User login
Beta-blockers for hypertension: Are they going out of style?
In recent years the role of beta-blockers as a primary tool to treat hypertension has come under question. These drugs have shown disappointing results when used as antihypertensive therapy in patients without heart disease, ie, when used as primary prevention. At the same time, beta-blockers clearly reduce the risk of future cardiovascular events in patients who already have heart disease, eg, who already have had a myocardial infarction or who have congestive heart failure.
Several meta-analyses and a few clinical trials have shown that beta-blockers may have no advantage over other antihypertensive drugs, and in fact may not reduce the risk of stroke as effectively as other classes of blood pressure medications.
Why should this be? Is it that the patients in the antihypertensive trials were mostly older, and that beta-blockers do not work as well in older patients as in younger ones? Or does it have to do with the fact that atenolol (Tenormin) was the drug most often used in the trials? Would newer, different beta-blockers be better?
Hypertension experts currently disagree on how to interpret the available data, and this has led to conflict and confusion among clinicians as to the role of beta-blockers in managing hypertension. Current evidence suggests that older beta-blockers may not be the preferred first-line antihypertensive drugs for hypertensive patients who have no compelling indications for them (eg, heart failure, myocardial infarction, diabetes, high risk of coronary heart disease). However, newer beta-blockers with vasodilatory properties should be considered in cases of uncontrolled or resistant hypertension, especially in younger patients.
Further, while controversy and debate continue over the benefits and adverse effects of one class of antihypertensive drugs vs another, it is indisputable that controlling arterial blood pressure to the recommended goal offers major protection against cardiovascular and renal events in patients with hypertension.1,2
MECHANISM OF ACTION OF BETA-BLOCKERS
Beta-blockers effectively reduce blood pressure in both systolic-diastolic hypertension and isolated systolic hypertension.3–5 Exactly how is not known, but it has been proposed that they may do so by:
Reducing the heart rate and cardiac output. When catecholamines activate beta-1 receptors in the heart, the heart rate and myocardial contractility increase. By blocking beta-1 receptors, beta-blockers reduce the heart rate and myocardial contractility, thus lowering cardiac output and arterial blood pressure.6
Inhibiting renin release. Activation of the renin-angiotensin system is another major pathway that can lead to elevated arterial blood pressure. Renin release is mediated through the sympathetic nervous system via beta-1 receptors on the juxtaglomerular cells of the kidney. Beta-blockers can therefore lower blood pressure by inhibiting renin release.7
Inhibiting central nervous sympathetic outflow, thereby inducing presynaptic blockade, which in turn reduces the release of catecholamines.
Reducing venous return and plasma volume.
Generating nitric oxide, thus reducing peripheral vascular resistance (some agents).8
Reducing vasomotor tone.
Reducing vascular tone.
Improving vascular compliance.
Resetting baroreceptor levels.
Attenuating the pressor response to catecholamines with exercise and stress.
HETEROGENEITY OF BETA-BLOCKERS
Selectivity
Beta-blockers are not all the same. They can be classified into three categories.
Nonselective beta-blockers block both beta-1 and beta-2 adrenergic receptors. It is generally accepted that beta-blockers exert their primary antihypertensive effect by blocking beta-1 adrenergic receptors.6 Of interest, nonselective beta-blockers inhibit beta-2 receptors on arteries and thus cause an unopposed alpha-adrenergic effect, leading to increased peripheral vascular resistance.9 Examples of this category:
- Nadolol (Corgard)
- Pindolol (Visken)
- Propranolol (Inderal)
- Timolol (Blocadren).
Selective beta-blockers specifically block beta-1 receptors alone, although they are known to be nonselective at higher doses. Examples:
- Atenolol (Tenormin)
- Betaxolol (Kerlone)
- Bisoprolol (Zebeta)
- Esmolol (Brevibloc)
- Metoprolol (Lopressor, Toprol).
Beta-blockers with peripheral vasodilatatory effects act either via antagonism of the alpha-1 receptor, as with labetolol (Normodyne) and carvedilol (Coreg),10 or via enhanced release of nitric oxide, as with nebivolol (Bystolic).8
Lipid and water solubility
The lipid solubility and water solubility of each beta-blocker determine its bioavailability and side-effect profile.
Lipid solubility determines the degree to which a beta-blocker penetrates the blood-brain barrier and thereby leads to central nervous system side effects such as lethargy, nightmares, confusion, and depression. Propranolol is highly lipid-soluble; metoprolol and labetalol are moderately so.
Water-soluble beta-blockers such as atenolol have less tissue permeation, have a longer half-life, and cause fewer central nervous system effects and symptoms.11
Routes of elimination
Beta-blockers also differ in their route of elimination.
Atenolol and nadolol are eliminated by the kidney and require dose adjustment in patients with impaired renal function.12,13
On the other hand, propranolol, metoprolol, labetalol, carvedilol, and nebivolol are excreted primarily via hepatic metabolism.13
BETA-BLOCKERS IN THE MANAGEMENT OF HYPERTENSION
Beta-blockers were initially used to treat arrhythmias, but by the early 1970s they were also widely accepted for managing hypertension. 14 Their initial acceptance as one of the first-line classes of drugs for hypertension was based on their better side-effect profile compared with other antihypertensive drugs available at that time.
In the 1980s and 1990s, beta-blockers were listed as preferred first-line antihypertensive drugs along with diuretics in national hypertension guidelines.15 Subsequent updates of the guidelines favored diuretics as initial therapy and relegated all other classes of antihypertensive medications to be alternatives to diuretics.16 Although beta-blockers remain alternative first-line drugs in the latest guidelines (published in 2003; see reference 66), they are the preferred antihypertensive agents for patients with cardiac disease.
The current recommendations reflect the findings from hypertension trials in which patients with myocardial infarction and congestive heart failure had better cardiovascular outcomes if they received these drugs,17–19 including a lower risk of death.20,21 It was widely assumed that beta-blockers would also prevent first episodes of cardiovascular events.
However, to date, there is no evidence that beta-blockers are effective as primary prevention. Several large randomized controlled trials showed no benefit with beta-blockers compared with other antihypertensive drugs—in fact, there were more cardiovascular events with beta-blockers (see below).
Beta-blockers are well tolerated in clinical practice, although they can have side effects that include fatigue, depression, impaired exercise tolerance, sexual dysfunction, and asthma attacks.
Wiysonge et al22 analyzed how many patients withdrew from randomized trials of antihypertensive treatment because of drug-related adverse events. There was no significant difference in the incidence of fatigue, depressive symptoms, or sexual dysfunction with beta-blockers compared with placebo, and trial participants on a beta-blocker were not statistically significantly more likely to discontinue treatment than those receiving a placebo in three trials with 22,729 participants (relative risk [RR] 2.34, 95% confidence interval [CI] 0.84–6.52).
THE CONTROVERSY: WHAT THE TRIALS SHOWED
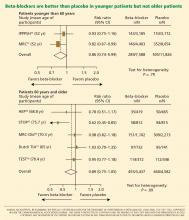
Messerli et al23 performed a meta-analysis published in 1998 that suggested that beta-blockers may not be as effective as diuretics in preventing cardiovascular events when used as first-line antihypertensive therapy in elderly patients. In 10 randomized controlled trials in 16,164 patients who were treated with either a diuretic or a beta-blocker (atenolol), blood pressure was normalized in two-thirds of diuretic-treated patients but only one-third of patients treated with atenolol as monotherapy. Diuretic therapy was superior with regard to all end points, and beta-blockers were found to be ineffective except in reducing cerebrovascular events.
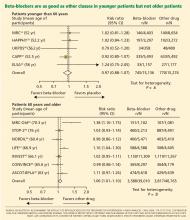
The LIFE study (Losartan Intervention for Endpoint Reduction in Hypertension)24 compared the angiotensin-receptor blocker losartan (Cozaar) and atenolol in 9,193 patients with hypertension and left ventricular hypertrophy. At 4 years of follow-up, the rate of primary cardiovascular events (death, myocardial infarction, or stroke) was lower in the losartan group than in the atenolol group. The difference was mainly due to a 25% lower incidence of stroke, which was statistically significant. The rates of myocardial infarction and death from cardiovascular causes were not significantly different between the two treatment groups. The systolic blood pressure was 1 mm Hg lower in the losartan group than in the atenolol group, which was statistically significant.
Carlberg et al25 performed another important meta-analysis that questioned whether atenolol reduces rates of cardiovascular morbidity and death in hypertensive patients. The results were surprising: eight randomized controlled trials including more than 6,000 patients and comparing atenolol with placebo or no treatment showed no differences between the treatment groups with regard to the outcomes of all-cause mortality (RR 1.01, 95% CI 0.89–1.15), cardiovascular mortality (RR 0.99, 95% CI 0.83–1.18), or myocardial infarction (RR 0.99, 95% CI 0.83–1.19).
In addition, when atenolol was compared with other antihypertensives in five other randomized controlled trials that included more than 14,000 patients, those treated with atenolol had a higher risk of stroke (RR 1.30, 95% CI 1.12–1.50) and death (RR 1.13, 95% CI 1.02–1.25).
The ASCOT-BPLA trial (Anglo-Scandinavian Cardiac Outcomes Trial—Blood Pressure Lowering Arm)26 had similar results. This trial compared the combination of atenolol plus the diuretic bendroflumethiazide against the combination of the calcium channel blocker amlodipine (Norvasc) plus the angiotensin-converting enzyme (ACE) inhibitor perindopril (Aceon). Although no significant difference was seen in the primary outcome of nonfatal myocardial infarction or fatal coronary heart disease (unadjusted hazard ratio [HR] with amlodipine-perindopril 0.90, 95% CI 0.79–1.02, P = .1052), the amlodipine-plus-perindopril group had significantly fewer strokes (327 vs 422, HR 0.77, 95% CI 0.66–0.89, P = .0003), fewer total cardiovascular events (1,362 vs 1,602, HR 0.84, 95% CI 0.78–0.90, P = .0001), and fewer deaths from any cause (738 vs 820; HR 0.89, 95% CI 0.81–0.99, P = .025).
Lindholm et al27 performed a meta-analysis that included studies of selective beta-blockers (including atenolol) and nonselective beta-blockers, with a follow-up time of more than 2 years. Compared with placebo or no treatment, beta-blockers reduced the risk of stroke by 19% but had no effect on myocardial infarction or all-cause mortality. Compared with other antihypertensive drugs, beta-blockers were less than optimum, and the relative risk of stroke was 16% higher. Atenolol was the beta-blocker used in most of the randomized clinical trials included in this meta-analysis.
The Cochrane group22 found beta-blockers to be inferior to all other antihypertensive drugs with respect to the ability to lower the risk of stroke.
WHY WERE THE RESULTS SO DISAPPOINTING?
Problems with atenolol
Most of the trials in the meta-analyses discussed above used atenolol and other beta-blockers that had no vasodilatory properties.
Further, in most of the trials atenolol was used in a once-daily dosage, whereas ideally it needs to be taken more frequently, based on its pharmacokinetic and pharmacodynamic properties (a half-life of 6–9 hours).3 Neutel et al28 confirmed that atenolol, when taken once daily, leaves the patient unprotected in the last 6 hours of a 24-hour period, as demonstrated by 24-hour ambulatory blood pressure monitoring. It is possible that this short duration of action of atenolol may have contributed to the results observed in clinical trials that used atenolol to treat hypertension.
Differences between older and younger patients
Another possible reason for the disappointing results is that the trials included many elderly patients, in whom beta-blockers may not be as effective. The pathophysiology of hypertension in younger people is different from that in older patients.29 Hemodynamic characteristics of younger hypertensive patients include a high cardiac output and hyperdynamic circulation with a low pulse pressure, while older patients have lower arterial compliance with an elevated vascular resistance.
The notion of choosing antihypertensive medications on the basis of age and age-related pathophysiology is supported by several clinical studies. Randomized controlled trials appear to show that beta-blockers are effective in younger hypertensive patients.30
Conversely, the CAFE (Conduit Artery Function Evaluation) trial,31 a substudy of the main ASCOT trial,26 indicated that betablocker-based therapy was less effective in reducing central aortic pressure than were regimens based on an ACE inhibitor or a calcium channel blocker.
The CAFE researchers recruited 2,073 patients from five ASCOT centers and used radial artery applanation tonometry and pulse-wave analysis to derive central aortic pressures and hemodynamic indices during study visits up to a period of 4 years. Although the two treatment groups achieved similar brachial systolic blood pressures, the central aortic systolic pressure was 4.3 mm Hg lower in the amlodipine group (95% CI 3.3–5.4; P < .0001), and the central aortic pulse pressure was 3.0 mm Hg lower (95% CI 2.1–3.9; P < .0001).
Pulse-wave dyssynchrony
Bangalore et al47 offer an interesting hypothesis to explain the probable adverse effect of beta-blockers. Their theory concerns the effect of these drugs on the arterial pulse wave.
Normally, with each contraction of the left ventricle during systole, an arterial pulse wave is generated and propagated forward to the peripheral arteries. This wave is then reflected back to the heart from the branching points of peripheral arteries. The final form of the pressure wave at the aortic root is a synchronized summation of the forward-traveling wave and the backward-reflected wave.
In healthy people with normal arteries, the reflected wave merges with the forward-traveling wave in diastole and augments coronary blood flow. In patients whose arteries are stiff due to aging or vascular comorbidities, the reflected wave returns faster and merges with the incident wave in systole, resulting in higher left ventricular afterload and less coronary perfusion.48
Bangalore et al47 propose that artificially reducing the heart rate with beta-blockers may further dyssynchronize the pulse wave, adversely affecting coronary perfusion and leading to an increased risk of cardiovascular events and death.
Metabolic side effects
Older beta-blockers, and especially atenolol, have well-known metabolic adverse effects, particularly impairment of glycemic control. This adverse effect appears to occur only with beta-blockers that do not possess vasodilatory properties and thus increase peripheral vascular resistance, which results in lower glucose availability and reduced uptake by skeletal muscles.49
Bangalore et al50 evaluated the effect of beta-blockers in a meta-analysis of 12 studies in 94,492 patients followed up for more than 1 year. Beta-blocker therapy resulted in a 22% higher risk of new-onset diabetes mellitus (RR 1.22, 95% CI 1.12–1.33) than with other nondiuretic antihypertensive agents.
Of note, however, the meta-analysis did not show a significantly higher risk of the onset of diabetes with propranolol or metoprolol than with other nondiuretic antihypertensives when studies of these beta-blockers were separated from atenolol-based studies.
Further, the United Kingdom Prospective Diabetes Study40 found that cardiovascular outcomes in patients with good blood pressure control were similar when atenolol-based therapy was compared with therapy with the ACE inhibitor captopril (Capoten).
A meta-analysis conducted by Balamuthusamy et al51 in 2009 found no higher risk of stroke in patients with hypertension and diabetes mellitus who received beta-blockers than in those who received other antihypertensive medications. However, beta-blockers were associated with a higher risk of death from cardiovascular causes (RR 1.39, 95% CI 1.07–1.804; P < .01) compared with reninangiotensin blockade.
NEWER BETA-BLOCKERS MAY BE BETTER
In the United States, more than 40 million prescriptions for atenolol are written every year, making it by far the most commonly used beta-blocker for the treatment of hypertension. 52 It is clear, however, that atenolol is not an ideal representative of this class of antihypertensive medications.
Preliminary data from studies of newer beta-blockers that possess beneficial vasodilatory properties are encouraging. Animal studies and preliminary human studies find that these new-generation beta-blockers cause fewer adverse metabolic effects and improve endothelial function, measures of arterial stiffness, and cardiovascular outcomes.
Carvedilol
Carvedilol is a nonselective beta-blocker with vasodilatory effects that are thought to be due to its ability to concurrently block alpha-1 receptors in addition to beta receptors. 53 In experiments in vitro and in trials in patients with diabetes and hypertension, carvedilol increased endothelial vasodilation and reduced inflammation and platelet aggregation. These effects may be achieved though antioxidant actions, thereby preserving nitric oxide bioactivity.54,55
In the Glycemic Effects in Diabetes Mellitus: Carvedilol-Metoprolol Comparison in Hypertensives (GEMINI) trial,56 carvedilol was associated with better maintenance of glycemic control in diabetic hypertensive patients than was metoprolol. Insulin sensitivity improved with carvedilol but not with metoprolol, and fewer patients on carvedilol progressed to microalbuminuria.
Nebivolol
Nebivolol is a novel selective beta-blocker with a much higher affinity for beta-1 adrenergic receptors than for beta-2 adrenergic receptors. Among all the beta-blockers in clinical use today, nebivolol has the highest selectivity for beta-1 receptors.8
Nebivolol causes vasodilation through activation of the l-arginine/nitric oxide pathway.57–59 Blockade of synthesis of nitric oxide leads to local arterial stiffness. Endothelial dysfunction is characterized by decreased bioavailability of nitric oxide and has been shown to be a strong predictor of cardiovascular outcomes. By generating nitric oxide, nebivolol reduces peripheral vascular resistance, overcoming a significant side effect of earlier beta-blockers that lowered blood pressure but ultimately increased peripheral vascular tone and resistance.8
In an experiment in a bovine model,60 nebivolol significantly reduced the pulse-wave velocity (a measure of arterial stiffness), while atenolol had no effect. Moreover, evidence for the role of the l-arginine/nitric oxide pathway in the vasodilatory effect of nebivolol was demonstrated by co-infusion of NG-monomethyl-L-arginine, a specific endothelial nitric oxide synthetase inhibitor that attenuated the reduction of pulse-wave velocity by nebivolol.
In studies in hypertensive patients, nebivolol was associated with a better metabolic profile than atenolol, with none of the adverse effects on insulin sensitivity that atenolol had.61 In the Study of Effects of Nebivolol Interventions on Outcomes and Rehospitalization in Seniors With Heart Failure (SENIORS) trial, significantly fewer patients receiving nebivolol died or were admitted to the hospital for cardiovascular reasons compared with those receiving placebo.62
Although these findings are encouraging, we do not yet know if these effects will translate into a significant reduction in cardiovascular outcomes in clinical trials. Large, prospective hypertension outcome trials, particularly to evaluate primary prevention of cardiovascular outcomes, are needed for an evidence-based approach to using the newer beta-blockers as preferred first-line therapy for hypertension.
WHAT RECENT GUIDELINES SAY ABOUT BETA-BLOCKERS
The British National Institute for Health and Clinical Excellence and the British Hypertension Society, in their 2004 guidelines, recommended beta-blockers as one of several first-line antihypertensive medications in young, nonblack patients.63 On the other hand, they advised clinicians to be aware of the reported increase in onset of diabetes mellitus in patients treated with these medications. After the LIFE24 and ASCOT26 study results were published, these guidelines were amended to exclude beta-blockers as preferred routine initial therapy for hypertension.64
More recently, the 2007 European Society of Hypertension and European Society of Cardiology reconsidered the role of beta-blockers, recommending them as an option in both initial and subsequent antihypertensive treatment strategies.65
The current guidelines from the National Heart, Lung, and Blood Institute,66 which were published in 2003, were highly influenced by the results of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT),2 and favor diuretics as the first-line therapy. However, they indicate that beta-blockers are a suitable alternative, particularly when a compelling cardiac indication is present.53 We hope that the next update, expected late in 2009, will re-address this issue in the light of more recent data.
- Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet 2001; 358:1305–1315.
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288:2981–2997.
- Neutel JM, Smith DH, Ram CV, et al. Application of ambulatory blood pressure monitoring in differentiating between antihypertensive agents. Am J Med 1993; 94:181–187.
- Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med 1993; 328:914–921.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Frishman W, Silverman R. Clinical pharmacology of the new beta-adrenergic blocking drugs. Part 2. Physiologic and metabolic effects. Am Heart J 1979; 97:797–807.
- Garrett BN, Kaplan NM. Plasma renin activity suppression: duration after withdrawal from beta-adrenergic blockade. Arch Intern Med 1980; 140:1316–1318.
- Pedersen ME, Cockcroft JR. The latest generation of beta-blockers: new pharmacologic properties. Curr Hypertens Rep 2006; 8:279–286.
- Man in’t Veld AJ, Van den Meiracker AH, Schalekamp MA. Do beta-blockers really increase peripheral vascular resistance? Review of the literature and new observations under basal conditions. Am J Hypertens 1988; 1:91–96.
- Pearce CJ, Wallin JD. Labetalol and other agents that block both alpha- and beta-adrenergic receptors. Cleve Clin J Med 1994; 61:59–69.
- Dimsdale JE, Newton RP, Joist T. Neuropsychological side effects of beta-blockers. Arch Intern Med 1989; 149:514–525.
- Agarwal R. Supervised atenolol therapy in the management of hemodialysis hypertension. Kidney Int 1999; 55:1528–1535.
- Sica DA, Black HR. Pharmacologic considerations in the positioning of beta-blockers in antihypertensive therapy. Curr Hypertens Rep 2008; 10:330–335.
- Prichard BN, Gillam GP. Use of propranolol (Inderal) in treatment of hypertension. Br Med J 1964; 19; 2:725–727.
- The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V). Arch Intern Med 1993; 153:154–183.
- Moser M. Evolution of the treatment of hypertension from the 1940s to JNC V. Am J Hypertens 1997; 10:2S–8S.
- Houghton T, Freemantle N, Cleland JG. Are beta-blockers effective in patients who develop heart failure soon after myocardial infarction? A meta-regression analysis of randomised trials. Eur J Heart Fail 2000; 2:333–340.
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999; 353:9–13.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 1985; 27:335–371.
- Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med 2001; 134:550–560.
- Wiysonge CS, Bradley H, Mayosi BM, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev 2007;CD002003.
- Messerli FH, Grossman E, Goldbourt U. Are beta-blockers efficacious as first-line therapy for hypertension in the elderly? A systematic review. JAMA 1998; 279:1903–1907.
- Dahlöf B, Devereux RB, Kjeldsen SE, et al; for the LIFE study group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint Reduction in Hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–1003.
- Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice? Lancet 2004; 364:1684–1689.
- Dahlöf B, Sever PS, Poulter NR, et al; ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005; 366:895–906.
- Lindholm LH, Carlberg B, Samuelsson O. Should beta-blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005; 366:1545–1553.
- Neutel JM, Schnaper H, Cheung DG, Graettinger WF, Weber MA. Antihypertensive effects of beta-blockers administered once daily: 24-hour measurements. Am Heart J 1990; 120:166–171.
- Franklin SS, Gustin W, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997; 96:308–315.
- The IPPPSH Collaborative Group. Cardiovascular risk and risk factors in a randomized trial of treatment based on the beta-blocker oxprenolol: the International Prospective Primary Prevention Study in Hypertension (IPPPSH). J Hypertens 1985; 3:379–392.
- Williams B, Lacy PS, Thom SM, et al; CAFE Investigators. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006; 113:1213–1225.
- Khan N, McAlister FA. Re-examining the efficacy of beta-blockers for the treatment of hypertension: a meta-analysis. CMAJ 2006; 174:1737–1742.
- Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. BMJ 1985; 291:97–104.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. BMJ 1986; 293:1145–1151.
- Dahlöf B, Lindholm LH, Hansson L, et al. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- MRC Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ 1992; 304:405–412.
- The Dutch TIA Study Group. Trial of secondary prevention with atenolol after transient ischemic attack or nondisabling ischemic stroke. Stroke 1993; 24:543–548.
- Eriksson S, Olofsson B-O, Wester P-O; for the TEST Study Group. Atenolol in secondary prevention after stroke. Cerebrovasc Dis 1995; 5:21–25.
- Wilhelmsen L, Berglund G, Elmfeldt D, et al. Beta-blockers versus diuretics in hypertensive men: main results from the HAPPHY trial. J Hypertens 1987; 5:561–572.
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317:713–720.
- Hansson L, Lindholm LH, Niskanen L, et al. Effect of angiotensin-converting enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPP) randomised trial. Lancet 1999; 353:611–616.
- Lanchetti A, Bond MG, Henning M, et al. Calcium antagonist lacidipine slow down progression of asymptomatic carotid atherosclerosis. Principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double-blind, long-term trial. Circulation 2002; 106:2422–2427.
- Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity in the Swedish Trial in Old Patients with Hypertension-2 study. Lancet 1999; 354:1751–1756.
- Hansson L, Hedner T, Lund-Johansen P, et al. Randomised trial of effects of calcium antagonists compared with diuretics and ß blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet 2000; 356:359–365.
- Pepine CJ, Handsberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003; 290:2805–2816.
- Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) Trial. JAMA 2003; 289:2073–2082.
- Bangalore S, Sawhney S, Messerli FH. Relation of beta-blocker-induced heart rate lowering and cardioprotection in hypertension. J Am Coll Cardiol 2008; 52:1482–1489.
- Boutouyrie P, Vermersch S, Laurent S, Briet M. Cardiovascular risk assessment through target organ damage: role of carotid to femoral pulse wave velocity. Clin Exp Pharmacol Physiol 2008; 35:530–533.
- Kveiborg B, Christiansen B, Major-Petersen A, Torp-Pedersen C. Metabolic effects of beta-adrenoceptor antagonists with special emphasis on carvedilol. Am J Cardiovasc Drugs 2006; 6:209–217.
- Bangalore S, Parkar S, Grossman E, Messerli FH. A meta-analysis of 94,492 patients with hypertension treated with beta-blockers to determine the risk of new-onset diabetes mellitus. Am J Cardiol 2007; 100:1254–1262.
- Balamuthusamy S, Molnar J, Adigopula S, Arora R. Comparative analysis of beta-blockers with other antihypertensive agents on cardiovascular outcomes in hypertensive patients with diabetes mellitus: a systematic review and meta-analysis. Am J Ther 2009; 16:133–142.
- Berenson A. Big drug makers see sales decline with their image. New York Times 2005 Nov 14.
- Bristow MR. Beta-adrenergic receptor blockade in chronic heart failure. Circulation 2000; 101:558–569.
- Giugliano D, Marfella R, Acampora R, Giunta R, Coppola L, D’Onofrio F. Effects of perindopril and carvedilol on endothelium-dependent vascular functions in patients with diabetes and hypertension. Diabetes Care 1998; 21:631–636.
- Lopez BL, Christopher TA, Yue TL, Ruffolo R, Feuerstein GZ, Ma XL. Carvedilol, a new beta-adrenoreceptor blocker antihypertensive drug, protects against free-radical-induced endothelial dysfunction. Pharmacology 1995; 51:165–173.
- Bakris GL, Fonseca V, Katholi RE, et al; GEMINI Investigators. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA 2004; 292:2227–2236.
- Georgescu A, Pluteanu F, Flonta ML, Badila E, Dorobantu M, Popov D. The cellular mechanisms involved in the vasodilator effect of nebivolol on the renal artery. Eur J Pharmacol 2005; 508:159–166.
- Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, et al. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation 2003; 107:2747–2752.
- Cockcroft JR, Chowienczyk PJ, Brett SE, et al. Nebivolol vasodilates human forearm vasculature: evidence for an L-arginine/NO-dependent mechanism. J Pharmacol Exp Ther 1995; 274:1067–1071.
- McEniery CM, Schmitt M, Qasem A, et al. Nebivolol increases arterial distensibility in vivo. Hypertension 2004; 44:305–310.
- Poirier L, Cleroux J, Nadeau A, Lacourciere Y. Effects of nebivolol and atenolol on insulin sensitivity and haemodynamics in hypertensive patients. J Hypertens 2001; 19:1429–1435.
- Flather MD, Shibata MC, Coats AJ, et al; SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005; 26:215–225.
- Williams B, Poulter NR, Brown MJ, et al; BHS guidelines working party, for the British Hypertension Society. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ 2004; 328:634–640.
- Sever P. New hypertension guidelines from the National Institute for Health and Clinical Excellence and the British Hypertension Society. J Renin Angiotensin Aldosterone Syst 2006; 7:61–63.
- Mancia G, De Backer G, Dominiczak A, et al; Management of Arterial Hypertension of the European Society of Hypertension. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25:1105–1187.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
In recent years the role of beta-blockers as a primary tool to treat hypertension has come under question. These drugs have shown disappointing results when used as antihypertensive therapy in patients without heart disease, ie, when used as primary prevention. At the same time, beta-blockers clearly reduce the risk of future cardiovascular events in patients who already have heart disease, eg, who already have had a myocardial infarction or who have congestive heart failure.
Several meta-analyses and a few clinical trials have shown that beta-blockers may have no advantage over other antihypertensive drugs, and in fact may not reduce the risk of stroke as effectively as other classes of blood pressure medications.
Why should this be? Is it that the patients in the antihypertensive trials were mostly older, and that beta-blockers do not work as well in older patients as in younger ones? Or does it have to do with the fact that atenolol (Tenormin) was the drug most often used in the trials? Would newer, different beta-blockers be better?
Hypertension experts currently disagree on how to interpret the available data, and this has led to conflict and confusion among clinicians as to the role of beta-blockers in managing hypertension. Current evidence suggests that older beta-blockers may not be the preferred first-line antihypertensive drugs for hypertensive patients who have no compelling indications for them (eg, heart failure, myocardial infarction, diabetes, high risk of coronary heart disease). However, newer beta-blockers with vasodilatory properties should be considered in cases of uncontrolled or resistant hypertension, especially in younger patients.
Further, while controversy and debate continue over the benefits and adverse effects of one class of antihypertensive drugs vs another, it is indisputable that controlling arterial blood pressure to the recommended goal offers major protection against cardiovascular and renal events in patients with hypertension.1,2
MECHANISM OF ACTION OF BETA-BLOCKERS
Beta-blockers effectively reduce blood pressure in both systolic-diastolic hypertension and isolated systolic hypertension.3–5 Exactly how is not known, but it has been proposed that they may do so by:
Reducing the heart rate and cardiac output. When catecholamines activate beta-1 receptors in the heart, the heart rate and myocardial contractility increase. By blocking beta-1 receptors, beta-blockers reduce the heart rate and myocardial contractility, thus lowering cardiac output and arterial blood pressure.6
Inhibiting renin release. Activation of the renin-angiotensin system is another major pathway that can lead to elevated arterial blood pressure. Renin release is mediated through the sympathetic nervous system via beta-1 receptors on the juxtaglomerular cells of the kidney. Beta-blockers can therefore lower blood pressure by inhibiting renin release.7
Inhibiting central nervous sympathetic outflow, thereby inducing presynaptic blockade, which in turn reduces the release of catecholamines.
Reducing venous return and plasma volume.
Generating nitric oxide, thus reducing peripheral vascular resistance (some agents).8
Reducing vasomotor tone.
Reducing vascular tone.
Improving vascular compliance.
Resetting baroreceptor levels.
Attenuating the pressor response to catecholamines with exercise and stress.
HETEROGENEITY OF BETA-BLOCKERS
Selectivity
Beta-blockers are not all the same. They can be classified into three categories.
Nonselective beta-blockers block both beta-1 and beta-2 adrenergic receptors. It is generally accepted that beta-blockers exert their primary antihypertensive effect by blocking beta-1 adrenergic receptors.6 Of interest, nonselective beta-blockers inhibit beta-2 receptors on arteries and thus cause an unopposed alpha-adrenergic effect, leading to increased peripheral vascular resistance.9 Examples of this category:
- Nadolol (Corgard)
- Pindolol (Visken)
- Propranolol (Inderal)
- Timolol (Blocadren).
Selective beta-blockers specifically block beta-1 receptors alone, although they are known to be nonselective at higher doses. Examples:
- Atenolol (Tenormin)
- Betaxolol (Kerlone)
- Bisoprolol (Zebeta)
- Esmolol (Brevibloc)
- Metoprolol (Lopressor, Toprol).
Beta-blockers with peripheral vasodilatatory effects act either via antagonism of the alpha-1 receptor, as with labetolol (Normodyne) and carvedilol (Coreg),10 or via enhanced release of nitric oxide, as with nebivolol (Bystolic).8
Lipid and water solubility
The lipid solubility and water solubility of each beta-blocker determine its bioavailability and side-effect profile.
Lipid solubility determines the degree to which a beta-blocker penetrates the blood-brain barrier and thereby leads to central nervous system side effects such as lethargy, nightmares, confusion, and depression. Propranolol is highly lipid-soluble; metoprolol and labetalol are moderately so.
Water-soluble beta-blockers such as atenolol have less tissue permeation, have a longer half-life, and cause fewer central nervous system effects and symptoms.11
Routes of elimination
Beta-blockers also differ in their route of elimination.
Atenolol and nadolol are eliminated by the kidney and require dose adjustment in patients with impaired renal function.12,13
On the other hand, propranolol, metoprolol, labetalol, carvedilol, and nebivolol are excreted primarily via hepatic metabolism.13
BETA-BLOCKERS IN THE MANAGEMENT OF HYPERTENSION
Beta-blockers were initially used to treat arrhythmias, but by the early 1970s they were also widely accepted for managing hypertension. 14 Their initial acceptance as one of the first-line classes of drugs for hypertension was based on their better side-effect profile compared with other antihypertensive drugs available at that time.
In the 1980s and 1990s, beta-blockers were listed as preferred first-line antihypertensive drugs along with diuretics in national hypertension guidelines.15 Subsequent updates of the guidelines favored diuretics as initial therapy and relegated all other classes of antihypertensive medications to be alternatives to diuretics.16 Although beta-blockers remain alternative first-line drugs in the latest guidelines (published in 2003; see reference 66), they are the preferred antihypertensive agents for patients with cardiac disease.
The current recommendations reflect the findings from hypertension trials in which patients with myocardial infarction and congestive heart failure had better cardiovascular outcomes if they received these drugs,17–19 including a lower risk of death.20,21 It was widely assumed that beta-blockers would also prevent first episodes of cardiovascular events.
However, to date, there is no evidence that beta-blockers are effective as primary prevention. Several large randomized controlled trials showed no benefit with beta-blockers compared with other antihypertensive drugs—in fact, there were more cardiovascular events with beta-blockers (see below).
Beta-blockers are well tolerated in clinical practice, although they can have side effects that include fatigue, depression, impaired exercise tolerance, sexual dysfunction, and asthma attacks.
Wiysonge et al22 analyzed how many patients withdrew from randomized trials of antihypertensive treatment because of drug-related adverse events. There was no significant difference in the incidence of fatigue, depressive symptoms, or sexual dysfunction with beta-blockers compared with placebo, and trial participants on a beta-blocker were not statistically significantly more likely to discontinue treatment than those receiving a placebo in three trials with 22,729 participants (relative risk [RR] 2.34, 95% confidence interval [CI] 0.84–6.52).
THE CONTROVERSY: WHAT THE TRIALS SHOWED
Messerli et al23 performed a meta-analysis published in 1998 that suggested that beta-blockers may not be as effective as diuretics in preventing cardiovascular events when used as first-line antihypertensive therapy in elderly patients. In 10 randomized controlled trials in 16,164 patients who were treated with either a diuretic or a beta-blocker (atenolol), blood pressure was normalized in two-thirds of diuretic-treated patients but only one-third of patients treated with atenolol as monotherapy. Diuretic therapy was superior with regard to all end points, and beta-blockers were found to be ineffective except in reducing cerebrovascular events.
The LIFE study (Losartan Intervention for Endpoint Reduction in Hypertension)24 compared the angiotensin-receptor blocker losartan (Cozaar) and atenolol in 9,193 patients with hypertension and left ventricular hypertrophy. At 4 years of follow-up, the rate of primary cardiovascular events (death, myocardial infarction, or stroke) was lower in the losartan group than in the atenolol group. The difference was mainly due to a 25% lower incidence of stroke, which was statistically significant. The rates of myocardial infarction and death from cardiovascular causes were not significantly different between the two treatment groups. The systolic blood pressure was 1 mm Hg lower in the losartan group than in the atenolol group, which was statistically significant.
Carlberg et al25 performed another important meta-analysis that questioned whether atenolol reduces rates of cardiovascular morbidity and death in hypertensive patients. The results were surprising: eight randomized controlled trials including more than 6,000 patients and comparing atenolol with placebo or no treatment showed no differences between the treatment groups with regard to the outcomes of all-cause mortality (RR 1.01, 95% CI 0.89–1.15), cardiovascular mortality (RR 0.99, 95% CI 0.83–1.18), or myocardial infarction (RR 0.99, 95% CI 0.83–1.19).
In addition, when atenolol was compared with other antihypertensives in five other randomized controlled trials that included more than 14,000 patients, those treated with atenolol had a higher risk of stroke (RR 1.30, 95% CI 1.12–1.50) and death (RR 1.13, 95% CI 1.02–1.25).
The ASCOT-BPLA trial (Anglo-Scandinavian Cardiac Outcomes Trial—Blood Pressure Lowering Arm)26 had similar results. This trial compared the combination of atenolol plus the diuretic bendroflumethiazide against the combination of the calcium channel blocker amlodipine (Norvasc) plus the angiotensin-converting enzyme (ACE) inhibitor perindopril (Aceon). Although no significant difference was seen in the primary outcome of nonfatal myocardial infarction or fatal coronary heart disease (unadjusted hazard ratio [HR] with amlodipine-perindopril 0.90, 95% CI 0.79–1.02, P = .1052), the amlodipine-plus-perindopril group had significantly fewer strokes (327 vs 422, HR 0.77, 95% CI 0.66–0.89, P = .0003), fewer total cardiovascular events (1,362 vs 1,602, HR 0.84, 95% CI 0.78–0.90, P = .0001), and fewer deaths from any cause (738 vs 820; HR 0.89, 95% CI 0.81–0.99, P = .025).
Lindholm et al27 performed a meta-analysis that included studies of selective beta-blockers (including atenolol) and nonselective beta-blockers, with a follow-up time of more than 2 years. Compared with placebo or no treatment, beta-blockers reduced the risk of stroke by 19% but had no effect on myocardial infarction or all-cause mortality. Compared with other antihypertensive drugs, beta-blockers were less than optimum, and the relative risk of stroke was 16% higher. Atenolol was the beta-blocker used in most of the randomized clinical trials included in this meta-analysis.
The Cochrane group22 found beta-blockers to be inferior to all other antihypertensive drugs with respect to the ability to lower the risk of stroke.
WHY WERE THE RESULTS SO DISAPPOINTING?
Problems with atenolol
Most of the trials in the meta-analyses discussed above used atenolol and other beta-blockers that had no vasodilatory properties.
Further, in most of the trials atenolol was used in a once-daily dosage, whereas ideally it needs to be taken more frequently, based on its pharmacokinetic and pharmacodynamic properties (a half-life of 6–9 hours).3 Neutel et al28 confirmed that atenolol, when taken once daily, leaves the patient unprotected in the last 6 hours of a 24-hour period, as demonstrated by 24-hour ambulatory blood pressure monitoring. It is possible that this short duration of action of atenolol may have contributed to the results observed in clinical trials that used atenolol to treat hypertension.
Differences between older and younger patients
Another possible reason for the disappointing results is that the trials included many elderly patients, in whom beta-blockers may not be as effective. The pathophysiology of hypertension in younger people is different from that in older patients.29 Hemodynamic characteristics of younger hypertensive patients include a high cardiac output and hyperdynamic circulation with a low pulse pressure, while older patients have lower arterial compliance with an elevated vascular resistance.
The notion of choosing antihypertensive medications on the basis of age and age-related pathophysiology is supported by several clinical studies. Randomized controlled trials appear to show that beta-blockers are effective in younger hypertensive patients.30
Conversely, the CAFE (Conduit Artery Function Evaluation) trial,31 a substudy of the main ASCOT trial,26 indicated that betablocker-based therapy was less effective in reducing central aortic pressure than were regimens based on an ACE inhibitor or a calcium channel blocker.
The CAFE researchers recruited 2,073 patients from five ASCOT centers and used radial artery applanation tonometry and pulse-wave analysis to derive central aortic pressures and hemodynamic indices during study visits up to a period of 4 years. Although the two treatment groups achieved similar brachial systolic blood pressures, the central aortic systolic pressure was 4.3 mm Hg lower in the amlodipine group (95% CI 3.3–5.4; P < .0001), and the central aortic pulse pressure was 3.0 mm Hg lower (95% CI 2.1–3.9; P < .0001).
Pulse-wave dyssynchrony
Bangalore et al47 offer an interesting hypothesis to explain the probable adverse effect of beta-blockers. Their theory concerns the effect of these drugs on the arterial pulse wave.
Normally, with each contraction of the left ventricle during systole, an arterial pulse wave is generated and propagated forward to the peripheral arteries. This wave is then reflected back to the heart from the branching points of peripheral arteries. The final form of the pressure wave at the aortic root is a synchronized summation of the forward-traveling wave and the backward-reflected wave.
In healthy people with normal arteries, the reflected wave merges with the forward-traveling wave in diastole and augments coronary blood flow. In patients whose arteries are stiff due to aging or vascular comorbidities, the reflected wave returns faster and merges with the incident wave in systole, resulting in higher left ventricular afterload and less coronary perfusion.48
Bangalore et al47 propose that artificially reducing the heart rate with beta-blockers may further dyssynchronize the pulse wave, adversely affecting coronary perfusion and leading to an increased risk of cardiovascular events and death.
Metabolic side effects
Older beta-blockers, and especially atenolol, have well-known metabolic adverse effects, particularly impairment of glycemic control. This adverse effect appears to occur only with beta-blockers that do not possess vasodilatory properties and thus increase peripheral vascular resistance, which results in lower glucose availability and reduced uptake by skeletal muscles.49
Bangalore et al50 evaluated the effect of beta-blockers in a meta-analysis of 12 studies in 94,492 patients followed up for more than 1 year. Beta-blocker therapy resulted in a 22% higher risk of new-onset diabetes mellitus (RR 1.22, 95% CI 1.12–1.33) than with other nondiuretic antihypertensive agents.
Of note, however, the meta-analysis did not show a significantly higher risk of the onset of diabetes with propranolol or metoprolol than with other nondiuretic antihypertensives when studies of these beta-blockers were separated from atenolol-based studies.
Further, the United Kingdom Prospective Diabetes Study40 found that cardiovascular outcomes in patients with good blood pressure control were similar when atenolol-based therapy was compared with therapy with the ACE inhibitor captopril (Capoten).
A meta-analysis conducted by Balamuthusamy et al51 in 2009 found no higher risk of stroke in patients with hypertension and diabetes mellitus who received beta-blockers than in those who received other antihypertensive medications. However, beta-blockers were associated with a higher risk of death from cardiovascular causes (RR 1.39, 95% CI 1.07–1.804; P < .01) compared with reninangiotensin blockade.
NEWER BETA-BLOCKERS MAY BE BETTER
In the United States, more than 40 million prescriptions for atenolol are written every year, making it by far the most commonly used beta-blocker for the treatment of hypertension. 52 It is clear, however, that atenolol is not an ideal representative of this class of antihypertensive medications.
Preliminary data from studies of newer beta-blockers that possess beneficial vasodilatory properties are encouraging. Animal studies and preliminary human studies find that these new-generation beta-blockers cause fewer adverse metabolic effects and improve endothelial function, measures of arterial stiffness, and cardiovascular outcomes.
Carvedilol
Carvedilol is a nonselective beta-blocker with vasodilatory effects that are thought to be due to its ability to concurrently block alpha-1 receptors in addition to beta receptors. 53 In experiments in vitro and in trials in patients with diabetes and hypertension, carvedilol increased endothelial vasodilation and reduced inflammation and platelet aggregation. These effects may be achieved though antioxidant actions, thereby preserving nitric oxide bioactivity.54,55
In the Glycemic Effects in Diabetes Mellitus: Carvedilol-Metoprolol Comparison in Hypertensives (GEMINI) trial,56 carvedilol was associated with better maintenance of glycemic control in diabetic hypertensive patients than was metoprolol. Insulin sensitivity improved with carvedilol but not with metoprolol, and fewer patients on carvedilol progressed to microalbuminuria.
Nebivolol
Nebivolol is a novel selective beta-blocker with a much higher affinity for beta-1 adrenergic receptors than for beta-2 adrenergic receptors. Among all the beta-blockers in clinical use today, nebivolol has the highest selectivity for beta-1 receptors.8
Nebivolol causes vasodilation through activation of the l-arginine/nitric oxide pathway.57–59 Blockade of synthesis of nitric oxide leads to local arterial stiffness. Endothelial dysfunction is characterized by decreased bioavailability of nitric oxide and has been shown to be a strong predictor of cardiovascular outcomes. By generating nitric oxide, nebivolol reduces peripheral vascular resistance, overcoming a significant side effect of earlier beta-blockers that lowered blood pressure but ultimately increased peripheral vascular tone and resistance.8
In an experiment in a bovine model,60 nebivolol significantly reduced the pulse-wave velocity (a measure of arterial stiffness), while atenolol had no effect. Moreover, evidence for the role of the l-arginine/nitric oxide pathway in the vasodilatory effect of nebivolol was demonstrated by co-infusion of NG-monomethyl-L-arginine, a specific endothelial nitric oxide synthetase inhibitor that attenuated the reduction of pulse-wave velocity by nebivolol.
In studies in hypertensive patients, nebivolol was associated with a better metabolic profile than atenolol, with none of the adverse effects on insulin sensitivity that atenolol had.61 In the Study of Effects of Nebivolol Interventions on Outcomes and Rehospitalization in Seniors With Heart Failure (SENIORS) trial, significantly fewer patients receiving nebivolol died or were admitted to the hospital for cardiovascular reasons compared with those receiving placebo.62
Although these findings are encouraging, we do not yet know if these effects will translate into a significant reduction in cardiovascular outcomes in clinical trials. Large, prospective hypertension outcome trials, particularly to evaluate primary prevention of cardiovascular outcomes, are needed for an evidence-based approach to using the newer beta-blockers as preferred first-line therapy for hypertension.
WHAT RECENT GUIDELINES SAY ABOUT BETA-BLOCKERS
The British National Institute for Health and Clinical Excellence and the British Hypertension Society, in their 2004 guidelines, recommended beta-blockers as one of several first-line antihypertensive medications in young, nonblack patients.63 On the other hand, they advised clinicians to be aware of the reported increase in onset of diabetes mellitus in patients treated with these medications. After the LIFE24 and ASCOT26 study results were published, these guidelines were amended to exclude beta-blockers as preferred routine initial therapy for hypertension.64
More recently, the 2007 European Society of Hypertension and European Society of Cardiology reconsidered the role of beta-blockers, recommending them as an option in both initial and subsequent antihypertensive treatment strategies.65
The current guidelines from the National Heart, Lung, and Blood Institute,66 which were published in 2003, were highly influenced by the results of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT),2 and favor diuretics as the first-line therapy. However, they indicate that beta-blockers are a suitable alternative, particularly when a compelling cardiac indication is present.53 We hope that the next update, expected late in 2009, will re-address this issue in the light of more recent data.
In recent years the role of beta-blockers as a primary tool to treat hypertension has come under question. These drugs have shown disappointing results when used as antihypertensive therapy in patients without heart disease, ie, when used as primary prevention. At the same time, beta-blockers clearly reduce the risk of future cardiovascular events in patients who already have heart disease, eg, who already have had a myocardial infarction or who have congestive heart failure.
Several meta-analyses and a few clinical trials have shown that beta-blockers may have no advantage over other antihypertensive drugs, and in fact may not reduce the risk of stroke as effectively as other classes of blood pressure medications.
Why should this be? Is it that the patients in the antihypertensive trials were mostly older, and that beta-blockers do not work as well in older patients as in younger ones? Or does it have to do with the fact that atenolol (Tenormin) was the drug most often used in the trials? Would newer, different beta-blockers be better?
Hypertension experts currently disagree on how to interpret the available data, and this has led to conflict and confusion among clinicians as to the role of beta-blockers in managing hypertension. Current evidence suggests that older beta-blockers may not be the preferred first-line antihypertensive drugs for hypertensive patients who have no compelling indications for them (eg, heart failure, myocardial infarction, diabetes, high risk of coronary heart disease). However, newer beta-blockers with vasodilatory properties should be considered in cases of uncontrolled or resistant hypertension, especially in younger patients.
Further, while controversy and debate continue over the benefits and adverse effects of one class of antihypertensive drugs vs another, it is indisputable that controlling arterial blood pressure to the recommended goal offers major protection against cardiovascular and renal events in patients with hypertension.1,2
MECHANISM OF ACTION OF BETA-BLOCKERS
Beta-blockers effectively reduce blood pressure in both systolic-diastolic hypertension and isolated systolic hypertension.3–5 Exactly how is not known, but it has been proposed that they may do so by:
Reducing the heart rate and cardiac output. When catecholamines activate beta-1 receptors in the heart, the heart rate and myocardial contractility increase. By blocking beta-1 receptors, beta-blockers reduce the heart rate and myocardial contractility, thus lowering cardiac output and arterial blood pressure.6
Inhibiting renin release. Activation of the renin-angiotensin system is another major pathway that can lead to elevated arterial blood pressure. Renin release is mediated through the sympathetic nervous system via beta-1 receptors on the juxtaglomerular cells of the kidney. Beta-blockers can therefore lower blood pressure by inhibiting renin release.7
Inhibiting central nervous sympathetic outflow, thereby inducing presynaptic blockade, which in turn reduces the release of catecholamines.
Reducing venous return and plasma volume.
Generating nitric oxide, thus reducing peripheral vascular resistance (some agents).8
Reducing vasomotor tone.
Reducing vascular tone.
Improving vascular compliance.
Resetting baroreceptor levels.
Attenuating the pressor response to catecholamines with exercise and stress.
HETEROGENEITY OF BETA-BLOCKERS
Selectivity
Beta-blockers are not all the same. They can be classified into three categories.
Nonselective beta-blockers block both beta-1 and beta-2 adrenergic receptors. It is generally accepted that beta-blockers exert their primary antihypertensive effect by blocking beta-1 adrenergic receptors.6 Of interest, nonselective beta-blockers inhibit beta-2 receptors on arteries and thus cause an unopposed alpha-adrenergic effect, leading to increased peripheral vascular resistance.9 Examples of this category:
- Nadolol (Corgard)
- Pindolol (Visken)
- Propranolol (Inderal)
- Timolol (Blocadren).
Selective beta-blockers specifically block beta-1 receptors alone, although they are known to be nonselective at higher doses. Examples:
- Atenolol (Tenormin)
- Betaxolol (Kerlone)
- Bisoprolol (Zebeta)
- Esmolol (Brevibloc)
- Metoprolol (Lopressor, Toprol).
Beta-blockers with peripheral vasodilatatory effects act either via antagonism of the alpha-1 receptor, as with labetolol (Normodyne) and carvedilol (Coreg),10 or via enhanced release of nitric oxide, as with nebivolol (Bystolic).8
Lipid and water solubility
The lipid solubility and water solubility of each beta-blocker determine its bioavailability and side-effect profile.
Lipid solubility determines the degree to which a beta-blocker penetrates the blood-brain barrier and thereby leads to central nervous system side effects such as lethargy, nightmares, confusion, and depression. Propranolol is highly lipid-soluble; metoprolol and labetalol are moderately so.
Water-soluble beta-blockers such as atenolol have less tissue permeation, have a longer half-life, and cause fewer central nervous system effects and symptoms.11
Routes of elimination
Beta-blockers also differ in their route of elimination.
Atenolol and nadolol are eliminated by the kidney and require dose adjustment in patients with impaired renal function.12,13
On the other hand, propranolol, metoprolol, labetalol, carvedilol, and nebivolol are excreted primarily via hepatic metabolism.13
BETA-BLOCKERS IN THE MANAGEMENT OF HYPERTENSION
Beta-blockers were initially used to treat arrhythmias, but by the early 1970s they were also widely accepted for managing hypertension. 14 Their initial acceptance as one of the first-line classes of drugs for hypertension was based on their better side-effect profile compared with other antihypertensive drugs available at that time.
In the 1980s and 1990s, beta-blockers were listed as preferred first-line antihypertensive drugs along with diuretics in national hypertension guidelines.15 Subsequent updates of the guidelines favored diuretics as initial therapy and relegated all other classes of antihypertensive medications to be alternatives to diuretics.16 Although beta-blockers remain alternative first-line drugs in the latest guidelines (published in 2003; see reference 66), they are the preferred antihypertensive agents for patients with cardiac disease.
The current recommendations reflect the findings from hypertension trials in which patients with myocardial infarction and congestive heart failure had better cardiovascular outcomes if they received these drugs,17–19 including a lower risk of death.20,21 It was widely assumed that beta-blockers would also prevent first episodes of cardiovascular events.
However, to date, there is no evidence that beta-blockers are effective as primary prevention. Several large randomized controlled trials showed no benefit with beta-blockers compared with other antihypertensive drugs—in fact, there were more cardiovascular events with beta-blockers (see below).
Beta-blockers are well tolerated in clinical practice, although they can have side effects that include fatigue, depression, impaired exercise tolerance, sexual dysfunction, and asthma attacks.
Wiysonge et al22 analyzed how many patients withdrew from randomized trials of antihypertensive treatment because of drug-related adverse events. There was no significant difference in the incidence of fatigue, depressive symptoms, or sexual dysfunction with beta-blockers compared with placebo, and trial participants on a beta-blocker were not statistically significantly more likely to discontinue treatment than those receiving a placebo in three trials with 22,729 participants (relative risk [RR] 2.34, 95% confidence interval [CI] 0.84–6.52).
THE CONTROVERSY: WHAT THE TRIALS SHOWED
Messerli et al23 performed a meta-analysis published in 1998 that suggested that beta-blockers may not be as effective as diuretics in preventing cardiovascular events when used as first-line antihypertensive therapy in elderly patients. In 10 randomized controlled trials in 16,164 patients who were treated with either a diuretic or a beta-blocker (atenolol), blood pressure was normalized in two-thirds of diuretic-treated patients but only one-third of patients treated with atenolol as monotherapy. Diuretic therapy was superior with regard to all end points, and beta-blockers were found to be ineffective except in reducing cerebrovascular events.
The LIFE study (Losartan Intervention for Endpoint Reduction in Hypertension)24 compared the angiotensin-receptor blocker losartan (Cozaar) and atenolol in 9,193 patients with hypertension and left ventricular hypertrophy. At 4 years of follow-up, the rate of primary cardiovascular events (death, myocardial infarction, or stroke) was lower in the losartan group than in the atenolol group. The difference was mainly due to a 25% lower incidence of stroke, which was statistically significant. The rates of myocardial infarction and death from cardiovascular causes were not significantly different between the two treatment groups. The systolic blood pressure was 1 mm Hg lower in the losartan group than in the atenolol group, which was statistically significant.
Carlberg et al25 performed another important meta-analysis that questioned whether atenolol reduces rates of cardiovascular morbidity and death in hypertensive patients. The results were surprising: eight randomized controlled trials including more than 6,000 patients and comparing atenolol with placebo or no treatment showed no differences between the treatment groups with regard to the outcomes of all-cause mortality (RR 1.01, 95% CI 0.89–1.15), cardiovascular mortality (RR 0.99, 95% CI 0.83–1.18), or myocardial infarction (RR 0.99, 95% CI 0.83–1.19).
In addition, when atenolol was compared with other antihypertensives in five other randomized controlled trials that included more than 14,000 patients, those treated with atenolol had a higher risk of stroke (RR 1.30, 95% CI 1.12–1.50) and death (RR 1.13, 95% CI 1.02–1.25).
The ASCOT-BPLA trial (Anglo-Scandinavian Cardiac Outcomes Trial—Blood Pressure Lowering Arm)26 had similar results. This trial compared the combination of atenolol plus the diuretic bendroflumethiazide against the combination of the calcium channel blocker amlodipine (Norvasc) plus the angiotensin-converting enzyme (ACE) inhibitor perindopril (Aceon). Although no significant difference was seen in the primary outcome of nonfatal myocardial infarction or fatal coronary heart disease (unadjusted hazard ratio [HR] with amlodipine-perindopril 0.90, 95% CI 0.79–1.02, P = .1052), the amlodipine-plus-perindopril group had significantly fewer strokes (327 vs 422, HR 0.77, 95% CI 0.66–0.89, P = .0003), fewer total cardiovascular events (1,362 vs 1,602, HR 0.84, 95% CI 0.78–0.90, P = .0001), and fewer deaths from any cause (738 vs 820; HR 0.89, 95% CI 0.81–0.99, P = .025).
Lindholm et al27 performed a meta-analysis that included studies of selective beta-blockers (including atenolol) and nonselective beta-blockers, with a follow-up time of more than 2 years. Compared with placebo or no treatment, beta-blockers reduced the risk of stroke by 19% but had no effect on myocardial infarction or all-cause mortality. Compared with other antihypertensive drugs, beta-blockers were less than optimum, and the relative risk of stroke was 16% higher. Atenolol was the beta-blocker used in most of the randomized clinical trials included in this meta-analysis.
The Cochrane group22 found beta-blockers to be inferior to all other antihypertensive drugs with respect to the ability to lower the risk of stroke.
WHY WERE THE RESULTS SO DISAPPOINTING?
Problems with atenolol
Most of the trials in the meta-analyses discussed above used atenolol and other beta-blockers that had no vasodilatory properties.
Further, in most of the trials atenolol was used in a once-daily dosage, whereas ideally it needs to be taken more frequently, based on its pharmacokinetic and pharmacodynamic properties (a half-life of 6–9 hours).3 Neutel et al28 confirmed that atenolol, when taken once daily, leaves the patient unprotected in the last 6 hours of a 24-hour period, as demonstrated by 24-hour ambulatory blood pressure monitoring. It is possible that this short duration of action of atenolol may have contributed to the results observed in clinical trials that used atenolol to treat hypertension.
Differences between older and younger patients
Another possible reason for the disappointing results is that the trials included many elderly patients, in whom beta-blockers may not be as effective. The pathophysiology of hypertension in younger people is different from that in older patients.29 Hemodynamic characteristics of younger hypertensive patients include a high cardiac output and hyperdynamic circulation with a low pulse pressure, while older patients have lower arterial compliance with an elevated vascular resistance.
The notion of choosing antihypertensive medications on the basis of age and age-related pathophysiology is supported by several clinical studies. Randomized controlled trials appear to show that beta-blockers are effective in younger hypertensive patients.30
Conversely, the CAFE (Conduit Artery Function Evaluation) trial,31 a substudy of the main ASCOT trial,26 indicated that betablocker-based therapy was less effective in reducing central aortic pressure than were regimens based on an ACE inhibitor or a calcium channel blocker.
The CAFE researchers recruited 2,073 patients from five ASCOT centers and used radial artery applanation tonometry and pulse-wave analysis to derive central aortic pressures and hemodynamic indices during study visits up to a period of 4 years. Although the two treatment groups achieved similar brachial systolic blood pressures, the central aortic systolic pressure was 4.3 mm Hg lower in the amlodipine group (95% CI 3.3–5.4; P < .0001), and the central aortic pulse pressure was 3.0 mm Hg lower (95% CI 2.1–3.9; P < .0001).
Pulse-wave dyssynchrony
Bangalore et al47 offer an interesting hypothesis to explain the probable adverse effect of beta-blockers. Their theory concerns the effect of these drugs on the arterial pulse wave.
Normally, with each contraction of the left ventricle during systole, an arterial pulse wave is generated and propagated forward to the peripheral arteries. This wave is then reflected back to the heart from the branching points of peripheral arteries. The final form of the pressure wave at the aortic root is a synchronized summation of the forward-traveling wave and the backward-reflected wave.
In healthy people with normal arteries, the reflected wave merges with the forward-traveling wave in diastole and augments coronary blood flow. In patients whose arteries are stiff due to aging or vascular comorbidities, the reflected wave returns faster and merges with the incident wave in systole, resulting in higher left ventricular afterload and less coronary perfusion.48
Bangalore et al47 propose that artificially reducing the heart rate with beta-blockers may further dyssynchronize the pulse wave, adversely affecting coronary perfusion and leading to an increased risk of cardiovascular events and death.
Metabolic side effects
Older beta-blockers, and especially atenolol, have well-known metabolic adverse effects, particularly impairment of glycemic control. This adverse effect appears to occur only with beta-blockers that do not possess vasodilatory properties and thus increase peripheral vascular resistance, which results in lower glucose availability and reduced uptake by skeletal muscles.49
Bangalore et al50 evaluated the effect of beta-blockers in a meta-analysis of 12 studies in 94,492 patients followed up for more than 1 year. Beta-blocker therapy resulted in a 22% higher risk of new-onset diabetes mellitus (RR 1.22, 95% CI 1.12–1.33) than with other nondiuretic antihypertensive agents.
Of note, however, the meta-analysis did not show a significantly higher risk of the onset of diabetes with propranolol or metoprolol than with other nondiuretic antihypertensives when studies of these beta-blockers were separated from atenolol-based studies.
Further, the United Kingdom Prospective Diabetes Study40 found that cardiovascular outcomes in patients with good blood pressure control were similar when atenolol-based therapy was compared with therapy with the ACE inhibitor captopril (Capoten).
A meta-analysis conducted by Balamuthusamy et al51 in 2009 found no higher risk of stroke in patients with hypertension and diabetes mellitus who received beta-blockers than in those who received other antihypertensive medications. However, beta-blockers were associated with a higher risk of death from cardiovascular causes (RR 1.39, 95% CI 1.07–1.804; P < .01) compared with reninangiotensin blockade.
NEWER BETA-BLOCKERS MAY BE BETTER
In the United States, more than 40 million prescriptions for atenolol are written every year, making it by far the most commonly used beta-blocker for the treatment of hypertension. 52 It is clear, however, that atenolol is not an ideal representative of this class of antihypertensive medications.
Preliminary data from studies of newer beta-blockers that possess beneficial vasodilatory properties are encouraging. Animal studies and preliminary human studies find that these new-generation beta-blockers cause fewer adverse metabolic effects and improve endothelial function, measures of arterial stiffness, and cardiovascular outcomes.
Carvedilol
Carvedilol is a nonselective beta-blocker with vasodilatory effects that are thought to be due to its ability to concurrently block alpha-1 receptors in addition to beta receptors. 53 In experiments in vitro and in trials in patients with diabetes and hypertension, carvedilol increased endothelial vasodilation and reduced inflammation and platelet aggregation. These effects may be achieved though antioxidant actions, thereby preserving nitric oxide bioactivity.54,55
In the Glycemic Effects in Diabetes Mellitus: Carvedilol-Metoprolol Comparison in Hypertensives (GEMINI) trial,56 carvedilol was associated with better maintenance of glycemic control in diabetic hypertensive patients than was metoprolol. Insulin sensitivity improved with carvedilol but not with metoprolol, and fewer patients on carvedilol progressed to microalbuminuria.
Nebivolol
Nebivolol is a novel selective beta-blocker with a much higher affinity for beta-1 adrenergic receptors than for beta-2 adrenergic receptors. Among all the beta-blockers in clinical use today, nebivolol has the highest selectivity for beta-1 receptors.8
Nebivolol causes vasodilation through activation of the l-arginine/nitric oxide pathway.57–59 Blockade of synthesis of nitric oxide leads to local arterial stiffness. Endothelial dysfunction is characterized by decreased bioavailability of nitric oxide and has been shown to be a strong predictor of cardiovascular outcomes. By generating nitric oxide, nebivolol reduces peripheral vascular resistance, overcoming a significant side effect of earlier beta-blockers that lowered blood pressure but ultimately increased peripheral vascular tone and resistance.8
In an experiment in a bovine model,60 nebivolol significantly reduced the pulse-wave velocity (a measure of arterial stiffness), while atenolol had no effect. Moreover, evidence for the role of the l-arginine/nitric oxide pathway in the vasodilatory effect of nebivolol was demonstrated by co-infusion of NG-monomethyl-L-arginine, a specific endothelial nitric oxide synthetase inhibitor that attenuated the reduction of pulse-wave velocity by nebivolol.
In studies in hypertensive patients, nebivolol was associated with a better metabolic profile than atenolol, with none of the adverse effects on insulin sensitivity that atenolol had.61 In the Study of Effects of Nebivolol Interventions on Outcomes and Rehospitalization in Seniors With Heart Failure (SENIORS) trial, significantly fewer patients receiving nebivolol died or were admitted to the hospital for cardiovascular reasons compared with those receiving placebo.62
Although these findings are encouraging, we do not yet know if these effects will translate into a significant reduction in cardiovascular outcomes in clinical trials. Large, prospective hypertension outcome trials, particularly to evaluate primary prevention of cardiovascular outcomes, are needed for an evidence-based approach to using the newer beta-blockers as preferred first-line therapy for hypertension.
WHAT RECENT GUIDELINES SAY ABOUT BETA-BLOCKERS
The British National Institute for Health and Clinical Excellence and the British Hypertension Society, in their 2004 guidelines, recommended beta-blockers as one of several first-line antihypertensive medications in young, nonblack patients.63 On the other hand, they advised clinicians to be aware of the reported increase in onset of diabetes mellitus in patients treated with these medications. After the LIFE24 and ASCOT26 study results were published, these guidelines were amended to exclude beta-blockers as preferred routine initial therapy for hypertension.64
More recently, the 2007 European Society of Hypertension and European Society of Cardiology reconsidered the role of beta-blockers, recommending them as an option in both initial and subsequent antihypertensive treatment strategies.65
The current guidelines from the National Heart, Lung, and Blood Institute,66 which were published in 2003, were highly influenced by the results of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT),2 and favor diuretics as the first-line therapy. However, they indicate that beta-blockers are a suitable alternative, particularly when a compelling cardiac indication is present.53 We hope that the next update, expected late in 2009, will re-address this issue in the light of more recent data.
- Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet 2001; 358:1305–1315.
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288:2981–2997.
- Neutel JM, Smith DH, Ram CV, et al. Application of ambulatory blood pressure monitoring in differentiating between antihypertensive agents. Am J Med 1993; 94:181–187.
- Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med 1993; 328:914–921.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Frishman W, Silverman R. Clinical pharmacology of the new beta-adrenergic blocking drugs. Part 2. Physiologic and metabolic effects. Am Heart J 1979; 97:797–807.
- Garrett BN, Kaplan NM. Plasma renin activity suppression: duration after withdrawal from beta-adrenergic blockade. Arch Intern Med 1980; 140:1316–1318.
- Pedersen ME, Cockcroft JR. The latest generation of beta-blockers: new pharmacologic properties. Curr Hypertens Rep 2006; 8:279–286.
- Man in’t Veld AJ, Van den Meiracker AH, Schalekamp MA. Do beta-blockers really increase peripheral vascular resistance? Review of the literature and new observations under basal conditions. Am J Hypertens 1988; 1:91–96.
- Pearce CJ, Wallin JD. Labetalol and other agents that block both alpha- and beta-adrenergic receptors. Cleve Clin J Med 1994; 61:59–69.
- Dimsdale JE, Newton RP, Joist T. Neuropsychological side effects of beta-blockers. Arch Intern Med 1989; 149:514–525.
- Agarwal R. Supervised atenolol therapy in the management of hemodialysis hypertension. Kidney Int 1999; 55:1528–1535.
- Sica DA, Black HR. Pharmacologic considerations in the positioning of beta-blockers in antihypertensive therapy. Curr Hypertens Rep 2008; 10:330–335.
- Prichard BN, Gillam GP. Use of propranolol (Inderal) in treatment of hypertension. Br Med J 1964; 19; 2:725–727.
- The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V). Arch Intern Med 1993; 153:154–183.
- Moser M. Evolution of the treatment of hypertension from the 1940s to JNC V. Am J Hypertens 1997; 10:2S–8S.
- Houghton T, Freemantle N, Cleland JG. Are beta-blockers effective in patients who develop heart failure soon after myocardial infarction? A meta-regression analysis of randomised trials. Eur J Heart Fail 2000; 2:333–340.
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999; 353:9–13.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 1985; 27:335–371.
- Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med 2001; 134:550–560.
- Wiysonge CS, Bradley H, Mayosi BM, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev 2007;CD002003.
- Messerli FH, Grossman E, Goldbourt U. Are beta-blockers efficacious as first-line therapy for hypertension in the elderly? A systematic review. JAMA 1998; 279:1903–1907.
- Dahlöf B, Devereux RB, Kjeldsen SE, et al; for the LIFE study group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint Reduction in Hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–1003.
- Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice? Lancet 2004; 364:1684–1689.
- Dahlöf B, Sever PS, Poulter NR, et al; ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005; 366:895–906.
- Lindholm LH, Carlberg B, Samuelsson O. Should beta-blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005; 366:1545–1553.
- Neutel JM, Schnaper H, Cheung DG, Graettinger WF, Weber MA. Antihypertensive effects of beta-blockers administered once daily: 24-hour measurements. Am Heart J 1990; 120:166–171.
- Franklin SS, Gustin W, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997; 96:308–315.
- The IPPPSH Collaborative Group. Cardiovascular risk and risk factors in a randomized trial of treatment based on the beta-blocker oxprenolol: the International Prospective Primary Prevention Study in Hypertension (IPPPSH). J Hypertens 1985; 3:379–392.
- Williams B, Lacy PS, Thom SM, et al; CAFE Investigators. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006; 113:1213–1225.
- Khan N, McAlister FA. Re-examining the efficacy of beta-blockers for the treatment of hypertension: a meta-analysis. CMAJ 2006; 174:1737–1742.
- Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. BMJ 1985; 291:97–104.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. BMJ 1986; 293:1145–1151.
- Dahlöf B, Lindholm LH, Hansson L, et al. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- MRC Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ 1992; 304:405–412.
- The Dutch TIA Study Group. Trial of secondary prevention with atenolol after transient ischemic attack or nondisabling ischemic stroke. Stroke 1993; 24:543–548.
- Eriksson S, Olofsson B-O, Wester P-O; for the TEST Study Group. Atenolol in secondary prevention after stroke. Cerebrovasc Dis 1995; 5:21–25.
- Wilhelmsen L, Berglund G, Elmfeldt D, et al. Beta-blockers versus diuretics in hypertensive men: main results from the HAPPHY trial. J Hypertens 1987; 5:561–572.
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317:713–720.
- Hansson L, Lindholm LH, Niskanen L, et al. Effect of angiotensin-converting enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPP) randomised trial. Lancet 1999; 353:611–616.
- Lanchetti A, Bond MG, Henning M, et al. Calcium antagonist lacidipine slow down progression of asymptomatic carotid atherosclerosis. Principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double-blind, long-term trial. Circulation 2002; 106:2422–2427.
- Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity in the Swedish Trial in Old Patients with Hypertension-2 study. Lancet 1999; 354:1751–1756.
- Hansson L, Hedner T, Lund-Johansen P, et al. Randomised trial of effects of calcium antagonists compared with diuretics and ß blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet 2000; 356:359–365.
- Pepine CJ, Handsberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003; 290:2805–2816.
- Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) Trial. JAMA 2003; 289:2073–2082.
- Bangalore S, Sawhney S, Messerli FH. Relation of beta-blocker-induced heart rate lowering and cardioprotection in hypertension. J Am Coll Cardiol 2008; 52:1482–1489.
- Boutouyrie P, Vermersch S, Laurent S, Briet M. Cardiovascular risk assessment through target organ damage: role of carotid to femoral pulse wave velocity. Clin Exp Pharmacol Physiol 2008; 35:530–533.
- Kveiborg B, Christiansen B, Major-Petersen A, Torp-Pedersen C. Metabolic effects of beta-adrenoceptor antagonists with special emphasis on carvedilol. Am J Cardiovasc Drugs 2006; 6:209–217.
- Bangalore S, Parkar S, Grossman E, Messerli FH. A meta-analysis of 94,492 patients with hypertension treated with beta-blockers to determine the risk of new-onset diabetes mellitus. Am J Cardiol 2007; 100:1254–1262.
- Balamuthusamy S, Molnar J, Adigopula S, Arora R. Comparative analysis of beta-blockers with other antihypertensive agents on cardiovascular outcomes in hypertensive patients with diabetes mellitus: a systematic review and meta-analysis. Am J Ther 2009; 16:133–142.
- Berenson A. Big drug makers see sales decline with their image. New York Times 2005 Nov 14.
- Bristow MR. Beta-adrenergic receptor blockade in chronic heart failure. Circulation 2000; 101:558–569.
- Giugliano D, Marfella R, Acampora R, Giunta R, Coppola L, D’Onofrio F. Effects of perindopril and carvedilol on endothelium-dependent vascular functions in patients with diabetes and hypertension. Diabetes Care 1998; 21:631–636.
- Lopez BL, Christopher TA, Yue TL, Ruffolo R, Feuerstein GZ, Ma XL. Carvedilol, a new beta-adrenoreceptor blocker antihypertensive drug, protects against free-radical-induced endothelial dysfunction. Pharmacology 1995; 51:165–173.
- Bakris GL, Fonseca V, Katholi RE, et al; GEMINI Investigators. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA 2004; 292:2227–2236.
- Georgescu A, Pluteanu F, Flonta ML, Badila E, Dorobantu M, Popov D. The cellular mechanisms involved in the vasodilator effect of nebivolol on the renal artery. Eur J Pharmacol 2005; 508:159–166.
- Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, et al. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation 2003; 107:2747–2752.
- Cockcroft JR, Chowienczyk PJ, Brett SE, et al. Nebivolol vasodilates human forearm vasculature: evidence for an L-arginine/NO-dependent mechanism. J Pharmacol Exp Ther 1995; 274:1067–1071.
- McEniery CM, Schmitt M, Qasem A, et al. Nebivolol increases arterial distensibility in vivo. Hypertension 2004; 44:305–310.
- Poirier L, Cleroux J, Nadeau A, Lacourciere Y. Effects of nebivolol and atenolol on insulin sensitivity and haemodynamics in hypertensive patients. J Hypertens 2001; 19:1429–1435.
- Flather MD, Shibata MC, Coats AJ, et al; SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005; 26:215–225.
- Williams B, Poulter NR, Brown MJ, et al; BHS guidelines working party, for the British Hypertension Society. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ 2004; 328:634–640.
- Sever P. New hypertension guidelines from the National Institute for Health and Clinical Excellence and the British Hypertension Society. J Renin Angiotensin Aldosterone Syst 2006; 7:61–63.
- Mancia G, De Backer G, Dominiczak A, et al; Management of Arterial Hypertension of the European Society of Hypertension. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25:1105–1187.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet 2001; 358:1305–1315.
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288:2981–2997.
- Neutel JM, Smith DH, Ram CV, et al. Application of ambulatory blood pressure monitoring in differentiating between antihypertensive agents. Am J Med 1993; 94:181–187.
- Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med 1993; 328:914–921.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Frishman W, Silverman R. Clinical pharmacology of the new beta-adrenergic blocking drugs. Part 2. Physiologic and metabolic effects. Am Heart J 1979; 97:797–807.
- Garrett BN, Kaplan NM. Plasma renin activity suppression: duration after withdrawal from beta-adrenergic blockade. Arch Intern Med 1980; 140:1316–1318.
- Pedersen ME, Cockcroft JR. The latest generation of beta-blockers: new pharmacologic properties. Curr Hypertens Rep 2006; 8:279–286.
- Man in’t Veld AJ, Van den Meiracker AH, Schalekamp MA. Do beta-blockers really increase peripheral vascular resistance? Review of the literature and new observations under basal conditions. Am J Hypertens 1988; 1:91–96.
- Pearce CJ, Wallin JD. Labetalol and other agents that block both alpha- and beta-adrenergic receptors. Cleve Clin J Med 1994; 61:59–69.
- Dimsdale JE, Newton RP, Joist T. Neuropsychological side effects of beta-blockers. Arch Intern Med 1989; 149:514–525.
- Agarwal R. Supervised atenolol therapy in the management of hemodialysis hypertension. Kidney Int 1999; 55:1528–1535.
- Sica DA, Black HR. Pharmacologic considerations in the positioning of beta-blockers in antihypertensive therapy. Curr Hypertens Rep 2008; 10:330–335.
- Prichard BN, Gillam GP. Use of propranolol (Inderal) in treatment of hypertension. Br Med J 1964; 19; 2:725–727.
- The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V). Arch Intern Med 1993; 153:154–183.
- Moser M. Evolution of the treatment of hypertension from the 1940s to JNC V. Am J Hypertens 1997; 10:2S–8S.
- Houghton T, Freemantle N, Cleland JG. Are beta-blockers effective in patients who develop heart failure soon after myocardial infarction? A meta-regression analysis of randomised trials. Eur J Heart Fail 2000; 2:333–340.
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999; 353:9–13.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 1985; 27:335–371.
- Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med 2001; 134:550–560.
- Wiysonge CS, Bradley H, Mayosi BM, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev 2007;CD002003.
- Messerli FH, Grossman E, Goldbourt U. Are beta-blockers efficacious as first-line therapy for hypertension in the elderly? A systematic review. JAMA 1998; 279:1903–1907.
- Dahlöf B, Devereux RB, Kjeldsen SE, et al; for the LIFE study group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint Reduction in Hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–1003.
- Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice? Lancet 2004; 364:1684–1689.
- Dahlöf B, Sever PS, Poulter NR, et al; ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005; 366:895–906.
- Lindholm LH, Carlberg B, Samuelsson O. Should beta-blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005; 366:1545–1553.
- Neutel JM, Schnaper H, Cheung DG, Graettinger WF, Weber MA. Antihypertensive effects of beta-blockers administered once daily: 24-hour measurements. Am Heart J 1990; 120:166–171.
- Franklin SS, Gustin W, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997; 96:308–315.
- The IPPPSH Collaborative Group. Cardiovascular risk and risk factors in a randomized trial of treatment based on the beta-blocker oxprenolol: the International Prospective Primary Prevention Study in Hypertension (IPPPSH). J Hypertens 1985; 3:379–392.
- Williams B, Lacy PS, Thom SM, et al; CAFE Investigators. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006; 113:1213–1225.
- Khan N, McAlister FA. Re-examining the efficacy of beta-blockers for the treatment of hypertension: a meta-analysis. CMAJ 2006; 174:1737–1742.
- Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. BMJ 1985; 291:97–104.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. BMJ 1986; 293:1145–1151.
- Dahlöf B, Lindholm LH, Hansson L, et al. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- MRC Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ 1992; 304:405–412.
- The Dutch TIA Study Group. Trial of secondary prevention with atenolol after transient ischemic attack or nondisabling ischemic stroke. Stroke 1993; 24:543–548.
- Eriksson S, Olofsson B-O, Wester P-O; for the TEST Study Group. Atenolol in secondary prevention after stroke. Cerebrovasc Dis 1995; 5:21–25.
- Wilhelmsen L, Berglund G, Elmfeldt D, et al. Beta-blockers versus diuretics in hypertensive men: main results from the HAPPHY trial. J Hypertens 1987; 5:561–572.
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317:713–720.
- Hansson L, Lindholm LH, Niskanen L, et al. Effect of angiotensin-converting enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPP) randomised trial. Lancet 1999; 353:611–616.
- Lanchetti A, Bond MG, Henning M, et al. Calcium antagonist lacidipine slow down progression of asymptomatic carotid atherosclerosis. Principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double-blind, long-term trial. Circulation 2002; 106:2422–2427.
- Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity in the Swedish Trial in Old Patients with Hypertension-2 study. Lancet 1999; 354:1751–1756.
- Hansson L, Hedner T, Lund-Johansen P, et al. Randomised trial of effects of calcium antagonists compared with diuretics and ß blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet 2000; 356:359–365.
- Pepine CJ, Handsberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003; 290:2805–2816.
- Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) Trial. JAMA 2003; 289:2073–2082.
- Bangalore S, Sawhney S, Messerli FH. Relation of beta-blocker-induced heart rate lowering and cardioprotection in hypertension. J Am Coll Cardiol 2008; 52:1482–1489.
- Boutouyrie P, Vermersch S, Laurent S, Briet M. Cardiovascular risk assessment through target organ damage: role of carotid to femoral pulse wave velocity. Clin Exp Pharmacol Physiol 2008; 35:530–533.
- Kveiborg B, Christiansen B, Major-Petersen A, Torp-Pedersen C. Metabolic effects of beta-adrenoceptor antagonists with special emphasis on carvedilol. Am J Cardiovasc Drugs 2006; 6:209–217.
- Bangalore S, Parkar S, Grossman E, Messerli FH. A meta-analysis of 94,492 patients with hypertension treated with beta-blockers to determine the risk of new-onset diabetes mellitus. Am J Cardiol 2007; 100:1254–1262.
- Balamuthusamy S, Molnar J, Adigopula S, Arora R. Comparative analysis of beta-blockers with other antihypertensive agents on cardiovascular outcomes in hypertensive patients with diabetes mellitus: a systematic review and meta-analysis. Am J Ther 2009; 16:133–142.
- Berenson A. Big drug makers see sales decline with their image. New York Times 2005 Nov 14.
- Bristow MR. Beta-adrenergic receptor blockade in chronic heart failure. Circulation 2000; 101:558–569.
- Giugliano D, Marfella R, Acampora R, Giunta R, Coppola L, D’Onofrio F. Effects of perindopril and carvedilol on endothelium-dependent vascular functions in patients with diabetes and hypertension. Diabetes Care 1998; 21:631–636.
- Lopez BL, Christopher TA, Yue TL, Ruffolo R, Feuerstein GZ, Ma XL. Carvedilol, a new beta-adrenoreceptor blocker antihypertensive drug, protects against free-radical-induced endothelial dysfunction. Pharmacology 1995; 51:165–173.
- Bakris GL, Fonseca V, Katholi RE, et al; GEMINI Investigators. Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA 2004; 292:2227–2236.
- Georgescu A, Pluteanu F, Flonta ML, Badila E, Dorobantu M, Popov D. The cellular mechanisms involved in the vasodilator effect of nebivolol on the renal artery. Eur J Pharmacol 2005; 508:159–166.
- Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, et al. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation 2003; 107:2747–2752.
- Cockcroft JR, Chowienczyk PJ, Brett SE, et al. Nebivolol vasodilates human forearm vasculature: evidence for an L-arginine/NO-dependent mechanism. J Pharmacol Exp Ther 1995; 274:1067–1071.
- McEniery CM, Schmitt M, Qasem A, et al. Nebivolol increases arterial distensibility in vivo. Hypertension 2004; 44:305–310.
- Poirier L, Cleroux J, Nadeau A, Lacourciere Y. Effects of nebivolol and atenolol on insulin sensitivity and haemodynamics in hypertensive patients. J Hypertens 2001; 19:1429–1435.
- Flather MD, Shibata MC, Coats AJ, et al; SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005; 26:215–225.
- Williams B, Poulter NR, Brown MJ, et al; BHS guidelines working party, for the British Hypertension Society. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ 2004; 328:634–640.
- Sever P. New hypertension guidelines from the National Institute for Health and Clinical Excellence and the British Hypertension Society. J Renin Angiotensin Aldosterone Syst 2006; 7:61–63.
- Mancia G, De Backer G, Dominiczak A, et al; Management of Arterial Hypertension of the European Society of Hypertension. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25:1105–1187.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
KEY POINTS
- No evidence exists that beta-blockers prevent first episodes of cardiovascular events in patients with hypertension, and in some trials, outcomes were worse with beta-blockers than with antihypertensive drugs of other classes.
- Younger hypertensive patients have hemodynamic characteristics that would seem to be amenable to beta-blocker therapy. However, most clinical trials of beta blockers did not stratify patients by age.
- Most trials of the antihypertensive effects of beta-blockers used atenolol (Tenormin), which is not an ideal representative of this class of drugs.
- Newer beta-blockers with vasodilatory properties may overcome the adverse effect of increased peripheral vascular resistance that occurs with older agents such as atenolol.
Vertebroplasty, evidence, and health care reform: What is quality care?
Should only evidence-based medicine be reimbursed?
In this time of intense discussion about ways to reduce health costs, and of President Obama’s desire to include efficacy and safety outcome data in the dialogue of how to deliver health services to everyone (although perhaps not every possible health service to everyone), the practical and philosophical implications of studies like these are worth pondering. Like it or not, the concept that all health care services will be paid for on demand by third-party payers is not a sustainable model of health care.
Randomized placebo-controlled trials are the cornerstone of evidence-based medicine. But at their best they provide only an approximation of the truth. Sample size is always a limitation. Patients and physicians in the office or operating suite do not always behave exactly like those in clinical trials. Yet, well-designed clinical trials are often considered to be the best we can do. Practice guidelines and US Food and Drug Administration approvals are based more on the results of randomized clinical trials and less on information from clinical registries and real-world observational outcome studies (which have technical foibles of their own). Approval for devices and procedures does not historically get the same type of regulatory scrutiny, but health care payers in the future may be less likely to cover the cost of procedures that lack proof of efficacy from rigorously conducted outcome studies. The development of quality care measures also depends on appropriate conduct and application of these trials.
The deadly sins and decision-making
We physicians generally take umbrage with external oversight of our decision-making. It is our job and our responsibility to balance the science (evidence-based medicine) and the art (experience and gestalt) of clinical care for the benefit of our patients. But as I thought about the impact these new studies may exert on vertebroplasty utilization, I also wondered about the factors that influence our decision-making process. For example, we have long had solid data on the value of treating systolic hypertension (we undertreat), and of treating uncomplicated urinary tract infections with only 3 days of antibiotics (we overtreat). Performance indicators suggest that this solid evidence has only a modest influence on practice patterns. Why?
I recently heard Dr. Louis B. Rice, Professor of Medicine at Case Western Reserve University and Chief of the Medical Service at the Louis Stokes Cleveland VA Medical Center, discuss the possible impact of some of the seven deadly sins on clinical decision-making. A similar analysis applies when thinking about why some treatments continue to be offered despite good evidence of only limited efficacy.
Pride plays a role. We believe that our own clinical skills will permit us to select the ideal patient to undergo a procedure or therapy, whereas such cherry-picking of patients does not generally occur in large clinical trials. This argument (and others of “external validity,” in the lingo of evidence-based medicine) has been put forth to defend the continued use of some procedures that may not have fared well against sham controls in clinical trials, and these procedures continue to flourish.
Pride may also apply to the feeling we physicians have for doing something right for our patients. This feeling may push us to believe we can succeed where an impersonal clinical trial failed. I suspect this is most keenly felt when the therapy is a procedure that depends on our own individual skills. I suspect that internists and subspecialists with special interest in osteoporosis will interpret these trials differently than surgeons and interventional radiologists who are routinely performing these procedures.
Avarice must be considered, and regulatory controls in the future may limit financial gain from these therapies. But I am not convinced that monetary greed drives all clinical decisions that go against the grain of evidence-based medicine.
And then there is gluttony: we and our patients want it all. We do not want to hear that our patient cannot be provided the most recent therapeutic advance—it might work.
Placebo effect, other issues in ‘negative’ studies
A number of factors in these trials of vertebroplasty need to be dissected and discussed. Not the least is the apparent salubrious effect of the sham procedure. This was documented previously with intra-articular injections of saline (placebo) in studies of hyaluronate joint injections for the pain of knee osteoarthritis,3 in which either type of injection provided significant pain relief. Are these truly markedly positive effects of the sham but invasive maneuvers in the vertebroplasty studies, or are we witnessing the natural history of pain resolution in these disorders (in the absence of a true nonintervention control group that could help make this distinction)?
Crossover issues in one of the vertebroplasty papers will certainly generate letters to the editor. Were patients really blinded to their procedure throughout? Which subsets of patients might have responded better or worse? What about balloon kyphoplasty?
We plan to publish commentaries from proceduralists and medical experts in osteoporosis to critique these key clinical trials for us and to put these issues into clinical perspective.
What role for evidence-based medicine?
In the meantime, I urge you to peruse these papers along with the op-ed pieces in your local newspapers as catalysts to reconsider the role evidence-based medicine should play in our daily one-on-one routine with patients, as well as in the redesign of our health care delivery and reimbursement systems. I don’t think that clinical conundrums can be resolved with a simple look at P values and confidence intervals; clinical trials are not the total story. As physicians, we always need to put the trial results into a clinical perspective. Nonetheless, our personal belief of efficacy (or lack of efficacy) also should not be the total story as we make decisions with individual patients and allocate resources within the health care system.
In the end, it should be all about giving high-quality care to the patient sitting in front of us. A question to be addressed is how well we can assess the quality of a given treatment prior to its administration.
- Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009; 361:569–579.
- Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009; 361:557–568.
- Lundsgaard C, Dufour N, Fallentin E, Winkel P, Gluud C. Intra-articular sodium hyaluronate 2 mL versus physiological saline 20 mL versus physiological saline 2 mL for painful knee osteoarthritis: a randomized clinical trial. Scand J Rheumatol 2008; 37:142–150.
Should only evidence-based medicine be reimbursed?
In this time of intense discussion about ways to reduce health costs, and of President Obama’s desire to include efficacy and safety outcome data in the dialogue of how to deliver health services to everyone (although perhaps not every possible health service to everyone), the practical and philosophical implications of studies like these are worth pondering. Like it or not, the concept that all health care services will be paid for on demand by third-party payers is not a sustainable model of health care.
Randomized placebo-controlled trials are the cornerstone of evidence-based medicine. But at their best they provide only an approximation of the truth. Sample size is always a limitation. Patients and physicians in the office or operating suite do not always behave exactly like those in clinical trials. Yet, well-designed clinical trials are often considered to be the best we can do. Practice guidelines and US Food and Drug Administration approvals are based more on the results of randomized clinical trials and less on information from clinical registries and real-world observational outcome studies (which have technical foibles of their own). Approval for devices and procedures does not historically get the same type of regulatory scrutiny, but health care payers in the future may be less likely to cover the cost of procedures that lack proof of efficacy from rigorously conducted outcome studies. The development of quality care measures also depends on appropriate conduct and application of these trials.
The deadly sins and decision-making
We physicians generally take umbrage with external oversight of our decision-making. It is our job and our responsibility to balance the science (evidence-based medicine) and the art (experience and gestalt) of clinical care for the benefit of our patients. But as I thought about the impact these new studies may exert on vertebroplasty utilization, I also wondered about the factors that influence our decision-making process. For example, we have long had solid data on the value of treating systolic hypertension (we undertreat), and of treating uncomplicated urinary tract infections with only 3 days of antibiotics (we overtreat). Performance indicators suggest that this solid evidence has only a modest influence on practice patterns. Why?
I recently heard Dr. Louis B. Rice, Professor of Medicine at Case Western Reserve University and Chief of the Medical Service at the Louis Stokes Cleveland VA Medical Center, discuss the possible impact of some of the seven deadly sins on clinical decision-making. A similar analysis applies when thinking about why some treatments continue to be offered despite good evidence of only limited efficacy.
Pride plays a role. We believe that our own clinical skills will permit us to select the ideal patient to undergo a procedure or therapy, whereas such cherry-picking of patients does not generally occur in large clinical trials. This argument (and others of “external validity,” in the lingo of evidence-based medicine) has been put forth to defend the continued use of some procedures that may not have fared well against sham controls in clinical trials, and these procedures continue to flourish.
Pride may also apply to the feeling we physicians have for doing something right for our patients. This feeling may push us to believe we can succeed where an impersonal clinical trial failed. I suspect this is most keenly felt when the therapy is a procedure that depends on our own individual skills. I suspect that internists and subspecialists with special interest in osteoporosis will interpret these trials differently than surgeons and interventional radiologists who are routinely performing these procedures.
Avarice must be considered, and regulatory controls in the future may limit financial gain from these therapies. But I am not convinced that monetary greed drives all clinical decisions that go against the grain of evidence-based medicine.
And then there is gluttony: we and our patients want it all. We do not want to hear that our patient cannot be provided the most recent therapeutic advance—it might work.
Placebo effect, other issues in ‘negative’ studies
A number of factors in these trials of vertebroplasty need to be dissected and discussed. Not the least is the apparent salubrious effect of the sham procedure. This was documented previously with intra-articular injections of saline (placebo) in studies of hyaluronate joint injections for the pain of knee osteoarthritis,3 in which either type of injection provided significant pain relief. Are these truly markedly positive effects of the sham but invasive maneuvers in the vertebroplasty studies, or are we witnessing the natural history of pain resolution in these disorders (in the absence of a true nonintervention control group that could help make this distinction)?
Crossover issues in one of the vertebroplasty papers will certainly generate letters to the editor. Were patients really blinded to their procedure throughout? Which subsets of patients might have responded better or worse? What about balloon kyphoplasty?
We plan to publish commentaries from proceduralists and medical experts in osteoporosis to critique these key clinical trials for us and to put these issues into clinical perspective.
What role for evidence-based medicine?
In the meantime, I urge you to peruse these papers along with the op-ed pieces in your local newspapers as catalysts to reconsider the role evidence-based medicine should play in our daily one-on-one routine with patients, as well as in the redesign of our health care delivery and reimbursement systems. I don’t think that clinical conundrums can be resolved with a simple look at P values and confidence intervals; clinical trials are not the total story. As physicians, we always need to put the trial results into a clinical perspective. Nonetheless, our personal belief of efficacy (or lack of efficacy) also should not be the total story as we make decisions with individual patients and allocate resources within the health care system.
In the end, it should be all about giving high-quality care to the patient sitting in front of us. A question to be addressed is how well we can assess the quality of a given treatment prior to its administration.
Should only evidence-based medicine be reimbursed?
In this time of intense discussion about ways to reduce health costs, and of President Obama’s desire to include efficacy and safety outcome data in the dialogue of how to deliver health services to everyone (although perhaps not every possible health service to everyone), the practical and philosophical implications of studies like these are worth pondering. Like it or not, the concept that all health care services will be paid for on demand by third-party payers is not a sustainable model of health care.
Randomized placebo-controlled trials are the cornerstone of evidence-based medicine. But at their best they provide only an approximation of the truth. Sample size is always a limitation. Patients and physicians in the office or operating suite do not always behave exactly like those in clinical trials. Yet, well-designed clinical trials are often considered to be the best we can do. Practice guidelines and US Food and Drug Administration approvals are based more on the results of randomized clinical trials and less on information from clinical registries and real-world observational outcome studies (which have technical foibles of their own). Approval for devices and procedures does not historically get the same type of regulatory scrutiny, but health care payers in the future may be less likely to cover the cost of procedures that lack proof of efficacy from rigorously conducted outcome studies. The development of quality care measures also depends on appropriate conduct and application of these trials.
The deadly sins and decision-making
We physicians generally take umbrage with external oversight of our decision-making. It is our job and our responsibility to balance the science (evidence-based medicine) and the art (experience and gestalt) of clinical care for the benefit of our patients. But as I thought about the impact these new studies may exert on vertebroplasty utilization, I also wondered about the factors that influence our decision-making process. For example, we have long had solid data on the value of treating systolic hypertension (we undertreat), and of treating uncomplicated urinary tract infections with only 3 days of antibiotics (we overtreat). Performance indicators suggest that this solid evidence has only a modest influence on practice patterns. Why?
I recently heard Dr. Louis B. Rice, Professor of Medicine at Case Western Reserve University and Chief of the Medical Service at the Louis Stokes Cleveland VA Medical Center, discuss the possible impact of some of the seven deadly sins on clinical decision-making. A similar analysis applies when thinking about why some treatments continue to be offered despite good evidence of only limited efficacy.
Pride plays a role. We believe that our own clinical skills will permit us to select the ideal patient to undergo a procedure or therapy, whereas such cherry-picking of patients does not generally occur in large clinical trials. This argument (and others of “external validity,” in the lingo of evidence-based medicine) has been put forth to defend the continued use of some procedures that may not have fared well against sham controls in clinical trials, and these procedures continue to flourish.
Pride may also apply to the feeling we physicians have for doing something right for our patients. This feeling may push us to believe we can succeed where an impersonal clinical trial failed. I suspect this is most keenly felt when the therapy is a procedure that depends on our own individual skills. I suspect that internists and subspecialists with special interest in osteoporosis will interpret these trials differently than surgeons and interventional radiologists who are routinely performing these procedures.
Avarice must be considered, and regulatory controls in the future may limit financial gain from these therapies. But I am not convinced that monetary greed drives all clinical decisions that go against the grain of evidence-based medicine.
And then there is gluttony: we and our patients want it all. We do not want to hear that our patient cannot be provided the most recent therapeutic advance—it might work.
Placebo effect, other issues in ‘negative’ studies
A number of factors in these trials of vertebroplasty need to be dissected and discussed. Not the least is the apparent salubrious effect of the sham procedure. This was documented previously with intra-articular injections of saline (placebo) in studies of hyaluronate joint injections for the pain of knee osteoarthritis,3 in which either type of injection provided significant pain relief. Are these truly markedly positive effects of the sham but invasive maneuvers in the vertebroplasty studies, or are we witnessing the natural history of pain resolution in these disorders (in the absence of a true nonintervention control group that could help make this distinction)?
Crossover issues in one of the vertebroplasty papers will certainly generate letters to the editor. Were patients really blinded to their procedure throughout? Which subsets of patients might have responded better or worse? What about balloon kyphoplasty?
We plan to publish commentaries from proceduralists and medical experts in osteoporosis to critique these key clinical trials for us and to put these issues into clinical perspective.
What role for evidence-based medicine?
In the meantime, I urge you to peruse these papers along with the op-ed pieces in your local newspapers as catalysts to reconsider the role evidence-based medicine should play in our daily one-on-one routine with patients, as well as in the redesign of our health care delivery and reimbursement systems. I don’t think that clinical conundrums can be resolved with a simple look at P values and confidence intervals; clinical trials are not the total story. As physicians, we always need to put the trial results into a clinical perspective. Nonetheless, our personal belief of efficacy (or lack of efficacy) also should not be the total story as we make decisions with individual patients and allocate resources within the health care system.
In the end, it should be all about giving high-quality care to the patient sitting in front of us. A question to be addressed is how well we can assess the quality of a given treatment prior to its administration.
- Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009; 361:569–579.
- Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009; 361:557–568.
- Lundsgaard C, Dufour N, Fallentin E, Winkel P, Gluud C. Intra-articular sodium hyaluronate 2 mL versus physiological saline 20 mL versus physiological saline 2 mL for painful knee osteoarthritis: a randomized clinical trial. Scand J Rheumatol 2008; 37:142–150.
- Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009; 361:569–579.
- Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009; 361:557–568.
- Lundsgaard C, Dufour N, Fallentin E, Winkel P, Gluud C. Intra-articular sodium hyaluronate 2 mL versus physiological saline 20 mL versus physiological saline 2 mL for painful knee osteoarthritis: a randomized clinical trial. Scand J Rheumatol 2008; 37:142–150.
Tinea Cruris in Children
Fungal Foes: Rhinosporidium seeberi
That time of year: Turn back the clock, watch H1N1 flu return, and adopt a new ICD-9 code set
Additions and revision to this year’s International Classification of Diseases, Clinical Modification (ICD-9-CM)—which go into effect on October 1—reflect tinkering with existing codes and expansion of others to boost granularity and clarity in your reporting of diagnostic work. To that add a number of new codes—including one that acknowledges the arrival of the H1N1 (swine flu) virus nationwide.
In obstetrics, there are now specific codes for different types of puerperal infection and a requirement for more diagnostic information when a patient has venous complications during pregnancy and intrapartum.
On the gynecology side, changes include the way you report a finding of endometrial intraepithelial neoplasia. New codes have been created to report:
- visits and procedures for fertility preservation
- inconclusive mammography
- preprocedural laboratory testing.
Remember: On October 1, 2009, the new and revised codes discussed here, plus others, will be added to the national ICD-9-CM code set. Be cautioned that, as in past years, there is no grace period!
Changes to obstetric codes
PUERPERAL INFECTIONS
Before October 1, 2009, all puerperal infections were lumped into one code: 670.0 (Major puerperal infection). This changes now: You’ll be required to document, more specifically, the type of infection that your patient has.
Continue to report code 670.0 for an unspecified puerperal infection; but, if you admit the patient to the hospital, using that unspecified code may lead to a first-submission denial of claim. A fifth digit is also required for the unspecified and new more specific codes: 0 (unspecified as to episode of care or not applicable), 2 (delivered with mention of postpartum complication), or 4 (postpartum condition or complication) (to be reported only once the patient is discharged after delivery).
670.1x [0,2,4] Puerperal endometritis
670.2x [0,2,4] Puerperal sepsis
670.3x [0,2,4] Puerperal septic thrombophlebitis
670.8x [0,2,4] Other major puerperal infection
VENOUS COMPLICATIONS IN PREGNANCY AND PUERPERIUM
Code category 671 (venous complications in pregnancy and the puerperium) retains its current codes, but ICD-9 has added notes to clarify that additional information is required.
For example: When a patient has deep-vein thrombosis, either antepartum (671.3x) or postpartum (671.4x), assign a secondary diagnosis from code category 453 (Other venous embolism and thrombosis). If, in addition, the patient has been taking an anticoagulant for a long time and is currently taking it, report code V56.81, as well, to indicate this.
Gyn code changes
HYPERPLASIA
Over time, codes for hyperplasia have evolved from a system that described mild, moderate, severe, or atypical, to one in which hyperplasia was subdivided by architectural complexity, such as simple versus complex and whether or not atypia were present. Even this terminology fails, however, to adequately identify patients’ risk of cancer to improve therapeutic triaging.
In more recent years, physicians and pathologists have begun to distinguish benign hormonal effects of unopposed estrogen, classified as benign hyperplasia, from pre-cancerous lesions classified as endometrial intraepithelial neoplasia (EIN). To capture this newer terminology, ICD-9 has added two new codes.
ICD-9 has elected to retain existing codes in this area of diagnosis and assessment because the old terminology is still used by many older practicing physicians. The hope, however, is that, over time, more accurate distinctions between the types of hyperplasia will replace the older distinctions.
A note in ICD-9 will instruct providers that older codes may not be reported if one of the newer codes is assigned.
An additional note that accompanies the EIN diagnosis indicates that, if a patient is given a diagnosis of malignant neoplasm of the endometrium with endometrial intraepithelial neoplasia, the code for the malignancy (182.0, Malignant neoplasm of body of uterus; corpus uteri, except isthmus) would be reported instead of the EIN code.
621.34 Benign endometrial hyperplasia
621.35 Endometrial intraepithelial neoplasia
Routine mammograms are, as you know, sometimes labeled “inconclusive” because of what are termed “dense breasts.” This finding isn’t considered to represent an abnormal condition, but it does require further testing to confirm that no malignant condition exists that cannot be seen on mammogram.
Because many payers cover a repeat mammogram only when an abnormal finding is reported, a new code has been needed—and has now been added—to explain the reason for a second mammogram.
Because of the added code, ICD-9 also decided to revise wording for the 793 code category (until now, it’s been Nonspecific abnormal findings on radiological and other examination of body structure) to a more general heading of Nonspecific findings, which covers inconclusive and abnormal findings.
793.82 Inconclusive mammogram
FERTILITY PRESERVATION PRIOR TO ANTINEOPLASTIC THERAPY
Two new codes have been added to this area of practice at the request of the American Society for Reproductive Medicine (ASRM) and ACOG. They allow you to report visits and procedures aimed at preserving fertility in women who must undergo chemotherapy, surgery, or radiation therapy that might otherwise leave them sterile.
The codes reflect that, before a patient is treated, you may discuss a range of options that can increase her chances of becoming pregnant, including:
- conception before cancer treatment
- banking of sperm, eggs, ovarian tissue, and embryos
- protecting the ovaries during radiation therapy
- modifying surgery to spare the uterus.
V26.42 Encounter for fertility preservation counseling
V26.82 Encounter for fertility preservation procedure
PREPROCEDURAL EVALUATIONS
Code category V72.6 has been expanded from four to five digits to better capture reasons for ordering or performing laboratory tests that are not specifically linked to a medical diagnosis.
For example: If you order routine tests as part of a routine, general medical or gyn annual examination, report code V72.62. For routine preoperative lab tests, report V72.63 instead.
ICD-9 has clarified that V72.61 can be reported for testing of immune status, and that current code V72.83 (Other specified pre-operative examination) is the one to report when an exam precedes chemotherapy.
Note: ICD-9 rules require that you list the preprocedural examination code as the primary diagnosis, followed by the code that represents the reason for the surgery or procedure.
V72.60 Laboratory examination, unspecified
V72.61 Antibody response examination
V72.62 Laboratory examination ordered as part of a routine general medical examination
V72.63 Preprocedural laboratory examination
V72.69 Other laboratory examination
PERSONAL HISTORY CODES
A history of drug therapy can affect the care that you are giving a patient now, and may require testing from time to time to assess the consequences of such therapy.
Two examples are long-term estrogen therapy, which may increase a woman’s risk of developing breast cancer, and inhaled steroids, which can decrease bone density. In the absence of a known problem with these (or other) therapies in a given patient, new history codes listed below may be useful in communicating with a payer about ongoing follow-up care or testing that you are providing.
V87.43 Personal history of estrogen therapy
V87.44 Personal history of inhaled steroid therapy
V87.45 Personal history of systemic steroid therapy
V87.46 Personal history of immunosuppressive therapy
Plus a number of miscellaneous additions and changes
Here are few more new codes that may better explain why you saw a patient, provided:
- the new code for swine flu is reported only for a confirmed case, per ICD-9 rules
- the new V codes are reported only if the personal history or family circumstance affected treatment at the time of the visit, or if the patient was receiving counseling concerning only those issues.
995.24 Failed moderate sedation during procedure
V10.90 Personal history of unspecified type of malignant neoplasm
V15.80 Personal history of failed moderate sedation
V61.07 Family disruption due to death of family member
V61.08 Family disruption due to other extended absence of a family member
V61.42 Substance abuse in family
Additions and revision to this year’s International Classification of Diseases, Clinical Modification (ICD-9-CM)—which go into effect on October 1—reflect tinkering with existing codes and expansion of others to boost granularity and clarity in your reporting of diagnostic work. To that add a number of new codes—including one that acknowledges the arrival of the H1N1 (swine flu) virus nationwide.
In obstetrics, there are now specific codes for different types of puerperal infection and a requirement for more diagnostic information when a patient has venous complications during pregnancy and intrapartum.
On the gynecology side, changes include the way you report a finding of endometrial intraepithelial neoplasia. New codes have been created to report:
- visits and procedures for fertility preservation
- inconclusive mammography
- preprocedural laboratory testing.
Remember: On October 1, 2009, the new and revised codes discussed here, plus others, will be added to the national ICD-9-CM code set. Be cautioned that, as in past years, there is no grace period!
Changes to obstetric codes
PUERPERAL INFECTIONS
Before October 1, 2009, all puerperal infections were lumped into one code: 670.0 (Major puerperal infection). This changes now: You’ll be required to document, more specifically, the type of infection that your patient has.
Continue to report code 670.0 for an unspecified puerperal infection; but, if you admit the patient to the hospital, using that unspecified code may lead to a first-submission denial of claim. A fifth digit is also required for the unspecified and new more specific codes: 0 (unspecified as to episode of care or not applicable), 2 (delivered with mention of postpartum complication), or 4 (postpartum condition or complication) (to be reported only once the patient is discharged after delivery).
670.1x [0,2,4] Puerperal endometritis
670.2x [0,2,4] Puerperal sepsis
670.3x [0,2,4] Puerperal septic thrombophlebitis
670.8x [0,2,4] Other major puerperal infection
VENOUS COMPLICATIONS IN PREGNANCY AND PUERPERIUM
Code category 671 (venous complications in pregnancy and the puerperium) retains its current codes, but ICD-9 has added notes to clarify that additional information is required.
For example: When a patient has deep-vein thrombosis, either antepartum (671.3x) or postpartum (671.4x), assign a secondary diagnosis from code category 453 (Other venous embolism and thrombosis). If, in addition, the patient has been taking an anticoagulant for a long time and is currently taking it, report code V56.81, as well, to indicate this.
Gyn code changes
HYPERPLASIA
Over time, codes for hyperplasia have evolved from a system that described mild, moderate, severe, or atypical, to one in which hyperplasia was subdivided by architectural complexity, such as simple versus complex and whether or not atypia were present. Even this terminology fails, however, to adequately identify patients’ risk of cancer to improve therapeutic triaging.
In more recent years, physicians and pathologists have begun to distinguish benign hormonal effects of unopposed estrogen, classified as benign hyperplasia, from pre-cancerous lesions classified as endometrial intraepithelial neoplasia (EIN). To capture this newer terminology, ICD-9 has added two new codes.
ICD-9 has elected to retain existing codes in this area of diagnosis and assessment because the old terminology is still used by many older practicing physicians. The hope, however, is that, over time, more accurate distinctions between the types of hyperplasia will replace the older distinctions.
A note in ICD-9 will instruct providers that older codes may not be reported if one of the newer codes is assigned.
An additional note that accompanies the EIN diagnosis indicates that, if a patient is given a diagnosis of malignant neoplasm of the endometrium with endometrial intraepithelial neoplasia, the code for the malignancy (182.0, Malignant neoplasm of body of uterus; corpus uteri, except isthmus) would be reported instead of the EIN code.
621.34 Benign endometrial hyperplasia
621.35 Endometrial intraepithelial neoplasia
Routine mammograms are, as you know, sometimes labeled “inconclusive” because of what are termed “dense breasts.” This finding isn’t considered to represent an abnormal condition, but it does require further testing to confirm that no malignant condition exists that cannot be seen on mammogram.
Because many payers cover a repeat mammogram only when an abnormal finding is reported, a new code has been needed—and has now been added—to explain the reason for a second mammogram.
Because of the added code, ICD-9 also decided to revise wording for the 793 code category (until now, it’s been Nonspecific abnormal findings on radiological and other examination of body structure) to a more general heading of Nonspecific findings, which covers inconclusive and abnormal findings.
793.82 Inconclusive mammogram
FERTILITY PRESERVATION PRIOR TO ANTINEOPLASTIC THERAPY
Two new codes have been added to this area of practice at the request of the American Society for Reproductive Medicine (ASRM) and ACOG. They allow you to report visits and procedures aimed at preserving fertility in women who must undergo chemotherapy, surgery, or radiation therapy that might otherwise leave them sterile.
The codes reflect that, before a patient is treated, you may discuss a range of options that can increase her chances of becoming pregnant, including:
- conception before cancer treatment
- banking of sperm, eggs, ovarian tissue, and embryos
- protecting the ovaries during radiation therapy
- modifying surgery to spare the uterus.
V26.42 Encounter for fertility preservation counseling
V26.82 Encounter for fertility preservation procedure
PREPROCEDURAL EVALUATIONS
Code category V72.6 has been expanded from four to five digits to better capture reasons for ordering or performing laboratory tests that are not specifically linked to a medical diagnosis.
For example: If you order routine tests as part of a routine, general medical or gyn annual examination, report code V72.62. For routine preoperative lab tests, report V72.63 instead.
ICD-9 has clarified that V72.61 can be reported for testing of immune status, and that current code V72.83 (Other specified pre-operative examination) is the one to report when an exam precedes chemotherapy.
Note: ICD-9 rules require that you list the preprocedural examination code as the primary diagnosis, followed by the code that represents the reason for the surgery or procedure.
V72.60 Laboratory examination, unspecified
V72.61 Antibody response examination
V72.62 Laboratory examination ordered as part of a routine general medical examination
V72.63 Preprocedural laboratory examination
V72.69 Other laboratory examination
PERSONAL HISTORY CODES
A history of drug therapy can affect the care that you are giving a patient now, and may require testing from time to time to assess the consequences of such therapy.
Two examples are long-term estrogen therapy, which may increase a woman’s risk of developing breast cancer, and inhaled steroids, which can decrease bone density. In the absence of a known problem with these (or other) therapies in a given patient, new history codes listed below may be useful in communicating with a payer about ongoing follow-up care or testing that you are providing.
V87.43 Personal history of estrogen therapy
V87.44 Personal history of inhaled steroid therapy
V87.45 Personal history of systemic steroid therapy
V87.46 Personal history of immunosuppressive therapy
Plus a number of miscellaneous additions and changes
Here are few more new codes that may better explain why you saw a patient, provided:
- the new code for swine flu is reported only for a confirmed case, per ICD-9 rules
- the new V codes are reported only if the personal history or family circumstance affected treatment at the time of the visit, or if the patient was receiving counseling concerning only those issues.
995.24 Failed moderate sedation during procedure
V10.90 Personal history of unspecified type of malignant neoplasm
V15.80 Personal history of failed moderate sedation
V61.07 Family disruption due to death of family member
V61.08 Family disruption due to other extended absence of a family member
V61.42 Substance abuse in family
Additions and revision to this year’s International Classification of Diseases, Clinical Modification (ICD-9-CM)—which go into effect on October 1—reflect tinkering with existing codes and expansion of others to boost granularity and clarity in your reporting of diagnostic work. To that add a number of new codes—including one that acknowledges the arrival of the H1N1 (swine flu) virus nationwide.
In obstetrics, there are now specific codes for different types of puerperal infection and a requirement for more diagnostic information when a patient has venous complications during pregnancy and intrapartum.
On the gynecology side, changes include the way you report a finding of endometrial intraepithelial neoplasia. New codes have been created to report:
- visits and procedures for fertility preservation
- inconclusive mammography
- preprocedural laboratory testing.
Remember: On October 1, 2009, the new and revised codes discussed here, plus others, will be added to the national ICD-9-CM code set. Be cautioned that, as in past years, there is no grace period!
Changes to obstetric codes
PUERPERAL INFECTIONS
Before October 1, 2009, all puerperal infections were lumped into one code: 670.0 (Major puerperal infection). This changes now: You’ll be required to document, more specifically, the type of infection that your patient has.
Continue to report code 670.0 for an unspecified puerperal infection; but, if you admit the patient to the hospital, using that unspecified code may lead to a first-submission denial of claim. A fifth digit is also required for the unspecified and new more specific codes: 0 (unspecified as to episode of care or not applicable), 2 (delivered with mention of postpartum complication), or 4 (postpartum condition or complication) (to be reported only once the patient is discharged after delivery).
670.1x [0,2,4] Puerperal endometritis
670.2x [0,2,4] Puerperal sepsis
670.3x [0,2,4] Puerperal septic thrombophlebitis
670.8x [0,2,4] Other major puerperal infection
VENOUS COMPLICATIONS IN PREGNANCY AND PUERPERIUM
Code category 671 (venous complications in pregnancy and the puerperium) retains its current codes, but ICD-9 has added notes to clarify that additional information is required.
For example: When a patient has deep-vein thrombosis, either antepartum (671.3x) or postpartum (671.4x), assign a secondary diagnosis from code category 453 (Other venous embolism and thrombosis). If, in addition, the patient has been taking an anticoagulant for a long time and is currently taking it, report code V56.81, as well, to indicate this.
Gyn code changes
HYPERPLASIA
Over time, codes for hyperplasia have evolved from a system that described mild, moderate, severe, or atypical, to one in which hyperplasia was subdivided by architectural complexity, such as simple versus complex and whether or not atypia were present. Even this terminology fails, however, to adequately identify patients’ risk of cancer to improve therapeutic triaging.
In more recent years, physicians and pathologists have begun to distinguish benign hormonal effects of unopposed estrogen, classified as benign hyperplasia, from pre-cancerous lesions classified as endometrial intraepithelial neoplasia (EIN). To capture this newer terminology, ICD-9 has added two new codes.
ICD-9 has elected to retain existing codes in this area of diagnosis and assessment because the old terminology is still used by many older practicing physicians. The hope, however, is that, over time, more accurate distinctions between the types of hyperplasia will replace the older distinctions.
A note in ICD-9 will instruct providers that older codes may not be reported if one of the newer codes is assigned.
An additional note that accompanies the EIN diagnosis indicates that, if a patient is given a diagnosis of malignant neoplasm of the endometrium with endometrial intraepithelial neoplasia, the code for the malignancy (182.0, Malignant neoplasm of body of uterus; corpus uteri, except isthmus) would be reported instead of the EIN code.
621.34 Benign endometrial hyperplasia
621.35 Endometrial intraepithelial neoplasia
Routine mammograms are, as you know, sometimes labeled “inconclusive” because of what are termed “dense breasts.” This finding isn’t considered to represent an abnormal condition, but it does require further testing to confirm that no malignant condition exists that cannot be seen on mammogram.
Because many payers cover a repeat mammogram only when an abnormal finding is reported, a new code has been needed—and has now been added—to explain the reason for a second mammogram.
Because of the added code, ICD-9 also decided to revise wording for the 793 code category (until now, it’s been Nonspecific abnormal findings on radiological and other examination of body structure) to a more general heading of Nonspecific findings, which covers inconclusive and abnormal findings.
793.82 Inconclusive mammogram
FERTILITY PRESERVATION PRIOR TO ANTINEOPLASTIC THERAPY
Two new codes have been added to this area of practice at the request of the American Society for Reproductive Medicine (ASRM) and ACOG. They allow you to report visits and procedures aimed at preserving fertility in women who must undergo chemotherapy, surgery, or radiation therapy that might otherwise leave them sterile.
The codes reflect that, before a patient is treated, you may discuss a range of options that can increase her chances of becoming pregnant, including:
- conception before cancer treatment
- banking of sperm, eggs, ovarian tissue, and embryos
- protecting the ovaries during radiation therapy
- modifying surgery to spare the uterus.
V26.42 Encounter for fertility preservation counseling
V26.82 Encounter for fertility preservation procedure
PREPROCEDURAL EVALUATIONS
Code category V72.6 has been expanded from four to five digits to better capture reasons for ordering or performing laboratory tests that are not specifically linked to a medical diagnosis.
For example: If you order routine tests as part of a routine, general medical or gyn annual examination, report code V72.62. For routine preoperative lab tests, report V72.63 instead.
ICD-9 has clarified that V72.61 can be reported for testing of immune status, and that current code V72.83 (Other specified pre-operative examination) is the one to report when an exam precedes chemotherapy.
Note: ICD-9 rules require that you list the preprocedural examination code as the primary diagnosis, followed by the code that represents the reason for the surgery or procedure.
V72.60 Laboratory examination, unspecified
V72.61 Antibody response examination
V72.62 Laboratory examination ordered as part of a routine general medical examination
V72.63 Preprocedural laboratory examination
V72.69 Other laboratory examination
PERSONAL HISTORY CODES
A history of drug therapy can affect the care that you are giving a patient now, and may require testing from time to time to assess the consequences of such therapy.
Two examples are long-term estrogen therapy, which may increase a woman’s risk of developing breast cancer, and inhaled steroids, which can decrease bone density. In the absence of a known problem with these (or other) therapies in a given patient, new history codes listed below may be useful in communicating with a payer about ongoing follow-up care or testing that you are providing.
V87.43 Personal history of estrogen therapy
V87.44 Personal history of inhaled steroid therapy
V87.45 Personal history of systemic steroid therapy
V87.46 Personal history of immunosuppressive therapy
Plus a number of miscellaneous additions and changes
Here are few more new codes that may better explain why you saw a patient, provided:
- the new code for swine flu is reported only for a confirmed case, per ICD-9 rules
- the new V codes are reported only if the personal history or family circumstance affected treatment at the time of the visit, or if the patient was receiving counseling concerning only those issues.
995.24 Failed moderate sedation during procedure
V10.90 Personal history of unspecified type of malignant neoplasm
V15.80 Personal history of failed moderate sedation
V61.07 Family disruption due to death of family member
V61.08 Family disruption due to other extended absence of a family member
V61.42 Substance abuse in family
Pandemic and seasonal flu: What you need to know
This coming flu season will be interesting—and confusing. As of August 6, 2009, the Centers for Disease Control and Prevention (CDC) reported 6506 hospitalized cases and 436 deaths from the pandemic H1N1 flu virus since the first US cases were reported in April 2009.1 (Reporting on individual confirmed and probable cases has been discontinued.) On July 31, the World Health Organization reported pandemic influenza in 168 countries, with 162,380 reported cases and 1154 deaths.2 At the same time the pandemic was developing, the seasonal flu of 2009—a relatively mild year—was tapering off. The pandemic influenza has continued to cause widespread disease in the United States throughout the summer, a somewhat unusual pattern for influenza.
So far, pandemic H1N1 flu is relatively benign, treatable
The pandemic virus, though highly infectious, has had a low case fatality rate up to now. Deaths have occurred predominantly in those with underlying medical conditions that put them at high risk of infection. Attack rates for those older than age 65 have been lower than expected, indicating that this age group may have some immunity based on past infection. The pandemic virus so far has been sensitive to both oseltamivir (Tamiflu) and zanamivir (Relenza). The resistance patterns of the key viruses from last flu season showed that the H1N1 seasonal virus was resistant to oseltamivir but sensitive to zanamivir and the adamantanes (rimantadine and amantadine), while the H3N2 virus that circulated last year was sensitive to oseltamivir.3
Fall flu season: Be prepared
So, what can you expect this fall? With pandemic H1N1 still causing illness and strains of seasonal virus circulating elsewhere in the world, no one knows for sure. But it is very likely that we will experience much higher rates of pandemic influenza once schools reopen and children begin to congregate. It is also likely we will have pandemic influenza circulating along with seasonal influenza viruses this fall and into 2010.
Immunize for seasonal flu, now
The 2009-2010 seasonal influenza vaccine will contain antigens from 3 strains: a nonpandemic H1N1 influenza A strain, an H3N2 influenza A strain, and an influenza B strain.4 These 3 antigens will be in all seasonal influenza vaccine products, whether they are the trivalent influenza vaccine given by injection or the live attenuated influenza vaccine provided as a nasal spray. The CDC is recommending immunization against seasonal influenza as soon as the vaccine is available.
The groups for whom seasonal influenza vaccine is recommended have not changed from last year. The recommendations are summarized in the TABLE.
TABLE
Who should get seasonal flu vaccine, 2009-2010?
| All children and adolescents ages 6 months through 18 years |
| Adults ≥50 years of age |
| Individuals at risk for medical complications |
| Women who will be pregnant during the influenza season |
| Adults and children who have chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, hematologic, or metabolic disorders (including diabetes mellitus) |
| Adults and children who have immunosuppression (including immunosuppression caused by medications or by HIV) |
| Adults and children who have any condition (eg, cognitive dysfunction, spinal cord injury, seizure disorder, or other neuromuscular disorder) that can compromise respiratory function or the handling of respiratory secretions or increase the risk for aspiration |
| Residents of nursing homes and other chronic-care facilities |
| Individuals who live with, or care for, people at high risk for influenza-related complications |
| Health care personnel |
| Healthy household contacts (including children) and caregivers of children <5 years of age and adults ≥50 years |
| Healthy household contacts (including children) of individuals with medical conditions that put them at higher risk for severe complications from influenza. |
| Source: Centers for Disease Control and Prevention. MMWR Recomm Rep. 2009.4 |
Pandemic flu vaccine will be available in the fall
The vaccine for pandemic H1N1 is being produced, and the Department of Health and Human Services is projecting it to be available starting in mid- to late October. The supply will be limited at first, with increasing quantities produced as time progresses. The intent is to produce 600 million doses, or 2 per US resident, since 2 doses will be required.
Who should get the vaccine for pandemic H1N1? At its meeting at the end of July, the Advisory Committee on Immunization Practices (ACIP) recommended that vaccination efforts focus on 5 key populations:
- pregnant women
- people who live with, or care for, children <6 months of age
- health care and emergency services personnel
- individuals between the ages of 6 months and 24 years
- individuals 25 to 64 years of age who are at higher risk for novel H1N1 because of chronic health disorders or compromised immune systems.
In the event of initial shortages of the vaccine, the first 3 groups listed above should be given priority, along with children 6 months through 4 years of age and children 5 through 18 years who have chronic medical conditions.5 In the event of a vaccine surplus (due to low demand and/or faster-than-expected supply), prioritization will not apply and the vaccine should be administered to anyone requesting it who does not have a contraindication.
It is not known how the pandemic influenza vaccine will be distributed and administered. The extent of involvement by physician offices and clinics is undetermined and may vary by locale. There may be extensive use of mass immunization clinics and school clinics to administer the vaccine quickly. Administration will be complicated by the need for 2 doses for protection and a perception by the public that the pandemic virus is not a major concern.
Medical practices may be administering 2 influenza vaccines with different dose requirements: a single dose for seasonal influenza vaccine (except for children <9 years who are being vaccinated for the first time; they get 2 doses), and 2 doses for pandemic vaccine.
Antivirals protect vulnerable patients
Antiviral medications can be used for chemoprophylaxis, both to prevent infection in patients with a high-risk medical condition who are not, or cannot be, vaccinated (chemoprevention), and for post-exposure prophylaxis (PEP) for those who are at risk for complications or want to avoid illness. PEP is time limited (5 days), while chemoprevention may be needed for the duration of potential exposure during an outbreak or epidemic.
PEP should be considered for residents in an assisted living facility during an influenza outbreak, and for individuals who are at higher risk for influenza-related complications and who have had recent household or other close contact with a person with laboratory-confirmed influenza. Chemoprevention is an option with limited applicability at this time. If the pandemic virus were to become more virulent, it might be considered for health care workers until they had received 2 doses of vaccine.
Follow recommendations for antiviral treatment
Because resistance patterns differ among flu viruses, the decision on which antiviral or combination of antivirals to use depends on the predominant viruses circulating in the community and on laboratory tests from the infected patient to determine the influenza type involved. Current recommendations for seasonal influenza can be found at http://www2a.cdc.gov/han/ArchiveSys/ViewMsgV.asp?AlertNum=00279, and recommendations for pandemic influenza are at http://www.cdc.gov/h1n1flu/recommendations.htm#table1. These recommendations may change as the season progresses and viral resistance patterns are determined.
Consider antiviral treatment for those at high risk for complications from the virus. These include anyone hospitalized for influenza, children <5 years of age (especially those <2 years), adults ≥65 years of age, and individuals with the following conditions:
- chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, hematologic (including sickle cell disease), neurologic, neuromuscular, or metabolic disorders (including diabetes mellitus)
- immunosuppression, including that caused by medications or by HIV
- pregnant women
- individuals <19 years of age who are receiving long-term aspirin therapy
- residents of nursing homes and other chronic-care facilities.
The evidence for antiviral effectiveness is strongest if it is given within the first 48 hours of symptom onset, although in hospitalized patients, there is some evidence of effectiveness if started after this time.
Be diligent about infection control
Physicians and other health care workers will need to practice good infection control this flu season. This has been the topic of a previous Practice Alert.6 All health care workers should be fully immunized against influenza—seasonal and pandemic. In addition, each clinical practice should plan on implementing policies to prevent the spread of infection within the clinic or office. Such policies might include scheduling patients with respiratory illnesses for later in the day, separating patients with respiratory illnesses from other patients, requiring patients to cover their nose and mouth when they cough or sneeze, and providing tissues and hand sanitizers for patients and staff.
Physicians and staff will need to take measures to protect themselves from infection by frequent hand washing, avoiding work when ill, and using personal protective equipment when there is potential exposure to respiratory droplets.7 It will also be important to teach families to follow infection control practices at home whenever a household member has an influenza-like illness. Recommendations for home care can be found at www.cdc.gov/h1n1flu/guidance_homecare.htm/?x_cid=ccu071309_HomeCareGuidance_e.
Stay on top of the situation
As this influenza season progresses, keeping current about influenza recommendations will be crucial. The 3 issues to say on top of are:
- Who should receive the vaccine for pandemic influenza and where will it be administered?
- What influenza viruses are circulating in the community?
- What is happening to antiviral resistance patterns and how are changes in these patterns affecting recommendations for treatment and chemoprophylaxis?
Web sites that will keep you up to date
- The CDC influenza Web site: http://www.cdc.gov/flu
- Your local and state public health department Web sites
- The American Academy of Family Physicians (AAFP) Web site: http://www.aafp.org/online/en/home.html.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; [email protected]
1. CDC. Novel H1N1 flu situation update: August 6, 2009. Available at: http://www.cdc.gov/h1n1flu/update.htm. Accessed August 12, 2009.
2. WHO. Pandemic (H1N1) 2009-update 60. July 31, 2009. Available at: http://www.who.int/csr/don/2009_08_04/en/index.html. Accessed August 5, 2009.
3. CDC. CDC issues interim recommendations for the use of influenza antiviral medications in the setting of oseltamivir resistance among circulating influenza A (H1N1) viruses, 2008-09 influenza season [CDC health advisory]. December 19, 2008. Available at: http://www2a.cdc.gov/han/Archivesys/ViewMsgV.asp?AlertNum=00279. Accessed August 5, 2009.
4. CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-8):1-52. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5808a1.htm. Accessed August 5, 2009.
5. CDC. CDC advisors make recommendations for use of vaccine against novel H1N1 [press release]. July 29, 2009. Available at: http://www.cdc.gov/media/pressrel/2009/r090729b.htm. Accessed August 5, 2009.
6. Campos-Outcalt D. Infection control in the outpatient setting. J Fam Pract. 2004;53:485-488.
7. CDC. 10 steps you can take: actions for novel H1N1 influenza planning and response for medical offices and outpatient facilities. July 14, 2009. Available at: http://www.cdc.gov/h1n1flu/10steps.htm. Accessed August 3, 2009.
This coming flu season will be interesting—and confusing. As of August 6, 2009, the Centers for Disease Control and Prevention (CDC) reported 6506 hospitalized cases and 436 deaths from the pandemic H1N1 flu virus since the first US cases were reported in April 2009.1 (Reporting on individual confirmed and probable cases has been discontinued.) On July 31, the World Health Organization reported pandemic influenza in 168 countries, with 162,380 reported cases and 1154 deaths.2 At the same time the pandemic was developing, the seasonal flu of 2009—a relatively mild year—was tapering off. The pandemic influenza has continued to cause widespread disease in the United States throughout the summer, a somewhat unusual pattern for influenza.
So far, pandemic H1N1 flu is relatively benign, treatable
The pandemic virus, though highly infectious, has had a low case fatality rate up to now. Deaths have occurred predominantly in those with underlying medical conditions that put them at high risk of infection. Attack rates for those older than age 65 have been lower than expected, indicating that this age group may have some immunity based on past infection. The pandemic virus so far has been sensitive to both oseltamivir (Tamiflu) and zanamivir (Relenza). The resistance patterns of the key viruses from last flu season showed that the H1N1 seasonal virus was resistant to oseltamivir but sensitive to zanamivir and the adamantanes (rimantadine and amantadine), while the H3N2 virus that circulated last year was sensitive to oseltamivir.3
Fall flu season: Be prepared
So, what can you expect this fall? With pandemic H1N1 still causing illness and strains of seasonal virus circulating elsewhere in the world, no one knows for sure. But it is very likely that we will experience much higher rates of pandemic influenza once schools reopen and children begin to congregate. It is also likely we will have pandemic influenza circulating along with seasonal influenza viruses this fall and into 2010.
Immunize for seasonal flu, now
The 2009-2010 seasonal influenza vaccine will contain antigens from 3 strains: a nonpandemic H1N1 influenza A strain, an H3N2 influenza A strain, and an influenza B strain.4 These 3 antigens will be in all seasonal influenza vaccine products, whether they are the trivalent influenza vaccine given by injection or the live attenuated influenza vaccine provided as a nasal spray. The CDC is recommending immunization against seasonal influenza as soon as the vaccine is available.
The groups for whom seasonal influenza vaccine is recommended have not changed from last year. The recommendations are summarized in the TABLE.
TABLE
Who should get seasonal flu vaccine, 2009-2010?
| All children and adolescents ages 6 months through 18 years |
| Adults ≥50 years of age |
| Individuals at risk for medical complications |
| Women who will be pregnant during the influenza season |
| Adults and children who have chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, hematologic, or metabolic disorders (including diabetes mellitus) |
| Adults and children who have immunosuppression (including immunosuppression caused by medications or by HIV) |
| Adults and children who have any condition (eg, cognitive dysfunction, spinal cord injury, seizure disorder, or other neuromuscular disorder) that can compromise respiratory function or the handling of respiratory secretions or increase the risk for aspiration |
| Residents of nursing homes and other chronic-care facilities |
| Individuals who live with, or care for, people at high risk for influenza-related complications |
| Health care personnel |
| Healthy household contacts (including children) and caregivers of children <5 years of age and adults ≥50 years |
| Healthy household contacts (including children) of individuals with medical conditions that put them at higher risk for severe complications from influenza. |
| Source: Centers for Disease Control and Prevention. MMWR Recomm Rep. 2009.4 |
Pandemic flu vaccine will be available in the fall
The vaccine for pandemic H1N1 is being produced, and the Department of Health and Human Services is projecting it to be available starting in mid- to late October. The supply will be limited at first, with increasing quantities produced as time progresses. The intent is to produce 600 million doses, or 2 per US resident, since 2 doses will be required.
Who should get the vaccine for pandemic H1N1? At its meeting at the end of July, the Advisory Committee on Immunization Practices (ACIP) recommended that vaccination efforts focus on 5 key populations:
- pregnant women
- people who live with, or care for, children <6 months of age
- health care and emergency services personnel
- individuals between the ages of 6 months and 24 years
- individuals 25 to 64 years of age who are at higher risk for novel H1N1 because of chronic health disorders or compromised immune systems.
In the event of initial shortages of the vaccine, the first 3 groups listed above should be given priority, along with children 6 months through 4 years of age and children 5 through 18 years who have chronic medical conditions.5 In the event of a vaccine surplus (due to low demand and/or faster-than-expected supply), prioritization will not apply and the vaccine should be administered to anyone requesting it who does not have a contraindication.
It is not known how the pandemic influenza vaccine will be distributed and administered. The extent of involvement by physician offices and clinics is undetermined and may vary by locale. There may be extensive use of mass immunization clinics and school clinics to administer the vaccine quickly. Administration will be complicated by the need for 2 doses for protection and a perception by the public that the pandemic virus is not a major concern.
Medical practices may be administering 2 influenza vaccines with different dose requirements: a single dose for seasonal influenza vaccine (except for children <9 years who are being vaccinated for the first time; they get 2 doses), and 2 doses for pandemic vaccine.
Antivirals protect vulnerable patients
Antiviral medications can be used for chemoprophylaxis, both to prevent infection in patients with a high-risk medical condition who are not, or cannot be, vaccinated (chemoprevention), and for post-exposure prophylaxis (PEP) for those who are at risk for complications or want to avoid illness. PEP is time limited (5 days), while chemoprevention may be needed for the duration of potential exposure during an outbreak or epidemic.
PEP should be considered for residents in an assisted living facility during an influenza outbreak, and for individuals who are at higher risk for influenza-related complications and who have had recent household or other close contact with a person with laboratory-confirmed influenza. Chemoprevention is an option with limited applicability at this time. If the pandemic virus were to become more virulent, it might be considered for health care workers until they had received 2 doses of vaccine.
Follow recommendations for antiviral treatment
Because resistance patterns differ among flu viruses, the decision on which antiviral or combination of antivirals to use depends on the predominant viruses circulating in the community and on laboratory tests from the infected patient to determine the influenza type involved. Current recommendations for seasonal influenza can be found at http://www2a.cdc.gov/han/ArchiveSys/ViewMsgV.asp?AlertNum=00279, and recommendations for pandemic influenza are at http://www.cdc.gov/h1n1flu/recommendations.htm#table1. These recommendations may change as the season progresses and viral resistance patterns are determined.
Consider antiviral treatment for those at high risk for complications from the virus. These include anyone hospitalized for influenza, children <5 years of age (especially those <2 years), adults ≥65 years of age, and individuals with the following conditions:
- chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, hematologic (including sickle cell disease), neurologic, neuromuscular, or metabolic disorders (including diabetes mellitus)
- immunosuppression, including that caused by medications or by HIV
- pregnant women
- individuals <19 years of age who are receiving long-term aspirin therapy
- residents of nursing homes and other chronic-care facilities.
The evidence for antiviral effectiveness is strongest if it is given within the first 48 hours of symptom onset, although in hospitalized patients, there is some evidence of effectiveness if started after this time.
Be diligent about infection control
Physicians and other health care workers will need to practice good infection control this flu season. This has been the topic of a previous Practice Alert.6 All health care workers should be fully immunized against influenza—seasonal and pandemic. In addition, each clinical practice should plan on implementing policies to prevent the spread of infection within the clinic or office. Such policies might include scheduling patients with respiratory illnesses for later in the day, separating patients with respiratory illnesses from other patients, requiring patients to cover their nose and mouth when they cough or sneeze, and providing tissues and hand sanitizers for patients and staff.
Physicians and staff will need to take measures to protect themselves from infection by frequent hand washing, avoiding work when ill, and using personal protective equipment when there is potential exposure to respiratory droplets.7 It will also be important to teach families to follow infection control practices at home whenever a household member has an influenza-like illness. Recommendations for home care can be found at www.cdc.gov/h1n1flu/guidance_homecare.htm/?x_cid=ccu071309_HomeCareGuidance_e.
Stay on top of the situation
As this influenza season progresses, keeping current about influenza recommendations will be crucial. The 3 issues to say on top of are:
- Who should receive the vaccine for pandemic influenza and where will it be administered?
- What influenza viruses are circulating in the community?
- What is happening to antiviral resistance patterns and how are changes in these patterns affecting recommendations for treatment and chemoprophylaxis?
Web sites that will keep you up to date
- The CDC influenza Web site: http://www.cdc.gov/flu
- Your local and state public health department Web sites
- The American Academy of Family Physicians (AAFP) Web site: http://www.aafp.org/online/en/home.html.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; [email protected]
This coming flu season will be interesting—and confusing. As of August 6, 2009, the Centers for Disease Control and Prevention (CDC) reported 6506 hospitalized cases and 436 deaths from the pandemic H1N1 flu virus since the first US cases were reported in April 2009.1 (Reporting on individual confirmed and probable cases has been discontinued.) On July 31, the World Health Organization reported pandemic influenza in 168 countries, with 162,380 reported cases and 1154 deaths.2 At the same time the pandemic was developing, the seasonal flu of 2009—a relatively mild year—was tapering off. The pandemic influenza has continued to cause widespread disease in the United States throughout the summer, a somewhat unusual pattern for influenza.
So far, pandemic H1N1 flu is relatively benign, treatable
The pandemic virus, though highly infectious, has had a low case fatality rate up to now. Deaths have occurred predominantly in those with underlying medical conditions that put them at high risk of infection. Attack rates for those older than age 65 have been lower than expected, indicating that this age group may have some immunity based on past infection. The pandemic virus so far has been sensitive to both oseltamivir (Tamiflu) and zanamivir (Relenza). The resistance patterns of the key viruses from last flu season showed that the H1N1 seasonal virus was resistant to oseltamivir but sensitive to zanamivir and the adamantanes (rimantadine and amantadine), while the H3N2 virus that circulated last year was sensitive to oseltamivir.3
Fall flu season: Be prepared
So, what can you expect this fall? With pandemic H1N1 still causing illness and strains of seasonal virus circulating elsewhere in the world, no one knows for sure. But it is very likely that we will experience much higher rates of pandemic influenza once schools reopen and children begin to congregate. It is also likely we will have pandemic influenza circulating along with seasonal influenza viruses this fall and into 2010.
Immunize for seasonal flu, now
The 2009-2010 seasonal influenza vaccine will contain antigens from 3 strains: a nonpandemic H1N1 influenza A strain, an H3N2 influenza A strain, and an influenza B strain.4 These 3 antigens will be in all seasonal influenza vaccine products, whether they are the trivalent influenza vaccine given by injection or the live attenuated influenza vaccine provided as a nasal spray. The CDC is recommending immunization against seasonal influenza as soon as the vaccine is available.
The groups for whom seasonal influenza vaccine is recommended have not changed from last year. The recommendations are summarized in the TABLE.
TABLE
Who should get seasonal flu vaccine, 2009-2010?
| All children and adolescents ages 6 months through 18 years |
| Adults ≥50 years of age |
| Individuals at risk for medical complications |
| Women who will be pregnant during the influenza season |
| Adults and children who have chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, hematologic, or metabolic disorders (including diabetes mellitus) |
| Adults and children who have immunosuppression (including immunosuppression caused by medications or by HIV) |
| Adults and children who have any condition (eg, cognitive dysfunction, spinal cord injury, seizure disorder, or other neuromuscular disorder) that can compromise respiratory function or the handling of respiratory secretions or increase the risk for aspiration |
| Residents of nursing homes and other chronic-care facilities |
| Individuals who live with, or care for, people at high risk for influenza-related complications |
| Health care personnel |
| Healthy household contacts (including children) and caregivers of children <5 years of age and adults ≥50 years |
| Healthy household contacts (including children) of individuals with medical conditions that put them at higher risk for severe complications from influenza. |
| Source: Centers for Disease Control and Prevention. MMWR Recomm Rep. 2009.4 |
Pandemic flu vaccine will be available in the fall
The vaccine for pandemic H1N1 is being produced, and the Department of Health and Human Services is projecting it to be available starting in mid- to late October. The supply will be limited at first, with increasing quantities produced as time progresses. The intent is to produce 600 million doses, or 2 per US resident, since 2 doses will be required.
Who should get the vaccine for pandemic H1N1? At its meeting at the end of July, the Advisory Committee on Immunization Practices (ACIP) recommended that vaccination efforts focus on 5 key populations:
- pregnant women
- people who live with, or care for, children <6 months of age
- health care and emergency services personnel
- individuals between the ages of 6 months and 24 years
- individuals 25 to 64 years of age who are at higher risk for novel H1N1 because of chronic health disorders or compromised immune systems.
In the event of initial shortages of the vaccine, the first 3 groups listed above should be given priority, along with children 6 months through 4 years of age and children 5 through 18 years who have chronic medical conditions.5 In the event of a vaccine surplus (due to low demand and/or faster-than-expected supply), prioritization will not apply and the vaccine should be administered to anyone requesting it who does not have a contraindication.
It is not known how the pandemic influenza vaccine will be distributed and administered. The extent of involvement by physician offices and clinics is undetermined and may vary by locale. There may be extensive use of mass immunization clinics and school clinics to administer the vaccine quickly. Administration will be complicated by the need for 2 doses for protection and a perception by the public that the pandemic virus is not a major concern.
Medical practices may be administering 2 influenza vaccines with different dose requirements: a single dose for seasonal influenza vaccine (except for children <9 years who are being vaccinated for the first time; they get 2 doses), and 2 doses for pandemic vaccine.
Antivirals protect vulnerable patients
Antiviral medications can be used for chemoprophylaxis, both to prevent infection in patients with a high-risk medical condition who are not, or cannot be, vaccinated (chemoprevention), and for post-exposure prophylaxis (PEP) for those who are at risk for complications or want to avoid illness. PEP is time limited (5 days), while chemoprevention may be needed for the duration of potential exposure during an outbreak or epidemic.
PEP should be considered for residents in an assisted living facility during an influenza outbreak, and for individuals who are at higher risk for influenza-related complications and who have had recent household or other close contact with a person with laboratory-confirmed influenza. Chemoprevention is an option with limited applicability at this time. If the pandemic virus were to become more virulent, it might be considered for health care workers until they had received 2 doses of vaccine.
Follow recommendations for antiviral treatment
Because resistance patterns differ among flu viruses, the decision on which antiviral or combination of antivirals to use depends on the predominant viruses circulating in the community and on laboratory tests from the infected patient to determine the influenza type involved. Current recommendations for seasonal influenza can be found at http://www2a.cdc.gov/han/ArchiveSys/ViewMsgV.asp?AlertNum=00279, and recommendations for pandemic influenza are at http://www.cdc.gov/h1n1flu/recommendations.htm#table1. These recommendations may change as the season progresses and viral resistance patterns are determined.
Consider antiviral treatment for those at high risk for complications from the virus. These include anyone hospitalized for influenza, children <5 years of age (especially those <2 years), adults ≥65 years of age, and individuals with the following conditions:
- chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, hematologic (including sickle cell disease), neurologic, neuromuscular, or metabolic disorders (including diabetes mellitus)
- immunosuppression, including that caused by medications or by HIV
- pregnant women
- individuals <19 years of age who are receiving long-term aspirin therapy
- residents of nursing homes and other chronic-care facilities.
The evidence for antiviral effectiveness is strongest if it is given within the first 48 hours of symptom onset, although in hospitalized patients, there is some evidence of effectiveness if started after this time.
Be diligent about infection control
Physicians and other health care workers will need to practice good infection control this flu season. This has been the topic of a previous Practice Alert.6 All health care workers should be fully immunized against influenza—seasonal and pandemic. In addition, each clinical practice should plan on implementing policies to prevent the spread of infection within the clinic or office. Such policies might include scheduling patients with respiratory illnesses for later in the day, separating patients with respiratory illnesses from other patients, requiring patients to cover their nose and mouth when they cough or sneeze, and providing tissues and hand sanitizers for patients and staff.
Physicians and staff will need to take measures to protect themselves from infection by frequent hand washing, avoiding work when ill, and using personal protective equipment when there is potential exposure to respiratory droplets.7 It will also be important to teach families to follow infection control practices at home whenever a household member has an influenza-like illness. Recommendations for home care can be found at www.cdc.gov/h1n1flu/guidance_homecare.htm/?x_cid=ccu071309_HomeCareGuidance_e.
Stay on top of the situation
As this influenza season progresses, keeping current about influenza recommendations will be crucial. The 3 issues to say on top of are:
- Who should receive the vaccine for pandemic influenza and where will it be administered?
- What influenza viruses are circulating in the community?
- What is happening to antiviral resistance patterns and how are changes in these patterns affecting recommendations for treatment and chemoprophylaxis?
Web sites that will keep you up to date
- The CDC influenza Web site: http://www.cdc.gov/flu
- Your local and state public health department Web sites
- The American Academy of Family Physicians (AAFP) Web site: http://www.aafp.org/online/en/home.html.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; [email protected]
1. CDC. Novel H1N1 flu situation update: August 6, 2009. Available at: http://www.cdc.gov/h1n1flu/update.htm. Accessed August 12, 2009.
2. WHO. Pandemic (H1N1) 2009-update 60. July 31, 2009. Available at: http://www.who.int/csr/don/2009_08_04/en/index.html. Accessed August 5, 2009.
3. CDC. CDC issues interim recommendations for the use of influenza antiviral medications in the setting of oseltamivir resistance among circulating influenza A (H1N1) viruses, 2008-09 influenza season [CDC health advisory]. December 19, 2008. Available at: http://www2a.cdc.gov/han/Archivesys/ViewMsgV.asp?AlertNum=00279. Accessed August 5, 2009.
4. CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-8):1-52. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5808a1.htm. Accessed August 5, 2009.
5. CDC. CDC advisors make recommendations for use of vaccine against novel H1N1 [press release]. July 29, 2009. Available at: http://www.cdc.gov/media/pressrel/2009/r090729b.htm. Accessed August 5, 2009.
6. Campos-Outcalt D. Infection control in the outpatient setting. J Fam Pract. 2004;53:485-488.
7. CDC. 10 steps you can take: actions for novel H1N1 influenza planning and response for medical offices and outpatient facilities. July 14, 2009. Available at: http://www.cdc.gov/h1n1flu/10steps.htm. Accessed August 3, 2009.
1. CDC. Novel H1N1 flu situation update: August 6, 2009. Available at: http://www.cdc.gov/h1n1flu/update.htm. Accessed August 12, 2009.
2. WHO. Pandemic (H1N1) 2009-update 60. July 31, 2009. Available at: http://www.who.int/csr/don/2009_08_04/en/index.html. Accessed August 5, 2009.
3. CDC. CDC issues interim recommendations for the use of influenza antiviral medications in the setting of oseltamivir resistance among circulating influenza A (H1N1) viruses, 2008-09 influenza season [CDC health advisory]. December 19, 2008. Available at: http://www2a.cdc.gov/han/Archivesys/ViewMsgV.asp?AlertNum=00279. Accessed August 5, 2009.
4. CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-8):1-52. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5808a1.htm. Accessed August 5, 2009.
5. CDC. CDC advisors make recommendations for use of vaccine against novel H1N1 [press release]. July 29, 2009. Available at: http://www.cdc.gov/media/pressrel/2009/r090729b.htm. Accessed August 5, 2009.
6. Campos-Outcalt D. Infection control in the outpatient setting. J Fam Pract. 2004;53:485-488.
7. CDC. 10 steps you can take: actions for novel H1N1 influenza planning and response for medical offices and outpatient facilities. July 14, 2009. Available at: http://www.cdc.gov/h1n1flu/10steps.htm. Accessed August 3, 2009.
When an athlete can’t catch his breath
- Don’t rely on self-reported symptoms to diagnose exercise-induced bronchoconstriction (EIB) (A).
- Indirect testing is the best way to diagnose EIB in patients who do not have underlying asthma (A).
- Short-acting β2-agonists should be first-line management in EIB (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Luke, a 16-year-old basketball player, complains that he can’t finish a game without running out of breath. He says things are at their worst when the game is close and when it’s nearing the end. He doesn’t have the problem during practice, or when he is playing other sports. The team physician suggested using an albuterol inhaler half an hour before game time and when he has symptoms, but he gets only minimal relief. Now he has come to you.
His vital signs, lung exam, and cardiac exam are normal. Results of pulmonary function tests with pre- and post-albuterol challenge done a year ago were also normal. Does Luke have exercise-induced bronchoconstriction (EIB)? How can you be sure? And what can you do to help?
Symptoms like Luke’s are common among athletes of all abilities. They may add up to EIB, a condition with an estimated prevalence of 6% to 12% in the general population—or they may not.1 One study showed that only a third of athletes with symptoms or prior diagnosis of EIB had positive objective testing for the condition, and current studies show that reported symptoms are not an accurate guide in athletes like Luke who do not have underlying asthma.2,3 To treat him correctly, you will need to nail down the diagnosis with additional tests.3,4
Shortness of breath that’s worse than expected
EIB can have many different presentations. The most common symptom is cough associated with exercise.3 Other common signs and symptoms include wheezing, chest tightness, and more severe than expected or worsening shortness of breath. More unusual symptoms include a decrease in performance or fatigue out of proportion to workload. Often patients with EIB have other associated medical conditions, such as allergic rhinitis.
Bronchoconstriction usually occurs with maximal or near maximal exertion. Generally, it takes 5 to 8 minutes of exercising at 80% of maximal heart rate to trigger EIB. Classically, the symptoms peak 5 to 10 minutes after exercise begins.5
Rule out cardiac problems. If EIB is the correct diagnosis, the physical exam is usually normal. The importance of the physical exam is to evaluate for other diagnoses with similar presentations. Conditions to rule out include cardiac problems, exercise-induced hyperventilation, upper and lower respiratory infections or abnormalities, exercise-induced laryngeal dysfunction, exercise-induced anaphylaxis, and gastroesophageal reflux disease (GERD). The differential diagnosis for EIB is summarized in TABLE 1.
Test for asthma. Once you have gone through the differential diagnosis and are comfortable that the symptoms are respiratory, the next step should be pulmonary function tests (PFT), pre- and post-albuterol challenge. Findings of obstruction, such as reduced forced expiratory volume in 1 second (FEV1) or increased lung volume, are consistent with a diagnosis of asthma. In that case, no further workup is needed—unless the patient is unresponsive to asthma treatment. In athletes like Luke who do not have asthma and have a normal nonprovocative spirometry, you can move on to either provocative spirometry or empiric treatment.
TABLE 1
Is it EIB, or something else?
| ETIOLOGY | POSSIBLE DIAGNOSES |
|---|---|
| Pulmonary | Exercise-induced hyperventilation (pseudo-asthma syndrome) Restrictive lung disease Cystic fibrosis Upper and lower respiratory infections Foreign body aspiration |
| Cardiac | Coronary artery disease Congenital and acquired heart defects Cardiomyopathy Congestive heart failure |
| Laryngeal | Exercise-induced laryngeal dysfunction Vocal cord dysfunction Laryngeal prolapse Laryngomalacia |
| Gastroesophageal | Gastroesophageal reflux disease |
| Allergic | Exercise-induced anaphylaxis |
| Other | Athlete is out of shape |
| EIB, exercise-induced bronchoconstriction. | |
| Source: Weiler JM, et al. J Allergy Clin Immunol. 2007.4 | |
Perform provocative spirometry
Direct spirometry is commonly done with a methacholine challenge. This test is less sensitive than indirect testing for EIB patients who do not have underlying asthma.
The gold standard for indirect testing is eucapnic voluntary hyperventilation (EVH). Because EVH requires special equipment, however, it may not be an option in your office. The more reasonable choice is exercise challenge testing, which can be done either in your office or in the milieu—the basketball court, for example—where the athlete’s symptoms usually occur. In an exercise challenge, you get a baseline spirometry measurement, have the athlete exercise to 80% to 90% of maximal heart rate, and then repeat spirometry at short intervals after exercise ends. If you do an exercise challenge in the office, you can reduce false-negative results by maintaining an ambient temperature between 68° and 77°F (20°-25°C) with a relative humidity of less than 50%.6,7
Or try empiric treatment
Empiric treatment is a reasonable strategy for athletes with EIB symptoms, worth trying both for athletes who have underlying asthma and for those who do not. If the athlete with asthma responds to treatment, the problem is solved. For the athlete who does not have asthma, however, there are some exceptions to this approach—specifically, the elite athlete.
In the elite athlete, you will need to confirm the diagnosis because many of the substances used to treat EIB are restricted by governing bodies such as the International Olympic Committee (IOC) and require provocative testing to obtain a therapeutic use exemption.8 There is some debate as to whether nonelite athletes also need bronchoprovocative testing. Some recommendations advise testing all elite and competitive athletes and restricting empiric treatment to recreational athletes.1 For more information on banned or restricted medications, see “Is that drug banned from competition?”.
If you take the empiric approach and the athlete does not respond to treatment, consider further testing to rule out other, more serious problems. In Luke’s case, where empiric treatment with albuterol has failed, indirect testing would be the next step.
Certain medications used in the treatment of asthma and exercise-induced bronchoconstriction (EIB) are considered performance-enhancing drugs and either banned or restricted in athletic competition. The regulatory bodies that make these designations in the United States are the National Collegiate Athletic Association (NCAA) and the International Olympic Committee World Anti-Doping Agency (IOC-WADA). These organizations update their list of banned substances yearly and make the current list available on the Web. You can find the NCAA list at www.pace.edu/emplibrary/NCAA%20LIST%20OF%20BANNED%20SUBSTANCESb.doc and the IOC-WADA list at www.wada-ama.org/rtecontent/document/2009_Prohibited_List_ENG_Final_20_Sept_08.pdf.
The IOC-WADA allows competing athletes to use inhaled corticosteroids and β2 agonists, but requires athletes with asthma to provide documentation that the medication is for therapeutic use. Glucocorticosteroids and oral β2 agonists remain prohibited by the IOC-WADA, but only oral β2 agonists are banned by the NCAA. The NCAA warns that student athletes are responsible for knowing which substances are on the banned list and advises them to consult www.drugfreesport.com for more information. To avoid disqualifying a patient from sports participation, check medications you prescribe with the official lists and be sure your EIB patient has the documentation he or she needs to qualify for a therapeutic use exemption.
Medicate before exercise: SABAs and LABAs
Prophylaxis for EIB usually starts with an inhaled short-acting β2 agonist (SABA) such as albuterol or pirbuterol, taken 15 minutes before starting to exercise.9,10 The effectiveness of both short- and long-acting β2 agonists decreases with frequent use, which may be Luke’s problem. For that reason, patients with mild EIB may choose to use pretreatment medication only for more demanding exercise sessions.11 Advise EIB patients who need daily pretreatment to try adjunctive maintenance therapy (discussed at greater length, below.)
Longer-acting β2 agonists (LABAs) such as salmeterol or formoterol may be effective for prolonged or all-day exercise, but may lose their prophylactic effect with prolonged use.12 Furthermore, the US Food and Drug Administration (FDA) has advised against using LABAs alone because of the possibility of severe asthma episodes or death. LABAs should be used only in conjunction with daily maintenance therapy with inhaled corticosteroids. The properties of these and other EIB medications are summarized in TABLE 2.
TABLE 2
EIB medications
| MEDICATION | INDICATION | DOSE | CAUTIONS | COMMENT |
|---|---|---|---|---|
| Short-acting β2 agonists (SABAs) | ||||
| Albuterol, pirbuterol | Pre-exercise prophylaxis, acute treatment | 2 puffs pre-exercise or 2 puffs every 4-6 h as needed | May cause tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | First-line treatment |
| Mast cell stabilizers | ||||
| Cromolyn | Pre-exercise treatment | 2 puffs 30-45 min before exercise | None | Best combined with SABA. Tell patients not to use for rescue. |
| Inhaled corticosteroids | ||||
| Flunisolide, fluticasone, budesonide, triamcinolone, beclomethasone, mometasone | Daily maintenance | Variable | Can cause oral candidiasis, hoarseness. | Tell patients this is not a rescue inhaler. |
| Leukotriene inhibitors | ||||
| Zafirlukast | Daily maintenance | 20 mg PO, bid | None | Variable response. Works well with inhaled corticosteroids. Low side-effect profile. |
| Montelukast | Daily maintenance, pre-exercise prophylaxis | 10 mg PO daily or up to 2 h pre-exercise | None | Variable response. Works well with inhaled corticosteroids. Low side-effect profile. |
| Zileuton | Daily maintenance | 1200 mg PO, bid | Risk of elevated liver function tests. | Variable response. Low side-effect profile. |
| Combinations | ||||
| Inhaled fluticasone and salmeterol | Daily maintenance | Variable doses (100/50, 250/50, 500/50 mcg/spray); 1 puff bid | Can cause oral candidiasis, hoarseness, tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | Tell patients this is not a rescue inhaler. |
| Inhaled budesonide and formoterol | Daily maintenance | Variable doses (80/4.5, 160/4.5 mcg/spray); 1 puff bid | Can cause oral candidiasis, hoarseness, tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | Tell patients this is not a rescue inhaler. |
| EIB, exercise-induced bronchoconstriction. | ||||
| Adapted from the National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma.9 | ||||
Cromolyn, antileukotrienes are options, too
Mast cell stabilizers (cromolyn) can be used with β2 agonists as prophylactic therapy. When these agents are used together, they have an additive effect.13 The athlete may take them 10 minutes to an hour before exercise. Make sure your patient knows that mast cell stabilizers cannot be used as a rescue inhaler or bronchodilator.
Inhaled corticosteroids (flunisolide, fluticasone, others) may be needed for athletes with poorly controlled chronic asthma; they can also be used as adjunct preventive treatment for athletes who have EIB with no underlying chronic asthma.14-16 Often, inhaled corticosteroids are used as combination therapy with a LABA or an antileukotriene agent (montelukast, zafirlukast; see below). Recent research shows that montelukast in combination with inhaled corticosteroids is more efficacious than LABA with inhaled corticosteroids.14,17
Antileukotriene agents can be especially helpful for EIB in patients with mild, stable asthma.18 Patients who do respond to antileukotriene agents usually respond very favorably. Antileukotrienes offer a reasonable alternative to inhaled corticosteroids and LABAs. They have a low side-effect profile and should be considered as daily prophylaxis.19,20 The effects of montelukast are evident as early as 2 hours after administration, and bronchoprotective effects can last as long as 24 hours.21,22 For that reason, montelukast is especially useful in children whose exercise patterns are not always predictable.
Be prepared for acute exacerbations. Prophylactic medication does not always prevent acute exacerbations. When that happens, your EIB patient will need to use a β2 agonist as rescue therapy. Make sure your patient knows that none of the other medications are effective bronchodilators in acute exacerbations.
Remember, too, that EIB cannot be effectively treated if the athlete has poorly controlled chronic asthma. Underlying causes of asthma exacerbations like allergies or respiratory infections must be addressed and stabilized first, following guidelines of the National Asthma Education and Prevention Program (NAEPP).9 You can access the guidelines at www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
These tips can help the athlete
Encourage athletes with EIB to keep up their exercise routines, because cardiovascular fitness has a beneficial effect on this condition. Fit individuals breathe more slowly, which reduces the likelihood of exacerbations. Of note, though: Certain sports are easier on patients with EIB. Patients may want to keep this in mind when deciding which team they want to go out for. Specifically, indoor sports, where air temperature, humidity, and exposure to allergens are controlled, and sports like baseball, sprinting, or football, which require less prolonged aerobic endurance, are good options.
Tell athletes whose sports require cold, dry conditions—ice skating, or skiing, for instance—to try breathing through a scarf or mask to keep inspired air warm and less irritating.
And tell all athletes with EIB to warm up properly before they start to compete.23 That means a 15-minute warm-up at moderate exertion, followed by a 15- to 30-minute rest period. The rest period is the time to take their medication.
When therapy fails
When an EIB patient fails to respond despite multiple drug therapy, it’s time to reconsider other diagnoses, such as vocal cord dysfunction and severe GERD, which may mimic symptoms of EIB.
On the horizon. Other therapies for possible treatment of EIB are being studied. These include omega-3 fatty acid dietary supplementation and inhaled enoxaparin.24,25 Data are currently insufficient to recommend use of these agents in clinical practice.
As for Luke, indirect testing via exercise challenge was positive for EIB. Adjunctive therapy with montelukast was added to his albuterol inhaler, and the combination has worked well for him. He’s still playing basketball, and enjoying it.
Acknowledgments
The authors thank Ken Rundell, PhD, for reviewing this article. Dr. Rundell is director of the Human Physiology Laboratory at the Keith J. O’Neill Center of Marywood University, Scranton, Pa.
CORRESPONDENCE
Michael A. Krafczyk, MD, FAAFP, St. Luke’s Sports Medicine, 153 Brodhead Rd, Bethlehem, PA 18017; [email protected]
1. Holzer K, Brukner P. Screening of athletes for exercise-induced bronchoconstriction. Clin J Sport Med. 2004;14:134-138.
2. Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr. 2002;141:343-348.
3. Rundell KW, Mayers LB, Wilber RL, et al. Self-reported symptoms of exercise-induced asthma in the elite athlete. Med Sci Sports Exerc. 2001;33:208-213.
4. Weiler JM, Bonini S, Coifman R, et al. Ad Hoc Committee of Sports Medicine Committee, American Academy of Allergy, Asthma, and Immunology Work Group Report: exercise-induced asthma. J Allergy Clin Immunol. 2007;119:1349-1358.
5. Parsons JP, Mastronarde JG. Exercise-induced bronchoconstriction in athletes. Chest. 2005;128:3966-3974.
6. Rundell KW, Slee JB. Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J Allergy Clin Immunol. 2008;122:238-246.
7. Rundell KW, Wilber RL, Szmedra L, et al. Exercise-induced asthma screening of elite athletes: field versus laboratory exercise challenges. Med Sci Sports Exerc. 2000;32:309-316.
8. Fitch KD, Sue-Chu M, Anderson SD, et al. Asthma and the elite athlete: summary of the International Olympic Committee’s Consensus Conference, Lausanne Switzerland. January 22-24, 2008. J Allergy Clin Immunol. 2008;122:254-260.
9. National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. NIH publication no. 08-4051. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed September 1, 2007.
10. Anderson S, Seale JP, Ferris L, et al. An evaluation of pharmacotherapy for exercise-induced asthma. J Allergy Clin Immunol. 1979;64:612-624.
11. Hancox RJ, Subbarao P, Kamada D, et al. β2-Agonist tolerance and exercise-induced bronchospasm. Am Respir Crit Care Med. 2002;165:1068-1070.
12. Inman M, O’Byrne PM. The effect of regular inhaled albuterol on exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 1996;153:65-69.
13. Latimer KM, O’Byrne PM, Morris MM, et al. Bronchoconstriction stimulated by airway cooling: better protection with combined inhalation of terbutaline sulphate and cromolyn sodium than with either alone. Am Rev Respir Dis. 1983;128:440-443.
14. Stelmach I, Grzelewski T, Majak P, et al. Effect of different antiasthmatic treatments on exercise-induced bronchoconstriction in children with asthma. J Allergy Clin Immunol. 2008;121:383-389.
15. Koh MS, Tee A, Lasserson TJ, et al. Inhaled corticosteroids compared to placebo for prevention of exercise induced bronchoconstriction. Cochrane Database Syst Rev. 2007;(3):CD002739.-
16. Jonasson G, Carlsen KH, Hultquist C. Low-dose budesonide improves exercise-induced bronchospasm in schoolchildren. Pediatr Allergy Immunol. 2000;11:120-125.
17. Storms W, Chervinsky P, Ghannam AF, et al. Challenge-Rescue Study Group. Respir Med. 2004;98:1051-1062.
18. Leff JA, Busse WW, Pearlman D, et al. Montelukast, a leukotriene-receptor antagonist for the treatment of mild asthma and exercise-induced bronchoconstriction. N Engl J Med. 1998;339:147-152.
19. Steinshamn S, Sandsund M, Sue-Chu M, et al. Effects of montelukast and salmeterol on physical performance and exercise economy in adult asthmatics with exercise-induced bronchoconstriction. Chest. 2004;126:1154-1160.
20. Storms W. Update on montelukast and its role in the treatment of asthma, allergic rhinitis, and exercise-induced bronchoconstriction. Expert Opin Pharmacother. 2007;8:2173-2187.
21. Pearlman DS, van Adelsberg J, Philip G, et al. Onset and duration of protection against exercise-induced bronchoconstriction by a single oral dose of montelukast. Ann Allergy Asthma Immunol. 2006;97:98-104.
22. Philip G, Villaran C, Pearlman DS, et al. Protection against exercise-induced bronchoconstriction two hours after a single oral dose of montelukast. J Asthma. 2007;44:213-217.
23. Storms WW. Review of exercise-induced asthma. Med Sci Sports Exerc. 2003;35:1464-1470.
24. Mickleborough TD, Lindley MR, Ionescu AA, et al. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006;129:39-49.
25. Ahmed T, Gonzalez BJ, Danta I. Prevention of exercise-induced bronchoconstriction by inhaled low-molecular-weight heparin. Am J Respir Crit Care Med. 1999;160:576-581.
- Don’t rely on self-reported symptoms to diagnose exercise-induced bronchoconstriction (EIB) (A).
- Indirect testing is the best way to diagnose EIB in patients who do not have underlying asthma (A).
- Short-acting β2-agonists should be first-line management in EIB (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Luke, a 16-year-old basketball player, complains that he can’t finish a game without running out of breath. He says things are at their worst when the game is close and when it’s nearing the end. He doesn’t have the problem during practice, or when he is playing other sports. The team physician suggested using an albuterol inhaler half an hour before game time and when he has symptoms, but he gets only minimal relief. Now he has come to you.
His vital signs, lung exam, and cardiac exam are normal. Results of pulmonary function tests with pre- and post-albuterol challenge done a year ago were also normal. Does Luke have exercise-induced bronchoconstriction (EIB)? How can you be sure? And what can you do to help?
Symptoms like Luke’s are common among athletes of all abilities. They may add up to EIB, a condition with an estimated prevalence of 6% to 12% in the general population—or they may not.1 One study showed that only a third of athletes with symptoms or prior diagnosis of EIB had positive objective testing for the condition, and current studies show that reported symptoms are not an accurate guide in athletes like Luke who do not have underlying asthma.2,3 To treat him correctly, you will need to nail down the diagnosis with additional tests.3,4
Shortness of breath that’s worse than expected
EIB can have many different presentations. The most common symptom is cough associated with exercise.3 Other common signs and symptoms include wheezing, chest tightness, and more severe than expected or worsening shortness of breath. More unusual symptoms include a decrease in performance or fatigue out of proportion to workload. Often patients with EIB have other associated medical conditions, such as allergic rhinitis.
Bronchoconstriction usually occurs with maximal or near maximal exertion. Generally, it takes 5 to 8 minutes of exercising at 80% of maximal heart rate to trigger EIB. Classically, the symptoms peak 5 to 10 minutes after exercise begins.5
Rule out cardiac problems. If EIB is the correct diagnosis, the physical exam is usually normal. The importance of the physical exam is to evaluate for other diagnoses with similar presentations. Conditions to rule out include cardiac problems, exercise-induced hyperventilation, upper and lower respiratory infections or abnormalities, exercise-induced laryngeal dysfunction, exercise-induced anaphylaxis, and gastroesophageal reflux disease (GERD). The differential diagnosis for EIB is summarized in TABLE 1.
Test for asthma. Once you have gone through the differential diagnosis and are comfortable that the symptoms are respiratory, the next step should be pulmonary function tests (PFT), pre- and post-albuterol challenge. Findings of obstruction, such as reduced forced expiratory volume in 1 second (FEV1) or increased lung volume, are consistent with a diagnosis of asthma. In that case, no further workup is needed—unless the patient is unresponsive to asthma treatment. In athletes like Luke who do not have asthma and have a normal nonprovocative spirometry, you can move on to either provocative spirometry or empiric treatment.
TABLE 1
Is it EIB, or something else?
| ETIOLOGY | POSSIBLE DIAGNOSES |
|---|---|
| Pulmonary | Exercise-induced hyperventilation (pseudo-asthma syndrome) Restrictive lung disease Cystic fibrosis Upper and lower respiratory infections Foreign body aspiration |
| Cardiac | Coronary artery disease Congenital and acquired heart defects Cardiomyopathy Congestive heart failure |
| Laryngeal | Exercise-induced laryngeal dysfunction Vocal cord dysfunction Laryngeal prolapse Laryngomalacia |
| Gastroesophageal | Gastroesophageal reflux disease |
| Allergic | Exercise-induced anaphylaxis |
| Other | Athlete is out of shape |
| EIB, exercise-induced bronchoconstriction. | |
| Source: Weiler JM, et al. J Allergy Clin Immunol. 2007.4 | |
Perform provocative spirometry
Direct spirometry is commonly done with a methacholine challenge. This test is less sensitive than indirect testing for EIB patients who do not have underlying asthma.
The gold standard for indirect testing is eucapnic voluntary hyperventilation (EVH). Because EVH requires special equipment, however, it may not be an option in your office. The more reasonable choice is exercise challenge testing, which can be done either in your office or in the milieu—the basketball court, for example—where the athlete’s symptoms usually occur. In an exercise challenge, you get a baseline spirometry measurement, have the athlete exercise to 80% to 90% of maximal heart rate, and then repeat spirometry at short intervals after exercise ends. If you do an exercise challenge in the office, you can reduce false-negative results by maintaining an ambient temperature between 68° and 77°F (20°-25°C) with a relative humidity of less than 50%.6,7
Or try empiric treatment
Empiric treatment is a reasonable strategy for athletes with EIB symptoms, worth trying both for athletes who have underlying asthma and for those who do not. If the athlete with asthma responds to treatment, the problem is solved. For the athlete who does not have asthma, however, there are some exceptions to this approach—specifically, the elite athlete.
In the elite athlete, you will need to confirm the diagnosis because many of the substances used to treat EIB are restricted by governing bodies such as the International Olympic Committee (IOC) and require provocative testing to obtain a therapeutic use exemption.8 There is some debate as to whether nonelite athletes also need bronchoprovocative testing. Some recommendations advise testing all elite and competitive athletes and restricting empiric treatment to recreational athletes.1 For more information on banned or restricted medications, see “Is that drug banned from competition?”.
If you take the empiric approach and the athlete does not respond to treatment, consider further testing to rule out other, more serious problems. In Luke’s case, where empiric treatment with albuterol has failed, indirect testing would be the next step.
Certain medications used in the treatment of asthma and exercise-induced bronchoconstriction (EIB) are considered performance-enhancing drugs and either banned or restricted in athletic competition. The regulatory bodies that make these designations in the United States are the National Collegiate Athletic Association (NCAA) and the International Olympic Committee World Anti-Doping Agency (IOC-WADA). These organizations update their list of banned substances yearly and make the current list available on the Web. You can find the NCAA list at www.pace.edu/emplibrary/NCAA%20LIST%20OF%20BANNED%20SUBSTANCESb.doc and the IOC-WADA list at www.wada-ama.org/rtecontent/document/2009_Prohibited_List_ENG_Final_20_Sept_08.pdf.
The IOC-WADA allows competing athletes to use inhaled corticosteroids and β2 agonists, but requires athletes with asthma to provide documentation that the medication is for therapeutic use. Glucocorticosteroids and oral β2 agonists remain prohibited by the IOC-WADA, but only oral β2 agonists are banned by the NCAA. The NCAA warns that student athletes are responsible for knowing which substances are on the banned list and advises them to consult www.drugfreesport.com for more information. To avoid disqualifying a patient from sports participation, check medications you prescribe with the official lists and be sure your EIB patient has the documentation he or she needs to qualify for a therapeutic use exemption.
Medicate before exercise: SABAs and LABAs
Prophylaxis for EIB usually starts with an inhaled short-acting β2 agonist (SABA) such as albuterol or pirbuterol, taken 15 minutes before starting to exercise.9,10 The effectiveness of both short- and long-acting β2 agonists decreases with frequent use, which may be Luke’s problem. For that reason, patients with mild EIB may choose to use pretreatment medication only for more demanding exercise sessions.11 Advise EIB patients who need daily pretreatment to try adjunctive maintenance therapy (discussed at greater length, below.)
Longer-acting β2 agonists (LABAs) such as salmeterol or formoterol may be effective for prolonged or all-day exercise, but may lose their prophylactic effect with prolonged use.12 Furthermore, the US Food and Drug Administration (FDA) has advised against using LABAs alone because of the possibility of severe asthma episodes or death. LABAs should be used only in conjunction with daily maintenance therapy with inhaled corticosteroids. The properties of these and other EIB medications are summarized in TABLE 2.
TABLE 2
EIB medications
| MEDICATION | INDICATION | DOSE | CAUTIONS | COMMENT |
|---|---|---|---|---|
| Short-acting β2 agonists (SABAs) | ||||
| Albuterol, pirbuterol | Pre-exercise prophylaxis, acute treatment | 2 puffs pre-exercise or 2 puffs every 4-6 h as needed | May cause tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | First-line treatment |
| Mast cell stabilizers | ||||
| Cromolyn | Pre-exercise treatment | 2 puffs 30-45 min before exercise | None | Best combined with SABA. Tell patients not to use for rescue. |
| Inhaled corticosteroids | ||||
| Flunisolide, fluticasone, budesonide, triamcinolone, beclomethasone, mometasone | Daily maintenance | Variable | Can cause oral candidiasis, hoarseness. | Tell patients this is not a rescue inhaler. |
| Leukotriene inhibitors | ||||
| Zafirlukast | Daily maintenance | 20 mg PO, bid | None | Variable response. Works well with inhaled corticosteroids. Low side-effect profile. |
| Montelukast | Daily maintenance, pre-exercise prophylaxis | 10 mg PO daily or up to 2 h pre-exercise | None | Variable response. Works well with inhaled corticosteroids. Low side-effect profile. |
| Zileuton | Daily maintenance | 1200 mg PO, bid | Risk of elevated liver function tests. | Variable response. Low side-effect profile. |
| Combinations | ||||
| Inhaled fluticasone and salmeterol | Daily maintenance | Variable doses (100/50, 250/50, 500/50 mcg/spray); 1 puff bid | Can cause oral candidiasis, hoarseness, tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | Tell patients this is not a rescue inhaler. |
| Inhaled budesonide and formoterol | Daily maintenance | Variable doses (80/4.5, 160/4.5 mcg/spray); 1 puff bid | Can cause oral candidiasis, hoarseness, tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | Tell patients this is not a rescue inhaler. |
| EIB, exercise-induced bronchoconstriction. | ||||
| Adapted from the National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma.9 | ||||
Cromolyn, antileukotrienes are options, too
Mast cell stabilizers (cromolyn) can be used with β2 agonists as prophylactic therapy. When these agents are used together, they have an additive effect.13 The athlete may take them 10 minutes to an hour before exercise. Make sure your patient knows that mast cell stabilizers cannot be used as a rescue inhaler or bronchodilator.
Inhaled corticosteroids (flunisolide, fluticasone, others) may be needed for athletes with poorly controlled chronic asthma; they can also be used as adjunct preventive treatment for athletes who have EIB with no underlying chronic asthma.14-16 Often, inhaled corticosteroids are used as combination therapy with a LABA or an antileukotriene agent (montelukast, zafirlukast; see below). Recent research shows that montelukast in combination with inhaled corticosteroids is more efficacious than LABA with inhaled corticosteroids.14,17
Antileukotriene agents can be especially helpful for EIB in patients with mild, stable asthma.18 Patients who do respond to antileukotriene agents usually respond very favorably. Antileukotrienes offer a reasonable alternative to inhaled corticosteroids and LABAs. They have a low side-effect profile and should be considered as daily prophylaxis.19,20 The effects of montelukast are evident as early as 2 hours after administration, and bronchoprotective effects can last as long as 24 hours.21,22 For that reason, montelukast is especially useful in children whose exercise patterns are not always predictable.
Be prepared for acute exacerbations. Prophylactic medication does not always prevent acute exacerbations. When that happens, your EIB patient will need to use a β2 agonist as rescue therapy. Make sure your patient knows that none of the other medications are effective bronchodilators in acute exacerbations.
Remember, too, that EIB cannot be effectively treated if the athlete has poorly controlled chronic asthma. Underlying causes of asthma exacerbations like allergies or respiratory infections must be addressed and stabilized first, following guidelines of the National Asthma Education and Prevention Program (NAEPP).9 You can access the guidelines at www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
These tips can help the athlete
Encourage athletes with EIB to keep up their exercise routines, because cardiovascular fitness has a beneficial effect on this condition. Fit individuals breathe more slowly, which reduces the likelihood of exacerbations. Of note, though: Certain sports are easier on patients with EIB. Patients may want to keep this in mind when deciding which team they want to go out for. Specifically, indoor sports, where air temperature, humidity, and exposure to allergens are controlled, and sports like baseball, sprinting, or football, which require less prolonged aerobic endurance, are good options.
Tell athletes whose sports require cold, dry conditions—ice skating, or skiing, for instance—to try breathing through a scarf or mask to keep inspired air warm and less irritating.
And tell all athletes with EIB to warm up properly before they start to compete.23 That means a 15-minute warm-up at moderate exertion, followed by a 15- to 30-minute rest period. The rest period is the time to take their medication.
When therapy fails
When an EIB patient fails to respond despite multiple drug therapy, it’s time to reconsider other diagnoses, such as vocal cord dysfunction and severe GERD, which may mimic symptoms of EIB.
On the horizon. Other therapies for possible treatment of EIB are being studied. These include omega-3 fatty acid dietary supplementation and inhaled enoxaparin.24,25 Data are currently insufficient to recommend use of these agents in clinical practice.
As for Luke, indirect testing via exercise challenge was positive for EIB. Adjunctive therapy with montelukast was added to his albuterol inhaler, and the combination has worked well for him. He’s still playing basketball, and enjoying it.
Acknowledgments
The authors thank Ken Rundell, PhD, for reviewing this article. Dr. Rundell is director of the Human Physiology Laboratory at the Keith J. O’Neill Center of Marywood University, Scranton, Pa.
CORRESPONDENCE
Michael A. Krafczyk, MD, FAAFP, St. Luke’s Sports Medicine, 153 Brodhead Rd, Bethlehem, PA 18017; [email protected]
- Don’t rely on self-reported symptoms to diagnose exercise-induced bronchoconstriction (EIB) (A).
- Indirect testing is the best way to diagnose EIB in patients who do not have underlying asthma (A).
- Short-acting β2-agonists should be first-line management in EIB (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Luke, a 16-year-old basketball player, complains that he can’t finish a game without running out of breath. He says things are at their worst when the game is close and when it’s nearing the end. He doesn’t have the problem during practice, or when he is playing other sports. The team physician suggested using an albuterol inhaler half an hour before game time and when he has symptoms, but he gets only minimal relief. Now he has come to you.
His vital signs, lung exam, and cardiac exam are normal. Results of pulmonary function tests with pre- and post-albuterol challenge done a year ago were also normal. Does Luke have exercise-induced bronchoconstriction (EIB)? How can you be sure? And what can you do to help?
Symptoms like Luke’s are common among athletes of all abilities. They may add up to EIB, a condition with an estimated prevalence of 6% to 12% in the general population—or they may not.1 One study showed that only a third of athletes with symptoms or prior diagnosis of EIB had positive objective testing for the condition, and current studies show that reported symptoms are not an accurate guide in athletes like Luke who do not have underlying asthma.2,3 To treat him correctly, you will need to nail down the diagnosis with additional tests.3,4
Shortness of breath that’s worse than expected
EIB can have many different presentations. The most common symptom is cough associated with exercise.3 Other common signs and symptoms include wheezing, chest tightness, and more severe than expected or worsening shortness of breath. More unusual symptoms include a decrease in performance or fatigue out of proportion to workload. Often patients with EIB have other associated medical conditions, such as allergic rhinitis.
Bronchoconstriction usually occurs with maximal or near maximal exertion. Generally, it takes 5 to 8 minutes of exercising at 80% of maximal heart rate to trigger EIB. Classically, the symptoms peak 5 to 10 minutes after exercise begins.5
Rule out cardiac problems. If EIB is the correct diagnosis, the physical exam is usually normal. The importance of the physical exam is to evaluate for other diagnoses with similar presentations. Conditions to rule out include cardiac problems, exercise-induced hyperventilation, upper and lower respiratory infections or abnormalities, exercise-induced laryngeal dysfunction, exercise-induced anaphylaxis, and gastroesophageal reflux disease (GERD). The differential diagnosis for EIB is summarized in TABLE 1.
Test for asthma. Once you have gone through the differential diagnosis and are comfortable that the symptoms are respiratory, the next step should be pulmonary function tests (PFT), pre- and post-albuterol challenge. Findings of obstruction, such as reduced forced expiratory volume in 1 second (FEV1) or increased lung volume, are consistent with a diagnosis of asthma. In that case, no further workup is needed—unless the patient is unresponsive to asthma treatment. In athletes like Luke who do not have asthma and have a normal nonprovocative spirometry, you can move on to either provocative spirometry or empiric treatment.
TABLE 1
Is it EIB, or something else?
| ETIOLOGY | POSSIBLE DIAGNOSES |
|---|---|
| Pulmonary | Exercise-induced hyperventilation (pseudo-asthma syndrome) Restrictive lung disease Cystic fibrosis Upper and lower respiratory infections Foreign body aspiration |
| Cardiac | Coronary artery disease Congenital and acquired heart defects Cardiomyopathy Congestive heart failure |
| Laryngeal | Exercise-induced laryngeal dysfunction Vocal cord dysfunction Laryngeal prolapse Laryngomalacia |
| Gastroesophageal | Gastroesophageal reflux disease |
| Allergic | Exercise-induced anaphylaxis |
| Other | Athlete is out of shape |
| EIB, exercise-induced bronchoconstriction. | |
| Source: Weiler JM, et al. J Allergy Clin Immunol. 2007.4 | |
Perform provocative spirometry
Direct spirometry is commonly done with a methacholine challenge. This test is less sensitive than indirect testing for EIB patients who do not have underlying asthma.
The gold standard for indirect testing is eucapnic voluntary hyperventilation (EVH). Because EVH requires special equipment, however, it may not be an option in your office. The more reasonable choice is exercise challenge testing, which can be done either in your office or in the milieu—the basketball court, for example—where the athlete’s symptoms usually occur. In an exercise challenge, you get a baseline spirometry measurement, have the athlete exercise to 80% to 90% of maximal heart rate, and then repeat spirometry at short intervals after exercise ends. If you do an exercise challenge in the office, you can reduce false-negative results by maintaining an ambient temperature between 68° and 77°F (20°-25°C) with a relative humidity of less than 50%.6,7
Or try empiric treatment
Empiric treatment is a reasonable strategy for athletes with EIB symptoms, worth trying both for athletes who have underlying asthma and for those who do not. If the athlete with asthma responds to treatment, the problem is solved. For the athlete who does not have asthma, however, there are some exceptions to this approach—specifically, the elite athlete.
In the elite athlete, you will need to confirm the diagnosis because many of the substances used to treat EIB are restricted by governing bodies such as the International Olympic Committee (IOC) and require provocative testing to obtain a therapeutic use exemption.8 There is some debate as to whether nonelite athletes also need bronchoprovocative testing. Some recommendations advise testing all elite and competitive athletes and restricting empiric treatment to recreational athletes.1 For more information on banned or restricted medications, see “Is that drug banned from competition?”.
If you take the empiric approach and the athlete does not respond to treatment, consider further testing to rule out other, more serious problems. In Luke’s case, where empiric treatment with albuterol has failed, indirect testing would be the next step.
Certain medications used in the treatment of asthma and exercise-induced bronchoconstriction (EIB) are considered performance-enhancing drugs and either banned or restricted in athletic competition. The regulatory bodies that make these designations in the United States are the National Collegiate Athletic Association (NCAA) and the International Olympic Committee World Anti-Doping Agency (IOC-WADA). These organizations update their list of banned substances yearly and make the current list available on the Web. You can find the NCAA list at www.pace.edu/emplibrary/NCAA%20LIST%20OF%20BANNED%20SUBSTANCESb.doc and the IOC-WADA list at www.wada-ama.org/rtecontent/document/2009_Prohibited_List_ENG_Final_20_Sept_08.pdf.
The IOC-WADA allows competing athletes to use inhaled corticosteroids and β2 agonists, but requires athletes with asthma to provide documentation that the medication is for therapeutic use. Glucocorticosteroids and oral β2 agonists remain prohibited by the IOC-WADA, but only oral β2 agonists are banned by the NCAA. The NCAA warns that student athletes are responsible for knowing which substances are on the banned list and advises them to consult www.drugfreesport.com for more information. To avoid disqualifying a patient from sports participation, check medications you prescribe with the official lists and be sure your EIB patient has the documentation he or she needs to qualify for a therapeutic use exemption.
Medicate before exercise: SABAs and LABAs
Prophylaxis for EIB usually starts with an inhaled short-acting β2 agonist (SABA) such as albuterol or pirbuterol, taken 15 minutes before starting to exercise.9,10 The effectiveness of both short- and long-acting β2 agonists decreases with frequent use, which may be Luke’s problem. For that reason, patients with mild EIB may choose to use pretreatment medication only for more demanding exercise sessions.11 Advise EIB patients who need daily pretreatment to try adjunctive maintenance therapy (discussed at greater length, below.)
Longer-acting β2 agonists (LABAs) such as salmeterol or formoterol may be effective for prolonged or all-day exercise, but may lose their prophylactic effect with prolonged use.12 Furthermore, the US Food and Drug Administration (FDA) has advised against using LABAs alone because of the possibility of severe asthma episodes or death. LABAs should be used only in conjunction with daily maintenance therapy with inhaled corticosteroids. The properties of these and other EIB medications are summarized in TABLE 2.
TABLE 2
EIB medications
| MEDICATION | INDICATION | DOSE | CAUTIONS | COMMENT |
|---|---|---|---|---|
| Short-acting β2 agonists (SABAs) | ||||
| Albuterol, pirbuterol | Pre-exercise prophylaxis, acute treatment | 2 puffs pre-exercise or 2 puffs every 4-6 h as needed | May cause tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | First-line treatment |
| Mast cell stabilizers | ||||
| Cromolyn | Pre-exercise treatment | 2 puffs 30-45 min before exercise | None | Best combined with SABA. Tell patients not to use for rescue. |
| Inhaled corticosteroids | ||||
| Flunisolide, fluticasone, budesonide, triamcinolone, beclomethasone, mometasone | Daily maintenance | Variable | Can cause oral candidiasis, hoarseness. | Tell patients this is not a rescue inhaler. |
| Leukotriene inhibitors | ||||
| Zafirlukast | Daily maintenance | 20 mg PO, bid | None | Variable response. Works well with inhaled corticosteroids. Low side-effect profile. |
| Montelukast | Daily maintenance, pre-exercise prophylaxis | 10 mg PO daily or up to 2 h pre-exercise | None | Variable response. Works well with inhaled corticosteroids. Low side-effect profile. |
| Zileuton | Daily maintenance | 1200 mg PO, bid | Risk of elevated liver function tests. | Variable response. Low side-effect profile. |
| Combinations | ||||
| Inhaled fluticasone and salmeterol | Daily maintenance | Variable doses (100/50, 250/50, 500/50 mcg/spray); 1 puff bid | Can cause oral candidiasis, hoarseness, tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | Tell patients this is not a rescue inhaler. |
| Inhaled budesonide and formoterol | Daily maintenance | Variable doses (80/4.5, 160/4.5 mcg/spray); 1 puff bid | Can cause oral candidiasis, hoarseness, tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | Tell patients this is not a rescue inhaler. |
| EIB, exercise-induced bronchoconstriction. | ||||
| Adapted from the National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma.9 | ||||
Cromolyn, antileukotrienes are options, too
Mast cell stabilizers (cromolyn) can be used with β2 agonists as prophylactic therapy. When these agents are used together, they have an additive effect.13 The athlete may take them 10 minutes to an hour before exercise. Make sure your patient knows that mast cell stabilizers cannot be used as a rescue inhaler or bronchodilator.
Inhaled corticosteroids (flunisolide, fluticasone, others) may be needed for athletes with poorly controlled chronic asthma; they can also be used as adjunct preventive treatment for athletes who have EIB with no underlying chronic asthma.14-16 Often, inhaled corticosteroids are used as combination therapy with a LABA or an antileukotriene agent (montelukast, zafirlukast; see below). Recent research shows that montelukast in combination with inhaled corticosteroids is more efficacious than LABA with inhaled corticosteroids.14,17
Antileukotriene agents can be especially helpful for EIB in patients with mild, stable asthma.18 Patients who do respond to antileukotriene agents usually respond very favorably. Antileukotrienes offer a reasonable alternative to inhaled corticosteroids and LABAs. They have a low side-effect profile and should be considered as daily prophylaxis.19,20 The effects of montelukast are evident as early as 2 hours after administration, and bronchoprotective effects can last as long as 24 hours.21,22 For that reason, montelukast is especially useful in children whose exercise patterns are not always predictable.
Be prepared for acute exacerbations. Prophylactic medication does not always prevent acute exacerbations. When that happens, your EIB patient will need to use a β2 agonist as rescue therapy. Make sure your patient knows that none of the other medications are effective bronchodilators in acute exacerbations.
Remember, too, that EIB cannot be effectively treated if the athlete has poorly controlled chronic asthma. Underlying causes of asthma exacerbations like allergies or respiratory infections must be addressed and stabilized first, following guidelines of the National Asthma Education and Prevention Program (NAEPP).9 You can access the guidelines at www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
These tips can help the athlete
Encourage athletes with EIB to keep up their exercise routines, because cardiovascular fitness has a beneficial effect on this condition. Fit individuals breathe more slowly, which reduces the likelihood of exacerbations. Of note, though: Certain sports are easier on patients with EIB. Patients may want to keep this in mind when deciding which team they want to go out for. Specifically, indoor sports, where air temperature, humidity, and exposure to allergens are controlled, and sports like baseball, sprinting, or football, which require less prolonged aerobic endurance, are good options.
Tell athletes whose sports require cold, dry conditions—ice skating, or skiing, for instance—to try breathing through a scarf or mask to keep inspired air warm and less irritating.
And tell all athletes with EIB to warm up properly before they start to compete.23 That means a 15-minute warm-up at moderate exertion, followed by a 15- to 30-minute rest period. The rest period is the time to take their medication.
When therapy fails
When an EIB patient fails to respond despite multiple drug therapy, it’s time to reconsider other diagnoses, such as vocal cord dysfunction and severe GERD, which may mimic symptoms of EIB.
On the horizon. Other therapies for possible treatment of EIB are being studied. These include omega-3 fatty acid dietary supplementation and inhaled enoxaparin.24,25 Data are currently insufficient to recommend use of these agents in clinical practice.
As for Luke, indirect testing via exercise challenge was positive for EIB. Adjunctive therapy with montelukast was added to his albuterol inhaler, and the combination has worked well for him. He’s still playing basketball, and enjoying it.
Acknowledgments
The authors thank Ken Rundell, PhD, for reviewing this article. Dr. Rundell is director of the Human Physiology Laboratory at the Keith J. O’Neill Center of Marywood University, Scranton, Pa.
CORRESPONDENCE
Michael A. Krafczyk, MD, FAAFP, St. Luke’s Sports Medicine, 153 Brodhead Rd, Bethlehem, PA 18017; [email protected]
1. Holzer K, Brukner P. Screening of athletes for exercise-induced bronchoconstriction. Clin J Sport Med. 2004;14:134-138.
2. Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr. 2002;141:343-348.
3. Rundell KW, Mayers LB, Wilber RL, et al. Self-reported symptoms of exercise-induced asthma in the elite athlete. Med Sci Sports Exerc. 2001;33:208-213.
4. Weiler JM, Bonini S, Coifman R, et al. Ad Hoc Committee of Sports Medicine Committee, American Academy of Allergy, Asthma, and Immunology Work Group Report: exercise-induced asthma. J Allergy Clin Immunol. 2007;119:1349-1358.
5. Parsons JP, Mastronarde JG. Exercise-induced bronchoconstriction in athletes. Chest. 2005;128:3966-3974.
6. Rundell KW, Slee JB. Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J Allergy Clin Immunol. 2008;122:238-246.
7. Rundell KW, Wilber RL, Szmedra L, et al. Exercise-induced asthma screening of elite athletes: field versus laboratory exercise challenges. Med Sci Sports Exerc. 2000;32:309-316.
8. Fitch KD, Sue-Chu M, Anderson SD, et al. Asthma and the elite athlete: summary of the International Olympic Committee’s Consensus Conference, Lausanne Switzerland. January 22-24, 2008. J Allergy Clin Immunol. 2008;122:254-260.
9. National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. NIH publication no. 08-4051. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed September 1, 2007.
10. Anderson S, Seale JP, Ferris L, et al. An evaluation of pharmacotherapy for exercise-induced asthma. J Allergy Clin Immunol. 1979;64:612-624.
11. Hancox RJ, Subbarao P, Kamada D, et al. β2-Agonist tolerance and exercise-induced bronchospasm. Am Respir Crit Care Med. 2002;165:1068-1070.
12. Inman M, O’Byrne PM. The effect of regular inhaled albuterol on exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 1996;153:65-69.
13. Latimer KM, O’Byrne PM, Morris MM, et al. Bronchoconstriction stimulated by airway cooling: better protection with combined inhalation of terbutaline sulphate and cromolyn sodium than with either alone. Am Rev Respir Dis. 1983;128:440-443.
14. Stelmach I, Grzelewski T, Majak P, et al. Effect of different antiasthmatic treatments on exercise-induced bronchoconstriction in children with asthma. J Allergy Clin Immunol. 2008;121:383-389.
15. Koh MS, Tee A, Lasserson TJ, et al. Inhaled corticosteroids compared to placebo for prevention of exercise induced bronchoconstriction. Cochrane Database Syst Rev. 2007;(3):CD002739.-
16. Jonasson G, Carlsen KH, Hultquist C. Low-dose budesonide improves exercise-induced bronchospasm in schoolchildren. Pediatr Allergy Immunol. 2000;11:120-125.
17. Storms W, Chervinsky P, Ghannam AF, et al. Challenge-Rescue Study Group. Respir Med. 2004;98:1051-1062.
18. Leff JA, Busse WW, Pearlman D, et al. Montelukast, a leukotriene-receptor antagonist for the treatment of mild asthma and exercise-induced bronchoconstriction. N Engl J Med. 1998;339:147-152.
19. Steinshamn S, Sandsund M, Sue-Chu M, et al. Effects of montelukast and salmeterol on physical performance and exercise economy in adult asthmatics with exercise-induced bronchoconstriction. Chest. 2004;126:1154-1160.
20. Storms W. Update on montelukast and its role in the treatment of asthma, allergic rhinitis, and exercise-induced bronchoconstriction. Expert Opin Pharmacother. 2007;8:2173-2187.
21. Pearlman DS, van Adelsberg J, Philip G, et al. Onset and duration of protection against exercise-induced bronchoconstriction by a single oral dose of montelukast. Ann Allergy Asthma Immunol. 2006;97:98-104.
22. Philip G, Villaran C, Pearlman DS, et al. Protection against exercise-induced bronchoconstriction two hours after a single oral dose of montelukast. J Asthma. 2007;44:213-217.
23. Storms WW. Review of exercise-induced asthma. Med Sci Sports Exerc. 2003;35:1464-1470.
24. Mickleborough TD, Lindley MR, Ionescu AA, et al. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006;129:39-49.
25. Ahmed T, Gonzalez BJ, Danta I. Prevention of exercise-induced bronchoconstriction by inhaled low-molecular-weight heparin. Am J Respir Crit Care Med. 1999;160:576-581.
1. Holzer K, Brukner P. Screening of athletes for exercise-induced bronchoconstriction. Clin J Sport Med. 2004;14:134-138.
2. Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr. 2002;141:343-348.
3. Rundell KW, Mayers LB, Wilber RL, et al. Self-reported symptoms of exercise-induced asthma in the elite athlete. Med Sci Sports Exerc. 2001;33:208-213.
4. Weiler JM, Bonini S, Coifman R, et al. Ad Hoc Committee of Sports Medicine Committee, American Academy of Allergy, Asthma, and Immunology Work Group Report: exercise-induced asthma. J Allergy Clin Immunol. 2007;119:1349-1358.
5. Parsons JP, Mastronarde JG. Exercise-induced bronchoconstriction in athletes. Chest. 2005;128:3966-3974.
6. Rundell KW, Slee JB. Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J Allergy Clin Immunol. 2008;122:238-246.
7. Rundell KW, Wilber RL, Szmedra L, et al. Exercise-induced asthma screening of elite athletes: field versus laboratory exercise challenges. Med Sci Sports Exerc. 2000;32:309-316.
8. Fitch KD, Sue-Chu M, Anderson SD, et al. Asthma and the elite athlete: summary of the International Olympic Committee’s Consensus Conference, Lausanne Switzerland. January 22-24, 2008. J Allergy Clin Immunol. 2008;122:254-260.
9. National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. NIH publication no. 08-4051. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed September 1, 2007.
10. Anderson S, Seale JP, Ferris L, et al. An evaluation of pharmacotherapy for exercise-induced asthma. J Allergy Clin Immunol. 1979;64:612-624.
11. Hancox RJ, Subbarao P, Kamada D, et al. β2-Agonist tolerance and exercise-induced bronchospasm. Am Respir Crit Care Med. 2002;165:1068-1070.
12. Inman M, O’Byrne PM. The effect of regular inhaled albuterol on exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 1996;153:65-69.
13. Latimer KM, O’Byrne PM, Morris MM, et al. Bronchoconstriction stimulated by airway cooling: better protection with combined inhalation of terbutaline sulphate and cromolyn sodium than with either alone. Am Rev Respir Dis. 1983;128:440-443.
14. Stelmach I, Grzelewski T, Majak P, et al. Effect of different antiasthmatic treatments on exercise-induced bronchoconstriction in children with asthma. J Allergy Clin Immunol. 2008;121:383-389.
15. Koh MS, Tee A, Lasserson TJ, et al. Inhaled corticosteroids compared to placebo for prevention of exercise induced bronchoconstriction. Cochrane Database Syst Rev. 2007;(3):CD002739.-
16. Jonasson G, Carlsen KH, Hultquist C. Low-dose budesonide improves exercise-induced bronchospasm in schoolchildren. Pediatr Allergy Immunol. 2000;11:120-125.
17. Storms W, Chervinsky P, Ghannam AF, et al. Challenge-Rescue Study Group. Respir Med. 2004;98:1051-1062.
18. Leff JA, Busse WW, Pearlman D, et al. Montelukast, a leukotriene-receptor antagonist for the treatment of mild asthma and exercise-induced bronchoconstriction. N Engl J Med. 1998;339:147-152.
19. Steinshamn S, Sandsund M, Sue-Chu M, et al. Effects of montelukast and salmeterol on physical performance and exercise economy in adult asthmatics with exercise-induced bronchoconstriction. Chest. 2004;126:1154-1160.
20. Storms W. Update on montelukast and its role in the treatment of asthma, allergic rhinitis, and exercise-induced bronchoconstriction. Expert Opin Pharmacother. 2007;8:2173-2187.
21. Pearlman DS, van Adelsberg J, Philip G, et al. Onset and duration of protection against exercise-induced bronchoconstriction by a single oral dose of montelukast. Ann Allergy Asthma Immunol. 2006;97:98-104.
22. Philip G, Villaran C, Pearlman DS, et al. Protection against exercise-induced bronchoconstriction two hours after a single oral dose of montelukast. J Asthma. 2007;44:213-217.
23. Storms WW. Review of exercise-induced asthma. Med Sci Sports Exerc. 2003;35:1464-1470.
24. Mickleborough TD, Lindley MR, Ionescu AA, et al. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006;129:39-49.
25. Ahmed T, Gonzalez BJ, Danta I. Prevention of exercise-induced bronchoconstriction by inhaled low-molecular-weight heparin. Am J Respir Crit Care Med. 1999;160:576-581.
Consider PTSD subtypes in patient workup
Posttraumatic stress disorder (PTSD) is a confusing diagnostic category because it includes victims of trauma as well as individuals exposed to trauma. Also, PTSD encompasses exposure to different types of trauma, which can have significant implications for symptom development and treatment.
Consider the treatment history of a male combat veteran who exhibits multiple PTSD symptoms, including nightmares, flashbacks, social isolation, anger, and guilt related to his war experiences. Several psychiatrists saw the patient, which resulted in multiple medication changes but little benefit. On further assessment, the practitioners noted that the veteran’s war experiences were minimally problematic; the prominent nightmares, ruminations, flashbacks, and guilt were related to his witnessing a civilian female being sexually assaulted. The veteran’s guilt about not intervening was the basis of his PTSD. This led to a change in treatment from pharmacotherapy to a focus on supportive therapy.
Conceptualizing subtypes of PTSD—similar to many DSM-IV-TR diagnoses such as phobias or delusional disorders—might help better define the diagnosis. Each sub-type, as conceptualized below, might have its own prognosis and treatment. Our hope is that this strategy will benefit the patient by improving research and evidence-based practice.
PTSD subtypes
Victim-related trauma. Related to witnessing a criminal act or being a victim of a criminal act such as rape or assault. The patient is in a passive role.
Natural disasters, such as a tornado, earthquake, or hurricane.
Survivor guilt. The patient is not a perpetrator and might have been exposed to trauma, but symptoms are related to surviving while others close to the patient did not.
Perpetrator guilt. It is debatable whether this should be a PTSD subtype but our experience suggests that this pattern severely complicates PTSD diagnosis and treatment. It often is not initially disclosed by patients but surfaces when treatment is not working despite a strong therapeutic alliance.
PTSD not otherwise specified. This subtype is typical in patients who were not directly involved in a traumatic event but experienced symptoms related to it. Examples include picking up dead bodies, cleaning up a tornado site, or observing siblings being beaten. This category also may reflect an unclear picture if no primary subtype accounts for the majority of symptoms.
Qualifiers
Individuals who previously have been exposed to trauma are more vulnerable to subsequent trauma. Experiencing ongoing multiple traumatic events—such as in military combat—can have a cumulative effect. Thus, identifying episodes of trauma also should be part of the PTSD assessment.
Single event. The patient is exposed to a single traumatic episode, such as being the victim of a crime.
Multiple events/single episode. The patient is exposed to repeated, related traumatic events. Examples include ongoing military combat or child abuse.
Multiple events. The patient is exposed to ≥2 separate traumatic events. A combination such as this might include a serious motor vehicle accident followed by a natural disaster.
As the diagnosis of PTSD evolves, utilizing subtypes and qualifiers might clarify treatment strategies because some subtypes might be more amenable to certain psychopharmacologic or psychotherapeutic treatment regimens.
Diagnostic confusion
Some researchers question whether traumatic stress causes PTSD syndrome,1 whereas others recommend “tightening” the diagnostic criteria.2 Concerns regarding PTSD diagnosis are multiple and include:
- the importance of ruling out malingering3
- the effects of different diagnostic criteria resulting in disparate prevalence rates
- emphasizing the importance of dysfunction as a criterion for PTSD.4
Conceptual inconsistencies in DSM-IV-TR diagnostic criteria also can lead to confusion. Although there is a category of arousal symptoms, Criterion B4 (intense psychological distress) and Criterion B5 (physiological reactivity) are listed as re-experiencing symptoms rather than arousal symptoms. Finally, the criteria presented do not follow a logical progression. Research suggests that re-experiencing symptoms do not lead to avoidance but result in arousal symptoms, which in turn trigger avoidance.5
1. Bodkin JA, Pope HG, Detke MJ, et al. Is PTSD caused by traumatic stress? J Anxiety Disord. 2007;21:176-182.
2. Spitzer RL, First MB, Wakefield JC. Saving PTSD from itself in DSM-V. J Anxiety Disord. 2007;21:233-241.
3. Rosen GM, Taylor S. Pseudo-PTSD. J Anxiety Disord 2007;21:201-210.
4. Zahava S, Horesh D. Changes in diagnostic criteria for PTSD: implications from two prospective longitudinal studies. Am J Orthopsychiatry. 2007;77:182-188.
5. Resick PA, Monson CM, Chard KM. Cognitive processing therapy: veteran/military version. Washington, DC: Department of Veterans Affairs; 2007.
Posttraumatic stress disorder (PTSD) is a confusing diagnostic category because it includes victims of trauma as well as individuals exposed to trauma. Also, PTSD encompasses exposure to different types of trauma, which can have significant implications for symptom development and treatment.
Consider the treatment history of a male combat veteran who exhibits multiple PTSD symptoms, including nightmares, flashbacks, social isolation, anger, and guilt related to his war experiences. Several psychiatrists saw the patient, which resulted in multiple medication changes but little benefit. On further assessment, the practitioners noted that the veteran’s war experiences were minimally problematic; the prominent nightmares, ruminations, flashbacks, and guilt were related to his witnessing a civilian female being sexually assaulted. The veteran’s guilt about not intervening was the basis of his PTSD. This led to a change in treatment from pharmacotherapy to a focus on supportive therapy.
Conceptualizing subtypes of PTSD—similar to many DSM-IV-TR diagnoses such as phobias or delusional disorders—might help better define the diagnosis. Each sub-type, as conceptualized below, might have its own prognosis and treatment. Our hope is that this strategy will benefit the patient by improving research and evidence-based practice.
PTSD subtypes
Victim-related trauma. Related to witnessing a criminal act or being a victim of a criminal act such as rape or assault. The patient is in a passive role.
Natural disasters, such as a tornado, earthquake, or hurricane.
Survivor guilt. The patient is not a perpetrator and might have been exposed to trauma, but symptoms are related to surviving while others close to the patient did not.
Perpetrator guilt. It is debatable whether this should be a PTSD subtype but our experience suggests that this pattern severely complicates PTSD diagnosis and treatment. It often is not initially disclosed by patients but surfaces when treatment is not working despite a strong therapeutic alliance.
PTSD not otherwise specified. This subtype is typical in patients who were not directly involved in a traumatic event but experienced symptoms related to it. Examples include picking up dead bodies, cleaning up a tornado site, or observing siblings being beaten. This category also may reflect an unclear picture if no primary subtype accounts for the majority of symptoms.
Qualifiers
Individuals who previously have been exposed to trauma are more vulnerable to subsequent trauma. Experiencing ongoing multiple traumatic events—such as in military combat—can have a cumulative effect. Thus, identifying episodes of trauma also should be part of the PTSD assessment.
Single event. The patient is exposed to a single traumatic episode, such as being the victim of a crime.
Multiple events/single episode. The patient is exposed to repeated, related traumatic events. Examples include ongoing military combat or child abuse.
Multiple events. The patient is exposed to ≥2 separate traumatic events. A combination such as this might include a serious motor vehicle accident followed by a natural disaster.
As the diagnosis of PTSD evolves, utilizing subtypes and qualifiers might clarify treatment strategies because some subtypes might be more amenable to certain psychopharmacologic or psychotherapeutic treatment regimens.
Diagnostic confusion
Some researchers question whether traumatic stress causes PTSD syndrome,1 whereas others recommend “tightening” the diagnostic criteria.2 Concerns regarding PTSD diagnosis are multiple and include:
- the importance of ruling out malingering3
- the effects of different diagnostic criteria resulting in disparate prevalence rates
- emphasizing the importance of dysfunction as a criterion for PTSD.4
Conceptual inconsistencies in DSM-IV-TR diagnostic criteria also can lead to confusion. Although there is a category of arousal symptoms, Criterion B4 (intense psychological distress) and Criterion B5 (physiological reactivity) are listed as re-experiencing symptoms rather than arousal symptoms. Finally, the criteria presented do not follow a logical progression. Research suggests that re-experiencing symptoms do not lead to avoidance but result in arousal symptoms, which in turn trigger avoidance.5
Posttraumatic stress disorder (PTSD) is a confusing diagnostic category because it includes victims of trauma as well as individuals exposed to trauma. Also, PTSD encompasses exposure to different types of trauma, which can have significant implications for symptom development and treatment.
Consider the treatment history of a male combat veteran who exhibits multiple PTSD symptoms, including nightmares, flashbacks, social isolation, anger, and guilt related to his war experiences. Several psychiatrists saw the patient, which resulted in multiple medication changes but little benefit. On further assessment, the practitioners noted that the veteran’s war experiences were minimally problematic; the prominent nightmares, ruminations, flashbacks, and guilt were related to his witnessing a civilian female being sexually assaulted. The veteran’s guilt about not intervening was the basis of his PTSD. This led to a change in treatment from pharmacotherapy to a focus on supportive therapy.
Conceptualizing subtypes of PTSD—similar to many DSM-IV-TR diagnoses such as phobias or delusional disorders—might help better define the diagnosis. Each sub-type, as conceptualized below, might have its own prognosis and treatment. Our hope is that this strategy will benefit the patient by improving research and evidence-based practice.
PTSD subtypes
Victim-related trauma. Related to witnessing a criminal act or being a victim of a criminal act such as rape or assault. The patient is in a passive role.
Natural disasters, such as a tornado, earthquake, or hurricane.
Survivor guilt. The patient is not a perpetrator and might have been exposed to trauma, but symptoms are related to surviving while others close to the patient did not.
Perpetrator guilt. It is debatable whether this should be a PTSD subtype but our experience suggests that this pattern severely complicates PTSD diagnosis and treatment. It often is not initially disclosed by patients but surfaces when treatment is not working despite a strong therapeutic alliance.
PTSD not otherwise specified. This subtype is typical in patients who were not directly involved in a traumatic event but experienced symptoms related to it. Examples include picking up dead bodies, cleaning up a tornado site, or observing siblings being beaten. This category also may reflect an unclear picture if no primary subtype accounts for the majority of symptoms.
Qualifiers
Individuals who previously have been exposed to trauma are more vulnerable to subsequent trauma. Experiencing ongoing multiple traumatic events—such as in military combat—can have a cumulative effect. Thus, identifying episodes of trauma also should be part of the PTSD assessment.
Single event. The patient is exposed to a single traumatic episode, such as being the victim of a crime.
Multiple events/single episode. The patient is exposed to repeated, related traumatic events. Examples include ongoing military combat or child abuse.
Multiple events. The patient is exposed to ≥2 separate traumatic events. A combination such as this might include a serious motor vehicle accident followed by a natural disaster.
As the diagnosis of PTSD evolves, utilizing subtypes and qualifiers might clarify treatment strategies because some subtypes might be more amenable to certain psychopharmacologic or psychotherapeutic treatment regimens.
Diagnostic confusion
Some researchers question whether traumatic stress causes PTSD syndrome,1 whereas others recommend “tightening” the diagnostic criteria.2 Concerns regarding PTSD diagnosis are multiple and include:
- the importance of ruling out malingering3
- the effects of different diagnostic criteria resulting in disparate prevalence rates
- emphasizing the importance of dysfunction as a criterion for PTSD.4
Conceptual inconsistencies in DSM-IV-TR diagnostic criteria also can lead to confusion. Although there is a category of arousal symptoms, Criterion B4 (intense psychological distress) and Criterion B5 (physiological reactivity) are listed as re-experiencing symptoms rather than arousal symptoms. Finally, the criteria presented do not follow a logical progression. Research suggests that re-experiencing symptoms do not lead to avoidance but result in arousal symptoms, which in turn trigger avoidance.5
1. Bodkin JA, Pope HG, Detke MJ, et al. Is PTSD caused by traumatic stress? J Anxiety Disord. 2007;21:176-182.
2. Spitzer RL, First MB, Wakefield JC. Saving PTSD from itself in DSM-V. J Anxiety Disord. 2007;21:233-241.
3. Rosen GM, Taylor S. Pseudo-PTSD. J Anxiety Disord 2007;21:201-210.
4. Zahava S, Horesh D. Changes in diagnostic criteria for PTSD: implications from two prospective longitudinal studies. Am J Orthopsychiatry. 2007;77:182-188.
5. Resick PA, Monson CM, Chard KM. Cognitive processing therapy: veteran/military version. Washington, DC: Department of Veterans Affairs; 2007.
1. Bodkin JA, Pope HG, Detke MJ, et al. Is PTSD caused by traumatic stress? J Anxiety Disord. 2007;21:176-182.
2. Spitzer RL, First MB, Wakefield JC. Saving PTSD from itself in DSM-V. J Anxiety Disord. 2007;21:233-241.
3. Rosen GM, Taylor S. Pseudo-PTSD. J Anxiety Disord 2007;21:201-210.
4. Zahava S, Horesh D. Changes in diagnostic criteria for PTSD: implications from two prospective longitudinal studies. Am J Orthopsychiatry. 2007;77:182-188.
5. Resick PA, Monson CM, Chard KM. Cognitive processing therapy: veteran/military version. Washington, DC: Department of Veterans Affairs; 2007.
Chest Pain in the Child and Adolescent
Chest pain is extremely common in children and adolescents—as many as 70% of healthy children experience chest pain. In most instances, following a thorough history and physical examination, no intervention is required.
The incidence of chest pain with a cardiac etiology is extraordinarily low, less than 1%. Patient and family histories and physical examination dictate when management by a general pediatrician is appropriate. First, determine through history if the child has exercise-induced pain. Patients with noncardiac chest pain often have sharp stabbing pain that lasts a few seconds to 1-2 minutes, and the pain is not associated with exercise.
Exercise-induced chest pain is concerning, but the most common cause is exercise-induced bronchospasm. Ask the child or adolescent to describe the painful episodes. If the patient says: “I run, and then I feel like there is an elephant sitting on my chest,” that should prompt referral to a cardiologist. In contrast, if the patient says: “I run, and I feel like I cannot breathe and/or I cough,” that is more likely exercise-induced asthma.
There are red flags in the history and physical examination that prompt referral of the child to a specialist. But keep in mind that overreferral is a concern. Many general pediatricians understandably are scared when a patient presents with chest pain, but most communities do not have the resources to support widespread referral nor is it warranted in most cases.
Anticipatory guidance is critical for pediatricians managing most children and adolescents with chest pain. Inform the typical patient with sharp, stabbing pain and the family members that such episodes are likely to continue in the future. The patient does not necessarily need to return or go to the emergency department every time the pain recurs.
In contrast, a patient with a history of exercise-induced chest pain or who reports passing out during exercise is more of a concern. Ask patients about any extreme fatigue associated with exercise that is different from what their peers experience. Also, children with an unexplained seizure disorder or a history of passing out after an emotional startle (from a loud noise) might have long QT syndrome. Referral to a specialist is warranted. Although Kawasaki disease is rare, consider it in your differential diagnosis; keep in mind that some patients might experience chest pain associated with Marfan syndrome.
In terms of family history, ask if any relatives were diagnosed with long QT syndrome or hypertrophic cardiomyopathy. Family history also is relevant if there were any unexpected or unexplained deaths before age 50 years. A family member who died of cardiac causes before age 50, especially in the absence of typical risk factors, is also a concern. Listen for a murmur, especially a murmur that gets louder when the patient stands. This feature could be consistent with hypertrophic cardiomyopathy.
I do not recommend an electrocardiogram for most patients with a history of sedentary chest pain because there is a high false-positive rate with this test. Also, I generally do not recommend exercise stress tests because they are not helpful in the setting of routine chest pain. For the minority of patients with true exercise-induced chest pain, however, these tests can be useful, but they should be ordered by a cardiologist.
Children's Healthcare of Atlanta provides a Pediatric Sudden Cardiac Death Risk Assessment Form online for any health care provider. This tool can be accessed at www.choa.org/default.aspx?id=7317
Chest pain is extremely common in children and adolescents—as many as 70% of healthy children experience chest pain. In most instances, following a thorough history and physical examination, no intervention is required.
The incidence of chest pain with a cardiac etiology is extraordinarily low, less than 1%. Patient and family histories and physical examination dictate when management by a general pediatrician is appropriate. First, determine through history if the child has exercise-induced pain. Patients with noncardiac chest pain often have sharp stabbing pain that lasts a few seconds to 1-2 minutes, and the pain is not associated with exercise.
Exercise-induced chest pain is concerning, but the most common cause is exercise-induced bronchospasm. Ask the child or adolescent to describe the painful episodes. If the patient says: “I run, and then I feel like there is an elephant sitting on my chest,” that should prompt referral to a cardiologist. In contrast, if the patient says: “I run, and I feel like I cannot breathe and/or I cough,” that is more likely exercise-induced asthma.
There are red flags in the history and physical examination that prompt referral of the child to a specialist. But keep in mind that overreferral is a concern. Many general pediatricians understandably are scared when a patient presents with chest pain, but most communities do not have the resources to support widespread referral nor is it warranted in most cases.
Anticipatory guidance is critical for pediatricians managing most children and adolescents with chest pain. Inform the typical patient with sharp, stabbing pain and the family members that such episodes are likely to continue in the future. The patient does not necessarily need to return or go to the emergency department every time the pain recurs.
In contrast, a patient with a history of exercise-induced chest pain or who reports passing out during exercise is more of a concern. Ask patients about any extreme fatigue associated with exercise that is different from what their peers experience. Also, children with an unexplained seizure disorder or a history of passing out after an emotional startle (from a loud noise) might have long QT syndrome. Referral to a specialist is warranted. Although Kawasaki disease is rare, consider it in your differential diagnosis; keep in mind that some patients might experience chest pain associated with Marfan syndrome.
In terms of family history, ask if any relatives were diagnosed with long QT syndrome or hypertrophic cardiomyopathy. Family history also is relevant if there were any unexpected or unexplained deaths before age 50 years. A family member who died of cardiac causes before age 50, especially in the absence of typical risk factors, is also a concern. Listen for a murmur, especially a murmur that gets louder when the patient stands. This feature could be consistent with hypertrophic cardiomyopathy.
I do not recommend an electrocardiogram for most patients with a history of sedentary chest pain because there is a high false-positive rate with this test. Also, I generally do not recommend exercise stress tests because they are not helpful in the setting of routine chest pain. For the minority of patients with true exercise-induced chest pain, however, these tests can be useful, but they should be ordered by a cardiologist.
Children's Healthcare of Atlanta provides a Pediatric Sudden Cardiac Death Risk Assessment Form online for any health care provider. This tool can be accessed at www.choa.org/default.aspx?id=7317
Chest pain is extremely common in children and adolescents—as many as 70% of healthy children experience chest pain. In most instances, following a thorough history and physical examination, no intervention is required.
The incidence of chest pain with a cardiac etiology is extraordinarily low, less than 1%. Patient and family histories and physical examination dictate when management by a general pediatrician is appropriate. First, determine through history if the child has exercise-induced pain. Patients with noncardiac chest pain often have sharp stabbing pain that lasts a few seconds to 1-2 minutes, and the pain is not associated with exercise.
Exercise-induced chest pain is concerning, but the most common cause is exercise-induced bronchospasm. Ask the child or adolescent to describe the painful episodes. If the patient says: “I run, and then I feel like there is an elephant sitting on my chest,” that should prompt referral to a cardiologist. In contrast, if the patient says: “I run, and I feel like I cannot breathe and/or I cough,” that is more likely exercise-induced asthma.
There are red flags in the history and physical examination that prompt referral of the child to a specialist. But keep in mind that overreferral is a concern. Many general pediatricians understandably are scared when a patient presents with chest pain, but most communities do not have the resources to support widespread referral nor is it warranted in most cases.
Anticipatory guidance is critical for pediatricians managing most children and adolescents with chest pain. Inform the typical patient with sharp, stabbing pain and the family members that such episodes are likely to continue in the future. The patient does not necessarily need to return or go to the emergency department every time the pain recurs.
In contrast, a patient with a history of exercise-induced chest pain or who reports passing out during exercise is more of a concern. Ask patients about any extreme fatigue associated with exercise that is different from what their peers experience. Also, children with an unexplained seizure disorder or a history of passing out after an emotional startle (from a loud noise) might have long QT syndrome. Referral to a specialist is warranted. Although Kawasaki disease is rare, consider it in your differential diagnosis; keep in mind that some patients might experience chest pain associated with Marfan syndrome.
In terms of family history, ask if any relatives were diagnosed with long QT syndrome or hypertrophic cardiomyopathy. Family history also is relevant if there were any unexpected or unexplained deaths before age 50 years. A family member who died of cardiac causes before age 50, especially in the absence of typical risk factors, is also a concern. Listen for a murmur, especially a murmur that gets louder when the patient stands. This feature could be consistent with hypertrophic cardiomyopathy.
I do not recommend an electrocardiogram for most patients with a history of sedentary chest pain because there is a high false-positive rate with this test. Also, I generally do not recommend exercise stress tests because they are not helpful in the setting of routine chest pain. For the minority of patients with true exercise-induced chest pain, however, these tests can be useful, but they should be ordered by a cardiologist.
Children's Healthcare of Atlanta provides a Pediatric Sudden Cardiac Death Risk Assessment Form online for any health care provider. This tool can be accessed at www.choa.org/default.aspx?id=7317
Ticagrelor tops clopidogrel in recent study
Results of the PLATO trial suggest the antiplatelet therapy ticagrelor is superior to clopidogrel in patients with acute coronary syndromes, with or without ST-segment elevation.
Ticagrelor significantly reduced the rate of death from vascular causes, myocardial infarction, or stroke without an increase in major bleeding.
Investigators published the results of the study in The New England Journal of Medicine.
Ticagrelor is an oral, reversible, direct-acting inhibitor of the adenosine diphosphate receptor P2Y12. It has a faster onset and greater platelet inhibition than clopidogrel, which also blocks the adenosine diphosphate receptor P2Y12, but irreversibly.
Lars Wallentin, MD, PhD, of Uppsala Clinical Research Center in Sweden, and his colleagues set out to ascertain whether ticagrelor is superior to clopidogrel for the prevention of vascular events and death.
They enrolled 18,624 patients from 862 centers in 43 countries from October 2006 through July 2008. They randomized the patients to receive 90 mg of ticagrelor twice daily after a loading dose of 180 mg, or 75 mg of clopidogrel after a 300 mg loading dose. Patients in both cohorts took 75-100 mg aspirin daily.
Baseline patient characteristics were similar in the 2 groups.
After a 12-month follow-up, investigators determined that patients in the ticagrelor group experienced significantly fewer deaths from vascular causes, myocardial infarction, or stroke (9.8%) than those in the clopidogrel group (11.7%; P<0.001). And the treatment effect was noticeable within the first 30 days.
Investigators also observed that ticagrelor significantly reduced myocardial infarction alone (5.8% vs 6.9%; P=0.005), death from vascular causes (4.0% vs 5.1%; P=0.001), and death from any cause (4.5% vs 5.9%; P<0.001).
However, ticagrelor did not reduce stroke alone compared to clopidogrel (P=0.22). And patients on tricagrelor experienced more hemorrhagic strokes than those on clopidogrel.
The investigators also determined that ticagrelor did not increase the rate of overall bleeding. However, it did increase the rate of non-procedure-related bleeding.
The investigators noted more dyspnea in the ticagrelor group than the clopidogrel group (14.2% vs 9.2%; P<0.001). The investigators pointed out that few patients dropped out of the study, however, because of dyspnea.
In an accompanying editorial, Albert Schömig, MD, of the Deutsches Herzzentrum München in Germany, emphasized that the absence of increased bleeding with ticagrelor “highlights the important advantage of reversibility in the mechanism of action of ticagrelor.”
Dr Schömig felt that the study would have been stronger, however, if ticagrelor had been administered for at least a year, if the clopidogrel loading dose had been used for all patients in that arm irrespective of whether they had previously been treated with clopidogrel, and if patients had used proton-pump inhibitors less frequently after randomization.
Nevertheless, Dr Schömig concluded that “efforts to develop new effective and safe antithrombotic drug regimens should not be discouraged by the perception that an increase in antithrombotic efficacy is necessarily associated with a higher risk of bleeding.”
The study was supported by AstraZeneca, the company developing ticagrelor. The drug is not yet on the market. ![]()
Results of the PLATO trial suggest the antiplatelet therapy ticagrelor is superior to clopidogrel in patients with acute coronary syndromes, with or without ST-segment elevation.
Ticagrelor significantly reduced the rate of death from vascular causes, myocardial infarction, or stroke without an increase in major bleeding.
Investigators published the results of the study in The New England Journal of Medicine.
Ticagrelor is an oral, reversible, direct-acting inhibitor of the adenosine diphosphate receptor P2Y12. It has a faster onset and greater platelet inhibition than clopidogrel, which also blocks the adenosine diphosphate receptor P2Y12, but irreversibly.
Lars Wallentin, MD, PhD, of Uppsala Clinical Research Center in Sweden, and his colleagues set out to ascertain whether ticagrelor is superior to clopidogrel for the prevention of vascular events and death.
They enrolled 18,624 patients from 862 centers in 43 countries from October 2006 through July 2008. They randomized the patients to receive 90 mg of ticagrelor twice daily after a loading dose of 180 mg, or 75 mg of clopidogrel after a 300 mg loading dose. Patients in both cohorts took 75-100 mg aspirin daily.
Baseline patient characteristics were similar in the 2 groups.
After a 12-month follow-up, investigators determined that patients in the ticagrelor group experienced significantly fewer deaths from vascular causes, myocardial infarction, or stroke (9.8%) than those in the clopidogrel group (11.7%; P<0.001). And the treatment effect was noticeable within the first 30 days.
Investigators also observed that ticagrelor significantly reduced myocardial infarction alone (5.8% vs 6.9%; P=0.005), death from vascular causes (4.0% vs 5.1%; P=0.001), and death from any cause (4.5% vs 5.9%; P<0.001).
However, ticagrelor did not reduce stroke alone compared to clopidogrel (P=0.22). And patients on tricagrelor experienced more hemorrhagic strokes than those on clopidogrel.
The investigators also determined that ticagrelor did not increase the rate of overall bleeding. However, it did increase the rate of non-procedure-related bleeding.
The investigators noted more dyspnea in the ticagrelor group than the clopidogrel group (14.2% vs 9.2%; P<0.001). The investigators pointed out that few patients dropped out of the study, however, because of dyspnea.
In an accompanying editorial, Albert Schömig, MD, of the Deutsches Herzzentrum München in Germany, emphasized that the absence of increased bleeding with ticagrelor “highlights the important advantage of reversibility in the mechanism of action of ticagrelor.”
Dr Schömig felt that the study would have been stronger, however, if ticagrelor had been administered for at least a year, if the clopidogrel loading dose had been used for all patients in that arm irrespective of whether they had previously been treated with clopidogrel, and if patients had used proton-pump inhibitors less frequently after randomization.
Nevertheless, Dr Schömig concluded that “efforts to develop new effective and safe antithrombotic drug regimens should not be discouraged by the perception that an increase in antithrombotic efficacy is necessarily associated with a higher risk of bleeding.”
The study was supported by AstraZeneca, the company developing ticagrelor. The drug is not yet on the market. ![]()
Results of the PLATO trial suggest the antiplatelet therapy ticagrelor is superior to clopidogrel in patients with acute coronary syndromes, with or without ST-segment elevation.
Ticagrelor significantly reduced the rate of death from vascular causes, myocardial infarction, or stroke without an increase in major bleeding.
Investigators published the results of the study in The New England Journal of Medicine.
Ticagrelor is an oral, reversible, direct-acting inhibitor of the adenosine diphosphate receptor P2Y12. It has a faster onset and greater platelet inhibition than clopidogrel, which also blocks the adenosine diphosphate receptor P2Y12, but irreversibly.
Lars Wallentin, MD, PhD, of Uppsala Clinical Research Center in Sweden, and his colleagues set out to ascertain whether ticagrelor is superior to clopidogrel for the prevention of vascular events and death.
They enrolled 18,624 patients from 862 centers in 43 countries from October 2006 through July 2008. They randomized the patients to receive 90 mg of ticagrelor twice daily after a loading dose of 180 mg, or 75 mg of clopidogrel after a 300 mg loading dose. Patients in both cohorts took 75-100 mg aspirin daily.
Baseline patient characteristics were similar in the 2 groups.
After a 12-month follow-up, investigators determined that patients in the ticagrelor group experienced significantly fewer deaths from vascular causes, myocardial infarction, or stroke (9.8%) than those in the clopidogrel group (11.7%; P<0.001). And the treatment effect was noticeable within the first 30 days.
Investigators also observed that ticagrelor significantly reduced myocardial infarction alone (5.8% vs 6.9%; P=0.005), death from vascular causes (4.0% vs 5.1%; P=0.001), and death from any cause (4.5% vs 5.9%; P<0.001).
However, ticagrelor did not reduce stroke alone compared to clopidogrel (P=0.22). And patients on tricagrelor experienced more hemorrhagic strokes than those on clopidogrel.
The investigators also determined that ticagrelor did not increase the rate of overall bleeding. However, it did increase the rate of non-procedure-related bleeding.
The investigators noted more dyspnea in the ticagrelor group than the clopidogrel group (14.2% vs 9.2%; P<0.001). The investigators pointed out that few patients dropped out of the study, however, because of dyspnea.
In an accompanying editorial, Albert Schömig, MD, of the Deutsches Herzzentrum München in Germany, emphasized that the absence of increased bleeding with ticagrelor “highlights the important advantage of reversibility in the mechanism of action of ticagrelor.”
Dr Schömig felt that the study would have been stronger, however, if ticagrelor had been administered for at least a year, if the clopidogrel loading dose had been used for all patients in that arm irrespective of whether they had previously been treated with clopidogrel, and if patients had used proton-pump inhibitors less frequently after randomization.
Nevertheless, Dr Schömig concluded that “efforts to develop new effective and safe antithrombotic drug regimens should not be discouraged by the perception that an increase in antithrombotic efficacy is necessarily associated with a higher risk of bleeding.”
The study was supported by AstraZeneca, the company developing ticagrelor. The drug is not yet on the market. ![]()