User login
Primary Hepatic Lymphoma: A Rare Form of Diffuse Large B-Cell Lymphoma of the Liver
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
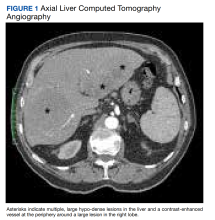
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
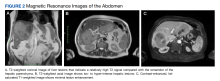
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
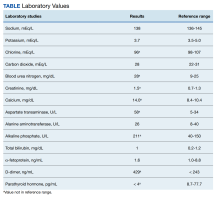
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
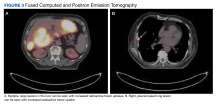
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
Watching feasible for asymptomatic kidney stones
Many patients with asymptomatic renal stones can qualify for an active surveillance program, Swiss researchers report at the American Urological Association 2023 Annual Meeting.
Kevin Stritt, MD, chief resident in the urology department at Lausanne University Hospital, said kidney stones often pass without symptoms. But until now, data on the frequency of asymptomatic, spontaneous passage of stones have been lacking.
The new data come from the NOSTONE trial, a prospective, multicenter, double-blind, placebo-controlled randomized trial to assess the efficacy of hydrochlorothiazide in the prevention of recurrence in patients with recurrent calcium-containing kidney stones.
Dr. Stritt and colleagues evaluated the natural history of asymptomatic renal stones during a median follow-up of 35 months. “We found for the first time that a relevant number of kidney stone passages [39%] were asymptomatic, spontaneous stone passages,” Dr. Stritt told this news organization.
All asymptomatic spontaneous stone passages were analyzed in a comparison of the total number of kidney stones on low-dose, nonintravenous contrast CT imaging at the beginning and end of the 3-year follow-up.
Of the 403 stones passed spontaneously, 61% (245) were symptomatic stone passages and 39% (158) were asymptomatic stone passages, Dr. Stritt told this news organization.
Asymptomatic stones were a median size of 2.4 mm, and symptomatic stones were 2.15 mm, which was not significantly different (P = .366), according to the researchers. Dr. Stritt said the spontaneous passage of asymptomatic stones was largely influenced by a higher number of stones on CT imaging at randomization (P = .001) and a lower total stone volume (P = .001).
Ephrem Olweny, MD, an assistant professor of urology and section chief of endourology at Rush University Medical Center in Chicago, said previous studies have found that the rate of spontaneous passage of kidney stones ranges from 3% to 29%.
“But this secondary analysis of data from a prior multicenter prospective randomized trial offers higher-quality data that will be of value in guiding patient counseling,” Dr. Olweny said.
“Observation should be initially offered to these patients. However, patients should be informed that 52% are likely to develop symptoms, and some may indeed opt for preemptive surgical removal,” he added.
David Schulsinger, MD, an associate professor in the department of urology at Stony Brook (N.Y.) University Hospital, said the incidence of kidney stones has been increasing worldwide, affecting approximately 12% of men and 6% of women. Dehydration and diets high in sodium and calcium are major factors, he said.
Patients with a history of stones have a 50% risk of recurrence in the next 5 years, and an 80% risk in their lifetime, he added.
Dr. Schulsinger said the message from the Swiss study is that urologists can be “comfortable” watching small stones, those averaging 2.4 mm or less in size. “But if a patient has a 7- or 8-mm stone, you might be more inclined to manage that patient a little bit more aggressively.”
Roughly half of patients with stones less than 2 mm will pass it in about 8 days, he said.
Dr. Olweny noted that the study was a secondary analysis of data from a randomized controlled trial that evaluated the efficacy of thiazides in preventing the recurrence of calcium stones. “The original study was not specifically designed to look at asymptomatic stone passage rates for small renal stones, and therefore, the observed rates may not reflect the most precise estimates,” he said.
Dr. Stritt said his group has not studied the size limit of stones that pass spontaneously without symptoms. “This study could serve to construct recurrence prediction models based on medical history and stone burden on CT imaging. More well-designed research on this topic is urgently needed,” he said. “These results should encourage urologists to counsel patients about the possibility of an active surveillance strategy when smaller kidney stones are present.”
The author and independent commentators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many patients with asymptomatic renal stones can qualify for an active surveillance program, Swiss researchers report at the American Urological Association 2023 Annual Meeting.
Kevin Stritt, MD, chief resident in the urology department at Lausanne University Hospital, said kidney stones often pass without symptoms. But until now, data on the frequency of asymptomatic, spontaneous passage of stones have been lacking.
The new data come from the NOSTONE trial, a prospective, multicenter, double-blind, placebo-controlled randomized trial to assess the efficacy of hydrochlorothiazide in the prevention of recurrence in patients with recurrent calcium-containing kidney stones.
Dr. Stritt and colleagues evaluated the natural history of asymptomatic renal stones during a median follow-up of 35 months. “We found for the first time that a relevant number of kidney stone passages [39%] were asymptomatic, spontaneous stone passages,” Dr. Stritt told this news organization.
All asymptomatic spontaneous stone passages were analyzed in a comparison of the total number of kidney stones on low-dose, nonintravenous contrast CT imaging at the beginning and end of the 3-year follow-up.
Of the 403 stones passed spontaneously, 61% (245) were symptomatic stone passages and 39% (158) were asymptomatic stone passages, Dr. Stritt told this news organization.
Asymptomatic stones were a median size of 2.4 mm, and symptomatic stones were 2.15 mm, which was not significantly different (P = .366), according to the researchers. Dr. Stritt said the spontaneous passage of asymptomatic stones was largely influenced by a higher number of stones on CT imaging at randomization (P = .001) and a lower total stone volume (P = .001).
Ephrem Olweny, MD, an assistant professor of urology and section chief of endourology at Rush University Medical Center in Chicago, said previous studies have found that the rate of spontaneous passage of kidney stones ranges from 3% to 29%.
“But this secondary analysis of data from a prior multicenter prospective randomized trial offers higher-quality data that will be of value in guiding patient counseling,” Dr. Olweny said.
“Observation should be initially offered to these patients. However, patients should be informed that 52% are likely to develop symptoms, and some may indeed opt for preemptive surgical removal,” he added.
David Schulsinger, MD, an associate professor in the department of urology at Stony Brook (N.Y.) University Hospital, said the incidence of kidney stones has been increasing worldwide, affecting approximately 12% of men and 6% of women. Dehydration and diets high in sodium and calcium are major factors, he said.
Patients with a history of stones have a 50% risk of recurrence in the next 5 years, and an 80% risk in their lifetime, he added.
Dr. Schulsinger said the message from the Swiss study is that urologists can be “comfortable” watching small stones, those averaging 2.4 mm or less in size. “But if a patient has a 7- or 8-mm stone, you might be more inclined to manage that patient a little bit more aggressively.”
Roughly half of patients with stones less than 2 mm will pass it in about 8 days, he said.
Dr. Olweny noted that the study was a secondary analysis of data from a randomized controlled trial that evaluated the efficacy of thiazides in preventing the recurrence of calcium stones. “The original study was not specifically designed to look at asymptomatic stone passage rates for small renal stones, and therefore, the observed rates may not reflect the most precise estimates,” he said.
Dr. Stritt said his group has not studied the size limit of stones that pass spontaneously without symptoms. “This study could serve to construct recurrence prediction models based on medical history and stone burden on CT imaging. More well-designed research on this topic is urgently needed,” he said. “These results should encourage urologists to counsel patients about the possibility of an active surveillance strategy when smaller kidney stones are present.”
The author and independent commentators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many patients with asymptomatic renal stones can qualify for an active surveillance program, Swiss researchers report at the American Urological Association 2023 Annual Meeting.
Kevin Stritt, MD, chief resident in the urology department at Lausanne University Hospital, said kidney stones often pass without symptoms. But until now, data on the frequency of asymptomatic, spontaneous passage of stones have been lacking.
The new data come from the NOSTONE trial, a prospective, multicenter, double-blind, placebo-controlled randomized trial to assess the efficacy of hydrochlorothiazide in the prevention of recurrence in patients with recurrent calcium-containing kidney stones.
Dr. Stritt and colleagues evaluated the natural history of asymptomatic renal stones during a median follow-up of 35 months. “We found for the first time that a relevant number of kidney stone passages [39%] were asymptomatic, spontaneous stone passages,” Dr. Stritt told this news organization.
All asymptomatic spontaneous stone passages were analyzed in a comparison of the total number of kidney stones on low-dose, nonintravenous contrast CT imaging at the beginning and end of the 3-year follow-up.
Of the 403 stones passed spontaneously, 61% (245) were symptomatic stone passages and 39% (158) were asymptomatic stone passages, Dr. Stritt told this news organization.
Asymptomatic stones were a median size of 2.4 mm, and symptomatic stones were 2.15 mm, which was not significantly different (P = .366), according to the researchers. Dr. Stritt said the spontaneous passage of asymptomatic stones was largely influenced by a higher number of stones on CT imaging at randomization (P = .001) and a lower total stone volume (P = .001).
Ephrem Olweny, MD, an assistant professor of urology and section chief of endourology at Rush University Medical Center in Chicago, said previous studies have found that the rate of spontaneous passage of kidney stones ranges from 3% to 29%.
“But this secondary analysis of data from a prior multicenter prospective randomized trial offers higher-quality data that will be of value in guiding patient counseling,” Dr. Olweny said.
“Observation should be initially offered to these patients. However, patients should be informed that 52% are likely to develop symptoms, and some may indeed opt for preemptive surgical removal,” he added.
David Schulsinger, MD, an associate professor in the department of urology at Stony Brook (N.Y.) University Hospital, said the incidence of kidney stones has been increasing worldwide, affecting approximately 12% of men and 6% of women. Dehydration and diets high in sodium and calcium are major factors, he said.
Patients with a history of stones have a 50% risk of recurrence in the next 5 years, and an 80% risk in their lifetime, he added.
Dr. Schulsinger said the message from the Swiss study is that urologists can be “comfortable” watching small stones, those averaging 2.4 mm or less in size. “But if a patient has a 7- or 8-mm stone, you might be more inclined to manage that patient a little bit more aggressively.”
Roughly half of patients with stones less than 2 mm will pass it in about 8 days, he said.
Dr. Olweny noted that the study was a secondary analysis of data from a randomized controlled trial that evaluated the efficacy of thiazides in preventing the recurrence of calcium stones. “The original study was not specifically designed to look at asymptomatic stone passage rates for small renal stones, and therefore, the observed rates may not reflect the most precise estimates,” he said.
Dr. Stritt said his group has not studied the size limit of stones that pass spontaneously without symptoms. “This study could serve to construct recurrence prediction models based on medical history and stone burden on CT imaging. More well-designed research on this topic is urgently needed,” he said. “These results should encourage urologists to counsel patients about the possibility of an active surveillance strategy when smaller kidney stones are present.”
The author and independent commentators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Diagnosis of Indolent Clonorchis sinensis and Opisthorchis viverrini Infections as Risk Factors for Cholangiocarcinoma: An Unmet Medical Need
Cholangiocarcinoma is a heterogeneous, highly aggressive cancer of the biliary tract epithelium with an overall 5-year relative survival rate of only 9%.1,2 Although surgical resection of localized, intrahepatic cholangiocarcinoma is associated with improved overall survival, most patients present with advanced disease not amenable to surgery due to a late onset of symptoms.2 Recently, an increased incidence of cholangiocarcinoma has been reported in the United States.3 Although relatively rare in the US, cholangiocarcinoma is prevalent across large parts of Asia, including China, Vietnam, Thailand, South Korea, and Taiwan.2
Risk Factors
To date, risk factors for developing cholangiocarcinoma have not been elucidated. 4,5 However, a growing body of literature suggests that chronic infection of genetically susceptible human subjects with Clonorchis sinensis ( C sinensis ) and Opisthorchis viverrini ( O viverrini ) plays a role. 6,7 The life cycle of these food-borne zoonotic trematodes involves eggs discharged in the stool of infected humans, the definitive host. 6,7 In nature, these eggs are ingested by freshwater snails, the intermediate host, where they undergo several developmental stages to form cercariae. Once released from snails into the water, free-swimming cercariae come in contact and penetrate freshwater fish where they encyst as metacercariae. Infection of humans occurs by ingesting undercooked, salted, pickled, or smoked freshwater fish infested with metacercariae. After ingestion, metacercariae excyst in the duodenum and ascend the biliary tract through the ampulla of Vater. They then mature into adult flukes that reside in small- and medium-sized intrahepatic biliary ducts. 6,7
Although most infected people remain asymptomatic, untreated indolent infections with C sinensis and O viverrini may persist in peripheral intrahepatic bile ducts for as long as 30 years, which is the lifespan of the trematodes.6,7 During this prolonged period, C sinensis and O viverrini feeding activities and their excretory-secretory products may damage bile duct epithelium and promote intense local inflammation.6,7 Conceivably, these pathological processes could then provoke the epithelial desquamation, adenomatous hyperplasia, goblet cell metaplasia, periductal fibrosis, and granuloma formation that are conducive to initiation and progression of cholangiocarcinoma in genetically susceptible people.8 Accordingly, the International Agency for Research on Cancer (IARC) has determined that there is sufficient evidence for the carcinogenicity of chronic infections with C sinensis and O viverrini in humans and that chronic infections with these trematodes cause cholangiocarcinoma.9 The IARC concluded that chronic infections with C sinensis and O viverrini are carcinogenic to humans (Group 1).9
Diagnosis
Presently, the diagnosis of C sinensis and O viverrini infection is based on microscopic identification and enumeration of the parasites’ eggs in weighted stool specimens using a formalin-ethyl acetate sedimentation concentration technique. 6,7 This approach requires a labor-intensive test that is conducted by an experienced technician. The test has low specificity and sensitivity because eggs could be confused with those of nonpathogenic intestinal flukes that are morphologically similar and because eggs are not present in feces during all stages of the infection. Although diffuse dilatation of intrahepatic bile ducts by screening sonography is used to diagnose clonorchiasis in endemic areas, it has low sensitivity, particularly in patients with low-level C sinensis and O viverrin i infections. 10
To address the current diagnostic gap, several enzyme-linked immunosorbent assays (ELISA) have been developed for the diagnosis of C sinensis, including monoclonal antibody-based (mAb) ELISA and indirect antibody ELISA.11,12 However, both have important limitations. The mAb ELISA detects only active infections while indirect antibody ELISA cross-reacts with other liver flukes.11,12 Taken together, these data illustrate the difficulties in diagnosing asymptomatic individuals with low-burden C sinensis or O viverrini infections by existing laboratory methods.
Timely serodiagnosis of indolent C sinensis and O viverrini infections is important because these parasites have recently been raised as a risk factor for cholangiocarcinoma in veterans who served in Vietnam.13 The American War Library estimates that as of February 28, 2019, about 610,000 Americans who served on land in Vietnam or in the air over Vietnam between 1954 and 1975 are alive, and about 164,000 Americans who served at sea in Vietnam waters are alive.14 To that end, Psevdos and colleagues screened 97 US veterans who served in Vietnam and identified 50 who reported exposure to raw or undercooked fish while there.13 None had evidence of active C sinensis or O viverrini infection. Blood samples obtained from these veterans were analyzed for circulating C sinensis and O viverrini antibodies using an ELISA developed in South Korea and 12 blood samples tested positive for the trematodes. Imaging of extrahepatic and intrahepatic bile ducts was unyielding in all cases. One veteran diagnosed with cholangiocarcinoma had repeated negative tests. However, the results of this study were challenged by several experts in this field because the authors did not report the sensitivity and specificity of the ELISA assay used.15
Serologic testing of US veterans who served in C sinensis and O viverrini–endemic countries for indolent infections with these parasites is not recommended at present.15 Nevertheless, there is an urgent need to develop sensitive and specific serologic assays, such as ELISA tests with recombinant antigens, to detect both acute and indolent infections caused by each biliary liver fluke in the US, including in patients diagnosed with cholangiocarcinoma. We posit that testing and treatment of high-risk populations could lead to earlier detection and treatment of cholangiocarcinoma, leading to improved overall survival in the population at risk.
1. American Cancer Society. Survival rates for bile duct cancer. Updated March 1, 2023. Accessed March 17, 2023. https://www.cancer.org/cancer/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html
2. Vij M, Puri Y, Rammohan A, et al. Pathological, molecular, and clinical characteristics of cholangiocarcinoma: A comprehensive review. World J Gastrointest Oncol. 2022;14(3):607-627. doi:10.4251/wjgo.v14.i3.607
3. Yao KJ, Jabbour S, Parekh N, Lin Y, Moss RA. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016;16(1):117. Published 2016 Sep 21. doi:10.1186/s12876-016-0527-z
4. Rustagi T, Dasanu CA. Risk factors for gallbladder cancer and cholangiocarcinoma: similarities, differences and updates. J Gastrointest Cancer. 2012;43(2):137-147. doi:10.1007/s12029-011-9284-y
5. Maemura K, Natsugoe S, Takao S. Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat Sci. 2014;21(10):754-760. doi:10.1002/jhbp.126
6. Steele JA, Richter CH, Echaubard P, et al. Thinking beyond Opisthorchis viverrini for risk of cholangiocarcinoma in the lower Mekong region: a systematic review and meta-analysis. Infect Dis Poverty. 2018;7(1):44. Published 2018 May 17. doi:10.1186/s40249-018-0434-3.
7. Kim TS, Pak JH, Kim JB, Bahk YY. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review. BMB Rep. 2016;49(11):590-597. doi:10.5483/bmbrep.2016.49.11.109
8. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. Published 2018 Oct 20. doi:10.1186/s12199-018-0740-1
9. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt B):1-441.
10. Mairiang E, Laha T, Bethony JM, et al. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol Int. 2012;61(1):208-211. doi:10.1016/j.parint.2011.07.009
11. Li HM, Qian MB, Yang YC, et al. Performance evaluation of existing immunoassays for Clonorchis sinensis infection in China. Parasit Vectors. 2018;11(1):35. Published 2018 Jan 15. doi:10.1186/s13071-018-2612-3
12. Hughes T, O’Connor T, Techasen A, et al. Opisthorchiasis and cholangiocarcinoma in Southeast Asia: an unresolved problem. Int J Gen Med. 2017;10:227-237. Published 2017 Aug 10. doi:10.2147/IJGM.S133292
13. Psevdos G, Ford FM, Hong ST. Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war. Infect Dis Clin Pract (Baltim Md). 2018;26(4):208-210. doi:10.1097/IPC.0000000000000611
14. American War Library. In harm’s way... How many real Vietnam vets are alive today? Updated February 28, 2019. Accessed March 17, 2023. https://www.americanwarlibrary.com/personnel/vietvet.htm
15. Nash TE, Sullivan D, Mitre E, et al. Comments on “Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war”. Infect Dis Clin Pract (Baltim Md). 2018;26(4):240-241. doi:10.1097/IPC.0000000000000659
Cholangiocarcinoma is a heterogeneous, highly aggressive cancer of the biliary tract epithelium with an overall 5-year relative survival rate of only 9%.1,2 Although surgical resection of localized, intrahepatic cholangiocarcinoma is associated with improved overall survival, most patients present with advanced disease not amenable to surgery due to a late onset of symptoms.2 Recently, an increased incidence of cholangiocarcinoma has been reported in the United States.3 Although relatively rare in the US, cholangiocarcinoma is prevalent across large parts of Asia, including China, Vietnam, Thailand, South Korea, and Taiwan.2
Risk Factors
To date, risk factors for developing cholangiocarcinoma have not been elucidated. 4,5 However, a growing body of literature suggests that chronic infection of genetically susceptible human subjects with Clonorchis sinensis ( C sinensis ) and Opisthorchis viverrini ( O viverrini ) plays a role. 6,7 The life cycle of these food-borne zoonotic trematodes involves eggs discharged in the stool of infected humans, the definitive host. 6,7 In nature, these eggs are ingested by freshwater snails, the intermediate host, where they undergo several developmental stages to form cercariae. Once released from snails into the water, free-swimming cercariae come in contact and penetrate freshwater fish where they encyst as metacercariae. Infection of humans occurs by ingesting undercooked, salted, pickled, or smoked freshwater fish infested with metacercariae. After ingestion, metacercariae excyst in the duodenum and ascend the biliary tract through the ampulla of Vater. They then mature into adult flukes that reside in small- and medium-sized intrahepatic biliary ducts. 6,7
Although most infected people remain asymptomatic, untreated indolent infections with C sinensis and O viverrini may persist in peripheral intrahepatic bile ducts for as long as 30 years, which is the lifespan of the trematodes.6,7 During this prolonged period, C sinensis and O viverrini feeding activities and their excretory-secretory products may damage bile duct epithelium and promote intense local inflammation.6,7 Conceivably, these pathological processes could then provoke the epithelial desquamation, adenomatous hyperplasia, goblet cell metaplasia, periductal fibrosis, and granuloma formation that are conducive to initiation and progression of cholangiocarcinoma in genetically susceptible people.8 Accordingly, the International Agency for Research on Cancer (IARC) has determined that there is sufficient evidence for the carcinogenicity of chronic infections with C sinensis and O viverrini in humans and that chronic infections with these trematodes cause cholangiocarcinoma.9 The IARC concluded that chronic infections with C sinensis and O viverrini are carcinogenic to humans (Group 1).9
Diagnosis
Presently, the diagnosis of C sinensis and O viverrini infection is based on microscopic identification and enumeration of the parasites’ eggs in weighted stool specimens using a formalin-ethyl acetate sedimentation concentration technique. 6,7 This approach requires a labor-intensive test that is conducted by an experienced technician. The test has low specificity and sensitivity because eggs could be confused with those of nonpathogenic intestinal flukes that are morphologically similar and because eggs are not present in feces during all stages of the infection. Although diffuse dilatation of intrahepatic bile ducts by screening sonography is used to diagnose clonorchiasis in endemic areas, it has low sensitivity, particularly in patients with low-level C sinensis and O viverrin i infections. 10
To address the current diagnostic gap, several enzyme-linked immunosorbent assays (ELISA) have been developed for the diagnosis of C sinensis, including monoclonal antibody-based (mAb) ELISA and indirect antibody ELISA.11,12 However, both have important limitations. The mAb ELISA detects only active infections while indirect antibody ELISA cross-reacts with other liver flukes.11,12 Taken together, these data illustrate the difficulties in diagnosing asymptomatic individuals with low-burden C sinensis or O viverrini infections by existing laboratory methods.
Timely serodiagnosis of indolent C sinensis and O viverrini infections is important because these parasites have recently been raised as a risk factor for cholangiocarcinoma in veterans who served in Vietnam.13 The American War Library estimates that as of February 28, 2019, about 610,000 Americans who served on land in Vietnam or in the air over Vietnam between 1954 and 1975 are alive, and about 164,000 Americans who served at sea in Vietnam waters are alive.14 To that end, Psevdos and colleagues screened 97 US veterans who served in Vietnam and identified 50 who reported exposure to raw or undercooked fish while there.13 None had evidence of active C sinensis or O viverrini infection. Blood samples obtained from these veterans were analyzed for circulating C sinensis and O viverrini antibodies using an ELISA developed in South Korea and 12 blood samples tested positive for the trematodes. Imaging of extrahepatic and intrahepatic bile ducts was unyielding in all cases. One veteran diagnosed with cholangiocarcinoma had repeated negative tests. However, the results of this study were challenged by several experts in this field because the authors did not report the sensitivity and specificity of the ELISA assay used.15
Serologic testing of US veterans who served in C sinensis and O viverrini–endemic countries for indolent infections with these parasites is not recommended at present.15 Nevertheless, there is an urgent need to develop sensitive and specific serologic assays, such as ELISA tests with recombinant antigens, to detect both acute and indolent infections caused by each biliary liver fluke in the US, including in patients diagnosed with cholangiocarcinoma. We posit that testing and treatment of high-risk populations could lead to earlier detection and treatment of cholangiocarcinoma, leading to improved overall survival in the population at risk.
Cholangiocarcinoma is a heterogeneous, highly aggressive cancer of the biliary tract epithelium with an overall 5-year relative survival rate of only 9%.1,2 Although surgical resection of localized, intrahepatic cholangiocarcinoma is associated with improved overall survival, most patients present with advanced disease not amenable to surgery due to a late onset of symptoms.2 Recently, an increased incidence of cholangiocarcinoma has been reported in the United States.3 Although relatively rare in the US, cholangiocarcinoma is prevalent across large parts of Asia, including China, Vietnam, Thailand, South Korea, and Taiwan.2
Risk Factors
To date, risk factors for developing cholangiocarcinoma have not been elucidated. 4,5 However, a growing body of literature suggests that chronic infection of genetically susceptible human subjects with Clonorchis sinensis ( C sinensis ) and Opisthorchis viverrini ( O viverrini ) plays a role. 6,7 The life cycle of these food-borne zoonotic trematodes involves eggs discharged in the stool of infected humans, the definitive host. 6,7 In nature, these eggs are ingested by freshwater snails, the intermediate host, where they undergo several developmental stages to form cercariae. Once released from snails into the water, free-swimming cercariae come in contact and penetrate freshwater fish where they encyst as metacercariae. Infection of humans occurs by ingesting undercooked, salted, pickled, or smoked freshwater fish infested with metacercariae. After ingestion, metacercariae excyst in the duodenum and ascend the biliary tract through the ampulla of Vater. They then mature into adult flukes that reside in small- and medium-sized intrahepatic biliary ducts. 6,7
Although most infected people remain asymptomatic, untreated indolent infections with C sinensis and O viverrini may persist in peripheral intrahepatic bile ducts for as long as 30 years, which is the lifespan of the trematodes.6,7 During this prolonged period, C sinensis and O viverrini feeding activities and their excretory-secretory products may damage bile duct epithelium and promote intense local inflammation.6,7 Conceivably, these pathological processes could then provoke the epithelial desquamation, adenomatous hyperplasia, goblet cell metaplasia, periductal fibrosis, and granuloma formation that are conducive to initiation and progression of cholangiocarcinoma in genetically susceptible people.8 Accordingly, the International Agency for Research on Cancer (IARC) has determined that there is sufficient evidence for the carcinogenicity of chronic infections with C sinensis and O viverrini in humans and that chronic infections with these trematodes cause cholangiocarcinoma.9 The IARC concluded that chronic infections with C sinensis and O viverrini are carcinogenic to humans (Group 1).9
Diagnosis
Presently, the diagnosis of C sinensis and O viverrini infection is based on microscopic identification and enumeration of the parasites’ eggs in weighted stool specimens using a formalin-ethyl acetate sedimentation concentration technique. 6,7 This approach requires a labor-intensive test that is conducted by an experienced technician. The test has low specificity and sensitivity because eggs could be confused with those of nonpathogenic intestinal flukes that are morphologically similar and because eggs are not present in feces during all stages of the infection. Although diffuse dilatation of intrahepatic bile ducts by screening sonography is used to diagnose clonorchiasis in endemic areas, it has low sensitivity, particularly in patients with low-level C sinensis and O viverrin i infections. 10
To address the current diagnostic gap, several enzyme-linked immunosorbent assays (ELISA) have been developed for the diagnosis of C sinensis, including monoclonal antibody-based (mAb) ELISA and indirect antibody ELISA.11,12 However, both have important limitations. The mAb ELISA detects only active infections while indirect antibody ELISA cross-reacts with other liver flukes.11,12 Taken together, these data illustrate the difficulties in diagnosing asymptomatic individuals with low-burden C sinensis or O viverrini infections by existing laboratory methods.
Timely serodiagnosis of indolent C sinensis and O viverrini infections is important because these parasites have recently been raised as a risk factor for cholangiocarcinoma in veterans who served in Vietnam.13 The American War Library estimates that as of February 28, 2019, about 610,000 Americans who served on land in Vietnam or in the air over Vietnam between 1954 and 1975 are alive, and about 164,000 Americans who served at sea in Vietnam waters are alive.14 To that end, Psevdos and colleagues screened 97 US veterans who served in Vietnam and identified 50 who reported exposure to raw or undercooked fish while there.13 None had evidence of active C sinensis or O viverrini infection. Blood samples obtained from these veterans were analyzed for circulating C sinensis and O viverrini antibodies using an ELISA developed in South Korea and 12 blood samples tested positive for the trematodes. Imaging of extrahepatic and intrahepatic bile ducts was unyielding in all cases. One veteran diagnosed with cholangiocarcinoma had repeated negative tests. However, the results of this study were challenged by several experts in this field because the authors did not report the sensitivity and specificity of the ELISA assay used.15
Serologic testing of US veterans who served in C sinensis and O viverrini–endemic countries for indolent infections with these parasites is not recommended at present.15 Nevertheless, there is an urgent need to develop sensitive and specific serologic assays, such as ELISA tests with recombinant antigens, to detect both acute and indolent infections caused by each biliary liver fluke in the US, including in patients diagnosed with cholangiocarcinoma. We posit that testing and treatment of high-risk populations could lead to earlier detection and treatment of cholangiocarcinoma, leading to improved overall survival in the population at risk.
1. American Cancer Society. Survival rates for bile duct cancer. Updated March 1, 2023. Accessed March 17, 2023. https://www.cancer.org/cancer/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html
2. Vij M, Puri Y, Rammohan A, et al. Pathological, molecular, and clinical characteristics of cholangiocarcinoma: A comprehensive review. World J Gastrointest Oncol. 2022;14(3):607-627. doi:10.4251/wjgo.v14.i3.607
3. Yao KJ, Jabbour S, Parekh N, Lin Y, Moss RA. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016;16(1):117. Published 2016 Sep 21. doi:10.1186/s12876-016-0527-z
4. Rustagi T, Dasanu CA. Risk factors for gallbladder cancer and cholangiocarcinoma: similarities, differences and updates. J Gastrointest Cancer. 2012;43(2):137-147. doi:10.1007/s12029-011-9284-y
5. Maemura K, Natsugoe S, Takao S. Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat Sci. 2014;21(10):754-760. doi:10.1002/jhbp.126
6. Steele JA, Richter CH, Echaubard P, et al. Thinking beyond Opisthorchis viverrini for risk of cholangiocarcinoma in the lower Mekong region: a systematic review and meta-analysis. Infect Dis Poverty. 2018;7(1):44. Published 2018 May 17. doi:10.1186/s40249-018-0434-3.
7. Kim TS, Pak JH, Kim JB, Bahk YY. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review. BMB Rep. 2016;49(11):590-597. doi:10.5483/bmbrep.2016.49.11.109
8. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. Published 2018 Oct 20. doi:10.1186/s12199-018-0740-1
9. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt B):1-441.
10. Mairiang E, Laha T, Bethony JM, et al. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol Int. 2012;61(1):208-211. doi:10.1016/j.parint.2011.07.009
11. Li HM, Qian MB, Yang YC, et al. Performance evaluation of existing immunoassays for Clonorchis sinensis infection in China. Parasit Vectors. 2018;11(1):35. Published 2018 Jan 15. doi:10.1186/s13071-018-2612-3
12. Hughes T, O’Connor T, Techasen A, et al. Opisthorchiasis and cholangiocarcinoma in Southeast Asia: an unresolved problem. Int J Gen Med. 2017;10:227-237. Published 2017 Aug 10. doi:10.2147/IJGM.S133292
13. Psevdos G, Ford FM, Hong ST. Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war. Infect Dis Clin Pract (Baltim Md). 2018;26(4):208-210. doi:10.1097/IPC.0000000000000611
14. American War Library. In harm’s way... How many real Vietnam vets are alive today? Updated February 28, 2019. Accessed March 17, 2023. https://www.americanwarlibrary.com/personnel/vietvet.htm
15. Nash TE, Sullivan D, Mitre E, et al. Comments on “Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war”. Infect Dis Clin Pract (Baltim Md). 2018;26(4):240-241. doi:10.1097/IPC.0000000000000659
1. American Cancer Society. Survival rates for bile duct cancer. Updated March 1, 2023. Accessed March 17, 2023. https://www.cancer.org/cancer/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html
2. Vij M, Puri Y, Rammohan A, et al. Pathological, molecular, and clinical characteristics of cholangiocarcinoma: A comprehensive review. World J Gastrointest Oncol. 2022;14(3):607-627. doi:10.4251/wjgo.v14.i3.607
3. Yao KJ, Jabbour S, Parekh N, Lin Y, Moss RA. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016;16(1):117. Published 2016 Sep 21. doi:10.1186/s12876-016-0527-z
4. Rustagi T, Dasanu CA. Risk factors for gallbladder cancer and cholangiocarcinoma: similarities, differences and updates. J Gastrointest Cancer. 2012;43(2):137-147. doi:10.1007/s12029-011-9284-y
5. Maemura K, Natsugoe S, Takao S. Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat Sci. 2014;21(10):754-760. doi:10.1002/jhbp.126
6. Steele JA, Richter CH, Echaubard P, et al. Thinking beyond Opisthorchis viverrini for risk of cholangiocarcinoma in the lower Mekong region: a systematic review and meta-analysis. Infect Dis Poverty. 2018;7(1):44. Published 2018 May 17. doi:10.1186/s40249-018-0434-3.
7. Kim TS, Pak JH, Kim JB, Bahk YY. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review. BMB Rep. 2016;49(11):590-597. doi:10.5483/bmbrep.2016.49.11.109
8. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. Published 2018 Oct 20. doi:10.1186/s12199-018-0740-1
9. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt B):1-441.
10. Mairiang E, Laha T, Bethony JM, et al. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol Int. 2012;61(1):208-211. doi:10.1016/j.parint.2011.07.009
11. Li HM, Qian MB, Yang YC, et al. Performance evaluation of existing immunoassays for Clonorchis sinensis infection in China. Parasit Vectors. 2018;11(1):35. Published 2018 Jan 15. doi:10.1186/s13071-018-2612-3
12. Hughes T, O’Connor T, Techasen A, et al. Opisthorchiasis and cholangiocarcinoma in Southeast Asia: an unresolved problem. Int J Gen Med. 2017;10:227-237. Published 2017 Aug 10. doi:10.2147/IJGM.S133292
13. Psevdos G, Ford FM, Hong ST. Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war. Infect Dis Clin Pract (Baltim Md). 2018;26(4):208-210. doi:10.1097/IPC.0000000000000611
14. American War Library. In harm’s way... How many real Vietnam vets are alive today? Updated February 28, 2019. Accessed March 17, 2023. https://www.americanwarlibrary.com/personnel/vietvet.htm
15. Nash TE, Sullivan D, Mitre E, et al. Comments on “Screening US Vietnam veterans for liver fluke exposure 5 decades after the end of the war”. Infect Dis Clin Pract (Baltim Md). 2018;26(4):240-241. doi:10.1097/IPC.0000000000000659
Study of hospitalizations in Canada quantifies benefit of COVID-19 vaccine to reduce death, ICU admissions
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
Longitudinal Dynamic in Weight Loss Impacts Clinical Outcomes for Veterans Undergoing Curative Surgery for Colorectal Cancer
In patients with gastrointestinal (GI) malignancies, malnutrition is common. In addition, it has various negative implications, including high risk for surgical complications, prolonged hospitalization, decreased quality of life (QOL), increased mortality, and poor tolerance for treatments such as chemotherapy and radiotherapy.1
A 2014 French study of 1903 patients hospitalized for cancer reported a 39% overall prevalence of malnutrition; 39% in patients with cancers of the colon/rectum, 60% for pancreatic cancer, and 67% for cancers of the esophagus/stomach.2 Malnutrition was defined as body mass index (BMI) < 18.5 for individuals aged < 75 years or BMI < 21 for individuals aged ≥ 75 years, and/or weight loss > 10% since disease onset. Malnutrition also was strongly associated with worsened performance status.
The etiology of malnutrition in GI cancers is often multifactorial. It includes systemic tumor effects, such as inflammatory mediators contributing to hypermetabolism and cachexia, local tumor-associated mechanical obstruction, GI toxicities caused by antineoplastic therapy or other medications, and psychological factors that contribute to anorexia.3 Patient-related risk factors such as older age, other chronic diseases, and history of other GI surgeries also play a role.1
Other studies have demonstrated that malnutrition in patients with GI malignancies undergoing surgical resection is associated with high rates of severe postoperative complications, increased length of stay (LOS) and time on a ventilator for patients treated in the intensive care unit, and poor QOL in the postoperative survival period.4-6 Several randomized controlled trials conducted in patients with GI cancers have shown that enteral and parenteral nutrition supplementations in the perioperative period improve various outcomes, such as reduction of postoperative complication rates, fewer readmissions, improved chemotherapy tolerance, and improved QOL.7-10 Thus, in the management of patients with GI malignancies, it is highly important to implement early nutritional screening and establish a diagnosis of malnutrition to intervene and reduce postoperative morbidity and mortality.1
However, tools and predictors of malnutrition are often imperfect. The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (AND/ASPEN) weight-based criteria define malnutrition and nutritionally-at-risk as BMI < 18.5, involuntary loss of at least 10% of body weight within 6 months or 5% within 1 month, or loss of 10 lb within 6 months.11 While the ASPEN criteria are often used to define malnourishment, they may not fully capture the population at risk, and there does not exist a gold-standard tool for nutritional screening. A 2002 study that performed a critical appraisal of 44 nutritional screening tools found that no single tool was fully sufficient for application, development, evaluation, and consistent screening.12 As such, consistently screening for malnutrition to target interventions in the perioperative period for GI surgical oncology has been challenging.13 More recent tools such as the perioperative nutrition screen (PONS) have been validated as rapid, effective screening tools to predict postoperative outcomes.14 Additionally, implementation of perioperative nutritional protocols, such as enhanced recovery after surgery (ERAS) in colon cancer (CC) surgery, also has shown improved perioperative care and outcomes.15
Preoperative nutritional interventions have been implemented in practice and have focused mostly on the immediate perioperative period. This has been shown to improve surgical outcomes. The Veterans Health Administration (VHA) provides comprehensive care to patients in a single-payer system, allowing for capture of perioperative data and the opportunity for focused preoperative interventions to improve outcomes.
Methods
This was a retrospective record review of colorectal malignancies treated with curative intent at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan between January 1, 2015, and December 31, 2019. We examined nutritional status, degree of longitudinal weight loss, and subsequent clinical outcomes, including delayed postoperative recovery and delays in chemotherapy in 115 patients with CC and 33 patients with rectal cancer (RC) undergoing curative surgical resection at VAAAHS. To avoid additional confounding effects of advanced cancer, only early-stage, curable disease was included. This study was approved by the VAAAHS Institutional Review Board.
Patients with postoperative follow-up outside of VAAAHS were excluded. Patients were excluded if their surgery had noncurative intent or if they had distant metastatic disease. Data on patient weights, laboratory results, nutrition consultations, postoperative complications, delayed recovery, readmissions, and chemotherapy tolerance were abstracted by patient chart review in the VHA Computerized Patient Record System and Joint Legacy Viewer by 2 researchers.
Delayed recovery was defined as any abnormal clinical development described in inpatient progress notes, outpatient follow-up notes within 60 days, or in hospital discharge summaries. Excluded were psychiatric events without additional medical complications, postoperative bleeding not requiring an invasive intervention, urinary retention, postoperative glycemic control difficulties, cardiac events that happened before postoperative hospital discharge and not requiring readmission, and postoperative alcohol withdrawal. Complications were defined similarly to delayed recovery but excluded isolated prolonged postoperative ileus. LOS was defined in days as time from admission to discharge.
Adjuvant management course was derived from reviewing documentation from medical oncology consultations and progress notes. In patients for whom adjuvant chemotherapy was indicated and prescribed, chemotherapy was considered complete if chemotherapy was started and completed as indicated. Adjuvant chemotherapy was considered incomplete if the patient declined chemotherapy, if chemotherapy was not started when indicated, or if chemotherapy was not completed as indicated. Neoadjuvant therapy data were abstracted from medical and radiation oncology notes.
Recorded data were collected on both weight and BMI. Weights were extracted as follows: Weight 1 year before time of diagnosis, ± 4 months; weight 6 months before diagnosis ± 3 months; weight at time of diagnosis ± 2 weeks; weight at time of surgery ± 2 weeks; weight 30 days postsurgery ± 2 weeks; weight 60 days postsurgery ± 2 weeks; weight 1 year postsurgery ± 4 months. Mean percent change in weight was calculated from recorded weights between each allocated time point. A weight loss of ≥ 3% was found to be clinically relevant and was chosen as the minimal cutoff value when analyzing outcomes associated with weight trends.
Nutrition consultations were abstracted as follows: Preoperative nutrition consultations were defined as occurring between time of cancer diagnosis and surgery in either the inpatient or outpatient setting; inpatient postoperative nutrition consultations occurred during admission for surgery; readmission nutrition consultations occurred on readmission in inpatient setting, if applicable; outpatient postoperative nutrition consultations were defined as occurring up to 2 months postdischarge in the outpatient setting.
Albumin values were extracted as follows: Preoperative albumin levels were defined as up to 4 months prior to diagnosis, and postoperative albumin levels were defined as 2 to 6 months after surgery.
Analysis
The data were described using mean (SD) for continuous variables and number and percentages for categorical variables. Where appropriate, Fisher exact test, Pearson χ2 test, Spearman ρ, and Mann-Whitney U test were used for tests of significance. SAS (SAS Institute) was utilized for multivariable analysis. The significance level was P = .05 for all tests.
Results
There were 115 patients in the CC cohort and 33 in the RC cohort. The mean (SD) age at diagnosis was 70 (9.1) for CC group and 59 (1.4) for RC group (Table 1).
Weight Trends
From 1 year to 6 months before diagnosis, 40 of 80 patients lost weight in the CC cohort (mean change, +1.9%) and 6 of 22 patients lost weight in the RC cohort (mean change, + 0.5%). From 6 months before diagnosis to time of diagnosis, 47 of 74 patients lost weight in the CC cohort (mean change, -1.5%) and 14 of 21 patients lost weight in the RC cohort (mean change, -2.5%). From time of diagnosis to time of surgery, 36 of 104 patients with CC and 14 of 32 patients with RC lost weight with a mean weight change of and +0.1% and -0.3%, respectively. In the 6 months before surgery, any amount of weight loss was observed in 58 patients (66%) in the CC group and in 12 patients (57%) in the RC group. In this time frame, in the CC cohort, 32 patients (36%) were observed to have at least 3% weight loss, and 23 (26%) were observed to have at least 5% weight loss (Table 3).
In patients who completed adjuvant chemotherapy in the CC group, mean (SD) BMI at the beginning and end of chemotherapy was 32.6 (8.6) and 33.1 (8.7), respectively, and a -0.3% mean change in weight was observed. In the RC group, mean (SD) BMI was 28.2 (5.0) at the initiation of adjuvant chemotherapy and 28.4 (5.0) at its completion, with a +2.6% mean change in weight.
In the immediate postoperative period, most patients were losing weight in both the CC and RC groups (mean, -3.5% and -7.0% at 1 month postoperative, respectively). At 1-year after surgery, patients had modest mean increases in weight: +1.3% for patients with CC and +4.9% for patients with RC.
A relatively large proportion of patients had missing data on weights at various data points (Table 4).
Nutrition Consultations
In the CC group, preoperative nutrition consultations (either inpatient or outpatient) occurred in 17 patients (15%). Inpatient postoperative nutrition evaluations occurred in 110 patients (96%) (Table 5).
In the RC group, preoperative inpatient or outpatient nutrition consultations occurred in 12 patients (36%). Eight of those occurred before initiation of neoadjuvant chemoradiotherapy. All 33 patients received an inpatient postoperative nutrition evaluation during admission. Oral or enteral nutrition supplements were prescribed 19 times (58%). Postoperative outpatient nutrition consultations occurred for 24 patients (73%). Of the 19 patients who were readmitted to the hospital, 3 (16%) had a nutrition reconsultation on readmission.
Outcomes
The primary outcomes observed were delayed recovery, hospital readmission and LOS, and completion of adjuvant chemotherapy as indicated. Delayed recovery was observed in 35 patients with CC (40%) and 21 patients with RC (64%). Multivariable analysis in the CC cohort demonstrated that weight change was significantly associated with delayed recovery. Among those with ≥ 3% weight loss in the 6-month preoperative period (the weight measurement 6 months prior to diagnosis to date of surgery), 20 patients (63%) had delayed recovery compared with 15 patients (27%) without ≥ 3% weight loss who experienced delayed recovery (χ2 = 10.84; P < .001).
Weight loss of ≥ 3% in the 6-month preoperative period also was significantly associated with complications. Of patients with at least 3% preoperative weight loss, 16 (50%) experienced complications, while 8 (14%) with < 3% preoperative weight loss experienced complications (χ2 = 11.20; P < .001). Notably, ≥ 3% weight loss in the 1-year preoperative period before surgery was not significantly associated with delayed recovery. Any degree of 30-day postoperative weight loss was not correlated with delayed recovery. Finally, low preoperative albumin also was not correlated with delayed recovery (Fisher exact; P = .13). Table 3 displays differences based on presence of delayed recovery in the 88 patients with CC 6 months before surgery. Of note, ≥ 10-lb weight loss in the 6 months preceding surgery also correlated with delayed recovery (P = .01).In our cohort, 3% weight loss over 6 months had a sensitivity of 57%, specificity of 77%, positive predictive value 63%, and negative predictive value 73% for delayed recovery. By comparison, a 10-lb weight loss in 6 months per ASPEN criteria had a sensitivity of 40%, specificity of 85%, positive predictive value 64%, and negative predictive value 68% for delayed recovery.
Hospital Readmissions and LOS
Hospital readmissions occurred within the first 30 days in 11 patients (10%) in the CC cohort and 12 patients (36%) in the RC cohort. Readmissions occurred between 31 and 60 days in 4 (3%) and 7 (21%) of CC and RC cohorts, respectively. The presence of ≥ 3% weight loss in the 6-month
Mean (SD) LOS was 6.4 (4.7) days (range, 1-28) for patients with CC and 8.8 (5.1) days (range, 3-23) for patients with RC. Mean (SD) LOS increased to 10.2 (4.3) days and 9.7 (6.0) days in patients with delayed recovery in the CC and RC cohorts, respectively. The mean (SD) LOS was 5.2 (2.8) days and 6.3 (2.2) days in patients without delayed recovery in the CC and RC cohorts, respectively. There was no significant difference when examining association between percent weight change and LOS for either initial admission (rs = -0.1409; 2-tailed P = .19) or for initial and readmission combined (rs = -0.13532; 2-tailed P = .21) within the CC cohort.
Chemotherapy
Within the CC cohort, 31 patients (27%) had an indication for adjuvant chemotherapy. Of these, 25 of 31 (81%) started chemotherapy within 12 weeks of surgical resection, and of these, 17 of 25 patients (68%) completed chemotherapy as indicated. Within the RC cohort all 33 patients had an indication for adjuvant chemotherapy, of these 18 of 33 patients (55%) began within 12 weeks of surgical resection, and 10 of 18 (56%) completed chemotherapy as indicated.
Among the CC cohort who began but did not complete adjuvant chemotherapy, there was no significant association between completion of chemotherapy and
Discussion
This study highlights several important findings. There were no patients in our cohort that met ASPEN malnourishment criteria with a BMI < 18.5. Twenty percent of patients lost at least 10 lb in 6 months before the operation. Notably, patients had significant associations with adverse outcomes with less pronounced weight loss than previously noted. As has been established previously, malnourishment can be difficult to screen for, and BMI also is often an imprecise tool.12 In the CC cohort, weight loss
Our findings imply that the effects of even mild malnutrition are even more profound than previously thought. Significantly, this applies to overweight and obese patients as well, as these constituted a significant fraction of our cohort. A finding of ≥ 3% weight loss at the time of CC diagnosis may provide an opportunity for a focused nutrition intervention up to the time of surgery. Second, although nutrition consultation was frequent in the inpatient setting during the hospital admission (96%-100%), rates of nutrition evaluation were as low as 15% before surgery and 12% after surgery, representing a key area for improvement and focused intervention. An optimal time for intervention and nutrition prehabilitation would be at time of diagnosis before surgery with plans for continued aggressive monitoring and subsequent follow-up. Our finding seems to provide a more sensitive tool to identify patients at risk for delayed recovery compared with the ASPEN-driven assessment. Given the simplicity and the clinical significance, our test consisting of 3% weight loss over 6 months, with its sensitivity of 57%, may be superior to the ASPEN 10-lb weight loss, with its sensitivity of 40% in our cohort.
Previous Studies
Our findings are consistent with previous studies that have demonstrated that perioperative weight loss and malnutrition are correlated with delayed recovery and complications, such as wound healing, in patients with GI cancer.2,4,5,8 In a retrospective study of more than 7000 patients with CC, those who were overweight or obese were found to have an improved overall survival compared with other BMI categories, and those who were underweight had an increased 30-day mortality and postoperative complications.16
In another retrospective study of 3799 patients with CC, those who were overweight and obese had an improved 5-year survival rate compared with patients whose weight was normal or underweight. Outcomes were found to be stage dependent.17 In this study cohort, all patients were either overweight or obese and remained in that category even with weight loss. This may have contributed to overall improved outcomes.
Implications and Next Steps
Our study has several implications. One is that BMI criteria < 18.5 may not be a good measure for malnutrition given that about 75% of the patients in our cohort were overweight or obese and none were underweight. We also show a concrete, easily identifiable finding of percent weight change that could be addressed as an automated electronic notification and potentially identify a patient at risk and serve as a trigger for both timely and early nutrition intervention. It seems to be more sensitive than the ASPEN criterion of 10-lb weight loss in 6 months before surgery. Sensitivity is especially appealing given the ease and potential of embedding this tool in an electronic health record and the clinical importance of the consequent intervention. Preoperative as opposed to perioperative nutrition optimization at time of CC diagnosis is essential, as it may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Finally, although our study found that rates of inpatient postoperative nutrition consultation were high, rates of outpatient nutrition consultation in the preoperative period were low. This represents a missed opportunity for intervention before surgery. Similarly, rates of postoperative nutrition follow-up period were low, which points to an area for improvement in longitudinal and holistic care.
We suggest modifications to nutrition intervention protocols, such as ERAS, which should start at the time of GI malignancy diagnosis.18 Other suggestions include standard involvement of nutritionists in inpatient and outpatient settings with longitudinal follow-up in the preoperative and postoperative periods and patient enrollment in a nutrition program with monitoring at time of diagnosis at the VHA. Our findings as well as previous literature suggest that the preoperative period is the most important time to intervene with regard to nutrition optimization and represents an opportunity for intensive prehabilitation. Future areas of research include incorporating other important measures of malnourishment independent of BMI into future study designs, such as sarcopenia and adipose tissue density, to better assess body composition and predict prognostic risk in CC.18,19
Strengths and Limitations
This study is limited by its single-center, retrospective design and small sample sizes, and we acknowledge the limitations of our data set. However, the strength of this VHA-based study is that the single-payer system allows for complete capture of perioperative data as well as the opportunity for focused preoperative interventions to improve outcomes. To our knowledge, there is no currently existing literature on improving nutrition protocols at the VHA for patients with a GI malignancy. These retrospective data will help inform current gaps in quality improvement and supportive oncology as it relates to optimizing malnourishment in veterans undergoing surgical resection for their cancer.
Conclusions
In the CC cohort, weight loss of ≥ 3% from 6 months prior to time of surgery was significantly associated with delayed recovery, complications, and hospital readmissions. Our findings suggest that patients with CC undergoing surgery may benefit from an intensive, early nutrition prehabilitation. Preoperative nutrition optimization may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Further research would be able to clarify these hypotheses.
1. Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg. 2015;152:S3-S7. doi:10.1016/S1878-7886(15)30003-5
2. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enter Nutr. 2014;38(2):196-204. doi:10.1177/0148607113502674
3. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9:S51-S63. doi:10.1016/j.ejon.2005.09.007
4. Nishiyama VKG, Albertini SM, de Moraes CMZG, et al. Malnutrition and clinical outcomes in surgical patients with colorectal disease. Arq Gastroenterol. 2018;55(4):397-402. doi:10.1590/s0004-2803.201800000-85
5. Shpata V, Prendushi X, Kreka M, Kola I, Kurti F, Ohri I. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med Arch Sarajevo Bosnia Herzeg. 2014;68(4):263-267. doi:10.5455/medarh.2014.68.263-267
6. Lim HS, Cho GS, Park YH, Kim SK. Comparison of quality of life and nutritional status in gastric cancer patients undergoing gastrectomies. Clin Nutr Res. 2015;4(3):153-159. doi:10.7762/cnr.2015.4.3.153
7. Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. J Parenter Enter Nutr. 2000;24(1):7-14. doi:10.1177/014860710002400107
8. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26(6):698-709. doi:10.1016/j.clnu.2007.06.009
9. Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001; 358(9292):1487-1492. doi:10.1016/S0140-6736(01)06578-3
10. Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr Edinb Scotl. 2021;40(1):40-46. doi:10.1016/j.clnu.2020.04.043 start
11. White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. doi:10.1016/j.jand.2012.03.012
12. Jones JM. The methodology of nutritional screening and assessment tools. J Hum Nutr Diet. 2002;15(1):59-71. doi:10.1046/j.1365-277X.2002.00327.x
13. Williams J, Wischmeyer P. Assessment of perioperative nutrition practices and attitudes—a national survey of colorectal and GI surgical oncology programs. Am J Surg. 2017;213(6):1010-1018. doi:10.1016/j.amjsurg.2016.10.008
14. Williams DG, Aronson S, Murray S, et al. Validation of the perioperative nutrition screen for prediction of postoperative outcomes. JPEN J Parenter Enteral Nutr. 2022;46(6):1307-1315. doi:10.1002/jpen.2310
15. Besson AJ, Kei C, Djordjevic A, Carter V, Deftereos I, Yeung J. Does implementation of and adherence to enhanced recovery after surgery improve perioperative nutritional management in colorectal cancer surgery? ANZ J Surg. 2022;92(6):1382-1387. doi:10.1111/ans.17599
16. Arkenbosch JHC, van Erning FN, Rutten HJ, Zimmerman D, de Wilt JHW, Beijer S. The association between body mass index and postoperative complications, 30-day mortality and long-term survival in Dutch patients with colorectal cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2019;45(2):160-166. doi:10.1016/j.ejso.2018.09.012
17. Shahjehan F, Merchea A, Cochuyt JJ, Li Z, Colibaseanu DT, Kasi PM. Body mass index and long-term outcomes in patients with colorectal cancer. Front Oncol. 2018;8:620. doi:10.3389/fonc.2018.00620
18. Nishigori T, Obama K, Sakai Y. Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer. Transl Gastroenterol Hepatol. 2020;5:22. doi:10.21037/tgh.2019.10.13
19. Feliciano EMC, Winkels RM, Meyerhardt JA, Prado CM, Afman LA, Caan BJ. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am J Clin Nutr. 2021;114(6):1917-1924. doi:10.1093/ajcn/nqab285
In patients with gastrointestinal (GI) malignancies, malnutrition is common. In addition, it has various negative implications, including high risk for surgical complications, prolonged hospitalization, decreased quality of life (QOL), increased mortality, and poor tolerance for treatments such as chemotherapy and radiotherapy.1
A 2014 French study of 1903 patients hospitalized for cancer reported a 39% overall prevalence of malnutrition; 39% in patients with cancers of the colon/rectum, 60% for pancreatic cancer, and 67% for cancers of the esophagus/stomach.2 Malnutrition was defined as body mass index (BMI) < 18.5 for individuals aged < 75 years or BMI < 21 for individuals aged ≥ 75 years, and/or weight loss > 10% since disease onset. Malnutrition also was strongly associated with worsened performance status.
The etiology of malnutrition in GI cancers is often multifactorial. It includes systemic tumor effects, such as inflammatory mediators contributing to hypermetabolism and cachexia, local tumor-associated mechanical obstruction, GI toxicities caused by antineoplastic therapy or other medications, and psychological factors that contribute to anorexia.3 Patient-related risk factors such as older age, other chronic diseases, and history of other GI surgeries also play a role.1
Other studies have demonstrated that malnutrition in patients with GI malignancies undergoing surgical resection is associated with high rates of severe postoperative complications, increased length of stay (LOS) and time on a ventilator for patients treated in the intensive care unit, and poor QOL in the postoperative survival period.4-6 Several randomized controlled trials conducted in patients with GI cancers have shown that enteral and parenteral nutrition supplementations in the perioperative period improve various outcomes, such as reduction of postoperative complication rates, fewer readmissions, improved chemotherapy tolerance, and improved QOL.7-10 Thus, in the management of patients with GI malignancies, it is highly important to implement early nutritional screening and establish a diagnosis of malnutrition to intervene and reduce postoperative morbidity and mortality.1
However, tools and predictors of malnutrition are often imperfect. The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (AND/ASPEN) weight-based criteria define malnutrition and nutritionally-at-risk as BMI < 18.5, involuntary loss of at least 10% of body weight within 6 months or 5% within 1 month, or loss of 10 lb within 6 months.11 While the ASPEN criteria are often used to define malnourishment, they may not fully capture the population at risk, and there does not exist a gold-standard tool for nutritional screening. A 2002 study that performed a critical appraisal of 44 nutritional screening tools found that no single tool was fully sufficient for application, development, evaluation, and consistent screening.12 As such, consistently screening for malnutrition to target interventions in the perioperative period for GI surgical oncology has been challenging.13 More recent tools such as the perioperative nutrition screen (PONS) have been validated as rapid, effective screening tools to predict postoperative outcomes.14 Additionally, implementation of perioperative nutritional protocols, such as enhanced recovery after surgery (ERAS) in colon cancer (CC) surgery, also has shown improved perioperative care and outcomes.15
Preoperative nutritional interventions have been implemented in practice and have focused mostly on the immediate perioperative period. This has been shown to improve surgical outcomes. The Veterans Health Administration (VHA) provides comprehensive care to patients in a single-payer system, allowing for capture of perioperative data and the opportunity for focused preoperative interventions to improve outcomes.
Methods
This was a retrospective record review of colorectal malignancies treated with curative intent at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan between January 1, 2015, and December 31, 2019. We examined nutritional status, degree of longitudinal weight loss, and subsequent clinical outcomes, including delayed postoperative recovery and delays in chemotherapy in 115 patients with CC and 33 patients with rectal cancer (RC) undergoing curative surgical resection at VAAAHS. To avoid additional confounding effects of advanced cancer, only early-stage, curable disease was included. This study was approved by the VAAAHS Institutional Review Board.
Patients with postoperative follow-up outside of VAAAHS were excluded. Patients were excluded if their surgery had noncurative intent or if they had distant metastatic disease. Data on patient weights, laboratory results, nutrition consultations, postoperative complications, delayed recovery, readmissions, and chemotherapy tolerance were abstracted by patient chart review in the VHA Computerized Patient Record System and Joint Legacy Viewer by 2 researchers.
Delayed recovery was defined as any abnormal clinical development described in inpatient progress notes, outpatient follow-up notes within 60 days, or in hospital discharge summaries. Excluded were psychiatric events without additional medical complications, postoperative bleeding not requiring an invasive intervention, urinary retention, postoperative glycemic control difficulties, cardiac events that happened before postoperative hospital discharge and not requiring readmission, and postoperative alcohol withdrawal. Complications were defined similarly to delayed recovery but excluded isolated prolonged postoperative ileus. LOS was defined in days as time from admission to discharge.
Adjuvant management course was derived from reviewing documentation from medical oncology consultations and progress notes. In patients for whom adjuvant chemotherapy was indicated and prescribed, chemotherapy was considered complete if chemotherapy was started and completed as indicated. Adjuvant chemotherapy was considered incomplete if the patient declined chemotherapy, if chemotherapy was not started when indicated, or if chemotherapy was not completed as indicated. Neoadjuvant therapy data were abstracted from medical and radiation oncology notes.
Recorded data were collected on both weight and BMI. Weights were extracted as follows: Weight 1 year before time of diagnosis, ± 4 months; weight 6 months before diagnosis ± 3 months; weight at time of diagnosis ± 2 weeks; weight at time of surgery ± 2 weeks; weight 30 days postsurgery ± 2 weeks; weight 60 days postsurgery ± 2 weeks; weight 1 year postsurgery ± 4 months. Mean percent change in weight was calculated from recorded weights between each allocated time point. A weight loss of ≥ 3% was found to be clinically relevant and was chosen as the minimal cutoff value when analyzing outcomes associated with weight trends.
Nutrition consultations were abstracted as follows: Preoperative nutrition consultations were defined as occurring between time of cancer diagnosis and surgery in either the inpatient or outpatient setting; inpatient postoperative nutrition consultations occurred during admission for surgery; readmission nutrition consultations occurred on readmission in inpatient setting, if applicable; outpatient postoperative nutrition consultations were defined as occurring up to 2 months postdischarge in the outpatient setting.
Albumin values were extracted as follows: Preoperative albumin levels were defined as up to 4 months prior to diagnosis, and postoperative albumin levels were defined as 2 to 6 months after surgery.
Analysis
The data were described using mean (SD) for continuous variables and number and percentages for categorical variables. Where appropriate, Fisher exact test, Pearson χ2 test, Spearman ρ, and Mann-Whitney U test were used for tests of significance. SAS (SAS Institute) was utilized for multivariable analysis. The significance level was P = .05 for all tests.
Results
There were 115 patients in the CC cohort and 33 in the RC cohort. The mean (SD) age at diagnosis was 70 (9.1) for CC group and 59 (1.4) for RC group (Table 1).
Weight Trends
From 1 year to 6 months before diagnosis, 40 of 80 patients lost weight in the CC cohort (mean change, +1.9%) and 6 of 22 patients lost weight in the RC cohort (mean change, + 0.5%). From 6 months before diagnosis to time of diagnosis, 47 of 74 patients lost weight in the CC cohort (mean change, -1.5%) and 14 of 21 patients lost weight in the RC cohort (mean change, -2.5%). From time of diagnosis to time of surgery, 36 of 104 patients with CC and 14 of 32 patients with RC lost weight with a mean weight change of and +0.1% and -0.3%, respectively. In the 6 months before surgery, any amount of weight loss was observed in 58 patients (66%) in the CC group and in 12 patients (57%) in the RC group. In this time frame, in the CC cohort, 32 patients (36%) were observed to have at least 3% weight loss, and 23 (26%) were observed to have at least 5% weight loss (Table 3).
In patients who completed adjuvant chemotherapy in the CC group, mean (SD) BMI at the beginning and end of chemotherapy was 32.6 (8.6) and 33.1 (8.7), respectively, and a -0.3% mean change in weight was observed. In the RC group, mean (SD) BMI was 28.2 (5.0) at the initiation of adjuvant chemotherapy and 28.4 (5.0) at its completion, with a +2.6% mean change in weight.
In the immediate postoperative period, most patients were losing weight in both the CC and RC groups (mean, -3.5% and -7.0% at 1 month postoperative, respectively). At 1-year after surgery, patients had modest mean increases in weight: +1.3% for patients with CC and +4.9% for patients with RC.
A relatively large proportion of patients had missing data on weights at various data points (Table 4).
Nutrition Consultations
In the CC group, preoperative nutrition consultations (either inpatient or outpatient) occurred in 17 patients (15%). Inpatient postoperative nutrition evaluations occurred in 110 patients (96%) (Table 5).
In the RC group, preoperative inpatient or outpatient nutrition consultations occurred in 12 patients (36%). Eight of those occurred before initiation of neoadjuvant chemoradiotherapy. All 33 patients received an inpatient postoperative nutrition evaluation during admission. Oral or enteral nutrition supplements were prescribed 19 times (58%). Postoperative outpatient nutrition consultations occurred for 24 patients (73%). Of the 19 patients who were readmitted to the hospital, 3 (16%) had a nutrition reconsultation on readmission.
Outcomes
The primary outcomes observed were delayed recovery, hospital readmission and LOS, and completion of adjuvant chemotherapy as indicated. Delayed recovery was observed in 35 patients with CC (40%) and 21 patients with RC (64%). Multivariable analysis in the CC cohort demonstrated that weight change was significantly associated with delayed recovery. Among those with ≥ 3% weight loss in the 6-month preoperative period (the weight measurement 6 months prior to diagnosis to date of surgery), 20 patients (63%) had delayed recovery compared with 15 patients (27%) without ≥ 3% weight loss who experienced delayed recovery (χ2 = 10.84; P < .001).
Weight loss of ≥ 3% in the 6-month preoperative period also was significantly associated with complications. Of patients with at least 3% preoperative weight loss, 16 (50%) experienced complications, while 8 (14%) with < 3% preoperative weight loss experienced complications (χ2 = 11.20; P < .001). Notably, ≥ 3% weight loss in the 1-year preoperative period before surgery was not significantly associated with delayed recovery. Any degree of 30-day postoperative weight loss was not correlated with delayed recovery. Finally, low preoperative albumin also was not correlated with delayed recovery (Fisher exact; P = .13). Table 3 displays differences based on presence of delayed recovery in the 88 patients with CC 6 months before surgery. Of note, ≥ 10-lb weight loss in the 6 months preceding surgery also correlated with delayed recovery (P = .01).In our cohort, 3% weight loss over 6 months had a sensitivity of 57%, specificity of 77%, positive predictive value 63%, and negative predictive value 73% for delayed recovery. By comparison, a 10-lb weight loss in 6 months per ASPEN criteria had a sensitivity of 40%, specificity of 85%, positive predictive value 64%, and negative predictive value 68% for delayed recovery.
Hospital Readmissions and LOS
Hospital readmissions occurred within the first 30 days in 11 patients (10%) in the CC cohort and 12 patients (36%) in the RC cohort. Readmissions occurred between 31 and 60 days in 4 (3%) and 7 (21%) of CC and RC cohorts, respectively. The presence of ≥ 3% weight loss in the 6-month
Mean (SD) LOS was 6.4 (4.7) days (range, 1-28) for patients with CC and 8.8 (5.1) days (range, 3-23) for patients with RC. Mean (SD) LOS increased to 10.2 (4.3) days and 9.7 (6.0) days in patients with delayed recovery in the CC and RC cohorts, respectively. The mean (SD) LOS was 5.2 (2.8) days and 6.3 (2.2) days in patients without delayed recovery in the CC and RC cohorts, respectively. There was no significant difference when examining association between percent weight change and LOS for either initial admission (rs = -0.1409; 2-tailed P = .19) or for initial and readmission combined (rs = -0.13532; 2-tailed P = .21) within the CC cohort.
Chemotherapy
Within the CC cohort, 31 patients (27%) had an indication for adjuvant chemotherapy. Of these, 25 of 31 (81%) started chemotherapy within 12 weeks of surgical resection, and of these, 17 of 25 patients (68%) completed chemotherapy as indicated. Within the RC cohort all 33 patients had an indication for adjuvant chemotherapy, of these 18 of 33 patients (55%) began within 12 weeks of surgical resection, and 10 of 18 (56%) completed chemotherapy as indicated.
Among the CC cohort who began but did not complete adjuvant chemotherapy, there was no significant association between completion of chemotherapy and
Discussion
This study highlights several important findings. There were no patients in our cohort that met ASPEN malnourishment criteria with a BMI < 18.5. Twenty percent of patients lost at least 10 lb in 6 months before the operation. Notably, patients had significant associations with adverse outcomes with less pronounced weight loss than previously noted. As has been established previously, malnourishment can be difficult to screen for, and BMI also is often an imprecise tool.12 In the CC cohort, weight loss
Our findings imply that the effects of even mild malnutrition are even more profound than previously thought. Significantly, this applies to overweight and obese patients as well, as these constituted a significant fraction of our cohort. A finding of ≥ 3% weight loss at the time of CC diagnosis may provide an opportunity for a focused nutrition intervention up to the time of surgery. Second, although nutrition consultation was frequent in the inpatient setting during the hospital admission (96%-100%), rates of nutrition evaluation were as low as 15% before surgery and 12% after surgery, representing a key area for improvement and focused intervention. An optimal time for intervention and nutrition prehabilitation would be at time of diagnosis before surgery with plans for continued aggressive monitoring and subsequent follow-up. Our finding seems to provide a more sensitive tool to identify patients at risk for delayed recovery compared with the ASPEN-driven assessment. Given the simplicity and the clinical significance, our test consisting of 3% weight loss over 6 months, with its sensitivity of 57%, may be superior to the ASPEN 10-lb weight loss, with its sensitivity of 40% in our cohort.
Previous Studies
Our findings are consistent with previous studies that have demonstrated that perioperative weight loss and malnutrition are correlated with delayed recovery and complications, such as wound healing, in patients with GI cancer.2,4,5,8 In a retrospective study of more than 7000 patients with CC, those who were overweight or obese were found to have an improved overall survival compared with other BMI categories, and those who were underweight had an increased 30-day mortality and postoperative complications.16
In another retrospective study of 3799 patients with CC, those who were overweight and obese had an improved 5-year survival rate compared with patients whose weight was normal or underweight. Outcomes were found to be stage dependent.17 In this study cohort, all patients were either overweight or obese and remained in that category even with weight loss. This may have contributed to overall improved outcomes.
Implications and Next Steps
Our study has several implications. One is that BMI criteria < 18.5 may not be a good measure for malnutrition given that about 75% of the patients in our cohort were overweight or obese and none were underweight. We also show a concrete, easily identifiable finding of percent weight change that could be addressed as an automated electronic notification and potentially identify a patient at risk and serve as a trigger for both timely and early nutrition intervention. It seems to be more sensitive than the ASPEN criterion of 10-lb weight loss in 6 months before surgery. Sensitivity is especially appealing given the ease and potential of embedding this tool in an electronic health record and the clinical importance of the consequent intervention. Preoperative as opposed to perioperative nutrition optimization at time of CC diagnosis is essential, as it may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Finally, although our study found that rates of inpatient postoperative nutrition consultation were high, rates of outpatient nutrition consultation in the preoperative period were low. This represents a missed opportunity for intervention before surgery. Similarly, rates of postoperative nutrition follow-up period were low, which points to an area for improvement in longitudinal and holistic care.
We suggest modifications to nutrition intervention protocols, such as ERAS, which should start at the time of GI malignancy diagnosis.18 Other suggestions include standard involvement of nutritionists in inpatient and outpatient settings with longitudinal follow-up in the preoperative and postoperative periods and patient enrollment in a nutrition program with monitoring at time of diagnosis at the VHA. Our findings as well as previous literature suggest that the preoperative period is the most important time to intervene with regard to nutrition optimization and represents an opportunity for intensive prehabilitation. Future areas of research include incorporating other important measures of malnourishment independent of BMI into future study designs, such as sarcopenia and adipose tissue density, to better assess body composition and predict prognostic risk in CC.18,19
Strengths and Limitations
This study is limited by its single-center, retrospective design and small sample sizes, and we acknowledge the limitations of our data set. However, the strength of this VHA-based study is that the single-payer system allows for complete capture of perioperative data as well as the opportunity for focused preoperative interventions to improve outcomes. To our knowledge, there is no currently existing literature on improving nutrition protocols at the VHA for patients with a GI malignancy. These retrospective data will help inform current gaps in quality improvement and supportive oncology as it relates to optimizing malnourishment in veterans undergoing surgical resection for their cancer.
Conclusions
In the CC cohort, weight loss of ≥ 3% from 6 months prior to time of surgery was significantly associated with delayed recovery, complications, and hospital readmissions. Our findings suggest that patients with CC undergoing surgery may benefit from an intensive, early nutrition prehabilitation. Preoperative nutrition optimization may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Further research would be able to clarify these hypotheses.
In patients with gastrointestinal (GI) malignancies, malnutrition is common. In addition, it has various negative implications, including high risk for surgical complications, prolonged hospitalization, decreased quality of life (QOL), increased mortality, and poor tolerance for treatments such as chemotherapy and radiotherapy.1
A 2014 French study of 1903 patients hospitalized for cancer reported a 39% overall prevalence of malnutrition; 39% in patients with cancers of the colon/rectum, 60% for pancreatic cancer, and 67% for cancers of the esophagus/stomach.2 Malnutrition was defined as body mass index (BMI) < 18.5 for individuals aged < 75 years or BMI < 21 for individuals aged ≥ 75 years, and/or weight loss > 10% since disease onset. Malnutrition also was strongly associated with worsened performance status.
The etiology of malnutrition in GI cancers is often multifactorial. It includes systemic tumor effects, such as inflammatory mediators contributing to hypermetabolism and cachexia, local tumor-associated mechanical obstruction, GI toxicities caused by antineoplastic therapy or other medications, and psychological factors that contribute to anorexia.3 Patient-related risk factors such as older age, other chronic diseases, and history of other GI surgeries also play a role.1
Other studies have demonstrated that malnutrition in patients with GI malignancies undergoing surgical resection is associated with high rates of severe postoperative complications, increased length of stay (LOS) and time on a ventilator for patients treated in the intensive care unit, and poor QOL in the postoperative survival period.4-6 Several randomized controlled trials conducted in patients with GI cancers have shown that enteral and parenteral nutrition supplementations in the perioperative period improve various outcomes, such as reduction of postoperative complication rates, fewer readmissions, improved chemotherapy tolerance, and improved QOL.7-10 Thus, in the management of patients with GI malignancies, it is highly important to implement early nutritional screening and establish a diagnosis of malnutrition to intervene and reduce postoperative morbidity and mortality.1
However, tools and predictors of malnutrition are often imperfect. The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (AND/ASPEN) weight-based criteria define malnutrition and nutritionally-at-risk as BMI < 18.5, involuntary loss of at least 10% of body weight within 6 months or 5% within 1 month, or loss of 10 lb within 6 months.11 While the ASPEN criteria are often used to define malnourishment, they may not fully capture the population at risk, and there does not exist a gold-standard tool for nutritional screening. A 2002 study that performed a critical appraisal of 44 nutritional screening tools found that no single tool was fully sufficient for application, development, evaluation, and consistent screening.12 As such, consistently screening for malnutrition to target interventions in the perioperative period for GI surgical oncology has been challenging.13 More recent tools such as the perioperative nutrition screen (PONS) have been validated as rapid, effective screening tools to predict postoperative outcomes.14 Additionally, implementation of perioperative nutritional protocols, such as enhanced recovery after surgery (ERAS) in colon cancer (CC) surgery, also has shown improved perioperative care and outcomes.15
Preoperative nutritional interventions have been implemented in practice and have focused mostly on the immediate perioperative period. This has been shown to improve surgical outcomes. The Veterans Health Administration (VHA) provides comprehensive care to patients in a single-payer system, allowing for capture of perioperative data and the opportunity for focused preoperative interventions to improve outcomes.
Methods
This was a retrospective record review of colorectal malignancies treated with curative intent at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan between January 1, 2015, and December 31, 2019. We examined nutritional status, degree of longitudinal weight loss, and subsequent clinical outcomes, including delayed postoperative recovery and delays in chemotherapy in 115 patients with CC and 33 patients with rectal cancer (RC) undergoing curative surgical resection at VAAAHS. To avoid additional confounding effects of advanced cancer, only early-stage, curable disease was included. This study was approved by the VAAAHS Institutional Review Board.
Patients with postoperative follow-up outside of VAAAHS were excluded. Patients were excluded if their surgery had noncurative intent or if they had distant metastatic disease. Data on patient weights, laboratory results, nutrition consultations, postoperative complications, delayed recovery, readmissions, and chemotherapy tolerance were abstracted by patient chart review in the VHA Computerized Patient Record System and Joint Legacy Viewer by 2 researchers.
Delayed recovery was defined as any abnormal clinical development described in inpatient progress notes, outpatient follow-up notes within 60 days, or in hospital discharge summaries. Excluded were psychiatric events without additional medical complications, postoperative bleeding not requiring an invasive intervention, urinary retention, postoperative glycemic control difficulties, cardiac events that happened before postoperative hospital discharge and not requiring readmission, and postoperative alcohol withdrawal. Complications were defined similarly to delayed recovery but excluded isolated prolonged postoperative ileus. LOS was defined in days as time from admission to discharge.
Adjuvant management course was derived from reviewing documentation from medical oncology consultations and progress notes. In patients for whom adjuvant chemotherapy was indicated and prescribed, chemotherapy was considered complete if chemotherapy was started and completed as indicated. Adjuvant chemotherapy was considered incomplete if the patient declined chemotherapy, if chemotherapy was not started when indicated, or if chemotherapy was not completed as indicated. Neoadjuvant therapy data were abstracted from medical and radiation oncology notes.
Recorded data were collected on both weight and BMI. Weights were extracted as follows: Weight 1 year before time of diagnosis, ± 4 months; weight 6 months before diagnosis ± 3 months; weight at time of diagnosis ± 2 weeks; weight at time of surgery ± 2 weeks; weight 30 days postsurgery ± 2 weeks; weight 60 days postsurgery ± 2 weeks; weight 1 year postsurgery ± 4 months. Mean percent change in weight was calculated from recorded weights between each allocated time point. A weight loss of ≥ 3% was found to be clinically relevant and was chosen as the minimal cutoff value when analyzing outcomes associated with weight trends.
Nutrition consultations were abstracted as follows: Preoperative nutrition consultations were defined as occurring between time of cancer diagnosis and surgery in either the inpatient or outpatient setting; inpatient postoperative nutrition consultations occurred during admission for surgery; readmission nutrition consultations occurred on readmission in inpatient setting, if applicable; outpatient postoperative nutrition consultations were defined as occurring up to 2 months postdischarge in the outpatient setting.
Albumin values were extracted as follows: Preoperative albumin levels were defined as up to 4 months prior to diagnosis, and postoperative albumin levels were defined as 2 to 6 months after surgery.
Analysis
The data were described using mean (SD) for continuous variables and number and percentages for categorical variables. Where appropriate, Fisher exact test, Pearson χ2 test, Spearman ρ, and Mann-Whitney U test were used for tests of significance. SAS (SAS Institute) was utilized for multivariable analysis. The significance level was P = .05 for all tests.
Results
There were 115 patients in the CC cohort and 33 in the RC cohort. The mean (SD) age at diagnosis was 70 (9.1) for CC group and 59 (1.4) for RC group (Table 1).
Weight Trends
From 1 year to 6 months before diagnosis, 40 of 80 patients lost weight in the CC cohort (mean change, +1.9%) and 6 of 22 patients lost weight in the RC cohort (mean change, + 0.5%). From 6 months before diagnosis to time of diagnosis, 47 of 74 patients lost weight in the CC cohort (mean change, -1.5%) and 14 of 21 patients lost weight in the RC cohort (mean change, -2.5%). From time of diagnosis to time of surgery, 36 of 104 patients with CC and 14 of 32 patients with RC lost weight with a mean weight change of and +0.1% and -0.3%, respectively. In the 6 months before surgery, any amount of weight loss was observed in 58 patients (66%) in the CC group and in 12 patients (57%) in the RC group. In this time frame, in the CC cohort, 32 patients (36%) were observed to have at least 3% weight loss, and 23 (26%) were observed to have at least 5% weight loss (Table 3).
In patients who completed adjuvant chemotherapy in the CC group, mean (SD) BMI at the beginning and end of chemotherapy was 32.6 (8.6) and 33.1 (8.7), respectively, and a -0.3% mean change in weight was observed. In the RC group, mean (SD) BMI was 28.2 (5.0) at the initiation of adjuvant chemotherapy and 28.4 (5.0) at its completion, with a +2.6% mean change in weight.
In the immediate postoperative period, most patients were losing weight in both the CC and RC groups (mean, -3.5% and -7.0% at 1 month postoperative, respectively). At 1-year after surgery, patients had modest mean increases in weight: +1.3% for patients with CC and +4.9% for patients with RC.
A relatively large proportion of patients had missing data on weights at various data points (Table 4).
Nutrition Consultations
In the CC group, preoperative nutrition consultations (either inpatient or outpatient) occurred in 17 patients (15%). Inpatient postoperative nutrition evaluations occurred in 110 patients (96%) (Table 5).
In the RC group, preoperative inpatient or outpatient nutrition consultations occurred in 12 patients (36%). Eight of those occurred before initiation of neoadjuvant chemoradiotherapy. All 33 patients received an inpatient postoperative nutrition evaluation during admission. Oral or enteral nutrition supplements were prescribed 19 times (58%). Postoperative outpatient nutrition consultations occurred for 24 patients (73%). Of the 19 patients who were readmitted to the hospital, 3 (16%) had a nutrition reconsultation on readmission.
Outcomes
The primary outcomes observed were delayed recovery, hospital readmission and LOS, and completion of adjuvant chemotherapy as indicated. Delayed recovery was observed in 35 patients with CC (40%) and 21 patients with RC (64%). Multivariable analysis in the CC cohort demonstrated that weight change was significantly associated with delayed recovery. Among those with ≥ 3% weight loss in the 6-month preoperative period (the weight measurement 6 months prior to diagnosis to date of surgery), 20 patients (63%) had delayed recovery compared with 15 patients (27%) without ≥ 3% weight loss who experienced delayed recovery (χ2 = 10.84; P < .001).
Weight loss of ≥ 3% in the 6-month preoperative period also was significantly associated with complications. Of patients with at least 3% preoperative weight loss, 16 (50%) experienced complications, while 8 (14%) with < 3% preoperative weight loss experienced complications (χ2 = 11.20; P < .001). Notably, ≥ 3% weight loss in the 1-year preoperative period before surgery was not significantly associated with delayed recovery. Any degree of 30-day postoperative weight loss was not correlated with delayed recovery. Finally, low preoperative albumin also was not correlated with delayed recovery (Fisher exact; P = .13). Table 3 displays differences based on presence of delayed recovery in the 88 patients with CC 6 months before surgery. Of note, ≥ 10-lb weight loss in the 6 months preceding surgery also correlated with delayed recovery (P = .01).In our cohort, 3% weight loss over 6 months had a sensitivity of 57%, specificity of 77%, positive predictive value 63%, and negative predictive value 73% for delayed recovery. By comparison, a 10-lb weight loss in 6 months per ASPEN criteria had a sensitivity of 40%, specificity of 85%, positive predictive value 64%, and negative predictive value 68% for delayed recovery.
Hospital Readmissions and LOS
Hospital readmissions occurred within the first 30 days in 11 patients (10%) in the CC cohort and 12 patients (36%) in the RC cohort. Readmissions occurred between 31 and 60 days in 4 (3%) and 7 (21%) of CC and RC cohorts, respectively. The presence of ≥ 3% weight loss in the 6-month
Mean (SD) LOS was 6.4 (4.7) days (range, 1-28) for patients with CC and 8.8 (5.1) days (range, 3-23) for patients with RC. Mean (SD) LOS increased to 10.2 (4.3) days and 9.7 (6.0) days in patients with delayed recovery in the CC and RC cohorts, respectively. The mean (SD) LOS was 5.2 (2.8) days and 6.3 (2.2) days in patients without delayed recovery in the CC and RC cohorts, respectively. There was no significant difference when examining association between percent weight change and LOS for either initial admission (rs = -0.1409; 2-tailed P = .19) or for initial and readmission combined (rs = -0.13532; 2-tailed P = .21) within the CC cohort.
Chemotherapy
Within the CC cohort, 31 patients (27%) had an indication for adjuvant chemotherapy. Of these, 25 of 31 (81%) started chemotherapy within 12 weeks of surgical resection, and of these, 17 of 25 patients (68%) completed chemotherapy as indicated. Within the RC cohort all 33 patients had an indication for adjuvant chemotherapy, of these 18 of 33 patients (55%) began within 12 weeks of surgical resection, and 10 of 18 (56%) completed chemotherapy as indicated.
Among the CC cohort who began but did not complete adjuvant chemotherapy, there was no significant association between completion of chemotherapy and
Discussion
This study highlights several important findings. There were no patients in our cohort that met ASPEN malnourishment criteria with a BMI < 18.5. Twenty percent of patients lost at least 10 lb in 6 months before the operation. Notably, patients had significant associations with adverse outcomes with less pronounced weight loss than previously noted. As has been established previously, malnourishment can be difficult to screen for, and BMI also is often an imprecise tool.12 In the CC cohort, weight loss
Our findings imply that the effects of even mild malnutrition are even more profound than previously thought. Significantly, this applies to overweight and obese patients as well, as these constituted a significant fraction of our cohort. A finding of ≥ 3% weight loss at the time of CC diagnosis may provide an opportunity for a focused nutrition intervention up to the time of surgery. Second, although nutrition consultation was frequent in the inpatient setting during the hospital admission (96%-100%), rates of nutrition evaluation were as low as 15% before surgery and 12% after surgery, representing a key area for improvement and focused intervention. An optimal time for intervention and nutrition prehabilitation would be at time of diagnosis before surgery with plans for continued aggressive monitoring and subsequent follow-up. Our finding seems to provide a more sensitive tool to identify patients at risk for delayed recovery compared with the ASPEN-driven assessment. Given the simplicity and the clinical significance, our test consisting of 3% weight loss over 6 months, with its sensitivity of 57%, may be superior to the ASPEN 10-lb weight loss, with its sensitivity of 40% in our cohort.
Previous Studies
Our findings are consistent with previous studies that have demonstrated that perioperative weight loss and malnutrition are correlated with delayed recovery and complications, such as wound healing, in patients with GI cancer.2,4,5,8 In a retrospective study of more than 7000 patients with CC, those who were overweight or obese were found to have an improved overall survival compared with other BMI categories, and those who were underweight had an increased 30-day mortality and postoperative complications.16
In another retrospective study of 3799 patients with CC, those who were overweight and obese had an improved 5-year survival rate compared with patients whose weight was normal or underweight. Outcomes were found to be stage dependent.17 In this study cohort, all patients were either overweight or obese and remained in that category even with weight loss. This may have contributed to overall improved outcomes.
Implications and Next Steps
Our study has several implications. One is that BMI criteria < 18.5 may not be a good measure for malnutrition given that about 75% of the patients in our cohort were overweight or obese and none were underweight. We also show a concrete, easily identifiable finding of percent weight change that could be addressed as an automated electronic notification and potentially identify a patient at risk and serve as a trigger for both timely and early nutrition intervention. It seems to be more sensitive than the ASPEN criterion of 10-lb weight loss in 6 months before surgery. Sensitivity is especially appealing given the ease and potential of embedding this tool in an electronic health record and the clinical importance of the consequent intervention. Preoperative as opposed to perioperative nutrition optimization at time of CC diagnosis is essential, as it may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Finally, although our study found that rates of inpatient postoperative nutrition consultation were high, rates of outpatient nutrition consultation in the preoperative period were low. This represents a missed opportunity for intervention before surgery. Similarly, rates of postoperative nutrition follow-up period were low, which points to an area for improvement in longitudinal and holistic care.
We suggest modifications to nutrition intervention protocols, such as ERAS, which should start at the time of GI malignancy diagnosis.18 Other suggestions include standard involvement of nutritionists in inpatient and outpatient settings with longitudinal follow-up in the preoperative and postoperative periods and patient enrollment in a nutrition program with monitoring at time of diagnosis at the VHA. Our findings as well as previous literature suggest that the preoperative period is the most important time to intervene with regard to nutrition optimization and represents an opportunity for intensive prehabilitation. Future areas of research include incorporating other important measures of malnourishment independent of BMI into future study designs, such as sarcopenia and adipose tissue density, to better assess body composition and predict prognostic risk in CC.18,19
Strengths and Limitations
This study is limited by its single-center, retrospective design and small sample sizes, and we acknowledge the limitations of our data set. However, the strength of this VHA-based study is that the single-payer system allows for complete capture of perioperative data as well as the opportunity for focused preoperative interventions to improve outcomes. To our knowledge, there is no currently existing literature on improving nutrition protocols at the VHA for patients with a GI malignancy. These retrospective data will help inform current gaps in quality improvement and supportive oncology as it relates to optimizing malnourishment in veterans undergoing surgical resection for their cancer.
Conclusions
In the CC cohort, weight loss of ≥ 3% from 6 months prior to time of surgery was significantly associated with delayed recovery, complications, and hospital readmissions. Our findings suggest that patients with CC undergoing surgery may benefit from an intensive, early nutrition prehabilitation. Preoperative nutrition optimization may help improve postsurgical outcomes as well as oncologic outcomes, including completion of adjuvant chemotherapy. Further research would be able to clarify these hypotheses.
1. Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg. 2015;152:S3-S7. doi:10.1016/S1878-7886(15)30003-5
2. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enter Nutr. 2014;38(2):196-204. doi:10.1177/0148607113502674
3. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9:S51-S63. doi:10.1016/j.ejon.2005.09.007
4. Nishiyama VKG, Albertini SM, de Moraes CMZG, et al. Malnutrition and clinical outcomes in surgical patients with colorectal disease. Arq Gastroenterol. 2018;55(4):397-402. doi:10.1590/s0004-2803.201800000-85
5. Shpata V, Prendushi X, Kreka M, Kola I, Kurti F, Ohri I. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med Arch Sarajevo Bosnia Herzeg. 2014;68(4):263-267. doi:10.5455/medarh.2014.68.263-267
6. Lim HS, Cho GS, Park YH, Kim SK. Comparison of quality of life and nutritional status in gastric cancer patients undergoing gastrectomies. Clin Nutr Res. 2015;4(3):153-159. doi:10.7762/cnr.2015.4.3.153
7. Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. J Parenter Enter Nutr. 2000;24(1):7-14. doi:10.1177/014860710002400107
8. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26(6):698-709. doi:10.1016/j.clnu.2007.06.009
9. Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001; 358(9292):1487-1492. doi:10.1016/S0140-6736(01)06578-3
10. Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr Edinb Scotl. 2021;40(1):40-46. doi:10.1016/j.clnu.2020.04.043 start
11. White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. doi:10.1016/j.jand.2012.03.012
12. Jones JM. The methodology of nutritional screening and assessment tools. J Hum Nutr Diet. 2002;15(1):59-71. doi:10.1046/j.1365-277X.2002.00327.x
13. Williams J, Wischmeyer P. Assessment of perioperative nutrition practices and attitudes—a national survey of colorectal and GI surgical oncology programs. Am J Surg. 2017;213(6):1010-1018. doi:10.1016/j.amjsurg.2016.10.008
14. Williams DG, Aronson S, Murray S, et al. Validation of the perioperative nutrition screen for prediction of postoperative outcomes. JPEN J Parenter Enteral Nutr. 2022;46(6):1307-1315. doi:10.1002/jpen.2310
15. Besson AJ, Kei C, Djordjevic A, Carter V, Deftereos I, Yeung J. Does implementation of and adherence to enhanced recovery after surgery improve perioperative nutritional management in colorectal cancer surgery? ANZ J Surg. 2022;92(6):1382-1387. doi:10.1111/ans.17599
16. Arkenbosch JHC, van Erning FN, Rutten HJ, Zimmerman D, de Wilt JHW, Beijer S. The association between body mass index and postoperative complications, 30-day mortality and long-term survival in Dutch patients with colorectal cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2019;45(2):160-166. doi:10.1016/j.ejso.2018.09.012
17. Shahjehan F, Merchea A, Cochuyt JJ, Li Z, Colibaseanu DT, Kasi PM. Body mass index and long-term outcomes in patients with colorectal cancer. Front Oncol. 2018;8:620. doi:10.3389/fonc.2018.00620
18. Nishigori T, Obama K, Sakai Y. Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer. Transl Gastroenterol Hepatol. 2020;5:22. doi:10.21037/tgh.2019.10.13
19. Feliciano EMC, Winkels RM, Meyerhardt JA, Prado CM, Afman LA, Caan BJ. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am J Clin Nutr. 2021;114(6):1917-1924. doi:10.1093/ajcn/nqab285
1. Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg. 2015;152:S3-S7. doi:10.1016/S1878-7886(15)30003-5
2. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enter Nutr. 2014;38(2):196-204. doi:10.1177/0148607113502674
3. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9:S51-S63. doi:10.1016/j.ejon.2005.09.007
4. Nishiyama VKG, Albertini SM, de Moraes CMZG, et al. Malnutrition and clinical outcomes in surgical patients with colorectal disease. Arq Gastroenterol. 2018;55(4):397-402. doi:10.1590/s0004-2803.201800000-85
5. Shpata V, Prendushi X, Kreka M, Kola I, Kurti F, Ohri I. Malnutrition at the time of surgery affects negatively the clinical outcome of critically ill patients with gastrointestinal cancer. Med Arch Sarajevo Bosnia Herzeg. 2014;68(4):263-267. doi:10.5455/medarh.2014.68.263-267
6. Lim HS, Cho GS, Park YH, Kim SK. Comparison of quality of life and nutritional status in gastric cancer patients undergoing gastrectomies. Clin Nutr Res. 2015;4(3):153-159. doi:10.7762/cnr.2015.4.3.153
7. Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. J Parenter Enter Nutr. 2000;24(1):7-14. doi:10.1177/014860710002400107
8. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26(6):698-709. doi:10.1016/j.clnu.2007.06.009
9. Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001; 358(9292):1487-1492. doi:10.1016/S0140-6736(01)06578-3
10. Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr Edinb Scotl. 2021;40(1):40-46. doi:10.1016/j.clnu.2020.04.043 start
11. White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. doi:10.1016/j.jand.2012.03.012
12. Jones JM. The methodology of nutritional screening and assessment tools. J Hum Nutr Diet. 2002;15(1):59-71. doi:10.1046/j.1365-277X.2002.00327.x
13. Williams J, Wischmeyer P. Assessment of perioperative nutrition practices and attitudes—a national survey of colorectal and GI surgical oncology programs. Am J Surg. 2017;213(6):1010-1018. doi:10.1016/j.amjsurg.2016.10.008
14. Williams DG, Aronson S, Murray S, et al. Validation of the perioperative nutrition screen for prediction of postoperative outcomes. JPEN J Parenter Enteral Nutr. 2022;46(6):1307-1315. doi:10.1002/jpen.2310
15. Besson AJ, Kei C, Djordjevic A, Carter V, Deftereos I, Yeung J. Does implementation of and adherence to enhanced recovery after surgery improve perioperative nutritional management in colorectal cancer surgery? ANZ J Surg. 2022;92(6):1382-1387. doi:10.1111/ans.17599
16. Arkenbosch JHC, van Erning FN, Rutten HJ, Zimmerman D, de Wilt JHW, Beijer S. The association between body mass index and postoperative complications, 30-day mortality and long-term survival in Dutch patients with colorectal cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2019;45(2):160-166. doi:10.1016/j.ejso.2018.09.012
17. Shahjehan F, Merchea A, Cochuyt JJ, Li Z, Colibaseanu DT, Kasi PM. Body mass index and long-term outcomes in patients with colorectal cancer. Front Oncol. 2018;8:620. doi:10.3389/fonc.2018.00620
18. Nishigori T, Obama K, Sakai Y. Assessment of body composition and impact of sarcopenia and sarcopenic obesity in patients with gastric cancer. Transl Gastroenterol Hepatol. 2020;5:22. doi:10.21037/tgh.2019.10.13
19. Feliciano EMC, Winkels RM, Meyerhardt JA, Prado CM, Afman LA, Caan BJ. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am J Clin Nutr. 2021;114(6):1917-1924. doi:10.1093/ajcn/nqab285
Home sleep apnea test: Peripheral arterial tonometry
Sleep Medicine Network
Respiratory-related Sleep Disorders Section
Home sleep apnea test: Peripheral arterial tonometry
OSA is associated with serious health consequences and increased health care utilization (Kapur V, et al. Sleep. 1999:22[6]:749).
Polysomnography (PSG) is the gold standard for diagnosis, but is expensive, cumbersome, and inconsistently accessible. (Kapur VK, et al. J Clin Sleep Med. 2017;13[3]:479; Skomro RP, et al. Chest. 2010;138[2]:257).
Utilization of HSAT devices has increased in recent years, partly due to the COVID-19 pandemic and limitations in insurance reimbursement for PSG as the initial diagnostic test. But while there are benefits to home testing with respect to convenience and increased access, we must take the clinical context into account.
Peripheral arterial tonometry (PAT) is a commonly used HSAT technology, which measures peripheral arterial vascular tone using plethysmography at the fingertip. It has a sensitivity of 80% and specificity of 83% for detecting OSA in patients without significant comorbidities and high pretest probability of OSA compared to PSG (Ward KL, et al. J Clin Sleep Med. 2015;11[4]:433). But PAT has also been criticized for lacking diagnostic accuracy, particularly when including patients with mild OSA in analysis (Ichikawa M, et al. J Sleep Res. 2022;31[6]:e13682).
HSAT devices using PAT technology have been studied in patients with atrial fibrillation (Tauman R, et al. Nat Sci Sleep. 2020;12:1115), adolescents (Choi JH, et al. J Clin Sleep Med. 2018;14[10]:1741), and pregnant women (O’Brien LM, et al. J Clin Sleep Med. 2012;8[3]:287), and to assess OSA treatment adequacy with varying sensitivity and specificity. Study in special populations may allow for increased access to testing with the benefit of increased recognition of a generally underdiagnosed disorder. But it’s important to use HSAT alongside awareness of its limitations and it should not replace good clinical judgment when making treatment decisions.
Dimple Tejwani, MD
Member-at-Large
Kara Dupuy-McCauley, MD
Member-at-Large
Sleep Medicine Network
Respiratory-related Sleep Disorders Section
Home sleep apnea test: Peripheral arterial tonometry
OSA is associated with serious health consequences and increased health care utilization (Kapur V, et al. Sleep. 1999:22[6]:749).
Polysomnography (PSG) is the gold standard for diagnosis, but is expensive, cumbersome, and inconsistently accessible. (Kapur VK, et al. J Clin Sleep Med. 2017;13[3]:479; Skomro RP, et al. Chest. 2010;138[2]:257).
Utilization of HSAT devices has increased in recent years, partly due to the COVID-19 pandemic and limitations in insurance reimbursement for PSG as the initial diagnostic test. But while there are benefits to home testing with respect to convenience and increased access, we must take the clinical context into account.
Peripheral arterial tonometry (PAT) is a commonly used HSAT technology, which measures peripheral arterial vascular tone using plethysmography at the fingertip. It has a sensitivity of 80% and specificity of 83% for detecting OSA in patients without significant comorbidities and high pretest probability of OSA compared to PSG (Ward KL, et al. J Clin Sleep Med. 2015;11[4]:433). But PAT has also been criticized for lacking diagnostic accuracy, particularly when including patients with mild OSA in analysis (Ichikawa M, et al. J Sleep Res. 2022;31[6]:e13682).
HSAT devices using PAT technology have been studied in patients with atrial fibrillation (Tauman R, et al. Nat Sci Sleep. 2020;12:1115), adolescents (Choi JH, et al. J Clin Sleep Med. 2018;14[10]:1741), and pregnant women (O’Brien LM, et al. J Clin Sleep Med. 2012;8[3]:287), and to assess OSA treatment adequacy with varying sensitivity and specificity. Study in special populations may allow for increased access to testing with the benefit of increased recognition of a generally underdiagnosed disorder. But it’s important to use HSAT alongside awareness of its limitations and it should not replace good clinical judgment when making treatment decisions.
Dimple Tejwani, MD
Member-at-Large
Kara Dupuy-McCauley, MD
Member-at-Large
Sleep Medicine Network
Respiratory-related Sleep Disorders Section
Home sleep apnea test: Peripheral arterial tonometry
OSA is associated with serious health consequences and increased health care utilization (Kapur V, et al. Sleep. 1999:22[6]:749).
Polysomnography (PSG) is the gold standard for diagnosis, but is expensive, cumbersome, and inconsistently accessible. (Kapur VK, et al. J Clin Sleep Med. 2017;13[3]:479; Skomro RP, et al. Chest. 2010;138[2]:257).
Utilization of HSAT devices has increased in recent years, partly due to the COVID-19 pandemic and limitations in insurance reimbursement for PSG as the initial diagnostic test. But while there are benefits to home testing with respect to convenience and increased access, we must take the clinical context into account.
Peripheral arterial tonometry (PAT) is a commonly used HSAT technology, which measures peripheral arterial vascular tone using plethysmography at the fingertip. It has a sensitivity of 80% and specificity of 83% for detecting OSA in patients without significant comorbidities and high pretest probability of OSA compared to PSG (Ward KL, et al. J Clin Sleep Med. 2015;11[4]:433). But PAT has also been criticized for lacking diagnostic accuracy, particularly when including patients with mild OSA in analysis (Ichikawa M, et al. J Sleep Res. 2022;31[6]:e13682).
HSAT devices using PAT technology have been studied in patients with atrial fibrillation (Tauman R, et al. Nat Sci Sleep. 2020;12:1115), adolescents (Choi JH, et al. J Clin Sleep Med. 2018;14[10]:1741), and pregnant women (O’Brien LM, et al. J Clin Sleep Med. 2012;8[3]:287), and to assess OSA treatment adequacy with varying sensitivity and specificity. Study in special populations may allow for increased access to testing with the benefit of increased recognition of a generally underdiagnosed disorder. But it’s important to use HSAT alongside awareness of its limitations and it should not replace good clinical judgment when making treatment decisions.
Dimple Tejwani, MD
Member-at-Large
Kara Dupuy-McCauley, MD
Member-at-Large
Emerging role of tele-rehab: Efficacy and challenges
Diffuse Lung Disease and Transplant Network
Pulmonary Physiology and Rehabilitation Section
Pulmonary rehabilitation (PR) is an essential component of the management of chronic pulmonary disease. Interest in alternate PR delivery methods has grown in recent years. The official workshop report of the American Thoracic Society (Holland AE, et al. Ann Am Thorac Soc. 2021;18[5]:e12) identified 13 essential components of PR in response to new program models. They encompass patient assessment, program content, method of delivery, and quality assurance, and serve as a guide for successful implementation of emerging programs.
A recent study reported significant improvement in COPD Assessment Test (CAT) scores after PR in both in-person (n=383) and virtual programs (n=171). Similar improvements were found in health outcomes, attendance, and dropout rate (Huynh VC, et al. Chest. 2023;163[3]:529). Another concurrent 3-year prospective study enrolled COPD patients in standard PR (n=89) or community based tele-PR (n=177) at seven tele-sites and one standard site (Alwakeel AJ, et al. Ann Am Thorac Soc. 2022;19[1]:39).
This study established the accessibility, feasibility, and safety of a community based tele-PR program and noted no differences between groups in 6-minute walk test or CAT score improvement.
Ongoing challenges with tele-PR include standardization of programs and of initial clinical evaluations that determine eligibility for them. Patients on home oxygen and those with exercise desaturation are often excluded, but they have the most potential for improvement. Studies are needed to determine the characteristics of patients who would benefit most from non-traditional models of PR.
Fatima Zeba, MD
Fellow-in-Training
Rania Abdallah, MD
Member-at-Large
Malik Khurram Khan, MD
Member-at-Large
Diffuse Lung Disease and Transplant Network
Pulmonary Physiology and Rehabilitation Section
Pulmonary rehabilitation (PR) is an essential component of the management of chronic pulmonary disease. Interest in alternate PR delivery methods has grown in recent years. The official workshop report of the American Thoracic Society (Holland AE, et al. Ann Am Thorac Soc. 2021;18[5]:e12) identified 13 essential components of PR in response to new program models. They encompass patient assessment, program content, method of delivery, and quality assurance, and serve as a guide for successful implementation of emerging programs.
A recent study reported significant improvement in COPD Assessment Test (CAT) scores after PR in both in-person (n=383) and virtual programs (n=171). Similar improvements were found in health outcomes, attendance, and dropout rate (Huynh VC, et al. Chest. 2023;163[3]:529). Another concurrent 3-year prospective study enrolled COPD patients in standard PR (n=89) or community based tele-PR (n=177) at seven tele-sites and one standard site (Alwakeel AJ, et al. Ann Am Thorac Soc. 2022;19[1]:39).
This study established the accessibility, feasibility, and safety of a community based tele-PR program and noted no differences between groups in 6-minute walk test or CAT score improvement.
Ongoing challenges with tele-PR include standardization of programs and of initial clinical evaluations that determine eligibility for them. Patients on home oxygen and those with exercise desaturation are often excluded, but they have the most potential for improvement. Studies are needed to determine the characteristics of patients who would benefit most from non-traditional models of PR.
Fatima Zeba, MD
Fellow-in-Training
Rania Abdallah, MD
Member-at-Large
Malik Khurram Khan, MD
Member-at-Large
Diffuse Lung Disease and Transplant Network
Pulmonary Physiology and Rehabilitation Section
Pulmonary rehabilitation (PR) is an essential component of the management of chronic pulmonary disease. Interest in alternate PR delivery methods has grown in recent years. The official workshop report of the American Thoracic Society (Holland AE, et al. Ann Am Thorac Soc. 2021;18[5]:e12) identified 13 essential components of PR in response to new program models. They encompass patient assessment, program content, method of delivery, and quality assurance, and serve as a guide for successful implementation of emerging programs.
A recent study reported significant improvement in COPD Assessment Test (CAT) scores after PR in both in-person (n=383) and virtual programs (n=171). Similar improvements were found in health outcomes, attendance, and dropout rate (Huynh VC, et al. Chest. 2023;163[3]:529). Another concurrent 3-year prospective study enrolled COPD patients in standard PR (n=89) or community based tele-PR (n=177) at seven tele-sites and one standard site (Alwakeel AJ, et al. Ann Am Thorac Soc. 2022;19[1]:39).
This study established the accessibility, feasibility, and safety of a community based tele-PR program and noted no differences between groups in 6-minute walk test or CAT score improvement.
Ongoing challenges with tele-PR include standardization of programs and of initial clinical evaluations that determine eligibility for them. Patients on home oxygen and those with exercise desaturation are often excluded, but they have the most potential for improvement. Studies are needed to determine the characteristics of patients who would benefit most from non-traditional models of PR.
Fatima Zeba, MD
Fellow-in-Training
Rania Abdallah, MD
Member-at-Large
Malik Khurram Khan, MD
Member-at-Large
Study shifts burden of IgG4-related disease to women
The incidence and prevalence of IgG4-related disease each rose considerably from 2015 to 2019 in the United States, and the risk of death in those with the immune-mediated condition is about 2.5 times higher than those who are not affected, based on an analysis of claims data from commercially insured adults.
The first population-based study of IgG4-RD incidence, prevalence, and mortality establishes “key benchmarks for informing the diagnosis and management of patients” with a condition “that causes fibrosing inflammatory lesions at nearly any anatomic site,” and wasn’t initially described until 2001, Zachary S. Wallace, MD, and associates said in Annals of the Rheumatic Diseases.
The increases in incidence and prevalence likely reflected increased disease awareness, they suggested. Overall U.S. incidence was 1.2 per 100,000 person-years for the 5-year period of 2015-2019, rising 86% from 0.78 per 100,000 person-years to 1.45 in 2018 before dropping to 1.39 in 2019. The change in prevalence was even greater, increasing 122% from 2.41 per 100,000 persons in 2015 to 5.34 per 100,000 in 2019, the investigators said.
Previous studies had indicated that the majority of patients with IgG4-RD were male, but the current study, using Optum’s Clinformatics Data Mart, which includes commercial health plan and Medicare Advantage members in all 50 states, showed that both incidence and prevalence (see graph) were higher among women, noted Dr. Wallace of Massachusetts General Hospital, Boston, and associates. They identified 524 patients (57.6% female) in the database who met the criteria for IgG4-RD from Jan. 1, 2010, to Dec. 31, 2019.
Incidence over the course of the study “was similar in patients identified as Asian or White but lower in those identified as Black or Hispanic,” they noted, adding that “the prevalence of IgG4-RD during this period reflected similar trends.” A jump in prevalence from 2018 to 2019, however, left White patients with a much higher rate (6.13 per 100,000 persons) than Asian patients (4.54 per 100,000), Black patients (3.42), and Hispanic patients (3.02).
For the mortality analysis, 516 patients with IgG4-RD were age-, sex-, and race-matched with 5,160 patients without IgG4-RD. Mortality was 3.42 and 1.46 per 100 person-years, respectively, over the 5.5 years of follow-up, so IgG4-RD was associated with a 2.5-fold higher risk of death. “The association of IgG4-RD with a higher risk of death was observed across the age spectrum and among both male and female patients,” the researchers said.
“Clinicians across specialties should be aware of IgG4-RD given the incidence, prevalence, and excess risk of death associated with this condition. ... Additional studies are urgently needed to define optimal management strategies to improve survival,” they wrote.
The study was supported by a grant to Massachusetts General Hospital from Sanofi, and Dr. Wallace received funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation. He has received research support and consulting fees from several companies, and four coinvestigators are employees of Sanofi.
The incidence and prevalence of IgG4-related disease each rose considerably from 2015 to 2019 in the United States, and the risk of death in those with the immune-mediated condition is about 2.5 times higher than those who are not affected, based on an analysis of claims data from commercially insured adults.
The first population-based study of IgG4-RD incidence, prevalence, and mortality establishes “key benchmarks for informing the diagnosis and management of patients” with a condition “that causes fibrosing inflammatory lesions at nearly any anatomic site,” and wasn’t initially described until 2001, Zachary S. Wallace, MD, and associates said in Annals of the Rheumatic Diseases.
The increases in incidence and prevalence likely reflected increased disease awareness, they suggested. Overall U.S. incidence was 1.2 per 100,000 person-years for the 5-year period of 2015-2019, rising 86% from 0.78 per 100,000 person-years to 1.45 in 2018 before dropping to 1.39 in 2019. The change in prevalence was even greater, increasing 122% from 2.41 per 100,000 persons in 2015 to 5.34 per 100,000 in 2019, the investigators said.
Previous studies had indicated that the majority of patients with IgG4-RD were male, but the current study, using Optum’s Clinformatics Data Mart, which includes commercial health plan and Medicare Advantage members in all 50 states, showed that both incidence and prevalence (see graph) were higher among women, noted Dr. Wallace of Massachusetts General Hospital, Boston, and associates. They identified 524 patients (57.6% female) in the database who met the criteria for IgG4-RD from Jan. 1, 2010, to Dec. 31, 2019.
Incidence over the course of the study “was similar in patients identified as Asian or White but lower in those identified as Black or Hispanic,” they noted, adding that “the prevalence of IgG4-RD during this period reflected similar trends.” A jump in prevalence from 2018 to 2019, however, left White patients with a much higher rate (6.13 per 100,000 persons) than Asian patients (4.54 per 100,000), Black patients (3.42), and Hispanic patients (3.02).
For the mortality analysis, 516 patients with IgG4-RD were age-, sex-, and race-matched with 5,160 patients without IgG4-RD. Mortality was 3.42 and 1.46 per 100 person-years, respectively, over the 5.5 years of follow-up, so IgG4-RD was associated with a 2.5-fold higher risk of death. “The association of IgG4-RD with a higher risk of death was observed across the age spectrum and among both male and female patients,” the researchers said.
“Clinicians across specialties should be aware of IgG4-RD given the incidence, prevalence, and excess risk of death associated with this condition. ... Additional studies are urgently needed to define optimal management strategies to improve survival,” they wrote.
The study was supported by a grant to Massachusetts General Hospital from Sanofi, and Dr. Wallace received funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation. He has received research support and consulting fees from several companies, and four coinvestigators are employees of Sanofi.
The incidence and prevalence of IgG4-related disease each rose considerably from 2015 to 2019 in the United States, and the risk of death in those with the immune-mediated condition is about 2.5 times higher than those who are not affected, based on an analysis of claims data from commercially insured adults.
The first population-based study of IgG4-RD incidence, prevalence, and mortality establishes “key benchmarks for informing the diagnosis and management of patients” with a condition “that causes fibrosing inflammatory lesions at nearly any anatomic site,” and wasn’t initially described until 2001, Zachary S. Wallace, MD, and associates said in Annals of the Rheumatic Diseases.
The increases in incidence and prevalence likely reflected increased disease awareness, they suggested. Overall U.S. incidence was 1.2 per 100,000 person-years for the 5-year period of 2015-2019, rising 86% from 0.78 per 100,000 person-years to 1.45 in 2018 before dropping to 1.39 in 2019. The change in prevalence was even greater, increasing 122% from 2.41 per 100,000 persons in 2015 to 5.34 per 100,000 in 2019, the investigators said.
Previous studies had indicated that the majority of patients with IgG4-RD were male, but the current study, using Optum’s Clinformatics Data Mart, which includes commercial health plan and Medicare Advantage members in all 50 states, showed that both incidence and prevalence (see graph) were higher among women, noted Dr. Wallace of Massachusetts General Hospital, Boston, and associates. They identified 524 patients (57.6% female) in the database who met the criteria for IgG4-RD from Jan. 1, 2010, to Dec. 31, 2019.
Incidence over the course of the study “was similar in patients identified as Asian or White but lower in those identified as Black or Hispanic,” they noted, adding that “the prevalence of IgG4-RD during this period reflected similar trends.” A jump in prevalence from 2018 to 2019, however, left White patients with a much higher rate (6.13 per 100,000 persons) than Asian patients (4.54 per 100,000), Black patients (3.42), and Hispanic patients (3.02).
For the mortality analysis, 516 patients with IgG4-RD were age-, sex-, and race-matched with 5,160 patients without IgG4-RD. Mortality was 3.42 and 1.46 per 100 person-years, respectively, over the 5.5 years of follow-up, so IgG4-RD was associated with a 2.5-fold higher risk of death. “The association of IgG4-RD with a higher risk of death was observed across the age spectrum and among both male and female patients,” the researchers said.
“Clinicians across specialties should be aware of IgG4-RD given the incidence, prevalence, and excess risk of death associated with this condition. ... Additional studies are urgently needed to define optimal management strategies to improve survival,” they wrote.
The study was supported by a grant to Massachusetts General Hospital from Sanofi, and Dr. Wallace received funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation. He has received research support and consulting fees from several companies, and four coinvestigators are employees of Sanofi.
FROM ANNALS OF THE RHEUMATIC DISEASES
New USPSTF draft suggests mammography start at 40, not 50
The major change: USPSTF proposed reducing the recommended start age for routine screening mammograms from age 50 to age 40. The latest recommendation, which carries a B grade, also calls for screening every other year and sets a cutoff age of 74.
The task force’s A and B ratings indicate strong confidence in the evidence for benefit, meaning that clinicians should encourage their patients to get these services as appropriate.
The influential federal advisory panel last updated these recommendations in 2016. At the time, USPSTF recommended routine screening mammograms starting at age 50, and gave a C grade to starting before that.
In the 2016 recommendations, “we felt a woman could start screening in her 40s depending on how she feels about the harms and benefits in an individualized personal decision,” USPSTF member John Wong, MD, chief of clinical decision making and a primary care physician at Tufts Medical Center in Boston, said in an interview. “In this draft recommendation, we now recommend that all women get screened starting at age 40.”
Two major factors prompted the change, explained Dr. Wong. One is that more women are being diagnosed with breast cancer in their 40s. The other is that a growing body of evidence showing that Black women get breast cancer younger, are more likely to die of breast cancer, and would benefit from earlier screening.
“It is now clear that screening every other year starting at age 40 has the potential to save about 20% more lives among all women and there is even greater potential benefit for Black women, who are much more likely to die from breast cancer,” Dr. Wong said.
The American Cancer Society (ACS) called the draft recommendations a “significant positive change,” while noting that the task force recommendations only apply to women at average risk for breast cancer.
The American College of Radiology (ACR) already recommends yearly mammograms for average risk women starting at age 40. Its latest guidelines on mammography call for women at higher-than-average risk for breast cancer to undergo a risk assessment by age 25 to determine if screening before age 40 is needed.
When asked about the differing views, Debra Monticciolo, MD, division chief for breast imaging at Massachusetts General Hospital, said annual screenings that follow ACR recommendations would save more lives than the every-other-year approach backed by the task force. Dr. Monticciolo also highlighted that the available scientific evidence supports earlier assessment as well as augmented and earlier-than-age-40 screening of many women – particularly Black women.
“These evidence-based updates should spur more-informed doctor–patient conversations and help providers save more lives,” Dr. Monticciolo said in a press release.
Insurance access
Typically, upgrading a USPSTF recommendation from C to B leads to better access and insurance coverage for patients. The Affordable Care Act (ACA) of 2010 requires insurers to cover the cost of services that get A and B recommendations from the USPSTF without charging copays – a mandate intended to promote greater use for highly regarded services.
But Congress created a special workaround that effectively makes the ACA mandate apply to the 2002 task force recommendations on mammography. In those recommendations, the task force gave a B grade to screening mammograms every 1 or 2 years starting at age 40 without an age limit.
Federal lawmakers have sought to provide copay-free access to mammograms for this entire population even when the USPSTF recommendations in 2009 and 2016 gave a C grade to routine screening for women under 50.
Still, “it is important to note that our recommendation is based solely on the science of what works to prevent breast cancer and it is not a recommendation for or against insurance coverage,” the task force acknowledged when unveiling the new draft update. “Coverage decisions involve considerations beyond the evidence about clinical benefit, and in the end, these decisions are the responsibility of payors, regulators, and legislators.”
Uncertainties persist
The new draft recommendations also highlight the persistent gaps in knowledge about the uses of mammography, despite years of widespread use of this screening tool.
The updated draft recommendations emphasize the lack of sufficient evidence to address major areas of concern related to screening and treating Black women, older women, women with dense breasts, and those with ductal carcinoma in situ (DCIS).
The task force called for more research addressing the underlying causes of elevated breast cancer mortality rates among Black women.
The USPSTF also issued an ‘I’ statement for providing women with dense breasts additional screening with breast ultrasound or MRI and for screening women older than 75 for breast cancer. Such statements indicate that the available evidence is lacking, poor quality, or conflicting, and thus the USPSTF can’t assess the benefits and harms or make a recommendation for or against providing the preventive service.
“Nearly half of all women have dense breasts, which increases their risk for breast cancer and means that mammograms may not work as well for them. We need to know more about whether and how additional screening might help women with dense breasts stay healthy,” the task force explained.
The task force also called for more research on approaches to reduce the risk for overdiagnosis and overtreatment for breast lesions, such as DCIS, which are identified through screening.
One analysis – the COMET study – is currently underway to assess whether women could be spared surgery for DCIS and opt for watchful waiting instead.
“If we can find that monitoring them carefully, either with or without some sort of endocrine therapy, is just as effective in keeping patients free of invasive cancer as surgery, then I think we could help to de-escalate treatment for this very low-risk group of patients,” Shelley Hwang, MD, MPH, principal investigator of the COMET study, told this news organization in December.
The task force will accept comments from the public on this draft update through June 5.
A version of this article first appeared on Medscape.com.
The major change: USPSTF proposed reducing the recommended start age for routine screening mammograms from age 50 to age 40. The latest recommendation, which carries a B grade, also calls for screening every other year and sets a cutoff age of 74.
The task force’s A and B ratings indicate strong confidence in the evidence for benefit, meaning that clinicians should encourage their patients to get these services as appropriate.
The influential federal advisory panel last updated these recommendations in 2016. At the time, USPSTF recommended routine screening mammograms starting at age 50, and gave a C grade to starting before that.
In the 2016 recommendations, “we felt a woman could start screening in her 40s depending on how she feels about the harms and benefits in an individualized personal decision,” USPSTF member John Wong, MD, chief of clinical decision making and a primary care physician at Tufts Medical Center in Boston, said in an interview. “In this draft recommendation, we now recommend that all women get screened starting at age 40.”
Two major factors prompted the change, explained Dr. Wong. One is that more women are being diagnosed with breast cancer in their 40s. The other is that a growing body of evidence showing that Black women get breast cancer younger, are more likely to die of breast cancer, and would benefit from earlier screening.
“It is now clear that screening every other year starting at age 40 has the potential to save about 20% more lives among all women and there is even greater potential benefit for Black women, who are much more likely to die from breast cancer,” Dr. Wong said.
The American Cancer Society (ACS) called the draft recommendations a “significant positive change,” while noting that the task force recommendations only apply to women at average risk for breast cancer.
The American College of Radiology (ACR) already recommends yearly mammograms for average risk women starting at age 40. Its latest guidelines on mammography call for women at higher-than-average risk for breast cancer to undergo a risk assessment by age 25 to determine if screening before age 40 is needed.
When asked about the differing views, Debra Monticciolo, MD, division chief for breast imaging at Massachusetts General Hospital, said annual screenings that follow ACR recommendations would save more lives than the every-other-year approach backed by the task force. Dr. Monticciolo also highlighted that the available scientific evidence supports earlier assessment as well as augmented and earlier-than-age-40 screening of many women – particularly Black women.
“These evidence-based updates should spur more-informed doctor–patient conversations and help providers save more lives,” Dr. Monticciolo said in a press release.
Insurance access
Typically, upgrading a USPSTF recommendation from C to B leads to better access and insurance coverage for patients. The Affordable Care Act (ACA) of 2010 requires insurers to cover the cost of services that get A and B recommendations from the USPSTF without charging copays – a mandate intended to promote greater use for highly regarded services.
But Congress created a special workaround that effectively makes the ACA mandate apply to the 2002 task force recommendations on mammography. In those recommendations, the task force gave a B grade to screening mammograms every 1 or 2 years starting at age 40 without an age limit.
Federal lawmakers have sought to provide copay-free access to mammograms for this entire population even when the USPSTF recommendations in 2009 and 2016 gave a C grade to routine screening for women under 50.
Still, “it is important to note that our recommendation is based solely on the science of what works to prevent breast cancer and it is not a recommendation for or against insurance coverage,” the task force acknowledged when unveiling the new draft update. “Coverage decisions involve considerations beyond the evidence about clinical benefit, and in the end, these decisions are the responsibility of payors, regulators, and legislators.”
Uncertainties persist
The new draft recommendations also highlight the persistent gaps in knowledge about the uses of mammography, despite years of widespread use of this screening tool.
The updated draft recommendations emphasize the lack of sufficient evidence to address major areas of concern related to screening and treating Black women, older women, women with dense breasts, and those with ductal carcinoma in situ (DCIS).
The task force called for more research addressing the underlying causes of elevated breast cancer mortality rates among Black women.
The USPSTF also issued an ‘I’ statement for providing women with dense breasts additional screening with breast ultrasound or MRI and for screening women older than 75 for breast cancer. Such statements indicate that the available evidence is lacking, poor quality, or conflicting, and thus the USPSTF can’t assess the benefits and harms or make a recommendation for or against providing the preventive service.
“Nearly half of all women have dense breasts, which increases their risk for breast cancer and means that mammograms may not work as well for them. We need to know more about whether and how additional screening might help women with dense breasts stay healthy,” the task force explained.
The task force also called for more research on approaches to reduce the risk for overdiagnosis and overtreatment for breast lesions, such as DCIS, which are identified through screening.
One analysis – the COMET study – is currently underway to assess whether women could be spared surgery for DCIS and opt for watchful waiting instead.
“If we can find that monitoring them carefully, either with or without some sort of endocrine therapy, is just as effective in keeping patients free of invasive cancer as surgery, then I think we could help to de-escalate treatment for this very low-risk group of patients,” Shelley Hwang, MD, MPH, principal investigator of the COMET study, told this news organization in December.
The task force will accept comments from the public on this draft update through June 5.
A version of this article first appeared on Medscape.com.
The major change: USPSTF proposed reducing the recommended start age for routine screening mammograms from age 50 to age 40. The latest recommendation, which carries a B grade, also calls for screening every other year and sets a cutoff age of 74.
The task force’s A and B ratings indicate strong confidence in the evidence for benefit, meaning that clinicians should encourage their patients to get these services as appropriate.
The influential federal advisory panel last updated these recommendations in 2016. At the time, USPSTF recommended routine screening mammograms starting at age 50, and gave a C grade to starting before that.
In the 2016 recommendations, “we felt a woman could start screening in her 40s depending on how she feels about the harms and benefits in an individualized personal decision,” USPSTF member John Wong, MD, chief of clinical decision making and a primary care physician at Tufts Medical Center in Boston, said in an interview. “In this draft recommendation, we now recommend that all women get screened starting at age 40.”
Two major factors prompted the change, explained Dr. Wong. One is that more women are being diagnosed with breast cancer in their 40s. The other is that a growing body of evidence showing that Black women get breast cancer younger, are more likely to die of breast cancer, and would benefit from earlier screening.
“It is now clear that screening every other year starting at age 40 has the potential to save about 20% more lives among all women and there is even greater potential benefit for Black women, who are much more likely to die from breast cancer,” Dr. Wong said.
The American Cancer Society (ACS) called the draft recommendations a “significant positive change,” while noting that the task force recommendations only apply to women at average risk for breast cancer.
The American College of Radiology (ACR) already recommends yearly mammograms for average risk women starting at age 40. Its latest guidelines on mammography call for women at higher-than-average risk for breast cancer to undergo a risk assessment by age 25 to determine if screening before age 40 is needed.
When asked about the differing views, Debra Monticciolo, MD, division chief for breast imaging at Massachusetts General Hospital, said annual screenings that follow ACR recommendations would save more lives than the every-other-year approach backed by the task force. Dr. Monticciolo also highlighted that the available scientific evidence supports earlier assessment as well as augmented and earlier-than-age-40 screening of many women – particularly Black women.
“These evidence-based updates should spur more-informed doctor–patient conversations and help providers save more lives,” Dr. Monticciolo said in a press release.
Insurance access
Typically, upgrading a USPSTF recommendation from C to B leads to better access and insurance coverage for patients. The Affordable Care Act (ACA) of 2010 requires insurers to cover the cost of services that get A and B recommendations from the USPSTF without charging copays – a mandate intended to promote greater use for highly regarded services.
But Congress created a special workaround that effectively makes the ACA mandate apply to the 2002 task force recommendations on mammography. In those recommendations, the task force gave a B grade to screening mammograms every 1 or 2 years starting at age 40 without an age limit.
Federal lawmakers have sought to provide copay-free access to mammograms for this entire population even when the USPSTF recommendations in 2009 and 2016 gave a C grade to routine screening for women under 50.
Still, “it is important to note that our recommendation is based solely on the science of what works to prevent breast cancer and it is not a recommendation for or against insurance coverage,” the task force acknowledged when unveiling the new draft update. “Coverage decisions involve considerations beyond the evidence about clinical benefit, and in the end, these decisions are the responsibility of payors, regulators, and legislators.”
Uncertainties persist
The new draft recommendations also highlight the persistent gaps in knowledge about the uses of mammography, despite years of widespread use of this screening tool.
The updated draft recommendations emphasize the lack of sufficient evidence to address major areas of concern related to screening and treating Black women, older women, women with dense breasts, and those with ductal carcinoma in situ (DCIS).
The task force called for more research addressing the underlying causes of elevated breast cancer mortality rates among Black women.
The USPSTF also issued an ‘I’ statement for providing women with dense breasts additional screening with breast ultrasound or MRI and for screening women older than 75 for breast cancer. Such statements indicate that the available evidence is lacking, poor quality, or conflicting, and thus the USPSTF can’t assess the benefits and harms or make a recommendation for or against providing the preventive service.
“Nearly half of all women have dense breasts, which increases their risk for breast cancer and means that mammograms may not work as well for them. We need to know more about whether and how additional screening might help women with dense breasts stay healthy,” the task force explained.
The task force also called for more research on approaches to reduce the risk for overdiagnosis and overtreatment for breast lesions, such as DCIS, which are identified through screening.
One analysis – the COMET study – is currently underway to assess whether women could be spared surgery for DCIS and opt for watchful waiting instead.
“If we can find that monitoring them carefully, either with or without some sort of endocrine therapy, is just as effective in keeping patients free of invasive cancer as surgery, then I think we could help to de-escalate treatment for this very low-risk group of patients,” Shelley Hwang, MD, MPH, principal investigator of the COMET study, told this news organization in December.
The task force will accept comments from the public on this draft update through June 5.
A version of this article first appeared on Medscape.com.
Axial spondyloarthritis versus axial psoriatic arthritis: Different entities?
Are there clinically significant differences between axial spondyloarthritis with psoriasis and psoriatic arthritis with axial symptoms? Does it matter?
It all depends on whom you ask, but right now the evidence seems to be tipping in favor of the “splitters” who cite evidence supporting their contention that axial spondyloarthritis (axSpA)/ankylosing spondylitis (AS) with psoriasis and psoriatic arthritis (PsA) with axial symptoms are distinct clinical entities that require more precise diagnosis and treatment.
“Lumpers,” in contrast, argue that they are different points on the same clinical spectrum.
The debate is not just of academic interest, but has real consequences for patients, say specialists on both sides of the aisle.
Overlapping features, different presentations
“Axial SpA and axPsA have overlapping features but also meaningful differences in genetics, clinical presentation, imaging, and immunophenotype. Efforts are underway to develop classification criteria for axPsA to aid research efforts as well as clinical diagnosis and management,” Philip J. Mease, MD, director of rheumatology research at Swedish Medical Center/Providence–St. Joseph Health in Seattle, and colleagues contend.
In an editorial published in the International Journal of Rheumatic Diseases, Dr. Mease and colleagues noted that, although HLA-B*27 is a genetic risk factor for both axPsA and axSpA, some HLA-B alleles are significantly associated with axPsA, whereas other alleles are associated with axSpA.
In addition, while genes in the interleukin-23 and IL-17 pathway are associated with increased risk for axSpA, genes in the IL-13 pathway have been identified as risk markers for axPsA, they noted.
Two cohorts better than one?
Dafna Gladman, MD, professor of medicine at the University of Toronto and senior scientist at the Schroeder Arthritis Institute at Toronto Western Hospital, and colleagues have a unique perspective on the similarities and differences between the disease entities.
Her group’s research uses data on cohorts of patients treated in two separate clinics at Toronto Western Hospital: one for patients with PsA, and one for patients with axial spondyloarthritis, including those with ankylosing spondylitis, nonradiographic axSpA, and spondylitis associated with inflammatory bowel disease.
“Our work has shown that there are differences, and one of the reasons that it’s now important is that the anti–IL-23 medications, both the IL-12/23 inhibitor ustekinumab [Stelara] and the IL-23 inhibitor guselkumab [Tremfya] work for psoriatic arthritis, whereas IL-23 did not work in ankylosing spondylitis, so that provided further impetus to look into the distinction between the two groups,” Dr. Gladman said in an interview.
Dr. Gladman and colleagues published a study in Rheumatology in which they compared clinical presentations and features of patients with AS with or without psoriasis with patients with axPsA.
They found that patients with AS with or without psoriasis tended to be younger, had a higher proportion of males to females, and were more likely to be positive for HLA-B*27. Patients with AS also had more back pain at presentation, worse axial disease activity scores, worse global assessments by physicians, and higher grades of sacroiliitis, and they were more likely to be taking biologic agents.
“What that showed, right off the top, that whether we’re looking at the total group or we’re looking specifically at those patients who have psoriasis or don’t have psoriasis, they are different from those with psoriatic arthritis with axial disease,” she said.
They concluded that “axPsA seems to be a distinct entity.”
Two clinics, same presentation
Because the aforementioned study included all patients with PsA with or without peripheral disease, the investigators decided to filter out some of the background noise and conduct a second study in which they compared patients who presented to the two clinics with the same presentation, either with spinal disease and psoriasis to the spondylitis clinic, or with psoriasis and isolated axial disease to the PsA clinic.
The results, published in Annals of the Rheumatic Diseases, showed that just 2.03% of patients with PsA had isolated axial disease, and an additional 29.38% had axial and peripheral disease.
In this study, “you can see that even in that group there are distinct differences. The patients that are labeled psoriatic spondylitis are different from those that are labeled ankylosing spondylitis with psoriasis,” Dr. Gladman said.
Isolated axial disease in patients with PsA was associated with HLA-B*27 positivity and lower Health Assessment Questionnaire scores. In addition, patients who were HLA-B*27 positive also had a nearly eightfold higher risk for developing peripheral disease over time.
Patients with isolated axial PsA were significantly more likely to be diagnosed at an older age (mean, 37.44 vs. 29.65 years), had higher Psoriasis Area Severity Index scores and a higher likelihood of having psoriatic nail lesions than patients with AS with isolated axial disease and psoriasis.
In contrast, patients with isolated axSpA with psoriasis were more likely to have inflammatory back pain, spinal pain, joint pain/swelling, and areas of localized tenderness, and they had greater severity of morning stiffness.
Dr. Gladman noted that, although AS and PsA are associated with the same gene that encodes for the IL-23 receptor, each condition is associated with a different single-nucleotide polymorphism.
Same disease, different flavors?
But as Mark Twain said, it is difference of opinion that makes horse races, and some specialists in rheumatology say that axSpA amd axPsA are just two sides of the same coin.
“There are always different schools of thought. I believe that they are not different diseases, but a spectrum of diseases,” said Shailendra Singh, MD, a rheumatologist at Unity Health Medical Center in Searcy, Ark., and past president of the Arkansas Rheumatology Association.
In an interview, Dr. Singh said that the spectrum ranges from diseases with primarily axial involvement, such as AS, to those with primarily peripheral involvement, such as reactive arthritis.
He pointed out that these conditions have overlapping symptoms, including enthesitis, dactylitis, and uveitis, and inflammatory arthritis.
Daniel Wendling, MD, PhD, from the Centre Hospitalier Régional Universitaire de Besançon (France), Université de Franche-Comté, and colleagues agreed.
“The criteria currently available for both SpA [ASAS (Assessment of Spondyloarthritis International Society) criteria] and PsA [CASPAR (Classification for Psoriatic Arthritis) criteria] are classification criteria, not diagnostic criteria. They are not very stringent and are not exclusive. Thus, the same patient can easily be classified simultaneously in both entities, making the distinction between axSpA with psoriasis and axPsA theoretical,” they wrote in an editorial published in Joint Bone Spine.
They cited as an example of the allegedly fuzzy criteria a prospective study conducted by the investigators in Bath, England, in which modified New York criteria for AS were met by 24% of patients with AS, and CASPAR criteria for PsA were met by an equal number of patients with AS.
Therapeutic implications
Dr. Wendling and colleagues acknowledge the differences cited in studies by Dr. Gladman, Dr. Mease, and others between patients with axPsA and those with axSpA, but argue that the differences are not that great and not so clear.
“It should also be emphasized that, although some differences between axPsA and axSpA reach statistical significance, they are mostly at the margin, with low odd ratios,” they wrote.
“It is also important to consider the variability in the definition of axPsA, sometimes simply ‘physician reported’ and elsewhere based on the modified New York radiographic criteria; the latter are only present late in the course of the disease, and this may induce bias,” they continued.
Dr. Singh agreed that, as noted by Dr. Gladman, some patients will respond to anti–IL-17, anti–IL-23, and anti–IL-12/23 agents, whereas others will have better responses with tumor necrosis factor (TNF) inhibitors, and still others, such as those with peripheral involvement in the hands and feet may fare better with nonbiologic disease-modifying antirheumatic drugs such as methotrexate.
Answers to come?
Dr. Gladman noted that the information available to date about the efficacy of IL-23 inhibition in axPsA is based on a post hoc analysis of the PSUMMIT 1 and 2 controlled trials in PsA, and is not definitive.
The randomized, controlled STAR trial, currently recruiting patients, is designed to see whether guselkumab can reduce axial symptoms and inflammation in patients with active axPsA.
“What I say is, there is a rationale for [anti–IL-23] to work in psoriatic arthritis, and not work in ankylosing spondylitis,” she said.
In contrast, IL-17 inhibitors, anti-TNF agents, and Janus kinase inhibitors show efficacy against both axPsA and AS. Rituximab is ineffective against PsA, but has shown efficacy against AS, especially in patients with neurologic complications from anti-TNF agents.
“There may be other medications that would work more specifically in axial psoriatic arthritis that don’t work in ankylosing spondylitis, but at least recognizing that there may be some differences, and that therefore a correct diagnosis should be obtained, might be important,” she said.
Ideally, the picture will become clearer with results from the ongoing Axial Involvement in Psoriatic Arthritis cohort, a joint project of ASAS and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. The multinational, cross-sectional study is designed “to systematically evaluate clinical and imaging manifestations indicative of axial involvement in patients with PsA and to develop classification criteria and a unified nomenclature for axial involvement in PsA that would allow defining a homogeneous subgroup of patients for research.”
Stay tuned.
Dr. Gladman’s research is supported by a grant from the Krembil Foundation. Dr. Singh disclosed research support from various companies. Funding sources and conflict of interest disclosures from other works cited are contained in their respective references.
Are there clinically significant differences between axial spondyloarthritis with psoriasis and psoriatic arthritis with axial symptoms? Does it matter?
It all depends on whom you ask, but right now the evidence seems to be tipping in favor of the “splitters” who cite evidence supporting their contention that axial spondyloarthritis (axSpA)/ankylosing spondylitis (AS) with psoriasis and psoriatic arthritis (PsA) with axial symptoms are distinct clinical entities that require more precise diagnosis and treatment.
“Lumpers,” in contrast, argue that they are different points on the same clinical spectrum.
The debate is not just of academic interest, but has real consequences for patients, say specialists on both sides of the aisle.
Overlapping features, different presentations
“Axial SpA and axPsA have overlapping features but also meaningful differences in genetics, clinical presentation, imaging, and immunophenotype. Efforts are underway to develop classification criteria for axPsA to aid research efforts as well as clinical diagnosis and management,” Philip J. Mease, MD, director of rheumatology research at Swedish Medical Center/Providence–St. Joseph Health in Seattle, and colleagues contend.
In an editorial published in the International Journal of Rheumatic Diseases, Dr. Mease and colleagues noted that, although HLA-B*27 is a genetic risk factor for both axPsA and axSpA, some HLA-B alleles are significantly associated with axPsA, whereas other alleles are associated with axSpA.
In addition, while genes in the interleukin-23 and IL-17 pathway are associated with increased risk for axSpA, genes in the IL-13 pathway have been identified as risk markers for axPsA, they noted.
Two cohorts better than one?
Dafna Gladman, MD, professor of medicine at the University of Toronto and senior scientist at the Schroeder Arthritis Institute at Toronto Western Hospital, and colleagues have a unique perspective on the similarities and differences between the disease entities.
Her group’s research uses data on cohorts of patients treated in two separate clinics at Toronto Western Hospital: one for patients with PsA, and one for patients with axial spondyloarthritis, including those with ankylosing spondylitis, nonradiographic axSpA, and spondylitis associated with inflammatory bowel disease.
“Our work has shown that there are differences, and one of the reasons that it’s now important is that the anti–IL-23 medications, both the IL-12/23 inhibitor ustekinumab [Stelara] and the IL-23 inhibitor guselkumab [Tremfya] work for psoriatic arthritis, whereas IL-23 did not work in ankylosing spondylitis, so that provided further impetus to look into the distinction between the two groups,” Dr. Gladman said in an interview.
Dr. Gladman and colleagues published a study in Rheumatology in which they compared clinical presentations and features of patients with AS with or without psoriasis with patients with axPsA.
They found that patients with AS with or without psoriasis tended to be younger, had a higher proportion of males to females, and were more likely to be positive for HLA-B*27. Patients with AS also had more back pain at presentation, worse axial disease activity scores, worse global assessments by physicians, and higher grades of sacroiliitis, and they were more likely to be taking biologic agents.
“What that showed, right off the top, that whether we’re looking at the total group or we’re looking specifically at those patients who have psoriasis or don’t have psoriasis, they are different from those with psoriatic arthritis with axial disease,” she said.
They concluded that “axPsA seems to be a distinct entity.”
Two clinics, same presentation
Because the aforementioned study included all patients with PsA with or without peripheral disease, the investigators decided to filter out some of the background noise and conduct a second study in which they compared patients who presented to the two clinics with the same presentation, either with spinal disease and psoriasis to the spondylitis clinic, or with psoriasis and isolated axial disease to the PsA clinic.
The results, published in Annals of the Rheumatic Diseases, showed that just 2.03% of patients with PsA had isolated axial disease, and an additional 29.38% had axial and peripheral disease.
In this study, “you can see that even in that group there are distinct differences. The patients that are labeled psoriatic spondylitis are different from those that are labeled ankylosing spondylitis with psoriasis,” Dr. Gladman said.
Isolated axial disease in patients with PsA was associated with HLA-B*27 positivity and lower Health Assessment Questionnaire scores. In addition, patients who were HLA-B*27 positive also had a nearly eightfold higher risk for developing peripheral disease over time.
Patients with isolated axial PsA were significantly more likely to be diagnosed at an older age (mean, 37.44 vs. 29.65 years), had higher Psoriasis Area Severity Index scores and a higher likelihood of having psoriatic nail lesions than patients with AS with isolated axial disease and psoriasis.
In contrast, patients with isolated axSpA with psoriasis were more likely to have inflammatory back pain, spinal pain, joint pain/swelling, and areas of localized tenderness, and they had greater severity of morning stiffness.
Dr. Gladman noted that, although AS and PsA are associated with the same gene that encodes for the IL-23 receptor, each condition is associated with a different single-nucleotide polymorphism.
Same disease, different flavors?
But as Mark Twain said, it is difference of opinion that makes horse races, and some specialists in rheumatology say that axSpA amd axPsA are just two sides of the same coin.
“There are always different schools of thought. I believe that they are not different diseases, but a spectrum of diseases,” said Shailendra Singh, MD, a rheumatologist at Unity Health Medical Center in Searcy, Ark., and past president of the Arkansas Rheumatology Association.
In an interview, Dr. Singh said that the spectrum ranges from diseases with primarily axial involvement, such as AS, to those with primarily peripheral involvement, such as reactive arthritis.
He pointed out that these conditions have overlapping symptoms, including enthesitis, dactylitis, and uveitis, and inflammatory arthritis.
Daniel Wendling, MD, PhD, from the Centre Hospitalier Régional Universitaire de Besançon (France), Université de Franche-Comté, and colleagues agreed.
“The criteria currently available for both SpA [ASAS (Assessment of Spondyloarthritis International Society) criteria] and PsA [CASPAR (Classification for Psoriatic Arthritis) criteria] are classification criteria, not diagnostic criteria. They are not very stringent and are not exclusive. Thus, the same patient can easily be classified simultaneously in both entities, making the distinction between axSpA with psoriasis and axPsA theoretical,” they wrote in an editorial published in Joint Bone Spine.
They cited as an example of the allegedly fuzzy criteria a prospective study conducted by the investigators in Bath, England, in which modified New York criteria for AS were met by 24% of patients with AS, and CASPAR criteria for PsA were met by an equal number of patients with AS.
Therapeutic implications
Dr. Wendling and colleagues acknowledge the differences cited in studies by Dr. Gladman, Dr. Mease, and others between patients with axPsA and those with axSpA, but argue that the differences are not that great and not so clear.
“It should also be emphasized that, although some differences between axPsA and axSpA reach statistical significance, they are mostly at the margin, with low odd ratios,” they wrote.
“It is also important to consider the variability in the definition of axPsA, sometimes simply ‘physician reported’ and elsewhere based on the modified New York radiographic criteria; the latter are only present late in the course of the disease, and this may induce bias,” they continued.
Dr. Singh agreed that, as noted by Dr. Gladman, some patients will respond to anti–IL-17, anti–IL-23, and anti–IL-12/23 agents, whereas others will have better responses with tumor necrosis factor (TNF) inhibitors, and still others, such as those with peripheral involvement in the hands and feet may fare better with nonbiologic disease-modifying antirheumatic drugs such as methotrexate.
Answers to come?
Dr. Gladman noted that the information available to date about the efficacy of IL-23 inhibition in axPsA is based on a post hoc analysis of the PSUMMIT 1 and 2 controlled trials in PsA, and is not definitive.
The randomized, controlled STAR trial, currently recruiting patients, is designed to see whether guselkumab can reduce axial symptoms and inflammation in patients with active axPsA.
“What I say is, there is a rationale for [anti–IL-23] to work in psoriatic arthritis, and not work in ankylosing spondylitis,” she said.
In contrast, IL-17 inhibitors, anti-TNF agents, and Janus kinase inhibitors show efficacy against both axPsA and AS. Rituximab is ineffective against PsA, but has shown efficacy against AS, especially in patients with neurologic complications from anti-TNF agents.
“There may be other medications that would work more specifically in axial psoriatic arthritis that don’t work in ankylosing spondylitis, but at least recognizing that there may be some differences, and that therefore a correct diagnosis should be obtained, might be important,” she said.
Ideally, the picture will become clearer with results from the ongoing Axial Involvement in Psoriatic Arthritis cohort, a joint project of ASAS and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. The multinational, cross-sectional study is designed “to systematically evaluate clinical and imaging manifestations indicative of axial involvement in patients with PsA and to develop classification criteria and a unified nomenclature for axial involvement in PsA that would allow defining a homogeneous subgroup of patients for research.”
Stay tuned.
Dr. Gladman’s research is supported by a grant from the Krembil Foundation. Dr. Singh disclosed research support from various companies. Funding sources and conflict of interest disclosures from other works cited are contained in their respective references.
Are there clinically significant differences between axial spondyloarthritis with psoriasis and psoriatic arthritis with axial symptoms? Does it matter?
It all depends on whom you ask, but right now the evidence seems to be tipping in favor of the “splitters” who cite evidence supporting their contention that axial spondyloarthritis (axSpA)/ankylosing spondylitis (AS) with psoriasis and psoriatic arthritis (PsA) with axial symptoms are distinct clinical entities that require more precise diagnosis and treatment.
“Lumpers,” in contrast, argue that they are different points on the same clinical spectrum.
The debate is not just of academic interest, but has real consequences for patients, say specialists on both sides of the aisle.
Overlapping features, different presentations
“Axial SpA and axPsA have overlapping features but also meaningful differences in genetics, clinical presentation, imaging, and immunophenotype. Efforts are underway to develop classification criteria for axPsA to aid research efforts as well as clinical diagnosis and management,” Philip J. Mease, MD, director of rheumatology research at Swedish Medical Center/Providence–St. Joseph Health in Seattle, and colleagues contend.
In an editorial published in the International Journal of Rheumatic Diseases, Dr. Mease and colleagues noted that, although HLA-B*27 is a genetic risk factor for both axPsA and axSpA, some HLA-B alleles are significantly associated with axPsA, whereas other alleles are associated with axSpA.
In addition, while genes in the interleukin-23 and IL-17 pathway are associated with increased risk for axSpA, genes in the IL-13 pathway have been identified as risk markers for axPsA, they noted.
Two cohorts better than one?
Dafna Gladman, MD, professor of medicine at the University of Toronto and senior scientist at the Schroeder Arthritis Institute at Toronto Western Hospital, and colleagues have a unique perspective on the similarities and differences between the disease entities.
Her group’s research uses data on cohorts of patients treated in two separate clinics at Toronto Western Hospital: one for patients with PsA, and one for patients with axial spondyloarthritis, including those with ankylosing spondylitis, nonradiographic axSpA, and spondylitis associated with inflammatory bowel disease.
“Our work has shown that there are differences, and one of the reasons that it’s now important is that the anti–IL-23 medications, both the IL-12/23 inhibitor ustekinumab [Stelara] and the IL-23 inhibitor guselkumab [Tremfya] work for psoriatic arthritis, whereas IL-23 did not work in ankylosing spondylitis, so that provided further impetus to look into the distinction between the two groups,” Dr. Gladman said in an interview.
Dr. Gladman and colleagues published a study in Rheumatology in which they compared clinical presentations and features of patients with AS with or without psoriasis with patients with axPsA.
They found that patients with AS with or without psoriasis tended to be younger, had a higher proportion of males to females, and were more likely to be positive for HLA-B*27. Patients with AS also had more back pain at presentation, worse axial disease activity scores, worse global assessments by physicians, and higher grades of sacroiliitis, and they were more likely to be taking biologic agents.
“What that showed, right off the top, that whether we’re looking at the total group or we’re looking specifically at those patients who have psoriasis or don’t have psoriasis, they are different from those with psoriatic arthritis with axial disease,” she said.
They concluded that “axPsA seems to be a distinct entity.”
Two clinics, same presentation
Because the aforementioned study included all patients with PsA with or without peripheral disease, the investigators decided to filter out some of the background noise and conduct a second study in which they compared patients who presented to the two clinics with the same presentation, either with spinal disease and psoriasis to the spondylitis clinic, or with psoriasis and isolated axial disease to the PsA clinic.
The results, published in Annals of the Rheumatic Diseases, showed that just 2.03% of patients with PsA had isolated axial disease, and an additional 29.38% had axial and peripheral disease.
In this study, “you can see that even in that group there are distinct differences. The patients that are labeled psoriatic spondylitis are different from those that are labeled ankylosing spondylitis with psoriasis,” Dr. Gladman said.
Isolated axial disease in patients with PsA was associated with HLA-B*27 positivity and lower Health Assessment Questionnaire scores. In addition, patients who were HLA-B*27 positive also had a nearly eightfold higher risk for developing peripheral disease over time.
Patients with isolated axial PsA were significantly more likely to be diagnosed at an older age (mean, 37.44 vs. 29.65 years), had higher Psoriasis Area Severity Index scores and a higher likelihood of having psoriatic nail lesions than patients with AS with isolated axial disease and psoriasis.
In contrast, patients with isolated axSpA with psoriasis were more likely to have inflammatory back pain, spinal pain, joint pain/swelling, and areas of localized tenderness, and they had greater severity of morning stiffness.
Dr. Gladman noted that, although AS and PsA are associated with the same gene that encodes for the IL-23 receptor, each condition is associated with a different single-nucleotide polymorphism.
Same disease, different flavors?
But as Mark Twain said, it is difference of opinion that makes horse races, and some specialists in rheumatology say that axSpA amd axPsA are just two sides of the same coin.
“There are always different schools of thought. I believe that they are not different diseases, but a spectrum of diseases,” said Shailendra Singh, MD, a rheumatologist at Unity Health Medical Center in Searcy, Ark., and past president of the Arkansas Rheumatology Association.
In an interview, Dr. Singh said that the spectrum ranges from diseases with primarily axial involvement, such as AS, to those with primarily peripheral involvement, such as reactive arthritis.
He pointed out that these conditions have overlapping symptoms, including enthesitis, dactylitis, and uveitis, and inflammatory arthritis.
Daniel Wendling, MD, PhD, from the Centre Hospitalier Régional Universitaire de Besançon (France), Université de Franche-Comté, and colleagues agreed.
“The criteria currently available for both SpA [ASAS (Assessment of Spondyloarthritis International Society) criteria] and PsA [CASPAR (Classification for Psoriatic Arthritis) criteria] are classification criteria, not diagnostic criteria. They are not very stringent and are not exclusive. Thus, the same patient can easily be classified simultaneously in both entities, making the distinction between axSpA with psoriasis and axPsA theoretical,” they wrote in an editorial published in Joint Bone Spine.
They cited as an example of the allegedly fuzzy criteria a prospective study conducted by the investigators in Bath, England, in which modified New York criteria for AS were met by 24% of patients with AS, and CASPAR criteria for PsA were met by an equal number of patients with AS.
Therapeutic implications
Dr. Wendling and colleagues acknowledge the differences cited in studies by Dr. Gladman, Dr. Mease, and others between patients with axPsA and those with axSpA, but argue that the differences are not that great and not so clear.
“It should also be emphasized that, although some differences between axPsA and axSpA reach statistical significance, they are mostly at the margin, with low odd ratios,” they wrote.
“It is also important to consider the variability in the definition of axPsA, sometimes simply ‘physician reported’ and elsewhere based on the modified New York radiographic criteria; the latter are only present late in the course of the disease, and this may induce bias,” they continued.
Dr. Singh agreed that, as noted by Dr. Gladman, some patients will respond to anti–IL-17, anti–IL-23, and anti–IL-12/23 agents, whereas others will have better responses with tumor necrosis factor (TNF) inhibitors, and still others, such as those with peripheral involvement in the hands and feet may fare better with nonbiologic disease-modifying antirheumatic drugs such as methotrexate.
Answers to come?
Dr. Gladman noted that the information available to date about the efficacy of IL-23 inhibition in axPsA is based on a post hoc analysis of the PSUMMIT 1 and 2 controlled trials in PsA, and is not definitive.
The randomized, controlled STAR trial, currently recruiting patients, is designed to see whether guselkumab can reduce axial symptoms and inflammation in patients with active axPsA.
“What I say is, there is a rationale for [anti–IL-23] to work in psoriatic arthritis, and not work in ankylosing spondylitis,” she said.
In contrast, IL-17 inhibitors, anti-TNF agents, and Janus kinase inhibitors show efficacy against both axPsA and AS. Rituximab is ineffective against PsA, but has shown efficacy against AS, especially in patients with neurologic complications from anti-TNF agents.
“There may be other medications that would work more specifically in axial psoriatic arthritis that don’t work in ankylosing spondylitis, but at least recognizing that there may be some differences, and that therefore a correct diagnosis should be obtained, might be important,” she said.
Ideally, the picture will become clearer with results from the ongoing Axial Involvement in Psoriatic Arthritis cohort, a joint project of ASAS and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. The multinational, cross-sectional study is designed “to systematically evaluate clinical and imaging manifestations indicative of axial involvement in patients with PsA and to develop classification criteria and a unified nomenclature for axial involvement in PsA that would allow defining a homogeneous subgroup of patients for research.”
Stay tuned.
Dr. Gladman’s research is supported by a grant from the Krembil Foundation. Dr. Singh disclosed research support from various companies. Funding sources and conflict of interest disclosures from other works cited are contained in their respective references.