID-19 on neurosurgical OR efficiency, institutional perioperative databases at a single high-volume center were queried for operations performed from December 2019 until October 2021. March 12, 2020, was chosen as the onset of COVID-19 for analytic purposes, as this was the date when the state of Tennessee declared a state of emergency. The 90-day periods before and after this date were used for comparative analysis for pre-COVID-19, peak COVID-19, and post-peak-restrictions time periods. The peak COVID-19 period was defined as the 90-day period following the initial onset of COVID-19 and the surge of cases. For comparison purposes, post-peak COVID-19 was defined as the months following the first peak until October 2021 (approximately 17 months). COVID-19 burden was determined using a COVID-19 single-institution census of confirmed cases by polymerase chain reaction (PCR) for which the average number of cases of COVID-19 during a given month was determined. This number is a scaled trend, and a true number of COVID-19 cases in our hospital was not reported.
Neurosurgical and neuroendovascular cases were included in the analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases, defined as the time from the patient leaving the room until the next patient entered the room. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover, which is a standard for our single-institution perioperative center). Statistical analyses, including data aggregation, were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Patients’ demographic and clinical characteristics were analyzed using an independent 2-sample t-test for interval variables and a chi-square test for categorical variables. Significance was defined as P < .05.
Results
First-Start Time
First-start time was analyzed in 426 pre-COVID-19, 357 peak-COVID-19, and 2304 post-peak-COVID-19 cases. The unadjusted mean delay length was significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004) (Table 1). 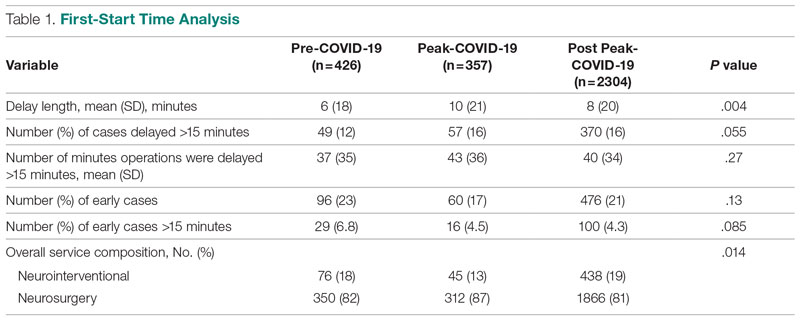
The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different, but they have been slightly higher since the onset of COVID-19. The proportion of cases that have started early, as well as significantly early past a 15-minute threshold, have also trended down since the onset of the COVID-19 pandemic, but this difference was again not significant. The temporal relationship of first-start delay, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 1. The trend of increasing delay is loosely associated with the COVID-19 burden experienced by our hospital. The start of COVID-19 as well as both COVID-19 peaks have been associated with increased delays in our hospital.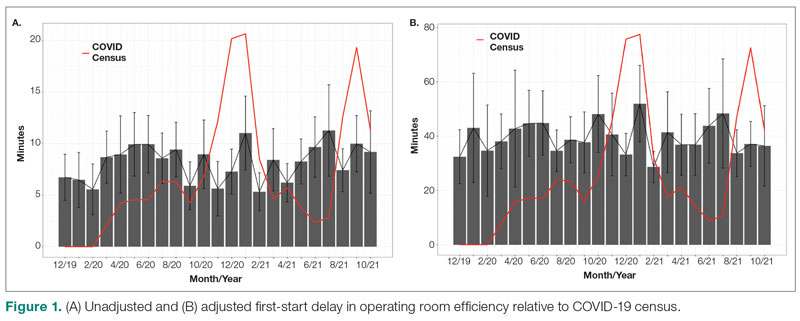
Turnover Time
Turnover time was assessed in 437 pre-COVID-19, 278 peak-restrictions, and 2411 post-peak-restrictions cases. Turnover time during peak restrictions was not significantly different from pre-COVID-19 (88 [100] vs 85 [95]) and has since remained relatively unchanged (83 [87], P = .78). A similar trend held for comparisons of proportion of cases with turnover time past 90 minutes and average times past the 90-minute threshold (Table 2). The temporal relationship between COVID-19 burden and turnover time, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 2. Both figures demonstrate a slight initial increase in turnover time delay at the start of COVID-19, which stabilized with little variation thereafter.
Discussion
We analyzed the OR efficiency metrics of first-start and turnover time during the 90-day period before COVID-19 (pre-COVID-19), the 90 days following Tennessee declaring a state of emergency (peak COVID-19), and the time following this period (post-COVID-19) for all neurosurgical and neuroendovascular cases at Vanderbilt University Medical Center (VUMC). We found a significant difference in unadjusted mean delay length in first-start time between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes for pre-COVID-19, peak-COVID-19, and post-COVID-19: 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). No significant increase in turnover time between cases was found between these 3 time periods. Based on metrics from first-start delay and turnover time, our center was able to maintain OR efficiency before, during, and after peak COVID-19.
After the Centers for Disease Control and Prevention released guidelines recommending deferring elective procedures to conserve beds and PPE, VUMC made the decision to suspend all elective surgical procedures from March 18 to April 24, 2020. Prior research conducted during the COVID-19 pandemic has demonstrated more than 400 types of surgical procedures with negatively impacted outcomes when compared to surgical outcomes from the same time frame in 2018 and 2019.4 For more than 20 of these types of procedures, there was a significant association between procedure delay and adverse patient outcomes.4 Testing protocols for patients prior to surgery varied throughout the pandemic based on vaccination status and type of procedure. Before vaccines became widely available, all patients were required to obtain a PCR SARS-CoV-2 test within 48 to 72 hours of the scheduled procedure. If the patient’s procedure was urgent and testing was not feasible, the patient was treated as a SARS-CoV-2–positive patient, which required all health care workers involved in the case to wear gowns, gloves, surgical masks, and eye protection. Testing patients preoperatively likely helped to maintain OR efficiency since not all patients received test results prior to the scheduled procedure, leading to cancellations of cases and therefore more staff available for fewer cases.
After vaccines became widely available to the public, testing requirements for patients preoperatively were relaxed, and only patients who were not fully vaccinated or severely immunocompromised were required to test prior to procedures. However, approximately 40% of the population in Tennessee was fully vaccinated in 2021, which reflects the patient population of VUMC.5 This means that many patients who received care at VUMC were still tested prior to procedures.
Adopting adequate safety protocols was found to be key for OR efficiency during the COVID-19 pandemic since performing surgery increased the risk of infection for each health care worker in the OR.6 VUMC protocols identified procedures that required enhanced safety measures to prevent infection of health care workers and avoid staffing shortages, which would decrease OR efficiency. Protocols mandated that only anesthesia team members were allowed to be in the OR during intubation and extubation of patients, which could be one factor leading to increased delays and decreased efficiency for some institutions. Methods for neurosurgeons to decrease risk of infection in the OR include postponing all nonurgent cases, reappraising the necessity for general anesthesia and endotracheal intubation, considering alternative surgical approaches that avoid the respiratory tract, and limiting the use of aerosol-generating instruments.7,8 VUMC’s success in implementing these protocols likely explains why our center was able to maintain OR efficiency throughout the COVID-19 pandemic.
A study conducted by Andreata et al showed a significantly increased mean first-case delay and a nonsignificant increased turnover time in orthopedic surgeries in Northern Italy when comparing surgeries performed during the COVID-19 pandemic to those performed prior to COVID-19.2 Other studies have indicated a similar trend in decreased OR efficiency during COVID-19 in other areas around the world.9,10 These findings are not consistent with our own findings for neurosurgical and neuroendovascular surgeries at VUMC, and any change at our institution was relatively immaterial. Factors that threatened to change OR efficiency—but did not result in meaningful changes in our institutional experience—include delays due to pending COVID-19 test results, safety procedures such as PPE donning, and planning difficulties to ensure the existence of teams with non-overlapping providers in the case of a surgeon being infected.2,11-13
Globally, many surgery centers halted all elective surgeries during the initial COVID-19 spike to prevent a PPE shortage and mitigate risk of infection of patients and health care workers.8,12,14 However, there is no centralized definition of which neurosurgical procedures are elective, so that decision was made on a surgeon or center level, which could lead to variability in efficiency trends.14 One study on neurosurgical procedures during COVID-19 found a 30% decline in all cases and a 23% decline in emergent procedures, showing that the decrease in volume was not only due to cancellation of elective procedures.15 This decrease in elective and emergent surgeries created a backlog of surgeries as well as a loss in health care revenue, and caused many patients to go without adequate health care.10 Looking forward, it is imperative that surgical centers study trends in OR efficiency from COVID-19 and learn how to better maintain OR efficiency during future pandemic conditions to prevent a backlog of cases, loss of health care revenue, and decreased health care access.
Limitations
Our data are from a single center and therefore may not be representative of experiences of other hospitals due to different populations and different impacts from COVID-19. However, given our center’s high volume and diverse patient population, we believe our analysis highlights important trends in neurosurgery practice. Notably, data for patient and OR timing are digitally generated and are entered manually by nurses in the electronic medical record, making it prone to errors and variability. This is in our experience, and if any error is present, we believe it is minimal.
Conclusion
The COVID-19 pandemic has had far-reaching effects on health care worldwide, including neurosurgical care. OR efficiency across the United States generally worsened given the stresses of supply chain issues, staffing shortages, and cancellations. At our institution, we were able to maintain OR efficiency during the known COVID-19 peaks until October 2021. Continually functional neurosurgical ORs are important in preventing delays in care and maintaining a steady revenue in order for hospitals and other health care entities to remain solvent. Further study of OR efficiency is needed for health care systems to prepare for future pandemics and other resource-straining events in order to provide optimal patient care.
Corresponding author: Campbell Liles, MD, Vanderbilt University Medical Center, Department of Neurological Surgery, 1161 21st Ave. South, T4224 Medical Center North, Nashville, TN 37232-2380; [email protected]
Disclosures: None reported.
1. Koester SW, Catapano JS, Ma KL, et al. COVID-19 and neurosurgery consultation call volume at a single large tertiary center with a propensity- adjusted analysis. World Neurosurg. 2021;146:e768-e772. doi:10.1016/j.wneu.2020.11.017
2. Andreata M, Faraldi M, Bucci E, Lombardi G, Zagra L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int Orthop. 2020;44(12):2499-2504. doi:10.1007/s00264-020-04772-x
3. Dexter F, Abouleish AE, Epstein RH, et al. Use of operating room information system data to predict the impact of reducing turnover times on staffing costs. Anesth Analg. 2003;97(4):1119-1126. doi:10.1213/01.ANE.0000082520.68800.79
4. Zheng NS, Warner JL, Osterman TJ, et al. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J Biomed Inform. 2021;113:103657. doi:10.1016/j.jbi.2020.103657
5. Alcendor DJ. Targeting COVID-19 vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID- 19 pandemic in the South. Vaccines (Basel). 2021;9(11):1279. doi:10.3390/vaccines9111279
6. Perrone G, Giuffrida M, Bellini V, et al. Operating room setup: how to improve health care professionals safety during pandemic COVID- 19: a quality improvement study. J Laparoendosc Adv Surg Tech A. 2021;31(1):85-89. doi:10.1089/lap.2020.0592
7. Iorio-Morin C, Hodaie M, Sarica C, et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87(2):E178-E185. doi:10.1093/ neuros/nyaa157
8. Gupta P, Muthukumar N, Rajshekhar V, et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68(2):246-254. doi:10.4103/0028-3886.283130
9. Mercer ST, Agarwal R, Dayananda KSS, et al. A comparative study looking at trauma and orthopaedic operating efficiency in the COVID-19 era. Perioper Care Oper Room Manag. 2020;21:100142. doi:10.1016/j.pcorm.2020.100142
10. Rozario N, Rozario D. Can machine learning optimize the efficiency of the operating room in the era of COVID-19? Can J Surg. 2020;63(6):E527-E529. doi:10.1503/cjs.016520
11. Toh KHQ, Barazanchi A, Rajaretnam NS, et al. COVID-19 response by New Zealand general surgical departments in tertiary metropolitan hospitals. ANZ J Surg. 2021;91(7-8):1352-1357. doi:10.1111/ ans.17044
12. Moorthy RK, Rajshekhar V. Impact of COVID-19 pandemic on neurosurgical practice in India: a survey on personal protective equipment usage, testing, and perceptions on disease transmission. Neurol India. 2020;68(5):1133-1138. doi:10.4103/0028- 3886.299173
13. Meneghini RM. Techniques and strategies to optimize efficiencies in the office and operating room: getting through the patient backlog and preserving hospital resources. J Arthroplasty. 2021;36(7S):S49-S51. doi:10.1016/j.arth.2021.03.010
14. Jean WC, Ironside NT, Sack KD, et al. The impact of COVID- 19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien). 2020;162(6):1229-1240. doi:10.1007/s00701-020- 04342-5
15. Raneri F, Rustemi O, Zambon G, et al. Neurosurgery in times of a pandemic: a survey of neurosurgical services during the COVID-19 outbreak in the Veneto region in Italy. Neurosurg Focus. 2020;49(6):E9. doi:10.3171/2020.9.FOCUS20691
From the Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN (Stefan W. Koester, Puja Jagasia, and Drs. Liles, Dambrino IV, Feldman, and Chambless), and the Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN (Drs. Mathews and Tiwari).
ABSTRACT
Background: The COVID-19 pandemic has had broad effects on surgical care, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and newly implemented anti-infective measures. Our aim was to assess neurosurgery OR efficiency before the COVID-19 pandemic, during peak COVID-19, and during current times.
Methods: Institutional perioperative databases at a single, high-volume neurosurgical center were queried for operations performed from December 2019 until October 2021. March 12, 2020, the day that the state of Tennessee declared a state of emergency, was chosen as the onset of the COVID-19 pandemic. The 90-day periods before and after this day were used to define the pre-COVID-19, peak-COVID-19, and post-peak restrictions time periods for comparative analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover). Univariate analysis used Wilcoxon rank-sum test for continuous outcomes, while chi-square test and Fisher’s exact test were used for categorical comparisons. Significance was defined as P < .05.
Results: First-start time was analyzed in 426 pre-COVID-19, 357 peak-restrictions, and 2304 post-peak-restrictions cases. The unadjusted mean delay length was found to be significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different. The proportion of cases that started early, as well as significantly early past a 15-minute threshold, have not been impacted. There was no significant change in turnover time during peak restrictions relative to the pre-COVID-19 period (88 [100] minutes vs 85 [95] minutes), and turnover time has since remained unchanged (83 [87] minutes).
Conclusion: Our center was able to maintain OR efficiency before, during, and after peak restrictions even while instituting advanced infection-control strategies. While there were significant changes, delays were relatively small in magnitude.
Keywords: operating room timing, hospital efficiency, socioeconomics, pandemic.
The COVID-19 pandemic has led to major changes in patient care both from a surgical perspective and in regard to inpatient hospital course. Safety protocols nationwide have been implemented to protect both patients and providers. Some elements of surgical care have drastically changed, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and increased sterilization measures. Furloughs, layoffs, and reassignments due to the focus on nonelective and COVID-19–related cases challenged OR staffing and efficiency. Operating room staff with COVID-19 exposures or COVID-19 infections also caused last-minute changes in staffing. All of these scenarios can cause issues due to actual understaffing or due to staff members being pushed into highly specialized areas, such as neurosurgery, in which they have very little experience. A further obstacle to OR efficiency included policy changes involving PPE utilization, sterilization measures, and supply chain shortages of necessary resources such as PPE.
Neurosurgery in particular has been susceptible to COVID-19–related system-wide changes given operator proximity to the patient’s respiratory passages, frequency of emergent cases, and varying anesthetic needs, as well as the high level of specialization needed to perform neurosurgical care. Previous studies have shown a change in the makeup of neurosurgical patients seeking care, as well as in the acuity of neurological consult of these patients.1 A study in orthopedic surgery by Andreata et al demonstrated worsened OR efficiency, with significantly increased first-start and turnover times.2 In the COVID-19 era, OR quality and safety are crucially important to both patients and providers. Providing this safe and effective care in an efficient manner is important for optimal neurosurgical management in the long term.3 Moreover, the financial burden of implementing new protocols and standards can be compounded by additional financial losses due to reduced OR efficiency.
Methods
To analyze the effect of COVID-19 on neurosurgical OR efficiency, institutional perioperative databases at a single high-volume center were queried for operations performed from December 2019 until October 2021. March 12, 2020, was chosen as the onset of COVID-19 for analytic purposes, as this was the date when the state of Tennessee declared a state of emergency. The 90-day periods before and after this date were used for comparative analysis for pre-COVID-19, peak COVID-19, and post-peak-restrictions time periods. The peak COVID-19 period was defined as the 90-day period following the initial onset of COVID-19 and the surge of cases. For comparison purposes, post-peak COVID-19 was defined as the months following the first peak until October 2021 (approximately 17 months). COVID-19 burden was determined using a COVID-19 single-institution census of confirmed cases by polymerase chain reaction (PCR) for which the average number of cases of COVID-19 during a given month was determined. This number is a scaled trend, and a true number of COVID-19 cases in our hospital was not reported.
Neurosurgical and neuroendovascular cases were included in the analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases, defined as the time from the patient leaving the room until the next patient entered the room. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover, which is a standard for our single-institution perioperative center). Statistical analyses, including data aggregation, were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Patients’ demographic and clinical characteristics were analyzed using an independent 2-sample t-test for interval variables and a chi-square test for categorical variables. Significance was defined as P < .05.
Results
First-Start Time
First-start time was analyzed in 426 pre-COVID-19, 357 peak-COVID-19, and 2304 post-peak-COVID-19 cases. The unadjusted mean delay length was significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004) (Table 1). 
The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different, but they have been slightly higher since the onset of COVID-19. The proportion of cases that have started early, as well as significantly early past a 15-minute threshold, have also trended down since the onset of the COVID-19 pandemic, but this difference was again not significant. The temporal relationship of first-start delay, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 1. The trend of increasing delay is loosely associated with the COVID-19 burden experienced by our hospital. The start of COVID-19 as well as both COVID-19 peaks have been associated with increased delays in our hospital.
Turnover Time
Turnover time was assessed in 437 pre-COVID-19, 278 peak-restrictions, and 2411 post-peak-restrictions cases. Turnover time during peak restrictions was not significantly different from pre-COVID-19 (88 [100] vs 85 [95]) and has since remained relatively unchanged (83 [87], P = .78). A similar trend held for comparisons of proportion of cases with turnover time past 90 minutes and average times past the 90-minute threshold (Table 2). The temporal relationship between COVID-19 burden and turnover time, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 2. Both figures demonstrate a slight initial increase in turnover time delay at the start of COVID-19, which stabilized with little variation thereafter.


Discussion
We analyzed the OR efficiency metrics of first-start and turnover time during the 90-day period before COVID-19 (pre-COVID-19), the 90 days following Tennessee declaring a state of emergency (peak COVID-19), and the time following this period (post-COVID-19) for all neurosurgical and neuroendovascular cases at Vanderbilt University Medical Center (VUMC). We found a significant difference in unadjusted mean delay length in first-start time between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes for pre-COVID-19, peak-COVID-19, and post-COVID-19: 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). No significant increase in turnover time between cases was found between these 3 time periods. Based on metrics from first-start delay and turnover time, our center was able to maintain OR efficiency before, during, and after peak COVID-19.
After the Centers for Disease Control and Prevention released guidelines recommending deferring elective procedures to conserve beds and PPE, VUMC made the decision to suspend all elective surgical procedures from March 18 to April 24, 2020. Prior research conducted during the COVID-19 pandemic has demonstrated more than 400 types of surgical procedures with negatively impacted outcomes when compared to surgical outcomes from the same time frame in 2018 and 2019.4 For more than 20 of these types of procedures, there was a significant association between procedure delay and adverse patient outcomes.4 Testing protocols for patients prior to surgery varied throughout the pandemic based on vaccination status and type of procedure. Before vaccines became widely available, all patients were required to obtain a PCR SARS-CoV-2 test within 48 to 72 hours of the scheduled procedure. If the patient’s procedure was urgent and testing was not feasible, the patient was treated as a SARS-CoV-2–positive patient, which required all health care workers involved in the case to wear gowns, gloves, surgical masks, and eye protection. Testing patients preoperatively likely helped to maintain OR efficiency since not all patients received test results prior to the scheduled procedure, leading to cancellations of cases and therefore more staff available for fewer cases.
After vaccines became widely available to the public, testing requirements for patients preoperatively were relaxed, and only patients who were not fully vaccinated or severely immunocompromised were required to test prior to procedures. However, approximately 40% of the population in Tennessee was fully vaccinated in 2021, which reflects the patient population of VUMC.5 This means that many patients who received care at VUMC were still tested prior to procedures.
Adopting adequate safety protocols was found to be key for OR efficiency during the COVID-19 pandemic since performing surgery increased the risk of infection for each health care worker in the OR.6 VUMC protocols identified procedures that required enhanced safety measures to prevent infection of health care workers and avoid staffing shortages, which would decrease OR efficiency. Protocols mandated that only anesthesia team members were allowed to be in the OR during intubation and extubation of patients, which could be one factor leading to increased delays and decreased efficiency for some institutions. Methods for neurosurgeons to decrease risk of infection in the OR include postponing all nonurgent cases, reappraising the necessity for general anesthesia and endotracheal intubation, considering alternative surgical approaches that avoid the respiratory tract, and limiting the use of aerosol-generating instruments.7,8 VUMC’s success in implementing these protocols likely explains why our center was able to maintain OR efficiency throughout the COVID-19 pandemic.
A study conducted by Andreata et al showed a significantly increased mean first-case delay and a nonsignificant increased turnover time in orthopedic surgeries in Northern Italy when comparing surgeries performed during the COVID-19 pandemic to those performed prior to COVID-19.2 Other studies have indicated a similar trend in decreased OR efficiency during COVID-19 in other areas around the world.9,10 These findings are not consistent with our own findings for neurosurgical and neuroendovascular surgeries at VUMC, and any change at our institution was relatively immaterial. Factors that threatened to change OR efficiency—but did not result in meaningful changes in our institutional experience—include delays due to pending COVID-19 test results, safety procedures such as PPE donning, and planning difficulties to ensure the existence of teams with non-overlapping providers in the case of a surgeon being infected.2,11-13
Globally, many surgery centers halted all elective surgeries during the initial COVID-19 spike to prevent a PPE shortage and mitigate risk of infection of patients and health care workers.8,12,14 However, there is no centralized definition of which neurosurgical procedures are elective, so that decision was made on a surgeon or center level, which could lead to variability in efficiency trends.14 One study on neurosurgical procedures during COVID-19 found a 30% decline in all cases and a 23% decline in emergent procedures, showing that the decrease in volume was not only due to cancellation of elective procedures.15 This decrease in elective and emergent surgeries created a backlog of surgeries as well as a loss in health care revenue, and caused many patients to go without adequate health care.10 Looking forward, it is imperative that surgical centers study trends in OR efficiency from COVID-19 and learn how to better maintain OR efficiency during future pandemic conditions to prevent a backlog of cases, loss of health care revenue, and decreased health care access.
Limitations
Our data are from a single center and therefore may not be representative of experiences of other hospitals due to different populations and different impacts from COVID-19. However, given our center’s high volume and diverse patient population, we believe our analysis highlights important trends in neurosurgery practice. Notably, data for patient and OR timing are digitally generated and are entered manually by nurses in the electronic medical record, making it prone to errors and variability. This is in our experience, and if any error is present, we believe it is minimal.
Conclusion
The COVID-19 pandemic has had far-reaching effects on health care worldwide, including neurosurgical care. OR efficiency across the United States generally worsened given the stresses of supply chain issues, staffing shortages, and cancellations. At our institution, we were able to maintain OR efficiency during the known COVID-19 peaks until October 2021. Continually functional neurosurgical ORs are important in preventing delays in care and maintaining a steady revenue in order for hospitals and other health care entities to remain solvent. Further study of OR efficiency is needed for health care systems to prepare for future pandemics and other resource-straining events in order to provide optimal patient care.
Corresponding author: Campbell Liles, MD, Vanderbilt University Medical Center, Department of Neurological Surgery, 1161 21st Ave. South, T4224 Medical Center North, Nashville, TN 37232-2380; [email protected]
Disclosures: None reported.
From the Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN (Stefan W. Koester, Puja Jagasia, and Drs. Liles, Dambrino IV, Feldman, and Chambless), and the Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN (Drs. Mathews and Tiwari).
ABSTRACT
Background: The COVID-19 pandemic has had broad effects on surgical care, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and newly implemented anti-infective measures. Our aim was to assess neurosurgery OR efficiency before the COVID-19 pandemic, during peak COVID-19, and during current times.
Methods: Institutional perioperative databases at a single, high-volume neurosurgical center were queried for operations performed from December 2019 until October 2021. March 12, 2020, the day that the state of Tennessee declared a state of emergency, was chosen as the onset of the COVID-19 pandemic. The 90-day periods before and after this day were used to define the pre-COVID-19, peak-COVID-19, and post-peak restrictions time periods for comparative analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover). Univariate analysis used Wilcoxon rank-sum test for continuous outcomes, while chi-square test and Fisher’s exact test were used for categorical comparisons. Significance was defined as P < .05.
Results: First-start time was analyzed in 426 pre-COVID-19, 357 peak-restrictions, and 2304 post-peak-restrictions cases. The unadjusted mean delay length was found to be significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different. The proportion of cases that started early, as well as significantly early past a 15-minute threshold, have not been impacted. There was no significant change in turnover time during peak restrictions relative to the pre-COVID-19 period (88 [100] minutes vs 85 [95] minutes), and turnover time has since remained unchanged (83 [87] minutes).
Conclusion: Our center was able to maintain OR efficiency before, during, and after peak restrictions even while instituting advanced infection-control strategies. While there were significant changes, delays were relatively small in magnitude.
Keywords: operating room timing, hospital efficiency, socioeconomics, pandemic.
The COVID-19 pandemic has led to major changes in patient care both from a surgical perspective and in regard to inpatient hospital course. Safety protocols nationwide have been implemented to protect both patients and providers. Some elements of surgical care have drastically changed, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and increased sterilization measures. Furloughs, layoffs, and reassignments due to the focus on nonelective and COVID-19–related cases challenged OR staffing and efficiency. Operating room staff with COVID-19 exposures or COVID-19 infections also caused last-minute changes in staffing. All of these scenarios can cause issues due to actual understaffing or due to staff members being pushed into highly specialized areas, such as neurosurgery, in which they have very little experience. A further obstacle to OR efficiency included policy changes involving PPE utilization, sterilization measures, and supply chain shortages of necessary resources such as PPE.
Neurosurgery in particular has been susceptible to COVID-19–related system-wide changes given operator proximity to the patient’s respiratory passages, frequency of emergent cases, and varying anesthetic needs, as well as the high level of specialization needed to perform neurosurgical care. Previous studies have shown a change in the makeup of neurosurgical patients seeking care, as well as in the acuity of neurological consult of these patients.1 A study in orthopedic surgery by Andreata et al demonstrated worsened OR efficiency, with significantly increased first-start and turnover times.2 In the COVID-19 era, OR quality and safety are crucially important to both patients and providers. Providing this safe and effective care in an efficient manner is important for optimal neurosurgical management in the long term.3 Moreover, the financial burden of implementing new protocols and standards can be compounded by additional financial losses due to reduced OR efficiency.
Methods
To analyze the effect of COVID-19 on neurosurgical OR efficiency, institutional perioperative databases at a single high-volume center were queried for operations performed from December 2019 until October 2021. March 12, 2020, was chosen as the onset of COVID-19 for analytic purposes, as this was the date when the state of Tennessee declared a state of emergency. The 90-day periods before and after this date were used for comparative analysis for pre-COVID-19, peak COVID-19, and post-peak-restrictions time periods. The peak COVID-19 period was defined as the 90-day period following the initial onset of COVID-19 and the surge of cases. For comparison purposes, post-peak COVID-19 was defined as the months following the first peak until October 2021 (approximately 17 months). COVID-19 burden was determined using a COVID-19 single-institution census of confirmed cases by polymerase chain reaction (PCR) for which the average number of cases of COVID-19 during a given month was determined. This number is a scaled trend, and a true number of COVID-19 cases in our hospital was not reported.
Neurosurgical and neuroendovascular cases were included in the analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases, defined as the time from the patient leaving the room until the next patient entered the room. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover, which is a standard for our single-institution perioperative center). Statistical analyses, including data aggregation, were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Patients’ demographic and clinical characteristics were analyzed using an independent 2-sample t-test for interval variables and a chi-square test for categorical variables. Significance was defined as P < .05.
Results
First-Start Time
First-start time was analyzed in 426 pre-COVID-19, 357 peak-COVID-19, and 2304 post-peak-COVID-19 cases. The unadjusted mean delay length was significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004) (Table 1). 
The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different, but they have been slightly higher since the onset of COVID-19. The proportion of cases that have started early, as well as significantly early past a 15-minute threshold, have also trended down since the onset of the COVID-19 pandemic, but this difference was again not significant. The temporal relationship of first-start delay, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 1. The trend of increasing delay is loosely associated with the COVID-19 burden experienced by our hospital. The start of COVID-19 as well as both COVID-19 peaks have been associated with increased delays in our hospital.
Turnover Time
Turnover time was assessed in 437 pre-COVID-19, 278 peak-restrictions, and 2411 post-peak-restrictions cases. Turnover time during peak restrictions was not significantly different from pre-COVID-19 (88 [100] vs 85 [95]) and has since remained relatively unchanged (83 [87], P = .78). A similar trend held for comparisons of proportion of cases with turnover time past 90 minutes and average times past the 90-minute threshold (Table 2). The temporal relationship between COVID-19 burden and turnover time, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 2. Both figures demonstrate a slight initial increase in turnover time delay at the start of COVID-19, which stabilized with little variation thereafter.


Discussion
We analyzed the OR efficiency metrics of first-start and turnover time during the 90-day period before COVID-19 (pre-COVID-19), the 90 days following Tennessee declaring a state of emergency (peak COVID-19), and the time following this period (post-COVID-19) for all neurosurgical and neuroendovascular cases at Vanderbilt University Medical Center (VUMC). We found a significant difference in unadjusted mean delay length in first-start time between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes for pre-COVID-19, peak-COVID-19, and post-COVID-19: 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). No significant increase in turnover time between cases was found between these 3 time periods. Based on metrics from first-start delay and turnover time, our center was able to maintain OR efficiency before, during, and after peak COVID-19.
After the Centers for Disease Control and Prevention released guidelines recommending deferring elective procedures to conserve beds and PPE, VUMC made the decision to suspend all elective surgical procedures from March 18 to April 24, 2020. Prior research conducted during the COVID-19 pandemic has demonstrated more than 400 types of surgical procedures with negatively impacted outcomes when compared to surgical outcomes from the same time frame in 2018 and 2019.4 For more than 20 of these types of procedures, there was a significant association between procedure delay and adverse patient outcomes.4 Testing protocols for patients prior to surgery varied throughout the pandemic based on vaccination status and type of procedure. Before vaccines became widely available, all patients were required to obtain a PCR SARS-CoV-2 test within 48 to 72 hours of the scheduled procedure. If the patient’s procedure was urgent and testing was not feasible, the patient was treated as a SARS-CoV-2–positive patient, which required all health care workers involved in the case to wear gowns, gloves, surgical masks, and eye protection. Testing patients preoperatively likely helped to maintain OR efficiency since not all patients received test results prior to the scheduled procedure, leading to cancellations of cases and therefore more staff available for fewer cases.
After vaccines became widely available to the public, testing requirements for patients preoperatively were relaxed, and only patients who were not fully vaccinated or severely immunocompromised were required to test prior to procedures. However, approximately 40% of the population in Tennessee was fully vaccinated in 2021, which reflects the patient population of VUMC.5 This means that many patients who received care at VUMC were still tested prior to procedures.
Adopting adequate safety protocols was found to be key for OR efficiency during the COVID-19 pandemic since performing surgery increased the risk of infection for each health care worker in the OR.6 VUMC protocols identified procedures that required enhanced safety measures to prevent infection of health care workers and avoid staffing shortages, which would decrease OR efficiency. Protocols mandated that only anesthesia team members were allowed to be in the OR during intubation and extubation of patients, which could be one factor leading to increased delays and decreased efficiency for some institutions. Methods for neurosurgeons to decrease risk of infection in the OR include postponing all nonurgent cases, reappraising the necessity for general anesthesia and endotracheal intubation, considering alternative surgical approaches that avoid the respiratory tract, and limiting the use of aerosol-generating instruments.7,8 VUMC’s success in implementing these protocols likely explains why our center was able to maintain OR efficiency throughout the COVID-19 pandemic.
A study conducted by Andreata et al showed a significantly increased mean first-case delay and a nonsignificant increased turnover time in orthopedic surgeries in Northern Italy when comparing surgeries performed during the COVID-19 pandemic to those performed prior to COVID-19.2 Other studies have indicated a similar trend in decreased OR efficiency during COVID-19 in other areas around the world.9,10 These findings are not consistent with our own findings for neurosurgical and neuroendovascular surgeries at VUMC, and any change at our institution was relatively immaterial. Factors that threatened to change OR efficiency—but did not result in meaningful changes in our institutional experience—include delays due to pending COVID-19 test results, safety procedures such as PPE donning, and planning difficulties to ensure the existence of teams with non-overlapping providers in the case of a surgeon being infected.2,11-13
Globally, many surgery centers halted all elective surgeries during the initial COVID-19 spike to prevent a PPE shortage and mitigate risk of infection of patients and health care workers.8,12,14 However, there is no centralized definition of which neurosurgical procedures are elective, so that decision was made on a surgeon or center level, which could lead to variability in efficiency trends.14 One study on neurosurgical procedures during COVID-19 found a 30% decline in all cases and a 23% decline in emergent procedures, showing that the decrease in volume was not only due to cancellation of elective procedures.15 This decrease in elective and emergent surgeries created a backlog of surgeries as well as a loss in health care revenue, and caused many patients to go without adequate health care.10 Looking forward, it is imperative that surgical centers study trends in OR efficiency from COVID-19 and learn how to better maintain OR efficiency during future pandemic conditions to prevent a backlog of cases, loss of health care revenue, and decreased health care access.
Limitations
Our data are from a single center and therefore may not be representative of experiences of other hospitals due to different populations and different impacts from COVID-19. However, given our center’s high volume and diverse patient population, we believe our analysis highlights important trends in neurosurgery practice. Notably, data for patient and OR timing are digitally generated and are entered manually by nurses in the electronic medical record, making it prone to errors and variability. This is in our experience, and if any error is present, we believe it is minimal.
Conclusion
The COVID-19 pandemic has had far-reaching effects on health care worldwide, including neurosurgical care. OR efficiency across the United States generally worsened given the stresses of supply chain issues, staffing shortages, and cancellations. At our institution, we were able to maintain OR efficiency during the known COVID-19 peaks until October 2021. Continually functional neurosurgical ORs are important in preventing delays in care and maintaining a steady revenue in order for hospitals and other health care entities to remain solvent. Further study of OR efficiency is needed for health care systems to prepare for future pandemics and other resource-straining events in order to provide optimal patient care.
Corresponding author: Campbell Liles, MD, Vanderbilt University Medical Center, Department of Neurological Surgery, 1161 21st Ave. South, T4224 Medical Center North, Nashville, TN 37232-2380; [email protected]
Disclosures: None reported.
1. Koester SW, Catapano JS, Ma KL, et al. COVID-19 and neurosurgery consultation call volume at a single large tertiary center with a propensity- adjusted analysis. World Neurosurg. 2021;146:e768-e772. doi:10.1016/j.wneu.2020.11.017
2. Andreata M, Faraldi M, Bucci E, Lombardi G, Zagra L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int Orthop. 2020;44(12):2499-2504. doi:10.1007/s00264-020-04772-x
3. Dexter F, Abouleish AE, Epstein RH, et al. Use of operating room information system data to predict the impact of reducing turnover times on staffing costs. Anesth Analg. 2003;97(4):1119-1126. doi:10.1213/01.ANE.0000082520.68800.79
4. Zheng NS, Warner JL, Osterman TJ, et al. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J Biomed Inform. 2021;113:103657. doi:10.1016/j.jbi.2020.103657
5. Alcendor DJ. Targeting COVID-19 vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID- 19 pandemic in the South. Vaccines (Basel). 2021;9(11):1279. doi:10.3390/vaccines9111279
6. Perrone G, Giuffrida M, Bellini V, et al. Operating room setup: how to improve health care professionals safety during pandemic COVID- 19: a quality improvement study. J Laparoendosc Adv Surg Tech A. 2021;31(1):85-89. doi:10.1089/lap.2020.0592
7. Iorio-Morin C, Hodaie M, Sarica C, et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87(2):E178-E185. doi:10.1093/ neuros/nyaa157
8. Gupta P, Muthukumar N, Rajshekhar V, et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68(2):246-254. doi:10.4103/0028-3886.283130
9. Mercer ST, Agarwal R, Dayananda KSS, et al. A comparative study looking at trauma and orthopaedic operating efficiency in the COVID-19 era. Perioper Care Oper Room Manag. 2020;21:100142. doi:10.1016/j.pcorm.2020.100142
10. Rozario N, Rozario D. Can machine learning optimize the efficiency of the operating room in the era of COVID-19? Can J Surg. 2020;63(6):E527-E529. doi:10.1503/cjs.016520
11. Toh KHQ, Barazanchi A, Rajaretnam NS, et al. COVID-19 response by New Zealand general surgical departments in tertiary metropolitan hospitals. ANZ J Surg. 2021;91(7-8):1352-1357. doi:10.1111/ ans.17044
12. Moorthy RK, Rajshekhar V. Impact of COVID-19 pandemic on neurosurgical practice in India: a survey on personal protective equipment usage, testing, and perceptions on disease transmission. Neurol India. 2020;68(5):1133-1138. doi:10.4103/0028- 3886.299173
13. Meneghini RM. Techniques and strategies to optimize efficiencies in the office and operating room: getting through the patient backlog and preserving hospital resources. J Arthroplasty. 2021;36(7S):S49-S51. doi:10.1016/j.arth.2021.03.010
14. Jean WC, Ironside NT, Sack KD, et al. The impact of COVID- 19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien). 2020;162(6):1229-1240. doi:10.1007/s00701-020- 04342-5
15. Raneri F, Rustemi O, Zambon G, et al. Neurosurgery in times of a pandemic: a survey of neurosurgical services during the COVID-19 outbreak in the Veneto region in Italy. Neurosurg Focus. 2020;49(6):E9. doi:10.3171/2020.9.FOCUS20691
1. Koester SW, Catapano JS, Ma KL, et al. COVID-19 and neurosurgery consultation call volume at a single large tertiary center with a propensity- adjusted analysis. World Neurosurg. 2021;146:e768-e772. doi:10.1016/j.wneu.2020.11.017
2. Andreata M, Faraldi M, Bucci E, Lombardi G, Zagra L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int Orthop. 2020;44(12):2499-2504. doi:10.1007/s00264-020-04772-x
3. Dexter F, Abouleish AE, Epstein RH, et al. Use of operating room information system data to predict the impact of reducing turnover times on staffing costs. Anesth Analg. 2003;97(4):1119-1126. doi:10.1213/01.ANE.0000082520.68800.79
4. Zheng NS, Warner JL, Osterman TJ, et al. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J Biomed Inform. 2021;113:103657. doi:10.1016/j.jbi.2020.103657
5. Alcendor DJ. Targeting COVID-19 vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID- 19 pandemic in the South. Vaccines (Basel). 2021;9(11):1279. doi:10.3390/vaccines9111279
6. Perrone G, Giuffrida M, Bellini V, et al. Operating room setup: how to improve health care professionals safety during pandemic COVID- 19: a quality improvement study. J Laparoendosc Adv Surg Tech A. 2021;31(1):85-89. doi:10.1089/lap.2020.0592
7. Iorio-Morin C, Hodaie M, Sarica C, et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87(2):E178-E185. doi:10.1093/ neuros/nyaa157
8. Gupta P, Muthukumar N, Rajshekhar V, et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68(2):246-254. doi:10.4103/0028-3886.283130
9. Mercer ST, Agarwal R, Dayananda KSS, et al. A comparative study looking at trauma and orthopaedic operating efficiency in the COVID-19 era. Perioper Care Oper Room Manag. 2020;21:100142. doi:10.1016/j.pcorm.2020.100142
10. Rozario N, Rozario D. Can machine learning optimize the efficiency of the operating room in the era of COVID-19? Can J Surg. 2020;63(6):E527-E529. doi:10.1503/cjs.016520
11. Toh KHQ, Barazanchi A, Rajaretnam NS, et al. COVID-19 response by New Zealand general surgical departments in tertiary metropolitan hospitals. ANZ J Surg. 2021;91(7-8):1352-1357. doi:10.1111/ ans.17044
12. Moorthy RK, Rajshekhar V. Impact of COVID-19 pandemic on neurosurgical practice in India: a survey on personal protective equipment usage, testing, and perceptions on disease transmission. Neurol India. 2020;68(5):1133-1138. doi:10.4103/0028- 3886.299173
13. Meneghini RM. Techniques and strategies to optimize efficiencies in the office and operating room: getting through the patient backlog and preserving hospital resources. J Arthroplasty. 2021;36(7S):S49-S51. doi:10.1016/j.arth.2021.03.010
14. Jean WC, Ironside NT, Sack KD, et al. The impact of COVID- 19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien). 2020;162(6):1229-1240. doi:10.1007/s00701-020- 04342-5
15. Raneri F, Rustemi O, Zambon G, et al. Neurosurgery in times of a pandemic: a survey of neurosurgical services during the COVID-19 outbreak in the Veneto region in Italy. Neurosurg Focus. 2020;49(6):E9. doi:10.3171/2020.9.FOCUS20691
‘A huge deal’: Millions have long COVID, and more are expected
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
Psoriasiform Dermatitis Associated With the Moderna COVID-19 Messenger RNA Vaccine
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.
A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.
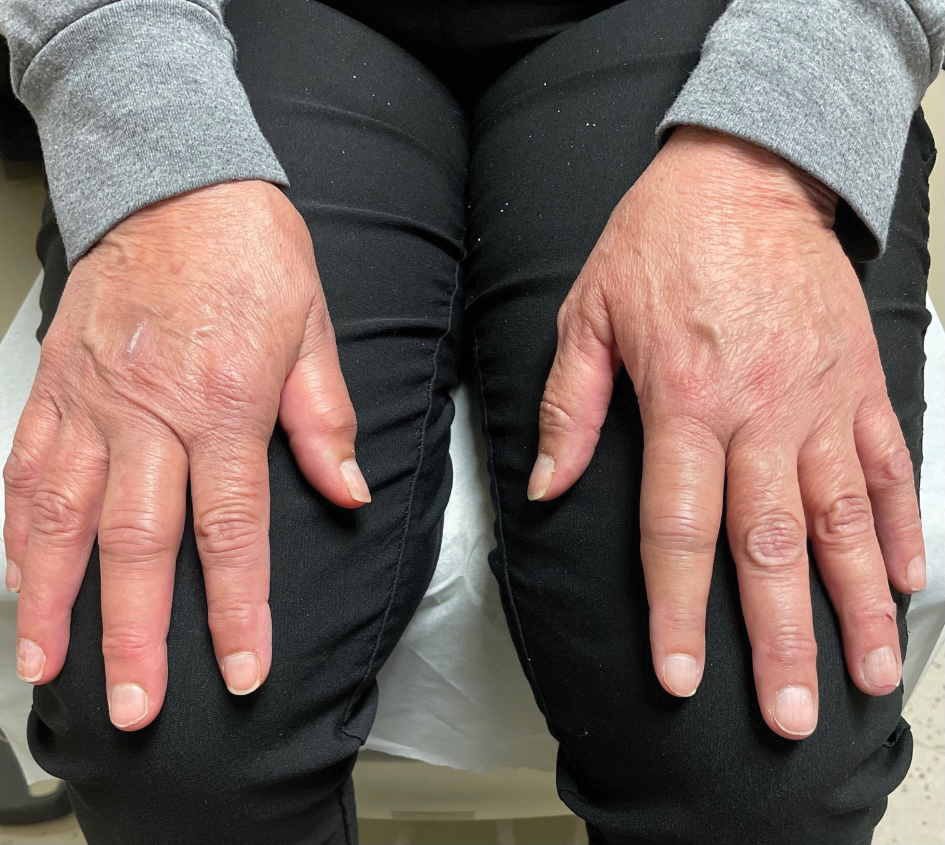
Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).
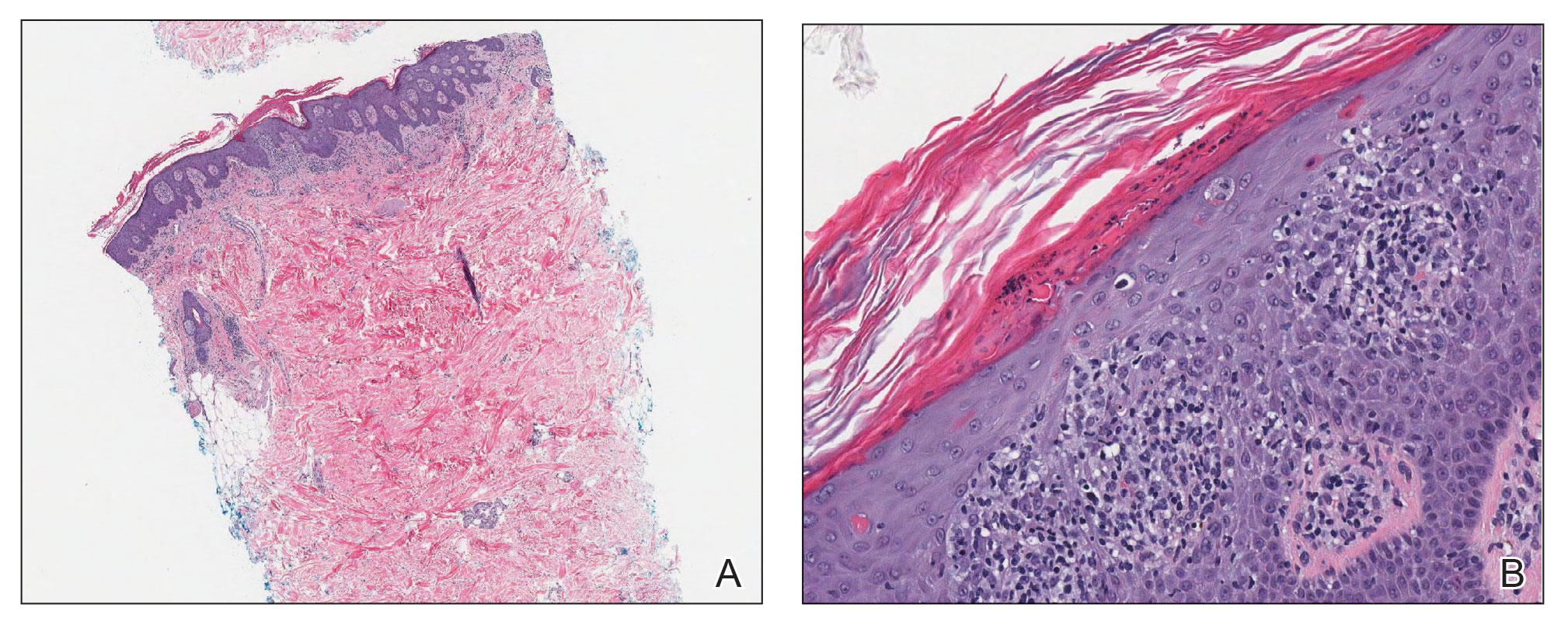
The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
PRACTICE POINTS
- The differential diagnosis for a new-onset psoriasiform rash in an elderly patient should include a vaccine-related rash.
- A rash following vaccination that necessitates systemic corticosteroid therapy can decrease vaccine efficacy.
Children and COVID: Weekly cases continue to hold fairly steady
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.
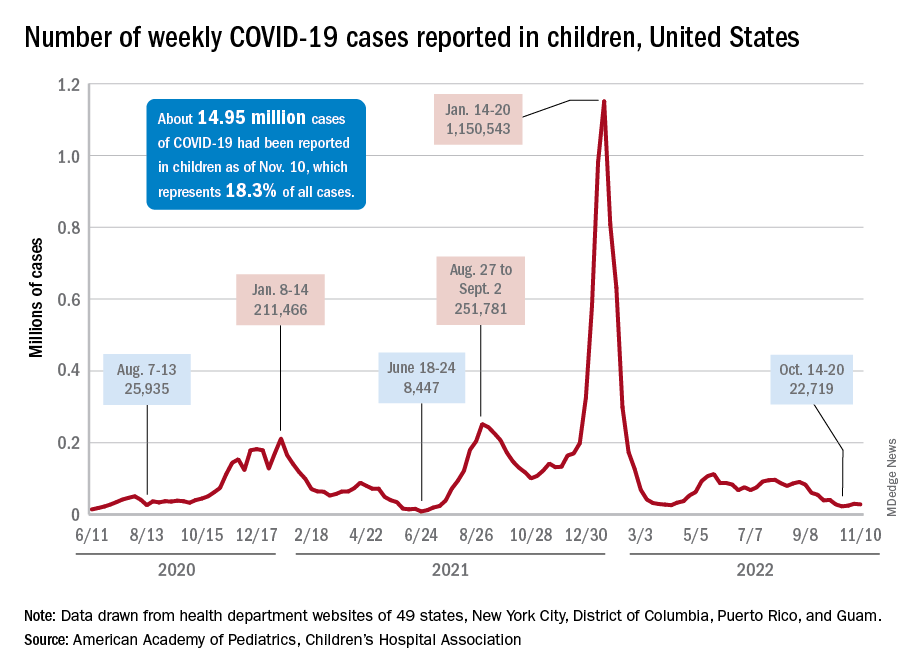
The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.

The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.

The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
Love them or hate them, masks in schools work
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
On March 26, 2022, Hawaii became the last state in the United States to lift its indoor mask mandate. By the time the current school year started, there were essentially no public school mask mandates either.
Whether you viewed the mask as an emblem of stalwart defiance against a rampaging virus, or a scarlet letter emblematic of the overreaches of public policy, you probably aren’t seeing them much anymore.
And yet, the debate about masks still rages. Who was right, who was wrong? Who trusted science, and what does the science even say? If we brought our country into marriage counseling, would we be told it is time to move on? To look forward, not backward? To plan for our bright future together?
Perhaps. But this question isn’t really moot just because masks have largely disappeared in the United States. Variants may emerge that lead to more infection waves – and other pandemics may occur in the future. And so I think it is important to discuss a study that, with quite rigorous analysis, attempts to answer the following question: Did masking in schools lower students’ and teachers’ risk of COVID?
We are talking about this study, appearing in the New England Journal of Medicine. The short version goes like this.
Researchers had access to two important sources of data. One – an accounting of all the teachers and students (more than 300,000 of them) in 79 public, noncharter school districts in Eastern Massachusetts who tested positive for COVID every week. Two – the date that each of those school districts lifted their mask mandates or (in the case of two districts) didn’t.
Right away, I’m sure you’re thinking of potential issues. Districts that kept masks even when the statewide ban was lifted are likely quite a bit different from districts that dropped masks right away. You’re right, of course – hold on to that thought; we’ll get there.
But first – the big question – would districts that kept their masks on longer do better when it comes to the rate of COVID infection?
When everyone was masking, COVID case rates were pretty similar. Statewide mandates are lifted in late February – and most school districts remove their mandates within a few weeks – the black line are the two districts (Boston and Chelsea) where mask mandates remained in place.
Prior to the mask mandate lifting, you see very similar COVID rates in districts that would eventually remove the mandate and those that would not, with a bit of noise around the initial Omicron wave which saw just a huge amount of people get infected.
And then, after the mandate was lifted, separation. Districts that held on to masks longer had lower rates of COVID infection.
In all, over the 15-weeks of the study, there were roughly 12,000 extra cases of COVID in the mask-free school districts, which corresponds to about 35% of the total COVID burden during that time. And, yes, kids do well with COVID – on average. But 12,000 extra cases is enough to translate into a significant number of important clinical outcomes – think hospitalizations and post-COVID syndromes. And of course, maybe most importantly, missed school days. Positive kids were not allowed in class no matter what district they were in.
Okay – I promised we’d address confounders. This was not a cluster-randomized trial, where some school districts had their mandates removed based on the vicissitudes of a virtual coin flip, as much as many of us would have been interested to see that. The decision to remove masks was up to the various school boards – and they had a lot of pressure on them from many different directions. But all we need to worry about is whether any of those things that pressure a school board to keep masks on would ALSO lead to fewer COVID cases. That’s how confounders work, and how you can get false results in a study like this.
And yes – districts that kept the masks on longer were different than those who took them right off. But check out how they were different.
The districts that kept masks on longer had more low-income students. More Black and Latino students. More students per classroom. These are all risk factors that increase the risk of COVID infection. In other words, the confounding here goes in the opposite direction of the results. If anything, these factors should make you more certain that masking works.
The authors also adjusted for other factors – the community transmission of COVID-19, vaccination rates, school district sizes, and so on. No major change in the results.
One concern I addressed to Dr. Ellie Murray, the biostatistician on the study – could districts that removed masks simply have been testing more to compensate, leading to increased capturing of cases?
If anything, the schools that kept masks on were testing more than the schools that took them off – again that would tend to imply that the results are even stronger than what was reported.
Is this a perfect study? Of course not – it’s one study, it’s from one state. And the relatively large effects from keeping masks on for one or 2 weeks require us to really embrace the concept of exponential growth of infections, but, if COVID has taught us anything, it is that small changes in initial conditions can have pretty big effects.
My daughter, who goes to a public school here in Connecticut, unmasked, was home with COVID this past week. She’s fine. But you know what? She missed a week of school. I worked from home to be with her – though I didn’t test positive. And that is a real cost to both of us that I think we need to consider when we consider the value of masks. Yes, they’re annoying – but if they keep kids in school, might they be worth it? Perhaps not for now, as cases aren’t surging. But in the future, be it a particularly concerning variant, or a whole new pandemic, we should not discount the simple, cheap, and apparently beneficial act of wearing masks to decrease transmission.
Dr. Perry Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The body of evidence for Paxlovid therapy
Dear Colleagues,
We have a mismatch. The evidence supporting treatment for Paxlovid is compelling for people aged 60 or over, but the older patients in the United States are much less likely to be treated. Not only was there a randomized, placebo-controlled trial of high-risk patients which showed 89% reduction of hospitalizations and deaths (median age, 45), but there have been multiple real-world effectiveness studies subsequently published that have partitioned the benefit for age 65 or older, such as the ones from Israel and Hong Kong (age 60+). Overall, the real-world effectiveness in the first month after treatment is at least as good, if not better, than in the high-risk randomized trial.
We’re doing the current survey to find out, but the most likely reasons include (1) lack of confidence of benefit; (2) medication interactions; and (3) concerns over rebound.
Let me address each of these briefly. The lack of confidence in benefit stems from the fact that the initial high-risk trial was in unvaccinated individuals. That concern can now be put aside because all of the several real-world studies confirming the protective benefit against hospitalizations and deaths are in people who have been vaccinated, and a significant proportion received booster shots.
The potential medication interactions due to the ritonavir component of the Paxlovid drug combination, attributable to its cytochrome P450 3A4 inhibition, have been unduly emphasized. There are many drug-interaction checkers for Paxlovid, but this one from the University of Liverpool is user friendly, color- and icon-coded, and shows that the vast majority of interactions can be sidestepped by discontinuing the medication of concern for the length of the Paxlovid treatment, 5 days. The simple chart is provided in my recent substack newsletter.
As far as rebound, this problem has unfortunately been exaggerated because of lack of prospective systematic studies and appreciation that a positive test of clinical symptom rebound can occur without Paxlovid. There are soon to be multiple reports that the incidence of Paxlovid rebound is fairly low, in the range of 10%. That concern should not be a reason to withhold treatment.
Now the plot thickens. A new preprint report from the Veterans Health Administration, the largest health care system in the United States, looks at 90-day outcomes of about 9,000 Paxlovid-treated patients and approximately 47,000 controls. Not only was there a 26% reduction in long COVID, but of the breakdown of 12 organs/systems and symptoms, 10 of 12 were significantly reduced with Paxlovid, including pulmonary embolism, deep vein thrombosis, and neurocognitive impairment. There was also a 48% reduction in death and a 30% reduction in hospitalizations after the first 30 days. I have reviewed all of these data and put them in context in a recent newsletter. A key point is that the magnitude of benefit was unaffected by vaccination or booster status, or prior COVID infections, or unvaccinated status. Also, it was the same for men and women, as well as for age > 70 and age < 60. These findings all emphasize a new reason to be using Paxlovid therapy, and if replicated, Paxlovid may even be indicated for younger patients (who are at low risk for hospitalizations and deaths but at increased risk for long COVID).
In summary, for older patients, we should be thinking of why we should be using Paxlovid rather than the reason not to treat. We’ll be interested in the survey results to understand the mismatch better, and we look forward to your ideas and feedback to make better use of this treatment for the people who need it the most.
Sincerely yours, Eric J. Topol, MD
Dr. Topol reports no conflicts of interest with Pfizer; he receives no honoraria or speaker fees, does not serve in an advisory role, and has no financial association with the company.
A version of this article first appeared on Medscape.com.
Dear Colleagues,
We have a mismatch. The evidence supporting treatment for Paxlovid is compelling for people aged 60 or over, but the older patients in the United States are much less likely to be treated. Not only was there a randomized, placebo-controlled trial of high-risk patients which showed 89% reduction of hospitalizations and deaths (median age, 45), but there have been multiple real-world effectiveness studies subsequently published that have partitioned the benefit for age 65 or older, such as the ones from Israel and Hong Kong (age 60+). Overall, the real-world effectiveness in the first month after treatment is at least as good, if not better, than in the high-risk randomized trial.
We’re doing the current survey to find out, but the most likely reasons include (1) lack of confidence of benefit; (2) medication interactions; and (3) concerns over rebound.
Let me address each of these briefly. The lack of confidence in benefit stems from the fact that the initial high-risk trial was in unvaccinated individuals. That concern can now be put aside because all of the several real-world studies confirming the protective benefit against hospitalizations and deaths are in people who have been vaccinated, and a significant proportion received booster shots.
The potential medication interactions due to the ritonavir component of the Paxlovid drug combination, attributable to its cytochrome P450 3A4 inhibition, have been unduly emphasized. There are many drug-interaction checkers for Paxlovid, but this one from the University of Liverpool is user friendly, color- and icon-coded, and shows that the vast majority of interactions can be sidestepped by discontinuing the medication of concern for the length of the Paxlovid treatment, 5 days. The simple chart is provided in my recent substack newsletter.
As far as rebound, this problem has unfortunately been exaggerated because of lack of prospective systematic studies and appreciation that a positive test of clinical symptom rebound can occur without Paxlovid. There are soon to be multiple reports that the incidence of Paxlovid rebound is fairly low, in the range of 10%. That concern should not be a reason to withhold treatment.
Now the plot thickens. A new preprint report from the Veterans Health Administration, the largest health care system in the United States, looks at 90-day outcomes of about 9,000 Paxlovid-treated patients and approximately 47,000 controls. Not only was there a 26% reduction in long COVID, but of the breakdown of 12 organs/systems and symptoms, 10 of 12 were significantly reduced with Paxlovid, including pulmonary embolism, deep vein thrombosis, and neurocognitive impairment. There was also a 48% reduction in death and a 30% reduction in hospitalizations after the first 30 days. I have reviewed all of these data and put them in context in a recent newsletter. A key point is that the magnitude of benefit was unaffected by vaccination or booster status, or prior COVID infections, or unvaccinated status. Also, it was the same for men and women, as well as for age > 70 and age < 60. These findings all emphasize a new reason to be using Paxlovid therapy, and if replicated, Paxlovid may even be indicated for younger patients (who are at low risk for hospitalizations and deaths but at increased risk for long COVID).
In summary, for older patients, we should be thinking of why we should be using Paxlovid rather than the reason not to treat. We’ll be interested in the survey results to understand the mismatch better, and we look forward to your ideas and feedback to make better use of this treatment for the people who need it the most.
Sincerely yours, Eric J. Topol, MD
Dr. Topol reports no conflicts of interest with Pfizer; he receives no honoraria or speaker fees, does not serve in an advisory role, and has no financial association with the company.
A version of this article first appeared on Medscape.com.
Dear Colleagues,
We have a mismatch. The evidence supporting treatment for Paxlovid is compelling for people aged 60 or over, but the older patients in the United States are much less likely to be treated. Not only was there a randomized, placebo-controlled trial of high-risk patients which showed 89% reduction of hospitalizations and deaths (median age, 45), but there have been multiple real-world effectiveness studies subsequently published that have partitioned the benefit for age 65 or older, such as the ones from Israel and Hong Kong (age 60+). Overall, the real-world effectiveness in the first month after treatment is at least as good, if not better, than in the high-risk randomized trial.
We’re doing the current survey to find out, but the most likely reasons include (1) lack of confidence of benefit; (2) medication interactions; and (3) concerns over rebound.
Let me address each of these briefly. The lack of confidence in benefit stems from the fact that the initial high-risk trial was in unvaccinated individuals. That concern can now be put aside because all of the several real-world studies confirming the protective benefit against hospitalizations and deaths are in people who have been vaccinated, and a significant proportion received booster shots.
The potential medication interactions due to the ritonavir component of the Paxlovid drug combination, attributable to its cytochrome P450 3A4 inhibition, have been unduly emphasized. There are many drug-interaction checkers for Paxlovid, but this one from the University of Liverpool is user friendly, color- and icon-coded, and shows that the vast majority of interactions can be sidestepped by discontinuing the medication of concern for the length of the Paxlovid treatment, 5 days. The simple chart is provided in my recent substack newsletter.
As far as rebound, this problem has unfortunately been exaggerated because of lack of prospective systematic studies and appreciation that a positive test of clinical symptom rebound can occur without Paxlovid. There are soon to be multiple reports that the incidence of Paxlovid rebound is fairly low, in the range of 10%. That concern should not be a reason to withhold treatment.
Now the plot thickens. A new preprint report from the Veterans Health Administration, the largest health care system in the United States, looks at 90-day outcomes of about 9,000 Paxlovid-treated patients and approximately 47,000 controls. Not only was there a 26% reduction in long COVID, but of the breakdown of 12 organs/systems and symptoms, 10 of 12 were significantly reduced with Paxlovid, including pulmonary embolism, deep vein thrombosis, and neurocognitive impairment. There was also a 48% reduction in death and a 30% reduction in hospitalizations after the first 30 days. I have reviewed all of these data and put them in context in a recent newsletter. A key point is that the magnitude of benefit was unaffected by vaccination or booster status, or prior COVID infections, or unvaccinated status. Also, it was the same for men and women, as well as for age > 70 and age < 60. These findings all emphasize a new reason to be using Paxlovid therapy, and if replicated, Paxlovid may even be indicated for younger patients (who are at low risk for hospitalizations and deaths but at increased risk for long COVID).
In summary, for older patients, we should be thinking of why we should be using Paxlovid rather than the reason not to treat. We’ll be interested in the survey results to understand the mismatch better, and we look forward to your ideas and feedback to make better use of this treatment for the people who need it the most.
Sincerely yours, Eric J. Topol, MD
Dr. Topol reports no conflicts of interest with Pfizer; he receives no honoraria or speaker fees, does not serve in an advisory role, and has no financial association with the company.
A version of this article first appeared on Medscape.com.
Repeat COVID infection doubles mortality risk
Getting COVID-19 a second time doubles a person’s chance of dying and triples the likelihood of being hospitalized in the next 6 months, a new study found.
Vaccination and booster status did not improve survival or hospitalization rates among people who were infected more than once.
“Reinfection with COVID-19 increases the risk of both acute outcomes and long COVID,” study author Ziyad Al-Aly, MD, told Reuters. “This was evident in unvaccinated, vaccinated and boosted people.”
The study was published in the journal Nature Medicine.
Researchers analyzed U.S. Department of Veterans Affairs data, including 443,588 people with a first infection of SARS-CoV-2, 40,947 people who were infected two or more times, and 5.3 million people who had not been infected with coronavirus, whose data served as the control group.
“During the past few months, there’s been an air of invincibility among people who have had COVID-19 or their vaccinations and boosters, and especially among people who have had an infection and also received vaccines; some people started to [refer] to these individuals as having a sort of superimmunity to the virus,” Dr. Al-Aly said in a press release from Washington University in St. Louis. “Without ambiguity, our research showed that getting an infection a second, third or fourth time contributes to additional health risks in the acute phase, meaning the first 30 days after infection, and in the months beyond, meaning the long COVID phase.”
Being infected with COVID-19 more than once also dramatically increased the risk of developing lung problems, heart conditions, or brain conditions. The heightened risks persisted for 6 months.
Researchers said a limitation of their study was that data primarily came from White males.
An expert not involved in the study told Reuters that the Veterans Affairs population does not reflect the general population. Patients at VA health facilities are generally older with more than normal health complications, said John Moore, PhD, a professor of microbiology and immunology at Weill Cornell Medicine, New York.
Dr. Al-Aly encouraged people to be vigilant as they plan for the holiday season, Reuters reported.
“We had started seeing a lot of patients coming to the clinic with an air of invincibility,” he told Reuters. “They wondered, ‘Does getting a reinfection really matter?’ The answer is yes, it absolutely does.”
A version of this article first appeared on WebMD.com.
Getting COVID-19 a second time doubles a person’s chance of dying and triples the likelihood of being hospitalized in the next 6 months, a new study found.
Vaccination and booster status did not improve survival or hospitalization rates among people who were infected more than once.
“Reinfection with COVID-19 increases the risk of both acute outcomes and long COVID,” study author Ziyad Al-Aly, MD, told Reuters. “This was evident in unvaccinated, vaccinated and boosted people.”
The study was published in the journal Nature Medicine.
Researchers analyzed U.S. Department of Veterans Affairs data, including 443,588 people with a first infection of SARS-CoV-2, 40,947 people who were infected two or more times, and 5.3 million people who had not been infected with coronavirus, whose data served as the control group.
“During the past few months, there’s been an air of invincibility among people who have had COVID-19 or their vaccinations and boosters, and especially among people who have had an infection and also received vaccines; some people started to [refer] to these individuals as having a sort of superimmunity to the virus,” Dr. Al-Aly said in a press release from Washington University in St. Louis. “Without ambiguity, our research showed that getting an infection a second, third or fourth time contributes to additional health risks in the acute phase, meaning the first 30 days after infection, and in the months beyond, meaning the long COVID phase.”
Being infected with COVID-19 more than once also dramatically increased the risk of developing lung problems, heart conditions, or brain conditions. The heightened risks persisted for 6 months.
Researchers said a limitation of their study was that data primarily came from White males.
An expert not involved in the study told Reuters that the Veterans Affairs population does not reflect the general population. Patients at VA health facilities are generally older with more than normal health complications, said John Moore, PhD, a professor of microbiology and immunology at Weill Cornell Medicine, New York.
Dr. Al-Aly encouraged people to be vigilant as they plan for the holiday season, Reuters reported.
“We had started seeing a lot of patients coming to the clinic with an air of invincibility,” he told Reuters. “They wondered, ‘Does getting a reinfection really matter?’ The answer is yes, it absolutely does.”
A version of this article first appeared on WebMD.com.
Getting COVID-19 a second time doubles a person’s chance of dying and triples the likelihood of being hospitalized in the next 6 months, a new study found.
Vaccination and booster status did not improve survival or hospitalization rates among people who were infected more than once.
“Reinfection with COVID-19 increases the risk of both acute outcomes and long COVID,” study author Ziyad Al-Aly, MD, told Reuters. “This was evident in unvaccinated, vaccinated and boosted people.”
The study was published in the journal Nature Medicine.
Researchers analyzed U.S. Department of Veterans Affairs data, including 443,588 people with a first infection of SARS-CoV-2, 40,947 people who were infected two or more times, and 5.3 million people who had not been infected with coronavirus, whose data served as the control group.
“During the past few months, there’s been an air of invincibility among people who have had COVID-19 or their vaccinations and boosters, and especially among people who have had an infection and also received vaccines; some people started to [refer] to these individuals as having a sort of superimmunity to the virus,” Dr. Al-Aly said in a press release from Washington University in St. Louis. “Without ambiguity, our research showed that getting an infection a second, third or fourth time contributes to additional health risks in the acute phase, meaning the first 30 days after infection, and in the months beyond, meaning the long COVID phase.”
Being infected with COVID-19 more than once also dramatically increased the risk of developing lung problems, heart conditions, or brain conditions. The heightened risks persisted for 6 months.
Researchers said a limitation of their study was that data primarily came from White males.
An expert not involved in the study told Reuters that the Veterans Affairs population does not reflect the general population. Patients at VA health facilities are generally older with more than normal health complications, said John Moore, PhD, a professor of microbiology and immunology at Weill Cornell Medicine, New York.
Dr. Al-Aly encouraged people to be vigilant as they plan for the holiday season, Reuters reported.
“We had started seeing a lot of patients coming to the clinic with an air of invincibility,” he told Reuters. “They wondered, ‘Does getting a reinfection really matter?’ The answer is yes, it absolutely does.”
A version of this article first appeared on WebMD.com.
FROM NATURE MEDICINE