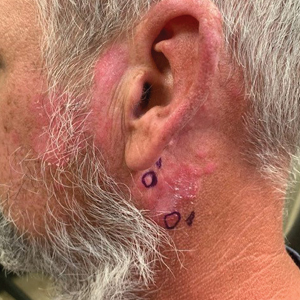User login
‘Financial toxicity’ from breast cancer is a worldwide phenomenon
Women across the world face high levels of financial burden from breast cancer, a new systematic review and analysis finds. While the burden of the disease is much higher in less-developed countries, about a third of women in Western nations like the United States say the disease has hurt their financial well-being.
When it comes to financial burden, patients with breast cancer are “a highly vulnerable patient population,” said study coauthor Kavitha Ranganathan, MD, of Brigham and Women’s Hospital, Boston, in an interview. “We need to be both strategic and comprehensive with our approach and use evidence-based methods to come up with these comprehensive solutions,” said Dr. Ranganathan, who noted that she’s hearing more from patients who face monetary hurdles.
The findings were published online in JAMA Network Open.
The researchers believe their analysis is the first to attempt to understand financial toxicity (FT) – excessive financial burden – in breast cancer on a global level. This turned out to be a challenge since there’s no standard way to measure FT.
One approach is to look at financial burden in terms of whether patients are suffering from “catastrophic expenditure,” Dr. Ranganathan said. “That’s what the World Bank and other top health and economic organizations have focused on. It means that the cost of care and – whatever it takes to get care – exceeds 10% of total annual household income.”
Another approach is more subjective and based on patient-reported outcomes, she said: “Are patients having to forgo basic subsistence needs like rent and food?”
For the report, researchers analyzed studies that use both approaches to measure FT from breast cancer. The studies came from high-income countries (n = 24, including 19 from the United States) and middle- and low-income countries (n = 10), and ranged in size from 5 to 2,445 subjects.
The analyzed studies were a range of cross-sectional (n = 26), prospective (n = 7), and retrospective designs (n = 1).
The authors pooled the data from 18 studies and estimated that the rate of patients with FT was 35.3% (14 studies, 27.3%-44.4%) in high-income countries and 78.8% (4 studies, 60.4%-90.0%) in the other countries.
The researchers also conducted a separate pooled analysis of only the U.S. studies (n = 11). It found that 34% (27%-43%) of subjects reported FT. The researchers also conducted a new analysis of Canada-only studies (n = 2) and found that 19% (9%-35%) reported FT.
The researchers weren’t able to provide insight into trends in FT in the United States prior to the period of the studies (2014-2021). But raw numbers suggest the percentage of patients facing financial challenges rose over that time, suggesting a possible increase in burden.
Previous research has suggested that breast cancer poses a higher financial burden than other chronic conditions. “Breast cancer care in particular may be associated with high FT given the need for screening and diagnosis, multidisciplinary care, and longitudinal follow-up,” the researchers write. They add that “notably, gender also affects financial security.”
As for limitations, the researchers report that they only analyzed studies in English, and there was a wide variation in approaches used to analyze FT. The analysis “did not account for different health care systems or control for health care–dedicated gross domestic product,” meaning that there’s no way to know for sure that rates were lower in nations with universal health care.
How could the new findings be useful? “They’re eye-opening for health policymakers. Whenever they see these numbers, they will say, ‘Wow, it is really a problem,’ and they’ll start thinking about solutions,” said study coauthor Rania A. Mekary, PhD, MSc, MSc, of Massachusetts College of Pharmacy and Health Sciences in Boston. “When you give them evidence-based data, then they will take it more seriously.”
The researchers call for interventions in several areas including education about early diagnosis and treatment of breast cancer, expansion of health care coverage, programs to help with nonmedical costs, and better resources for breast cancer care.
In an interview, Mary C. Politi, PhD, of Washington University, St. Louis, said the new report is useful “because it examines financial hardship internationally. Some people wonder whether financial hardship is a U.S. problem because of our health care system, which often relies on insurance and a lot of cost-sharing between insurance and patients. However, financial toxicity is prevalent across countries.”
And, she said, “the study is also useful because it encourages us to measure financial hardship and burden in a more uniform way so we can better compare and pool studies.”
Dr. Politi noted that there are ways to help patients now. “Most hospitals and health centers have staff who can talk to patients about their bills. Sometimes, a payment plan can be set up to space out payments,” she said. “Health care teams can try to consolidate care for patients on the same day to reduce parking expenses or time off for work or child care. Sometimes, changing to less expensive but effective generic medications is an option.”
The study authors received support from the National Cancer Institute, the United Nations Institute for Training and Research, the Global Surgery Foundation, the Harvard Global Health Institute, the Connors Center for Women’s Health and Gender Biology, the Center for Surgery and Public Health, and the National Endowment for Plastic Surgery. Dr. Ranganathan and Dr. Mekary report no disclosures. One coauthor reported a patent (BREAST-Q) and codevelopment of QPROMS, owned by Memorial Sloan Kettering Cancer Center. Another author reports salary support from Blue Cross Blue Shield of Michigan through the collaborative quality initiative known as Michigan Social Health Interventions to Eliminate Disparities. Dr. Politi has no disclosures.
Women across the world face high levels of financial burden from breast cancer, a new systematic review and analysis finds. While the burden of the disease is much higher in less-developed countries, about a third of women in Western nations like the United States say the disease has hurt their financial well-being.
When it comes to financial burden, patients with breast cancer are “a highly vulnerable patient population,” said study coauthor Kavitha Ranganathan, MD, of Brigham and Women’s Hospital, Boston, in an interview. “We need to be both strategic and comprehensive with our approach and use evidence-based methods to come up with these comprehensive solutions,” said Dr. Ranganathan, who noted that she’s hearing more from patients who face monetary hurdles.
The findings were published online in JAMA Network Open.
The researchers believe their analysis is the first to attempt to understand financial toxicity (FT) – excessive financial burden – in breast cancer on a global level. This turned out to be a challenge since there’s no standard way to measure FT.
One approach is to look at financial burden in terms of whether patients are suffering from “catastrophic expenditure,” Dr. Ranganathan said. “That’s what the World Bank and other top health and economic organizations have focused on. It means that the cost of care and – whatever it takes to get care – exceeds 10% of total annual household income.”
Another approach is more subjective and based on patient-reported outcomes, she said: “Are patients having to forgo basic subsistence needs like rent and food?”
For the report, researchers analyzed studies that use both approaches to measure FT from breast cancer. The studies came from high-income countries (n = 24, including 19 from the United States) and middle- and low-income countries (n = 10), and ranged in size from 5 to 2,445 subjects.
The analyzed studies were a range of cross-sectional (n = 26), prospective (n = 7), and retrospective designs (n = 1).
The authors pooled the data from 18 studies and estimated that the rate of patients with FT was 35.3% (14 studies, 27.3%-44.4%) in high-income countries and 78.8% (4 studies, 60.4%-90.0%) in the other countries.
The researchers also conducted a separate pooled analysis of only the U.S. studies (n = 11). It found that 34% (27%-43%) of subjects reported FT. The researchers also conducted a new analysis of Canada-only studies (n = 2) and found that 19% (9%-35%) reported FT.
The researchers weren’t able to provide insight into trends in FT in the United States prior to the period of the studies (2014-2021). But raw numbers suggest the percentage of patients facing financial challenges rose over that time, suggesting a possible increase in burden.
Previous research has suggested that breast cancer poses a higher financial burden than other chronic conditions. “Breast cancer care in particular may be associated with high FT given the need for screening and diagnosis, multidisciplinary care, and longitudinal follow-up,” the researchers write. They add that “notably, gender also affects financial security.”
As for limitations, the researchers report that they only analyzed studies in English, and there was a wide variation in approaches used to analyze FT. The analysis “did not account for different health care systems or control for health care–dedicated gross domestic product,” meaning that there’s no way to know for sure that rates were lower in nations with universal health care.
How could the new findings be useful? “They’re eye-opening for health policymakers. Whenever they see these numbers, they will say, ‘Wow, it is really a problem,’ and they’ll start thinking about solutions,” said study coauthor Rania A. Mekary, PhD, MSc, MSc, of Massachusetts College of Pharmacy and Health Sciences in Boston. “When you give them evidence-based data, then they will take it more seriously.”
The researchers call for interventions in several areas including education about early diagnosis and treatment of breast cancer, expansion of health care coverage, programs to help with nonmedical costs, and better resources for breast cancer care.
In an interview, Mary C. Politi, PhD, of Washington University, St. Louis, said the new report is useful “because it examines financial hardship internationally. Some people wonder whether financial hardship is a U.S. problem because of our health care system, which often relies on insurance and a lot of cost-sharing between insurance and patients. However, financial toxicity is prevalent across countries.”
And, she said, “the study is also useful because it encourages us to measure financial hardship and burden in a more uniform way so we can better compare and pool studies.”
Dr. Politi noted that there are ways to help patients now. “Most hospitals and health centers have staff who can talk to patients about their bills. Sometimes, a payment plan can be set up to space out payments,” she said. “Health care teams can try to consolidate care for patients on the same day to reduce parking expenses or time off for work or child care. Sometimes, changing to less expensive but effective generic medications is an option.”
The study authors received support from the National Cancer Institute, the United Nations Institute for Training and Research, the Global Surgery Foundation, the Harvard Global Health Institute, the Connors Center for Women’s Health and Gender Biology, the Center for Surgery and Public Health, and the National Endowment for Plastic Surgery. Dr. Ranganathan and Dr. Mekary report no disclosures. One coauthor reported a patent (BREAST-Q) and codevelopment of QPROMS, owned by Memorial Sloan Kettering Cancer Center. Another author reports salary support from Blue Cross Blue Shield of Michigan through the collaborative quality initiative known as Michigan Social Health Interventions to Eliminate Disparities. Dr. Politi has no disclosures.
Women across the world face high levels of financial burden from breast cancer, a new systematic review and analysis finds. While the burden of the disease is much higher in less-developed countries, about a third of women in Western nations like the United States say the disease has hurt their financial well-being.
When it comes to financial burden, patients with breast cancer are “a highly vulnerable patient population,” said study coauthor Kavitha Ranganathan, MD, of Brigham and Women’s Hospital, Boston, in an interview. “We need to be both strategic and comprehensive with our approach and use evidence-based methods to come up with these comprehensive solutions,” said Dr. Ranganathan, who noted that she’s hearing more from patients who face monetary hurdles.
The findings were published online in JAMA Network Open.
The researchers believe their analysis is the first to attempt to understand financial toxicity (FT) – excessive financial burden – in breast cancer on a global level. This turned out to be a challenge since there’s no standard way to measure FT.
One approach is to look at financial burden in terms of whether patients are suffering from “catastrophic expenditure,” Dr. Ranganathan said. “That’s what the World Bank and other top health and economic organizations have focused on. It means that the cost of care and – whatever it takes to get care – exceeds 10% of total annual household income.”
Another approach is more subjective and based on patient-reported outcomes, she said: “Are patients having to forgo basic subsistence needs like rent and food?”
For the report, researchers analyzed studies that use both approaches to measure FT from breast cancer. The studies came from high-income countries (n = 24, including 19 from the United States) and middle- and low-income countries (n = 10), and ranged in size from 5 to 2,445 subjects.
The analyzed studies were a range of cross-sectional (n = 26), prospective (n = 7), and retrospective designs (n = 1).
The authors pooled the data from 18 studies and estimated that the rate of patients with FT was 35.3% (14 studies, 27.3%-44.4%) in high-income countries and 78.8% (4 studies, 60.4%-90.0%) in the other countries.
The researchers also conducted a separate pooled analysis of only the U.S. studies (n = 11). It found that 34% (27%-43%) of subjects reported FT. The researchers also conducted a new analysis of Canada-only studies (n = 2) and found that 19% (9%-35%) reported FT.
The researchers weren’t able to provide insight into trends in FT in the United States prior to the period of the studies (2014-2021). But raw numbers suggest the percentage of patients facing financial challenges rose over that time, suggesting a possible increase in burden.
Previous research has suggested that breast cancer poses a higher financial burden than other chronic conditions. “Breast cancer care in particular may be associated with high FT given the need for screening and diagnosis, multidisciplinary care, and longitudinal follow-up,” the researchers write. They add that “notably, gender also affects financial security.”
As for limitations, the researchers report that they only analyzed studies in English, and there was a wide variation in approaches used to analyze FT. The analysis “did not account for different health care systems or control for health care–dedicated gross domestic product,” meaning that there’s no way to know for sure that rates were lower in nations with universal health care.
How could the new findings be useful? “They’re eye-opening for health policymakers. Whenever they see these numbers, they will say, ‘Wow, it is really a problem,’ and they’ll start thinking about solutions,” said study coauthor Rania A. Mekary, PhD, MSc, MSc, of Massachusetts College of Pharmacy and Health Sciences in Boston. “When you give them evidence-based data, then they will take it more seriously.”
The researchers call for interventions in several areas including education about early diagnosis and treatment of breast cancer, expansion of health care coverage, programs to help with nonmedical costs, and better resources for breast cancer care.
In an interview, Mary C. Politi, PhD, of Washington University, St. Louis, said the new report is useful “because it examines financial hardship internationally. Some people wonder whether financial hardship is a U.S. problem because of our health care system, which often relies on insurance and a lot of cost-sharing between insurance and patients. However, financial toxicity is prevalent across countries.”
And, she said, “the study is also useful because it encourages us to measure financial hardship and burden in a more uniform way so we can better compare and pool studies.”
Dr. Politi noted that there are ways to help patients now. “Most hospitals and health centers have staff who can talk to patients about their bills. Sometimes, a payment plan can be set up to space out payments,” she said. “Health care teams can try to consolidate care for patients on the same day to reduce parking expenses or time off for work or child care. Sometimes, changing to less expensive but effective generic medications is an option.”
The study authors received support from the National Cancer Institute, the United Nations Institute for Training and Research, the Global Surgery Foundation, the Harvard Global Health Institute, the Connors Center for Women’s Health and Gender Biology, the Center for Surgery and Public Health, and the National Endowment for Plastic Surgery. Dr. Ranganathan and Dr. Mekary report no disclosures. One coauthor reported a patent (BREAST-Q) and codevelopment of QPROMS, owned by Memorial Sloan Kettering Cancer Center. Another author reports salary support from Blue Cross Blue Shield of Michigan through the collaborative quality initiative known as Michigan Social Health Interventions to Eliminate Disparities. Dr. Politi has no disclosures.
FROM JAMA NETWORK OPEN
What does the future of psoriasis treatment look like?
HONOLULU – During office visits with Andrew Blauvelt, MD, MBA, many patients well controlled on biologic therapy for their moderate to severe psoriasis often ask him when their scheduled injections can stop.
The most common question he hears is, “ ‘Why do I have to keep doing this? I’ve been clear for 2 or 3 years,’ ” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “We have terrific drugs for psoriasis, but how can we do better?”
Development of oral biologics. At least two companies are developing a peptide-type small molecule that blocks interleukin (IL)-17 or IL-23 signaling, but would be given as a pill, he said. Another concept in the works is a robotic pill for drug delivery. The pill, which is being developed by Rani Therapeutics, protects the biotherapeutic drug payload from digestion in the GI tract and auto-injects it into the wall of the small intestine, according to a report of two studies that demonstrated the safety and tolerability of the robotic pill in healthy humans.
In an animal study, the same researchers showed that delivering monoclonal antibodies with the robotic pill achieved bioavailability on par with that obtained by standard subcutaneous injections.
Identifying “super responders” who require less frequent dosing of medication. “There’s data to suggest that we can kind of back off treatment in these patients,” Dr. Blauvelt said.
Hitting treatment hard and early. “There’s a concept in medicine of hitting disease hard and hitting it early, before the disease can establish itself and cause damage,” he said.
Targeting tissue resident memory T cells. In psoriasis, the idea is that if you treat earlier, when patients are just diagnosed, “perhaps you might be able to decrease resident memory T cells that set up shop in the skin and are responsible for disease recurrences,” Dr. Blauvelt said. “Research has shown that IL-23 blockers decrease tissue resident memory T cells, and IL-17 blockers don’t. This could explain why we see long remissions in this class of drug because we’re getting at these resident memory T cells and knocking them down,” he explained. “Our hypothesis is that hitting hard and early in the treatment course with high-dose IL-23 blockade may be an effective strategy to induce long-term remissions and possible cure, what we call ‘knock-out therapy.’ ”
In a pilot study of 20 patients, Dr. Blauvelt and colleagues are evaluating whether higher initial doses of the IL-23 antagonist risankizumab (300 mg and 600 mg, 2 times and 4 times the standard initial doses for plaque psoriasis) can more effectively target resident memory T cells. “This involves dosing at weeks 0, 4, and 16, then stopping and measuring resident T cells in the tissue to see how long we can induce psoriasis remissions,” Dr. Blauvelt said.
“I have no data to share, but I think we have the potential for unprecedented PASI-100 numbers with no added safety concerns, and the potential to break away from established regular dosing patterns,” such as the possibility of yearly dosing, the possibility of long-term remissions, and the possibility of cure in some patients, he noted.
Inducing tolerance. This refers to efforts aimed at increasing regulatory T cells, which are natural T cells that calm inflammation. He described it as “revving up our natural anti-inflammatory T cells to help balance the immune system.”
Gene editing. This involves using CRISPR gene editing technology to cut genes as a way to cure disease. “What if we cut the IL-23 receptor?” Dr. Blauvelt asked. “You would get rid of that whole signaling pathway. Would the patient be fine?”
In an interview a the meeting, Linda Stein Gold, MD, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit, said that Dr. Blauvelt “has a very exciting view” of the future of psoriasis treatments. “I think that some of it will come true; we’ll have to see which,” Dr. Stein Gold said. “The idea that we might be able to change the trajectory of disease by being aggressive upfront, and possibly modify the course, is exciting. That would be a wonderful new treatment approach.”
Dr. Blauvelt disclosed ties with AbbVie, Abcentra, Affibody, Aligos, Almirall, Alumis, Amgen, AnaptysBio, Arcutis, Arena, ASLAN Pharma, Athenex, Bluefin, Boehringer Ingelheim, Bristol Myers Squibb, Cara, Dermavant, EcoR1, Escient, Evelo, Evommune, Forte, Galderma, Highlightll, Incyte, Innovent Bio, Janssen, Landos, Leo, Lilly, Merck, Novartis, Pfizer, Rapt, Regeneron, Sanofi-Genzyme, Spherix, Sun Pharmaceuticals Industries, TLL Pharmaceutical, TrialSpark, UCB Pharma, Vibliome, and Xencor.
Dr. Stein Gold disclosed ties with Almirall, Cutera, Dermata, Galderma, Novartis, Ortho Dermatologics, and Sun Pharmaceutical Industries, Ltd.
Medscape and this news organization are owned by the same parent company.
HONOLULU – During office visits with Andrew Blauvelt, MD, MBA, many patients well controlled on biologic therapy for their moderate to severe psoriasis often ask him when their scheduled injections can stop.
The most common question he hears is, “ ‘Why do I have to keep doing this? I’ve been clear for 2 or 3 years,’ ” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “We have terrific drugs for psoriasis, but how can we do better?”
Development of oral biologics. At least two companies are developing a peptide-type small molecule that blocks interleukin (IL)-17 or IL-23 signaling, but would be given as a pill, he said. Another concept in the works is a robotic pill for drug delivery. The pill, which is being developed by Rani Therapeutics, protects the biotherapeutic drug payload from digestion in the GI tract and auto-injects it into the wall of the small intestine, according to a report of two studies that demonstrated the safety and tolerability of the robotic pill in healthy humans.
In an animal study, the same researchers showed that delivering monoclonal antibodies with the robotic pill achieved bioavailability on par with that obtained by standard subcutaneous injections.
Identifying “super responders” who require less frequent dosing of medication. “There’s data to suggest that we can kind of back off treatment in these patients,” Dr. Blauvelt said.
Hitting treatment hard and early. “There’s a concept in medicine of hitting disease hard and hitting it early, before the disease can establish itself and cause damage,” he said.
Targeting tissue resident memory T cells. In psoriasis, the idea is that if you treat earlier, when patients are just diagnosed, “perhaps you might be able to decrease resident memory T cells that set up shop in the skin and are responsible for disease recurrences,” Dr. Blauvelt said. “Research has shown that IL-23 blockers decrease tissue resident memory T cells, and IL-17 blockers don’t. This could explain why we see long remissions in this class of drug because we’re getting at these resident memory T cells and knocking them down,” he explained. “Our hypothesis is that hitting hard and early in the treatment course with high-dose IL-23 blockade may be an effective strategy to induce long-term remissions and possible cure, what we call ‘knock-out therapy.’ ”
In a pilot study of 20 patients, Dr. Blauvelt and colleagues are evaluating whether higher initial doses of the IL-23 antagonist risankizumab (300 mg and 600 mg, 2 times and 4 times the standard initial doses for plaque psoriasis) can more effectively target resident memory T cells. “This involves dosing at weeks 0, 4, and 16, then stopping and measuring resident T cells in the tissue to see how long we can induce psoriasis remissions,” Dr. Blauvelt said.
“I have no data to share, but I think we have the potential for unprecedented PASI-100 numbers with no added safety concerns, and the potential to break away from established regular dosing patterns,” such as the possibility of yearly dosing, the possibility of long-term remissions, and the possibility of cure in some patients, he noted.
Inducing tolerance. This refers to efforts aimed at increasing regulatory T cells, which are natural T cells that calm inflammation. He described it as “revving up our natural anti-inflammatory T cells to help balance the immune system.”
Gene editing. This involves using CRISPR gene editing technology to cut genes as a way to cure disease. “What if we cut the IL-23 receptor?” Dr. Blauvelt asked. “You would get rid of that whole signaling pathway. Would the patient be fine?”
In an interview a the meeting, Linda Stein Gold, MD, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit, said that Dr. Blauvelt “has a very exciting view” of the future of psoriasis treatments. “I think that some of it will come true; we’ll have to see which,” Dr. Stein Gold said. “The idea that we might be able to change the trajectory of disease by being aggressive upfront, and possibly modify the course, is exciting. That would be a wonderful new treatment approach.”
Dr. Blauvelt disclosed ties with AbbVie, Abcentra, Affibody, Aligos, Almirall, Alumis, Amgen, AnaptysBio, Arcutis, Arena, ASLAN Pharma, Athenex, Bluefin, Boehringer Ingelheim, Bristol Myers Squibb, Cara, Dermavant, EcoR1, Escient, Evelo, Evommune, Forte, Galderma, Highlightll, Incyte, Innovent Bio, Janssen, Landos, Leo, Lilly, Merck, Novartis, Pfizer, Rapt, Regeneron, Sanofi-Genzyme, Spherix, Sun Pharmaceuticals Industries, TLL Pharmaceutical, TrialSpark, UCB Pharma, Vibliome, and Xencor.
Dr. Stein Gold disclosed ties with Almirall, Cutera, Dermata, Galderma, Novartis, Ortho Dermatologics, and Sun Pharmaceutical Industries, Ltd.
Medscape and this news organization are owned by the same parent company.
HONOLULU – During office visits with Andrew Blauvelt, MD, MBA, many patients well controlled on biologic therapy for their moderate to severe psoriasis often ask him when their scheduled injections can stop.
The most common question he hears is, “ ‘Why do I have to keep doing this? I’ve been clear for 2 or 3 years,’ ” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “We have terrific drugs for psoriasis, but how can we do better?”
Development of oral biologics. At least two companies are developing a peptide-type small molecule that blocks interleukin (IL)-17 or IL-23 signaling, but would be given as a pill, he said. Another concept in the works is a robotic pill for drug delivery. The pill, which is being developed by Rani Therapeutics, protects the biotherapeutic drug payload from digestion in the GI tract and auto-injects it into the wall of the small intestine, according to a report of two studies that demonstrated the safety and tolerability of the robotic pill in healthy humans.
In an animal study, the same researchers showed that delivering monoclonal antibodies with the robotic pill achieved bioavailability on par with that obtained by standard subcutaneous injections.
Identifying “super responders” who require less frequent dosing of medication. “There’s data to suggest that we can kind of back off treatment in these patients,” Dr. Blauvelt said.
Hitting treatment hard and early. “There’s a concept in medicine of hitting disease hard and hitting it early, before the disease can establish itself and cause damage,” he said.
Targeting tissue resident memory T cells. In psoriasis, the idea is that if you treat earlier, when patients are just diagnosed, “perhaps you might be able to decrease resident memory T cells that set up shop in the skin and are responsible for disease recurrences,” Dr. Blauvelt said. “Research has shown that IL-23 blockers decrease tissue resident memory T cells, and IL-17 blockers don’t. This could explain why we see long remissions in this class of drug because we’re getting at these resident memory T cells and knocking them down,” he explained. “Our hypothesis is that hitting hard and early in the treatment course with high-dose IL-23 blockade may be an effective strategy to induce long-term remissions and possible cure, what we call ‘knock-out therapy.’ ”
In a pilot study of 20 patients, Dr. Blauvelt and colleagues are evaluating whether higher initial doses of the IL-23 antagonist risankizumab (300 mg and 600 mg, 2 times and 4 times the standard initial doses for plaque psoriasis) can more effectively target resident memory T cells. “This involves dosing at weeks 0, 4, and 16, then stopping and measuring resident T cells in the tissue to see how long we can induce psoriasis remissions,” Dr. Blauvelt said.
“I have no data to share, but I think we have the potential for unprecedented PASI-100 numbers with no added safety concerns, and the potential to break away from established regular dosing patterns,” such as the possibility of yearly dosing, the possibility of long-term remissions, and the possibility of cure in some patients, he noted.
Inducing tolerance. This refers to efforts aimed at increasing regulatory T cells, which are natural T cells that calm inflammation. He described it as “revving up our natural anti-inflammatory T cells to help balance the immune system.”
Gene editing. This involves using CRISPR gene editing technology to cut genes as a way to cure disease. “What if we cut the IL-23 receptor?” Dr. Blauvelt asked. “You would get rid of that whole signaling pathway. Would the patient be fine?”
In an interview a the meeting, Linda Stein Gold, MD, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit, said that Dr. Blauvelt “has a very exciting view” of the future of psoriasis treatments. “I think that some of it will come true; we’ll have to see which,” Dr. Stein Gold said. “The idea that we might be able to change the trajectory of disease by being aggressive upfront, and possibly modify the course, is exciting. That would be a wonderful new treatment approach.”
Dr. Blauvelt disclosed ties with AbbVie, Abcentra, Affibody, Aligos, Almirall, Alumis, Amgen, AnaptysBio, Arcutis, Arena, ASLAN Pharma, Athenex, Bluefin, Boehringer Ingelheim, Bristol Myers Squibb, Cara, Dermavant, EcoR1, Escient, Evelo, Evommune, Forte, Galderma, Highlightll, Incyte, Innovent Bio, Janssen, Landos, Leo, Lilly, Merck, Novartis, Pfizer, Rapt, Regeneron, Sanofi-Genzyme, Spherix, Sun Pharmaceuticals Industries, TLL Pharmaceutical, TrialSpark, UCB Pharma, Vibliome, and Xencor.
Dr. Stein Gold disclosed ties with Almirall, Cutera, Dermata, Galderma, Novartis, Ortho Dermatologics, and Sun Pharmaceutical Industries, Ltd.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPE LIVE! HAWAII DERMATOLOGY SEMINAR
How to get started with prescribing and advising on CGM
Continuous glucose monitoring (CGM) is gaining ground with both patients and providers because of an array of driving forces, including broadening eligibility, insulin price caps, public awareness, and an increasing number of educational initiatives for doctors.
While professional organizations aim to familiarize doctors with this relatively new technology, more patients are learning independently that finger sticks may be optional, leading them to request CGM from their provider, according to Neil Skolnik, MD.
“We in primary care are being shepherded into this space by our patients who have seen an advertisement or talked to a friend about the benefits of CGM, and then asked us to prescribe it,” said Dr. Skolnik, professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health.
Systemic factors are also accelerating CGM uptake, he added, highlighting recent Medicare rule changes to expand eligibility, with insurance companies beginning to follow suit.
Warren A. Jones, MD, FAAFP, professor emeritus at the University of Mississippi, Jackson, and past president of the AAFP, said that insulin price regulations have also opened doors to CGM.
“When you had patients trying to determine whether they were going to buy food or pay for high-priced insulin, that was a big challenge,” Dr. Jones said in an interview. “But that barrier has recently been removed, so we’re at the dawn of a new era.”
Like any paradigm shift, however,
Overview of online resources and navigating coverage
The latest learning resource on CGM for physicians comes from the American Academy of Family Physicians in the form of a new online educational hub with a 2-credit, ACCME-accredited course. It offers comprehensive guidance for employing CGM in daily practice. Topics include both medical and practical considerations, from interpretation of curves and glucose goal-setting to choosing a device and navigating coverage.
The AAFP’s new offering joins a growing number of similar educational efforts launched over the past few years by the Association of Diabetes Care & Education Specialists, the American Pharmacists Association, the American Diabetes Association, and the American Association of Clinical Endocrinologists.
Checking for coverage is a key first step when considering CGM for a particular patient, Dr. Jones said, noting that CGM, like any new form of care, presents unique challenges with coding and claims that must be overcome to get reimbursed.
“No margin, no mission,” Dr. Jones said. “If you are not able to pay your bills, you can’t be available for your patients. Our goal at the AAFP is to make sure that physicians get this knowledge [about reimbursement].”
To this end, the AAFP’s new online educational hub and the guide provided by APhA present CGM eligibility criteria for various patient groups, including those with Medicare, Medicaid, private insurance, and without coverage.
Medicare criteria include a diagnosis of diabetes, treatment with three or more daily administrations of insulin or continuous infusion via a pump, frequent adjustment to insulin treatment based on glucose readings, and presentation for diabetes in the past 6 months.
Once these requirements are clearly documented in the patient’s record, providers need to write the script, complete a certificate of medical necessity, and choose a supplier. Medicare covers CGM as a durable medical equipment benefit instead of a pharmacy benefit, according to the AAFP and APhA.
Exact coverage criteria and reimbursement processes for non-Medicare patients follow similar paths, although details vary by state and insurer, so personalized investigation is required.
When exploring coverage, the AAFP recommends paying attention to information needed for prior authorization, the patient’s diabetes type and age, and other medical requirements, such as minimum number of daily finger sticks or insulin doses per day.
Looking ahead, Dr. Jones predicted that authorization obstacles stemming from short-term cost concerns are going to fade as long-term savings are uncovered.
“I think pharmacy benefit managers and payers are going to recognize that we have better patient compliance, and that continuous glucose monitoring is going to bring the cost of care down and decrease the rate of hospitalizations,” Dr. Jones said. “So I think they’re going to be willing to pay clinicians to engage in this more readily over time.”
Patients who fail to qualify for personal CGM can still benefit from professional CGM, in which they borrow necessary equipment on a short-term basis. This avenue typically requires minimal or no insurance authorization. In addition, providers have the “opportunity to cover/exceed expenses by enhancing revenue with separately billable procedures, which can be billed in addition to [evaluation and management] if done on same day,” according to the AAFP guide, which goes on to provide appropriate codes.
Learning CGM through first-hand experience
Getting started with CGM can be intimidating for providers, Dr. Skolnik said, although he offered some reassurance, suggesting that the learning process may be more forgiving than prescribing a new drug for the first time.
“I think the best way to figure out CGM is to prescribe it to a couple of patients and learn with them,” Dr. Skolnik said. “You can’t do that with medicines. With medicines, you need to know what you’re doing before you choose who to give a medicine to.”
Instead of “reading everything under the sun” about CGM, he recommends starting with several of the ADA’s resources focusing on time in range, including an article, webinar, and podcast.
After that, physicians can learn on the job. A beginner’s mindset to CGM is well received by patients, he said, especially if you share your natural curiosity with them.
“Share your patients’ wonder at what they see,” Dr. Skolnik said. “They’ll open the app and you’ll look at their time and range and together you’ll go, ‘Wow, isn’t that something? I wonder why?’ ”
With this approach, providers and patients can join forces to explore trends and troubleshoot anomalous readings.
“Together you’ll go: ‘Hmm, I wonder why on Thursday, that graph is looking so far off from the other days? Wow. And then the patient remembers: they ate out on Thursday. They had a big pasta meal, perhaps. Everyone’s different in how they respond to different carbs. And you’ll both have this epiphany together about: ‘Wow, what I do matters.’ And I think that’s actually the best way to jump in.”
According to the AAFP, ADCES, and APhA resources, providers should first address time below range, as hypoglycemia can be imminently dangerous.
Next, providers should consider time in range, average glucose, and glucose management indicator, the latter of which acts as a surrogate for HbA1c. The first couple weeks of monitoring should be viewed as an information gathering phase, after which specific targets can be addressed through behavioral modifications and insulin adjustments, the AAFP advises.
The ADA guide highlights CGM usage, glucose variability, time in range, time above range, and average glucose as key metrics to monitor and offers corresponding actions when targets are unmet.
Encouraging patients to start CGM
Like providers, patients may also be intimidated by CGM, Dr. Jones said, typically because they don’t know how it works, or it seems complicated. Fortunately, he said, these fears are easily overcome when patients learn that they don’t need to stick themselves, record any of their readings, or really do anything at all for the first few weeks.
“You don’t even worry about it,” Dr. Jones tells his patients, who typically feel “more in control and engaged in their own care” after experiencing CGM for themselves.
Dr. Jones speaks from both professional and personal experience. A member of his family recently started CGM after being discharged from the hospital, and the benefits have been significant for everyone involved.
“I see how effectively we can control [my family member’s] blood pressure and insulin requirements, as opposed to several months ago when we didn’t have it,” Dr. Jones said. “So I’m giving it to you from two perspectives: one, of the clinician who knows, intellectually, what should go on, and two, experientially, from a family trying to take care of someone they love.”
Dr. Skolnik disclosed relationships with AstraZeneca, Teva, Lilly, Boehringer Ingelheim, Sanofi, GSK, Bayer, Genentech, Abbott, Idorsia, Merck, Novartis, Heartland, and Novo Nordisk. Dr Jones disclosed no relevant conflicts of interest.
Continuous glucose monitoring (CGM) is gaining ground with both patients and providers because of an array of driving forces, including broadening eligibility, insulin price caps, public awareness, and an increasing number of educational initiatives for doctors.
While professional organizations aim to familiarize doctors with this relatively new technology, more patients are learning independently that finger sticks may be optional, leading them to request CGM from their provider, according to Neil Skolnik, MD.
“We in primary care are being shepherded into this space by our patients who have seen an advertisement or talked to a friend about the benefits of CGM, and then asked us to prescribe it,” said Dr. Skolnik, professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health.
Systemic factors are also accelerating CGM uptake, he added, highlighting recent Medicare rule changes to expand eligibility, with insurance companies beginning to follow suit.
Warren A. Jones, MD, FAAFP, professor emeritus at the University of Mississippi, Jackson, and past president of the AAFP, said that insulin price regulations have also opened doors to CGM.
“When you had patients trying to determine whether they were going to buy food or pay for high-priced insulin, that was a big challenge,” Dr. Jones said in an interview. “But that barrier has recently been removed, so we’re at the dawn of a new era.”
Like any paradigm shift, however,
Overview of online resources and navigating coverage
The latest learning resource on CGM for physicians comes from the American Academy of Family Physicians in the form of a new online educational hub with a 2-credit, ACCME-accredited course. It offers comprehensive guidance for employing CGM in daily practice. Topics include both medical and practical considerations, from interpretation of curves and glucose goal-setting to choosing a device and navigating coverage.
The AAFP’s new offering joins a growing number of similar educational efforts launched over the past few years by the Association of Diabetes Care & Education Specialists, the American Pharmacists Association, the American Diabetes Association, and the American Association of Clinical Endocrinologists.
Checking for coverage is a key first step when considering CGM for a particular patient, Dr. Jones said, noting that CGM, like any new form of care, presents unique challenges with coding and claims that must be overcome to get reimbursed.
“No margin, no mission,” Dr. Jones said. “If you are not able to pay your bills, you can’t be available for your patients. Our goal at the AAFP is to make sure that physicians get this knowledge [about reimbursement].”
To this end, the AAFP’s new online educational hub and the guide provided by APhA present CGM eligibility criteria for various patient groups, including those with Medicare, Medicaid, private insurance, and without coverage.
Medicare criteria include a diagnosis of diabetes, treatment with three or more daily administrations of insulin or continuous infusion via a pump, frequent adjustment to insulin treatment based on glucose readings, and presentation for diabetes in the past 6 months.
Once these requirements are clearly documented in the patient’s record, providers need to write the script, complete a certificate of medical necessity, and choose a supplier. Medicare covers CGM as a durable medical equipment benefit instead of a pharmacy benefit, according to the AAFP and APhA.
Exact coverage criteria and reimbursement processes for non-Medicare patients follow similar paths, although details vary by state and insurer, so personalized investigation is required.
When exploring coverage, the AAFP recommends paying attention to information needed for prior authorization, the patient’s diabetes type and age, and other medical requirements, such as minimum number of daily finger sticks or insulin doses per day.
Looking ahead, Dr. Jones predicted that authorization obstacles stemming from short-term cost concerns are going to fade as long-term savings are uncovered.
“I think pharmacy benefit managers and payers are going to recognize that we have better patient compliance, and that continuous glucose monitoring is going to bring the cost of care down and decrease the rate of hospitalizations,” Dr. Jones said. “So I think they’re going to be willing to pay clinicians to engage in this more readily over time.”
Patients who fail to qualify for personal CGM can still benefit from professional CGM, in which they borrow necessary equipment on a short-term basis. This avenue typically requires minimal or no insurance authorization. In addition, providers have the “opportunity to cover/exceed expenses by enhancing revenue with separately billable procedures, which can be billed in addition to [evaluation and management] if done on same day,” according to the AAFP guide, which goes on to provide appropriate codes.
Learning CGM through first-hand experience
Getting started with CGM can be intimidating for providers, Dr. Skolnik said, although he offered some reassurance, suggesting that the learning process may be more forgiving than prescribing a new drug for the first time.
“I think the best way to figure out CGM is to prescribe it to a couple of patients and learn with them,” Dr. Skolnik said. “You can’t do that with medicines. With medicines, you need to know what you’re doing before you choose who to give a medicine to.”
Instead of “reading everything under the sun” about CGM, he recommends starting with several of the ADA’s resources focusing on time in range, including an article, webinar, and podcast.
After that, physicians can learn on the job. A beginner’s mindset to CGM is well received by patients, he said, especially if you share your natural curiosity with them.
“Share your patients’ wonder at what they see,” Dr. Skolnik said. “They’ll open the app and you’ll look at their time and range and together you’ll go, ‘Wow, isn’t that something? I wonder why?’ ”
With this approach, providers and patients can join forces to explore trends and troubleshoot anomalous readings.
“Together you’ll go: ‘Hmm, I wonder why on Thursday, that graph is looking so far off from the other days? Wow. And then the patient remembers: they ate out on Thursday. They had a big pasta meal, perhaps. Everyone’s different in how they respond to different carbs. And you’ll both have this epiphany together about: ‘Wow, what I do matters.’ And I think that’s actually the best way to jump in.”
According to the AAFP, ADCES, and APhA resources, providers should first address time below range, as hypoglycemia can be imminently dangerous.
Next, providers should consider time in range, average glucose, and glucose management indicator, the latter of which acts as a surrogate for HbA1c. The first couple weeks of monitoring should be viewed as an information gathering phase, after which specific targets can be addressed through behavioral modifications and insulin adjustments, the AAFP advises.
The ADA guide highlights CGM usage, glucose variability, time in range, time above range, and average glucose as key metrics to monitor and offers corresponding actions when targets are unmet.
Encouraging patients to start CGM
Like providers, patients may also be intimidated by CGM, Dr. Jones said, typically because they don’t know how it works, or it seems complicated. Fortunately, he said, these fears are easily overcome when patients learn that they don’t need to stick themselves, record any of their readings, or really do anything at all for the first few weeks.
“You don’t even worry about it,” Dr. Jones tells his patients, who typically feel “more in control and engaged in their own care” after experiencing CGM for themselves.
Dr. Jones speaks from both professional and personal experience. A member of his family recently started CGM after being discharged from the hospital, and the benefits have been significant for everyone involved.
“I see how effectively we can control [my family member’s] blood pressure and insulin requirements, as opposed to several months ago when we didn’t have it,” Dr. Jones said. “So I’m giving it to you from two perspectives: one, of the clinician who knows, intellectually, what should go on, and two, experientially, from a family trying to take care of someone they love.”
Dr. Skolnik disclosed relationships with AstraZeneca, Teva, Lilly, Boehringer Ingelheim, Sanofi, GSK, Bayer, Genentech, Abbott, Idorsia, Merck, Novartis, Heartland, and Novo Nordisk. Dr Jones disclosed no relevant conflicts of interest.
Continuous glucose monitoring (CGM) is gaining ground with both patients and providers because of an array of driving forces, including broadening eligibility, insulin price caps, public awareness, and an increasing number of educational initiatives for doctors.
While professional organizations aim to familiarize doctors with this relatively new technology, more patients are learning independently that finger sticks may be optional, leading them to request CGM from their provider, according to Neil Skolnik, MD.
“We in primary care are being shepherded into this space by our patients who have seen an advertisement or talked to a friend about the benefits of CGM, and then asked us to prescribe it,” said Dr. Skolnik, professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health.
Systemic factors are also accelerating CGM uptake, he added, highlighting recent Medicare rule changes to expand eligibility, with insurance companies beginning to follow suit.
Warren A. Jones, MD, FAAFP, professor emeritus at the University of Mississippi, Jackson, and past president of the AAFP, said that insulin price regulations have also opened doors to CGM.
“When you had patients trying to determine whether they were going to buy food or pay for high-priced insulin, that was a big challenge,” Dr. Jones said in an interview. “But that barrier has recently been removed, so we’re at the dawn of a new era.”
Like any paradigm shift, however,
Overview of online resources and navigating coverage
The latest learning resource on CGM for physicians comes from the American Academy of Family Physicians in the form of a new online educational hub with a 2-credit, ACCME-accredited course. It offers comprehensive guidance for employing CGM in daily practice. Topics include both medical and practical considerations, from interpretation of curves and glucose goal-setting to choosing a device and navigating coverage.
The AAFP’s new offering joins a growing number of similar educational efforts launched over the past few years by the Association of Diabetes Care & Education Specialists, the American Pharmacists Association, the American Diabetes Association, and the American Association of Clinical Endocrinologists.
Checking for coverage is a key first step when considering CGM for a particular patient, Dr. Jones said, noting that CGM, like any new form of care, presents unique challenges with coding and claims that must be overcome to get reimbursed.
“No margin, no mission,” Dr. Jones said. “If you are not able to pay your bills, you can’t be available for your patients. Our goal at the AAFP is to make sure that physicians get this knowledge [about reimbursement].”
To this end, the AAFP’s new online educational hub and the guide provided by APhA present CGM eligibility criteria for various patient groups, including those with Medicare, Medicaid, private insurance, and without coverage.
Medicare criteria include a diagnosis of diabetes, treatment with three or more daily administrations of insulin or continuous infusion via a pump, frequent adjustment to insulin treatment based on glucose readings, and presentation for diabetes in the past 6 months.
Once these requirements are clearly documented in the patient’s record, providers need to write the script, complete a certificate of medical necessity, and choose a supplier. Medicare covers CGM as a durable medical equipment benefit instead of a pharmacy benefit, according to the AAFP and APhA.
Exact coverage criteria and reimbursement processes for non-Medicare patients follow similar paths, although details vary by state and insurer, so personalized investigation is required.
When exploring coverage, the AAFP recommends paying attention to information needed for prior authorization, the patient’s diabetes type and age, and other medical requirements, such as minimum number of daily finger sticks or insulin doses per day.
Looking ahead, Dr. Jones predicted that authorization obstacles stemming from short-term cost concerns are going to fade as long-term savings are uncovered.
“I think pharmacy benefit managers and payers are going to recognize that we have better patient compliance, and that continuous glucose monitoring is going to bring the cost of care down and decrease the rate of hospitalizations,” Dr. Jones said. “So I think they’re going to be willing to pay clinicians to engage in this more readily over time.”
Patients who fail to qualify for personal CGM can still benefit from professional CGM, in which they borrow necessary equipment on a short-term basis. This avenue typically requires minimal or no insurance authorization. In addition, providers have the “opportunity to cover/exceed expenses by enhancing revenue with separately billable procedures, which can be billed in addition to [evaluation and management] if done on same day,” according to the AAFP guide, which goes on to provide appropriate codes.
Learning CGM through first-hand experience
Getting started with CGM can be intimidating for providers, Dr. Skolnik said, although he offered some reassurance, suggesting that the learning process may be more forgiving than prescribing a new drug for the first time.
“I think the best way to figure out CGM is to prescribe it to a couple of patients and learn with them,” Dr. Skolnik said. “You can’t do that with medicines. With medicines, you need to know what you’re doing before you choose who to give a medicine to.”
Instead of “reading everything under the sun” about CGM, he recommends starting with several of the ADA’s resources focusing on time in range, including an article, webinar, and podcast.
After that, physicians can learn on the job. A beginner’s mindset to CGM is well received by patients, he said, especially if you share your natural curiosity with them.
“Share your patients’ wonder at what they see,” Dr. Skolnik said. “They’ll open the app and you’ll look at their time and range and together you’ll go, ‘Wow, isn’t that something? I wonder why?’ ”
With this approach, providers and patients can join forces to explore trends and troubleshoot anomalous readings.
“Together you’ll go: ‘Hmm, I wonder why on Thursday, that graph is looking so far off from the other days? Wow. And then the patient remembers: they ate out on Thursday. They had a big pasta meal, perhaps. Everyone’s different in how they respond to different carbs. And you’ll both have this epiphany together about: ‘Wow, what I do matters.’ And I think that’s actually the best way to jump in.”
According to the AAFP, ADCES, and APhA resources, providers should first address time below range, as hypoglycemia can be imminently dangerous.
Next, providers should consider time in range, average glucose, and glucose management indicator, the latter of which acts as a surrogate for HbA1c. The first couple weeks of monitoring should be viewed as an information gathering phase, after which specific targets can be addressed through behavioral modifications and insulin adjustments, the AAFP advises.
The ADA guide highlights CGM usage, glucose variability, time in range, time above range, and average glucose as key metrics to monitor and offers corresponding actions when targets are unmet.
Encouraging patients to start CGM
Like providers, patients may also be intimidated by CGM, Dr. Jones said, typically because they don’t know how it works, or it seems complicated. Fortunately, he said, these fears are easily overcome when patients learn that they don’t need to stick themselves, record any of their readings, or really do anything at all for the first few weeks.
“You don’t even worry about it,” Dr. Jones tells his patients, who typically feel “more in control and engaged in their own care” after experiencing CGM for themselves.
Dr. Jones speaks from both professional and personal experience. A member of his family recently started CGM after being discharged from the hospital, and the benefits have been significant for everyone involved.
“I see how effectively we can control [my family member’s] blood pressure and insulin requirements, as opposed to several months ago when we didn’t have it,” Dr. Jones said. “So I’m giving it to you from two perspectives: one, of the clinician who knows, intellectually, what should go on, and two, experientially, from a family trying to take care of someone they love.”
Dr. Skolnik disclosed relationships with AstraZeneca, Teva, Lilly, Boehringer Ingelheim, Sanofi, GSK, Bayer, Genentech, Abbott, Idorsia, Merck, Novartis, Heartland, and Novo Nordisk. Dr Jones disclosed no relevant conflicts of interest.
Chronic Erythematous Plaques Around the Ears
The Diagnosis: Discoid Lupus Erythematosus
The biopsies demonstrated vacuolar interface changes with superficial and deep perivascular and periadnexal inflammation as well as increased background mucin deposition. The clinical morphology and distributions of the plaques limited to the photoexposed areas of the head suggested a diagnosis of discoid lupus erythematosus (DLE). The interface changes on histopathology supported this clinical impression. Our patient was treated with limited application of triamcinolone ointment 0.1% twice daily around the ears and neck, tacrolimus ointment 0.1% twice daily on the face, and hydroxychloroquine, as well as sun protection instructions. Smoking cessation was strongly advised.
Discoid lupus erythematosus is a disorder with chronic, erythematous, scaly, coin-shaped (discoid) plaques and is the most common form of chronic cutaneous lupus erythematosus.1 Lesions usually present on sun-exposed areas of the face, scalp, neck, ears, lips, or upper torso. They expand slowly with an active peripheral margin and a central scar that can result in induration, pigmentation changes, telangiectases, pruritus, or tenderness. Hair-bearing areas may be involved, causing hair loss due to follicular plugging; irreversible scarring alopecia can result. Facial DLE often spares the nasolabial folds. Ear involvement characteristically includes the conchal bowl and the outer external auditory canal. Discoid lupus erythematosus is considered localized if most of the head and neck region is involved or generalized if lesions also are present below the neck. Risk factors for DLE include genetic and environmental factors such as UV exposure, hormones, or exposure to toxins such as cigarette smoke.1 The disorder most commonly affects females and has a higher prevalence in patients of African descent than in Asian and White patients. Disease can occur at any age but usually occurs between 20 and 40 years of age.2 Discoid lupus erythematosus and other forms of chronic cutaneous lupus can occur independently or in conjunction with systemic lupus erythematosus (SLE), and approximately 15% to 30% of SLE patients develop DLE.1
Discoid lupus erythematosus is clinically diagnosed by the presentation of plaques in the characteristic distribution with confirmation via skin biopsy.1 Elman et al3 created a system for DLE classification that was only clinical and did not involve histopathology. Histologically, DLE often includes basement membrane thickening, follicular keratin plugs, mucin deposition, and vacuolar change with an interface, and a perivascular and periadnexal lymphocytic infiltrate.3,4 Antibodies such as antinuclear antibodies, rheumatoid factor, anti–double-stranded DNA, anti-Smith, and Sjögren syndrome A and B antibodies may be present (albeit with low positive frequency) in cutaneous lupus erythematosus.4 Characteristics of SLE also may be present, helping to confirm the diagnosis. Because there is an association of DLE with SLE, various laboratory tests should be ordered, including complete blood cell count, renal function panel, inflammatory markers, antibodies, and urinalysis for proteinuria.2,4
Treatment of DLE consists of preventative measures, such as sun protection with vitamin D supplementation, avoidance of drug triggers, and smoking cessation, as well as pharmacotherapy. The importance of wearing sun protective hats and garments with sunscreen use cannot be understated.1 Smoking cessation should be advised because smoking reduces the efficacy of antimalarial treatment and potentially increases the likelihood of patients requiring a second antimalarial drug. Quinacrine often is noted in both the dermatology and rheumatology literature to be used for escalating cutaneous lupus erythematosus care when hydroxychloroquine is ineffective or not tolerated, but no US manufacturer produces this medication; thus, compounding is required, which may be financially prohibitive, making this recommendation difficult to translate into clinical practice.5 Firstline therapy for acute flares is high-potency topical corticosteroids. If lesions are primarily on areas other than the face, a medium-potency topical steroid may be used. Topical calcineurin inhibitors or intralesional corticosteroids may be used if minimal improvement is seen after initial topical corticosteroid therapy. Treatment for widespread disease or disease that is resistant to local treatment is systemic therapy with antimalarial agents, followed by antimetabolites, systemic retinoids, thalidomide, or dapsone.1,2 The Cutaneous Lupus Erythematosus Disease Area and Severity Index is a valid tool to gauge the degree of disease and to help with disease progression and treatment response by noting the features of the plaques.1
Patients also should be educated that this disease can last for years, and long-standing DLE plaques infrequently can give rise to squamous cell carcinoma. In addition, isolated DLE can progress to SLE in 5% to 28% of patients.2
The differential diagnosis in our patient included other diseases with violaceous annular lesions and central clearing. Majocchi granuloma usually presents in areas of prior trauma, possibly due to shaving the face in our patient, or in the setting of topical corticosteroid use or immunosuppression. Scaling often is present within lesions, and histology shows fungal elements.6 Cutaneous sarcoidosis usually presents on the face, with scarring alopecia when appearing on the scalp; histology shows noncaseating granulomas, and 70% of patients with cutaneous symptoms will have systemic sarcoidosis.7 Granuloma annulare most commonly presents on the extremities, and histology shows lymphohistiocytic granulomas in a palisaded or interstitial pattern with connective-tissue degeneration and mucinous deposits.8 Annular psoriasis often is scaly and symmetric with parakeratosis, epidermal hyperplasia, dilated dermal capillaries, loss of granular layer, perivascular mononuclear cell infiltrate, and elongation of rete ridges on histology.9 Drug-induced lupus erythematosus always should be considered in patients taking triggering drugs such as antihypertensives, lipid-lowering drugs, antifungals, anti–tumor necrosis factor drugs, and proton pump inhibitors—the latter being a drug our patient was taking.10
- Sontheimer CJ, Costner MI, Sontheimer RD. Lupus erythematosus. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:1037-1060.
- Lee KC. Discoid lupus. In: Ferri FF, ed. Ferri’s Clinical Advisor 2021. Elsevier; 2021:477.e15-477.e18.
- Elman SA, Joyce C, Braudis K, et al. Creation and validation of classification criteria for discoid lupus erythematosus. JAMA Dermatol. 2020;156:901-906. doi:10.1001/jamadermatol.2020.1698
- Patel P, Werth V. Cutaneous lupus erythematosus: a review. Dermatol Clin. 2002;20:373-385, v. doi:10.1016/s0733-8635(02)00016-5
- Mittal L, Werth VP. The quinacrine experience in a population of patients with cutaneous lupus erythematosus and dermatomyositis. J Am Acad Dermatol. 2017;77:374-377. doi:10.1016/j .jaad.2017.03.027
- Craddock LN, Schieke SM. Superficial fungal infection. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:2925-2951.
- Tan J, Vleugels R. Dermatologic findings in systemic disease. In: McKean S, Dressler D, Ross J, et al, eds. Principles and Practice of Hospital Medicine. 2nd ed. McGraw Hill; 2017:1145-1170.
- Prendiville JS. Granuloma annulare. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:564-571.
- Gudjonsson JE, Elder JT. Psoriasis. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:457-497.
- He Y, Sawalha AH. Drug-induced lupus erythematosus: an update on drugs and mechanisms. Curr Opin Rheumatol. 2018;30:490-497. doi:10.1097/BOR.0000000000000522
The Diagnosis: Discoid Lupus Erythematosus
The biopsies demonstrated vacuolar interface changes with superficial and deep perivascular and periadnexal inflammation as well as increased background mucin deposition. The clinical morphology and distributions of the plaques limited to the photoexposed areas of the head suggested a diagnosis of discoid lupus erythematosus (DLE). The interface changes on histopathology supported this clinical impression. Our patient was treated with limited application of triamcinolone ointment 0.1% twice daily around the ears and neck, tacrolimus ointment 0.1% twice daily on the face, and hydroxychloroquine, as well as sun protection instructions. Smoking cessation was strongly advised.
Discoid lupus erythematosus is a disorder with chronic, erythematous, scaly, coin-shaped (discoid) plaques and is the most common form of chronic cutaneous lupus erythematosus.1 Lesions usually present on sun-exposed areas of the face, scalp, neck, ears, lips, or upper torso. They expand slowly with an active peripheral margin and a central scar that can result in induration, pigmentation changes, telangiectases, pruritus, or tenderness. Hair-bearing areas may be involved, causing hair loss due to follicular plugging; irreversible scarring alopecia can result. Facial DLE often spares the nasolabial folds. Ear involvement characteristically includes the conchal bowl and the outer external auditory canal. Discoid lupus erythematosus is considered localized if most of the head and neck region is involved or generalized if lesions also are present below the neck. Risk factors for DLE include genetic and environmental factors such as UV exposure, hormones, or exposure to toxins such as cigarette smoke.1 The disorder most commonly affects females and has a higher prevalence in patients of African descent than in Asian and White patients. Disease can occur at any age but usually occurs between 20 and 40 years of age.2 Discoid lupus erythematosus and other forms of chronic cutaneous lupus can occur independently or in conjunction with systemic lupus erythematosus (SLE), and approximately 15% to 30% of SLE patients develop DLE.1
Discoid lupus erythematosus is clinically diagnosed by the presentation of plaques in the characteristic distribution with confirmation via skin biopsy.1 Elman et al3 created a system for DLE classification that was only clinical and did not involve histopathology. Histologically, DLE often includes basement membrane thickening, follicular keratin plugs, mucin deposition, and vacuolar change with an interface, and a perivascular and periadnexal lymphocytic infiltrate.3,4 Antibodies such as antinuclear antibodies, rheumatoid factor, anti–double-stranded DNA, anti-Smith, and Sjögren syndrome A and B antibodies may be present (albeit with low positive frequency) in cutaneous lupus erythematosus.4 Characteristics of SLE also may be present, helping to confirm the diagnosis. Because there is an association of DLE with SLE, various laboratory tests should be ordered, including complete blood cell count, renal function panel, inflammatory markers, antibodies, and urinalysis for proteinuria.2,4
Treatment of DLE consists of preventative measures, such as sun protection with vitamin D supplementation, avoidance of drug triggers, and smoking cessation, as well as pharmacotherapy. The importance of wearing sun protective hats and garments with sunscreen use cannot be understated.1 Smoking cessation should be advised because smoking reduces the efficacy of antimalarial treatment and potentially increases the likelihood of patients requiring a second antimalarial drug. Quinacrine often is noted in both the dermatology and rheumatology literature to be used for escalating cutaneous lupus erythematosus care when hydroxychloroquine is ineffective or not tolerated, but no US manufacturer produces this medication; thus, compounding is required, which may be financially prohibitive, making this recommendation difficult to translate into clinical practice.5 Firstline therapy for acute flares is high-potency topical corticosteroids. If lesions are primarily on areas other than the face, a medium-potency topical steroid may be used. Topical calcineurin inhibitors or intralesional corticosteroids may be used if minimal improvement is seen after initial topical corticosteroid therapy. Treatment for widespread disease or disease that is resistant to local treatment is systemic therapy with antimalarial agents, followed by antimetabolites, systemic retinoids, thalidomide, or dapsone.1,2 The Cutaneous Lupus Erythematosus Disease Area and Severity Index is a valid tool to gauge the degree of disease and to help with disease progression and treatment response by noting the features of the plaques.1
Patients also should be educated that this disease can last for years, and long-standing DLE plaques infrequently can give rise to squamous cell carcinoma. In addition, isolated DLE can progress to SLE in 5% to 28% of patients.2
The differential diagnosis in our patient included other diseases with violaceous annular lesions and central clearing. Majocchi granuloma usually presents in areas of prior trauma, possibly due to shaving the face in our patient, or in the setting of topical corticosteroid use or immunosuppression. Scaling often is present within lesions, and histology shows fungal elements.6 Cutaneous sarcoidosis usually presents on the face, with scarring alopecia when appearing on the scalp; histology shows noncaseating granulomas, and 70% of patients with cutaneous symptoms will have systemic sarcoidosis.7 Granuloma annulare most commonly presents on the extremities, and histology shows lymphohistiocytic granulomas in a palisaded or interstitial pattern with connective-tissue degeneration and mucinous deposits.8 Annular psoriasis often is scaly and symmetric with parakeratosis, epidermal hyperplasia, dilated dermal capillaries, loss of granular layer, perivascular mononuclear cell infiltrate, and elongation of rete ridges on histology.9 Drug-induced lupus erythematosus always should be considered in patients taking triggering drugs such as antihypertensives, lipid-lowering drugs, antifungals, anti–tumor necrosis factor drugs, and proton pump inhibitors—the latter being a drug our patient was taking.10
The Diagnosis: Discoid Lupus Erythematosus
The biopsies demonstrated vacuolar interface changes with superficial and deep perivascular and periadnexal inflammation as well as increased background mucin deposition. The clinical morphology and distributions of the plaques limited to the photoexposed areas of the head suggested a diagnosis of discoid lupus erythematosus (DLE). The interface changes on histopathology supported this clinical impression. Our patient was treated with limited application of triamcinolone ointment 0.1% twice daily around the ears and neck, tacrolimus ointment 0.1% twice daily on the face, and hydroxychloroquine, as well as sun protection instructions. Smoking cessation was strongly advised.
Discoid lupus erythematosus is a disorder with chronic, erythematous, scaly, coin-shaped (discoid) plaques and is the most common form of chronic cutaneous lupus erythematosus.1 Lesions usually present on sun-exposed areas of the face, scalp, neck, ears, lips, or upper torso. They expand slowly with an active peripheral margin and a central scar that can result in induration, pigmentation changes, telangiectases, pruritus, or tenderness. Hair-bearing areas may be involved, causing hair loss due to follicular plugging; irreversible scarring alopecia can result. Facial DLE often spares the nasolabial folds. Ear involvement characteristically includes the conchal bowl and the outer external auditory canal. Discoid lupus erythematosus is considered localized if most of the head and neck region is involved or generalized if lesions also are present below the neck. Risk factors for DLE include genetic and environmental factors such as UV exposure, hormones, or exposure to toxins such as cigarette smoke.1 The disorder most commonly affects females and has a higher prevalence in patients of African descent than in Asian and White patients. Disease can occur at any age but usually occurs between 20 and 40 years of age.2 Discoid lupus erythematosus and other forms of chronic cutaneous lupus can occur independently or in conjunction with systemic lupus erythematosus (SLE), and approximately 15% to 30% of SLE patients develop DLE.1
Discoid lupus erythematosus is clinically diagnosed by the presentation of plaques in the characteristic distribution with confirmation via skin biopsy.1 Elman et al3 created a system for DLE classification that was only clinical and did not involve histopathology. Histologically, DLE often includes basement membrane thickening, follicular keratin plugs, mucin deposition, and vacuolar change with an interface, and a perivascular and periadnexal lymphocytic infiltrate.3,4 Antibodies such as antinuclear antibodies, rheumatoid factor, anti–double-stranded DNA, anti-Smith, and Sjögren syndrome A and B antibodies may be present (albeit with low positive frequency) in cutaneous lupus erythematosus.4 Characteristics of SLE also may be present, helping to confirm the diagnosis. Because there is an association of DLE with SLE, various laboratory tests should be ordered, including complete blood cell count, renal function panel, inflammatory markers, antibodies, and urinalysis for proteinuria.2,4
Treatment of DLE consists of preventative measures, such as sun protection with vitamin D supplementation, avoidance of drug triggers, and smoking cessation, as well as pharmacotherapy. The importance of wearing sun protective hats and garments with sunscreen use cannot be understated.1 Smoking cessation should be advised because smoking reduces the efficacy of antimalarial treatment and potentially increases the likelihood of patients requiring a second antimalarial drug. Quinacrine often is noted in both the dermatology and rheumatology literature to be used for escalating cutaneous lupus erythematosus care when hydroxychloroquine is ineffective or not tolerated, but no US manufacturer produces this medication; thus, compounding is required, which may be financially prohibitive, making this recommendation difficult to translate into clinical practice.5 Firstline therapy for acute flares is high-potency topical corticosteroids. If lesions are primarily on areas other than the face, a medium-potency topical steroid may be used. Topical calcineurin inhibitors or intralesional corticosteroids may be used if minimal improvement is seen after initial topical corticosteroid therapy. Treatment for widespread disease or disease that is resistant to local treatment is systemic therapy with antimalarial agents, followed by antimetabolites, systemic retinoids, thalidomide, or dapsone.1,2 The Cutaneous Lupus Erythematosus Disease Area and Severity Index is a valid tool to gauge the degree of disease and to help with disease progression and treatment response by noting the features of the plaques.1
Patients also should be educated that this disease can last for years, and long-standing DLE plaques infrequently can give rise to squamous cell carcinoma. In addition, isolated DLE can progress to SLE in 5% to 28% of patients.2
The differential diagnosis in our patient included other diseases with violaceous annular lesions and central clearing. Majocchi granuloma usually presents in areas of prior trauma, possibly due to shaving the face in our patient, or in the setting of topical corticosteroid use or immunosuppression. Scaling often is present within lesions, and histology shows fungal elements.6 Cutaneous sarcoidosis usually presents on the face, with scarring alopecia when appearing on the scalp; histology shows noncaseating granulomas, and 70% of patients with cutaneous symptoms will have systemic sarcoidosis.7 Granuloma annulare most commonly presents on the extremities, and histology shows lymphohistiocytic granulomas in a palisaded or interstitial pattern with connective-tissue degeneration and mucinous deposits.8 Annular psoriasis often is scaly and symmetric with parakeratosis, epidermal hyperplasia, dilated dermal capillaries, loss of granular layer, perivascular mononuclear cell infiltrate, and elongation of rete ridges on histology.9 Drug-induced lupus erythematosus always should be considered in patients taking triggering drugs such as antihypertensives, lipid-lowering drugs, antifungals, anti–tumor necrosis factor drugs, and proton pump inhibitors—the latter being a drug our patient was taking.10
- Sontheimer CJ, Costner MI, Sontheimer RD. Lupus erythematosus. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:1037-1060.
- Lee KC. Discoid lupus. In: Ferri FF, ed. Ferri’s Clinical Advisor 2021. Elsevier; 2021:477.e15-477.e18.
- Elman SA, Joyce C, Braudis K, et al. Creation and validation of classification criteria for discoid lupus erythematosus. JAMA Dermatol. 2020;156:901-906. doi:10.1001/jamadermatol.2020.1698
- Patel P, Werth V. Cutaneous lupus erythematosus: a review. Dermatol Clin. 2002;20:373-385, v. doi:10.1016/s0733-8635(02)00016-5
- Mittal L, Werth VP. The quinacrine experience in a population of patients with cutaneous lupus erythematosus and dermatomyositis. J Am Acad Dermatol. 2017;77:374-377. doi:10.1016/j .jaad.2017.03.027
- Craddock LN, Schieke SM. Superficial fungal infection. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:2925-2951.
- Tan J, Vleugels R. Dermatologic findings in systemic disease. In: McKean S, Dressler D, Ross J, et al, eds. Principles and Practice of Hospital Medicine. 2nd ed. McGraw Hill; 2017:1145-1170.
- Prendiville JS. Granuloma annulare. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:564-571.
- Gudjonsson JE, Elder JT. Psoriasis. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:457-497.
- He Y, Sawalha AH. Drug-induced lupus erythematosus: an update on drugs and mechanisms. Curr Opin Rheumatol. 2018;30:490-497. doi:10.1097/BOR.0000000000000522
- Sontheimer CJ, Costner MI, Sontheimer RD. Lupus erythematosus. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:1037-1060.
- Lee KC. Discoid lupus. In: Ferri FF, ed. Ferri’s Clinical Advisor 2021. Elsevier; 2021:477.e15-477.e18.
- Elman SA, Joyce C, Braudis K, et al. Creation and validation of classification criteria for discoid lupus erythematosus. JAMA Dermatol. 2020;156:901-906. doi:10.1001/jamadermatol.2020.1698
- Patel P, Werth V. Cutaneous lupus erythematosus: a review. Dermatol Clin. 2002;20:373-385, v. doi:10.1016/s0733-8635(02)00016-5
- Mittal L, Werth VP. The quinacrine experience in a population of patients with cutaneous lupus erythematosus and dermatomyositis. J Am Acad Dermatol. 2017;77:374-377. doi:10.1016/j .jaad.2017.03.027
- Craddock LN, Schieke SM. Superficial fungal infection. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:2925-2951.
- Tan J, Vleugels R. Dermatologic findings in systemic disease. In: McKean S, Dressler D, Ross J, et al, eds. Principles and Practice of Hospital Medicine. 2nd ed. McGraw Hill; 2017:1145-1170.
- Prendiville JS. Granuloma annulare. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:564-571.
- Gudjonsson JE, Elder JT. Psoriasis. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:457-497.
- He Y, Sawalha AH. Drug-induced lupus erythematosus: an update on drugs and mechanisms. Curr Opin Rheumatol. 2018;30:490-497. doi:10.1097/BOR.0000000000000522
A 41-year-old man presented to the dermatology clinic with erythematous, pruritic, and painful plaques around the ears of 6 years’ duration. He reported that application of topical steroids, antifungals, and most recently a topical calcineurin inhibitor did not change the appearance or symptoms. His medical history was notable for tobacco smoking and gastroesophageal reflux disease, for which he was taking omeprazole for the last 3 years. He was unsure if the lesions changed with UV exposure. He was an active-duty US military service member, and his job required frequently working outdoors. A review of systems was otherwise unremarkable. Physical examination revealed annular, erythematous, indurated plaques on both the preauricular and postauricular skin on the left ear with associated central atrophy and hypopigmentation. No alopecia was appreciated. The remainder of the skin examination was unremarkable. Ancillary laboratory test results were notable for a negative antinuclear antibody screen but positive (low titer) for Sjögren syndrome A and B antibodies. Two punch biopsies were performed.
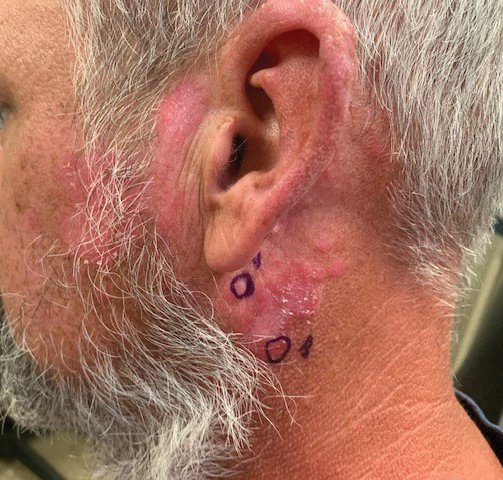
Steak dinners, sales reps, and risky procedures: Inside the big business of clogged arteries
On June 14, 2017, just before noon, a doctor made an incision near a patient’s groin. Kari Kirk, a representative for the world’s largest medical device company, Medtronic, looked on and began texting her colleague a play-by-play.
“Fixing both legs from the ankles,” she wrote.
It was a fairly common procedure at the Robert J. Dole Veterans Affairs Medical Center in Wichita, Kansas, performed to treat blockages in the leg vessels.
Within reach were an array of Medtronic products: tubes with blades attached to shave hardened deposits off of artery walls; stents to widen blood vessels; balloons coated with therapeutic drugs.
Each time a doctor puts a foreign device in someone’s body, it carries a risk of complication, which can include clots or even require amputation. So medical experts, research and even Medtronic’s own device instructions urge doctors to use as few as are necessary.
“Just used 12 [drug-coated balloons]!!” Kirk texted her colleague.
“Does that mean I owe u $$,” he responded.
“Thats what I’m thinking!!!” she said. “And now 14 balloons?!”
“but only one stent so far??”
“So far!”
As the texting continued, her colleague replied, “U are going to want to start going to the VA all the time.”
The messages, recently unsealed in an ongoing whistleblower lawsuit, give a window into the way money and medicine mingle in the booming business of peripheral artery disease, a condition that afflicts 6.5 million Americans over age 40 and is caused when fatty plaque builds up in arteries, blocking blood flow to the legs.
Representatives from companies are often present during vascular procedures to guide doctors on how to use their complex devices. This kind of access has the potential to influence treatment plans, as companies and their representatives profit when more of their product is used.
The suit, filed in 2017 by a sales representative for a competing medical device firm, alleges an illegal kickback scheme between Medtronic and hospital employees. According to the complaint and documents released in the suit, between 2011 and 2018, VA health care workers received steakhouse dinners, Apple electronics, and NASCAR tickets, and in turn, Medtronic secured a lucrative contract with the hospital. Meanwhile, the company’s representatives allegedly “groomed and trained” physicians at the facility, who then deployed the company’s devices even when it was not medically indicated.
Independently from the whistleblower suit, internal investigators at the Wichita facility have also examined the treatment patterns of its vascular patients in recent years and found numerous cases where medical devices were used excessively. While it’s not uncommon to deploy several devices, a medical expert on the investigation team found that the VA doctors sometimes used more than 15 at a time – one used 33 – deviating from the standard of care.
“It is unconscionable – there can be no valid medically acceptable basis to cram so many devices into a human being,” wrote attorneys representing the whistleblower in legal filings from January 2023. “This is not medical treatment. This is abuse.”
Dr. Kim Hodgson, former president of the Society for Vascular Surgery and an expert retained by the plaintiff, said the findings of the internal review of patient data raise “a high level of concern regarding necessity of treatment provided,” according to case documents.
Medtronic declined to respond to ProPublica’s questions, citing the ongoing litigation. “These allegations are false and Medtronic is defending against these claims in court,” said Boua Xiong, a spokesperson for the company. Medtronic representative Kirk declined to respond to ProPublica’s request for comment.
The hospital investigation found that amputations increased sixfold in the same time frame as the procedures in question, according to internal emails, but made no conclusion about whether those two things were connected. ProPublica reached out to the VA to ask whether any patients had been harmed.
The VA is “conducting an extensive review of patient care” at the Kansas hospital, “including the number of devices used on patients – to make sure that Veterans were not harmed by any procedures,” press secretary Terrence Hayes said in a statement. So far, the VA’s investigation has found no “quality of care issues,” he said, and the investigation will continue “until every Veteran’s case has been reviewed.” (Read the full statement here.) Neither the department nor the hospital has taken formal action against the medical providers, Hayes said.
The medical group that had a contract with the VA for vascular interventions, Wichita Radiological Group, did not respond to ProPublica’s requests for comment, nor did the doctors named in the suit: Dr. Shaun Gonda, Dr. Bret Winblad, and Dr. Kermit Rust. It is unclear from the case documents which doctors conducted which procedures. Eric Barth, an attorney for the medical group, denied the allegations in recent legal filings, calling the claims “baseless” and the lawsuit a “witch hunt.”
The lawsuit comes amid growing concern about one of these procedures – atherectomies – after researchers and doctors have uncovered patterns of excessive and inappropriate use. Recent research has found that this procedure, a common but costly treatment to shave or laser plaque from blood vessels, is not more effective than cheaper alternatives and may even be associated with a higher risk of complications including amputation. In recent years, several doctors and clinics have been investigated for allegedly taking advantage of Medicare’s reimbursement rates, and one study found that many doctors are resorting to atherectomies in the earliest stages of peripheral artery disease, against best practices that urge noninvasive treatment.
“Atherectomy is important in certain settings. But it’s being used in a way that is entirely inappropriate and it’s largely driven by the incentive structure,” said Dr. Caitlin Hicks, the lead author of the study and an associate professor of surgery at Johns Hopkins University School of Medicine.
Although different payment structures govern the care of veterans, the whistleblower lawsuit alleges that outside physicians, paid hourly by the Dole VA, were motivated to conduct longer and more complex procedures that would earn them higher payment.
Under different circumstances, the patient in the procedure room on that summer day could have been done after 2 hours.
But, 150 minutes in, those Medtronic representatives were still texting. At that point, more than 15 of their vascular devices had been used, including stents, balloons, and those for atherectomy.
“Long case!” Kirk’s colleague texted. “Is it looking ok??”
“It is,” she said. “Thought we were done a few times! Now he’s going back in to cut again!”
A little while later, she texted: “....17!”
He texted back [with laughing emoticons].
Hospital leaders had been scrutinizing the use of these procedures at the Dole VA for years.
In 2017, shortly after Rick Ament was hired to lead the facility, he noticed something was amiss. While the longtime hospital administrator was poring over the finances, he was alarmed to discover that the relatively small Dole VA had one of the most expensive cardiac programs in the country. As Ament dug deeper, he realized vascular interventions were the reason.
“It just did not make sense that the acuity level of our patients would generate such extreme cost variances from the norm,” he testified in December, in a deposition for the whistleblower case. “It was so significant, we needed to get to the bottom of it.”
Ament, a second generation Air Force veteran, quietly assembled a task force to investigate why the facility had purchased so many medical devices for these procedures. After they examined inventory records, calculating the total number of medical devices and the cost of devices per patient, they grew concerned.
“We were more expensive than, I believe it was, the top 10 hospitals in the VA combined,” he said. “My feeling was that we either had very, very bad providers or we had product walking out the door.”
Ament enlisted experts from other VA hospitals to help his team investigate, including an administrative officer who could understand finances and a respected interventional radiologist who could examine records. The task force gathered a list of patients from 2016 to 2018, according to internal emails, and analyzed their medical charts.
According to internal VA documents released through the whistleblower suit, the review found a number of clinical failings: Evidence-based medicine had not been followed in the majority of cases reviewed. Procedures were over-aggressive, treating lesions that should have been left alone. And there was a total disregard for established best practices for treating peripheral artery disease.
One of the experts on the investigative team explained to Ament that while it was not uncommon for doctors to use a couple of devices in one intervention, the total number of devices in many of the procedures at his facility went into the double digits, sometimes five times the expected amount.
In one encounter, a doctor deployed 33 devices in one procedure – 3 atherectomy devices, 9 stents, and 21 balloons.
This use of devices was exorbitant, Ament came to understand. “I want to say the term ‘egregious’ was used,” he testified. “It was kind of like validation, but I really wish I was wrong.”
“Did it make you concerned for patient care?” a lawyer asked during the deposition.
“It did,” Ament replied.
A member of his task force pulled data for veterans who had leg amputations due to vascular disease. Over 5 years, the number of veterans who had amputations increased, from about 6 in 2013 to 38 in 2018, according to internal emails released in the suit. The VA did not respond to ProPublica’s questions about the rise in amputations or whether it was due to complications from the procedures.
Even though Ament testified in December 2022 that he became aware of the excessive use of devices during his investigation that began about 5 years ago, neither he nor the VA have publicly acknowledged these findings outside of the lawsuit. It is unclear whether VA representatives informed the patients whose records were reviewed about their findings. ProPublica reached out to more than half a dozen veteran community groups in the Wichita area and none were aware of the investigation nor the allegations of overuse of vascular procedures at the facility.
The VA says that if its ongoing review finds instances of substandard care, it will reach out to affected patients and inform them about possible complications and benefits they may be entitled to. The press secretary said the review will take several months. Ament declined to respond to ProPublica’s questions, citing the ongoing case.
In 2018, Ament turned over his findings to the criminal division of the VA’s Office of Inspector General. He also shut down interventional radiology procedures at the facility’s catheter lab.
Federal agents separately opened an investigation into the same unit in the facility, looking into allegations of kickbacks.
More than 40 pages of expense reports from Medtronic, revealed in the whistleblower case, show sales representatives treating Dole health care workers to hundreds of meals over several years – lunches at Dempsey’s Biscuit Co.; business meals at the Scotch & Sirloin steakhouse; dinner at Chester’s Chophouse & Wine Bar, price per attendee: $122.39.
Federal agents obtained the receipts.
“Robert J. Dole VAMC employees may have received improper gratuities, in the forms of paid lunches, dinners, etc., from sales representatives from Medtronic,” Nathen Howard, a special agent in the VA OIG, wrote in an investigation memo from February 2019.
This kind of relationship could violate VA policy, which forbids federal employees from receiving any gifts, including meals, from people who do business or seek to do business with a federal institution. For health care workers, violating this policy could have serious implications for patients. Numerous studies have shown that even modest industry-sponsored gifts, including meals, may influence prescribing or treatment behavior of health care professionals.
The agents opened their investigation into kickbacks at the Wichita facility in response to the whistleblower lawsuit, which was filed by Thomas Schroeder in 2017. The VA OIG would not confirm or deny whether it was continuing to investigate kickbacks at the facility. The VA did not directly answer ProPublica’s questions about kickbacks at the Dole VA, but it said that every employee must complete an annual ethics training, which covers gift rules.
In recent years, Medtronic has settled a handful of other cases that have alleged kickbacks between company representatives and health care professionals.
In 2018, Medtronic’s subsidiary Covidien paid $13 million to settle claims with the U.S. Department of Justice that it paid kickbacks to health care institutions that used its mechanical blood clot devices. In 2019, the same subsidiary paid $17 million to resolve allegations that it provided in-kind marketing support to doctors using its vein products. And in 2020, Medtronic paid more than $8 million to settle claims that representatives had paid kickbacks to a neurosurgeon, including scores of lavish meals at a restaurant that the doctor owned, to induce him to purchase the company’s medication pumps.
Schroeder’s lawsuit is not the first time Medtronic’s vascular devices were named in an alleged kickback scheme. In early 2015, Medtronic acquired Covidien, and shortly after the merger, its subsidiary ev3 Inc. agreed to pay $1.25 million to resolve allegations that it had paid doctors who were “high volume users” of its atherectomy devices to act as evangelists for the company, and had provided physicians with company shares to participate in clinical trials for their tools.
The whistleblower in this earlier case, a former sales representative for the company, also alleged that the subsidiary was gaming Medicare’s payment system. Hospitals were often hesitant to conduct atherectomy procedures because of the low reimbursement rates. According to the suit, sales representatives encouraged doctors to admit patients for longer stays to reap greater reimbursements and make a profit, even though such stays were often not medically indicated.
“Medical device makers that try to boost their profits by causing patients to be admitted for unnecessary and expensive inpatient hospital stays will be held accountable,” special agent Thomas O’Donnell, from the Office of Inspector General at the U.S. Department of Health and Human Services, said in a press release for the settlement. “Both patients and taxpayers deserve to have medical decisions made based on what is medically appropriate.”
Medtronic spokesperson Xiong said that in each case, the company “cooperated fully with the DOJ to resolve its concerns and, where wrongdoing was found, took appropriate remedial action.”
Seton Hall Law School professor Jacob Elberg, a former assistant U.S. attorney for the District of New Jersey who led its health care and government fraud unit, is concerned by the frequency of such settlements in the last 2 decades. “There are, at this point, real questions as to whether the sanctions imposed by DOJ are sufficient to deter wrongdoing and to lead to meaningful change, especially within the medical device industry.”
Although the Department of Justice has declined to intervene in the lawsuit involving the Dole VA at this time, the case is ongoing and further depositions with Medtronic sales representatives and a former VA employee are scheduled for this month.
VA employees and doctors named in the suit declined to comment or did not respond to ProPublica’s questions about the alleged kickbacks and whether sales representatives may have influenced veterans’ treatment plans. In interviews with federal investigators, according to released transcripts, several of the employees who were questioned denied receiving frequent meals from sales representatives, contradicting Medtronic’s expense reports.
Their statements also stand in contrast to Medtronic representative Kari Kirk’s final text messages during that procedure in June 2017, which ultimately lasted more than 3 hours.
“Now u done??” her colleague asked.
“Just finished,” she texted. “Running to get them lunch!”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
On June 14, 2017, just before noon, a doctor made an incision near a patient’s groin. Kari Kirk, a representative for the world’s largest medical device company, Medtronic, looked on and began texting her colleague a play-by-play.
“Fixing both legs from the ankles,” she wrote.
It was a fairly common procedure at the Robert J. Dole Veterans Affairs Medical Center in Wichita, Kansas, performed to treat blockages in the leg vessels.
Within reach were an array of Medtronic products: tubes with blades attached to shave hardened deposits off of artery walls; stents to widen blood vessels; balloons coated with therapeutic drugs.
Each time a doctor puts a foreign device in someone’s body, it carries a risk of complication, which can include clots or even require amputation. So medical experts, research and even Medtronic’s own device instructions urge doctors to use as few as are necessary.
“Just used 12 [drug-coated balloons]!!” Kirk texted her colleague.
“Does that mean I owe u $$,” he responded.
“Thats what I’m thinking!!!” she said. “And now 14 balloons?!”
“but only one stent so far??”
“So far!”
As the texting continued, her colleague replied, “U are going to want to start going to the VA all the time.”
The messages, recently unsealed in an ongoing whistleblower lawsuit, give a window into the way money and medicine mingle in the booming business of peripheral artery disease, a condition that afflicts 6.5 million Americans over age 40 and is caused when fatty plaque builds up in arteries, blocking blood flow to the legs.
Representatives from companies are often present during vascular procedures to guide doctors on how to use their complex devices. This kind of access has the potential to influence treatment plans, as companies and their representatives profit when more of their product is used.
The suit, filed in 2017 by a sales representative for a competing medical device firm, alleges an illegal kickback scheme between Medtronic and hospital employees. According to the complaint and documents released in the suit, between 2011 and 2018, VA health care workers received steakhouse dinners, Apple electronics, and NASCAR tickets, and in turn, Medtronic secured a lucrative contract with the hospital. Meanwhile, the company’s representatives allegedly “groomed and trained” physicians at the facility, who then deployed the company’s devices even when it was not medically indicated.
Independently from the whistleblower suit, internal investigators at the Wichita facility have also examined the treatment patterns of its vascular patients in recent years and found numerous cases where medical devices were used excessively. While it’s not uncommon to deploy several devices, a medical expert on the investigation team found that the VA doctors sometimes used more than 15 at a time – one used 33 – deviating from the standard of care.
“It is unconscionable – there can be no valid medically acceptable basis to cram so many devices into a human being,” wrote attorneys representing the whistleblower in legal filings from January 2023. “This is not medical treatment. This is abuse.”
Dr. Kim Hodgson, former president of the Society for Vascular Surgery and an expert retained by the plaintiff, said the findings of the internal review of patient data raise “a high level of concern regarding necessity of treatment provided,” according to case documents.
Medtronic declined to respond to ProPublica’s questions, citing the ongoing litigation. “These allegations are false and Medtronic is defending against these claims in court,” said Boua Xiong, a spokesperson for the company. Medtronic representative Kirk declined to respond to ProPublica’s request for comment.
The hospital investigation found that amputations increased sixfold in the same time frame as the procedures in question, according to internal emails, but made no conclusion about whether those two things were connected. ProPublica reached out to the VA to ask whether any patients had been harmed.
The VA is “conducting an extensive review of patient care” at the Kansas hospital, “including the number of devices used on patients – to make sure that Veterans were not harmed by any procedures,” press secretary Terrence Hayes said in a statement. So far, the VA’s investigation has found no “quality of care issues,” he said, and the investigation will continue “until every Veteran’s case has been reviewed.” (Read the full statement here.) Neither the department nor the hospital has taken formal action against the medical providers, Hayes said.
The medical group that had a contract with the VA for vascular interventions, Wichita Radiological Group, did not respond to ProPublica’s requests for comment, nor did the doctors named in the suit: Dr. Shaun Gonda, Dr. Bret Winblad, and Dr. Kermit Rust. It is unclear from the case documents which doctors conducted which procedures. Eric Barth, an attorney for the medical group, denied the allegations in recent legal filings, calling the claims “baseless” and the lawsuit a “witch hunt.”
The lawsuit comes amid growing concern about one of these procedures – atherectomies – after researchers and doctors have uncovered patterns of excessive and inappropriate use. Recent research has found that this procedure, a common but costly treatment to shave or laser plaque from blood vessels, is not more effective than cheaper alternatives and may even be associated with a higher risk of complications including amputation. In recent years, several doctors and clinics have been investigated for allegedly taking advantage of Medicare’s reimbursement rates, and one study found that many doctors are resorting to atherectomies in the earliest stages of peripheral artery disease, against best practices that urge noninvasive treatment.
“Atherectomy is important in certain settings. But it’s being used in a way that is entirely inappropriate and it’s largely driven by the incentive structure,” said Dr. Caitlin Hicks, the lead author of the study and an associate professor of surgery at Johns Hopkins University School of Medicine.
Although different payment structures govern the care of veterans, the whistleblower lawsuit alleges that outside physicians, paid hourly by the Dole VA, were motivated to conduct longer and more complex procedures that would earn them higher payment.
Under different circumstances, the patient in the procedure room on that summer day could have been done after 2 hours.
But, 150 minutes in, those Medtronic representatives were still texting. At that point, more than 15 of their vascular devices had been used, including stents, balloons, and those for atherectomy.
“Long case!” Kirk’s colleague texted. “Is it looking ok??”
“It is,” she said. “Thought we were done a few times! Now he’s going back in to cut again!”
A little while later, she texted: “....17!”
He texted back [with laughing emoticons].
Hospital leaders had been scrutinizing the use of these procedures at the Dole VA for years.
In 2017, shortly after Rick Ament was hired to lead the facility, he noticed something was amiss. While the longtime hospital administrator was poring over the finances, he was alarmed to discover that the relatively small Dole VA had one of the most expensive cardiac programs in the country. As Ament dug deeper, he realized vascular interventions were the reason.
“It just did not make sense that the acuity level of our patients would generate such extreme cost variances from the norm,” he testified in December, in a deposition for the whistleblower case. “It was so significant, we needed to get to the bottom of it.”
Ament, a second generation Air Force veteran, quietly assembled a task force to investigate why the facility had purchased so many medical devices for these procedures. After they examined inventory records, calculating the total number of medical devices and the cost of devices per patient, they grew concerned.
“We were more expensive than, I believe it was, the top 10 hospitals in the VA combined,” he said. “My feeling was that we either had very, very bad providers or we had product walking out the door.”
Ament enlisted experts from other VA hospitals to help his team investigate, including an administrative officer who could understand finances and a respected interventional radiologist who could examine records. The task force gathered a list of patients from 2016 to 2018, according to internal emails, and analyzed their medical charts.
According to internal VA documents released through the whistleblower suit, the review found a number of clinical failings: Evidence-based medicine had not been followed in the majority of cases reviewed. Procedures were over-aggressive, treating lesions that should have been left alone. And there was a total disregard for established best practices for treating peripheral artery disease.
One of the experts on the investigative team explained to Ament that while it was not uncommon for doctors to use a couple of devices in one intervention, the total number of devices in many of the procedures at his facility went into the double digits, sometimes five times the expected amount.
In one encounter, a doctor deployed 33 devices in one procedure – 3 atherectomy devices, 9 stents, and 21 balloons.
This use of devices was exorbitant, Ament came to understand. “I want to say the term ‘egregious’ was used,” he testified. “It was kind of like validation, but I really wish I was wrong.”
“Did it make you concerned for patient care?” a lawyer asked during the deposition.
“It did,” Ament replied.
A member of his task force pulled data for veterans who had leg amputations due to vascular disease. Over 5 years, the number of veterans who had amputations increased, from about 6 in 2013 to 38 in 2018, according to internal emails released in the suit. The VA did not respond to ProPublica’s questions about the rise in amputations or whether it was due to complications from the procedures.
Even though Ament testified in December 2022 that he became aware of the excessive use of devices during his investigation that began about 5 years ago, neither he nor the VA have publicly acknowledged these findings outside of the lawsuit. It is unclear whether VA representatives informed the patients whose records were reviewed about their findings. ProPublica reached out to more than half a dozen veteran community groups in the Wichita area and none were aware of the investigation nor the allegations of overuse of vascular procedures at the facility.
The VA says that if its ongoing review finds instances of substandard care, it will reach out to affected patients and inform them about possible complications and benefits they may be entitled to. The press secretary said the review will take several months. Ament declined to respond to ProPublica’s questions, citing the ongoing case.
In 2018, Ament turned over his findings to the criminal division of the VA’s Office of Inspector General. He also shut down interventional radiology procedures at the facility’s catheter lab.
Federal agents separately opened an investigation into the same unit in the facility, looking into allegations of kickbacks.
More than 40 pages of expense reports from Medtronic, revealed in the whistleblower case, show sales representatives treating Dole health care workers to hundreds of meals over several years – lunches at Dempsey’s Biscuit Co.; business meals at the Scotch & Sirloin steakhouse; dinner at Chester’s Chophouse & Wine Bar, price per attendee: $122.39.
Federal agents obtained the receipts.
“Robert J. Dole VAMC employees may have received improper gratuities, in the forms of paid lunches, dinners, etc., from sales representatives from Medtronic,” Nathen Howard, a special agent in the VA OIG, wrote in an investigation memo from February 2019.
This kind of relationship could violate VA policy, which forbids federal employees from receiving any gifts, including meals, from people who do business or seek to do business with a federal institution. For health care workers, violating this policy could have serious implications for patients. Numerous studies have shown that even modest industry-sponsored gifts, including meals, may influence prescribing or treatment behavior of health care professionals.
The agents opened their investigation into kickbacks at the Wichita facility in response to the whistleblower lawsuit, which was filed by Thomas Schroeder in 2017. The VA OIG would not confirm or deny whether it was continuing to investigate kickbacks at the facility. The VA did not directly answer ProPublica’s questions about kickbacks at the Dole VA, but it said that every employee must complete an annual ethics training, which covers gift rules.
In recent years, Medtronic has settled a handful of other cases that have alleged kickbacks between company representatives and health care professionals.
In 2018, Medtronic’s subsidiary Covidien paid $13 million to settle claims with the U.S. Department of Justice that it paid kickbacks to health care institutions that used its mechanical blood clot devices. In 2019, the same subsidiary paid $17 million to resolve allegations that it provided in-kind marketing support to doctors using its vein products. And in 2020, Medtronic paid more than $8 million to settle claims that representatives had paid kickbacks to a neurosurgeon, including scores of lavish meals at a restaurant that the doctor owned, to induce him to purchase the company’s medication pumps.
Schroeder’s lawsuit is not the first time Medtronic’s vascular devices were named in an alleged kickback scheme. In early 2015, Medtronic acquired Covidien, and shortly after the merger, its subsidiary ev3 Inc. agreed to pay $1.25 million to resolve allegations that it had paid doctors who were “high volume users” of its atherectomy devices to act as evangelists for the company, and had provided physicians with company shares to participate in clinical trials for their tools.
The whistleblower in this earlier case, a former sales representative for the company, also alleged that the subsidiary was gaming Medicare’s payment system. Hospitals were often hesitant to conduct atherectomy procedures because of the low reimbursement rates. According to the suit, sales representatives encouraged doctors to admit patients for longer stays to reap greater reimbursements and make a profit, even though such stays were often not medically indicated.
“Medical device makers that try to boost their profits by causing patients to be admitted for unnecessary and expensive inpatient hospital stays will be held accountable,” special agent Thomas O’Donnell, from the Office of Inspector General at the U.S. Department of Health and Human Services, said in a press release for the settlement. “Both patients and taxpayers deserve to have medical decisions made based on what is medically appropriate.”
Medtronic spokesperson Xiong said that in each case, the company “cooperated fully with the DOJ to resolve its concerns and, where wrongdoing was found, took appropriate remedial action.”
Seton Hall Law School professor Jacob Elberg, a former assistant U.S. attorney for the District of New Jersey who led its health care and government fraud unit, is concerned by the frequency of such settlements in the last 2 decades. “There are, at this point, real questions as to whether the sanctions imposed by DOJ are sufficient to deter wrongdoing and to lead to meaningful change, especially within the medical device industry.”
Although the Department of Justice has declined to intervene in the lawsuit involving the Dole VA at this time, the case is ongoing and further depositions with Medtronic sales representatives and a former VA employee are scheduled for this month.
VA employees and doctors named in the suit declined to comment or did not respond to ProPublica’s questions about the alleged kickbacks and whether sales representatives may have influenced veterans’ treatment plans. In interviews with federal investigators, according to released transcripts, several of the employees who were questioned denied receiving frequent meals from sales representatives, contradicting Medtronic’s expense reports.
Their statements also stand in contrast to Medtronic representative Kari Kirk’s final text messages during that procedure in June 2017, which ultimately lasted more than 3 hours.
“Now u done??” her colleague asked.
“Just finished,” she texted. “Running to get them lunch!”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
On June 14, 2017, just before noon, a doctor made an incision near a patient’s groin. Kari Kirk, a representative for the world’s largest medical device company, Medtronic, looked on and began texting her colleague a play-by-play.
“Fixing both legs from the ankles,” she wrote.
It was a fairly common procedure at the Robert J. Dole Veterans Affairs Medical Center in Wichita, Kansas, performed to treat blockages in the leg vessels.
Within reach were an array of Medtronic products: tubes with blades attached to shave hardened deposits off of artery walls; stents to widen blood vessels; balloons coated with therapeutic drugs.
Each time a doctor puts a foreign device in someone’s body, it carries a risk of complication, which can include clots or even require amputation. So medical experts, research and even Medtronic’s own device instructions urge doctors to use as few as are necessary.
“Just used 12 [drug-coated balloons]!!” Kirk texted her colleague.
“Does that mean I owe u $$,” he responded.
“Thats what I’m thinking!!!” she said. “And now 14 balloons?!”
“but only one stent so far??”
“So far!”
As the texting continued, her colleague replied, “U are going to want to start going to the VA all the time.”
The messages, recently unsealed in an ongoing whistleblower lawsuit, give a window into the way money and medicine mingle in the booming business of peripheral artery disease, a condition that afflicts 6.5 million Americans over age 40 and is caused when fatty plaque builds up in arteries, blocking blood flow to the legs.
Representatives from companies are often present during vascular procedures to guide doctors on how to use their complex devices. This kind of access has the potential to influence treatment plans, as companies and their representatives profit when more of their product is used.
The suit, filed in 2017 by a sales representative for a competing medical device firm, alleges an illegal kickback scheme between Medtronic and hospital employees. According to the complaint and documents released in the suit, between 2011 and 2018, VA health care workers received steakhouse dinners, Apple electronics, and NASCAR tickets, and in turn, Medtronic secured a lucrative contract with the hospital. Meanwhile, the company’s representatives allegedly “groomed and trained” physicians at the facility, who then deployed the company’s devices even when it was not medically indicated.
Independently from the whistleblower suit, internal investigators at the Wichita facility have also examined the treatment patterns of its vascular patients in recent years and found numerous cases where medical devices were used excessively. While it’s not uncommon to deploy several devices, a medical expert on the investigation team found that the VA doctors sometimes used more than 15 at a time – one used 33 – deviating from the standard of care.
“It is unconscionable – there can be no valid medically acceptable basis to cram so many devices into a human being,” wrote attorneys representing the whistleblower in legal filings from January 2023. “This is not medical treatment. This is abuse.”
Dr. Kim Hodgson, former president of the Society for Vascular Surgery and an expert retained by the plaintiff, said the findings of the internal review of patient data raise “a high level of concern regarding necessity of treatment provided,” according to case documents.
Medtronic declined to respond to ProPublica’s questions, citing the ongoing litigation. “These allegations are false and Medtronic is defending against these claims in court,” said Boua Xiong, a spokesperson for the company. Medtronic representative Kirk declined to respond to ProPublica’s request for comment.
The hospital investigation found that amputations increased sixfold in the same time frame as the procedures in question, according to internal emails, but made no conclusion about whether those two things were connected. ProPublica reached out to the VA to ask whether any patients had been harmed.
The VA is “conducting an extensive review of patient care” at the Kansas hospital, “including the number of devices used on patients – to make sure that Veterans were not harmed by any procedures,” press secretary Terrence Hayes said in a statement. So far, the VA’s investigation has found no “quality of care issues,” he said, and the investigation will continue “until every Veteran’s case has been reviewed.” (Read the full statement here.) Neither the department nor the hospital has taken formal action against the medical providers, Hayes said.
The medical group that had a contract with the VA for vascular interventions, Wichita Radiological Group, did not respond to ProPublica’s requests for comment, nor did the doctors named in the suit: Dr. Shaun Gonda, Dr. Bret Winblad, and Dr. Kermit Rust. It is unclear from the case documents which doctors conducted which procedures. Eric Barth, an attorney for the medical group, denied the allegations in recent legal filings, calling the claims “baseless” and the lawsuit a “witch hunt.”
The lawsuit comes amid growing concern about one of these procedures – atherectomies – after researchers and doctors have uncovered patterns of excessive and inappropriate use. Recent research has found that this procedure, a common but costly treatment to shave or laser plaque from blood vessels, is not more effective than cheaper alternatives and may even be associated with a higher risk of complications including amputation. In recent years, several doctors and clinics have been investigated for allegedly taking advantage of Medicare’s reimbursement rates, and one study found that many doctors are resorting to atherectomies in the earliest stages of peripheral artery disease, against best practices that urge noninvasive treatment.
“Atherectomy is important in certain settings. But it’s being used in a way that is entirely inappropriate and it’s largely driven by the incentive structure,” said Dr. Caitlin Hicks, the lead author of the study and an associate professor of surgery at Johns Hopkins University School of Medicine.
Although different payment structures govern the care of veterans, the whistleblower lawsuit alleges that outside physicians, paid hourly by the Dole VA, were motivated to conduct longer and more complex procedures that would earn them higher payment.
Under different circumstances, the patient in the procedure room on that summer day could have been done after 2 hours.
But, 150 minutes in, those Medtronic representatives were still texting. At that point, more than 15 of their vascular devices had been used, including stents, balloons, and those for atherectomy.
“Long case!” Kirk’s colleague texted. “Is it looking ok??”
“It is,” she said. “Thought we were done a few times! Now he’s going back in to cut again!”
A little while later, she texted: “....17!”
He texted back [with laughing emoticons].
Hospital leaders had been scrutinizing the use of these procedures at the Dole VA for years.
In 2017, shortly after Rick Ament was hired to lead the facility, he noticed something was amiss. While the longtime hospital administrator was poring over the finances, he was alarmed to discover that the relatively small Dole VA had one of the most expensive cardiac programs in the country. As Ament dug deeper, he realized vascular interventions were the reason.
“It just did not make sense that the acuity level of our patients would generate such extreme cost variances from the norm,” he testified in December, in a deposition for the whistleblower case. “It was so significant, we needed to get to the bottom of it.”
Ament, a second generation Air Force veteran, quietly assembled a task force to investigate why the facility had purchased so many medical devices for these procedures. After they examined inventory records, calculating the total number of medical devices and the cost of devices per patient, they grew concerned.
“We were more expensive than, I believe it was, the top 10 hospitals in the VA combined,” he said. “My feeling was that we either had very, very bad providers or we had product walking out the door.”
Ament enlisted experts from other VA hospitals to help his team investigate, including an administrative officer who could understand finances and a respected interventional radiologist who could examine records. The task force gathered a list of patients from 2016 to 2018, according to internal emails, and analyzed their medical charts.
According to internal VA documents released through the whistleblower suit, the review found a number of clinical failings: Evidence-based medicine had not been followed in the majority of cases reviewed. Procedures were over-aggressive, treating lesions that should have been left alone. And there was a total disregard for established best practices for treating peripheral artery disease.
One of the experts on the investigative team explained to Ament that while it was not uncommon for doctors to use a couple of devices in one intervention, the total number of devices in many of the procedures at his facility went into the double digits, sometimes five times the expected amount.
In one encounter, a doctor deployed 33 devices in one procedure – 3 atherectomy devices, 9 stents, and 21 balloons.
This use of devices was exorbitant, Ament came to understand. “I want to say the term ‘egregious’ was used,” he testified. “It was kind of like validation, but I really wish I was wrong.”
“Did it make you concerned for patient care?” a lawyer asked during the deposition.
“It did,” Ament replied.
A member of his task force pulled data for veterans who had leg amputations due to vascular disease. Over 5 years, the number of veterans who had amputations increased, from about 6 in 2013 to 38 in 2018, according to internal emails released in the suit. The VA did not respond to ProPublica’s questions about the rise in amputations or whether it was due to complications from the procedures.
Even though Ament testified in December 2022 that he became aware of the excessive use of devices during his investigation that began about 5 years ago, neither he nor the VA have publicly acknowledged these findings outside of the lawsuit. It is unclear whether VA representatives informed the patients whose records were reviewed about their findings. ProPublica reached out to more than half a dozen veteran community groups in the Wichita area and none were aware of the investigation nor the allegations of overuse of vascular procedures at the facility.
The VA says that if its ongoing review finds instances of substandard care, it will reach out to affected patients and inform them about possible complications and benefits they may be entitled to. The press secretary said the review will take several months. Ament declined to respond to ProPublica’s questions, citing the ongoing case.
In 2018, Ament turned over his findings to the criminal division of the VA’s Office of Inspector General. He also shut down interventional radiology procedures at the facility’s catheter lab.
Federal agents separately opened an investigation into the same unit in the facility, looking into allegations of kickbacks.
More than 40 pages of expense reports from Medtronic, revealed in the whistleblower case, show sales representatives treating Dole health care workers to hundreds of meals over several years – lunches at Dempsey’s Biscuit Co.; business meals at the Scotch & Sirloin steakhouse; dinner at Chester’s Chophouse & Wine Bar, price per attendee: $122.39.
Federal agents obtained the receipts.
“Robert J. Dole VAMC employees may have received improper gratuities, in the forms of paid lunches, dinners, etc., from sales representatives from Medtronic,” Nathen Howard, a special agent in the VA OIG, wrote in an investigation memo from February 2019.
This kind of relationship could violate VA policy, which forbids federal employees from receiving any gifts, including meals, from people who do business or seek to do business with a federal institution. For health care workers, violating this policy could have serious implications for patients. Numerous studies have shown that even modest industry-sponsored gifts, including meals, may influence prescribing or treatment behavior of health care professionals.
The agents opened their investigation into kickbacks at the Wichita facility in response to the whistleblower lawsuit, which was filed by Thomas Schroeder in 2017. The VA OIG would not confirm or deny whether it was continuing to investigate kickbacks at the facility. The VA did not directly answer ProPublica’s questions about kickbacks at the Dole VA, but it said that every employee must complete an annual ethics training, which covers gift rules.
In recent years, Medtronic has settled a handful of other cases that have alleged kickbacks between company representatives and health care professionals.
In 2018, Medtronic’s subsidiary Covidien paid $13 million to settle claims with the U.S. Department of Justice that it paid kickbacks to health care institutions that used its mechanical blood clot devices. In 2019, the same subsidiary paid $17 million to resolve allegations that it provided in-kind marketing support to doctors using its vein products. And in 2020, Medtronic paid more than $8 million to settle claims that representatives had paid kickbacks to a neurosurgeon, including scores of lavish meals at a restaurant that the doctor owned, to induce him to purchase the company’s medication pumps.
Schroeder’s lawsuit is not the first time Medtronic’s vascular devices were named in an alleged kickback scheme. In early 2015, Medtronic acquired Covidien, and shortly after the merger, its subsidiary ev3 Inc. agreed to pay $1.25 million to resolve allegations that it had paid doctors who were “high volume users” of its atherectomy devices to act as evangelists for the company, and had provided physicians with company shares to participate in clinical trials for their tools.
The whistleblower in this earlier case, a former sales representative for the company, also alleged that the subsidiary was gaming Medicare’s payment system. Hospitals were often hesitant to conduct atherectomy procedures because of the low reimbursement rates. According to the suit, sales representatives encouraged doctors to admit patients for longer stays to reap greater reimbursements and make a profit, even though such stays were often not medically indicated.
“Medical device makers that try to boost their profits by causing patients to be admitted for unnecessary and expensive inpatient hospital stays will be held accountable,” special agent Thomas O’Donnell, from the Office of Inspector General at the U.S. Department of Health and Human Services, said in a press release for the settlement. “Both patients and taxpayers deserve to have medical decisions made based on what is medically appropriate.”
Medtronic spokesperson Xiong said that in each case, the company “cooperated fully with the DOJ to resolve its concerns and, where wrongdoing was found, took appropriate remedial action.”
Seton Hall Law School professor Jacob Elberg, a former assistant U.S. attorney for the District of New Jersey who led its health care and government fraud unit, is concerned by the frequency of such settlements in the last 2 decades. “There are, at this point, real questions as to whether the sanctions imposed by DOJ are sufficient to deter wrongdoing and to lead to meaningful change, especially within the medical device industry.”
Although the Department of Justice has declined to intervene in the lawsuit involving the Dole VA at this time, the case is ongoing and further depositions with Medtronic sales representatives and a former VA employee are scheduled for this month.
VA employees and doctors named in the suit declined to comment or did not respond to ProPublica’s questions about the alleged kickbacks and whether sales representatives may have influenced veterans’ treatment plans. In interviews with federal investigators, according to released transcripts, several of the employees who were questioned denied receiving frequent meals from sales representatives, contradicting Medtronic’s expense reports.
Their statements also stand in contrast to Medtronic representative Kari Kirk’s final text messages during that procedure in June 2017, which ultimately lasted more than 3 hours.
“Now u done??” her colleague asked.
“Just finished,” she texted. “Running to get them lunch!”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
Eight-week TB treatment strategy shows potential
A strategy for the
“We found that if we use the strategy of a bedaquiline-linezolid five-drug regimen for 8 weeks and then followed patients for 96 weeks, [the regimen] was noninferior, clinically, to the standard regimen in terms of the number of people alive, free of TB disease, and not on treatment,” said lead author Nicholas Paton, MD, of the National University of Singapore, in a press conference held during the Conference on Retroviruses & Opportunistic Infections.
“The total time on treatment was reduced by half – instead of 160 days, it was 85 days for the total duration.”
Commenting on the study, which was published concurrently in the New England Journal of Medicine, Richard E. Chaisson, MD, noted that, although more needs to be understood, the high number of responses is nevertheless encouraging.
“Clinicians will not feel comfortable with the short regimens at this point, but it is remarkable that so many patients did well with shorter treatments,” Dr. Chaisson, who is a professor of medicine, epidemiology, and international health and director of the Johns Hopkins University Center for Tuberculosis Research, Baltimore, said in an interview.
Importantly, the study should help push forward “future studies [that] will stratify patients according to their likelihood of responding to shorter treatments,” he said.
The current global standard for TB treatment, practiced for 4 decades, has been a 6-month rifampin-based regimen. Although the regimen performs well, curing more than 95% of cases in clinical trials, in real-world practice, the prolonged duration can be problematic, with issues of nonadherence and loss of patients to follow-up.
Previous research has shown that shorter regimens have potential, with some studies showing as many as 85% of patients cured with 3- and 4-month regimens, and some promising 2-month regimens showing efficacy specifically for those with smear-negative TB.
These efforts suggest that “the current 6-month regimen may lead to overtreatment in the majority of persons in order to prevent relapse in a minority of persons,” the authors asserted.
TRUNCATE-TB
To investigate a suitable shorter-term alternative, the authors conducted the phase 2-3, prospective, open-label TRUNCATE-TB trial, in which 674 patients with rifampin-susceptible pulmonary TB were enrolled at 18 sites in Asia and Africa.
The patients were randomly assigned to receive either the standard treatment regimen (rifampin and isoniazid for 24 weeks with pyrazinamide and ethambutol for the first 8 weeks; n = 181), or one of four novel five-drug regimens to be administered over 8 weeks, along with extended treatment for persistent clinical disease of up to 12 weeks, if needed, and a plan for retreatment in the case of relapse (n = 493).
Two of the regimens were dropped because of logistic criteria; the two remaining shorter-course groups included in the study involved either high-dose rifampin plus linezolid or bedaquiline plus linezolid, each combined with isoniazid, pyrazinamide, and ethambutol.
Of the patients, 62% were male, and four withdrew or were lost to follow-up by the end of the study at a final follow-up at week 96.
Among patients assigned to the 8-week regimens, 80% stopped at exactly 8 weeks, while 9% wound up having extended treatment to 10 weeks and 3% were extended to 12 weeks.
For the primary endpoint, a composite of death, ongoing treatment, or active disease at week 96, the rate was lowest in the standard 24-week therapy group, occurring in 7 of 181 patients (3.9%), compared with 21 of 184 patients (11.4%) in the rifampin plus linezolid group (adjusted difference, 7.4 percentage points, which did not meet noninferiority criterion), and 11 of 189 (5.8%) in the group in the bedaquiline plus linezolid group (adjusted difference, 0.8 percentage points, meeting noninferiority criterion).
The mean total duration of treatment through week 96 in the standard treatment group was 180 days versus 106 days in the rifampin–linezolid group, and 85 days in the bedaquiline-linezolid group.
The results were consistent across multiple subgroups defined according to baseline characteristics, including some that could be linked to severe disease and a high risk for relapse.
In terms of safety, there were no significant differences between the groups in terms of grade 3 or 4 adverse events.
Of note, only two patients (1.1%) in the bedaquiline plus linezolid group acquired a resistance, which Dr. Paton said was “encouraging,” because of concerns about resistance to that drug.
‘Unfavorable’ composite also evaluated
In an updated analysis of the study that Dr. Paton presented at the meeting, the authors looked at a revised “unfavorable” primary outcome – a composite including treatment failure, relapse, death, or nonattendance at week 96 without evidence of prior disease clearance.
The rate remained lowest in the standard 24-week therapy group (3.9%) versus 25% in the rifampin plus linezolid group, and 13.8% in the bedaquiline plus linezolid group.
Though the lower rate with the standard treatment was expected, Dr. Paton said the results nevertheless hold promise, at least for some patients, for successful treatment with the 8-week bedaquiline plus linezolid strategy.
“What the trial has told us is that even with that 13.8% relapse rate, we can manage patients within this strategy and people can do fine at the end, because with some simple clinical biomarkers, we can pick the people who may have a high chance of achieving a cure.”
Dr. Chaisson expressed concern over the higher unfavorable rates, but said the results help pave the way for refining a workable-shorter term strategy.
“TRUNCATE-TB did find that most patients could be successfully treated in 2 months with the novel regimen of bedaquiline plus linezolid, but the failure rate was still unacceptably high,” he said.
“This regimen will not be widely adapted at this point, but additional analyses may identify subsets of patients who will do well with shorter regimens, and future studies will stratify patients according to their likelihood of responding to shorter treatments.”
The authors of an accompanying editorial further commented that the benefits of a shorter treatment strategy could very well outweigh possible shortcomings.
“Treatment algorithms such as that used in the TRUNCATE-TB trial are fundamental to tuberculosis control,” wrote Véronique Dartois, PhD, Center for Discovery and Innovation, Nutley, N.J., and Eric J. Rubin, MD, PhD, the editor-in-chief of NEJM. “Although implementing them could be a challenge, any added burden might be offset by reduced costs, better adherence, and increased patient satisfaction. Thus, for tuberculosis, a strategy might be more than just a regimen.”
The good news, as summed up by CROI vice-chair Landon Myer, MD, PhD, in the press conference, is that “we’re moving closer and closer to the holy grail of a short, efficacious regimen for TB treatment. We’re getting there slowly, but we’re getting there.”
The study received grant funding from the Singapore National Medical Research Council; a grant from the Department of Health and Social Care; the Foreign, Commonwealth, and Development Office; the Medical Research Council; and Wellcome Trust; as well as a grant from the UK Research and Innovation Medical Research Council. Dr. Dartois reported no relevant financial relationships. Dr. Chaisson had no disclosures to report.
A version of this article originally appeared on Medscape.com.
A strategy for the
“We found that if we use the strategy of a bedaquiline-linezolid five-drug regimen for 8 weeks and then followed patients for 96 weeks, [the regimen] was noninferior, clinically, to the standard regimen in terms of the number of people alive, free of TB disease, and not on treatment,” said lead author Nicholas Paton, MD, of the National University of Singapore, in a press conference held during the Conference on Retroviruses & Opportunistic Infections.
“The total time on treatment was reduced by half – instead of 160 days, it was 85 days for the total duration.”
Commenting on the study, which was published concurrently in the New England Journal of Medicine, Richard E. Chaisson, MD, noted that, although more needs to be understood, the high number of responses is nevertheless encouraging.
“Clinicians will not feel comfortable with the short regimens at this point, but it is remarkable that so many patients did well with shorter treatments,” Dr. Chaisson, who is a professor of medicine, epidemiology, and international health and director of the Johns Hopkins University Center for Tuberculosis Research, Baltimore, said in an interview.
Importantly, the study should help push forward “future studies [that] will stratify patients according to their likelihood of responding to shorter treatments,” he said.
The current global standard for TB treatment, practiced for 4 decades, has been a 6-month rifampin-based regimen. Although the regimen performs well, curing more than 95% of cases in clinical trials, in real-world practice, the prolonged duration can be problematic, with issues of nonadherence and loss of patients to follow-up.
Previous research has shown that shorter regimens have potential, with some studies showing as many as 85% of patients cured with 3- and 4-month regimens, and some promising 2-month regimens showing efficacy specifically for those with smear-negative TB.
These efforts suggest that “the current 6-month regimen may lead to overtreatment in the majority of persons in order to prevent relapse in a minority of persons,” the authors asserted.
TRUNCATE-TB
To investigate a suitable shorter-term alternative, the authors conducted the phase 2-3, prospective, open-label TRUNCATE-TB trial, in which 674 patients with rifampin-susceptible pulmonary TB were enrolled at 18 sites in Asia and Africa.
The patients were randomly assigned to receive either the standard treatment regimen (rifampin and isoniazid for 24 weeks with pyrazinamide and ethambutol for the first 8 weeks; n = 181), or one of four novel five-drug regimens to be administered over 8 weeks, along with extended treatment for persistent clinical disease of up to 12 weeks, if needed, and a plan for retreatment in the case of relapse (n = 493).
Two of the regimens were dropped because of logistic criteria; the two remaining shorter-course groups included in the study involved either high-dose rifampin plus linezolid or bedaquiline plus linezolid, each combined with isoniazid, pyrazinamide, and ethambutol.
Of the patients, 62% were male, and four withdrew or were lost to follow-up by the end of the study at a final follow-up at week 96.
Among patients assigned to the 8-week regimens, 80% stopped at exactly 8 weeks, while 9% wound up having extended treatment to 10 weeks and 3% were extended to 12 weeks.
For the primary endpoint, a composite of death, ongoing treatment, or active disease at week 96, the rate was lowest in the standard 24-week therapy group, occurring in 7 of 181 patients (3.9%), compared with 21 of 184 patients (11.4%) in the rifampin plus linezolid group (adjusted difference, 7.4 percentage points, which did not meet noninferiority criterion), and 11 of 189 (5.8%) in the group in the bedaquiline plus linezolid group (adjusted difference, 0.8 percentage points, meeting noninferiority criterion).
The mean total duration of treatment through week 96 in the standard treatment group was 180 days versus 106 days in the rifampin–linezolid group, and 85 days in the bedaquiline-linezolid group.
The results were consistent across multiple subgroups defined according to baseline characteristics, including some that could be linked to severe disease and a high risk for relapse.
In terms of safety, there were no significant differences between the groups in terms of grade 3 or 4 adverse events.
Of note, only two patients (1.1%) in the bedaquiline plus linezolid group acquired a resistance, which Dr. Paton said was “encouraging,” because of concerns about resistance to that drug.
‘Unfavorable’ composite also evaluated
In an updated analysis of the study that Dr. Paton presented at the meeting, the authors looked at a revised “unfavorable” primary outcome – a composite including treatment failure, relapse, death, or nonattendance at week 96 without evidence of prior disease clearance.
The rate remained lowest in the standard 24-week therapy group (3.9%) versus 25% in the rifampin plus linezolid group, and 13.8% in the bedaquiline plus linezolid group.
Though the lower rate with the standard treatment was expected, Dr. Paton said the results nevertheless hold promise, at least for some patients, for successful treatment with the 8-week bedaquiline plus linezolid strategy.
“What the trial has told us is that even with that 13.8% relapse rate, we can manage patients within this strategy and people can do fine at the end, because with some simple clinical biomarkers, we can pick the people who may have a high chance of achieving a cure.”
Dr. Chaisson expressed concern over the higher unfavorable rates, but said the results help pave the way for refining a workable-shorter term strategy.
“TRUNCATE-TB did find that most patients could be successfully treated in 2 months with the novel regimen of bedaquiline plus linezolid, but the failure rate was still unacceptably high,” he said.
“This regimen will not be widely adapted at this point, but additional analyses may identify subsets of patients who will do well with shorter regimens, and future studies will stratify patients according to their likelihood of responding to shorter treatments.”
The authors of an accompanying editorial further commented that the benefits of a shorter treatment strategy could very well outweigh possible shortcomings.
“Treatment algorithms such as that used in the TRUNCATE-TB trial are fundamental to tuberculosis control,” wrote Véronique Dartois, PhD, Center for Discovery and Innovation, Nutley, N.J., and Eric J. Rubin, MD, PhD, the editor-in-chief of NEJM. “Although implementing them could be a challenge, any added burden might be offset by reduced costs, better adherence, and increased patient satisfaction. Thus, for tuberculosis, a strategy might be more than just a regimen.”
The good news, as summed up by CROI vice-chair Landon Myer, MD, PhD, in the press conference, is that “we’re moving closer and closer to the holy grail of a short, efficacious regimen for TB treatment. We’re getting there slowly, but we’re getting there.”
The study received grant funding from the Singapore National Medical Research Council; a grant from the Department of Health and Social Care; the Foreign, Commonwealth, and Development Office; the Medical Research Council; and Wellcome Trust; as well as a grant from the UK Research and Innovation Medical Research Council. Dr. Dartois reported no relevant financial relationships. Dr. Chaisson had no disclosures to report.
A version of this article originally appeared on Medscape.com.
A strategy for the
“We found that if we use the strategy of a bedaquiline-linezolid five-drug regimen for 8 weeks and then followed patients for 96 weeks, [the regimen] was noninferior, clinically, to the standard regimen in terms of the number of people alive, free of TB disease, and not on treatment,” said lead author Nicholas Paton, MD, of the National University of Singapore, in a press conference held during the Conference on Retroviruses & Opportunistic Infections.
“The total time on treatment was reduced by half – instead of 160 days, it was 85 days for the total duration.”
Commenting on the study, which was published concurrently in the New England Journal of Medicine, Richard E. Chaisson, MD, noted that, although more needs to be understood, the high number of responses is nevertheless encouraging.
“Clinicians will not feel comfortable with the short regimens at this point, but it is remarkable that so many patients did well with shorter treatments,” Dr. Chaisson, who is a professor of medicine, epidemiology, and international health and director of the Johns Hopkins University Center for Tuberculosis Research, Baltimore, said in an interview.
Importantly, the study should help push forward “future studies [that] will stratify patients according to their likelihood of responding to shorter treatments,” he said.
The current global standard for TB treatment, practiced for 4 decades, has been a 6-month rifampin-based regimen. Although the regimen performs well, curing more than 95% of cases in clinical trials, in real-world practice, the prolonged duration can be problematic, with issues of nonadherence and loss of patients to follow-up.
Previous research has shown that shorter regimens have potential, with some studies showing as many as 85% of patients cured with 3- and 4-month regimens, and some promising 2-month regimens showing efficacy specifically for those with smear-negative TB.
These efforts suggest that “the current 6-month regimen may lead to overtreatment in the majority of persons in order to prevent relapse in a minority of persons,” the authors asserted.
TRUNCATE-TB
To investigate a suitable shorter-term alternative, the authors conducted the phase 2-3, prospective, open-label TRUNCATE-TB trial, in which 674 patients with rifampin-susceptible pulmonary TB were enrolled at 18 sites in Asia and Africa.
The patients were randomly assigned to receive either the standard treatment regimen (rifampin and isoniazid for 24 weeks with pyrazinamide and ethambutol for the first 8 weeks; n = 181), or one of four novel five-drug regimens to be administered over 8 weeks, along with extended treatment for persistent clinical disease of up to 12 weeks, if needed, and a plan for retreatment in the case of relapse (n = 493).
Two of the regimens were dropped because of logistic criteria; the two remaining shorter-course groups included in the study involved either high-dose rifampin plus linezolid or bedaquiline plus linezolid, each combined with isoniazid, pyrazinamide, and ethambutol.
Of the patients, 62% were male, and four withdrew or were lost to follow-up by the end of the study at a final follow-up at week 96.
Among patients assigned to the 8-week regimens, 80% stopped at exactly 8 weeks, while 9% wound up having extended treatment to 10 weeks and 3% were extended to 12 weeks.
For the primary endpoint, a composite of death, ongoing treatment, or active disease at week 96, the rate was lowest in the standard 24-week therapy group, occurring in 7 of 181 patients (3.9%), compared with 21 of 184 patients (11.4%) in the rifampin plus linezolid group (adjusted difference, 7.4 percentage points, which did not meet noninferiority criterion), and 11 of 189 (5.8%) in the group in the bedaquiline plus linezolid group (adjusted difference, 0.8 percentage points, meeting noninferiority criterion).
The mean total duration of treatment through week 96 in the standard treatment group was 180 days versus 106 days in the rifampin–linezolid group, and 85 days in the bedaquiline-linezolid group.
The results were consistent across multiple subgroups defined according to baseline characteristics, including some that could be linked to severe disease and a high risk for relapse.
In terms of safety, there were no significant differences between the groups in terms of grade 3 or 4 adverse events.
Of note, only two patients (1.1%) in the bedaquiline plus linezolid group acquired a resistance, which Dr. Paton said was “encouraging,” because of concerns about resistance to that drug.
‘Unfavorable’ composite also evaluated
In an updated analysis of the study that Dr. Paton presented at the meeting, the authors looked at a revised “unfavorable” primary outcome – a composite including treatment failure, relapse, death, or nonattendance at week 96 without evidence of prior disease clearance.
The rate remained lowest in the standard 24-week therapy group (3.9%) versus 25% in the rifampin plus linezolid group, and 13.8% in the bedaquiline plus linezolid group.
Though the lower rate with the standard treatment was expected, Dr. Paton said the results nevertheless hold promise, at least for some patients, for successful treatment with the 8-week bedaquiline plus linezolid strategy.
“What the trial has told us is that even with that 13.8% relapse rate, we can manage patients within this strategy and people can do fine at the end, because with some simple clinical biomarkers, we can pick the people who may have a high chance of achieving a cure.”
Dr. Chaisson expressed concern over the higher unfavorable rates, but said the results help pave the way for refining a workable-shorter term strategy.
“TRUNCATE-TB did find that most patients could be successfully treated in 2 months with the novel regimen of bedaquiline plus linezolid, but the failure rate was still unacceptably high,” he said.
“This regimen will not be widely adapted at this point, but additional analyses may identify subsets of patients who will do well with shorter regimens, and future studies will stratify patients according to their likelihood of responding to shorter treatments.”
The authors of an accompanying editorial further commented that the benefits of a shorter treatment strategy could very well outweigh possible shortcomings.
“Treatment algorithms such as that used in the TRUNCATE-TB trial are fundamental to tuberculosis control,” wrote Véronique Dartois, PhD, Center for Discovery and Innovation, Nutley, N.J., and Eric J. Rubin, MD, PhD, the editor-in-chief of NEJM. “Although implementing them could be a challenge, any added burden might be offset by reduced costs, better adherence, and increased patient satisfaction. Thus, for tuberculosis, a strategy might be more than just a regimen.”
The good news, as summed up by CROI vice-chair Landon Myer, MD, PhD, in the press conference, is that “we’re moving closer and closer to the holy grail of a short, efficacious regimen for TB treatment. We’re getting there slowly, but we’re getting there.”
The study received grant funding from the Singapore National Medical Research Council; a grant from the Department of Health and Social Care; the Foreign, Commonwealth, and Development Office; the Medical Research Council; and Wellcome Trust; as well as a grant from the UK Research and Innovation Medical Research Council. Dr. Dartois reported no relevant financial relationships. Dr. Chaisson had no disclosures to report.
A version of this article originally appeared on Medscape.com.
FROM CROI 2023
Alzheimer’s Disease Pathophysiology
How to recognize and treat hidden inflammation
“This then leads to inflammation, the healing of which the body is unable to keep under control,” explained Ulf Müller-Ladner, MD, PhD, chairperson of the German Society of Internal Medicine.
At the DGIM annual press conference, Dr. Müller-Ladner, who is also director of the department of rheumatology and clinical immunology at the Kerckhoff Clinic in Bad Nauheim, Germany, explained how IgG4 inflammation is triggered throughout the body and what therapeutic options are available.
Many manifestations
IgG4-associated inflammation can affect one or more organs or the surrounding connective tissue and cause fibrosis. As a result of fibrosis, the organ gradually loses function and is eventually transformed completely into scarred connective tissue.
“In the case of IgG4-associated inflammation, these fibroses have a histological structure, but extracting a sample is not possible from every affected organ,” said Dr. Müller-Ladner. Liver, bile ducts, blood vessels, skin, eyes, or even the central nervous system – practically every organ system can be affected by these inflammatory reactions.
IgG4-associated diseases have likely been around for some time, but it is only in the past 10 years that awareness has grown that, despite various manifestations, “they are all one and the same disease,” said Dr. Müller-Ladner.
IgG4-associated chronic, inflammatory, fibrosing diseases were only classified together as a single entity in the past few years. In terms of pathophysiology, B lymphocytes, IgG4-positive plasma cells, follicular T-helper cells, cytotoxic CD4-positive T cells, and macrophages work together and trigger an inflammatory reaction, which then encourages fibroblasts to overproduce connective tissue.
Beware inexplicable inflammation
It is estimated that 1 in 100,000 people suffer from the disease, but the number of incorrectly categorized patients may be significantly higher.
The diagnostic challenge lies in the fact that IgG4-associated inflammation occurs in almost every organ. It can cause different symptoms, depending on the organ affected.
Dr. Müller-Ladner provided the following take-home message: “Every inexplicable inflammation event and every organ dysfunction, especially if associated with an increase in connective tissue, could be an IgG4-associated disease. Keeping this in mind is the key to recovery.”
With most people, the inflammation persists for many years before any symptoms of the disease develop. Highly acute courses of progression are also possible.
Classic symptoms, such as fever, are not so characteristic of the latent inflammatory reaction, and according to classification criteria published by specialist rheumatology societies, they are an exclusion criterion. This is true with respect to the differential diagnosis for vasculitis, which also occurs throughout the body.
Histology is key
Blood levels of IgG4 and imaging are not always enough to confirm the diagnosis. In such cases, the histology is often a crucial factor in making a definitive diagnosis. Dominant organs in IgG4-associated diseases are the pancreas, the liver, the gallbladder, the intestines, the retroperitoneum, large blood vessels, the kidneys, the heart, the brain, saliva, tear ducts, as well all of the body’s connective tissue.
The kidneys play host to inflammation in the connective tissue and space-occupying masses in particular. “If the pancreas is affected, the signs can vary from diffuse swelling to the onset of diabetes mellitus. In contrast, if the aorta is affected, then the inflammation is characterized through a thickening of the vessel walls, aneurysms, and the corresponding circulation disorders,” said Dr. Müller-Ladner.
Because of the long period before the diagnosis is made, more than 50% of patients exhibit irreversible organ damage at the time of diagnosis, he added.
Glucocorticoids and immunosuppressants
Despite therapeutic intervention, the disease can have a fatal outcome, even if the patient is young, said Dr. Müller-Ladner. Glucocorticoids are the current therapy of choice. The dose is more than 0.5 mg of prednisolone equivalent per kg of body weight. “This usually leads to a rapid improvement in the inflammation. Subsequently, every organ is thoroughly diagnosed to assess the severity of the disease and to plan further treatment steps.”
In the long term, proven immunosuppressants, such as azathioprine, mycophenolate, leflunomide, and methotrexate, can be used, just as for many other chronic inflammatory diseases. Cyclophosphamide or cyclosporine is used more rarely, owing to their side effect profiles.
Because of the B-cell dominance, B-cell–depleting therapy with rituximab is currently a highly effective therapeutic option but one that must be applied for, because such use is off label. “If the body responds well to the medication, organ function often recovers,” said Dr. Müller-Ladner.
This article was translated from the Medscape German edition. A version appeared on Medscape.com.
“This then leads to inflammation, the healing of which the body is unable to keep under control,” explained Ulf Müller-Ladner, MD, PhD, chairperson of the German Society of Internal Medicine.
At the DGIM annual press conference, Dr. Müller-Ladner, who is also director of the department of rheumatology and clinical immunology at the Kerckhoff Clinic in Bad Nauheim, Germany, explained how IgG4 inflammation is triggered throughout the body and what therapeutic options are available.
Many manifestations
IgG4-associated inflammation can affect one or more organs or the surrounding connective tissue and cause fibrosis. As a result of fibrosis, the organ gradually loses function and is eventually transformed completely into scarred connective tissue.
“In the case of IgG4-associated inflammation, these fibroses have a histological structure, but extracting a sample is not possible from every affected organ,” said Dr. Müller-Ladner. Liver, bile ducts, blood vessels, skin, eyes, or even the central nervous system – practically every organ system can be affected by these inflammatory reactions.
IgG4-associated diseases have likely been around for some time, but it is only in the past 10 years that awareness has grown that, despite various manifestations, “they are all one and the same disease,” said Dr. Müller-Ladner.
IgG4-associated chronic, inflammatory, fibrosing diseases were only classified together as a single entity in the past few years. In terms of pathophysiology, B lymphocytes, IgG4-positive plasma cells, follicular T-helper cells, cytotoxic CD4-positive T cells, and macrophages work together and trigger an inflammatory reaction, which then encourages fibroblasts to overproduce connective tissue.
Beware inexplicable inflammation
It is estimated that 1 in 100,000 people suffer from the disease, but the number of incorrectly categorized patients may be significantly higher.
The diagnostic challenge lies in the fact that IgG4-associated inflammation occurs in almost every organ. It can cause different symptoms, depending on the organ affected.
Dr. Müller-Ladner provided the following take-home message: “Every inexplicable inflammation event and every organ dysfunction, especially if associated with an increase in connective tissue, could be an IgG4-associated disease. Keeping this in mind is the key to recovery.”
With most people, the inflammation persists for many years before any symptoms of the disease develop. Highly acute courses of progression are also possible.
Classic symptoms, such as fever, are not so characteristic of the latent inflammatory reaction, and according to classification criteria published by specialist rheumatology societies, they are an exclusion criterion. This is true with respect to the differential diagnosis for vasculitis, which also occurs throughout the body.
Histology is key
Blood levels of IgG4 and imaging are not always enough to confirm the diagnosis. In such cases, the histology is often a crucial factor in making a definitive diagnosis. Dominant organs in IgG4-associated diseases are the pancreas, the liver, the gallbladder, the intestines, the retroperitoneum, large blood vessels, the kidneys, the heart, the brain, saliva, tear ducts, as well all of the body’s connective tissue.
The kidneys play host to inflammation in the connective tissue and space-occupying masses in particular. “If the pancreas is affected, the signs can vary from diffuse swelling to the onset of diabetes mellitus. In contrast, if the aorta is affected, then the inflammation is characterized through a thickening of the vessel walls, aneurysms, and the corresponding circulation disorders,” said Dr. Müller-Ladner.
Because of the long period before the diagnosis is made, more than 50% of patients exhibit irreversible organ damage at the time of diagnosis, he added.
Glucocorticoids and immunosuppressants
Despite therapeutic intervention, the disease can have a fatal outcome, even if the patient is young, said Dr. Müller-Ladner. Glucocorticoids are the current therapy of choice. The dose is more than 0.5 mg of prednisolone equivalent per kg of body weight. “This usually leads to a rapid improvement in the inflammation. Subsequently, every organ is thoroughly diagnosed to assess the severity of the disease and to plan further treatment steps.”
In the long term, proven immunosuppressants, such as azathioprine, mycophenolate, leflunomide, and methotrexate, can be used, just as for many other chronic inflammatory diseases. Cyclophosphamide or cyclosporine is used more rarely, owing to their side effect profiles.
Because of the B-cell dominance, B-cell–depleting therapy with rituximab is currently a highly effective therapeutic option but one that must be applied for, because such use is off label. “If the body responds well to the medication, organ function often recovers,” said Dr. Müller-Ladner.
This article was translated from the Medscape German edition. A version appeared on Medscape.com.
“This then leads to inflammation, the healing of which the body is unable to keep under control,” explained Ulf Müller-Ladner, MD, PhD, chairperson of the German Society of Internal Medicine.
At the DGIM annual press conference, Dr. Müller-Ladner, who is also director of the department of rheumatology and clinical immunology at the Kerckhoff Clinic in Bad Nauheim, Germany, explained how IgG4 inflammation is triggered throughout the body and what therapeutic options are available.
Many manifestations
IgG4-associated inflammation can affect one or more organs or the surrounding connective tissue and cause fibrosis. As a result of fibrosis, the organ gradually loses function and is eventually transformed completely into scarred connective tissue.
“In the case of IgG4-associated inflammation, these fibroses have a histological structure, but extracting a sample is not possible from every affected organ,” said Dr. Müller-Ladner. Liver, bile ducts, blood vessels, skin, eyes, or even the central nervous system – practically every organ system can be affected by these inflammatory reactions.
IgG4-associated diseases have likely been around for some time, but it is only in the past 10 years that awareness has grown that, despite various manifestations, “they are all one and the same disease,” said Dr. Müller-Ladner.
IgG4-associated chronic, inflammatory, fibrosing diseases were only classified together as a single entity in the past few years. In terms of pathophysiology, B lymphocytes, IgG4-positive plasma cells, follicular T-helper cells, cytotoxic CD4-positive T cells, and macrophages work together and trigger an inflammatory reaction, which then encourages fibroblasts to overproduce connective tissue.
Beware inexplicable inflammation
It is estimated that 1 in 100,000 people suffer from the disease, but the number of incorrectly categorized patients may be significantly higher.
The diagnostic challenge lies in the fact that IgG4-associated inflammation occurs in almost every organ. It can cause different symptoms, depending on the organ affected.
Dr. Müller-Ladner provided the following take-home message: “Every inexplicable inflammation event and every organ dysfunction, especially if associated with an increase in connective tissue, could be an IgG4-associated disease. Keeping this in mind is the key to recovery.”
With most people, the inflammation persists for many years before any symptoms of the disease develop. Highly acute courses of progression are also possible.
Classic symptoms, such as fever, are not so characteristic of the latent inflammatory reaction, and according to classification criteria published by specialist rheumatology societies, they are an exclusion criterion. This is true with respect to the differential diagnosis for vasculitis, which also occurs throughout the body.
Histology is key
Blood levels of IgG4 and imaging are not always enough to confirm the diagnosis. In such cases, the histology is often a crucial factor in making a definitive diagnosis. Dominant organs in IgG4-associated diseases are the pancreas, the liver, the gallbladder, the intestines, the retroperitoneum, large blood vessels, the kidneys, the heart, the brain, saliva, tear ducts, as well all of the body’s connective tissue.
The kidneys play host to inflammation in the connective tissue and space-occupying masses in particular. “If the pancreas is affected, the signs can vary from diffuse swelling to the onset of diabetes mellitus. In contrast, if the aorta is affected, then the inflammation is characterized through a thickening of the vessel walls, aneurysms, and the corresponding circulation disorders,” said Dr. Müller-Ladner.
Because of the long period before the diagnosis is made, more than 50% of patients exhibit irreversible organ damage at the time of diagnosis, he added.
Glucocorticoids and immunosuppressants
Despite therapeutic intervention, the disease can have a fatal outcome, even if the patient is young, said Dr. Müller-Ladner. Glucocorticoids are the current therapy of choice. The dose is more than 0.5 mg of prednisolone equivalent per kg of body weight. “This usually leads to a rapid improvement in the inflammation. Subsequently, every organ is thoroughly diagnosed to assess the severity of the disease and to plan further treatment steps.”
In the long term, proven immunosuppressants, such as azathioprine, mycophenolate, leflunomide, and methotrexate, can be used, just as for many other chronic inflammatory diseases. Cyclophosphamide or cyclosporine is used more rarely, owing to their side effect profiles.
Because of the B-cell dominance, B-cell–depleting therapy with rituximab is currently a highly effective therapeutic option but one that must be applied for, because such use is off label. “If the body responds well to the medication, organ function often recovers,” said Dr. Müller-Ladner.
This article was translated from the Medscape German edition. A version appeared on Medscape.com.
COVID infection provides immunity equal to vaccination: Study
People who’ve been infected with COVID reduced their chances of hospitalization and death by 88% over 10 months compared to somebody who hasn’t been infected, according to the study, published in The Lancet.
The natural immunity provided by infection was “at least as high, if not higher” than the immunity provided by two doses of Moderna or Pfizer mRNA vaccines against the ancestral, Alpha, Delta, and Omicron BA.1 variants, the researchers reported.
But protection against the BA.1 subvariant of Omicron was not as high – 36% at 10 months after infection, wrote the research team from the Institute for Health Metrics and Evaluation at the University of Washington.
They examined 65 studies from 19 countries through Sept. 31, 2022. They did not study data about infection from Omicron XBB and its sub-lineages. People who had immunity from both infection and vaccination, known as hybrid immunity, were not studied.
The findings don’t mean people should skip the vaccines and get COVID on purpose, one of the researchers told NBC News.
“The problem of saying ‘I’m gonna get infected to get immunity’ is you might be one of those people that end up in the hospital or die,” said Christopher Murray, MD, DPhil, director of the IHME. “Why would you take the risk when you can get immunity through vaccination quite safely?”
The findings could help people figure out the most effective time to get vaccinated or boosted and guide officials in setting policies on workplace vaccine mandates and rules for high-occupancy indoor settings, the researchers concluded.
This was the largest meta-analysis of immunity following infection to date, NBC News reports.
A version of this article originally appeared on WebMD.com.
People who’ve been infected with COVID reduced their chances of hospitalization and death by 88% over 10 months compared to somebody who hasn’t been infected, according to the study, published in The Lancet.
The natural immunity provided by infection was “at least as high, if not higher” than the immunity provided by two doses of Moderna or Pfizer mRNA vaccines against the ancestral, Alpha, Delta, and Omicron BA.1 variants, the researchers reported.
But protection against the BA.1 subvariant of Omicron was not as high – 36% at 10 months after infection, wrote the research team from the Institute for Health Metrics and Evaluation at the University of Washington.
They examined 65 studies from 19 countries through Sept. 31, 2022. They did not study data about infection from Omicron XBB and its sub-lineages. People who had immunity from both infection and vaccination, known as hybrid immunity, were not studied.
The findings don’t mean people should skip the vaccines and get COVID on purpose, one of the researchers told NBC News.
“The problem of saying ‘I’m gonna get infected to get immunity’ is you might be one of those people that end up in the hospital or die,” said Christopher Murray, MD, DPhil, director of the IHME. “Why would you take the risk when you can get immunity through vaccination quite safely?”
The findings could help people figure out the most effective time to get vaccinated or boosted and guide officials in setting policies on workplace vaccine mandates and rules for high-occupancy indoor settings, the researchers concluded.
This was the largest meta-analysis of immunity following infection to date, NBC News reports.
A version of this article originally appeared on WebMD.com.
People who’ve been infected with COVID reduced their chances of hospitalization and death by 88% over 10 months compared to somebody who hasn’t been infected, according to the study, published in The Lancet.
The natural immunity provided by infection was “at least as high, if not higher” than the immunity provided by two doses of Moderna or Pfizer mRNA vaccines against the ancestral, Alpha, Delta, and Omicron BA.1 variants, the researchers reported.
But protection against the BA.1 subvariant of Omicron was not as high – 36% at 10 months after infection, wrote the research team from the Institute for Health Metrics and Evaluation at the University of Washington.
They examined 65 studies from 19 countries through Sept. 31, 2022. They did not study data about infection from Omicron XBB and its sub-lineages. People who had immunity from both infection and vaccination, known as hybrid immunity, were not studied.
The findings don’t mean people should skip the vaccines and get COVID on purpose, one of the researchers told NBC News.
“The problem of saying ‘I’m gonna get infected to get immunity’ is you might be one of those people that end up in the hospital or die,” said Christopher Murray, MD, DPhil, director of the IHME. “Why would you take the risk when you can get immunity through vaccination quite safely?”
The findings could help people figure out the most effective time to get vaccinated or boosted and guide officials in setting policies on workplace vaccine mandates and rules for high-occupancy indoor settings, the researchers concluded.
This was the largest meta-analysis of immunity following infection to date, NBC News reports.
A version of this article originally appeared on WebMD.com.
FROM THE LANCET
How to manage isotretinoin’s bothersome mucocutaneous side effects
HONOLULU –
“If they don’t have dry lips, you have to wonder if they’re even absorbing isotretinoin,” Dr. Barbieri, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “Everyone is going to get dry lips.”
According to a retrospective review of 1,743 patients started on isotretinoin, other common mucocutaneous side effects include eczema, nose bleeds, and eye problems. Emerging research suggests that there may be a role for oral omega-3 in decreasing such side effects of the drug. In a case control study, 118 patients were randomized to isotretinoin alone or isotretinoin plus 1 g/day of oral omega-3 for 16 weeks. At week 16, the rate of dry lips was 26% in the isoretinoin only group compared with 14% in the combination group; similar trends were seen with dry nose (11% vs. 0 %, respectively) and dry skin (11% vs. 2%).
“Omega-3 is a simple thing that we can think about recommending for patients,” Dr. Barbieri said. “It’s very safe, inexpensive, and it may help us manage these common sides effect we run into.”
Another potential side effect of isotretinoin that he characterized as underappreciated is chronic dry eye and other ocular changes. One retrospective cohort study of 14,682 adolescents and young adults in Israel found that use of the drug resulted in reduced tear production and reduced tear quality. In another study, a review and meta-analysis of 21 publications involving 1,105 eyes of 842 patients, isotretinoin use was associated with increased conjunctival fluorescein staining, decreased corneal thickness, and worse patient-reported ocular surface disease index scores.
“These changes may be mediated by meibomian gland dysfunction and atrophy,” Dr. Barbieri said. “Fortunately, many of these tear film changes appear to resolve after treatment. Those changes in corneal thickness do seem to get better. That’s reassuring.”
In a study of 54 patients treated with isotretinoin, tear production and quality returned to baseline within 6 months of treatment completion. “But some changes in the meibomian gland may be persistent,” Dr. Barbieri said. “At 6 and 12 months after the end of treatment, you can still see changes in the meibomian glands of patients who were treated with a standard course of 120 to 150 mg/kg isotretinoin,” he said, referring to the results of a study of 88 patients .
One study investigated the effects of omega-3 fatty acids and punctal plugs on tear film and ocular surface parameters in 90 patients receiving systemic isotretinoin therapy. They were divided into three groups: Those who received a soft preloaded silicone plug that was inserted in the inferior punctum of both eyes and received oral omega-3 fatty acid capsules twice daily for a total dose of 1,040 mg/day for 6 months; those who received a soft preloaded silicone plug and oral placebo, and those who received isotretinoin alone. At 6 months’ follow-up, those who were treated with omega-3 combined with the preloaded silicone plug had better meibomian gland function than did those who received isotretinoin alone or isotretinoin with the preloaded silicone plug.
Dr. Barbieri also noted that antihistamines may play a role in enhancing the effect of isotretinoin. In one study, 20 patients were treated with isotretinoin 0.4 mg/kg per day and 20 patients were also treated with an antihistamine, desloratadine 5 mg/day for 12 weeks. At week 12, patients in the group treated with isotretinoin and the antihistamine showed a more statistically significant decrease in acne lesion counts, compared with the isotretinoin-only group (reductions of 44.8% vs. 17.8%, respectively, in noninflammatory lesions; 55.8% vs. 22.9% in inflammatory lesions, and 45.6% vs. 18.7% in total lesions (P < .05 for all associations).
A subsequent larger study yielded similar findings. There were also lower rates of initial flaring and higher rates of patient satisfaction in the antihistamine groups in both studies.
In an interview at the meeting, Lawrence F. Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, described Dr. Barbieri as “a leader in taking a comprehensive view on what the history and latest information is on isotretinoin. His fresh approach is something everyone should consider and figure out what they can use in their practice.”
Dr. Barbieri disclosed that he receives consulting fees from Dexcel for work unrelated to his presentation. Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics. Medscape and this news organization are owned by the same parent company.
HONOLULU –
“If they don’t have dry lips, you have to wonder if they’re even absorbing isotretinoin,” Dr. Barbieri, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “Everyone is going to get dry lips.”
According to a retrospective review of 1,743 patients started on isotretinoin, other common mucocutaneous side effects include eczema, nose bleeds, and eye problems. Emerging research suggests that there may be a role for oral omega-3 in decreasing such side effects of the drug. In a case control study, 118 patients were randomized to isotretinoin alone or isotretinoin plus 1 g/day of oral omega-3 for 16 weeks. At week 16, the rate of dry lips was 26% in the isoretinoin only group compared with 14% in the combination group; similar trends were seen with dry nose (11% vs. 0 %, respectively) and dry skin (11% vs. 2%).
“Omega-3 is a simple thing that we can think about recommending for patients,” Dr. Barbieri said. “It’s very safe, inexpensive, and it may help us manage these common sides effect we run into.”
Another potential side effect of isotretinoin that he characterized as underappreciated is chronic dry eye and other ocular changes. One retrospective cohort study of 14,682 adolescents and young adults in Israel found that use of the drug resulted in reduced tear production and reduced tear quality. In another study, a review and meta-analysis of 21 publications involving 1,105 eyes of 842 patients, isotretinoin use was associated with increased conjunctival fluorescein staining, decreased corneal thickness, and worse patient-reported ocular surface disease index scores.
“These changes may be mediated by meibomian gland dysfunction and atrophy,” Dr. Barbieri said. “Fortunately, many of these tear film changes appear to resolve after treatment. Those changes in corneal thickness do seem to get better. That’s reassuring.”
In a study of 54 patients treated with isotretinoin, tear production and quality returned to baseline within 6 months of treatment completion. “But some changes in the meibomian gland may be persistent,” Dr. Barbieri said. “At 6 and 12 months after the end of treatment, you can still see changes in the meibomian glands of patients who were treated with a standard course of 120 to 150 mg/kg isotretinoin,” he said, referring to the results of a study of 88 patients .
One study investigated the effects of omega-3 fatty acids and punctal plugs on tear film and ocular surface parameters in 90 patients receiving systemic isotretinoin therapy. They were divided into three groups: Those who received a soft preloaded silicone plug that was inserted in the inferior punctum of both eyes and received oral omega-3 fatty acid capsules twice daily for a total dose of 1,040 mg/day for 6 months; those who received a soft preloaded silicone plug and oral placebo, and those who received isotretinoin alone. At 6 months’ follow-up, those who were treated with omega-3 combined with the preloaded silicone plug had better meibomian gland function than did those who received isotretinoin alone or isotretinoin with the preloaded silicone plug.
Dr. Barbieri also noted that antihistamines may play a role in enhancing the effect of isotretinoin. In one study, 20 patients were treated with isotretinoin 0.4 mg/kg per day and 20 patients were also treated with an antihistamine, desloratadine 5 mg/day for 12 weeks. At week 12, patients in the group treated with isotretinoin and the antihistamine showed a more statistically significant decrease in acne lesion counts, compared with the isotretinoin-only group (reductions of 44.8% vs. 17.8%, respectively, in noninflammatory lesions; 55.8% vs. 22.9% in inflammatory lesions, and 45.6% vs. 18.7% in total lesions (P < .05 for all associations).
A subsequent larger study yielded similar findings. There were also lower rates of initial flaring and higher rates of patient satisfaction in the antihistamine groups in both studies.
In an interview at the meeting, Lawrence F. Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, described Dr. Barbieri as “a leader in taking a comprehensive view on what the history and latest information is on isotretinoin. His fresh approach is something everyone should consider and figure out what they can use in their practice.”
Dr. Barbieri disclosed that he receives consulting fees from Dexcel for work unrelated to his presentation. Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics. Medscape and this news organization are owned by the same parent company.
HONOLULU –
“If they don’t have dry lips, you have to wonder if they’re even absorbing isotretinoin,” Dr. Barbieri, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “Everyone is going to get dry lips.”
According to a retrospective review of 1,743 patients started on isotretinoin, other common mucocutaneous side effects include eczema, nose bleeds, and eye problems. Emerging research suggests that there may be a role for oral omega-3 in decreasing such side effects of the drug. In a case control study, 118 patients were randomized to isotretinoin alone or isotretinoin plus 1 g/day of oral omega-3 for 16 weeks. At week 16, the rate of dry lips was 26% in the isoretinoin only group compared with 14% in the combination group; similar trends were seen with dry nose (11% vs. 0 %, respectively) and dry skin (11% vs. 2%).
“Omega-3 is a simple thing that we can think about recommending for patients,” Dr. Barbieri said. “It’s very safe, inexpensive, and it may help us manage these common sides effect we run into.”
Another potential side effect of isotretinoin that he characterized as underappreciated is chronic dry eye and other ocular changes. One retrospective cohort study of 14,682 adolescents and young adults in Israel found that use of the drug resulted in reduced tear production and reduced tear quality. In another study, a review and meta-analysis of 21 publications involving 1,105 eyes of 842 patients, isotretinoin use was associated with increased conjunctival fluorescein staining, decreased corneal thickness, and worse patient-reported ocular surface disease index scores.
“These changes may be mediated by meibomian gland dysfunction and atrophy,” Dr. Barbieri said. “Fortunately, many of these tear film changes appear to resolve after treatment. Those changes in corneal thickness do seem to get better. That’s reassuring.”
In a study of 54 patients treated with isotretinoin, tear production and quality returned to baseline within 6 months of treatment completion. “But some changes in the meibomian gland may be persistent,” Dr. Barbieri said. “At 6 and 12 months after the end of treatment, you can still see changes in the meibomian glands of patients who were treated with a standard course of 120 to 150 mg/kg isotretinoin,” he said, referring to the results of a study of 88 patients .
One study investigated the effects of omega-3 fatty acids and punctal plugs on tear film and ocular surface parameters in 90 patients receiving systemic isotretinoin therapy. They were divided into three groups: Those who received a soft preloaded silicone plug that was inserted in the inferior punctum of both eyes and received oral omega-3 fatty acid capsules twice daily for a total dose of 1,040 mg/day for 6 months; those who received a soft preloaded silicone plug and oral placebo, and those who received isotretinoin alone. At 6 months’ follow-up, those who were treated with omega-3 combined with the preloaded silicone plug had better meibomian gland function than did those who received isotretinoin alone or isotretinoin with the preloaded silicone plug.
Dr. Barbieri also noted that antihistamines may play a role in enhancing the effect of isotretinoin. In one study, 20 patients were treated with isotretinoin 0.4 mg/kg per day and 20 patients were also treated with an antihistamine, desloratadine 5 mg/day for 12 weeks. At week 12, patients in the group treated with isotretinoin and the antihistamine showed a more statistically significant decrease in acne lesion counts, compared with the isotretinoin-only group (reductions of 44.8% vs. 17.8%, respectively, in noninflammatory lesions; 55.8% vs. 22.9% in inflammatory lesions, and 45.6% vs. 18.7% in total lesions (P < .05 for all associations).
A subsequent larger study yielded similar findings. There were also lower rates of initial flaring and higher rates of patient satisfaction in the antihistamine groups in both studies.
In an interview at the meeting, Lawrence F. Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, described Dr. Barbieri as “a leader in taking a comprehensive view on what the history and latest information is on isotretinoin. His fresh approach is something everyone should consider and figure out what they can use in their practice.”
Dr. Barbieri disclosed that he receives consulting fees from Dexcel for work unrelated to his presentation. Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics. Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPE LIVE! HAWAII DERMATOLOGY SEMINAR