User login
CDC update: Guidelines for treating STDs
In 2010, the CDC released an update of its Sexually Transmitted Diseases (STD) Treatment Guidelines,1 which were last updated in 2006. The guidelines are widely viewed as the most authoritative source of information on the diagnosis, treatment, and follow-up of STDs, and they are the standard for publicly and privately funded clinics focusing on sexual health.
What’s new
Some of the notable changes made since the last update in 2006 appear in TABLE 1.1,2
Uncomplicated gonorrhea. Cephalosporins are the only class of antibiotic recommended as first-line treatment for gonorrhea. (In a 2007 recommendation revision, the CDC opted to no longer recommend quinolone antibiotics for the treatment of gonorrhea, because of widespread bacterial resistance.3) Preference is now given to ceftriaxone because of its proven effectiveness against pharyngeal infection, which is often asymptomatic, difficult to detect, and difficult to eradicate. Additionally, the 2010 update has increased the recommended dose of ceftriazone from 125 to 250 mg intramuscularly. The larger dose is more effective against pharyngeal infection; it is also a safeguard against decreased bacterial susceptibility to cephalosporins, which, although still very low, has been reported in more cases recently.
The guidelines still recommend that azithromycin, 1 g orally in a single dose, be given with ceftriaxone because of the relatively high rate of co-infection with Chlamydia trachomatis and the potential for azithromycin to assist with eradicating any gonorrhea with decreased susceptibility to ceftriaxone.
Pelvic inflammatory disease. Quinolones have also been removed from the list of options for outpatient treatment of pelvic inflammatory disease. All recommended regimens now specify a parenteral cephalosporin as a single injection with doxycycline 100 mg PO twice a day for 14 days, with or without metronidazole 500 mg PO twice a day for 14 days.
Bacterial vaginosis. Tinidazole, 2 g orally once a day for 2 days or 1 g orally once a day for 5 days, is now an alternative agent for bacterial vaginosis. However, preferred treatments remain metronidazole 500 mg orally twice a day for 7 days, metronidazole gel intravaginally once a day for 5 days, or clindamycin cream intravaginally at bedtime for 7 days.
Newborn gonococcal eye infection. A relatively minor change is the elimination of tetracycline as prophylaxis for newborn gonococcal eye infections, leaving only erythromycin ointment to prevent the condition.
TABLE 1
2010 vs 2006: How have the CDC recommendations for STD treatment changed?1,2
Uncomplicated gonococcal infections of the cervix, urethra, rectum, and pharynx
|
| Pelvic inflammatory disease Parenteral regimens
|
Bacterial vaginosis
|
Prophylaxis for gonococcal eye infection in a newborn
|
Single-dose therapy preferred among equivalent options
Single-dose therapy (TABLE 2), while often more expensive than other options, increases compliance and helps ensure treatment completion. Single-dose therapy administered in your office is essentially directly observed treatment, an intervention that has become the standard of care for other diseases such as tuberculosis. If the single-dose agent is as effective as alternative medications, directly observed on-site administration is the preferred option for treating STDs.
TABLE 2
Single-dose therapies for specific STDs1
| Infection or condition | Single-dose therapy |
|---|---|
| Candida | Miconazole 1200 mg vaginal suppository or Tioconazole 6.5% ointment 5 g intravaginally or Butoconazole 2% cream 5 g intravaginally or Fluconazole 150 mg PO |
| Cervicitis | Azithromycin 1 g PO |
| Chancroid | Azithromycin 1 g PO or Ceftriaxone 250 mg IM |
| Chlamydia urogenital infection | Azithromycin 1 g PO |
| Gonorrhea: conjunctivitis | Ceftriaxone 1 g IM |
| Gonorrhea: uncomplicated infection of the cervix, urethra, rectum | Ceftriaxone 250 mg IM (preferred) or Cefixime 400 mg PO or Single-dose injectable cephalosporin plus Azithromycin 1 g PO |
| Gonorrhea: uncomplicated infection of the pharynx | Ceftriaxone 250 mg IM plus Azithromycin 1 g PO |
| Nongonococcal urethritis | Azithromycin 1 g PO |
| Post-sexual assault prophylaxis | Ceftriaxone 250 mg IM or Cefixime 400 mg PO plus Metronidazole 2 g PO plus Azithromycin 1 g PO |
| Recurrent, persistent nongonococcal urethritis | Metronidazole 2 g PO or Tinidazole 2 g PO plus Azithromycin 1 g PO (if not used for initial episode) |
| Syphilis: primary, secondary, and early latent | Benzathine penicillin G 2.4 million units IM |
| Trichomoniasis | Metronidazole 2 g PO or Tinidazole 2 g PO |
Other guideline recommendations
The CDC’s STD treatment guidelines contain a wealth of useful information beyond treatment advice: recommended methods of confirming diagnoses, analyses of the usefulness of various diagnostic tests, recommendations on how to manage sex partners of those infected, guidance on STD prevention counseling, and considerations for special populations and circumstances.
Additionally, there is a section on screening for STDs reflecting recommendations of the US Preventive Services Task Force (USPSTF); it also includes recommendations from the American College of Obstetricians and Gynecologists. In at least one instance, though, the USPSTF recommendation on screening for HIV infection contradicts other CDC sources.4,5 Also included is guidance on using vaccines to prevent hepatitis A, hepatitis B, and human papillomavirus (HPV), which follows the recommendations of the Advisory Committee on Immunization Practices. When to use DNA testing to detect HPV is described briefly.
A shortcoming of the CDC guidelines
Although the CDC’s STD guidelines remain the most authoritative source of information on the diagnosis and treatment of STDs, they do not seem to use a consistent method for assessing and describing the strength of the evidence behind the recommendations, which family physicians have come to expect. (However, it is sometimes possible to discern the type and strength of evidence for a particular recommendation from the written discussion.)
The new guidelines state that a series of papers to be published in Clinical Infectious Diseases will describe more fully the evidence behind some of the recommendations and include evidence tables. However, in future guideline updates, it would be helpful if the CDC were to include a brief description of the quantity and strength of evidence alongside each recommended treatment option in the tables.
How best to keep up to date
Although the new guidelines summarize the current status of recommendations on the diagnosis, treatment, and prevention of STDs and are a useful resource for family physicians, we cannot stay current simply by referring to them alone over the next 4 to 5 years until a new edition is published. As new evidence develops, changes in recommendations will be published in the Morbidity and Mortality Weekly Report.
Case in point: new interim HIV recommendations. Interim recommendations were recently released on pre-exposure prophylaxis for men who have sex with men.6 (For more on these recommendations, check out this month’s audiocast at jfponline.com.) Final recommendations are expected later this year. Recommendations for post-exposure prophylaxis to prevent HIV infection are also expected soon.
1. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
2. Centers for Disease Control and Prevention, Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
3. Campos-Outcalt D. Practice alert: CDC no longer recommends quinolones for treatment of gonorrhea. J Fam Pract. 2007;56:554-558.
4. Branson BM, Handsfield HH, Lampe MA, et al. for the Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
5. Campos-Outcalt D. Time to revise your HIV testing routine. J Fam Pract. 2007;56:283-284.
6. Centers for Disease Control and Prevention (CDC). Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65-68.
In 2010, the CDC released an update of its Sexually Transmitted Diseases (STD) Treatment Guidelines,1 which were last updated in 2006. The guidelines are widely viewed as the most authoritative source of information on the diagnosis, treatment, and follow-up of STDs, and they are the standard for publicly and privately funded clinics focusing on sexual health.
What’s new
Some of the notable changes made since the last update in 2006 appear in TABLE 1.1,2
Uncomplicated gonorrhea. Cephalosporins are the only class of antibiotic recommended as first-line treatment for gonorrhea. (In a 2007 recommendation revision, the CDC opted to no longer recommend quinolone antibiotics for the treatment of gonorrhea, because of widespread bacterial resistance.3) Preference is now given to ceftriaxone because of its proven effectiveness against pharyngeal infection, which is often asymptomatic, difficult to detect, and difficult to eradicate. Additionally, the 2010 update has increased the recommended dose of ceftriazone from 125 to 250 mg intramuscularly. The larger dose is more effective against pharyngeal infection; it is also a safeguard against decreased bacterial susceptibility to cephalosporins, which, although still very low, has been reported in more cases recently.
The guidelines still recommend that azithromycin, 1 g orally in a single dose, be given with ceftriaxone because of the relatively high rate of co-infection with Chlamydia trachomatis and the potential for azithromycin to assist with eradicating any gonorrhea with decreased susceptibility to ceftriaxone.
Pelvic inflammatory disease. Quinolones have also been removed from the list of options for outpatient treatment of pelvic inflammatory disease. All recommended regimens now specify a parenteral cephalosporin as a single injection with doxycycline 100 mg PO twice a day for 14 days, with or without metronidazole 500 mg PO twice a day for 14 days.
Bacterial vaginosis. Tinidazole, 2 g orally once a day for 2 days or 1 g orally once a day for 5 days, is now an alternative agent for bacterial vaginosis. However, preferred treatments remain metronidazole 500 mg orally twice a day for 7 days, metronidazole gel intravaginally once a day for 5 days, or clindamycin cream intravaginally at bedtime for 7 days.
Newborn gonococcal eye infection. A relatively minor change is the elimination of tetracycline as prophylaxis for newborn gonococcal eye infections, leaving only erythromycin ointment to prevent the condition.
TABLE 1
2010 vs 2006: How have the CDC recommendations for STD treatment changed?1,2
Uncomplicated gonococcal infections of the cervix, urethra, rectum, and pharynx
|
| Pelvic inflammatory disease Parenteral regimens
|
Bacterial vaginosis
|
Prophylaxis for gonococcal eye infection in a newborn
|
Single-dose therapy preferred among equivalent options
Single-dose therapy (TABLE 2), while often more expensive than other options, increases compliance and helps ensure treatment completion. Single-dose therapy administered in your office is essentially directly observed treatment, an intervention that has become the standard of care for other diseases such as tuberculosis. If the single-dose agent is as effective as alternative medications, directly observed on-site administration is the preferred option for treating STDs.
TABLE 2
Single-dose therapies for specific STDs1
| Infection or condition | Single-dose therapy |
|---|---|
| Candida | Miconazole 1200 mg vaginal suppository or Tioconazole 6.5% ointment 5 g intravaginally or Butoconazole 2% cream 5 g intravaginally or Fluconazole 150 mg PO |
| Cervicitis | Azithromycin 1 g PO |
| Chancroid | Azithromycin 1 g PO or Ceftriaxone 250 mg IM |
| Chlamydia urogenital infection | Azithromycin 1 g PO |
| Gonorrhea: conjunctivitis | Ceftriaxone 1 g IM |
| Gonorrhea: uncomplicated infection of the cervix, urethra, rectum | Ceftriaxone 250 mg IM (preferred) or Cefixime 400 mg PO or Single-dose injectable cephalosporin plus Azithromycin 1 g PO |
| Gonorrhea: uncomplicated infection of the pharynx | Ceftriaxone 250 mg IM plus Azithromycin 1 g PO |
| Nongonococcal urethritis | Azithromycin 1 g PO |
| Post-sexual assault prophylaxis | Ceftriaxone 250 mg IM or Cefixime 400 mg PO plus Metronidazole 2 g PO plus Azithromycin 1 g PO |
| Recurrent, persistent nongonococcal urethritis | Metronidazole 2 g PO or Tinidazole 2 g PO plus Azithromycin 1 g PO (if not used for initial episode) |
| Syphilis: primary, secondary, and early latent | Benzathine penicillin G 2.4 million units IM |
| Trichomoniasis | Metronidazole 2 g PO or Tinidazole 2 g PO |
Other guideline recommendations
The CDC’s STD treatment guidelines contain a wealth of useful information beyond treatment advice: recommended methods of confirming diagnoses, analyses of the usefulness of various diagnostic tests, recommendations on how to manage sex partners of those infected, guidance on STD prevention counseling, and considerations for special populations and circumstances.
Additionally, there is a section on screening for STDs reflecting recommendations of the US Preventive Services Task Force (USPSTF); it also includes recommendations from the American College of Obstetricians and Gynecologists. In at least one instance, though, the USPSTF recommendation on screening for HIV infection contradicts other CDC sources.4,5 Also included is guidance on using vaccines to prevent hepatitis A, hepatitis B, and human papillomavirus (HPV), which follows the recommendations of the Advisory Committee on Immunization Practices. When to use DNA testing to detect HPV is described briefly.
A shortcoming of the CDC guidelines
Although the CDC’s STD guidelines remain the most authoritative source of information on the diagnosis and treatment of STDs, they do not seem to use a consistent method for assessing and describing the strength of the evidence behind the recommendations, which family physicians have come to expect. (However, it is sometimes possible to discern the type and strength of evidence for a particular recommendation from the written discussion.)
The new guidelines state that a series of papers to be published in Clinical Infectious Diseases will describe more fully the evidence behind some of the recommendations and include evidence tables. However, in future guideline updates, it would be helpful if the CDC were to include a brief description of the quantity and strength of evidence alongside each recommended treatment option in the tables.
How best to keep up to date
Although the new guidelines summarize the current status of recommendations on the diagnosis, treatment, and prevention of STDs and are a useful resource for family physicians, we cannot stay current simply by referring to them alone over the next 4 to 5 years until a new edition is published. As new evidence develops, changes in recommendations will be published in the Morbidity and Mortality Weekly Report.
Case in point: new interim HIV recommendations. Interim recommendations were recently released on pre-exposure prophylaxis for men who have sex with men.6 (For more on these recommendations, check out this month’s audiocast at jfponline.com.) Final recommendations are expected later this year. Recommendations for post-exposure prophylaxis to prevent HIV infection are also expected soon.
In 2010, the CDC released an update of its Sexually Transmitted Diseases (STD) Treatment Guidelines,1 which were last updated in 2006. The guidelines are widely viewed as the most authoritative source of information on the diagnosis, treatment, and follow-up of STDs, and they are the standard for publicly and privately funded clinics focusing on sexual health.
What’s new
Some of the notable changes made since the last update in 2006 appear in TABLE 1.1,2
Uncomplicated gonorrhea. Cephalosporins are the only class of antibiotic recommended as first-line treatment for gonorrhea. (In a 2007 recommendation revision, the CDC opted to no longer recommend quinolone antibiotics for the treatment of gonorrhea, because of widespread bacterial resistance.3) Preference is now given to ceftriaxone because of its proven effectiveness against pharyngeal infection, which is often asymptomatic, difficult to detect, and difficult to eradicate. Additionally, the 2010 update has increased the recommended dose of ceftriazone from 125 to 250 mg intramuscularly. The larger dose is more effective against pharyngeal infection; it is also a safeguard against decreased bacterial susceptibility to cephalosporins, which, although still very low, has been reported in more cases recently.
The guidelines still recommend that azithromycin, 1 g orally in a single dose, be given with ceftriaxone because of the relatively high rate of co-infection with Chlamydia trachomatis and the potential for azithromycin to assist with eradicating any gonorrhea with decreased susceptibility to ceftriaxone.
Pelvic inflammatory disease. Quinolones have also been removed from the list of options for outpatient treatment of pelvic inflammatory disease. All recommended regimens now specify a parenteral cephalosporin as a single injection with doxycycline 100 mg PO twice a day for 14 days, with or without metronidazole 500 mg PO twice a day for 14 days.
Bacterial vaginosis. Tinidazole, 2 g orally once a day for 2 days or 1 g orally once a day for 5 days, is now an alternative agent for bacterial vaginosis. However, preferred treatments remain metronidazole 500 mg orally twice a day for 7 days, metronidazole gel intravaginally once a day for 5 days, or clindamycin cream intravaginally at bedtime for 7 days.
Newborn gonococcal eye infection. A relatively minor change is the elimination of tetracycline as prophylaxis for newborn gonococcal eye infections, leaving only erythromycin ointment to prevent the condition.
TABLE 1
2010 vs 2006: How have the CDC recommendations for STD treatment changed?1,2
Uncomplicated gonococcal infections of the cervix, urethra, rectum, and pharynx
|
| Pelvic inflammatory disease Parenteral regimens
|
Bacterial vaginosis
|
Prophylaxis for gonococcal eye infection in a newborn
|
Single-dose therapy preferred among equivalent options
Single-dose therapy (TABLE 2), while often more expensive than other options, increases compliance and helps ensure treatment completion. Single-dose therapy administered in your office is essentially directly observed treatment, an intervention that has become the standard of care for other diseases such as tuberculosis. If the single-dose agent is as effective as alternative medications, directly observed on-site administration is the preferred option for treating STDs.
TABLE 2
Single-dose therapies for specific STDs1
| Infection or condition | Single-dose therapy |
|---|---|
| Candida | Miconazole 1200 mg vaginal suppository or Tioconazole 6.5% ointment 5 g intravaginally or Butoconazole 2% cream 5 g intravaginally or Fluconazole 150 mg PO |
| Cervicitis | Azithromycin 1 g PO |
| Chancroid | Azithromycin 1 g PO or Ceftriaxone 250 mg IM |
| Chlamydia urogenital infection | Azithromycin 1 g PO |
| Gonorrhea: conjunctivitis | Ceftriaxone 1 g IM |
| Gonorrhea: uncomplicated infection of the cervix, urethra, rectum | Ceftriaxone 250 mg IM (preferred) or Cefixime 400 mg PO or Single-dose injectable cephalosporin plus Azithromycin 1 g PO |
| Gonorrhea: uncomplicated infection of the pharynx | Ceftriaxone 250 mg IM plus Azithromycin 1 g PO |
| Nongonococcal urethritis | Azithromycin 1 g PO |
| Post-sexual assault prophylaxis | Ceftriaxone 250 mg IM or Cefixime 400 mg PO plus Metronidazole 2 g PO plus Azithromycin 1 g PO |
| Recurrent, persistent nongonococcal urethritis | Metronidazole 2 g PO or Tinidazole 2 g PO plus Azithromycin 1 g PO (if not used for initial episode) |
| Syphilis: primary, secondary, and early latent | Benzathine penicillin G 2.4 million units IM |
| Trichomoniasis | Metronidazole 2 g PO or Tinidazole 2 g PO |
Other guideline recommendations
The CDC’s STD treatment guidelines contain a wealth of useful information beyond treatment advice: recommended methods of confirming diagnoses, analyses of the usefulness of various diagnostic tests, recommendations on how to manage sex partners of those infected, guidance on STD prevention counseling, and considerations for special populations and circumstances.
Additionally, there is a section on screening for STDs reflecting recommendations of the US Preventive Services Task Force (USPSTF); it also includes recommendations from the American College of Obstetricians and Gynecologists. In at least one instance, though, the USPSTF recommendation on screening for HIV infection contradicts other CDC sources.4,5 Also included is guidance on using vaccines to prevent hepatitis A, hepatitis B, and human papillomavirus (HPV), which follows the recommendations of the Advisory Committee on Immunization Practices. When to use DNA testing to detect HPV is described briefly.
A shortcoming of the CDC guidelines
Although the CDC’s STD guidelines remain the most authoritative source of information on the diagnosis and treatment of STDs, they do not seem to use a consistent method for assessing and describing the strength of the evidence behind the recommendations, which family physicians have come to expect. (However, it is sometimes possible to discern the type and strength of evidence for a particular recommendation from the written discussion.)
The new guidelines state that a series of papers to be published in Clinical Infectious Diseases will describe more fully the evidence behind some of the recommendations and include evidence tables. However, in future guideline updates, it would be helpful if the CDC were to include a brief description of the quantity and strength of evidence alongside each recommended treatment option in the tables.
How best to keep up to date
Although the new guidelines summarize the current status of recommendations on the diagnosis, treatment, and prevention of STDs and are a useful resource for family physicians, we cannot stay current simply by referring to them alone over the next 4 to 5 years until a new edition is published. As new evidence develops, changes in recommendations will be published in the Morbidity and Mortality Weekly Report.
Case in point: new interim HIV recommendations. Interim recommendations were recently released on pre-exposure prophylaxis for men who have sex with men.6 (For more on these recommendations, check out this month’s audiocast at jfponline.com.) Final recommendations are expected later this year. Recommendations for post-exposure prophylaxis to prevent HIV infection are also expected soon.
1. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
2. Centers for Disease Control and Prevention, Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
3. Campos-Outcalt D. Practice alert: CDC no longer recommends quinolones for treatment of gonorrhea. J Fam Pract. 2007;56:554-558.
4. Branson BM, Handsfield HH, Lampe MA, et al. for the Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
5. Campos-Outcalt D. Time to revise your HIV testing routine. J Fam Pract. 2007;56:283-284.
6. Centers for Disease Control and Prevention (CDC). Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65-68.
1. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
2. Centers for Disease Control and Prevention, Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
3. Campos-Outcalt D. Practice alert: CDC no longer recommends quinolones for treatment of gonorrhea. J Fam Pract. 2007;56:554-558.
4. Branson BM, Handsfield HH, Lampe MA, et al. for the Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
5. Campos-Outcalt D. Time to revise your HIV testing routine. J Fam Pract. 2007;56:283-284.
6. Centers for Disease Control and Prevention (CDC). Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65-68.
Urine drug testing: An unproven risk management tool?
As a member of the editorial board of the Journal of Pain & Palliative Care Pharmacotherapy, an author of numerous scholarly articles about chronic pain (some of which are cited here), and a person who lives with chronic pain, I would like to comment on “Is it time to drug test your chronic pain patient?” (J Fam Pract. 2010;59:628-633). Drs. McBane and Weigle recommend the use of pain agreements and drug testing for every patient with noncancer chronic pain, but fail to mention that there is insufficient evidence of the efficacy of both adherence monitoring tools.1 In addition, a recent article in The American Journal of Bioethics recommends against the “universal application of pain agreements” and suggests that they can harm the patient/physician relationship.2 Consent for drug testing often comes from the pain contract3—agreements that have been called “unconscionable adhesion contracts” and may be unenforceable.4
The authors also suggest that urine drug testing is noninvasive. Nothing could be further from the truth. Drug testing of people with pain may be considered a suspicionless and warrantless search of bodily fluids and in certain cases may be unconstitutional.5 There is no question that drug misuse, abuse, addiction, and overdose are devastating to individuals, families, and society. However, using unproven risk management tools that may cause greater harm than good is just bad medicine.
Mark Collen
Sacramento, Calif
1. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152:712-720.
2. Payne R, Anderson E, Arnold R, et al. A rose by any other name: pain contracts/agreements. Am J Bioeth. 2010;10:5-12.
3. Collen M. Analysis of controlled substance agreements from private practice physicians. J Pain Palliat Care Pharmacother. 2009;23:357-364.
4. Collen M. Opioid contracts and random drug testing for people with chronic pain—think twice. J Law Med Ethics. 2009;37:841-845.
5. Collen M. The Fourth Amendment and random drug testing of people with chronic pain. J Pain Palliat Care Pharmacother. 2011;25:in press.
As a member of the editorial board of the Journal of Pain & Palliative Care Pharmacotherapy, an author of numerous scholarly articles about chronic pain (some of which are cited here), and a person who lives with chronic pain, I would like to comment on “Is it time to drug test your chronic pain patient?” (J Fam Pract. 2010;59:628-633). Drs. McBane and Weigle recommend the use of pain agreements and drug testing for every patient with noncancer chronic pain, but fail to mention that there is insufficient evidence of the efficacy of both adherence monitoring tools.1 In addition, a recent article in The American Journal of Bioethics recommends against the “universal application of pain agreements” and suggests that they can harm the patient/physician relationship.2 Consent for drug testing often comes from the pain contract3—agreements that have been called “unconscionable adhesion contracts” and may be unenforceable.4
The authors also suggest that urine drug testing is noninvasive. Nothing could be further from the truth. Drug testing of people with pain may be considered a suspicionless and warrantless search of bodily fluids and in certain cases may be unconstitutional.5 There is no question that drug misuse, abuse, addiction, and overdose are devastating to individuals, families, and society. However, using unproven risk management tools that may cause greater harm than good is just bad medicine.
Mark Collen
Sacramento, Calif
As a member of the editorial board of the Journal of Pain & Palliative Care Pharmacotherapy, an author of numerous scholarly articles about chronic pain (some of which are cited here), and a person who lives with chronic pain, I would like to comment on “Is it time to drug test your chronic pain patient?” (J Fam Pract. 2010;59:628-633). Drs. McBane and Weigle recommend the use of pain agreements and drug testing for every patient with noncancer chronic pain, but fail to mention that there is insufficient evidence of the efficacy of both adherence monitoring tools.1 In addition, a recent article in The American Journal of Bioethics recommends against the “universal application of pain agreements” and suggests that they can harm the patient/physician relationship.2 Consent for drug testing often comes from the pain contract3—agreements that have been called “unconscionable adhesion contracts” and may be unenforceable.4
The authors also suggest that urine drug testing is noninvasive. Nothing could be further from the truth. Drug testing of people with pain may be considered a suspicionless and warrantless search of bodily fluids and in certain cases may be unconstitutional.5 There is no question that drug misuse, abuse, addiction, and overdose are devastating to individuals, families, and society. However, using unproven risk management tools that may cause greater harm than good is just bad medicine.
Mark Collen
Sacramento, Calif
1. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152:712-720.
2. Payne R, Anderson E, Arnold R, et al. A rose by any other name: pain contracts/agreements. Am J Bioeth. 2010;10:5-12.
3. Collen M. Analysis of controlled substance agreements from private practice physicians. J Pain Palliat Care Pharmacother. 2009;23:357-364.
4. Collen M. Opioid contracts and random drug testing for people with chronic pain—think twice. J Law Med Ethics. 2009;37:841-845.
5. Collen M. The Fourth Amendment and random drug testing of people with chronic pain. J Pain Palliat Care Pharmacother. 2011;25:in press.
1. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152:712-720.
2. Payne R, Anderson E, Arnold R, et al. A rose by any other name: pain contracts/agreements. Am J Bioeth. 2010;10:5-12.
3. Collen M. Analysis of controlled substance agreements from private practice physicians. J Pain Palliat Care Pharmacother. 2009;23:357-364.
4. Collen M. Opioid contracts and random drug testing for people with chronic pain—think twice. J Law Med Ethics. 2009;37:841-845.
5. Collen M. The Fourth Amendment and random drug testing of people with chronic pain. J Pain Palliat Care Pharmacother. 2011;25:in press.
Diffuse abdominal pain, vomiting
• Use the APACHE-II scoring system early on to help predict the severity of pancreatitis.
• Consider early enteral nutrition in patients with severe disease; taking this step has been linked to lower infection rates and shorter lengths of stay.
• Consider patient factors and the risk of severe infection when deciding whether or not to use prophylactic antibiotics in cases of severe necrotizing pancreatitis.
CASE A 57-year-old Caucasian woman sought care at our emergency department (ED) for diffuse abdominal pain and nausea. She said that the pain began after eating lunch earlier that day, and localized periumbilically, with radiation to the back. She had several episodes of nonbilious, nonbloody vomiting, but denied fever, chills, or diarrhea.
Her past medical history was notable only for an episode of gallstone pancreatitis 11 years earlier, after which she underwent a cholecystectomy. Her only medications were ibandronate sodium (Boniva) taken for osteoporosis (diagnosed 2 years earlier), a multivitamin, calcium, magnesium, and vitamin E supplements. Her family history was notable for a brother who had pancreatic cancer in his 50s. The patient reported infrequent alcohol use.
The abdominal exam was notable for diffuse tenderness to palpation, most prominent in the epigastric region. The patient exhibited voluntary guarding, without rebound, and positive bowel sounds throughout.
The patient’s laboratory studies on admission included leukocytosis of 21,300 cells/mcL and hemoglobin and hematocrit of 17.3 g/dL and 52.1%, respectively. She had an amylase of 1733 U/L and lipase of 4288 U/L. Lactate and lactic dehydrogenase were 1.83 mg/dL and 265 U/L, respectively. Liver function tests and a basic metabolic panel were within normal limits. A noncontrast computed tomography (CT) scan of the abdomen and pelvis was notable for an enlarged pancreas with peripancreatic edema and free fluid in the abdomen.
The patient underwent aggressive fluid resuscitation throughout the first 6 hours of her hospital stay. Urine output was noted to be incongruent with fluid intake, at just over 60 cc/h. Over the next 4 hours, she became progressively tachycardic, tachypneic, and somnolent, with increasing abdominal tenderness. Her serum potassium level rose to 4.9 mEq/L, while serum bicarbonate declined to 13 mEq/L and serum calcium, to 6.2 mg/dL. Arterial blood gas revealed metabolic acidosis with a pH of 7.22.
Our patient was subsequently transferred to the medical intensive care unit, where she required endotracheal intubation.
WHAT IS THE MOST LIKELY EXPLANATION FOR HER CONDITION?
Acute necrotizing pancreatitis
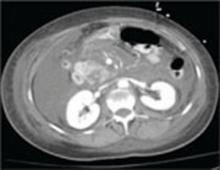
A repeat CT scan of the abdomen and pelvis with IV contrast taken on the second day of admission revealed extensive pancreatitis with complete disintegration of the pancreatic tissue and absence of pancreatic enhancement (FIGURE), as well as a large amount of abdominal ascites.
Pancreatitis is a common inpatient diagnosis, with approximately 200,000 hospitalizations yearly.1 Most cases are mild and self-limiting, requiring minimal intervention including parenteral fluid resuscitation, pain control, and restriction of oral intake. Most cases can be attributed to gallstones or excessive alcohol use, but approximately 25% of cases are idiopathic.1 Other causes include hypertriglyceridemia, infection, hypercalcemia, and medications such as azathioprine, 6-mercaptopurine, trimethoprim sulfa-methoxazole, and furosemide. Severe necrotizing pancreatitis represents about 20% of all cases, but carries a mortality rate of between 10% and 30%.1
Diagnosis is based on clinical features in conjunction with biochemical markers. Amylase is nonspecific, but levels 3 times the upper limit of normal are usually diagnostic of acute pancreatitis. Lipase is 85% to 100% sensitive for pancreatitis, and is more specific than amylase. Alanine aminotransferase >150 IU/L is 96% specific for gallstone pancreatitis.2 Of note: there is no evidence to support daily monitoring of these enzyme levels as predictors of clinical improvement or disease severity.
FIGURE
CT scan of abdomen taken on second day of admission
Predicting severity at time of presentation can be difficult
As was true with our patient, predicting the severity of acute pancreatitis at the time of presentation can be difficult. Scoring systems that are commonly used to evaluate disease severity include Ranson’s score, APACHE-II (Acute Physiology and Chronic Health Evaluation-II), and CT severity index, among others (TABLE). Of these, the APACHE-II score has been found to be most predictive of progression to severe disease, with accuracy of up to 75%.3
Recent studies have shown that a body mass index >30 kg/m2 is an independent risk factor for progression to severe pancreatitis.4 Other clinical predictors include poor urine output, rising hematocrit, agitation or confusion, and lack of improvement in symptoms within 48 hours.1
Though our patient came in with symptoms that were initially mild, she quickly manifested several clinical predictors for severe pancreatitis, including poor urine output and increasing confusion, as well as an APACHE-II score of 12 at 6 hours after presentation (values ≥8 indicate high risk for progression to severe disease).
TABLE
Predictors for progression to severe pancreatitis1
| Ranson score ≥3 |
| APACHE-II score ≥8 |
| CT severity index (CT grade + necrosis score) >6 |
| Body mass index >30 kg/m2 |
| Hematocrit >44% (clearly increases risk for pancreatic necrosis) |
Clinical findings:
|
| Lack of improvement in symptoms within the first 48 hours |
| APACHE, Acute Physiology and Chronic Health Evaluation; CT, computed tomography. |
Role of antibiotics? A source of debate
Infection represents the leading cause of morbidity and mortality in patients with pancreatic necrosis. Approximately 40% of patients with necrosis develop infection, with a 20% mortality rate.5 Signs of infection usually develop relatively late in the clinical course and rates increase drastically each week a patient remains hospitalized (71% of patients have signs of infection at 3 weeks).5
Interestingly, the role for antibiotics in such patients has been a source of debate in practice, as well as in the medical literature. Two recent large meta-analyses came to different conclusions regarding the use of antibiotics. A 2006 study by Heinrich et al concluded that patients with pancreatic necrosis demonstrated by contrast-enhanced CT scans should receive antibiotic prophylaxis with imipenem or meropenem for 14 days, and that prophylactic antibiotics do not increase rates of subsequent fungal infection.6 Conversely, as noted in a 2008 study published in the American Journal of Gastroenterology, “prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in patients with acute necrotizing pancreatitis.”7
Two leading professional groups have similarly contradictory recommendations on the topic, with the American Gastroenterological Association (AGA) supporting antibiotic use for patients with >30% pancreatic necrosis noted on CT and the American College of Gastroenterology (ACG) recommending against the use of prophylactic antibiotics.8
As with any clinical dilemma, it seems prudent to make the decision for or against prophylactic antibiotics based on available clinical information and the particular patient’s risk factors. Clearly, in the most high-risk patients, it would be difficult to justify withholding antibiotic therapy.
Complete bowel rest—or not?
In the past, it was thought necessary to allow for complete bowel rest and suppression of pancreatic exocrine secretion during acute pancreatitis by providing total parenteral nutrition.6,9 More recently, though, the use of early nasojejunal enteral feeding (which was initiated for our patient) has been advocated by several large meta-analyses,6 as well as by the AGA and ACG.2
The use of enteral feeding has been associated with improved outcomes, including lower infection rates (due to maintenance of the intestinal barrier and prevention of bacterial translocation), decreased length of stay, reduced rates of organ failure, and fewer deaths among patients who require surgical intervention.6
A lengthy road to recovery for our patient
After 7 days of mechanical ventilation, our patient was extubated. However, she developed significant bilateral pleural effusions as a result of fluid third spacing, and required thoracentesis.
She completed a 14-day course of imipenem, followed by an additional 10-day course due to hypotension and a suspected infected pseudocyst. Subsequent imaging studies confirmed our suspicions: She had developed a large pseudocyst (>13 cm), which remained under observation by both a gastroenterologist and general surgeon. Six weeks after admission, our patient was discharged to home with family.
But what was the cause? Although we were unable to clearly delineate an inciting cause for her pancreatitis during the admission, she was to undergo further investigation as an outpatient. There were also plans to drain the pseudocyst 6 weeks after discharge.
A learning opportunity. This patient’s case provided an excellent opportunity for our team to review the important clinical predictors for progression to severe pancreatitis, and the rapid nature of clinical decline in such patients. In hindsight, the predictors of severity in our patient were few, but included the rapid onset and clinical progression of her symptoms, as well as her elevated hematocrit on presentation and poor urine output over the first 6 hours of admission.
1. Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142-2150.
2. Vege SS, Whitcomb DC, Ginsburg CH. Clinical manifestations and diagnosis of acute pancreatitis. In: Basow DS. ed. UpTo-Date [online database]. Version 18.2. Waltham, Mass: UpTo-Date; 2010.
3. Vege SS, Whitcomb DC, Ginsburg CH. Predicting severity of acute pancreatitis. In: Basow DS, ed. UpToDate [online database]. Version 18.2. Waltham, Mass: UpToDate; 2010.
4. Skipworth JRA, Pereira SP. Acute pancreatitis. Curr Opin Crit Care. 2008;14:172-178.
5. Windsor JA, Schweder P. Complications of acute pancreatitis (including pseudocysts). In: Zinner MJ, Ashley SW, eds. Main-got’s Abdominal Operations. 11th ed. New York: McGraw-Hill; 2007:chap 37. Available at: http://www.accesssurgery.com/content.aspx?filename="6003JFP_HospitalRounds" aid=130125. Accessed November 30, 2010.
6. Heinrich S, Shafer M, Rousson V, et al. Evidenced-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg. 2006;243:154-168.
7. Bai Y, Gao J, Zou DW, et al. Prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in acute necrotizing pancreatitis: evidence from a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2008;103:104-110.
8. Vege SS, Whitcomb DC, Ginsburg CH. Treatment of acute pancreatitis. In: Basow DS, ed. UpToDate [online database]. Version 18.2. Waltham, Mass: UpToDate; 2010.
9. Haney JC, Pappas TN. Necrotizing pancreatitis: diagnosis and management. Surg Clin North Am. 2007;87:1431-1446.
• Use the APACHE-II scoring system early on to help predict the severity of pancreatitis.
• Consider early enteral nutrition in patients with severe disease; taking this step has been linked to lower infection rates and shorter lengths of stay.
• Consider patient factors and the risk of severe infection when deciding whether or not to use prophylactic antibiotics in cases of severe necrotizing pancreatitis.
CASE A 57-year-old Caucasian woman sought care at our emergency department (ED) for diffuse abdominal pain and nausea. She said that the pain began after eating lunch earlier that day, and localized periumbilically, with radiation to the back. She had several episodes of nonbilious, nonbloody vomiting, but denied fever, chills, or diarrhea.
Her past medical history was notable only for an episode of gallstone pancreatitis 11 years earlier, after which she underwent a cholecystectomy. Her only medications were ibandronate sodium (Boniva) taken for osteoporosis (diagnosed 2 years earlier), a multivitamin, calcium, magnesium, and vitamin E supplements. Her family history was notable for a brother who had pancreatic cancer in his 50s. The patient reported infrequent alcohol use.
The abdominal exam was notable for diffuse tenderness to palpation, most prominent in the epigastric region. The patient exhibited voluntary guarding, without rebound, and positive bowel sounds throughout.
The patient’s laboratory studies on admission included leukocytosis of 21,300 cells/mcL and hemoglobin and hematocrit of 17.3 g/dL and 52.1%, respectively. She had an amylase of 1733 U/L and lipase of 4288 U/L. Lactate and lactic dehydrogenase were 1.83 mg/dL and 265 U/L, respectively. Liver function tests and a basic metabolic panel were within normal limits. A noncontrast computed tomography (CT) scan of the abdomen and pelvis was notable for an enlarged pancreas with peripancreatic edema and free fluid in the abdomen.
The patient underwent aggressive fluid resuscitation throughout the first 6 hours of her hospital stay. Urine output was noted to be incongruent with fluid intake, at just over 60 cc/h. Over the next 4 hours, she became progressively tachycardic, tachypneic, and somnolent, with increasing abdominal tenderness. Her serum potassium level rose to 4.9 mEq/L, while serum bicarbonate declined to 13 mEq/L and serum calcium, to 6.2 mg/dL. Arterial blood gas revealed metabolic acidosis with a pH of 7.22.
Our patient was subsequently transferred to the medical intensive care unit, where she required endotracheal intubation.
WHAT IS THE MOST LIKELY EXPLANATION FOR HER CONDITION?
Acute necrotizing pancreatitis
A repeat CT scan of the abdomen and pelvis with IV contrast taken on the second day of admission revealed extensive pancreatitis with complete disintegration of the pancreatic tissue and absence of pancreatic enhancement (FIGURE), as well as a large amount of abdominal ascites.
Pancreatitis is a common inpatient diagnosis, with approximately 200,000 hospitalizations yearly.1 Most cases are mild and self-limiting, requiring minimal intervention including parenteral fluid resuscitation, pain control, and restriction of oral intake. Most cases can be attributed to gallstones or excessive alcohol use, but approximately 25% of cases are idiopathic.1 Other causes include hypertriglyceridemia, infection, hypercalcemia, and medications such as azathioprine, 6-mercaptopurine, trimethoprim sulfa-methoxazole, and furosemide. Severe necrotizing pancreatitis represents about 20% of all cases, but carries a mortality rate of between 10% and 30%.1
Diagnosis is based on clinical features in conjunction with biochemical markers. Amylase is nonspecific, but levels 3 times the upper limit of normal are usually diagnostic of acute pancreatitis. Lipase is 85% to 100% sensitive for pancreatitis, and is more specific than amylase. Alanine aminotransferase >150 IU/L is 96% specific for gallstone pancreatitis.2 Of note: there is no evidence to support daily monitoring of these enzyme levels as predictors of clinical improvement or disease severity.
FIGURE
CT scan of abdomen taken on second day of admission
Predicting severity at time of presentation can be difficult
As was true with our patient, predicting the severity of acute pancreatitis at the time of presentation can be difficult. Scoring systems that are commonly used to evaluate disease severity include Ranson’s score, APACHE-II (Acute Physiology and Chronic Health Evaluation-II), and CT severity index, among others (TABLE). Of these, the APACHE-II score has been found to be most predictive of progression to severe disease, with accuracy of up to 75%.3
Recent studies have shown that a body mass index >30 kg/m2 is an independent risk factor for progression to severe pancreatitis.4 Other clinical predictors include poor urine output, rising hematocrit, agitation or confusion, and lack of improvement in symptoms within 48 hours.1
Though our patient came in with symptoms that were initially mild, she quickly manifested several clinical predictors for severe pancreatitis, including poor urine output and increasing confusion, as well as an APACHE-II score of 12 at 6 hours after presentation (values ≥8 indicate high risk for progression to severe disease).
TABLE
Predictors for progression to severe pancreatitis1
| Ranson score ≥3 |
| APACHE-II score ≥8 |
| CT severity index (CT grade + necrosis score) >6 |
| Body mass index >30 kg/m2 |
| Hematocrit >44% (clearly increases risk for pancreatic necrosis) |
Clinical findings:
|
| Lack of improvement in symptoms within the first 48 hours |
| APACHE, Acute Physiology and Chronic Health Evaluation; CT, computed tomography. |
Role of antibiotics? A source of debate
Infection represents the leading cause of morbidity and mortality in patients with pancreatic necrosis. Approximately 40% of patients with necrosis develop infection, with a 20% mortality rate.5 Signs of infection usually develop relatively late in the clinical course and rates increase drastically each week a patient remains hospitalized (71% of patients have signs of infection at 3 weeks).5
Interestingly, the role for antibiotics in such patients has been a source of debate in practice, as well as in the medical literature. Two recent large meta-analyses came to different conclusions regarding the use of antibiotics. A 2006 study by Heinrich et al concluded that patients with pancreatic necrosis demonstrated by contrast-enhanced CT scans should receive antibiotic prophylaxis with imipenem or meropenem for 14 days, and that prophylactic antibiotics do not increase rates of subsequent fungal infection.6 Conversely, as noted in a 2008 study published in the American Journal of Gastroenterology, “prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in patients with acute necrotizing pancreatitis.”7
Two leading professional groups have similarly contradictory recommendations on the topic, with the American Gastroenterological Association (AGA) supporting antibiotic use for patients with >30% pancreatic necrosis noted on CT and the American College of Gastroenterology (ACG) recommending against the use of prophylactic antibiotics.8
As with any clinical dilemma, it seems prudent to make the decision for or against prophylactic antibiotics based on available clinical information and the particular patient’s risk factors. Clearly, in the most high-risk patients, it would be difficult to justify withholding antibiotic therapy.
Complete bowel rest—or not?
In the past, it was thought necessary to allow for complete bowel rest and suppression of pancreatic exocrine secretion during acute pancreatitis by providing total parenteral nutrition.6,9 More recently, though, the use of early nasojejunal enteral feeding (which was initiated for our patient) has been advocated by several large meta-analyses,6 as well as by the AGA and ACG.2
The use of enteral feeding has been associated with improved outcomes, including lower infection rates (due to maintenance of the intestinal barrier and prevention of bacterial translocation), decreased length of stay, reduced rates of organ failure, and fewer deaths among patients who require surgical intervention.6
A lengthy road to recovery for our patient
After 7 days of mechanical ventilation, our patient was extubated. However, she developed significant bilateral pleural effusions as a result of fluid third spacing, and required thoracentesis.
She completed a 14-day course of imipenem, followed by an additional 10-day course due to hypotension and a suspected infected pseudocyst. Subsequent imaging studies confirmed our suspicions: She had developed a large pseudocyst (>13 cm), which remained under observation by both a gastroenterologist and general surgeon. Six weeks after admission, our patient was discharged to home with family.
But what was the cause? Although we were unable to clearly delineate an inciting cause for her pancreatitis during the admission, she was to undergo further investigation as an outpatient. There were also plans to drain the pseudocyst 6 weeks after discharge.
A learning opportunity. This patient’s case provided an excellent opportunity for our team to review the important clinical predictors for progression to severe pancreatitis, and the rapid nature of clinical decline in such patients. In hindsight, the predictors of severity in our patient were few, but included the rapid onset and clinical progression of her symptoms, as well as her elevated hematocrit on presentation and poor urine output over the first 6 hours of admission.
• Use the APACHE-II scoring system early on to help predict the severity of pancreatitis.
• Consider early enteral nutrition in patients with severe disease; taking this step has been linked to lower infection rates and shorter lengths of stay.
• Consider patient factors and the risk of severe infection when deciding whether or not to use prophylactic antibiotics in cases of severe necrotizing pancreatitis.
CASE A 57-year-old Caucasian woman sought care at our emergency department (ED) for diffuse abdominal pain and nausea. She said that the pain began after eating lunch earlier that day, and localized periumbilically, with radiation to the back. She had several episodes of nonbilious, nonbloody vomiting, but denied fever, chills, or diarrhea.
Her past medical history was notable only for an episode of gallstone pancreatitis 11 years earlier, after which she underwent a cholecystectomy. Her only medications were ibandronate sodium (Boniva) taken for osteoporosis (diagnosed 2 years earlier), a multivitamin, calcium, magnesium, and vitamin E supplements. Her family history was notable for a brother who had pancreatic cancer in his 50s. The patient reported infrequent alcohol use.
The abdominal exam was notable for diffuse tenderness to palpation, most prominent in the epigastric region. The patient exhibited voluntary guarding, without rebound, and positive bowel sounds throughout.
The patient’s laboratory studies on admission included leukocytosis of 21,300 cells/mcL and hemoglobin and hematocrit of 17.3 g/dL and 52.1%, respectively. She had an amylase of 1733 U/L and lipase of 4288 U/L. Lactate and lactic dehydrogenase were 1.83 mg/dL and 265 U/L, respectively. Liver function tests and a basic metabolic panel were within normal limits. A noncontrast computed tomography (CT) scan of the abdomen and pelvis was notable for an enlarged pancreas with peripancreatic edema and free fluid in the abdomen.
The patient underwent aggressive fluid resuscitation throughout the first 6 hours of her hospital stay. Urine output was noted to be incongruent with fluid intake, at just over 60 cc/h. Over the next 4 hours, she became progressively tachycardic, tachypneic, and somnolent, with increasing abdominal tenderness. Her serum potassium level rose to 4.9 mEq/L, while serum bicarbonate declined to 13 mEq/L and serum calcium, to 6.2 mg/dL. Arterial blood gas revealed metabolic acidosis with a pH of 7.22.
Our patient was subsequently transferred to the medical intensive care unit, where she required endotracheal intubation.
WHAT IS THE MOST LIKELY EXPLANATION FOR HER CONDITION?
Acute necrotizing pancreatitis
A repeat CT scan of the abdomen and pelvis with IV contrast taken on the second day of admission revealed extensive pancreatitis with complete disintegration of the pancreatic tissue and absence of pancreatic enhancement (FIGURE), as well as a large amount of abdominal ascites.
Pancreatitis is a common inpatient diagnosis, with approximately 200,000 hospitalizations yearly.1 Most cases are mild and self-limiting, requiring minimal intervention including parenteral fluid resuscitation, pain control, and restriction of oral intake. Most cases can be attributed to gallstones or excessive alcohol use, but approximately 25% of cases are idiopathic.1 Other causes include hypertriglyceridemia, infection, hypercalcemia, and medications such as azathioprine, 6-mercaptopurine, trimethoprim sulfa-methoxazole, and furosemide. Severe necrotizing pancreatitis represents about 20% of all cases, but carries a mortality rate of between 10% and 30%.1
Diagnosis is based on clinical features in conjunction with biochemical markers. Amylase is nonspecific, but levels 3 times the upper limit of normal are usually diagnostic of acute pancreatitis. Lipase is 85% to 100% sensitive for pancreatitis, and is more specific than amylase. Alanine aminotransferase >150 IU/L is 96% specific for gallstone pancreatitis.2 Of note: there is no evidence to support daily monitoring of these enzyme levels as predictors of clinical improvement or disease severity.
FIGURE
CT scan of abdomen taken on second day of admission
Predicting severity at time of presentation can be difficult
As was true with our patient, predicting the severity of acute pancreatitis at the time of presentation can be difficult. Scoring systems that are commonly used to evaluate disease severity include Ranson’s score, APACHE-II (Acute Physiology and Chronic Health Evaluation-II), and CT severity index, among others (TABLE). Of these, the APACHE-II score has been found to be most predictive of progression to severe disease, with accuracy of up to 75%.3
Recent studies have shown that a body mass index >30 kg/m2 is an independent risk factor for progression to severe pancreatitis.4 Other clinical predictors include poor urine output, rising hematocrit, agitation or confusion, and lack of improvement in symptoms within 48 hours.1
Though our patient came in with symptoms that were initially mild, she quickly manifested several clinical predictors for severe pancreatitis, including poor urine output and increasing confusion, as well as an APACHE-II score of 12 at 6 hours after presentation (values ≥8 indicate high risk for progression to severe disease).
TABLE
Predictors for progression to severe pancreatitis1
| Ranson score ≥3 |
| APACHE-II score ≥8 |
| CT severity index (CT grade + necrosis score) >6 |
| Body mass index >30 kg/m2 |
| Hematocrit >44% (clearly increases risk for pancreatic necrosis) |
Clinical findings:
|
| Lack of improvement in symptoms within the first 48 hours |
| APACHE, Acute Physiology and Chronic Health Evaluation; CT, computed tomography. |
Role of antibiotics? A source of debate
Infection represents the leading cause of morbidity and mortality in patients with pancreatic necrosis. Approximately 40% of patients with necrosis develop infection, with a 20% mortality rate.5 Signs of infection usually develop relatively late in the clinical course and rates increase drastically each week a patient remains hospitalized (71% of patients have signs of infection at 3 weeks).5
Interestingly, the role for antibiotics in such patients has been a source of debate in practice, as well as in the medical literature. Two recent large meta-analyses came to different conclusions regarding the use of antibiotics. A 2006 study by Heinrich et al concluded that patients with pancreatic necrosis demonstrated by contrast-enhanced CT scans should receive antibiotic prophylaxis with imipenem or meropenem for 14 days, and that prophylactic antibiotics do not increase rates of subsequent fungal infection.6 Conversely, as noted in a 2008 study published in the American Journal of Gastroenterology, “prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in patients with acute necrotizing pancreatitis.”7
Two leading professional groups have similarly contradictory recommendations on the topic, with the American Gastroenterological Association (AGA) supporting antibiotic use for patients with >30% pancreatic necrosis noted on CT and the American College of Gastroenterology (ACG) recommending against the use of prophylactic antibiotics.8
As with any clinical dilemma, it seems prudent to make the decision for or against prophylactic antibiotics based on available clinical information and the particular patient’s risk factors. Clearly, in the most high-risk patients, it would be difficult to justify withholding antibiotic therapy.
Complete bowel rest—or not?
In the past, it was thought necessary to allow for complete bowel rest and suppression of pancreatic exocrine secretion during acute pancreatitis by providing total parenteral nutrition.6,9 More recently, though, the use of early nasojejunal enteral feeding (which was initiated for our patient) has been advocated by several large meta-analyses,6 as well as by the AGA and ACG.2
The use of enteral feeding has been associated with improved outcomes, including lower infection rates (due to maintenance of the intestinal barrier and prevention of bacterial translocation), decreased length of stay, reduced rates of organ failure, and fewer deaths among patients who require surgical intervention.6
A lengthy road to recovery for our patient
After 7 days of mechanical ventilation, our patient was extubated. However, she developed significant bilateral pleural effusions as a result of fluid third spacing, and required thoracentesis.
She completed a 14-day course of imipenem, followed by an additional 10-day course due to hypotension and a suspected infected pseudocyst. Subsequent imaging studies confirmed our suspicions: She had developed a large pseudocyst (>13 cm), which remained under observation by both a gastroenterologist and general surgeon. Six weeks after admission, our patient was discharged to home with family.
But what was the cause? Although we were unable to clearly delineate an inciting cause for her pancreatitis during the admission, she was to undergo further investigation as an outpatient. There were also plans to drain the pseudocyst 6 weeks after discharge.
A learning opportunity. This patient’s case provided an excellent opportunity for our team to review the important clinical predictors for progression to severe pancreatitis, and the rapid nature of clinical decline in such patients. In hindsight, the predictors of severity in our patient were few, but included the rapid onset and clinical progression of her symptoms, as well as her elevated hematocrit on presentation and poor urine output over the first 6 hours of admission.
1. Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142-2150.
2. Vege SS, Whitcomb DC, Ginsburg CH. Clinical manifestations and diagnosis of acute pancreatitis. In: Basow DS. ed. UpTo-Date [online database]. Version 18.2. Waltham, Mass: UpTo-Date; 2010.
3. Vege SS, Whitcomb DC, Ginsburg CH. Predicting severity of acute pancreatitis. In: Basow DS, ed. UpToDate [online database]. Version 18.2. Waltham, Mass: UpToDate; 2010.
4. Skipworth JRA, Pereira SP. Acute pancreatitis. Curr Opin Crit Care. 2008;14:172-178.
5. Windsor JA, Schweder P. Complications of acute pancreatitis (including pseudocysts). In: Zinner MJ, Ashley SW, eds. Main-got’s Abdominal Operations. 11th ed. New York: McGraw-Hill; 2007:chap 37. Available at: http://www.accesssurgery.com/content.aspx?filename="6003JFP_HospitalRounds" aid=130125. Accessed November 30, 2010.
6. Heinrich S, Shafer M, Rousson V, et al. Evidenced-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg. 2006;243:154-168.
7. Bai Y, Gao J, Zou DW, et al. Prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in acute necrotizing pancreatitis: evidence from a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2008;103:104-110.
8. Vege SS, Whitcomb DC, Ginsburg CH. Treatment of acute pancreatitis. In: Basow DS, ed. UpToDate [online database]. Version 18.2. Waltham, Mass: UpToDate; 2010.
9. Haney JC, Pappas TN. Necrotizing pancreatitis: diagnosis and management. Surg Clin North Am. 2007;87:1431-1446.
1. Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142-2150.
2. Vege SS, Whitcomb DC, Ginsburg CH. Clinical manifestations and diagnosis of acute pancreatitis. In: Basow DS. ed. UpTo-Date [online database]. Version 18.2. Waltham, Mass: UpTo-Date; 2010.
3. Vege SS, Whitcomb DC, Ginsburg CH. Predicting severity of acute pancreatitis. In: Basow DS, ed. UpToDate [online database]. Version 18.2. Waltham, Mass: UpToDate; 2010.
4. Skipworth JRA, Pereira SP. Acute pancreatitis. Curr Opin Crit Care. 2008;14:172-178.
5. Windsor JA, Schweder P. Complications of acute pancreatitis (including pseudocysts). In: Zinner MJ, Ashley SW, eds. Main-got’s Abdominal Operations. 11th ed. New York: McGraw-Hill; 2007:chap 37. Available at: http://www.accesssurgery.com/content.aspx?filename="6003JFP_HospitalRounds" aid=130125. Accessed November 30, 2010.
6. Heinrich S, Shafer M, Rousson V, et al. Evidenced-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg. 2006;243:154-168.
7. Bai Y, Gao J, Zou DW, et al. Prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in acute necrotizing pancreatitis: evidence from a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2008;103:104-110.
8. Vege SS, Whitcomb DC, Ginsburg CH. Treatment of acute pancreatitis. In: Basow DS, ed. UpToDate [online database]. Version 18.2. Waltham, Mass: UpToDate; 2010.
9. Haney JC, Pappas TN. Necrotizing pancreatitis: diagnosis and management. Surg Clin North Am. 2007;87:1431-1446.
How best to diagnose asthma in infants and toddlers?
NO RELIABLE WAY EXISTS TO DIAGNOSE ASTHMA IN INFANTS AND TODDLERS. Recurrent wheezing, especially apart from colds, combined with physician-diagnosed eczema or atopic dermatitis, eosinophilia, and a parental history of asthma, increase the probability of a subsequent asthma diagnosis in the absence of other causes (strength of recommendation: B, 2 good-quality cohort studies).
Evidence summary
Wheezing in children is common and the differential diagnosis is broad. The many potential causes include upper respiratory infection, asthma, cystic fibrosis, foreign body aspiration, vascular ring, tracheomalacia, primary immunodeficiency, and congenital heart disease.1
Outpatient primary care cohort studies estimate that about half of children wheeze before they reach school age. Only one-third of children who wheeze during the first 3 years of life, however, continue to wheeze into later childhood and young adulthood.2-4
These findings have led some experts to suggest that not all wheezing in children is asthma and that asthma exists in variant forms.5-7 Variant wheezing patterns include transient early wheezing, which seems to be most prevalent in the first 3 years of life; wheezing without atopy, which occurs most often at 3 to 6 years of age; and wheezing with immunoglobulin E-associated atopy, which gradually increases in prevalence from birth and dominates in the over-6 age group. It is children in this last group whom we generally consider to have asthma.
Objective measures of lung function are challenging to perform in young children. Clinical signs and symptoms thus suggest the diagnosis of asthma.
Atopy, rhinitis, and eczema most often accompany persistent wheezing
Primary care cohort studies provide the best available evidence on which findings in infants and toddlers most likely predict persistent airway disease in childhood. A whole-population cohort study followed nearly all children born on the Isle of Wight from January 1989 through February 1990 to evaluate the natural history of childhood wheezing and to study associated risk factors.8 Children were seen at birth and at 1, 2, 4, and 10 years of age.
Findings most associated with current wheezing (within the last year) in 10-year-olds were atopy (odds ratio [OR]=4.38; 95% confidence interval [CI], 3.07-6.25), rhinitis (OR=3.72; 95% CI, 2.21-6.27), and eczema (OR=3.04; 95% CI, 2.05-4.51).8
An index to predict asthma
Since 1980, the Tucson Children’s Respiratory Study has followed 1246 healthy newborns seen by pediatricians affiliated with a large HMO in Tucson, Arizona. Questionnaires about parental asthma history and prenatal smoking history were obtained at enrollment. Childhood wheezing and its frequency, as well as physician-diagnosed allergies or asthma, were assessed at ages 2 and 3. If the child had wheezed in the past year, then the child was considered to be an “early wheezer.” If the frequency was 3 or more on a 5-point scale, then the child was considered to be an “early frequent wheezer.” Questionnaires were re-administered at ages 6, 8, 11, and 13. Three episodes of wheezing within the past year or a physician diagnosis of asthma with symptoms in the past year was considered “active asthma.” Blood specimens for eosinophils were obtained at age 10.
Using these data, the researchers developed stringent and loose criteria (TABLE 1) and odds ratios (TABLES 2 and 3) for childhood factors most predictive of an asthma diagnosis at an older age. The findings of the study may help clinicians care for wheezing infants and toddlers.9
TABLE 1
A clinical index of asthma risk9*
| Major criteria | Minor criteria |
|---|---|
| Parental asthma (history of physician diagnosis of asthma in a parent) | Allergic rhinitis (physician diagnosis of allergic rhinitis as reported in questionnaires at ages 2 or 3 y) |
| Eczema (physician diagnosis of atopic dermatitis as reported in questionnaires at ages 2 or 3 y) | Wheezing apart from colds |
| Eosinophilia (≥4%) | |
| *Stringent index for predicting asthma: Child has early, frequent wheezing plus at least 1 of the 2 major criteria or 2 of the 3 minor criteria. Loose index for predicting asthma: Child has early wheezing plus at least 1 of the 2 major criteria or 2 of the 3 minor criteria. | |
TABLE 2
Likelihood of active asthma predicted by stringent index9
| Active asthma | OR (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|---|
| At 6 y | 9.8 (5.6-17.2) | 27.5 (24.6-30.4) | 96.3 (95.1-97.5) | 47.5 (44.3-50.7) | 91.6 (89.8-93.4) |
| At 8 y | 5.8 (2.9-11.2) | 16.3 (13.7-18.9) | 96.7 (95.4-98.0) | 43.6 (40.1-47.1) | 88.2 (85.9-90.5) |
| At 11 y | 4.3 (2.4-7.8) | 15 (12.6-17.4) | 96.1 (94.8-97.4) | 42.0 (38.7-45.3) | 85.6 (83.3-87.9) |
| At 13 y | 5.7 (2.8-11.6) | 14.8 (12.1-17.5) | 97.0 (95.7-98.3) | 51.5 (47.7-55.3) | 84.2 (81.4-87.0) |
| CI, confidence interval; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value. | |||||
TABLE 3
Likelihood of active asthma predicted by loose index9
| Active asthma | OR (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|---|
| At 6 y | 5.5 (3.5-8.4) | 56.6 (53.3-59.9) | 80.8 (78.3-83.3) | 26.2 (23.4-29.0) | 93.9 (92.4-95.4) |
| At 8 y | 4.4 (2.8-6.8) | 50.5 (47.0-54.0) | 81.1 (78.3-83.9) | 29.4 (26.2-32.6) | 91.3 (89.3-93.3) |
| At 11 y | 2.6 (1.8-3.8) | 40.1 (36.8-43.4) | 79.6 (76.9-82.3) | 27.1 (24.1-30.1) | 87.5 (85.3-89.7) |
| At 13 y | 3.0 (1.9-4.6) | 39.3 (35.5-43.1) | 82.1 (79.1-85.1) | 31.7 (28.1-35.3) | 86.5 (83.9-89.1) |
| CI, confidence interval; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value. | |||||
Recommendations
A European and United States expert panel guide to the diagnosis and treatment of asthma in childhood, PRACTALL, states that “asthma should be suspected in any infant with recurrent wheezing and cough episodes. Frequently, diagnosis is possible only through long-term follow-up, consideration of the extensive differential diagnoses, and by observing the child’s response to bronchodilator and/or anti-inflammatory treatment.”10
The National Asthma Education and Prevention Program’s Expert Panel Report 3 (EPR-3) notes that diagnostic evaluation for asthma in children 0 to 4 years of age should include history, symptoms, physical examination, and assessment of quality of life.1
1. National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma. NIH publication 07-4051. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed June 20, 2008.
2. Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133-138.
3. Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414-1422.
4. Jenkins MA, Hopper JL, Bowes G, et al. Factors in childhood as predictors of asthma in adult life. BMJ. 1994;309:90-93.
5. Rusconi F, Galassi C, Corbo GM, et al. Risk factors for early, persistent, and late-onset wheezing in young children. SIDRIA Collaborative Group. Am J Respir Crit Care Med. 1999;167:1617-1622.
6. Stein RT, Martinez FD. Asthma phenotypes in childhood: lessons from an epidemiologic approach. Paediatr Respir Rev. 2004;5:155-161.
7. Stein RT, Holberg CJ, Morgan WJ, et al. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax. 1997;52:946-952.
8. Arshad SH, Kurukulaaratchy RJ, Fenn M, et al. Early life risk factors for current wheeze, asthma, and bronchial hyper-responsiveness at 10 years of age. Chest. 2005;127:502-508.
9. Castro-Rodriguez JA, Holberg CJ, Wright AL, et al. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403-1406.
10. Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63:5-34.
NO RELIABLE WAY EXISTS TO DIAGNOSE ASTHMA IN INFANTS AND TODDLERS. Recurrent wheezing, especially apart from colds, combined with physician-diagnosed eczema or atopic dermatitis, eosinophilia, and a parental history of asthma, increase the probability of a subsequent asthma diagnosis in the absence of other causes (strength of recommendation: B, 2 good-quality cohort studies).
Evidence summary
Wheezing in children is common and the differential diagnosis is broad. The many potential causes include upper respiratory infection, asthma, cystic fibrosis, foreign body aspiration, vascular ring, tracheomalacia, primary immunodeficiency, and congenital heart disease.1
Outpatient primary care cohort studies estimate that about half of children wheeze before they reach school age. Only one-third of children who wheeze during the first 3 years of life, however, continue to wheeze into later childhood and young adulthood.2-4
These findings have led some experts to suggest that not all wheezing in children is asthma and that asthma exists in variant forms.5-7 Variant wheezing patterns include transient early wheezing, which seems to be most prevalent in the first 3 years of life; wheezing without atopy, which occurs most often at 3 to 6 years of age; and wheezing with immunoglobulin E-associated atopy, which gradually increases in prevalence from birth and dominates in the over-6 age group. It is children in this last group whom we generally consider to have asthma.
Objective measures of lung function are challenging to perform in young children. Clinical signs and symptoms thus suggest the diagnosis of asthma.
Atopy, rhinitis, and eczema most often accompany persistent wheezing
Primary care cohort studies provide the best available evidence on which findings in infants and toddlers most likely predict persistent airway disease in childhood. A whole-population cohort study followed nearly all children born on the Isle of Wight from January 1989 through February 1990 to evaluate the natural history of childhood wheezing and to study associated risk factors.8 Children were seen at birth and at 1, 2, 4, and 10 years of age.
Findings most associated with current wheezing (within the last year) in 10-year-olds were atopy (odds ratio [OR]=4.38; 95% confidence interval [CI], 3.07-6.25), rhinitis (OR=3.72; 95% CI, 2.21-6.27), and eczema (OR=3.04; 95% CI, 2.05-4.51).8
An index to predict asthma
Since 1980, the Tucson Children’s Respiratory Study has followed 1246 healthy newborns seen by pediatricians affiliated with a large HMO in Tucson, Arizona. Questionnaires about parental asthma history and prenatal smoking history were obtained at enrollment. Childhood wheezing and its frequency, as well as physician-diagnosed allergies or asthma, were assessed at ages 2 and 3. If the child had wheezed in the past year, then the child was considered to be an “early wheezer.” If the frequency was 3 or more on a 5-point scale, then the child was considered to be an “early frequent wheezer.” Questionnaires were re-administered at ages 6, 8, 11, and 13. Three episodes of wheezing within the past year or a physician diagnosis of asthma with symptoms in the past year was considered “active asthma.” Blood specimens for eosinophils were obtained at age 10.
Using these data, the researchers developed stringent and loose criteria (TABLE 1) and odds ratios (TABLES 2 and 3) for childhood factors most predictive of an asthma diagnosis at an older age. The findings of the study may help clinicians care for wheezing infants and toddlers.9
TABLE 1
A clinical index of asthma risk9*
| Major criteria | Minor criteria |
|---|---|
| Parental asthma (history of physician diagnosis of asthma in a parent) | Allergic rhinitis (physician diagnosis of allergic rhinitis as reported in questionnaires at ages 2 or 3 y) |
| Eczema (physician diagnosis of atopic dermatitis as reported in questionnaires at ages 2 or 3 y) | Wheezing apart from colds |
| Eosinophilia (≥4%) | |
| *Stringent index for predicting asthma: Child has early, frequent wheezing plus at least 1 of the 2 major criteria or 2 of the 3 minor criteria. Loose index for predicting asthma: Child has early wheezing plus at least 1 of the 2 major criteria or 2 of the 3 minor criteria. | |
TABLE 2
Likelihood of active asthma predicted by stringent index9
| Active asthma | OR (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|---|
| At 6 y | 9.8 (5.6-17.2) | 27.5 (24.6-30.4) | 96.3 (95.1-97.5) | 47.5 (44.3-50.7) | 91.6 (89.8-93.4) |
| At 8 y | 5.8 (2.9-11.2) | 16.3 (13.7-18.9) | 96.7 (95.4-98.0) | 43.6 (40.1-47.1) | 88.2 (85.9-90.5) |
| At 11 y | 4.3 (2.4-7.8) | 15 (12.6-17.4) | 96.1 (94.8-97.4) | 42.0 (38.7-45.3) | 85.6 (83.3-87.9) |
| At 13 y | 5.7 (2.8-11.6) | 14.8 (12.1-17.5) | 97.0 (95.7-98.3) | 51.5 (47.7-55.3) | 84.2 (81.4-87.0) |
| CI, confidence interval; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value. | |||||
TABLE 3
Likelihood of active asthma predicted by loose index9
| Active asthma | OR (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|---|
| At 6 y | 5.5 (3.5-8.4) | 56.6 (53.3-59.9) | 80.8 (78.3-83.3) | 26.2 (23.4-29.0) | 93.9 (92.4-95.4) |
| At 8 y | 4.4 (2.8-6.8) | 50.5 (47.0-54.0) | 81.1 (78.3-83.9) | 29.4 (26.2-32.6) | 91.3 (89.3-93.3) |
| At 11 y | 2.6 (1.8-3.8) | 40.1 (36.8-43.4) | 79.6 (76.9-82.3) | 27.1 (24.1-30.1) | 87.5 (85.3-89.7) |
| At 13 y | 3.0 (1.9-4.6) | 39.3 (35.5-43.1) | 82.1 (79.1-85.1) | 31.7 (28.1-35.3) | 86.5 (83.9-89.1) |
| CI, confidence interval; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value. | |||||
Recommendations
A European and United States expert panel guide to the diagnosis and treatment of asthma in childhood, PRACTALL, states that “asthma should be suspected in any infant with recurrent wheezing and cough episodes. Frequently, diagnosis is possible only through long-term follow-up, consideration of the extensive differential diagnoses, and by observing the child’s response to bronchodilator and/or anti-inflammatory treatment.”10
The National Asthma Education and Prevention Program’s Expert Panel Report 3 (EPR-3) notes that diagnostic evaluation for asthma in children 0 to 4 years of age should include history, symptoms, physical examination, and assessment of quality of life.1
NO RELIABLE WAY EXISTS TO DIAGNOSE ASTHMA IN INFANTS AND TODDLERS. Recurrent wheezing, especially apart from colds, combined with physician-diagnosed eczema or atopic dermatitis, eosinophilia, and a parental history of asthma, increase the probability of a subsequent asthma diagnosis in the absence of other causes (strength of recommendation: B, 2 good-quality cohort studies).
Evidence summary
Wheezing in children is common and the differential diagnosis is broad. The many potential causes include upper respiratory infection, asthma, cystic fibrosis, foreign body aspiration, vascular ring, tracheomalacia, primary immunodeficiency, and congenital heart disease.1
Outpatient primary care cohort studies estimate that about half of children wheeze before they reach school age. Only one-third of children who wheeze during the first 3 years of life, however, continue to wheeze into later childhood and young adulthood.2-4
These findings have led some experts to suggest that not all wheezing in children is asthma and that asthma exists in variant forms.5-7 Variant wheezing patterns include transient early wheezing, which seems to be most prevalent in the first 3 years of life; wheezing without atopy, which occurs most often at 3 to 6 years of age; and wheezing with immunoglobulin E-associated atopy, which gradually increases in prevalence from birth and dominates in the over-6 age group. It is children in this last group whom we generally consider to have asthma.
Objective measures of lung function are challenging to perform in young children. Clinical signs and symptoms thus suggest the diagnosis of asthma.
Atopy, rhinitis, and eczema most often accompany persistent wheezing
Primary care cohort studies provide the best available evidence on which findings in infants and toddlers most likely predict persistent airway disease in childhood. A whole-population cohort study followed nearly all children born on the Isle of Wight from January 1989 through February 1990 to evaluate the natural history of childhood wheezing and to study associated risk factors.8 Children were seen at birth and at 1, 2, 4, and 10 years of age.
Findings most associated with current wheezing (within the last year) in 10-year-olds were atopy (odds ratio [OR]=4.38; 95% confidence interval [CI], 3.07-6.25), rhinitis (OR=3.72; 95% CI, 2.21-6.27), and eczema (OR=3.04; 95% CI, 2.05-4.51).8
An index to predict asthma
Since 1980, the Tucson Children’s Respiratory Study has followed 1246 healthy newborns seen by pediatricians affiliated with a large HMO in Tucson, Arizona. Questionnaires about parental asthma history and prenatal smoking history were obtained at enrollment. Childhood wheezing and its frequency, as well as physician-diagnosed allergies or asthma, were assessed at ages 2 and 3. If the child had wheezed in the past year, then the child was considered to be an “early wheezer.” If the frequency was 3 or more on a 5-point scale, then the child was considered to be an “early frequent wheezer.” Questionnaires were re-administered at ages 6, 8, 11, and 13. Three episodes of wheezing within the past year or a physician diagnosis of asthma with symptoms in the past year was considered “active asthma.” Blood specimens for eosinophils were obtained at age 10.
Using these data, the researchers developed stringent and loose criteria (TABLE 1) and odds ratios (TABLES 2 and 3) for childhood factors most predictive of an asthma diagnosis at an older age. The findings of the study may help clinicians care for wheezing infants and toddlers.9
TABLE 1
A clinical index of asthma risk9*
| Major criteria | Minor criteria |
|---|---|
| Parental asthma (history of physician diagnosis of asthma in a parent) | Allergic rhinitis (physician diagnosis of allergic rhinitis as reported in questionnaires at ages 2 or 3 y) |
| Eczema (physician diagnosis of atopic dermatitis as reported in questionnaires at ages 2 or 3 y) | Wheezing apart from colds |
| Eosinophilia (≥4%) | |
| *Stringent index for predicting asthma: Child has early, frequent wheezing plus at least 1 of the 2 major criteria or 2 of the 3 minor criteria. Loose index for predicting asthma: Child has early wheezing plus at least 1 of the 2 major criteria or 2 of the 3 minor criteria. | |
TABLE 2
Likelihood of active asthma predicted by stringent index9
| Active asthma | OR (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|---|
| At 6 y | 9.8 (5.6-17.2) | 27.5 (24.6-30.4) | 96.3 (95.1-97.5) | 47.5 (44.3-50.7) | 91.6 (89.8-93.4) |
| At 8 y | 5.8 (2.9-11.2) | 16.3 (13.7-18.9) | 96.7 (95.4-98.0) | 43.6 (40.1-47.1) | 88.2 (85.9-90.5) |
| At 11 y | 4.3 (2.4-7.8) | 15 (12.6-17.4) | 96.1 (94.8-97.4) | 42.0 (38.7-45.3) | 85.6 (83.3-87.9) |
| At 13 y | 5.7 (2.8-11.6) | 14.8 (12.1-17.5) | 97.0 (95.7-98.3) | 51.5 (47.7-55.3) | 84.2 (81.4-87.0) |
| CI, confidence interval; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value. | |||||
TABLE 3
Likelihood of active asthma predicted by loose index9
| Active asthma | OR (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|---|
| At 6 y | 5.5 (3.5-8.4) | 56.6 (53.3-59.9) | 80.8 (78.3-83.3) | 26.2 (23.4-29.0) | 93.9 (92.4-95.4) |
| At 8 y | 4.4 (2.8-6.8) | 50.5 (47.0-54.0) | 81.1 (78.3-83.9) | 29.4 (26.2-32.6) | 91.3 (89.3-93.3) |
| At 11 y | 2.6 (1.8-3.8) | 40.1 (36.8-43.4) | 79.6 (76.9-82.3) | 27.1 (24.1-30.1) | 87.5 (85.3-89.7) |
| At 13 y | 3.0 (1.9-4.6) | 39.3 (35.5-43.1) | 82.1 (79.1-85.1) | 31.7 (28.1-35.3) | 86.5 (83.9-89.1) |
| CI, confidence interval; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value. | |||||
Recommendations
A European and United States expert panel guide to the diagnosis and treatment of asthma in childhood, PRACTALL, states that “asthma should be suspected in any infant with recurrent wheezing and cough episodes. Frequently, diagnosis is possible only through long-term follow-up, consideration of the extensive differential diagnoses, and by observing the child’s response to bronchodilator and/or anti-inflammatory treatment.”10
The National Asthma Education and Prevention Program’s Expert Panel Report 3 (EPR-3) notes that diagnostic evaluation for asthma in children 0 to 4 years of age should include history, symptoms, physical examination, and assessment of quality of life.1
1. National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma. NIH publication 07-4051. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed June 20, 2008.
2. Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133-138.
3. Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414-1422.
4. Jenkins MA, Hopper JL, Bowes G, et al. Factors in childhood as predictors of asthma in adult life. BMJ. 1994;309:90-93.
5. Rusconi F, Galassi C, Corbo GM, et al. Risk factors for early, persistent, and late-onset wheezing in young children. SIDRIA Collaborative Group. Am J Respir Crit Care Med. 1999;167:1617-1622.
6. Stein RT, Martinez FD. Asthma phenotypes in childhood: lessons from an epidemiologic approach. Paediatr Respir Rev. 2004;5:155-161.
7. Stein RT, Holberg CJ, Morgan WJ, et al. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax. 1997;52:946-952.
8. Arshad SH, Kurukulaaratchy RJ, Fenn M, et al. Early life risk factors for current wheeze, asthma, and bronchial hyper-responsiveness at 10 years of age. Chest. 2005;127:502-508.
9. Castro-Rodriguez JA, Holberg CJ, Wright AL, et al. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403-1406.
10. Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63:5-34.
1. National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma. NIH publication 07-4051. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed June 20, 2008.
2. Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133-138.
3. Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414-1422.
4. Jenkins MA, Hopper JL, Bowes G, et al. Factors in childhood as predictors of asthma in adult life. BMJ. 1994;309:90-93.
5. Rusconi F, Galassi C, Corbo GM, et al. Risk factors for early, persistent, and late-onset wheezing in young children. SIDRIA Collaborative Group. Am J Respir Crit Care Med. 1999;167:1617-1622.
6. Stein RT, Martinez FD. Asthma phenotypes in childhood: lessons from an epidemiologic approach. Paediatr Respir Rev. 2004;5:155-161.
7. Stein RT, Holberg CJ, Morgan WJ, et al. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax. 1997;52:946-952.
8. Arshad SH, Kurukulaaratchy RJ, Fenn M, et al. Early life risk factors for current wheeze, asthma, and bronchial hyper-responsiveness at 10 years of age. Chest. 2005;127:502-508.
9. Castro-Rodriguez JA, Holberg CJ, Wright AL, et al. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403-1406.
10. Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63:5-34.
Evidence-based answers from the Family Physicians Inquiries Network
Is C difficile to blame for your patient’s diarrhea?
• A C difficile diagnosis should be made by one of several widely available testing protocols, including a 2-step method using the common antigen assay to determine whether C difficile is present, followed by an enzyme immunoassay for toxins A and B to improve specificity. B
• Oral metronidazole should be used for initial treatment of mild to moderate C difficile infection, and oral vancomycin and possibly intravenous metronidazole for severe cases. A
• Metronidazole should not be used after an initial recurrence or for long-term therapy because of the risk of neurotoxicity. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Mary S, an 82-year-old patient you recently treated for bronchitis with a 3-day course of levofloxacin, calls your office complaining of diarrhea and abdominal cramps. She describes the diarrhea as nonbloody and particularly foul smelling and asks if she can take loperamide for her symptoms.
If Mary S were your patient, what would you tell her?
The incidence of Clostridium difficile infection (CDI) has been on the rise since 2000, when a common epidemic strain began circulating in North America.1 Although hospitalization or residency in a long-term care facility remains a classic risk factor for CDI, physicians in out-patient settings are increasingly likely to see patients with community-acquired CDI.
Recently updated guidelines from the Society for Health-care Epidemiology of America (SHEA) and the Infectious Diseases Society of America define CDI as the presence of diarrhea (≥3 unformed stools in 24 hours) and either a positive stool test for toxigenic C difficile or its toxins or colonoscopic or histopathologic findings demonstrating pseudomembranous colitis.2 That said, the clinical features of CDI are nonspecific and many patients do not fit the classic profile. So diagnosing CDI requires a high index of suspicion.
The text and tables that follow detail some surprising things about who is likely to develop CDI and which treatment options to employ (and, in some cases, avoid).
Is it CDI? Looking beyond the obvious
Antibiotic use and advanced age, like hospitalization, are classic risk factors for CDI.3 Diarrhea typically begins during or shortly after a course of antibiotics, but may develop as long as 8 weeks after treatment is completed. While any antibiotic, including metronidazole, can precipitate CDI, clindamycin, cephalosporins, extended-spectrum penicillins, and quinolones are most frequently implicated.4 Epidemiologic studies have suggested an association between gastric acid-reducing agents—primarily proton-pump inhibitors—and CDI.4-7 But this link remains controversial, as other investigations have not found a clear relationship.8
In addition to diarrhea, approximately 28% of patients with CDI develop a fever (as high as 104°F); 50% develop leukocytosis (up to 50,000 cells/mcL); and 22% develop abdominal pain, usually localized to the lower quadrants.9 These symptoms, however, are not specific to C difficile, and could be due to a different enteric pathogen, intra-abdominal sepsis, inflammatory bowel disease, or adverse effects of medication, among other causes.9
Markers for severe CDI include age >70 years, leukocyte count >20,000 cells/mcL, albumin level <2.5 g/dL, small-bowel obstruction or ileus, and a computed tomography (CT) scan showing colorectal inflammation.10 Severe CDI can lead to toxic megacolon, bowel perforation, sepsis, and even death.
In addition to considering CDI in patients with nonspecific symptoms, it is important to include it in the differential diagnosis of patients who do not fit the classic profile. In a recent study of patients with CDI at 4 Veterans Affairs facilities, almost half (49%) of those studied had no exposure to antimicrobial drugs. The researchers further found that the median age of patients with CDI was 61 years—younger than that found in previous studies—and that 20% of the cases were community-acquired.11
Consider CDI in children, too. Risk factors for CDI in pediatric patients include disruption of the normal microflora of the gastrointestinal tract, compromised immune status, poor diet, underlying health conditions, concurrent infections, and cancer.12
Diagnostic testing: Consider a 2-step assay
Patients with symptoms suggestive of CDI should undergo laboratory testing to confirm the diagnosis. TABLE 1 lists the tests that are widely available in the United States.3 Only liquid stools should be tested and just one sample should be sent to the lab, as multiple samples do not increase the diagnostic yield.13 In addition, tests should be used only for diagnosis, and not as a “test of cure.” This is because patients can shed C difficile toxin and spores for several weeks after completing treatment, and there are wide variations in the sensitivity of toxin assays.
Infants <1 year old have high rates of asymptomatic toxigenic strains of C difficile, and until 2008, recommendations from SHEA discouraged testing the stools of such young patients. Because of the difficulty in differentiating incidental colonization from true CDI in this patient population, the authors of a recent review suggested using more than one diagnostic approach when testing children <1 year of age.14
We advocate a 2-step assay—that is, testing for both glutamate dehydrogenase (GDH)—an antigen common to all strains of C difficile—and C difficile toxins A and B. The common antigen test is sensitive, but may detect carriers who do not have active disease. The enzyme immunoassay (EIA) for toxins A and B helps to improve specificity. Therefore, positive results of both tests would be considered a positive finding, negative results of both tests would be considered a negative finding, and one positive result with one negative result would require another test for toxin detection.3
The reverse-transcriptase polymerase chain reaction (RT-PCR) assay, which detects the toxin B gene of C difficile, is the newest test for CDI. The RT-PCR assay detects only toxigenic strains of C difficile, and all toxigenic strains produce toxin B, making it more specific than testing for the common antigen. The RT-PCR assay also has better sensitivity than the cytotoxin assay, which also tests for toxin B. The major limitation of the RT-PCR assay is the frequency of false-positive results in hospitalized patients with a high incidence of C difficile colonization.3
Routine laboratory studies, including a complete blood count with differential and a complete metabolic panel, are often useful to ascertain the presence and degree of leukocytosis, dehydration, and other metabolic abnormalities and to test for hypoalbuminemia. Fecal leukocytes can be seen in colitis and may be useful in select cases.
Imaging studies such as radiography, CT, and endoscopy have largely been superseded by lab testing for CDI. Plain radiographs are usually normal in patients with CDI, unless the patient has an ileus or toxic megacolon. CT is useful, however, in suspected cases of fulminant CDI or toxic megacolon, and may reveal colonic-wall thickening, pericolonic stranding, or ascites.9 Colonoscopy is preferred over sigmoidoscopy because up to one-third of patients with pseudomembranous colitis will have involvement of the right colon only.9 However, this test carries the risk of perforation in patients with fulminant colitis.
TABLE 1
Lab tests for C difficile infection
| Test | Substance detected | Time needed | Sensitivity | Specificity |
|---|---|---|---|---|
| Cytotoxin | Toxin B | 1-3 d | 95% | 90%-95% |
| Toxin culture | Toxigenic C difficile† | 3-5 d | >95% | 80%-90% |
| EIA toxin A or A/B | Toxin A or A/B | Hours | 75%-80% | 97%-98% |
| EIA GDH* | C difficile | Hours | 95%-100% | 70%-80% |
| EIA GDH* and toxin A/B | C difficile and C difficile toxin | Hours | 95%-100% | 97%-98% |
| RT-PCR | Toxigenic C difficile† | Hours | >98% | 80%-99% |
| *GDH is the common C difficile antigen. †All toxigenic strains produce toxin B. EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; RT-PCR, reverse-transcriptase polymerase chain reaction. Adapted from: Bartlett JG. Infect Control Hosp Epidemiol. 2010.3 | ||||
Treatment: What to consider, what to avoid
Of the 2 antibiotics most commonly used to treat CDI—metronidazole and vancomycin—only the latter has been approved by the US Food and Drug Administration for this indication. Nevertheless, metronidazole is generally recommended as first-line therapy and has the advantage of being much less expensive than vancomycin. However, an RCT found that oral vancomycin was superior to metronidazole in patients with severe disease.15 The time to resolution of diarrhea may be shorter with oral vancomycin than with metronidazole, as well.16
Recent guidelines suggest that clinicians consider 3 factors in deciding how to treat a first episode of CDI: the patient’s age, peak white blood cell count, and peak serum creatinine level.2 TABLE 2 presents an overview of treatment recommendations for both an initial episode of CDI and recurrences.
Treat severe CDI without delay. For patients with suspected severe CDI, treatment should be started empirically, without waiting for test results. Avoid antiperistaltic agents, which can obscure symptoms and precipitate toxic megacolon.2 Discontinue an antibiotic, if the patient is taking one, as soon as possible after the original infection has been adequately treated. If other infections need to be treated concurrently, we recommend that the course of treatment for CDI be extended until after the other antibiotic regimens have been stopped.
Avoid probiotics in this group. The use of probiotics, both for prevention and to help restore normal bowel flora in patients with CDI, has been advocated for many years. One RCT showed that a yogurt drink containing Lactobacillus and other bacteria reduced the risk of CDI in individuals ≥50 years of age who were taking antibiotics,17 but the guideline development panel recommended against using probiotics until larger trials have been completed.2
Probiotics are not without risk, and several cases of bacteremia have been reported.18,19 Immunocompromised patients appear to be at comparably higher risk, and probiotics should be avoided in this group. Numerous adjunctive agents, including intraluminal toxin binders, biotherapeutic agents, monoclonal antibodies, and a C difficile vaccine, are in various stages of development.2
How to handle recurrences
Relapse rates for CDI range from 6% to 25%,2 and affect patients who receive either vancomycin or metronidazole for the initial treatment. The mechanism relates to either relapse of the original infection or reinfection of susceptible patients with a new strain of C difficile.
Risk of relapse. Elderly patients treated with metronidazole seem to be particularly susceptible to CDI relapse.20 Other risk factors include the administration of non-C difficile antibiotics during or after treatment of CDI, a defective immune response against toxin A, glucocorticoid use, prior stroke, and concurrent use of a proton-pump inhibitor.21-25
TABLE 2 lists tapering and/or pulsed dosing of oral vancomycin as treatment for patients with a second recurrence. We often prescribe the following 6-week regimen, telling patients to take 125 mg vancomycin:
- 4 times a day for one week,
- then 2 times a day for one week,
- then once a day for one week,
- then every other day for one week, and
- finally, every 72 hours for 2 weeks.
Oral metronidazole should not be used beyond the first recurrence or for long-term therapy because of cumulative neurotoxicity, which can be irreversible.2
Management of patients whose CDI recurs after a long course of vancomycin is challenging. Oral rifaximin therapy (400 mg twice a day for 14 days), started immediately at the end of the oral vancomycin course, was shown to cure 7 of 8 patients with multiple relapses.26 Other potential treatment options are oral nitazoxanide, IV tigecycline, or IV immunoglobulin.
CASE You explain to Mary S that diagnostic tests are needed before you can determine whether she can safely take loperamide. When she comes in later that day, you collect a stool sample for C difficile antigen and toxin testing, and order a complete blood count and electrolyte panel.
The patient’s C difficile tests come back positive, her white blood cell count is <15,000 cells/mcL, and her creatinine level is ≤1.5 times her baseline, so you start her on oral metronidazole 500 mg every 8 hours for 14 days. (If the antigen assay had been positive and the toxin negative, you would have either repeated the test or treated Mary S empirically with metronidazole. If the initial antigen assay had been negative, you would have advised her to take the loperamide.)
You schedule a follow-up visit a day or 2 after starting therapy. If the patient is dehydrated or her symptoms have not improved by then, hospitalization may be required.
TABLE 2
Treatment recommendations for C difficile infection
| Clinical description | Clinical evidence | Recommended treatment |
|---|---|---|
| Initial episode (mild or moderate) | Leukocytosis with a white cell count <15,000 cells/mcL and creatinine <1.5 times premorbid level | Metronidazole (oral) 500 mg TID for 10-14 d |
| Initial episode (severe) | Leukocytosis with a white cell count ≥15,000 cells/mcL or creatinine ≥1.5 times premorbid level | Vancomycin (oral) 125 mg QID for 10-14 d |
| Initial episode (severe, complicated) | Hypotension or shock, ileus, megacolon | Vancomycin 500 mg QID (oral or by NG tube) plus metronidazole 500 mg (IV). If complete ileus, consider adding rectal instillation of vancomycin |
| First recurrence | Same as initial episode | |
| Second recurrence | Vancomycin in a tapered and/or pulsed regimen | |
| NG, nasogastric. Adapted from: Cohen SH, et al. Infect Control Hosp Epidemiol. 2010.2 | ||
CORRESPONDENCE
Richard R. Watkins, MD, MS, Division of Infectious Diseases, Akron General Medical Center, 224 West Exchange Street, Suite 290, Akron, OH 44302; [email protected]
1. Gerding DN. Global epidemiology of Clostridium difficile infection in 2010. Infect Control Hosp Epidemiol. 2010;31(suppl 1):S32-S34.
2. Cohen SH, Gerding DH, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
3. Bartlett JG. Detection of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2010;31(suppl 1):S35-S37.
4. Dial S, Alrasadi K, Manoukian C, et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171:33-38.
5. Dial S, Delaney JA, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989-2995.
6. Cunningham R, Dale B, Undy B, et al. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003;54:243-245.
7. Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784-790.
8. Shah S, Lewis A, Leopold D, et al. Gastric acid suppression does not promote clostridial diarrhoea in the elderly. QJM. 2000;93:175-181.
9. Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. 2008;46(suppl 1):S12-S18.
10. Henrich TJ, Krakower D, Bitton A, et al. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15:415-422.
11. Kutty PK, Woods CW, Sena AC, et al. Risk factors for and estimated incidence of community-associated Clostridium difficile infection, North Carolina, USA. Emerg Infect Dis. 2010;16:197-204.
12. Pituch H. Clostridium difficile is no longer just a nosocomial infection or an infection of adults. Int J Antimicrob Agents. 2009;33(suppl 1):S42-S45.
13. Mohan SS, McDermott BP, Parchuri S, et al. Lack of value of repeat stool testing for Clostridium difficile toxin. Am J Med. 2006;119:356.e7-e8.
14. Bryant K, McDonald LC. Clostridium difficile infections in children. Pediatr Infect Dis J. 2009;28:145-146.
15. Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
16. Belmares J, Gerding DN, Parada JP, et al. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect. 2007;55:495-501.
17. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80.-
18. Ledoux D, Labombardi VJ, Karter D. Lactobacillus acidophilus bacteraemia after use of a probiotic in a patient with AIDS and Hodgkin’s disease. Int J STD AIDS. 2006;17:280-282.
19. Hammerman C, Bin-Nun A, Kaplan M. Safety of probiotics: comparison of two popular strains. BMJ. 2006;333:1006-1008.
20. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590.
21. Nair S, Yadav D, Corpuz M, et al. Clostridium difficile colitis: factors influencing treatment failure and relapse—a prospective evaluation. Am J Gastroenterol. 1998;93:1873-1876.
22. Garey KW, Sethi S, Yadav Y, et al. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70:298-304.
23. Das R, Feuerstadt P, Brandt LJ. Glucocorticoids are associated with increased risk of short-term mortality in hospitalized patients with Clostridium difficile-associated disease. Am J Gastroenterol. 2010;105:2040-2049.
24. Cadena J, Thompson GR, 3rd, Patterson JE, et al. Clinical predictors and risk factors for relapsing Clostridium difficile infection. Am J Med Sci. 2010;339:350-355.
25. Linsky A, Gupta K, Lawler EV, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170:772-778.
26. Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007;44:846-848.
• A C difficile diagnosis should be made by one of several widely available testing protocols, including a 2-step method using the common antigen assay to determine whether C difficile is present, followed by an enzyme immunoassay for toxins A and B to improve specificity. B
• Oral metronidazole should be used for initial treatment of mild to moderate C difficile infection, and oral vancomycin and possibly intravenous metronidazole for severe cases. A
• Metronidazole should not be used after an initial recurrence or for long-term therapy because of the risk of neurotoxicity. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Mary S, an 82-year-old patient you recently treated for bronchitis with a 3-day course of levofloxacin, calls your office complaining of diarrhea and abdominal cramps. She describes the diarrhea as nonbloody and particularly foul smelling and asks if she can take loperamide for her symptoms.
If Mary S were your patient, what would you tell her?
The incidence of Clostridium difficile infection (CDI) has been on the rise since 2000, when a common epidemic strain began circulating in North America.1 Although hospitalization or residency in a long-term care facility remains a classic risk factor for CDI, physicians in out-patient settings are increasingly likely to see patients with community-acquired CDI.
Recently updated guidelines from the Society for Health-care Epidemiology of America (SHEA) and the Infectious Diseases Society of America define CDI as the presence of diarrhea (≥3 unformed stools in 24 hours) and either a positive stool test for toxigenic C difficile or its toxins or colonoscopic or histopathologic findings demonstrating pseudomembranous colitis.2 That said, the clinical features of CDI are nonspecific and many patients do not fit the classic profile. So diagnosing CDI requires a high index of suspicion.
The text and tables that follow detail some surprising things about who is likely to develop CDI and which treatment options to employ (and, in some cases, avoid).
Is it CDI? Looking beyond the obvious
Antibiotic use and advanced age, like hospitalization, are classic risk factors for CDI.3 Diarrhea typically begins during or shortly after a course of antibiotics, but may develop as long as 8 weeks after treatment is completed. While any antibiotic, including metronidazole, can precipitate CDI, clindamycin, cephalosporins, extended-spectrum penicillins, and quinolones are most frequently implicated.4 Epidemiologic studies have suggested an association between gastric acid-reducing agents—primarily proton-pump inhibitors—and CDI.4-7 But this link remains controversial, as other investigations have not found a clear relationship.8
In addition to diarrhea, approximately 28% of patients with CDI develop a fever (as high as 104°F); 50% develop leukocytosis (up to 50,000 cells/mcL); and 22% develop abdominal pain, usually localized to the lower quadrants.9 These symptoms, however, are not specific to C difficile, and could be due to a different enteric pathogen, intra-abdominal sepsis, inflammatory bowel disease, or adverse effects of medication, among other causes.9
Markers for severe CDI include age >70 years, leukocyte count >20,000 cells/mcL, albumin level <2.5 g/dL, small-bowel obstruction or ileus, and a computed tomography (CT) scan showing colorectal inflammation.10 Severe CDI can lead to toxic megacolon, bowel perforation, sepsis, and even death.
In addition to considering CDI in patients with nonspecific symptoms, it is important to include it in the differential diagnosis of patients who do not fit the classic profile. In a recent study of patients with CDI at 4 Veterans Affairs facilities, almost half (49%) of those studied had no exposure to antimicrobial drugs. The researchers further found that the median age of patients with CDI was 61 years—younger than that found in previous studies—and that 20% of the cases were community-acquired.11
Consider CDI in children, too. Risk factors for CDI in pediatric patients include disruption of the normal microflora of the gastrointestinal tract, compromised immune status, poor diet, underlying health conditions, concurrent infections, and cancer.12
Diagnostic testing: Consider a 2-step assay
Patients with symptoms suggestive of CDI should undergo laboratory testing to confirm the diagnosis. TABLE 1 lists the tests that are widely available in the United States.3 Only liquid stools should be tested and just one sample should be sent to the lab, as multiple samples do not increase the diagnostic yield.13 In addition, tests should be used only for diagnosis, and not as a “test of cure.” This is because patients can shed C difficile toxin and spores for several weeks after completing treatment, and there are wide variations in the sensitivity of toxin assays.
Infants <1 year old have high rates of asymptomatic toxigenic strains of C difficile, and until 2008, recommendations from SHEA discouraged testing the stools of such young patients. Because of the difficulty in differentiating incidental colonization from true CDI in this patient population, the authors of a recent review suggested using more than one diagnostic approach when testing children <1 year of age.14
We advocate a 2-step assay—that is, testing for both glutamate dehydrogenase (GDH)—an antigen common to all strains of C difficile—and C difficile toxins A and B. The common antigen test is sensitive, but may detect carriers who do not have active disease. The enzyme immunoassay (EIA) for toxins A and B helps to improve specificity. Therefore, positive results of both tests would be considered a positive finding, negative results of both tests would be considered a negative finding, and one positive result with one negative result would require another test for toxin detection.3
The reverse-transcriptase polymerase chain reaction (RT-PCR) assay, which detects the toxin B gene of C difficile, is the newest test for CDI. The RT-PCR assay detects only toxigenic strains of C difficile, and all toxigenic strains produce toxin B, making it more specific than testing for the common antigen. The RT-PCR assay also has better sensitivity than the cytotoxin assay, which also tests for toxin B. The major limitation of the RT-PCR assay is the frequency of false-positive results in hospitalized patients with a high incidence of C difficile colonization.3
Routine laboratory studies, including a complete blood count with differential and a complete metabolic panel, are often useful to ascertain the presence and degree of leukocytosis, dehydration, and other metabolic abnormalities and to test for hypoalbuminemia. Fecal leukocytes can be seen in colitis and may be useful in select cases.
Imaging studies such as radiography, CT, and endoscopy have largely been superseded by lab testing for CDI. Plain radiographs are usually normal in patients with CDI, unless the patient has an ileus or toxic megacolon. CT is useful, however, in suspected cases of fulminant CDI or toxic megacolon, and may reveal colonic-wall thickening, pericolonic stranding, or ascites.9 Colonoscopy is preferred over sigmoidoscopy because up to one-third of patients with pseudomembranous colitis will have involvement of the right colon only.9 However, this test carries the risk of perforation in patients with fulminant colitis.
TABLE 1
Lab tests for C difficile infection
| Test | Substance detected | Time needed | Sensitivity | Specificity |
|---|---|---|---|---|
| Cytotoxin | Toxin B | 1-3 d | 95% | 90%-95% |
| Toxin culture | Toxigenic C difficile† | 3-5 d | >95% | 80%-90% |
| EIA toxin A or A/B | Toxin A or A/B | Hours | 75%-80% | 97%-98% |
| EIA GDH* | C difficile | Hours | 95%-100% | 70%-80% |
| EIA GDH* and toxin A/B | C difficile and C difficile toxin | Hours | 95%-100% | 97%-98% |
| RT-PCR | Toxigenic C difficile† | Hours | >98% | 80%-99% |
| *GDH is the common C difficile antigen. †All toxigenic strains produce toxin B. EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; RT-PCR, reverse-transcriptase polymerase chain reaction. Adapted from: Bartlett JG. Infect Control Hosp Epidemiol. 2010.3 | ||||
Treatment: What to consider, what to avoid
Of the 2 antibiotics most commonly used to treat CDI—metronidazole and vancomycin—only the latter has been approved by the US Food and Drug Administration for this indication. Nevertheless, metronidazole is generally recommended as first-line therapy and has the advantage of being much less expensive than vancomycin. However, an RCT found that oral vancomycin was superior to metronidazole in patients with severe disease.15 The time to resolution of diarrhea may be shorter with oral vancomycin than with metronidazole, as well.16
Recent guidelines suggest that clinicians consider 3 factors in deciding how to treat a first episode of CDI: the patient’s age, peak white blood cell count, and peak serum creatinine level.2 TABLE 2 presents an overview of treatment recommendations for both an initial episode of CDI and recurrences.
Treat severe CDI without delay. For patients with suspected severe CDI, treatment should be started empirically, without waiting for test results. Avoid antiperistaltic agents, which can obscure symptoms and precipitate toxic megacolon.2 Discontinue an antibiotic, if the patient is taking one, as soon as possible after the original infection has been adequately treated. If other infections need to be treated concurrently, we recommend that the course of treatment for CDI be extended until after the other antibiotic regimens have been stopped.
Avoid probiotics in this group. The use of probiotics, both for prevention and to help restore normal bowel flora in patients with CDI, has been advocated for many years. One RCT showed that a yogurt drink containing Lactobacillus and other bacteria reduced the risk of CDI in individuals ≥50 years of age who were taking antibiotics,17 but the guideline development panel recommended against using probiotics until larger trials have been completed.2
Probiotics are not without risk, and several cases of bacteremia have been reported.18,19 Immunocompromised patients appear to be at comparably higher risk, and probiotics should be avoided in this group. Numerous adjunctive agents, including intraluminal toxin binders, biotherapeutic agents, monoclonal antibodies, and a C difficile vaccine, are in various stages of development.2
How to handle recurrences
Relapse rates for CDI range from 6% to 25%,2 and affect patients who receive either vancomycin or metronidazole for the initial treatment. The mechanism relates to either relapse of the original infection or reinfection of susceptible patients with a new strain of C difficile.
Risk of relapse. Elderly patients treated with metronidazole seem to be particularly susceptible to CDI relapse.20 Other risk factors include the administration of non-C difficile antibiotics during or after treatment of CDI, a defective immune response against toxin A, glucocorticoid use, prior stroke, and concurrent use of a proton-pump inhibitor.21-25
TABLE 2 lists tapering and/or pulsed dosing of oral vancomycin as treatment for patients with a second recurrence. We often prescribe the following 6-week regimen, telling patients to take 125 mg vancomycin:
- 4 times a day for one week,
- then 2 times a day for one week,
- then once a day for one week,
- then every other day for one week, and
- finally, every 72 hours for 2 weeks.
Oral metronidazole should not be used beyond the first recurrence or for long-term therapy because of cumulative neurotoxicity, which can be irreversible.2
Management of patients whose CDI recurs after a long course of vancomycin is challenging. Oral rifaximin therapy (400 mg twice a day for 14 days), started immediately at the end of the oral vancomycin course, was shown to cure 7 of 8 patients with multiple relapses.26 Other potential treatment options are oral nitazoxanide, IV tigecycline, or IV immunoglobulin.
CASE You explain to Mary S that diagnostic tests are needed before you can determine whether she can safely take loperamide. When she comes in later that day, you collect a stool sample for C difficile antigen and toxin testing, and order a complete blood count and electrolyte panel.
The patient’s C difficile tests come back positive, her white blood cell count is <15,000 cells/mcL, and her creatinine level is ≤1.5 times her baseline, so you start her on oral metronidazole 500 mg every 8 hours for 14 days. (If the antigen assay had been positive and the toxin negative, you would have either repeated the test or treated Mary S empirically with metronidazole. If the initial antigen assay had been negative, you would have advised her to take the loperamide.)
You schedule a follow-up visit a day or 2 after starting therapy. If the patient is dehydrated or her symptoms have not improved by then, hospitalization may be required.
TABLE 2
Treatment recommendations for C difficile infection
| Clinical description | Clinical evidence | Recommended treatment |
|---|---|---|
| Initial episode (mild or moderate) | Leukocytosis with a white cell count <15,000 cells/mcL and creatinine <1.5 times premorbid level | Metronidazole (oral) 500 mg TID for 10-14 d |
| Initial episode (severe) | Leukocytosis with a white cell count ≥15,000 cells/mcL or creatinine ≥1.5 times premorbid level | Vancomycin (oral) 125 mg QID for 10-14 d |
| Initial episode (severe, complicated) | Hypotension or shock, ileus, megacolon | Vancomycin 500 mg QID (oral or by NG tube) plus metronidazole 500 mg (IV). If complete ileus, consider adding rectal instillation of vancomycin |
| First recurrence | Same as initial episode | |
| Second recurrence | Vancomycin in a tapered and/or pulsed regimen | |
| NG, nasogastric. Adapted from: Cohen SH, et al. Infect Control Hosp Epidemiol. 2010.2 | ||
CORRESPONDENCE
Richard R. Watkins, MD, MS, Division of Infectious Diseases, Akron General Medical Center, 224 West Exchange Street, Suite 290, Akron, OH 44302; [email protected]
• A C difficile diagnosis should be made by one of several widely available testing protocols, including a 2-step method using the common antigen assay to determine whether C difficile is present, followed by an enzyme immunoassay for toxins A and B to improve specificity. B
• Oral metronidazole should be used for initial treatment of mild to moderate C difficile infection, and oral vancomycin and possibly intravenous metronidazole for severe cases. A
• Metronidazole should not be used after an initial recurrence or for long-term therapy because of the risk of neurotoxicity. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Mary S, an 82-year-old patient you recently treated for bronchitis with a 3-day course of levofloxacin, calls your office complaining of diarrhea and abdominal cramps. She describes the diarrhea as nonbloody and particularly foul smelling and asks if she can take loperamide for her symptoms.
If Mary S were your patient, what would you tell her?
The incidence of Clostridium difficile infection (CDI) has been on the rise since 2000, when a common epidemic strain began circulating in North America.1 Although hospitalization or residency in a long-term care facility remains a classic risk factor for CDI, physicians in out-patient settings are increasingly likely to see patients with community-acquired CDI.
Recently updated guidelines from the Society for Health-care Epidemiology of America (SHEA) and the Infectious Diseases Society of America define CDI as the presence of diarrhea (≥3 unformed stools in 24 hours) and either a positive stool test for toxigenic C difficile or its toxins or colonoscopic or histopathologic findings demonstrating pseudomembranous colitis.2 That said, the clinical features of CDI are nonspecific and many patients do not fit the classic profile. So diagnosing CDI requires a high index of suspicion.
The text and tables that follow detail some surprising things about who is likely to develop CDI and which treatment options to employ (and, in some cases, avoid).
Is it CDI? Looking beyond the obvious
Antibiotic use and advanced age, like hospitalization, are classic risk factors for CDI.3 Diarrhea typically begins during or shortly after a course of antibiotics, but may develop as long as 8 weeks after treatment is completed. While any antibiotic, including metronidazole, can precipitate CDI, clindamycin, cephalosporins, extended-spectrum penicillins, and quinolones are most frequently implicated.4 Epidemiologic studies have suggested an association between gastric acid-reducing agents—primarily proton-pump inhibitors—and CDI.4-7 But this link remains controversial, as other investigations have not found a clear relationship.8
In addition to diarrhea, approximately 28% of patients with CDI develop a fever (as high as 104°F); 50% develop leukocytosis (up to 50,000 cells/mcL); and 22% develop abdominal pain, usually localized to the lower quadrants.9 These symptoms, however, are not specific to C difficile, and could be due to a different enteric pathogen, intra-abdominal sepsis, inflammatory bowel disease, or adverse effects of medication, among other causes.9
Markers for severe CDI include age >70 years, leukocyte count >20,000 cells/mcL, albumin level <2.5 g/dL, small-bowel obstruction or ileus, and a computed tomography (CT) scan showing colorectal inflammation.10 Severe CDI can lead to toxic megacolon, bowel perforation, sepsis, and even death.
In addition to considering CDI in patients with nonspecific symptoms, it is important to include it in the differential diagnosis of patients who do not fit the classic profile. In a recent study of patients with CDI at 4 Veterans Affairs facilities, almost half (49%) of those studied had no exposure to antimicrobial drugs. The researchers further found that the median age of patients with CDI was 61 years—younger than that found in previous studies—and that 20% of the cases were community-acquired.11
Consider CDI in children, too. Risk factors for CDI in pediatric patients include disruption of the normal microflora of the gastrointestinal tract, compromised immune status, poor diet, underlying health conditions, concurrent infections, and cancer.12
Diagnostic testing: Consider a 2-step assay
Patients with symptoms suggestive of CDI should undergo laboratory testing to confirm the diagnosis. TABLE 1 lists the tests that are widely available in the United States.3 Only liquid stools should be tested and just one sample should be sent to the lab, as multiple samples do not increase the diagnostic yield.13 In addition, tests should be used only for diagnosis, and not as a “test of cure.” This is because patients can shed C difficile toxin and spores for several weeks after completing treatment, and there are wide variations in the sensitivity of toxin assays.
Infants <1 year old have high rates of asymptomatic toxigenic strains of C difficile, and until 2008, recommendations from SHEA discouraged testing the stools of such young patients. Because of the difficulty in differentiating incidental colonization from true CDI in this patient population, the authors of a recent review suggested using more than one diagnostic approach when testing children <1 year of age.14
We advocate a 2-step assay—that is, testing for both glutamate dehydrogenase (GDH)—an antigen common to all strains of C difficile—and C difficile toxins A and B. The common antigen test is sensitive, but may detect carriers who do not have active disease. The enzyme immunoassay (EIA) for toxins A and B helps to improve specificity. Therefore, positive results of both tests would be considered a positive finding, negative results of both tests would be considered a negative finding, and one positive result with one negative result would require another test for toxin detection.3
The reverse-transcriptase polymerase chain reaction (RT-PCR) assay, which detects the toxin B gene of C difficile, is the newest test for CDI. The RT-PCR assay detects only toxigenic strains of C difficile, and all toxigenic strains produce toxin B, making it more specific than testing for the common antigen. The RT-PCR assay also has better sensitivity than the cytotoxin assay, which also tests for toxin B. The major limitation of the RT-PCR assay is the frequency of false-positive results in hospitalized patients with a high incidence of C difficile colonization.3
Routine laboratory studies, including a complete blood count with differential and a complete metabolic panel, are often useful to ascertain the presence and degree of leukocytosis, dehydration, and other metabolic abnormalities and to test for hypoalbuminemia. Fecal leukocytes can be seen in colitis and may be useful in select cases.
Imaging studies such as radiography, CT, and endoscopy have largely been superseded by lab testing for CDI. Plain radiographs are usually normal in patients with CDI, unless the patient has an ileus or toxic megacolon. CT is useful, however, in suspected cases of fulminant CDI or toxic megacolon, and may reveal colonic-wall thickening, pericolonic stranding, or ascites.9 Colonoscopy is preferred over sigmoidoscopy because up to one-third of patients with pseudomembranous colitis will have involvement of the right colon only.9 However, this test carries the risk of perforation in patients with fulminant colitis.
TABLE 1
Lab tests for C difficile infection
| Test | Substance detected | Time needed | Sensitivity | Specificity |
|---|---|---|---|---|
| Cytotoxin | Toxin B | 1-3 d | 95% | 90%-95% |
| Toxin culture | Toxigenic C difficile† | 3-5 d | >95% | 80%-90% |
| EIA toxin A or A/B | Toxin A or A/B | Hours | 75%-80% | 97%-98% |
| EIA GDH* | C difficile | Hours | 95%-100% | 70%-80% |
| EIA GDH* and toxin A/B | C difficile and C difficile toxin | Hours | 95%-100% | 97%-98% |
| RT-PCR | Toxigenic C difficile† | Hours | >98% | 80%-99% |
| *GDH is the common C difficile antigen. †All toxigenic strains produce toxin B. EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; RT-PCR, reverse-transcriptase polymerase chain reaction. Adapted from: Bartlett JG. Infect Control Hosp Epidemiol. 2010.3 | ||||
Treatment: What to consider, what to avoid
Of the 2 antibiotics most commonly used to treat CDI—metronidazole and vancomycin—only the latter has been approved by the US Food and Drug Administration for this indication. Nevertheless, metronidazole is generally recommended as first-line therapy and has the advantage of being much less expensive than vancomycin. However, an RCT found that oral vancomycin was superior to metronidazole in patients with severe disease.15 The time to resolution of diarrhea may be shorter with oral vancomycin than with metronidazole, as well.16
Recent guidelines suggest that clinicians consider 3 factors in deciding how to treat a first episode of CDI: the patient’s age, peak white blood cell count, and peak serum creatinine level.2 TABLE 2 presents an overview of treatment recommendations for both an initial episode of CDI and recurrences.
Treat severe CDI without delay. For patients with suspected severe CDI, treatment should be started empirically, without waiting for test results. Avoid antiperistaltic agents, which can obscure symptoms and precipitate toxic megacolon.2 Discontinue an antibiotic, if the patient is taking one, as soon as possible after the original infection has been adequately treated. If other infections need to be treated concurrently, we recommend that the course of treatment for CDI be extended until after the other antibiotic regimens have been stopped.
Avoid probiotics in this group. The use of probiotics, both for prevention and to help restore normal bowel flora in patients with CDI, has been advocated for many years. One RCT showed that a yogurt drink containing Lactobacillus and other bacteria reduced the risk of CDI in individuals ≥50 years of age who were taking antibiotics,17 but the guideline development panel recommended against using probiotics until larger trials have been completed.2
Probiotics are not without risk, and several cases of bacteremia have been reported.18,19 Immunocompromised patients appear to be at comparably higher risk, and probiotics should be avoided in this group. Numerous adjunctive agents, including intraluminal toxin binders, biotherapeutic agents, monoclonal antibodies, and a C difficile vaccine, are in various stages of development.2
How to handle recurrences
Relapse rates for CDI range from 6% to 25%,2 and affect patients who receive either vancomycin or metronidazole for the initial treatment. The mechanism relates to either relapse of the original infection or reinfection of susceptible patients with a new strain of C difficile.
Risk of relapse. Elderly patients treated with metronidazole seem to be particularly susceptible to CDI relapse.20 Other risk factors include the administration of non-C difficile antibiotics during or after treatment of CDI, a defective immune response against toxin A, glucocorticoid use, prior stroke, and concurrent use of a proton-pump inhibitor.21-25
TABLE 2 lists tapering and/or pulsed dosing of oral vancomycin as treatment for patients with a second recurrence. We often prescribe the following 6-week regimen, telling patients to take 125 mg vancomycin:
- 4 times a day for one week,
- then 2 times a day for one week,
- then once a day for one week,
- then every other day for one week, and
- finally, every 72 hours for 2 weeks.
Oral metronidazole should not be used beyond the first recurrence or for long-term therapy because of cumulative neurotoxicity, which can be irreversible.2
Management of patients whose CDI recurs after a long course of vancomycin is challenging. Oral rifaximin therapy (400 mg twice a day for 14 days), started immediately at the end of the oral vancomycin course, was shown to cure 7 of 8 patients with multiple relapses.26 Other potential treatment options are oral nitazoxanide, IV tigecycline, or IV immunoglobulin.
CASE You explain to Mary S that diagnostic tests are needed before you can determine whether she can safely take loperamide. When she comes in later that day, you collect a stool sample for C difficile antigen and toxin testing, and order a complete blood count and electrolyte panel.
The patient’s C difficile tests come back positive, her white blood cell count is <15,000 cells/mcL, and her creatinine level is ≤1.5 times her baseline, so you start her on oral metronidazole 500 mg every 8 hours for 14 days. (If the antigen assay had been positive and the toxin negative, you would have either repeated the test or treated Mary S empirically with metronidazole. If the initial antigen assay had been negative, you would have advised her to take the loperamide.)
You schedule a follow-up visit a day or 2 after starting therapy. If the patient is dehydrated or her symptoms have not improved by then, hospitalization may be required.
TABLE 2
Treatment recommendations for C difficile infection
| Clinical description | Clinical evidence | Recommended treatment |
|---|---|---|
| Initial episode (mild or moderate) | Leukocytosis with a white cell count <15,000 cells/mcL and creatinine <1.5 times premorbid level | Metronidazole (oral) 500 mg TID for 10-14 d |
| Initial episode (severe) | Leukocytosis with a white cell count ≥15,000 cells/mcL or creatinine ≥1.5 times premorbid level | Vancomycin (oral) 125 mg QID for 10-14 d |
| Initial episode (severe, complicated) | Hypotension or shock, ileus, megacolon | Vancomycin 500 mg QID (oral or by NG tube) plus metronidazole 500 mg (IV). If complete ileus, consider adding rectal instillation of vancomycin |
| First recurrence | Same as initial episode | |
| Second recurrence | Vancomycin in a tapered and/or pulsed regimen | |
| NG, nasogastric. Adapted from: Cohen SH, et al. Infect Control Hosp Epidemiol. 2010.2 | ||
CORRESPONDENCE
Richard R. Watkins, MD, MS, Division of Infectious Diseases, Akron General Medical Center, 224 West Exchange Street, Suite 290, Akron, OH 44302; [email protected]
1. Gerding DN. Global epidemiology of Clostridium difficile infection in 2010. Infect Control Hosp Epidemiol. 2010;31(suppl 1):S32-S34.
2. Cohen SH, Gerding DH, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
3. Bartlett JG. Detection of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2010;31(suppl 1):S35-S37.
4. Dial S, Alrasadi K, Manoukian C, et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171:33-38.
5. Dial S, Delaney JA, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989-2995.
6. Cunningham R, Dale B, Undy B, et al. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003;54:243-245.
7. Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784-790.
8. Shah S, Lewis A, Leopold D, et al. Gastric acid suppression does not promote clostridial diarrhoea in the elderly. QJM. 2000;93:175-181.
9. Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. 2008;46(suppl 1):S12-S18.
10. Henrich TJ, Krakower D, Bitton A, et al. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15:415-422.
11. Kutty PK, Woods CW, Sena AC, et al. Risk factors for and estimated incidence of community-associated Clostridium difficile infection, North Carolina, USA. Emerg Infect Dis. 2010;16:197-204.
12. Pituch H. Clostridium difficile is no longer just a nosocomial infection or an infection of adults. Int J Antimicrob Agents. 2009;33(suppl 1):S42-S45.
13. Mohan SS, McDermott BP, Parchuri S, et al. Lack of value of repeat stool testing for Clostridium difficile toxin. Am J Med. 2006;119:356.e7-e8.
14. Bryant K, McDonald LC. Clostridium difficile infections in children. Pediatr Infect Dis J. 2009;28:145-146.
15. Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
16. Belmares J, Gerding DN, Parada JP, et al. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect. 2007;55:495-501.
17. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80.-
18. Ledoux D, Labombardi VJ, Karter D. Lactobacillus acidophilus bacteraemia after use of a probiotic in a patient with AIDS and Hodgkin’s disease. Int J STD AIDS. 2006;17:280-282.
19. Hammerman C, Bin-Nun A, Kaplan M. Safety of probiotics: comparison of two popular strains. BMJ. 2006;333:1006-1008.
20. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590.
21. Nair S, Yadav D, Corpuz M, et al. Clostridium difficile colitis: factors influencing treatment failure and relapse—a prospective evaluation. Am J Gastroenterol. 1998;93:1873-1876.
22. Garey KW, Sethi S, Yadav Y, et al. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70:298-304.
23. Das R, Feuerstadt P, Brandt LJ. Glucocorticoids are associated with increased risk of short-term mortality in hospitalized patients with Clostridium difficile-associated disease. Am J Gastroenterol. 2010;105:2040-2049.
24. Cadena J, Thompson GR, 3rd, Patterson JE, et al. Clinical predictors and risk factors for relapsing Clostridium difficile infection. Am J Med Sci. 2010;339:350-355.
25. Linsky A, Gupta K, Lawler EV, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170:772-778.
26. Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007;44:846-848.
1. Gerding DN. Global epidemiology of Clostridium difficile infection in 2010. Infect Control Hosp Epidemiol. 2010;31(suppl 1):S32-S34.
2. Cohen SH, Gerding DH, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
3. Bartlett JG. Detection of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2010;31(suppl 1):S35-S37.
4. Dial S, Alrasadi K, Manoukian C, et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171:33-38.
5. Dial S, Delaney JA, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989-2995.
6. Cunningham R, Dale B, Undy B, et al. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003;54:243-245.
7. Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784-790.
8. Shah S, Lewis A, Leopold D, et al. Gastric acid suppression does not promote clostridial diarrhoea in the elderly. QJM. 2000;93:175-181.
9. Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. 2008;46(suppl 1):S12-S18.
10. Henrich TJ, Krakower D, Bitton A, et al. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15:415-422.
11. Kutty PK, Woods CW, Sena AC, et al. Risk factors for and estimated incidence of community-associated Clostridium difficile infection, North Carolina, USA. Emerg Infect Dis. 2010;16:197-204.
12. Pituch H. Clostridium difficile is no longer just a nosocomial infection or an infection of adults. Int J Antimicrob Agents. 2009;33(suppl 1):S42-S45.
13. Mohan SS, McDermott BP, Parchuri S, et al. Lack of value of repeat stool testing for Clostridium difficile toxin. Am J Med. 2006;119:356.e7-e8.
14. Bryant K, McDonald LC. Clostridium difficile infections in children. Pediatr Infect Dis J. 2009;28:145-146.
15. Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302-307.
16. Belmares J, Gerding DN, Parada JP, et al. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect. 2007;55:495-501.
17. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80.-
18. Ledoux D, Labombardi VJ, Karter D. Lactobacillus acidophilus bacteraemia after use of a probiotic in a patient with AIDS and Hodgkin’s disease. Int J STD AIDS. 2006;17:280-282.
19. Hammerman C, Bin-Nun A, Kaplan M. Safety of probiotics: comparison of two popular strains. BMJ. 2006;333:1006-1008.
20. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586-1590.
21. Nair S, Yadav D, Corpuz M, et al. Clostridium difficile colitis: factors influencing treatment failure and relapse—a prospective evaluation. Am J Gastroenterol. 1998;93:1873-1876.
22. Garey KW, Sethi S, Yadav Y, et al. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70:298-304.
23. Das R, Feuerstadt P, Brandt LJ. Glucocorticoids are associated with increased risk of short-term mortality in hospitalized patients with Clostridium difficile-associated disease. Am J Gastroenterol. 2010;105:2040-2049.
24. Cadena J, Thompson GR, 3rd, Patterson JE, et al. Clinical predictors and risk factors for relapsing Clostridium difficile infection. Am J Med Sci. 2010;339:350-355.
25. Linsky A, Gupta K, Lawler EV, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170:772-778.
26. Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007;44:846-848.
Hydroxyzine: Rational choice for inpatients with insomnia
Many physicians prescribe hypnotics for hospitalized patients with insomnia. Frequently used medications include temazepam, diphenhydramine, quetiapine, and trazodone. We have found hydroxyzine, 25 mg to 100 mg nightly, to be effective in adults and geriatric patients and feel it is a more rational choice.
Temazepam and other benzodiazepines may cause behavioral disinhibition, delirium (particularly in geriatric patients), and development of tolerance,1 which may lead to withdrawal symptoms after discharge. Diphenhydramine, quetiapine, and trazodone are effective as hypnotics through antihistaminergic mechanisms, but efficacy can be compromised by adverse effects mediated by non-histaminergic receptor activity. For example, at doses used for sleep, quetiapine can cause weight gain,2 extrapyramidal symptoms, and orthostasis. Trazodone also causes orthostasis and, infrequently, priapism. Because of its relatively high affinity for acetylcholine receptors, diphenhydramine can cause constipation and urinary retention, worsen cognitive function, and exacerbate delirium, particularly in geriatric patients.3
Hydroxyzine is a more selective anti-histamine than the aforementioned molecules,4 which leads to the sleep-promoting benefits of Hl-receptor blockade without significant alpha-1 adrenergic antagonism or anticholinergic side effects. The ratio of affinity for H1 receptors to cerebral acetylcholine receptors is more than 10 times greater for hydroxyzine than for diphenhydramine. Similarly, hydroxyzine has greater affinity for H1 receptors than alpha-1 adrenergic receptors, while trazodone has greater affinity for alpha-1 adrenergic receptors than for H1 receptors. Additionally, hydroxyzine does not lead to tolerance.5 Finally, it has a potential economic advantage over on-patent drugs such as quetiapine.
A disadvantage to hydroxyzine is its comparatively long half-life of 20 hours. Although this can lead to daytime sedation after nighttime dosing, we have not found this to be clinically significant for most patients.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Rosenberg RP. Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies. Ann Clin Psychiatry. 2006;18:49-56.
2. Cates ME, Jackson CW, Feldman JM, et al. Metabolic consequences of using low-dose quetiapine for insomnia in psychiatric patients. Community Ment Health J. 2009;45:251-254.
3. Agostini JV, Leo-Summers LS, Inouye SK. Cognitive and other adverse effects of diphenhydramine use in hospitalized older patients. Arch Intern Med. 2001;161:2091-2097.
4. Kubo N, Shirakawa O, Kuno T, et al. Antimuscarinic effects of antihistamines: quantitative evaluation by receptor-binding assay. Jpn J Pharmacol. 1987;43:277-282.
5. Ferreri M, Hantouche EG. Recent clinical trials of hydroxyzine in generalized anxiety disorder. Acta Psychiatr Scand Suppl. 1998;393:102-108.
Many physicians prescribe hypnotics for hospitalized patients with insomnia. Frequently used medications include temazepam, diphenhydramine, quetiapine, and trazodone. We have found hydroxyzine, 25 mg to 100 mg nightly, to be effective in adults and geriatric patients and feel it is a more rational choice.
Temazepam and other benzodiazepines may cause behavioral disinhibition, delirium (particularly in geriatric patients), and development of tolerance,1 which may lead to withdrawal symptoms after discharge. Diphenhydramine, quetiapine, and trazodone are effective as hypnotics through antihistaminergic mechanisms, but efficacy can be compromised by adverse effects mediated by non-histaminergic receptor activity. For example, at doses used for sleep, quetiapine can cause weight gain,2 extrapyramidal symptoms, and orthostasis. Trazodone also causes orthostasis and, infrequently, priapism. Because of its relatively high affinity for acetylcholine receptors, diphenhydramine can cause constipation and urinary retention, worsen cognitive function, and exacerbate delirium, particularly in geriatric patients.3
Hydroxyzine is a more selective anti-histamine than the aforementioned molecules,4 which leads to the sleep-promoting benefits of Hl-receptor blockade without significant alpha-1 adrenergic antagonism or anticholinergic side effects. The ratio of affinity for H1 receptors to cerebral acetylcholine receptors is more than 10 times greater for hydroxyzine than for diphenhydramine. Similarly, hydroxyzine has greater affinity for H1 receptors than alpha-1 adrenergic receptors, while trazodone has greater affinity for alpha-1 adrenergic receptors than for H1 receptors. Additionally, hydroxyzine does not lead to tolerance.5 Finally, it has a potential economic advantage over on-patent drugs such as quetiapine.
A disadvantage to hydroxyzine is its comparatively long half-life of 20 hours. Although this can lead to daytime sedation after nighttime dosing, we have not found this to be clinically significant for most patients.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Many physicians prescribe hypnotics for hospitalized patients with insomnia. Frequently used medications include temazepam, diphenhydramine, quetiapine, and trazodone. We have found hydroxyzine, 25 mg to 100 mg nightly, to be effective in adults and geriatric patients and feel it is a more rational choice.
Temazepam and other benzodiazepines may cause behavioral disinhibition, delirium (particularly in geriatric patients), and development of tolerance,1 which may lead to withdrawal symptoms after discharge. Diphenhydramine, quetiapine, and trazodone are effective as hypnotics through antihistaminergic mechanisms, but efficacy can be compromised by adverse effects mediated by non-histaminergic receptor activity. For example, at doses used for sleep, quetiapine can cause weight gain,2 extrapyramidal symptoms, and orthostasis. Trazodone also causes orthostasis and, infrequently, priapism. Because of its relatively high affinity for acetylcholine receptors, diphenhydramine can cause constipation and urinary retention, worsen cognitive function, and exacerbate delirium, particularly in geriatric patients.3
Hydroxyzine is a more selective anti-histamine than the aforementioned molecules,4 which leads to the sleep-promoting benefits of Hl-receptor blockade without significant alpha-1 adrenergic antagonism or anticholinergic side effects. The ratio of affinity for H1 receptors to cerebral acetylcholine receptors is more than 10 times greater for hydroxyzine than for diphenhydramine. Similarly, hydroxyzine has greater affinity for H1 receptors than alpha-1 adrenergic receptors, while trazodone has greater affinity for alpha-1 adrenergic receptors than for H1 receptors. Additionally, hydroxyzine does not lead to tolerance.5 Finally, it has a potential economic advantage over on-patent drugs such as quetiapine.
A disadvantage to hydroxyzine is its comparatively long half-life of 20 hours. Although this can lead to daytime sedation after nighttime dosing, we have not found this to be clinically significant for most patients.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Rosenberg RP. Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies. Ann Clin Psychiatry. 2006;18:49-56.
2. Cates ME, Jackson CW, Feldman JM, et al. Metabolic consequences of using low-dose quetiapine for insomnia in psychiatric patients. Community Ment Health J. 2009;45:251-254.
3. Agostini JV, Leo-Summers LS, Inouye SK. Cognitive and other adverse effects of diphenhydramine use in hospitalized older patients. Arch Intern Med. 2001;161:2091-2097.
4. Kubo N, Shirakawa O, Kuno T, et al. Antimuscarinic effects of antihistamines: quantitative evaluation by receptor-binding assay. Jpn J Pharmacol. 1987;43:277-282.
5. Ferreri M, Hantouche EG. Recent clinical trials of hydroxyzine in generalized anxiety disorder. Acta Psychiatr Scand Suppl. 1998;393:102-108.
1. Rosenberg RP. Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies. Ann Clin Psychiatry. 2006;18:49-56.
2. Cates ME, Jackson CW, Feldman JM, et al. Metabolic consequences of using low-dose quetiapine for insomnia in psychiatric patients. Community Ment Health J. 2009;45:251-254.
3. Agostini JV, Leo-Summers LS, Inouye SK. Cognitive and other adverse effects of diphenhydramine use in hospitalized older patients. Arch Intern Med. 2001;161:2091-2097.
4. Kubo N, Shirakawa O, Kuno T, et al. Antimuscarinic effects of antihistamines: quantitative evaluation by receptor-binding assay. Jpn J Pharmacol. 1987;43:277-282.
5. Ferreri M, Hantouche EG. Recent clinical trials of hydroxyzine in generalized anxiety disorder. Acta Psychiatr Scand Suppl. 1998;393:102-108.
Possession obsession: Help hoarders escape their own prisons
The popularity of TV shows such as A&E’s Hoarders,TLC’s Hoarding: buried alive,and Planet Green’s Gutted has increased public awareness of problems caused by hoarding. Seventy-five communities across the United States have coordinated hoarding task forces that include therapists, social workers, police, fire departments, and child protective services, and that number is rising quickly.1 Therefore, psychiatrists likely will be encountering growing numbers of hoarders and their families seeking treatment.
Hoarding behavior
Affecting 2% to 5% of the population, compulsive hoarding is persistent difficulty parting with possessions—even useless items or those of limited value—that results in cluttered personal surroundings and impaired functioning.2 Hoarders may be threatened with eviction or the possibility of losing custody of their children.1 Hoarding can create fire hazards, fall risks, and unsanitary environments.3
Causes
Hoarding behavior usually is a result of fear of losing items that may be needed later or making the “wrong” decision about what should be kept or discarded.4Symptoms generally start at age 12 or 13, begin interfering with daily functioning in the mid-30s, and increase in severity with age.2 Clutter can accumulate with or without excessive acquisition of goods, through buying, collecting, or stealing.2Hoarding often is ego-syntonic, and many patients do not believe their behaviors are problematic.3
A new diagnosis?
Hoarding often is associated with obsessive-compulsive disorder (OCD), and DSM-IV-TR lists hoarding behavior as a criterion for obsessive-compulsive personality disorder. However, hoarding behavior has been observed in other neuropsychiatric disorders, including schizophrenia, depression, social phobia, dementia, eating disorders, brain injury, and mental retardation, and in nonclinical populations.3,4
Most research has focused on the connections between hoarding and OCD, but genetics, brain lesions, neuroimaging, and neuropsychological studies suggest that “hoarding disorder” should be a separate entity in DSM-5.2A proposed set of diagnostic criteria states that the hoarding behavior not be restricted to the symptoms of another mental disorder.2 For example, the behavior is not caused by intrusive or recurrent thoughts from OCD or secondary to apathy in depression. The proposed criteria specify if the hoarding is associated with excessive acquisition of items, as well as how much insight the patient has into his or her problem.2
Treatment
Because hoarding behavior frequently is ego-syntonic, patients may be brought in by family members or friends. Treatment should begin with a thorough neuropsychiatric evaluation to determine the etiology of the behavior, such as another axis I disorder.4Assess the amount of clutter, types of items saved, usability of living and work spaces, potential health and safety hazards, beliefs about possessions, information processing deficits, avoidance behaviors, insight, motivation for treatment, social and occupational functioning, and activities of daily living.4
Studies of selective serotonin reuptake inhibitors and cognitive-behavioral therapy (CBT) in OCD patients with hoarding symptoms have produced mixed results.4 In some cases, paroxetine monotherapy, specialized CBT protocols, and combined treatments have proven effective.4 Studies examining those treatments’ efficacy for hoarding in the absence of OCD are underway. Whichever strategy is employed, it is important to involve family members or friends in treatment2 and to identify other available resources, such as community hoarding task forces.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Webley K. Cleaning house. How community task forces are dealing with hoarding one pile of junk at a time. Time. 2010;176:43-44.
2. Mataix-Cols D, Frost R, Pertussa A, et al. Hoarding disorder: a new diagnosis for DSM-V? Depress Anxiety. 2010;27:556-572.
3. Steketee G, Frost R. Compulsive hoarding: current status of the research. Clin Psychol Rev. 2003;23:905-927.
4. Saxena S. Neurobiology and treatment of compulsive hoarding. CNS Spectr. 2008;13:29-36.
The popularity of TV shows such as A&E’s Hoarders,TLC’s Hoarding: buried alive,and Planet Green’s Gutted has increased public awareness of problems caused by hoarding. Seventy-five communities across the United States have coordinated hoarding task forces that include therapists, social workers, police, fire departments, and child protective services, and that number is rising quickly.1 Therefore, psychiatrists likely will be encountering growing numbers of hoarders and their families seeking treatment.
Hoarding behavior
Affecting 2% to 5% of the population, compulsive hoarding is persistent difficulty parting with possessions—even useless items or those of limited value—that results in cluttered personal surroundings and impaired functioning.2 Hoarders may be threatened with eviction or the possibility of losing custody of their children.1 Hoarding can create fire hazards, fall risks, and unsanitary environments.3
Causes
Hoarding behavior usually is a result of fear of losing items that may be needed later or making the “wrong” decision about what should be kept or discarded.4Symptoms generally start at age 12 or 13, begin interfering with daily functioning in the mid-30s, and increase in severity with age.2 Clutter can accumulate with or without excessive acquisition of goods, through buying, collecting, or stealing.2Hoarding often is ego-syntonic, and many patients do not believe their behaviors are problematic.3
A new diagnosis?
Hoarding often is associated with obsessive-compulsive disorder (OCD), and DSM-IV-TR lists hoarding behavior as a criterion for obsessive-compulsive personality disorder. However, hoarding behavior has been observed in other neuropsychiatric disorders, including schizophrenia, depression, social phobia, dementia, eating disorders, brain injury, and mental retardation, and in nonclinical populations.3,4
Most research has focused on the connections between hoarding and OCD, but genetics, brain lesions, neuroimaging, and neuropsychological studies suggest that “hoarding disorder” should be a separate entity in DSM-5.2A proposed set of diagnostic criteria states that the hoarding behavior not be restricted to the symptoms of another mental disorder.2 For example, the behavior is not caused by intrusive or recurrent thoughts from OCD or secondary to apathy in depression. The proposed criteria specify if the hoarding is associated with excessive acquisition of items, as well as how much insight the patient has into his or her problem.2
Treatment
Because hoarding behavior frequently is ego-syntonic, patients may be brought in by family members or friends. Treatment should begin with a thorough neuropsychiatric evaluation to determine the etiology of the behavior, such as another axis I disorder.4Assess the amount of clutter, types of items saved, usability of living and work spaces, potential health and safety hazards, beliefs about possessions, information processing deficits, avoidance behaviors, insight, motivation for treatment, social and occupational functioning, and activities of daily living.4
Studies of selective serotonin reuptake inhibitors and cognitive-behavioral therapy (CBT) in OCD patients with hoarding symptoms have produced mixed results.4 In some cases, paroxetine monotherapy, specialized CBT protocols, and combined treatments have proven effective.4 Studies examining those treatments’ efficacy for hoarding in the absence of OCD are underway. Whichever strategy is employed, it is important to involve family members or friends in treatment2 and to identify other available resources, such as community hoarding task forces.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
The popularity of TV shows such as A&E’s Hoarders,TLC’s Hoarding: buried alive,and Planet Green’s Gutted has increased public awareness of problems caused by hoarding. Seventy-five communities across the United States have coordinated hoarding task forces that include therapists, social workers, police, fire departments, and child protective services, and that number is rising quickly.1 Therefore, psychiatrists likely will be encountering growing numbers of hoarders and their families seeking treatment.
Hoarding behavior
Affecting 2% to 5% of the population, compulsive hoarding is persistent difficulty parting with possessions—even useless items or those of limited value—that results in cluttered personal surroundings and impaired functioning.2 Hoarders may be threatened with eviction or the possibility of losing custody of their children.1 Hoarding can create fire hazards, fall risks, and unsanitary environments.3
Causes
Hoarding behavior usually is a result of fear of losing items that may be needed later or making the “wrong” decision about what should be kept or discarded.4Symptoms generally start at age 12 or 13, begin interfering with daily functioning in the mid-30s, and increase in severity with age.2 Clutter can accumulate with or without excessive acquisition of goods, through buying, collecting, or stealing.2Hoarding often is ego-syntonic, and many patients do not believe their behaviors are problematic.3
A new diagnosis?
Hoarding often is associated with obsessive-compulsive disorder (OCD), and DSM-IV-TR lists hoarding behavior as a criterion for obsessive-compulsive personality disorder. However, hoarding behavior has been observed in other neuropsychiatric disorders, including schizophrenia, depression, social phobia, dementia, eating disorders, brain injury, and mental retardation, and in nonclinical populations.3,4
Most research has focused on the connections between hoarding and OCD, but genetics, brain lesions, neuroimaging, and neuropsychological studies suggest that “hoarding disorder” should be a separate entity in DSM-5.2A proposed set of diagnostic criteria states that the hoarding behavior not be restricted to the symptoms of another mental disorder.2 For example, the behavior is not caused by intrusive or recurrent thoughts from OCD or secondary to apathy in depression. The proposed criteria specify if the hoarding is associated with excessive acquisition of items, as well as how much insight the patient has into his or her problem.2
Treatment
Because hoarding behavior frequently is ego-syntonic, patients may be brought in by family members or friends. Treatment should begin with a thorough neuropsychiatric evaluation to determine the etiology of the behavior, such as another axis I disorder.4Assess the amount of clutter, types of items saved, usability of living and work spaces, potential health and safety hazards, beliefs about possessions, information processing deficits, avoidance behaviors, insight, motivation for treatment, social and occupational functioning, and activities of daily living.4
Studies of selective serotonin reuptake inhibitors and cognitive-behavioral therapy (CBT) in OCD patients with hoarding symptoms have produced mixed results.4 In some cases, paroxetine monotherapy, specialized CBT protocols, and combined treatments have proven effective.4 Studies examining those treatments’ efficacy for hoarding in the absence of OCD are underway. Whichever strategy is employed, it is important to involve family members or friends in treatment2 and to identify other available resources, such as community hoarding task forces.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Webley K. Cleaning house. How community task forces are dealing with hoarding one pile of junk at a time. Time. 2010;176:43-44.
2. Mataix-Cols D, Frost R, Pertussa A, et al. Hoarding disorder: a new diagnosis for DSM-V? Depress Anxiety. 2010;27:556-572.
3. Steketee G, Frost R. Compulsive hoarding: current status of the research. Clin Psychol Rev. 2003;23:905-927.
4. Saxena S. Neurobiology and treatment of compulsive hoarding. CNS Spectr. 2008;13:29-36.
1. Webley K. Cleaning house. How community task forces are dealing with hoarding one pile of junk at a time. Time. 2010;176:43-44.
2. Mataix-Cols D, Frost R, Pertussa A, et al. Hoarding disorder: a new diagnosis for DSM-V? Depress Anxiety. 2010;27:556-572.
3. Steketee G, Frost R. Compulsive hoarding: current status of the research. Clin Psychol Rev. 2003;23:905-927.
4. Saxena S. Neurobiology and treatment of compulsive hoarding. CNS Spectr. 2008;13:29-36.
‘Boxed in’ or ‘boxed out’? Prescribing atypicals for dementia
Dear Dr. Mossman:
Some of my older patients with dementia develop severe behavioral disturbances, and when other treatments don’t work, I sometimes use second-generation antipsychotics (SGAs) to help them cope better. But I worry about the liability I might face because of the “black-box” warning about prescribing SGAs to these patients. How can I minimize the legal risks of doing this?—Submitted by “Dr. K”
“Black-box” warning. The phrase sounds scary, and it’s meant to frighten you—or at least get your attention.
However, the FDA has put boxed warnings on all antidepressants and many other psychotropic drugs. This doesn’t mean you should quit practicing psychopharmacology. Instead, the FDA just wants you to hesitate and be careful when you prescribe certain drugs in certain situations. One such situation is using SGAs to address behavioral problems that often occur in older persons with dementia.
When it comes to prescribing SGAs for patients with dementia, you can respond to your fear of the “black box” with something that isn’t scary at all: doing what’s best for your older patient. In this article, we’ll explain how, as we cover:
- the scope of the clinical problem
- what a “black-box” warning is
- the significance of the boxed warning for SGA use for dementia-related behavioral disturbances
- how to minimize medicolegal liability when prescribing SGAs.
Aging boomers
As the “baby boom” generation enters its 7th and 8th decades, psychiatrists should expect to treat many older individuals who have dementia and behavioral problems. In the United States, approximately 4 million individuals age >60 have dementia,1 and this number will rise rapidly in the next few years.2 Rates of dementia-related agitation and aggression range from 20% to 80%.3,4 Such behavior—always distressing to patients, family members, and caregivers—can lead to physical injuries, increased caregiver burden, premature institutionalization, physical restraint, and over-medication.
No medication has received FDA approval for treatment of dementia-related agitation. Currently, doctors try a variety of medications, such as memantine, cholinesterase inhibitors, anticonvulsants, and selective serotonin reuptake inhibitors.5 Nearly one-third of nursing home residents with dementia receive antipsychotic drugs.6 Thus, despite the “black-box” warning, SGAs commonly are prescribed to cognitively impaired older persons for behavioral agitation and/or psychosis.
What’s a ‘black-box’ warning?
Almost every prescription drug has dozens of possible adverse effects. “Black-box” warning is a colloquialism that refers to the FDA’s format for describing particularly important potential complications or precautions necessary when prescribing a drug. (For the official definition of a “boxed warning, “ see Box ).7
Certain contraindications or serious warnings, particularly those that may lead to death or serious injury, may be required by the FDA to be presented in a box on the drug’s prescribing information. The box must contain, in uppercase letters, a heading inside the box that includes the word “WARNING” and conveys the general focus of the information in the box. The box must briefly explain the risk and refer to more detailed information in the “Contraindications” or “Warnings and Precautions” section for more detailed information.
Source: Reference 7
Understanding the warning
In April 2005, the FDA mandated a boxed warning for SGAs after placebo-controlled studies showed a significantly higher death rate—mostly from cardiovascular accidents or infections—in geriatric patients who received SGA treatment for dementia-related psychoses.8 The warning does not forbid you from using SGAs when treating older patients with dementia—but you must think carefully about this off-label treatment (ie, prescribing SGAs for an indication that is not FDA-approved).
In patients with dementia, medical conditions may be expressed as behavioral problems that should be addressed with behavioral therapies or appropriate medical therapy ( Table 1 ).9,10 You can feel better about starting SGA therapy if a thorough medical, cognitive, and functional workup has ruled out nonpsychiatric reasons for disruptive behavior.9,11,12 The workup should look for cardiovascular, cerebrovascular, pulmonary, and metabolic risk factors, along with medication side effects.
If medical and situational problems are ruled out, or if aggressive, assaultive, or disruptive behavior threatens the physical safety of patients or others, careful consideration of therapeutic alternatives may show that SGAs are the best treatment choice. Once this decision is reached, clinicians can minimize legal liabilities in several ways.
Table 1
Questions to ask before starting SGAs for dementia-related behavioral problems
| Questions | Comments |
|---|---|
| Is the behavior dangerous? | Nonviolent behavior (eg, foul language, inappropriate voiding, hoarding, or refusing to bathe) can be addressed with nonpharmacologic interventions |
| What about treatable medical problems? | A demented person’s behavioral outbursts may stem from pain (ingrown toenail, acid reflux), misinterpretations caused by hearing or vision problems, delirium from infections or drug interactions, etc |
| Would a nonpharmacologic approach work? | Possibilities include eliminating environmental stressors, increasing interpersonal attention, more frequent reorientation, or music or art therapy |
| SGAs: second-generation antipsychotics | |
| Source: References 9,10 | |
Informed consent: A process
Informed consent is an essential feature of most medical treatment ( Table 2 ). 13 Informed consent is especially important when—as with using SGAs for behavioral disturbances in dementia—you want to prescribe a drug off-label in a context for which the FDA has required a boxed warning.
Patients in early stages of dementia may retain their decision-making capacity and ability to give informed consent.14 If the opportunity presents itself, this is an ideal time to discuss the possible future need for SGAs and to make sure the patient has designated a proxy decision-maker who can make treatment choices if the patient loses capacity. If a patient’s decision-making capacity is questionable, obtain consent from a surrogate (often a relative).
Informed consent is a process, not a printed form. It involves taking time to be sure that the patient or surrogate decision-maker understands and accepts the risks associated with a proposed treatment. Thinking of informed consent as a process facilitates communication, acceptance of treatment, and trust between prescribers and recipients of care.
Table 2
Essentials of informed consent
| Elements | Patient has the capacity to consent, has received adequate information about the proposed treatment, and has not been coerced |
| Information to disclose about the proposed treatment | Expected benefits, risks (common and serious side effects), alternatives, and expected outcomes of treatment and no treatment |
| Source: Reference 13 | |
Involving family
When appropriate, include a patient’s family in informed consent and treatment planning processes. Providing written material, such as Treatment of dementia and agitation: a guide for families and caregivers, 15 can help educate persons whose loved ones suffer from dementia. These resources often improve care and build relationships with family members that sustain treatment alliances when adverse outcomes occur. Also, well-engaged and informed families are less likely to initiate malpractice lawsuits when adverse events occur.16
Monitor for side effects
Older patients are especially vulnerable to physical harm during agitated or aggressive behavior, but they’re also quite vulnerable to medication side effects. Before starting SGAs, note the patient’s alertness, activities of daily living, movement abnormalities, and EKG abnormalities. Knowing this “baseline” helps you assess the effects of medication and monitor for side effects. Brief assessment scales—such as the Montreal Cognitive Assessment Test,17 the Abnormal Involuntary Movement Scale,18 and the Instrumental Activities of Daily Living Scale19 —can help you quantify baseline functioning, monitor symptom response, and detect adverse effects.
For patients receiving SGA therapy, reassess benefits and risks at least every 3 months, and preferably more often.11 In geriatric patients, titrate dosages slowly, maintain medications at the lowest effective levels, and discontinue them once they are no longer necessary. When doubt arises about the effectiveness of SGA therapy, stop the drug.12
Remember to document
Because older patients have high rates of medical problems and medication side effects, negative outcomes always are a risk. Good documentation is a key risk management strategy that can help if a bad outcome requires you to defend your treatment plan in court ( Table 3 ).2 ”
Table 3
What to document
| Your reasons for starting treatment with SGAs |
| Other treatments that were tried unsuccessfully |
| Other treatments that were considered but deemed inappropriate |
| The patient’s baseline medical, physical, cognitive, and functional status, including consideration of cardiovascular, cerebrovascular, pulmonary, and metabolic risk factors |
| How and when you will monitor side effects |
| For a patient who resides outside a nursing home, send a letter to the primary care provider stating that the patient is on SGA therapy and requesting assistance in monitoring for medical problems |
| For a patient who resides in a nursing home, describe the monitoring system for identifying adverse events (eg, regular EKGs, pulmonary exams, and lab tests) |
| Collect and keep copies of literature supporting your treatment choice |
| SGAs: second-generation antipsychotics |
| Source: Adapted from reference 20 |
Drug Brand Name
- Memantine • Namenda
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Plassman BL, Langa KM, Fischer GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125-132.
2. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112-2117.
3. Tractenberg RE, Weiner MF, Patterson MB, et al. Comorbidity of psychopathological domains in community-dwelling persons with Alzheimer‘s disease. J Geriatr Psychiatry Neurol. 2003;16:94-99.
4. Ryu SH, Katona C, Rive B, et al. Persistence of and changes in neuropsychiatric symptoms in Alzheimer’s disease over 6 months: the LASER-AD study. Am J Geriatr Psychiatry. 2005;13:976-983.
5. Ballard C, Corbett A, Chitramohan R, et al. Management of agitation and aggression associated with Alzheimer’s disease: controversies and possible solutions. Curr Opin Psychiatry. 2009;22:532-540.
6. Kamble P, Chen H, Sherer JT, et al. Use of antipsychotics among elderly nursing home residents with dementia in the US: an analysis of National Survey Data. Drugs Aging. 2009;26:483-492.
7. 21 CFR § 201.57(c)(1). Specific requirements on content and format of labeling for human prescription drugs. Available at: http://edocket.access.gpo.gov/cfr_2006/aprqtr/pdf/21cfr201.57.pdf. Accessed January 24 2011.
8. Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. Available at: this link. Accessed January 2 2011.
9. Salzman C, Jeste DV, Meyer RE. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options clinical trials, methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
10. Dewing J. Responding to agitation in people with dementia. Nursing Older People. 2010;22:18-25.
11. American Psychiatric Association. Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Available at: http://www.psychiatryonline.com/pracGuide/pracGuideChapToc_3.aspx. Accessed January 3 2011.
12. Hermann N, Lanctot K. Atypical antipsychotics for neuropsychiatric symptoms of dementia. Malignant or maligned? Drug Safety. 2006;29:833-843.
13. Bernat JL. Informed consent. Muscle Nerve. 2001;24:614-621.
14. Fellows L. Competency and consent in dementia. J Am Geriatr Soc. 1998;46:922-926.
15. Treatmentof dementia and agitation: a guide for families and caregivers J Psychiatr Practice. 2007;13:207-216.
16. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication. The relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
17. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
18. Munetz MR, Benjamin S. How to examine patients using the abnormal involuntary movement scale. Hosp Comm Psychiatry. 1988;39:1172-1177.
19. Mathuranath PS, George A, Cherian PJ, et al. Instrumental activities of daily living scale for dementia screening in elderly people. Int Psychogeriatr. 2005;17:461-474.
20. Recupero PR, Rainey SE. Managing risk when considering the use of atypical antipsychotics for elderly patients with dementia-related psychosis. J Psychiatr Practice. 2007;13:143-152.
Dear Dr. Mossman:
Some of my older patients with dementia develop severe behavioral disturbances, and when other treatments don’t work, I sometimes use second-generation antipsychotics (SGAs) to help them cope better. But I worry about the liability I might face because of the “black-box” warning about prescribing SGAs to these patients. How can I minimize the legal risks of doing this?—Submitted by “Dr. K”
“Black-box” warning. The phrase sounds scary, and it’s meant to frighten you—or at least get your attention.
However, the FDA has put boxed warnings on all antidepressants and many other psychotropic drugs. This doesn’t mean you should quit practicing psychopharmacology. Instead, the FDA just wants you to hesitate and be careful when you prescribe certain drugs in certain situations. One such situation is using SGAs to address behavioral problems that often occur in older persons with dementia.
When it comes to prescribing SGAs for patients with dementia, you can respond to your fear of the “black box” with something that isn’t scary at all: doing what’s best for your older patient. In this article, we’ll explain how, as we cover:
- the scope of the clinical problem
- what a “black-box” warning is
- the significance of the boxed warning for SGA use for dementia-related behavioral disturbances
- how to minimize medicolegal liability when prescribing SGAs.
Aging boomers
As the “baby boom” generation enters its 7th and 8th decades, psychiatrists should expect to treat many older individuals who have dementia and behavioral problems. In the United States, approximately 4 million individuals age >60 have dementia,1 and this number will rise rapidly in the next few years.2 Rates of dementia-related agitation and aggression range from 20% to 80%.3,4 Such behavior—always distressing to patients, family members, and caregivers—can lead to physical injuries, increased caregiver burden, premature institutionalization, physical restraint, and over-medication.
No medication has received FDA approval for treatment of dementia-related agitation. Currently, doctors try a variety of medications, such as memantine, cholinesterase inhibitors, anticonvulsants, and selective serotonin reuptake inhibitors.5 Nearly one-third of nursing home residents with dementia receive antipsychotic drugs.6 Thus, despite the “black-box” warning, SGAs commonly are prescribed to cognitively impaired older persons for behavioral agitation and/or psychosis.
What’s a ‘black-box’ warning?
Almost every prescription drug has dozens of possible adverse effects. “Black-box” warning is a colloquialism that refers to the FDA’s format for describing particularly important potential complications or precautions necessary when prescribing a drug. (For the official definition of a “boxed warning, “ see Box ).7
Certain contraindications or serious warnings, particularly those that may lead to death or serious injury, may be required by the FDA to be presented in a box on the drug’s prescribing information. The box must contain, in uppercase letters, a heading inside the box that includes the word “WARNING” and conveys the general focus of the information in the box. The box must briefly explain the risk and refer to more detailed information in the “Contraindications” or “Warnings and Precautions” section for more detailed information.
Source: Reference 7
Understanding the warning
In April 2005, the FDA mandated a boxed warning for SGAs after placebo-controlled studies showed a significantly higher death rate—mostly from cardiovascular accidents or infections—in geriatric patients who received SGA treatment for dementia-related psychoses.8 The warning does not forbid you from using SGAs when treating older patients with dementia—but you must think carefully about this off-label treatment (ie, prescribing SGAs for an indication that is not FDA-approved).
In patients with dementia, medical conditions may be expressed as behavioral problems that should be addressed with behavioral therapies or appropriate medical therapy ( Table 1 ).9,10 You can feel better about starting SGA therapy if a thorough medical, cognitive, and functional workup has ruled out nonpsychiatric reasons for disruptive behavior.9,11,12 The workup should look for cardiovascular, cerebrovascular, pulmonary, and metabolic risk factors, along with medication side effects.
If medical and situational problems are ruled out, or if aggressive, assaultive, or disruptive behavior threatens the physical safety of patients or others, careful consideration of therapeutic alternatives may show that SGAs are the best treatment choice. Once this decision is reached, clinicians can minimize legal liabilities in several ways.
Table 1
Questions to ask before starting SGAs for dementia-related behavioral problems
| Questions | Comments |
|---|---|
| Is the behavior dangerous? | Nonviolent behavior (eg, foul language, inappropriate voiding, hoarding, or refusing to bathe) can be addressed with nonpharmacologic interventions |
| What about treatable medical problems? | A demented person’s behavioral outbursts may stem from pain (ingrown toenail, acid reflux), misinterpretations caused by hearing or vision problems, delirium from infections or drug interactions, etc |
| Would a nonpharmacologic approach work? | Possibilities include eliminating environmental stressors, increasing interpersonal attention, more frequent reorientation, or music or art therapy |
| SGAs: second-generation antipsychotics | |
| Source: References 9,10 | |
Informed consent: A process
Informed consent is an essential feature of most medical treatment ( Table 2 ). 13 Informed consent is especially important when—as with using SGAs for behavioral disturbances in dementia—you want to prescribe a drug off-label in a context for which the FDA has required a boxed warning.
Patients in early stages of dementia may retain their decision-making capacity and ability to give informed consent.14 If the opportunity presents itself, this is an ideal time to discuss the possible future need for SGAs and to make sure the patient has designated a proxy decision-maker who can make treatment choices if the patient loses capacity. If a patient’s decision-making capacity is questionable, obtain consent from a surrogate (often a relative).
Informed consent is a process, not a printed form. It involves taking time to be sure that the patient or surrogate decision-maker understands and accepts the risks associated with a proposed treatment. Thinking of informed consent as a process facilitates communication, acceptance of treatment, and trust between prescribers and recipients of care.
Table 2
Essentials of informed consent
| Elements | Patient has the capacity to consent, has received adequate information about the proposed treatment, and has not been coerced |
| Information to disclose about the proposed treatment | Expected benefits, risks (common and serious side effects), alternatives, and expected outcomes of treatment and no treatment |
| Source: Reference 13 | |
Involving family
When appropriate, include a patient’s family in informed consent and treatment planning processes. Providing written material, such as Treatment of dementia and agitation: a guide for families and caregivers, 15 can help educate persons whose loved ones suffer from dementia. These resources often improve care and build relationships with family members that sustain treatment alliances when adverse outcomes occur. Also, well-engaged and informed families are less likely to initiate malpractice lawsuits when adverse events occur.16
Monitor for side effects
Older patients are especially vulnerable to physical harm during agitated or aggressive behavior, but they’re also quite vulnerable to medication side effects. Before starting SGAs, note the patient’s alertness, activities of daily living, movement abnormalities, and EKG abnormalities. Knowing this “baseline” helps you assess the effects of medication and monitor for side effects. Brief assessment scales—such as the Montreal Cognitive Assessment Test,17 the Abnormal Involuntary Movement Scale,18 and the Instrumental Activities of Daily Living Scale19 —can help you quantify baseline functioning, monitor symptom response, and detect adverse effects.
For patients receiving SGA therapy, reassess benefits and risks at least every 3 months, and preferably more often.11 In geriatric patients, titrate dosages slowly, maintain medications at the lowest effective levels, and discontinue them once they are no longer necessary. When doubt arises about the effectiveness of SGA therapy, stop the drug.12
Remember to document
Because older patients have high rates of medical problems and medication side effects, negative outcomes always are a risk. Good documentation is a key risk management strategy that can help if a bad outcome requires you to defend your treatment plan in court ( Table 3 ).2 ”
Table 3
What to document
| Your reasons for starting treatment with SGAs |
| Other treatments that were tried unsuccessfully |
| Other treatments that were considered but deemed inappropriate |
| The patient’s baseline medical, physical, cognitive, and functional status, including consideration of cardiovascular, cerebrovascular, pulmonary, and metabolic risk factors |
| How and when you will monitor side effects |
| For a patient who resides outside a nursing home, send a letter to the primary care provider stating that the patient is on SGA therapy and requesting assistance in monitoring for medical problems |
| For a patient who resides in a nursing home, describe the monitoring system for identifying adverse events (eg, regular EKGs, pulmonary exams, and lab tests) |
| Collect and keep copies of literature supporting your treatment choice |
| SGAs: second-generation antipsychotics |
| Source: Adapted from reference 20 |
Drug Brand Name
- Memantine • Namenda
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dear Dr. Mossman:
Some of my older patients with dementia develop severe behavioral disturbances, and when other treatments don’t work, I sometimes use second-generation antipsychotics (SGAs) to help them cope better. But I worry about the liability I might face because of the “black-box” warning about prescribing SGAs to these patients. How can I minimize the legal risks of doing this?—Submitted by “Dr. K”
“Black-box” warning. The phrase sounds scary, and it’s meant to frighten you—or at least get your attention.
However, the FDA has put boxed warnings on all antidepressants and many other psychotropic drugs. This doesn’t mean you should quit practicing psychopharmacology. Instead, the FDA just wants you to hesitate and be careful when you prescribe certain drugs in certain situations. One such situation is using SGAs to address behavioral problems that often occur in older persons with dementia.
When it comes to prescribing SGAs for patients with dementia, you can respond to your fear of the “black box” with something that isn’t scary at all: doing what’s best for your older patient. In this article, we’ll explain how, as we cover:
- the scope of the clinical problem
- what a “black-box” warning is
- the significance of the boxed warning for SGA use for dementia-related behavioral disturbances
- how to minimize medicolegal liability when prescribing SGAs.
Aging boomers
As the “baby boom” generation enters its 7th and 8th decades, psychiatrists should expect to treat many older individuals who have dementia and behavioral problems. In the United States, approximately 4 million individuals age >60 have dementia,1 and this number will rise rapidly in the next few years.2 Rates of dementia-related agitation and aggression range from 20% to 80%.3,4 Such behavior—always distressing to patients, family members, and caregivers—can lead to physical injuries, increased caregiver burden, premature institutionalization, physical restraint, and over-medication.
No medication has received FDA approval for treatment of dementia-related agitation. Currently, doctors try a variety of medications, such as memantine, cholinesterase inhibitors, anticonvulsants, and selective serotonin reuptake inhibitors.5 Nearly one-third of nursing home residents with dementia receive antipsychotic drugs.6 Thus, despite the “black-box” warning, SGAs commonly are prescribed to cognitively impaired older persons for behavioral agitation and/or psychosis.
What’s a ‘black-box’ warning?
Almost every prescription drug has dozens of possible adverse effects. “Black-box” warning is a colloquialism that refers to the FDA’s format for describing particularly important potential complications or precautions necessary when prescribing a drug. (For the official definition of a “boxed warning, “ see Box ).7
Certain contraindications or serious warnings, particularly those that may lead to death or serious injury, may be required by the FDA to be presented in a box on the drug’s prescribing information. The box must contain, in uppercase letters, a heading inside the box that includes the word “WARNING” and conveys the general focus of the information in the box. The box must briefly explain the risk and refer to more detailed information in the “Contraindications” or “Warnings and Precautions” section for more detailed information.
Source: Reference 7
Understanding the warning
In April 2005, the FDA mandated a boxed warning for SGAs after placebo-controlled studies showed a significantly higher death rate—mostly from cardiovascular accidents or infections—in geriatric patients who received SGA treatment for dementia-related psychoses.8 The warning does not forbid you from using SGAs when treating older patients with dementia—but you must think carefully about this off-label treatment (ie, prescribing SGAs for an indication that is not FDA-approved).
In patients with dementia, medical conditions may be expressed as behavioral problems that should be addressed with behavioral therapies or appropriate medical therapy ( Table 1 ).9,10 You can feel better about starting SGA therapy if a thorough medical, cognitive, and functional workup has ruled out nonpsychiatric reasons for disruptive behavior.9,11,12 The workup should look for cardiovascular, cerebrovascular, pulmonary, and metabolic risk factors, along with medication side effects.
If medical and situational problems are ruled out, or if aggressive, assaultive, or disruptive behavior threatens the physical safety of patients or others, careful consideration of therapeutic alternatives may show that SGAs are the best treatment choice. Once this decision is reached, clinicians can minimize legal liabilities in several ways.
Table 1
Questions to ask before starting SGAs for dementia-related behavioral problems
| Questions | Comments |
|---|---|
| Is the behavior dangerous? | Nonviolent behavior (eg, foul language, inappropriate voiding, hoarding, or refusing to bathe) can be addressed with nonpharmacologic interventions |
| What about treatable medical problems? | A demented person’s behavioral outbursts may stem from pain (ingrown toenail, acid reflux), misinterpretations caused by hearing or vision problems, delirium from infections or drug interactions, etc |
| Would a nonpharmacologic approach work? | Possibilities include eliminating environmental stressors, increasing interpersonal attention, more frequent reorientation, or music or art therapy |
| SGAs: second-generation antipsychotics | |
| Source: References 9,10 | |
Informed consent: A process
Informed consent is an essential feature of most medical treatment ( Table 2 ). 13 Informed consent is especially important when—as with using SGAs for behavioral disturbances in dementia—you want to prescribe a drug off-label in a context for which the FDA has required a boxed warning.
Patients in early stages of dementia may retain their decision-making capacity and ability to give informed consent.14 If the opportunity presents itself, this is an ideal time to discuss the possible future need for SGAs and to make sure the patient has designated a proxy decision-maker who can make treatment choices if the patient loses capacity. If a patient’s decision-making capacity is questionable, obtain consent from a surrogate (often a relative).
Informed consent is a process, not a printed form. It involves taking time to be sure that the patient or surrogate decision-maker understands and accepts the risks associated with a proposed treatment. Thinking of informed consent as a process facilitates communication, acceptance of treatment, and trust between prescribers and recipients of care.
Table 2
Essentials of informed consent
| Elements | Patient has the capacity to consent, has received adequate information about the proposed treatment, and has not been coerced |
| Information to disclose about the proposed treatment | Expected benefits, risks (common and serious side effects), alternatives, and expected outcomes of treatment and no treatment |
| Source: Reference 13 | |
Involving family
When appropriate, include a patient’s family in informed consent and treatment planning processes. Providing written material, such as Treatment of dementia and agitation: a guide for families and caregivers, 15 can help educate persons whose loved ones suffer from dementia. These resources often improve care and build relationships with family members that sustain treatment alliances when adverse outcomes occur. Also, well-engaged and informed families are less likely to initiate malpractice lawsuits when adverse events occur.16
Monitor for side effects
Older patients are especially vulnerable to physical harm during agitated or aggressive behavior, but they’re also quite vulnerable to medication side effects. Before starting SGAs, note the patient’s alertness, activities of daily living, movement abnormalities, and EKG abnormalities. Knowing this “baseline” helps you assess the effects of medication and monitor for side effects. Brief assessment scales—such as the Montreal Cognitive Assessment Test,17 the Abnormal Involuntary Movement Scale,18 and the Instrumental Activities of Daily Living Scale19 —can help you quantify baseline functioning, monitor symptom response, and detect adverse effects.
For patients receiving SGA therapy, reassess benefits and risks at least every 3 months, and preferably more often.11 In geriatric patients, titrate dosages slowly, maintain medications at the lowest effective levels, and discontinue them once they are no longer necessary. When doubt arises about the effectiveness of SGA therapy, stop the drug.12
Remember to document
Because older patients have high rates of medical problems and medication side effects, negative outcomes always are a risk. Good documentation is a key risk management strategy that can help if a bad outcome requires you to defend your treatment plan in court ( Table 3 ).2 ”
Table 3
What to document
| Your reasons for starting treatment with SGAs |
| Other treatments that were tried unsuccessfully |
| Other treatments that were considered but deemed inappropriate |
| The patient’s baseline medical, physical, cognitive, and functional status, including consideration of cardiovascular, cerebrovascular, pulmonary, and metabolic risk factors |
| How and when you will monitor side effects |
| For a patient who resides outside a nursing home, send a letter to the primary care provider stating that the patient is on SGA therapy and requesting assistance in monitoring for medical problems |
| For a patient who resides in a nursing home, describe the monitoring system for identifying adverse events (eg, regular EKGs, pulmonary exams, and lab tests) |
| Collect and keep copies of literature supporting your treatment choice |
| SGAs: second-generation antipsychotics |
| Source: Adapted from reference 20 |
Drug Brand Name
- Memantine • Namenda
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Plassman BL, Langa KM, Fischer GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125-132.
2. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112-2117.
3. Tractenberg RE, Weiner MF, Patterson MB, et al. Comorbidity of psychopathological domains in community-dwelling persons with Alzheimer‘s disease. J Geriatr Psychiatry Neurol. 2003;16:94-99.
4. Ryu SH, Katona C, Rive B, et al. Persistence of and changes in neuropsychiatric symptoms in Alzheimer’s disease over 6 months: the LASER-AD study. Am J Geriatr Psychiatry. 2005;13:976-983.
5. Ballard C, Corbett A, Chitramohan R, et al. Management of agitation and aggression associated with Alzheimer’s disease: controversies and possible solutions. Curr Opin Psychiatry. 2009;22:532-540.
6. Kamble P, Chen H, Sherer JT, et al. Use of antipsychotics among elderly nursing home residents with dementia in the US: an analysis of National Survey Data. Drugs Aging. 2009;26:483-492.
7. 21 CFR § 201.57(c)(1). Specific requirements on content and format of labeling for human prescription drugs. Available at: http://edocket.access.gpo.gov/cfr_2006/aprqtr/pdf/21cfr201.57.pdf. Accessed January 24 2011.
8. Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. Available at: this link. Accessed January 2 2011.
9. Salzman C, Jeste DV, Meyer RE. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options clinical trials, methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
10. Dewing J. Responding to agitation in people with dementia. Nursing Older People. 2010;22:18-25.
11. American Psychiatric Association. Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Available at: http://www.psychiatryonline.com/pracGuide/pracGuideChapToc_3.aspx. Accessed January 3 2011.
12. Hermann N, Lanctot K. Atypical antipsychotics for neuropsychiatric symptoms of dementia. Malignant or maligned? Drug Safety. 2006;29:833-843.
13. Bernat JL. Informed consent. Muscle Nerve. 2001;24:614-621.
14. Fellows L. Competency and consent in dementia. J Am Geriatr Soc. 1998;46:922-926.
15. Treatmentof dementia and agitation: a guide for families and caregivers J Psychiatr Practice. 2007;13:207-216.
16. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication. The relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
17. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
18. Munetz MR, Benjamin S. How to examine patients using the abnormal involuntary movement scale. Hosp Comm Psychiatry. 1988;39:1172-1177.
19. Mathuranath PS, George A, Cherian PJ, et al. Instrumental activities of daily living scale for dementia screening in elderly people. Int Psychogeriatr. 2005;17:461-474.
20. Recupero PR, Rainey SE. Managing risk when considering the use of atypical antipsychotics for elderly patients with dementia-related psychosis. J Psychiatr Practice. 2007;13:143-152.
1. Plassman BL, Langa KM, Fischer GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125-132.
2. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112-2117.
3. Tractenberg RE, Weiner MF, Patterson MB, et al. Comorbidity of psychopathological domains in community-dwelling persons with Alzheimer‘s disease. J Geriatr Psychiatry Neurol. 2003;16:94-99.
4. Ryu SH, Katona C, Rive B, et al. Persistence of and changes in neuropsychiatric symptoms in Alzheimer’s disease over 6 months: the LASER-AD study. Am J Geriatr Psychiatry. 2005;13:976-983.
5. Ballard C, Corbett A, Chitramohan R, et al. Management of agitation and aggression associated with Alzheimer’s disease: controversies and possible solutions. Curr Opin Psychiatry. 2009;22:532-540.
6. Kamble P, Chen H, Sherer JT, et al. Use of antipsychotics among elderly nursing home residents with dementia in the US: an analysis of National Survey Data. Drugs Aging. 2009;26:483-492.
7. 21 CFR § 201.57(c)(1). Specific requirements on content and format of labeling for human prescription drugs. Available at: http://edocket.access.gpo.gov/cfr_2006/aprqtr/pdf/21cfr201.57.pdf. Accessed January 24 2011.
8. Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. Available at: this link. Accessed January 2 2011.
9. Salzman C, Jeste DV, Meyer RE. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options clinical trials, methodology, and policy. J Clin Psychiatry. 2008;69:889-898.
10. Dewing J. Responding to agitation in people with dementia. Nursing Older People. 2010;22:18-25.
11. American Psychiatric Association. Practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Available at: http://www.psychiatryonline.com/pracGuide/pracGuideChapToc_3.aspx. Accessed January 3 2011.
12. Hermann N, Lanctot K. Atypical antipsychotics for neuropsychiatric symptoms of dementia. Malignant or maligned? Drug Safety. 2006;29:833-843.
13. Bernat JL. Informed consent. Muscle Nerve. 2001;24:614-621.
14. Fellows L. Competency and consent in dementia. J Am Geriatr Soc. 1998;46:922-926.
15. Treatmentof dementia and agitation: a guide for families and caregivers J Psychiatr Practice. 2007;13:207-216.
16. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication. The relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
17. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
18. Munetz MR, Benjamin S. How to examine patients using the abnormal involuntary movement scale. Hosp Comm Psychiatry. 1988;39:1172-1177.
19. Mathuranath PS, George A, Cherian PJ, et al. Instrumental activities of daily living scale for dementia screening in elderly people. Int Psychogeriatr. 2005;17:461-474.
20. Recupero PR, Rainey SE. Managing risk when considering the use of atypical antipsychotics for elderly patients with dementia-related psychosis. J Psychiatr Practice. 2007;13:143-152.
How anxiety presents differently in older adults
Discuss this article at http://currentpsychiatry.blogspot.com/2011/03/how-anxiety-presents-differently-in.html#comments
Although anxiety disorders are common at all ages, there is a misconception that their prevalence drastically declines with age. For this reason anxiety disorders often are underdiagnosed and undertreated in geriatric patients, especially when the clinical presentation of these disorders in older patients differs from that seen in younger adults.
In older persons, anxiety symptoms often overlap with medical conditions such as hyperthyroidism and geriatric patients tend to express anxiety symptoms as medical or somatic problems such as pain rather than as psychological distress.1 As a result, older adults often seek treatment for depressive or anxiety symptoms from their primary care physician instead of a psychiatrist. Unfortunately, primary care physicians often miss psychiatric illness, including anxiety disorders, in geriatric patients.
Anxiety may be a symptom of an underlying psychiatric disturbance, secondary to a general medical condition, or induced by dietary substances, substances of abuse, or medications. Late-life anxiety often is comorbid with major depressive disorder (MDD) ( Box ) and other psychological stressors as older adults recognize declining cognitive and physical functioning.2 Anxiety disorders commonly begin in early adulthood, tend to be chronic and interspersed with remissions and relapses, and usually continue into old age.3 In generalized anxiety disorder (GAD), there is a bimodal distribution of onset; approximately two-thirds of patients experience onset between the late teens and late 20s and one-third develop the disorder for the first time after age 50.3
Prevalence rates for anxiety disorders among older adults (age ≥55) range from 3. 5% to 10. 2%.4 These rates are slightly lower than those for younger adults.5 Among older adults, presence of a 12-month anxiety disorder was associated with female sex, lower education, being unmarried, and having ≥3 or more chronic conditions.6
The Longitudinal Aging Study Amsterdam study—one of the largest epidemiologic studies to examine comorbidity of anxiety disorders and depression in patients age 55 to 85—found that 48% of older persons with primary major depressive disorder (MDD) also had a comorbid anxiety disorder, whereas approximately one-fourth of those with anxiety disorders also had MDD.a Pre-existing anxiety disorders, such as social phobia, obsessive-compulsive disorder, specific phobia, agoraphobia, and panic disorder, increase the risk of developing depression.b Rates of comorbid anxiety and depression increase with age.c
Late-life MDD comorbid with generalized anxiety disorder or panic disorder is associated with greater memory decline than MDD alone.d In addition, comorbid anxiety and depression is associated with greater symptom severity and persistence, greater functional impairment, substance dependence, poorer compliance and response to treatment, worse overall prognosis and outcome than patients with either disorder alone,e and greater likelihood of suicidal ideation in older men.f
References
a. Beekman AT, de Beurs E, van Balkom AJ, et al. Anxiety and depression in later life: co-occurrence and communality of risk factors. Am J Psychiatry. 2000; 157(1): 89-95.
b. Goodwin RD. Anxiety disorders and the onset of depression among adults in the community. Psychol Med. 2002; 32: 1121-1124.
c. Merikangas KR, Zhang H, Avenevoli S, et al. Longitudinal trajectories of depression and anxiety in a prospective community study: the Zurich Cohort Study. Arch Gen Psychiatry. 2003; 60: 993-1000.
d. DeLuca AK, Lenze EJ, Mulsant BH, et al. Comorbid anxiety disorder in late life depression: association with memory decline over four years. Int J Geriatr Psychiatry. 2005; 20(9): 848-854.
e. Merikangas KR, Kalaydjian A. Magnitude and impact of comorbidity of mental disorders from epidemiologic surveys. Curr Opin Psychiatry. 2007; 20: 353-358.
f. Lenze E, Mulsant BH, Shear MK, et al. Comorbid anxiety disorders in depressed elderly patients. Am J Psychiatry. 2000; 157: 722-728.
Anxiety and disability risk
Anxiety disorders affect geriatric patients more profoundly than their younger counterparts. Persons age ≥65 who have an anxiety disorder are 3 to 10 times more likely to be hospitalized than younger individuals.1 Anxiety is associated with high rates of medically unexplained symptoms, increased use of health care resources, chronic medical illness, low levels of physical health-related quality of life, and physical disability.7,8
Anxiety symptoms may predict progressing physical disability among older women and reduced ability to perform activities of daily living over 1 year.9 Anxious geriatric patients are less independent and increase the burden on family and caregivers.10 Anxiety disorders are associated with lower compliance with medical treatment, which could worsen chronic medical conditions and increase the risk for nursing home admission.11 Anxious older adults report decreased life satisfaction, memory impairment, poorer self perception of health, and increased loneliness.12
Generalized anxiety disorder
Although GAD is the most common anxiety disorder among geriatric patients, with a prevalence of 0. 7% to 9%,13 it remains underdiagnosed and undertreated.14 In a cross-sectional observational study of 439 adults age ≥55 with lifetime GAD, approximately one-half experienced onset after age 50.15 Late onset is associated with more frequent hypertension and a poorer health-related quality of life than early onset.15
Compared with younger individuals, older persons with GAD have a greater variety of worry topics, including memory loss, medical illnesses, and fear of falls,16 but worry less about the future and work than younger patients. This type of anxiety is largely situational and temporary, and often accompanies comorbid medical problems (Table 1) .
Obsessive-compulsive disorder
A study comparing older (age ≥60) and younger obsessive-compulsive disorder (OCD) patients found that the clinical presentation of the disorder does not substantially differ between age groups; however, geriatric patients had fewer concerns about symmetry, needing to know, and counting rituals. Handwashing and fear of having sinned were more common.17
OCD is fairly uncommon in geriatric patients. Prevalence rates decrease with age, ranging between 0% and 0. 8% among persons age ≥60.18 OCD seldom begins in late life; most geriatric patients with OCD have had symptoms for decades. By late life, most individuals with OCD improve, although they may continue to experience clinical or subclinical symptoms.19 However, 1 report found a second peak of incidence of OCD in women age ≥65.20 Case reports of late-onset OCD have found evidence of cerebral lesions, often in the basal ganglia, which suggests a possible neurodegenerative pathophysiology.21
Table 1
DSM-IV-TR criteria for generalized anxiety disorder
| A. | Excessive anxiety and worry (apprehensive expectation), occurring more days than not for at least 6 months, about a number of events or activities (such as work or school performance) |
| B. | The person finds it difficult to control the worry |
| C. | The anxiety and worry are associated with 3 or more of the following symptoms with at least some symptoms present for more days than not for the past 6 months:
|
| D. | The focus of the anxiety and worry is not confined to features of an axis I disorder |
| E. | The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| F. | The disturbance is not due to the direct physiological effects of a substance or a general medical condition and does not occur exclusively during a mood disorder, a psychotic disorder, or a pervasive developmental disorder |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 | |
Posttraumatic stress disorder
Untreated posttraumatic stress disorder (PTSD) often is assumed to be a chronic disorder. Recollections of past trauma may lead to new PTSD symptoms in older patients. Neurodegeneration of memory pathways and cognitive impairment associated with Alzheimer‘s disease or vascular or alcohol-related dementia may disinhibit PTSD symptoms in patients whose PTSD was fairly well controlled.22
Life events associated with aging—death of a spouse, financial and physical decline, chronic pain, or diminished cognitive coping resources—may precipitate or revive PTSD symptoms associated with earlier exposure to severe psychological trauma.23 These life changes also may precipitate socalled delayed PTSD, when symptoms relating to past traumatic experiences present for the first time. Geriatric patients may be more likely than younger persons to deny their PTSD symptoms if their cultural background emphasizes stoicism and fortitude.24
Phobias
Specific phobias. The prevalence of specific phobias drops dramatically in late life, although older patients might underreport symptoms. Many older persons are afraid of falling. Approximately 60% of older adults with a history of falling—and 30% of older individuals with no such history— report this fear. Fear of falling is more prevalent in women and increases with age.25,26 This fear may be a protective response to a real threat that prevents older persons from attempting high-risk activities, but it also can cause patients to restrict their activities, which can result in decreased social, physical, or cognitive functioning and loss of in-dependence.25
Social phobias (social anxiety disorder).
Among older adults, common social phobias include eating food around strangers, and—especially in men—being unable to urinate in public bathrooms. In a cross-sectional observational study, social anxiety disorder (SAD) was more common among older persons who reported stressful life events, such as death of a spouse.27 MDD, specific phobia, and personality disorder are associated with SAD in geriatric patients.27 Prevalence rates of SAD appear to slightly decrease with age, although the condition remains common in geriatric patients—5% of older adults report lifetime prevalence—and its presentation is similar to that seen in younger adults.27
Agoraphobia. In older persons the prevalence of agoraphobia is 0. 6%.28 Most cases are of early onset but the condition can present de novo following a stroke or other medical event and can inhibit activities needed for successful rehabilitation. Agoraphobia can present within the context of panic attacks as is seen in younger adults but most geriatric patients with agoraphobia do not have concurrent panic disorder. This phobia is more common in women, widowed or divorced individuals, patients with chronic health conditions, and those with comorbid psychiatric disorders.29
Panic disorder
Panic disorder (PD) rarely starts for the first time after age 60, and most late-onset panic attacks are associated with medical and psychiatric comorbidities. PD tends to be less severe in older individuals than in younger adults.30 Recent stressful life events or losses can predict onset and maintenance of PD. Older patients may present with panic symptoms, such as shortness of breath, dizziness, or trembling, that overlap with age-related medical conditions. PD may be prevalent in older patients with chest pain and no evidence of coronary artery disease.31 Panic symptoms that are secondary to underlying medical conditions, such as chronic obstructive pulmonary disease exacerbation, usually wax and wane.32
Treatment
Treatment for anxiety disorders in geriatric patients may involve a combination of psychotherapy, pharmacotherapy, and complementary and alternative therapies. Treatment may be complicated if patients have ≥1 anxiety disorder or suffer from comorbid depression, substance abuse, or medical problems. As is seen with younger adults, the course of anxiety disorders in older patients waxes and wanes, but most disorders are unlikely to remit completely.33 Aging may influence the effects of psychotropic medications in older patients. Increased distribution and decreased metabolism and clearance of medications results in higher medication plasma levels and longer elimination half-lives. Medication compliance in older patients may be complicated by:
- older patients’ sensitivity to anticho-linergic side effects
- coexisting medical illnesses
- polypharmacy, particularly in institutionalized settings
- sensory and cognitive deficits.34
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) generally are safe and produce fewer side effects compared with tricyclic antidepressants (TCAs), especially in geriatric patients. SSRIs and SNRIs may be useful for GAD, PD, OCD, and PTSD in older patients.35 TCAs can effectively treat anxiety symptoms but may be cardiotoxic and their anticholinergic properties can lead to serious side effects. Benzodiazepines often are used for acute or short-term anxiety management, but chronic use in geriatric patients can cause cognitive impairment, falls, and other serious side effects. Buspirone may be beneficial for GAD but is not effective for PD.36 The drug is well tolerated in older persons, but may take 2 to 4 weeks to be effective ( Table 2 ).35
Pharmacotherapy for anxiety disorders in geriatric patients often is used in conjunction with psychotherapy. Psychotherapeutic approaches include cognitive-behavioral therapy (CBT), exposure therapy, dialectical behavioral therapy, and interpersonal therapy. Increasing evidence supports the effectiveness of psychotherapy in treating anxiety disorders in younger adults as well as in older patients, often in combination with pharmacotherapy.37 In older patients with GAD, CBT is associated with a greater improvement in worry severity, depressive symptoms, and overall mental health compared with usual care.38
In addition to traditional pharmacotherapy, complementary and alternative therapies often are used for late-life anxiety. These therapies include biofeedback, progressive relaxation, acupuncture, yoga, massage therapy, art, music, or dance therapy, meditation, prayer, and spiritual counseling.
Table 2
Pharmacotherapy for anxiety disorders in older adults
| Medication | Comments |
|---|---|
| Selective serotonin reuptake inhibitors | May be useful for GAD, panic disorder, OCD, and PTSD |
| Serotonin-norepinephrine reuptake inhibitors | May be useful for GAD, panic disorder, OCD, and PTSD |
| Tricyclic antidepressants | Potential for cardiotoxicity and overdose, anticholinergic properties |
| Benzodiazepines | Chronic use can lead to cognitive impairment, falls |
| Buspirone | Effective for GAD, but not panic disorder; may take 2 to 4 weeks to be effective |
| GAD: generalized anxiety disorder; OCD: obsessive-compulsive disorder; PTSD: posttraumatic stress disorder | |
| Source: Reference 35 | |
Related Resources
- Wetherell JL, Lenze EJ, Stanley MA. Evidence-based treatment of geriatric anxiety disorders. Psychiatr Clin North Am. 2005; 28(4): 871-896, ix.
- Lenze EJ, Wetherell JL. Anxiety disorders. In: Blazer DG, Steffens DC, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. Arlington, VA: American Psychiatric Publishing, Inc; 2009: 333-345.
Drug Brand Name
- Buspirone • BuSpar
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Fuentes K, Cox BJ. Prevalence of anxiety disorders in elderly adults: a critical analysis. J Behav Ther Exp Psychiatry. 1997;28:269-279.
2. Préville M, Hérbert R, Bravo G, et al. Predisposing and facilitating factors of severe psychological distress among frail elderly. Can J Aging. 2002;21:195-204.
3. Le Roux H, Gatz M, Wetherell JL. Age at onset of generalized anxiety disorder in older adults. Am J Geriatr Psychiatry. 2005;13:23-30.
4. Beekman AT, Bremmer MA, Deeg DJ, et al. Anxiety disorders in later life: a report from the Longitudinal Aging Study Amsterdam. Int J Geriatr Psychiatry. 1998;13:717-726.
5. Regier DA, Rae DS, Narrow WE, et al. Prevalence of anxiety disorders and their comorbidity with mood and addictive disorders. Br J Psychiatry Suppl. 1998;34:24-28.
6. Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood anxiety, and substance-abuse disorders for older Americans in the national comorbidity survey-replication. Am J Geriatr Psychiatry. 2009;17(9):769-781.
7. Sareen J, Jacobi F, Cox BJ, et al. Disability and poor quality of life associated with comorbid anxiety disorders and physical conditions. Arch Intern Med. 2006;166:2109-2116.
8. Porensky EK, Dew MA, Karp JF, et al. The burden of late-life generalized anxiety disorder: effects on disability, health-related quality of life, and healthcare utilization. Am J Geriatr Psychiatry. 2009;17(6):473-482.
9. Tinetti ME, Inouye SK, Gill TM, et al. Shared risk-factors for falls, incontinence, and functional dependence: unifying the approach to geriatric syndromes. JAMA. 1995;273:1348-1353.
10. Lenze EJ, Karp JF, Mulsant BH, et al. Somatic symptoms in late-life anxiety: treatment issues. J Geriatr Psychiatry Neurol. 2005;18:89-96.
11. Gibbons LE, Teri L, Logsdon R, et al. Anxiety symptoms as predictors of nursing home placement in patients with Alzheimer’s disease. Journal of Clinical Geropsychology. 2002;4:335-342.
12. de Beurs E, Beekman AT, van Balkom AJ, et al. Consequences of anxiety in older persons: its effect on disability, well-being and use of health services. Psychol Med. 1999;29(3):583-593.
13. Schoevers RA, Beekman AT, Deeg DJ, et al. Comorbidity and risk-patterns of depression, generalised anxiety disorder and mixed anxiety-depression in later life: results from the AMSTEL study. Int J Geriatr Psychiatry. 2003;18:944-1001.
14. Wilk J, West J, Narrow W, et al. Are anxiety disorders underdiagnosed and undertreated in routine psychiatric practice? Poster presented at: AcademyHealth Annual Meeting; June 8, 2004; San Diego, CA.
15. Chou KL. Age at onset of generalized anxiety disorder in older adults. Am J Geriatr Psychiatry. 2009;17(6):455-464.
16. Howland J, Peterson EW, Levin WC, et al. Fear of falling among the community-dwelling elderly. J Aging Health. 1993;5(2):229-243.
17. Kohn R, Westlake RJ, Rasmussen SA, et al. Clinical features of obsessive-compulsive disorder in elderly patients. Am J Geriatr Psychiatry. 1997;5(3):211-215.
18. Flint AJ. Epidemiology and comorbidity of anxiety disorders in the elderly. Am J Psychiatry. 1994;151:640-649.
19. Skoog G, Skoog I. A 40-year follow-up of patients with obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;56(2):121-127.
20. Nestadt G, Bienvenu OJ, Cai G, et al. Incidence of obsessive-compulsive disorder in adults. J Nerv Ment Dis. 1998;186:401-406.
21. Chacko RC, Corbin MA, Harper RG. Acquired obsessive-compulsive disorder associated with basal ganglia lesions. J Neuropsychiatry Clin Neurosci. 2000;12:269-272.
22. Mittal D, Torres R, Abashidze A, et al. Worsening of post-traumatic stress disorder symptoms with cognitive decline: case series. J Geriatr Psychiatry Neurol. 2001;14(1):17-20.
23. Tedstone JE, Tarrier N. Posttraumatic stress disorder following medical illness and treatment. Clin Psychol Rev. 2003;23(3):409-448.
24. Creamer M, Parslow R. Trauma exposure and posttraumatic stress disorder in the elderly: a community prevalence study. Am J Ger Psychiatry. 2008;16:853-856.
25. Alcalde Tirado P. Fear of falling. Rev Esp Geriatr Gerontol. 2010;45(1):38-44.
26. Boyd R, Stevens JA. Falls and fear of falling: burden beliefs and behaviours. Age Ageing. 2009;38(4):423-428.
27. Cairney J, McCabe L, Veldhuizen S, et al. Epidemiology of social phobia in later life. Am J Geriatr Psychiatry. 2007;15(3):224-233.
28. Pontillo DC, Lang AJ, Stein MB. Management and treatment of anxiety disorders in the older patient. Clinical Geriatrics. 2002;10(10):38-49.
29. McCabe L, Cairney J, Veldhuizen S, et al. Prevalence and correlates of agoraphobia in older adults. Am J Geriatr Psychiatry. 2006;14(6):515-522.
30. Hassan R, Pollard CA. Late-life-onset panic disorder: clinical and demographic characteristics of a patient sample. J Geriatr Psychiatry Neurol. 1994;7:86-90.
31. Beitman BD, Kushner M, Grossberg GT. Late onset panic disorder: evidence from a study of patients with chest pain and normal cardiac evaluations. Int J Psychiatry Med. 1991;21(1):29-35.
32. Garvey MJ. Panic disorder: guidelines to safe use of benzodiazepines. Geriatrics. 1993;48(7):49-58.
33. Schuurmans J, Comijs HC, Beekman AT, et al. The outcome of anxiety disorders in older people at six-year follow-up: results from the Longitudinal Aging Study Amsterdam. Acta Psychiatr Scand. 2005;111:420-428.
34. Von Moltke LL, Abernethy DR, Greenblatt DJ. Kinetics and dynamics of psychotropic drugs in the elderly. In: Salzman C ed. Clinical geriatric psychopharmacology. 3rd ed. Baltimore, MD: Williams and Wilkins; 1998:70-93.
35. Baldwin DS, Anderson IM, Nutt DJ, et al and the British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2005;19(6):567-596.
36. Sheehan DV, Raj AB, Sheehan KH, et al. Is buspirone effective for panic disorder? J Clin Psychopharmacol. 1990;10(1):3-11.
37. Black DW. Efficacy of combined pharmacotherapy and psychotherapy versus monotherapy in the treatment of anxiety disorders. CNS Spectr. 2006;11(10 suppl 12):29-33.
38. Stanley MA, Wilson NL, Novy DM, et al. Cognitive behavior therapy for generalized anxiety disorder among older adults in primary care: a randomized clinical trial. JAMA. 2009;301(14):1460-1467.
Discuss this article at http://currentpsychiatry.blogspot.com/2011/03/how-anxiety-presents-differently-in.html#comments
Although anxiety disorders are common at all ages, there is a misconception that their prevalence drastically declines with age. For this reason anxiety disorders often are underdiagnosed and undertreated in geriatric patients, especially when the clinical presentation of these disorders in older patients differs from that seen in younger adults.
In older persons, anxiety symptoms often overlap with medical conditions such as hyperthyroidism and geriatric patients tend to express anxiety symptoms as medical or somatic problems such as pain rather than as psychological distress.1 As a result, older adults often seek treatment for depressive or anxiety symptoms from their primary care physician instead of a psychiatrist. Unfortunately, primary care physicians often miss psychiatric illness, including anxiety disorders, in geriatric patients.
Anxiety may be a symptom of an underlying psychiatric disturbance, secondary to a general medical condition, or induced by dietary substances, substances of abuse, or medications. Late-life anxiety often is comorbid with major depressive disorder (MDD) ( Box ) and other psychological stressors as older adults recognize declining cognitive and physical functioning.2 Anxiety disorders commonly begin in early adulthood, tend to be chronic and interspersed with remissions and relapses, and usually continue into old age.3 In generalized anxiety disorder (GAD), there is a bimodal distribution of onset; approximately two-thirds of patients experience onset between the late teens and late 20s and one-third develop the disorder for the first time after age 50.3
Prevalence rates for anxiety disorders among older adults (age ≥55) range from 3. 5% to 10. 2%.4 These rates are slightly lower than those for younger adults.5 Among older adults, presence of a 12-month anxiety disorder was associated with female sex, lower education, being unmarried, and having ≥3 or more chronic conditions.6
The Longitudinal Aging Study Amsterdam study—one of the largest epidemiologic studies to examine comorbidity of anxiety disorders and depression in patients age 55 to 85—found that 48% of older persons with primary major depressive disorder (MDD) also had a comorbid anxiety disorder, whereas approximately one-fourth of those with anxiety disorders also had MDD.a Pre-existing anxiety disorders, such as social phobia, obsessive-compulsive disorder, specific phobia, agoraphobia, and panic disorder, increase the risk of developing depression.b Rates of comorbid anxiety and depression increase with age.c
Late-life MDD comorbid with generalized anxiety disorder or panic disorder is associated with greater memory decline than MDD alone.d In addition, comorbid anxiety and depression is associated with greater symptom severity and persistence, greater functional impairment, substance dependence, poorer compliance and response to treatment, worse overall prognosis and outcome than patients with either disorder alone,e and greater likelihood of suicidal ideation in older men.f
References
a. Beekman AT, de Beurs E, van Balkom AJ, et al. Anxiety and depression in later life: co-occurrence and communality of risk factors. Am J Psychiatry. 2000; 157(1): 89-95.
b. Goodwin RD. Anxiety disorders and the onset of depression among adults in the community. Psychol Med. 2002; 32: 1121-1124.
c. Merikangas KR, Zhang H, Avenevoli S, et al. Longitudinal trajectories of depression and anxiety in a prospective community study: the Zurich Cohort Study. Arch Gen Psychiatry. 2003; 60: 993-1000.
d. DeLuca AK, Lenze EJ, Mulsant BH, et al. Comorbid anxiety disorder in late life depression: association with memory decline over four years. Int J Geriatr Psychiatry. 2005; 20(9): 848-854.
e. Merikangas KR, Kalaydjian A. Magnitude and impact of comorbidity of mental disorders from epidemiologic surveys. Curr Opin Psychiatry. 2007; 20: 353-358.
f. Lenze E, Mulsant BH, Shear MK, et al. Comorbid anxiety disorders in depressed elderly patients. Am J Psychiatry. 2000; 157: 722-728.
Anxiety and disability risk
Anxiety disorders affect geriatric patients more profoundly than their younger counterparts. Persons age ≥65 who have an anxiety disorder are 3 to 10 times more likely to be hospitalized than younger individuals.1 Anxiety is associated with high rates of medically unexplained symptoms, increased use of health care resources, chronic medical illness, low levels of physical health-related quality of life, and physical disability.7,8
Anxiety symptoms may predict progressing physical disability among older women and reduced ability to perform activities of daily living over 1 year.9 Anxious geriatric patients are less independent and increase the burden on family and caregivers.10 Anxiety disorders are associated with lower compliance with medical treatment, which could worsen chronic medical conditions and increase the risk for nursing home admission.11 Anxious older adults report decreased life satisfaction, memory impairment, poorer self perception of health, and increased loneliness.12
Generalized anxiety disorder
Although GAD is the most common anxiety disorder among geriatric patients, with a prevalence of 0. 7% to 9%,13 it remains underdiagnosed and undertreated.14 In a cross-sectional observational study of 439 adults age ≥55 with lifetime GAD, approximately one-half experienced onset after age 50.15 Late onset is associated with more frequent hypertension and a poorer health-related quality of life than early onset.15
Compared with younger individuals, older persons with GAD have a greater variety of worry topics, including memory loss, medical illnesses, and fear of falls,16 but worry less about the future and work than younger patients. This type of anxiety is largely situational and temporary, and often accompanies comorbid medical problems (Table 1) .
Obsessive-compulsive disorder
A study comparing older (age ≥60) and younger obsessive-compulsive disorder (OCD) patients found that the clinical presentation of the disorder does not substantially differ between age groups; however, geriatric patients had fewer concerns about symmetry, needing to know, and counting rituals. Handwashing and fear of having sinned were more common.17
OCD is fairly uncommon in geriatric patients. Prevalence rates decrease with age, ranging between 0% and 0. 8% among persons age ≥60.18 OCD seldom begins in late life; most geriatric patients with OCD have had symptoms for decades. By late life, most individuals with OCD improve, although they may continue to experience clinical or subclinical symptoms.19 However, 1 report found a second peak of incidence of OCD in women age ≥65.20 Case reports of late-onset OCD have found evidence of cerebral lesions, often in the basal ganglia, which suggests a possible neurodegenerative pathophysiology.21
Table 1
DSM-IV-TR criteria for generalized anxiety disorder
| A. | Excessive anxiety and worry (apprehensive expectation), occurring more days than not for at least 6 months, about a number of events or activities (such as work or school performance) |
| B. | The person finds it difficult to control the worry |
| C. | The anxiety and worry are associated with 3 or more of the following symptoms with at least some symptoms present for more days than not for the past 6 months:
|
| D. | The focus of the anxiety and worry is not confined to features of an axis I disorder |
| E. | The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| F. | The disturbance is not due to the direct physiological effects of a substance or a general medical condition and does not occur exclusively during a mood disorder, a psychotic disorder, or a pervasive developmental disorder |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 | |
Posttraumatic stress disorder
Untreated posttraumatic stress disorder (PTSD) often is assumed to be a chronic disorder. Recollections of past trauma may lead to new PTSD symptoms in older patients. Neurodegeneration of memory pathways and cognitive impairment associated with Alzheimer‘s disease or vascular or alcohol-related dementia may disinhibit PTSD symptoms in patients whose PTSD was fairly well controlled.22
Life events associated with aging—death of a spouse, financial and physical decline, chronic pain, or diminished cognitive coping resources—may precipitate or revive PTSD symptoms associated with earlier exposure to severe psychological trauma.23 These life changes also may precipitate socalled delayed PTSD, when symptoms relating to past traumatic experiences present for the first time. Geriatric patients may be more likely than younger persons to deny their PTSD symptoms if their cultural background emphasizes stoicism and fortitude.24
Phobias
Specific phobias. The prevalence of specific phobias drops dramatically in late life, although older patients might underreport symptoms. Many older persons are afraid of falling. Approximately 60% of older adults with a history of falling—and 30% of older individuals with no such history— report this fear. Fear of falling is more prevalent in women and increases with age.25,26 This fear may be a protective response to a real threat that prevents older persons from attempting high-risk activities, but it also can cause patients to restrict their activities, which can result in decreased social, physical, or cognitive functioning and loss of in-dependence.25
Social phobias (social anxiety disorder).
Among older adults, common social phobias include eating food around strangers, and—especially in men—being unable to urinate in public bathrooms. In a cross-sectional observational study, social anxiety disorder (SAD) was more common among older persons who reported stressful life events, such as death of a spouse.27 MDD, specific phobia, and personality disorder are associated with SAD in geriatric patients.27 Prevalence rates of SAD appear to slightly decrease with age, although the condition remains common in geriatric patients—5% of older adults report lifetime prevalence—and its presentation is similar to that seen in younger adults.27
Agoraphobia. In older persons the prevalence of agoraphobia is 0. 6%.28 Most cases are of early onset but the condition can present de novo following a stroke or other medical event and can inhibit activities needed for successful rehabilitation. Agoraphobia can present within the context of panic attacks as is seen in younger adults but most geriatric patients with agoraphobia do not have concurrent panic disorder. This phobia is more common in women, widowed or divorced individuals, patients with chronic health conditions, and those with comorbid psychiatric disorders.29
Panic disorder
Panic disorder (PD) rarely starts for the first time after age 60, and most late-onset panic attacks are associated with medical and psychiatric comorbidities. PD tends to be less severe in older individuals than in younger adults.30 Recent stressful life events or losses can predict onset and maintenance of PD. Older patients may present with panic symptoms, such as shortness of breath, dizziness, or trembling, that overlap with age-related medical conditions. PD may be prevalent in older patients with chest pain and no evidence of coronary artery disease.31 Panic symptoms that are secondary to underlying medical conditions, such as chronic obstructive pulmonary disease exacerbation, usually wax and wane.32
Treatment
Treatment for anxiety disorders in geriatric patients may involve a combination of psychotherapy, pharmacotherapy, and complementary and alternative therapies. Treatment may be complicated if patients have ≥1 anxiety disorder or suffer from comorbid depression, substance abuse, or medical problems. As is seen with younger adults, the course of anxiety disorders in older patients waxes and wanes, but most disorders are unlikely to remit completely.33 Aging may influence the effects of psychotropic medications in older patients. Increased distribution and decreased metabolism and clearance of medications results in higher medication plasma levels and longer elimination half-lives. Medication compliance in older patients may be complicated by:
- older patients’ sensitivity to anticho-linergic side effects
- coexisting medical illnesses
- polypharmacy, particularly in institutionalized settings
- sensory and cognitive deficits.34
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) generally are safe and produce fewer side effects compared with tricyclic antidepressants (TCAs), especially in geriatric patients. SSRIs and SNRIs may be useful for GAD, PD, OCD, and PTSD in older patients.35 TCAs can effectively treat anxiety symptoms but may be cardiotoxic and their anticholinergic properties can lead to serious side effects. Benzodiazepines often are used for acute or short-term anxiety management, but chronic use in geriatric patients can cause cognitive impairment, falls, and other serious side effects. Buspirone may be beneficial for GAD but is not effective for PD.36 The drug is well tolerated in older persons, but may take 2 to 4 weeks to be effective ( Table 2 ).35
Pharmacotherapy for anxiety disorders in geriatric patients often is used in conjunction with psychotherapy. Psychotherapeutic approaches include cognitive-behavioral therapy (CBT), exposure therapy, dialectical behavioral therapy, and interpersonal therapy. Increasing evidence supports the effectiveness of psychotherapy in treating anxiety disorders in younger adults as well as in older patients, often in combination with pharmacotherapy.37 In older patients with GAD, CBT is associated with a greater improvement in worry severity, depressive symptoms, and overall mental health compared with usual care.38
In addition to traditional pharmacotherapy, complementary and alternative therapies often are used for late-life anxiety. These therapies include biofeedback, progressive relaxation, acupuncture, yoga, massage therapy, art, music, or dance therapy, meditation, prayer, and spiritual counseling.
Table 2
Pharmacotherapy for anxiety disorders in older adults
| Medication | Comments |
|---|---|
| Selective serotonin reuptake inhibitors | May be useful for GAD, panic disorder, OCD, and PTSD |
| Serotonin-norepinephrine reuptake inhibitors | May be useful for GAD, panic disorder, OCD, and PTSD |
| Tricyclic antidepressants | Potential for cardiotoxicity and overdose, anticholinergic properties |
| Benzodiazepines | Chronic use can lead to cognitive impairment, falls |
| Buspirone | Effective for GAD, but not panic disorder; may take 2 to 4 weeks to be effective |
| GAD: generalized anxiety disorder; OCD: obsessive-compulsive disorder; PTSD: posttraumatic stress disorder | |
| Source: Reference 35 | |
Related Resources
- Wetherell JL, Lenze EJ, Stanley MA. Evidence-based treatment of geriatric anxiety disorders. Psychiatr Clin North Am. 2005; 28(4): 871-896, ix.
- Lenze EJ, Wetherell JL. Anxiety disorders. In: Blazer DG, Steffens DC, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. Arlington, VA: American Psychiatric Publishing, Inc; 2009: 333-345.
Drug Brand Name
- Buspirone • BuSpar
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Discuss this article at http://currentpsychiatry.blogspot.com/2011/03/how-anxiety-presents-differently-in.html#comments
Although anxiety disorders are common at all ages, there is a misconception that their prevalence drastically declines with age. For this reason anxiety disorders often are underdiagnosed and undertreated in geriatric patients, especially when the clinical presentation of these disorders in older patients differs from that seen in younger adults.
In older persons, anxiety symptoms often overlap with medical conditions such as hyperthyroidism and geriatric patients tend to express anxiety symptoms as medical or somatic problems such as pain rather than as psychological distress.1 As a result, older adults often seek treatment for depressive or anxiety symptoms from their primary care physician instead of a psychiatrist. Unfortunately, primary care physicians often miss psychiatric illness, including anxiety disorders, in geriatric patients.
Anxiety may be a symptom of an underlying psychiatric disturbance, secondary to a general medical condition, or induced by dietary substances, substances of abuse, or medications. Late-life anxiety often is comorbid with major depressive disorder (MDD) ( Box ) and other psychological stressors as older adults recognize declining cognitive and physical functioning.2 Anxiety disorders commonly begin in early adulthood, tend to be chronic and interspersed with remissions and relapses, and usually continue into old age.3 In generalized anxiety disorder (GAD), there is a bimodal distribution of onset; approximately two-thirds of patients experience onset between the late teens and late 20s and one-third develop the disorder for the first time after age 50.3
Prevalence rates for anxiety disorders among older adults (age ≥55) range from 3. 5% to 10. 2%.4 These rates are slightly lower than those for younger adults.5 Among older adults, presence of a 12-month anxiety disorder was associated with female sex, lower education, being unmarried, and having ≥3 or more chronic conditions.6
The Longitudinal Aging Study Amsterdam study—one of the largest epidemiologic studies to examine comorbidity of anxiety disorders and depression in patients age 55 to 85—found that 48% of older persons with primary major depressive disorder (MDD) also had a comorbid anxiety disorder, whereas approximately one-fourth of those with anxiety disorders also had MDD.a Pre-existing anxiety disorders, such as social phobia, obsessive-compulsive disorder, specific phobia, agoraphobia, and panic disorder, increase the risk of developing depression.b Rates of comorbid anxiety and depression increase with age.c
Late-life MDD comorbid with generalized anxiety disorder or panic disorder is associated with greater memory decline than MDD alone.d In addition, comorbid anxiety and depression is associated with greater symptom severity and persistence, greater functional impairment, substance dependence, poorer compliance and response to treatment, worse overall prognosis and outcome than patients with either disorder alone,e and greater likelihood of suicidal ideation in older men.f
References
a. Beekman AT, de Beurs E, van Balkom AJ, et al. Anxiety and depression in later life: co-occurrence and communality of risk factors. Am J Psychiatry. 2000; 157(1): 89-95.
b. Goodwin RD. Anxiety disorders and the onset of depression among adults in the community. Psychol Med. 2002; 32: 1121-1124.
c. Merikangas KR, Zhang H, Avenevoli S, et al. Longitudinal trajectories of depression and anxiety in a prospective community study: the Zurich Cohort Study. Arch Gen Psychiatry. 2003; 60: 993-1000.
d. DeLuca AK, Lenze EJ, Mulsant BH, et al. Comorbid anxiety disorder in late life depression: association with memory decline over four years. Int J Geriatr Psychiatry. 2005; 20(9): 848-854.
e. Merikangas KR, Kalaydjian A. Magnitude and impact of comorbidity of mental disorders from epidemiologic surveys. Curr Opin Psychiatry. 2007; 20: 353-358.
f. Lenze E, Mulsant BH, Shear MK, et al. Comorbid anxiety disorders in depressed elderly patients. Am J Psychiatry. 2000; 157: 722-728.
Anxiety and disability risk
Anxiety disorders affect geriatric patients more profoundly than their younger counterparts. Persons age ≥65 who have an anxiety disorder are 3 to 10 times more likely to be hospitalized than younger individuals.1 Anxiety is associated with high rates of medically unexplained symptoms, increased use of health care resources, chronic medical illness, low levels of physical health-related quality of life, and physical disability.7,8
Anxiety symptoms may predict progressing physical disability among older women and reduced ability to perform activities of daily living over 1 year.9 Anxious geriatric patients are less independent and increase the burden on family and caregivers.10 Anxiety disorders are associated with lower compliance with medical treatment, which could worsen chronic medical conditions and increase the risk for nursing home admission.11 Anxious older adults report decreased life satisfaction, memory impairment, poorer self perception of health, and increased loneliness.12
Generalized anxiety disorder
Although GAD is the most common anxiety disorder among geriatric patients, with a prevalence of 0. 7% to 9%,13 it remains underdiagnosed and undertreated.14 In a cross-sectional observational study of 439 adults age ≥55 with lifetime GAD, approximately one-half experienced onset after age 50.15 Late onset is associated with more frequent hypertension and a poorer health-related quality of life than early onset.15
Compared with younger individuals, older persons with GAD have a greater variety of worry topics, including memory loss, medical illnesses, and fear of falls,16 but worry less about the future and work than younger patients. This type of anxiety is largely situational and temporary, and often accompanies comorbid medical problems (Table 1) .
Obsessive-compulsive disorder
A study comparing older (age ≥60) and younger obsessive-compulsive disorder (OCD) patients found that the clinical presentation of the disorder does not substantially differ between age groups; however, geriatric patients had fewer concerns about symmetry, needing to know, and counting rituals. Handwashing and fear of having sinned were more common.17
OCD is fairly uncommon in geriatric patients. Prevalence rates decrease with age, ranging between 0% and 0. 8% among persons age ≥60.18 OCD seldom begins in late life; most geriatric patients with OCD have had symptoms for decades. By late life, most individuals with OCD improve, although they may continue to experience clinical or subclinical symptoms.19 However, 1 report found a second peak of incidence of OCD in women age ≥65.20 Case reports of late-onset OCD have found evidence of cerebral lesions, often in the basal ganglia, which suggests a possible neurodegenerative pathophysiology.21
Table 1
DSM-IV-TR criteria for generalized anxiety disorder
| A. | Excessive anxiety and worry (apprehensive expectation), occurring more days than not for at least 6 months, about a number of events or activities (such as work or school performance) |
| B. | The person finds it difficult to control the worry |
| C. | The anxiety and worry are associated with 3 or more of the following symptoms with at least some symptoms present for more days than not for the past 6 months:
|
| D. | The focus of the anxiety and worry is not confined to features of an axis I disorder |
| E. | The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| F. | The disturbance is not due to the direct physiological effects of a substance or a general medical condition and does not occur exclusively during a mood disorder, a psychotic disorder, or a pervasive developmental disorder |
| Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000 | |
Posttraumatic stress disorder
Untreated posttraumatic stress disorder (PTSD) often is assumed to be a chronic disorder. Recollections of past trauma may lead to new PTSD symptoms in older patients. Neurodegeneration of memory pathways and cognitive impairment associated with Alzheimer‘s disease or vascular or alcohol-related dementia may disinhibit PTSD symptoms in patients whose PTSD was fairly well controlled.22
Life events associated with aging—death of a spouse, financial and physical decline, chronic pain, or diminished cognitive coping resources—may precipitate or revive PTSD symptoms associated with earlier exposure to severe psychological trauma.23 These life changes also may precipitate socalled delayed PTSD, when symptoms relating to past traumatic experiences present for the first time. Geriatric patients may be more likely than younger persons to deny their PTSD symptoms if their cultural background emphasizes stoicism and fortitude.24
Phobias
Specific phobias. The prevalence of specific phobias drops dramatically in late life, although older patients might underreport symptoms. Many older persons are afraid of falling. Approximately 60% of older adults with a history of falling—and 30% of older individuals with no such history— report this fear. Fear of falling is more prevalent in women and increases with age.25,26 This fear may be a protective response to a real threat that prevents older persons from attempting high-risk activities, but it also can cause patients to restrict their activities, which can result in decreased social, physical, or cognitive functioning and loss of in-dependence.25
Social phobias (social anxiety disorder).
Among older adults, common social phobias include eating food around strangers, and—especially in men—being unable to urinate in public bathrooms. In a cross-sectional observational study, social anxiety disorder (SAD) was more common among older persons who reported stressful life events, such as death of a spouse.27 MDD, specific phobia, and personality disorder are associated with SAD in geriatric patients.27 Prevalence rates of SAD appear to slightly decrease with age, although the condition remains common in geriatric patients—5% of older adults report lifetime prevalence—and its presentation is similar to that seen in younger adults.27
Agoraphobia. In older persons the prevalence of agoraphobia is 0. 6%.28 Most cases are of early onset but the condition can present de novo following a stroke or other medical event and can inhibit activities needed for successful rehabilitation. Agoraphobia can present within the context of panic attacks as is seen in younger adults but most geriatric patients with agoraphobia do not have concurrent panic disorder. This phobia is more common in women, widowed or divorced individuals, patients with chronic health conditions, and those with comorbid psychiatric disorders.29
Panic disorder
Panic disorder (PD) rarely starts for the first time after age 60, and most late-onset panic attacks are associated with medical and psychiatric comorbidities. PD tends to be less severe in older individuals than in younger adults.30 Recent stressful life events or losses can predict onset and maintenance of PD. Older patients may present with panic symptoms, such as shortness of breath, dizziness, or trembling, that overlap with age-related medical conditions. PD may be prevalent in older patients with chest pain and no evidence of coronary artery disease.31 Panic symptoms that are secondary to underlying medical conditions, such as chronic obstructive pulmonary disease exacerbation, usually wax and wane.32
Treatment
Treatment for anxiety disorders in geriatric patients may involve a combination of psychotherapy, pharmacotherapy, and complementary and alternative therapies. Treatment may be complicated if patients have ≥1 anxiety disorder or suffer from comorbid depression, substance abuse, or medical problems. As is seen with younger adults, the course of anxiety disorders in older patients waxes and wanes, but most disorders are unlikely to remit completely.33 Aging may influence the effects of psychotropic medications in older patients. Increased distribution and decreased metabolism and clearance of medications results in higher medication plasma levels and longer elimination half-lives. Medication compliance in older patients may be complicated by:
- older patients’ sensitivity to anticho-linergic side effects
- coexisting medical illnesses
- polypharmacy, particularly in institutionalized settings
- sensory and cognitive deficits.34
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) generally are safe and produce fewer side effects compared with tricyclic antidepressants (TCAs), especially in geriatric patients. SSRIs and SNRIs may be useful for GAD, PD, OCD, and PTSD in older patients.35 TCAs can effectively treat anxiety symptoms but may be cardiotoxic and their anticholinergic properties can lead to serious side effects. Benzodiazepines often are used for acute or short-term anxiety management, but chronic use in geriatric patients can cause cognitive impairment, falls, and other serious side effects. Buspirone may be beneficial for GAD but is not effective for PD.36 The drug is well tolerated in older persons, but may take 2 to 4 weeks to be effective ( Table 2 ).35
Pharmacotherapy for anxiety disorders in geriatric patients often is used in conjunction with psychotherapy. Psychotherapeutic approaches include cognitive-behavioral therapy (CBT), exposure therapy, dialectical behavioral therapy, and interpersonal therapy. Increasing evidence supports the effectiveness of psychotherapy in treating anxiety disorders in younger adults as well as in older patients, often in combination with pharmacotherapy.37 In older patients with GAD, CBT is associated with a greater improvement in worry severity, depressive symptoms, and overall mental health compared with usual care.38
In addition to traditional pharmacotherapy, complementary and alternative therapies often are used for late-life anxiety. These therapies include biofeedback, progressive relaxation, acupuncture, yoga, massage therapy, art, music, or dance therapy, meditation, prayer, and spiritual counseling.
Table 2
Pharmacotherapy for anxiety disorders in older adults
| Medication | Comments |
|---|---|
| Selective serotonin reuptake inhibitors | May be useful for GAD, panic disorder, OCD, and PTSD |
| Serotonin-norepinephrine reuptake inhibitors | May be useful for GAD, panic disorder, OCD, and PTSD |
| Tricyclic antidepressants | Potential for cardiotoxicity and overdose, anticholinergic properties |
| Benzodiazepines | Chronic use can lead to cognitive impairment, falls |
| Buspirone | Effective for GAD, but not panic disorder; may take 2 to 4 weeks to be effective |
| GAD: generalized anxiety disorder; OCD: obsessive-compulsive disorder; PTSD: posttraumatic stress disorder | |
| Source: Reference 35 | |
Related Resources
- Wetherell JL, Lenze EJ, Stanley MA. Evidence-based treatment of geriatric anxiety disorders. Psychiatr Clin North Am. 2005; 28(4): 871-896, ix.
- Lenze EJ, Wetherell JL. Anxiety disorders. In: Blazer DG, Steffens DC, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. Arlington, VA: American Psychiatric Publishing, Inc; 2009: 333-345.
Drug Brand Name
- Buspirone • BuSpar
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Fuentes K, Cox BJ. Prevalence of anxiety disorders in elderly adults: a critical analysis. J Behav Ther Exp Psychiatry. 1997;28:269-279.
2. Préville M, Hérbert R, Bravo G, et al. Predisposing and facilitating factors of severe psychological distress among frail elderly. Can J Aging. 2002;21:195-204.
3. Le Roux H, Gatz M, Wetherell JL. Age at onset of generalized anxiety disorder in older adults. Am J Geriatr Psychiatry. 2005;13:23-30.
4. Beekman AT, Bremmer MA, Deeg DJ, et al. Anxiety disorders in later life: a report from the Longitudinal Aging Study Amsterdam. Int J Geriatr Psychiatry. 1998;13:717-726.
5. Regier DA, Rae DS, Narrow WE, et al. Prevalence of anxiety disorders and their comorbidity with mood and addictive disorders. Br J Psychiatry Suppl. 1998;34:24-28.
6. Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood anxiety, and substance-abuse disorders for older Americans in the national comorbidity survey-replication. Am J Geriatr Psychiatry. 2009;17(9):769-781.
7. Sareen J, Jacobi F, Cox BJ, et al. Disability and poor quality of life associated with comorbid anxiety disorders and physical conditions. Arch Intern Med. 2006;166:2109-2116.
8. Porensky EK, Dew MA, Karp JF, et al. The burden of late-life generalized anxiety disorder: effects on disability, health-related quality of life, and healthcare utilization. Am J Geriatr Psychiatry. 2009;17(6):473-482.
9. Tinetti ME, Inouye SK, Gill TM, et al. Shared risk-factors for falls, incontinence, and functional dependence: unifying the approach to geriatric syndromes. JAMA. 1995;273:1348-1353.
10. Lenze EJ, Karp JF, Mulsant BH, et al. Somatic symptoms in late-life anxiety: treatment issues. J Geriatr Psychiatry Neurol. 2005;18:89-96.
11. Gibbons LE, Teri L, Logsdon R, et al. Anxiety symptoms as predictors of nursing home placement in patients with Alzheimer’s disease. Journal of Clinical Geropsychology. 2002;4:335-342.
12. de Beurs E, Beekman AT, van Balkom AJ, et al. Consequences of anxiety in older persons: its effect on disability, well-being and use of health services. Psychol Med. 1999;29(3):583-593.
13. Schoevers RA, Beekman AT, Deeg DJ, et al. Comorbidity and risk-patterns of depression, generalised anxiety disorder and mixed anxiety-depression in later life: results from the AMSTEL study. Int J Geriatr Psychiatry. 2003;18:944-1001.
14. Wilk J, West J, Narrow W, et al. Are anxiety disorders underdiagnosed and undertreated in routine psychiatric practice? Poster presented at: AcademyHealth Annual Meeting; June 8, 2004; San Diego, CA.
15. Chou KL. Age at onset of generalized anxiety disorder in older adults. Am J Geriatr Psychiatry. 2009;17(6):455-464.
16. Howland J, Peterson EW, Levin WC, et al. Fear of falling among the community-dwelling elderly. J Aging Health. 1993;5(2):229-243.
17. Kohn R, Westlake RJ, Rasmussen SA, et al. Clinical features of obsessive-compulsive disorder in elderly patients. Am J Geriatr Psychiatry. 1997;5(3):211-215.
18. Flint AJ. Epidemiology and comorbidity of anxiety disorders in the elderly. Am J Psychiatry. 1994;151:640-649.
19. Skoog G, Skoog I. A 40-year follow-up of patients with obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;56(2):121-127.
20. Nestadt G, Bienvenu OJ, Cai G, et al. Incidence of obsessive-compulsive disorder in adults. J Nerv Ment Dis. 1998;186:401-406.
21. Chacko RC, Corbin MA, Harper RG. Acquired obsessive-compulsive disorder associated with basal ganglia lesions. J Neuropsychiatry Clin Neurosci. 2000;12:269-272.
22. Mittal D, Torres R, Abashidze A, et al. Worsening of post-traumatic stress disorder symptoms with cognitive decline: case series. J Geriatr Psychiatry Neurol. 2001;14(1):17-20.
23. Tedstone JE, Tarrier N. Posttraumatic stress disorder following medical illness and treatment. Clin Psychol Rev. 2003;23(3):409-448.
24. Creamer M, Parslow R. Trauma exposure and posttraumatic stress disorder in the elderly: a community prevalence study. Am J Ger Psychiatry. 2008;16:853-856.
25. Alcalde Tirado P. Fear of falling. Rev Esp Geriatr Gerontol. 2010;45(1):38-44.
26. Boyd R, Stevens JA. Falls and fear of falling: burden beliefs and behaviours. Age Ageing. 2009;38(4):423-428.
27. Cairney J, McCabe L, Veldhuizen S, et al. Epidemiology of social phobia in later life. Am J Geriatr Psychiatry. 2007;15(3):224-233.
28. Pontillo DC, Lang AJ, Stein MB. Management and treatment of anxiety disorders in the older patient. Clinical Geriatrics. 2002;10(10):38-49.
29. McCabe L, Cairney J, Veldhuizen S, et al. Prevalence and correlates of agoraphobia in older adults. Am J Geriatr Psychiatry. 2006;14(6):515-522.
30. Hassan R, Pollard CA. Late-life-onset panic disorder: clinical and demographic characteristics of a patient sample. J Geriatr Psychiatry Neurol. 1994;7:86-90.
31. Beitman BD, Kushner M, Grossberg GT. Late onset panic disorder: evidence from a study of patients with chest pain and normal cardiac evaluations. Int J Psychiatry Med. 1991;21(1):29-35.
32. Garvey MJ. Panic disorder: guidelines to safe use of benzodiazepines. Geriatrics. 1993;48(7):49-58.
33. Schuurmans J, Comijs HC, Beekman AT, et al. The outcome of anxiety disorders in older people at six-year follow-up: results from the Longitudinal Aging Study Amsterdam. Acta Psychiatr Scand. 2005;111:420-428.
34. Von Moltke LL, Abernethy DR, Greenblatt DJ. Kinetics and dynamics of psychotropic drugs in the elderly. In: Salzman C ed. Clinical geriatric psychopharmacology. 3rd ed. Baltimore, MD: Williams and Wilkins; 1998:70-93.
35. Baldwin DS, Anderson IM, Nutt DJ, et al and the British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2005;19(6):567-596.
36. Sheehan DV, Raj AB, Sheehan KH, et al. Is buspirone effective for panic disorder? J Clin Psychopharmacol. 1990;10(1):3-11.
37. Black DW. Efficacy of combined pharmacotherapy and psychotherapy versus monotherapy in the treatment of anxiety disorders. CNS Spectr. 2006;11(10 suppl 12):29-33.
38. Stanley MA, Wilson NL, Novy DM, et al. Cognitive behavior therapy for generalized anxiety disorder among older adults in primary care: a randomized clinical trial. JAMA. 2009;301(14):1460-1467.
1. Fuentes K, Cox BJ. Prevalence of anxiety disorders in elderly adults: a critical analysis. J Behav Ther Exp Psychiatry. 1997;28:269-279.
2. Préville M, Hérbert R, Bravo G, et al. Predisposing and facilitating factors of severe psychological distress among frail elderly. Can J Aging. 2002;21:195-204.
3. Le Roux H, Gatz M, Wetherell JL. Age at onset of generalized anxiety disorder in older adults. Am J Geriatr Psychiatry. 2005;13:23-30.
4. Beekman AT, Bremmer MA, Deeg DJ, et al. Anxiety disorders in later life: a report from the Longitudinal Aging Study Amsterdam. Int J Geriatr Psychiatry. 1998;13:717-726.
5. Regier DA, Rae DS, Narrow WE, et al. Prevalence of anxiety disorders and their comorbidity with mood and addictive disorders. Br J Psychiatry Suppl. 1998;34:24-28.
6. Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood anxiety, and substance-abuse disorders for older Americans in the national comorbidity survey-replication. Am J Geriatr Psychiatry. 2009;17(9):769-781.
7. Sareen J, Jacobi F, Cox BJ, et al. Disability and poor quality of life associated with comorbid anxiety disorders and physical conditions. Arch Intern Med. 2006;166:2109-2116.
8. Porensky EK, Dew MA, Karp JF, et al. The burden of late-life generalized anxiety disorder: effects on disability, health-related quality of life, and healthcare utilization. Am J Geriatr Psychiatry. 2009;17(6):473-482.
9. Tinetti ME, Inouye SK, Gill TM, et al. Shared risk-factors for falls, incontinence, and functional dependence: unifying the approach to geriatric syndromes. JAMA. 1995;273:1348-1353.
10. Lenze EJ, Karp JF, Mulsant BH, et al. Somatic symptoms in late-life anxiety: treatment issues. J Geriatr Psychiatry Neurol. 2005;18:89-96.
11. Gibbons LE, Teri L, Logsdon R, et al. Anxiety symptoms as predictors of nursing home placement in patients with Alzheimer’s disease. Journal of Clinical Geropsychology. 2002;4:335-342.
12. de Beurs E, Beekman AT, van Balkom AJ, et al. Consequences of anxiety in older persons: its effect on disability, well-being and use of health services. Psychol Med. 1999;29(3):583-593.
13. Schoevers RA, Beekman AT, Deeg DJ, et al. Comorbidity and risk-patterns of depression, generalised anxiety disorder and mixed anxiety-depression in later life: results from the AMSTEL study. Int J Geriatr Psychiatry. 2003;18:944-1001.
14. Wilk J, West J, Narrow W, et al. Are anxiety disorders underdiagnosed and undertreated in routine psychiatric practice? Poster presented at: AcademyHealth Annual Meeting; June 8, 2004; San Diego, CA.
15. Chou KL. Age at onset of generalized anxiety disorder in older adults. Am J Geriatr Psychiatry. 2009;17(6):455-464.
16. Howland J, Peterson EW, Levin WC, et al. Fear of falling among the community-dwelling elderly. J Aging Health. 1993;5(2):229-243.
17. Kohn R, Westlake RJ, Rasmussen SA, et al. Clinical features of obsessive-compulsive disorder in elderly patients. Am J Geriatr Psychiatry. 1997;5(3):211-215.
18. Flint AJ. Epidemiology and comorbidity of anxiety disorders in the elderly. Am J Psychiatry. 1994;151:640-649.
19. Skoog G, Skoog I. A 40-year follow-up of patients with obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;56(2):121-127.
20. Nestadt G, Bienvenu OJ, Cai G, et al. Incidence of obsessive-compulsive disorder in adults. J Nerv Ment Dis. 1998;186:401-406.
21. Chacko RC, Corbin MA, Harper RG. Acquired obsessive-compulsive disorder associated with basal ganglia lesions. J Neuropsychiatry Clin Neurosci. 2000;12:269-272.
22. Mittal D, Torres R, Abashidze A, et al. Worsening of post-traumatic stress disorder symptoms with cognitive decline: case series. J Geriatr Psychiatry Neurol. 2001;14(1):17-20.
23. Tedstone JE, Tarrier N. Posttraumatic stress disorder following medical illness and treatment. Clin Psychol Rev. 2003;23(3):409-448.
24. Creamer M, Parslow R. Trauma exposure and posttraumatic stress disorder in the elderly: a community prevalence study. Am J Ger Psychiatry. 2008;16:853-856.
25. Alcalde Tirado P. Fear of falling. Rev Esp Geriatr Gerontol. 2010;45(1):38-44.
26. Boyd R, Stevens JA. Falls and fear of falling: burden beliefs and behaviours. Age Ageing. 2009;38(4):423-428.
27. Cairney J, McCabe L, Veldhuizen S, et al. Epidemiology of social phobia in later life. Am J Geriatr Psychiatry. 2007;15(3):224-233.
28. Pontillo DC, Lang AJ, Stein MB. Management and treatment of anxiety disorders in the older patient. Clinical Geriatrics. 2002;10(10):38-49.
29. McCabe L, Cairney J, Veldhuizen S, et al. Prevalence and correlates of agoraphobia in older adults. Am J Geriatr Psychiatry. 2006;14(6):515-522.
30. Hassan R, Pollard CA. Late-life-onset panic disorder: clinical and demographic characteristics of a patient sample. J Geriatr Psychiatry Neurol. 1994;7:86-90.
31. Beitman BD, Kushner M, Grossberg GT. Late onset panic disorder: evidence from a study of patients with chest pain and normal cardiac evaluations. Int J Psychiatry Med. 1991;21(1):29-35.
32. Garvey MJ. Panic disorder: guidelines to safe use of benzodiazepines. Geriatrics. 1993;48(7):49-58.
33. Schuurmans J, Comijs HC, Beekman AT, et al. The outcome of anxiety disorders in older people at six-year follow-up: results from the Longitudinal Aging Study Amsterdam. Acta Psychiatr Scand. 2005;111:420-428.
34. Von Moltke LL, Abernethy DR, Greenblatt DJ. Kinetics and dynamics of psychotropic drugs in the elderly. In: Salzman C ed. Clinical geriatric psychopharmacology. 3rd ed. Baltimore, MD: Williams and Wilkins; 1998:70-93.
35. Baldwin DS, Anderson IM, Nutt DJ, et al and the British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2005;19(6):567-596.
36. Sheehan DV, Raj AB, Sheehan KH, et al. Is buspirone effective for panic disorder? J Clin Psychopharmacol. 1990;10(1):3-11.
37. Black DW. Efficacy of combined pharmacotherapy and psychotherapy versus monotherapy in the treatment of anxiety disorders. CNS Spectr. 2006;11(10 suppl 12):29-33.
38. Stanley MA, Wilson NL, Novy DM, et al. Cognitive behavior therapy for generalized anxiety disorder among older adults in primary care: a randomized clinical trial. JAMA. 2009;301(14):1460-1467.
Guidelines and ICDs
A recent analysis of the ICD Registry from the National Cardiovascular Data Registry raises significant concerns about the effectiveness of the treatment guidelines for implantable cardioverter defibrillators for the primary prevention of sudden cardiac arrhythmic death.
That study indicates that, although the guidelines as proposed by the sponsoring societies were adhered to in most implantations, a disappointing one-quarter of the implantations were outside of the guidelines. Of the over 111,707 ICDs implanted between 2006 and 2009, 25,145 (22.5%) were implanted in patients who were outside of the recommended guidelines. Certainly one can make a case for the fact that these are only guidelines, and doctor should have the prerogative to make clinical decisions, but there have been few guidelines that have been as carefully explored as those for ICD implantation. They emphasize not only the benefit of the devices implanted within the guidelines, but the hazards of the implantation outside of the guidelines (JAMA 2011;305:43-9).
Four guideline deviations were identified and included implantation carried out within 40 days of an acute MI, in 37%; within 3 months of coronary artery bypass surgery, in 3%; in patients with New York Heart Association class IV symptoms, in 12%; and in newly diagnosed heart failure, in 62%. There are adequate randomized clinical trials that clearly show the lack of benefit or increased risks of implantation in these four classes of patients.
Both the cardiology community and the device manufacturers have emphasized the importance of ICD therapy in as many individuals who fit the criteria for implantation as possible. Despite this effort, the implantation of ICDs on the potential candidates with systolic heart failure has significantly lagged. These results may have a further cooling effect on the rate of future implantation.
There are adequate randomized clinical trials that clearly demonstrate the lack of benefit or increased risks of implantation in these four classes of heart failure patients. The delay in implantation in new heart failure patients has been dictated by the observations that many individuals improve cardiac function after an acute event. In addition, a number of studies have shown that implantation at time of surgery is without merit and with some risk, and we have learned that the mode of death in NYHA class IV patients is dominated by progressive heart failure and not primary arrhythmias.
The use of ICDs within 40 days of an acute MI is of particular importance. Several studies have raised concern about the increase in heart failure mortality in patients who have experienced both appropriate and inappropriate ICD discharge for arrhythmias. It is unclear whether the increased heart failure precedes or is a result of ICD discharge. Similar observations were made in patients in whom an ICD was implanted early after an acute MI for the primary prevention of arrhythmic deaths. A recent study of the Defibrillation in Acute Myocardial Infarction Trial (DINAMIT) re-examines the observation that that heart failure mortality increased in patients who received an ICD shock (Circulation 2010;122:2645-52). DINAMIT enrolled patients 6–40 days after an acute MI in patients with left ventricular ejection fraction of less than 35%. Although ICD therapy decreased arrhythmic deaths, there was an increase in heart failure mortality in those patients who had an ICD shock, which resulted in a net increased mortality.
Both the American College of Cardiology and the Heart Rhythm Society have expressed concern about the recent report and plan to further emphasize ICD guidelines. The HRS is cooperating with the Department of Justice in an investigation of the inappropriate Medicare payments of ICDs “to lend expertise concerning the proper guidelines for clinical decision making,” according to a statement. Should the Justice Department become involved in the appropriate use of medical guidelines, an entirely new disturbing dimension would be introduced in regard to guideline application. In the meantime, both cardiologists and noncardiologists should rethink their decisions in regard to the use of ICDs. They clearly represent an important medical advance that has saved the lives of many of our patients, but their use does have significant risks which must be balanced against their benefit.
A recent analysis of the ICD Registry from the National Cardiovascular Data Registry raises significant concerns about the effectiveness of the treatment guidelines for implantable cardioverter defibrillators for the primary prevention of sudden cardiac arrhythmic death.
That study indicates that, although the guidelines as proposed by the sponsoring societies were adhered to in most implantations, a disappointing one-quarter of the implantations were outside of the guidelines. Of the over 111,707 ICDs implanted between 2006 and 2009, 25,145 (22.5%) were implanted in patients who were outside of the recommended guidelines. Certainly one can make a case for the fact that these are only guidelines, and doctor should have the prerogative to make clinical decisions, but there have been few guidelines that have been as carefully explored as those for ICD implantation. They emphasize not only the benefit of the devices implanted within the guidelines, but the hazards of the implantation outside of the guidelines (JAMA 2011;305:43-9).
Four guideline deviations were identified and included implantation carried out within 40 days of an acute MI, in 37%; within 3 months of coronary artery bypass surgery, in 3%; in patients with New York Heart Association class IV symptoms, in 12%; and in newly diagnosed heart failure, in 62%. There are adequate randomized clinical trials that clearly show the lack of benefit or increased risks of implantation in these four classes of patients.
Both the cardiology community and the device manufacturers have emphasized the importance of ICD therapy in as many individuals who fit the criteria for implantation as possible. Despite this effort, the implantation of ICDs on the potential candidates with systolic heart failure has significantly lagged. These results may have a further cooling effect on the rate of future implantation.
There are adequate randomized clinical trials that clearly demonstrate the lack of benefit or increased risks of implantation in these four classes of heart failure patients. The delay in implantation in new heart failure patients has been dictated by the observations that many individuals improve cardiac function after an acute event. In addition, a number of studies have shown that implantation at time of surgery is without merit and with some risk, and we have learned that the mode of death in NYHA class IV patients is dominated by progressive heart failure and not primary arrhythmias.
The use of ICDs within 40 days of an acute MI is of particular importance. Several studies have raised concern about the increase in heart failure mortality in patients who have experienced both appropriate and inappropriate ICD discharge for arrhythmias. It is unclear whether the increased heart failure precedes or is a result of ICD discharge. Similar observations were made in patients in whom an ICD was implanted early after an acute MI for the primary prevention of arrhythmic deaths. A recent study of the Defibrillation in Acute Myocardial Infarction Trial (DINAMIT) re-examines the observation that that heart failure mortality increased in patients who received an ICD shock (Circulation 2010;122:2645-52). DINAMIT enrolled patients 6–40 days after an acute MI in patients with left ventricular ejection fraction of less than 35%. Although ICD therapy decreased arrhythmic deaths, there was an increase in heart failure mortality in those patients who had an ICD shock, which resulted in a net increased mortality.
Both the American College of Cardiology and the Heart Rhythm Society have expressed concern about the recent report and plan to further emphasize ICD guidelines. The HRS is cooperating with the Department of Justice in an investigation of the inappropriate Medicare payments of ICDs “to lend expertise concerning the proper guidelines for clinical decision making,” according to a statement. Should the Justice Department become involved in the appropriate use of medical guidelines, an entirely new disturbing dimension would be introduced in regard to guideline application. In the meantime, both cardiologists and noncardiologists should rethink their decisions in regard to the use of ICDs. They clearly represent an important medical advance that has saved the lives of many of our patients, but their use does have significant risks which must be balanced against their benefit.
A recent analysis of the ICD Registry from the National Cardiovascular Data Registry raises significant concerns about the effectiveness of the treatment guidelines for implantable cardioverter defibrillators for the primary prevention of sudden cardiac arrhythmic death.
That study indicates that, although the guidelines as proposed by the sponsoring societies were adhered to in most implantations, a disappointing one-quarter of the implantations were outside of the guidelines. Of the over 111,707 ICDs implanted between 2006 and 2009, 25,145 (22.5%) were implanted in patients who were outside of the recommended guidelines. Certainly one can make a case for the fact that these are only guidelines, and doctor should have the prerogative to make clinical decisions, but there have been few guidelines that have been as carefully explored as those for ICD implantation. They emphasize not only the benefit of the devices implanted within the guidelines, but the hazards of the implantation outside of the guidelines (JAMA 2011;305:43-9).
Four guideline deviations were identified and included implantation carried out within 40 days of an acute MI, in 37%; within 3 months of coronary artery bypass surgery, in 3%; in patients with New York Heart Association class IV symptoms, in 12%; and in newly diagnosed heart failure, in 62%. There are adequate randomized clinical trials that clearly show the lack of benefit or increased risks of implantation in these four classes of patients.
Both the cardiology community and the device manufacturers have emphasized the importance of ICD therapy in as many individuals who fit the criteria for implantation as possible. Despite this effort, the implantation of ICDs on the potential candidates with systolic heart failure has significantly lagged. These results may have a further cooling effect on the rate of future implantation.
There are adequate randomized clinical trials that clearly demonstrate the lack of benefit or increased risks of implantation in these four classes of heart failure patients. The delay in implantation in new heart failure patients has been dictated by the observations that many individuals improve cardiac function after an acute event. In addition, a number of studies have shown that implantation at time of surgery is without merit and with some risk, and we have learned that the mode of death in NYHA class IV patients is dominated by progressive heart failure and not primary arrhythmias.
The use of ICDs within 40 days of an acute MI is of particular importance. Several studies have raised concern about the increase in heart failure mortality in patients who have experienced both appropriate and inappropriate ICD discharge for arrhythmias. It is unclear whether the increased heart failure precedes or is a result of ICD discharge. Similar observations were made in patients in whom an ICD was implanted early after an acute MI for the primary prevention of arrhythmic deaths. A recent study of the Defibrillation in Acute Myocardial Infarction Trial (DINAMIT) re-examines the observation that that heart failure mortality increased in patients who received an ICD shock (Circulation 2010;122:2645-52). DINAMIT enrolled patients 6–40 days after an acute MI in patients with left ventricular ejection fraction of less than 35%. Although ICD therapy decreased arrhythmic deaths, there was an increase in heart failure mortality in those patients who had an ICD shock, which resulted in a net increased mortality.
Both the American College of Cardiology and the Heart Rhythm Society have expressed concern about the recent report and plan to further emphasize ICD guidelines. The HRS is cooperating with the Department of Justice in an investigation of the inappropriate Medicare payments of ICDs “to lend expertise concerning the proper guidelines for clinical decision making,” according to a statement. Should the Justice Department become involved in the appropriate use of medical guidelines, an entirely new disturbing dimension would be introduced in regard to guideline application. In the meantime, both cardiologists and noncardiologists should rethink their decisions in regard to the use of ICDs. They clearly represent an important medical advance that has saved the lives of many of our patients, but their use does have significant risks which must be balanced against their benefit.