User login
PDAC surveillance in high-risk cases improves outcomes
shows a new study published in Gastroenterology.
While other studies have shown potential benefit in screening high-risk individuals, “a concern is that in absence of sufficiently large control groups with unscreened controls,” the outcomes may be influenced by lead-time bias. The current study is the first to address that important limitation.
The study, which was led by Derk C.F. Klatte, MD, of the department of gastroenterology and hepatology at Leiden University Medical Center, the Netherlands, included 43,762 patients from the Netherlands Cancer Registry who were diagnosed with PDAC between January 2000 and December 2020. Using a 1:5 ratio, researchers matched 31 patients who were diagnosed in the pancreatic cancer surveillance cohort against 155 patients in the non-surveillance group.
“We show that surveillance for PDAC in high-risk individuals results in significant earlier detection, increased resectability, and improved survival as compared with average-risk individuals diagnosed with PDAC not under surveillance. This reaffirms that pancreatic surveillance for certain in high-risk individuals is beneficial and could have a meaningful impact on disease course,” the authors wrote.
PDAC has the worst outcomes all cancers and is on pace to become the second-leading cause of cancer-related mortality. By the time a tumor is detected, it is usually unresectable or has developed distant metastases. In principle, early detection could improve outcomes, but there is no test that is adequate for population-wide screening. Surveillance must therefore concentrate on individuals deemed to be at heightened risk. Prospective studies have shown a benefit of pancreatic cancer screening in patients who are at high-risk. Such studies may be misleading, however, due to the potential for lead-time bias. This can occur when a condition is detected at an earlier time than it would have been identified based on clinical signs, as usually occurs in nonscreened populations, and this asymptomatic lag time between diagnosis and initial symptoms does not get incorporated into a survival analysis. The result can be an artificially longer survival time following diagnosis in the screened population.
Guidelines from the International Cancer of the Pancreas Screening (CAPS) consortium, the American Society for Gastrointestinal Endoscopy, and American Society of Clinical Oncology recommend surveillance in high-risk cases.
In this study, researchers conducted a propensity score matched cohort analysis of patients from the general population with primary PDAC who were diagnosed outside of a screening program, with carriers of a germline CDKN2A/p16 mutation who were diagnosed after surveillance.
The surveillance group received a stage 1 diagnosis in 38.7% of cases, versus 5.8% of those outside of surveillance (odds ratio [OR], 0.09; 95% confidence interval [CI], 0.04-0.19). Surgical resection occurred in 71.0% of surveillance patients, versus 18.7% of non-surveillance patients (OR, 10.62; 95% CI, 4.56-26.63), and stage 4 diagnoses were much more common in the nonsurveillance population (61.3% versus 9.7%). Among the patients who did not undergo surveillance, 61.3% were diagnosed with stage 4 disease compared with 9.7% of those in the surveillance group.
The 5-year survival rate (unadjusted for lead-time) in the surveillance group was 32.4% and 4.3% in the nonsurveillance group. The median overall survival was 26.8 months in the surveillance group compared with 5.2 months in the nonsurveillance group, (hazard ratio, 0.22; 95% CI, 0.14-0.36). The mortality rate per 100 person-years was 114.5 (95% CI, 96.2–135.3) in nonsurveillance patients and 21.9 (95% CI, 13.4–33.8) in surveillance patients.
Despite the apparent benefit of screening, there is room for improvement. “Although the outcomes presented here are encouraging and endorse our earlier findings, a significant proportion of surveillance patients (61%) still had poor outcomes because of diagnosis in a late stage (T2–4N0M0 and nodal or distant metastatic PDAC), with a 5-year survival of 16%,” the authors wrote.
The study received no funding and the authors declared no conflicts.
shows a new study published in Gastroenterology.
While other studies have shown potential benefit in screening high-risk individuals, “a concern is that in absence of sufficiently large control groups with unscreened controls,” the outcomes may be influenced by lead-time bias. The current study is the first to address that important limitation.
The study, which was led by Derk C.F. Klatte, MD, of the department of gastroenterology and hepatology at Leiden University Medical Center, the Netherlands, included 43,762 patients from the Netherlands Cancer Registry who were diagnosed with PDAC between January 2000 and December 2020. Using a 1:5 ratio, researchers matched 31 patients who were diagnosed in the pancreatic cancer surveillance cohort against 155 patients in the non-surveillance group.
“We show that surveillance for PDAC in high-risk individuals results in significant earlier detection, increased resectability, and improved survival as compared with average-risk individuals diagnosed with PDAC not under surveillance. This reaffirms that pancreatic surveillance for certain in high-risk individuals is beneficial and could have a meaningful impact on disease course,” the authors wrote.
PDAC has the worst outcomes all cancers and is on pace to become the second-leading cause of cancer-related mortality. By the time a tumor is detected, it is usually unresectable or has developed distant metastases. In principle, early detection could improve outcomes, but there is no test that is adequate for population-wide screening. Surveillance must therefore concentrate on individuals deemed to be at heightened risk. Prospective studies have shown a benefit of pancreatic cancer screening in patients who are at high-risk. Such studies may be misleading, however, due to the potential for lead-time bias. This can occur when a condition is detected at an earlier time than it would have been identified based on clinical signs, as usually occurs in nonscreened populations, and this asymptomatic lag time between diagnosis and initial symptoms does not get incorporated into a survival analysis. The result can be an artificially longer survival time following diagnosis in the screened population.
Guidelines from the International Cancer of the Pancreas Screening (CAPS) consortium, the American Society for Gastrointestinal Endoscopy, and American Society of Clinical Oncology recommend surveillance in high-risk cases.
In this study, researchers conducted a propensity score matched cohort analysis of patients from the general population with primary PDAC who were diagnosed outside of a screening program, with carriers of a germline CDKN2A/p16 mutation who were diagnosed after surveillance.
The surveillance group received a stage 1 diagnosis in 38.7% of cases, versus 5.8% of those outside of surveillance (odds ratio [OR], 0.09; 95% confidence interval [CI], 0.04-0.19). Surgical resection occurred in 71.0% of surveillance patients, versus 18.7% of non-surveillance patients (OR, 10.62; 95% CI, 4.56-26.63), and stage 4 diagnoses were much more common in the nonsurveillance population (61.3% versus 9.7%). Among the patients who did not undergo surveillance, 61.3% were diagnosed with stage 4 disease compared with 9.7% of those in the surveillance group.
The 5-year survival rate (unadjusted for lead-time) in the surveillance group was 32.4% and 4.3% in the nonsurveillance group. The median overall survival was 26.8 months in the surveillance group compared with 5.2 months in the nonsurveillance group, (hazard ratio, 0.22; 95% CI, 0.14-0.36). The mortality rate per 100 person-years was 114.5 (95% CI, 96.2–135.3) in nonsurveillance patients and 21.9 (95% CI, 13.4–33.8) in surveillance patients.
Despite the apparent benefit of screening, there is room for improvement. “Although the outcomes presented here are encouraging and endorse our earlier findings, a significant proportion of surveillance patients (61%) still had poor outcomes because of diagnosis in a late stage (T2–4N0M0 and nodal or distant metastatic PDAC), with a 5-year survival of 16%,” the authors wrote.
The study received no funding and the authors declared no conflicts.
shows a new study published in Gastroenterology.
While other studies have shown potential benefit in screening high-risk individuals, “a concern is that in absence of sufficiently large control groups with unscreened controls,” the outcomes may be influenced by lead-time bias. The current study is the first to address that important limitation.
The study, which was led by Derk C.F. Klatte, MD, of the department of gastroenterology and hepatology at Leiden University Medical Center, the Netherlands, included 43,762 patients from the Netherlands Cancer Registry who were diagnosed with PDAC between January 2000 and December 2020. Using a 1:5 ratio, researchers matched 31 patients who were diagnosed in the pancreatic cancer surveillance cohort against 155 patients in the non-surveillance group.
“We show that surveillance for PDAC in high-risk individuals results in significant earlier detection, increased resectability, and improved survival as compared with average-risk individuals diagnosed with PDAC not under surveillance. This reaffirms that pancreatic surveillance for certain in high-risk individuals is beneficial and could have a meaningful impact on disease course,” the authors wrote.
PDAC has the worst outcomes all cancers and is on pace to become the second-leading cause of cancer-related mortality. By the time a tumor is detected, it is usually unresectable or has developed distant metastases. In principle, early detection could improve outcomes, but there is no test that is adequate for population-wide screening. Surveillance must therefore concentrate on individuals deemed to be at heightened risk. Prospective studies have shown a benefit of pancreatic cancer screening in patients who are at high-risk. Such studies may be misleading, however, due to the potential for lead-time bias. This can occur when a condition is detected at an earlier time than it would have been identified based on clinical signs, as usually occurs in nonscreened populations, and this asymptomatic lag time between diagnosis and initial symptoms does not get incorporated into a survival analysis. The result can be an artificially longer survival time following diagnosis in the screened population.
Guidelines from the International Cancer of the Pancreas Screening (CAPS) consortium, the American Society for Gastrointestinal Endoscopy, and American Society of Clinical Oncology recommend surveillance in high-risk cases.
In this study, researchers conducted a propensity score matched cohort analysis of patients from the general population with primary PDAC who were diagnosed outside of a screening program, with carriers of a germline CDKN2A/p16 mutation who were diagnosed after surveillance.
The surveillance group received a stage 1 diagnosis in 38.7% of cases, versus 5.8% of those outside of surveillance (odds ratio [OR], 0.09; 95% confidence interval [CI], 0.04-0.19). Surgical resection occurred in 71.0% of surveillance patients, versus 18.7% of non-surveillance patients (OR, 10.62; 95% CI, 4.56-26.63), and stage 4 diagnoses were much more common in the nonsurveillance population (61.3% versus 9.7%). Among the patients who did not undergo surveillance, 61.3% were diagnosed with stage 4 disease compared with 9.7% of those in the surveillance group.
The 5-year survival rate (unadjusted for lead-time) in the surveillance group was 32.4% and 4.3% in the nonsurveillance group. The median overall survival was 26.8 months in the surveillance group compared with 5.2 months in the nonsurveillance group, (hazard ratio, 0.22; 95% CI, 0.14-0.36). The mortality rate per 100 person-years was 114.5 (95% CI, 96.2–135.3) in nonsurveillance patients and 21.9 (95% CI, 13.4–33.8) in surveillance patients.
Despite the apparent benefit of screening, there is room for improvement. “Although the outcomes presented here are encouraging and endorse our earlier findings, a significant proportion of surveillance patients (61%) still had poor outcomes because of diagnosis in a late stage (T2–4N0M0 and nodal or distant metastatic PDAC), with a 5-year survival of 16%,” the authors wrote.
The study received no funding and the authors declared no conflicts.
FROM GASTROENTEROLOGY
High cholesterol in seniors: Use statins for primary prevention?
LONG BEACH, CALIF. – For years, clinicians have debated whether prescribing statins to patients older than 75 for the prevention of cardiovascular events is appropriate.
In 2022, the U.S. Preventive Services Task Force concluded that scientific evidence was insufficient to assess the balance between the benefits and harms of the therapy for this older population.
At a session of the annual meeting of the American Geriatrics Society, experts laid out new preliminary recommendations of the AGS and the National Lipid Association on assessing risk and deciding on treatment.
The group concluded that LDL cholesterol levels are associated with incident arteriosclerotic cardiovascular disease (ASCVD), that the coronary artery calcium (CAC) score can be a valuable measure, and that statins may be reasonable to prescribe, even given the risks that have been linked to statins, such as that for muscle pain. Final recommendations are expected by fall 2023.
“This is still a work in progress,” said Daniel E. Forman, MD, professor of medicine and chair of geriatric cardiology at the University of Pittsburgh.
The AGS-NLA panel concluded that, for those aged 75 or older without established ASCVD, LDL cholesterol is associated with incident ASCVD, the only recommendation to be given a class I (strong) rating; others were classed as moderate or weak.
Dr. Forman reviewed the evidence for lowering LDL cholesterol to decrease ASCVD, citing a 2018 study that concluded, “Reverse causation may contribute to the association of lower TC with higher mortality in nonrandomized studies.”
However, research overall shows that, as LDL cholesterol levels increase, patients are more likely to experience a heart event.
Dr. Forman noted that the utility of equations for assessing 5- or 10-year risk of ASCVD is uncertain. However, he said, traditional risk factors, such as family history and ethnicity, still have value.
Assessing risk “has been enriched in the past few years by the introduction of the coronary artery calcium [CAC] score,” he said.
Lower scores predict lower rates of CVD events, Forman said. The AGS-NLA recommends measuring CAC if clinical uncertainty exists about the value of statins.
“It’s reasonable to measure CAC and to withhold statins when the CAC is zero,” Dr. Forman said. “When the CAC score is zero ... the risk of having a cardiovascular event is really next to nil. Patients are happy to know they have a CAC of zero.”
Likewise, patients appreciate knowing whether their score is high, which would indicate increased risk. He said the CAC score is underused by geriatric physicians.
The group also determined, after reviewing the research, that starting treatment is reasonable for patients with an LDL cholesterol level of 70-189 if they have no life-limiting illness and their life expectancy is over 5 years.
Other preliminary recommendations include the use of statins for those aged 75 and older, irrespective of risk for statin-associated muscle symptoms, type 2 diabetes, or impaired cognition. These associations are often weak, Dr. Forman added.
Focusing on person-centered decisions
Ariel Green, MD, MPH, PhD, associate professor of medicine at Johns Hopkins University, Baltimore, said statin therapy “should be individualized” to weigh benefits, noncardiac risks, and other considerations.
Clinicians can incorporate life expectancy into prevention decisions using tools such as ePrognosis, from the University of California, San Francisco, Dr. Green said.
If life expectancy is greater than the time to benefit, statin therapy may help. Dr. Green cited research that showed that 2.5 years of statin therapy was needed to prevent one major adverse cardiovascular event (MACE) per 100 patients in a population aged 50-75. Other data show reductions in MACE for those older than 75, but overall, the data are limited in this population.
The proposed recommendation is to use tools such as life tables that include comorbid conditions and functional status to guide clinical decisions.
“Another aspect of assessing net benefits of statin therapy is to consider competing health risks,” Dr. Green said.
The group recommends considering using competing risk-adjusted CVD models, though these are not widely used.
The group also recommends integrating screenings for frailty (Clinical Frailty Scale), dementia (Mini-Cog), and functional status (Vulnerable Elders Scale–13) into assessments.
“The presence of these syndromes should prompt elicitation of patient values and preferences related to prevention and medication use,” Dr. Green said.
Clinicians can use decision aids, but these are not always practical, owing to obstacles such as patients’ cognitive problems, Dr. Green said.
“Another approach is asking people to prioritize a set of universal health outcomes that apply across health conditions, such as maintaining independence, staying alive, reducing, or eliminating symptoms and focusing on comfort,” Dr. Green said.
She addressed the evidence about deprescribing statins, with a focus on those with a life expectancy of less than a year. Researchers have found an increase in quality of life and no increases in cardiovascular events or death when statins were deprescribed.
A welcome framework
Cory Krueger, MD, an internal medicine and geriatric physician in Cornville, Ariz., who attended the talk, said he welcomed the presentation, in which preliminary recommendations were explained.
“This has been a controversial area in geriatrics,” Dr. Krueger said. “At least this gave me a framework for discussing this with my patients in a reasonable way.”
Dr. Forman and Dr. Krueger disclosed no relevant financial relationships. Dr. Green receives funding from the National Institute of Aging and Impact Collaboratory.
A version of this article first appeared on Medscape.com.
LONG BEACH, CALIF. – For years, clinicians have debated whether prescribing statins to patients older than 75 for the prevention of cardiovascular events is appropriate.
In 2022, the U.S. Preventive Services Task Force concluded that scientific evidence was insufficient to assess the balance between the benefits and harms of the therapy for this older population.
At a session of the annual meeting of the American Geriatrics Society, experts laid out new preliminary recommendations of the AGS and the National Lipid Association on assessing risk and deciding on treatment.
The group concluded that LDL cholesterol levels are associated with incident arteriosclerotic cardiovascular disease (ASCVD), that the coronary artery calcium (CAC) score can be a valuable measure, and that statins may be reasonable to prescribe, even given the risks that have been linked to statins, such as that for muscle pain. Final recommendations are expected by fall 2023.
“This is still a work in progress,” said Daniel E. Forman, MD, professor of medicine and chair of geriatric cardiology at the University of Pittsburgh.
The AGS-NLA panel concluded that, for those aged 75 or older without established ASCVD, LDL cholesterol is associated with incident ASCVD, the only recommendation to be given a class I (strong) rating; others were classed as moderate or weak.
Dr. Forman reviewed the evidence for lowering LDL cholesterol to decrease ASCVD, citing a 2018 study that concluded, “Reverse causation may contribute to the association of lower TC with higher mortality in nonrandomized studies.”
However, research overall shows that, as LDL cholesterol levels increase, patients are more likely to experience a heart event.
Dr. Forman noted that the utility of equations for assessing 5- or 10-year risk of ASCVD is uncertain. However, he said, traditional risk factors, such as family history and ethnicity, still have value.
Assessing risk “has been enriched in the past few years by the introduction of the coronary artery calcium [CAC] score,” he said.
Lower scores predict lower rates of CVD events, Forman said. The AGS-NLA recommends measuring CAC if clinical uncertainty exists about the value of statins.
“It’s reasonable to measure CAC and to withhold statins when the CAC is zero,” Dr. Forman said. “When the CAC score is zero ... the risk of having a cardiovascular event is really next to nil. Patients are happy to know they have a CAC of zero.”
Likewise, patients appreciate knowing whether their score is high, which would indicate increased risk. He said the CAC score is underused by geriatric physicians.
The group also determined, after reviewing the research, that starting treatment is reasonable for patients with an LDL cholesterol level of 70-189 if they have no life-limiting illness and their life expectancy is over 5 years.
Other preliminary recommendations include the use of statins for those aged 75 and older, irrespective of risk for statin-associated muscle symptoms, type 2 diabetes, or impaired cognition. These associations are often weak, Dr. Forman added.
Focusing on person-centered decisions
Ariel Green, MD, MPH, PhD, associate professor of medicine at Johns Hopkins University, Baltimore, said statin therapy “should be individualized” to weigh benefits, noncardiac risks, and other considerations.
Clinicians can incorporate life expectancy into prevention decisions using tools such as ePrognosis, from the University of California, San Francisco, Dr. Green said.
If life expectancy is greater than the time to benefit, statin therapy may help. Dr. Green cited research that showed that 2.5 years of statin therapy was needed to prevent one major adverse cardiovascular event (MACE) per 100 patients in a population aged 50-75. Other data show reductions in MACE for those older than 75, but overall, the data are limited in this population.
The proposed recommendation is to use tools such as life tables that include comorbid conditions and functional status to guide clinical decisions.
“Another aspect of assessing net benefits of statin therapy is to consider competing health risks,” Dr. Green said.
The group recommends considering using competing risk-adjusted CVD models, though these are not widely used.
The group also recommends integrating screenings for frailty (Clinical Frailty Scale), dementia (Mini-Cog), and functional status (Vulnerable Elders Scale–13) into assessments.
“The presence of these syndromes should prompt elicitation of patient values and preferences related to prevention and medication use,” Dr. Green said.
Clinicians can use decision aids, but these are not always practical, owing to obstacles such as patients’ cognitive problems, Dr. Green said.
“Another approach is asking people to prioritize a set of universal health outcomes that apply across health conditions, such as maintaining independence, staying alive, reducing, or eliminating symptoms and focusing on comfort,” Dr. Green said.
She addressed the evidence about deprescribing statins, with a focus on those with a life expectancy of less than a year. Researchers have found an increase in quality of life and no increases in cardiovascular events or death when statins were deprescribed.
A welcome framework
Cory Krueger, MD, an internal medicine and geriatric physician in Cornville, Ariz., who attended the talk, said he welcomed the presentation, in which preliminary recommendations were explained.
“This has been a controversial area in geriatrics,” Dr. Krueger said. “At least this gave me a framework for discussing this with my patients in a reasonable way.”
Dr. Forman and Dr. Krueger disclosed no relevant financial relationships. Dr. Green receives funding from the National Institute of Aging and Impact Collaboratory.
A version of this article first appeared on Medscape.com.
LONG BEACH, CALIF. – For years, clinicians have debated whether prescribing statins to patients older than 75 for the prevention of cardiovascular events is appropriate.
In 2022, the U.S. Preventive Services Task Force concluded that scientific evidence was insufficient to assess the balance between the benefits and harms of the therapy for this older population.
At a session of the annual meeting of the American Geriatrics Society, experts laid out new preliminary recommendations of the AGS and the National Lipid Association on assessing risk and deciding on treatment.
The group concluded that LDL cholesterol levels are associated with incident arteriosclerotic cardiovascular disease (ASCVD), that the coronary artery calcium (CAC) score can be a valuable measure, and that statins may be reasonable to prescribe, even given the risks that have been linked to statins, such as that for muscle pain. Final recommendations are expected by fall 2023.
“This is still a work in progress,” said Daniel E. Forman, MD, professor of medicine and chair of geriatric cardiology at the University of Pittsburgh.
The AGS-NLA panel concluded that, for those aged 75 or older without established ASCVD, LDL cholesterol is associated with incident ASCVD, the only recommendation to be given a class I (strong) rating; others were classed as moderate or weak.
Dr. Forman reviewed the evidence for lowering LDL cholesterol to decrease ASCVD, citing a 2018 study that concluded, “Reverse causation may contribute to the association of lower TC with higher mortality in nonrandomized studies.”
However, research overall shows that, as LDL cholesterol levels increase, patients are more likely to experience a heart event.
Dr. Forman noted that the utility of equations for assessing 5- or 10-year risk of ASCVD is uncertain. However, he said, traditional risk factors, such as family history and ethnicity, still have value.
Assessing risk “has been enriched in the past few years by the introduction of the coronary artery calcium [CAC] score,” he said.
Lower scores predict lower rates of CVD events, Forman said. The AGS-NLA recommends measuring CAC if clinical uncertainty exists about the value of statins.
“It’s reasonable to measure CAC and to withhold statins when the CAC is zero,” Dr. Forman said. “When the CAC score is zero ... the risk of having a cardiovascular event is really next to nil. Patients are happy to know they have a CAC of zero.”
Likewise, patients appreciate knowing whether their score is high, which would indicate increased risk. He said the CAC score is underused by geriatric physicians.
The group also determined, after reviewing the research, that starting treatment is reasonable for patients with an LDL cholesterol level of 70-189 if they have no life-limiting illness and their life expectancy is over 5 years.
Other preliminary recommendations include the use of statins for those aged 75 and older, irrespective of risk for statin-associated muscle symptoms, type 2 diabetes, or impaired cognition. These associations are often weak, Dr. Forman added.
Focusing on person-centered decisions
Ariel Green, MD, MPH, PhD, associate professor of medicine at Johns Hopkins University, Baltimore, said statin therapy “should be individualized” to weigh benefits, noncardiac risks, and other considerations.
Clinicians can incorporate life expectancy into prevention decisions using tools such as ePrognosis, from the University of California, San Francisco, Dr. Green said.
If life expectancy is greater than the time to benefit, statin therapy may help. Dr. Green cited research that showed that 2.5 years of statin therapy was needed to prevent one major adverse cardiovascular event (MACE) per 100 patients in a population aged 50-75. Other data show reductions in MACE for those older than 75, but overall, the data are limited in this population.
The proposed recommendation is to use tools such as life tables that include comorbid conditions and functional status to guide clinical decisions.
“Another aspect of assessing net benefits of statin therapy is to consider competing health risks,” Dr. Green said.
The group recommends considering using competing risk-adjusted CVD models, though these are not widely used.
The group also recommends integrating screenings for frailty (Clinical Frailty Scale), dementia (Mini-Cog), and functional status (Vulnerable Elders Scale–13) into assessments.
“The presence of these syndromes should prompt elicitation of patient values and preferences related to prevention and medication use,” Dr. Green said.
Clinicians can use decision aids, but these are not always practical, owing to obstacles such as patients’ cognitive problems, Dr. Green said.
“Another approach is asking people to prioritize a set of universal health outcomes that apply across health conditions, such as maintaining independence, staying alive, reducing, or eliminating symptoms and focusing on comfort,” Dr. Green said.
She addressed the evidence about deprescribing statins, with a focus on those with a life expectancy of less than a year. Researchers have found an increase in quality of life and no increases in cardiovascular events or death when statins were deprescribed.
A welcome framework
Cory Krueger, MD, an internal medicine and geriatric physician in Cornville, Ariz., who attended the talk, said he welcomed the presentation, in which preliminary recommendations were explained.
“This has been a controversial area in geriatrics,” Dr. Krueger said. “At least this gave me a framework for discussing this with my patients in a reasonable way.”
Dr. Forman and Dr. Krueger disclosed no relevant financial relationships. Dr. Green receives funding from the National Institute of Aging and Impact Collaboratory.
A version of this article first appeared on Medscape.com.
AT AGS 2023
Risk assessment first urged for fragility fracture screening
A new Canadian guideline on screening for the primary prevention of fragility fractures recommends risk assessment first, before bone mineral density (BMD) testing, for women aged 65 and older. For younger women and men aged 40 and older, screening is not recommended.
To develop the guideline, a writing group from Canadian Task Force on Preventive Health Care commissioned systematic reviews of studies on the benefits and harms of fragility fracture screenings; the predictive accuracy of current risk-assessment tools; patient acceptability; and benefits of treatment. Treatment harms were analyzed via a rapid overview of reviews.
The guideline, published online in the Canadian Medical Association Journal, is aimed at primary care practitioners for their community-dwelling patients aged 40 and older. The recommendations do not apply to people already taking preventive drugs.
Nondrug treatments were beyond the scope of the current guideline, but guidelines on the prevention of falls and other strategies are planned, Roland Grad, MD, a guideline author and associate professor at McGill University in Montreal, told this news organization.
The new guideline says that women aged 65 and older may be able to avoid fracture through screening and preventive medication. An individual’s fracture risk can be estimated with a new Fragility Fractures Decision Aid, which uses the Canadian FRAX risk-assessment tool.
“A risk assessment–first approach promotes shared decision-making with the patient, based on best medical evidence,” Dr. Grad said.
“To help clinicians, we have created an infographic with visuals to communicate the time spent on BMD vs risk assessment first.”
New evidence
“At least three things motivated this new guideline,” Dr. Grad said. “When we started work on this prior to the pandemic, we saw a need for updated guidance on screening to prevent fragility fractures. We were also aware of new evidence from the publication of screening trials in females older than 65.”
To conduct the risk assessment in older women, clinicians are advised to do the following:
- Use the decision aid (which patients can also use on their own).
- Use the 10-year absolute risk of major osteoporotic fracture to facilitate shared decision-making about possible benefits and harms of preventive pharmacotherapy.
- If pharmacotherapy is being considered, request a BMD using DXA of the femoral neck, then reestimate the fracture risk by adding the BMD T-score into the FRAX.
Potential harms associated with various treatments, with varying levels of evidence, include the following: with alendronate and denosumab, nonserious gastrointestinal adverse events; with denosumab, rash, eczema, and infections; with zoledronic acid, nonserious events, such as headache and flulike symptoms; and with alendronate and bisphosphonates, rare but serious harms of atypical femoral fracture and osteonecrosis of the jaw.
“These recommendations emphasize the importance of good clinical practice, where clinicians are alert to changes in physical health and patient well-being,” the authors wrote. “Clinicians should also be aware of the importance of secondary prevention (i.e., after fracture) and manage patients accordingly.”
“This is an important topic,” Dr. Grad said. “Fragility fractures are consequential for individuals and for our publicly funded health care system. We anticipate questions from clinicians about the time needed to screen with the risk assessment–first strategy. Our modeling work suggests time savings with [this] strategy compared to a strategy of BMD testing first. Following our recommendations may lead to a reduction in BMD testing.”
To promote the guideline, the CMAJ has recorded a podcast and will use other strategies to increase awareness, Dr. Grad said. “The Canadian Task Force has a communications strategy that includes outreach to primary care, stakeholder webinars, social media, partnerships, and other tactics. The College of Family Physicians of Canada has endorsed the guideline and will help promote to its members.”
Other at-risk groups?
Aliya Khan, MD, FRCPC, FACP, FACE, professor in the divisions of endocrinology and metabolism and geriatrics and director of the fellowship in metabolic bone diseases at McMaster University in Hamilton, Ont., told this news organization she agrees with the strategy of evaluating women aged 65 and older for fracture risk.
“The decision aid is useful, but I would like to see it expanded to other circumstances and situations,” she said.
For example, Dr. Khan would like to see recommendations for younger women and for men of all ages regarding secondary causes of osteoporosis or medications known to have a detrimental effect on bone health. By not addressing these patients, she said, “we may miss patients who would benefit from a fracture risk assessment and potentially treatment to prevent low-trauma fractures.”
A recommendation for younger postmenopausal women was included in the most recent Society of Obstetricians and Gynaecologists Canada guideline, she noted.
Overall, she said, “I believe these recommendations will reduce the excess or inappropriate use of BMD testing and that is welcome.”
Funding for the Canadian Task Force on Preventive Health Care is provided by the Public Health Agency of Canada. The task force members report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new Canadian guideline on screening for the primary prevention of fragility fractures recommends risk assessment first, before bone mineral density (BMD) testing, for women aged 65 and older. For younger women and men aged 40 and older, screening is not recommended.
To develop the guideline, a writing group from Canadian Task Force on Preventive Health Care commissioned systematic reviews of studies on the benefits and harms of fragility fracture screenings; the predictive accuracy of current risk-assessment tools; patient acceptability; and benefits of treatment. Treatment harms were analyzed via a rapid overview of reviews.
The guideline, published online in the Canadian Medical Association Journal, is aimed at primary care practitioners for their community-dwelling patients aged 40 and older. The recommendations do not apply to people already taking preventive drugs.
Nondrug treatments were beyond the scope of the current guideline, but guidelines on the prevention of falls and other strategies are planned, Roland Grad, MD, a guideline author and associate professor at McGill University in Montreal, told this news organization.
The new guideline says that women aged 65 and older may be able to avoid fracture through screening and preventive medication. An individual’s fracture risk can be estimated with a new Fragility Fractures Decision Aid, which uses the Canadian FRAX risk-assessment tool.
“A risk assessment–first approach promotes shared decision-making with the patient, based on best medical evidence,” Dr. Grad said.
“To help clinicians, we have created an infographic with visuals to communicate the time spent on BMD vs risk assessment first.”
New evidence
“At least three things motivated this new guideline,” Dr. Grad said. “When we started work on this prior to the pandemic, we saw a need for updated guidance on screening to prevent fragility fractures. We were also aware of new evidence from the publication of screening trials in females older than 65.”
To conduct the risk assessment in older women, clinicians are advised to do the following:
- Use the decision aid (which patients can also use on their own).
- Use the 10-year absolute risk of major osteoporotic fracture to facilitate shared decision-making about possible benefits and harms of preventive pharmacotherapy.
- If pharmacotherapy is being considered, request a BMD using DXA of the femoral neck, then reestimate the fracture risk by adding the BMD T-score into the FRAX.
Potential harms associated with various treatments, with varying levels of evidence, include the following: with alendronate and denosumab, nonserious gastrointestinal adverse events; with denosumab, rash, eczema, and infections; with zoledronic acid, nonserious events, such as headache and flulike symptoms; and with alendronate and bisphosphonates, rare but serious harms of atypical femoral fracture and osteonecrosis of the jaw.
“These recommendations emphasize the importance of good clinical practice, where clinicians are alert to changes in physical health and patient well-being,” the authors wrote. “Clinicians should also be aware of the importance of secondary prevention (i.e., after fracture) and manage patients accordingly.”
“This is an important topic,” Dr. Grad said. “Fragility fractures are consequential for individuals and for our publicly funded health care system. We anticipate questions from clinicians about the time needed to screen with the risk assessment–first strategy. Our modeling work suggests time savings with [this] strategy compared to a strategy of BMD testing first. Following our recommendations may lead to a reduction in BMD testing.”
To promote the guideline, the CMAJ has recorded a podcast and will use other strategies to increase awareness, Dr. Grad said. “The Canadian Task Force has a communications strategy that includes outreach to primary care, stakeholder webinars, social media, partnerships, and other tactics. The College of Family Physicians of Canada has endorsed the guideline and will help promote to its members.”
Other at-risk groups?
Aliya Khan, MD, FRCPC, FACP, FACE, professor in the divisions of endocrinology and metabolism and geriatrics and director of the fellowship in metabolic bone diseases at McMaster University in Hamilton, Ont., told this news organization she agrees with the strategy of evaluating women aged 65 and older for fracture risk.
“The decision aid is useful, but I would like to see it expanded to other circumstances and situations,” she said.
For example, Dr. Khan would like to see recommendations for younger women and for men of all ages regarding secondary causes of osteoporosis or medications known to have a detrimental effect on bone health. By not addressing these patients, she said, “we may miss patients who would benefit from a fracture risk assessment and potentially treatment to prevent low-trauma fractures.”
A recommendation for younger postmenopausal women was included in the most recent Society of Obstetricians and Gynaecologists Canada guideline, she noted.
Overall, she said, “I believe these recommendations will reduce the excess or inappropriate use of BMD testing and that is welcome.”
Funding for the Canadian Task Force on Preventive Health Care is provided by the Public Health Agency of Canada. The task force members report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new Canadian guideline on screening for the primary prevention of fragility fractures recommends risk assessment first, before bone mineral density (BMD) testing, for women aged 65 and older. For younger women and men aged 40 and older, screening is not recommended.
To develop the guideline, a writing group from Canadian Task Force on Preventive Health Care commissioned systematic reviews of studies on the benefits and harms of fragility fracture screenings; the predictive accuracy of current risk-assessment tools; patient acceptability; and benefits of treatment. Treatment harms were analyzed via a rapid overview of reviews.
The guideline, published online in the Canadian Medical Association Journal, is aimed at primary care practitioners for their community-dwelling patients aged 40 and older. The recommendations do not apply to people already taking preventive drugs.
Nondrug treatments were beyond the scope of the current guideline, but guidelines on the prevention of falls and other strategies are planned, Roland Grad, MD, a guideline author and associate professor at McGill University in Montreal, told this news organization.
The new guideline says that women aged 65 and older may be able to avoid fracture through screening and preventive medication. An individual’s fracture risk can be estimated with a new Fragility Fractures Decision Aid, which uses the Canadian FRAX risk-assessment tool.
“A risk assessment–first approach promotes shared decision-making with the patient, based on best medical evidence,” Dr. Grad said.
“To help clinicians, we have created an infographic with visuals to communicate the time spent on BMD vs risk assessment first.”
New evidence
“At least three things motivated this new guideline,” Dr. Grad said. “When we started work on this prior to the pandemic, we saw a need for updated guidance on screening to prevent fragility fractures. We were also aware of new evidence from the publication of screening trials in females older than 65.”
To conduct the risk assessment in older women, clinicians are advised to do the following:
- Use the decision aid (which patients can also use on their own).
- Use the 10-year absolute risk of major osteoporotic fracture to facilitate shared decision-making about possible benefits and harms of preventive pharmacotherapy.
- If pharmacotherapy is being considered, request a BMD using DXA of the femoral neck, then reestimate the fracture risk by adding the BMD T-score into the FRAX.
Potential harms associated with various treatments, with varying levels of evidence, include the following: with alendronate and denosumab, nonserious gastrointestinal adverse events; with denosumab, rash, eczema, and infections; with zoledronic acid, nonserious events, such as headache and flulike symptoms; and with alendronate and bisphosphonates, rare but serious harms of atypical femoral fracture and osteonecrosis of the jaw.
“These recommendations emphasize the importance of good clinical practice, where clinicians are alert to changes in physical health and patient well-being,” the authors wrote. “Clinicians should also be aware of the importance of secondary prevention (i.e., after fracture) and manage patients accordingly.”
“This is an important topic,” Dr. Grad said. “Fragility fractures are consequential for individuals and for our publicly funded health care system. We anticipate questions from clinicians about the time needed to screen with the risk assessment–first strategy. Our modeling work suggests time savings with [this] strategy compared to a strategy of BMD testing first. Following our recommendations may lead to a reduction in BMD testing.”
To promote the guideline, the CMAJ has recorded a podcast and will use other strategies to increase awareness, Dr. Grad said. “The Canadian Task Force has a communications strategy that includes outreach to primary care, stakeholder webinars, social media, partnerships, and other tactics. The College of Family Physicians of Canada has endorsed the guideline and will help promote to its members.”
Other at-risk groups?
Aliya Khan, MD, FRCPC, FACP, FACE, professor in the divisions of endocrinology and metabolism and geriatrics and director of the fellowship in metabolic bone diseases at McMaster University in Hamilton, Ont., told this news organization she agrees with the strategy of evaluating women aged 65 and older for fracture risk.
“The decision aid is useful, but I would like to see it expanded to other circumstances and situations,” she said.
For example, Dr. Khan would like to see recommendations for younger women and for men of all ages regarding secondary causes of osteoporosis or medications known to have a detrimental effect on bone health. By not addressing these patients, she said, “we may miss patients who would benefit from a fracture risk assessment and potentially treatment to prevent low-trauma fractures.”
A recommendation for younger postmenopausal women was included in the most recent Society of Obstetricians and Gynaecologists Canada guideline, she noted.
Overall, she said, “I believe these recommendations will reduce the excess or inappropriate use of BMD testing and that is welcome.”
Funding for the Canadian Task Force on Preventive Health Care is provided by the Public Health Agency of Canada. The task force members report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Can this tool forecast peanut allergies?
Pediatricians may have a new aid to better predict peanut allergies among infants with atopic dermatitis.
Their study of the implementation of the scorecard was presented at the Pediatric Academic Societies annual meeting.
Infants with atopic dermatitis or eczema are six times more likely to have an egg allergy and eleven times more likely to have a peanut allergy at age 12 months than are infants without atopic dermatitis.
The scorecard reflects recent directives from the National Institute of Allergy and Infectious Diseases to help combat the public health problem.
“When the NIAID prevention of peanut allergy guidelines first came out, it asked pediatricians to serve as frontline practitioners in implementing them by identifying children at risk for peanut allergy and guiding families on what to do next,” said Waheeda Samady, MD, professor of pediatrics at Northwestern University, Chicago. “The impetus for the study was to further support pediatricians in this role.”
Although pediatricians are trained to identify and even treat mild to moderate cases of atopic dermatitis, little emphasis has gone to categorizing the condition on the basis of severity and to correlating peanut allergy risk.
The predictive scorecard captures 14 images from one infant of mixed race, two White infants, two Black infants, and two Hispanic infants.
To create the card, two in-house pediatric dermatologists assessed 58 images from 13 children and categorized images from 0 (no signs of atopic dermatitis) to 4 (severe signs of atopic dermatitis). After a first pass on categorization, the doctors agreed on 84% of images.
Of 189 pediatricians who used the card, fewer than half reported that they “sometimes,” “very often,” or “always” used the scorecard for atopic dermatitis evaluation. A little fewer than three-quarters reported that their ability to diagnose and categorize atopic dermatitis improved.
“Severity staging of atopic dermatitis is not something that the general pediatrician necessarily performs on a day-to-day basis,” said Kawaljit Brar, MD, professor of pediatrics in the division of allergy and immunology at Hassenfeld Children’s Hospital in New York.
Dr. Brar explained that children who are identified as being at high risk are often referred to specialists such as her, who then perform allergy screenings and can determine whether introduction of food at home is safe or whether office feedings supervised by an allergist are necessary. Researchers have found that early introduction to peanuts for children with moderate to severe atopic dermatitis could prevent peanut allergy.
“This represents a wonderful initiative to educate pediatricians so that they understand which patients require screening for peanut allergy and which patients don’t and can just get introduced to peanuts at home,” Dr. Brar said.
The atopic dermatitis scorecard reflects a growing recognition that varying skin tones show levels of severity incongruously.
“Many of us in clinical practice have recognized that our education has not always been inclusive of patients with varying skin tones,” Dr. Samady said. “When we looked for photos of patients with different skin tones, we simply could not find any that we thought were appropriate. So we decided to take some ourselves, and we’re currently continuing to take photos in order to improve the scorecard we currently have.”
The study was funded by the National Institute of Health and Food Allergy Research and Education. Dr. Samady and Dr. Brar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pediatricians may have a new aid to better predict peanut allergies among infants with atopic dermatitis.
Their study of the implementation of the scorecard was presented at the Pediatric Academic Societies annual meeting.
Infants with atopic dermatitis or eczema are six times more likely to have an egg allergy and eleven times more likely to have a peanut allergy at age 12 months than are infants without atopic dermatitis.
The scorecard reflects recent directives from the National Institute of Allergy and Infectious Diseases to help combat the public health problem.
“When the NIAID prevention of peanut allergy guidelines first came out, it asked pediatricians to serve as frontline practitioners in implementing them by identifying children at risk for peanut allergy and guiding families on what to do next,” said Waheeda Samady, MD, professor of pediatrics at Northwestern University, Chicago. “The impetus for the study was to further support pediatricians in this role.”
Although pediatricians are trained to identify and even treat mild to moderate cases of atopic dermatitis, little emphasis has gone to categorizing the condition on the basis of severity and to correlating peanut allergy risk.
The predictive scorecard captures 14 images from one infant of mixed race, two White infants, two Black infants, and two Hispanic infants.
To create the card, two in-house pediatric dermatologists assessed 58 images from 13 children and categorized images from 0 (no signs of atopic dermatitis) to 4 (severe signs of atopic dermatitis). After a first pass on categorization, the doctors agreed on 84% of images.
Of 189 pediatricians who used the card, fewer than half reported that they “sometimes,” “very often,” or “always” used the scorecard for atopic dermatitis evaluation. A little fewer than three-quarters reported that their ability to diagnose and categorize atopic dermatitis improved.
“Severity staging of atopic dermatitis is not something that the general pediatrician necessarily performs on a day-to-day basis,” said Kawaljit Brar, MD, professor of pediatrics in the division of allergy and immunology at Hassenfeld Children’s Hospital in New York.
Dr. Brar explained that children who are identified as being at high risk are often referred to specialists such as her, who then perform allergy screenings and can determine whether introduction of food at home is safe or whether office feedings supervised by an allergist are necessary. Researchers have found that early introduction to peanuts for children with moderate to severe atopic dermatitis could prevent peanut allergy.
“This represents a wonderful initiative to educate pediatricians so that they understand which patients require screening for peanut allergy and which patients don’t and can just get introduced to peanuts at home,” Dr. Brar said.
The atopic dermatitis scorecard reflects a growing recognition that varying skin tones show levels of severity incongruously.
“Many of us in clinical practice have recognized that our education has not always been inclusive of patients with varying skin tones,” Dr. Samady said. “When we looked for photos of patients with different skin tones, we simply could not find any that we thought were appropriate. So we decided to take some ourselves, and we’re currently continuing to take photos in order to improve the scorecard we currently have.”
The study was funded by the National Institute of Health and Food Allergy Research and Education. Dr. Samady and Dr. Brar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pediatricians may have a new aid to better predict peanut allergies among infants with atopic dermatitis.
Their study of the implementation of the scorecard was presented at the Pediatric Academic Societies annual meeting.
Infants with atopic dermatitis or eczema are six times more likely to have an egg allergy and eleven times more likely to have a peanut allergy at age 12 months than are infants without atopic dermatitis.
The scorecard reflects recent directives from the National Institute of Allergy and Infectious Diseases to help combat the public health problem.
“When the NIAID prevention of peanut allergy guidelines first came out, it asked pediatricians to serve as frontline practitioners in implementing them by identifying children at risk for peanut allergy and guiding families on what to do next,” said Waheeda Samady, MD, professor of pediatrics at Northwestern University, Chicago. “The impetus for the study was to further support pediatricians in this role.”
Although pediatricians are trained to identify and even treat mild to moderate cases of atopic dermatitis, little emphasis has gone to categorizing the condition on the basis of severity and to correlating peanut allergy risk.
The predictive scorecard captures 14 images from one infant of mixed race, two White infants, two Black infants, and two Hispanic infants.
To create the card, two in-house pediatric dermatologists assessed 58 images from 13 children and categorized images from 0 (no signs of atopic dermatitis) to 4 (severe signs of atopic dermatitis). After a first pass on categorization, the doctors agreed on 84% of images.
Of 189 pediatricians who used the card, fewer than half reported that they “sometimes,” “very often,” or “always” used the scorecard for atopic dermatitis evaluation. A little fewer than three-quarters reported that their ability to diagnose and categorize atopic dermatitis improved.
“Severity staging of atopic dermatitis is not something that the general pediatrician necessarily performs on a day-to-day basis,” said Kawaljit Brar, MD, professor of pediatrics in the division of allergy and immunology at Hassenfeld Children’s Hospital in New York.
Dr. Brar explained that children who are identified as being at high risk are often referred to specialists such as her, who then perform allergy screenings and can determine whether introduction of food at home is safe or whether office feedings supervised by an allergist are necessary. Researchers have found that early introduction to peanuts for children with moderate to severe atopic dermatitis could prevent peanut allergy.
“This represents a wonderful initiative to educate pediatricians so that they understand which patients require screening for peanut allergy and which patients don’t and can just get introduced to peanuts at home,” Dr. Brar said.
The atopic dermatitis scorecard reflects a growing recognition that varying skin tones show levels of severity incongruously.
“Many of us in clinical practice have recognized that our education has not always been inclusive of patients with varying skin tones,” Dr. Samady said. “When we looked for photos of patients with different skin tones, we simply could not find any that we thought were appropriate. So we decided to take some ourselves, and we’re currently continuing to take photos in order to improve the scorecard we currently have.”
The study was funded by the National Institute of Health and Food Allergy Research and Education. Dr. Samady and Dr. Brar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PAS 2023
Medical students gain momentum in effort to ban legacy admissions
, which they say offer preferential treatment to applicants based on their association with donors or alumni.
While an estimated 25% of public colleges and universities still use legacy admissions, a growing list of top medical schools have moved away from the practice over the last decade, including Johns Hopkins University, Baltimore, and Tufts University, Medford, Mass.
Legacy admissions contradict schools’ more inclusive policies, Senila Yasmin, MPH, a second-year medical student at Tufts University, said in an interview. While Tufts maintains legacy admissions for its undergraduate applicants, the medical school stopped the practice in 2021, said Ms. Yasmin, a member of a student group that lobbied against the school’s legacy preferences.
Describing herself as a low-income, first-generation Muslim-Pakistani American, Ms. Yasmin wants to use her experience at Tufts to improve accessibility for students like herself.
As a member of the American Medical Association (AMA) Medical Student Section, she coauthored a resolution stating that legacy admissions go against the AMA’s strategic plan to advance racial justice and health equity. The Student Section passed the resolution in November, and in June, the AMA House of Delegates will vote on whether to adopt the policy.
Along with a Supreme Court decision that could strike down race-conscious college admissions, an AMA policy could convince medical schools to rethink legacy admissions and how to maintain diverse student bodies. In June, the court is expected to issue a decision in the Students for Fair Admissions lawsuit against Harvard University, Cambridge, Mass., and the University of North Carolina, Chapel Hill, which alleges that considering race in holistic admissions constitutes racial discrimination and violates the Equal Protection Clause.
Opponents of legacy admissions, like Ms. Yasmin, say it penalizes students from racial minorities and lower socioeconomic backgrounds, hampering a fair and equitable admissions process that attracts diverse medical school admissions.
Diversity of medical applicants
Diversity in medical schools continued to increase last year with more Black, Hispanic, and female students applying and enrolling, according to a recent report by the Association of American Medical Colleges (AAMC). However, universities often include nonacademic criteria in their admission assessments to improve educational access for underrepresented minorities.
Medical schools carefully consider each applicant’s background “to yield a diverse class of students,” Geoffrey Young, PhD, AAMC’s senior director of transforming the health care workforce, told this news organization.
Some schools, such as Morehouse School of Medicine, Atlanta, the University of Virginia School of Medicine, Charlottesville, and the University of Arizona College of Medicine, Tucson, perform a thorough review of candidates while offering admissions practices designed specifically for legacy applicants. The schools assert that legacy designation doesn’t factor into the student’s likelihood of acceptance.
The arrangement may show that schools want to commit to equity and fairness but have trouble moving away from entrenched traditions, two professors from Penn State College of Medicine, Hershey, Pa., who sit on separate medical admissions subcommittees, wrote last year in Bioethics Today.
Legislation may hasten legacies’ end
In December, Ms. Yasmin and a group of Massachusetts Medical Society student-members presented another resolution to the state medical society, which adopted it.
The society’s new policy opposes the use of legacy status in medical school admissions and supports mechanisms to eliminate its inclusion from the application process, Theodore Calianos II, MD, FACS, president of the Massachusetts Medical Society, said in an interview.
“Legacy preferences limit racial and socioeconomic diversity on campuses, so we asked, ‘What can we do so that everyone has equal access to medical education?’ It is exciting to see the students and young physicians – the future of medicine – become involved in policymaking.”
Proposed laws may also hasten the end of legacy admissions. Last year, the U.S. Senate began considering a bill prohibiting colleges receiving federal financial aid from giving preferential treatment to students based on their relations to donors or alumni. However, the bill allows the Department of Education to make exceptions for institutions serving historically underrepresented groups.
The New York State Senate and the New York State Assembly also are reviewing bills that ban legacy and early admissions policies at public and private universities. Connecticut announced similar legislation last year. Massachusetts legislators are considering two bills: one that would ban the practice at the state’s public universities and another that would require all schools using legacy status to pay a “public service fee” equal to a percentage of its endowment. Colleges with endowment assets exceeding $2 billion must pay at least $2 million, according to the bill’s text.
At schools like Harvard, whose endowment surpasses $50 billion, the option to pay the penalty will make the law moot, Michael Walls, DO, MPH, president of the American Medical Student Association (AMSA), said in an interview. “Smaller schools wouldn’t be able to afford the fine and are less likely to be doing [legacy admissions] anyway,” he said. “The schools that want to continue doing it could just pay the fine.”
Dr. Walls said AMSA supports race-conscious admissions processes and anything that increases fairness for medical school applicants. “Whatever [fair] means is up for interpretation, but it would be great to eliminate legacy admissions,” he said.
A version of this article originally appeared on Medscape.com.
, which they say offer preferential treatment to applicants based on their association with donors or alumni.
While an estimated 25% of public colleges and universities still use legacy admissions, a growing list of top medical schools have moved away from the practice over the last decade, including Johns Hopkins University, Baltimore, and Tufts University, Medford, Mass.
Legacy admissions contradict schools’ more inclusive policies, Senila Yasmin, MPH, a second-year medical student at Tufts University, said in an interview. While Tufts maintains legacy admissions for its undergraduate applicants, the medical school stopped the practice in 2021, said Ms. Yasmin, a member of a student group that lobbied against the school’s legacy preferences.
Describing herself as a low-income, first-generation Muslim-Pakistani American, Ms. Yasmin wants to use her experience at Tufts to improve accessibility for students like herself.
As a member of the American Medical Association (AMA) Medical Student Section, she coauthored a resolution stating that legacy admissions go against the AMA’s strategic plan to advance racial justice and health equity. The Student Section passed the resolution in November, and in June, the AMA House of Delegates will vote on whether to adopt the policy.
Along with a Supreme Court decision that could strike down race-conscious college admissions, an AMA policy could convince medical schools to rethink legacy admissions and how to maintain diverse student bodies. In June, the court is expected to issue a decision in the Students for Fair Admissions lawsuit against Harvard University, Cambridge, Mass., and the University of North Carolina, Chapel Hill, which alleges that considering race in holistic admissions constitutes racial discrimination and violates the Equal Protection Clause.
Opponents of legacy admissions, like Ms. Yasmin, say it penalizes students from racial minorities and lower socioeconomic backgrounds, hampering a fair and equitable admissions process that attracts diverse medical school admissions.
Diversity of medical applicants
Diversity in medical schools continued to increase last year with more Black, Hispanic, and female students applying and enrolling, according to a recent report by the Association of American Medical Colleges (AAMC). However, universities often include nonacademic criteria in their admission assessments to improve educational access for underrepresented minorities.
Medical schools carefully consider each applicant’s background “to yield a diverse class of students,” Geoffrey Young, PhD, AAMC’s senior director of transforming the health care workforce, told this news organization.
Some schools, such as Morehouse School of Medicine, Atlanta, the University of Virginia School of Medicine, Charlottesville, and the University of Arizona College of Medicine, Tucson, perform a thorough review of candidates while offering admissions practices designed specifically for legacy applicants. The schools assert that legacy designation doesn’t factor into the student’s likelihood of acceptance.
The arrangement may show that schools want to commit to equity and fairness but have trouble moving away from entrenched traditions, two professors from Penn State College of Medicine, Hershey, Pa., who sit on separate medical admissions subcommittees, wrote last year in Bioethics Today.
Legislation may hasten legacies’ end
In December, Ms. Yasmin and a group of Massachusetts Medical Society student-members presented another resolution to the state medical society, which adopted it.
The society’s new policy opposes the use of legacy status in medical school admissions and supports mechanisms to eliminate its inclusion from the application process, Theodore Calianos II, MD, FACS, president of the Massachusetts Medical Society, said in an interview.
“Legacy preferences limit racial and socioeconomic diversity on campuses, so we asked, ‘What can we do so that everyone has equal access to medical education?’ It is exciting to see the students and young physicians – the future of medicine – become involved in policymaking.”
Proposed laws may also hasten the end of legacy admissions. Last year, the U.S. Senate began considering a bill prohibiting colleges receiving federal financial aid from giving preferential treatment to students based on their relations to donors or alumni. However, the bill allows the Department of Education to make exceptions for institutions serving historically underrepresented groups.
The New York State Senate and the New York State Assembly also are reviewing bills that ban legacy and early admissions policies at public and private universities. Connecticut announced similar legislation last year. Massachusetts legislators are considering two bills: one that would ban the practice at the state’s public universities and another that would require all schools using legacy status to pay a “public service fee” equal to a percentage of its endowment. Colleges with endowment assets exceeding $2 billion must pay at least $2 million, according to the bill’s text.
At schools like Harvard, whose endowment surpasses $50 billion, the option to pay the penalty will make the law moot, Michael Walls, DO, MPH, president of the American Medical Student Association (AMSA), said in an interview. “Smaller schools wouldn’t be able to afford the fine and are less likely to be doing [legacy admissions] anyway,” he said. “The schools that want to continue doing it could just pay the fine.”
Dr. Walls said AMSA supports race-conscious admissions processes and anything that increases fairness for medical school applicants. “Whatever [fair] means is up for interpretation, but it would be great to eliminate legacy admissions,” he said.
A version of this article originally appeared on Medscape.com.
, which they say offer preferential treatment to applicants based on their association with donors or alumni.
While an estimated 25% of public colleges and universities still use legacy admissions, a growing list of top medical schools have moved away from the practice over the last decade, including Johns Hopkins University, Baltimore, and Tufts University, Medford, Mass.
Legacy admissions contradict schools’ more inclusive policies, Senila Yasmin, MPH, a second-year medical student at Tufts University, said in an interview. While Tufts maintains legacy admissions for its undergraduate applicants, the medical school stopped the practice in 2021, said Ms. Yasmin, a member of a student group that lobbied against the school’s legacy preferences.
Describing herself as a low-income, first-generation Muslim-Pakistani American, Ms. Yasmin wants to use her experience at Tufts to improve accessibility for students like herself.
As a member of the American Medical Association (AMA) Medical Student Section, she coauthored a resolution stating that legacy admissions go against the AMA’s strategic plan to advance racial justice and health equity. The Student Section passed the resolution in November, and in June, the AMA House of Delegates will vote on whether to adopt the policy.
Along with a Supreme Court decision that could strike down race-conscious college admissions, an AMA policy could convince medical schools to rethink legacy admissions and how to maintain diverse student bodies. In June, the court is expected to issue a decision in the Students for Fair Admissions lawsuit against Harvard University, Cambridge, Mass., and the University of North Carolina, Chapel Hill, which alleges that considering race in holistic admissions constitutes racial discrimination and violates the Equal Protection Clause.
Opponents of legacy admissions, like Ms. Yasmin, say it penalizes students from racial minorities and lower socioeconomic backgrounds, hampering a fair and equitable admissions process that attracts diverse medical school admissions.
Diversity of medical applicants
Diversity in medical schools continued to increase last year with more Black, Hispanic, and female students applying and enrolling, according to a recent report by the Association of American Medical Colleges (AAMC). However, universities often include nonacademic criteria in their admission assessments to improve educational access for underrepresented minorities.
Medical schools carefully consider each applicant’s background “to yield a diverse class of students,” Geoffrey Young, PhD, AAMC’s senior director of transforming the health care workforce, told this news organization.
Some schools, such as Morehouse School of Medicine, Atlanta, the University of Virginia School of Medicine, Charlottesville, and the University of Arizona College of Medicine, Tucson, perform a thorough review of candidates while offering admissions practices designed specifically for legacy applicants. The schools assert that legacy designation doesn’t factor into the student’s likelihood of acceptance.
The arrangement may show that schools want to commit to equity and fairness but have trouble moving away from entrenched traditions, two professors from Penn State College of Medicine, Hershey, Pa., who sit on separate medical admissions subcommittees, wrote last year in Bioethics Today.
Legislation may hasten legacies’ end
In December, Ms. Yasmin and a group of Massachusetts Medical Society student-members presented another resolution to the state medical society, which adopted it.
The society’s new policy opposes the use of legacy status in medical school admissions and supports mechanisms to eliminate its inclusion from the application process, Theodore Calianos II, MD, FACS, president of the Massachusetts Medical Society, said in an interview.
“Legacy preferences limit racial and socioeconomic diversity on campuses, so we asked, ‘What can we do so that everyone has equal access to medical education?’ It is exciting to see the students and young physicians – the future of medicine – become involved in policymaking.”
Proposed laws may also hasten the end of legacy admissions. Last year, the U.S. Senate began considering a bill prohibiting colleges receiving federal financial aid from giving preferential treatment to students based on their relations to donors or alumni. However, the bill allows the Department of Education to make exceptions for institutions serving historically underrepresented groups.
The New York State Senate and the New York State Assembly also are reviewing bills that ban legacy and early admissions policies at public and private universities. Connecticut announced similar legislation last year. Massachusetts legislators are considering two bills: one that would ban the practice at the state’s public universities and another that would require all schools using legacy status to pay a “public service fee” equal to a percentage of its endowment. Colleges with endowment assets exceeding $2 billion must pay at least $2 million, according to the bill’s text.
At schools like Harvard, whose endowment surpasses $50 billion, the option to pay the penalty will make the law moot, Michael Walls, DO, MPH, president of the American Medical Student Association (AMSA), said in an interview. “Smaller schools wouldn’t be able to afford the fine and are less likely to be doing [legacy admissions] anyway,” he said. “The schools that want to continue doing it could just pay the fine.”
Dr. Walls said AMSA supports race-conscious admissions processes and anything that increases fairness for medical school applicants. “Whatever [fair] means is up for interpretation, but it would be great to eliminate legacy admissions,” he said.
A version of this article originally appeared on Medscape.com.
Picosecond laser applications continue to expand
PHOENIX – Ever since PicoSure became the first picosecond laser cleared by the Food and Drug Administration for the treatment of unwanted tattoos and pigmented lesions in 2012, new uses for this technology continue to expand.
Now, These include PicoWay, PicoSure, Enlighten, PicoPlus, PiQo4, and Quanta Pico, among others.
“PicoWay technology has integrated nicely into my practice in Houston, the most ethnically diverse city in the country, with its ability to safely treat a number of various benign, congenital, and acquired epidermal and dermal pigmented lesions with ultrashort pulse duration and low thermal impact, which greatly reduces the risk of postinflammatory hyperpigmentation even in darker skin types,” Paul M. Friedman, MD, director of the Dermatology and Laser Surgery Center, Houston, said at the annual conference of the American Society for Laser Medicine and Surgery.
He emphasized the importance of therapeutic clinical endpoints, noting that with q-switched lasers, “you’re looking for immediate whitening, whereas with picosecond lasers, your endpoint is slight whitening or slight darkening depending on wavelength, indication, and skin type. The ability to fractionate picosecond pulses has also allowed us to utilize this technology for photoaging as well as acne scarring.”
The PicoWay system includes a 730-nm picosecond titanium sapphire handpiece, which is FDA cleared for treatment of benign pigmented lesions and blue and green tattoo removal. Dr. Friedman said that he has seen good clinical results using the handpiece for café-au-lait macules, particularly in skin of color.
In an abstract presented at the ASLMS meeting, he and his colleagues presented a retrospective review of 12 patients with café-au-lait macules with Fitzpatrick skin types III-VI who were treated with the PicoWay 730 nm handpiece between April 2021 and January 2023. Patients received a mean of 3.1 treatments at intervals that ranged from 5 to 40 weeks. Clinical photographs were graded by three board-certified dermatologists using a 5-point visual analogue scale.
Overall, patients were rated to have a mean improvement of 26%-50%. Two patients achieved 100% clearance after four to five treatment sessions. “Café-au-lait macules with smooth borders responded less well to laser treatment, confirming prior studies at our center,” he said. “We often educate parents that café-au-lait macules may recur over time, especially with repeated sun exposure.”
Treating melasma
Dr. Friedman’s go-to devices for melasma include the low-density, low-energy 1,927-nm fractional diode laser; the 1,064 nm picosecond Nd:YAG, the low-fluence 1,064 nm Q-switched Nd:YAG with a nanosecond pulse duration, and the 595-nm pulsed dye laser for lesions exhibiting underlying vascularity. He said that combining therapies that target pigment and vasculature may be ideal to prevent relapses. “Melasma is a multifactorial condition so by improving patient education and expectation alongside advances in laser treatment of melasma, we have ultimately improved our ability to treat this condition,” he said.
“We’re approaching it from all angles, with ultraviolet photography and spectrocolorimetry, behavioral modifications, topical skin-lightening agents, broad spectrum sunscreens with protection against visible light, and oral tranexamic acid in advanced cases. Then, we intervene with these energy-based modalities, and the bottom line is, less energy and density is more, with lengthened treatment intervals. In 2023, we’re better than we’ve ever been in terms of our ability to safely and effectively improve melasma.”
Novel lasers
Dr. Friedman also described the UltraClear, a novel ablative fractional 2,910-nm erbium-doped glass fiber laser that delivers a customized blend of ablation and coagulation based on the patient’s condition, skin type, and tolerability for down time. He provided an overview of the versatility of what he described as highly customizable technology for conditions such as photoaging and dyschromia in patients of various skin types, making it a very versatile platform in his practice.
The AVAVA MIRIA system is a “next generation” laser “where you’re able to use a focal point. Basically, you’re treating the skin from the inside out in a 3D manner and you’re able to focus intradermally up to 1 mm with high energy 1,064 nm or 1,550 nm,” he said. “It’s a unique conical geometry that spares the epidermis, combined with sapphire tip cooling and images the skin at the same time with the potential for personalized treatments of dyschromia and photoaging in all skin types. It’s truly remarkable where the technology is heading.”
Dr. Friedman disclosed that he has received consulting fees from Allergan, Galderma, Acclaro, Merz Aesthetics, Solta Medical, and Cytrellis. He has conducted contracted research for Sofwave and is a member of the speakers bureau for Solta Medical and Candela.
PHOENIX – Ever since PicoSure became the first picosecond laser cleared by the Food and Drug Administration for the treatment of unwanted tattoos and pigmented lesions in 2012, new uses for this technology continue to expand.
Now, These include PicoWay, PicoSure, Enlighten, PicoPlus, PiQo4, and Quanta Pico, among others.
“PicoWay technology has integrated nicely into my practice in Houston, the most ethnically diverse city in the country, with its ability to safely treat a number of various benign, congenital, and acquired epidermal and dermal pigmented lesions with ultrashort pulse duration and low thermal impact, which greatly reduces the risk of postinflammatory hyperpigmentation even in darker skin types,” Paul M. Friedman, MD, director of the Dermatology and Laser Surgery Center, Houston, said at the annual conference of the American Society for Laser Medicine and Surgery.
He emphasized the importance of therapeutic clinical endpoints, noting that with q-switched lasers, “you’re looking for immediate whitening, whereas with picosecond lasers, your endpoint is slight whitening or slight darkening depending on wavelength, indication, and skin type. The ability to fractionate picosecond pulses has also allowed us to utilize this technology for photoaging as well as acne scarring.”
The PicoWay system includes a 730-nm picosecond titanium sapphire handpiece, which is FDA cleared for treatment of benign pigmented lesions and blue and green tattoo removal. Dr. Friedman said that he has seen good clinical results using the handpiece for café-au-lait macules, particularly in skin of color.
In an abstract presented at the ASLMS meeting, he and his colleagues presented a retrospective review of 12 patients with café-au-lait macules with Fitzpatrick skin types III-VI who were treated with the PicoWay 730 nm handpiece between April 2021 and January 2023. Patients received a mean of 3.1 treatments at intervals that ranged from 5 to 40 weeks. Clinical photographs were graded by three board-certified dermatologists using a 5-point visual analogue scale.
Overall, patients were rated to have a mean improvement of 26%-50%. Two patients achieved 100% clearance after four to five treatment sessions. “Café-au-lait macules with smooth borders responded less well to laser treatment, confirming prior studies at our center,” he said. “We often educate parents that café-au-lait macules may recur over time, especially with repeated sun exposure.”
Treating melasma
Dr. Friedman’s go-to devices for melasma include the low-density, low-energy 1,927-nm fractional diode laser; the 1,064 nm picosecond Nd:YAG, the low-fluence 1,064 nm Q-switched Nd:YAG with a nanosecond pulse duration, and the 595-nm pulsed dye laser for lesions exhibiting underlying vascularity. He said that combining therapies that target pigment and vasculature may be ideal to prevent relapses. “Melasma is a multifactorial condition so by improving patient education and expectation alongside advances in laser treatment of melasma, we have ultimately improved our ability to treat this condition,” he said.
“We’re approaching it from all angles, with ultraviolet photography and spectrocolorimetry, behavioral modifications, topical skin-lightening agents, broad spectrum sunscreens with protection against visible light, and oral tranexamic acid in advanced cases. Then, we intervene with these energy-based modalities, and the bottom line is, less energy and density is more, with lengthened treatment intervals. In 2023, we’re better than we’ve ever been in terms of our ability to safely and effectively improve melasma.”
Novel lasers
Dr. Friedman also described the UltraClear, a novel ablative fractional 2,910-nm erbium-doped glass fiber laser that delivers a customized blend of ablation and coagulation based on the patient’s condition, skin type, and tolerability for down time. He provided an overview of the versatility of what he described as highly customizable technology for conditions such as photoaging and dyschromia in patients of various skin types, making it a very versatile platform in his practice.
The AVAVA MIRIA system is a “next generation” laser “where you’re able to use a focal point. Basically, you’re treating the skin from the inside out in a 3D manner and you’re able to focus intradermally up to 1 mm with high energy 1,064 nm or 1,550 nm,” he said. “It’s a unique conical geometry that spares the epidermis, combined with sapphire tip cooling and images the skin at the same time with the potential for personalized treatments of dyschromia and photoaging in all skin types. It’s truly remarkable where the technology is heading.”
Dr. Friedman disclosed that he has received consulting fees from Allergan, Galderma, Acclaro, Merz Aesthetics, Solta Medical, and Cytrellis. He has conducted contracted research for Sofwave and is a member of the speakers bureau for Solta Medical and Candela.
PHOENIX – Ever since PicoSure became the first picosecond laser cleared by the Food and Drug Administration for the treatment of unwanted tattoos and pigmented lesions in 2012, new uses for this technology continue to expand.
Now, These include PicoWay, PicoSure, Enlighten, PicoPlus, PiQo4, and Quanta Pico, among others.
“PicoWay technology has integrated nicely into my practice in Houston, the most ethnically diverse city in the country, with its ability to safely treat a number of various benign, congenital, and acquired epidermal and dermal pigmented lesions with ultrashort pulse duration and low thermal impact, which greatly reduces the risk of postinflammatory hyperpigmentation even in darker skin types,” Paul M. Friedman, MD, director of the Dermatology and Laser Surgery Center, Houston, said at the annual conference of the American Society for Laser Medicine and Surgery.
He emphasized the importance of therapeutic clinical endpoints, noting that with q-switched lasers, “you’re looking for immediate whitening, whereas with picosecond lasers, your endpoint is slight whitening or slight darkening depending on wavelength, indication, and skin type. The ability to fractionate picosecond pulses has also allowed us to utilize this technology for photoaging as well as acne scarring.”
The PicoWay system includes a 730-nm picosecond titanium sapphire handpiece, which is FDA cleared for treatment of benign pigmented lesions and blue and green tattoo removal. Dr. Friedman said that he has seen good clinical results using the handpiece for café-au-lait macules, particularly in skin of color.
In an abstract presented at the ASLMS meeting, he and his colleagues presented a retrospective review of 12 patients with café-au-lait macules with Fitzpatrick skin types III-VI who were treated with the PicoWay 730 nm handpiece between April 2021 and January 2023. Patients received a mean of 3.1 treatments at intervals that ranged from 5 to 40 weeks. Clinical photographs were graded by three board-certified dermatologists using a 5-point visual analogue scale.
Overall, patients were rated to have a mean improvement of 26%-50%. Two patients achieved 100% clearance after four to five treatment sessions. “Café-au-lait macules with smooth borders responded less well to laser treatment, confirming prior studies at our center,” he said. “We often educate parents that café-au-lait macules may recur over time, especially with repeated sun exposure.”
Treating melasma
Dr. Friedman’s go-to devices for melasma include the low-density, low-energy 1,927-nm fractional diode laser; the 1,064 nm picosecond Nd:YAG, the low-fluence 1,064 nm Q-switched Nd:YAG with a nanosecond pulse duration, and the 595-nm pulsed dye laser for lesions exhibiting underlying vascularity. He said that combining therapies that target pigment and vasculature may be ideal to prevent relapses. “Melasma is a multifactorial condition so by improving patient education and expectation alongside advances in laser treatment of melasma, we have ultimately improved our ability to treat this condition,” he said.
“We’re approaching it from all angles, with ultraviolet photography and spectrocolorimetry, behavioral modifications, topical skin-lightening agents, broad spectrum sunscreens with protection against visible light, and oral tranexamic acid in advanced cases. Then, we intervene with these energy-based modalities, and the bottom line is, less energy and density is more, with lengthened treatment intervals. In 2023, we’re better than we’ve ever been in terms of our ability to safely and effectively improve melasma.”
Novel lasers
Dr. Friedman also described the UltraClear, a novel ablative fractional 2,910-nm erbium-doped glass fiber laser that delivers a customized blend of ablation and coagulation based on the patient’s condition, skin type, and tolerability for down time. He provided an overview of the versatility of what he described as highly customizable technology for conditions such as photoaging and dyschromia in patients of various skin types, making it a very versatile platform in his practice.
The AVAVA MIRIA system is a “next generation” laser “where you’re able to use a focal point. Basically, you’re treating the skin from the inside out in a 3D manner and you’re able to focus intradermally up to 1 mm with high energy 1,064 nm or 1,550 nm,” he said. “It’s a unique conical geometry that spares the epidermis, combined with sapphire tip cooling and images the skin at the same time with the potential for personalized treatments of dyschromia and photoaging in all skin types. It’s truly remarkable where the technology is heading.”
Dr. Friedman disclosed that he has received consulting fees from Allergan, Galderma, Acclaro, Merz Aesthetics, Solta Medical, and Cytrellis. He has conducted contracted research for Sofwave and is a member of the speakers bureau for Solta Medical and Candela.
FROM ASLMS 2023
Five ways docs may qualify for discounts on medical malpractice premiums
Getting a better deal might simply mean taking advantage of incentives and discounts your insurer may already offer. These include claims-free, new-to-practice, and working part-time discounts.
However, if you decide to shop around, keep in mind that discounts are just one factor that can affect your premium price – insurers look at your specialty, location, and claims history.
One of the most common ways physicians can earn discounts is by participating in risk management programs. With this type of program, physicians evaluate elements of their practice and documentation practices and identify areas that might leave them at risk for a lawsuit. While they save money, physician risk management programs also are designed to reduce malpractice claims, which ultimately minimizes the potential for bigger financial losses, insurance experts say.
“It’s a win-win situation when liability insurers and physicians work together to minimize risk, and it’s a win for patients,” said Gary Price, MD, president of The Physicians Foundation.
Doctors in private practice or employed by small hospitals that are not self-insured can qualify for these discounts, said David Zetter, president of Zetter HealthCare Management Consultants.
“I do a lot of work with medical malpractice companies trying to find clients policies. All the carriers are transparent about what physicians have to do to lower their premiums. Physicians can receive the discounts if they follow through and meet the insurer’s requirements,” said Mr. Zetter.
State insurance departments regulate medical malpractice insurance, including the premium credits insurers offer. Most states cap discounts at 25%, but some go as high as 70%, according to The Doctors Company, a national physician-owned medical malpractice insurer.
Insurers typically offer doctors several ways to earn discounts. The size of the discount also can depend on whether a doctor is new to a practice, remains claims free, or takes risk management courses.
In addition to the premium discount, some online risk management classes and webinars are eligible for CME credits.
“The credits can add up and they can be used for recertification or relicensure,” said Susan Boisvert, senior patient safety risk manager at The Doctors Company.
Here are five ways you may qualify for discounts with your insurer.
1. Make use of discounts available to new doctors
Doctors can earn hefty discounts on their premiums when they are no longer interns or residents and start practicing medicine. The Doctors Company usually gives a 50% discount on member premiums the first year they’re in practice and a 25% discount credit in their second year. The discounts end after that.
Other insurance carriers offer similar discounts to doctors starting to practice medicine. The deepest one is offered in the first year (at least 50%) and a smaller one (20%-25%) the second year, according to medical malpractice brokers.
“The new-to-practice discount is based solely on when the physician left their formal training to begin their practice for the first time; it is not based on claim-free history,” explained Mr. Zetter.
This is a very common discount used by different insurer carriers, said Dr. Price. “New physicians don’t have the same amount of risk of a lawsuit when they’re starting out. It’s unlikely they will have a claim and most liability actions have a 2-year time limit from the date of injury to be filed.”
2. Take advantage of being claims free
If you’ve been claims free for at least a few years, you may be eligible for a large discount.
“Doctors without claims are a better risk. Once a doctor has one claim, they’re likely to have a second, which the research shows,” said Mr. Zetter.
The most common credit The Doctors Company offers is 3 years of being claim free – this earns doctors up to 25%, he said. Mr. Zetter explained that the criteria and size of The Doctors Company credit may depend on the state where physicians practice.
“We allowed insurance carriers that we acquired to continue with their own claim-free discount program such as Florida’s First Professionals Insurance Company we acquired in 2011,” he said.
Doctors with other medical malpractice insurers may also be eligible for a credit up to 25%. In some instances, they may have to be claims free for 5 or 10 years, say insurance experts.
It pays to shop around before purchasing insurance.
3. If you work part time, make sure your premium reflects that
Physicians who see patients part time can receive up to a 75% discount on their medical liability insurance premiums.
The discounts are based on the hours the physician works per week. The fewer hours worked, the larger the discount. This type of discount does not vary by specialty.
According to The Doctors Company, working 10 hours or less per week may entitle doctors to a 75% discount; working 11-20 hours per week may entitle them to a 50% discount, and working 21-30 hours per week may entitle them to a 25% discount. If you are in this situation, it pays to ask your insurer if there is a discount available to you.
4. Look into your professional medical society insurance company
“I would look at your state medical association [or] state specialty society and talk to your colleagues to learn what premiums they’re paying and about any discounts they’re getting,” advised Mr. Zetter.
Some state medical societies have formed their own liability companies and offer lower premiums to their members because “they’re organized and managed by doctors, which makes their premiums more competitive,” Dr. Price said.
Other state medical societies endorse specific insurance carriers and offer their members a 5% discount for enrolling with them.
5. Enroll in a risk management program
Most insurers offer online educational activities designed to improve patient safety and reduce the risk of a lawsuit. Physicians may be eligible for both premium discounts and CME credits.
Medical Liability Mutual Insurance Company, owned by Berkshire Hathaway, operates in New York and offers physicians a premium discount of up to 5%, CME credit, and maintenance of certification credit for successfully completing its risk management program every other year.
ProAssurance members nationwide can earn 5% in premium discounts if they complete a 2-hour video series called “Back to Basics: Loss Prevention and Navigating Everyday Risks: Using Data to Drive Change.”
They can earn one credit for completing each webinar on topics such as “Medication Management: Minimizing Errors and Improving Safety” and “Opioid Prescribing: Keeping Patients Safe.”
MagMutual offers its insured physicians 1 CME credit for completing their specialty’s risk assessment and courses, which may be applied toward their premium discounts.
The Doctors Company offers its members a 5% premium discount if they complete 4 CME credits. One of its most popular courses is “How To Get Rid of a Difficult Patient.”
“Busy residents like the shorter case studies worth one-quarter credit that they can complete in 15 minutes,” said Ms. Boisvert.
“This is a good bargain from the physician’s standpoint and the fact that risk management education is offered online makes it a lot easier than going to a seminar in person,” said Dr. Price.
A version of this article first appeared on Medscape.com.
Getting a better deal might simply mean taking advantage of incentives and discounts your insurer may already offer. These include claims-free, new-to-practice, and working part-time discounts.
However, if you decide to shop around, keep in mind that discounts are just one factor that can affect your premium price – insurers look at your specialty, location, and claims history.
One of the most common ways physicians can earn discounts is by participating in risk management programs. With this type of program, physicians evaluate elements of their practice and documentation practices and identify areas that might leave them at risk for a lawsuit. While they save money, physician risk management programs also are designed to reduce malpractice claims, which ultimately minimizes the potential for bigger financial losses, insurance experts say.
“It’s a win-win situation when liability insurers and physicians work together to minimize risk, and it’s a win for patients,” said Gary Price, MD, president of The Physicians Foundation.
Doctors in private practice or employed by small hospitals that are not self-insured can qualify for these discounts, said David Zetter, president of Zetter HealthCare Management Consultants.
“I do a lot of work with medical malpractice companies trying to find clients policies. All the carriers are transparent about what physicians have to do to lower their premiums. Physicians can receive the discounts if they follow through and meet the insurer’s requirements,” said Mr. Zetter.
State insurance departments regulate medical malpractice insurance, including the premium credits insurers offer. Most states cap discounts at 25%, but some go as high as 70%, according to The Doctors Company, a national physician-owned medical malpractice insurer.
Insurers typically offer doctors several ways to earn discounts. The size of the discount also can depend on whether a doctor is new to a practice, remains claims free, or takes risk management courses.
In addition to the premium discount, some online risk management classes and webinars are eligible for CME credits.
“The credits can add up and they can be used for recertification or relicensure,” said Susan Boisvert, senior patient safety risk manager at The Doctors Company.
Here are five ways you may qualify for discounts with your insurer.
1. Make use of discounts available to new doctors
Doctors can earn hefty discounts on their premiums when they are no longer interns or residents and start practicing medicine. The Doctors Company usually gives a 50% discount on member premiums the first year they’re in practice and a 25% discount credit in their second year. The discounts end after that.
Other insurance carriers offer similar discounts to doctors starting to practice medicine. The deepest one is offered in the first year (at least 50%) and a smaller one (20%-25%) the second year, according to medical malpractice brokers.
“The new-to-practice discount is based solely on when the physician left their formal training to begin their practice for the first time; it is not based on claim-free history,” explained Mr. Zetter.
This is a very common discount used by different insurer carriers, said Dr. Price. “New physicians don’t have the same amount of risk of a lawsuit when they’re starting out. It’s unlikely they will have a claim and most liability actions have a 2-year time limit from the date of injury to be filed.”
2. Take advantage of being claims free
If you’ve been claims free for at least a few years, you may be eligible for a large discount.
“Doctors without claims are a better risk. Once a doctor has one claim, they’re likely to have a second, which the research shows,” said Mr. Zetter.
The most common credit The Doctors Company offers is 3 years of being claim free – this earns doctors up to 25%, he said. Mr. Zetter explained that the criteria and size of The Doctors Company credit may depend on the state where physicians practice.
“We allowed insurance carriers that we acquired to continue with their own claim-free discount program such as Florida’s First Professionals Insurance Company we acquired in 2011,” he said.
Doctors with other medical malpractice insurers may also be eligible for a credit up to 25%. In some instances, they may have to be claims free for 5 or 10 years, say insurance experts.
It pays to shop around before purchasing insurance.
3. If you work part time, make sure your premium reflects that
Physicians who see patients part time can receive up to a 75% discount on their medical liability insurance premiums.
The discounts are based on the hours the physician works per week. The fewer hours worked, the larger the discount. This type of discount does not vary by specialty.
According to The Doctors Company, working 10 hours or less per week may entitle doctors to a 75% discount; working 11-20 hours per week may entitle them to a 50% discount, and working 21-30 hours per week may entitle them to a 25% discount. If you are in this situation, it pays to ask your insurer if there is a discount available to you.
4. Look into your professional medical society insurance company
“I would look at your state medical association [or] state specialty society and talk to your colleagues to learn what premiums they’re paying and about any discounts they’re getting,” advised Mr. Zetter.
Some state medical societies have formed their own liability companies and offer lower premiums to their members because “they’re organized and managed by doctors, which makes their premiums more competitive,” Dr. Price said.
Other state medical societies endorse specific insurance carriers and offer their members a 5% discount for enrolling with them.
5. Enroll in a risk management program
Most insurers offer online educational activities designed to improve patient safety and reduce the risk of a lawsuit. Physicians may be eligible for both premium discounts and CME credits.
Medical Liability Mutual Insurance Company, owned by Berkshire Hathaway, operates in New York and offers physicians a premium discount of up to 5%, CME credit, and maintenance of certification credit for successfully completing its risk management program every other year.
ProAssurance members nationwide can earn 5% in premium discounts if they complete a 2-hour video series called “Back to Basics: Loss Prevention and Navigating Everyday Risks: Using Data to Drive Change.”
They can earn one credit for completing each webinar on topics such as “Medication Management: Minimizing Errors and Improving Safety” and “Opioid Prescribing: Keeping Patients Safe.”
MagMutual offers its insured physicians 1 CME credit for completing their specialty’s risk assessment and courses, which may be applied toward their premium discounts.
The Doctors Company offers its members a 5% premium discount if they complete 4 CME credits. One of its most popular courses is “How To Get Rid of a Difficult Patient.”
“Busy residents like the shorter case studies worth one-quarter credit that they can complete in 15 minutes,” said Ms. Boisvert.
“This is a good bargain from the physician’s standpoint and the fact that risk management education is offered online makes it a lot easier than going to a seminar in person,” said Dr. Price.
A version of this article first appeared on Medscape.com.
Getting a better deal might simply mean taking advantage of incentives and discounts your insurer may already offer. These include claims-free, new-to-practice, and working part-time discounts.
However, if you decide to shop around, keep in mind that discounts are just one factor that can affect your premium price – insurers look at your specialty, location, and claims history.
One of the most common ways physicians can earn discounts is by participating in risk management programs. With this type of program, physicians evaluate elements of their practice and documentation practices and identify areas that might leave them at risk for a lawsuit. While they save money, physician risk management programs also are designed to reduce malpractice claims, which ultimately minimizes the potential for bigger financial losses, insurance experts say.
“It’s a win-win situation when liability insurers and physicians work together to minimize risk, and it’s a win for patients,” said Gary Price, MD, president of The Physicians Foundation.
Doctors in private practice or employed by small hospitals that are not self-insured can qualify for these discounts, said David Zetter, president of Zetter HealthCare Management Consultants.
“I do a lot of work with medical malpractice companies trying to find clients policies. All the carriers are transparent about what physicians have to do to lower their premiums. Physicians can receive the discounts if they follow through and meet the insurer’s requirements,” said Mr. Zetter.
State insurance departments regulate medical malpractice insurance, including the premium credits insurers offer. Most states cap discounts at 25%, but some go as high as 70%, according to The Doctors Company, a national physician-owned medical malpractice insurer.
Insurers typically offer doctors several ways to earn discounts. The size of the discount also can depend on whether a doctor is new to a practice, remains claims free, or takes risk management courses.
In addition to the premium discount, some online risk management classes and webinars are eligible for CME credits.
“The credits can add up and they can be used for recertification or relicensure,” said Susan Boisvert, senior patient safety risk manager at The Doctors Company.
Here are five ways you may qualify for discounts with your insurer.
1. Make use of discounts available to new doctors
Doctors can earn hefty discounts on their premiums when they are no longer interns or residents and start practicing medicine. The Doctors Company usually gives a 50% discount on member premiums the first year they’re in practice and a 25% discount credit in their second year. The discounts end after that.
Other insurance carriers offer similar discounts to doctors starting to practice medicine. The deepest one is offered in the first year (at least 50%) and a smaller one (20%-25%) the second year, according to medical malpractice brokers.
“The new-to-practice discount is based solely on when the physician left their formal training to begin their practice for the first time; it is not based on claim-free history,” explained Mr. Zetter.
This is a very common discount used by different insurer carriers, said Dr. Price. “New physicians don’t have the same amount of risk of a lawsuit when they’re starting out. It’s unlikely they will have a claim and most liability actions have a 2-year time limit from the date of injury to be filed.”
2. Take advantage of being claims free
If you’ve been claims free for at least a few years, you may be eligible for a large discount.
“Doctors without claims are a better risk. Once a doctor has one claim, they’re likely to have a second, which the research shows,” said Mr. Zetter.
The most common credit The Doctors Company offers is 3 years of being claim free – this earns doctors up to 25%, he said. Mr. Zetter explained that the criteria and size of The Doctors Company credit may depend on the state where physicians practice.
“We allowed insurance carriers that we acquired to continue with their own claim-free discount program such as Florida’s First Professionals Insurance Company we acquired in 2011,” he said.
Doctors with other medical malpractice insurers may also be eligible for a credit up to 25%. In some instances, they may have to be claims free for 5 or 10 years, say insurance experts.
It pays to shop around before purchasing insurance.
3. If you work part time, make sure your premium reflects that
Physicians who see patients part time can receive up to a 75% discount on their medical liability insurance premiums.
The discounts are based on the hours the physician works per week. The fewer hours worked, the larger the discount. This type of discount does not vary by specialty.
According to The Doctors Company, working 10 hours or less per week may entitle doctors to a 75% discount; working 11-20 hours per week may entitle them to a 50% discount, and working 21-30 hours per week may entitle them to a 25% discount. If you are in this situation, it pays to ask your insurer if there is a discount available to you.
4. Look into your professional medical society insurance company
“I would look at your state medical association [or] state specialty society and talk to your colleagues to learn what premiums they’re paying and about any discounts they’re getting,” advised Mr. Zetter.
Some state medical societies have formed their own liability companies and offer lower premiums to their members because “they’re organized and managed by doctors, which makes their premiums more competitive,” Dr. Price said.
Other state medical societies endorse specific insurance carriers and offer their members a 5% discount for enrolling with them.
5. Enroll in a risk management program
Most insurers offer online educational activities designed to improve patient safety and reduce the risk of a lawsuit. Physicians may be eligible for both premium discounts and CME credits.
Medical Liability Mutual Insurance Company, owned by Berkshire Hathaway, operates in New York and offers physicians a premium discount of up to 5%, CME credit, and maintenance of certification credit for successfully completing its risk management program every other year.
ProAssurance members nationwide can earn 5% in premium discounts if they complete a 2-hour video series called “Back to Basics: Loss Prevention and Navigating Everyday Risks: Using Data to Drive Change.”
They can earn one credit for completing each webinar on topics such as “Medication Management: Minimizing Errors and Improving Safety” and “Opioid Prescribing: Keeping Patients Safe.”
MagMutual offers its insured physicians 1 CME credit for completing their specialty’s risk assessment and courses, which may be applied toward their premium discounts.
The Doctors Company offers its members a 5% premium discount if they complete 4 CME credits. One of its most popular courses is “How To Get Rid of a Difficult Patient.”
“Busy residents like the shorter case studies worth one-quarter credit that they can complete in 15 minutes,” said Ms. Boisvert.
“This is a good bargain from the physician’s standpoint and the fact that risk management education is offered online makes it a lot easier than going to a seminar in person,” said Dr. Price.
A version of this article first appeared on Medscape.com.
AGA clinical practice update: Extraesophageal gastroesophageal reflux disease
Extraesophageal reflux (EER) symptoms are a subset of gastroesophageal reflux disease (GERD) that can be difficult to diagnose because of its heterogeneous nature and symptoms that overlap with other conditions.
That puts the onus on physicians to take all symptoms into account and work across disciplines to diagnose, manage, and treat the condition, according to a new clinical practice update from the American Gastroenterological Association, which was published in Clinical Gastroenterology and Hepatology.
GERD is becoming increasingly common, which in turn has led to greater awareness and consideration of EER symptoms. EER symptoms can present a challenge because they may vary considerably and are not unique to GERD. The symptoms often do not respond well to proton pump inhibitor (PPI) therapy.
EER symptoms can include cough, laryngeal hoarseness, dysphonia, pulmonary fibrosis, asthma, dental erosions/caries, sinus disease, ear disease, postnasal drip, and throat clearing. Some patients with EER symptoms do not report heartburn or regurgitation, which leaves it up to the physician to determine if acid reflux is present and contributing to symptoms.
“The concept of extraesophageal symptoms secondary to GERD is complex and often controversial, leading to diagnostic and therapeutic challenges. Several extraesophageal symptoms have been associated with GERD, although the strength of evidence to support a causal relation varies,” wrote the authors, who were led by Joan W. Chen, MD, MS, a gastroenterologist with the University of Michigan, Ann Arbor.
There is also debate over whether fluid refluxate is the source of damage that causes EER symptoms, and if so, whether it is sufficient that the fluid be acidic or that pepsin be present, or if the cause is related to neurogenic signaling and resulting inflammation. Because of these questions, a PPI trial will not necessarily provide insight into the role of acid reflux in EER symptoms.
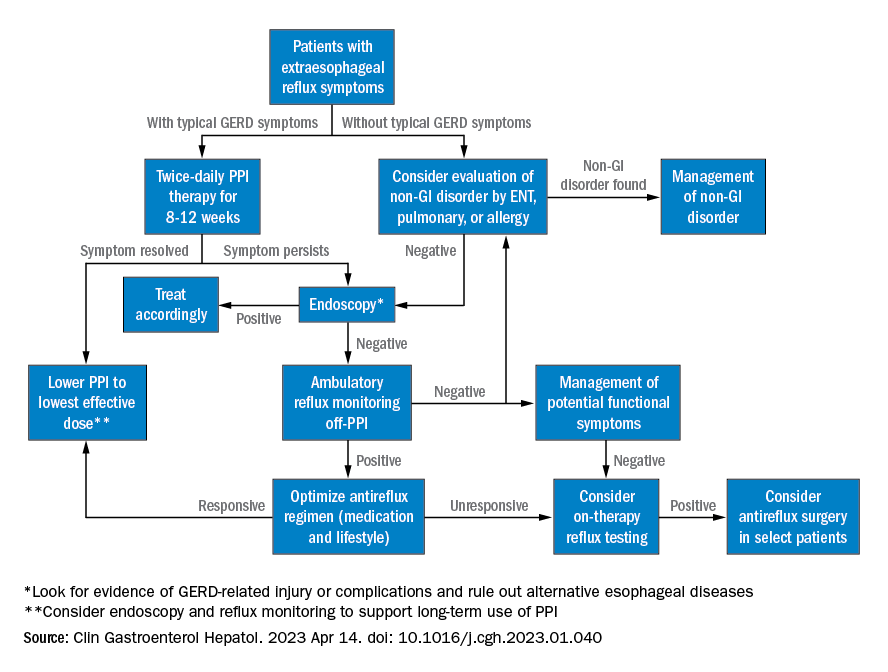
Best practice advice 1: The authors emphasized that gastroenterologists need to be aware of the potential extraesophageal symptoms of GERD. They should inquire with GERD patients to determine if laryngitis, chronic cough, asthma, and dental erosions are present.
Best practice advice 2: Consider a multidisciplinary approach to EER manifestations. Cases may require input from non-GI specialties. Tests performed by other specialists, such as bronchoscopy, thoracic imaging, or laryngoscopy, should be taken into account, since patients will also seek out multiple specialists to address their symptoms.
Best practice advice 3: There is no specific diagnostic test available to determine if GER is the cause of EER symptoms. Instead, physicians should interpret patient symptoms, response to GER therapy, and input from endoscopy and reflux tests.
Best practice advice 4: Rather than subject the patient to the cost and potential for even rare adverse events of a PPI trial, physicians should first consider conducting reflux testing. A PPI trial has clinical value but is insufficient on its own to help diagnose or manage EER. Initial single-dose PPI trial, titrating up to twice daily in those with typical GERD symptoms, is reasonable.
Best practice advice 5: The inconsistent therapeutic response to PPI therapy means that positive effects of PPI therapy on EER symptoms can’t confirm a GERD diagnosis because a placebo effect may be involved, and because symptom improvement can occur through mechanisms other than acid suppression. A meta-analysis found that a PPI trial has a sensitivity of 71%-78% and a specificity of 41%-54% with typical symptoms of heartburn and regurgitation. “Considering the greater variation expected with PPI response for extraesophageal symptoms, the diagnostic performance of empiric PPI trial for a diagnosis of EER would be anticipated to be substantially lower,” the authors wrote.
Best practice advice 6: When EER symptoms related to GERD are suspected and a PPI trial of up to 12 weeks does not lead to adequate improvement, the physician should consider testing for pathologic GER. Additional trials employing other PPIs are unlikely to succeed.
Best practice advice 7: Initial testing to evaluate for reflux should be tailored to patients’ clinical presentation. Potential methods to evaluate reflux include upper endoscopy and ambulatory reflux monitoring studies of acid suppressive therapy, which can assist with a GERD diagnosis, particularly when nonerosive reflux is present.
Best practice advice 8: About 50%-60% of patients with EER symptoms will not have GERD. Testing can be considered for those with an established objective diagnosis of GERD who do not respond well to high doses of acid suppression. Cost-effectiveness studies have confirmed the value of starting with ambulatory reflux monitoring, which can include a catheter-based pH sensor, pH impedance, or wireless pH capsule.
Ambulatory esophageal pH monitoring can also assist in making a GERD diagnosis, but it does not indicate whether GERD may be contributing to EER symptoms.
“Whichever the reflux testing modality, the strongest confidence for EER is achieved after ambulatory reflux testing showing pathologic acid exposure and a positive symptom-reflux association for EER symptoms,” the authors wrote. They also pointed out that ambulatory reflux monitoring in EER patients should be done in the absence of acid suppression unless there is already objective evidence for the presence of GERD.
Best practice advice 9: Aside from acid suppression, EER symptoms can also be managed through other means, including lifestyle modifications, such as eating avoidance prior to lying down, elevation of the head of the bed, sleeping on the left side, and weight loss. Or, alginate containing antacids, external upper esophageal sphincter compression device, cognitive behavioral therapy, and neuromodulators.
Best practice advice 10: In cases where the EER patient has objectively defined evidence of GERD, physicians should employ shared decision-making before considering anti-reflux surgery. If the patient did not respond to PPI therapy, this predicts a lack of response to antireflux surgery.
All four authors reported financial ties to multiple pharmaceutical companies.
Extraesophageal reflux (EER) symptoms are a subset of gastroesophageal reflux disease (GERD) that can be difficult to diagnose because of its heterogeneous nature and symptoms that overlap with other conditions.
That puts the onus on physicians to take all symptoms into account and work across disciplines to diagnose, manage, and treat the condition, according to a new clinical practice update from the American Gastroenterological Association, which was published in Clinical Gastroenterology and Hepatology.
GERD is becoming increasingly common, which in turn has led to greater awareness and consideration of EER symptoms. EER symptoms can present a challenge because they may vary considerably and are not unique to GERD. The symptoms often do not respond well to proton pump inhibitor (PPI) therapy.
EER symptoms can include cough, laryngeal hoarseness, dysphonia, pulmonary fibrosis, asthma, dental erosions/caries, sinus disease, ear disease, postnasal drip, and throat clearing. Some patients with EER symptoms do not report heartburn or regurgitation, which leaves it up to the physician to determine if acid reflux is present and contributing to symptoms.
“The concept of extraesophageal symptoms secondary to GERD is complex and often controversial, leading to diagnostic and therapeutic challenges. Several extraesophageal symptoms have been associated with GERD, although the strength of evidence to support a causal relation varies,” wrote the authors, who were led by Joan W. Chen, MD, MS, a gastroenterologist with the University of Michigan, Ann Arbor.
There is also debate over whether fluid refluxate is the source of damage that causes EER symptoms, and if so, whether it is sufficient that the fluid be acidic or that pepsin be present, or if the cause is related to neurogenic signaling and resulting inflammation. Because of these questions, a PPI trial will not necessarily provide insight into the role of acid reflux in EER symptoms.

Best practice advice 1: The authors emphasized that gastroenterologists need to be aware of the potential extraesophageal symptoms of GERD. They should inquire with GERD patients to determine if laryngitis, chronic cough, asthma, and dental erosions are present.
Best practice advice 2: Consider a multidisciplinary approach to EER manifestations. Cases may require input from non-GI specialties. Tests performed by other specialists, such as bronchoscopy, thoracic imaging, or laryngoscopy, should be taken into account, since patients will also seek out multiple specialists to address their symptoms.
Best practice advice 3: There is no specific diagnostic test available to determine if GER is the cause of EER symptoms. Instead, physicians should interpret patient symptoms, response to GER therapy, and input from endoscopy and reflux tests.
Best practice advice 4: Rather than subject the patient to the cost and potential for even rare adverse events of a PPI trial, physicians should first consider conducting reflux testing. A PPI trial has clinical value but is insufficient on its own to help diagnose or manage EER. Initial single-dose PPI trial, titrating up to twice daily in those with typical GERD symptoms, is reasonable.
Best practice advice 5: The inconsistent therapeutic response to PPI therapy means that positive effects of PPI therapy on EER symptoms can’t confirm a GERD diagnosis because a placebo effect may be involved, and because symptom improvement can occur through mechanisms other than acid suppression. A meta-analysis found that a PPI trial has a sensitivity of 71%-78% and a specificity of 41%-54% with typical symptoms of heartburn and regurgitation. “Considering the greater variation expected with PPI response for extraesophageal symptoms, the diagnostic performance of empiric PPI trial for a diagnosis of EER would be anticipated to be substantially lower,” the authors wrote.
Best practice advice 6: When EER symptoms related to GERD are suspected and a PPI trial of up to 12 weeks does not lead to adequate improvement, the physician should consider testing for pathologic GER. Additional trials employing other PPIs are unlikely to succeed.
Best practice advice 7: Initial testing to evaluate for reflux should be tailored to patients’ clinical presentation. Potential methods to evaluate reflux include upper endoscopy and ambulatory reflux monitoring studies of acid suppressive therapy, which can assist with a GERD diagnosis, particularly when nonerosive reflux is present.
Best practice advice 8: About 50%-60% of patients with EER symptoms will not have GERD. Testing can be considered for those with an established objective diagnosis of GERD who do not respond well to high doses of acid suppression. Cost-effectiveness studies have confirmed the value of starting with ambulatory reflux monitoring, which can include a catheter-based pH sensor, pH impedance, or wireless pH capsule.
Ambulatory esophageal pH monitoring can also assist in making a GERD diagnosis, but it does not indicate whether GERD may be contributing to EER symptoms.
“Whichever the reflux testing modality, the strongest confidence for EER is achieved after ambulatory reflux testing showing pathologic acid exposure and a positive symptom-reflux association for EER symptoms,” the authors wrote. They also pointed out that ambulatory reflux monitoring in EER patients should be done in the absence of acid suppression unless there is already objective evidence for the presence of GERD.
Best practice advice 9: Aside from acid suppression, EER symptoms can also be managed through other means, including lifestyle modifications, such as eating avoidance prior to lying down, elevation of the head of the bed, sleeping on the left side, and weight loss. Or, alginate containing antacids, external upper esophageal sphincter compression device, cognitive behavioral therapy, and neuromodulators.
Best practice advice 10: In cases where the EER patient has objectively defined evidence of GERD, physicians should employ shared decision-making before considering anti-reflux surgery. If the patient did not respond to PPI therapy, this predicts a lack of response to antireflux surgery.
All four authors reported financial ties to multiple pharmaceutical companies.
Extraesophageal reflux (EER) symptoms are a subset of gastroesophageal reflux disease (GERD) that can be difficult to diagnose because of its heterogeneous nature and symptoms that overlap with other conditions.
That puts the onus on physicians to take all symptoms into account and work across disciplines to diagnose, manage, and treat the condition, according to a new clinical practice update from the American Gastroenterological Association, which was published in Clinical Gastroenterology and Hepatology.
GERD is becoming increasingly common, which in turn has led to greater awareness and consideration of EER symptoms. EER symptoms can present a challenge because they may vary considerably and are not unique to GERD. The symptoms often do not respond well to proton pump inhibitor (PPI) therapy.
EER symptoms can include cough, laryngeal hoarseness, dysphonia, pulmonary fibrosis, asthma, dental erosions/caries, sinus disease, ear disease, postnasal drip, and throat clearing. Some patients with EER symptoms do not report heartburn or regurgitation, which leaves it up to the physician to determine if acid reflux is present and contributing to symptoms.
“The concept of extraesophageal symptoms secondary to GERD is complex and often controversial, leading to diagnostic and therapeutic challenges. Several extraesophageal symptoms have been associated with GERD, although the strength of evidence to support a causal relation varies,” wrote the authors, who were led by Joan W. Chen, MD, MS, a gastroenterologist with the University of Michigan, Ann Arbor.
There is also debate over whether fluid refluxate is the source of damage that causes EER symptoms, and if so, whether it is sufficient that the fluid be acidic or that pepsin be present, or if the cause is related to neurogenic signaling and resulting inflammation. Because of these questions, a PPI trial will not necessarily provide insight into the role of acid reflux in EER symptoms.

Best practice advice 1: The authors emphasized that gastroenterologists need to be aware of the potential extraesophageal symptoms of GERD. They should inquire with GERD patients to determine if laryngitis, chronic cough, asthma, and dental erosions are present.
Best practice advice 2: Consider a multidisciplinary approach to EER manifestations. Cases may require input from non-GI specialties. Tests performed by other specialists, such as bronchoscopy, thoracic imaging, or laryngoscopy, should be taken into account, since patients will also seek out multiple specialists to address their symptoms.
Best practice advice 3: There is no specific diagnostic test available to determine if GER is the cause of EER symptoms. Instead, physicians should interpret patient symptoms, response to GER therapy, and input from endoscopy and reflux tests.
Best practice advice 4: Rather than subject the patient to the cost and potential for even rare adverse events of a PPI trial, physicians should first consider conducting reflux testing. A PPI trial has clinical value but is insufficient on its own to help diagnose or manage EER. Initial single-dose PPI trial, titrating up to twice daily in those with typical GERD symptoms, is reasonable.
Best practice advice 5: The inconsistent therapeutic response to PPI therapy means that positive effects of PPI therapy on EER symptoms can’t confirm a GERD diagnosis because a placebo effect may be involved, and because symptom improvement can occur through mechanisms other than acid suppression. A meta-analysis found that a PPI trial has a sensitivity of 71%-78% and a specificity of 41%-54% with typical symptoms of heartburn and regurgitation. “Considering the greater variation expected with PPI response for extraesophageal symptoms, the diagnostic performance of empiric PPI trial for a diagnosis of EER would be anticipated to be substantially lower,” the authors wrote.
Best practice advice 6: When EER symptoms related to GERD are suspected and a PPI trial of up to 12 weeks does not lead to adequate improvement, the physician should consider testing for pathologic GER. Additional trials employing other PPIs are unlikely to succeed.
Best practice advice 7: Initial testing to evaluate for reflux should be tailored to patients’ clinical presentation. Potential methods to evaluate reflux include upper endoscopy and ambulatory reflux monitoring studies of acid suppressive therapy, which can assist with a GERD diagnosis, particularly when nonerosive reflux is present.
Best practice advice 8: About 50%-60% of patients with EER symptoms will not have GERD. Testing can be considered for those with an established objective diagnosis of GERD who do not respond well to high doses of acid suppression. Cost-effectiveness studies have confirmed the value of starting with ambulatory reflux monitoring, which can include a catheter-based pH sensor, pH impedance, or wireless pH capsule.
Ambulatory esophageal pH monitoring can also assist in making a GERD diagnosis, but it does not indicate whether GERD may be contributing to EER symptoms.
“Whichever the reflux testing modality, the strongest confidence for EER is achieved after ambulatory reflux testing showing pathologic acid exposure and a positive symptom-reflux association for EER symptoms,” the authors wrote. They also pointed out that ambulatory reflux monitoring in EER patients should be done in the absence of acid suppression unless there is already objective evidence for the presence of GERD.
Best practice advice 9: Aside from acid suppression, EER symptoms can also be managed through other means, including lifestyle modifications, such as eating avoidance prior to lying down, elevation of the head of the bed, sleeping on the left side, and weight loss. Or, alginate containing antacids, external upper esophageal sphincter compression device, cognitive behavioral therapy, and neuromodulators.
Best practice advice 10: In cases where the EER patient has objectively defined evidence of GERD, physicians should employ shared decision-making before considering anti-reflux surgery. If the patient did not respond to PPI therapy, this predicts a lack of response to antireflux surgery.
All four authors reported financial ties to multiple pharmaceutical companies.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Two phase 3 trials show benefits of dupilumab for prurigo nodularis
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
FROM NATURE MEDICINE
Bundled strategy increased preteen lipid screening
WASHINGTON – A bundled intervention combining point-of-care testing, electronic medical record support, and provider education significantly improved lipid screening rates in children aged 9-11 years, according to data from approximately 100 monthly visits over a 3-year period.
Guidelines from the National Heart, Lung, and Blood Institute currently recommend universal lipid screening for children aged 9-11 years, but screening rates in clinical practice remain low, according to Ruth E. Gardner, MD, of Penn State University, Hershey, and colleagues.
In a poster presented at the Pediatric Academic Societies annual meeting, Dr. Gardner and colleagues shared results of the implementation of a bundled testing protocol designed to improve screening.
The researchers reviewed data on lipid testing within 30 days for all 9- to 11-year-old well child visits at a single center between May 2019 and February 2022. The bundled intervention was introduced in May 2021.
The bundled protocol included in-office capillary testing and provider education. In addition, electronic medical record templates were modified to include prompts for lipid screening at relevant ages, and EMR orders were adjusted to include lipid testing. The researchers also collected targeted provider feedback on individualized screening rates in February 2022.
Screening rates were plotted monthly. For the period from May 2019 through May 2021, the rates averaged 6.5%. However, after the introduction of the bundled intervention, the rate increased to 29.9%. Following targeted provider feedback in February 2022, the researchers found an additional shift to 52.1% through March and April 2022.
The findings were limited by the use of data from a single center, and the researchers used an extended study period to account for disruptions to well-child care in the spring of 2020 related to the COVID-19 pandemic.
However, the results support the effectiveness of a bundled intervention for improving lipid screening rates in children aged 9-11 years, the researchers said, and targeted provider feedback and education could yield additional improvements, they concluded.
Preteen years are an optimal time for screening
“The current study is important because atherosclerosis begins in childhood, and screening at ages 9-11 is an optimal time to begin lifestyle changes to improve overall health and reduce risks of heart disease,” said Margaret Thew, DNP, FNP-BC, of the Medical College of Wisconsin, Milwaukee, in an interview.
Ms. Thew, who was not involved in the study, said, “The number of recommended and required screening items needed in pediatrics is vast, so many providers have to select which items to focus on for their health screenings with these ages.”
Overall, “I was impressed with the improvements that were made in this quality improvement study,” said Ms. Thew.
Barriers to lipid screening in this population include the reduced number of health screenings and immunizations recommended for this age group; the consequence is that access is limited to discuss preventive care opportunities, said Ms. Thew in an interview. Steps to overcome these barriers could include the use of many of the screening tools introduced in the current study, such as point-of-care testing in the office, use of the EMR to remind providers of testing, which can be done during well visits or school physicals, and educating providers about the current guidelines, she noted.
Other strategies to increase screening include moving the immunization series to provide more frequent appointments to children aged 9-11 years to offer education and preventive care, Ms. Thew added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
WASHINGTON – A bundled intervention combining point-of-care testing, electronic medical record support, and provider education significantly improved lipid screening rates in children aged 9-11 years, according to data from approximately 100 monthly visits over a 3-year period.
Guidelines from the National Heart, Lung, and Blood Institute currently recommend universal lipid screening for children aged 9-11 years, but screening rates in clinical practice remain low, according to Ruth E. Gardner, MD, of Penn State University, Hershey, and colleagues.
In a poster presented at the Pediatric Academic Societies annual meeting, Dr. Gardner and colleagues shared results of the implementation of a bundled testing protocol designed to improve screening.
The researchers reviewed data on lipid testing within 30 days for all 9- to 11-year-old well child visits at a single center between May 2019 and February 2022. The bundled intervention was introduced in May 2021.
The bundled protocol included in-office capillary testing and provider education. In addition, electronic medical record templates were modified to include prompts for lipid screening at relevant ages, and EMR orders were adjusted to include lipid testing. The researchers also collected targeted provider feedback on individualized screening rates in February 2022.
Screening rates were plotted monthly. For the period from May 2019 through May 2021, the rates averaged 6.5%. However, after the introduction of the bundled intervention, the rate increased to 29.9%. Following targeted provider feedback in February 2022, the researchers found an additional shift to 52.1% through March and April 2022.
The findings were limited by the use of data from a single center, and the researchers used an extended study period to account for disruptions to well-child care in the spring of 2020 related to the COVID-19 pandemic.
However, the results support the effectiveness of a bundled intervention for improving lipid screening rates in children aged 9-11 years, the researchers said, and targeted provider feedback and education could yield additional improvements, they concluded.
Preteen years are an optimal time for screening
“The current study is important because atherosclerosis begins in childhood, and screening at ages 9-11 is an optimal time to begin lifestyle changes to improve overall health and reduce risks of heart disease,” said Margaret Thew, DNP, FNP-BC, of the Medical College of Wisconsin, Milwaukee, in an interview.
Ms. Thew, who was not involved in the study, said, “The number of recommended and required screening items needed in pediatrics is vast, so many providers have to select which items to focus on for their health screenings with these ages.”
Overall, “I was impressed with the improvements that were made in this quality improvement study,” said Ms. Thew.
Barriers to lipid screening in this population include the reduced number of health screenings and immunizations recommended for this age group; the consequence is that access is limited to discuss preventive care opportunities, said Ms. Thew in an interview. Steps to overcome these barriers could include the use of many of the screening tools introduced in the current study, such as point-of-care testing in the office, use of the EMR to remind providers of testing, which can be done during well visits or school physicals, and educating providers about the current guidelines, she noted.
Other strategies to increase screening include moving the immunization series to provide more frequent appointments to children aged 9-11 years to offer education and preventive care, Ms. Thew added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
WASHINGTON – A bundled intervention combining point-of-care testing, electronic medical record support, and provider education significantly improved lipid screening rates in children aged 9-11 years, according to data from approximately 100 monthly visits over a 3-year period.
Guidelines from the National Heart, Lung, and Blood Institute currently recommend universal lipid screening for children aged 9-11 years, but screening rates in clinical practice remain low, according to Ruth E. Gardner, MD, of Penn State University, Hershey, and colleagues.
In a poster presented at the Pediatric Academic Societies annual meeting, Dr. Gardner and colleagues shared results of the implementation of a bundled testing protocol designed to improve screening.
The researchers reviewed data on lipid testing within 30 days for all 9- to 11-year-old well child visits at a single center between May 2019 and February 2022. The bundled intervention was introduced in May 2021.
The bundled protocol included in-office capillary testing and provider education. In addition, electronic medical record templates were modified to include prompts for lipid screening at relevant ages, and EMR orders were adjusted to include lipid testing. The researchers also collected targeted provider feedback on individualized screening rates in February 2022.
Screening rates were plotted monthly. For the period from May 2019 through May 2021, the rates averaged 6.5%. However, after the introduction of the bundled intervention, the rate increased to 29.9%. Following targeted provider feedback in February 2022, the researchers found an additional shift to 52.1% through March and April 2022.
The findings were limited by the use of data from a single center, and the researchers used an extended study period to account for disruptions to well-child care in the spring of 2020 related to the COVID-19 pandemic.
However, the results support the effectiveness of a bundled intervention for improving lipid screening rates in children aged 9-11 years, the researchers said, and targeted provider feedback and education could yield additional improvements, they concluded.
Preteen years are an optimal time for screening
“The current study is important because atherosclerosis begins in childhood, and screening at ages 9-11 is an optimal time to begin lifestyle changes to improve overall health and reduce risks of heart disease,” said Margaret Thew, DNP, FNP-BC, of the Medical College of Wisconsin, Milwaukee, in an interview.
Ms. Thew, who was not involved in the study, said, “The number of recommended and required screening items needed in pediatrics is vast, so many providers have to select which items to focus on for their health screenings with these ages.”
Overall, “I was impressed with the improvements that were made in this quality improvement study,” said Ms. Thew.
Barriers to lipid screening in this population include the reduced number of health screenings and immunizations recommended for this age group; the consequence is that access is limited to discuss preventive care opportunities, said Ms. Thew in an interview. Steps to overcome these barriers could include the use of many of the screening tools introduced in the current study, such as point-of-care testing in the office, use of the EMR to remind providers of testing, which can be done during well visits or school physicals, and educating providers about the current guidelines, she noted.
Other strategies to increase screening include moving the immunization series to provide more frequent appointments to children aged 9-11 years to offer education and preventive care, Ms. Thew added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
FROM PAS 2023





