User login
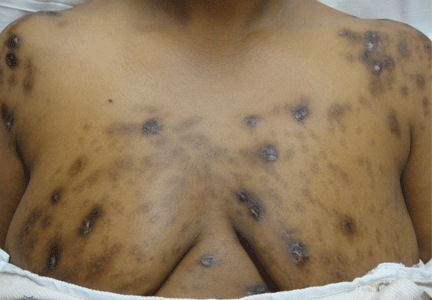
A 40-year-old woman with excoriated skin lesions
She denies any history of itching, insect bites, exposure to new medications or jewelry, allergies, recent change in medications, travel, or intravenous drug abuse.
Q: Which is the most likely diagnosis?
- Allergic contact dermatitis
- Xerosis
- Dermatotillomania
- Folliculitis
- Infestation (scabies)
A: Dermatotillomania, ie, pathologic skin picking, is the correct diagnosis. On further questioning, the patient reveals that the wounds have been self-inflicted over many years, starting in her adolescence. The wounds are located only in areas she can reach. She admits that social and emotional stressors had made the condition significantly worse and that lately she had lost control of her skin-picking. She denies nail-biting, trichotillomania, or obsessive-compulsive behavior.
As for the other possible diagnoses:
Allergic contact dermatitis occurs when contact with a particular substance elicits a hypersensitivity reaction. This reaction is of the delayed type (type IV). The affected individual can develop skin erythema and swelling with vesicles that are intensely pruritic at the contact site. The erythema may become evident hours after exposure, or not until weeks later, which can make the diagnosis difficult at times.
Our patient’s lesions were not pruritic, and she denied recent exposure to allergens.
Xerosis. Xerotic (dry) skin is usually rough, with fine scales and fissures. Xerosis can affect people of all ages and is often more intense during the winter. It affects mainly the arms, legs, and hands. Patients note pruritus, which can be treated with liberal use of emollients and tepid water baths.
Our patient’s lesions were scarred, hyperpigmented, and nonpruritic.
Folliculitis is a superficial infection of the hair follicle that presents as an erythematous pustule on the extremities, buttocks, or scalp. The pustule can be tender to palpation and can progress to an abscess that requires incision and drainage and intravenous antibiotics. A moist environment and poor hygiene are predisposing factors. Staphylococcus aureus is the culprit in most cases.
Our patient’s lesions were on the chest and upper back, where hair follicles were sparse or absent, and there was no erythema or tenderness.
Scabies is a skin infestation with Sarcoptes scabiei mites, which burrow in the skin and cause intense pruritus, especially at night. Scabies usually affects the sides and webs of the fingers and skin folds. Sexual contact is a common way of transmission; however, transmission can also occur by sharing beds and towels.
Patients with dermatotillomania lack intense pruritus, and skin-picking occurs during the day, while the patient is awake.
SELF-INFLICTED WOUNDS
Pathologic skin-picking, neurotic excoriation, excoriated acne, or dermatotillomania results from scratching, picking, gouging, or squeezing of one’s skin via teeth, fingernails, tweezers, or other objects.1–3 Lesions are usually found on skin that the patient can easily reach, such as the face, chest, upper and lower extremities, and upper back.4
The prevalence of pathologic skin-picking is estimated at 2% in dermatology patients.5 The overall prevalence of psychiatric disorders in all dermatology outpatients is estimated at 30% to 40%. Women outnumber men with this disorder.6
Dermatotillomania is thought to be on the spectrum of obsessive-compulsive disorder, in which patients exhibit impulses and compulsions.5 It starts in childhood or early adulthood, with an average lifetime duration of 21 years.7 It is usually associated with anxiety, depression, obsessive-compulsive traits, eating disorders, body dysmorphic disorders, or hypochondriasis. Psychosocial stress is the main trigger. Patients report feelings of tension and stress before picking and relief while picking; there is no suicidal ideation.8
Treatments are both pharmacologic and behavioral.9 Cognitive behavioral therapy and habit reversal therapy have each been successful when used alone.8 In addition, several case reports10 and double-blind studies11,12 have shown that treatment with a selective serotonin-reuptake inhibitor (SSRI) can reduce pathologic skin-picking.13,14 However, SSRIs have also been reported to induce or aggravate this behavior in patients with underlying mild skin-picking and a family history of skin-picking.15 Thus, it is pertinent to extract a detailed history from the patient before prescribing an SSRI.
We referred our patient for behavioral therapy and prescribed fluoxetine (Prozac) 20 mg daily. She showed improvement in symptoms in 4 weeks and has since stopped skin-picking completely.
- Arnold LM. Phenomenology and therapeutic options for dermatotillomania. Expert Rev Neurother 2002; 2:725–730.
- Bohne A, Keuthen N, Wilhelm S. Pathologic hairpulling, skin picking, and nail biting. Ann Clin Psychiatry 2005; 17:227–232.
- Gattu S, Rashid RM, Khachemoune A. Self-induced skin lesions: a review of dermatitis artefacta. Cutis 2009; 84:247–251.
- Keuthen NJ, Deckersbach T, Wilhelm S, et al. Repetitive skin-picking in a student population and comparison with a sample of self-injurious skin-pickers. Psychosomatics 2000; 41:210–215.
- Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology and approaches to treatment. CNS Drugs 2001; 15:351–359.
- Wilhelm S, Keuthen NJ, Deckersbach T, et al. Self-injurious skin picking: clinical characteristics and comorbidity. J Clin Psychiatry 1999; 60:454–459.
- Gupta MA, Gupta AK, Haberman HF. Neurotic excoriations: a review and some new perspectives. Compr Psychiatry 1986; 27:381–386.
- Rosenbaum MS, Ayllon T. The behavioral treatment of neurodermatitis through habit-reversal. Behav Res Ther 1981; 19:313–318.
- Deckersbach T, Wilhelm S, Keuthen N. Self-injurious skin picking: clinical characteristics, assessment methods, and treatment modalities. Brief Treatment and Crisis Intervention 2003; 3:249–260.
- Sharma H. Psychogenic excoriation responding to fluoxetine: a case report. J Indian Med Assoc 2008; 106:245,262.
- Bloch MR, Elliott M, Thompson H, Koran LM. Fluoxetine in pathologic skin-picking: open-label and double-blind results. Psychosomatics 2001; 42:314–319.
- Simeon D, Stein DJ, Gross S, Islam N, Schmeidler J, Hollander E. A double-blind trial of fluoxetine in pathologic skin picking. J Clin Psychiatry 1997; 58:341–347.
- Gupta MA, Gupta AK. The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol 2001; 15:512–518.
- Keuthen NJ, Jameson M, Loh R, Deckersbach T, Wilhelm S, Dougherty DD. Open-label escitalopram treatment for pathological skin picking. Int Clin Psychopharmacol 2007; 22:268–274.
- Denys D, van Megen HJ, Westenberg HG. Emerging skin-picking behaviour after serotonin reuptake inhibitor-treatment in patients with obsessive-compulsive disorder: possible mechanisms and implications for clinical care. J Psychopharmacol 2003; 17:127–129.
She denies any history of itching, insect bites, exposure to new medications or jewelry, allergies, recent change in medications, travel, or intravenous drug abuse.
Q: Which is the most likely diagnosis?
- Allergic contact dermatitis
- Xerosis
- Dermatotillomania
- Folliculitis
- Infestation (scabies)
A: Dermatotillomania, ie, pathologic skin picking, is the correct diagnosis. On further questioning, the patient reveals that the wounds have been self-inflicted over many years, starting in her adolescence. The wounds are located only in areas she can reach. She admits that social and emotional stressors had made the condition significantly worse and that lately she had lost control of her skin-picking. She denies nail-biting, trichotillomania, or obsessive-compulsive behavior.
As for the other possible diagnoses:
Allergic contact dermatitis occurs when contact with a particular substance elicits a hypersensitivity reaction. This reaction is of the delayed type (type IV). The affected individual can develop skin erythema and swelling with vesicles that are intensely pruritic at the contact site. The erythema may become evident hours after exposure, or not until weeks later, which can make the diagnosis difficult at times.
Our patient’s lesions were not pruritic, and she denied recent exposure to allergens.
Xerosis. Xerotic (dry) skin is usually rough, with fine scales and fissures. Xerosis can affect people of all ages and is often more intense during the winter. It affects mainly the arms, legs, and hands. Patients note pruritus, which can be treated with liberal use of emollients and tepid water baths.
Our patient’s lesions were scarred, hyperpigmented, and nonpruritic.
Folliculitis is a superficial infection of the hair follicle that presents as an erythematous pustule on the extremities, buttocks, or scalp. The pustule can be tender to palpation and can progress to an abscess that requires incision and drainage and intravenous antibiotics. A moist environment and poor hygiene are predisposing factors. Staphylococcus aureus is the culprit in most cases.
Our patient’s lesions were on the chest and upper back, where hair follicles were sparse or absent, and there was no erythema or tenderness.
Scabies is a skin infestation with Sarcoptes scabiei mites, which burrow in the skin and cause intense pruritus, especially at night. Scabies usually affects the sides and webs of the fingers and skin folds. Sexual contact is a common way of transmission; however, transmission can also occur by sharing beds and towels.
Patients with dermatotillomania lack intense pruritus, and skin-picking occurs during the day, while the patient is awake.
SELF-INFLICTED WOUNDS
Pathologic skin-picking, neurotic excoriation, excoriated acne, or dermatotillomania results from scratching, picking, gouging, or squeezing of one’s skin via teeth, fingernails, tweezers, or other objects.1–3 Lesions are usually found on skin that the patient can easily reach, such as the face, chest, upper and lower extremities, and upper back.4
The prevalence of pathologic skin-picking is estimated at 2% in dermatology patients.5 The overall prevalence of psychiatric disorders in all dermatology outpatients is estimated at 30% to 40%. Women outnumber men with this disorder.6
Dermatotillomania is thought to be on the spectrum of obsessive-compulsive disorder, in which patients exhibit impulses and compulsions.5 It starts in childhood or early adulthood, with an average lifetime duration of 21 years.7 It is usually associated with anxiety, depression, obsessive-compulsive traits, eating disorders, body dysmorphic disorders, or hypochondriasis. Psychosocial stress is the main trigger. Patients report feelings of tension and stress before picking and relief while picking; there is no suicidal ideation.8
Treatments are both pharmacologic and behavioral.9 Cognitive behavioral therapy and habit reversal therapy have each been successful when used alone.8 In addition, several case reports10 and double-blind studies11,12 have shown that treatment with a selective serotonin-reuptake inhibitor (SSRI) can reduce pathologic skin-picking.13,14 However, SSRIs have also been reported to induce or aggravate this behavior in patients with underlying mild skin-picking and a family history of skin-picking.15 Thus, it is pertinent to extract a detailed history from the patient before prescribing an SSRI.
We referred our patient for behavioral therapy and prescribed fluoxetine (Prozac) 20 mg daily. She showed improvement in symptoms in 4 weeks and has since stopped skin-picking completely.
She denies any history of itching, insect bites, exposure to new medications or jewelry, allergies, recent change in medications, travel, or intravenous drug abuse.
Q: Which is the most likely diagnosis?
- Allergic contact dermatitis
- Xerosis
- Dermatotillomania
- Folliculitis
- Infestation (scabies)
A: Dermatotillomania, ie, pathologic skin picking, is the correct diagnosis. On further questioning, the patient reveals that the wounds have been self-inflicted over many years, starting in her adolescence. The wounds are located only in areas she can reach. She admits that social and emotional stressors had made the condition significantly worse and that lately she had lost control of her skin-picking. She denies nail-biting, trichotillomania, or obsessive-compulsive behavior.
As for the other possible diagnoses:
Allergic contact dermatitis occurs when contact with a particular substance elicits a hypersensitivity reaction. This reaction is of the delayed type (type IV). The affected individual can develop skin erythema and swelling with vesicles that are intensely pruritic at the contact site. The erythema may become evident hours after exposure, or not until weeks later, which can make the diagnosis difficult at times.
Our patient’s lesions were not pruritic, and she denied recent exposure to allergens.
Xerosis. Xerotic (dry) skin is usually rough, with fine scales and fissures. Xerosis can affect people of all ages and is often more intense during the winter. It affects mainly the arms, legs, and hands. Patients note pruritus, which can be treated with liberal use of emollients and tepid water baths.
Our patient’s lesions were scarred, hyperpigmented, and nonpruritic.
Folliculitis is a superficial infection of the hair follicle that presents as an erythematous pustule on the extremities, buttocks, or scalp. The pustule can be tender to palpation and can progress to an abscess that requires incision and drainage and intravenous antibiotics. A moist environment and poor hygiene are predisposing factors. Staphylococcus aureus is the culprit in most cases.
Our patient’s lesions were on the chest and upper back, where hair follicles were sparse or absent, and there was no erythema or tenderness.
Scabies is a skin infestation with Sarcoptes scabiei mites, which burrow in the skin and cause intense pruritus, especially at night. Scabies usually affects the sides and webs of the fingers and skin folds. Sexual contact is a common way of transmission; however, transmission can also occur by sharing beds and towels.
Patients with dermatotillomania lack intense pruritus, and skin-picking occurs during the day, while the patient is awake.
SELF-INFLICTED WOUNDS
Pathologic skin-picking, neurotic excoriation, excoriated acne, or dermatotillomania results from scratching, picking, gouging, or squeezing of one’s skin via teeth, fingernails, tweezers, or other objects.1–3 Lesions are usually found on skin that the patient can easily reach, such as the face, chest, upper and lower extremities, and upper back.4
The prevalence of pathologic skin-picking is estimated at 2% in dermatology patients.5 The overall prevalence of psychiatric disorders in all dermatology outpatients is estimated at 30% to 40%. Women outnumber men with this disorder.6
Dermatotillomania is thought to be on the spectrum of obsessive-compulsive disorder, in which patients exhibit impulses and compulsions.5 It starts in childhood or early adulthood, with an average lifetime duration of 21 years.7 It is usually associated with anxiety, depression, obsessive-compulsive traits, eating disorders, body dysmorphic disorders, or hypochondriasis. Psychosocial stress is the main trigger. Patients report feelings of tension and stress before picking and relief while picking; there is no suicidal ideation.8
Treatments are both pharmacologic and behavioral.9 Cognitive behavioral therapy and habit reversal therapy have each been successful when used alone.8 In addition, several case reports10 and double-blind studies11,12 have shown that treatment with a selective serotonin-reuptake inhibitor (SSRI) can reduce pathologic skin-picking.13,14 However, SSRIs have also been reported to induce or aggravate this behavior in patients with underlying mild skin-picking and a family history of skin-picking.15 Thus, it is pertinent to extract a detailed history from the patient before prescribing an SSRI.
We referred our patient for behavioral therapy and prescribed fluoxetine (Prozac) 20 mg daily. She showed improvement in symptoms in 4 weeks and has since stopped skin-picking completely.
- Arnold LM. Phenomenology and therapeutic options for dermatotillomania. Expert Rev Neurother 2002; 2:725–730.
- Bohne A, Keuthen N, Wilhelm S. Pathologic hairpulling, skin picking, and nail biting. Ann Clin Psychiatry 2005; 17:227–232.
- Gattu S, Rashid RM, Khachemoune A. Self-induced skin lesions: a review of dermatitis artefacta. Cutis 2009; 84:247–251.
- Keuthen NJ, Deckersbach T, Wilhelm S, et al. Repetitive skin-picking in a student population and comparison with a sample of self-injurious skin-pickers. Psychosomatics 2000; 41:210–215.
- Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology and approaches to treatment. CNS Drugs 2001; 15:351–359.
- Wilhelm S, Keuthen NJ, Deckersbach T, et al. Self-injurious skin picking: clinical characteristics and comorbidity. J Clin Psychiatry 1999; 60:454–459.
- Gupta MA, Gupta AK, Haberman HF. Neurotic excoriations: a review and some new perspectives. Compr Psychiatry 1986; 27:381–386.
- Rosenbaum MS, Ayllon T. The behavioral treatment of neurodermatitis through habit-reversal. Behav Res Ther 1981; 19:313–318.
- Deckersbach T, Wilhelm S, Keuthen N. Self-injurious skin picking: clinical characteristics, assessment methods, and treatment modalities. Brief Treatment and Crisis Intervention 2003; 3:249–260.
- Sharma H. Psychogenic excoriation responding to fluoxetine: a case report. J Indian Med Assoc 2008; 106:245,262.
- Bloch MR, Elliott M, Thompson H, Koran LM. Fluoxetine in pathologic skin-picking: open-label and double-blind results. Psychosomatics 2001; 42:314–319.
- Simeon D, Stein DJ, Gross S, Islam N, Schmeidler J, Hollander E. A double-blind trial of fluoxetine in pathologic skin picking. J Clin Psychiatry 1997; 58:341–347.
- Gupta MA, Gupta AK. The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol 2001; 15:512–518.
- Keuthen NJ, Jameson M, Loh R, Deckersbach T, Wilhelm S, Dougherty DD. Open-label escitalopram treatment for pathological skin picking. Int Clin Psychopharmacol 2007; 22:268–274.
- Denys D, van Megen HJ, Westenberg HG. Emerging skin-picking behaviour after serotonin reuptake inhibitor-treatment in patients with obsessive-compulsive disorder: possible mechanisms and implications for clinical care. J Psychopharmacol 2003; 17:127–129.
- Arnold LM. Phenomenology and therapeutic options for dermatotillomania. Expert Rev Neurother 2002; 2:725–730.
- Bohne A, Keuthen N, Wilhelm S. Pathologic hairpulling, skin picking, and nail biting. Ann Clin Psychiatry 2005; 17:227–232.
- Gattu S, Rashid RM, Khachemoune A. Self-induced skin lesions: a review of dermatitis artefacta. Cutis 2009; 84:247–251.
- Keuthen NJ, Deckersbach T, Wilhelm S, et al. Repetitive skin-picking in a student population and comparison with a sample of self-injurious skin-pickers. Psychosomatics 2000; 41:210–215.
- Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology and approaches to treatment. CNS Drugs 2001; 15:351–359.
- Wilhelm S, Keuthen NJ, Deckersbach T, et al. Self-injurious skin picking: clinical characteristics and comorbidity. J Clin Psychiatry 1999; 60:454–459.
- Gupta MA, Gupta AK, Haberman HF. Neurotic excoriations: a review and some new perspectives. Compr Psychiatry 1986; 27:381–386.
- Rosenbaum MS, Ayllon T. The behavioral treatment of neurodermatitis through habit-reversal. Behav Res Ther 1981; 19:313–318.
- Deckersbach T, Wilhelm S, Keuthen N. Self-injurious skin picking: clinical characteristics, assessment methods, and treatment modalities. Brief Treatment and Crisis Intervention 2003; 3:249–260.
- Sharma H. Psychogenic excoriation responding to fluoxetine: a case report. J Indian Med Assoc 2008; 106:245,262.
- Bloch MR, Elliott M, Thompson H, Koran LM. Fluoxetine in pathologic skin-picking: open-label and double-blind results. Psychosomatics 2001; 42:314–319.
- Simeon D, Stein DJ, Gross S, Islam N, Schmeidler J, Hollander E. A double-blind trial of fluoxetine in pathologic skin picking. J Clin Psychiatry 1997; 58:341–347.
- Gupta MA, Gupta AK. The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol 2001; 15:512–518.
- Keuthen NJ, Jameson M, Loh R, Deckersbach T, Wilhelm S, Dougherty DD. Open-label escitalopram treatment for pathological skin picking. Int Clin Psychopharmacol 2007; 22:268–274.
- Denys D, van Megen HJ, Westenberg HG. Emerging skin-picking behaviour after serotonin reuptake inhibitor-treatment in patients with obsessive-compulsive disorder: possible mechanisms and implications for clinical care. J Psychopharmacol 2003; 17:127–129.
The urge to know: What does iron have to do with infection?
If you are one who avoids giving iron to patients with infection, you will be interested in knowing why Daoud et al argue that giving iron is OK. If you hadn’t thought about it recently, this is an excellent opportunity to consider why there has been concern. And why does the serum iron level drop with infection?
Patients with hemochromatosis, characterized by total body overload of iron, are reported to be at risk of overwhelming infection from Vibrio vulnificus. This may well hold true for patients with chronic severe liver disease of other etiologies as well. Vibrio and certain other bacteria (Listeria, Yersinia, Legionella) can demonstrate rapid growth and increased intracellular resistance to killing in the setting of excess iron. Macrophages, in the setting of chronic infection or inflammation, retain excess iron, which may reduce their bactericidal functions. Thus, there has been concern about iron supplementation (including transfusion) in the setting of infection, even in patients with low iron levels and anemia.
The circulating level of the liver protein hepcidin increases as part of the acute-phase response to infection, perhaps with the physiologic “goal” of reducing the availability of free iron to microbial invaders. Hepcidin binds and blocks the function of the membrane iron exporter ferroportin, and iron is functionally trapped within intestinal enterocytes (reducing its absorption) and macrophages (reducing its availability to erythrocyte precursors).
For some of us out of medical school for more than 10 years, the work of Ganz and others1,2 describing the seminal role of hepcidin in iron metabolism may be only partially known. The short article by Daoud and colleagues may urge us to read more about the pathophysiologic foundation of a clinical conundrum. I hope so, for it is that urge to understand that helps define our professional identity as physicians.
- Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med 2011; 62:347–360.
- Lee PL, Beutler E. Regulation of hepcidin and iron-overload disease. Annu Rev Pathol 2009; 4:489–515.
If you are one who avoids giving iron to patients with infection, you will be interested in knowing why Daoud et al argue that giving iron is OK. If you hadn’t thought about it recently, this is an excellent opportunity to consider why there has been concern. And why does the serum iron level drop with infection?
Patients with hemochromatosis, characterized by total body overload of iron, are reported to be at risk of overwhelming infection from Vibrio vulnificus. This may well hold true for patients with chronic severe liver disease of other etiologies as well. Vibrio and certain other bacteria (Listeria, Yersinia, Legionella) can demonstrate rapid growth and increased intracellular resistance to killing in the setting of excess iron. Macrophages, in the setting of chronic infection or inflammation, retain excess iron, which may reduce their bactericidal functions. Thus, there has been concern about iron supplementation (including transfusion) in the setting of infection, even in patients with low iron levels and anemia.
The circulating level of the liver protein hepcidin increases as part of the acute-phase response to infection, perhaps with the physiologic “goal” of reducing the availability of free iron to microbial invaders. Hepcidin binds and blocks the function of the membrane iron exporter ferroportin, and iron is functionally trapped within intestinal enterocytes (reducing its absorption) and macrophages (reducing its availability to erythrocyte precursors).
For some of us out of medical school for more than 10 years, the work of Ganz and others1,2 describing the seminal role of hepcidin in iron metabolism may be only partially known. The short article by Daoud and colleagues may urge us to read more about the pathophysiologic foundation of a clinical conundrum. I hope so, for it is that urge to understand that helps define our professional identity as physicians.
If you are one who avoids giving iron to patients with infection, you will be interested in knowing why Daoud et al argue that giving iron is OK. If you hadn’t thought about it recently, this is an excellent opportunity to consider why there has been concern. And why does the serum iron level drop with infection?
Patients with hemochromatosis, characterized by total body overload of iron, are reported to be at risk of overwhelming infection from Vibrio vulnificus. This may well hold true for patients with chronic severe liver disease of other etiologies as well. Vibrio and certain other bacteria (Listeria, Yersinia, Legionella) can demonstrate rapid growth and increased intracellular resistance to killing in the setting of excess iron. Macrophages, in the setting of chronic infection or inflammation, retain excess iron, which may reduce their bactericidal functions. Thus, there has been concern about iron supplementation (including transfusion) in the setting of infection, even in patients with low iron levels and anemia.
The circulating level of the liver protein hepcidin increases as part of the acute-phase response to infection, perhaps with the physiologic “goal” of reducing the availability of free iron to microbial invaders. Hepcidin binds and blocks the function of the membrane iron exporter ferroportin, and iron is functionally trapped within intestinal enterocytes (reducing its absorption) and macrophages (reducing its availability to erythrocyte precursors).
For some of us out of medical school for more than 10 years, the work of Ganz and others1,2 describing the seminal role of hepcidin in iron metabolism may be only partially known. The short article by Daoud and colleagues may urge us to read more about the pathophysiologic foundation of a clinical conundrum. I hope so, for it is that urge to understand that helps define our professional identity as physicians.
- Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med 2011; 62:347–360.
- Lee PL, Beutler E. Regulation of hepcidin and iron-overload disease. Annu Rev Pathol 2009; 4:489–515.
- Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med 2011; 62:347–360.
- Lee PL, Beutler E. Regulation of hepcidin and iron-overload disease. Annu Rev Pathol 2009; 4:489–515.
Is iron therapy for anemia harmful in the setting of infection?
The harmful effects of iron therapy in the setting of infection are more theoretical than observed, with no irrefutable data to support them. On the other hand, there are also no convincing data to support the benefit of this therapy. If iron is to be used, frequent monitoring of serum iron markers is prudent to avoid iron overload during treatment.
ANEMIA OF INFLAMMATION IS COMPLEX
Anemia that develops in the hospital, especially in the setting of infection or inflammation, is similar hematologically to anemia of chronic disease, except for its acute onset.1
The pathogenesis of anemia in such settings is complex, but the most important causes of this common syndrome include shortening of red cell survival, impaired erythropoietin production, blunted responsiveness of the bone marrow to endogenous erythropoietin, and impaired iron metabolism mediated through the action of inflammatory cytokines.2,3 Other important causes include nutritional deficiencies (iron, vitamin B12, and folic acid)4 and blood loss.5,6
Moreover, anemia of inflammation may be difficult to differentiate from iron-deficiency anemia because the serum iron markers are unreliable in inflammation.1
The reported prevalence of anemia during hospitalization has ranged from 55% on hospital wards7 to 95% in intensive care units.8
Transfusion of packed red blood cells is the fastest treatment for anemia in hospitalized patients and it is the one traditionally used, but many concerns have been raised about its efficacy and adverse effects.9 Erythropoietin, with or without iron therapy, has emerged as an alternative in treating anemia of inflammation.10,11
IRON THERAPY
Iron is widely used to treat anemia, especially in hospitalized patients and those with chronic kidney disease.2 The intravenous route is more commonly used than the oral route, since it has faster action, is better tolerated, and has better bioavailability.1,2
Controversy over benefit
Whether iron supplementation increases the red blood cell mass and reduces the need for blood transfusion is controversial.10,12 Pieracci et al13 documented these benefits in critically ill surgical patients, whereas van Iperen et al11 did not find such benefits in critically ill patients receiving intravenous iron and erythropoietin.
Harmful effects
Some authors1,14 object to giving iron to hospitalized patients (especially critically ill patients) who have infections on the grounds that it is risky, although definitive evidence is lacking.15
Most of the harmful effects of iron have been linked to elevated serum ferritin levels and to non–transferrin-bound iron, more than to iron per se.16 Ferritin is an acute-phase reactant; thus, ferritin levels may be elevated in inflammation and infection regardless of the body iron status.1
Anaphylactic reaction. This rare complication of iron dextran therapy is not much of a concern at present with the newer formulations of iron such as iron gluconate and iron sucrose.16
Oxidative stress. Iron-derived free radicals can cause a rise in inflammatory cytokine levels, especially if the ferritin level is elevated (> 500 μg/L). This cytokine rise is worrisome, as it may have acute detrimental effects on cellular homeostasis, leading to tissue injury,15 while chronically it might be related to enhanced atherosclerosis and cardiac disease.16
Iron overload. In vitro and animal studies have documented an association between elevated ferritin levels (500–650 μg/L) and decreases in T-cell function, polymorphonuclear neutrophil migration, phagocytosis, and bacterial eradication.15 Studies in hemodialysis patients have identified iron overload as an independent risk factor for bacterial infection, but the confounding role of the dialysis process cannot be disregarded.17,18
Bacterial growth. Many bacteria depend on iron for their growth; examples are Escherichia coli; Klebsiella, Pseudomonas, Salmonella, Yersinia, Listeria, and Staphylococcus species; and Haemophilus influenzae. In vitro studies have linked increased bacterial growth with increased transferrin saturation in plasma.15,19
Iron therapy and infection risk
The theory linking iron with risk of infection arose from the observation that patients with hemochromatosis are more susceptible to certain bacterial infections, especially Vibrio vulnificus.20 A few human studies, most of them in chronic hemodialysis patients, have examined the relation between iron therapy and infection risk, with conflicting results.21–26 Multiple studies13,19,21,22,25–27 found no relation between iron therapy and risk of infection or death.
Canziani et al23 found that the risk of infection was higher with higher intravenous doses of iron than with lower doses.
Collins et al24 found a higher risk of sepsis and hospitalization in patients who received iron for a prolonged duration (5–6 months) than in those who did not.
Feldman et al,27 in their report of a study of iron therapy in hemodialysis patients, suggested that previously observed associations between iron administration and higher death rates may have been confounded by other factors.
Iron therapy in concurrent infection
There are no data in humans on the effects of iron therapy on outcomes during concurrent infection or sepsis.15,28 However, mice with sepsis had worse outcomes when treated with intravenous iron.28
A CONUNDRUM IN CLINICAL PRACTICE
After reviewing the available literature, we concur with most of the authors1,15,16,18,19,29 that despite the worrisome theoretical adverse effects of iron therapy in patients with infections, there are no convincing data to support those fears. On the other hand, there are also no convincing data to favor its benefit.
More definitive studies are needed to answer this question, which has been a conundrum in clinical practice. Patients who might benefit from iron therapy should not be deprived of it on the basis of the available data. Frequent monitoring of serum iron markers during therapy to avoid iron overload seems prudent.
- Pieracci FM, Barie PS. Diagnosis and management of iron-related anemias in critical illness. Crit Care Med 2006; 34:1898–1905.
- Krantz SB. Pathogenesis and treatment of the anemia of chronic disease. Am J Med Sci 1994; 307:353–359.
- Price EA, Schrier SL. Unexplained aspects of anemia of inflammation. Review article. Adv Hematol 2010; 2010:508739.
- Rodriguez RM, Corwin HL, Gettinger A, Corwin MJ, Gubler D, Pearl RG. Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness. J Crit Care 2001; 16:36–41.
- Wong P, Intragumtornchai T. Hospital-acquired anemia. J Med Assoc Thai 2006; 89:63–67.
- Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med 2005; 20:520–524.
- Reade MC, Weissfeld L, Angus DC, Kellum JA, Milbrandt EB. The prevalence of anemia and its association with 90-day mortality in hospitalized community-acquired pneumonia. BMC Pulm Med 2010; 10:15.
- Debellis RJ. Anemia in critical care patients: incidence, etiology, impact, management, and use of treatment guidelines and protocols. Am J Health Syst Pharm 2007; 64:S14–S21.
- Marik PE. The hazards of blood transfusion. Br J Hosp Med (Lond) 2009; 70:12–15.
- Corwin HL, Gettinger A, Fabian TC, et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med 2007; 357:965–976.
- van Iperen CE, Gaillard CA, Kraaijenhagen RJ, Braam BG, Marx JJ, van de Wiel A. Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients. Crit Care Med 2000; 28:2773–2778.
- Muñoz M, Breymann C, García-Erce JA, Gómez-Ramirez S, Comin J, Bisbe E. Efficacy and safety of intravenous iron therapy as an alternative/adjunct to allogeneic blood transfusion. Vox Sang 2008; 94:172–183.
- Pieracci FM, Henderson P, Rodney JR, et al. Randomized, double-blind, placebo-controlled trial of effects of enteral iron supplementation on anemia and risk of infection during surgical critical illness. Surg Infect 2009; 10:9–19.
- Pieracci FM, Barie PS. Iron and the risk of infection. Surg Infect 2005; 6(suppl 1):S41–S46.
- Maynor L, Brophy DF. Risk of infections with intravenous iron therapy. Ann Pharmacother 2007; 41:1476–1480.
- Cavill I. Intravenous iron as adjuvant therapy: a two-edged sword? Nephrol Dial Transplant 2003; 18(suppl 8):viii24–viii28.
- Kessler M, Hoen B, Mayeux D, Hestin D, Fontenaille C. Bacteremia in patients on chronic hemodialysis. A multicenter prospective survey. Nephron 1993; 64:95–100.
- Hoen B, Kessler M, Hestin D, Mayeux D. Risk factors for bacterial infections in chronic haemodialysis adult patients: a multicentre prospective survey. Nephrol Dial Transplant 1995; 10:377–381.
- Cieri E. Does iron cause bacterial infections in patients with end stage renal disease? ANNA J 1999; 26:591–596.
- Jurado RL. Iron, infections, and anemia of inflammation. Clin Infect Dis 1997; 25:888–895.
- Brewster UC, Coca SG, Reilly RF, Perazella MA. Effect of intravenous iron on hemodialysis catheter microbial colonization and blood-borne infection. Nephrology 2005; 10:124–128.
- Aronoff GR, Bennett WM, Blumenthal S, et al; United States Iron Sucrose (Venofer) Clinical Trials Group. Iron sucrose in hemodialysis patients: safety of replacement and maintenance regimens. Kidney Int 2004; 66:1193–1198.
- Canziani ME, Yumiya ST, Rangel EB, Manfredi SR, Neto MC, Draibe SA. Risk of bacterial infection in patients under intravenous iron therapy: dose versus length of treatment. Artif Organs 2001; 25:866–869.
- Collins A, Ma J, Xia H, et al. I.V. iron dosing patterns and hospitalization. J Am Soc Nephrol 1998; 9:204A.
- Burns DL, Mascioli EA, Bistrian BR. Effect of iron-supplemented total parenteral nutrition in patients with iron deficiency anemia. Nutrition 1996; 12:411–415.
- Olijhoek G, Megens JG, Musto P, et al. Role of oral versus IV iron supplementation in the erythropoietic response to rHuEPO: a randomized, placebo-controlled trial. Transfusion 2001; 41:957–963.
- Feldman HI, Joffe M, Robinson B, et al. Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc Nephrol 2004; 15:1623–1632.
- Javadi P, Buchman TG, Stromberg PE, et al. High-dose exogenous iron following cecal ligation and puncture increases mortality rate in mice and is associated with an increase in gut epithelial and splenic apoptosis. Crit Care Med 2004; 32:1178–1185.
- Lapointe M. Iron supplementation in the intensive care unit: when, how much, and by what route? Crit Care 2004; 8(suppl 2):S37–S41.
The harmful effects of iron therapy in the setting of infection are more theoretical than observed, with no irrefutable data to support them. On the other hand, there are also no convincing data to support the benefit of this therapy. If iron is to be used, frequent monitoring of serum iron markers is prudent to avoid iron overload during treatment.
ANEMIA OF INFLAMMATION IS COMPLEX
Anemia that develops in the hospital, especially in the setting of infection or inflammation, is similar hematologically to anemia of chronic disease, except for its acute onset.1
The pathogenesis of anemia in such settings is complex, but the most important causes of this common syndrome include shortening of red cell survival, impaired erythropoietin production, blunted responsiveness of the bone marrow to endogenous erythropoietin, and impaired iron metabolism mediated through the action of inflammatory cytokines.2,3 Other important causes include nutritional deficiencies (iron, vitamin B12, and folic acid)4 and blood loss.5,6
Moreover, anemia of inflammation may be difficult to differentiate from iron-deficiency anemia because the serum iron markers are unreliable in inflammation.1
The reported prevalence of anemia during hospitalization has ranged from 55% on hospital wards7 to 95% in intensive care units.8
Transfusion of packed red blood cells is the fastest treatment for anemia in hospitalized patients and it is the one traditionally used, but many concerns have been raised about its efficacy and adverse effects.9 Erythropoietin, with or without iron therapy, has emerged as an alternative in treating anemia of inflammation.10,11
IRON THERAPY
Iron is widely used to treat anemia, especially in hospitalized patients and those with chronic kidney disease.2 The intravenous route is more commonly used than the oral route, since it has faster action, is better tolerated, and has better bioavailability.1,2
Controversy over benefit
Whether iron supplementation increases the red blood cell mass and reduces the need for blood transfusion is controversial.10,12 Pieracci et al13 documented these benefits in critically ill surgical patients, whereas van Iperen et al11 did not find such benefits in critically ill patients receiving intravenous iron and erythropoietin.
Harmful effects
Some authors1,14 object to giving iron to hospitalized patients (especially critically ill patients) who have infections on the grounds that it is risky, although definitive evidence is lacking.15
Most of the harmful effects of iron have been linked to elevated serum ferritin levels and to non–transferrin-bound iron, more than to iron per se.16 Ferritin is an acute-phase reactant; thus, ferritin levels may be elevated in inflammation and infection regardless of the body iron status.1
Anaphylactic reaction. This rare complication of iron dextran therapy is not much of a concern at present with the newer formulations of iron such as iron gluconate and iron sucrose.16
Oxidative stress. Iron-derived free radicals can cause a rise in inflammatory cytokine levels, especially if the ferritin level is elevated (> 500 μg/L). This cytokine rise is worrisome, as it may have acute detrimental effects on cellular homeostasis, leading to tissue injury,15 while chronically it might be related to enhanced atherosclerosis and cardiac disease.16
Iron overload. In vitro and animal studies have documented an association between elevated ferritin levels (500–650 μg/L) and decreases in T-cell function, polymorphonuclear neutrophil migration, phagocytosis, and bacterial eradication.15 Studies in hemodialysis patients have identified iron overload as an independent risk factor for bacterial infection, but the confounding role of the dialysis process cannot be disregarded.17,18
Bacterial growth. Many bacteria depend on iron for their growth; examples are Escherichia coli; Klebsiella, Pseudomonas, Salmonella, Yersinia, Listeria, and Staphylococcus species; and Haemophilus influenzae. In vitro studies have linked increased bacterial growth with increased transferrin saturation in plasma.15,19
Iron therapy and infection risk
The theory linking iron with risk of infection arose from the observation that patients with hemochromatosis are more susceptible to certain bacterial infections, especially Vibrio vulnificus.20 A few human studies, most of them in chronic hemodialysis patients, have examined the relation between iron therapy and infection risk, with conflicting results.21–26 Multiple studies13,19,21,22,25–27 found no relation between iron therapy and risk of infection or death.
Canziani et al23 found that the risk of infection was higher with higher intravenous doses of iron than with lower doses.
Collins et al24 found a higher risk of sepsis and hospitalization in patients who received iron for a prolonged duration (5–6 months) than in those who did not.
Feldman et al,27 in their report of a study of iron therapy in hemodialysis patients, suggested that previously observed associations between iron administration and higher death rates may have been confounded by other factors.
Iron therapy in concurrent infection
There are no data in humans on the effects of iron therapy on outcomes during concurrent infection or sepsis.15,28 However, mice with sepsis had worse outcomes when treated with intravenous iron.28
A CONUNDRUM IN CLINICAL PRACTICE
After reviewing the available literature, we concur with most of the authors1,15,16,18,19,29 that despite the worrisome theoretical adverse effects of iron therapy in patients with infections, there are no convincing data to support those fears. On the other hand, there are also no convincing data to favor its benefit.
More definitive studies are needed to answer this question, which has been a conundrum in clinical practice. Patients who might benefit from iron therapy should not be deprived of it on the basis of the available data. Frequent monitoring of serum iron markers during therapy to avoid iron overload seems prudent.
The harmful effects of iron therapy in the setting of infection are more theoretical than observed, with no irrefutable data to support them. On the other hand, there are also no convincing data to support the benefit of this therapy. If iron is to be used, frequent monitoring of serum iron markers is prudent to avoid iron overload during treatment.
ANEMIA OF INFLAMMATION IS COMPLEX
Anemia that develops in the hospital, especially in the setting of infection or inflammation, is similar hematologically to anemia of chronic disease, except for its acute onset.1
The pathogenesis of anemia in such settings is complex, but the most important causes of this common syndrome include shortening of red cell survival, impaired erythropoietin production, blunted responsiveness of the bone marrow to endogenous erythropoietin, and impaired iron metabolism mediated through the action of inflammatory cytokines.2,3 Other important causes include nutritional deficiencies (iron, vitamin B12, and folic acid)4 and blood loss.5,6
Moreover, anemia of inflammation may be difficult to differentiate from iron-deficiency anemia because the serum iron markers are unreliable in inflammation.1
The reported prevalence of anemia during hospitalization has ranged from 55% on hospital wards7 to 95% in intensive care units.8
Transfusion of packed red blood cells is the fastest treatment for anemia in hospitalized patients and it is the one traditionally used, but many concerns have been raised about its efficacy and adverse effects.9 Erythropoietin, with or without iron therapy, has emerged as an alternative in treating anemia of inflammation.10,11
IRON THERAPY
Iron is widely used to treat anemia, especially in hospitalized patients and those with chronic kidney disease.2 The intravenous route is more commonly used than the oral route, since it has faster action, is better tolerated, and has better bioavailability.1,2
Controversy over benefit
Whether iron supplementation increases the red blood cell mass and reduces the need for blood transfusion is controversial.10,12 Pieracci et al13 documented these benefits in critically ill surgical patients, whereas van Iperen et al11 did not find such benefits in critically ill patients receiving intravenous iron and erythropoietin.
Harmful effects
Some authors1,14 object to giving iron to hospitalized patients (especially critically ill patients) who have infections on the grounds that it is risky, although definitive evidence is lacking.15
Most of the harmful effects of iron have been linked to elevated serum ferritin levels and to non–transferrin-bound iron, more than to iron per se.16 Ferritin is an acute-phase reactant; thus, ferritin levels may be elevated in inflammation and infection regardless of the body iron status.1
Anaphylactic reaction. This rare complication of iron dextran therapy is not much of a concern at present with the newer formulations of iron such as iron gluconate and iron sucrose.16
Oxidative stress. Iron-derived free radicals can cause a rise in inflammatory cytokine levels, especially if the ferritin level is elevated (> 500 μg/L). This cytokine rise is worrisome, as it may have acute detrimental effects on cellular homeostasis, leading to tissue injury,15 while chronically it might be related to enhanced atherosclerosis and cardiac disease.16
Iron overload. In vitro and animal studies have documented an association between elevated ferritin levels (500–650 μg/L) and decreases in T-cell function, polymorphonuclear neutrophil migration, phagocytosis, and bacterial eradication.15 Studies in hemodialysis patients have identified iron overload as an independent risk factor for bacterial infection, but the confounding role of the dialysis process cannot be disregarded.17,18
Bacterial growth. Many bacteria depend on iron for their growth; examples are Escherichia coli; Klebsiella, Pseudomonas, Salmonella, Yersinia, Listeria, and Staphylococcus species; and Haemophilus influenzae. In vitro studies have linked increased bacterial growth with increased transferrin saturation in plasma.15,19
Iron therapy and infection risk
The theory linking iron with risk of infection arose from the observation that patients with hemochromatosis are more susceptible to certain bacterial infections, especially Vibrio vulnificus.20 A few human studies, most of them in chronic hemodialysis patients, have examined the relation between iron therapy and infection risk, with conflicting results.21–26 Multiple studies13,19,21,22,25–27 found no relation between iron therapy and risk of infection or death.
Canziani et al23 found that the risk of infection was higher with higher intravenous doses of iron than with lower doses.
Collins et al24 found a higher risk of sepsis and hospitalization in patients who received iron for a prolonged duration (5–6 months) than in those who did not.
Feldman et al,27 in their report of a study of iron therapy in hemodialysis patients, suggested that previously observed associations between iron administration and higher death rates may have been confounded by other factors.
Iron therapy in concurrent infection
There are no data in humans on the effects of iron therapy on outcomes during concurrent infection or sepsis.15,28 However, mice with sepsis had worse outcomes when treated with intravenous iron.28
A CONUNDRUM IN CLINICAL PRACTICE
After reviewing the available literature, we concur with most of the authors1,15,16,18,19,29 that despite the worrisome theoretical adverse effects of iron therapy in patients with infections, there are no convincing data to support those fears. On the other hand, there are also no convincing data to favor its benefit.
More definitive studies are needed to answer this question, which has been a conundrum in clinical practice. Patients who might benefit from iron therapy should not be deprived of it on the basis of the available data. Frequent monitoring of serum iron markers during therapy to avoid iron overload seems prudent.
- Pieracci FM, Barie PS. Diagnosis and management of iron-related anemias in critical illness. Crit Care Med 2006; 34:1898–1905.
- Krantz SB. Pathogenesis and treatment of the anemia of chronic disease. Am J Med Sci 1994; 307:353–359.
- Price EA, Schrier SL. Unexplained aspects of anemia of inflammation. Review article. Adv Hematol 2010; 2010:508739.
- Rodriguez RM, Corwin HL, Gettinger A, Corwin MJ, Gubler D, Pearl RG. Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness. J Crit Care 2001; 16:36–41.
- Wong P, Intragumtornchai T. Hospital-acquired anemia. J Med Assoc Thai 2006; 89:63–67.
- Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med 2005; 20:520–524.
- Reade MC, Weissfeld L, Angus DC, Kellum JA, Milbrandt EB. The prevalence of anemia and its association with 90-day mortality in hospitalized community-acquired pneumonia. BMC Pulm Med 2010; 10:15.
- Debellis RJ. Anemia in critical care patients: incidence, etiology, impact, management, and use of treatment guidelines and protocols. Am J Health Syst Pharm 2007; 64:S14–S21.
- Marik PE. The hazards of blood transfusion. Br J Hosp Med (Lond) 2009; 70:12–15.
- Corwin HL, Gettinger A, Fabian TC, et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med 2007; 357:965–976.
- van Iperen CE, Gaillard CA, Kraaijenhagen RJ, Braam BG, Marx JJ, van de Wiel A. Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients. Crit Care Med 2000; 28:2773–2778.
- Muñoz M, Breymann C, García-Erce JA, Gómez-Ramirez S, Comin J, Bisbe E. Efficacy and safety of intravenous iron therapy as an alternative/adjunct to allogeneic blood transfusion. Vox Sang 2008; 94:172–183.
- Pieracci FM, Henderson P, Rodney JR, et al. Randomized, double-blind, placebo-controlled trial of effects of enteral iron supplementation on anemia and risk of infection during surgical critical illness. Surg Infect 2009; 10:9–19.
- Pieracci FM, Barie PS. Iron and the risk of infection. Surg Infect 2005; 6(suppl 1):S41–S46.
- Maynor L, Brophy DF. Risk of infections with intravenous iron therapy. Ann Pharmacother 2007; 41:1476–1480.
- Cavill I. Intravenous iron as adjuvant therapy: a two-edged sword? Nephrol Dial Transplant 2003; 18(suppl 8):viii24–viii28.
- Kessler M, Hoen B, Mayeux D, Hestin D, Fontenaille C. Bacteremia in patients on chronic hemodialysis. A multicenter prospective survey. Nephron 1993; 64:95–100.
- Hoen B, Kessler M, Hestin D, Mayeux D. Risk factors for bacterial infections in chronic haemodialysis adult patients: a multicentre prospective survey. Nephrol Dial Transplant 1995; 10:377–381.
- Cieri E. Does iron cause bacterial infections in patients with end stage renal disease? ANNA J 1999; 26:591–596.
- Jurado RL. Iron, infections, and anemia of inflammation. Clin Infect Dis 1997; 25:888–895.
- Brewster UC, Coca SG, Reilly RF, Perazella MA. Effect of intravenous iron on hemodialysis catheter microbial colonization and blood-borne infection. Nephrology 2005; 10:124–128.
- Aronoff GR, Bennett WM, Blumenthal S, et al; United States Iron Sucrose (Venofer) Clinical Trials Group. Iron sucrose in hemodialysis patients: safety of replacement and maintenance regimens. Kidney Int 2004; 66:1193–1198.
- Canziani ME, Yumiya ST, Rangel EB, Manfredi SR, Neto MC, Draibe SA. Risk of bacterial infection in patients under intravenous iron therapy: dose versus length of treatment. Artif Organs 2001; 25:866–869.
- Collins A, Ma J, Xia H, et al. I.V. iron dosing patterns and hospitalization. J Am Soc Nephrol 1998; 9:204A.
- Burns DL, Mascioli EA, Bistrian BR. Effect of iron-supplemented total parenteral nutrition in patients with iron deficiency anemia. Nutrition 1996; 12:411–415.
- Olijhoek G, Megens JG, Musto P, et al. Role of oral versus IV iron supplementation in the erythropoietic response to rHuEPO: a randomized, placebo-controlled trial. Transfusion 2001; 41:957–963.
- Feldman HI, Joffe M, Robinson B, et al. Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc Nephrol 2004; 15:1623–1632.
- Javadi P, Buchman TG, Stromberg PE, et al. High-dose exogenous iron following cecal ligation and puncture increases mortality rate in mice and is associated with an increase in gut epithelial and splenic apoptosis. Crit Care Med 2004; 32:1178–1185.
- Lapointe M. Iron supplementation in the intensive care unit: when, how much, and by what route? Crit Care 2004; 8(suppl 2):S37–S41.
- Pieracci FM, Barie PS. Diagnosis and management of iron-related anemias in critical illness. Crit Care Med 2006; 34:1898–1905.
- Krantz SB. Pathogenesis and treatment of the anemia of chronic disease. Am J Med Sci 1994; 307:353–359.
- Price EA, Schrier SL. Unexplained aspects of anemia of inflammation. Review article. Adv Hematol 2010; 2010:508739.
- Rodriguez RM, Corwin HL, Gettinger A, Corwin MJ, Gubler D, Pearl RG. Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness. J Crit Care 2001; 16:36–41.
- Wong P, Intragumtornchai T. Hospital-acquired anemia. J Med Assoc Thai 2006; 89:63–67.
- Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med 2005; 20:520–524.
- Reade MC, Weissfeld L, Angus DC, Kellum JA, Milbrandt EB. The prevalence of anemia and its association with 90-day mortality in hospitalized community-acquired pneumonia. BMC Pulm Med 2010; 10:15.
- Debellis RJ. Anemia in critical care patients: incidence, etiology, impact, management, and use of treatment guidelines and protocols. Am J Health Syst Pharm 2007; 64:S14–S21.
- Marik PE. The hazards of blood transfusion. Br J Hosp Med (Lond) 2009; 70:12–15.
- Corwin HL, Gettinger A, Fabian TC, et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med 2007; 357:965–976.
- van Iperen CE, Gaillard CA, Kraaijenhagen RJ, Braam BG, Marx JJ, van de Wiel A. Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients. Crit Care Med 2000; 28:2773–2778.
- Muñoz M, Breymann C, García-Erce JA, Gómez-Ramirez S, Comin J, Bisbe E. Efficacy and safety of intravenous iron therapy as an alternative/adjunct to allogeneic blood transfusion. Vox Sang 2008; 94:172–183.
- Pieracci FM, Henderson P, Rodney JR, et al. Randomized, double-blind, placebo-controlled trial of effects of enteral iron supplementation on anemia and risk of infection during surgical critical illness. Surg Infect 2009; 10:9–19.
- Pieracci FM, Barie PS. Iron and the risk of infection. Surg Infect 2005; 6(suppl 1):S41–S46.
- Maynor L, Brophy DF. Risk of infections with intravenous iron therapy. Ann Pharmacother 2007; 41:1476–1480.
- Cavill I. Intravenous iron as adjuvant therapy: a two-edged sword? Nephrol Dial Transplant 2003; 18(suppl 8):viii24–viii28.
- Kessler M, Hoen B, Mayeux D, Hestin D, Fontenaille C. Bacteremia in patients on chronic hemodialysis. A multicenter prospective survey. Nephron 1993; 64:95–100.
- Hoen B, Kessler M, Hestin D, Mayeux D. Risk factors for bacterial infections in chronic haemodialysis adult patients: a multicentre prospective survey. Nephrol Dial Transplant 1995; 10:377–381.
- Cieri E. Does iron cause bacterial infections in patients with end stage renal disease? ANNA J 1999; 26:591–596.
- Jurado RL. Iron, infections, and anemia of inflammation. Clin Infect Dis 1997; 25:888–895.
- Brewster UC, Coca SG, Reilly RF, Perazella MA. Effect of intravenous iron on hemodialysis catheter microbial colonization and blood-borne infection. Nephrology 2005; 10:124–128.
- Aronoff GR, Bennett WM, Blumenthal S, et al; United States Iron Sucrose (Venofer) Clinical Trials Group. Iron sucrose in hemodialysis patients: safety of replacement and maintenance regimens. Kidney Int 2004; 66:1193–1198.
- Canziani ME, Yumiya ST, Rangel EB, Manfredi SR, Neto MC, Draibe SA. Risk of bacterial infection in patients under intravenous iron therapy: dose versus length of treatment. Artif Organs 2001; 25:866–869.
- Collins A, Ma J, Xia H, et al. I.V. iron dosing patterns and hospitalization. J Am Soc Nephrol 1998; 9:204A.
- Burns DL, Mascioli EA, Bistrian BR. Effect of iron-supplemented total parenteral nutrition in patients with iron deficiency anemia. Nutrition 1996; 12:411–415.
- Olijhoek G, Megens JG, Musto P, et al. Role of oral versus IV iron supplementation in the erythropoietic response to rHuEPO: a randomized, placebo-controlled trial. Transfusion 2001; 41:957–963.
- Feldman HI, Joffe M, Robinson B, et al. Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc Nephrol 2004; 15:1623–1632.
- Javadi P, Buchman TG, Stromberg PE, et al. High-dose exogenous iron following cecal ligation and puncture increases mortality rate in mice and is associated with an increase in gut epithelial and splenic apoptosis. Crit Care Med 2004; 32:1178–1185.
- Lapointe M. Iron supplementation in the intensive care unit: when, how much, and by what route? Crit Care 2004; 8(suppl 2):S37–S41.
Good Late Outcomes Seen After CABG Plus Adult CHD Repair
SAN DIEGO - More and more patients with congenital heart disease are surviving into adulthood, resulting in a growing number of operations performed to repair adult congenital heart disease (ACHD). Many of these patients also have atherosclerotic coronary artery disease that may need to be addressed at the time of ACHD surgery, but data on the prevalence of coronary artery disease in this population, as well as outcomes after such surgery, are limited.
To address this issue, Dr. John M. Stulak of the Mayo Medical School, Rochester, Minn., and his associates conducted a study of 122 patients (77 male) who underwent concomitant coronary artery bypass grafting (CABG) for atherosclerotic coronary artery disease (CAD) at the time of ACHD repair. Dr. Stulak presented the results at the annual meeting of the Society of Thoracic Surgeons.
Dr. Stulak noted that, based on his findings, "Concomitant CABG may be required at the time of repair of ACHD. Disease of the LAD [left anterior descending coronary artery] is most common, and survival is higher when a LIMA [left internal mammary artery] graft is used. Late functional outcome is good with a low incidence of late angina, MI, or the need for percutaneous coronary intervention."
The patients, mean age 64 years, had surgery between February 1972 and August 2009. A total of 25% had angina, 6% had prior myocardial infarction, and 5% had previous percutaneous intervention.
The most common primary cardiac diagnoses were secundum atrial septal defect (ASD) in 60%, Ebstein anomaly in 11%, partial anomalous pulmonary venous connection (PAPVC) in 7%, and ventricular septal defect (VSD) in 6%. A total of 17% of the patients had a prior cardiac operation.
The most common operations included ASD repair in 64%; tricuspid valve surgery (11%), pulmonary valve surgery (8%), VSD repair (8%), and PAPVC repair (7%). A single bypass graft was performed in 69 patients, 2 grafts in 32 patients, 3 grafts in 14 patients, 4 grafts in 5 patients, and 5 grafts in 2 patients. The LIMA was used in 57 of 82 patients (70%) with LAD disease.
The median follow-up was 6 years for 111 available patients. During that time, recurrent CAD was reported in 9 patients (8%); 8 patients (7%) had angina, and 5 (4%) had an MI. Six (5%) patients underwent intervention. All but 11 patients achieved NYHA functional class 1 or 2. The overall survival observed was 76% at 5 years, 56% at 10 years, and 33% at 15 years. In those patients with LAD disease, 10-year survival was significantly higher when LIMA was used (66% vs. 36%).
Dr. Stulak added the importance of this study is also to stress that each treatment approach should be individualized whether it is conventional CABG, off-pump CABG, or a staged hybrid technique with percutaneous coronary intervention for CAD.
Dr. Stulak and his colleagues had no disclosures.
SAN DIEGO - More and more patients with congenital heart disease are surviving into adulthood, resulting in a growing number of operations performed to repair adult congenital heart disease (ACHD). Many of these patients also have atherosclerotic coronary artery disease that may need to be addressed at the time of ACHD surgery, but data on the prevalence of coronary artery disease in this population, as well as outcomes after such surgery, are limited.
To address this issue, Dr. John M. Stulak of the Mayo Medical School, Rochester, Minn., and his associates conducted a study of 122 patients (77 male) who underwent concomitant coronary artery bypass grafting (CABG) for atherosclerotic coronary artery disease (CAD) at the time of ACHD repair. Dr. Stulak presented the results at the annual meeting of the Society of Thoracic Surgeons.
Dr. Stulak noted that, based on his findings, "Concomitant CABG may be required at the time of repair of ACHD. Disease of the LAD [left anterior descending coronary artery] is most common, and survival is higher when a LIMA [left internal mammary artery] graft is used. Late functional outcome is good with a low incidence of late angina, MI, or the need for percutaneous coronary intervention."
The patients, mean age 64 years, had surgery between February 1972 and August 2009. A total of 25% had angina, 6% had prior myocardial infarction, and 5% had previous percutaneous intervention.
The most common primary cardiac diagnoses were secundum atrial septal defect (ASD) in 60%, Ebstein anomaly in 11%, partial anomalous pulmonary venous connection (PAPVC) in 7%, and ventricular septal defect (VSD) in 6%. A total of 17% of the patients had a prior cardiac operation.
The most common operations included ASD repair in 64%; tricuspid valve surgery (11%), pulmonary valve surgery (8%), VSD repair (8%), and PAPVC repair (7%). A single bypass graft was performed in 69 patients, 2 grafts in 32 patients, 3 grafts in 14 patients, 4 grafts in 5 patients, and 5 grafts in 2 patients. The LIMA was used in 57 of 82 patients (70%) with LAD disease.
The median follow-up was 6 years for 111 available patients. During that time, recurrent CAD was reported in 9 patients (8%); 8 patients (7%) had angina, and 5 (4%) had an MI. Six (5%) patients underwent intervention. All but 11 patients achieved NYHA functional class 1 or 2. The overall survival observed was 76% at 5 years, 56% at 10 years, and 33% at 15 years. In those patients with LAD disease, 10-year survival was significantly higher when LIMA was used (66% vs. 36%).
Dr. Stulak added the importance of this study is also to stress that each treatment approach should be individualized whether it is conventional CABG, off-pump CABG, or a staged hybrid technique with percutaneous coronary intervention for CAD.
Dr. Stulak and his colleagues had no disclosures.
SAN DIEGO - More and more patients with congenital heart disease are surviving into adulthood, resulting in a growing number of operations performed to repair adult congenital heart disease (ACHD). Many of these patients also have atherosclerotic coronary artery disease that may need to be addressed at the time of ACHD surgery, but data on the prevalence of coronary artery disease in this population, as well as outcomes after such surgery, are limited.
To address this issue, Dr. John M. Stulak of the Mayo Medical School, Rochester, Minn., and his associates conducted a study of 122 patients (77 male) who underwent concomitant coronary artery bypass grafting (CABG) for atherosclerotic coronary artery disease (CAD) at the time of ACHD repair. Dr. Stulak presented the results at the annual meeting of the Society of Thoracic Surgeons.
Dr. Stulak noted that, based on his findings, "Concomitant CABG may be required at the time of repair of ACHD. Disease of the LAD [left anterior descending coronary artery] is most common, and survival is higher when a LIMA [left internal mammary artery] graft is used. Late functional outcome is good with a low incidence of late angina, MI, or the need for percutaneous coronary intervention."
The patients, mean age 64 years, had surgery between February 1972 and August 2009. A total of 25% had angina, 6% had prior myocardial infarction, and 5% had previous percutaneous intervention.
The most common primary cardiac diagnoses were secundum atrial septal defect (ASD) in 60%, Ebstein anomaly in 11%, partial anomalous pulmonary venous connection (PAPVC) in 7%, and ventricular septal defect (VSD) in 6%. A total of 17% of the patients had a prior cardiac operation.
The most common operations included ASD repair in 64%; tricuspid valve surgery (11%), pulmonary valve surgery (8%), VSD repair (8%), and PAPVC repair (7%). A single bypass graft was performed in 69 patients, 2 grafts in 32 patients, 3 grafts in 14 patients, 4 grafts in 5 patients, and 5 grafts in 2 patients. The LIMA was used in 57 of 82 patients (70%) with LAD disease.
The median follow-up was 6 years for 111 available patients. During that time, recurrent CAD was reported in 9 patients (8%); 8 patients (7%) had angina, and 5 (4%) had an MI. Six (5%) patients underwent intervention. All but 11 patients achieved NYHA functional class 1 or 2. The overall survival observed was 76% at 5 years, 56% at 10 years, and 33% at 15 years. In those patients with LAD disease, 10-year survival was significantly higher when LIMA was used (66% vs. 36%).
Dr. Stulak added the importance of this study is also to stress that each treatment approach should be individualized whether it is conventional CABG, off-pump CABG, or a staged hybrid technique with percutaneous coronary intervention for CAD.
Dr. Stulak and his colleagues had no disclosures.
Good Late Outcomes Seen After CABG Plus Adult CHD Repair
SAN DIEGO - More and more patients with congenital heart disease are surviving into adulthood, resulting in a growing number of operations performed to repair adult congenital heart disease (ACHD). Many of these patients also have atherosclerotic coronary artery disease that may need to be addressed at the time of ACHD surgery, but data on the prevalence of coronary artery disease in this population, as well as outcomes after such surgery, are limited.
To address this issue, Dr. John M. Stulak of the Mayo Medical School, Rochester, Minn., and his associates conducted a study of 122 patients (77 male) who underwent concomitant coronary artery bypass grafting (CABG) for atherosclerotic coronary artery disease (CAD) at the time of ACHD repair. Dr. Stulak presented the results at the annual meeting of the Society of Thoracic Surgeons.
Dr. Stulak noted that, based on his findings, "Concomitant CABG may be required at the time of repair of ACHD. Disease of the LAD [left anterior descending coronary artery] is most common, and survival is higher when a LIMA [left internal mammary artery] graft is used. Late functional outcome is good with a low incidence of late angina, MI, or the need for percutaneous coronary intervention."
The patients, mean age 64 years, had surgery between February 1972 and August 2009. A total of 25% had angina, 6% had prior myocardial infarction, and 5% had previous percutaneous intervention.
The most common primary cardiac diagnoses were secundum atrial septal defect (ASD) in 60%, Ebstein anomaly in 11%, partial anomalous pulmonary venous connection (PAPVC) in 7%, and ventricular septal defect (VSD) in 6%. A total of 17% of the patients had a prior cardiac operation.
The most common operations included ASD repair in 64%; tricuspid valve surgery (11%), pulmonary valve surgery (8%), VSD repair (8%), and PAPVC repair (7%). A single bypass graft was performed in 69 patients, 2 grafts in 32 patients, 3 grafts in 14 patients, 4 grafts in 5 patients, and 5 grafts in 2 patients. The LIMA was used in 57 of 82 patients (70%) with LAD disease.
The median follow-up was 6 years for 111 available patients. During that time, recurrent CAD was reported in 9 patients (8%); 8 patients (7%) had angina, and 5 (4%) had an MI. Six (5%) patients underwent intervention. All but 11 patients achieved NYHA functional class 1 or 2. The overall survival observed was 76% at 5 years, 56% at 10 years, and 33% at 15 years. In those patients with LAD disease, 10-year survival was significantly higher when LIMA was used (66% vs. 36%).
Dr. Stulak added the importance of this study is also to stress that each treatment approach should be individualized whether it is conventional CABG, off-pump CABG, or a staged hybrid technique with percutaneous coronary intervention for CAD.
Dr. Stulak and his colleagues had no disclosures.
SAN DIEGO - More and more patients with congenital heart disease are surviving into adulthood, resulting in a growing number of operations performed to repair adult congenital heart disease (ACHD). Many of these patients also have atherosclerotic coronary artery disease that may need to be addressed at the time of ACHD surgery, but data on the prevalence of coronary artery disease in this population, as well as outcomes after such surgery, are limited.
To address this issue, Dr. John M. Stulak of the Mayo Medical School, Rochester, Minn., and his associates conducted a study of 122 patients (77 male) who underwent concomitant coronary artery bypass grafting (CABG) for atherosclerotic coronary artery disease (CAD) at the time of ACHD repair. Dr. Stulak presented the results at the annual meeting of the Society of Thoracic Surgeons.
Dr. Stulak noted that, based on his findings, "Concomitant CABG may be required at the time of repair of ACHD. Disease of the LAD [left anterior descending coronary artery] is most common, and survival is higher when a LIMA [left internal mammary artery] graft is used. Late functional outcome is good with a low incidence of late angina, MI, or the need for percutaneous coronary intervention."
The patients, mean age 64 years, had surgery between February 1972 and August 2009. A total of 25% had angina, 6% had prior myocardial infarction, and 5% had previous percutaneous intervention.
The most common primary cardiac diagnoses were secundum atrial septal defect (ASD) in 60%, Ebstein anomaly in 11%, partial anomalous pulmonary venous connection (PAPVC) in 7%, and ventricular septal defect (VSD) in 6%. A total of 17% of the patients had a prior cardiac operation.
The most common operations included ASD repair in 64%; tricuspid valve surgery (11%), pulmonary valve surgery (8%), VSD repair (8%), and PAPVC repair (7%). A single bypass graft was performed in 69 patients, 2 grafts in 32 patients, 3 grafts in 14 patients, 4 grafts in 5 patients, and 5 grafts in 2 patients. The LIMA was used in 57 of 82 patients (70%) with LAD disease.
The median follow-up was 6 years for 111 available patients. During that time, recurrent CAD was reported in 9 patients (8%); 8 patients (7%) had angina, and 5 (4%) had an MI. Six (5%) patients underwent intervention. All but 11 patients achieved NYHA functional class 1 or 2. The overall survival observed was 76% at 5 years, 56% at 10 years, and 33% at 15 years. In those patients with LAD disease, 10-year survival was significantly higher when LIMA was used (66% vs. 36%).
Dr. Stulak added the importance of this study is also to stress that each treatment approach should be individualized whether it is conventional CABG, off-pump CABG, or a staged hybrid technique with percutaneous coronary intervention for CAD.
Dr. Stulak and his colleagues had no disclosures.
SAN DIEGO - More and more patients with congenital heart disease are surviving into adulthood, resulting in a growing number of operations performed to repair adult congenital heart disease (ACHD). Many of these patients also have atherosclerotic coronary artery disease that may need to be addressed at the time of ACHD surgery, but data on the prevalence of coronary artery disease in this population, as well as outcomes after such surgery, are limited.
To address this issue, Dr. John M. Stulak of the Mayo Medical School, Rochester, Minn., and his associates conducted a study of 122 patients (77 male) who underwent concomitant coronary artery bypass grafting (CABG) for atherosclerotic coronary artery disease (CAD) at the time of ACHD repair. Dr. Stulak presented the results at the annual meeting of the Society of Thoracic Surgeons.
Dr. Stulak noted that, based on his findings, "Concomitant CABG may be required at the time of repair of ACHD. Disease of the LAD [left anterior descending coronary artery] is most common, and survival is higher when a LIMA [left internal mammary artery] graft is used. Late functional outcome is good with a low incidence of late angina, MI, or the need for percutaneous coronary intervention."
The patients, mean age 64 years, had surgery between February 1972 and August 2009. A total of 25% had angina, 6% had prior myocardial infarction, and 5% had previous percutaneous intervention.
The most common primary cardiac diagnoses were secundum atrial septal defect (ASD) in 60%, Ebstein anomaly in 11%, partial anomalous pulmonary venous connection (PAPVC) in 7%, and ventricular septal defect (VSD) in 6%. A total of 17% of the patients had a prior cardiac operation.
The most common operations included ASD repair in 64%; tricuspid valve surgery (11%), pulmonary valve surgery (8%), VSD repair (8%), and PAPVC repair (7%). A single bypass graft was performed in 69 patients, 2 grafts in 32 patients, 3 grafts in 14 patients, 4 grafts in 5 patients, and 5 grafts in 2 patients. The LIMA was used in 57 of 82 patients (70%) with LAD disease.
The median follow-up was 6 years for 111 available patients. During that time, recurrent CAD was reported in 9 patients (8%); 8 patients (7%) had angina, and 5 (4%) had an MI. Six (5%) patients underwent intervention. All but 11 patients achieved NYHA functional class 1 or 2. The overall survival observed was 76% at 5 years, 56% at 10 years, and 33% at 15 years. In those patients with LAD disease, 10-year survival was significantly higher when LIMA was used (66% vs. 36%).
Dr. Stulak added the importance of this study is also to stress that each treatment approach should be individualized whether it is conventional CABG, off-pump CABG, or a staged hybrid technique with percutaneous coronary intervention for CAD.
Dr. Stulak and his colleagues had no disclosures.
Changing Indications In Pediatric Transplants
SAN DIEGO - Over the past 24 years, the prevalence of indications for pediatric heart transplantation resulting from congenital heart disease has changed. Transplantation for failed SV palliation, including failed Fontan procedure, has now become the predominant indication, according to the observations of a single-center experience reported in the J. Maxwell Chamberlain Memorial Paper for Congenital Heart Surgery at the annual meeting of the Society of Thoracic Surgeons.
Heart transplantation is the only viable treatment for children with end-stage heart failure resulting from either congenital heart disease (CHD) or cardiomyopathy. The purpose of this study by Dr. Rochus K. Voeller and his colleagues at Washington University in St. Louis was to review the trends in the indications for transplant and survival following transplant, using a retrospective review of all 307 orthotopic heart transplants performed at St. Louis Children's Hospital from January 1986 to December 2009. Combined heart-lung transplants were excluded from the study.
The indications for transplantation in 1986-2009 were 39% cardiomyopathy, 57% CHD, and 4% retransplant. Of the 174 patients with CHD, 80% had single-ventricle anomalies (SV). In the CHD group, transplantation for failed SV palliation, including the failed Fontan procedure, became the predominant indication in the latest 8-year interval of their program (increasing from 11% in the 1984-1993 period to 60% in the 2002-2009 period). The rate of retransplantation remained low and unchanged across the various time periods, according to Dr. Voeller.
The mean recipient age was 6.1 years, with 41% of the recipients aged younger than 1 year at the time of transplantation. Nearly one-third of all patients had prior surgical procedures or surgery ranging from banding to Fontan operations; 55% of the patients were boys; 8% of patients were bridged with either ECMO (extracorporeal circulation membrane oxygenation) or VAD (ventricular assist devices).
Overall survival of transplant patients was 81%, 76%, 72%, and 65% at 1, 3, 5, and 10 years, respectively. Survival was best in those patients who were transplanted for cardiomyopathy (1-, 3-, 5-, and 10-year survival of 90%, 84%, 81%, and 81%, respectively) and worst in patients with failed palliations for SV anomalies, especially failed Fontan procedures (1-, 3-, 5-, and 10-year survival of 66%, 61%, 61%, and 53%, respectively).
"Our results demonstrate the high-risk nature of transplants in patients with failed palliations for SV anomalies, including Fontan procedures performed during infancy. As the survival with early palliation for SV anomaly patients improves, more centers will be referred with these patients who will require transplantation at some point," said Dr. Voeller in an interview.
"This will not only impact pediatric heart transplant programs, but it will also influence adult transplant programs as well. Patients following SV palliation, including Fontan procedure, are much more difficult patients to transplant because of a variety of factors. Risk factor analysis will be needed to determine which patients might benefit from earlier transplant referral and how to better prepare these patient for transplant in order to reduce the risk of the procedure," he concluded.
Dr. Voeller reported that none of the authors had any financial disclosures.
SAN DIEGO - Over the past 24 years, the prevalence of indications for pediatric heart transplantation resulting from congenital heart disease has changed. Transplantation for failed SV palliation, including failed Fontan procedure, has now become the predominant indication, according to the observations of a single-center experience reported in the J. Maxwell Chamberlain Memorial Paper for Congenital Heart Surgery at the annual meeting of the Society of Thoracic Surgeons.
Heart transplantation is the only viable treatment for children with end-stage heart failure resulting from either congenital heart disease (CHD) or cardiomyopathy. The purpose of this study by Dr. Rochus K. Voeller and his colleagues at Washington University in St. Louis was to review the trends in the indications for transplant and survival following transplant, using a retrospective review of all 307 orthotopic heart transplants performed at St. Louis Children's Hospital from January 1986 to December 2009. Combined heart-lung transplants were excluded from the study.
The indications for transplantation in 1986-2009 were 39% cardiomyopathy, 57% CHD, and 4% retransplant. Of the 174 patients with CHD, 80% had single-ventricle anomalies (SV). In the CHD group, transplantation for failed SV palliation, including the failed Fontan procedure, became the predominant indication in the latest 8-year interval of their program (increasing from 11% in the 1984-1993 period to 60% in the 2002-2009 period). The rate of retransplantation remained low and unchanged across the various time periods, according to Dr. Voeller.
The mean recipient age was 6.1 years, with 41% of the recipients aged younger than 1 year at the time of transplantation. Nearly one-third of all patients had prior surgical procedures or surgery ranging from banding to Fontan operations; 55% of the patients were boys; 8% of patients were bridged with either ECMO (extracorporeal circulation membrane oxygenation) or VAD (ventricular assist devices).
Overall survival of transplant patients was 81%, 76%, 72%, and 65% at 1, 3, 5, and 10 years, respectively. Survival was best in those patients who were transplanted for cardiomyopathy (1-, 3-, 5-, and 10-year survival of 90%, 84%, 81%, and 81%, respectively) and worst in patients with failed palliations for SV anomalies, especially failed Fontan procedures (1-, 3-, 5-, and 10-year survival of 66%, 61%, 61%, and 53%, respectively).
"Our results demonstrate the high-risk nature of transplants in patients with failed palliations for SV anomalies, including Fontan procedures performed during infancy. As the survival with early palliation for SV anomaly patients improves, more centers will be referred with these patients who will require transplantation at some point," said Dr. Voeller in an interview.
"This will not only impact pediatric heart transplant programs, but it will also influence adult transplant programs as well. Patients following SV palliation, including Fontan procedure, are much more difficult patients to transplant because of a variety of factors. Risk factor analysis will be needed to determine which patients might benefit from earlier transplant referral and how to better prepare these patient for transplant in order to reduce the risk of the procedure," he concluded.
Dr. Voeller reported that none of the authors had any financial disclosures.
SAN DIEGO - Over the past 24 years, the prevalence of indications for pediatric heart transplantation resulting from congenital heart disease has changed. Transplantation for failed SV palliation, including failed Fontan procedure, has now become the predominant indication, according to the observations of a single-center experience reported in the J. Maxwell Chamberlain Memorial Paper for Congenital Heart Surgery at the annual meeting of the Society of Thoracic Surgeons.
Heart transplantation is the only viable treatment for children with end-stage heart failure resulting from either congenital heart disease (CHD) or cardiomyopathy. The purpose of this study by Dr. Rochus K. Voeller and his colleagues at Washington University in St. Louis was to review the trends in the indications for transplant and survival following transplant, using a retrospective review of all 307 orthotopic heart transplants performed at St. Louis Children's Hospital from January 1986 to December 2009. Combined heart-lung transplants were excluded from the study.
The indications for transplantation in 1986-2009 were 39% cardiomyopathy, 57% CHD, and 4% retransplant. Of the 174 patients with CHD, 80% had single-ventricle anomalies (SV). In the CHD group, transplantation for failed SV palliation, including the failed Fontan procedure, became the predominant indication in the latest 8-year interval of their program (increasing from 11% in the 1984-1993 period to 60% in the 2002-2009 period). The rate of retransplantation remained low and unchanged across the various time periods, according to Dr. Voeller.
The mean recipient age was 6.1 years, with 41% of the recipients aged younger than 1 year at the time of transplantation. Nearly one-third of all patients had prior surgical procedures or surgery ranging from banding to Fontan operations; 55% of the patients were boys; 8% of patients were bridged with either ECMO (extracorporeal circulation membrane oxygenation) or VAD (ventricular assist devices).
Overall survival of transplant patients was 81%, 76%, 72%, and 65% at 1, 3, 5, and 10 years, respectively. Survival was best in those patients who were transplanted for cardiomyopathy (1-, 3-, 5-, and 10-year survival of 90%, 84%, 81%, and 81%, respectively) and worst in patients with failed palliations for SV anomalies, especially failed Fontan procedures (1-, 3-, 5-, and 10-year survival of 66%, 61%, 61%, and 53%, respectively).
"Our results demonstrate the high-risk nature of transplants in patients with failed palliations for SV anomalies, including Fontan procedures performed during infancy. As the survival with early palliation for SV anomaly patients improves, more centers will be referred with these patients who will require transplantation at some point," said Dr. Voeller in an interview.
"This will not only impact pediatric heart transplant programs, but it will also influence adult transplant programs as well. Patients following SV palliation, including Fontan procedure, are much more difficult patients to transplant because of a variety of factors. Risk factor analysis will be needed to determine which patients might benefit from earlier transplant referral and how to better prepare these patient for transplant in order to reduce the risk of the procedure," he concluded.
Dr. Voeller reported that none of the authors had any financial disclosures.