User login
Seek and treat: HIV update 2011
With early treatment of human immunodeficiency virus (HIV) infection, we can now expect patients to live a much longer life and, in some situations, have a near-normal lifespan.1 Unfortunately, in screening for HIV infection, the United States lags behind many regions of the world, and infection is often not diagnosed until patients present with advanced disease, ie, the acquired immunodeficiency syndrome (AIDS). In this country there is a critical need to make HIV screening a routine part of medical care in all health settings in order to give patients their best chance for a healthy life, to prevent mother-to-child transmission, and to reduce the spread of HIV in the community.
HIV infection meets the criteria that justify routine screening, as laid out by the World Health Organization2:
- It is a serious health disorder that can be detected before symptoms develop
- Treatment is more beneficial if begun before symptoms develop
- Reliable, inexpensive, and acceptable screening tests exist
- The costs of screening are reasonable in relation to the anticipated benefits.
This article will review the epidemiology of the HIV epidemic, present the benefits of early treatment, and make the case for widely expanding screening for HIV infection in the US health care system.
HIV INFECTION CONTINUES TO BE A LARGE BURDEN
In 2008, an estimated 33.4 million people worldwide were HIV-positive. The vast majority of infected people—more than 22 million—live in sub-Saharan Africa.3
The United States has approximately 1.2 million cases.4 Although this is a small proportion of cases worldwide, it still represents a significant health care burden. In this country, the number of AIDS cases peaked in 1993, and the rate of deaths from AIDS began to decrease over the ensuing years as adequate therapy for HIV was developed. Standard therapy then and now consists of at least three drugs from two different classes.
Unfortunately, we have made little progress on the incidence of this disease. The estimated number of new HIV infections in the United States in 2008 was 56,000 and had remained about the same over the previous 15 years.5,6 Because of improved rates of survival, the prevalence has risen steadily since the mid-1990s to the current estimate of 1.2 million persons living with HIV/AIDS in the US.
About 25% of people infected with HIV are unaware of it. This group accounts for more than half of all new infections annually, which highlights the importance of enhanced screening. Once people know they are infected, they tend to change their behavior and are less likely to spread the disease.7
HIV disproportionately affects minority populations and gay men
Cases of HIV infection are reported among all age groups, although most patients tend to have been infected as young adults. Currently, the largest age group living with HIV is middle-aged. As this cohort grows older, an increasing burden of comorbidities due to aging can be expected. In 5 years, about half of the people with HIV in this country are expected to be 50 years of age or older. Although survival rates have steadily increased due to better treatment, survival tends to be shorter for older people newly diagnosed with HIV.
Worldwide, about an equal number of men and women are infected with HIV, but in the United States infected men outnumber women. In this country, about half the cases of HIV transmission among adults are by male-to-male sexual contact, about 30% are by high-risk heterosexual contact (ie, with a partner known to be HIV-infected or at high risk for being infected), and about 10% are by injection drug use.
In the United States, AIDS is predominantly and disproportionately a disease of minorities and those who live in poverty. African Americans account for the largest number of cases, followed by whites and then by Hispanics. Combined, African Americans and Hispanics account for two-thirds to three-fourths of all new cases, although they make up less than one-fourth of the US population. The incidence rate is nearly 137 per 100,000 for African Americans, 56 per 100,000 for Hispanics, and 19 per 100,000 for whites. The incidence is highest in New York and in the southeast, the geographic areas where the greatest number of minorities and people living in poverty reside. These groups also often lack access to health care.
HIV TREATMENT IS MORE EFFECTIVE IF STARTED EARLY
Treatment guidelines from the US Department of Health and Human Services (DHHS) have changed over the years. When effective medications were first introduced in the 1990s, the trend was to treat everyone as soon as they were diagnosed. As the burden of therapy began to unfold (side effects, cost, adherence, and drug resistance), the consensus was to wait until the CD4 T-cell count dropped to a lower level. As the medications have improved and have become better tolerated, the pendulum has swung back to treating earlier in the course of the disease. Currently, the DHHS recommends that therapy be started at CD4 counts of 350 cells/mL or lower (level of evidence: A1).8 It also recommends therapy for CD4 counts between 350 and 500 cells/mL, but the level of evidence is lower.8
The CD4 T cell is the prime target of the HIV virus and also an important marker of the health of the immune system. The lower the CD4 count at the start of therapy, the more challenging it is to normalize.9 If HIV infection is diagnosed early and therapy is started early, the likelihood is higher of normalizing the CD4 count and preserving immune function.
Progress is being made toward diagnosing HIV earlier. The CD4 count at presentation is increasing, but patients in the United States still present for care later than in other countries. In 1997, the median CD4 count at presentation was 234 cells/mL; in 2007, it was 327 (normal is about 550–1,000). Although this is a significant improvement, more than 50% of patients still have fewer than 350 cells/mL at presentation, which is the current threshold for beginning therapy, according to the most recent guidelines.10
Before triple therapy was available, almost all HIV-infected patients died of AIDS-related diseases. Now, about half of treated HIV-infected patients in Europe and North America die of other causes.11 However, many diseases not previously attributed to AIDS are now also known to be exacerbated by HIV infection.
Cancer risk increases with lower CD4 counts
The cumulative incidence of AIDS-defining cancers (Kaposi sarcoma, non-Hodgkin lymphoma, cervical carcinoma) has decreased steadily from 8.7% in the 1980s to 6.4% during the years 1990 to 1995, and to 2.1% between 1996 and 2006. This is attributable to improved immune function as a result of treatment success with antiviral therapy.12
But the incidence of non-AIDS-defining cancers (Hodgkin disease, anal cancer, oral and respiratory cancers) has increased.11 As therapy has regenerated the immune system, patients are surviving longer and are developing the more common cancers but with higher rates than in the general population.
Higher cancer risk is attributed to reduced immune surveillance. Many of these cancers are associated with viruses, such as human papillomavirus (anal and oral or pharyngeal cancers) and Epstein-Barr virus (Hodgkin disease), which can usually be controlled by a fully functioning immune system. The lower the CD4 count, the higher the risk of cancer, which highlights the need to diagnose HIV and start treatment early.13
Cardiovascular disease increases with lower CD4 counts
Associations have recently been identified between coronary disease and HIV as well as with HIV medications. Protease inhibitors tend to raise the levels of triglycerides, low-density lipoprotein cholesterol, and total cholesterol and increase the risk of heart attack.14
Regardless of therapy, HIV appears to be an independent risk factor for coronary disease. Arterial stiffness, as measured by carotid femoral pulse-wave velocity, was found to be increased among a sample of 80 HIV-infected men. This was associated with the usual risk factors of increasing age, blood pressure, and diabetes, as well as with lower nadir CD4 count.15
Fractures and neurocognitive disorders increase with HIV
Osteoporotic fractures are also more common in patients with HIV than in the general population. Risk factors include the traditional risks of older age, hepatitis C infection, diabetes, and substance abuse, but also nadir CD4 count less than 200.16
The risk of neurocognitive disorders is also associated with lower nadir CD4 counts. The lower the CD4 count, the higher the risk of developing neurocognitive deficits.17 The potential benefits of earlier diagnosis and treatment are obvious based upon the multiple recent findings outlined above.
CLINICAL PRESENTATION OF PRIMARY HIV INFECTION
During primary HIV infection, when patients are first infected, 50% to 90% are symptomatic. Symptoms usually appear in the first 6 weeks. The viral load tends to be highest at this time. Higher viral loads appear directly correlated with the degree of infectivity, highlighting the urgency of finding and treating new infections promptly to help avoid transmission to others.18
The clinical picture during primary infection is similar to that of acute mononucleosis. Signs and symptoms include fever, fatigue, rash, headache, lymphadenopathy, sore throat, and muscle aches. Although this presentation is common to many viral infections, questioning the patient about high-risk behavior (unprotected sex, multiple partners, intravenous drug use) will lead the astute physician to the correct testing and diagnosis.
Other early manifestations include mucocutaneous signs, such as seborrheic dermatitis, psoriasis, folliculitis, and thrush. Laboratory test results demonstrating leukopenia, thrombocytopenia, elevated total protein levels, proteinuria, and transaminitis are also suggestive of HIV infection.
THE CASE FOR INCREASED TESTING AND TREATMENT
The estimated prevalence of HIV in the United States is approximately 0.3%. However, its prevalence in Washington, DC, is 3%, which rivals rates in some areas of the developing world. From 2004 to 2008, health officials made a concerted effort in Washington, DC, to screen more people, particularly those at high risk. The number of publicly funded HIV tests performed increased by a factor of 3.7, and the number of newly reported cases increased by 17%. There was also a significant increase in the median CD4 count at the time of HIV diagnosis and a significant delay in time to progression to AIDS after HIV diagnosis.19
A study in British Columbia expanded access to highly active antiretroviral therapy during 2004 through 2009. High-risk individuals were targeted for increased screening. All those diagnosed with HIV were provided free medication. This resulted in a 50% reduction in new diagnoses of HIV infection throughout the community, especially among injectable drug users, a usually marginalized population. The proportion of patients with HIV-1 RNA levels above 1,500 copies/mL fell from about 50% to about 20%, indicating that the viral load—a measure of infectivity throughout the community—was reduced. Interestingly, this trend occurred during a time of increased rates of gonorrhea, syphilis, and other sexually transmitted diseases known to be associated with enhanced HIV transmission.20
In Africa, antiretroviral therapy was offered to discordant couples (one partner was infected with HIV and the other was not). Among those who chose therapy, the rate of HIV transmission was 92% lower than in those not receiving antiretroviral drugs,21 once again demonstrating that control of HIV by treatment can lead to decreased transmission.
US HIV testing is inadequate
The current state of HIV testing in the United States needs to be improved. Testing is not performed routinely, leading to delayed diagnosis when patients present with symptomatic, advanced disease. Patients who are tested late (within 12 months before being diagnosed with AIDS) tend to be younger and less educated and are more likely to be heterosexual and either African American or Hispanic than patients who are tested earlier.22 When retrospectively evaluated, these patients often have been in the health care system but not tested. Routine universal screening and targeted testing could lead to a much earlier diagnosis and potential better long-term outcomes.
A 1996 survey of 95 academic emergency departments found that for patients with suspected sexually transmitted infections, 93% of physicians said they screen for gonorrhea, 88% for Chlamydia infection, 58% for syphilis, but only 3% for HIV.23 Sexually transmitted infections and HIV are often transmitted together.
A similar 2002 survey of 154 emergency department providers who saw an average of 13 patients with sexually transmitted infections per week found that only 10% always recommend HIV testing to these patients. Reasons given for not testing were concern about follow-up (51%), not having a “certified” counselor (45%), HIV testing being too time-consuming (19%), and HIV testing being unavailable (27%).24
Although most HIV tests are given by private doctors and health maintenance organizations, the likelihood of finding patients with HIV is greatest in hospitals, emergency departments, outpatient clinics, and public community clinics.
The Advancing HIV Prevention initiative of the US Centers for Disease Control and Prevention (CDC) has four priorities:
- To make voluntary HIV testing a routine part of medical care
- To implement new models for diagnosing HIV infection outside medical settings
- To prevent HIV infection by working with patients with HIV and their partners
- To further decrease the rate of perinatal HIV transmission.
Rapid tests for HIV are available
There is a public health need to have rapid HIV testing available in all health care settings. With standard HIV tests, which can take 48 to 72 hours to run, about one-third of patients do not return for results. Subsequently locating them can be a huge challenge and is sometimes impossible. The ability to have rapid test results can improve this situation. It is especially important in prenatal care settings, where the mother can be immediately treated to reduce the risk of transmission to the child. Rapid testing increases the feasibility of testing in multiple venues, particularly acute-care settings with almost immediate results and linkage to care.
Several rapid tests are available and can be performed on whole blood, serum, plasma, and oral fluid. The tests provide reliable results in minutes, with 99% sensitivity and specificity. Positive results must be confirmed by subsequent two-stage laboratory testing, enzyme-linked immunosorbent assay, and Western blot. Patients who have negative or have indeterminate results on Western blot testing should be tested again after 4 weeks.
The cost-effectiveness of routine screening for HIV, even in populations with a low prevalence, is similar to that of commonly accepted interventions.25 In populations with a 1% prevalence of HIV, the cost is $15,078 per quality-adjusted life-year.26 Even if the prevalence is less than 0.05%, the cost is less than $50,000 per quality-adjusted life-year, which is normally the cutoff for acceptability for screening tests.25,26
‘OPT-OUT’ TESTING
In the past, patients were asked if they would like to have HIV testing (“opt-in” testing). It is now recommended that physicians request testing to be performed (“opt-out” testing). This still allows the patient to decline but also conveys a “matter of fact” nonjudgmental message, indicative of a routine procedure no different than other screening tests. When testing was done on an opt-in basis, only 35% of pregnant women agreed to be tested. Some women felt that accepting an HIV test indicated that they engage in high-risk behavior. When testing was instead offered as routine but with an opportunity to decline, 88% accepted testing, and they were significantly less anxious about testing.27
CDC RECOMMENDATIONS
The CDC now recommends that routine, voluntary HIV screening be done for all persons ages 13 to 64 in health care settings, regardless of risk.28 Screening should be repeated at least annually in persons with known risk. Screening should be done on an opt-out basis, with the opportunity to ask questions and the option to decline. Consent for HIV testing should be included with general consent for care. A separate signed informed consent is not recommended, and verbal consent can merely be documented in the medical record. Prevention counseling in conjunction with HIV screening in health care settings is not required.
Testing should be done in all health care settings, including primary care settings, inpatient services, emergency departments, urgent care clinics, and sexually transmitted disease clinics. Test results should be communicated in the same manner as other diagnostic and screening care. Clinical HIV care should be available onsite or reliable referral to qualified providers should be established.
For pregnant women, the CDC recommends universal opt-out HIV screening, with HIV testing as part of the routine panel of prenatal screening tests. The consent for prenatal care includes HIV testing, with notification and the option to decline. Women should be tested again in the third trimester if they are known to be at risk for HIV, and in areas and health care facilities in which the prevalence of HIV is high.
In women whose HIV status is undocumented in labor and delivery, opt-out rapid testing should be performed, and antiretroviral prophylaxis should be given on the basis of the rapid test result. Rapid testing of the newborn is recommended if the mother’s status is unknown at delivery, and antiretroviral prophylaxis should be started within 12 hours of birth on the basis of the rapid test result.
Widespread routine screening and earlier treatment could significantly reduce the incidence and improve the outcomes of HIV in this country. Health care providers are encouraged to adopt these practices.
- Van Sighem A, Gras L, Reiss P, Brinkman K, de Wolf F, and ATHENA Natl Observational Cohort Study. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 526.
- World Health Organization. Principles and Practice of Screening for Disease. WHO Public Health Paper, 1968.
- Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO). Global Facts & Figures 09. http://data.unaids.org/pub/FactSheet/2009/20091124_FS_global_en.pdf. Accessed 1/4/2011.
- World Health Organization. Epidemiological Fact Sheet on HIV and AIDS. Core data on epidemiology and response. United States of America. 2008 Update. http://apps.who.int/globalatlas/predefinedReports/EFS2008/full/EFS2008_US.pdf. Accessed 1/4/2011.
- US Centers for Disease Control and Prevention. HIV Surveillance Report, 2008; vol. 20. http://www.cdc.gov/hiv/topics/surveillance/resources/reports/. Published June 2010. Accessed 8/7/2010.
- Hall HI, Song R, Rhodes P, et al; HIV Incidence Surveillance Group. Estimation of HIV incidence in the United States. JAMA 2008; 300:520–529.
- Marks G, Crepaz N, Janssen RS. Estimated sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 2006; 20:1447–1450.
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. December 1, 2009;1–161. http://www.aidsinfo.nih.gov/ContentFiles/AdultsandAdolescentGL.pdf. Accessed 1/4/2011.
- Palella F, Armon C, Buchacz , et al; the HOPS Investigators. CD4 at HAART initiation predicts long term CD4 responses and mortality from AIDS and non-AIDS causes in the HIV Outpatient Study (HOPS). Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 983.
- Althoff K, Gange S, Klein M, et al; the North American-AIDS Cohort Collaboration on Res and Design. Late presentation for HIV care in the United States and Canada. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 982.
- Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis 2010; 50:1387–1396.
- Simard E, Pfeiffer R, Engels E. Cancer incidence and cancer-attributable mortality among persons with AIDS in the United States. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 27.
- Silverberg M, Xu L, Chao C, et al. Immunodeficiency, HIV RNA levels, and risk of non-AIDS-defining cancers. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 28.
- DAD Study Group, Friis-Møller N, Reiss P, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–1735.
- Ho J, Deeks S, Hecht F, et al. Earlier initiation of antiretroviral therapy in HIV-infected individuals is associated with reduced arterial stiffness. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 707.
- Dao C, Young B, Buchacz K, Baker R, Brooks J, and the HIV Outpatient Study Investigators. Higher and increasing rates of fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared to the general US population 1994 to 2008. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 128.
- Ellis R, Heaton R, Letendre S, et al; the CHARTER Group. Higher CD4 nadir is associated with reduced rates of HIV-associated neurocognitive disorders in the CHARTER study: potential implications for early treatment initiation. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 429.
- Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med 1996; 125:257–264.
- Castel A, Samala R, Griffin A, et al. Monitoring the impact of expanded HIV testing in the District of Columbia using population-based HIV/AIDS surveillance data. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 34.
- Montaner J, Wood E, Kerr T, et al. Association of expanded HAART coverage with a decrease in new HIV diagnoses, particularly mong injection drug users in British Columbia, Canada. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 88LB.
- Donnell D, Kiarie J, Thomas K, et al. ART and risk of heterosexual HIV-1 transmissin in HIV-1 serodiscordant African couples: a multinational prospective study. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 136.
- Centers for Disease Control and Prevention. Late versus early testing of HIV—16 sites, United States, 2000–2003. MMWR Morb Mortal Wkly Rep 2003; Jun 27; 52( 25):581–586.
- Wilson SR, Mitchell C, Bradbury DR, Chavez J. Testing for HIV: current practies in the academic ED. Am J Emerg Med 1999; 17:346–356.
- Fincher-Mergi M, Cartone KJ, Mischler J, Pasieka P, Lerner EB, Billittier AJ. Assessment of emergency department heatlh care professionals’ behaviors regaridng HIV testing and referral for patients with STDs. AIDS Patient Care STDs 2002; 16:549–553.
- Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. N Engl J Med 2005; 352:586–595.
- Sanders GD, Gayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med 2005; 352:570–585.
- Simpson WM, Johnstone FD, Goldberg DJ, Gormley SM, Hart GJ. Antenatal HIV testing: assessment of a routine voluntary approach. BMJ 1999; 318:1660–1661.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55(RR-14):1–17.
With early treatment of human immunodeficiency virus (HIV) infection, we can now expect patients to live a much longer life and, in some situations, have a near-normal lifespan.1 Unfortunately, in screening for HIV infection, the United States lags behind many regions of the world, and infection is often not diagnosed until patients present with advanced disease, ie, the acquired immunodeficiency syndrome (AIDS). In this country there is a critical need to make HIV screening a routine part of medical care in all health settings in order to give patients their best chance for a healthy life, to prevent mother-to-child transmission, and to reduce the spread of HIV in the community.
HIV infection meets the criteria that justify routine screening, as laid out by the World Health Organization2:
- It is a serious health disorder that can be detected before symptoms develop
- Treatment is more beneficial if begun before symptoms develop
- Reliable, inexpensive, and acceptable screening tests exist
- The costs of screening are reasonable in relation to the anticipated benefits.
This article will review the epidemiology of the HIV epidemic, present the benefits of early treatment, and make the case for widely expanding screening for HIV infection in the US health care system.
HIV INFECTION CONTINUES TO BE A LARGE BURDEN
In 2008, an estimated 33.4 million people worldwide were HIV-positive. The vast majority of infected people—more than 22 million—live in sub-Saharan Africa.3
The United States has approximately 1.2 million cases.4 Although this is a small proportion of cases worldwide, it still represents a significant health care burden. In this country, the number of AIDS cases peaked in 1993, and the rate of deaths from AIDS began to decrease over the ensuing years as adequate therapy for HIV was developed. Standard therapy then and now consists of at least three drugs from two different classes.
Unfortunately, we have made little progress on the incidence of this disease. The estimated number of new HIV infections in the United States in 2008 was 56,000 and had remained about the same over the previous 15 years.5,6 Because of improved rates of survival, the prevalence has risen steadily since the mid-1990s to the current estimate of 1.2 million persons living with HIV/AIDS in the US.
About 25% of people infected with HIV are unaware of it. This group accounts for more than half of all new infections annually, which highlights the importance of enhanced screening. Once people know they are infected, they tend to change their behavior and are less likely to spread the disease.7
HIV disproportionately affects minority populations and gay men
Cases of HIV infection are reported among all age groups, although most patients tend to have been infected as young adults. Currently, the largest age group living with HIV is middle-aged. As this cohort grows older, an increasing burden of comorbidities due to aging can be expected. In 5 years, about half of the people with HIV in this country are expected to be 50 years of age or older. Although survival rates have steadily increased due to better treatment, survival tends to be shorter for older people newly diagnosed with HIV.
Worldwide, about an equal number of men and women are infected with HIV, but in the United States infected men outnumber women. In this country, about half the cases of HIV transmission among adults are by male-to-male sexual contact, about 30% are by high-risk heterosexual contact (ie, with a partner known to be HIV-infected or at high risk for being infected), and about 10% are by injection drug use.
In the United States, AIDS is predominantly and disproportionately a disease of minorities and those who live in poverty. African Americans account for the largest number of cases, followed by whites and then by Hispanics. Combined, African Americans and Hispanics account for two-thirds to three-fourths of all new cases, although they make up less than one-fourth of the US population. The incidence rate is nearly 137 per 100,000 for African Americans, 56 per 100,000 for Hispanics, and 19 per 100,000 for whites. The incidence is highest in New York and in the southeast, the geographic areas where the greatest number of minorities and people living in poverty reside. These groups also often lack access to health care.
HIV TREATMENT IS MORE EFFECTIVE IF STARTED EARLY
Treatment guidelines from the US Department of Health and Human Services (DHHS) have changed over the years. When effective medications were first introduced in the 1990s, the trend was to treat everyone as soon as they were diagnosed. As the burden of therapy began to unfold (side effects, cost, adherence, and drug resistance), the consensus was to wait until the CD4 T-cell count dropped to a lower level. As the medications have improved and have become better tolerated, the pendulum has swung back to treating earlier in the course of the disease. Currently, the DHHS recommends that therapy be started at CD4 counts of 350 cells/mL or lower (level of evidence: A1).8 It also recommends therapy for CD4 counts between 350 and 500 cells/mL, but the level of evidence is lower.8
The CD4 T cell is the prime target of the HIV virus and also an important marker of the health of the immune system. The lower the CD4 count at the start of therapy, the more challenging it is to normalize.9 If HIV infection is diagnosed early and therapy is started early, the likelihood is higher of normalizing the CD4 count and preserving immune function.
Progress is being made toward diagnosing HIV earlier. The CD4 count at presentation is increasing, but patients in the United States still present for care later than in other countries. In 1997, the median CD4 count at presentation was 234 cells/mL; in 2007, it was 327 (normal is about 550–1,000). Although this is a significant improvement, more than 50% of patients still have fewer than 350 cells/mL at presentation, which is the current threshold for beginning therapy, according to the most recent guidelines.10
Before triple therapy was available, almost all HIV-infected patients died of AIDS-related diseases. Now, about half of treated HIV-infected patients in Europe and North America die of other causes.11 However, many diseases not previously attributed to AIDS are now also known to be exacerbated by HIV infection.
Cancer risk increases with lower CD4 counts
The cumulative incidence of AIDS-defining cancers (Kaposi sarcoma, non-Hodgkin lymphoma, cervical carcinoma) has decreased steadily from 8.7% in the 1980s to 6.4% during the years 1990 to 1995, and to 2.1% between 1996 and 2006. This is attributable to improved immune function as a result of treatment success with antiviral therapy.12
But the incidence of non-AIDS-defining cancers (Hodgkin disease, anal cancer, oral and respiratory cancers) has increased.11 As therapy has regenerated the immune system, patients are surviving longer and are developing the more common cancers but with higher rates than in the general population.
Higher cancer risk is attributed to reduced immune surveillance. Many of these cancers are associated with viruses, such as human papillomavirus (anal and oral or pharyngeal cancers) and Epstein-Barr virus (Hodgkin disease), which can usually be controlled by a fully functioning immune system. The lower the CD4 count, the higher the risk of cancer, which highlights the need to diagnose HIV and start treatment early.13
Cardiovascular disease increases with lower CD4 counts
Associations have recently been identified between coronary disease and HIV as well as with HIV medications. Protease inhibitors tend to raise the levels of triglycerides, low-density lipoprotein cholesterol, and total cholesterol and increase the risk of heart attack.14
Regardless of therapy, HIV appears to be an independent risk factor for coronary disease. Arterial stiffness, as measured by carotid femoral pulse-wave velocity, was found to be increased among a sample of 80 HIV-infected men. This was associated with the usual risk factors of increasing age, blood pressure, and diabetes, as well as with lower nadir CD4 count.15
Fractures and neurocognitive disorders increase with HIV
Osteoporotic fractures are also more common in patients with HIV than in the general population. Risk factors include the traditional risks of older age, hepatitis C infection, diabetes, and substance abuse, but also nadir CD4 count less than 200.16
The risk of neurocognitive disorders is also associated with lower nadir CD4 counts. The lower the CD4 count, the higher the risk of developing neurocognitive deficits.17 The potential benefits of earlier diagnosis and treatment are obvious based upon the multiple recent findings outlined above.
CLINICAL PRESENTATION OF PRIMARY HIV INFECTION
During primary HIV infection, when patients are first infected, 50% to 90% are symptomatic. Symptoms usually appear in the first 6 weeks. The viral load tends to be highest at this time. Higher viral loads appear directly correlated with the degree of infectivity, highlighting the urgency of finding and treating new infections promptly to help avoid transmission to others.18
The clinical picture during primary infection is similar to that of acute mononucleosis. Signs and symptoms include fever, fatigue, rash, headache, lymphadenopathy, sore throat, and muscle aches. Although this presentation is common to many viral infections, questioning the patient about high-risk behavior (unprotected sex, multiple partners, intravenous drug use) will lead the astute physician to the correct testing and diagnosis.
Other early manifestations include mucocutaneous signs, such as seborrheic dermatitis, psoriasis, folliculitis, and thrush. Laboratory test results demonstrating leukopenia, thrombocytopenia, elevated total protein levels, proteinuria, and transaminitis are also suggestive of HIV infection.
THE CASE FOR INCREASED TESTING AND TREATMENT
The estimated prevalence of HIV in the United States is approximately 0.3%. However, its prevalence in Washington, DC, is 3%, which rivals rates in some areas of the developing world. From 2004 to 2008, health officials made a concerted effort in Washington, DC, to screen more people, particularly those at high risk. The number of publicly funded HIV tests performed increased by a factor of 3.7, and the number of newly reported cases increased by 17%. There was also a significant increase in the median CD4 count at the time of HIV diagnosis and a significant delay in time to progression to AIDS after HIV diagnosis.19
A study in British Columbia expanded access to highly active antiretroviral therapy during 2004 through 2009. High-risk individuals were targeted for increased screening. All those diagnosed with HIV were provided free medication. This resulted in a 50% reduction in new diagnoses of HIV infection throughout the community, especially among injectable drug users, a usually marginalized population. The proportion of patients with HIV-1 RNA levels above 1,500 copies/mL fell from about 50% to about 20%, indicating that the viral load—a measure of infectivity throughout the community—was reduced. Interestingly, this trend occurred during a time of increased rates of gonorrhea, syphilis, and other sexually transmitted diseases known to be associated with enhanced HIV transmission.20
In Africa, antiretroviral therapy was offered to discordant couples (one partner was infected with HIV and the other was not). Among those who chose therapy, the rate of HIV transmission was 92% lower than in those not receiving antiretroviral drugs,21 once again demonstrating that control of HIV by treatment can lead to decreased transmission.
US HIV testing is inadequate
The current state of HIV testing in the United States needs to be improved. Testing is not performed routinely, leading to delayed diagnosis when patients present with symptomatic, advanced disease. Patients who are tested late (within 12 months before being diagnosed with AIDS) tend to be younger and less educated and are more likely to be heterosexual and either African American or Hispanic than patients who are tested earlier.22 When retrospectively evaluated, these patients often have been in the health care system but not tested. Routine universal screening and targeted testing could lead to a much earlier diagnosis and potential better long-term outcomes.
A 1996 survey of 95 academic emergency departments found that for patients with suspected sexually transmitted infections, 93% of physicians said they screen for gonorrhea, 88% for Chlamydia infection, 58% for syphilis, but only 3% for HIV.23 Sexually transmitted infections and HIV are often transmitted together.
A similar 2002 survey of 154 emergency department providers who saw an average of 13 patients with sexually transmitted infections per week found that only 10% always recommend HIV testing to these patients. Reasons given for not testing were concern about follow-up (51%), not having a “certified” counselor (45%), HIV testing being too time-consuming (19%), and HIV testing being unavailable (27%).24
Although most HIV tests are given by private doctors and health maintenance organizations, the likelihood of finding patients with HIV is greatest in hospitals, emergency departments, outpatient clinics, and public community clinics.
The Advancing HIV Prevention initiative of the US Centers for Disease Control and Prevention (CDC) has four priorities:
- To make voluntary HIV testing a routine part of medical care
- To implement new models for diagnosing HIV infection outside medical settings
- To prevent HIV infection by working with patients with HIV and their partners
- To further decrease the rate of perinatal HIV transmission.
Rapid tests for HIV are available
There is a public health need to have rapid HIV testing available in all health care settings. With standard HIV tests, which can take 48 to 72 hours to run, about one-third of patients do not return for results. Subsequently locating them can be a huge challenge and is sometimes impossible. The ability to have rapid test results can improve this situation. It is especially important in prenatal care settings, where the mother can be immediately treated to reduce the risk of transmission to the child. Rapid testing increases the feasibility of testing in multiple venues, particularly acute-care settings with almost immediate results and linkage to care.
Several rapid tests are available and can be performed on whole blood, serum, plasma, and oral fluid. The tests provide reliable results in minutes, with 99% sensitivity and specificity. Positive results must be confirmed by subsequent two-stage laboratory testing, enzyme-linked immunosorbent assay, and Western blot. Patients who have negative or have indeterminate results on Western blot testing should be tested again after 4 weeks.
The cost-effectiveness of routine screening for HIV, even in populations with a low prevalence, is similar to that of commonly accepted interventions.25 In populations with a 1% prevalence of HIV, the cost is $15,078 per quality-adjusted life-year.26 Even if the prevalence is less than 0.05%, the cost is less than $50,000 per quality-adjusted life-year, which is normally the cutoff for acceptability for screening tests.25,26
‘OPT-OUT’ TESTING
In the past, patients were asked if they would like to have HIV testing (“opt-in” testing). It is now recommended that physicians request testing to be performed (“opt-out” testing). This still allows the patient to decline but also conveys a “matter of fact” nonjudgmental message, indicative of a routine procedure no different than other screening tests. When testing was done on an opt-in basis, only 35% of pregnant women agreed to be tested. Some women felt that accepting an HIV test indicated that they engage in high-risk behavior. When testing was instead offered as routine but with an opportunity to decline, 88% accepted testing, and they were significantly less anxious about testing.27
CDC RECOMMENDATIONS
The CDC now recommends that routine, voluntary HIV screening be done for all persons ages 13 to 64 in health care settings, regardless of risk.28 Screening should be repeated at least annually in persons with known risk. Screening should be done on an opt-out basis, with the opportunity to ask questions and the option to decline. Consent for HIV testing should be included with general consent for care. A separate signed informed consent is not recommended, and verbal consent can merely be documented in the medical record. Prevention counseling in conjunction with HIV screening in health care settings is not required.
Testing should be done in all health care settings, including primary care settings, inpatient services, emergency departments, urgent care clinics, and sexually transmitted disease clinics. Test results should be communicated in the same manner as other diagnostic and screening care. Clinical HIV care should be available onsite or reliable referral to qualified providers should be established.
For pregnant women, the CDC recommends universal opt-out HIV screening, with HIV testing as part of the routine panel of prenatal screening tests. The consent for prenatal care includes HIV testing, with notification and the option to decline. Women should be tested again in the third trimester if they are known to be at risk for HIV, and in areas and health care facilities in which the prevalence of HIV is high.
In women whose HIV status is undocumented in labor and delivery, opt-out rapid testing should be performed, and antiretroviral prophylaxis should be given on the basis of the rapid test result. Rapid testing of the newborn is recommended if the mother’s status is unknown at delivery, and antiretroviral prophylaxis should be started within 12 hours of birth on the basis of the rapid test result.
Widespread routine screening and earlier treatment could significantly reduce the incidence and improve the outcomes of HIV in this country. Health care providers are encouraged to adopt these practices.
With early treatment of human immunodeficiency virus (HIV) infection, we can now expect patients to live a much longer life and, in some situations, have a near-normal lifespan.1 Unfortunately, in screening for HIV infection, the United States lags behind many regions of the world, and infection is often not diagnosed until patients present with advanced disease, ie, the acquired immunodeficiency syndrome (AIDS). In this country there is a critical need to make HIV screening a routine part of medical care in all health settings in order to give patients their best chance for a healthy life, to prevent mother-to-child transmission, and to reduce the spread of HIV in the community.
HIV infection meets the criteria that justify routine screening, as laid out by the World Health Organization2:
- It is a serious health disorder that can be detected before symptoms develop
- Treatment is more beneficial if begun before symptoms develop
- Reliable, inexpensive, and acceptable screening tests exist
- The costs of screening are reasonable in relation to the anticipated benefits.
This article will review the epidemiology of the HIV epidemic, present the benefits of early treatment, and make the case for widely expanding screening for HIV infection in the US health care system.
HIV INFECTION CONTINUES TO BE A LARGE BURDEN
In 2008, an estimated 33.4 million people worldwide were HIV-positive. The vast majority of infected people—more than 22 million—live in sub-Saharan Africa.3
The United States has approximately 1.2 million cases.4 Although this is a small proportion of cases worldwide, it still represents a significant health care burden. In this country, the number of AIDS cases peaked in 1993, and the rate of deaths from AIDS began to decrease over the ensuing years as adequate therapy for HIV was developed. Standard therapy then and now consists of at least three drugs from two different classes.
Unfortunately, we have made little progress on the incidence of this disease. The estimated number of new HIV infections in the United States in 2008 was 56,000 and had remained about the same over the previous 15 years.5,6 Because of improved rates of survival, the prevalence has risen steadily since the mid-1990s to the current estimate of 1.2 million persons living with HIV/AIDS in the US.
About 25% of people infected with HIV are unaware of it. This group accounts for more than half of all new infections annually, which highlights the importance of enhanced screening. Once people know they are infected, they tend to change their behavior and are less likely to spread the disease.7
HIV disproportionately affects minority populations and gay men
Cases of HIV infection are reported among all age groups, although most patients tend to have been infected as young adults. Currently, the largest age group living with HIV is middle-aged. As this cohort grows older, an increasing burden of comorbidities due to aging can be expected. In 5 years, about half of the people with HIV in this country are expected to be 50 years of age or older. Although survival rates have steadily increased due to better treatment, survival tends to be shorter for older people newly diagnosed with HIV.
Worldwide, about an equal number of men and women are infected with HIV, but in the United States infected men outnumber women. In this country, about half the cases of HIV transmission among adults are by male-to-male sexual contact, about 30% are by high-risk heterosexual contact (ie, with a partner known to be HIV-infected or at high risk for being infected), and about 10% are by injection drug use.
In the United States, AIDS is predominantly and disproportionately a disease of minorities and those who live in poverty. African Americans account for the largest number of cases, followed by whites and then by Hispanics. Combined, African Americans and Hispanics account for two-thirds to three-fourths of all new cases, although they make up less than one-fourth of the US population. The incidence rate is nearly 137 per 100,000 for African Americans, 56 per 100,000 for Hispanics, and 19 per 100,000 for whites. The incidence is highest in New York and in the southeast, the geographic areas where the greatest number of minorities and people living in poverty reside. These groups also often lack access to health care.
HIV TREATMENT IS MORE EFFECTIVE IF STARTED EARLY
Treatment guidelines from the US Department of Health and Human Services (DHHS) have changed over the years. When effective medications were first introduced in the 1990s, the trend was to treat everyone as soon as they were diagnosed. As the burden of therapy began to unfold (side effects, cost, adherence, and drug resistance), the consensus was to wait until the CD4 T-cell count dropped to a lower level. As the medications have improved and have become better tolerated, the pendulum has swung back to treating earlier in the course of the disease. Currently, the DHHS recommends that therapy be started at CD4 counts of 350 cells/mL or lower (level of evidence: A1).8 It also recommends therapy for CD4 counts between 350 and 500 cells/mL, but the level of evidence is lower.8
The CD4 T cell is the prime target of the HIV virus and also an important marker of the health of the immune system. The lower the CD4 count at the start of therapy, the more challenging it is to normalize.9 If HIV infection is diagnosed early and therapy is started early, the likelihood is higher of normalizing the CD4 count and preserving immune function.
Progress is being made toward diagnosing HIV earlier. The CD4 count at presentation is increasing, but patients in the United States still present for care later than in other countries. In 1997, the median CD4 count at presentation was 234 cells/mL; in 2007, it was 327 (normal is about 550–1,000). Although this is a significant improvement, more than 50% of patients still have fewer than 350 cells/mL at presentation, which is the current threshold for beginning therapy, according to the most recent guidelines.10
Before triple therapy was available, almost all HIV-infected patients died of AIDS-related diseases. Now, about half of treated HIV-infected patients in Europe and North America die of other causes.11 However, many diseases not previously attributed to AIDS are now also known to be exacerbated by HIV infection.
Cancer risk increases with lower CD4 counts
The cumulative incidence of AIDS-defining cancers (Kaposi sarcoma, non-Hodgkin lymphoma, cervical carcinoma) has decreased steadily from 8.7% in the 1980s to 6.4% during the years 1990 to 1995, and to 2.1% between 1996 and 2006. This is attributable to improved immune function as a result of treatment success with antiviral therapy.12
But the incidence of non-AIDS-defining cancers (Hodgkin disease, anal cancer, oral and respiratory cancers) has increased.11 As therapy has regenerated the immune system, patients are surviving longer and are developing the more common cancers but with higher rates than in the general population.
Higher cancer risk is attributed to reduced immune surveillance. Many of these cancers are associated with viruses, such as human papillomavirus (anal and oral or pharyngeal cancers) and Epstein-Barr virus (Hodgkin disease), which can usually be controlled by a fully functioning immune system. The lower the CD4 count, the higher the risk of cancer, which highlights the need to diagnose HIV and start treatment early.13
Cardiovascular disease increases with lower CD4 counts
Associations have recently been identified between coronary disease and HIV as well as with HIV medications. Protease inhibitors tend to raise the levels of triglycerides, low-density lipoprotein cholesterol, and total cholesterol and increase the risk of heart attack.14
Regardless of therapy, HIV appears to be an independent risk factor for coronary disease. Arterial stiffness, as measured by carotid femoral pulse-wave velocity, was found to be increased among a sample of 80 HIV-infected men. This was associated with the usual risk factors of increasing age, blood pressure, and diabetes, as well as with lower nadir CD4 count.15
Fractures and neurocognitive disorders increase with HIV
Osteoporotic fractures are also more common in patients with HIV than in the general population. Risk factors include the traditional risks of older age, hepatitis C infection, diabetes, and substance abuse, but also nadir CD4 count less than 200.16
The risk of neurocognitive disorders is also associated with lower nadir CD4 counts. The lower the CD4 count, the higher the risk of developing neurocognitive deficits.17 The potential benefits of earlier diagnosis and treatment are obvious based upon the multiple recent findings outlined above.
CLINICAL PRESENTATION OF PRIMARY HIV INFECTION
During primary HIV infection, when patients are first infected, 50% to 90% are symptomatic. Symptoms usually appear in the first 6 weeks. The viral load tends to be highest at this time. Higher viral loads appear directly correlated with the degree of infectivity, highlighting the urgency of finding and treating new infections promptly to help avoid transmission to others.18
The clinical picture during primary infection is similar to that of acute mononucleosis. Signs and symptoms include fever, fatigue, rash, headache, lymphadenopathy, sore throat, and muscle aches. Although this presentation is common to many viral infections, questioning the patient about high-risk behavior (unprotected sex, multiple partners, intravenous drug use) will lead the astute physician to the correct testing and diagnosis.
Other early manifestations include mucocutaneous signs, such as seborrheic dermatitis, psoriasis, folliculitis, and thrush. Laboratory test results demonstrating leukopenia, thrombocytopenia, elevated total protein levels, proteinuria, and transaminitis are also suggestive of HIV infection.
THE CASE FOR INCREASED TESTING AND TREATMENT
The estimated prevalence of HIV in the United States is approximately 0.3%. However, its prevalence in Washington, DC, is 3%, which rivals rates in some areas of the developing world. From 2004 to 2008, health officials made a concerted effort in Washington, DC, to screen more people, particularly those at high risk. The number of publicly funded HIV tests performed increased by a factor of 3.7, and the number of newly reported cases increased by 17%. There was also a significant increase in the median CD4 count at the time of HIV diagnosis and a significant delay in time to progression to AIDS after HIV diagnosis.19
A study in British Columbia expanded access to highly active antiretroviral therapy during 2004 through 2009. High-risk individuals were targeted for increased screening. All those diagnosed with HIV were provided free medication. This resulted in a 50% reduction in new diagnoses of HIV infection throughout the community, especially among injectable drug users, a usually marginalized population. The proportion of patients with HIV-1 RNA levels above 1,500 copies/mL fell from about 50% to about 20%, indicating that the viral load—a measure of infectivity throughout the community—was reduced. Interestingly, this trend occurred during a time of increased rates of gonorrhea, syphilis, and other sexually transmitted diseases known to be associated with enhanced HIV transmission.20
In Africa, antiretroviral therapy was offered to discordant couples (one partner was infected with HIV and the other was not). Among those who chose therapy, the rate of HIV transmission was 92% lower than in those not receiving antiretroviral drugs,21 once again demonstrating that control of HIV by treatment can lead to decreased transmission.
US HIV testing is inadequate
The current state of HIV testing in the United States needs to be improved. Testing is not performed routinely, leading to delayed diagnosis when patients present with symptomatic, advanced disease. Patients who are tested late (within 12 months before being diagnosed with AIDS) tend to be younger and less educated and are more likely to be heterosexual and either African American or Hispanic than patients who are tested earlier.22 When retrospectively evaluated, these patients often have been in the health care system but not tested. Routine universal screening and targeted testing could lead to a much earlier diagnosis and potential better long-term outcomes.
A 1996 survey of 95 academic emergency departments found that for patients with suspected sexually transmitted infections, 93% of physicians said they screen for gonorrhea, 88% for Chlamydia infection, 58% for syphilis, but only 3% for HIV.23 Sexually transmitted infections and HIV are often transmitted together.
A similar 2002 survey of 154 emergency department providers who saw an average of 13 patients with sexually transmitted infections per week found that only 10% always recommend HIV testing to these patients. Reasons given for not testing were concern about follow-up (51%), not having a “certified” counselor (45%), HIV testing being too time-consuming (19%), and HIV testing being unavailable (27%).24
Although most HIV tests are given by private doctors and health maintenance organizations, the likelihood of finding patients with HIV is greatest in hospitals, emergency departments, outpatient clinics, and public community clinics.
The Advancing HIV Prevention initiative of the US Centers for Disease Control and Prevention (CDC) has four priorities:
- To make voluntary HIV testing a routine part of medical care
- To implement new models for diagnosing HIV infection outside medical settings
- To prevent HIV infection by working with patients with HIV and their partners
- To further decrease the rate of perinatal HIV transmission.
Rapid tests for HIV are available
There is a public health need to have rapid HIV testing available in all health care settings. With standard HIV tests, which can take 48 to 72 hours to run, about one-third of patients do not return for results. Subsequently locating them can be a huge challenge and is sometimes impossible. The ability to have rapid test results can improve this situation. It is especially important in prenatal care settings, where the mother can be immediately treated to reduce the risk of transmission to the child. Rapid testing increases the feasibility of testing in multiple venues, particularly acute-care settings with almost immediate results and linkage to care.
Several rapid tests are available and can be performed on whole blood, serum, plasma, and oral fluid. The tests provide reliable results in minutes, with 99% sensitivity and specificity. Positive results must be confirmed by subsequent two-stage laboratory testing, enzyme-linked immunosorbent assay, and Western blot. Patients who have negative or have indeterminate results on Western blot testing should be tested again after 4 weeks.
The cost-effectiveness of routine screening for HIV, even in populations with a low prevalence, is similar to that of commonly accepted interventions.25 In populations with a 1% prevalence of HIV, the cost is $15,078 per quality-adjusted life-year.26 Even if the prevalence is less than 0.05%, the cost is less than $50,000 per quality-adjusted life-year, which is normally the cutoff for acceptability for screening tests.25,26
‘OPT-OUT’ TESTING
In the past, patients were asked if they would like to have HIV testing (“opt-in” testing). It is now recommended that physicians request testing to be performed (“opt-out” testing). This still allows the patient to decline but also conveys a “matter of fact” nonjudgmental message, indicative of a routine procedure no different than other screening tests. When testing was done on an opt-in basis, only 35% of pregnant women agreed to be tested. Some women felt that accepting an HIV test indicated that they engage in high-risk behavior. When testing was instead offered as routine but with an opportunity to decline, 88% accepted testing, and they were significantly less anxious about testing.27
CDC RECOMMENDATIONS
The CDC now recommends that routine, voluntary HIV screening be done for all persons ages 13 to 64 in health care settings, regardless of risk.28 Screening should be repeated at least annually in persons with known risk. Screening should be done on an opt-out basis, with the opportunity to ask questions and the option to decline. Consent for HIV testing should be included with general consent for care. A separate signed informed consent is not recommended, and verbal consent can merely be documented in the medical record. Prevention counseling in conjunction with HIV screening in health care settings is not required.
Testing should be done in all health care settings, including primary care settings, inpatient services, emergency departments, urgent care clinics, and sexually transmitted disease clinics. Test results should be communicated in the same manner as other diagnostic and screening care. Clinical HIV care should be available onsite or reliable referral to qualified providers should be established.
For pregnant women, the CDC recommends universal opt-out HIV screening, with HIV testing as part of the routine panel of prenatal screening tests. The consent for prenatal care includes HIV testing, with notification and the option to decline. Women should be tested again in the third trimester if they are known to be at risk for HIV, and in areas and health care facilities in which the prevalence of HIV is high.
In women whose HIV status is undocumented in labor and delivery, opt-out rapid testing should be performed, and antiretroviral prophylaxis should be given on the basis of the rapid test result. Rapid testing of the newborn is recommended if the mother’s status is unknown at delivery, and antiretroviral prophylaxis should be started within 12 hours of birth on the basis of the rapid test result.
Widespread routine screening and earlier treatment could significantly reduce the incidence and improve the outcomes of HIV in this country. Health care providers are encouraged to adopt these practices.
- Van Sighem A, Gras L, Reiss P, Brinkman K, de Wolf F, and ATHENA Natl Observational Cohort Study. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 526.
- World Health Organization. Principles and Practice of Screening for Disease. WHO Public Health Paper, 1968.
- Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO). Global Facts & Figures 09. http://data.unaids.org/pub/FactSheet/2009/20091124_FS_global_en.pdf. Accessed 1/4/2011.
- World Health Organization. Epidemiological Fact Sheet on HIV and AIDS. Core data on epidemiology and response. United States of America. 2008 Update. http://apps.who.int/globalatlas/predefinedReports/EFS2008/full/EFS2008_US.pdf. Accessed 1/4/2011.
- US Centers for Disease Control and Prevention. HIV Surveillance Report, 2008; vol. 20. http://www.cdc.gov/hiv/topics/surveillance/resources/reports/. Published June 2010. Accessed 8/7/2010.
- Hall HI, Song R, Rhodes P, et al; HIV Incidence Surveillance Group. Estimation of HIV incidence in the United States. JAMA 2008; 300:520–529.
- Marks G, Crepaz N, Janssen RS. Estimated sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 2006; 20:1447–1450.
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. December 1, 2009;1–161. http://www.aidsinfo.nih.gov/ContentFiles/AdultsandAdolescentGL.pdf. Accessed 1/4/2011.
- Palella F, Armon C, Buchacz , et al; the HOPS Investigators. CD4 at HAART initiation predicts long term CD4 responses and mortality from AIDS and non-AIDS causes in the HIV Outpatient Study (HOPS). Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 983.
- Althoff K, Gange S, Klein M, et al; the North American-AIDS Cohort Collaboration on Res and Design. Late presentation for HIV care in the United States and Canada. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 982.
- Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis 2010; 50:1387–1396.
- Simard E, Pfeiffer R, Engels E. Cancer incidence and cancer-attributable mortality among persons with AIDS in the United States. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 27.
- Silverberg M, Xu L, Chao C, et al. Immunodeficiency, HIV RNA levels, and risk of non-AIDS-defining cancers. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 28.
- DAD Study Group, Friis-Møller N, Reiss P, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–1735.
- Ho J, Deeks S, Hecht F, et al. Earlier initiation of antiretroviral therapy in HIV-infected individuals is associated with reduced arterial stiffness. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 707.
- Dao C, Young B, Buchacz K, Baker R, Brooks J, and the HIV Outpatient Study Investigators. Higher and increasing rates of fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared to the general US population 1994 to 2008. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 128.
- Ellis R, Heaton R, Letendre S, et al; the CHARTER Group. Higher CD4 nadir is associated with reduced rates of HIV-associated neurocognitive disorders in the CHARTER study: potential implications for early treatment initiation. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 429.
- Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med 1996; 125:257–264.
- Castel A, Samala R, Griffin A, et al. Monitoring the impact of expanded HIV testing in the District of Columbia using population-based HIV/AIDS surveillance data. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 34.
- Montaner J, Wood E, Kerr T, et al. Association of expanded HAART coverage with a decrease in new HIV diagnoses, particularly mong injection drug users in British Columbia, Canada. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 88LB.
- Donnell D, Kiarie J, Thomas K, et al. ART and risk of heterosexual HIV-1 transmissin in HIV-1 serodiscordant African couples: a multinational prospective study. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 136.
- Centers for Disease Control and Prevention. Late versus early testing of HIV—16 sites, United States, 2000–2003. MMWR Morb Mortal Wkly Rep 2003; Jun 27; 52( 25):581–586.
- Wilson SR, Mitchell C, Bradbury DR, Chavez J. Testing for HIV: current practies in the academic ED. Am J Emerg Med 1999; 17:346–356.
- Fincher-Mergi M, Cartone KJ, Mischler J, Pasieka P, Lerner EB, Billittier AJ. Assessment of emergency department heatlh care professionals’ behaviors regaridng HIV testing and referral for patients with STDs. AIDS Patient Care STDs 2002; 16:549–553.
- Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. N Engl J Med 2005; 352:586–595.
- Sanders GD, Gayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med 2005; 352:570–585.
- Simpson WM, Johnstone FD, Goldberg DJ, Gormley SM, Hart GJ. Antenatal HIV testing: assessment of a routine voluntary approach. BMJ 1999; 318:1660–1661.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55(RR-14):1–17.
- Van Sighem A, Gras L, Reiss P, Brinkman K, de Wolf F, and ATHENA Natl Observational Cohort Study. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 526.
- World Health Organization. Principles and Practice of Screening for Disease. WHO Public Health Paper, 1968.
- Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO). Global Facts & Figures 09. http://data.unaids.org/pub/FactSheet/2009/20091124_FS_global_en.pdf. Accessed 1/4/2011.
- World Health Organization. Epidemiological Fact Sheet on HIV and AIDS. Core data on epidemiology and response. United States of America. 2008 Update. http://apps.who.int/globalatlas/predefinedReports/EFS2008/full/EFS2008_US.pdf. Accessed 1/4/2011.
- US Centers for Disease Control and Prevention. HIV Surveillance Report, 2008; vol. 20. http://www.cdc.gov/hiv/topics/surveillance/resources/reports/. Published June 2010. Accessed 8/7/2010.
- Hall HI, Song R, Rhodes P, et al; HIV Incidence Surveillance Group. Estimation of HIV incidence in the United States. JAMA 2008; 300:520–529.
- Marks G, Crepaz N, Janssen RS. Estimated sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 2006; 20:1447–1450.
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. December 1, 2009;1–161. http://www.aidsinfo.nih.gov/ContentFiles/AdultsandAdolescentGL.pdf. Accessed 1/4/2011.
- Palella F, Armon C, Buchacz , et al; the HOPS Investigators. CD4 at HAART initiation predicts long term CD4 responses and mortality from AIDS and non-AIDS causes in the HIV Outpatient Study (HOPS). Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 983.
- Althoff K, Gange S, Klein M, et al; the North American-AIDS Cohort Collaboration on Res and Design. Late presentation for HIV care in the United States and Canada. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 982.
- Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis 2010; 50:1387–1396.
- Simard E, Pfeiffer R, Engels E. Cancer incidence and cancer-attributable mortality among persons with AIDS in the United States. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 27.
- Silverberg M, Xu L, Chao C, et al. Immunodeficiency, HIV RNA levels, and risk of non-AIDS-defining cancers. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 28.
- DAD Study Group, Friis-Møller N, Reiss P, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–1735.
- Ho J, Deeks S, Hecht F, et al. Earlier initiation of antiretroviral therapy in HIV-infected individuals is associated with reduced arterial stiffness. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 707.
- Dao C, Young B, Buchacz K, Baker R, Brooks J, and the HIV Outpatient Study Investigators. Higher and increasing rates of fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared to the general US population 1994 to 2008. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 128.
- Ellis R, Heaton R, Letendre S, et al; the CHARTER Group. Higher CD4 nadir is associated with reduced rates of HIV-associated neurocognitive disorders in the CHARTER study: potential implications for early treatment initiation. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 429.
- Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med 1996; 125:257–264.
- Castel A, Samala R, Griffin A, et al. Monitoring the impact of expanded HIV testing in the District of Columbia using population-based HIV/AIDS surveillance data. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 34.
- Montaner J, Wood E, Kerr T, et al. Association of expanded HAART coverage with a decrease in new HIV diagnoses, particularly mong injection drug users in British Columbia, Canada. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 88LB.
- Donnell D, Kiarie J, Thomas K, et al. ART and risk of heterosexual HIV-1 transmissin in HIV-1 serodiscordant African couples: a multinational prospective study. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, February 16–19, 2010. Abstract 136.
- Centers for Disease Control and Prevention. Late versus early testing of HIV—16 sites, United States, 2000–2003. MMWR Morb Mortal Wkly Rep 2003; Jun 27; 52( 25):581–586.
- Wilson SR, Mitchell C, Bradbury DR, Chavez J. Testing for HIV: current practies in the academic ED. Am J Emerg Med 1999; 17:346–356.
- Fincher-Mergi M, Cartone KJ, Mischler J, Pasieka P, Lerner EB, Billittier AJ. Assessment of emergency department heatlh care professionals’ behaviors regaridng HIV testing and referral for patients with STDs. AIDS Patient Care STDs 2002; 16:549–553.
- Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. N Engl J Med 2005; 352:586–595.
- Sanders GD, Gayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med 2005; 352:570–585.
- Simpson WM, Johnstone FD, Goldberg DJ, Gormley SM, Hart GJ. Antenatal HIV testing: assessment of a routine voluntary approach. BMJ 1999; 318:1660–1661.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55(RR-14):1–17.
KEY POINTS
- Recommendations from the US Centers for Disease Control and Prevention call for routine HIV screening for all people ages 13 to 64 at least once regardless of their risk profile, and annual testing for people with known risk factors for acquiring HIV.
- Early treatment of HIV infection may reduce the risk of cancer, cardiovascular disease, neurocognitive disorders, and osteoporotic fractures and improve the rate of survival compared with patients treated late in the course of HIV infection.
- Finding and treating patients early in the course of infection has the potential to reduce infectivity in the community.
- Reliable rapid testing is now available to screen for HIV in community settings, emergency departments, and public health clinics, and during labor for those not tested in the prenatal period. It is also useful when follow-up is uncertain.
Exercises for air travel
These exercises should be repeated every hour on a flight when you are awake.
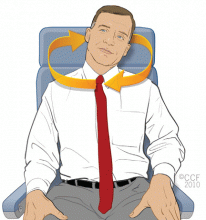
Neck roll
With your shoulders and arms relaxed and hanging down, tilt your head to your left, hold for a few seconds, then slowly roll your head toward your back and hold for a few seconds, then slowly roll your head toward your right shoulder and hold for a few seconds, and then slowly roll your head toward your chest and hold for a few seconds. Repeat this exercise for a total of five times clockwise and then five times counterclockwise.
Shoulder roll
While in your seat with your arms on the arm rests, move both shoulders in a circular motion from front to back five times and then repeat in the opposite direction.
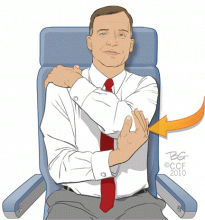
Shoulder stretch
While in your seat, put your left hand on your right shoulder. With your right hand, grasp your elbow and pull your left elbow toward your right side. Hold this position for 15 seconds and then switch arms and repeat the stretch with the opposite side. Repeat these stretches five times with each arm.
Knee-to-chest stretch
While in your seat, lean forward slightly and grab your knee just below the joint. Slowly pull your knee toward your chest and hold for 15 seconds. Repeat the stretch with your other knee. Repeat the cycle five times.
Ankle circles
Raise your feet off the floor and rotate them in a circular motion five times clockwise and then five times counterclockwise.
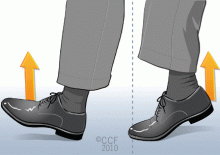
Foot pumps
With your heels on the floor, first raise your toes as high as you can and hold that position for 10 seconds. Then lower your toes until they touch the floor, and then raise your heels off the floor as much as you can, keeping your toes on the floor, and hold that position for 10 seconds. Repeat this exercise five times.
Adapted with permission from Continental Airlines
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints are available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Web site, www.clevelandclinic.org/health
These exercises should be repeated every hour on a flight when you are awake.
Neck roll
With your shoulders and arms relaxed and hanging down, tilt your head to your left, hold for a few seconds, then slowly roll your head toward your back and hold for a few seconds, then slowly roll your head toward your right shoulder and hold for a few seconds, and then slowly roll your head toward your chest and hold for a few seconds. Repeat this exercise for a total of five times clockwise and then five times counterclockwise.
Shoulder roll
While in your seat with your arms on the arm rests, move both shoulders in a circular motion from front to back five times and then repeat in the opposite direction.
Shoulder stretch
While in your seat, put your left hand on your right shoulder. With your right hand, grasp your elbow and pull your left elbow toward your right side. Hold this position for 15 seconds and then switch arms and repeat the stretch with the opposite side. Repeat these stretches five times with each arm.
Knee-to-chest stretch
While in your seat, lean forward slightly and grab your knee just below the joint. Slowly pull your knee toward your chest and hold for 15 seconds. Repeat the stretch with your other knee. Repeat the cycle five times.
Ankle circles
Raise your feet off the floor and rotate them in a circular motion five times clockwise and then five times counterclockwise.
Foot pumps
With your heels on the floor, first raise your toes as high as you can and hold that position for 10 seconds. Then lower your toes until they touch the floor, and then raise your heels off the floor as much as you can, keeping your toes on the floor, and hold that position for 10 seconds. Repeat this exercise five times.
Adapted with permission from Continental Airlines
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints are available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Web site, www.clevelandclinic.org/health
These exercises should be repeated every hour on a flight when you are awake.
Neck roll
With your shoulders and arms relaxed and hanging down, tilt your head to your left, hold for a few seconds, then slowly roll your head toward your back and hold for a few seconds, then slowly roll your head toward your right shoulder and hold for a few seconds, and then slowly roll your head toward your chest and hold for a few seconds. Repeat this exercise for a total of five times clockwise and then five times counterclockwise.
Shoulder roll
While in your seat with your arms on the arm rests, move both shoulders in a circular motion from front to back five times and then repeat in the opposite direction.
Shoulder stretch
While in your seat, put your left hand on your right shoulder. With your right hand, grasp your elbow and pull your left elbow toward your right side. Hold this position for 15 seconds and then switch arms and repeat the stretch with the opposite side. Repeat these stretches five times with each arm.
Knee-to-chest stretch
While in your seat, lean forward slightly and grab your knee just below the joint. Slowly pull your knee toward your chest and hold for 15 seconds. Repeat the stretch with your other knee. Repeat the cycle five times.
Ankle circles
Raise your feet off the floor and rotate them in a circular motion five times clockwise and then five times counterclockwise.
Foot pumps
With your heels on the floor, first raise your toes as high as you can and hold that position for 10 seconds. Then lower your toes until they touch the floor, and then raise your heels off the floor as much as you can, keeping your toes on the floor, and hold that position for 10 seconds. Repeat this exercise five times.
Adapted with permission from Continental Airlines
This information is provided by your physician and the Cleveland Clinic Journal of Medicine. It is not designed to replace a physician’s medical assessment and judgment.
This page may be reproduced noncommercially to share with patients. Any other reproduction is subject to Cleveland Clinic Journal of Medicine approval. Bulk color reprints are available by calling 216-444-2661.
For patient information on hundreds of health topics, see the Web site, www.clevelandclinic.org/health
REV, Metras Beat Rastelli for TGA, VSD, and LVOTO
Optimal surgical management of patients with transposition of the great arteries, ventricular septal defect, and left ventricular outflow obstruction is still considered controversial. Although the Rastelli operation is the most commonly performed procedure, the Réparation à l'Etage Ventriculaire procedure and Metras modification yielded the best long-term results for both survival and event-free survival, according to a retrospective study of 146 patients who underwent surgery from 1980 to 2008 in eight European hospitals.
The multicenter study compared use and outcomes of several different surgical operations for transposition of the great arteries (TGA), ventricular septal defect (VSD), and left ventricular outflow obstruction (LVOTO), according to a report published in the European Journal of Cardio-thoracic Surgery.
A total of 141 patients had TGA, VSD, and LVOTO; 5 patients had the TGA type of double-outlet right ventricle (DORV) with LVOTO. Only those patients for whom the surgical method chosen was equivalent to those for TGA, VSD, and LVOTO were included in the study; all other DORV types were excluded, according to Dr. Mark Gerard Hazekamp of Leids Universitair Medisch Centrum, Leiden, the Netherlands, and his colleagues from various European universities on behalf of the European Congenital Heart Surgeons Association.
The procedures investigated were the Rastelli (82 patients), arterial (24) and atrial (5) switch operation with relief of LVOTO, Reparation l'Etage Ventriculaire (REV) procedure (7), and Metras modification (24), as well as the Nikaidoh (4). The type of surgery used has traditionally been different in different countries, they said, with the REV procedure and Metras modification mainly in France and the Rastelli procedure being the norm in most other countries.
Patients had a median age at operation of 21.5 months (range 0.2-165.1 months) and a median weight of 10 kg (range 2.0-41.0 kg). Pulmonary stenosis was found in 119 patients, while 27 had pulmonary atresia. LVOTO was solely valvar in 24% of the patients, only subvalvar in 37% of patients, and multilevel in 39%.
The location of the most important VSD was known in 143 patients, with outlet septum in 102, inlet septum in 14, trabecular septum in 3, and a combination of the three in 24 patients. The great majority of the 140 patients for whom data were available had great artery commitment of the biggest VSD: to the aorta in 60, the pulmonary artery in 32, and doubly committed to both in 19. Only 29 patients had noncommitment of one of the great arteries to the VSD.
Overall postoperative survival was 92% at 1 month, 88% at 1 year, 88% at 10 years, and 58% at 20 years. Events were followed as an outcome and were defined as death, reoperation, transcatheter intervention, or cardiac transplantation. The frequent necessity of reintervention (40.7% over follow-up) caused the overall event-free survival to be lower: 85% at 1 month, 80% at 1 year, 45% at 10 years, and 26% at 20 years (Euro. J. Cardiothorac. Surg. 2010;38:699-706).
There were 41 surgical reinterventions and 20 percutaneous procedures, with the most frequent cause of reoperation being RVOT obstruction, including conduit failure (25.0%), followed by LVOT obstruction (7.9%), residual VSD closure (7.1%), and pulmonary artery plasty (4.3%).
In multivariate analysis, age at the corrective surgery, year of the operation, and type of operation were significant predictors for reoperation and trans-catheter intervention, in general, as well as for RVOT reoperation/intervention. The younger the patient at the time of operation, the higher the risk of later reoperation, leading the researchers to speculate that the more recent the surgery, the less the probability that a patient would undergo reoperation.
Reoperation for RVOTO was most common in patients with a Rastelli operation, according to the authors.
"Although there are some differences between Rastelli outcomes among different groups, the all-over rates of freedom from reoperation and, especially, event-free survival, are not satisfactory with event-free survival rates at 10 years that vary from 24% to 49%," they said.
"The Rastelli procedure was a significant independent risk factor for re-operation, with the REV/Metras and the Nikaidoh having the lowest re-intervention rates," they wrote.
They indicated more patients need to be studied with longer follow-up, especially for the Nikaidoh technique.
The authors had no disclosures.
Optimal surgical management of patients with transposition of the great arteries, ventricular septal defect, and left ventricular outflow obstruction is still considered controversial. Although the Rastelli operation is the most commonly performed procedure, the Réparation à l'Etage Ventriculaire procedure and Metras modification yielded the best long-term results for both survival and event-free survival, according to a retrospective study of 146 patients who underwent surgery from 1980 to 2008 in eight European hospitals.
The multicenter study compared use and outcomes of several different surgical operations for transposition of the great arteries (TGA), ventricular septal defect (VSD), and left ventricular outflow obstruction (LVOTO), according to a report published in the European Journal of Cardio-thoracic Surgery.
A total of 141 patients had TGA, VSD, and LVOTO; 5 patients had the TGA type of double-outlet right ventricle (DORV) with LVOTO. Only those patients for whom the surgical method chosen was equivalent to those for TGA, VSD, and LVOTO were included in the study; all other DORV types were excluded, according to Dr. Mark Gerard Hazekamp of Leids Universitair Medisch Centrum, Leiden, the Netherlands, and his colleagues from various European universities on behalf of the European Congenital Heart Surgeons Association.
The procedures investigated were the Rastelli (82 patients), arterial (24) and atrial (5) switch operation with relief of LVOTO, Reparation l'Etage Ventriculaire (REV) procedure (7), and Metras modification (24), as well as the Nikaidoh (4). The type of surgery used has traditionally been different in different countries, they said, with the REV procedure and Metras modification mainly in France and the Rastelli procedure being the norm in most other countries.
Patients had a median age at operation of 21.5 months (range 0.2-165.1 months) and a median weight of 10 kg (range 2.0-41.0 kg). Pulmonary stenosis was found in 119 patients, while 27 had pulmonary atresia. LVOTO was solely valvar in 24% of the patients, only subvalvar in 37% of patients, and multilevel in 39%.
The location of the most important VSD was known in 143 patients, with outlet septum in 102, inlet septum in 14, trabecular septum in 3, and a combination of the three in 24 patients. The great majority of the 140 patients for whom data were available had great artery commitment of the biggest VSD: to the aorta in 60, the pulmonary artery in 32, and doubly committed to both in 19. Only 29 patients had noncommitment of one of the great arteries to the VSD.
Overall postoperative survival was 92% at 1 month, 88% at 1 year, 88% at 10 years, and 58% at 20 years. Events were followed as an outcome and were defined as death, reoperation, transcatheter intervention, or cardiac transplantation. The frequent necessity of reintervention (40.7% over follow-up) caused the overall event-free survival to be lower: 85% at 1 month, 80% at 1 year, 45% at 10 years, and 26% at 20 years (Euro. J. Cardiothorac. Surg. 2010;38:699-706).
There were 41 surgical reinterventions and 20 percutaneous procedures, with the most frequent cause of reoperation being RVOT obstruction, including conduit failure (25.0%), followed by LVOT obstruction (7.9%), residual VSD closure (7.1%), and pulmonary artery plasty (4.3%).
In multivariate analysis, age at the corrective surgery, year of the operation, and type of operation were significant predictors for reoperation and trans-catheter intervention, in general, as well as for RVOT reoperation/intervention. The younger the patient at the time of operation, the higher the risk of later reoperation, leading the researchers to speculate that the more recent the surgery, the less the probability that a patient would undergo reoperation.
Reoperation for RVOTO was most common in patients with a Rastelli operation, according to the authors.
"Although there are some differences between Rastelli outcomes among different groups, the all-over rates of freedom from reoperation and, especially, event-free survival, are not satisfactory with event-free survival rates at 10 years that vary from 24% to 49%," they said.
"The Rastelli procedure was a significant independent risk factor for re-operation, with the REV/Metras and the Nikaidoh having the lowest re-intervention rates," they wrote.
They indicated more patients need to be studied with longer follow-up, especially for the Nikaidoh technique.
The authors had no disclosures.
Optimal surgical management of patients with transposition of the great arteries, ventricular septal defect, and left ventricular outflow obstruction is still considered controversial. Although the Rastelli operation is the most commonly performed procedure, the Réparation à l'Etage Ventriculaire procedure and Metras modification yielded the best long-term results for both survival and event-free survival, according to a retrospective study of 146 patients who underwent surgery from 1980 to 2008 in eight European hospitals.
The multicenter study compared use and outcomes of several different surgical operations for transposition of the great arteries (TGA), ventricular septal defect (VSD), and left ventricular outflow obstruction (LVOTO), according to a report published in the European Journal of Cardio-thoracic Surgery.
A total of 141 patients had TGA, VSD, and LVOTO; 5 patients had the TGA type of double-outlet right ventricle (DORV) with LVOTO. Only those patients for whom the surgical method chosen was equivalent to those for TGA, VSD, and LVOTO were included in the study; all other DORV types were excluded, according to Dr. Mark Gerard Hazekamp of Leids Universitair Medisch Centrum, Leiden, the Netherlands, and his colleagues from various European universities on behalf of the European Congenital Heart Surgeons Association.
The procedures investigated were the Rastelli (82 patients), arterial (24) and atrial (5) switch operation with relief of LVOTO, Reparation l'Etage Ventriculaire (REV) procedure (7), and Metras modification (24), as well as the Nikaidoh (4). The type of surgery used has traditionally been different in different countries, they said, with the REV procedure and Metras modification mainly in France and the Rastelli procedure being the norm in most other countries.
Patients had a median age at operation of 21.5 months (range 0.2-165.1 months) and a median weight of 10 kg (range 2.0-41.0 kg). Pulmonary stenosis was found in 119 patients, while 27 had pulmonary atresia. LVOTO was solely valvar in 24% of the patients, only subvalvar in 37% of patients, and multilevel in 39%.
The location of the most important VSD was known in 143 patients, with outlet septum in 102, inlet septum in 14, trabecular septum in 3, and a combination of the three in 24 patients. The great majority of the 140 patients for whom data were available had great artery commitment of the biggest VSD: to the aorta in 60, the pulmonary artery in 32, and doubly committed to both in 19. Only 29 patients had noncommitment of one of the great arteries to the VSD.
Overall postoperative survival was 92% at 1 month, 88% at 1 year, 88% at 10 years, and 58% at 20 years. Events were followed as an outcome and were defined as death, reoperation, transcatheter intervention, or cardiac transplantation. The frequent necessity of reintervention (40.7% over follow-up) caused the overall event-free survival to be lower: 85% at 1 month, 80% at 1 year, 45% at 10 years, and 26% at 20 years (Euro. J. Cardiothorac. Surg. 2010;38:699-706).
There were 41 surgical reinterventions and 20 percutaneous procedures, with the most frequent cause of reoperation being RVOT obstruction, including conduit failure (25.0%), followed by LVOT obstruction (7.9%), residual VSD closure (7.1%), and pulmonary artery plasty (4.3%).
In multivariate analysis, age at the corrective surgery, year of the operation, and type of operation were significant predictors for reoperation and trans-catheter intervention, in general, as well as for RVOT reoperation/intervention. The younger the patient at the time of operation, the higher the risk of later reoperation, leading the researchers to speculate that the more recent the surgery, the less the probability that a patient would undergo reoperation.
Reoperation for RVOTO was most common in patients with a Rastelli operation, according to the authors.
"Although there are some differences between Rastelli outcomes among different groups, the all-over rates of freedom from reoperation and, especially, event-free survival, are not satisfactory with event-free survival rates at 10 years that vary from 24% to 49%," they said.
"The Rastelli procedure was a significant independent risk factor for re-operation, with the REV/Metras and the Nikaidoh having the lowest re-intervention rates," they wrote.
They indicated more patients need to be studied with longer follow-up, especially for the Nikaidoh technique.
The authors had no disclosures.
Neutropenia and the White Blood Cells
VA Launches Program to Assist Dying Veterans
Anomalous Motor Learning May Be Specific to Children With Autism
Children with autism rely heavily on proprioception, unlike children with other developmental motor impairments or typically developing children.
PROVIDENCE, RI—Children with autism spectrum disorder form a representation of internal models that places an unusually strong reliance on proprioception, according to research presented at the 39th National Meeting of the Child Neurology Society.
“This anomalous motor learning is specific to autism spectrum disorder, rather than a general deficit of all populations with developmental motor impairments, as children with ADHD did not generalize differently than typically developing children,” reported Stewart H. Mostofsky, MD, research scientist at the Kennedy Krieger Institute and Associate Professor of Neurology, Johns Hopkins University School of Medicine, Baltimore, and colleagues. “Our results suggest that autism-associated impairment in understanding actions of others may be a consequence of the fact that in learning to perform actions, children with autism place a greater than normal reliance on their own proprioception while discounting the visual consequences of their actions.”
The researchers analyzed 25 children with autism (mean age, 10.31), 16 with ADHD (mean age, 10.66), and 39 typically developing children (mean age, 10.82). As part of a game, each child held the handle of a robotic arm, trying to capture animals that had escaped from a zoo. An animal would appear at a target location 8 cm away; if the child reached the target in time, the animal was captured and the child was given a point.
“Analyses revealed that all groups were able to effectively adapt their arm movement,” stated Dr. Mostofsky’s group. “However, generalization patterns were markedly different. There was a significant interaction between diagnostic group and relative generalization to targets 2 and 3. Posthoc analyses revealed this difference was due to significantly greater generalization of the autism group in the intrinsic (proprioceptive) coordinate system as compared to typically developing children. In contrast, there was no significant difference in generalization between ADHD and typically developing children.”
Regression analyses revealed that among all groups, generalization in the intrinsic (proprioceptive) coordinate system (ie, to target 3) was a significant predictor of social ability, such that greater social impairment was predicted by increased force for target 3, noted Dr. Mostofsky and colleagues. “Further,” the researchers concluded, “for the children with autism, increased force for target 3 predicted impaired social interaction. In addition, increased generalization to target 3 also predicted impaired imitation ability, as assessed on a praxis examination, as well as impairment in motor control.”
Children with autism rely heavily on proprioception, unlike children with other developmental motor impairments or typically developing children.
PROVIDENCE, RI—Children with autism spectrum disorder form a representation of internal models that places an unusually strong reliance on proprioception, according to research presented at the 39th National Meeting of the Child Neurology Society.
“This anomalous motor learning is specific to autism spectrum disorder, rather than a general deficit of all populations with developmental motor impairments, as children with ADHD did not generalize differently than typically developing children,” reported Stewart H. Mostofsky, MD, research scientist at the Kennedy Krieger Institute and Associate Professor of Neurology, Johns Hopkins University School of Medicine, Baltimore, and colleagues. “Our results suggest that autism-associated impairment in understanding actions of others may be a consequence of the fact that in learning to perform actions, children with autism place a greater than normal reliance on their own proprioception while discounting the visual consequences of their actions.”
The researchers analyzed 25 children with autism (mean age, 10.31), 16 with ADHD (mean age, 10.66), and 39 typically developing children (mean age, 10.82). As part of a game, each child held the handle of a robotic arm, trying to capture animals that had escaped from a zoo. An animal would appear at a target location 8 cm away; if the child reached the target in time, the animal was captured and the child was given a point.
“Analyses revealed that all groups were able to effectively adapt their arm movement,” stated Dr. Mostofsky’s group. “However, generalization patterns were markedly different. There was a significant interaction between diagnostic group and relative generalization to targets 2 and 3. Posthoc analyses revealed this difference was due to significantly greater generalization of the autism group in the intrinsic (proprioceptive) coordinate system as compared to typically developing children. In contrast, there was no significant difference in generalization between ADHD and typically developing children.”
Regression analyses revealed that among all groups, generalization in the intrinsic (proprioceptive) coordinate system (ie, to target 3) was a significant predictor of social ability, such that greater social impairment was predicted by increased force for target 3, noted Dr. Mostofsky and colleagues. “Further,” the researchers concluded, “for the children with autism, increased force for target 3 predicted impaired social interaction. In addition, increased generalization to target 3 also predicted impaired imitation ability, as assessed on a praxis examination, as well as impairment in motor control.”
Children with autism rely heavily on proprioception, unlike children with other developmental motor impairments or typically developing children.
PROVIDENCE, RI—Children with autism spectrum disorder form a representation of internal models that places an unusually strong reliance on proprioception, according to research presented at the 39th National Meeting of the Child Neurology Society.
“This anomalous motor learning is specific to autism spectrum disorder, rather than a general deficit of all populations with developmental motor impairments, as children with ADHD did not generalize differently than typically developing children,” reported Stewart H. Mostofsky, MD, research scientist at the Kennedy Krieger Institute and Associate Professor of Neurology, Johns Hopkins University School of Medicine, Baltimore, and colleagues. “Our results suggest that autism-associated impairment in understanding actions of others may be a consequence of the fact that in learning to perform actions, children with autism place a greater than normal reliance on their own proprioception while discounting the visual consequences of their actions.”
The researchers analyzed 25 children with autism (mean age, 10.31), 16 with ADHD (mean age, 10.66), and 39 typically developing children (mean age, 10.82). As part of a game, each child held the handle of a robotic arm, trying to capture animals that had escaped from a zoo. An animal would appear at a target location 8 cm away; if the child reached the target in time, the animal was captured and the child was given a point.
“Analyses revealed that all groups were able to effectively adapt their arm movement,” stated Dr. Mostofsky’s group. “However, generalization patterns were markedly different. There was a significant interaction between diagnostic group and relative generalization to targets 2 and 3. Posthoc analyses revealed this difference was due to significantly greater generalization of the autism group in the intrinsic (proprioceptive) coordinate system as compared to typically developing children. In contrast, there was no significant difference in generalization between ADHD and typically developing children.”
Regression analyses revealed that among all groups, generalization in the intrinsic (proprioceptive) coordinate system (ie, to target 3) was a significant predictor of social ability, such that greater social impairment was predicted by increased force for target 3, noted Dr. Mostofsky and colleagues. “Further,” the researchers concluded, “for the children with autism, increased force for target 3 predicted impaired social interaction. In addition, increased generalization to target 3 also predicted impaired imitation ability, as assessed on a praxis examination, as well as impairment in motor control.”
Self-Monitoring of Glucose in Diabetes
Despite therapeutic advances in diabetes management, the majority of patients with diabetes are unable to achieve glycemic targets proven to reduce the burden of the disease. This burden not only involves the quality of life of patients with diabetes who experience the complications of this disease; it also includes the burden to society. One out of every five health care dollars is spent on caring for someone with diabetes—the majority on treating the complications.1
Major barriers to patients’ ability to achieve glycemic goals include the need to make behavioral changes, lack of awareness of glycemic levels, and fear of hypoglycemia.2
Q: Is self-monitoring of blood glucose worthwhile in diabetes?
Studies have shown a benefit from self-monitoring of blood glucose (SMBG) in patients using insulin but not in those taking oral antidiabetic drugs. However, the American Diabetes Association recommends that patients with diabetes monitor their glucose once daily if they are being treated with noninsulin therapy and at least three times daily if they are taking insulin.3
Guidelines from the American Association of Clinical Endocrinologists (AACE) state that patients taking noninsulin or once-daily insulin therapy who have not achieved A1C targets should monitor at least twice daily, while those at target should monitor at least once daily. Those taking multiple daily injections should perform SMBG at least three times per day. If patients experience frequent hypoglycemia, AACE suggests monitoring glucose more often.4
The A1C test provides the “big picture,” the average daily glucose level during the previous 90 to 120 days, and correlates with end-organ impact. It does not identify glycemic variability, hypoglycemia, or hyperglycemia.
By contrast, SMBG patterns provide day-to-day data that can be used to select and manage glucose control programs and ultimately optimize a patient’s A1C. SMBG provides a measure of the specific pharmacologic impact of medications and, through feedback, allows design and implementation of physiologic insulin-replacement programs.
One example of SMBG is to have patients monitor glucose in pairs (ie, pick a meal each day and do a premeal and two-hour postmeal reading) and ask them to keep a log or download the data from their meter in the office. This type of monitoring can be enlightening and self-empowering for the patient in that it can provide valuable information regarding the glycemic response to the particular meal.
Intensive glycemic management has been shown to reduce the incidence and progression of diabetic complications. However, it is associated with an increase in severe hypoglycemia. This is worrisome for both patients and providers, as severe hypoglycemia has been associated with an increase in risk for mortality. SMBG can assist patients in understanding how their lifestyle affects their diabetes, as well as identifying hypoglycemia for those who may have hypoglycemia unawareness (ie, who lack the relevant symptoms).
Q: What is continuous glucose monitoring (CGM)?
CGM devices give real-time readouts of current glucose levels. They utilize a subcutaneous sensor that is inserted in the abdomen and worn for 3 to 7 days (depending on which device is used). The sensor sends an electronic signal to a receiver worn by the patient.
There are three major CGM devices that have been approved by the FDA and are available for both personal and professional use. Health care providers can purchase the units and have patients wear them for retrospective analysis; this is a reimbursable expense. All available CGM devices measure glucose values in the interstitial fluid. The sensor reads electrical current produced by the same glucose-oxidase reaction that is utilized by glucose meters that patients use to perform fingersticks for home monitoring.
Currently available CGM systems need to be calibrated at least twice daily. Sensor calibration entails the pairing of the fingerstick value with the sensor value from the interstitial space. Calibration confirms sensor accuracy during various points by “teaching” the sensor the glucose value that corresponds with the electrical current signal.
There is a known physiologic lag time that occurs between fingerstick and sensor values. This lag time is typically up to 15 minutes but is increased with rapidly changing glucose values.
Q: What are the benefits of CGM?
Recent studies have shown CGM can improve A1C without increasing the incidence of hypoglycemia.5
CGM systems have both low and high glucose threshold alarms that can be set to alert once the threshold is reached. The newest generation devices can also predict hypoglycemia or hyperglycemia by tracking rate of change, and users can be alerted to a potential event. This would then allow them to take appropriate action, such as consuming food or carbohydrates or taking insulin as necessary. (Before taking any action, the glucose should first be confirmed by SMBG.)
Software programs allow for review of glucose data, which can assist in identifying trends not appreciated by typical SMBG testing (such as nocturnal hypoglycemia and meal-time excursions). This allows for adjustment of insulin regimens to reduce the incidence of these events.
Q: Can CGM replace SMBG?
While CGM can provide much more detail regarding glucose trends and patterns, it is not a replacement for SMBG. CGM should not be used as a replacement for SMBG to dose insulin for meal- or activity-related adjustments. All dosing decisions should be based on the SMBG.
Currently, CGM is indicated for patients 18 or older, in conjunction with SMBG for the purpose of improving glycemic control:
• to identify and aid in management of glycemic patterns not recognized with typical SMBG
• to prevent glycemic excursions of hypoglycemia and hyperglycemia.
Its use is supported by ADA and AACE guidelines for glucose monitoring.
Q: Who would benefit from CGM?
Suitable candidates for CGM include those with a high degree of glycemic variability, those with hypoglycemic unawareness, shift workers, patients who use insulin pumps, athletes, and women who are planning to become or are pregnant. Patients should work closely with their health care team and perform regular SMBG.
It has been suggested that patients need comprehensive training and follow-up visits to fully understand the large amount of data that they can be confronted with, in order to fully benefit from these devices.6 While the accuracy is improving, there are a few limitations to this technology, including false alarms. Studies have also shown a positive correlation between sensor wear time (hours per week) and greater reductions in A1C.5
Conclusion
Glucose monitoring is a necessary tool—for patients as well as providers—that assists in identifying how patients’ lifestyles affect their diabetes.
1. American Diabetes Association. Economic costs of diabetes in the US in 2007. Diabetes Care. 2008;31(3):596-615.
2. Hirsch IB, Armstrong D, Bergenstal RM, et al. Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM). Diabetes Technol Ther. 2008;10(4):232-246.
3. American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2010;34(suppl 1):S11-S61.
4. American Association of Clinical Endocrinologists. Medical guidelines for clinical practice for the management of diabetes mellitus. Endocrin Prac. 2007;13(suppl 1):1-68.
5. Bergenstal RM, Tamberlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320.
6. Fabiato K, Buse J, Duclos M, et al. Clinical experience with continuous glucose monitoring in adults. Diabetes Technol Ther. 2009;11(suppl 1):S93-S103.
Despite therapeutic advances in diabetes management, the majority of patients with diabetes are unable to achieve glycemic targets proven to reduce the burden of the disease. This burden not only involves the quality of life of patients with diabetes who experience the complications of this disease; it also includes the burden to society. One out of every five health care dollars is spent on caring for someone with diabetes—the majority on treating the complications.1
Major barriers to patients’ ability to achieve glycemic goals include the need to make behavioral changes, lack of awareness of glycemic levels, and fear of hypoglycemia.2
Q: Is self-monitoring of blood glucose worthwhile in diabetes?
Studies have shown a benefit from self-monitoring of blood glucose (SMBG) in patients using insulin but not in those taking oral antidiabetic drugs. However, the American Diabetes Association recommends that patients with diabetes monitor their glucose once daily if they are being treated with noninsulin therapy and at least three times daily if they are taking insulin.3
Guidelines from the American Association of Clinical Endocrinologists (AACE) state that patients taking noninsulin or once-daily insulin therapy who have not achieved A1C targets should monitor at least twice daily, while those at target should monitor at least once daily. Those taking multiple daily injections should perform SMBG at least three times per day. If patients experience frequent hypoglycemia, AACE suggests monitoring glucose more often.4
The A1C test provides the “big picture,” the average daily glucose level during the previous 90 to 120 days, and correlates with end-organ impact. It does not identify glycemic variability, hypoglycemia, or hyperglycemia.
By contrast, SMBG patterns provide day-to-day data that can be used to select and manage glucose control programs and ultimately optimize a patient’s A1C. SMBG provides a measure of the specific pharmacologic impact of medications and, through feedback, allows design and implementation of physiologic insulin-replacement programs.
One example of SMBG is to have patients monitor glucose in pairs (ie, pick a meal each day and do a premeal and two-hour postmeal reading) and ask them to keep a log or download the data from their meter in the office. This type of monitoring can be enlightening and self-empowering for the patient in that it can provide valuable information regarding the glycemic response to the particular meal.
Intensive glycemic management has been shown to reduce the incidence and progression of diabetic complications. However, it is associated with an increase in severe hypoglycemia. This is worrisome for both patients and providers, as severe hypoglycemia has been associated with an increase in risk for mortality. SMBG can assist patients in understanding how their lifestyle affects their diabetes, as well as identifying hypoglycemia for those who may have hypoglycemia unawareness (ie, who lack the relevant symptoms).
Q: What is continuous glucose monitoring (CGM)?
CGM devices give real-time readouts of current glucose levels. They utilize a subcutaneous sensor that is inserted in the abdomen and worn for 3 to 7 days (depending on which device is used). The sensor sends an electronic signal to a receiver worn by the patient.
There are three major CGM devices that have been approved by the FDA and are available for both personal and professional use. Health care providers can purchase the units and have patients wear them for retrospective analysis; this is a reimbursable expense. All available CGM devices measure glucose values in the interstitial fluid. The sensor reads electrical current produced by the same glucose-oxidase reaction that is utilized by glucose meters that patients use to perform fingersticks for home monitoring.
Currently available CGM systems need to be calibrated at least twice daily. Sensor calibration entails the pairing of the fingerstick value with the sensor value from the interstitial space. Calibration confirms sensor accuracy during various points by “teaching” the sensor the glucose value that corresponds with the electrical current signal.
There is a known physiologic lag time that occurs between fingerstick and sensor values. This lag time is typically up to 15 minutes but is increased with rapidly changing glucose values.
Q: What are the benefits of CGM?
Recent studies have shown CGM can improve A1C without increasing the incidence of hypoglycemia.5
CGM systems have both low and high glucose threshold alarms that can be set to alert once the threshold is reached. The newest generation devices can also predict hypoglycemia or hyperglycemia by tracking rate of change, and users can be alerted to a potential event. This would then allow them to take appropriate action, such as consuming food or carbohydrates or taking insulin as necessary. (Before taking any action, the glucose should first be confirmed by SMBG.)
Software programs allow for review of glucose data, which can assist in identifying trends not appreciated by typical SMBG testing (such as nocturnal hypoglycemia and meal-time excursions). This allows for adjustment of insulin regimens to reduce the incidence of these events.
Q: Can CGM replace SMBG?
While CGM can provide much more detail regarding glucose trends and patterns, it is not a replacement for SMBG. CGM should not be used as a replacement for SMBG to dose insulin for meal- or activity-related adjustments. All dosing decisions should be based on the SMBG.
Currently, CGM is indicated for patients 18 or older, in conjunction with SMBG for the purpose of improving glycemic control:
• to identify and aid in management of glycemic patterns not recognized with typical SMBG
• to prevent glycemic excursions of hypoglycemia and hyperglycemia.
Its use is supported by ADA and AACE guidelines for glucose monitoring.
Q: Who would benefit from CGM?
Suitable candidates for CGM include those with a high degree of glycemic variability, those with hypoglycemic unawareness, shift workers, patients who use insulin pumps, athletes, and women who are planning to become or are pregnant. Patients should work closely with their health care team and perform regular SMBG.
It has been suggested that patients need comprehensive training and follow-up visits to fully understand the large amount of data that they can be confronted with, in order to fully benefit from these devices.6 While the accuracy is improving, there are a few limitations to this technology, including false alarms. Studies have also shown a positive correlation between sensor wear time (hours per week) and greater reductions in A1C.5
Conclusion
Glucose monitoring is a necessary tool—for patients as well as providers—that assists in identifying how patients’ lifestyles affect their diabetes.
Despite therapeutic advances in diabetes management, the majority of patients with diabetes are unable to achieve glycemic targets proven to reduce the burden of the disease. This burden not only involves the quality of life of patients with diabetes who experience the complications of this disease; it also includes the burden to society. One out of every five health care dollars is spent on caring for someone with diabetes—the majority on treating the complications.1
Major barriers to patients’ ability to achieve glycemic goals include the need to make behavioral changes, lack of awareness of glycemic levels, and fear of hypoglycemia.2
Q: Is self-monitoring of blood glucose worthwhile in diabetes?
Studies have shown a benefit from self-monitoring of blood glucose (SMBG) in patients using insulin but not in those taking oral antidiabetic drugs. However, the American Diabetes Association recommends that patients with diabetes monitor their glucose once daily if they are being treated with noninsulin therapy and at least three times daily if they are taking insulin.3
Guidelines from the American Association of Clinical Endocrinologists (AACE) state that patients taking noninsulin or once-daily insulin therapy who have not achieved A1C targets should monitor at least twice daily, while those at target should monitor at least once daily. Those taking multiple daily injections should perform SMBG at least three times per day. If patients experience frequent hypoglycemia, AACE suggests monitoring glucose more often.4
The A1C test provides the “big picture,” the average daily glucose level during the previous 90 to 120 days, and correlates with end-organ impact. It does not identify glycemic variability, hypoglycemia, or hyperglycemia.
By contrast, SMBG patterns provide day-to-day data that can be used to select and manage glucose control programs and ultimately optimize a patient’s A1C. SMBG provides a measure of the specific pharmacologic impact of medications and, through feedback, allows design and implementation of physiologic insulin-replacement programs.
One example of SMBG is to have patients monitor glucose in pairs (ie, pick a meal each day and do a premeal and two-hour postmeal reading) and ask them to keep a log or download the data from their meter in the office. This type of monitoring can be enlightening and self-empowering for the patient in that it can provide valuable information regarding the glycemic response to the particular meal.
Intensive glycemic management has been shown to reduce the incidence and progression of diabetic complications. However, it is associated with an increase in severe hypoglycemia. This is worrisome for both patients and providers, as severe hypoglycemia has been associated with an increase in risk for mortality. SMBG can assist patients in understanding how their lifestyle affects their diabetes, as well as identifying hypoglycemia for those who may have hypoglycemia unawareness (ie, who lack the relevant symptoms).
Q: What is continuous glucose monitoring (CGM)?
CGM devices give real-time readouts of current glucose levels. They utilize a subcutaneous sensor that is inserted in the abdomen and worn for 3 to 7 days (depending on which device is used). The sensor sends an electronic signal to a receiver worn by the patient.
There are three major CGM devices that have been approved by the FDA and are available for both personal and professional use. Health care providers can purchase the units and have patients wear them for retrospective analysis; this is a reimbursable expense. All available CGM devices measure glucose values in the interstitial fluid. The sensor reads electrical current produced by the same glucose-oxidase reaction that is utilized by glucose meters that patients use to perform fingersticks for home monitoring.
Currently available CGM systems need to be calibrated at least twice daily. Sensor calibration entails the pairing of the fingerstick value with the sensor value from the interstitial space. Calibration confirms sensor accuracy during various points by “teaching” the sensor the glucose value that corresponds with the electrical current signal.
There is a known physiologic lag time that occurs between fingerstick and sensor values. This lag time is typically up to 15 minutes but is increased with rapidly changing glucose values.
Q: What are the benefits of CGM?
Recent studies have shown CGM can improve A1C without increasing the incidence of hypoglycemia.5
CGM systems have both low and high glucose threshold alarms that can be set to alert once the threshold is reached. The newest generation devices can also predict hypoglycemia or hyperglycemia by tracking rate of change, and users can be alerted to a potential event. This would then allow them to take appropriate action, such as consuming food or carbohydrates or taking insulin as necessary. (Before taking any action, the glucose should first be confirmed by SMBG.)
Software programs allow for review of glucose data, which can assist in identifying trends not appreciated by typical SMBG testing (such as nocturnal hypoglycemia and meal-time excursions). This allows for adjustment of insulin regimens to reduce the incidence of these events.
Q: Can CGM replace SMBG?
While CGM can provide much more detail regarding glucose trends and patterns, it is not a replacement for SMBG. CGM should not be used as a replacement for SMBG to dose insulin for meal- or activity-related adjustments. All dosing decisions should be based on the SMBG.
Currently, CGM is indicated for patients 18 or older, in conjunction with SMBG for the purpose of improving glycemic control:
• to identify and aid in management of glycemic patterns not recognized with typical SMBG
• to prevent glycemic excursions of hypoglycemia and hyperglycemia.
Its use is supported by ADA and AACE guidelines for glucose monitoring.
Q: Who would benefit from CGM?
Suitable candidates for CGM include those with a high degree of glycemic variability, those with hypoglycemic unawareness, shift workers, patients who use insulin pumps, athletes, and women who are planning to become or are pregnant. Patients should work closely with their health care team and perform regular SMBG.
It has been suggested that patients need comprehensive training and follow-up visits to fully understand the large amount of data that they can be confronted with, in order to fully benefit from these devices.6 While the accuracy is improving, there are a few limitations to this technology, including false alarms. Studies have also shown a positive correlation between sensor wear time (hours per week) and greater reductions in A1C.5
Conclusion
Glucose monitoring is a necessary tool—for patients as well as providers—that assists in identifying how patients’ lifestyles affect their diabetes.
1. American Diabetes Association. Economic costs of diabetes in the US in 2007. Diabetes Care. 2008;31(3):596-615.
2. Hirsch IB, Armstrong D, Bergenstal RM, et al. Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM). Diabetes Technol Ther. 2008;10(4):232-246.
3. American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2010;34(suppl 1):S11-S61.
4. American Association of Clinical Endocrinologists. Medical guidelines for clinical practice for the management of diabetes mellitus. Endocrin Prac. 2007;13(suppl 1):1-68.
5. Bergenstal RM, Tamberlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320.
6. Fabiato K, Buse J, Duclos M, et al. Clinical experience with continuous glucose monitoring in adults. Diabetes Technol Ther. 2009;11(suppl 1):S93-S103.
1. American Diabetes Association. Economic costs of diabetes in the US in 2007. Diabetes Care. 2008;31(3):596-615.
2. Hirsch IB, Armstrong D, Bergenstal RM, et al. Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM). Diabetes Technol Ther. 2008;10(4):232-246.
3. American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2010;34(suppl 1):S11-S61.
4. American Association of Clinical Endocrinologists. Medical guidelines for clinical practice for the management of diabetes mellitus. Endocrin Prac. 2007;13(suppl 1):1-68.
5. Bergenstal RM, Tamberlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320.
6. Fabiato K, Buse J, Duclos M, et al. Clinical experience with continuous glucose monitoring in adults. Diabetes Technol Ther. 2009;11(suppl 1):S93-S103.
Erratum (2010;86:239-240)
Pityriasis Alba Revisited: Perspectives on an Enigmatic Disorder of Childhood
Drug testing is inexpensive? Not always, says this doc
As a recently retired physician who worked in family practice, palliative care, and occupational medicine for more than 30 years, I read “Is it time to drug test your chronic pain patient?” (J Fam Pract. 2010;59:628-633) with interest. I myself suffer from arthritis in the neck and low back, for which a medical school colleague prescribed a very low dose of hydrocodone prn several years ago. I believe my resident physician may have read your article right before my last office visit; he suggested a drug screen was appropriate for me because hydrocodone is a “high-risk” medication.
I understood, of course, and readily agreed. After all, I did thousands of these screens during my years of practice—at a cost of about $5 per test. The authors of your article apparently did not research the average retail cost of the test, stating only that it is “inexpensive.” Imagine my surprise when I saw the bill—$676 for the drug screen alone. My insurer readily paid its portion of the “allowable” charge ($434).
The medication itself is wonderful; it helps keep me functioning and costs me about 8 cents per pill, so I won’t complain too much. But I suspect that most of your readers would be surprised by the true cost of this “inexpensive” test at a major medical school.
Mack Tyner, MD
Gainesville, Fla
As a recently retired physician who worked in family practice, palliative care, and occupational medicine for more than 30 years, I read “Is it time to drug test your chronic pain patient?” (J Fam Pract. 2010;59:628-633) with interest. I myself suffer from arthritis in the neck and low back, for which a medical school colleague prescribed a very low dose of hydrocodone prn several years ago. I believe my resident physician may have read your article right before my last office visit; he suggested a drug screen was appropriate for me because hydrocodone is a “high-risk” medication.
I understood, of course, and readily agreed. After all, I did thousands of these screens during my years of practice—at a cost of about $5 per test. The authors of your article apparently did not research the average retail cost of the test, stating only that it is “inexpensive.” Imagine my surprise when I saw the bill—$676 for the drug screen alone. My insurer readily paid its portion of the “allowable” charge ($434).
The medication itself is wonderful; it helps keep me functioning and costs me about 8 cents per pill, so I won’t complain too much. But I suspect that most of your readers would be surprised by the true cost of this “inexpensive” test at a major medical school.
Mack Tyner, MD
Gainesville, Fla
As a recently retired physician who worked in family practice, palliative care, and occupational medicine for more than 30 years, I read “Is it time to drug test your chronic pain patient?” (J Fam Pract. 2010;59:628-633) with interest. I myself suffer from arthritis in the neck and low back, for which a medical school colleague prescribed a very low dose of hydrocodone prn several years ago. I believe my resident physician may have read your article right before my last office visit; he suggested a drug screen was appropriate for me because hydrocodone is a “high-risk” medication.
I understood, of course, and readily agreed. After all, I did thousands of these screens during my years of practice—at a cost of about $5 per test. The authors of your article apparently did not research the average retail cost of the test, stating only that it is “inexpensive.” Imagine my surprise when I saw the bill—$676 for the drug screen alone. My insurer readily paid its portion of the “allowable” charge ($434).
The medication itself is wonderful; it helps keep me functioning and costs me about 8 cents per pill, so I won’t complain too much. But I suspect that most of your readers would be surprised by the true cost of this “inexpensive” test at a major medical school.
Mack Tyner, MD
Gainesville, Fla