User login
Multiple sclerosis, natalizumab, and PML: Helping patients decide
Multiple sclerosis (MS) is an autoimmune disease whose inflammatory process causes demyelination, axonal loss, and neurodegeneration, all of which can lead to progressive neurologic disability. Without treatment, the risk of progressive disability 15 to 20 years after the onset of MS has been estimated to be as high as 50%.1
Seven drugs have been approved by the US Food and Drug Administration (FDA) for the treatment of MS. Of these, interferon beta drugs and glatiramer acetate are generally considered as first-line agents based on extensive experience and relative safety. Indeed, reports with followup approaching 20 years have not identified significant safety concerns. However, these agents have only modest efficacy, reducing by approximately 30% the frequency of relapse in patients with relapsing-remitting MS.2–6
Natalizumab, and more recently, fingolimod, are generally used as second-line agents. Fingolimod, the first oral agent to receive FDA approval for the treatment of relapsing-remitting MS, is a functional antagonist of sphingosine-1-phosphate receptors. The reductions in annualized relapse rates in two phase 3 controlled trials of fingolimod were approximately 55% compared with placebo7 or intramuscular interferon beta-1a.8 Because of its more convenient oral route of administration and its documented efficacy, widespread use of fingolimod is anticipated. However, adverse reactions affecting more than 10% of patients include headache, influenza, diarrhea, back pain, liver transaminase elevations, and cough.9 Because sphingosine-1-phosphate receptors are widespread in many body tissues, off-target effects of fingolimod may be problematic and long-term toxicity is unknown.
In addition to natalizumab and fingolimod, which are currently available for use as second-line agents, several other MS therapies are showing promise. Oral cladribine, teriflunomide, and laquinimod have reported positive phase 3 results in publication or at national meetings, and several other drugs are in late stages of development (alemtuzumab, BG-12, ocrelizumab) based on encouraging phase 2 results.10–12 Thus, the options for MS patients are expanding, but drugs with higher efficacy also may pose greater risk.
NATALIZUMAB: ROBUST BENEFITS BUT ASSOCIATED RISK
Natalizumab is a humanized monoclonal antibody that binds to alpha-4 integrin on leukocytes. By inhibiting alpha-4 integrin, natalizumab, the first of a new class of selective adhesion-molecule inhibitors, impedes migration of activated mononuclear leukocytes into the brain and gut.13
Significant efficacy
Two phase 3 studies demonstrated more robust efficacy of natalizumab in patients with relapsing-remitting MS than had been observed in prior studies with other agents.14,15 In the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study, which followed patients over 2 years of treatment, natalizumab was associated with a 68% reduction in the annualized relapse rate14; a 92% reduction in gadolinium-enhanced lesions on magnetic resonance imaging (MRI), which indicate new, active lesions16; and an 83% reduction in the mean number of new or enlarging T2 lesions16 compared with placebo. The likelihood of confirmed worsening on the Kurtzke Expanded Disability Status Score, which is the standard measure for MS-related disability, was also 42% lower in patients assigned to natalizumab compared with placebo.14
Other reported benefits from natalizumab therapy include a significantly increased probability of maintaining disease-free status17 and clinically significant improvements on patient-reported quality-of-life measures.18 Although there have been no head-to-head studies of natalizumab with interferon beta, glatiramer acetate, or fingolimod, there is a widespread view that treatment benefits of natalizumab exceed those of other disease-modifying drugs. In clinical practice, patients with MS who experience breakthrough disease on standard disease-modifying drugs are routinely observed to achieve disease control after switching to natalizumab. Thus, based purely on efficacy, patient-reported outcomes, and the convenience of once-monthly intravenous infusion, natalizumab represents an extremely attractive treatment option for patients with relapsing-remitting MS.
Use discontinued in 2005
Natalizumab was approved for treatment of relapsing-remitting MS in November 2004, using the FDA accelerated review pathway. The approval was based on the first-year results of the AFFIRM14 and the Natalizumab plus Interferon Beta-1a for Relapsing Remitting Multiple Sclerosis (SENTINEL)19 studies, both of which were completed in February 2005. In the 3 to 4 months between the drug’s approval and completion of the AFFIRM and SENTINEL studies, approximately 7,000 patients with relapsing-remitting MS received treatment with natalizumab. In February 2005, shortly after the release of the 2-year data, three cases of progressive multifocal leukoencephalopathy (PML) were identified in natalizumab-treated patients (one with Crohn disease, the other two with MS). Clinical and research use of natalizumab was abruptly suspended that month, pending a comprehensive safety review.
A safety study evaluated 3,116 patients who had received natalizumab over a mean exposure of 17.9 monthly doses.20 The study failed to identify any additional cases of PML and concluded that the risk of PML was approximately 1 in 1,000 patients. Abrupt discontinuation of natalizumab also allowed systematic assessment of disease behavior following treatment interruption. In 1,866 patients who had received natalizumab during clinical trials but who discontinued natalizumab after PML was recognized, MS relapses and gadolinium-enhancing lesions returned approximately to baseline levels within 4 to 7 months of natalizumab suspension. Reactivation of MS disease activity was observed even in patients who instituted one of the first-line disease-modifying drugs as substitute therapy.21
Based on the strong efficacy data and the extensive safety review, an FDA advisory committee recommended reintroduction, and natalizumab was returned to the market in June 2006. Natalizumab may be administered only in accredited infusion centers that agree to a monthly reporting regimen designed to identify all cases of PML. In the United States, natalizumab is available to patients only through the Tysabri Outreach Unified Commitment to Health (TOUCH) Prescribing Program, a restricted distribution program. Aggressive monitoring and reporting is also required in other regions of the world, so that ascertainment of PML associated with natalizumab is thought to be relatively complete.
Risk related to duration of therapy
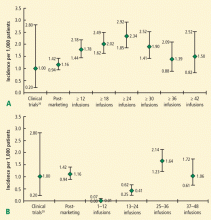
Of the first 35 cases of natalizumab-associated PML, 10 cases (29%) were fatal. Among surviving patients, the level of disability was found to be severe in 48%; moderate in 36%; and mild in 16%.23 Improved survival was associated with younger age, less MS-related disability prior to PML, more localized disease on brain MRI at diagnosis, and shorter time from symptom onset to PML diagnosis.23 As of August 4, 2011, there were 150 confirmed cases of natalizumab-associated PML (58 in the United States, 85 in Europe, and 7 from the rest of the world); of these, 29 (19%) have died.22
VARIATION IN PATIENT RISK TOLERANCE
The postmarketing surveillance of natalizumab clearly demonstrates that risk is associated with administration of the drug, but risk tolerance varies considerably among individuals with MS. Some patients elect to use natalizumab despite the risk of PML, even when they have relatively mild MS. Other patients decline use of natalizumab even when their MS is severe and has responded poorly to other disease-modifying drugs.
In most cases, based on my experience, patients accept the risk of natalizumab-associated PML if MS disease is their primary consideration. Another major factor is the patient’s prior experiences with disease-modifying drugs; patients who have experienced breakthrough disease activity despite treatment with first-line drugs commonly opt for natalizumab regardless of the risk of PML.
Interestingly, the treating neurologist’s perception of MS severity and risk of PML may differ from the patient’s perception. In a study of 69 natalizumab-treated MS patients and 66 neurologists, Heesen et al found that patients had a significantly worse perception of their disease and were more willing to assume treatment risks and continue natalizumab therapy than their neurologists were.24 About one-half of the neurologists said that they would discontinue natalizumab at a risk level of 1 in 5,000 or lower, whereas only 17% of the patients would stop at this risk level. This finding has significant implications for clinical practice and implies that the neurologist should discuss concerns about MS and risk of treatment with the patient in order to tailor the decision to the patient’s concerns.
Interest in identifying biomarkers to aid in quantifying risk is ongoing. Chen et al found that subclinical reactivation of the JC virus (JCV) occurred frequently in 19 natalizumab-treated MS patients.25 Another study of 24 natalizumab-treated MS patients found no JCV DNA in the blood, although JCV DNA was found in the urine in 25% of patients.26 A large survey of blood and urine from natalizumab-treated MS patients found low sensitivity and specificity for JCV DNA as a predictor for subsequent PML.27 In this study of more than 1,000 natalizumab-treated patients, JCV DNA was detected in 0.3% of patients’ plasma and in 26% of patients’ urine, but PML did not develop in any patient who was JCV-positive. Among five natalizumab-treated patients who developed PML, JCV DNA was detected in none before the advent of symptoms. The presence of JCV DNA in bodily fluids is important for the diagnosis of PML, but it currently holds no predictive clinical value. At present, measuring JCV DNA in blood, cells, or urine as a predictive biomarker for natalizumab-associated PML does not appear to be clinically useful.
Stratifying risk by measuring JCV serology, however, does appear to be a useful strategy. Investigators using a two-step assay to detect and quantify JCV antibodies found 53.6% of MS patients to be seropositive, with a false-negative rate of 2.5%. Of most interest, all 17 natalizumab-associated PML patients who had available blood samples taken an average of 2 years before onset of PML tested positive for JCV antibodies.28 Although studies are ongoing, classification according to JCV-antibody status may be helpful in advising patients. Patients who are JCV-antibody seronegative (about one-half of patients) appear to be at extraordinarily low risk for PML. In these patients, use of natalizumab could be liberalized and continued as long as the JCV-antibody status remains negative. In patients who are seropositive for JCV antibodies, caution is recommended, particularly for patients who had prior immunosuppressive drug therapy and for patients who have received treatment for more than 24 months. Even in JCV-antibody–seropositive patients, use of natalizumab may be advisable depending on disease severity, available options, and the patient’s risk tolerance. JCV-antibody testing is a rare example of a clinically useful biomarker that can guide specific treatment decisions in the field of neurology.
CURRENT PRACTICE: A PERSONAL MANAGEMENT ALGORITHM
Based on current evidence, the following opinion on the use of natalizumab for the treatment of MS is offered as a supplement to approved prescribing information. The neurologist must individualize the treatment decision for each patient and recognize that these general comments represent a personal opinion. Several factors affect decisions about the use of disease-modifying drugs in MS, and specifically use of natalizumab: How severe is the disease, and what is the prognosis for future disease progression from the neurologist’s perspective? How concerned is the patient about current or future MS symptoms and disability? What is the patient’s tolerance for medication side effects? For risk taking? Has there been prior immunosuppressive therapy? What is the JCV antibody status? What other options are available for disease management?
These issues require discussion among the neurologist, the patient, and the patient’s family. The neurologist should provide input on disease status, an opinion about prognosis, and a description of appropriate options for disease management. Many patients also want a global recommendation (ie, “Tell me what you think I should do”). The neurologist must tailor that global recommendation to the patient’s perceptions of his or her MS, its treatment, and preferences regarding treatment options.
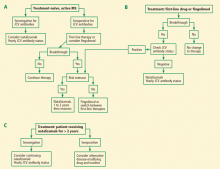
For patients who are already receiving treatment with a first-line drug and whose disease is well controlled, I make no changes in treatment until a breakthrough occurs, at which time I recommend switching to natalizumab with JCV antibody status assessed yearly (Figure 2B). If a patient has been taking natalizumab for more than 2 years and is seronegative, I advise continuing natalizumab (Figure 2C). If seropositive after 2 or more years of natalizumab therapy, I recommend switching to fingolimod and monitoring for disease reactivation.
DISCUSSION
Dr. Calabrese: Have you perceived growing concern over PML among the MS patient population over the past 2 to 3 years?
Dr. Rudick: I would say that it’s pretty stable. Patients who are risk intolerant select out of natalizumab. Some patients would just as soon take their chances with MS rather than deal with additional risk. Since we talk about the risk of PML with patients prior to treatment, the patients who choose natalizumab are able to deal with the risk. The difficult cases are those patients who will be severely disabled before long but choose not to go on natalizumab because they’re very risk averse. It gets even more complicated when the closest family member (parents or spouse) want their relative to use natalizumab but the patient is risk averse. This can become quite complicated—for example, I’ve seen situations where the patient is a minor, and one parent wants their child to use natalizumab but the other is risk-averse. Spouses often see risk differently, and this has led to interesting and difficult discussions. In the case of a child with MS, I listen to preferences from family members, but otherwise I empower the patient to drive the decision.
Dr. Major: Our data seem to suggest a higher percentage of individuals who are seropositive—about 56%. The issue, however, is a lack of a standard to define seropositive and seronegative. I suggested that the natalizumab manufacturer collect a bank of samples and allocate them to laboratories with no vested interest for polymerase chain reaction (PCR) assays, from which consensus definitions of seropositive and seronegative could be developed.
Dr. Calabrese: Why is there no confirmatory immunoassay for this virus? We don’t have false positives for hepatitis B or human immunodeficiency virus. We still seem to be relying on older technologies.
Dr. Major: To determine the level of antibody, an enzyme-linked immunosorbent assay is just fine.
Dr. Calabrese: That’s for sensitivity, but what about specificity?
Dr. Major: Specificity is quite good. Everybody now uses the same antigen, the polyoma capsid antigen, VP1, to detect productive viral infection, but there are no set standards for the cutpoints to classify as seropositive and seronegative. Certainly, there’s high sensitivity with PCR to detect JCV DNA in cerebrospinal fluid (CSF), because we’re able to detect very low copy levels of JCV DNA in the CSF.
Dr. Simpson: I would like to see a quantitative measure of risk versus benefit for all of the drugs used in MS, not just natalizumab. When you look at any clinical trial, you see a table of adverse events and you see efficacy measures, but you don’t see the two combined. This really is necessary to compare drug A with drug B. Instead, we end up making decisions based on risk tolerance and rather soft criteria. One could argue that we don’t want to be so algorithmic that we take the art out of medicine, but the criteria we use to make decisions are quite soft. I wonder whether you have any suggestions on a more quantitative approach.
Dr. Rudick: This is an important point, but a difficult problem. We have much more information about PML associated with natalizumab than we do about many serious adverse events. For example, there are rare adverse events with interferon beta—severe depression, liver injury, and so forth. But we don’t have precise quantitative data on most adverse drug effects, and in general adverse events are underreported in clinical practice.
The natalizumab-PML situation is somewhat unique. PML is a dramatic, often fatal, disease that is virtually never observed spontaneously in MS, and the strict reporting requirements have resulted in near-complete ascertainment and more precise risk estimates. This situation doesn’t apply to most adverse events associated with other therapies—even for some severe adverse events. But you are correct—focusing exclusively on the risk of PML seems somewhat simplistic because there are clear risks with other drugs, and these need to be factored into treatment decisions.
Dr. Molloy: Do you have good tools that predict how a patient with MS will do over time?
Dr. Fox: We have fair tools, not great tools.
Dr. Rudick: We’re diagnosing MS earlier, sometimes at the first symptom. We’re even beginning to recognize it in patients without symptoms who have MS observed as an incidental MRI finding. It is difficult at the earliest stage of MS to predict severity with any confidence. The best predictor we have is the severity of the disease by MRI criteria. This can provide a general guide to treatment decisions, but it is an imprecise predictor.
- Weinshenker BG, Bass B, Rice GPA, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 1999; 112:133–146.
- Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol 1996; 39:285–294.
- The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis I: clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993; 43:655–661.
- IFNB Multiple Sclerosis Study Group, The University of British Columbia MS/MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. The IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. Neurology 1995; 45:1277–1285.
- Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind, placebo-controlled trial. Neurology 1995; 45:1268–1276.
- PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 1998; 352:1498–1504.
- Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362:387–401.
- Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362:402–415.
- Gilenya [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011.
- Coles AJ, Fox E, Vladic A, et al. Alemtuzumab versus interferon beta-1a in early relapsing-remitting multiple sclerosis: post-hoc and subset analyses of clinical efficacy outcomes. Lancet Neurol 2011; 10:338–348.
- Nicholas R, Giannetti P, Alsanousi A, Friede T, Muraro PA. Development of oral immunomodulatory agents in the management of multiple sclerosis. Drug Des Devel Ther 2011; 5:255–274.
- Consortium of Multiple Sclerosis Centers (CMSC). Phase II study with ocrelizumab shows significant reduction in disease activity. CMSC Web site. http://mscare.org/cmsc/index.php?option=com_content&task=view&id=1081&Itemid=1465. Published October 15, 2010. Accessed August 26, 2011.
- Tysabri [package insert]. South San Francisco, CA: Elan Pharmaceuticals, Inc.; 2011.
- Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006; 354:899–910.
- Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2003; 348:15–23.
- Miller DH, Soon D, Fernando KT, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology 2007; 68:1390–1401.
- Havrdova E, Galetta S, Hutchinson M, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol 2009; 8:254–260.
- Rudick RA, Miller D, Hass S, et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol 2007; 62:335–346.
- Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multipls sclerosis. N Engl J Med 2006; 354:911–923.
- Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 2006; 354:924–933.
- O’Connor PW, Goodman A, Kappos L, et al. Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis [published online ahead of print May 4, 2011]. Neurology 2011; 76:1858–1865.
- Update on Tysabri and PML. National Multiple Sclerosis Society Web site. http://www.nationalmssociety.org/news/news-detail/index.aspx?nid=2308. Published April 11, 2011. Updated May 23, 2011. Accessed June 22, 2011.
- Vermersch P, Kappos L, Gold R, et al. Clinical outcomes of natalizumab-associated progressive multifocal leukoencephalopathy. Neurology 2011; 76:1697–1704.
- Heesen C, Kleiter I, Nguyen F, et al. Risk perception in natalizumab-treated multiple sclerosis patients and their neurologists. Mult Scler 2010; 16:1507–1512.
- Chen Y, Bord E, Tompkins T, et al. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med 2009; 361:1067–1074.
- Jilek S, Jaquiery E, Hirsch HH, et al. Immune responses to JC virus in patients with multiple sclerosis treated with natalizumab: a cross-sectional and longitudinal study. Lancet Neurol 2010; 9:264–272.
- Rudick RA, O’Connor PW, Polman CH, et al. Assessment of JC virus DNA in blood and urine from natalizumab-treated patients. Ann Neurol 2010; 68:304–310.
- Gorelik L, Lerner M, Bixler S, et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol 2010; 68:295–303.
Multiple sclerosis (MS) is an autoimmune disease whose inflammatory process causes demyelination, axonal loss, and neurodegeneration, all of which can lead to progressive neurologic disability. Without treatment, the risk of progressive disability 15 to 20 years after the onset of MS has been estimated to be as high as 50%.1
Seven drugs have been approved by the US Food and Drug Administration (FDA) for the treatment of MS. Of these, interferon beta drugs and glatiramer acetate are generally considered as first-line agents based on extensive experience and relative safety. Indeed, reports with followup approaching 20 years have not identified significant safety concerns. However, these agents have only modest efficacy, reducing by approximately 30% the frequency of relapse in patients with relapsing-remitting MS.2–6
Natalizumab, and more recently, fingolimod, are generally used as second-line agents. Fingolimod, the first oral agent to receive FDA approval for the treatment of relapsing-remitting MS, is a functional antagonist of sphingosine-1-phosphate receptors. The reductions in annualized relapse rates in two phase 3 controlled trials of fingolimod were approximately 55% compared with placebo7 or intramuscular interferon beta-1a.8 Because of its more convenient oral route of administration and its documented efficacy, widespread use of fingolimod is anticipated. However, adverse reactions affecting more than 10% of patients include headache, influenza, diarrhea, back pain, liver transaminase elevations, and cough.9 Because sphingosine-1-phosphate receptors are widespread in many body tissues, off-target effects of fingolimod may be problematic and long-term toxicity is unknown.
In addition to natalizumab and fingolimod, which are currently available for use as second-line agents, several other MS therapies are showing promise. Oral cladribine, teriflunomide, and laquinimod have reported positive phase 3 results in publication or at national meetings, and several other drugs are in late stages of development (alemtuzumab, BG-12, ocrelizumab) based on encouraging phase 2 results.10–12 Thus, the options for MS patients are expanding, but drugs with higher efficacy also may pose greater risk.
NATALIZUMAB: ROBUST BENEFITS BUT ASSOCIATED RISK
Natalizumab is a humanized monoclonal antibody that binds to alpha-4 integrin on leukocytes. By inhibiting alpha-4 integrin, natalizumab, the first of a new class of selective adhesion-molecule inhibitors, impedes migration of activated mononuclear leukocytes into the brain and gut.13
Significant efficacy
Two phase 3 studies demonstrated more robust efficacy of natalizumab in patients with relapsing-remitting MS than had been observed in prior studies with other agents.14,15 In the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study, which followed patients over 2 years of treatment, natalizumab was associated with a 68% reduction in the annualized relapse rate14; a 92% reduction in gadolinium-enhanced lesions on magnetic resonance imaging (MRI), which indicate new, active lesions16; and an 83% reduction in the mean number of new or enlarging T2 lesions16 compared with placebo. The likelihood of confirmed worsening on the Kurtzke Expanded Disability Status Score, which is the standard measure for MS-related disability, was also 42% lower in patients assigned to natalizumab compared with placebo.14
Other reported benefits from natalizumab therapy include a significantly increased probability of maintaining disease-free status17 and clinically significant improvements on patient-reported quality-of-life measures.18 Although there have been no head-to-head studies of natalizumab with interferon beta, glatiramer acetate, or fingolimod, there is a widespread view that treatment benefits of natalizumab exceed those of other disease-modifying drugs. In clinical practice, patients with MS who experience breakthrough disease on standard disease-modifying drugs are routinely observed to achieve disease control after switching to natalizumab. Thus, based purely on efficacy, patient-reported outcomes, and the convenience of once-monthly intravenous infusion, natalizumab represents an extremely attractive treatment option for patients with relapsing-remitting MS.
Use discontinued in 2005
Natalizumab was approved for treatment of relapsing-remitting MS in November 2004, using the FDA accelerated review pathway. The approval was based on the first-year results of the AFFIRM14 and the Natalizumab plus Interferon Beta-1a for Relapsing Remitting Multiple Sclerosis (SENTINEL)19 studies, both of which were completed in February 2005. In the 3 to 4 months between the drug’s approval and completion of the AFFIRM and SENTINEL studies, approximately 7,000 patients with relapsing-remitting MS received treatment with natalizumab. In February 2005, shortly after the release of the 2-year data, three cases of progressive multifocal leukoencephalopathy (PML) were identified in natalizumab-treated patients (one with Crohn disease, the other two with MS). Clinical and research use of natalizumab was abruptly suspended that month, pending a comprehensive safety review.
A safety study evaluated 3,116 patients who had received natalizumab over a mean exposure of 17.9 monthly doses.20 The study failed to identify any additional cases of PML and concluded that the risk of PML was approximately 1 in 1,000 patients. Abrupt discontinuation of natalizumab also allowed systematic assessment of disease behavior following treatment interruption. In 1,866 patients who had received natalizumab during clinical trials but who discontinued natalizumab after PML was recognized, MS relapses and gadolinium-enhancing lesions returned approximately to baseline levels within 4 to 7 months of natalizumab suspension. Reactivation of MS disease activity was observed even in patients who instituted one of the first-line disease-modifying drugs as substitute therapy.21
Based on the strong efficacy data and the extensive safety review, an FDA advisory committee recommended reintroduction, and natalizumab was returned to the market in June 2006. Natalizumab may be administered only in accredited infusion centers that agree to a monthly reporting regimen designed to identify all cases of PML. In the United States, natalizumab is available to patients only through the Tysabri Outreach Unified Commitment to Health (TOUCH) Prescribing Program, a restricted distribution program. Aggressive monitoring and reporting is also required in other regions of the world, so that ascertainment of PML associated with natalizumab is thought to be relatively complete.
Risk related to duration of therapy
Of the first 35 cases of natalizumab-associated PML, 10 cases (29%) were fatal. Among surviving patients, the level of disability was found to be severe in 48%; moderate in 36%; and mild in 16%.23 Improved survival was associated with younger age, less MS-related disability prior to PML, more localized disease on brain MRI at diagnosis, and shorter time from symptom onset to PML diagnosis.23 As of August 4, 2011, there were 150 confirmed cases of natalizumab-associated PML (58 in the United States, 85 in Europe, and 7 from the rest of the world); of these, 29 (19%) have died.22
VARIATION IN PATIENT RISK TOLERANCE
The postmarketing surveillance of natalizumab clearly demonstrates that risk is associated with administration of the drug, but risk tolerance varies considerably among individuals with MS. Some patients elect to use natalizumab despite the risk of PML, even when they have relatively mild MS. Other patients decline use of natalizumab even when their MS is severe and has responded poorly to other disease-modifying drugs.
In most cases, based on my experience, patients accept the risk of natalizumab-associated PML if MS disease is their primary consideration. Another major factor is the patient’s prior experiences with disease-modifying drugs; patients who have experienced breakthrough disease activity despite treatment with first-line drugs commonly opt for natalizumab regardless of the risk of PML.
Interestingly, the treating neurologist’s perception of MS severity and risk of PML may differ from the patient’s perception. In a study of 69 natalizumab-treated MS patients and 66 neurologists, Heesen et al found that patients had a significantly worse perception of their disease and were more willing to assume treatment risks and continue natalizumab therapy than their neurologists were.24 About one-half of the neurologists said that they would discontinue natalizumab at a risk level of 1 in 5,000 or lower, whereas only 17% of the patients would stop at this risk level. This finding has significant implications for clinical practice and implies that the neurologist should discuss concerns about MS and risk of treatment with the patient in order to tailor the decision to the patient’s concerns.
Interest in identifying biomarkers to aid in quantifying risk is ongoing. Chen et al found that subclinical reactivation of the JC virus (JCV) occurred frequently in 19 natalizumab-treated MS patients.25 Another study of 24 natalizumab-treated MS patients found no JCV DNA in the blood, although JCV DNA was found in the urine in 25% of patients.26 A large survey of blood and urine from natalizumab-treated MS patients found low sensitivity and specificity for JCV DNA as a predictor for subsequent PML.27 In this study of more than 1,000 natalizumab-treated patients, JCV DNA was detected in 0.3% of patients’ plasma and in 26% of patients’ urine, but PML did not develop in any patient who was JCV-positive. Among five natalizumab-treated patients who developed PML, JCV DNA was detected in none before the advent of symptoms. The presence of JCV DNA in bodily fluids is important for the diagnosis of PML, but it currently holds no predictive clinical value. At present, measuring JCV DNA in blood, cells, or urine as a predictive biomarker for natalizumab-associated PML does not appear to be clinically useful.
Stratifying risk by measuring JCV serology, however, does appear to be a useful strategy. Investigators using a two-step assay to detect and quantify JCV antibodies found 53.6% of MS patients to be seropositive, with a false-negative rate of 2.5%. Of most interest, all 17 natalizumab-associated PML patients who had available blood samples taken an average of 2 years before onset of PML tested positive for JCV antibodies.28 Although studies are ongoing, classification according to JCV-antibody status may be helpful in advising patients. Patients who are JCV-antibody seronegative (about one-half of patients) appear to be at extraordinarily low risk for PML. In these patients, use of natalizumab could be liberalized and continued as long as the JCV-antibody status remains negative. In patients who are seropositive for JCV antibodies, caution is recommended, particularly for patients who had prior immunosuppressive drug therapy and for patients who have received treatment for more than 24 months. Even in JCV-antibody–seropositive patients, use of natalizumab may be advisable depending on disease severity, available options, and the patient’s risk tolerance. JCV-antibody testing is a rare example of a clinically useful biomarker that can guide specific treatment decisions in the field of neurology.
CURRENT PRACTICE: A PERSONAL MANAGEMENT ALGORITHM
Based on current evidence, the following opinion on the use of natalizumab for the treatment of MS is offered as a supplement to approved prescribing information. The neurologist must individualize the treatment decision for each patient and recognize that these general comments represent a personal opinion. Several factors affect decisions about the use of disease-modifying drugs in MS, and specifically use of natalizumab: How severe is the disease, and what is the prognosis for future disease progression from the neurologist’s perspective? How concerned is the patient about current or future MS symptoms and disability? What is the patient’s tolerance for medication side effects? For risk taking? Has there been prior immunosuppressive therapy? What is the JCV antibody status? What other options are available for disease management?
These issues require discussion among the neurologist, the patient, and the patient’s family. The neurologist should provide input on disease status, an opinion about prognosis, and a description of appropriate options for disease management. Many patients also want a global recommendation (ie, “Tell me what you think I should do”). The neurologist must tailor that global recommendation to the patient’s perceptions of his or her MS, its treatment, and preferences regarding treatment options.
For patients who are already receiving treatment with a first-line drug and whose disease is well controlled, I make no changes in treatment until a breakthrough occurs, at which time I recommend switching to natalizumab with JCV antibody status assessed yearly (Figure 2B). If a patient has been taking natalizumab for more than 2 years and is seronegative, I advise continuing natalizumab (Figure 2C). If seropositive after 2 or more years of natalizumab therapy, I recommend switching to fingolimod and monitoring for disease reactivation.
DISCUSSION
Dr. Calabrese: Have you perceived growing concern over PML among the MS patient population over the past 2 to 3 years?
Dr. Rudick: I would say that it’s pretty stable. Patients who are risk intolerant select out of natalizumab. Some patients would just as soon take their chances with MS rather than deal with additional risk. Since we talk about the risk of PML with patients prior to treatment, the patients who choose natalizumab are able to deal with the risk. The difficult cases are those patients who will be severely disabled before long but choose not to go on natalizumab because they’re very risk averse. It gets even more complicated when the closest family member (parents or spouse) want their relative to use natalizumab but the patient is risk averse. This can become quite complicated—for example, I’ve seen situations where the patient is a minor, and one parent wants their child to use natalizumab but the other is risk-averse. Spouses often see risk differently, and this has led to interesting and difficult discussions. In the case of a child with MS, I listen to preferences from family members, but otherwise I empower the patient to drive the decision.
Dr. Major: Our data seem to suggest a higher percentage of individuals who are seropositive—about 56%. The issue, however, is a lack of a standard to define seropositive and seronegative. I suggested that the natalizumab manufacturer collect a bank of samples and allocate them to laboratories with no vested interest for polymerase chain reaction (PCR) assays, from which consensus definitions of seropositive and seronegative could be developed.
Dr. Calabrese: Why is there no confirmatory immunoassay for this virus? We don’t have false positives for hepatitis B or human immunodeficiency virus. We still seem to be relying on older technologies.
Dr. Major: To determine the level of antibody, an enzyme-linked immunosorbent assay is just fine.
Dr. Calabrese: That’s for sensitivity, but what about specificity?
Dr. Major: Specificity is quite good. Everybody now uses the same antigen, the polyoma capsid antigen, VP1, to detect productive viral infection, but there are no set standards for the cutpoints to classify as seropositive and seronegative. Certainly, there’s high sensitivity with PCR to detect JCV DNA in cerebrospinal fluid (CSF), because we’re able to detect very low copy levels of JCV DNA in the CSF.
Dr. Simpson: I would like to see a quantitative measure of risk versus benefit for all of the drugs used in MS, not just natalizumab. When you look at any clinical trial, you see a table of adverse events and you see efficacy measures, but you don’t see the two combined. This really is necessary to compare drug A with drug B. Instead, we end up making decisions based on risk tolerance and rather soft criteria. One could argue that we don’t want to be so algorithmic that we take the art out of medicine, but the criteria we use to make decisions are quite soft. I wonder whether you have any suggestions on a more quantitative approach.
Dr. Rudick: This is an important point, but a difficult problem. We have much more information about PML associated with natalizumab than we do about many serious adverse events. For example, there are rare adverse events with interferon beta—severe depression, liver injury, and so forth. But we don’t have precise quantitative data on most adverse drug effects, and in general adverse events are underreported in clinical practice.
The natalizumab-PML situation is somewhat unique. PML is a dramatic, often fatal, disease that is virtually never observed spontaneously in MS, and the strict reporting requirements have resulted in near-complete ascertainment and more precise risk estimates. This situation doesn’t apply to most adverse events associated with other therapies—even for some severe adverse events. But you are correct—focusing exclusively on the risk of PML seems somewhat simplistic because there are clear risks with other drugs, and these need to be factored into treatment decisions.
Dr. Molloy: Do you have good tools that predict how a patient with MS will do over time?
Dr. Fox: We have fair tools, not great tools.
Dr. Rudick: We’re diagnosing MS earlier, sometimes at the first symptom. We’re even beginning to recognize it in patients without symptoms who have MS observed as an incidental MRI finding. It is difficult at the earliest stage of MS to predict severity with any confidence. The best predictor we have is the severity of the disease by MRI criteria. This can provide a general guide to treatment decisions, but it is an imprecise predictor.
Multiple sclerosis (MS) is an autoimmune disease whose inflammatory process causes demyelination, axonal loss, and neurodegeneration, all of which can lead to progressive neurologic disability. Without treatment, the risk of progressive disability 15 to 20 years after the onset of MS has been estimated to be as high as 50%.1
Seven drugs have been approved by the US Food and Drug Administration (FDA) for the treatment of MS. Of these, interferon beta drugs and glatiramer acetate are generally considered as first-line agents based on extensive experience and relative safety. Indeed, reports with followup approaching 20 years have not identified significant safety concerns. However, these agents have only modest efficacy, reducing by approximately 30% the frequency of relapse in patients with relapsing-remitting MS.2–6
Natalizumab, and more recently, fingolimod, are generally used as second-line agents. Fingolimod, the first oral agent to receive FDA approval for the treatment of relapsing-remitting MS, is a functional antagonist of sphingosine-1-phosphate receptors. The reductions in annualized relapse rates in two phase 3 controlled trials of fingolimod were approximately 55% compared with placebo7 or intramuscular interferon beta-1a.8 Because of its more convenient oral route of administration and its documented efficacy, widespread use of fingolimod is anticipated. However, adverse reactions affecting more than 10% of patients include headache, influenza, diarrhea, back pain, liver transaminase elevations, and cough.9 Because sphingosine-1-phosphate receptors are widespread in many body tissues, off-target effects of fingolimod may be problematic and long-term toxicity is unknown.
In addition to natalizumab and fingolimod, which are currently available for use as second-line agents, several other MS therapies are showing promise. Oral cladribine, teriflunomide, and laquinimod have reported positive phase 3 results in publication or at national meetings, and several other drugs are in late stages of development (alemtuzumab, BG-12, ocrelizumab) based on encouraging phase 2 results.10–12 Thus, the options for MS patients are expanding, but drugs with higher efficacy also may pose greater risk.
NATALIZUMAB: ROBUST BENEFITS BUT ASSOCIATED RISK
Natalizumab is a humanized monoclonal antibody that binds to alpha-4 integrin on leukocytes. By inhibiting alpha-4 integrin, natalizumab, the first of a new class of selective adhesion-molecule inhibitors, impedes migration of activated mononuclear leukocytes into the brain and gut.13
Significant efficacy
Two phase 3 studies demonstrated more robust efficacy of natalizumab in patients with relapsing-remitting MS than had been observed in prior studies with other agents.14,15 In the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study, which followed patients over 2 years of treatment, natalizumab was associated with a 68% reduction in the annualized relapse rate14; a 92% reduction in gadolinium-enhanced lesions on magnetic resonance imaging (MRI), which indicate new, active lesions16; and an 83% reduction in the mean number of new or enlarging T2 lesions16 compared with placebo. The likelihood of confirmed worsening on the Kurtzke Expanded Disability Status Score, which is the standard measure for MS-related disability, was also 42% lower in patients assigned to natalizumab compared with placebo.14
Other reported benefits from natalizumab therapy include a significantly increased probability of maintaining disease-free status17 and clinically significant improvements on patient-reported quality-of-life measures.18 Although there have been no head-to-head studies of natalizumab with interferon beta, glatiramer acetate, or fingolimod, there is a widespread view that treatment benefits of natalizumab exceed those of other disease-modifying drugs. In clinical practice, patients with MS who experience breakthrough disease on standard disease-modifying drugs are routinely observed to achieve disease control after switching to natalizumab. Thus, based purely on efficacy, patient-reported outcomes, and the convenience of once-monthly intravenous infusion, natalizumab represents an extremely attractive treatment option for patients with relapsing-remitting MS.
Use discontinued in 2005
Natalizumab was approved for treatment of relapsing-remitting MS in November 2004, using the FDA accelerated review pathway. The approval was based on the first-year results of the AFFIRM14 and the Natalizumab plus Interferon Beta-1a for Relapsing Remitting Multiple Sclerosis (SENTINEL)19 studies, both of which were completed in February 2005. In the 3 to 4 months between the drug’s approval and completion of the AFFIRM and SENTINEL studies, approximately 7,000 patients with relapsing-remitting MS received treatment with natalizumab. In February 2005, shortly after the release of the 2-year data, three cases of progressive multifocal leukoencephalopathy (PML) were identified in natalizumab-treated patients (one with Crohn disease, the other two with MS). Clinical and research use of natalizumab was abruptly suspended that month, pending a comprehensive safety review.
A safety study evaluated 3,116 patients who had received natalizumab over a mean exposure of 17.9 monthly doses.20 The study failed to identify any additional cases of PML and concluded that the risk of PML was approximately 1 in 1,000 patients. Abrupt discontinuation of natalizumab also allowed systematic assessment of disease behavior following treatment interruption. In 1,866 patients who had received natalizumab during clinical trials but who discontinued natalizumab after PML was recognized, MS relapses and gadolinium-enhancing lesions returned approximately to baseline levels within 4 to 7 months of natalizumab suspension. Reactivation of MS disease activity was observed even in patients who instituted one of the first-line disease-modifying drugs as substitute therapy.21
Based on the strong efficacy data and the extensive safety review, an FDA advisory committee recommended reintroduction, and natalizumab was returned to the market in June 2006. Natalizumab may be administered only in accredited infusion centers that agree to a monthly reporting regimen designed to identify all cases of PML. In the United States, natalizumab is available to patients only through the Tysabri Outreach Unified Commitment to Health (TOUCH) Prescribing Program, a restricted distribution program. Aggressive monitoring and reporting is also required in other regions of the world, so that ascertainment of PML associated with natalizumab is thought to be relatively complete.
Risk related to duration of therapy
Of the first 35 cases of natalizumab-associated PML, 10 cases (29%) were fatal. Among surviving patients, the level of disability was found to be severe in 48%; moderate in 36%; and mild in 16%.23 Improved survival was associated with younger age, less MS-related disability prior to PML, more localized disease on brain MRI at diagnosis, and shorter time from symptom onset to PML diagnosis.23 As of August 4, 2011, there were 150 confirmed cases of natalizumab-associated PML (58 in the United States, 85 in Europe, and 7 from the rest of the world); of these, 29 (19%) have died.22
VARIATION IN PATIENT RISK TOLERANCE
The postmarketing surveillance of natalizumab clearly demonstrates that risk is associated with administration of the drug, but risk tolerance varies considerably among individuals with MS. Some patients elect to use natalizumab despite the risk of PML, even when they have relatively mild MS. Other patients decline use of natalizumab even when their MS is severe and has responded poorly to other disease-modifying drugs.
In most cases, based on my experience, patients accept the risk of natalizumab-associated PML if MS disease is their primary consideration. Another major factor is the patient’s prior experiences with disease-modifying drugs; patients who have experienced breakthrough disease activity despite treatment with first-line drugs commonly opt for natalizumab regardless of the risk of PML.
Interestingly, the treating neurologist’s perception of MS severity and risk of PML may differ from the patient’s perception. In a study of 69 natalizumab-treated MS patients and 66 neurologists, Heesen et al found that patients had a significantly worse perception of their disease and were more willing to assume treatment risks and continue natalizumab therapy than their neurologists were.24 About one-half of the neurologists said that they would discontinue natalizumab at a risk level of 1 in 5,000 or lower, whereas only 17% of the patients would stop at this risk level. This finding has significant implications for clinical practice and implies that the neurologist should discuss concerns about MS and risk of treatment with the patient in order to tailor the decision to the patient’s concerns.
Interest in identifying biomarkers to aid in quantifying risk is ongoing. Chen et al found that subclinical reactivation of the JC virus (JCV) occurred frequently in 19 natalizumab-treated MS patients.25 Another study of 24 natalizumab-treated MS patients found no JCV DNA in the blood, although JCV DNA was found in the urine in 25% of patients.26 A large survey of blood and urine from natalizumab-treated MS patients found low sensitivity and specificity for JCV DNA as a predictor for subsequent PML.27 In this study of more than 1,000 natalizumab-treated patients, JCV DNA was detected in 0.3% of patients’ plasma and in 26% of patients’ urine, but PML did not develop in any patient who was JCV-positive. Among five natalizumab-treated patients who developed PML, JCV DNA was detected in none before the advent of symptoms. The presence of JCV DNA in bodily fluids is important for the diagnosis of PML, but it currently holds no predictive clinical value. At present, measuring JCV DNA in blood, cells, or urine as a predictive biomarker for natalizumab-associated PML does not appear to be clinically useful.
Stratifying risk by measuring JCV serology, however, does appear to be a useful strategy. Investigators using a two-step assay to detect and quantify JCV antibodies found 53.6% of MS patients to be seropositive, with a false-negative rate of 2.5%. Of most interest, all 17 natalizumab-associated PML patients who had available blood samples taken an average of 2 years before onset of PML tested positive for JCV antibodies.28 Although studies are ongoing, classification according to JCV-antibody status may be helpful in advising patients. Patients who are JCV-antibody seronegative (about one-half of patients) appear to be at extraordinarily low risk for PML. In these patients, use of natalizumab could be liberalized and continued as long as the JCV-antibody status remains negative. In patients who are seropositive for JCV antibodies, caution is recommended, particularly for patients who had prior immunosuppressive drug therapy and for patients who have received treatment for more than 24 months. Even in JCV-antibody–seropositive patients, use of natalizumab may be advisable depending on disease severity, available options, and the patient’s risk tolerance. JCV-antibody testing is a rare example of a clinically useful biomarker that can guide specific treatment decisions in the field of neurology.
CURRENT PRACTICE: A PERSONAL MANAGEMENT ALGORITHM
Based on current evidence, the following opinion on the use of natalizumab for the treatment of MS is offered as a supplement to approved prescribing information. The neurologist must individualize the treatment decision for each patient and recognize that these general comments represent a personal opinion. Several factors affect decisions about the use of disease-modifying drugs in MS, and specifically use of natalizumab: How severe is the disease, and what is the prognosis for future disease progression from the neurologist’s perspective? How concerned is the patient about current or future MS symptoms and disability? What is the patient’s tolerance for medication side effects? For risk taking? Has there been prior immunosuppressive therapy? What is the JCV antibody status? What other options are available for disease management?
These issues require discussion among the neurologist, the patient, and the patient’s family. The neurologist should provide input on disease status, an opinion about prognosis, and a description of appropriate options for disease management. Many patients also want a global recommendation (ie, “Tell me what you think I should do”). The neurologist must tailor that global recommendation to the patient’s perceptions of his or her MS, its treatment, and preferences regarding treatment options.
For patients who are already receiving treatment with a first-line drug and whose disease is well controlled, I make no changes in treatment until a breakthrough occurs, at which time I recommend switching to natalizumab with JCV antibody status assessed yearly (Figure 2B). If a patient has been taking natalizumab for more than 2 years and is seronegative, I advise continuing natalizumab (Figure 2C). If seropositive after 2 or more years of natalizumab therapy, I recommend switching to fingolimod and monitoring for disease reactivation.
DISCUSSION
Dr. Calabrese: Have you perceived growing concern over PML among the MS patient population over the past 2 to 3 years?
Dr. Rudick: I would say that it’s pretty stable. Patients who are risk intolerant select out of natalizumab. Some patients would just as soon take their chances with MS rather than deal with additional risk. Since we talk about the risk of PML with patients prior to treatment, the patients who choose natalizumab are able to deal with the risk. The difficult cases are those patients who will be severely disabled before long but choose not to go on natalizumab because they’re very risk averse. It gets even more complicated when the closest family member (parents or spouse) want their relative to use natalizumab but the patient is risk averse. This can become quite complicated—for example, I’ve seen situations where the patient is a minor, and one parent wants their child to use natalizumab but the other is risk-averse. Spouses often see risk differently, and this has led to interesting and difficult discussions. In the case of a child with MS, I listen to preferences from family members, but otherwise I empower the patient to drive the decision.
Dr. Major: Our data seem to suggest a higher percentage of individuals who are seropositive—about 56%. The issue, however, is a lack of a standard to define seropositive and seronegative. I suggested that the natalizumab manufacturer collect a bank of samples and allocate them to laboratories with no vested interest for polymerase chain reaction (PCR) assays, from which consensus definitions of seropositive and seronegative could be developed.
Dr. Calabrese: Why is there no confirmatory immunoassay for this virus? We don’t have false positives for hepatitis B or human immunodeficiency virus. We still seem to be relying on older technologies.
Dr. Major: To determine the level of antibody, an enzyme-linked immunosorbent assay is just fine.
Dr. Calabrese: That’s for sensitivity, but what about specificity?
Dr. Major: Specificity is quite good. Everybody now uses the same antigen, the polyoma capsid antigen, VP1, to detect productive viral infection, but there are no set standards for the cutpoints to classify as seropositive and seronegative. Certainly, there’s high sensitivity with PCR to detect JCV DNA in cerebrospinal fluid (CSF), because we’re able to detect very low copy levels of JCV DNA in the CSF.
Dr. Simpson: I would like to see a quantitative measure of risk versus benefit for all of the drugs used in MS, not just natalizumab. When you look at any clinical trial, you see a table of adverse events and you see efficacy measures, but you don’t see the two combined. This really is necessary to compare drug A with drug B. Instead, we end up making decisions based on risk tolerance and rather soft criteria. One could argue that we don’t want to be so algorithmic that we take the art out of medicine, but the criteria we use to make decisions are quite soft. I wonder whether you have any suggestions on a more quantitative approach.
Dr. Rudick: This is an important point, but a difficult problem. We have much more information about PML associated with natalizumab than we do about many serious adverse events. For example, there are rare adverse events with interferon beta—severe depression, liver injury, and so forth. But we don’t have precise quantitative data on most adverse drug effects, and in general adverse events are underreported in clinical practice.
The natalizumab-PML situation is somewhat unique. PML is a dramatic, often fatal, disease that is virtually never observed spontaneously in MS, and the strict reporting requirements have resulted in near-complete ascertainment and more precise risk estimates. This situation doesn’t apply to most adverse events associated with other therapies—even for some severe adverse events. But you are correct—focusing exclusively on the risk of PML seems somewhat simplistic because there are clear risks with other drugs, and these need to be factored into treatment decisions.
Dr. Molloy: Do you have good tools that predict how a patient with MS will do over time?
Dr. Fox: We have fair tools, not great tools.
Dr. Rudick: We’re diagnosing MS earlier, sometimes at the first symptom. We’re even beginning to recognize it in patients without symptoms who have MS observed as an incidental MRI finding. It is difficult at the earliest stage of MS to predict severity with any confidence. The best predictor we have is the severity of the disease by MRI criteria. This can provide a general guide to treatment decisions, but it is an imprecise predictor.
- Weinshenker BG, Bass B, Rice GPA, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 1999; 112:133–146.
- Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol 1996; 39:285–294.
- The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis I: clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993; 43:655–661.
- IFNB Multiple Sclerosis Study Group, The University of British Columbia MS/MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. The IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. Neurology 1995; 45:1277–1285.
- Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind, placebo-controlled trial. Neurology 1995; 45:1268–1276.
- PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 1998; 352:1498–1504.
- Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362:387–401.
- Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362:402–415.
- Gilenya [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011.
- Coles AJ, Fox E, Vladic A, et al. Alemtuzumab versus interferon beta-1a in early relapsing-remitting multiple sclerosis: post-hoc and subset analyses of clinical efficacy outcomes. Lancet Neurol 2011; 10:338–348.
- Nicholas R, Giannetti P, Alsanousi A, Friede T, Muraro PA. Development of oral immunomodulatory agents in the management of multiple sclerosis. Drug Des Devel Ther 2011; 5:255–274.
- Consortium of Multiple Sclerosis Centers (CMSC). Phase II study with ocrelizumab shows significant reduction in disease activity. CMSC Web site. http://mscare.org/cmsc/index.php?option=com_content&task=view&id=1081&Itemid=1465. Published October 15, 2010. Accessed August 26, 2011.
- Tysabri [package insert]. South San Francisco, CA: Elan Pharmaceuticals, Inc.; 2011.
- Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006; 354:899–910.
- Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2003; 348:15–23.
- Miller DH, Soon D, Fernando KT, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology 2007; 68:1390–1401.
- Havrdova E, Galetta S, Hutchinson M, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol 2009; 8:254–260.
- Rudick RA, Miller D, Hass S, et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol 2007; 62:335–346.
- Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multipls sclerosis. N Engl J Med 2006; 354:911–923.
- Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 2006; 354:924–933.
- O’Connor PW, Goodman A, Kappos L, et al. Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis [published online ahead of print May 4, 2011]. Neurology 2011; 76:1858–1865.
- Update on Tysabri and PML. National Multiple Sclerosis Society Web site. http://www.nationalmssociety.org/news/news-detail/index.aspx?nid=2308. Published April 11, 2011. Updated May 23, 2011. Accessed June 22, 2011.
- Vermersch P, Kappos L, Gold R, et al. Clinical outcomes of natalizumab-associated progressive multifocal leukoencephalopathy. Neurology 2011; 76:1697–1704.
- Heesen C, Kleiter I, Nguyen F, et al. Risk perception in natalizumab-treated multiple sclerosis patients and their neurologists. Mult Scler 2010; 16:1507–1512.
- Chen Y, Bord E, Tompkins T, et al. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med 2009; 361:1067–1074.
- Jilek S, Jaquiery E, Hirsch HH, et al. Immune responses to JC virus in patients with multiple sclerosis treated with natalizumab: a cross-sectional and longitudinal study. Lancet Neurol 2010; 9:264–272.
- Rudick RA, O’Connor PW, Polman CH, et al. Assessment of JC virus DNA in blood and urine from natalizumab-treated patients. Ann Neurol 2010; 68:304–310.
- Gorelik L, Lerner M, Bixler S, et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol 2010; 68:295–303.
- Weinshenker BG, Bass B, Rice GPA, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 1999; 112:133–146.
- Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol 1996; 39:285–294.
- The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis I: clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993; 43:655–661.
- IFNB Multiple Sclerosis Study Group, The University of British Columbia MS/MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. The IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. Neurology 1995; 45:1277–1285.
- Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind, placebo-controlled trial. Neurology 1995; 45:1268–1276.
- PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 1998; 352:1498–1504.
- Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362:387–401.
- Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362:402–415.
- Gilenya [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2011.
- Coles AJ, Fox E, Vladic A, et al. Alemtuzumab versus interferon beta-1a in early relapsing-remitting multiple sclerosis: post-hoc and subset analyses of clinical efficacy outcomes. Lancet Neurol 2011; 10:338–348.
- Nicholas R, Giannetti P, Alsanousi A, Friede T, Muraro PA. Development of oral immunomodulatory agents in the management of multiple sclerosis. Drug Des Devel Ther 2011; 5:255–274.
- Consortium of Multiple Sclerosis Centers (CMSC). Phase II study with ocrelizumab shows significant reduction in disease activity. CMSC Web site. http://mscare.org/cmsc/index.php?option=com_content&task=view&id=1081&Itemid=1465. Published October 15, 2010. Accessed August 26, 2011.
- Tysabri [package insert]. South San Francisco, CA: Elan Pharmaceuticals, Inc.; 2011.
- Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006; 354:899–910.
- Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2003; 348:15–23.
- Miller DH, Soon D, Fernando KT, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology 2007; 68:1390–1401.
- Havrdova E, Galetta S, Hutchinson M, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol 2009; 8:254–260.
- Rudick RA, Miller D, Hass S, et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol 2007; 62:335–346.
- Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multipls sclerosis. N Engl J Med 2006; 354:911–923.
- Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 2006; 354:924–933.
- O’Connor PW, Goodman A, Kappos L, et al. Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis [published online ahead of print May 4, 2011]. Neurology 2011; 76:1858–1865.
- Update on Tysabri and PML. National Multiple Sclerosis Society Web site. http://www.nationalmssociety.org/news/news-detail/index.aspx?nid=2308. Published April 11, 2011. Updated May 23, 2011. Accessed June 22, 2011.
- Vermersch P, Kappos L, Gold R, et al. Clinical outcomes of natalizumab-associated progressive multifocal leukoencephalopathy. Neurology 2011; 76:1697–1704.
- Heesen C, Kleiter I, Nguyen F, et al. Risk perception in natalizumab-treated multiple sclerosis patients and their neurologists. Mult Scler 2010; 16:1507–1512.
- Chen Y, Bord E, Tompkins T, et al. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med 2009; 361:1067–1074.
- Jilek S, Jaquiery E, Hirsch HH, et al. Immune responses to JC virus in patients with multiple sclerosis treated with natalizumab: a cross-sectional and longitudinal study. Lancet Neurol 2010; 9:264–272.
- Rudick RA, O’Connor PW, Polman CH, et al. Assessment of JC virus DNA in blood and urine from natalizumab-treated patients. Ann Neurol 2010; 68:304–310.
- Gorelik L, Lerner M, Bixler S, et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol 2010; 68:295–303.