User login
Pediatric Hospitalist Role in Transition
Optimal health care is achieved when every person at every age receives health care that is medically and developmentally appropriate.1 For healthy patients, medically and developmentally appropriate care is usually available, but for children with special healthcare needs (SHCN), receiving this care can be a challenge that is magnified as a child with SHCN grows into a teenager and then a young adult. For these children, simply transferring care, which is essentially a handoff of responsibility, to adult providers is insufficient to meet the needs of a special healthcare population.1 Transition is the purposeful planned movement of adolescents and young adults with chronic medical conditions and disabilities from pediatric (child‐centered) to adult‐oriented providers and facilities.2
The 2005‐2006 National Survey of Children with SHCN identified 4 component measures of transition which included discussions between the patient and the healthcare provider about: 1) shifting to adult providers, 2) adult healthcare needs, 3) health insurance, and 4) encouraging the patient to take responsibility for his/her care.34 Overall, only 41% of youth with SHCN met the core outcomes.4 The survey also found that those most affected by their health conditions were less likely to have transition discussions compared with less‐affected youth.3
The importance of healthcare transition also resonates with pediatric hospitalists. The results of a 1988 National Health Survey revealed that 4% of all children with SHCN were hospitalized, and 2% of those with severe chronic conditions accounted for 27% of all hospital bed days.5 Or, stated differently, a small percentage of patients are admitted most frequently and stay the longest in the hospital. Further, transition for those with significant cognitive delay was more difficult, because of the lack of adult‐oriented providers who are willing to care for the patient and work collaboratively with the family.6 This has a significant implication for pediatric hospitalists, because it increases the likelihood that a severely affected 21 to 25‐year‐old patient will be admitted to the pediatric hospitalist service having not yet made a successful transition to an adult‐oriented provider.
Clearly, transition of care should have its roots in the outpatient medical home; however, often a patient with a chronic medical condition will spend extended periods of time in the hospital and away from their identified medical home. Although not a widely accepted concept, some may consider the pediatric hospitalist service to be an extension of the medical home for inpatients. The pediatric hospitalist is often the physician who cares for inpatients with SHCN because of their complexity. There are components of the transition process that specifically deal with hospitalization. Pediatric hospitalists should understand transition and their role in it, so that the process does not stop when patients are hospitalized.
Our hypothesis is that pediatric hospitalists are well poised to provide inpatient transition services, but insufficient understanding of the concepts and practical processes related to transition limit involvement. Through this exploratory survey, we hope to understand current attitudes and knowledge about transition. We survey the degree to which pediatric hospitalists want to participate in the process, the level of support that healthcare transition services receive from the institutions in which pediatric hospitalists practice, and potential barriers and benefits of their participation.
METHODS
Participants
After a review of current literature, we developed an exploratory survey for pediatric hospitalists that was approved by our institutional review board, and was reviewed for content and face validity by a qualitative expert on transition of care. Using Survey Monkey, the survey was piloted with a small group of pediatric hospitalists for feedback regarding the clarity of the survey questions before it was introduced to the American Academy of Pediatrics (AAP)/Pediatric Hospital Medicine Listserv. The Listserv is available to any pediatric hospitalist that joins. The exact number of pediatric hospitalists is unknown. A reasonable approximation of Listserv members at the time the survey was introduced is 1800. A fraction of that number is active on the Listserv, as defined by multiple postings during the course of an academic year. Pediatric hospitalists were the targeted group because of their expertise in the care of the adolescent and, often, young adult, with SHCN. The survey was voluntary and anonymous, and was reintroduced 3 times to capture as many participants as possible. The purpose of the survey was to gauge the interest, attitudes, and understanding of healthcare transition in a cross section of pediatric hospitalists.
Definitions
For clarity, in this survey, transfer is defined as an event, a handoff of responsibility for the management of a patient from one physician to another. Transition is defined as the purposeful planned movement of adolescents and young adults, with chronic medical conditions and disabilities, from pediatric (child‐centered) to adult‐oriented providers and facilities.2
Survey
This questionnaire contained 33 items and included a mixture of open‐ended questions, yes and no questions, and questions with responses that used a modified Likert scale. The survey questions were not adapted from another study or survey; they were developed in conjunction with 2 well‐published experts on the subject of transition. The demographic questions were used to help determine whether patterns related to transition services and knowledge could be detected based on age, gender, or type of practice, whether academic or community. The survey content included several areas: 1) Who is responsible for the care of young adults and adolescents; 2) Hospital‐based transition services; 3) Benefits or challenges for pediatric hospitalists who become involved in health care transition (HCT); 4) Knowledge of HCT and education opportunities. Informed consent was the first page; if consent was obtained, the participant could move forward in the survey (Table 1).
| Who is primarily responsible for inpatient care of patients 16‐21? |
| Please rate your knowledge of healthcare transition. |
| Are there healthcare transition services in your hospital? |
| How old are patients when they first receive inpatient‐oriented healthcare transition services? |
| How beneficial are these services to the patient and the provider if available? |
| Does your hospital have a policy that mandates the age by which an adolescent/young adult patient must be transferred from Pediatrics to adult providers and facilities? |
| What factors determine age of transfer? |
| How big a problem is it transferring adolescent/young adult patients from Pediatrics to adult providers and facilities? |
| How prepared are adolescents and young adults in your institution for transition to adult services? |
| Should pediatric hospitalists be involved in providing healthcare transition services and supports to patients with chronic healthcare conditions in the inpatient setting? |
| How often have you been asked by a subspecialist to provide healthcare transition services and supports to patients with chronic health conditions in the inpatient setting? |
| Who is best qualified to provide healthcare transition services? |
| What are the biggest impediments and benefits to hospitalist involvement in healthcare transition services? |
| If there was an online educational training module about healthcare transitions, would you take it? |
RESULTS
There were 131 participants who consented to participate in, and completed, the survey.
Demographics
Of all participants, 42.5% identified their primary practice site as a pediatric hospital; 40.8% identified their primary practice setting as a children's hospital within a general hospital, and 15.8% identified their primary practice setting as a general hospital with pediatric beds but no designation as a children's hospital. The participants came from nearly every state in the United States; 69.2% were women and 30.8% were men. The ages ranged from 27 to 67, with the majority of participants in their mid‐30s and 40s. Most were boarded in Pediatrics and/or Internal Medicine with some subspecialties such as Physical Medical and Rehabilitation, Cardiology, Critical Care, Pulmonology, and Developmental and Behavioral Pediatrics represented. Although the sample size is small, it is representative of the larger population of pediatric hospitalists. There were no patterns detected based on demographics relative to the knowledge or participation in healthcare transition (Table 2).
| Male | Female | |
|---|---|---|
| Age range | 3267 years | 2761 years |
| Boarded in Pediatrics/Pediatric subspecialty | 92% | 99% |
| Practice setting | ||
| Pediatric hospital | 51.4% | 38.6% |
| General hospital with a pediatric hospital within it | 27% | 47% |
| General hospital with pediatric beds but no children's hospital designation | 21.6% | 13.3% |
| Other | 8.1% | 7.2% |
Which Groups of Physicians Are Caring for Adolescent and Young Adult Patients?
Establishing whether pediatric subspecialists, adult providers, or pediatric hospitalists are the primary caregivers for adolescents and young adults with SCHN is important to determine whether the pediatric hospitalist is really well poised to deal with transition issues. If the pediatric hospitalist does not care for these patients, then developing modules to educate them about healthcare transition may not be necessary. As expected, pediatric hospitalists believe they care for adolescent and young adult patients with special healthcare needs in the vast majority of cases. Table 3 illustrates in more detail who is specifically responsible for their care.
| Patient Age | ||
|---|---|---|
| 1617 | 1820 | |
| ||
| Inpatient care provider | ||
| Pediatric hospitalist | 70.1%* | 36.8% |
| Adult hospitalist | 0.9% | 27.4% |
| Pediatric subspecialist | 27.4% | 25.6% |
| Adult subspecialist | 0.9% | 2.6% |
| Other/not sure | 7.7% | 0.9% |
Knowledge of Healthcare Transition
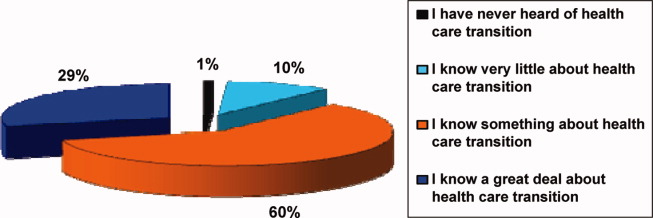
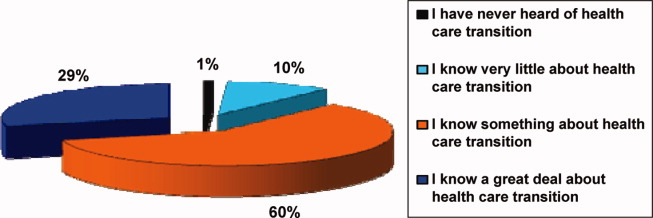
Participants in the survey were provided definitions of healthcare transition; they were asked to rate their knowledge of healthcare transition on a modified Likert scale, given the definition provided. The results can be seen in Figure 1.
Transition Programs
Of all participants, 60.9% did not know if their hospital had inpatient‐oriented healthcare transition services. Another 27.8% only had informal or unstructured services for some patients with a chronic condition, and less than 1% of all respondents said they had a formal or structured program at their institution for inpatients with any chronic medical condition. Eighty percent thought transferring adolescent or young adult patients from pediatric to adult providers was a moderate to major problem.
Of those who responded to the survey, 97.6% feel that inpatient‐oriented healthcare transition services would be beneficial to adolescent and young adult patients, and 92.2% felt that these supports would be beneficial to pediatric providers. This is consistent with the data from another question in which respondents felt that only 1% of patients were quite a bit prepared for transition to inpatient adult providers, and that over half were only a little bit or not at all prepared.
Institutional Mandates
Nearly 40% of institutions have a mandated age by which adolescent and young adult patients must be transferred to adult providers and facilities. Additionally, only 5.2% of those institutions have a written procedure or protocol that describes how these pediatric patients will be transferred to adult providers and institutions.
Pediatric Hospitalist Participation in Transition
Sixty‐eight percent of respondents believe that the patient's primary care provider is the most qualified to discuss healthcare transition issues, followed by their pediatric subspecialists. However, more than 75% of respondents agree or strongly agree that pediatric hospitalists should be involved in providing healthcare transition services and supports to inpatients with chronic health conditions. Please refer to Table 4. Despite this, 58% of pediatric hospitalists are rarely, if ever, asked to participate in healthcare transition by their subspecialist counterparts.
| Respondents, % (No.) | |
|---|---|
| Strongly agree | 28.6 (28) |
| Agree | 50.0 (49) |
| Neither agree or disagree | 12.2 (12) |
| Disagree | 6.1 (6) |
| Strongly disagree | 3.1 (3) |
Barriers to Pediatric Hospitalist Participation in Transition
The survey participants were given a list of potential barriers to participation in healthcare transition and were asked to rank 3 of the choices in order of significance, with 1 being the biggest perceived impediment and 3 being the least significant. Seventeen percent ranked lack of familiarity with healthcare transition resources as the biggest barrier in their setting. Thirteen percent indicated that lack of support from pediatric and adult subspecialists is the major barrier, and 13% felt that insufficient time to provide transition services and supports would be the most significant barrier to their participation in transition. Interestingly, billing and reimbursement issues were not seen as obstacles (see Supporting Information 1/Table 5 in the online version of this article).
Advantages to Pediatric Hospitalist Participation in Transition
The participants were given a set of potential benefits that might result from the pediatric hospitalist participation in transition of care. They were asked to rank 3 of the choices in order of importance, with 1 being the most important positive outcome to 3 being a lesser, but still positive, outcome. Twenty‐three percent of respondents ranked improved communication between pediatric and adult providers and facilities as being the most significant advantage. Twenty‐one percent ranked both better continuity of care in the inpatient setting, and better quality of care for adolescents and young adults with chronic healthcare conditions, as the most important potential advantages of pediatric hospitalist involvement in healthcare transition. However, most felt that improved cost effectiveness would not be an important result (see Supporting Information 2/Table 6 in the online version of this article).
Educational Process
If an educational module was offered, over half of the respondents would definitely or probably take the training, and another 22% might take the training.
LIMITATIONS
This is an exploratory study which is limited by the small number of participants. Although the demographics of the participants include both young and experienced hospitalists, as well as academic and community institutions, it is difficult to determine whether the results are truly representative of the larger pediatric hospitalist population. The survey was also too long which may have deterred participation. Given the lack of experience and literature on pediatric hospitalist involvement in transition of care, it is difficult to construct a concise survey that addresses all of the concerns of the diverse hospitalist population. This is a new area of exploration for pediatric hospitalists, and ideally new questions will arise out of these preliminary findings, despite the limitations in the survey. Future surveys should focus on singular issues related to transition, and every attempt should be made to increase participation in the survey.
DISCUSSION
The survey demonstrates that the majority of the pediatric hospitalists believe providing transition services is important, but that transition programs are, for all practical purposes, nonexistent. Hospitalists believe the primary care doctor or the subspecialists should direct the transition process, but most clearly believe that their participation in the process would be beneficial for their patients, as evidenced by a 97.6% positive response to that question in the survey. Transition of care should be handled predominantly in the medical home.1 At this point, there is no literature that describes a pediatric hospitalist service as an inpatient medical home. However, pediatric hospitalists, not pediatric subspecialists, care for the majority of patients with SHCN in the transition age range while they are hospitalized; therefore, continuing the transition discussion while a patient is hospitalized may be a key component to its success. Better quality and continuity of care for the inpatient with SHCN is a potential advantage, as is coordination of services. Having the support of the pediatric subspecialists and the pediatric primary care provider is not only important, but it is critical in successful transition. Further, most pediatric hospitalists identify transfer of pediatric patients to adult providers as a major problem, and the perception is that only 1% of patients are adequately prepared for this transfer of care. Few institutions have a formalized process by which patients are transferred to adult care providers; however, many institutions have a mandated age at which they expect transfer of care to occur.
The literature about transition of care highlights the issues in the outpatient setting. Reiss and Gibson used focus groups comprised of caregivers, and youth and young adults with SHCN, to explore the issues related to transition of care.7 They identified several factors associated with successful transition that are pertinent to inpatient pediatrics. Involving the patient as a responsible member of the treatment team is important because it fosters independence and problem solving.7 Advocating this in the inpatient setting gives the patient and caregivers confidence that the patient can participate in the healthcare process, thereby making it a habit.7 A second important factor is attending to the patient's personal preferences and interpersonal dynamics.7 Many adolescents and young adult patients prefer to be cared for by their pediatric providers on adult wards away from crying infants and children.7 This may be their first indication that they'd like to explore the adult medical world.7
Pediatric hospitalists should be prepared to meet the needs of adolescent and young adult patients with SCHN by becoming familiar with the components of the transition process. Saidi and Kovacs provide a checklist of practical transition strategies that are helpful to review, and many are quite pertinent to the practice of pediatric hospital medicine.8 Education is a fundamental aspect of the identity of the pediatric hospitalist and is also the foundation of the transition process. Identifying established institutional transition resources, developing educational tools for faculty and residents to learn about transition, as well as adapting the transition checklist to inpatient needs are useful tools for developing a culture of effective healthcare transition. Simple strategies, such as speaking to young patients on their own, and displaying a public commitment to transition are other easy changes that can be made to the everyday activities of the pediatric hospitalist.8
Other key issues that would be important for the pediatric hospitalist to address are the adolescents' understanding of his/her disease, current treatments, long‐term complications, and the impact of healthy and unhealthy behaviors.9 Because these issues can directly affect his/her hospitalization, the pediatric hospitalist should play a role in discussing these issues and reaffirming their importance in the overall health of the patient. This affirmation will also support the process of transition, and will give further confidence to the patient and family that the patient is becoming a responsible member of the healthcare team.
Many of the strategies espoused by experts in transition are part of what the pediatric hospitalist does regularly. The pediatric hospitalist is a resource for patients, families, and subspecialists, because of their comfort and expertise managing complex pediatric patients and because of their understanding of the hospital and how it functions. The process of transition of care should be part of what pediatric hospitalists are prepared to teach, because of the numbers of adolescent and young adult patients that are in their care. The current knowledge base for most pediatric hospitalists seems to be a basic understanding of what transition of care means, but little knowledge about how to go about engaging in the process. More and more transition is relevant to primary care doctors and hospitalists, as the medically complex patient survives into adolescence and adulthood.
CONCLUSION
The survey provides a snapshot of the current attitudes and beliefs of pediatric hospitalists relative to involvement in healthcare transition. This article addresses what we believe to be important questions for the pediatric hospitalist to ask, prior to becoming involved in healthcare transition. Our hypothesis, that pediatric hospitalists are well poised to provide inpatient transition services but are limited by lack of understanding of the concepts and process, is supported by the responses in the survey which show that pediatric hospitalists are interested in participating in healthcare transition but feel impeded by time, support, and understanding of the process of transition. A larger sample size is needed to strengthen the data and lend support to these observations. Additionally, more research to compare current models of transition services and a hospitalist model could be important in realizing the potential positive outcomes predicted in this survey. Education and resources for transition of care are inadequate. Targeted educational modules might provide a foundation for pediatric hospitalists to build their scope of practice to include transition services. The next step for interested pediatric hospitalists might be developing a web‐based module that addresses the unique needs of the inpatient provider and the chronically ill pediatric patient who spends a great deal of time as an inpatient. The measurable outcomes for such an intervention might well be the feeling of preparation that the family and patient have as they move into the adult provider world.
- A consensus statement on health care transitions for young adults with special health care needs.Pediatrics.2002;110(6 pt 2):1304–1306.
- ,,,,,.Perceptions of transitional care needs and experiences in pediatric heart transplant recipients.Am J Transplant.2009;9(3):614–619.
- ,,,,,.Planning for health care transitions: results from the 2005–2006 National Survey of Children With Special Health Care Needs.Pediatrics.2009;123(1):e145–e152.
- US Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Bureau.The National Survey of Children With Special Health Care Needs, Chartbook 2005–2006.Rockville, MD:US Department of Health and Human Services;2008.
- ,,,,,.Changes in hospitalization patterns among patients with congenital heart disease during the transition from adolescence to adulthood.J Am Coll Cardiol.2007;49(8):875–882.
- ,,.Health care transition: youth, family, and provider perspectives.Pediatrics.2005;115(1):112–120.
- ,.Health care transition: destinations unknown.Pediatrics.2002;110(6 pt 2):1307–1314.
- ,.Developing a transition program from pediatric‐ to adult‐focused cardiology care: practical considerations.Congenit Heart Dis.2009;4(4):204–215.
- .Transition for youth with chronic conditions: primary care physicians' approaches.Pediatrics.2002;110(6 pt 2):1315–1321.
Optimal health care is achieved when every person at every age receives health care that is medically and developmentally appropriate.1 For healthy patients, medically and developmentally appropriate care is usually available, but for children with special healthcare needs (SHCN), receiving this care can be a challenge that is magnified as a child with SHCN grows into a teenager and then a young adult. For these children, simply transferring care, which is essentially a handoff of responsibility, to adult providers is insufficient to meet the needs of a special healthcare population.1 Transition is the purposeful planned movement of adolescents and young adults with chronic medical conditions and disabilities from pediatric (child‐centered) to adult‐oriented providers and facilities.2
The 2005‐2006 National Survey of Children with SHCN identified 4 component measures of transition which included discussions between the patient and the healthcare provider about: 1) shifting to adult providers, 2) adult healthcare needs, 3) health insurance, and 4) encouraging the patient to take responsibility for his/her care.34 Overall, only 41% of youth with SHCN met the core outcomes.4 The survey also found that those most affected by their health conditions were less likely to have transition discussions compared with less‐affected youth.3
The importance of healthcare transition also resonates with pediatric hospitalists. The results of a 1988 National Health Survey revealed that 4% of all children with SHCN were hospitalized, and 2% of those with severe chronic conditions accounted for 27% of all hospital bed days.5 Or, stated differently, a small percentage of patients are admitted most frequently and stay the longest in the hospital. Further, transition for those with significant cognitive delay was more difficult, because of the lack of adult‐oriented providers who are willing to care for the patient and work collaboratively with the family.6 This has a significant implication for pediatric hospitalists, because it increases the likelihood that a severely affected 21 to 25‐year‐old patient will be admitted to the pediatric hospitalist service having not yet made a successful transition to an adult‐oriented provider.
Clearly, transition of care should have its roots in the outpatient medical home; however, often a patient with a chronic medical condition will spend extended periods of time in the hospital and away from their identified medical home. Although not a widely accepted concept, some may consider the pediatric hospitalist service to be an extension of the medical home for inpatients. The pediatric hospitalist is often the physician who cares for inpatients with SHCN because of their complexity. There are components of the transition process that specifically deal with hospitalization. Pediatric hospitalists should understand transition and their role in it, so that the process does not stop when patients are hospitalized.
Our hypothesis is that pediatric hospitalists are well poised to provide inpatient transition services, but insufficient understanding of the concepts and practical processes related to transition limit involvement. Through this exploratory survey, we hope to understand current attitudes and knowledge about transition. We survey the degree to which pediatric hospitalists want to participate in the process, the level of support that healthcare transition services receive from the institutions in which pediatric hospitalists practice, and potential barriers and benefits of their participation.
METHODS
Participants
After a review of current literature, we developed an exploratory survey for pediatric hospitalists that was approved by our institutional review board, and was reviewed for content and face validity by a qualitative expert on transition of care. Using Survey Monkey, the survey was piloted with a small group of pediatric hospitalists for feedback regarding the clarity of the survey questions before it was introduced to the American Academy of Pediatrics (AAP)/Pediatric Hospital Medicine Listserv. The Listserv is available to any pediatric hospitalist that joins. The exact number of pediatric hospitalists is unknown. A reasonable approximation of Listserv members at the time the survey was introduced is 1800. A fraction of that number is active on the Listserv, as defined by multiple postings during the course of an academic year. Pediatric hospitalists were the targeted group because of their expertise in the care of the adolescent and, often, young adult, with SHCN. The survey was voluntary and anonymous, and was reintroduced 3 times to capture as many participants as possible. The purpose of the survey was to gauge the interest, attitudes, and understanding of healthcare transition in a cross section of pediatric hospitalists.
Definitions
For clarity, in this survey, transfer is defined as an event, a handoff of responsibility for the management of a patient from one physician to another. Transition is defined as the purposeful planned movement of adolescents and young adults, with chronic medical conditions and disabilities, from pediatric (child‐centered) to adult‐oriented providers and facilities.2
Survey
This questionnaire contained 33 items and included a mixture of open‐ended questions, yes and no questions, and questions with responses that used a modified Likert scale. The survey questions were not adapted from another study or survey; they were developed in conjunction with 2 well‐published experts on the subject of transition. The demographic questions were used to help determine whether patterns related to transition services and knowledge could be detected based on age, gender, or type of practice, whether academic or community. The survey content included several areas: 1) Who is responsible for the care of young adults and adolescents; 2) Hospital‐based transition services; 3) Benefits or challenges for pediatric hospitalists who become involved in health care transition (HCT); 4) Knowledge of HCT and education opportunities. Informed consent was the first page; if consent was obtained, the participant could move forward in the survey (Table 1).
| Who is primarily responsible for inpatient care of patients 16‐21? |
| Please rate your knowledge of healthcare transition. |
| Are there healthcare transition services in your hospital? |
| How old are patients when they first receive inpatient‐oriented healthcare transition services? |
| How beneficial are these services to the patient and the provider if available? |
| Does your hospital have a policy that mandates the age by which an adolescent/young adult patient must be transferred from Pediatrics to adult providers and facilities? |
| What factors determine age of transfer? |
| How big a problem is it transferring adolescent/young adult patients from Pediatrics to adult providers and facilities? |
| How prepared are adolescents and young adults in your institution for transition to adult services? |
| Should pediatric hospitalists be involved in providing healthcare transition services and supports to patients with chronic healthcare conditions in the inpatient setting? |
| How often have you been asked by a subspecialist to provide healthcare transition services and supports to patients with chronic health conditions in the inpatient setting? |
| Who is best qualified to provide healthcare transition services? |
| What are the biggest impediments and benefits to hospitalist involvement in healthcare transition services? |
| If there was an online educational training module about healthcare transitions, would you take it? |
RESULTS
There were 131 participants who consented to participate in, and completed, the survey.
Demographics
Of all participants, 42.5% identified their primary practice site as a pediatric hospital; 40.8% identified their primary practice setting as a children's hospital within a general hospital, and 15.8% identified their primary practice setting as a general hospital with pediatric beds but no designation as a children's hospital. The participants came from nearly every state in the United States; 69.2% were women and 30.8% were men. The ages ranged from 27 to 67, with the majority of participants in their mid‐30s and 40s. Most were boarded in Pediatrics and/or Internal Medicine with some subspecialties such as Physical Medical and Rehabilitation, Cardiology, Critical Care, Pulmonology, and Developmental and Behavioral Pediatrics represented. Although the sample size is small, it is representative of the larger population of pediatric hospitalists. There were no patterns detected based on demographics relative to the knowledge or participation in healthcare transition (Table 2).
| Male | Female | |
|---|---|---|
| Age range | 3267 years | 2761 years |
| Boarded in Pediatrics/Pediatric subspecialty | 92% | 99% |
| Practice setting | ||
| Pediatric hospital | 51.4% | 38.6% |
| General hospital with a pediatric hospital within it | 27% | 47% |
| General hospital with pediatric beds but no children's hospital designation | 21.6% | 13.3% |
| Other | 8.1% | 7.2% |
Which Groups of Physicians Are Caring for Adolescent and Young Adult Patients?
Establishing whether pediatric subspecialists, adult providers, or pediatric hospitalists are the primary caregivers for adolescents and young adults with SCHN is important to determine whether the pediatric hospitalist is really well poised to deal with transition issues. If the pediatric hospitalist does not care for these patients, then developing modules to educate them about healthcare transition may not be necessary. As expected, pediatric hospitalists believe they care for adolescent and young adult patients with special healthcare needs in the vast majority of cases. Table 3 illustrates in more detail who is specifically responsible for their care.
| Patient Age | ||
|---|---|---|
| 1617 | 1820 | |
| ||
| Inpatient care provider | ||
| Pediatric hospitalist | 70.1%* | 36.8% |
| Adult hospitalist | 0.9% | 27.4% |
| Pediatric subspecialist | 27.4% | 25.6% |
| Adult subspecialist | 0.9% | 2.6% |
| Other/not sure | 7.7% | 0.9% |
Knowledge of Healthcare Transition
Participants in the survey were provided definitions of healthcare transition; they were asked to rate their knowledge of healthcare transition on a modified Likert scale, given the definition provided. The results can be seen in Figure 1.

Transition Programs
Of all participants, 60.9% did not know if their hospital had inpatient‐oriented healthcare transition services. Another 27.8% only had informal or unstructured services for some patients with a chronic condition, and less than 1% of all respondents said they had a formal or structured program at their institution for inpatients with any chronic medical condition. Eighty percent thought transferring adolescent or young adult patients from pediatric to adult providers was a moderate to major problem.
Of those who responded to the survey, 97.6% feel that inpatient‐oriented healthcare transition services would be beneficial to adolescent and young adult patients, and 92.2% felt that these supports would be beneficial to pediatric providers. This is consistent with the data from another question in which respondents felt that only 1% of patients were quite a bit prepared for transition to inpatient adult providers, and that over half were only a little bit or not at all prepared.
Institutional Mandates
Nearly 40% of institutions have a mandated age by which adolescent and young adult patients must be transferred to adult providers and facilities. Additionally, only 5.2% of those institutions have a written procedure or protocol that describes how these pediatric patients will be transferred to adult providers and institutions.
Pediatric Hospitalist Participation in Transition
Sixty‐eight percent of respondents believe that the patient's primary care provider is the most qualified to discuss healthcare transition issues, followed by their pediatric subspecialists. However, more than 75% of respondents agree or strongly agree that pediatric hospitalists should be involved in providing healthcare transition services and supports to inpatients with chronic health conditions. Please refer to Table 4. Despite this, 58% of pediatric hospitalists are rarely, if ever, asked to participate in healthcare transition by their subspecialist counterparts.
| Respondents, % (No.) | |
|---|---|
| Strongly agree | 28.6 (28) |
| Agree | 50.0 (49) |
| Neither agree or disagree | 12.2 (12) |
| Disagree | 6.1 (6) |
| Strongly disagree | 3.1 (3) |
Barriers to Pediatric Hospitalist Participation in Transition
The survey participants were given a list of potential barriers to participation in healthcare transition and were asked to rank 3 of the choices in order of significance, with 1 being the biggest perceived impediment and 3 being the least significant. Seventeen percent ranked lack of familiarity with healthcare transition resources as the biggest barrier in their setting. Thirteen percent indicated that lack of support from pediatric and adult subspecialists is the major barrier, and 13% felt that insufficient time to provide transition services and supports would be the most significant barrier to their participation in transition. Interestingly, billing and reimbursement issues were not seen as obstacles (see Supporting Information 1/Table 5 in the online version of this article).
Advantages to Pediatric Hospitalist Participation in Transition
The participants were given a set of potential benefits that might result from the pediatric hospitalist participation in transition of care. They were asked to rank 3 of the choices in order of importance, with 1 being the most important positive outcome to 3 being a lesser, but still positive, outcome. Twenty‐three percent of respondents ranked improved communication between pediatric and adult providers and facilities as being the most significant advantage. Twenty‐one percent ranked both better continuity of care in the inpatient setting, and better quality of care for adolescents and young adults with chronic healthcare conditions, as the most important potential advantages of pediatric hospitalist involvement in healthcare transition. However, most felt that improved cost effectiveness would not be an important result (see Supporting Information 2/Table 6 in the online version of this article).
Educational Process
If an educational module was offered, over half of the respondents would definitely or probably take the training, and another 22% might take the training.
LIMITATIONS
This is an exploratory study which is limited by the small number of participants. Although the demographics of the participants include both young and experienced hospitalists, as well as academic and community institutions, it is difficult to determine whether the results are truly representative of the larger pediatric hospitalist population. The survey was also too long which may have deterred participation. Given the lack of experience and literature on pediatric hospitalist involvement in transition of care, it is difficult to construct a concise survey that addresses all of the concerns of the diverse hospitalist population. This is a new area of exploration for pediatric hospitalists, and ideally new questions will arise out of these preliminary findings, despite the limitations in the survey. Future surveys should focus on singular issues related to transition, and every attempt should be made to increase participation in the survey.
DISCUSSION
The survey demonstrates that the majority of the pediatric hospitalists believe providing transition services is important, but that transition programs are, for all practical purposes, nonexistent. Hospitalists believe the primary care doctor or the subspecialists should direct the transition process, but most clearly believe that their participation in the process would be beneficial for their patients, as evidenced by a 97.6% positive response to that question in the survey. Transition of care should be handled predominantly in the medical home.1 At this point, there is no literature that describes a pediatric hospitalist service as an inpatient medical home. However, pediatric hospitalists, not pediatric subspecialists, care for the majority of patients with SHCN in the transition age range while they are hospitalized; therefore, continuing the transition discussion while a patient is hospitalized may be a key component to its success. Better quality and continuity of care for the inpatient with SHCN is a potential advantage, as is coordination of services. Having the support of the pediatric subspecialists and the pediatric primary care provider is not only important, but it is critical in successful transition. Further, most pediatric hospitalists identify transfer of pediatric patients to adult providers as a major problem, and the perception is that only 1% of patients are adequately prepared for this transfer of care. Few institutions have a formalized process by which patients are transferred to adult care providers; however, many institutions have a mandated age at which they expect transfer of care to occur.
The literature about transition of care highlights the issues in the outpatient setting. Reiss and Gibson used focus groups comprised of caregivers, and youth and young adults with SHCN, to explore the issues related to transition of care.7 They identified several factors associated with successful transition that are pertinent to inpatient pediatrics. Involving the patient as a responsible member of the treatment team is important because it fosters independence and problem solving.7 Advocating this in the inpatient setting gives the patient and caregivers confidence that the patient can participate in the healthcare process, thereby making it a habit.7 A second important factor is attending to the patient's personal preferences and interpersonal dynamics.7 Many adolescents and young adult patients prefer to be cared for by their pediatric providers on adult wards away from crying infants and children.7 This may be their first indication that they'd like to explore the adult medical world.7
Pediatric hospitalists should be prepared to meet the needs of adolescent and young adult patients with SCHN by becoming familiar with the components of the transition process. Saidi and Kovacs provide a checklist of practical transition strategies that are helpful to review, and many are quite pertinent to the practice of pediatric hospital medicine.8 Education is a fundamental aspect of the identity of the pediatric hospitalist and is also the foundation of the transition process. Identifying established institutional transition resources, developing educational tools for faculty and residents to learn about transition, as well as adapting the transition checklist to inpatient needs are useful tools for developing a culture of effective healthcare transition. Simple strategies, such as speaking to young patients on their own, and displaying a public commitment to transition are other easy changes that can be made to the everyday activities of the pediatric hospitalist.8
Other key issues that would be important for the pediatric hospitalist to address are the adolescents' understanding of his/her disease, current treatments, long‐term complications, and the impact of healthy and unhealthy behaviors.9 Because these issues can directly affect his/her hospitalization, the pediatric hospitalist should play a role in discussing these issues and reaffirming their importance in the overall health of the patient. This affirmation will also support the process of transition, and will give further confidence to the patient and family that the patient is becoming a responsible member of the healthcare team.
Many of the strategies espoused by experts in transition are part of what the pediatric hospitalist does regularly. The pediatric hospitalist is a resource for patients, families, and subspecialists, because of their comfort and expertise managing complex pediatric patients and because of their understanding of the hospital and how it functions. The process of transition of care should be part of what pediatric hospitalists are prepared to teach, because of the numbers of adolescent and young adult patients that are in their care. The current knowledge base for most pediatric hospitalists seems to be a basic understanding of what transition of care means, but little knowledge about how to go about engaging in the process. More and more transition is relevant to primary care doctors and hospitalists, as the medically complex patient survives into adolescence and adulthood.
CONCLUSION
The survey provides a snapshot of the current attitudes and beliefs of pediatric hospitalists relative to involvement in healthcare transition. This article addresses what we believe to be important questions for the pediatric hospitalist to ask, prior to becoming involved in healthcare transition. Our hypothesis, that pediatric hospitalists are well poised to provide inpatient transition services but are limited by lack of understanding of the concepts and process, is supported by the responses in the survey which show that pediatric hospitalists are interested in participating in healthcare transition but feel impeded by time, support, and understanding of the process of transition. A larger sample size is needed to strengthen the data and lend support to these observations. Additionally, more research to compare current models of transition services and a hospitalist model could be important in realizing the potential positive outcomes predicted in this survey. Education and resources for transition of care are inadequate. Targeted educational modules might provide a foundation for pediatric hospitalists to build their scope of practice to include transition services. The next step for interested pediatric hospitalists might be developing a web‐based module that addresses the unique needs of the inpatient provider and the chronically ill pediatric patient who spends a great deal of time as an inpatient. The measurable outcomes for such an intervention might well be the feeling of preparation that the family and patient have as they move into the adult provider world.
Optimal health care is achieved when every person at every age receives health care that is medically and developmentally appropriate.1 For healthy patients, medically and developmentally appropriate care is usually available, but for children with special healthcare needs (SHCN), receiving this care can be a challenge that is magnified as a child with SHCN grows into a teenager and then a young adult. For these children, simply transferring care, which is essentially a handoff of responsibility, to adult providers is insufficient to meet the needs of a special healthcare population.1 Transition is the purposeful planned movement of adolescents and young adults with chronic medical conditions and disabilities from pediatric (child‐centered) to adult‐oriented providers and facilities.2
The 2005‐2006 National Survey of Children with SHCN identified 4 component measures of transition which included discussions between the patient and the healthcare provider about: 1) shifting to adult providers, 2) adult healthcare needs, 3) health insurance, and 4) encouraging the patient to take responsibility for his/her care.34 Overall, only 41% of youth with SHCN met the core outcomes.4 The survey also found that those most affected by their health conditions were less likely to have transition discussions compared with less‐affected youth.3
The importance of healthcare transition also resonates with pediatric hospitalists. The results of a 1988 National Health Survey revealed that 4% of all children with SHCN were hospitalized, and 2% of those with severe chronic conditions accounted for 27% of all hospital bed days.5 Or, stated differently, a small percentage of patients are admitted most frequently and stay the longest in the hospital. Further, transition for those with significant cognitive delay was more difficult, because of the lack of adult‐oriented providers who are willing to care for the patient and work collaboratively with the family.6 This has a significant implication for pediatric hospitalists, because it increases the likelihood that a severely affected 21 to 25‐year‐old patient will be admitted to the pediatric hospitalist service having not yet made a successful transition to an adult‐oriented provider.
Clearly, transition of care should have its roots in the outpatient medical home; however, often a patient with a chronic medical condition will spend extended periods of time in the hospital and away from their identified medical home. Although not a widely accepted concept, some may consider the pediatric hospitalist service to be an extension of the medical home for inpatients. The pediatric hospitalist is often the physician who cares for inpatients with SHCN because of their complexity. There are components of the transition process that specifically deal with hospitalization. Pediatric hospitalists should understand transition and their role in it, so that the process does not stop when patients are hospitalized.
Our hypothesis is that pediatric hospitalists are well poised to provide inpatient transition services, but insufficient understanding of the concepts and practical processes related to transition limit involvement. Through this exploratory survey, we hope to understand current attitudes and knowledge about transition. We survey the degree to which pediatric hospitalists want to participate in the process, the level of support that healthcare transition services receive from the institutions in which pediatric hospitalists practice, and potential barriers and benefits of their participation.
METHODS
Participants
After a review of current literature, we developed an exploratory survey for pediatric hospitalists that was approved by our institutional review board, and was reviewed for content and face validity by a qualitative expert on transition of care. Using Survey Monkey, the survey was piloted with a small group of pediatric hospitalists for feedback regarding the clarity of the survey questions before it was introduced to the American Academy of Pediatrics (AAP)/Pediatric Hospital Medicine Listserv. The Listserv is available to any pediatric hospitalist that joins. The exact number of pediatric hospitalists is unknown. A reasonable approximation of Listserv members at the time the survey was introduced is 1800. A fraction of that number is active on the Listserv, as defined by multiple postings during the course of an academic year. Pediatric hospitalists were the targeted group because of their expertise in the care of the adolescent and, often, young adult, with SHCN. The survey was voluntary and anonymous, and was reintroduced 3 times to capture as many participants as possible. The purpose of the survey was to gauge the interest, attitudes, and understanding of healthcare transition in a cross section of pediatric hospitalists.
Definitions
For clarity, in this survey, transfer is defined as an event, a handoff of responsibility for the management of a patient from one physician to another. Transition is defined as the purposeful planned movement of adolescents and young adults, with chronic medical conditions and disabilities, from pediatric (child‐centered) to adult‐oriented providers and facilities.2
Survey
This questionnaire contained 33 items and included a mixture of open‐ended questions, yes and no questions, and questions with responses that used a modified Likert scale. The survey questions were not adapted from another study or survey; they were developed in conjunction with 2 well‐published experts on the subject of transition. The demographic questions were used to help determine whether patterns related to transition services and knowledge could be detected based on age, gender, or type of practice, whether academic or community. The survey content included several areas: 1) Who is responsible for the care of young adults and adolescents; 2) Hospital‐based transition services; 3) Benefits or challenges for pediatric hospitalists who become involved in health care transition (HCT); 4) Knowledge of HCT and education opportunities. Informed consent was the first page; if consent was obtained, the participant could move forward in the survey (Table 1).
| Who is primarily responsible for inpatient care of patients 16‐21? |
| Please rate your knowledge of healthcare transition. |
| Are there healthcare transition services in your hospital? |
| How old are patients when they first receive inpatient‐oriented healthcare transition services? |
| How beneficial are these services to the patient and the provider if available? |
| Does your hospital have a policy that mandates the age by which an adolescent/young adult patient must be transferred from Pediatrics to adult providers and facilities? |
| What factors determine age of transfer? |
| How big a problem is it transferring adolescent/young adult patients from Pediatrics to adult providers and facilities? |
| How prepared are adolescents and young adults in your institution for transition to adult services? |
| Should pediatric hospitalists be involved in providing healthcare transition services and supports to patients with chronic healthcare conditions in the inpatient setting? |
| How often have you been asked by a subspecialist to provide healthcare transition services and supports to patients with chronic health conditions in the inpatient setting? |
| Who is best qualified to provide healthcare transition services? |
| What are the biggest impediments and benefits to hospitalist involvement in healthcare transition services? |
| If there was an online educational training module about healthcare transitions, would you take it? |
RESULTS
There were 131 participants who consented to participate in, and completed, the survey.
Demographics
Of all participants, 42.5% identified their primary practice site as a pediatric hospital; 40.8% identified their primary practice setting as a children's hospital within a general hospital, and 15.8% identified their primary practice setting as a general hospital with pediatric beds but no designation as a children's hospital. The participants came from nearly every state in the United States; 69.2% were women and 30.8% were men. The ages ranged from 27 to 67, with the majority of participants in their mid‐30s and 40s. Most were boarded in Pediatrics and/or Internal Medicine with some subspecialties such as Physical Medical and Rehabilitation, Cardiology, Critical Care, Pulmonology, and Developmental and Behavioral Pediatrics represented. Although the sample size is small, it is representative of the larger population of pediatric hospitalists. There were no patterns detected based on demographics relative to the knowledge or participation in healthcare transition (Table 2).
| Male | Female | |
|---|---|---|
| Age range | 3267 years | 2761 years |
| Boarded in Pediatrics/Pediatric subspecialty | 92% | 99% |
| Practice setting | ||
| Pediatric hospital | 51.4% | 38.6% |
| General hospital with a pediatric hospital within it | 27% | 47% |
| General hospital with pediatric beds but no children's hospital designation | 21.6% | 13.3% |
| Other | 8.1% | 7.2% |
Which Groups of Physicians Are Caring for Adolescent and Young Adult Patients?
Establishing whether pediatric subspecialists, adult providers, or pediatric hospitalists are the primary caregivers for adolescents and young adults with SCHN is important to determine whether the pediatric hospitalist is really well poised to deal with transition issues. If the pediatric hospitalist does not care for these patients, then developing modules to educate them about healthcare transition may not be necessary. As expected, pediatric hospitalists believe they care for adolescent and young adult patients with special healthcare needs in the vast majority of cases. Table 3 illustrates in more detail who is specifically responsible for their care.
| Patient Age | ||
|---|---|---|
| 1617 | 1820 | |
| ||
| Inpatient care provider | ||
| Pediatric hospitalist | 70.1%* | 36.8% |
| Adult hospitalist | 0.9% | 27.4% |
| Pediatric subspecialist | 27.4% | 25.6% |
| Adult subspecialist | 0.9% | 2.6% |
| Other/not sure | 7.7% | 0.9% |
Knowledge of Healthcare Transition
Participants in the survey were provided definitions of healthcare transition; they were asked to rate their knowledge of healthcare transition on a modified Likert scale, given the definition provided. The results can be seen in Figure 1.

Transition Programs
Of all participants, 60.9% did not know if their hospital had inpatient‐oriented healthcare transition services. Another 27.8% only had informal or unstructured services for some patients with a chronic condition, and less than 1% of all respondents said they had a formal or structured program at their institution for inpatients with any chronic medical condition. Eighty percent thought transferring adolescent or young adult patients from pediatric to adult providers was a moderate to major problem.
Of those who responded to the survey, 97.6% feel that inpatient‐oriented healthcare transition services would be beneficial to adolescent and young adult patients, and 92.2% felt that these supports would be beneficial to pediatric providers. This is consistent with the data from another question in which respondents felt that only 1% of patients were quite a bit prepared for transition to inpatient adult providers, and that over half were only a little bit or not at all prepared.
Institutional Mandates
Nearly 40% of institutions have a mandated age by which adolescent and young adult patients must be transferred to adult providers and facilities. Additionally, only 5.2% of those institutions have a written procedure or protocol that describes how these pediatric patients will be transferred to adult providers and institutions.
Pediatric Hospitalist Participation in Transition
Sixty‐eight percent of respondents believe that the patient's primary care provider is the most qualified to discuss healthcare transition issues, followed by their pediatric subspecialists. However, more than 75% of respondents agree or strongly agree that pediatric hospitalists should be involved in providing healthcare transition services and supports to inpatients with chronic health conditions. Please refer to Table 4. Despite this, 58% of pediatric hospitalists are rarely, if ever, asked to participate in healthcare transition by their subspecialist counterparts.
| Respondents, % (No.) | |
|---|---|
| Strongly agree | 28.6 (28) |
| Agree | 50.0 (49) |
| Neither agree or disagree | 12.2 (12) |
| Disagree | 6.1 (6) |
| Strongly disagree | 3.1 (3) |
Barriers to Pediatric Hospitalist Participation in Transition
The survey participants were given a list of potential barriers to participation in healthcare transition and were asked to rank 3 of the choices in order of significance, with 1 being the biggest perceived impediment and 3 being the least significant. Seventeen percent ranked lack of familiarity with healthcare transition resources as the biggest barrier in their setting. Thirteen percent indicated that lack of support from pediatric and adult subspecialists is the major barrier, and 13% felt that insufficient time to provide transition services and supports would be the most significant barrier to their participation in transition. Interestingly, billing and reimbursement issues were not seen as obstacles (see Supporting Information 1/Table 5 in the online version of this article).
Advantages to Pediatric Hospitalist Participation in Transition
The participants were given a set of potential benefits that might result from the pediatric hospitalist participation in transition of care. They were asked to rank 3 of the choices in order of importance, with 1 being the most important positive outcome to 3 being a lesser, but still positive, outcome. Twenty‐three percent of respondents ranked improved communication between pediatric and adult providers and facilities as being the most significant advantage. Twenty‐one percent ranked both better continuity of care in the inpatient setting, and better quality of care for adolescents and young adults with chronic healthcare conditions, as the most important potential advantages of pediatric hospitalist involvement in healthcare transition. However, most felt that improved cost effectiveness would not be an important result (see Supporting Information 2/Table 6 in the online version of this article).
Educational Process
If an educational module was offered, over half of the respondents would definitely or probably take the training, and another 22% might take the training.
LIMITATIONS
This is an exploratory study which is limited by the small number of participants. Although the demographics of the participants include both young and experienced hospitalists, as well as academic and community institutions, it is difficult to determine whether the results are truly representative of the larger pediatric hospitalist population. The survey was also too long which may have deterred participation. Given the lack of experience and literature on pediatric hospitalist involvement in transition of care, it is difficult to construct a concise survey that addresses all of the concerns of the diverse hospitalist population. This is a new area of exploration for pediatric hospitalists, and ideally new questions will arise out of these preliminary findings, despite the limitations in the survey. Future surveys should focus on singular issues related to transition, and every attempt should be made to increase participation in the survey.
DISCUSSION
The survey demonstrates that the majority of the pediatric hospitalists believe providing transition services is important, but that transition programs are, for all practical purposes, nonexistent. Hospitalists believe the primary care doctor or the subspecialists should direct the transition process, but most clearly believe that their participation in the process would be beneficial for their patients, as evidenced by a 97.6% positive response to that question in the survey. Transition of care should be handled predominantly in the medical home.1 At this point, there is no literature that describes a pediatric hospitalist service as an inpatient medical home. However, pediatric hospitalists, not pediatric subspecialists, care for the majority of patients with SHCN in the transition age range while they are hospitalized; therefore, continuing the transition discussion while a patient is hospitalized may be a key component to its success. Better quality and continuity of care for the inpatient with SHCN is a potential advantage, as is coordination of services. Having the support of the pediatric subspecialists and the pediatric primary care provider is not only important, but it is critical in successful transition. Further, most pediatric hospitalists identify transfer of pediatric patients to adult providers as a major problem, and the perception is that only 1% of patients are adequately prepared for this transfer of care. Few institutions have a formalized process by which patients are transferred to adult care providers; however, many institutions have a mandated age at which they expect transfer of care to occur.
The literature about transition of care highlights the issues in the outpatient setting. Reiss and Gibson used focus groups comprised of caregivers, and youth and young adults with SHCN, to explore the issues related to transition of care.7 They identified several factors associated with successful transition that are pertinent to inpatient pediatrics. Involving the patient as a responsible member of the treatment team is important because it fosters independence and problem solving.7 Advocating this in the inpatient setting gives the patient and caregivers confidence that the patient can participate in the healthcare process, thereby making it a habit.7 A second important factor is attending to the patient's personal preferences and interpersonal dynamics.7 Many adolescents and young adult patients prefer to be cared for by their pediatric providers on adult wards away from crying infants and children.7 This may be their first indication that they'd like to explore the adult medical world.7
Pediatric hospitalists should be prepared to meet the needs of adolescent and young adult patients with SCHN by becoming familiar with the components of the transition process. Saidi and Kovacs provide a checklist of practical transition strategies that are helpful to review, and many are quite pertinent to the practice of pediatric hospital medicine.8 Education is a fundamental aspect of the identity of the pediatric hospitalist and is also the foundation of the transition process. Identifying established institutional transition resources, developing educational tools for faculty and residents to learn about transition, as well as adapting the transition checklist to inpatient needs are useful tools for developing a culture of effective healthcare transition. Simple strategies, such as speaking to young patients on their own, and displaying a public commitment to transition are other easy changes that can be made to the everyday activities of the pediatric hospitalist.8
Other key issues that would be important for the pediatric hospitalist to address are the adolescents' understanding of his/her disease, current treatments, long‐term complications, and the impact of healthy and unhealthy behaviors.9 Because these issues can directly affect his/her hospitalization, the pediatric hospitalist should play a role in discussing these issues and reaffirming their importance in the overall health of the patient. This affirmation will also support the process of transition, and will give further confidence to the patient and family that the patient is becoming a responsible member of the healthcare team.
Many of the strategies espoused by experts in transition are part of what the pediatric hospitalist does regularly. The pediatric hospitalist is a resource for patients, families, and subspecialists, because of their comfort and expertise managing complex pediatric patients and because of their understanding of the hospital and how it functions. The process of transition of care should be part of what pediatric hospitalists are prepared to teach, because of the numbers of adolescent and young adult patients that are in their care. The current knowledge base for most pediatric hospitalists seems to be a basic understanding of what transition of care means, but little knowledge about how to go about engaging in the process. More and more transition is relevant to primary care doctors and hospitalists, as the medically complex patient survives into adolescence and adulthood.
CONCLUSION
The survey provides a snapshot of the current attitudes and beliefs of pediatric hospitalists relative to involvement in healthcare transition. This article addresses what we believe to be important questions for the pediatric hospitalist to ask, prior to becoming involved in healthcare transition. Our hypothesis, that pediatric hospitalists are well poised to provide inpatient transition services but are limited by lack of understanding of the concepts and process, is supported by the responses in the survey which show that pediatric hospitalists are interested in participating in healthcare transition but feel impeded by time, support, and understanding of the process of transition. A larger sample size is needed to strengthen the data and lend support to these observations. Additionally, more research to compare current models of transition services and a hospitalist model could be important in realizing the potential positive outcomes predicted in this survey. Education and resources for transition of care are inadequate. Targeted educational modules might provide a foundation for pediatric hospitalists to build their scope of practice to include transition services. The next step for interested pediatric hospitalists might be developing a web‐based module that addresses the unique needs of the inpatient provider and the chronically ill pediatric patient who spends a great deal of time as an inpatient. The measurable outcomes for such an intervention might well be the feeling of preparation that the family and patient have as they move into the adult provider world.
- A consensus statement on health care transitions for young adults with special health care needs.Pediatrics.2002;110(6 pt 2):1304–1306.
- ,,,,,.Perceptions of transitional care needs and experiences in pediatric heart transplant recipients.Am J Transplant.2009;9(3):614–619.
- ,,,,,.Planning for health care transitions: results from the 2005–2006 National Survey of Children With Special Health Care Needs.Pediatrics.2009;123(1):e145–e152.
- US Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Bureau.The National Survey of Children With Special Health Care Needs, Chartbook 2005–2006.Rockville, MD:US Department of Health and Human Services;2008.
- ,,,,,.Changes in hospitalization patterns among patients with congenital heart disease during the transition from adolescence to adulthood.J Am Coll Cardiol.2007;49(8):875–882.
- ,,.Health care transition: youth, family, and provider perspectives.Pediatrics.2005;115(1):112–120.
- ,.Health care transition: destinations unknown.Pediatrics.2002;110(6 pt 2):1307–1314.
- ,.Developing a transition program from pediatric‐ to adult‐focused cardiology care: practical considerations.Congenit Heart Dis.2009;4(4):204–215.
- .Transition for youth with chronic conditions: primary care physicians' approaches.Pediatrics.2002;110(6 pt 2):1315–1321.
- A consensus statement on health care transitions for young adults with special health care needs.Pediatrics.2002;110(6 pt 2):1304–1306.
- ,,,,,.Perceptions of transitional care needs and experiences in pediatric heart transplant recipients.Am J Transplant.2009;9(3):614–619.
- ,,,,,.Planning for health care transitions: results from the 2005–2006 National Survey of Children With Special Health Care Needs.Pediatrics.2009;123(1):e145–e152.
- US Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Bureau.The National Survey of Children With Special Health Care Needs, Chartbook 2005–2006.Rockville, MD:US Department of Health and Human Services;2008.
- ,,,,,.Changes in hospitalization patterns among patients with congenital heart disease during the transition from adolescence to adulthood.J Am Coll Cardiol.2007;49(8):875–882.
- ,,.Health care transition: youth, family, and provider perspectives.Pediatrics.2005;115(1):112–120.
- ,.Health care transition: destinations unknown.Pediatrics.2002;110(6 pt 2):1307–1314.
- ,.Developing a transition program from pediatric‐ to adult‐focused cardiology care: practical considerations.Congenit Heart Dis.2009;4(4):204–215.
- .Transition for youth with chronic conditions: primary care physicians' approaches.Pediatrics.2002;110(6 pt 2):1315–1321.
Copyright © 2011 Society of Hospital Medicine
HPS: Benefits, Safety Persist With Long-Term Statin Use
The protection against vascular morbidity and mortality that resulted from lowering LDL cholesterol with simvastatin over a period of about 5 years in the Heart Protection Study persisted for nearly 6 additional years after study treatment ended, according to findings from an extended follow-up of the large randomized controlled trial.
Furthermore, no evidence of emerging safety concerns was apparent during the follow-up, the Heart Protection Study Collaborative Group reported online on Nov. 23 in the Lancet.
Participants in the Medical Research Council and British Heart Foundation Heart Protection Study (HPS) was composed of 20,536 adults aged 40-80 years who were at increased risk of vascular events and who were enrolled between July 1994 and May 1997. Those allocated to receive simvastatin experienced a mean reduction in LDL cholesterol of 1.0 mmol/L and a proportional reduction of 23% in major cardiovascular events during the 5-year study period.
The findings, along with those from other major trials of statins, provided "compelling evidence" of the value of lowering LDL cholesterol – and led to the widespread use of long-term statin treatment – but evidence from observational studies has raised concerns about possible increases in the risk of certain types of cancer and other nonvascular morbidity and mortality in patients with lower blood cholesterol concentrations, according to the study group.
The findings of the HPS extended follow-up appear to lay those concerns to rest.
At a mean of 5.3 years (for a total HPS follow-up of 11 years), the rate of first vascular events in previously event-free participants was similar in both the initial simvastatin group and the initial placebo (21.7% and 22.5%, respectively; risk ratio 0.95), the investigators said (Lancet 2011 Nov. 23 [doi:10.1016/S0140-6736(11)61125-2]).
Although a further 14% decrease in vascular events occurred in the first year in the simvastatin group, little difference was seen between the treatment and placebo groups thereafter. Similar patterns were seen for major coronary events, strokes, and revascularization procedures.
Vascular mortality was also similar in the two groups during the post-trial follow-up (11.5% and 11.6% in the simvastatin and placebo groups). During the in-trial period, an 18% proportional reduction in vascular mortality was seen in the treatment group, so the follow-up findings indicate that the in-trial survival gains persisted, they investigators said.
Nonvascular mortality rates were also similar in the two groups during the post-trial follow-up (10.6% and 10.9%). There were no differences in deaths from cancer, respiratory disease, or nonmedical causes.
"When the 11 years of in-trial and post-trial follow-up are considered together, allocation to about 5 years of statin treatment was not associated with any increase in nonvascular mortality, either overall [14.8% vs. 15.1%] or for any prespecified category of death," they said.
As for first diagnoses of any type of cancer, rates were also similar in the in-trial and post-trial periods, for a combined incidence of 17% in each group.
"Indeed, even during the later years of this prolonged follow-up, no suggestion was noted of any emerging difference in the overall incidence of cancer," the investigators said, noting that the large numbers of incident cancer that occurred during the entire in-trial and post-trial period allowed for reliable assessment of the effects of the substantial 5-year reduction in cholesterol on 11-year risks of common cancer types. No significant differences were seen in the incidences of genitourinary, gastrointestinal, respiratory, hematologic, or any other malignant disease, even in those aged 70 years or older at baseline, and in those with below-average pretreatment cholesterol concentrations.
Statin use was encouraged in all HPS participants following the initial study treatment period and was similar in both treatment groups during the post-trial follow-up at about 59% in the first year and increasing to about 84% in the fifth year. LDL cholesterol concentrations were also similar at 2.6 mmol/L in both groups at 3.2 years, the investigators noted.
Although 11 years might not be long enough to fully discern all cancer and other risks in this study population, it is notable that no adverse trend was noted, even during the later years of follow-up, the researchers wrote.
The findings are consistent with those from four other large randomized trials of statin treatment, and, taken together, the data support the prompt initiation and long-term continuation of statin treatment in individuals at increased risk of vascular events, they concluded.
This study was supported by the UK Medical Research Council, the British Heart Foundation, Merck & Co., and Roche Vitamins. The HPS Collaborative Study Group is bound by a policy of not accepting honoraria or other payments from the pharmaceutical industry other than reimbursement of costs to participate in scientific meetings. As a result, the only disclosures of the group relate to such reimbursement.
In light of the findings of the HPS Collaborative Study Group, doctors should feel reassured about the long-term safety of statin use to lower LDL cholesterol, Dr. Payal Kohli and Dr. Christopher P. Cannon wrote in an editorial.
Given that follow-up in most prior studies did not extend beyond 5 years and that some data have suggested an increase in cancer risk in patients with very-low cholesterol levels – and possibly with prolonged statin treatment – concerns about an increased risk of cancers that take longer than 5 years to emerge have persisted, they said.
The finding of the HPS extended follow-up, however, provide "contemporary and confirmatory" evidence to the contrary, they added (Lancet 2011 Nov. 23[doi.10.1016/S0140-6736(11)61544-4]).
The findings indicate the risk of cancer and nonvascular mortality is not increased with extended statin use, even among elderly patients.
The original concerns about statin safety were from observational data, which were most likely heavily confounded, they said.
"We now have strong evidence from HPS and several other randomized controlled trials that prolonged treatment with statins is indeed efficacious, safe, and has long-lasting beneficial effects, even after discontinuation of therapy," they concluded.
Dr. Kohli and Dr. Cannon are with the TIMI Study Group, Cardiovascular Division, Brigham and Women’s Hospital, Boston. Dr. Cannon disclosed that he has received research funding from Accumetrics, AstraZeneca, GlaxoSmithKline, Merck, and Takeda, and that he has received honoraria from Pfizer and AstraZeneca. He has also participated in advisory boards for Bristol-Myers Squibb/Sanofi, Novartis, and Alnylam, and he has equity in Automedics Medical Systems. Dr. Kohli had no disclosures.
In light of the findings of the HPS Collaborative Study Group, doctors should feel reassured about the long-term safety of statin use to lower LDL cholesterol, Dr. Payal Kohli and Dr. Christopher P. Cannon wrote in an editorial.
Given that follow-up in most prior studies did not extend beyond 5 years and that some data have suggested an increase in cancer risk in patients with very-low cholesterol levels – and possibly with prolonged statin treatment – concerns about an increased risk of cancers that take longer than 5 years to emerge have persisted, they said.
The finding of the HPS extended follow-up, however, provide "contemporary and confirmatory" evidence to the contrary, they added (Lancet 2011 Nov. 23[doi.10.1016/S0140-6736(11)61544-4]).
The findings indicate the risk of cancer and nonvascular mortality is not increased with extended statin use, even among elderly patients.
The original concerns about statin safety were from observational data, which were most likely heavily confounded, they said.
"We now have strong evidence from HPS and several other randomized controlled trials that prolonged treatment with statins is indeed efficacious, safe, and has long-lasting beneficial effects, even after discontinuation of therapy," they concluded.
Dr. Kohli and Dr. Cannon are with the TIMI Study Group, Cardiovascular Division, Brigham and Women’s Hospital, Boston. Dr. Cannon disclosed that he has received research funding from Accumetrics, AstraZeneca, GlaxoSmithKline, Merck, and Takeda, and that he has received honoraria from Pfizer and AstraZeneca. He has also participated in advisory boards for Bristol-Myers Squibb/Sanofi, Novartis, and Alnylam, and he has equity in Automedics Medical Systems. Dr. Kohli had no disclosures.
In light of the findings of the HPS Collaborative Study Group, doctors should feel reassured about the long-term safety of statin use to lower LDL cholesterol, Dr. Payal Kohli and Dr. Christopher P. Cannon wrote in an editorial.
Given that follow-up in most prior studies did not extend beyond 5 years and that some data have suggested an increase in cancer risk in patients with very-low cholesterol levels – and possibly with prolonged statin treatment – concerns about an increased risk of cancers that take longer than 5 years to emerge have persisted, they said.
The finding of the HPS extended follow-up, however, provide "contemporary and confirmatory" evidence to the contrary, they added (Lancet 2011 Nov. 23[doi.10.1016/S0140-6736(11)61544-4]).
The findings indicate the risk of cancer and nonvascular mortality is not increased with extended statin use, even among elderly patients.
The original concerns about statin safety were from observational data, which were most likely heavily confounded, they said.
"We now have strong evidence from HPS and several other randomized controlled trials that prolonged treatment with statins is indeed efficacious, safe, and has long-lasting beneficial effects, even after discontinuation of therapy," they concluded.
Dr. Kohli and Dr. Cannon are with the TIMI Study Group, Cardiovascular Division, Brigham and Women’s Hospital, Boston. Dr. Cannon disclosed that he has received research funding from Accumetrics, AstraZeneca, GlaxoSmithKline, Merck, and Takeda, and that he has received honoraria from Pfizer and AstraZeneca. He has also participated in advisory boards for Bristol-Myers Squibb/Sanofi, Novartis, and Alnylam, and he has equity in Automedics Medical Systems. Dr. Kohli had no disclosures.
The protection against vascular morbidity and mortality that resulted from lowering LDL cholesterol with simvastatin over a period of about 5 years in the Heart Protection Study persisted for nearly 6 additional years after study treatment ended, according to findings from an extended follow-up of the large randomized controlled trial.
Furthermore, no evidence of emerging safety concerns was apparent during the follow-up, the Heart Protection Study Collaborative Group reported online on Nov. 23 in the Lancet.
Participants in the Medical Research Council and British Heart Foundation Heart Protection Study (HPS) was composed of 20,536 adults aged 40-80 years who were at increased risk of vascular events and who were enrolled between July 1994 and May 1997. Those allocated to receive simvastatin experienced a mean reduction in LDL cholesterol of 1.0 mmol/L and a proportional reduction of 23% in major cardiovascular events during the 5-year study period.
The findings, along with those from other major trials of statins, provided "compelling evidence" of the value of lowering LDL cholesterol – and led to the widespread use of long-term statin treatment – but evidence from observational studies has raised concerns about possible increases in the risk of certain types of cancer and other nonvascular morbidity and mortality in patients with lower blood cholesterol concentrations, according to the study group.
The findings of the HPS extended follow-up appear to lay those concerns to rest.
At a mean of 5.3 years (for a total HPS follow-up of 11 years), the rate of first vascular events in previously event-free participants was similar in both the initial simvastatin group and the initial placebo (21.7% and 22.5%, respectively; risk ratio 0.95), the investigators said (Lancet 2011 Nov. 23 [doi:10.1016/S0140-6736(11)61125-2]).
Although a further 14% decrease in vascular events occurred in the first year in the simvastatin group, little difference was seen between the treatment and placebo groups thereafter. Similar patterns were seen for major coronary events, strokes, and revascularization procedures.
Vascular mortality was also similar in the two groups during the post-trial follow-up (11.5% and 11.6% in the simvastatin and placebo groups). During the in-trial period, an 18% proportional reduction in vascular mortality was seen in the treatment group, so the follow-up findings indicate that the in-trial survival gains persisted, they investigators said.
Nonvascular mortality rates were also similar in the two groups during the post-trial follow-up (10.6% and 10.9%). There were no differences in deaths from cancer, respiratory disease, or nonmedical causes.
"When the 11 years of in-trial and post-trial follow-up are considered together, allocation to about 5 years of statin treatment was not associated with any increase in nonvascular mortality, either overall [14.8% vs. 15.1%] or for any prespecified category of death," they said.
As for first diagnoses of any type of cancer, rates were also similar in the in-trial and post-trial periods, for a combined incidence of 17% in each group.
"Indeed, even during the later years of this prolonged follow-up, no suggestion was noted of any emerging difference in the overall incidence of cancer," the investigators said, noting that the large numbers of incident cancer that occurred during the entire in-trial and post-trial period allowed for reliable assessment of the effects of the substantial 5-year reduction in cholesterol on 11-year risks of common cancer types. No significant differences were seen in the incidences of genitourinary, gastrointestinal, respiratory, hematologic, or any other malignant disease, even in those aged 70 years or older at baseline, and in those with below-average pretreatment cholesterol concentrations.
Statin use was encouraged in all HPS participants following the initial study treatment period and was similar in both treatment groups during the post-trial follow-up at about 59% in the first year and increasing to about 84% in the fifth year. LDL cholesterol concentrations were also similar at 2.6 mmol/L in both groups at 3.2 years, the investigators noted.
Although 11 years might not be long enough to fully discern all cancer and other risks in this study population, it is notable that no adverse trend was noted, even during the later years of follow-up, the researchers wrote.
The findings are consistent with those from four other large randomized trials of statin treatment, and, taken together, the data support the prompt initiation and long-term continuation of statin treatment in individuals at increased risk of vascular events, they concluded.
This study was supported by the UK Medical Research Council, the British Heart Foundation, Merck & Co., and Roche Vitamins. The HPS Collaborative Study Group is bound by a policy of not accepting honoraria or other payments from the pharmaceutical industry other than reimbursement of costs to participate in scientific meetings. As a result, the only disclosures of the group relate to such reimbursement.
The protection against vascular morbidity and mortality that resulted from lowering LDL cholesterol with simvastatin over a period of about 5 years in the Heart Protection Study persisted for nearly 6 additional years after study treatment ended, according to findings from an extended follow-up of the large randomized controlled trial.
Furthermore, no evidence of emerging safety concerns was apparent during the follow-up, the Heart Protection Study Collaborative Group reported online on Nov. 23 in the Lancet.
Participants in the Medical Research Council and British Heart Foundation Heart Protection Study (HPS) was composed of 20,536 adults aged 40-80 years who were at increased risk of vascular events and who were enrolled between July 1994 and May 1997. Those allocated to receive simvastatin experienced a mean reduction in LDL cholesterol of 1.0 mmol/L and a proportional reduction of 23% in major cardiovascular events during the 5-year study period.
The findings, along with those from other major trials of statins, provided "compelling evidence" of the value of lowering LDL cholesterol – and led to the widespread use of long-term statin treatment – but evidence from observational studies has raised concerns about possible increases in the risk of certain types of cancer and other nonvascular morbidity and mortality in patients with lower blood cholesterol concentrations, according to the study group.
The findings of the HPS extended follow-up appear to lay those concerns to rest.
At a mean of 5.3 years (for a total HPS follow-up of 11 years), the rate of first vascular events in previously event-free participants was similar in both the initial simvastatin group and the initial placebo (21.7% and 22.5%, respectively; risk ratio 0.95), the investigators said (Lancet 2011 Nov. 23 [doi:10.1016/S0140-6736(11)61125-2]).
Although a further 14% decrease in vascular events occurred in the first year in the simvastatin group, little difference was seen between the treatment and placebo groups thereafter. Similar patterns were seen for major coronary events, strokes, and revascularization procedures.
Vascular mortality was also similar in the two groups during the post-trial follow-up (11.5% and 11.6% in the simvastatin and placebo groups). During the in-trial period, an 18% proportional reduction in vascular mortality was seen in the treatment group, so the follow-up findings indicate that the in-trial survival gains persisted, they investigators said.
Nonvascular mortality rates were also similar in the two groups during the post-trial follow-up (10.6% and 10.9%). There were no differences in deaths from cancer, respiratory disease, or nonmedical causes.
"When the 11 years of in-trial and post-trial follow-up are considered together, allocation to about 5 years of statin treatment was not associated with any increase in nonvascular mortality, either overall [14.8% vs. 15.1%] or for any prespecified category of death," they said.
As for first diagnoses of any type of cancer, rates were also similar in the in-trial and post-trial periods, for a combined incidence of 17% in each group.
"Indeed, even during the later years of this prolonged follow-up, no suggestion was noted of any emerging difference in the overall incidence of cancer," the investigators said, noting that the large numbers of incident cancer that occurred during the entire in-trial and post-trial period allowed for reliable assessment of the effects of the substantial 5-year reduction in cholesterol on 11-year risks of common cancer types. No significant differences were seen in the incidences of genitourinary, gastrointestinal, respiratory, hematologic, or any other malignant disease, even in those aged 70 years or older at baseline, and in those with below-average pretreatment cholesterol concentrations.
Statin use was encouraged in all HPS participants following the initial study treatment period and was similar in both treatment groups during the post-trial follow-up at about 59% in the first year and increasing to about 84% in the fifth year. LDL cholesterol concentrations were also similar at 2.6 mmol/L in both groups at 3.2 years, the investigators noted.
Although 11 years might not be long enough to fully discern all cancer and other risks in this study population, it is notable that no adverse trend was noted, even during the later years of follow-up, the researchers wrote.
The findings are consistent with those from four other large randomized trials of statin treatment, and, taken together, the data support the prompt initiation and long-term continuation of statin treatment in individuals at increased risk of vascular events, they concluded.
This study was supported by the UK Medical Research Council, the British Heart Foundation, Merck & Co., and Roche Vitamins. The HPS Collaborative Study Group is bound by a policy of not accepting honoraria or other payments from the pharmaceutical industry other than reimbursement of costs to participate in scientific meetings. As a result, the only disclosures of the group relate to such reimbursement.
FROM THE LANCET
Major Finding: At a mean of 5.3 years (for a total HPS follow-up of 11 years), the rate of first vascular events in previously event-free participants was similar in both the initial simvastatin group and the initial placebo (21.7% and 22.5%, respectively; risk ratio, 0.95).Similar patterns were seen for major coronary events (RR, 0.96), strokes (RR 0.98), revascularization procedures (RR, 0.93), vascular mortality (RR, 0.98), nonvascular mortality (RR, 0.97), and cancer incidence (RR, 0.98).
Data Source: An extended follow-up of the randomized controlled Health Protection Study
Disclosures: This study was supported by the UK Medical Research Council, the British Heart Foundation, Merck & Co., and Roche Vitamins. The HPS Collaborative Study Group is bound by a policy of not accepting honoraria or other payments from the pharmaceutical industry other than reimbursement of costs to participate in scientific meetings. As a result, the only disclosures of the group relate to such reimbursement.
Branching Out
Michele Torres, MD, a family-medicine-trained hospitalist at Good Samaritan Hospital in Dayton, Ohio, wanted to diversify her medical practice. So she created Youthinity, a cosmetic spa that utilizes laser lipolysis to sculpt, slim, and contour women’s bodies.
Launched in September following 18 months of business planning, the spa includes other cosmetic treatments, such as Botox and Juvederm, for wrinkles in a relaxed spa environment. The business employs a full-time office manager and a per-diem nurse to help with procedures.
“I love hospital medicine and don’t want to leave it, but I don’t want to have all my eggs in one basket, either,” Dr. Torres says.
Dr. Torres has been a hospitalist for five years and founded an HM program at another hospital. But hospital mergers, the uncertainties of healthcare reform, and hospitalist scheduling demands made her want to establish a second medical practice that didn’t tie her down to as many hospital shifts. She currently works a seven-on, seven-off HM schedule, and is able to schedule laser procedures during the off weeks or after hours. She also hopes to reduce her HM hours as the business grows.
Another driving force behind the business was the desire as a female physician to offer procedures that are primarily—although not exclusively—sought by women.
"It's really a very different experience than hospital medicine. You need to enjoy doing procedures," she adds. "In a business like this, it's also very market-driven and hands-on. People having the procedures done want to know their doctors. If someone is paying for this procedure, they want to feel pampered."
Michele Torres, MD, a family-medicine-trained hospitalist at Good Samaritan Hospital in Dayton, Ohio, wanted to diversify her medical practice. So she created Youthinity, a cosmetic spa that utilizes laser lipolysis to sculpt, slim, and contour women’s bodies.
Launched in September following 18 months of business planning, the spa includes other cosmetic treatments, such as Botox and Juvederm, for wrinkles in a relaxed spa environment. The business employs a full-time office manager and a per-diem nurse to help with procedures.
“I love hospital medicine and don’t want to leave it, but I don’t want to have all my eggs in one basket, either,” Dr. Torres says.
Dr. Torres has been a hospitalist for five years and founded an HM program at another hospital. But hospital mergers, the uncertainties of healthcare reform, and hospitalist scheduling demands made her want to establish a second medical practice that didn’t tie her down to as many hospital shifts. She currently works a seven-on, seven-off HM schedule, and is able to schedule laser procedures during the off weeks or after hours. She also hopes to reduce her HM hours as the business grows.
Another driving force behind the business was the desire as a female physician to offer procedures that are primarily—although not exclusively—sought by women.
"It's really a very different experience than hospital medicine. You need to enjoy doing procedures," she adds. "In a business like this, it's also very market-driven and hands-on. People having the procedures done want to know their doctors. If someone is paying for this procedure, they want to feel pampered."
Michele Torres, MD, a family-medicine-trained hospitalist at Good Samaritan Hospital in Dayton, Ohio, wanted to diversify her medical practice. So she created Youthinity, a cosmetic spa that utilizes laser lipolysis to sculpt, slim, and contour women’s bodies.
Launched in September following 18 months of business planning, the spa includes other cosmetic treatments, such as Botox and Juvederm, for wrinkles in a relaxed spa environment. The business employs a full-time office manager and a per-diem nurse to help with procedures.
“I love hospital medicine and don’t want to leave it, but I don’t want to have all my eggs in one basket, either,” Dr. Torres says.
Dr. Torres has been a hospitalist for five years and founded an HM program at another hospital. But hospital mergers, the uncertainties of healthcare reform, and hospitalist scheduling demands made her want to establish a second medical practice that didn’t tie her down to as many hospital shifts. She currently works a seven-on, seven-off HM schedule, and is able to schedule laser procedures during the off weeks or after hours. She also hopes to reduce her HM hours as the business grows.
Another driving force behind the business was the desire as a female physician to offer procedures that are primarily—although not exclusively—sought by women.
"It's really a very different experience than hospital medicine. You need to enjoy doing procedures," she adds. "In a business like this, it's also very market-driven and hands-on. People having the procedures done want to know their doctors. If someone is paying for this procedure, they want to feel pampered."
FDA Approves Chemotherapeutic Enzyme L-Asparaginase
The Food and Drug Administration on Nov. 18 granted orphan drug status to asparaginase Erwinia chrysanthemi, a chemotherapeutic enzyme indicated for patients with acute lymphoblastic leukemia* who have become allergic to pegaspargase or asparaginase derived from Escherichia coli.
The new drug (Erwinaze) is derived from Erwinia chrysanthemi, a gram-negative bacillus related to E. coli.
It works by the same mechanism as the two previously approved agents – blocking asparagine, a protein necessary for the proliferation of neoplastic cells, according to an FDA press statement.
"The approval of Erwinaze underscores the FDA’s commitment to the approval of drugs for conditions with limited patient populations with unmet medical needs using novel trial end points," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement.
According to the prescribing information, asparaginase Erwinia chrysanthemi is indicated as part of a multi-agent treatment regimen for patients with hypersensitivity to E. coli–derived asparaginase.
To substitute for a dose of pegaspargase, patients should receive 25,000 IU/m2 of asparaginase Erwinia chrysanthemi administered intramuscularly three times a week (six doses for each planned dose of pegaspargase). To substitute for a dose of native E. coli asparaginase, the recommended dose of asparaginase Erwinia chrysanthemi is 25,000 IU/m2 intramuscularly for each scheduled dose of the E. coli–derived drug.
Two pivotal trials influenced the approval, the press statement noted. These included the ongoing Erwinase Master Treatment Protocol with full data on 843 patients, and a completed trial of 58 patients.
In each study, the main end point was the number of patients with sustained asparaginase activity levels that have been correlated with better disease control and survival, according to a statement on the National Cancer Institute Web site.
"The major efficacy outcome was attainment of sustained serum asparaginase activity levels of 0.1 IU/mL or higher, which has been demonstrated to correlate with asparagine depletion and to serum levels that predict clinical efficacy," according to the prescribing information. Among 48 patients with available samples, all achieved this threshold trough level of asparaginase activity.
The most common side effect associated with the drug was allergic reaction (17%), the prescribing information notes. Other side effects included pancreatitis (4%), coagulation abnormalities (3%), abnormal liver function (4%), and hyperglycemia (2%).
Nausea, vomiting, or abdominal pain was reported in 5%, while headache, diarrhea, or seizure occurred in 1% each.
EUSA Pharma Inc. of Langhorne, Pa., manufactures the drug. A patient education page is available.
*CORRECTION 12/22/11: This sentence originally stated that the enzyme was indicated for chronic lymphoblastic anemia. The sentence has been corrected to chronic lymphoblastic leukemia.
The Food and Drug Administration on Nov. 18 granted orphan drug status to asparaginase Erwinia chrysanthemi, a chemotherapeutic enzyme indicated for patients with acute lymphoblastic leukemia* who have become allergic to pegaspargase or asparaginase derived from Escherichia coli.
The new drug (Erwinaze) is derived from Erwinia chrysanthemi, a gram-negative bacillus related to E. coli.
It works by the same mechanism as the two previously approved agents – blocking asparagine, a protein necessary for the proliferation of neoplastic cells, according to an FDA press statement.
"The approval of Erwinaze underscores the FDA’s commitment to the approval of drugs for conditions with limited patient populations with unmet medical needs using novel trial end points," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement.
According to the prescribing information, asparaginase Erwinia chrysanthemi is indicated as part of a multi-agent treatment regimen for patients with hypersensitivity to E. coli–derived asparaginase.
To substitute for a dose of pegaspargase, patients should receive 25,000 IU/m2 of asparaginase Erwinia chrysanthemi administered intramuscularly three times a week (six doses for each planned dose of pegaspargase). To substitute for a dose of native E. coli asparaginase, the recommended dose of asparaginase Erwinia chrysanthemi is 25,000 IU/m2 intramuscularly for each scheduled dose of the E. coli–derived drug.
Two pivotal trials influenced the approval, the press statement noted. These included the ongoing Erwinase Master Treatment Protocol with full data on 843 patients, and a completed trial of 58 patients.
In each study, the main end point was the number of patients with sustained asparaginase activity levels that have been correlated with better disease control and survival, according to a statement on the National Cancer Institute Web site.
"The major efficacy outcome was attainment of sustained serum asparaginase activity levels of 0.1 IU/mL or higher, which has been demonstrated to correlate with asparagine depletion and to serum levels that predict clinical efficacy," according to the prescribing information. Among 48 patients with available samples, all achieved this threshold trough level of asparaginase activity.
The most common side effect associated with the drug was allergic reaction (17%), the prescribing information notes. Other side effects included pancreatitis (4%), coagulation abnormalities (3%), abnormal liver function (4%), and hyperglycemia (2%).
Nausea, vomiting, or abdominal pain was reported in 5%, while headache, diarrhea, or seizure occurred in 1% each.
EUSA Pharma Inc. of Langhorne, Pa., manufactures the drug. A patient education page is available.
*CORRECTION 12/22/11: This sentence originally stated that the enzyme was indicated for chronic lymphoblastic anemia. The sentence has been corrected to chronic lymphoblastic leukemia.
The Food and Drug Administration on Nov. 18 granted orphan drug status to asparaginase Erwinia chrysanthemi, a chemotherapeutic enzyme indicated for patients with acute lymphoblastic leukemia* who have become allergic to pegaspargase or asparaginase derived from Escherichia coli.
The new drug (Erwinaze) is derived from Erwinia chrysanthemi, a gram-negative bacillus related to E. coli.
It works by the same mechanism as the two previously approved agents – blocking asparagine, a protein necessary for the proliferation of neoplastic cells, according to an FDA press statement.
"The approval of Erwinaze underscores the FDA’s commitment to the approval of drugs for conditions with limited patient populations with unmet medical needs using novel trial end points," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement.
According to the prescribing information, asparaginase Erwinia chrysanthemi is indicated as part of a multi-agent treatment regimen for patients with hypersensitivity to E. coli–derived asparaginase.
To substitute for a dose of pegaspargase, patients should receive 25,000 IU/m2 of asparaginase Erwinia chrysanthemi administered intramuscularly three times a week (six doses for each planned dose of pegaspargase). To substitute for a dose of native E. coli asparaginase, the recommended dose of asparaginase Erwinia chrysanthemi is 25,000 IU/m2 intramuscularly for each scheduled dose of the E. coli–derived drug.
Two pivotal trials influenced the approval, the press statement noted. These included the ongoing Erwinase Master Treatment Protocol with full data on 843 patients, and a completed trial of 58 patients.
In each study, the main end point was the number of patients with sustained asparaginase activity levels that have been correlated with better disease control and survival, according to a statement on the National Cancer Institute Web site.
"The major efficacy outcome was attainment of sustained serum asparaginase activity levels of 0.1 IU/mL or higher, which has been demonstrated to correlate with asparagine depletion and to serum levels that predict clinical efficacy," according to the prescribing information. Among 48 patients with available samples, all achieved this threshold trough level of asparaginase activity.
The most common side effect associated with the drug was allergic reaction (17%), the prescribing information notes. Other side effects included pancreatitis (4%), coagulation abnormalities (3%), abnormal liver function (4%), and hyperglycemia (2%).
Nausea, vomiting, or abdominal pain was reported in 5%, while headache, diarrhea, or seizure occurred in 1% each.
EUSA Pharma Inc. of Langhorne, Pa., manufactures the drug. A patient education page is available.
*CORRECTION 12/22/11: This sentence originally stated that the enzyme was indicated for chronic lymphoblastic anemia. The sentence has been corrected to chronic lymphoblastic leukemia.
Novel Gout Treatment Boosts Response in Allopurinol Nonresponders
CHICAGO – Combined treatment with allopurinol and the novel purine nucleoside phosphorylase inhibitor BCX4208 significantly lowered serum uric acid levels in gout patients who had failed to respond to allopurinol alone, according to findings from a randomized placebo-controlled trial involving 278 adults.
Between 33% and 49% of patients who received 12 weeks of combined treatment with BCX4208 at doses ranging from 5 to 40 mg/day plus allopurinol at 300 mg/day achieved the goal serum uric acid level of less than 6.0 mg/dL, compared with only 18% of those receiving placebo and allopurinol, Dr. William P. Sheridan reported during a late-breaking abstract session at the annual meeting of the American College of Rheumatology.
The response rates differed by BCX4208 dose, with 45%, 33%, 39%, and 49% of those receiving 5, 10, 20, and 40 mg, respectively, achieving goal serum uric acid levels. The differences between the 5, 20, and 40 mg groups and the placebo arm were statistically significant, said Dr. Sheridan of BioCryst Pharmaceuticals, Durham, N.C., which is the maker of BCX4208 and the sponsor of the study.
Gout flares occurred in 5%-11% of patient in each BCX4208 arm and in 5% of patient in the placebo group, and both the severity and frequency of adverse events were evenly distributed across dose groups. No opportunistic infections occurred.
Also, dose-related reductions in lymphocytes and lymphocyte subsets occurred during drug administration, but these appeared to plateau within 12 weeks, Dr. Sheridan said.
Discontinuations occurred in both the 20- and 40-mg groups because of confirmed CD4+ cell counts less than 350 cells/mm3. No patients in the other groups discontinued because of low CD4+ cell counts, he said.
Study participants were adults with a mean age of 49 years with a gout diagnosis and serum uric acid levels of 6.0 mg/dL or less who had not responded sufficiently after treatment with at least 2 weeks of allopurinol at 300 mg/day. All patients received colchicine or naproxen as prophylaxis for gout flares.
The participants had a high mean body mass index of 36 kg/m2, and comorbidities were common; 58% had hypertension, 16% had diabetes, and 39% had hypercholesterolemia.
The findings suggest that BCX4208 is generally safe and well tolerated when combined with allopurinol, and that the combination improves the likelihood that gout patient will reach goal serum uric acid levels, Dr. Sheridan said, noting that the findings are important given that few alternatives have existed for patients who fail to reach serum uric acid goal range with a xanthine oxidase inhibitor alone.
BCX4208 appears shows promise for those patients, and an extension phase of the study is underway, he said.
"The safety and tolerability profile on BCX4208 was quite satisfactory and certainly adequate for consideration of further clinical research," he concluded, noting that phase III studies are in development.
Dr. Sheridan disclosed that he is employed by BioCryst Pharmaceuticals, which is the maker of BCX4208 and the sponsor of this study.
CHICAGO – Combined treatment with allopurinol and the novel purine nucleoside phosphorylase inhibitor BCX4208 significantly lowered serum uric acid levels in gout patients who had failed to respond to allopurinol alone, according to findings from a randomized placebo-controlled trial involving 278 adults.
Between 33% and 49% of patients who received 12 weeks of combined treatment with BCX4208 at doses ranging from 5 to 40 mg/day plus allopurinol at 300 mg/day achieved the goal serum uric acid level of less than 6.0 mg/dL, compared with only 18% of those receiving placebo and allopurinol, Dr. William P. Sheridan reported during a late-breaking abstract session at the annual meeting of the American College of Rheumatology.
The response rates differed by BCX4208 dose, with 45%, 33%, 39%, and 49% of those receiving 5, 10, 20, and 40 mg, respectively, achieving goal serum uric acid levels. The differences between the 5, 20, and 40 mg groups and the placebo arm were statistically significant, said Dr. Sheridan of BioCryst Pharmaceuticals, Durham, N.C., which is the maker of BCX4208 and the sponsor of the study.
Gout flares occurred in 5%-11% of patient in each BCX4208 arm and in 5% of patient in the placebo group, and both the severity and frequency of adverse events were evenly distributed across dose groups. No opportunistic infections occurred.
Also, dose-related reductions in lymphocytes and lymphocyte subsets occurred during drug administration, but these appeared to plateau within 12 weeks, Dr. Sheridan said.
Discontinuations occurred in both the 20- and 40-mg groups because of confirmed CD4+ cell counts less than 350 cells/mm3. No patients in the other groups discontinued because of low CD4+ cell counts, he said.
Study participants were adults with a mean age of 49 years with a gout diagnosis and serum uric acid levels of 6.0 mg/dL or less who had not responded sufficiently after treatment with at least 2 weeks of allopurinol at 300 mg/day. All patients received colchicine or naproxen as prophylaxis for gout flares.
The participants had a high mean body mass index of 36 kg/m2, and comorbidities were common; 58% had hypertension, 16% had diabetes, and 39% had hypercholesterolemia.
The findings suggest that BCX4208 is generally safe and well tolerated when combined with allopurinol, and that the combination improves the likelihood that gout patient will reach goal serum uric acid levels, Dr. Sheridan said, noting that the findings are important given that few alternatives have existed for patients who fail to reach serum uric acid goal range with a xanthine oxidase inhibitor alone.
BCX4208 appears shows promise for those patients, and an extension phase of the study is underway, he said.
"The safety and tolerability profile on BCX4208 was quite satisfactory and certainly adequate for consideration of further clinical research," he concluded, noting that phase III studies are in development.
Dr. Sheridan disclosed that he is employed by BioCryst Pharmaceuticals, which is the maker of BCX4208 and the sponsor of this study.
CHICAGO – Combined treatment with allopurinol and the novel purine nucleoside phosphorylase inhibitor BCX4208 significantly lowered serum uric acid levels in gout patients who had failed to respond to allopurinol alone, according to findings from a randomized placebo-controlled trial involving 278 adults.
Between 33% and 49% of patients who received 12 weeks of combined treatment with BCX4208 at doses ranging from 5 to 40 mg/day plus allopurinol at 300 mg/day achieved the goal serum uric acid level of less than 6.0 mg/dL, compared with only 18% of those receiving placebo and allopurinol, Dr. William P. Sheridan reported during a late-breaking abstract session at the annual meeting of the American College of Rheumatology.
The response rates differed by BCX4208 dose, with 45%, 33%, 39%, and 49% of those receiving 5, 10, 20, and 40 mg, respectively, achieving goal serum uric acid levels. The differences between the 5, 20, and 40 mg groups and the placebo arm were statistically significant, said Dr. Sheridan of BioCryst Pharmaceuticals, Durham, N.C., which is the maker of BCX4208 and the sponsor of the study.
Gout flares occurred in 5%-11% of patient in each BCX4208 arm and in 5% of patient in the placebo group, and both the severity and frequency of adverse events were evenly distributed across dose groups. No opportunistic infections occurred.
Also, dose-related reductions in lymphocytes and lymphocyte subsets occurred during drug administration, but these appeared to plateau within 12 weeks, Dr. Sheridan said.
Discontinuations occurred in both the 20- and 40-mg groups because of confirmed CD4+ cell counts less than 350 cells/mm3. No patients in the other groups discontinued because of low CD4+ cell counts, he said.
Study participants were adults with a mean age of 49 years with a gout diagnosis and serum uric acid levels of 6.0 mg/dL or less who had not responded sufficiently after treatment with at least 2 weeks of allopurinol at 300 mg/day. All patients received colchicine or naproxen as prophylaxis for gout flares.
The participants had a high mean body mass index of 36 kg/m2, and comorbidities were common; 58% had hypertension, 16% had diabetes, and 39% had hypercholesterolemia.
The findings suggest that BCX4208 is generally safe and well tolerated when combined with allopurinol, and that the combination improves the likelihood that gout patient will reach goal serum uric acid levels, Dr. Sheridan said, noting that the findings are important given that few alternatives have existed for patients who fail to reach serum uric acid goal range with a xanthine oxidase inhibitor alone.
BCX4208 appears shows promise for those patients, and an extension phase of the study is underway, he said.
"The safety and tolerability profile on BCX4208 was quite satisfactory and certainly adequate for consideration of further clinical research," he concluded, noting that phase III studies are in development.
Dr. Sheridan disclosed that he is employed by BioCryst Pharmaceuticals, which is the maker of BCX4208 and the sponsor of this study.
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF RHEUMATOLOGY
Major Finding: Between 33% and 49% of patients who received 12 weeks of combined treatment with BCX4208 at doses ranging from 5 to 40 mg/day plus allopurinol at 300 mg/day achieved the goal serum uric acid level of less than 6.0 mg/dL, compared with only 18% of those receiving placebo and allopurinol.
Data Source: A randomized placebo-controlled study.
Disclosures: Dr. Sheridan disclosed that he is employed by BioCryst Pharmaceuticals, which is the maker of BCX4208 and the sponsor of this study.
VTE Prophylaxis in Focus
Heparin prophylaxis had no significant impact on mortality rates in medical patients, according to a new report in Annals of Internal Medicine. And one of the authors suggests that the results should push hospitalists to use a more critical eye when considering pharmacological prophylaxis.
"Venous Thromboembolism Prophylaxis in Hospitalized Medical Patients and Those With Stroke: A Background Review for an American College of Physicians Clinical Practice Guideline" showed that in medical patients, heparin prophylaxis had no "statistically significant effect on any outcome in patients with acute stroke except for an increase in major bleeding events" (OR, 1.66 [CI, 1.20 to 2.28]). The authors concluded that precautionary use of heparin might have reduced pulmonary embolisms in both medical and stroke patients, but combined with upticks in bleeding and major bleeding events, the overall outcome results "in little or no net benefit."
"Our results do not really decide the issue for a physician and a patient whether prophylaxis should be used but rather show that this is a question that is still very much up in the air," says author Frank Lederle, MD, professor of medicine at the Minneapolis VA Medical Center. "That there may be good reasons to use prophylaxis, but it certainly shouldn't be something we're mandating or trying to achieve uniformity on when we don't have the evidence to do so."
Dr. Lederle adds that while some researchers are publishing papers on how well hospitalists and other physicians adhere to prophylactic procedures, he'd like to see more evidence-based analysis of the tactic's efficacy.
"The reason that this is not universally accepted by physicians is that people are aware that the data aren't that supportive of it," he says. "And that, really, the question should go back to 'Does it work and in whom does it work?' not 'Why are physicians failing to follow a guideline?'"
Heparin prophylaxis had no significant impact on mortality rates in medical patients, according to a new report in Annals of Internal Medicine. And one of the authors suggests that the results should push hospitalists to use a more critical eye when considering pharmacological prophylaxis.
"Venous Thromboembolism Prophylaxis in Hospitalized Medical Patients and Those With Stroke: A Background Review for an American College of Physicians Clinical Practice Guideline" showed that in medical patients, heparin prophylaxis had no "statistically significant effect on any outcome in patients with acute stroke except for an increase in major bleeding events" (OR, 1.66 [CI, 1.20 to 2.28]). The authors concluded that precautionary use of heparin might have reduced pulmonary embolisms in both medical and stroke patients, but combined with upticks in bleeding and major bleeding events, the overall outcome results "in little or no net benefit."
"Our results do not really decide the issue for a physician and a patient whether prophylaxis should be used but rather show that this is a question that is still very much up in the air," says author Frank Lederle, MD, professor of medicine at the Minneapolis VA Medical Center. "That there may be good reasons to use prophylaxis, but it certainly shouldn't be something we're mandating or trying to achieve uniformity on when we don't have the evidence to do so."
Dr. Lederle adds that while some researchers are publishing papers on how well hospitalists and other physicians adhere to prophylactic procedures, he'd like to see more evidence-based analysis of the tactic's efficacy.
"The reason that this is not universally accepted by physicians is that people are aware that the data aren't that supportive of it," he says. "And that, really, the question should go back to 'Does it work and in whom does it work?' not 'Why are physicians failing to follow a guideline?'"
Heparin prophylaxis had no significant impact on mortality rates in medical patients, according to a new report in Annals of Internal Medicine. And one of the authors suggests that the results should push hospitalists to use a more critical eye when considering pharmacological prophylaxis.
"Venous Thromboembolism Prophylaxis in Hospitalized Medical Patients and Those With Stroke: A Background Review for an American College of Physicians Clinical Practice Guideline" showed that in medical patients, heparin prophylaxis had no "statistically significant effect on any outcome in patients with acute stroke except for an increase in major bleeding events" (OR, 1.66 [CI, 1.20 to 2.28]). The authors concluded that precautionary use of heparin might have reduced pulmonary embolisms in both medical and stroke patients, but combined with upticks in bleeding and major bleeding events, the overall outcome results "in little or no net benefit."
"Our results do not really decide the issue for a physician and a patient whether prophylaxis should be used but rather show that this is a question that is still very much up in the air," says author Frank Lederle, MD, professor of medicine at the Minneapolis VA Medical Center. "That there may be good reasons to use prophylaxis, but it certainly shouldn't be something we're mandating or trying to achieve uniformity on when we don't have the evidence to do so."
Dr. Lederle adds that while some researchers are publishing papers on how well hospitalists and other physicians adhere to prophylactic procedures, he'd like to see more evidence-based analysis of the tactic's efficacy.
"The reason that this is not universally accepted by physicians is that people are aware that the data aren't that supportive of it," he says. "And that, really, the question should go back to 'Does it work and in whom does it work?' not 'Why are physicians failing to follow a guideline?'"
Improving Stroke Alert Response Time
In‐hospital strokes account for a significant proportion of the almost 800,000 cerebrovascular accidents that occur each year in the United States.1 Although inpatient strokes are thought to be under‐recognized and under‐reported, between 4% and 17% of all stroke patients in the hospital experienced stroke onset during hospitalization.2, 3 Estimates place the number of in‐hospital strokes at 35,000‐75,000 each year in the United States.4
As a result of the exquisite sensitivity of brain tissue to ischemic events, stroke is a medical emergency and time‐to‐treatment is of the essence. With each minute of ischemia, 1.9 million neurons are destroyed.5 Evidence suggests benefit of treatment with intravenous thrombolysis up to 4.5 hours after symptom onset, with lower disability associated with more rapid initiation of therapy.6, 7 To facilitate timely thrombolytic therapy, the American Stroke Association (ASA) recommends that imaging of the brain be initiated within 25 minutes of presentation for patients with suspected stroke.8
Studies demonstrate greater delays in the evaluation of hospitalized patients suffering from stroke compared to stroke patients presenting to the Emergency Department (ED).9, 10 Performance of timely evaluation of in‐hospital stroke rarely meets ASA goals. Analysis of a Michigan stroke registry found that only 3.1% of patients with in‐hospital strokes received computed tomography (CT) scan within 25 minutes of symptom recognition, and a Colorado stroke registry found time‐to‐evaluation to be more than twice the recommended benchmark.11, 12 Data from a multicenter stroke registry in Spain showed that half of all thrombolysis‐eligible, in‐hospital stroke patients could not be treated due to delays in evaluation.13
Our prior work demonstrated that the use of an in‐hospital stroke response team significantly reduced time to evaluation for true ischemic strokes.10 Even with this rapid response mechanism, the evaluation time for in‐hospital stroke was still more than twice that observed in the ED despite using the same team to respond to both settings. Hospital rapid response systems, specifically for patients with suspected stroke, have been described in the literature and outline in‐hospital response systems capable of meeting evaluation time goals.1415 How to optimize a stroke response system has not been previously described. The aim of this quality improvement (QI) initiative was to reduce time‐to‐evaluation for strokes occurring in patients already hospitalized using systems analysis and modification. We describe key elements and tools for implementing institutional QI for in‐hospital stroke.
METHODS
The QI initiative was implemented at the University of Colorado Hospital (UCH), a tertiary care academic medical center. The Colorado Multiple Institutional Review Board determined this project to be in the exempt category. UCH uses a protocol in which all stroke alerts undergo non‐contrast CT of the brain. If no intracranial bleeding is found, and the patient is a thrombolytic candidate, advanced CT imaging including CT perfusion and CT angiogram will also be performed during the alert. Magnetic resonance imaging (MRI) with diffusion weighted imaging is done non‐emergently for subsequent stroke evaluation, but is not part of the stroke alert protocol. The primary endpoint of time from alert to initiation of CT was chosen because it represents an unambiguous interval which is present for all stroke alerts. Pre‐intervention data was gathered for 6 months, from September 2008 to February 2009. During this period, the process through which in‐hospital strokes were identified, referred for evaluation, and treated was mapped to identify inefficient or unreliable steps, and the process was redesigned to enhance efficiency. The intervention was rolled out over a 3‐month period from March 2009 to May 2009. During the intervention roll‐out period, the refined stroke alert process and a checklist containing the optimal in‐hospital stroke alert response system was implemented. An education campaign was initiated, for acute stroke team members and nursing staff, on signs of stroke and each individual's role in response to symptoms of in‐hospital stroke based on the new process. During the roll‐out period, each unit in the hospital was provided in‐hospital stroke alert posters and a packet containing specific stroke education on the in‐hospital stroke alert process. Unit educators were empowered to determine how to best deliver the education to their staff, and many chose to invite the stroke program coordinator to give an hour‐long presentation on stroke prior to shift or during lunch. Each unit educator kept record of the stroke instruction provided and submitted staff signatures to the stroke program. Nursing staff was also provided with in‐hospital stroke protocol badge cards that outlined optimal approach to stroke identification and treatment using the revised protocol. Interventions were being implemented in a progressive fashion throughout the roll‐out period. Starting during the roll‐out and continuing into the post‐intervention period, feedback on all in‐hospital stroke alerts was provided to the stroke team and front‐line providers. The impact of the intervention was followed for 6 months post‐intervention from June 2009 to November 2009. The QI tools used in this project are well described by the Institute of Healthcare Improvement, and each step in the QI process is outlined in detail below:16
Step 1: Process Map With Identification of Unreliable and Reliably Slow Steps
A detailed process map was created to outline steps in the existing stroke alert process (see Supporting Figures, Process Maps, in the online version of this article). One investigator (R.Z.) interviewed key members of the multidisciplinary stroke team, including representatives from the departments of neurology, nursing, hospital medicine, neurosurgery, radiology, and transportation. Interviews with key stakeholders and frequent participants in stroke alerts revealed evidence of episodic unreliable steps. Stakeholders were noted to have slightly different conceptions of how the process flow was intended to occur, and where responsibility lay for certain tasks. The interviews aided in identification of pitfalls, bottlenecks, misconceptions, and areas that needed clarification or change in the alert process.
Examples of unreliable and bottleneck steps include: In the pre‐intervention process, the transportation department was responsible for moving patients to radiology; this step was identified as reliably slow. Investigation revealed that the transportation department did not have a mechanism for rapid response to emergency transport requests. Analysis also revealed that 2 key steps necessary for treating in‐hospital stroke were occasionally neglected: ensuring adequate intravenous (IV) access, and ordering of the correct panel of laboratory tests. Finally, a process communication deficit was identified, with CT technicians periodically unaware of the pending arrival of an in‐hospital stroke patient, thus preventing the scan from being cleared for the emergent stroke imaging.
Direct observation of real‐time stroke alerts in both the inpatient and ED settings was also employed to outline the process and identify areas of inefficiency. Direct observation of stroke alerts in progress verified the unified picture of process flow developed from stakeholder interviews (see Supporting Figures, Process Maps, in the online version of this article). Particular note was made of differences between the stroke alert process in the ED and the inpatient setting.
Step 2: System Redesign With Input From All Stakeholders
Proposed interventions were presented to hospital governing councils, including the interdisciplinary Stroke Council and Nurse Managers Council. After verification of the shortcomings of the existing alert process and obtaining buy‐in from key participants and governing departments, a new process was designed (see Supporting Figures, Process Maps, in the online version of this article). Specific changes include the following examples: First, electrocardiogram was moved to occur after CT scan. Second, investigation revealed that the transportation department within the hospital was designed for non‐emergent transportation and not amenable to change. The mechanism of patient transportation was changed such that, rather than using the transportation department, patients were now transported by the neurology resident responding to the stroke alert, accompanied by the patient's ward nurse. This both removed a bottleneck step and assured critical staff presence during the transportation of a potentially unstable patient. Third, to ensure effective communication, CT technicians were provided with stroke alert pagers that receive text messages regarding incoming in‐hospital stroke alert patients. Fourth, a time limit was set for IV attempts prior to transportation. The new protocol, along with explicit expectations for the role of the patient's nurse in in‐hospital stroke alerts, was described in a hospital‐wide nursing stroke education initiative.
Step 3: In‐Hospital Stroke Alert Checklist
A new standardized protocol for optimal in‐hospital stroke care was detailed on a laminated pocket card. The checklist described exactly what steps were to be performed, by whom, how to make them occur, and in what order. The checklist was designed to reduce the incidence of omitted steps, such as ordering of correct laboratory evaluations. The laminated cards highlighted the benchmark time to evaluation of 25 minutes. Process checklist cards were distributed to all members of the acute stroke alert response team, and short versions designed specifically for nursing staff were distributed as badge cards and posted on clinical care units (Supporting Information Appendix I).
Step 4: Real‐Time Feedback
During the intervention roll‐out and post‐intervention periods, feedback was provided from the stroke program to the front‐line providers following each in‐hospital stroke alert. The clinicians involved were notified of the final diagnosis and patient outcome, and were provided with feedback about how the patient's evaluation times compared with benchmark goals. Feedback may serve to motivate, based on clinician professionalism, but performance in the alert was not tied to rewards or penalties for the providers involved. The feedback process was designed to be bi‐directional, with requests for input from staff on barriers to rapid evaluation experienced and suggestions for future process improvement (Supporting Information Appendix II).
Statistical Analysis
The primary outcome was the change in time from stroke alert to CT scan (alert‐to‐CT), comparing pre‐intervention and post‐intervention periods. This time interval was chosen because its calculation involved unambiguous time points, which are available for all patients for whom an in‐hospital alert is called. It is a measure of process efficiency, with minimal expected variation based on differences in patient characteristics (ie, hemorrhagic vs ischemic stroke). Non‐overlapping Kaplan‐Meier curves confirmed the proportional hazards assumption for 2 Cox proportional hazards models: unadjusted and adjusted by group characteristics with P‐value 0.10. Relative hazards and estimates for the percent of patients with alert‐to‐CT scan 25 minutes, according to intervention groups, were obtained from these models. For analyses, admit unit was re‐categorized as intensive care unit (ICU), Med/Surg, or Other. Analyses were conducted using SAS Version 9.2 (SAS Institute, Inc, Cary, NC).
RESULTS
During the study intervals, there were 82 inpatient stroke alerts. Of these alerts, 75 were included in the analysis. Seven were excluded for the following reasons: alert canceled by the stroke team (3), time of alert was not recorded (1), patient identifiers not recorded (1), or stroke alert was preceded by CT imaging (2).
During the 6 months prior to intervention, the median inpatient stroke alert‐to‐CT time (n = 31) was 69.0 minutes (Table 1). Nineteen percent of these alerts met the goal of 25 minutes from alert‐to‐CT time. During the 6‐month post‐intervention period, the median inpatient alert‐to‐CT time (n = 44) was 29.5 minutes. Thirty‐two percent of these alerts met the 25‐minute alert‐to‐CT time benchmark. In the unadjusted model, patients during the post‐intervention period were significantly more likely to have alert‐to‐CT scan time 25 minutes compared to patients prior to the intervention (post‐intervention compared to pre‐intervention, Relative Hazard (RH): 3.03; 95% confidence interval [CI]: 1.76‐5.20; log‐rank P 0.0001). This remained significant after adjustment for hyperlipidemia, active cancer, final diagnosis of ischemic brain injury, and final diagnosis of stroke mimic (RH: 4.96; 95% CI: 2.65‐9.32; P 0.0001); data not shown. Admit unit was not included in the adjusted model since there was no indication of differences in the 3‐level variable according to intervention group (P = 0.27). In addition to reduction in median response times, the variability of response times was markedly reduced, and no patient in the 6‐month post‐intervention period had delay to CT sufficient to preclude use of IV thrombolysis (Figure 1).
| Pre‐Intervention (n = 31) | Post‐Intervention (n = 44) | P Value | |
|---|---|---|---|
| |||
| Stroke alert to CT time, median [95% CI] | 69 min [34, 103] | 29.5 min [26, 40] | P 0.0001 |
| Age, median [IQR] | 61.0 [54.0, 70.0] | 60.5 [48.5, 70.5] | 0.94 |
| Female (%) | 19 (61.3) | 23 (52.3) | 0.44 |
| Race (%) | |||
| Asian | 1 (3.2) | 1 (2.3) | 0.31 |
| Black | 4 (12.9) | 6 (13.6) | |
| Caucasian | 21 (67.7) | 27 (61.4) | |
| Hispanic | 3 (9.7) | 10 (22.7) | |
| Unknown | 2 (6.5) | 0 (0) | |
| Admit unit (%) | |||
| Intensive care | 12 (38.7) | 10 (22.7) | 0.07 |
| Medicine/surgery | 15 (48.4) | 24 (54.6) | |
| Neurology | 0 (0) | 5 (11.4) | |
| Post‐acute care | 3 (9.7) | 0 (0) | |
| Rehabilitation | 1 (3.2) | 2 (4.6) | |
| Women's and maternal care | 0 (0) | 2 (4.6) | |
| Cardiology | 0 (0) | 1 (2.3) | |
| Case mix index, median [IQR] | n = 29 2.6 [1.1, 5.0] | n = 42 2.2 [1.6, 4.5] | 0.82 |
| Prior cerebrovascular accident (%) | 5 (16.1) | 8 (18.2) | 0.82 |
| Hypertension (%) | 17 (54.8) | 24 (54.6) | 0.98 |
| Diabetes mellitus (%) | 7 (22.6) | 11 (25.0) | 0.81 |
| Hyperlipidemia (%) | 15 (48.4) | 9 (20.5) | 0.01 |
| Tobacco abuse, current (%) | 4 (12.9) | 1 (2.3) | 0.15 |
| Alcohol abuse (%) | 2 (6.5) | 0 (0) | 0.17 |
| Active cancer (%) | 8 (25.8) | 5 (11.4) | 0.10 |
| Peripheral vascular disease (%) | 2 (6.5) | 3 (6.8) | 1.0 |
| Coronary artery disease (%) | 6 (19.4) | 7 (15.9) | 0.70 |
| Congestive heart failure (%) | n = 30 5 (16.7) | 4 (9.1) | 0.47 |
| Valvulopathy (%) | 0 (0) | 1 (2.3) | 1.0 |
| Atrial fibrillation (%) | 3 (9.7) | 10 (22.7) | 0.14 |
| Anticoagulation (%) | 7 (22.6) | 7 (15.9) | 0.47 |
| Final diagnosis ischemic brain injury (%) | 15 (48.4) | 11 (25.0) | 0.04 |
| Final diagnosis hemorrhagic brain injury (%) | 3 (9.7) | 4 (9.1) | 1.0 |
| Final diagnosis stroke mimic (symptoms not due to ischemic or hemorrhagic brain injury) (%) | 13 (41.9) | 29 (65.9) | 0.04 |
CONCLUSIONS
In‐hospital strokes represent an emergency for which response time is critical. Neurologic injury progresses with every minute of ischemia, and current recommendations offer a limited time window for intravenous thrombolysis. For stroke with symptom onset in the monitored setting of the hospital, there is a compelling imperative to reduce all delays from system inefficiencies. The findings of the current QI initiative suggest that dramatic improvements are possible through systematic evaluation and redesign of hospital response processes, a checklist for in‐hospital stroke carried by front‐line responders, and ongoing real‐time feedback.
Limitations of this study include a prepost design. The necessity of implementing system change hospital‐wide precluded use of a concurrent control group. The time goals for evaluation are derived from American Stroke Association targets for patients arriving in the Emergency Department. There are differences in process between the hospital ward and the Emergency Department, but the fundamental concept of minimizing time to evaluation once patient symptoms are recognized by hospital staff remains valid.
The possibility of system improvements not due to this QI initiative cannot be excluded. In 2006, this hospital expanded the responsibility of the stroke response team to include acute neurologic deficits outside of the ED without other changes to the in‐hospital stroke alert process. This reduced time to evaluation for in‐hospital ischemic strokes compared to usual care, but even with the same acute stroke response team responding to stroke alerts in both settings, in‐hospital stroke response times remained significantly longer than response times for stroke in the ED.10 The presence of an in‐hospital stroke alert response team alone was not capable of reducing evaluation times to goal. Minimal improvement in median in‐hospital stroke alert evaluation time was seen in the intervening year, following the completion of our previously published analysis, suggesting explicit system QI was necessary.
The Hawthorne effect, in which individuals who know they are being observed modify behavior while such monitoring is in effect, is a major limitation of interpreting QI initiatives. By committing to continuous and ongoing feedback to front‐line providers, this phenomenon can be harnessed to sustain improvement.17 In effect, the study of efficient response to the in‐hospital stroke never ceases. UCH has continued to employ the post‐intervention stroke alert protocol and engage in ongoing feedback after each stroke alert. In the 12 months following the conclusion of this study, the median response time to in‐hospital strokes continues to be 30 minutes, and 7 additional in‐hospital stroke patients have been treated with thrombolysis.
This inpatient stroke alert initiative decreased median inpatient alert‐to‐CT time by 57%, and demonstrates that quality of in‐hospital stroke care can be improved. Decrease in stroke alert‐to‐CT time facilitates earlier thrombolytic therapy. Analysis of treatment and patient outcomes was outside of the scope of the current study, but earlier treatment has potential to significantly improve clinical outcomes.
The Society of Hospital Medicine defines one of the goals of QI to be the change in processes with reduction in variation, thus improving the care for all patients rather than focusing exclusively on outlier events.18 This initiative markedly reduced evaluation variability, allowing a greater percentage of patients to be eligible for treatment within the critical time window. Prior to the intervention, almost a quarter of patients had delays in evaluation sufficient to preclude IV thrombolysis, whereas in the 6 months after the intervention was initiated, not a single patient had evaluation delayed to the point that IV thrombolysis would not have been an option (Figure 1). The goal of in‐hospital stroke QI must be to improve the speed of the process for all patients, and assure that no patient is denied the potential for therapy as a result of inefficiencies in hospital systems.
Acknowledgements
The authors thank Traci Yamashita, PRA, for her work in the statistical analysis for this publication, and Dr Jeffrey Glasheen for development of the University of Colorado Hospital's Hospitalist Training Track Quality Improvement Program of which this work is a product.
- ,,, et al.Heart disease and stroke statistics—2010 update: a report from the American Heart Association.Circulation.2010;121:e46–e215.
- ,,.Characteristics of in‐hospital onset ischemic stroke.Eur Neurol.2006;55:155–159.
- ,.Inpatient and community ischemic strokes in a community hospital.Neuroepidemiology.2007;28:86–92.
- .In‐hospital stroke.Lancet Neurol.2003;2:741–746.
- .Time is brain‐quantified.Stroke.2006;37:263–266.
- ,,, et al.Ultra‐early thrombolysis in acute ischemic stroke is associated with better outcomes and lower mortality.Stroke.2010;41:712–716.
- ,,,.Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association.Stroke.2009;40:2945–2948.
- ,,, et al.Guidelines for early management of adults with ischemic stroke.Stroke.2007;38;1655–1711.
- ,,, et al.In‐hospital stroke treated with intravenous tissue plasminogen activator.Stroke.2008;39:2614–2616.
- ,,,,.Stroke alert program improves recognition and evaluation time of in‐hospital ischemic stroke.J Stroke Cerebrovasc Dis.2009;19:494–496.
- ,,,,,.In‐hospital stroke in a statewide stroke registry.Cerebrovasc Dis.2008;25:12–20.
- ,,,,.Quality of care for in‐hospital stroke: analysis of a statewide registry.Stroke.2011;42:207–210.
- ,,, et al.In‐hospital stroke: a multi‐center prospective registry.Eur J Neurol.2011;18:170–176.
- ,,.Code Gray—an organized approach to inpatient stroke.Crit Care Nurs Q.2003;26:296–302.
- ,,.ID, stat‐rapid response to in‐hospital stroke patients.Nurs Manage.2009;40:34–38.
- Institute of Healthcare Improvement. Quality Improvement Tools. Available at: http://www.ihi.org/IHI/Topics/Improvement/ImprovementMethods/Tools/. Accessed December 1,2010.
- ,,,,,.Variability in the Hawthorne effect with regard to hand hygiene performance in high‐ and low‐performing inpatient care units.Infect Control Hosp Epidemiol.2009;30:222–225.
- Society of Hospital Medicine Quality Improvement Resources. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/32. Accessed December 1,2010.
In‐hospital strokes account for a significant proportion of the almost 800,000 cerebrovascular accidents that occur each year in the United States.1 Although inpatient strokes are thought to be under‐recognized and under‐reported, between 4% and 17% of all stroke patients in the hospital experienced stroke onset during hospitalization.2, 3 Estimates place the number of in‐hospital strokes at 35,000‐75,000 each year in the United States.4
As a result of the exquisite sensitivity of brain tissue to ischemic events, stroke is a medical emergency and time‐to‐treatment is of the essence. With each minute of ischemia, 1.9 million neurons are destroyed.5 Evidence suggests benefit of treatment with intravenous thrombolysis up to 4.5 hours after symptom onset, with lower disability associated with more rapid initiation of therapy.6, 7 To facilitate timely thrombolytic therapy, the American Stroke Association (ASA) recommends that imaging of the brain be initiated within 25 minutes of presentation for patients with suspected stroke.8
Studies demonstrate greater delays in the evaluation of hospitalized patients suffering from stroke compared to stroke patients presenting to the Emergency Department (ED).9, 10 Performance of timely evaluation of in‐hospital stroke rarely meets ASA goals. Analysis of a Michigan stroke registry found that only 3.1% of patients with in‐hospital strokes received computed tomography (CT) scan within 25 minutes of symptom recognition, and a Colorado stroke registry found time‐to‐evaluation to be more than twice the recommended benchmark.11, 12 Data from a multicenter stroke registry in Spain showed that half of all thrombolysis‐eligible, in‐hospital stroke patients could not be treated due to delays in evaluation.13
Our prior work demonstrated that the use of an in‐hospital stroke response team significantly reduced time to evaluation for true ischemic strokes.10 Even with this rapid response mechanism, the evaluation time for in‐hospital stroke was still more than twice that observed in the ED despite using the same team to respond to both settings. Hospital rapid response systems, specifically for patients with suspected stroke, have been described in the literature and outline in‐hospital response systems capable of meeting evaluation time goals.1415 How to optimize a stroke response system has not been previously described. The aim of this quality improvement (QI) initiative was to reduce time‐to‐evaluation for strokes occurring in patients already hospitalized using systems analysis and modification. We describe key elements and tools for implementing institutional QI for in‐hospital stroke.
METHODS
The QI initiative was implemented at the University of Colorado Hospital (UCH), a tertiary care academic medical center. The Colorado Multiple Institutional Review Board determined this project to be in the exempt category. UCH uses a protocol in which all stroke alerts undergo non‐contrast CT of the brain. If no intracranial bleeding is found, and the patient is a thrombolytic candidate, advanced CT imaging including CT perfusion and CT angiogram will also be performed during the alert. Magnetic resonance imaging (MRI) with diffusion weighted imaging is done non‐emergently for subsequent stroke evaluation, but is not part of the stroke alert protocol. The primary endpoint of time from alert to initiation of CT was chosen because it represents an unambiguous interval which is present for all stroke alerts. Pre‐intervention data was gathered for 6 months, from September 2008 to February 2009. During this period, the process through which in‐hospital strokes were identified, referred for evaluation, and treated was mapped to identify inefficient or unreliable steps, and the process was redesigned to enhance efficiency. The intervention was rolled out over a 3‐month period from March 2009 to May 2009. During the intervention roll‐out period, the refined stroke alert process and a checklist containing the optimal in‐hospital stroke alert response system was implemented. An education campaign was initiated, for acute stroke team members and nursing staff, on signs of stroke and each individual's role in response to symptoms of in‐hospital stroke based on the new process. During the roll‐out period, each unit in the hospital was provided in‐hospital stroke alert posters and a packet containing specific stroke education on the in‐hospital stroke alert process. Unit educators were empowered to determine how to best deliver the education to their staff, and many chose to invite the stroke program coordinator to give an hour‐long presentation on stroke prior to shift or during lunch. Each unit educator kept record of the stroke instruction provided and submitted staff signatures to the stroke program. Nursing staff was also provided with in‐hospital stroke protocol badge cards that outlined optimal approach to stroke identification and treatment using the revised protocol. Interventions were being implemented in a progressive fashion throughout the roll‐out period. Starting during the roll‐out and continuing into the post‐intervention period, feedback on all in‐hospital stroke alerts was provided to the stroke team and front‐line providers. The impact of the intervention was followed for 6 months post‐intervention from June 2009 to November 2009. The QI tools used in this project are well described by the Institute of Healthcare Improvement, and each step in the QI process is outlined in detail below:16
Step 1: Process Map With Identification of Unreliable and Reliably Slow Steps
A detailed process map was created to outline steps in the existing stroke alert process (see Supporting Figures, Process Maps, in the online version of this article). One investigator (R.Z.) interviewed key members of the multidisciplinary stroke team, including representatives from the departments of neurology, nursing, hospital medicine, neurosurgery, radiology, and transportation. Interviews with key stakeholders and frequent participants in stroke alerts revealed evidence of episodic unreliable steps. Stakeholders were noted to have slightly different conceptions of how the process flow was intended to occur, and where responsibility lay for certain tasks. The interviews aided in identification of pitfalls, bottlenecks, misconceptions, and areas that needed clarification or change in the alert process.
Examples of unreliable and bottleneck steps include: In the pre‐intervention process, the transportation department was responsible for moving patients to radiology; this step was identified as reliably slow. Investigation revealed that the transportation department did not have a mechanism for rapid response to emergency transport requests. Analysis also revealed that 2 key steps necessary for treating in‐hospital stroke were occasionally neglected: ensuring adequate intravenous (IV) access, and ordering of the correct panel of laboratory tests. Finally, a process communication deficit was identified, with CT technicians periodically unaware of the pending arrival of an in‐hospital stroke patient, thus preventing the scan from being cleared for the emergent stroke imaging.
Direct observation of real‐time stroke alerts in both the inpatient and ED settings was also employed to outline the process and identify areas of inefficiency. Direct observation of stroke alerts in progress verified the unified picture of process flow developed from stakeholder interviews (see Supporting Figures, Process Maps, in the online version of this article). Particular note was made of differences between the stroke alert process in the ED and the inpatient setting.
Step 2: System Redesign With Input From All Stakeholders
Proposed interventions were presented to hospital governing councils, including the interdisciplinary Stroke Council and Nurse Managers Council. After verification of the shortcomings of the existing alert process and obtaining buy‐in from key participants and governing departments, a new process was designed (see Supporting Figures, Process Maps, in the online version of this article). Specific changes include the following examples: First, electrocardiogram was moved to occur after CT scan. Second, investigation revealed that the transportation department within the hospital was designed for non‐emergent transportation and not amenable to change. The mechanism of patient transportation was changed such that, rather than using the transportation department, patients were now transported by the neurology resident responding to the stroke alert, accompanied by the patient's ward nurse. This both removed a bottleneck step and assured critical staff presence during the transportation of a potentially unstable patient. Third, to ensure effective communication, CT technicians were provided with stroke alert pagers that receive text messages regarding incoming in‐hospital stroke alert patients. Fourth, a time limit was set for IV attempts prior to transportation. The new protocol, along with explicit expectations for the role of the patient's nurse in in‐hospital stroke alerts, was described in a hospital‐wide nursing stroke education initiative.
Step 3: In‐Hospital Stroke Alert Checklist
A new standardized protocol for optimal in‐hospital stroke care was detailed on a laminated pocket card. The checklist described exactly what steps were to be performed, by whom, how to make them occur, and in what order. The checklist was designed to reduce the incidence of omitted steps, such as ordering of correct laboratory evaluations. The laminated cards highlighted the benchmark time to evaluation of 25 minutes. Process checklist cards were distributed to all members of the acute stroke alert response team, and short versions designed specifically for nursing staff were distributed as badge cards and posted on clinical care units (Supporting Information Appendix I).
Step 4: Real‐Time Feedback
During the intervention roll‐out and post‐intervention periods, feedback was provided from the stroke program to the front‐line providers following each in‐hospital stroke alert. The clinicians involved were notified of the final diagnosis and patient outcome, and were provided with feedback about how the patient's evaluation times compared with benchmark goals. Feedback may serve to motivate, based on clinician professionalism, but performance in the alert was not tied to rewards or penalties for the providers involved. The feedback process was designed to be bi‐directional, with requests for input from staff on barriers to rapid evaluation experienced and suggestions for future process improvement (Supporting Information Appendix II).
Statistical Analysis
The primary outcome was the change in time from stroke alert to CT scan (alert‐to‐CT), comparing pre‐intervention and post‐intervention periods. This time interval was chosen because its calculation involved unambiguous time points, which are available for all patients for whom an in‐hospital alert is called. It is a measure of process efficiency, with minimal expected variation based on differences in patient characteristics (ie, hemorrhagic vs ischemic stroke). Non‐overlapping Kaplan‐Meier curves confirmed the proportional hazards assumption for 2 Cox proportional hazards models: unadjusted and adjusted by group characteristics with P‐value 0.10. Relative hazards and estimates for the percent of patients with alert‐to‐CT scan 25 minutes, according to intervention groups, were obtained from these models. For analyses, admit unit was re‐categorized as intensive care unit (ICU), Med/Surg, or Other. Analyses were conducted using SAS Version 9.2 (SAS Institute, Inc, Cary, NC).
RESULTS
During the study intervals, there were 82 inpatient stroke alerts. Of these alerts, 75 were included in the analysis. Seven were excluded for the following reasons: alert canceled by the stroke team (3), time of alert was not recorded (1), patient identifiers not recorded (1), or stroke alert was preceded by CT imaging (2).
During the 6 months prior to intervention, the median inpatient stroke alert‐to‐CT time (n = 31) was 69.0 minutes (Table 1). Nineteen percent of these alerts met the goal of 25 minutes from alert‐to‐CT time. During the 6‐month post‐intervention period, the median inpatient alert‐to‐CT time (n = 44) was 29.5 minutes. Thirty‐two percent of these alerts met the 25‐minute alert‐to‐CT time benchmark. In the unadjusted model, patients during the post‐intervention period were significantly more likely to have alert‐to‐CT scan time 25 minutes compared to patients prior to the intervention (post‐intervention compared to pre‐intervention, Relative Hazard (RH): 3.03; 95% confidence interval [CI]: 1.76‐5.20; log‐rank P 0.0001). This remained significant after adjustment for hyperlipidemia, active cancer, final diagnosis of ischemic brain injury, and final diagnosis of stroke mimic (RH: 4.96; 95% CI: 2.65‐9.32; P 0.0001); data not shown. Admit unit was not included in the adjusted model since there was no indication of differences in the 3‐level variable according to intervention group (P = 0.27). In addition to reduction in median response times, the variability of response times was markedly reduced, and no patient in the 6‐month post‐intervention period had delay to CT sufficient to preclude use of IV thrombolysis (Figure 1).
| Pre‐Intervention (n = 31) | Post‐Intervention (n = 44) | P Value | |
|---|---|---|---|
| |||
| Stroke alert to CT time, median [95% CI] | 69 min [34, 103] | 29.5 min [26, 40] | P 0.0001 |
| Age, median [IQR] | 61.0 [54.0, 70.0] | 60.5 [48.5, 70.5] | 0.94 |
| Female (%) | 19 (61.3) | 23 (52.3) | 0.44 |
| Race (%) | |||
| Asian | 1 (3.2) | 1 (2.3) | 0.31 |
| Black | 4 (12.9) | 6 (13.6) | |
| Caucasian | 21 (67.7) | 27 (61.4) | |
| Hispanic | 3 (9.7) | 10 (22.7) | |
| Unknown | 2 (6.5) | 0 (0) | |
| Admit unit (%) | |||
| Intensive care | 12 (38.7) | 10 (22.7) | 0.07 |
| Medicine/surgery | 15 (48.4) | 24 (54.6) | |
| Neurology | 0 (0) | 5 (11.4) | |
| Post‐acute care | 3 (9.7) | 0 (0) | |
| Rehabilitation | 1 (3.2) | 2 (4.6) | |
| Women's and maternal care | 0 (0) | 2 (4.6) | |
| Cardiology | 0 (0) | 1 (2.3) | |
| Case mix index, median [IQR] | n = 29 2.6 [1.1, 5.0] | n = 42 2.2 [1.6, 4.5] | 0.82 |
| Prior cerebrovascular accident (%) | 5 (16.1) | 8 (18.2) | 0.82 |
| Hypertension (%) | 17 (54.8) | 24 (54.6) | 0.98 |
| Diabetes mellitus (%) | 7 (22.6) | 11 (25.0) | 0.81 |
| Hyperlipidemia (%) | 15 (48.4) | 9 (20.5) | 0.01 |
| Tobacco abuse, current (%) | 4 (12.9) | 1 (2.3) | 0.15 |
| Alcohol abuse (%) | 2 (6.5) | 0 (0) | 0.17 |
| Active cancer (%) | 8 (25.8) | 5 (11.4) | 0.10 |
| Peripheral vascular disease (%) | 2 (6.5) | 3 (6.8) | 1.0 |
| Coronary artery disease (%) | 6 (19.4) | 7 (15.9) | 0.70 |
| Congestive heart failure (%) | n = 30 5 (16.7) | 4 (9.1) | 0.47 |
| Valvulopathy (%) | 0 (0) | 1 (2.3) | 1.0 |
| Atrial fibrillation (%) | 3 (9.7) | 10 (22.7) | 0.14 |
| Anticoagulation (%) | 7 (22.6) | 7 (15.9) | 0.47 |
| Final diagnosis ischemic brain injury (%) | 15 (48.4) | 11 (25.0) | 0.04 |
| Final diagnosis hemorrhagic brain injury (%) | 3 (9.7) | 4 (9.1) | 1.0 |
| Final diagnosis stroke mimic (symptoms not due to ischemic or hemorrhagic brain injury) (%) | 13 (41.9) | 29 (65.9) | 0.04 |

CONCLUSIONS
In‐hospital strokes represent an emergency for which response time is critical. Neurologic injury progresses with every minute of ischemia, and current recommendations offer a limited time window for intravenous thrombolysis. For stroke with symptom onset in the monitored setting of the hospital, there is a compelling imperative to reduce all delays from system inefficiencies. The findings of the current QI initiative suggest that dramatic improvements are possible through systematic evaluation and redesign of hospital response processes, a checklist for in‐hospital stroke carried by front‐line responders, and ongoing real‐time feedback.
Limitations of this study include a prepost design. The necessity of implementing system change hospital‐wide precluded use of a concurrent control group. The time goals for evaluation are derived from American Stroke Association targets for patients arriving in the Emergency Department. There are differences in process between the hospital ward and the Emergency Department, but the fundamental concept of minimizing time to evaluation once patient symptoms are recognized by hospital staff remains valid.
The possibility of system improvements not due to this QI initiative cannot be excluded. In 2006, this hospital expanded the responsibility of the stroke response team to include acute neurologic deficits outside of the ED without other changes to the in‐hospital stroke alert process. This reduced time to evaluation for in‐hospital ischemic strokes compared to usual care, but even with the same acute stroke response team responding to stroke alerts in both settings, in‐hospital stroke response times remained significantly longer than response times for stroke in the ED.10 The presence of an in‐hospital stroke alert response team alone was not capable of reducing evaluation times to goal. Minimal improvement in median in‐hospital stroke alert evaluation time was seen in the intervening year, following the completion of our previously published analysis, suggesting explicit system QI was necessary.
The Hawthorne effect, in which individuals who know they are being observed modify behavior while such monitoring is in effect, is a major limitation of interpreting QI initiatives. By committing to continuous and ongoing feedback to front‐line providers, this phenomenon can be harnessed to sustain improvement.17 In effect, the study of efficient response to the in‐hospital stroke never ceases. UCH has continued to employ the post‐intervention stroke alert protocol and engage in ongoing feedback after each stroke alert. In the 12 months following the conclusion of this study, the median response time to in‐hospital strokes continues to be 30 minutes, and 7 additional in‐hospital stroke patients have been treated with thrombolysis.
This inpatient stroke alert initiative decreased median inpatient alert‐to‐CT time by 57%, and demonstrates that quality of in‐hospital stroke care can be improved. Decrease in stroke alert‐to‐CT time facilitates earlier thrombolytic therapy. Analysis of treatment and patient outcomes was outside of the scope of the current study, but earlier treatment has potential to significantly improve clinical outcomes.
The Society of Hospital Medicine defines one of the goals of QI to be the change in processes with reduction in variation, thus improving the care for all patients rather than focusing exclusively on outlier events.18 This initiative markedly reduced evaluation variability, allowing a greater percentage of patients to be eligible for treatment within the critical time window. Prior to the intervention, almost a quarter of patients had delays in evaluation sufficient to preclude IV thrombolysis, whereas in the 6 months after the intervention was initiated, not a single patient had evaluation delayed to the point that IV thrombolysis would not have been an option (Figure 1). The goal of in‐hospital stroke QI must be to improve the speed of the process for all patients, and assure that no patient is denied the potential for therapy as a result of inefficiencies in hospital systems.
Acknowledgements
The authors thank Traci Yamashita, PRA, for her work in the statistical analysis for this publication, and Dr Jeffrey Glasheen for development of the University of Colorado Hospital's Hospitalist Training Track Quality Improvement Program of which this work is a product.
In‐hospital strokes account for a significant proportion of the almost 800,000 cerebrovascular accidents that occur each year in the United States.1 Although inpatient strokes are thought to be under‐recognized and under‐reported, between 4% and 17% of all stroke patients in the hospital experienced stroke onset during hospitalization.2, 3 Estimates place the number of in‐hospital strokes at 35,000‐75,000 each year in the United States.4
As a result of the exquisite sensitivity of brain tissue to ischemic events, stroke is a medical emergency and time‐to‐treatment is of the essence. With each minute of ischemia, 1.9 million neurons are destroyed.5 Evidence suggests benefit of treatment with intravenous thrombolysis up to 4.5 hours after symptom onset, with lower disability associated with more rapid initiation of therapy.6, 7 To facilitate timely thrombolytic therapy, the American Stroke Association (ASA) recommends that imaging of the brain be initiated within 25 minutes of presentation for patients with suspected stroke.8
Studies demonstrate greater delays in the evaluation of hospitalized patients suffering from stroke compared to stroke patients presenting to the Emergency Department (ED).9, 10 Performance of timely evaluation of in‐hospital stroke rarely meets ASA goals. Analysis of a Michigan stroke registry found that only 3.1% of patients with in‐hospital strokes received computed tomography (CT) scan within 25 minutes of symptom recognition, and a Colorado stroke registry found time‐to‐evaluation to be more than twice the recommended benchmark.11, 12 Data from a multicenter stroke registry in Spain showed that half of all thrombolysis‐eligible, in‐hospital stroke patients could not be treated due to delays in evaluation.13
Our prior work demonstrated that the use of an in‐hospital stroke response team significantly reduced time to evaluation for true ischemic strokes.10 Even with this rapid response mechanism, the evaluation time for in‐hospital stroke was still more than twice that observed in the ED despite using the same team to respond to both settings. Hospital rapid response systems, specifically for patients with suspected stroke, have been described in the literature and outline in‐hospital response systems capable of meeting evaluation time goals.1415 How to optimize a stroke response system has not been previously described. The aim of this quality improvement (QI) initiative was to reduce time‐to‐evaluation for strokes occurring in patients already hospitalized using systems analysis and modification. We describe key elements and tools for implementing institutional QI for in‐hospital stroke.
METHODS
The QI initiative was implemented at the University of Colorado Hospital (UCH), a tertiary care academic medical center. The Colorado Multiple Institutional Review Board determined this project to be in the exempt category. UCH uses a protocol in which all stroke alerts undergo non‐contrast CT of the brain. If no intracranial bleeding is found, and the patient is a thrombolytic candidate, advanced CT imaging including CT perfusion and CT angiogram will also be performed during the alert. Magnetic resonance imaging (MRI) with diffusion weighted imaging is done non‐emergently for subsequent stroke evaluation, but is not part of the stroke alert protocol. The primary endpoint of time from alert to initiation of CT was chosen because it represents an unambiguous interval which is present for all stroke alerts. Pre‐intervention data was gathered for 6 months, from September 2008 to February 2009. During this period, the process through which in‐hospital strokes were identified, referred for evaluation, and treated was mapped to identify inefficient or unreliable steps, and the process was redesigned to enhance efficiency. The intervention was rolled out over a 3‐month period from March 2009 to May 2009. During the intervention roll‐out period, the refined stroke alert process and a checklist containing the optimal in‐hospital stroke alert response system was implemented. An education campaign was initiated, for acute stroke team members and nursing staff, on signs of stroke and each individual's role in response to symptoms of in‐hospital stroke based on the new process. During the roll‐out period, each unit in the hospital was provided in‐hospital stroke alert posters and a packet containing specific stroke education on the in‐hospital stroke alert process. Unit educators were empowered to determine how to best deliver the education to their staff, and many chose to invite the stroke program coordinator to give an hour‐long presentation on stroke prior to shift or during lunch. Each unit educator kept record of the stroke instruction provided and submitted staff signatures to the stroke program. Nursing staff was also provided with in‐hospital stroke protocol badge cards that outlined optimal approach to stroke identification and treatment using the revised protocol. Interventions were being implemented in a progressive fashion throughout the roll‐out period. Starting during the roll‐out and continuing into the post‐intervention period, feedback on all in‐hospital stroke alerts was provided to the stroke team and front‐line providers. The impact of the intervention was followed for 6 months post‐intervention from June 2009 to November 2009. The QI tools used in this project are well described by the Institute of Healthcare Improvement, and each step in the QI process is outlined in detail below:16
Step 1: Process Map With Identification of Unreliable and Reliably Slow Steps
A detailed process map was created to outline steps in the existing stroke alert process (see Supporting Figures, Process Maps, in the online version of this article). One investigator (R.Z.) interviewed key members of the multidisciplinary stroke team, including representatives from the departments of neurology, nursing, hospital medicine, neurosurgery, radiology, and transportation. Interviews with key stakeholders and frequent participants in stroke alerts revealed evidence of episodic unreliable steps. Stakeholders were noted to have slightly different conceptions of how the process flow was intended to occur, and where responsibility lay for certain tasks. The interviews aided in identification of pitfalls, bottlenecks, misconceptions, and areas that needed clarification or change in the alert process.
Examples of unreliable and bottleneck steps include: In the pre‐intervention process, the transportation department was responsible for moving patients to radiology; this step was identified as reliably slow. Investigation revealed that the transportation department did not have a mechanism for rapid response to emergency transport requests. Analysis also revealed that 2 key steps necessary for treating in‐hospital stroke were occasionally neglected: ensuring adequate intravenous (IV) access, and ordering of the correct panel of laboratory tests. Finally, a process communication deficit was identified, with CT technicians periodically unaware of the pending arrival of an in‐hospital stroke patient, thus preventing the scan from being cleared for the emergent stroke imaging.
Direct observation of real‐time stroke alerts in both the inpatient and ED settings was also employed to outline the process and identify areas of inefficiency. Direct observation of stroke alerts in progress verified the unified picture of process flow developed from stakeholder interviews (see Supporting Figures, Process Maps, in the online version of this article). Particular note was made of differences between the stroke alert process in the ED and the inpatient setting.
Step 2: System Redesign With Input From All Stakeholders
Proposed interventions were presented to hospital governing councils, including the interdisciplinary Stroke Council and Nurse Managers Council. After verification of the shortcomings of the existing alert process and obtaining buy‐in from key participants and governing departments, a new process was designed (see Supporting Figures, Process Maps, in the online version of this article). Specific changes include the following examples: First, electrocardiogram was moved to occur after CT scan. Second, investigation revealed that the transportation department within the hospital was designed for non‐emergent transportation and not amenable to change. The mechanism of patient transportation was changed such that, rather than using the transportation department, patients were now transported by the neurology resident responding to the stroke alert, accompanied by the patient's ward nurse. This both removed a bottleneck step and assured critical staff presence during the transportation of a potentially unstable patient. Third, to ensure effective communication, CT technicians were provided with stroke alert pagers that receive text messages regarding incoming in‐hospital stroke alert patients. Fourth, a time limit was set for IV attempts prior to transportation. The new protocol, along with explicit expectations for the role of the patient's nurse in in‐hospital stroke alerts, was described in a hospital‐wide nursing stroke education initiative.
Step 3: In‐Hospital Stroke Alert Checklist
A new standardized protocol for optimal in‐hospital stroke care was detailed on a laminated pocket card. The checklist described exactly what steps were to be performed, by whom, how to make them occur, and in what order. The checklist was designed to reduce the incidence of omitted steps, such as ordering of correct laboratory evaluations. The laminated cards highlighted the benchmark time to evaluation of 25 minutes. Process checklist cards were distributed to all members of the acute stroke alert response team, and short versions designed specifically for nursing staff were distributed as badge cards and posted on clinical care units (Supporting Information Appendix I).
Step 4: Real‐Time Feedback
During the intervention roll‐out and post‐intervention periods, feedback was provided from the stroke program to the front‐line providers following each in‐hospital stroke alert. The clinicians involved were notified of the final diagnosis and patient outcome, and were provided with feedback about how the patient's evaluation times compared with benchmark goals. Feedback may serve to motivate, based on clinician professionalism, but performance in the alert was not tied to rewards or penalties for the providers involved. The feedback process was designed to be bi‐directional, with requests for input from staff on barriers to rapid evaluation experienced and suggestions for future process improvement (Supporting Information Appendix II).
Statistical Analysis
The primary outcome was the change in time from stroke alert to CT scan (alert‐to‐CT), comparing pre‐intervention and post‐intervention periods. This time interval was chosen because its calculation involved unambiguous time points, which are available for all patients for whom an in‐hospital alert is called. It is a measure of process efficiency, with minimal expected variation based on differences in patient characteristics (ie, hemorrhagic vs ischemic stroke). Non‐overlapping Kaplan‐Meier curves confirmed the proportional hazards assumption for 2 Cox proportional hazards models: unadjusted and adjusted by group characteristics with P‐value 0.10. Relative hazards and estimates for the percent of patients with alert‐to‐CT scan 25 minutes, according to intervention groups, were obtained from these models. For analyses, admit unit was re‐categorized as intensive care unit (ICU), Med/Surg, or Other. Analyses were conducted using SAS Version 9.2 (SAS Institute, Inc, Cary, NC).
RESULTS
During the study intervals, there were 82 inpatient stroke alerts. Of these alerts, 75 were included in the analysis. Seven were excluded for the following reasons: alert canceled by the stroke team (3), time of alert was not recorded (1), patient identifiers not recorded (1), or stroke alert was preceded by CT imaging (2).
During the 6 months prior to intervention, the median inpatient stroke alert‐to‐CT time (n = 31) was 69.0 minutes (Table 1). Nineteen percent of these alerts met the goal of 25 minutes from alert‐to‐CT time. During the 6‐month post‐intervention period, the median inpatient alert‐to‐CT time (n = 44) was 29.5 minutes. Thirty‐two percent of these alerts met the 25‐minute alert‐to‐CT time benchmark. In the unadjusted model, patients during the post‐intervention period were significantly more likely to have alert‐to‐CT scan time 25 minutes compared to patients prior to the intervention (post‐intervention compared to pre‐intervention, Relative Hazard (RH): 3.03; 95% confidence interval [CI]: 1.76‐5.20; log‐rank P 0.0001). This remained significant after adjustment for hyperlipidemia, active cancer, final diagnosis of ischemic brain injury, and final diagnosis of stroke mimic (RH: 4.96; 95% CI: 2.65‐9.32; P 0.0001); data not shown. Admit unit was not included in the adjusted model since there was no indication of differences in the 3‐level variable according to intervention group (P = 0.27). In addition to reduction in median response times, the variability of response times was markedly reduced, and no patient in the 6‐month post‐intervention period had delay to CT sufficient to preclude use of IV thrombolysis (Figure 1).
| Pre‐Intervention (n = 31) | Post‐Intervention (n = 44) | P Value | |
|---|---|---|---|
| |||
| Stroke alert to CT time, median [95% CI] | 69 min [34, 103] | 29.5 min [26, 40] | P 0.0001 |
| Age, median [IQR] | 61.0 [54.0, 70.0] | 60.5 [48.5, 70.5] | 0.94 |
| Female (%) | 19 (61.3) | 23 (52.3) | 0.44 |
| Race (%) | |||
| Asian | 1 (3.2) | 1 (2.3) | 0.31 |
| Black | 4 (12.9) | 6 (13.6) | |
| Caucasian | 21 (67.7) | 27 (61.4) | |
| Hispanic | 3 (9.7) | 10 (22.7) | |
| Unknown | 2 (6.5) | 0 (0) | |
| Admit unit (%) | |||
| Intensive care | 12 (38.7) | 10 (22.7) | 0.07 |
| Medicine/surgery | 15 (48.4) | 24 (54.6) | |
| Neurology | 0 (0) | 5 (11.4) | |
| Post‐acute care | 3 (9.7) | 0 (0) | |
| Rehabilitation | 1 (3.2) | 2 (4.6) | |
| Women's and maternal care | 0 (0) | 2 (4.6) | |
| Cardiology | 0 (0) | 1 (2.3) | |
| Case mix index, median [IQR] | n = 29 2.6 [1.1, 5.0] | n = 42 2.2 [1.6, 4.5] | 0.82 |
| Prior cerebrovascular accident (%) | 5 (16.1) | 8 (18.2) | 0.82 |
| Hypertension (%) | 17 (54.8) | 24 (54.6) | 0.98 |
| Diabetes mellitus (%) | 7 (22.6) | 11 (25.0) | 0.81 |
| Hyperlipidemia (%) | 15 (48.4) | 9 (20.5) | 0.01 |
| Tobacco abuse, current (%) | 4 (12.9) | 1 (2.3) | 0.15 |
| Alcohol abuse (%) | 2 (6.5) | 0 (0) | 0.17 |
| Active cancer (%) | 8 (25.8) | 5 (11.4) | 0.10 |
| Peripheral vascular disease (%) | 2 (6.5) | 3 (6.8) | 1.0 |
| Coronary artery disease (%) | 6 (19.4) | 7 (15.9) | 0.70 |
| Congestive heart failure (%) | n = 30 5 (16.7) | 4 (9.1) | 0.47 |
| Valvulopathy (%) | 0 (0) | 1 (2.3) | 1.0 |
| Atrial fibrillation (%) | 3 (9.7) | 10 (22.7) | 0.14 |
| Anticoagulation (%) | 7 (22.6) | 7 (15.9) | 0.47 |
| Final diagnosis ischemic brain injury (%) | 15 (48.4) | 11 (25.0) | 0.04 |
| Final diagnosis hemorrhagic brain injury (%) | 3 (9.7) | 4 (9.1) | 1.0 |
| Final diagnosis stroke mimic (symptoms not due to ischemic or hemorrhagic brain injury) (%) | 13 (41.9) | 29 (65.9) | 0.04 |

CONCLUSIONS
In‐hospital strokes represent an emergency for which response time is critical. Neurologic injury progresses with every minute of ischemia, and current recommendations offer a limited time window for intravenous thrombolysis. For stroke with symptom onset in the monitored setting of the hospital, there is a compelling imperative to reduce all delays from system inefficiencies. The findings of the current QI initiative suggest that dramatic improvements are possible through systematic evaluation and redesign of hospital response processes, a checklist for in‐hospital stroke carried by front‐line responders, and ongoing real‐time feedback.
Limitations of this study include a prepost design. The necessity of implementing system change hospital‐wide precluded use of a concurrent control group. The time goals for evaluation are derived from American Stroke Association targets for patients arriving in the Emergency Department. There are differences in process between the hospital ward and the Emergency Department, but the fundamental concept of minimizing time to evaluation once patient symptoms are recognized by hospital staff remains valid.
The possibility of system improvements not due to this QI initiative cannot be excluded. In 2006, this hospital expanded the responsibility of the stroke response team to include acute neurologic deficits outside of the ED without other changes to the in‐hospital stroke alert process. This reduced time to evaluation for in‐hospital ischemic strokes compared to usual care, but even with the same acute stroke response team responding to stroke alerts in both settings, in‐hospital stroke response times remained significantly longer than response times for stroke in the ED.10 The presence of an in‐hospital stroke alert response team alone was not capable of reducing evaluation times to goal. Minimal improvement in median in‐hospital stroke alert evaluation time was seen in the intervening year, following the completion of our previously published analysis, suggesting explicit system QI was necessary.
The Hawthorne effect, in which individuals who know they are being observed modify behavior while such monitoring is in effect, is a major limitation of interpreting QI initiatives. By committing to continuous and ongoing feedback to front‐line providers, this phenomenon can be harnessed to sustain improvement.17 In effect, the study of efficient response to the in‐hospital stroke never ceases. UCH has continued to employ the post‐intervention stroke alert protocol and engage in ongoing feedback after each stroke alert. In the 12 months following the conclusion of this study, the median response time to in‐hospital strokes continues to be 30 minutes, and 7 additional in‐hospital stroke patients have been treated with thrombolysis.
This inpatient stroke alert initiative decreased median inpatient alert‐to‐CT time by 57%, and demonstrates that quality of in‐hospital stroke care can be improved. Decrease in stroke alert‐to‐CT time facilitates earlier thrombolytic therapy. Analysis of treatment and patient outcomes was outside of the scope of the current study, but earlier treatment has potential to significantly improve clinical outcomes.
The Society of Hospital Medicine defines one of the goals of QI to be the change in processes with reduction in variation, thus improving the care for all patients rather than focusing exclusively on outlier events.18 This initiative markedly reduced evaluation variability, allowing a greater percentage of patients to be eligible for treatment within the critical time window. Prior to the intervention, almost a quarter of patients had delays in evaluation sufficient to preclude IV thrombolysis, whereas in the 6 months after the intervention was initiated, not a single patient had evaluation delayed to the point that IV thrombolysis would not have been an option (Figure 1). The goal of in‐hospital stroke QI must be to improve the speed of the process for all patients, and assure that no patient is denied the potential for therapy as a result of inefficiencies in hospital systems.
Acknowledgements
The authors thank Traci Yamashita, PRA, for her work in the statistical analysis for this publication, and Dr Jeffrey Glasheen for development of the University of Colorado Hospital's Hospitalist Training Track Quality Improvement Program of which this work is a product.
- ,,, et al.Heart disease and stroke statistics—2010 update: a report from the American Heart Association.Circulation.2010;121:e46–e215.
- ,,.Characteristics of in‐hospital onset ischemic stroke.Eur Neurol.2006;55:155–159.
- ,.Inpatient and community ischemic strokes in a community hospital.Neuroepidemiology.2007;28:86–92.
- .In‐hospital stroke.Lancet Neurol.2003;2:741–746.
- .Time is brain‐quantified.Stroke.2006;37:263–266.
- ,,, et al.Ultra‐early thrombolysis in acute ischemic stroke is associated with better outcomes and lower mortality.Stroke.2010;41:712–716.
- ,,,.Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association.Stroke.2009;40:2945–2948.
- ,,, et al.Guidelines for early management of adults with ischemic stroke.Stroke.2007;38;1655–1711.
- ,,, et al.In‐hospital stroke treated with intravenous tissue plasminogen activator.Stroke.2008;39:2614–2616.
- ,,,,.Stroke alert program improves recognition and evaluation time of in‐hospital ischemic stroke.J Stroke Cerebrovasc Dis.2009;19:494–496.
- ,,,,,.In‐hospital stroke in a statewide stroke registry.Cerebrovasc Dis.2008;25:12–20.
- ,,,,.Quality of care for in‐hospital stroke: analysis of a statewide registry.Stroke.2011;42:207–210.
- ,,, et al.In‐hospital stroke: a multi‐center prospective registry.Eur J Neurol.2011;18:170–176.
- ,,.Code Gray—an organized approach to inpatient stroke.Crit Care Nurs Q.2003;26:296–302.
- ,,.ID, stat‐rapid response to in‐hospital stroke patients.Nurs Manage.2009;40:34–38.
- Institute of Healthcare Improvement. Quality Improvement Tools. Available at: http://www.ihi.org/IHI/Topics/Improvement/ImprovementMethods/Tools/. Accessed December 1,2010.
- ,,,,,.Variability in the Hawthorne effect with regard to hand hygiene performance in high‐ and low‐performing inpatient care units.Infect Control Hosp Epidemiol.2009;30:222–225.
- Society of Hospital Medicine Quality Improvement Resources. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/32. Accessed December 1,2010.
- ,,, et al.Heart disease and stroke statistics—2010 update: a report from the American Heart Association.Circulation.2010;121:e46–e215.
- ,,.Characteristics of in‐hospital onset ischemic stroke.Eur Neurol.2006;55:155–159.
- ,.Inpatient and community ischemic strokes in a community hospital.Neuroepidemiology.2007;28:86–92.
- .In‐hospital stroke.Lancet Neurol.2003;2:741–746.
- .Time is brain‐quantified.Stroke.2006;37:263–266.
- ,,, et al.Ultra‐early thrombolysis in acute ischemic stroke is associated with better outcomes and lower mortality.Stroke.2010;41:712–716.
- ,,,.Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association.Stroke.2009;40:2945–2948.
- ,,, et al.Guidelines for early management of adults with ischemic stroke.Stroke.2007;38;1655–1711.
- ,,, et al.In‐hospital stroke treated with intravenous tissue plasminogen activator.Stroke.2008;39:2614–2616.
- ,,,,.Stroke alert program improves recognition and evaluation time of in‐hospital ischemic stroke.J Stroke Cerebrovasc Dis.2009;19:494–496.
- ,,,,,.In‐hospital stroke in a statewide stroke registry.Cerebrovasc Dis.2008;25:12–20.
- ,,,,.Quality of care for in‐hospital stroke: analysis of a statewide registry.Stroke.2011;42:207–210.
- ,,, et al.In‐hospital stroke: a multi‐center prospective registry.Eur J Neurol.2011;18:170–176.
- ,,.Code Gray—an organized approach to inpatient stroke.Crit Care Nurs Q.2003;26:296–302.
- ,,.ID, stat‐rapid response to in‐hospital stroke patients.Nurs Manage.2009;40:34–38.
- Institute of Healthcare Improvement. Quality Improvement Tools. Available at: http://www.ihi.org/IHI/Topics/Improvement/ImprovementMethods/Tools/. Accessed December 1,2010.
- ,,,,,.Variability in the Hawthorne effect with regard to hand hygiene performance in high‐ and low‐performing inpatient care units.Infect Control Hosp Epidemiol.2009;30:222–225.
- Society of Hospital Medicine Quality Improvement Resources. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/32. Accessed December 1,2010.
Toxin Assay and ICD‐9 for Reporting
With an increased incidence of 13.1 per 1000 inpatients1, 2 and an attributable mortality of 6.1%,3 in 2006 the Canadian government added Clostridium difficileassociated disease (CDAD) to its list of reportable diseases.4 The Centers for Disease Control and Prevention and the Infectious Disease Society of America subsequently created definitions of the disease and recommended surveillance of rates of health care facilityassociated CDAD, which has been found to double a patient's length of stay and cost of hospitalization.57 While not included in the current list of hospital‐acquired conditions for which payment is declined,8 the Centers for Medicare & Medicaid Services (CMS) has noted CDAD as under consideration for addition to the list,9 which would require hospital reporting of CDAD rates. This reporting is typically a labor‐intensive, medical record review process that increases the cost of delivering health care. Health care institutions have reported spending as much as $21 million per year or $400 per discharged patient on quality improvement.10 These costs are passed on to payers such as those governed by the CMS that are projected to pay for more than half of all national health spending in 2018.11 Therefore, it is prudent to examine alternatives for determining rates of disease for public reporting and quality improvement initiatives such as infection control and antibiotic stewardship programs.
There are 3 methods that can be used for this reporting. First, medical record review has been used for determining the case rate of CDAD by published reports.1, 2 This procedure allows the CMS to define the desired data to be collected, but it is labor‐intensive. Second, the use of International Statistical Classification of Diseases and Related Health Problems, 9th Edition (ICD‐9) codes from hospital databases offers the speed of database query. However, due to diagnostic and coding errors, it may report inaccurate rates of disease. Previous reports include a university‐based hospital that found a sensitivity of 78% and specificity of 99.7%,12 whereas a Veterans Administration medical center found nearly two‐thirds of patients with CDAD did not have the ICD‐9 code for C. difficile infection noted in their database.13 Therefore, the accuracy of ICD‐9 code at a community hospital that better represents the majority of United States hospitals is needed. The third method of identifying CDAD is the presence of toxin identified in the microbiology laboratory. Although this method offers ease of obtainment, it suffers from potential inaccuracy arising from duplicate patient samples, positive samples in patients who are symptom‐free carriers, and patients with CDAD despite having a negative toxin.
The same organizations that recommend surveillance for CDAD have identified that there are inadequate data on which to base a decision regarding how to proceed with routine community and hospital surveillance.6 In the setting of public reporting and nonpayment, the method of identifying CDAD cases must be accurate to ensure fairness, while being inexpensive. To identify the potential value of these less labor‐intensive methods of reporting the incidence of CDAD, we evaluated the use of ICD‐9 codes and C. difficile toxin to accurately report the incidence of CDAD at a community hospital, as well as the labor hours required for each reporting method.
METHODS
Patients >18 years of age and potentially having CDAD were identified via database queries for ICD‐9 codes and positive C. difficile toxin assays at our institution from November 1, 2006, through August 31, 2007. Our institution is a 379‐bed university‐affiliated community teaching hospital in a socio‐economically diverse area of Baltimorewith approximately 110,000 emergency department visits, 30,000 discharges, and 110,000 inpatient days each yearthat uses a handwritten paper chart for all provider orders and patient documentation. The C. difficile toxin assay method used during the study period was an enzyme immunoassay that detects both toxins A and B (Meridan Bioscience, Cincinnati, OH). ICD‐9 codes were queried in the CareScience database(Premier, Inc; Charlotte, NC), while C. difficile toxin was queried via the hospital laboratory database. All identified patients underwent a medical record review to confirm the diagnosis of CDAD and the patient's location at the time of disease acquisition. To eliminate duplicate reporting of a single episode of disease, all duplicate patient visits with a diagnosis of CDAD within 30 days were removed.
Our diagnostic criteria (Table 1) used the recommended criteria of a combination of symptoms and positive toxin assay, visualization of pseudomembranes on colonoscopy or pathology‐proven CDAD.6 Based on the presence of CDAD in 25% of patients with a white blood cell count >30,000 and the recommendation that CDAD be considered in all patients with a white blood cell count >15,000, we included the criterion of clinical findings with severe leukocytosis to identify patients with toxin‐negative CDAD.14
| Chart Review Criteria for CDAD | Chart Review Criteria for CDAD Obtained While Patient Was Not at Our Hospital | Chart Review Criteria for CDAD Obtained While Patient Was at Our Hospital |
|---|---|---|
| ||
| Pseudomembranous colitis seen during endoscopy OR biopsy or resection with surgical pathology consistent with CDAD | One of the above symptoms or objective criteria positive within the first 3 days of hospitalization AND patient was not cared for at our insitution within the last 7 days | Patient was cared for at our institution within 7 days of presentation OR the patient's symptoms were not present upon arrival at the hospital and the symptoms began after the third day of hospitalization |
| If neither of the above are present, then Clostridium difficile toxin or WBC count >25,000 plus one of the following: | ||
| Diarrhea | ||
| Fever without other cause | ||
| Abdominal pain without other cause | ||
| Colonic ileus without other cause | ||
Patients were identified by querying the CareScience database for patients having an ICD‐9 code of 008.45. Duplicate cases for the same patient within 30 days were removed. These cases were compared with the cases of CDAD determined by the medical record review for analysis. Patients were identified via positive C. difficile toxin by querying the microbiology laboratory database. A query of all patients with a positive C. difficile toxin was performed. Duplicate samples for the same patient within 30 days were removed. Patients are considered to be outside the hospital when CDAD was acquired if their toxin was positive within the first 3 days of arrival to the hospital, and they were not hospitalized at our institution within 1 week before arrival. All other cases were considered to be acquired while in our hospital. The number of patients with a toxin‐positive stool sample was compared with the medical record review.
Recognizing that it is unrealistic to review the medical records of the 23,495 discharges during the 10‐month study period, a random sample review of 500 charts not included in our CDAD‐identified patient list was performed to identify the rate at which CDAD patients failed to be identified by our methods. From a list of discharges during the study period, as long as they were not in our study population, every thirtieth patient was selected for review up to a total of 500 patient charts. Then, the identified case rate was used to predict the prevalence of disease at our institution during the study period.
Sample selection was based on the absence of previous studies evaluating the performance of C. difficile toxin assay, and the desire to have the same assay used during the entire study. The lack of data from which to perform sample size determination would result in an inaccurate estimate. Therefore, we chose a period that offered the maximum sample size, during which a single assay was used at our institution. Compared with medical record review, the accuracy and positive predictive values of the ICD‐9 code and positive stool toxin methods to identify the cases of CDAD were compared. A true positive was when the ICD‐9 or laboratory query method identified a patient who had CDAD based on chart review; a false positive was when the ICD‐9 or laboratory query method identified a patient who did not have CDAD based on chart review. Rates of over‐ or underdiagnosis, case rates, and acquisition location were determined. Statistical analysis of the case rates and acquisition location were performed via chi‐square test. The time to perform these queries was collected with accuracy to the minute.
RESULTS
Of the 23,495 discharges during the study period, the combination of ICD‐9 and C. difficile toxin assay identified a total cohort of 496 patients, 319 of whom were identified by both the ICD‐9 method and the toxin assay, 50 of whom were identified only by the toxin assay, and 127 of whom were identified only by the ICD‐9 method. Chart review confirmed the presence of CDAD in 384 of these 496 cases, for a case rate of 16.3 per 1000 discharged patients (Table 2). The diagnostic criteria for each of these confirmed cases are listed in Table 3. Of the 384 confirmed CDAD cases, 319 were identified by both the ICD‐9 and toxin assay, 50 were identified only by the toxin assay, and 15 were identified only by the ICD‐9 query. Of the 50 cases identified by the toxin assay that were not identified via the ICD‐9 method, all 50 (100%) were confirmed to have CDAD by chart review. In contrast, of the 127 cases identified via the ICD‐9 method that were not found via the toxin assay, only 15 (11.8%) were confirmed to have CDAD by chart review (Figure 1).
| ICD‐9 | Toxin Assay | Chart Review | |
|---|---|---|---|
| |||
| No. of patients identified | 446 | 369 | 384 |
| Case rate per 1000 discharges* | 19.0 | 15.7 | 16.3 |
| 95% confidence interval | 17.320.8 | 14.117.4 | NA |
| Case rate compared with chart review, P | P = 0.001 | P = 0.440 | NA |
| CDAD rate reported compared with chart review | 116.1% | 96.1% | NA |
| Accuracy | 83.9% | 96.1% | NA |
| PPV | 74.9% | 100% | NA |
| Portion of cases acquired at our hospital, % | NA | 48.2% | 40.6% |
| Portion of cases acquired at our hospital, P | NA | P = 0.003 | NA |
| Minutes consumed for data collection | 312 | 842 | 21,899 |
| Estimated annual cost per hospital | $234.00 | $631.50 | $16,424.25 |
| Criterion | No. of Cases | Case Rate* |
|---|---|---|
| ||
| Endoscopy | 2 | 0.5% |
| Surgical pathology | 9 | 2.3% |
| Positive toxin and diarrhea | 369 | 96.1% |
| WBC >25.0 and diarrhea | 51 | 13.3% |
| WBC >25.0 and fever without other source | 9 | 2.3% |
| WBC >25.0 and unexplained abdominal pain | 17 | 4.4% |
| WBC >25.0 and colonic ileus | 2 | 0.5% |
Of these 384 cases, 369 were identified via the toxin assay for a case rate of 15.7 per 1000 patient discharges, which was not found to be different from the rate of 16.3 determined by chart review (P = 0.440; 95% confidence interval [CI], 14.117.4). Compared with chart review, the toxin assay reported 96.1% (369/384) of cases. Chart review demonstrated that every patient who had a positive toxin assay met the diagnostic criteria for CDAD for a positive predictive value (PPV) of 100%.
The ICD‐9 method identified 446 patients thought to have CDAD, 334 of whom were confirmed by chart review for a PPV of 74.9% (334/446). Compared with chart review, the ICD‐9 method reported 116.1% (446/384) of cases for a case rate of 19.0 per 1000 discharges and was significantly different than the rate of 16.3 reported by chart review (P = 0.001; 95% CI, 17.320.8).
Chart review identified 156 of 384 (40.6%) patients who acquired CDAD while at our hospital and 228 of 384 (59.4%) who acquired it elsewhere. In comparison, the toxin assay criteria identified 369 cases of CDAD, of which 48.2% (178/369) were acquired while at our hospital and 51.8% (191/369) were acquired elsewhere (P = 0.003).
The time for data extraction via these 3 methods differed greatly. The ICD‐9 method only consumed 312 minutes and the toxin assay method 842 minutes, whereas the chart review method consumed 21,899 minutes. These times reported include the database query and data analysis for the ICD‐9 and toxin assay methods, while it includes the database query and list generation along with the manual chart review and data analysis for the chart review method. The review of the random sample of patients believed not to have CDAD was not included in any of the reported times. Chart review on a random sample of 500 patients not previously identified for review found no additional cases of CDAD.
DISCUSSION
Our study demonstrates that use of positive C. difficile toxin assay data from the microbiology laboratory alone is an efficient method of identifying patients with CDAD. This method only consumed 842 minutes versus 21,899 minutes consumed by the chart review method. The C. difficile toxin method reduces the workforce required to collect and analyze this data, but more importantly, it was found to be reliable by reporting an institutional case rate that is similar to that of chart review.
In contrast, the ICD‐9 method was efficient but less reliable. It only consumed 312 minutes, but it overreported the institutional rate of CDAD by 16.1%, had a PPV of only 74.9%, and of those patients who were identified by ICD‐9 but not toxin assay, only 11.8% actually had CDAD. This finding is in conflict with the previously noted underreporting of this method.13, 15 We believe this difference to be associated with institutional differences, because previous reports originated from a veterans hospital and an academic medical center, and previous authors have failed to use predefined diagnostic criteria and a complete chart review to confirm cases of CDAD. Similar to our study, an academic medical center identified that listing of CDAD in a patient's medical history in their chart was associated with a false‐positive ICD‐9 code for CDAD.15 This observation appears to bring clarity to one of the causes of the variance of ICD‐9 code accuracy between institutions. Institutions seem to vary on the method of attaching diagnoses to the patient's final hospital record. Some institutions include only what is listed as an active problem, whereas others list diagnoses listed in the chart as previous problems and those listed as a potential diagnosis without confirmation. Another potential cause of the ICD‐9 inaccuracy is the potential of clinicians to diagnose and treat a patient for CDAD in the absence of the diagnostic criteria used for chart review. Physician practices such as these are known to vary between institutions leading to a variance in the ICD‐9 code accuracy.
In total, 15 cases of CDAD were identified in the absence of a positive toxin assay, and of these, 12 cases were identified using leukocytosis‐based criteria (Table 4). This resulted in 3.1% of our cases being toxin‐negative, based on leukocytosis criteria, and is lower than the previously identified 35% of cases.14 Because it was the only assay available at the time, this previous research used a toxin Aonly assay, which is more likely to have false‐negative results than the toxin A/B assay used at our institution during the study period. The investigators also required all toxin‐negative patients to have been recently treated with antibiotics. Based on the increasing rates of community‐acquired CDAD, including those that are antibiotic‐nave patients, we felt a history of antibiotic exposure was no longer a prerequisite for CDAD and thus excluded it from our diagnostic criteria.16, 17 Based on these differences, we feel our results are likely an accurate reflection of the number of cases identified by ICD‐9 query in the absence of toxin positivity. However, concerns should be further alleviated through the realization that nonuse of this strategy improves the accuracy of the toxin assay method, while reducing the accuracy of the ICD‐9 method and thereby strengthening the validity of our conclusions. Mathematically, this would result in 369 of 369 patients identified by toxin assay and 369 patients identified by the ICD‐9 method. This would reduce the case rate of the chart review method to the same 15.7 of the toxin assay, while the ICD‐9 method would remain at 19.0.
| Criterion | No. of Cases* |
|---|---|
| |
| Endoscopy | 0 |
| Surgical pathology | 3 |
| WBC >25.0 and diarrhea | 10 |
| WBC >25.0 and fever without other source | 1 |
| WBC >25.0 and unexplained abdominal pain | 3 |
| WBC >25.0 and colonic ileus | 1 |
We considered whether the toxin assay method may overestimate the number of cases due to a C. difficileasymptomatic carrier rate as high as 50% of hospitalized patients.6 However, we found no difference in the case rate when compared with that of chart review, and there were no false‐positive cases. We believe this is attributable to the 30‐day window that was used to identify a single episode of CDAD and the absence of toxin assays being performed on asymptomatic individuals. To avoid overrepresentation of the actual number of CDAD cases, we chose to label all repeat positive toxins and repeat hospitalizations within 30 days as a single episode of CDAD. This was based on the identification that 56% of patients remained toxin‐positive 26 weeks after adequate treatment for CDAD.18 The enzyme‐linked immunosorbent assay (ELISA) method used by our laboratory is the method used to report 94% of CDAD cases in a national point prevalence study that collected data from United States acute care hospitals with representation from 47 states1, 6, 19 (Table 5). This use of ELISA in the majority of United States hospitals suggests that our data can be extrapolated for use throughout the United States. While laboratories are increasing their use of 2‐step algorithms involving glutamate dehydrogenase antigen assay followed by cytotoxin neutralization, and more recently beginning the use of polymerase chain reaction assays, both of these methods have been found to increase the accuracy of detecting C. difficile compared with ELISA.20 Therefore, as laboratories evolve to use more accurate assays to detect CDAD, the methods described herein will be expected to increase in reliability.
| Method | Sensitivity | Specificity | Cost | Ease of Performance | Typical Results Reporting | Notes |
|---|---|---|---|---|---|---|
| ||||||
| Culture | Gold standard | NA | $$$$ | Difficult | Days | Slow turnaround time; is the standard upon which other test methods are based, but not all organisms are toxin‐producing |
| Cell cytotoxicity assay | 67%100% | Gold standard | $$$ | Intermediate | Next day | Is the standard upon which other test methods are based to identify toxin‐producing stains of Clostridium difficile |
| EIA for toxin A/B | 63%94% | 75%100% | $ | Easy | Same day | Used by >90% of laboratories in the United States |
| EIA for detection of Clostridium difficile common antigen (GDH) | 85%95% | 89%99% | $ | Easy | Same day | Provides no information regarding the toxigenicity of the isolate, typically used in combination with cell cytotoxicity assay to identify toxin‐producing strains |
| Polymerase chain reaction | 96%100% | 88%91% | $$ | Intermediate | Same day | More data are needed before recommendation for routine testing |
The toxin assay methodology used to determine the rate of CDAD cases acquired while the patient was at our hospital overreported these cases. Based on this result, identification of individual cases of CDAD that are obtained at a specific hospital would continue to require manual chart review. This expensive method may be avoided by instead choosing to use institutional case rates for reporting, monitoring, and incentivizing hospitals. However, a discussion of the methods of this approach and its confluence with our societal goal to move toward Accountable Care Organizations is beyond the scope of this discussion section.
Although it appears that we identified all cases of CDAD occurring at our institution, a limitation of this study is its inability to review all charts during the 10‐month study period. We used a combination of ICD‐9 and positive C. difficile toxin assay data to identify all possible cases of C. difficile. The current approach to case identification for reported hospital conditions is limited to an ICD‐9 database query. This query is followed by chart review to collect data for hospital performance that is published in locations such as
Increased automation is expected in the future of reporting. The Centers for Disease Control and Prevention found increased rates of disease reporting and increased accuracy when reporting is electronically automated via their software system, Electronic Support for Public Health, which is designed to communicate with and perform automated data queries on providers' electronic medical records.21 While use of this model is creeping into the health system for reporting to public health authorities,22 universal hospital electronic medical record implementation and full connectivity with such reporting systems is many years from fruition. In addition to its practical use for reporting CDAD in our current health system, our work easily transitions into automated reporting within an electronically integrated health system once achieved.
In conclusion, ICD‐9 data were found to be unreliable, and consideration must be given to cessation of their use for CDAD case rate research and reporting. Use of a positive C. difficile toxin assay accurately reports the institutional incidence of disease, can be used by individual institutions to self‐monitor case rates or by the government to determine regionally acceptable intuitional rates of CDAD on which incentives and penalties can be based, and will increase in efficiency as reporting continues to be automated. This process can be instituted at a fraction of the cost of the standard chart review that is currently used for most reporting.
- ,,,.National point prevalence of Clostridium difficile in US health care facility inpatients, 2008.Am J Infect Control.2009;37:263–270.
- ,,.Secular trends in hospital‐acquired Clostridium difficile disease in the United States, 1987–2001.J Infect Dis.2004;189:1585–1589.
- ,,, et al.Analysis of 30‐day mortality for Clostridium difficile‐associated disease in the ICU setting.Chest.2007;132:418–424.
- .Final report and recommendations from the National Notifiable Diseases Working Group.Can Commun Dis Rep.2006;32:211–225.
- ,,, et al.Recommendations for surveillance of Clostridium difficile‐associated disease.Infect Control Hosp Epidemiol.2007;28:140–145.
- ,,, et al.Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA).Infect Control Hosp Epidemiol.2010;31:431–455.
- ,.Review of current literature on the economic burden of Clostridium difficile infection.Infect Control Hosp Epidemiol.2009;30:57–66.
- Centers for Medicare 35:544–550.
- ,,, et al.Health spending projections through 2018: recession effects add uncertainty to the outlook.Health Aff (Millwood).2009;28:w346–w357.
- ,,,.ICD‐9 codes and surveillance for Clostridium difficile‐associated disease.Emerg Infect Dis.2006;12:1576–1579.
- ,,,.Fluoroquinolone use and risk factors for Clostridium difficile‐associated disease within a Veterans Administration health care system.Clin Infect Dis.2007;45:1141–1151.
- ,,.Conditions associated with leukocytosis in a tertiary care hospital, with particular attention to the role of infection caused by clostridium difficile.Clin Infect Dis.2002;34:1585–1592.
- ,,,.Accuracy of ICD‐9 coding for Clostridium difficile infections: a retrospective cohort.Epidemiol Infect.2007;135:1010–1013.
- ,,,.Implications of the changing face of Clostridium difficile disease for health care practitioners.Am J Infect Control.2007;35:237–253.
- .Clostridium difficile is no longer just a nosocomial infection or an infection of adults.Int J Antimicrob Agents.2009;33(suppl 1):S42–S45.
- ,,,,.Treatment of antibiotic‐associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens.Am J Med.1989;86:15–19.
- ,,, et al.Comparison of real‐time PCR techniques to cytotoxigenic culture methods for diagnosing Clostridium difficile infection.J Clin Microbiol.2011;49:227–231.
- ,,,.Comparison of BD GeneOhm Cdiff real‐time PCR assay with a two‐step algorithm and a toxin A/B enzyme‐linked immunosorbent assay for diagnosis of toxigenic Clostridium difficile infection.J Clin Microbiol.2010;48:109–114.
- Centers for Disease Control and Prevention.Automated detection and reporting of notifiable diseases using electronic medical records versus passive surveillance—Massachusetts, June 2006‐July 2007.MMWR Morb Mortal Wkly Rep.2008;57:373–376.
- ,,, et al.Development of an electronic public health case report using HL7 v2.5 to meet public health needs.J Am Med Inform Assoc.2010;17:34–41.
With an increased incidence of 13.1 per 1000 inpatients1, 2 and an attributable mortality of 6.1%,3 in 2006 the Canadian government added Clostridium difficileassociated disease (CDAD) to its list of reportable diseases.4 The Centers for Disease Control and Prevention and the Infectious Disease Society of America subsequently created definitions of the disease and recommended surveillance of rates of health care facilityassociated CDAD, which has been found to double a patient's length of stay and cost of hospitalization.57 While not included in the current list of hospital‐acquired conditions for which payment is declined,8 the Centers for Medicare & Medicaid Services (CMS) has noted CDAD as under consideration for addition to the list,9 which would require hospital reporting of CDAD rates. This reporting is typically a labor‐intensive, medical record review process that increases the cost of delivering health care. Health care institutions have reported spending as much as $21 million per year or $400 per discharged patient on quality improvement.10 These costs are passed on to payers such as those governed by the CMS that are projected to pay for more than half of all national health spending in 2018.11 Therefore, it is prudent to examine alternatives for determining rates of disease for public reporting and quality improvement initiatives such as infection control and antibiotic stewardship programs.
There are 3 methods that can be used for this reporting. First, medical record review has been used for determining the case rate of CDAD by published reports.1, 2 This procedure allows the CMS to define the desired data to be collected, but it is labor‐intensive. Second, the use of International Statistical Classification of Diseases and Related Health Problems, 9th Edition (ICD‐9) codes from hospital databases offers the speed of database query. However, due to diagnostic and coding errors, it may report inaccurate rates of disease. Previous reports include a university‐based hospital that found a sensitivity of 78% and specificity of 99.7%,12 whereas a Veterans Administration medical center found nearly two‐thirds of patients with CDAD did not have the ICD‐9 code for C. difficile infection noted in their database.13 Therefore, the accuracy of ICD‐9 code at a community hospital that better represents the majority of United States hospitals is needed. The third method of identifying CDAD is the presence of toxin identified in the microbiology laboratory. Although this method offers ease of obtainment, it suffers from potential inaccuracy arising from duplicate patient samples, positive samples in patients who are symptom‐free carriers, and patients with CDAD despite having a negative toxin.
The same organizations that recommend surveillance for CDAD have identified that there are inadequate data on which to base a decision regarding how to proceed with routine community and hospital surveillance.6 In the setting of public reporting and nonpayment, the method of identifying CDAD cases must be accurate to ensure fairness, while being inexpensive. To identify the potential value of these less labor‐intensive methods of reporting the incidence of CDAD, we evaluated the use of ICD‐9 codes and C. difficile toxin to accurately report the incidence of CDAD at a community hospital, as well as the labor hours required for each reporting method.
METHODS
Patients >18 years of age and potentially having CDAD were identified via database queries for ICD‐9 codes and positive C. difficile toxin assays at our institution from November 1, 2006, through August 31, 2007. Our institution is a 379‐bed university‐affiliated community teaching hospital in a socio‐economically diverse area of Baltimorewith approximately 110,000 emergency department visits, 30,000 discharges, and 110,000 inpatient days each yearthat uses a handwritten paper chart for all provider orders and patient documentation. The C. difficile toxin assay method used during the study period was an enzyme immunoassay that detects both toxins A and B (Meridan Bioscience, Cincinnati, OH). ICD‐9 codes were queried in the CareScience database(Premier, Inc; Charlotte, NC), while C. difficile toxin was queried via the hospital laboratory database. All identified patients underwent a medical record review to confirm the diagnosis of CDAD and the patient's location at the time of disease acquisition. To eliminate duplicate reporting of a single episode of disease, all duplicate patient visits with a diagnosis of CDAD within 30 days were removed.
Our diagnostic criteria (Table 1) used the recommended criteria of a combination of symptoms and positive toxin assay, visualization of pseudomembranes on colonoscopy or pathology‐proven CDAD.6 Based on the presence of CDAD in 25% of patients with a white blood cell count >30,000 and the recommendation that CDAD be considered in all patients with a white blood cell count >15,000, we included the criterion of clinical findings with severe leukocytosis to identify patients with toxin‐negative CDAD.14
| Chart Review Criteria for CDAD | Chart Review Criteria for CDAD Obtained While Patient Was Not at Our Hospital | Chart Review Criteria for CDAD Obtained While Patient Was at Our Hospital |
|---|---|---|
| ||
| Pseudomembranous colitis seen during endoscopy OR biopsy or resection with surgical pathology consistent with CDAD | One of the above symptoms or objective criteria positive within the first 3 days of hospitalization AND patient was not cared for at our insitution within the last 7 days | Patient was cared for at our institution within 7 days of presentation OR the patient's symptoms were not present upon arrival at the hospital and the symptoms began after the third day of hospitalization |
| If neither of the above are present, then Clostridium difficile toxin or WBC count >25,000 plus one of the following: | ||
| Diarrhea | ||
| Fever without other cause | ||
| Abdominal pain without other cause | ||
| Colonic ileus without other cause | ||
Patients were identified by querying the CareScience database for patients having an ICD‐9 code of 008.45. Duplicate cases for the same patient within 30 days were removed. These cases were compared with the cases of CDAD determined by the medical record review for analysis. Patients were identified via positive C. difficile toxin by querying the microbiology laboratory database. A query of all patients with a positive C. difficile toxin was performed. Duplicate samples for the same patient within 30 days were removed. Patients are considered to be outside the hospital when CDAD was acquired if their toxin was positive within the first 3 days of arrival to the hospital, and they were not hospitalized at our institution within 1 week before arrival. All other cases were considered to be acquired while in our hospital. The number of patients with a toxin‐positive stool sample was compared with the medical record review.
Recognizing that it is unrealistic to review the medical records of the 23,495 discharges during the 10‐month study period, a random sample review of 500 charts not included in our CDAD‐identified patient list was performed to identify the rate at which CDAD patients failed to be identified by our methods. From a list of discharges during the study period, as long as they were not in our study population, every thirtieth patient was selected for review up to a total of 500 patient charts. Then, the identified case rate was used to predict the prevalence of disease at our institution during the study period.
Sample selection was based on the absence of previous studies evaluating the performance of C. difficile toxin assay, and the desire to have the same assay used during the entire study. The lack of data from which to perform sample size determination would result in an inaccurate estimate. Therefore, we chose a period that offered the maximum sample size, during which a single assay was used at our institution. Compared with medical record review, the accuracy and positive predictive values of the ICD‐9 code and positive stool toxin methods to identify the cases of CDAD were compared. A true positive was when the ICD‐9 or laboratory query method identified a patient who had CDAD based on chart review; a false positive was when the ICD‐9 or laboratory query method identified a patient who did not have CDAD based on chart review. Rates of over‐ or underdiagnosis, case rates, and acquisition location were determined. Statistical analysis of the case rates and acquisition location were performed via chi‐square test. The time to perform these queries was collected with accuracy to the minute.
RESULTS
Of the 23,495 discharges during the study period, the combination of ICD‐9 and C. difficile toxin assay identified a total cohort of 496 patients, 319 of whom were identified by both the ICD‐9 method and the toxin assay, 50 of whom were identified only by the toxin assay, and 127 of whom were identified only by the ICD‐9 method. Chart review confirmed the presence of CDAD in 384 of these 496 cases, for a case rate of 16.3 per 1000 discharged patients (Table 2). The diagnostic criteria for each of these confirmed cases are listed in Table 3. Of the 384 confirmed CDAD cases, 319 were identified by both the ICD‐9 and toxin assay, 50 were identified only by the toxin assay, and 15 were identified only by the ICD‐9 query. Of the 50 cases identified by the toxin assay that were not identified via the ICD‐9 method, all 50 (100%) were confirmed to have CDAD by chart review. In contrast, of the 127 cases identified via the ICD‐9 method that were not found via the toxin assay, only 15 (11.8%) were confirmed to have CDAD by chart review (Figure 1).

| ICD‐9 | Toxin Assay | Chart Review | |
|---|---|---|---|
| |||
| No. of patients identified | 446 | 369 | 384 |
| Case rate per 1000 discharges* | 19.0 | 15.7 | 16.3 |
| 95% confidence interval | 17.320.8 | 14.117.4 | NA |
| Case rate compared with chart review, P | P = 0.001 | P = 0.440 | NA |
| CDAD rate reported compared with chart review | 116.1% | 96.1% | NA |
| Accuracy | 83.9% | 96.1% | NA |
| PPV | 74.9% | 100% | NA |
| Portion of cases acquired at our hospital, % | NA | 48.2% | 40.6% |
| Portion of cases acquired at our hospital, P | NA | P = 0.003 | NA |
| Minutes consumed for data collection | 312 | 842 | 21,899 |
| Estimated annual cost per hospital | $234.00 | $631.50 | $16,424.25 |
| Criterion | No. of Cases | Case Rate* |
|---|---|---|
| ||
| Endoscopy | 2 | 0.5% |
| Surgical pathology | 9 | 2.3% |
| Positive toxin and diarrhea | 369 | 96.1% |
| WBC >25.0 and diarrhea | 51 | 13.3% |
| WBC >25.0 and fever without other source | 9 | 2.3% |
| WBC >25.0 and unexplained abdominal pain | 17 | 4.4% |
| WBC >25.0 and colonic ileus | 2 | 0.5% |
Of these 384 cases, 369 were identified via the toxin assay for a case rate of 15.7 per 1000 patient discharges, which was not found to be different from the rate of 16.3 determined by chart review (P = 0.440; 95% confidence interval [CI], 14.117.4). Compared with chart review, the toxin assay reported 96.1% (369/384) of cases. Chart review demonstrated that every patient who had a positive toxin assay met the diagnostic criteria for CDAD for a positive predictive value (PPV) of 100%.
The ICD‐9 method identified 446 patients thought to have CDAD, 334 of whom were confirmed by chart review for a PPV of 74.9% (334/446). Compared with chart review, the ICD‐9 method reported 116.1% (446/384) of cases for a case rate of 19.0 per 1000 discharges and was significantly different than the rate of 16.3 reported by chart review (P = 0.001; 95% CI, 17.320.8).
Chart review identified 156 of 384 (40.6%) patients who acquired CDAD while at our hospital and 228 of 384 (59.4%) who acquired it elsewhere. In comparison, the toxin assay criteria identified 369 cases of CDAD, of which 48.2% (178/369) were acquired while at our hospital and 51.8% (191/369) were acquired elsewhere (P = 0.003).
The time for data extraction via these 3 methods differed greatly. The ICD‐9 method only consumed 312 minutes and the toxin assay method 842 minutes, whereas the chart review method consumed 21,899 minutes. These times reported include the database query and data analysis for the ICD‐9 and toxin assay methods, while it includes the database query and list generation along with the manual chart review and data analysis for the chart review method. The review of the random sample of patients believed not to have CDAD was not included in any of the reported times. Chart review on a random sample of 500 patients not previously identified for review found no additional cases of CDAD.
DISCUSSION
Our study demonstrates that use of positive C. difficile toxin assay data from the microbiology laboratory alone is an efficient method of identifying patients with CDAD. This method only consumed 842 minutes versus 21,899 minutes consumed by the chart review method. The C. difficile toxin method reduces the workforce required to collect and analyze this data, but more importantly, it was found to be reliable by reporting an institutional case rate that is similar to that of chart review.
In contrast, the ICD‐9 method was efficient but less reliable. It only consumed 312 minutes, but it overreported the institutional rate of CDAD by 16.1%, had a PPV of only 74.9%, and of those patients who were identified by ICD‐9 but not toxin assay, only 11.8% actually had CDAD. This finding is in conflict with the previously noted underreporting of this method.13, 15 We believe this difference to be associated with institutional differences, because previous reports originated from a veterans hospital and an academic medical center, and previous authors have failed to use predefined diagnostic criteria and a complete chart review to confirm cases of CDAD. Similar to our study, an academic medical center identified that listing of CDAD in a patient's medical history in their chart was associated with a false‐positive ICD‐9 code for CDAD.15 This observation appears to bring clarity to one of the causes of the variance of ICD‐9 code accuracy between institutions. Institutions seem to vary on the method of attaching diagnoses to the patient's final hospital record. Some institutions include only what is listed as an active problem, whereas others list diagnoses listed in the chart as previous problems and those listed as a potential diagnosis without confirmation. Another potential cause of the ICD‐9 inaccuracy is the potential of clinicians to diagnose and treat a patient for CDAD in the absence of the diagnostic criteria used for chart review. Physician practices such as these are known to vary between institutions leading to a variance in the ICD‐9 code accuracy.
In total, 15 cases of CDAD were identified in the absence of a positive toxin assay, and of these, 12 cases were identified using leukocytosis‐based criteria (Table 4). This resulted in 3.1% of our cases being toxin‐negative, based on leukocytosis criteria, and is lower than the previously identified 35% of cases.14 Because it was the only assay available at the time, this previous research used a toxin Aonly assay, which is more likely to have false‐negative results than the toxin A/B assay used at our institution during the study period. The investigators also required all toxin‐negative patients to have been recently treated with antibiotics. Based on the increasing rates of community‐acquired CDAD, including those that are antibiotic‐nave patients, we felt a history of antibiotic exposure was no longer a prerequisite for CDAD and thus excluded it from our diagnostic criteria.16, 17 Based on these differences, we feel our results are likely an accurate reflection of the number of cases identified by ICD‐9 query in the absence of toxin positivity. However, concerns should be further alleviated through the realization that nonuse of this strategy improves the accuracy of the toxin assay method, while reducing the accuracy of the ICD‐9 method and thereby strengthening the validity of our conclusions. Mathematically, this would result in 369 of 369 patients identified by toxin assay and 369 patients identified by the ICD‐9 method. This would reduce the case rate of the chart review method to the same 15.7 of the toxin assay, while the ICD‐9 method would remain at 19.0.
| Criterion | No. of Cases* |
|---|---|
| |
| Endoscopy | 0 |
| Surgical pathology | 3 |
| WBC >25.0 and diarrhea | 10 |
| WBC >25.0 and fever without other source | 1 |
| WBC >25.0 and unexplained abdominal pain | 3 |
| WBC >25.0 and colonic ileus | 1 |
We considered whether the toxin assay method may overestimate the number of cases due to a C. difficileasymptomatic carrier rate as high as 50% of hospitalized patients.6 However, we found no difference in the case rate when compared with that of chart review, and there were no false‐positive cases. We believe this is attributable to the 30‐day window that was used to identify a single episode of CDAD and the absence of toxin assays being performed on asymptomatic individuals. To avoid overrepresentation of the actual number of CDAD cases, we chose to label all repeat positive toxins and repeat hospitalizations within 30 days as a single episode of CDAD. This was based on the identification that 56% of patients remained toxin‐positive 26 weeks after adequate treatment for CDAD.18 The enzyme‐linked immunosorbent assay (ELISA) method used by our laboratory is the method used to report 94% of CDAD cases in a national point prevalence study that collected data from United States acute care hospitals with representation from 47 states1, 6, 19 (Table 5). This use of ELISA in the majority of United States hospitals suggests that our data can be extrapolated for use throughout the United States. While laboratories are increasing their use of 2‐step algorithms involving glutamate dehydrogenase antigen assay followed by cytotoxin neutralization, and more recently beginning the use of polymerase chain reaction assays, both of these methods have been found to increase the accuracy of detecting C. difficile compared with ELISA.20 Therefore, as laboratories evolve to use more accurate assays to detect CDAD, the methods described herein will be expected to increase in reliability.
| Method | Sensitivity | Specificity | Cost | Ease of Performance | Typical Results Reporting | Notes |
|---|---|---|---|---|---|---|
| ||||||
| Culture | Gold standard | NA | $$$$ | Difficult | Days | Slow turnaround time; is the standard upon which other test methods are based, but not all organisms are toxin‐producing |
| Cell cytotoxicity assay | 67%100% | Gold standard | $$$ | Intermediate | Next day | Is the standard upon which other test methods are based to identify toxin‐producing stains of Clostridium difficile |
| EIA for toxin A/B | 63%94% | 75%100% | $ | Easy | Same day | Used by >90% of laboratories in the United States |
| EIA for detection of Clostridium difficile common antigen (GDH) | 85%95% | 89%99% | $ | Easy | Same day | Provides no information regarding the toxigenicity of the isolate, typically used in combination with cell cytotoxicity assay to identify toxin‐producing strains |
| Polymerase chain reaction | 96%100% | 88%91% | $$ | Intermediate | Same day | More data are needed before recommendation for routine testing |
The toxin assay methodology used to determine the rate of CDAD cases acquired while the patient was at our hospital overreported these cases. Based on this result, identification of individual cases of CDAD that are obtained at a specific hospital would continue to require manual chart review. This expensive method may be avoided by instead choosing to use institutional case rates for reporting, monitoring, and incentivizing hospitals. However, a discussion of the methods of this approach and its confluence with our societal goal to move toward Accountable Care Organizations is beyond the scope of this discussion section.
Although it appears that we identified all cases of CDAD occurring at our institution, a limitation of this study is its inability to review all charts during the 10‐month study period. We used a combination of ICD‐9 and positive C. difficile toxin assay data to identify all possible cases of C. difficile. The current approach to case identification for reported hospital conditions is limited to an ICD‐9 database query. This query is followed by chart review to collect data for hospital performance that is published in locations such as
Increased automation is expected in the future of reporting. The Centers for Disease Control and Prevention found increased rates of disease reporting and increased accuracy when reporting is electronically automated via their software system, Electronic Support for Public Health, which is designed to communicate with and perform automated data queries on providers' electronic medical records.21 While use of this model is creeping into the health system for reporting to public health authorities,22 universal hospital electronic medical record implementation and full connectivity with such reporting systems is many years from fruition. In addition to its practical use for reporting CDAD in our current health system, our work easily transitions into automated reporting within an electronically integrated health system once achieved.
In conclusion, ICD‐9 data were found to be unreliable, and consideration must be given to cessation of their use for CDAD case rate research and reporting. Use of a positive C. difficile toxin assay accurately reports the institutional incidence of disease, can be used by individual institutions to self‐monitor case rates or by the government to determine regionally acceptable intuitional rates of CDAD on which incentives and penalties can be based, and will increase in efficiency as reporting continues to be automated. This process can be instituted at a fraction of the cost of the standard chart review that is currently used for most reporting.
With an increased incidence of 13.1 per 1000 inpatients1, 2 and an attributable mortality of 6.1%,3 in 2006 the Canadian government added Clostridium difficileassociated disease (CDAD) to its list of reportable diseases.4 The Centers for Disease Control and Prevention and the Infectious Disease Society of America subsequently created definitions of the disease and recommended surveillance of rates of health care facilityassociated CDAD, which has been found to double a patient's length of stay and cost of hospitalization.57 While not included in the current list of hospital‐acquired conditions for which payment is declined,8 the Centers for Medicare & Medicaid Services (CMS) has noted CDAD as under consideration for addition to the list,9 which would require hospital reporting of CDAD rates. This reporting is typically a labor‐intensive, medical record review process that increases the cost of delivering health care. Health care institutions have reported spending as much as $21 million per year or $400 per discharged patient on quality improvement.10 These costs are passed on to payers such as those governed by the CMS that are projected to pay for more than half of all national health spending in 2018.11 Therefore, it is prudent to examine alternatives for determining rates of disease for public reporting and quality improvement initiatives such as infection control and antibiotic stewardship programs.
There are 3 methods that can be used for this reporting. First, medical record review has been used for determining the case rate of CDAD by published reports.1, 2 This procedure allows the CMS to define the desired data to be collected, but it is labor‐intensive. Second, the use of International Statistical Classification of Diseases and Related Health Problems, 9th Edition (ICD‐9) codes from hospital databases offers the speed of database query. However, due to diagnostic and coding errors, it may report inaccurate rates of disease. Previous reports include a university‐based hospital that found a sensitivity of 78% and specificity of 99.7%,12 whereas a Veterans Administration medical center found nearly two‐thirds of patients with CDAD did not have the ICD‐9 code for C. difficile infection noted in their database.13 Therefore, the accuracy of ICD‐9 code at a community hospital that better represents the majority of United States hospitals is needed. The third method of identifying CDAD is the presence of toxin identified in the microbiology laboratory. Although this method offers ease of obtainment, it suffers from potential inaccuracy arising from duplicate patient samples, positive samples in patients who are symptom‐free carriers, and patients with CDAD despite having a negative toxin.
The same organizations that recommend surveillance for CDAD have identified that there are inadequate data on which to base a decision regarding how to proceed with routine community and hospital surveillance.6 In the setting of public reporting and nonpayment, the method of identifying CDAD cases must be accurate to ensure fairness, while being inexpensive. To identify the potential value of these less labor‐intensive methods of reporting the incidence of CDAD, we evaluated the use of ICD‐9 codes and C. difficile toxin to accurately report the incidence of CDAD at a community hospital, as well as the labor hours required for each reporting method.
METHODS
Patients >18 years of age and potentially having CDAD were identified via database queries for ICD‐9 codes and positive C. difficile toxin assays at our institution from November 1, 2006, through August 31, 2007. Our institution is a 379‐bed university‐affiliated community teaching hospital in a socio‐economically diverse area of Baltimorewith approximately 110,000 emergency department visits, 30,000 discharges, and 110,000 inpatient days each yearthat uses a handwritten paper chart for all provider orders and patient documentation. The C. difficile toxin assay method used during the study period was an enzyme immunoassay that detects both toxins A and B (Meridan Bioscience, Cincinnati, OH). ICD‐9 codes were queried in the CareScience database(Premier, Inc; Charlotte, NC), while C. difficile toxin was queried via the hospital laboratory database. All identified patients underwent a medical record review to confirm the diagnosis of CDAD and the patient's location at the time of disease acquisition. To eliminate duplicate reporting of a single episode of disease, all duplicate patient visits with a diagnosis of CDAD within 30 days were removed.
Our diagnostic criteria (Table 1) used the recommended criteria of a combination of symptoms and positive toxin assay, visualization of pseudomembranes on colonoscopy or pathology‐proven CDAD.6 Based on the presence of CDAD in 25% of patients with a white blood cell count >30,000 and the recommendation that CDAD be considered in all patients with a white blood cell count >15,000, we included the criterion of clinical findings with severe leukocytosis to identify patients with toxin‐negative CDAD.14
| Chart Review Criteria for CDAD | Chart Review Criteria for CDAD Obtained While Patient Was Not at Our Hospital | Chart Review Criteria for CDAD Obtained While Patient Was at Our Hospital |
|---|---|---|
| ||
| Pseudomembranous colitis seen during endoscopy OR biopsy or resection with surgical pathology consistent with CDAD | One of the above symptoms or objective criteria positive within the first 3 days of hospitalization AND patient was not cared for at our insitution within the last 7 days | Patient was cared for at our institution within 7 days of presentation OR the patient's symptoms were not present upon arrival at the hospital and the symptoms began after the third day of hospitalization |
| If neither of the above are present, then Clostridium difficile toxin or WBC count >25,000 plus one of the following: | ||
| Diarrhea | ||
| Fever without other cause | ||
| Abdominal pain without other cause | ||
| Colonic ileus without other cause | ||
Patients were identified by querying the CareScience database for patients having an ICD‐9 code of 008.45. Duplicate cases for the same patient within 30 days were removed. These cases were compared with the cases of CDAD determined by the medical record review for analysis. Patients were identified via positive C. difficile toxin by querying the microbiology laboratory database. A query of all patients with a positive C. difficile toxin was performed. Duplicate samples for the same patient within 30 days were removed. Patients are considered to be outside the hospital when CDAD was acquired if their toxin was positive within the first 3 days of arrival to the hospital, and they were not hospitalized at our institution within 1 week before arrival. All other cases were considered to be acquired while in our hospital. The number of patients with a toxin‐positive stool sample was compared with the medical record review.
Recognizing that it is unrealistic to review the medical records of the 23,495 discharges during the 10‐month study period, a random sample review of 500 charts not included in our CDAD‐identified patient list was performed to identify the rate at which CDAD patients failed to be identified by our methods. From a list of discharges during the study period, as long as they were not in our study population, every thirtieth patient was selected for review up to a total of 500 patient charts. Then, the identified case rate was used to predict the prevalence of disease at our institution during the study period.
Sample selection was based on the absence of previous studies evaluating the performance of C. difficile toxin assay, and the desire to have the same assay used during the entire study. The lack of data from which to perform sample size determination would result in an inaccurate estimate. Therefore, we chose a period that offered the maximum sample size, during which a single assay was used at our institution. Compared with medical record review, the accuracy and positive predictive values of the ICD‐9 code and positive stool toxin methods to identify the cases of CDAD were compared. A true positive was when the ICD‐9 or laboratory query method identified a patient who had CDAD based on chart review; a false positive was when the ICD‐9 or laboratory query method identified a patient who did not have CDAD based on chart review. Rates of over‐ or underdiagnosis, case rates, and acquisition location were determined. Statistical analysis of the case rates and acquisition location were performed via chi‐square test. The time to perform these queries was collected with accuracy to the minute.
RESULTS
Of the 23,495 discharges during the study period, the combination of ICD‐9 and C. difficile toxin assay identified a total cohort of 496 patients, 319 of whom were identified by both the ICD‐9 method and the toxin assay, 50 of whom were identified only by the toxin assay, and 127 of whom were identified only by the ICD‐9 method. Chart review confirmed the presence of CDAD in 384 of these 496 cases, for a case rate of 16.3 per 1000 discharged patients (Table 2). The diagnostic criteria for each of these confirmed cases are listed in Table 3. Of the 384 confirmed CDAD cases, 319 were identified by both the ICD‐9 and toxin assay, 50 were identified only by the toxin assay, and 15 were identified only by the ICD‐9 query. Of the 50 cases identified by the toxin assay that were not identified via the ICD‐9 method, all 50 (100%) were confirmed to have CDAD by chart review. In contrast, of the 127 cases identified via the ICD‐9 method that were not found via the toxin assay, only 15 (11.8%) were confirmed to have CDAD by chart review (Figure 1).

| ICD‐9 | Toxin Assay | Chart Review | |
|---|---|---|---|
| |||
| No. of patients identified | 446 | 369 | 384 |
| Case rate per 1000 discharges* | 19.0 | 15.7 | 16.3 |
| 95% confidence interval | 17.320.8 | 14.117.4 | NA |
| Case rate compared with chart review, P | P = 0.001 | P = 0.440 | NA |
| CDAD rate reported compared with chart review | 116.1% | 96.1% | NA |
| Accuracy | 83.9% | 96.1% | NA |
| PPV | 74.9% | 100% | NA |
| Portion of cases acquired at our hospital, % | NA | 48.2% | 40.6% |
| Portion of cases acquired at our hospital, P | NA | P = 0.003 | NA |
| Minutes consumed for data collection | 312 | 842 | 21,899 |
| Estimated annual cost per hospital | $234.00 | $631.50 | $16,424.25 |
| Criterion | No. of Cases | Case Rate* |
|---|---|---|
| ||
| Endoscopy | 2 | 0.5% |
| Surgical pathology | 9 | 2.3% |
| Positive toxin and diarrhea | 369 | 96.1% |
| WBC >25.0 and diarrhea | 51 | 13.3% |
| WBC >25.0 and fever without other source | 9 | 2.3% |
| WBC >25.0 and unexplained abdominal pain | 17 | 4.4% |
| WBC >25.0 and colonic ileus | 2 | 0.5% |
Of these 384 cases, 369 were identified via the toxin assay for a case rate of 15.7 per 1000 patient discharges, which was not found to be different from the rate of 16.3 determined by chart review (P = 0.440; 95% confidence interval [CI], 14.117.4). Compared with chart review, the toxin assay reported 96.1% (369/384) of cases. Chart review demonstrated that every patient who had a positive toxin assay met the diagnostic criteria for CDAD for a positive predictive value (PPV) of 100%.
The ICD‐9 method identified 446 patients thought to have CDAD, 334 of whom were confirmed by chart review for a PPV of 74.9% (334/446). Compared with chart review, the ICD‐9 method reported 116.1% (446/384) of cases for a case rate of 19.0 per 1000 discharges and was significantly different than the rate of 16.3 reported by chart review (P = 0.001; 95% CI, 17.320.8).
Chart review identified 156 of 384 (40.6%) patients who acquired CDAD while at our hospital and 228 of 384 (59.4%) who acquired it elsewhere. In comparison, the toxin assay criteria identified 369 cases of CDAD, of which 48.2% (178/369) were acquired while at our hospital and 51.8% (191/369) were acquired elsewhere (P = 0.003).
The time for data extraction via these 3 methods differed greatly. The ICD‐9 method only consumed 312 minutes and the toxin assay method 842 minutes, whereas the chart review method consumed 21,899 minutes. These times reported include the database query and data analysis for the ICD‐9 and toxin assay methods, while it includes the database query and list generation along with the manual chart review and data analysis for the chart review method. The review of the random sample of patients believed not to have CDAD was not included in any of the reported times. Chart review on a random sample of 500 patients not previously identified for review found no additional cases of CDAD.
DISCUSSION
Our study demonstrates that use of positive C. difficile toxin assay data from the microbiology laboratory alone is an efficient method of identifying patients with CDAD. This method only consumed 842 minutes versus 21,899 minutes consumed by the chart review method. The C. difficile toxin method reduces the workforce required to collect and analyze this data, but more importantly, it was found to be reliable by reporting an institutional case rate that is similar to that of chart review.
In contrast, the ICD‐9 method was efficient but less reliable. It only consumed 312 minutes, but it overreported the institutional rate of CDAD by 16.1%, had a PPV of only 74.9%, and of those patients who were identified by ICD‐9 but not toxin assay, only 11.8% actually had CDAD. This finding is in conflict with the previously noted underreporting of this method.13, 15 We believe this difference to be associated with institutional differences, because previous reports originated from a veterans hospital and an academic medical center, and previous authors have failed to use predefined diagnostic criteria and a complete chart review to confirm cases of CDAD. Similar to our study, an academic medical center identified that listing of CDAD in a patient's medical history in their chart was associated with a false‐positive ICD‐9 code for CDAD.15 This observation appears to bring clarity to one of the causes of the variance of ICD‐9 code accuracy between institutions. Institutions seem to vary on the method of attaching diagnoses to the patient's final hospital record. Some institutions include only what is listed as an active problem, whereas others list diagnoses listed in the chart as previous problems and those listed as a potential diagnosis without confirmation. Another potential cause of the ICD‐9 inaccuracy is the potential of clinicians to diagnose and treat a patient for CDAD in the absence of the diagnostic criteria used for chart review. Physician practices such as these are known to vary between institutions leading to a variance in the ICD‐9 code accuracy.
In total, 15 cases of CDAD were identified in the absence of a positive toxin assay, and of these, 12 cases were identified using leukocytosis‐based criteria (Table 4). This resulted in 3.1% of our cases being toxin‐negative, based on leukocytosis criteria, and is lower than the previously identified 35% of cases.14 Because it was the only assay available at the time, this previous research used a toxin Aonly assay, which is more likely to have false‐negative results than the toxin A/B assay used at our institution during the study period. The investigators also required all toxin‐negative patients to have been recently treated with antibiotics. Based on the increasing rates of community‐acquired CDAD, including those that are antibiotic‐nave patients, we felt a history of antibiotic exposure was no longer a prerequisite for CDAD and thus excluded it from our diagnostic criteria.16, 17 Based on these differences, we feel our results are likely an accurate reflection of the number of cases identified by ICD‐9 query in the absence of toxin positivity. However, concerns should be further alleviated through the realization that nonuse of this strategy improves the accuracy of the toxin assay method, while reducing the accuracy of the ICD‐9 method and thereby strengthening the validity of our conclusions. Mathematically, this would result in 369 of 369 patients identified by toxin assay and 369 patients identified by the ICD‐9 method. This would reduce the case rate of the chart review method to the same 15.7 of the toxin assay, while the ICD‐9 method would remain at 19.0.
| Criterion | No. of Cases* |
|---|---|
| |
| Endoscopy | 0 |
| Surgical pathology | 3 |
| WBC >25.0 and diarrhea | 10 |
| WBC >25.0 and fever without other source | 1 |
| WBC >25.0 and unexplained abdominal pain | 3 |
| WBC >25.0 and colonic ileus | 1 |
We considered whether the toxin assay method may overestimate the number of cases due to a C. difficileasymptomatic carrier rate as high as 50% of hospitalized patients.6 However, we found no difference in the case rate when compared with that of chart review, and there were no false‐positive cases. We believe this is attributable to the 30‐day window that was used to identify a single episode of CDAD and the absence of toxin assays being performed on asymptomatic individuals. To avoid overrepresentation of the actual number of CDAD cases, we chose to label all repeat positive toxins and repeat hospitalizations within 30 days as a single episode of CDAD. This was based on the identification that 56% of patients remained toxin‐positive 26 weeks after adequate treatment for CDAD.18 The enzyme‐linked immunosorbent assay (ELISA) method used by our laboratory is the method used to report 94% of CDAD cases in a national point prevalence study that collected data from United States acute care hospitals with representation from 47 states1, 6, 19 (Table 5). This use of ELISA in the majority of United States hospitals suggests that our data can be extrapolated for use throughout the United States. While laboratories are increasing their use of 2‐step algorithms involving glutamate dehydrogenase antigen assay followed by cytotoxin neutralization, and more recently beginning the use of polymerase chain reaction assays, both of these methods have been found to increase the accuracy of detecting C. difficile compared with ELISA.20 Therefore, as laboratories evolve to use more accurate assays to detect CDAD, the methods described herein will be expected to increase in reliability.
| Method | Sensitivity | Specificity | Cost | Ease of Performance | Typical Results Reporting | Notes |
|---|---|---|---|---|---|---|
| ||||||
| Culture | Gold standard | NA | $$$$ | Difficult | Days | Slow turnaround time; is the standard upon which other test methods are based, but not all organisms are toxin‐producing |
| Cell cytotoxicity assay | 67%100% | Gold standard | $$$ | Intermediate | Next day | Is the standard upon which other test methods are based to identify toxin‐producing stains of Clostridium difficile |
| EIA for toxin A/B | 63%94% | 75%100% | $ | Easy | Same day | Used by >90% of laboratories in the United States |
| EIA for detection of Clostridium difficile common antigen (GDH) | 85%95% | 89%99% | $ | Easy | Same day | Provides no information regarding the toxigenicity of the isolate, typically used in combination with cell cytotoxicity assay to identify toxin‐producing strains |
| Polymerase chain reaction | 96%100% | 88%91% | $$ | Intermediate | Same day | More data are needed before recommendation for routine testing |
The toxin assay methodology used to determine the rate of CDAD cases acquired while the patient was at our hospital overreported these cases. Based on this result, identification of individual cases of CDAD that are obtained at a specific hospital would continue to require manual chart review. This expensive method may be avoided by instead choosing to use institutional case rates for reporting, monitoring, and incentivizing hospitals. However, a discussion of the methods of this approach and its confluence with our societal goal to move toward Accountable Care Organizations is beyond the scope of this discussion section.
Although it appears that we identified all cases of CDAD occurring at our institution, a limitation of this study is its inability to review all charts during the 10‐month study period. We used a combination of ICD‐9 and positive C. difficile toxin assay data to identify all possible cases of C. difficile. The current approach to case identification for reported hospital conditions is limited to an ICD‐9 database query. This query is followed by chart review to collect data for hospital performance that is published in locations such as
Increased automation is expected in the future of reporting. The Centers for Disease Control and Prevention found increased rates of disease reporting and increased accuracy when reporting is electronically automated via their software system, Electronic Support for Public Health, which is designed to communicate with and perform automated data queries on providers' electronic medical records.21 While use of this model is creeping into the health system for reporting to public health authorities,22 universal hospital electronic medical record implementation and full connectivity with such reporting systems is many years from fruition. In addition to its practical use for reporting CDAD in our current health system, our work easily transitions into automated reporting within an electronically integrated health system once achieved.
In conclusion, ICD‐9 data were found to be unreliable, and consideration must be given to cessation of their use for CDAD case rate research and reporting. Use of a positive C. difficile toxin assay accurately reports the institutional incidence of disease, can be used by individual institutions to self‐monitor case rates or by the government to determine regionally acceptable intuitional rates of CDAD on which incentives and penalties can be based, and will increase in efficiency as reporting continues to be automated. This process can be instituted at a fraction of the cost of the standard chart review that is currently used for most reporting.
- ,,,.National point prevalence of Clostridium difficile in US health care facility inpatients, 2008.Am J Infect Control.2009;37:263–270.
- ,,.Secular trends in hospital‐acquired Clostridium difficile disease in the United States, 1987–2001.J Infect Dis.2004;189:1585–1589.
- ,,, et al.Analysis of 30‐day mortality for Clostridium difficile‐associated disease in the ICU setting.Chest.2007;132:418–424.
- .Final report and recommendations from the National Notifiable Diseases Working Group.Can Commun Dis Rep.2006;32:211–225.
- ,,, et al.Recommendations for surveillance of Clostridium difficile‐associated disease.Infect Control Hosp Epidemiol.2007;28:140–145.
- ,,, et al.Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA).Infect Control Hosp Epidemiol.2010;31:431–455.
- ,.Review of current literature on the economic burden of Clostridium difficile infection.Infect Control Hosp Epidemiol.2009;30:57–66.
- Centers for Medicare 35:544–550.
- ,,, et al.Health spending projections through 2018: recession effects add uncertainty to the outlook.Health Aff (Millwood).2009;28:w346–w357.
- ,,,.ICD‐9 codes and surveillance for Clostridium difficile‐associated disease.Emerg Infect Dis.2006;12:1576–1579.
- ,,,.Fluoroquinolone use and risk factors for Clostridium difficile‐associated disease within a Veterans Administration health care system.Clin Infect Dis.2007;45:1141–1151.
- ,,.Conditions associated with leukocytosis in a tertiary care hospital, with particular attention to the role of infection caused by clostridium difficile.Clin Infect Dis.2002;34:1585–1592.
- ,,,.Accuracy of ICD‐9 coding for Clostridium difficile infections: a retrospective cohort.Epidemiol Infect.2007;135:1010–1013.
- ,,,.Implications of the changing face of Clostridium difficile disease for health care practitioners.Am J Infect Control.2007;35:237–253.
- .Clostridium difficile is no longer just a nosocomial infection or an infection of adults.Int J Antimicrob Agents.2009;33(suppl 1):S42–S45.
- ,,,,.Treatment of antibiotic‐associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens.Am J Med.1989;86:15–19.
- ,,, et al.Comparison of real‐time PCR techniques to cytotoxigenic culture methods for diagnosing Clostridium difficile infection.J Clin Microbiol.2011;49:227–231.
- ,,,.Comparison of BD GeneOhm Cdiff real‐time PCR assay with a two‐step algorithm and a toxin A/B enzyme‐linked immunosorbent assay for diagnosis of toxigenic Clostridium difficile infection.J Clin Microbiol.2010;48:109–114.
- Centers for Disease Control and Prevention.Automated detection and reporting of notifiable diseases using electronic medical records versus passive surveillance—Massachusetts, June 2006‐July 2007.MMWR Morb Mortal Wkly Rep.2008;57:373–376.
- ,,, et al.Development of an electronic public health case report using HL7 v2.5 to meet public health needs.J Am Med Inform Assoc.2010;17:34–41.
- ,,,.National point prevalence of Clostridium difficile in US health care facility inpatients, 2008.Am J Infect Control.2009;37:263–270.
- ,,.Secular trends in hospital‐acquired Clostridium difficile disease in the United States, 1987–2001.J Infect Dis.2004;189:1585–1589.
- ,,, et al.Analysis of 30‐day mortality for Clostridium difficile‐associated disease in the ICU setting.Chest.2007;132:418–424.
- .Final report and recommendations from the National Notifiable Diseases Working Group.Can Commun Dis Rep.2006;32:211–225.
- ,,, et al.Recommendations for surveillance of Clostridium difficile‐associated disease.Infect Control Hosp Epidemiol.2007;28:140–145.
- ,,, et al.Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA).Infect Control Hosp Epidemiol.2010;31:431–455.
- ,.Review of current literature on the economic burden of Clostridium difficile infection.Infect Control Hosp Epidemiol.2009;30:57–66.
- Centers for Medicare 35:544–550.
- ,,, et al.Health spending projections through 2018: recession effects add uncertainty to the outlook.Health Aff (Millwood).2009;28:w346–w357.
- ,,,.ICD‐9 codes and surveillance for Clostridium difficile‐associated disease.Emerg Infect Dis.2006;12:1576–1579.
- ,,,.Fluoroquinolone use and risk factors for Clostridium difficile‐associated disease within a Veterans Administration health care system.Clin Infect Dis.2007;45:1141–1151.
- ,,.Conditions associated with leukocytosis in a tertiary care hospital, with particular attention to the role of infection caused by clostridium difficile.Clin Infect Dis.2002;34:1585–1592.
- ,,,.Accuracy of ICD‐9 coding for Clostridium difficile infections: a retrospective cohort.Epidemiol Infect.2007;135:1010–1013.
- ,,,.Implications of the changing face of Clostridium difficile disease for health care practitioners.Am J Infect Control.2007;35:237–253.
- .Clostridium difficile is no longer just a nosocomial infection or an infection of adults.Int J Antimicrob Agents.2009;33(suppl 1):S42–S45.
- ,,,,.Treatment of antibiotic‐associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens.Am J Med.1989;86:15–19.
- ,,, et al.Comparison of real‐time PCR techniques to cytotoxigenic culture methods for diagnosing Clostridium difficile infection.J Clin Microbiol.2011;49:227–231.
- ,,,.Comparison of BD GeneOhm Cdiff real‐time PCR assay with a two‐step algorithm and a toxin A/B enzyme‐linked immunosorbent assay for diagnosis of toxigenic Clostridium difficile infection.J Clin Microbiol.2010;48:109–114.
- Centers for Disease Control and Prevention.Automated detection and reporting of notifiable diseases using electronic medical records versus passive surveillance—Massachusetts, June 2006‐July 2007.MMWR Morb Mortal Wkly Rep.2008;57:373–376.
- ,,, et al.Development of an electronic public health case report using HL7 v2.5 to meet public health needs.J Am Med Inform Assoc.2010;17:34–41.
Copyright © 2011 Society of Hospital Medicine
Bed Utilization in the PICU
Patient flow refers to the management and movement of patients in health care settings and is linked to quality, safety, and cost.16 The intensive care unit (ICU) is crucial in patient flow.7, 8 The limited number of beds and the resource‐intensive services and staffing associated with them require that hospitals optimize their utilization, as is increasingly true of all hospital resources. To maximize delivery of services to patients who need them and minimize real and opportunity losses (eg, postponed surgery, diverted transports, or inability to accept patients), patients in ICU beds should receive critical care medicine/nursing services while there and be transferred or discharged when appropriate.
The time between arrival and departure from any area of the hospital, including the ICU, is considered the time when a patient is receiving needed clinical carethe value‐added portion of health care operationsand time waiting to move on to the next step.911 This period includes both necessary logistics (eg, signing out a patient or waiting a reasonable amount of time for room cleaning) and nonvalue‐added time (eg, an excessively long amount of time for room cleaning). Operations management labels nonvalue‐added time as waste, and its reduction is vital for high‐quality health care.9, 12, 13 As in other industries, one important way to understand value versus waste is through direct observation.11, 14 Although operating rooms have been the subject of several published process improvement projects to improve efficiency,1518 inpatient beds have not been the subject of such scrutiny. The objectives of this study were to generate a direct observation method and use it to describe pediatric ICU (PICU) bed utilization from a value‐added perspective.
METHODS
An interdisciplinary work group of physicians, nurses, quality improvement specialists, and 1 operations management expert developed an Excel spreadsheet to categorize hour‐by‐hour status of PICU beds. The clinicians generated a list of 27 activities. A critical care nurse trained in quality improvement piloted the list for 3 separate 4‐hour blocks over 2 weeks adding 18 activities; 2 additional activities were added during the 5 weeks of observation (Table 1). (The recording tool is provided in the Supporting Information Appendix.) Three observers with knowledge of medical terminology (2 third‐year medical students and 1 premedical student with years of experience as an emergency medical technician) were trained over 12 hours to conduct the observations. Prior to the observations, the 3 observers also spent time in the PICU, and terminology used for recordings was reviewed. Interobserver reliability was checked during 3 sets of observation circuits by all 3 observers and the principal investigator, as well as by spot checks during the study.
| Activity Description | Activity Code | Total Hours Over 5 Weeks | % Total Hours Over 5 Weeks* | Mean Hours per Week* |
|---|---|---|---|---|
| ||||
| Ventilated patient | Vent | 8996 | 45 | 1799 |
| CCSs not otherwise specified | NOS | 2982 | 15 | 596 |
| Neurosurgery patient with ICU needs | NeurosurgICU | 1534 | 8 | 307 |
| Room empty and unassigned | Empty‐unassigned | 1511 | 8 | 302 |
| Patient on continuous infusion | ContinInfus | 958 | 5 | 192 |
| Awaiting floor bed assignment | Floorbedassign | 919 | 5 | 184 |
| Patient with arterial line | ArtLine | 508 | 3 | 102 |
| Patient on high‐flow nasal cannula | HFNC | 475 | 2 | 95 |
| Room cleaning | EVS | 318 | 2 | 64 |
| Patient <12 hours after extubation | PostVent | 226 | 1 | 45 |
| Patient in OR, bed being held | OR | 210 | 1 | 42 |
| Neurosurgery patient, post‐ICU needs | NeurosurgPostICU | 164 | 0.8 | 33 |
| No clear ICU need, but no other accepting floor or service | Unclear | 163 | 0.8 | 33 |
| Patient at procedure, bed being held | Proced | 133 | 0.7 | 27 |
| Patient awaiting a rehabilitation bed | Rehab | 99 | 0.5 | 20 |
| Patient with ventriculostomy | Ventriculostomy | 82 | 0.4 | 16 |
| Patient eligible to be in NICU | NICU | 76 | 0.4 | 15 |
| Patient awaiting social work, case management, prescriptions before discharge | AwaitingOtherServ | 66 | 0.3 | 13 |
| Empty bed, assigned to ED patient | Empty‐ED | 40 | 0.2 | 8 |
| Empty bed, assigned to incoming transport patient | Empty‐Transport | 37 | 0.2 | 7 |
| Patient awaiting transport to another facility | Transport | 37 | 0.2 | 7 |
| Patient awaiting consult to determine transfer | Consult | 33 | 0.2 | 7 |
| Patient awaiting physician or NP sign‐out to floor before transfer | CallMDNP | 30 | 0.2 | 6 |
| PICU room needs a bed for next patient | Bed | 26 | 0.1 | 5 |
| Patient eligible to be in CCU | CCU | 24 | 0.1 | 5 |
| Patient eligible to be in CICU | CICU | 24 | 0.1 | 5 |
| Patient awaiting laboratory result to determine transfer or discharge | LabResult | 21 | 0.1 | 4 |
| Patient awaiting a ride home | Ride | 21 | 0.1 | 4 |
| Empty bed, assigned to floor patient | Empty‐floor | 19 | 0.1 | 4 |
| Patient awaiting nursing report to floor for transfer | Callnurse | 18 | 0.1 | 4 |
| Patient eligible to be in PCU | PCU | 18 | 0.1 | 4 |
| Patient on cardiac pressor | Pressor | 16 | 0.1 | 3 |
| Patient actively coding | Code | 15 | 0.1 | 3 |
| Patient on continuous veno‐venous hemofiltration | CVVH | 15 | 0.1 | 3 |
| Nursing work needed to enable transfer out | Nursing | 11 | 0.1 | 2 |
| Patient awaiting order for transfer to floor | Order | 11 | 0.1 | 2 |
| Patient in interventional radiology, bed being held | IR | 10 | 0.1 | 2 |
| Patient deceased in PICU room | Deceased | 9 | 0.1 | 2 |
| Awaiting radiology result to clear transfer or discharge | RadResult | 9 | 0.1 | 2 |
| Patient awaiting a floor bed to be cleaned for transfer out | Floorbedclean | 7 | <0.1 | 1 |
| Other logistical need for an empty room | Logistics | 7 | <0.1 | 1 |
| Disagreement among services for disposition | Disagreement | 4 | <0.1 | 1 |
| Family request to stay in PICU | Family | 3 | <0.1 | 1 |
| Awaiting accepting attending/fellow for transfer out | Accept | 1 | <0.1 | <1 |
| PICU room needs a crib for next patient | Crib | 1 | <0.1 | <1 |
| Patient with preventable reason for being in PICU | Prev | 0 | 0 | 0 |
| PICU room needs specialty bed for next patient | SpecialBed | 0 | 0 | 0 |
| Total | 19,887 | 100 | ||
The targeted area included 24 single‐patient rooms. The activity of each bed was recorded hourly. Real‐time recording in to the Excel spreadsheet on a dedicated laptop occurred from 8:00 AM until 11:00 PM. The most visible or critical event was recorded. Although some activities were not mutually exclusive (eg, a patient could be ventilated and on a continuous infusion simultaneously), the objective was to identify when a room was being used for any critical care service, not enumerate all of them. The observers noted overnight events that occurred from 11:00 PM to 8:00 AM in the morning by reviewing the bedside record and talking to the staff to complete each day's 24‐hour recording. The observers also recorded the hospital‐wide census and the census for the other half of the PICU every 4 hours. The observations occurred over 5 noncontiguous weeks between January 2009 and April 2009.
After all observations were complete, activities were classified as critical care services (CCS) or noncritical‐care services (NCCS). NCCSs were further divided into necessary logistics (defined for analysis purposes as the first hour of any NCCS activity) or nonvalue‐added (the second or greater hour of NCCS). A time limit of 1 hour was chosen to define necessary logistics based on a consensus that nonclinical activities optimally would not take more than 1 hour each. We also analyzed results with 2 hours as the cutoff for necessary logistics. Admission, discharge, and transfer records were reviewed to check for returns to the PICU or hospital within 48 hours of transfer or discharge from the PICU.
Analyses were conducted using Microsoft Excel (Microsoft, Redmond, WA) and Stata 10.0 (StataCorp, College Station, TX). The study was approved by the Children's Hospital of Philadelphia Institutional Review Board with waiver of consent.
RESULTS
A total of 824 hours of recordings included 19,887 bed‐hours with 219 unique patients; among them, 2 remained from the first day of recording in January to the last day in April (sample recording in Figure 1). A total of 50 patients (range, 812 per week) stayed for the entirety of each 1‐week observation period. Of the 47 possible activities, 45 of them were recorded for at least 1 hour in the 5 weeks. Overall, 14 activities accounted for 95% of the observed bed‐hours and 31 activities accounted for the remaining 5%. CCS accounted for 82% of observed bed‐hours, NCCS accounted for 10.4%, and empty unassigned accounted for 8% (Figure 2). Using the 1‐hour cutoff for necessary services, 77% of NCCS time was nonvalue‐added, whereas 23% of it was necessary logistics; using the 2‐hour cutoff, 54% was nonvalue‐added, and 46% was necessary logistics.
During the observation period, <1% of bed‐hours were used for CCS for overflow patients from the neonatal ICU (NICU), cardiac care unit (CCU), cardiac ICU (CICU), or progressive care unit (PCU; tracheostomy/ventilator unit). Although only 4 patients required transport to a rehabilitation facility, their wait time comprised 99 hours (<1%) of total recordings. Eight patients waited a mean of 2.6 hours for transportation home (maximum, 10 hours).
To demonstrate the cycle of room use, activities were divided into 4 categories: room preparation, critical care services, disposition pending, and postcritical care services (Figure 3). As an example of detailed data revealed by direct observation, we identified 102 instances totaling 919 hours when a patient was waiting for a bed assignment on another floor (5% of all bed‐hours). The mean wait time was 9 hours (range, 188 hours) and the median time was 5.5 hours. There were only 15 instances when floor bed assignment took 1 hour or less, and only 9 instances when it took 12 hours. Similarly, considerable time was spent on cleaning rooms between patients: only 66 of 146 instances of cleaning took 1 hour or less. The mean time for cleaning was 2.2 hours (range, 115), and the median was 2 hours. (There were 136 recorded instances of room cleaning and 10 additional episodes that were not recorded but had to be completed for the room to turnover from one patient to the next, yielding a total of 146 instances of cleaning.)
From the 824 hours of recording, we identified 200 hours (25% of time) when there were zero empty unassigned beds available in the section of the PICU being observed. Episodes of full occupancy occurred mostly on weekdays, with 23% of hours of full capacity on Thursdays, 21% on Mondays, and 21% on Wednesdays; only 8% were on Saturdays and <1% on Sundays. These 200 hours fell into 36 separate episodes of complete occupancy, each lasting 122 hours. Each patient, on average, received 3.1 hours of NCCS during each episode of full occupancy (range, 111 hours). Within these 200 hours at capacity, we identified only 15 hours (8%) when all 24 beds were used for CCS. For 72% of the time, there was at least 1 bed with NCCS, and for 37% at least 2 beds. A small portion of the time (7%), the lack of beds was affected by occupancy by patients who should have been in the NICU, CICU, CCU, or PCU.
Data collected through direct observation can be used to understand aggregated and averaged experiences, but also more specific time periods. For example, we identified 1 week with the highest consistent level of occupancy and turnover: March 915 had empty unassigned beds for only 4% of the week. Of the 168 hours in the week, 68 (40%) had full capacity. However, for 90% of the time, at least 1 bed was used for a NCCS. Other analytic options included varying the assumptions around time needed for logistics. Overall, NCCS time on necessary logistics changes from 23% to 46% using 1 hour versus 2 hours as the cutoff. For floor bed assignments, assuming that the first hour of this activity is necessary logistics and any hour thereafter is not, 817 hours were wasted. Even after assuming 2 hours of necessary logistical time (which may also include steps such as nursing and physician sign‐out to the receiving team, often not recorded in the observations), this left 715 hours of NCCS time in which patients waited to be placed elsewhere in the hospital. For room cleaning, because recordings were hourly, but room cleaning could take less time, we performed a sensitivity analysis, converting all 1‐hour recordings to half‐hour recordings to half‐hour recordings (an exaggerated shortening since industry‐standard cleaning may take longer).
Of the 219 patients directly observed, 15 were noted to be waiting for a transfer out of the PICU but experienced a change in disposition before the transfer. On average, these patients waited 8 hours for a floor bed assignment (range, 221) before reverting to a CCS, which then lasted an average of 16.5 hours (range, 149). (Included in this group are 2 patients who experienced this change in disposition twice.) In post hoc review across the 5 weeks, no patients were transferred back to the PICU within 48 hours after being transferred out. During the study period, 19 patients were discharged directly from the PICU (8 to home, 7 by transport to another facility, and 4 to rehabilitation). One patient returned to the hospital (but not the PICU) within 48 hours of being discharged home from the PICU.
During the study period, using the highest census value for recorded for each 24‐hour period and the number of beds available that day, median hospital‐wide occupancy was 93% (interquartile range, 90%96%). During the 35 days of observation, 71% of the days had occupancy >90%, 29% of days had occupancy >95%, and 3% of days had occupancy >100%.
DISCUSSION
In this direct observation of a PICU, we found high usage of beds for delivery of CCS. We identified many episodes in which the half of the PICU we observed was fully occupied (200 of 824 hours), but not necessarily delivering PICU‐level care to all patients. In fact, 75% of the full‐capacity hours had at least 1 patient receiving NCCS and 37% had at least 2. Patients waiting for a floor bed assignment represented nearly 5% of bed‐hours observed (mean 9 hours per patient). That full occupancy was not random, but rather clustered on weekdays, is consistent with other work showing that hospitals are at greater risk for midweek crowding due to the way in which scheduled admissions enter and leave.1925
Our methods provide the basis for operational analysis and improvement to patient flow, such as value stream mapping.9, 26 Process improvement work could be directed to areas of delay uncovered through this analysis and inform clinical and nonclinical management. For example, one of the key problems faced by the PICU was finding floor bed assignments for patients leaving the unit. Simply building more beds in the PICU will not solve this problemand at an estimated cost of $2 million to add a bed, it is likely not an efficient means of responding to poor flow. In these cases, the problem seems to lie downstream, and could suggest shortage of regular floor beds or inefficient bed assignment procedures within the hospital. The output also suggests that variation in nonclinical processes should be a target for improvement, such as time to clean rooms, because variation is known to be a source of nonvalue‐added time in many operations.9, 26 High occupancy on weekdays but low occupancy on weekends also emphasizes the potential for smoothing occupancy to reduce the risk of midweek crowding and to better manage bed utilization and staffing.24, 25
When seeking to reduce nonvalue‐added time, one must weigh the risks of increased efficiency against clinical outcomes. For example, if patients could be transferred out of the PICU faster, would the risk of returns to the PICU be higher? In this study, 15 patients (7%) had a change in disposition from awaiting transfer back to a CCS. The fact that transfers did not happen instantaneously may serve as a safety check to reduce rapid returns, but it is not possible for us to evaluate the reasons why patients did not actually complete the pending transfers. Specifically, we cannot determine whether the patient's clinical status objectively deteriorated, the ICU team made a judgment call to hold the patient, or the floor team refused to accept the transfer. Given this fact, although it appears in this study (and in the health care system more broadly) that there are opportunities to increase efficiency and reduce nonvalue‐added time, it is not realistic (nor advisable) that such time be reduced to zero. Along this line, one must consider separately purely nonclinical functions such as room cleaning and those that include some clinical element, such as time waiting for a patient to be transferred.
Beyond the direct findings of this study, the method should be replicable in other settings and can reveal important information about health care efficiency, capacity, and flexibility. The bottlenecks identified would have been difficult to identify through administrative record review. The exact amount of time to spend on observation may vary from place to place and would depend on the expected variation over time and the level of detail sought. In general, the more common the event and the less variation, the less time needed to observe it.
This study has several limitations that should be considered in terms of interpreting the results and in seeking to reproduce the approach. First, hourly recordings may not be discrete enough for events that took less than 1 hour. To assess the degree to which this would affect our results, we reanalyzed all NCCS by subtracting 30 minutes (0.5 hour) from all recordings, which increased total CCS from 82% to 87% and decreased NCCS by the same 5 percentage points. In a related fashion, our recordings were truncated at the start and end of each 1‐week period, so we could only observe a maximum of 168 hours for any given activity and did not record how long an activity was happening before or after the recordings started or stopped, respectively. Second, each recording could only be for 1 activity per hour. Separate from the level of granularity already noted, this also limits interpretation of critical care activities that may have been simultaneous. However, because the goal of the study was not to describe the provision of critical care services, but rather the times when they were not being delivered, this does not influence our conclusions. For movement of patients, however, we missed instances of physician and nursing calling sign‐out on patients to receiving units, as these events last less than 1 hour (and in the case of surgical patients, generally do not occur as the team provides continuous coverage). The time for such events is then included in other activities. To the extent that this may influence the results, it would increase the perceived time for nonvalue‐added services, but to a limited degree, and never by more than 59 minutes. Third, the overnight hours (11:00 PM to 8:00 AM) were not directly observed, but retrospectively recorded each morning by reviewing the records and discussing the overnight events with the clinical staff. For example, if a patient was intubated at 11:00 PM and at 8:00 AM, the observer would confirm this and record that status for the intervening hours. This is unlikely to result in a substantial impact on the findings, because the overnight hours have a relative degree of stability even for unstable patients in terms of their status of needing or not needing a CCS. Fourth, we did not evaluate the appropriateness of CCS delivered (eg, how long a patient was ventilated). Our definitions for CCS and NCSS were based on Children's Hospital of Philadelphia practices, which may not be the same as those of other facilities. The categorization of CCS was objective for activities such as ventilation or continuous infusion, but was less clear for the not otherwise specified recordings, which represented patients with a complex illness or projected organ, respiratory, cardiac, or neurological failure. These patients were not receiving a specific critical care intervention, but were deemed to need to be in the PICU as opposed to a regular floor (eg, for frequent monitoring of potential respiratory failure). It would also include patients receiving combinations of therapies more efficiently delivered in the PICU. For that, the observers relied on the judgment of clinicians (primarily nurses) to determine whether the patient needed to be in the PICU or not; if no specific reason could be provided, not otherwise specified was applied. These 192 instances accounted for 2982 aggregate bed‐hours (15% of total). It is difficult to judge the direction of bias, because overestimation of need to be in the PICU may be as likely to occur as underestimation. Fifth, the very presence of the observers may have changed behavior. Knowing that they were being observed staff may have acted with greater efficiency than otherwise. We expect that such a finding would lead to less time appearing as necessary logistics or NCCS. Finally, results may not be generalizable to other hospitals or hospital settings. There are clearly important contextual factors, not only for the location but also for the duration. For example, staffing was never an issue during the 5 weeks of observation, but there are locations where an empty bed is not necessarily usable due to lack of staffing. Nonetheless, we believe the results provide a generalizable approach and methodology for other settings (and staffing could be a reason for an empty bed).
In terms of the setting, as noted, we observed one discrete 24‐bed unit, which comprises half of the total PICU. Thus, statements that the PICU was at full capacity must be interpreted in the context that additional rooms may have been available on the other side. Patients are generally admitted alternately to each unit, so the occupancies should parallel each other. We recorded the census every 4 hours for both sides from the electronic system (Sunrise Clinical Manager [SCM]). However, this only accounts for patients physically in beds, not beds held for patients in other locations. Thus, we would expect a discrepancy between direct observation and the SCM value. Through analysis of the entire pediatric intensive care unit,* that part which observed directly, and that which we did not observe directly using census data, we think it reasonable to assert that both units of the total PICU had constrained capacity during the times we directly observed and recorded such constraint on one side.
This study demonstrates the use of direct observation for inpatient settings to learn about resource utilization and identification of value‐added services. PubMed searches for the terms efficiency, flow, process redesign, and time management bring up many more references for operating rooms than for ICUs or inpatient beds. Some examples of ICU‐directed work include videography of an ICU in Australia27 and human factor analysis in ICU nursing.5 Time‐motion studies have also been conducted on clinical staff, such as physicians.28, 29
In conclusion, we found that direct observation provided important insights into the utilization of patient rooms in an important inpatient setting. Data such as these are valuable for clinical and process improvement work, as well as understanding how best to match capacity to patient need. Finally, the methodology is reproducible for other settings and would be an additional tool to measuring and improving the efficiency and value of the health system. When appropriate, this approach can also evaluate the effectiveness of process improvement, help identify and reduce waste,13 and contribute to the growing field that merges operations management with hospital administration and clinical care: in other words, evidence‐based management.30
Acknowledgements
The authors thank Paula Agosto, Patricia Hubbs, Heidi Martin, and Annette Bollig for contributions to the study design.
In comparing direct observation to the SCM count, we found perfect concordance for 110 hours (55%) during which 0 beds were available. For the other 90 hours, SCM reported 1 bed being available in 46 hours (23%), 2 beds being available in 24 hours (12%), 3 beds being available in 17 hours (9%), and 4 beds being available in 3 hours (2%)all while we directly observed 0 beds being available. Thus, cumulatively, 90% of the hours observed with no beds had an SCM report availability of 02 beds; 99% of the time that was 03 beds. Applying this rate of mismatch to the unit that we did not observe directly, SCM reported 0 beds for 46 (23%) of the 200 hours the observation unit was full; SCM reported 1 bed available in 70 hours (35%), 2 beds open in 42 hours (21%), 3 beds open in 26 hours (13%), and 4 beds open in 16 hours (8%). Cumulatively, that is 79% of the time with 02 beds and 92% at 03 beds. From this, we conclude that the combined PICU for both sides was likely functionally full at least 158 of the 200 hours that the side we observed was full (79% 200 hours) and likely had very constrained capacity during the other 42 hours.
- ,,,,.The effect of hospital occupancy on emergency department length of stay and patient disposition.Acad Emerg Med.2003;10:127–133.
- ,,,.The effect of hospital bed occupancy on throughput in the pediatric emergency department.Ann Emerg Med.2009;53:767–776.
- ,,,.A Comparison of in‐hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza.Medical Care.2010;48:224–232.
- .Intensive care unit occupancy: making room for more patients.Crit Care Med.2009;37:1794–1795.
- ,.A human factors engineering conceptual framework of nursing workload and patient safety in intensive care units.Intensive Crit Care Nurs.2005;21:284–301.
- ,,, et al.High level of burnout in intensivists: prevalence and associated factors.Am J Respir Crit Care Med.2007;175:686–692.
- ,,.Length of stay and efficiency in pediatric intensive care units.J Pediatr.1998;133:79–85.
- ,.Variability in duration of stay in pediatric intensive care units: a multiinstitutional study.J Pediatr.1996;128:35–44.
- ,.Matching Supply with Demand: An Introduction to Operations Management.New York, NY:McGraw‐Hill;2006.
- ,.Impact of workload on service time and patient safety: an econometric analysis of hospital operations.Management Science.2009;55:1486–1498.
- .OPIM 631: Operations Management.Philadelphia, PA:Wharton School, University of Pennsylvania;2008.
- ,,.From waste to value in health care.JAMA.2008;299:568–571.
- .Eliminating “waste” in health care.JAMA.2009;302:2481–2482.
- .Toyota Production System: Beyond Large‐scale Production.London, UK:Productivity Press;1995.
- ,.Interdisciplinary work flow assessment and redesign decreases operating room turnover time and allows for additional caseload.Arch Surg.2006;141:65–69.
- ,,,.Improving operating room efficiency through process redesign.Surgery.2006;140:509–514.
- ,,,.Successful strategies for improving operating room efficiency at academic institutions.Anesth Analg.1998year="1998"1998;86:896–906.
- ,,.Efficiency of the operating room suite.Am J Surg.2003;185:244–250.
- ,,, et al.Children's hospitals do not acutely respond to high occupancy.Pediatrics.2010;125:974–981.
- Institute for Healthcare Improvement. Smoothing elective surgical admissions. Available at: http://www.ihi.org/IHI/Topics/Flow/Patient Flow/EmergingContent/SmoothingElectiveSurgicalAdmissions.htm. Accessed October 24,2008.
- Boston Hospital Sees Big Impact from Smoothing Elective Schedule.OR Manager. Volume 20, no. 12,2004.
- ,.Rethinking rapid response teams.JAMA.2010;304:1375–1376.
- Litvak E, ed.Managing Patient Flow in Hospitals: Strategies and Solutions.2nd ed.Oak Brook, IL:Joint Commission Resources;2009.
- ,,,,,.Scheduled admissions and high occupancy at a children's hospital.J Hosp Med.2011;6:81–87.
- ,,, et al.Addressing inpatient crowding by smoothing occupancy at children's hospitals.J Hosp Med.2011;6:466–473.
- ,.Learning to See: Value Stream Mapping to Add Value and Eliminate MUDA.Cambridge, MA:Lean Enterprise Institute;1999.
- ,,.Reshaping ICU ward round practices using video‐reflexive ethnography.Qual Health Res.2008;18:380–390.
- ,,.How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- ,,, et al.Where did the day go? A time‐motion study of hospitalists.J Hosp Med2010;5:323–238.
- ,,.Improving patient care by linking evidence‐based medicine and evidence‐based management.JAMA.2007;298:673–676.
Patient flow refers to the management and movement of patients in health care settings and is linked to quality, safety, and cost.16 The intensive care unit (ICU) is crucial in patient flow.7, 8 The limited number of beds and the resource‐intensive services and staffing associated with them require that hospitals optimize their utilization, as is increasingly true of all hospital resources. To maximize delivery of services to patients who need them and minimize real and opportunity losses (eg, postponed surgery, diverted transports, or inability to accept patients), patients in ICU beds should receive critical care medicine/nursing services while there and be transferred or discharged when appropriate.
The time between arrival and departure from any area of the hospital, including the ICU, is considered the time when a patient is receiving needed clinical carethe value‐added portion of health care operationsand time waiting to move on to the next step.911 This period includes both necessary logistics (eg, signing out a patient or waiting a reasonable amount of time for room cleaning) and nonvalue‐added time (eg, an excessively long amount of time for room cleaning). Operations management labels nonvalue‐added time as waste, and its reduction is vital for high‐quality health care.9, 12, 13 As in other industries, one important way to understand value versus waste is through direct observation.11, 14 Although operating rooms have been the subject of several published process improvement projects to improve efficiency,1518 inpatient beds have not been the subject of such scrutiny. The objectives of this study were to generate a direct observation method and use it to describe pediatric ICU (PICU) bed utilization from a value‐added perspective.
METHODS
An interdisciplinary work group of physicians, nurses, quality improvement specialists, and 1 operations management expert developed an Excel spreadsheet to categorize hour‐by‐hour status of PICU beds. The clinicians generated a list of 27 activities. A critical care nurse trained in quality improvement piloted the list for 3 separate 4‐hour blocks over 2 weeks adding 18 activities; 2 additional activities were added during the 5 weeks of observation (Table 1). (The recording tool is provided in the Supporting Information Appendix.) Three observers with knowledge of medical terminology (2 third‐year medical students and 1 premedical student with years of experience as an emergency medical technician) were trained over 12 hours to conduct the observations. Prior to the observations, the 3 observers also spent time in the PICU, and terminology used for recordings was reviewed. Interobserver reliability was checked during 3 sets of observation circuits by all 3 observers and the principal investigator, as well as by spot checks during the study.
| Activity Description | Activity Code | Total Hours Over 5 Weeks | % Total Hours Over 5 Weeks* | Mean Hours per Week* |
|---|---|---|---|---|
| ||||
| Ventilated patient | Vent | 8996 | 45 | 1799 |
| CCSs not otherwise specified | NOS | 2982 | 15 | 596 |
| Neurosurgery patient with ICU needs | NeurosurgICU | 1534 | 8 | 307 |
| Room empty and unassigned | Empty‐unassigned | 1511 | 8 | 302 |
| Patient on continuous infusion | ContinInfus | 958 | 5 | 192 |
| Awaiting floor bed assignment | Floorbedassign | 919 | 5 | 184 |
| Patient with arterial line | ArtLine | 508 | 3 | 102 |
| Patient on high‐flow nasal cannula | HFNC | 475 | 2 | 95 |
| Room cleaning | EVS | 318 | 2 | 64 |
| Patient <12 hours after extubation | PostVent | 226 | 1 | 45 |
| Patient in OR, bed being held | OR | 210 | 1 | 42 |
| Neurosurgery patient, post‐ICU needs | NeurosurgPostICU | 164 | 0.8 | 33 |
| No clear ICU need, but no other accepting floor or service | Unclear | 163 | 0.8 | 33 |
| Patient at procedure, bed being held | Proced | 133 | 0.7 | 27 |
| Patient awaiting a rehabilitation bed | Rehab | 99 | 0.5 | 20 |
| Patient with ventriculostomy | Ventriculostomy | 82 | 0.4 | 16 |
| Patient eligible to be in NICU | NICU | 76 | 0.4 | 15 |
| Patient awaiting social work, case management, prescriptions before discharge | AwaitingOtherServ | 66 | 0.3 | 13 |
| Empty bed, assigned to ED patient | Empty‐ED | 40 | 0.2 | 8 |
| Empty bed, assigned to incoming transport patient | Empty‐Transport | 37 | 0.2 | 7 |
| Patient awaiting transport to another facility | Transport | 37 | 0.2 | 7 |
| Patient awaiting consult to determine transfer | Consult | 33 | 0.2 | 7 |
| Patient awaiting physician or NP sign‐out to floor before transfer | CallMDNP | 30 | 0.2 | 6 |
| PICU room needs a bed for next patient | Bed | 26 | 0.1 | 5 |
| Patient eligible to be in CCU | CCU | 24 | 0.1 | 5 |
| Patient eligible to be in CICU | CICU | 24 | 0.1 | 5 |
| Patient awaiting laboratory result to determine transfer or discharge | LabResult | 21 | 0.1 | 4 |
| Patient awaiting a ride home | Ride | 21 | 0.1 | 4 |
| Empty bed, assigned to floor patient | Empty‐floor | 19 | 0.1 | 4 |
| Patient awaiting nursing report to floor for transfer | Callnurse | 18 | 0.1 | 4 |
| Patient eligible to be in PCU | PCU | 18 | 0.1 | 4 |
| Patient on cardiac pressor | Pressor | 16 | 0.1 | 3 |
| Patient actively coding | Code | 15 | 0.1 | 3 |
| Patient on continuous veno‐venous hemofiltration | CVVH | 15 | 0.1 | 3 |
| Nursing work needed to enable transfer out | Nursing | 11 | 0.1 | 2 |
| Patient awaiting order for transfer to floor | Order | 11 | 0.1 | 2 |
| Patient in interventional radiology, bed being held | IR | 10 | 0.1 | 2 |
| Patient deceased in PICU room | Deceased | 9 | 0.1 | 2 |
| Awaiting radiology result to clear transfer or discharge | RadResult | 9 | 0.1 | 2 |
| Patient awaiting a floor bed to be cleaned for transfer out | Floorbedclean | 7 | <0.1 | 1 |
| Other logistical need for an empty room | Logistics | 7 | <0.1 | 1 |
| Disagreement among services for disposition | Disagreement | 4 | <0.1 | 1 |
| Family request to stay in PICU | Family | 3 | <0.1 | 1 |
| Awaiting accepting attending/fellow for transfer out | Accept | 1 | <0.1 | <1 |
| PICU room needs a crib for next patient | Crib | 1 | <0.1 | <1 |
| Patient with preventable reason for being in PICU | Prev | 0 | 0 | 0 |
| PICU room needs specialty bed for next patient | SpecialBed | 0 | 0 | 0 |
| Total | 19,887 | 100 | ||
The targeted area included 24 single‐patient rooms. The activity of each bed was recorded hourly. Real‐time recording in to the Excel spreadsheet on a dedicated laptop occurred from 8:00 AM until 11:00 PM. The most visible or critical event was recorded. Although some activities were not mutually exclusive (eg, a patient could be ventilated and on a continuous infusion simultaneously), the objective was to identify when a room was being used for any critical care service, not enumerate all of them. The observers noted overnight events that occurred from 11:00 PM to 8:00 AM in the morning by reviewing the bedside record and talking to the staff to complete each day's 24‐hour recording. The observers also recorded the hospital‐wide census and the census for the other half of the PICU every 4 hours. The observations occurred over 5 noncontiguous weeks between January 2009 and April 2009.
After all observations were complete, activities were classified as critical care services (CCS) or noncritical‐care services (NCCS). NCCSs were further divided into necessary logistics (defined for analysis purposes as the first hour of any NCCS activity) or nonvalue‐added (the second or greater hour of NCCS). A time limit of 1 hour was chosen to define necessary logistics based on a consensus that nonclinical activities optimally would not take more than 1 hour each. We also analyzed results with 2 hours as the cutoff for necessary logistics. Admission, discharge, and transfer records were reviewed to check for returns to the PICU or hospital within 48 hours of transfer or discharge from the PICU.
Analyses were conducted using Microsoft Excel (Microsoft, Redmond, WA) and Stata 10.0 (StataCorp, College Station, TX). The study was approved by the Children's Hospital of Philadelphia Institutional Review Board with waiver of consent.
RESULTS
A total of 824 hours of recordings included 19,887 bed‐hours with 219 unique patients; among them, 2 remained from the first day of recording in January to the last day in April (sample recording in Figure 1). A total of 50 patients (range, 812 per week) stayed for the entirety of each 1‐week observation period. Of the 47 possible activities, 45 of them were recorded for at least 1 hour in the 5 weeks. Overall, 14 activities accounted for 95% of the observed bed‐hours and 31 activities accounted for the remaining 5%. CCS accounted for 82% of observed bed‐hours, NCCS accounted for 10.4%, and empty unassigned accounted for 8% (Figure 2). Using the 1‐hour cutoff for necessary services, 77% of NCCS time was nonvalue‐added, whereas 23% of it was necessary logistics; using the 2‐hour cutoff, 54% was nonvalue‐added, and 46% was necessary logistics.


During the observation period, <1% of bed‐hours were used for CCS for overflow patients from the neonatal ICU (NICU), cardiac care unit (CCU), cardiac ICU (CICU), or progressive care unit (PCU; tracheostomy/ventilator unit). Although only 4 patients required transport to a rehabilitation facility, their wait time comprised 99 hours (<1%) of total recordings. Eight patients waited a mean of 2.6 hours for transportation home (maximum, 10 hours).
To demonstrate the cycle of room use, activities were divided into 4 categories: room preparation, critical care services, disposition pending, and postcritical care services (Figure 3). As an example of detailed data revealed by direct observation, we identified 102 instances totaling 919 hours when a patient was waiting for a bed assignment on another floor (5% of all bed‐hours). The mean wait time was 9 hours (range, 188 hours) and the median time was 5.5 hours. There were only 15 instances when floor bed assignment took 1 hour or less, and only 9 instances when it took 12 hours. Similarly, considerable time was spent on cleaning rooms between patients: only 66 of 146 instances of cleaning took 1 hour or less. The mean time for cleaning was 2.2 hours (range, 115), and the median was 2 hours. (There were 136 recorded instances of room cleaning and 10 additional episodes that were not recorded but had to be completed for the room to turnover from one patient to the next, yielding a total of 146 instances of cleaning.)

From the 824 hours of recording, we identified 200 hours (25% of time) when there were zero empty unassigned beds available in the section of the PICU being observed. Episodes of full occupancy occurred mostly on weekdays, with 23% of hours of full capacity on Thursdays, 21% on Mondays, and 21% on Wednesdays; only 8% were on Saturdays and <1% on Sundays. These 200 hours fell into 36 separate episodes of complete occupancy, each lasting 122 hours. Each patient, on average, received 3.1 hours of NCCS during each episode of full occupancy (range, 111 hours). Within these 200 hours at capacity, we identified only 15 hours (8%) when all 24 beds were used for CCS. For 72% of the time, there was at least 1 bed with NCCS, and for 37% at least 2 beds. A small portion of the time (7%), the lack of beds was affected by occupancy by patients who should have been in the NICU, CICU, CCU, or PCU.
Data collected through direct observation can be used to understand aggregated and averaged experiences, but also more specific time periods. For example, we identified 1 week with the highest consistent level of occupancy and turnover: March 915 had empty unassigned beds for only 4% of the week. Of the 168 hours in the week, 68 (40%) had full capacity. However, for 90% of the time, at least 1 bed was used for a NCCS. Other analytic options included varying the assumptions around time needed for logistics. Overall, NCCS time on necessary logistics changes from 23% to 46% using 1 hour versus 2 hours as the cutoff. For floor bed assignments, assuming that the first hour of this activity is necessary logistics and any hour thereafter is not, 817 hours were wasted. Even after assuming 2 hours of necessary logistical time (which may also include steps such as nursing and physician sign‐out to the receiving team, often not recorded in the observations), this left 715 hours of NCCS time in which patients waited to be placed elsewhere in the hospital. For room cleaning, because recordings were hourly, but room cleaning could take less time, we performed a sensitivity analysis, converting all 1‐hour recordings to half‐hour recordings to half‐hour recordings (an exaggerated shortening since industry‐standard cleaning may take longer).
Of the 219 patients directly observed, 15 were noted to be waiting for a transfer out of the PICU but experienced a change in disposition before the transfer. On average, these patients waited 8 hours for a floor bed assignment (range, 221) before reverting to a CCS, which then lasted an average of 16.5 hours (range, 149). (Included in this group are 2 patients who experienced this change in disposition twice.) In post hoc review across the 5 weeks, no patients were transferred back to the PICU within 48 hours after being transferred out. During the study period, 19 patients were discharged directly from the PICU (8 to home, 7 by transport to another facility, and 4 to rehabilitation). One patient returned to the hospital (but not the PICU) within 48 hours of being discharged home from the PICU.
During the study period, using the highest census value for recorded for each 24‐hour period and the number of beds available that day, median hospital‐wide occupancy was 93% (interquartile range, 90%96%). During the 35 days of observation, 71% of the days had occupancy >90%, 29% of days had occupancy >95%, and 3% of days had occupancy >100%.
DISCUSSION
In this direct observation of a PICU, we found high usage of beds for delivery of CCS. We identified many episodes in which the half of the PICU we observed was fully occupied (200 of 824 hours), but not necessarily delivering PICU‐level care to all patients. In fact, 75% of the full‐capacity hours had at least 1 patient receiving NCCS and 37% had at least 2. Patients waiting for a floor bed assignment represented nearly 5% of bed‐hours observed (mean 9 hours per patient). That full occupancy was not random, but rather clustered on weekdays, is consistent with other work showing that hospitals are at greater risk for midweek crowding due to the way in which scheduled admissions enter and leave.1925
Our methods provide the basis for operational analysis and improvement to patient flow, such as value stream mapping.9, 26 Process improvement work could be directed to areas of delay uncovered through this analysis and inform clinical and nonclinical management. For example, one of the key problems faced by the PICU was finding floor bed assignments for patients leaving the unit. Simply building more beds in the PICU will not solve this problemand at an estimated cost of $2 million to add a bed, it is likely not an efficient means of responding to poor flow. In these cases, the problem seems to lie downstream, and could suggest shortage of regular floor beds or inefficient bed assignment procedures within the hospital. The output also suggests that variation in nonclinical processes should be a target for improvement, such as time to clean rooms, because variation is known to be a source of nonvalue‐added time in many operations.9, 26 High occupancy on weekdays but low occupancy on weekends also emphasizes the potential for smoothing occupancy to reduce the risk of midweek crowding and to better manage bed utilization and staffing.24, 25
When seeking to reduce nonvalue‐added time, one must weigh the risks of increased efficiency against clinical outcomes. For example, if patients could be transferred out of the PICU faster, would the risk of returns to the PICU be higher? In this study, 15 patients (7%) had a change in disposition from awaiting transfer back to a CCS. The fact that transfers did not happen instantaneously may serve as a safety check to reduce rapid returns, but it is not possible for us to evaluate the reasons why patients did not actually complete the pending transfers. Specifically, we cannot determine whether the patient's clinical status objectively deteriorated, the ICU team made a judgment call to hold the patient, or the floor team refused to accept the transfer. Given this fact, although it appears in this study (and in the health care system more broadly) that there are opportunities to increase efficiency and reduce nonvalue‐added time, it is not realistic (nor advisable) that such time be reduced to zero. Along this line, one must consider separately purely nonclinical functions such as room cleaning and those that include some clinical element, such as time waiting for a patient to be transferred.
Beyond the direct findings of this study, the method should be replicable in other settings and can reveal important information about health care efficiency, capacity, and flexibility. The bottlenecks identified would have been difficult to identify through administrative record review. The exact amount of time to spend on observation may vary from place to place and would depend on the expected variation over time and the level of detail sought. In general, the more common the event and the less variation, the less time needed to observe it.
This study has several limitations that should be considered in terms of interpreting the results and in seeking to reproduce the approach. First, hourly recordings may not be discrete enough for events that took less than 1 hour. To assess the degree to which this would affect our results, we reanalyzed all NCCS by subtracting 30 minutes (0.5 hour) from all recordings, which increased total CCS from 82% to 87% and decreased NCCS by the same 5 percentage points. In a related fashion, our recordings were truncated at the start and end of each 1‐week period, so we could only observe a maximum of 168 hours for any given activity and did not record how long an activity was happening before or after the recordings started or stopped, respectively. Second, each recording could only be for 1 activity per hour. Separate from the level of granularity already noted, this also limits interpretation of critical care activities that may have been simultaneous. However, because the goal of the study was not to describe the provision of critical care services, but rather the times when they were not being delivered, this does not influence our conclusions. For movement of patients, however, we missed instances of physician and nursing calling sign‐out on patients to receiving units, as these events last less than 1 hour (and in the case of surgical patients, generally do not occur as the team provides continuous coverage). The time for such events is then included in other activities. To the extent that this may influence the results, it would increase the perceived time for nonvalue‐added services, but to a limited degree, and never by more than 59 minutes. Third, the overnight hours (11:00 PM to 8:00 AM) were not directly observed, but retrospectively recorded each morning by reviewing the records and discussing the overnight events with the clinical staff. For example, if a patient was intubated at 11:00 PM and at 8:00 AM, the observer would confirm this and record that status for the intervening hours. This is unlikely to result in a substantial impact on the findings, because the overnight hours have a relative degree of stability even for unstable patients in terms of their status of needing or not needing a CCS. Fourth, we did not evaluate the appropriateness of CCS delivered (eg, how long a patient was ventilated). Our definitions for CCS and NCSS were based on Children's Hospital of Philadelphia practices, which may not be the same as those of other facilities. The categorization of CCS was objective for activities such as ventilation or continuous infusion, but was less clear for the not otherwise specified recordings, which represented patients with a complex illness or projected organ, respiratory, cardiac, or neurological failure. These patients were not receiving a specific critical care intervention, but were deemed to need to be in the PICU as opposed to a regular floor (eg, for frequent monitoring of potential respiratory failure). It would also include patients receiving combinations of therapies more efficiently delivered in the PICU. For that, the observers relied on the judgment of clinicians (primarily nurses) to determine whether the patient needed to be in the PICU or not; if no specific reason could be provided, not otherwise specified was applied. These 192 instances accounted for 2982 aggregate bed‐hours (15% of total). It is difficult to judge the direction of bias, because overestimation of need to be in the PICU may be as likely to occur as underestimation. Fifth, the very presence of the observers may have changed behavior. Knowing that they were being observed staff may have acted with greater efficiency than otherwise. We expect that such a finding would lead to less time appearing as necessary logistics or NCCS. Finally, results may not be generalizable to other hospitals or hospital settings. There are clearly important contextual factors, not only for the location but also for the duration. For example, staffing was never an issue during the 5 weeks of observation, but there are locations where an empty bed is not necessarily usable due to lack of staffing. Nonetheless, we believe the results provide a generalizable approach and methodology for other settings (and staffing could be a reason for an empty bed).
In terms of the setting, as noted, we observed one discrete 24‐bed unit, which comprises half of the total PICU. Thus, statements that the PICU was at full capacity must be interpreted in the context that additional rooms may have been available on the other side. Patients are generally admitted alternately to each unit, so the occupancies should parallel each other. We recorded the census every 4 hours for both sides from the electronic system (Sunrise Clinical Manager [SCM]). However, this only accounts for patients physically in beds, not beds held for patients in other locations. Thus, we would expect a discrepancy between direct observation and the SCM value. Through analysis of the entire pediatric intensive care unit,* that part which observed directly, and that which we did not observe directly using census data, we think it reasonable to assert that both units of the total PICU had constrained capacity during the times we directly observed and recorded such constraint on one side.
This study demonstrates the use of direct observation for inpatient settings to learn about resource utilization and identification of value‐added services. PubMed searches for the terms efficiency, flow, process redesign, and time management bring up many more references for operating rooms than for ICUs or inpatient beds. Some examples of ICU‐directed work include videography of an ICU in Australia27 and human factor analysis in ICU nursing.5 Time‐motion studies have also been conducted on clinical staff, such as physicians.28, 29
In conclusion, we found that direct observation provided important insights into the utilization of patient rooms in an important inpatient setting. Data such as these are valuable for clinical and process improvement work, as well as understanding how best to match capacity to patient need. Finally, the methodology is reproducible for other settings and would be an additional tool to measuring and improving the efficiency and value of the health system. When appropriate, this approach can also evaluate the effectiveness of process improvement, help identify and reduce waste,13 and contribute to the growing field that merges operations management with hospital administration and clinical care: in other words, evidence‐based management.30
Acknowledgements
The authors thank Paula Agosto, Patricia Hubbs, Heidi Martin, and Annette Bollig for contributions to the study design.
In comparing direct observation to the SCM count, we found perfect concordance for 110 hours (55%) during which 0 beds were available. For the other 90 hours, SCM reported 1 bed being available in 46 hours (23%), 2 beds being available in 24 hours (12%), 3 beds being available in 17 hours (9%), and 4 beds being available in 3 hours (2%)all while we directly observed 0 beds being available. Thus, cumulatively, 90% of the hours observed with no beds had an SCM report availability of 02 beds; 99% of the time that was 03 beds. Applying this rate of mismatch to the unit that we did not observe directly, SCM reported 0 beds for 46 (23%) of the 200 hours the observation unit was full; SCM reported 1 bed available in 70 hours (35%), 2 beds open in 42 hours (21%), 3 beds open in 26 hours (13%), and 4 beds open in 16 hours (8%). Cumulatively, that is 79% of the time with 02 beds and 92% at 03 beds. From this, we conclude that the combined PICU for both sides was likely functionally full at least 158 of the 200 hours that the side we observed was full (79% 200 hours) and likely had very constrained capacity during the other 42 hours.
Patient flow refers to the management and movement of patients in health care settings and is linked to quality, safety, and cost.16 The intensive care unit (ICU) is crucial in patient flow.7, 8 The limited number of beds and the resource‐intensive services and staffing associated with them require that hospitals optimize their utilization, as is increasingly true of all hospital resources. To maximize delivery of services to patients who need them and minimize real and opportunity losses (eg, postponed surgery, diverted transports, or inability to accept patients), patients in ICU beds should receive critical care medicine/nursing services while there and be transferred or discharged when appropriate.
The time between arrival and departure from any area of the hospital, including the ICU, is considered the time when a patient is receiving needed clinical carethe value‐added portion of health care operationsand time waiting to move on to the next step.911 This period includes both necessary logistics (eg, signing out a patient or waiting a reasonable amount of time for room cleaning) and nonvalue‐added time (eg, an excessively long amount of time for room cleaning). Operations management labels nonvalue‐added time as waste, and its reduction is vital for high‐quality health care.9, 12, 13 As in other industries, one important way to understand value versus waste is through direct observation.11, 14 Although operating rooms have been the subject of several published process improvement projects to improve efficiency,1518 inpatient beds have not been the subject of such scrutiny. The objectives of this study were to generate a direct observation method and use it to describe pediatric ICU (PICU) bed utilization from a value‐added perspective.
METHODS
An interdisciplinary work group of physicians, nurses, quality improvement specialists, and 1 operations management expert developed an Excel spreadsheet to categorize hour‐by‐hour status of PICU beds. The clinicians generated a list of 27 activities. A critical care nurse trained in quality improvement piloted the list for 3 separate 4‐hour blocks over 2 weeks adding 18 activities; 2 additional activities were added during the 5 weeks of observation (Table 1). (The recording tool is provided in the Supporting Information Appendix.) Three observers with knowledge of medical terminology (2 third‐year medical students and 1 premedical student with years of experience as an emergency medical technician) were trained over 12 hours to conduct the observations. Prior to the observations, the 3 observers also spent time in the PICU, and terminology used for recordings was reviewed. Interobserver reliability was checked during 3 sets of observation circuits by all 3 observers and the principal investigator, as well as by spot checks during the study.
| Activity Description | Activity Code | Total Hours Over 5 Weeks | % Total Hours Over 5 Weeks* | Mean Hours per Week* |
|---|---|---|---|---|
| ||||
| Ventilated patient | Vent | 8996 | 45 | 1799 |
| CCSs not otherwise specified | NOS | 2982 | 15 | 596 |
| Neurosurgery patient with ICU needs | NeurosurgICU | 1534 | 8 | 307 |
| Room empty and unassigned | Empty‐unassigned | 1511 | 8 | 302 |
| Patient on continuous infusion | ContinInfus | 958 | 5 | 192 |
| Awaiting floor bed assignment | Floorbedassign | 919 | 5 | 184 |
| Patient with arterial line | ArtLine | 508 | 3 | 102 |
| Patient on high‐flow nasal cannula | HFNC | 475 | 2 | 95 |
| Room cleaning | EVS | 318 | 2 | 64 |
| Patient <12 hours after extubation | PostVent | 226 | 1 | 45 |
| Patient in OR, bed being held | OR | 210 | 1 | 42 |
| Neurosurgery patient, post‐ICU needs | NeurosurgPostICU | 164 | 0.8 | 33 |
| No clear ICU need, but no other accepting floor or service | Unclear | 163 | 0.8 | 33 |
| Patient at procedure, bed being held | Proced | 133 | 0.7 | 27 |
| Patient awaiting a rehabilitation bed | Rehab | 99 | 0.5 | 20 |
| Patient with ventriculostomy | Ventriculostomy | 82 | 0.4 | 16 |
| Patient eligible to be in NICU | NICU | 76 | 0.4 | 15 |
| Patient awaiting social work, case management, prescriptions before discharge | AwaitingOtherServ | 66 | 0.3 | 13 |
| Empty bed, assigned to ED patient | Empty‐ED | 40 | 0.2 | 8 |
| Empty bed, assigned to incoming transport patient | Empty‐Transport | 37 | 0.2 | 7 |
| Patient awaiting transport to another facility | Transport | 37 | 0.2 | 7 |
| Patient awaiting consult to determine transfer | Consult | 33 | 0.2 | 7 |
| Patient awaiting physician or NP sign‐out to floor before transfer | CallMDNP | 30 | 0.2 | 6 |
| PICU room needs a bed for next patient | Bed | 26 | 0.1 | 5 |
| Patient eligible to be in CCU | CCU | 24 | 0.1 | 5 |
| Patient eligible to be in CICU | CICU | 24 | 0.1 | 5 |
| Patient awaiting laboratory result to determine transfer or discharge | LabResult | 21 | 0.1 | 4 |
| Patient awaiting a ride home | Ride | 21 | 0.1 | 4 |
| Empty bed, assigned to floor patient | Empty‐floor | 19 | 0.1 | 4 |
| Patient awaiting nursing report to floor for transfer | Callnurse | 18 | 0.1 | 4 |
| Patient eligible to be in PCU | PCU | 18 | 0.1 | 4 |
| Patient on cardiac pressor | Pressor | 16 | 0.1 | 3 |
| Patient actively coding | Code | 15 | 0.1 | 3 |
| Patient on continuous veno‐venous hemofiltration | CVVH | 15 | 0.1 | 3 |
| Nursing work needed to enable transfer out | Nursing | 11 | 0.1 | 2 |
| Patient awaiting order for transfer to floor | Order | 11 | 0.1 | 2 |
| Patient in interventional radiology, bed being held | IR | 10 | 0.1 | 2 |
| Patient deceased in PICU room | Deceased | 9 | 0.1 | 2 |
| Awaiting radiology result to clear transfer or discharge | RadResult | 9 | 0.1 | 2 |
| Patient awaiting a floor bed to be cleaned for transfer out | Floorbedclean | 7 | <0.1 | 1 |
| Other logistical need for an empty room | Logistics | 7 | <0.1 | 1 |
| Disagreement among services for disposition | Disagreement | 4 | <0.1 | 1 |
| Family request to stay in PICU | Family | 3 | <0.1 | 1 |
| Awaiting accepting attending/fellow for transfer out | Accept | 1 | <0.1 | <1 |
| PICU room needs a crib for next patient | Crib | 1 | <0.1 | <1 |
| Patient with preventable reason for being in PICU | Prev | 0 | 0 | 0 |
| PICU room needs specialty bed for next patient | SpecialBed | 0 | 0 | 0 |
| Total | 19,887 | 100 | ||
The targeted area included 24 single‐patient rooms. The activity of each bed was recorded hourly. Real‐time recording in to the Excel spreadsheet on a dedicated laptop occurred from 8:00 AM until 11:00 PM. The most visible or critical event was recorded. Although some activities were not mutually exclusive (eg, a patient could be ventilated and on a continuous infusion simultaneously), the objective was to identify when a room was being used for any critical care service, not enumerate all of them. The observers noted overnight events that occurred from 11:00 PM to 8:00 AM in the morning by reviewing the bedside record and talking to the staff to complete each day's 24‐hour recording. The observers also recorded the hospital‐wide census and the census for the other half of the PICU every 4 hours. The observations occurred over 5 noncontiguous weeks between January 2009 and April 2009.
After all observations were complete, activities were classified as critical care services (CCS) or noncritical‐care services (NCCS). NCCSs were further divided into necessary logistics (defined for analysis purposes as the first hour of any NCCS activity) or nonvalue‐added (the second or greater hour of NCCS). A time limit of 1 hour was chosen to define necessary logistics based on a consensus that nonclinical activities optimally would not take more than 1 hour each. We also analyzed results with 2 hours as the cutoff for necessary logistics. Admission, discharge, and transfer records were reviewed to check for returns to the PICU or hospital within 48 hours of transfer or discharge from the PICU.
Analyses were conducted using Microsoft Excel (Microsoft, Redmond, WA) and Stata 10.0 (StataCorp, College Station, TX). The study was approved by the Children's Hospital of Philadelphia Institutional Review Board with waiver of consent.
RESULTS
A total of 824 hours of recordings included 19,887 bed‐hours with 219 unique patients; among them, 2 remained from the first day of recording in January to the last day in April (sample recording in Figure 1). A total of 50 patients (range, 812 per week) stayed for the entirety of each 1‐week observation period. Of the 47 possible activities, 45 of them were recorded for at least 1 hour in the 5 weeks. Overall, 14 activities accounted for 95% of the observed bed‐hours and 31 activities accounted for the remaining 5%. CCS accounted for 82% of observed bed‐hours, NCCS accounted for 10.4%, and empty unassigned accounted for 8% (Figure 2). Using the 1‐hour cutoff for necessary services, 77% of NCCS time was nonvalue‐added, whereas 23% of it was necessary logistics; using the 2‐hour cutoff, 54% was nonvalue‐added, and 46% was necessary logistics.


During the observation period, <1% of bed‐hours were used for CCS for overflow patients from the neonatal ICU (NICU), cardiac care unit (CCU), cardiac ICU (CICU), or progressive care unit (PCU; tracheostomy/ventilator unit). Although only 4 patients required transport to a rehabilitation facility, their wait time comprised 99 hours (<1%) of total recordings. Eight patients waited a mean of 2.6 hours for transportation home (maximum, 10 hours).
To demonstrate the cycle of room use, activities were divided into 4 categories: room preparation, critical care services, disposition pending, and postcritical care services (Figure 3). As an example of detailed data revealed by direct observation, we identified 102 instances totaling 919 hours when a patient was waiting for a bed assignment on another floor (5% of all bed‐hours). The mean wait time was 9 hours (range, 188 hours) and the median time was 5.5 hours. There were only 15 instances when floor bed assignment took 1 hour or less, and only 9 instances when it took 12 hours. Similarly, considerable time was spent on cleaning rooms between patients: only 66 of 146 instances of cleaning took 1 hour or less. The mean time for cleaning was 2.2 hours (range, 115), and the median was 2 hours. (There were 136 recorded instances of room cleaning and 10 additional episodes that were not recorded but had to be completed for the room to turnover from one patient to the next, yielding a total of 146 instances of cleaning.)

From the 824 hours of recording, we identified 200 hours (25% of time) when there were zero empty unassigned beds available in the section of the PICU being observed. Episodes of full occupancy occurred mostly on weekdays, with 23% of hours of full capacity on Thursdays, 21% on Mondays, and 21% on Wednesdays; only 8% were on Saturdays and <1% on Sundays. These 200 hours fell into 36 separate episodes of complete occupancy, each lasting 122 hours. Each patient, on average, received 3.1 hours of NCCS during each episode of full occupancy (range, 111 hours). Within these 200 hours at capacity, we identified only 15 hours (8%) when all 24 beds were used for CCS. For 72% of the time, there was at least 1 bed with NCCS, and for 37% at least 2 beds. A small portion of the time (7%), the lack of beds was affected by occupancy by patients who should have been in the NICU, CICU, CCU, or PCU.
Data collected through direct observation can be used to understand aggregated and averaged experiences, but also more specific time periods. For example, we identified 1 week with the highest consistent level of occupancy and turnover: March 915 had empty unassigned beds for only 4% of the week. Of the 168 hours in the week, 68 (40%) had full capacity. However, for 90% of the time, at least 1 bed was used for a NCCS. Other analytic options included varying the assumptions around time needed for logistics. Overall, NCCS time on necessary logistics changes from 23% to 46% using 1 hour versus 2 hours as the cutoff. For floor bed assignments, assuming that the first hour of this activity is necessary logistics and any hour thereafter is not, 817 hours were wasted. Even after assuming 2 hours of necessary logistical time (which may also include steps such as nursing and physician sign‐out to the receiving team, often not recorded in the observations), this left 715 hours of NCCS time in which patients waited to be placed elsewhere in the hospital. For room cleaning, because recordings were hourly, but room cleaning could take less time, we performed a sensitivity analysis, converting all 1‐hour recordings to half‐hour recordings to half‐hour recordings (an exaggerated shortening since industry‐standard cleaning may take longer).
Of the 219 patients directly observed, 15 were noted to be waiting for a transfer out of the PICU but experienced a change in disposition before the transfer. On average, these patients waited 8 hours for a floor bed assignment (range, 221) before reverting to a CCS, which then lasted an average of 16.5 hours (range, 149). (Included in this group are 2 patients who experienced this change in disposition twice.) In post hoc review across the 5 weeks, no patients were transferred back to the PICU within 48 hours after being transferred out. During the study period, 19 patients were discharged directly from the PICU (8 to home, 7 by transport to another facility, and 4 to rehabilitation). One patient returned to the hospital (but not the PICU) within 48 hours of being discharged home from the PICU.
During the study period, using the highest census value for recorded for each 24‐hour period and the number of beds available that day, median hospital‐wide occupancy was 93% (interquartile range, 90%96%). During the 35 days of observation, 71% of the days had occupancy >90%, 29% of days had occupancy >95%, and 3% of days had occupancy >100%.
DISCUSSION
In this direct observation of a PICU, we found high usage of beds for delivery of CCS. We identified many episodes in which the half of the PICU we observed was fully occupied (200 of 824 hours), but not necessarily delivering PICU‐level care to all patients. In fact, 75% of the full‐capacity hours had at least 1 patient receiving NCCS and 37% had at least 2. Patients waiting for a floor bed assignment represented nearly 5% of bed‐hours observed (mean 9 hours per patient). That full occupancy was not random, but rather clustered on weekdays, is consistent with other work showing that hospitals are at greater risk for midweek crowding due to the way in which scheduled admissions enter and leave.1925
Our methods provide the basis for operational analysis and improvement to patient flow, such as value stream mapping.9, 26 Process improvement work could be directed to areas of delay uncovered through this analysis and inform clinical and nonclinical management. For example, one of the key problems faced by the PICU was finding floor bed assignments for patients leaving the unit. Simply building more beds in the PICU will not solve this problemand at an estimated cost of $2 million to add a bed, it is likely not an efficient means of responding to poor flow. In these cases, the problem seems to lie downstream, and could suggest shortage of regular floor beds or inefficient bed assignment procedures within the hospital. The output also suggests that variation in nonclinical processes should be a target for improvement, such as time to clean rooms, because variation is known to be a source of nonvalue‐added time in many operations.9, 26 High occupancy on weekdays but low occupancy on weekends also emphasizes the potential for smoothing occupancy to reduce the risk of midweek crowding and to better manage bed utilization and staffing.24, 25
When seeking to reduce nonvalue‐added time, one must weigh the risks of increased efficiency against clinical outcomes. For example, if patients could be transferred out of the PICU faster, would the risk of returns to the PICU be higher? In this study, 15 patients (7%) had a change in disposition from awaiting transfer back to a CCS. The fact that transfers did not happen instantaneously may serve as a safety check to reduce rapid returns, but it is not possible for us to evaluate the reasons why patients did not actually complete the pending transfers. Specifically, we cannot determine whether the patient's clinical status objectively deteriorated, the ICU team made a judgment call to hold the patient, or the floor team refused to accept the transfer. Given this fact, although it appears in this study (and in the health care system more broadly) that there are opportunities to increase efficiency and reduce nonvalue‐added time, it is not realistic (nor advisable) that such time be reduced to zero. Along this line, one must consider separately purely nonclinical functions such as room cleaning and those that include some clinical element, such as time waiting for a patient to be transferred.
Beyond the direct findings of this study, the method should be replicable in other settings and can reveal important information about health care efficiency, capacity, and flexibility. The bottlenecks identified would have been difficult to identify through administrative record review. The exact amount of time to spend on observation may vary from place to place and would depend on the expected variation over time and the level of detail sought. In general, the more common the event and the less variation, the less time needed to observe it.
This study has several limitations that should be considered in terms of interpreting the results and in seeking to reproduce the approach. First, hourly recordings may not be discrete enough for events that took less than 1 hour. To assess the degree to which this would affect our results, we reanalyzed all NCCS by subtracting 30 minutes (0.5 hour) from all recordings, which increased total CCS from 82% to 87% and decreased NCCS by the same 5 percentage points. In a related fashion, our recordings were truncated at the start and end of each 1‐week period, so we could only observe a maximum of 168 hours for any given activity and did not record how long an activity was happening before or after the recordings started or stopped, respectively. Second, each recording could only be for 1 activity per hour. Separate from the level of granularity already noted, this also limits interpretation of critical care activities that may have been simultaneous. However, because the goal of the study was not to describe the provision of critical care services, but rather the times when they were not being delivered, this does not influence our conclusions. For movement of patients, however, we missed instances of physician and nursing calling sign‐out on patients to receiving units, as these events last less than 1 hour (and in the case of surgical patients, generally do not occur as the team provides continuous coverage). The time for such events is then included in other activities. To the extent that this may influence the results, it would increase the perceived time for nonvalue‐added services, but to a limited degree, and never by more than 59 minutes. Third, the overnight hours (11:00 PM to 8:00 AM) were not directly observed, but retrospectively recorded each morning by reviewing the records and discussing the overnight events with the clinical staff. For example, if a patient was intubated at 11:00 PM and at 8:00 AM, the observer would confirm this and record that status for the intervening hours. This is unlikely to result in a substantial impact on the findings, because the overnight hours have a relative degree of stability even for unstable patients in terms of their status of needing or not needing a CCS. Fourth, we did not evaluate the appropriateness of CCS delivered (eg, how long a patient was ventilated). Our definitions for CCS and NCSS were based on Children's Hospital of Philadelphia practices, which may not be the same as those of other facilities. The categorization of CCS was objective for activities such as ventilation or continuous infusion, but was less clear for the not otherwise specified recordings, which represented patients with a complex illness or projected organ, respiratory, cardiac, or neurological failure. These patients were not receiving a specific critical care intervention, but were deemed to need to be in the PICU as opposed to a regular floor (eg, for frequent monitoring of potential respiratory failure). It would also include patients receiving combinations of therapies more efficiently delivered in the PICU. For that, the observers relied on the judgment of clinicians (primarily nurses) to determine whether the patient needed to be in the PICU or not; if no specific reason could be provided, not otherwise specified was applied. These 192 instances accounted for 2982 aggregate bed‐hours (15% of total). It is difficult to judge the direction of bias, because overestimation of need to be in the PICU may be as likely to occur as underestimation. Fifth, the very presence of the observers may have changed behavior. Knowing that they were being observed staff may have acted with greater efficiency than otherwise. We expect that such a finding would lead to less time appearing as necessary logistics or NCCS. Finally, results may not be generalizable to other hospitals or hospital settings. There are clearly important contextual factors, not only for the location but also for the duration. For example, staffing was never an issue during the 5 weeks of observation, but there are locations where an empty bed is not necessarily usable due to lack of staffing. Nonetheless, we believe the results provide a generalizable approach and methodology for other settings (and staffing could be a reason for an empty bed).
In terms of the setting, as noted, we observed one discrete 24‐bed unit, which comprises half of the total PICU. Thus, statements that the PICU was at full capacity must be interpreted in the context that additional rooms may have been available on the other side. Patients are generally admitted alternately to each unit, so the occupancies should parallel each other. We recorded the census every 4 hours for both sides from the electronic system (Sunrise Clinical Manager [SCM]). However, this only accounts for patients physically in beds, not beds held for patients in other locations. Thus, we would expect a discrepancy between direct observation and the SCM value. Through analysis of the entire pediatric intensive care unit,* that part which observed directly, and that which we did not observe directly using census data, we think it reasonable to assert that both units of the total PICU had constrained capacity during the times we directly observed and recorded such constraint on one side.
This study demonstrates the use of direct observation for inpatient settings to learn about resource utilization and identification of value‐added services. PubMed searches for the terms efficiency, flow, process redesign, and time management bring up many more references for operating rooms than for ICUs or inpatient beds. Some examples of ICU‐directed work include videography of an ICU in Australia27 and human factor analysis in ICU nursing.5 Time‐motion studies have also been conducted on clinical staff, such as physicians.28, 29
In conclusion, we found that direct observation provided important insights into the utilization of patient rooms in an important inpatient setting. Data such as these are valuable for clinical and process improvement work, as well as understanding how best to match capacity to patient need. Finally, the methodology is reproducible for other settings and would be an additional tool to measuring and improving the efficiency and value of the health system. When appropriate, this approach can also evaluate the effectiveness of process improvement, help identify and reduce waste,13 and contribute to the growing field that merges operations management with hospital administration and clinical care: in other words, evidence‐based management.30
Acknowledgements
The authors thank Paula Agosto, Patricia Hubbs, Heidi Martin, and Annette Bollig for contributions to the study design.
In comparing direct observation to the SCM count, we found perfect concordance for 110 hours (55%) during which 0 beds were available. For the other 90 hours, SCM reported 1 bed being available in 46 hours (23%), 2 beds being available in 24 hours (12%), 3 beds being available in 17 hours (9%), and 4 beds being available in 3 hours (2%)all while we directly observed 0 beds being available. Thus, cumulatively, 90% of the hours observed with no beds had an SCM report availability of 02 beds; 99% of the time that was 03 beds. Applying this rate of mismatch to the unit that we did not observe directly, SCM reported 0 beds for 46 (23%) of the 200 hours the observation unit was full; SCM reported 1 bed available in 70 hours (35%), 2 beds open in 42 hours (21%), 3 beds open in 26 hours (13%), and 4 beds open in 16 hours (8%). Cumulatively, that is 79% of the time with 02 beds and 92% at 03 beds. From this, we conclude that the combined PICU for both sides was likely functionally full at least 158 of the 200 hours that the side we observed was full (79% 200 hours) and likely had very constrained capacity during the other 42 hours.
- ,,,,.The effect of hospital occupancy on emergency department length of stay and patient disposition.Acad Emerg Med.2003;10:127–133.
- ,,,.The effect of hospital bed occupancy on throughput in the pediatric emergency department.Ann Emerg Med.2009;53:767–776.
- ,,,.A Comparison of in‐hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza.Medical Care.2010;48:224–232.
- .Intensive care unit occupancy: making room for more patients.Crit Care Med.2009;37:1794–1795.
- ,.A human factors engineering conceptual framework of nursing workload and patient safety in intensive care units.Intensive Crit Care Nurs.2005;21:284–301.
- ,,, et al.High level of burnout in intensivists: prevalence and associated factors.Am J Respir Crit Care Med.2007;175:686–692.
- ,,.Length of stay and efficiency in pediatric intensive care units.J Pediatr.1998;133:79–85.
- ,.Variability in duration of stay in pediatric intensive care units: a multiinstitutional study.J Pediatr.1996;128:35–44.
- ,.Matching Supply with Demand: An Introduction to Operations Management.New York, NY:McGraw‐Hill;2006.
- ,.Impact of workload on service time and patient safety: an econometric analysis of hospital operations.Management Science.2009;55:1486–1498.
- .OPIM 631: Operations Management.Philadelphia, PA:Wharton School, University of Pennsylvania;2008.
- ,,.From waste to value in health care.JAMA.2008;299:568–571.
- .Eliminating “waste” in health care.JAMA.2009;302:2481–2482.
- .Toyota Production System: Beyond Large‐scale Production.London, UK:Productivity Press;1995.
- ,.Interdisciplinary work flow assessment and redesign decreases operating room turnover time and allows for additional caseload.Arch Surg.2006;141:65–69.
- ,,,.Improving operating room efficiency through process redesign.Surgery.2006;140:509–514.
- ,,,.Successful strategies for improving operating room efficiency at academic institutions.Anesth Analg.1998year="1998"1998;86:896–906.
- ,,.Efficiency of the operating room suite.Am J Surg.2003;185:244–250.
- ,,, et al.Children's hospitals do not acutely respond to high occupancy.Pediatrics.2010;125:974–981.
- Institute for Healthcare Improvement. Smoothing elective surgical admissions. Available at: http://www.ihi.org/IHI/Topics/Flow/Patient Flow/EmergingContent/SmoothingElectiveSurgicalAdmissions.htm. Accessed October 24,2008.
- Boston Hospital Sees Big Impact from Smoothing Elective Schedule.OR Manager. Volume 20, no. 12,2004.
- ,.Rethinking rapid response teams.JAMA.2010;304:1375–1376.
- Litvak E, ed.Managing Patient Flow in Hospitals: Strategies and Solutions.2nd ed.Oak Brook, IL:Joint Commission Resources;2009.
- ,,,,,.Scheduled admissions and high occupancy at a children's hospital.J Hosp Med.2011;6:81–87.
- ,,, et al.Addressing inpatient crowding by smoothing occupancy at children's hospitals.J Hosp Med.2011;6:466–473.
- ,.Learning to See: Value Stream Mapping to Add Value and Eliminate MUDA.Cambridge, MA:Lean Enterprise Institute;1999.
- ,,.Reshaping ICU ward round practices using video‐reflexive ethnography.Qual Health Res.2008;18:380–390.
- ,,.How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- ,,, et al.Where did the day go? A time‐motion study of hospitalists.J Hosp Med2010;5:323–238.
- ,,.Improving patient care by linking evidence‐based medicine and evidence‐based management.JAMA.2007;298:673–676.
- ,,,,.The effect of hospital occupancy on emergency department length of stay and patient disposition.Acad Emerg Med.2003;10:127–133.
- ,,,.The effect of hospital bed occupancy on throughput in the pediatric emergency department.Ann Emerg Med.2009;53:767–776.
- ,,,.A Comparison of in‐hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza.Medical Care.2010;48:224–232.
- .Intensive care unit occupancy: making room for more patients.Crit Care Med.2009;37:1794–1795.
- ,.A human factors engineering conceptual framework of nursing workload and patient safety in intensive care units.Intensive Crit Care Nurs.2005;21:284–301.
- ,,, et al.High level of burnout in intensivists: prevalence and associated factors.Am J Respir Crit Care Med.2007;175:686–692.
- ,,.Length of stay and efficiency in pediatric intensive care units.J Pediatr.1998;133:79–85.
- ,.Variability in duration of stay in pediatric intensive care units: a multiinstitutional study.J Pediatr.1996;128:35–44.
- ,.Matching Supply with Demand: An Introduction to Operations Management.New York, NY:McGraw‐Hill;2006.
- ,.Impact of workload on service time and patient safety: an econometric analysis of hospital operations.Management Science.2009;55:1486–1498.
- .OPIM 631: Operations Management.Philadelphia, PA:Wharton School, University of Pennsylvania;2008.
- ,,.From waste to value in health care.JAMA.2008;299:568–571.
- .Eliminating “waste” in health care.JAMA.2009;302:2481–2482.
- .Toyota Production System: Beyond Large‐scale Production.London, UK:Productivity Press;1995.
- ,.Interdisciplinary work flow assessment and redesign decreases operating room turnover time and allows for additional caseload.Arch Surg.2006;141:65–69.
- ,,,.Improving operating room efficiency through process redesign.Surgery.2006;140:509–514.
- ,,,.Successful strategies for improving operating room efficiency at academic institutions.Anesth Analg.1998year="1998"1998;86:896–906.
- ,,.Efficiency of the operating room suite.Am J Surg.2003;185:244–250.
- ,,, et al.Children's hospitals do not acutely respond to high occupancy.Pediatrics.2010;125:974–981.
- Institute for Healthcare Improvement. Smoothing elective surgical admissions. Available at: http://www.ihi.org/IHI/Topics/Flow/Patient Flow/EmergingContent/SmoothingElectiveSurgicalAdmissions.htm. Accessed October 24,2008.
- Boston Hospital Sees Big Impact from Smoothing Elective Schedule.OR Manager. Volume 20, no. 12,2004.
- ,.Rethinking rapid response teams.JAMA.2010;304:1375–1376.
- Litvak E, ed.Managing Patient Flow in Hospitals: Strategies and Solutions.2nd ed.Oak Brook, IL:Joint Commission Resources;2009.
- ,,,,,.Scheduled admissions and high occupancy at a children's hospital.J Hosp Med.2011;6:81–87.
- ,,, et al.Addressing inpatient crowding by smoothing occupancy at children's hospitals.J Hosp Med.2011;6:466–473.
- ,.Learning to See: Value Stream Mapping to Add Value and Eliminate MUDA.Cambridge, MA:Lean Enterprise Institute;1999.
- ,,.Reshaping ICU ward round practices using video‐reflexive ethnography.Qual Health Res.2008;18:380–390.
- ,,.How hospitalists spend their time: insights on efficiency and safety.J Hosp Med.2006;1:88–93.
- ,,, et al.Where did the day go? A time‐motion study of hospitalists.J Hosp Med2010;5:323–238.
- ,,.Improving patient care by linking evidence‐based medicine and evidence‐based management.JAMA.2007;298:673–676.
Copyright © 2011 Society of Hospital Medicine
2.0
Ten years ago, leaders in Hospital Medicine saw the need for a peer‐reviewed Hospital Medicine journal, a key step in the growth of the field. However, there was no small amount of uncertainty as to whether there was room for another medical publication, or whether Hospital Medicine was ready for its own journal.
It's clear now that we should not have been worried. Our specialty has grown in size and influence, and the Journal of Hospital Medicine's growth has progressed along a similar track, linked to the success of the many leaders in our field, including the founders of the Society of Hospital Medicine: John Nelson, MD, MHM, Win Whitcomb, MD, MHM, and Bob Wachter, MD, MHM. Support from them in selecting the Founding Editor, Mark V. Williams, ensured his success in assembling an outstanding editorial team, developing JHM's editorial process, and setting this journal as the bestand not just the onlyjournal for hospitalists to publish their work. JHM serves as both a beacon and a mirror for the field of Hospital Medicine, and I am honored for the opportunity to lead this dynamic journal. I also owe special thanks to the Society of Hospital Medicine and the outstanding team at Wiley‐Blackwell, who have made my transition to this role a smooth one.
After the transition, JHM will continue to be a mirror for Hospital Medicine in that it will reflect the scholarship and innovation of hospitalists' scholarly work in research, quality improvement, education, and clinical excellence. From a practical standpoint, this means JHM will continue to do what it has done so successfully to date: provide fair, insightful, and rapid evaluation and publication of articles that are scientifically rigorous and have an impact on hospitalists and their patients. Being an effective mirror also means the journal will need to be in tune with technological advances in publication and learning. Few of us read paper journals any longer, and the move from print to digital and mobile media provides an important opportunity for this journal. Expanding the means by which we disseminate JHM's findings, highlight evidence, and promote knowledge that impacts our field is a clear direction for the journal.
At the transition from JHM 1.0 to JHM 2.0, the journal is positioned to be a beacon for the field by publishing papers that address new and rapidly evolving issues that will affect hospitalists and their patients. JHM and my editorial team eagerly seek submission of manuscripts on these issues delineated below.
Even if health care reform legislation evolves or changes after the 2012 elections, the need to improve health care value across multiple phases of care is unlikely to disappear. The medical home and accountable care organizations will prompt hospitalists to work with outpatient partners to achieve improvements; focus on readmissions and high‐utilization patients may catalyze integration even without larger changes. This evolution plays to hospitalists' traditional strengths as innovators and leaders of health system innovations while erasing the boundaries between inpatient and outpatient phases of care. How the field adapts toor even better, anticipateschanges in care delivery is a momentous opportunity.
Hospitalists will continue to be leaders in quality and safety improvement, but the need to develop innovations that are effective, scalable, and widely adoptable is growing even more acute. Stated alternately, we need to develop innovations quickly and rigorously, so that neither time nor resources are wasted. Fortunately, there is likely to be financial support for projects that link improvement and evaluation from the Center for Medicare and Medicaid Innovations (CMMI). It is a fair bet that a large number of the CMMI's target issues will be ones that hospitalists also find important, and which are ripe for inquiry.
Shifting from quality to outcomes will prompt a revisiting of how we measure our success as hospitalists. Achieving success in process benchmarks will no longer be sufficient, as our practices will increasingly be measured by our patients' experience, functional status, quality of life, and clinical events (of which measures of safety are a part)both within the walls of the hospital and afterwardrather than solely relying on whether patients appropriately received a drug or procedure during their stay. The need to improve outcomes will immediately bump up against the disappointingly small proportion of measures or evidence that apply to the typical Hospital Medicine patient. Developing these new measures, and the evidence for how to improve them, will be a key challenge for the field of Hospital Medicine. Outcome development and comparisons are a clear focus of the Patient‐Centered Outcomes Research Institute. Again, studies documenting such research will find a welcome home at JHM.
The role of information technology in how hospitalists provide care to patients, decide on best practices, communicate with physicians and patients, and manage their practices is becoming central. A huge, nationwide natural experiment is underway as health systems work to meet meaningful use criteria, and oftentimes hospitalists are central to these efforts. Disseminating best practices, implementing innovative systems, and creating workflows that meet the needs of hospitalists' patients is a key short‐term need, and one our field is uniquely positioned to address.
Finally, the practice of Hospital Medicine continues to evolve. In teaching centers, hospitalists are leading educators of medical students and residents; developing training models that reflect newer thinking about how to teach a 21st‐century physician is a key need for the field. The importance of adaptations to work‐hour reductions for residents cannot be overstated, but attention must be paid to how hospitalists' work hours impact patient care as well. Comanagement systemswhether for medical subspecialties (ie, cancer or heart failure) or surgical specialtieshave yet to fulfill their promise, yet demand for comanagement grows. How might comanagement systems be adapted and targeted so that they become more effective?
Not being a futurist or even slightly omniscient, I am sure this list is neither exhaustive nor final. In my 15 or so years in Hospital Medicine, I know firsthand that the field is vigorous, innovative, and full of surprises. Fortunately, JHM is attuned to changes happening now as well as issues on the horizon, and will always strive to be an even better messenger for Hospital Medicine as a professional and academic specialty.1 In that way, JHM 2.0 will be the same as JHM 1.0. I'm excited to shepherd JHM's ongoing growth and look forward to my years at the helm.
Acknowledgements
Funding Source: Dr. Auerbach is supported by National Heart, Lung, and Blood Institute Grant K24 K24HL098372.
Disclosure: The author discloses no relevant or financial conflicts of interest.
- .Editor transition—getting up off the couch and walking out the door.J Hosp Med.2011;6:485–486.
Ten years ago, leaders in Hospital Medicine saw the need for a peer‐reviewed Hospital Medicine journal, a key step in the growth of the field. However, there was no small amount of uncertainty as to whether there was room for another medical publication, or whether Hospital Medicine was ready for its own journal.
It's clear now that we should not have been worried. Our specialty has grown in size and influence, and the Journal of Hospital Medicine's growth has progressed along a similar track, linked to the success of the many leaders in our field, including the founders of the Society of Hospital Medicine: John Nelson, MD, MHM, Win Whitcomb, MD, MHM, and Bob Wachter, MD, MHM. Support from them in selecting the Founding Editor, Mark V. Williams, ensured his success in assembling an outstanding editorial team, developing JHM's editorial process, and setting this journal as the bestand not just the onlyjournal for hospitalists to publish their work. JHM serves as both a beacon and a mirror for the field of Hospital Medicine, and I am honored for the opportunity to lead this dynamic journal. I also owe special thanks to the Society of Hospital Medicine and the outstanding team at Wiley‐Blackwell, who have made my transition to this role a smooth one.
After the transition, JHM will continue to be a mirror for Hospital Medicine in that it will reflect the scholarship and innovation of hospitalists' scholarly work in research, quality improvement, education, and clinical excellence. From a practical standpoint, this means JHM will continue to do what it has done so successfully to date: provide fair, insightful, and rapid evaluation and publication of articles that are scientifically rigorous and have an impact on hospitalists and their patients. Being an effective mirror also means the journal will need to be in tune with technological advances in publication and learning. Few of us read paper journals any longer, and the move from print to digital and mobile media provides an important opportunity for this journal. Expanding the means by which we disseminate JHM's findings, highlight evidence, and promote knowledge that impacts our field is a clear direction for the journal.
At the transition from JHM 1.0 to JHM 2.0, the journal is positioned to be a beacon for the field by publishing papers that address new and rapidly evolving issues that will affect hospitalists and their patients. JHM and my editorial team eagerly seek submission of manuscripts on these issues delineated below.
Even if health care reform legislation evolves or changes after the 2012 elections, the need to improve health care value across multiple phases of care is unlikely to disappear. The medical home and accountable care organizations will prompt hospitalists to work with outpatient partners to achieve improvements; focus on readmissions and high‐utilization patients may catalyze integration even without larger changes. This evolution plays to hospitalists' traditional strengths as innovators and leaders of health system innovations while erasing the boundaries between inpatient and outpatient phases of care. How the field adapts toor even better, anticipateschanges in care delivery is a momentous opportunity.
Hospitalists will continue to be leaders in quality and safety improvement, but the need to develop innovations that are effective, scalable, and widely adoptable is growing even more acute. Stated alternately, we need to develop innovations quickly and rigorously, so that neither time nor resources are wasted. Fortunately, there is likely to be financial support for projects that link improvement and evaluation from the Center for Medicare and Medicaid Innovations (CMMI). It is a fair bet that a large number of the CMMI's target issues will be ones that hospitalists also find important, and which are ripe for inquiry.
Shifting from quality to outcomes will prompt a revisiting of how we measure our success as hospitalists. Achieving success in process benchmarks will no longer be sufficient, as our practices will increasingly be measured by our patients' experience, functional status, quality of life, and clinical events (of which measures of safety are a part)both within the walls of the hospital and afterwardrather than solely relying on whether patients appropriately received a drug or procedure during their stay. The need to improve outcomes will immediately bump up against the disappointingly small proportion of measures or evidence that apply to the typical Hospital Medicine patient. Developing these new measures, and the evidence for how to improve them, will be a key challenge for the field of Hospital Medicine. Outcome development and comparisons are a clear focus of the Patient‐Centered Outcomes Research Institute. Again, studies documenting such research will find a welcome home at JHM.
The role of information technology in how hospitalists provide care to patients, decide on best practices, communicate with physicians and patients, and manage their practices is becoming central. A huge, nationwide natural experiment is underway as health systems work to meet meaningful use criteria, and oftentimes hospitalists are central to these efforts. Disseminating best practices, implementing innovative systems, and creating workflows that meet the needs of hospitalists' patients is a key short‐term need, and one our field is uniquely positioned to address.
Finally, the practice of Hospital Medicine continues to evolve. In teaching centers, hospitalists are leading educators of medical students and residents; developing training models that reflect newer thinking about how to teach a 21st‐century physician is a key need for the field. The importance of adaptations to work‐hour reductions for residents cannot be overstated, but attention must be paid to how hospitalists' work hours impact patient care as well. Comanagement systemswhether for medical subspecialties (ie, cancer or heart failure) or surgical specialtieshave yet to fulfill their promise, yet demand for comanagement grows. How might comanagement systems be adapted and targeted so that they become more effective?
Not being a futurist or even slightly omniscient, I am sure this list is neither exhaustive nor final. In my 15 or so years in Hospital Medicine, I know firsthand that the field is vigorous, innovative, and full of surprises. Fortunately, JHM is attuned to changes happening now as well as issues on the horizon, and will always strive to be an even better messenger for Hospital Medicine as a professional and academic specialty.1 In that way, JHM 2.0 will be the same as JHM 1.0. I'm excited to shepherd JHM's ongoing growth and look forward to my years at the helm.
Acknowledgements
Funding Source: Dr. Auerbach is supported by National Heart, Lung, and Blood Institute Grant K24 K24HL098372.
Disclosure: The author discloses no relevant or financial conflicts of interest.
Ten years ago, leaders in Hospital Medicine saw the need for a peer‐reviewed Hospital Medicine journal, a key step in the growth of the field. However, there was no small amount of uncertainty as to whether there was room for another medical publication, or whether Hospital Medicine was ready for its own journal.
It's clear now that we should not have been worried. Our specialty has grown in size and influence, and the Journal of Hospital Medicine's growth has progressed along a similar track, linked to the success of the many leaders in our field, including the founders of the Society of Hospital Medicine: John Nelson, MD, MHM, Win Whitcomb, MD, MHM, and Bob Wachter, MD, MHM. Support from them in selecting the Founding Editor, Mark V. Williams, ensured his success in assembling an outstanding editorial team, developing JHM's editorial process, and setting this journal as the bestand not just the onlyjournal for hospitalists to publish their work. JHM serves as both a beacon and a mirror for the field of Hospital Medicine, and I am honored for the opportunity to lead this dynamic journal. I also owe special thanks to the Society of Hospital Medicine and the outstanding team at Wiley‐Blackwell, who have made my transition to this role a smooth one.
After the transition, JHM will continue to be a mirror for Hospital Medicine in that it will reflect the scholarship and innovation of hospitalists' scholarly work in research, quality improvement, education, and clinical excellence. From a practical standpoint, this means JHM will continue to do what it has done so successfully to date: provide fair, insightful, and rapid evaluation and publication of articles that are scientifically rigorous and have an impact on hospitalists and their patients. Being an effective mirror also means the journal will need to be in tune with technological advances in publication and learning. Few of us read paper journals any longer, and the move from print to digital and mobile media provides an important opportunity for this journal. Expanding the means by which we disseminate JHM's findings, highlight evidence, and promote knowledge that impacts our field is a clear direction for the journal.
At the transition from JHM 1.0 to JHM 2.0, the journal is positioned to be a beacon for the field by publishing papers that address new and rapidly evolving issues that will affect hospitalists and their patients. JHM and my editorial team eagerly seek submission of manuscripts on these issues delineated below.
Even if health care reform legislation evolves or changes after the 2012 elections, the need to improve health care value across multiple phases of care is unlikely to disappear. The medical home and accountable care organizations will prompt hospitalists to work with outpatient partners to achieve improvements; focus on readmissions and high‐utilization patients may catalyze integration even without larger changes. This evolution plays to hospitalists' traditional strengths as innovators and leaders of health system innovations while erasing the boundaries between inpatient and outpatient phases of care. How the field adapts toor even better, anticipateschanges in care delivery is a momentous opportunity.
Hospitalists will continue to be leaders in quality and safety improvement, but the need to develop innovations that are effective, scalable, and widely adoptable is growing even more acute. Stated alternately, we need to develop innovations quickly and rigorously, so that neither time nor resources are wasted. Fortunately, there is likely to be financial support for projects that link improvement and evaluation from the Center for Medicare and Medicaid Innovations (CMMI). It is a fair bet that a large number of the CMMI's target issues will be ones that hospitalists also find important, and which are ripe for inquiry.
Shifting from quality to outcomes will prompt a revisiting of how we measure our success as hospitalists. Achieving success in process benchmarks will no longer be sufficient, as our practices will increasingly be measured by our patients' experience, functional status, quality of life, and clinical events (of which measures of safety are a part)both within the walls of the hospital and afterwardrather than solely relying on whether patients appropriately received a drug or procedure during their stay. The need to improve outcomes will immediately bump up against the disappointingly small proportion of measures or evidence that apply to the typical Hospital Medicine patient. Developing these new measures, and the evidence for how to improve them, will be a key challenge for the field of Hospital Medicine. Outcome development and comparisons are a clear focus of the Patient‐Centered Outcomes Research Institute. Again, studies documenting such research will find a welcome home at JHM.
The role of information technology in how hospitalists provide care to patients, decide on best practices, communicate with physicians and patients, and manage their practices is becoming central. A huge, nationwide natural experiment is underway as health systems work to meet meaningful use criteria, and oftentimes hospitalists are central to these efforts. Disseminating best practices, implementing innovative systems, and creating workflows that meet the needs of hospitalists' patients is a key short‐term need, and one our field is uniquely positioned to address.
Finally, the practice of Hospital Medicine continues to evolve. In teaching centers, hospitalists are leading educators of medical students and residents; developing training models that reflect newer thinking about how to teach a 21st‐century physician is a key need for the field. The importance of adaptations to work‐hour reductions for residents cannot be overstated, but attention must be paid to how hospitalists' work hours impact patient care as well. Comanagement systemswhether for medical subspecialties (ie, cancer or heart failure) or surgical specialtieshave yet to fulfill their promise, yet demand for comanagement grows. How might comanagement systems be adapted and targeted so that they become more effective?
Not being a futurist or even slightly omniscient, I am sure this list is neither exhaustive nor final. In my 15 or so years in Hospital Medicine, I know firsthand that the field is vigorous, innovative, and full of surprises. Fortunately, JHM is attuned to changes happening now as well as issues on the horizon, and will always strive to be an even better messenger for Hospital Medicine as a professional and academic specialty.1 In that way, JHM 2.0 will be the same as JHM 1.0. I'm excited to shepherd JHM's ongoing growth and look forward to my years at the helm.
Acknowledgements
Funding Source: Dr. Auerbach is supported by National Heart, Lung, and Blood Institute Grant K24 K24HL098372.
Disclosure: The author discloses no relevant or financial conflicts of interest.
- .Editor transition—getting up off the couch and walking out the door.J Hosp Med.2011;6:485–486.
- .Editor transition—getting up off the couch and walking out the door.J Hosp Med.2011;6:485–486.