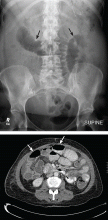
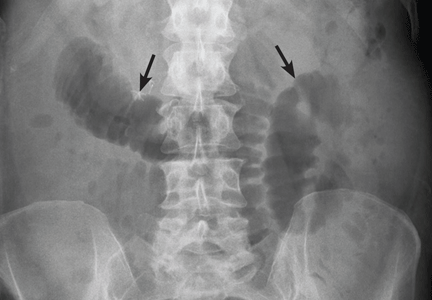
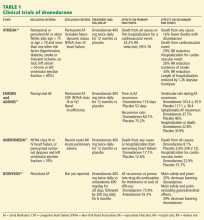
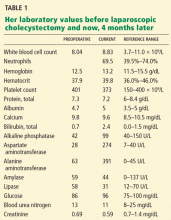
User login
Minivan, Major Lesson
I recently visited my parents in my ancestral home of Wisconsin. As parents of a certain age, they inexplicably are genetically predisposed to owning a minivan. Another quirk of their DNA is that they must own a new minivan. No sooner has the last wisp of new-car smell osmosed from the burled walnut interior than they are trading up to the newest, tricked-out minivan. Perhaps more puzzling is the manner of pride they display in their minivan.
Now, my dad, as if not readily apparent, is not cool. And to see him folded into the driver’s seat, his furry-ear-to-furry-ear grin signaling a self-satisfaction customarily reserved for his grandchildren, painstakingly recounting glory-day stories and 4:30 p.m. dinner buffets, further solidifies his place in the Annals of Uncool.
When I’m home, they tend to employ my chauffeur services (most likely in retribution for my peri-pubescent years), and on the first day back home, I stopped their newest ride near the back door of the house, foot idling on the brake while this exchange occurred: “That’s a fascinating story about how much more challenging the world was when you were my age, Dad. You are a true American hero. Would you like to get out here or in the garage?”
“Here,” he replied.
“OK, then get out,” I countered.
“I can’t,” he responded knowingly.
“Why not?” I queried, the patience seeping from my voice.
“Because the door’s not open,” he answered, seemingly mocking me.
“Then open it,” I replied, silently recounting the evidence for his institutionalization.
“I can’t,” he responded.
“Why not?” I replied again, this time calculating the likelihood that I was adopted.
“Because it’s locked,” came his retort.
“Then unlock it,” I answered, reconfirming my decision to move away for college.
“I can’t,” he replied, ostensibly encouraging parenticide.
“Why not?” I queried, strongly contemplating parenticide.
“Because you haven’t put the car in park,” he responded triumphantly.
A System So Safe
As a safety feature, the minivan needed to be in park before you could open the door to exit. I’ve never heard of anyone actually falling out of a moving car, but recollecting high school, I can fathom the right mix, type, and number of teenagers where possibility would meet inevitability. But, apparently, enough people are falling out of moving vehicles that car engineers have built a system that is so safe, this can’t happen. So no matter how hard someone tries, it just isn’t possible to fall out of a moving car (believe me, toward the end of a week of my father’s car stories, my mind had worked every possible angle).
Likewise, newer vehicles employ occupant-sensitive sensors that detect the weight, size, and position of the passenger to determine if the airbag should deploy. Rather than depending on the driver to turn the passenger-side airbag on or off, the car does it for you: heavy enough to trigger the sensor, and the airbag will deploy; too light, and the car assumes you are a child and doesn’t deploy. It’s a system that is so safe because it doesn’t depend on the operator to get it right.
Ditto motion sensors that detect objects behind the car while reversing (avoiding accidental back-overs), antilock brakes (to maintain control during panicked braking), traction control (improves stability during acceleration), electronic stability control (foils spinouts), tire-pressure-monitoring systems (avoids blowouts), daytime running lights (ensures others see you), rollover airbags (they stay inflated to keep you in the car), lane-departure warning (alerts you if you stray from your lane), and doors that automatically lock after the car starts (again, falling out of cars).
For all the negative press of late, car manufacturers understand safety.
A System Not So Safe
Contrast this to healthcare, in which 10% of patients will suffer a serious, preventable, adverse event during their hospital stay.1 Read that sentence again. That’s 10%; that’s preventable; that’s a number that has largely remained unchanged in the past decade. If 1 in 10 drivers suffered a serious adverse preventable auto accident, Congress would do nothing but hold automotive safety hearings.
In medicine, we still largely employ unsafe systems in which even the best doctors can, and do, hurt patients. Sure, we have made strides in this arena (oxygen tubing that only works if hooked up properly, smart pumps that avert IV dosing errors, CO2 monitors to detect proper endotracheal tube placement), but remarkably, in this era of patient safety, we still utilize systems that largely depend on the heroism of the individual.
As physicians, we are famously autonomous and value our professional independence, even to the degree that it might harm patients. We generally eschew standardization, believing that each patient is inherently different. In fact, the thrill of the improvisational theater that follows every patient’s chief compliant is one of the great satisfiers in medicine. We love that feeling that comes from sleuthing each case, deftly enacting a plan of action to shepherd the patient to health.
To suggest following protocols, guidelines, and checklists is derisively dismissed as “cookbook medicine.” To work in teams in which certain tasks are delegated to others is seen as weakness—we don’t need a system that utilizes a pharmacist; rather, we should know the doses of all medicines, their interactions, and the effect of renal and liver impairment on their clearance. To suggest otherwise is an insult to our Oslerian roots. To examine our errors, our system breakdowns, our patient harms is anathema to our practice, an admission of failure.
The result is that most of us continue to toil in systems that have become exponentially unsafe as healthcare has become more complex. Today, we still have a system that will more or less allow us to kill a patient by doing nothing more than forgetting the letter “g.” I can go to my hospital today and intend to write “4 grams of magnesium sulfate (MgSO4)” and inadvertently forget the “g” in “Mg.” This could result in an order for a lethal dose of morphine sulfate (MSO4). It’s that easy to hurt a patient. Now, you might say that would never happen, because the pharmacy would catch it. And this is likely. But is it guaranteed? Can you 100% ensure it wouldn’t happen? Consider that nearly 20% of medication doses administered in a hospital are done so incorrectly.2 Nearly 1 in 5. This is the type of system we are employing to stop this lethal overdose. Is this system, which depends on another human to prevent an error, foolproof, or just a snare waiting to prove you the fool?
This represents our opportunity. As hospitalists, the hospital is our tapestry, our system of care, our responsibility. Few others are as well-positioned to ensure that the systems that envelop our patients are highly functional, reliable, and safe. This will take work—work that will feel burdensome, underappreciated, undercompensated. And, fully recognizing that none of us went into medicine to become systems engineers, this will be hard.
However, if not us, who? Who will ensure that our fathers, our mothers, our children will be as safe in the hospital as they are on the drive to the hospital? TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
References
- Global health leaders join the World Health Organization to announce accelerated efforts to improve patient safety. World Health Organization website. Available at: www.who.int/mediacentre/news/releases/2004/pr74/en/. Accessed Feb. 14, 2011.
- Barker KN, Flynn EA, Pepper GA, Bates DW, Mikeal RL. Medication errors observed in 36 health care facilities. Arch Intern Med. 2002;162(16):1897-1903.
I recently visited my parents in my ancestral home of Wisconsin. As parents of a certain age, they inexplicably are genetically predisposed to owning a minivan. Another quirk of their DNA is that they must own a new minivan. No sooner has the last wisp of new-car smell osmosed from the burled walnut interior than they are trading up to the newest, tricked-out minivan. Perhaps more puzzling is the manner of pride they display in their minivan.
Now, my dad, as if not readily apparent, is not cool. And to see him folded into the driver’s seat, his furry-ear-to-furry-ear grin signaling a self-satisfaction customarily reserved for his grandchildren, painstakingly recounting glory-day stories and 4:30 p.m. dinner buffets, further solidifies his place in the Annals of Uncool.
When I’m home, they tend to employ my chauffeur services (most likely in retribution for my peri-pubescent years), and on the first day back home, I stopped their newest ride near the back door of the house, foot idling on the brake while this exchange occurred: “That’s a fascinating story about how much more challenging the world was when you were my age, Dad. You are a true American hero. Would you like to get out here or in the garage?”
“Here,” he replied.
“OK, then get out,” I countered.
“I can’t,” he responded knowingly.
“Why not?” I queried, the patience seeping from my voice.
“Because the door’s not open,” he answered, seemingly mocking me.
“Then open it,” I replied, silently recounting the evidence for his institutionalization.
“I can’t,” he responded.
“Why not?” I replied again, this time calculating the likelihood that I was adopted.
“Because it’s locked,” came his retort.
“Then unlock it,” I answered, reconfirming my decision to move away for college.
“I can’t,” he replied, ostensibly encouraging parenticide.
“Why not?” I queried, strongly contemplating parenticide.
“Because you haven’t put the car in park,” he responded triumphantly.
A System So Safe
As a safety feature, the minivan needed to be in park before you could open the door to exit. I’ve never heard of anyone actually falling out of a moving car, but recollecting high school, I can fathom the right mix, type, and number of teenagers where possibility would meet inevitability. But, apparently, enough people are falling out of moving vehicles that car engineers have built a system that is so safe, this can’t happen. So no matter how hard someone tries, it just isn’t possible to fall out of a moving car (believe me, toward the end of a week of my father’s car stories, my mind had worked every possible angle).
Likewise, newer vehicles employ occupant-sensitive sensors that detect the weight, size, and position of the passenger to determine if the airbag should deploy. Rather than depending on the driver to turn the passenger-side airbag on or off, the car does it for you: heavy enough to trigger the sensor, and the airbag will deploy; too light, and the car assumes you are a child and doesn’t deploy. It’s a system that is so safe because it doesn’t depend on the operator to get it right.
Ditto motion sensors that detect objects behind the car while reversing (avoiding accidental back-overs), antilock brakes (to maintain control during panicked braking), traction control (improves stability during acceleration), electronic stability control (foils spinouts), tire-pressure-monitoring systems (avoids blowouts), daytime running lights (ensures others see you), rollover airbags (they stay inflated to keep you in the car), lane-departure warning (alerts you if you stray from your lane), and doors that automatically lock after the car starts (again, falling out of cars).
For all the negative press of late, car manufacturers understand safety.
A System Not So Safe
Contrast this to healthcare, in which 10% of patients will suffer a serious, preventable, adverse event during their hospital stay.1 Read that sentence again. That’s 10%; that’s preventable; that’s a number that has largely remained unchanged in the past decade. If 1 in 10 drivers suffered a serious adverse preventable auto accident, Congress would do nothing but hold automotive safety hearings.
In medicine, we still largely employ unsafe systems in which even the best doctors can, and do, hurt patients. Sure, we have made strides in this arena (oxygen tubing that only works if hooked up properly, smart pumps that avert IV dosing errors, CO2 monitors to detect proper endotracheal tube placement), but remarkably, in this era of patient safety, we still utilize systems that largely depend on the heroism of the individual.
As physicians, we are famously autonomous and value our professional independence, even to the degree that it might harm patients. We generally eschew standardization, believing that each patient is inherently different. In fact, the thrill of the improvisational theater that follows every patient’s chief compliant is one of the great satisfiers in medicine. We love that feeling that comes from sleuthing each case, deftly enacting a plan of action to shepherd the patient to health.
To suggest following protocols, guidelines, and checklists is derisively dismissed as “cookbook medicine.” To work in teams in which certain tasks are delegated to others is seen as weakness—we don’t need a system that utilizes a pharmacist; rather, we should know the doses of all medicines, their interactions, and the effect of renal and liver impairment on their clearance. To suggest otherwise is an insult to our Oslerian roots. To examine our errors, our system breakdowns, our patient harms is anathema to our practice, an admission of failure.
The result is that most of us continue to toil in systems that have become exponentially unsafe as healthcare has become more complex. Today, we still have a system that will more or less allow us to kill a patient by doing nothing more than forgetting the letter “g.” I can go to my hospital today and intend to write “4 grams of magnesium sulfate (MgSO4)” and inadvertently forget the “g” in “Mg.” This could result in an order for a lethal dose of morphine sulfate (MSO4). It’s that easy to hurt a patient. Now, you might say that would never happen, because the pharmacy would catch it. And this is likely. But is it guaranteed? Can you 100% ensure it wouldn’t happen? Consider that nearly 20% of medication doses administered in a hospital are done so incorrectly.2 Nearly 1 in 5. This is the type of system we are employing to stop this lethal overdose. Is this system, which depends on another human to prevent an error, foolproof, or just a snare waiting to prove you the fool?
This represents our opportunity. As hospitalists, the hospital is our tapestry, our system of care, our responsibility. Few others are as well-positioned to ensure that the systems that envelop our patients are highly functional, reliable, and safe. This will take work—work that will feel burdensome, underappreciated, undercompensated. And, fully recognizing that none of us went into medicine to become systems engineers, this will be hard.
However, if not us, who? Who will ensure that our fathers, our mothers, our children will be as safe in the hospital as they are on the drive to the hospital? TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
References
- Global health leaders join the World Health Organization to announce accelerated efforts to improve patient safety. World Health Organization website. Available at: www.who.int/mediacentre/news/releases/2004/pr74/en/. Accessed Feb. 14, 2011.
- Barker KN, Flynn EA, Pepper GA, Bates DW, Mikeal RL. Medication errors observed in 36 health care facilities. Arch Intern Med. 2002;162(16):1897-1903.
I recently visited my parents in my ancestral home of Wisconsin. As parents of a certain age, they inexplicably are genetically predisposed to owning a minivan. Another quirk of their DNA is that they must own a new minivan. No sooner has the last wisp of new-car smell osmosed from the burled walnut interior than they are trading up to the newest, tricked-out minivan. Perhaps more puzzling is the manner of pride they display in their minivan.
Now, my dad, as if not readily apparent, is not cool. And to see him folded into the driver’s seat, his furry-ear-to-furry-ear grin signaling a self-satisfaction customarily reserved for his grandchildren, painstakingly recounting glory-day stories and 4:30 p.m. dinner buffets, further solidifies his place in the Annals of Uncool.
When I’m home, they tend to employ my chauffeur services (most likely in retribution for my peri-pubescent years), and on the first day back home, I stopped their newest ride near the back door of the house, foot idling on the brake while this exchange occurred: “That’s a fascinating story about how much more challenging the world was when you were my age, Dad. You are a true American hero. Would you like to get out here or in the garage?”
“Here,” he replied.
“OK, then get out,” I countered.
“I can’t,” he responded knowingly.
“Why not?” I queried, the patience seeping from my voice.
“Because the door’s not open,” he answered, seemingly mocking me.
“Then open it,” I replied, silently recounting the evidence for his institutionalization.
“I can’t,” he responded.
“Why not?” I replied again, this time calculating the likelihood that I was adopted.
“Because it’s locked,” came his retort.
“Then unlock it,” I answered, reconfirming my decision to move away for college.
“I can’t,” he replied, ostensibly encouraging parenticide.
“Why not?” I queried, strongly contemplating parenticide.
“Because you haven’t put the car in park,” he responded triumphantly.
A System So Safe
As a safety feature, the minivan needed to be in park before you could open the door to exit. I’ve never heard of anyone actually falling out of a moving car, but recollecting high school, I can fathom the right mix, type, and number of teenagers where possibility would meet inevitability. But, apparently, enough people are falling out of moving vehicles that car engineers have built a system that is so safe, this can’t happen. So no matter how hard someone tries, it just isn’t possible to fall out of a moving car (believe me, toward the end of a week of my father’s car stories, my mind had worked every possible angle).
Likewise, newer vehicles employ occupant-sensitive sensors that detect the weight, size, and position of the passenger to determine if the airbag should deploy. Rather than depending on the driver to turn the passenger-side airbag on or off, the car does it for you: heavy enough to trigger the sensor, and the airbag will deploy; too light, and the car assumes you are a child and doesn’t deploy. It’s a system that is so safe because it doesn’t depend on the operator to get it right.
Ditto motion sensors that detect objects behind the car while reversing (avoiding accidental back-overs), antilock brakes (to maintain control during panicked braking), traction control (improves stability during acceleration), electronic stability control (foils spinouts), tire-pressure-monitoring systems (avoids blowouts), daytime running lights (ensures others see you), rollover airbags (they stay inflated to keep you in the car), lane-departure warning (alerts you if you stray from your lane), and doors that automatically lock after the car starts (again, falling out of cars).
For all the negative press of late, car manufacturers understand safety.
A System Not So Safe
Contrast this to healthcare, in which 10% of patients will suffer a serious, preventable, adverse event during their hospital stay.1 Read that sentence again. That’s 10%; that’s preventable; that’s a number that has largely remained unchanged in the past decade. If 1 in 10 drivers suffered a serious adverse preventable auto accident, Congress would do nothing but hold automotive safety hearings.
In medicine, we still largely employ unsafe systems in which even the best doctors can, and do, hurt patients. Sure, we have made strides in this arena (oxygen tubing that only works if hooked up properly, smart pumps that avert IV dosing errors, CO2 monitors to detect proper endotracheal tube placement), but remarkably, in this era of patient safety, we still utilize systems that largely depend on the heroism of the individual.
As physicians, we are famously autonomous and value our professional independence, even to the degree that it might harm patients. We generally eschew standardization, believing that each patient is inherently different. In fact, the thrill of the improvisational theater that follows every patient’s chief compliant is one of the great satisfiers in medicine. We love that feeling that comes from sleuthing each case, deftly enacting a plan of action to shepherd the patient to health.
To suggest following protocols, guidelines, and checklists is derisively dismissed as “cookbook medicine.” To work in teams in which certain tasks are delegated to others is seen as weakness—we don’t need a system that utilizes a pharmacist; rather, we should know the doses of all medicines, their interactions, and the effect of renal and liver impairment on their clearance. To suggest otherwise is an insult to our Oslerian roots. To examine our errors, our system breakdowns, our patient harms is anathema to our practice, an admission of failure.
The result is that most of us continue to toil in systems that have become exponentially unsafe as healthcare has become more complex. Today, we still have a system that will more or less allow us to kill a patient by doing nothing more than forgetting the letter “g.” I can go to my hospital today and intend to write “4 grams of magnesium sulfate (MgSO4)” and inadvertently forget the “g” in “Mg.” This could result in an order for a lethal dose of morphine sulfate (MSO4). It’s that easy to hurt a patient. Now, you might say that would never happen, because the pharmacy would catch it. And this is likely. But is it guaranteed? Can you 100% ensure it wouldn’t happen? Consider that nearly 20% of medication doses administered in a hospital are done so incorrectly.2 Nearly 1 in 5. This is the type of system we are employing to stop this lethal overdose. Is this system, which depends on another human to prevent an error, foolproof, or just a snare waiting to prove you the fool?
This represents our opportunity. As hospitalists, the hospital is our tapestry, our system of care, our responsibility. Few others are as well-positioned to ensure that the systems that envelop our patients are highly functional, reliable, and safe. This will take work—work that will feel burdensome, underappreciated, undercompensated. And, fully recognizing that none of us went into medicine to become systems engineers, this will be hard.
However, if not us, who? Who will ensure that our fathers, our mothers, our children will be as safe in the hospital as they are on the drive to the hospital? TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
References
- Global health leaders join the World Health Organization to announce accelerated efforts to improve patient safety. World Health Organization website. Available at: www.who.int/mediacentre/news/releases/2004/pr74/en/. Accessed Feb. 14, 2011.
- Barker KN, Flynn EA, Pepper GA, Bates DW, Mikeal RL. Medication errors observed in 36 health care facilities. Arch Intern Med. 2002;162(16):1897-1903.
Par Excellence
I’m often asked about the attributes associated with high-functioning practices, so I thought I’d offer a list of them in this column. I’ve written entire columns about some of them in prior issues of The Hospitalist, so I will provide only brief commentary about each of them here.
I think this list can serve as a valuable frame of reference for any hospitalist practice, though it is geared more toward nonacademic settings. It is based on my own career as a hospitalist, which spans more than 20 years, and 15 years’ work as a consultant with nearly 300 institutions around the country. While I think my experience has given me a valuable perspective, others might reasonably omit some attributes listed here or add others.
I believe the single most important measure of a practice is excellent outcomes for its patients. That said, all of the attributes I describe here have more to do with excellent operational, or business, performance. It is possible for a practice to have all of these attributes and still provide disappointing clinical quality for its patients, but that seems really unlikely to me. And even a practice that provides superior clinical care probably won’t be able to do so for long without high-functioning business operations.
I think each of these attributes might be a cause of a practice’s excellent performance, but it is possible that some are a result of it. They are listed in no particular order.
A culture of practice ownership. The most important attribute associated with a high-functioning practice is that the providers in the group maintain a mindset of practice ownership. Even if you are employees of a hospital or other organization, you should think of yourselves as owners of the practice’s performance. When problems arise, you shouldn’t simply assume it is up to the practice leader alone, or an administrator outside of the practice to solve it. Instead, each doctor should always be thinking about how to improve the practice and taking action to make it happen. For more, see “Foster Ownership Culture” in the August 2008 issue, or visit my website and take a quiz (http://nelsonflores.com/html/quiz.html) to assess your ownership culture.
An effective group leader. All groups need a leader who takes the role seriously and doesn’t just view the job description as making the work schedule and attending more meetings than the other hospitalists. (Unfortunately, my experience is that this is precisely what a lot of leaders think.) Of the many markers that effective leaders display, one that seems pretty reliable to me is whether the group has routinely scheduled meetings, with an agenda provided in advance and minutes circulated a few days later. I wrote about effective group leaders in a June 2008 column titled “Follow the Money.”
Autonomy in making decisions. Even when you are an employee of a larger entity, the practice should be structured so that hospitalists have as much autonomy in decision-making as possible. For example, you should always be able to adjust the group’s work schedule (e.g. when shifts start and stop). You also should have a lot of say about your staffing and workload. The latter typically requires that the group is connected to the financial consequences of its choices, which usually means a compensation system based, to a significant degree, on productivity.
While still common for hospitalists, when the largest salary component is fixed, it will always follow that someone outside the group (e.g. an administrator at the hospital) will end up deciding how hard you will have to work to justify the promised salary. And the hospitalists will almost always find fault with that person’s decision—a recipe for constant frustration that inhibits the development of an ownership culture, among other things.
Each doctor sees the job as more than just providing care to patients. In addition to providing quality care for your patients, each hospitalist in the group must work to improve the performance of the hospital. This means work on clinical protocols, medical staff functions (e.g. credentials committee), documentation and coding for both CPT and DRG billing, etc.
Strong social connections. Every high-performing practice I’ve worked with is notable for the social connections between the hospitalists themselves, as well as between hospitalists and other physicians, nursing staff, and administrators. This shouldn’t be taken lightly. Social connections matter—a lot. And while the hospitalists in most groups feel reasonably connected with one another, too often they feel isolated from the other doctors and administrators at the hospital. I wrote some more thoughts about this in a June 2010 column, “Square Peg, Square Hole.”
Hospitalists actively involved in recruiting for their practice. The hospitalists themselves—at a minimum, the group leader—should be very involved in recruiting new members for the group. Professional recruiters are very valuable but can be a lot more effective if the hospitalists themselves participate in the process. The group will land better candidates that way. For more, see “We’re Hiring,” from July 2008.
Hospitalists know data about their performance. Too many practices fail to provide routine data about each provider’s clinical and financial performance. Make sure your group isn’t in this category. Develop a routine report of key metrics for your practice. Usually it is fine, and best, to provide to the whole group unblinded performance data about each individual hospitalist. For more information, check out “Measuring Hospitalist Performance: Metrics, Reports, and Dashboards” on the SHM website.
Don’t rely solely on consensus-based decision-making. Relying on consensus is reasonable for most decisions, if a group has about eight to 10 members. Larger groups need to decide how they’ll make decisions if consensus can’t be reached easily. And they need to have the discipline to stick to their agreed upon process, usually a vote. For more on this subject, see “Play by the Rules” in the December 2007 issue. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
I’m often asked about the attributes associated with high-functioning practices, so I thought I’d offer a list of them in this column. I’ve written entire columns about some of them in prior issues of The Hospitalist, so I will provide only brief commentary about each of them here.
I think this list can serve as a valuable frame of reference for any hospitalist practice, though it is geared more toward nonacademic settings. It is based on my own career as a hospitalist, which spans more than 20 years, and 15 years’ work as a consultant with nearly 300 institutions around the country. While I think my experience has given me a valuable perspective, others might reasonably omit some attributes listed here or add others.
I believe the single most important measure of a practice is excellent outcomes for its patients. That said, all of the attributes I describe here have more to do with excellent operational, or business, performance. It is possible for a practice to have all of these attributes and still provide disappointing clinical quality for its patients, but that seems really unlikely to me. And even a practice that provides superior clinical care probably won’t be able to do so for long without high-functioning business operations.
I think each of these attributes might be a cause of a practice’s excellent performance, but it is possible that some are a result of it. They are listed in no particular order.
A culture of practice ownership. The most important attribute associated with a high-functioning practice is that the providers in the group maintain a mindset of practice ownership. Even if you are employees of a hospital or other organization, you should think of yourselves as owners of the practice’s performance. When problems arise, you shouldn’t simply assume it is up to the practice leader alone, or an administrator outside of the practice to solve it. Instead, each doctor should always be thinking about how to improve the practice and taking action to make it happen. For more, see “Foster Ownership Culture” in the August 2008 issue, or visit my website and take a quiz (http://nelsonflores.com/html/quiz.html) to assess your ownership culture.
An effective group leader. All groups need a leader who takes the role seriously and doesn’t just view the job description as making the work schedule and attending more meetings than the other hospitalists. (Unfortunately, my experience is that this is precisely what a lot of leaders think.) Of the many markers that effective leaders display, one that seems pretty reliable to me is whether the group has routinely scheduled meetings, with an agenda provided in advance and minutes circulated a few days later. I wrote about effective group leaders in a June 2008 column titled “Follow the Money.”
Autonomy in making decisions. Even when you are an employee of a larger entity, the practice should be structured so that hospitalists have as much autonomy in decision-making as possible. For example, you should always be able to adjust the group’s work schedule (e.g. when shifts start and stop). You also should have a lot of say about your staffing and workload. The latter typically requires that the group is connected to the financial consequences of its choices, which usually means a compensation system based, to a significant degree, on productivity.
While still common for hospitalists, when the largest salary component is fixed, it will always follow that someone outside the group (e.g. an administrator at the hospital) will end up deciding how hard you will have to work to justify the promised salary. And the hospitalists will almost always find fault with that person’s decision—a recipe for constant frustration that inhibits the development of an ownership culture, among other things.
Each doctor sees the job as more than just providing care to patients. In addition to providing quality care for your patients, each hospitalist in the group must work to improve the performance of the hospital. This means work on clinical protocols, medical staff functions (e.g. credentials committee), documentation and coding for both CPT and DRG billing, etc.
Strong social connections. Every high-performing practice I’ve worked with is notable for the social connections between the hospitalists themselves, as well as between hospitalists and other physicians, nursing staff, and administrators. This shouldn’t be taken lightly. Social connections matter—a lot. And while the hospitalists in most groups feel reasonably connected with one another, too often they feel isolated from the other doctors and administrators at the hospital. I wrote some more thoughts about this in a June 2010 column, “Square Peg, Square Hole.”
Hospitalists actively involved in recruiting for their practice. The hospitalists themselves—at a minimum, the group leader—should be very involved in recruiting new members for the group. Professional recruiters are very valuable but can be a lot more effective if the hospitalists themselves participate in the process. The group will land better candidates that way. For more, see “We’re Hiring,” from July 2008.
Hospitalists know data about their performance. Too many practices fail to provide routine data about each provider’s clinical and financial performance. Make sure your group isn’t in this category. Develop a routine report of key metrics for your practice. Usually it is fine, and best, to provide to the whole group unblinded performance data about each individual hospitalist. For more information, check out “Measuring Hospitalist Performance: Metrics, Reports, and Dashboards” on the SHM website.
Don’t rely solely on consensus-based decision-making. Relying on consensus is reasonable for most decisions, if a group has about eight to 10 members. Larger groups need to decide how they’ll make decisions if consensus can’t be reached easily. And they need to have the discipline to stick to their agreed upon process, usually a vote. For more on this subject, see “Play by the Rules” in the December 2007 issue. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
I’m often asked about the attributes associated with high-functioning practices, so I thought I’d offer a list of them in this column. I’ve written entire columns about some of them in prior issues of The Hospitalist, so I will provide only brief commentary about each of them here.
I think this list can serve as a valuable frame of reference for any hospitalist practice, though it is geared more toward nonacademic settings. It is based on my own career as a hospitalist, which spans more than 20 years, and 15 years’ work as a consultant with nearly 300 institutions around the country. While I think my experience has given me a valuable perspective, others might reasonably omit some attributes listed here or add others.
I believe the single most important measure of a practice is excellent outcomes for its patients. That said, all of the attributes I describe here have more to do with excellent operational, or business, performance. It is possible for a practice to have all of these attributes and still provide disappointing clinical quality for its patients, but that seems really unlikely to me. And even a practice that provides superior clinical care probably won’t be able to do so for long without high-functioning business operations.
I think each of these attributes might be a cause of a practice’s excellent performance, but it is possible that some are a result of it. They are listed in no particular order.
A culture of practice ownership. The most important attribute associated with a high-functioning practice is that the providers in the group maintain a mindset of practice ownership. Even if you are employees of a hospital or other organization, you should think of yourselves as owners of the practice’s performance. When problems arise, you shouldn’t simply assume it is up to the practice leader alone, or an administrator outside of the practice to solve it. Instead, each doctor should always be thinking about how to improve the practice and taking action to make it happen. For more, see “Foster Ownership Culture” in the August 2008 issue, or visit my website and take a quiz (http://nelsonflores.com/html/quiz.html) to assess your ownership culture.
An effective group leader. All groups need a leader who takes the role seriously and doesn’t just view the job description as making the work schedule and attending more meetings than the other hospitalists. (Unfortunately, my experience is that this is precisely what a lot of leaders think.) Of the many markers that effective leaders display, one that seems pretty reliable to me is whether the group has routinely scheduled meetings, with an agenda provided in advance and minutes circulated a few days later. I wrote about effective group leaders in a June 2008 column titled “Follow the Money.”
Autonomy in making decisions. Even when you are an employee of a larger entity, the practice should be structured so that hospitalists have as much autonomy in decision-making as possible. For example, you should always be able to adjust the group’s work schedule (e.g. when shifts start and stop). You also should have a lot of say about your staffing and workload. The latter typically requires that the group is connected to the financial consequences of its choices, which usually means a compensation system based, to a significant degree, on productivity.
While still common for hospitalists, when the largest salary component is fixed, it will always follow that someone outside the group (e.g. an administrator at the hospital) will end up deciding how hard you will have to work to justify the promised salary. And the hospitalists will almost always find fault with that person’s decision—a recipe for constant frustration that inhibits the development of an ownership culture, among other things.
Each doctor sees the job as more than just providing care to patients. In addition to providing quality care for your patients, each hospitalist in the group must work to improve the performance of the hospital. This means work on clinical protocols, medical staff functions (e.g. credentials committee), documentation and coding for both CPT and DRG billing, etc.
Strong social connections. Every high-performing practice I’ve worked with is notable for the social connections between the hospitalists themselves, as well as between hospitalists and other physicians, nursing staff, and administrators. This shouldn’t be taken lightly. Social connections matter—a lot. And while the hospitalists in most groups feel reasonably connected with one another, too often they feel isolated from the other doctors and administrators at the hospital. I wrote some more thoughts about this in a June 2010 column, “Square Peg, Square Hole.”
Hospitalists actively involved in recruiting for their practice. The hospitalists themselves—at a minimum, the group leader—should be very involved in recruiting new members for the group. Professional recruiters are very valuable but can be a lot more effective if the hospitalists themselves participate in the process. The group will land better candidates that way. For more, see “We’re Hiring,” from July 2008.
Hospitalists know data about their performance. Too many practices fail to provide routine data about each provider’s clinical and financial performance. Make sure your group isn’t in this category. Develop a routine report of key metrics for your practice. Usually it is fine, and best, to provide to the whole group unblinded performance data about each individual hospitalist. For more information, check out “Measuring Hospitalist Performance: Metrics, Reports, and Dashboards” on the SHM website.
Don’t rely solely on consensus-based decision-making. Relying on consensus is reasonable for most decisions, if a group has about eight to 10 members. Larger groups need to decide how they’ll make decisions if consensus can’t be reached easily. And they need to have the discipline to stick to their agreed upon process, usually a vote. For more on this subject, see “Play by the Rules” in the December 2007 issue. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants, a national hospitalist practice management consulting firm (www.nelsonflores.com). He is also course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Burned by bait-and-switch contract? Best approach is to avoid burning bridges
I am leaving my current hospitalist company, and they are telling me that I have to pay them back the $10,000 sign-on/relocation bonus that was originally given to me. When I looked at the contract that I was given at the onset of our interview meetings, there was no clause stating a timeframe or any mention of me paying this back if I left the company. However, when I stated that I was giving my three-month notice, they said that their reasoning was based on a clause in the contract that says if I left before the end of the first year, then I would need to return the bonus.
I have worked for this company for nine months and moved to this city specifically for the purpose of this job. I didn’t believe what they were saying because I had read over the initial contract very well ... but then I compared that initial contract and the one that I actually signed. They changed some things that we never talked about; for instance, they changed the insurance from occurrence type to claims made, and they added a sentence at the end of the paragraph discussing the bonus.
I just think this was not very honest of them. Do I have any recourse? I wouldn’t even mind paying back a portion of it, but it makes me so mad that they obviously were being sneaky in changing the 14-page contract.
Cindy Nichols, MD
Austin, Texas
Dr. Hospitalist responds: I am sorry to hear of the troubles. When I read your note, I thought unfortunately of the English playwright Noel Coward, who once said, “It is discouraging to think how many people are shocked by honesty and how few by deceit.” Unfortunately, this happens all too commonly. Sounds like you are the unfortunate victim of nothing more than a bait-and-switch. I am not a lawyer and am not offering legal advice, but I suggest you contact a healthcare attorney familiar with employment contracts in Texas. But it would seem to me that since you did sign the contract, I suspect you have little recourse.
Although you are going to be out some money, I actually think this is more damaging to your employer than it is to you. You might have to repay the $10,000, but this could cost your employer much more. In today’s environment, in which anyone who has access to the Internet or a smartphone, one’s reputation can be reviled or revered at the whim of one’s keystroke. It does not seem to me that it makes much sense to engage in business in such a deceitful manner. Word can spread easily about a dishonest employer, even though the contract handed to you could have been the action of a single dishonest employee, rather than a corporate strategy. If the company is fortunate, you will take the time to let someone senior in the company know what happened to you. With this information, they can review their practice and assure that it doesn’t happen to another employee.
More likely, I expect that you are not likely to waste your time speaking with anyone else in the company, but that instead you will tell friends and colleagues about how you were victimized by this employer. It is a small world and this company’s image will suffer immeasurably from these water-cooler discussions. But I encourage you to take the high road: Contact a higher-up in the company and hold a professional discussion with them.
Best-case scenario: They respect your feedback and honor the original contract. Worst-case scenario: You walk away from people you really don’t want to work with, and you walk away with your dignity intact. TH
I am leaving my current hospitalist company, and they are telling me that I have to pay them back the $10,000 sign-on/relocation bonus that was originally given to me. When I looked at the contract that I was given at the onset of our interview meetings, there was no clause stating a timeframe or any mention of me paying this back if I left the company. However, when I stated that I was giving my three-month notice, they said that their reasoning was based on a clause in the contract that says if I left before the end of the first year, then I would need to return the bonus.
I have worked for this company for nine months and moved to this city specifically for the purpose of this job. I didn’t believe what they were saying because I had read over the initial contract very well ... but then I compared that initial contract and the one that I actually signed. They changed some things that we never talked about; for instance, they changed the insurance from occurrence type to claims made, and they added a sentence at the end of the paragraph discussing the bonus.
I just think this was not very honest of them. Do I have any recourse? I wouldn’t even mind paying back a portion of it, but it makes me so mad that they obviously were being sneaky in changing the 14-page contract.
Cindy Nichols, MD
Austin, Texas
Dr. Hospitalist responds: I am sorry to hear of the troubles. When I read your note, I thought unfortunately of the English playwright Noel Coward, who once said, “It is discouraging to think how many people are shocked by honesty and how few by deceit.” Unfortunately, this happens all too commonly. Sounds like you are the unfortunate victim of nothing more than a bait-and-switch. I am not a lawyer and am not offering legal advice, but I suggest you contact a healthcare attorney familiar with employment contracts in Texas. But it would seem to me that since you did sign the contract, I suspect you have little recourse.
Although you are going to be out some money, I actually think this is more damaging to your employer than it is to you. You might have to repay the $10,000, but this could cost your employer much more. In today’s environment, in which anyone who has access to the Internet or a smartphone, one’s reputation can be reviled or revered at the whim of one’s keystroke. It does not seem to me that it makes much sense to engage in business in such a deceitful manner. Word can spread easily about a dishonest employer, even though the contract handed to you could have been the action of a single dishonest employee, rather than a corporate strategy. If the company is fortunate, you will take the time to let someone senior in the company know what happened to you. With this information, they can review their practice and assure that it doesn’t happen to another employee.
More likely, I expect that you are not likely to waste your time speaking with anyone else in the company, but that instead you will tell friends and colleagues about how you were victimized by this employer. It is a small world and this company’s image will suffer immeasurably from these water-cooler discussions. But I encourage you to take the high road: Contact a higher-up in the company and hold a professional discussion with them.
Best-case scenario: They respect your feedback and honor the original contract. Worst-case scenario: You walk away from people you really don’t want to work with, and you walk away with your dignity intact. TH
I am leaving my current hospitalist company, and they are telling me that I have to pay them back the $10,000 sign-on/relocation bonus that was originally given to me. When I looked at the contract that I was given at the onset of our interview meetings, there was no clause stating a timeframe or any mention of me paying this back if I left the company. However, when I stated that I was giving my three-month notice, they said that their reasoning was based on a clause in the contract that says if I left before the end of the first year, then I would need to return the bonus.
I have worked for this company for nine months and moved to this city specifically for the purpose of this job. I didn’t believe what they were saying because I had read over the initial contract very well ... but then I compared that initial contract and the one that I actually signed. They changed some things that we never talked about; for instance, they changed the insurance from occurrence type to claims made, and they added a sentence at the end of the paragraph discussing the bonus.
I just think this was not very honest of them. Do I have any recourse? I wouldn’t even mind paying back a portion of it, but it makes me so mad that they obviously were being sneaky in changing the 14-page contract.
Cindy Nichols, MD
Austin, Texas
Dr. Hospitalist responds: I am sorry to hear of the troubles. When I read your note, I thought unfortunately of the English playwright Noel Coward, who once said, “It is discouraging to think how many people are shocked by honesty and how few by deceit.” Unfortunately, this happens all too commonly. Sounds like you are the unfortunate victim of nothing more than a bait-and-switch. I am not a lawyer and am not offering legal advice, but I suggest you contact a healthcare attorney familiar with employment contracts in Texas. But it would seem to me that since you did sign the contract, I suspect you have little recourse.
Although you are going to be out some money, I actually think this is more damaging to your employer than it is to you. You might have to repay the $10,000, but this could cost your employer much more. In today’s environment, in which anyone who has access to the Internet or a smartphone, one’s reputation can be reviled or revered at the whim of one’s keystroke. It does not seem to me that it makes much sense to engage in business in such a deceitful manner. Word can spread easily about a dishonest employer, even though the contract handed to you could have been the action of a single dishonest employee, rather than a corporate strategy. If the company is fortunate, you will take the time to let someone senior in the company know what happened to you. With this information, they can review their practice and assure that it doesn’t happen to another employee.
More likely, I expect that you are not likely to waste your time speaking with anyone else in the company, but that instead you will tell friends and colleagues about how you were victimized by this employer. It is a small world and this company’s image will suffer immeasurably from these water-cooler discussions. But I encourage you to take the high road: Contact a higher-up in the company and hold a professional discussion with them.
Best-case scenario: They respect your feedback and honor the original contract. Worst-case scenario: You walk away from people you really don’t want to work with, and you walk away with your dignity intact. TH
ONLINE EXCLUSIVE: Rep. Giffords, shooting survivors recover nicely
Enter text here
Enter text here
Enter text here
ONLINE EXCLUSIVE: Opinions Mixed on Application of Touchscreen Technology at Bedside
It sounds counterintuitive, but could touchscreen technology make HM more personal? Satish Misra, MD, thinks so. A first-year internal-medicine resident at Johns Hopkins School of Medicine in Baltimore, he also serves as a senior editor for iMedicalApps.com, a blog that publishes commentary and reviews of mobile medical technology and applications in the healthcare realm.
Dr. Misra envisions smartphones and tablet computers as a way of connecting more with patients. “It’s just a much more interactive platform than your standard keyboard and screen,” he says. “It’s something that’s easy to put in front of both myself and the patient, as opposed to between us. It sort of demystifies a lot of what you’re trying to say.
“You can imagine if you’re trying to explain endoscopy to a patient. It’s one thing to use yourself as an example … it’s another thing to have a 3-D image you can rotate with your finger and enlarge in certain places.”
Not all patients are ready to see those details, though. And that’s where other intangible lessons come in handy for hospitalists, says Steven Peskin, MD, MBA, FACP, executive vice president and chief medical officer of Yardley, Pa.-based MediMedia USA. He agrees there are times when the use of touchscreen technology to educate might enhance the patient encounter. But hospitalists need to use their judgment in deciding which patients can tolerate learning—for example, the exact path a catheterization tube takes on its way north from entry in that patient’s groin.
“Some might be scared to death; others might be reassured,” Dr. Peskin says. “The personalization is still largely in the personal skills, the very-old-school bedside manner.
The power of digital media to pictorially or visually or graphically show somebody something—sure, absolutely, that’s valuable. I wouldn’t equate that with patient-physician communication or healthcare professional communication. I think that still relies more on eye contact, empathy, and listening.”
Richard Quinn is a freelance writer based in New Jersey.
It sounds counterintuitive, but could touchscreen technology make HM more personal? Satish Misra, MD, thinks so. A first-year internal-medicine resident at Johns Hopkins School of Medicine in Baltimore, he also serves as a senior editor for iMedicalApps.com, a blog that publishes commentary and reviews of mobile medical technology and applications in the healthcare realm.
Dr. Misra envisions smartphones and tablet computers as a way of connecting more with patients. “It’s just a much more interactive platform than your standard keyboard and screen,” he says. “It’s something that’s easy to put in front of both myself and the patient, as opposed to between us. It sort of demystifies a lot of what you’re trying to say.
“You can imagine if you’re trying to explain endoscopy to a patient. It’s one thing to use yourself as an example … it’s another thing to have a 3-D image you can rotate with your finger and enlarge in certain places.”
Not all patients are ready to see those details, though. And that’s where other intangible lessons come in handy for hospitalists, says Steven Peskin, MD, MBA, FACP, executive vice president and chief medical officer of Yardley, Pa.-based MediMedia USA. He agrees there are times when the use of touchscreen technology to educate might enhance the patient encounter. But hospitalists need to use their judgment in deciding which patients can tolerate learning—for example, the exact path a catheterization tube takes on its way north from entry in that patient’s groin.
“Some might be scared to death; others might be reassured,” Dr. Peskin says. “The personalization is still largely in the personal skills, the very-old-school bedside manner.
The power of digital media to pictorially or visually or graphically show somebody something—sure, absolutely, that’s valuable. I wouldn’t equate that with patient-physician communication or healthcare professional communication. I think that still relies more on eye contact, empathy, and listening.”
Richard Quinn is a freelance writer based in New Jersey.
It sounds counterintuitive, but could touchscreen technology make HM more personal? Satish Misra, MD, thinks so. A first-year internal-medicine resident at Johns Hopkins School of Medicine in Baltimore, he also serves as a senior editor for iMedicalApps.com, a blog that publishes commentary and reviews of mobile medical technology and applications in the healthcare realm.
Dr. Misra envisions smartphones and tablet computers as a way of connecting more with patients. “It’s just a much more interactive platform than your standard keyboard and screen,” he says. “It’s something that’s easy to put in front of both myself and the patient, as opposed to between us. It sort of demystifies a lot of what you’re trying to say.
“You can imagine if you’re trying to explain endoscopy to a patient. It’s one thing to use yourself as an example … it’s another thing to have a 3-D image you can rotate with your finger and enlarge in certain places.”
Not all patients are ready to see those details, though. And that’s where other intangible lessons come in handy for hospitalists, says Steven Peskin, MD, MBA, FACP, executive vice president and chief medical officer of Yardley, Pa.-based MediMedia USA. He agrees there are times when the use of touchscreen technology to educate might enhance the patient encounter. But hospitalists need to use their judgment in deciding which patients can tolerate learning—for example, the exact path a catheterization tube takes on its way north from entry in that patient’s groin.
“Some might be scared to death; others might be reassured,” Dr. Peskin says. “The personalization is still largely in the personal skills, the very-old-school bedside manner.
The power of digital media to pictorially or visually or graphically show somebody something—sure, absolutely, that’s valuable. I wouldn’t equate that with patient-physician communication or healthcare professional communication. I think that still relies more on eye contact, empathy, and listening.”
Richard Quinn is a freelance writer based in New Jersey.
Teaching suicidal adolescents how to "walk the middle path"
Perioperative Medicine Summit 2011
Summit Director:
Amir K. Jaffer, MD
Contents
Abstract 1: Application of 2007 ACC/AHA guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery using decision support tools
BobbieJean Sweitzer, Michael Vigoda, Vicente Behrens, Nikola Miljkovic, and Kris Arheart
Abstract 2: Prevalence of obstructive dleep spnea in patients presenting for hip or knee replacement surgery
Micah Beachy, DO; Jason Shiffermiller, MD; and Chad Vokoun, MD
Abstract 3: A protocol to triage preoperative assessments to either nurses or nurse practitioners/physician assistants
Anthony Basil, RN; Pamela Pennigar, FNP; David R. Wright, MD; and Ronald P. Olson, MD
Abstract 4: Application of 2007 ACC/AHA guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery using decision support tools
BobbieJean Sweitzer, Michael Vigoda, Vicente Behrens, Nikola Miljkovic, and Kris Arheart
Abstract 5: Most anesthesiologists don’t correctly apply 2007 ACC/AHA guidelines on perioperative cardiac evaluation
BobbieJean Sweitzer, Michael Vigoda, Vicente Behrens, Nikola Miljkovic, Kris Arheart, and Richard Dutton
Abstract 6: Anesthesiology residents do not agree with their training programs on the degree to which the 2007 ACC/AHA guidelines are emphasized
BobbieJean Sweitzer, Michael Vigoda, Vicente Behrens, Nikola Miljkovic, and Kris Arheart
Abstract 7: Prevalence of obstructive sleep apnea in patients presenting for hip or knee replacement surgery
Micah Beachy, DO; Jason Shiffermiller, MD; and Chad Vokoun, MD
Abstract 8: A protocol to triage preoperative assessments to either nurses or nurse practitioners/physician assistants
Anthony Basil, RN; Pamela Pennigar, FNP; David R. Wright, MD; and Ronald P. Olson, MD
Abstract 9: Do ACEIs on the morning of surgery increase risk of intraoperative hypotension?
Steven L. Cohn, MD, and Kalia Skeete, MD
Abstract 10: One-year incidence of postoperative troponin revations in patients undergoing major orthopedic surgery
Michael Urban, MD, PhD; Stephen Wolfe, BS; Niel Sanghevi, BS; and Steven Magid, MD
Abstract 11: A review of preoperative clinic cardiology referrals for adults undergoing intermediate- and low-risk surgery
Susan Calderwood, MD; Jennifer Lee Morse, MS; and Damon R. Michaels, CCRP
Abstract 12: Patterns of preoperative consultation by risk and surgical specialty in a large health care system
Stephan Thilen, MD, MS; Christopher Bryson, MD, MS; Robert Reid, MD, PhD; and Miriam Treggiari, MD, MPH, PhD
Abstract 13: One-year incidence for admission to a critical care unit after major orthopedic surgery
Michael Urban, MD, PhD; Steven Magid, MD; and Michele Mangini, DNP
Abstract 14: Determination of the causes of long patient wait times in a preoperative evaluation clinic
Jean Kwo, MD; Devon Price, BS; Mary Elizabeth Ellbeg, RN; and Retsef Levi, PhD
Abstract 15: Does perioperative statin treatment affect hospital and ICU length of stay rollowing cardiac surgery: A systematic review
Vineet Chopra, MD, FACP, FHM; David Wesorick, MD; and Kim A. Eagle, MD
Abstract 16: Assessment of patient satisfaction of nurse screening vs complete preoperative assessment
Ronald Olson, MD, and Kathy Bock, RN
Abstract 17: Traumatic subdural hematoma: An update on morbidity
Rachel Thompson, MD; Christina Ryan, MD; Nancy Temkin, PhD; Richard Ellenbogen, MD; and Joann G. Elmore, MD, MPH
Abstract 18: Lipid emulsion as a lifesaving treatment for local anesthetic systemic toxicity (LAST)
Deepti Sachdev and Guy Weinberg, MD
Abstract 19: Perioperative ACLS recommendations should be modified for the treatment of local anesthetic toxicity
Adam Haas, MD, and Alexia Beccue, MD
Abstract 20: Preoperative EMR containing smart-set reminders improve accuracy of documentation by nonanesthesia clinicians during preoperative assessments
Angela Edwards, MD; Jill Grant, PA; and Ruth Hyde, MD
Abstract 21: POET: Procedure outcomes evaluation tool
Ahmad AbuSalah, MSc, and Terrence Adam, MD, PhD
Abstract 22: Results of a multidisciplinary preoperative assessment process for high-risk orthopedic patients
Terrence Adam, MD, PhD; Connie Parenti, MD; Terence Gioe, MD; and Karen Ringsred, MD
Abstract 23: Practical algorithm for preoperative evaluation of patients with liver disease
Madalina A. Vlase, PA-C, and Deborah C. Richman, MBChB, FFA(SA)
Abstract 24: Evaluation and management of isolated elevated aPTT
Sheila Hassan, MSN, NP; Patricia Kidik, MSN, NP; Catherine McGowan, MSN, NP; and Angela M. Bader, MD
Abstract 25: A perioperative triage plan for obstructive sleep apnea patients
Christian Altman, MD; R. Michael Boyer, DO; and Peter G. Kallas, MD
Abstract 26: Quantitative evaluation of handoff checklists
Jay Joshi, MD, and David Mayer, MD
Abstract 27: To deflate or not to deflate: Lap-Band® management in subsequent surgeries
Arjun Reddy, MD, and Deborah C. Richman, MBChB, FFA(SA)
Abstract 28: Takotsubo cardiomyopathy and resultant cardiogenic shock after mitral valve repair
Adam Evans, MD, MBA; Daniel B. Sims, MD; Nir Uriel, MD; Ulrich P. Jorde, MD; and Craig R. Smith, MD
Abstract 29: Intravenous vitamin K: Rapid reversal of warfarin and lack of subsequent warfarin resistance
Feras Abdul Khalek, MD; Interdeep Dhaliwal, MD; and Twylla Tassava, MD
Abstract 30: Cervical spine surgery: When not to extubate postoperatively
Carlos Mateo Mijares, MD; Doris Debs, ARNP, MSN-BC; Nicole Martin, MD; and Ronald Lee Samson, MD
Abstract 31: Total occlusion of oral cavity by mandibular sarcoma for resection: To intubate nasally or proceed to an awake tracheostomy?
Carlos Mateo Mijares, MD, and Maria DeLapena, MD
Abstract 32: Perioperative fatal embolic stroke associated with iron deficiency anemia and thrombocytosis
Carlos Mateo Mijares, MD; Nicole Martin, MD; and Ricardo Martinez-Ruiz, MD
Abstract 33: Conservative approach saves the day anesthesia-wise and surgical-wise
Carlos Mateo Mijares, MD; Bradley Shore, MD; Edward Zalkind, CRNA; and Nicole Martin, MD
Abstract 34: Predictors of acute kidney injury in patients undergoing total knee replacement surgery
Vishal Sehgal, MD; Pardeep Bansal, MD; Praveen Reddy, MD; Vishal Sharma, MD; Samuel Lesko, MD; John H. Doherty, MD; Theodore Tomaszewski, MD; Jack Prior, MD; Roger Getts, MD; and Jeremiah Eagan, MD
Abstract 35: Perioperative medical management of the Marfan patient undergoing repeat cardiothoracic surgery
Aashish Shah, MD, and Adam Skrzynski, MD
Summit Director:
Amir K. Jaffer, MD
Contents
Abstract 1: Application of 2007 ACC/AHA guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery using decision support tools
BobbieJean Sweitzer, Michael Vigoda, Vicente Behrens, Nikola Miljkovic, and Kris Arheart
Abstract 2: Prevalence of obstructive dleep spnea in patients presenting for hip or knee replacement surgery
Micah Beachy, DO; Jason Shiffermiller, MD; and Chad Vokoun, MD
Abstract 3: A protocol to triage preoperative assessments to either nurses or nurse practitioners/physician assistants
Anthony Basil, RN; Pamela Pennigar, FNP; David R. Wright, MD; and Ronald P. Olson, MD
Abstract 4: Application of 2007 ACC/AHA guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery using decision support tools
BobbieJean Sweitzer, Michael Vigoda, Vicente Behrens, Nikola Miljkovic, and Kris Arheart
Abstract 5: Most anesthesiologists don’t correctly apply 2007 ACC/AHA guidelines on perioperative cardiac evaluation
BobbieJean Sweitzer, Michael Vigoda, Vicente Behrens, Nikola Miljkovic, Kris Arheart, and Richard Dutton
Abstract 6: Anesthesiology residents do not agree with their training programs on the degree to which the 2007 ACC/AHA guidelines are emphasized
BobbieJean Sweitzer, Michael Vigoda, Vicente Behrens, Nikola Miljkovic, and Kris Arheart
Abstract 7: Prevalence of obstructive sleep apnea in patients presenting for hip or knee replacement surgery
Micah Beachy, DO; Jason Shiffermiller, MD; and Chad Vokoun, MD
Abstract 8: A protocol to triage preoperative assessments to either nurses or nurse practitioners/physician assistants
Anthony Basil, RN; Pamela Pennigar, FNP; David R. Wright, MD; and Ronald P. Olson, MD
Abstract 9: Do ACEIs on the morning of surgery increase risk of intraoperative hypotension?
Steven L. Cohn, MD, and Kalia Skeete, MD
Abstract 10: One-year incidence of postoperative troponin revations in patients undergoing major orthopedic surgery
Michael Urban, MD, PhD; Stephen Wolfe, BS; Niel Sanghevi, BS; and Steven Magid, MD
Abstract 11: A review of preoperative clinic cardiology referrals for adults undergoing intermediate- and low-risk surgery
Susan Calderwood, MD; Jennifer Lee Morse, MS; and Damon R. Michaels, CCRP
Abstract 12: Patterns of preoperative consultation by risk and surgical specialty in a large health care system
Stephan Thilen, MD, MS; Christopher Bryson, MD, MS; Robert Reid, MD, PhD; and Miriam Treggiari, MD, MPH, PhD
Abstract 13: One-year incidence for admission to a critical care unit after major orthopedic surgery
Michael Urban, MD, PhD; Steven Magid, MD; and Michele Mangini, DNP
Abstract 14: Determination of the causes of long patient wait times in a preoperative evaluation clinic
Jean Kwo, MD; Devon Price, BS; Mary Elizabeth Ellbeg, RN; and Retsef Levi, PhD
Abstract 15: Does perioperative statin treatment affect hospital and ICU length of stay rollowing cardiac surgery: A systematic review
Vineet Chopra, MD, FACP, FHM; David Wesorick, MD; and Kim A. Eagle, MD
Abstract 16: Assessment of patient satisfaction of nurse screening vs complete preoperative assessment
Ronald Olson, MD, and Kathy Bock, RN
Abstract 17: Traumatic subdural hematoma: An update on morbidity
Rachel Thompson, MD; Christina Ryan, MD; Nancy Temkin, PhD; Richard Ellenbogen, MD; and Joann G. Elmore, MD, MPH
Abstract 18: Lipid emulsion as a lifesaving treatment for local anesthetic systemic toxicity (LAST)
Deepti Sachdev and Guy Weinberg, MD
Abstract 19: Perioperative ACLS recommendations should be modified for the treatment of local anesthetic toxicity
Adam Haas, MD, and Alexia Beccue, MD
Abstract 20: Preoperative EMR containing smart-set reminders improve accuracy of documentation by nonanesthesia clinicians during preoperative assessments
Angela Edwards, MD; Jill Grant, PA; and Ruth Hyde, MD
Abstract 21: POET: Procedure outcomes evaluation tool
Ahmad AbuSalah, MSc, and Terrence Adam, MD, PhD
Abstract 22: Results of a multidisciplinary preoperative assessment process for high-risk orthopedic patients
Terrence Adam, MD, PhD; Connie Parenti, MD; Terence Gioe, MD; and Karen Ringsred, MD
Abstract 23: Practical algorithm for preoperative evaluation of patients with liver disease
Madalina A. Vlase, PA-C, and Deborah C. Richman, MBChB, FFA(SA)
Abstract 24: Evaluation and management of isolated elevated aPTT
Sheila Hassan, MSN, NP; Patricia Kidik, MSN, NP; Catherine McGowan, MSN, NP; and Angela M. Bader, MD
Abstract 25: A perioperative triage plan for obstructive sleep apnea patients
Christian Altman, MD; R. Michael Boyer, DO; and Peter G. Kallas, MD
Abstract 26: Quantitative evaluation of handoff checklists
Jay Joshi, MD, and David Mayer, MD
Abstract 27: To deflate or not to deflate: Lap-Band® management in subsequent surgeries
Arjun Reddy, MD, and Deborah C. Richman, MBChB, FFA(SA)
Abstract 28: Takotsubo cardiomyopathy and resultant cardiogenic shock after mitral valve repair
Adam Evans, MD, MBA; Daniel B. Sims, MD; Nir Uriel, MD; Ulrich P. Jorde, MD; and Craig R. Smith, MD
Abstract 29: Intravenous vitamin K: Rapid reversal of warfarin and lack of subsequent warfarin resistance
Feras Abdul Khalek, MD; Interdeep Dhaliwal, MD; and Twylla Tassava, MD
Abstract 30: Cervical spine surgery: When not to extubate postoperatively
Carlos Mateo Mijares, MD; Doris Debs, ARNP, MSN-BC; Nicole Martin, MD; and Ronald Lee Samson, MD
Abstract 31: Total occlusion of oral cavity by mandibular sarcoma for resection: To intubate nasally or proceed to an awake tracheostomy?
Carlos Mateo Mijares, MD, and Maria DeLapena, MD
Abstract 32: Perioperative fatal embolic stroke associated with iron deficiency anemia and thrombocytosis
Carlos Mateo Mijares, MD; Nicole Martin, MD; and Ricardo Martinez-Ruiz, MD
Abstract 33: Conservative approach saves the day anesthesia-wise and surgical-wise
Carlos Mateo Mijares, MD; Bradley Shore, MD; Edward Zalkind, CRNA; and Nicole Martin, MD
Abstract 34: Predictors of acute kidney injury in patients undergoing total knee replacement surgery
Vishal Sehgal, MD; Pardeep Bansal, MD; Praveen Reddy, MD; Vishal Sharma, MD; Samuel Lesko, MD; John H. Doherty, MD; Theodore Tomaszewski, MD; Jack Prior, MD; Roger Getts, MD; and Jeremiah Eagan, MD
Abstract 35: Perioperative medical management of the Marfan patient undergoing repeat cardiothoracic surgery
Aashish Shah, MD, and Adam Skrzynski, MD
Summit Director:
Amir K. Jaffer, MD
Contents
Abstract 1: Application of 2007 ACC/AHA guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery using decision support tools
BobbieJean Sweitzer, Michael Vigoda, Vicente Behrens, Nikola Miljkovic, and Kris Arheart
Abstract 2: Prevalence of obstructive dleep spnea in patients presenting for hip or knee replacement surgery
Micah Beachy, DO; Jason Shiffermiller, MD; and Chad Vokoun, MD
Abstract 3: A protocol to triage preoperative assessments to either nurses or nurse practitioners/physician assistants
Anthony Basil, RN; Pamela Pennigar, FNP; David R. Wright, MD; and Ronald P. Olson, MD
Abstract 4: Application of 2007 ACC/AHA guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery using decision support tools
BobbieJean Sweitzer, Michael Vigoda, Vicente Behrens, Nikola Miljkovic, and Kris Arheart
Abstract 5: Most anesthesiologists don’t correctly apply 2007 ACC/AHA guidelines on perioperative cardiac evaluation
BobbieJean Sweitzer, Michael Vigoda, Vicente Behrens, Nikola Miljkovic, Kris Arheart, and Richard Dutton
Abstract 6: Anesthesiology residents do not agree with their training programs on the degree to which the 2007 ACC/AHA guidelines are emphasized
BobbieJean Sweitzer, Michael Vigoda, Vicente Behrens, Nikola Miljkovic, and Kris Arheart
Abstract 7: Prevalence of obstructive sleep apnea in patients presenting for hip or knee replacement surgery
Micah Beachy, DO; Jason Shiffermiller, MD; and Chad Vokoun, MD
Abstract 8: A protocol to triage preoperative assessments to either nurses or nurse practitioners/physician assistants
Anthony Basil, RN; Pamela Pennigar, FNP; David R. Wright, MD; and Ronald P. Olson, MD
Abstract 9: Do ACEIs on the morning of surgery increase risk of intraoperative hypotension?
Steven L. Cohn, MD, and Kalia Skeete, MD
Abstract 10: One-year incidence of postoperative troponin revations in patients undergoing major orthopedic surgery
Michael Urban, MD, PhD; Stephen Wolfe, BS; Niel Sanghevi, BS; and Steven Magid, MD
Abstract 11: A review of preoperative clinic cardiology referrals for adults undergoing intermediate- and low-risk surgery
Susan Calderwood, MD; Jennifer Lee Morse, MS; and Damon R. Michaels, CCRP
Abstract 12: Patterns of preoperative consultation by risk and surgical specialty in a large health care system
Stephan Thilen, MD, MS; Christopher Bryson, MD, MS; Robert Reid, MD, PhD; and Miriam Treggiari, MD, MPH, PhD
Abstract 13: One-year incidence for admission to a critical care unit after major orthopedic surgery
Michael Urban, MD, PhD; Steven Magid, MD; and Michele Mangini, DNP
Abstract 14: Determination of the causes of long patient wait times in a preoperative evaluation clinic
Jean Kwo, MD; Devon Price, BS; Mary Elizabeth Ellbeg, RN; and Retsef Levi, PhD
Abstract 15: Does perioperative statin treatment affect hospital and ICU length of stay rollowing cardiac surgery: A systematic review
Vineet Chopra, MD, FACP, FHM; David Wesorick, MD; and Kim A. Eagle, MD
Abstract 16: Assessment of patient satisfaction of nurse screening vs complete preoperative assessment
Ronald Olson, MD, and Kathy Bock, RN
Abstract 17: Traumatic subdural hematoma: An update on morbidity
Rachel Thompson, MD; Christina Ryan, MD; Nancy Temkin, PhD; Richard Ellenbogen, MD; and Joann G. Elmore, MD, MPH
Abstract 18: Lipid emulsion as a lifesaving treatment for local anesthetic systemic toxicity (LAST)
Deepti Sachdev and Guy Weinberg, MD
Abstract 19: Perioperative ACLS recommendations should be modified for the treatment of local anesthetic toxicity
Adam Haas, MD, and Alexia Beccue, MD
Abstract 20: Preoperative EMR containing smart-set reminders improve accuracy of documentation by nonanesthesia clinicians during preoperative assessments
Angela Edwards, MD; Jill Grant, PA; and Ruth Hyde, MD
Abstract 21: POET: Procedure outcomes evaluation tool
Ahmad AbuSalah, MSc, and Terrence Adam, MD, PhD
Abstract 22: Results of a multidisciplinary preoperative assessment process for high-risk orthopedic patients
Terrence Adam, MD, PhD; Connie Parenti, MD; Terence Gioe, MD; and Karen Ringsred, MD
Abstract 23: Practical algorithm for preoperative evaluation of patients with liver disease
Madalina A. Vlase, PA-C, and Deborah C. Richman, MBChB, FFA(SA)
Abstract 24: Evaluation and management of isolated elevated aPTT
Sheila Hassan, MSN, NP; Patricia Kidik, MSN, NP; Catherine McGowan, MSN, NP; and Angela M. Bader, MD
Abstract 25: A perioperative triage plan for obstructive sleep apnea patients
Christian Altman, MD; R. Michael Boyer, DO; and Peter G. Kallas, MD
Abstract 26: Quantitative evaluation of handoff checklists
Jay Joshi, MD, and David Mayer, MD
Abstract 27: To deflate or not to deflate: Lap-Band® management in subsequent surgeries
Arjun Reddy, MD, and Deborah C. Richman, MBChB, FFA(SA)
Abstract 28: Takotsubo cardiomyopathy and resultant cardiogenic shock after mitral valve repair
Adam Evans, MD, MBA; Daniel B. Sims, MD; Nir Uriel, MD; Ulrich P. Jorde, MD; and Craig R. Smith, MD
Abstract 29: Intravenous vitamin K: Rapid reversal of warfarin and lack of subsequent warfarin resistance
Feras Abdul Khalek, MD; Interdeep Dhaliwal, MD; and Twylla Tassava, MD
Abstract 30: Cervical spine surgery: When not to extubate postoperatively
Carlos Mateo Mijares, MD; Doris Debs, ARNP, MSN-BC; Nicole Martin, MD; and Ronald Lee Samson, MD
Abstract 31: Total occlusion of oral cavity by mandibular sarcoma for resection: To intubate nasally or proceed to an awake tracheostomy?
Carlos Mateo Mijares, MD, and Maria DeLapena, MD
Abstract 32: Perioperative fatal embolic stroke associated with iron deficiency anemia and thrombocytosis
Carlos Mateo Mijares, MD; Nicole Martin, MD; and Ricardo Martinez-Ruiz, MD
Abstract 33: Conservative approach saves the day anesthesia-wise and surgical-wise
Carlos Mateo Mijares, MD; Bradley Shore, MD; Edward Zalkind, CRNA; and Nicole Martin, MD
Abstract 34: Predictors of acute kidney injury in patients undergoing total knee replacement surgery
Vishal Sehgal, MD; Pardeep Bansal, MD; Praveen Reddy, MD; Vishal Sharma, MD; Samuel Lesko, MD; John H. Doherty, MD; Theodore Tomaszewski, MD; Jack Prior, MD; Roger Getts, MD; and Jeremiah Eagan, MD
Abstract 35: Perioperative medical management of the Marfan patient undergoing repeat cardiothoracic surgery
Aashish Shah, MD, and Adam Skrzynski, MD
Malignant bowel obstruction: Individualized treatment near the end of life
Malignant bowel obstruction occurs in 5% to 51% of women with ovarian cancer and in 10% to 28% of patients with gastrointestinal cancer, predominantly in the advanced stages.1 Median survival after its onset ranges from 30 to 90 days.2–5
Its symptoms are challenging to manage, since nausea, vomiting, colic, and abdominal pain, which are common, cause significant physical distress and demoralization. The decision whether to correct it with surgery requires an individualized approach and a clear understanding of the goals of care and expected survival in the individual patient.
This review focuses on the management of inoperable malignant bowel obstruction and includes discussion of hydration, nutrition, and endoscopic palliative options.
WHAT ARE THE DIFFERENT TYPES OF OBSTRUCTION?
Bowel obstruction may be mechanical or functional, partial or complete, and may occur at one or at many sites. Tumors can impair bowel function in several ways6–8:
- Intraluminal tumors can occlude the lumen or act as a point of intussusception.
- Intramural tumors can extend to the mucosa and obstruct the lumen or impair peristalsis.
- Mesenteric and omental masses or malignant adhesions can kink or angulate the bowel, creating an extramural obstruction.
- Tumors that infiltrate into the mesentery, bowel muscle, or the enteric or celiac plexus can cause dysmotility.
Cholangiocarcinoma, pancreatic carcinoma, and gallbladder carcinoma are the most common tumors causing duodenal obstruction.9 Distal obstruction is caused mainly by colon and ovarian cancer.
Obstruction can be due to treatment
In a minority of patients, obstruction is unrelated to the cancer and is instead due to adhesions arising from surgery, radiation therapy (causing enteritis and strictures), desmoplastic reactions to intraperitoneal chemotherapy, torsion, or internal hernias.10–12
In rare cases, a patient has intestinal pseudo-obstruction from paraneoplastic destruction of enteric neurons, or severe ileus from anticholinergic or sympathomimetic drugs, as seen with acute colonic pseudo-obstruction (Ogilvie syndrome).13
Physiologic reactions to obstruction
Malignant bowel obstruction stimulates gastric, biliary, pancreatic, and intestinal secretions, decreases intraluminal sodium and water reabsorption, and increases mucosal sodium and water secretion.6,14 In response to the obstruction, peristalsis increases, and prostaglandin, vasoactive intestinal peptide, and nociceptive mediators are released. Vasoactive intestinal polypeptide perpetuates a cycle of secretion, distention, and contraction that leads to intestinal hyperemia, bowel edema, and accumulation of fluid in the lumen.8,10,11,15
Signs and symptoms depend on the site
The site of obstruction determines the signs and symptoms patients experience.7,14 Obstructions high in the gastrointestinal tract are associated with greater symptoms but fewer signs than colonic obstructions.1 Patients with proximal small-bowel obstruction have more severe nausea and a greater number of episodes of emesis, but they have relatively normal plain radiographs of the abdomen, which do not have the characteristic air-fluid levels commonly seen with distal small-bowel obstruction.
Most malignant obstructions remain partial, but increasing abdominal distention, worsening nausea, vomiting, abdominal pain, and obstipation over 1 to 2 weeks1 suggest progression to complete obstruction.
IMAGING TESTS FOR MALIGNANT BOWEL OBSTRUCTION
What is the value of plain radiography?
Despite these limitations, plain radiography is useful in assessing constipation and its severity as a potential cause of symptoms, and thus it remains an important initial imaging study in almost all patients with suspected malignant bowel obstruction.17,18 It is also used to assess response to treatment.
When do you need contrast radiographs?
Contrast radiography (barium swallow or barium or Gastrograffin enema) is helpful in patients with symptoms of dysmotility from suspected bowel obstruction. It defines the site or sites of obstruction and the extent of the obstruction with a fair degree of accuracy.7,19 Single-contrast studies, if positive, exclude opioid-induced bowel dysfunction or pseudo-obstruction in 83% of patients, with a sensitivity and specificity of 96% and 98%, respectively.8,20 Small-bowel follow-through with barium is more appropriate for low-grade obstructions or for symptomatic patients with a normal kidney, ureter, bladder radiograph.19
However, contrast radiography is limited by the patient’s ability to swallow barium or water-soluble contrast agents, and it can worsen nausea or vomiting.17,18 Also, barium is not absorbed systemically and may interfere with subsequent radiologic studies. Large volumes of contrast agents increase the risk of aspiration pneumonia in patients with poorly controlled nausea and can lead to severe impaction proximal to the obstructed site.8
Is enteroclysis better than barium swallow?
Enteroclysis, ie, injecting radiographic contrast into the bowel via a nasoduodenal tube, has some advantages over the barium swallow technique for detecting partial small-bowel obstruction, since it bypasses the stomach and allows for therapeutic decompression as well as direct visualization of the area of concern.17,18 Enteroclysis radiography objectively gauges severity of intestinal obstruction and bowel wall distensibility, which is an advantage over other imaging studies. Its sensitivity is 100% and specificity 88% in experienced hands.17 Enteroclysis studies also detect nonobstructing intraluminal tumors when computed tomography (CT) is not diagnostic.17,18,21
The drawbacks to enteroclysis are that it is technically difficult to perform and that few radiologists are trained in it.
When is CT useful?
CT is the primary imaging study for patients with obstructive symptoms and a history of abdominal malignancy or a palpable abdominal mass17,20,22,23 (Figure 1). It has a specificity of 100% and a sensitivity of 94%. It plays a major role in decision-making regarding surgery, endoscopy, or palliative interventions,7,19 as it locates the obstruction and differentiates benign from malignant causes with a fair degree of precision.22
CT findings in malignant bowel obstruction may include:
- A mass at the site of obstruction or within the original surgical field
- Lymphadenopathy
- Abrupt transitions in luminal diameter or irregular thickening of the bowel wall at the site or sites of obstruction.7
SURGERY: A DIFFICULT DECISION
Is the patient fit for surgery?
Surgery for malignant bowel obstruction should not be done in patients who have advanced malignancies with bulky intra-abdominal metastases or cancer that has spread outside the abdominal cavity without taking into account treatment options for the cancer, the patient’s nutritional status, and the goals of care.
The role of abdominal surgery (debulking, resection, or bypass) in advanced cancer remains unclear and controversial.24 From 42% to 80% of patients report that symptoms improve after surgery, but recurrent obstruction occurs in 10% to 50%.10 Even in patients with low tumor bulk and good nutritional status, 30-day mortality rates range from 5% to 40%, and complication rates range from 9% to 90%.3,4,6,7,10,14
Outcomes after surgery depend on patient selection criteria perhaps as much as on the surgeon’s experience and skill. Patients with more advanced cancer who have had multiple surgical procedures and those with cancer that does not respond to chemotherapy and radiation present the greatest challenge to surgeons.23
What is the benefit of surgery?
Reports of palliative surgery have included information about 60-day survival rates after the operation, but a number of factors may be more meaningful in this context, such as postoperative symptoms, the patient’s overall wellbeing, how the original symptoms respond to the surgery, complications, and length of hospitalization.14 The paucity of published, validated, patient-related outcome data on which to gauge the value of surgery and the lack of a standard definition of “benefit” further confuse the objective determination of whether these patients benefit from surgery.
In a cohort with advanced ovarian cancer and bowel obstruction, surgery was detrimental to survival and quality of life for all subgroups, and most patients died in the hospital.6
The risk of surgery for malignant bowel obstruction is presumably higher than for abdominal surgery for other indications, since many of the patients are debilitated from their cancer and chemotherapy, and many are malnourished.23 Even when taking into account a potential selection bias in favor of surgery, several studies have reported no significant difference in 30-day mortality rates or median survival between operative and nonoperative groups.2,12 Neither the type of obstruction nor the extent of the surgery influenced outcomes. Surgical outcomes are best in patients with a benign cause of obstruction; little benefit is seen in operating on those with abdominal carcinomatosis.12
Nevertheless, surgery is beneficial in a select few. For patients with a good performance status, slowly progressive cancer, and an expected survival of more than 6 months, surgical bypass or resection is preferred.7,12,25 The challenge is to identify these surgical candidates, taking into account prognostic factors such as nutritional status, tumor burden, performance status, presence of ascites, advanced age, extensive prior chemotherapy or radiotherapy, and diffuse carcinomatosis.3,10,12,20,23
Is surgery consistent with the goals of care?
Crucial to decision-making are the goals of care. Since palliative surgery carries a low level of evidence for benefit in terms of quality of life and survival, time should be set aside to thoroughly review the patient’s medical condition, to explore options, and to clarify expectations and goals of care.3,10 Family members should be invited to be present during these discussions and to be involved in the decision-making process.
WHAT IS THE BENEFIT OF GASTRIC OR COLONIC STENTING?
Endoscopic procedures are alternatives to surgery and offer a palliative option in malignant bowel obstruction. Endoscopic procedures are associated with a shorter hospital stay and quicker recovery than after laparotomy.9,26–30 In certain situations, stenting serves as a bridge to surgery, allowing time to mitigate comorbid conditions, to enhance nutrition, and to complete staging, while relieving symptoms.27–29,31,32 Definitive surgery can be done as a single-stage procedure without a diverting enterostomy.
Self-expanding metal stents for gastric outlet, small-bowel, and colonic obstructions are an option in patients who have incurable metastatic disease who are unfit for surgery, in patients with a single point of obstruction or locally extensive disease, or in patients who do not want to undergo laparotomy.28–30
Technical and clinical success rates for colorectal stenting are high (88% to 93%).26,27 Stenting is more successful for left-sided colonic obstructions than for proximal colonic obstructions. Even for patients with extracolonic malignancies such as ovarian cancer, the technical success rate of colorectal stenting is 87%.26 However, patients with unrecognized peritoneal carcinomatosis or multifocal bowel obstruction are less likely to have symptomatic relief even after successful stenting.6,9
Contraindications to stenting
Absolute contraindications to stenting are colonic or tumor perforation with peritonitis. A relative contraindication is a rectal tumor within 2 cm of the anal margin. Stenting in this circumstance leads to tenesmus and incontinence.33
Complications of stenting
Death rates during colorectal stent insertion are less than 1%. The hospital stay and incidence of complications are significantly less than with surgery.26,30
Stent migration occurs in 10% of cases and is asymptomatic, but half of patients with this complication require a repeat intervention. The risk of migration is greater if chemotherapy or radiation therapy succeeds in shrinking the tumor.
Bleeding occurs in 5% of cases, usually from the underlying tumor.
Perforation occurs in 4%, but the rate increases to 10% with the use of dilatation before stent placement.
The rate of recurrent obstruction from tumor ingrowth, overgrowth, or fecal impaction is 10%.9,26,29 Recurrent obstruction may be treated with additional stents inserted within the original stent.9
GASTRIC OUTLET OBSTRUCTION: SURGERY VS STENTING
Gastrojejunostomy has in the past been the treatment of choice for gastric outlet obstruction. Certainly, patients with slow-growing tumors and an expected survival of greater than 60 days may be considered for this bypass procedure; those with a short tumor length, a single site of obstruction (preferably in the pylorus or proximal duodenum), a good performance status, and a life expectancy greater than 30 days are good candidates.7 Nevertheless, for patients with advanced cancer and poor performance status, gastroenterostomy carries a significant risk of morbidity and death.28
Endoscopic stenting of gastric outlet obstruction has a greater success rate, a shorter time to oral intake, a lower morbidity rate, a lower incidence of delayed gastric emptying, and a shorter hospital stay compared with gastroenterostomy.28,29 Technical success rates of stenting are 90%, and 75% of patients have resolution of nausea and vomiting.7 Stenting is generally not possible if the obstruction occurs beyond the ligament of Treitz.
Patients who are expected to survive less than 1 month or who have rapidly progressive disease, overt ascites, carcinomatosis, or multiple sites of obstruction should be managed with percutaneous, endoscopically placed gastrostomy tubes.7
Late complications of stenting for gastric outlet obstruction are occlusion with food or ingrowth of tumor through or around the wire mesh.7 This may require laser therapy or placement of a second stent, or both.
DRUG THERAPY
Medical therapy can palliate symptoms of malignant bowel obstruction for most patients.34 Recommendations have been published by the Working Group of the European Association for Palliative Care.24 Symptom management is focused on pain, nausea, and vomiting.
Which drugs can I use for abdominal pain?
Patients experience two types of abdominal pain: continuous and colic. Each type of pain requires different treatment approaches and classes of drugs.
Potent opioids such as morphine, hydromorphone (Dilaudid), and fentanyl (Fentora) are used to relieve continuous abdominal pain.7 The dose is titrated for adequate relief. Subcutaneous, intravenous, sublingual, and transdermal routes can be used if nausea and vomiting prevent oral administration.
However, opioids can aggravate colic by stimulating circular smooth muscle, leading to segmental contractions. Opioid-sparing adjuvant drugs such as ketorolac (Toradol) may improve colic and continuous pain and prevent a partial obstruction from becoming a complete obstruction by sparing opioid doses.35
Colic may persist or worsen with the use of opioids. Drugs that reduce colic include the scopolamine drugs hyoscine butylbromide and hyoscine hydrobromide, glycopyrrolate (Robinul), and octreotide (Sandostatin).7,34–37
Which drugs are appropriate for reducing nausea and vomiting?
Phenothiazines reduce nausea and control vomiting. Chlorpromazine (Thorazine), prochlorperazine (Compro, Compazine), and promethazine (Phenergan) have all been reported to treat nausea successfully.35,37
Haloperidol (Haldol), a butyrophenone selective dopamine D2-receptor antagonist, has negligible anticholinergic activity. At low doses it produces less sedation than phenothiazines and is an ideal agent for patients with nausea and delirium.35 Doses range from 5 to 15 mg/day, given in divided doses or as intermittent or continuous intravenous infusions.
Anticholinergics, with or without somatostatin analogues, reduce gastrointestinal secretions, fluid accumulation, and vomiting. Anticholinergics bind to muscarinic receptors on enteric neurons in the myenteric and the submucosal plexus. Dosages:
- Hyoscine butylbromide 40 to 120 mg/day.
- Hyoscine hydrobromide 0.2 to 0.9 mg/day.7,34
Glycopyrrolate, a quaternary ammonium anticholinergic, has minimal central nervous system penetration and is less likely to cause delirium or cardiac side effects compared with tertiary amine anticholinergics such as atropine and scopolamine.38 The recommended dose is 0.1 to 0.2 mg subcutaneously or intravenously three to four times daily.
Octreotide, an analogue of somatostatin, blocks the release of vasoactive intestinal polypeptide, which is increased in malignant bowel obstruction.14,15 It reduces the excretion of water, sodium, and chloride into the bowel lumen and increases the absorption of electrolytes and water. It also inhibits pancreatic enzyme secretion and splanchnic blood flow. The result of all these effects is reduced luminal content, reduced motility, reduced vascular congestion of the bowel wall, and, in certain circumstances, reduced ascites.39
In small randomized trials, octreotide was more successful than anticholinergics at improving nausea, vomiting, and colic in patients requiring a nasogastric tube and in those whose symptoms were refractory to standard medical treatment.5,34,40–43 A recent case report found octreotide helpful in resolving symptoms of partial bowel obstruction that were unresponsive to standard measures.44
Octreotide is well tolerated and reduces the time patients require a nasogastric tube without significantly worsening xerostomia. High cost limits its use in American hospice care due to the Medicare capitated system of reimbursement for drugs and services, and as a result it is a second-tier drug despite evidence of its efficacy.
Octreotide doses are 100 to 200 mg every 8 hours.
Metoclopramide (Reglan), a dopaminergic antagonist, a 5HT4 receptor agonist, and a 5HT3 receptor blocker at doses greater than 120 mg/day, combines the action of a phenothiazine, which blocks D2 receptors in the central chemoreceptor trigger zone, with promotility actions through serotonin receptors (5HT4).35,37
Metoclopramide should not be used with anticholinergics or in patients with colic or complete obstruction.35,45 In some centers it is the first-line drug for functional or partial bowel obstruction.7 Dosages range from 40 to 240 mg/day.
Olanzapine (Zyprexa), an atypical antipsychotic, blocks multiple neurotransmitter receptors (D2, H1, Ach, 5HT3) responsible for initiating emesis. It is an option in patients whose nausea and vomiting fail to respond to standard antiemetics.46 Dosages range from 2.5 to 20 mg/day.
Dissolvable tablets are given sublingually, which makes olanzapine a versatile antiemetic in cases of intractable nausea. Our unpublished experience is that the sublingual route reduces nausea associated with malignant bowel obstruction and obviates the need for subcutaneous injections or intravenous antiemetic infusions.
Corticosteroids. Although how corticosteroids relieve malignant bowel obstruction is unknown, they are presumed to act centrally.37,45 In addition, they reduce peritumoral edema and luminal salt and water, and they also have antiemetic and analgesic properties.
Evidence from a meta-analysis found that 6 to 16 mg of parenteral dexamethasone per day reduced symptoms and improved bowel function in 60% of patients but did not change the prognosis.11
A trial of 4 or 5 days is adequate to determine response. If there is no response, the corticosteroid should be rapidly tapered. Side effects are minimal when corticosteroids are used short-term.
Combination therapy. Only rarely does a single drug resolve symptoms of malignant bowel obstruction. Antiemetics, analgesics, corticosteroids, antisecretory anticholinergics, and octreotide are often required in combination to achieve acceptable symptom relief.3,5,7,47
In a small prospective case series, the combination of metoclopramide 60 mg/day, octreotide 0.3 mg/day, and dexamethasone 12 mg/day with a single bolus of amidotrizoic acid (a contrast agent) improved intestinal transit within 1 to 5 days and resolved vomiting within 24 hours.45
Compatibility and the route of administration of medications are key considerations when choosing drug combinations.
WHEN TO CONSIDER A VENTING GASTROSTOMY
Patients with a poor performance status, rapidly progressive disease, peritoneal carcinomatosis, a life expectancy of less than 30 days, or multiple levels of obstruction benefit from placement of a percutaneous endoscopic gastrostomy tube (ie, a venting gastrostomy) rather than surgery if symptoms do not respond to drug therapy.7,48 There is compelling evidence that this procedure relieves nausea and vomiting in 80% to 90% of patients and restores some level of oral intake in many.5,6,48,49 A venting gastrostomy tube can be placed during surgical exploration, percutaneously with fluoroscopy, or endoscopically.9
There are no absolute contraindications to gastrostomy tube placement. It is feasible even in patients with tumors encasing the stomach, diffuse carcinomatosis, and ascites.48 However, massive ascites, previous upper abdominal surgery, or a large mass attached to the abdominal wall make tube placement difficult.
Complications are often local. Patients experience transient abdominal wall pain after the procedure. Dislodgement, bleeding, catheter migration, peritonitis, and necrotizing fasciitis are early complications. Others include skin excoriation from leakage of gastric contents, leakage of ascitic fluid from the site, and obstruction or dislodgement of the tube.48,49
Patients can be discharged from the hospital soon after the tube is placed, usually with fewer medications than for patients who undergo surgery.48 This is particularly important for patients with a short expected survival. Some patients at home benefit from hydration (less than 2 L/day) via an existing central venous port or peripherally inserted central catheter, or by hypodermoclysis.
WHEN IS A NASOGASTRIC TUBE APPROPRIATE?
Some patients with malignant bowel obstruction require a nasogastric tube early in their hospital course.12 Unfortunately, nasogastric tubes, if left in place, cause nose and throat pain, sinusitis, abscess formation, erosion of nasal cartilage, aspiration, esophageal erosion, pharyngitis, and social isolation.5,6
Nasogastric tubes should be a temporizing measure to vent gastrointestinal secretions, reduce abdominal distension, and improve nausea and vomiting while a decision about surgery is being made.13,24 If surgery is not feasible, one can avoid the long-term complications and discomfort of a nasogastric tube via medical management and earlier evaluation for venting gastrostomy in those with symptoms that respond poorly to optimal medical management.49
WHICH PATIENTS BENEFIT FROM TOTAL PARENTERAL NUTRITION?
The use of total parenteral nutrition in patients with incurable malignancies is controversial. Enteral and parenteral feeding can increase muscle mass and improve functional status and quality of life in a subset of patients who are not suffering from cancer-related cachexia.2,50,51 However, for those whose weight loss and malnutrition are consequences of tumor-mediated cachexia, as demonstrated by anorexia and an elevated C-reactive protein level, parenteral nutrition is unlikely to improve the outcome.51 For most terminally ill patients, retrospective studies have failed to show that parenteral nutrition improves overall survival, performance status, or quality of life.2,48,50–54
Total parenteral nutrition poses risks: it is invasive and requires central venous access, which predisposes to infection; it requires frequent monitoring of hydration and electrolytes; and it predisposes to thrombosis, diarrhea, hyperglycemia, and liver failure.50–56
Total parenteral nutrition may be justified in patients with minimal tumor burden who are candidates for definitive surgery, or in those with a good performance status early in the disease course who have not had chemotherapy or whose cancer responds to chemotherapy.2,50–56
The American College of Physicians discourages the routine use of parenteral nutrition in those with advanced cancer who are undergoing palliative chemotherapy, since few patients benefit and many experience side effects.53
Total parenteral nutrition is much like a medical intervention in that it should be offered or continued only if it provides benefit. Conversations at the time that it is begun must include adverse effects that will lead to its discontinuation, and criteria for response. In certain situations, a limited trial of parenteral nutrition may be considered for patients with an uncertain prognosis or for those who have potentially reversible conditions that limit oral intake.51 In such cases, there should be a clear understanding between patient and physician that parenteral nutrition will be discontinued if it fails to show benefit.53
ADDITIONAL CONCERNS OF PATIENTS AND FAMILIES
‘Will I starve to death?’
Starvation is a fear echoed by patients and families. Ethical discourse on the continuation of nutrition and hydration for the terminally ill has been polarizing.57–60 Withdrawal of nutrition can be perceived as euthanasia.
Advanced cancer patients in general do not experience hunger, and those who do require only small amounts of food for satiation.61 In one report, most patients died of their advanced cancer and not from starvation.52 Artificial hydration and nutrition will thus not influence survival and can even be a burden without benefit in the imminently dying.60 These patients should be encouraged to take food orally for pleasure, as long as it is tolerated, without consideration of end points such as weight gain, body mass index, or albumin levels.
Complaints of thirst and dryness of the mouth are relieved with mouth care, ice chips, lubrication to the lips, and sips of fluid, rather than by parenteral nutrition.59 Patients with a terminal illness experience relief from thirst with minimal intake. The symptom of thirst may be relieved without hydration.34,61 Adequate hydration requires smaller fluid volumes due to decreased body weight, decreased renal clearance of free water, and decreased insensible water losses from reduced physical activity.58
‘Can we continue intravenous hydration so he won’t die of thirst?’
Overzealous intravenous hydration may worsen the symptoms of malignant bowel obstruction. Overhydration can increase secretions in the gut lumen and worsen the secretion-distention-contraction cycle, leading to greater abdominal pain and to nausea and vomiting.7 There is a greater risk of fluid overload in these patients, since they have edema and excessive interstitial fluid. Most have a low serum albumin level, which results in movement of fluid from intravascular to interstitial spaces due to reduced colloid osmotic pressure. In these instances, overzealous hydration can lead to respiratory insufficiency and worsening edema.
In spite of numerous discussions in the medical literature of the benefits and burdens of continual hydration, there is no consensus or guideline. When a patient has limited oral intake, the decision to hydrate should be individualized, with careful assessment of the risks and benefits and in accordance with the patient’s or family’s wishes.57,58
Is treatment at home feasible?
Discharging patients with inoperable malignant bowel obstruction requires careful planning. Patients and family members need to be educated on the use of around-the-clock medications and symptom-targeted, as-needed drugs. Days before discharge, questions about diet need to be clarified. Education about total parenteral nutrition and gastrostomy tube care should be completed before discharge from the hospital.
Drug management should be simplified, or compatible medications should be combined into a single infusion. For example, morphine, glycopyrrolate, and haloperidol or metoclopramide are chemically compatible in standard intravenous solutions and can be combined.
Families feel less anxious about the foreseen and the possible unforeseen course of the illness if they can talk with hospice workers early on. This early involvement also facilitates the transition to home hospice care.
SUMMARY OF IMPORTANT POINTS
- Patients with malignant bowel obstruction need a highly individualized approach, tailored to their medical condition, the prognosis, and the goals of care.
- Surgery should not be routinely undertaken; less-invasive approaches such as gastric or colonic stenting should be considered first.
- Combinations of analgesics, antisecretory drugs, and antiemetics can provide acceptable symptom relief in the inoperable patient.
- A venting gastrostomy should be considered if drug therapy fails to reduce nausea and vomiting to an acceptable level.
- A nasogastric tube should be used only as a temporizing measure, until symptoms are controlled medically or a venting gastrostomy is placed.
- Total parenteral nutrition is of benefit only in patients with intermediate life expectancy who may otherwise die of starvation rather than from the cancer itself.
- Mercadante S. Intestinal dysfunction and obstruction. In:Walsh D, editor. Palliative Medicine. Philadelphia, PA: Saunders/Elsevier, 2009:1267–1275.
- Pasanisi F, Orban A, Scalfi L, et al. Predictors of survival in terminal-cancer patients with irreversible bowel obstruction receiving home parenteral nutrition. Nutrition 2001; 17:581–584.
- Pameijer CR, Mahvi DM, Stewart JA, Weber SM. Bowel obstruction in patients with metastatic cancer: does intervention influence outcome? Int J Gastrointest Cancer 2005; 35:127–133.
- Bais JMJ, Schilthuis MS, Ansink AC. Palliative management of intestinal obstruction in patients with advanced gynaecological cancer. J Gynecol Oncol 2002; 7:299–305.
- Laval G, Arvieux C, Stefani L, Villard ML, Mestrallet JP, Cardin N. Protocol for the treatment of malignant inoperable bowel obstruction: a prospective study of 80 cases at Grenoble University Hospital Center. J Pain Symptom Manage 2006; 31:502–512.
- Jatoi A, Podratz KC, Gill P, Hartmann LC. Pathophysiology and palliation of inoperable bowel obstruction in patients with ovarian cancer. J Support Oncol 2004; 2:323–334.
- Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer 2008; 44:1105–1115.
- Roeland E, von Gunten CF. Current concepts in malignant bowel obstruction management. Curr Oncol Rep 2009; 11:298–303.
- Baron TH. Interventional palliative strategies for malignant bowel obstruction. Curr Oncol Rep 2009; 11:293–297.
- Feuer DJ, Broadley KE, Shepherd JH, Barton DP. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD002764.
- Feuer DJ, Broadley KE. Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD001219.
- Woolfson RG, Jennings K, Whalen GF. Management of bowel obstruction in patients with abdominal cancer. Arch Surg 1997; 132:1093–1097.
- Saunders MD, Kimmey MB. Systematic review: acute colonic pseudo-obstruction. Aliment Pharmacol Ther 2005; 22:917–925.
- Ripamonti C, Bruera E. Palliative management of malignant bowel obstruction. Int J Gynecol Cancer 2002; 12:135–143.
- Nellgård P, Bojö L, Cassuto J. Importance of vasoactive intestinal peptide and somatostatin for fluid losses in small-bowel obstruction. Scand J Gastroenterol 1995; 30:464–469.
- Böhner H, Yang Q, Franke C, Verreet PR, Ohmann C. Simple data from history and physical examination help to exclude bowel obstruction and to avoid radiographic studies in patients with acute abdominal pain. Eur J Surg 1998; 164:777–784.
- Maglinte DD, Kelvin FM, Sandrasegaran K, et al. Radiology of small bowel obstruction: contemporary approach and controversies. Abdom Imaging 2005; 30:160–178.
- Maglinte DD, Howard TJ, Lillemoe KD, Sandrasegaran K, Rex DK. Small-bowel obstruction: state-of-the-art imaging and its role in clinical management. Clin Gastroenterol Hepatol 2008; 6:130–139.
- Silva AC, Pimenta M, Guimarães LS. Small bowel obstruction: what to look for. Radiographics 2009; 29:423–439.
- Finan PJ, Campbell S, Verma R, et al. The management of malignant large bowel obstruction: ACPGBI position statement. Colorectal Dis 2007; 9(suppl 4):1–17.
- Kohli MD, Maglinte DD. CT enteroclysis in incomplete small bowel obstruction. Abdom Imaging 2009; 34:321–327.
- Ha HK, Shin BS, Lee SI, et al. Usefulness of CT in patients with intestinal obstruction who have undergone abdominal surgery for malignancy. AJR Am J Roentgenol 1998; 171:1587–1593.
- DeBernardo R. Surgical management of malignant bowel obstruction: strategies toward palliation of patients with advanced cancer. Curr Oncol Rep 2009; 11:287–292.
- Ripamonti C, Twycross R, Baines M, et al; Working Group of the European Association for Palliative Care. Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Support Care Cancer 2001; 9:223–233.
- Mangili G, Aletti G, Frigerio L, et al. Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. Int J Gynecol Cancer 2005; 15:830–835.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg 2002; 89:1096–1102.
- Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol 2007; 42:283–290.
- Del Piano M, Ballarè M, Montino F, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc 2005; 61:421–426.
- Tilney HS, Lovegrove RE, Purkayastha S, et al. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc 2007; 21:225–233.
- Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc 2004; 60:1010–1017.
- Dastur JK, Forshaw MJ, Modarai B, Solkar MM, Raymond T, Parker MC. Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech Coloproctol 2008; 12:51–55.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Ripamonti C, Mercadante S, Groff L, Zecca E, De Conno F, Casuccio A. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: a prospective randomized trial. J Pain Symptom Manage 2000; 19:23–34.
- Davis MP, Walsh D. Treatment of nausea and vomiting in advanced cancer. Support Care Cancer 2000; 8:444–452.
- Bicanovsky LK, Lagman RL, Davis MP, Walsh D. Managing nonmalignant chronic abdominal pain and malignant bowel obstruction. Gastroenterol Clin North Am 2006; 35:131–142.
- Glare P, Pereira G, Kristjanson LJ, Stockler M, Tattersall M. Systematic review of the efficacy of antiemetics in the treatment of nausea in patients with far-advanced cancer. Support Care Cancer 2004; 12:432–440.
- Davis MP, Furste A. Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage 1999; 18:153–154.
- Ripamonti C, Mercadante S. How to use octreotide for malignant bowel obstruction. J Support Oncol 2004; 2:357–364.
- Shima Y, Ohtsu A, Shirao K, Sasaki Y. Clinical efficacy and safety of octreotide (SMS201-995) in terminally ill Japanese cancer patients with malignant bowel obstruction. Jpn J Clin Oncol 2008; 38:354–359.
- Mercadante S, Casuccio A, Mangione S. Medical treatment for inoperable malignant bowel obstruction: a qualitative systematic review. J Pain Symptom Manage 2007; 33:217–223.
- Mystakidou K, Tsilika E, Kalaidopoulou O, Chondros K, Georgaki S, Papadimitriou L. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double-blind, controlled clinical trial. Anticancer Res 2002; 22:1187–1192.
- Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer 2000; 8:188–191.
- Myers J, Tamber A, Farhadian M. Management of treatment-related intermittent partial small bowel obstruction: the use of octreotide. J Pain Symptom Manage 2010; 39:e1–e3.
- Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage 2004; 28:412–416.
- Srivastava M, Brito-Dellan N, Davis MP, Leach M, Lagman R. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage 2003; 25:578–582.
- Bentley A, Boyd K. Use of clinical pictures in the management of nausea and vomiting: a prospective audit. Palliat Med 2001; 15:247–253.
- Pothuri B, Montemarano M, Gerardi M, et al. Percutaneous endoscopic gastrostomy tube placement in patients with malignant bowel obstruction due to ovarian carcinoma. Gynecol Oncol 2005; 96:330–334.
- Brooksbank MA, Game PA, Ashby MA. Palliative venting gastrostomy in malignant intestinal obstruction. Palliat Med 2002; 16:520–526.
- Wang MY, Wu MH, Hsieh DY, et al. Home parenteral nutrition support in adults: experience of a medical center in Asia. JPEN J Parenter Enteral Nutr 2007; 31:306–310.
- Dy SM. Enteral and parenteral nutrition in terminally ill cancer patients: a review of the literature. Am J Hosp Palliat Care 2006; 23:369–377.
- Whitworth MK, Whitfield A, Holm S, Shaffer J, Makin W, Jayson GC. Doctor, does this mean I’m going to starve to death? J Clin Oncol 2004; 22:199–201.
- Hoda D, Jatoi A, Burnes J, Loprinzi C, Kelly D. Should patients with advanced, incurable cancers ever be sent home with total parenteral nutrition? A single institution’s 20-year experience. Cancer 2005; 103:863–868.
- Philip J, Depczynski B. The role of total parenteral nutrition for patients with irreversible bowel obstruction secondary to gynecological malignancy. J Pain Symptom Manage 1997; 13:104–111.
- August DA, Thorn D, Fisher RL, Welchek CM. Home parenteral nutrition for patients with inoperable malignant bowel obstruction. JPEN J Parenter Enteral Nutr 1991; 15:323–327.
- Abu-Rustum NR, Barakat RR, Venkatraman E, Spriggs D. Chemotherapy and total parenteral nutrition for advanced ovarian cancer with bowel obstruction. Gynecol Oncol 1997; 64:493–495.
- Fainsinger RL, Bruera E. When to treat dehydration in a terminally ill patient? Support Care Cancer 1997; 5:205–211.
- Steiner N, Bruera E. Methods of hydration in palliative care patients. J Palliat Care 1998; 14:6–13.
- Slomka J. Withholding nutrition at the end of life: clinical and ethical issues. Cleve Clin J Med 2003; 70:548–552.
- Chiu TY, Hu WY, Chuang RB, Chen CY. Nutrition and hydration for terminal cancer patients in Taiwan. Support Care Cancer 2002; 10:630–636.
- McCann RM, Hall WJ, Groth-Juncker A. Comfort care for terminally ill patients. The appropriate use of nutrition and hydration. JAMA 1994; 272:1263–1266.
Malignant bowel obstruction occurs in 5% to 51% of women with ovarian cancer and in 10% to 28% of patients with gastrointestinal cancer, predominantly in the advanced stages.1 Median survival after its onset ranges from 30 to 90 days.2–5
Its symptoms are challenging to manage, since nausea, vomiting, colic, and abdominal pain, which are common, cause significant physical distress and demoralization. The decision whether to correct it with surgery requires an individualized approach and a clear understanding of the goals of care and expected survival in the individual patient.
This review focuses on the management of inoperable malignant bowel obstruction and includes discussion of hydration, nutrition, and endoscopic palliative options.
WHAT ARE THE DIFFERENT TYPES OF OBSTRUCTION?
Bowel obstruction may be mechanical or functional, partial or complete, and may occur at one or at many sites. Tumors can impair bowel function in several ways6–8:
- Intraluminal tumors can occlude the lumen or act as a point of intussusception.
- Intramural tumors can extend to the mucosa and obstruct the lumen or impair peristalsis.
- Mesenteric and omental masses or malignant adhesions can kink or angulate the bowel, creating an extramural obstruction.
- Tumors that infiltrate into the mesentery, bowel muscle, or the enteric or celiac plexus can cause dysmotility.
Cholangiocarcinoma, pancreatic carcinoma, and gallbladder carcinoma are the most common tumors causing duodenal obstruction.9 Distal obstruction is caused mainly by colon and ovarian cancer.
Obstruction can be due to treatment
In a minority of patients, obstruction is unrelated to the cancer and is instead due to adhesions arising from surgery, radiation therapy (causing enteritis and strictures), desmoplastic reactions to intraperitoneal chemotherapy, torsion, or internal hernias.10–12
In rare cases, a patient has intestinal pseudo-obstruction from paraneoplastic destruction of enteric neurons, or severe ileus from anticholinergic or sympathomimetic drugs, as seen with acute colonic pseudo-obstruction (Ogilvie syndrome).13
Physiologic reactions to obstruction
Malignant bowel obstruction stimulates gastric, biliary, pancreatic, and intestinal secretions, decreases intraluminal sodium and water reabsorption, and increases mucosal sodium and water secretion.6,14 In response to the obstruction, peristalsis increases, and prostaglandin, vasoactive intestinal peptide, and nociceptive mediators are released. Vasoactive intestinal polypeptide perpetuates a cycle of secretion, distention, and contraction that leads to intestinal hyperemia, bowel edema, and accumulation of fluid in the lumen.8,10,11,15
Signs and symptoms depend on the site
The site of obstruction determines the signs and symptoms patients experience.7,14 Obstructions high in the gastrointestinal tract are associated with greater symptoms but fewer signs than colonic obstructions.1 Patients with proximal small-bowel obstruction have more severe nausea and a greater number of episodes of emesis, but they have relatively normal plain radiographs of the abdomen, which do not have the characteristic air-fluid levels commonly seen with distal small-bowel obstruction.
Most malignant obstructions remain partial, but increasing abdominal distention, worsening nausea, vomiting, abdominal pain, and obstipation over 1 to 2 weeks1 suggest progression to complete obstruction.
IMAGING TESTS FOR MALIGNANT BOWEL OBSTRUCTION
What is the value of plain radiography?
Despite these limitations, plain radiography is useful in assessing constipation and its severity as a potential cause of symptoms, and thus it remains an important initial imaging study in almost all patients with suspected malignant bowel obstruction.17,18 It is also used to assess response to treatment.
When do you need contrast radiographs?
Contrast radiography (barium swallow or barium or Gastrograffin enema) is helpful in patients with symptoms of dysmotility from suspected bowel obstruction. It defines the site or sites of obstruction and the extent of the obstruction with a fair degree of accuracy.7,19 Single-contrast studies, if positive, exclude opioid-induced bowel dysfunction or pseudo-obstruction in 83% of patients, with a sensitivity and specificity of 96% and 98%, respectively.8,20 Small-bowel follow-through with barium is more appropriate for low-grade obstructions or for symptomatic patients with a normal kidney, ureter, bladder radiograph.19
However, contrast radiography is limited by the patient’s ability to swallow barium or water-soluble contrast agents, and it can worsen nausea or vomiting.17,18 Also, barium is not absorbed systemically and may interfere with subsequent radiologic studies. Large volumes of contrast agents increase the risk of aspiration pneumonia in patients with poorly controlled nausea and can lead to severe impaction proximal to the obstructed site.8
Is enteroclysis better than barium swallow?
Enteroclysis, ie, injecting radiographic contrast into the bowel via a nasoduodenal tube, has some advantages over the barium swallow technique for detecting partial small-bowel obstruction, since it bypasses the stomach and allows for therapeutic decompression as well as direct visualization of the area of concern.17,18 Enteroclysis radiography objectively gauges severity of intestinal obstruction and bowel wall distensibility, which is an advantage over other imaging studies. Its sensitivity is 100% and specificity 88% in experienced hands.17 Enteroclysis studies also detect nonobstructing intraluminal tumors when computed tomography (CT) is not diagnostic.17,18,21
The drawbacks to enteroclysis are that it is technically difficult to perform and that few radiologists are trained in it.
When is CT useful?
CT is the primary imaging study for patients with obstructive symptoms and a history of abdominal malignancy or a palpable abdominal mass17,20,22,23 (Figure 1). It has a specificity of 100% and a sensitivity of 94%. It plays a major role in decision-making regarding surgery, endoscopy, or palliative interventions,7,19 as it locates the obstruction and differentiates benign from malignant causes with a fair degree of precision.22
CT findings in malignant bowel obstruction may include:
- A mass at the site of obstruction or within the original surgical field
- Lymphadenopathy
- Abrupt transitions in luminal diameter or irregular thickening of the bowel wall at the site or sites of obstruction.7
SURGERY: A DIFFICULT DECISION
Is the patient fit for surgery?
Surgery for malignant bowel obstruction should not be done in patients who have advanced malignancies with bulky intra-abdominal metastases or cancer that has spread outside the abdominal cavity without taking into account treatment options for the cancer, the patient’s nutritional status, and the goals of care.
The role of abdominal surgery (debulking, resection, or bypass) in advanced cancer remains unclear and controversial.24 From 42% to 80% of patients report that symptoms improve after surgery, but recurrent obstruction occurs in 10% to 50%.10 Even in patients with low tumor bulk and good nutritional status, 30-day mortality rates range from 5% to 40%, and complication rates range from 9% to 90%.3,4,6,7,10,14
Outcomes after surgery depend on patient selection criteria perhaps as much as on the surgeon’s experience and skill. Patients with more advanced cancer who have had multiple surgical procedures and those with cancer that does not respond to chemotherapy and radiation present the greatest challenge to surgeons.23
What is the benefit of surgery?
Reports of palliative surgery have included information about 60-day survival rates after the operation, but a number of factors may be more meaningful in this context, such as postoperative symptoms, the patient’s overall wellbeing, how the original symptoms respond to the surgery, complications, and length of hospitalization.14 The paucity of published, validated, patient-related outcome data on which to gauge the value of surgery and the lack of a standard definition of “benefit” further confuse the objective determination of whether these patients benefit from surgery.
In a cohort with advanced ovarian cancer and bowel obstruction, surgery was detrimental to survival and quality of life for all subgroups, and most patients died in the hospital.6
The risk of surgery for malignant bowel obstruction is presumably higher than for abdominal surgery for other indications, since many of the patients are debilitated from their cancer and chemotherapy, and many are malnourished.23 Even when taking into account a potential selection bias in favor of surgery, several studies have reported no significant difference in 30-day mortality rates or median survival between operative and nonoperative groups.2,12 Neither the type of obstruction nor the extent of the surgery influenced outcomes. Surgical outcomes are best in patients with a benign cause of obstruction; little benefit is seen in operating on those with abdominal carcinomatosis.12
Nevertheless, surgery is beneficial in a select few. For patients with a good performance status, slowly progressive cancer, and an expected survival of more than 6 months, surgical bypass or resection is preferred.7,12,25 The challenge is to identify these surgical candidates, taking into account prognostic factors such as nutritional status, tumor burden, performance status, presence of ascites, advanced age, extensive prior chemotherapy or radiotherapy, and diffuse carcinomatosis.3,10,12,20,23
Is surgery consistent with the goals of care?
Crucial to decision-making are the goals of care. Since palliative surgery carries a low level of evidence for benefit in terms of quality of life and survival, time should be set aside to thoroughly review the patient’s medical condition, to explore options, and to clarify expectations and goals of care.3,10 Family members should be invited to be present during these discussions and to be involved in the decision-making process.
WHAT IS THE BENEFIT OF GASTRIC OR COLONIC STENTING?
Endoscopic procedures are alternatives to surgery and offer a palliative option in malignant bowel obstruction. Endoscopic procedures are associated with a shorter hospital stay and quicker recovery than after laparotomy.9,26–30 In certain situations, stenting serves as a bridge to surgery, allowing time to mitigate comorbid conditions, to enhance nutrition, and to complete staging, while relieving symptoms.27–29,31,32 Definitive surgery can be done as a single-stage procedure without a diverting enterostomy.
Self-expanding metal stents for gastric outlet, small-bowel, and colonic obstructions are an option in patients who have incurable metastatic disease who are unfit for surgery, in patients with a single point of obstruction or locally extensive disease, or in patients who do not want to undergo laparotomy.28–30
Technical and clinical success rates for colorectal stenting are high (88% to 93%).26,27 Stenting is more successful for left-sided colonic obstructions than for proximal colonic obstructions. Even for patients with extracolonic malignancies such as ovarian cancer, the technical success rate of colorectal stenting is 87%.26 However, patients with unrecognized peritoneal carcinomatosis or multifocal bowel obstruction are less likely to have symptomatic relief even after successful stenting.6,9
Contraindications to stenting
Absolute contraindications to stenting are colonic or tumor perforation with peritonitis. A relative contraindication is a rectal tumor within 2 cm of the anal margin. Stenting in this circumstance leads to tenesmus and incontinence.33
Complications of stenting
Death rates during colorectal stent insertion are less than 1%. The hospital stay and incidence of complications are significantly less than with surgery.26,30
Stent migration occurs in 10% of cases and is asymptomatic, but half of patients with this complication require a repeat intervention. The risk of migration is greater if chemotherapy or radiation therapy succeeds in shrinking the tumor.
Bleeding occurs in 5% of cases, usually from the underlying tumor.
Perforation occurs in 4%, but the rate increases to 10% with the use of dilatation before stent placement.
The rate of recurrent obstruction from tumor ingrowth, overgrowth, or fecal impaction is 10%.9,26,29 Recurrent obstruction may be treated with additional stents inserted within the original stent.9
GASTRIC OUTLET OBSTRUCTION: SURGERY VS STENTING
Gastrojejunostomy has in the past been the treatment of choice for gastric outlet obstruction. Certainly, patients with slow-growing tumors and an expected survival of greater than 60 days may be considered for this bypass procedure; those with a short tumor length, a single site of obstruction (preferably in the pylorus or proximal duodenum), a good performance status, and a life expectancy greater than 30 days are good candidates.7 Nevertheless, for patients with advanced cancer and poor performance status, gastroenterostomy carries a significant risk of morbidity and death.28
Endoscopic stenting of gastric outlet obstruction has a greater success rate, a shorter time to oral intake, a lower morbidity rate, a lower incidence of delayed gastric emptying, and a shorter hospital stay compared with gastroenterostomy.28,29 Technical success rates of stenting are 90%, and 75% of patients have resolution of nausea and vomiting.7 Stenting is generally not possible if the obstruction occurs beyond the ligament of Treitz.
Patients who are expected to survive less than 1 month or who have rapidly progressive disease, overt ascites, carcinomatosis, or multiple sites of obstruction should be managed with percutaneous, endoscopically placed gastrostomy tubes.7
Late complications of stenting for gastric outlet obstruction are occlusion with food or ingrowth of tumor through or around the wire mesh.7 This may require laser therapy or placement of a second stent, or both.
DRUG THERAPY
Medical therapy can palliate symptoms of malignant bowel obstruction for most patients.34 Recommendations have been published by the Working Group of the European Association for Palliative Care.24 Symptom management is focused on pain, nausea, and vomiting.
Which drugs can I use for abdominal pain?
Patients experience two types of abdominal pain: continuous and colic. Each type of pain requires different treatment approaches and classes of drugs.
Potent opioids such as morphine, hydromorphone (Dilaudid), and fentanyl (Fentora) are used to relieve continuous abdominal pain.7 The dose is titrated for adequate relief. Subcutaneous, intravenous, sublingual, and transdermal routes can be used if nausea and vomiting prevent oral administration.
However, opioids can aggravate colic by stimulating circular smooth muscle, leading to segmental contractions. Opioid-sparing adjuvant drugs such as ketorolac (Toradol) may improve colic and continuous pain and prevent a partial obstruction from becoming a complete obstruction by sparing opioid doses.35
Colic may persist or worsen with the use of opioids. Drugs that reduce colic include the scopolamine drugs hyoscine butylbromide and hyoscine hydrobromide, glycopyrrolate (Robinul), and octreotide (Sandostatin).7,34–37
Which drugs are appropriate for reducing nausea and vomiting?
Phenothiazines reduce nausea and control vomiting. Chlorpromazine (Thorazine), prochlorperazine (Compro, Compazine), and promethazine (Phenergan) have all been reported to treat nausea successfully.35,37
Haloperidol (Haldol), a butyrophenone selective dopamine D2-receptor antagonist, has negligible anticholinergic activity. At low doses it produces less sedation than phenothiazines and is an ideal agent for patients with nausea and delirium.35 Doses range from 5 to 15 mg/day, given in divided doses or as intermittent or continuous intravenous infusions.
Anticholinergics, with or without somatostatin analogues, reduce gastrointestinal secretions, fluid accumulation, and vomiting. Anticholinergics bind to muscarinic receptors on enteric neurons in the myenteric and the submucosal plexus. Dosages:
- Hyoscine butylbromide 40 to 120 mg/day.
- Hyoscine hydrobromide 0.2 to 0.9 mg/day.7,34
Glycopyrrolate, a quaternary ammonium anticholinergic, has minimal central nervous system penetration and is less likely to cause delirium or cardiac side effects compared with tertiary amine anticholinergics such as atropine and scopolamine.38 The recommended dose is 0.1 to 0.2 mg subcutaneously or intravenously three to four times daily.
Octreotide, an analogue of somatostatin, blocks the release of vasoactive intestinal polypeptide, which is increased in malignant bowel obstruction.14,15 It reduces the excretion of water, sodium, and chloride into the bowel lumen and increases the absorption of electrolytes and water. It also inhibits pancreatic enzyme secretion and splanchnic blood flow. The result of all these effects is reduced luminal content, reduced motility, reduced vascular congestion of the bowel wall, and, in certain circumstances, reduced ascites.39
In small randomized trials, octreotide was more successful than anticholinergics at improving nausea, vomiting, and colic in patients requiring a nasogastric tube and in those whose symptoms were refractory to standard medical treatment.5,34,40–43 A recent case report found octreotide helpful in resolving symptoms of partial bowel obstruction that were unresponsive to standard measures.44
Octreotide is well tolerated and reduces the time patients require a nasogastric tube without significantly worsening xerostomia. High cost limits its use in American hospice care due to the Medicare capitated system of reimbursement for drugs and services, and as a result it is a second-tier drug despite evidence of its efficacy.
Octreotide doses are 100 to 200 mg every 8 hours.
Metoclopramide (Reglan), a dopaminergic antagonist, a 5HT4 receptor agonist, and a 5HT3 receptor blocker at doses greater than 120 mg/day, combines the action of a phenothiazine, which blocks D2 receptors in the central chemoreceptor trigger zone, with promotility actions through serotonin receptors (5HT4).35,37
Metoclopramide should not be used with anticholinergics or in patients with colic or complete obstruction.35,45 In some centers it is the first-line drug for functional or partial bowel obstruction.7 Dosages range from 40 to 240 mg/day.
Olanzapine (Zyprexa), an atypical antipsychotic, blocks multiple neurotransmitter receptors (D2, H1, Ach, 5HT3) responsible for initiating emesis. It is an option in patients whose nausea and vomiting fail to respond to standard antiemetics.46 Dosages range from 2.5 to 20 mg/day.
Dissolvable tablets are given sublingually, which makes olanzapine a versatile antiemetic in cases of intractable nausea. Our unpublished experience is that the sublingual route reduces nausea associated with malignant bowel obstruction and obviates the need for subcutaneous injections or intravenous antiemetic infusions.
Corticosteroids. Although how corticosteroids relieve malignant bowel obstruction is unknown, they are presumed to act centrally.37,45 In addition, they reduce peritumoral edema and luminal salt and water, and they also have antiemetic and analgesic properties.
Evidence from a meta-analysis found that 6 to 16 mg of parenteral dexamethasone per day reduced symptoms and improved bowel function in 60% of patients but did not change the prognosis.11
A trial of 4 or 5 days is adequate to determine response. If there is no response, the corticosteroid should be rapidly tapered. Side effects are minimal when corticosteroids are used short-term.
Combination therapy. Only rarely does a single drug resolve symptoms of malignant bowel obstruction. Antiemetics, analgesics, corticosteroids, antisecretory anticholinergics, and octreotide are often required in combination to achieve acceptable symptom relief.3,5,7,47
In a small prospective case series, the combination of metoclopramide 60 mg/day, octreotide 0.3 mg/day, and dexamethasone 12 mg/day with a single bolus of amidotrizoic acid (a contrast agent) improved intestinal transit within 1 to 5 days and resolved vomiting within 24 hours.45
Compatibility and the route of administration of medications are key considerations when choosing drug combinations.
WHEN TO CONSIDER A VENTING GASTROSTOMY
Patients with a poor performance status, rapidly progressive disease, peritoneal carcinomatosis, a life expectancy of less than 30 days, or multiple levels of obstruction benefit from placement of a percutaneous endoscopic gastrostomy tube (ie, a venting gastrostomy) rather than surgery if symptoms do not respond to drug therapy.7,48 There is compelling evidence that this procedure relieves nausea and vomiting in 80% to 90% of patients and restores some level of oral intake in many.5,6,48,49 A venting gastrostomy tube can be placed during surgical exploration, percutaneously with fluoroscopy, or endoscopically.9
There are no absolute contraindications to gastrostomy tube placement. It is feasible even in patients with tumors encasing the stomach, diffuse carcinomatosis, and ascites.48 However, massive ascites, previous upper abdominal surgery, or a large mass attached to the abdominal wall make tube placement difficult.
Complications are often local. Patients experience transient abdominal wall pain after the procedure. Dislodgement, bleeding, catheter migration, peritonitis, and necrotizing fasciitis are early complications. Others include skin excoriation from leakage of gastric contents, leakage of ascitic fluid from the site, and obstruction or dislodgement of the tube.48,49
Patients can be discharged from the hospital soon after the tube is placed, usually with fewer medications than for patients who undergo surgery.48 This is particularly important for patients with a short expected survival. Some patients at home benefit from hydration (less than 2 L/day) via an existing central venous port or peripherally inserted central catheter, or by hypodermoclysis.
WHEN IS A NASOGASTRIC TUBE APPROPRIATE?
Some patients with malignant bowel obstruction require a nasogastric tube early in their hospital course.12 Unfortunately, nasogastric tubes, if left in place, cause nose and throat pain, sinusitis, abscess formation, erosion of nasal cartilage, aspiration, esophageal erosion, pharyngitis, and social isolation.5,6
Nasogastric tubes should be a temporizing measure to vent gastrointestinal secretions, reduce abdominal distension, and improve nausea and vomiting while a decision about surgery is being made.13,24 If surgery is not feasible, one can avoid the long-term complications and discomfort of a nasogastric tube via medical management and earlier evaluation for venting gastrostomy in those with symptoms that respond poorly to optimal medical management.49
WHICH PATIENTS BENEFIT FROM TOTAL PARENTERAL NUTRITION?
The use of total parenteral nutrition in patients with incurable malignancies is controversial. Enteral and parenteral feeding can increase muscle mass and improve functional status and quality of life in a subset of patients who are not suffering from cancer-related cachexia.2,50,51 However, for those whose weight loss and malnutrition are consequences of tumor-mediated cachexia, as demonstrated by anorexia and an elevated C-reactive protein level, parenteral nutrition is unlikely to improve the outcome.51 For most terminally ill patients, retrospective studies have failed to show that parenteral nutrition improves overall survival, performance status, or quality of life.2,48,50–54
Total parenteral nutrition poses risks: it is invasive and requires central venous access, which predisposes to infection; it requires frequent monitoring of hydration and electrolytes; and it predisposes to thrombosis, diarrhea, hyperglycemia, and liver failure.50–56
Total parenteral nutrition may be justified in patients with minimal tumor burden who are candidates for definitive surgery, or in those with a good performance status early in the disease course who have not had chemotherapy or whose cancer responds to chemotherapy.2,50–56
The American College of Physicians discourages the routine use of parenteral nutrition in those with advanced cancer who are undergoing palliative chemotherapy, since few patients benefit and many experience side effects.53
Total parenteral nutrition is much like a medical intervention in that it should be offered or continued only if it provides benefit. Conversations at the time that it is begun must include adverse effects that will lead to its discontinuation, and criteria for response. In certain situations, a limited trial of parenteral nutrition may be considered for patients with an uncertain prognosis or for those who have potentially reversible conditions that limit oral intake.51 In such cases, there should be a clear understanding between patient and physician that parenteral nutrition will be discontinued if it fails to show benefit.53
ADDITIONAL CONCERNS OF PATIENTS AND FAMILIES
‘Will I starve to death?’
Starvation is a fear echoed by patients and families. Ethical discourse on the continuation of nutrition and hydration for the terminally ill has been polarizing.57–60 Withdrawal of nutrition can be perceived as euthanasia.
Advanced cancer patients in general do not experience hunger, and those who do require only small amounts of food for satiation.61 In one report, most patients died of their advanced cancer and not from starvation.52 Artificial hydration and nutrition will thus not influence survival and can even be a burden without benefit in the imminently dying.60 These patients should be encouraged to take food orally for pleasure, as long as it is tolerated, without consideration of end points such as weight gain, body mass index, or albumin levels.
Complaints of thirst and dryness of the mouth are relieved with mouth care, ice chips, lubrication to the lips, and sips of fluid, rather than by parenteral nutrition.59 Patients with a terminal illness experience relief from thirst with minimal intake. The symptom of thirst may be relieved without hydration.34,61 Adequate hydration requires smaller fluid volumes due to decreased body weight, decreased renal clearance of free water, and decreased insensible water losses from reduced physical activity.58
‘Can we continue intravenous hydration so he won’t die of thirst?’
Overzealous intravenous hydration may worsen the symptoms of malignant bowel obstruction. Overhydration can increase secretions in the gut lumen and worsen the secretion-distention-contraction cycle, leading to greater abdominal pain and to nausea and vomiting.7 There is a greater risk of fluid overload in these patients, since they have edema and excessive interstitial fluid. Most have a low serum albumin level, which results in movement of fluid from intravascular to interstitial spaces due to reduced colloid osmotic pressure. In these instances, overzealous hydration can lead to respiratory insufficiency and worsening edema.
In spite of numerous discussions in the medical literature of the benefits and burdens of continual hydration, there is no consensus or guideline. When a patient has limited oral intake, the decision to hydrate should be individualized, with careful assessment of the risks and benefits and in accordance with the patient’s or family’s wishes.57,58
Is treatment at home feasible?
Discharging patients with inoperable malignant bowel obstruction requires careful planning. Patients and family members need to be educated on the use of around-the-clock medications and symptom-targeted, as-needed drugs. Days before discharge, questions about diet need to be clarified. Education about total parenteral nutrition and gastrostomy tube care should be completed before discharge from the hospital.
Drug management should be simplified, or compatible medications should be combined into a single infusion. For example, morphine, glycopyrrolate, and haloperidol or metoclopramide are chemically compatible in standard intravenous solutions and can be combined.
Families feel less anxious about the foreseen and the possible unforeseen course of the illness if they can talk with hospice workers early on. This early involvement also facilitates the transition to home hospice care.
SUMMARY OF IMPORTANT POINTS
- Patients with malignant bowel obstruction need a highly individualized approach, tailored to their medical condition, the prognosis, and the goals of care.
- Surgery should not be routinely undertaken; less-invasive approaches such as gastric or colonic stenting should be considered first.
- Combinations of analgesics, antisecretory drugs, and antiemetics can provide acceptable symptom relief in the inoperable patient.
- A venting gastrostomy should be considered if drug therapy fails to reduce nausea and vomiting to an acceptable level.
- A nasogastric tube should be used only as a temporizing measure, until symptoms are controlled medically or a venting gastrostomy is placed.
- Total parenteral nutrition is of benefit only in patients with intermediate life expectancy who may otherwise die of starvation rather than from the cancer itself.
Malignant bowel obstruction occurs in 5% to 51% of women with ovarian cancer and in 10% to 28% of patients with gastrointestinal cancer, predominantly in the advanced stages.1 Median survival after its onset ranges from 30 to 90 days.2–5
Its symptoms are challenging to manage, since nausea, vomiting, colic, and abdominal pain, which are common, cause significant physical distress and demoralization. The decision whether to correct it with surgery requires an individualized approach and a clear understanding of the goals of care and expected survival in the individual patient.
This review focuses on the management of inoperable malignant bowel obstruction and includes discussion of hydration, nutrition, and endoscopic palliative options.
WHAT ARE THE DIFFERENT TYPES OF OBSTRUCTION?
Bowel obstruction may be mechanical or functional, partial or complete, and may occur at one or at many sites. Tumors can impair bowel function in several ways6–8:
- Intraluminal tumors can occlude the lumen or act as a point of intussusception.
- Intramural tumors can extend to the mucosa and obstruct the lumen or impair peristalsis.
- Mesenteric and omental masses or malignant adhesions can kink or angulate the bowel, creating an extramural obstruction.
- Tumors that infiltrate into the mesentery, bowel muscle, or the enteric or celiac plexus can cause dysmotility.
Cholangiocarcinoma, pancreatic carcinoma, and gallbladder carcinoma are the most common tumors causing duodenal obstruction.9 Distal obstruction is caused mainly by colon and ovarian cancer.
Obstruction can be due to treatment
In a minority of patients, obstruction is unrelated to the cancer and is instead due to adhesions arising from surgery, radiation therapy (causing enteritis and strictures), desmoplastic reactions to intraperitoneal chemotherapy, torsion, or internal hernias.10–12
In rare cases, a patient has intestinal pseudo-obstruction from paraneoplastic destruction of enteric neurons, or severe ileus from anticholinergic or sympathomimetic drugs, as seen with acute colonic pseudo-obstruction (Ogilvie syndrome).13
Physiologic reactions to obstruction
Malignant bowel obstruction stimulates gastric, biliary, pancreatic, and intestinal secretions, decreases intraluminal sodium and water reabsorption, and increases mucosal sodium and water secretion.6,14 In response to the obstruction, peristalsis increases, and prostaglandin, vasoactive intestinal peptide, and nociceptive mediators are released. Vasoactive intestinal polypeptide perpetuates a cycle of secretion, distention, and contraction that leads to intestinal hyperemia, bowel edema, and accumulation of fluid in the lumen.8,10,11,15
Signs and symptoms depend on the site
The site of obstruction determines the signs and symptoms patients experience.7,14 Obstructions high in the gastrointestinal tract are associated with greater symptoms but fewer signs than colonic obstructions.1 Patients with proximal small-bowel obstruction have more severe nausea and a greater number of episodes of emesis, but they have relatively normal plain radiographs of the abdomen, which do not have the characteristic air-fluid levels commonly seen with distal small-bowel obstruction.
Most malignant obstructions remain partial, but increasing abdominal distention, worsening nausea, vomiting, abdominal pain, and obstipation over 1 to 2 weeks1 suggest progression to complete obstruction.
IMAGING TESTS FOR MALIGNANT BOWEL OBSTRUCTION
What is the value of plain radiography?
Despite these limitations, plain radiography is useful in assessing constipation and its severity as a potential cause of symptoms, and thus it remains an important initial imaging study in almost all patients with suspected malignant bowel obstruction.17,18 It is also used to assess response to treatment.
When do you need contrast radiographs?
Contrast radiography (barium swallow or barium or Gastrograffin enema) is helpful in patients with symptoms of dysmotility from suspected bowel obstruction. It defines the site or sites of obstruction and the extent of the obstruction with a fair degree of accuracy.7,19 Single-contrast studies, if positive, exclude opioid-induced bowel dysfunction or pseudo-obstruction in 83% of patients, with a sensitivity and specificity of 96% and 98%, respectively.8,20 Small-bowel follow-through with barium is more appropriate for low-grade obstructions or for symptomatic patients with a normal kidney, ureter, bladder radiograph.19
However, contrast radiography is limited by the patient’s ability to swallow barium or water-soluble contrast agents, and it can worsen nausea or vomiting.17,18 Also, barium is not absorbed systemically and may interfere with subsequent radiologic studies. Large volumes of contrast agents increase the risk of aspiration pneumonia in patients with poorly controlled nausea and can lead to severe impaction proximal to the obstructed site.8
Is enteroclysis better than barium swallow?
Enteroclysis, ie, injecting radiographic contrast into the bowel via a nasoduodenal tube, has some advantages over the barium swallow technique for detecting partial small-bowel obstruction, since it bypasses the stomach and allows for therapeutic decompression as well as direct visualization of the area of concern.17,18 Enteroclysis radiography objectively gauges severity of intestinal obstruction and bowel wall distensibility, which is an advantage over other imaging studies. Its sensitivity is 100% and specificity 88% in experienced hands.17 Enteroclysis studies also detect nonobstructing intraluminal tumors when computed tomography (CT) is not diagnostic.17,18,21
The drawbacks to enteroclysis are that it is technically difficult to perform and that few radiologists are trained in it.
When is CT useful?
CT is the primary imaging study for patients with obstructive symptoms and a history of abdominal malignancy or a palpable abdominal mass17,20,22,23 (Figure 1). It has a specificity of 100% and a sensitivity of 94%. It plays a major role in decision-making regarding surgery, endoscopy, or palliative interventions,7,19 as it locates the obstruction and differentiates benign from malignant causes with a fair degree of precision.22
CT findings in malignant bowel obstruction may include:
- A mass at the site of obstruction or within the original surgical field
- Lymphadenopathy
- Abrupt transitions in luminal diameter or irregular thickening of the bowel wall at the site or sites of obstruction.7
SURGERY: A DIFFICULT DECISION
Is the patient fit for surgery?
Surgery for malignant bowel obstruction should not be done in patients who have advanced malignancies with bulky intra-abdominal metastases or cancer that has spread outside the abdominal cavity without taking into account treatment options for the cancer, the patient’s nutritional status, and the goals of care.
The role of abdominal surgery (debulking, resection, or bypass) in advanced cancer remains unclear and controversial.24 From 42% to 80% of patients report that symptoms improve after surgery, but recurrent obstruction occurs in 10% to 50%.10 Even in patients with low tumor bulk and good nutritional status, 30-day mortality rates range from 5% to 40%, and complication rates range from 9% to 90%.3,4,6,7,10,14
Outcomes after surgery depend on patient selection criteria perhaps as much as on the surgeon’s experience and skill. Patients with more advanced cancer who have had multiple surgical procedures and those with cancer that does not respond to chemotherapy and radiation present the greatest challenge to surgeons.23
What is the benefit of surgery?
Reports of palliative surgery have included information about 60-day survival rates after the operation, but a number of factors may be more meaningful in this context, such as postoperative symptoms, the patient’s overall wellbeing, how the original symptoms respond to the surgery, complications, and length of hospitalization.14 The paucity of published, validated, patient-related outcome data on which to gauge the value of surgery and the lack of a standard definition of “benefit” further confuse the objective determination of whether these patients benefit from surgery.
In a cohort with advanced ovarian cancer and bowel obstruction, surgery was detrimental to survival and quality of life for all subgroups, and most patients died in the hospital.6
The risk of surgery for malignant bowel obstruction is presumably higher than for abdominal surgery for other indications, since many of the patients are debilitated from their cancer and chemotherapy, and many are malnourished.23 Even when taking into account a potential selection bias in favor of surgery, several studies have reported no significant difference in 30-day mortality rates or median survival between operative and nonoperative groups.2,12 Neither the type of obstruction nor the extent of the surgery influenced outcomes. Surgical outcomes are best in patients with a benign cause of obstruction; little benefit is seen in operating on those with abdominal carcinomatosis.12
Nevertheless, surgery is beneficial in a select few. For patients with a good performance status, slowly progressive cancer, and an expected survival of more than 6 months, surgical bypass or resection is preferred.7,12,25 The challenge is to identify these surgical candidates, taking into account prognostic factors such as nutritional status, tumor burden, performance status, presence of ascites, advanced age, extensive prior chemotherapy or radiotherapy, and diffuse carcinomatosis.3,10,12,20,23
Is surgery consistent with the goals of care?
Crucial to decision-making are the goals of care. Since palliative surgery carries a low level of evidence for benefit in terms of quality of life and survival, time should be set aside to thoroughly review the patient’s medical condition, to explore options, and to clarify expectations and goals of care.3,10 Family members should be invited to be present during these discussions and to be involved in the decision-making process.
WHAT IS THE BENEFIT OF GASTRIC OR COLONIC STENTING?
Endoscopic procedures are alternatives to surgery and offer a palliative option in malignant bowel obstruction. Endoscopic procedures are associated with a shorter hospital stay and quicker recovery than after laparotomy.9,26–30 In certain situations, stenting serves as a bridge to surgery, allowing time to mitigate comorbid conditions, to enhance nutrition, and to complete staging, while relieving symptoms.27–29,31,32 Definitive surgery can be done as a single-stage procedure without a diverting enterostomy.
Self-expanding metal stents for gastric outlet, small-bowel, and colonic obstructions are an option in patients who have incurable metastatic disease who are unfit for surgery, in patients with a single point of obstruction or locally extensive disease, or in patients who do not want to undergo laparotomy.28–30
Technical and clinical success rates for colorectal stenting are high (88% to 93%).26,27 Stenting is more successful for left-sided colonic obstructions than for proximal colonic obstructions. Even for patients with extracolonic malignancies such as ovarian cancer, the technical success rate of colorectal stenting is 87%.26 However, patients with unrecognized peritoneal carcinomatosis or multifocal bowel obstruction are less likely to have symptomatic relief even after successful stenting.6,9
Contraindications to stenting
Absolute contraindications to stenting are colonic or tumor perforation with peritonitis. A relative contraindication is a rectal tumor within 2 cm of the anal margin. Stenting in this circumstance leads to tenesmus and incontinence.33
Complications of stenting
Death rates during colorectal stent insertion are less than 1%. The hospital stay and incidence of complications are significantly less than with surgery.26,30
Stent migration occurs in 10% of cases and is asymptomatic, but half of patients with this complication require a repeat intervention. The risk of migration is greater if chemotherapy or radiation therapy succeeds in shrinking the tumor.
Bleeding occurs in 5% of cases, usually from the underlying tumor.
Perforation occurs in 4%, but the rate increases to 10% with the use of dilatation before stent placement.
The rate of recurrent obstruction from tumor ingrowth, overgrowth, or fecal impaction is 10%.9,26,29 Recurrent obstruction may be treated with additional stents inserted within the original stent.9
GASTRIC OUTLET OBSTRUCTION: SURGERY VS STENTING
Gastrojejunostomy has in the past been the treatment of choice for gastric outlet obstruction. Certainly, patients with slow-growing tumors and an expected survival of greater than 60 days may be considered for this bypass procedure; those with a short tumor length, a single site of obstruction (preferably in the pylorus or proximal duodenum), a good performance status, and a life expectancy greater than 30 days are good candidates.7 Nevertheless, for patients with advanced cancer and poor performance status, gastroenterostomy carries a significant risk of morbidity and death.28
Endoscopic stenting of gastric outlet obstruction has a greater success rate, a shorter time to oral intake, a lower morbidity rate, a lower incidence of delayed gastric emptying, and a shorter hospital stay compared with gastroenterostomy.28,29 Technical success rates of stenting are 90%, and 75% of patients have resolution of nausea and vomiting.7 Stenting is generally not possible if the obstruction occurs beyond the ligament of Treitz.
Patients who are expected to survive less than 1 month or who have rapidly progressive disease, overt ascites, carcinomatosis, or multiple sites of obstruction should be managed with percutaneous, endoscopically placed gastrostomy tubes.7
Late complications of stenting for gastric outlet obstruction are occlusion with food or ingrowth of tumor through or around the wire mesh.7 This may require laser therapy or placement of a second stent, or both.
DRUG THERAPY
Medical therapy can palliate symptoms of malignant bowel obstruction for most patients.34 Recommendations have been published by the Working Group of the European Association for Palliative Care.24 Symptom management is focused on pain, nausea, and vomiting.
Which drugs can I use for abdominal pain?
Patients experience two types of abdominal pain: continuous and colic. Each type of pain requires different treatment approaches and classes of drugs.
Potent opioids such as morphine, hydromorphone (Dilaudid), and fentanyl (Fentora) are used to relieve continuous abdominal pain.7 The dose is titrated for adequate relief. Subcutaneous, intravenous, sublingual, and transdermal routes can be used if nausea and vomiting prevent oral administration.
However, opioids can aggravate colic by stimulating circular smooth muscle, leading to segmental contractions. Opioid-sparing adjuvant drugs such as ketorolac (Toradol) may improve colic and continuous pain and prevent a partial obstruction from becoming a complete obstruction by sparing opioid doses.35
Colic may persist or worsen with the use of opioids. Drugs that reduce colic include the scopolamine drugs hyoscine butylbromide and hyoscine hydrobromide, glycopyrrolate (Robinul), and octreotide (Sandostatin).7,34–37
Which drugs are appropriate for reducing nausea and vomiting?
Phenothiazines reduce nausea and control vomiting. Chlorpromazine (Thorazine), prochlorperazine (Compro, Compazine), and promethazine (Phenergan) have all been reported to treat nausea successfully.35,37
Haloperidol (Haldol), a butyrophenone selective dopamine D2-receptor antagonist, has negligible anticholinergic activity. At low doses it produces less sedation than phenothiazines and is an ideal agent for patients with nausea and delirium.35 Doses range from 5 to 15 mg/day, given in divided doses or as intermittent or continuous intravenous infusions.
Anticholinergics, with or without somatostatin analogues, reduce gastrointestinal secretions, fluid accumulation, and vomiting. Anticholinergics bind to muscarinic receptors on enteric neurons in the myenteric and the submucosal plexus. Dosages:
- Hyoscine butylbromide 40 to 120 mg/day.
- Hyoscine hydrobromide 0.2 to 0.9 mg/day.7,34
Glycopyrrolate, a quaternary ammonium anticholinergic, has minimal central nervous system penetration and is less likely to cause delirium or cardiac side effects compared with tertiary amine anticholinergics such as atropine and scopolamine.38 The recommended dose is 0.1 to 0.2 mg subcutaneously or intravenously three to four times daily.
Octreotide, an analogue of somatostatin, blocks the release of vasoactive intestinal polypeptide, which is increased in malignant bowel obstruction.14,15 It reduces the excretion of water, sodium, and chloride into the bowel lumen and increases the absorption of electrolytes and water. It also inhibits pancreatic enzyme secretion and splanchnic blood flow. The result of all these effects is reduced luminal content, reduced motility, reduced vascular congestion of the bowel wall, and, in certain circumstances, reduced ascites.39
In small randomized trials, octreotide was more successful than anticholinergics at improving nausea, vomiting, and colic in patients requiring a nasogastric tube and in those whose symptoms were refractory to standard medical treatment.5,34,40–43 A recent case report found octreotide helpful in resolving symptoms of partial bowel obstruction that were unresponsive to standard measures.44
Octreotide is well tolerated and reduces the time patients require a nasogastric tube without significantly worsening xerostomia. High cost limits its use in American hospice care due to the Medicare capitated system of reimbursement for drugs and services, and as a result it is a second-tier drug despite evidence of its efficacy.
Octreotide doses are 100 to 200 mg every 8 hours.
Metoclopramide (Reglan), a dopaminergic antagonist, a 5HT4 receptor agonist, and a 5HT3 receptor blocker at doses greater than 120 mg/day, combines the action of a phenothiazine, which blocks D2 receptors in the central chemoreceptor trigger zone, with promotility actions through serotonin receptors (5HT4).35,37
Metoclopramide should not be used with anticholinergics or in patients with colic or complete obstruction.35,45 In some centers it is the first-line drug for functional or partial bowel obstruction.7 Dosages range from 40 to 240 mg/day.
Olanzapine (Zyprexa), an atypical antipsychotic, blocks multiple neurotransmitter receptors (D2, H1, Ach, 5HT3) responsible for initiating emesis. It is an option in patients whose nausea and vomiting fail to respond to standard antiemetics.46 Dosages range from 2.5 to 20 mg/day.
Dissolvable tablets are given sublingually, which makes olanzapine a versatile antiemetic in cases of intractable nausea. Our unpublished experience is that the sublingual route reduces nausea associated with malignant bowel obstruction and obviates the need for subcutaneous injections or intravenous antiemetic infusions.
Corticosteroids. Although how corticosteroids relieve malignant bowel obstruction is unknown, they are presumed to act centrally.37,45 In addition, they reduce peritumoral edema and luminal salt and water, and they also have antiemetic and analgesic properties.
Evidence from a meta-analysis found that 6 to 16 mg of parenteral dexamethasone per day reduced symptoms and improved bowel function in 60% of patients but did not change the prognosis.11
A trial of 4 or 5 days is adequate to determine response. If there is no response, the corticosteroid should be rapidly tapered. Side effects are minimal when corticosteroids are used short-term.
Combination therapy. Only rarely does a single drug resolve symptoms of malignant bowel obstruction. Antiemetics, analgesics, corticosteroids, antisecretory anticholinergics, and octreotide are often required in combination to achieve acceptable symptom relief.3,5,7,47
In a small prospective case series, the combination of metoclopramide 60 mg/day, octreotide 0.3 mg/day, and dexamethasone 12 mg/day with a single bolus of amidotrizoic acid (a contrast agent) improved intestinal transit within 1 to 5 days and resolved vomiting within 24 hours.45
Compatibility and the route of administration of medications are key considerations when choosing drug combinations.
WHEN TO CONSIDER A VENTING GASTROSTOMY
Patients with a poor performance status, rapidly progressive disease, peritoneal carcinomatosis, a life expectancy of less than 30 days, or multiple levels of obstruction benefit from placement of a percutaneous endoscopic gastrostomy tube (ie, a venting gastrostomy) rather than surgery if symptoms do not respond to drug therapy.7,48 There is compelling evidence that this procedure relieves nausea and vomiting in 80% to 90% of patients and restores some level of oral intake in many.5,6,48,49 A venting gastrostomy tube can be placed during surgical exploration, percutaneously with fluoroscopy, or endoscopically.9
There are no absolute contraindications to gastrostomy tube placement. It is feasible even in patients with tumors encasing the stomach, diffuse carcinomatosis, and ascites.48 However, massive ascites, previous upper abdominal surgery, or a large mass attached to the abdominal wall make tube placement difficult.
Complications are often local. Patients experience transient abdominal wall pain after the procedure. Dislodgement, bleeding, catheter migration, peritonitis, and necrotizing fasciitis are early complications. Others include skin excoriation from leakage of gastric contents, leakage of ascitic fluid from the site, and obstruction or dislodgement of the tube.48,49
Patients can be discharged from the hospital soon after the tube is placed, usually with fewer medications than for patients who undergo surgery.48 This is particularly important for patients with a short expected survival. Some patients at home benefit from hydration (less than 2 L/day) via an existing central venous port or peripherally inserted central catheter, or by hypodermoclysis.
WHEN IS A NASOGASTRIC TUBE APPROPRIATE?
Some patients with malignant bowel obstruction require a nasogastric tube early in their hospital course.12 Unfortunately, nasogastric tubes, if left in place, cause nose and throat pain, sinusitis, abscess formation, erosion of nasal cartilage, aspiration, esophageal erosion, pharyngitis, and social isolation.5,6
Nasogastric tubes should be a temporizing measure to vent gastrointestinal secretions, reduce abdominal distension, and improve nausea and vomiting while a decision about surgery is being made.13,24 If surgery is not feasible, one can avoid the long-term complications and discomfort of a nasogastric tube via medical management and earlier evaluation for venting gastrostomy in those with symptoms that respond poorly to optimal medical management.49
WHICH PATIENTS BENEFIT FROM TOTAL PARENTERAL NUTRITION?
The use of total parenteral nutrition in patients with incurable malignancies is controversial. Enteral and parenteral feeding can increase muscle mass and improve functional status and quality of life in a subset of patients who are not suffering from cancer-related cachexia.2,50,51 However, for those whose weight loss and malnutrition are consequences of tumor-mediated cachexia, as demonstrated by anorexia and an elevated C-reactive protein level, parenteral nutrition is unlikely to improve the outcome.51 For most terminally ill patients, retrospective studies have failed to show that parenteral nutrition improves overall survival, performance status, or quality of life.2,48,50–54
Total parenteral nutrition poses risks: it is invasive and requires central venous access, which predisposes to infection; it requires frequent monitoring of hydration and electrolytes; and it predisposes to thrombosis, diarrhea, hyperglycemia, and liver failure.50–56
Total parenteral nutrition may be justified in patients with minimal tumor burden who are candidates for definitive surgery, or in those with a good performance status early in the disease course who have not had chemotherapy or whose cancer responds to chemotherapy.2,50–56
The American College of Physicians discourages the routine use of parenteral nutrition in those with advanced cancer who are undergoing palliative chemotherapy, since few patients benefit and many experience side effects.53
Total parenteral nutrition is much like a medical intervention in that it should be offered or continued only if it provides benefit. Conversations at the time that it is begun must include adverse effects that will lead to its discontinuation, and criteria for response. In certain situations, a limited trial of parenteral nutrition may be considered for patients with an uncertain prognosis or for those who have potentially reversible conditions that limit oral intake.51 In such cases, there should be a clear understanding between patient and physician that parenteral nutrition will be discontinued if it fails to show benefit.53
ADDITIONAL CONCERNS OF PATIENTS AND FAMILIES
‘Will I starve to death?’
Starvation is a fear echoed by patients and families. Ethical discourse on the continuation of nutrition and hydration for the terminally ill has been polarizing.57–60 Withdrawal of nutrition can be perceived as euthanasia.
Advanced cancer patients in general do not experience hunger, and those who do require only small amounts of food for satiation.61 In one report, most patients died of their advanced cancer and not from starvation.52 Artificial hydration and nutrition will thus not influence survival and can even be a burden without benefit in the imminently dying.60 These patients should be encouraged to take food orally for pleasure, as long as it is tolerated, without consideration of end points such as weight gain, body mass index, or albumin levels.
Complaints of thirst and dryness of the mouth are relieved with mouth care, ice chips, lubrication to the lips, and sips of fluid, rather than by parenteral nutrition.59 Patients with a terminal illness experience relief from thirst with minimal intake. The symptom of thirst may be relieved without hydration.34,61 Adequate hydration requires smaller fluid volumes due to decreased body weight, decreased renal clearance of free water, and decreased insensible water losses from reduced physical activity.58
‘Can we continue intravenous hydration so he won’t die of thirst?’
Overzealous intravenous hydration may worsen the symptoms of malignant bowel obstruction. Overhydration can increase secretions in the gut lumen and worsen the secretion-distention-contraction cycle, leading to greater abdominal pain and to nausea and vomiting.7 There is a greater risk of fluid overload in these patients, since they have edema and excessive interstitial fluid. Most have a low serum albumin level, which results in movement of fluid from intravascular to interstitial spaces due to reduced colloid osmotic pressure. In these instances, overzealous hydration can lead to respiratory insufficiency and worsening edema.
In spite of numerous discussions in the medical literature of the benefits and burdens of continual hydration, there is no consensus or guideline. When a patient has limited oral intake, the decision to hydrate should be individualized, with careful assessment of the risks and benefits and in accordance with the patient’s or family’s wishes.57,58
Is treatment at home feasible?
Discharging patients with inoperable malignant bowel obstruction requires careful planning. Patients and family members need to be educated on the use of around-the-clock medications and symptom-targeted, as-needed drugs. Days before discharge, questions about diet need to be clarified. Education about total parenteral nutrition and gastrostomy tube care should be completed before discharge from the hospital.
Drug management should be simplified, or compatible medications should be combined into a single infusion. For example, morphine, glycopyrrolate, and haloperidol or metoclopramide are chemically compatible in standard intravenous solutions and can be combined.
Families feel less anxious about the foreseen and the possible unforeseen course of the illness if they can talk with hospice workers early on. This early involvement also facilitates the transition to home hospice care.
SUMMARY OF IMPORTANT POINTS
- Patients with malignant bowel obstruction need a highly individualized approach, tailored to their medical condition, the prognosis, and the goals of care.
- Surgery should not be routinely undertaken; less-invasive approaches such as gastric or colonic stenting should be considered first.
- Combinations of analgesics, antisecretory drugs, and antiemetics can provide acceptable symptom relief in the inoperable patient.
- A venting gastrostomy should be considered if drug therapy fails to reduce nausea and vomiting to an acceptable level.
- A nasogastric tube should be used only as a temporizing measure, until symptoms are controlled medically or a venting gastrostomy is placed.
- Total parenteral nutrition is of benefit only in patients with intermediate life expectancy who may otherwise die of starvation rather than from the cancer itself.
- Mercadante S. Intestinal dysfunction and obstruction. In:Walsh D, editor. Palliative Medicine. Philadelphia, PA: Saunders/Elsevier, 2009:1267–1275.
- Pasanisi F, Orban A, Scalfi L, et al. Predictors of survival in terminal-cancer patients with irreversible bowel obstruction receiving home parenteral nutrition. Nutrition 2001; 17:581–584.
- Pameijer CR, Mahvi DM, Stewart JA, Weber SM. Bowel obstruction in patients with metastatic cancer: does intervention influence outcome? Int J Gastrointest Cancer 2005; 35:127–133.
- Bais JMJ, Schilthuis MS, Ansink AC. Palliative management of intestinal obstruction in patients with advanced gynaecological cancer. J Gynecol Oncol 2002; 7:299–305.
- Laval G, Arvieux C, Stefani L, Villard ML, Mestrallet JP, Cardin N. Protocol for the treatment of malignant inoperable bowel obstruction: a prospective study of 80 cases at Grenoble University Hospital Center. J Pain Symptom Manage 2006; 31:502–512.
- Jatoi A, Podratz KC, Gill P, Hartmann LC. Pathophysiology and palliation of inoperable bowel obstruction in patients with ovarian cancer. J Support Oncol 2004; 2:323–334.
- Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer 2008; 44:1105–1115.
- Roeland E, von Gunten CF. Current concepts in malignant bowel obstruction management. Curr Oncol Rep 2009; 11:298–303.
- Baron TH. Interventional palliative strategies for malignant bowel obstruction. Curr Oncol Rep 2009; 11:293–297.
- Feuer DJ, Broadley KE, Shepherd JH, Barton DP. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD002764.
- Feuer DJ, Broadley KE. Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD001219.
- Woolfson RG, Jennings K, Whalen GF. Management of bowel obstruction in patients with abdominal cancer. Arch Surg 1997; 132:1093–1097.
- Saunders MD, Kimmey MB. Systematic review: acute colonic pseudo-obstruction. Aliment Pharmacol Ther 2005; 22:917–925.
- Ripamonti C, Bruera E. Palliative management of malignant bowel obstruction. Int J Gynecol Cancer 2002; 12:135–143.
- Nellgård P, Bojö L, Cassuto J. Importance of vasoactive intestinal peptide and somatostatin for fluid losses in small-bowel obstruction. Scand J Gastroenterol 1995; 30:464–469.
- Böhner H, Yang Q, Franke C, Verreet PR, Ohmann C. Simple data from history and physical examination help to exclude bowel obstruction and to avoid radiographic studies in patients with acute abdominal pain. Eur J Surg 1998; 164:777–784.
- Maglinte DD, Kelvin FM, Sandrasegaran K, et al. Radiology of small bowel obstruction: contemporary approach and controversies. Abdom Imaging 2005; 30:160–178.
- Maglinte DD, Howard TJ, Lillemoe KD, Sandrasegaran K, Rex DK. Small-bowel obstruction: state-of-the-art imaging and its role in clinical management. Clin Gastroenterol Hepatol 2008; 6:130–139.
- Silva AC, Pimenta M, Guimarães LS. Small bowel obstruction: what to look for. Radiographics 2009; 29:423–439.
- Finan PJ, Campbell S, Verma R, et al. The management of malignant large bowel obstruction: ACPGBI position statement. Colorectal Dis 2007; 9(suppl 4):1–17.
- Kohli MD, Maglinte DD. CT enteroclysis in incomplete small bowel obstruction. Abdom Imaging 2009; 34:321–327.
- Ha HK, Shin BS, Lee SI, et al. Usefulness of CT in patients with intestinal obstruction who have undergone abdominal surgery for malignancy. AJR Am J Roentgenol 1998; 171:1587–1593.
- DeBernardo R. Surgical management of malignant bowel obstruction: strategies toward palliation of patients with advanced cancer. Curr Oncol Rep 2009; 11:287–292.
- Ripamonti C, Twycross R, Baines M, et al; Working Group of the European Association for Palliative Care. Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Support Care Cancer 2001; 9:223–233.
- Mangili G, Aletti G, Frigerio L, et al. Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. Int J Gynecol Cancer 2005; 15:830–835.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg 2002; 89:1096–1102.
- Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol 2007; 42:283–290.
- Del Piano M, Ballarè M, Montino F, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc 2005; 61:421–426.
- Tilney HS, Lovegrove RE, Purkayastha S, et al. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc 2007; 21:225–233.
- Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc 2004; 60:1010–1017.
- Dastur JK, Forshaw MJ, Modarai B, Solkar MM, Raymond T, Parker MC. Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech Coloproctol 2008; 12:51–55.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Ripamonti C, Mercadante S, Groff L, Zecca E, De Conno F, Casuccio A. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: a prospective randomized trial. J Pain Symptom Manage 2000; 19:23–34.
- Davis MP, Walsh D. Treatment of nausea and vomiting in advanced cancer. Support Care Cancer 2000; 8:444–452.
- Bicanovsky LK, Lagman RL, Davis MP, Walsh D. Managing nonmalignant chronic abdominal pain and malignant bowel obstruction. Gastroenterol Clin North Am 2006; 35:131–142.
- Glare P, Pereira G, Kristjanson LJ, Stockler M, Tattersall M. Systematic review of the efficacy of antiemetics in the treatment of nausea in patients with far-advanced cancer. Support Care Cancer 2004; 12:432–440.
- Davis MP, Furste A. Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage 1999; 18:153–154.
- Ripamonti C, Mercadante S. How to use octreotide for malignant bowel obstruction. J Support Oncol 2004; 2:357–364.
- Shima Y, Ohtsu A, Shirao K, Sasaki Y. Clinical efficacy and safety of octreotide (SMS201-995) in terminally ill Japanese cancer patients with malignant bowel obstruction. Jpn J Clin Oncol 2008; 38:354–359.
- Mercadante S, Casuccio A, Mangione S. Medical treatment for inoperable malignant bowel obstruction: a qualitative systematic review. J Pain Symptom Manage 2007; 33:217–223.
- Mystakidou K, Tsilika E, Kalaidopoulou O, Chondros K, Georgaki S, Papadimitriou L. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double-blind, controlled clinical trial. Anticancer Res 2002; 22:1187–1192.
- Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer 2000; 8:188–191.
- Myers J, Tamber A, Farhadian M. Management of treatment-related intermittent partial small bowel obstruction: the use of octreotide. J Pain Symptom Manage 2010; 39:e1–e3.
- Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage 2004; 28:412–416.
- Srivastava M, Brito-Dellan N, Davis MP, Leach M, Lagman R. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage 2003; 25:578–582.
- Bentley A, Boyd K. Use of clinical pictures in the management of nausea and vomiting: a prospective audit. Palliat Med 2001; 15:247–253.
- Pothuri B, Montemarano M, Gerardi M, et al. Percutaneous endoscopic gastrostomy tube placement in patients with malignant bowel obstruction due to ovarian carcinoma. Gynecol Oncol 2005; 96:330–334.
- Brooksbank MA, Game PA, Ashby MA. Palliative venting gastrostomy in malignant intestinal obstruction. Palliat Med 2002; 16:520–526.
- Wang MY, Wu MH, Hsieh DY, et al. Home parenteral nutrition support in adults: experience of a medical center in Asia. JPEN J Parenter Enteral Nutr 2007; 31:306–310.
- Dy SM. Enteral and parenteral nutrition in terminally ill cancer patients: a review of the literature. Am J Hosp Palliat Care 2006; 23:369–377.
- Whitworth MK, Whitfield A, Holm S, Shaffer J, Makin W, Jayson GC. Doctor, does this mean I’m going to starve to death? J Clin Oncol 2004; 22:199–201.
- Hoda D, Jatoi A, Burnes J, Loprinzi C, Kelly D. Should patients with advanced, incurable cancers ever be sent home with total parenteral nutrition? A single institution’s 20-year experience. Cancer 2005; 103:863–868.
- Philip J, Depczynski B. The role of total parenteral nutrition for patients with irreversible bowel obstruction secondary to gynecological malignancy. J Pain Symptom Manage 1997; 13:104–111.
- August DA, Thorn D, Fisher RL, Welchek CM. Home parenteral nutrition for patients with inoperable malignant bowel obstruction. JPEN J Parenter Enteral Nutr 1991; 15:323–327.
- Abu-Rustum NR, Barakat RR, Venkatraman E, Spriggs D. Chemotherapy and total parenteral nutrition for advanced ovarian cancer with bowel obstruction. Gynecol Oncol 1997; 64:493–495.
- Fainsinger RL, Bruera E. When to treat dehydration in a terminally ill patient? Support Care Cancer 1997; 5:205–211.
- Steiner N, Bruera E. Methods of hydration in palliative care patients. J Palliat Care 1998; 14:6–13.
- Slomka J. Withholding nutrition at the end of life: clinical and ethical issues. Cleve Clin J Med 2003; 70:548–552.
- Chiu TY, Hu WY, Chuang RB, Chen CY. Nutrition and hydration for terminal cancer patients in Taiwan. Support Care Cancer 2002; 10:630–636.
- McCann RM, Hall WJ, Groth-Juncker A. Comfort care for terminally ill patients. The appropriate use of nutrition and hydration. JAMA 1994; 272:1263–1266.
- Mercadante S. Intestinal dysfunction and obstruction. In:Walsh D, editor. Palliative Medicine. Philadelphia, PA: Saunders/Elsevier, 2009:1267–1275.
- Pasanisi F, Orban A, Scalfi L, et al. Predictors of survival in terminal-cancer patients with irreversible bowel obstruction receiving home parenteral nutrition. Nutrition 2001; 17:581–584.
- Pameijer CR, Mahvi DM, Stewart JA, Weber SM. Bowel obstruction in patients with metastatic cancer: does intervention influence outcome? Int J Gastrointest Cancer 2005; 35:127–133.
- Bais JMJ, Schilthuis MS, Ansink AC. Palliative management of intestinal obstruction in patients with advanced gynaecological cancer. J Gynecol Oncol 2002; 7:299–305.
- Laval G, Arvieux C, Stefani L, Villard ML, Mestrallet JP, Cardin N. Protocol for the treatment of malignant inoperable bowel obstruction: a prospective study of 80 cases at Grenoble University Hospital Center. J Pain Symptom Manage 2006; 31:502–512.
- Jatoi A, Podratz KC, Gill P, Hartmann LC. Pathophysiology and palliation of inoperable bowel obstruction in patients with ovarian cancer. J Support Oncol 2004; 2:323–334.
- Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer 2008; 44:1105–1115.
- Roeland E, von Gunten CF. Current concepts in malignant bowel obstruction management. Curr Oncol Rep 2009; 11:298–303.
- Baron TH. Interventional palliative strategies for malignant bowel obstruction. Curr Oncol Rep 2009; 11:293–297.
- Feuer DJ, Broadley KE, Shepherd JH, Barton DP. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD002764.
- Feuer DJ, Broadley KE. Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000;CD001219.
- Woolfson RG, Jennings K, Whalen GF. Management of bowel obstruction in patients with abdominal cancer. Arch Surg 1997; 132:1093–1097.
- Saunders MD, Kimmey MB. Systematic review: acute colonic pseudo-obstruction. Aliment Pharmacol Ther 2005; 22:917–925.
- Ripamonti C, Bruera E. Palliative management of malignant bowel obstruction. Int J Gynecol Cancer 2002; 12:135–143.
- Nellgård P, Bojö L, Cassuto J. Importance of vasoactive intestinal peptide and somatostatin for fluid losses in small-bowel obstruction. Scand J Gastroenterol 1995; 30:464–469.
- Böhner H, Yang Q, Franke C, Verreet PR, Ohmann C. Simple data from history and physical examination help to exclude bowel obstruction and to avoid radiographic studies in patients with acute abdominal pain. Eur J Surg 1998; 164:777–784.
- Maglinte DD, Kelvin FM, Sandrasegaran K, et al. Radiology of small bowel obstruction: contemporary approach and controversies. Abdom Imaging 2005; 30:160–178.
- Maglinte DD, Howard TJ, Lillemoe KD, Sandrasegaran K, Rex DK. Small-bowel obstruction: state-of-the-art imaging and its role in clinical management. Clin Gastroenterol Hepatol 2008; 6:130–139.
- Silva AC, Pimenta M, Guimarães LS. Small bowel obstruction: what to look for. Radiographics 2009; 29:423–439.
- Finan PJ, Campbell S, Verma R, et al. The management of malignant large bowel obstruction: ACPGBI position statement. Colorectal Dis 2007; 9(suppl 4):1–17.
- Kohli MD, Maglinte DD. CT enteroclysis in incomplete small bowel obstruction. Abdom Imaging 2009; 34:321–327.
- Ha HK, Shin BS, Lee SI, et al. Usefulness of CT in patients with intestinal obstruction who have undergone abdominal surgery for malignancy. AJR Am J Roentgenol 1998; 171:1587–1593.
- DeBernardo R. Surgical management of malignant bowel obstruction: strategies toward palliation of patients with advanced cancer. Curr Oncol Rep 2009; 11:287–292.
- Ripamonti C, Twycross R, Baines M, et al; Working Group of the European Association for Palliative Care. Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Support Care Cancer 2001; 9:223–233.
- Mangili G, Aletti G, Frigerio L, et al. Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. Int J Gynecol Cancer 2005; 15:830–835.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg 2002; 89:1096–1102.
- Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol 2007; 42:283–290.
- Del Piano M, Ballarè M, Montino F, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc 2005; 61:421–426.
- Tilney HS, Lovegrove RE, Purkayastha S, et al. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc 2007; 21:225–233.
- Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self-expanding metallic stents: the treatment of choice? Gastrointest Endosc 2004; 60:1010–1017.
- Dastur JK, Forshaw MJ, Modarai B, Solkar MM, Raymond T, Parker MC. Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech Coloproctol 2008; 12:51–55.
- Turner J, Cummin T, Bennett A, Swift G, Green J. Stents and stentability: treatment for malignant bowel obstruction. Br J Hosp Med (Lond) 2008; 69:676–680.
- Ripamonti C, Mercadante S, Groff L, Zecca E, De Conno F, Casuccio A. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: a prospective randomized trial. J Pain Symptom Manage 2000; 19:23–34.
- Davis MP, Walsh D. Treatment of nausea and vomiting in advanced cancer. Support Care Cancer 2000; 8:444–452.
- Bicanovsky LK, Lagman RL, Davis MP, Walsh D. Managing nonmalignant chronic abdominal pain and malignant bowel obstruction. Gastroenterol Clin North Am 2006; 35:131–142.
- Glare P, Pereira G, Kristjanson LJ, Stockler M, Tattersall M. Systematic review of the efficacy of antiemetics in the treatment of nausea in patients with far-advanced cancer. Support Care Cancer 2004; 12:432–440.
- Davis MP, Furste A. Glycopyrrolate: a useful drug in the palliation of mechanical bowel obstruction. J Pain Symptom Manage 1999; 18:153–154.
- Ripamonti C, Mercadante S. How to use octreotide for malignant bowel obstruction. J Support Oncol 2004; 2:357–364.
- Shima Y, Ohtsu A, Shirao K, Sasaki Y. Clinical efficacy and safety of octreotide (SMS201-995) in terminally ill Japanese cancer patients with malignant bowel obstruction. Jpn J Clin Oncol 2008; 38:354–359.
- Mercadante S, Casuccio A, Mangione S. Medical treatment for inoperable malignant bowel obstruction: a qualitative systematic review. J Pain Symptom Manage 2007; 33:217–223.
- Mystakidou K, Tsilika E, Kalaidopoulou O, Chondros K, Georgaki S, Papadimitriou L. Comparison of octreotide administration vs conservative treatment in the management of inoperable bowel obstruction in patients with far advanced cancer: a randomized, double-blind, controlled clinical trial. Anticancer Res 2002; 22:1187–1192.
- Mercadante S, Ripamonti C, Casuccio A, Zecca E, Groff L. Comparison of octreotide and hyoscine butylbromide in controlling gastrointestinal symptoms due to malignant inoperable bowel obstruction. Support Care Cancer 2000; 8:188–191.
- Myers J, Tamber A, Farhadian M. Management of treatment-related intermittent partial small bowel obstruction: the use of octreotide. J Pain Symptom Manage 2010; 39:e1–e3.
- Mercadante S, Ferrera P, Villari P, Marrazzo A. Aggressive pharmacological treatment for reversing malignant bowel obstruction. J Pain Symptom Manage 2004; 28:412–416.
- Srivastava M, Brito-Dellan N, Davis MP, Leach M, Lagman R. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage 2003; 25:578–582.
- Bentley A, Boyd K. Use of clinical pictures in the management of nausea and vomiting: a prospective audit. Palliat Med 2001; 15:247–253.
- Pothuri B, Montemarano M, Gerardi M, et al. Percutaneous endoscopic gastrostomy tube placement in patients with malignant bowel obstruction due to ovarian carcinoma. Gynecol Oncol 2005; 96:330–334.
- Brooksbank MA, Game PA, Ashby MA. Palliative venting gastrostomy in malignant intestinal obstruction. Palliat Med 2002; 16:520–526.
- Wang MY, Wu MH, Hsieh DY, et al. Home parenteral nutrition support in adults: experience of a medical center in Asia. JPEN J Parenter Enteral Nutr 2007; 31:306–310.
- Dy SM. Enteral and parenteral nutrition in terminally ill cancer patients: a review of the literature. Am J Hosp Palliat Care 2006; 23:369–377.
- Whitworth MK, Whitfield A, Holm S, Shaffer J, Makin W, Jayson GC. Doctor, does this mean I’m going to starve to death? J Clin Oncol 2004; 22:199–201.
- Hoda D, Jatoi A, Burnes J, Loprinzi C, Kelly D. Should patients with advanced, incurable cancers ever be sent home with total parenteral nutrition? A single institution’s 20-year experience. Cancer 2005; 103:863–868.
- Philip J, Depczynski B. The role of total parenteral nutrition for patients with irreversible bowel obstruction secondary to gynecological malignancy. J Pain Symptom Manage 1997; 13:104–111.
- August DA, Thorn D, Fisher RL, Welchek CM. Home parenteral nutrition for patients with inoperable malignant bowel obstruction. JPEN J Parenter Enteral Nutr 1991; 15:323–327.
- Abu-Rustum NR, Barakat RR, Venkatraman E, Spriggs D. Chemotherapy and total parenteral nutrition for advanced ovarian cancer with bowel obstruction. Gynecol Oncol 1997; 64:493–495.
- Fainsinger RL, Bruera E. When to treat dehydration in a terminally ill patient? Support Care Cancer 1997; 5:205–211.
- Steiner N, Bruera E. Methods of hydration in palliative care patients. J Palliat Care 1998; 14:6–13.
- Slomka J. Withholding nutrition at the end of life: clinical and ethical issues. Cleve Clin J Med 2003; 70:548–552.
- Chiu TY, Hu WY, Chuang RB, Chen CY. Nutrition and hydration for terminal cancer patients in Taiwan. Support Care Cancer 2002; 10:630–636.
- McCann RM, Hall WJ, Groth-Juncker A. Comfort care for terminally ill patients. The appropriate use of nutrition and hydration. JAMA 1994; 272:1263–1266.
KEY POINTS
- Combinations of analgesics, antisecretory drugs, and antiemetics can provide acceptable symptom relief.
- A venting gastrostomy should be considered if drug therapy fails to reduce nausea and vomiting to an acceptable level.
- A nasogastric tube should be used only as a temporizing measure, until symptoms are controlled medically or a venting gastrostomy is placed.
- Total parenteral nutrition is beneficial only in patients with intermediate life expectancy who may otherwise die of starvation rather than the cancer itself.
Dronedarone for atrial fibrillation: How does it compare with amiodarone?
Dronedarone (Multaq), approved by the US Food and Drug Administration in July 2009, is a congener of the antiarrhythmic drug amiodarone (Cordarone). Designed in the hope that it would be safer than amiodarone, its official indication is to lower the risk of hospitalization in patients with paroxysmal or persistent atrial fibrillation or atrial flutter. However, its precise role in the management of atrial fibrillation is yet to be defined. If dronedarone remains well tolerated, it may permit clinicians to pursue a rhythm control strategy more often. In this article, we present a progress report on this new agent.
BETTER ANTIARRHYTHMIC DRUGS ARE NEEDED
Atrial fibrillation increases the risk of stroke fivefold and accounts for 15% to 20% of all strokes.1 It also increases the risk of heart failure. Drugs are the mainstay of therapy, but many antiarrhythmic drugs are not very effective and cause cardiac and extracardiac toxicity. Thus, the need for safe and effective new drugs.2
Much effort is going into the development of drugs that target specific ion channels or proteins expressed predominantly in atrial myocardium. The rationale is to avoid the unwanted effects of ionic currents on the ventricle and thus avoid ventricular proarrhythmic effects. At the same time, alternatives to the multiple channel blocker amiodarone, the mainstay of heart rhythm control therapy in atrial fibrillation, are being developed to retain the electrophysiologic efficacy of the mother compound but avoid its extracardiac toxicity.
RATE CONTROL VS RHYTHM CONTROL
In the acute care setting, heart rate control with atrioventricular nodal agents (beta-blockers, calcium channel blockers, and digitalis) is the preferred initial strategy in most hemodynamically stable patients presenting with new-onset atrial fibrillation.3
Since we lack an effective method for maintaining sinus rhythm without incurring significant adverse effects, rate control is also often chosen for chronic management of atrial fibrillation. This is particularly true for patients who have no symptoms or only minimal symptoms and in whom adequate rate control is easily attained. Indeed, results of large clinical trials suggest that rate control is satisfactory for many patients.
The main purpose of rate control is to control symptoms as opposed to merely lowering the ventricular rate. Effective rate control often prevents hemodynamic instability in patients with underlying heart disease who present acutely with atrial fibrillation. In patients with permanent atrial fibrillation, the RACE II study4 (Rate Control Efficacy in Permanent Atrial Fibrillation: a Comparison between Lenient Versus Strict Rate Control II), during a 3-year follow-up, showed that lenient rate control (resting heart rate < 110 beats per minute) is not inferior to strict rate control (resting heart rate < 80 beats per minute) in preventing major cardiovascular events (heart failure, stroke) or arrhythmic events such as syncope and sustained ventricular tachycardia.4
As a long-term strategy, rate control also prevents tachycardia-induced cardiomyopathy, reduces the risk of worsening of underlying heart failure, and can improve symptoms and quality of life.
Although maintenance of sinus rhythm is most likely associated with a survival benefit, heart rhythm control with antiarrhythmic drugs has not shown an advantage over rate control in overall or cardiovascular death rates, thromboembolic complications, or impact on heart failure. Indeed, a rhythm control strategy has been associated only with better exercise tolerance and, although less clear, with better quality of life.5
One possible explanation as to why a rhythm control strategy has not been shown to be superior to a rate control strategy is the side effects of the presently available drugs for rhythm control.
In a subgroup analysis of the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial,6 antiarrhythmic therapy was associated with a 49% increase in the mortality rate that offset the benefits of conversion and maintenance of sinus rhythm, which was associated with a 53% reduction in mortality rates.
The hope is that newer drugs with less toxicity may produce better outcomes for patients treated with rhythm control.
AN ANALOGUE OF AMIODARONE, WITHOUT THE IODINE
Dronedarone is a structurally modified version of amiodarone, the antiarrhythmic drug that has shown the greatest efficacy at maintaining sinus rhythm in patients with paroxysmal atrial fibrillation. Although historically amiodarone has been effective in maintaining sinus rhythm and has been used safely in patients with advanced heart failure, its use has been limited by cumulative and often irreversible extracardiac organ toxicity.
Dronedarone was designed to match amiodarone’s efficacy but with a better safety profile. An iodine radical makes up more than one-third of amiodarone’s molecular weight. The omission of iodine in dronedarone was intended to reduce the likelihood of toxic side effects.
Dronedarone is a benzofuran derivative pharmacologically related to amiodarone, with the addition of a methylsulfonamide group. This reduces lipophilicity and the propensity to cross the blood-brain barrier; over a 2-year period this drug has not been shown to have neurotoxic effects.7
Dronedarone has proved efficacious without toxic or proarrhythmic effects and has minimal side effects, but concerns remain regarding its use in advanced heart failure. To date, its adverse-event profile appears comparable to that of placebo. However, whether its efficacy and incidence of adverse effects are comparable to what has been reported in the literature may take time to assess.
DRONEDARONE’S PHARMACOLOGY
Dronedarone, like amiodarone, blocks multiple sodium and potassium ion channels. It also exerts an antiadrenergic effect by noncompetitive binding to beta-adrenergic receptors as well as by inhibiting an agonist-induced increase in adenylate cyclase activity.8 Compared with amiodarone, dronedarone is a more potent blocker of peak sodium current.
Dronedarone is largely metabolized by the hepatic enzyme cytochrome P450 3A4 isoform (CYP3A4). Only 6% of dronedarone is excreted renally; however, no trial has yet assessed dronedarone’s safety in patients with marked kidney dysfunction.89
Dronedarone’s steady-state terminal elimination half-life is approximately 30 hours. When taken twice a day, it achieves steady-state concentrations in 5 to 7 days.
Dronedarone is available only for oral administration at 400 mg twice daily. Dose adjustment or titration is not recommended.
CLINICAL TRIALS OF DRONEDARONE
Dronedarone vs placebo
ATHENA (A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death From any Cause in Patients With Atrial Fibrillation/Atrial Flutter)10 was a prospective, double-blind study to assess morbidity and death rates in 4,628 patients with atrial fibrillation or atrial flutter and at least one other cardiovascular risk factor.
EURIDIS and ADONIS. Two trials,11 EURIDIS (European Trial in Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm) and ADONIS (American-Australian Trial With Dronedarone in Atrial Fibrillation or Flutter Patients for the Maintenance of Sinus Rhythm), enrolled a total of more than 1,200 patients and showed that dronedarone 400 mg twice a day produced a significantly lower rate of recurrence of atrial fibrillation after electrical cardioversion compared with placebo.
Overall, treatment with dronedarone significantly reduced the risk of a first recurrence of atrial fibrillation by 22% (ADONIS) and 27.5% (EURIDIS) (Table 1).
ERATO (Efficacy and Safety of Dronedarone for the Control of Ventricular Rate During Atrial Fibrillation),12 an additional phase III study, showed that dronedarone controlled the heart rate in patients with persistently accelerated ventricular rates despite concomitant standard therapy with a beta-blocker, digitalis, or a calcium-channel blocker. Dronedarone reduced the mean 24-hour heart rate by 11.7 beats per minute and the maximal exercise ventricular rate by 24.5 beats per minute at the 14th day.
ANDROMEDA (Anti-arrhythmic Trial With Dronedarone in Moderate to Severe CHF Evaluating Morbidity Decrease)13 was a study not of patients with atrial fibrillation but rather of patients with symptomatic congestive heart failure, a left ventricular ejection fraction of 35% or less, and recent hospitalization with new or worsening heart failure. The study was terminated early because of a higher rate of death with dronedarone13 (Table 1).
Dronedarone vs amiodarone
DIONYSOS (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Atrial Fibrillation)14 was a randomized double-blind trial. It evaluated the efficacy and safety of dronedarone (400 mg twice daily) or amiodarone (600 mg daily for 28 days, then 200 mg daily thereafter) for at least 6 months for the maintenance of sinus rhythm in patients with atrial fibrillation. It enrolled 504 patients with persistent atrial fibrillation; patients had not previously taken amiodarone. Dronedarone was less effective than amiodarone in maintaining sinus rhythm: the rate of recurrent atrial fibrillation was 63% with dronedarone and 42% with amiodarone. But dronedarone was associated with fewer adverse effects and less need for premature discontinuation of drug treatment at a mean follow-up of 7 months (Table 1).
WHERE DOES DRONEDARONE FIT IN ATRIAL FIBRILLATION MANAGEMENT?
Dronedarone is indicated in persistent or paroxysmal atrial fibrillation, based on the observed reduction of the rate of hospitalization. It is indicated for the maintenance of sinus rhythm and may be used in patients with persistent or paroxysmal atrial fibrillation and flutter who are in sinus rhythm or will be undergoing cardioversion soon after starting the drug. Dronedarone has no role in the acute management of atrial fibrillation, such as in cardioversion to sinus rhythm in the emergency department.
We do not have substantial evidence of the efficacy of dronedarone in patients with resistant atrial fibrillation, in whom multiple antiarrhythmics have failed to maintain sinus rhythm, and no published trial has used the inclusion criterion of treatment failure with other antiarrhythmic drugs.
The role of dronedarone in heart failure with preserved systolic function is unclear. Patients taking dronedarone are twice as likely as those taking amiodarone to have a recurrence of atrial fibrillation.
The main advantage of dronedarone is its lower adverse effect profile. However, this statement is based on only a few years of observation. If the patient has developed adverse effects with amiodarone, or if the clinician is concerned about the risk of serious adverse effects, dronedarone presents an alternative for those patients without heart failure or significant left ventricular dysfunction. One such group may be younger patients, because of concerns about the cumulative effects of amiodarone taken over a lifetime.
Dronedarone may represent an acceptable alternative to many of the current antiarrhythmic drugs. Based on the results of the Cardiac Arrhythmia Suppression Trial (CAST),15 class IC antiarrhythmics such as flecainide (Tambocor) are generally avoided in patients with prior myocardial infarction or with known or even suspected coronary artery disease. Similarly, sotalol (Betapace) is generally avoided in patients with marked left ventricular hypertrophy because of adverse effects.16 Dofetilide (Tikosyn) and often sotalol require hospitalization with telemetric monitoring for QTc prolongation and the risk of proarrhythmia with torsades de pointes. Dronedarone, however, generally can be safely started in the outpatient setting.
As when considering prescribing any antiarrhythmic, the clinician must assess the patient’s thromboembolic risk, since this risk persists with a rhythm control strategy.
There is substantial evidence from the ATHENA trial,10 in which 30% of the patients had coronary artery disease, that dronedarone is safe and effective in patients with coronary artery disease. Its use in patients who have undergone coronary artery bypass surgery remains to be defined.
WHEN SHOULD WE SWITCH PATIENTS TO DRONEDARONE?
Preliminary experience suggests that dronedarone, unlike most antiarrhythmic drugs, can be safely started about 48 hours after amiodarone is discontinued. Cumulative toxicity has not been noted with dronedarone. Caution should be exercised when switching if the patient has baseline bradycardia or QT interval prolongation. No algorithm has been developed for switching from other antiarrhythmic drugs to dronedarone.
CONTRAINDICATIONS TO DRONEDARONE
Dronedarone is contraindicated in:
- Patients with New York Heart Association (NYHA) class IV heart failure or NYHA class II or III heart failure with recent decompensation requiring hospitalization or referral to a specialized heart failure clinic
- Patients with second- or third-degree atrioventricular block or sick sinus syndrome (except when used in conjunction with a functioning pacemaker) or bradycardia (a heart rate < 50 beats per minute)
- Patients with a QTc interval of 500 ms or longer
- Patients with severe hepatic impairment
- Women who are pregnant, are attempting to become pregnant, or are breast-feeding
- Patients taking potent CYP3A inhibitors—antifungals like ketoconazole (Nizoral), itraconazole (Sporanox), or voriconazole (VFEND); macrolide antibiotics like telithromycin (Ketek) or clarithromycin (Biaxin); protease inhibitors; or other drugs that prolong the QT interval.
In patients with new or worsening heart failure, one should consider suspending or stopping dronedarone therapy.
DRONEDARONE’S ADVERSE EFFECTS
In trials to date, dronedarone has not shown evidence of proarrhythmia (tachyarrhythmia or bradyarrythmia), torsades de pointes, or amiodarone-like organ toxicity affecting the thyroid or the lungs. Recently, rare cases of severe hepatic injury were associated with dronedarone; therefore, periodic liver function testing is advised for patients taking dronedarone, especially during the first 6 months of therapy.
Dronedarone has been associated with higher rates of diarrhea, nausea, bradycardia, QT interval prolongation, and cutaneous rash compared with placebo. In DAFNE (Dronedarone Atrial Fibrillation Study After Electrical Cardioversion),17 10.8% of patients taking dronedarone had to stop taking it because of adverse events. With 800 mg daily, the discontinuation rate was only 3.9%. The most common cause of drug discontinuation was gastrointestinal effects. Anecdotal reports suggest that the gastrointestinal side effects may be self-limited and may not always require discontinuation of the drug.
Serum creatinine levels increase by about 0.1 mg/dL after the start of treatment. This elevation occurs after 1 to 2 days, reaches a plateau after 7 days, and is reversible. The mechanism is thought to be that dronedarone partially inhibits tubular organic cation transporters, which in turn reduces renal creatinine clearance by about 18%, but with no evidence of an effect on glomerular filtration, renal plasma flow, or electrolyte exchanges.18 A limited increase in serum creatinine is, therefore, expected with dronedarone treatment, but this does not mean there is a decline in renal function.
DRONEDARONE AND POTENTIAL DRUG INTERACTIONS
Warfarin. Dronedarone does not increase the international normalized ratio when used with warfarin (Coumadin).
Verapamil, diltiazem. Dose reduction is required to avoid bradyarrhythmias with co-administration of moderate CYP3A4 inhibitors such as verapamil (Calan, Verelan) and diltiazem (Cardizem).
Simvastatin. Dronedarone increases levels of simvastatin (Zocor), a CYP3A4 substrate, two to four times, thus increasing the risk of statin-induced myopathy.
Digoxin. Dronedarone increases the serum digoxin concentration about 2.5 times, and this necessitates monitoring the digoxin level and possibly reducing the digoxin dose.13
Diuretics. Hypokalemia and hypomagnesemia may occur with concomitant administration of potassium-depleting diuretics. Potassium levels should be maintained in the normal range before and during administration of dronedarone.
Tacrolimus, sirolimus. Dronedarone may increase levels of tacrolimus (Prograf) or sirolimus (Rapamune) in posttransplantation patients. This requires dose monitoring and adjustment in concomitant therapy with these agents.
COST VARIES
The cost of dronedarone varies based on factors that include location. Dronedarone’s retail cost ranges from $3.20 to $4.00 per pill (approximately $7.20 per day). It is not available in generic form. It is presently covered by many health plans as a tier 2 drug, representing a $15 to $40 monthly copay.
MORE DATA NEEDED
Dronedarone represents the first in what may well be a number of new antiarrhythmic drugs for the treatment of patients with paroxysmal atrial fibrillation. Although less efficacious then amiodarone, dronedarone appears to be better tolerated and have less serious side effects. It is contraindicated in patients with severe systolic dysfunction and in those with recent heart failure decompensation. It appears safe in coronary artery disease and marked left ventricular hypertrophy, unlike flecainide, propafenone (Rythmol), and sotalol.
To further understand how dronedarone will fare against other antiarrhythmic drugs, more studies with longer follow-up are needed. These studies need to demonstrate superior tolerability of dronedarone, acceptable quality of life without unacceptable loss of efficacy, or a decrease in morbidity or mortality rates compared with amiodarone.
Dronedarone can be safely started in most patients on an outpatient basis. The risk of proarrhythmia with dronedarone appears to be very low.
- Mathew ST, Patel J, Joseph S. Atrial fibrillation: mechanistic insights and treatment options. Eur J Intern Med 2009; 20:672–681.
- Schmitt J, Ehrlich JR, Hohnloser SH. New antiarrhythmic drugs for the treatment of atrial fibrillation. Herz 2008; 33:562–567.
- Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347:1825–1833.
- Van Gelder IC, Groenveld HF, Crijns HJ, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010; 362:1363–1373.
- Singh BN, Singh SN, Reda DJ, et al; Sotalol Amiodarone Atrial Fibrillation Efficacy Trial (SAFE-T) Investigators. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med 2005; 352:1861–1872.
- The AFFIRM Investigators. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. Circulation 2004; 109:1509–1513.
- Van Beeren HC, Jong WM, Kaptein E, Visser TJ, Bakker O, Wiersinga WM. Dronerarone [sic] acts as a selective inhibitor of 3,5,3′-triiodothyronine binding to thyroid hormone receptor-alpha1: in vitro and in vivo evidence. Endocrinology 2003; 144:552–558.
- Patel C, Yan GX, Kowey PR. Dronedarone. Circulation 2009; 120:636–644.
- Dale KM, White CM. Dronedarone: an amiodarone analog for the treatment of atrial fibrillation and atrial flutter. Ann Pharmacother 2007; 41:599–605.
- Hohnloser SH, Crijns HJ, van Eickels M; ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009; 360:668–678.
- Singh BN, Connolly SJ, Crijns HJ, et al; EURIDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007; 357:987–999.
- Davy JM, Herold M, Hoglund CERATO Study Investigators. Dronedarone for the control of ventricular rate in permanent atrial fibrillation: the Efficacy and safety of dRonedArone for the cOntrol of ventricular rate during atrial fibrillation (ERATO) study. Am Heart J 2008; 156:527.e1–e9.
- Køber L, Torp-Pedersen C, McMurray JJ; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008; 358:2678–2687.
- Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol 2010; 21:597–605.
- The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989; 321:406–412.
- Pratt CM. Clinical implications of the Survival With Oral D-sotalol (SWORD) trial: an investigation of patients with left ventricular dysfunction after myocardial infarction. Card Electrophysiol Rev 1998; 2:28–29.
- Touboul P, Brugada J, Capucci A, Crijns HJ, Edvardsson N, Hohnloser SH. Dronedarone for prevention of atrial fibrillation: a dose-ranging study. Eur Heart J 2003; 24:1481–1487.
- Tschuppert Y, Buclin T, Rothuizen LE, et al. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol 2007; 64:785–791.
Dronedarone (Multaq), approved by the US Food and Drug Administration in July 2009, is a congener of the antiarrhythmic drug amiodarone (Cordarone). Designed in the hope that it would be safer than amiodarone, its official indication is to lower the risk of hospitalization in patients with paroxysmal or persistent atrial fibrillation or atrial flutter. However, its precise role in the management of atrial fibrillation is yet to be defined. If dronedarone remains well tolerated, it may permit clinicians to pursue a rhythm control strategy more often. In this article, we present a progress report on this new agent.
BETTER ANTIARRHYTHMIC DRUGS ARE NEEDED
Atrial fibrillation increases the risk of stroke fivefold and accounts for 15% to 20% of all strokes.1 It also increases the risk of heart failure. Drugs are the mainstay of therapy, but many antiarrhythmic drugs are not very effective and cause cardiac and extracardiac toxicity. Thus, the need for safe and effective new drugs.2
Much effort is going into the development of drugs that target specific ion channels or proteins expressed predominantly in atrial myocardium. The rationale is to avoid the unwanted effects of ionic currents on the ventricle and thus avoid ventricular proarrhythmic effects. At the same time, alternatives to the multiple channel blocker amiodarone, the mainstay of heart rhythm control therapy in atrial fibrillation, are being developed to retain the electrophysiologic efficacy of the mother compound but avoid its extracardiac toxicity.
RATE CONTROL VS RHYTHM CONTROL
In the acute care setting, heart rate control with atrioventricular nodal agents (beta-blockers, calcium channel blockers, and digitalis) is the preferred initial strategy in most hemodynamically stable patients presenting with new-onset atrial fibrillation.3
Since we lack an effective method for maintaining sinus rhythm without incurring significant adverse effects, rate control is also often chosen for chronic management of atrial fibrillation. This is particularly true for patients who have no symptoms or only minimal symptoms and in whom adequate rate control is easily attained. Indeed, results of large clinical trials suggest that rate control is satisfactory for many patients.
The main purpose of rate control is to control symptoms as opposed to merely lowering the ventricular rate. Effective rate control often prevents hemodynamic instability in patients with underlying heart disease who present acutely with atrial fibrillation. In patients with permanent atrial fibrillation, the RACE II study4 (Rate Control Efficacy in Permanent Atrial Fibrillation: a Comparison between Lenient Versus Strict Rate Control II), during a 3-year follow-up, showed that lenient rate control (resting heart rate < 110 beats per minute) is not inferior to strict rate control (resting heart rate < 80 beats per minute) in preventing major cardiovascular events (heart failure, stroke) or arrhythmic events such as syncope and sustained ventricular tachycardia.4
As a long-term strategy, rate control also prevents tachycardia-induced cardiomyopathy, reduces the risk of worsening of underlying heart failure, and can improve symptoms and quality of life.
Although maintenance of sinus rhythm is most likely associated with a survival benefit, heart rhythm control with antiarrhythmic drugs has not shown an advantage over rate control in overall or cardiovascular death rates, thromboembolic complications, or impact on heart failure. Indeed, a rhythm control strategy has been associated only with better exercise tolerance and, although less clear, with better quality of life.5
One possible explanation as to why a rhythm control strategy has not been shown to be superior to a rate control strategy is the side effects of the presently available drugs for rhythm control.
In a subgroup analysis of the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial,6 antiarrhythmic therapy was associated with a 49% increase in the mortality rate that offset the benefits of conversion and maintenance of sinus rhythm, which was associated with a 53% reduction in mortality rates.
The hope is that newer drugs with less toxicity may produce better outcomes for patients treated with rhythm control.
AN ANALOGUE OF AMIODARONE, WITHOUT THE IODINE
Dronedarone is a structurally modified version of amiodarone, the antiarrhythmic drug that has shown the greatest efficacy at maintaining sinus rhythm in patients with paroxysmal atrial fibrillation. Although historically amiodarone has been effective in maintaining sinus rhythm and has been used safely in patients with advanced heart failure, its use has been limited by cumulative and often irreversible extracardiac organ toxicity.
Dronedarone was designed to match amiodarone’s efficacy but with a better safety profile. An iodine radical makes up more than one-third of amiodarone’s molecular weight. The omission of iodine in dronedarone was intended to reduce the likelihood of toxic side effects.
Dronedarone is a benzofuran derivative pharmacologically related to amiodarone, with the addition of a methylsulfonamide group. This reduces lipophilicity and the propensity to cross the blood-brain barrier; over a 2-year period this drug has not been shown to have neurotoxic effects.7
Dronedarone has proved efficacious without toxic or proarrhythmic effects and has minimal side effects, but concerns remain regarding its use in advanced heart failure. To date, its adverse-event profile appears comparable to that of placebo. However, whether its efficacy and incidence of adverse effects are comparable to what has been reported in the literature may take time to assess.
DRONEDARONE’S PHARMACOLOGY
Dronedarone, like amiodarone, blocks multiple sodium and potassium ion channels. It also exerts an antiadrenergic effect by noncompetitive binding to beta-adrenergic receptors as well as by inhibiting an agonist-induced increase in adenylate cyclase activity.8 Compared with amiodarone, dronedarone is a more potent blocker of peak sodium current.
Dronedarone is largely metabolized by the hepatic enzyme cytochrome P450 3A4 isoform (CYP3A4). Only 6% of dronedarone is excreted renally; however, no trial has yet assessed dronedarone’s safety in patients with marked kidney dysfunction.89
Dronedarone’s steady-state terminal elimination half-life is approximately 30 hours. When taken twice a day, it achieves steady-state concentrations in 5 to 7 days.
Dronedarone is available only for oral administration at 400 mg twice daily. Dose adjustment or titration is not recommended.
CLINICAL TRIALS OF DRONEDARONE
Dronedarone vs placebo
ATHENA (A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death From any Cause in Patients With Atrial Fibrillation/Atrial Flutter)10 was a prospective, double-blind study to assess morbidity and death rates in 4,628 patients with atrial fibrillation or atrial flutter and at least one other cardiovascular risk factor.
EURIDIS and ADONIS. Two trials,11 EURIDIS (European Trial in Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm) and ADONIS (American-Australian Trial With Dronedarone in Atrial Fibrillation or Flutter Patients for the Maintenance of Sinus Rhythm), enrolled a total of more than 1,200 patients and showed that dronedarone 400 mg twice a day produced a significantly lower rate of recurrence of atrial fibrillation after electrical cardioversion compared with placebo.
Overall, treatment with dronedarone significantly reduced the risk of a first recurrence of atrial fibrillation by 22% (ADONIS) and 27.5% (EURIDIS) (Table 1).
ERATO (Efficacy and Safety of Dronedarone for the Control of Ventricular Rate During Atrial Fibrillation),12 an additional phase III study, showed that dronedarone controlled the heart rate in patients with persistently accelerated ventricular rates despite concomitant standard therapy with a beta-blocker, digitalis, or a calcium-channel blocker. Dronedarone reduced the mean 24-hour heart rate by 11.7 beats per minute and the maximal exercise ventricular rate by 24.5 beats per minute at the 14th day.
ANDROMEDA (Anti-arrhythmic Trial With Dronedarone in Moderate to Severe CHF Evaluating Morbidity Decrease)13 was a study not of patients with atrial fibrillation but rather of patients with symptomatic congestive heart failure, a left ventricular ejection fraction of 35% or less, and recent hospitalization with new or worsening heart failure. The study was terminated early because of a higher rate of death with dronedarone13 (Table 1).
Dronedarone vs amiodarone
DIONYSOS (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Atrial Fibrillation)14 was a randomized double-blind trial. It evaluated the efficacy and safety of dronedarone (400 mg twice daily) or amiodarone (600 mg daily for 28 days, then 200 mg daily thereafter) for at least 6 months for the maintenance of sinus rhythm in patients with atrial fibrillation. It enrolled 504 patients with persistent atrial fibrillation; patients had not previously taken amiodarone. Dronedarone was less effective than amiodarone in maintaining sinus rhythm: the rate of recurrent atrial fibrillation was 63% with dronedarone and 42% with amiodarone. But dronedarone was associated with fewer adverse effects and less need for premature discontinuation of drug treatment at a mean follow-up of 7 months (Table 1).
WHERE DOES DRONEDARONE FIT IN ATRIAL FIBRILLATION MANAGEMENT?
Dronedarone is indicated in persistent or paroxysmal atrial fibrillation, based on the observed reduction of the rate of hospitalization. It is indicated for the maintenance of sinus rhythm and may be used in patients with persistent or paroxysmal atrial fibrillation and flutter who are in sinus rhythm or will be undergoing cardioversion soon after starting the drug. Dronedarone has no role in the acute management of atrial fibrillation, such as in cardioversion to sinus rhythm in the emergency department.
We do not have substantial evidence of the efficacy of dronedarone in patients with resistant atrial fibrillation, in whom multiple antiarrhythmics have failed to maintain sinus rhythm, and no published trial has used the inclusion criterion of treatment failure with other antiarrhythmic drugs.
The role of dronedarone in heart failure with preserved systolic function is unclear. Patients taking dronedarone are twice as likely as those taking amiodarone to have a recurrence of atrial fibrillation.
The main advantage of dronedarone is its lower adverse effect profile. However, this statement is based on only a few years of observation. If the patient has developed adverse effects with amiodarone, or if the clinician is concerned about the risk of serious adverse effects, dronedarone presents an alternative for those patients without heart failure or significant left ventricular dysfunction. One such group may be younger patients, because of concerns about the cumulative effects of amiodarone taken over a lifetime.
Dronedarone may represent an acceptable alternative to many of the current antiarrhythmic drugs. Based on the results of the Cardiac Arrhythmia Suppression Trial (CAST),15 class IC antiarrhythmics such as flecainide (Tambocor) are generally avoided in patients with prior myocardial infarction or with known or even suspected coronary artery disease. Similarly, sotalol (Betapace) is generally avoided in patients with marked left ventricular hypertrophy because of adverse effects.16 Dofetilide (Tikosyn) and often sotalol require hospitalization with telemetric monitoring for QTc prolongation and the risk of proarrhythmia with torsades de pointes. Dronedarone, however, generally can be safely started in the outpatient setting.
As when considering prescribing any antiarrhythmic, the clinician must assess the patient’s thromboembolic risk, since this risk persists with a rhythm control strategy.
There is substantial evidence from the ATHENA trial,10 in which 30% of the patients had coronary artery disease, that dronedarone is safe and effective in patients with coronary artery disease. Its use in patients who have undergone coronary artery bypass surgery remains to be defined.
WHEN SHOULD WE SWITCH PATIENTS TO DRONEDARONE?
Preliminary experience suggests that dronedarone, unlike most antiarrhythmic drugs, can be safely started about 48 hours after amiodarone is discontinued. Cumulative toxicity has not been noted with dronedarone. Caution should be exercised when switching if the patient has baseline bradycardia or QT interval prolongation. No algorithm has been developed for switching from other antiarrhythmic drugs to dronedarone.
CONTRAINDICATIONS TO DRONEDARONE
Dronedarone is contraindicated in:
- Patients with New York Heart Association (NYHA) class IV heart failure or NYHA class II or III heart failure with recent decompensation requiring hospitalization or referral to a specialized heart failure clinic
- Patients with second- or third-degree atrioventricular block or sick sinus syndrome (except when used in conjunction with a functioning pacemaker) or bradycardia (a heart rate < 50 beats per minute)
- Patients with a QTc interval of 500 ms or longer
- Patients with severe hepatic impairment
- Women who are pregnant, are attempting to become pregnant, or are breast-feeding
- Patients taking potent CYP3A inhibitors—antifungals like ketoconazole (Nizoral), itraconazole (Sporanox), or voriconazole (VFEND); macrolide antibiotics like telithromycin (Ketek) or clarithromycin (Biaxin); protease inhibitors; or other drugs that prolong the QT interval.
In patients with new or worsening heart failure, one should consider suspending or stopping dronedarone therapy.
DRONEDARONE’S ADVERSE EFFECTS
In trials to date, dronedarone has not shown evidence of proarrhythmia (tachyarrhythmia or bradyarrythmia), torsades de pointes, or amiodarone-like organ toxicity affecting the thyroid or the lungs. Recently, rare cases of severe hepatic injury were associated with dronedarone; therefore, periodic liver function testing is advised for patients taking dronedarone, especially during the first 6 months of therapy.
Dronedarone has been associated with higher rates of diarrhea, nausea, bradycardia, QT interval prolongation, and cutaneous rash compared with placebo. In DAFNE (Dronedarone Atrial Fibrillation Study After Electrical Cardioversion),17 10.8% of patients taking dronedarone had to stop taking it because of adverse events. With 800 mg daily, the discontinuation rate was only 3.9%. The most common cause of drug discontinuation was gastrointestinal effects. Anecdotal reports suggest that the gastrointestinal side effects may be self-limited and may not always require discontinuation of the drug.
Serum creatinine levels increase by about 0.1 mg/dL after the start of treatment. This elevation occurs after 1 to 2 days, reaches a plateau after 7 days, and is reversible. The mechanism is thought to be that dronedarone partially inhibits tubular organic cation transporters, which in turn reduces renal creatinine clearance by about 18%, but with no evidence of an effect on glomerular filtration, renal plasma flow, or electrolyte exchanges.18 A limited increase in serum creatinine is, therefore, expected with dronedarone treatment, but this does not mean there is a decline in renal function.
DRONEDARONE AND POTENTIAL DRUG INTERACTIONS
Warfarin. Dronedarone does not increase the international normalized ratio when used with warfarin (Coumadin).
Verapamil, diltiazem. Dose reduction is required to avoid bradyarrhythmias with co-administration of moderate CYP3A4 inhibitors such as verapamil (Calan, Verelan) and diltiazem (Cardizem).
Simvastatin. Dronedarone increases levels of simvastatin (Zocor), a CYP3A4 substrate, two to four times, thus increasing the risk of statin-induced myopathy.
Digoxin. Dronedarone increases the serum digoxin concentration about 2.5 times, and this necessitates monitoring the digoxin level and possibly reducing the digoxin dose.13
Diuretics. Hypokalemia and hypomagnesemia may occur with concomitant administration of potassium-depleting diuretics. Potassium levels should be maintained in the normal range before and during administration of dronedarone.
Tacrolimus, sirolimus. Dronedarone may increase levels of tacrolimus (Prograf) or sirolimus (Rapamune) in posttransplantation patients. This requires dose monitoring and adjustment in concomitant therapy with these agents.
COST VARIES
The cost of dronedarone varies based on factors that include location. Dronedarone’s retail cost ranges from $3.20 to $4.00 per pill (approximately $7.20 per day). It is not available in generic form. It is presently covered by many health plans as a tier 2 drug, representing a $15 to $40 monthly copay.
MORE DATA NEEDED
Dronedarone represents the first in what may well be a number of new antiarrhythmic drugs for the treatment of patients with paroxysmal atrial fibrillation. Although less efficacious then amiodarone, dronedarone appears to be better tolerated and have less serious side effects. It is contraindicated in patients with severe systolic dysfunction and in those with recent heart failure decompensation. It appears safe in coronary artery disease and marked left ventricular hypertrophy, unlike flecainide, propafenone (Rythmol), and sotalol.
To further understand how dronedarone will fare against other antiarrhythmic drugs, more studies with longer follow-up are needed. These studies need to demonstrate superior tolerability of dronedarone, acceptable quality of life without unacceptable loss of efficacy, or a decrease in morbidity or mortality rates compared with amiodarone.
Dronedarone can be safely started in most patients on an outpatient basis. The risk of proarrhythmia with dronedarone appears to be very low.
Dronedarone (Multaq), approved by the US Food and Drug Administration in July 2009, is a congener of the antiarrhythmic drug amiodarone (Cordarone). Designed in the hope that it would be safer than amiodarone, its official indication is to lower the risk of hospitalization in patients with paroxysmal or persistent atrial fibrillation or atrial flutter. However, its precise role in the management of atrial fibrillation is yet to be defined. If dronedarone remains well tolerated, it may permit clinicians to pursue a rhythm control strategy more often. In this article, we present a progress report on this new agent.
BETTER ANTIARRHYTHMIC DRUGS ARE NEEDED
Atrial fibrillation increases the risk of stroke fivefold and accounts for 15% to 20% of all strokes.1 It also increases the risk of heart failure. Drugs are the mainstay of therapy, but many antiarrhythmic drugs are not very effective and cause cardiac and extracardiac toxicity. Thus, the need for safe and effective new drugs.2
Much effort is going into the development of drugs that target specific ion channels or proteins expressed predominantly in atrial myocardium. The rationale is to avoid the unwanted effects of ionic currents on the ventricle and thus avoid ventricular proarrhythmic effects. At the same time, alternatives to the multiple channel blocker amiodarone, the mainstay of heart rhythm control therapy in atrial fibrillation, are being developed to retain the electrophysiologic efficacy of the mother compound but avoid its extracardiac toxicity.
RATE CONTROL VS RHYTHM CONTROL
In the acute care setting, heart rate control with atrioventricular nodal agents (beta-blockers, calcium channel blockers, and digitalis) is the preferred initial strategy in most hemodynamically stable patients presenting with new-onset atrial fibrillation.3
Since we lack an effective method for maintaining sinus rhythm without incurring significant adverse effects, rate control is also often chosen for chronic management of atrial fibrillation. This is particularly true for patients who have no symptoms or only minimal symptoms and in whom adequate rate control is easily attained. Indeed, results of large clinical trials suggest that rate control is satisfactory for many patients.
The main purpose of rate control is to control symptoms as opposed to merely lowering the ventricular rate. Effective rate control often prevents hemodynamic instability in patients with underlying heart disease who present acutely with atrial fibrillation. In patients with permanent atrial fibrillation, the RACE II study4 (Rate Control Efficacy in Permanent Atrial Fibrillation: a Comparison between Lenient Versus Strict Rate Control II), during a 3-year follow-up, showed that lenient rate control (resting heart rate < 110 beats per minute) is not inferior to strict rate control (resting heart rate < 80 beats per minute) in preventing major cardiovascular events (heart failure, stroke) or arrhythmic events such as syncope and sustained ventricular tachycardia.4
As a long-term strategy, rate control also prevents tachycardia-induced cardiomyopathy, reduces the risk of worsening of underlying heart failure, and can improve symptoms and quality of life.
Although maintenance of sinus rhythm is most likely associated with a survival benefit, heart rhythm control with antiarrhythmic drugs has not shown an advantage over rate control in overall or cardiovascular death rates, thromboembolic complications, or impact on heart failure. Indeed, a rhythm control strategy has been associated only with better exercise tolerance and, although less clear, with better quality of life.5
One possible explanation as to why a rhythm control strategy has not been shown to be superior to a rate control strategy is the side effects of the presently available drugs for rhythm control.
In a subgroup analysis of the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial,6 antiarrhythmic therapy was associated with a 49% increase in the mortality rate that offset the benefits of conversion and maintenance of sinus rhythm, which was associated with a 53% reduction in mortality rates.
The hope is that newer drugs with less toxicity may produce better outcomes for patients treated with rhythm control.
AN ANALOGUE OF AMIODARONE, WITHOUT THE IODINE
Dronedarone is a structurally modified version of amiodarone, the antiarrhythmic drug that has shown the greatest efficacy at maintaining sinus rhythm in patients with paroxysmal atrial fibrillation. Although historically amiodarone has been effective in maintaining sinus rhythm and has been used safely in patients with advanced heart failure, its use has been limited by cumulative and often irreversible extracardiac organ toxicity.
Dronedarone was designed to match amiodarone’s efficacy but with a better safety profile. An iodine radical makes up more than one-third of amiodarone’s molecular weight. The omission of iodine in dronedarone was intended to reduce the likelihood of toxic side effects.
Dronedarone is a benzofuran derivative pharmacologically related to amiodarone, with the addition of a methylsulfonamide group. This reduces lipophilicity and the propensity to cross the blood-brain barrier; over a 2-year period this drug has not been shown to have neurotoxic effects.7
Dronedarone has proved efficacious without toxic or proarrhythmic effects and has minimal side effects, but concerns remain regarding its use in advanced heart failure. To date, its adverse-event profile appears comparable to that of placebo. However, whether its efficacy and incidence of adverse effects are comparable to what has been reported in the literature may take time to assess.
DRONEDARONE’S PHARMACOLOGY
Dronedarone, like amiodarone, blocks multiple sodium and potassium ion channels. It also exerts an antiadrenergic effect by noncompetitive binding to beta-adrenergic receptors as well as by inhibiting an agonist-induced increase in adenylate cyclase activity.8 Compared with amiodarone, dronedarone is a more potent blocker of peak sodium current.
Dronedarone is largely metabolized by the hepatic enzyme cytochrome P450 3A4 isoform (CYP3A4). Only 6% of dronedarone is excreted renally; however, no trial has yet assessed dronedarone’s safety in patients with marked kidney dysfunction.89
Dronedarone’s steady-state terminal elimination half-life is approximately 30 hours. When taken twice a day, it achieves steady-state concentrations in 5 to 7 days.
Dronedarone is available only for oral administration at 400 mg twice daily. Dose adjustment or titration is not recommended.
CLINICAL TRIALS OF DRONEDARONE
Dronedarone vs placebo
ATHENA (A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death From any Cause in Patients With Atrial Fibrillation/Atrial Flutter)10 was a prospective, double-blind study to assess morbidity and death rates in 4,628 patients with atrial fibrillation or atrial flutter and at least one other cardiovascular risk factor.
EURIDIS and ADONIS. Two trials,11 EURIDIS (European Trial in Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm) and ADONIS (American-Australian Trial With Dronedarone in Atrial Fibrillation or Flutter Patients for the Maintenance of Sinus Rhythm), enrolled a total of more than 1,200 patients and showed that dronedarone 400 mg twice a day produced a significantly lower rate of recurrence of atrial fibrillation after electrical cardioversion compared with placebo.
Overall, treatment with dronedarone significantly reduced the risk of a first recurrence of atrial fibrillation by 22% (ADONIS) and 27.5% (EURIDIS) (Table 1).
ERATO (Efficacy and Safety of Dronedarone for the Control of Ventricular Rate During Atrial Fibrillation),12 an additional phase III study, showed that dronedarone controlled the heart rate in patients with persistently accelerated ventricular rates despite concomitant standard therapy with a beta-blocker, digitalis, or a calcium-channel blocker. Dronedarone reduced the mean 24-hour heart rate by 11.7 beats per minute and the maximal exercise ventricular rate by 24.5 beats per minute at the 14th day.
ANDROMEDA (Anti-arrhythmic Trial With Dronedarone in Moderate to Severe CHF Evaluating Morbidity Decrease)13 was a study not of patients with atrial fibrillation but rather of patients with symptomatic congestive heart failure, a left ventricular ejection fraction of 35% or less, and recent hospitalization with new or worsening heart failure. The study was terminated early because of a higher rate of death with dronedarone13 (Table 1).
Dronedarone vs amiodarone
DIONYSOS (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Atrial Fibrillation)14 was a randomized double-blind trial. It evaluated the efficacy and safety of dronedarone (400 mg twice daily) or amiodarone (600 mg daily for 28 days, then 200 mg daily thereafter) for at least 6 months for the maintenance of sinus rhythm in patients with atrial fibrillation. It enrolled 504 patients with persistent atrial fibrillation; patients had not previously taken amiodarone. Dronedarone was less effective than amiodarone in maintaining sinus rhythm: the rate of recurrent atrial fibrillation was 63% with dronedarone and 42% with amiodarone. But dronedarone was associated with fewer adverse effects and less need for premature discontinuation of drug treatment at a mean follow-up of 7 months (Table 1).
WHERE DOES DRONEDARONE FIT IN ATRIAL FIBRILLATION MANAGEMENT?
Dronedarone is indicated in persistent or paroxysmal atrial fibrillation, based on the observed reduction of the rate of hospitalization. It is indicated for the maintenance of sinus rhythm and may be used in patients with persistent or paroxysmal atrial fibrillation and flutter who are in sinus rhythm or will be undergoing cardioversion soon after starting the drug. Dronedarone has no role in the acute management of atrial fibrillation, such as in cardioversion to sinus rhythm in the emergency department.
We do not have substantial evidence of the efficacy of dronedarone in patients with resistant atrial fibrillation, in whom multiple antiarrhythmics have failed to maintain sinus rhythm, and no published trial has used the inclusion criterion of treatment failure with other antiarrhythmic drugs.
The role of dronedarone in heart failure with preserved systolic function is unclear. Patients taking dronedarone are twice as likely as those taking amiodarone to have a recurrence of atrial fibrillation.
The main advantage of dronedarone is its lower adverse effect profile. However, this statement is based on only a few years of observation. If the patient has developed adverse effects with amiodarone, or if the clinician is concerned about the risk of serious adverse effects, dronedarone presents an alternative for those patients without heart failure or significant left ventricular dysfunction. One such group may be younger patients, because of concerns about the cumulative effects of amiodarone taken over a lifetime.
Dronedarone may represent an acceptable alternative to many of the current antiarrhythmic drugs. Based on the results of the Cardiac Arrhythmia Suppression Trial (CAST),15 class IC antiarrhythmics such as flecainide (Tambocor) are generally avoided in patients with prior myocardial infarction or with known or even suspected coronary artery disease. Similarly, sotalol (Betapace) is generally avoided in patients with marked left ventricular hypertrophy because of adverse effects.16 Dofetilide (Tikosyn) and often sotalol require hospitalization with telemetric monitoring for QTc prolongation and the risk of proarrhythmia with torsades de pointes. Dronedarone, however, generally can be safely started in the outpatient setting.
As when considering prescribing any antiarrhythmic, the clinician must assess the patient’s thromboembolic risk, since this risk persists with a rhythm control strategy.
There is substantial evidence from the ATHENA trial,10 in which 30% of the patients had coronary artery disease, that dronedarone is safe and effective in patients with coronary artery disease. Its use in patients who have undergone coronary artery bypass surgery remains to be defined.
WHEN SHOULD WE SWITCH PATIENTS TO DRONEDARONE?
Preliminary experience suggests that dronedarone, unlike most antiarrhythmic drugs, can be safely started about 48 hours after amiodarone is discontinued. Cumulative toxicity has not been noted with dronedarone. Caution should be exercised when switching if the patient has baseline bradycardia or QT interval prolongation. No algorithm has been developed for switching from other antiarrhythmic drugs to dronedarone.
CONTRAINDICATIONS TO DRONEDARONE
Dronedarone is contraindicated in:
- Patients with New York Heart Association (NYHA) class IV heart failure or NYHA class II or III heart failure with recent decompensation requiring hospitalization or referral to a specialized heart failure clinic
- Patients with second- or third-degree atrioventricular block or sick sinus syndrome (except when used in conjunction with a functioning pacemaker) or bradycardia (a heart rate < 50 beats per minute)
- Patients with a QTc interval of 500 ms or longer
- Patients with severe hepatic impairment
- Women who are pregnant, are attempting to become pregnant, or are breast-feeding
- Patients taking potent CYP3A inhibitors—antifungals like ketoconazole (Nizoral), itraconazole (Sporanox), or voriconazole (VFEND); macrolide antibiotics like telithromycin (Ketek) or clarithromycin (Biaxin); protease inhibitors; or other drugs that prolong the QT interval.
In patients with new or worsening heart failure, one should consider suspending or stopping dronedarone therapy.
DRONEDARONE’S ADVERSE EFFECTS
In trials to date, dronedarone has not shown evidence of proarrhythmia (tachyarrhythmia or bradyarrythmia), torsades de pointes, or amiodarone-like organ toxicity affecting the thyroid or the lungs. Recently, rare cases of severe hepatic injury were associated with dronedarone; therefore, periodic liver function testing is advised for patients taking dronedarone, especially during the first 6 months of therapy.
Dronedarone has been associated with higher rates of diarrhea, nausea, bradycardia, QT interval prolongation, and cutaneous rash compared with placebo. In DAFNE (Dronedarone Atrial Fibrillation Study After Electrical Cardioversion),17 10.8% of patients taking dronedarone had to stop taking it because of adverse events. With 800 mg daily, the discontinuation rate was only 3.9%. The most common cause of drug discontinuation was gastrointestinal effects. Anecdotal reports suggest that the gastrointestinal side effects may be self-limited and may not always require discontinuation of the drug.
Serum creatinine levels increase by about 0.1 mg/dL after the start of treatment. This elevation occurs after 1 to 2 days, reaches a plateau after 7 days, and is reversible. The mechanism is thought to be that dronedarone partially inhibits tubular organic cation transporters, which in turn reduces renal creatinine clearance by about 18%, but with no evidence of an effect on glomerular filtration, renal plasma flow, or electrolyte exchanges.18 A limited increase in serum creatinine is, therefore, expected with dronedarone treatment, but this does not mean there is a decline in renal function.
DRONEDARONE AND POTENTIAL DRUG INTERACTIONS
Warfarin. Dronedarone does not increase the international normalized ratio when used with warfarin (Coumadin).
Verapamil, diltiazem. Dose reduction is required to avoid bradyarrhythmias with co-administration of moderate CYP3A4 inhibitors such as verapamil (Calan, Verelan) and diltiazem (Cardizem).
Simvastatin. Dronedarone increases levels of simvastatin (Zocor), a CYP3A4 substrate, two to four times, thus increasing the risk of statin-induced myopathy.
Digoxin. Dronedarone increases the serum digoxin concentration about 2.5 times, and this necessitates monitoring the digoxin level and possibly reducing the digoxin dose.13
Diuretics. Hypokalemia and hypomagnesemia may occur with concomitant administration of potassium-depleting diuretics. Potassium levels should be maintained in the normal range before and during administration of dronedarone.
Tacrolimus, sirolimus. Dronedarone may increase levels of tacrolimus (Prograf) or sirolimus (Rapamune) in posttransplantation patients. This requires dose monitoring and adjustment in concomitant therapy with these agents.
COST VARIES
The cost of dronedarone varies based on factors that include location. Dronedarone’s retail cost ranges from $3.20 to $4.00 per pill (approximately $7.20 per day). It is not available in generic form. It is presently covered by many health plans as a tier 2 drug, representing a $15 to $40 monthly copay.
MORE DATA NEEDED
Dronedarone represents the first in what may well be a number of new antiarrhythmic drugs for the treatment of patients with paroxysmal atrial fibrillation. Although less efficacious then amiodarone, dronedarone appears to be better tolerated and have less serious side effects. It is contraindicated in patients with severe systolic dysfunction and in those with recent heart failure decompensation. It appears safe in coronary artery disease and marked left ventricular hypertrophy, unlike flecainide, propafenone (Rythmol), and sotalol.
To further understand how dronedarone will fare against other antiarrhythmic drugs, more studies with longer follow-up are needed. These studies need to demonstrate superior tolerability of dronedarone, acceptable quality of life without unacceptable loss of efficacy, or a decrease in morbidity or mortality rates compared with amiodarone.
Dronedarone can be safely started in most patients on an outpatient basis. The risk of proarrhythmia with dronedarone appears to be very low.
- Mathew ST, Patel J, Joseph S. Atrial fibrillation: mechanistic insights and treatment options. Eur J Intern Med 2009; 20:672–681.
- Schmitt J, Ehrlich JR, Hohnloser SH. New antiarrhythmic drugs for the treatment of atrial fibrillation. Herz 2008; 33:562–567.
- Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347:1825–1833.
- Van Gelder IC, Groenveld HF, Crijns HJ, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010; 362:1363–1373.
- Singh BN, Singh SN, Reda DJ, et al; Sotalol Amiodarone Atrial Fibrillation Efficacy Trial (SAFE-T) Investigators. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med 2005; 352:1861–1872.
- The AFFIRM Investigators. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. Circulation 2004; 109:1509–1513.
- Van Beeren HC, Jong WM, Kaptein E, Visser TJ, Bakker O, Wiersinga WM. Dronerarone [sic] acts as a selective inhibitor of 3,5,3′-triiodothyronine binding to thyroid hormone receptor-alpha1: in vitro and in vivo evidence. Endocrinology 2003; 144:552–558.
- Patel C, Yan GX, Kowey PR. Dronedarone. Circulation 2009; 120:636–644.
- Dale KM, White CM. Dronedarone: an amiodarone analog for the treatment of atrial fibrillation and atrial flutter. Ann Pharmacother 2007; 41:599–605.
- Hohnloser SH, Crijns HJ, van Eickels M; ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009; 360:668–678.
- Singh BN, Connolly SJ, Crijns HJ, et al; EURIDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007; 357:987–999.
- Davy JM, Herold M, Hoglund CERATO Study Investigators. Dronedarone for the control of ventricular rate in permanent atrial fibrillation: the Efficacy and safety of dRonedArone for the cOntrol of ventricular rate during atrial fibrillation (ERATO) study. Am Heart J 2008; 156:527.e1–e9.
- Køber L, Torp-Pedersen C, McMurray JJ; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008; 358:2678–2687.
- Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol 2010; 21:597–605.
- The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989; 321:406–412.
- Pratt CM. Clinical implications of the Survival With Oral D-sotalol (SWORD) trial: an investigation of patients with left ventricular dysfunction after myocardial infarction. Card Electrophysiol Rev 1998; 2:28–29.
- Touboul P, Brugada J, Capucci A, Crijns HJ, Edvardsson N, Hohnloser SH. Dronedarone for prevention of atrial fibrillation: a dose-ranging study. Eur Heart J 2003; 24:1481–1487.
- Tschuppert Y, Buclin T, Rothuizen LE, et al. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol 2007; 64:785–791.
- Mathew ST, Patel J, Joseph S. Atrial fibrillation: mechanistic insights and treatment options. Eur J Intern Med 2009; 20:672–681.
- Schmitt J, Ehrlich JR, Hohnloser SH. New antiarrhythmic drugs for the treatment of atrial fibrillation. Herz 2008; 33:562–567.
- Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347:1825–1833.
- Van Gelder IC, Groenveld HF, Crijns HJ, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010; 362:1363–1373.
- Singh BN, Singh SN, Reda DJ, et al; Sotalol Amiodarone Atrial Fibrillation Efficacy Trial (SAFE-T) Investigators. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med 2005; 352:1861–1872.
- The AFFIRM Investigators. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. Circulation 2004; 109:1509–1513.
- Van Beeren HC, Jong WM, Kaptein E, Visser TJ, Bakker O, Wiersinga WM. Dronerarone [sic] acts as a selective inhibitor of 3,5,3′-triiodothyronine binding to thyroid hormone receptor-alpha1: in vitro and in vivo evidence. Endocrinology 2003; 144:552–558.
- Patel C, Yan GX, Kowey PR. Dronedarone. Circulation 2009; 120:636–644.
- Dale KM, White CM. Dronedarone: an amiodarone analog for the treatment of atrial fibrillation and atrial flutter. Ann Pharmacother 2007; 41:599–605.
- Hohnloser SH, Crijns HJ, van Eickels M; ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009; 360:668–678.
- Singh BN, Connolly SJ, Crijns HJ, et al; EURIDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007; 357:987–999.
- Davy JM, Herold M, Hoglund CERATO Study Investigators. Dronedarone for the control of ventricular rate in permanent atrial fibrillation: the Efficacy and safety of dRonedArone for the cOntrol of ventricular rate during atrial fibrillation (ERATO) study. Am Heart J 2008; 156:527.e1–e9.
- Køber L, Torp-Pedersen C, McMurray JJ; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008; 358:2678–2687.
- Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol 2010; 21:597–605.
- The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989; 321:406–412.
- Pratt CM. Clinical implications of the Survival With Oral D-sotalol (SWORD) trial: an investigation of patients with left ventricular dysfunction after myocardial infarction. Card Electrophysiol Rev 1998; 2:28–29.
- Touboul P, Brugada J, Capucci A, Crijns HJ, Edvardsson N, Hohnloser SH. Dronedarone for prevention of atrial fibrillation: a dose-ranging study. Eur Heart J 2003; 24:1481–1487.
- Tschuppert Y, Buclin T, Rothuizen LE, et al. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol 2007; 64:785–791.
KEY POINTS
- Patients with persistent or paroxysmal atrial fibrillation are candidates for dronedarone therapy if they are in sinus rhythm or will be cardioverted soon after starting. This drug is not indicated for the acute management of atrial fibrillation, for example, in the emergency department.
- Dronedarone is an option if a patient cannot tolerate amiodarone or has an underlying condition such as pulmonary or thyroid disease that is a contraindication to amiodarone.
- Dronedarone is contraindicated in patients with significant left ventricular dysfunction or heart failure with recent decompensation.
- The ultimate role for dronedarone is yet to be defined. Little evidence exists as to whether it will succeed when other drugs have failed.
Recurrent abdominal pain after laparoscopic cholecystectomy
Four months after undergoing laparoscopic cholecystectomy for symptomatic gallstones, an otherwise healthy 26-year-old woman begins to have episodes of epigastric and back pain similar to what she experienced before the surgery. The surgery was without complications, and her classic biliary colic disappeared afterward. Histologic evaluation of the surgical specimen revealed chronic cholecystitis with multiple small, mixed gallstones.
Now she describes a burning pain in her epigastrium and mid to upper back, starting about 30 minutes after a meal and lasting up to 4 hours. Sometimes it awakens her at night. She avoids eating for fear of inducing the pain. She has occasional chills but no fever, nausea, vomiting, jaundice, or changes in urine or stool color.
Three years ago she was diagnosed with a gastric ulcer induced by taking a nonsteroidal anti-inflammatory drug (NSAID). The ulcer was treated with a proton pump inhibitor for 1 month. She says the ulcer pain was dull and aching, different from her current pain.
Upper endoscopy 4 months ago (ie, before her laparoscopic cholecystectomy) showed no evidence of esophagitis or peptic ulcer disease.
Apart from her gallbladder operation, she has had no other surgery. According to the surgeon’s notes, intraoperative cholangiography was not performed, and no macroscopic changes of acute cholecystitis or difficult biliary anatomy were noted.
The patient does not smoke, does not drink alcohol, is not currently taking any medications, including NSAIDs or over-the-counter medications, and has not taken any recently. Her mother also had symptomatic gallstones requiring cholecystectomy.
On physical examination, only fever
On examination, her temperature is 101.2°F (38.4°C), blood pressure 117/80 mm Hg, heart rate 82 beats per minute, and blood oxygen saturation 99% on room air. Her weight is 138 lb (62.6 kg), height 5 feet 6 inches (168 cm).
There is no jaundice or pallor. Her heart and lung examinations are normal.
No costovertebral angle or spinal tenderness can be elicited.
Her laboratory values are shown in Table 1.
POSTCHOLECYSTECTOMY SYNDROME
1. After cholecystectomy, preoperative symptoms recur in what percentage of patients?
- 10% to 40%
- 50%
- 60%
- 80%
Postcholecystectomy syndrome—the recurrence of symptoms similar to those before the procedure—occurs in 10% to 40% of patients. The time to the onset of symptoms can range from 2 days to up to 25 years.1–4 Women may be at higher risk, with symptoms recurring in 43% vs 28% in men.5
Postcholecystectomy syndrome can have a biliary or a nonbiliary cause. Biliary causes include strictures, retained calculi, dropped calculi, tumors, sphincter of Oddi dysfunction, and calculi in the cystic duct remnant. Nonbiliary causes include functional and organic disorders such as peptic ulcer disease, gastroesophageal reflux, pancreatic disease, hepatocellular disorders, coronary artery disease, irritable bowel syndrome, and intercostal neuritis.
WHAT IS THE NEXT STEP?
2. Which is the most appropriate next step in the workup of this patient?
- Ultrasonography of the right upper quadrant
- Magnetic resonance cholangiopancreatography (MRCP)
- Endoscopic retrograde cholangiopancreatography (ERCP)
- Observation and reassurance
- Review the operative record and consult with the surgeon
Although the patient is presenting with pain and fever, two features of the classic Charcot triad (pain, fever, jaundice) seen in cholangitis (infection of a bile duct), and although cholangitis almost confirms the diagnosis of common bile duct stones in a patient with gallstones (before or after cholecystectomy), other diagnoses to consider are bile duct injury, bile leak, and biloma.
Biloma can be detected with ultrasonography. Bile duct injuries are identified intraoperatively in up to 25% of patients. For those with an unrecognized injury, the clinical presentation is variable and depends on the type of injury. If a bile leak is present, patients present early, at a median of 3 days postoperatively. However, our patient presented with symptoms 4 months after her surgery. Patients with bile duct strictures without bile leak have a longer symptom-free interval and usually present with signs of biliary obstruction. Ultrasonography can then detect biliary dilatation.6
It would be very helpful to review the operative record and to talk to the surgeon to confirm that intraoperative cholangiography had not been done and to determine the level of difficulty of the surgery. (Intraoperative cholangiography involves the introduction of contrast dye into the biliary system by cannulation of the cystic duct or by direct injection into the common bile duct. An intraoperative cholangiogram is considered normal if the entire intrahepatic and extrahepatic biliary tree is seen to be filled with contrast.) A normal cholangiogram has a negative predictive value of 99.8% for the detection of ductal stones. Thus, a normal intraoperative cholangiogram can prevent unnecessary postoperative ECRP, since it almost always indicates a clean bile duct.7
Ultrasonography of the right upper quadrant has a low sensitivity (< 50%) for detecting common bile duct stones. However, it is highly operator-dependent, and it may be twice as sensitive if done by expert radiologists than by less experienced ones. Its limitations include poor visualization of the distal portion of the duct and low sensitivity in patients in whom the common bile duct is minimally dilated and also in patients with small stones. In most studies, however, it had a very high specificity—ie, greater than 95%.8
MRCP has a sensitivity of 82.6% and a specificity of 97.5% in detecting stones in the common bile duct.9 Therefore, normal results on abdominal ultrasonography and MRCP do not completely rule out stones.
Although this patient has a high pretest probability of having common bile duct stones, ERCP should be done only after a thorough review of the previous operative procedure.
Observation and reassurance are not appropriate in a patient with cholangitis, such as this patient, because waiting increases the risk of septicemia.
The patient undergoes ERCP with stone removal
Review of the operative report and discussion with the surgeon confirm that the laparoscopic procedure was uneventful and that intraoperative cholangiography was not done.
Therefore, the patient undergoes ERCP. The major papilla is normal. Cholangiography reveals nondilated common bile and intrahepatic ducts, with faint filling defects in the mid to distal common bile duct. Endoscopic sphincterotomy is performed, and three small stones are extracted from the common bile duct. Repeat balloon-occlusion cholangiography is normal.
The patient tolerates the procedure well and resumes a normal diet and normal activities.
Her pain persists, prompting an emergency room visit
Five days after her ERCP procedure, however, the same burning epigastric pain returns. As before, the pain occurs after eating and does not occur with fasting. At this time, she has no fever or chills.
WHAT IS CAUSING HER PAIN?
3. Which is the most likely cause of her persistent pain?
- Acute pancreatitis after ERCP
- Peptic ulcer disease
- Sphincter of Oddi dysfunction
- Biliary stones
The most likely cause is persistent biliary stones. The common bile duct was recently explored and stones were removed, but she may still have stones in the intrahepatic ducts or in the cystic duct remnant, both of which were unopacified during the ERCP procedure, indicating that either the test was incomplete or a stone is obstructing the passage of contrast. Her persistent symptoms warrant repeating her liver function tests.
Acute pancreatitis is the most common and feared complication of ERCP, and it should be suspected in any patient who develops abdominal pain within 6 hours of the procedure. It is much less likely to develop after 12 hours, however. Risk factors for post-ERCP pancreatitis include patient factors (young age, female sex, history of recurrent pancreatitis), procedural factors (difficult cannulation, minor papilla sphincterotomy), and, less likely, operator-related factors.10–13 In general, the more likely a patient is to have an abnormal and irregular common bile duct or pancreatic duct, the lower the risk of post-ERCP pancreatitis. The importance of operator-dependent factors is not yet clear.10–13
Despite the postprandial pattern of our patient’s pain and her history of gastric ulcer, peptic ulcer disease is unlikely in view of a normal esophagogastroduodenoscopic examination done 4 months earlier, and since she has no recent exposure to NSAIDs.
Sphincter of Oddi dysfunction may explain her symptoms, but she recently underwent endoscopic sphincterotomy, which is regarded as the most definitive treatment.14
WHAT SHOULD BE DONE NEXT?
4. What would be the best next step in her management?
- Repeat ERCP
- MRCP
- Endoscopic ultrasonography
- Observation and reassurance
MRCP is the most appropriate next step, given her recurrent symptoms. Repeat ERCP is not appropriate, since there is no evidence of cholangitis, and since her liver function tests had completely normalized.
A recent systematic review of endoscopic ultrasonography and MRCP for diagnosing choledocholithiasis found both tests to be highly accurate, with no statistically significant differences in sensitivity or specificity between the two.15 However, MRCP has the advantage of being noninvasive and of being able to show intrahepatic stones.
Park et al,16 in a prospective study of 66 patients with primary intrahepatic stones, concluded that MRCP findings were comparable to those of percutaneous transhepatic cholangioscopy, the reference standard for locating intrahepatic stones. The sensitivity, specificity, and accuracy of MRCP for detecting and locating intrahepatic stones were high (97%, 99%, and 98%, respectively).16 However, after sphincterotomy, pneumobilia may create an appearance that can be mistaken for intraductal stones.
She undergoes MRCP
The patient continues to have pain, and she has lost 5 pounds because she is still avoiding eating. At this point, she is beginning to wonder if her symptoms are psychogenic, since all the test results have been normal.
ERCP, MRCP, ULTRASONOGRAPHY?
5. What would be the best next step?
- Reassurance
- Referral to a psychiatrist
- Referral to a pain management clinic
- Endoscopic ultrasonography
- Repeat ERCP
Endoscopic ultrasonography is needed to look for cystic duct stones. Although several tests have shown normal results, the patient’s pain continues as in the previous episodes, making stone disease the most likely cause.
Although no stones were seen on MRCP and ultrasonography, a detailed evaluation for stones in a cystic duct or retained gallbladder remnant was not done satisfactorily.
Reassurance and referral to a psychiatrist or pain management clinic are not appropriate, since an organic cause of her pain has not been completely ruled out.
Findings on endoscopic ultrasonography
Endoscopic ultrasonography is performed and reveals a large (7-mm) stone in the area of the cystic duct remnant or gallbladder remnant (Figure 3). The common bile duct is normal.
CAUSES OF RETAINED GALLBLADDER AND CYSTIC DUCT REMNANT
6. What may have predisposed this patient to a retained gallbladder or cystic duct remnant after her surgery?
- Laparoscopic cholecystectomy
- Not doing intraoperative cholangiography
- Cholecystectomy for acute cholecystitis
- All of the above
All of the above may have contributed.
Postcholecystectomy syndrome can pose a diagnostic and therapeutic challenge, as in our patient. Although it has been reported since the advent of the operation, it is more common after laparoscopic cholecystectomy than after open surgery. One possible cause is stones in a cystic duct remnant, ie, a stub longer than 1 cm.
During open cholecystectomy, the cystic duct is ligated and cut as close to the common bile duct as possible, leaving only a small remnant. In laparoscopic cholecystectomy, it is divided closer to the gallbladder to avoid iatrogenic injury to the common bile duct, leaving a longer remnant. A long cystic duct remnant can be prevented by accurately locating the junction of the gallbladder and the cystic duct during cholecystectomy and by routinely doing intraoperative cholangiography. The presence of stones in a cystic duct or retained gallbladder remnant is a rare cause of postcholecystectomy syndrome, and suspicion is required to make the diagnosis.17–19
We should note that stones may also lurk in the short cystic duct remnant left after open cholecystectomy. In fact, the first case of cystic duct remnant, the so-called reformed gallbladder containing stones, was described in 1912 by Flörcken.20
Intraoperative cholangiography was introduced in 1931 by Mirizzi,21 who recommended its routine use. Since the advent of laparoscopic cholecystectomy in 1988, the routine use of intraoperative cholangiography has been debated. Advocates point to its ability to detect unsuspected calculi and to delineate the biliary anatomy, thus reducing the risk of biliary duct injury.7,22–25 Those who argue against its routine use emphasize the low reported rates of unsuspected stones in the common bile duct (2% to 3%), a longer operative time, the additional cost, and false-positive results that may lead to unnecessary common bile duct exploration. Another argument against its routine use is that most small ductal stones pass spontaneously without significant sequelae.26–28 Surgeons who use intraoperative cholangiography only selectively use it in patients with unclear biliary anatomy and preoperative biochemical or radiologic evidence of choledocholithiasis.
Case continued: She undergoes repeat ERCP
IF STONES ARE DIFFICULT TO EXTRACT
7. If the cystic duct stone were not amenable to endoscopic extraction, what would be the best alternative?
- Extracorporeal shock-wave lithotripsy (ESWL)
- Endoscopic biliary laser lithotripsy
- Repeat laparoscopic cholecystectomy
- All of the above
All of the above are alternatives.
A symptomatic stone in a cystic duct remnant is uncommon and is mentioned in the literature only in case series and case reports.
ESWL is effective for treating bile duct calculi.29 In a cohort of 239 patients with bile duct stones treated by ESWL, Benninger et al30 concluded that endoscopy plus ESWL was a definitive treatment for all patients except one, who subsequently underwent cholecystectomy. Once fragmented, the stones are extracted endoscopically.
Another fragmentation technique that can be offered to patients with stones in the cystic duct that are difficult to extract is contact fragmentation with a holmium laser placed in a transpapillary position under visual guidance.17
Repeat cholecystectomy with removal of stones in the cystic duct remnant (and removal of retained gallbladder remnants and reduction of the cystic duct remnant) has good postoperative results.17,18,31,32
After incomplete cholecystectomy, the cystic duct remnant and the Calot (cystohepatic) triangle are surrounded by inflamed scar tissue, and this was thought to make laparoscopic reoperation difficult.33 However, with advances in surgical technique and increasing experience of surgeons, repeat cholecystectomy can be done laparoscopically. It has now been suggested that laparoscopic exploration to remove the gallbladder remnants is safe and feasible in such patients.34,35
Discharge and follow-up
The patient is discharged home after the procedure. She is still free of symptoms 31 months later.
LESSONS LEARNED
Remnant cystic duct stones are uncommon
The estimated incidence of a retained calculus within the cystic duct remnant after cholecystectomy is less than 2.5%.2,36 In a series of 322 patients who underwent repeat surgery because of postcholecystectomy syndrome, Rogy et al36 found only 8 who had a stone in the cystic duct or gallbladder remnant, and in a series of 371 patients, Zhou el al2 found 4 who had a stone in the cystic duct remnant.
Stones in the cystic duct remnant are difficult to diagnose
Diagnosing stones in surgical remnants of the cystic duct or gallbladder can be difficult. The sensitivity of abdominal ultrasonography in detecting cystic duct stones is low—only 27% in one study, with a specificity of 100% and an accuracy of 75%.37 Ultrasonography may occasionally suggest cystic duct stones by showing an acoustic shadow in the anatomic region of the cystic duct. However, the results should be interpreted with caution.
Determining the accuracy of ERCP and MRCP in detecting cystic duct remnant stones is also difficult, as few cases have been reported and data may be conflicting. In a review of seven patients confirmed to have retained stones in a surgical remnant, Walsh et al17 found that ERCP correctly diagnosed the retained stone in only four out of six patients; MRCP was done in one patient, and it was read as normal.
In three cases of stones in a postsurgical gallbladder remnant, Hassan and Vilmann38 reported that ERCP and MRCP failed to identify the gallbladder remnant in two out of three cases, likely because the remaining structures are small. The diagnosis was finally made by endoscopic ultrasonography, which the authors concluded was a valuable method to visualize a small gallbladder remnant with stones.
Greater suspicion is needed in patients with typical biliary colic after cholecystectomy
Retained gallbladder remnant is described in the literature as a latent complication. The main problem is not the remnant itself but the chance that it harbors retained stones, which can lead to dilatation and inflammation of the remnant.
The patient can develop symptoms of acute cholecystitis or even acute cholangitis if the stone migrates to the common bile duct. Symptoms can develop as early as 2 weeks or as late as 25 years after laparoscopic cholecystectomy.
Endoscopic ultrasonography may be the best way to look for these remnant stones and to evaluate the bile duct and pancreas. Therefore, it should be part of the diagnostic algorithm in the evaluation of postcholecystectomy pain.
Mixed results with ERCP for extracting cystic duct stones
In case reports of cystic duct calculi after cholecystectomy, ERCP by itself has had mixed results. This traditional means of removing stones may succeed, as in our case. However, the success rate depends largely on anatomic factors such as the position of the stone in the cystic duct, the degree of stone impaction, the diameter of the cystic duct, and the number of valves in the duct.17
Stones in the cystic duct that cannot be extracted with ERCP may benefit from fragmentation techniques in situ via holmium laser followed by endoscopic extraction.
Repeat cholecystectomy is generally advised for any residual gallbladder, and it can be done laparoscopically.
- Lehman GA, Sherman S. Sphincter of Oddi dysfunction (postcholecystectomy syndrome). In:Yamada T, editor. Textbook of Gastroenterology. 2nd ed. Philadelphia: Lippincott; 1995:2251–2262.
- Zhou PH, Liu FL, Yao LQ, Qin XY. Endoscopic diagnosis and treatment of post-cholecystectomy syndrome. Hepatobiliary Pancreat Dis Int 2003; 2:117–120.
- Mergener K, Clavien PA, Branch MS, Baillie J. A stone in a grossly dilated cystic duct stump: a rare cause of postcholecystectomy pain. Am J Gastroenterol 1999; 94:229–231.
- Goenka MK, Kochhar R, Nagi B, Bhasin DK, Chowdhury A, Singh K. Endoscopic retrograde cholangiopancreatography in postcholecystectomy syndrome. J Assoc Physicians India 1996; 44:119–122.
- Bodvall B, Overgaard B. Cystic duct remnant after cholecystectomy: incidence studied by cholegraphy in 500 cases, and significance in 103 reoperations. Ann Surg 1966; 163:382–390.
- Bergman JJ, van den Brink GR, Rauws EA, et al. Treatment of bile duct lesions after laparoscopic cholecystectomy. Gut 1996; 38:141–147.
- Nickkholgh A, Soltaniyekta S, Kalbasi H. Routine versus selective intraoperative cholangiography during laparoscopic cholecystectomy: a survey of 2,130 patients undergoing laparoscopic cholecystectomy. Surg Endosc 2006; 20:868–874.
- Gandolfi L, Torresan F, Solmi L, Puccetti A. The role of ultrasound in biliary and pancreatic diseases. Eur J Ultrasound 2003; 16:141–159.
- Al Samaraee A, Khan U, Almashta Z, Yiannakou Y. Preoperative diagnosis of choledocholithiasis: the role of MRCP. Br J Hosp Med (Lond) 2009; 70:339–343.
- Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc 2001; 54:425–434.
- Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol 2006; 101:139–147.
- Mehta SN, Pavone E, Barkun JS, Bouchard S, Barkun AN. Predictors of post-ERCP complications in patients with suspected choledocholithiasis. Endoscopy 1998; 30:457–463.
- Badalov N, Tenner S, Baillie J. The prevention, recognition and treatment of post-ERCP pancreatitis. JOP 2009; 10:88–97.
- Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989; 320:82–87.
- Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64:248–254.
- Park DH, Kim MH, Lee SS, et al. Accuracy of magnetic resonance cholangiopancreatography for locating hepatolithiasis and detecting accompanying biliary strictures. Endoscopy 2004; 36:987–992.
- Walsh RM, Ponsky JL, Dumot J. Retained gallbladder/cystic duct remnant calculi as a cause of postcholecystectomy pain. Surg Endosc 2002; 16:981–984.
- Tantia O, Jain M, Khanna S, Sen B. Post cholecystectomy syndrome: role of cystic duct stump and re-intervention by laparoscopic surgery. J Minim Access Surg 2008; 4:71–75.
- Palanivelu C, Rangarajan M, Jategaonkar PA, Madankumar MV, Anand NV. Laparoscopic management of remnant cystic duct calculi: a retrospective study. Ann R Coll Surg Engl 2009; 91:25–29.
- Flörcken H. Gallenblasenregeneration mit Steinrecidiv nach Cholecystectomie. Deutsch Z Chir 1912; 113:604.
- Mirizzi PL. La colangiografía durante las operaciones de las vias biliares. Bol Soc Cirug Buenos Aires 1932; 16:1113.
- Soper NJ, Brunt LM. The case for routine operative cholangiography during laparoscopic cholecystectomy. Surg Clin North Am 1994; 74:953–959.
- Cuschieri A, Shimi S, Banting S, Nathanson LK, Pietrabissa A. Intraoperative cholangiography during laparoscopic cholecystectomy. Routine vs selective policy. Surg Endosc 1994; 8:302–305.
- Woods MS, Traverso LW, Kozarek RA, et al. Biliary tract complications of laparoscopic cholecystectomy are detected more frequently with routine intraoperative cholangiography. Surg Endosc 1995; 9:1076–1080.
- Vezakis A, Davides D, Ammori BJ, Martin IG, Larvin M, McMahon MJ. Intraoperative cholangiography during laparoscopic cholecystectomy. Surg Endosc 2000; 14:1118–1122.
- Ladocsi LT, Benitez LD, Filippone DR, Nance FC. Intraoperative cholangiography in laparoscopic cholecystectomy: a review of 734 consecutive cases. Am Surg 1997; 63:150–156.
- Clair DG, Brooks DC. Laparoscopic cholangiography. The case for a selective approach. Surg Clin North Am 1994; 74:961–966.
- Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg 2004; 239:28–33.
- Ponsky LE, Geisinger MA, Ponsky JL, Streem SB. Contemporary ‘urologic’ intervention in the pancreaticobiliary tree. Urology 2001; 57:21–25.
- Benninger J, Rabenstein T, Farnbacher M, Keppler J, Hahn EG, Schneider HT. Extracorporeal shockwave lithotripsy of gallstones in cystic duct remnants and Mirizzi syndrome. Gastrointest Endosc 2004; 60:454–459.
- Demetriades H, Pramateftakis MG, Kanellos I, Angelopoulos S, Mantzoros I, Betsis D. Retained gallbladder remnant after laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A 2008; 18:276–279.
- Shaw C, O’Hanlon DM, Fenlon HM, McEntee GP. Cystic duct remnant and the ‘post-cholecystectomy syndrome. ’ Hepatogastroenterology 2004; 51:36–38.
- Rozsos I, Magyaródi Z, Orbán P. Cystic duct syndrome and minimally invasive surgery. [Hungarian] Orv Hetil 1997; 138:2397–2401.
- Chowbey PK, Bandyopadhyay SK, Sharma A, Khullar R, Soni V, Baijal M. Laparoscopic reintervention for residual gallstone disease. Surg Laparosc Endosc Percutan Tech 2003; 13:31–35.
- Clemente G, Giuliante F, Cadeddu F, Nuzzo G. Laparoscopic removal of gallbladder remnant and long cystic stump. Endoscopy 2001; 33:814–815.
- Rogy MA, Függer R, Herbst F, Schulz F. Reoperation after cholecystectomy. The role of the cystic duct stump. HPB Surg 1991; 4:129–134.
- Laing FC, Jeffrey RB. Choledocholithiasis and cystic duct obstruction: difficult ultrasonographic diagnosis. Radiology 1983; 146:475–479.
- Hassan H, Vilmann P. Insufficient cholecystectomy diagnosed by endoscopic ultrasonography. Endoscopy 2004; 36:236–238.
Four months after undergoing laparoscopic cholecystectomy for symptomatic gallstones, an otherwise healthy 26-year-old woman begins to have episodes of epigastric and back pain similar to what she experienced before the surgery. The surgery was without complications, and her classic biliary colic disappeared afterward. Histologic evaluation of the surgical specimen revealed chronic cholecystitis with multiple small, mixed gallstones.
Now she describes a burning pain in her epigastrium and mid to upper back, starting about 30 minutes after a meal and lasting up to 4 hours. Sometimes it awakens her at night. She avoids eating for fear of inducing the pain. She has occasional chills but no fever, nausea, vomiting, jaundice, or changes in urine or stool color.
Three years ago she was diagnosed with a gastric ulcer induced by taking a nonsteroidal anti-inflammatory drug (NSAID). The ulcer was treated with a proton pump inhibitor for 1 month. She says the ulcer pain was dull and aching, different from her current pain.
Upper endoscopy 4 months ago (ie, before her laparoscopic cholecystectomy) showed no evidence of esophagitis or peptic ulcer disease.
Apart from her gallbladder operation, she has had no other surgery. According to the surgeon’s notes, intraoperative cholangiography was not performed, and no macroscopic changes of acute cholecystitis or difficult biliary anatomy were noted.
The patient does not smoke, does not drink alcohol, is not currently taking any medications, including NSAIDs or over-the-counter medications, and has not taken any recently. Her mother also had symptomatic gallstones requiring cholecystectomy.
On physical examination, only fever
On examination, her temperature is 101.2°F (38.4°C), blood pressure 117/80 mm Hg, heart rate 82 beats per minute, and blood oxygen saturation 99% on room air. Her weight is 138 lb (62.6 kg), height 5 feet 6 inches (168 cm).
There is no jaundice or pallor. Her heart and lung examinations are normal.
No costovertebral angle or spinal tenderness can be elicited.
Her laboratory values are shown in Table 1.
POSTCHOLECYSTECTOMY SYNDROME
1. After cholecystectomy, preoperative symptoms recur in what percentage of patients?
- 10% to 40%
- 50%
- 60%
- 80%
Postcholecystectomy syndrome—the recurrence of symptoms similar to those before the procedure—occurs in 10% to 40% of patients. The time to the onset of symptoms can range from 2 days to up to 25 years.1–4 Women may be at higher risk, with symptoms recurring in 43% vs 28% in men.5
Postcholecystectomy syndrome can have a biliary or a nonbiliary cause. Biliary causes include strictures, retained calculi, dropped calculi, tumors, sphincter of Oddi dysfunction, and calculi in the cystic duct remnant. Nonbiliary causes include functional and organic disorders such as peptic ulcer disease, gastroesophageal reflux, pancreatic disease, hepatocellular disorders, coronary artery disease, irritable bowel syndrome, and intercostal neuritis.
WHAT IS THE NEXT STEP?
2. Which is the most appropriate next step in the workup of this patient?
- Ultrasonography of the right upper quadrant
- Magnetic resonance cholangiopancreatography (MRCP)
- Endoscopic retrograde cholangiopancreatography (ERCP)
- Observation and reassurance
- Review the operative record and consult with the surgeon
Although the patient is presenting with pain and fever, two features of the classic Charcot triad (pain, fever, jaundice) seen in cholangitis (infection of a bile duct), and although cholangitis almost confirms the diagnosis of common bile duct stones in a patient with gallstones (before or after cholecystectomy), other diagnoses to consider are bile duct injury, bile leak, and biloma.
Biloma can be detected with ultrasonography. Bile duct injuries are identified intraoperatively in up to 25% of patients. For those with an unrecognized injury, the clinical presentation is variable and depends on the type of injury. If a bile leak is present, patients present early, at a median of 3 days postoperatively. However, our patient presented with symptoms 4 months after her surgery. Patients with bile duct strictures without bile leak have a longer symptom-free interval and usually present with signs of biliary obstruction. Ultrasonography can then detect biliary dilatation.6
It would be very helpful to review the operative record and to talk to the surgeon to confirm that intraoperative cholangiography had not been done and to determine the level of difficulty of the surgery. (Intraoperative cholangiography involves the introduction of contrast dye into the biliary system by cannulation of the cystic duct or by direct injection into the common bile duct. An intraoperative cholangiogram is considered normal if the entire intrahepatic and extrahepatic biliary tree is seen to be filled with contrast.) A normal cholangiogram has a negative predictive value of 99.8% for the detection of ductal stones. Thus, a normal intraoperative cholangiogram can prevent unnecessary postoperative ECRP, since it almost always indicates a clean bile duct.7
Ultrasonography of the right upper quadrant has a low sensitivity (< 50%) for detecting common bile duct stones. However, it is highly operator-dependent, and it may be twice as sensitive if done by expert radiologists than by less experienced ones. Its limitations include poor visualization of the distal portion of the duct and low sensitivity in patients in whom the common bile duct is minimally dilated and also in patients with small stones. In most studies, however, it had a very high specificity—ie, greater than 95%.8
MRCP has a sensitivity of 82.6% and a specificity of 97.5% in detecting stones in the common bile duct.9 Therefore, normal results on abdominal ultrasonography and MRCP do not completely rule out stones.
Although this patient has a high pretest probability of having common bile duct stones, ERCP should be done only after a thorough review of the previous operative procedure.
Observation and reassurance are not appropriate in a patient with cholangitis, such as this patient, because waiting increases the risk of septicemia.
The patient undergoes ERCP with stone removal
Review of the operative report and discussion with the surgeon confirm that the laparoscopic procedure was uneventful and that intraoperative cholangiography was not done.
Therefore, the patient undergoes ERCP. The major papilla is normal. Cholangiography reveals nondilated common bile and intrahepatic ducts, with faint filling defects in the mid to distal common bile duct. Endoscopic sphincterotomy is performed, and three small stones are extracted from the common bile duct. Repeat balloon-occlusion cholangiography is normal.
The patient tolerates the procedure well and resumes a normal diet and normal activities.
Her pain persists, prompting an emergency room visit
Five days after her ERCP procedure, however, the same burning epigastric pain returns. As before, the pain occurs after eating and does not occur with fasting. At this time, she has no fever or chills.
WHAT IS CAUSING HER PAIN?
3. Which is the most likely cause of her persistent pain?
- Acute pancreatitis after ERCP
- Peptic ulcer disease
- Sphincter of Oddi dysfunction
- Biliary stones
The most likely cause is persistent biliary stones. The common bile duct was recently explored and stones were removed, but she may still have stones in the intrahepatic ducts or in the cystic duct remnant, both of which were unopacified during the ERCP procedure, indicating that either the test was incomplete or a stone is obstructing the passage of contrast. Her persistent symptoms warrant repeating her liver function tests.
Acute pancreatitis is the most common and feared complication of ERCP, and it should be suspected in any patient who develops abdominal pain within 6 hours of the procedure. It is much less likely to develop after 12 hours, however. Risk factors for post-ERCP pancreatitis include patient factors (young age, female sex, history of recurrent pancreatitis), procedural factors (difficult cannulation, minor papilla sphincterotomy), and, less likely, operator-related factors.10–13 In general, the more likely a patient is to have an abnormal and irregular common bile duct or pancreatic duct, the lower the risk of post-ERCP pancreatitis. The importance of operator-dependent factors is not yet clear.10–13
Despite the postprandial pattern of our patient’s pain and her history of gastric ulcer, peptic ulcer disease is unlikely in view of a normal esophagogastroduodenoscopic examination done 4 months earlier, and since she has no recent exposure to NSAIDs.
Sphincter of Oddi dysfunction may explain her symptoms, but she recently underwent endoscopic sphincterotomy, which is regarded as the most definitive treatment.14
WHAT SHOULD BE DONE NEXT?
4. What would be the best next step in her management?
- Repeat ERCP
- MRCP
- Endoscopic ultrasonography
- Observation and reassurance
MRCP is the most appropriate next step, given her recurrent symptoms. Repeat ERCP is not appropriate, since there is no evidence of cholangitis, and since her liver function tests had completely normalized.
A recent systematic review of endoscopic ultrasonography and MRCP for diagnosing choledocholithiasis found both tests to be highly accurate, with no statistically significant differences in sensitivity or specificity between the two.15 However, MRCP has the advantage of being noninvasive and of being able to show intrahepatic stones.
Park et al,16 in a prospective study of 66 patients with primary intrahepatic stones, concluded that MRCP findings were comparable to those of percutaneous transhepatic cholangioscopy, the reference standard for locating intrahepatic stones. The sensitivity, specificity, and accuracy of MRCP for detecting and locating intrahepatic stones were high (97%, 99%, and 98%, respectively).16 However, after sphincterotomy, pneumobilia may create an appearance that can be mistaken for intraductal stones.
She undergoes MRCP
The patient continues to have pain, and she has lost 5 pounds because she is still avoiding eating. At this point, she is beginning to wonder if her symptoms are psychogenic, since all the test results have been normal.
ERCP, MRCP, ULTRASONOGRAPHY?
5. What would be the best next step?
- Reassurance
- Referral to a psychiatrist
- Referral to a pain management clinic
- Endoscopic ultrasonography
- Repeat ERCP
Endoscopic ultrasonography is needed to look for cystic duct stones. Although several tests have shown normal results, the patient’s pain continues as in the previous episodes, making stone disease the most likely cause.
Although no stones were seen on MRCP and ultrasonography, a detailed evaluation for stones in a cystic duct or retained gallbladder remnant was not done satisfactorily.
Reassurance and referral to a psychiatrist or pain management clinic are not appropriate, since an organic cause of her pain has not been completely ruled out.
Findings on endoscopic ultrasonography
Endoscopic ultrasonography is performed and reveals a large (7-mm) stone in the area of the cystic duct remnant or gallbladder remnant (Figure 3). The common bile duct is normal.
CAUSES OF RETAINED GALLBLADDER AND CYSTIC DUCT REMNANT
6. What may have predisposed this patient to a retained gallbladder or cystic duct remnant after her surgery?
- Laparoscopic cholecystectomy
- Not doing intraoperative cholangiography
- Cholecystectomy for acute cholecystitis
- All of the above
All of the above may have contributed.
Postcholecystectomy syndrome can pose a diagnostic and therapeutic challenge, as in our patient. Although it has been reported since the advent of the operation, it is more common after laparoscopic cholecystectomy than after open surgery. One possible cause is stones in a cystic duct remnant, ie, a stub longer than 1 cm.
During open cholecystectomy, the cystic duct is ligated and cut as close to the common bile duct as possible, leaving only a small remnant. In laparoscopic cholecystectomy, it is divided closer to the gallbladder to avoid iatrogenic injury to the common bile duct, leaving a longer remnant. A long cystic duct remnant can be prevented by accurately locating the junction of the gallbladder and the cystic duct during cholecystectomy and by routinely doing intraoperative cholangiography. The presence of stones in a cystic duct or retained gallbladder remnant is a rare cause of postcholecystectomy syndrome, and suspicion is required to make the diagnosis.17–19
We should note that stones may also lurk in the short cystic duct remnant left after open cholecystectomy. In fact, the first case of cystic duct remnant, the so-called reformed gallbladder containing stones, was described in 1912 by Flörcken.20
Intraoperative cholangiography was introduced in 1931 by Mirizzi,21 who recommended its routine use. Since the advent of laparoscopic cholecystectomy in 1988, the routine use of intraoperative cholangiography has been debated. Advocates point to its ability to detect unsuspected calculi and to delineate the biliary anatomy, thus reducing the risk of biliary duct injury.7,22–25 Those who argue against its routine use emphasize the low reported rates of unsuspected stones in the common bile duct (2% to 3%), a longer operative time, the additional cost, and false-positive results that may lead to unnecessary common bile duct exploration. Another argument against its routine use is that most small ductal stones pass spontaneously without significant sequelae.26–28 Surgeons who use intraoperative cholangiography only selectively use it in patients with unclear biliary anatomy and preoperative biochemical or radiologic evidence of choledocholithiasis.
Case continued: She undergoes repeat ERCP
IF STONES ARE DIFFICULT TO EXTRACT
7. If the cystic duct stone were not amenable to endoscopic extraction, what would be the best alternative?
- Extracorporeal shock-wave lithotripsy (ESWL)
- Endoscopic biliary laser lithotripsy
- Repeat laparoscopic cholecystectomy
- All of the above
All of the above are alternatives.
A symptomatic stone in a cystic duct remnant is uncommon and is mentioned in the literature only in case series and case reports.
ESWL is effective for treating bile duct calculi.29 In a cohort of 239 patients with bile duct stones treated by ESWL, Benninger et al30 concluded that endoscopy plus ESWL was a definitive treatment for all patients except one, who subsequently underwent cholecystectomy. Once fragmented, the stones are extracted endoscopically.
Another fragmentation technique that can be offered to patients with stones in the cystic duct that are difficult to extract is contact fragmentation with a holmium laser placed in a transpapillary position under visual guidance.17
Repeat cholecystectomy with removal of stones in the cystic duct remnant (and removal of retained gallbladder remnants and reduction of the cystic duct remnant) has good postoperative results.17,18,31,32
After incomplete cholecystectomy, the cystic duct remnant and the Calot (cystohepatic) triangle are surrounded by inflamed scar tissue, and this was thought to make laparoscopic reoperation difficult.33 However, with advances in surgical technique and increasing experience of surgeons, repeat cholecystectomy can be done laparoscopically. It has now been suggested that laparoscopic exploration to remove the gallbladder remnants is safe and feasible in such patients.34,35
Discharge and follow-up
The patient is discharged home after the procedure. She is still free of symptoms 31 months later.
LESSONS LEARNED
Remnant cystic duct stones are uncommon
The estimated incidence of a retained calculus within the cystic duct remnant after cholecystectomy is less than 2.5%.2,36 In a series of 322 patients who underwent repeat surgery because of postcholecystectomy syndrome, Rogy et al36 found only 8 who had a stone in the cystic duct or gallbladder remnant, and in a series of 371 patients, Zhou el al2 found 4 who had a stone in the cystic duct remnant.
Stones in the cystic duct remnant are difficult to diagnose
Diagnosing stones in surgical remnants of the cystic duct or gallbladder can be difficult. The sensitivity of abdominal ultrasonography in detecting cystic duct stones is low—only 27% in one study, with a specificity of 100% and an accuracy of 75%.37 Ultrasonography may occasionally suggest cystic duct stones by showing an acoustic shadow in the anatomic region of the cystic duct. However, the results should be interpreted with caution.
Determining the accuracy of ERCP and MRCP in detecting cystic duct remnant stones is also difficult, as few cases have been reported and data may be conflicting. In a review of seven patients confirmed to have retained stones in a surgical remnant, Walsh et al17 found that ERCP correctly diagnosed the retained stone in only four out of six patients; MRCP was done in one patient, and it was read as normal.
In three cases of stones in a postsurgical gallbladder remnant, Hassan and Vilmann38 reported that ERCP and MRCP failed to identify the gallbladder remnant in two out of three cases, likely because the remaining structures are small. The diagnosis was finally made by endoscopic ultrasonography, which the authors concluded was a valuable method to visualize a small gallbladder remnant with stones.
Greater suspicion is needed in patients with typical biliary colic after cholecystectomy
Retained gallbladder remnant is described in the literature as a latent complication. The main problem is not the remnant itself but the chance that it harbors retained stones, which can lead to dilatation and inflammation of the remnant.
The patient can develop symptoms of acute cholecystitis or even acute cholangitis if the stone migrates to the common bile duct. Symptoms can develop as early as 2 weeks or as late as 25 years after laparoscopic cholecystectomy.
Endoscopic ultrasonography may be the best way to look for these remnant stones and to evaluate the bile duct and pancreas. Therefore, it should be part of the diagnostic algorithm in the evaluation of postcholecystectomy pain.
Mixed results with ERCP for extracting cystic duct stones
In case reports of cystic duct calculi after cholecystectomy, ERCP by itself has had mixed results. This traditional means of removing stones may succeed, as in our case. However, the success rate depends largely on anatomic factors such as the position of the stone in the cystic duct, the degree of stone impaction, the diameter of the cystic duct, and the number of valves in the duct.17
Stones in the cystic duct that cannot be extracted with ERCP may benefit from fragmentation techniques in situ via holmium laser followed by endoscopic extraction.
Repeat cholecystectomy is generally advised for any residual gallbladder, and it can be done laparoscopically.
Four months after undergoing laparoscopic cholecystectomy for symptomatic gallstones, an otherwise healthy 26-year-old woman begins to have episodes of epigastric and back pain similar to what she experienced before the surgery. The surgery was without complications, and her classic biliary colic disappeared afterward. Histologic evaluation of the surgical specimen revealed chronic cholecystitis with multiple small, mixed gallstones.
Now she describes a burning pain in her epigastrium and mid to upper back, starting about 30 minutes after a meal and lasting up to 4 hours. Sometimes it awakens her at night. She avoids eating for fear of inducing the pain. She has occasional chills but no fever, nausea, vomiting, jaundice, or changes in urine or stool color.
Three years ago she was diagnosed with a gastric ulcer induced by taking a nonsteroidal anti-inflammatory drug (NSAID). The ulcer was treated with a proton pump inhibitor for 1 month. She says the ulcer pain was dull and aching, different from her current pain.
Upper endoscopy 4 months ago (ie, before her laparoscopic cholecystectomy) showed no evidence of esophagitis or peptic ulcer disease.
Apart from her gallbladder operation, she has had no other surgery. According to the surgeon’s notes, intraoperative cholangiography was not performed, and no macroscopic changes of acute cholecystitis or difficult biliary anatomy were noted.
The patient does not smoke, does not drink alcohol, is not currently taking any medications, including NSAIDs or over-the-counter medications, and has not taken any recently. Her mother also had symptomatic gallstones requiring cholecystectomy.
On physical examination, only fever
On examination, her temperature is 101.2°F (38.4°C), blood pressure 117/80 mm Hg, heart rate 82 beats per minute, and blood oxygen saturation 99% on room air. Her weight is 138 lb (62.6 kg), height 5 feet 6 inches (168 cm).
There is no jaundice or pallor. Her heart and lung examinations are normal.
No costovertebral angle or spinal tenderness can be elicited.
Her laboratory values are shown in Table 1.
POSTCHOLECYSTECTOMY SYNDROME
1. After cholecystectomy, preoperative symptoms recur in what percentage of patients?
- 10% to 40%
- 50%
- 60%
- 80%
Postcholecystectomy syndrome—the recurrence of symptoms similar to those before the procedure—occurs in 10% to 40% of patients. The time to the onset of symptoms can range from 2 days to up to 25 years.1–4 Women may be at higher risk, with symptoms recurring in 43% vs 28% in men.5
Postcholecystectomy syndrome can have a biliary or a nonbiliary cause. Biliary causes include strictures, retained calculi, dropped calculi, tumors, sphincter of Oddi dysfunction, and calculi in the cystic duct remnant. Nonbiliary causes include functional and organic disorders such as peptic ulcer disease, gastroesophageal reflux, pancreatic disease, hepatocellular disorders, coronary artery disease, irritable bowel syndrome, and intercostal neuritis.
WHAT IS THE NEXT STEP?
2. Which is the most appropriate next step in the workup of this patient?
- Ultrasonography of the right upper quadrant
- Magnetic resonance cholangiopancreatography (MRCP)
- Endoscopic retrograde cholangiopancreatography (ERCP)
- Observation and reassurance
- Review the operative record and consult with the surgeon
Although the patient is presenting with pain and fever, two features of the classic Charcot triad (pain, fever, jaundice) seen in cholangitis (infection of a bile duct), and although cholangitis almost confirms the diagnosis of common bile duct stones in a patient with gallstones (before or after cholecystectomy), other diagnoses to consider are bile duct injury, bile leak, and biloma.
Biloma can be detected with ultrasonography. Bile duct injuries are identified intraoperatively in up to 25% of patients. For those with an unrecognized injury, the clinical presentation is variable and depends on the type of injury. If a bile leak is present, patients present early, at a median of 3 days postoperatively. However, our patient presented with symptoms 4 months after her surgery. Patients with bile duct strictures without bile leak have a longer symptom-free interval and usually present with signs of biliary obstruction. Ultrasonography can then detect biliary dilatation.6
It would be very helpful to review the operative record and to talk to the surgeon to confirm that intraoperative cholangiography had not been done and to determine the level of difficulty of the surgery. (Intraoperative cholangiography involves the introduction of contrast dye into the biliary system by cannulation of the cystic duct or by direct injection into the common bile duct. An intraoperative cholangiogram is considered normal if the entire intrahepatic and extrahepatic biliary tree is seen to be filled with contrast.) A normal cholangiogram has a negative predictive value of 99.8% for the detection of ductal stones. Thus, a normal intraoperative cholangiogram can prevent unnecessary postoperative ECRP, since it almost always indicates a clean bile duct.7
Ultrasonography of the right upper quadrant has a low sensitivity (< 50%) for detecting common bile duct stones. However, it is highly operator-dependent, and it may be twice as sensitive if done by expert radiologists than by less experienced ones. Its limitations include poor visualization of the distal portion of the duct and low sensitivity in patients in whom the common bile duct is minimally dilated and also in patients with small stones. In most studies, however, it had a very high specificity—ie, greater than 95%.8
MRCP has a sensitivity of 82.6% and a specificity of 97.5% in detecting stones in the common bile duct.9 Therefore, normal results on abdominal ultrasonography and MRCP do not completely rule out stones.
Although this patient has a high pretest probability of having common bile duct stones, ERCP should be done only after a thorough review of the previous operative procedure.
Observation and reassurance are not appropriate in a patient with cholangitis, such as this patient, because waiting increases the risk of septicemia.
The patient undergoes ERCP with stone removal
Review of the operative report and discussion with the surgeon confirm that the laparoscopic procedure was uneventful and that intraoperative cholangiography was not done.
Therefore, the patient undergoes ERCP. The major papilla is normal. Cholangiography reveals nondilated common bile and intrahepatic ducts, with faint filling defects in the mid to distal common bile duct. Endoscopic sphincterotomy is performed, and three small stones are extracted from the common bile duct. Repeat balloon-occlusion cholangiography is normal.
The patient tolerates the procedure well and resumes a normal diet and normal activities.
Her pain persists, prompting an emergency room visit
Five days after her ERCP procedure, however, the same burning epigastric pain returns. As before, the pain occurs after eating and does not occur with fasting. At this time, she has no fever or chills.
WHAT IS CAUSING HER PAIN?
3. Which is the most likely cause of her persistent pain?
- Acute pancreatitis after ERCP
- Peptic ulcer disease
- Sphincter of Oddi dysfunction
- Biliary stones
The most likely cause is persistent biliary stones. The common bile duct was recently explored and stones were removed, but she may still have stones in the intrahepatic ducts or in the cystic duct remnant, both of which were unopacified during the ERCP procedure, indicating that either the test was incomplete or a stone is obstructing the passage of contrast. Her persistent symptoms warrant repeating her liver function tests.
Acute pancreatitis is the most common and feared complication of ERCP, and it should be suspected in any patient who develops abdominal pain within 6 hours of the procedure. It is much less likely to develop after 12 hours, however. Risk factors for post-ERCP pancreatitis include patient factors (young age, female sex, history of recurrent pancreatitis), procedural factors (difficult cannulation, minor papilla sphincterotomy), and, less likely, operator-related factors.10–13 In general, the more likely a patient is to have an abnormal and irregular common bile duct or pancreatic duct, the lower the risk of post-ERCP pancreatitis. The importance of operator-dependent factors is not yet clear.10–13
Despite the postprandial pattern of our patient’s pain and her history of gastric ulcer, peptic ulcer disease is unlikely in view of a normal esophagogastroduodenoscopic examination done 4 months earlier, and since she has no recent exposure to NSAIDs.
Sphincter of Oddi dysfunction may explain her symptoms, but she recently underwent endoscopic sphincterotomy, which is regarded as the most definitive treatment.14
WHAT SHOULD BE DONE NEXT?
4. What would be the best next step in her management?
- Repeat ERCP
- MRCP
- Endoscopic ultrasonography
- Observation and reassurance
MRCP is the most appropriate next step, given her recurrent symptoms. Repeat ERCP is not appropriate, since there is no evidence of cholangitis, and since her liver function tests had completely normalized.
A recent systematic review of endoscopic ultrasonography and MRCP for diagnosing choledocholithiasis found both tests to be highly accurate, with no statistically significant differences in sensitivity or specificity between the two.15 However, MRCP has the advantage of being noninvasive and of being able to show intrahepatic stones.
Park et al,16 in a prospective study of 66 patients with primary intrahepatic stones, concluded that MRCP findings were comparable to those of percutaneous transhepatic cholangioscopy, the reference standard for locating intrahepatic stones. The sensitivity, specificity, and accuracy of MRCP for detecting and locating intrahepatic stones were high (97%, 99%, and 98%, respectively).16 However, after sphincterotomy, pneumobilia may create an appearance that can be mistaken for intraductal stones.
She undergoes MRCP
The patient continues to have pain, and she has lost 5 pounds because she is still avoiding eating. At this point, she is beginning to wonder if her symptoms are psychogenic, since all the test results have been normal.
ERCP, MRCP, ULTRASONOGRAPHY?
5. What would be the best next step?
- Reassurance
- Referral to a psychiatrist
- Referral to a pain management clinic
- Endoscopic ultrasonography
- Repeat ERCP
Endoscopic ultrasonography is needed to look for cystic duct stones. Although several tests have shown normal results, the patient’s pain continues as in the previous episodes, making stone disease the most likely cause.
Although no stones were seen on MRCP and ultrasonography, a detailed evaluation for stones in a cystic duct or retained gallbladder remnant was not done satisfactorily.
Reassurance and referral to a psychiatrist or pain management clinic are not appropriate, since an organic cause of her pain has not been completely ruled out.
Findings on endoscopic ultrasonography
Endoscopic ultrasonography is performed and reveals a large (7-mm) stone in the area of the cystic duct remnant or gallbladder remnant (Figure 3). The common bile duct is normal.
CAUSES OF RETAINED GALLBLADDER AND CYSTIC DUCT REMNANT
6. What may have predisposed this patient to a retained gallbladder or cystic duct remnant after her surgery?
- Laparoscopic cholecystectomy
- Not doing intraoperative cholangiography
- Cholecystectomy for acute cholecystitis
- All of the above
All of the above may have contributed.
Postcholecystectomy syndrome can pose a diagnostic and therapeutic challenge, as in our patient. Although it has been reported since the advent of the operation, it is more common after laparoscopic cholecystectomy than after open surgery. One possible cause is stones in a cystic duct remnant, ie, a stub longer than 1 cm.
During open cholecystectomy, the cystic duct is ligated and cut as close to the common bile duct as possible, leaving only a small remnant. In laparoscopic cholecystectomy, it is divided closer to the gallbladder to avoid iatrogenic injury to the common bile duct, leaving a longer remnant. A long cystic duct remnant can be prevented by accurately locating the junction of the gallbladder and the cystic duct during cholecystectomy and by routinely doing intraoperative cholangiography. The presence of stones in a cystic duct or retained gallbladder remnant is a rare cause of postcholecystectomy syndrome, and suspicion is required to make the diagnosis.17–19
We should note that stones may also lurk in the short cystic duct remnant left after open cholecystectomy. In fact, the first case of cystic duct remnant, the so-called reformed gallbladder containing stones, was described in 1912 by Flörcken.20
Intraoperative cholangiography was introduced in 1931 by Mirizzi,21 who recommended its routine use. Since the advent of laparoscopic cholecystectomy in 1988, the routine use of intraoperative cholangiography has been debated. Advocates point to its ability to detect unsuspected calculi and to delineate the biliary anatomy, thus reducing the risk of biliary duct injury.7,22–25 Those who argue against its routine use emphasize the low reported rates of unsuspected stones in the common bile duct (2% to 3%), a longer operative time, the additional cost, and false-positive results that may lead to unnecessary common bile duct exploration. Another argument against its routine use is that most small ductal stones pass spontaneously without significant sequelae.26–28 Surgeons who use intraoperative cholangiography only selectively use it in patients with unclear biliary anatomy and preoperative biochemical or radiologic evidence of choledocholithiasis.
Case continued: She undergoes repeat ERCP
IF STONES ARE DIFFICULT TO EXTRACT
7. If the cystic duct stone were not amenable to endoscopic extraction, what would be the best alternative?
- Extracorporeal shock-wave lithotripsy (ESWL)
- Endoscopic biliary laser lithotripsy
- Repeat laparoscopic cholecystectomy
- All of the above
All of the above are alternatives.
A symptomatic stone in a cystic duct remnant is uncommon and is mentioned in the literature only in case series and case reports.
ESWL is effective for treating bile duct calculi.29 In a cohort of 239 patients with bile duct stones treated by ESWL, Benninger et al30 concluded that endoscopy plus ESWL was a definitive treatment for all patients except one, who subsequently underwent cholecystectomy. Once fragmented, the stones are extracted endoscopically.
Another fragmentation technique that can be offered to patients with stones in the cystic duct that are difficult to extract is contact fragmentation with a holmium laser placed in a transpapillary position under visual guidance.17
Repeat cholecystectomy with removal of stones in the cystic duct remnant (and removal of retained gallbladder remnants and reduction of the cystic duct remnant) has good postoperative results.17,18,31,32
After incomplete cholecystectomy, the cystic duct remnant and the Calot (cystohepatic) triangle are surrounded by inflamed scar tissue, and this was thought to make laparoscopic reoperation difficult.33 However, with advances in surgical technique and increasing experience of surgeons, repeat cholecystectomy can be done laparoscopically. It has now been suggested that laparoscopic exploration to remove the gallbladder remnants is safe and feasible in such patients.34,35
Discharge and follow-up
The patient is discharged home after the procedure. She is still free of symptoms 31 months later.
LESSONS LEARNED
Remnant cystic duct stones are uncommon
The estimated incidence of a retained calculus within the cystic duct remnant after cholecystectomy is less than 2.5%.2,36 In a series of 322 patients who underwent repeat surgery because of postcholecystectomy syndrome, Rogy et al36 found only 8 who had a stone in the cystic duct or gallbladder remnant, and in a series of 371 patients, Zhou el al2 found 4 who had a stone in the cystic duct remnant.
Stones in the cystic duct remnant are difficult to diagnose
Diagnosing stones in surgical remnants of the cystic duct or gallbladder can be difficult. The sensitivity of abdominal ultrasonography in detecting cystic duct stones is low—only 27% in one study, with a specificity of 100% and an accuracy of 75%.37 Ultrasonography may occasionally suggest cystic duct stones by showing an acoustic shadow in the anatomic region of the cystic duct. However, the results should be interpreted with caution.
Determining the accuracy of ERCP and MRCP in detecting cystic duct remnant stones is also difficult, as few cases have been reported and data may be conflicting. In a review of seven patients confirmed to have retained stones in a surgical remnant, Walsh et al17 found that ERCP correctly diagnosed the retained stone in only four out of six patients; MRCP was done in one patient, and it was read as normal.
In three cases of stones in a postsurgical gallbladder remnant, Hassan and Vilmann38 reported that ERCP and MRCP failed to identify the gallbladder remnant in two out of three cases, likely because the remaining structures are small. The diagnosis was finally made by endoscopic ultrasonography, which the authors concluded was a valuable method to visualize a small gallbladder remnant with stones.
Greater suspicion is needed in patients with typical biliary colic after cholecystectomy
Retained gallbladder remnant is described in the literature as a latent complication. The main problem is not the remnant itself but the chance that it harbors retained stones, which can lead to dilatation and inflammation of the remnant.
The patient can develop symptoms of acute cholecystitis or even acute cholangitis if the stone migrates to the common bile duct. Symptoms can develop as early as 2 weeks or as late as 25 years after laparoscopic cholecystectomy.
Endoscopic ultrasonography may be the best way to look for these remnant stones and to evaluate the bile duct and pancreas. Therefore, it should be part of the diagnostic algorithm in the evaluation of postcholecystectomy pain.
Mixed results with ERCP for extracting cystic duct stones
In case reports of cystic duct calculi after cholecystectomy, ERCP by itself has had mixed results. This traditional means of removing stones may succeed, as in our case. However, the success rate depends largely on anatomic factors such as the position of the stone in the cystic duct, the degree of stone impaction, the diameter of the cystic duct, and the number of valves in the duct.17
Stones in the cystic duct that cannot be extracted with ERCP may benefit from fragmentation techniques in situ via holmium laser followed by endoscopic extraction.
Repeat cholecystectomy is generally advised for any residual gallbladder, and it can be done laparoscopically.
- Lehman GA, Sherman S. Sphincter of Oddi dysfunction (postcholecystectomy syndrome). In:Yamada T, editor. Textbook of Gastroenterology. 2nd ed. Philadelphia: Lippincott; 1995:2251–2262.
- Zhou PH, Liu FL, Yao LQ, Qin XY. Endoscopic diagnosis and treatment of post-cholecystectomy syndrome. Hepatobiliary Pancreat Dis Int 2003; 2:117–120.
- Mergener K, Clavien PA, Branch MS, Baillie J. A stone in a grossly dilated cystic duct stump: a rare cause of postcholecystectomy pain. Am J Gastroenterol 1999; 94:229–231.
- Goenka MK, Kochhar R, Nagi B, Bhasin DK, Chowdhury A, Singh K. Endoscopic retrograde cholangiopancreatography in postcholecystectomy syndrome. J Assoc Physicians India 1996; 44:119–122.
- Bodvall B, Overgaard B. Cystic duct remnant after cholecystectomy: incidence studied by cholegraphy in 500 cases, and significance in 103 reoperations. Ann Surg 1966; 163:382–390.
- Bergman JJ, van den Brink GR, Rauws EA, et al. Treatment of bile duct lesions after laparoscopic cholecystectomy. Gut 1996; 38:141–147.
- Nickkholgh A, Soltaniyekta S, Kalbasi H. Routine versus selective intraoperative cholangiography during laparoscopic cholecystectomy: a survey of 2,130 patients undergoing laparoscopic cholecystectomy. Surg Endosc 2006; 20:868–874.
- Gandolfi L, Torresan F, Solmi L, Puccetti A. The role of ultrasound in biliary and pancreatic diseases. Eur J Ultrasound 2003; 16:141–159.
- Al Samaraee A, Khan U, Almashta Z, Yiannakou Y. Preoperative diagnosis of choledocholithiasis: the role of MRCP. Br J Hosp Med (Lond) 2009; 70:339–343.
- Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc 2001; 54:425–434.
- Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol 2006; 101:139–147.
- Mehta SN, Pavone E, Barkun JS, Bouchard S, Barkun AN. Predictors of post-ERCP complications in patients with suspected choledocholithiasis. Endoscopy 1998; 30:457–463.
- Badalov N, Tenner S, Baillie J. The prevention, recognition and treatment of post-ERCP pancreatitis. JOP 2009; 10:88–97.
- Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989; 320:82–87.
- Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64:248–254.
- Park DH, Kim MH, Lee SS, et al. Accuracy of magnetic resonance cholangiopancreatography for locating hepatolithiasis and detecting accompanying biliary strictures. Endoscopy 2004; 36:987–992.
- Walsh RM, Ponsky JL, Dumot J. Retained gallbladder/cystic duct remnant calculi as a cause of postcholecystectomy pain. Surg Endosc 2002; 16:981–984.
- Tantia O, Jain M, Khanna S, Sen B. Post cholecystectomy syndrome: role of cystic duct stump and re-intervention by laparoscopic surgery. J Minim Access Surg 2008; 4:71–75.
- Palanivelu C, Rangarajan M, Jategaonkar PA, Madankumar MV, Anand NV. Laparoscopic management of remnant cystic duct calculi: a retrospective study. Ann R Coll Surg Engl 2009; 91:25–29.
- Flörcken H. Gallenblasenregeneration mit Steinrecidiv nach Cholecystectomie. Deutsch Z Chir 1912; 113:604.
- Mirizzi PL. La colangiografía durante las operaciones de las vias biliares. Bol Soc Cirug Buenos Aires 1932; 16:1113.
- Soper NJ, Brunt LM. The case for routine operative cholangiography during laparoscopic cholecystectomy. Surg Clin North Am 1994; 74:953–959.
- Cuschieri A, Shimi S, Banting S, Nathanson LK, Pietrabissa A. Intraoperative cholangiography during laparoscopic cholecystectomy. Routine vs selective policy. Surg Endosc 1994; 8:302–305.
- Woods MS, Traverso LW, Kozarek RA, et al. Biliary tract complications of laparoscopic cholecystectomy are detected more frequently with routine intraoperative cholangiography. Surg Endosc 1995; 9:1076–1080.
- Vezakis A, Davides D, Ammori BJ, Martin IG, Larvin M, McMahon MJ. Intraoperative cholangiography during laparoscopic cholecystectomy. Surg Endosc 2000; 14:1118–1122.
- Ladocsi LT, Benitez LD, Filippone DR, Nance FC. Intraoperative cholangiography in laparoscopic cholecystectomy: a review of 734 consecutive cases. Am Surg 1997; 63:150–156.
- Clair DG, Brooks DC. Laparoscopic cholangiography. The case for a selective approach. Surg Clin North Am 1994; 74:961–966.
- Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg 2004; 239:28–33.
- Ponsky LE, Geisinger MA, Ponsky JL, Streem SB. Contemporary ‘urologic’ intervention in the pancreaticobiliary tree. Urology 2001; 57:21–25.
- Benninger J, Rabenstein T, Farnbacher M, Keppler J, Hahn EG, Schneider HT. Extracorporeal shockwave lithotripsy of gallstones in cystic duct remnants and Mirizzi syndrome. Gastrointest Endosc 2004; 60:454–459.
- Demetriades H, Pramateftakis MG, Kanellos I, Angelopoulos S, Mantzoros I, Betsis D. Retained gallbladder remnant after laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A 2008; 18:276–279.
- Shaw C, O’Hanlon DM, Fenlon HM, McEntee GP. Cystic duct remnant and the ‘post-cholecystectomy syndrome. ’ Hepatogastroenterology 2004; 51:36–38.
- Rozsos I, Magyaródi Z, Orbán P. Cystic duct syndrome and minimally invasive surgery. [Hungarian] Orv Hetil 1997; 138:2397–2401.
- Chowbey PK, Bandyopadhyay SK, Sharma A, Khullar R, Soni V, Baijal M. Laparoscopic reintervention for residual gallstone disease. Surg Laparosc Endosc Percutan Tech 2003; 13:31–35.
- Clemente G, Giuliante F, Cadeddu F, Nuzzo G. Laparoscopic removal of gallbladder remnant and long cystic stump. Endoscopy 2001; 33:814–815.
- Rogy MA, Függer R, Herbst F, Schulz F. Reoperation after cholecystectomy. The role of the cystic duct stump. HPB Surg 1991; 4:129–134.
- Laing FC, Jeffrey RB. Choledocholithiasis and cystic duct obstruction: difficult ultrasonographic diagnosis. Radiology 1983; 146:475–479.
- Hassan H, Vilmann P. Insufficient cholecystectomy diagnosed by endoscopic ultrasonography. Endoscopy 2004; 36:236–238.
- Lehman GA, Sherman S. Sphincter of Oddi dysfunction (postcholecystectomy syndrome). In:Yamada T, editor. Textbook of Gastroenterology. 2nd ed. Philadelphia: Lippincott; 1995:2251–2262.
- Zhou PH, Liu FL, Yao LQ, Qin XY. Endoscopic diagnosis and treatment of post-cholecystectomy syndrome. Hepatobiliary Pancreat Dis Int 2003; 2:117–120.
- Mergener K, Clavien PA, Branch MS, Baillie J. A stone in a grossly dilated cystic duct stump: a rare cause of postcholecystectomy pain. Am J Gastroenterol 1999; 94:229–231.
- Goenka MK, Kochhar R, Nagi B, Bhasin DK, Chowdhury A, Singh K. Endoscopic retrograde cholangiopancreatography in postcholecystectomy syndrome. J Assoc Physicians India 1996; 44:119–122.
- Bodvall B, Overgaard B. Cystic duct remnant after cholecystectomy: incidence studied by cholegraphy in 500 cases, and significance in 103 reoperations. Ann Surg 1966; 163:382–390.
- Bergman JJ, van den Brink GR, Rauws EA, et al. Treatment of bile duct lesions after laparoscopic cholecystectomy. Gut 1996; 38:141–147.
- Nickkholgh A, Soltaniyekta S, Kalbasi H. Routine versus selective intraoperative cholangiography during laparoscopic cholecystectomy: a survey of 2,130 patients undergoing laparoscopic cholecystectomy. Surg Endosc 2006; 20:868–874.
- Gandolfi L, Torresan F, Solmi L, Puccetti A. The role of ultrasound in biliary and pancreatic diseases. Eur J Ultrasound 2003; 16:141–159.
- Al Samaraee A, Khan U, Almashta Z, Yiannakou Y. Preoperative diagnosis of choledocholithiasis: the role of MRCP. Br J Hosp Med (Lond) 2009; 70:339–343.
- Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc 2001; 54:425–434.
- Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol 2006; 101:139–147.
- Mehta SN, Pavone E, Barkun JS, Bouchard S, Barkun AN. Predictors of post-ERCP complications in patients with suspected choledocholithiasis. Endoscopy 1998; 30:457–463.
- Badalov N, Tenner S, Baillie J. The prevention, recognition and treatment of post-ERCP pancreatitis. JOP 2009; 10:88–97.
- Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Venu RP. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter-of-Oddi dysfunction. N Engl J Med 1989; 320:82–87.
- Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc 2006; 64:248–254.
- Park DH, Kim MH, Lee SS, et al. Accuracy of magnetic resonance cholangiopancreatography for locating hepatolithiasis and detecting accompanying biliary strictures. Endoscopy 2004; 36:987–992.
- Walsh RM, Ponsky JL, Dumot J. Retained gallbladder/cystic duct remnant calculi as a cause of postcholecystectomy pain. Surg Endosc 2002; 16:981–984.
- Tantia O, Jain M, Khanna S, Sen B. Post cholecystectomy syndrome: role of cystic duct stump and re-intervention by laparoscopic surgery. J Minim Access Surg 2008; 4:71–75.
- Palanivelu C, Rangarajan M, Jategaonkar PA, Madankumar MV, Anand NV. Laparoscopic management of remnant cystic duct calculi: a retrospective study. Ann R Coll Surg Engl 2009; 91:25–29.
- Flörcken H. Gallenblasenregeneration mit Steinrecidiv nach Cholecystectomie. Deutsch Z Chir 1912; 113:604.
- Mirizzi PL. La colangiografía durante las operaciones de las vias biliares. Bol Soc Cirug Buenos Aires 1932; 16:1113.
- Soper NJ, Brunt LM. The case for routine operative cholangiography during laparoscopic cholecystectomy. Surg Clin North Am 1994; 74:953–959.
- Cuschieri A, Shimi S, Banting S, Nathanson LK, Pietrabissa A. Intraoperative cholangiography during laparoscopic cholecystectomy. Routine vs selective policy. Surg Endosc 1994; 8:302–305.
- Woods MS, Traverso LW, Kozarek RA, et al. Biliary tract complications of laparoscopic cholecystectomy are detected more frequently with routine intraoperative cholangiography. Surg Endosc 1995; 9:1076–1080.
- Vezakis A, Davides D, Ammori BJ, Martin IG, Larvin M, McMahon MJ. Intraoperative cholangiography during laparoscopic cholecystectomy. Surg Endosc 2000; 14:1118–1122.
- Ladocsi LT, Benitez LD, Filippone DR, Nance FC. Intraoperative cholangiography in laparoscopic cholecystectomy: a review of 734 consecutive cases. Am Surg 1997; 63:150–156.
- Clair DG, Brooks DC. Laparoscopic cholangiography. The case for a selective approach. Surg Clin North Am 1994; 74:961–966.
- Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg 2004; 239:28–33.
- Ponsky LE, Geisinger MA, Ponsky JL, Streem SB. Contemporary ‘urologic’ intervention in the pancreaticobiliary tree. Urology 2001; 57:21–25.
- Benninger J, Rabenstein T, Farnbacher M, Keppler J, Hahn EG, Schneider HT. Extracorporeal shockwave lithotripsy of gallstones in cystic duct remnants and Mirizzi syndrome. Gastrointest Endosc 2004; 60:454–459.
- Demetriades H, Pramateftakis MG, Kanellos I, Angelopoulos S, Mantzoros I, Betsis D. Retained gallbladder remnant after laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A 2008; 18:276–279.
- Shaw C, O’Hanlon DM, Fenlon HM, McEntee GP. Cystic duct remnant and the ‘post-cholecystectomy syndrome. ’ Hepatogastroenterology 2004; 51:36–38.
- Rozsos I, Magyaródi Z, Orbán P. Cystic duct syndrome and minimally invasive surgery. [Hungarian] Orv Hetil 1997; 138:2397–2401.
- Chowbey PK, Bandyopadhyay SK, Sharma A, Khullar R, Soni V, Baijal M. Laparoscopic reintervention for residual gallstone disease. Surg Laparosc Endosc Percutan Tech 2003; 13:31–35.
- Clemente G, Giuliante F, Cadeddu F, Nuzzo G. Laparoscopic removal of gallbladder remnant and long cystic stump. Endoscopy 2001; 33:814–815.
- Rogy MA, Függer R, Herbst F, Schulz F. Reoperation after cholecystectomy. The role of the cystic duct stump. HPB Surg 1991; 4:129–134.
- Laing FC, Jeffrey RB. Choledocholithiasis and cystic duct obstruction: difficult ultrasonographic diagnosis. Radiology 1983; 146:475–479.
- Hassan H, Vilmann P. Insufficient cholecystectomy diagnosed by endoscopic ultrasonography. Endoscopy 2004; 36:236–238.