User login
‘Infuriating’ prescription denial leaves patient without antiemetics
It was Friday, and oncologist Coral Olazagasti, MD, faced a ticking clock.
The patient – a man with HPV-related oropharyngeal cancer – was experiencing severe side effects from standard chemoradiation with weekly cisplatin. Intense nausea and grade 3 mucositis, in particular, left him struggling to swallow or take in any food or fluids.
He was on 8 mg of ondansetron (Zofran) every 8 hours, as needed, to keep the nausea at bay. The pills along with a feeding tube helped, but his symptoms were so intense, neither was quite enough.
“He still needed to be hospitalized twice for dehydration,” said Dr. Olazagasti, who specializes in head and neck medical cancer at Sylvester Comprehensive Cancer Center in Miami.
But when it came time to renew his ondansetron prescription, his insurance company denied it.
The reasoning: “The company had only approved 30 tablets a month and, for them, it was unjustifiable to approve anything above that amount,” Dr. Olazagasti explained.
After Dr. Olazagasti called the insurance company to resolve the issue, a company representative told her to fill out a prior authorization form.
But it was already after 7:30 p.m. ET on Friday.
At that point, finding the prior authorization documents, filling them out, and submitting them would take more time – and the paperwork couldn’t be filed until Monday.
“My patient was at home with zero tablets left and horrible symptoms. He couldn’t keep anything down,” Dr. Olazagasti said.
On Monday, the oncology team sent the prior authorization request, and her patient received his medication a few days later.
“My patient had to wait about 5 days to get the nausea meds he needed,” she said. In the meantime, he was in pain. “Having a refill of this simple supportive care medication rejected was infuriating.”
When Dr. Olazagasti vented her frustrations on Twitter, several people chimed in, suggesting purchasing the drug at a discount through GoodRx or Cost Plus instead of going through the insurance company.
At Cost Plus, for instance, 30 8-mg pills would cost $6.30, but ordering from the online pharmacy would mean waiting several days for delivery.
Discounts through GoodRx may provide a potentially faster solution in a pinch, but the pharmacy matters. In Miami, 30 8-mg pills would cost $19.99 at Costco with a GoodRx coupon, but $233.56 at CVS and $253.60 at Walgreens.
Although potentially useful, these options may not be the obvious choice for oncologists and patients, especially when a drug has already been approved and covered by the insurer. In this case, the denial was also a surprise, which left Dr. Olazagasti and her patient scrambling right before the weekend.
In addition, companies providing discounted generic drugs may only have a limited number of oncology-related medications. Cost Plus, for instance, now sells more than 1,000 generic prescription drugs at a fraction of what insurance companies charge, but only about 7 are cancer drugs.
On a broader level, Dr. Olazagasti noted, “insurance companies have a responsibility to cover these drugs. If we all get so fed up that we start relying on alternate routes to get patients their treatments, then insurance companies are let off the hook.”
However, using an alternative option like GoodRx or CostPlus could mean bypassing insurance company obstacles in certain cases.
“The hurdles someone may have to go through to get a generic drug approved are very frustrating,” said Stacie B. Dusetzina, PhD, professor of health policy and a professor of cancer research at Vanderbilt University in Nashville, Tenn.
In a weekend emergency situation, if the drug is discounted through GoodRx, “it can be a good backup strategy to send the prescription to the pharmacy” and more generally “worth it for patients to check if they can get a better deal on generic drugs through these companies.”
A version of this article first appeared on Medscape.com.
It was Friday, and oncologist Coral Olazagasti, MD, faced a ticking clock.
The patient – a man with HPV-related oropharyngeal cancer – was experiencing severe side effects from standard chemoradiation with weekly cisplatin. Intense nausea and grade 3 mucositis, in particular, left him struggling to swallow or take in any food or fluids.
He was on 8 mg of ondansetron (Zofran) every 8 hours, as needed, to keep the nausea at bay. The pills along with a feeding tube helped, but his symptoms were so intense, neither was quite enough.
“He still needed to be hospitalized twice for dehydration,” said Dr. Olazagasti, who specializes in head and neck medical cancer at Sylvester Comprehensive Cancer Center in Miami.
But when it came time to renew his ondansetron prescription, his insurance company denied it.
The reasoning: “The company had only approved 30 tablets a month and, for them, it was unjustifiable to approve anything above that amount,” Dr. Olazagasti explained.
After Dr. Olazagasti called the insurance company to resolve the issue, a company representative told her to fill out a prior authorization form.
But it was already after 7:30 p.m. ET on Friday.
At that point, finding the prior authorization documents, filling them out, and submitting them would take more time – and the paperwork couldn’t be filed until Monday.
“My patient was at home with zero tablets left and horrible symptoms. He couldn’t keep anything down,” Dr. Olazagasti said.
On Monday, the oncology team sent the prior authorization request, and her patient received his medication a few days later.
“My patient had to wait about 5 days to get the nausea meds he needed,” she said. In the meantime, he was in pain. “Having a refill of this simple supportive care medication rejected was infuriating.”
When Dr. Olazagasti vented her frustrations on Twitter, several people chimed in, suggesting purchasing the drug at a discount through GoodRx or Cost Plus instead of going through the insurance company.
At Cost Plus, for instance, 30 8-mg pills would cost $6.30, but ordering from the online pharmacy would mean waiting several days for delivery.
Discounts through GoodRx may provide a potentially faster solution in a pinch, but the pharmacy matters. In Miami, 30 8-mg pills would cost $19.99 at Costco with a GoodRx coupon, but $233.56 at CVS and $253.60 at Walgreens.
Although potentially useful, these options may not be the obvious choice for oncologists and patients, especially when a drug has already been approved and covered by the insurer. In this case, the denial was also a surprise, which left Dr. Olazagasti and her patient scrambling right before the weekend.
In addition, companies providing discounted generic drugs may only have a limited number of oncology-related medications. Cost Plus, for instance, now sells more than 1,000 generic prescription drugs at a fraction of what insurance companies charge, but only about 7 are cancer drugs.
On a broader level, Dr. Olazagasti noted, “insurance companies have a responsibility to cover these drugs. If we all get so fed up that we start relying on alternate routes to get patients their treatments, then insurance companies are let off the hook.”
However, using an alternative option like GoodRx or CostPlus could mean bypassing insurance company obstacles in certain cases.
“The hurdles someone may have to go through to get a generic drug approved are very frustrating,” said Stacie B. Dusetzina, PhD, professor of health policy and a professor of cancer research at Vanderbilt University in Nashville, Tenn.
In a weekend emergency situation, if the drug is discounted through GoodRx, “it can be a good backup strategy to send the prescription to the pharmacy” and more generally “worth it for patients to check if they can get a better deal on generic drugs through these companies.”
A version of this article first appeared on Medscape.com.
It was Friday, and oncologist Coral Olazagasti, MD, faced a ticking clock.
The patient – a man with HPV-related oropharyngeal cancer – was experiencing severe side effects from standard chemoradiation with weekly cisplatin. Intense nausea and grade 3 mucositis, in particular, left him struggling to swallow or take in any food or fluids.
He was on 8 mg of ondansetron (Zofran) every 8 hours, as needed, to keep the nausea at bay. The pills along with a feeding tube helped, but his symptoms were so intense, neither was quite enough.
“He still needed to be hospitalized twice for dehydration,” said Dr. Olazagasti, who specializes in head and neck medical cancer at Sylvester Comprehensive Cancer Center in Miami.
But when it came time to renew his ondansetron prescription, his insurance company denied it.
The reasoning: “The company had only approved 30 tablets a month and, for them, it was unjustifiable to approve anything above that amount,” Dr. Olazagasti explained.
After Dr. Olazagasti called the insurance company to resolve the issue, a company representative told her to fill out a prior authorization form.
But it was already after 7:30 p.m. ET on Friday.
At that point, finding the prior authorization documents, filling them out, and submitting them would take more time – and the paperwork couldn’t be filed until Monday.
“My patient was at home with zero tablets left and horrible symptoms. He couldn’t keep anything down,” Dr. Olazagasti said.
On Monday, the oncology team sent the prior authorization request, and her patient received his medication a few days later.
“My patient had to wait about 5 days to get the nausea meds he needed,” she said. In the meantime, he was in pain. “Having a refill of this simple supportive care medication rejected was infuriating.”
When Dr. Olazagasti vented her frustrations on Twitter, several people chimed in, suggesting purchasing the drug at a discount through GoodRx or Cost Plus instead of going through the insurance company.
At Cost Plus, for instance, 30 8-mg pills would cost $6.30, but ordering from the online pharmacy would mean waiting several days for delivery.
Discounts through GoodRx may provide a potentially faster solution in a pinch, but the pharmacy matters. In Miami, 30 8-mg pills would cost $19.99 at Costco with a GoodRx coupon, but $233.56 at CVS and $253.60 at Walgreens.
Although potentially useful, these options may not be the obvious choice for oncologists and patients, especially when a drug has already been approved and covered by the insurer. In this case, the denial was also a surprise, which left Dr. Olazagasti and her patient scrambling right before the weekend.
In addition, companies providing discounted generic drugs may only have a limited number of oncology-related medications. Cost Plus, for instance, now sells more than 1,000 generic prescription drugs at a fraction of what insurance companies charge, but only about 7 are cancer drugs.
On a broader level, Dr. Olazagasti noted, “insurance companies have a responsibility to cover these drugs. If we all get so fed up that we start relying on alternate routes to get patients their treatments, then insurance companies are let off the hook.”
However, using an alternative option like GoodRx or CostPlus could mean bypassing insurance company obstacles in certain cases.
“The hurdles someone may have to go through to get a generic drug approved are very frustrating,” said Stacie B. Dusetzina, PhD, professor of health policy and a professor of cancer research at Vanderbilt University in Nashville, Tenn.
In a weekend emergency situation, if the drug is discounted through GoodRx, “it can be a good backup strategy to send the prescription to the pharmacy” and more generally “worth it for patients to check if they can get a better deal on generic drugs through these companies.”
A version of this article first appeared on Medscape.com.
Omit radiation in older women with low-risk, ER+ breast cancer
say researchers reporting 10-year outcomes from the large phase 3 trial known as PRIME II.
“Our trial provides robust evidence indicating that irradiation can be safely omitted in women 65 years of age or older who have grade 1 or 2 ER-high cancers treated by breast-conserving therapy, provided that they receive 5 years of adjuvant endocrine therapy,” concluded investigators led by Ian Kunkler, MB, a clinical oncology professor at the University of Edinburgh.
The trial randomly assigned 1,326 women who had undergone a lumpectomy to either whole-breast irradiation or no radiation on a background of tamoxifen.
The incidence of local recurrence was lower with radiation (0.9% vs. 9.5%), but there was no significant difference in distant metastases or breast cancer–specific or overall survival.
The findings will “help clinicians guide older patients on whether this particular aspect of early breast cancer treatment can be omitted,” Dr. Kunkler said in a press release. Radiation carries risks of heart and lung damage, and these results show that skipping it does not increase the odds of dying from breast cancer.
The new study was published in the New England Journal of Medicine.
“Any doubt that radiotherapy cannot be omitted in women” who meet the criteria “can be put to rest,” commented breast radiation oncologists Alice Ho, MD, of Duke University in Durham, N.C., and Jennifer Bellon, MD, of Harvard Medical School, Boston, in an accompanying editorial.
Clinical guidelines already support omitting radiation therapy in older women with low-risk tumors treated with lumpectomy and endocrine therapy, but the move has been controversial owing to a lack of long-term data, and use of radiation for such women remains common in the United States, the investigators explain.
The “highly anticipated” results for 10-year outcomes from this trial should help address that issue, as well as “the long-standing problem of overtreatment in older women with low-risk breast cancer,” the editorialists comment.
Study details
PRIME II was conducted from 2003 to 2009 mainly in the United Kingdom. Participants were aged 65 years or older and had T1 or T2 ER-positive tumors no larger than 3 cm and were without nodal involvement.
Following lumpectomies with clear margins, the women underwent endocrine therapy; the investigators recommended tamoxifen at 20 mg/day for 5 years.
Women who were randomly assigned to radiation also received 40-50 Gy of whole-breast irradiation in 20-25 fractions over 3-5 weeks.
At 10 years, 1.6% of women in the no-radiation arm had distant metastases as their first recurrence vs. 3% of women who underwent radiation.
Ten-year breast cancer–specific survival was 97.9% with radiation and 97.4% with no radiation. Ten-year overall survival was 80.7% in the radiotherapy arm vs. 80.8% in the no-radiotherapy group.
In addition, the recurrence rate was lower after radiation. The investigators suggest that lower adherence to endocrine therapy and lower levels of ER positivity increased the risk of local recurrence among women who didn’t receive radiation.
Almost 10% of the women who did not receive radiation had local recurrences by 10 years, but the investigators note that if tumors do recur locally, women still have the option of a second lumpectomy, and if they so choose, they can then receive radiation, so local recurrence “does not necessarily mean loss of the breast.”
PRIME II was funded by the Scottish Government’s chief scientist office and the Breast Cancer Institute at Western General Hospital, Edinburgh. Dr. Kunkler reported no conflicts of interest. A coauthor has acted as a speaker, adviser, and/or researcher for many companies, including Hoffmann-La Roche, Exact Sciences, and Eli Lilly. Dr. Ho reported grants from and/or being a consultant for GlaxoSmithKline, Roche, Merck, and others. Dr. Bellon reported ties to Varian Medical Systems and Veracyte.
A version of this article originally appeared on Medscape.com.
say researchers reporting 10-year outcomes from the large phase 3 trial known as PRIME II.
“Our trial provides robust evidence indicating that irradiation can be safely omitted in women 65 years of age or older who have grade 1 or 2 ER-high cancers treated by breast-conserving therapy, provided that they receive 5 years of adjuvant endocrine therapy,” concluded investigators led by Ian Kunkler, MB, a clinical oncology professor at the University of Edinburgh.
The trial randomly assigned 1,326 women who had undergone a lumpectomy to either whole-breast irradiation or no radiation on a background of tamoxifen.
The incidence of local recurrence was lower with radiation (0.9% vs. 9.5%), but there was no significant difference in distant metastases or breast cancer–specific or overall survival.
The findings will “help clinicians guide older patients on whether this particular aspect of early breast cancer treatment can be omitted,” Dr. Kunkler said in a press release. Radiation carries risks of heart and lung damage, and these results show that skipping it does not increase the odds of dying from breast cancer.
The new study was published in the New England Journal of Medicine.
“Any doubt that radiotherapy cannot be omitted in women” who meet the criteria “can be put to rest,” commented breast radiation oncologists Alice Ho, MD, of Duke University in Durham, N.C., and Jennifer Bellon, MD, of Harvard Medical School, Boston, in an accompanying editorial.
Clinical guidelines already support omitting radiation therapy in older women with low-risk tumors treated with lumpectomy and endocrine therapy, but the move has been controversial owing to a lack of long-term data, and use of radiation for such women remains common in the United States, the investigators explain.
The “highly anticipated” results for 10-year outcomes from this trial should help address that issue, as well as “the long-standing problem of overtreatment in older women with low-risk breast cancer,” the editorialists comment.
Study details
PRIME II was conducted from 2003 to 2009 mainly in the United Kingdom. Participants were aged 65 years or older and had T1 or T2 ER-positive tumors no larger than 3 cm and were without nodal involvement.
Following lumpectomies with clear margins, the women underwent endocrine therapy; the investigators recommended tamoxifen at 20 mg/day for 5 years.
Women who were randomly assigned to radiation also received 40-50 Gy of whole-breast irradiation in 20-25 fractions over 3-5 weeks.
At 10 years, 1.6% of women in the no-radiation arm had distant metastases as their first recurrence vs. 3% of women who underwent radiation.
Ten-year breast cancer–specific survival was 97.9% with radiation and 97.4% with no radiation. Ten-year overall survival was 80.7% in the radiotherapy arm vs. 80.8% in the no-radiotherapy group.
In addition, the recurrence rate was lower after radiation. The investigators suggest that lower adherence to endocrine therapy and lower levels of ER positivity increased the risk of local recurrence among women who didn’t receive radiation.
Almost 10% of the women who did not receive radiation had local recurrences by 10 years, but the investigators note that if tumors do recur locally, women still have the option of a second lumpectomy, and if they so choose, they can then receive radiation, so local recurrence “does not necessarily mean loss of the breast.”
PRIME II was funded by the Scottish Government’s chief scientist office and the Breast Cancer Institute at Western General Hospital, Edinburgh. Dr. Kunkler reported no conflicts of interest. A coauthor has acted as a speaker, adviser, and/or researcher for many companies, including Hoffmann-La Roche, Exact Sciences, and Eli Lilly. Dr. Ho reported grants from and/or being a consultant for GlaxoSmithKline, Roche, Merck, and others. Dr. Bellon reported ties to Varian Medical Systems and Veracyte.
A version of this article originally appeared on Medscape.com.
say researchers reporting 10-year outcomes from the large phase 3 trial known as PRIME II.
“Our trial provides robust evidence indicating that irradiation can be safely omitted in women 65 years of age or older who have grade 1 or 2 ER-high cancers treated by breast-conserving therapy, provided that they receive 5 years of adjuvant endocrine therapy,” concluded investigators led by Ian Kunkler, MB, a clinical oncology professor at the University of Edinburgh.
The trial randomly assigned 1,326 women who had undergone a lumpectomy to either whole-breast irradiation or no radiation on a background of tamoxifen.
The incidence of local recurrence was lower with radiation (0.9% vs. 9.5%), but there was no significant difference in distant metastases or breast cancer–specific or overall survival.
The findings will “help clinicians guide older patients on whether this particular aspect of early breast cancer treatment can be omitted,” Dr. Kunkler said in a press release. Radiation carries risks of heart and lung damage, and these results show that skipping it does not increase the odds of dying from breast cancer.
The new study was published in the New England Journal of Medicine.
“Any doubt that radiotherapy cannot be omitted in women” who meet the criteria “can be put to rest,” commented breast radiation oncologists Alice Ho, MD, of Duke University in Durham, N.C., and Jennifer Bellon, MD, of Harvard Medical School, Boston, in an accompanying editorial.
Clinical guidelines already support omitting radiation therapy in older women with low-risk tumors treated with lumpectomy and endocrine therapy, but the move has been controversial owing to a lack of long-term data, and use of radiation for such women remains common in the United States, the investigators explain.
The “highly anticipated” results for 10-year outcomes from this trial should help address that issue, as well as “the long-standing problem of overtreatment in older women with low-risk breast cancer,” the editorialists comment.
Study details
PRIME II was conducted from 2003 to 2009 mainly in the United Kingdom. Participants were aged 65 years or older and had T1 or T2 ER-positive tumors no larger than 3 cm and were without nodal involvement.
Following lumpectomies with clear margins, the women underwent endocrine therapy; the investigators recommended tamoxifen at 20 mg/day for 5 years.
Women who were randomly assigned to radiation also received 40-50 Gy of whole-breast irradiation in 20-25 fractions over 3-5 weeks.
At 10 years, 1.6% of women in the no-radiation arm had distant metastases as their first recurrence vs. 3% of women who underwent radiation.
Ten-year breast cancer–specific survival was 97.9% with radiation and 97.4% with no radiation. Ten-year overall survival was 80.7% in the radiotherapy arm vs. 80.8% in the no-radiotherapy group.
In addition, the recurrence rate was lower after radiation. The investigators suggest that lower adherence to endocrine therapy and lower levels of ER positivity increased the risk of local recurrence among women who didn’t receive radiation.
Almost 10% of the women who did not receive radiation had local recurrences by 10 years, but the investigators note that if tumors do recur locally, women still have the option of a second lumpectomy, and if they so choose, they can then receive radiation, so local recurrence “does not necessarily mean loss of the breast.”
PRIME II was funded by the Scottish Government’s chief scientist office and the Breast Cancer Institute at Western General Hospital, Edinburgh. Dr. Kunkler reported no conflicts of interest. A coauthor has acted as a speaker, adviser, and/or researcher for many companies, including Hoffmann-La Roche, Exact Sciences, and Eli Lilly. Dr. Ho reported grants from and/or being a consultant for GlaxoSmithKline, Roche, Merck, and others. Dr. Bellon reported ties to Varian Medical Systems and Veracyte.
A version of this article originally appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Thrombolysis not necessary in mild nondisabling stroke: ARAMIS
in the ARAMIS trial.
The trial was presented by Thanh Nguyen, MD, Boston Medical Center, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
“Given the ease of administration, less intensive monitoring, low cost, and safety profile of dual antiplatelet therapy, the current findings support the use of dual antiplatelet in this population,” Dr. Nguyen concluded.
In a comment on the trial, Pooja Khatri, MD, professor of neurology at the University of Cincinnati, and lead investigator of the previous PRISMS study of tissue plasminogen activator (tPA) or alteplase in mild stroke, said the results reinforced the current recommendations of giving dual antiplatelet therapy but not alteplase to these patients.
Noting that the standard of care is now to give dual antiplatelet therapy to these patients, Dr. Khatri said: “These data reassure that this remains the right way to go.”
She added that her take-home message from the study would be: “Keep giving dual antiplatelet therapy, and we may be doing more harm than good with alteplase in this patient population.”
Introducing her presentation, Dr. Nguyen explained that mild ischemic stroke, defined as having a National Institutes of Health Stroke Scale (NIHSS) score of 5 or less, comprises half of ischemic stroke patients in the United States. But the benefit of thrombolysis in patients with minor ischemic stroke that is not disabling is unknown.
A subgroup analysis of one of the major thrombolysis trials (IST-3) found that a higher proportion of patients with mild ischemic stroke who were treated within 3 hours of symptom onset were alive and independent at 6 months if they had been given thrombolysis (84%), compared to 65% in the control group who received standard medical treatment.
This led to the first randomized trial (PRISMS) dedicated to patients with mild nondisabling stroke, which found that alteplase given within 3 hours of symptom onset did not increase the likelihood of a good functional outcome at 90 days in comparison with single-agent aspirin. The study was unfortunately terminated early for administrative reasons, and no definitive conclusions could be drawn on the basis of these results, Dr. Nguyen reported.
In 2018, the American Heart Association/American Stroke Association guidelines indicated that for patients who present within 3 hours of symptom onset with mild ischemic stroke that was judged to be nondisabling, thrombolysis with intravenous alteplase could be considered, she noted.
In the meantime, dual antiplatelet therapy was shown to be safe and effective in the POINT and CHANCE trials in patients presenting with minor stroke within 12 or 24 hours, and the CHANCE trial also found a benefit in reducing recurrent stroke that was most effective in the first 2 weeks.
The current ARAMIS trial was therefore conducted to evaluate dual antiplatelet therapy in comparison with thrombolysis for patients with acute minor stroke (NIHSS 5 or less) who presented within 4.5 hours of symptom onset and were without clearly disabling deficit.
The trial was conducted in 38 hospitals in China and included 760 patients (median NIHSS score of 2) who were randomly assigned to receive intravenous alteplase at the standard dose of 0.9 mg/kg, followed by guideline-based antiplatelet treatment, or dual antiplatelet therapy (clopidogrel 300 mg plus 100 mg aspirin loading dose followed by 10 to 14 days of aspirin 100 mg and clopidogrel 75 mg).
The trial was designed to assess noninferiority of dual antiplatelet therapy to alteplase with noninferiority margin of –4.5%.
In the modified intention-to-treat analysis, which included 722 patients, the primary outcome (excellent functional outcome, defined as a Modified Rankin Scale score of 0 or 1 at 90 days) occurred in 93.8% of patients in the dual antiplatelet therapy group and in 91.4% of the alteplase group. This gave a difference of 2.4%, which fell within the limits for noninferiority (P = .0002 for noninferiority test).
“Therefore, this was a positive trial,” Dr. Nguyen stated.
About 20% of patients crossed over from the dual antiplatelet group to the thrombolysis group, and about 16% of patients crossed over from the thrombolysis group to the dual antiplatelet group. But a per-protocol and an “as treated” analysis showed results similar to those of the main intention-to-treat analysis.
Secondary outcomes were largely similar between the two groups other than early neurologic deterioration, which was less common in the dual antiplatelet therapy group.
In terms of safety, symptomatic intracranial hemorrhage occurred in 0.3% (1/369) in the dual antiplatelet group and in 0.9% (3/350) in the alteplase group, a nonsignificant difference.
Events of “any bleeding” occurred in more patients in the thrombolysis group (5.4%) than in the dual antiplatelet therapy group (1.6%), and this difference was significant (P = .01).
Subgroup analysis showed a trend toward benefit of alteplase for patients with higher NIHSS score at baseline (NIHSS > 3). Otherwise, the other subgroups looked similar to the main results.
Dr. Nguyen pointed out one limitation of the study – that dual antiplatelet therapy was updated to standard treatment in this target population in the 2019 AHA/ASA guidelines.
In her discussion of the study, Dr. Khatri suggested that the ARAMIS results were what might have been expected.
“Dual antiplatelet therapy is designed to prevent stroke. Even in the POINT trial, dual antiplatelet therapy showed no effect on 90-day functional outcome. It was really about prevention. The PRISMS trial suggested that alteplase was also unlikely to improve 90-day functional outcome in this population of patients with mild and not clearly disabling stroke. So, it is not surprising that dual antiplatelet therapy was noninferior to alteplase for 90-day functional outcome for both those reasons,” she explained.
“That being said, while designed as a noninferiority study, it is interesting to note that alteplase again showed no evidence of treatment effect compared to antiplatelet therapy, affirming what was observed in the prematurely terminated PRISMS trial,” Dr. Khatri added.
In a discussion of the study at an ISC 2023 highlights session, ISC program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., said: “This is very important data and it’s actually the first completed trial that examines this question.”
But, he added, “I think we need to refine our knowledge about what a nondisabling stroke actually is. You could argue that every stroke is disabling. I think we need more clarity on this definition, as in practice, many clinicians still give tPA on account of these mild strokes still being disabling.”
The ARAMIS trial was funded by the National Key R&D Program of China and the Science and Technology Project Plan of Liaoning Province. Dr. Nguyen reports research support from Medtronic that was not related to the current study.
A version of this article first appeared on Medscape.com.
in the ARAMIS trial.
The trial was presented by Thanh Nguyen, MD, Boston Medical Center, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
“Given the ease of administration, less intensive monitoring, low cost, and safety profile of dual antiplatelet therapy, the current findings support the use of dual antiplatelet in this population,” Dr. Nguyen concluded.
In a comment on the trial, Pooja Khatri, MD, professor of neurology at the University of Cincinnati, and lead investigator of the previous PRISMS study of tissue plasminogen activator (tPA) or alteplase in mild stroke, said the results reinforced the current recommendations of giving dual antiplatelet therapy but not alteplase to these patients.
Noting that the standard of care is now to give dual antiplatelet therapy to these patients, Dr. Khatri said: “These data reassure that this remains the right way to go.”
She added that her take-home message from the study would be: “Keep giving dual antiplatelet therapy, and we may be doing more harm than good with alteplase in this patient population.”
Introducing her presentation, Dr. Nguyen explained that mild ischemic stroke, defined as having a National Institutes of Health Stroke Scale (NIHSS) score of 5 or less, comprises half of ischemic stroke patients in the United States. But the benefit of thrombolysis in patients with minor ischemic stroke that is not disabling is unknown.
A subgroup analysis of one of the major thrombolysis trials (IST-3) found that a higher proportion of patients with mild ischemic stroke who were treated within 3 hours of symptom onset were alive and independent at 6 months if they had been given thrombolysis (84%), compared to 65% in the control group who received standard medical treatment.
This led to the first randomized trial (PRISMS) dedicated to patients with mild nondisabling stroke, which found that alteplase given within 3 hours of symptom onset did not increase the likelihood of a good functional outcome at 90 days in comparison with single-agent aspirin. The study was unfortunately terminated early for administrative reasons, and no definitive conclusions could be drawn on the basis of these results, Dr. Nguyen reported.
In 2018, the American Heart Association/American Stroke Association guidelines indicated that for patients who present within 3 hours of symptom onset with mild ischemic stroke that was judged to be nondisabling, thrombolysis with intravenous alteplase could be considered, she noted.
In the meantime, dual antiplatelet therapy was shown to be safe and effective in the POINT and CHANCE trials in patients presenting with minor stroke within 12 or 24 hours, and the CHANCE trial also found a benefit in reducing recurrent stroke that was most effective in the first 2 weeks.
The current ARAMIS trial was therefore conducted to evaluate dual antiplatelet therapy in comparison with thrombolysis for patients with acute minor stroke (NIHSS 5 or less) who presented within 4.5 hours of symptom onset and were without clearly disabling deficit.
The trial was conducted in 38 hospitals in China and included 760 patients (median NIHSS score of 2) who were randomly assigned to receive intravenous alteplase at the standard dose of 0.9 mg/kg, followed by guideline-based antiplatelet treatment, or dual antiplatelet therapy (clopidogrel 300 mg plus 100 mg aspirin loading dose followed by 10 to 14 days of aspirin 100 mg and clopidogrel 75 mg).
The trial was designed to assess noninferiority of dual antiplatelet therapy to alteplase with noninferiority margin of –4.5%.
In the modified intention-to-treat analysis, which included 722 patients, the primary outcome (excellent functional outcome, defined as a Modified Rankin Scale score of 0 or 1 at 90 days) occurred in 93.8% of patients in the dual antiplatelet therapy group and in 91.4% of the alteplase group. This gave a difference of 2.4%, which fell within the limits for noninferiority (P = .0002 for noninferiority test).
“Therefore, this was a positive trial,” Dr. Nguyen stated.
About 20% of patients crossed over from the dual antiplatelet group to the thrombolysis group, and about 16% of patients crossed over from the thrombolysis group to the dual antiplatelet group. But a per-protocol and an “as treated” analysis showed results similar to those of the main intention-to-treat analysis.
Secondary outcomes were largely similar between the two groups other than early neurologic deterioration, which was less common in the dual antiplatelet therapy group.
In terms of safety, symptomatic intracranial hemorrhage occurred in 0.3% (1/369) in the dual antiplatelet group and in 0.9% (3/350) in the alteplase group, a nonsignificant difference.
Events of “any bleeding” occurred in more patients in the thrombolysis group (5.4%) than in the dual antiplatelet therapy group (1.6%), and this difference was significant (P = .01).
Subgroup analysis showed a trend toward benefit of alteplase for patients with higher NIHSS score at baseline (NIHSS > 3). Otherwise, the other subgroups looked similar to the main results.
Dr. Nguyen pointed out one limitation of the study – that dual antiplatelet therapy was updated to standard treatment in this target population in the 2019 AHA/ASA guidelines.
In her discussion of the study, Dr. Khatri suggested that the ARAMIS results were what might have been expected.
“Dual antiplatelet therapy is designed to prevent stroke. Even in the POINT trial, dual antiplatelet therapy showed no effect on 90-day functional outcome. It was really about prevention. The PRISMS trial suggested that alteplase was also unlikely to improve 90-day functional outcome in this population of patients with mild and not clearly disabling stroke. So, it is not surprising that dual antiplatelet therapy was noninferior to alteplase for 90-day functional outcome for both those reasons,” she explained.
“That being said, while designed as a noninferiority study, it is interesting to note that alteplase again showed no evidence of treatment effect compared to antiplatelet therapy, affirming what was observed in the prematurely terminated PRISMS trial,” Dr. Khatri added.
In a discussion of the study at an ISC 2023 highlights session, ISC program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., said: “This is very important data and it’s actually the first completed trial that examines this question.”
But, he added, “I think we need to refine our knowledge about what a nondisabling stroke actually is. You could argue that every stroke is disabling. I think we need more clarity on this definition, as in practice, many clinicians still give tPA on account of these mild strokes still being disabling.”
The ARAMIS trial was funded by the National Key R&D Program of China and the Science and Technology Project Plan of Liaoning Province. Dr. Nguyen reports research support from Medtronic that was not related to the current study.
A version of this article first appeared on Medscape.com.
in the ARAMIS trial.
The trial was presented by Thanh Nguyen, MD, Boston Medical Center, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
“Given the ease of administration, less intensive monitoring, low cost, and safety profile of dual antiplatelet therapy, the current findings support the use of dual antiplatelet in this population,” Dr. Nguyen concluded.
In a comment on the trial, Pooja Khatri, MD, professor of neurology at the University of Cincinnati, and lead investigator of the previous PRISMS study of tissue plasminogen activator (tPA) or alteplase in mild stroke, said the results reinforced the current recommendations of giving dual antiplatelet therapy but not alteplase to these patients.
Noting that the standard of care is now to give dual antiplatelet therapy to these patients, Dr. Khatri said: “These data reassure that this remains the right way to go.”
She added that her take-home message from the study would be: “Keep giving dual antiplatelet therapy, and we may be doing more harm than good with alteplase in this patient population.”
Introducing her presentation, Dr. Nguyen explained that mild ischemic stroke, defined as having a National Institutes of Health Stroke Scale (NIHSS) score of 5 or less, comprises half of ischemic stroke patients in the United States. But the benefit of thrombolysis in patients with minor ischemic stroke that is not disabling is unknown.
A subgroup analysis of one of the major thrombolysis trials (IST-3) found that a higher proportion of patients with mild ischemic stroke who were treated within 3 hours of symptom onset were alive and independent at 6 months if they had been given thrombolysis (84%), compared to 65% in the control group who received standard medical treatment.
This led to the first randomized trial (PRISMS) dedicated to patients with mild nondisabling stroke, which found that alteplase given within 3 hours of symptom onset did not increase the likelihood of a good functional outcome at 90 days in comparison with single-agent aspirin. The study was unfortunately terminated early for administrative reasons, and no definitive conclusions could be drawn on the basis of these results, Dr. Nguyen reported.
In 2018, the American Heart Association/American Stroke Association guidelines indicated that for patients who present within 3 hours of symptom onset with mild ischemic stroke that was judged to be nondisabling, thrombolysis with intravenous alteplase could be considered, she noted.
In the meantime, dual antiplatelet therapy was shown to be safe and effective in the POINT and CHANCE trials in patients presenting with minor stroke within 12 or 24 hours, and the CHANCE trial also found a benefit in reducing recurrent stroke that was most effective in the first 2 weeks.
The current ARAMIS trial was therefore conducted to evaluate dual antiplatelet therapy in comparison with thrombolysis for patients with acute minor stroke (NIHSS 5 or less) who presented within 4.5 hours of symptom onset and were without clearly disabling deficit.
The trial was conducted in 38 hospitals in China and included 760 patients (median NIHSS score of 2) who were randomly assigned to receive intravenous alteplase at the standard dose of 0.9 mg/kg, followed by guideline-based antiplatelet treatment, or dual antiplatelet therapy (clopidogrel 300 mg plus 100 mg aspirin loading dose followed by 10 to 14 days of aspirin 100 mg and clopidogrel 75 mg).
The trial was designed to assess noninferiority of dual antiplatelet therapy to alteplase with noninferiority margin of –4.5%.
In the modified intention-to-treat analysis, which included 722 patients, the primary outcome (excellent functional outcome, defined as a Modified Rankin Scale score of 0 or 1 at 90 days) occurred in 93.8% of patients in the dual antiplatelet therapy group and in 91.4% of the alteplase group. This gave a difference of 2.4%, which fell within the limits for noninferiority (P = .0002 for noninferiority test).
“Therefore, this was a positive trial,” Dr. Nguyen stated.
About 20% of patients crossed over from the dual antiplatelet group to the thrombolysis group, and about 16% of patients crossed over from the thrombolysis group to the dual antiplatelet group. But a per-protocol and an “as treated” analysis showed results similar to those of the main intention-to-treat analysis.
Secondary outcomes were largely similar between the two groups other than early neurologic deterioration, which was less common in the dual antiplatelet therapy group.
In terms of safety, symptomatic intracranial hemorrhage occurred in 0.3% (1/369) in the dual antiplatelet group and in 0.9% (3/350) in the alteplase group, a nonsignificant difference.
Events of “any bleeding” occurred in more patients in the thrombolysis group (5.4%) than in the dual antiplatelet therapy group (1.6%), and this difference was significant (P = .01).
Subgroup analysis showed a trend toward benefit of alteplase for patients with higher NIHSS score at baseline (NIHSS > 3). Otherwise, the other subgroups looked similar to the main results.
Dr. Nguyen pointed out one limitation of the study – that dual antiplatelet therapy was updated to standard treatment in this target population in the 2019 AHA/ASA guidelines.
In her discussion of the study, Dr. Khatri suggested that the ARAMIS results were what might have been expected.
“Dual antiplatelet therapy is designed to prevent stroke. Even in the POINT trial, dual antiplatelet therapy showed no effect on 90-day functional outcome. It was really about prevention. The PRISMS trial suggested that alteplase was also unlikely to improve 90-day functional outcome in this population of patients with mild and not clearly disabling stroke. So, it is not surprising that dual antiplatelet therapy was noninferior to alteplase for 90-day functional outcome for both those reasons,” she explained.
“That being said, while designed as a noninferiority study, it is interesting to note that alteplase again showed no evidence of treatment effect compared to antiplatelet therapy, affirming what was observed in the prematurely terminated PRISMS trial,” Dr. Khatri added.
In a discussion of the study at an ISC 2023 highlights session, ISC program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., said: “This is very important data and it’s actually the first completed trial that examines this question.”
But, he added, “I think we need to refine our knowledge about what a nondisabling stroke actually is. You could argue that every stroke is disabling. I think we need more clarity on this definition, as in practice, many clinicians still give tPA on account of these mild strokes still being disabling.”
The ARAMIS trial was funded by the National Key R&D Program of China and the Science and Technology Project Plan of Liaoning Province. Dr. Nguyen reports research support from Medtronic that was not related to the current study.
A version of this article first appeared on Medscape.com.
FROM ISC 2023
Diabetes drug tied to lower dementia risk
new research suggests.
Overall, in a large cohort study from South Korea, patients who took pioglitazone were 16% less likely to develop dementia over an average of 10 years than peers who did not take the drug.
However, the dementia risk reduction was 54% among those with ischemic heart disease and 43% among those with a history of stroke.
“Our study was to see the association between pioglitazone use and incidence of dementia, not how (with what mechanisms) this drug can suppress dementia pathology,” coinvestigator Eosu Kim, MD, PhD, Yonsei University, Seoul, South Korea, said in an interview.
However, “as we found this drug is more effective in diabetic patients who have blood circulation problems in the heart or brain than in those without such problems, we speculate that pioglitazone’s antidementia action may be related to improving blood vessel’s health,” Dr. Kim said.
This finding suggests that pioglitazone could be used as a personalized treatment approach for dementia prevention in this subgroup of patients with diabetes, the researchers noted.
The results were published online in Neurology.
Dose-response relationship
Risk for dementia is doubled in adults with T2DM, the investigators wrote. Prior studies have suggested that pioglitazone may protect against dementia, as well as a first or recurrent stroke, in patients with T2DM.
This led Dr. Kim and colleagues to examine the effects of pioglitazone on dementia risk overall and in relation to stroke and ischemic heart disease.
Using the national Korean health database, the researchers identified 91,218 adults aged 50 and older with new-onset T2DM who did not have dementia. A total of 3,467 were treated with pioglitazone.
Pioglitazone exposure was defined as a total cumulative daily dose of 90 or more calculated from all dispensations during 4 years after T2DM diagnosis, with outcomes assessed after this period.
Over an average of 10 years, 8.3% of pioglitazone users developed dementia, compared with 10.0% of nonusers.
There was a statistically significant 16% lower risk for developing all-cause dementia among pioglitazone users than among nonusers (adjusted hazard ratio, 0.84; 95% confidence interval, 0.75-0.95).
A dose-response relationship was evident; pioglitazone users who received the highest cumulative daily dose were at lower risk for dementia (aHR, 0.72; 95% CI, 0.55-0.94).
Several limitations
The reduced risk for dementia was more pronounced among patients who used pioglitazone for 4 years in comparison with patients who did not use the drug (aHR, 0.63; 95% CI, 0.44-0.90).
The apparent protective effect of pioglitazone with regard to dementia was greater among those with a history of ischemic heart disease (aHR, 0.46; 95% CI, 0.24-0.90) or stroke (aHR, 0.57; 95% CI, 0.38-0.86) before diabetes diagnosis.
The incidence of stroke was also reduced with pioglitazone use (aHR, 0.81; 95% CI, 0.66-1.0).
“These results provide valuable information on who could potentially benefit from pioglitazone use for prevention of dementia,” Dr. Kim said in a news release.
However, “the risk and benefit balance of long-term use of this drug to prevent dementia should be prospectively assessed,” he said in an interview.
The researchers cautioned that the study was observational; hence, the reported associations cannot address causal relationships. Also, because of the use of claims data, drug compliance could not be guaranteed, and exposure may have been overestimated.
There is also the potential for selection bias, and no information on apolipoprotein E was available, they noted.
More data needed
In an accompanying editorial, Colleen J. Maxwell, PhD, University of Waterloo (Ont.), and colleagues wrote that the results “not only support previous studies showing the potential cognitive benefit of pioglitazone but also extend our understanding of this benefit through the mediating effect of reducing ischemic stroke.”
However, because of their associated risks, which include fractures, weight gain, heart failure, and bladder cancer, thiazolidinediones are not currently favored in diabetes management guidelines – and their use has significantly declined since the mid to late 2000s, the editorialists noted.
They agreed that it will be important to reassess the risk-benefit profile of pioglitazone in T2DM as additional findings emerge.
They also noted that sodium-glucose cotransporter-2 inhibitors, which have significant cardiovascular and renal benefits and minimal side effects, may also lower the risk for dementia.
“As both pioglitazone and SGLT-2 inhibitors are second-line options for physicians, the current decision would easily be in favor of SGLT-2 inhibitors given their safety profile,” Dr. Maxwell and colleagues wrote.
For now, pioglitazone “should not be used to prevent dementia in patients with T2DM,” they concluded.
The study was supported by grants from the National Research Foundation of Korea funded by the Korean government and the Ministry of Health and Welfare. The investigators and editorialists report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Overall, in a large cohort study from South Korea, patients who took pioglitazone were 16% less likely to develop dementia over an average of 10 years than peers who did not take the drug.
However, the dementia risk reduction was 54% among those with ischemic heart disease and 43% among those with a history of stroke.
“Our study was to see the association between pioglitazone use and incidence of dementia, not how (with what mechanisms) this drug can suppress dementia pathology,” coinvestigator Eosu Kim, MD, PhD, Yonsei University, Seoul, South Korea, said in an interview.
However, “as we found this drug is more effective in diabetic patients who have blood circulation problems in the heart or brain than in those without such problems, we speculate that pioglitazone’s antidementia action may be related to improving blood vessel’s health,” Dr. Kim said.
This finding suggests that pioglitazone could be used as a personalized treatment approach for dementia prevention in this subgroup of patients with diabetes, the researchers noted.
The results were published online in Neurology.
Dose-response relationship
Risk for dementia is doubled in adults with T2DM, the investigators wrote. Prior studies have suggested that pioglitazone may protect against dementia, as well as a first or recurrent stroke, in patients with T2DM.
This led Dr. Kim and colleagues to examine the effects of pioglitazone on dementia risk overall and in relation to stroke and ischemic heart disease.
Using the national Korean health database, the researchers identified 91,218 adults aged 50 and older with new-onset T2DM who did not have dementia. A total of 3,467 were treated with pioglitazone.
Pioglitazone exposure was defined as a total cumulative daily dose of 90 or more calculated from all dispensations during 4 years after T2DM diagnosis, with outcomes assessed after this period.
Over an average of 10 years, 8.3% of pioglitazone users developed dementia, compared with 10.0% of nonusers.
There was a statistically significant 16% lower risk for developing all-cause dementia among pioglitazone users than among nonusers (adjusted hazard ratio, 0.84; 95% confidence interval, 0.75-0.95).
A dose-response relationship was evident; pioglitazone users who received the highest cumulative daily dose were at lower risk for dementia (aHR, 0.72; 95% CI, 0.55-0.94).
Several limitations
The reduced risk for dementia was more pronounced among patients who used pioglitazone for 4 years in comparison with patients who did not use the drug (aHR, 0.63; 95% CI, 0.44-0.90).
The apparent protective effect of pioglitazone with regard to dementia was greater among those with a history of ischemic heart disease (aHR, 0.46; 95% CI, 0.24-0.90) or stroke (aHR, 0.57; 95% CI, 0.38-0.86) before diabetes diagnosis.
The incidence of stroke was also reduced with pioglitazone use (aHR, 0.81; 95% CI, 0.66-1.0).
“These results provide valuable information on who could potentially benefit from pioglitazone use for prevention of dementia,” Dr. Kim said in a news release.
However, “the risk and benefit balance of long-term use of this drug to prevent dementia should be prospectively assessed,” he said in an interview.
The researchers cautioned that the study was observational; hence, the reported associations cannot address causal relationships. Also, because of the use of claims data, drug compliance could not be guaranteed, and exposure may have been overestimated.
There is also the potential for selection bias, and no information on apolipoprotein E was available, they noted.
More data needed
In an accompanying editorial, Colleen J. Maxwell, PhD, University of Waterloo (Ont.), and colleagues wrote that the results “not only support previous studies showing the potential cognitive benefit of pioglitazone but also extend our understanding of this benefit through the mediating effect of reducing ischemic stroke.”
However, because of their associated risks, which include fractures, weight gain, heart failure, and bladder cancer, thiazolidinediones are not currently favored in diabetes management guidelines – and their use has significantly declined since the mid to late 2000s, the editorialists noted.
They agreed that it will be important to reassess the risk-benefit profile of pioglitazone in T2DM as additional findings emerge.
They also noted that sodium-glucose cotransporter-2 inhibitors, which have significant cardiovascular and renal benefits and minimal side effects, may also lower the risk for dementia.
“As both pioglitazone and SGLT-2 inhibitors are second-line options for physicians, the current decision would easily be in favor of SGLT-2 inhibitors given their safety profile,” Dr. Maxwell and colleagues wrote.
For now, pioglitazone “should not be used to prevent dementia in patients with T2DM,” they concluded.
The study was supported by grants from the National Research Foundation of Korea funded by the Korean government and the Ministry of Health and Welfare. The investigators and editorialists report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Overall, in a large cohort study from South Korea, patients who took pioglitazone were 16% less likely to develop dementia over an average of 10 years than peers who did not take the drug.
However, the dementia risk reduction was 54% among those with ischemic heart disease and 43% among those with a history of stroke.
“Our study was to see the association between pioglitazone use and incidence of dementia, not how (with what mechanisms) this drug can suppress dementia pathology,” coinvestigator Eosu Kim, MD, PhD, Yonsei University, Seoul, South Korea, said in an interview.
However, “as we found this drug is more effective in diabetic patients who have blood circulation problems in the heart or brain than in those without such problems, we speculate that pioglitazone’s antidementia action may be related to improving blood vessel’s health,” Dr. Kim said.
This finding suggests that pioglitazone could be used as a personalized treatment approach for dementia prevention in this subgroup of patients with diabetes, the researchers noted.
The results were published online in Neurology.
Dose-response relationship
Risk for dementia is doubled in adults with T2DM, the investigators wrote. Prior studies have suggested that pioglitazone may protect against dementia, as well as a first or recurrent stroke, in patients with T2DM.
This led Dr. Kim and colleagues to examine the effects of pioglitazone on dementia risk overall and in relation to stroke and ischemic heart disease.
Using the national Korean health database, the researchers identified 91,218 adults aged 50 and older with new-onset T2DM who did not have dementia. A total of 3,467 were treated with pioglitazone.
Pioglitazone exposure was defined as a total cumulative daily dose of 90 or more calculated from all dispensations during 4 years after T2DM diagnosis, with outcomes assessed after this period.
Over an average of 10 years, 8.3% of pioglitazone users developed dementia, compared with 10.0% of nonusers.
There was a statistically significant 16% lower risk for developing all-cause dementia among pioglitazone users than among nonusers (adjusted hazard ratio, 0.84; 95% confidence interval, 0.75-0.95).
A dose-response relationship was evident; pioglitazone users who received the highest cumulative daily dose were at lower risk for dementia (aHR, 0.72; 95% CI, 0.55-0.94).
Several limitations
The reduced risk for dementia was more pronounced among patients who used pioglitazone for 4 years in comparison with patients who did not use the drug (aHR, 0.63; 95% CI, 0.44-0.90).
The apparent protective effect of pioglitazone with regard to dementia was greater among those with a history of ischemic heart disease (aHR, 0.46; 95% CI, 0.24-0.90) or stroke (aHR, 0.57; 95% CI, 0.38-0.86) before diabetes diagnosis.
The incidence of stroke was also reduced with pioglitazone use (aHR, 0.81; 95% CI, 0.66-1.0).
“These results provide valuable information on who could potentially benefit from pioglitazone use for prevention of dementia,” Dr. Kim said in a news release.
However, “the risk and benefit balance of long-term use of this drug to prevent dementia should be prospectively assessed,” he said in an interview.
The researchers cautioned that the study was observational; hence, the reported associations cannot address causal relationships. Also, because of the use of claims data, drug compliance could not be guaranteed, and exposure may have been overestimated.
There is also the potential for selection bias, and no information on apolipoprotein E was available, they noted.
More data needed
In an accompanying editorial, Colleen J. Maxwell, PhD, University of Waterloo (Ont.), and colleagues wrote that the results “not only support previous studies showing the potential cognitive benefit of pioglitazone but also extend our understanding of this benefit through the mediating effect of reducing ischemic stroke.”
However, because of their associated risks, which include fractures, weight gain, heart failure, and bladder cancer, thiazolidinediones are not currently favored in diabetes management guidelines – and their use has significantly declined since the mid to late 2000s, the editorialists noted.
They agreed that it will be important to reassess the risk-benefit profile of pioglitazone in T2DM as additional findings emerge.
They also noted that sodium-glucose cotransporter-2 inhibitors, which have significant cardiovascular and renal benefits and minimal side effects, may also lower the risk for dementia.
“As both pioglitazone and SGLT-2 inhibitors are second-line options for physicians, the current decision would easily be in favor of SGLT-2 inhibitors given their safety profile,” Dr. Maxwell and colleagues wrote.
For now, pioglitazone “should not be used to prevent dementia in patients with T2DM,” they concluded.
The study was supported by grants from the National Research Foundation of Korea funded by the Korean government and the Ministry of Health and Welfare. The investigators and editorialists report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM NEUROLOGY
‘Only a sociopath could work for a large health system,’ doc says sardonically
A frustrated physician recently voiced some strong words in Medscape’s US Physician Burnout & Depression Report: “Only a sociopath could work for a large health system and not be burned out. Anyone who cares about patients is doomed to burnout.”
Medscape’s report showed that 53% of physicians feel burned out by job requirements; 65% say that burnout has impacted their relationships, and other statistics say that physicians are leaving clinical medicine because of all this pressure.
What is it about being employed by large organizations that can be so negative? In another study, MEMO – Minimizing Error, Maximizing Outcomes – researchers at the University of Wisconsin surveyed more than 400 doctors to learn about how their working environments corresponded with medical errors. More than half of the physicians reported time pressures when conducting physical examinations. Nearly a third felt they needed at least 50% more time than was allotted for this patient care function, and nearly a quarter said they needed at least 50% more time for follow-up appointments.
Some have asked: Can anyone, then, thrive in today’s health care environment and avoid burnout?
Although the frustrated physician noted above may sardonically say that a doctor needs to be sociopathic to enjoy it – lacking in feelings for others – “It’s a very small number of doctors who get in it for the wrong reasons and therefore care about their own benefit and not their patients,” said psychiatrist Wendy Dean, MD, CEO and cofounder of Moral Injury of Healthcare, a nonprofit organization addressing workforce distress in health care. “Those are the outliers.”
The vast majority of physicians do care about their patients – deeply, said Dr. Dean. They struggle under the weight of the health care system and yet must find ways to get through. Today, thriving in an imperfect system requires honing new skills, asking for help when needed, and pushing for systemic and cultural change.
“We’ve been assessing and trying to address burnout for half a century,” said Dr. Dean. “Despite all the good intentions, and people dedicating their entire careers to solving the issue, we’ve barely made a dent.”
With the advent of new technological requirements on the job and more demands from increasingly larger health care organizations, the risk for burnout is higher than ever before. “There’s an increased burden of regulatory-mandated and cumbersome administrative workload per patient,” said Shomron Ben-Horin, MD, cofounder of Evinature. “Often the computer/paperwork before and after a procedure is much longer than the procedure itself.”
Meeting insurance requirements is increasingly cumbersome, too, and preauthorizations and debates with payers over medical approval may put physicians frustratingly in the middle.
“This increases the psychological burden for physicians who may feel responsible for wrongdoing no matter which option they deem better,” Dr. Ben-Horin said. “Add in physician accessibility around the clock via mobile phones, emails, and apps, and you end up on call even if you’re not officially on call.”
Why some physicians suffer more
Some physicians are more likely to suffer burnout than others, said Jessi Gold, MD, assistant professor in the department of psychiatry at Washington University in St. Louis. “The self-valuation concept comes into play here,” she said. “If you make a mistake, do you blame yourself or see it as a growth opportunity? If it’s the former, you’re more likely to burn out.”
Dr. Ben-Horin added that the most patient-centric doctors are the ones who struggle most. “These are the doctors we’d all love to have as a patient,” he said. “But they are burdened by the extra tasks of the job, and they are the most stressed by the environment.”
So too are those physicians who never master compartmentalizing their feelings and emotions. “We learn in training to compartmentalize our emotions,” said Dr. Dean. “You can’t allow yourself to get emotional while performing chest compressions on an 18-year-old kid. So you shut it all away; otherwise, you might lose the patient.”
This turn-off switch becomes automatic, but it also comes at a cost. “When doctors were interviewed about [Buffalo Bills player] Damar Hamlin going into cardiac arrest on the football field, they talked about how a life-and-death situation is so common that they have to put the emotions away, work on the patient, and move onto the next,” said Dr. Dean. “The next patient needs you just as much. We must lock away our feelings and manage the situation.”
Dr. Gold explained that burying feelings, however, is a symptom of burnout. “We have to remove ourselves from the situation to protect ourselves,” she said. “We can’t cry in these situations, but we can’t bury our feelings either.”
Instead, Dr. Gold suggested, a good medium may exist. “You may not be able to address them in the moment, but you should sometime after,” she said.
This is just a starting point on how to remain a dedicated, caring physician without burning out. “The system is pretty broken, and to survive it first means wanting to survive it,” Dr. Gold said. “There’s a lot of focus on resiliency and lack thereof if a physician expresses burnout, but that’s a false notion. Doctors are a resilient bunch but even they get burned out.”
Change for the better must come from several places. One is asking for help, something that can be hard for a group conditioned to keeping a stiff upper lip. “Just because your peers might look healthy (emotionally) doesn’t mean they are,” said Dr. Gold. “We’ve normalized this culture of burying feelings, but that doesn’t mean it’s right.”
Dr. Ben-Horin also advocates diversifying your work. This might include engaging in research and academics, for instance. “This not only makes you a better broad-perspective doctor but allows you to psychologically switch gears on research days,” he said.
The biggest place to make change, however, is within the health care system culture itself. The AMA created a series of recommendations to address burnout at the resident and fellow level, a good starting point to carry through into staff work. The steps include creating a well-being framework, gathering a team to support a well-being program, developing the program in a way to foster fun and connectivity among the staff, fostering individual well-being that addresses emotional and physical well-being, and confronting burnout and creating a sustainable culture of well-being.
On a personal level, it’s essential that physicians keep close tabs on themselves and peers. “Understand the signs and symptoms of burnout by taking stock of where you are emotionally,” said Dr. Gold. “Have a place and time at the end of a hard day to reflect or find a ritual that helps you and stay with it.”
You might also reach out to a therapist or a peer when you’re struggling. Having honest conversations with peers can go a long way. “Find a confidant that allows you to be vulnerable,” Dr. Gold recommended. “Acknowledge that this is hard and that you might need help taking care of yourself. The system needs to change, but we can also learn to survive in the meantime. You don’t have to be a sociopath to make it.”
A version of this article originally appeared on Medscape.com.
A frustrated physician recently voiced some strong words in Medscape’s US Physician Burnout & Depression Report: “Only a sociopath could work for a large health system and not be burned out. Anyone who cares about patients is doomed to burnout.”
Medscape’s report showed that 53% of physicians feel burned out by job requirements; 65% say that burnout has impacted their relationships, and other statistics say that physicians are leaving clinical medicine because of all this pressure.
What is it about being employed by large organizations that can be so negative? In another study, MEMO – Minimizing Error, Maximizing Outcomes – researchers at the University of Wisconsin surveyed more than 400 doctors to learn about how their working environments corresponded with medical errors. More than half of the physicians reported time pressures when conducting physical examinations. Nearly a third felt they needed at least 50% more time than was allotted for this patient care function, and nearly a quarter said they needed at least 50% more time for follow-up appointments.
Some have asked: Can anyone, then, thrive in today’s health care environment and avoid burnout?
Although the frustrated physician noted above may sardonically say that a doctor needs to be sociopathic to enjoy it – lacking in feelings for others – “It’s a very small number of doctors who get in it for the wrong reasons and therefore care about their own benefit and not their patients,” said psychiatrist Wendy Dean, MD, CEO and cofounder of Moral Injury of Healthcare, a nonprofit organization addressing workforce distress in health care. “Those are the outliers.”
The vast majority of physicians do care about their patients – deeply, said Dr. Dean. They struggle under the weight of the health care system and yet must find ways to get through. Today, thriving in an imperfect system requires honing new skills, asking for help when needed, and pushing for systemic and cultural change.
“We’ve been assessing and trying to address burnout for half a century,” said Dr. Dean. “Despite all the good intentions, and people dedicating their entire careers to solving the issue, we’ve barely made a dent.”
With the advent of new technological requirements on the job and more demands from increasingly larger health care organizations, the risk for burnout is higher than ever before. “There’s an increased burden of regulatory-mandated and cumbersome administrative workload per patient,” said Shomron Ben-Horin, MD, cofounder of Evinature. “Often the computer/paperwork before and after a procedure is much longer than the procedure itself.”
Meeting insurance requirements is increasingly cumbersome, too, and preauthorizations and debates with payers over medical approval may put physicians frustratingly in the middle.
“This increases the psychological burden for physicians who may feel responsible for wrongdoing no matter which option they deem better,” Dr. Ben-Horin said. “Add in physician accessibility around the clock via mobile phones, emails, and apps, and you end up on call even if you’re not officially on call.”
Why some physicians suffer more
Some physicians are more likely to suffer burnout than others, said Jessi Gold, MD, assistant professor in the department of psychiatry at Washington University in St. Louis. “The self-valuation concept comes into play here,” she said. “If you make a mistake, do you blame yourself or see it as a growth opportunity? If it’s the former, you’re more likely to burn out.”
Dr. Ben-Horin added that the most patient-centric doctors are the ones who struggle most. “These are the doctors we’d all love to have as a patient,” he said. “But they are burdened by the extra tasks of the job, and they are the most stressed by the environment.”
So too are those physicians who never master compartmentalizing their feelings and emotions. “We learn in training to compartmentalize our emotions,” said Dr. Dean. “You can’t allow yourself to get emotional while performing chest compressions on an 18-year-old kid. So you shut it all away; otherwise, you might lose the patient.”
This turn-off switch becomes automatic, but it also comes at a cost. “When doctors were interviewed about [Buffalo Bills player] Damar Hamlin going into cardiac arrest on the football field, they talked about how a life-and-death situation is so common that they have to put the emotions away, work on the patient, and move onto the next,” said Dr. Dean. “The next patient needs you just as much. We must lock away our feelings and manage the situation.”
Dr. Gold explained that burying feelings, however, is a symptom of burnout. “We have to remove ourselves from the situation to protect ourselves,” she said. “We can’t cry in these situations, but we can’t bury our feelings either.”
Instead, Dr. Gold suggested, a good medium may exist. “You may not be able to address them in the moment, but you should sometime after,” she said.
This is just a starting point on how to remain a dedicated, caring physician without burning out. “The system is pretty broken, and to survive it first means wanting to survive it,” Dr. Gold said. “There’s a lot of focus on resiliency and lack thereof if a physician expresses burnout, but that’s a false notion. Doctors are a resilient bunch but even they get burned out.”
Change for the better must come from several places. One is asking for help, something that can be hard for a group conditioned to keeping a stiff upper lip. “Just because your peers might look healthy (emotionally) doesn’t mean they are,” said Dr. Gold. “We’ve normalized this culture of burying feelings, but that doesn’t mean it’s right.”
Dr. Ben-Horin also advocates diversifying your work. This might include engaging in research and academics, for instance. “This not only makes you a better broad-perspective doctor but allows you to psychologically switch gears on research days,” he said.
The biggest place to make change, however, is within the health care system culture itself. The AMA created a series of recommendations to address burnout at the resident and fellow level, a good starting point to carry through into staff work. The steps include creating a well-being framework, gathering a team to support a well-being program, developing the program in a way to foster fun and connectivity among the staff, fostering individual well-being that addresses emotional and physical well-being, and confronting burnout and creating a sustainable culture of well-being.
On a personal level, it’s essential that physicians keep close tabs on themselves and peers. “Understand the signs and symptoms of burnout by taking stock of where you are emotionally,” said Dr. Gold. “Have a place and time at the end of a hard day to reflect or find a ritual that helps you and stay with it.”
You might also reach out to a therapist or a peer when you’re struggling. Having honest conversations with peers can go a long way. “Find a confidant that allows you to be vulnerable,” Dr. Gold recommended. “Acknowledge that this is hard and that you might need help taking care of yourself. The system needs to change, but we can also learn to survive in the meantime. You don’t have to be a sociopath to make it.”
A version of this article originally appeared on Medscape.com.
A frustrated physician recently voiced some strong words in Medscape’s US Physician Burnout & Depression Report: “Only a sociopath could work for a large health system and not be burned out. Anyone who cares about patients is doomed to burnout.”
Medscape’s report showed that 53% of physicians feel burned out by job requirements; 65% say that burnout has impacted their relationships, and other statistics say that physicians are leaving clinical medicine because of all this pressure.
What is it about being employed by large organizations that can be so negative? In another study, MEMO – Minimizing Error, Maximizing Outcomes – researchers at the University of Wisconsin surveyed more than 400 doctors to learn about how their working environments corresponded with medical errors. More than half of the physicians reported time pressures when conducting physical examinations. Nearly a third felt they needed at least 50% more time than was allotted for this patient care function, and nearly a quarter said they needed at least 50% more time for follow-up appointments.
Some have asked: Can anyone, then, thrive in today’s health care environment and avoid burnout?
Although the frustrated physician noted above may sardonically say that a doctor needs to be sociopathic to enjoy it – lacking in feelings for others – “It’s a very small number of doctors who get in it for the wrong reasons and therefore care about their own benefit and not their patients,” said psychiatrist Wendy Dean, MD, CEO and cofounder of Moral Injury of Healthcare, a nonprofit organization addressing workforce distress in health care. “Those are the outliers.”
The vast majority of physicians do care about their patients – deeply, said Dr. Dean. They struggle under the weight of the health care system and yet must find ways to get through. Today, thriving in an imperfect system requires honing new skills, asking for help when needed, and pushing for systemic and cultural change.
“We’ve been assessing and trying to address burnout for half a century,” said Dr. Dean. “Despite all the good intentions, and people dedicating their entire careers to solving the issue, we’ve barely made a dent.”
With the advent of new technological requirements on the job and more demands from increasingly larger health care organizations, the risk for burnout is higher than ever before. “There’s an increased burden of regulatory-mandated and cumbersome administrative workload per patient,” said Shomron Ben-Horin, MD, cofounder of Evinature. “Often the computer/paperwork before and after a procedure is much longer than the procedure itself.”
Meeting insurance requirements is increasingly cumbersome, too, and preauthorizations and debates with payers over medical approval may put physicians frustratingly in the middle.
“This increases the psychological burden for physicians who may feel responsible for wrongdoing no matter which option they deem better,” Dr. Ben-Horin said. “Add in physician accessibility around the clock via mobile phones, emails, and apps, and you end up on call even if you’re not officially on call.”
Why some physicians suffer more
Some physicians are more likely to suffer burnout than others, said Jessi Gold, MD, assistant professor in the department of psychiatry at Washington University in St. Louis. “The self-valuation concept comes into play here,” she said. “If you make a mistake, do you blame yourself or see it as a growth opportunity? If it’s the former, you’re more likely to burn out.”
Dr. Ben-Horin added that the most patient-centric doctors are the ones who struggle most. “These are the doctors we’d all love to have as a patient,” he said. “But they are burdened by the extra tasks of the job, and they are the most stressed by the environment.”
So too are those physicians who never master compartmentalizing their feelings and emotions. “We learn in training to compartmentalize our emotions,” said Dr. Dean. “You can’t allow yourself to get emotional while performing chest compressions on an 18-year-old kid. So you shut it all away; otherwise, you might lose the patient.”
This turn-off switch becomes automatic, but it also comes at a cost. “When doctors were interviewed about [Buffalo Bills player] Damar Hamlin going into cardiac arrest on the football field, they talked about how a life-and-death situation is so common that they have to put the emotions away, work on the patient, and move onto the next,” said Dr. Dean. “The next patient needs you just as much. We must lock away our feelings and manage the situation.”
Dr. Gold explained that burying feelings, however, is a symptom of burnout. “We have to remove ourselves from the situation to protect ourselves,” she said. “We can’t cry in these situations, but we can’t bury our feelings either.”
Instead, Dr. Gold suggested, a good medium may exist. “You may not be able to address them in the moment, but you should sometime after,” she said.
This is just a starting point on how to remain a dedicated, caring physician without burning out. “The system is pretty broken, and to survive it first means wanting to survive it,” Dr. Gold said. “There’s a lot of focus on resiliency and lack thereof if a physician expresses burnout, but that’s a false notion. Doctors are a resilient bunch but even they get burned out.”
Change for the better must come from several places. One is asking for help, something that can be hard for a group conditioned to keeping a stiff upper lip. “Just because your peers might look healthy (emotionally) doesn’t mean they are,” said Dr. Gold. “We’ve normalized this culture of burying feelings, but that doesn’t mean it’s right.”
Dr. Ben-Horin also advocates diversifying your work. This might include engaging in research and academics, for instance. “This not only makes you a better broad-perspective doctor but allows you to psychologically switch gears on research days,” he said.
The biggest place to make change, however, is within the health care system culture itself. The AMA created a series of recommendations to address burnout at the resident and fellow level, a good starting point to carry through into staff work. The steps include creating a well-being framework, gathering a team to support a well-being program, developing the program in a way to foster fun and connectivity among the staff, fostering individual well-being that addresses emotional and physical well-being, and confronting burnout and creating a sustainable culture of well-being.
On a personal level, it’s essential that physicians keep close tabs on themselves and peers. “Understand the signs and symptoms of burnout by taking stock of where you are emotionally,” said Dr. Gold. “Have a place and time at the end of a hard day to reflect or find a ritual that helps you and stay with it.”
You might also reach out to a therapist or a peer when you’re struggling. Having honest conversations with peers can go a long way. “Find a confidant that allows you to be vulnerable,” Dr. Gold recommended. “Acknowledge that this is hard and that you might need help taking care of yourself. The system needs to change, but we can also learn to survive in the meantime. You don’t have to be a sociopath to make it.”
A version of this article originally appeared on Medscape.com.
Not always implemented or enforced: Harassment policies at work
Many companies, government agencies, and organizations have implemented policies and procedures to shield employees from sexual and other forms of harassment. The U.S. Department of Health & Human Services and the American Medical Association are just two examples.
Employers can tap a rich lode of guidance and resources to craft these antiharassment policies. The National Institutes of Health’s resource page is a good site for hospitals to check out.
But how effective have official policies proved in deterring harassment in medical workplaces? After all, in a study by the American Association of Medical Colleges, 34% of female faculty said they had experienced sexual harassment irrespective of such policies. And in a recent Medscape survey of more than 3,000 physicians, 27% reported that they had either witnessed or been subjected to sexual harassment or misconduct at work during the past 4 years.
When policies are absent or unenforced
She believes employer rules and policies generally are helpful in establishing who fields harassment complaints and in creating at least some accountability.
On the other hand, policies that don’t recognize anonymous complaints effectively discourage harassment victims from coming forward, Dr. Rohr-Kirchgraber argues. Even those policies that do allow anonymous complaints may have limitations.
For example, the NIH policy on reporting harassment acknowledges that “officials must follow up on all allegations of harassment and cannot guarantee that your identity will not become apparent during the process. Please note that if you remain anonymous, key details about the allegation or concern [may] be omitted. This will limit the NIH’s ability to conduct an inquiry and take corrective action as warranted.”
Risks in pressing a harassment case
A complainant whose name becomes public risks getting a reputation as a problem employee or suffering workplace retaliation, according to Dr. Rohr-Kirchgraber. She recalls a colleague who was on a clinical education track until she lodged a harassment complaint. Abruptly, she was told she was needed on a service with fewer teaching opportunities.
With such risks in mind, respondents to the Medscape survey advised employees in medical workplaces to familiarize themselves with policies and procedures before pressing a case.
“Document everything,” an ophthalmologist urged, including time, place, offender, and witnesses. Present that information to your supervisor, and if nothing is done, hire a lawyer, a gastroenterologist suggested.
But taking the situation to the Equal Employment Opportunity Commission can be complicated, Roberta Gebhard, DO, past AMWA president and founder of its Gender Equity Task Force, told this news organization.
“They talk to the employer and get the employer’s side of the story and eventually render a decision about whether you have a case you can put through and file a lawsuit,” she said. “I don’t know of any other situation in which you need ‘permission’ to file a lawsuit.”
Nevertheless, an attorney can be helpful with cases, and when someone is terminated, a lawyer can possibly have it overturned or converted to a resignation, Dr. Gebhard said.
“And always have a lawyer review your contract before you take the job,” she advised. The lawyer might adjust the contract’s verbiage in ways that can protect one down the road in the event of a potential termination. “It’s money very well spent.”
More education needed
Dr. Rohr-Kirchgraber said that protection against harassment goes beyond the employer’s policies and procedures. Building an overall consciousness of what harassment is should begin with employee onboarding, she said.
“The harasser may not even recognize that what they’re doing or saying is a form of harassment, so we need better education,” Dr. Rohr-Kirchgraber emphasized.
A version of this article originally appeared on Medscape.com.
Many companies, government agencies, and organizations have implemented policies and procedures to shield employees from sexual and other forms of harassment. The U.S. Department of Health & Human Services and the American Medical Association are just two examples.
Employers can tap a rich lode of guidance and resources to craft these antiharassment policies. The National Institutes of Health’s resource page is a good site for hospitals to check out.
But how effective have official policies proved in deterring harassment in medical workplaces? After all, in a study by the American Association of Medical Colleges, 34% of female faculty said they had experienced sexual harassment irrespective of such policies. And in a recent Medscape survey of more than 3,000 physicians, 27% reported that they had either witnessed or been subjected to sexual harassment or misconduct at work during the past 4 years.
When policies are absent or unenforced
She believes employer rules and policies generally are helpful in establishing who fields harassment complaints and in creating at least some accountability.
On the other hand, policies that don’t recognize anonymous complaints effectively discourage harassment victims from coming forward, Dr. Rohr-Kirchgraber argues. Even those policies that do allow anonymous complaints may have limitations.
For example, the NIH policy on reporting harassment acknowledges that “officials must follow up on all allegations of harassment and cannot guarantee that your identity will not become apparent during the process. Please note that if you remain anonymous, key details about the allegation or concern [may] be omitted. This will limit the NIH’s ability to conduct an inquiry and take corrective action as warranted.”
Risks in pressing a harassment case
A complainant whose name becomes public risks getting a reputation as a problem employee or suffering workplace retaliation, according to Dr. Rohr-Kirchgraber. She recalls a colleague who was on a clinical education track until she lodged a harassment complaint. Abruptly, she was told she was needed on a service with fewer teaching opportunities.
With such risks in mind, respondents to the Medscape survey advised employees in medical workplaces to familiarize themselves with policies and procedures before pressing a case.
“Document everything,” an ophthalmologist urged, including time, place, offender, and witnesses. Present that information to your supervisor, and if nothing is done, hire a lawyer, a gastroenterologist suggested.
But taking the situation to the Equal Employment Opportunity Commission can be complicated, Roberta Gebhard, DO, past AMWA president and founder of its Gender Equity Task Force, told this news organization.
“They talk to the employer and get the employer’s side of the story and eventually render a decision about whether you have a case you can put through and file a lawsuit,” she said. “I don’t know of any other situation in which you need ‘permission’ to file a lawsuit.”
Nevertheless, an attorney can be helpful with cases, and when someone is terminated, a lawyer can possibly have it overturned or converted to a resignation, Dr. Gebhard said.
“And always have a lawyer review your contract before you take the job,” she advised. The lawyer might adjust the contract’s verbiage in ways that can protect one down the road in the event of a potential termination. “It’s money very well spent.”
More education needed
Dr. Rohr-Kirchgraber said that protection against harassment goes beyond the employer’s policies and procedures. Building an overall consciousness of what harassment is should begin with employee onboarding, she said.
“The harasser may not even recognize that what they’re doing or saying is a form of harassment, so we need better education,” Dr. Rohr-Kirchgraber emphasized.
A version of this article originally appeared on Medscape.com.
Many companies, government agencies, and organizations have implemented policies and procedures to shield employees from sexual and other forms of harassment. The U.S. Department of Health & Human Services and the American Medical Association are just two examples.
Employers can tap a rich lode of guidance and resources to craft these antiharassment policies. The National Institutes of Health’s resource page is a good site for hospitals to check out.
But how effective have official policies proved in deterring harassment in medical workplaces? After all, in a study by the American Association of Medical Colleges, 34% of female faculty said they had experienced sexual harassment irrespective of such policies. And in a recent Medscape survey of more than 3,000 physicians, 27% reported that they had either witnessed or been subjected to sexual harassment or misconduct at work during the past 4 years.
When policies are absent or unenforced
She believes employer rules and policies generally are helpful in establishing who fields harassment complaints and in creating at least some accountability.
On the other hand, policies that don’t recognize anonymous complaints effectively discourage harassment victims from coming forward, Dr. Rohr-Kirchgraber argues. Even those policies that do allow anonymous complaints may have limitations.
For example, the NIH policy on reporting harassment acknowledges that “officials must follow up on all allegations of harassment and cannot guarantee that your identity will not become apparent during the process. Please note that if you remain anonymous, key details about the allegation or concern [may] be omitted. This will limit the NIH’s ability to conduct an inquiry and take corrective action as warranted.”
Risks in pressing a harassment case
A complainant whose name becomes public risks getting a reputation as a problem employee or suffering workplace retaliation, according to Dr. Rohr-Kirchgraber. She recalls a colleague who was on a clinical education track until she lodged a harassment complaint. Abruptly, she was told she was needed on a service with fewer teaching opportunities.
With such risks in mind, respondents to the Medscape survey advised employees in medical workplaces to familiarize themselves with policies and procedures before pressing a case.
“Document everything,” an ophthalmologist urged, including time, place, offender, and witnesses. Present that information to your supervisor, and if nothing is done, hire a lawyer, a gastroenterologist suggested.
But taking the situation to the Equal Employment Opportunity Commission can be complicated, Roberta Gebhard, DO, past AMWA president and founder of its Gender Equity Task Force, told this news organization.
“They talk to the employer and get the employer’s side of the story and eventually render a decision about whether you have a case you can put through and file a lawsuit,” she said. “I don’t know of any other situation in which you need ‘permission’ to file a lawsuit.”
Nevertheless, an attorney can be helpful with cases, and when someone is terminated, a lawyer can possibly have it overturned or converted to a resignation, Dr. Gebhard said.
“And always have a lawyer review your contract before you take the job,” she advised. The lawyer might adjust the contract’s verbiage in ways that can protect one down the road in the event of a potential termination. “It’s money very well spent.”
More education needed
Dr. Rohr-Kirchgraber said that protection against harassment goes beyond the employer’s policies and procedures. Building an overall consciousness of what harassment is should begin with employee onboarding, she said.
“The harasser may not even recognize that what they’re doing or saying is a form of harassment, so we need better education,” Dr. Rohr-Kirchgraber emphasized.
A version of this article originally appeared on Medscape.com.
Screen high-risk individuals for NAFLD, urges guidance
from the American Association for the Study of Liver Diseases.
The guidance, published online in Hepatology, also reflects recent advances in the diagnosis and management of NAFLD, particularly noninvasive testing, lead author Mary E. Rinella, MD, University of Chicago, told this news organization.
The “biggest change” from the previous guidance, published 5 years ago, is “that we are explicitly recommending that people in high-risk categories get screened in primary care,” she said.
NAFLD is “a silent disease ... you could easily have somebody develop cirrhosis,” Dr. Rinella said. By identifying patients earlier, physicians would be able to “prevent or reduce the number of people turning up decompensated or at an advanced stage,” she added.
The other message that Dr. Rinella wants clinicians to take away from the guidance is that “something can be done” for patients with NAFLD. “It’s very common, and the often-cited reason why patients aren’t referred to specialty care is that there’s ‘nothing that can be done.’ ”
Although there is no U.S. Food and Drug Administration–approved drug for NAFLD, clinicians can still support their patients and suggest lifestyle changes, she added.
The guidelines also are designed to prepare the groundwork for novel drugs in the pipeline, as well as discuss those that are already available for conditions such as obesity and diabetes and that benefit people with liver disease, Dr. Rinella said.
Screening and evaluation
The guidance covers all aspects of NAFLD, including the latest developments in understanding of the epidemiology and natural history of the disease, and its molecular and cellular pathogenesis.
The guidance continues to recommend against population-based screening for NAFLD. In addition to the aforementioned screening for advanced fibrosis in high-risk individuals, it calls for a primary risk assessment with FIB-4 to be performed every 1-2 years in patients with pre-diabetes, type 2 diabetes, two or more metabolic risk factors, or imaging evidence of hepatic steatosis.
Patients with nonalcoholic steatohepatitis (NASH) cirrhosis require routine monitoring, as they are at the highest risk for liver-related outcomes, the guidance adds. Those with suspected NASH should be referred for evaluation by a specialist.
In assessments of liver fibrosis in patients, findings such as highly elevated liver stiffness, FIB-4, and enhanced liver fibrosis scores can predict an increased risk for hepatic decompensation and mortality, the authors write.
Intervention guidance
Patients with NAFLD who are overweight or obese should be prescribed a reduced calorie diet in a multidisciplinary setting because weight loss “improves hepatic steatosis, NASH, and hepatic fibrosis in a dose-dependent manner,” the guidance states.
Bariatric surgery should be considered in appropriate patients because of its effectiveness in resolving NAFLD and NASH in most patients without cirrhosis, the guidance says. However, in patients with well-compensated NASH cirrhosis, the type, safety, and efficacy of bariatric surgery is not established, so a “careful benefit-risk assessment by a multidisciplinary team of experts that includes a hepatologist” is needed, the authors note.
The guidance discusses alcohol consumption’s role in the progression of fatty liver disease and recommends that intake be assessed on a regular basis in patients with NAFLD. Patients with clinically significant hepatic fibrosis should abstain completely, it adds. Abstinence, particularly for patients with moderate to heavy alcohol intake, may lower the risk of fibrosis progression and hepatic and extrahepatic malignancies, the authors note.
Additionally, drinking at least three cups of coffee, caffeinated or not, per day is “associated with less-advanced liver disease,” they write.
The guidance also sets out key considerations for people with comorbid conditions. It states that patients with NAFLD should be screened for type 2 diabetes and that statins can be safely used for cardiovascular risk reduction “across the disease spectrum, including compensated cirrhosis.”
While noting the lack of approved medications for NAFLD, the guidance states that some drugs prescribed for comorbidities also benefit patients with NASH. These are glucagon-like peptide 1 agonist semaglutide (Ozempic), pioglitazone (Actos), and vitamin E supplementation in select patients.
Available data on these same drugs, however, do not show an antifibrotic benefit and haven’t been studied in patients with cirrhosis, the guidance states.
Additionally, metformin, ursodeoxycholic acid, dipeptidyl peptidase-4 inhibitors, silymarin, and statins do not offer meaningful histologic benefit and shouldn’t be used as a treatment for NASH.
Help against an ‘evolving epidemic’
The guidance is “timely and long awaited,” Jamile Wakim-Fleming, MD, director of the fatty liver disease program at the Cleveland Clinic, said in an interview. NAFLD is an “evolving epidemic,” she added.
Numerous recent studies have “led to new modalities for diagnosis and therapy, and a better understanding” of the epidemiology and pathophysiology of NAFLD, she said. “More specifically, advancements in noninvasive testing, risk stratifications, and therapeutic modalities are now available and worth disseminating.”
NAFLD’s complexity and the lack of an FDA-approved therapy specifically targeting the liver means that managing the disease “requires expertise in multiple disciplines and knowledge of the latest developments,” Dr. Wakim-Fleming noted.
“This guidance describes preventive and treatment strategies for the metabolic conditions associated with NAFLD and is very useful for physicians in different specialties who treat individuals with these conditions,” she said.
No funding was declared. Dr. Rinella and Dr. Wakim-Fleming have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
from the American Association for the Study of Liver Diseases.
The guidance, published online in Hepatology, also reflects recent advances in the diagnosis and management of NAFLD, particularly noninvasive testing, lead author Mary E. Rinella, MD, University of Chicago, told this news organization.
The “biggest change” from the previous guidance, published 5 years ago, is “that we are explicitly recommending that people in high-risk categories get screened in primary care,” she said.
NAFLD is “a silent disease ... you could easily have somebody develop cirrhosis,” Dr. Rinella said. By identifying patients earlier, physicians would be able to “prevent or reduce the number of people turning up decompensated or at an advanced stage,” she added.
The other message that Dr. Rinella wants clinicians to take away from the guidance is that “something can be done” for patients with NAFLD. “It’s very common, and the often-cited reason why patients aren’t referred to specialty care is that there’s ‘nothing that can be done.’ ”
Although there is no U.S. Food and Drug Administration–approved drug for NAFLD, clinicians can still support their patients and suggest lifestyle changes, she added.
The guidelines also are designed to prepare the groundwork for novel drugs in the pipeline, as well as discuss those that are already available for conditions such as obesity and diabetes and that benefit people with liver disease, Dr. Rinella said.
Screening and evaluation
The guidance covers all aspects of NAFLD, including the latest developments in understanding of the epidemiology and natural history of the disease, and its molecular and cellular pathogenesis.
The guidance continues to recommend against population-based screening for NAFLD. In addition to the aforementioned screening for advanced fibrosis in high-risk individuals, it calls for a primary risk assessment with FIB-4 to be performed every 1-2 years in patients with pre-diabetes, type 2 diabetes, two or more metabolic risk factors, or imaging evidence of hepatic steatosis.
Patients with nonalcoholic steatohepatitis (NASH) cirrhosis require routine monitoring, as they are at the highest risk for liver-related outcomes, the guidance adds. Those with suspected NASH should be referred for evaluation by a specialist.
In assessments of liver fibrosis in patients, findings such as highly elevated liver stiffness, FIB-4, and enhanced liver fibrosis scores can predict an increased risk for hepatic decompensation and mortality, the authors write.
Intervention guidance
Patients with NAFLD who are overweight or obese should be prescribed a reduced calorie diet in a multidisciplinary setting because weight loss “improves hepatic steatosis, NASH, and hepatic fibrosis in a dose-dependent manner,” the guidance states.
Bariatric surgery should be considered in appropriate patients because of its effectiveness in resolving NAFLD and NASH in most patients without cirrhosis, the guidance says. However, in patients with well-compensated NASH cirrhosis, the type, safety, and efficacy of bariatric surgery is not established, so a “careful benefit-risk assessment by a multidisciplinary team of experts that includes a hepatologist” is needed, the authors note.
The guidance discusses alcohol consumption’s role in the progression of fatty liver disease and recommends that intake be assessed on a regular basis in patients with NAFLD. Patients with clinically significant hepatic fibrosis should abstain completely, it adds. Abstinence, particularly for patients with moderate to heavy alcohol intake, may lower the risk of fibrosis progression and hepatic and extrahepatic malignancies, the authors note.
Additionally, drinking at least three cups of coffee, caffeinated or not, per day is “associated with less-advanced liver disease,” they write.
The guidance also sets out key considerations for people with comorbid conditions. It states that patients with NAFLD should be screened for type 2 diabetes and that statins can be safely used for cardiovascular risk reduction “across the disease spectrum, including compensated cirrhosis.”
While noting the lack of approved medications for NAFLD, the guidance states that some drugs prescribed for comorbidities also benefit patients with NASH. These are glucagon-like peptide 1 agonist semaglutide (Ozempic), pioglitazone (Actos), and vitamin E supplementation in select patients.
Available data on these same drugs, however, do not show an antifibrotic benefit and haven’t been studied in patients with cirrhosis, the guidance states.
Additionally, metformin, ursodeoxycholic acid, dipeptidyl peptidase-4 inhibitors, silymarin, and statins do not offer meaningful histologic benefit and shouldn’t be used as a treatment for NASH.
Help against an ‘evolving epidemic’
The guidance is “timely and long awaited,” Jamile Wakim-Fleming, MD, director of the fatty liver disease program at the Cleveland Clinic, said in an interview. NAFLD is an “evolving epidemic,” she added.
Numerous recent studies have “led to new modalities for diagnosis and therapy, and a better understanding” of the epidemiology and pathophysiology of NAFLD, she said. “More specifically, advancements in noninvasive testing, risk stratifications, and therapeutic modalities are now available and worth disseminating.”
NAFLD’s complexity and the lack of an FDA-approved therapy specifically targeting the liver means that managing the disease “requires expertise in multiple disciplines and knowledge of the latest developments,” Dr. Wakim-Fleming noted.
“This guidance describes preventive and treatment strategies for the metabolic conditions associated with NAFLD and is very useful for physicians in different specialties who treat individuals with these conditions,” she said.
No funding was declared. Dr. Rinella and Dr. Wakim-Fleming have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
from the American Association for the Study of Liver Diseases.
The guidance, published online in Hepatology, also reflects recent advances in the diagnosis and management of NAFLD, particularly noninvasive testing, lead author Mary E. Rinella, MD, University of Chicago, told this news organization.
The “biggest change” from the previous guidance, published 5 years ago, is “that we are explicitly recommending that people in high-risk categories get screened in primary care,” she said.
NAFLD is “a silent disease ... you could easily have somebody develop cirrhosis,” Dr. Rinella said. By identifying patients earlier, physicians would be able to “prevent or reduce the number of people turning up decompensated or at an advanced stage,” she added.
The other message that Dr. Rinella wants clinicians to take away from the guidance is that “something can be done” for patients with NAFLD. “It’s very common, and the often-cited reason why patients aren’t referred to specialty care is that there’s ‘nothing that can be done.’ ”
Although there is no U.S. Food and Drug Administration–approved drug for NAFLD, clinicians can still support their patients and suggest lifestyle changes, she added.
The guidelines also are designed to prepare the groundwork for novel drugs in the pipeline, as well as discuss those that are already available for conditions such as obesity and diabetes and that benefit people with liver disease, Dr. Rinella said.
Screening and evaluation
The guidance covers all aspects of NAFLD, including the latest developments in understanding of the epidemiology and natural history of the disease, and its molecular and cellular pathogenesis.
The guidance continues to recommend against population-based screening for NAFLD. In addition to the aforementioned screening for advanced fibrosis in high-risk individuals, it calls for a primary risk assessment with FIB-4 to be performed every 1-2 years in patients with pre-diabetes, type 2 diabetes, two or more metabolic risk factors, or imaging evidence of hepatic steatosis.
Patients with nonalcoholic steatohepatitis (NASH) cirrhosis require routine monitoring, as they are at the highest risk for liver-related outcomes, the guidance adds. Those with suspected NASH should be referred for evaluation by a specialist.
In assessments of liver fibrosis in patients, findings such as highly elevated liver stiffness, FIB-4, and enhanced liver fibrosis scores can predict an increased risk for hepatic decompensation and mortality, the authors write.
Intervention guidance
Patients with NAFLD who are overweight or obese should be prescribed a reduced calorie diet in a multidisciplinary setting because weight loss “improves hepatic steatosis, NASH, and hepatic fibrosis in a dose-dependent manner,” the guidance states.
Bariatric surgery should be considered in appropriate patients because of its effectiveness in resolving NAFLD and NASH in most patients without cirrhosis, the guidance says. However, in patients with well-compensated NASH cirrhosis, the type, safety, and efficacy of bariatric surgery is not established, so a “careful benefit-risk assessment by a multidisciplinary team of experts that includes a hepatologist” is needed, the authors note.
The guidance discusses alcohol consumption’s role in the progression of fatty liver disease and recommends that intake be assessed on a regular basis in patients with NAFLD. Patients with clinically significant hepatic fibrosis should abstain completely, it adds. Abstinence, particularly for patients with moderate to heavy alcohol intake, may lower the risk of fibrosis progression and hepatic and extrahepatic malignancies, the authors note.
Additionally, drinking at least three cups of coffee, caffeinated or not, per day is “associated with less-advanced liver disease,” they write.
The guidance also sets out key considerations for people with comorbid conditions. It states that patients with NAFLD should be screened for type 2 diabetes and that statins can be safely used for cardiovascular risk reduction “across the disease spectrum, including compensated cirrhosis.”
While noting the lack of approved medications for NAFLD, the guidance states that some drugs prescribed for comorbidities also benefit patients with NASH. These are glucagon-like peptide 1 agonist semaglutide (Ozempic), pioglitazone (Actos), and vitamin E supplementation in select patients.
Available data on these same drugs, however, do not show an antifibrotic benefit and haven’t been studied in patients with cirrhosis, the guidance states.
Additionally, metformin, ursodeoxycholic acid, dipeptidyl peptidase-4 inhibitors, silymarin, and statins do not offer meaningful histologic benefit and shouldn’t be used as a treatment for NASH.
Help against an ‘evolving epidemic’
The guidance is “timely and long awaited,” Jamile Wakim-Fleming, MD, director of the fatty liver disease program at the Cleveland Clinic, said in an interview. NAFLD is an “evolving epidemic,” she added.
Numerous recent studies have “led to new modalities for diagnosis and therapy, and a better understanding” of the epidemiology and pathophysiology of NAFLD, she said. “More specifically, advancements in noninvasive testing, risk stratifications, and therapeutic modalities are now available and worth disseminating.”
NAFLD’s complexity and the lack of an FDA-approved therapy specifically targeting the liver means that managing the disease “requires expertise in multiple disciplines and knowledge of the latest developments,” Dr. Wakim-Fleming noted.
“This guidance describes preventive and treatment strategies for the metabolic conditions associated with NAFLD and is very useful for physicians in different specialties who treat individuals with these conditions,” she said.
No funding was declared. Dr. Rinella and Dr. Wakim-Fleming have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM HEPATOLOGY
Is there still a role for tubal surgery in the modern world of IVF?
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.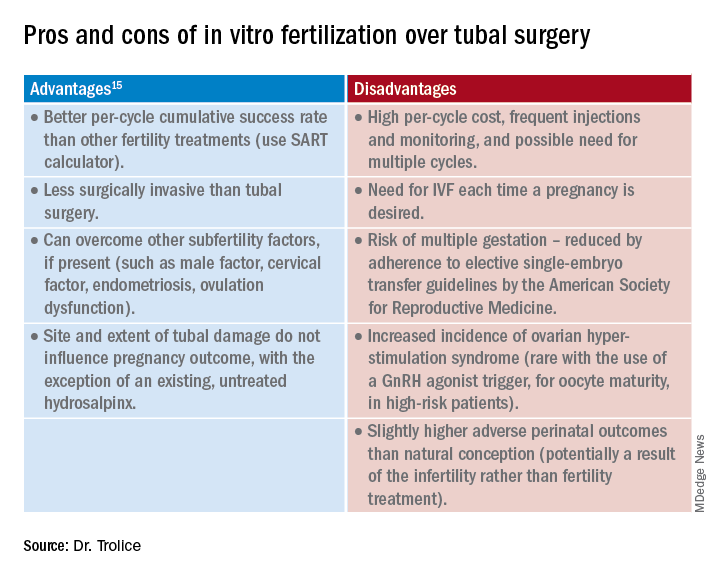
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
According to the Centers for Disease Control and Preventions, in 2019 2.1% of all infants born in the United States were conceived by assisted reproductive technology (ART). Now 45 years old, ART, namely in vitro fertilization (IVF), is offered in nearly 500 clinics in the United States, contributing to over 300,000 treatment cycles per year.
A tubal factor is responsible for 30% of female infertility and may involve proximal and/or distal tubal occlusion, irrespective of pelvic adhesions.1 Before the advent of IVF, the sole approach to the treatment of a tubal factor had been surgery. Given its success and minimal invasiveness, IVF is increasingly being offered to circumvent a tubal factor for infertility. This month we examine the utility of surgical treatment of tubal factor infertility. The options for fertility with a history of bilateral tubal ligation was covered in a prior Reproductive Rounds column.
Tubal disease and pelvic adhesions prevent the normal transport of the oocyte and sperm through the fallopian tube. The primary etiology of tubal factor infertility is pelvic inflammatory disease, mainly caused by chlamydia or gonorrhea. Other conditions that may interfere with tubal transport include severe endometriosis, adhesions from previous surgery, or nontubal infection (for example, appendicitis, inflammatory bowel disease), pelvic tuberculosis, and salpingitis isthmica nodosa (that is, diverticulosis of the fallopian tube).
Proximal tubal occlusion
During a hysterosalpingogram (HSG), transient uterine cornual spasm can result if a woman experiences significant uterine cramping, thereby resulting in a false-positive diagnosis of proximal tubal occlusion. When a repeat HSG is gently performed with slow instillation of contrast, uterine cramping is less likely, and the tubal patency rate is 60%. PTO may also result from plugs of mucus and amorphous debris, but this is not true occlusion.2 In cases with unilateral PTO, controlled ovarian hyperstimulation with intrauterine insemination has resulted in pregnancy rates similar to those in patients with unexplained infertility.3
Reconstructive surgery for bilateral PTO has limited effectiveness and the risk of subsequent ectopic pregnancy is as high as 20%.4 A more successful option is fluoroscopic tubal catheterization (FTC), an outpatient procedure performed in a radiology or infertility center. FTC uses a coaxial catheter system where the outer catheter is guided through the tubal ostium and an inner catheter is atraumatically advanced to overcome the blockage. This procedure is 85% successful for tubal patency with 50% of patients conceiving in the first 12 months; one-third of time the tubes reocclude. After the reestablishment of patency with FTC, the chance of achieving a live birth is 22% and the risk of ectopic pregnancy is 4%.5
Treatment of distal tubal occlusion – the hydrosalpinx
Surgery for treating tubal factor infertility is most successful in women with distal tubal obstruction (DTO), often caused by a hydrosalpinx. Fimbrioplasty is the lysis of fimbrial adhesions or dilatation of fimbrial strictures; the tube is patent, but there are adhesive bands that surround the terminal end with preserved tubal rugae. Gentle introduction of an alligator laparoscopic forceps into the tubal ostium followed by opening and withdrawal of the forceps helps to stretch the tube and release minor degrees of fimbrial agglutination.6
A hydrosalpinx is diagnosed by DTO with dilation and intraluminal fluid accumulation along with the reduction/loss of endothelial cilia. Left untreated, a hydrosalpinx can lead to a 50% reduction in IVF pregnancy rates.7 Tube-sparing treatment involves neosalpingostomy to create a new tubal opening. A nonsurgical approach, ultrasound-guided aspiration of hydrosalpinges, has not been shown to significantly increase the rate of clinical pregnancy. Efficacy for improving fertility is generally poor, but depends upon tubal wall thickness, ampullary dilation, presence of mucosal folds, percentage of ciliated cells in the fimbrial end, and peritubal adhesions.8
Evidence supports that laparoscopic salpingectomy in women with hydrosalpinges improves the outcomes of IVF treatment, compared with no surgical intervention.9 The improvement in pregnancy and live birth rates likely stems from the elimination of the retrograde flow of embryotoxic fluid that disrupts implantation. Endometrial receptivity markers (endometrial cell adhesion molecules, integrins, and HOXA10) have been shown to be reduced in the presence of hydrosalpinx.10 A small, randomized trial demonstrated that bipolar diathermy prior to IVF improved pregnancy outcomes.11 PTO was not more effective than salpingectomy. Conceptions, without IVF, have been reported following salpingectomy for unilateral hydrosalpinx.12
In a series including 434 patients with DTO who underwent laparoscopic fimbrioplasty (enlargement of the ostium) or neosalpingostomy (creation of a new ostium) by a single surgeon, 5-year actuarial delivery rates decreased as the severity of tubal occlusion increased; the ectopic rate was stable at approximately 15%.13 A prospective study reported that the relative increase in the pregnancy rate after salpingectomy was greatest in women with a large hydrosalpinx visible on ultrasound.14
Because of the possible risks of decreased ovarian reserve secondary to interruption of ovarian blood supply, salpingectomy should be done with minimal thermal injury and very close to the fallopian tube.
Summary
Surgery may be considered for young women with mild distal tubal disease as one surgical procedure can lead to several pregnancies whereas IVF must be performed each time pregnancy is desired. IVF is more likely than surgery to be successful in women with bilateral hydrosalpinx, in those with pelvic adhesions, in older reproductive aged women, and for both proximal and distal tubal occlusion.15 An online prediction calculator from the Society for Assisted Reproductive Technology (SART) can be helpful in counseling patients on personalized expectations for IVF pregnancy outcomes.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Ambildhuke K et al. Cureus. 2022;1:14(11):e30990.
2. Fatemeh Z et al. Br J Radiol. 2021 Jun 1;94(1122):20201386.
3. Farhi J et al. Fertil Steril. 2007 Aug;88(2):396.
4. Honoré GM et al. Fertil Steril. 1999;71(5):785.
5. De Silva PM et al. Hum Reprod. 2017;32(4):836.
6. Namnoum A and Murphy A. “Diagnostic and Operative Laparoscopy,” in Te Linde’s Operative Gynecology, 8th ed. Philadelphia: Lippincott-Raven, 1997, pp. 389.
7. Camus E et al.Hum Reprod. 1999;14(5):1243.
8. Marana R et al. Hum Reprod. 1999;14(12):2991-5.
9. Johnson N et al. Cochrane Database Syst Rev. 2010 Jan 20;2010(1):CD002125.
10. Savaris RF et al. Fertil Steril. 2006 Jan;85(1):188.
11. Kontoravdis A et al. Fertil Steril. 2006;86(6):1642.
12. Sagoskin AW et al. Hum Reprod. 2003;18(12):2634.
13. Audebert A et al. Fertil Steril. 2014;102(4):1203.
14. Bildirici I et al. Hum Reprod. 2001;16(11):2422.
15. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;97(3):539.
Treatment of several nail disorders reviewed
ORLANDO – at the ODAC Dermatology, Aesthetic, & Surgical Conference.
Dr. Hinshaw, professor of dermatology at the University of Wisconsin, Madison, reviewed several disorders and provided guidance on diagnosis, and achieving the best outcomes for patients.
Retronychia: This is an ingrowth of the proximal nail plate into the proximal nail fold, which mimics chronic paronychia, or nail inflammation. A key to the diagnosis is elevation of the proximal nail plate, Dr. Hinshaw said, along with yellowing of the nail. In some cases, a second or even third nail can be seen growing under the nail plate, she said.
“There has been traumatic lifting of the central portion of the nail plate over the matrix,” she explained. “The body thinks it needs to make a new nail plate, so it starts to do that while the primary nail plate has not yet let go.”
Sometimes, treatment with topical steroids will be effective, she said, but there might be secondary changes that require further treatment. She referred to a systematic review and a suggested treatment algorithm for retronychia, published in 2022, which can be helpful. “Even though this entity is not very well studied, there are at least some consensus approaches that the proximal nail plate needs to be removed, if not the entire nail plate,” she said.
Onycholysis: Essential to treatment of this disorder – separation of the nail from the nail bed – is knowing when it is secondary to another issue, whether it is a fungal infection, psoriasis, or tumor under the nail.
When a patient has primary onycholysis “and there’s nothing else going on in the nail, remember to try retinoids,” Dr. Hinshaw said. She suggested clipping back the nail and treating the nail bed every night with tretinoin 0.025%. If the nail bed becomes irritated, patients can pause treatment for a few days, she said.
If onycholysis has been present for 6-12 months, it can become permanent. But she said she has had success treating patients who’ve had it for a year or even a little longer, “so what we don’t want to do is give up hope for patients.”
Pyogenic granuloma (PG) in the nail: These are benign vascular tumors that can mimic more serious conditions, Dr. Hinshaw said. In adults, PG requires a histologic diagnosis, she said.
“So these all really should have a biopsy,” because of potential confusion with amelanotic melanoma or squamous cell carcinoma, she said, although in children, a biopsy is likely not necessary.
Treatment with topical beta-blockers can be effective for PG, she said, and avoids the scarring seen with surgical removal. “These are benign conditions – we want them to go away, but we want these patients to have a functional nail thereafter.”
Periungual or subungual warts: For these warts, which are alongside or under the nail, destructive approaches can cause scarring of the nail bed and are far from optimal, she said.
“We’d like to avoid that, of course.” Therefore, treatments such as lasers and liquid nitrogen “would be much further down, if at all, on my list,” she said.
Injections of the antiviral cidofovir, into the dermis right under the wart, can be highly effective, and one or two treatments is often enough, Dr. Hinshaw said. Sometimes, local anesthesia isn’t even needed for the injection, she said. “This is a wonderful option,” she added.
Dr. Hinshaw is co-owner and chief medical officer of Acure.
ORLANDO – at the ODAC Dermatology, Aesthetic, & Surgical Conference.
Dr. Hinshaw, professor of dermatology at the University of Wisconsin, Madison, reviewed several disorders and provided guidance on diagnosis, and achieving the best outcomes for patients.
Retronychia: This is an ingrowth of the proximal nail plate into the proximal nail fold, which mimics chronic paronychia, or nail inflammation. A key to the diagnosis is elevation of the proximal nail plate, Dr. Hinshaw said, along with yellowing of the nail. In some cases, a second or even third nail can be seen growing under the nail plate, she said.
“There has been traumatic lifting of the central portion of the nail plate over the matrix,” she explained. “The body thinks it needs to make a new nail plate, so it starts to do that while the primary nail plate has not yet let go.”
Sometimes, treatment with topical steroids will be effective, she said, but there might be secondary changes that require further treatment. She referred to a systematic review and a suggested treatment algorithm for retronychia, published in 2022, which can be helpful. “Even though this entity is not very well studied, there are at least some consensus approaches that the proximal nail plate needs to be removed, if not the entire nail plate,” she said.
Onycholysis: Essential to treatment of this disorder – separation of the nail from the nail bed – is knowing when it is secondary to another issue, whether it is a fungal infection, psoriasis, or tumor under the nail.
When a patient has primary onycholysis “and there’s nothing else going on in the nail, remember to try retinoids,” Dr. Hinshaw said. She suggested clipping back the nail and treating the nail bed every night with tretinoin 0.025%. If the nail bed becomes irritated, patients can pause treatment for a few days, she said.
If onycholysis has been present for 6-12 months, it can become permanent. But she said she has had success treating patients who’ve had it for a year or even a little longer, “so what we don’t want to do is give up hope for patients.”
Pyogenic granuloma (PG) in the nail: These are benign vascular tumors that can mimic more serious conditions, Dr. Hinshaw said. In adults, PG requires a histologic diagnosis, she said.
“So these all really should have a biopsy,” because of potential confusion with amelanotic melanoma or squamous cell carcinoma, she said, although in children, a biopsy is likely not necessary.
Treatment with topical beta-blockers can be effective for PG, she said, and avoids the scarring seen with surgical removal. “These are benign conditions – we want them to go away, but we want these patients to have a functional nail thereafter.”
Periungual or subungual warts: For these warts, which are alongside or under the nail, destructive approaches can cause scarring of the nail bed and are far from optimal, she said.
“We’d like to avoid that, of course.” Therefore, treatments such as lasers and liquid nitrogen “would be much further down, if at all, on my list,” she said.
Injections of the antiviral cidofovir, into the dermis right under the wart, can be highly effective, and one or two treatments is often enough, Dr. Hinshaw said. Sometimes, local anesthesia isn’t even needed for the injection, she said. “This is a wonderful option,” she added.
Dr. Hinshaw is co-owner and chief medical officer of Acure.
ORLANDO – at the ODAC Dermatology, Aesthetic, & Surgical Conference.
Dr. Hinshaw, professor of dermatology at the University of Wisconsin, Madison, reviewed several disorders and provided guidance on diagnosis, and achieving the best outcomes for patients.
Retronychia: This is an ingrowth of the proximal nail plate into the proximal nail fold, which mimics chronic paronychia, or nail inflammation. A key to the diagnosis is elevation of the proximal nail plate, Dr. Hinshaw said, along with yellowing of the nail. In some cases, a second or even third nail can be seen growing under the nail plate, she said.
“There has been traumatic lifting of the central portion of the nail plate over the matrix,” she explained. “The body thinks it needs to make a new nail plate, so it starts to do that while the primary nail plate has not yet let go.”
Sometimes, treatment with topical steroids will be effective, she said, but there might be secondary changes that require further treatment. She referred to a systematic review and a suggested treatment algorithm for retronychia, published in 2022, which can be helpful. “Even though this entity is not very well studied, there are at least some consensus approaches that the proximal nail plate needs to be removed, if not the entire nail plate,” she said.
Onycholysis: Essential to treatment of this disorder – separation of the nail from the nail bed – is knowing when it is secondary to another issue, whether it is a fungal infection, psoriasis, or tumor under the nail.
When a patient has primary onycholysis “and there’s nothing else going on in the nail, remember to try retinoids,” Dr. Hinshaw said. She suggested clipping back the nail and treating the nail bed every night with tretinoin 0.025%. If the nail bed becomes irritated, patients can pause treatment for a few days, she said.
If onycholysis has been present for 6-12 months, it can become permanent. But she said she has had success treating patients who’ve had it for a year or even a little longer, “so what we don’t want to do is give up hope for patients.”
Pyogenic granuloma (PG) in the nail: These are benign vascular tumors that can mimic more serious conditions, Dr. Hinshaw said. In adults, PG requires a histologic diagnosis, she said.
“So these all really should have a biopsy,” because of potential confusion with amelanotic melanoma or squamous cell carcinoma, she said, although in children, a biopsy is likely not necessary.
Treatment with topical beta-blockers can be effective for PG, she said, and avoids the scarring seen with surgical removal. “These are benign conditions – we want them to go away, but we want these patients to have a functional nail thereafter.”
Periungual or subungual warts: For these warts, which are alongside or under the nail, destructive approaches can cause scarring of the nail bed and are far from optimal, she said.
“We’d like to avoid that, of course.” Therefore, treatments such as lasers and liquid nitrogen “would be much further down, if at all, on my list,” she said.
Injections of the antiviral cidofovir, into the dermis right under the wart, can be highly effective, and one or two treatments is often enough, Dr. Hinshaw said. Sometimes, local anesthesia isn’t even needed for the injection, she said. “This is a wonderful option,” she added.
Dr. Hinshaw is co-owner and chief medical officer of Acure.
AT ODAC 2023
How prevalent is pediatric melanoma?
SAN DIEGO – When parents bring their children to Caroline Piggott, MD, to evaluate a suspicious mole on the scalp or other body location, the vast majority turn out to be benign, because the incidence of melanoma is rare, especially before puberty.
“Only 1%-2% of all melanomas in the world are in children, so most of my job is to provide reassurance,” Dr. Piggott, a pediatric dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “
To help parents identify melanoma, clinicians typically recommend the “ABCDE” rule, for Asymmetry, Border irregularity, Color variation (especially dark or multiple colors), Diameter greater than 6 mm, and Evolving (is it changing, bleeding or painful?).
While Dr. Piggott considers the standard ABCDE rules as important – especially in older children and teenagers – researchers led by Kelly M. Cordoro, MD, professor of dermatology at the University of California, San Francisco, proposed a modified ABCD criteria based on evaluating a cohort of 60 children who were diagnosed with melanoma and 10 who were diagnosed with ambiguous melanocytic tumors treated as melanoma before age 20 years at UCSF from 1984 to 2009.
The researchers divided patients into two groups: those aged 0-10 years (19; group A) and those aged 11-19 years (51; group B), and found that 60% of children in group A and 40% of those in group B did not present with conventional ABCDE criteria for children. Of the 60 melanoma patients, 10 died. Of these, 9 were older than age 10, and 70% had amelanotic lesions. Based on their analysis of clinical, histopathologic, and outcomes data, Dr. Cordoro and colleagues proposed additional ABCD criteria in which A stands for stands Amelanotic; B for Bleeding or Bump; C for Color uniformity, and D for De novo or any Diameter.
“This doesn’t mean you throw the old ABCDE criteria out the window,” Dr. Piggott said. “It means that you use this modified criteria in conjunction with the conventional ABCDE rules.”
Risk factors for melanoma in children are like those in adults, and include a family history of melanoma, large/giant congenital nevi, the presence of many atypical appearing nevi, having Fitzpatrick skin types I or II, a history of blistering sunburns, and the presence of genetic anomalies such as xeroderma pigmentosum.
According to an analysis of data from the Surveillance, Epidemiology, and End Results (SEER) Program, melanoma incidence increased in all individuals in the United States aged 0-19 years from 1973 to 2009. Key risk factors included White race, female sex, and living in a SEER registry categorized as low UVB exposure. Over the study period, boys experienced increased incidence rates of melanoma on the face and trunk, while girls experienced increased incidence rates of melanoma on the lower limbs and hip.
More recently, researchers extracted data from 988,103 cases of invasive melanoma in the 2001-2015 SEER database to determine the age-specific incidence of melanoma in the United States. In 2015, 83,362 cases of invasive melanoma were reported for all ages. Of these, only 67 cases were younger than age 10, while 251 were between the ages of 10 and 19 and 1,973 were young adults between the ages of 20 and 29.
In other findings, between 2006 and 2015, the overall incidence of invasive melanoma for all ages increased from 200 million to 229 cases per million person-years. “However, there were statistically significant decreases in melanoma incidence for individuals aged 10-19 years and for those aged 10-29 years,” said Dr. Piggott, who was not involved with the study. “The hypothesis is that public health efforts encouraging against sun exposure and tanning bed use may be influencing melanoma incidence in younger populations. What is interesting, though, is that young adult women have twice the melanoma risk as young adult men.”
In a separate study, researchers prospectively followed 60 melanoma-prone families for up to 40 years to evaluate the risk of pediatric melanoma in those with and without cyclin-dependent kinase inhibitor 2A (CDKN2A) mutations. Regardless of their CDKN2A status, the percentage of pediatric melanoma cases was 6- to 28-fold higher among melanoma-prone families, compared with the general population. In addition, families who were CDKN2A positive had a significantly higher rate of pediatric melanoma cases compared with those who were CDKN2A negative (11.1% vs. 2.5%; P = .004).
As for treating pediatric melanoma, the standard of care is similar to that for adults: usually wide local surgical excision of the primary lesion, depending on depth. Clinicians typically follow adult parameters for sentinel lymph node biopsy, such as lesion depth and ulceration.
“We know that a positive sentinel node does have prognostic value, but there is great debate on whether to do a lymph node dissection if the sentinel lymph node is positive,” Dr. Piggott said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “This is determined on a case-by-case basis. We consider factors such as, are the nodes palpable? Is there evidence on ultrasound? But there are no formal guidelines.”
Limited studies of systemic therapy in children exist because this population is excluded from most melanoma clinical trials. “In the past, interferon was sometimes used,” she said. “But in recent years, as with adults, we have started to use targeted immunologic therapy. This is usually managed by a tertiary academic oncology center.”
The chance of surviving pediatric melanoma is good if caught early. As in adults, the stage correlates strongly with survival, and distant metastases carry a poor prognosis.
In 2020, researchers published a retrospective, multicenter review of 38 cases of fatal pediatric melanoma between 1994 and 2017. The analysis was limited to individuals 20 years of age and younger who were cared for at 12 academic medical centers. Of the 38 patients, 42% were male, 58% were female, and 57% were White. In addition, 19% were Hispanic, “which is a larger percentage than fatalities in adult [Hispanic] populations with melanoma,” said Dr. Piggott, who was not involved in the study.
The mean age at diagnosis was 12.7 years, the mean age at death was 15.6 , and the mean survival time after diagnosis was about 35 months. Of the 16 cases with known identifiable subtypes, 50% were nodular, 31% were superficial spreading, and 19% were spitzoid melanoma. In addition, one-quarter of melanomas arose in association with congenital melanocytic nevi.
“The good news is that there are only 38 total cases of fatal pediatric melanoma between 12 academic centers over a 23-year period,” Dr. Piggott said. “Thanks goodness the number is that low.”
Dr. Piggott reported having no relevant disclosures.
SAN DIEGO – When parents bring their children to Caroline Piggott, MD, to evaluate a suspicious mole on the scalp or other body location, the vast majority turn out to be benign, because the incidence of melanoma is rare, especially before puberty.
“Only 1%-2% of all melanomas in the world are in children, so most of my job is to provide reassurance,” Dr. Piggott, a pediatric dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “
To help parents identify melanoma, clinicians typically recommend the “ABCDE” rule, for Asymmetry, Border irregularity, Color variation (especially dark or multiple colors), Diameter greater than 6 mm, and Evolving (is it changing, bleeding or painful?).
While Dr. Piggott considers the standard ABCDE rules as important – especially in older children and teenagers – researchers led by Kelly M. Cordoro, MD, professor of dermatology at the University of California, San Francisco, proposed a modified ABCD criteria based on evaluating a cohort of 60 children who were diagnosed with melanoma and 10 who were diagnosed with ambiguous melanocytic tumors treated as melanoma before age 20 years at UCSF from 1984 to 2009.
The researchers divided patients into two groups: those aged 0-10 years (19; group A) and those aged 11-19 years (51; group B), and found that 60% of children in group A and 40% of those in group B did not present with conventional ABCDE criteria for children. Of the 60 melanoma patients, 10 died. Of these, 9 were older than age 10, and 70% had amelanotic lesions. Based on their analysis of clinical, histopathologic, and outcomes data, Dr. Cordoro and colleagues proposed additional ABCD criteria in which A stands for stands Amelanotic; B for Bleeding or Bump; C for Color uniformity, and D for De novo or any Diameter.
“This doesn’t mean you throw the old ABCDE criteria out the window,” Dr. Piggott said. “It means that you use this modified criteria in conjunction with the conventional ABCDE rules.”
Risk factors for melanoma in children are like those in adults, and include a family history of melanoma, large/giant congenital nevi, the presence of many atypical appearing nevi, having Fitzpatrick skin types I or II, a history of blistering sunburns, and the presence of genetic anomalies such as xeroderma pigmentosum.
According to an analysis of data from the Surveillance, Epidemiology, and End Results (SEER) Program, melanoma incidence increased in all individuals in the United States aged 0-19 years from 1973 to 2009. Key risk factors included White race, female sex, and living in a SEER registry categorized as low UVB exposure. Over the study period, boys experienced increased incidence rates of melanoma on the face and trunk, while girls experienced increased incidence rates of melanoma on the lower limbs and hip.
More recently, researchers extracted data from 988,103 cases of invasive melanoma in the 2001-2015 SEER database to determine the age-specific incidence of melanoma in the United States. In 2015, 83,362 cases of invasive melanoma were reported for all ages. Of these, only 67 cases were younger than age 10, while 251 were between the ages of 10 and 19 and 1,973 were young adults between the ages of 20 and 29.
In other findings, between 2006 and 2015, the overall incidence of invasive melanoma for all ages increased from 200 million to 229 cases per million person-years. “However, there were statistically significant decreases in melanoma incidence for individuals aged 10-19 years and for those aged 10-29 years,” said Dr. Piggott, who was not involved with the study. “The hypothesis is that public health efforts encouraging against sun exposure and tanning bed use may be influencing melanoma incidence in younger populations. What is interesting, though, is that young adult women have twice the melanoma risk as young adult men.”
In a separate study, researchers prospectively followed 60 melanoma-prone families for up to 40 years to evaluate the risk of pediatric melanoma in those with and without cyclin-dependent kinase inhibitor 2A (CDKN2A) mutations. Regardless of their CDKN2A status, the percentage of pediatric melanoma cases was 6- to 28-fold higher among melanoma-prone families, compared with the general population. In addition, families who were CDKN2A positive had a significantly higher rate of pediatric melanoma cases compared with those who were CDKN2A negative (11.1% vs. 2.5%; P = .004).
As for treating pediatric melanoma, the standard of care is similar to that for adults: usually wide local surgical excision of the primary lesion, depending on depth. Clinicians typically follow adult parameters for sentinel lymph node biopsy, such as lesion depth and ulceration.
“We know that a positive sentinel node does have prognostic value, but there is great debate on whether to do a lymph node dissection if the sentinel lymph node is positive,” Dr. Piggott said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “This is determined on a case-by-case basis. We consider factors such as, are the nodes palpable? Is there evidence on ultrasound? But there are no formal guidelines.”
Limited studies of systemic therapy in children exist because this population is excluded from most melanoma clinical trials. “In the past, interferon was sometimes used,” she said. “But in recent years, as with adults, we have started to use targeted immunologic therapy. This is usually managed by a tertiary academic oncology center.”
The chance of surviving pediatric melanoma is good if caught early. As in adults, the stage correlates strongly with survival, and distant metastases carry a poor prognosis.
In 2020, researchers published a retrospective, multicenter review of 38 cases of fatal pediatric melanoma between 1994 and 2017. The analysis was limited to individuals 20 years of age and younger who were cared for at 12 academic medical centers. Of the 38 patients, 42% were male, 58% were female, and 57% were White. In addition, 19% were Hispanic, “which is a larger percentage than fatalities in adult [Hispanic] populations with melanoma,” said Dr. Piggott, who was not involved in the study.
The mean age at diagnosis was 12.7 years, the mean age at death was 15.6 , and the mean survival time after diagnosis was about 35 months. Of the 16 cases with known identifiable subtypes, 50% were nodular, 31% were superficial spreading, and 19% were spitzoid melanoma. In addition, one-quarter of melanomas arose in association with congenital melanocytic nevi.
“The good news is that there are only 38 total cases of fatal pediatric melanoma between 12 academic centers over a 23-year period,” Dr. Piggott said. “Thanks goodness the number is that low.”
Dr. Piggott reported having no relevant disclosures.
SAN DIEGO – When parents bring their children to Caroline Piggott, MD, to evaluate a suspicious mole on the scalp or other body location, the vast majority turn out to be benign, because the incidence of melanoma is rare, especially before puberty.
“Only 1%-2% of all melanomas in the world are in children, so most of my job is to provide reassurance,” Dr. Piggott, a pediatric dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “
To help parents identify melanoma, clinicians typically recommend the “ABCDE” rule, for Asymmetry, Border irregularity, Color variation (especially dark or multiple colors), Diameter greater than 6 mm, and Evolving (is it changing, bleeding or painful?).
While Dr. Piggott considers the standard ABCDE rules as important – especially in older children and teenagers – researchers led by Kelly M. Cordoro, MD, professor of dermatology at the University of California, San Francisco, proposed a modified ABCD criteria based on evaluating a cohort of 60 children who were diagnosed with melanoma and 10 who were diagnosed with ambiguous melanocytic tumors treated as melanoma before age 20 years at UCSF from 1984 to 2009.
The researchers divided patients into two groups: those aged 0-10 years (19; group A) and those aged 11-19 years (51; group B), and found that 60% of children in group A and 40% of those in group B did not present with conventional ABCDE criteria for children. Of the 60 melanoma patients, 10 died. Of these, 9 were older than age 10, and 70% had amelanotic lesions. Based on their analysis of clinical, histopathologic, and outcomes data, Dr. Cordoro and colleagues proposed additional ABCD criteria in which A stands for stands Amelanotic; B for Bleeding or Bump; C for Color uniformity, and D for De novo or any Diameter.
“This doesn’t mean you throw the old ABCDE criteria out the window,” Dr. Piggott said. “It means that you use this modified criteria in conjunction with the conventional ABCDE rules.”
Risk factors for melanoma in children are like those in adults, and include a family history of melanoma, large/giant congenital nevi, the presence of many atypical appearing nevi, having Fitzpatrick skin types I or II, a history of blistering sunburns, and the presence of genetic anomalies such as xeroderma pigmentosum.
According to an analysis of data from the Surveillance, Epidemiology, and End Results (SEER) Program, melanoma incidence increased in all individuals in the United States aged 0-19 years from 1973 to 2009. Key risk factors included White race, female sex, and living in a SEER registry categorized as low UVB exposure. Over the study period, boys experienced increased incidence rates of melanoma on the face and trunk, while girls experienced increased incidence rates of melanoma on the lower limbs and hip.
More recently, researchers extracted data from 988,103 cases of invasive melanoma in the 2001-2015 SEER database to determine the age-specific incidence of melanoma in the United States. In 2015, 83,362 cases of invasive melanoma were reported for all ages. Of these, only 67 cases were younger than age 10, while 251 were between the ages of 10 and 19 and 1,973 were young adults between the ages of 20 and 29.
In other findings, between 2006 and 2015, the overall incidence of invasive melanoma for all ages increased from 200 million to 229 cases per million person-years. “However, there were statistically significant decreases in melanoma incidence for individuals aged 10-19 years and for those aged 10-29 years,” said Dr. Piggott, who was not involved with the study. “The hypothesis is that public health efforts encouraging against sun exposure and tanning bed use may be influencing melanoma incidence in younger populations. What is interesting, though, is that young adult women have twice the melanoma risk as young adult men.”
In a separate study, researchers prospectively followed 60 melanoma-prone families for up to 40 years to evaluate the risk of pediatric melanoma in those with and without cyclin-dependent kinase inhibitor 2A (CDKN2A) mutations. Regardless of their CDKN2A status, the percentage of pediatric melanoma cases was 6- to 28-fold higher among melanoma-prone families, compared with the general population. In addition, families who were CDKN2A positive had a significantly higher rate of pediatric melanoma cases compared with those who were CDKN2A negative (11.1% vs. 2.5%; P = .004).
As for treating pediatric melanoma, the standard of care is similar to that for adults: usually wide local surgical excision of the primary lesion, depending on depth. Clinicians typically follow adult parameters for sentinel lymph node biopsy, such as lesion depth and ulceration.
“We know that a positive sentinel node does have prognostic value, but there is great debate on whether to do a lymph node dissection if the sentinel lymph node is positive,” Dr. Piggott said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “This is determined on a case-by-case basis. We consider factors such as, are the nodes palpable? Is there evidence on ultrasound? But there are no formal guidelines.”
Limited studies of systemic therapy in children exist because this population is excluded from most melanoma clinical trials. “In the past, interferon was sometimes used,” she said. “But in recent years, as with adults, we have started to use targeted immunologic therapy. This is usually managed by a tertiary academic oncology center.”
The chance of surviving pediatric melanoma is good if caught early. As in adults, the stage correlates strongly with survival, and distant metastases carry a poor prognosis.
In 2020, researchers published a retrospective, multicenter review of 38 cases of fatal pediatric melanoma between 1994 and 2017. The analysis was limited to individuals 20 years of age and younger who were cared for at 12 academic medical centers. Of the 38 patients, 42% were male, 58% were female, and 57% were White. In addition, 19% were Hispanic, “which is a larger percentage than fatalities in adult [Hispanic] populations with melanoma,” said Dr. Piggott, who was not involved in the study.
The mean age at diagnosis was 12.7 years, the mean age at death was 15.6 , and the mean survival time after diagnosis was about 35 months. Of the 16 cases with known identifiable subtypes, 50% were nodular, 31% were superficial spreading, and 19% were spitzoid melanoma. In addition, one-quarter of melanomas arose in association with congenital melanocytic nevi.
“The good news is that there are only 38 total cases of fatal pediatric melanoma between 12 academic centers over a 23-year period,” Dr. Piggott said. “Thanks goodness the number is that low.”
Dr. Piggott reported having no relevant disclosures.
AT MELANOMA 2023





