User login
Caution Urged in Interpreting Glargine Cancer Risk
A series of studies reported last month by a European health organization has questioned whether the use of insulin glargine, known commercially as Lantus, inflates a patient’s risk of cancer. But according to one source, hospitalists with a large census of diabetic or hypoglycemic patients shouldn’t pull their patients off the treatment just yet.
Four different population-based studies were reported on the Web site for Diabetologia, the journal of the European Association of the Study of Diabetes. A German study of 127,000 patients in an insurance database found that for every 100 patients taking Lantus, there was one more person diagnosed with cancer when compared with 100 patients taking similar doses of human insulin. The risk increased with the dosage, the study reported.
But the American Diabetes Association quickly released a statement calling the studies “conflicting and confusing.”
Hospitalist Jeffrey Schnipper, MD, MPH, FHM, who estimates one-third of his patients are either diabetic or hypoglycemic, agrees, saying that observational studies make it hard to draw any conclusions.
“I tell patients we don’t know enough,” says Dr. Schnipper, director of clinical research and associate physician in the Division of General Medicine at Brigham and Women’s Hospital Hospitalist Service in Boston. “Right now, there’s no good strong evidence that Lantus is worse than any other alternative.”
Dr. Schnipper cautioned fellow hospitalists to not overreact to the reports, noting that without a randomized trial to follow through on the hypotheses raised, there is no resolution to the confounding-by-indication bias that can plague observational studies.
“There’s an adage in medicine that you never want to be the first person or the last person to use a drug,” Dr. Schnipper says. “I would say you should never be the first person or the last person to stop using a drug.”
A series of studies reported last month by a European health organization has questioned whether the use of insulin glargine, known commercially as Lantus, inflates a patient’s risk of cancer. But according to one source, hospitalists with a large census of diabetic or hypoglycemic patients shouldn’t pull their patients off the treatment just yet.
Four different population-based studies were reported on the Web site for Diabetologia, the journal of the European Association of the Study of Diabetes. A German study of 127,000 patients in an insurance database found that for every 100 patients taking Lantus, there was one more person diagnosed with cancer when compared with 100 patients taking similar doses of human insulin. The risk increased with the dosage, the study reported.
But the American Diabetes Association quickly released a statement calling the studies “conflicting and confusing.”
Hospitalist Jeffrey Schnipper, MD, MPH, FHM, who estimates one-third of his patients are either diabetic or hypoglycemic, agrees, saying that observational studies make it hard to draw any conclusions.
“I tell patients we don’t know enough,” says Dr. Schnipper, director of clinical research and associate physician in the Division of General Medicine at Brigham and Women’s Hospital Hospitalist Service in Boston. “Right now, there’s no good strong evidence that Lantus is worse than any other alternative.”
Dr. Schnipper cautioned fellow hospitalists to not overreact to the reports, noting that without a randomized trial to follow through on the hypotheses raised, there is no resolution to the confounding-by-indication bias that can plague observational studies.
“There’s an adage in medicine that you never want to be the first person or the last person to use a drug,” Dr. Schnipper says. “I would say you should never be the first person or the last person to stop using a drug.”
A series of studies reported last month by a European health organization has questioned whether the use of insulin glargine, known commercially as Lantus, inflates a patient’s risk of cancer. But according to one source, hospitalists with a large census of diabetic or hypoglycemic patients shouldn’t pull their patients off the treatment just yet.
Four different population-based studies were reported on the Web site for Diabetologia, the journal of the European Association of the Study of Diabetes. A German study of 127,000 patients in an insurance database found that for every 100 patients taking Lantus, there was one more person diagnosed with cancer when compared with 100 patients taking similar doses of human insulin. The risk increased with the dosage, the study reported.
But the American Diabetes Association quickly released a statement calling the studies “conflicting and confusing.”
Hospitalist Jeffrey Schnipper, MD, MPH, FHM, who estimates one-third of his patients are either diabetic or hypoglycemic, agrees, saying that observational studies make it hard to draw any conclusions.
“I tell patients we don’t know enough,” says Dr. Schnipper, director of clinical research and associate physician in the Division of General Medicine at Brigham and Women’s Hospital Hospitalist Service in Boston. “Right now, there’s no good strong evidence that Lantus is worse than any other alternative.”
Dr. Schnipper cautioned fellow hospitalists to not overreact to the reports, noting that without a randomized trial to follow through on the hypotheses raised, there is no resolution to the confounding-by-indication bias that can plague observational studies.
“There’s an adage in medicine that you never want to be the first person or the last person to use a drug,” Dr. Schnipper says. “I would say you should never be the first person or the last person to stop using a drug.”
JHM Names Thomas Baudendistel, MD, New CME Editor
Some physicians have an interest in teaching; some are really good at it, and some make a career out of it. For Thomas Baudendistel, MD, teaching comes second nature and, as one of his former colleagues says, is a definition of who he is.
It’s those qualities, his experience in both academic and community hospital settings, and a passion for innovation that Dr. Baudendistel, the internal-medicine residency program director for Kaiser Permanente in Oakland, Calif., hopes to infuse as CME editor of the Journal of Hospital Medicine (JHM). He was appointed to the new position in June; the first issue containing article-level CME, the answers to which will be submitted online, is scheduled to appear in October.
“Tom is a superlative educator. He has defined himself that way. He has a passion for it and a talent for it,” says Brian J. Harte, MD, FHM, chair of the department of hospital medicine at The Cleveland Clinic and a deputy editor of JHM.
Dr. Harte, who first met Dr. Baudendistel in 1996 during his residency at the University of California at San Francisco, says his former mentor “can take a submission, drill down to the most important teaching point, and challenge the readership.”
Dr. Baudendistel, who admits teaching is why he “gets out of bed in the morning,” says he wants to take advantage of the young, tech-savvy nature of most HM physicians. “JHM has been an innovative journal. I see the CME piece as being equally innovative,” he says. “I’d like to move [CME] past the pencil-and-paper phase.”
Some physicians have an interest in teaching; some are really good at it, and some make a career out of it. For Thomas Baudendistel, MD, teaching comes second nature and, as one of his former colleagues says, is a definition of who he is.
It’s those qualities, his experience in both academic and community hospital settings, and a passion for innovation that Dr. Baudendistel, the internal-medicine residency program director for Kaiser Permanente in Oakland, Calif., hopes to infuse as CME editor of the Journal of Hospital Medicine (JHM). He was appointed to the new position in June; the first issue containing article-level CME, the answers to which will be submitted online, is scheduled to appear in October.
“Tom is a superlative educator. He has defined himself that way. He has a passion for it and a talent for it,” says Brian J. Harte, MD, FHM, chair of the department of hospital medicine at The Cleveland Clinic and a deputy editor of JHM.
Dr. Harte, who first met Dr. Baudendistel in 1996 during his residency at the University of California at San Francisco, says his former mentor “can take a submission, drill down to the most important teaching point, and challenge the readership.”
Dr. Baudendistel, who admits teaching is why he “gets out of bed in the morning,” says he wants to take advantage of the young, tech-savvy nature of most HM physicians. “JHM has been an innovative journal. I see the CME piece as being equally innovative,” he says. “I’d like to move [CME] past the pencil-and-paper phase.”
Some physicians have an interest in teaching; some are really good at it, and some make a career out of it. For Thomas Baudendistel, MD, teaching comes second nature and, as one of his former colleagues says, is a definition of who he is.
It’s those qualities, his experience in both academic and community hospital settings, and a passion for innovation that Dr. Baudendistel, the internal-medicine residency program director for Kaiser Permanente in Oakland, Calif., hopes to infuse as CME editor of the Journal of Hospital Medicine (JHM). He was appointed to the new position in June; the first issue containing article-level CME, the answers to which will be submitted online, is scheduled to appear in October.
“Tom is a superlative educator. He has defined himself that way. He has a passion for it and a talent for it,” says Brian J. Harte, MD, FHM, chair of the department of hospital medicine at The Cleveland Clinic and a deputy editor of JHM.
Dr. Harte, who first met Dr. Baudendistel in 1996 during his residency at the University of California at San Francisco, says his former mentor “can take a submission, drill down to the most important teaching point, and challenge the readership.”
Dr. Baudendistel, who admits teaching is why he “gets out of bed in the morning,” says he wants to take advantage of the young, tech-savvy nature of most HM physicians. “JHM has been an innovative journal. I see the CME piece as being equally innovative,” he says. “I’d like to move [CME] past the pencil-and-paper phase.”
Perceptions of Hospital Discharge Software
During the transition from inpatient to outpatient care, patients are vulnerable to adverse events.1 Poor communication between hospital personnel and either the patient or the outpatient primary care physician has been associated with preventable or ameliorable adverse events after discharge.1 Systematic reviews confirm that discharge communication is often delayed, inaccurate, or ineffective.2, 3
Discharge communication failures may occur if hospital processes rely on dictated discharge summaries.2 For several reasons, discharge summaries are inadequate for communication. Most patients complete their initial posthospital clinic visit before their primary care physician receives the discharge summary.4 For many patients, the discharge summary is unavailable for all posthospital visits.4 Discharge summaries often fail as communication because they are not generated or transmitted.4
Recommendations to improve discharge communication include the use of health information technology.2, 5 The benefits of computer‐generated discharge summaries include decreases in delivery time for discharge communications.2 The benefits of computerized physician order entry (CPOE) include reduction of medical errors.6 These theoretical benefits create a rationale for clinical trials to measure improvements after discharge software applications with CPOE.5
In an effort to improve discharge communication and clinically relevant outcomes, we performed a cluster‐randomized trial to assess the value of a discharge software application of CPOE. The clustered design followed recommendations from a systematic review of discharge interventions.3 We applied our research intervention at the physician level and measured outcomes at the patient level. Our objective was to assess the benefit of discharge software with CPOE vs. usual care when used to discharge patients at high risk for repeat admission. In a previous work, we reported that discharge software did not reduce rates of hospital readmission, emergency department visits, or postdischarge adverse events due to medical management.7 In the present article, we compare secondary outcomes after the research intervention: perceptions of the discharge from the perspectives of patients, primary care physicians, and hospital physicians.
Methods
The trial design was a cluster randomized, controlled trial. The setting was the postdischarge environment following index hospitalization at a 730‐bed, tertiary care, teaching hospital in central Illinois. The Peoria Institutional Review Board approved the protocol for human research.
Participants
We enrolled consenting hospital physicians and their patients between November 2004 and January 2007. The hospital physician defined the cluster. Patients discharged by the physician comprised the cluster. The eligibility criteria for hospital physicians required internal medicine resident or attending physicians with assignments to inpatient duties for at least 2 months during the 27‐month enrollment period. After achieving informed consent from physicians, research personnel screened all consecutive, adult inpatients who were discharged to home. Patient inclusion required a probability of repeat admission (Pra) equal to or greater than 0.40.8, 9 The purpose of the inclusion criterion was to enrich the sample with patients likely to benefit from interventions to improve discharge processes. Furthermore, hospital readmission was the primary endpoint of the study, as reported separately.7 The Pra came from a predictive model with scores for age, gender, prior hospitalizations, prior doctor visits, self‐rated health status, presence of informal caregiver in the home, and comorbid coronary heart disease and diabetes mellitus. Research coordinators calculated the Pra within 2 days before discharge from the index hospitalization.
Exclusion Criteria
We excluded patients previously enrolled in the study, candidates for hospice, and patients unable to participate in outcome ascertainment. Cognitive impairment was a conditional exclusion criterion for patients. We defined cognitive impairment as a score less than 9 on the 10‐point clock test.10 Patients with cognitive impairment participated only with consent from their legally authorized representative. We enrolled patients with cognitive impairment only if a proxy spent at least 3 hours daily with the patient and the proxy agreed to answer postdischarge interviews. If a patient's outpatient primary care physician treated the patient during the index hospitalization, then there was no perceived barrier in physician‐to‐physician communication and we excluded the patient.
Intervention
The research intervention was discharge software with CPOE. Detailed description of the software appeared previously.5 In summary, the CPOE software application facilitated communication at the time of hospital discharge to patients, retail pharmacists, and community physicians. The application had basic levels of clinical decision support, required fields, pick lists, standard drug doses, alerts, reminders, and online reference information. The software addressed deficiencies in the usual care discharge process reported globally and reviewed previously.5 For example, 1 deficiency occurred when inpatient physicians failed to warn outpatient physicians about diagnostic tests with results pending at discharge.11 Another deficiency was discharge medication error.12 The software prompted the discharging physician to enter pending tests, order tests after discharge, and perform medication reconciliation. On the day of discharge, hospital physicians used the software to automatically generate discharge documents and reconcile prescriptions for the patient, primary care physician, retail pharmacist, and the ward nurse. The discharge letter went to the outpatient practitioner via facsimile transmission plus a duplicate via U.S. mail.
The control intervention was the usual care, handwritten discharge process commonly used by hospitalists.2 Hospital physicians and ward nurses completed handwritten discharge forms on the day of discharge. The forms contained blanks for discharge diagnoses, discharge medications, medication instructions, postdischarge activities and restrictions, postdischarge diet, postdischarge diagnostic and therapeutic interventions, and appointments. Patients received handwritten copies of the forms, 1 page of which also included medication instructions and prescriptions. In a previous publication, we provided details about the usual care discharge process as well as the standard care available to all study patients regardless of intervention.5
Randomization
The hospital physician who completed the discharge process was the unit of randomization. Random allocation was to discharge software or usual care discharge process, with a randomization ratio of 1:1 and block size of 2. We concealed allocation with the following process. An investigator who was not involved with hospital physician recruitment generated the randomization sequence with a computerized random number generator. The randomization list was maintained in a secure location. Another investigator who was unaware of the next random assignment performed the hospital physician recruitment and informed consent. After confirming eligibility and obtaining informed consent from physicians, the blinded investigator requested the next random assignment from the custodian of the randomization list. Hospital physicians subsequently used their randomly assigned process when discharging their patients who enrolled in the study. After random allocation, it was not possible to conceal the test or control intervention from physicians or their patients.
Hospital physicians underwent training on the software or usual care discharge process; the details appeared previously.7 Physicians assigned to usual care did not receive training on the discharge software and were blocked from using the software. Patients were passive recipients of the research intervention performed by their discharging physician. Patients received the research intervention on the day of discharge of the index hospitalization.
Baseline Assessment
During the index hospitalization, trained data abstractors recorded baseline patient demographic data plus variables to calculate the Pra for probability for repeat admission. We recorded the availability of an informal caregiver in response to the question, Is there a friend, relative, or neighbor who would take care of you for a few days, if necessary? Data came from the patient or proxy for physical functioning, mental health,13 heart failure, and number of previous emergency department visits. Other data came from hospital records for chronic obstructive pulmonary disease, number of discharge medications, and length of stay for the index hospitalization.
Outcome Assessment
We assessed the patient's perception of the discharge with 2 validated survey instruments. One week after discharge, research personnel performed telephone interviews with patients or proxies. While following a script, interviewers instructed patients to avoid mentioning the discharge process. Interviewers read items from the B‐PREPARED questionnaire.14, 15 and the Satisfaction with Information About Medicines Scale (SIMS).16 The B‐PREPARED scale assessed 3 principal components of patient preparedness for discharge: self‐care information for medications and activities, equipment and services, and confidence. The scale demonstrated internal consistency, construct validity, and predictive validity. High scale values reflected high perceptions of discharge preparedness from the patient perspective.15 SIMS measured patient satisfaction with information about discharge medications. Validation studies revealed SIMS had internal consistency, test‐retest reliability, and criterion‐related validity.16 Interviewers recorded responses to calculate a total SIMS score. Patients with high total SIMS scores had high satisfaction. While assessing B‐PREPARED and SIMS, interviewers were blind to intervention assignment. We evaluated the adequacy of blinding by asking interviewers to guess the patient's intervention assignment.
We measured the quality of hospital discharge from the outpatient physician perspective. During the index hospitalization, patients designated an outpatient primary care practitioner to receive discharge reports and results of diagnostic tests. Ten days after discharge, research personnel mailed the Physician‐PREPARED questionnaire to the designated community practitioner.17 The sum of item responses comprised the Modified Physician‐PREPARED scale and demonstrated internal consistency and construct validity. The principal components of the Modified Physician‐PREPARED were timeliness of communication and adequacy of discharge plan/transmission. High scale values reflected high perceptions of discharge quality.17 Outpatient practitioners gave implied consent when they completed and returned questionnaires. We requested 1 questionnaire for each enrolled patient, so the outcome assessment was at the patient level. The assessment was not blinded because primary care physicians received the output of discharge software or usual care discharge.
We assessed the satisfaction of hospital physicians who used the discharge software and the usual care. After hospital physicians participated in the trial for 6 months, they rated their assigned discharge process on Likert scales. The first question was, On a scale of 1 to 10, indicate your satisfaction with your portion of the discharge process. The scale anchors were 1 for very dissatisfied and 10 for very satisfied. The second question was, On a scale of 1 to 10, indicate the effort to complete your portion of the discharge process. For the second question, the scale anchors were 1 for very difficult and 10 for very easy. It was not possible to mask the hospital physicians after they received their intervention assignment. Consequently, their outcome assessment was not blinded.
Statistical Methods
The cluster number and size were selected to maintain test significance level, 1‐sided alpha less than 0.05, and power greater than 80%. We previously published the assumptions and rationale for 35 hospital physician clusters per intervention and 9 patients per cluster.7 We did not perform separate sample size estimates for the secondary outcomes reported herein.
The statistical analyses employed SPSS PC (Version 15.0.1; SPSS, Inc., Chicago, IL). Statistical procedures for baseline variables were descriptive and included means and standard deviations (SDs) for interval variables and percentages for categorical variables. For all analyses, we employed the principle of intention‐to‐treat. We assumed patient or physician exposure to the intervention randomly assigned to the discharging physician. Analyses employed standard tests for normal distribution, homogeneity of variance, and linearity of relationships between independent and dependent variables. If assumptions failed, then we stratified variables or performed transformations. We accepted P < 0.05 as significant.
We tested hypotheses for patient‐level outcomes with generalized estimating equations (GEEs) that corrected for clustering by hospital physician. We employed GEEs because they provide unbiased estimates of standard errors for parameters even with incorrect specification of the intracluster dependence structure.18 Each patient‐level outcome was the dependent variable in a separate GEE. The intervention variable for each GEE was discharge software vs. usual care, handwritten discharge. The statistic of interest was the coefficient for the intervention variable. The null hypothesis was no difference between discharge software and usual care. The statistical definition of the null hypothesis was an intervention variable coefficient with a 95% confidence interval (CI) that included 0.
For analyses that were unaffected by the cluster assumption, we performed standard tests. The hypothesis for hospital physicians was significantly higher satisfaction for discharge software users and the inferential procedure was the t test. When we assessed the success of the study blinding, we assumed no association between true intervention allocation and guesses by outcome assessors. We used chi‐square for assessment of the blinding.
Results
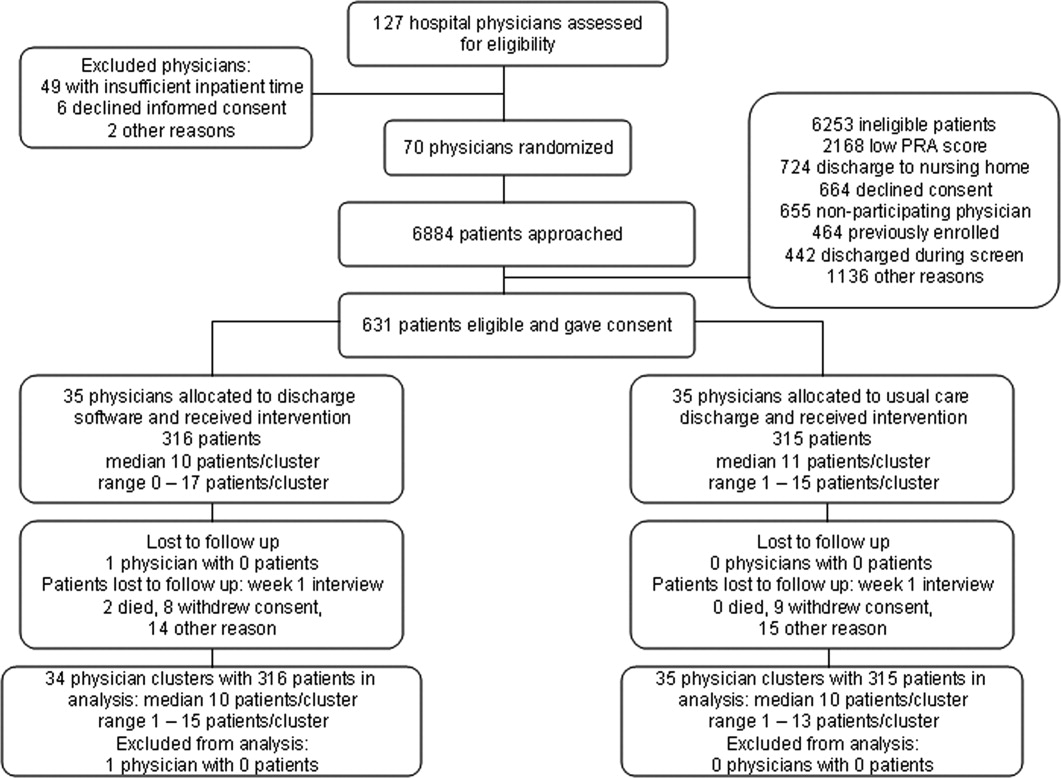
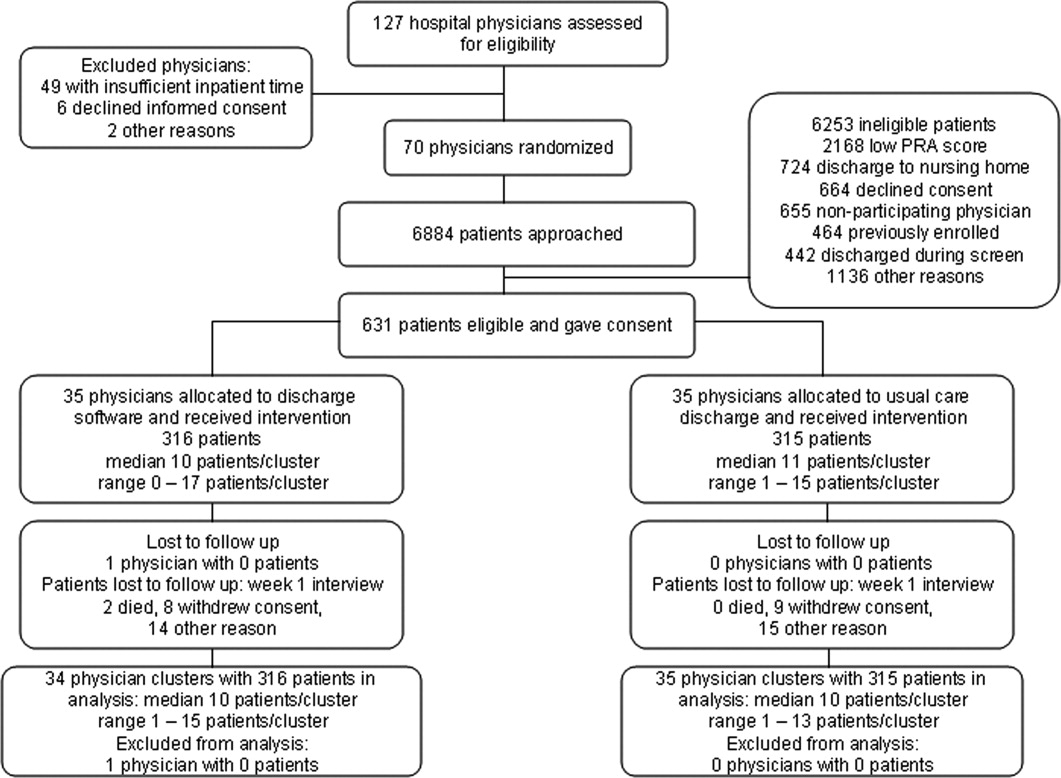
We screened 127 physicians who were general internal medicine hospital physicians. Seventy physicians consented and received random allocation to discharge software or usual care. We excluded 57 physicians for reasons shown in the trial flow diagram (Figure 1). We approached 6884 patients during their index hospitalization. After excluding 6253 ineligible patients, we enrolled 631 willing patients (Supplementary Appendix). As depicted in Figure 1, the most common reason for ineligibility occurred for patients with Pra score <0.40 (2168/6253 exclusions; 34.7%). We followed 631 patients who received the discharge intervention (Figure 1). There was no differential dropout between the interventions. Protocol deviations were rare, 0.5% (3/631). Three patients erroneously received usual care discharge from physicians assigned to discharge software. All 631 patients were included in the intention‐to‐treat analysis. The baseline characteristics of the randomly assigned hospital physicians and their patients are in Table 1. Most of the hospital physicians were residents in the first year of postgraduate training.
| Discharge Software | Usual Care | |
|---|---|---|
| ||
| Hospital physician characteristics, n (%) | n = 35 | n = 35 |
| Postgraduate year 1 | 18 (51.4) | 23 (65.7) |
| Postgraduate years 2‐4 | 10 (28.6) | 7 (20.0) |
| Attending physician | 7 (20.0) | 5 (14.3) |
| Patient characteristics, n (%) | n = 316 | n = 315 |
| Gender, male | 136 (43.0) | 147 (46.7) |
| Age, years | ||
| 18‐44 | 68 (21.5) | 95 (30.2) |
| 45‐54 | 79 (25.0) | 76 (24.1) |
| 55‐64 | 86 (27.2) | 74 (23.5) |
| 65‐98 | 83 (26.3) | 70 (22.2) |
| Self‐rated health status | ||
| Poor | 82 (25.9) | 108 (34.3) |
| Fair | 169 (53.5) | 147 (46.7) |
| Good | 54 (17.1) | 46 (14.6) |
| Very good | 10 (3.2) | 11 (3.5) |
| Excellent | 1 (0.3) | 3 (1.0) |
| Diabetes mellitus | 172 (54.4) | 177 (56.2) |
| Chronic obstructive pulmonary disease | ||
| None | 259 (82.0) | 257 (81.6) |
| Without oral steroid or home oxygen | 28 (8.9) | 26 (8.3) |
| With chronic oral steroid | 10 (3.2) | 8 (2.5) |
| With home oxygen oral steroid | 19 (6.0) | 24 (7.6) |
| Coronary heart disease | 133 (42.1) | 120 (38.1) |
| Heart failure | 80 (25.3) | 67 (21.3) |
| Physical Functioning from SF‐36 | ||
| Lowest third | 128 (40.5) | 121 (38.4) |
| Upper two‐thirds | 188 (59.5) | 194 (61.6) |
| Mental Health from SF‐36 | ||
| Lowest one‐third | 113 (35.8) | 117 (37.1)* |
| Upper two‐thirds | 203 (64.2) | 197 (62.5)* |
| Emergency department visits during 6 months before index admission | ||
| 0 or 1 | 194 (61.4) | 168 (53.3) |
| 2 or more | 122 (38.6) | 147 (46.7) |
| Mean (SD) | ||
| Number of discharge medications | 10.5 (4.8) | 9.9 (5.1) |
| Index hospital length of stay, days | 3.9 (3.5) | 3.5 (3.5) |
| Pra | 0.486 (0.072) | 0.495 (0.076) |
We assessed the patient's perception of discharge preparedness. One week after discharge, research personnel interviewed 92.4% (292/316) of patients in the discharge software group and 92.4% (291/315) in the usual care group. The mean (SD) B‐PREPARED scores for discharge preparedness were 17.7 (4.1) in the discharge software group and 17.2 (4.0) in the usual care group. In the generalized estimating equation that accounted for potential clustering within hospital physicians, the parameter estimate for the intervention variable coefficient was small but significant (P = 0.042; Table 2). Patients in the discharge software group had slightly better perceptions of their discharge preparedness.
| Outcome Variable | Discharge Software [mean (SD)] | Usual Care [mean (SD)] | Parameter Estimate Without Cluster Correction (95% CI) | P Value | Parameter Estimate with Cluster Correction (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Patient perception of discharge preparedness (B‐PREPARED) | 17.7 (4.1) | 17.2 (4.0) | 0.147* (0.006‐0.288) | 0.040 | 0.147* (0.005‐0.289) | 0.042 |
| Patient satisfaction with medication information score (SIMS) | 12.3 (4.8) | 12.1 (4.6) | 0.212 (0.978‐0.554) | 0.587 | 0.212 (0.937‐0.513) | 0.567 |
| Outpatient physician perception (Modified Physician‐PREPARED) | 17.2 (3.8) | 16.5 (3.9) | 0.133 (0.012‐0.254) | 0.031 | 0.133 (0.015‐0.251) | 0.027 |
Another outcome was the patient's satisfaction with information about discharge medications (Table 2). One week after discharge, mean (SD) SIMS scores for satisfaction were 12.3 (4.8) in the discharge software group and 12.1 (4.6) in the usual care group. The generalized estimating equation revealed an insignificant coefficient for the intervention variable (P = 0.567; Table 2).
We assessed the outpatient physician perception of the discharge with a questionnaire sent 10 days after discharge. We received 496 out of 631 questionnaires (78.6%) from outpatient practitioners and the median response time was 19 days after the date of discharge. The practitioner specialty was internal medicine for 38.9% (193/496), family medicine for 27.2% (135/496), medicine‐pediatrics for 27.0% (134/496), advance practice nurse for 4.4% (22/496), other physician specialties for 2.0% (10/496), and physician assistant for 0.4% (2/496). We excluded 18 questionnaires from analysis because outpatient practitioners failed to answer 2 or more items in the Modified Physician‐PREPARED scale. When we compared baseline characteristics for patients who had complete questionnaires vs. patients with nonrespondent or excluded questionnaires, we found no significant differences (data available upon request). Among the discharge software group, 72.2% (228/316) of patients had complete questionnaires from their outpatient physicians. The response rate with complete questionnaires was 79.4% (250/315) of patients assigned to usual care. On the Modified Physician‐PREPARED scale, the mean (SD) scores were 17.2 (3.8) for the discharge software group and 16.5 (3.9) for the usual care group. The parameter estimate from the generalized estimating equation was significant (P = 0.027; Table 2). Outpatient physicians had slightly better perception of discharge quality for patients assigned to discharge software.
In the questionnaire sent to outpatient practitioners, we requested additional information about discharge communication. When asked about timeliness, outpatient physicians perceived no faster communication with the discharge software (Table 3). We asked about the media for discharge information exchange. It was uncommon for community physicians to receive discharge information via electronic mail (Table 3). Outpatient physicians acknowledged receipt of a minority of facsimile transmissions with no significant difference between discharge software vs. usual care (Table 3). Investigators documented facsimile transmission of the output from the discharge software to outpatient practitioners. Transmission occurred on the first business day after discharge. Despite the documentation of all facsimile transmissions, only 23.4% of patients assigned to discharge software had community practitioners who acknowledged receipt.
| Discharge Software (n = 316) [n (%)] | Usual Care (n = 315) [n (%)] | |
|---|---|---|
| ||
| Question: How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? | ||
| Within 1‐2 days | 72 (22.8) | 55 (17.5) |
| Within 1 week | 105 (33.2) | 125 (39.7) |
| Longer than 1 week | 36 (11.4) | 41 (13.0) |
| Not received | 20 (6.3) | 26 (8.3) |
| Other | 4 (1.3) | 7 (2.2) |
| Question: How did you receive discharge health status information? (Check all that apply)* | ||
| Written/typed letter | 106 (33.5) | 89 (28.3) |
| Telephone call | 82 (25.9) | 67 (21.3) |
| Fax (facsimile transmission) | 74 (23.4) | 90 (28.6) |
| Electronic mail | 8 (2.5) | 23 (7.3) |
| Other | 15 (4.7) | 15 (4.8) |
In exploratory analyses, we evaluated the effect of hospital physician level of training. We wondered if discharging physician experience or seniority affected perceptions of patients or primary care physicians. We entered level of training as a covariate in generalized estimating equations. When patient perception of discharge preparedness (B‐PREPARED) was the dependent variable, then physician level of training had a nonsignificant coefficient (P > 0.219). Likewise, physician level of training was nonsignificant in models of patient satisfaction with medication information, SIMS (P > 0.068), and outpatient physician perception, Modified Physician‐PREPARED (P > 0.177). We concluded that physician level of training had no influence on the patient‐level outcomes assessed in our study.
We compared the satisfaction of hospital physicians who used the discharge software and the usual care discharge. The proportions of hospital physicians who returned questionnaires were 85.7% (30/35) in the discharge software group and 97% (34/35) in the usual care group. After using their assigned discharge process for at least 6 months, discharge software users had mean (SD) satisfaction 7.4 (1.4) vs. 7.9 (1.4) for usual care physicians (difference = 0.5; 95% CI = 0.2‐1.3; P = 0.129). The effort for discharge software users was more difficult than the effort for usual care (mean [SD] effort = 6.5 [1.9] vs. 7.9 [2.1], respectively). The mean difference in effort was significant (difference = 1.4; 95% CI = 0.3‐2.4; P = 0.011). We reviewed free‐text comments on hospital physician questionnaires. The common theme was software users spent more time to complete discharges. We did not perform time‐motion assessments so we cannot confirm or refute these impressions. Even though hospital physicians found the discharge software significantly more difficult, they did not report a significant decrease in their satisfaction between the 2 discharge interventions.
The cluster design of our trial assumed variance in outcomes measured at the patient level. We predicted some variance attributable to clustering by hospital physician. After the trial, we calculated the intracluster correlation coefficients for B‐PREPARED, SIMS, and Modified Physician‐PREPARED. For all of these outcome variables, the intracluster correlation coefficients were negligible. We also evaluated generalized estimating equations with and without correction for hospital physician cluster. We confirmed the negligible cluster effect on CIs for intervention coefficients (Table 2).
We evaluated the adequacy of the blind for outcome assessors who interviewed patients for B‐PREPARED and SIMS. The guesses of outcomes assessors were unrelated to true intervention assignment (P = 0.253). We interpreted the blind as adequate.
Discussion
We performed a cluster‐randomized clinical trial to measure the effects of discharge software vs. usual care discharge. The discharge software incorporated the ASTM (American Society for Testing and Material) Continuity of Care Record (CCR) standards.19 The CCR is a patient health summary standard with widespread support from medical and specialty organizations. The rationale for the CCR was the need for continuity of care from 1 provider or practitioner to any other practitioner. Our discharge software had the same rationale as the CCR and included a subset of the clinical content specified by the CCR. Like the CCR, our discharge software produced concise reports, and emphasized a brief, postdischarge, care plan. Since we included clinical data elements recommended by the CCR, we hypothesized our discharge software would produce clinically relevant improvements.
Our discharge software also implemented elements of high‐quality discharge planning and communication endorsed by the Society of Hospital Medicine.20 For example, the discharge software produced a legible, typed, discharge plan for the patient or caregiver with medication instructions, follow‐up tests, studies, and appointments. The discharge software generated a discharge summary for the outpatient primary care physician and other clinicians who provided postdischarge care. The summary included discharge diagnoses, key findings and test results, follow‐up appointments, pending diagnostic tests, documentation of patient education, reconciled medication list, and contact information for the hospital physician. The discharge software compiled data for purposes of benchmarking, measurement, and continuous quality improvement. We thought our implementation of discharge software would lead to improved outcomes.
Despite our deployment of recommended strategies, we detected only small increases in patient perceptions of discharge preparedness. We do not know if small changes in B‐PREPARED values were clinically important. We found no improvement in patient satisfaction with medication information. Our results are consistent with systematic reviews that revealed limited benefit of interventions other than discharge planning with postdischarge support.21 Since our discharge software was added to robust discharge planning and support, we possibly had limited ability to detect benefit unless the intervention had a large effect size.
Our discharge software caused a small increase in positive perception reported by outpatient physicians. Small changes in the Modified Physician‐PREPARED had uncertain clinical relevance. Potential delays imposed by our distribution method may have contributed to our findings. Output from our discharge software went to community physicians via facsimile transmission with backup copies via standard U.S. mail. Our distribution system responded to several realities. Most community physicians in our area had no access to interoperable electronic medical records or secured e‐mail. In addition, electronic transmittal of prescriptions was not commonplace. Our discharge intervention did not control the flow of information inside the offices of outpatient physicians. We did not know if our facsimile transmissions joined piles of unread laboratory and imaging reports on the desks of busy primary care physicians. Despite the limited technology available to community physicians, they perceived communication generated by the software to be an improvement over the handwritten process. Our results support previous studies in which physicians preferred computer‐generated discharge summaries and summaries in standardized formats.2224
One of the limitations of our trial design was the unmasked intervention. Hospital physicians assigned to usual care might have improved their handwritten and verbal discharge communication after observation of their colleagues assigned to discharge software. This phenomenon is encountered in unmasked trials and is called contamination. We attempted to minimize contamination when we blocked usual care physicians from access to the discharge software. However, we could not eliminate cross‐talk among unmasked hospital physicians who worked together in close proximity during 27 months of patient enrollment. Some contamination was inevitable. When contamination occurred, there was bias toward the null (increased type II error).
Another limitation was the large proportion of hospital physicians in the first year of postgraduate training. There was a potential for variance from multilevel clusters with patient‐level outcomes clustered within first‐year hospital physicians who were clustered within teams supervised by senior resident or attending physicians. Our results argued against hierarchical clusters because intracluster correlation coefficients were negligible. Furthermore, our exploratory analysis suggested physician training level had no influence on patient outcomes measured in our study. We speculate the highly structured discharge process for both usual care and software minimized variance attributable to physician training level.
The research intervention in our trial was a stand‐alone software application. The discharge software did not integrate with the hospital electronic medical record. Consequently, hospital physician users had to reenter patient demographic data and prescription data that already existed in the electronic record. Data reentry probably caused hospital physicians to attribute greater effort to the discharge software.
In our study, hospital physicians incorporated discharge software with CPOE into their clinical workflow without deterioration in their satisfaction. Our experience may inform the decisions of hospital personnel who design health information systems. When designing discharge functions, developers should consider medication reconciliation and the standards of the CCR.19 Modules within the discharge software would likely be more efficient with prepopulated data from the electronic record. Then users could shift their work from data entry to data verification and possibly mitigate their perceived effort. Software developers may wish to explore options for data transmission to community physicians: secure e‐mail, automated fax servers, and direct digital file transfer. Future studies should test the acceptability of discharge functions incorporated within electronic health records with robust clinical decision support.
Our results apply to a population of adults of all ages with high risk for readmission. The results may not generalize to children, surgical patients, or people with low risk for readmission. All of the patients in our study were discharged to home. The exclusion of other discharge destinations helped us to enroll a homogenous cohort. However, the exclusion criteria did not allow us to generalize our results to patients discharged to nursing homes, inpatient rehabilitation units, or other acute care facilities. We designed the intervention to apply to the hospitalist model, in which responsibility for patient care transitions to a different physician after discharge. The results of our study do not apply when the inpatient and outpatient physician are the same. Since we enrolled general internal medicine hospital physicians, our results may not generalize to care provided by other specialists.
Conclusions
A discharge software application with CPOE improved perceptions of the hospital discharge process for patients and their outpatient physicians. When compared to the handwritten discharge process, the improvements were small in magnitude. Hospital physicians who used the discharge software reported more effort but otherwise no decrement in their satisfaction with the discharge process.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2004;(1):CD000313.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,.Software design to facilitate information transfer at hospital discharge.Inform Prim Care.2006;14:109–119.
- ,.Computer physician order entry: benefits, costs, and issues.Ann Intern Med.2003;139:31–39.
- ,,, et al.Patient readmissions, emergency visits, and adverse events after software‐assisted discharge from hospital: cluster randomized trial.J Hosp Med.2009; DOI: 10.1002/jhm.459. PMID: 19479782.
- ,,.Predictive validity of a questionnaire that identifies older persons at risk for hospital admission.J Am Geriatr Soc.1995;43:374–377.
- ,.Prediction of early readmission in medical inpatients using the Probability of Repeated Admission instrument.Nurs Res.2008;57:406–415.
- ,.The ten point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients.Int J Psychiatry Med.1994;24:229–244.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- .SF‐36 health survey update.Spine.2000;25:3130–3139.
- ,.The development, validity and application of a new instrument to assess the quality of discharge planning activities from the community perspective.Int J Qual Health Care.2001;13:109–116.
- ,,.Brief scale measuring patient preparedness for hospital discharge to home: psychometric properties.J Hosp Med.2008;3:446–454.
- ,,.The Satisfaction with Information about Medicines Scale (SIMS): a new measurement tool for audit and research.Qual Health Care.2001;10:135–140.
- ,,.Discharge planning scale: community physicians' perspective.J Hosp Med.2008;3:455–464.
- ,.An introduction to generalized estimating equations and an application to assess selectivity effects in a longitudinal study on very old individuals.J Educ Behav Stat.2004;29:421–437. Available at: http://jeb.sagepub.com/cgi/content/abstract/29/4/421. Accessed June 2009.
- ASTM. E2369‐05 Standard Specification for Continuity of Care Record (CCR). Available at: http://www.astm.org/Standards/E2369.htm. Accessed June2009.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1:354–360.
- ,,.Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta‐review.BMC Health Serv Res.2007;7:47.
- ,,,,,.Evaluation of a computer‐generated discharge summary for patients with acute coronary syndromes.Br J Gen Pract.1998;48:1163–1164.
- ,,,.Standardized or narrative discharge summaries. Which do family physicians prefer?Can Fam Physician.1998;44:62–69.
- ,,,.Dictated versus database‐generated discharge summaries: a randomized clinical trial.CMAJ.1999;160:319–326.
During the transition from inpatient to outpatient care, patients are vulnerable to adverse events.1 Poor communication between hospital personnel and either the patient or the outpatient primary care physician has been associated with preventable or ameliorable adverse events after discharge.1 Systematic reviews confirm that discharge communication is often delayed, inaccurate, or ineffective.2, 3
Discharge communication failures may occur if hospital processes rely on dictated discharge summaries.2 For several reasons, discharge summaries are inadequate for communication. Most patients complete their initial posthospital clinic visit before their primary care physician receives the discharge summary.4 For many patients, the discharge summary is unavailable for all posthospital visits.4 Discharge summaries often fail as communication because they are not generated or transmitted.4
Recommendations to improve discharge communication include the use of health information technology.2, 5 The benefits of computer‐generated discharge summaries include decreases in delivery time for discharge communications.2 The benefits of computerized physician order entry (CPOE) include reduction of medical errors.6 These theoretical benefits create a rationale for clinical trials to measure improvements after discharge software applications with CPOE.5
In an effort to improve discharge communication and clinically relevant outcomes, we performed a cluster‐randomized trial to assess the value of a discharge software application of CPOE. The clustered design followed recommendations from a systematic review of discharge interventions.3 We applied our research intervention at the physician level and measured outcomes at the patient level. Our objective was to assess the benefit of discharge software with CPOE vs. usual care when used to discharge patients at high risk for repeat admission. In a previous work, we reported that discharge software did not reduce rates of hospital readmission, emergency department visits, or postdischarge adverse events due to medical management.7 In the present article, we compare secondary outcomes after the research intervention: perceptions of the discharge from the perspectives of patients, primary care physicians, and hospital physicians.
Methods
The trial design was a cluster randomized, controlled trial. The setting was the postdischarge environment following index hospitalization at a 730‐bed, tertiary care, teaching hospital in central Illinois. The Peoria Institutional Review Board approved the protocol for human research.
Participants
We enrolled consenting hospital physicians and their patients between November 2004 and January 2007. The hospital physician defined the cluster. Patients discharged by the physician comprised the cluster. The eligibility criteria for hospital physicians required internal medicine resident or attending physicians with assignments to inpatient duties for at least 2 months during the 27‐month enrollment period. After achieving informed consent from physicians, research personnel screened all consecutive, adult inpatients who were discharged to home. Patient inclusion required a probability of repeat admission (Pra) equal to or greater than 0.40.8, 9 The purpose of the inclusion criterion was to enrich the sample with patients likely to benefit from interventions to improve discharge processes. Furthermore, hospital readmission was the primary endpoint of the study, as reported separately.7 The Pra came from a predictive model with scores for age, gender, prior hospitalizations, prior doctor visits, self‐rated health status, presence of informal caregiver in the home, and comorbid coronary heart disease and diabetes mellitus. Research coordinators calculated the Pra within 2 days before discharge from the index hospitalization.
Exclusion Criteria
We excluded patients previously enrolled in the study, candidates for hospice, and patients unable to participate in outcome ascertainment. Cognitive impairment was a conditional exclusion criterion for patients. We defined cognitive impairment as a score less than 9 on the 10‐point clock test.10 Patients with cognitive impairment participated only with consent from their legally authorized representative. We enrolled patients with cognitive impairment only if a proxy spent at least 3 hours daily with the patient and the proxy agreed to answer postdischarge interviews. If a patient's outpatient primary care physician treated the patient during the index hospitalization, then there was no perceived barrier in physician‐to‐physician communication and we excluded the patient.
Intervention
The research intervention was discharge software with CPOE. Detailed description of the software appeared previously.5 In summary, the CPOE software application facilitated communication at the time of hospital discharge to patients, retail pharmacists, and community physicians. The application had basic levels of clinical decision support, required fields, pick lists, standard drug doses, alerts, reminders, and online reference information. The software addressed deficiencies in the usual care discharge process reported globally and reviewed previously.5 For example, 1 deficiency occurred when inpatient physicians failed to warn outpatient physicians about diagnostic tests with results pending at discharge.11 Another deficiency was discharge medication error.12 The software prompted the discharging physician to enter pending tests, order tests after discharge, and perform medication reconciliation. On the day of discharge, hospital physicians used the software to automatically generate discharge documents and reconcile prescriptions for the patient, primary care physician, retail pharmacist, and the ward nurse. The discharge letter went to the outpatient practitioner via facsimile transmission plus a duplicate via U.S. mail.
The control intervention was the usual care, handwritten discharge process commonly used by hospitalists.2 Hospital physicians and ward nurses completed handwritten discharge forms on the day of discharge. The forms contained blanks for discharge diagnoses, discharge medications, medication instructions, postdischarge activities and restrictions, postdischarge diet, postdischarge diagnostic and therapeutic interventions, and appointments. Patients received handwritten copies of the forms, 1 page of which also included medication instructions and prescriptions. In a previous publication, we provided details about the usual care discharge process as well as the standard care available to all study patients regardless of intervention.5
Randomization
The hospital physician who completed the discharge process was the unit of randomization. Random allocation was to discharge software or usual care discharge process, with a randomization ratio of 1:1 and block size of 2. We concealed allocation with the following process. An investigator who was not involved with hospital physician recruitment generated the randomization sequence with a computerized random number generator. The randomization list was maintained in a secure location. Another investigator who was unaware of the next random assignment performed the hospital physician recruitment and informed consent. After confirming eligibility and obtaining informed consent from physicians, the blinded investigator requested the next random assignment from the custodian of the randomization list. Hospital physicians subsequently used their randomly assigned process when discharging their patients who enrolled in the study. After random allocation, it was not possible to conceal the test or control intervention from physicians or their patients.
Hospital physicians underwent training on the software or usual care discharge process; the details appeared previously.7 Physicians assigned to usual care did not receive training on the discharge software and were blocked from using the software. Patients were passive recipients of the research intervention performed by their discharging physician. Patients received the research intervention on the day of discharge of the index hospitalization.
Baseline Assessment
During the index hospitalization, trained data abstractors recorded baseline patient demographic data plus variables to calculate the Pra for probability for repeat admission. We recorded the availability of an informal caregiver in response to the question, Is there a friend, relative, or neighbor who would take care of you for a few days, if necessary? Data came from the patient or proxy for physical functioning, mental health,13 heart failure, and number of previous emergency department visits. Other data came from hospital records for chronic obstructive pulmonary disease, number of discharge medications, and length of stay for the index hospitalization.
Outcome Assessment
We assessed the patient's perception of the discharge with 2 validated survey instruments. One week after discharge, research personnel performed telephone interviews with patients or proxies. While following a script, interviewers instructed patients to avoid mentioning the discharge process. Interviewers read items from the B‐PREPARED questionnaire.14, 15 and the Satisfaction with Information About Medicines Scale (SIMS).16 The B‐PREPARED scale assessed 3 principal components of patient preparedness for discharge: self‐care information for medications and activities, equipment and services, and confidence. The scale demonstrated internal consistency, construct validity, and predictive validity. High scale values reflected high perceptions of discharge preparedness from the patient perspective.15 SIMS measured patient satisfaction with information about discharge medications. Validation studies revealed SIMS had internal consistency, test‐retest reliability, and criterion‐related validity.16 Interviewers recorded responses to calculate a total SIMS score. Patients with high total SIMS scores had high satisfaction. While assessing B‐PREPARED and SIMS, interviewers were blind to intervention assignment. We evaluated the adequacy of blinding by asking interviewers to guess the patient's intervention assignment.
We measured the quality of hospital discharge from the outpatient physician perspective. During the index hospitalization, patients designated an outpatient primary care practitioner to receive discharge reports and results of diagnostic tests. Ten days after discharge, research personnel mailed the Physician‐PREPARED questionnaire to the designated community practitioner.17 The sum of item responses comprised the Modified Physician‐PREPARED scale and demonstrated internal consistency and construct validity. The principal components of the Modified Physician‐PREPARED were timeliness of communication and adequacy of discharge plan/transmission. High scale values reflected high perceptions of discharge quality.17 Outpatient practitioners gave implied consent when they completed and returned questionnaires. We requested 1 questionnaire for each enrolled patient, so the outcome assessment was at the patient level. The assessment was not blinded because primary care physicians received the output of discharge software or usual care discharge.
We assessed the satisfaction of hospital physicians who used the discharge software and the usual care. After hospital physicians participated in the trial for 6 months, they rated their assigned discharge process on Likert scales. The first question was, On a scale of 1 to 10, indicate your satisfaction with your portion of the discharge process. The scale anchors were 1 for very dissatisfied and 10 for very satisfied. The second question was, On a scale of 1 to 10, indicate the effort to complete your portion of the discharge process. For the second question, the scale anchors were 1 for very difficult and 10 for very easy. It was not possible to mask the hospital physicians after they received their intervention assignment. Consequently, their outcome assessment was not blinded.
Statistical Methods
The cluster number and size were selected to maintain test significance level, 1‐sided alpha less than 0.05, and power greater than 80%. We previously published the assumptions and rationale for 35 hospital physician clusters per intervention and 9 patients per cluster.7 We did not perform separate sample size estimates for the secondary outcomes reported herein.
The statistical analyses employed SPSS PC (Version 15.0.1; SPSS, Inc., Chicago, IL). Statistical procedures for baseline variables were descriptive and included means and standard deviations (SDs) for interval variables and percentages for categorical variables. For all analyses, we employed the principle of intention‐to‐treat. We assumed patient or physician exposure to the intervention randomly assigned to the discharging physician. Analyses employed standard tests for normal distribution, homogeneity of variance, and linearity of relationships between independent and dependent variables. If assumptions failed, then we stratified variables or performed transformations. We accepted P < 0.05 as significant.
We tested hypotheses for patient‐level outcomes with generalized estimating equations (GEEs) that corrected for clustering by hospital physician. We employed GEEs because they provide unbiased estimates of standard errors for parameters even with incorrect specification of the intracluster dependence structure.18 Each patient‐level outcome was the dependent variable in a separate GEE. The intervention variable for each GEE was discharge software vs. usual care, handwritten discharge. The statistic of interest was the coefficient for the intervention variable. The null hypothesis was no difference between discharge software and usual care. The statistical definition of the null hypothesis was an intervention variable coefficient with a 95% confidence interval (CI) that included 0.
For analyses that were unaffected by the cluster assumption, we performed standard tests. The hypothesis for hospital physicians was significantly higher satisfaction for discharge software users and the inferential procedure was the t test. When we assessed the success of the study blinding, we assumed no association between true intervention allocation and guesses by outcome assessors. We used chi‐square for assessment of the blinding.
Results
We screened 127 physicians who were general internal medicine hospital physicians. Seventy physicians consented and received random allocation to discharge software or usual care. We excluded 57 physicians for reasons shown in the trial flow diagram (Figure 1). We approached 6884 patients during their index hospitalization. After excluding 6253 ineligible patients, we enrolled 631 willing patients (Supplementary Appendix). As depicted in Figure 1, the most common reason for ineligibility occurred for patients with Pra score <0.40 (2168/6253 exclusions; 34.7%). We followed 631 patients who received the discharge intervention (Figure 1). There was no differential dropout between the interventions. Protocol deviations were rare, 0.5% (3/631). Three patients erroneously received usual care discharge from physicians assigned to discharge software. All 631 patients were included in the intention‐to‐treat analysis. The baseline characteristics of the randomly assigned hospital physicians and their patients are in Table 1. Most of the hospital physicians were residents in the first year of postgraduate training.

| Discharge Software | Usual Care | |
|---|---|---|
| ||
| Hospital physician characteristics, n (%) | n = 35 | n = 35 |
| Postgraduate year 1 | 18 (51.4) | 23 (65.7) |
| Postgraduate years 2‐4 | 10 (28.6) | 7 (20.0) |
| Attending physician | 7 (20.0) | 5 (14.3) |
| Patient characteristics, n (%) | n = 316 | n = 315 |
| Gender, male | 136 (43.0) | 147 (46.7) |
| Age, years | ||
| 18‐44 | 68 (21.5) | 95 (30.2) |
| 45‐54 | 79 (25.0) | 76 (24.1) |
| 55‐64 | 86 (27.2) | 74 (23.5) |
| 65‐98 | 83 (26.3) | 70 (22.2) |
| Self‐rated health status | ||
| Poor | 82 (25.9) | 108 (34.3) |
| Fair | 169 (53.5) | 147 (46.7) |
| Good | 54 (17.1) | 46 (14.6) |
| Very good | 10 (3.2) | 11 (3.5) |
| Excellent | 1 (0.3) | 3 (1.0) |
| Diabetes mellitus | 172 (54.4) | 177 (56.2) |
| Chronic obstructive pulmonary disease | ||
| None | 259 (82.0) | 257 (81.6) |
| Without oral steroid or home oxygen | 28 (8.9) | 26 (8.3) |
| With chronic oral steroid | 10 (3.2) | 8 (2.5) |
| With home oxygen oral steroid | 19 (6.0) | 24 (7.6) |
| Coronary heart disease | 133 (42.1) | 120 (38.1) |
| Heart failure | 80 (25.3) | 67 (21.3) |
| Physical Functioning from SF‐36 | ||
| Lowest third | 128 (40.5) | 121 (38.4) |
| Upper two‐thirds | 188 (59.5) | 194 (61.6) |
| Mental Health from SF‐36 | ||
| Lowest one‐third | 113 (35.8) | 117 (37.1)* |
| Upper two‐thirds | 203 (64.2) | 197 (62.5)* |
| Emergency department visits during 6 months before index admission | ||
| 0 or 1 | 194 (61.4) | 168 (53.3) |
| 2 or more | 122 (38.6) | 147 (46.7) |
| Mean (SD) | ||
| Number of discharge medications | 10.5 (4.8) | 9.9 (5.1) |
| Index hospital length of stay, days | 3.9 (3.5) | 3.5 (3.5) |
| Pra | 0.486 (0.072) | 0.495 (0.076) |
We assessed the patient's perception of discharge preparedness. One week after discharge, research personnel interviewed 92.4% (292/316) of patients in the discharge software group and 92.4% (291/315) in the usual care group. The mean (SD) B‐PREPARED scores for discharge preparedness were 17.7 (4.1) in the discharge software group and 17.2 (4.0) in the usual care group. In the generalized estimating equation that accounted for potential clustering within hospital physicians, the parameter estimate for the intervention variable coefficient was small but significant (P = 0.042; Table 2). Patients in the discharge software group had slightly better perceptions of their discharge preparedness.
| Outcome Variable | Discharge Software [mean (SD)] | Usual Care [mean (SD)] | Parameter Estimate Without Cluster Correction (95% CI) | P Value | Parameter Estimate with Cluster Correction (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Patient perception of discharge preparedness (B‐PREPARED) | 17.7 (4.1) | 17.2 (4.0) | 0.147* (0.006‐0.288) | 0.040 | 0.147* (0.005‐0.289) | 0.042 |
| Patient satisfaction with medication information score (SIMS) | 12.3 (4.8) | 12.1 (4.6) | 0.212 (0.978‐0.554) | 0.587 | 0.212 (0.937‐0.513) | 0.567 |
| Outpatient physician perception (Modified Physician‐PREPARED) | 17.2 (3.8) | 16.5 (3.9) | 0.133 (0.012‐0.254) | 0.031 | 0.133 (0.015‐0.251) | 0.027 |
Another outcome was the patient's satisfaction with information about discharge medications (Table 2). One week after discharge, mean (SD) SIMS scores for satisfaction were 12.3 (4.8) in the discharge software group and 12.1 (4.6) in the usual care group. The generalized estimating equation revealed an insignificant coefficient for the intervention variable (P = 0.567; Table 2).
We assessed the outpatient physician perception of the discharge with a questionnaire sent 10 days after discharge. We received 496 out of 631 questionnaires (78.6%) from outpatient practitioners and the median response time was 19 days after the date of discharge. The practitioner specialty was internal medicine for 38.9% (193/496), family medicine for 27.2% (135/496), medicine‐pediatrics for 27.0% (134/496), advance practice nurse for 4.4% (22/496), other physician specialties for 2.0% (10/496), and physician assistant for 0.4% (2/496). We excluded 18 questionnaires from analysis because outpatient practitioners failed to answer 2 or more items in the Modified Physician‐PREPARED scale. When we compared baseline characteristics for patients who had complete questionnaires vs. patients with nonrespondent or excluded questionnaires, we found no significant differences (data available upon request). Among the discharge software group, 72.2% (228/316) of patients had complete questionnaires from their outpatient physicians. The response rate with complete questionnaires was 79.4% (250/315) of patients assigned to usual care. On the Modified Physician‐PREPARED scale, the mean (SD) scores were 17.2 (3.8) for the discharge software group and 16.5 (3.9) for the usual care group. The parameter estimate from the generalized estimating equation was significant (P = 0.027; Table 2). Outpatient physicians had slightly better perception of discharge quality for patients assigned to discharge software.
In the questionnaire sent to outpatient practitioners, we requested additional information about discharge communication. When asked about timeliness, outpatient physicians perceived no faster communication with the discharge software (Table 3). We asked about the media for discharge information exchange. It was uncommon for community physicians to receive discharge information via electronic mail (Table 3). Outpatient physicians acknowledged receipt of a minority of facsimile transmissions with no significant difference between discharge software vs. usual care (Table 3). Investigators documented facsimile transmission of the output from the discharge software to outpatient practitioners. Transmission occurred on the first business day after discharge. Despite the documentation of all facsimile transmissions, only 23.4% of patients assigned to discharge software had community practitioners who acknowledged receipt.
| Discharge Software (n = 316) [n (%)] | Usual Care (n = 315) [n (%)] | |
|---|---|---|
| ||
| Question: How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? | ||
| Within 1‐2 days | 72 (22.8) | 55 (17.5) |
| Within 1 week | 105 (33.2) | 125 (39.7) |
| Longer than 1 week | 36 (11.4) | 41 (13.0) |
| Not received | 20 (6.3) | 26 (8.3) |
| Other | 4 (1.3) | 7 (2.2) |
| Question: How did you receive discharge health status information? (Check all that apply)* | ||
| Written/typed letter | 106 (33.5) | 89 (28.3) |
| Telephone call | 82 (25.9) | 67 (21.3) |
| Fax (facsimile transmission) | 74 (23.4) | 90 (28.6) |
| Electronic mail | 8 (2.5) | 23 (7.3) |
| Other | 15 (4.7) | 15 (4.8) |
In exploratory analyses, we evaluated the effect of hospital physician level of training. We wondered if discharging physician experience or seniority affected perceptions of patients or primary care physicians. We entered level of training as a covariate in generalized estimating equations. When patient perception of discharge preparedness (B‐PREPARED) was the dependent variable, then physician level of training had a nonsignificant coefficient (P > 0.219). Likewise, physician level of training was nonsignificant in models of patient satisfaction with medication information, SIMS (P > 0.068), and outpatient physician perception, Modified Physician‐PREPARED (P > 0.177). We concluded that physician level of training had no influence on the patient‐level outcomes assessed in our study.
We compared the satisfaction of hospital physicians who used the discharge software and the usual care discharge. The proportions of hospital physicians who returned questionnaires were 85.7% (30/35) in the discharge software group and 97% (34/35) in the usual care group. After using their assigned discharge process for at least 6 months, discharge software users had mean (SD) satisfaction 7.4 (1.4) vs. 7.9 (1.4) for usual care physicians (difference = 0.5; 95% CI = 0.2‐1.3; P = 0.129). The effort for discharge software users was more difficult than the effort for usual care (mean [SD] effort = 6.5 [1.9] vs. 7.9 [2.1], respectively). The mean difference in effort was significant (difference = 1.4; 95% CI = 0.3‐2.4; P = 0.011). We reviewed free‐text comments on hospital physician questionnaires. The common theme was software users spent more time to complete discharges. We did not perform time‐motion assessments so we cannot confirm or refute these impressions. Even though hospital physicians found the discharge software significantly more difficult, they did not report a significant decrease in their satisfaction between the 2 discharge interventions.
The cluster design of our trial assumed variance in outcomes measured at the patient level. We predicted some variance attributable to clustering by hospital physician. After the trial, we calculated the intracluster correlation coefficients for B‐PREPARED, SIMS, and Modified Physician‐PREPARED. For all of these outcome variables, the intracluster correlation coefficients were negligible. We also evaluated generalized estimating equations with and without correction for hospital physician cluster. We confirmed the negligible cluster effect on CIs for intervention coefficients (Table 2).
We evaluated the adequacy of the blind for outcome assessors who interviewed patients for B‐PREPARED and SIMS. The guesses of outcomes assessors were unrelated to true intervention assignment (P = 0.253). We interpreted the blind as adequate.
Discussion
We performed a cluster‐randomized clinical trial to measure the effects of discharge software vs. usual care discharge. The discharge software incorporated the ASTM (American Society for Testing and Material) Continuity of Care Record (CCR) standards.19 The CCR is a patient health summary standard with widespread support from medical and specialty organizations. The rationale for the CCR was the need for continuity of care from 1 provider or practitioner to any other practitioner. Our discharge software had the same rationale as the CCR and included a subset of the clinical content specified by the CCR. Like the CCR, our discharge software produced concise reports, and emphasized a brief, postdischarge, care plan. Since we included clinical data elements recommended by the CCR, we hypothesized our discharge software would produce clinically relevant improvements.
Our discharge software also implemented elements of high‐quality discharge planning and communication endorsed by the Society of Hospital Medicine.20 For example, the discharge software produced a legible, typed, discharge plan for the patient or caregiver with medication instructions, follow‐up tests, studies, and appointments. The discharge software generated a discharge summary for the outpatient primary care physician and other clinicians who provided postdischarge care. The summary included discharge diagnoses, key findings and test results, follow‐up appointments, pending diagnostic tests, documentation of patient education, reconciled medication list, and contact information for the hospital physician. The discharge software compiled data for purposes of benchmarking, measurement, and continuous quality improvement. We thought our implementation of discharge software would lead to improved outcomes.
Despite our deployment of recommended strategies, we detected only small increases in patient perceptions of discharge preparedness. We do not know if small changes in B‐PREPARED values were clinically important. We found no improvement in patient satisfaction with medication information. Our results are consistent with systematic reviews that revealed limited benefit of interventions other than discharge planning with postdischarge support.21 Since our discharge software was added to robust discharge planning and support, we possibly had limited ability to detect benefit unless the intervention had a large effect size.
Our discharge software caused a small increase in positive perception reported by outpatient physicians. Small changes in the Modified Physician‐PREPARED had uncertain clinical relevance. Potential delays imposed by our distribution method may have contributed to our findings. Output from our discharge software went to community physicians via facsimile transmission with backup copies via standard U.S. mail. Our distribution system responded to several realities. Most community physicians in our area had no access to interoperable electronic medical records or secured e‐mail. In addition, electronic transmittal of prescriptions was not commonplace. Our discharge intervention did not control the flow of information inside the offices of outpatient physicians. We did not know if our facsimile transmissions joined piles of unread laboratory and imaging reports on the desks of busy primary care physicians. Despite the limited technology available to community physicians, they perceived communication generated by the software to be an improvement over the handwritten process. Our results support previous studies in which physicians preferred computer‐generated discharge summaries and summaries in standardized formats.2224
One of the limitations of our trial design was the unmasked intervention. Hospital physicians assigned to usual care might have improved their handwritten and verbal discharge communication after observation of their colleagues assigned to discharge software. This phenomenon is encountered in unmasked trials and is called contamination. We attempted to minimize contamination when we blocked usual care physicians from access to the discharge software. However, we could not eliminate cross‐talk among unmasked hospital physicians who worked together in close proximity during 27 months of patient enrollment. Some contamination was inevitable. When contamination occurred, there was bias toward the null (increased type II error).
Another limitation was the large proportion of hospital physicians in the first year of postgraduate training. There was a potential for variance from multilevel clusters with patient‐level outcomes clustered within first‐year hospital physicians who were clustered within teams supervised by senior resident or attending physicians. Our results argued against hierarchical clusters because intracluster correlation coefficients were negligible. Furthermore, our exploratory analysis suggested physician training level had no influence on patient outcomes measured in our study. We speculate the highly structured discharge process for both usual care and software minimized variance attributable to physician training level.
The research intervention in our trial was a stand‐alone software application. The discharge software did not integrate with the hospital electronic medical record. Consequently, hospital physician users had to reenter patient demographic data and prescription data that already existed in the electronic record. Data reentry probably caused hospital physicians to attribute greater effort to the discharge software.
In our study, hospital physicians incorporated discharge software with CPOE into their clinical workflow without deterioration in their satisfaction. Our experience may inform the decisions of hospital personnel who design health information systems. When designing discharge functions, developers should consider medication reconciliation and the standards of the CCR.19 Modules within the discharge software would likely be more efficient with prepopulated data from the electronic record. Then users could shift their work from data entry to data verification and possibly mitigate their perceived effort. Software developers may wish to explore options for data transmission to community physicians: secure e‐mail, automated fax servers, and direct digital file transfer. Future studies should test the acceptability of discharge functions incorporated within electronic health records with robust clinical decision support.
Our results apply to a population of adults of all ages with high risk for readmission. The results may not generalize to children, surgical patients, or people with low risk for readmission. All of the patients in our study were discharged to home. The exclusion of other discharge destinations helped us to enroll a homogenous cohort. However, the exclusion criteria did not allow us to generalize our results to patients discharged to nursing homes, inpatient rehabilitation units, or other acute care facilities. We designed the intervention to apply to the hospitalist model, in which responsibility for patient care transitions to a different physician after discharge. The results of our study do not apply when the inpatient and outpatient physician are the same. Since we enrolled general internal medicine hospital physicians, our results may not generalize to care provided by other specialists.
Conclusions
A discharge software application with CPOE improved perceptions of the hospital discharge process for patients and their outpatient physicians. When compared to the handwritten discharge process, the improvements were small in magnitude. Hospital physicians who used the discharge software reported more effort but otherwise no decrement in their satisfaction with the discharge process.
During the transition from inpatient to outpatient care, patients are vulnerable to adverse events.1 Poor communication between hospital personnel and either the patient or the outpatient primary care physician has been associated with preventable or ameliorable adverse events after discharge.1 Systematic reviews confirm that discharge communication is often delayed, inaccurate, or ineffective.2, 3
Discharge communication failures may occur if hospital processes rely on dictated discharge summaries.2 For several reasons, discharge summaries are inadequate for communication. Most patients complete their initial posthospital clinic visit before their primary care physician receives the discharge summary.4 For many patients, the discharge summary is unavailable for all posthospital visits.4 Discharge summaries often fail as communication because they are not generated or transmitted.4
Recommendations to improve discharge communication include the use of health information technology.2, 5 The benefits of computer‐generated discharge summaries include decreases in delivery time for discharge communications.2 The benefits of computerized physician order entry (CPOE) include reduction of medical errors.6 These theoretical benefits create a rationale for clinical trials to measure improvements after discharge software applications with CPOE.5
In an effort to improve discharge communication and clinically relevant outcomes, we performed a cluster‐randomized trial to assess the value of a discharge software application of CPOE. The clustered design followed recommendations from a systematic review of discharge interventions.3 We applied our research intervention at the physician level and measured outcomes at the patient level. Our objective was to assess the benefit of discharge software with CPOE vs. usual care when used to discharge patients at high risk for repeat admission. In a previous work, we reported that discharge software did not reduce rates of hospital readmission, emergency department visits, or postdischarge adverse events due to medical management.7 In the present article, we compare secondary outcomes after the research intervention: perceptions of the discharge from the perspectives of patients, primary care physicians, and hospital physicians.
Methods
The trial design was a cluster randomized, controlled trial. The setting was the postdischarge environment following index hospitalization at a 730‐bed, tertiary care, teaching hospital in central Illinois. The Peoria Institutional Review Board approved the protocol for human research.
Participants
We enrolled consenting hospital physicians and their patients between November 2004 and January 2007. The hospital physician defined the cluster. Patients discharged by the physician comprised the cluster. The eligibility criteria for hospital physicians required internal medicine resident or attending physicians with assignments to inpatient duties for at least 2 months during the 27‐month enrollment period. After achieving informed consent from physicians, research personnel screened all consecutive, adult inpatients who were discharged to home. Patient inclusion required a probability of repeat admission (Pra) equal to or greater than 0.40.8, 9 The purpose of the inclusion criterion was to enrich the sample with patients likely to benefit from interventions to improve discharge processes. Furthermore, hospital readmission was the primary endpoint of the study, as reported separately.7 The Pra came from a predictive model with scores for age, gender, prior hospitalizations, prior doctor visits, self‐rated health status, presence of informal caregiver in the home, and comorbid coronary heart disease and diabetes mellitus. Research coordinators calculated the Pra within 2 days before discharge from the index hospitalization.
Exclusion Criteria
We excluded patients previously enrolled in the study, candidates for hospice, and patients unable to participate in outcome ascertainment. Cognitive impairment was a conditional exclusion criterion for patients. We defined cognitive impairment as a score less than 9 on the 10‐point clock test.10 Patients with cognitive impairment participated only with consent from their legally authorized representative. We enrolled patients with cognitive impairment only if a proxy spent at least 3 hours daily with the patient and the proxy agreed to answer postdischarge interviews. If a patient's outpatient primary care physician treated the patient during the index hospitalization, then there was no perceived barrier in physician‐to‐physician communication and we excluded the patient.
Intervention
The research intervention was discharge software with CPOE. Detailed description of the software appeared previously.5 In summary, the CPOE software application facilitated communication at the time of hospital discharge to patients, retail pharmacists, and community physicians. The application had basic levels of clinical decision support, required fields, pick lists, standard drug doses, alerts, reminders, and online reference information. The software addressed deficiencies in the usual care discharge process reported globally and reviewed previously.5 For example, 1 deficiency occurred when inpatient physicians failed to warn outpatient physicians about diagnostic tests with results pending at discharge.11 Another deficiency was discharge medication error.12 The software prompted the discharging physician to enter pending tests, order tests after discharge, and perform medication reconciliation. On the day of discharge, hospital physicians used the software to automatically generate discharge documents and reconcile prescriptions for the patient, primary care physician, retail pharmacist, and the ward nurse. The discharge letter went to the outpatient practitioner via facsimile transmission plus a duplicate via U.S. mail.
The control intervention was the usual care, handwritten discharge process commonly used by hospitalists.2 Hospital physicians and ward nurses completed handwritten discharge forms on the day of discharge. The forms contained blanks for discharge diagnoses, discharge medications, medication instructions, postdischarge activities and restrictions, postdischarge diet, postdischarge diagnostic and therapeutic interventions, and appointments. Patients received handwritten copies of the forms, 1 page of which also included medication instructions and prescriptions. In a previous publication, we provided details about the usual care discharge process as well as the standard care available to all study patients regardless of intervention.5
Randomization
The hospital physician who completed the discharge process was the unit of randomization. Random allocation was to discharge software or usual care discharge process, with a randomization ratio of 1:1 and block size of 2. We concealed allocation with the following process. An investigator who was not involved with hospital physician recruitment generated the randomization sequence with a computerized random number generator. The randomization list was maintained in a secure location. Another investigator who was unaware of the next random assignment performed the hospital physician recruitment and informed consent. After confirming eligibility and obtaining informed consent from physicians, the blinded investigator requested the next random assignment from the custodian of the randomization list. Hospital physicians subsequently used their randomly assigned process when discharging their patients who enrolled in the study. After random allocation, it was not possible to conceal the test or control intervention from physicians or their patients.
Hospital physicians underwent training on the software or usual care discharge process; the details appeared previously.7 Physicians assigned to usual care did not receive training on the discharge software and were blocked from using the software. Patients were passive recipients of the research intervention performed by their discharging physician. Patients received the research intervention on the day of discharge of the index hospitalization.
Baseline Assessment
During the index hospitalization, trained data abstractors recorded baseline patient demographic data plus variables to calculate the Pra for probability for repeat admission. We recorded the availability of an informal caregiver in response to the question, Is there a friend, relative, or neighbor who would take care of you for a few days, if necessary? Data came from the patient or proxy for physical functioning, mental health,13 heart failure, and number of previous emergency department visits. Other data came from hospital records for chronic obstructive pulmonary disease, number of discharge medications, and length of stay for the index hospitalization.
Outcome Assessment
We assessed the patient's perception of the discharge with 2 validated survey instruments. One week after discharge, research personnel performed telephone interviews with patients or proxies. While following a script, interviewers instructed patients to avoid mentioning the discharge process. Interviewers read items from the B‐PREPARED questionnaire.14, 15 and the Satisfaction with Information About Medicines Scale (SIMS).16 The B‐PREPARED scale assessed 3 principal components of patient preparedness for discharge: self‐care information for medications and activities, equipment and services, and confidence. The scale demonstrated internal consistency, construct validity, and predictive validity. High scale values reflected high perceptions of discharge preparedness from the patient perspective.15 SIMS measured patient satisfaction with information about discharge medications. Validation studies revealed SIMS had internal consistency, test‐retest reliability, and criterion‐related validity.16 Interviewers recorded responses to calculate a total SIMS score. Patients with high total SIMS scores had high satisfaction. While assessing B‐PREPARED and SIMS, interviewers were blind to intervention assignment. We evaluated the adequacy of blinding by asking interviewers to guess the patient's intervention assignment.
We measured the quality of hospital discharge from the outpatient physician perspective. During the index hospitalization, patients designated an outpatient primary care practitioner to receive discharge reports and results of diagnostic tests. Ten days after discharge, research personnel mailed the Physician‐PREPARED questionnaire to the designated community practitioner.17 The sum of item responses comprised the Modified Physician‐PREPARED scale and demonstrated internal consistency and construct validity. The principal components of the Modified Physician‐PREPARED were timeliness of communication and adequacy of discharge plan/transmission. High scale values reflected high perceptions of discharge quality.17 Outpatient practitioners gave implied consent when they completed and returned questionnaires. We requested 1 questionnaire for each enrolled patient, so the outcome assessment was at the patient level. The assessment was not blinded because primary care physicians received the output of discharge software or usual care discharge.
We assessed the satisfaction of hospital physicians who used the discharge software and the usual care. After hospital physicians participated in the trial for 6 months, they rated their assigned discharge process on Likert scales. The first question was, On a scale of 1 to 10, indicate your satisfaction with your portion of the discharge process. The scale anchors were 1 for very dissatisfied and 10 for very satisfied. The second question was, On a scale of 1 to 10, indicate the effort to complete your portion of the discharge process. For the second question, the scale anchors were 1 for very difficult and 10 for very easy. It was not possible to mask the hospital physicians after they received their intervention assignment. Consequently, their outcome assessment was not blinded.
Statistical Methods
The cluster number and size were selected to maintain test significance level, 1‐sided alpha less than 0.05, and power greater than 80%. We previously published the assumptions and rationale for 35 hospital physician clusters per intervention and 9 patients per cluster.7 We did not perform separate sample size estimates for the secondary outcomes reported herein.
The statistical analyses employed SPSS PC (Version 15.0.1; SPSS, Inc., Chicago, IL). Statistical procedures for baseline variables were descriptive and included means and standard deviations (SDs) for interval variables and percentages for categorical variables. For all analyses, we employed the principle of intention‐to‐treat. We assumed patient or physician exposure to the intervention randomly assigned to the discharging physician. Analyses employed standard tests for normal distribution, homogeneity of variance, and linearity of relationships between independent and dependent variables. If assumptions failed, then we stratified variables or performed transformations. We accepted P < 0.05 as significant.
We tested hypotheses for patient‐level outcomes with generalized estimating equations (GEEs) that corrected for clustering by hospital physician. We employed GEEs because they provide unbiased estimates of standard errors for parameters even with incorrect specification of the intracluster dependence structure.18 Each patient‐level outcome was the dependent variable in a separate GEE. The intervention variable for each GEE was discharge software vs. usual care, handwritten discharge. The statistic of interest was the coefficient for the intervention variable. The null hypothesis was no difference between discharge software and usual care. The statistical definition of the null hypothesis was an intervention variable coefficient with a 95% confidence interval (CI) that included 0.
For analyses that were unaffected by the cluster assumption, we performed standard tests. The hypothesis for hospital physicians was significantly higher satisfaction for discharge software users and the inferential procedure was the t test. When we assessed the success of the study blinding, we assumed no association between true intervention allocation and guesses by outcome assessors. We used chi‐square for assessment of the blinding.
Results
We screened 127 physicians who were general internal medicine hospital physicians. Seventy physicians consented and received random allocation to discharge software or usual care. We excluded 57 physicians for reasons shown in the trial flow diagram (Figure 1). We approached 6884 patients during their index hospitalization. After excluding 6253 ineligible patients, we enrolled 631 willing patients (Supplementary Appendix). As depicted in Figure 1, the most common reason for ineligibility occurred for patients with Pra score <0.40 (2168/6253 exclusions; 34.7%). We followed 631 patients who received the discharge intervention (Figure 1). There was no differential dropout between the interventions. Protocol deviations were rare, 0.5% (3/631). Three patients erroneously received usual care discharge from physicians assigned to discharge software. All 631 patients were included in the intention‐to‐treat analysis. The baseline characteristics of the randomly assigned hospital physicians and their patients are in Table 1. Most of the hospital physicians were residents in the first year of postgraduate training.

| Discharge Software | Usual Care | |
|---|---|---|
| ||
| Hospital physician characteristics, n (%) | n = 35 | n = 35 |
| Postgraduate year 1 | 18 (51.4) | 23 (65.7) |
| Postgraduate years 2‐4 | 10 (28.6) | 7 (20.0) |
| Attending physician | 7 (20.0) | 5 (14.3) |
| Patient characteristics, n (%) | n = 316 | n = 315 |
| Gender, male | 136 (43.0) | 147 (46.7) |
| Age, years | ||
| 18‐44 | 68 (21.5) | 95 (30.2) |
| 45‐54 | 79 (25.0) | 76 (24.1) |
| 55‐64 | 86 (27.2) | 74 (23.5) |
| 65‐98 | 83 (26.3) | 70 (22.2) |
| Self‐rated health status | ||
| Poor | 82 (25.9) | 108 (34.3) |
| Fair | 169 (53.5) | 147 (46.7) |
| Good | 54 (17.1) | 46 (14.6) |
| Very good | 10 (3.2) | 11 (3.5) |
| Excellent | 1 (0.3) | 3 (1.0) |
| Diabetes mellitus | 172 (54.4) | 177 (56.2) |
| Chronic obstructive pulmonary disease | ||
| None | 259 (82.0) | 257 (81.6) |
| Without oral steroid or home oxygen | 28 (8.9) | 26 (8.3) |
| With chronic oral steroid | 10 (3.2) | 8 (2.5) |
| With home oxygen oral steroid | 19 (6.0) | 24 (7.6) |
| Coronary heart disease | 133 (42.1) | 120 (38.1) |
| Heart failure | 80 (25.3) | 67 (21.3) |
| Physical Functioning from SF‐36 | ||
| Lowest third | 128 (40.5) | 121 (38.4) |
| Upper two‐thirds | 188 (59.5) | 194 (61.6) |
| Mental Health from SF‐36 | ||
| Lowest one‐third | 113 (35.8) | 117 (37.1)* |
| Upper two‐thirds | 203 (64.2) | 197 (62.5)* |
| Emergency department visits during 6 months before index admission | ||
| 0 or 1 | 194 (61.4) | 168 (53.3) |
| 2 or more | 122 (38.6) | 147 (46.7) |
| Mean (SD) | ||
| Number of discharge medications | 10.5 (4.8) | 9.9 (5.1) |
| Index hospital length of stay, days | 3.9 (3.5) | 3.5 (3.5) |
| Pra | 0.486 (0.072) | 0.495 (0.076) |
We assessed the patient's perception of discharge preparedness. One week after discharge, research personnel interviewed 92.4% (292/316) of patients in the discharge software group and 92.4% (291/315) in the usual care group. The mean (SD) B‐PREPARED scores for discharge preparedness were 17.7 (4.1) in the discharge software group and 17.2 (4.0) in the usual care group. In the generalized estimating equation that accounted for potential clustering within hospital physicians, the parameter estimate for the intervention variable coefficient was small but significant (P = 0.042; Table 2). Patients in the discharge software group had slightly better perceptions of their discharge preparedness.
| Outcome Variable | Discharge Software [mean (SD)] | Usual Care [mean (SD)] | Parameter Estimate Without Cluster Correction (95% CI) | P Value | Parameter Estimate with Cluster Correction (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Patient perception of discharge preparedness (B‐PREPARED) | 17.7 (4.1) | 17.2 (4.0) | 0.147* (0.006‐0.288) | 0.040 | 0.147* (0.005‐0.289) | 0.042 |
| Patient satisfaction with medication information score (SIMS) | 12.3 (4.8) | 12.1 (4.6) | 0.212 (0.978‐0.554) | 0.587 | 0.212 (0.937‐0.513) | 0.567 |
| Outpatient physician perception (Modified Physician‐PREPARED) | 17.2 (3.8) | 16.5 (3.9) | 0.133 (0.012‐0.254) | 0.031 | 0.133 (0.015‐0.251) | 0.027 |
Another outcome was the patient's satisfaction with information about discharge medications (Table 2). One week after discharge, mean (SD) SIMS scores for satisfaction were 12.3 (4.8) in the discharge software group and 12.1 (4.6) in the usual care group. The generalized estimating equation revealed an insignificant coefficient for the intervention variable (P = 0.567; Table 2).
We assessed the outpatient physician perception of the discharge with a questionnaire sent 10 days after discharge. We received 496 out of 631 questionnaires (78.6%) from outpatient practitioners and the median response time was 19 days after the date of discharge. The practitioner specialty was internal medicine for 38.9% (193/496), family medicine for 27.2% (135/496), medicine‐pediatrics for 27.0% (134/496), advance practice nurse for 4.4% (22/496), other physician specialties for 2.0% (10/496), and physician assistant for 0.4% (2/496). We excluded 18 questionnaires from analysis because outpatient practitioners failed to answer 2 or more items in the Modified Physician‐PREPARED scale. When we compared baseline characteristics for patients who had complete questionnaires vs. patients with nonrespondent or excluded questionnaires, we found no significant differences (data available upon request). Among the discharge software group, 72.2% (228/316) of patients had complete questionnaires from their outpatient physicians. The response rate with complete questionnaires was 79.4% (250/315) of patients assigned to usual care. On the Modified Physician‐PREPARED scale, the mean (SD) scores were 17.2 (3.8) for the discharge software group and 16.5 (3.9) for the usual care group. The parameter estimate from the generalized estimating equation was significant (P = 0.027; Table 2). Outpatient physicians had slightly better perception of discharge quality for patients assigned to discharge software.
In the questionnaire sent to outpatient practitioners, we requested additional information about discharge communication. When asked about timeliness, outpatient physicians perceived no faster communication with the discharge software (Table 3). We asked about the media for discharge information exchange. It was uncommon for community physicians to receive discharge information via electronic mail (Table 3). Outpatient physicians acknowledged receipt of a minority of facsimile transmissions with no significant difference between discharge software vs. usual care (Table 3). Investigators documented facsimile transmission of the output from the discharge software to outpatient practitioners. Transmission occurred on the first business day after discharge. Despite the documentation of all facsimile transmissions, only 23.4% of patients assigned to discharge software had community practitioners who acknowledged receipt.
| Discharge Software (n = 316) [n (%)] | Usual Care (n = 315) [n (%)] | |
|---|---|---|
| ||
| Question: How soon after discharge did you receive any information (in any form) relating to this patient's hospital admission and discharge plans? | ||
| Within 1‐2 days | 72 (22.8) | 55 (17.5) |
| Within 1 week | 105 (33.2) | 125 (39.7) |
| Longer than 1 week | 36 (11.4) | 41 (13.0) |
| Not received | 20 (6.3) | 26 (8.3) |
| Other | 4 (1.3) | 7 (2.2) |
| Question: How did you receive discharge health status information? (Check all that apply)* | ||
| Written/typed letter | 106 (33.5) | 89 (28.3) |
| Telephone call | 82 (25.9) | 67 (21.3) |
| Fax (facsimile transmission) | 74 (23.4) | 90 (28.6) |
| Electronic mail | 8 (2.5) | 23 (7.3) |
| Other | 15 (4.7) | 15 (4.8) |
In exploratory analyses, we evaluated the effect of hospital physician level of training. We wondered if discharging physician experience or seniority affected perceptions of patients or primary care physicians. We entered level of training as a covariate in generalized estimating equations. When patient perception of discharge preparedness (B‐PREPARED) was the dependent variable, then physician level of training had a nonsignificant coefficient (P > 0.219). Likewise, physician level of training was nonsignificant in models of patient satisfaction with medication information, SIMS (P > 0.068), and outpatient physician perception, Modified Physician‐PREPARED (P > 0.177). We concluded that physician level of training had no influence on the patient‐level outcomes assessed in our study.
We compared the satisfaction of hospital physicians who used the discharge software and the usual care discharge. The proportions of hospital physicians who returned questionnaires were 85.7% (30/35) in the discharge software group and 97% (34/35) in the usual care group. After using their assigned discharge process for at least 6 months, discharge software users had mean (SD) satisfaction 7.4 (1.4) vs. 7.9 (1.4) for usual care physicians (difference = 0.5; 95% CI = 0.2‐1.3; P = 0.129). The effort for discharge software users was more difficult than the effort for usual care (mean [SD] effort = 6.5 [1.9] vs. 7.9 [2.1], respectively). The mean difference in effort was significant (difference = 1.4; 95% CI = 0.3‐2.4; P = 0.011). We reviewed free‐text comments on hospital physician questionnaires. The common theme was software users spent more time to complete discharges. We did not perform time‐motion assessments so we cannot confirm or refute these impressions. Even though hospital physicians found the discharge software significantly more difficult, they did not report a significant decrease in their satisfaction between the 2 discharge interventions.
The cluster design of our trial assumed variance in outcomes measured at the patient level. We predicted some variance attributable to clustering by hospital physician. After the trial, we calculated the intracluster correlation coefficients for B‐PREPARED, SIMS, and Modified Physician‐PREPARED. For all of these outcome variables, the intracluster correlation coefficients were negligible. We also evaluated generalized estimating equations with and without correction for hospital physician cluster. We confirmed the negligible cluster effect on CIs for intervention coefficients (Table 2).
We evaluated the adequacy of the blind for outcome assessors who interviewed patients for B‐PREPARED and SIMS. The guesses of outcomes assessors were unrelated to true intervention assignment (P = 0.253). We interpreted the blind as adequate.
Discussion
We performed a cluster‐randomized clinical trial to measure the effects of discharge software vs. usual care discharge. The discharge software incorporated the ASTM (American Society for Testing and Material) Continuity of Care Record (CCR) standards.19 The CCR is a patient health summary standard with widespread support from medical and specialty organizations. The rationale for the CCR was the need for continuity of care from 1 provider or practitioner to any other practitioner. Our discharge software had the same rationale as the CCR and included a subset of the clinical content specified by the CCR. Like the CCR, our discharge software produced concise reports, and emphasized a brief, postdischarge, care plan. Since we included clinical data elements recommended by the CCR, we hypothesized our discharge software would produce clinically relevant improvements.
Our discharge software also implemented elements of high‐quality discharge planning and communication endorsed by the Society of Hospital Medicine.20 For example, the discharge software produced a legible, typed, discharge plan for the patient or caregiver with medication instructions, follow‐up tests, studies, and appointments. The discharge software generated a discharge summary for the outpatient primary care physician and other clinicians who provided postdischarge care. The summary included discharge diagnoses, key findings and test results, follow‐up appointments, pending diagnostic tests, documentation of patient education, reconciled medication list, and contact information for the hospital physician. The discharge software compiled data for purposes of benchmarking, measurement, and continuous quality improvement. We thought our implementation of discharge software would lead to improved outcomes.
Despite our deployment of recommended strategies, we detected only small increases in patient perceptions of discharge preparedness. We do not know if small changes in B‐PREPARED values were clinically important. We found no improvement in patient satisfaction with medication information. Our results are consistent with systematic reviews that revealed limited benefit of interventions other than discharge planning with postdischarge support.21 Since our discharge software was added to robust discharge planning and support, we possibly had limited ability to detect benefit unless the intervention had a large effect size.
Our discharge software caused a small increase in positive perception reported by outpatient physicians. Small changes in the Modified Physician‐PREPARED had uncertain clinical relevance. Potential delays imposed by our distribution method may have contributed to our findings. Output from our discharge software went to community physicians via facsimile transmission with backup copies via standard U.S. mail. Our distribution system responded to several realities. Most community physicians in our area had no access to interoperable electronic medical records or secured e‐mail. In addition, electronic transmittal of prescriptions was not commonplace. Our discharge intervention did not control the flow of information inside the offices of outpatient physicians. We did not know if our facsimile transmissions joined piles of unread laboratory and imaging reports on the desks of busy primary care physicians. Despite the limited technology available to community physicians, they perceived communication generated by the software to be an improvement over the handwritten process. Our results support previous studies in which physicians preferred computer‐generated discharge summaries and summaries in standardized formats.2224
One of the limitations of our trial design was the unmasked intervention. Hospital physicians assigned to usual care might have improved their handwritten and verbal discharge communication after observation of their colleagues assigned to discharge software. This phenomenon is encountered in unmasked trials and is called contamination. We attempted to minimize contamination when we blocked usual care physicians from access to the discharge software. However, we could not eliminate cross‐talk among unmasked hospital physicians who worked together in close proximity during 27 months of patient enrollment. Some contamination was inevitable. When contamination occurred, there was bias toward the null (increased type II error).
Another limitation was the large proportion of hospital physicians in the first year of postgraduate training. There was a potential for variance from multilevel clusters with patient‐level outcomes clustered within first‐year hospital physicians who were clustered within teams supervised by senior resident or attending physicians. Our results argued against hierarchical clusters because intracluster correlation coefficients were negligible. Furthermore, our exploratory analysis suggested physician training level had no influence on patient outcomes measured in our study. We speculate the highly structured discharge process for both usual care and software minimized variance attributable to physician training level.
The research intervention in our trial was a stand‐alone software application. The discharge software did not integrate with the hospital electronic medical record. Consequently, hospital physician users had to reenter patient demographic data and prescription data that already existed in the electronic record. Data reentry probably caused hospital physicians to attribute greater effort to the discharge software.
In our study, hospital physicians incorporated discharge software with CPOE into their clinical workflow without deterioration in their satisfaction. Our experience may inform the decisions of hospital personnel who design health information systems. When designing discharge functions, developers should consider medication reconciliation and the standards of the CCR.19 Modules within the discharge software would likely be more efficient with prepopulated data from the electronic record. Then users could shift their work from data entry to data verification and possibly mitigate their perceived effort. Software developers may wish to explore options for data transmission to community physicians: secure e‐mail, automated fax servers, and direct digital file transfer. Future studies should test the acceptability of discharge functions incorporated within electronic health records with robust clinical decision support.
Our results apply to a population of adults of all ages with high risk for readmission. The results may not generalize to children, surgical patients, or people with low risk for readmission. All of the patients in our study were discharged to home. The exclusion of other discharge destinations helped us to enroll a homogenous cohort. However, the exclusion criteria did not allow us to generalize our results to patients discharged to nursing homes, inpatient rehabilitation units, or other acute care facilities. We designed the intervention to apply to the hospitalist model, in which responsibility for patient care transitions to a different physician after discharge. The results of our study do not apply when the inpatient and outpatient physician are the same. Since we enrolled general internal medicine hospital physicians, our results may not generalize to care provided by other specialists.
Conclusions
A discharge software application with CPOE improved perceptions of the hospital discharge process for patients and their outpatient physicians. When compared to the handwritten discharge process, the improvements were small in magnitude. Hospital physicians who used the discharge software reported more effort but otherwise no decrement in their satisfaction with the discharge process.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2004;(1):CD000313.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,.Software design to facilitate information transfer at hospital discharge.Inform Prim Care.2006;14:109–119.
- ,.Computer physician order entry: benefits, costs, and issues.Ann Intern Med.2003;139:31–39.
- ,,, et al.Patient readmissions, emergency visits, and adverse events after software‐assisted discharge from hospital: cluster randomized trial.J Hosp Med.2009; DOI: 10.1002/jhm.459. PMID: 19479782.
- ,,.Predictive validity of a questionnaire that identifies older persons at risk for hospital admission.J Am Geriatr Soc.1995;43:374–377.
- ,.Prediction of early readmission in medical inpatients using the Probability of Repeated Admission instrument.Nurs Res.2008;57:406–415.
- ,.The ten point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients.Int J Psychiatry Med.1994;24:229–244.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- .SF‐36 health survey update.Spine.2000;25:3130–3139.
- ,.The development, validity and application of a new instrument to assess the quality of discharge planning activities from the community perspective.Int J Qual Health Care.2001;13:109–116.
- ,,.Brief scale measuring patient preparedness for hospital discharge to home: psychometric properties.J Hosp Med.2008;3:446–454.
- ,,.The Satisfaction with Information about Medicines Scale (SIMS): a new measurement tool for audit and research.Qual Health Care.2001;10:135–140.
- ,,.Discharge planning scale: community physicians' perspective.J Hosp Med.2008;3:455–464.
- ,.An introduction to generalized estimating equations and an application to assess selectivity effects in a longitudinal study on very old individuals.J Educ Behav Stat.2004;29:421–437. Available at: http://jeb.sagepub.com/cgi/content/abstract/29/4/421. Accessed June 2009.
- ASTM. E2369‐05 Standard Specification for Continuity of Care Record (CCR). Available at: http://www.astm.org/Standards/E2369.htm. Accessed June2009.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1:354–360.
- ,,.Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta‐review.BMC Health Serv Res.2007;7:47.
- ,,,,,.Evaluation of a computer‐generated discharge summary for patients with acute coronary syndromes.Br J Gen Pract.1998;48:1163–1164.
- ,,,.Standardized or narrative discharge summaries. Which do family physicians prefer?Can Fam Physician.1998;44:62–69.
- ,,,.Dictated versus database‐generated discharge summaries: a randomized clinical trial.CMAJ.1999;160:319–326.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,,.Discharge planning from hospital to home.Cochrane Database Syst Rev.2004;(1):CD000313.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,.Software design to facilitate information transfer at hospital discharge.Inform Prim Care.2006;14:109–119.
- ,.Computer physician order entry: benefits, costs, and issues.Ann Intern Med.2003;139:31–39.
- ,,, et al.Patient readmissions, emergency visits, and adverse events after software‐assisted discharge from hospital: cluster randomized trial.J Hosp Med.2009; DOI: 10.1002/jhm.459. PMID: 19479782.
- ,,.Predictive validity of a questionnaire that identifies older persons at risk for hospital admission.J Am Geriatr Soc.1995;43:374–377.
- ,.Prediction of early readmission in medical inpatients using the Probability of Repeated Admission instrument.Nurs Res.2008;57:406–415.
- ,.The ten point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients.Int J Psychiatry Med.1994;24:229–244.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166:565–571.
- .SF‐36 health survey update.Spine.2000;25:3130–3139.
- ,.The development, validity and application of a new instrument to assess the quality of discharge planning activities from the community perspective.Int J Qual Health Care.2001;13:109–116.
- ,,.Brief scale measuring patient preparedness for hospital discharge to home: psychometric properties.J Hosp Med.2008;3:446–454.
- ,,.The Satisfaction with Information about Medicines Scale (SIMS): a new measurement tool for audit and research.Qual Health Care.2001;10:135–140.
- ,,.Discharge planning scale: community physicians' perspective.J Hosp Med.2008;3:455–464.
- ,.An introduction to generalized estimating equations and an application to assess selectivity effects in a longitudinal study on very old individuals.J Educ Behav Stat.2004;29:421–437. Available at: http://jeb.sagepub.com/cgi/content/abstract/29/4/421. Accessed June 2009.
- ASTM. E2369‐05 Standard Specification for Continuity of Care Record (CCR). Available at: http://www.astm.org/Standards/E2369.htm. Accessed June2009.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1:354–360.
- ,,.Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta‐review.BMC Health Serv Res.2007;7:47.
- ,,,,,.Evaluation of a computer‐generated discharge summary for patients with acute coronary syndromes.Br J Gen Pract.1998;48:1163–1164.
- ,,,.Standardized or narrative discharge summaries. Which do family physicians prefer?Can Fam Physician.1998;44:62–69.
- ,,,.Dictated versus database‐generated discharge summaries: a randomized clinical trial.CMAJ.1999;160:319–326.
Copyright © 2009 Society of Hospital Medicine
H1N1 Pandemic Should Increase Adult Vaccination Awareness
The H1N1 pandemic has contributed to more than 200 U.S. deaths and the Center for Disease Control reports more than 37,000 probable or confirmed cases nationwide, which highlights the need for increased education for adult immunizations.
The H1N1 vaccination is expected to be available this fall and has attracted the attention of inpatient and outpatient physicians alike, says hospitalist and infectious-disease specialist John J. Ross, MD, of Brigham and Women's Hospital in Boston. Dr. Ross expects a spike in adult vaccinations similar to the increase witnessed after the Sept. 11 attacks. "You saw an increase in vaccinations because of increased panic," he says. "I expect that to be the same this fall with the introduction of the swine flu shot.”
The American College of Physicians (ACP) and the Infectious Diseases Society of America (IDSA) also express the need for heightened awareness. The medical groups recently issued a joint statement calling for a "renewed and stronger" emphasis on adult vaccinations. The statement, also supported by SHM, emphasizes an increase in patient education and documentation. It suggests hospital workers review and update their own immunizations, with particular attention to annual influenza immunizations.
Though most vaccinations are administered in the outpatient setting, hospitalists are required to update their patients’ vaccination status. "While hospitalists are doing a good job of handling pneumonia and influenza vaccinations, they could do a better job of handling some of the less common disease vaccinations,” says Ross.
Dr. Ross says hospitalists should remain knowledgeable in all of the immunization categories, a complete list of which is available at the CDC Web site. "The more knowledgeable the hospitalist is on vaccinations, the more they can educate their patients," he says.
The H1N1 pandemic has contributed to more than 200 U.S. deaths and the Center for Disease Control reports more than 37,000 probable or confirmed cases nationwide, which highlights the need for increased education for adult immunizations.
The H1N1 vaccination is expected to be available this fall and has attracted the attention of inpatient and outpatient physicians alike, says hospitalist and infectious-disease specialist John J. Ross, MD, of Brigham and Women's Hospital in Boston. Dr. Ross expects a spike in adult vaccinations similar to the increase witnessed after the Sept. 11 attacks. "You saw an increase in vaccinations because of increased panic," he says. "I expect that to be the same this fall with the introduction of the swine flu shot.”
The American College of Physicians (ACP) and the Infectious Diseases Society of America (IDSA) also express the need for heightened awareness. The medical groups recently issued a joint statement calling for a "renewed and stronger" emphasis on adult vaccinations. The statement, also supported by SHM, emphasizes an increase in patient education and documentation. It suggests hospital workers review and update their own immunizations, with particular attention to annual influenza immunizations.
Though most vaccinations are administered in the outpatient setting, hospitalists are required to update their patients’ vaccination status. "While hospitalists are doing a good job of handling pneumonia and influenza vaccinations, they could do a better job of handling some of the less common disease vaccinations,” says Ross.
Dr. Ross says hospitalists should remain knowledgeable in all of the immunization categories, a complete list of which is available at the CDC Web site. "The more knowledgeable the hospitalist is on vaccinations, the more they can educate their patients," he says.
The H1N1 pandemic has contributed to more than 200 U.S. deaths and the Center for Disease Control reports more than 37,000 probable or confirmed cases nationwide, which highlights the need for increased education for adult immunizations.
The H1N1 vaccination is expected to be available this fall and has attracted the attention of inpatient and outpatient physicians alike, says hospitalist and infectious-disease specialist John J. Ross, MD, of Brigham and Women's Hospital in Boston. Dr. Ross expects a spike in adult vaccinations similar to the increase witnessed after the Sept. 11 attacks. "You saw an increase in vaccinations because of increased panic," he says. "I expect that to be the same this fall with the introduction of the swine flu shot.”
The American College of Physicians (ACP) and the Infectious Diseases Society of America (IDSA) also express the need for heightened awareness. The medical groups recently issued a joint statement calling for a "renewed and stronger" emphasis on adult vaccinations. The statement, also supported by SHM, emphasizes an increase in patient education and documentation. It suggests hospital workers review and update their own immunizations, with particular attention to annual influenza immunizations.
Though most vaccinations are administered in the outpatient setting, hospitalists are required to update their patients’ vaccination status. "While hospitalists are doing a good job of handling pneumonia and influenza vaccinations, they could do a better job of handling some of the less common disease vaccinations,” says Ross.
Dr. Ross says hospitalists should remain knowledgeable in all of the immunization categories, a complete list of which is available at the CDC Web site. "The more knowledgeable the hospitalist is on vaccinations, the more they can educate their patients," he says.
In the Literature: Research You Need to Know
Clinical question: Can one or both antiplatelet agents be held in a patient requiring surgery who is on dual antiplatelet therapy to manage a drug-eluting stent?
Background: Data suggest that cessation of antiplatelet therapy within one year of placement of a drug-eluting stent, even for brief time periods, is associated with rapid development of stent thrombosis (ST). However, continuation of antiplatelet agents increases perioperative risk related to hemostasis. No published data is available regarding how long one or both antiplatelet agents can be stopped to allow for safer surgery.
Study design: Case review.
Setting: Cases identified through literature search.
Synopsis: In patients with drug-eluting stents, stopping aspirin presents more risk than stopping thienopyridines. Patients who stopped both antiplatelet agents had ST at seven days (median time to event). If a patient stopped thienopyridines but not aspirin, mean time to ST was 122 days. If a patient stopped thienopyridines then subsequently stopped aspirin, the median time to ST was seven days. This suggests a potential seven-day window when both agents might be stopped, and a longer window when thienopyridines might be stopped if aspirin is continued.
The study only looked at ST cases, not the incidence of ST in all patients undergoing surgery for drug-eluting stents while on dual-antiplatelet therapy, so these results only describe the small fraction of patients who actually have surgery when antiplatelet agents are stopped.
The study design, analyzing existing cases without controls, is limited by the infrequency of the event, resulting in huge sample sizes needed to detect a significant result. The study also describes a median time to ST, which does not provide a true window of protection for the entire duration of the window.
Bottom line: The risks of bleeding complications need to be weighed against the risk of stent thrombosis in individual patients. This study also suggests that the risk of thrombosis is lower if a patient is continued on aspirin while thienopyridines are held.
Citation Eisenberg MJ, Richard PR, Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation. 2009;119:1634-1642.
Reviewed for TH eWire by Erin A. Egan, MD, JD, Ethan Cumbler, MD, Jodie Grout, RN, MS, ANP-C, Jeannette Guerrasio, MD, Nichole Zehnder, MD, and Judy Zerzan, MD, MPH, Hospital Medicine Group, University of Colorado Denver.
Clinical question: Can one or both antiplatelet agents be held in a patient requiring surgery who is on dual antiplatelet therapy to manage a drug-eluting stent?
Background: Data suggest that cessation of antiplatelet therapy within one year of placement of a drug-eluting stent, even for brief time periods, is associated with rapid development of stent thrombosis (ST). However, continuation of antiplatelet agents increases perioperative risk related to hemostasis. No published data is available regarding how long one or both antiplatelet agents can be stopped to allow for safer surgery.
Study design: Case review.
Setting: Cases identified through literature search.
Synopsis: In patients with drug-eluting stents, stopping aspirin presents more risk than stopping thienopyridines. Patients who stopped both antiplatelet agents had ST at seven days (median time to event). If a patient stopped thienopyridines but not aspirin, mean time to ST was 122 days. If a patient stopped thienopyridines then subsequently stopped aspirin, the median time to ST was seven days. This suggests a potential seven-day window when both agents might be stopped, and a longer window when thienopyridines might be stopped if aspirin is continued.
The study only looked at ST cases, not the incidence of ST in all patients undergoing surgery for drug-eluting stents while on dual-antiplatelet therapy, so these results only describe the small fraction of patients who actually have surgery when antiplatelet agents are stopped.
The study design, analyzing existing cases without controls, is limited by the infrequency of the event, resulting in huge sample sizes needed to detect a significant result. The study also describes a median time to ST, which does not provide a true window of protection for the entire duration of the window.
Bottom line: The risks of bleeding complications need to be weighed against the risk of stent thrombosis in individual patients. This study also suggests that the risk of thrombosis is lower if a patient is continued on aspirin while thienopyridines are held.
Citation Eisenberg MJ, Richard PR, Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation. 2009;119:1634-1642.
Reviewed for TH eWire by Erin A. Egan, MD, JD, Ethan Cumbler, MD, Jodie Grout, RN, MS, ANP-C, Jeannette Guerrasio, MD, Nichole Zehnder, MD, and Judy Zerzan, MD, MPH, Hospital Medicine Group, University of Colorado Denver.
Clinical question: Can one or both antiplatelet agents be held in a patient requiring surgery who is on dual antiplatelet therapy to manage a drug-eluting stent?
Background: Data suggest that cessation of antiplatelet therapy within one year of placement of a drug-eluting stent, even for brief time periods, is associated with rapid development of stent thrombosis (ST). However, continuation of antiplatelet agents increases perioperative risk related to hemostasis. No published data is available regarding how long one or both antiplatelet agents can be stopped to allow for safer surgery.
Study design: Case review.
Setting: Cases identified through literature search.
Synopsis: In patients with drug-eluting stents, stopping aspirin presents more risk than stopping thienopyridines. Patients who stopped both antiplatelet agents had ST at seven days (median time to event). If a patient stopped thienopyridines but not aspirin, mean time to ST was 122 days. If a patient stopped thienopyridines then subsequently stopped aspirin, the median time to ST was seven days. This suggests a potential seven-day window when both agents might be stopped, and a longer window when thienopyridines might be stopped if aspirin is continued.
The study only looked at ST cases, not the incidence of ST in all patients undergoing surgery for drug-eluting stents while on dual-antiplatelet therapy, so these results only describe the small fraction of patients who actually have surgery when antiplatelet agents are stopped.
The study design, analyzing existing cases without controls, is limited by the infrequency of the event, resulting in huge sample sizes needed to detect a significant result. The study also describes a median time to ST, which does not provide a true window of protection for the entire duration of the window.
Bottom line: The risks of bleeding complications need to be weighed against the risk of stent thrombosis in individual patients. This study also suggests that the risk of thrombosis is lower if a patient is continued on aspirin while thienopyridines are held.
Citation Eisenberg MJ, Richard PR, Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation. 2009;119:1634-1642.
Reviewed for TH eWire by Erin A. Egan, MD, JD, Ethan Cumbler, MD, Jodie Grout, RN, MS, ANP-C, Jeannette Guerrasio, MD, Nichole Zehnder, MD, and Judy Zerzan, MD, MPH, Hospital Medicine Group, University of Colorado Denver.
A Sickle Cell Primer
Sickle cell anemia is a prototypical single gene deletion disorder familiar to medical students everywhere. Most physicians recall that it is a devastating disorder that starts with a single substitution of valine for glutamic acid in the beta-globin gene of the hemoglobin molecule, rendering the hemoglobin molecule unstable in its de-oxygenated state. This leads to polymerization of hemoglobin molecules within the red cell, deformation of the cell membrane, and sickling. Sickling, in turn, causes blood vessel damage, vaso-occlusion, and various other physiologic effects, which lead to both micro- and macro-vascular protean complications.1
Despite extensive basic research in sickle cell disease (SCD), clinical research into the optimal management of this complex disease has lagged behind. Many adult hospitalists may be unfamiliar with the care of adult SCD patients because most of these patients are cared for in academic centers by hematologists.
In this article we review the common complications of sickle cell anemia in adult patients, the management of associated conditions, and the evidence base for treatment guidelines.
Overview
Sickle cell anemia is not just one disorder, but is, rather, a collection of related disorders involving mutations of the hemoglobin molecule. (These disorders are categorized in Table 1, p. 40) In the United States, SCD primarily affects black Americans, with about 9% of this population having sickle trait.2 One in 600 black Americans has sickle cell anemia, also known as hemoglobin SS disease, and there are an estimated 72,000 patients with SCD in the United States.2
In the early 1970s, the average life expectancy for patients with SCD was estimated to be 14.3 years.3 At that time, more than half of SCD patients died before age five, primarily from infectious complications such as pneumococcal sepsis. Twenty years later, the Cooperative Study of SCD documented a much better life expectancy: an average of 42 for males and 48 for females with homozygous S disease (the set of patients with two copies of the defective hemoglobin S mutation).
On average, patients with hemoglobin SCD routinely survive into their 60s. Many factors, including earlier diagnosis of SCD through universal screening programs, better vaccines and vaccination rates, prophylactic antibiotics in infancy and young childhood, and aggressive treatment of fever in infants, as well as other advances in care, have contributed to this progress. A longer life expectancy in this population has heralded the extension of common complications into adulthood, however, along with the emergence of other adult-specific complications of SCD. (See Table 2, p. 40.) In order to best treat adults with SCD, hospitalists should be aware of these complications, as well as of the advances that have been achieved.
Extension of Pediatric Complications
Perhaps the most common manifestation of SCD in children and adults is the painful crisis. This manifests in infants as a painful swelling in the digits of the hands and feet known as dactylitis. In older children and adults, pain occurs more often in the long bones of the arms and legs and in the sternum, vertebrae, and pelvic bones. Risk factors for painful crises include higher hematocrit values and higher sickle fractions, typically greater than 30%.4 Frequent painful crises are a marker of disease severity and an independent risk factor for death in SCD.
Hospitalists who regularly admit SCD patients are familiar with a subset of “frequent flyers” who experience recurrent painful crises. Remember that this is a small fraction of the total SCD population. In fact, 40% of SCD patients don’t suffer any painful crises requiring medical attention in a given year, and only 1% face more than six such events.5 Pain management in SCD is perhaps beyond the scope of this article, and there are no widely accepted guidelines. A few expert reviews are available for guidance, however.6,7
Treatment Options for Painful Crises
The most beneficial new treatment for painful crisis in the past two decades has been the use of hydroxyurea. After early studies showed promise, a phase III clinical trial in adults with SCD who suffered frequent painful crises (i.e., more than four per year) and who were given doses of up to 35 mg/kg/day of hydroxyurea showed a dramatic decrease in painful episodes. These patients also endured fewer episodes of acute chest syndrome, with a relative risk of 0.44, and experienced a reduced need for transfusions, with a relative risk of 0.64. This trial was stopped early (at 21 months average follow-up) due to the striking findings.8 Subsequent longer-term trials have not shown any of the feared theoretical complications of hydroxyurea, including cytopenias (when the production of one or more blood cell types ceases or is greatly reduced) or secondary malignancies.9 (See Table 3, p. 40.)
Childhood Complications
Some SCD complications are more prominent or more severe in early childhood than in adulthood. Two examples of this phenomenon are infectious complications and splenic sequestration. Pediatricians and parents of children with SCD live in fear of febrile illness because overwhelming infections such as pneumococcal sepsis remain the primary reason for death in children with SCD. This was especially true before Hemophilus influenza type b (Hib) and pneumococcal conjugate vaccines were introduced and before penicillin prophylaxis from birth through age five became universal.
Though functionally asplenic, adult patients are at a relatively low risk for overwhelming infections because their immune systems have matured enough to allow type-specific antibody production to polysaccharide antigens.7 Still, adults with SCD should be aggressively evaluated for infectious etiology of any febrile illness and routinely administered empiric antibiotics to cover strep species.
Splenic sequestration occurs when sickled cells are caught up in the spleen; this causes massive hemolysis, splenic enlargement, and cardiovascular compromise, and it is most common in SCD patients younger than five. Thankfully, this is rare in adults with SS and SB0Thal; however, the notable exception to this rule is in patients with hemoglobin SC disease or SB+Thal, where complete splenic infarction does not routinely occur.
Acute chest syndrome (ACS) is perhaps the most feared complication in both children and adults with SCD—and with good reason. It is the second most common cause for hospital admission in SCD patients, and it is an independent risk factor for death in SCD.7,10 Occurring more often in children, ACS is typically more severe in adults, in whom ACS can progress rapidly to an acute respiratory distress syndrome (ARDS)-like picture, with mortality reaching 5%-9%.11
ACS involves a classic triad of fever, chest pain, and new pulmonary infiltrates on chest X-ray, but patients are invariably hypoxic and dyspneic as well. The etiology of ACS varies. In one series, 54% of patients had an identified infectious pathogen, most commonly chlamydia or mycoplasma. Bronchoalveolar lavage (BAL) showed lipid-laden macrophages in about 16%, suggesting fat embolism after bony infarcts. The remainder of patients were presumed to have primary pulmonary infarctions.11 The treatment of ACS involves broad-spectrum antibiotics, especially atypical coverage, along with transfusion (simple versus exchange), supplemental oxygen, pain control, and judicious use of IV fluids.
The second most feared complication in SCD is stroke, which can be devastating in both children and young adults. Eleven percent of patients younger than 20 have ischemic strokes, with a risk of stroke that is 200 times higher than that of age-matched peers.9,12 The peak incidence of ischemic stroke occurs before 10 and after 30. These patients are also susceptible to hemorrhagic strokes, the incidence of which peaks in the third decade, for reasons that are unclear.13
Management of acute ischemic stroke in patients with SCD is similar to that used with general patients: antiplatelet therapy, careful attention to normo-glycemia and normovolemia, and maintenance of cerebral perfusion pressure. In addition, SCD patients should receive emergent transfusion to reduce the sickle fraction to less than 30%. Patients with prior transient ischemic attack (TIA) or stroke should be on a chronic monthly transfusion regimen. Also, based on the results of the 1997 STOP I trial (Stroke Prevention Trial in Sickle Cell Anemia), children with SCD should be screened with transcranial Doppler for high velocity flow (>200 cm/second) in the internal jugular and vertebral arteries.14 Children with high velocity flow who were treated with preventative transfusion regimens had a 90% absolute risk reduction in the incidence of first stroke.
In 2001-2006, a follow-up trial (STOP 2) revealed that among patients receiving at least 30 months of chronic transfusion to prevent a first stroke 39% of those randomized to discontinue transfusion had a reversion to high risk Doppler or suffered a stroke within an average of 4.5 months. This has led many to believe that prophylactic transfusion should be a lifelong treatment.15
Complications Seen Primarily in Adults
As the SCD population in this country has grown older, some previously uncommon complications have become more prominent. One predominantly adult complication of SCD is avascular necrosis of the femoral and humeral heads. Though clearly recognized in younger children in whom the incidence is estimated at about 3%, the major burden of femoral osteonecrosis is seen in adults older than 35, in whom prevalence reaches 50%.16 Necrosis of the humeral head can affect nearly 20% of SCD adults as well.17
It is important to recognize this complication as a new or different type of pain, separate from the vaso-occlusive pain usually experienced by SCD patients because it benefits from different therapies. Diagnosis by plain radiography is possible in the late stages, when evidence of remodeling, cystic changes, and sclerosis can be seen, but MRI has become the gold standard, with an estimated diagnostic accuracy of 90%.16 Conservative treatments include NSAIDs and steroid joint injections, but many afflicted patients may need orthopedic referral for joint replacement.
While acute chest syndrome remains a primary cause of mortality in SCD, adults with SCD are also at high risk for the chronic effects of pulmonary arterial hypertension (PAH). Thought to be uncommon in children, PAH affects up to one-third of adult SCD patients.18 Suspicion of this condition can be based on worsened fatigue, new resting hypoxemia, or increased painful crises, but experts advocate universal screening using transthoracic echocardiography. Patients with a tricuspid regurgitant jet velocity of 2.5 m/sec meet diagnostic criteria, and it is notable that the relative risk of death is 7.4 compared with SCD patients without PAH. This correlates with only moderate elevation of pulmonary pressures, suggesting that SCD patients tolerate PAH less well than other populations. Treatment options advocated include hydroxyurea, chronic transfusions, oxygen, pulmonary vasodilators such as prostacyclin and bosentan, and phosphodiesterase inhibitors such as sildenafil.
As SCD patients age, kidney disease is seen with increasing frequency, and three primary mechanisms are recognized. First, ischemic damage in the tubules causes tubular necrosis, which leads to hematuria.19 This condition can range from microscopic to severe gross hematuria, threatening urinary obstruction. Second, damage in the collecting duct impairs the body’s ability to concentrate urine, a condition that is called hyposthenuria. This condition makes SCD patients susceptible to dehydration, especially during physical exertion or in hot weather.
Interestingly, these first two mechanisms of kidney damage are also seen in patients with sickle cell trait. Finally, and most importantly, medullary interstitial fibrosis damages the glomerulus. Clinically, this is the most important mechanism because it leads to nephritic syndrome and chronic kidney disease as well as end-stage renal disease (ESRD).19
Priapism is the persistent, painful erection of the penis in post-pubertal SCD males not associated with sexual desire or relieved by orgasm; this condition is typically defined as lasting more than four to six hours.20 It is particularly common in younger males, with a yearly incidence of 6%-27%; the incidence of priapism in adults approaches 42%. In addition to being extremely painful, prolonged or repeated episodes can lead to impotence. Unfortunately, there are few well-studied treatment options; expert opinion suggests supportive care, including IV fluids, oxygen, ice, elevation, narcotic pain control, and even red cell transfusion in selected cases. Medications used include oral terbutaline, pseudoephedrine, and even stilboestrol, as well as injected phenylephrine, epinephrine, methylene blue, and tenecteplase (TNK-tPA). Surgical procedures attempted have included dorsal penile nerve block, cavernous aspiration, and the Winter procedure, which involves creating a vascular shunt from the corpora cavernosa to the glans penis. Urology consultation is often required for severe cases.
Several forms of eye disease occur in SCD patients. They can be broadly grouped into non-proliferative and proliferative diseases. In the former group, ocular trauma should be recognized as a visual emergency because patients are at risk for developing hyphema, occlusion of the trabeculae in the anterior chamber, and acute glaucoma. SCD patients of all ages are also at risk for acute retinal artery occlusion. Older SCD patients are at greatest risk, however, for a proliferative retinopathy similar to that seen in diabetes.21
Though red blood cell transfusions are helpful in many SCD patients, repeated transfusions can result in infections, immunologic consequences, and iron overload. (See Table 4, p. 41.) Infections that may result include parvovirus B19, HIV, human T-lymphotropic viruses (HTLV) I and II, and viral hepatitides. Immunologic consequences include alloimmunization, which occurs in up to 50% of SCD patients, and potentially fatal acute hemolytic reactions.22 Finally, patients who require frequent transfusions develop iron overload, which in turn causes fatigue, cardiomyopathy, diabetes mellitus, and cirrhosis. This necessitates iron chelators such as deferoxamine, which are disappointingly difficult to administer, requiring either IV or subcutaneous infusion over 12-24 hours daily. One bright note is the recent approval of deferasirox, an iron chelator that is taken orally once daily.
Conclusion
SCD is a complex genetic disease that affects multiple organs. Advances in medical care have increased longevity for SCD patients, and there are currently more adults living with the disease than ever before. Though patients often receive their care in academic centers, hospitalists may encounter them in routine practice. It is useful to have an understanding of the complications of SCD commonly seen in adults and to review the evidence base for care. TH
References
- Platt OS. Preventing stroke in sickle cell anemia. N Engl J Med. 2005 Dec 29;353(26): 2743-2745.
- Quinn CT, Rogers ZR, Buchanan GR. Survival of children with sickle cell disease. Blood. 2004 Jun 1;103(11):4023-4027.
- Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. [See comment.] N Engl J Med. 1994 Oct 13;331(15):1022-1023.
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease: rates and risk factors. N Engl J Med. 1991 Jul 4;325(1):11-16.
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. rates and risk factors. [See comment.] N Engl J Med. 1991;325(24):1747-1748.
- Ballas SK. Pain management of sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):785-802.
- National Institutes of Health. The Management of Sickle Cell Disease. 4th ed. Washington, DC: National Heart, Lung, and Blood Institute; 2002. NIH publication No. 02-2117.
- Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. [See comment.] N Engl J Med. 1995 May 18;332(20):1317-1322.
- Okpala IE. New therapies for sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):975-987, ix. Review.
- Johnson CS. The acute chest syndrome. Hematol Oncol Clin North Am. 2005;19(5):857-879, vi-vii.
- Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000 Sep 14;342(25):1855-65.
- Platt OS. Preventing stroke in sickle cell anemia. N Engl J Med. 2005 Dec 29;353(26): 2743-2745.
- Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998 Jan;91(1):288-294.
- Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. [See comment.] N Engl J Med. 1998 Jul;339(20):1477-1478.
- Adams RJ, Brambilla D. Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP 2) Trial Investigators. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. [See comment.] N Engl J Med. 2005;353(26):2743-2745.
- Aguilar C, Vichinsky E, Neumayr L. Bone and joint disease in sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):929-941, viii. Review.
- Milner PF, Kraus AP, Sebes JI, et al. Osteonecrosis of the humeral head in sickle cell disease. Clin Orthop Relat Res. 1993;289:136-143.
- Castro O, Gladwin MT. Pulmonary hypertension in sickle cell disease: mechanisms, diagnosis, and management. Hematol Oncol Clin North Am. 2005;19(5):881-896, vii.
- Saborio P, Scheinman JI. Sickle cell nephropathy. J Am Soc Nephrol. 1999 Jan;10(1):187-192. Review.
- Vilke GM, Harrigan RA, Ufberg JW, et al. Emergency evaluation and treatment of priapism. J Emerg Med. 2004 Apr;26(3):325-329. Review.
- Charache S. Eye disease in sickling disorders. Hematol Oncol Clin North Am. 1996;10(6): 1357-1362.
- Wanko SO, Telen MJ. Transfusion management in sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):803-826, v-vi.
- Ohene-Frempong K. Indications for red cell transfusion in sickle cell disease. Semin Hematol. 2001 Jan;38(1 Suppl 1):5-13.
- Vichinsky EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. [See comment.] N Engl J Med. 1995 Jul 27;333(4):206-213.
- Piga A, Galanello R, Cappellini, MD, et al. Phase II study of oral chelator ICL670 in thalassaemia patients with transfusional iron overload: efficacy, safety, pharmacokinetics (PK) and pharmacodynamics (PD) after 6 months of therapy. Blood. 2002;100:5a (abstract).
Sickle cell anemia is a prototypical single gene deletion disorder familiar to medical students everywhere. Most physicians recall that it is a devastating disorder that starts with a single substitution of valine for glutamic acid in the beta-globin gene of the hemoglobin molecule, rendering the hemoglobin molecule unstable in its de-oxygenated state. This leads to polymerization of hemoglobin molecules within the red cell, deformation of the cell membrane, and sickling. Sickling, in turn, causes blood vessel damage, vaso-occlusion, and various other physiologic effects, which lead to both micro- and macro-vascular protean complications.1
Despite extensive basic research in sickle cell disease (SCD), clinical research into the optimal management of this complex disease has lagged behind. Many adult hospitalists may be unfamiliar with the care of adult SCD patients because most of these patients are cared for in academic centers by hematologists.
In this article we review the common complications of sickle cell anemia in adult patients, the management of associated conditions, and the evidence base for treatment guidelines.
Overview
Sickle cell anemia is not just one disorder, but is, rather, a collection of related disorders involving mutations of the hemoglobin molecule. (These disorders are categorized in Table 1, p. 40) In the United States, SCD primarily affects black Americans, with about 9% of this population having sickle trait.2 One in 600 black Americans has sickle cell anemia, also known as hemoglobin SS disease, and there are an estimated 72,000 patients with SCD in the United States.2
In the early 1970s, the average life expectancy for patients with SCD was estimated to be 14.3 years.3 At that time, more than half of SCD patients died before age five, primarily from infectious complications such as pneumococcal sepsis. Twenty years later, the Cooperative Study of SCD documented a much better life expectancy: an average of 42 for males and 48 for females with homozygous S disease (the set of patients with two copies of the defective hemoglobin S mutation).
On average, patients with hemoglobin SCD routinely survive into their 60s. Many factors, including earlier diagnosis of SCD through universal screening programs, better vaccines and vaccination rates, prophylactic antibiotics in infancy and young childhood, and aggressive treatment of fever in infants, as well as other advances in care, have contributed to this progress. A longer life expectancy in this population has heralded the extension of common complications into adulthood, however, along with the emergence of other adult-specific complications of SCD. (See Table 2, p. 40.) In order to best treat adults with SCD, hospitalists should be aware of these complications, as well as of the advances that have been achieved.
Extension of Pediatric Complications
Perhaps the most common manifestation of SCD in children and adults is the painful crisis. This manifests in infants as a painful swelling in the digits of the hands and feet known as dactylitis. In older children and adults, pain occurs more often in the long bones of the arms and legs and in the sternum, vertebrae, and pelvic bones. Risk factors for painful crises include higher hematocrit values and higher sickle fractions, typically greater than 30%.4 Frequent painful crises are a marker of disease severity and an independent risk factor for death in SCD.
Hospitalists who regularly admit SCD patients are familiar with a subset of “frequent flyers” who experience recurrent painful crises. Remember that this is a small fraction of the total SCD population. In fact, 40% of SCD patients don’t suffer any painful crises requiring medical attention in a given year, and only 1% face more than six such events.5 Pain management in SCD is perhaps beyond the scope of this article, and there are no widely accepted guidelines. A few expert reviews are available for guidance, however.6,7
Treatment Options for Painful Crises
The most beneficial new treatment for painful crisis in the past two decades has been the use of hydroxyurea. After early studies showed promise, a phase III clinical trial in adults with SCD who suffered frequent painful crises (i.e., more than four per year) and who were given doses of up to 35 mg/kg/day of hydroxyurea showed a dramatic decrease in painful episodes. These patients also endured fewer episodes of acute chest syndrome, with a relative risk of 0.44, and experienced a reduced need for transfusions, with a relative risk of 0.64. This trial was stopped early (at 21 months average follow-up) due to the striking findings.8 Subsequent longer-term trials have not shown any of the feared theoretical complications of hydroxyurea, including cytopenias (when the production of one or more blood cell types ceases or is greatly reduced) or secondary malignancies.9 (See Table 3, p. 40.)
Childhood Complications
Some SCD complications are more prominent or more severe in early childhood than in adulthood. Two examples of this phenomenon are infectious complications and splenic sequestration. Pediatricians and parents of children with SCD live in fear of febrile illness because overwhelming infections such as pneumococcal sepsis remain the primary reason for death in children with SCD. This was especially true before Hemophilus influenza type b (Hib) and pneumococcal conjugate vaccines were introduced and before penicillin prophylaxis from birth through age five became universal.
Though functionally asplenic, adult patients are at a relatively low risk for overwhelming infections because their immune systems have matured enough to allow type-specific antibody production to polysaccharide antigens.7 Still, adults with SCD should be aggressively evaluated for infectious etiology of any febrile illness and routinely administered empiric antibiotics to cover strep species.
Splenic sequestration occurs when sickled cells are caught up in the spleen; this causes massive hemolysis, splenic enlargement, and cardiovascular compromise, and it is most common in SCD patients younger than five. Thankfully, this is rare in adults with SS and SB0Thal; however, the notable exception to this rule is in patients with hemoglobin SC disease or SB+Thal, where complete splenic infarction does not routinely occur.
Acute chest syndrome (ACS) is perhaps the most feared complication in both children and adults with SCD—and with good reason. It is the second most common cause for hospital admission in SCD patients, and it is an independent risk factor for death in SCD.7,10 Occurring more often in children, ACS is typically more severe in adults, in whom ACS can progress rapidly to an acute respiratory distress syndrome (ARDS)-like picture, with mortality reaching 5%-9%.11
ACS involves a classic triad of fever, chest pain, and new pulmonary infiltrates on chest X-ray, but patients are invariably hypoxic and dyspneic as well. The etiology of ACS varies. In one series, 54% of patients had an identified infectious pathogen, most commonly chlamydia or mycoplasma. Bronchoalveolar lavage (BAL) showed lipid-laden macrophages in about 16%, suggesting fat embolism after bony infarcts. The remainder of patients were presumed to have primary pulmonary infarctions.11 The treatment of ACS involves broad-spectrum antibiotics, especially atypical coverage, along with transfusion (simple versus exchange), supplemental oxygen, pain control, and judicious use of IV fluids.
The second most feared complication in SCD is stroke, which can be devastating in both children and young adults. Eleven percent of patients younger than 20 have ischemic strokes, with a risk of stroke that is 200 times higher than that of age-matched peers.9,12 The peak incidence of ischemic stroke occurs before 10 and after 30. These patients are also susceptible to hemorrhagic strokes, the incidence of which peaks in the third decade, for reasons that are unclear.13
Management of acute ischemic stroke in patients with SCD is similar to that used with general patients: antiplatelet therapy, careful attention to normo-glycemia and normovolemia, and maintenance of cerebral perfusion pressure. In addition, SCD patients should receive emergent transfusion to reduce the sickle fraction to less than 30%. Patients with prior transient ischemic attack (TIA) or stroke should be on a chronic monthly transfusion regimen. Also, based on the results of the 1997 STOP I trial (Stroke Prevention Trial in Sickle Cell Anemia), children with SCD should be screened with transcranial Doppler for high velocity flow (>200 cm/second) in the internal jugular and vertebral arteries.14 Children with high velocity flow who were treated with preventative transfusion regimens had a 90% absolute risk reduction in the incidence of first stroke.
In 2001-2006, a follow-up trial (STOP 2) revealed that among patients receiving at least 30 months of chronic transfusion to prevent a first stroke 39% of those randomized to discontinue transfusion had a reversion to high risk Doppler or suffered a stroke within an average of 4.5 months. This has led many to believe that prophylactic transfusion should be a lifelong treatment.15
Complications Seen Primarily in Adults
As the SCD population in this country has grown older, some previously uncommon complications have become more prominent. One predominantly adult complication of SCD is avascular necrosis of the femoral and humeral heads. Though clearly recognized in younger children in whom the incidence is estimated at about 3%, the major burden of femoral osteonecrosis is seen in adults older than 35, in whom prevalence reaches 50%.16 Necrosis of the humeral head can affect nearly 20% of SCD adults as well.17
It is important to recognize this complication as a new or different type of pain, separate from the vaso-occlusive pain usually experienced by SCD patients because it benefits from different therapies. Diagnosis by plain radiography is possible in the late stages, when evidence of remodeling, cystic changes, and sclerosis can be seen, but MRI has become the gold standard, with an estimated diagnostic accuracy of 90%.16 Conservative treatments include NSAIDs and steroid joint injections, but many afflicted patients may need orthopedic referral for joint replacement.
While acute chest syndrome remains a primary cause of mortality in SCD, adults with SCD are also at high risk for the chronic effects of pulmonary arterial hypertension (PAH). Thought to be uncommon in children, PAH affects up to one-third of adult SCD patients.18 Suspicion of this condition can be based on worsened fatigue, new resting hypoxemia, or increased painful crises, but experts advocate universal screening using transthoracic echocardiography. Patients with a tricuspid regurgitant jet velocity of 2.5 m/sec meet diagnostic criteria, and it is notable that the relative risk of death is 7.4 compared with SCD patients without PAH. This correlates with only moderate elevation of pulmonary pressures, suggesting that SCD patients tolerate PAH less well than other populations. Treatment options advocated include hydroxyurea, chronic transfusions, oxygen, pulmonary vasodilators such as prostacyclin and bosentan, and phosphodiesterase inhibitors such as sildenafil.
As SCD patients age, kidney disease is seen with increasing frequency, and three primary mechanisms are recognized. First, ischemic damage in the tubules causes tubular necrosis, which leads to hematuria.19 This condition can range from microscopic to severe gross hematuria, threatening urinary obstruction. Second, damage in the collecting duct impairs the body’s ability to concentrate urine, a condition that is called hyposthenuria. This condition makes SCD patients susceptible to dehydration, especially during physical exertion or in hot weather.
Interestingly, these first two mechanisms of kidney damage are also seen in patients with sickle cell trait. Finally, and most importantly, medullary interstitial fibrosis damages the glomerulus. Clinically, this is the most important mechanism because it leads to nephritic syndrome and chronic kidney disease as well as end-stage renal disease (ESRD).19
Priapism is the persistent, painful erection of the penis in post-pubertal SCD males not associated with sexual desire or relieved by orgasm; this condition is typically defined as lasting more than four to six hours.20 It is particularly common in younger males, with a yearly incidence of 6%-27%; the incidence of priapism in adults approaches 42%. In addition to being extremely painful, prolonged or repeated episodes can lead to impotence. Unfortunately, there are few well-studied treatment options; expert opinion suggests supportive care, including IV fluids, oxygen, ice, elevation, narcotic pain control, and even red cell transfusion in selected cases. Medications used include oral terbutaline, pseudoephedrine, and even stilboestrol, as well as injected phenylephrine, epinephrine, methylene blue, and tenecteplase (TNK-tPA). Surgical procedures attempted have included dorsal penile nerve block, cavernous aspiration, and the Winter procedure, which involves creating a vascular shunt from the corpora cavernosa to the glans penis. Urology consultation is often required for severe cases.
Several forms of eye disease occur in SCD patients. They can be broadly grouped into non-proliferative and proliferative diseases. In the former group, ocular trauma should be recognized as a visual emergency because patients are at risk for developing hyphema, occlusion of the trabeculae in the anterior chamber, and acute glaucoma. SCD patients of all ages are also at risk for acute retinal artery occlusion. Older SCD patients are at greatest risk, however, for a proliferative retinopathy similar to that seen in diabetes.21
Though red blood cell transfusions are helpful in many SCD patients, repeated transfusions can result in infections, immunologic consequences, and iron overload. (See Table 4, p. 41.) Infections that may result include parvovirus B19, HIV, human T-lymphotropic viruses (HTLV) I and II, and viral hepatitides. Immunologic consequences include alloimmunization, which occurs in up to 50% of SCD patients, and potentially fatal acute hemolytic reactions.22 Finally, patients who require frequent transfusions develop iron overload, which in turn causes fatigue, cardiomyopathy, diabetes mellitus, and cirrhosis. This necessitates iron chelators such as deferoxamine, which are disappointingly difficult to administer, requiring either IV or subcutaneous infusion over 12-24 hours daily. One bright note is the recent approval of deferasirox, an iron chelator that is taken orally once daily.
Conclusion
SCD is a complex genetic disease that affects multiple organs. Advances in medical care have increased longevity for SCD patients, and there are currently more adults living with the disease than ever before. Though patients often receive their care in academic centers, hospitalists may encounter them in routine practice. It is useful to have an understanding of the complications of SCD commonly seen in adults and to review the evidence base for care. TH
References
- Platt OS. Preventing stroke in sickle cell anemia. N Engl J Med. 2005 Dec 29;353(26): 2743-2745.
- Quinn CT, Rogers ZR, Buchanan GR. Survival of children with sickle cell disease. Blood. 2004 Jun 1;103(11):4023-4027.
- Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. [See comment.] N Engl J Med. 1994 Oct 13;331(15):1022-1023.
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease: rates and risk factors. N Engl J Med. 1991 Jul 4;325(1):11-16.
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. rates and risk factors. [See comment.] N Engl J Med. 1991;325(24):1747-1748.
- Ballas SK. Pain management of sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):785-802.
- National Institutes of Health. The Management of Sickle Cell Disease. 4th ed. Washington, DC: National Heart, Lung, and Blood Institute; 2002. NIH publication No. 02-2117.
- Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. [See comment.] N Engl J Med. 1995 May 18;332(20):1317-1322.
- Okpala IE. New therapies for sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):975-987, ix. Review.
- Johnson CS. The acute chest syndrome. Hematol Oncol Clin North Am. 2005;19(5):857-879, vi-vii.
- Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000 Sep 14;342(25):1855-65.
- Platt OS. Preventing stroke in sickle cell anemia. N Engl J Med. 2005 Dec 29;353(26): 2743-2745.
- Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998 Jan;91(1):288-294.
- Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. [See comment.] N Engl J Med. 1998 Jul;339(20):1477-1478.
- Adams RJ, Brambilla D. Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP 2) Trial Investigators. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. [See comment.] N Engl J Med. 2005;353(26):2743-2745.
- Aguilar C, Vichinsky E, Neumayr L. Bone and joint disease in sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):929-941, viii. Review.
- Milner PF, Kraus AP, Sebes JI, et al. Osteonecrosis of the humeral head in sickle cell disease. Clin Orthop Relat Res. 1993;289:136-143.
- Castro O, Gladwin MT. Pulmonary hypertension in sickle cell disease: mechanisms, diagnosis, and management. Hematol Oncol Clin North Am. 2005;19(5):881-896, vii.
- Saborio P, Scheinman JI. Sickle cell nephropathy. J Am Soc Nephrol. 1999 Jan;10(1):187-192. Review.
- Vilke GM, Harrigan RA, Ufberg JW, et al. Emergency evaluation and treatment of priapism. J Emerg Med. 2004 Apr;26(3):325-329. Review.
- Charache S. Eye disease in sickling disorders. Hematol Oncol Clin North Am. 1996;10(6): 1357-1362.
- Wanko SO, Telen MJ. Transfusion management in sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):803-826, v-vi.
- Ohene-Frempong K. Indications for red cell transfusion in sickle cell disease. Semin Hematol. 2001 Jan;38(1 Suppl 1):5-13.
- Vichinsky EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. [See comment.] N Engl J Med. 1995 Jul 27;333(4):206-213.
- Piga A, Galanello R, Cappellini, MD, et al. Phase II study of oral chelator ICL670 in thalassaemia patients with transfusional iron overload: efficacy, safety, pharmacokinetics (PK) and pharmacodynamics (PD) after 6 months of therapy. Blood. 2002;100:5a (abstract).
Sickle cell anemia is a prototypical single gene deletion disorder familiar to medical students everywhere. Most physicians recall that it is a devastating disorder that starts with a single substitution of valine for glutamic acid in the beta-globin gene of the hemoglobin molecule, rendering the hemoglobin molecule unstable in its de-oxygenated state. This leads to polymerization of hemoglobin molecules within the red cell, deformation of the cell membrane, and sickling. Sickling, in turn, causes blood vessel damage, vaso-occlusion, and various other physiologic effects, which lead to both micro- and macro-vascular protean complications.1
Despite extensive basic research in sickle cell disease (SCD), clinical research into the optimal management of this complex disease has lagged behind. Many adult hospitalists may be unfamiliar with the care of adult SCD patients because most of these patients are cared for in academic centers by hematologists.
In this article we review the common complications of sickle cell anemia in adult patients, the management of associated conditions, and the evidence base for treatment guidelines.
Overview
Sickle cell anemia is not just one disorder, but is, rather, a collection of related disorders involving mutations of the hemoglobin molecule. (These disorders are categorized in Table 1, p. 40) In the United States, SCD primarily affects black Americans, with about 9% of this population having sickle trait.2 One in 600 black Americans has sickle cell anemia, also known as hemoglobin SS disease, and there are an estimated 72,000 patients with SCD in the United States.2
In the early 1970s, the average life expectancy for patients with SCD was estimated to be 14.3 years.3 At that time, more than half of SCD patients died before age five, primarily from infectious complications such as pneumococcal sepsis. Twenty years later, the Cooperative Study of SCD documented a much better life expectancy: an average of 42 for males and 48 for females with homozygous S disease (the set of patients with two copies of the defective hemoglobin S mutation).
On average, patients with hemoglobin SCD routinely survive into their 60s. Many factors, including earlier diagnosis of SCD through universal screening programs, better vaccines and vaccination rates, prophylactic antibiotics in infancy and young childhood, and aggressive treatment of fever in infants, as well as other advances in care, have contributed to this progress. A longer life expectancy in this population has heralded the extension of common complications into adulthood, however, along with the emergence of other adult-specific complications of SCD. (See Table 2, p. 40.) In order to best treat adults with SCD, hospitalists should be aware of these complications, as well as of the advances that have been achieved.
Extension of Pediatric Complications
Perhaps the most common manifestation of SCD in children and adults is the painful crisis. This manifests in infants as a painful swelling in the digits of the hands and feet known as dactylitis. In older children and adults, pain occurs more often in the long bones of the arms and legs and in the sternum, vertebrae, and pelvic bones. Risk factors for painful crises include higher hematocrit values and higher sickle fractions, typically greater than 30%.4 Frequent painful crises are a marker of disease severity and an independent risk factor for death in SCD.
Hospitalists who regularly admit SCD patients are familiar with a subset of “frequent flyers” who experience recurrent painful crises. Remember that this is a small fraction of the total SCD population. In fact, 40% of SCD patients don’t suffer any painful crises requiring medical attention in a given year, and only 1% face more than six such events.5 Pain management in SCD is perhaps beyond the scope of this article, and there are no widely accepted guidelines. A few expert reviews are available for guidance, however.6,7
Treatment Options for Painful Crises
The most beneficial new treatment for painful crisis in the past two decades has been the use of hydroxyurea. After early studies showed promise, a phase III clinical trial in adults with SCD who suffered frequent painful crises (i.e., more than four per year) and who were given doses of up to 35 mg/kg/day of hydroxyurea showed a dramatic decrease in painful episodes. These patients also endured fewer episodes of acute chest syndrome, with a relative risk of 0.44, and experienced a reduced need for transfusions, with a relative risk of 0.64. This trial was stopped early (at 21 months average follow-up) due to the striking findings.8 Subsequent longer-term trials have not shown any of the feared theoretical complications of hydroxyurea, including cytopenias (when the production of one or more blood cell types ceases or is greatly reduced) or secondary malignancies.9 (See Table 3, p. 40.)
Childhood Complications
Some SCD complications are more prominent or more severe in early childhood than in adulthood. Two examples of this phenomenon are infectious complications and splenic sequestration. Pediatricians and parents of children with SCD live in fear of febrile illness because overwhelming infections such as pneumococcal sepsis remain the primary reason for death in children with SCD. This was especially true before Hemophilus influenza type b (Hib) and pneumococcal conjugate vaccines were introduced and before penicillin prophylaxis from birth through age five became universal.
Though functionally asplenic, adult patients are at a relatively low risk for overwhelming infections because their immune systems have matured enough to allow type-specific antibody production to polysaccharide antigens.7 Still, adults with SCD should be aggressively evaluated for infectious etiology of any febrile illness and routinely administered empiric antibiotics to cover strep species.
Splenic sequestration occurs when sickled cells are caught up in the spleen; this causes massive hemolysis, splenic enlargement, and cardiovascular compromise, and it is most common in SCD patients younger than five. Thankfully, this is rare in adults with SS and SB0Thal; however, the notable exception to this rule is in patients with hemoglobin SC disease or SB+Thal, where complete splenic infarction does not routinely occur.
Acute chest syndrome (ACS) is perhaps the most feared complication in both children and adults with SCD—and with good reason. It is the second most common cause for hospital admission in SCD patients, and it is an independent risk factor for death in SCD.7,10 Occurring more often in children, ACS is typically more severe in adults, in whom ACS can progress rapidly to an acute respiratory distress syndrome (ARDS)-like picture, with mortality reaching 5%-9%.11
ACS involves a classic triad of fever, chest pain, and new pulmonary infiltrates on chest X-ray, but patients are invariably hypoxic and dyspneic as well. The etiology of ACS varies. In one series, 54% of patients had an identified infectious pathogen, most commonly chlamydia or mycoplasma. Bronchoalveolar lavage (BAL) showed lipid-laden macrophages in about 16%, suggesting fat embolism after bony infarcts. The remainder of patients were presumed to have primary pulmonary infarctions.11 The treatment of ACS involves broad-spectrum antibiotics, especially atypical coverage, along with transfusion (simple versus exchange), supplemental oxygen, pain control, and judicious use of IV fluids.
The second most feared complication in SCD is stroke, which can be devastating in both children and young adults. Eleven percent of patients younger than 20 have ischemic strokes, with a risk of stroke that is 200 times higher than that of age-matched peers.9,12 The peak incidence of ischemic stroke occurs before 10 and after 30. These patients are also susceptible to hemorrhagic strokes, the incidence of which peaks in the third decade, for reasons that are unclear.13
Management of acute ischemic stroke in patients with SCD is similar to that used with general patients: antiplatelet therapy, careful attention to normo-glycemia and normovolemia, and maintenance of cerebral perfusion pressure. In addition, SCD patients should receive emergent transfusion to reduce the sickle fraction to less than 30%. Patients with prior transient ischemic attack (TIA) or stroke should be on a chronic monthly transfusion regimen. Also, based on the results of the 1997 STOP I trial (Stroke Prevention Trial in Sickle Cell Anemia), children with SCD should be screened with transcranial Doppler for high velocity flow (>200 cm/second) in the internal jugular and vertebral arteries.14 Children with high velocity flow who were treated with preventative transfusion regimens had a 90% absolute risk reduction in the incidence of first stroke.
In 2001-2006, a follow-up trial (STOP 2) revealed that among patients receiving at least 30 months of chronic transfusion to prevent a first stroke 39% of those randomized to discontinue transfusion had a reversion to high risk Doppler or suffered a stroke within an average of 4.5 months. This has led many to believe that prophylactic transfusion should be a lifelong treatment.15
Complications Seen Primarily in Adults
As the SCD population in this country has grown older, some previously uncommon complications have become more prominent. One predominantly adult complication of SCD is avascular necrosis of the femoral and humeral heads. Though clearly recognized in younger children in whom the incidence is estimated at about 3%, the major burden of femoral osteonecrosis is seen in adults older than 35, in whom prevalence reaches 50%.16 Necrosis of the humeral head can affect nearly 20% of SCD adults as well.17
It is important to recognize this complication as a new or different type of pain, separate from the vaso-occlusive pain usually experienced by SCD patients because it benefits from different therapies. Diagnosis by plain radiography is possible in the late stages, when evidence of remodeling, cystic changes, and sclerosis can be seen, but MRI has become the gold standard, with an estimated diagnostic accuracy of 90%.16 Conservative treatments include NSAIDs and steroid joint injections, but many afflicted patients may need orthopedic referral for joint replacement.
While acute chest syndrome remains a primary cause of mortality in SCD, adults with SCD are also at high risk for the chronic effects of pulmonary arterial hypertension (PAH). Thought to be uncommon in children, PAH affects up to one-third of adult SCD patients.18 Suspicion of this condition can be based on worsened fatigue, new resting hypoxemia, or increased painful crises, but experts advocate universal screening using transthoracic echocardiography. Patients with a tricuspid regurgitant jet velocity of 2.5 m/sec meet diagnostic criteria, and it is notable that the relative risk of death is 7.4 compared with SCD patients without PAH. This correlates with only moderate elevation of pulmonary pressures, suggesting that SCD patients tolerate PAH less well than other populations. Treatment options advocated include hydroxyurea, chronic transfusions, oxygen, pulmonary vasodilators such as prostacyclin and bosentan, and phosphodiesterase inhibitors such as sildenafil.
As SCD patients age, kidney disease is seen with increasing frequency, and three primary mechanisms are recognized. First, ischemic damage in the tubules causes tubular necrosis, which leads to hematuria.19 This condition can range from microscopic to severe gross hematuria, threatening urinary obstruction. Second, damage in the collecting duct impairs the body’s ability to concentrate urine, a condition that is called hyposthenuria. This condition makes SCD patients susceptible to dehydration, especially during physical exertion or in hot weather.
Interestingly, these first two mechanisms of kidney damage are also seen in patients with sickle cell trait. Finally, and most importantly, medullary interstitial fibrosis damages the glomerulus. Clinically, this is the most important mechanism because it leads to nephritic syndrome and chronic kidney disease as well as end-stage renal disease (ESRD).19
Priapism is the persistent, painful erection of the penis in post-pubertal SCD males not associated with sexual desire or relieved by orgasm; this condition is typically defined as lasting more than four to six hours.20 It is particularly common in younger males, with a yearly incidence of 6%-27%; the incidence of priapism in adults approaches 42%. In addition to being extremely painful, prolonged or repeated episodes can lead to impotence. Unfortunately, there are few well-studied treatment options; expert opinion suggests supportive care, including IV fluids, oxygen, ice, elevation, narcotic pain control, and even red cell transfusion in selected cases. Medications used include oral terbutaline, pseudoephedrine, and even stilboestrol, as well as injected phenylephrine, epinephrine, methylene blue, and tenecteplase (TNK-tPA). Surgical procedures attempted have included dorsal penile nerve block, cavernous aspiration, and the Winter procedure, which involves creating a vascular shunt from the corpora cavernosa to the glans penis. Urology consultation is often required for severe cases.
Several forms of eye disease occur in SCD patients. They can be broadly grouped into non-proliferative and proliferative diseases. In the former group, ocular trauma should be recognized as a visual emergency because patients are at risk for developing hyphema, occlusion of the trabeculae in the anterior chamber, and acute glaucoma. SCD patients of all ages are also at risk for acute retinal artery occlusion. Older SCD patients are at greatest risk, however, for a proliferative retinopathy similar to that seen in diabetes.21
Though red blood cell transfusions are helpful in many SCD patients, repeated transfusions can result in infections, immunologic consequences, and iron overload. (See Table 4, p. 41.) Infections that may result include parvovirus B19, HIV, human T-lymphotropic viruses (HTLV) I and II, and viral hepatitides. Immunologic consequences include alloimmunization, which occurs in up to 50% of SCD patients, and potentially fatal acute hemolytic reactions.22 Finally, patients who require frequent transfusions develop iron overload, which in turn causes fatigue, cardiomyopathy, diabetes mellitus, and cirrhosis. This necessitates iron chelators such as deferoxamine, which are disappointingly difficult to administer, requiring either IV or subcutaneous infusion over 12-24 hours daily. One bright note is the recent approval of deferasirox, an iron chelator that is taken orally once daily.
Conclusion
SCD is a complex genetic disease that affects multiple organs. Advances in medical care have increased longevity for SCD patients, and there are currently more adults living with the disease than ever before. Though patients often receive their care in academic centers, hospitalists may encounter them in routine practice. It is useful to have an understanding of the complications of SCD commonly seen in adults and to review the evidence base for care. TH
References
- Platt OS. Preventing stroke in sickle cell anemia. N Engl J Med. 2005 Dec 29;353(26): 2743-2745.
- Quinn CT, Rogers ZR, Buchanan GR. Survival of children with sickle cell disease. Blood. 2004 Jun 1;103(11):4023-4027.
- Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. [See comment.] N Engl J Med. 1994 Oct 13;331(15):1022-1023.
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease: rates and risk factors. N Engl J Med. 1991 Jul 4;325(1):11-16.
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. rates and risk factors. [See comment.] N Engl J Med. 1991;325(24):1747-1748.
- Ballas SK. Pain management of sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):785-802.
- National Institutes of Health. The Management of Sickle Cell Disease. 4th ed. Washington, DC: National Heart, Lung, and Blood Institute; 2002. NIH publication No. 02-2117.
- Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. [See comment.] N Engl J Med. 1995 May 18;332(20):1317-1322.
- Okpala IE. New therapies for sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):975-987, ix. Review.
- Johnson CS. The acute chest syndrome. Hematol Oncol Clin North Am. 2005;19(5):857-879, vi-vii.
- Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000 Sep 14;342(25):1855-65.
- Platt OS. Preventing stroke in sickle cell anemia. N Engl J Med. 2005 Dec 29;353(26): 2743-2745.
- Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998 Jan;91(1):288-294.
- Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. [See comment.] N Engl J Med. 1998 Jul;339(20):1477-1478.
- Adams RJ, Brambilla D. Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP 2) Trial Investigators. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. [See comment.] N Engl J Med. 2005;353(26):2743-2745.
- Aguilar C, Vichinsky E, Neumayr L. Bone and joint disease in sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):929-941, viii. Review.
- Milner PF, Kraus AP, Sebes JI, et al. Osteonecrosis of the humeral head in sickle cell disease. Clin Orthop Relat Res. 1993;289:136-143.
- Castro O, Gladwin MT. Pulmonary hypertension in sickle cell disease: mechanisms, diagnosis, and management. Hematol Oncol Clin North Am. 2005;19(5):881-896, vii.
- Saborio P, Scheinman JI. Sickle cell nephropathy. J Am Soc Nephrol. 1999 Jan;10(1):187-192. Review.
- Vilke GM, Harrigan RA, Ufberg JW, et al. Emergency evaluation and treatment of priapism. J Emerg Med. 2004 Apr;26(3):325-329. Review.
- Charache S. Eye disease in sickling disorders. Hematol Oncol Clin North Am. 1996;10(6): 1357-1362.
- Wanko SO, Telen MJ. Transfusion management in sickle cell disease. Hematol Oncol Clin North Am. 2005 Oct;19(5):803-826, v-vi.
- Ohene-Frempong K. Indications for red cell transfusion in sickle cell disease. Semin Hematol. 2001 Jan;38(1 Suppl 1):5-13.
- Vichinsky EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. [See comment.] N Engl J Med. 1995 Jul 27;333(4):206-213.
- Piga A, Galanello R, Cappellini, MD, et al. Phase II study of oral chelator ICL670 in thalassaemia patients with transfusional iron overload: efficacy, safety, pharmacokinetics (PK) and pharmacodynamics (PD) after 6 months of therapy. Blood. 2002;100:5a (abstract).
Initial Impact
The Journal of Hospital Medicine’s debut impact factor (IF) ranks it in the top 20% of its cohort—no small achievement for a peer-reviewed medical journal in its fourth year of publication.
JHM’s 2008 IF is 3.163, a stronger-than-expected showing that Editor-in-Chief Mark V. Williams, MD, FACP, FHM, hopes will translate into increased submissions.
JHM ranks No. 21 out of 107 journals in the Medicine, General, and Internal subject category. Impact factors are an industry metric used as a rough average of citations received by peer-reviewed journals. For comparison's sake, the IF for the Journal of General Internal Medicine is 2.72; the IF for the Annals of Internal Medicine is 17.457; and the IF for the New England Journal of Medicine is 50.017.
“In context of other journals of similar editorial scope … an [IF score] does indicate something about its influence,” says James Testa, senior director of editorial development and publisher relations for Thomson Reuters, which calculates the score.
Dr. Williams is a little more effusive. The IF, he says, “tells us we need to keep doing what we’re doing. ... There are journals that are 20 years old that don’t have impact factors as high as we do.” Dr. Williams is professor and chief of the Division of Hospital Medicine at Northwestern University Feinberg School of Medicine in Chicago. “Hopefully, this will lead to academicians across the world being interested in submitting their scholarly work.”
JHM's top-cited source is Core Competencies in Hospital Medicine: A Framework for Curriculum Development, a supplement published with Volume 1, Issue 1. The supplement's clinical content is an essential tool for practicing hospitalists, providing guidance in the areas of clinical conditions, procedures, healthcare systems, development and methodologies.
"SHM is very proud of our journal, JHM, and getting such a sterling impact factor is a further acknowledgement of JHM’s credibility, reach, and world-class content,” says Larry Wellikson, MD, FHM, CEO of SHM.
The Journal of Hospital Medicine’s debut impact factor (IF) ranks it in the top 20% of its cohort—no small achievement for a peer-reviewed medical journal in its fourth year of publication.
JHM’s 2008 IF is 3.163, a stronger-than-expected showing that Editor-in-Chief Mark V. Williams, MD, FACP, FHM, hopes will translate into increased submissions.
JHM ranks No. 21 out of 107 journals in the Medicine, General, and Internal subject category. Impact factors are an industry metric used as a rough average of citations received by peer-reviewed journals. For comparison's sake, the IF for the Journal of General Internal Medicine is 2.72; the IF for the Annals of Internal Medicine is 17.457; and the IF for the New England Journal of Medicine is 50.017.
“In context of other journals of similar editorial scope … an [IF score] does indicate something about its influence,” says James Testa, senior director of editorial development and publisher relations for Thomson Reuters, which calculates the score.
Dr. Williams is a little more effusive. The IF, he says, “tells us we need to keep doing what we’re doing. ... There are journals that are 20 years old that don’t have impact factors as high as we do.” Dr. Williams is professor and chief of the Division of Hospital Medicine at Northwestern University Feinberg School of Medicine in Chicago. “Hopefully, this will lead to academicians across the world being interested in submitting their scholarly work.”
JHM's top-cited source is Core Competencies in Hospital Medicine: A Framework for Curriculum Development, a supplement published with Volume 1, Issue 1. The supplement's clinical content is an essential tool for practicing hospitalists, providing guidance in the areas of clinical conditions, procedures, healthcare systems, development and methodologies.
"SHM is very proud of our journal, JHM, and getting such a sterling impact factor is a further acknowledgement of JHM’s credibility, reach, and world-class content,” says Larry Wellikson, MD, FHM, CEO of SHM.
The Journal of Hospital Medicine’s debut impact factor (IF) ranks it in the top 20% of its cohort—no small achievement for a peer-reviewed medical journal in its fourth year of publication.
JHM’s 2008 IF is 3.163, a stronger-than-expected showing that Editor-in-Chief Mark V. Williams, MD, FACP, FHM, hopes will translate into increased submissions.
JHM ranks No. 21 out of 107 journals in the Medicine, General, and Internal subject category. Impact factors are an industry metric used as a rough average of citations received by peer-reviewed journals. For comparison's sake, the IF for the Journal of General Internal Medicine is 2.72; the IF for the Annals of Internal Medicine is 17.457; and the IF for the New England Journal of Medicine is 50.017.
“In context of other journals of similar editorial scope … an [IF score] does indicate something about its influence,” says James Testa, senior director of editorial development and publisher relations for Thomson Reuters, which calculates the score.
Dr. Williams is a little more effusive. The IF, he says, “tells us we need to keep doing what we’re doing. ... There are journals that are 20 years old that don’t have impact factors as high as we do.” Dr. Williams is professor and chief of the Division of Hospital Medicine at Northwestern University Feinberg School of Medicine in Chicago. “Hopefully, this will lead to academicians across the world being interested in submitting their scholarly work.”
JHM's top-cited source is Core Competencies in Hospital Medicine: A Framework for Curriculum Development, a supplement published with Volume 1, Issue 1. The supplement's clinical content is an essential tool for practicing hospitalists, providing guidance in the areas of clinical conditions, procedures, healthcare systems, development and methodologies.
"SHM is very proud of our journal, JHM, and getting such a sterling impact factor is a further acknowledgement of JHM’s credibility, reach, and world-class content,” says Larry Wellikson, MD, FHM, CEO of SHM.
Pharma Promises Price Reductions
An $80 billion deal to help reduce out-of-pocket drug costs for Medicare beneficiaries has elicited mixed reactions on what it might mean for patients, as well as calls for hospitalists to remain vigilant about prescription drug expenses.
Under a pledge negotiated with the White House and congressional Democrats, the pharmaceutical industry has promised a 50% discount for name-brand drugs to beneficiaries stuck in the notorious gap of the Medicare Part D prescription drug plan, commonly called the “doughnut hole.” In 2009, the gap in coverage kicks in after $2,700 in total drug costs and persists until $6,154 in total costs, by which point patients have spent as much as $4,350 of their own money for prescription drugs.
President Obama says the gap “has been placing a crushing burden on many older Americans who live on fixed incomes and can’t afford thousands of dollars in out-of-pocket expenses.” The AARP hails the “unique solution” as a “major step forward,” though other industry observers have taken a more circumspect stance and said they want to see tougher cost-control measures in writing.
“You don’t want to look an $80 billion gift horse in the mouth, but there’s some halitosis in this mouth,” says Bill Vaughan, a health policy analyst for Consumers Union in Washington, D.C. “It’s not as pure and altruistic as it seems at first blush, and people need to keep pushing for generics because these [brand-name drugs] are grossly overpriced.”
The deal includes several caveats: The pledge doesn’t address the cost of brand-name drugs before or after the coverage gap, and the doughnut-hole price reduction would go into effect only if Congress enacts healthcare reform legislation.
William D. Atchley Jr., MD, FACP, FHM, says hospitalists need to know what’s available in the hospital pharmacy and maintain an open line of communication with their patients in terms of their access and ability to pay for prescriptions.
“You need to understand patients’ economic status. You need to know if they get their medications from Walmart or the VA hospital pharmacy,” says Dr. Atchley, chief of the division of hospital medicine for Sentara Medical Group in Norfolk, Va., and a member of SHM’s Public Policy Committee. “Cost is an issue to our Medicare patients, and it’s important to collaborate with them to make sure they can afford the drug. If they can’t, you need to work with them to find another affordable drug that will provide the same benefit.”
An $80 billion deal to help reduce out-of-pocket drug costs for Medicare beneficiaries has elicited mixed reactions on what it might mean for patients, as well as calls for hospitalists to remain vigilant about prescription drug expenses.
Under a pledge negotiated with the White House and congressional Democrats, the pharmaceutical industry has promised a 50% discount for name-brand drugs to beneficiaries stuck in the notorious gap of the Medicare Part D prescription drug plan, commonly called the “doughnut hole.” In 2009, the gap in coverage kicks in after $2,700 in total drug costs and persists until $6,154 in total costs, by which point patients have spent as much as $4,350 of their own money for prescription drugs.
President Obama says the gap “has been placing a crushing burden on many older Americans who live on fixed incomes and can’t afford thousands of dollars in out-of-pocket expenses.” The AARP hails the “unique solution” as a “major step forward,” though other industry observers have taken a more circumspect stance and said they want to see tougher cost-control measures in writing.
“You don’t want to look an $80 billion gift horse in the mouth, but there’s some halitosis in this mouth,” says Bill Vaughan, a health policy analyst for Consumers Union in Washington, D.C. “It’s not as pure and altruistic as it seems at first blush, and people need to keep pushing for generics because these [brand-name drugs] are grossly overpriced.”
The deal includes several caveats: The pledge doesn’t address the cost of brand-name drugs before or after the coverage gap, and the doughnut-hole price reduction would go into effect only if Congress enacts healthcare reform legislation.
William D. Atchley Jr., MD, FACP, FHM, says hospitalists need to know what’s available in the hospital pharmacy and maintain an open line of communication with their patients in terms of their access and ability to pay for prescriptions.
“You need to understand patients’ economic status. You need to know if they get their medications from Walmart or the VA hospital pharmacy,” says Dr. Atchley, chief of the division of hospital medicine for Sentara Medical Group in Norfolk, Va., and a member of SHM’s Public Policy Committee. “Cost is an issue to our Medicare patients, and it’s important to collaborate with them to make sure they can afford the drug. If they can’t, you need to work with them to find another affordable drug that will provide the same benefit.”
An $80 billion deal to help reduce out-of-pocket drug costs for Medicare beneficiaries has elicited mixed reactions on what it might mean for patients, as well as calls for hospitalists to remain vigilant about prescription drug expenses.
Under a pledge negotiated with the White House and congressional Democrats, the pharmaceutical industry has promised a 50% discount for name-brand drugs to beneficiaries stuck in the notorious gap of the Medicare Part D prescription drug plan, commonly called the “doughnut hole.” In 2009, the gap in coverage kicks in after $2,700 in total drug costs and persists until $6,154 in total costs, by which point patients have spent as much as $4,350 of their own money for prescription drugs.
President Obama says the gap “has been placing a crushing burden on many older Americans who live on fixed incomes and can’t afford thousands of dollars in out-of-pocket expenses.” The AARP hails the “unique solution” as a “major step forward,” though other industry observers have taken a more circumspect stance and said they want to see tougher cost-control measures in writing.
“You don’t want to look an $80 billion gift horse in the mouth, but there’s some halitosis in this mouth,” says Bill Vaughan, a health policy analyst for Consumers Union in Washington, D.C. “It’s not as pure and altruistic as it seems at first blush, and people need to keep pushing for generics because these [brand-name drugs] are grossly overpriced.”
The deal includes several caveats: The pledge doesn’t address the cost of brand-name drugs before or after the coverage gap, and the doughnut-hole price reduction would go into effect only if Congress enacts healthcare reform legislation.
William D. Atchley Jr., MD, FACP, FHM, says hospitalists need to know what’s available in the hospital pharmacy and maintain an open line of communication with their patients in terms of their access and ability to pay for prescriptions.
“You need to understand patients’ economic status. You need to know if they get their medications from Walmart or the VA hospital pharmacy,” says Dr. Atchley, chief of the division of hospital medicine for Sentara Medical Group in Norfolk, Va., and a member of SHM’s Public Policy Committee. “Cost is an issue to our Medicare patients, and it’s important to collaborate with them to make sure they can afford the drug. If they can’t, you need to work with them to find another affordable drug that will provide the same benefit.”
Bouncebacks
Mr. D is an 80-year-old gentleman who is treated in the hospital for congestive heart failure (CHF) with a question of pneumonia. He is deemed de-conditioned secondary to his medical illness and is sent to an extended care facility. He returns from the extended care facility 18 hours later with fever and shortness of breath. The emergency department (ED) attending speaks to the primary care attending who took care of him during his previous admission; the primary care physician now wants the patient on the hospitalist service.
Does this scenario sound familiar? We have all dealt with such requests during our hospitalist careers and have wondered what the potential repercussions might be.
There is a danger—always present—that the hospitalist service will be used as a receptacle for undesirable patients. The word undesirable is used loosely here to include complicated patients, patients who keep returning to the hospital with recurrent problems, patients with no insurance or poor insurance coverage, and, of course, problematic patients with problematic families.
Complicated Patients, Complicated Consequences
In the scenario described above, the patient comes back with the same diagnoses but now winds up on the hospitalist service. From an ethical perspective alone this seems objectionable. What about the legal ramifications of such a situation? Two different admissions with the same diagnoses occur within a short time frame, but different physician groups are involved in caring for the patient. Additionally, such scenarios often cause patient dissatisfaction and even hostility. Surely it doesn’t make the patient happy to hear that the primary care physician no longer wants the patient on her service.
The hospitalist service usually deals with more complicated patients than the average physician. Often, the primary physicians, who have more constraints on their time, want hospitalists to take care of the more complicated patients. This becomes a problem when a patient who was on a physician’s service is readmitted. Naturally, the primary doctors are frustrated with the recurrent admissions. A case could be made to admit to the hospitalist service when the readmitting diagnosis is different from the previous discharge diagnosis, but when the discharge and readmission diagnoses are the same, the jury is hung.
Loss of Revenue
To many physicians’ minds, a readmission occurs less than 24 hours after the previous discharge; to others, however, a readmission means something else. For example, Medicaid, considers a readmission one that occurs within fewer than seven days of the previous admission. This situation brings about an automatic readmission review, and the readmission is denied if it is perceived that the patient discharge was not appropriate or was premature.
In the case of Mr. D, the hospitalists might end up getting a denial—and suffering a loss of revenue—for a readmission that had nothing to do with them. Hospitalists are seldom cognizant of such repercussions because we are programmed to perform patient care without contemplating the financial implications.
The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) collects data for their core measures program. A part of this effort also involves reporting the readmission rate within 14 days—both for the same diagnosis and for a different one.
Looking again at the case of Mr. D, the core measures would have included estimation of ejection fraction, use of angiotensin-converting enzyme inhibitors, and appropriateness and timing of antibiotics in pneumonia. The hospitalists, who had no control of these indices on the first admission, might have been penalized if these particular measures were not carried out and their omission contributed to the patient’s readmission.
Resident Overflow
Another potential point of contention is the practice of admitting patients to the hospitalist service once the resident teaching service is capped. This is institution-specific. In cases where the entire resident overflow is admitted to the hospitalist service, patients seldom go back to the teaching service because ED doctors label them as belonging to the hospitalist service. Many of these patients either have no insurance (or have Medicaid); in addition, they often have multiple health problems, and noncompliance runs rife. Because unscheduled readmissions are viewed negatively under current guidelines, patients who are handed off in this manner can cause resulting penalties for the hospitalists who end up serving them.
Extended Care Readmissions
Patients with established primary care physicians often go to extended-care facilities where there is another physician of record. At readmission, the new attending is recorded as the patient’s physician. The prior primary attending might have wanted to follow the patient during the readmission. Unfortunately, the ED physician will typically call the newly assigned attending because that is the name that appears on the transfer note. If, at this time, the new attending decides to admit the patient to the hospitalist service, a misunderstanding may ensue. The original primary care attending may view this as an attempt on the part of the hospitalist service to appropriate patients, though the decision to admit to the hospitalist service is seldom made by the hospitalist.
The pitfalls of these practices are accentuated when the readmission occurs within a relatively short time frame. Another downside may arise if the new attending, who knows little of the patient’s history, orders another extensive inpatient workup. This example highlights a potential, and avoidable, cause of spiraling healthcare costs.
The Hospitalist’s Role
We practice in a milieu of increasing scrutiny. Pay for performance is gaining momentum and acceptance. Two years from now, non-compliance with specific indicators, such as readmission rates, will be met with financial penalties. Hospitals complain of decreasing reimbursements. Unscheduled readmissions to the hospital continue to be a source of lost revenue and patient dissatisfaction.
The hospitalist plays a central role in the management of the patient from a medical standpoint. Rules of admission to the hospitalist service vary widely amongst different institutions. Often, depending on patient load and available staffing, these rules are in flux even within institutions. Procedures run the gamut from the so-called “closed system,” in which only specific physicians can admit patients to the hospitalist service, to the “open system,” in which everyone is welcome, on a voluntary basis, to admit to the hospitalist service. The potential pitfalls of the open system will become more and more apparent in the years to come, and many of us will be forced to rethink our models of healthcare delivery. TH
Dr. Chabria is a hospitalist at Waterbury Hospital, Conn., and a clinical instructor at Yale University School of Medicine, New Haven, Conn.
Mr. D is an 80-year-old gentleman who is treated in the hospital for congestive heart failure (CHF) with a question of pneumonia. He is deemed de-conditioned secondary to his medical illness and is sent to an extended care facility. He returns from the extended care facility 18 hours later with fever and shortness of breath. The emergency department (ED) attending speaks to the primary care attending who took care of him during his previous admission; the primary care physician now wants the patient on the hospitalist service.
Does this scenario sound familiar? We have all dealt with such requests during our hospitalist careers and have wondered what the potential repercussions might be.
There is a danger—always present—that the hospitalist service will be used as a receptacle for undesirable patients. The word undesirable is used loosely here to include complicated patients, patients who keep returning to the hospital with recurrent problems, patients with no insurance or poor insurance coverage, and, of course, problematic patients with problematic families.
Complicated Patients, Complicated Consequences
In the scenario described above, the patient comes back with the same diagnoses but now winds up on the hospitalist service. From an ethical perspective alone this seems objectionable. What about the legal ramifications of such a situation? Two different admissions with the same diagnoses occur within a short time frame, but different physician groups are involved in caring for the patient. Additionally, such scenarios often cause patient dissatisfaction and even hostility. Surely it doesn’t make the patient happy to hear that the primary care physician no longer wants the patient on her service.
The hospitalist service usually deals with more complicated patients than the average physician. Often, the primary physicians, who have more constraints on their time, want hospitalists to take care of the more complicated patients. This becomes a problem when a patient who was on a physician’s service is readmitted. Naturally, the primary doctors are frustrated with the recurrent admissions. A case could be made to admit to the hospitalist service when the readmitting diagnosis is different from the previous discharge diagnosis, but when the discharge and readmission diagnoses are the same, the jury is hung.
Loss of Revenue
To many physicians’ minds, a readmission occurs less than 24 hours after the previous discharge; to others, however, a readmission means something else. For example, Medicaid, considers a readmission one that occurs within fewer than seven days of the previous admission. This situation brings about an automatic readmission review, and the readmission is denied if it is perceived that the patient discharge was not appropriate or was premature.
In the case of Mr. D, the hospitalists might end up getting a denial—and suffering a loss of revenue—for a readmission that had nothing to do with them. Hospitalists are seldom cognizant of such repercussions because we are programmed to perform patient care without contemplating the financial implications.
The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) collects data for their core measures program. A part of this effort also involves reporting the readmission rate within 14 days—both for the same diagnosis and for a different one.
Looking again at the case of Mr. D, the core measures would have included estimation of ejection fraction, use of angiotensin-converting enzyme inhibitors, and appropriateness and timing of antibiotics in pneumonia. The hospitalists, who had no control of these indices on the first admission, might have been penalized if these particular measures were not carried out and their omission contributed to the patient’s readmission.
Resident Overflow
Another potential point of contention is the practice of admitting patients to the hospitalist service once the resident teaching service is capped. This is institution-specific. In cases where the entire resident overflow is admitted to the hospitalist service, patients seldom go back to the teaching service because ED doctors label them as belonging to the hospitalist service. Many of these patients either have no insurance (or have Medicaid); in addition, they often have multiple health problems, and noncompliance runs rife. Because unscheduled readmissions are viewed negatively under current guidelines, patients who are handed off in this manner can cause resulting penalties for the hospitalists who end up serving them.
Extended Care Readmissions
Patients with established primary care physicians often go to extended-care facilities where there is another physician of record. At readmission, the new attending is recorded as the patient’s physician. The prior primary attending might have wanted to follow the patient during the readmission. Unfortunately, the ED physician will typically call the newly assigned attending because that is the name that appears on the transfer note. If, at this time, the new attending decides to admit the patient to the hospitalist service, a misunderstanding may ensue. The original primary care attending may view this as an attempt on the part of the hospitalist service to appropriate patients, though the decision to admit to the hospitalist service is seldom made by the hospitalist.
The pitfalls of these practices are accentuated when the readmission occurs within a relatively short time frame. Another downside may arise if the new attending, who knows little of the patient’s history, orders another extensive inpatient workup. This example highlights a potential, and avoidable, cause of spiraling healthcare costs.
The Hospitalist’s Role
We practice in a milieu of increasing scrutiny. Pay for performance is gaining momentum and acceptance. Two years from now, non-compliance with specific indicators, such as readmission rates, will be met with financial penalties. Hospitals complain of decreasing reimbursements. Unscheduled readmissions to the hospital continue to be a source of lost revenue and patient dissatisfaction.
The hospitalist plays a central role in the management of the patient from a medical standpoint. Rules of admission to the hospitalist service vary widely amongst different institutions. Often, depending on patient load and available staffing, these rules are in flux even within institutions. Procedures run the gamut from the so-called “closed system,” in which only specific physicians can admit patients to the hospitalist service, to the “open system,” in which everyone is welcome, on a voluntary basis, to admit to the hospitalist service. The potential pitfalls of the open system will become more and more apparent in the years to come, and many of us will be forced to rethink our models of healthcare delivery. TH
Dr. Chabria is a hospitalist at Waterbury Hospital, Conn., and a clinical instructor at Yale University School of Medicine, New Haven, Conn.
Mr. D is an 80-year-old gentleman who is treated in the hospital for congestive heart failure (CHF) with a question of pneumonia. He is deemed de-conditioned secondary to his medical illness and is sent to an extended care facility. He returns from the extended care facility 18 hours later with fever and shortness of breath. The emergency department (ED) attending speaks to the primary care attending who took care of him during his previous admission; the primary care physician now wants the patient on the hospitalist service.
Does this scenario sound familiar? We have all dealt with such requests during our hospitalist careers and have wondered what the potential repercussions might be.
There is a danger—always present—that the hospitalist service will be used as a receptacle for undesirable patients. The word undesirable is used loosely here to include complicated patients, patients who keep returning to the hospital with recurrent problems, patients with no insurance or poor insurance coverage, and, of course, problematic patients with problematic families.
Complicated Patients, Complicated Consequences
In the scenario described above, the patient comes back with the same diagnoses but now winds up on the hospitalist service. From an ethical perspective alone this seems objectionable. What about the legal ramifications of such a situation? Two different admissions with the same diagnoses occur within a short time frame, but different physician groups are involved in caring for the patient. Additionally, such scenarios often cause patient dissatisfaction and even hostility. Surely it doesn’t make the patient happy to hear that the primary care physician no longer wants the patient on her service.
The hospitalist service usually deals with more complicated patients than the average physician. Often, the primary physicians, who have more constraints on their time, want hospitalists to take care of the more complicated patients. This becomes a problem when a patient who was on a physician’s service is readmitted. Naturally, the primary doctors are frustrated with the recurrent admissions. A case could be made to admit to the hospitalist service when the readmitting diagnosis is different from the previous discharge diagnosis, but when the discharge and readmission diagnoses are the same, the jury is hung.
Loss of Revenue
To many physicians’ minds, a readmission occurs less than 24 hours after the previous discharge; to others, however, a readmission means something else. For example, Medicaid, considers a readmission one that occurs within fewer than seven days of the previous admission. This situation brings about an automatic readmission review, and the readmission is denied if it is perceived that the patient discharge was not appropriate or was premature.
In the case of Mr. D, the hospitalists might end up getting a denial—and suffering a loss of revenue—for a readmission that had nothing to do with them. Hospitalists are seldom cognizant of such repercussions because we are programmed to perform patient care without contemplating the financial implications.
The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) collects data for their core measures program. A part of this effort also involves reporting the readmission rate within 14 days—both for the same diagnosis and for a different one.
Looking again at the case of Mr. D, the core measures would have included estimation of ejection fraction, use of angiotensin-converting enzyme inhibitors, and appropriateness and timing of antibiotics in pneumonia. The hospitalists, who had no control of these indices on the first admission, might have been penalized if these particular measures were not carried out and their omission contributed to the patient’s readmission.
Resident Overflow
Another potential point of contention is the practice of admitting patients to the hospitalist service once the resident teaching service is capped. This is institution-specific. In cases where the entire resident overflow is admitted to the hospitalist service, patients seldom go back to the teaching service because ED doctors label them as belonging to the hospitalist service. Many of these patients either have no insurance (or have Medicaid); in addition, they often have multiple health problems, and noncompliance runs rife. Because unscheduled readmissions are viewed negatively under current guidelines, patients who are handed off in this manner can cause resulting penalties for the hospitalists who end up serving them.
Extended Care Readmissions
Patients with established primary care physicians often go to extended-care facilities where there is another physician of record. At readmission, the new attending is recorded as the patient’s physician. The prior primary attending might have wanted to follow the patient during the readmission. Unfortunately, the ED physician will typically call the newly assigned attending because that is the name that appears on the transfer note. If, at this time, the new attending decides to admit the patient to the hospitalist service, a misunderstanding may ensue. The original primary care attending may view this as an attempt on the part of the hospitalist service to appropriate patients, though the decision to admit to the hospitalist service is seldom made by the hospitalist.
The pitfalls of these practices are accentuated when the readmission occurs within a relatively short time frame. Another downside may arise if the new attending, who knows little of the patient’s history, orders another extensive inpatient workup. This example highlights a potential, and avoidable, cause of spiraling healthcare costs.
The Hospitalist’s Role
We practice in a milieu of increasing scrutiny. Pay for performance is gaining momentum and acceptance. Two years from now, non-compliance with specific indicators, such as readmission rates, will be met with financial penalties. Hospitals complain of decreasing reimbursements. Unscheduled readmissions to the hospital continue to be a source of lost revenue and patient dissatisfaction.
The hospitalist plays a central role in the management of the patient from a medical standpoint. Rules of admission to the hospitalist service vary widely amongst different institutions. Often, depending on patient load and available staffing, these rules are in flux even within institutions. Procedures run the gamut from the so-called “closed system,” in which only specific physicians can admit patients to the hospitalist service, to the “open system,” in which everyone is welcome, on a voluntary basis, to admit to the hospitalist service. The potential pitfalls of the open system will become more and more apparent in the years to come, and many of us will be forced to rethink our models of healthcare delivery. TH
Dr. Chabria is a hospitalist at Waterbury Hospital, Conn., and a clinical instructor at Yale University School of Medicine, New Haven, Conn.
Relational Growth
A new survey conducted by SHM and the American Medical Association’s (AMA) Organized Medical Staff Section finds that while more than 90% of hospitalists feel an HM presence improves the quality of hospital care, less than half of primary-care physicians (PCPs) feel the same way. On the bright side, the percentage of PCPs with favorable views of HM is climbing.
“There seems to be a general trend toward improvement of how primary-care physicians view hospitalists,” says Chad Whelan, MD, FHM, associate professor of medicine and director of the division of hospital medicine at Loyola University Chicago Stritch School of Medicine and chair of SHM’s Career Satisfaction Task Force. “But there are still very different views. ... It’s a narrower gap, but it’s still a gap.”
The data come from a recent survey of 874 hospitalists and 497 PCPs. The "Survey on the Growing Hospitalist Trend" is a follow-up to a similar study conducted two years ago to gauge the effects of HM on the primary-care model, and vice versa. The study also looks to define perceptions of the hospitalist model on the care of shared patients.
One key issue the survey examined was viewpoints on how well hospitalists and PCPs communicate with each other. Upon admission, the survey shows, half of hospitalists feel they communicate effectively with PCPs, but only 25% of PCPs feel the same way about hospitalists. On discharge, the disparity grows, with 70% of hospitalists saying they feel they communicate well with PCPs; however, only 35% of PCPs agree.
Still, 46% of PCPs agree or strongly agree that hospitalists have improved the overall quality of hospital care. That number is up from 40% two years ago.
“Everyone can learn from this,” says James DeNuccio, director of the AMA’s Organized Medical Staff Services and Physicians in Practice. “If the hospitalists and PCPs both can learn something from this, that helps them adjust their practice. In the end, patients will benefit.”
A new survey conducted by SHM and the American Medical Association’s (AMA) Organized Medical Staff Section finds that while more than 90% of hospitalists feel an HM presence improves the quality of hospital care, less than half of primary-care physicians (PCPs) feel the same way. On the bright side, the percentage of PCPs with favorable views of HM is climbing.
“There seems to be a general trend toward improvement of how primary-care physicians view hospitalists,” says Chad Whelan, MD, FHM, associate professor of medicine and director of the division of hospital medicine at Loyola University Chicago Stritch School of Medicine and chair of SHM’s Career Satisfaction Task Force. “But there are still very different views. ... It’s a narrower gap, but it’s still a gap.”
The data come from a recent survey of 874 hospitalists and 497 PCPs. The "Survey on the Growing Hospitalist Trend" is a follow-up to a similar study conducted two years ago to gauge the effects of HM on the primary-care model, and vice versa. The study also looks to define perceptions of the hospitalist model on the care of shared patients.
One key issue the survey examined was viewpoints on how well hospitalists and PCPs communicate with each other. Upon admission, the survey shows, half of hospitalists feel they communicate effectively with PCPs, but only 25% of PCPs feel the same way about hospitalists. On discharge, the disparity grows, with 70% of hospitalists saying they feel they communicate well with PCPs; however, only 35% of PCPs agree.
Still, 46% of PCPs agree or strongly agree that hospitalists have improved the overall quality of hospital care. That number is up from 40% two years ago.
“Everyone can learn from this,” says James DeNuccio, director of the AMA’s Organized Medical Staff Services and Physicians in Practice. “If the hospitalists and PCPs both can learn something from this, that helps them adjust their practice. In the end, patients will benefit.”
A new survey conducted by SHM and the American Medical Association’s (AMA) Organized Medical Staff Section finds that while more than 90% of hospitalists feel an HM presence improves the quality of hospital care, less than half of primary-care physicians (PCPs) feel the same way. On the bright side, the percentage of PCPs with favorable views of HM is climbing.
“There seems to be a general trend toward improvement of how primary-care physicians view hospitalists,” says Chad Whelan, MD, FHM, associate professor of medicine and director of the division of hospital medicine at Loyola University Chicago Stritch School of Medicine and chair of SHM’s Career Satisfaction Task Force. “But there are still very different views. ... It’s a narrower gap, but it’s still a gap.”
The data come from a recent survey of 874 hospitalists and 497 PCPs. The "Survey on the Growing Hospitalist Trend" is a follow-up to a similar study conducted two years ago to gauge the effects of HM on the primary-care model, and vice versa. The study also looks to define perceptions of the hospitalist model on the care of shared patients.
One key issue the survey examined was viewpoints on how well hospitalists and PCPs communicate with each other. Upon admission, the survey shows, half of hospitalists feel they communicate effectively with PCPs, but only 25% of PCPs feel the same way about hospitalists. On discharge, the disparity grows, with 70% of hospitalists saying they feel they communicate well with PCPs; however, only 35% of PCPs agree.
Still, 46% of PCPs agree or strongly agree that hospitalists have improved the overall quality of hospital care. That number is up from 40% two years ago.
“Everyone can learn from this,” says James DeNuccio, director of the AMA’s Organized Medical Staff Services and Physicians in Practice. “If the hospitalists and PCPs both can learn something from this, that helps them adjust their practice. In the end, patients will benefit.”