User login
Evidence-Based Medicine and the Hospitalist
The terms “hospital medicine” and “evidence-based medicine” (EBM) are both recent arrivals in the history of medicine. Both have spread through medicine at a rapid pace, highlighting the attraction and fundamental soundness of their core ideas. Much has been written about the benefits of the hospitalist movement regarding quality, patient throughput and financial indicators. The next phase in the revolution of patient care is the confluence of technology, EBM, and hospital medicine. One of the pillars for the continued success of the hospital medicine movement will be EBM. EBM must become an integral part of the skill set for all hospitalists.
EBM is an analytical approach with a fundamental knowledge base and a set of tools. The exponential growth of clinical information requires that physicians use an analytical approach for answering clinical questions and keeping up-to-date. This may be easier if you work at an academic center rather than a non-teaching hospital, although this is not guaranteed. Regardless of the working environment, an analytical approach will be needed if we are to build on initial success and unrealized potential to improve quality and patient safety.
The term “EBM” was introduced by a group of clinician researchers and educators at McMaster University during the early 1990s. It was initially defined as “a systemic approach to analyzed published research as the basis of clinical decision making.” Subsequently, as the EBM movement matured as a discipline, the early proponents and developers provided a more complete definition: “Evidence based medicine is the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients. The practice of evidence-based medicine means integrating individual clinical expertise with the best available external clinical evidence from systematic research (1).”
Of course, the concept of practicing medicine based on the scientific method has been around for years. The days of bloodletting with leeches are behind us, but rigorous scientific evaluation of medicine reached critical mass only in the last century. The first double-blind randomized controlled trial (RCT) was conducted in 1931; the study tested the use of sanocrysin for treatment of tuberculosis (2). Since then, there has been an exponential growth in clinical trials. This information explosion requires new approaches to integrating the ever-increasing knowledge with patient care; the concurrent revolution in information technology provides opportunities limited only by our own imaginations.
EBM is not without its critics: “It is cookbook medicine,” “It focuses on cost efficiency,” “I can’t find an RCT that fits my patient,” and “It doesn’t take into account the clinician’s experience” are often argued points. If EBM is not used effectively, all the criticisms are appropriate. Sacket, one of the earliest proponents and likely EBM’s most eloquent champion, refuted the claim that EBM is “cookbook medicine.” He argued that EBM requires a bottom-up approach that integrates the best external evidence with individual clinical expertise and patients’ choice. External clinical evidence informs, but does not replace, individual clinical expertise. The physician must decide whether the external evidence applies to the individual patient at all and, if so, how it should be integrated into a clinical decision (1).
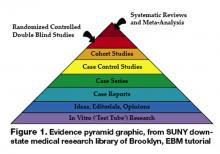
Neither is EBM strictly about RCTs and meta-analysis. It’s about tracking down the best available evidence for your question and, thus, your patient. Sometimes a cohort study is best when you want to find the prognosis of a certain illness. Similarly, a cross-sectional study may be most appropriate when you’re trying to determine the sensitivity and specificity of a test. If a disease once thought universally fatal is proven otherwise in a case report, then a randomized control trial is hardly necessary. Finally, an RCT or meta-analysis is not always going to be available for the disease process you are dealing with, but EBM gives us the skill set to look down the evidence pyramid and find the next best thing (Figure 1).
It is also clear that EBM is not about cost cutting, although many hospital medicine programs were started with this as the primary goal, given the current healthcare environment. Certainly fears exist that EBM is being used by healthcare managers, organizations, and administrators as a cost-efficiency tool. It may be that good evidence is cost-efficient in certain situations, while in others it may require the healthcare system to invest more in itself if available evidence supports doing so. Thus it is imperative that hospitalists accept the challenge of incorporating EBM into their daily practice and become leaders in its application, with patient safety and quality of care as primary goals. If we don’t, others will define the role of EBM for us, with a potential for poor outcomes for the patient and the profession.
The EBM skill set and its tools are being continuously refined, with the evidence pyramid as one of the most basic principles (3). This evidence pyramid is a model for grading the evidence. It puts in perspective the different grades of evidence or study designs. For example, a systematic review of randomized controlled trials that show consistent results provides the highest quality evidence and is ranked accordingly in the pyramid. In contrast, a case report or case series of a treatment would be ranked much lower.
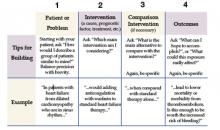
The first step in incorporating EBM into one’s daily practice requires an understanding of its analytical approach and access to the necessary tools. The process begins with asking a question that is answerable. A well-built clinical question is one that benefits the patient and clinician. Such questions are directly relevant to patient problems and phrased in ways that direct your search to relevant and precise answers.
In forming the question the following process, referred to as the PICO method, is helpful (4).
With the question formed, consider what type of question you have. This is often referred to as the typology of the question.
- Clinical Findings: Gathering and interpreting findings from the history, clinical examination, and test results.
- Etiology: Identifying causes for disease.
- Differential Diagnosis: Ranking by likelihood, seriousness, and treatability of the patients problem.
- Prognosis: Figuring out how to estimate the likely clinical course and complications over time of the disease
- Therapy: Selecting treatments to offer that do more good than harm and that are worth the effort and cost of using them.
- Prevention: Reducing the chance of disease by identifying and modifying risk factors and how to diagnose disease early by screening.
- Self-improvement: Keeping up-to-date, improve your clinical skills, and run a better, more efficient clinical practice.
The types of questions can next be matched to the type of research that may provide the answer:
- Diagnosis: prospective cohort study with good quality validation against “gold standard.”
- Prognosis: prospective cohort study.
- Therapy or prevention: prospective, randomized controlled clinical trial (RCT).
- Harm/Etiology: RCT, cohort or case-control study (probably retrospective).
- Economic: analysis of sensible costs against evidence-based outcome.
Once the question has been formed, the following steps lie ahead: finding the evidence, critically appraising the evidence, acting on the evidence, and, finally, evaluating one’s performance. Very much like the formation of the question, each of the subsequent steps involves an analytical approach that can be mastered. Technology—particularly personal computers, the Internet and PDAs—has made the task of mastering EBM easier in many ways. The additional steps in using EBM effectively will be addressed in future articles. A list of useful links is provided below.
http://library.downstate.edu/EBM2/contents.htm
http://healthsystem.virginia.edu/internet/library/collections/ebm/index.cfm
Dr. Kathuria may be reached at [email protected].
Endnotes
- Guyatt GH, Haynes RB, Jaeschke RZ, et al. Users’ guides to the medical literature: XXV. Evidence-based medicine: principles for applying the users’ guides to patient care. Evidence-Based Medicine Working Group. JAMA. 2000;284:1290-6.
- Claridge, J, Fabian, T. History and Development of Evidence Based Medicine. World Journal of Surgery 2005
- Guyatt GH, Haynes RB, et. al. Users’ guides to the medical literature: XXV. Evidence-Based Medicine: Principles for Applying the Users’ Guides to Patient Care. 2000;284:1290-1296
- Sackett DL, Richardson WS, Rosenberg W, Haynes RB (1997). Evidence-based medicine: How to practice and teach EBM. New York: Churchill Livingston
The terms “hospital medicine” and “evidence-based medicine” (EBM) are both recent arrivals in the history of medicine. Both have spread through medicine at a rapid pace, highlighting the attraction and fundamental soundness of their core ideas. Much has been written about the benefits of the hospitalist movement regarding quality, patient throughput and financial indicators. The next phase in the revolution of patient care is the confluence of technology, EBM, and hospital medicine. One of the pillars for the continued success of the hospital medicine movement will be EBM. EBM must become an integral part of the skill set for all hospitalists.
EBM is an analytical approach with a fundamental knowledge base and a set of tools. The exponential growth of clinical information requires that physicians use an analytical approach for answering clinical questions and keeping up-to-date. This may be easier if you work at an academic center rather than a non-teaching hospital, although this is not guaranteed. Regardless of the working environment, an analytical approach will be needed if we are to build on initial success and unrealized potential to improve quality and patient safety.
The term “EBM” was introduced by a group of clinician researchers and educators at McMaster University during the early 1990s. It was initially defined as “a systemic approach to analyzed published research as the basis of clinical decision making.” Subsequently, as the EBM movement matured as a discipline, the early proponents and developers provided a more complete definition: “Evidence based medicine is the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients. The practice of evidence-based medicine means integrating individual clinical expertise with the best available external clinical evidence from systematic research (1).”
Of course, the concept of practicing medicine based on the scientific method has been around for years. The days of bloodletting with leeches are behind us, but rigorous scientific evaluation of medicine reached critical mass only in the last century. The first double-blind randomized controlled trial (RCT) was conducted in 1931; the study tested the use of sanocrysin for treatment of tuberculosis (2). Since then, there has been an exponential growth in clinical trials. This information explosion requires new approaches to integrating the ever-increasing knowledge with patient care; the concurrent revolution in information technology provides opportunities limited only by our own imaginations.
EBM is not without its critics: “It is cookbook medicine,” “It focuses on cost efficiency,” “I can’t find an RCT that fits my patient,” and “It doesn’t take into account the clinician’s experience” are often argued points. If EBM is not used effectively, all the criticisms are appropriate. Sacket, one of the earliest proponents and likely EBM’s most eloquent champion, refuted the claim that EBM is “cookbook medicine.” He argued that EBM requires a bottom-up approach that integrates the best external evidence with individual clinical expertise and patients’ choice. External clinical evidence informs, but does not replace, individual clinical expertise. The physician must decide whether the external evidence applies to the individual patient at all and, if so, how it should be integrated into a clinical decision (1).
Neither is EBM strictly about RCTs and meta-analysis. It’s about tracking down the best available evidence for your question and, thus, your patient. Sometimes a cohort study is best when you want to find the prognosis of a certain illness. Similarly, a cross-sectional study may be most appropriate when you’re trying to determine the sensitivity and specificity of a test. If a disease once thought universally fatal is proven otherwise in a case report, then a randomized control trial is hardly necessary. Finally, an RCT or meta-analysis is not always going to be available for the disease process you are dealing with, but EBM gives us the skill set to look down the evidence pyramid and find the next best thing (Figure 1).
It is also clear that EBM is not about cost cutting, although many hospital medicine programs were started with this as the primary goal, given the current healthcare environment. Certainly fears exist that EBM is being used by healthcare managers, organizations, and administrators as a cost-efficiency tool. It may be that good evidence is cost-efficient in certain situations, while in others it may require the healthcare system to invest more in itself if available evidence supports doing so. Thus it is imperative that hospitalists accept the challenge of incorporating EBM into their daily practice and become leaders in its application, with patient safety and quality of care as primary goals. If we don’t, others will define the role of EBM for us, with a potential for poor outcomes for the patient and the profession.
The EBM skill set and its tools are being continuously refined, with the evidence pyramid as one of the most basic principles (3). This evidence pyramid is a model for grading the evidence. It puts in perspective the different grades of evidence or study designs. For example, a systematic review of randomized controlled trials that show consistent results provides the highest quality evidence and is ranked accordingly in the pyramid. In contrast, a case report or case series of a treatment would be ranked much lower.
The first step in incorporating EBM into one’s daily practice requires an understanding of its analytical approach and access to the necessary tools. The process begins with asking a question that is answerable. A well-built clinical question is one that benefits the patient and clinician. Such questions are directly relevant to patient problems and phrased in ways that direct your search to relevant and precise answers.
In forming the question the following process, referred to as the PICO method, is helpful (4).
With the question formed, consider what type of question you have. This is often referred to as the typology of the question.
- Clinical Findings: Gathering and interpreting findings from the history, clinical examination, and test results.
- Etiology: Identifying causes for disease.
- Differential Diagnosis: Ranking by likelihood, seriousness, and treatability of the patients problem.
- Prognosis: Figuring out how to estimate the likely clinical course and complications over time of the disease
- Therapy: Selecting treatments to offer that do more good than harm and that are worth the effort and cost of using them.
- Prevention: Reducing the chance of disease by identifying and modifying risk factors and how to diagnose disease early by screening.
- Self-improvement: Keeping up-to-date, improve your clinical skills, and run a better, more efficient clinical practice.
The types of questions can next be matched to the type of research that may provide the answer:
- Diagnosis: prospective cohort study with good quality validation against “gold standard.”
- Prognosis: prospective cohort study.
- Therapy or prevention: prospective, randomized controlled clinical trial (RCT).
- Harm/Etiology: RCT, cohort or case-control study (probably retrospective).
- Economic: analysis of sensible costs against evidence-based outcome.
Once the question has been formed, the following steps lie ahead: finding the evidence, critically appraising the evidence, acting on the evidence, and, finally, evaluating one’s performance. Very much like the formation of the question, each of the subsequent steps involves an analytical approach that can be mastered. Technology—particularly personal computers, the Internet and PDAs—has made the task of mastering EBM easier in many ways. The additional steps in using EBM effectively will be addressed in future articles. A list of useful links is provided below.
http://library.downstate.edu/EBM2/contents.htm
http://healthsystem.virginia.edu/internet/library/collections/ebm/index.cfm
Dr. Kathuria may be reached at [email protected].
Endnotes
- Guyatt GH, Haynes RB, Jaeschke RZ, et al. Users’ guides to the medical literature: XXV. Evidence-based medicine: principles for applying the users’ guides to patient care. Evidence-Based Medicine Working Group. JAMA. 2000;284:1290-6.
- Claridge, J, Fabian, T. History and Development of Evidence Based Medicine. World Journal of Surgery 2005
- Guyatt GH, Haynes RB, et. al. Users’ guides to the medical literature: XXV. Evidence-Based Medicine: Principles for Applying the Users’ Guides to Patient Care. 2000;284:1290-1296
- Sackett DL, Richardson WS, Rosenberg W, Haynes RB (1997). Evidence-based medicine: How to practice and teach EBM. New York: Churchill Livingston
The terms “hospital medicine” and “evidence-based medicine” (EBM) are both recent arrivals in the history of medicine. Both have spread through medicine at a rapid pace, highlighting the attraction and fundamental soundness of their core ideas. Much has been written about the benefits of the hospitalist movement regarding quality, patient throughput and financial indicators. The next phase in the revolution of patient care is the confluence of technology, EBM, and hospital medicine. One of the pillars for the continued success of the hospital medicine movement will be EBM. EBM must become an integral part of the skill set for all hospitalists.
EBM is an analytical approach with a fundamental knowledge base and a set of tools. The exponential growth of clinical information requires that physicians use an analytical approach for answering clinical questions and keeping up-to-date. This may be easier if you work at an academic center rather than a non-teaching hospital, although this is not guaranteed. Regardless of the working environment, an analytical approach will be needed if we are to build on initial success and unrealized potential to improve quality and patient safety.
The term “EBM” was introduced by a group of clinician researchers and educators at McMaster University during the early 1990s. It was initially defined as “a systemic approach to analyzed published research as the basis of clinical decision making.” Subsequently, as the EBM movement matured as a discipline, the early proponents and developers provided a more complete definition: “Evidence based medicine is the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients. The practice of evidence-based medicine means integrating individual clinical expertise with the best available external clinical evidence from systematic research (1).”
Of course, the concept of practicing medicine based on the scientific method has been around for years. The days of bloodletting with leeches are behind us, but rigorous scientific evaluation of medicine reached critical mass only in the last century. The first double-blind randomized controlled trial (RCT) was conducted in 1931; the study tested the use of sanocrysin for treatment of tuberculosis (2). Since then, there has been an exponential growth in clinical trials. This information explosion requires new approaches to integrating the ever-increasing knowledge with patient care; the concurrent revolution in information technology provides opportunities limited only by our own imaginations.
EBM is not without its critics: “It is cookbook medicine,” “It focuses on cost efficiency,” “I can’t find an RCT that fits my patient,” and “It doesn’t take into account the clinician’s experience” are often argued points. If EBM is not used effectively, all the criticisms are appropriate. Sacket, one of the earliest proponents and likely EBM’s most eloquent champion, refuted the claim that EBM is “cookbook medicine.” He argued that EBM requires a bottom-up approach that integrates the best external evidence with individual clinical expertise and patients’ choice. External clinical evidence informs, but does not replace, individual clinical expertise. The physician must decide whether the external evidence applies to the individual patient at all and, if so, how it should be integrated into a clinical decision (1).
Neither is EBM strictly about RCTs and meta-analysis. It’s about tracking down the best available evidence for your question and, thus, your patient. Sometimes a cohort study is best when you want to find the prognosis of a certain illness. Similarly, a cross-sectional study may be most appropriate when you’re trying to determine the sensitivity and specificity of a test. If a disease once thought universally fatal is proven otherwise in a case report, then a randomized control trial is hardly necessary. Finally, an RCT or meta-analysis is not always going to be available for the disease process you are dealing with, but EBM gives us the skill set to look down the evidence pyramid and find the next best thing (Figure 1).
It is also clear that EBM is not about cost cutting, although many hospital medicine programs were started with this as the primary goal, given the current healthcare environment. Certainly fears exist that EBM is being used by healthcare managers, organizations, and administrators as a cost-efficiency tool. It may be that good evidence is cost-efficient in certain situations, while in others it may require the healthcare system to invest more in itself if available evidence supports doing so. Thus it is imperative that hospitalists accept the challenge of incorporating EBM into their daily practice and become leaders in its application, with patient safety and quality of care as primary goals. If we don’t, others will define the role of EBM for us, with a potential for poor outcomes for the patient and the profession.
The EBM skill set and its tools are being continuously refined, with the evidence pyramid as one of the most basic principles (3). This evidence pyramid is a model for grading the evidence. It puts in perspective the different grades of evidence or study designs. For example, a systematic review of randomized controlled trials that show consistent results provides the highest quality evidence and is ranked accordingly in the pyramid. In contrast, a case report or case series of a treatment would be ranked much lower.
The first step in incorporating EBM into one’s daily practice requires an understanding of its analytical approach and access to the necessary tools. The process begins with asking a question that is answerable. A well-built clinical question is one that benefits the patient and clinician. Such questions are directly relevant to patient problems and phrased in ways that direct your search to relevant and precise answers.
In forming the question the following process, referred to as the PICO method, is helpful (4).
With the question formed, consider what type of question you have. This is often referred to as the typology of the question.
- Clinical Findings: Gathering and interpreting findings from the history, clinical examination, and test results.
- Etiology: Identifying causes for disease.
- Differential Diagnosis: Ranking by likelihood, seriousness, and treatability of the patients problem.
- Prognosis: Figuring out how to estimate the likely clinical course and complications over time of the disease
- Therapy: Selecting treatments to offer that do more good than harm and that are worth the effort and cost of using them.
- Prevention: Reducing the chance of disease by identifying and modifying risk factors and how to diagnose disease early by screening.
- Self-improvement: Keeping up-to-date, improve your clinical skills, and run a better, more efficient clinical practice.
The types of questions can next be matched to the type of research that may provide the answer:
- Diagnosis: prospective cohort study with good quality validation against “gold standard.”
- Prognosis: prospective cohort study.
- Therapy or prevention: prospective, randomized controlled clinical trial (RCT).
- Harm/Etiology: RCT, cohort or case-control study (probably retrospective).
- Economic: analysis of sensible costs against evidence-based outcome.
Once the question has been formed, the following steps lie ahead: finding the evidence, critically appraising the evidence, acting on the evidence, and, finally, evaluating one’s performance. Very much like the formation of the question, each of the subsequent steps involves an analytical approach that can be mastered. Technology—particularly personal computers, the Internet and PDAs—has made the task of mastering EBM easier in many ways. The additional steps in using EBM effectively will be addressed in future articles. A list of useful links is provided below.
http://library.downstate.edu/EBM2/contents.htm
http://healthsystem.virginia.edu/internet/library/collections/ebm/index.cfm
Dr. Kathuria may be reached at [email protected].
Endnotes
- Guyatt GH, Haynes RB, Jaeschke RZ, et al. Users’ guides to the medical literature: XXV. Evidence-based medicine: principles for applying the users’ guides to patient care. Evidence-Based Medicine Working Group. JAMA. 2000;284:1290-6.
- Claridge, J, Fabian, T. History and Development of Evidence Based Medicine. World Journal of Surgery 2005
- Guyatt GH, Haynes RB, et. al. Users’ guides to the medical literature: XXV. Evidence-Based Medicine: Principles for Applying the Users’ Guides to Patient Care. 2000;284:1290-1296
- Sackett DL, Richardson WS, Rosenberg W, Haynes RB (1997). Evidence-based medicine: How to practice and teach EBM. New York: Churchill Livingston
What Is a Laborist?
One of the interesting things about hospital medicine is our diversity. It is an evolutionary construct and can be both a strength and a concern as we all try to create and define our new specialty.
Most hospitalists are trained as general internists. This wasn’t always so. As recently as 1997 almost 50% of hospitalists were internal medical subspecialists. The thinking at that time was that because infectious disease docs, pulmonologists, intensivists, and others were already in the hospital seeing ill patients, why couldn’t they just also be hospitalists?
Well, it turned out they wanted to be infectious disease specialists and pulmonologists and they soon found out that being a hospitalist is somewhat different than these other specialties.
Now hospital medicine is a popular career path for those finishing a general internal medicine residency, as well as for those who are finding a career as a hospitalist preferable to their original choice as in traditional internal medicine. (See “Trendwatch: The Specialization of Hospital Medicine,” p. 27.)
PEDIATRICIANS AND FAMILY PRACTICE
At the same time, even though only 3% of hospitalists are family practitioners, more than 90% of hospitalists in Canada come out of family practice training. Increasingly, young graduates of family practice residency programs are choosing to become hospitalists.
And let’s not forget the pediatricians. Pediatricians comprise about 9% of all hospitalists, and more than 200 pediatric hospitalists got together for the largest pediatric hospital medicine meeting ever in Denver at the end of July. (See the “Pediatric Special Section,” p. 33.) It was an impressive community of pediatric hospitalists. Most children’s hospitals and many community hospitals now have pediatric hospital medicine groups. Most of the pediatric inpatient care in this country is now provided by hospitalists and pediatric subspecialists.
In fact, those who have taken training in med-peds are finding that a career as a hospitalist is a nice fit, and they are welcomed by those who care for children and adults in the hospital.
But hospital medicine is not only about physician caregivers. More than 5% of hospitalists in this country are nonphysician providers, either nurse practitioners or physician assistants. As the demand for hospitalists rapidly increases many hospital medicine groups find that adding nurse practitioners or physician assistants helps them to complete the workforce they need to have in place to meet their clinical and administrative demands.
And hospital medicine includes pharmacists, case managers, and administrators to round out the inpatient team. Each of these professions is developing “hospital medicine specialists” looking for skills and experiences to allow them to help facilitate the work of the hospitalists and to use a team approach to achieve the rapidly expanded expectations of hospital medicine groups.
EDUCATION AND CERTIFICATION ISSUES
This growing conglomeration of healthcare professionals in one specialty presents unique issues. Some of these come in the form of diverse and expanding educational needs. The patient wants to be assured that no matter where the individual hospitalist started his or her training, the hospitalist will bring to the bedside the appropriate skills for their acute medical problems. This leads to having SHM develop courses in critical care skills, perioperative medicine, leadership, and the like.
Yet even though the endpoint may need to be similar for all hospitalists, it takes a fine touch and significant customization to craft educational materials when many hospitalists may start from a different base point.
When it comes to potential credentialing in hospital medicine, there is not a clear path to create a certification in hospital medicine. The solution for the 80% of hospitalists trained in internal medicine may very well be through the American Board of Internal Medicine (ABIM), but the pediatric and family practice solution will need to involve the American Board of Pediatrics (ABP) and the American Board of Family Medicine (ABFP), respectively. And this doesn’t even begin to address the credentialing needs of the nonphysicians.
Further, as SHM looks to represent all of the diverse elements that form the fabric of hospital medicine, we need to be in touch with the American College of Physicians, the American Academy of Pediatrics, the American Academy of Family Physicians, the Society of General Internal Medicine, the Ambulatory Pediatric Association, the American Association of Critical Care Nurses, the American Society of Health System Pharmacists, the American
Academy of Physicians Assistants, the American Academy of Nurse Practitioners, and many other important substantial medical professional societies; members of each believe that their organization relates to a segment of hospital medicine.
DIVERSITY CREATES NEW SOLUTIONS
The good part of this diversity is that as SHM helps to build this new specialty we are able to include so many unique vantage points. This strategy of inclusion allows for new ideas to percolate to the surface and leads to innovation and creativity. In fashioning the hospital of the future, “old think” must not rule the day. One way to change the outcome is to change those who are at the table.
If hospital medicine is to be part of the process of creating a hospital that is patient-centered, relies on measurable quality improvement, and delivers care by teams of healthcare professionals, then we need to open the tent and let in different perspectives. In SHM’s ongoing quality improvement efforts in heart failure and glycemic control, this is our approach—with meaningful input from hospitalists, subspecialists, nurses, pharmacists, and many other stakeholders in hospital medicine. This will lead to a different and—let’s hope—better outcome.
LABORISTS AND SURGICALISTS
And there are more wrinkles in the hospitalist world all the time. Recently USA Today wrote a story about “laborists” as hospitals try to solve access to obstetrical services by having contracted laborists on site 24/7. Some hospitals have to be creative when their community surgeons aren’t available for trauma care and some hospitals have contracted with orthopedists and general surgeons as “surgicalists.”
Are these the latest additions to the roll call of hospital medicine or just a footnote or an asterisk? Time and the marketplace will tell.
Besides the basic training for hospitalists there are many variations determined by site of practice and employment model. Whether we are talking about the differences between academic
hospital medicine or that practiced in community hospitals, or the uniqueness of a small group of hospitalists at only one hospital or a large multistate group of hospitalists in 10 states with 400 hospitalists, we are all part of hospital medicine.
In the end, hospital medicine is defined more by its common goals and its common values regardless of initial training or mode of practice. At this time in healthcare, many are looking for healthcare professionals to have the skills and the energy to create the hospital
of the future that will be a better place to work and to get the best care. SHM is committed to harnessing the diversity of our specialty to do our part to create a better future. With your help, we can get there. TH
Dr. Wellikson has been the CEO of SHM since 2000.
One of the interesting things about hospital medicine is our diversity. It is an evolutionary construct and can be both a strength and a concern as we all try to create and define our new specialty.
Most hospitalists are trained as general internists. This wasn’t always so. As recently as 1997 almost 50% of hospitalists were internal medical subspecialists. The thinking at that time was that because infectious disease docs, pulmonologists, intensivists, and others were already in the hospital seeing ill patients, why couldn’t they just also be hospitalists?
Well, it turned out they wanted to be infectious disease specialists and pulmonologists and they soon found out that being a hospitalist is somewhat different than these other specialties.
Now hospital medicine is a popular career path for those finishing a general internal medicine residency, as well as for those who are finding a career as a hospitalist preferable to their original choice as in traditional internal medicine. (See “Trendwatch: The Specialization of Hospital Medicine,” p. 27.)
PEDIATRICIANS AND FAMILY PRACTICE
At the same time, even though only 3% of hospitalists are family practitioners, more than 90% of hospitalists in Canada come out of family practice training. Increasingly, young graduates of family practice residency programs are choosing to become hospitalists.
And let’s not forget the pediatricians. Pediatricians comprise about 9% of all hospitalists, and more than 200 pediatric hospitalists got together for the largest pediatric hospital medicine meeting ever in Denver at the end of July. (See the “Pediatric Special Section,” p. 33.) It was an impressive community of pediatric hospitalists. Most children’s hospitals and many community hospitals now have pediatric hospital medicine groups. Most of the pediatric inpatient care in this country is now provided by hospitalists and pediatric subspecialists.
In fact, those who have taken training in med-peds are finding that a career as a hospitalist is a nice fit, and they are welcomed by those who care for children and adults in the hospital.
But hospital medicine is not only about physician caregivers. More than 5% of hospitalists in this country are nonphysician providers, either nurse practitioners or physician assistants. As the demand for hospitalists rapidly increases many hospital medicine groups find that adding nurse practitioners or physician assistants helps them to complete the workforce they need to have in place to meet their clinical and administrative demands.
And hospital medicine includes pharmacists, case managers, and administrators to round out the inpatient team. Each of these professions is developing “hospital medicine specialists” looking for skills and experiences to allow them to help facilitate the work of the hospitalists and to use a team approach to achieve the rapidly expanded expectations of hospital medicine groups.
EDUCATION AND CERTIFICATION ISSUES
This growing conglomeration of healthcare professionals in one specialty presents unique issues. Some of these come in the form of diverse and expanding educational needs. The patient wants to be assured that no matter where the individual hospitalist started his or her training, the hospitalist will bring to the bedside the appropriate skills for their acute medical problems. This leads to having SHM develop courses in critical care skills, perioperative medicine, leadership, and the like.
Yet even though the endpoint may need to be similar for all hospitalists, it takes a fine touch and significant customization to craft educational materials when many hospitalists may start from a different base point.
When it comes to potential credentialing in hospital medicine, there is not a clear path to create a certification in hospital medicine. The solution for the 80% of hospitalists trained in internal medicine may very well be through the American Board of Internal Medicine (ABIM), but the pediatric and family practice solution will need to involve the American Board of Pediatrics (ABP) and the American Board of Family Medicine (ABFP), respectively. And this doesn’t even begin to address the credentialing needs of the nonphysicians.
Further, as SHM looks to represent all of the diverse elements that form the fabric of hospital medicine, we need to be in touch with the American College of Physicians, the American Academy of Pediatrics, the American Academy of Family Physicians, the Society of General Internal Medicine, the Ambulatory Pediatric Association, the American Association of Critical Care Nurses, the American Society of Health System Pharmacists, the American
Academy of Physicians Assistants, the American Academy of Nurse Practitioners, and many other important substantial medical professional societies; members of each believe that their organization relates to a segment of hospital medicine.
DIVERSITY CREATES NEW SOLUTIONS
The good part of this diversity is that as SHM helps to build this new specialty we are able to include so many unique vantage points. This strategy of inclusion allows for new ideas to percolate to the surface and leads to innovation and creativity. In fashioning the hospital of the future, “old think” must not rule the day. One way to change the outcome is to change those who are at the table.
If hospital medicine is to be part of the process of creating a hospital that is patient-centered, relies on measurable quality improvement, and delivers care by teams of healthcare professionals, then we need to open the tent and let in different perspectives. In SHM’s ongoing quality improvement efforts in heart failure and glycemic control, this is our approach—with meaningful input from hospitalists, subspecialists, nurses, pharmacists, and many other stakeholders in hospital medicine. This will lead to a different and—let’s hope—better outcome.
LABORISTS AND SURGICALISTS
And there are more wrinkles in the hospitalist world all the time. Recently USA Today wrote a story about “laborists” as hospitals try to solve access to obstetrical services by having contracted laborists on site 24/7. Some hospitals have to be creative when their community surgeons aren’t available for trauma care and some hospitals have contracted with orthopedists and general surgeons as “surgicalists.”
Are these the latest additions to the roll call of hospital medicine or just a footnote or an asterisk? Time and the marketplace will tell.
Besides the basic training for hospitalists there are many variations determined by site of practice and employment model. Whether we are talking about the differences between academic
hospital medicine or that practiced in community hospitals, or the uniqueness of a small group of hospitalists at only one hospital or a large multistate group of hospitalists in 10 states with 400 hospitalists, we are all part of hospital medicine.
In the end, hospital medicine is defined more by its common goals and its common values regardless of initial training or mode of practice. At this time in healthcare, many are looking for healthcare professionals to have the skills and the energy to create the hospital
of the future that will be a better place to work and to get the best care. SHM is committed to harnessing the diversity of our specialty to do our part to create a better future. With your help, we can get there. TH
Dr. Wellikson has been the CEO of SHM since 2000.
One of the interesting things about hospital medicine is our diversity. It is an evolutionary construct and can be both a strength and a concern as we all try to create and define our new specialty.
Most hospitalists are trained as general internists. This wasn’t always so. As recently as 1997 almost 50% of hospitalists were internal medical subspecialists. The thinking at that time was that because infectious disease docs, pulmonologists, intensivists, and others were already in the hospital seeing ill patients, why couldn’t they just also be hospitalists?
Well, it turned out they wanted to be infectious disease specialists and pulmonologists and they soon found out that being a hospitalist is somewhat different than these other specialties.
Now hospital medicine is a popular career path for those finishing a general internal medicine residency, as well as for those who are finding a career as a hospitalist preferable to their original choice as in traditional internal medicine. (See “Trendwatch: The Specialization of Hospital Medicine,” p. 27.)
PEDIATRICIANS AND FAMILY PRACTICE
At the same time, even though only 3% of hospitalists are family practitioners, more than 90% of hospitalists in Canada come out of family practice training. Increasingly, young graduates of family practice residency programs are choosing to become hospitalists.
And let’s not forget the pediatricians. Pediatricians comprise about 9% of all hospitalists, and more than 200 pediatric hospitalists got together for the largest pediatric hospital medicine meeting ever in Denver at the end of July. (See the “Pediatric Special Section,” p. 33.) It was an impressive community of pediatric hospitalists. Most children’s hospitals and many community hospitals now have pediatric hospital medicine groups. Most of the pediatric inpatient care in this country is now provided by hospitalists and pediatric subspecialists.
In fact, those who have taken training in med-peds are finding that a career as a hospitalist is a nice fit, and they are welcomed by those who care for children and adults in the hospital.
But hospital medicine is not only about physician caregivers. More than 5% of hospitalists in this country are nonphysician providers, either nurse practitioners or physician assistants. As the demand for hospitalists rapidly increases many hospital medicine groups find that adding nurse practitioners or physician assistants helps them to complete the workforce they need to have in place to meet their clinical and administrative demands.
And hospital medicine includes pharmacists, case managers, and administrators to round out the inpatient team. Each of these professions is developing “hospital medicine specialists” looking for skills and experiences to allow them to help facilitate the work of the hospitalists and to use a team approach to achieve the rapidly expanded expectations of hospital medicine groups.
EDUCATION AND CERTIFICATION ISSUES
This growing conglomeration of healthcare professionals in one specialty presents unique issues. Some of these come in the form of diverse and expanding educational needs. The patient wants to be assured that no matter where the individual hospitalist started his or her training, the hospitalist will bring to the bedside the appropriate skills for their acute medical problems. This leads to having SHM develop courses in critical care skills, perioperative medicine, leadership, and the like.
Yet even though the endpoint may need to be similar for all hospitalists, it takes a fine touch and significant customization to craft educational materials when many hospitalists may start from a different base point.
When it comes to potential credentialing in hospital medicine, there is not a clear path to create a certification in hospital medicine. The solution for the 80% of hospitalists trained in internal medicine may very well be through the American Board of Internal Medicine (ABIM), but the pediatric and family practice solution will need to involve the American Board of Pediatrics (ABP) and the American Board of Family Medicine (ABFP), respectively. And this doesn’t even begin to address the credentialing needs of the nonphysicians.
Further, as SHM looks to represent all of the diverse elements that form the fabric of hospital medicine, we need to be in touch with the American College of Physicians, the American Academy of Pediatrics, the American Academy of Family Physicians, the Society of General Internal Medicine, the Ambulatory Pediatric Association, the American Association of Critical Care Nurses, the American Society of Health System Pharmacists, the American
Academy of Physicians Assistants, the American Academy of Nurse Practitioners, and many other important substantial medical professional societies; members of each believe that their organization relates to a segment of hospital medicine.
DIVERSITY CREATES NEW SOLUTIONS
The good part of this diversity is that as SHM helps to build this new specialty we are able to include so many unique vantage points. This strategy of inclusion allows for new ideas to percolate to the surface and leads to innovation and creativity. In fashioning the hospital of the future, “old think” must not rule the day. One way to change the outcome is to change those who are at the table.
If hospital medicine is to be part of the process of creating a hospital that is patient-centered, relies on measurable quality improvement, and delivers care by teams of healthcare professionals, then we need to open the tent and let in different perspectives. In SHM’s ongoing quality improvement efforts in heart failure and glycemic control, this is our approach—with meaningful input from hospitalists, subspecialists, nurses, pharmacists, and many other stakeholders in hospital medicine. This will lead to a different and—let’s hope—better outcome.
LABORISTS AND SURGICALISTS
And there are more wrinkles in the hospitalist world all the time. Recently USA Today wrote a story about “laborists” as hospitals try to solve access to obstetrical services by having contracted laborists on site 24/7. Some hospitals have to be creative when their community surgeons aren’t available for trauma care and some hospitals have contracted with orthopedists and general surgeons as “surgicalists.”
Are these the latest additions to the roll call of hospital medicine or just a footnote or an asterisk? Time and the marketplace will tell.
Besides the basic training for hospitalists there are many variations determined by site of practice and employment model. Whether we are talking about the differences between academic
hospital medicine or that practiced in community hospitals, or the uniqueness of a small group of hospitalists at only one hospital or a large multistate group of hospitalists in 10 states with 400 hospitalists, we are all part of hospital medicine.
In the end, hospital medicine is defined more by its common goals and its common values regardless of initial training or mode of practice. At this time in healthcare, many are looking for healthcare professionals to have the skills and the energy to create the hospital
of the future that will be a better place to work and to get the best care. SHM is committed to harnessing the diversity of our specialty to do our part to create a better future. With your help, we can get there. TH
Dr. Wellikson has been the CEO of SHM since 2000.
Hurricane Katrina: Tragedy and Hope
My mind has been very much on New Orleans and the Gulf Coast the past couple of weeks. The utter devastation wrought by Hurricane Katrina, and the horrific images of stranded people clinging to rooftops, are shocking. Sitting in San Francisco it was hard to imagine just how terrifying and chaotic it could be.
What a different image than the one I had during my first visit to New Orleans in 1999 for the 2nd Annual Meeting of the National Association of Inpatient Physicians. I remember being enchanted by New Orleans and thinking how it seemed more European than American and the most foreign of any American city I had visited.
I brought my family to the meeting, and we had a wonderful visit. We stayed near the convention center and enjoyed strolling the streets of the French Quarter, eating beignets, and riding the streetcar. I had an unforgettable dinner at Emeril’s that still ranks among the finest I’ve ever had. These memories are completely at odds with the images in the newspaper and on television. Some of the most vivid stories I read of Hurricane Katrina were e-mails from hospitalists on the ground in New Orleans and from others helping to care for sick patients evacuated from hospitals in Louisiana.
The first-hand accounts from hospitalists in New Orleans were gripping. I read the now-familiar stories of trying to live in a city with no electricity, no safe drinking water, no sewer system, and no government. I read of one physician entering a darkened pharmacy under police escort so that he could gather life-saving medicines for people whose prescriptions were destroyed along with their homes. Steve Deitzelsweig, MD, FACP, a hospitalist at Ochsner Clinic in New Orleans described the fear of epidemics in the Wall Street Journal, as well as in The Hospitalist (see October, p. 1). The possibility of a typhoid outbreak in 21st century America seemed more like a plot from a bad movie than a headline in a major newspaper. Even hospitalists far from New Orleans enlisted in aiding evacuees.
E-mail dispatches from Pat Cawley, MD, in South Carolina and others described hospitalists helping care for the sick airlifted from Louisiana, Mississippi, and Alabama. Jeanne Huddleston, MD, SHM’s immediate past president, described her role leading a team of doctors from Mayo Clinic who went to Louisiana to care for the sick and injured.
Perhaps the most frightening images were of doctors and nurses caring for critically ill patients without the help of monitors, ventilators, or other equipment when the emergency power went out. The images of patients waiting for helicopters to airlift them to safety were harrowing. Also striking were reports from hospitalists whose families had been evacuated, their homes destroyed—and they were at the hospital caring for those who were sick prior to and because of the hurricane.
My gratitude and admiration go out to all of the doctors, nurses, respiratory therapists, pharmacists, social workers, chaplains, and others in the hurricane-devastated regions and elsewhere who worked so valiantly to help patients in the face of chaos. As president of SHM I am proud of the efforts of our members and of all hospitalists who continue to assist in the face of this tragedy.
The medical crisis caused by Hurricane Katrina is perhaps most noteworthy because it happened in America. Of course, physicians and nurses struggle to help their patients under similar or worse conditions every day across the world without the ability to airlift their patients anywhere. Nonetheless, to watch this happen in New Orleans was shocking and offered insights into what it must be like in war zones and the developing world.
In addition, this was the fist time I recall hospitalists playing a prominent role in the medical response (see “Tours of Duty,” p. 1 and “The Red Badge of Katrina,” p. 13). To be sure, hospitals and healthcare personnel responded actively to tragedies like this one before hospitalists. But to the many advantages we bring as hospitalists, we can now add being in place—in the hospital—when disaster strikes. I do not pretend that this reason will convince many hospitals to start hospitalist programs—there are better and more pressing reasons to do so. But the ability to respond to disaster is clearly a benefit of a hospitalist program.
Included among the many e-mails circulating on the SHM listserv and among hospitalists was the question of whether hospitalists were included in official disaster response plans including those by FEMA and other agencies. After Hurricane Katrina, we will be.
Dire Inequities
Among the many tragedies revealed by Hurricane Katrina perhaps none was so striking as the inequities in our society. Even if we are willing to accept that in a free-market society some have more than others, the desperate situation faced by so many in New Orleans who were left behind is an indictment of a system that pays too little attention to those who have no resources.
We are aware of inequities in healthcare evidenced in part by the fact that millions of Americans have no health insurance. This tragedy showed that, in addition to not having health insurance, being poor exposes you to the brunt of a natural disaster that those with money can escape. The buses that arrived days after Hurricane Katrina to take people to Houston and elsewhere should have been there days before the hurricane.
What role do we play in changing this system? I can’t say that I have easy answers. Many of us contributed our skills after the tragedy to help those in need. Some of us farther away contributed money or goods to assist those affected by the hurricane. Some of us will begin or continue to advocate for a more just system.
While some of these issues are beyond the scope of SHM, during our planned legislative day preceding the 2006 Annual Meeting in Washington, D.C., we will have the opportunity to meet with our elected representatives to tell them about hospitalists and hospital medicine. We should share with them our experience from the frontline of American healthcare: Every day we care for many people who present to the hospital with illnesses that could have been prevented or significantly ameliorated by earlier intervention if they had only had access to healthcare. We are direct witnesses to what befalls those who lack health insurance and have poor access to healthcare. I hope that one of the messages we bring to Congress is that all Americans should have access to healthcare with health insurance.
The scenes of the hurricane-ravaged Gulf Coast also led me to reflect on the fragility of life and its precarious balance. Here in San Francisco we are safe from hurricanes, but at the mercy of earthquakes. It is still true that anyone who experienced the 1989 earthquake here in San Francisco can tell you exactly where they were and what they were doing at the time.
Final Thoughts
In the wake of Hurricane Katrina my wife and I have been talking a lot about earthquakes and how to ensure that we are prepared—if such a thing is even possible. The news reports tell us to have 72 hours’ worth of food and water, a battery operated radio, gas in the car, flashlights, and other necessities. We promise ourselves to get all the supplies we need and believe we will do so. But I also realize that denial is part of life and that in living near an earthquake fault denial might be necessary; just as living on the Gulf Coast may require a certain denial about the destructive power of hurricanes. But as one e-mail correspondent from New Orleans wrote, “Despite it all, this is a soul-edifying experience.”
Perhaps the tragedy that hit the Gulf Coast will help each of us edify our souls through less drastic measures and remind us that any day can be our last. This knowledge is a gift to help us spend our time in the best way possible. Those who are helping the people whose lives were ravaged by Hurricane Katrina remind us of the good that we can do in the world. As hospitalists we get to experience this good every day at the bedside through the privilege of patient care. May we cherish this opportunity and fulfill it with dignity and pride. To all those involved in hurricane relief efforts, thank you, and to all those whose lives were ravaged by the hurricane I wish you strength and recovery. TH
SHM President Dr. Pantilat is an associate professor of clinical medicine at the University of California at San Francisco.
My mind has been very much on New Orleans and the Gulf Coast the past couple of weeks. The utter devastation wrought by Hurricane Katrina, and the horrific images of stranded people clinging to rooftops, are shocking. Sitting in San Francisco it was hard to imagine just how terrifying and chaotic it could be.
What a different image than the one I had during my first visit to New Orleans in 1999 for the 2nd Annual Meeting of the National Association of Inpatient Physicians. I remember being enchanted by New Orleans and thinking how it seemed more European than American and the most foreign of any American city I had visited.
I brought my family to the meeting, and we had a wonderful visit. We stayed near the convention center and enjoyed strolling the streets of the French Quarter, eating beignets, and riding the streetcar. I had an unforgettable dinner at Emeril’s that still ranks among the finest I’ve ever had. These memories are completely at odds with the images in the newspaper and on television. Some of the most vivid stories I read of Hurricane Katrina were e-mails from hospitalists on the ground in New Orleans and from others helping to care for sick patients evacuated from hospitals in Louisiana.
The first-hand accounts from hospitalists in New Orleans were gripping. I read the now-familiar stories of trying to live in a city with no electricity, no safe drinking water, no sewer system, and no government. I read of one physician entering a darkened pharmacy under police escort so that he could gather life-saving medicines for people whose prescriptions were destroyed along with their homes. Steve Deitzelsweig, MD, FACP, a hospitalist at Ochsner Clinic in New Orleans described the fear of epidemics in the Wall Street Journal, as well as in The Hospitalist (see October, p. 1). The possibility of a typhoid outbreak in 21st century America seemed more like a plot from a bad movie than a headline in a major newspaper. Even hospitalists far from New Orleans enlisted in aiding evacuees.
E-mail dispatches from Pat Cawley, MD, in South Carolina and others described hospitalists helping care for the sick airlifted from Louisiana, Mississippi, and Alabama. Jeanne Huddleston, MD, SHM’s immediate past president, described her role leading a team of doctors from Mayo Clinic who went to Louisiana to care for the sick and injured.
Perhaps the most frightening images were of doctors and nurses caring for critically ill patients without the help of monitors, ventilators, or other equipment when the emergency power went out. The images of patients waiting for helicopters to airlift them to safety were harrowing. Also striking were reports from hospitalists whose families had been evacuated, their homes destroyed—and they were at the hospital caring for those who were sick prior to and because of the hurricane.
My gratitude and admiration go out to all of the doctors, nurses, respiratory therapists, pharmacists, social workers, chaplains, and others in the hurricane-devastated regions and elsewhere who worked so valiantly to help patients in the face of chaos. As president of SHM I am proud of the efforts of our members and of all hospitalists who continue to assist in the face of this tragedy.
The medical crisis caused by Hurricane Katrina is perhaps most noteworthy because it happened in America. Of course, physicians and nurses struggle to help their patients under similar or worse conditions every day across the world without the ability to airlift their patients anywhere. Nonetheless, to watch this happen in New Orleans was shocking and offered insights into what it must be like in war zones and the developing world.
In addition, this was the fist time I recall hospitalists playing a prominent role in the medical response (see “Tours of Duty,” p. 1 and “The Red Badge of Katrina,” p. 13). To be sure, hospitals and healthcare personnel responded actively to tragedies like this one before hospitalists. But to the many advantages we bring as hospitalists, we can now add being in place—in the hospital—when disaster strikes. I do not pretend that this reason will convince many hospitals to start hospitalist programs—there are better and more pressing reasons to do so. But the ability to respond to disaster is clearly a benefit of a hospitalist program.
Included among the many e-mails circulating on the SHM listserv and among hospitalists was the question of whether hospitalists were included in official disaster response plans including those by FEMA and other agencies. After Hurricane Katrina, we will be.
Dire Inequities
Among the many tragedies revealed by Hurricane Katrina perhaps none was so striking as the inequities in our society. Even if we are willing to accept that in a free-market society some have more than others, the desperate situation faced by so many in New Orleans who were left behind is an indictment of a system that pays too little attention to those who have no resources.
We are aware of inequities in healthcare evidenced in part by the fact that millions of Americans have no health insurance. This tragedy showed that, in addition to not having health insurance, being poor exposes you to the brunt of a natural disaster that those with money can escape. The buses that arrived days after Hurricane Katrina to take people to Houston and elsewhere should have been there days before the hurricane.
What role do we play in changing this system? I can’t say that I have easy answers. Many of us contributed our skills after the tragedy to help those in need. Some of us farther away contributed money or goods to assist those affected by the hurricane. Some of us will begin or continue to advocate for a more just system.
While some of these issues are beyond the scope of SHM, during our planned legislative day preceding the 2006 Annual Meeting in Washington, D.C., we will have the opportunity to meet with our elected representatives to tell them about hospitalists and hospital medicine. We should share with them our experience from the frontline of American healthcare: Every day we care for many people who present to the hospital with illnesses that could have been prevented or significantly ameliorated by earlier intervention if they had only had access to healthcare. We are direct witnesses to what befalls those who lack health insurance and have poor access to healthcare. I hope that one of the messages we bring to Congress is that all Americans should have access to healthcare with health insurance.
The scenes of the hurricane-ravaged Gulf Coast also led me to reflect on the fragility of life and its precarious balance. Here in San Francisco we are safe from hurricanes, but at the mercy of earthquakes. It is still true that anyone who experienced the 1989 earthquake here in San Francisco can tell you exactly where they were and what they were doing at the time.
Final Thoughts
In the wake of Hurricane Katrina my wife and I have been talking a lot about earthquakes and how to ensure that we are prepared—if such a thing is even possible. The news reports tell us to have 72 hours’ worth of food and water, a battery operated radio, gas in the car, flashlights, and other necessities. We promise ourselves to get all the supplies we need and believe we will do so. But I also realize that denial is part of life and that in living near an earthquake fault denial might be necessary; just as living on the Gulf Coast may require a certain denial about the destructive power of hurricanes. But as one e-mail correspondent from New Orleans wrote, “Despite it all, this is a soul-edifying experience.”
Perhaps the tragedy that hit the Gulf Coast will help each of us edify our souls through less drastic measures and remind us that any day can be our last. This knowledge is a gift to help us spend our time in the best way possible. Those who are helping the people whose lives were ravaged by Hurricane Katrina remind us of the good that we can do in the world. As hospitalists we get to experience this good every day at the bedside through the privilege of patient care. May we cherish this opportunity and fulfill it with dignity and pride. To all those involved in hurricane relief efforts, thank you, and to all those whose lives were ravaged by the hurricane I wish you strength and recovery. TH
SHM President Dr. Pantilat is an associate professor of clinical medicine at the University of California at San Francisco.
My mind has been very much on New Orleans and the Gulf Coast the past couple of weeks. The utter devastation wrought by Hurricane Katrina, and the horrific images of stranded people clinging to rooftops, are shocking. Sitting in San Francisco it was hard to imagine just how terrifying and chaotic it could be.
What a different image than the one I had during my first visit to New Orleans in 1999 for the 2nd Annual Meeting of the National Association of Inpatient Physicians. I remember being enchanted by New Orleans and thinking how it seemed more European than American and the most foreign of any American city I had visited.
I brought my family to the meeting, and we had a wonderful visit. We stayed near the convention center and enjoyed strolling the streets of the French Quarter, eating beignets, and riding the streetcar. I had an unforgettable dinner at Emeril’s that still ranks among the finest I’ve ever had. These memories are completely at odds with the images in the newspaper and on television. Some of the most vivid stories I read of Hurricane Katrina were e-mails from hospitalists on the ground in New Orleans and from others helping to care for sick patients evacuated from hospitals in Louisiana.
The first-hand accounts from hospitalists in New Orleans were gripping. I read the now-familiar stories of trying to live in a city with no electricity, no safe drinking water, no sewer system, and no government. I read of one physician entering a darkened pharmacy under police escort so that he could gather life-saving medicines for people whose prescriptions were destroyed along with their homes. Steve Deitzelsweig, MD, FACP, a hospitalist at Ochsner Clinic in New Orleans described the fear of epidemics in the Wall Street Journal, as well as in The Hospitalist (see October, p. 1). The possibility of a typhoid outbreak in 21st century America seemed more like a plot from a bad movie than a headline in a major newspaper. Even hospitalists far from New Orleans enlisted in aiding evacuees.
E-mail dispatches from Pat Cawley, MD, in South Carolina and others described hospitalists helping care for the sick airlifted from Louisiana, Mississippi, and Alabama. Jeanne Huddleston, MD, SHM’s immediate past president, described her role leading a team of doctors from Mayo Clinic who went to Louisiana to care for the sick and injured.
Perhaps the most frightening images were of doctors and nurses caring for critically ill patients without the help of monitors, ventilators, or other equipment when the emergency power went out. The images of patients waiting for helicopters to airlift them to safety were harrowing. Also striking were reports from hospitalists whose families had been evacuated, their homes destroyed—and they were at the hospital caring for those who were sick prior to and because of the hurricane.
My gratitude and admiration go out to all of the doctors, nurses, respiratory therapists, pharmacists, social workers, chaplains, and others in the hurricane-devastated regions and elsewhere who worked so valiantly to help patients in the face of chaos. As president of SHM I am proud of the efforts of our members and of all hospitalists who continue to assist in the face of this tragedy.
The medical crisis caused by Hurricane Katrina is perhaps most noteworthy because it happened in America. Of course, physicians and nurses struggle to help their patients under similar or worse conditions every day across the world without the ability to airlift their patients anywhere. Nonetheless, to watch this happen in New Orleans was shocking and offered insights into what it must be like in war zones and the developing world.
In addition, this was the fist time I recall hospitalists playing a prominent role in the medical response (see “Tours of Duty,” p. 1 and “The Red Badge of Katrina,” p. 13). To be sure, hospitals and healthcare personnel responded actively to tragedies like this one before hospitalists. But to the many advantages we bring as hospitalists, we can now add being in place—in the hospital—when disaster strikes. I do not pretend that this reason will convince many hospitals to start hospitalist programs—there are better and more pressing reasons to do so. But the ability to respond to disaster is clearly a benefit of a hospitalist program.
Included among the many e-mails circulating on the SHM listserv and among hospitalists was the question of whether hospitalists were included in official disaster response plans including those by FEMA and other agencies. After Hurricane Katrina, we will be.
Dire Inequities
Among the many tragedies revealed by Hurricane Katrina perhaps none was so striking as the inequities in our society. Even if we are willing to accept that in a free-market society some have more than others, the desperate situation faced by so many in New Orleans who were left behind is an indictment of a system that pays too little attention to those who have no resources.
We are aware of inequities in healthcare evidenced in part by the fact that millions of Americans have no health insurance. This tragedy showed that, in addition to not having health insurance, being poor exposes you to the brunt of a natural disaster that those with money can escape. The buses that arrived days after Hurricane Katrina to take people to Houston and elsewhere should have been there days before the hurricane.
What role do we play in changing this system? I can’t say that I have easy answers. Many of us contributed our skills after the tragedy to help those in need. Some of us farther away contributed money or goods to assist those affected by the hurricane. Some of us will begin or continue to advocate for a more just system.
While some of these issues are beyond the scope of SHM, during our planned legislative day preceding the 2006 Annual Meeting in Washington, D.C., we will have the opportunity to meet with our elected representatives to tell them about hospitalists and hospital medicine. We should share with them our experience from the frontline of American healthcare: Every day we care for many people who present to the hospital with illnesses that could have been prevented or significantly ameliorated by earlier intervention if they had only had access to healthcare. We are direct witnesses to what befalls those who lack health insurance and have poor access to healthcare. I hope that one of the messages we bring to Congress is that all Americans should have access to healthcare with health insurance.
The scenes of the hurricane-ravaged Gulf Coast also led me to reflect on the fragility of life and its precarious balance. Here in San Francisco we are safe from hurricanes, but at the mercy of earthquakes. It is still true that anyone who experienced the 1989 earthquake here in San Francisco can tell you exactly where they were and what they were doing at the time.
Final Thoughts
In the wake of Hurricane Katrina my wife and I have been talking a lot about earthquakes and how to ensure that we are prepared—if such a thing is even possible. The news reports tell us to have 72 hours’ worth of food and water, a battery operated radio, gas in the car, flashlights, and other necessities. We promise ourselves to get all the supplies we need and believe we will do so. But I also realize that denial is part of life and that in living near an earthquake fault denial might be necessary; just as living on the Gulf Coast may require a certain denial about the destructive power of hurricanes. But as one e-mail correspondent from New Orleans wrote, “Despite it all, this is a soul-edifying experience.”
Perhaps the tragedy that hit the Gulf Coast will help each of us edify our souls through less drastic measures and remind us that any day can be our last. This knowledge is a gift to help us spend our time in the best way possible. Those who are helping the people whose lives were ravaged by Hurricane Katrina remind us of the good that we can do in the world. As hospitalists we get to experience this good every day at the bedside through the privilege of patient care. May we cherish this opportunity and fulfill it with dignity and pride. To all those involved in hurricane relief efforts, thank you, and to all those whose lives were ravaged by the hurricane I wish you strength and recovery. TH
SHM President Dr. Pantilat is an associate professor of clinical medicine at the University of California at San Francisco.
Detail-Oriented
While President Obama made a splash last week with his first stump speeches for healthcare reform, SHM policy leaders want to hear more—a lot more. Those hospitalists say HM advocates need to stay on top of the details that will emerge in coming months about “accountable healthcare,” the bundling of Medicare reimbursement payments, and other sensitive issues.
"Reform is going to happen," says Eric Siegal, MD, FHM, a critical-care fellow at the University of Wisconsin School of Medicine and Public Health in Madison and the chair of SHM’s Public Policy Committee. “The scope of what it is remains to be seen. ... The devil is absolutely in the details."
Dr. Siegal resisted focusing on Obama's opposition to cap malpractice awards. "While tort reform is important and should be addressed, medical malpractice is not the root cause of our dysfunctional healthcare system." Dr. Siegal agrees "conceptually" with the administration’s plans to bundle payments, encourage more medical students to enter primary care, and hold healthcare organizations accountable. But he and other SHM leaders want pilot programs to test the efficacy of such initiatives before any broad changes are implemented.
Some policy wonks wonder how fast HM leaders might see the impact of Obama’s proposal to cut $600 billion from Medicare and Medicaid by 2019. SHM policy committee member Bradley Flansbaum, DO, MPH, said if cuts are implemented quickly, then hospitalists could see hospital subsidies to their programs reduced, or could simply be competing with other specialists for a shrinking fiscal pie.
“Short-term, I would be concerned,” says Dr. Flansbaum, chief of hospitalist services at Lenox Hill Hospital in New York City. “What he’s proposing is more or less ... a haircut. Depending on how quickly the system is worked out, there could be a lot of pain on the hospital medicine side.”
For more public policy information, visit SHM’s Advocacy Website.
While President Obama made a splash last week with his first stump speeches for healthcare reform, SHM policy leaders want to hear more—a lot more. Those hospitalists say HM advocates need to stay on top of the details that will emerge in coming months about “accountable healthcare,” the bundling of Medicare reimbursement payments, and other sensitive issues.
"Reform is going to happen," says Eric Siegal, MD, FHM, a critical-care fellow at the University of Wisconsin School of Medicine and Public Health in Madison and the chair of SHM’s Public Policy Committee. “The scope of what it is remains to be seen. ... The devil is absolutely in the details."
Dr. Siegal resisted focusing on Obama's opposition to cap malpractice awards. "While tort reform is important and should be addressed, medical malpractice is not the root cause of our dysfunctional healthcare system." Dr. Siegal agrees "conceptually" with the administration’s plans to bundle payments, encourage more medical students to enter primary care, and hold healthcare organizations accountable. But he and other SHM leaders want pilot programs to test the efficacy of such initiatives before any broad changes are implemented.
Some policy wonks wonder how fast HM leaders might see the impact of Obama’s proposal to cut $600 billion from Medicare and Medicaid by 2019. SHM policy committee member Bradley Flansbaum, DO, MPH, said if cuts are implemented quickly, then hospitalists could see hospital subsidies to their programs reduced, or could simply be competing with other specialists for a shrinking fiscal pie.
“Short-term, I would be concerned,” says Dr. Flansbaum, chief of hospitalist services at Lenox Hill Hospital in New York City. “What he’s proposing is more or less ... a haircut. Depending on how quickly the system is worked out, there could be a lot of pain on the hospital medicine side.”
For more public policy information, visit SHM’s Advocacy Website.
While President Obama made a splash last week with his first stump speeches for healthcare reform, SHM policy leaders want to hear more—a lot more. Those hospitalists say HM advocates need to stay on top of the details that will emerge in coming months about “accountable healthcare,” the bundling of Medicare reimbursement payments, and other sensitive issues.
"Reform is going to happen," says Eric Siegal, MD, FHM, a critical-care fellow at the University of Wisconsin School of Medicine and Public Health in Madison and the chair of SHM’s Public Policy Committee. “The scope of what it is remains to be seen. ... The devil is absolutely in the details."
Dr. Siegal resisted focusing on Obama's opposition to cap malpractice awards. "While tort reform is important and should be addressed, medical malpractice is not the root cause of our dysfunctional healthcare system." Dr. Siegal agrees "conceptually" with the administration’s plans to bundle payments, encourage more medical students to enter primary care, and hold healthcare organizations accountable. But he and other SHM leaders want pilot programs to test the efficacy of such initiatives before any broad changes are implemented.
Some policy wonks wonder how fast HM leaders might see the impact of Obama’s proposal to cut $600 billion from Medicare and Medicaid by 2019. SHM policy committee member Bradley Flansbaum, DO, MPH, said if cuts are implemented quickly, then hospitalists could see hospital subsidies to their programs reduced, or could simply be competing with other specialists for a shrinking fiscal pie.
“Short-term, I would be concerned,” says Dr. Flansbaum, chief of hospitalist services at Lenox Hill Hospital in New York City. “What he’s proposing is more or less ... a haircut. Depending on how quickly the system is worked out, there could be a lot of pain on the hospital medicine side.”
For more public policy information, visit SHM’s Advocacy Website.
The Blog Rounds
2008-09 apparently was a good time to find a job in HM. The Wall Street Journal’s Health Blog cites a new report from Merritt Hawkins & Associates (download PDF) that shows from April 2008 to March 2009, 85% of searches offered signing bonuses averaging $24,850. That’s in contrast to 46% of searches in 2005-2006, when the average bonus was $14,030.
The report also included average salaries for HM, which was the third-most-requested search assignment following family medicine and general internal medicine. The average salary for HM during that time period, excluding benefits or productivity bonuses, was $201,000, according to the report. It represents a 14.8% increase since 2005-2006, when the annual average annual salary for a hospitalist was $175,000, according to the report.
Fun & Games
On his blog, Running a Hospital, Paul Levy, president and CEO of Beth Israel Deaconess Medical Center in Boston, offers physicians a game to help them better understand the value of standardized medicine. Interestingly, the game involves drawing a pig. For instructions, visit http://runningahospital.blogspot.com/2009/06/pig-part-1.html.
Work-Life Balance
In her Well blog, New York Times writer Tara Parker Pope cites a recent article from Times columnist Pauline Chen, MD, in which Dr. Chen recalls the toll her intense medical training took on her temperament and personal relationships.
Doctors responded to the post with a variety of viewpoints. Here’s what one old-timer had to say:
“I am from the era of 120-hour weeks, every second or third night on call. No ‘cap’ on the numbers of patients we admitted or carried on our service. I remember still being in the hospital at 10 p.m. after a night on call (40 hours straight). I learned how to manage the sickest patients through the entire course of their hospitalization. My residency and fellowship after medical school was seven years in duration, and I loved it. The ONLY regret I have after all these years is reading the NYT comments and blogs about all the doctor-haters and disgruntled patients who think that medicine is an easy path to riches.”
2008-09 apparently was a good time to find a job in HM. The Wall Street Journal’s Health Blog cites a new report from Merritt Hawkins & Associates (download PDF) that shows from April 2008 to March 2009, 85% of searches offered signing bonuses averaging $24,850. That’s in contrast to 46% of searches in 2005-2006, when the average bonus was $14,030.
The report also included average salaries for HM, which was the third-most-requested search assignment following family medicine and general internal medicine. The average salary for HM during that time period, excluding benefits or productivity bonuses, was $201,000, according to the report. It represents a 14.8% increase since 2005-2006, when the annual average annual salary for a hospitalist was $175,000, according to the report.
Fun & Games
On his blog, Running a Hospital, Paul Levy, president and CEO of Beth Israel Deaconess Medical Center in Boston, offers physicians a game to help them better understand the value of standardized medicine. Interestingly, the game involves drawing a pig. For instructions, visit http://runningahospital.blogspot.com/2009/06/pig-part-1.html.
Work-Life Balance
In her Well blog, New York Times writer Tara Parker Pope cites a recent article from Times columnist Pauline Chen, MD, in which Dr. Chen recalls the toll her intense medical training took on her temperament and personal relationships.
Doctors responded to the post with a variety of viewpoints. Here’s what one old-timer had to say:
“I am from the era of 120-hour weeks, every second or third night on call. No ‘cap’ on the numbers of patients we admitted or carried on our service. I remember still being in the hospital at 10 p.m. after a night on call (40 hours straight). I learned how to manage the sickest patients through the entire course of their hospitalization. My residency and fellowship after medical school was seven years in duration, and I loved it. The ONLY regret I have after all these years is reading the NYT comments and blogs about all the doctor-haters and disgruntled patients who think that medicine is an easy path to riches.”
2008-09 apparently was a good time to find a job in HM. The Wall Street Journal’s Health Blog cites a new report from Merritt Hawkins & Associates (download PDF) that shows from April 2008 to March 2009, 85% of searches offered signing bonuses averaging $24,850. That’s in contrast to 46% of searches in 2005-2006, when the average bonus was $14,030.
The report also included average salaries for HM, which was the third-most-requested search assignment following family medicine and general internal medicine. The average salary for HM during that time period, excluding benefits or productivity bonuses, was $201,000, according to the report. It represents a 14.8% increase since 2005-2006, when the annual average annual salary for a hospitalist was $175,000, according to the report.
Fun & Games
On his blog, Running a Hospital, Paul Levy, president and CEO of Beth Israel Deaconess Medical Center in Boston, offers physicians a game to help them better understand the value of standardized medicine. Interestingly, the game involves drawing a pig. For instructions, visit http://runningahospital.blogspot.com/2009/06/pig-part-1.html.
Work-Life Balance
In her Well blog, New York Times writer Tara Parker Pope cites a recent article from Times columnist Pauline Chen, MD, in which Dr. Chen recalls the toll her intense medical training took on her temperament and personal relationships.
Doctors responded to the post with a variety of viewpoints. Here’s what one old-timer had to say:
“I am from the era of 120-hour weeks, every second or third night on call. No ‘cap’ on the numbers of patients we admitted or carried on our service. I remember still being in the hospital at 10 p.m. after a night on call (40 hours straight). I learned how to manage the sickest patients through the entire course of their hospitalization. My residency and fellowship after medical school was seven years in duration, and I loved it. The ONLY regret I have after all these years is reading the NYT comments and blogs about all the doctor-haters and disgruntled patients who think that medicine is an easy path to riches.”
Ofatumumab
Dr. William Wierda discusses ofatumumab, an investigational monoclonal antibody that targets B cells and has been studied in the treatment of chronic lymphocytic leukemia. Mitchel Zoler of the Global Medical News Network (GMNN) reports from the annual congress of the European Hematology Association in Berlin.
Dr. William Wierda discusses ofatumumab, an investigational monoclonal antibody that targets B cells and has been studied in the treatment of chronic lymphocytic leukemia. Mitchel Zoler of the Global Medical News Network (GMNN) reports from the annual congress of the European Hematology Association in Berlin.
Dr. William Wierda discusses ofatumumab, an investigational monoclonal antibody that targets B cells and has been studied in the treatment of chronic lymphocytic leukemia. Mitchel Zoler of the Global Medical News Network (GMNN) reports from the annual congress of the European Hematology Association in Berlin.
A Hospitalist Is Born
The hospitalist business model has been adopted nationwide to help stem the tide of obstetrician/gynecologists who forgo delivering babies at hospitals—or providing emergency obstetric care—because of skyrocketing malpractice costs and a frustration with on-call schedules. Like the familiar HM model, a new class of OB-GYN has cropped up to work in medical centers.
Called laborists or OB hospitalists, this breed resembles hospital-based physicians: They work out of their respective institutions but don’t have private-practice patients. Hospitals like the arrangement because they have trained delivery staff in-house, and private-practice physicians don't mind giving up hospital calls because they can earn more cycling patients through their office.
The OB Hospitalist Group of Greenville, S.C., already has placed 60 OB hospitalists in six states, including Texas, California, and Florida. Group president Chris Swain, MD, hopes to place 100 by year's end, but only if he can find enough qualified candidates to manage both deliveries and OB emergencies.
"If you're going to be in a hospital 24 hours a day, you want to be able to handle all the emergencies that come in," Dr. Swain says. “You can't take time to look it up. You need to know it."
Dr. Swain's group pays for that knowledge. The average OB-GYN makes about $280,000 per year; Dr. Swain's OB hospitalists earn roughly $300,000 a year. In exchange for the higher salaries and defined work schedules, OB Hospitalist Group requires board certification, monitoring courses, and continued training. "We want our doctors to be the experts in emergency obstetrics care," Dr. Swain says. "We pay our doctors more; we expect more out of them."
The hospitalist business model has been adopted nationwide to help stem the tide of obstetrician/gynecologists who forgo delivering babies at hospitals—or providing emergency obstetric care—because of skyrocketing malpractice costs and a frustration with on-call schedules. Like the familiar HM model, a new class of OB-GYN has cropped up to work in medical centers.
Called laborists or OB hospitalists, this breed resembles hospital-based physicians: They work out of their respective institutions but don’t have private-practice patients. Hospitals like the arrangement because they have trained delivery staff in-house, and private-practice physicians don't mind giving up hospital calls because they can earn more cycling patients through their office.
The OB Hospitalist Group of Greenville, S.C., already has placed 60 OB hospitalists in six states, including Texas, California, and Florida. Group president Chris Swain, MD, hopes to place 100 by year's end, but only if he can find enough qualified candidates to manage both deliveries and OB emergencies.
"If you're going to be in a hospital 24 hours a day, you want to be able to handle all the emergencies that come in," Dr. Swain says. “You can't take time to look it up. You need to know it."
Dr. Swain's group pays for that knowledge. The average OB-GYN makes about $280,000 per year; Dr. Swain's OB hospitalists earn roughly $300,000 a year. In exchange for the higher salaries and defined work schedules, OB Hospitalist Group requires board certification, monitoring courses, and continued training. "We want our doctors to be the experts in emergency obstetrics care," Dr. Swain says. "We pay our doctors more; we expect more out of them."
The hospitalist business model has been adopted nationwide to help stem the tide of obstetrician/gynecologists who forgo delivering babies at hospitals—or providing emergency obstetric care—because of skyrocketing malpractice costs and a frustration with on-call schedules. Like the familiar HM model, a new class of OB-GYN has cropped up to work in medical centers.
Called laborists or OB hospitalists, this breed resembles hospital-based physicians: They work out of their respective institutions but don’t have private-practice patients. Hospitals like the arrangement because they have trained delivery staff in-house, and private-practice physicians don't mind giving up hospital calls because they can earn more cycling patients through their office.
The OB Hospitalist Group of Greenville, S.C., already has placed 60 OB hospitalists in six states, including Texas, California, and Florida. Group president Chris Swain, MD, hopes to place 100 by year's end, but only if he can find enough qualified candidates to manage both deliveries and OB emergencies.
"If you're going to be in a hospital 24 hours a day, you want to be able to handle all the emergencies that come in," Dr. Swain says. “You can't take time to look it up. You need to know it."
Dr. Swain's group pays for that knowledge. The average OB-GYN makes about $280,000 per year; Dr. Swain's OB hospitalists earn roughly $300,000 a year. In exchange for the higher salaries and defined work schedules, OB Hospitalist Group requires board certification, monitoring courses, and continued training. "We want our doctors to be the experts in emergency obstetrics care," Dr. Swain says. "We pay our doctors more; we expect more out of them."
In the Literature: The Latest Research You Need to Know
Clinical question: Does intensive glucose control reduce mortality at 90 days in adult ICU patients?
Background: The American Diabetic Association currently recommends tight glucose control for patients admitted to an ICU, despite conflicting evidence in the literature about the benefits of this practice.
Study design: Randomized controlled trial.
Setting: Medical and surgical ICUs at 42 hospitals in Australia, Canada, and New Zealand.
Synopsis: More than 6,000 medical and surgical ICU patients were randomly assigned to receive either intensive (target blood sugar range of 81 mg/dL to 108 mg/dL) or conventional (target blood sugar of <180 mg/dL) glucose control. Eligible patients were expected to stay at least three days in the ICU. Mortality at 90 days for the intensive treatment group was 27.5% versus 24.9% in the conventional treatment group, with an absolute difference in mortality of 2.6% resulting in a number needed to harm of 38.5. This difference in mortality remained significant whether the patients were in a SICU or MICU, diabetic or nondiabetic, with or without sepsis, and with APACHE II scores of above or below 25. The excess deaths were attributed to cardiovascular causes, but more investigation is needed. This study demonstrates that there is no additional benefit and that there may be harm in pursuing aggressive glucose control in ICU patients.
Bottom line: When compared with conventional practice in adult ICU patients, intensive glucose control resulted in an increase in 90-day mortality.
Citation: NICE-SUGAR Study Investigators, Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283-1297.
Clinical question: Does intensive glucose control reduce mortality at 90 days in adult ICU patients?
Background: The American Diabetic Association currently recommends tight glucose control for patients admitted to an ICU, despite conflicting evidence in the literature about the benefits of this practice.
Study design: Randomized controlled trial.
Setting: Medical and surgical ICUs at 42 hospitals in Australia, Canada, and New Zealand.
Synopsis: More than 6,000 medical and surgical ICU patients were randomly assigned to receive either intensive (target blood sugar range of 81 mg/dL to 108 mg/dL) or conventional (target blood sugar of <180 mg/dL) glucose control. Eligible patients were expected to stay at least three days in the ICU. Mortality at 90 days for the intensive treatment group was 27.5% versus 24.9% in the conventional treatment group, with an absolute difference in mortality of 2.6% resulting in a number needed to harm of 38.5. This difference in mortality remained significant whether the patients were in a SICU or MICU, diabetic or nondiabetic, with or without sepsis, and with APACHE II scores of above or below 25. The excess deaths were attributed to cardiovascular causes, but more investigation is needed. This study demonstrates that there is no additional benefit and that there may be harm in pursuing aggressive glucose control in ICU patients.
Bottom line: When compared with conventional practice in adult ICU patients, intensive glucose control resulted in an increase in 90-day mortality.
Citation: NICE-SUGAR Study Investigators, Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283-1297.
Clinical question: Does intensive glucose control reduce mortality at 90 days in adult ICU patients?
Background: The American Diabetic Association currently recommends tight glucose control for patients admitted to an ICU, despite conflicting evidence in the literature about the benefits of this practice.
Study design: Randomized controlled trial.
Setting: Medical and surgical ICUs at 42 hospitals in Australia, Canada, and New Zealand.
Synopsis: More than 6,000 medical and surgical ICU patients were randomly assigned to receive either intensive (target blood sugar range of 81 mg/dL to 108 mg/dL) or conventional (target blood sugar of <180 mg/dL) glucose control. Eligible patients were expected to stay at least three days in the ICU. Mortality at 90 days for the intensive treatment group was 27.5% versus 24.9% in the conventional treatment group, with an absolute difference in mortality of 2.6% resulting in a number needed to harm of 38.5. This difference in mortality remained significant whether the patients were in a SICU or MICU, diabetic or nondiabetic, with or without sepsis, and with APACHE II scores of above or below 25. The excess deaths were attributed to cardiovascular causes, but more investigation is needed. This study demonstrates that there is no additional benefit and that there may be harm in pursuing aggressive glucose control in ICU patients.
Bottom line: When compared with conventional practice in adult ICU patients, intensive glucose control resulted in an increase in 90-day mortality.
Citation: NICE-SUGAR Study Investigators, Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283-1297.
Team Approach to Patient Care
Hospitalists find themselves working with nonphysician providers (NPP) more and more as institutions spread workloads and cut costs. The work arrangement can be panaceas when they work well, barely palatable when they don’t.
Several sessions at HM09 in Chicago explored the best practices of HM groups that use nurse practitioners (NPs) and physician assistants (PAs). A particularly popular session examined case studies that showed two main trends: NPP programs run into trouble when the people involved view their positions as competition; programs succeed when there is buy-in from all stakeholders.
“There are things DOs and MDs do better than NPs and PAs, and there are things NPs and PAs do better than DOs and MDs,” says Mitch Wilson, MD, FHM, corporate medical director for Eagle Hospital Physicians in Atlanta. "It’s all about understanding the cumulative skill set.”
Dr. Wilson and Tracy Cardin, ACNP, at the University of Chicago Medical Center, used care stories as teaching tools for hospitals looking to implement new programs. The first cautionary tale failed because an “old school” culture left a new NPP so isolated from physicians and nursing staff that she quit. Two other cases showed NPPs integrate more easily because of mutual respect, defined responsibilities, and an alignment of expectations between both hospitalists and NPPs.
Mac McCormick, MD, vice president of clinical services at Eagle Hospital Physicians in Atlanta, offers the following suggestions to best utilize NPPs:
•Conduct an analysis of your practice environment, bylaws, staff experience, and pre-existing attitudes to identify potential barriers and optimal opportunities in hiring NPPs;
•Avoid pigeonholing NPPs into such narrow roles as completing discharge summaries or collecting data. Tasks that tend not to utilize their skills set might lead to professional dissatisfaction and likely aren't the most cost-efficient use of resources; and
•Approach things in a team model. Shared visits are one way to accomplish this. Keep communication lines open to make sure accurate and timely information is available to all.
Hospitalists find themselves working with nonphysician providers (NPP) more and more as institutions spread workloads and cut costs. The work arrangement can be panaceas when they work well, barely palatable when they don’t.
Several sessions at HM09 in Chicago explored the best practices of HM groups that use nurse practitioners (NPs) and physician assistants (PAs). A particularly popular session examined case studies that showed two main trends: NPP programs run into trouble when the people involved view their positions as competition; programs succeed when there is buy-in from all stakeholders.
“There are things DOs and MDs do better than NPs and PAs, and there are things NPs and PAs do better than DOs and MDs,” says Mitch Wilson, MD, FHM, corporate medical director for Eagle Hospital Physicians in Atlanta. "It’s all about understanding the cumulative skill set.”
Dr. Wilson and Tracy Cardin, ACNP, at the University of Chicago Medical Center, used care stories as teaching tools for hospitals looking to implement new programs. The first cautionary tale failed because an “old school” culture left a new NPP so isolated from physicians and nursing staff that she quit. Two other cases showed NPPs integrate more easily because of mutual respect, defined responsibilities, and an alignment of expectations between both hospitalists and NPPs.
Mac McCormick, MD, vice president of clinical services at Eagle Hospital Physicians in Atlanta, offers the following suggestions to best utilize NPPs:
•Conduct an analysis of your practice environment, bylaws, staff experience, and pre-existing attitudes to identify potential barriers and optimal opportunities in hiring NPPs;
•Avoid pigeonholing NPPs into such narrow roles as completing discharge summaries or collecting data. Tasks that tend not to utilize their skills set might lead to professional dissatisfaction and likely aren't the most cost-efficient use of resources; and
•Approach things in a team model. Shared visits are one way to accomplish this. Keep communication lines open to make sure accurate and timely information is available to all.
Hospitalists find themselves working with nonphysician providers (NPP) more and more as institutions spread workloads and cut costs. The work arrangement can be panaceas when they work well, barely palatable when they don’t.
Several sessions at HM09 in Chicago explored the best practices of HM groups that use nurse practitioners (NPs) and physician assistants (PAs). A particularly popular session examined case studies that showed two main trends: NPP programs run into trouble when the people involved view their positions as competition; programs succeed when there is buy-in from all stakeholders.
“There are things DOs and MDs do better than NPs and PAs, and there are things NPs and PAs do better than DOs and MDs,” says Mitch Wilson, MD, FHM, corporate medical director for Eagle Hospital Physicians in Atlanta. "It’s all about understanding the cumulative skill set.”
Dr. Wilson and Tracy Cardin, ACNP, at the University of Chicago Medical Center, used care stories as teaching tools for hospitals looking to implement new programs. The first cautionary tale failed because an “old school” culture left a new NPP so isolated from physicians and nursing staff that she quit. Two other cases showed NPPs integrate more easily because of mutual respect, defined responsibilities, and an alignment of expectations between both hospitalists and NPPs.
Mac McCormick, MD, vice president of clinical services at Eagle Hospital Physicians in Atlanta, offers the following suggestions to best utilize NPPs:
•Conduct an analysis of your practice environment, bylaws, staff experience, and pre-existing attitudes to identify potential barriers and optimal opportunities in hiring NPPs;
•Avoid pigeonholing NPPs into such narrow roles as completing discharge summaries or collecting data. Tasks that tend not to utilize their skills set might lead to professional dissatisfaction and likely aren't the most cost-efficient use of resources; and
•Approach things in a team model. Shared visits are one way to accomplish this. Keep communication lines open to make sure accurate and timely information is available to all.
Tackle Medical School Debt
Overwhelmed by medical school debt? You're not alone. A 2008 American Medical Association survey showed that the average 2007 medical school graduate was left with a $139,000 debt burden and a powerful incentive to avoid primary care.
But according to Renee Zerehi, the American College of Physicians' manager of health policy, increasing numbers of students choose HM because of flexible scheduling and opportunities to reduce their debt. Zerehi and Bijo Chacko, MD, FHM, a member of SHM's Young Physicians Committee and hospitalist program medical director for Preferred Health Partners in New York City, offer these strategies for debt reduction.
•Understand your debt portfolio: Talk to a financial consultant to assess debt, your family situation, and lifestyle issues. "A strong, keen understanding of how debt impacts your budget is essential," Dr. Chacko says. Medical school loans often come with different interest rates and grace periods, so try to pay off the high-interest loans immediately, he explains.
•Consolidate your debt: Loans from different lenders with different balances, interest rates, and due dates may best be handled by a federal consolidation loan. The AMA explains it all in
"The Ins and Outs of Student Loan Consolidation."
•Student loan forgiveness: A number of hospitalist programs offer loan repayment programs. The National Health Service Corps, the Health Professions Scholarship Program, and state loan repayment programs offer loan forgiveness for physicians practicing in underserved areas. Visit the AAMC Web site for a comprehensive list.
•NIH Faculty Loan Forgiveness: For academic hospitalists doing research, the National Institutes of Health (NIH) offers up to $35,000 a year for loan repayment and tax reimbursement for each year of service.
For more information, visit SHM's Young Physician microsite.
Overwhelmed by medical school debt? You're not alone. A 2008 American Medical Association survey showed that the average 2007 medical school graduate was left with a $139,000 debt burden and a powerful incentive to avoid primary care.
But according to Renee Zerehi, the American College of Physicians' manager of health policy, increasing numbers of students choose HM because of flexible scheduling and opportunities to reduce their debt. Zerehi and Bijo Chacko, MD, FHM, a member of SHM's Young Physicians Committee and hospitalist program medical director for Preferred Health Partners in New York City, offer these strategies for debt reduction.
•Understand your debt portfolio: Talk to a financial consultant to assess debt, your family situation, and lifestyle issues. "A strong, keen understanding of how debt impacts your budget is essential," Dr. Chacko says. Medical school loans often come with different interest rates and grace periods, so try to pay off the high-interest loans immediately, he explains.
•Consolidate your debt: Loans from different lenders with different balances, interest rates, and due dates may best be handled by a federal consolidation loan. The AMA explains it all in
"The Ins and Outs of Student Loan Consolidation."
•Student loan forgiveness: A number of hospitalist programs offer loan repayment programs. The National Health Service Corps, the Health Professions Scholarship Program, and state loan repayment programs offer loan forgiveness for physicians practicing in underserved areas. Visit the AAMC Web site for a comprehensive list.
•NIH Faculty Loan Forgiveness: For academic hospitalists doing research, the National Institutes of Health (NIH) offers up to $35,000 a year for loan repayment and tax reimbursement for each year of service.
For more information, visit SHM's Young Physician microsite.
Overwhelmed by medical school debt? You're not alone. A 2008 American Medical Association survey showed that the average 2007 medical school graduate was left with a $139,000 debt burden and a powerful incentive to avoid primary care.
But according to Renee Zerehi, the American College of Physicians' manager of health policy, increasing numbers of students choose HM because of flexible scheduling and opportunities to reduce their debt. Zerehi and Bijo Chacko, MD, FHM, a member of SHM's Young Physicians Committee and hospitalist program medical director for Preferred Health Partners in New York City, offer these strategies for debt reduction.
•Understand your debt portfolio: Talk to a financial consultant to assess debt, your family situation, and lifestyle issues. "A strong, keen understanding of how debt impacts your budget is essential," Dr. Chacko says. Medical school loans often come with different interest rates and grace periods, so try to pay off the high-interest loans immediately, he explains.
•Consolidate your debt: Loans from different lenders with different balances, interest rates, and due dates may best be handled by a federal consolidation loan. The AMA explains it all in
"The Ins and Outs of Student Loan Consolidation."
•Student loan forgiveness: A number of hospitalist programs offer loan repayment programs. The National Health Service Corps, the Health Professions Scholarship Program, and state loan repayment programs offer loan forgiveness for physicians practicing in underserved areas. Visit the AAMC Web site for a comprehensive list.
•NIH Faculty Loan Forgiveness: For academic hospitalists doing research, the National Institutes of Health (NIH) offers up to $35,000 a year for loan repayment and tax reimbursement for each year of service.
For more information, visit SHM's Young Physician microsite.