User login
The blade, the flea, and the colon
As Elder et al point out in this issue of the Journal, the management of ischemic colitis presents an interesting clinical paradox: the internist makes the diagnosis of potentially life-threatening impending tissue necrosis, while the surgeon, consulted to act, tends to be a cheerleader for temperate observation.
Ischemic colitis may account for 1 in 1,000 hospitalizations. Many patients present with a combination of focal lower abdominal pain and some bloody diarrhea. The examiner often localizes the tender colon either by anterior palpation or by rectal examination, unlike the scenario of life-threatening small bowel ischemia, in which severe pain may be accompanied by a fairly “benign” examination.
Some cases of ischemic colitis require resection of a gangrenous colon or become chronic and lead to the development of a stricture. But far more often the ischemic episode resolves after several days of watchful waiting. The typical but not specific endoscopic findings and the thumb-printing and thickening seen on radiographic imaging resolve.
Whatever the assumed cause (a specific one is often not found), ischemic colitis gives the internist and the surgeon a chance to commiserate on the power of informed watchful waiting.
As Elder et al point out in this issue of the Journal, the management of ischemic colitis presents an interesting clinical paradox: the internist makes the diagnosis of potentially life-threatening impending tissue necrosis, while the surgeon, consulted to act, tends to be a cheerleader for temperate observation.
Ischemic colitis may account for 1 in 1,000 hospitalizations. Many patients present with a combination of focal lower abdominal pain and some bloody diarrhea. The examiner often localizes the tender colon either by anterior palpation or by rectal examination, unlike the scenario of life-threatening small bowel ischemia, in which severe pain may be accompanied by a fairly “benign” examination.
Some cases of ischemic colitis require resection of a gangrenous colon or become chronic and lead to the development of a stricture. But far more often the ischemic episode resolves after several days of watchful waiting. The typical but not specific endoscopic findings and the thumb-printing and thickening seen on radiographic imaging resolve.
Whatever the assumed cause (a specific one is often not found), ischemic colitis gives the internist and the surgeon a chance to commiserate on the power of informed watchful waiting.
As Elder et al point out in this issue of the Journal, the management of ischemic colitis presents an interesting clinical paradox: the internist makes the diagnosis of potentially life-threatening impending tissue necrosis, while the surgeon, consulted to act, tends to be a cheerleader for temperate observation.
Ischemic colitis may account for 1 in 1,000 hospitalizations. Many patients present with a combination of focal lower abdominal pain and some bloody diarrhea. The examiner often localizes the tender colon either by anterior palpation or by rectal examination, unlike the scenario of life-threatening small bowel ischemia, in which severe pain may be accompanied by a fairly “benign” examination.
Some cases of ischemic colitis require resection of a gangrenous colon or become chronic and lead to the development of a stricture. But far more often the ischemic episode resolves after several days of watchful waiting. The typical but not specific endoscopic findings and the thumb-printing and thickening seen on radiographic imaging resolve.
Whatever the assumed cause (a specific one is often not found), ischemic colitis gives the internist and the surgeon a chance to commiserate on the power of informed watchful waiting.
Clinical approach to colonic ischemia
Ischemic colitis is one of the diagnoses that should be considered when patients present with abdominal pain, diarrhea, and intestinal bleeding (others are infectious colitis, inflammatory bowel disease, diverticulitis, and colon cancer). Its incidence is difficult to determine, as many mild cases are transient and are either not reported or misdiagnosed. However, it is the most common type of intestinal ischemia1 and is responsible for an estimated 1 in 2,000 hospital admissions.2
In this review, we review the main causes of and risk factors for colonic ischemia and discuss how to diagnose and treat it.
BLOOD SUPPLY IS TENUOUS IN ‘WATERSHED’ AREAS
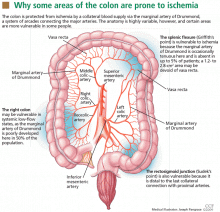
The superior and inferior mesenteric arteries have an extensive network of collateral blood vessels at both the base and border of the mesentery, called the arch of Riolan and the marginal artery of Drummond, respectively.
MANY POSSIBLE CAUSES AND FACTORS
Age. Ischemic colitis most often occurs in elderly people; the average age is 70 years.6 Binns and Isaacson7 suggest that age-related tortuosity of the colonic arteries increases vascular resistance and contributes to colonic ischemia in elderly patients.
Hypotension and hypovolemia are the most common mechanisms of colonic ischemia. Hypotension can be due to sepsis or impaired left ventricular function, and hypovolemia can be caused by dehydration or bleeding. These result in systemic hypoperfusion, triggering a mesenteric vasoconstrictive reflex. Once the hypoperfusion resolves and blood flow to the ulcerated portions resumes, bleeding develops from reperfusion injury.8
Cardiac thromboembolism can also contribute to colonic ischemia. Hourmand-Ollivier et al9 found a cardiac source of embolism in almost one-third of patients who had ischemic colitis, suggesting the need for routine screening with electrocardiography, Holter monitoring, and transthoracic echocardiography.
Myocardial infarction. Cappell10 found, upon colonoscopic examination, that about 14% of patients who developed hematochezia after a myocardial infarction had ischemic colitis. These patients had more complications and a worse in-hospital prognosis than did patients who had ischemic colitis due to other causes.11
Major vascular surgical procedures can disrupt the colonic blood supply, and in two case series,12,13 up to 7% of patients who underwent endoscopy after open aortoiliac reconstructive surgery had evidence of ischemic colitis. In other series,14,15 the segment most often affected was the distal left colon, and the cause was iatrogenic ligation of the inferior mesenteric artery or intraoperative hypoperfusion in patients with chronic occlusion of this artery. Endovascular repair of aortoiliac aneurysm also carries a risk of ischemic colitis, though this risk is smaller (< 2%).16
Hypercoagulable states. The role of acquired or hereditary hypercoagulable states in colonic ischemia has not been extensively investigated and remains poorly understood.
Conditions that increase clotting can cause thrombotic occlusion of small vessels that supply the colon, leading to ischemia. In small retrospective studies and case reports,17–26 28% to 74% of patients who had ischemic colitis had abnormal results on tests for protein C deficiency, protein S deficiency, antithrombin III deficiency, antiphospholipid antibodies, the factor V Leiden mutation, and the prothrombin G20210A mutation. However, in what percentage of cases the abnormality actually caused the ischemic colitis remains unknown.
Arnott et al27 reported that 9 of 24 patients with ischemic colitis had abnormal results on testing for hypercoagulable conditions. Three patients had mildly persistent elevation in anticardiolipin antibodies, but none had the factor V Leiden mutation or a deficiency of protein C, protein S, or antithrombin.
Koutroubakis et al20 reported significantly higher prevalences of antiphospholipid antibodies and heterogeneity for the factor V Leiden mutation in 35 patients with a history of ischemic colitis than in 18 patients with diverticulitis and 52 healthy controls (19.4% vs 0 and 1.9%, 22.2% vs 0 and 3.8%, respectively). Overall, 26 (72%) of 36 patients had at least one abnormal hypercoagulable test result.
Most patients with ischemic colitis are relatively old (over 60 years), and many have multiple concomitant vascular risk factors, suggesting that many factors contribute to ischemic colitis and that thrombophilia is not necessarily the direct cause. Hypercoagulable states may play a more important role in young, healthy patients who present with chronic or recurrent colonic ischemia.
Because no large clinical trials have been done and data are scarce and limited to case reports and small retrospective studies, a hypercoagulable evaluation is reserved for younger patients and those with recurrent, unexplained ischemic colitis.
Even if we detect thrombophilia, nobody yet knows what the appropriate medical treatment should be. Although some cases of ischemic colitis with associated thrombophilia have been successfully treated with anticoagulants,28,29 the benefit of diagnosing and treating a hypercoagulable state in ischemic colitis is still unproven. Therefore, oral anticoagulation should be used only in those in whom a hypercoagulable state is the most likely cause of severe or recurrent colonic ischemia.
There are no official guidelines on the duration of anticoagulation in such patients, but we treat for 6 months and consider indefinite treatment if the ischemic colitis recurs.
Medications that should always be considered as possible culprits include:
- Alosetron (Lotronex), which was temporarily withdrawn by the US Food and Drug Administration because of its association with ischemic colitis when prescribed to treat diarrhea-predominant irritable bowel syndrome.30 It was later reinstated, with some restrictions.
- Digitalis
- Diuretics
- Estrogens
- Danazol (Danocrine)
- Nonsteroidal anti-inflammatory drugs
- Tegaserod (Zelnorm)
- Paclitaxel (Abraxane)
- Carboplatin (Paraplatin)
- Sumatriptan (Imitrex)
- Simvastatin (Zocor)
- Interferon-ribavirin31
- Pseudoephedrine (eg, Sudafed).32
Endoscopic retrograde cholangiopancreatography can cause ischemic colitis if the rare life-threatening complication of mesenteric hematoma occurs.33
Chronic constipation can lead to colonic ischemia by increasing intraluminal pressure, which hinders blood flow and reduces the arteriovenous oxygen gradient in the colonic wall.34,35
Long-distance running can cause sustained bouts of ischemia, likely due to shunting of blood away from the splanchnic circulation, along with dehydration and electrolyte abnormalities. Affected runners present with lower abdominal pain and hematochezia. The colitis usually resolves without sequelae with rehydration and electrolyte correction.36
Vasospasm has been described as a cause of ischemia. During systemic hypoperfusion, vasoactive substances shunt blood from the gut to the brain through mesenteric vasoconstriction.37 This phenomenon can occur in dehydration-induced hypotension, heart failure, septic shock, or exposure to drugs such as antihypertensive medications, digoxin, or cocaine. Necrosis of the villous layer and transmural infarctions can occur with uninterrupted ischemia lasting more than 8 hours.38
Snake venom. The bite of Agkistrodon blomhoffii brevicaudus, a pit viper found in China and Korea, was recently reported to cause transient ischemic colitis due to disseminated intravascular coagulation. The condition resolved in about 10 days after treatment with polyvalent antivenom solution, transfusion of platelets and fresh frozen plasma, and empirically chosen antibiotics, ie, ampicillin-sulbactam (Unasyn) and metronidazole (Flagyl).39
CLINICAL MANIFESTATIONS
As stated above, ischemic colitis should be included in the differential diagnosis when assessing patients with abdominal pain, diarrhea, or bloody stools.
Typical presentation
The typical presentation of acute colonic ischemia includes:
- Rapid onset of mild abdominal pain
- Tenderness over the affected bowel area, usually on the left side near the splenic flexure or the rectosigmoid junction
- Mild to moderate hematochezia beginning within 1 day of the onset of abdominal pain. The bleeding is often not profuse and does not cause hemodynamic instability or require transfusion.40
Differentiate from mesenteric ischemia
It is important to differentiate between ischemic colitis and mesenteric ischemia, which is more serious and affects the small bowel.
Most patients with acute mesenteric ischemia complain of sudden onset of severe abdominal pain out of proportion to the tenderness on physical examination, they appear profoundly ill, and they do not have bloody stools until the late stages. They need urgent mesenteric angiography or another fast imaging test.4
In contrast, many patients with chronic mesenteric ischemia (or “abdominal angina”) report recurrent severe postprandial abdominal pain, leading to fear of food and weight loss.
Varies in severity
The severity of ischemic colitis varies widely, with hypoperfusion affecting as little as a single segment or as much as the entire colon. Over three-fourths of cases are the milder, nongangrenous form, which is temporary and rarely causes long-term complications such as persistent segmental colitis or strictures.41 In contrast, gangrenous colonic ischemia, which accounts for about 15% of cases, can be life-threatening.
Colonic ischemia can be categorized according to its severity and clinical presentation42:
- Reversible colonopathy (submucosal or intramural hemorrhage)
- Transient colitis (45% of cases were reversible or transient in a 1978 report by Boley et al43)
- Chronic colitis (19% of cases)
- Stricture (13%)
- Gangrene (19%)
- Fulminant universal colitis.
The resulting ischemic injury can range from superficial mucosal damage to mural or even full-thickness transmural infarction.44
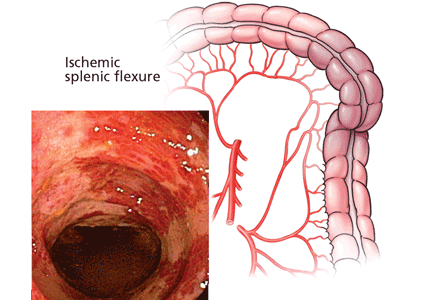
Although most cases involve the left colon, about one-fourth involve the right. Right-sided colonic ischemia tends to be more severe: about 60% of patients require surgery (five times more than with colitis of other regions), and the death rate is twice as high (close to 23%).45
DIAGNOSIS DEPENDS ON SUSPICION
The diagnosis of colonic ischemia largely depends on clinical suspicion, especially since many other conditions (eg, infectious colitis, inflammatory bowel disease, diverticulitis, colon cancer) present with abdominal pain, diarrhea, and hematochezia. One study showed that a clinical presentation of lower abdominal pain or bleeding, or both, was 100% predictive of ischemic colitis when accompanied by four or more of the following risk factors: age over 60, hemodialysis, hypertension, hypoalbuminemia, diabetes mellitus, or drug-induced constipation. 46
Stool studies can identify organisms
Invasive infections with Salmonella, Shigella, and Campylobacter species and Eschericia coli O157:H7 should be identified early with stool studies if the patient presents as an outpatient or has been hospitalized less than 72 hours. Parasites such as Entamoeba histolytica and Angiostrongylus costaricensis and viruses such as cytomegalovirus should be considered in the differential diagnosis, as they can cause ischemic colitis.41,47Clostridium difficile should be excluded in those exposed to antibiotics.
Blood tests may indicate tissue injury
Although no laboratory marker is specific for ischemic colitis, elevated serum levels of lactate, lactate dehydrogenase, creatine kinase, or amylase may indicate tissue injury. The combination of abdominal pain, a white blood cell count greater than 20 × 109/L, and metabolic acidosis suggests intestinal ischemia and infarction.
Endoscopy is the test of choice
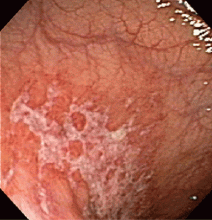
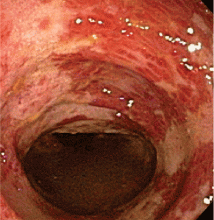
Endoscopy has become the diagnostic test of choice in establishing the diagnosis of ischemic colitis, although computed tomography (CT) can provide suggestive findings and exclude other illnesses. Colonoscopy has almost completely replaced radiography with bariumenema contrast as a diagnostic tool because it is more sensitive for detecting mucosal changes, it directly visualizes the mucosa, and it can be used to obtain biopsy specimens.48
Colonoscopy is performed without bowel preparation to prevent hypoperfusion caused by dehydrating cathartics. In addition, the scope should not be advanced beyond the affected area, and minimal air insufflation should be used to prevent perforation.
Endoscopic findings can help differentiate ischemic colitis from other, clinically similar diseases. For instance, unlike irritable bowel disease, ischemic colitis tends to affect a discrete segment with a clear delineation between affected and normal mucosa, it spares the rectum, the mucosa heals rapidly as seen on serial colonoscopic examinations, and a single linear ulcer, termed the “single-stripe” sign, runs along the longitudinal axis of the colon.49,50
Biopsy features are not specific, as findings of hemorrhage, capillary thrombosis, granulation tissue with crypt abscesses, and pseudopolyps can also be seen in other conditions, such as Crohn disease.54,55
Imaging studies are not specific
Imaging studies are often used, but the findings lack specificity.
Plain abdominal radiography can help only in advanced ischemia, in which distention or pneumatosis can be seen.
CT with contrast can reveal thickening of the colon wall in a segmental pattern in ischemic colitis, but this finding also can be present in infectious and Crohn colitis. CT findings of colonic ischemia also include pericolic streakiness and free fluid. Pneumatosis coli often signifies infarcted bowel.56 However, CT findings can be completely normal in mild cases or if done early in the course.
Angiography in severe cases
Since colonic ischemia is most often transient, mesenteric angiography is not indicated in mild cases. Angiography is only considered in more severe cases, especially when only the right colon is involved, the diagnosis of colonic ischemia has not yet been determined, and acute mesenteric ischemia needs to be excluded. A focal lesion is often seen in mesenteric ischemia, but not often in colonic ischemia.
Looking for the underlying cause
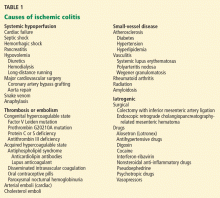
Once the diagnosis of ischemic colitis is made, an effort should be made to identify the cause (Table 1). The initial step can be to remove or treat reversible causes such as medications or infections. As mentioned earlier, electrocardiography, Holter monitoring, and transthoracic echocardiography should be considered in patients with ischemic colitis to rule out cardiac embolic sources.9 A hypercoagulable workup can be done, but only in young patients without other clear causes or patients with recurrent events.
CONSERVATIVE TREATMENT IS ENOUGH FOR MOST
Empirically chosen broad-spectrum antibiotics that cover both aerobic and anaerobic coliform bacteria are reserved for patients with moderate to severe colitis to minimize bacterial translocation and sepsis.
Whenever symptomatic ileus is present, a nasogastric tube should be placed to alleviate vomiting and abdominal discomfort.
Antiplatelet agents have not been evaluated in treating ischemic colitis and are generally not used. As mentioned earlier, anticoagulation has been used in patients who have been proven to have hypercoagulable conditions,28,29 but its benefit is not yet proven. Currently, if the coagulation profile is abnormal, anticoagulation should be used only in cases of recurrent colonic ischemia or in young patients with severe cases in the absence of a clear cause. Anticoagulation should also be used in confirmed cases of cardiac embolization.
Surgery for some
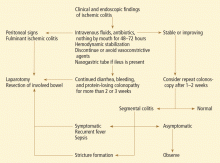
Exploratory laparotomy with possible subtotal or segmental colectomy may be needed in acute, subacute, or chronic settings.42 Acute indications include peritoneal signs, massive bleeding, and fulminant ischemic colitis. Subacute indications are lack of resolution, with symptoms that persist for more than 2 or 3 weeks, or malnutrition or hypoalbuminemia due to protein-losing colonopathy. Colon stricture can be chronic and becomes an indication for surgery only when symptomatic, as some strictures resolve with time (months to years).
Right hemicolectomy and primary anastomosis of viable remaining colon is performed for right-sided colonic ischemia and necrosis, while left-sided colonic ischemia is managed with a proximal stoma and distal mucous fistula, or Hartmann procedure. Re-anastomosis and ostomy closure are usually done after 4 to 6 months.57 However, resection and primary anastomosis can also be an option for patients with isolated ischemia of the sigmoid colon.58 Transendoscopic dilation or stenting of short strictures can be an alternative to surgery, although experience with this is limited.59,60
THE PROGNOSIS IS USUALLY GOOD
The prognosis depends on the extent of injury and comorbidities. Transient, self-limited ischemia involving the mucosa and submucosa has a good prognosis, while fulminant ischemia with transmural infarction carries a poor one, as it can progress to necrosis and death.
Although up to 85% of cases of ischemic colitis managed conservatively improve within 1 or 2 days and resolve completely within 1 or 2 weeks, close to one-fifth of patients develop peritonitis or deteriorate clinically and require surgery.61,62 Surgical resection is required when irreversible ischemic injury and chronic colitis develop, as both can lead to bacteremia and sepsis, colonic stricture, persistent abdominal pain and bloody diarrhea, and protein-losing enteropathy.40
- Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther 2004; 19:729–738.
- Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 7th ed. Philadelphia, PA: Saunders; 2002.
- Gandhi SK, Hanson MM, Vernava AM, Kaminski DL, Longo WE. Ischemic colitis. Dis Colon Rectum 1996; 39:88–100.
- Greenwald DA, Brandt LJ, Reinus JF. Ischemic bowel disease in the elderly. Gastroenterol Clin North Am 2001; 30:445–473.
- Reeders JW, Tytgat GN, Rosenbusch G, et al. Ischaemic colitis. The Hague: Martinus Nijhoff, 1984;17.
- Brandt L, Boley S, Goldberg L, Mitsudo S, Berman A. Colitis in the elderly. A reappraisal. Am J Gastroenterol 1981; 76:239–245.
- Binns JC, Isaacson P. Age-related changes in the colonic blood supply: their relevance to ischaemic colitis. Gut 1978; 19:384–390.
- Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am 1992; 72:65–83.
- Hourmand-Ollivier I, Bouin M, Saloux E, et al. Cardiac sources of embolism should be routinely screened in ischemic colitis. Am J Gastroenterol 2003; 98:1573–1577.
- Cappell MS. Safety and efficacy of colonoscopy after myocardial infarction: an analysis of 100 study patients and 100 control patients at two tertiary cardiac referral hospitals. Gastrointest Endosc 2004; 60:901–909.
- Cappell MS, Mahajan D, Kurupath V. Characterization of ischemic colitis associated with myocardial infarction: an analysis of 23 patients. Am J Med 2006; 119:527.e1–e9.
- Hagihara PF, Ernst CB, Griffen WO. Incidence of ischemic colitis following abdominal aortic reconstruction. Surg Gynecol Obstet 1979; 149:571–573.
- Brewster DC, Franklin DP, Cambria RP, et al. Intestinal ischemia complicating abdominal aortic surgery. Surgery 1991; 109:447–454.
- Piotrowski JJ, Ripepi AJ, Yuhas JP, Alexander JJ, Brandt CP. Colonic ischemia: the Achilles heel of ruptured aortic aneurysm repair. Am Surg 1996; 62:557–560.
- Ernst CB. Colonic ischemia following aortic reconstruction. In: Rutherford RB, editor. Vascular Surgery. 4th ed. Philadelphia, PA: WB Saunders; 1995:1312–1320.
- Geroghty PS, Sanchez LA, Rubin BG, et al. Overt ischemic colitis after endovascular repair of aortoiliac aneurysm. J Vasc Surg 2004; 40:413–418.
- Klestzick HN, McPhedran P, Cipolla D, Berry WA, DiCorato M, Denowitz J. The antiphospholipid syndrome and ischemic colitis. Gastroenterologist 1995; 3:249–256.
- Knot EA, ten Cate JW, Bruin T, Iburg AH, Tytgat GN. Antithrombin III metabolism in two colitis patients with acquired antithrombin III deficiency. Gastroenterology 1985; 89:421–425.
- Maloisel F. Role of coagulation disorders in mesenteric ischemia. J Chir (Paris) 1996; 133:442–447.
- Koutroubakis IE, Sfiridaki A, Theodoropoulou A, Kouroumalis EA. Role of acquired and hereditary thrombotic risk factors in colon ischemia of ambulatory patients. Gastroenterology 2001; 121:561–565.
- Midian-Singh R, Polen A, Durishin C, Crock RD, Whittier FC, Fahmy N. Ischemic colitis revisited: a prospective study identifying hypercoagulability as a risk factor. South Med J 2004; 97:120–123.
- Blanc P, Bories P, Donadio D, et al. Ischemic colitis and recurrent venous thrombosis caused by familial protein S deficiency. Gastroenterol Clin Biol 1989; 13:945.
- Verger P, Blanc C, Feydy P, Boey S. Ischemic colitis caused by protein S deficiency. Presse Med 1996; 25:1350.
- Ludwig D, Stahl M, David-Walek T, et al. Ischemic colitis, pulmonary embolism, and atrial thrombosis in a patient with inherited resistance to activated protein C. Dig Dis Sci 1998; 43:1362–1367.
- Yee NS, Guerry D, Lichtenstein GR. Ischemic colitis associated with factor V Leiden mutation. Ann Intern Med 2000; 132:595–596.
- Balian A, Veyradier A, Naveau S, et al. Prothrombin 20210G/A mutation in two patients with mesenteric ischemia. Dig Dis Sci 1999; 44:1910–1913.
- Arnott ID, Ghosh S, Ferguson A. The spectrum of ischaemic colitis. Eur J Gastroenterol Hepatol 1999; 11:295–303.
- Chin BW, Greenberg D, Wilson RB, Meredith CG. A case of ischemic colitis associated with factor V Leiden mutation: successful treatment with anticoagulation. Gastrointest Endosc 2007; 66:416–418.
- Heyn J, Buhmann S, Ladurner R, et al. Recurrent ischemic colitis in a patient with Leiden factor V mutation and systemic lupus erythematosus with antiphospholipid syndrome. Eur J Med Res 2008; 13:182–184.
- Chang L, Chey WD, Harris L, Olden K, Surawicz C, Schoenfeld P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol 2006; 101:1069–1079.
- Punnam SR, Pothula VR, Gourineni N, Punnam A, Ranganathan V. Interferon-ribavirin-associated ischemic colitis. J Clin Gastroenterol 2008; 42:323–325.
- Dowd J, Bailey D, Moussa K, Nair S, Doyle R, Culpepper-Morgan JA. Ischemic colitis associated with pseudoephedrine: four cases. Am J Gastroenterol 1999; 94:2430–2434.
- Kingsley DD, Schermer CR, Jamal MM. Rare complications of endoscopic retrograde cholangiopancreatography: two case reports. JSLS 2001; 5:171–173.
- Boley SJ, Agrawal GP, Warren AR, et al. Pathophysiologic effects of bowel distension on intestinal blood flow. Am J Surg 1969; 117:228–234.
- Reinus JF, Brandt LJ, Boley SJ. Ischemic diseases of the bowel. Gastroenterol Clin North Am 1990; 19:319–343.
- Moses FM. Exercise-associated intestinal ischemia. Curr Sports Med Rep 2005; 4:91–95.
- Rosenblum JD, Boyle CM, Schwartz LB. The mesenteric circulation. Anatomy and physiology. Surg Clin North Am 1997; 77:289–306.
- Haglund U, Bulkley GB, Granger DN. On the pathophysiology of intestinal ischemic injury. Clinical review. Acta Chir Scand 1987; 153:321–324.
- Kim MK, Cho YS, Kim HK, Kim JS, Kim SS, Chae HS. Transient ischemic colitis after a pit viper bite (Agkistrodon blomhoffii brevicaudus). J Clin Gastroenterol 2008; 42:111–112.
- Cappell MS. Intestinal (mesenteric) vasculopathy. II. Ischemic colitis and chronic mesenteric ischemia. Gastroenterol Clin North Am 1998; 27:827–860.
- Greenwald DA, Brandt LJ. Colonic ischemia. J Clin Gastroenterol 1998; 27:122–128.
- Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology 2000; 118:954–968.
- Boley SJ, Brandt LJ, Veith FJ. Ischemic disorders of the intestines. Curr Probl Surg 1978; 15:1–85.
- Schuler JG, Hudlin MM. Cecal necrosis: infrequent variant of ischemic colitis. Report of five cases. Dis Colon Rectum 2000; 43:708–712.
- Sotiriadis J, Brandt LJ, Behin DS, Southern WN. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol 2007; 102:2247–2252.
- Park CJ, Jang MK, Shin WG, et al. Can we predict the development of ischemic colitis among patients with lower abdominal pain? Dis Colon Rectum 2007; 50:232–238.
- Su C, Brandt LJ, Sigal SH, et al. The immunohistological diagnosis of E. coli 0157:H7 colitis: possible association with colonic ischemia. Am J Gastroenterol 1998; 93:1055–1059.
- Scowcroft CW, Sanowski RA, Kozarek RA. Colonoscopy in ischemic colitis. Gastrointest Endosc 1981; 27:156–161.
- Rogers AI, David S. Intestinal blood flow and diseases of vascular impairment. In: Haubrich WS, Schaffner F, Berk JE, editors. Gastroenterology. 5th ed. Philadelphia: WB Saunders; 1995:1212–1234.
- Zuckerman GR, Prakash C, Merriman RB, Sawhney MS, DeSchryver-Kecskemeti K, Clouse RE. The colon single-stripe sign and its relationship to ischemic colitis. Am J Gastroenterol 2003; 98:2018–2022.
- Green BT, Tendler DA. Ischemic colitis: a clinical review. South Med J 2005; 98:217–222.
- Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis. Cleve Clin J Med 2003; 70:920–930.
- Habu Y, Tahashi Y, Kiyota K, et al. Reevaluation of clinical features of ischemic colitis: analysis of 68 consecutive cases diagnosed by early colonoscopy. Scand J Gastroenterol 1996; 31:881–886.
- Mitsudo S, Brandt LJ. Pathology of intestinal ischemia. Surg Clin North Am 1992; 72:43–63.
- Price AB. Ischaemic colitis. Curr Top Pathol 1990; 81:229–246.
- Balthazar EJ, Yen BC, Gordon RB. Ischemic colitis: CT evaluation of 54 cases. Radiology 1999; 211:381–388.
- Mosdell DM, Doberneck RC. Morbidity and mortality of ostomy closure. Am J Surg 1991; 162:633–636.
- Iqbal T, Zarin M, Iqbal A, et al. Results of primary closure in the management of gangrenous and viable sigmoid volvulus. Pak J Surg 2007; 23:118–121.
- Oz MC, Forde KA. Endoscopic alternatives in the management of colonic strictures. Surgery 1990; 108:513–519.
- Profili S, Bifulco V, Meloni GB, Demelas L, Niolu P, Manzoni MA. A case of ischemic stenosis of the colon-sigmoid treated with self-expandable uncoated metallic prosthesis. Radiol Med 1996; 91:665–667.
- Brandt LJ, Boley SJ. Colonic ischemia. Surg Clin North Am 1992; 72:203–229.
- Boley SJ. 1989 David H. Sun lecture. Colonic ischemia—25 years later. Am J Gastroenterol 1990; 85:931–934.
Ischemic colitis is one of the diagnoses that should be considered when patients present with abdominal pain, diarrhea, and intestinal bleeding (others are infectious colitis, inflammatory bowel disease, diverticulitis, and colon cancer). Its incidence is difficult to determine, as many mild cases are transient and are either not reported or misdiagnosed. However, it is the most common type of intestinal ischemia1 and is responsible for an estimated 1 in 2,000 hospital admissions.2
In this review, we review the main causes of and risk factors for colonic ischemia and discuss how to diagnose and treat it.
BLOOD SUPPLY IS TENUOUS IN ‘WATERSHED’ AREAS
The superior and inferior mesenteric arteries have an extensive network of collateral blood vessels at both the base and border of the mesentery, called the arch of Riolan and the marginal artery of Drummond, respectively.
MANY POSSIBLE CAUSES AND FACTORS
Age. Ischemic colitis most often occurs in elderly people; the average age is 70 years.6 Binns and Isaacson7 suggest that age-related tortuosity of the colonic arteries increases vascular resistance and contributes to colonic ischemia in elderly patients.
Hypotension and hypovolemia are the most common mechanisms of colonic ischemia. Hypotension can be due to sepsis or impaired left ventricular function, and hypovolemia can be caused by dehydration or bleeding. These result in systemic hypoperfusion, triggering a mesenteric vasoconstrictive reflex. Once the hypoperfusion resolves and blood flow to the ulcerated portions resumes, bleeding develops from reperfusion injury.8
Cardiac thromboembolism can also contribute to colonic ischemia. Hourmand-Ollivier et al9 found a cardiac source of embolism in almost one-third of patients who had ischemic colitis, suggesting the need for routine screening with electrocardiography, Holter monitoring, and transthoracic echocardiography.
Myocardial infarction. Cappell10 found, upon colonoscopic examination, that about 14% of patients who developed hematochezia after a myocardial infarction had ischemic colitis. These patients had more complications and a worse in-hospital prognosis than did patients who had ischemic colitis due to other causes.11
Major vascular surgical procedures can disrupt the colonic blood supply, and in two case series,12,13 up to 7% of patients who underwent endoscopy after open aortoiliac reconstructive surgery had evidence of ischemic colitis. In other series,14,15 the segment most often affected was the distal left colon, and the cause was iatrogenic ligation of the inferior mesenteric artery or intraoperative hypoperfusion in patients with chronic occlusion of this artery. Endovascular repair of aortoiliac aneurysm also carries a risk of ischemic colitis, though this risk is smaller (< 2%).16
Hypercoagulable states. The role of acquired or hereditary hypercoagulable states in colonic ischemia has not been extensively investigated and remains poorly understood.
Conditions that increase clotting can cause thrombotic occlusion of small vessels that supply the colon, leading to ischemia. In small retrospective studies and case reports,17–26 28% to 74% of patients who had ischemic colitis had abnormal results on tests for protein C deficiency, protein S deficiency, antithrombin III deficiency, antiphospholipid antibodies, the factor V Leiden mutation, and the prothrombin G20210A mutation. However, in what percentage of cases the abnormality actually caused the ischemic colitis remains unknown.
Arnott et al27 reported that 9 of 24 patients with ischemic colitis had abnormal results on testing for hypercoagulable conditions. Three patients had mildly persistent elevation in anticardiolipin antibodies, but none had the factor V Leiden mutation or a deficiency of protein C, protein S, or antithrombin.
Koutroubakis et al20 reported significantly higher prevalences of antiphospholipid antibodies and heterogeneity for the factor V Leiden mutation in 35 patients with a history of ischemic colitis than in 18 patients with diverticulitis and 52 healthy controls (19.4% vs 0 and 1.9%, 22.2% vs 0 and 3.8%, respectively). Overall, 26 (72%) of 36 patients had at least one abnormal hypercoagulable test result.
Most patients with ischemic colitis are relatively old (over 60 years), and many have multiple concomitant vascular risk factors, suggesting that many factors contribute to ischemic colitis and that thrombophilia is not necessarily the direct cause. Hypercoagulable states may play a more important role in young, healthy patients who present with chronic or recurrent colonic ischemia.
Because no large clinical trials have been done and data are scarce and limited to case reports and small retrospective studies, a hypercoagulable evaluation is reserved for younger patients and those with recurrent, unexplained ischemic colitis.
Even if we detect thrombophilia, nobody yet knows what the appropriate medical treatment should be. Although some cases of ischemic colitis with associated thrombophilia have been successfully treated with anticoagulants,28,29 the benefit of diagnosing and treating a hypercoagulable state in ischemic colitis is still unproven. Therefore, oral anticoagulation should be used only in those in whom a hypercoagulable state is the most likely cause of severe or recurrent colonic ischemia.
There are no official guidelines on the duration of anticoagulation in such patients, but we treat for 6 months and consider indefinite treatment if the ischemic colitis recurs.
Medications that should always be considered as possible culprits include:
- Alosetron (Lotronex), which was temporarily withdrawn by the US Food and Drug Administration because of its association with ischemic colitis when prescribed to treat diarrhea-predominant irritable bowel syndrome.30 It was later reinstated, with some restrictions.
- Digitalis
- Diuretics
- Estrogens
- Danazol (Danocrine)
- Nonsteroidal anti-inflammatory drugs
- Tegaserod (Zelnorm)
- Paclitaxel (Abraxane)
- Carboplatin (Paraplatin)
- Sumatriptan (Imitrex)
- Simvastatin (Zocor)
- Interferon-ribavirin31
- Pseudoephedrine (eg, Sudafed).32
Endoscopic retrograde cholangiopancreatography can cause ischemic colitis if the rare life-threatening complication of mesenteric hematoma occurs.33
Chronic constipation can lead to colonic ischemia by increasing intraluminal pressure, which hinders blood flow and reduces the arteriovenous oxygen gradient in the colonic wall.34,35
Long-distance running can cause sustained bouts of ischemia, likely due to shunting of blood away from the splanchnic circulation, along with dehydration and electrolyte abnormalities. Affected runners present with lower abdominal pain and hematochezia. The colitis usually resolves without sequelae with rehydration and electrolyte correction.36
Vasospasm has been described as a cause of ischemia. During systemic hypoperfusion, vasoactive substances shunt blood from the gut to the brain through mesenteric vasoconstriction.37 This phenomenon can occur in dehydration-induced hypotension, heart failure, septic shock, or exposure to drugs such as antihypertensive medications, digoxin, or cocaine. Necrosis of the villous layer and transmural infarctions can occur with uninterrupted ischemia lasting more than 8 hours.38
Snake venom. The bite of Agkistrodon blomhoffii brevicaudus, a pit viper found in China and Korea, was recently reported to cause transient ischemic colitis due to disseminated intravascular coagulation. The condition resolved in about 10 days after treatment with polyvalent antivenom solution, transfusion of platelets and fresh frozen plasma, and empirically chosen antibiotics, ie, ampicillin-sulbactam (Unasyn) and metronidazole (Flagyl).39
CLINICAL MANIFESTATIONS
As stated above, ischemic colitis should be included in the differential diagnosis when assessing patients with abdominal pain, diarrhea, or bloody stools.
Typical presentation
The typical presentation of acute colonic ischemia includes:
- Rapid onset of mild abdominal pain
- Tenderness over the affected bowel area, usually on the left side near the splenic flexure or the rectosigmoid junction
- Mild to moderate hematochezia beginning within 1 day of the onset of abdominal pain. The bleeding is often not profuse and does not cause hemodynamic instability or require transfusion.40
Differentiate from mesenteric ischemia
It is important to differentiate between ischemic colitis and mesenteric ischemia, which is more serious and affects the small bowel.
Most patients with acute mesenteric ischemia complain of sudden onset of severe abdominal pain out of proportion to the tenderness on physical examination, they appear profoundly ill, and they do not have bloody stools until the late stages. They need urgent mesenteric angiography or another fast imaging test.4
In contrast, many patients with chronic mesenteric ischemia (or “abdominal angina”) report recurrent severe postprandial abdominal pain, leading to fear of food and weight loss.
Varies in severity
The severity of ischemic colitis varies widely, with hypoperfusion affecting as little as a single segment or as much as the entire colon. Over three-fourths of cases are the milder, nongangrenous form, which is temporary and rarely causes long-term complications such as persistent segmental colitis or strictures.41 In contrast, gangrenous colonic ischemia, which accounts for about 15% of cases, can be life-threatening.
Colonic ischemia can be categorized according to its severity and clinical presentation42:
- Reversible colonopathy (submucosal or intramural hemorrhage)
- Transient colitis (45% of cases were reversible or transient in a 1978 report by Boley et al43)
- Chronic colitis (19% of cases)
- Stricture (13%)
- Gangrene (19%)
- Fulminant universal colitis.
The resulting ischemic injury can range from superficial mucosal damage to mural or even full-thickness transmural infarction.44
Although most cases involve the left colon, about one-fourth involve the right. Right-sided colonic ischemia tends to be more severe: about 60% of patients require surgery (five times more than with colitis of other regions), and the death rate is twice as high (close to 23%).45
DIAGNOSIS DEPENDS ON SUSPICION
The diagnosis of colonic ischemia largely depends on clinical suspicion, especially since many other conditions (eg, infectious colitis, inflammatory bowel disease, diverticulitis, colon cancer) present with abdominal pain, diarrhea, and hematochezia. One study showed that a clinical presentation of lower abdominal pain or bleeding, or both, was 100% predictive of ischemic colitis when accompanied by four or more of the following risk factors: age over 60, hemodialysis, hypertension, hypoalbuminemia, diabetes mellitus, or drug-induced constipation. 46
Stool studies can identify organisms
Invasive infections with Salmonella, Shigella, and Campylobacter species and Eschericia coli O157:H7 should be identified early with stool studies if the patient presents as an outpatient or has been hospitalized less than 72 hours. Parasites such as Entamoeba histolytica and Angiostrongylus costaricensis and viruses such as cytomegalovirus should be considered in the differential diagnosis, as they can cause ischemic colitis.41,47Clostridium difficile should be excluded in those exposed to antibiotics.
Blood tests may indicate tissue injury
Although no laboratory marker is specific for ischemic colitis, elevated serum levels of lactate, lactate dehydrogenase, creatine kinase, or amylase may indicate tissue injury. The combination of abdominal pain, a white blood cell count greater than 20 × 109/L, and metabolic acidosis suggests intestinal ischemia and infarction.
Endoscopy is the test of choice
Endoscopy has become the diagnostic test of choice in establishing the diagnosis of ischemic colitis, although computed tomography (CT) can provide suggestive findings and exclude other illnesses. Colonoscopy has almost completely replaced radiography with bariumenema contrast as a diagnostic tool because it is more sensitive for detecting mucosal changes, it directly visualizes the mucosa, and it can be used to obtain biopsy specimens.48
Colonoscopy is performed without bowel preparation to prevent hypoperfusion caused by dehydrating cathartics. In addition, the scope should not be advanced beyond the affected area, and minimal air insufflation should be used to prevent perforation.
Endoscopic findings can help differentiate ischemic colitis from other, clinically similar diseases. For instance, unlike irritable bowel disease, ischemic colitis tends to affect a discrete segment with a clear delineation between affected and normal mucosa, it spares the rectum, the mucosa heals rapidly as seen on serial colonoscopic examinations, and a single linear ulcer, termed the “single-stripe” sign, runs along the longitudinal axis of the colon.49,50
Biopsy features are not specific, as findings of hemorrhage, capillary thrombosis, granulation tissue with crypt abscesses, and pseudopolyps can also be seen in other conditions, such as Crohn disease.54,55
Imaging studies are not specific
Imaging studies are often used, but the findings lack specificity.
Plain abdominal radiography can help only in advanced ischemia, in which distention or pneumatosis can be seen.
CT with contrast can reveal thickening of the colon wall in a segmental pattern in ischemic colitis, but this finding also can be present in infectious and Crohn colitis. CT findings of colonic ischemia also include pericolic streakiness and free fluid. Pneumatosis coli often signifies infarcted bowel.56 However, CT findings can be completely normal in mild cases or if done early in the course.
Angiography in severe cases
Since colonic ischemia is most often transient, mesenteric angiography is not indicated in mild cases. Angiography is only considered in more severe cases, especially when only the right colon is involved, the diagnosis of colonic ischemia has not yet been determined, and acute mesenteric ischemia needs to be excluded. A focal lesion is often seen in mesenteric ischemia, but not often in colonic ischemia.
Looking for the underlying cause
Once the diagnosis of ischemic colitis is made, an effort should be made to identify the cause (Table 1). The initial step can be to remove or treat reversible causes such as medications or infections. As mentioned earlier, electrocardiography, Holter monitoring, and transthoracic echocardiography should be considered in patients with ischemic colitis to rule out cardiac embolic sources.9 A hypercoagulable workup can be done, but only in young patients without other clear causes or patients with recurrent events.
CONSERVATIVE TREATMENT IS ENOUGH FOR MOST
Empirically chosen broad-spectrum antibiotics that cover both aerobic and anaerobic coliform bacteria are reserved for patients with moderate to severe colitis to minimize bacterial translocation and sepsis.
Whenever symptomatic ileus is present, a nasogastric tube should be placed to alleviate vomiting and abdominal discomfort.
Antiplatelet agents have not been evaluated in treating ischemic colitis and are generally not used. As mentioned earlier, anticoagulation has been used in patients who have been proven to have hypercoagulable conditions,28,29 but its benefit is not yet proven. Currently, if the coagulation profile is abnormal, anticoagulation should be used only in cases of recurrent colonic ischemia or in young patients with severe cases in the absence of a clear cause. Anticoagulation should also be used in confirmed cases of cardiac embolization.
Surgery for some
Exploratory laparotomy with possible subtotal or segmental colectomy may be needed in acute, subacute, or chronic settings.42 Acute indications include peritoneal signs, massive bleeding, and fulminant ischemic colitis. Subacute indications are lack of resolution, with symptoms that persist for more than 2 or 3 weeks, or malnutrition or hypoalbuminemia due to protein-losing colonopathy. Colon stricture can be chronic and becomes an indication for surgery only when symptomatic, as some strictures resolve with time (months to years).
Right hemicolectomy and primary anastomosis of viable remaining colon is performed for right-sided colonic ischemia and necrosis, while left-sided colonic ischemia is managed with a proximal stoma and distal mucous fistula, or Hartmann procedure. Re-anastomosis and ostomy closure are usually done after 4 to 6 months.57 However, resection and primary anastomosis can also be an option for patients with isolated ischemia of the sigmoid colon.58 Transendoscopic dilation or stenting of short strictures can be an alternative to surgery, although experience with this is limited.59,60
THE PROGNOSIS IS USUALLY GOOD
The prognosis depends on the extent of injury and comorbidities. Transient, self-limited ischemia involving the mucosa and submucosa has a good prognosis, while fulminant ischemia with transmural infarction carries a poor one, as it can progress to necrosis and death.
Although up to 85% of cases of ischemic colitis managed conservatively improve within 1 or 2 days and resolve completely within 1 or 2 weeks, close to one-fifth of patients develop peritonitis or deteriorate clinically and require surgery.61,62 Surgical resection is required when irreversible ischemic injury and chronic colitis develop, as both can lead to bacteremia and sepsis, colonic stricture, persistent abdominal pain and bloody diarrhea, and protein-losing enteropathy.40
Ischemic colitis is one of the diagnoses that should be considered when patients present with abdominal pain, diarrhea, and intestinal bleeding (others are infectious colitis, inflammatory bowel disease, diverticulitis, and colon cancer). Its incidence is difficult to determine, as many mild cases are transient and are either not reported or misdiagnosed. However, it is the most common type of intestinal ischemia1 and is responsible for an estimated 1 in 2,000 hospital admissions.2
In this review, we review the main causes of and risk factors for colonic ischemia and discuss how to diagnose and treat it.
BLOOD SUPPLY IS TENUOUS IN ‘WATERSHED’ AREAS
The superior and inferior mesenteric arteries have an extensive network of collateral blood vessels at both the base and border of the mesentery, called the arch of Riolan and the marginal artery of Drummond, respectively.
MANY POSSIBLE CAUSES AND FACTORS
Age. Ischemic colitis most often occurs in elderly people; the average age is 70 years.6 Binns and Isaacson7 suggest that age-related tortuosity of the colonic arteries increases vascular resistance and contributes to colonic ischemia in elderly patients.
Hypotension and hypovolemia are the most common mechanisms of colonic ischemia. Hypotension can be due to sepsis or impaired left ventricular function, and hypovolemia can be caused by dehydration or bleeding. These result in systemic hypoperfusion, triggering a mesenteric vasoconstrictive reflex. Once the hypoperfusion resolves and blood flow to the ulcerated portions resumes, bleeding develops from reperfusion injury.8
Cardiac thromboembolism can also contribute to colonic ischemia. Hourmand-Ollivier et al9 found a cardiac source of embolism in almost one-third of patients who had ischemic colitis, suggesting the need for routine screening with electrocardiography, Holter monitoring, and transthoracic echocardiography.
Myocardial infarction. Cappell10 found, upon colonoscopic examination, that about 14% of patients who developed hematochezia after a myocardial infarction had ischemic colitis. These patients had more complications and a worse in-hospital prognosis than did patients who had ischemic colitis due to other causes.11
Major vascular surgical procedures can disrupt the colonic blood supply, and in two case series,12,13 up to 7% of patients who underwent endoscopy after open aortoiliac reconstructive surgery had evidence of ischemic colitis. In other series,14,15 the segment most often affected was the distal left colon, and the cause was iatrogenic ligation of the inferior mesenteric artery or intraoperative hypoperfusion in patients with chronic occlusion of this artery. Endovascular repair of aortoiliac aneurysm also carries a risk of ischemic colitis, though this risk is smaller (< 2%).16
Hypercoagulable states. The role of acquired or hereditary hypercoagulable states in colonic ischemia has not been extensively investigated and remains poorly understood.
Conditions that increase clotting can cause thrombotic occlusion of small vessels that supply the colon, leading to ischemia. In small retrospective studies and case reports,17–26 28% to 74% of patients who had ischemic colitis had abnormal results on tests for protein C deficiency, protein S deficiency, antithrombin III deficiency, antiphospholipid antibodies, the factor V Leiden mutation, and the prothrombin G20210A mutation. However, in what percentage of cases the abnormality actually caused the ischemic colitis remains unknown.
Arnott et al27 reported that 9 of 24 patients with ischemic colitis had abnormal results on testing for hypercoagulable conditions. Three patients had mildly persistent elevation in anticardiolipin antibodies, but none had the factor V Leiden mutation or a deficiency of protein C, protein S, or antithrombin.
Koutroubakis et al20 reported significantly higher prevalences of antiphospholipid antibodies and heterogeneity for the factor V Leiden mutation in 35 patients with a history of ischemic colitis than in 18 patients with diverticulitis and 52 healthy controls (19.4% vs 0 and 1.9%, 22.2% vs 0 and 3.8%, respectively). Overall, 26 (72%) of 36 patients had at least one abnormal hypercoagulable test result.
Most patients with ischemic colitis are relatively old (over 60 years), and many have multiple concomitant vascular risk factors, suggesting that many factors contribute to ischemic colitis and that thrombophilia is not necessarily the direct cause. Hypercoagulable states may play a more important role in young, healthy patients who present with chronic or recurrent colonic ischemia.
Because no large clinical trials have been done and data are scarce and limited to case reports and small retrospective studies, a hypercoagulable evaluation is reserved for younger patients and those with recurrent, unexplained ischemic colitis.
Even if we detect thrombophilia, nobody yet knows what the appropriate medical treatment should be. Although some cases of ischemic colitis with associated thrombophilia have been successfully treated with anticoagulants,28,29 the benefit of diagnosing and treating a hypercoagulable state in ischemic colitis is still unproven. Therefore, oral anticoagulation should be used only in those in whom a hypercoagulable state is the most likely cause of severe or recurrent colonic ischemia.
There are no official guidelines on the duration of anticoagulation in such patients, but we treat for 6 months and consider indefinite treatment if the ischemic colitis recurs.
Medications that should always be considered as possible culprits include:
- Alosetron (Lotronex), which was temporarily withdrawn by the US Food and Drug Administration because of its association with ischemic colitis when prescribed to treat diarrhea-predominant irritable bowel syndrome.30 It was later reinstated, with some restrictions.
- Digitalis
- Diuretics
- Estrogens
- Danazol (Danocrine)
- Nonsteroidal anti-inflammatory drugs
- Tegaserod (Zelnorm)
- Paclitaxel (Abraxane)
- Carboplatin (Paraplatin)
- Sumatriptan (Imitrex)
- Simvastatin (Zocor)
- Interferon-ribavirin31
- Pseudoephedrine (eg, Sudafed).32
Endoscopic retrograde cholangiopancreatography can cause ischemic colitis if the rare life-threatening complication of mesenteric hematoma occurs.33
Chronic constipation can lead to colonic ischemia by increasing intraluminal pressure, which hinders blood flow and reduces the arteriovenous oxygen gradient in the colonic wall.34,35
Long-distance running can cause sustained bouts of ischemia, likely due to shunting of blood away from the splanchnic circulation, along with dehydration and electrolyte abnormalities. Affected runners present with lower abdominal pain and hematochezia. The colitis usually resolves without sequelae with rehydration and electrolyte correction.36
Vasospasm has been described as a cause of ischemia. During systemic hypoperfusion, vasoactive substances shunt blood from the gut to the brain through mesenteric vasoconstriction.37 This phenomenon can occur in dehydration-induced hypotension, heart failure, septic shock, or exposure to drugs such as antihypertensive medications, digoxin, or cocaine. Necrosis of the villous layer and transmural infarctions can occur with uninterrupted ischemia lasting more than 8 hours.38
Snake venom. The bite of Agkistrodon blomhoffii brevicaudus, a pit viper found in China and Korea, was recently reported to cause transient ischemic colitis due to disseminated intravascular coagulation. The condition resolved in about 10 days after treatment with polyvalent antivenom solution, transfusion of platelets and fresh frozen plasma, and empirically chosen antibiotics, ie, ampicillin-sulbactam (Unasyn) and metronidazole (Flagyl).39
CLINICAL MANIFESTATIONS
As stated above, ischemic colitis should be included in the differential diagnosis when assessing patients with abdominal pain, diarrhea, or bloody stools.
Typical presentation
The typical presentation of acute colonic ischemia includes:
- Rapid onset of mild abdominal pain
- Tenderness over the affected bowel area, usually on the left side near the splenic flexure or the rectosigmoid junction
- Mild to moderate hematochezia beginning within 1 day of the onset of abdominal pain. The bleeding is often not profuse and does not cause hemodynamic instability or require transfusion.40
Differentiate from mesenteric ischemia
It is important to differentiate between ischemic colitis and mesenteric ischemia, which is more serious and affects the small bowel.
Most patients with acute mesenteric ischemia complain of sudden onset of severe abdominal pain out of proportion to the tenderness on physical examination, they appear profoundly ill, and they do not have bloody stools until the late stages. They need urgent mesenteric angiography or another fast imaging test.4
In contrast, many patients with chronic mesenteric ischemia (or “abdominal angina”) report recurrent severe postprandial abdominal pain, leading to fear of food and weight loss.
Varies in severity
The severity of ischemic colitis varies widely, with hypoperfusion affecting as little as a single segment or as much as the entire colon. Over three-fourths of cases are the milder, nongangrenous form, which is temporary and rarely causes long-term complications such as persistent segmental colitis or strictures.41 In contrast, gangrenous colonic ischemia, which accounts for about 15% of cases, can be life-threatening.
Colonic ischemia can be categorized according to its severity and clinical presentation42:
- Reversible colonopathy (submucosal or intramural hemorrhage)
- Transient colitis (45% of cases were reversible or transient in a 1978 report by Boley et al43)
- Chronic colitis (19% of cases)
- Stricture (13%)
- Gangrene (19%)
- Fulminant universal colitis.
The resulting ischemic injury can range from superficial mucosal damage to mural or even full-thickness transmural infarction.44
Although most cases involve the left colon, about one-fourth involve the right. Right-sided colonic ischemia tends to be more severe: about 60% of patients require surgery (five times more than with colitis of other regions), and the death rate is twice as high (close to 23%).45
DIAGNOSIS DEPENDS ON SUSPICION
The diagnosis of colonic ischemia largely depends on clinical suspicion, especially since many other conditions (eg, infectious colitis, inflammatory bowel disease, diverticulitis, colon cancer) present with abdominal pain, diarrhea, and hematochezia. One study showed that a clinical presentation of lower abdominal pain or bleeding, or both, was 100% predictive of ischemic colitis when accompanied by four or more of the following risk factors: age over 60, hemodialysis, hypertension, hypoalbuminemia, diabetes mellitus, or drug-induced constipation. 46
Stool studies can identify organisms
Invasive infections with Salmonella, Shigella, and Campylobacter species and Eschericia coli O157:H7 should be identified early with stool studies if the patient presents as an outpatient or has been hospitalized less than 72 hours. Parasites such as Entamoeba histolytica and Angiostrongylus costaricensis and viruses such as cytomegalovirus should be considered in the differential diagnosis, as they can cause ischemic colitis.41,47Clostridium difficile should be excluded in those exposed to antibiotics.
Blood tests may indicate tissue injury
Although no laboratory marker is specific for ischemic colitis, elevated serum levels of lactate, lactate dehydrogenase, creatine kinase, or amylase may indicate tissue injury. The combination of abdominal pain, a white blood cell count greater than 20 × 109/L, and metabolic acidosis suggests intestinal ischemia and infarction.
Endoscopy is the test of choice
Endoscopy has become the diagnostic test of choice in establishing the diagnosis of ischemic colitis, although computed tomography (CT) can provide suggestive findings and exclude other illnesses. Colonoscopy has almost completely replaced radiography with bariumenema contrast as a diagnostic tool because it is more sensitive for detecting mucosal changes, it directly visualizes the mucosa, and it can be used to obtain biopsy specimens.48
Colonoscopy is performed without bowel preparation to prevent hypoperfusion caused by dehydrating cathartics. In addition, the scope should not be advanced beyond the affected area, and minimal air insufflation should be used to prevent perforation.
Endoscopic findings can help differentiate ischemic colitis from other, clinically similar diseases. For instance, unlike irritable bowel disease, ischemic colitis tends to affect a discrete segment with a clear delineation between affected and normal mucosa, it spares the rectum, the mucosa heals rapidly as seen on serial colonoscopic examinations, and a single linear ulcer, termed the “single-stripe” sign, runs along the longitudinal axis of the colon.49,50
Biopsy features are not specific, as findings of hemorrhage, capillary thrombosis, granulation tissue with crypt abscesses, and pseudopolyps can also be seen in other conditions, such as Crohn disease.54,55
Imaging studies are not specific
Imaging studies are often used, but the findings lack specificity.
Plain abdominal radiography can help only in advanced ischemia, in which distention or pneumatosis can be seen.
CT with contrast can reveal thickening of the colon wall in a segmental pattern in ischemic colitis, but this finding also can be present in infectious and Crohn colitis. CT findings of colonic ischemia also include pericolic streakiness and free fluid. Pneumatosis coli often signifies infarcted bowel.56 However, CT findings can be completely normal in mild cases or if done early in the course.
Angiography in severe cases
Since colonic ischemia is most often transient, mesenteric angiography is not indicated in mild cases. Angiography is only considered in more severe cases, especially when only the right colon is involved, the diagnosis of colonic ischemia has not yet been determined, and acute mesenteric ischemia needs to be excluded. A focal lesion is often seen in mesenteric ischemia, but not often in colonic ischemia.
Looking for the underlying cause
Once the diagnosis of ischemic colitis is made, an effort should be made to identify the cause (Table 1). The initial step can be to remove or treat reversible causes such as medications or infections. As mentioned earlier, electrocardiography, Holter monitoring, and transthoracic echocardiography should be considered in patients with ischemic colitis to rule out cardiac embolic sources.9 A hypercoagulable workup can be done, but only in young patients without other clear causes or patients with recurrent events.
CONSERVATIVE TREATMENT IS ENOUGH FOR MOST
Empirically chosen broad-spectrum antibiotics that cover both aerobic and anaerobic coliform bacteria are reserved for patients with moderate to severe colitis to minimize bacterial translocation and sepsis.
Whenever symptomatic ileus is present, a nasogastric tube should be placed to alleviate vomiting and abdominal discomfort.
Antiplatelet agents have not been evaluated in treating ischemic colitis and are generally not used. As mentioned earlier, anticoagulation has been used in patients who have been proven to have hypercoagulable conditions,28,29 but its benefit is not yet proven. Currently, if the coagulation profile is abnormal, anticoagulation should be used only in cases of recurrent colonic ischemia or in young patients with severe cases in the absence of a clear cause. Anticoagulation should also be used in confirmed cases of cardiac embolization.
Surgery for some
Exploratory laparotomy with possible subtotal or segmental colectomy may be needed in acute, subacute, or chronic settings.42 Acute indications include peritoneal signs, massive bleeding, and fulminant ischemic colitis. Subacute indications are lack of resolution, with symptoms that persist for more than 2 or 3 weeks, or malnutrition or hypoalbuminemia due to protein-losing colonopathy. Colon stricture can be chronic and becomes an indication for surgery only when symptomatic, as some strictures resolve with time (months to years).
Right hemicolectomy and primary anastomosis of viable remaining colon is performed for right-sided colonic ischemia and necrosis, while left-sided colonic ischemia is managed with a proximal stoma and distal mucous fistula, or Hartmann procedure. Re-anastomosis and ostomy closure are usually done after 4 to 6 months.57 However, resection and primary anastomosis can also be an option for patients with isolated ischemia of the sigmoid colon.58 Transendoscopic dilation or stenting of short strictures can be an alternative to surgery, although experience with this is limited.59,60
THE PROGNOSIS IS USUALLY GOOD
The prognosis depends on the extent of injury and comorbidities. Transient, self-limited ischemia involving the mucosa and submucosa has a good prognosis, while fulminant ischemia with transmural infarction carries a poor one, as it can progress to necrosis and death.
Although up to 85% of cases of ischemic colitis managed conservatively improve within 1 or 2 days and resolve completely within 1 or 2 weeks, close to one-fifth of patients develop peritonitis or deteriorate clinically and require surgery.61,62 Surgical resection is required when irreversible ischemic injury and chronic colitis develop, as both can lead to bacteremia and sepsis, colonic stricture, persistent abdominal pain and bloody diarrhea, and protein-losing enteropathy.40
- Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther 2004; 19:729–738.
- Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 7th ed. Philadelphia, PA: Saunders; 2002.
- Gandhi SK, Hanson MM, Vernava AM, Kaminski DL, Longo WE. Ischemic colitis. Dis Colon Rectum 1996; 39:88–100.
- Greenwald DA, Brandt LJ, Reinus JF. Ischemic bowel disease in the elderly. Gastroenterol Clin North Am 2001; 30:445–473.
- Reeders JW, Tytgat GN, Rosenbusch G, et al. Ischaemic colitis. The Hague: Martinus Nijhoff, 1984;17.
- Brandt L, Boley S, Goldberg L, Mitsudo S, Berman A. Colitis in the elderly. A reappraisal. Am J Gastroenterol 1981; 76:239–245.
- Binns JC, Isaacson P. Age-related changes in the colonic blood supply: their relevance to ischaemic colitis. Gut 1978; 19:384–390.
- Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am 1992; 72:65–83.
- Hourmand-Ollivier I, Bouin M, Saloux E, et al. Cardiac sources of embolism should be routinely screened in ischemic colitis. Am J Gastroenterol 2003; 98:1573–1577.
- Cappell MS. Safety and efficacy of colonoscopy after myocardial infarction: an analysis of 100 study patients and 100 control patients at two tertiary cardiac referral hospitals. Gastrointest Endosc 2004; 60:901–909.
- Cappell MS, Mahajan D, Kurupath V. Characterization of ischemic colitis associated with myocardial infarction: an analysis of 23 patients. Am J Med 2006; 119:527.e1–e9.
- Hagihara PF, Ernst CB, Griffen WO. Incidence of ischemic colitis following abdominal aortic reconstruction. Surg Gynecol Obstet 1979; 149:571–573.
- Brewster DC, Franklin DP, Cambria RP, et al. Intestinal ischemia complicating abdominal aortic surgery. Surgery 1991; 109:447–454.
- Piotrowski JJ, Ripepi AJ, Yuhas JP, Alexander JJ, Brandt CP. Colonic ischemia: the Achilles heel of ruptured aortic aneurysm repair. Am Surg 1996; 62:557–560.
- Ernst CB. Colonic ischemia following aortic reconstruction. In: Rutherford RB, editor. Vascular Surgery. 4th ed. Philadelphia, PA: WB Saunders; 1995:1312–1320.
- Geroghty PS, Sanchez LA, Rubin BG, et al. Overt ischemic colitis after endovascular repair of aortoiliac aneurysm. J Vasc Surg 2004; 40:413–418.
- Klestzick HN, McPhedran P, Cipolla D, Berry WA, DiCorato M, Denowitz J. The antiphospholipid syndrome and ischemic colitis. Gastroenterologist 1995; 3:249–256.
- Knot EA, ten Cate JW, Bruin T, Iburg AH, Tytgat GN. Antithrombin III metabolism in two colitis patients with acquired antithrombin III deficiency. Gastroenterology 1985; 89:421–425.
- Maloisel F. Role of coagulation disorders in mesenteric ischemia. J Chir (Paris) 1996; 133:442–447.
- Koutroubakis IE, Sfiridaki A, Theodoropoulou A, Kouroumalis EA. Role of acquired and hereditary thrombotic risk factors in colon ischemia of ambulatory patients. Gastroenterology 2001; 121:561–565.
- Midian-Singh R, Polen A, Durishin C, Crock RD, Whittier FC, Fahmy N. Ischemic colitis revisited: a prospective study identifying hypercoagulability as a risk factor. South Med J 2004; 97:120–123.
- Blanc P, Bories P, Donadio D, et al. Ischemic colitis and recurrent venous thrombosis caused by familial protein S deficiency. Gastroenterol Clin Biol 1989; 13:945.
- Verger P, Blanc C, Feydy P, Boey S. Ischemic colitis caused by protein S deficiency. Presse Med 1996; 25:1350.
- Ludwig D, Stahl M, David-Walek T, et al. Ischemic colitis, pulmonary embolism, and atrial thrombosis in a patient with inherited resistance to activated protein C. Dig Dis Sci 1998; 43:1362–1367.
- Yee NS, Guerry D, Lichtenstein GR. Ischemic colitis associated with factor V Leiden mutation. Ann Intern Med 2000; 132:595–596.
- Balian A, Veyradier A, Naveau S, et al. Prothrombin 20210G/A mutation in two patients with mesenteric ischemia. Dig Dis Sci 1999; 44:1910–1913.
- Arnott ID, Ghosh S, Ferguson A. The spectrum of ischaemic colitis. Eur J Gastroenterol Hepatol 1999; 11:295–303.
- Chin BW, Greenberg D, Wilson RB, Meredith CG. A case of ischemic colitis associated with factor V Leiden mutation: successful treatment with anticoagulation. Gastrointest Endosc 2007; 66:416–418.
- Heyn J, Buhmann S, Ladurner R, et al. Recurrent ischemic colitis in a patient with Leiden factor V mutation and systemic lupus erythematosus with antiphospholipid syndrome. Eur J Med Res 2008; 13:182–184.
- Chang L, Chey WD, Harris L, Olden K, Surawicz C, Schoenfeld P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol 2006; 101:1069–1079.
- Punnam SR, Pothula VR, Gourineni N, Punnam A, Ranganathan V. Interferon-ribavirin-associated ischemic colitis. J Clin Gastroenterol 2008; 42:323–325.
- Dowd J, Bailey D, Moussa K, Nair S, Doyle R, Culpepper-Morgan JA. Ischemic colitis associated with pseudoephedrine: four cases. Am J Gastroenterol 1999; 94:2430–2434.
- Kingsley DD, Schermer CR, Jamal MM. Rare complications of endoscopic retrograde cholangiopancreatography: two case reports. JSLS 2001; 5:171–173.
- Boley SJ, Agrawal GP, Warren AR, et al. Pathophysiologic effects of bowel distension on intestinal blood flow. Am J Surg 1969; 117:228–234.
- Reinus JF, Brandt LJ, Boley SJ. Ischemic diseases of the bowel. Gastroenterol Clin North Am 1990; 19:319–343.
- Moses FM. Exercise-associated intestinal ischemia. Curr Sports Med Rep 2005; 4:91–95.
- Rosenblum JD, Boyle CM, Schwartz LB. The mesenteric circulation. Anatomy and physiology. Surg Clin North Am 1997; 77:289–306.
- Haglund U, Bulkley GB, Granger DN. On the pathophysiology of intestinal ischemic injury. Clinical review. Acta Chir Scand 1987; 153:321–324.
- Kim MK, Cho YS, Kim HK, Kim JS, Kim SS, Chae HS. Transient ischemic colitis after a pit viper bite (Agkistrodon blomhoffii brevicaudus). J Clin Gastroenterol 2008; 42:111–112.
- Cappell MS. Intestinal (mesenteric) vasculopathy. II. Ischemic colitis and chronic mesenteric ischemia. Gastroenterol Clin North Am 1998; 27:827–860.
- Greenwald DA, Brandt LJ. Colonic ischemia. J Clin Gastroenterol 1998; 27:122–128.
- Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology 2000; 118:954–968.
- Boley SJ, Brandt LJ, Veith FJ. Ischemic disorders of the intestines. Curr Probl Surg 1978; 15:1–85.
- Schuler JG, Hudlin MM. Cecal necrosis: infrequent variant of ischemic colitis. Report of five cases. Dis Colon Rectum 2000; 43:708–712.
- Sotiriadis J, Brandt LJ, Behin DS, Southern WN. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol 2007; 102:2247–2252.
- Park CJ, Jang MK, Shin WG, et al. Can we predict the development of ischemic colitis among patients with lower abdominal pain? Dis Colon Rectum 2007; 50:232–238.
- Su C, Brandt LJ, Sigal SH, et al. The immunohistological diagnosis of E. coli 0157:H7 colitis: possible association with colonic ischemia. Am J Gastroenterol 1998; 93:1055–1059.
- Scowcroft CW, Sanowski RA, Kozarek RA. Colonoscopy in ischemic colitis. Gastrointest Endosc 1981; 27:156–161.
- Rogers AI, David S. Intestinal blood flow and diseases of vascular impairment. In: Haubrich WS, Schaffner F, Berk JE, editors. Gastroenterology. 5th ed. Philadelphia: WB Saunders; 1995:1212–1234.
- Zuckerman GR, Prakash C, Merriman RB, Sawhney MS, DeSchryver-Kecskemeti K, Clouse RE. The colon single-stripe sign and its relationship to ischemic colitis. Am J Gastroenterol 2003; 98:2018–2022.
- Green BT, Tendler DA. Ischemic colitis: a clinical review. South Med J 2005; 98:217–222.
- Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis. Cleve Clin J Med 2003; 70:920–930.
- Habu Y, Tahashi Y, Kiyota K, et al. Reevaluation of clinical features of ischemic colitis: analysis of 68 consecutive cases diagnosed by early colonoscopy. Scand J Gastroenterol 1996; 31:881–886.
- Mitsudo S, Brandt LJ. Pathology of intestinal ischemia. Surg Clin North Am 1992; 72:43–63.
- Price AB. Ischaemic colitis. Curr Top Pathol 1990; 81:229–246.
- Balthazar EJ, Yen BC, Gordon RB. Ischemic colitis: CT evaluation of 54 cases. Radiology 1999; 211:381–388.
- Mosdell DM, Doberneck RC. Morbidity and mortality of ostomy closure. Am J Surg 1991; 162:633–636.
- Iqbal T, Zarin M, Iqbal A, et al. Results of primary closure in the management of gangrenous and viable sigmoid volvulus. Pak J Surg 2007; 23:118–121.
- Oz MC, Forde KA. Endoscopic alternatives in the management of colonic strictures. Surgery 1990; 108:513–519.
- Profili S, Bifulco V, Meloni GB, Demelas L, Niolu P, Manzoni MA. A case of ischemic stenosis of the colon-sigmoid treated with self-expandable uncoated metallic prosthesis. Radiol Med 1996; 91:665–667.
- Brandt LJ, Boley SJ. Colonic ischemia. Surg Clin North Am 1992; 72:203–229.
- Boley SJ. 1989 David H. Sun lecture. Colonic ischemia—25 years later. Am J Gastroenterol 1990; 85:931–934.
- Higgins PD, Davis KJ, Laine L. Systematic review: the epidemiology of ischaemic colitis. Aliment Pharmacol Ther 2004; 19:729–738.
- Feldman M, Friedman LS, Sleisenger MH, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 7th ed. Philadelphia, PA: Saunders; 2002.
- Gandhi SK, Hanson MM, Vernava AM, Kaminski DL, Longo WE. Ischemic colitis. Dis Colon Rectum 1996; 39:88–100.
- Greenwald DA, Brandt LJ, Reinus JF. Ischemic bowel disease in the elderly. Gastroenterol Clin North Am 2001; 30:445–473.
- Reeders JW, Tytgat GN, Rosenbusch G, et al. Ischaemic colitis. The Hague: Martinus Nijhoff, 1984;17.
- Brandt L, Boley S, Goldberg L, Mitsudo S, Berman A. Colitis in the elderly. A reappraisal. Am J Gastroenterol 1981; 76:239–245.
- Binns JC, Isaacson P. Age-related changes in the colonic blood supply: their relevance to ischaemic colitis. Gut 1978; 19:384–390.
- Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am 1992; 72:65–83.
- Hourmand-Ollivier I, Bouin M, Saloux E, et al. Cardiac sources of embolism should be routinely screened in ischemic colitis. Am J Gastroenterol 2003; 98:1573–1577.
- Cappell MS. Safety and efficacy of colonoscopy after myocardial infarction: an analysis of 100 study patients and 100 control patients at two tertiary cardiac referral hospitals. Gastrointest Endosc 2004; 60:901–909.
- Cappell MS, Mahajan D, Kurupath V. Characterization of ischemic colitis associated with myocardial infarction: an analysis of 23 patients. Am J Med 2006; 119:527.e1–e9.
- Hagihara PF, Ernst CB, Griffen WO. Incidence of ischemic colitis following abdominal aortic reconstruction. Surg Gynecol Obstet 1979; 149:571–573.
- Brewster DC, Franklin DP, Cambria RP, et al. Intestinal ischemia complicating abdominal aortic surgery. Surgery 1991; 109:447–454.
- Piotrowski JJ, Ripepi AJ, Yuhas JP, Alexander JJ, Brandt CP. Colonic ischemia: the Achilles heel of ruptured aortic aneurysm repair. Am Surg 1996; 62:557–560.
- Ernst CB. Colonic ischemia following aortic reconstruction. In: Rutherford RB, editor. Vascular Surgery. 4th ed. Philadelphia, PA: WB Saunders; 1995:1312–1320.
- Geroghty PS, Sanchez LA, Rubin BG, et al. Overt ischemic colitis after endovascular repair of aortoiliac aneurysm. J Vasc Surg 2004; 40:413–418.
- Klestzick HN, McPhedran P, Cipolla D, Berry WA, DiCorato M, Denowitz J. The antiphospholipid syndrome and ischemic colitis. Gastroenterologist 1995; 3:249–256.
- Knot EA, ten Cate JW, Bruin T, Iburg AH, Tytgat GN. Antithrombin III metabolism in two colitis patients with acquired antithrombin III deficiency. Gastroenterology 1985; 89:421–425.
- Maloisel F. Role of coagulation disorders in mesenteric ischemia. J Chir (Paris) 1996; 133:442–447.
- Koutroubakis IE, Sfiridaki A, Theodoropoulou A, Kouroumalis EA. Role of acquired and hereditary thrombotic risk factors in colon ischemia of ambulatory patients. Gastroenterology 2001; 121:561–565.
- Midian-Singh R, Polen A, Durishin C, Crock RD, Whittier FC, Fahmy N. Ischemic colitis revisited: a prospective study identifying hypercoagulability as a risk factor. South Med J 2004; 97:120–123.
- Blanc P, Bories P, Donadio D, et al. Ischemic colitis and recurrent venous thrombosis caused by familial protein S deficiency. Gastroenterol Clin Biol 1989; 13:945.
- Verger P, Blanc C, Feydy P, Boey S. Ischemic colitis caused by protein S deficiency. Presse Med 1996; 25:1350.
- Ludwig D, Stahl M, David-Walek T, et al. Ischemic colitis, pulmonary embolism, and atrial thrombosis in a patient with inherited resistance to activated protein C. Dig Dis Sci 1998; 43:1362–1367.
- Yee NS, Guerry D, Lichtenstein GR. Ischemic colitis associated with factor V Leiden mutation. Ann Intern Med 2000; 132:595–596.
- Balian A, Veyradier A, Naveau S, et al. Prothrombin 20210G/A mutation in two patients with mesenteric ischemia. Dig Dis Sci 1999; 44:1910–1913.
- Arnott ID, Ghosh S, Ferguson A. The spectrum of ischaemic colitis. Eur J Gastroenterol Hepatol 1999; 11:295–303.
- Chin BW, Greenberg D, Wilson RB, Meredith CG. A case of ischemic colitis associated with factor V Leiden mutation: successful treatment with anticoagulation. Gastrointest Endosc 2007; 66:416–418.
- Heyn J, Buhmann S, Ladurner R, et al. Recurrent ischemic colitis in a patient with Leiden factor V mutation and systemic lupus erythematosus with antiphospholipid syndrome. Eur J Med Res 2008; 13:182–184.
- Chang L, Chey WD, Harris L, Olden K, Surawicz C, Schoenfeld P. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol 2006; 101:1069–1079.
- Punnam SR, Pothula VR, Gourineni N, Punnam A, Ranganathan V. Interferon-ribavirin-associated ischemic colitis. J Clin Gastroenterol 2008; 42:323–325.
- Dowd J, Bailey D, Moussa K, Nair S, Doyle R, Culpepper-Morgan JA. Ischemic colitis associated with pseudoephedrine: four cases. Am J Gastroenterol 1999; 94:2430–2434.
- Kingsley DD, Schermer CR, Jamal MM. Rare complications of endoscopic retrograde cholangiopancreatography: two case reports. JSLS 2001; 5:171–173.
- Boley SJ, Agrawal GP, Warren AR, et al. Pathophysiologic effects of bowel distension on intestinal blood flow. Am J Surg 1969; 117:228–234.
- Reinus JF, Brandt LJ, Boley SJ. Ischemic diseases of the bowel. Gastroenterol Clin North Am 1990; 19:319–343.
- Moses FM. Exercise-associated intestinal ischemia. Curr Sports Med Rep 2005; 4:91–95.
- Rosenblum JD, Boyle CM, Schwartz LB. The mesenteric circulation. Anatomy and physiology. Surg Clin North Am 1997; 77:289–306.
- Haglund U, Bulkley GB, Granger DN. On the pathophysiology of intestinal ischemic injury. Clinical review. Acta Chir Scand 1987; 153:321–324.
- Kim MK, Cho YS, Kim HK, Kim JS, Kim SS, Chae HS. Transient ischemic colitis after a pit viper bite (Agkistrodon blomhoffii brevicaudus). J Clin Gastroenterol 2008; 42:111–112.
- Cappell MS. Intestinal (mesenteric) vasculopathy. II. Ischemic colitis and chronic mesenteric ischemia. Gastroenterol Clin North Am 1998; 27:827–860.
- Greenwald DA, Brandt LJ. Colonic ischemia. J Clin Gastroenterol 1998; 27:122–128.
- Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology 2000; 118:954–968.
- Boley SJ, Brandt LJ, Veith FJ. Ischemic disorders of the intestines. Curr Probl Surg 1978; 15:1–85.
- Schuler JG, Hudlin MM. Cecal necrosis: infrequent variant of ischemic colitis. Report of five cases. Dis Colon Rectum 2000; 43:708–712.
- Sotiriadis J, Brandt LJ, Behin DS, Southern WN. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol 2007; 102:2247–2252.
- Park CJ, Jang MK, Shin WG, et al. Can we predict the development of ischemic colitis among patients with lower abdominal pain? Dis Colon Rectum 2007; 50:232–238.
- Su C, Brandt LJ, Sigal SH, et al. The immunohistological diagnosis of E. coli 0157:H7 colitis: possible association with colonic ischemia. Am J Gastroenterol 1998; 93:1055–1059.
- Scowcroft CW, Sanowski RA, Kozarek RA. Colonoscopy in ischemic colitis. Gastrointest Endosc 1981; 27:156–161.
- Rogers AI, David S. Intestinal blood flow and diseases of vascular impairment. In: Haubrich WS, Schaffner F, Berk JE, editors. Gastroenterology. 5th ed. Philadelphia: WB Saunders; 1995:1212–1234.
- Zuckerman GR, Prakash C, Merriman RB, Sawhney MS, DeSchryver-Kecskemeti K, Clouse RE. The colon single-stripe sign and its relationship to ischemic colitis. Am J Gastroenterol 2003; 98:2018–2022.
- Green BT, Tendler DA. Ischemic colitis: a clinical review. South Med J 2005; 98:217–222.
- Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis. Cleve Clin J Med 2003; 70:920–930.
- Habu Y, Tahashi Y, Kiyota K, et al. Reevaluation of clinical features of ischemic colitis: analysis of 68 consecutive cases diagnosed by early colonoscopy. Scand J Gastroenterol 1996; 31:881–886.
- Mitsudo S, Brandt LJ. Pathology of intestinal ischemia. Surg Clin North Am 1992; 72:43–63.
- Price AB. Ischaemic colitis. Curr Top Pathol 1990; 81:229–246.
- Balthazar EJ, Yen BC, Gordon RB. Ischemic colitis: CT evaluation of 54 cases. Radiology 1999; 211:381–388.
- Mosdell DM, Doberneck RC. Morbidity and mortality of ostomy closure. Am J Surg 1991; 162:633–636.
- Iqbal T, Zarin M, Iqbal A, et al. Results of primary closure in the management of gangrenous and viable sigmoid volvulus. Pak J Surg 2007; 23:118–121.
- Oz MC, Forde KA. Endoscopic alternatives in the management of colonic strictures. Surgery 1990; 108:513–519.
- Profili S, Bifulco V, Meloni GB, Demelas L, Niolu P, Manzoni MA. A case of ischemic stenosis of the colon-sigmoid treated with self-expandable uncoated metallic prosthesis. Radiol Med 1996; 91:665–667.
- Brandt LJ, Boley SJ. Colonic ischemia. Surg Clin North Am 1992; 72:203–229.
- Boley SJ. 1989 David H. Sun lecture. Colonic ischemia—25 years later. Am J Gastroenterol 1990; 85:931–934.
KEY POINTS
- The incidence of colonic ischemia is difficult to ascertain, as most cases are transient and either not reported or misdiagnosed.
- Most cases are in the elderly.
- The clinical presentation is not specific, as other conditions also present with abdominal pain and hematochezia.
- The most common mechanisms are hypotension and hypovolemia caused by dehydration or bleeding that results in systemic hypoperfusion.
- Endoscopy has become the diagnostic procedure of choice.
- Although most patients can be treated conservatively with intravenous fluids, bowel rest, and antibiotics, some develop peritonitis or clinically deteriorate and require surgery.
VA Report Says Endoscope Problems Continue
Grand Rounds: Man, 60, With Abdominal Pain
A 60-year-old white man with a history of hyperlipidemia, hypertension, and anxiety presented with complaints of abdominal pain, localized to an area left of the umbilicus. He described the pain as constant and rated it 6 on a scale of 1 to 10. He said the pain had been present for longer than three weeks.
The man said he had been seen by another health care provider shortly after the pain began, but he did not think the provider took his complaint seriously. At that visit, antacids were prescribed, blood work was ordered, and the man was told to return if there was no improvement. He felt that because he was being treated for anxiety, the provider believed he was just imagining the pain.
At the current visit, the review of systems revealed additional complaints of shakiness and nausea without vomiting, with other findings unremarkable. The persistent pain did not seem related to eating, and the patient had no history of any surgeries that might help explain his current complaints. He had smoked a pack of cigarettes daily for 40 years and had a history of heavy alcohol use, although he denied having consumed any alcohol during the previous five years.
His prescribed medications included gemfibrozil 600 mg per day, hydrochlorothiazide 25 mg each morning, and diazepam 5 mg twice daily, with an OTC antacid.
The patient’s recent laboratory results were normal; they included a complete blood count, comprehensive metabolic panel, liver enzyme levels, and a serum amylase level. The patient weighed 280 lb and his height was 5’10”; his BMI was 40. His temperature was 97.7°F, with a regular heart rate of 88 beats/min; blood pressure, 140/90 mm Hg; and respiratory rate, 18 breaths/min.
The patient did not appear to be in acute distress. A bruit was heard in the indicated area of pain. No mass was palpated, and the width of his aorta could not be determined because of his obesity. His physical exam was otherwise normal.
Abdominal ultrasonography (US) revealed a 5.5-cm abdominal aortic aneurysm (AAA), and the man was referred for immediate surgery. The aneurysm was repaired in an open abdominal procedure with a polyester prosthetic graft. The surgery was successful.
Discussion
AAA is a permanent bulging area of the aorta that exceeds 3.0 cm in diameter (see Figure 1). It is a potentially life-threatening condition due to the possibility of rupture. Often an aneurysm is asymptomatic until it ruptures, making this a difficult illness to diagnose.1
Each year, an estimated 10,000 deaths result from a ruptured AAA, making this condition the 14th leading cause of death in the United States.2,3 Incidence of AAA appears to have increased over the past two decades. Causes for this may include the aging of the US population, an increase in the number of smokers, and a trend toward diets that are higher in fat.
Prognosis among patients with AAA can be improved with increased awareness of the disease among health care providers, earlier detection of AAAs at risk for rupture, and timely, effective interventions.
Symptomatology
In about one-third of patients with a ruptured AAA, a clinical triad of symptoms is present: abdominal and/or back pain, a pulsatile abdominal mass, and hypotension.4,5 In these cases, according to the American College of Cardiology/American Heart Association (ACC/AHA),4 immediate surgical evaluation is indicated.
Prior to the rupture of an AAA, the patient may feel a pulsing sensation in the abdomen or may experience no symptoms at all. Some patients report vague complaints, such as back, flank, groin, or abdominal pain. Syncope may be the chief complaint as the aneurysm expands, so it is important for primary care providers to be alert to progressive symptoms, including this signal that an aneurysm may exist and may be expanding.6
Pain may also be abrupt and severe in the lower abdomen and back, including tenderness in the area over the aneurysm. Shock can develop rapidly and symptoms such as cyanosis, mottling, altered mental status, tachycardia, and hypotension may be present.1,4
Since symptoms may be vague, the differential diagnosis can be broad (see Table 14,7,8), necessitating a detailed patient history and a careful physical examination. In an elderly patient, low back pain should be evaluated for AAA.9 In addition, acute abdominal pain in a patient older than 50 should be presumed to be a ruptured AAA.8
Risk Factors
A clinician should be familiar with the risk factors for AAA so that diagnosis can be made before a rupture occurs. Male gender and age greater than 65 are important risk factors for AAA, but one of the most important environmental risks is cigarette smoking.9,10 Current smokers are more than seven times more likely than nonsmokers to have an aneurysm.10 Atherosclerosis, which weakens the wall of the aorta, is also believed to contribute to the risk for AAA.11
Other contributing factors include hypertension, chronic obstructive pulmonary disease, hyperlipidemia, and family history. Chronic infection, inflammatory illnesses, and connective tissue disorders (eg, Marfan syndrome) can also increase the risk for aneurysm. Less frequent causes of AAA are trauma and infectious diseases, such as syphilis.1,12
In 85% of patients with femoral aneurysms, AAA has been found to coexist, as it has in 62% of patients with popliteal aneurysms. Patients previously diagnosed with these conditions should be screened for AAA.4,13,14
Diagnosis
An abdominal bruit or a pulsating mass may be found on palpation, but the sensitivity for detection of AAA is related to its size. An aneurysm greater than 5.0 cm has an 82% chance of detection by palpation.15 To assess for the presence of an abdominal aneurysm, the examiner should press the midline between the xiphoid and umbilicus bimanually, firmly but gently.12 There is no evidence to suggest that palpating the abdomen can cause an aneurysm to rupture.
The most useful tests for diagnosis of AAA are US, CT, and MRI.6 US is the simplest and least costly of these diagnostic procedures; it is noninvasive and has a sensitivity of 95% and specificity of nearly 100%. Bedside US can provide a rapid diagnosis in an unstable patient.16
CT is nearly 100% effective in diagnosing AAA and is usually used to help decide on appropriate treatment, as it can determine the size and shape of the aneurysm.17 However, CT should not be used for unstable patients.
MRI is useful in diagnosing AAA, but it is expensive, and inappropriate for unstable patients. Currently, conventional aortography is rarely used for preoperative assessment but may still be used for placement of endovascular devices or in patients with renal complications.1,12
Screening Recommendations
The US Preventive Services Task Force (USPSTF) recommends that all men ages 65 to 74 who have a lifelong history of smoking at least 100 cigarettes should be screened for AAA with abdominal US.3,18 Screening is not recommended for those younger than 65 who have never smoked, but this decision must be individualized to the patient, with other risk factors considered.
The ACC/AHA4 advises that men whose parents or siblings have a history of AAA and who are older than 60 should undergo physical examination and screening US for AAA. In addition, patients with a small AAA should receive US surveillance until the aneurysm reaches 5.5 cm in diameter; survival has not been shown to improve if an AAA is repaired before it reaches this size.1,2,19 In consideration of increased comorbidities and decreased life expectancy, screening is not recommended for men older than 75, but this too should be determined individually.3
Screening for women is not recommended by the USPSTF.3,18 The document states that the prevalence of large AAAs in women is low and that screening may lead to an increased number of unnecessary surgeries with associated morbidity and mortality. Clinical judgment must be used in making this decision, however, as several studies have shown that women have an AAA rupture rate that is three times higher than that in men; they also have an increased in-hospital mortality rate when rupture does occur. Thus, women are less likely to experience AAA but have a worse prognosis when AAA does develop.20-22
Management
The size of an AAA is the most important predictor of rupture. According to the ACC/AHA,4 the associated risk for rupture is about 20% for aneurysms that measure 5.0 cm in diameter, 40% for those measuring at least 6.0 cm, and at least 50% for aneurysms exceeding 7.0 cm.4,23,24 Regarding surveillance of known aneurysms, it is recommended that a patient with an aneurysm smaller than 3.0 cm in diameter requires no further testing. If an AAA measures 3.0 to 4.0 cm, US should be performed yearly; if it is 4.0 to 4.9 cm, US should be performed every six months.4,25
If an identified AAA is larger than 4.5 cm, or if any segment of the aorta is more than 1.5 times the diameter of an adjacent section, referral to a vascular surgeon for further evaluation is indicated. The vascular surgeon should be consulted immediately regarding a symptomatic patient with an AAA, or one with an aneurysm that measures 5.5 cm or larger, as the risk for rupture is high.4,26
Preventing rupture of an AAA is the primary aim in management. Beta-blockers may be used to reduce systolic hypertension in cardiac patients, thus slowing the rate of expansion in those with aortic aneurysms. Patients with a known AAA should undergo frequent monitoring for blood pressure and lipid levels and be advised to stop smoking. Smoking cessation interventions such as behavior modification, nicotine replacement, or bupropion should be offered.27,28
There is evidence that statin use may reduce the size of aneurysms, even in patients without hypercholesterolemia, possibly due to statins’ anti-inflammatory properties.22,29 ACE inhibitors may also be beneficial in reducing AAA growth and in lowering blood pressure. Antiplatelet medications are important in general cardiovascular risk reduction in the patient with AAA. Aspirin is the drug of choice.27,29
Surgical Repair
AAAs are usually repaired by one of two types of surgery: endovascular repair (EVR) or open surgery. Open surgical repair, the more traditional method, involves an incision into the abdomen from the breastbone to below the navel. The weakened area is replaced with a graft made of synthetic material. Open repair of an intact AAA, performed under general anesthesia, takes from three to six hours, and the patient must be hospitalized for five to eight days.30
In EVR, the patient is given epidural anesthesia and an incision is made in the right groin, allowing a synthetic stent graft to be threaded by way of a catheter through the femoral artery to repair the lesion (see Figure 2). EVR generally takes two to five hours, followed by a two- to five-day hospital stay. EVR is usually recommended for patients who are at high risk for complications from open operations because of severe cardiopulmonary disease or other risk factors, such as advanced age, morbid obesity, or a history of multiple abdominal operations.1,2,4,19
Prognosis
Patients with a ruptured AAA have a survival rate of less than 50%, with most deaths occurring before surgical repair has been attempted.3,31 In patients with kidney failure resulting from AAA (whether ruptured or unruptured, an AAA can disrupt renal blood flow), the chance for survival is poor. By contrast, the risk for death during surgical graft repair of an AAA is only about 2% to 8%.1,12
In a systematic review, EVR was associated with a lower 30-day mortality rate compared with open surgical repair (1.6% vs 4.7%, respectively), but this reduction did not persist over two years’ follow-up; neither did EVR improve overall survival or quality of life, compared with open surgery.1 Additionally, EVR requires periodic imaging throughout the patient’s life, which is associated with more reinterventions.1,19
Patient Education
Clinicians should encourage all patients to stop smoking, follow a low-cholesterol diet, control hypertension, and exercise regularly to lower the risk for AAAs. Screening recommendations should be explained to patients at risk, as should the signs and symptoms of an aneurysm. These patients should be instructed to call their health care provider immediately if they suspect a problem.
Conclusion
The incidence of AAA is increasing, and primary care providers must be prepared to act promptly in any case of suspected AAA to ensure a safe outcome. For aneurysms measuring greater than 5.5 cm in diameter, open or endovascular surgical repair should be considered. Patients with smaller aneurysms or contraindications for surgery should receive careful medical management and education to reduce the risks of AAA expansion leading to possible rupture.
1. Wilt TJ, Lederle FA, MacDonald R, et al; Agency for Healthcare Research and Quality. Comparison of Endovascular and Open Surgical Repairs for Abdominal Aortic Aneurysm. Rockville, MD: Agency for Healthcare Research and Quality; 2006. AHRQ publication 06-E107. Evidence Report/Technology Assessment 144. www.ahrq.gov/CLINIC/tp/aaareptp.htm. Accessed June 23, 2009.
2. Birkmeyer JD, Upchurch GR Jr. Evidence-based screening and management of abdominal aortic aneurysm. Ann Intern Med. 2007;146(10):749-750.
3. Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005;142(3):203-211.
4. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47(6):1239-1312.
5. Kiell CS, Ernst CB. Advances in management of abdominal aortic aneurysm. Adv Surg. 1993;26:73–98.
6. O’Connor RE. Aneurysm, abdominal. http://emedicine.medscape.com/article/756735-overview. Accessed June 23, 2009.
7. Lederle FA, Parenti CM, Chute EP. Ruptured abdominal aortic aneurysm: the internist as diagnostician. Am J Med. 1994;96:163-167.
8. Cartwright SL, Knudson MP. Evaluation of acute abdominal pain in adults. Am Fam Physician. 2008;77(7): 971-978.
9. Lyon C, Clark DC. Diagnosis of acute abdominal pain in older patients. Am Fam Physician. 2006;74(9):1537-1544.
10. Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999;30(6):1099-1105.
11. Palazzuoli P, Gallotta M, Guerrieri G, et al. Prevalence of risk factors, coronary and systemic atherosclerosis in abdominal aortic aneurysm: comparison with high cardiovascular risk population. Vasc Health Risk Manag. 2008;4(4):877-883.
12. Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365(9470):1577-1589.
13. Graham LM, Zelenock GB, Whitehouse WM Jr, et al. Clinical significance of arteriosclerotic femoral artery aneurysms. Arch Surg. 1980;115(4):502–507.
14. Whitehouse WM Jr, Wakefield TW, Graham LM, et al. Limb-threatening potential of arteriosclerotic popliteal artery aneurysms. Surgery. 1983;93(5):694–699.
15. Fink HA, Lederle FA, Roth CS, et al. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med. 2000;160:833-836.
16. Bentz S, Jones J. Accuracy of emergency department ultrasound scanning in detecting abdominal aortic aneurysm. Emerg Med J. 2006;23(10):803-804.
17. Kvilekval KH, Best IM, Mason RA, et al. The value of computed tomography in the management of symptomatic abdominal aortic aneurysm. J Vasc Surg. 1990;12(1):28-33.
18. US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med. 2005;142(3):198-202.
19. Lederle FA, Kane RL, MacDonald R, Wilt TJ. Systematic review: repair of unruptured abdominal aortic aneurysm. Ann Intern Med. 2007;146(10):735-741.
20. McPhee JT, Hill JS, Elami MH. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001-2004. J Vasc Surg. 2007;45(5):891-899.
21. Mofidi R, Goldie VJ, Kelman J, et al. Influence of sex on expansion rate of abdominal aortic aneurysms. Br J Surg. 2007;94(3):310-314.
22. Norman PE, Powell JT. Abdominal aortic aneurysm: the prognosis in women is worse than in men. Circulation. 2007;115(22):2865-2869.
23. Englund R, Hudson P, Hanel K, Stanton A. Expansion rates of small abdominal aortic aneurysms. Aust N Z J Surg. 1998;68(1):21–24.
24. Conway KP, Byrne J, Townsend M, Lane IF. Prognosis of patients turned down for conventional abdominal aortic aneurysm repair in the endovascular and sonographic era: Szilagyi revisited? J Vasc Surg. 2001;33(4):752–757.
25. Cook TA, Galland RB. A prospective study to define the optimum rescreening interval for small abdominal aortic aneurysm. Cardiovasc Surg. 1996;4(4):441–444.
26. Kent KC, Zwolak RM, Jaff MR, et al; Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine and Biology. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg. 2004;39(1):267-269.
27. Golledge J, Powell JT. Medical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2007;4(3):267-273.
28. Sule S, Aronow WS. Management of abdominal aortic aneurysms. Compr Ther. 2009;35(1):3-8.
29. Powell JT. Non-operative or medical management of abdominal aortic aneurysm. Scand J Surg. 2008;97(2): 121-124.
30. Huber TS, Wang JG, Derrow AE, et al. Experience in the United States with intact abdominal aortic aneurysm repair. J Vasc Surg. 2001;33(2):304-310.
31. Adam DJ, Mohan IV, Stuart WP, et al. Community and hospital outcome from ruptured abdominal aortic aneurysm within the catchment area of a regional vascular surgical service. J Vasc Surg. 1999;30(5):922-928.
A 60-year-old white man with a history of hyperlipidemia, hypertension, and anxiety presented with complaints of abdominal pain, localized to an area left of the umbilicus. He described the pain as constant and rated it 6 on a scale of 1 to 10. He said the pain had been present for longer than three weeks.
The man said he had been seen by another health care provider shortly after the pain began, but he did not think the provider took his complaint seriously. At that visit, antacids were prescribed, blood work was ordered, and the man was told to return if there was no improvement. He felt that because he was being treated for anxiety, the provider believed he was just imagining the pain.
At the current visit, the review of systems revealed additional complaints of shakiness and nausea without vomiting, with other findings unremarkable. The persistent pain did not seem related to eating, and the patient had no history of any surgeries that might help explain his current complaints. He had smoked a pack of cigarettes daily for 40 years and had a history of heavy alcohol use, although he denied having consumed any alcohol during the previous five years.
His prescribed medications included gemfibrozil 600 mg per day, hydrochlorothiazide 25 mg each morning, and diazepam 5 mg twice daily, with an OTC antacid.
The patient’s recent laboratory results were normal; they included a complete blood count, comprehensive metabolic panel, liver enzyme levels, and a serum amylase level. The patient weighed 280 lb and his height was 5’10”; his BMI was 40. His temperature was 97.7°F, with a regular heart rate of 88 beats/min; blood pressure, 140/90 mm Hg; and respiratory rate, 18 breaths/min.
The patient did not appear to be in acute distress. A bruit was heard in the indicated area of pain. No mass was palpated, and the width of his aorta could not be determined because of his obesity. His physical exam was otherwise normal.
Abdominal ultrasonography (US) revealed a 5.5-cm abdominal aortic aneurysm (AAA), and the man was referred for immediate surgery. The aneurysm was repaired in an open abdominal procedure with a polyester prosthetic graft. The surgery was successful.
Discussion
AAA is a permanent bulging area of the aorta that exceeds 3.0 cm in diameter (see Figure 1). It is a potentially life-threatening condition due to the possibility of rupture. Often an aneurysm is asymptomatic until it ruptures, making this a difficult illness to diagnose.1
Each year, an estimated 10,000 deaths result from a ruptured AAA, making this condition the 14th leading cause of death in the United States.2,3 Incidence of AAA appears to have increased over the past two decades. Causes for this may include the aging of the US population, an increase in the number of smokers, and a trend toward diets that are higher in fat.
Prognosis among patients with AAA can be improved with increased awareness of the disease among health care providers, earlier detection of AAAs at risk for rupture, and timely, effective interventions.
Symptomatology
In about one-third of patients with a ruptured AAA, a clinical triad of symptoms is present: abdominal and/or back pain, a pulsatile abdominal mass, and hypotension.4,5 In these cases, according to the American College of Cardiology/American Heart Association (ACC/AHA),4 immediate surgical evaluation is indicated.
Prior to the rupture of an AAA, the patient may feel a pulsing sensation in the abdomen or may experience no symptoms at all. Some patients report vague complaints, such as back, flank, groin, or abdominal pain. Syncope may be the chief complaint as the aneurysm expands, so it is important for primary care providers to be alert to progressive symptoms, including this signal that an aneurysm may exist and may be expanding.6
Pain may also be abrupt and severe in the lower abdomen and back, including tenderness in the area over the aneurysm. Shock can develop rapidly and symptoms such as cyanosis, mottling, altered mental status, tachycardia, and hypotension may be present.1,4
Since symptoms may be vague, the differential diagnosis can be broad (see Table 14,7,8), necessitating a detailed patient history and a careful physical examination. In an elderly patient, low back pain should be evaluated for AAA.9 In addition, acute abdominal pain in a patient older than 50 should be presumed to be a ruptured AAA.8
Risk Factors
A clinician should be familiar with the risk factors for AAA so that diagnosis can be made before a rupture occurs. Male gender and age greater than 65 are important risk factors for AAA, but one of the most important environmental risks is cigarette smoking.9,10 Current smokers are more than seven times more likely than nonsmokers to have an aneurysm.10 Atherosclerosis, which weakens the wall of the aorta, is also believed to contribute to the risk for AAA.11
Other contributing factors include hypertension, chronic obstructive pulmonary disease, hyperlipidemia, and family history. Chronic infection, inflammatory illnesses, and connective tissue disorders (eg, Marfan syndrome) can also increase the risk for aneurysm. Less frequent causes of AAA are trauma and infectious diseases, such as syphilis.1,12
In 85% of patients with femoral aneurysms, AAA has been found to coexist, as it has in 62% of patients with popliteal aneurysms. Patients previously diagnosed with these conditions should be screened for AAA.4,13,14
Diagnosis
An abdominal bruit or a pulsating mass may be found on palpation, but the sensitivity for detection of AAA is related to its size. An aneurysm greater than 5.0 cm has an 82% chance of detection by palpation.15 To assess for the presence of an abdominal aneurysm, the examiner should press the midline between the xiphoid and umbilicus bimanually, firmly but gently.12 There is no evidence to suggest that palpating the abdomen can cause an aneurysm to rupture.
The most useful tests for diagnosis of AAA are US, CT, and MRI.6 US is the simplest and least costly of these diagnostic procedures; it is noninvasive and has a sensitivity of 95% and specificity of nearly 100%. Bedside US can provide a rapid diagnosis in an unstable patient.16
CT is nearly 100% effective in diagnosing AAA and is usually used to help decide on appropriate treatment, as it can determine the size and shape of the aneurysm.17 However, CT should not be used for unstable patients.
MRI is useful in diagnosing AAA, but it is expensive, and inappropriate for unstable patients. Currently, conventional aortography is rarely used for preoperative assessment but may still be used for placement of endovascular devices or in patients with renal complications.1,12
Screening Recommendations
The US Preventive Services Task Force (USPSTF) recommends that all men ages 65 to 74 who have a lifelong history of smoking at least 100 cigarettes should be screened for AAA with abdominal US.3,18 Screening is not recommended for those younger than 65 who have never smoked, but this decision must be individualized to the patient, with other risk factors considered.
The ACC/AHA4 advises that men whose parents or siblings have a history of AAA and who are older than 60 should undergo physical examination and screening US for AAA. In addition, patients with a small AAA should receive US surveillance until the aneurysm reaches 5.5 cm in diameter; survival has not been shown to improve if an AAA is repaired before it reaches this size.1,2,19 In consideration of increased comorbidities and decreased life expectancy, screening is not recommended for men older than 75, but this too should be determined individually.3
Screening for women is not recommended by the USPSTF.3,18 The document states that the prevalence of large AAAs in women is low and that screening may lead to an increased number of unnecessary surgeries with associated morbidity and mortality. Clinical judgment must be used in making this decision, however, as several studies have shown that women have an AAA rupture rate that is three times higher than that in men; they also have an increased in-hospital mortality rate when rupture does occur. Thus, women are less likely to experience AAA but have a worse prognosis when AAA does develop.20-22
Management
The size of an AAA is the most important predictor of rupture. According to the ACC/AHA,4 the associated risk for rupture is about 20% for aneurysms that measure 5.0 cm in diameter, 40% for those measuring at least 6.0 cm, and at least 50% for aneurysms exceeding 7.0 cm.4,23,24 Regarding surveillance of known aneurysms, it is recommended that a patient with an aneurysm smaller than 3.0 cm in diameter requires no further testing. If an AAA measures 3.0 to 4.0 cm, US should be performed yearly; if it is 4.0 to 4.9 cm, US should be performed every six months.4,25
If an identified AAA is larger than 4.5 cm, or if any segment of the aorta is more than 1.5 times the diameter of an adjacent section, referral to a vascular surgeon for further evaluation is indicated. The vascular surgeon should be consulted immediately regarding a symptomatic patient with an AAA, or one with an aneurysm that measures 5.5 cm or larger, as the risk for rupture is high.4,26
Preventing rupture of an AAA is the primary aim in management. Beta-blockers may be used to reduce systolic hypertension in cardiac patients, thus slowing the rate of expansion in those with aortic aneurysms. Patients with a known AAA should undergo frequent monitoring for blood pressure and lipid levels and be advised to stop smoking. Smoking cessation interventions such as behavior modification, nicotine replacement, or bupropion should be offered.27,28
There is evidence that statin use may reduce the size of aneurysms, even in patients without hypercholesterolemia, possibly due to statins’ anti-inflammatory properties.22,29 ACE inhibitors may also be beneficial in reducing AAA growth and in lowering blood pressure. Antiplatelet medications are important in general cardiovascular risk reduction in the patient with AAA. Aspirin is the drug of choice.27,29
Surgical Repair
AAAs are usually repaired by one of two types of surgery: endovascular repair (EVR) or open surgery. Open surgical repair, the more traditional method, involves an incision into the abdomen from the breastbone to below the navel. The weakened area is replaced with a graft made of synthetic material. Open repair of an intact AAA, performed under general anesthesia, takes from three to six hours, and the patient must be hospitalized for five to eight days.30
In EVR, the patient is given epidural anesthesia and an incision is made in the right groin, allowing a synthetic stent graft to be threaded by way of a catheter through the femoral artery to repair the lesion (see Figure 2). EVR generally takes two to five hours, followed by a two- to five-day hospital stay. EVR is usually recommended for patients who are at high risk for complications from open operations because of severe cardiopulmonary disease or other risk factors, such as advanced age, morbid obesity, or a history of multiple abdominal operations.1,2,4,19
Prognosis
Patients with a ruptured AAA have a survival rate of less than 50%, with most deaths occurring before surgical repair has been attempted.3,31 In patients with kidney failure resulting from AAA (whether ruptured or unruptured, an AAA can disrupt renal blood flow), the chance for survival is poor. By contrast, the risk for death during surgical graft repair of an AAA is only about 2% to 8%.1,12
In a systematic review, EVR was associated with a lower 30-day mortality rate compared with open surgical repair (1.6% vs 4.7%, respectively), but this reduction did not persist over two years’ follow-up; neither did EVR improve overall survival or quality of life, compared with open surgery.1 Additionally, EVR requires periodic imaging throughout the patient’s life, which is associated with more reinterventions.1,19
Patient Education
Clinicians should encourage all patients to stop smoking, follow a low-cholesterol diet, control hypertension, and exercise regularly to lower the risk for AAAs. Screening recommendations should be explained to patients at risk, as should the signs and symptoms of an aneurysm. These patients should be instructed to call their health care provider immediately if they suspect a problem.
Conclusion
The incidence of AAA is increasing, and primary care providers must be prepared to act promptly in any case of suspected AAA to ensure a safe outcome. For aneurysms measuring greater than 5.5 cm in diameter, open or endovascular surgical repair should be considered. Patients with smaller aneurysms or contraindications for surgery should receive careful medical management and education to reduce the risks of AAA expansion leading to possible rupture.
A 60-year-old white man with a history of hyperlipidemia, hypertension, and anxiety presented with complaints of abdominal pain, localized to an area left of the umbilicus. He described the pain as constant and rated it 6 on a scale of 1 to 10. He said the pain had been present for longer than three weeks.
The man said he had been seen by another health care provider shortly after the pain began, but he did not think the provider took his complaint seriously. At that visit, antacids were prescribed, blood work was ordered, and the man was told to return if there was no improvement. He felt that because he was being treated for anxiety, the provider believed he was just imagining the pain.
At the current visit, the review of systems revealed additional complaints of shakiness and nausea without vomiting, with other findings unremarkable. The persistent pain did not seem related to eating, and the patient had no history of any surgeries that might help explain his current complaints. He had smoked a pack of cigarettes daily for 40 years and had a history of heavy alcohol use, although he denied having consumed any alcohol during the previous five years.
His prescribed medications included gemfibrozil 600 mg per day, hydrochlorothiazide 25 mg each morning, and diazepam 5 mg twice daily, with an OTC antacid.
The patient’s recent laboratory results were normal; they included a complete blood count, comprehensive metabolic panel, liver enzyme levels, and a serum amylase level. The patient weighed 280 lb and his height was 5’10”; his BMI was 40. His temperature was 97.7°F, with a regular heart rate of 88 beats/min; blood pressure, 140/90 mm Hg; and respiratory rate, 18 breaths/min.
The patient did not appear to be in acute distress. A bruit was heard in the indicated area of pain. No mass was palpated, and the width of his aorta could not be determined because of his obesity. His physical exam was otherwise normal.
Abdominal ultrasonography (US) revealed a 5.5-cm abdominal aortic aneurysm (AAA), and the man was referred for immediate surgery. The aneurysm was repaired in an open abdominal procedure with a polyester prosthetic graft. The surgery was successful.
Discussion
AAA is a permanent bulging area of the aorta that exceeds 3.0 cm in diameter (see Figure 1). It is a potentially life-threatening condition due to the possibility of rupture. Often an aneurysm is asymptomatic until it ruptures, making this a difficult illness to diagnose.1
Each year, an estimated 10,000 deaths result from a ruptured AAA, making this condition the 14th leading cause of death in the United States.2,3 Incidence of AAA appears to have increased over the past two decades. Causes for this may include the aging of the US population, an increase in the number of smokers, and a trend toward diets that are higher in fat.
Prognosis among patients with AAA can be improved with increased awareness of the disease among health care providers, earlier detection of AAAs at risk for rupture, and timely, effective interventions.
Symptomatology
In about one-third of patients with a ruptured AAA, a clinical triad of symptoms is present: abdominal and/or back pain, a pulsatile abdominal mass, and hypotension.4,5 In these cases, according to the American College of Cardiology/American Heart Association (ACC/AHA),4 immediate surgical evaluation is indicated.
Prior to the rupture of an AAA, the patient may feel a pulsing sensation in the abdomen or may experience no symptoms at all. Some patients report vague complaints, such as back, flank, groin, or abdominal pain. Syncope may be the chief complaint as the aneurysm expands, so it is important for primary care providers to be alert to progressive symptoms, including this signal that an aneurysm may exist and may be expanding.6
Pain may also be abrupt and severe in the lower abdomen and back, including tenderness in the area over the aneurysm. Shock can develop rapidly and symptoms such as cyanosis, mottling, altered mental status, tachycardia, and hypotension may be present.1,4
Since symptoms may be vague, the differential diagnosis can be broad (see Table 14,7,8), necessitating a detailed patient history and a careful physical examination. In an elderly patient, low back pain should be evaluated for AAA.9 In addition, acute abdominal pain in a patient older than 50 should be presumed to be a ruptured AAA.8
Risk Factors
A clinician should be familiar with the risk factors for AAA so that diagnosis can be made before a rupture occurs. Male gender and age greater than 65 are important risk factors for AAA, but one of the most important environmental risks is cigarette smoking.9,10 Current smokers are more than seven times more likely than nonsmokers to have an aneurysm.10 Atherosclerosis, which weakens the wall of the aorta, is also believed to contribute to the risk for AAA.11
Other contributing factors include hypertension, chronic obstructive pulmonary disease, hyperlipidemia, and family history. Chronic infection, inflammatory illnesses, and connective tissue disorders (eg, Marfan syndrome) can also increase the risk for aneurysm. Less frequent causes of AAA are trauma and infectious diseases, such as syphilis.1,12
In 85% of patients with femoral aneurysms, AAA has been found to coexist, as it has in 62% of patients with popliteal aneurysms. Patients previously diagnosed with these conditions should be screened for AAA.4,13,14
Diagnosis
An abdominal bruit or a pulsating mass may be found on palpation, but the sensitivity for detection of AAA is related to its size. An aneurysm greater than 5.0 cm has an 82% chance of detection by palpation.15 To assess for the presence of an abdominal aneurysm, the examiner should press the midline between the xiphoid and umbilicus bimanually, firmly but gently.12 There is no evidence to suggest that palpating the abdomen can cause an aneurysm to rupture.
The most useful tests for diagnosis of AAA are US, CT, and MRI.6 US is the simplest and least costly of these diagnostic procedures; it is noninvasive and has a sensitivity of 95% and specificity of nearly 100%. Bedside US can provide a rapid diagnosis in an unstable patient.16
CT is nearly 100% effective in diagnosing AAA and is usually used to help decide on appropriate treatment, as it can determine the size and shape of the aneurysm.17 However, CT should not be used for unstable patients.
MRI is useful in diagnosing AAA, but it is expensive, and inappropriate for unstable patients. Currently, conventional aortography is rarely used for preoperative assessment but may still be used for placement of endovascular devices or in patients with renal complications.1,12
Screening Recommendations
The US Preventive Services Task Force (USPSTF) recommends that all men ages 65 to 74 who have a lifelong history of smoking at least 100 cigarettes should be screened for AAA with abdominal US.3,18 Screening is not recommended for those younger than 65 who have never smoked, but this decision must be individualized to the patient, with other risk factors considered.
The ACC/AHA4 advises that men whose parents or siblings have a history of AAA and who are older than 60 should undergo physical examination and screening US for AAA. In addition, patients with a small AAA should receive US surveillance until the aneurysm reaches 5.5 cm in diameter; survival has not been shown to improve if an AAA is repaired before it reaches this size.1,2,19 In consideration of increased comorbidities and decreased life expectancy, screening is not recommended for men older than 75, but this too should be determined individually.3
Screening for women is not recommended by the USPSTF.3,18 The document states that the prevalence of large AAAs in women is low and that screening may lead to an increased number of unnecessary surgeries with associated morbidity and mortality. Clinical judgment must be used in making this decision, however, as several studies have shown that women have an AAA rupture rate that is three times higher than that in men; they also have an increased in-hospital mortality rate when rupture does occur. Thus, women are less likely to experience AAA but have a worse prognosis when AAA does develop.20-22
Management
The size of an AAA is the most important predictor of rupture. According to the ACC/AHA,4 the associated risk for rupture is about 20% for aneurysms that measure 5.0 cm in diameter, 40% for those measuring at least 6.0 cm, and at least 50% for aneurysms exceeding 7.0 cm.4,23,24 Regarding surveillance of known aneurysms, it is recommended that a patient with an aneurysm smaller than 3.0 cm in diameter requires no further testing. If an AAA measures 3.0 to 4.0 cm, US should be performed yearly; if it is 4.0 to 4.9 cm, US should be performed every six months.4,25
If an identified AAA is larger than 4.5 cm, or if any segment of the aorta is more than 1.5 times the diameter of an adjacent section, referral to a vascular surgeon for further evaluation is indicated. The vascular surgeon should be consulted immediately regarding a symptomatic patient with an AAA, or one with an aneurysm that measures 5.5 cm or larger, as the risk for rupture is high.4,26
Preventing rupture of an AAA is the primary aim in management. Beta-blockers may be used to reduce systolic hypertension in cardiac patients, thus slowing the rate of expansion in those with aortic aneurysms. Patients with a known AAA should undergo frequent monitoring for blood pressure and lipid levels and be advised to stop smoking. Smoking cessation interventions such as behavior modification, nicotine replacement, or bupropion should be offered.27,28
There is evidence that statin use may reduce the size of aneurysms, even in patients without hypercholesterolemia, possibly due to statins’ anti-inflammatory properties.22,29 ACE inhibitors may also be beneficial in reducing AAA growth and in lowering blood pressure. Antiplatelet medications are important in general cardiovascular risk reduction in the patient with AAA. Aspirin is the drug of choice.27,29
Surgical Repair
AAAs are usually repaired by one of two types of surgery: endovascular repair (EVR) or open surgery. Open surgical repair, the more traditional method, involves an incision into the abdomen from the breastbone to below the navel. The weakened area is replaced with a graft made of synthetic material. Open repair of an intact AAA, performed under general anesthesia, takes from three to six hours, and the patient must be hospitalized for five to eight days.30
In EVR, the patient is given epidural anesthesia and an incision is made in the right groin, allowing a synthetic stent graft to be threaded by way of a catheter through the femoral artery to repair the lesion (see Figure 2). EVR generally takes two to five hours, followed by a two- to five-day hospital stay. EVR is usually recommended for patients who are at high risk for complications from open operations because of severe cardiopulmonary disease or other risk factors, such as advanced age, morbid obesity, or a history of multiple abdominal operations.1,2,4,19
Prognosis
Patients with a ruptured AAA have a survival rate of less than 50%, with most deaths occurring before surgical repair has been attempted.3,31 In patients with kidney failure resulting from AAA (whether ruptured or unruptured, an AAA can disrupt renal blood flow), the chance for survival is poor. By contrast, the risk for death during surgical graft repair of an AAA is only about 2% to 8%.1,12
In a systematic review, EVR was associated with a lower 30-day mortality rate compared with open surgical repair (1.6% vs 4.7%, respectively), but this reduction did not persist over two years’ follow-up; neither did EVR improve overall survival or quality of life, compared with open surgery.1 Additionally, EVR requires periodic imaging throughout the patient’s life, which is associated with more reinterventions.1,19
Patient Education
Clinicians should encourage all patients to stop smoking, follow a low-cholesterol diet, control hypertension, and exercise regularly to lower the risk for AAAs. Screening recommendations should be explained to patients at risk, as should the signs and symptoms of an aneurysm. These patients should be instructed to call their health care provider immediately if they suspect a problem.
Conclusion
The incidence of AAA is increasing, and primary care providers must be prepared to act promptly in any case of suspected AAA to ensure a safe outcome. For aneurysms measuring greater than 5.5 cm in diameter, open or endovascular surgical repair should be considered. Patients with smaller aneurysms or contraindications for surgery should receive careful medical management and education to reduce the risks of AAA expansion leading to possible rupture.
1. Wilt TJ, Lederle FA, MacDonald R, et al; Agency for Healthcare Research and Quality. Comparison of Endovascular and Open Surgical Repairs for Abdominal Aortic Aneurysm. Rockville, MD: Agency for Healthcare Research and Quality; 2006. AHRQ publication 06-E107. Evidence Report/Technology Assessment 144. www.ahrq.gov/CLINIC/tp/aaareptp.htm. Accessed June 23, 2009.
2. Birkmeyer JD, Upchurch GR Jr. Evidence-based screening and management of abdominal aortic aneurysm. Ann Intern Med. 2007;146(10):749-750.
3. Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005;142(3):203-211.
4. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47(6):1239-1312.
5. Kiell CS, Ernst CB. Advances in management of abdominal aortic aneurysm. Adv Surg. 1993;26:73–98.
6. O’Connor RE. Aneurysm, abdominal. http://emedicine.medscape.com/article/756735-overview. Accessed June 23, 2009.
7. Lederle FA, Parenti CM, Chute EP. Ruptured abdominal aortic aneurysm: the internist as diagnostician. Am J Med. 1994;96:163-167.
8. Cartwright SL, Knudson MP. Evaluation of acute abdominal pain in adults. Am Fam Physician. 2008;77(7): 971-978.
9. Lyon C, Clark DC. Diagnosis of acute abdominal pain in older patients. Am Fam Physician. 2006;74(9):1537-1544.
10. Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999;30(6):1099-1105.
11. Palazzuoli P, Gallotta M, Guerrieri G, et al. Prevalence of risk factors, coronary and systemic atherosclerosis in abdominal aortic aneurysm: comparison with high cardiovascular risk population. Vasc Health Risk Manag. 2008;4(4):877-883.
12. Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365(9470):1577-1589.
13. Graham LM, Zelenock GB, Whitehouse WM Jr, et al. Clinical significance of arteriosclerotic femoral artery aneurysms. Arch Surg. 1980;115(4):502–507.
14. Whitehouse WM Jr, Wakefield TW, Graham LM, et al. Limb-threatening potential of arteriosclerotic popliteal artery aneurysms. Surgery. 1983;93(5):694–699.
15. Fink HA, Lederle FA, Roth CS, et al. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med. 2000;160:833-836.
16. Bentz S, Jones J. Accuracy of emergency department ultrasound scanning in detecting abdominal aortic aneurysm. Emerg Med J. 2006;23(10):803-804.
17. Kvilekval KH, Best IM, Mason RA, et al. The value of computed tomography in the management of symptomatic abdominal aortic aneurysm. J Vasc Surg. 1990;12(1):28-33.
18. US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med. 2005;142(3):198-202.
19. Lederle FA, Kane RL, MacDonald R, Wilt TJ. Systematic review: repair of unruptured abdominal aortic aneurysm. Ann Intern Med. 2007;146(10):735-741.
20. McPhee JT, Hill JS, Elami MH. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001-2004. J Vasc Surg. 2007;45(5):891-899.
21. Mofidi R, Goldie VJ, Kelman J, et al. Influence of sex on expansion rate of abdominal aortic aneurysms. Br J Surg. 2007;94(3):310-314.
22. Norman PE, Powell JT. Abdominal aortic aneurysm: the prognosis in women is worse than in men. Circulation. 2007;115(22):2865-2869.
23. Englund R, Hudson P, Hanel K, Stanton A. Expansion rates of small abdominal aortic aneurysms. Aust N Z J Surg. 1998;68(1):21–24.
24. Conway KP, Byrne J, Townsend M, Lane IF. Prognosis of patients turned down for conventional abdominal aortic aneurysm repair in the endovascular and sonographic era: Szilagyi revisited? J Vasc Surg. 2001;33(4):752–757.
25. Cook TA, Galland RB. A prospective study to define the optimum rescreening interval for small abdominal aortic aneurysm. Cardiovasc Surg. 1996;4(4):441–444.
26. Kent KC, Zwolak RM, Jaff MR, et al; Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine and Biology. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg. 2004;39(1):267-269.
27. Golledge J, Powell JT. Medical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2007;4(3):267-273.
28. Sule S, Aronow WS. Management of abdominal aortic aneurysms. Compr Ther. 2009;35(1):3-8.
29. Powell JT. Non-operative or medical management of abdominal aortic aneurysm. Scand J Surg. 2008;97(2): 121-124.
30. Huber TS, Wang JG, Derrow AE, et al. Experience in the United States with intact abdominal aortic aneurysm repair. J Vasc Surg. 2001;33(2):304-310.
31. Adam DJ, Mohan IV, Stuart WP, et al. Community and hospital outcome from ruptured abdominal aortic aneurysm within the catchment area of a regional vascular surgical service. J Vasc Surg. 1999;30(5):922-928.
1. Wilt TJ, Lederle FA, MacDonald R, et al; Agency for Healthcare Research and Quality. Comparison of Endovascular and Open Surgical Repairs for Abdominal Aortic Aneurysm. Rockville, MD: Agency for Healthcare Research and Quality; 2006. AHRQ publication 06-E107. Evidence Report/Technology Assessment 144. www.ahrq.gov/CLINIC/tp/aaareptp.htm. Accessed June 23, 2009.
2. Birkmeyer JD, Upchurch GR Jr. Evidence-based screening and management of abdominal aortic aneurysm. Ann Intern Med. 2007;146(10):749-750.
3. Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med. 2005;142(3):203-211.
4. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47(6):1239-1312.
5. Kiell CS, Ernst CB. Advances in management of abdominal aortic aneurysm. Adv Surg. 1993;26:73–98.
6. O’Connor RE. Aneurysm, abdominal. http://emedicine.medscape.com/article/756735-overview. Accessed June 23, 2009.
7. Lederle FA, Parenti CM, Chute EP. Ruptured abdominal aortic aneurysm: the internist as diagnostician. Am J Med. 1994;96:163-167.
8. Cartwright SL, Knudson MP. Evaluation of acute abdominal pain in adults. Am Fam Physician. 2008;77(7): 971-978.
9. Lyon C, Clark DC. Diagnosis of acute abdominal pain in older patients. Am Fam Physician. 2006;74(9):1537-1544.
10. Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999;30(6):1099-1105.
11. Palazzuoli P, Gallotta M, Guerrieri G, et al. Prevalence of risk factors, coronary and systemic atherosclerosis in abdominal aortic aneurysm: comparison with high cardiovascular risk population. Vasc Health Risk Manag. 2008;4(4):877-883.
12. Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365(9470):1577-1589.
13. Graham LM, Zelenock GB, Whitehouse WM Jr, et al. Clinical significance of arteriosclerotic femoral artery aneurysms. Arch Surg. 1980;115(4):502–507.
14. Whitehouse WM Jr, Wakefield TW, Graham LM, et al. Limb-threatening potential of arteriosclerotic popliteal artery aneurysms. Surgery. 1983;93(5):694–699.
15. Fink HA, Lederle FA, Roth CS, et al. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med. 2000;160:833-836.
16. Bentz S, Jones J. Accuracy of emergency department ultrasound scanning in detecting abdominal aortic aneurysm. Emerg Med J. 2006;23(10):803-804.
17. Kvilekval KH, Best IM, Mason RA, et al. The value of computed tomography in the management of symptomatic abdominal aortic aneurysm. J Vasc Surg. 1990;12(1):28-33.
18. US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med. 2005;142(3):198-202.
19. Lederle FA, Kane RL, MacDonald R, Wilt TJ. Systematic review: repair of unruptured abdominal aortic aneurysm. Ann Intern Med. 2007;146(10):735-741.
20. McPhee JT, Hill JS, Elami MH. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001-2004. J Vasc Surg. 2007;45(5):891-899.
21. Mofidi R, Goldie VJ, Kelman J, et al. Influence of sex on expansion rate of abdominal aortic aneurysms. Br J Surg. 2007;94(3):310-314.
22. Norman PE, Powell JT. Abdominal aortic aneurysm: the prognosis in women is worse than in men. Circulation. 2007;115(22):2865-2869.
23. Englund R, Hudson P, Hanel K, Stanton A. Expansion rates of small abdominal aortic aneurysms. Aust N Z J Surg. 1998;68(1):21–24.
24. Conway KP, Byrne J, Townsend M, Lane IF. Prognosis of patients turned down for conventional abdominal aortic aneurysm repair in the endovascular and sonographic era: Szilagyi revisited? J Vasc Surg. 2001;33(4):752–757.
25. Cook TA, Galland RB. A prospective study to define the optimum rescreening interval for small abdominal aortic aneurysm. Cardiovasc Surg. 1996;4(4):441–444.
26. Kent KC, Zwolak RM, Jaff MR, et al; Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine and Biology. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg. 2004;39(1):267-269.
27. Golledge J, Powell JT. Medical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2007;4(3):267-273.
28. Sule S, Aronow WS. Management of abdominal aortic aneurysms. Compr Ther. 2009;35(1):3-8.
29. Powell JT. Non-operative or medical management of abdominal aortic aneurysm. Scand J Surg. 2008;97(2): 121-124.
30. Huber TS, Wang JG, Derrow AE, et al. Experience in the United States with intact abdominal aortic aneurysm repair. J Vasc Surg. 2001;33(2):304-310.
31. Adam DJ, Mohan IV, Stuart WP, et al. Community and hospital outcome from ruptured abdominal aortic aneurysm within the catchment area of a regional vascular surgical service. J Vasc Surg. 1999;30(5):922-928.
Botanical Briefs: Common Ivy (Hedera helix)
Multiple Congenital Plaquelike Glomuvenous Malformations With Type 2 Segmental Involvement
Potential caregivers for homebound elderly: More numerous than supposed?
Background This qualitative study examined the experiences and perspectives of caregivers of homebound elderly patients.
Methods We performed in-depth, semistructured interviews with 22 caregivers (average age 59 years) of homebound elderly patients and analyzed them to determine major themes. The homebound patients were part of a house call program of a US academic medical center in Baltimore, Maryland.
Results Caregiver relationships in our study were diverse: 41% were spouses or children, and 41% were unrelated to the homebound patient; 36% were male. We identified 3 themes: (1) caregiving has both positive and negative aspects, (2) caregiver motivation is heterogeneous, and (3) caregivers sometimes undergo transformation as a result of their caregiving experience.
Conclusion Caregiver experience is varied. Interviewees reported a variety of motivations for becoming caregivers and both positive and negative aspects of the experience. Caregivers in this study were diverse with respect to sex and relationship to the patient, suggesting the pool of potential caregivers may be larger than previously thought.
When thinking about long-term home care for the chronically ill elderly, many people automatically imagine a spouse or child as the primary caregiver. In our study, however, 41% of the caregivers interviewed were unrelated to the person receiving the care. In addition to this diversity, we found that motivations for providing care varied among participants; that their different experiences ranged from positive to negative, or a little of both; and that a few caregivers felt their attitudes changed for the better over the course of giving assistance.
We must plan for an aging population
In 2000, 35 million people, or 8% of the US population, were 65 years of age or older. In 2040, there will be 80 million seniors, or 20.4% of the US population.1 The National Long Term Care Survey found that in 1999, 3.9 million Medicare enrollees with a chronic disability were receiving care in their homes. A significant portion of caregiving burden is borne by patients’ relatives and friends; more than 90% of homebound patients were receiving some degree of informal, unpaid assistance.2 As the population ages, more caregivers will be needed to tend chronically ill elders, and most will be informal caregivers.
The reason for our study
Research over the past few decades has found that the burden is significant for those caring for older adults.3-6 Daily challenges and stressors increase the burden caregivers feel in their role.7,8 More recent work has also examined interventions to alleviate caregiver burden.9 Studies of caregivers—published predominately in the social science and nursing literature—have seldom reported on the positive aspects of the role.10-12 Only 1 study in the US medical literature, a national survey, noted positive aspects of the caregiving role.13
Through in-depth interviews, we sought to learn more fully about the experiences of caregivers of chronically ill homebound elderly people.
Methods
Design, setting, study population
This qualitative study of caregivers, a focused ethnography,14 was part of a larger project that interviewed the patients and their doctors. We conducted the study through the Johns Hopkins Geriatrics Center Elder Housecall Program (EHP) from 1997 to 2001. Since 1979, EHP has provided medical and nursing care to generally frail, homebound elderly (mean age 77), predominantly white (82%) and female (69%) patients in a largely blue-collar community in east Baltimore. Annual mortality for patients is 25%.15 We selected a qualitative approach because we wanted to learn more about the experiences and perspectives of the caregivers.
Sampling
The parent study used a purposive and probabilistic sampling strategy to select patients, as described elsewhere.16,17 We found subjects for our caregiver study through the patients, who identified the individuals who assist them. The range of caregiver responsibilities included, but was not limited to, coordinating services, managing medical and financial affairs, and directing such activities as bathing, dressing, and meal preparation. All caregivers we invited to participate did so.
Measurements
We conducted in-depth, semistructured interviews lasting approximately 1 hour. We also collected demographic information. As a starting point for each interview, we used the following brief guide:
- What has your experience as a caregiver for the patient been like?
- Do you recall any particular examples of rewarding aspects of the role?
- Do you recall any particular examples of difficult aspects of the role?
- Are there any particular challenges or important issues in your relationship with the patient that you wish to share?
An investigator trained in qualitative research (JC) asked additional questions, as needed, to further explore caregiver responses. We gave interviewees considerable latitude in commenting on points or topics they considered relevant.
Analysis
Two of this study’s authors (JC, JM) audiotaped, transcribed, and independently coded the interviews, and compared them for agreement. We used an editing style analysis.18 Thematic categories and subcategories became apparent during coding, and we modified them as the interviewing proceeded. We examined and conceptually organized the categories, using the qualitative research software program NUD*IST 4 (Qualitative Solutions and Research Pty Ltd, Victoria, Australia) to facilitate data management and analysis.
A consensus approach
At least 2 investigators participated in each step of the analysis (eg, reading and coding of transcripts; identification, modification, conceptual organization of categories; and selection of themes for presentation). The team made all decisions by consensus.
Human subjects research approval
A Johns Hopkins University School of Medicine Institutional Review Board approved this study, and we obtained written informed consent from all study participants.
Results
We interviewed 22 caregivers (TABLE 1). The number of caregivers per patient ranged from 0 to 3 (20 patients altogether). Most patients had 1 caregiver, but several had 2 or 3. Sixteen interviews involved 1 caregiver, and 3 involved caregiver teams. The average age of the caregivers was 59.3 years, and they had known the patients for an average of 37.4 years. Fourteen of the 22 caregivers (63.6%) were female (a finding similar to results from a Kaiser Family Foundation study in 1998 of 1002 caregivers, in which 64% of the 511 primary caregivers were female).19 Nine of the 22 (40.9%) participants were unrelated to the patient. Caregivers were primarily unpaid relatives or friends (77.3%), but compensated individuals were also included.
Three major themes emerged from analysis of the interview transcripts: (1) positive and negative experiences of caregiving, (2) caregiver motivation, and (3) caregiver transformation. Representative quotes are used to illustrate the themes presented. Specific examples of these themes are shown in TABLE 2.
TABLE 1
Characteristics of the caregivers we interviewed
| Sex | |
| Female | 14/22 (63.6%) |
| Average age | |
| 59.3 years | |
| Ethnicity | |
| Caucasian | 19/22 (86.4%) |
| African American | 3/22 (13.6%) |
| Average length of relationship with patient | |
| 37.4 years | |
| Relationship to patient | |
| Related | 13/22 (59.1%) |
| 1 wife 4 sons 4 daughters 2 grandchildren 1 daughter-in-law 1 grandson-in-law | |
| Unrelated | 9/22 (40.9%) |
| 4 friends (1 paid) 3 paid professional caregivers 1 paid nonprofessional caregiver 1 distant nonblood relative | |
TABLE 2
Common caregiver themes that emerged during interviews
Positive aspects of caregiving
| Negative aspects of caregiving
|
Caregiver motivations
| Caregiver transformation
|
Positive and negative aspects of caregiving
Caregivers described very different experiences of their roles—some only negative, some only positive, and others both positive and negative. Accounts of 6 of the 22 interviewees were essentially value-neutral.
Negative aspects of caregiving. Seven of 22 caregivers reported only negative feelings toward their role, including feeling burdened.
A 62-year-old retired son described how caring for his mother adversely affected his life:
Yeah, I sleep here. I don’t even go to my bed. I haven’t been to bed in over 3 years, because…if I don’t go down as soon as she rings the bell, she can’t hold her water.… I used to go out to Pennsylvania and go up in the battlefield there. I used to go down Skyline Drive, places like that. I can’t do that anymore.
Positive aspects of caregiving. Four caregivers addressed only positive aspects of caregiving.
A 71-year-old retired secretary who was asked if she had encountered any difficulties in caring for her friend said:
I haven’t had any. We’ve become very close friends and her friendship means a lot…I don’t think there’s any problems with going up there…Because I call her before I go to the grocery store to make sure she’s got everything on the list and then I go and just take it up to her and do from there.
Mixed experiences with caregiving. Five of our participants discussed both positive and negative aspects of caregiving; 4 of these 5 lived with the patient.
A 47-year-old machine technician who cared for his grandmother described the benefits his 16-year-old son was receiving from the caregiving arrangement:
It’s been a plus for him to have his great-grandmother living here. I think he enjoys her company, the little stories that go along and plus they always had a good relationship when he was a small child…I would hope he would realize the importance of family and I feel we’re losing that in our society, we’re losing our family. Seems like everybody is moving away and not being associated as close as probably we once were, and you know you realize that sometimes people need a little bit of help and not to be as selfish as you would want to be and maybe learn from that that we all kind of need one another at one time and not to be so independent, like it seems like our society has gone.
He also identified negative aspects of caregiving, including the burdens associated with selling his grandmother’s home and helping her settle into his family’s home:
For the last year it has been kind of hectic with trying to make things easier, doing what needs to be done, taking care of her house. Now that that’s out of the way, that’s a big burden out of the way.
Caregiver motivations
Eight caregivers shared their motivations for deciding to care for a homebound relative or friend. These comments were unsolicited and unexpected. Four caregivers believed they were repaying the patient for help received earlier in life.
A 69-year-old daughter-in-law said the following:
I say to her the same thing I said to my mother: “You took care of me when I was little and I am taking care of you. Now it is my turn.”…I mean we are put on this earth for a purpose and I figure this is our purpose. God put us down here to take care of someone or to help someone.
Potential for caregiver transformation
Another unexpected finding from our study was that 3 interviewees reported that they or their family members were changed by the caregiving experience. Transformations included changing one’s outlook on life, changing one’s views of the caregiving role, and being able to better cope with the death of others.
A 59-year-old homemaker related how her feelings about caregiving changed over time, and she felt she was repaying her mother for help she herself had received:
My major thing in the beginning was I really felt dumped on, like you have to do this whether you want to or not to prevent her from going in a place she didn’t want to go to. But then, after a while, I didn’t feel that way no more because she helped me when I needed help, when my kids were little. She was always there for me.
Discussion
While the medical literature to date has focused on the burdens and difficulties of caregiving, our study shows that caregivers have positive as well as negative experiences in their roles, and that, for some, the experience is a complex mixture of burdens and benefits. Interestingly, 4 of the 5 caregivers who experienced that mixture lived with the patient, suggesting that proximity and increased exposure may result in a more complex experience. In addition to these findings, some caregivers have different motivations for providing care. A small number even describe the experience as transformative.
These findings are consistent with a few studies from the nursing and social science literature that address the positive aspects of caregiving.11,20 For example, 2 studies found that caregivers of patients with dementia experienced both positive and negative aspects of their role.10,12 A recent analysis of a national survey of caregivers noted that two-thirds had feelings of personal reward.13
How can you support caregivers? A deeper understanding of caregivers’ diverse motivations and experiences can help physicians prepare others for this important role, and support and encourage those who are already caring for someone.
You can offer support by discussing with current and prospective caregivers the possibility that the role may bring both positive and negative experiences.
It may also be helpful to describe the potentially transformative nature of caregiving—to point out that some people report that their negative feelings have become more positive in time. In the end, care of dependent elderly patients may improve with such awareness.
Pool of potential caregivers larger than expected. Another finding of our study is the diversity of caregivers. Only 9 of the 22 caregivers interviewed were spouses or children, and only 5 of these 9 were wives or daughters. Among the children, there were just as many sons as daughters. Grandchildren were also represented, and 41% of the caregivers were unrelated to the patient.
Traditionally, many health professionals and the public have looked to female adult children or spouses to care for patients, and the literature on the caregiver experience often represents their views. However, some studies have noted that friends and others are also involved.21 Our finding adds to an evolving understanding that potential caregivers for the homebound elderly can be drawn from a broader pool than first-degree, female relatives.
Limitations of this study. The study sample was small—22 caregivers who live in a particular section of the greater Baltimore metropolitan area. In addition, most of the caregivers were Caucasian and thus do not reflect the ethnic diversity of the United States. As such, we must be cautious in extrapolating these findings to other caregivers in other settings. Nevertheless, we believe that aspects of the caregiver experience reported here will ring true to caregivers who live elsewhere.
Americans are living longer, and many of them have chronic medical problems. An increasing percentage of these elderly will require some level of caregiving to stay in their homes. Future studies might explore in more depth caregiver motivations and caregiver transformation to gain better insight into these important issues.
Acknowledgement
Dr. Carrese received support for this project from the Robert Wood Johnson Generalist Physician Faculty Scholars Program.
Correspondence
Laura A. Hanyok, MD, Johns Hopkins Bayview Medical Center, 5501 Hopkins Bayview Circle, Room 1B.45, Baltimore, MD 21224; [email protected].
1. Federal Interagency Forum on Aging-Related Statistics: 2006 older Americans update: key indicator of wellness. Available at: http://www.agingstats.gov/agingstatsdotnet/Main_Site/Data/Data_2006.aspx. Accessed June 13, 2008.
2. Wolff JL, Kasper JD. Caregivers of frail elders: updating a national profile. Gerontologist. 2006;46:344-356.
3. Brody EM. The Donald P. Kent Memorial Lecture. Parent care as a normative family stress. Gerontologist. 1986;25:19-29.
4. George LK, Gwyther LP. Caregiver well-being: A multidimensional examination of family caregivers of demented adults. Gerontologist. 1986;26:253-259.
5. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20:649-655.
6. Zarit SH, Todd PA, Zarit JM. Subjective burden of husbands and wives as caregivers: A longitudinal study. Gerontologist. 1986;26:260-266.
7. Öhman M, Seidenberg S. The experiences of close relatives living with a person with serious chronic illness. Qual Health Res. 2004;14:396-410.
8. Sawatzky JE, Fowler-Kerry S. Impact of caregiving: listening to the voice of informal caregivers. J Psychiatr Ment Health Nurs. 2003;10:277-286.
9. Yin T, Zhou Q, Bashford C. Burden on family members: Caring for frail elderly: a meta-analysis of interventions. Nurs Res. 2002;51:199-208.
10. Andrén S, Elmståhl S. Family caregivers’ subjective experiences of satisfaction in dementia care: aspects of burden, subjective health and sense of coherence. Scand J Caring Sci. 2005;19:157-168.
11. Riedel SE, Fredman L, Langenberg P. Associations among caregiving difficulties, burden, and rewards in caregivers to older post-rehabilitation patients. J Gerontol B Psychol Sci Soc Sci. 1998;53:165-174.
12. Sanders S. Is the glass half empty or half full? Reflections on strain and gain in caregivers of individuals with Alzheimer’s disease. Soc Work Health Care. 2005;40:57-73.
13. Wolff JL, Dy SM, Frick KD, et al. End-of-life care: findings from a national survey of informal caregivers. Arch Intern Med. 2007;167:40-46.
14. Muecke MA. On the evaluation of ethnographies. In: Morse J, ed. Critical Issues in Qualitative Research Methods. Thousand oaks, Calif: Sage Publications; 1994:198-199.
15. Tsuji I, Fox-Whalen S, Finucane TE. Predictors of nursing home placement in community-based long-term care. J Am Geriatr Soc. 1995;43:761-766.
16. Carrese JA, Mullaney JL, Faden RR, et al. Planning for death but not serious future illness: Qualitative study of housebound elderly patients. BMJ. 2002;325:125-127.
17. Russell BH. Research Methods in Anthropology: Qualitative and Quantitative Approaches. 2nd ed. Thousand oaks, Calif: Sage Publications; 1994:95-96.
18. Crabtree BF, Mille WL. Doing Qualitative Research. Newbury Park, Calif: Sage Publications; 1992:18.
19. Donelan K, Hill CA, Hoffman C, et al. Challenged to care: informal caregivers in a changing health system. Health Affairs. 2002. Available at: http://content.healthaffairs.org/cgi/content/full/21/4/222. Accessed June 9, 2009.
20. Jarvis A, Worth A, Porter M. The experience of caring for someone over 75 years of age: results from a Scottish General Practice. J Clin Nurs. 2006;15:1450-1459.
21. Grunfield E, Coyle D, Whelan T, et al. Family caregiver burden; Results of a longitudinal study of breast cancer patient and their principal caregivers. CMAJ. 2004;170:1795-1801.
Background This qualitative study examined the experiences and perspectives of caregivers of homebound elderly patients.
Methods We performed in-depth, semistructured interviews with 22 caregivers (average age 59 years) of homebound elderly patients and analyzed them to determine major themes. The homebound patients were part of a house call program of a US academic medical center in Baltimore, Maryland.
Results Caregiver relationships in our study were diverse: 41% were spouses or children, and 41% were unrelated to the homebound patient; 36% were male. We identified 3 themes: (1) caregiving has both positive and negative aspects, (2) caregiver motivation is heterogeneous, and (3) caregivers sometimes undergo transformation as a result of their caregiving experience.
Conclusion Caregiver experience is varied. Interviewees reported a variety of motivations for becoming caregivers and both positive and negative aspects of the experience. Caregivers in this study were diverse with respect to sex and relationship to the patient, suggesting the pool of potential caregivers may be larger than previously thought.
When thinking about long-term home care for the chronically ill elderly, many people automatically imagine a spouse or child as the primary caregiver. In our study, however, 41% of the caregivers interviewed were unrelated to the person receiving the care. In addition to this diversity, we found that motivations for providing care varied among participants; that their different experiences ranged from positive to negative, or a little of both; and that a few caregivers felt their attitudes changed for the better over the course of giving assistance.
We must plan for an aging population
In 2000, 35 million people, or 8% of the US population, were 65 years of age or older. In 2040, there will be 80 million seniors, or 20.4% of the US population.1 The National Long Term Care Survey found that in 1999, 3.9 million Medicare enrollees with a chronic disability were receiving care in their homes. A significant portion of caregiving burden is borne by patients’ relatives and friends; more than 90% of homebound patients were receiving some degree of informal, unpaid assistance.2 As the population ages, more caregivers will be needed to tend chronically ill elders, and most will be informal caregivers.
The reason for our study
Research over the past few decades has found that the burden is significant for those caring for older adults.3-6 Daily challenges and stressors increase the burden caregivers feel in their role.7,8 More recent work has also examined interventions to alleviate caregiver burden.9 Studies of caregivers—published predominately in the social science and nursing literature—have seldom reported on the positive aspects of the role.10-12 Only 1 study in the US medical literature, a national survey, noted positive aspects of the caregiving role.13
Through in-depth interviews, we sought to learn more fully about the experiences of caregivers of chronically ill homebound elderly people.
Methods
Design, setting, study population
This qualitative study of caregivers, a focused ethnography,14 was part of a larger project that interviewed the patients and their doctors. We conducted the study through the Johns Hopkins Geriatrics Center Elder Housecall Program (EHP) from 1997 to 2001. Since 1979, EHP has provided medical and nursing care to generally frail, homebound elderly (mean age 77), predominantly white (82%) and female (69%) patients in a largely blue-collar community in east Baltimore. Annual mortality for patients is 25%.15 We selected a qualitative approach because we wanted to learn more about the experiences and perspectives of the caregivers.
Sampling
The parent study used a purposive and probabilistic sampling strategy to select patients, as described elsewhere.16,17 We found subjects for our caregiver study through the patients, who identified the individuals who assist them. The range of caregiver responsibilities included, but was not limited to, coordinating services, managing medical and financial affairs, and directing such activities as bathing, dressing, and meal preparation. All caregivers we invited to participate did so.
Measurements
We conducted in-depth, semistructured interviews lasting approximately 1 hour. We also collected demographic information. As a starting point for each interview, we used the following brief guide:
- What has your experience as a caregiver for the patient been like?
- Do you recall any particular examples of rewarding aspects of the role?
- Do you recall any particular examples of difficult aspects of the role?
- Are there any particular challenges or important issues in your relationship with the patient that you wish to share?
An investigator trained in qualitative research (JC) asked additional questions, as needed, to further explore caregiver responses. We gave interviewees considerable latitude in commenting on points or topics they considered relevant.
Analysis
Two of this study’s authors (JC, JM) audiotaped, transcribed, and independently coded the interviews, and compared them for agreement. We used an editing style analysis.18 Thematic categories and subcategories became apparent during coding, and we modified them as the interviewing proceeded. We examined and conceptually organized the categories, using the qualitative research software program NUD*IST 4 (Qualitative Solutions and Research Pty Ltd, Victoria, Australia) to facilitate data management and analysis.
A consensus approach
At least 2 investigators participated in each step of the analysis (eg, reading and coding of transcripts; identification, modification, conceptual organization of categories; and selection of themes for presentation). The team made all decisions by consensus.
Human subjects research approval
A Johns Hopkins University School of Medicine Institutional Review Board approved this study, and we obtained written informed consent from all study participants.
Results
We interviewed 22 caregivers (TABLE 1). The number of caregivers per patient ranged from 0 to 3 (20 patients altogether). Most patients had 1 caregiver, but several had 2 or 3. Sixteen interviews involved 1 caregiver, and 3 involved caregiver teams. The average age of the caregivers was 59.3 years, and they had known the patients for an average of 37.4 years. Fourteen of the 22 caregivers (63.6%) were female (a finding similar to results from a Kaiser Family Foundation study in 1998 of 1002 caregivers, in which 64% of the 511 primary caregivers were female).19 Nine of the 22 (40.9%) participants were unrelated to the patient. Caregivers were primarily unpaid relatives or friends (77.3%), but compensated individuals were also included.
Three major themes emerged from analysis of the interview transcripts: (1) positive and negative experiences of caregiving, (2) caregiver motivation, and (3) caregiver transformation. Representative quotes are used to illustrate the themes presented. Specific examples of these themes are shown in TABLE 2.
TABLE 1
Characteristics of the caregivers we interviewed
| Sex | |
| Female | 14/22 (63.6%) |
| Average age | |
| 59.3 years | |
| Ethnicity | |
| Caucasian | 19/22 (86.4%) |
| African American | 3/22 (13.6%) |
| Average length of relationship with patient | |
| 37.4 years | |
| Relationship to patient | |
| Related | 13/22 (59.1%) |
| 1 wife 4 sons 4 daughters 2 grandchildren 1 daughter-in-law 1 grandson-in-law | |
| Unrelated | 9/22 (40.9%) |
| 4 friends (1 paid) 3 paid professional caregivers 1 paid nonprofessional caregiver 1 distant nonblood relative | |
TABLE 2
Common caregiver themes that emerged during interviews
Positive aspects of caregiving
| Negative aspects of caregiving
|
Caregiver motivations
| Caregiver transformation
|
Positive and negative aspects of caregiving
Caregivers described very different experiences of their roles—some only negative, some only positive, and others both positive and negative. Accounts of 6 of the 22 interviewees were essentially value-neutral.
Negative aspects of caregiving. Seven of 22 caregivers reported only negative feelings toward their role, including feeling burdened.
A 62-year-old retired son described how caring for his mother adversely affected his life:
Yeah, I sleep here. I don’t even go to my bed. I haven’t been to bed in over 3 years, because…if I don’t go down as soon as she rings the bell, she can’t hold her water.… I used to go out to Pennsylvania and go up in the battlefield there. I used to go down Skyline Drive, places like that. I can’t do that anymore.
Positive aspects of caregiving. Four caregivers addressed only positive aspects of caregiving.
A 71-year-old retired secretary who was asked if she had encountered any difficulties in caring for her friend said:
I haven’t had any. We’ve become very close friends and her friendship means a lot…I don’t think there’s any problems with going up there…Because I call her before I go to the grocery store to make sure she’s got everything on the list and then I go and just take it up to her and do from there.
Mixed experiences with caregiving. Five of our participants discussed both positive and negative aspects of caregiving; 4 of these 5 lived with the patient.
A 47-year-old machine technician who cared for his grandmother described the benefits his 16-year-old son was receiving from the caregiving arrangement:
It’s been a plus for him to have his great-grandmother living here. I think he enjoys her company, the little stories that go along and plus they always had a good relationship when he was a small child…I would hope he would realize the importance of family and I feel we’re losing that in our society, we’re losing our family. Seems like everybody is moving away and not being associated as close as probably we once were, and you know you realize that sometimes people need a little bit of help and not to be as selfish as you would want to be and maybe learn from that that we all kind of need one another at one time and not to be so independent, like it seems like our society has gone.
He also identified negative aspects of caregiving, including the burdens associated with selling his grandmother’s home and helping her settle into his family’s home:
For the last year it has been kind of hectic with trying to make things easier, doing what needs to be done, taking care of her house. Now that that’s out of the way, that’s a big burden out of the way.
Caregiver motivations
Eight caregivers shared their motivations for deciding to care for a homebound relative or friend. These comments were unsolicited and unexpected. Four caregivers believed they were repaying the patient for help received earlier in life.
A 69-year-old daughter-in-law said the following:
I say to her the same thing I said to my mother: “You took care of me when I was little and I am taking care of you. Now it is my turn.”…I mean we are put on this earth for a purpose and I figure this is our purpose. God put us down here to take care of someone or to help someone.
Potential for caregiver transformation
Another unexpected finding from our study was that 3 interviewees reported that they or their family members were changed by the caregiving experience. Transformations included changing one’s outlook on life, changing one’s views of the caregiving role, and being able to better cope with the death of others.
A 59-year-old homemaker related how her feelings about caregiving changed over time, and she felt she was repaying her mother for help she herself had received:
My major thing in the beginning was I really felt dumped on, like you have to do this whether you want to or not to prevent her from going in a place she didn’t want to go to. But then, after a while, I didn’t feel that way no more because she helped me when I needed help, when my kids were little. She was always there for me.
Discussion
While the medical literature to date has focused on the burdens and difficulties of caregiving, our study shows that caregivers have positive as well as negative experiences in their roles, and that, for some, the experience is a complex mixture of burdens and benefits. Interestingly, 4 of the 5 caregivers who experienced that mixture lived with the patient, suggesting that proximity and increased exposure may result in a more complex experience. In addition to these findings, some caregivers have different motivations for providing care. A small number even describe the experience as transformative.
These findings are consistent with a few studies from the nursing and social science literature that address the positive aspects of caregiving.11,20 For example, 2 studies found that caregivers of patients with dementia experienced both positive and negative aspects of their role.10,12 A recent analysis of a national survey of caregivers noted that two-thirds had feelings of personal reward.13
How can you support caregivers? A deeper understanding of caregivers’ diverse motivations and experiences can help physicians prepare others for this important role, and support and encourage those who are already caring for someone.
You can offer support by discussing with current and prospective caregivers the possibility that the role may bring both positive and negative experiences.
It may also be helpful to describe the potentially transformative nature of caregiving—to point out that some people report that their negative feelings have become more positive in time. In the end, care of dependent elderly patients may improve with such awareness.
Pool of potential caregivers larger than expected. Another finding of our study is the diversity of caregivers. Only 9 of the 22 caregivers interviewed were spouses or children, and only 5 of these 9 were wives or daughters. Among the children, there were just as many sons as daughters. Grandchildren were also represented, and 41% of the caregivers were unrelated to the patient.
Traditionally, many health professionals and the public have looked to female adult children or spouses to care for patients, and the literature on the caregiver experience often represents their views. However, some studies have noted that friends and others are also involved.21 Our finding adds to an evolving understanding that potential caregivers for the homebound elderly can be drawn from a broader pool than first-degree, female relatives.
Limitations of this study. The study sample was small—22 caregivers who live in a particular section of the greater Baltimore metropolitan area. In addition, most of the caregivers were Caucasian and thus do not reflect the ethnic diversity of the United States. As such, we must be cautious in extrapolating these findings to other caregivers in other settings. Nevertheless, we believe that aspects of the caregiver experience reported here will ring true to caregivers who live elsewhere.
Americans are living longer, and many of them have chronic medical problems. An increasing percentage of these elderly will require some level of caregiving to stay in their homes. Future studies might explore in more depth caregiver motivations and caregiver transformation to gain better insight into these important issues.
Acknowledgement
Dr. Carrese received support for this project from the Robert Wood Johnson Generalist Physician Faculty Scholars Program.
Correspondence
Laura A. Hanyok, MD, Johns Hopkins Bayview Medical Center, 5501 Hopkins Bayview Circle, Room 1B.45, Baltimore, MD 21224; [email protected].
Background This qualitative study examined the experiences and perspectives of caregivers of homebound elderly patients.
Methods We performed in-depth, semistructured interviews with 22 caregivers (average age 59 years) of homebound elderly patients and analyzed them to determine major themes. The homebound patients were part of a house call program of a US academic medical center in Baltimore, Maryland.
Results Caregiver relationships in our study were diverse: 41% were spouses or children, and 41% were unrelated to the homebound patient; 36% were male. We identified 3 themes: (1) caregiving has both positive and negative aspects, (2) caregiver motivation is heterogeneous, and (3) caregivers sometimes undergo transformation as a result of their caregiving experience.
Conclusion Caregiver experience is varied. Interviewees reported a variety of motivations for becoming caregivers and both positive and negative aspects of the experience. Caregivers in this study were diverse with respect to sex and relationship to the patient, suggesting the pool of potential caregivers may be larger than previously thought.
When thinking about long-term home care for the chronically ill elderly, many people automatically imagine a spouse or child as the primary caregiver. In our study, however, 41% of the caregivers interviewed were unrelated to the person receiving the care. In addition to this diversity, we found that motivations for providing care varied among participants; that their different experiences ranged from positive to negative, or a little of both; and that a few caregivers felt their attitudes changed for the better over the course of giving assistance.
We must plan for an aging population
In 2000, 35 million people, or 8% of the US population, were 65 years of age or older. In 2040, there will be 80 million seniors, or 20.4% of the US population.1 The National Long Term Care Survey found that in 1999, 3.9 million Medicare enrollees with a chronic disability were receiving care in their homes. A significant portion of caregiving burden is borne by patients’ relatives and friends; more than 90% of homebound patients were receiving some degree of informal, unpaid assistance.2 As the population ages, more caregivers will be needed to tend chronically ill elders, and most will be informal caregivers.
The reason for our study
Research over the past few decades has found that the burden is significant for those caring for older adults.3-6 Daily challenges and stressors increase the burden caregivers feel in their role.7,8 More recent work has also examined interventions to alleviate caregiver burden.9 Studies of caregivers—published predominately in the social science and nursing literature—have seldom reported on the positive aspects of the role.10-12 Only 1 study in the US medical literature, a national survey, noted positive aspects of the caregiving role.13
Through in-depth interviews, we sought to learn more fully about the experiences of caregivers of chronically ill homebound elderly people.
Methods
Design, setting, study population
This qualitative study of caregivers, a focused ethnography,14 was part of a larger project that interviewed the patients and their doctors. We conducted the study through the Johns Hopkins Geriatrics Center Elder Housecall Program (EHP) from 1997 to 2001. Since 1979, EHP has provided medical and nursing care to generally frail, homebound elderly (mean age 77), predominantly white (82%) and female (69%) patients in a largely blue-collar community in east Baltimore. Annual mortality for patients is 25%.15 We selected a qualitative approach because we wanted to learn more about the experiences and perspectives of the caregivers.
Sampling
The parent study used a purposive and probabilistic sampling strategy to select patients, as described elsewhere.16,17 We found subjects for our caregiver study through the patients, who identified the individuals who assist them. The range of caregiver responsibilities included, but was not limited to, coordinating services, managing medical and financial affairs, and directing such activities as bathing, dressing, and meal preparation. All caregivers we invited to participate did so.
Measurements
We conducted in-depth, semistructured interviews lasting approximately 1 hour. We also collected demographic information. As a starting point for each interview, we used the following brief guide:
- What has your experience as a caregiver for the patient been like?
- Do you recall any particular examples of rewarding aspects of the role?
- Do you recall any particular examples of difficult aspects of the role?
- Are there any particular challenges or important issues in your relationship with the patient that you wish to share?
An investigator trained in qualitative research (JC) asked additional questions, as needed, to further explore caregiver responses. We gave interviewees considerable latitude in commenting on points or topics they considered relevant.
Analysis
Two of this study’s authors (JC, JM) audiotaped, transcribed, and independently coded the interviews, and compared them for agreement. We used an editing style analysis.18 Thematic categories and subcategories became apparent during coding, and we modified them as the interviewing proceeded. We examined and conceptually organized the categories, using the qualitative research software program NUD*IST 4 (Qualitative Solutions and Research Pty Ltd, Victoria, Australia) to facilitate data management and analysis.
A consensus approach
At least 2 investigators participated in each step of the analysis (eg, reading and coding of transcripts; identification, modification, conceptual organization of categories; and selection of themes for presentation). The team made all decisions by consensus.
Human subjects research approval
A Johns Hopkins University School of Medicine Institutional Review Board approved this study, and we obtained written informed consent from all study participants.
Results
We interviewed 22 caregivers (TABLE 1). The number of caregivers per patient ranged from 0 to 3 (20 patients altogether). Most patients had 1 caregiver, but several had 2 or 3. Sixteen interviews involved 1 caregiver, and 3 involved caregiver teams. The average age of the caregivers was 59.3 years, and they had known the patients for an average of 37.4 years. Fourteen of the 22 caregivers (63.6%) were female (a finding similar to results from a Kaiser Family Foundation study in 1998 of 1002 caregivers, in which 64% of the 511 primary caregivers were female).19 Nine of the 22 (40.9%) participants were unrelated to the patient. Caregivers were primarily unpaid relatives or friends (77.3%), but compensated individuals were also included.
Three major themes emerged from analysis of the interview transcripts: (1) positive and negative experiences of caregiving, (2) caregiver motivation, and (3) caregiver transformation. Representative quotes are used to illustrate the themes presented. Specific examples of these themes are shown in TABLE 2.
TABLE 1
Characteristics of the caregivers we interviewed
| Sex | |
| Female | 14/22 (63.6%) |
| Average age | |
| 59.3 years | |
| Ethnicity | |
| Caucasian | 19/22 (86.4%) |
| African American | 3/22 (13.6%) |
| Average length of relationship with patient | |
| 37.4 years | |
| Relationship to patient | |
| Related | 13/22 (59.1%) |
| 1 wife 4 sons 4 daughters 2 grandchildren 1 daughter-in-law 1 grandson-in-law | |
| Unrelated | 9/22 (40.9%) |
| 4 friends (1 paid) 3 paid professional caregivers 1 paid nonprofessional caregiver 1 distant nonblood relative | |
TABLE 2
Common caregiver themes that emerged during interviews
Positive aspects of caregiving
| Negative aspects of caregiving
|
Caregiver motivations
| Caregiver transformation
|
Positive and negative aspects of caregiving
Caregivers described very different experiences of their roles—some only negative, some only positive, and others both positive and negative. Accounts of 6 of the 22 interviewees were essentially value-neutral.
Negative aspects of caregiving. Seven of 22 caregivers reported only negative feelings toward their role, including feeling burdened.
A 62-year-old retired son described how caring for his mother adversely affected his life:
Yeah, I sleep here. I don’t even go to my bed. I haven’t been to bed in over 3 years, because…if I don’t go down as soon as she rings the bell, she can’t hold her water.… I used to go out to Pennsylvania and go up in the battlefield there. I used to go down Skyline Drive, places like that. I can’t do that anymore.
Positive aspects of caregiving. Four caregivers addressed only positive aspects of caregiving.
A 71-year-old retired secretary who was asked if she had encountered any difficulties in caring for her friend said:
I haven’t had any. We’ve become very close friends and her friendship means a lot…I don’t think there’s any problems with going up there…Because I call her before I go to the grocery store to make sure she’s got everything on the list and then I go and just take it up to her and do from there.
Mixed experiences with caregiving. Five of our participants discussed both positive and negative aspects of caregiving; 4 of these 5 lived with the patient.
A 47-year-old machine technician who cared for his grandmother described the benefits his 16-year-old son was receiving from the caregiving arrangement:
It’s been a plus for him to have his great-grandmother living here. I think he enjoys her company, the little stories that go along and plus they always had a good relationship when he was a small child…I would hope he would realize the importance of family and I feel we’re losing that in our society, we’re losing our family. Seems like everybody is moving away and not being associated as close as probably we once were, and you know you realize that sometimes people need a little bit of help and not to be as selfish as you would want to be and maybe learn from that that we all kind of need one another at one time and not to be so independent, like it seems like our society has gone.
He also identified negative aspects of caregiving, including the burdens associated with selling his grandmother’s home and helping her settle into his family’s home:
For the last year it has been kind of hectic with trying to make things easier, doing what needs to be done, taking care of her house. Now that that’s out of the way, that’s a big burden out of the way.
Caregiver motivations
Eight caregivers shared their motivations for deciding to care for a homebound relative or friend. These comments were unsolicited and unexpected. Four caregivers believed they were repaying the patient for help received earlier in life.
A 69-year-old daughter-in-law said the following:
I say to her the same thing I said to my mother: “You took care of me when I was little and I am taking care of you. Now it is my turn.”…I mean we are put on this earth for a purpose and I figure this is our purpose. God put us down here to take care of someone or to help someone.
Potential for caregiver transformation
Another unexpected finding from our study was that 3 interviewees reported that they or their family members were changed by the caregiving experience. Transformations included changing one’s outlook on life, changing one’s views of the caregiving role, and being able to better cope with the death of others.
A 59-year-old homemaker related how her feelings about caregiving changed over time, and she felt she was repaying her mother for help she herself had received:
My major thing in the beginning was I really felt dumped on, like you have to do this whether you want to or not to prevent her from going in a place she didn’t want to go to. But then, after a while, I didn’t feel that way no more because she helped me when I needed help, when my kids were little. She was always there for me.
Discussion
While the medical literature to date has focused on the burdens and difficulties of caregiving, our study shows that caregivers have positive as well as negative experiences in their roles, and that, for some, the experience is a complex mixture of burdens and benefits. Interestingly, 4 of the 5 caregivers who experienced that mixture lived with the patient, suggesting that proximity and increased exposure may result in a more complex experience. In addition to these findings, some caregivers have different motivations for providing care. A small number even describe the experience as transformative.
These findings are consistent with a few studies from the nursing and social science literature that address the positive aspects of caregiving.11,20 For example, 2 studies found that caregivers of patients with dementia experienced both positive and negative aspects of their role.10,12 A recent analysis of a national survey of caregivers noted that two-thirds had feelings of personal reward.13
How can you support caregivers? A deeper understanding of caregivers’ diverse motivations and experiences can help physicians prepare others for this important role, and support and encourage those who are already caring for someone.
You can offer support by discussing with current and prospective caregivers the possibility that the role may bring both positive and negative experiences.
It may also be helpful to describe the potentially transformative nature of caregiving—to point out that some people report that their negative feelings have become more positive in time. In the end, care of dependent elderly patients may improve with such awareness.
Pool of potential caregivers larger than expected. Another finding of our study is the diversity of caregivers. Only 9 of the 22 caregivers interviewed were spouses or children, and only 5 of these 9 were wives or daughters. Among the children, there were just as many sons as daughters. Grandchildren were also represented, and 41% of the caregivers were unrelated to the patient.
Traditionally, many health professionals and the public have looked to female adult children or spouses to care for patients, and the literature on the caregiver experience often represents their views. However, some studies have noted that friends and others are also involved.21 Our finding adds to an evolving understanding that potential caregivers for the homebound elderly can be drawn from a broader pool than first-degree, female relatives.
Limitations of this study. The study sample was small—22 caregivers who live in a particular section of the greater Baltimore metropolitan area. In addition, most of the caregivers were Caucasian and thus do not reflect the ethnic diversity of the United States. As such, we must be cautious in extrapolating these findings to other caregivers in other settings. Nevertheless, we believe that aspects of the caregiver experience reported here will ring true to caregivers who live elsewhere.
Americans are living longer, and many of them have chronic medical problems. An increasing percentage of these elderly will require some level of caregiving to stay in their homes. Future studies might explore in more depth caregiver motivations and caregiver transformation to gain better insight into these important issues.
Acknowledgement
Dr. Carrese received support for this project from the Robert Wood Johnson Generalist Physician Faculty Scholars Program.
Correspondence
Laura A. Hanyok, MD, Johns Hopkins Bayview Medical Center, 5501 Hopkins Bayview Circle, Room 1B.45, Baltimore, MD 21224; [email protected].
1. Federal Interagency Forum on Aging-Related Statistics: 2006 older Americans update: key indicator of wellness. Available at: http://www.agingstats.gov/agingstatsdotnet/Main_Site/Data/Data_2006.aspx. Accessed June 13, 2008.
2. Wolff JL, Kasper JD. Caregivers of frail elders: updating a national profile. Gerontologist. 2006;46:344-356.
3. Brody EM. The Donald P. Kent Memorial Lecture. Parent care as a normative family stress. Gerontologist. 1986;25:19-29.
4. George LK, Gwyther LP. Caregiver well-being: A multidimensional examination of family caregivers of demented adults. Gerontologist. 1986;26:253-259.
5. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20:649-655.
6. Zarit SH, Todd PA, Zarit JM. Subjective burden of husbands and wives as caregivers: A longitudinal study. Gerontologist. 1986;26:260-266.
7. Öhman M, Seidenberg S. The experiences of close relatives living with a person with serious chronic illness. Qual Health Res. 2004;14:396-410.
8. Sawatzky JE, Fowler-Kerry S. Impact of caregiving: listening to the voice of informal caregivers. J Psychiatr Ment Health Nurs. 2003;10:277-286.
9. Yin T, Zhou Q, Bashford C. Burden on family members: Caring for frail elderly: a meta-analysis of interventions. Nurs Res. 2002;51:199-208.
10. Andrén S, Elmståhl S. Family caregivers’ subjective experiences of satisfaction in dementia care: aspects of burden, subjective health and sense of coherence. Scand J Caring Sci. 2005;19:157-168.
11. Riedel SE, Fredman L, Langenberg P. Associations among caregiving difficulties, burden, and rewards in caregivers to older post-rehabilitation patients. J Gerontol B Psychol Sci Soc Sci. 1998;53:165-174.
12. Sanders S. Is the glass half empty or half full? Reflections on strain and gain in caregivers of individuals with Alzheimer’s disease. Soc Work Health Care. 2005;40:57-73.
13. Wolff JL, Dy SM, Frick KD, et al. End-of-life care: findings from a national survey of informal caregivers. Arch Intern Med. 2007;167:40-46.
14. Muecke MA. On the evaluation of ethnographies. In: Morse J, ed. Critical Issues in Qualitative Research Methods. Thousand oaks, Calif: Sage Publications; 1994:198-199.
15. Tsuji I, Fox-Whalen S, Finucane TE. Predictors of nursing home placement in community-based long-term care. J Am Geriatr Soc. 1995;43:761-766.
16. Carrese JA, Mullaney JL, Faden RR, et al. Planning for death but not serious future illness: Qualitative study of housebound elderly patients. BMJ. 2002;325:125-127.
17. Russell BH. Research Methods in Anthropology: Qualitative and Quantitative Approaches. 2nd ed. Thousand oaks, Calif: Sage Publications; 1994:95-96.
18. Crabtree BF, Mille WL. Doing Qualitative Research. Newbury Park, Calif: Sage Publications; 1992:18.
19. Donelan K, Hill CA, Hoffman C, et al. Challenged to care: informal caregivers in a changing health system. Health Affairs. 2002. Available at: http://content.healthaffairs.org/cgi/content/full/21/4/222. Accessed June 9, 2009.
20. Jarvis A, Worth A, Porter M. The experience of caring for someone over 75 years of age: results from a Scottish General Practice. J Clin Nurs. 2006;15:1450-1459.
21. Grunfield E, Coyle D, Whelan T, et al. Family caregiver burden; Results of a longitudinal study of breast cancer patient and their principal caregivers. CMAJ. 2004;170:1795-1801.
1. Federal Interagency Forum on Aging-Related Statistics: 2006 older Americans update: key indicator of wellness. Available at: http://www.agingstats.gov/agingstatsdotnet/Main_Site/Data/Data_2006.aspx. Accessed June 13, 2008.
2. Wolff JL, Kasper JD. Caregivers of frail elders: updating a national profile. Gerontologist. 2006;46:344-356.
3. Brody EM. The Donald P. Kent Memorial Lecture. Parent care as a normative family stress. Gerontologist. 1986;25:19-29.
4. George LK, Gwyther LP. Caregiver well-being: A multidimensional examination of family caregivers of demented adults. Gerontologist. 1986;26:253-259.
5. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20:649-655.
6. Zarit SH, Todd PA, Zarit JM. Subjective burden of husbands and wives as caregivers: A longitudinal study. Gerontologist. 1986;26:260-266.
7. Öhman M, Seidenberg S. The experiences of close relatives living with a person with serious chronic illness. Qual Health Res. 2004;14:396-410.
8. Sawatzky JE, Fowler-Kerry S. Impact of caregiving: listening to the voice of informal caregivers. J Psychiatr Ment Health Nurs. 2003;10:277-286.
9. Yin T, Zhou Q, Bashford C. Burden on family members: Caring for frail elderly: a meta-analysis of interventions. Nurs Res. 2002;51:199-208.
10. Andrén S, Elmståhl S. Family caregivers’ subjective experiences of satisfaction in dementia care: aspects of burden, subjective health and sense of coherence. Scand J Caring Sci. 2005;19:157-168.
11. Riedel SE, Fredman L, Langenberg P. Associations among caregiving difficulties, burden, and rewards in caregivers to older post-rehabilitation patients. J Gerontol B Psychol Sci Soc Sci. 1998;53:165-174.
12. Sanders S. Is the glass half empty or half full? Reflections on strain and gain in caregivers of individuals with Alzheimer’s disease. Soc Work Health Care. 2005;40:57-73.
13. Wolff JL, Dy SM, Frick KD, et al. End-of-life care: findings from a national survey of informal caregivers. Arch Intern Med. 2007;167:40-46.
14. Muecke MA. On the evaluation of ethnographies. In: Morse J, ed. Critical Issues in Qualitative Research Methods. Thousand oaks, Calif: Sage Publications; 1994:198-199.
15. Tsuji I, Fox-Whalen S, Finucane TE. Predictors of nursing home placement in community-based long-term care. J Am Geriatr Soc. 1995;43:761-766.
16. Carrese JA, Mullaney JL, Faden RR, et al. Planning for death but not serious future illness: Qualitative study of housebound elderly patients. BMJ. 2002;325:125-127.
17. Russell BH. Research Methods in Anthropology: Qualitative and Quantitative Approaches. 2nd ed. Thousand oaks, Calif: Sage Publications; 1994:95-96.
18. Crabtree BF, Mille WL. Doing Qualitative Research. Newbury Park, Calif: Sage Publications; 1992:18.
19. Donelan K, Hill CA, Hoffman C, et al. Challenged to care: informal caregivers in a changing health system. Health Affairs. 2002. Available at: http://content.healthaffairs.org/cgi/content/full/21/4/222. Accessed June 9, 2009.
20. Jarvis A, Worth A, Porter M. The experience of caring for someone over 75 years of age: results from a Scottish General Practice. J Clin Nurs. 2006;15:1450-1459.
21. Grunfield E, Coyle D, Whelan T, et al. Family caregiver burden; Results of a longitudinal study of breast cancer patient and their principal caregivers. CMAJ. 2004;170:1795-1801.
Preventive services: The good, the bad, and the unproven
The past 12 months have been busy ones for the United States Preventive Services Task Force (USPSTF), which issued 34 new recommendations since our last Practice Alert on the group’s activity a year ago. Some recommendations address controversial topics, such as cholesterol screening, and several others—on topics such as prostate cancer screening and acceptable tests for detecting colorectal cancer—differ from those of such prominent groups as the American Cancer Society (ACS).
TABLE 1 provides a breakdown of the 5 categories of USPSTF recommendations (A, B, C, D, I). We’ll start with recent D recommendations (TABLE 2), services the Task Force recommends against, to emphasize that some preventive measures—even if they are widely touted—either provide no benefit or cause more harms than benefits.
TABLE 1
USPSTF recommendation categories
| A Recommendation: The Task Force recommends the service. There is high certainty that the net benefit is substantial. |
| B Recommendation: The Task Force recommends this service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
| C Recommendation: The Task Force recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small. |
| D Recommendation: The Task Force recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
| I Statement: The Task Force concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
TABLE 2
The USPSTF recommends AGAINST
|
What not to do
The most notable new D recommendations advise against screening men ≥75 years of age for prostate cancer and against screening for colorectal cancer after age 85. The Task Force also recommends against routine screening for colorectal cancer after age 75, although individual patient considerations may influence your decision about this screen for patients between ages 76 and 85. Bear in mind that the benefits of early detection of colon cancer decline after age 75 because of the time lag between early intervention and benefit and because of competing causes of morbidity and mortality.1
Cancer screening controversies. The recommendations for an age cutoff for prostate and colon cancer screening differ from those of the ACS, which lists no age cutoff for screening for either condition.2 In fact, the Task Force does not recommend screening for prostate cancer at all. Its rationale is that before age 75, the evidence is insufficient to evaluate benefits and harms, and after 75 there is good evidence that screening does more harm than good. The ACS no longer recommends routine prostate cancer screening, but does say that when a patient leaves the decision to the physician, screening should be performed.
Thumbs down on these, too. The Task Force now recommends against using spirometry to screen for chronic obstructive pulmonary disease and against using aspirin for preventing stroke in women <55 years and myocardial infarction (MI) in men <45 years. (See below for a fuller discussion of aspirin as a preventive measure.) The Task Force also recommends against screening for asymptomatic bacteriuria in men and nonpregnant women.
Recommended interventions
Now for the preventive interventions the USPSTF advises you to perform. They include:
Prescribing low-dose aspirin. The most complicated positive recommendations are those for low-dose aspirin to prevent MI in men and stroke in women. Aspirin is effective in preventing these conditions, but carries the risk of major gastrointestinal (GI) bleeding and cerebral hemorrhage. For younger patients, as we’ve seen in the previous section, the Task Force finds the risks of prophylactic low-dose aspirin therapy outweigh the benefits. But for older patients (men between the ages of 45 and 79 years and women ages 55-79), aspirin is recommended when the potential benefit of reducing the incidence of MI in men and stroke in women outweigh the harms. To assist clinicians in weighing the potential benefits and harms, the USPSTF provides a link to a coronary heart disease risk calculator, as well as several tables comparing numbers of prevented heart attacks for men and strokes for women by age and risk category, as well as risks of bleeding complications.3
Screening for hypercholesterolemia. The Task Force’s recommendations for dyslipidemia screening differ markedly from those of the American Heart Association and the Final Report of the National Cholesterol Education Program (NCEP) Expert Panel, which recommend routine screening for all adults starting at age 20 with no age cutoff.4 The USPSTF recommends deferring screening until patients are older, except for those at increased risk of coronary heart disease. This controversy was described in a 2008 Practice Alert.5
Screening for diabetes. The only asymptomatic patients the Task Force recommends screening for diabetes are those with a sustained blood pressure of more than 135/80 mm Hg, treated or untreated. The American Diabetes Association (ADA) would cast a wider net, recommending that you consider screening for prediabetes or diabetes in those ≥45 years of age, particularly in those with a body mass index of ≥25 kg/m2, and in overweight patients <45 years of age who have another risk factor for diabetes.6
Screening for colorectal cancer. The Task Force recommends screening adults starting at age 50 until age 75, using fecal occult blood testing, sigmoidoscopy, or colonoscopy. The ACS also recommends these screening modalities, but adds CT colonography and fecal DNA testing to the list of acceptable methods. The USPSTF found insufficient evidence to evaluate the benefits and harms of these newer tests and expressed concern over the high rate of incidental findings and the unknown long-term effects of radiation from CT colonography.
Screening adolescents. The Task Force is in favor of screening teenagers for major depressive disorder (MDD), as long as systems are in place to provide accurate diagnosis, therapy, and follow-up. High-intensity behavioral counseling for sexually active teens and adults at risk is also endorsed for the prevention of sexually transmitted infections. In both areas, however, the Task Force recognizes that adequately addressing these issues will require more than brief office- or clinic-based interventions.
Caring for pregnant women and newborns. According to the USPSTF, pregnant women should be screened for asymptomatic bacteriuria, advised to take a daily folic acid supplement, counseled about tobacco use, and encouraged to breastfeed. Newborns should be screened for congenital hypothyroidism, phenylketonuria, and hearing loss. These most recent A and B recommendations from the USPSTF are summarized in TABLE 3.
TABLE 3
The USPSTF recommends FOR
| CARDIOVASCULAR DISEASE PREVENTION |
|
| CANCER SCREENING |
|
| PREGNANCY |
|
| NEWBORNS |
|
| ADOLESCENTS |
|
Not proven
When evidence is not available, some organizations are willing to issue guidelines based on expert opinion or consensus. Not so the USPSTF. When the Task Force members find current evidence is not sufficient to make a judgment, they put the intervention into Category I, for Insufficient. The new I recommendations range from aspirin to prevent MI and stroke in those ≥80 years to screening children for MDD and performing whole body skin examinations to detect early manifestations of skin cancer. The new I recommendations are listed in TABLE 4.
TABLE 4
Evidence is INSUFFICIENT to recommend for or against
|
What’s the take-home message?
All of these recent Task Force decisions add substantially to the full set of Task Force recommendations, which can be found at www.ahrq.gov/CLINIC/uspstfix.htm. Given the large number of level A and B recommendations from the Task Force, clinicians are faced with the dilemma of limited time to accomplish all the recommendations. It is reasonable to concentrate on the positive recommendations and avoid performing the interventions recommended against. The interventions in the “I” category are not as clear-cut and clinicians will continue to struggle with them, particularly when other professional organizations recommend them.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; [email protected].
1. US Preventive Services Task Force. Screening for colorectal cancer. October 2008. Available at: www.ahrq.gov/clinic/uspstf/uspscolo.htm. Accessed June 3, 2009.
2. American Cancer Society guidelines for early detection of cancer. Last revised May 21, 2009. Available at: http://www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines_36.asp?sitearea=PED. Accessed June 3, 2009.
3. US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: recommendation statement. March 2009. Available at: http://www.ahrq.gov/clinic/uspstf09/aspirincvd/aspcvdrs.htm. Accessed June 3, 2009.
4. National Cholesterol Education Program. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143-3421.Available at: http://circ.ahajournals.org/cgi/content/full/106/25/3143. Accessed June 3, 2009.
5. Campos-Outcalt D. USPSTF scales back approach to lipid screening for women. J Fam Pract. 2008;57:740-742.
6. American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(suppl 1):S12-S54.
The past 12 months have been busy ones for the United States Preventive Services Task Force (USPSTF), which issued 34 new recommendations since our last Practice Alert on the group’s activity a year ago. Some recommendations address controversial topics, such as cholesterol screening, and several others—on topics such as prostate cancer screening and acceptable tests for detecting colorectal cancer—differ from those of such prominent groups as the American Cancer Society (ACS).
TABLE 1 provides a breakdown of the 5 categories of USPSTF recommendations (A, B, C, D, I). We’ll start with recent D recommendations (TABLE 2), services the Task Force recommends against, to emphasize that some preventive measures—even if they are widely touted—either provide no benefit or cause more harms than benefits.
TABLE 1
USPSTF recommendation categories
| A Recommendation: The Task Force recommends the service. There is high certainty that the net benefit is substantial. |
| B Recommendation: The Task Force recommends this service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
| C Recommendation: The Task Force recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small. |
| D Recommendation: The Task Force recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
| I Statement: The Task Force concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
TABLE 2
The USPSTF recommends AGAINST
|
What not to do
The most notable new D recommendations advise against screening men ≥75 years of age for prostate cancer and against screening for colorectal cancer after age 85. The Task Force also recommends against routine screening for colorectal cancer after age 75, although individual patient considerations may influence your decision about this screen for patients between ages 76 and 85. Bear in mind that the benefits of early detection of colon cancer decline after age 75 because of the time lag between early intervention and benefit and because of competing causes of morbidity and mortality.1
Cancer screening controversies. The recommendations for an age cutoff for prostate and colon cancer screening differ from those of the ACS, which lists no age cutoff for screening for either condition.2 In fact, the Task Force does not recommend screening for prostate cancer at all. Its rationale is that before age 75, the evidence is insufficient to evaluate benefits and harms, and after 75 there is good evidence that screening does more harm than good. The ACS no longer recommends routine prostate cancer screening, but does say that when a patient leaves the decision to the physician, screening should be performed.
Thumbs down on these, too. The Task Force now recommends against using spirometry to screen for chronic obstructive pulmonary disease and against using aspirin for preventing stroke in women <55 years and myocardial infarction (MI) in men <45 years. (See below for a fuller discussion of aspirin as a preventive measure.) The Task Force also recommends against screening for asymptomatic bacteriuria in men and nonpregnant women.
Recommended interventions
Now for the preventive interventions the USPSTF advises you to perform. They include:
Prescribing low-dose aspirin. The most complicated positive recommendations are those for low-dose aspirin to prevent MI in men and stroke in women. Aspirin is effective in preventing these conditions, but carries the risk of major gastrointestinal (GI) bleeding and cerebral hemorrhage. For younger patients, as we’ve seen in the previous section, the Task Force finds the risks of prophylactic low-dose aspirin therapy outweigh the benefits. But for older patients (men between the ages of 45 and 79 years and women ages 55-79), aspirin is recommended when the potential benefit of reducing the incidence of MI in men and stroke in women outweigh the harms. To assist clinicians in weighing the potential benefits and harms, the USPSTF provides a link to a coronary heart disease risk calculator, as well as several tables comparing numbers of prevented heart attacks for men and strokes for women by age and risk category, as well as risks of bleeding complications.3
Screening for hypercholesterolemia. The Task Force’s recommendations for dyslipidemia screening differ markedly from those of the American Heart Association and the Final Report of the National Cholesterol Education Program (NCEP) Expert Panel, which recommend routine screening for all adults starting at age 20 with no age cutoff.4 The USPSTF recommends deferring screening until patients are older, except for those at increased risk of coronary heart disease. This controversy was described in a 2008 Practice Alert.5
Screening for diabetes. The only asymptomatic patients the Task Force recommends screening for diabetes are those with a sustained blood pressure of more than 135/80 mm Hg, treated or untreated. The American Diabetes Association (ADA) would cast a wider net, recommending that you consider screening for prediabetes or diabetes in those ≥45 years of age, particularly in those with a body mass index of ≥25 kg/m2, and in overweight patients <45 years of age who have another risk factor for diabetes.6
Screening for colorectal cancer. The Task Force recommends screening adults starting at age 50 until age 75, using fecal occult blood testing, sigmoidoscopy, or colonoscopy. The ACS also recommends these screening modalities, but adds CT colonography and fecal DNA testing to the list of acceptable methods. The USPSTF found insufficient evidence to evaluate the benefits and harms of these newer tests and expressed concern over the high rate of incidental findings and the unknown long-term effects of radiation from CT colonography.
Screening adolescents. The Task Force is in favor of screening teenagers for major depressive disorder (MDD), as long as systems are in place to provide accurate diagnosis, therapy, and follow-up. High-intensity behavioral counseling for sexually active teens and adults at risk is also endorsed for the prevention of sexually transmitted infections. In both areas, however, the Task Force recognizes that adequately addressing these issues will require more than brief office- or clinic-based interventions.
Caring for pregnant women and newborns. According to the USPSTF, pregnant women should be screened for asymptomatic bacteriuria, advised to take a daily folic acid supplement, counseled about tobacco use, and encouraged to breastfeed. Newborns should be screened for congenital hypothyroidism, phenylketonuria, and hearing loss. These most recent A and B recommendations from the USPSTF are summarized in TABLE 3.
TABLE 3
The USPSTF recommends FOR
| CARDIOVASCULAR DISEASE PREVENTION |
|
| CANCER SCREENING |
|
| PREGNANCY |
|
| NEWBORNS |
|
| ADOLESCENTS |
|
Not proven
When evidence is not available, some organizations are willing to issue guidelines based on expert opinion or consensus. Not so the USPSTF. When the Task Force members find current evidence is not sufficient to make a judgment, they put the intervention into Category I, for Insufficient. The new I recommendations range from aspirin to prevent MI and stroke in those ≥80 years to screening children for MDD and performing whole body skin examinations to detect early manifestations of skin cancer. The new I recommendations are listed in TABLE 4.
TABLE 4
Evidence is INSUFFICIENT to recommend for or against
|
What’s the take-home message?
All of these recent Task Force decisions add substantially to the full set of Task Force recommendations, which can be found at www.ahrq.gov/CLINIC/uspstfix.htm. Given the large number of level A and B recommendations from the Task Force, clinicians are faced with the dilemma of limited time to accomplish all the recommendations. It is reasonable to concentrate on the positive recommendations and avoid performing the interventions recommended against. The interventions in the “I” category are not as clear-cut and clinicians will continue to struggle with them, particularly when other professional organizations recommend them.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; [email protected].
The past 12 months have been busy ones for the United States Preventive Services Task Force (USPSTF), which issued 34 new recommendations since our last Practice Alert on the group’s activity a year ago. Some recommendations address controversial topics, such as cholesterol screening, and several others—on topics such as prostate cancer screening and acceptable tests for detecting colorectal cancer—differ from those of such prominent groups as the American Cancer Society (ACS).
TABLE 1 provides a breakdown of the 5 categories of USPSTF recommendations (A, B, C, D, I). We’ll start with recent D recommendations (TABLE 2), services the Task Force recommends against, to emphasize that some preventive measures—even if they are widely touted—either provide no benefit or cause more harms than benefits.
TABLE 1
USPSTF recommendation categories
| A Recommendation: The Task Force recommends the service. There is high certainty that the net benefit is substantial. |
| B Recommendation: The Task Force recommends this service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
| C Recommendation: The Task Force recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small. |
| D Recommendation: The Task Force recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
| I Statement: The Task Force concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
TABLE 2
The USPSTF recommends AGAINST
|
What not to do
The most notable new D recommendations advise against screening men ≥75 years of age for prostate cancer and against screening for colorectal cancer after age 85. The Task Force also recommends against routine screening for colorectal cancer after age 75, although individual patient considerations may influence your decision about this screen for patients between ages 76 and 85. Bear in mind that the benefits of early detection of colon cancer decline after age 75 because of the time lag between early intervention and benefit and because of competing causes of morbidity and mortality.1
Cancer screening controversies. The recommendations for an age cutoff for prostate and colon cancer screening differ from those of the ACS, which lists no age cutoff for screening for either condition.2 In fact, the Task Force does not recommend screening for prostate cancer at all. Its rationale is that before age 75, the evidence is insufficient to evaluate benefits and harms, and after 75 there is good evidence that screening does more harm than good. The ACS no longer recommends routine prostate cancer screening, but does say that when a patient leaves the decision to the physician, screening should be performed.
Thumbs down on these, too. The Task Force now recommends against using spirometry to screen for chronic obstructive pulmonary disease and against using aspirin for preventing stroke in women <55 years and myocardial infarction (MI) in men <45 years. (See below for a fuller discussion of aspirin as a preventive measure.) The Task Force also recommends against screening for asymptomatic bacteriuria in men and nonpregnant women.
Recommended interventions
Now for the preventive interventions the USPSTF advises you to perform. They include:
Prescribing low-dose aspirin. The most complicated positive recommendations are those for low-dose aspirin to prevent MI in men and stroke in women. Aspirin is effective in preventing these conditions, but carries the risk of major gastrointestinal (GI) bleeding and cerebral hemorrhage. For younger patients, as we’ve seen in the previous section, the Task Force finds the risks of prophylactic low-dose aspirin therapy outweigh the benefits. But for older patients (men between the ages of 45 and 79 years and women ages 55-79), aspirin is recommended when the potential benefit of reducing the incidence of MI in men and stroke in women outweigh the harms. To assist clinicians in weighing the potential benefits and harms, the USPSTF provides a link to a coronary heart disease risk calculator, as well as several tables comparing numbers of prevented heart attacks for men and strokes for women by age and risk category, as well as risks of bleeding complications.3
Screening for hypercholesterolemia. The Task Force’s recommendations for dyslipidemia screening differ markedly from those of the American Heart Association and the Final Report of the National Cholesterol Education Program (NCEP) Expert Panel, which recommend routine screening for all adults starting at age 20 with no age cutoff.4 The USPSTF recommends deferring screening until patients are older, except for those at increased risk of coronary heart disease. This controversy was described in a 2008 Practice Alert.5
Screening for diabetes. The only asymptomatic patients the Task Force recommends screening for diabetes are those with a sustained blood pressure of more than 135/80 mm Hg, treated or untreated. The American Diabetes Association (ADA) would cast a wider net, recommending that you consider screening for prediabetes or diabetes in those ≥45 years of age, particularly in those with a body mass index of ≥25 kg/m2, and in overweight patients <45 years of age who have another risk factor for diabetes.6
Screening for colorectal cancer. The Task Force recommends screening adults starting at age 50 until age 75, using fecal occult blood testing, sigmoidoscopy, or colonoscopy. The ACS also recommends these screening modalities, but adds CT colonography and fecal DNA testing to the list of acceptable methods. The USPSTF found insufficient evidence to evaluate the benefits and harms of these newer tests and expressed concern over the high rate of incidental findings and the unknown long-term effects of radiation from CT colonography.
Screening adolescents. The Task Force is in favor of screening teenagers for major depressive disorder (MDD), as long as systems are in place to provide accurate diagnosis, therapy, and follow-up. High-intensity behavioral counseling for sexually active teens and adults at risk is also endorsed for the prevention of sexually transmitted infections. In both areas, however, the Task Force recognizes that adequately addressing these issues will require more than brief office- or clinic-based interventions.
Caring for pregnant women and newborns. According to the USPSTF, pregnant women should be screened for asymptomatic bacteriuria, advised to take a daily folic acid supplement, counseled about tobacco use, and encouraged to breastfeed. Newborns should be screened for congenital hypothyroidism, phenylketonuria, and hearing loss. These most recent A and B recommendations from the USPSTF are summarized in TABLE 3.
TABLE 3
The USPSTF recommends FOR
| CARDIOVASCULAR DISEASE PREVENTION |
|
| CANCER SCREENING |
|
| PREGNANCY |
|
| NEWBORNS |
|
| ADOLESCENTS |
|
Not proven
When evidence is not available, some organizations are willing to issue guidelines based on expert opinion or consensus. Not so the USPSTF. When the Task Force members find current evidence is not sufficient to make a judgment, they put the intervention into Category I, for Insufficient. The new I recommendations range from aspirin to prevent MI and stroke in those ≥80 years to screening children for MDD and performing whole body skin examinations to detect early manifestations of skin cancer. The new I recommendations are listed in TABLE 4.
TABLE 4
Evidence is INSUFFICIENT to recommend for or against
|
What’s the take-home message?
All of these recent Task Force decisions add substantially to the full set of Task Force recommendations, which can be found at www.ahrq.gov/CLINIC/uspstfix.htm. Given the large number of level A and B recommendations from the Task Force, clinicians are faced with the dilemma of limited time to accomplish all the recommendations. It is reasonable to concentrate on the positive recommendations and avoid performing the interventions recommended against. The interventions in the “I” category are not as clear-cut and clinicians will continue to struggle with them, particularly when other professional organizations recommend them.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; [email protected].
1. US Preventive Services Task Force. Screening for colorectal cancer. October 2008. Available at: www.ahrq.gov/clinic/uspstf/uspscolo.htm. Accessed June 3, 2009.
2. American Cancer Society guidelines for early detection of cancer. Last revised May 21, 2009. Available at: http://www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines_36.asp?sitearea=PED. Accessed June 3, 2009.
3. US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: recommendation statement. March 2009. Available at: http://www.ahrq.gov/clinic/uspstf09/aspirincvd/aspcvdrs.htm. Accessed June 3, 2009.
4. National Cholesterol Education Program. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143-3421.Available at: http://circ.ahajournals.org/cgi/content/full/106/25/3143. Accessed June 3, 2009.
5. Campos-Outcalt D. USPSTF scales back approach to lipid screening for women. J Fam Pract. 2008;57:740-742.
6. American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(suppl 1):S12-S54.
1. US Preventive Services Task Force. Screening for colorectal cancer. October 2008. Available at: www.ahrq.gov/clinic/uspstf/uspscolo.htm. Accessed June 3, 2009.
2. American Cancer Society guidelines for early detection of cancer. Last revised May 21, 2009. Available at: http://www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines_36.asp?sitearea=PED. Accessed June 3, 2009.
3. US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: recommendation statement. March 2009. Available at: http://www.ahrq.gov/clinic/uspstf09/aspirincvd/aspcvdrs.htm. Accessed June 3, 2009.
4. National Cholesterol Education Program. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143-3421.Available at: http://circ.ahajournals.org/cgi/content/full/106/25/3143. Accessed June 3, 2009.
5. Campos-Outcalt D. USPSTF scales back approach to lipid screening for women. J Fam Pract. 2008;57:740-742.
6. American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(suppl 1):S12-S54.
ACOG guidelines for HIV screening don’t always acknowledge coding reality
Routine screening for the human immunodeficiency virus (HIV) is recommended for all women 19 to 64 years old, according to guidelines issued in August 2008 by the American College of Obstetricians and Gynecologists (ACOG). In addition, ACOG recommends that women outside that age range who have a risk factor for HIV infection undergo targeted screening.
To accomplish these goals, ACOG suggests “opt-out” HIV screening, in which the patient is notified that HIV testing will be performed as a routine part of gynecologic and obstetric care unless she declines it.
Opt-out testing may not always be feasible, however, because many payers still require that you counsel the patient about the HIV test before it is performed, as well as have her sign a consent form.
Information about individual states’ requirements for testing, counseling, and informed consent can be found at the Compendium of State HIV Testing Laws, Quick Reference Guide for Clinicians (March 17, 2009), prepared by the National HIV/AIDS Clinicians’ Consultation Center at www.nccc.ucsf.edu/StateLaws/About%20Compendium/Quick%20Reference%20Guide.pdf.
The patient may be offered the test during any of the following:
- her preventive health checkup
- an office visit for a presenting problem
- a scheduled obstetric visit.
When you provide counseling, bill for it!
Counseling for HIV in the absence of the condition is considered a preventive service, which is reported using 99401–99404 (Preventive medicine counseling and/or risk factor reduction intervention(s) provided to an individual), based on total counseling time between 15 and 60 minutes (reported in 15-minute increments). Such preventive counseling can be reported in addition to a problem E/M service by adding the modifier -25 (Significant, separately identifiable evaluation and management service by the same physician on the same day of the procedure or other service) to the problem E/M code. It can also be reported separately at the time of an obstetric visit. However, such counseling is not covered when it is conducted during a preventive exam.
Include the proper diagnostic code
Diagnostic coding, following these ICD-9 rules, lets the payer know why the service is being rendered:
- Report V73.89 (Screening for other specified viral disease) if the patient is being seen to determine her HIV status.
- Report V69.8 (Other problems related to lifestyle) as a secondary diagnosis if the patient is in a group known to be at high risk of HIV infection.
- Report V65.44 (HIV counseling) for counseling provided during the encounter for the test, or use this code to report the visit at which the patient returns to discuss her result.
Just what constitutes “routine” testing?
The ACOG guidelines are unclear as to what, exactly, “routine” testing means. Is an ObGyn expected to test a patient once in her lifetime, annually, or any time her life partner changes?
These specifics are not addressed in the ACOG recommendations. Based on similar recommendations from the Centers for Disease Control and Prevention (CDC) and the US Preventive Services Task Force (USPSTF), however, you might surmise the following:
- Test all patients 19 to 64 years old for HIV at least once.
- Test all patients at high risk of contracting HIV annually. (High-risk groups include women who receive a blood transfusion, practice unsafe sex, or have a new sexual partner who has not been tested.)
- Test all women each time they become pregnant.
- HIV screening be carried out after the patient is notified that testing will be performed, unless she declines (opt-out screening)
- any person who is at high risk of contracting HIV be screened at least annually
- separate written consent for HIV testing not be required (general consent for medical care should be sufficient to encompass consent for HIV testing)
- prevention counseling not be required with HIV diagnostic testing or as part of HIV screening programs in health-care settings
- among pregnant women, HIV screening be included in the routine panel of prenatal screening tests
- every pregnant woman be screened for HIV after she is notified that testing will be performed, unless she declines (opt-out screening)
- separate written consent for HIV testing not be required for pregnant women (general consent for medical care should be sufficient to encompass consent for HIV testing)
- repeat screening in the third trimester be carried out in certain jurisdictions that have an elevated rate of HIV infection among pregnant women.
Obviously, the CDC’s call for opt-out screening and its recommendation against informed consent for HIV testing contradict the requirements of some states, so it is important to know the regulations where you practice.
Routine screening for the human immunodeficiency virus (HIV) is recommended for all women 19 to 64 years old, according to guidelines issued in August 2008 by the American College of Obstetricians and Gynecologists (ACOG). In addition, ACOG recommends that women outside that age range who have a risk factor for HIV infection undergo targeted screening.
To accomplish these goals, ACOG suggests “opt-out” HIV screening, in which the patient is notified that HIV testing will be performed as a routine part of gynecologic and obstetric care unless she declines it.
Opt-out testing may not always be feasible, however, because many payers still require that you counsel the patient about the HIV test before it is performed, as well as have her sign a consent form.
Information about individual states’ requirements for testing, counseling, and informed consent can be found at the Compendium of State HIV Testing Laws, Quick Reference Guide for Clinicians (March 17, 2009), prepared by the National HIV/AIDS Clinicians’ Consultation Center at www.nccc.ucsf.edu/StateLaws/About%20Compendium/Quick%20Reference%20Guide.pdf.
The patient may be offered the test during any of the following:
- her preventive health checkup
- an office visit for a presenting problem
- a scheduled obstetric visit.
When you provide counseling, bill for it!
Counseling for HIV in the absence of the condition is considered a preventive service, which is reported using 99401–99404 (Preventive medicine counseling and/or risk factor reduction intervention(s) provided to an individual), based on total counseling time between 15 and 60 minutes (reported in 15-minute increments). Such preventive counseling can be reported in addition to a problem E/M service by adding the modifier -25 (Significant, separately identifiable evaluation and management service by the same physician on the same day of the procedure or other service) to the problem E/M code. It can also be reported separately at the time of an obstetric visit. However, such counseling is not covered when it is conducted during a preventive exam.
Include the proper diagnostic code
Diagnostic coding, following these ICD-9 rules, lets the payer know why the service is being rendered:
- Report V73.89 (Screening for other specified viral disease) if the patient is being seen to determine her HIV status.
- Report V69.8 (Other problems related to lifestyle) as a secondary diagnosis if the patient is in a group known to be at high risk of HIV infection.
- Report V65.44 (HIV counseling) for counseling provided during the encounter for the test, or use this code to report the visit at which the patient returns to discuss her result.
Just what constitutes “routine” testing?
The ACOG guidelines are unclear as to what, exactly, “routine” testing means. Is an ObGyn expected to test a patient once in her lifetime, annually, or any time her life partner changes?
These specifics are not addressed in the ACOG recommendations. Based on similar recommendations from the Centers for Disease Control and Prevention (CDC) and the US Preventive Services Task Force (USPSTF), however, you might surmise the following:
- Test all patients 19 to 64 years old for HIV at least once.
- Test all patients at high risk of contracting HIV annually. (High-risk groups include women who receive a blood transfusion, practice unsafe sex, or have a new sexual partner who has not been tested.)
- Test all women each time they become pregnant.
- HIV screening be carried out after the patient is notified that testing will be performed, unless she declines (opt-out screening)
- any person who is at high risk of contracting HIV be screened at least annually
- separate written consent for HIV testing not be required (general consent for medical care should be sufficient to encompass consent for HIV testing)
- prevention counseling not be required with HIV diagnostic testing or as part of HIV screening programs in health-care settings
- among pregnant women, HIV screening be included in the routine panel of prenatal screening tests
- every pregnant woman be screened for HIV after she is notified that testing will be performed, unless she declines (opt-out screening)
- separate written consent for HIV testing not be required for pregnant women (general consent for medical care should be sufficient to encompass consent for HIV testing)
- repeat screening in the third trimester be carried out in certain jurisdictions that have an elevated rate of HIV infection among pregnant women.
Obviously, the CDC’s call for opt-out screening and its recommendation against informed consent for HIV testing contradict the requirements of some states, so it is important to know the regulations where you practice.
Routine screening for the human immunodeficiency virus (HIV) is recommended for all women 19 to 64 years old, according to guidelines issued in August 2008 by the American College of Obstetricians and Gynecologists (ACOG). In addition, ACOG recommends that women outside that age range who have a risk factor for HIV infection undergo targeted screening.
To accomplish these goals, ACOG suggests “opt-out” HIV screening, in which the patient is notified that HIV testing will be performed as a routine part of gynecologic and obstetric care unless she declines it.
Opt-out testing may not always be feasible, however, because many payers still require that you counsel the patient about the HIV test before it is performed, as well as have her sign a consent form.
Information about individual states’ requirements for testing, counseling, and informed consent can be found at the Compendium of State HIV Testing Laws, Quick Reference Guide for Clinicians (March 17, 2009), prepared by the National HIV/AIDS Clinicians’ Consultation Center at www.nccc.ucsf.edu/StateLaws/About%20Compendium/Quick%20Reference%20Guide.pdf.
The patient may be offered the test during any of the following:
- her preventive health checkup
- an office visit for a presenting problem
- a scheduled obstetric visit.
When you provide counseling, bill for it!
Counseling for HIV in the absence of the condition is considered a preventive service, which is reported using 99401–99404 (Preventive medicine counseling and/or risk factor reduction intervention(s) provided to an individual), based on total counseling time between 15 and 60 minutes (reported in 15-minute increments). Such preventive counseling can be reported in addition to a problem E/M service by adding the modifier -25 (Significant, separately identifiable evaluation and management service by the same physician on the same day of the procedure or other service) to the problem E/M code. It can also be reported separately at the time of an obstetric visit. However, such counseling is not covered when it is conducted during a preventive exam.
Include the proper diagnostic code
Diagnostic coding, following these ICD-9 rules, lets the payer know why the service is being rendered:
- Report V73.89 (Screening for other specified viral disease) if the patient is being seen to determine her HIV status.
- Report V69.8 (Other problems related to lifestyle) as a secondary diagnosis if the patient is in a group known to be at high risk of HIV infection.
- Report V65.44 (HIV counseling) for counseling provided during the encounter for the test, or use this code to report the visit at which the patient returns to discuss her result.
Just what constitutes “routine” testing?
The ACOG guidelines are unclear as to what, exactly, “routine” testing means. Is an ObGyn expected to test a patient once in her lifetime, annually, or any time her life partner changes?
These specifics are not addressed in the ACOG recommendations. Based on similar recommendations from the Centers for Disease Control and Prevention (CDC) and the US Preventive Services Task Force (USPSTF), however, you might surmise the following:
- Test all patients 19 to 64 years old for HIV at least once.
- Test all patients at high risk of contracting HIV annually. (High-risk groups include women who receive a blood transfusion, practice unsafe sex, or have a new sexual partner who has not been tested.)
- Test all women each time they become pregnant.
- HIV screening be carried out after the patient is notified that testing will be performed, unless she declines (opt-out screening)
- any person who is at high risk of contracting HIV be screened at least annually
- separate written consent for HIV testing not be required (general consent for medical care should be sufficient to encompass consent for HIV testing)
- prevention counseling not be required with HIV diagnostic testing or as part of HIV screening programs in health-care settings
- among pregnant women, HIV screening be included in the routine panel of prenatal screening tests
- every pregnant woman be screened for HIV after she is notified that testing will be performed, unless she declines (opt-out screening)
- separate written consent for HIV testing not be required for pregnant women (general consent for medical care should be sufficient to encompass consent for HIV testing)
- repeat screening in the third trimester be carried out in certain jurisdictions that have an elevated rate of HIV infection among pregnant women.
Obviously, the CDC’s call for opt-out screening and its recommendation against informed consent for HIV testing contradict the requirements of some states, so it is important to know the regulations where you practice.
The Child With Migraine
More often than not the child who presents with recurrent or chronic headaches will be experiencing migraines. Pediatric migraine is very common; in fact, it is one of the top five health problems for children. General pediatricians treat the majority of these children, up to 90%. There are not enough pediatric neurologists in the United States to take care of all of these children. For example, given the prevalence rate of 10%, an estimated 60,000 children and adolescents experience migraine out of the 2.4 million people in the Cincinnati draw area for my institution. Obviously, they cannot all be referred to a specialist.
The most important thing for you to do is to rule out secondary headaches. Differentiate primary headaches (such as migraine and tension headaches) from secondary ones, being skeptical of the secondary presentations. If a secondary etiology is suspected and the headaches do not resolve, then reassess, but the headaches may be primary ones.
Ask patients and parents about headache symptoms because often the symptoms are initially missed. Standardized criteria such as the American Academy of Neurology practice parameters on the treatment of migraine headache in children and adolescents can guide your diagnosis and management (Neurology 2004;63:2215–24). If the clinical picture does not fit these criteria, consider further evaluation of the child.
After your differential diagnosis, perform a complete neurologic examination. If findings are abnormal on the neurologic exam, consider an MRI. Such imaging also may be warranted for children with exclusively occipital headaches, if they experience a crescendo or abrupt change in headaches, or if they lack a relevant family history.
Almost all other tests are less useful and do not help prior to referral to a pediatric neurologist. We do not need EEGs, sinus x-rays, or CT exams, which have lots of unnecessary radiation. We routinely check riboflavin and coenzyme Q10 levels, but this may be beyond the level of general pediatricians.
Referral to a specialist is appropriate when the above strategies are not working and if the headaches do not improve. Also refer if a child has chronic daily headaches (defined as 15 or more days per month), if a child is missing a lot of school or other activities of daily living, and/or if the history and diagnosis do not seem to fit the presentation.
Optimal treatment is a standardized strategy that incorporates acute and preventive pharmacologic strategies, as well as biobehavioral treatments.
Appropriate NSAID use, for example, can aid a child with an acute presentation. For example, prescribe 10 mg/kg of ibuprofen at onset, and do not exceed three dosages per week. In addition, be familiar with at least one triptan at appropriate dosing (an adult dose for teenagers).
Also be comfortable with at least one age-appropriate, preventive agent. Amitriptyline is the easiest to use. The recommended dosage regimen is 1 mg/kg titrated up slowly (over a period of 8–10 weeks) for about 2–3 months at full dose to allow sufficient time for clinical effect. I also advise against the use of cyproheptadine for teenagers with migraines because of the appetite effect.
Adequate fluid intake without caffeine, exercise at least three to four times per week, and sufficient regular sleep (8–9 hours, for example) are important components of biobehavioral treatment. In addition, educate patients and parents about the importance of a healthy, balanced diet. Instruct patients not to skip meals. We do not recommend avoidance of particular foods, and the evidence supports this stance. It is more important to make healthy food choices.
More often than not the child who presents with recurrent or chronic headaches will be experiencing migraines. Pediatric migraine is very common; in fact, it is one of the top five health problems for children. General pediatricians treat the majority of these children, up to 90%. There are not enough pediatric neurologists in the United States to take care of all of these children. For example, given the prevalence rate of 10%, an estimated 60,000 children and adolescents experience migraine out of the 2.4 million people in the Cincinnati draw area for my institution. Obviously, they cannot all be referred to a specialist.
The most important thing for you to do is to rule out secondary headaches. Differentiate primary headaches (such as migraine and tension headaches) from secondary ones, being skeptical of the secondary presentations. If a secondary etiology is suspected and the headaches do not resolve, then reassess, but the headaches may be primary ones.
Ask patients and parents about headache symptoms because often the symptoms are initially missed. Standardized criteria such as the American Academy of Neurology practice parameters on the treatment of migraine headache in children and adolescents can guide your diagnosis and management (Neurology 2004;63:2215–24). If the clinical picture does not fit these criteria, consider further evaluation of the child.
After your differential diagnosis, perform a complete neurologic examination. If findings are abnormal on the neurologic exam, consider an MRI. Such imaging also may be warranted for children with exclusively occipital headaches, if they experience a crescendo or abrupt change in headaches, or if they lack a relevant family history.
Almost all other tests are less useful and do not help prior to referral to a pediatric neurologist. We do not need EEGs, sinus x-rays, or CT exams, which have lots of unnecessary radiation. We routinely check riboflavin and coenzyme Q10 levels, but this may be beyond the level of general pediatricians.
Referral to a specialist is appropriate when the above strategies are not working and if the headaches do not improve. Also refer if a child has chronic daily headaches (defined as 15 or more days per month), if a child is missing a lot of school or other activities of daily living, and/or if the history and diagnosis do not seem to fit the presentation.
Optimal treatment is a standardized strategy that incorporates acute and preventive pharmacologic strategies, as well as biobehavioral treatments.
Appropriate NSAID use, for example, can aid a child with an acute presentation. For example, prescribe 10 mg/kg of ibuprofen at onset, and do not exceed three dosages per week. In addition, be familiar with at least one triptan at appropriate dosing (an adult dose for teenagers).
Also be comfortable with at least one age-appropriate, preventive agent. Amitriptyline is the easiest to use. The recommended dosage regimen is 1 mg/kg titrated up slowly (over a period of 8–10 weeks) for about 2–3 months at full dose to allow sufficient time for clinical effect. I also advise against the use of cyproheptadine for teenagers with migraines because of the appetite effect.
Adequate fluid intake without caffeine, exercise at least three to four times per week, and sufficient regular sleep (8–9 hours, for example) are important components of biobehavioral treatment. In addition, educate patients and parents about the importance of a healthy, balanced diet. Instruct patients not to skip meals. We do not recommend avoidance of particular foods, and the evidence supports this stance. It is more important to make healthy food choices.
More often than not the child who presents with recurrent or chronic headaches will be experiencing migraines. Pediatric migraine is very common; in fact, it is one of the top five health problems for children. General pediatricians treat the majority of these children, up to 90%. There are not enough pediatric neurologists in the United States to take care of all of these children. For example, given the prevalence rate of 10%, an estimated 60,000 children and adolescents experience migraine out of the 2.4 million people in the Cincinnati draw area for my institution. Obviously, they cannot all be referred to a specialist.
The most important thing for you to do is to rule out secondary headaches. Differentiate primary headaches (such as migraine and tension headaches) from secondary ones, being skeptical of the secondary presentations. If a secondary etiology is suspected and the headaches do not resolve, then reassess, but the headaches may be primary ones.
Ask patients and parents about headache symptoms because often the symptoms are initially missed. Standardized criteria such as the American Academy of Neurology practice parameters on the treatment of migraine headache in children and adolescents can guide your diagnosis and management (Neurology 2004;63:2215–24). If the clinical picture does not fit these criteria, consider further evaluation of the child.
After your differential diagnosis, perform a complete neurologic examination. If findings are abnormal on the neurologic exam, consider an MRI. Such imaging also may be warranted for children with exclusively occipital headaches, if they experience a crescendo or abrupt change in headaches, or if they lack a relevant family history.
Almost all other tests are less useful and do not help prior to referral to a pediatric neurologist. We do not need EEGs, sinus x-rays, or CT exams, which have lots of unnecessary radiation. We routinely check riboflavin and coenzyme Q10 levels, but this may be beyond the level of general pediatricians.
Referral to a specialist is appropriate when the above strategies are not working and if the headaches do not improve. Also refer if a child has chronic daily headaches (defined as 15 or more days per month), if a child is missing a lot of school or other activities of daily living, and/or if the history and diagnosis do not seem to fit the presentation.
Optimal treatment is a standardized strategy that incorporates acute and preventive pharmacologic strategies, as well as biobehavioral treatments.
Appropriate NSAID use, for example, can aid a child with an acute presentation. For example, prescribe 10 mg/kg of ibuprofen at onset, and do not exceed three dosages per week. In addition, be familiar with at least one triptan at appropriate dosing (an adult dose for teenagers).
Also be comfortable with at least one age-appropriate, preventive agent. Amitriptyline is the easiest to use. The recommended dosage regimen is 1 mg/kg titrated up slowly (over a period of 8–10 weeks) for about 2–3 months at full dose to allow sufficient time for clinical effect. I also advise against the use of cyproheptadine for teenagers with migraines because of the appetite effect.
Adequate fluid intake without caffeine, exercise at least three to four times per week, and sufficient regular sleep (8–9 hours, for example) are important components of biobehavioral treatment. In addition, educate patients and parents about the importance of a healthy, balanced diet. Instruct patients not to skip meals. We do not recommend avoidance of particular foods, and the evidence supports this stance. It is more important to make healthy food choices.