User login
New Initiative: Defibrillator Delays
A new report that hints stress factors like case volume and academic status of a hospital do not explain the wide disparities in defibrillation response times in hospitals has at least one hospitalist convinced HM leaders can help solve the problem.
Traditional hospital pressures do not predict whether patients with cardiac arrest are likely to experience delays in receiving defibrillation, according to a July 27 report in the Archives of Internal Medicine (2009;169(14):1260-1261). Such factors as the number of beds and where the cardiac unit was located were found to have more impact, the study found.
“This is a very simple thing,” says hospitalist Jason Persoff, MD, FHM, assistant professor of medicine at the Mayo Clinic in Jacksonville, Fla. "What are the barriers to shocking the patient? This doesn’t require huge committees. The question is, 'Why isn’t this happening?' … This paper is a call to arms."
According to the study, rates of delayed defibrillation, which were defined as longer than the two-minute standard, ranged from 2.4% to 50.9%. The authors state that standardizing defibrillation times to meet the two-minute standard set by the American Hospital Association could be a quality initiative focus for HM groups.
“Now that we’ve identified the problem, that helps us identify how to move forward,” Dr. Persoff says. “We are in dire need of improving our system when it comes to cardiac care. The hospitalists are in the best position to do that because we are able to work closest with the nurses.”
Jane Kelly-Cummings, RN, CPHQ, SHM's senior director of quality initiatives, agrees there is room for improvement in the survival rate of in-hospital cardiac patients. "In order to make those improvements, hospitals will need to make changes to their cardiac resuscitation processes and procedures," she says. "Hospitalists are integral and central players on cardiac resuscitation teams at a great majority of hospitals with hospital medicine programs. They act as change agents at these and many other facilities."
For more information on HM's role in cardiac resuscitation of hospitalized patients, visit the Emergency Procedures section of the "Core Competencies in Hospital Medicine."a
A new report that hints stress factors like case volume and academic status of a hospital do not explain the wide disparities in defibrillation response times in hospitals has at least one hospitalist convinced HM leaders can help solve the problem.
Traditional hospital pressures do not predict whether patients with cardiac arrest are likely to experience delays in receiving defibrillation, according to a July 27 report in the Archives of Internal Medicine (2009;169(14):1260-1261). Such factors as the number of beds and where the cardiac unit was located were found to have more impact, the study found.
“This is a very simple thing,” says hospitalist Jason Persoff, MD, FHM, assistant professor of medicine at the Mayo Clinic in Jacksonville, Fla. "What are the barriers to shocking the patient? This doesn’t require huge committees. The question is, 'Why isn’t this happening?' … This paper is a call to arms."
According to the study, rates of delayed defibrillation, which were defined as longer than the two-minute standard, ranged from 2.4% to 50.9%. The authors state that standardizing defibrillation times to meet the two-minute standard set by the American Hospital Association could be a quality initiative focus for HM groups.
“Now that we’ve identified the problem, that helps us identify how to move forward,” Dr. Persoff says. “We are in dire need of improving our system when it comes to cardiac care. The hospitalists are in the best position to do that because we are able to work closest with the nurses.”
Jane Kelly-Cummings, RN, CPHQ, SHM's senior director of quality initiatives, agrees there is room for improvement in the survival rate of in-hospital cardiac patients. "In order to make those improvements, hospitals will need to make changes to their cardiac resuscitation processes and procedures," she says. "Hospitalists are integral and central players on cardiac resuscitation teams at a great majority of hospitals with hospital medicine programs. They act as change agents at these and many other facilities."
For more information on HM's role in cardiac resuscitation of hospitalized patients, visit the Emergency Procedures section of the "Core Competencies in Hospital Medicine."a
A new report that hints stress factors like case volume and academic status of a hospital do not explain the wide disparities in defibrillation response times in hospitals has at least one hospitalist convinced HM leaders can help solve the problem.
Traditional hospital pressures do not predict whether patients with cardiac arrest are likely to experience delays in receiving defibrillation, according to a July 27 report in the Archives of Internal Medicine (2009;169(14):1260-1261). Such factors as the number of beds and where the cardiac unit was located were found to have more impact, the study found.
“This is a very simple thing,” says hospitalist Jason Persoff, MD, FHM, assistant professor of medicine at the Mayo Clinic in Jacksonville, Fla. "What are the barriers to shocking the patient? This doesn’t require huge committees. The question is, 'Why isn’t this happening?' … This paper is a call to arms."
According to the study, rates of delayed defibrillation, which were defined as longer than the two-minute standard, ranged from 2.4% to 50.9%. The authors state that standardizing defibrillation times to meet the two-minute standard set by the American Hospital Association could be a quality initiative focus for HM groups.
“Now that we’ve identified the problem, that helps us identify how to move forward,” Dr. Persoff says. “We are in dire need of improving our system when it comes to cardiac care. The hospitalists are in the best position to do that because we are able to work closest with the nurses.”
Jane Kelly-Cummings, RN, CPHQ, SHM's senior director of quality initiatives, agrees there is room for improvement in the survival rate of in-hospital cardiac patients. "In order to make those improvements, hospitals will need to make changes to their cardiac resuscitation processes and procedures," she says. "Hospitalists are integral and central players on cardiac resuscitation teams at a great majority of hospitals with hospital medicine programs. They act as change agents at these and many other facilities."
For more information on HM's role in cardiac resuscitation of hospitalized patients, visit the Emergency Procedures section of the "Core Competencies in Hospital Medicine."a
In the Lit: Research You Need to Know
Clinical question: Does tailoring the duration of anticoagulation based on the persistence of residual thrombus following conventional duration therapy reduce rates of recurrent venous thromboembolism (VTE) in adults with proximal deep-venous thrombosis (DVT)?
Background: The optimal duration of oral anticoagulation therapy in adults with proximal DVT remains uncertain. This study was designed to ascertain if tailoring the duration of therapy based on ultrasonographic findings improves outcomes through a reduction in recurrent VTE.
Study design: Parallel, open-label, randomized trial with independent and blinded assessment of study outcomes.
Setting: Nine university and hospital centers in Italy.
Synopsis: Five hundred thirty-eight patients with proximal DVT who completed three months of anticoagulation were randomly assigned to fixed-duration or flexible-duration therapy. Patients in the fixed-duration group with provoked DVT received no further therapy; those with unprovoked DVT received an additional three months of anticoagulation. Patients in the flexible-duration group had no further therapy if ultrasonography demonstrated recanalized veins, and received further therapy (up to nine to 21 months for provoked and unprovoked DVT, respectively) if persistent thrombi were demonstrated. Patients were followed over three years for the primary outcomes of recurrent VTE and major bleeding events.
Significantly fewer recurrent VTE occurred in the ultrasound guided, flexible-duration treatment group (11.9% vs. 17.2%; HR 0.64; 95% CI 0.39 to 0.99). There was no significant difference in major bleeding events between the two groups.
Limitations of this study include the lack of a double-blind design and the relatively small sample size.
Bottom line: Tailoring the duration of anticoagulation in adults with proximal DVT based on ultrasonographic demonstration of residual thrombi reduces rates of recurrent VTE without increasing major bleeding events.
Citation: Prandoni P, Prins MH, Lensing AW, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med. 2009;150(9):577-585.
Reviewed for TH eWire by Alexander R. Carbo, MD, FHM; Suzanne Bertisch, MD, MPH; Lauren Doctoroff, MD; John Fani Srour, MD; Caleb Hale, MD; Nancy Torres-Finnerty, MD, FHM, Hospital Medicine Program, Beth Israel Deaconess Medical Center, Boston
Clinical question: Does tailoring the duration of anticoagulation based on the persistence of residual thrombus following conventional duration therapy reduce rates of recurrent venous thromboembolism (VTE) in adults with proximal deep-venous thrombosis (DVT)?
Background: The optimal duration of oral anticoagulation therapy in adults with proximal DVT remains uncertain. This study was designed to ascertain if tailoring the duration of therapy based on ultrasonographic findings improves outcomes through a reduction in recurrent VTE.
Study design: Parallel, open-label, randomized trial with independent and blinded assessment of study outcomes.
Setting: Nine university and hospital centers in Italy.
Synopsis: Five hundred thirty-eight patients with proximal DVT who completed three months of anticoagulation were randomly assigned to fixed-duration or flexible-duration therapy. Patients in the fixed-duration group with provoked DVT received no further therapy; those with unprovoked DVT received an additional three months of anticoagulation. Patients in the flexible-duration group had no further therapy if ultrasonography demonstrated recanalized veins, and received further therapy (up to nine to 21 months for provoked and unprovoked DVT, respectively) if persistent thrombi were demonstrated. Patients were followed over three years for the primary outcomes of recurrent VTE and major bleeding events.
Significantly fewer recurrent VTE occurred in the ultrasound guided, flexible-duration treatment group (11.9% vs. 17.2%; HR 0.64; 95% CI 0.39 to 0.99). There was no significant difference in major bleeding events between the two groups.
Limitations of this study include the lack of a double-blind design and the relatively small sample size.
Bottom line: Tailoring the duration of anticoagulation in adults with proximal DVT based on ultrasonographic demonstration of residual thrombi reduces rates of recurrent VTE without increasing major bleeding events.
Citation: Prandoni P, Prins MH, Lensing AW, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med. 2009;150(9):577-585.
Reviewed for TH eWire by Alexander R. Carbo, MD, FHM; Suzanne Bertisch, MD, MPH; Lauren Doctoroff, MD; John Fani Srour, MD; Caleb Hale, MD; Nancy Torres-Finnerty, MD, FHM, Hospital Medicine Program, Beth Israel Deaconess Medical Center, Boston
Clinical question: Does tailoring the duration of anticoagulation based on the persistence of residual thrombus following conventional duration therapy reduce rates of recurrent venous thromboembolism (VTE) in adults with proximal deep-venous thrombosis (DVT)?
Background: The optimal duration of oral anticoagulation therapy in adults with proximal DVT remains uncertain. This study was designed to ascertain if tailoring the duration of therapy based on ultrasonographic findings improves outcomes through a reduction in recurrent VTE.
Study design: Parallel, open-label, randomized trial with independent and blinded assessment of study outcomes.
Setting: Nine university and hospital centers in Italy.
Synopsis: Five hundred thirty-eight patients with proximal DVT who completed three months of anticoagulation were randomly assigned to fixed-duration or flexible-duration therapy. Patients in the fixed-duration group with provoked DVT received no further therapy; those with unprovoked DVT received an additional three months of anticoagulation. Patients in the flexible-duration group had no further therapy if ultrasonography demonstrated recanalized veins, and received further therapy (up to nine to 21 months for provoked and unprovoked DVT, respectively) if persistent thrombi were demonstrated. Patients were followed over three years for the primary outcomes of recurrent VTE and major bleeding events.
Significantly fewer recurrent VTE occurred in the ultrasound guided, flexible-duration treatment group (11.9% vs. 17.2%; HR 0.64; 95% CI 0.39 to 0.99). There was no significant difference in major bleeding events between the two groups.
Limitations of this study include the lack of a double-blind design and the relatively small sample size.
Bottom line: Tailoring the duration of anticoagulation in adults with proximal DVT based on ultrasonographic demonstration of residual thrombi reduces rates of recurrent VTE without increasing major bleeding events.
Citation: Prandoni P, Prins MH, Lensing AW, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med. 2009;150(9):577-585.
Reviewed for TH eWire by Alexander R. Carbo, MD, FHM; Suzanne Bertisch, MD, MPH; Lauren Doctoroff, MD; John Fani Srour, MD; Caleb Hale, MD; Nancy Torres-Finnerty, MD, FHM, Hospital Medicine Program, Beth Israel Deaconess Medical Center, Boston
Afraid in the Hospital
The Institute of Medicine report linking between 48,000 and 98,000 deaths annually to medical errors1 has raised awareness about medical errors across all areas of medicine. In pediatrics, medical errors in hospitalized children are associated with significant increases in length of stay, healthcare costs, and death.2, 3 While much attention has been paid to the use of hospital systems to prevent medical errors, there has been considerably less focus on the experiences of patients and their potential role in preventing errors.
Studies have suggested that a significant majority of adult patients are concerned about medical errors during hospitalization.4, 5 However, a similar assessment of parents' concerns about medical errors in pediatrics is lacking. Admittedly, for concern to be constructive it must be linked to action. The Joint Commission and the Agency for Healthcare Research and Quality (AHRQ) currently recommend that parents help prevent errors by becoming active, involved and informed members of their healthcare team and taking part in every decision about (their) child's health care.6, 7 However, the extent to which parental concern about medical errors is related to a parent's self‐efficacy, or confidence, interacting with physicians is unknown.
Self‐efficacy is a construct used in social cognitive theory to explain behavior change.8 It refers to an individual's belief in (his/her) capabilities to organize and execute the courses of action required to produce given attainments or a desired outcome.9 Self‐efficacy is not a general concept; it must be discussed in reference to a specific activity. In healthcare it has been associated not only with willingness to adopt preventive strategies,10 but also with treatment adherence,11, 12 behavior change,13 and with greater patient participation in healthcare decision‐making.14, 15
In this study we had 2 objectives. First, we sought to assess the proportion of parents of hospitalized children who are concerned about medical errors. Second, we attempted to examine whether a parent's self‐efficacy interacting with physicians was associated with their concern about medical errors for their child. Given that parents with greater self‐efficacy interacting with physicians might feel more empowered to prevent errors and, as such, be more inclined to take an active role to do so, we hypothesized that such parents would be less concerned about medical errors during a pediatric hospitalization.
Subjects and Methods
Population
We surveyed parents of children <18 years of age (including 2 grandparents who will hereafter be referred to as parents) who were admitted to the general medical service of the Children's Hospital & Regional Medical Center (CHRMC) in Seattle, WA, from July through September 2005. This study was approved by the CHRMC Institutional Review Board. Due to stipulations of the Health Insurance Portability and Accountability Act (HIPAA), we were unable to collect extensive information on those parents who were missed or those who refused to participate in the study.
Exclusions
We excluded parents if: (1) they did not feel comfortable answering a written survey in English or Spanish; (2) their child was transferred to the general medical unit either from the intensive care unit (ICU) or from the inpatient unit of another hospital; or (3) they were not present during the hospitalization.
Study Design
We conducted a cross‐sectional self‐administered written survey of parents. The survey was translated into Spanish by a certified Spanish translator. A second independent translator confirmed the accuracy of the translation. Informed consent was obtained from parents before administration of the survey.
Data Collection
Parents were surveyed with a consecutive sampling methodology Tuesday through Friday from July 2005 through September 2005. We surveyed parents within 48 hours of admission of their child to the hospital, but after they had an opportunity to speak with the inpatient medical team that was caring for their child. A more detailed discussion of the data collection process has been published previously.16
Dependent Variables
Parental Concern About the Need to Watch for Medical Errors
We assessed parental concerns about medical errors during hospitalization by measuring responses to the statement When my child is in the hospital I feel that I have to watch over the care that he/she is receiving to make sure that mistakes aren't made. Parents reported their agreement/disagreement with this statement using a 5‐point Likert scale. For our analysis we dichotomized the dependent variable into those parents who responded Strongly Agree or Agree vs. those who responded Strongly Disagree or Disagree. We chose to focus on parents who expressed a directional response (eg, agree or disagree) because we felt that such responses were more likely to be correlated with behavior. As a result, we excluded from our primary analysis those participants who responded Unsure. In order to determine the effect of exclusion on our results (given its size), we conducted separate post‐hoc analyses in which we included the Unsure respondents in Agree and Disagree categories, respectively.
Independent Variables
Self‐Efficacy in Patient‐Physician Interactions
The Joint Commission's Speak Up initiative7 recommends that patients and parents interact with their healthcare providers in order to prevent errors. We gauged a parent's confidence of interacting with healthcare providers using an adapted scale of the Perceived Efficacy in Patient‐Physician Interactions (PEPPI) self‐efficacy scale. The PEPPI is a 50‐point self‐efficacy scale that has been validated in older adults.17 The response to each question is recorded on a 5‐point Likert scale ranging from 1 to 5 where 1 represents not at all confident and 5 represents very confident. Higher scores on this scale have been associated with greater participation in treatment decisions by women with breast cancer.18 We adapted this scale for use in pediatrics (Appendix 1).
Covariates
Prior Hospitalizations and Chronic Illness History
We asked parents to report how many times their child had been hospitalized prior to this current admission (not including birth). We chose to query parents directly because a search of our institutions' medical record database would not capture hospitalizations at other facilities. We categorized the variable for previous hospitalizations as follows: none, 1, 2, 3.
We asked parents to report if prior to this hospitalization they had ever been told by a nurse or doctor that their child had any of a list of chronic medical conditions such as chronic respiratory disease, mental retardation, and seizure disorder, among others. We gave parents the opportunity to specify a medical condition not provided on this list. The list of conditions was the same as that used in the Child Health Questionnaire PF‐28,19 which has been used in national and international studies to measure quality of life in children with and without chronic conditions20, 21 (Appendix 2).
Limited English Proficiency
We assessed the potential for a language barrier to impede communication between parent and healthcare providers by asking parents the following question: How comfortable are you that you can express your concerns and ask questions of your child's doctors in English? We measured parental responses on a 5‐point Likert scale (very comfortable vs. somewhat comfortable, not sure, somewhat uncomfortable, very uncomfortable). For our analyses, we dichotomized responses into those who reported being very comfortable vs. those who chose any other response category.
Social Desirability
We measured the potential for social desirability to bias responses using the Marlowe‐Crowne 2(10) Scale of Social Desirability.22 The Marlowe‐Crowne 2(10) Scale of Social Desirability is a shorter, validated version of the Marlowe‐Crowne Scale.23 This scale has been recommended by the National Institutes of Health (NIH)'s Behavior Change Consortium for use in behavioral change research related to health.24 It has been used in previous studies to account for social desirability bias in studies which involve patient self‐report of attitudes and beliefs.25 We analyzed scores on a continuous scale with higher scores representing greater social desirability bias in responses.
Demographics
We collected the following demographic data on parents: age, gender, race/ethnicity (white non‐Hispanic vs. other), education (high school or less, some college, college or higher). We also recorded the child's age and gender.
Statistical Analysis
Parental Concern for Medical Errors
Univariate statistics were used to report proportions of parents who were concerned about medical errors and to summarize the data for covariates and demographics. We conducted bivariate logistic regression analyses to assess the association between our outcome variable and the independent variable and each covariate, respectively. We used a Fisher's exact test to examine the association between limited English proficiency and our outcome variable because the absence of participants (eg, a zero cell) who were not very comfortable with English and not concerned about errors precluded the use of bivariate logistic regression. Therefore, to explore the relationship between race and language we used a Fischer's exact test to compare concern about medical errors between white and non‐white participants who were very comfortable with English.
We used multivariate logistic regression to test our hypothesis that greater self‐efficacy would be associated with less concern about medical errors after adjusting for the aforementioned covariates and demographics, excluding child gender. We had no a priori reason to expect that child gender would affect parental self‐efficacy or parental concern about errors and did not include it in the regression model. In order to provide a more clinically relevant interpretation of our results, we calculated adjusted predicted probabilities for the 25%, 50%, and 75% PEPPI scores using the mean for all other variables in the model.
We conducted post‐hoc analysis using a likelihood‐ratio test to determine if the hospitalization variable was significant in the multivariate regression model. We conducted additional post‐hoc analysis using bivariate logistic regression to explore the relationship between concern about medical errors and the following independent variables: hospitalization for >3 days after birth (yes/no); previous hospitalization for >1 week (yes/no); parents' experience with the hospital system (a lot, some, not sure, a little, none); overall perception of child's health (excellent, very good, good, fair); previous hospitalizations for other children (yes, no, no other children); rating of care that child has received (excellent vs. other [very good, good, fair]). We incorporated any significant associations (P < 0.05) into our preexisting multivariate model.
Results
During the time period of our study, 278 parents were eligible to participate. Eighty‐five parents could not be surveyed either because they could not be reached despite multiple attempts (eg, out of the room, speaking with physicians) or because the child had already been discharged. Of the 193 parents approached, 130 agreed to take the survey. Two parents who agreed to complete the survey forgot to return it before their children were discharged. Demographics of respondents and nonparticipants are presented in Table 1. The distribution of self‐efficacy scores was skewed, with a mean score of 45 (median 46, range 5‐50) on a 50‐point scale, consistent with previous studies in adults.18
| Characteristics | Respondents (n = 130) | Number Missed (n = 85) | Number Refused (n = 61) |
|---|---|---|---|
| |||
| Parent's mean age | 34 years (range: 18‐51) | NA | NA |
| Parent sex female, n (%) | 105 (80.8) | NA | NA |
| Parental education, n (%) | |||
| College or higher | 65 (50.4) | ||
| Some college | 34 (26.4) | ||
| High school or less | 30 (23.3) | NA | NA |
| Parental race, n (%) | |||
| White | 86 (67.2) | ||
| Non‐White | 42 (32.8) | NA | NA |
| Parent's social desirability score, mean (SD) | 7.0 (2.0) | NA | NA |
| Child's median age | 21.4 months (range: 1 day‐17.8 years) | 24 months | 24 months |
| Child's sex female, n (%) | 63 (48.5) | 36 (42) | 35 (57) |
| Number of previous hospitalizations (child), n (%) | |||
| None | 68 (53.1) | ||
| 1 | 26 (20.3) | ||
| 2 | 19 (14.8) | ||
| 3 | 15 (11.7) | NA | NA |
| Number of chronic medical conditions (child), n (%) | |||
| None | 56 (48.7) | ||
| 1 | 34 (29.6) | ||
| 2 | 25 (21.8) | NA | NA |
| Parent's comfort expressing concerns in English, n (%) | NA | NA | |
| Very comfortable | 109 (83.9) | ||
| Less than very comfortable | 21 (16.1) | ||
| Self‐efficacy score (parent), mean (SD) | 45 (6.3) | NA | NA |
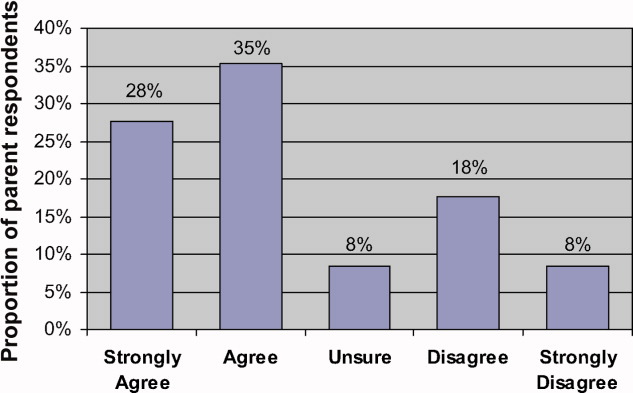
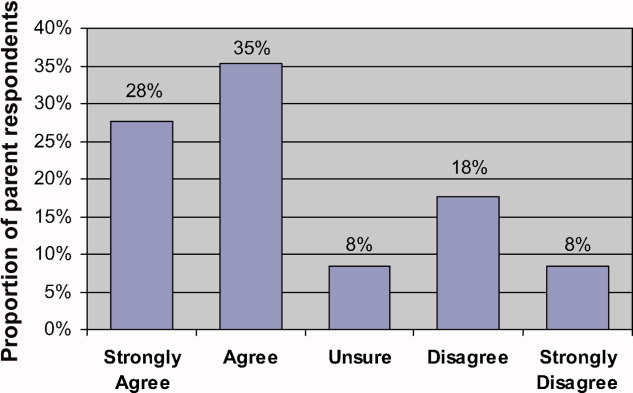
Eighty‐two parents (63% of respondents) Agreed or Strongly Agreed with the statement When my child is in the hospital I feel that I need to watch over his/her care in order to make sure that mistakes aren't made (Figure 1). In bivariate analyses, non‐white race (Table 2) and English proficiency (P = 0.002) were significantly associated with parental concern about medical errors. Notably, all respondents who were not very comfortable with English agreed that they felt the need to watch over their child's care to ensure that mistakes do not happen. The association between self‐efficacy with physician interactions and concern was nearly significant (Table 2).
| Variable | Crude Odds Ratio | Confidence Interval | P Value | Adjusted Odds Ratio | Confidence Interval | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Parent age (years) | 1.01 | 0.95‐1.06 | 0.79 | 1.05 | 0.95‐1.15 | 0.36 |
| Parent gender | ||||||
| Female | Referent | |||||
| Male | 0.86 | 0.32‐2.35 | 0.77 | 0.76 | 0.20‐2.79 | 0.68 |
| Age of child (months) | 1.00 | 0.99‐1.01 | 0.97 | 1.00 | 0.99‐1.01 | 0.83 |
| Parental education | ||||||
| College or higher | Referent | Referent | ||||
| Some college | 0.48 | 0.18‐1.26 | 0.14 | 0.3 | 0.07‐1.13 | 0.07 |
| High school or less | 1.39 | 0.48‐4.00 | 0.55 | 0.5 | 0.1‐2.2 | 0.38 |
| Parental race | ||||||
| White | Referent | Referent | ||||
| Non‐White | 5.00* | 1.61‐15.56 | 0.005 | 4.9 | 1.19‐20.4 | 0.03 |
| Previous hospitalization (child) | ||||||
| None | Referent | Referent | ||||
| 1 | 0.44 | 0.16‐1.20 | 0.11 | 0.16 | 0.04‐0.69 | 0.01 |
| 2 | 0.89 | 0.27‐2.87 | 0.84 | 0.60 | 0.13‐2.83 | 0.5 |
| 3 | 0.91 | 0.21‐3.84 | 0.90 | 0.79 | 0.12‐5.06 | 0.8 |
| Number of chronic medical conditions | 0.86 | 0.67‐1.11 | 0.26 | 1.05 | 0.74‐1.50 | 0.78 |
| Social desirability score (parent) | 0.99 | 0.81‐1.21 | 0.92 | 0.99 | 0.76‐1.29 | 0.9 |
| Self‐efficacy score (parent) | 0.90 | 0.81‐1.00 | 0.06 | 0.83 | 0.73‐0.95 | 0.006* |
In multivariate analysis, self‐efficacy was independently associated with parental report about the need to watch over a child's care (odds ratio [OR], 0.83; 95% confidence interval [CI], 0.72‐0.92). In prediction models, with self‐efficacy scores of 44 (25th percentile), 46 (50th percentile), and 49 (75th percentile), about 72.2% (59.1‐82.3), 64.2% (51.8‐75.0), and 50.8% (35.3‐66.2) of parents, respectively, would feel the need to watch over their child's care to prevent medical errors.
While respondents of non‐white race had the greatest independent odds of reporting a concern for medical errors occurring while their child was hospitalized (OR, 4.9; 95% CI, 1.19‐20.4), we could not reliably determine how much of this effect was due to language instead of to race because the vast majority of parents who reported being less than very comfortable with English were also non‐white (non‐white 90.5% vs. white 9.5%, P < 0.001). In additional analyses we were unable to find a difference in concern about medical errors between white and non‐white parents who were very comfortable with English (data not shown).
Of note, while having 1 hospitalization compared to none was significantly associated with having decreased concern about medical errors (Table 2), the variable hospitalizations was not significant in the model (P = 0.07).
In post‐hoc analysis, we found no association between hospitalization for >3 days after birth, previous hospitalization for >1 week, parents' experience with the hospital system, and overall perception of child's health, previous hospitalizations for other children. While rating of care that child received was significantly associated with parents' concern about medical errors in the bivariate analysis, it did not remain significant in multivariate analysis and did not substantially change the magnitude or significance of previous associations.
Discussion
In our study, we found that nearly two‐thirds of parents of children admitted to the general pediatric service of a tertiary care children's hospital felt the need to watch over their child's care to ensure that mistakes would not be made. We also found that a parent's self‐efficacy interacting with physicians was associated with less parental concern for medical errors.
To our knowledge, this is the first study to systematically survey parents' concerns about medical errors during a child's hospitalization and to evaluate factors associated with this concern. The immediate question prompted by our findings is whether the fact that 63% of parents are concerned is alarming because it is too low or too high. Some might contend that concern about medical errors is an appropriate and desirable response because it may motivate parents to become more vigilant about the medical care that their child is receiving. However, others may challenge that such concern may indicate a feeling of powerlessness to act to prevent potential errors. In our study, the relationship between higher self‐efficacy and less parental concern raises the possibility that parents with higher levels of self‐efficacy with physician interactions may feel more comfortable communicating with physicians, which in turn may temper parents' concerns about medical errors during hospitalization.
It is equally plausible that concern about medical errors during hospitalization may motivate parents to become involved in their child's medical care and, in turn, lead them to feel empowered to prevent medical errors and so ease their concerns. It is conceivable that experience with past medical errors may fuel a parent's need to watch over their child's care to prevent additional medical errors. Future studies should address the independent effect of past medical errors on parental concern about medical errors.
In this study, all parents who reported being very uncomfortable with English and parents of non‐white race felt the need to watch over their child's care to help prevent errors. A previous survey of a nationally representative sample of U.S. adults found greater proportion of non‐white adults were very concerned about errors or mistakes happening when receiving care at a hospital (blacks 62%, Hispanics 57%, whites 44%).26 However, in our study, the relationship between race and concern is likely mediated by language since many of the parents who described themselves as other than white also reported being not very comfortable with English and we could not find an effect of race on concern among parents who were very comfortable with English. Indeed, previous studies have linked decreased English proficiency to medical errors with potential clinical consequence.27
Given our previous investigation of the relationship between self‐efficacy and parent participation in medical decisions during a child's hospitalization, we conducted post‐hoc analyses exploring the association between parents' self‐report of participation in medical decisions and concern about medical errors during their child's hospitalization.16 Using a simple logistic regression we did not find any association. However, we advise caution in interpreting and generalizing these results because the study was not powered to adequately evaluate this association.
There are additional limitations in our study to be noted. First, this question has not been used previously to assess parental concern about medical errors, so future work will need to focus on assessing its reliability and validity. Second, it is also possible that parents' concern for medical errors is mitigated by the complexity of their child's healthcare.5 We attempted to address this issue by controlling for the child's number of chronic illnesses. However, it is possible that our metric did not capture the level of complexity associated with different types of chronic conditions. Moreover, additional variables such as health insurance type, parental physical and mental health, and quality of interactions with the nursing staff may confound the relationships that we observed. Future studies should examine the effect of these variables on parents' self‐efficacy and their concern about medical errors.
Third, we surveyed parents at a single institution and, as such, differences in demographics and hospital‐specific practices related to patient‐physician interactions may prevent generalization of our findings to other institutions. For example, the parents in our survey had a higher average education level than the general population and the racial makeup of our population was not nationally representative. Also, due to HIPAA constraints, we were unable to collect extensive demographic information on parents and children who were missed or those who refused to participate in the study, which also could conceivably influence the strength of our findings.
Fourth, we adapted a validated adult measure of self‐efficacy for use in pediatrics. The patient‐physician self‐efficacy scale, the PEPPI, did have a skewed distribution in our study, although this performance is consistent with adult studies18 and in post‐hoc analyses, outlier PEPPI scores did not have a significant effect on the magnitude of the relationship we observed between self‐efficacy and parental concern about medical errors. However, the reading level of this instrument is ninth grade, which may impact the generalization of our findings to populations with lower literacy levels.
Fifth, we excluded parents who were unsure about their concern from our analyses. In post‐hoc multivariate regression analyses, reassignment of unsure responses to either agree or disagree did not result in any change in odds ratio for any endpoint.
Finally, it is possible that parental concern was influenced by social desirability bias in that parents may have been less likely to report concern about medical errors during a hospitalization because of fear of the implications it might have for their child's care. We attempted to control for this effect by adjusting for social desirability bias using the Marlowe‐Crowne scale. This scale is commonly used in behavioral science research to account for such response bias and has been recommended by the NIH Consortium on Behavior Change for use in behavioral change research related to health.24
Within the context of these limitations, we feel that our study contributes an important first step toward characterizing the scope of parental concern about medical errors during pediatric hospitalizations and understanding the relationship of self‐efficacy with physician interactions to this concern. Devising a quality initiative program to improve parents' self‐efficacy interacting with physicians might help to temper parents' concerns about medical errors while also encouraging their involvement in their child's medical care. Such a program would likely prove most beneficial if it sought to improve self‐efficacy among parents with lower English proficiency given that this group had the highest concern for medical errors. Possible interventions might include more ready access to interpreters or use of visual aids.
- Institute of Medicine Committee on Quality of Health Care in America.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,,, et al.Medication errors and adverse drug events in pediatric inpatients.JAMA.2001;285(16):2114–2120.
- ,.Pediatric patient safety in hospitals: a national picture in 2000.Pediatrics.2004;113(6):1741–1746.
- ,,,,,.Brief report: hospitalized patients' attitudes about and participation in error prevention.J Gen Intern Med.2006;21(4):367–370.
- ,,, et al.Patients' concerns about medical errors during hospitalization.Jt Comm J Qual Patient Saf.2007;33(1):5–14.
- Agency for Healthcare Research and Quality.20 Tips to Help Prevent Medical Errors in Children. Patient Fact Sheet. 2009, AHRQ Publication No. 02‐P034.Rockville, MD:Agency for Healthcare Research and Quality.
- Joint Commission on Accreditation of Healthcare Organizations. Speak Up Initiatives. Available at: http://www.jointcommission.org/PatientSafety/SpeakUp. Accessed May 2009.
- .Self‐efficacy: toward a unifying theory of behavioral change.Psychol Rev.1977;84(2):191–215.
- .Self‐Efficacy: The Exercise of Control.New York, NY:W.H. Freeman and Company;1997.
- ,,,.Psychosocial correlates of physical activity in healthy children.Arch Pediatr Adolesc Med.2001;155(8):897–902.
- ,,,,.Self‐efficacy as a mediator variable for adolescents' adherence to treatment for insulin‐dependent diabetes mellitus.Children's Health Care.2000;29(1):47–63.
- ,,.Diabetes regimen behaviors. Predicting adherence.Med Care.1987;25(9):868–881.
- ,,,,,.Pediatrician self‐efficacy for counseling parents of asthmatic children to quit smoking.Pediatrics.2004;113(1 Pt 1):78–81.
- ,,.Examining the relationship of patients' attitudes and beliefs with their self‐reported level of participation in medical decision‐making.Med Care.2005;43(9):865–872.
- ,,,,,.Patient‐physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision.J Clin Oncol.2004;22(15):3091–3098.
- ,,.Toward family‐centered inpatient medical care: the role of parents as participants in medical decisions.J Pediatr.2007;151(6):690–695.
- ,,,,.Perceived efficacy in patient‐physician interactions (PEPPI): validation of an instrument in older persons.J Am Geriatr Soc.1998;46(7):889–894.
- ,,,.Determinants of participation in treatment decision‐making by older breast cancer patients.Breast Cancer Res Treat.2004;85(3):201–209.
- ,.The CHQ: A User's Manual (2nd printing).Boston, MA:HealthAct;1999.
- ,,,,.The health and well‐being of adolescents: a school‐based population study of the self‐report Child Health Questionnaire.J Adolesc Health.2001;29(2):140–149.
- ,,.The Child Health Questionnaire in children with diabetes: cross‐sectional survey of parent and adolescent‐reported functional health status.Diabet Med.2000;17(10):700–707.
- ,.Semantic style variance in personality questionnaires.J Psychol.1973;85:109–118.
- ,.A new scale of social desirability independent of psychopathology.J Consult Psychol.1960;24:349–354.
- National Institutes of Health. Behavior Change Consortium‐Recommended Nutrition Measures. Available at:http://www1.od.nih.gov/behaviorchange/measures/nutrition.htm. Accessed May 2009.
- ,,.Response bias influences mental health symptom reporting in patients with obstructive sleep apnea.Ann Behav Med.2001;23(4):313–317.
- Kaiser Family Foundation, Agency for Healthcare Research and Quality.Americans as Health Care Consumers: Update on the Role of Quality Information.Rockville, MD:Agency for Healthcare Research and Quality;2000.
- ,,, et al.Errors in medical interpretation and their potential clinical consequences in pediatric encounters.Pediatrics.2003;111(1):6–14.
The Institute of Medicine report linking between 48,000 and 98,000 deaths annually to medical errors1 has raised awareness about medical errors across all areas of medicine. In pediatrics, medical errors in hospitalized children are associated with significant increases in length of stay, healthcare costs, and death.2, 3 While much attention has been paid to the use of hospital systems to prevent medical errors, there has been considerably less focus on the experiences of patients and their potential role in preventing errors.
Studies have suggested that a significant majority of adult patients are concerned about medical errors during hospitalization.4, 5 However, a similar assessment of parents' concerns about medical errors in pediatrics is lacking. Admittedly, for concern to be constructive it must be linked to action. The Joint Commission and the Agency for Healthcare Research and Quality (AHRQ) currently recommend that parents help prevent errors by becoming active, involved and informed members of their healthcare team and taking part in every decision about (their) child's health care.6, 7 However, the extent to which parental concern about medical errors is related to a parent's self‐efficacy, or confidence, interacting with physicians is unknown.
Self‐efficacy is a construct used in social cognitive theory to explain behavior change.8 It refers to an individual's belief in (his/her) capabilities to organize and execute the courses of action required to produce given attainments or a desired outcome.9 Self‐efficacy is not a general concept; it must be discussed in reference to a specific activity. In healthcare it has been associated not only with willingness to adopt preventive strategies,10 but also with treatment adherence,11, 12 behavior change,13 and with greater patient participation in healthcare decision‐making.14, 15
In this study we had 2 objectives. First, we sought to assess the proportion of parents of hospitalized children who are concerned about medical errors. Second, we attempted to examine whether a parent's self‐efficacy interacting with physicians was associated with their concern about medical errors for their child. Given that parents with greater self‐efficacy interacting with physicians might feel more empowered to prevent errors and, as such, be more inclined to take an active role to do so, we hypothesized that such parents would be less concerned about medical errors during a pediatric hospitalization.
Subjects and Methods
Population
We surveyed parents of children <18 years of age (including 2 grandparents who will hereafter be referred to as parents) who were admitted to the general medical service of the Children's Hospital & Regional Medical Center (CHRMC) in Seattle, WA, from July through September 2005. This study was approved by the CHRMC Institutional Review Board. Due to stipulations of the Health Insurance Portability and Accountability Act (HIPAA), we were unable to collect extensive information on those parents who were missed or those who refused to participate in the study.
Exclusions
We excluded parents if: (1) they did not feel comfortable answering a written survey in English or Spanish; (2) their child was transferred to the general medical unit either from the intensive care unit (ICU) or from the inpatient unit of another hospital; or (3) they were not present during the hospitalization.
Study Design
We conducted a cross‐sectional self‐administered written survey of parents. The survey was translated into Spanish by a certified Spanish translator. A second independent translator confirmed the accuracy of the translation. Informed consent was obtained from parents before administration of the survey.
Data Collection
Parents were surveyed with a consecutive sampling methodology Tuesday through Friday from July 2005 through September 2005. We surveyed parents within 48 hours of admission of their child to the hospital, but after they had an opportunity to speak with the inpatient medical team that was caring for their child. A more detailed discussion of the data collection process has been published previously.16
Dependent Variables
Parental Concern About the Need to Watch for Medical Errors
We assessed parental concerns about medical errors during hospitalization by measuring responses to the statement When my child is in the hospital I feel that I have to watch over the care that he/she is receiving to make sure that mistakes aren't made. Parents reported their agreement/disagreement with this statement using a 5‐point Likert scale. For our analysis we dichotomized the dependent variable into those parents who responded Strongly Agree or Agree vs. those who responded Strongly Disagree or Disagree. We chose to focus on parents who expressed a directional response (eg, agree or disagree) because we felt that such responses were more likely to be correlated with behavior. As a result, we excluded from our primary analysis those participants who responded Unsure. In order to determine the effect of exclusion on our results (given its size), we conducted separate post‐hoc analyses in which we included the Unsure respondents in Agree and Disagree categories, respectively.
Independent Variables
Self‐Efficacy in Patient‐Physician Interactions
The Joint Commission's Speak Up initiative7 recommends that patients and parents interact with their healthcare providers in order to prevent errors. We gauged a parent's confidence of interacting with healthcare providers using an adapted scale of the Perceived Efficacy in Patient‐Physician Interactions (PEPPI) self‐efficacy scale. The PEPPI is a 50‐point self‐efficacy scale that has been validated in older adults.17 The response to each question is recorded on a 5‐point Likert scale ranging from 1 to 5 where 1 represents not at all confident and 5 represents very confident. Higher scores on this scale have been associated with greater participation in treatment decisions by women with breast cancer.18 We adapted this scale for use in pediatrics (Appendix 1).
Covariates
Prior Hospitalizations and Chronic Illness History
We asked parents to report how many times their child had been hospitalized prior to this current admission (not including birth). We chose to query parents directly because a search of our institutions' medical record database would not capture hospitalizations at other facilities. We categorized the variable for previous hospitalizations as follows: none, 1, 2, 3.
We asked parents to report if prior to this hospitalization they had ever been told by a nurse or doctor that their child had any of a list of chronic medical conditions such as chronic respiratory disease, mental retardation, and seizure disorder, among others. We gave parents the opportunity to specify a medical condition not provided on this list. The list of conditions was the same as that used in the Child Health Questionnaire PF‐28,19 which has been used in national and international studies to measure quality of life in children with and without chronic conditions20, 21 (Appendix 2).
Limited English Proficiency
We assessed the potential for a language barrier to impede communication between parent and healthcare providers by asking parents the following question: How comfortable are you that you can express your concerns and ask questions of your child's doctors in English? We measured parental responses on a 5‐point Likert scale (very comfortable vs. somewhat comfortable, not sure, somewhat uncomfortable, very uncomfortable). For our analyses, we dichotomized responses into those who reported being very comfortable vs. those who chose any other response category.
Social Desirability
We measured the potential for social desirability to bias responses using the Marlowe‐Crowne 2(10) Scale of Social Desirability.22 The Marlowe‐Crowne 2(10) Scale of Social Desirability is a shorter, validated version of the Marlowe‐Crowne Scale.23 This scale has been recommended by the National Institutes of Health (NIH)'s Behavior Change Consortium for use in behavioral change research related to health.24 It has been used in previous studies to account for social desirability bias in studies which involve patient self‐report of attitudes and beliefs.25 We analyzed scores on a continuous scale with higher scores representing greater social desirability bias in responses.
Demographics
We collected the following demographic data on parents: age, gender, race/ethnicity (white non‐Hispanic vs. other), education (high school or less, some college, college or higher). We also recorded the child's age and gender.
Statistical Analysis
Parental Concern for Medical Errors
Univariate statistics were used to report proportions of parents who were concerned about medical errors and to summarize the data for covariates and demographics. We conducted bivariate logistic regression analyses to assess the association between our outcome variable and the independent variable and each covariate, respectively. We used a Fisher's exact test to examine the association between limited English proficiency and our outcome variable because the absence of participants (eg, a zero cell) who were not very comfortable with English and not concerned about errors precluded the use of bivariate logistic regression. Therefore, to explore the relationship between race and language we used a Fischer's exact test to compare concern about medical errors between white and non‐white participants who were very comfortable with English.
We used multivariate logistic regression to test our hypothesis that greater self‐efficacy would be associated with less concern about medical errors after adjusting for the aforementioned covariates and demographics, excluding child gender. We had no a priori reason to expect that child gender would affect parental self‐efficacy or parental concern about errors and did not include it in the regression model. In order to provide a more clinically relevant interpretation of our results, we calculated adjusted predicted probabilities for the 25%, 50%, and 75% PEPPI scores using the mean for all other variables in the model.
We conducted post‐hoc analysis using a likelihood‐ratio test to determine if the hospitalization variable was significant in the multivariate regression model. We conducted additional post‐hoc analysis using bivariate logistic regression to explore the relationship between concern about medical errors and the following independent variables: hospitalization for >3 days after birth (yes/no); previous hospitalization for >1 week (yes/no); parents' experience with the hospital system (a lot, some, not sure, a little, none); overall perception of child's health (excellent, very good, good, fair); previous hospitalizations for other children (yes, no, no other children); rating of care that child has received (excellent vs. other [very good, good, fair]). We incorporated any significant associations (P < 0.05) into our preexisting multivariate model.
Results
During the time period of our study, 278 parents were eligible to participate. Eighty‐five parents could not be surveyed either because they could not be reached despite multiple attempts (eg, out of the room, speaking with physicians) or because the child had already been discharged. Of the 193 parents approached, 130 agreed to take the survey. Two parents who agreed to complete the survey forgot to return it before their children were discharged. Demographics of respondents and nonparticipants are presented in Table 1. The distribution of self‐efficacy scores was skewed, with a mean score of 45 (median 46, range 5‐50) on a 50‐point scale, consistent with previous studies in adults.18
| Characteristics | Respondents (n = 130) | Number Missed (n = 85) | Number Refused (n = 61) |
|---|---|---|---|
| |||
| Parent's mean age | 34 years (range: 18‐51) | NA | NA |
| Parent sex female, n (%) | 105 (80.8) | NA | NA |
| Parental education, n (%) | |||
| College or higher | 65 (50.4) | ||
| Some college | 34 (26.4) | ||
| High school or less | 30 (23.3) | NA | NA |
| Parental race, n (%) | |||
| White | 86 (67.2) | ||
| Non‐White | 42 (32.8) | NA | NA |
| Parent's social desirability score, mean (SD) | 7.0 (2.0) | NA | NA |
| Child's median age | 21.4 months (range: 1 day‐17.8 years) | 24 months | 24 months |
| Child's sex female, n (%) | 63 (48.5) | 36 (42) | 35 (57) |
| Number of previous hospitalizations (child), n (%) | |||
| None | 68 (53.1) | ||
| 1 | 26 (20.3) | ||
| 2 | 19 (14.8) | ||
| 3 | 15 (11.7) | NA | NA |
| Number of chronic medical conditions (child), n (%) | |||
| None | 56 (48.7) | ||
| 1 | 34 (29.6) | ||
| 2 | 25 (21.8) | NA | NA |
| Parent's comfort expressing concerns in English, n (%) | NA | NA | |
| Very comfortable | 109 (83.9) | ||
| Less than very comfortable | 21 (16.1) | ||
| Self‐efficacy score (parent), mean (SD) | 45 (6.3) | NA | NA |
Eighty‐two parents (63% of respondents) Agreed or Strongly Agreed with the statement When my child is in the hospital I feel that I need to watch over his/her care in order to make sure that mistakes aren't made (Figure 1). In bivariate analyses, non‐white race (Table 2) and English proficiency (P = 0.002) were significantly associated with parental concern about medical errors. Notably, all respondents who were not very comfortable with English agreed that they felt the need to watch over their child's care to ensure that mistakes do not happen. The association between self‐efficacy with physician interactions and concern was nearly significant (Table 2).

| Variable | Crude Odds Ratio | Confidence Interval | P Value | Adjusted Odds Ratio | Confidence Interval | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Parent age (years) | 1.01 | 0.95‐1.06 | 0.79 | 1.05 | 0.95‐1.15 | 0.36 |
| Parent gender | ||||||
| Female | Referent | |||||
| Male | 0.86 | 0.32‐2.35 | 0.77 | 0.76 | 0.20‐2.79 | 0.68 |
| Age of child (months) | 1.00 | 0.99‐1.01 | 0.97 | 1.00 | 0.99‐1.01 | 0.83 |
| Parental education | ||||||
| College or higher | Referent | Referent | ||||
| Some college | 0.48 | 0.18‐1.26 | 0.14 | 0.3 | 0.07‐1.13 | 0.07 |
| High school or less | 1.39 | 0.48‐4.00 | 0.55 | 0.5 | 0.1‐2.2 | 0.38 |
| Parental race | ||||||
| White | Referent | Referent | ||||
| Non‐White | 5.00* | 1.61‐15.56 | 0.005 | 4.9 | 1.19‐20.4 | 0.03 |
| Previous hospitalization (child) | ||||||
| None | Referent | Referent | ||||
| 1 | 0.44 | 0.16‐1.20 | 0.11 | 0.16 | 0.04‐0.69 | 0.01 |
| 2 | 0.89 | 0.27‐2.87 | 0.84 | 0.60 | 0.13‐2.83 | 0.5 |
| 3 | 0.91 | 0.21‐3.84 | 0.90 | 0.79 | 0.12‐5.06 | 0.8 |
| Number of chronic medical conditions | 0.86 | 0.67‐1.11 | 0.26 | 1.05 | 0.74‐1.50 | 0.78 |
| Social desirability score (parent) | 0.99 | 0.81‐1.21 | 0.92 | 0.99 | 0.76‐1.29 | 0.9 |
| Self‐efficacy score (parent) | 0.90 | 0.81‐1.00 | 0.06 | 0.83 | 0.73‐0.95 | 0.006* |
In multivariate analysis, self‐efficacy was independently associated with parental report about the need to watch over a child's care (odds ratio [OR], 0.83; 95% confidence interval [CI], 0.72‐0.92). In prediction models, with self‐efficacy scores of 44 (25th percentile), 46 (50th percentile), and 49 (75th percentile), about 72.2% (59.1‐82.3), 64.2% (51.8‐75.0), and 50.8% (35.3‐66.2) of parents, respectively, would feel the need to watch over their child's care to prevent medical errors.
While respondents of non‐white race had the greatest independent odds of reporting a concern for medical errors occurring while their child was hospitalized (OR, 4.9; 95% CI, 1.19‐20.4), we could not reliably determine how much of this effect was due to language instead of to race because the vast majority of parents who reported being less than very comfortable with English were also non‐white (non‐white 90.5% vs. white 9.5%, P < 0.001). In additional analyses we were unable to find a difference in concern about medical errors between white and non‐white parents who were very comfortable with English (data not shown).
Of note, while having 1 hospitalization compared to none was significantly associated with having decreased concern about medical errors (Table 2), the variable hospitalizations was not significant in the model (P = 0.07).
In post‐hoc analysis, we found no association between hospitalization for >3 days after birth, previous hospitalization for >1 week, parents' experience with the hospital system, and overall perception of child's health, previous hospitalizations for other children. While rating of care that child received was significantly associated with parents' concern about medical errors in the bivariate analysis, it did not remain significant in multivariate analysis and did not substantially change the magnitude or significance of previous associations.
Discussion
In our study, we found that nearly two‐thirds of parents of children admitted to the general pediatric service of a tertiary care children's hospital felt the need to watch over their child's care to ensure that mistakes would not be made. We also found that a parent's self‐efficacy interacting with physicians was associated with less parental concern for medical errors.
To our knowledge, this is the first study to systematically survey parents' concerns about medical errors during a child's hospitalization and to evaluate factors associated with this concern. The immediate question prompted by our findings is whether the fact that 63% of parents are concerned is alarming because it is too low or too high. Some might contend that concern about medical errors is an appropriate and desirable response because it may motivate parents to become more vigilant about the medical care that their child is receiving. However, others may challenge that such concern may indicate a feeling of powerlessness to act to prevent potential errors. In our study, the relationship between higher self‐efficacy and less parental concern raises the possibility that parents with higher levels of self‐efficacy with physician interactions may feel more comfortable communicating with physicians, which in turn may temper parents' concerns about medical errors during hospitalization.
It is equally plausible that concern about medical errors during hospitalization may motivate parents to become involved in their child's medical care and, in turn, lead them to feel empowered to prevent medical errors and so ease their concerns. It is conceivable that experience with past medical errors may fuel a parent's need to watch over their child's care to prevent additional medical errors. Future studies should address the independent effect of past medical errors on parental concern about medical errors.
In this study, all parents who reported being very uncomfortable with English and parents of non‐white race felt the need to watch over their child's care to help prevent errors. A previous survey of a nationally representative sample of U.S. adults found greater proportion of non‐white adults were very concerned about errors or mistakes happening when receiving care at a hospital (blacks 62%, Hispanics 57%, whites 44%).26 However, in our study, the relationship between race and concern is likely mediated by language since many of the parents who described themselves as other than white also reported being not very comfortable with English and we could not find an effect of race on concern among parents who were very comfortable with English. Indeed, previous studies have linked decreased English proficiency to medical errors with potential clinical consequence.27
Given our previous investigation of the relationship between self‐efficacy and parent participation in medical decisions during a child's hospitalization, we conducted post‐hoc analyses exploring the association between parents' self‐report of participation in medical decisions and concern about medical errors during their child's hospitalization.16 Using a simple logistic regression we did not find any association. However, we advise caution in interpreting and generalizing these results because the study was not powered to adequately evaluate this association.
There are additional limitations in our study to be noted. First, this question has not been used previously to assess parental concern about medical errors, so future work will need to focus on assessing its reliability and validity. Second, it is also possible that parents' concern for medical errors is mitigated by the complexity of their child's healthcare.5 We attempted to address this issue by controlling for the child's number of chronic illnesses. However, it is possible that our metric did not capture the level of complexity associated with different types of chronic conditions. Moreover, additional variables such as health insurance type, parental physical and mental health, and quality of interactions with the nursing staff may confound the relationships that we observed. Future studies should examine the effect of these variables on parents' self‐efficacy and their concern about medical errors.
Third, we surveyed parents at a single institution and, as such, differences in demographics and hospital‐specific practices related to patient‐physician interactions may prevent generalization of our findings to other institutions. For example, the parents in our survey had a higher average education level than the general population and the racial makeup of our population was not nationally representative. Also, due to HIPAA constraints, we were unable to collect extensive demographic information on parents and children who were missed or those who refused to participate in the study, which also could conceivably influence the strength of our findings.
Fourth, we adapted a validated adult measure of self‐efficacy for use in pediatrics. The patient‐physician self‐efficacy scale, the PEPPI, did have a skewed distribution in our study, although this performance is consistent with adult studies18 and in post‐hoc analyses, outlier PEPPI scores did not have a significant effect on the magnitude of the relationship we observed between self‐efficacy and parental concern about medical errors. However, the reading level of this instrument is ninth grade, which may impact the generalization of our findings to populations with lower literacy levels.
Fifth, we excluded parents who were unsure about their concern from our analyses. In post‐hoc multivariate regression analyses, reassignment of unsure responses to either agree or disagree did not result in any change in odds ratio for any endpoint.
Finally, it is possible that parental concern was influenced by social desirability bias in that parents may have been less likely to report concern about medical errors during a hospitalization because of fear of the implications it might have for their child's care. We attempted to control for this effect by adjusting for social desirability bias using the Marlowe‐Crowne scale. This scale is commonly used in behavioral science research to account for such response bias and has been recommended by the NIH Consortium on Behavior Change for use in behavioral change research related to health.24
Within the context of these limitations, we feel that our study contributes an important first step toward characterizing the scope of parental concern about medical errors during pediatric hospitalizations and understanding the relationship of self‐efficacy with physician interactions to this concern. Devising a quality initiative program to improve parents' self‐efficacy interacting with physicians might help to temper parents' concerns about medical errors while also encouraging their involvement in their child's medical care. Such a program would likely prove most beneficial if it sought to improve self‐efficacy among parents with lower English proficiency given that this group had the highest concern for medical errors. Possible interventions might include more ready access to interpreters or use of visual aids.
The Institute of Medicine report linking between 48,000 and 98,000 deaths annually to medical errors1 has raised awareness about medical errors across all areas of medicine. In pediatrics, medical errors in hospitalized children are associated with significant increases in length of stay, healthcare costs, and death.2, 3 While much attention has been paid to the use of hospital systems to prevent medical errors, there has been considerably less focus on the experiences of patients and their potential role in preventing errors.
Studies have suggested that a significant majority of adult patients are concerned about medical errors during hospitalization.4, 5 However, a similar assessment of parents' concerns about medical errors in pediatrics is lacking. Admittedly, for concern to be constructive it must be linked to action. The Joint Commission and the Agency for Healthcare Research and Quality (AHRQ) currently recommend that parents help prevent errors by becoming active, involved and informed members of their healthcare team and taking part in every decision about (their) child's health care.6, 7 However, the extent to which parental concern about medical errors is related to a parent's self‐efficacy, or confidence, interacting with physicians is unknown.
Self‐efficacy is a construct used in social cognitive theory to explain behavior change.8 It refers to an individual's belief in (his/her) capabilities to organize and execute the courses of action required to produce given attainments or a desired outcome.9 Self‐efficacy is not a general concept; it must be discussed in reference to a specific activity. In healthcare it has been associated not only with willingness to adopt preventive strategies,10 but also with treatment adherence,11, 12 behavior change,13 and with greater patient participation in healthcare decision‐making.14, 15
In this study we had 2 objectives. First, we sought to assess the proportion of parents of hospitalized children who are concerned about medical errors. Second, we attempted to examine whether a parent's self‐efficacy interacting with physicians was associated with their concern about medical errors for their child. Given that parents with greater self‐efficacy interacting with physicians might feel more empowered to prevent errors and, as such, be more inclined to take an active role to do so, we hypothesized that such parents would be less concerned about medical errors during a pediatric hospitalization.
Subjects and Methods
Population
We surveyed parents of children <18 years of age (including 2 grandparents who will hereafter be referred to as parents) who were admitted to the general medical service of the Children's Hospital & Regional Medical Center (CHRMC) in Seattle, WA, from July through September 2005. This study was approved by the CHRMC Institutional Review Board. Due to stipulations of the Health Insurance Portability and Accountability Act (HIPAA), we were unable to collect extensive information on those parents who were missed or those who refused to participate in the study.
Exclusions
We excluded parents if: (1) they did not feel comfortable answering a written survey in English or Spanish; (2) their child was transferred to the general medical unit either from the intensive care unit (ICU) or from the inpatient unit of another hospital; or (3) they were not present during the hospitalization.
Study Design
We conducted a cross‐sectional self‐administered written survey of parents. The survey was translated into Spanish by a certified Spanish translator. A second independent translator confirmed the accuracy of the translation. Informed consent was obtained from parents before administration of the survey.
Data Collection
Parents were surveyed with a consecutive sampling methodology Tuesday through Friday from July 2005 through September 2005. We surveyed parents within 48 hours of admission of their child to the hospital, but after they had an opportunity to speak with the inpatient medical team that was caring for their child. A more detailed discussion of the data collection process has been published previously.16
Dependent Variables
Parental Concern About the Need to Watch for Medical Errors
We assessed parental concerns about medical errors during hospitalization by measuring responses to the statement When my child is in the hospital I feel that I have to watch over the care that he/she is receiving to make sure that mistakes aren't made. Parents reported their agreement/disagreement with this statement using a 5‐point Likert scale. For our analysis we dichotomized the dependent variable into those parents who responded Strongly Agree or Agree vs. those who responded Strongly Disagree or Disagree. We chose to focus on parents who expressed a directional response (eg, agree or disagree) because we felt that such responses were more likely to be correlated with behavior. As a result, we excluded from our primary analysis those participants who responded Unsure. In order to determine the effect of exclusion on our results (given its size), we conducted separate post‐hoc analyses in which we included the Unsure respondents in Agree and Disagree categories, respectively.
Independent Variables
Self‐Efficacy in Patient‐Physician Interactions
The Joint Commission's Speak Up initiative7 recommends that patients and parents interact with their healthcare providers in order to prevent errors. We gauged a parent's confidence of interacting with healthcare providers using an adapted scale of the Perceived Efficacy in Patient‐Physician Interactions (PEPPI) self‐efficacy scale. The PEPPI is a 50‐point self‐efficacy scale that has been validated in older adults.17 The response to each question is recorded on a 5‐point Likert scale ranging from 1 to 5 where 1 represents not at all confident and 5 represents very confident. Higher scores on this scale have been associated with greater participation in treatment decisions by women with breast cancer.18 We adapted this scale for use in pediatrics (Appendix 1).
Covariates
Prior Hospitalizations and Chronic Illness History
We asked parents to report how many times their child had been hospitalized prior to this current admission (not including birth). We chose to query parents directly because a search of our institutions' medical record database would not capture hospitalizations at other facilities. We categorized the variable for previous hospitalizations as follows: none, 1, 2, 3.
We asked parents to report if prior to this hospitalization they had ever been told by a nurse or doctor that their child had any of a list of chronic medical conditions such as chronic respiratory disease, mental retardation, and seizure disorder, among others. We gave parents the opportunity to specify a medical condition not provided on this list. The list of conditions was the same as that used in the Child Health Questionnaire PF‐28,19 which has been used in national and international studies to measure quality of life in children with and without chronic conditions20, 21 (Appendix 2).
Limited English Proficiency
We assessed the potential for a language barrier to impede communication between parent and healthcare providers by asking parents the following question: How comfortable are you that you can express your concerns and ask questions of your child's doctors in English? We measured parental responses on a 5‐point Likert scale (very comfortable vs. somewhat comfortable, not sure, somewhat uncomfortable, very uncomfortable). For our analyses, we dichotomized responses into those who reported being very comfortable vs. those who chose any other response category.
Social Desirability
We measured the potential for social desirability to bias responses using the Marlowe‐Crowne 2(10) Scale of Social Desirability.22 The Marlowe‐Crowne 2(10) Scale of Social Desirability is a shorter, validated version of the Marlowe‐Crowne Scale.23 This scale has been recommended by the National Institutes of Health (NIH)'s Behavior Change Consortium for use in behavioral change research related to health.24 It has been used in previous studies to account for social desirability bias in studies which involve patient self‐report of attitudes and beliefs.25 We analyzed scores on a continuous scale with higher scores representing greater social desirability bias in responses.
Demographics
We collected the following demographic data on parents: age, gender, race/ethnicity (white non‐Hispanic vs. other), education (high school or less, some college, college or higher). We also recorded the child's age and gender.
Statistical Analysis
Parental Concern for Medical Errors
Univariate statistics were used to report proportions of parents who were concerned about medical errors and to summarize the data for covariates and demographics. We conducted bivariate logistic regression analyses to assess the association between our outcome variable and the independent variable and each covariate, respectively. We used a Fisher's exact test to examine the association between limited English proficiency and our outcome variable because the absence of participants (eg, a zero cell) who were not very comfortable with English and not concerned about errors precluded the use of bivariate logistic regression. Therefore, to explore the relationship between race and language we used a Fischer's exact test to compare concern about medical errors between white and non‐white participants who were very comfortable with English.
We used multivariate logistic regression to test our hypothesis that greater self‐efficacy would be associated with less concern about medical errors after adjusting for the aforementioned covariates and demographics, excluding child gender. We had no a priori reason to expect that child gender would affect parental self‐efficacy or parental concern about errors and did not include it in the regression model. In order to provide a more clinically relevant interpretation of our results, we calculated adjusted predicted probabilities for the 25%, 50%, and 75% PEPPI scores using the mean for all other variables in the model.
We conducted post‐hoc analysis using a likelihood‐ratio test to determine if the hospitalization variable was significant in the multivariate regression model. We conducted additional post‐hoc analysis using bivariate logistic regression to explore the relationship between concern about medical errors and the following independent variables: hospitalization for >3 days after birth (yes/no); previous hospitalization for >1 week (yes/no); parents' experience with the hospital system (a lot, some, not sure, a little, none); overall perception of child's health (excellent, very good, good, fair); previous hospitalizations for other children (yes, no, no other children); rating of care that child has received (excellent vs. other [very good, good, fair]). We incorporated any significant associations (P < 0.05) into our preexisting multivariate model.
Results
During the time period of our study, 278 parents were eligible to participate. Eighty‐five parents could not be surveyed either because they could not be reached despite multiple attempts (eg, out of the room, speaking with physicians) or because the child had already been discharged. Of the 193 parents approached, 130 agreed to take the survey. Two parents who agreed to complete the survey forgot to return it before their children were discharged. Demographics of respondents and nonparticipants are presented in Table 1. The distribution of self‐efficacy scores was skewed, with a mean score of 45 (median 46, range 5‐50) on a 50‐point scale, consistent with previous studies in adults.18
| Characteristics | Respondents (n = 130) | Number Missed (n = 85) | Number Refused (n = 61) |
|---|---|---|---|
| |||
| Parent's mean age | 34 years (range: 18‐51) | NA | NA |
| Parent sex female, n (%) | 105 (80.8) | NA | NA |
| Parental education, n (%) | |||
| College or higher | 65 (50.4) | ||
| Some college | 34 (26.4) | ||
| High school or less | 30 (23.3) | NA | NA |
| Parental race, n (%) | |||
| White | 86 (67.2) | ||
| Non‐White | 42 (32.8) | NA | NA |
| Parent's social desirability score, mean (SD) | 7.0 (2.0) | NA | NA |
| Child's median age | 21.4 months (range: 1 day‐17.8 years) | 24 months | 24 months |
| Child's sex female, n (%) | 63 (48.5) | 36 (42) | 35 (57) |
| Number of previous hospitalizations (child), n (%) | |||
| None | 68 (53.1) | ||
| 1 | 26 (20.3) | ||
| 2 | 19 (14.8) | ||
| 3 | 15 (11.7) | NA | NA |
| Number of chronic medical conditions (child), n (%) | |||
| None | 56 (48.7) | ||
| 1 | 34 (29.6) | ||
| 2 | 25 (21.8) | NA | NA |
| Parent's comfort expressing concerns in English, n (%) | NA | NA | |
| Very comfortable | 109 (83.9) | ||
| Less than very comfortable | 21 (16.1) | ||
| Self‐efficacy score (parent), mean (SD) | 45 (6.3) | NA | NA |
Eighty‐two parents (63% of respondents) Agreed or Strongly Agreed with the statement When my child is in the hospital I feel that I need to watch over his/her care in order to make sure that mistakes aren't made (Figure 1). In bivariate analyses, non‐white race (Table 2) and English proficiency (P = 0.002) were significantly associated with parental concern about medical errors. Notably, all respondents who were not very comfortable with English agreed that they felt the need to watch over their child's care to ensure that mistakes do not happen. The association between self‐efficacy with physician interactions and concern was nearly significant (Table 2).

| Variable | Crude Odds Ratio | Confidence Interval | P Value | Adjusted Odds Ratio | Confidence Interval | P Value |
|---|---|---|---|---|---|---|
| ||||||
| Parent age (years) | 1.01 | 0.95‐1.06 | 0.79 | 1.05 | 0.95‐1.15 | 0.36 |
| Parent gender | ||||||
| Female | Referent | |||||
| Male | 0.86 | 0.32‐2.35 | 0.77 | 0.76 | 0.20‐2.79 | 0.68 |
| Age of child (months) | 1.00 | 0.99‐1.01 | 0.97 | 1.00 | 0.99‐1.01 | 0.83 |
| Parental education | ||||||
| College or higher | Referent | Referent | ||||
| Some college | 0.48 | 0.18‐1.26 | 0.14 | 0.3 | 0.07‐1.13 | 0.07 |
| High school or less | 1.39 | 0.48‐4.00 | 0.55 | 0.5 | 0.1‐2.2 | 0.38 |
| Parental race | ||||||
| White | Referent | Referent | ||||
| Non‐White | 5.00* | 1.61‐15.56 | 0.005 | 4.9 | 1.19‐20.4 | 0.03 |
| Previous hospitalization (child) | ||||||
| None | Referent | Referent | ||||
| 1 | 0.44 | 0.16‐1.20 | 0.11 | 0.16 | 0.04‐0.69 | 0.01 |
| 2 | 0.89 | 0.27‐2.87 | 0.84 | 0.60 | 0.13‐2.83 | 0.5 |
| 3 | 0.91 | 0.21‐3.84 | 0.90 | 0.79 | 0.12‐5.06 | 0.8 |
| Number of chronic medical conditions | 0.86 | 0.67‐1.11 | 0.26 | 1.05 | 0.74‐1.50 | 0.78 |
| Social desirability score (parent) | 0.99 | 0.81‐1.21 | 0.92 | 0.99 | 0.76‐1.29 | 0.9 |
| Self‐efficacy score (parent) | 0.90 | 0.81‐1.00 | 0.06 | 0.83 | 0.73‐0.95 | 0.006* |
In multivariate analysis, self‐efficacy was independently associated with parental report about the need to watch over a child's care (odds ratio [OR], 0.83; 95% confidence interval [CI], 0.72‐0.92). In prediction models, with self‐efficacy scores of 44 (25th percentile), 46 (50th percentile), and 49 (75th percentile), about 72.2% (59.1‐82.3), 64.2% (51.8‐75.0), and 50.8% (35.3‐66.2) of parents, respectively, would feel the need to watch over their child's care to prevent medical errors.
While respondents of non‐white race had the greatest independent odds of reporting a concern for medical errors occurring while their child was hospitalized (OR, 4.9; 95% CI, 1.19‐20.4), we could not reliably determine how much of this effect was due to language instead of to race because the vast majority of parents who reported being less than very comfortable with English were also non‐white (non‐white 90.5% vs. white 9.5%, P < 0.001). In additional analyses we were unable to find a difference in concern about medical errors between white and non‐white parents who were very comfortable with English (data not shown).
Of note, while having 1 hospitalization compared to none was significantly associated with having decreased concern about medical errors (Table 2), the variable hospitalizations was not significant in the model (P = 0.07).
In post‐hoc analysis, we found no association between hospitalization for >3 days after birth, previous hospitalization for >1 week, parents' experience with the hospital system, and overall perception of child's health, previous hospitalizations for other children. While rating of care that child received was significantly associated with parents' concern about medical errors in the bivariate analysis, it did not remain significant in multivariate analysis and did not substantially change the magnitude or significance of previous associations.
Discussion
In our study, we found that nearly two‐thirds of parents of children admitted to the general pediatric service of a tertiary care children's hospital felt the need to watch over their child's care to ensure that mistakes would not be made. We also found that a parent's self‐efficacy interacting with physicians was associated with less parental concern for medical errors.
To our knowledge, this is the first study to systematically survey parents' concerns about medical errors during a child's hospitalization and to evaluate factors associated with this concern. The immediate question prompted by our findings is whether the fact that 63% of parents are concerned is alarming because it is too low or too high. Some might contend that concern about medical errors is an appropriate and desirable response because it may motivate parents to become more vigilant about the medical care that their child is receiving. However, others may challenge that such concern may indicate a feeling of powerlessness to act to prevent potential errors. In our study, the relationship between higher self‐efficacy and less parental concern raises the possibility that parents with higher levels of self‐efficacy with physician interactions may feel more comfortable communicating with physicians, which in turn may temper parents' concerns about medical errors during hospitalization.
It is equally plausible that concern about medical errors during hospitalization may motivate parents to become involved in their child's medical care and, in turn, lead them to feel empowered to prevent medical errors and so ease their concerns. It is conceivable that experience with past medical errors may fuel a parent's need to watch over their child's care to prevent additional medical errors. Future studies should address the independent effect of past medical errors on parental concern about medical errors.
In this study, all parents who reported being very uncomfortable with English and parents of non‐white race felt the need to watch over their child's care to help prevent errors. A previous survey of a nationally representative sample of U.S. adults found greater proportion of non‐white adults were very concerned about errors or mistakes happening when receiving care at a hospital (blacks 62%, Hispanics 57%, whites 44%).26 However, in our study, the relationship between race and concern is likely mediated by language since many of the parents who described themselves as other than white also reported being not very comfortable with English and we could not find an effect of race on concern among parents who were very comfortable with English. Indeed, previous studies have linked decreased English proficiency to medical errors with potential clinical consequence.27
Given our previous investigation of the relationship between self‐efficacy and parent participation in medical decisions during a child's hospitalization, we conducted post‐hoc analyses exploring the association between parents' self‐report of participation in medical decisions and concern about medical errors during their child's hospitalization.16 Using a simple logistic regression we did not find any association. However, we advise caution in interpreting and generalizing these results because the study was not powered to adequately evaluate this association.
There are additional limitations in our study to be noted. First, this question has not been used previously to assess parental concern about medical errors, so future work will need to focus on assessing its reliability and validity. Second, it is also possible that parents' concern for medical errors is mitigated by the complexity of their child's healthcare.5 We attempted to address this issue by controlling for the child's number of chronic illnesses. However, it is possible that our metric did not capture the level of complexity associated with different types of chronic conditions. Moreover, additional variables such as health insurance type, parental physical and mental health, and quality of interactions with the nursing staff may confound the relationships that we observed. Future studies should examine the effect of these variables on parents' self‐efficacy and their concern about medical errors.
Third, we surveyed parents at a single institution and, as such, differences in demographics and hospital‐specific practices related to patient‐physician interactions may prevent generalization of our findings to other institutions. For example, the parents in our survey had a higher average education level than the general population and the racial makeup of our population was not nationally representative. Also, due to HIPAA constraints, we were unable to collect extensive demographic information on parents and children who were missed or those who refused to participate in the study, which also could conceivably influence the strength of our findings.
Fourth, we adapted a validated adult measure of self‐efficacy for use in pediatrics. The patient‐physician self‐efficacy scale, the PEPPI, did have a skewed distribution in our study, although this performance is consistent with adult studies18 and in post‐hoc analyses, outlier PEPPI scores did not have a significant effect on the magnitude of the relationship we observed between self‐efficacy and parental concern about medical errors. However, the reading level of this instrument is ninth grade, which may impact the generalization of our findings to populations with lower literacy levels.
Fifth, we excluded parents who were unsure about their concern from our analyses. In post‐hoc multivariate regression analyses, reassignment of unsure responses to either agree or disagree did not result in any change in odds ratio for any endpoint.
Finally, it is possible that parental concern was influenced by social desirability bias in that parents may have been less likely to report concern about medical errors during a hospitalization because of fear of the implications it might have for their child's care. We attempted to control for this effect by adjusting for social desirability bias using the Marlowe‐Crowne scale. This scale is commonly used in behavioral science research to account for such response bias and has been recommended by the NIH Consortium on Behavior Change for use in behavioral change research related to health.24
Within the context of these limitations, we feel that our study contributes an important first step toward characterizing the scope of parental concern about medical errors during pediatric hospitalizations and understanding the relationship of self‐efficacy with physician interactions to this concern. Devising a quality initiative program to improve parents' self‐efficacy interacting with physicians might help to temper parents' concerns about medical errors while also encouraging their involvement in their child's medical care. Such a program would likely prove most beneficial if it sought to improve self‐efficacy among parents with lower English proficiency given that this group had the highest concern for medical errors. Possible interventions might include more ready access to interpreters or use of visual aids.
- Institute of Medicine Committee on Quality of Health Care in America.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,,, et al.Medication errors and adverse drug events in pediatric inpatients.JAMA.2001;285(16):2114–2120.
- ,.Pediatric patient safety in hospitals: a national picture in 2000.Pediatrics.2004;113(6):1741–1746.
- ,,,,,.Brief report: hospitalized patients' attitudes about and participation in error prevention.J Gen Intern Med.2006;21(4):367–370.
- ,,, et al.Patients' concerns about medical errors during hospitalization.Jt Comm J Qual Patient Saf.2007;33(1):5–14.
- Agency for Healthcare Research and Quality.20 Tips to Help Prevent Medical Errors in Children. Patient Fact Sheet. 2009, AHRQ Publication No. 02‐P034.Rockville, MD:Agency for Healthcare Research and Quality.
- Joint Commission on Accreditation of Healthcare Organizations. Speak Up Initiatives. Available at: http://www.jointcommission.org/PatientSafety/SpeakUp. Accessed May 2009.
- .Self‐efficacy: toward a unifying theory of behavioral change.Psychol Rev.1977;84(2):191–215.
- .Self‐Efficacy: The Exercise of Control.New York, NY:W.H. Freeman and Company;1997.
- ,,,.Psychosocial correlates of physical activity in healthy children.Arch Pediatr Adolesc Med.2001;155(8):897–902.
- ,,,,.Self‐efficacy as a mediator variable for adolescents' adherence to treatment for insulin‐dependent diabetes mellitus.Children's Health Care.2000;29(1):47–63.
- ,,.Diabetes regimen behaviors. Predicting adherence.Med Care.1987;25(9):868–881.
- ,,,,,.Pediatrician self‐efficacy for counseling parents of asthmatic children to quit smoking.Pediatrics.2004;113(1 Pt 1):78–81.
- ,,.Examining the relationship of patients' attitudes and beliefs with their self‐reported level of participation in medical decision‐making.Med Care.2005;43(9):865–872.
- ,,,,,.Patient‐physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision.J Clin Oncol.2004;22(15):3091–3098.
- ,,.Toward family‐centered inpatient medical care: the role of parents as participants in medical decisions.J Pediatr.2007;151(6):690–695.
- ,,,,.Perceived efficacy in patient‐physician interactions (PEPPI): validation of an instrument in older persons.J Am Geriatr Soc.1998;46(7):889–894.
- ,,,.Determinants of participation in treatment decision‐making by older breast cancer patients.Breast Cancer Res Treat.2004;85(3):201–209.
- ,.The CHQ: A User's Manual (2nd printing).Boston, MA:HealthAct;1999.
- ,,,,.The health and well‐being of adolescents: a school‐based population study of the self‐report Child Health Questionnaire.J Adolesc Health.2001;29(2):140–149.
- ,,.The Child Health Questionnaire in children with diabetes: cross‐sectional survey of parent and adolescent‐reported functional health status.Diabet Med.2000;17(10):700–707.
- ,.Semantic style variance in personality questionnaires.J Psychol.1973;85:109–118.
- ,.A new scale of social desirability independent of psychopathology.J Consult Psychol.1960;24:349–354.
- National Institutes of Health. Behavior Change Consortium‐Recommended Nutrition Measures. Available at:http://www1.od.nih.gov/behaviorchange/measures/nutrition.htm. Accessed May 2009.
- ,,.Response bias influences mental health symptom reporting in patients with obstructive sleep apnea.Ann Behav Med.2001;23(4):313–317.
- Kaiser Family Foundation, Agency for Healthcare Research and Quality.Americans as Health Care Consumers: Update on the Role of Quality Information.Rockville, MD:Agency for Healthcare Research and Quality;2000.
- ,,, et al.Errors in medical interpretation and their potential clinical consequences in pediatric encounters.Pediatrics.2003;111(1):6–14.
- Institute of Medicine Committee on Quality of Health Care in America.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,,, et al.Medication errors and adverse drug events in pediatric inpatients.JAMA.2001;285(16):2114–2120.
- ,.Pediatric patient safety in hospitals: a national picture in 2000.Pediatrics.2004;113(6):1741–1746.
- ,,,,,.Brief report: hospitalized patients' attitudes about and participation in error prevention.J Gen Intern Med.2006;21(4):367–370.
- ,,, et al.Patients' concerns about medical errors during hospitalization.Jt Comm J Qual Patient Saf.2007;33(1):5–14.
- Agency for Healthcare Research and Quality.20 Tips to Help Prevent Medical Errors in Children. Patient Fact Sheet. 2009, AHRQ Publication No. 02‐P034.Rockville, MD:Agency for Healthcare Research and Quality.
- Joint Commission on Accreditation of Healthcare Organizations. Speak Up Initiatives. Available at: http://www.jointcommission.org/PatientSafety/SpeakUp. Accessed May 2009.
- .Self‐efficacy: toward a unifying theory of behavioral change.Psychol Rev.1977;84(2):191–215.
- .Self‐Efficacy: The Exercise of Control.New York, NY:W.H. Freeman and Company;1997.
- ,,,.Psychosocial correlates of physical activity in healthy children.Arch Pediatr Adolesc Med.2001;155(8):897–902.
- ,,,,.Self‐efficacy as a mediator variable for adolescents' adherence to treatment for insulin‐dependent diabetes mellitus.Children's Health Care.2000;29(1):47–63.
- ,,.Diabetes regimen behaviors. Predicting adherence.Med Care.1987;25(9):868–881.
- ,,,,,.Pediatrician self‐efficacy for counseling parents of asthmatic children to quit smoking.Pediatrics.2004;113(1 Pt 1):78–81.
- ,,.Examining the relationship of patients' attitudes and beliefs with their self‐reported level of participation in medical decision‐making.Med Care.2005;43(9):865–872.
- ,,,,,.Patient‐physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision.J Clin Oncol.2004;22(15):3091–3098.
- ,,.Toward family‐centered inpatient medical care: the role of parents as participants in medical decisions.J Pediatr.2007;151(6):690–695.
- ,,,,.Perceived efficacy in patient‐physician interactions (PEPPI): validation of an instrument in older persons.J Am Geriatr Soc.1998;46(7):889–894.
- ,,,.Determinants of participation in treatment decision‐making by older breast cancer patients.Breast Cancer Res Treat.2004;85(3):201–209.
- ,.The CHQ: A User's Manual (2nd printing).Boston, MA:HealthAct;1999.
- ,,,,.The health and well‐being of adolescents: a school‐based population study of the self‐report Child Health Questionnaire.J Adolesc Health.2001;29(2):140–149.
- ,,.The Child Health Questionnaire in children with diabetes: cross‐sectional survey of parent and adolescent‐reported functional health status.Diabet Med.2000;17(10):700–707.
- ,.Semantic style variance in personality questionnaires.J Psychol.1973;85:109–118.
- ,.A new scale of social desirability independent of psychopathology.J Consult Psychol.1960;24:349–354.
- National Institutes of Health. Behavior Change Consortium‐Recommended Nutrition Measures. Available at:http://www1.od.nih.gov/behaviorchange/measures/nutrition.htm. Accessed May 2009.
- ,,.Response bias influences mental health symptom reporting in patients with obstructive sleep apnea.Ann Behav Med.2001;23(4):313–317.
- Kaiser Family Foundation, Agency for Healthcare Research and Quality.Americans as Health Care Consumers: Update on the Role of Quality Information.Rockville, MD:Agency for Healthcare Research and Quality;2000.
- ,,, et al.Errors in medical interpretation and their potential clinical consequences in pediatric encounters.Pediatrics.2003;111(1):6–14.
Copyright © 2009 Society of Hospital Medicine
Think Twice Before Accepting an Expert Witness Offer
Think Twice Before Accepting an Expert Witness Offer
A medical malpractice attorney recently contacted me, asking if I would be interested in reviewing a case. They are looking for a hospitalist “expert witness.” I’ve never done this before and don’t know if I’m qualified. Can you tell me about the benefits and risks of being a medical expert witness?
R. Jones, MD
Miami
Dr. Hospitalist responds: Most physicians complete medical school and postgraduate training without firsthand knowledge of our legal system. Unfortunately, a number of physicians become defendants in medical lawsuits during their professional careers. I hear with increasing frequency about hospitalists being sued for alleged medical malpractice. I am not surprised. This is not an indictment against hospital HM, but more a matter of probability. There are at least tenfold more hospitalists today than 10 years ago.
To be clear, I am not an attorney, nor do I have any formal legal training. I suggest you speak with an attorney if you have questions about the law.
Laws vary from state to state, but for the most part, plaintiff attorneys and defense attorneys retain expert witnesses to help them determine the merits of a lawsuit. Did the defendant have a duty to treat the patient? Was there a breach of the standard of care? What were the damages, and were they due to the defendant’s actions or lack of action?
Understand that our judicial system holds that a physician in the same field as the defendant is the most qualified to determine whether the defendant met the standard of care. Standard of care is what is reasonably expected of a physician in that field given the circumstances. So if the defendant is a hospitalist, the attorneys are looking for an expert witness who is also a hospitalist. Seems like a reasonable system, right? Individuals are judged by their peers. But the system is far from perfect.
Critics point out the system is inherently flawed when we rely on “experts” to help us determine the standard of care. Aside from working in a given field of medicine, there are no specific qualifications to be an expert witness. Unfortunately, not all experts are experts, and not all experts are completely honest. And there can be a lot of money at stake. Plaintiffs attorneys and defense attorneys, along with expert witnesses for both sides, stand to profit from lawsuits. All of this drives up the cost of medical malpractice premiums.
I will not tell you not to become an expert witness. Until we see real, sustainable tort reform, we have to live with the system. If I am sued, my defense attorney would seek an expert witness’s opinion. If a patient is hurt because of alleged negligence, the patient’s attorney would seek the opinion of an expert witness. So we need honest physicians to provide honest opinions as expert witnesses. This goes for defendants and plaintiffs.
Many expert witnesses find gratification in knowing they helped a patient or a physician. As I mentioned previously, an expert-witness gig can be financially lucrative, but it is not without its drawbacks. Expert witnesses are subject to the code of ethics set forth by the medical society and state board of registration in medicine. Any sworn testimony you provide is discoverable. It is easier than you might think for others (e.g., opposition attorneys) to believe you have contradicted yourself when you give your opinion on the same subject in more than one case. As an expert witness, know that you will be cross-examined by an attorney, either in deposition or at trial. Testifying under oath can be a grueling experience.
Most expert witnesses are reputable physicians in their fields. You should feel honored for being asked to participate as an expert witness, but think carefully before you accept the offer. Understand what is being asked of you before you take on this responsibility. TH
Think Twice Before Accepting an Expert Witness Offer
A medical malpractice attorney recently contacted me, asking if I would be interested in reviewing a case. They are looking for a hospitalist “expert witness.” I’ve never done this before and don’t know if I’m qualified. Can you tell me about the benefits and risks of being a medical expert witness?
R. Jones, MD
Miami
Dr. Hospitalist responds: Most physicians complete medical school and postgraduate training without firsthand knowledge of our legal system. Unfortunately, a number of physicians become defendants in medical lawsuits during their professional careers. I hear with increasing frequency about hospitalists being sued for alleged medical malpractice. I am not surprised. This is not an indictment against hospital HM, but more a matter of probability. There are at least tenfold more hospitalists today than 10 years ago.
To be clear, I am not an attorney, nor do I have any formal legal training. I suggest you speak with an attorney if you have questions about the law.
Laws vary from state to state, but for the most part, plaintiff attorneys and defense attorneys retain expert witnesses to help them determine the merits of a lawsuit. Did the defendant have a duty to treat the patient? Was there a breach of the standard of care? What were the damages, and were they due to the defendant’s actions or lack of action?
Understand that our judicial system holds that a physician in the same field as the defendant is the most qualified to determine whether the defendant met the standard of care. Standard of care is what is reasonably expected of a physician in that field given the circumstances. So if the defendant is a hospitalist, the attorneys are looking for an expert witness who is also a hospitalist. Seems like a reasonable system, right? Individuals are judged by their peers. But the system is far from perfect.
Critics point out the system is inherently flawed when we rely on “experts” to help us determine the standard of care. Aside from working in a given field of medicine, there are no specific qualifications to be an expert witness. Unfortunately, not all experts are experts, and not all experts are completely honest. And there can be a lot of money at stake. Plaintiffs attorneys and defense attorneys, along with expert witnesses for both sides, stand to profit from lawsuits. All of this drives up the cost of medical malpractice premiums.
I will not tell you not to become an expert witness. Until we see real, sustainable tort reform, we have to live with the system. If I am sued, my defense attorney would seek an expert witness’s opinion. If a patient is hurt because of alleged negligence, the patient’s attorney would seek the opinion of an expert witness. So we need honest physicians to provide honest opinions as expert witnesses. This goes for defendants and plaintiffs.
Many expert witnesses find gratification in knowing they helped a patient or a physician. As I mentioned previously, an expert-witness gig can be financially lucrative, but it is not without its drawbacks. Expert witnesses are subject to the code of ethics set forth by the medical society and state board of registration in medicine. Any sworn testimony you provide is discoverable. It is easier than you might think for others (e.g., opposition attorneys) to believe you have contradicted yourself when you give your opinion on the same subject in more than one case. As an expert witness, know that you will be cross-examined by an attorney, either in deposition or at trial. Testifying under oath can be a grueling experience.
Most expert witnesses are reputable physicians in their fields. You should feel honored for being asked to participate as an expert witness, but think carefully before you accept the offer. Understand what is being asked of you before you take on this responsibility. TH
Think Twice Before Accepting an Expert Witness Offer
A medical malpractice attorney recently contacted me, asking if I would be interested in reviewing a case. They are looking for a hospitalist “expert witness.” I’ve never done this before and don’t know if I’m qualified. Can you tell me about the benefits and risks of being a medical expert witness?
R. Jones, MD
Miami
Dr. Hospitalist responds: Most physicians complete medical school and postgraduate training without firsthand knowledge of our legal system. Unfortunately, a number of physicians become defendants in medical lawsuits during their professional careers. I hear with increasing frequency about hospitalists being sued for alleged medical malpractice. I am not surprised. This is not an indictment against hospital HM, but more a matter of probability. There are at least tenfold more hospitalists today than 10 years ago.
To be clear, I am not an attorney, nor do I have any formal legal training. I suggest you speak with an attorney if you have questions about the law.
Laws vary from state to state, but for the most part, plaintiff attorneys and defense attorneys retain expert witnesses to help them determine the merits of a lawsuit. Did the defendant have a duty to treat the patient? Was there a breach of the standard of care? What were the damages, and were they due to the defendant’s actions or lack of action?
Understand that our judicial system holds that a physician in the same field as the defendant is the most qualified to determine whether the defendant met the standard of care. Standard of care is what is reasonably expected of a physician in that field given the circumstances. So if the defendant is a hospitalist, the attorneys are looking for an expert witness who is also a hospitalist. Seems like a reasonable system, right? Individuals are judged by their peers. But the system is far from perfect.
Critics point out the system is inherently flawed when we rely on “experts” to help us determine the standard of care. Aside from working in a given field of medicine, there are no specific qualifications to be an expert witness. Unfortunately, not all experts are experts, and not all experts are completely honest. And there can be a lot of money at stake. Plaintiffs attorneys and defense attorneys, along with expert witnesses for both sides, stand to profit from lawsuits. All of this drives up the cost of medical malpractice premiums.
I will not tell you not to become an expert witness. Until we see real, sustainable tort reform, we have to live with the system. If I am sued, my defense attorney would seek an expert witness’s opinion. If a patient is hurt because of alleged negligence, the patient’s attorney would seek the opinion of an expert witness. So we need honest physicians to provide honest opinions as expert witnesses. This goes for defendants and plaintiffs.
Many expert witnesses find gratification in knowing they helped a patient or a physician. As I mentioned previously, an expert-witness gig can be financially lucrative, but it is not without its drawbacks. Expert witnesses are subject to the code of ethics set forth by the medical society and state board of registration in medicine. Any sworn testimony you provide is discoverable. It is easier than you might think for others (e.g., opposition attorneys) to believe you have contradicted yourself when you give your opinion on the same subject in more than one case. As an expert witness, know that you will be cross-examined by an attorney, either in deposition or at trial. Testifying under oath can be a grueling experience.
Most expert witnesses are reputable physicians in their fields. You should feel honored for being asked to participate as an expert witness, but think carefully before you accept the offer. Understand what is being asked of you before you take on this responsibility. TH
Volume Variables
At sold-out HM09 in Chicago in May, I had the pleasure of moderating a panel discussion titled “Who Says 15 Patients a Day is the Right Number?” As you might guess, each panelist (including me) said, in effect, “No one says 15 patients a day is the right number.”
Despite being a very important issue to SHM, the society doesn’t have an official position on the “right” or optimal daily patient volume or workload for a hospitalist. SHM generates and disseminates a lot of information to help each practice make decisions about workload, including SHM’s 2007-2008 “Bi-annual Survey on the State of the Hospital Medicine Movement,” articles available at www.hospitalmedicine.org, and articles in The Hospitalist. But all practices, and individual hospitalists, will have to decide what level of patient volume enables safe care, a sustainable and satisfying workload, and reasonable economic performance.
Workload Metrics
A problem that makes any workload discussion difficult is that many terms are sometimes used to mean different things. For example, “daily census” is used when comparing workloads between practices, but “daily encounters” is nearly always a more informative metric. Remember, daily encounters for a practice will always be higher (though on rare occasion, the same) than daily census.
The only definition of encounters that can be reliably compared between practices is billable encounters. Confusion arises when one person is reporting billable encounters, and another person is counting as one encounter each time a hospitalist interacted with a patient (e.g., went into the patient’s room or made a chart entry) and reports a higher encounter volume despite having the same workload and patient volume. Of course, billable encounters fail to perfectly describe our workloads, but until someone comes up with a better metric that is universally understood and applicable across all settings, billable encounters is the best metric we have.
Both billable encounters and census can be tricky. We might be convinced that because she averages 17 billable encounters per day, Dr. Krause has a higher workload than Dr. Palmer, who averages 15. But it turns out that Dr. Palmer works 210 shifts annually, generating 3,150 annual encounters; Dr. Krause’s 181 annual shifts generate 3,077 encounters. So while Dr. Krause does indeed work harder on the average day, she has lower annual productivity. My experience is that by failing to compare workloads over long periods, such as a year, many attempts to compare workloads yield misleading conclusions.
Comparing encounter volume from one practice to the next fails to capture other ways workloads differ. Dr. Krause may be the principal caregiver for a number of ICU patients; Dr. Plant might turn such patients over to intensivists. For this reason, work relative value units (wRVUs), which attempt to capture the complexity of each encounter, usually are a more meaningful—though still imperfect—metric.
Apples vs. Apples
Any truly valid method of comparing workloads should sum the annual workload for the entire practice and divide by the total provider full-time equivalents (FTEs). Yet problems arise because night shifts usually are less productive than day shifts. Consider a practice that has a distinct night shift worked by a doctor who does no day-shift work the day before or after. There is a tendency to leave this night shift out of the analysis of average workload per FTE, which makes the practice appear more productive than it really is.
For example, the practice I am part of has dedicated nocturnists who don’t work day shifts. So when thinking about how hard we’re working, we tend to sum each “day” doctor’s wRVUs for the year and divide by the number of day doctors. Calculated this way, our day doctors appear to be more productive than SHM data might indicate, but when including our nocturnists’ production and FTEs in the analysis, the overall workload per FTE in the practice is similar to the data.
Productivity Per FTE
While I think productivity per FTE per year is the best metric to use when comparing a practice to external survey data or comparing one practice to another, it is confounded by two sticky problems: inconsistent definitions of what constitutes an FTE, and the lack of an agreed-upon method of accounting for the contribution of nonphysician providers.
The most common definitions of what constitutes an FTE are based on the number of hours or shifts worked. One practice might define an FTE as 2,000 hours of work annually; another might use 180 annual shifts. Unless each shift has a clearly defined duration, it will be very difficult to reach conclusions. Although each practice might make sound decisions about how they define an FTE, they’re ultimately making arbitrary choices that aren’t consistent from one practice to the next. Controlling for this issue is very difficult.
Even trickier is how to compare the contributions of physician assistants (PAs) and nurse practitioners (NPs) from one practice to the next. In the above example, Dr. Plant’s practice has eight physician FTEs and four NPPs, and Dr. Krause’s group has eight MDs and no NPPs. How much more productive should we expect Dr. Plant’s practice to be? (NPPs make many valuable contributions, in addition to increasing productivity, but these are outside the scope of this article.)
Though survey data offer some clues, there is no established standard for increased productivity expected of PAs and NPs. One approach I use is to compare an NPP’s total compensation (salary and benefits) with that of the average physician FTE. If the cost of each NPP is 60% of an MD, then one could say that each NPP represents 0.6 “physician FTE equivalents” and could be expected to increase the productivity of the practice proportionately. So Dr. Plant’s group might be expected to have the productivity of 10.2 physician FTEs (4 NPPs X 0.6 = 2.2 “physician FTE equivalents,” added to the eight physician FTEs). (Note: While I think converting NPP FTEs into “physician equivalents” is useful in analyzing the effect of workloads on budgets, there are clearly many other very important issues when trying to quantify the contribution of NPPs to a practice.)
Judgment Rules
There are dozens of additional issues, such as the effect on productivity resulting from inefficient hospital systems (e.g., a clunky electronic health record, typed admit notes aren’t available for a few days compared with records available within two to four hours of dictation), activities such as attending Rapid Response Team activations, and differences in the social complexity of the patient population. None of these would show up in wRVU reports, so they are missing from the analysis I’ve described above.
I think it is impossible to control for all of the differences between practices that influence the definition of appropriate workload. This is the main reason SHM probably will never have a firm position on exactly what is the right or optimal number.
Although 15 patients a day is a reasonable starting point (I prefer to see fewer patients daily, but work more days/shifts annually), things get complicated in a hurry, and there will always be significant variation. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He also is faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
At sold-out HM09 in Chicago in May, I had the pleasure of moderating a panel discussion titled “Who Says 15 Patients a Day is the Right Number?” As you might guess, each panelist (including me) said, in effect, “No one says 15 patients a day is the right number.”
Despite being a very important issue to SHM, the society doesn’t have an official position on the “right” or optimal daily patient volume or workload for a hospitalist. SHM generates and disseminates a lot of information to help each practice make decisions about workload, including SHM’s 2007-2008 “Bi-annual Survey on the State of the Hospital Medicine Movement,” articles available at www.hospitalmedicine.org, and articles in The Hospitalist. But all practices, and individual hospitalists, will have to decide what level of patient volume enables safe care, a sustainable and satisfying workload, and reasonable economic performance.
Workload Metrics
A problem that makes any workload discussion difficult is that many terms are sometimes used to mean different things. For example, “daily census” is used when comparing workloads between practices, but “daily encounters” is nearly always a more informative metric. Remember, daily encounters for a practice will always be higher (though on rare occasion, the same) than daily census.
The only definition of encounters that can be reliably compared between practices is billable encounters. Confusion arises when one person is reporting billable encounters, and another person is counting as one encounter each time a hospitalist interacted with a patient (e.g., went into the patient’s room or made a chart entry) and reports a higher encounter volume despite having the same workload and patient volume. Of course, billable encounters fail to perfectly describe our workloads, but until someone comes up with a better metric that is universally understood and applicable across all settings, billable encounters is the best metric we have.
Both billable encounters and census can be tricky. We might be convinced that because she averages 17 billable encounters per day, Dr. Krause has a higher workload than Dr. Palmer, who averages 15. But it turns out that Dr. Palmer works 210 shifts annually, generating 3,150 annual encounters; Dr. Krause’s 181 annual shifts generate 3,077 encounters. So while Dr. Krause does indeed work harder on the average day, she has lower annual productivity. My experience is that by failing to compare workloads over long periods, such as a year, many attempts to compare workloads yield misleading conclusions.
Comparing encounter volume from one practice to the next fails to capture other ways workloads differ. Dr. Krause may be the principal caregiver for a number of ICU patients; Dr. Plant might turn such patients over to intensivists. For this reason, work relative value units (wRVUs), which attempt to capture the complexity of each encounter, usually are a more meaningful—though still imperfect—metric.
Apples vs. Apples
Any truly valid method of comparing workloads should sum the annual workload for the entire practice and divide by the total provider full-time equivalents (FTEs). Yet problems arise because night shifts usually are less productive than day shifts. Consider a practice that has a distinct night shift worked by a doctor who does no day-shift work the day before or after. There is a tendency to leave this night shift out of the analysis of average workload per FTE, which makes the practice appear more productive than it really is.
For example, the practice I am part of has dedicated nocturnists who don’t work day shifts. So when thinking about how hard we’re working, we tend to sum each “day” doctor’s wRVUs for the year and divide by the number of day doctors. Calculated this way, our day doctors appear to be more productive than SHM data might indicate, but when including our nocturnists’ production and FTEs in the analysis, the overall workload per FTE in the practice is similar to the data.
Productivity Per FTE
While I think productivity per FTE per year is the best metric to use when comparing a practice to external survey data or comparing one practice to another, it is confounded by two sticky problems: inconsistent definitions of what constitutes an FTE, and the lack of an agreed-upon method of accounting for the contribution of nonphysician providers.
The most common definitions of what constitutes an FTE are based on the number of hours or shifts worked. One practice might define an FTE as 2,000 hours of work annually; another might use 180 annual shifts. Unless each shift has a clearly defined duration, it will be very difficult to reach conclusions. Although each practice might make sound decisions about how they define an FTE, they’re ultimately making arbitrary choices that aren’t consistent from one practice to the next. Controlling for this issue is very difficult.
Even trickier is how to compare the contributions of physician assistants (PAs) and nurse practitioners (NPs) from one practice to the next. In the above example, Dr. Plant’s practice has eight physician FTEs and four NPPs, and Dr. Krause’s group has eight MDs and no NPPs. How much more productive should we expect Dr. Plant’s practice to be? (NPPs make many valuable contributions, in addition to increasing productivity, but these are outside the scope of this article.)
Though survey data offer some clues, there is no established standard for increased productivity expected of PAs and NPs. One approach I use is to compare an NPP’s total compensation (salary and benefits) with that of the average physician FTE. If the cost of each NPP is 60% of an MD, then one could say that each NPP represents 0.6 “physician FTE equivalents” and could be expected to increase the productivity of the practice proportionately. So Dr. Plant’s group might be expected to have the productivity of 10.2 physician FTEs (4 NPPs X 0.6 = 2.2 “physician FTE equivalents,” added to the eight physician FTEs). (Note: While I think converting NPP FTEs into “physician equivalents” is useful in analyzing the effect of workloads on budgets, there are clearly many other very important issues when trying to quantify the contribution of NPPs to a practice.)
Judgment Rules
There are dozens of additional issues, such as the effect on productivity resulting from inefficient hospital systems (e.g., a clunky electronic health record, typed admit notes aren’t available for a few days compared with records available within two to four hours of dictation), activities such as attending Rapid Response Team activations, and differences in the social complexity of the patient population. None of these would show up in wRVU reports, so they are missing from the analysis I’ve described above.
I think it is impossible to control for all of the differences between practices that influence the definition of appropriate workload. This is the main reason SHM probably will never have a firm position on exactly what is the right or optimal number.
Although 15 patients a day is a reasonable starting point (I prefer to see fewer patients daily, but work more days/shifts annually), things get complicated in a hurry, and there will always be significant variation. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He also is faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
At sold-out HM09 in Chicago in May, I had the pleasure of moderating a panel discussion titled “Who Says 15 Patients a Day is the Right Number?” As you might guess, each panelist (including me) said, in effect, “No one says 15 patients a day is the right number.”
Despite being a very important issue to SHM, the society doesn’t have an official position on the “right” or optimal daily patient volume or workload for a hospitalist. SHM generates and disseminates a lot of information to help each practice make decisions about workload, including SHM’s 2007-2008 “Bi-annual Survey on the State of the Hospital Medicine Movement,” articles available at www.hospitalmedicine.org, and articles in The Hospitalist. But all practices, and individual hospitalists, will have to decide what level of patient volume enables safe care, a sustainable and satisfying workload, and reasonable economic performance.
Workload Metrics
A problem that makes any workload discussion difficult is that many terms are sometimes used to mean different things. For example, “daily census” is used when comparing workloads between practices, but “daily encounters” is nearly always a more informative metric. Remember, daily encounters for a practice will always be higher (though on rare occasion, the same) than daily census.
The only definition of encounters that can be reliably compared between practices is billable encounters. Confusion arises when one person is reporting billable encounters, and another person is counting as one encounter each time a hospitalist interacted with a patient (e.g., went into the patient’s room or made a chart entry) and reports a higher encounter volume despite having the same workload and patient volume. Of course, billable encounters fail to perfectly describe our workloads, but until someone comes up with a better metric that is universally understood and applicable across all settings, billable encounters is the best metric we have.
Both billable encounters and census can be tricky. We might be convinced that because she averages 17 billable encounters per day, Dr. Krause has a higher workload than Dr. Palmer, who averages 15. But it turns out that Dr. Palmer works 210 shifts annually, generating 3,150 annual encounters; Dr. Krause’s 181 annual shifts generate 3,077 encounters. So while Dr. Krause does indeed work harder on the average day, she has lower annual productivity. My experience is that by failing to compare workloads over long periods, such as a year, many attempts to compare workloads yield misleading conclusions.
Comparing encounter volume from one practice to the next fails to capture other ways workloads differ. Dr. Krause may be the principal caregiver for a number of ICU patients; Dr. Plant might turn such patients over to intensivists. For this reason, work relative value units (wRVUs), which attempt to capture the complexity of each encounter, usually are a more meaningful—though still imperfect—metric.
Apples vs. Apples
Any truly valid method of comparing workloads should sum the annual workload for the entire practice and divide by the total provider full-time equivalents (FTEs). Yet problems arise because night shifts usually are less productive than day shifts. Consider a practice that has a distinct night shift worked by a doctor who does no day-shift work the day before or after. There is a tendency to leave this night shift out of the analysis of average workload per FTE, which makes the practice appear more productive than it really is.
For example, the practice I am part of has dedicated nocturnists who don’t work day shifts. So when thinking about how hard we’re working, we tend to sum each “day” doctor’s wRVUs for the year and divide by the number of day doctors. Calculated this way, our day doctors appear to be more productive than SHM data might indicate, but when including our nocturnists’ production and FTEs in the analysis, the overall workload per FTE in the practice is similar to the data.
Productivity Per FTE
While I think productivity per FTE per year is the best metric to use when comparing a practice to external survey data or comparing one practice to another, it is confounded by two sticky problems: inconsistent definitions of what constitutes an FTE, and the lack of an agreed-upon method of accounting for the contribution of nonphysician providers.
The most common definitions of what constitutes an FTE are based on the number of hours or shifts worked. One practice might define an FTE as 2,000 hours of work annually; another might use 180 annual shifts. Unless each shift has a clearly defined duration, it will be very difficult to reach conclusions. Although each practice might make sound decisions about how they define an FTE, they’re ultimately making arbitrary choices that aren’t consistent from one practice to the next. Controlling for this issue is very difficult.
Even trickier is how to compare the contributions of physician assistants (PAs) and nurse practitioners (NPs) from one practice to the next. In the above example, Dr. Plant’s practice has eight physician FTEs and four NPPs, and Dr. Krause’s group has eight MDs and no NPPs. How much more productive should we expect Dr. Plant’s practice to be? (NPPs make many valuable contributions, in addition to increasing productivity, but these are outside the scope of this article.)
Though survey data offer some clues, there is no established standard for increased productivity expected of PAs and NPs. One approach I use is to compare an NPP’s total compensation (salary and benefits) with that of the average physician FTE. If the cost of each NPP is 60% of an MD, then one could say that each NPP represents 0.6 “physician FTE equivalents” and could be expected to increase the productivity of the practice proportionately. So Dr. Plant’s group might be expected to have the productivity of 10.2 physician FTEs (4 NPPs X 0.6 = 2.2 “physician FTE equivalents,” added to the eight physician FTEs). (Note: While I think converting NPP FTEs into “physician equivalents” is useful in analyzing the effect of workloads on budgets, there are clearly many other very important issues when trying to quantify the contribution of NPPs to a practice.)
Judgment Rules
There are dozens of additional issues, such as the effect on productivity resulting from inefficient hospital systems (e.g., a clunky electronic health record, typed admit notes aren’t available for a few days compared with records available within two to four hours of dictation), activities such as attending Rapid Response Team activations, and differences in the social complexity of the patient population. None of these would show up in wRVU reports, so they are missing from the analysis I’ve described above.
I think it is impossible to control for all of the differences between practices that influence the definition of appropriate workload. This is the main reason SHM probably will never have a firm position on exactly what is the right or optimal number.
Although 15 patients a day is a reasonable starting point (I prefer to see fewer patients daily, but work more days/shifts annually), things get complicated in a hurry, and there will always be significant variation. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He also is faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
The Anvil of Indecision
Lying in bed, I’m jarred by what can only be an anvil dropping heavily upon my chest. Wakefulness reveals a more canine, cranium-like object. Staring deep into cataract-smudged eyes, I ponder the question that has occupied my mind for nearly two weeks: What would Hogan want?
My Dog Has Cancer
More accurately, he has a tumor—or, I guess, what appears to be a tumor on his chest X-ray. It was discovered, incidentally, on a liver ultrasound that was being done for abnormal liver function test results. That study revealed nothing wrong with his liver, but led to a follow-up radiograph showing a 4.9-cm, right-lower-lobe lung mass. Also uncovered in this process was a tangle of complex emotions, turmoil, and uncertainty surrounding my first personal foray into end-of-life decision-making.
Hogan, my now-presumed-cancer-ridden, 10-year-old Weimaraner, came into my life permanently when he was all of 8 weeks old. I first met him during a visit to the breeder when his litter was only three days old. Over the successive weeks, I visited him often, anxious for the day I’d be able to take my new companion home.
I picked up Hogan on Fourth of July weekend during my chief year of residency—sort of a gift for completing my grueling training. He was the first dog I raised, trained, and cared for by myself. And while we had our share of eaten walls, destroyed comforters, and chewed bits of Jeep Cherokee, this was no “Marley & Me” relationship. We were more like roommates, best friends. We hiked, camped, and went everywhere together—either an idyllic boy-and-his-dog relationship or a sad, pitifully lonely, soul-in-need-of-a-girlfriend existence, depending on your point of view, I suppose.
In the end, the two viewpoints melded as Hogan eventually brought my wife and I together, a story that shall not be printed in these pages.
Through the years, Hogan bore witness to many personal and family milestones. My chief residency, my first grand rounds (his constant audience during my preparation brought him unparalleled expertise in canine zoonoses), my first house, our marriage, a horribly flailing attempt to recapture the magic of my first dog through a second Weim named Grady (definitely “Marley & Me” mixed with a healthy dose of “Dumb & Dumber”), and the birth of our first child.
It was during this time that Hogan began a long journey toward today. He became a little long in the tooth, droopy in the belly, and slow on the trail. His limitless energy and boundless passion for chasing tennis balls gave way to such leisurely pursuits as park pooping and command disobedience. His fluid, sinew-laced limbs became arthritic shells of their former selves, betraying the youthful grace that still echoed inside of him. I distinctly recall the first time Grady beat him to a tennis ball, a moment that clearly represented a passing of the baton—a crestfallen 6-year-old canine eclipsed by the 2-year-old whippersnapper. The youngster sprinted back, bursting with a mouthful of tennis ball and pride. The elder took a decidedly more tortuous and tortured route back—a carriage of nonchalance that failed in its attempt to convey the message that “chasing tennis balls is stupid.”
On the advice of our veterinarian, we stopped throwing Frisbees at Hogan at age 8, out of concern that an awkward jump might result in a paralytic shift in his progressively stenosing spine. While Hogan is otherwise healthy, his hips and forepaws are riddled with osteoarthritis, his eyes carry the cottony haze of cataracts, and his abdomen and skin are home to lumpy lipomas. So, on the advent of his 10th birthday, we are asked to decide how many resources, how much physical distress, how much intervention we afford to an older, sleep-most-of-the-day arthritic dog.
This scenario is complicated by the idiosyncrasies and mores of veterinary medicine. Unlike human medicine, which is replete with tomes of data steeped in decades of experience, our veterinarian counterparts often are left with gaping treatment holes and inadequately studied interventions. This is not a knock against the profession. In fact, I have had nothing short of fantastic experiences with the veterinary professionals with whom I’ve interacted. Rather, there just aren’t prospective, randomized, controlled trials to inform whether intervention will enhance Hogan’s quantity and quality of life.
Then there are the economic realities of the situation. As one who has rarely been ill and always been insured, I was staggered by the cost of medicine for the uninsured. Two-hundred-dollar antibiotics, $500 ultrasounds, $1,500 CT scans, and up to $10,000 operative and surgical ICU stays would have invoked, “you’re joking, right?” exclamations from me prior to this experience. Now they are just another variable that complicates this already emotionally complex discussion—the variable that makes you feel hollow inside for considering it, foolhardy for not.
Questions Abound
What price would I pay to have another few years with my best friend? What if it’s only a year, six months? Would the money be better spent funding my child’s 529 account? What if this is a benign process and intervention is for naught? What if this tumor is metastatic and intervention is futile? Should we spend the extra money on an upfront staging CT scan that has much lower sensitivity than those we routinely utilize?
If we intervene, should we attempt a costly, CT-guided biopsy to rule in malignant disease, or go straight to lobectomy? What if the surgery negatively alters his quality of life? What if he dies on the table? What do I know about the surgical outcomes of the two centers I’m considering? Should we attempt an open or laparoscopic approach to this tumor?
Can we achieve a cure? If we do, what does that mean for a dog in the twilight years of his life? Should we just let the disease progress to its natural endpoint?
What Would Hogan Want?
These are the questions that haunt me. As I stare into Hogan’s eyes, a portal to my companion’s soul, I am tormented by the cauldron of emotions, the indecision bred by incomplete information and the guilt that comes from knowing that Hogan unconditionally trusts that I will do what is right for him.
Will I? My eyes continue to ask Hogan what he would want until finally the answer becomes obvious.
Hogan wants his breakfast. TH
Dr. Glasheen is associate professor of medicine and director of the hospital medicine group and hospitalist training program at the University of Colorado at Denver.
Lying in bed, I’m jarred by what can only be an anvil dropping heavily upon my chest. Wakefulness reveals a more canine, cranium-like object. Staring deep into cataract-smudged eyes, I ponder the question that has occupied my mind for nearly two weeks: What would Hogan want?
My Dog Has Cancer
More accurately, he has a tumor—or, I guess, what appears to be a tumor on his chest X-ray. It was discovered, incidentally, on a liver ultrasound that was being done for abnormal liver function test results. That study revealed nothing wrong with his liver, but led to a follow-up radiograph showing a 4.9-cm, right-lower-lobe lung mass. Also uncovered in this process was a tangle of complex emotions, turmoil, and uncertainty surrounding my first personal foray into end-of-life decision-making.
Hogan, my now-presumed-cancer-ridden, 10-year-old Weimaraner, came into my life permanently when he was all of 8 weeks old. I first met him during a visit to the breeder when his litter was only three days old. Over the successive weeks, I visited him often, anxious for the day I’d be able to take my new companion home.
I picked up Hogan on Fourth of July weekend during my chief year of residency—sort of a gift for completing my grueling training. He was the first dog I raised, trained, and cared for by myself. And while we had our share of eaten walls, destroyed comforters, and chewed bits of Jeep Cherokee, this was no “Marley & Me” relationship. We were more like roommates, best friends. We hiked, camped, and went everywhere together—either an idyllic boy-and-his-dog relationship or a sad, pitifully lonely, soul-in-need-of-a-girlfriend existence, depending on your point of view, I suppose.
In the end, the two viewpoints melded as Hogan eventually brought my wife and I together, a story that shall not be printed in these pages.
Through the years, Hogan bore witness to many personal and family milestones. My chief residency, my first grand rounds (his constant audience during my preparation brought him unparalleled expertise in canine zoonoses), my first house, our marriage, a horribly flailing attempt to recapture the magic of my first dog through a second Weim named Grady (definitely “Marley & Me” mixed with a healthy dose of “Dumb & Dumber”), and the birth of our first child.
It was during this time that Hogan began a long journey toward today. He became a little long in the tooth, droopy in the belly, and slow on the trail. His limitless energy and boundless passion for chasing tennis balls gave way to such leisurely pursuits as park pooping and command disobedience. His fluid, sinew-laced limbs became arthritic shells of their former selves, betraying the youthful grace that still echoed inside of him. I distinctly recall the first time Grady beat him to a tennis ball, a moment that clearly represented a passing of the baton—a crestfallen 6-year-old canine eclipsed by the 2-year-old whippersnapper. The youngster sprinted back, bursting with a mouthful of tennis ball and pride. The elder took a decidedly more tortuous and tortured route back—a carriage of nonchalance that failed in its attempt to convey the message that “chasing tennis balls is stupid.”
On the advice of our veterinarian, we stopped throwing Frisbees at Hogan at age 8, out of concern that an awkward jump might result in a paralytic shift in his progressively stenosing spine. While Hogan is otherwise healthy, his hips and forepaws are riddled with osteoarthritis, his eyes carry the cottony haze of cataracts, and his abdomen and skin are home to lumpy lipomas. So, on the advent of his 10th birthday, we are asked to decide how many resources, how much physical distress, how much intervention we afford to an older, sleep-most-of-the-day arthritic dog.
This scenario is complicated by the idiosyncrasies and mores of veterinary medicine. Unlike human medicine, which is replete with tomes of data steeped in decades of experience, our veterinarian counterparts often are left with gaping treatment holes and inadequately studied interventions. This is not a knock against the profession. In fact, I have had nothing short of fantastic experiences with the veterinary professionals with whom I’ve interacted. Rather, there just aren’t prospective, randomized, controlled trials to inform whether intervention will enhance Hogan’s quantity and quality of life.
Then there are the economic realities of the situation. As one who has rarely been ill and always been insured, I was staggered by the cost of medicine for the uninsured. Two-hundred-dollar antibiotics, $500 ultrasounds, $1,500 CT scans, and up to $10,000 operative and surgical ICU stays would have invoked, “you’re joking, right?” exclamations from me prior to this experience. Now they are just another variable that complicates this already emotionally complex discussion—the variable that makes you feel hollow inside for considering it, foolhardy for not.
Questions Abound
What price would I pay to have another few years with my best friend? What if it’s only a year, six months? Would the money be better spent funding my child’s 529 account? What if this is a benign process and intervention is for naught? What if this tumor is metastatic and intervention is futile? Should we spend the extra money on an upfront staging CT scan that has much lower sensitivity than those we routinely utilize?
If we intervene, should we attempt a costly, CT-guided biopsy to rule in malignant disease, or go straight to lobectomy? What if the surgery negatively alters his quality of life? What if he dies on the table? What do I know about the surgical outcomes of the two centers I’m considering? Should we attempt an open or laparoscopic approach to this tumor?
Can we achieve a cure? If we do, what does that mean for a dog in the twilight years of his life? Should we just let the disease progress to its natural endpoint?
What Would Hogan Want?
These are the questions that haunt me. As I stare into Hogan’s eyes, a portal to my companion’s soul, I am tormented by the cauldron of emotions, the indecision bred by incomplete information and the guilt that comes from knowing that Hogan unconditionally trusts that I will do what is right for him.
Will I? My eyes continue to ask Hogan what he would want until finally the answer becomes obvious.
Hogan wants his breakfast. TH
Dr. Glasheen is associate professor of medicine and director of the hospital medicine group and hospitalist training program at the University of Colorado at Denver.
Lying in bed, I’m jarred by what can only be an anvil dropping heavily upon my chest. Wakefulness reveals a more canine, cranium-like object. Staring deep into cataract-smudged eyes, I ponder the question that has occupied my mind for nearly two weeks: What would Hogan want?
My Dog Has Cancer
More accurately, he has a tumor—or, I guess, what appears to be a tumor on his chest X-ray. It was discovered, incidentally, on a liver ultrasound that was being done for abnormal liver function test results. That study revealed nothing wrong with his liver, but led to a follow-up radiograph showing a 4.9-cm, right-lower-lobe lung mass. Also uncovered in this process was a tangle of complex emotions, turmoil, and uncertainty surrounding my first personal foray into end-of-life decision-making.
Hogan, my now-presumed-cancer-ridden, 10-year-old Weimaraner, came into my life permanently when he was all of 8 weeks old. I first met him during a visit to the breeder when his litter was only three days old. Over the successive weeks, I visited him often, anxious for the day I’d be able to take my new companion home.
I picked up Hogan on Fourth of July weekend during my chief year of residency—sort of a gift for completing my grueling training. He was the first dog I raised, trained, and cared for by myself. And while we had our share of eaten walls, destroyed comforters, and chewed bits of Jeep Cherokee, this was no “Marley & Me” relationship. We were more like roommates, best friends. We hiked, camped, and went everywhere together—either an idyllic boy-and-his-dog relationship or a sad, pitifully lonely, soul-in-need-of-a-girlfriend existence, depending on your point of view, I suppose.
In the end, the two viewpoints melded as Hogan eventually brought my wife and I together, a story that shall not be printed in these pages.
Through the years, Hogan bore witness to many personal and family milestones. My chief residency, my first grand rounds (his constant audience during my preparation brought him unparalleled expertise in canine zoonoses), my first house, our marriage, a horribly flailing attempt to recapture the magic of my first dog through a second Weim named Grady (definitely “Marley & Me” mixed with a healthy dose of “Dumb & Dumber”), and the birth of our first child.
It was during this time that Hogan began a long journey toward today. He became a little long in the tooth, droopy in the belly, and slow on the trail. His limitless energy and boundless passion for chasing tennis balls gave way to such leisurely pursuits as park pooping and command disobedience. His fluid, sinew-laced limbs became arthritic shells of their former selves, betraying the youthful grace that still echoed inside of him. I distinctly recall the first time Grady beat him to a tennis ball, a moment that clearly represented a passing of the baton—a crestfallen 6-year-old canine eclipsed by the 2-year-old whippersnapper. The youngster sprinted back, bursting with a mouthful of tennis ball and pride. The elder took a decidedly more tortuous and tortured route back—a carriage of nonchalance that failed in its attempt to convey the message that “chasing tennis balls is stupid.”
On the advice of our veterinarian, we stopped throwing Frisbees at Hogan at age 8, out of concern that an awkward jump might result in a paralytic shift in his progressively stenosing spine. While Hogan is otherwise healthy, his hips and forepaws are riddled with osteoarthritis, his eyes carry the cottony haze of cataracts, and his abdomen and skin are home to lumpy lipomas. So, on the advent of his 10th birthday, we are asked to decide how many resources, how much physical distress, how much intervention we afford to an older, sleep-most-of-the-day arthritic dog.
This scenario is complicated by the idiosyncrasies and mores of veterinary medicine. Unlike human medicine, which is replete with tomes of data steeped in decades of experience, our veterinarian counterparts often are left with gaping treatment holes and inadequately studied interventions. This is not a knock against the profession. In fact, I have had nothing short of fantastic experiences with the veterinary professionals with whom I’ve interacted. Rather, there just aren’t prospective, randomized, controlled trials to inform whether intervention will enhance Hogan’s quantity and quality of life.
Then there are the economic realities of the situation. As one who has rarely been ill and always been insured, I was staggered by the cost of medicine for the uninsured. Two-hundred-dollar antibiotics, $500 ultrasounds, $1,500 CT scans, and up to $10,000 operative and surgical ICU stays would have invoked, “you’re joking, right?” exclamations from me prior to this experience. Now they are just another variable that complicates this already emotionally complex discussion—the variable that makes you feel hollow inside for considering it, foolhardy for not.
Questions Abound
What price would I pay to have another few years with my best friend? What if it’s only a year, six months? Would the money be better spent funding my child’s 529 account? What if this is a benign process and intervention is for naught? What if this tumor is metastatic and intervention is futile? Should we spend the extra money on an upfront staging CT scan that has much lower sensitivity than those we routinely utilize?
If we intervene, should we attempt a costly, CT-guided biopsy to rule in malignant disease, or go straight to lobectomy? What if the surgery negatively alters his quality of life? What if he dies on the table? What do I know about the surgical outcomes of the two centers I’m considering? Should we attempt an open or laparoscopic approach to this tumor?
Can we achieve a cure? If we do, what does that mean for a dog in the twilight years of his life? Should we just let the disease progress to its natural endpoint?
What Would Hogan Want?
These are the questions that haunt me. As I stare into Hogan’s eyes, a portal to my companion’s soul, I am tormented by the cauldron of emotions, the indecision bred by incomplete information and the guilt that comes from knowing that Hogan unconditionally trusts that I will do what is right for him.
Will I? My eyes continue to ask Hogan what he would want until finally the answer becomes obvious.
Hogan wants his breakfast. TH
Dr. Glasheen is associate professor of medicine and director of the hospital medicine group and hospitalist training program at the University of Colorado at Denver.
Life under the Big Tent
SHM prides itself on being a “big tent” organization—inclusive of care providers with different training backgrounds, from varied clinical settings, and representing a multitude of hospital roles. SHM’s diversity expands beyond care providers to include other key stakeholders, such as administrators of hospitalist programs or departments. The diversity was highlighted in the society’s 2007-2008 “Bi-annual Survey on the State of the Hospital Medicine Movement,” which shows that 40% of our members are hospital-employed, 20% are from academic settings, about 15% work in large multistate management companies, and the remaining 25% are equally split between multispecialty practices and local hospitalist groups. The diversity extends to internists, pediatricians, family practitioners, nurse practitioners, physician assistants, specialists—the list goes on.
All of us at SHM appreciate that diversity and routinely try to nurture it. Our board of directors includes physicians from all the aforementioned practice settings and includes dedicated seats for such key constituencies as pediatrics. We have more than 25 committees and task-force groups representing all the key factions of our membership. These groups address issues of relevance to every type of hospitalist and hospitalist group. Our annual meeting has evolved to meet the needs of this diverse membership by addressing an enormous volume of topics and incorporating a variety of tracks that cater to general hospitalists, quality experts, academics, and pediatricians.
One notable area in which we lack diversity: age. We are a young specialty; the average hospitalist is 40 years old.
SHM’s organizational diversity creates challenges; new issues surface every year. One key issue is the balance between academic hospitalists and community hospitalists. Academic hospitalists have wanted SHM to more aggressively support their interests. Community hospitalists want SHM to advocate for their interests, as well as develop programs and projects to meet their needs.
With my election as president and the recent election of another academic hospitalist to serve as SHM president in 2010-2011, there might be some concern that community hospitalists could get lost in an academic agenda. Interestingly, academic hospitalists might have raised similar concerns several years ago following the election of a second consecutive community hospitalist as society president. We have been fortunate to have leaders who can see the whole HM picture, regardless of their professional backgrounds.
One Tent, Many Spikes in the Ground
The issues are more complex than simply the differences between academic and community hospitalists. Appropriately, each of our members wants their groups’ issues discussed and addressed. Although workforce might be an issue for private-practice hospitalists, academic hospitalists share these same issues—just in a different environment. Pediatricians see SHM develop core competencies for adult medicine, then want their own pediatric core competencies; SHM needs to look for a way to make this happen. Nurse practitioners (NPs), physician assistants (PAs), and administrators have looked to SHM to represent not just physicians in HM, but also their interests.
As a result, SHM has developed committees and approaches to engage the professional societies representing PAs and NPs, along with the Medical Group Management Association, to design specific projects and programs. At any point in time, there might be a group of members who see a need for SHM to pay attention to “their” issue or perceive that a current approach, while relevant to one group, falls short of the needs of another. As our diversity grows, the frequency of these situations will increase.
So, is this worrisome? Quite the contrary. I believe it is healthy.
Having an established organization with members of differing opinions and backgrounds helps challenge our assumptions. It refines our approach to complex problems, highlights issues or concerns we did not anticipate, and, most importantly, guards against “groupthink”—the tendency to agree with one another all the time. SHM’s board of directors is committed to this type of inclusive leadership.
We do need to be cautious and think quite a bit about this issue in the coming years. The big tent is filling up quickly. It’s becoming more diverse by the week. The concern is that in trying to work at a level that keeps all our constituents happy, we might please no one. If all our activities have to be justified as being relevant to every distinct group that makes up SHM, then we might dilute our effectiveness.
Alternatively, we do not currently have the bandwidth as an organization to initiate in-depth projects in areas relevant to all our members. So far, our approach has been to focus on areas core to every hospitalist: quality and safety, process improvement, leadership, practice management, care transitions, networking, and education.
As unique problems or issues arise that are relevant to only a subset of members, we will weigh the importance. In many cases, we have created task-force groups to clarify and tackle the problem. We provide the support, but the members of the group create the solution. It has worked well so far.
One Voice, One Goal
But can we stick to this strategy as the diversity of membership expands and the number of relevant issues grows? I don’t know. What I do know is that there is strength in numbers, and even though we all have different issues we deem more important, there are times when it helps to come together and speak as one very big, very loud voice.
Older specialties like endocrinology, allergy, and others have split into a variety of organizations and potentially diluted their message. SHM needs to look for creative ways to be relevant to many constituencies within the specialty. In the meantime, we must pay close attention to the big-tent issues. An academic hospitalist in leadership needs to listen to the voices of hospitalists in the community, work to understand them, and support efforts to address problems relevant to them.
In the past, SHM leadership from the community hospitalist setting has worked to help address and solve issues relevant to academic hospitalists. We need to understand and respect the diversity within SHM’s tent, and we need to work to keep us all together. I firmly believe that is the way forward, and I assure you that is the goal of SHM’s leadership.
As President Kennedy said, “If we cannot end now our differences, at least we can help make the world safe for diversity.” I pledge to keep SHM your organization, regardless of how you were trained or where you practice HM. I can’t hope to know all of your important issues, but I can commit to stand ready to hear your concerns and do what SHM has always done—give your request a thoughtful response and all of our energy.
SHM is your organization. Let me know the direction you think SHM should go. Send me an e-mail at [email protected]. TH
Dr. Flanders is president of SHM.
SHM prides itself on being a “big tent” organization—inclusive of care providers with different training backgrounds, from varied clinical settings, and representing a multitude of hospital roles. SHM’s diversity expands beyond care providers to include other key stakeholders, such as administrators of hospitalist programs or departments. The diversity was highlighted in the society’s 2007-2008 “Bi-annual Survey on the State of the Hospital Medicine Movement,” which shows that 40% of our members are hospital-employed, 20% are from academic settings, about 15% work in large multistate management companies, and the remaining 25% are equally split between multispecialty practices and local hospitalist groups. The diversity extends to internists, pediatricians, family practitioners, nurse practitioners, physician assistants, specialists—the list goes on.
All of us at SHM appreciate that diversity and routinely try to nurture it. Our board of directors includes physicians from all the aforementioned practice settings and includes dedicated seats for such key constituencies as pediatrics. We have more than 25 committees and task-force groups representing all the key factions of our membership. These groups address issues of relevance to every type of hospitalist and hospitalist group. Our annual meeting has evolved to meet the needs of this diverse membership by addressing an enormous volume of topics and incorporating a variety of tracks that cater to general hospitalists, quality experts, academics, and pediatricians.
One notable area in which we lack diversity: age. We are a young specialty; the average hospitalist is 40 years old.
SHM’s organizational diversity creates challenges; new issues surface every year. One key issue is the balance between academic hospitalists and community hospitalists. Academic hospitalists have wanted SHM to more aggressively support their interests. Community hospitalists want SHM to advocate for their interests, as well as develop programs and projects to meet their needs.
With my election as president and the recent election of another academic hospitalist to serve as SHM president in 2010-2011, there might be some concern that community hospitalists could get lost in an academic agenda. Interestingly, academic hospitalists might have raised similar concerns several years ago following the election of a second consecutive community hospitalist as society president. We have been fortunate to have leaders who can see the whole HM picture, regardless of their professional backgrounds.
One Tent, Many Spikes in the Ground
The issues are more complex than simply the differences between academic and community hospitalists. Appropriately, each of our members wants their groups’ issues discussed and addressed. Although workforce might be an issue for private-practice hospitalists, academic hospitalists share these same issues—just in a different environment. Pediatricians see SHM develop core competencies for adult medicine, then want their own pediatric core competencies; SHM needs to look for a way to make this happen. Nurse practitioners (NPs), physician assistants (PAs), and administrators have looked to SHM to represent not just physicians in HM, but also their interests.
As a result, SHM has developed committees and approaches to engage the professional societies representing PAs and NPs, along with the Medical Group Management Association, to design specific projects and programs. At any point in time, there might be a group of members who see a need for SHM to pay attention to “their” issue or perceive that a current approach, while relevant to one group, falls short of the needs of another. As our diversity grows, the frequency of these situations will increase.
So, is this worrisome? Quite the contrary. I believe it is healthy.
Having an established organization with members of differing opinions and backgrounds helps challenge our assumptions. It refines our approach to complex problems, highlights issues or concerns we did not anticipate, and, most importantly, guards against “groupthink”—the tendency to agree with one another all the time. SHM’s board of directors is committed to this type of inclusive leadership.
We do need to be cautious and think quite a bit about this issue in the coming years. The big tent is filling up quickly. It’s becoming more diverse by the week. The concern is that in trying to work at a level that keeps all our constituents happy, we might please no one. If all our activities have to be justified as being relevant to every distinct group that makes up SHM, then we might dilute our effectiveness.
Alternatively, we do not currently have the bandwidth as an organization to initiate in-depth projects in areas relevant to all our members. So far, our approach has been to focus on areas core to every hospitalist: quality and safety, process improvement, leadership, practice management, care transitions, networking, and education.
As unique problems or issues arise that are relevant to only a subset of members, we will weigh the importance. In many cases, we have created task-force groups to clarify and tackle the problem. We provide the support, but the members of the group create the solution. It has worked well so far.
One Voice, One Goal
But can we stick to this strategy as the diversity of membership expands and the number of relevant issues grows? I don’t know. What I do know is that there is strength in numbers, and even though we all have different issues we deem more important, there are times when it helps to come together and speak as one very big, very loud voice.
Older specialties like endocrinology, allergy, and others have split into a variety of organizations and potentially diluted their message. SHM needs to look for creative ways to be relevant to many constituencies within the specialty. In the meantime, we must pay close attention to the big-tent issues. An academic hospitalist in leadership needs to listen to the voices of hospitalists in the community, work to understand them, and support efforts to address problems relevant to them.
In the past, SHM leadership from the community hospitalist setting has worked to help address and solve issues relevant to academic hospitalists. We need to understand and respect the diversity within SHM’s tent, and we need to work to keep us all together. I firmly believe that is the way forward, and I assure you that is the goal of SHM’s leadership.
As President Kennedy said, “If we cannot end now our differences, at least we can help make the world safe for diversity.” I pledge to keep SHM your organization, regardless of how you were trained or where you practice HM. I can’t hope to know all of your important issues, but I can commit to stand ready to hear your concerns and do what SHM has always done—give your request a thoughtful response and all of our energy.
SHM is your organization. Let me know the direction you think SHM should go. Send me an e-mail at [email protected]. TH
Dr. Flanders is president of SHM.
SHM prides itself on being a “big tent” organization—inclusive of care providers with different training backgrounds, from varied clinical settings, and representing a multitude of hospital roles. SHM’s diversity expands beyond care providers to include other key stakeholders, such as administrators of hospitalist programs or departments. The diversity was highlighted in the society’s 2007-2008 “Bi-annual Survey on the State of the Hospital Medicine Movement,” which shows that 40% of our members are hospital-employed, 20% are from academic settings, about 15% work in large multistate management companies, and the remaining 25% are equally split between multispecialty practices and local hospitalist groups. The diversity extends to internists, pediatricians, family practitioners, nurse practitioners, physician assistants, specialists—the list goes on.
All of us at SHM appreciate that diversity and routinely try to nurture it. Our board of directors includes physicians from all the aforementioned practice settings and includes dedicated seats for such key constituencies as pediatrics. We have more than 25 committees and task-force groups representing all the key factions of our membership. These groups address issues of relevance to every type of hospitalist and hospitalist group. Our annual meeting has evolved to meet the needs of this diverse membership by addressing an enormous volume of topics and incorporating a variety of tracks that cater to general hospitalists, quality experts, academics, and pediatricians.
One notable area in which we lack diversity: age. We are a young specialty; the average hospitalist is 40 years old.
SHM’s organizational diversity creates challenges; new issues surface every year. One key issue is the balance between academic hospitalists and community hospitalists. Academic hospitalists have wanted SHM to more aggressively support their interests. Community hospitalists want SHM to advocate for their interests, as well as develop programs and projects to meet their needs.
With my election as president and the recent election of another academic hospitalist to serve as SHM president in 2010-2011, there might be some concern that community hospitalists could get lost in an academic agenda. Interestingly, academic hospitalists might have raised similar concerns several years ago following the election of a second consecutive community hospitalist as society president. We have been fortunate to have leaders who can see the whole HM picture, regardless of their professional backgrounds.
One Tent, Many Spikes in the Ground
The issues are more complex than simply the differences between academic and community hospitalists. Appropriately, each of our members wants their groups’ issues discussed and addressed. Although workforce might be an issue for private-practice hospitalists, academic hospitalists share these same issues—just in a different environment. Pediatricians see SHM develop core competencies for adult medicine, then want their own pediatric core competencies; SHM needs to look for a way to make this happen. Nurse practitioners (NPs), physician assistants (PAs), and administrators have looked to SHM to represent not just physicians in HM, but also their interests.
As a result, SHM has developed committees and approaches to engage the professional societies representing PAs and NPs, along with the Medical Group Management Association, to design specific projects and programs. At any point in time, there might be a group of members who see a need for SHM to pay attention to “their” issue or perceive that a current approach, while relevant to one group, falls short of the needs of another. As our diversity grows, the frequency of these situations will increase.
So, is this worrisome? Quite the contrary. I believe it is healthy.
Having an established organization with members of differing opinions and backgrounds helps challenge our assumptions. It refines our approach to complex problems, highlights issues or concerns we did not anticipate, and, most importantly, guards against “groupthink”—the tendency to agree with one another all the time. SHM’s board of directors is committed to this type of inclusive leadership.
We do need to be cautious and think quite a bit about this issue in the coming years. The big tent is filling up quickly. It’s becoming more diverse by the week. The concern is that in trying to work at a level that keeps all our constituents happy, we might please no one. If all our activities have to be justified as being relevant to every distinct group that makes up SHM, then we might dilute our effectiveness.
Alternatively, we do not currently have the bandwidth as an organization to initiate in-depth projects in areas relevant to all our members. So far, our approach has been to focus on areas core to every hospitalist: quality and safety, process improvement, leadership, practice management, care transitions, networking, and education.
As unique problems or issues arise that are relevant to only a subset of members, we will weigh the importance. In many cases, we have created task-force groups to clarify and tackle the problem. We provide the support, but the members of the group create the solution. It has worked well so far.
One Voice, One Goal
But can we stick to this strategy as the diversity of membership expands and the number of relevant issues grows? I don’t know. What I do know is that there is strength in numbers, and even though we all have different issues we deem more important, there are times when it helps to come together and speak as one very big, very loud voice.
Older specialties like endocrinology, allergy, and others have split into a variety of organizations and potentially diluted their message. SHM needs to look for creative ways to be relevant to many constituencies within the specialty. In the meantime, we must pay close attention to the big-tent issues. An academic hospitalist in leadership needs to listen to the voices of hospitalists in the community, work to understand them, and support efforts to address problems relevant to them.
In the past, SHM leadership from the community hospitalist setting has worked to help address and solve issues relevant to academic hospitalists. We need to understand and respect the diversity within SHM’s tent, and we need to work to keep us all together. I firmly believe that is the way forward, and I assure you that is the goal of SHM’s leadership.
As President Kennedy said, “If we cannot end now our differences, at least we can help make the world safe for diversity.” I pledge to keep SHM your organization, regardless of how you were trained or where you practice HM. I can’t hope to know all of your important issues, but I can commit to stand ready to hear your concerns and do what SHM has always done—give your request a thoughtful response and all of our energy.
SHM is your organization. Let me know the direction you think SHM should go. Send me an e-mail at [email protected]. TH
Dr. Flanders is president of SHM.
How should hypertension in pregnant patients be managed?
Case
You are consulted on a 29-year-old gravida 1 at nine weeks gestation with a two-year history of Type 2 diabetes and hypertension. She is admitted to the obstetric inpatient service for glycemic control. Although prescribed metformin and lisinopril, she ran out of both four months ago. Her current hemoglobin A1C is 9%. Her blood pressure is 140/90 mmHg in both arms, with an appropriately sized manual cuff while seated. She does not have retinopathy, nephropathy, or neuropathy. The obstetric team will begin weight-based insulin to achieve glycemic targets, and they ask for your input regarding blood-pressure management. How should one approach a pregnant patient with hypertension?
Overview
The most common chronic medical issue in reproductive-age women, essential hypertension (termed chronic hypertension in obstetric literature) contributes significantly to maternal and perinatal morbidity and mortality, primarily via increased risk of preeclampsia.
Chronic hypertension complicates up to 5% of pregnancies in the U.S., or as many as 120,000 pregnant women per year.1 Rates of chronic hypertension are expected to increase with later childbearing and increased rate of obesity. Prior to and during pregnancy, hypertension is defined as blood pressure 140/90 mmHg or higher. Chronic hypertension can be either hypertension diagnosed prior to pregnancy or elevated blood pressures identified prior to 20 weeks gestation.2 Normal pregnancy physiology leads to decreased systemic vascular resistance by the end of the first trimester, dropping systolic and diastolic blood pressure between 10 and 15 mmHg, with maximal effect mid-pregnancy followed by a gradual return to baseline.3 Therefore, chronic hypertension might be masked in early pregnancy. Normal changes in pregnancy include renal vasodilatation and increased glomerular filtration rate, so the average serum creatinine (SCr) is 0.5 mg/dL.4
Newly identified hypertension or accelerating hypertension after 20 weeks warrants close evaluation for preeclampsia. Preeclampsia is a multisystem, life-threatening disorder characterized by hypertension and proteinuria (greater than 300 mg/day). Severe forms of preeclampsia include HELLP (hemolysis, elevated liver enzymes and low platelets) syndrome and eclampsia (seizures with no other attributable cause). Superimposed preeclampsia occurs in 20% to 25% of women with chronic hypertension.5 Women with hypertensive target organ damage have an even greater likelihood of preeclampsia as well as maternal and fetal complications. Unfortunately, blood-pressure control during pregnancy has not been shown to minimize the likelihood of developing superimposed preeclampsia or associated maternal and fetal complications.6 The goal of antihypertensive management during pregnancy is to avoid acute maternal or fetal complications of severe hypertension.
Review of the Data
Q: How are hypertensive disorders of pregnancy classified?
The American College of Obstetrics and Gynecology and the National High Blood Pressure Education Program guideline committees have classified hypertensive disorders of pregnancy into four categories: chronic hypertension, preeclampsia, preeclampsia superimposed on chronic hypertension, and gestational hypertension.2,7
Chronic hypertension is defined as blood pressure 140/90 mmHg or greater on two occasions before pregnancy, prior to 20 weeks of gestation, or persisting 12 weeks postpartum. Severe hypertension is defined as diastolic blood pressure ≥110 mm Hg. Hypertensive women tend to have a greater decline in blood pressure during early pregnancy than normotensive women.8
Secondary hypertension is an important consideration in women of reproductive age. A brief screen for secondary causes includes bilateral arm pressures and femoral pulse assessment, renal bruit assessment, inquiry into snoring, gasping, and daytime somnolence, as well as measurement of serum thyrotropin, potassium, calcium, creatinine, and urinalysis. This kind of evaluation will assess for coarctation of the aorta, renal artery stenosis, obstructive sleep apnea, hyper- or hypothyroidism, hyperaldosteronism, hyperparathyroidism, and underlying renal disease.9,10 Patient reports of episodic headache, palpitations, and diaphoresis should prompt investigation for pheochromocytoma.
Hyperaldosteronism, pheochromocytoma, or hyperthyroidism might be quiescent during pregnancy but flare in the postpartum period.
Women with severe chronic hypertension or target organ damage have higher rates of superimposed preeclampsia. In these individuals, preeclampsia is more likely to present early (before 34 weeks gestation) or with severe disease.1
Preeclampsia is defined as hypertension and proteinuria (greater than 300 mg/day) at or beyond 20 weeks gestation in a previously normotensive woman. Preeclampsia rates vary from 5% to 10% of nulliparous women, to much higher rates in women with medical comorbidities or fetal factors (e.g., multiple gestations, molar pregnancies, hydrops, or triploidy). Preeclampsia’s pathogenesis is attributed to abnormal placental implantation with abnormal maternal immune adaptation, altered angiogenic factors with increased systemic vascular resistance and endothelial dysfunction leading to the clinically apparent maternal syndrome.11
Severe preeclampsia criteria include any of the following: eclampsia, HELLP syndrome (platelets less than 100,000/mm³, transaminases more than twice the upper limit of normal, and/or epigastric pain), SBP ≥160 mmHg, DBP ≥110 mmHg, proteinuria ≥5 grams per day, oliguria, pulmonary edema, placental abruption, or fetal manifestations such as intrauterine growth restriction (≤10th percentile of expected fetal weight based on gestational age), decreased amniotic fluid index, or fetal demise.2,5,12
Maternal symptoms might include headache, visual disturbances, epigastric or right upper quadrant (RUQ) pain, rapid weight gain, and severe edema. Some women remain asymptomatic. Preeclampsia can rapidly progress from “less severe” to severe. Maternal symptoms and abnormal lab findings are more predictive of adverse pregnancy outcomes than the degree of hypertension and/or proteinuria.1
It is always in the mother’s interest to deliver when preeclampsia is diagnosed, because preeclampsia will not resolve until after delivery, with hypertension and lab abnormalities sometimes persisting for months postpartum. Preeclampsia might be diagnosed before fetal viability (approximately 24 weeks gestation), although the vast majority of cases occur near term.
Risks of premature delivery must be balanced with the risks of progressively severe manifestations for the mother and fetus. Guidelines for expectant management of early (<34 weeks) preeclampsia are based on available evidence and expert opinion.13 Magnesium sulfate has been shown to be the most effective agent to minimize the likelihood of seizure in preeclamptic women.14 With an initial bolus of 4 g to 6 g IV followed by infusion of 1-2 g/hour, magnesium sulfate is usually continued for 24 to 48 hours after delivery.
Preeclampsia can first appear postpartum, most likely in the first days to weeks. A growing body of literature links preeclampsia, particularly early and/or recurrent, to subsequent increased risk for cardiovascular disease and end-stage renal disease.15
Preeclampsia superimposed on chronic hypertension is defined as the new onset or markedly increasing proteinuria or accelerating hypertension in the latter half of pregnancy. Maternal symptoms, transaminase elevation, thrombocytopenia, or fetal manifestations further support this diagnosis.
Gestational hypertension, previously known as pregnancy-induced hypertension, is defined as hypertension in the absence of proteinuria in the latter half of pregnancy. Symptoms and lab abnormalities of preeclampsia will be absent. At least half of women with hypertension in the latter half of pregnancy progress to preeclampsia, so gestational hypertension should be considered a provisional diagnosis. Severe gestational hypertension, even without proteinuria or other lab abnormalities, carries increased perinatal risk.
Q: What factors contribute to increased preeclampsia risk?
Maternal factors include: first pregnancy, first pregnancy with a new father, maternal age >35, particularly >40, personal or family history of preeclampsia, chronic hypertension, diabetes mellitus (Type 1, 2 or gestational), systemic lupus erythematosus, antiphospholipid antibody syndrome, renal disease, and obesity. Fetal factors include: multiple gestations, molar pregnancies, hydrops, and triploidy.5,12
Q: When should antihypertensive medications be used in pregnancy?
Most women are hesitant to expose their fetus to medication, and thus must be in therapeutic alliance with their obstetrician and consultants. The overriding principle of medication use in pregnancy is that a healthy fetus requires a healthy mother, and medication use is justified when there is definite benefit to the mother. Due to increased metabolism during pregnancy, medications otherwise dosed once per day often require two doses per day, and those dosed twice daily often require every-eight-hour dosing to maintain efficacy. Additionally, titration up every few days may be required.
Therapy goals include avoiding maternal and fetal complications from severely elevated blood pressure, as well as avoiding fetal growth restriction due to impaired uteroplacental flow. The ideal blood pressure for a hypertensive pregnant woman has not been established, but recommendations are based upon available data and expert opinion.2,5,10,12 Maternal risk of intracerebral hemorrhage increases with SBP ≥160 mmHg.16 Diastolic BP ≥110 mmHg has been associated with greater risk of placental abruption and intrauterine growth restriction.
Pharmacologic treatment generally is initiated or adjusted to achieve SBP <160 mm Hg and DBP <100 to <105 mmHg, although some societies advocate treatment initiation at 140/90 mmHg.2,5,12,17,18 If a woman has target organ damage or concomitant medical issues warranting tighter control (e.g., diabetes or pre-existing renal disease), 130/80 mmHg is preferable.19 Activity limitation and/or bed rest, although commonly recommended, have not been shown to reduce maternal or fetal morbidity or mortality, or prolong time to delivery.
An ongoing, randomized, prospective trial will compare maternal and fetal outcomes in women with mild chronic hypertension with deliberate blood-pressure stratification (goal DBP 85 mmHg vs. goal DBP 100 mmHg).20
Q: What are reasonable treatment options for a woman with chronic hypertension during pregnancy?
Due to vasodilatation of pregnancy, antihypertensive agents often can be discontinued early in pregnancy with close, ongoing monitoring. The majority of women with mild chronic hypertension will have blood pressures <160/100 mmHg without medication during the first half of pregnancy.
If a woman has been using a pharmacologic agent not advisable during pregnancy, she could be switched to a preferred agent. If a woman has been using a pharmacologic agent preferred during pregnancy, she could continue this agent.
Q: What antihypertensives are favored during pregnancy?
Methyldopa and labetalol have been used extensively. Methyldopa has not been found to adversely affect cognitive development in children exposed in utero. On the maternal side, somnolence, dizziness, and dry mouth are common side effects.
Labetalol is widely used as a first- or second-line agent. It can be used intravenously or orally. Intravenous labetalol in escalating doses (10 mg, 20 mg, 40 mg, 80 mg) is the first line of acute treatment for severe hypertension/preeclampsia.
Atenolol and propranolol have been associated with fetal growth restriction, metoprolol to a lesser degree.
Metoprolol is useful in women with coronary artery disease, tachyarrhythmias, and/or requiring migraine prophylaxis during pregnancy.
Nifedipine is often used as a second-line agent, with extended-release preparation preferred. Short-acting nifedipine should be used with caution during pregnancy due to the potential for acute impairment of uteroplacental flow. However, short-acting nifedipine is used for tocolysis in pre-term labor.
Intravenous hydralazine is another option for acute treatment in the setting of severe hypertension/preeclampsia.
Angiotensin-converting enzyme (ACE) inhibitors are contraindicated during pregnancy due to association with increased rates of cardiovascular and central nervous system malformations when used in the first trimester, as well as fetal anuric renal failure when used later in pregnancy.21 Due to similar mechanisms of action, angiotensin receptor blockers (ARBs) are contraindicated.
In general, antihypertensive agents are considered compatible with lactation, with most minimally excreted into breast milk. Women requiring antihypertensive agents or almost any medication during lactation seek particular reassurance from caregivers.
It is essential to emphasize the benefit of breastfeeding for both mother and newborn, which far outweighs the risk of medication exposure to the newborn—with rare exceptions. Enalapril and captopril are considered compatible with breastfeeding by the American Academy of Pediatrics.22
Q: Can we identify and possibly prevent preeclampsia?
Escalating hypertension or maternal symptoms, especially in women with increased risk factors, warrant careful examination and laboratory assessment for preeclampsia. Physical findings may include retinal vasospasm, rales on pulmonary exam, cardiac gallop, RUQ or midepigastric tenderness from hepatic capsule stretching, nondependent edema (e.g., face, hands), or clonus on deep tendon reflex evaluation. Useful laboratory values include complete blood count, serum creatinine, hepatic transaminases, uric acid, and urinalysis.
Marked anemia or hemoconcentration, thrombocytopenia, SCr ≥0.8 mg/dL, transaminases above normal, uric acid ≥5.0 mg/dL, urine protein 1+ or greater on dipstick, are all suggestive of preeclampsia, particularly if worsened compared to prior values. Urine protein-to-creatinine ratios have not reliably correlated with 24-hour urine protein collections in preeclamptic patients, although very high or low values could be helpful.23
Women are typically admitted for fetal monitoring, 24-hour urine protein collection, and blood-pressure management during a preeclampsia evaluation.
Thus far, the only intervention shown to reduce the likelihood of preeclampsia in women at increased risk is low-dose aspirin. A recent meta-analysis noted 10% reduction of relative risk of preeclampsia and pre-term birth prior to 34 weeks in women with history of preeclampsia treated with aspirin from the second trimester onward.24 Other interventions in trials that have not displayed reduced risk include vitamin C, vitamin E, calcium, fish oil, zinc, magnesium, and antihypertensive therapy.
Back to the Case
Our patient has chronic hypertension and diabetes, so she should have a blood-pressure goal of <130/80 mmHg. She could be initiated on methyldopa or labetalol. She should have a screen for secondary hypertension via exam and serum thyrotropin, potassium, and calcium, as well as baseline “preeclampsia labs”: complete blood count, serum creatinine, transaminases, uric acid, and 24-hour urine protein assessment. Aspirin at 81 mg daily should be considered from 12 weeks gestation to delivery.
Glycemic control is critical in early gestation to avoid increased risk for congenital malformations and spontaneous abortion, and later on to minimize macrosomia. Close monitoring for maternal symptoms of preeclampsia and blood-pressure assessment is advisable. With medical comorbidities of hypertension and diabetes mellitus, the woman’s risk of preeclampsia is at least 25%. Her pregnancy dating should be confirmed by a first-trimester ultrasound.
Bottom Line
A pregnant woman with chronic hypertension should have evaluation for secondary causes of hypertension, adjustment or initiation of preferred antihypertensive agents to achieve blood pressures that minimize the risk for acute hypertensive complications and fetal growth impairment, and close monitoring for superimposed preeclampsia. TH
Dr. Hayes is an obstetric internist at Women & Infants’ Hospital and assistant professor of medicine (clinical) at the Warren Alpert Medical School of Brown University, Providence, R.I.
References
- Sibai BM. Caring for women with hypertension in pregnancy. JAMA. 2007;298(13):1566-1568.
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
- Christianson RE. Studies on blood pressure during pregnancy. I. Influence of parity and age. Am J Obstet Gynecol. 1976;125(4):509-513.
- Gibson P, Rosene-Montella K. Normal renal and vascular changes in pregnancy. In: Rosene-Montella K, Keely E, Barbour LA, Lee RV, eds. Medical Care of the Pregnant Patient. 2nd ed. Philadelphia: American College of Physicians; 2008:149-152.
- ACOG practice bulletin, No. 33, January 2002. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99(1):159-167.
- Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100(2):369-377.
- Mulrow CD, Chiquette E, Ferrer RL, et al. Management of Chronic Hypertension During Pregnancy. Rockville: Agency for Healthcare Research and Quality; 2000.
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Prof-essional and Public Education of the American Heart Association Council on high blood pressure Research. Circulation. 2005;111(5):697-716.
- Powrie RO. A 30-year-old woman with chronic hypertension trying to conceive. JAMA. 2007; 298(13):1548-1558.
- Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003:289(19):2560-2571.
- Widmer M, Villar J, Benigni A, Conde-Agudelo A, Karumanchi SA, Lindheimer M. Mapping the theories of preeclampsia and the role of angiogenic factors: a systematic review. Obstet Gynecol. 2007; 109(1):168-180.
- Magee LA, Helewa M, Moutquin JM, van Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. SOGC Clinical Practice Guideline. J Obstet Gynaecol Can. 2008; 30:S1-S48.
- Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol. 2007;196(6):514.e1-514.e1-9.
- Duley L, Gülmezoglu AM, Henderson-Smart DJ. Magnesium sulphate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev. 2003;(2):CD000025.
- McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918-930.
- Martin JN Jr., Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol. 2005;105(2): 246-254.
- Lindheimer MD, Taler SJ, Cunningham FG. Hyper-tension in pregnancy. J Am Soc Hypertens. 2008; 2(6):484-494.
- Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008; 51(4):960-969.
- Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care. 2008;31(5):1060-1079.
- Magee LA, von Dadelszen P, Chan S, et al. The Control of Hypertension In Pregnancy Study pilot trial. BJOG. 2007;114(6):770,e13-e20.
- Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006; 354(23):2442-2451.
- American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108(3):776-789.
- Papanna R, Mann LK, Kouides RW, Glantz JC. Protein/creatinine ratio in preeclampsia: a systematic review. Obstet Gynecol. 2008;112(1):135-144.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA, PARIS Collaborative group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575): 1791-1798.
Case
You are consulted on a 29-year-old gravida 1 at nine weeks gestation with a two-year history of Type 2 diabetes and hypertension. She is admitted to the obstetric inpatient service for glycemic control. Although prescribed metformin and lisinopril, she ran out of both four months ago. Her current hemoglobin A1C is 9%. Her blood pressure is 140/90 mmHg in both arms, with an appropriately sized manual cuff while seated. She does not have retinopathy, nephropathy, or neuropathy. The obstetric team will begin weight-based insulin to achieve glycemic targets, and they ask for your input regarding blood-pressure management. How should one approach a pregnant patient with hypertension?
Overview
The most common chronic medical issue in reproductive-age women, essential hypertension (termed chronic hypertension in obstetric literature) contributes significantly to maternal and perinatal morbidity and mortality, primarily via increased risk of preeclampsia.
Chronic hypertension complicates up to 5% of pregnancies in the U.S., or as many as 120,000 pregnant women per year.1 Rates of chronic hypertension are expected to increase with later childbearing and increased rate of obesity. Prior to and during pregnancy, hypertension is defined as blood pressure 140/90 mmHg or higher. Chronic hypertension can be either hypertension diagnosed prior to pregnancy or elevated blood pressures identified prior to 20 weeks gestation.2 Normal pregnancy physiology leads to decreased systemic vascular resistance by the end of the first trimester, dropping systolic and diastolic blood pressure between 10 and 15 mmHg, with maximal effect mid-pregnancy followed by a gradual return to baseline.3 Therefore, chronic hypertension might be masked in early pregnancy. Normal changes in pregnancy include renal vasodilatation and increased glomerular filtration rate, so the average serum creatinine (SCr) is 0.5 mg/dL.4
Newly identified hypertension or accelerating hypertension after 20 weeks warrants close evaluation for preeclampsia. Preeclampsia is a multisystem, life-threatening disorder characterized by hypertension and proteinuria (greater than 300 mg/day). Severe forms of preeclampsia include HELLP (hemolysis, elevated liver enzymes and low platelets) syndrome and eclampsia (seizures with no other attributable cause). Superimposed preeclampsia occurs in 20% to 25% of women with chronic hypertension.5 Women with hypertensive target organ damage have an even greater likelihood of preeclampsia as well as maternal and fetal complications. Unfortunately, blood-pressure control during pregnancy has not been shown to minimize the likelihood of developing superimposed preeclampsia or associated maternal and fetal complications.6 The goal of antihypertensive management during pregnancy is to avoid acute maternal or fetal complications of severe hypertension.
Review of the Data
Q: How are hypertensive disorders of pregnancy classified?
The American College of Obstetrics and Gynecology and the National High Blood Pressure Education Program guideline committees have classified hypertensive disorders of pregnancy into four categories: chronic hypertension, preeclampsia, preeclampsia superimposed on chronic hypertension, and gestational hypertension.2,7
Chronic hypertension is defined as blood pressure 140/90 mmHg or greater on two occasions before pregnancy, prior to 20 weeks of gestation, or persisting 12 weeks postpartum. Severe hypertension is defined as diastolic blood pressure ≥110 mm Hg. Hypertensive women tend to have a greater decline in blood pressure during early pregnancy than normotensive women.8
Secondary hypertension is an important consideration in women of reproductive age. A brief screen for secondary causes includes bilateral arm pressures and femoral pulse assessment, renal bruit assessment, inquiry into snoring, gasping, and daytime somnolence, as well as measurement of serum thyrotropin, potassium, calcium, creatinine, and urinalysis. This kind of evaluation will assess for coarctation of the aorta, renal artery stenosis, obstructive sleep apnea, hyper- or hypothyroidism, hyperaldosteronism, hyperparathyroidism, and underlying renal disease.9,10 Patient reports of episodic headache, palpitations, and diaphoresis should prompt investigation for pheochromocytoma.
Hyperaldosteronism, pheochromocytoma, or hyperthyroidism might be quiescent during pregnancy but flare in the postpartum period.
Women with severe chronic hypertension or target organ damage have higher rates of superimposed preeclampsia. In these individuals, preeclampsia is more likely to present early (before 34 weeks gestation) or with severe disease.1
Preeclampsia is defined as hypertension and proteinuria (greater than 300 mg/day) at or beyond 20 weeks gestation in a previously normotensive woman. Preeclampsia rates vary from 5% to 10% of nulliparous women, to much higher rates in women with medical comorbidities or fetal factors (e.g., multiple gestations, molar pregnancies, hydrops, or triploidy). Preeclampsia’s pathogenesis is attributed to abnormal placental implantation with abnormal maternal immune adaptation, altered angiogenic factors with increased systemic vascular resistance and endothelial dysfunction leading to the clinically apparent maternal syndrome.11
Severe preeclampsia criteria include any of the following: eclampsia, HELLP syndrome (platelets less than 100,000/mm³, transaminases more than twice the upper limit of normal, and/or epigastric pain), SBP ≥160 mmHg, DBP ≥110 mmHg, proteinuria ≥5 grams per day, oliguria, pulmonary edema, placental abruption, or fetal manifestations such as intrauterine growth restriction (≤10th percentile of expected fetal weight based on gestational age), decreased amniotic fluid index, or fetal demise.2,5,12
Maternal symptoms might include headache, visual disturbances, epigastric or right upper quadrant (RUQ) pain, rapid weight gain, and severe edema. Some women remain asymptomatic. Preeclampsia can rapidly progress from “less severe” to severe. Maternal symptoms and abnormal lab findings are more predictive of adverse pregnancy outcomes than the degree of hypertension and/or proteinuria.1
It is always in the mother’s interest to deliver when preeclampsia is diagnosed, because preeclampsia will not resolve until after delivery, with hypertension and lab abnormalities sometimes persisting for months postpartum. Preeclampsia might be diagnosed before fetal viability (approximately 24 weeks gestation), although the vast majority of cases occur near term.
Risks of premature delivery must be balanced with the risks of progressively severe manifestations for the mother and fetus. Guidelines for expectant management of early (<34 weeks) preeclampsia are based on available evidence and expert opinion.13 Magnesium sulfate has been shown to be the most effective agent to minimize the likelihood of seizure in preeclamptic women.14 With an initial bolus of 4 g to 6 g IV followed by infusion of 1-2 g/hour, magnesium sulfate is usually continued for 24 to 48 hours after delivery.
Preeclampsia can first appear postpartum, most likely in the first days to weeks. A growing body of literature links preeclampsia, particularly early and/or recurrent, to subsequent increased risk for cardiovascular disease and end-stage renal disease.15
Preeclampsia superimposed on chronic hypertension is defined as the new onset or markedly increasing proteinuria or accelerating hypertension in the latter half of pregnancy. Maternal symptoms, transaminase elevation, thrombocytopenia, or fetal manifestations further support this diagnosis.
Gestational hypertension, previously known as pregnancy-induced hypertension, is defined as hypertension in the absence of proteinuria in the latter half of pregnancy. Symptoms and lab abnormalities of preeclampsia will be absent. At least half of women with hypertension in the latter half of pregnancy progress to preeclampsia, so gestational hypertension should be considered a provisional diagnosis. Severe gestational hypertension, even without proteinuria or other lab abnormalities, carries increased perinatal risk.
Q: What factors contribute to increased preeclampsia risk?
Maternal factors include: first pregnancy, first pregnancy with a new father, maternal age >35, particularly >40, personal or family history of preeclampsia, chronic hypertension, diabetes mellitus (Type 1, 2 or gestational), systemic lupus erythematosus, antiphospholipid antibody syndrome, renal disease, and obesity. Fetal factors include: multiple gestations, molar pregnancies, hydrops, and triploidy.5,12
Q: When should antihypertensive medications be used in pregnancy?
Most women are hesitant to expose their fetus to medication, and thus must be in therapeutic alliance with their obstetrician and consultants. The overriding principle of medication use in pregnancy is that a healthy fetus requires a healthy mother, and medication use is justified when there is definite benefit to the mother. Due to increased metabolism during pregnancy, medications otherwise dosed once per day often require two doses per day, and those dosed twice daily often require every-eight-hour dosing to maintain efficacy. Additionally, titration up every few days may be required.
Therapy goals include avoiding maternal and fetal complications from severely elevated blood pressure, as well as avoiding fetal growth restriction due to impaired uteroplacental flow. The ideal blood pressure for a hypertensive pregnant woman has not been established, but recommendations are based upon available data and expert opinion.2,5,10,12 Maternal risk of intracerebral hemorrhage increases with SBP ≥160 mmHg.16 Diastolic BP ≥110 mmHg has been associated with greater risk of placental abruption and intrauterine growth restriction.
Pharmacologic treatment generally is initiated or adjusted to achieve SBP <160 mm Hg and DBP <100 to <105 mmHg, although some societies advocate treatment initiation at 140/90 mmHg.2,5,12,17,18 If a woman has target organ damage or concomitant medical issues warranting tighter control (e.g., diabetes or pre-existing renal disease), 130/80 mmHg is preferable.19 Activity limitation and/or bed rest, although commonly recommended, have not been shown to reduce maternal or fetal morbidity or mortality, or prolong time to delivery.
An ongoing, randomized, prospective trial will compare maternal and fetal outcomes in women with mild chronic hypertension with deliberate blood-pressure stratification (goal DBP 85 mmHg vs. goal DBP 100 mmHg).20
Q: What are reasonable treatment options for a woman with chronic hypertension during pregnancy?
Due to vasodilatation of pregnancy, antihypertensive agents often can be discontinued early in pregnancy with close, ongoing monitoring. The majority of women with mild chronic hypertension will have blood pressures <160/100 mmHg without medication during the first half of pregnancy.
If a woman has been using a pharmacologic agent not advisable during pregnancy, she could be switched to a preferred agent. If a woman has been using a pharmacologic agent preferred during pregnancy, she could continue this agent.
Q: What antihypertensives are favored during pregnancy?
Methyldopa and labetalol have been used extensively. Methyldopa has not been found to adversely affect cognitive development in children exposed in utero. On the maternal side, somnolence, dizziness, and dry mouth are common side effects.
Labetalol is widely used as a first- or second-line agent. It can be used intravenously or orally. Intravenous labetalol in escalating doses (10 mg, 20 mg, 40 mg, 80 mg) is the first line of acute treatment for severe hypertension/preeclampsia.
Atenolol and propranolol have been associated with fetal growth restriction, metoprolol to a lesser degree.
Metoprolol is useful in women with coronary artery disease, tachyarrhythmias, and/or requiring migraine prophylaxis during pregnancy.
Nifedipine is often used as a second-line agent, with extended-release preparation preferred. Short-acting nifedipine should be used with caution during pregnancy due to the potential for acute impairment of uteroplacental flow. However, short-acting nifedipine is used for tocolysis in pre-term labor.
Intravenous hydralazine is another option for acute treatment in the setting of severe hypertension/preeclampsia.
Angiotensin-converting enzyme (ACE) inhibitors are contraindicated during pregnancy due to association with increased rates of cardiovascular and central nervous system malformations when used in the first trimester, as well as fetal anuric renal failure when used later in pregnancy.21 Due to similar mechanisms of action, angiotensin receptor blockers (ARBs) are contraindicated.
In general, antihypertensive agents are considered compatible with lactation, with most minimally excreted into breast milk. Women requiring antihypertensive agents or almost any medication during lactation seek particular reassurance from caregivers.
It is essential to emphasize the benefit of breastfeeding for both mother and newborn, which far outweighs the risk of medication exposure to the newborn—with rare exceptions. Enalapril and captopril are considered compatible with breastfeeding by the American Academy of Pediatrics.22
Q: Can we identify and possibly prevent preeclampsia?
Escalating hypertension or maternal symptoms, especially in women with increased risk factors, warrant careful examination and laboratory assessment for preeclampsia. Physical findings may include retinal vasospasm, rales on pulmonary exam, cardiac gallop, RUQ or midepigastric tenderness from hepatic capsule stretching, nondependent edema (e.g., face, hands), or clonus on deep tendon reflex evaluation. Useful laboratory values include complete blood count, serum creatinine, hepatic transaminases, uric acid, and urinalysis.
Marked anemia or hemoconcentration, thrombocytopenia, SCr ≥0.8 mg/dL, transaminases above normal, uric acid ≥5.0 mg/dL, urine protein 1+ or greater on dipstick, are all suggestive of preeclampsia, particularly if worsened compared to prior values. Urine protein-to-creatinine ratios have not reliably correlated with 24-hour urine protein collections in preeclamptic patients, although very high or low values could be helpful.23
Women are typically admitted for fetal monitoring, 24-hour urine protein collection, and blood-pressure management during a preeclampsia evaluation.
Thus far, the only intervention shown to reduce the likelihood of preeclampsia in women at increased risk is low-dose aspirin. A recent meta-analysis noted 10% reduction of relative risk of preeclampsia and pre-term birth prior to 34 weeks in women with history of preeclampsia treated with aspirin from the second trimester onward.24 Other interventions in trials that have not displayed reduced risk include vitamin C, vitamin E, calcium, fish oil, zinc, magnesium, and antihypertensive therapy.
Back to the Case
Our patient has chronic hypertension and diabetes, so she should have a blood-pressure goal of <130/80 mmHg. She could be initiated on methyldopa or labetalol. She should have a screen for secondary hypertension via exam and serum thyrotropin, potassium, and calcium, as well as baseline “preeclampsia labs”: complete blood count, serum creatinine, transaminases, uric acid, and 24-hour urine protein assessment. Aspirin at 81 mg daily should be considered from 12 weeks gestation to delivery.
Glycemic control is critical in early gestation to avoid increased risk for congenital malformations and spontaneous abortion, and later on to minimize macrosomia. Close monitoring for maternal symptoms of preeclampsia and blood-pressure assessment is advisable. With medical comorbidities of hypertension and diabetes mellitus, the woman’s risk of preeclampsia is at least 25%. Her pregnancy dating should be confirmed by a first-trimester ultrasound.
Bottom Line
A pregnant woman with chronic hypertension should have evaluation for secondary causes of hypertension, adjustment or initiation of preferred antihypertensive agents to achieve blood pressures that minimize the risk for acute hypertensive complications and fetal growth impairment, and close monitoring for superimposed preeclampsia. TH
Dr. Hayes is an obstetric internist at Women & Infants’ Hospital and assistant professor of medicine (clinical) at the Warren Alpert Medical School of Brown University, Providence, R.I.
References
- Sibai BM. Caring for women with hypertension in pregnancy. JAMA. 2007;298(13):1566-1568.
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
- Christianson RE. Studies on blood pressure during pregnancy. I. Influence of parity and age. Am J Obstet Gynecol. 1976;125(4):509-513.
- Gibson P, Rosene-Montella K. Normal renal and vascular changes in pregnancy. In: Rosene-Montella K, Keely E, Barbour LA, Lee RV, eds. Medical Care of the Pregnant Patient. 2nd ed. Philadelphia: American College of Physicians; 2008:149-152.
- ACOG practice bulletin, No. 33, January 2002. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99(1):159-167.
- Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100(2):369-377.
- Mulrow CD, Chiquette E, Ferrer RL, et al. Management of Chronic Hypertension During Pregnancy. Rockville: Agency for Healthcare Research and Quality; 2000.
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Prof-essional and Public Education of the American Heart Association Council on high blood pressure Research. Circulation. 2005;111(5):697-716.
- Powrie RO. A 30-year-old woman with chronic hypertension trying to conceive. JAMA. 2007; 298(13):1548-1558.
- Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003:289(19):2560-2571.
- Widmer M, Villar J, Benigni A, Conde-Agudelo A, Karumanchi SA, Lindheimer M. Mapping the theories of preeclampsia and the role of angiogenic factors: a systematic review. Obstet Gynecol. 2007; 109(1):168-180.
- Magee LA, Helewa M, Moutquin JM, van Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. SOGC Clinical Practice Guideline. J Obstet Gynaecol Can. 2008; 30:S1-S48.
- Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol. 2007;196(6):514.e1-514.e1-9.
- Duley L, Gülmezoglu AM, Henderson-Smart DJ. Magnesium sulphate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev. 2003;(2):CD000025.
- McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918-930.
- Martin JN Jr., Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol. 2005;105(2): 246-254.
- Lindheimer MD, Taler SJ, Cunningham FG. Hyper-tension in pregnancy. J Am Soc Hypertens. 2008; 2(6):484-494.
- Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008; 51(4):960-969.
- Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care. 2008;31(5):1060-1079.
- Magee LA, von Dadelszen P, Chan S, et al. The Control of Hypertension In Pregnancy Study pilot trial. BJOG. 2007;114(6):770,e13-e20.
- Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006; 354(23):2442-2451.
- American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108(3):776-789.
- Papanna R, Mann LK, Kouides RW, Glantz JC. Protein/creatinine ratio in preeclampsia: a systematic review. Obstet Gynecol. 2008;112(1):135-144.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA, PARIS Collaborative group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575): 1791-1798.
Case
You are consulted on a 29-year-old gravida 1 at nine weeks gestation with a two-year history of Type 2 diabetes and hypertension. She is admitted to the obstetric inpatient service for glycemic control. Although prescribed metformin and lisinopril, she ran out of both four months ago. Her current hemoglobin A1C is 9%. Her blood pressure is 140/90 mmHg in both arms, with an appropriately sized manual cuff while seated. She does not have retinopathy, nephropathy, or neuropathy. The obstetric team will begin weight-based insulin to achieve glycemic targets, and they ask for your input regarding blood-pressure management. How should one approach a pregnant patient with hypertension?
Overview
The most common chronic medical issue in reproductive-age women, essential hypertension (termed chronic hypertension in obstetric literature) contributes significantly to maternal and perinatal morbidity and mortality, primarily via increased risk of preeclampsia.
Chronic hypertension complicates up to 5% of pregnancies in the U.S., or as many as 120,000 pregnant women per year.1 Rates of chronic hypertension are expected to increase with later childbearing and increased rate of obesity. Prior to and during pregnancy, hypertension is defined as blood pressure 140/90 mmHg or higher. Chronic hypertension can be either hypertension diagnosed prior to pregnancy or elevated blood pressures identified prior to 20 weeks gestation.2 Normal pregnancy physiology leads to decreased systemic vascular resistance by the end of the first trimester, dropping systolic and diastolic blood pressure between 10 and 15 mmHg, with maximal effect mid-pregnancy followed by a gradual return to baseline.3 Therefore, chronic hypertension might be masked in early pregnancy. Normal changes in pregnancy include renal vasodilatation and increased glomerular filtration rate, so the average serum creatinine (SCr) is 0.5 mg/dL.4
Newly identified hypertension or accelerating hypertension after 20 weeks warrants close evaluation for preeclampsia. Preeclampsia is a multisystem, life-threatening disorder characterized by hypertension and proteinuria (greater than 300 mg/day). Severe forms of preeclampsia include HELLP (hemolysis, elevated liver enzymes and low platelets) syndrome and eclampsia (seizures with no other attributable cause). Superimposed preeclampsia occurs in 20% to 25% of women with chronic hypertension.5 Women with hypertensive target organ damage have an even greater likelihood of preeclampsia as well as maternal and fetal complications. Unfortunately, blood-pressure control during pregnancy has not been shown to minimize the likelihood of developing superimposed preeclampsia or associated maternal and fetal complications.6 The goal of antihypertensive management during pregnancy is to avoid acute maternal or fetal complications of severe hypertension.
Review of the Data
Q: How are hypertensive disorders of pregnancy classified?
The American College of Obstetrics and Gynecology and the National High Blood Pressure Education Program guideline committees have classified hypertensive disorders of pregnancy into four categories: chronic hypertension, preeclampsia, preeclampsia superimposed on chronic hypertension, and gestational hypertension.2,7
Chronic hypertension is defined as blood pressure 140/90 mmHg or greater on two occasions before pregnancy, prior to 20 weeks of gestation, or persisting 12 weeks postpartum. Severe hypertension is defined as diastolic blood pressure ≥110 mm Hg. Hypertensive women tend to have a greater decline in blood pressure during early pregnancy than normotensive women.8
Secondary hypertension is an important consideration in women of reproductive age. A brief screen for secondary causes includes bilateral arm pressures and femoral pulse assessment, renal bruit assessment, inquiry into snoring, gasping, and daytime somnolence, as well as measurement of serum thyrotropin, potassium, calcium, creatinine, and urinalysis. This kind of evaluation will assess for coarctation of the aorta, renal artery stenosis, obstructive sleep apnea, hyper- or hypothyroidism, hyperaldosteronism, hyperparathyroidism, and underlying renal disease.9,10 Patient reports of episodic headache, palpitations, and diaphoresis should prompt investigation for pheochromocytoma.
Hyperaldosteronism, pheochromocytoma, or hyperthyroidism might be quiescent during pregnancy but flare in the postpartum period.
Women with severe chronic hypertension or target organ damage have higher rates of superimposed preeclampsia. In these individuals, preeclampsia is more likely to present early (before 34 weeks gestation) or with severe disease.1
Preeclampsia is defined as hypertension and proteinuria (greater than 300 mg/day) at or beyond 20 weeks gestation in a previously normotensive woman. Preeclampsia rates vary from 5% to 10% of nulliparous women, to much higher rates in women with medical comorbidities or fetal factors (e.g., multiple gestations, molar pregnancies, hydrops, or triploidy). Preeclampsia’s pathogenesis is attributed to abnormal placental implantation with abnormal maternal immune adaptation, altered angiogenic factors with increased systemic vascular resistance and endothelial dysfunction leading to the clinically apparent maternal syndrome.11
Severe preeclampsia criteria include any of the following: eclampsia, HELLP syndrome (platelets less than 100,000/mm³, transaminases more than twice the upper limit of normal, and/or epigastric pain), SBP ≥160 mmHg, DBP ≥110 mmHg, proteinuria ≥5 grams per day, oliguria, pulmonary edema, placental abruption, or fetal manifestations such as intrauterine growth restriction (≤10th percentile of expected fetal weight based on gestational age), decreased amniotic fluid index, or fetal demise.2,5,12
Maternal symptoms might include headache, visual disturbances, epigastric or right upper quadrant (RUQ) pain, rapid weight gain, and severe edema. Some women remain asymptomatic. Preeclampsia can rapidly progress from “less severe” to severe. Maternal symptoms and abnormal lab findings are more predictive of adverse pregnancy outcomes than the degree of hypertension and/or proteinuria.1
It is always in the mother’s interest to deliver when preeclampsia is diagnosed, because preeclampsia will not resolve until after delivery, with hypertension and lab abnormalities sometimes persisting for months postpartum. Preeclampsia might be diagnosed before fetal viability (approximately 24 weeks gestation), although the vast majority of cases occur near term.
Risks of premature delivery must be balanced with the risks of progressively severe manifestations for the mother and fetus. Guidelines for expectant management of early (<34 weeks) preeclampsia are based on available evidence and expert opinion.13 Magnesium sulfate has been shown to be the most effective agent to minimize the likelihood of seizure in preeclamptic women.14 With an initial bolus of 4 g to 6 g IV followed by infusion of 1-2 g/hour, magnesium sulfate is usually continued for 24 to 48 hours after delivery.
Preeclampsia can first appear postpartum, most likely in the first days to weeks. A growing body of literature links preeclampsia, particularly early and/or recurrent, to subsequent increased risk for cardiovascular disease and end-stage renal disease.15
Preeclampsia superimposed on chronic hypertension is defined as the new onset or markedly increasing proteinuria or accelerating hypertension in the latter half of pregnancy. Maternal symptoms, transaminase elevation, thrombocytopenia, or fetal manifestations further support this diagnosis.
Gestational hypertension, previously known as pregnancy-induced hypertension, is defined as hypertension in the absence of proteinuria in the latter half of pregnancy. Symptoms and lab abnormalities of preeclampsia will be absent. At least half of women with hypertension in the latter half of pregnancy progress to preeclampsia, so gestational hypertension should be considered a provisional diagnosis. Severe gestational hypertension, even without proteinuria or other lab abnormalities, carries increased perinatal risk.
Q: What factors contribute to increased preeclampsia risk?
Maternal factors include: first pregnancy, first pregnancy with a new father, maternal age >35, particularly >40, personal or family history of preeclampsia, chronic hypertension, diabetes mellitus (Type 1, 2 or gestational), systemic lupus erythematosus, antiphospholipid antibody syndrome, renal disease, and obesity. Fetal factors include: multiple gestations, molar pregnancies, hydrops, and triploidy.5,12
Q: When should antihypertensive medications be used in pregnancy?
Most women are hesitant to expose their fetus to medication, and thus must be in therapeutic alliance with their obstetrician and consultants. The overriding principle of medication use in pregnancy is that a healthy fetus requires a healthy mother, and medication use is justified when there is definite benefit to the mother. Due to increased metabolism during pregnancy, medications otherwise dosed once per day often require two doses per day, and those dosed twice daily often require every-eight-hour dosing to maintain efficacy. Additionally, titration up every few days may be required.
Therapy goals include avoiding maternal and fetal complications from severely elevated blood pressure, as well as avoiding fetal growth restriction due to impaired uteroplacental flow. The ideal blood pressure for a hypertensive pregnant woman has not been established, but recommendations are based upon available data and expert opinion.2,5,10,12 Maternal risk of intracerebral hemorrhage increases with SBP ≥160 mmHg.16 Diastolic BP ≥110 mmHg has been associated with greater risk of placental abruption and intrauterine growth restriction.
Pharmacologic treatment generally is initiated or adjusted to achieve SBP <160 mm Hg and DBP <100 to <105 mmHg, although some societies advocate treatment initiation at 140/90 mmHg.2,5,12,17,18 If a woman has target organ damage or concomitant medical issues warranting tighter control (e.g., diabetes or pre-existing renal disease), 130/80 mmHg is preferable.19 Activity limitation and/or bed rest, although commonly recommended, have not been shown to reduce maternal or fetal morbidity or mortality, or prolong time to delivery.
An ongoing, randomized, prospective trial will compare maternal and fetal outcomes in women with mild chronic hypertension with deliberate blood-pressure stratification (goal DBP 85 mmHg vs. goal DBP 100 mmHg).20
Q: What are reasonable treatment options for a woman with chronic hypertension during pregnancy?
Due to vasodilatation of pregnancy, antihypertensive agents often can be discontinued early in pregnancy with close, ongoing monitoring. The majority of women with mild chronic hypertension will have blood pressures <160/100 mmHg without medication during the first half of pregnancy.
If a woman has been using a pharmacologic agent not advisable during pregnancy, she could be switched to a preferred agent. If a woman has been using a pharmacologic agent preferred during pregnancy, she could continue this agent.
Q: What antihypertensives are favored during pregnancy?
Methyldopa and labetalol have been used extensively. Methyldopa has not been found to adversely affect cognitive development in children exposed in utero. On the maternal side, somnolence, dizziness, and dry mouth are common side effects.
Labetalol is widely used as a first- or second-line agent. It can be used intravenously or orally. Intravenous labetalol in escalating doses (10 mg, 20 mg, 40 mg, 80 mg) is the first line of acute treatment for severe hypertension/preeclampsia.
Atenolol and propranolol have been associated with fetal growth restriction, metoprolol to a lesser degree.
Metoprolol is useful in women with coronary artery disease, tachyarrhythmias, and/or requiring migraine prophylaxis during pregnancy.
Nifedipine is often used as a second-line agent, with extended-release preparation preferred. Short-acting nifedipine should be used with caution during pregnancy due to the potential for acute impairment of uteroplacental flow. However, short-acting nifedipine is used for tocolysis in pre-term labor.
Intravenous hydralazine is another option for acute treatment in the setting of severe hypertension/preeclampsia.
Angiotensin-converting enzyme (ACE) inhibitors are contraindicated during pregnancy due to association with increased rates of cardiovascular and central nervous system malformations when used in the first trimester, as well as fetal anuric renal failure when used later in pregnancy.21 Due to similar mechanisms of action, angiotensin receptor blockers (ARBs) are contraindicated.
In general, antihypertensive agents are considered compatible with lactation, with most minimally excreted into breast milk. Women requiring antihypertensive agents or almost any medication during lactation seek particular reassurance from caregivers.
It is essential to emphasize the benefit of breastfeeding for both mother and newborn, which far outweighs the risk of medication exposure to the newborn—with rare exceptions. Enalapril and captopril are considered compatible with breastfeeding by the American Academy of Pediatrics.22
Q: Can we identify and possibly prevent preeclampsia?
Escalating hypertension or maternal symptoms, especially in women with increased risk factors, warrant careful examination and laboratory assessment for preeclampsia. Physical findings may include retinal vasospasm, rales on pulmonary exam, cardiac gallop, RUQ or midepigastric tenderness from hepatic capsule stretching, nondependent edema (e.g., face, hands), or clonus on deep tendon reflex evaluation. Useful laboratory values include complete blood count, serum creatinine, hepatic transaminases, uric acid, and urinalysis.
Marked anemia or hemoconcentration, thrombocytopenia, SCr ≥0.8 mg/dL, transaminases above normal, uric acid ≥5.0 mg/dL, urine protein 1+ or greater on dipstick, are all suggestive of preeclampsia, particularly if worsened compared to prior values. Urine protein-to-creatinine ratios have not reliably correlated with 24-hour urine protein collections in preeclamptic patients, although very high or low values could be helpful.23
Women are typically admitted for fetal monitoring, 24-hour urine protein collection, and blood-pressure management during a preeclampsia evaluation.
Thus far, the only intervention shown to reduce the likelihood of preeclampsia in women at increased risk is low-dose aspirin. A recent meta-analysis noted 10% reduction of relative risk of preeclampsia and pre-term birth prior to 34 weeks in women with history of preeclampsia treated with aspirin from the second trimester onward.24 Other interventions in trials that have not displayed reduced risk include vitamin C, vitamin E, calcium, fish oil, zinc, magnesium, and antihypertensive therapy.
Back to the Case
Our patient has chronic hypertension and diabetes, so she should have a blood-pressure goal of <130/80 mmHg. She could be initiated on methyldopa or labetalol. She should have a screen for secondary hypertension via exam and serum thyrotropin, potassium, and calcium, as well as baseline “preeclampsia labs”: complete blood count, serum creatinine, transaminases, uric acid, and 24-hour urine protein assessment. Aspirin at 81 mg daily should be considered from 12 weeks gestation to delivery.
Glycemic control is critical in early gestation to avoid increased risk for congenital malformations and spontaneous abortion, and later on to minimize macrosomia. Close monitoring for maternal symptoms of preeclampsia and blood-pressure assessment is advisable. With medical comorbidities of hypertension and diabetes mellitus, the woman’s risk of preeclampsia is at least 25%. Her pregnancy dating should be confirmed by a first-trimester ultrasound.
Bottom Line
A pregnant woman with chronic hypertension should have evaluation for secondary causes of hypertension, adjustment or initiation of preferred antihypertensive agents to achieve blood pressures that minimize the risk for acute hypertensive complications and fetal growth impairment, and close monitoring for superimposed preeclampsia. TH
Dr. Hayes is an obstetric internist at Women & Infants’ Hospital and assistant professor of medicine (clinical) at the Warren Alpert Medical School of Brown University, Providence, R.I.
References
- Sibai BM. Caring for women with hypertension in pregnancy. JAMA. 2007;298(13):1566-1568.
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
- Christianson RE. Studies on blood pressure during pregnancy. I. Influence of parity and age. Am J Obstet Gynecol. 1976;125(4):509-513.
- Gibson P, Rosene-Montella K. Normal renal and vascular changes in pregnancy. In: Rosene-Montella K, Keely E, Barbour LA, Lee RV, eds. Medical Care of the Pregnant Patient. 2nd ed. Philadelphia: American College of Physicians; 2008:149-152.
- ACOG practice bulletin, No. 33, January 2002. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99(1):159-167.
- Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100(2):369-377.
- Mulrow CD, Chiquette E, Ferrer RL, et al. Management of Chronic Hypertension During Pregnancy. Rockville: Agency for Healthcare Research and Quality; 2000.
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Prof-essional and Public Education of the American Heart Association Council on high blood pressure Research. Circulation. 2005;111(5):697-716.
- Powrie RO. A 30-year-old woman with chronic hypertension trying to conceive. JAMA. 2007; 298(13):1548-1558.
- Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003:289(19):2560-2571.
- Widmer M, Villar J, Benigni A, Conde-Agudelo A, Karumanchi SA, Lindheimer M. Mapping the theories of preeclampsia and the role of angiogenic factors: a systematic review. Obstet Gynecol. 2007; 109(1):168-180.
- Magee LA, Helewa M, Moutquin JM, van Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. SOGC Clinical Practice Guideline. J Obstet Gynaecol Can. 2008; 30:S1-S48.
- Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol. 2007;196(6):514.e1-514.e1-9.
- Duley L, Gülmezoglu AM, Henderson-Smart DJ. Magnesium sulphate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev. 2003;(2):CD000025.
- McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918-930.
- Martin JN Jr., Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol. 2005;105(2): 246-254.
- Lindheimer MD, Taler SJ, Cunningham FG. Hyper-tension in pregnancy. J Am Soc Hypertens. 2008; 2(6):484-494.
- Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008; 51(4):960-969.
- Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care. 2008;31(5):1060-1079.
- Magee LA, von Dadelszen P, Chan S, et al. The Control of Hypertension In Pregnancy Study pilot trial. BJOG. 2007;114(6):770,e13-e20.
- Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006; 354(23):2442-2451.
- American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108(3):776-789.
- Papanna R, Mann LK, Kouides RW, Glantz JC. Protein/creatinine ratio in preeclampsia: a systematic review. Obstet Gynecol. 2008;112(1):135-144.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA, PARIS Collaborative group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575): 1791-1798.
Investigate Claim Denials
In order to recover the appropriate payment for services provided by hospitalists, the following must occur:
- The billing provider renders service fully, or jointly with a resident under the teaching physician guidelines or nonphysician provider under the shared/split billing rules;
- The service is completely and accurately documented in the medical record;
- The correct information is entered on the claim form that is submitted to the payor; and
- The service is determined to be a covered benefit and eligible for payment.
Claims frequently are rejected or denied. Even more frequently, the physician or billing staff does not understand the reason for the denial. The typical reaction to claim denial is twofold: “appeal with paper” and “write off.” In other words, send a copy of the physician notes to the payor and consider the claim unsuccessful and payment unable to be obtained.
Examining and understanding the payor’s initial claim determination might prompt a more successful response. Presuming the patient demographics are entered without error, the insurance information is correct, the patient is eligible for coverage, and all precertifications and authorizations were obtained, check for these other common errors.
Medical Necessity
Denials for “medical necessity” are not always what they seem. Individuals often assume that the physician reported an incorrect diagnosis code. Consider the service/procedure code when trying to formulate a response to the denial. When dealing with procedure codes, it is likely the denial is received for a mismatched diagnosis.
For example, a payor might deny a claim for cardiopulmonary resuscitation (92950) that is associated with a diagnosis code of congestive heart failure (428.0), despite this being the underlying condition that prompted the decline in the patient’s condition. The payor might only accept “cardiac arrest” (427.5) as the diagnosis for cardiopulmonary resuscitation because it was the direct reason for the procedure. After you ensure that the documentation supports the diagnosis, the claim should be resubmitted with the corrected diagnosis code.
If the “medical necessity” denial involves a covered evaluation and management (E/M) visit, it is less likely that the diagnosis code is the issue. When dealing with Medicare in particular, this type of denial likely is the result of a failure to respond to a prepayment request for documentation. Medicare issues prepayment requests for documentation for the following inpatient CPT codes: 99255, 99254, 99233, 99232, 99223, 99239, and 99292. If the documentation isn’t provided to the Medicare review department within the designated time frame, the claim is automatically denied. The reason for denial is cited as “not deemed a medical necessity.” Some providers misunderstand this remittance remark and assume that the physician assigned an incorrect diagnosis code. Although that might be true, it probably is due to a failure to respond to the prepayment documentation request. Appealing these claims requires the submission of documentation to the Medicare appeals department. Once the supporting documentation is reviewed, reimbursement is granted.
Bundling
The National Correct Coding Initiative (NCCI) identifies edits that ultimately affect claims submission and payment. The Column One/Column Two Correct Coding Edits and the Mutually Exclusive Edits list code pairs that should not be reported together on the same date by either a single physician or physicians of the same specialty within a provider group. Under some well-documented circumstances, the physician is allowed to “unbundle” the services by appending the appropriate modifier.
When services are denied as being “incidental/integral” to another reimbursed service (e.g., bundled), the claim should not automatically be resubmitted with a modifier appended to the “bundled” procedure code.
Documentation should be reviewed to determine if the denied service is separately reportable from the paid service. Only when supported by documentation can the physician append the appropriate modifier and resubmit the claim. For example, a hospitalist evaluated a patient with congestive heart failure and pleural effusions. The hospitalist determined that the patient requires placement of a central venous catheter (36556). Because the patient’s underlying condition was evaluated and resulted in the decision to place a central venous catheter, both the visit (99233) and the procedure (36556) can be reported. If submitted without modifiers, some payors may deny payment for the visit because it was not “integral” to the catheter placement. You should resubmit those claims with modifier 25.
Place of Service
Ensure that the place of service (POS) matches the service/procedure code. For example, say a hospitalist performs a consultation in the ED and determines that the patient does not need to be treated as an inpatient but provides recommendations for ED care and outpatient followup. Avoid a mismatch of the service code and the location. Consults performed in the ED should be reported with outpatient consultation codes (99241-99245) as appropriate. The correct POS should be the ED, not the inpatient hospital. Reporting outpatient codes with an inpatient POS (e.g., 21: inpatient hospital, 31: skilled nursing facility) will result in claim denial.
The same is true when trying to report inpatient consultation codes (99251-99255) in an outpatient location (e.g., 23-ED). The appropriate response for this type of denial is to resubmit the claim with the correct the POS and service/procedure code. A complete list of POS codes and corresponding definitions can be found in Chapter 26, Section 10.5 of the Medicare Claims Processing Manual, available at www.cms.hhs.gov/manuals/downloads/clm104c26.pdf.
Provider Enrollment
Provider enrollment issues occur when a physician’s national provider identifier (NPI) is not properly linked to the group practice. More often than not, the group practice receives claim rejections for enrollment issues when services involve nurse practitioners or physician assistants who have not enrolled with Medicare or cannot enroll with non-Medicare payors.
For example, a nurse practitioner independently provides a subsequent hospital-care service (e.g., 99232). The claim is submitted and Medicare reimburses the service at the correct amount as a primary insurer. The remaining balance is submitted to the secondary insurer. Because the submitted claim identifies the service provider as a nonphysician provider, who likely is not enrolled with the non-Medicare payor, the claim is rejected.
If the physician group has a contractual agreement to recognize nonphysician provider services by reporting them under the collaborating physician’s name, the claim can be resubmitted in the physician’s name. In absence of such an agreement, the claim should be written off. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is faculty of SHM’s inpatient coding course.
Reference
- Beebe M, Dalton J, Espronceda M, Evans D, Glenn R. Current Procedural Terminology Professional Edition. Chicago: American Medical Association Press; 2008.
In order to recover the appropriate payment for services provided by hospitalists, the following must occur:
- The billing provider renders service fully, or jointly with a resident under the teaching physician guidelines or nonphysician provider under the shared/split billing rules;
- The service is completely and accurately documented in the medical record;
- The correct information is entered on the claim form that is submitted to the payor; and
- The service is determined to be a covered benefit and eligible for payment.
Claims frequently are rejected or denied. Even more frequently, the physician or billing staff does not understand the reason for the denial. The typical reaction to claim denial is twofold: “appeal with paper” and “write off.” In other words, send a copy of the physician notes to the payor and consider the claim unsuccessful and payment unable to be obtained.
Examining and understanding the payor’s initial claim determination might prompt a more successful response. Presuming the patient demographics are entered without error, the insurance information is correct, the patient is eligible for coverage, and all precertifications and authorizations were obtained, check for these other common errors.
Medical Necessity
Denials for “medical necessity” are not always what they seem. Individuals often assume that the physician reported an incorrect diagnosis code. Consider the service/procedure code when trying to formulate a response to the denial. When dealing with procedure codes, it is likely the denial is received for a mismatched diagnosis.
For example, a payor might deny a claim for cardiopulmonary resuscitation (92950) that is associated with a diagnosis code of congestive heart failure (428.0), despite this being the underlying condition that prompted the decline in the patient’s condition. The payor might only accept “cardiac arrest” (427.5) as the diagnosis for cardiopulmonary resuscitation because it was the direct reason for the procedure. After you ensure that the documentation supports the diagnosis, the claim should be resubmitted with the corrected diagnosis code.
If the “medical necessity” denial involves a covered evaluation and management (E/M) visit, it is less likely that the diagnosis code is the issue. When dealing with Medicare in particular, this type of denial likely is the result of a failure to respond to a prepayment request for documentation. Medicare issues prepayment requests for documentation for the following inpatient CPT codes: 99255, 99254, 99233, 99232, 99223, 99239, and 99292. If the documentation isn’t provided to the Medicare review department within the designated time frame, the claim is automatically denied. The reason for denial is cited as “not deemed a medical necessity.” Some providers misunderstand this remittance remark and assume that the physician assigned an incorrect diagnosis code. Although that might be true, it probably is due to a failure to respond to the prepayment documentation request. Appealing these claims requires the submission of documentation to the Medicare appeals department. Once the supporting documentation is reviewed, reimbursement is granted.
Bundling
The National Correct Coding Initiative (NCCI) identifies edits that ultimately affect claims submission and payment. The Column One/Column Two Correct Coding Edits and the Mutually Exclusive Edits list code pairs that should not be reported together on the same date by either a single physician or physicians of the same specialty within a provider group. Under some well-documented circumstances, the physician is allowed to “unbundle” the services by appending the appropriate modifier.
When services are denied as being “incidental/integral” to another reimbursed service (e.g., bundled), the claim should not automatically be resubmitted with a modifier appended to the “bundled” procedure code.
Documentation should be reviewed to determine if the denied service is separately reportable from the paid service. Only when supported by documentation can the physician append the appropriate modifier and resubmit the claim. For example, a hospitalist evaluated a patient with congestive heart failure and pleural effusions. The hospitalist determined that the patient requires placement of a central venous catheter (36556). Because the patient’s underlying condition was evaluated and resulted in the decision to place a central venous catheter, both the visit (99233) and the procedure (36556) can be reported. If submitted without modifiers, some payors may deny payment for the visit because it was not “integral” to the catheter placement. You should resubmit those claims with modifier 25.
Place of Service
Ensure that the place of service (POS) matches the service/procedure code. For example, say a hospitalist performs a consultation in the ED and determines that the patient does not need to be treated as an inpatient but provides recommendations for ED care and outpatient followup. Avoid a mismatch of the service code and the location. Consults performed in the ED should be reported with outpatient consultation codes (99241-99245) as appropriate. The correct POS should be the ED, not the inpatient hospital. Reporting outpatient codes with an inpatient POS (e.g., 21: inpatient hospital, 31: skilled nursing facility) will result in claim denial.
The same is true when trying to report inpatient consultation codes (99251-99255) in an outpatient location (e.g., 23-ED). The appropriate response for this type of denial is to resubmit the claim with the correct the POS and service/procedure code. A complete list of POS codes and corresponding definitions can be found in Chapter 26, Section 10.5 of the Medicare Claims Processing Manual, available at www.cms.hhs.gov/manuals/downloads/clm104c26.pdf.
Provider Enrollment
Provider enrollment issues occur when a physician’s national provider identifier (NPI) is not properly linked to the group practice. More often than not, the group practice receives claim rejections for enrollment issues when services involve nurse practitioners or physician assistants who have not enrolled with Medicare or cannot enroll with non-Medicare payors.
For example, a nurse practitioner independently provides a subsequent hospital-care service (e.g., 99232). The claim is submitted and Medicare reimburses the service at the correct amount as a primary insurer. The remaining balance is submitted to the secondary insurer. Because the submitted claim identifies the service provider as a nonphysician provider, who likely is not enrolled with the non-Medicare payor, the claim is rejected.
If the physician group has a contractual agreement to recognize nonphysician provider services by reporting them under the collaborating physician’s name, the claim can be resubmitted in the physician’s name. In absence of such an agreement, the claim should be written off. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is faculty of SHM’s inpatient coding course.
Reference
- Beebe M, Dalton J, Espronceda M, Evans D, Glenn R. Current Procedural Terminology Professional Edition. Chicago: American Medical Association Press; 2008.
In order to recover the appropriate payment for services provided by hospitalists, the following must occur:
- The billing provider renders service fully, or jointly with a resident under the teaching physician guidelines or nonphysician provider under the shared/split billing rules;
- The service is completely and accurately documented in the medical record;
- The correct information is entered on the claim form that is submitted to the payor; and
- The service is determined to be a covered benefit and eligible for payment.
Claims frequently are rejected or denied. Even more frequently, the physician or billing staff does not understand the reason for the denial. The typical reaction to claim denial is twofold: “appeal with paper” and “write off.” In other words, send a copy of the physician notes to the payor and consider the claim unsuccessful and payment unable to be obtained.
Examining and understanding the payor’s initial claim determination might prompt a more successful response. Presuming the patient demographics are entered without error, the insurance information is correct, the patient is eligible for coverage, and all precertifications and authorizations were obtained, check for these other common errors.
Medical Necessity
Denials for “medical necessity” are not always what they seem. Individuals often assume that the physician reported an incorrect diagnosis code. Consider the service/procedure code when trying to formulate a response to the denial. When dealing with procedure codes, it is likely the denial is received for a mismatched diagnosis.
For example, a payor might deny a claim for cardiopulmonary resuscitation (92950) that is associated with a diagnosis code of congestive heart failure (428.0), despite this being the underlying condition that prompted the decline in the patient’s condition. The payor might only accept “cardiac arrest” (427.5) as the diagnosis for cardiopulmonary resuscitation because it was the direct reason for the procedure. After you ensure that the documentation supports the diagnosis, the claim should be resubmitted with the corrected diagnosis code.
If the “medical necessity” denial involves a covered evaluation and management (E/M) visit, it is less likely that the diagnosis code is the issue. When dealing with Medicare in particular, this type of denial likely is the result of a failure to respond to a prepayment request for documentation. Medicare issues prepayment requests for documentation for the following inpatient CPT codes: 99255, 99254, 99233, 99232, 99223, 99239, and 99292. If the documentation isn’t provided to the Medicare review department within the designated time frame, the claim is automatically denied. The reason for denial is cited as “not deemed a medical necessity.” Some providers misunderstand this remittance remark and assume that the physician assigned an incorrect diagnosis code. Although that might be true, it probably is due to a failure to respond to the prepayment documentation request. Appealing these claims requires the submission of documentation to the Medicare appeals department. Once the supporting documentation is reviewed, reimbursement is granted.
Bundling
The National Correct Coding Initiative (NCCI) identifies edits that ultimately affect claims submission and payment. The Column One/Column Two Correct Coding Edits and the Mutually Exclusive Edits list code pairs that should not be reported together on the same date by either a single physician or physicians of the same specialty within a provider group. Under some well-documented circumstances, the physician is allowed to “unbundle” the services by appending the appropriate modifier.
When services are denied as being “incidental/integral” to another reimbursed service (e.g., bundled), the claim should not automatically be resubmitted with a modifier appended to the “bundled” procedure code.
Documentation should be reviewed to determine if the denied service is separately reportable from the paid service. Only when supported by documentation can the physician append the appropriate modifier and resubmit the claim. For example, a hospitalist evaluated a patient with congestive heart failure and pleural effusions. The hospitalist determined that the patient requires placement of a central venous catheter (36556). Because the patient’s underlying condition was evaluated and resulted in the decision to place a central venous catheter, both the visit (99233) and the procedure (36556) can be reported. If submitted without modifiers, some payors may deny payment for the visit because it was not “integral” to the catheter placement. You should resubmit those claims with modifier 25.
Place of Service
Ensure that the place of service (POS) matches the service/procedure code. For example, say a hospitalist performs a consultation in the ED and determines that the patient does not need to be treated as an inpatient but provides recommendations for ED care and outpatient followup. Avoid a mismatch of the service code and the location. Consults performed in the ED should be reported with outpatient consultation codes (99241-99245) as appropriate. The correct POS should be the ED, not the inpatient hospital. Reporting outpatient codes with an inpatient POS (e.g., 21: inpatient hospital, 31: skilled nursing facility) will result in claim denial.
The same is true when trying to report inpatient consultation codes (99251-99255) in an outpatient location (e.g., 23-ED). The appropriate response for this type of denial is to resubmit the claim with the correct the POS and service/procedure code. A complete list of POS codes and corresponding definitions can be found in Chapter 26, Section 10.5 of the Medicare Claims Processing Manual, available at www.cms.hhs.gov/manuals/downloads/clm104c26.pdf.
Provider Enrollment
Provider enrollment issues occur when a physician’s national provider identifier (NPI) is not properly linked to the group practice. More often than not, the group practice receives claim rejections for enrollment issues when services involve nurse practitioners or physician assistants who have not enrolled with Medicare or cannot enroll with non-Medicare payors.
For example, a nurse practitioner independently provides a subsequent hospital-care service (e.g., 99232). The claim is submitted and Medicare reimburses the service at the correct amount as a primary insurer. The remaining balance is submitted to the secondary insurer. Because the submitted claim identifies the service provider as a nonphysician provider, who likely is not enrolled with the non-Medicare payor, the claim is rejected.
If the physician group has a contractual agreement to recognize nonphysician provider services by reporting them under the collaborating physician’s name, the claim can be resubmitted in the physician’s name. In absence of such an agreement, the claim should be written off. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is faculty of SHM’s inpatient coding course.
Reference
- Beebe M, Dalton J, Espronceda M, Evans D, Glenn R. Current Procedural Terminology Professional Edition. Chicago: American Medical Association Press; 2008.
Pharma Pledges Price Cut
The June 22 announcement of an $80 billion deal to help reduce out-of-pocket drug costs for Medicare beneficiaries has elicited mixed reactions on what it might mean for patients, and calls for hospitalists to remain vigilant about prescription drug expenses.
Under a pledge negotiated with the White House and congressional Democrats, the pharmaceutical industry has promised a 50% discount for name-brand drugs to beneficiaries stuck in the notorious gap of the Medicare Part D prescription drug plan, commonly called the “doughnut hole” (see “Beware the Doughnut Hole,” June 2009, p. 1) In 2009, the gap in coverage kicks in after $2,700 in total drug costs and persists until $6,154 in total costs, by which point patients have spent as much as $4,350 of their own money for prescription drugs.
President Obama says the gap “has been placing a crushing burden on many older Americans who live on fixed incomes and can’t afford thousands of dollars in out-of-pocket expenses.” The AARP hails the “unique solution” as a “major step forward,” though other industry observers have taken a more circumspect stance and say they want to see tougher cost-control measures in writing.
“You don’t want to look an $80 billion gift horse in the mouth, but there’s some halitosis in this mouth,” says Bill Vaughan, a health policy analyst for Consumers Union in Washington, D.C. “It’s not as pure and altruistic as it seems at first blush, and people need to keep pushing for generics because these [brand-name drugs] are grossly overpriced.”
Under the proposal, the cost for a 30-day supply of the beta-blocker drug carvedilol (Coreg) could be halved, from $142.79 to $71.40, based on Drugstore.com’s current retail prices. Even with the savings, the drug would still cost almost four and a half times more than a generic alternative, metoprolol tartrate (Lopressor), which costs $15.99 for a 30-day supply.
The deal also includes several caveats: The pledge doesn’t address the cost of brand-name drugs before or after the coverage gap, and the doughnut-hole price reduction would go into effect only if Congress enacts healthcare reform legislation.
Battles already are looming over the fight to make generics more accessible and to eliminate the doughnut hole entirely.
“I think the sense is that … everybody is going to have to give back a little bit,” says William D. Atchley Jr., MD, FACP, FHM, chief of the division of hospital medicine for Sentara Medical Group in Norfolk, Va., and a member of SHM’s Public Policy Committee. “This is a different landscape than the early 1990s. … Stakeholders are ready to be proactive and work with people.”
News accounts suggest that the pharmaceutical industry will offer an estimated $30 billion over 10 years toward narrowing the gap, while another $50 billion in concessions still must be worked out. Even so, consumer advocates like Vaughan say the need for hospitalists to help patients avoid unnecessary drug costs remains as high as ever.
Dr. Atchley, a former member of SHM’s board of directors, says hospitalists need to know what’s available in the hospital pharmacy and maintain an open line of communication with their patients in terms of their access and ability to pay for prescriptions.
“You need to understand patients’ economic status. You need to know if they get their medication from Walmart or the [Veterans Affairs] hospital pharmacy,” he says. “Cost is an issue to our Medicare patients, and it’s important to collaborate with them to make sure they can afford the drug. If they can’t, you need to work with them to find another affordable drug that will provide the same benefit.” TH
Bryn Nelson, PhD, is a freelance writer based in Seattle.
The June 22 announcement of an $80 billion deal to help reduce out-of-pocket drug costs for Medicare beneficiaries has elicited mixed reactions on what it might mean for patients, and calls for hospitalists to remain vigilant about prescription drug expenses.
Under a pledge negotiated with the White House and congressional Democrats, the pharmaceutical industry has promised a 50% discount for name-brand drugs to beneficiaries stuck in the notorious gap of the Medicare Part D prescription drug plan, commonly called the “doughnut hole” (see “Beware the Doughnut Hole,” June 2009, p. 1) In 2009, the gap in coverage kicks in after $2,700 in total drug costs and persists until $6,154 in total costs, by which point patients have spent as much as $4,350 of their own money for prescription drugs.
President Obama says the gap “has been placing a crushing burden on many older Americans who live on fixed incomes and can’t afford thousands of dollars in out-of-pocket expenses.” The AARP hails the “unique solution” as a “major step forward,” though other industry observers have taken a more circumspect stance and say they want to see tougher cost-control measures in writing.
“You don’t want to look an $80 billion gift horse in the mouth, but there’s some halitosis in this mouth,” says Bill Vaughan, a health policy analyst for Consumers Union in Washington, D.C. “It’s not as pure and altruistic as it seems at first blush, and people need to keep pushing for generics because these [brand-name drugs] are grossly overpriced.”
Under the proposal, the cost for a 30-day supply of the beta-blocker drug carvedilol (Coreg) could be halved, from $142.79 to $71.40, based on Drugstore.com’s current retail prices. Even with the savings, the drug would still cost almost four and a half times more than a generic alternative, metoprolol tartrate (Lopressor), which costs $15.99 for a 30-day supply.
The deal also includes several caveats: The pledge doesn’t address the cost of brand-name drugs before or after the coverage gap, and the doughnut-hole price reduction would go into effect only if Congress enacts healthcare reform legislation.
Battles already are looming over the fight to make generics more accessible and to eliminate the doughnut hole entirely.
“I think the sense is that … everybody is going to have to give back a little bit,” says William D. Atchley Jr., MD, FACP, FHM, chief of the division of hospital medicine for Sentara Medical Group in Norfolk, Va., and a member of SHM’s Public Policy Committee. “This is a different landscape than the early 1990s. … Stakeholders are ready to be proactive and work with people.”
News accounts suggest that the pharmaceutical industry will offer an estimated $30 billion over 10 years toward narrowing the gap, while another $50 billion in concessions still must be worked out. Even so, consumer advocates like Vaughan say the need for hospitalists to help patients avoid unnecessary drug costs remains as high as ever.
Dr. Atchley, a former member of SHM’s board of directors, says hospitalists need to know what’s available in the hospital pharmacy and maintain an open line of communication with their patients in terms of their access and ability to pay for prescriptions.
“You need to understand patients’ economic status. You need to know if they get their medication from Walmart or the [Veterans Affairs] hospital pharmacy,” he says. “Cost is an issue to our Medicare patients, and it’s important to collaborate with them to make sure they can afford the drug. If they can’t, you need to work with them to find another affordable drug that will provide the same benefit.” TH
Bryn Nelson, PhD, is a freelance writer based in Seattle.
The June 22 announcement of an $80 billion deal to help reduce out-of-pocket drug costs for Medicare beneficiaries has elicited mixed reactions on what it might mean for patients, and calls for hospitalists to remain vigilant about prescription drug expenses.
Under a pledge negotiated with the White House and congressional Democrats, the pharmaceutical industry has promised a 50% discount for name-brand drugs to beneficiaries stuck in the notorious gap of the Medicare Part D prescription drug plan, commonly called the “doughnut hole” (see “Beware the Doughnut Hole,” June 2009, p. 1) In 2009, the gap in coverage kicks in after $2,700 in total drug costs and persists until $6,154 in total costs, by which point patients have spent as much as $4,350 of their own money for prescription drugs.
President Obama says the gap “has been placing a crushing burden on many older Americans who live on fixed incomes and can’t afford thousands of dollars in out-of-pocket expenses.” The AARP hails the “unique solution” as a “major step forward,” though other industry observers have taken a more circumspect stance and say they want to see tougher cost-control measures in writing.
“You don’t want to look an $80 billion gift horse in the mouth, but there’s some halitosis in this mouth,” says Bill Vaughan, a health policy analyst for Consumers Union in Washington, D.C. “It’s not as pure and altruistic as it seems at first blush, and people need to keep pushing for generics because these [brand-name drugs] are grossly overpriced.”
Under the proposal, the cost for a 30-day supply of the beta-blocker drug carvedilol (Coreg) could be halved, from $142.79 to $71.40, based on Drugstore.com’s current retail prices. Even with the savings, the drug would still cost almost four and a half times more than a generic alternative, metoprolol tartrate (Lopressor), which costs $15.99 for a 30-day supply.
The deal also includes several caveats: The pledge doesn’t address the cost of brand-name drugs before or after the coverage gap, and the doughnut-hole price reduction would go into effect only if Congress enacts healthcare reform legislation.
Battles already are looming over the fight to make generics more accessible and to eliminate the doughnut hole entirely.
“I think the sense is that … everybody is going to have to give back a little bit,” says William D. Atchley Jr., MD, FACP, FHM, chief of the division of hospital medicine for Sentara Medical Group in Norfolk, Va., and a member of SHM’s Public Policy Committee. “This is a different landscape than the early 1990s. … Stakeholders are ready to be proactive and work with people.”
News accounts suggest that the pharmaceutical industry will offer an estimated $30 billion over 10 years toward narrowing the gap, while another $50 billion in concessions still must be worked out. Even so, consumer advocates like Vaughan say the need for hospitalists to help patients avoid unnecessary drug costs remains as high as ever.
Dr. Atchley, a former member of SHM’s board of directors, says hospitalists need to know what’s available in the hospital pharmacy and maintain an open line of communication with their patients in terms of their access and ability to pay for prescriptions.
“You need to understand patients’ economic status. You need to know if they get their medication from Walmart or the [Veterans Affairs] hospital pharmacy,” he says. “Cost is an issue to our Medicare patients, and it’s important to collaborate with them to make sure they can afford the drug. If they can’t, you need to work with them to find another affordable drug that will provide the same benefit.” TH
Bryn Nelson, PhD, is a freelance writer based in Seattle.