User login
Diabetes and Steroid Use
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
New Onset Diabetes in Adults: Type 1 and Type 2 Differences
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Home Monitoring of Glucose
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Hypoglycemia
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
To hear his lectures, including “Type 1 Diabetes: Multi-Dose Injections and Pump Therapy Dilemma—‘You’re All Alone and No One is Home’” and earn CE/CME credit, please attend the 2013 MEDS West in Irvine, CA, October 3-5. Click here for complete details.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
To hear his lectures, including “Type 1 Diabetes: Multi-Dose Injections and Pump Therapy Dilemma—‘You’re All Alone and No One is Home’” and earn CE/CME credit, please attend the 2013 MEDS West in Irvine, CA, October 3-5. Click here for complete details.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
To hear his lectures, including “Type 1 Diabetes: Multi-Dose Injections and Pump Therapy Dilemma—‘You’re All Alone and No One is Home’” and earn CE/CME credit, please attend the 2013 MEDS West in Irvine, CA, October 3-5. Click here for complete details.
Insulin Pump Therapy for the Patient With Diabetes
The FDA has reported that in 2007, 375,000 Americans with diabetes were using an insulin pump.1 Improvements in pump technology (including the addition of continuous glucose monitoring2,3), more data on the use of pumps in type 2 diabetes,4-7 improved insurance coverage,8 and the general population’s comfort with technology-based solutions may lead more patients with diabetes to choose a pump to deliver their insulin.
As a result, primary care practitioners will be increasingly likely to have patients who use continuous subcutaneous insulin infusion (CSII) pumps. While the initiation of pump therapy and follow-up with patients who use pumps have traditionally been the purview of endocrinologists, it is important for primary care providers to understand the basics of insulin pump therapy, enabling them to work collaboratively with the patient and the diabetes care team.
REVIEW OF INSULIN ACTION IN THE BODY
The body’s own (endogenous) insulin is secreted by the beta cells of the pancreas. Basal insulin is the background amount of insulin continuously released by the body in order to regulate hepatic glucose production and lipolysis. In response to food intake, the body releases additional insulin to match the glycemic effect of carbohydrates, fat, and protein.9
Insulin regimens attempt to mimic the body’s physiologic release of insulin by administering long-acting insulin to provide basal coverage, and rapid- or short-acting insulin to metabolize meals. The first long-acting insulin developed was NPH (still in use today), which was created by the addition of neutral protamine Hagedorn to regular insulin to delay insulin absorption.10
There are a few reasons that NPH use has fallen out of favor, especially for patients with type 1 diabetes, who do not produce endogenous insulin. One is a pronounced peak in action that requires patients to time their consumption of meals and snacks around peaks. If food consumption is insufficient, hypoglycemia may occur. Additionally, after the peak follows a valley in which low levels of exogenous insulin can lead to hyperglycemia.11
The second problem with NPH surrounds the inconsistency in both intersubject and intrasubject absorption rates. Absorption variability as great as 50% has been reported.12,13 This, of course, may lead to an increase in glycemic variability, that is, the magnitude of glucose fluctuation14 within a day. (Note: It has been hypothesized that, in conjunction with the A1C measure, glycemic variability may account in part for the long-term complications of diabetes.14)
Insulin Analogs
Radical advances in the potential for improved glucose control emerged with the development of insulin analogs.15,16 On the market now are three rapid-acting insulin analogs: lispro, aspart, and glulisine. Long-acting analogs are glargine and detemir, either of which may be given once or twice a day.17 Clinically, detemir is generally given twice per day in most patients with type 1 diabetes due to its shorter duration of action.
Most patients with type 1 diabetes are now managed with a once- or twice-daily injection of a long-acting insulin analog and one short-acting analog before each meal; thus, patients must take four or more insulin injections each day. While this multiple daily injection (MDI) insulin regimen approximates the body’s endogenous production of insulin more closely than the traditional split/mix regimens of NPH and regular insulin, there are still shortcomings. Once the long-acting insulin has been injected, it provides a steady level of insulin that cannot be adjusted for changes in the patient’s routine, such as exercise, delayed meals, or sleep, or for changes in the metabolic milieu, such as infection or other illness.18
PUMP BASICS
The CSII pump is a programmable insulin delivery system that provides continuous amounts of insulin and allows the user to deliver extra insulin to metabolize meals or to correct high blood glucose levels. Typically, rapid-acting insulin is used in the pump, although for patients with severe insulin resistance who need high doses of insulin, U-500 regular insulin may be used.19 U-500 regular insulin is a concentrated insulin dosed at one-fifth of U-100 regular insulin; that is, 100 units of regular insulin would equate to 20 units of U-500 insulin. (To clarify further, “U-100” refers to 100 U/mL, and “U-500” means 500 U/mL.) Understanding the indications for U-500 insulin and its adjustment is a specialized skill of the diabetologist experienced in its use.
A traditional pump consists of a beeper-sized device. It contains a reservoir that the user fills with insulin, then connects to an infusion set. The infusion set consists of tubing connected to a flexible catheter or needle that is inserted into the subcutaneous tissue. A mechanism to attach and detach the tubing from the infusion site is included so that users can disconnect it for bathing, sexual activity, contact sports, or other circumstances. Infusion sets are available in different sizes and styles to accommodate patients with different body weights, body fat levels, and lifestyle needs.
The “patch” or “wireless” pump, as it is sometimes called, is a self-contained unit or “pod” that resides directly on the skin, delivers insulin through a flexible catheter, and is disposed of or recycled every two to three days; it does not require tubing.
Potential pump users have a variety of manufacturers and models from which to choose, all providing similar features. Considerations for selecting a pump should include its particular features, cost of supplies, insurance coverage, customer service and support, and personal preference.
Basal and Bolus Insulin
The continuous background delivery in the pump is known as the basal; the rate at which the pump delivers the basal can be changed at different times of day to allow for changes in the patient’s routine and metabolic milieu (see Figure 1). Basal rates can be set anywhere between 0.025 and 35 units per hour. Most pumps allow for basal rates to last for as little as 30 minutes, but pump users commonly set one to four basal rates over 24 hours.
Some diabetic patients experience the dawn phenomenon, that is, fasting hyperglycemia resulting from the body’s release of growth hormone, cortisol, and adrenaline. These hormones, which produce a state of insulin resistance, are released beginning at about 2:00 to 3:00 am. A patient who is taking 24 units per day of total basal insulin might set a basal rate of one unit per hour; adjustments can be made if the patient needs less insulin at night and is prone to the dawn phenomenon.
All pumps begin basal rate programming at midnight. A basal rate profile for a patient who is prone to the dawn phenomenon and who needs 24 units/d of basal, goes to bed at 10:00 pm, and wakes up at 6:00 am, might look like this:
12:00 am: 0.8 units/h
3:00 am: 1.2 units/h
9:00 am: 1.0 units/h
10:00 pm: 0.8 units/h
Temporary basal rates may be set lower to account for exercise, or higher during times of illness. The pumper selects the amount released, the rate, and the length of time the temporary rate should last. After that selected time period is over, the pump automatically reverts to the regular basal rate. Setting lower basal rates before, during, or after exercise may benefit a patient who experiences exercise-related hypoglycemia. A patient who needs to adjust for exercise might select a temporary basal rate set to begin one hour before exercise and continue during exercise and for one hour after exercise.20
Even with these adjustments, however, low blood sugars may still occur, and delayed hypoglycemia may develop hours after exercise is completed.21 Muscle and liver tissue store excess sugar in the form of glycogen; glycogen stores are depleted during exercise, and blood sugar may decline when the muscle and liver take up blood sugar to replace the stores. In such instances, an extra carbohydrate snack and/or use of the temporary basal may prevent hypoglycemia.20
Setting temporary basal rates may also prevent hyperglycemia. In the absence of sufficient insulin, patients with type 1 diabetes produce ketones. Setting higher basal rates during illness, combined with frequent monitoring of blood sugars and ketones in the blood or urine, can prevent diabetic ketoacidosis (DKA). It is important for patients to understand that illness may increase their metabolic need for insulin.9 Even if the appetite decreases during illness, extra insulin may be needed. Frequent blood glucose monitoring and testing for ketones, when necessary, are essential for the patient with type 1 diabetes.
The supplemental insulin that patients take to compensate for carbohydrate intake and to correct high blood glucose is called the bolus. More recent pump technology, sometimes called the “smart” pump, includes features that will calculate for the patient how much of a bolus should be taken, based on three factors: carbohydrates ingested, current blood glucose level, and active insulin, that is, the amount of insulin that is still considered active after the patient takes a bolus based on the pharmacodynamic profile of the insulin.15,22
Carbohydrate-Counting
By the time a patient is started on an insulin pump, he or she should be proficient in carbohydrate-counting.8 There are several books on the market that list carbohydrate content of foods, including popular restaurant menu items and products sold in supermarkets. There are also apps available for PDAs and smart phones. The American Diabetes Association Web site (www.diabetes.org) offers carbohydrate-counting and menu-planning tools. For patients who eat out, it may be helpful to look up the restaurant’s menu online in advance and if possible, research the nutrition facts associated with each course selection. Even for patients with good carbohydrate-counting skills, it is often a challenge to get blood sugars into a desirable postprandial range.
The glycemic index is an important consideration. This 0-to-100–point scale is used to indicate how quickly specific foods raise blood sugar. Foods higher on the index (such as white potatoes and fruit juice) raise blood sugar more quickly than do foods that are lower on the index (whole grains, milk, nuts, and seeds).23 The caveat to using the glycemic index is that foods are often consumed as part of a mixed meal of proteins, fats, and carbohydrates—each of which will have a different effect on how rapidly blood sugars rise. Since fat slows digestion, meals that are high in both fats and carbohydrates can cause extended elevations in blood glucose.
To address this concern, smart pumps are equipped with an extended bolus feature that allows delivery of the bolus over a preset period of time.24 A percentage of the bolus may be delivered immediately and the remainder over time (dual-wave bolus). A typical split may be 50% now and 50% over the next two hours. This takes into account not only carbohydrate intake, but the components of a meal high in carbs and fat, such as pizza.
Several factors can impact the accuracy with which the patient calculates boluses to lower blood sugars for meals and other times of elevated blood glucose. Optimally, the total amount of correction insulin should represent only a small portion of the total daily bolus amount if the patient is being proactive. Ask patients about their carbohydrate-counting. Are they counting at a high skill level? Is their insulin-to-carbohydrate ratio correct? What about their inputs into their bolus calculator? Are their targets set too high or too low? Is their sensitivity correct? Is their active insulin time correct?
To assess postprandial control, patients should be instructed to check their blood glucose two hours after they started to ingest each meal.15 Consistent elevations mean that an adjustment to the insulin-to-carbohydrate ratio is indicated; alternatively, the patient may choose to consume fewer carbohydrates if weight is a concern.
If patients are not consistently measuring their foods (a discipline that is important but challenging to maintain), portion creep may be occurring. Encourage the patient to start measuring food again, at least for a while. A refresher visit to the nutritionist or diabetes educator may also be helpful. If patients are eating out often, they should be encouraged to limit themselves to meals with a known carbohydrate content.
Response to various foods is often idiosyncratic. Having patients keep a detailed log of foods eaten, exercise performed, and corresponding blood glucose levels will often clear up mysterious blood sugar elevations.
Correction Insulin
Depending on their personal approach and regimen, patients are taught to monitor their glucose either prior to eating, after eating, or both; and to take insulin to achieve a correction if the glucose is outside their target zone. A typical preprandial target range is 80 to 120 mg/dL, with 140 to 180 mg/dL two hours postprandial. (The American Association of Clinical Endocrinologists, while emphasizing the importance of individualizing glucose targets, suggests a fasting plasma glucose level below 110 mg/dL and a two-hour postprandial concentration below 140 mg/dL to achieve a target A1C level ≤ 6.5% in the nonpregnant adult.16 The American Diabetes Association’s comparable recommendations are 70 to 130 mg/dL preprandial and 180 mg/dL peak postprandial.15)
In today’s pumps, as part of the bolus calculation, a number can be programmed that represents the number of points in mg/dL by which one unit of insulin will reduce blood sugars. This may be known as a sensitivity or correction factor.
For example, a pumper who is making a postprandial correction and is not eating has a target blood sugar between 140 and 180 mg/dL and a sensitivity of 40; at present, his blood sugar is 230 mg/dL. Assuming no active insulin, the pump would calculate a dose of about 1.25 units of rapid-acting insulin.
How active insulin, or insulin on board, is calculated differs among pump manufacturers but is loosely based on original insulin pharmacodynamic studies.15,22 In smart pumps, the bolus calculator takes into consideration how much insulin is still active when it recommends a correction dose for hyperglycemia. This allows the user to address hyperglycemia quickly and effectively but prevents “stacking” of insulin doses and subsequent hypoglycemia.18
The active insulin time is preset in some pumps, but many diabetes clinicians use three or four hours (anecdotal evidence). The pumper can test the validity of active insulin by administering a correction bolus when blood glucose exceeds 250 mg/dL, then checking blood glucose levels every 30 to 60 minutes for six hours with no further food intake.9 The length of time it takes for blood sugars to return to target and remain steady is determined to be the correct duration of insulin action. In the example above, if the pump determined that there was active insulin remaining from the prior meal, it would subtract that amount from the calculation to prevent insulin stacking—which would most likely lead to overcorrection and hypoglycemia.
PUMP OPTIONS AND GLUCOSE MONITORING
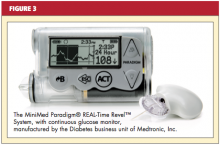
In addition to using wireless communication, current “disposable” (“patch”) pumps operate without the conventional infusion set and tubing. Some smart pumps are now equipped with a combination device: a diabetes-specific, PDA-like apparatus with an integrated glucose monitor that links to the pump through infrared technology (see Figure 2). Another pump has a “linked” glucose monitor (see Figure 3) that allows the user to test blood sugar, with the reading downloaded to the pump. The pump (or PDA) calculates the needed dose, based on programmed insulin-to-carbohydrate ratios and correction factors.22
A bolus calculator on the PDA helps the user calculate the bolus dose to be delivered to the pump without the user’s having to remove the pump to administer a dose. The pumper can discreetly administer insulin, even when the patient is eating out.
The one patch pump that is currently on the market requires the user to set the PDA to bolus, since the pod has no buttons with which to input instructions for a bolus. Use of the PDA or linked meter requires the pumper to use a particular brand of test strips. If insurance does not cover that brand, the patient can use another manufacturer’s monitor and enter blood sugar readings manually.
Potential Pump Problems
Patients who are considering any CSII pump must be willing to check blood glucose levels frequently. Malfunction of insulin pumps (including a blockage or pump failure) is associated with an increased risk for DKA,8 because the pump delivers only short-acting insulin. Without the presence of long-acting insulin as a backup, DKA can develop rapidly. All pumps have pressure-sensing alarms to detect blockages; in patients who receive small doses of insulin, however, it may take time for the alarm to be triggered. Thus, the importance of frequent blood glucose monitoring is evident. (Additionally, in case of pump failure, patients should know their basal rates and insulin-to-carbohydrate ratios or keep them written down.)
Pumpers are taught that if they experience two or more unexplained high glucose readings in a row to troubleshoot the infusion set for air bubbles or a clog. They should also take an injection of insulin to correct the high glucose and change the reservoir and infusion set.
Continuous Glucose Monitor
Some patients use continuous glucose monitoring (CGM) with a sensor that measures glucose levels in the interstitial fluid. At the time of this writing, one currently available pump is equipped with an integrated glucose sensor (see Figure 3). This device monitors interstitial fluid glucose levels continuously and can provide trend data, letting the user know whether blood sugars are rising or falling and how rapidly.25
Recently, researchers for the Sensor-Augmented Pump Therapy for A1C Reduction (STAR 3) study26,27 reported that use of sensor-augmented insulin pumps (SAP) reduces A1C without increasing hypoglycemia, compared with MDI insulin regimens. Additional markers of success in SAP use, compared with an MDI regimen, are sensor glucose values that are closer to target, bolus-calculator interactions, amount of sensor use, and lower glycemic variability—especially in patients who achieve lower A1C.28,29
Even when the patient is using a glucose sensor, however, blood sugars must still be tested at least twice a day to calibrate the sensor. There is a common misperception that CGM serves as a substitute for blood glucose (finger-stick) monitoring, but there is a lag time between interstitial fluid glucose levels and blood glucose levels.30 Blood glucose monitoring by finger-stick is still considered the gold standard for measuring glucose; sensor manufacturers recommend that patients not take a treatment action based on sensor data without confirming first with a finger-stick.
Barriers to effective glucose sensor use include the high cost of sensors and variability in insurance coverage, user factors (patients’ not using sensors daily, calibrating them inaccurately), insertion-site infections, and technology issues, like sensor failure.27,31
Memory Features
Pump technology makes it possible to review bolus history (ie, the amount of bolus and the time at which it was given) as well as the dosage of insulin taken throughout the day. Based on these data, several assessments can be made:
• Is the basal-to-bolus ratio appropriate? (It should be about 40% to 50% basal / 50% to 60% bolus.)8
• Is the amount of insulin adequate? Adults and prepubertal children with type 1 diabetes usually require 0.6 to 0.9 units/kg/d.15 During puberty, insulin requirements can rise to 1.5 units/kg/d32 due to increased insulin resistance, surges in pubertal hormones, and greater caloric intake during growth spurts. Pump manufacturers offer data management software that allows the user to produce graphs, track trends, and monitor basal-to-bolus ratios.
• Is the patient actually bolusing for meals? Does the patient check blood glucose postprandially and bolus when the reading is high?
PATIENT TRAINING BEFORE PUMP INITIATION
Insurance coverage is generally favorable for pumps and associated supplies, since their cost benefit (ie, decreases in use of antidiabetic drugs and of health care resources6) is well proven for patients with type 1 diabetes. For those with type 2 diabetes, obtaining coverage is more challenging5 but can be supported if the patient has a history of severe hypoglycemia, insulin resistance, or uncontrolled blood sugar levels.6,7
Pump manufacturers employ insurance specialists to work with patients and providers in order to facilitate the insurance reimbursement process. Payment plans are available for what insurance does not cover, and pump warranties last for several years. Many insurance plans do not cover an automatic upgrade to a newer pump when the warranty expires, but the patient may be eligible for a discount on a newer pump.
Before initiating pump use, patients receive instruction from certified pump trainers—generally health professionals, such as nurses and registered dietitians, who may be employees of the endocrine practice or of the pump manufacturing company, or independent contractors; many are certified diabetes educators (CDEs). Training may be conducted in the patient’s home or in the medical office. Typically, patients are given some sort of DVD or CD-ROM and workbook to complete before pump training takes place.
Pump trainers make sure patients understand the basics of carbohydrate-counting, sick day management, and treatment of hypoglycemia and hyperglycemia. Training may comprise two sessions, one during which the patient can wear the pump with a saline administration and practice with it for a few days before starting on insulin at the second session. Training should be individualized to allow for different speeds and styles of learning.
Careful patient selection is the key to successful pump use. Optimally, patients should be motivated to improve their blood glucose control, compliant with their current regimen, and proficient in their diabetes self-management skills, including carbohydrate-counting, troubleshooting, and making adjustments for day-to-day variability in their circumstances.8
Pump trainers can make suggestions to the patient’s diabetologist regarding basal rates, correction factors, and insulin-to-carbohydrate ratios, but a health care provider’s order is necessary to initiate use of a pump.
RESOURCES FOR PATIENTS
Referral to a CDE and/or an endocrinology specialist may be helpful for any patient who was started on a pump in the past but is now struggling with management or would like to fine-tune his or her control. Patients who are unable to reduce an A1C of 8% may also benefit from referral. Not all of these practitioners operate at an expert level in pump therapy, however.
Given the shortage of endocrinologists and CDEs, especially in rural areas, finding a qualified practitioner is sometimes challenging. The American Association of Diabetes Educators maintains a referral service at www.diabeteseduca tor.org/find or (800) TeamUp4.
Patients can also contact the pump manufacturer to ask about local resources. Classes in advanced pumping skills and local pump support groups are often sponsored by pump manufacturers. Additionally, excellent books have been published on pump therapy and carbohydrate-counting, including those listed below.
RISKS AND BENEFITS OF INSULIN PUMP THERAPY
Risks of insulin pump therapy include DKA, infusion-site problems, and infection leading to abscess.27,33,34
In the absence of insulin, patients with type 1 diabetes produce ketones; because a pump uses only rapid- or short-acting insulin, a failure to deliver that insulin can lead to acute insulin deficiency, resulting in DKA. All pumps are equipped with pressure-sensitive alarms that will alert the user to a blockage that may result in failure to deliver insulin. Pump users who receive low doses of insulin (the absence of which may not cause sufficient pressure to trigger the alarm) or who experience pump blockages that cause only partial delivery may experience alarm failure. It is crucial for patients to monitor their blood sugar frequently; those who are unwilling to do so are not good candidates for insulin pump therapy.8
Infusion-site problems can include skin reactions to tape or infections resulting from inadequate cleansing or from failure to cleanse the site and change the infusion set, as recommended by the manufacturer, every two to three days. Pump trainers or the pump manufacturer’s customer service team can offer solutions to infusion-site skin care problems. Scrupulous handwashing, timely site changes, and cleansing of the skin are essential to preventing site problems.
Benefits of insulin pump therapy include the opportunity to individualize therapy for specific medical needs and lifestyle differences. Basal profiles can be individually adjusted for metabolic concerns, such as the dawn phenomenon or temporary illness—as well as day-to-day changes, such as increased physical activity. Bolus calculators allow for more accurate matching of the insulin-to-carbohydrate ratio and the amount of correction insulin necessary before meals.
CONCLUSION
CSII pumps may offer significant benefits to diabetic patients, compared with their current MDI regimen. These include reduction in A1C without increases in hypoglycemia, greater flexibility, improved quality of life—and, especially when used in conjunction with continuous glucose monitoring, reduction in glycemic variability. Patients and providers must bear in mind, however, that the pump is a tool, not a substitute for what guarantees success in diabetes management—namely, attention to meal planning, carbohydrate-counting, frequent blood sugar monitoring, competent sick day management, regular exercise, and target blood sugar and A1C goals. The patient most likely to benefit from insulin pump use is one with the motivation to make the lifestyle changes that effective therapy requires.
REFERENCES
1. General Hospital and Personal Use Medical Devices Panel Meeting—March 5, 2010. www .fda.gov/downloads/AdvisoryCommittees/Com mitteesMeetingMaterials/MedicalDevices/
MedicalDevicesAdvisoryCommittee/General HospitalandPersonalUseDevicesPanel/
UCM203749.pdf. Accessed October 21, 2011.
2. Fabiato K, Buse J, Duclos M, et al. Clinical experience with continuous glucose monitoring in adults. Diabetes Technol Ther. 2009;11 suppl 1:S93-S103.
3. Battelino T, Phillip M, Bratina N, et al. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011; 34(4):795-800
4. Edelman SV, Bode BW, Bailey TS, et al. Insulin pump therapy in patients with type 2 diabetes safely improved glycemic control using a simple insulin dosing regimen. Diabetes Technol Ther. 2010;12(8):627-633.
5. Skladany MJ, Miller M, Guthermann JS, Ludwig CR. Patch-pump technology to manage type 2 diabetes mellitus: hurdles to market acceptance. J Diabetes Sci Technol. 2008;2(6): 1147-1150.
6. Lynch PM, Riedel AA, Samant N, et al. Resource utilization with insulin pump therapy for type 2 diabetes mellitus. Am J Manag Care. 2010;16(12):892-896.
7. Nielsen S, Kain D, Szudzik E, et al. Use of continuous subcutaneous insulin infusion pump in patients with type 2 diabetes mellitus. Diabetes Educ. 2005;31(6):843-848.
8. Scheiner G, Sobel RJ, Smith DE, et al. Insulin pump therapy: guidelines for successful outcomes. Diabetes Educ. 2009;35 suppl 2:29S-41S.
9. Unger J. Insulin pump therapy. In: Unger J. Diabetes Management in Primary Care. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:265-320.
10. Hagedorn HC. Modifications of insulin. Physician’s Bull. 1947;12(5):26-33.
11. Salemyr J, Bang P, Örtqvist E. Lower HbA1c after 1 year, in children with type 1 diabetes treated with insulin glargine vs. NPH insulin from diagnosis: a retrospective study. Pediatr Diabetes. 2011;12(5):501-505.
12. Lauritzen T, Pramming S, Deckert T, Binder C. Pharmacokinetics of continuous subcutaneous insulin infusion. Diabetologia. 1983;24(5): 326-329.
13. Binder C, Lauritzen T, Faber O, Pramming S. Insulin pharmacokinetics. Diabetes Care. 1984; 7(2):188-199.
14. Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c–independent risk factor for diabetic complications. JAMA. 2006;295(14): 1707-1708.
15. American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31 suppl 1:S12-S54.
16. Handelsman Y, Mechanick JI, Blonde L, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan. Endocrine Pract. 2011;17(suppl 2):1-53.
17. Kaufman F. Insulin treatment. In: Kaufman F, ed. Medical Management of Type 1 Diabetes. 5th ed. Alexandria, VA: American Diabetes Association; 2008:57-82.
18. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352(2):174-183.
19. Cochran E, Musso C, Gorden P. The use of U-500 in patients with extreme insulin resistance. Diabetes Care. 2005;28(5):1240-1244.
20. Diabetes Research in Children Network (DirecNet) Study Group. Prevention of hypoglycemia during exercise in children with type 1 diabetes by suspending basal insulin. Diabetes Care. 2006;29(10):2200-2204.
21. Maran A, Pavan P, Bonsembiante B, et al. Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high-intensity exercise in nontrained patients with type 1 diabetes. Diabetes Technol Ther. 2010; 12(10):763-768.
22. Davidson PC, Hebblewhite HR, Steed RD, Bode BW. Analysis of guidelines for basal-bolus insulin dosing: basal insulin, correction factor, and carbohydrate-to-insulin ratio. Endocr Pract. 2008;14(9):1095-1101.
23. Glycemic Index Foundation, Sydney University Glycemic Index Research Service (SUGiRS). GI testing and research. www.glycemicindex
.com. Accessed October 21, 2011.
24. Pankowska E, Błazik M. Bolus calculator with nutrition database software, a new concept of prandial insulin programming for pump users.
J Diabetes Sci Technol. 2010;4(3):571-576.
25. Davis SN, Horton ES, Battelino T, et al. STAR 3 randomized controlled trial to compare sensor-augmented insulin pump therapy with multiple daily injections in the treatment of type 1 diabetes: research design, methods, and baseline characteristics of enrolled subjects. Diabetes Technol Ther. 2010;12(4):249-255.
26. Buse JB, Dailey G, Ahmann AA, et al. Baseline predictors of A1C reduction in adults using sensor-augmented pump therapy or multiple daily injection therapy: the STAR 3 experience. Diabetes Technol Ther. 2011;13(6):601-606.
27. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Sensor-Augmented Pump Therapy for A1C Reduction (STAR 3) Study: results from the 6-month continuation phase. Diabetes Care. 2011 Sep 20. Epub ahead of print.
28. Green JB, Lane WS, Bergenstal RM, et al. Insulin Pump Settings Associated with Degree of Glycemic Improvement in Adults with Type 1 Diabetes Using Sensor-Augmented Pump Therapy. Presented at: Diabetes Technology Meeting; November 11-13, 2010; Bethesda, MD. Abstract A51.
29. Buse JB, Kidva YC, Guthrie RA, et al. Assessment of glycemic variability and CD40 ligand in the STAR 3 Study. Presented at: American Diabetes Association Scientific Sessions; June 2, 2011; San Diego, CA. Abstract 0923-P.
30. Hirsch IB. Algorithms for care in adults using continuous glucose monitoring. J Diabetes Sci Technol. 2007;1(1):126-129.
31. Wolpert HA. Continuous glucose monitoring: coming of age (editorial). N Engl J Med. 2010;363(4):383-384.
32. Wiegand S, Raile K, Reinehr T, et al; DPV-Wiss Study Group. Daily insulin requirement of children and adolescents with type 1 diabetes: effect of age, body mass index and mode of therapy. Eur J Endocrinol. 2008;158(4):543-549.
33. Hanas R, Ludvigsson J. Hypoglycemia and ketoacidosis with insulin pump therapy in children and adolescents. Pediatr Diabetes. 2006;7 suppl 4:32-38.
34. Unger J. A primary care approach to continuous subcutaneous insulin infusion. Clin Diabetes. 1999;17(3). http://journal.diabetes.org/clinicaldiabetes/V17N31999/Pg113.htm. Accessed October 21, 2011.
The FDA has reported that in 2007, 375,000 Americans with diabetes were using an insulin pump.1 Improvements in pump technology (including the addition of continuous glucose monitoring2,3), more data on the use of pumps in type 2 diabetes,4-7 improved insurance coverage,8 and the general population’s comfort with technology-based solutions may lead more patients with diabetes to choose a pump to deliver their insulin.
As a result, primary care practitioners will be increasingly likely to have patients who use continuous subcutaneous insulin infusion (CSII) pumps. While the initiation of pump therapy and follow-up with patients who use pumps have traditionally been the purview of endocrinologists, it is important for primary care providers to understand the basics of insulin pump therapy, enabling them to work collaboratively with the patient and the diabetes care team.
REVIEW OF INSULIN ACTION IN THE BODY
The body’s own (endogenous) insulin is secreted by the beta cells of the pancreas. Basal insulin is the background amount of insulin continuously released by the body in order to regulate hepatic glucose production and lipolysis. In response to food intake, the body releases additional insulin to match the glycemic effect of carbohydrates, fat, and protein.9
Insulin regimens attempt to mimic the body’s physiologic release of insulin by administering long-acting insulin to provide basal coverage, and rapid- or short-acting insulin to metabolize meals. The first long-acting insulin developed was NPH (still in use today), which was created by the addition of neutral protamine Hagedorn to regular insulin to delay insulin absorption.10
There are a few reasons that NPH use has fallen out of favor, especially for patients with type 1 diabetes, who do not produce endogenous insulin. One is a pronounced peak in action that requires patients to time their consumption of meals and snacks around peaks. If food consumption is insufficient, hypoglycemia may occur. Additionally, after the peak follows a valley in which low levels of exogenous insulin can lead to hyperglycemia.11
The second problem with NPH surrounds the inconsistency in both intersubject and intrasubject absorption rates. Absorption variability as great as 50% has been reported.12,13 This, of course, may lead to an increase in glycemic variability, that is, the magnitude of glucose fluctuation14 within a day. (Note: It has been hypothesized that, in conjunction with the A1C measure, glycemic variability may account in part for the long-term complications of diabetes.14)
Insulin Analogs
Radical advances in the potential for improved glucose control emerged with the development of insulin analogs.15,16 On the market now are three rapid-acting insulin analogs: lispro, aspart, and glulisine. Long-acting analogs are glargine and detemir, either of which may be given once or twice a day.17 Clinically, detemir is generally given twice per day in most patients with type 1 diabetes due to its shorter duration of action.
Most patients with type 1 diabetes are now managed with a once- or twice-daily injection of a long-acting insulin analog and one short-acting analog before each meal; thus, patients must take four or more insulin injections each day. While this multiple daily injection (MDI) insulin regimen approximates the body’s endogenous production of insulin more closely than the traditional split/mix regimens of NPH and regular insulin, there are still shortcomings. Once the long-acting insulin has been injected, it provides a steady level of insulin that cannot be adjusted for changes in the patient’s routine, such as exercise, delayed meals, or sleep, or for changes in the metabolic milieu, such as infection or other illness.18
PUMP BASICS
The CSII pump is a programmable insulin delivery system that provides continuous amounts of insulin and allows the user to deliver extra insulin to metabolize meals or to correct high blood glucose levels. Typically, rapid-acting insulin is used in the pump, although for patients with severe insulin resistance who need high doses of insulin, U-500 regular insulin may be used.19 U-500 regular insulin is a concentrated insulin dosed at one-fifth of U-100 regular insulin; that is, 100 units of regular insulin would equate to 20 units of U-500 insulin. (To clarify further, “U-100” refers to 100 U/mL, and “U-500” means 500 U/mL.) Understanding the indications for U-500 insulin and its adjustment is a specialized skill of the diabetologist experienced in its use.
A traditional pump consists of a beeper-sized device. It contains a reservoir that the user fills with insulin, then connects to an infusion set. The infusion set consists of tubing connected to a flexible catheter or needle that is inserted into the subcutaneous tissue. A mechanism to attach and detach the tubing from the infusion site is included so that users can disconnect it for bathing, sexual activity, contact sports, or other circumstances. Infusion sets are available in different sizes and styles to accommodate patients with different body weights, body fat levels, and lifestyle needs.
The “patch” or “wireless” pump, as it is sometimes called, is a self-contained unit or “pod” that resides directly on the skin, delivers insulin through a flexible catheter, and is disposed of or recycled every two to three days; it does not require tubing.
Potential pump users have a variety of manufacturers and models from which to choose, all providing similar features. Considerations for selecting a pump should include its particular features, cost of supplies, insurance coverage, customer service and support, and personal preference.
Basal and Bolus Insulin
The continuous background delivery in the pump is known as the basal; the rate at which the pump delivers the basal can be changed at different times of day to allow for changes in the patient’s routine and metabolic milieu (see Figure 1). Basal rates can be set anywhere between 0.025 and 35 units per hour. Most pumps allow for basal rates to last for as little as 30 minutes, but pump users commonly set one to four basal rates over 24 hours.
Some diabetic patients experience the dawn phenomenon, that is, fasting hyperglycemia resulting from the body’s release of growth hormone, cortisol, and adrenaline. These hormones, which produce a state of insulin resistance, are released beginning at about 2:00 to 3:00 am. A patient who is taking 24 units per day of total basal insulin might set a basal rate of one unit per hour; adjustments can be made if the patient needs less insulin at night and is prone to the dawn phenomenon.
All pumps begin basal rate programming at midnight. A basal rate profile for a patient who is prone to the dawn phenomenon and who needs 24 units/d of basal, goes to bed at 10:00 pm, and wakes up at 6:00 am, might look like this:
12:00 am: 0.8 units/h
3:00 am: 1.2 units/h
9:00 am: 1.0 units/h
10:00 pm: 0.8 units/h
Temporary basal rates may be set lower to account for exercise, or higher during times of illness. The pumper selects the amount released, the rate, and the length of time the temporary rate should last. After that selected time period is over, the pump automatically reverts to the regular basal rate. Setting lower basal rates before, during, or after exercise may benefit a patient who experiences exercise-related hypoglycemia. A patient who needs to adjust for exercise might select a temporary basal rate set to begin one hour before exercise and continue during exercise and for one hour after exercise.20
Even with these adjustments, however, low blood sugars may still occur, and delayed hypoglycemia may develop hours after exercise is completed.21 Muscle and liver tissue store excess sugar in the form of glycogen; glycogen stores are depleted during exercise, and blood sugar may decline when the muscle and liver take up blood sugar to replace the stores. In such instances, an extra carbohydrate snack and/or use of the temporary basal may prevent hypoglycemia.20
Setting temporary basal rates may also prevent hyperglycemia. In the absence of sufficient insulin, patients with type 1 diabetes produce ketones. Setting higher basal rates during illness, combined with frequent monitoring of blood sugars and ketones in the blood or urine, can prevent diabetic ketoacidosis (DKA). It is important for patients to understand that illness may increase their metabolic need for insulin.9 Even if the appetite decreases during illness, extra insulin may be needed. Frequent blood glucose monitoring and testing for ketones, when necessary, are essential for the patient with type 1 diabetes.
The supplemental insulin that patients take to compensate for carbohydrate intake and to correct high blood glucose is called the bolus. More recent pump technology, sometimes called the “smart” pump, includes features that will calculate for the patient how much of a bolus should be taken, based on three factors: carbohydrates ingested, current blood glucose level, and active insulin, that is, the amount of insulin that is still considered active after the patient takes a bolus based on the pharmacodynamic profile of the insulin.15,22
Carbohydrate-Counting
By the time a patient is started on an insulin pump, he or she should be proficient in carbohydrate-counting.8 There are several books on the market that list carbohydrate content of foods, including popular restaurant menu items and products sold in supermarkets. There are also apps available for PDAs and smart phones. The American Diabetes Association Web site (www.diabetes.org) offers carbohydrate-counting and menu-planning tools. For patients who eat out, it may be helpful to look up the restaurant’s menu online in advance and if possible, research the nutrition facts associated with each course selection. Even for patients with good carbohydrate-counting skills, it is often a challenge to get blood sugars into a desirable postprandial range.
The glycemic index is an important consideration. This 0-to-100–point scale is used to indicate how quickly specific foods raise blood sugar. Foods higher on the index (such as white potatoes and fruit juice) raise blood sugar more quickly than do foods that are lower on the index (whole grains, milk, nuts, and seeds).23 The caveat to using the glycemic index is that foods are often consumed as part of a mixed meal of proteins, fats, and carbohydrates—each of which will have a different effect on how rapidly blood sugars rise. Since fat slows digestion, meals that are high in both fats and carbohydrates can cause extended elevations in blood glucose.
To address this concern, smart pumps are equipped with an extended bolus feature that allows delivery of the bolus over a preset period of time.24 A percentage of the bolus may be delivered immediately and the remainder over time (dual-wave bolus). A typical split may be 50% now and 50% over the next two hours. This takes into account not only carbohydrate intake, but the components of a meal high in carbs and fat, such as pizza.
Several factors can impact the accuracy with which the patient calculates boluses to lower blood sugars for meals and other times of elevated blood glucose. Optimally, the total amount of correction insulin should represent only a small portion of the total daily bolus amount if the patient is being proactive. Ask patients about their carbohydrate-counting. Are they counting at a high skill level? Is their insulin-to-carbohydrate ratio correct? What about their inputs into their bolus calculator? Are their targets set too high or too low? Is their sensitivity correct? Is their active insulin time correct?
To assess postprandial control, patients should be instructed to check their blood glucose two hours after they started to ingest each meal.15 Consistent elevations mean that an adjustment to the insulin-to-carbohydrate ratio is indicated; alternatively, the patient may choose to consume fewer carbohydrates if weight is a concern.
If patients are not consistently measuring their foods (a discipline that is important but challenging to maintain), portion creep may be occurring. Encourage the patient to start measuring food again, at least for a while. A refresher visit to the nutritionist or diabetes educator may also be helpful. If patients are eating out often, they should be encouraged to limit themselves to meals with a known carbohydrate content.
Response to various foods is often idiosyncratic. Having patients keep a detailed log of foods eaten, exercise performed, and corresponding blood glucose levels will often clear up mysterious blood sugar elevations.
Correction Insulin
Depending on their personal approach and regimen, patients are taught to monitor their glucose either prior to eating, after eating, or both; and to take insulin to achieve a correction if the glucose is outside their target zone. A typical preprandial target range is 80 to 120 mg/dL, with 140 to 180 mg/dL two hours postprandial. (The American Association of Clinical Endocrinologists, while emphasizing the importance of individualizing glucose targets, suggests a fasting plasma glucose level below 110 mg/dL and a two-hour postprandial concentration below 140 mg/dL to achieve a target A1C level ≤ 6.5% in the nonpregnant adult.16 The American Diabetes Association’s comparable recommendations are 70 to 130 mg/dL preprandial and 180 mg/dL peak postprandial.15)
In today’s pumps, as part of the bolus calculation, a number can be programmed that represents the number of points in mg/dL by which one unit of insulin will reduce blood sugars. This may be known as a sensitivity or correction factor.
For example, a pumper who is making a postprandial correction and is not eating has a target blood sugar between 140 and 180 mg/dL and a sensitivity of 40; at present, his blood sugar is 230 mg/dL. Assuming no active insulin, the pump would calculate a dose of about 1.25 units of rapid-acting insulin.
How active insulin, or insulin on board, is calculated differs among pump manufacturers but is loosely based on original insulin pharmacodynamic studies.15,22 In smart pumps, the bolus calculator takes into consideration how much insulin is still active when it recommends a correction dose for hyperglycemia. This allows the user to address hyperglycemia quickly and effectively but prevents “stacking” of insulin doses and subsequent hypoglycemia.18
The active insulin time is preset in some pumps, but many diabetes clinicians use three or four hours (anecdotal evidence). The pumper can test the validity of active insulin by administering a correction bolus when blood glucose exceeds 250 mg/dL, then checking blood glucose levels every 30 to 60 minutes for six hours with no further food intake.9 The length of time it takes for blood sugars to return to target and remain steady is determined to be the correct duration of insulin action. In the example above, if the pump determined that there was active insulin remaining from the prior meal, it would subtract that amount from the calculation to prevent insulin stacking—which would most likely lead to overcorrection and hypoglycemia.
PUMP OPTIONS AND GLUCOSE MONITORING
In addition to using wireless communication, current “disposable” (“patch”) pumps operate without the conventional infusion set and tubing. Some smart pumps are now equipped with a combination device: a diabetes-specific, PDA-like apparatus with an integrated glucose monitor that links to the pump through infrared technology (see Figure 2). Another pump has a “linked” glucose monitor (see Figure 3) that allows the user to test blood sugar, with the reading downloaded to the pump. The pump (or PDA) calculates the needed dose, based on programmed insulin-to-carbohydrate ratios and correction factors.22
A bolus calculator on the PDA helps the user calculate the bolus dose to be delivered to the pump without the user’s having to remove the pump to administer a dose. The pumper can discreetly administer insulin, even when the patient is eating out.
The one patch pump that is currently on the market requires the user to set the PDA to bolus, since the pod has no buttons with which to input instructions for a bolus. Use of the PDA or linked meter requires the pumper to use a particular brand of test strips. If insurance does not cover that brand, the patient can use another manufacturer’s monitor and enter blood sugar readings manually.
Potential Pump Problems
Patients who are considering any CSII pump must be willing to check blood glucose levels frequently. Malfunction of insulin pumps (including a blockage or pump failure) is associated with an increased risk for DKA,8 because the pump delivers only short-acting insulin. Without the presence of long-acting insulin as a backup, DKA can develop rapidly. All pumps have pressure-sensing alarms to detect blockages; in patients who receive small doses of insulin, however, it may take time for the alarm to be triggered. Thus, the importance of frequent blood glucose monitoring is evident. (Additionally, in case of pump failure, patients should know their basal rates and insulin-to-carbohydrate ratios or keep them written down.)
Pumpers are taught that if they experience two or more unexplained high glucose readings in a row to troubleshoot the infusion set for air bubbles or a clog. They should also take an injection of insulin to correct the high glucose and change the reservoir and infusion set.
Continuous Glucose Monitor
Some patients use continuous glucose monitoring (CGM) with a sensor that measures glucose levels in the interstitial fluid. At the time of this writing, one currently available pump is equipped with an integrated glucose sensor (see Figure 3). This device monitors interstitial fluid glucose levels continuously and can provide trend data, letting the user know whether blood sugars are rising or falling and how rapidly.25
Recently, researchers for the Sensor-Augmented Pump Therapy for A1C Reduction (STAR 3) study26,27 reported that use of sensor-augmented insulin pumps (SAP) reduces A1C without increasing hypoglycemia, compared with MDI insulin regimens. Additional markers of success in SAP use, compared with an MDI regimen, are sensor glucose values that are closer to target, bolus-calculator interactions, amount of sensor use, and lower glycemic variability—especially in patients who achieve lower A1C.28,29
Even when the patient is using a glucose sensor, however, blood sugars must still be tested at least twice a day to calibrate the sensor. There is a common misperception that CGM serves as a substitute for blood glucose (finger-stick) monitoring, but there is a lag time between interstitial fluid glucose levels and blood glucose levels.30 Blood glucose monitoring by finger-stick is still considered the gold standard for measuring glucose; sensor manufacturers recommend that patients not take a treatment action based on sensor data without confirming first with a finger-stick.
Barriers to effective glucose sensor use include the high cost of sensors and variability in insurance coverage, user factors (patients’ not using sensors daily, calibrating them inaccurately), insertion-site infections, and technology issues, like sensor failure.27,31
Memory Features
Pump technology makes it possible to review bolus history (ie, the amount of bolus and the time at which it was given) as well as the dosage of insulin taken throughout the day. Based on these data, several assessments can be made:
• Is the basal-to-bolus ratio appropriate? (It should be about 40% to 50% basal / 50% to 60% bolus.)8
• Is the amount of insulin adequate? Adults and prepubertal children with type 1 diabetes usually require 0.6 to 0.9 units/kg/d.15 During puberty, insulin requirements can rise to 1.5 units/kg/d32 due to increased insulin resistance, surges in pubertal hormones, and greater caloric intake during growth spurts. Pump manufacturers offer data management software that allows the user to produce graphs, track trends, and monitor basal-to-bolus ratios.
• Is the patient actually bolusing for meals? Does the patient check blood glucose postprandially and bolus when the reading is high?
PATIENT TRAINING BEFORE PUMP INITIATION
Insurance coverage is generally favorable for pumps and associated supplies, since their cost benefit (ie, decreases in use of antidiabetic drugs and of health care resources6) is well proven for patients with type 1 diabetes. For those with type 2 diabetes, obtaining coverage is more challenging5 but can be supported if the patient has a history of severe hypoglycemia, insulin resistance, or uncontrolled blood sugar levels.6,7
Pump manufacturers employ insurance specialists to work with patients and providers in order to facilitate the insurance reimbursement process. Payment plans are available for what insurance does not cover, and pump warranties last for several years. Many insurance plans do not cover an automatic upgrade to a newer pump when the warranty expires, but the patient may be eligible for a discount on a newer pump.
Before initiating pump use, patients receive instruction from certified pump trainers—generally health professionals, such as nurses and registered dietitians, who may be employees of the endocrine practice or of the pump manufacturing company, or independent contractors; many are certified diabetes educators (CDEs). Training may be conducted in the patient’s home or in the medical office. Typically, patients are given some sort of DVD or CD-ROM and workbook to complete before pump training takes place.
Pump trainers make sure patients understand the basics of carbohydrate-counting, sick day management, and treatment of hypoglycemia and hyperglycemia. Training may comprise two sessions, one during which the patient can wear the pump with a saline administration and practice with it for a few days before starting on insulin at the second session. Training should be individualized to allow for different speeds and styles of learning.
Careful patient selection is the key to successful pump use. Optimally, patients should be motivated to improve their blood glucose control, compliant with their current regimen, and proficient in their diabetes self-management skills, including carbohydrate-counting, troubleshooting, and making adjustments for day-to-day variability in their circumstances.8
Pump trainers can make suggestions to the patient’s diabetologist regarding basal rates, correction factors, and insulin-to-carbohydrate ratios, but a health care provider’s order is necessary to initiate use of a pump.
RESOURCES FOR PATIENTS
Referral to a CDE and/or an endocrinology specialist may be helpful for any patient who was started on a pump in the past but is now struggling with management or would like to fine-tune his or her control. Patients who are unable to reduce an A1C of 8% may also benefit from referral. Not all of these practitioners operate at an expert level in pump therapy, however.
Given the shortage of endocrinologists and CDEs, especially in rural areas, finding a qualified practitioner is sometimes challenging. The American Association of Diabetes Educators maintains a referral service at www.diabeteseduca tor.org/find or (800) TeamUp4.
Patients can also contact the pump manufacturer to ask about local resources. Classes in advanced pumping skills and local pump support groups are often sponsored by pump manufacturers. Additionally, excellent books have been published on pump therapy and carbohydrate-counting, including those listed below.
RISKS AND BENEFITS OF INSULIN PUMP THERAPY
Risks of insulin pump therapy include DKA, infusion-site problems, and infection leading to abscess.27,33,34
In the absence of insulin, patients with type 1 diabetes produce ketones; because a pump uses only rapid- or short-acting insulin, a failure to deliver that insulin can lead to acute insulin deficiency, resulting in DKA. All pumps are equipped with pressure-sensitive alarms that will alert the user to a blockage that may result in failure to deliver insulin. Pump users who receive low doses of insulin (the absence of which may not cause sufficient pressure to trigger the alarm) or who experience pump blockages that cause only partial delivery may experience alarm failure. It is crucial for patients to monitor their blood sugar frequently; those who are unwilling to do so are not good candidates for insulin pump therapy.8
Infusion-site problems can include skin reactions to tape or infections resulting from inadequate cleansing or from failure to cleanse the site and change the infusion set, as recommended by the manufacturer, every two to three days. Pump trainers or the pump manufacturer’s customer service team can offer solutions to infusion-site skin care problems. Scrupulous handwashing, timely site changes, and cleansing of the skin are essential to preventing site problems.
Benefits of insulin pump therapy include the opportunity to individualize therapy for specific medical needs and lifestyle differences. Basal profiles can be individually adjusted for metabolic concerns, such as the dawn phenomenon or temporary illness—as well as day-to-day changes, such as increased physical activity. Bolus calculators allow for more accurate matching of the insulin-to-carbohydrate ratio and the amount of correction insulin necessary before meals.
CONCLUSION
CSII pumps may offer significant benefits to diabetic patients, compared with their current MDI regimen. These include reduction in A1C without increases in hypoglycemia, greater flexibility, improved quality of life—and, especially when used in conjunction with continuous glucose monitoring, reduction in glycemic variability. Patients and providers must bear in mind, however, that the pump is a tool, not a substitute for what guarantees success in diabetes management—namely, attention to meal planning, carbohydrate-counting, frequent blood sugar monitoring, competent sick day management, regular exercise, and target blood sugar and A1C goals. The patient most likely to benefit from insulin pump use is one with the motivation to make the lifestyle changes that effective therapy requires.
REFERENCES
1. General Hospital and Personal Use Medical Devices Panel Meeting—March 5, 2010. www .fda.gov/downloads/AdvisoryCommittees/Com mitteesMeetingMaterials/MedicalDevices/
MedicalDevicesAdvisoryCommittee/General HospitalandPersonalUseDevicesPanel/
UCM203749.pdf. Accessed October 21, 2011.
2. Fabiato K, Buse J, Duclos M, et al. Clinical experience with continuous glucose monitoring in adults. Diabetes Technol Ther. 2009;11 suppl 1:S93-S103.
3. Battelino T, Phillip M, Bratina N, et al. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011; 34(4):795-800
4. Edelman SV, Bode BW, Bailey TS, et al. Insulin pump therapy in patients with type 2 diabetes safely improved glycemic control using a simple insulin dosing regimen. Diabetes Technol Ther. 2010;12(8):627-633.
5. Skladany MJ, Miller M, Guthermann JS, Ludwig CR. Patch-pump technology to manage type 2 diabetes mellitus: hurdles to market acceptance. J Diabetes Sci Technol. 2008;2(6): 1147-1150.
6. Lynch PM, Riedel AA, Samant N, et al. Resource utilization with insulin pump therapy for type 2 diabetes mellitus. Am J Manag Care. 2010;16(12):892-896.
7. Nielsen S, Kain D, Szudzik E, et al. Use of continuous subcutaneous insulin infusion pump in patients with type 2 diabetes mellitus. Diabetes Educ. 2005;31(6):843-848.
8. Scheiner G, Sobel RJ, Smith DE, et al. Insulin pump therapy: guidelines for successful outcomes. Diabetes Educ. 2009;35 suppl 2:29S-41S.
9. Unger J. Insulin pump therapy. In: Unger J. Diabetes Management in Primary Care. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:265-320.
10. Hagedorn HC. Modifications of insulin. Physician’s Bull. 1947;12(5):26-33.
11. Salemyr J, Bang P, Örtqvist E. Lower HbA1c after 1 year, in children with type 1 diabetes treated with insulin glargine vs. NPH insulin from diagnosis: a retrospective study. Pediatr Diabetes. 2011;12(5):501-505.
12. Lauritzen T, Pramming S, Deckert T, Binder C. Pharmacokinetics of continuous subcutaneous insulin infusion. Diabetologia. 1983;24(5): 326-329.
13. Binder C, Lauritzen T, Faber O, Pramming S. Insulin pharmacokinetics. Diabetes Care. 1984; 7(2):188-199.
14. Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c–independent risk factor for diabetic complications. JAMA. 2006;295(14): 1707-1708.
15. American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31 suppl 1:S12-S54.
16. Handelsman Y, Mechanick JI, Blonde L, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan. Endocrine Pract. 2011;17(suppl 2):1-53.
17. Kaufman F. Insulin treatment. In: Kaufman F, ed. Medical Management of Type 1 Diabetes. 5th ed. Alexandria, VA: American Diabetes Association; 2008:57-82.
18. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352(2):174-183.
19. Cochran E, Musso C, Gorden P. The use of U-500 in patients with extreme insulin resistance. Diabetes Care. 2005;28(5):1240-1244.
20. Diabetes Research in Children Network (DirecNet) Study Group. Prevention of hypoglycemia during exercise in children with type 1 diabetes by suspending basal insulin. Diabetes Care. 2006;29(10):2200-2204.
21. Maran A, Pavan P, Bonsembiante B, et al. Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high-intensity exercise in nontrained patients with type 1 diabetes. Diabetes Technol Ther. 2010; 12(10):763-768.
22. Davidson PC, Hebblewhite HR, Steed RD, Bode BW. Analysis of guidelines for basal-bolus insulin dosing: basal insulin, correction factor, and carbohydrate-to-insulin ratio. Endocr Pract. 2008;14(9):1095-1101.
23. Glycemic Index Foundation, Sydney University Glycemic Index Research Service (SUGiRS). GI testing and research. www.glycemicindex
.com. Accessed October 21, 2011.
24. Pankowska E, Błazik M. Bolus calculator with nutrition database software, a new concept of prandial insulin programming for pump users.
J Diabetes Sci Technol. 2010;4(3):571-576.
25. Davis SN, Horton ES, Battelino T, et al. STAR 3 randomized controlled trial to compare sensor-augmented insulin pump therapy with multiple daily injections in the treatment of type 1 diabetes: research design, methods, and baseline characteristics of enrolled subjects. Diabetes Technol Ther. 2010;12(4):249-255.
26. Buse JB, Dailey G, Ahmann AA, et al. Baseline predictors of A1C reduction in adults using sensor-augmented pump therapy or multiple daily injection therapy: the STAR 3 experience. Diabetes Technol Ther. 2011;13(6):601-606.
27. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Sensor-Augmented Pump Therapy for A1C Reduction (STAR 3) Study: results from the 6-month continuation phase. Diabetes Care. 2011 Sep 20. Epub ahead of print.
28. Green JB, Lane WS, Bergenstal RM, et al. Insulin Pump Settings Associated with Degree of Glycemic Improvement in Adults with Type 1 Diabetes Using Sensor-Augmented Pump Therapy. Presented at: Diabetes Technology Meeting; November 11-13, 2010; Bethesda, MD. Abstract A51.
29. Buse JB, Kidva YC, Guthrie RA, et al. Assessment of glycemic variability and CD40 ligand in the STAR 3 Study. Presented at: American Diabetes Association Scientific Sessions; June 2, 2011; San Diego, CA. Abstract 0923-P.
30. Hirsch IB. Algorithms for care in adults using continuous glucose monitoring. J Diabetes Sci Technol. 2007;1(1):126-129.
31. Wolpert HA. Continuous glucose monitoring: coming of age (editorial). N Engl J Med. 2010;363(4):383-384.
32. Wiegand S, Raile K, Reinehr T, et al; DPV-Wiss Study Group. Daily insulin requirement of children and adolescents with type 1 diabetes: effect of age, body mass index and mode of therapy. Eur J Endocrinol. 2008;158(4):543-549.
33. Hanas R, Ludvigsson J. Hypoglycemia and ketoacidosis with insulin pump therapy in children and adolescents. Pediatr Diabetes. 2006;7 suppl 4:32-38.
34. Unger J. A primary care approach to continuous subcutaneous insulin infusion. Clin Diabetes. 1999;17(3). http://journal.diabetes.org/clinicaldiabetes/V17N31999/Pg113.htm. Accessed October 21, 2011.
The FDA has reported that in 2007, 375,000 Americans with diabetes were using an insulin pump.1 Improvements in pump technology (including the addition of continuous glucose monitoring2,3), more data on the use of pumps in type 2 diabetes,4-7 improved insurance coverage,8 and the general population’s comfort with technology-based solutions may lead more patients with diabetes to choose a pump to deliver their insulin.
As a result, primary care practitioners will be increasingly likely to have patients who use continuous subcutaneous insulin infusion (CSII) pumps. While the initiation of pump therapy and follow-up with patients who use pumps have traditionally been the purview of endocrinologists, it is important for primary care providers to understand the basics of insulin pump therapy, enabling them to work collaboratively with the patient and the diabetes care team.
REVIEW OF INSULIN ACTION IN THE BODY
The body’s own (endogenous) insulin is secreted by the beta cells of the pancreas. Basal insulin is the background amount of insulin continuously released by the body in order to regulate hepatic glucose production and lipolysis. In response to food intake, the body releases additional insulin to match the glycemic effect of carbohydrates, fat, and protein.9
Insulin regimens attempt to mimic the body’s physiologic release of insulin by administering long-acting insulin to provide basal coverage, and rapid- or short-acting insulin to metabolize meals. The first long-acting insulin developed was NPH (still in use today), which was created by the addition of neutral protamine Hagedorn to regular insulin to delay insulin absorption.10
There are a few reasons that NPH use has fallen out of favor, especially for patients with type 1 diabetes, who do not produce endogenous insulin. One is a pronounced peak in action that requires patients to time their consumption of meals and snacks around peaks. If food consumption is insufficient, hypoglycemia may occur. Additionally, after the peak follows a valley in which low levels of exogenous insulin can lead to hyperglycemia.11
The second problem with NPH surrounds the inconsistency in both intersubject and intrasubject absorption rates. Absorption variability as great as 50% has been reported.12,13 This, of course, may lead to an increase in glycemic variability, that is, the magnitude of glucose fluctuation14 within a day. (Note: It has been hypothesized that, in conjunction with the A1C measure, glycemic variability may account in part for the long-term complications of diabetes.14)
Insulin Analogs
Radical advances in the potential for improved glucose control emerged with the development of insulin analogs.15,16 On the market now are three rapid-acting insulin analogs: lispro, aspart, and glulisine. Long-acting analogs are glargine and detemir, either of which may be given once or twice a day.17 Clinically, detemir is generally given twice per day in most patients with type 1 diabetes due to its shorter duration of action.
Most patients with type 1 diabetes are now managed with a once- or twice-daily injection of a long-acting insulin analog and one short-acting analog before each meal; thus, patients must take four or more insulin injections each day. While this multiple daily injection (MDI) insulin regimen approximates the body’s endogenous production of insulin more closely than the traditional split/mix regimens of NPH and regular insulin, there are still shortcomings. Once the long-acting insulin has been injected, it provides a steady level of insulin that cannot be adjusted for changes in the patient’s routine, such as exercise, delayed meals, or sleep, or for changes in the metabolic milieu, such as infection or other illness.18
PUMP BASICS
The CSII pump is a programmable insulin delivery system that provides continuous amounts of insulin and allows the user to deliver extra insulin to metabolize meals or to correct high blood glucose levels. Typically, rapid-acting insulin is used in the pump, although for patients with severe insulin resistance who need high doses of insulin, U-500 regular insulin may be used.19 U-500 regular insulin is a concentrated insulin dosed at one-fifth of U-100 regular insulin; that is, 100 units of regular insulin would equate to 20 units of U-500 insulin. (To clarify further, “U-100” refers to 100 U/mL, and “U-500” means 500 U/mL.) Understanding the indications for U-500 insulin and its adjustment is a specialized skill of the diabetologist experienced in its use.
A traditional pump consists of a beeper-sized device. It contains a reservoir that the user fills with insulin, then connects to an infusion set. The infusion set consists of tubing connected to a flexible catheter or needle that is inserted into the subcutaneous tissue. A mechanism to attach and detach the tubing from the infusion site is included so that users can disconnect it for bathing, sexual activity, contact sports, or other circumstances. Infusion sets are available in different sizes and styles to accommodate patients with different body weights, body fat levels, and lifestyle needs.
The “patch” or “wireless” pump, as it is sometimes called, is a self-contained unit or “pod” that resides directly on the skin, delivers insulin through a flexible catheter, and is disposed of or recycled every two to three days; it does not require tubing.
Potential pump users have a variety of manufacturers and models from which to choose, all providing similar features. Considerations for selecting a pump should include its particular features, cost of supplies, insurance coverage, customer service and support, and personal preference.
Basal and Bolus Insulin
The continuous background delivery in the pump is known as the basal; the rate at which the pump delivers the basal can be changed at different times of day to allow for changes in the patient’s routine and metabolic milieu (see Figure 1). Basal rates can be set anywhere between 0.025 and 35 units per hour. Most pumps allow for basal rates to last for as little as 30 minutes, but pump users commonly set one to four basal rates over 24 hours.
Some diabetic patients experience the dawn phenomenon, that is, fasting hyperglycemia resulting from the body’s release of growth hormone, cortisol, and adrenaline. These hormones, which produce a state of insulin resistance, are released beginning at about 2:00 to 3:00 am. A patient who is taking 24 units per day of total basal insulin might set a basal rate of one unit per hour; adjustments can be made if the patient needs less insulin at night and is prone to the dawn phenomenon.
All pumps begin basal rate programming at midnight. A basal rate profile for a patient who is prone to the dawn phenomenon and who needs 24 units/d of basal, goes to bed at 10:00 pm, and wakes up at 6:00 am, might look like this:
12:00 am: 0.8 units/h
3:00 am: 1.2 units/h
9:00 am: 1.0 units/h
10:00 pm: 0.8 units/h
Temporary basal rates may be set lower to account for exercise, or higher during times of illness. The pumper selects the amount released, the rate, and the length of time the temporary rate should last. After that selected time period is over, the pump automatically reverts to the regular basal rate. Setting lower basal rates before, during, or after exercise may benefit a patient who experiences exercise-related hypoglycemia. A patient who needs to adjust for exercise might select a temporary basal rate set to begin one hour before exercise and continue during exercise and for one hour after exercise.20
Even with these adjustments, however, low blood sugars may still occur, and delayed hypoglycemia may develop hours after exercise is completed.21 Muscle and liver tissue store excess sugar in the form of glycogen; glycogen stores are depleted during exercise, and blood sugar may decline when the muscle and liver take up blood sugar to replace the stores. In such instances, an extra carbohydrate snack and/or use of the temporary basal may prevent hypoglycemia.20
Setting temporary basal rates may also prevent hyperglycemia. In the absence of sufficient insulin, patients with type 1 diabetes produce ketones. Setting higher basal rates during illness, combined with frequent monitoring of blood sugars and ketones in the blood or urine, can prevent diabetic ketoacidosis (DKA). It is important for patients to understand that illness may increase their metabolic need for insulin.9 Even if the appetite decreases during illness, extra insulin may be needed. Frequent blood glucose monitoring and testing for ketones, when necessary, are essential for the patient with type 1 diabetes.
The supplemental insulin that patients take to compensate for carbohydrate intake and to correct high blood glucose is called the bolus. More recent pump technology, sometimes called the “smart” pump, includes features that will calculate for the patient how much of a bolus should be taken, based on three factors: carbohydrates ingested, current blood glucose level, and active insulin, that is, the amount of insulin that is still considered active after the patient takes a bolus based on the pharmacodynamic profile of the insulin.15,22
Carbohydrate-Counting
By the time a patient is started on an insulin pump, he or she should be proficient in carbohydrate-counting.8 There are several books on the market that list carbohydrate content of foods, including popular restaurant menu items and products sold in supermarkets. There are also apps available for PDAs and smart phones. The American Diabetes Association Web site (www.diabetes.org) offers carbohydrate-counting and menu-planning tools. For patients who eat out, it may be helpful to look up the restaurant’s menu online in advance and if possible, research the nutrition facts associated with each course selection. Even for patients with good carbohydrate-counting skills, it is often a challenge to get blood sugars into a desirable postprandial range.
The glycemic index is an important consideration. This 0-to-100–point scale is used to indicate how quickly specific foods raise blood sugar. Foods higher on the index (such as white potatoes and fruit juice) raise blood sugar more quickly than do foods that are lower on the index (whole grains, milk, nuts, and seeds).23 The caveat to using the glycemic index is that foods are often consumed as part of a mixed meal of proteins, fats, and carbohydrates—each of which will have a different effect on how rapidly blood sugars rise. Since fat slows digestion, meals that are high in both fats and carbohydrates can cause extended elevations in blood glucose.
To address this concern, smart pumps are equipped with an extended bolus feature that allows delivery of the bolus over a preset period of time.24 A percentage of the bolus may be delivered immediately and the remainder over time (dual-wave bolus). A typical split may be 50% now and 50% over the next two hours. This takes into account not only carbohydrate intake, but the components of a meal high in carbs and fat, such as pizza.
Several factors can impact the accuracy with which the patient calculates boluses to lower blood sugars for meals and other times of elevated blood glucose. Optimally, the total amount of correction insulin should represent only a small portion of the total daily bolus amount if the patient is being proactive. Ask patients about their carbohydrate-counting. Are they counting at a high skill level? Is their insulin-to-carbohydrate ratio correct? What about their inputs into their bolus calculator? Are their targets set too high or too low? Is their sensitivity correct? Is their active insulin time correct?
To assess postprandial control, patients should be instructed to check their blood glucose two hours after they started to ingest each meal.15 Consistent elevations mean that an adjustment to the insulin-to-carbohydrate ratio is indicated; alternatively, the patient may choose to consume fewer carbohydrates if weight is a concern.
If patients are not consistently measuring their foods (a discipline that is important but challenging to maintain), portion creep may be occurring. Encourage the patient to start measuring food again, at least for a while. A refresher visit to the nutritionist or diabetes educator may also be helpful. If patients are eating out often, they should be encouraged to limit themselves to meals with a known carbohydrate content.
Response to various foods is often idiosyncratic. Having patients keep a detailed log of foods eaten, exercise performed, and corresponding blood glucose levels will often clear up mysterious blood sugar elevations.
Correction Insulin
Depending on their personal approach and regimen, patients are taught to monitor their glucose either prior to eating, after eating, or both; and to take insulin to achieve a correction if the glucose is outside their target zone. A typical preprandial target range is 80 to 120 mg/dL, with 140 to 180 mg/dL two hours postprandial. (The American Association of Clinical Endocrinologists, while emphasizing the importance of individualizing glucose targets, suggests a fasting plasma glucose level below 110 mg/dL and a two-hour postprandial concentration below 140 mg/dL to achieve a target A1C level ≤ 6.5% in the nonpregnant adult.16 The American Diabetes Association’s comparable recommendations are 70 to 130 mg/dL preprandial and 180 mg/dL peak postprandial.15)
In today’s pumps, as part of the bolus calculation, a number can be programmed that represents the number of points in mg/dL by which one unit of insulin will reduce blood sugars. This may be known as a sensitivity or correction factor.
For example, a pumper who is making a postprandial correction and is not eating has a target blood sugar between 140 and 180 mg/dL and a sensitivity of 40; at present, his blood sugar is 230 mg/dL. Assuming no active insulin, the pump would calculate a dose of about 1.25 units of rapid-acting insulin.
How active insulin, or insulin on board, is calculated differs among pump manufacturers but is loosely based on original insulin pharmacodynamic studies.15,22 In smart pumps, the bolus calculator takes into consideration how much insulin is still active when it recommends a correction dose for hyperglycemia. This allows the user to address hyperglycemia quickly and effectively but prevents “stacking” of insulin doses and subsequent hypoglycemia.18
The active insulin time is preset in some pumps, but many diabetes clinicians use three or four hours (anecdotal evidence). The pumper can test the validity of active insulin by administering a correction bolus when blood glucose exceeds 250 mg/dL, then checking blood glucose levels every 30 to 60 minutes for six hours with no further food intake.9 The length of time it takes for blood sugars to return to target and remain steady is determined to be the correct duration of insulin action. In the example above, if the pump determined that there was active insulin remaining from the prior meal, it would subtract that amount from the calculation to prevent insulin stacking—which would most likely lead to overcorrection and hypoglycemia.
PUMP OPTIONS AND GLUCOSE MONITORING
In addition to using wireless communication, current “disposable” (“patch”) pumps operate without the conventional infusion set and tubing. Some smart pumps are now equipped with a combination device: a diabetes-specific, PDA-like apparatus with an integrated glucose monitor that links to the pump through infrared technology (see Figure 2). Another pump has a “linked” glucose monitor (see Figure 3) that allows the user to test blood sugar, with the reading downloaded to the pump. The pump (or PDA) calculates the needed dose, based on programmed insulin-to-carbohydrate ratios and correction factors.22
A bolus calculator on the PDA helps the user calculate the bolus dose to be delivered to the pump without the user’s having to remove the pump to administer a dose. The pumper can discreetly administer insulin, even when the patient is eating out.
The one patch pump that is currently on the market requires the user to set the PDA to bolus, since the pod has no buttons with which to input instructions for a bolus. Use of the PDA or linked meter requires the pumper to use a particular brand of test strips. If insurance does not cover that brand, the patient can use another manufacturer’s monitor and enter blood sugar readings manually.
Potential Pump Problems
Patients who are considering any CSII pump must be willing to check blood glucose levels frequently. Malfunction of insulin pumps (including a blockage or pump failure) is associated with an increased risk for DKA,8 because the pump delivers only short-acting insulin. Without the presence of long-acting insulin as a backup, DKA can develop rapidly. All pumps have pressure-sensing alarms to detect blockages; in patients who receive small doses of insulin, however, it may take time for the alarm to be triggered. Thus, the importance of frequent blood glucose monitoring is evident. (Additionally, in case of pump failure, patients should know their basal rates and insulin-to-carbohydrate ratios or keep them written down.)
Pumpers are taught that if they experience two or more unexplained high glucose readings in a row to troubleshoot the infusion set for air bubbles or a clog. They should also take an injection of insulin to correct the high glucose and change the reservoir and infusion set.
Continuous Glucose Monitor
Some patients use continuous glucose monitoring (CGM) with a sensor that measures glucose levels in the interstitial fluid. At the time of this writing, one currently available pump is equipped with an integrated glucose sensor (see Figure 3). This device monitors interstitial fluid glucose levels continuously and can provide trend data, letting the user know whether blood sugars are rising or falling and how rapidly.25
Recently, researchers for the Sensor-Augmented Pump Therapy for A1C Reduction (STAR 3) study26,27 reported that use of sensor-augmented insulin pumps (SAP) reduces A1C without increasing hypoglycemia, compared with MDI insulin regimens. Additional markers of success in SAP use, compared with an MDI regimen, are sensor glucose values that are closer to target, bolus-calculator interactions, amount of sensor use, and lower glycemic variability—especially in patients who achieve lower A1C.28,29
Even when the patient is using a glucose sensor, however, blood sugars must still be tested at least twice a day to calibrate the sensor. There is a common misperception that CGM serves as a substitute for blood glucose (finger-stick) monitoring, but there is a lag time between interstitial fluid glucose levels and blood glucose levels.30 Blood glucose monitoring by finger-stick is still considered the gold standard for measuring glucose; sensor manufacturers recommend that patients not take a treatment action based on sensor data without confirming first with a finger-stick.
Barriers to effective glucose sensor use include the high cost of sensors and variability in insurance coverage, user factors (patients’ not using sensors daily, calibrating them inaccurately), insertion-site infections, and technology issues, like sensor failure.27,31
Memory Features
Pump technology makes it possible to review bolus history (ie, the amount of bolus and the time at which it was given) as well as the dosage of insulin taken throughout the day. Based on these data, several assessments can be made:
• Is the basal-to-bolus ratio appropriate? (It should be about 40% to 50% basal / 50% to 60% bolus.)8
• Is the amount of insulin adequate? Adults and prepubertal children with type 1 diabetes usually require 0.6 to 0.9 units/kg/d.15 During puberty, insulin requirements can rise to 1.5 units/kg/d32 due to increased insulin resistance, surges in pubertal hormones, and greater caloric intake during growth spurts. Pump manufacturers offer data management software that allows the user to produce graphs, track trends, and monitor basal-to-bolus ratios.
• Is the patient actually bolusing for meals? Does the patient check blood glucose postprandially and bolus when the reading is high?
PATIENT TRAINING BEFORE PUMP INITIATION
Insurance coverage is generally favorable for pumps and associated supplies, since their cost benefit (ie, decreases in use of antidiabetic drugs and of health care resources6) is well proven for patients with type 1 diabetes. For those with type 2 diabetes, obtaining coverage is more challenging5 but can be supported if the patient has a history of severe hypoglycemia, insulin resistance, or uncontrolled blood sugar levels.6,7
Pump manufacturers employ insurance specialists to work with patients and providers in order to facilitate the insurance reimbursement process. Payment plans are available for what insurance does not cover, and pump warranties last for several years. Many insurance plans do not cover an automatic upgrade to a newer pump when the warranty expires, but the patient may be eligible for a discount on a newer pump.
Before initiating pump use, patients receive instruction from certified pump trainers—generally health professionals, such as nurses and registered dietitians, who may be employees of the endocrine practice or of the pump manufacturing company, or independent contractors; many are certified diabetes educators (CDEs). Training may be conducted in the patient’s home or in the medical office. Typically, patients are given some sort of DVD or CD-ROM and workbook to complete before pump training takes place.
Pump trainers make sure patients understand the basics of carbohydrate-counting, sick day management, and treatment of hypoglycemia and hyperglycemia. Training may comprise two sessions, one during which the patient can wear the pump with a saline administration and practice with it for a few days before starting on insulin at the second session. Training should be individualized to allow for different speeds and styles of learning.
Careful patient selection is the key to successful pump use. Optimally, patients should be motivated to improve their blood glucose control, compliant with their current regimen, and proficient in their diabetes self-management skills, including carbohydrate-counting, troubleshooting, and making adjustments for day-to-day variability in their circumstances.8
Pump trainers can make suggestions to the patient’s diabetologist regarding basal rates, correction factors, and insulin-to-carbohydrate ratios, but a health care provider’s order is necessary to initiate use of a pump.
RESOURCES FOR PATIENTS
Referral to a CDE and/or an endocrinology specialist may be helpful for any patient who was started on a pump in the past but is now struggling with management or would like to fine-tune his or her control. Patients who are unable to reduce an A1C of 8% may also benefit from referral. Not all of these practitioners operate at an expert level in pump therapy, however.
Given the shortage of endocrinologists and CDEs, especially in rural areas, finding a qualified practitioner is sometimes challenging. The American Association of Diabetes Educators maintains a referral service at www.diabeteseduca tor.org/find or (800) TeamUp4.
Patients can also contact the pump manufacturer to ask about local resources. Classes in advanced pumping skills and local pump support groups are often sponsored by pump manufacturers. Additionally, excellent books have been published on pump therapy and carbohydrate-counting, including those listed below.
RISKS AND BENEFITS OF INSULIN PUMP THERAPY
Risks of insulin pump therapy include DKA, infusion-site problems, and infection leading to abscess.27,33,34
In the absence of insulin, patients with type 1 diabetes produce ketones; because a pump uses only rapid- or short-acting insulin, a failure to deliver that insulin can lead to acute insulin deficiency, resulting in DKA. All pumps are equipped with pressure-sensitive alarms that will alert the user to a blockage that may result in failure to deliver insulin. Pump users who receive low doses of insulin (the absence of which may not cause sufficient pressure to trigger the alarm) or who experience pump blockages that cause only partial delivery may experience alarm failure. It is crucial for patients to monitor their blood sugar frequently; those who are unwilling to do so are not good candidates for insulin pump therapy.8
Infusion-site problems can include skin reactions to tape or infections resulting from inadequate cleansing or from failure to cleanse the site and change the infusion set, as recommended by the manufacturer, every two to three days. Pump trainers or the pump manufacturer’s customer service team can offer solutions to infusion-site skin care problems. Scrupulous handwashing, timely site changes, and cleansing of the skin are essential to preventing site problems.
Benefits of insulin pump therapy include the opportunity to individualize therapy for specific medical needs and lifestyle differences. Basal profiles can be individually adjusted for metabolic concerns, such as the dawn phenomenon or temporary illness—as well as day-to-day changes, such as increased physical activity. Bolus calculators allow for more accurate matching of the insulin-to-carbohydrate ratio and the amount of correction insulin necessary before meals.
CONCLUSION
CSII pumps may offer significant benefits to diabetic patients, compared with their current MDI regimen. These include reduction in A1C without increases in hypoglycemia, greater flexibility, improved quality of life—and, especially when used in conjunction with continuous glucose monitoring, reduction in glycemic variability. Patients and providers must bear in mind, however, that the pump is a tool, not a substitute for what guarantees success in diabetes management—namely, attention to meal planning, carbohydrate-counting, frequent blood sugar monitoring, competent sick day management, regular exercise, and target blood sugar and A1C goals. The patient most likely to benefit from insulin pump use is one with the motivation to make the lifestyle changes that effective therapy requires.
REFERENCES
1. General Hospital and Personal Use Medical Devices Panel Meeting—March 5, 2010. www .fda.gov/downloads/AdvisoryCommittees/Com mitteesMeetingMaterials/MedicalDevices/
MedicalDevicesAdvisoryCommittee/General HospitalandPersonalUseDevicesPanel/
UCM203749.pdf. Accessed October 21, 2011.
2. Fabiato K, Buse J, Duclos M, et al. Clinical experience with continuous glucose monitoring in adults. Diabetes Technol Ther. 2009;11 suppl 1:S93-S103.
3. Battelino T, Phillip M, Bratina N, et al. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011; 34(4):795-800
4. Edelman SV, Bode BW, Bailey TS, et al. Insulin pump therapy in patients with type 2 diabetes safely improved glycemic control using a simple insulin dosing regimen. Diabetes Technol Ther. 2010;12(8):627-633.
5. Skladany MJ, Miller M, Guthermann JS, Ludwig CR. Patch-pump technology to manage type 2 diabetes mellitus: hurdles to market acceptance. J Diabetes Sci Technol. 2008;2(6): 1147-1150.
6. Lynch PM, Riedel AA, Samant N, et al. Resource utilization with insulin pump therapy for type 2 diabetes mellitus. Am J Manag Care. 2010;16(12):892-896.
7. Nielsen S, Kain D, Szudzik E, et al. Use of continuous subcutaneous insulin infusion pump in patients with type 2 diabetes mellitus. Diabetes Educ. 2005;31(6):843-848.
8. Scheiner G, Sobel RJ, Smith DE, et al. Insulin pump therapy: guidelines for successful outcomes. Diabetes Educ. 2009;35 suppl 2:29S-41S.
9. Unger J. Insulin pump therapy. In: Unger J. Diabetes Management in Primary Care. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:265-320.
10. Hagedorn HC. Modifications of insulin. Physician’s Bull. 1947;12(5):26-33.
11. Salemyr J, Bang P, Örtqvist E. Lower HbA1c after 1 year, in children with type 1 diabetes treated with insulin glargine vs. NPH insulin from diagnosis: a retrospective study. Pediatr Diabetes. 2011;12(5):501-505.
12. Lauritzen T, Pramming S, Deckert T, Binder C. Pharmacokinetics of continuous subcutaneous insulin infusion. Diabetologia. 1983;24(5): 326-329.
13. Binder C, Lauritzen T, Faber O, Pramming S. Insulin pharmacokinetics. Diabetes Care. 1984; 7(2):188-199.
14. Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c–independent risk factor for diabetic complications. JAMA. 2006;295(14): 1707-1708.
15. American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31 suppl 1:S12-S54.
16. Handelsman Y, Mechanick JI, Blonde L, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan. Endocrine Pract. 2011;17(suppl 2):1-53.
17. Kaufman F. Insulin treatment. In: Kaufman F, ed. Medical Management of Type 1 Diabetes. 5th ed. Alexandria, VA: American Diabetes Association; 2008:57-82.
18. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352(2):174-183.
19. Cochran E, Musso C, Gorden P. The use of U-500 in patients with extreme insulin resistance. Diabetes Care. 2005;28(5):1240-1244.
20. Diabetes Research in Children Network (DirecNet) Study Group. Prevention of hypoglycemia during exercise in children with type 1 diabetes by suspending basal insulin. Diabetes Care. 2006;29(10):2200-2204.
21. Maran A, Pavan P, Bonsembiante B, et al. Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high-intensity exercise in nontrained patients with type 1 diabetes. Diabetes Technol Ther. 2010; 12(10):763-768.
22. Davidson PC, Hebblewhite HR, Steed RD, Bode BW. Analysis of guidelines for basal-bolus insulin dosing: basal insulin, correction factor, and carbohydrate-to-insulin ratio. Endocr Pract. 2008;14(9):1095-1101.
23. Glycemic Index Foundation, Sydney University Glycemic Index Research Service (SUGiRS). GI testing and research. www.glycemicindex
.com. Accessed October 21, 2011.
24. Pankowska E, Błazik M. Bolus calculator with nutrition database software, a new concept of prandial insulin programming for pump users.
J Diabetes Sci Technol. 2010;4(3):571-576.
25. Davis SN, Horton ES, Battelino T, et al. STAR 3 randomized controlled trial to compare sensor-augmented insulin pump therapy with multiple daily injections in the treatment of type 1 diabetes: research design, methods, and baseline characteristics of enrolled subjects. Diabetes Technol Ther. 2010;12(4):249-255.
26. Buse JB, Dailey G, Ahmann AA, et al. Baseline predictors of A1C reduction in adults using sensor-augmented pump therapy or multiple daily injection therapy: the STAR 3 experience. Diabetes Technol Ther. 2011;13(6):601-606.
27. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Sensor-Augmented Pump Therapy for A1C Reduction (STAR 3) Study: results from the 6-month continuation phase. Diabetes Care. 2011 Sep 20. Epub ahead of print.
28. Green JB, Lane WS, Bergenstal RM, et al. Insulin Pump Settings Associated with Degree of Glycemic Improvement in Adults with Type 1 Diabetes Using Sensor-Augmented Pump Therapy. Presented at: Diabetes Technology Meeting; November 11-13, 2010; Bethesda, MD. Abstract A51.
29. Buse JB, Kidva YC, Guthrie RA, et al. Assessment of glycemic variability and CD40 ligand in the STAR 3 Study. Presented at: American Diabetes Association Scientific Sessions; June 2, 2011; San Diego, CA. Abstract 0923-P.
30. Hirsch IB. Algorithms for care in adults using continuous glucose monitoring. J Diabetes Sci Technol. 2007;1(1):126-129.
31. Wolpert HA. Continuous glucose monitoring: coming of age (editorial). N Engl J Med. 2010;363(4):383-384.
32. Wiegand S, Raile K, Reinehr T, et al; DPV-Wiss Study Group. Daily insulin requirement of children and adolescents with type 1 diabetes: effect of age, body mass index and mode of therapy. Eur J Endocrinol. 2008;158(4):543-549.
33. Hanas R, Ludvigsson J. Hypoglycemia and ketoacidosis with insulin pump therapy in children and adolescents. Pediatr Diabetes. 2006;7 suppl 4:32-38.
34. Unger J. A primary care approach to continuous subcutaneous insulin infusion. Clin Diabetes. 1999;17(3). http://journal.diabetes.org/clinicaldiabetes/V17N31999/Pg113.htm. Accessed October 21, 2011.
Self-Monitoring of Glucose in Diabetes
Despite therapeutic advances in diabetes management, the majority of patients with diabetes are unable to achieve glycemic targets proven to reduce the burden of the disease. This burden not only involves the quality of life of patients with diabetes who experience the complications of this disease; it also includes the burden to society. One out of every five health care dollars is spent on caring for someone with diabetes—the majority on treating the complications.1
Major barriers to patients’ ability to achieve glycemic goals include the need to make behavioral changes, lack of awareness of glycemic levels, and fear of hypoglycemia.2
Q: Is self-monitoring of blood glucose worthwhile in diabetes?
Studies have shown a benefit from self-monitoring of blood glucose (SMBG) in patients using insulin but not in those taking oral antidiabetic drugs. However, the American Diabetes Association recommends that patients with diabetes monitor their glucose once daily if they are being treated with noninsulin therapy and at least three times daily if they are taking insulin.3
Guidelines from the American Association of Clinical Endocrinologists (AACE) state that patients taking noninsulin or once-daily insulin therapy who have not achieved A1C targets should monitor at least twice daily, while those at target should monitor at least once daily. Those taking multiple daily injections should perform SMBG at least three times per day. If patients experience frequent hypoglycemia, AACE suggests monitoring glucose more often.4
The A1C test provides the “big picture,” the average daily glucose level during the previous 90 to 120 days, and correlates with end-organ impact. It does not identify glycemic variability, hypoglycemia, or hyperglycemia.
By contrast, SMBG patterns provide day-to-day data that can be used to select and manage glucose control programs and ultimately optimize a patient’s A1C. SMBG provides a measure of the specific pharmacologic impact of medications and, through feedback, allows design and implementation of physiologic insulin-replacement programs.
One example of SMBG is to have patients monitor glucose in pairs (ie, pick a meal each day and do a premeal and two-hour postmeal reading) and ask them to keep a log or download the data from their meter in the office. This type of monitoring can be enlightening and self-empowering for the patient in that it can provide valuable information regarding the glycemic response to the particular meal.
Intensive glycemic management has been shown to reduce the incidence and progression of diabetic complications. However, it is associated with an increase in severe hypoglycemia. This is worrisome for both patients and providers, as severe hypoglycemia has been associated with an increase in risk for mortality. SMBG can assist patients in understanding how their lifestyle affects their diabetes, as well as identifying hypoglycemia for those who may have hypoglycemia unawareness (ie, who lack the relevant symptoms).
Q: What is continuous glucose monitoring (CGM)?
CGM devices give real-time readouts of current glucose levels. They utilize a subcutaneous sensor that is inserted in the abdomen and worn for 3 to 7 days (depending on which device is used). The sensor sends an electronic signal to a receiver worn by the patient.
There are three major CGM devices that have been approved by the FDA and are available for both personal and professional use. Health care providers can purchase the units and have patients wear them for retrospective analysis; this is a reimbursable expense. All available CGM devices measure glucose values in the interstitial fluid. The sensor reads electrical current produced by the same glucose-oxidase reaction that is utilized by glucose meters that patients use to perform fingersticks for home monitoring.
Currently available CGM systems need to be calibrated at least twice daily. Sensor calibration entails the pairing of the fingerstick value with the sensor value from the interstitial space. Calibration confirms sensor accuracy during various points by “teaching” the sensor the glucose value that corresponds with the electrical current signal.
There is a known physiologic lag time that occurs between fingerstick and sensor values. This lag time is typically up to 15 minutes but is increased with rapidly changing glucose values.
Q: What are the benefits of CGM?
Recent studies have shown CGM can improve A1C without increasing the incidence of hypoglycemia.5
CGM systems have both low and high glucose threshold alarms that can be set to alert once the threshold is reached. The newest generation devices can also predict hypoglycemia or hyperglycemia by tracking rate of change, and users can be alerted to a potential event. This would then allow them to take appropriate action, such as consuming food or carbohydrates or taking insulin as necessary. (Before taking any action, the glucose should first be confirmed by SMBG.)
Software programs allow for review of glucose data, which can assist in identifying trends not appreciated by typical SMBG testing (such as nocturnal hypoglycemia and meal-time excursions). This allows for adjustment of insulin regimens to reduce the incidence of these events.
Q: Can CGM replace SMBG?
While CGM can provide much more detail regarding glucose trends and patterns, it is not a replacement for SMBG. CGM should not be used as a replacement for SMBG to dose insulin for meal- or activity-related adjustments. All dosing decisions should be based on the SMBG.
Currently, CGM is indicated for patients 18 or older, in conjunction with SMBG for the purpose of improving glycemic control:
• to identify and aid in management of glycemic patterns not recognized with typical SMBG
• to prevent glycemic excursions of hypoglycemia and hyperglycemia.
Its use is supported by ADA and AACE guidelines for glucose monitoring.
Q: Who would benefit from CGM?
Suitable candidates for CGM include those with a high degree of glycemic variability, those with hypoglycemic unawareness, shift workers, patients who use insulin pumps, athletes, and women who are planning to become or are pregnant. Patients should work closely with their health care team and perform regular SMBG.
It has been suggested that patients need comprehensive training and follow-up visits to fully understand the large amount of data that they can be confronted with, in order to fully benefit from these devices.6 While the accuracy is improving, there are a few limitations to this technology, including false alarms. Studies have also shown a positive correlation between sensor wear time (hours per week) and greater reductions in A1C.5
Conclusion
Glucose monitoring is a necessary tool—for patients as well as providers—that assists in identifying how patients’ lifestyles affect their diabetes.
1. American Diabetes Association. Economic costs of diabetes in the US in 2007. Diabetes Care. 2008;31(3):596-615.
2. Hirsch IB, Armstrong D, Bergenstal RM, et al. Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM). Diabetes Technol Ther. 2008;10(4):232-246.
3. American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2010;34(suppl 1):S11-S61.
4. American Association of Clinical Endocrinologists. Medical guidelines for clinical practice for the management of diabetes mellitus. Endocrin Prac. 2007;13(suppl 1):1-68.
5. Bergenstal RM, Tamberlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320.
6. Fabiato K, Buse J, Duclos M, et al. Clinical experience with continuous glucose monitoring in adults. Diabetes Technol Ther. 2009;11(suppl 1):S93-S103.
Despite therapeutic advances in diabetes management, the majority of patients with diabetes are unable to achieve glycemic targets proven to reduce the burden of the disease. This burden not only involves the quality of life of patients with diabetes who experience the complications of this disease; it also includes the burden to society. One out of every five health care dollars is spent on caring for someone with diabetes—the majority on treating the complications.1
Major barriers to patients’ ability to achieve glycemic goals include the need to make behavioral changes, lack of awareness of glycemic levels, and fear of hypoglycemia.2
Q: Is self-monitoring of blood glucose worthwhile in diabetes?
Studies have shown a benefit from self-monitoring of blood glucose (SMBG) in patients using insulin but not in those taking oral antidiabetic drugs. However, the American Diabetes Association recommends that patients with diabetes monitor their glucose once daily if they are being treated with noninsulin therapy and at least three times daily if they are taking insulin.3
Guidelines from the American Association of Clinical Endocrinologists (AACE) state that patients taking noninsulin or once-daily insulin therapy who have not achieved A1C targets should monitor at least twice daily, while those at target should monitor at least once daily. Those taking multiple daily injections should perform SMBG at least three times per day. If patients experience frequent hypoglycemia, AACE suggests monitoring glucose more often.4
The A1C test provides the “big picture,” the average daily glucose level during the previous 90 to 120 days, and correlates with end-organ impact. It does not identify glycemic variability, hypoglycemia, or hyperglycemia.
By contrast, SMBG patterns provide day-to-day data that can be used to select and manage glucose control programs and ultimately optimize a patient’s A1C. SMBG provides a measure of the specific pharmacologic impact of medications and, through feedback, allows design and implementation of physiologic insulin-replacement programs.
One example of SMBG is to have patients monitor glucose in pairs (ie, pick a meal each day and do a premeal and two-hour postmeal reading) and ask them to keep a log or download the data from their meter in the office. This type of monitoring can be enlightening and self-empowering for the patient in that it can provide valuable information regarding the glycemic response to the particular meal.
Intensive glycemic management has been shown to reduce the incidence and progression of diabetic complications. However, it is associated with an increase in severe hypoglycemia. This is worrisome for both patients and providers, as severe hypoglycemia has been associated with an increase in risk for mortality. SMBG can assist patients in understanding how their lifestyle affects their diabetes, as well as identifying hypoglycemia for those who may have hypoglycemia unawareness (ie, who lack the relevant symptoms).
Q: What is continuous glucose monitoring (CGM)?
CGM devices give real-time readouts of current glucose levels. They utilize a subcutaneous sensor that is inserted in the abdomen and worn for 3 to 7 days (depending on which device is used). The sensor sends an electronic signal to a receiver worn by the patient.
There are three major CGM devices that have been approved by the FDA and are available for both personal and professional use. Health care providers can purchase the units and have patients wear them for retrospective analysis; this is a reimbursable expense. All available CGM devices measure glucose values in the interstitial fluid. The sensor reads electrical current produced by the same glucose-oxidase reaction that is utilized by glucose meters that patients use to perform fingersticks for home monitoring.
Currently available CGM systems need to be calibrated at least twice daily. Sensor calibration entails the pairing of the fingerstick value with the sensor value from the interstitial space. Calibration confirms sensor accuracy during various points by “teaching” the sensor the glucose value that corresponds with the electrical current signal.
There is a known physiologic lag time that occurs between fingerstick and sensor values. This lag time is typically up to 15 minutes but is increased with rapidly changing glucose values.
Q: What are the benefits of CGM?
Recent studies have shown CGM can improve A1C without increasing the incidence of hypoglycemia.5
CGM systems have both low and high glucose threshold alarms that can be set to alert once the threshold is reached. The newest generation devices can also predict hypoglycemia or hyperglycemia by tracking rate of change, and users can be alerted to a potential event. This would then allow them to take appropriate action, such as consuming food or carbohydrates or taking insulin as necessary. (Before taking any action, the glucose should first be confirmed by SMBG.)
Software programs allow for review of glucose data, which can assist in identifying trends not appreciated by typical SMBG testing (such as nocturnal hypoglycemia and meal-time excursions). This allows for adjustment of insulin regimens to reduce the incidence of these events.
Q: Can CGM replace SMBG?
While CGM can provide much more detail regarding glucose trends and patterns, it is not a replacement for SMBG. CGM should not be used as a replacement for SMBG to dose insulin for meal- or activity-related adjustments. All dosing decisions should be based on the SMBG.
Currently, CGM is indicated for patients 18 or older, in conjunction with SMBG for the purpose of improving glycemic control:
• to identify and aid in management of glycemic patterns not recognized with typical SMBG
• to prevent glycemic excursions of hypoglycemia and hyperglycemia.
Its use is supported by ADA and AACE guidelines for glucose monitoring.
Q: Who would benefit from CGM?
Suitable candidates for CGM include those with a high degree of glycemic variability, those with hypoglycemic unawareness, shift workers, patients who use insulin pumps, athletes, and women who are planning to become or are pregnant. Patients should work closely with their health care team and perform regular SMBG.
It has been suggested that patients need comprehensive training and follow-up visits to fully understand the large amount of data that they can be confronted with, in order to fully benefit from these devices.6 While the accuracy is improving, there are a few limitations to this technology, including false alarms. Studies have also shown a positive correlation between sensor wear time (hours per week) and greater reductions in A1C.5
Conclusion
Glucose monitoring is a necessary tool—for patients as well as providers—that assists in identifying how patients’ lifestyles affect their diabetes.
Despite therapeutic advances in diabetes management, the majority of patients with diabetes are unable to achieve glycemic targets proven to reduce the burden of the disease. This burden not only involves the quality of life of patients with diabetes who experience the complications of this disease; it also includes the burden to society. One out of every five health care dollars is spent on caring for someone with diabetes—the majority on treating the complications.1
Major barriers to patients’ ability to achieve glycemic goals include the need to make behavioral changes, lack of awareness of glycemic levels, and fear of hypoglycemia.2
Q: Is self-monitoring of blood glucose worthwhile in diabetes?
Studies have shown a benefit from self-monitoring of blood glucose (SMBG) in patients using insulin but not in those taking oral antidiabetic drugs. However, the American Diabetes Association recommends that patients with diabetes monitor their glucose once daily if they are being treated with noninsulin therapy and at least three times daily if they are taking insulin.3
Guidelines from the American Association of Clinical Endocrinologists (AACE) state that patients taking noninsulin or once-daily insulin therapy who have not achieved A1C targets should monitor at least twice daily, while those at target should monitor at least once daily. Those taking multiple daily injections should perform SMBG at least three times per day. If patients experience frequent hypoglycemia, AACE suggests monitoring glucose more often.4
The A1C test provides the “big picture,” the average daily glucose level during the previous 90 to 120 days, and correlates with end-organ impact. It does not identify glycemic variability, hypoglycemia, or hyperglycemia.
By contrast, SMBG patterns provide day-to-day data that can be used to select and manage glucose control programs and ultimately optimize a patient’s A1C. SMBG provides a measure of the specific pharmacologic impact of medications and, through feedback, allows design and implementation of physiologic insulin-replacement programs.
One example of SMBG is to have patients monitor glucose in pairs (ie, pick a meal each day and do a premeal and two-hour postmeal reading) and ask them to keep a log or download the data from their meter in the office. This type of monitoring can be enlightening and self-empowering for the patient in that it can provide valuable information regarding the glycemic response to the particular meal.
Intensive glycemic management has been shown to reduce the incidence and progression of diabetic complications. However, it is associated with an increase in severe hypoglycemia. This is worrisome for both patients and providers, as severe hypoglycemia has been associated with an increase in risk for mortality. SMBG can assist patients in understanding how their lifestyle affects their diabetes, as well as identifying hypoglycemia for those who may have hypoglycemia unawareness (ie, who lack the relevant symptoms).
Q: What is continuous glucose monitoring (CGM)?
CGM devices give real-time readouts of current glucose levels. They utilize a subcutaneous sensor that is inserted in the abdomen and worn for 3 to 7 days (depending on which device is used). The sensor sends an electronic signal to a receiver worn by the patient.
There are three major CGM devices that have been approved by the FDA and are available for both personal and professional use. Health care providers can purchase the units and have patients wear them for retrospective analysis; this is a reimbursable expense. All available CGM devices measure glucose values in the interstitial fluid. The sensor reads electrical current produced by the same glucose-oxidase reaction that is utilized by glucose meters that patients use to perform fingersticks for home monitoring.
Currently available CGM systems need to be calibrated at least twice daily. Sensor calibration entails the pairing of the fingerstick value with the sensor value from the interstitial space. Calibration confirms sensor accuracy during various points by “teaching” the sensor the glucose value that corresponds with the electrical current signal.
There is a known physiologic lag time that occurs between fingerstick and sensor values. This lag time is typically up to 15 minutes but is increased with rapidly changing glucose values.
Q: What are the benefits of CGM?
Recent studies have shown CGM can improve A1C without increasing the incidence of hypoglycemia.5
CGM systems have both low and high glucose threshold alarms that can be set to alert once the threshold is reached. The newest generation devices can also predict hypoglycemia or hyperglycemia by tracking rate of change, and users can be alerted to a potential event. This would then allow them to take appropriate action, such as consuming food or carbohydrates or taking insulin as necessary. (Before taking any action, the glucose should first be confirmed by SMBG.)
Software programs allow for review of glucose data, which can assist in identifying trends not appreciated by typical SMBG testing (such as nocturnal hypoglycemia and meal-time excursions). This allows for adjustment of insulin regimens to reduce the incidence of these events.
Q: Can CGM replace SMBG?
While CGM can provide much more detail regarding glucose trends and patterns, it is not a replacement for SMBG. CGM should not be used as a replacement for SMBG to dose insulin for meal- or activity-related adjustments. All dosing decisions should be based on the SMBG.
Currently, CGM is indicated for patients 18 or older, in conjunction with SMBG for the purpose of improving glycemic control:
• to identify and aid in management of glycemic patterns not recognized with typical SMBG
• to prevent glycemic excursions of hypoglycemia and hyperglycemia.
Its use is supported by ADA and AACE guidelines for glucose monitoring.
Q: Who would benefit from CGM?
Suitable candidates for CGM include those with a high degree of glycemic variability, those with hypoglycemic unawareness, shift workers, patients who use insulin pumps, athletes, and women who are planning to become or are pregnant. Patients should work closely with their health care team and perform regular SMBG.
It has been suggested that patients need comprehensive training and follow-up visits to fully understand the large amount of data that they can be confronted with, in order to fully benefit from these devices.6 While the accuracy is improving, there are a few limitations to this technology, including false alarms. Studies have also shown a positive correlation between sensor wear time (hours per week) and greater reductions in A1C.5
Conclusion
Glucose monitoring is a necessary tool—for patients as well as providers—that assists in identifying how patients’ lifestyles affect their diabetes.
1. American Diabetes Association. Economic costs of diabetes in the US in 2007. Diabetes Care. 2008;31(3):596-615.
2. Hirsch IB, Armstrong D, Bergenstal RM, et al. Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM). Diabetes Technol Ther. 2008;10(4):232-246.
3. American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2010;34(suppl 1):S11-S61.
4. American Association of Clinical Endocrinologists. Medical guidelines for clinical practice for the management of diabetes mellitus. Endocrin Prac. 2007;13(suppl 1):1-68.
5. Bergenstal RM, Tamberlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320.
6. Fabiato K, Buse J, Duclos M, et al. Clinical experience with continuous glucose monitoring in adults. Diabetes Technol Ther. 2009;11(suppl 1):S93-S103.
1. American Diabetes Association. Economic costs of diabetes in the US in 2007. Diabetes Care. 2008;31(3):596-615.
2. Hirsch IB, Armstrong D, Bergenstal RM, et al. Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM). Diabetes Technol Ther. 2008;10(4):232-246.
3. American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2010;34(suppl 1):S11-S61.
4. American Association of Clinical Endocrinologists. Medical guidelines for clinical practice for the management of diabetes mellitus. Endocrin Prac. 2007;13(suppl 1):1-68.
5. Bergenstal RM, Tamberlane WV, Ahmann A, et al; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320.
6. Fabiato K, Buse J, Duclos M, et al. Clinical experience with continuous glucose monitoring in adults. Diabetes Technol Ther. 2009;11(suppl 1):S93-S103.