User login
For Most With Diabetes, Revascularization Can Wait
ORLANDO – Virtually no patients with type 2 diabetes and documented coronary artery disease and coronary ischemia benefit from immediate coronary revascularization, as long as they receive intensive medical management, based on the outcomes of more than 1,000 patients who were randomized to the deferred revascularization arm of the BARI 2D trial.
The only possible exception to this approach are the rare patients who initially present with severe or unstable angina and proximal left anterior descending (LAD) artery disease, a small group accounting for just 2% of these patients, Dr. Ronald J. Krone said at the annual scientific sessions of the American Heart Association. Even in this small subgroup with the worst chance of avoiding revascularization, eventual coronary bypass surgery or percutaneous coronary intervention (PCI) is not an absolute. Among the 21 patients with this initial presentation at study entry (of the total 1,192 who were randomized to the deferred revascularization arm), 50% continued to avoid revascularization 6 months later, and 29% had still not undergone revascularization 5 years after the study began, said Dr. Krone, an interventional cardiologist and professor of medicine at Washington University, St. Louis.
"What it comes down to is that there is no group you can identify up front" that unequivocally needs immediate revascularization," Dr. Krone said in an interview. "We could not identify patients who will need revascularization at a high enough rate to warrant initial revascularization, with the possible exception" of the small proximal LAD and severe angina subgroup. "Even in the worst patients, you can intervene later. We used to be afraid that if we didn’t [revascularize these patients] they would drop dead or have a big myocardial infarction, but that didn’t happen. These results give us confidence that you don’t need to intervene on every tight lesion."
Today, a physician or surgeon can’t say "’I have to revascularize, because it’s the best I can do’" for these patients. Instead, the onus is to intensively treat these patients medically, especially patients with diabetes, Dr. Krone said. This strategy includes optimal control of hypertension, lipids, glycemia, and intensive lifestyle intervention with exercise, diet, and smoking cessation.
The analysis he presented focused on patients enrolled in the BARI 2D (Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes), which randomized a total of 2,368 patients with diabetes and documented coronary ischemia and stenosis suitable for an elective intervention. The researchers put all these patients on an intensive medical management regimen, and also randomized them to either immediate or deferred revascularization. The study’s primary results showed absolutely identical 5-year outcomes in the two groups, with a mortality rate of 12% in each arm of the study, and a combined rate of death, MI, or stroke of 23% in the immediate revascularization patients and 24% in those with deferred intervention (N. Engl. J. Med. 2009;360:2503-15).
Among the 1,192 patients in the deferred subgroup, 13% required PCI or bypass surgery after 6 months, and 40% needed revascularization after 5 years of follow-up. Within the group who eventually had revascularization, 47% required it for worsening angina, 23% because of an acute coronary syndrome event, 18% for worsening ischemia, 6% for progression of their coronary disease, and the remaining 6% for another reason. The current analysis aimed to determine whether "we can identify patients with such a high likelihood of needing revascularization that it need not be deferred," Dr. Krone said.
The average age of the patients in the deferred revascularization group was 62 years; 30% were women, 28% were on insulin treatment, 17% had a left ventricular ejection fraction below 50%, and 13% had proximal LAD coronary disease. Their average duration of type 2 diabetes was 11 years.
A multivariate analysis that controlled for age, sex, race, and nationality identified five factors that were linked with a significantly increased rate of revascularization after 6 months: class III or IV stable angina, unstable angina, a systolic blood pressure of 100 mm Hg or less, a blood triglyceride level of 100 mg/dL or less, and proximal LAD disease. These factors were linked with anywhere from a 3.8-fold increased rate of revascularization (in patients with systolic hypotension, compared with patients with a systolic pressure greater than 100 mm Hg) to a 75% increased rate (in patients with proximal LAD disease, compared with those without LAD disease). However, none of these increased rates appeared to justify performing routine, upfront revascularization.
The 5-year multivariate analysis produced similar results. It identified nine baseline factors that each significantly linked with a significantly increased rate of revascularization during 5-year follow-up: class I or II stable angina, class III or IV stable angina, unstable angina, systolic blood pressure of 101-120 mm Hg, a systolic pressure of 100 mm HG or less, a blood triglyceride level of 100 mg/dL or greater, proximal LAD disease, having two diseased coronary regions, or having three diseased coronary regions. The increased rates associated with these features ranged from a 90% increased revascularization rate (in patients with class III or IV stable angina, compared with patients without angina), to a 28% increased revascularization rate (in patients with class I or II stable angina at baseline). Again, none of these increased rates appeared to justify uniform, upfront revascularization, Dr. Krone said.
The sole exception to this approach might possibly be the small number of patients who initially presented with both proximal LAD disease and either class III or IV stable angina or unstable angina, because eventually over 5 years 71% of these patients underwent revascularization. But these patients constituted only 2% of the total group studied, Dr. Krone noted. In general, more severe angina or stenosis was uncommon in these patients: Some 41% had no angina and 45% had class I or II angina at baseline, and 87% were free of proximal LAD disease at baseline.
Dr. Krone said that he had no disclosures.
ORLANDO – Virtually no patients with type 2 diabetes and documented coronary artery disease and coronary ischemia benefit from immediate coronary revascularization, as long as they receive intensive medical management, based on the outcomes of more than 1,000 patients who were randomized to the deferred revascularization arm of the BARI 2D trial.
The only possible exception to this approach are the rare patients who initially present with severe or unstable angina and proximal left anterior descending (LAD) artery disease, a small group accounting for just 2% of these patients, Dr. Ronald J. Krone said at the annual scientific sessions of the American Heart Association. Even in this small subgroup with the worst chance of avoiding revascularization, eventual coronary bypass surgery or percutaneous coronary intervention (PCI) is not an absolute. Among the 21 patients with this initial presentation at study entry (of the total 1,192 who were randomized to the deferred revascularization arm), 50% continued to avoid revascularization 6 months later, and 29% had still not undergone revascularization 5 years after the study began, said Dr. Krone, an interventional cardiologist and professor of medicine at Washington University, St. Louis.
"What it comes down to is that there is no group you can identify up front" that unequivocally needs immediate revascularization," Dr. Krone said in an interview. "We could not identify patients who will need revascularization at a high enough rate to warrant initial revascularization, with the possible exception" of the small proximal LAD and severe angina subgroup. "Even in the worst patients, you can intervene later. We used to be afraid that if we didn’t [revascularize these patients] they would drop dead or have a big myocardial infarction, but that didn’t happen. These results give us confidence that you don’t need to intervene on every tight lesion."
Today, a physician or surgeon can’t say "’I have to revascularize, because it’s the best I can do’" for these patients. Instead, the onus is to intensively treat these patients medically, especially patients with diabetes, Dr. Krone said. This strategy includes optimal control of hypertension, lipids, glycemia, and intensive lifestyle intervention with exercise, diet, and smoking cessation.
The analysis he presented focused on patients enrolled in the BARI 2D (Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes), which randomized a total of 2,368 patients with diabetes and documented coronary ischemia and stenosis suitable for an elective intervention. The researchers put all these patients on an intensive medical management regimen, and also randomized them to either immediate or deferred revascularization. The study’s primary results showed absolutely identical 5-year outcomes in the two groups, with a mortality rate of 12% in each arm of the study, and a combined rate of death, MI, or stroke of 23% in the immediate revascularization patients and 24% in those with deferred intervention (N. Engl. J. Med. 2009;360:2503-15).
Among the 1,192 patients in the deferred subgroup, 13% required PCI or bypass surgery after 6 months, and 40% needed revascularization after 5 years of follow-up. Within the group who eventually had revascularization, 47% required it for worsening angina, 23% because of an acute coronary syndrome event, 18% for worsening ischemia, 6% for progression of their coronary disease, and the remaining 6% for another reason. The current analysis aimed to determine whether "we can identify patients with such a high likelihood of needing revascularization that it need not be deferred," Dr. Krone said.
The average age of the patients in the deferred revascularization group was 62 years; 30% were women, 28% were on insulin treatment, 17% had a left ventricular ejection fraction below 50%, and 13% had proximal LAD coronary disease. Their average duration of type 2 diabetes was 11 years.
A multivariate analysis that controlled for age, sex, race, and nationality identified five factors that were linked with a significantly increased rate of revascularization after 6 months: class III or IV stable angina, unstable angina, a systolic blood pressure of 100 mm Hg or less, a blood triglyceride level of 100 mg/dL or less, and proximal LAD disease. These factors were linked with anywhere from a 3.8-fold increased rate of revascularization (in patients with systolic hypotension, compared with patients with a systolic pressure greater than 100 mm Hg) to a 75% increased rate (in patients with proximal LAD disease, compared with those without LAD disease). However, none of these increased rates appeared to justify performing routine, upfront revascularization.
The 5-year multivariate analysis produced similar results. It identified nine baseline factors that each significantly linked with a significantly increased rate of revascularization during 5-year follow-up: class I or II stable angina, class III or IV stable angina, unstable angina, systolic blood pressure of 101-120 mm Hg, a systolic pressure of 100 mm HG or less, a blood triglyceride level of 100 mg/dL or greater, proximal LAD disease, having two diseased coronary regions, or having three diseased coronary regions. The increased rates associated with these features ranged from a 90% increased revascularization rate (in patients with class III or IV stable angina, compared with patients without angina), to a 28% increased revascularization rate (in patients with class I or II stable angina at baseline). Again, none of these increased rates appeared to justify uniform, upfront revascularization, Dr. Krone said.
The sole exception to this approach might possibly be the small number of patients who initially presented with both proximal LAD disease and either class III or IV stable angina or unstable angina, because eventually over 5 years 71% of these patients underwent revascularization. But these patients constituted only 2% of the total group studied, Dr. Krone noted. In general, more severe angina or stenosis was uncommon in these patients: Some 41% had no angina and 45% had class I or II angina at baseline, and 87% were free of proximal LAD disease at baseline.
Dr. Krone said that he had no disclosures.
ORLANDO – Virtually no patients with type 2 diabetes and documented coronary artery disease and coronary ischemia benefit from immediate coronary revascularization, as long as they receive intensive medical management, based on the outcomes of more than 1,000 patients who were randomized to the deferred revascularization arm of the BARI 2D trial.
The only possible exception to this approach are the rare patients who initially present with severe or unstable angina and proximal left anterior descending (LAD) artery disease, a small group accounting for just 2% of these patients, Dr. Ronald J. Krone said at the annual scientific sessions of the American Heart Association. Even in this small subgroup with the worst chance of avoiding revascularization, eventual coronary bypass surgery or percutaneous coronary intervention (PCI) is not an absolute. Among the 21 patients with this initial presentation at study entry (of the total 1,192 who were randomized to the deferred revascularization arm), 50% continued to avoid revascularization 6 months later, and 29% had still not undergone revascularization 5 years after the study began, said Dr. Krone, an interventional cardiologist and professor of medicine at Washington University, St. Louis.
"What it comes down to is that there is no group you can identify up front" that unequivocally needs immediate revascularization," Dr. Krone said in an interview. "We could not identify patients who will need revascularization at a high enough rate to warrant initial revascularization, with the possible exception" of the small proximal LAD and severe angina subgroup. "Even in the worst patients, you can intervene later. We used to be afraid that if we didn’t [revascularize these patients] they would drop dead or have a big myocardial infarction, but that didn’t happen. These results give us confidence that you don’t need to intervene on every tight lesion."
Today, a physician or surgeon can’t say "’I have to revascularize, because it’s the best I can do’" for these patients. Instead, the onus is to intensively treat these patients medically, especially patients with diabetes, Dr. Krone said. This strategy includes optimal control of hypertension, lipids, glycemia, and intensive lifestyle intervention with exercise, diet, and smoking cessation.
The analysis he presented focused on patients enrolled in the BARI 2D (Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes), which randomized a total of 2,368 patients with diabetes and documented coronary ischemia and stenosis suitable for an elective intervention. The researchers put all these patients on an intensive medical management regimen, and also randomized them to either immediate or deferred revascularization. The study’s primary results showed absolutely identical 5-year outcomes in the two groups, with a mortality rate of 12% in each arm of the study, and a combined rate of death, MI, or stroke of 23% in the immediate revascularization patients and 24% in those with deferred intervention (N. Engl. J. Med. 2009;360:2503-15).
Among the 1,192 patients in the deferred subgroup, 13% required PCI or bypass surgery after 6 months, and 40% needed revascularization after 5 years of follow-up. Within the group who eventually had revascularization, 47% required it for worsening angina, 23% because of an acute coronary syndrome event, 18% for worsening ischemia, 6% for progression of their coronary disease, and the remaining 6% for another reason. The current analysis aimed to determine whether "we can identify patients with such a high likelihood of needing revascularization that it need not be deferred," Dr. Krone said.
The average age of the patients in the deferred revascularization group was 62 years; 30% were women, 28% were on insulin treatment, 17% had a left ventricular ejection fraction below 50%, and 13% had proximal LAD coronary disease. Their average duration of type 2 diabetes was 11 years.
A multivariate analysis that controlled for age, sex, race, and nationality identified five factors that were linked with a significantly increased rate of revascularization after 6 months: class III or IV stable angina, unstable angina, a systolic blood pressure of 100 mm Hg or less, a blood triglyceride level of 100 mg/dL or less, and proximal LAD disease. These factors were linked with anywhere from a 3.8-fold increased rate of revascularization (in patients with systolic hypotension, compared with patients with a systolic pressure greater than 100 mm Hg) to a 75% increased rate (in patients with proximal LAD disease, compared with those without LAD disease). However, none of these increased rates appeared to justify performing routine, upfront revascularization.
The 5-year multivariate analysis produced similar results. It identified nine baseline factors that each significantly linked with a significantly increased rate of revascularization during 5-year follow-up: class I or II stable angina, class III or IV stable angina, unstable angina, systolic blood pressure of 101-120 mm Hg, a systolic pressure of 100 mm HG or less, a blood triglyceride level of 100 mg/dL or greater, proximal LAD disease, having two diseased coronary regions, or having three diseased coronary regions. The increased rates associated with these features ranged from a 90% increased revascularization rate (in patients with class III or IV stable angina, compared with patients without angina), to a 28% increased revascularization rate (in patients with class I or II stable angina at baseline). Again, none of these increased rates appeared to justify uniform, upfront revascularization, Dr. Krone said.
The sole exception to this approach might possibly be the small number of patients who initially presented with both proximal LAD disease and either class III or IV stable angina or unstable angina, because eventually over 5 years 71% of these patients underwent revascularization. But these patients constituted only 2% of the total group studied, Dr. Krone noted. In general, more severe angina or stenosis was uncommon in these patients: Some 41% had no angina and 45% had class I or II angina at baseline, and 87% were free of proximal LAD disease at baseline.
Dr. Krone said that he had no disclosures.
FROM THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN HEART ASSOCIATION
Major Finding: Few patients with diabetes and documented ischemic coronary disease suitable for elective revascularization have features that predict a high risk for eventually requiring a procedure during the subsequent 5 years. The only possible exception is the 2% of patients with both proximal LAD coronary disease and severe or unstable angina at baseline, who had a 71% revascularization rate.
Data Source: A subgroup analysis of the BARI 2D study, which randomized 2,368 patients with type 2 diabetes and documented ischemic coronary disease suitable for elective revascularization to an immediate or deferred procedure. The new analysis focused on 1,192 patients initially randomized to the delayed revascularization arm.
Disclosures: Dr. Krone said that he had no disclosures.
JAK Inhibitor Ruxolitinib Wins First FDA Approval in Myelofibrosis
In a much-anticipated double milestone, the Food and Drug Administration has approved ruxolitinib for treatment of patients with myelofibrosis.
Ruxolitinib, an orphan drug to be marketed as Jakafi by Incyte Corp., becomes the first agent to be approved for the rare blood disease. The indication covers patients with intermediate or high-risk myelofibrosis (MF), including primary MF, post-polycythemia vera MF, and post-essential thrombocythemia MF, according to a statement from Wilmington, Del.–based Incyte.
The FDA decision also makes ruxolitinib the first approved agent in a new class of drugs called JAK (Janus-associated kinase) inhibitors. Deregulation of signaling in the JAK pathway is believed to be associated with the enlarged spleen and other symptoms of myelofibrosis. Ruxolitinib inhibits the tyrosine kinases JAK1 and JAK2, which are suspected of being up-regulated in various inflammatory disorders and malignancies.
"Jakafi represents another example of an increasing trend in oncology where a detailed scientific understanding of the mechanisms of a disease allows a drug to be directed toward specific molecular pathways," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the agency’s announcement.
"The clinical trials leading to this approval focused on problems that patients with myelofibrosis commonly encounter, including enlarged spleens and pain," he noted.
In the pivotal phase III COMFORT-I and COMFORT-II trials, ruxolitinib produced substantial symptom relief in patients who were resistant or refractory to available myelofibrosis therapy or ineligible for allogeneic bone marrow transplantation. All 528 patients in these studies had enlarged spleens (splenomegaly) and other disease-related symptoms. They were assigned to treatment with ruxolitinib, placebo, or best available therapy (usually hydroxyurea or glucocorticoids).
More patients on ruxolitinib had a greater-than-35% reduction in spleen size, compared with those given the alternatives, the FDA noted. Similarly, patients on ruxolitinib were more likely to have a more-than-50% reduction in MF-related symptoms, such as abdominal discomfort, night sweats, itching, and bone or muscle pain, compared with placebo.
The Incyte announcement noted that 41.9% of patients who were treated with ruxolitinib in the COMFORT-I trial had a 35% or greater reduction in spleen volume at 24 weeks, compared with 0.7% of patients taking placebo (P less than 0.0001). The median time to response was less than 4 weeks.
In the COMFORT-II trial, 28.5% of patients who were treated with ruxolitinib had a 35% or greater reduction in spleen volume at 48 weeks, compared with none of the patients in the best available therapy arm, Incyte said. COMFORT-II was conducted by Novartis, which is collaborating with Incyte outside the United States.
Incyte said that the ruxolitinib dosage should be adjusted based on safety and efficacy. The recommended starting dose of ruxolitinib for most patients of 15 mg or 20 mg given orally twice daily based on the patient’s platelet count. A blood cell count must be performed before initiation of therapy, the company said, and complete blood counts should be monitored every 2-4 weeks until doses are stabilized.
Thrombocytopenia, anemia, fatigue, diarrhea, dyspnea, headache, dizziness, and nausea were the most common side effects, according to the FDA.
In a much-anticipated double milestone, the Food and Drug Administration has approved ruxolitinib for treatment of patients with myelofibrosis.
Ruxolitinib, an orphan drug to be marketed as Jakafi by Incyte Corp., becomes the first agent to be approved for the rare blood disease. The indication covers patients with intermediate or high-risk myelofibrosis (MF), including primary MF, post-polycythemia vera MF, and post-essential thrombocythemia MF, according to a statement from Wilmington, Del.–based Incyte.
The FDA decision also makes ruxolitinib the first approved agent in a new class of drugs called JAK (Janus-associated kinase) inhibitors. Deregulation of signaling in the JAK pathway is believed to be associated with the enlarged spleen and other symptoms of myelofibrosis. Ruxolitinib inhibits the tyrosine kinases JAK1 and JAK2, which are suspected of being up-regulated in various inflammatory disorders and malignancies.
"Jakafi represents another example of an increasing trend in oncology where a detailed scientific understanding of the mechanisms of a disease allows a drug to be directed toward specific molecular pathways," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the agency’s announcement.
"The clinical trials leading to this approval focused on problems that patients with myelofibrosis commonly encounter, including enlarged spleens and pain," he noted.
In the pivotal phase III COMFORT-I and COMFORT-II trials, ruxolitinib produced substantial symptom relief in patients who were resistant or refractory to available myelofibrosis therapy or ineligible for allogeneic bone marrow transplantation. All 528 patients in these studies had enlarged spleens (splenomegaly) and other disease-related symptoms. They were assigned to treatment with ruxolitinib, placebo, or best available therapy (usually hydroxyurea or glucocorticoids).
More patients on ruxolitinib had a greater-than-35% reduction in spleen size, compared with those given the alternatives, the FDA noted. Similarly, patients on ruxolitinib were more likely to have a more-than-50% reduction in MF-related symptoms, such as abdominal discomfort, night sweats, itching, and bone or muscle pain, compared with placebo.
The Incyte announcement noted that 41.9% of patients who were treated with ruxolitinib in the COMFORT-I trial had a 35% or greater reduction in spleen volume at 24 weeks, compared with 0.7% of patients taking placebo (P less than 0.0001). The median time to response was less than 4 weeks.
In the COMFORT-II trial, 28.5% of patients who were treated with ruxolitinib had a 35% or greater reduction in spleen volume at 48 weeks, compared with none of the patients in the best available therapy arm, Incyte said. COMFORT-II was conducted by Novartis, which is collaborating with Incyte outside the United States.
Incyte said that the ruxolitinib dosage should be adjusted based on safety and efficacy. The recommended starting dose of ruxolitinib for most patients of 15 mg or 20 mg given orally twice daily based on the patient’s platelet count. A blood cell count must be performed before initiation of therapy, the company said, and complete blood counts should be monitored every 2-4 weeks until doses are stabilized.
Thrombocytopenia, anemia, fatigue, diarrhea, dyspnea, headache, dizziness, and nausea were the most common side effects, according to the FDA.
In a much-anticipated double milestone, the Food and Drug Administration has approved ruxolitinib for treatment of patients with myelofibrosis.
Ruxolitinib, an orphan drug to be marketed as Jakafi by Incyte Corp., becomes the first agent to be approved for the rare blood disease. The indication covers patients with intermediate or high-risk myelofibrosis (MF), including primary MF, post-polycythemia vera MF, and post-essential thrombocythemia MF, according to a statement from Wilmington, Del.–based Incyte.
The FDA decision also makes ruxolitinib the first approved agent in a new class of drugs called JAK (Janus-associated kinase) inhibitors. Deregulation of signaling in the JAK pathway is believed to be associated with the enlarged spleen and other symptoms of myelofibrosis. Ruxolitinib inhibits the tyrosine kinases JAK1 and JAK2, which are suspected of being up-regulated in various inflammatory disorders and malignancies.
"Jakafi represents another example of an increasing trend in oncology where a detailed scientific understanding of the mechanisms of a disease allows a drug to be directed toward specific molecular pathways," Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the agency’s announcement.
"The clinical trials leading to this approval focused on problems that patients with myelofibrosis commonly encounter, including enlarged spleens and pain," he noted.
In the pivotal phase III COMFORT-I and COMFORT-II trials, ruxolitinib produced substantial symptom relief in patients who were resistant or refractory to available myelofibrosis therapy or ineligible for allogeneic bone marrow transplantation. All 528 patients in these studies had enlarged spleens (splenomegaly) and other disease-related symptoms. They were assigned to treatment with ruxolitinib, placebo, or best available therapy (usually hydroxyurea or glucocorticoids).
More patients on ruxolitinib had a greater-than-35% reduction in spleen size, compared with those given the alternatives, the FDA noted. Similarly, patients on ruxolitinib were more likely to have a more-than-50% reduction in MF-related symptoms, such as abdominal discomfort, night sweats, itching, and bone or muscle pain, compared with placebo.
The Incyte announcement noted that 41.9% of patients who were treated with ruxolitinib in the COMFORT-I trial had a 35% or greater reduction in spleen volume at 24 weeks, compared with 0.7% of patients taking placebo (P less than 0.0001). The median time to response was less than 4 weeks.
In the COMFORT-II trial, 28.5% of patients who were treated with ruxolitinib had a 35% or greater reduction in spleen volume at 48 weeks, compared with none of the patients in the best available therapy arm, Incyte said. COMFORT-II was conducted by Novartis, which is collaborating with Incyte outside the United States.
Incyte said that the ruxolitinib dosage should be adjusted based on safety and efficacy. The recommended starting dose of ruxolitinib for most patients of 15 mg or 20 mg given orally twice daily based on the patient’s platelet count. A blood cell count must be performed before initiation of therapy, the company said, and complete blood counts should be monitored every 2-4 weeks until doses are stabilized.
Thrombocytopenia, anemia, fatigue, diarrhea, dyspnea, headache, dizziness, and nausea were the most common side effects, according to the FDA.
MGMA, ACMPE Name Hospitalist "Physician Executive of the Year"
Modesty comes naturally to IPC The Hospitalist Co. executive Dave Bowman, MD. He shies from the spotlight and seeks to downplay his own accomplishments in favor of talking about the results of those he works with.
That tack got a bit more difficult last month when Dr. Bowman, based in Tucson, Ariz., received the Medical Group Management Association (MGMA) and American College of Medical Practice Executives' (ACMPE) "Physician Executive of the Year" award for 2011. It's the second year in a row the honor went to an HM leader; last year's winner was IPC chief executive Adam Singer, MD.
Dr. Bowman was praised both for his professional skills and the heroic role he played providing medical aid in the immediate aftermath of the Jan. 8 shooting in Tucson that left six people dead and injured 13 others, including U.S. Rep. Gabrielle Giffords (D-Ariz.)
Dr. Bowman tried to downplay the award until it was presented at a conference last month in Las Vegas. "When I step back and look at it from a non-physician-jaundiced view, that was a pretty neat thing. I was very humbled and grateful," he says.
He quickly adds, though, that the award means those he works with are doing their jobs just as exceptionally.
"You have to have a team to take care of people," he says. "If you're a lone wolf, you can do a good job for your 16 patients that day. But what happens when you leave? ... You have to be part of a team to ensure the good work you’re doing is continued."
Dr. Bowman, IPC's executive director in Tucson, has grown his group's practice to more than 75 physicians and non-physician providers. He notes that all of his providers with at least one year of seniority sit on at least one committee at their institution.
But his most sage advice for hospitalist leaders?
"Get involved, be out there," he says. "Take night call because you have two letters after your name that says you can do it. ... Be involved clinically, not just administratively."
Modesty comes naturally to IPC The Hospitalist Co. executive Dave Bowman, MD. He shies from the spotlight and seeks to downplay his own accomplishments in favor of talking about the results of those he works with.
That tack got a bit more difficult last month when Dr. Bowman, based in Tucson, Ariz., received the Medical Group Management Association (MGMA) and American College of Medical Practice Executives' (ACMPE) "Physician Executive of the Year" award for 2011. It's the second year in a row the honor went to an HM leader; last year's winner was IPC chief executive Adam Singer, MD.
Dr. Bowman was praised both for his professional skills and the heroic role he played providing medical aid in the immediate aftermath of the Jan. 8 shooting in Tucson that left six people dead and injured 13 others, including U.S. Rep. Gabrielle Giffords (D-Ariz.)
Dr. Bowman tried to downplay the award until it was presented at a conference last month in Las Vegas. "When I step back and look at it from a non-physician-jaundiced view, that was a pretty neat thing. I was very humbled and grateful," he says.
He quickly adds, though, that the award means those he works with are doing their jobs just as exceptionally.
"You have to have a team to take care of people," he says. "If you're a lone wolf, you can do a good job for your 16 patients that day. But what happens when you leave? ... You have to be part of a team to ensure the good work you’re doing is continued."
Dr. Bowman, IPC's executive director in Tucson, has grown his group's practice to more than 75 physicians and non-physician providers. He notes that all of his providers with at least one year of seniority sit on at least one committee at their institution.
But his most sage advice for hospitalist leaders?
"Get involved, be out there," he says. "Take night call because you have two letters after your name that says you can do it. ... Be involved clinically, not just administratively."
Modesty comes naturally to IPC The Hospitalist Co. executive Dave Bowman, MD. He shies from the spotlight and seeks to downplay his own accomplishments in favor of talking about the results of those he works with.
That tack got a bit more difficult last month when Dr. Bowman, based in Tucson, Ariz., received the Medical Group Management Association (MGMA) and American College of Medical Practice Executives' (ACMPE) "Physician Executive of the Year" award for 2011. It's the second year in a row the honor went to an HM leader; last year's winner was IPC chief executive Adam Singer, MD.
Dr. Bowman was praised both for his professional skills and the heroic role he played providing medical aid in the immediate aftermath of the Jan. 8 shooting in Tucson that left six people dead and injured 13 others, including U.S. Rep. Gabrielle Giffords (D-Ariz.)
Dr. Bowman tried to downplay the award until it was presented at a conference last month in Las Vegas. "When I step back and look at it from a non-physician-jaundiced view, that was a pretty neat thing. I was very humbled and grateful," he says.
He quickly adds, though, that the award means those he works with are doing their jobs just as exceptionally.
"You have to have a team to take care of people," he says. "If you're a lone wolf, you can do a good job for your 16 patients that day. But what happens when you leave? ... You have to be part of a team to ensure the good work you’re doing is continued."
Dr. Bowman, IPC's executive director in Tucson, has grown his group's practice to more than 75 physicians and non-physician providers. He notes that all of his providers with at least one year of seniority sit on at least one committee at their institution.
But his most sage advice for hospitalist leaders?
"Get involved, be out there," he says. "Take night call because you have two letters after your name that says you can do it. ... Be involved clinically, not just administratively."
Pediatric Deterioration Risk Score
Thousands of hospitals have implemented rapid response systems in recent years in attempts to reduce mortality outside the intensive care unit (ICU).1 These systems have 2 components, a response arm and an identification arm. The response arm is usually comprised of a multidisciplinary critical care team that responds to calls for urgent assistance outside the ICU; this team is often called a rapid response team or a medical emergency team. The identification arm comes in 2 forms, predictive and detective. Predictive tools estimate a patient's risk of deterioration over time based on factors that are not rapidly changing, such as elements of the patient's history. In contrast, detective tools include highly time‐varying signs of active deterioration, such as vital sign abnormalities.2 To date, most pediatric studies have focused on developing detective tools, including several early warning scores.38
In this study, we sought to identify the characteristics that increase the probability that a hospitalized child will deteriorate, and combine these characteristics into a predictive score. Tools like this may be helpful in identifying and triaging the subset of high‐risk children who should be intensively monitored for early signs of deterioration at the time of admission, as well as in identifying very low‐risk children who, in the absence of other clinical concerns, may be monitored less intensively.
METHODS
Detailed methods, including the inclusion/exclusion criteria, the matching procedures, and a full description of the statistical analysis are provided as an appendix (see Supporting Online Appendix: Supplement to Methods Section in the online version of this article). An abbreviated version follows.
Design
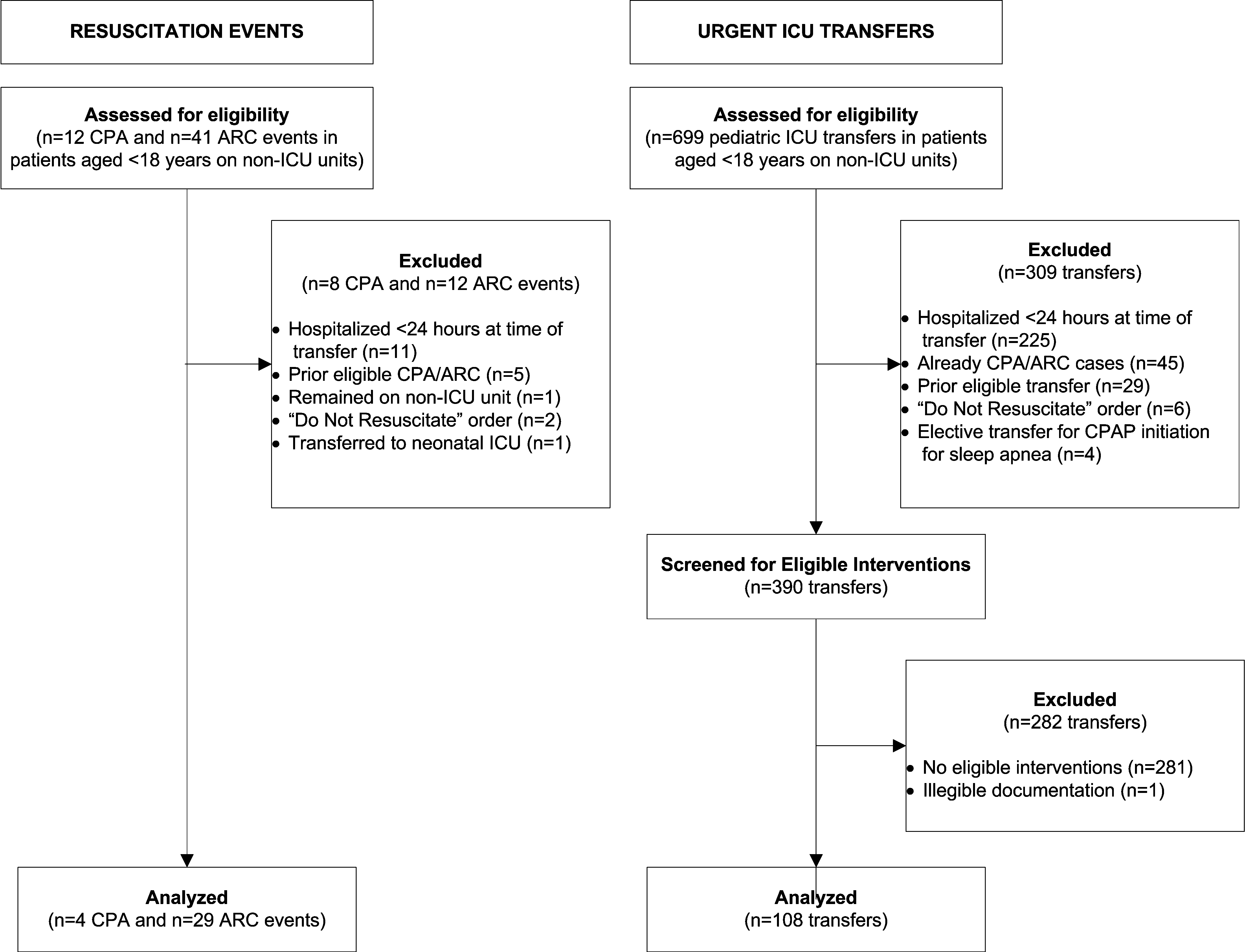
We performed a case‐control study among children, younger than 18 years old, hospitalized for >24 hours between January 1, 2005 and December 31, 2008. The case group consisted of children who experienced clinical deterioration, a composite outcome defined as cardiopulmonary arrest (CPA), acute respiratory compromise (ARC), or urgent ICU transfer, while on a non‐ICU unit. ICU transfers were considered urgent if they included at least one of the following outcomes in the 12 hours after transfer: death, CPA, intubation, initiation of noninvasive ventilation, or administration of a vasoactive medication infusion used for the treatment of shock. The control group consisted of a random sample of patients matched 3:1 to cases if they met the criteria of being on a non‐ICU unit at the same time as their matched case.
Variables and Measurements
We collected data on demographics, complex chronic conditions (CCCs), other patient characteristics, and laboratory studies. CCCs were specific diagnoses divided into the following 9 categories according to an established framework: neuromuscular, cardiovascular, respiratory, renal, gastrointestinal, hematologic/emmmunologic, metabolic, malignancy, and genetic/congenital defects.9 Other patient characteristics evaluated included age, weight‐for‐age, gestational age, history of transplant, time from hospital admission to event, recent ICU stays, administration of total parenteral nutrition, use of a patient‐controlled analgesia pump, and presence of medical devices including central venous lines and enteral tubes (naso‐gastric, gastrostomy, or jejunostomy).
Laboratory studies evaluated included hemoglobin value, white blood cell count, and blood culture drawn in the preceding 72 hours. We included these laboratory studies in this predictive score because we hypothesized that they represented factors that increased a child's risk of deterioration over time, as opposed to signs of acute deterioration that would be more appropriate for a detective score.
Statistical Analysis
We used conditional logistic regression for the bivariable and multivariable analyses to account for the matching. We derived the predictive score using an established method10 in which the regression coefficients for each covariate were divided by the smallest coefficient, and then rounded to the nearest integer, to establish each variable's sub‐score. We grouped the total scores into very low, low, intermediate, and high‐risk groups, calculated overall stratum‐specific likelihood ratios (SSLRs), and estimated stratum‐specific probabilities of deterioration for each group.
RESULTS
Patient Characteristics
We identified 12 CPAs, 41 ARCs, and 699 urgent ICU transfers during the study period. A total of 141 cases met our strict criteria for inclusion (see Figure in Supporting Online Appendix: Supplement to Methods Section in the online version of this article) among approximately 96,000 admissions during the study period, making the baseline incidence of events (pre‐test probability) approximately 0.15%. The case and control groups were similar in age, sex, and family‐reported race/ethnicity. Cases had been hospitalized longer than controls at the time of their event, were less likely to have been on a surgical service, and were less likely to survive to hospital discharge (Table 1). There was a high prevalence of CCCs among both cases and controls; 78% of cases and 52% of controls had at least 1 CCC.
| Cases (n = 141) | Controls (n = 423) | ||
|---|---|---|---|
| n (%) | n (%) | P Value | |
| |||
| Type of event | |||
| Cardiopulmonary arrest | 4 (3) | 0 | NA |
| Acute respiratory compromise | 29 (20) | 0 | NA |
| Urgent ICU transfer | 108 (77) | 0 | NA |
| Demographics | |||
| Age | 0.34 | ||
| 0‐6 mo | 17 (12) | 62 (15) | |
| 6‐12 mo | 22 (16) | 41 (10) | |
| 1‐4 yr | 34 (24) | 97 (23) | |
| 4‐10 yr | 26 (18) | 78 (18) | |
| 10‐18 yr | 42 (30) | 145 (34) | |
| Sex | 0.70 | ||
| Female | 60 (43) | 188 (44) | |
| Male | 81 (57) | 235 (56) | |
| Race | 0.40 | ||
| White | 69 (49) | 189 (45) | |
| Black/African‐American | 49 (35) | 163 (38) | |
| Asian/Pacific Islander | 0 (0) | 7 (2) | |
| Other | 23 (16) | 62 (15) | |
| Not reported | 0 (0) | 2 (1) | |
| Ethnicity | 0.53 | ||
| Non‐Hispanic | 127 (90) | 388 (92) | |
| Hispanic | 14 (10) | 33 (8) | |
| Unknown/not reported | 0 (0) | 2 (1) | |
| Hospitalization | |||
| Length of stay in days, median (interquartile range) | 7.8 (2.6‐18.2) | 3.9 (1.9‐11.2) | 0.001 |
| Surgical service | 4 (3) | 67 (16) | 0.001 |
| Survived to hospital discharge | 107 (76) | 421 (99.5) | 0.001 |
Unadjusted (Bivariable) Analysis
Results of bivariable analysis are shown in Table 2.
| Variable | Cases n (%) | Controls n (%) | OR* | 95% CI | P Value |
|---|---|---|---|---|---|
| |||||
| Complex chronic conditions categories | |||||
| Congenital/genetic | 19 (13) | 21 (5) | 3.0 | 1.6‐5.8 | 0.001 |
| Neuromuscular | 31 (22) | 48 (11) | 2.2 | 1.3‐3.7 | 0.002 |
| Respiratory | 18 (13) | 27 (6) | 2.0 | 1.1‐3.7 | 0.02 |
| Cardiovascular | 15 (10) | 24 (6) | 2.0 | 1.0‐3.9 | 0.05 |
| Metabolic | 5 (3) | 6 (1) | 2.5 | 0.8‐8.2 | 0.13 |
| Gastrointestinal | 10 (7) | 24 (6) | 1.3 | 0.6‐2.7 | 0.54 |
| Renal | 3 (2) | 8 (2) | 1.1 | 0.3‐4.2 | 0.86 |
| Hematology/emmmunodeficiency | 6 (4) | 19 (4) | 0.9 | 0.4‐2.4 | 0.91 |
| Specific conditions | |||||
| Mental retardation | 21 (15) | 25 (6) | 2.7 | 1.5‐4.9 | 0.001 |
| Malignancy | 49 (35) | 90 (21) | 1.9 | 1.3‐2.8 | 0.002 |
| Epilepsy | 22 (15) | 30 (7) | 2.4 | 1.3‐4.3 | 0.004 |
| Cardiac malformations | 14 (10) | 19 (4) | 2.2 | 1.1‐4.4 | 0.02 |
| Chronic respiratory disease arising in the perinatal period | 11 (8) | 15 (4) | 2.2 | 1.0‐4.8 | 0.05 |
| Cerebral palsy | 7 (5) | 13 (3) | 1.7 | 0.6‐4.2 | 0.30 |
| Cystic fibrosis | 1 (1) | 9 (2) | 0.3 | 0.1‐2.6 | 0.30 |
| Other patient characteristics | |||||
| Time from hospital admission to event 7 days | 74 (52) | 146 (35) | 2.1 | 1.4‐3.1 | 0.001 |
| History of any transplant | 27 (19) | 17 (4) | 5.7 | 2.9‐11.1 | 0.001 |
| Enteral tube | 65 (46) | 102 (24) | 2.6 | 1.8‐3.9 | 0.001 |
| Hospitalized in an intensive care unit during the same admission | 43 (31) | 77 (18) | 2.0 | 1.3‐3.1 | 0.002 |
| Administration of TPN in preceding 24 hr | 26 (18) | 36 (9) | 2.3 | 1.4‐3.9 | 0.002 |
| Administration of an opioid via a patient‐controlled analgesia pump in the preceding 24 hr | 14 (9) | 14 (3) | 3.6 | 1.6‐8.3 | 0.002 |
| Weight‐for‐age 5th percentile | 49 (35) | 94 (22) | 1.9 | 1.2‐2.9 | 0.003 |
| Central venous line | 55 (39) | 113 (27) | 1.8 | 1.2‐2.7 | 0.005 |
| Age 1 yr | 39 (28) | 103 (24) | 1.2 | 0.8‐1.9 | 0.42 |
| Gestational age 37 wk or documentation of prematurity | 21 (15) | 60 (14) | 1.1 | 0.6‐1.8 | 0.84 |
| Laboratory studies | |||||
| Hemoglobin in preceding 72 hr | |||||
| Not tested | 28 (20) | 190 (45) | 1.0 | [reference] | |
| 10 g/dL | 42 (30) | 144 (34) | 2.0 | 1.2‐3.5 | 0.01 |
| 10 g/dL | 71 (50) | 89 (21) | 5.6 | 3.3‐9.5 | 0.001 |
| White blood cell count in preceding 72 hr | |||||
| Not tested | 28 (20) | 190 (45) | 1.0 | [reference] | |
| 5000 to 15,000/l | 45 (32) | 131 (31) | 2.4 | 1.4‐4.1 | 0.001 |
| 15,000/l | 19 (13) | 25 (6) | 5.7 | 2.7‐12.0 | 0.001 |
| 5000/l | 49 (35) | 77 (18) | 4.5 | 2.6‐7.8 | 0.001 |
| Blood culture drawn in preceding 72 hr | 78 (55) | 85 (20) | 5.2 | 3.3‐8.1 | 0.001 |
Adjusted (Multivariable) Analysis
The multivariable conditional logistic regression model included 7 independent risk factors for deterioration (Table 3): age 1 year, epilepsy, congenital/genetic defects, history of transplant, enteral tubes, hemoglobin 10 g/dL, and blood culture drawn in the preceding 72 hours.
| Predictor | Adjusted OR (95% CI) | P Value | Regression Coefficient (95% CI) | Score* |
|---|---|---|---|---|
| ||||
| Age 1 yr | 1.9 (1.0‐3.4) | 0.038 | 0.6 (0.1‐1.2) | 1 |
| Epilepsy | 4.4 (1.9‐9.8) | 0.001 | 1.5 (0.7‐2.3) | 2 |
| Congenital/genetic defects | 2.1 (0.9‐4.9) | 0.075 | 0.8 (0.1‐1.6) | 1 |
| History of any transplant | 3.0 (1.3‐6.9) | 0.010 | 1.1 (0.3‐1.9) | 2 |
| Enteral tube | 2.1 (1.3‐3.6) | 0.003 | 0.8 (0.3‐1.3) | 1 |
| Hemoglobin 10 g/dL in preceding 72 hr | 3.0 (1.8‐5.1) | 0.001 | 1.1 (0.6‐1.6) | 2 |
| Blood culture drawn in preceding 72 hr | 5.8 (3.3‐10.3) | 0.001 | 1.8 (1.2‐2.3) | 3 |
Predictive Score
The range of the resulting predictive score was 0 to 12. The median score among cases was 4, and the median score among controls was 1 (P 0.001). The area under the receiver operating characteristic curve was 0.78 (95% confidence interval 0.74‐0.83).
We grouped the scores by SSLRs into 4 risk strata and calculated each group's estimated post‐test probability of deterioration based on the pre‐test probability of deterioration of 0.15% (Table 4). The very low‐risk group had a probability of deterioration of 0.06%, less than one‐half the pre‐test probability. The low‐risk group had a probability of deterioration of 0.18%, similar to the pre‐test probability. The intermediate‐risk group had a probability of deterioration of 0.39%, 2.6 times higher than the pre‐test probability. The high‐risk group had a probability of deterioration of 12.60%, 84 times higher than the pre‐test probability.
| Risk stratum | Score range | Cases in stratumn (%) | Controls in stratumn (%) | SSLR (95% CI) | Probability of deterioration (%)* |
|---|---|---|---|---|---|
| |||||
| Very low | 0‐2 | 37 (26) | 288 (68) | 0.4 (0.3‐0.5) | 0.06 |
| Low | 3‐4 | 37 (26) | 94 (22) | 1.2 (0.9‐1.6) | 0.2 |
| Intermediate | 5‐6 | 35 (25) | 40 (9) | 2.6 (1.7‐4.0) | 0.4 |
| High | 7‐12 | 32 (23) | 1 (1) | 96.0 (13.2‐696.2) | 12.6 |
DISCUSSION
Despite the widespread adoption of rapid response systems, we know little about the optimal methods to identify patients whose clinical characteristics alone put them at increased risk of deterioration, and triage the care they receive based on this risk. Pediatric case series have suggested that younger children and those with chronic illnesses are more likely to require assistance from a medical emergency team,1112 but this is the first study to measure their association with this outcome in children.
Most studies with the objective of identifying patients at risk have focused on tools designed to detect symptoms of deterioration that have already begun, using single‐parameter medical emergency team calling criteria1316 or multi‐parameter early warning scores.38 Rather than create a tool to detect deterioration that has already begun, we developed a predictive score that incorporates patient characteristics independently associated with deterioration in hospitalized children, including age 1 year, epilepsy, congenital/genetic defects, history of transplant, enteral tube, hemoglobin 10 g/dL, and blood culture drawn in the preceding 72 hours. The score has the potential to help clinicians identify the children at highest risk of deterioration who might benefit most from the use of vital sign‐based methods to detect deterioration, as well as the children at lowest risk for whom monitoring may be unnecessary. For example, this score could be performed at the time of admission, and those at very low risk of deterioration and without other clinically concerning findings might be considered for a low‐intensity schedule of vital signs and monitoring (such as vital signs every 8 hours, no continuous cardiorespiratory monitoring or pulse oximetry, and early warning score calculation daily), while patients in the intermediate and high‐risk groups might be considered for a more intensive schedule of vital signs and monitoring (such as vital signs every 4 hours, continuous cardiorespiratory monitoring and pulse oximetry, and early warning score calculation every 4 hours). It should be noted, however, that 37 cases (26%) fell into the very low‐risk category, raising the importance of external validation at the point of admission from the emergency department, before the score can be implemented for the potential clinical use described above. If the score performs well in validation studies, then its use in tailoring monitoring parameters has the potential to reduce the amount of time nurses spend responding to false monitor alarms and calculating early warning scores on patients at very low risk of deterioration.
Of note, we excluded children hospitalized for fewer than 24 hours, resulting in the exclusion of 31% of the potentially eligible events. We also excluded 40% of the potentially eligible ICU transfers because they did not meet urgent criteria. These may be limitations because: (1) the first 24 hours of hospitalization may be a high‐risk period; and (2) patients who were on trajectories toward severe deterioration and received interventions that prevented further deterioration, but did not meet urgent criteria, were excluded. It may be that the children we included as cases were at increased risk of deterioration that is either more difficult to recognize early, or more difficult to treat effectively without ICU interventions. In addition, the population of patients meeting urgent criteria may vary across hospitals, limiting generalizability of this score.
In summary, we developed a predictive score and risk stratification tool that may be useful in triaging the intensity of monitoring and surveillance for deterioration that children receive when hospitalized on non‐ICU units. External validation using the setting and frequency of score measurement that would be most valuable clinically (for example, in the emergency department at the time of admission) is needed before clinical use can be recommended.
Acknowledgements
The authors thank Annie Chung, BA, Emily Huang, and Susan Lipsett, MD, for their assistance with data collection.
- Institute for Healthcare Improvement. About IHI. Available at: http://www.ihi.org/ihi/about. Accessed July 18,2010.
- ,,, et al.“Identifying the hospitalised patient in crisis”—a consensus conference on the afferent limb of Rapid Response Systems.Resuscitation.2010;81(4):375–382.
- ,,.The Pediatric Early Warning System score: a severity of illness score to predict urgent medical need in hospitalized children.J Crit Care.2006;21(3):271–278.
- ,,.Development and initial validation of the Bedside Paediatric Early Warning System score.Crit Care.2009;13(4):R135.
- .Detecting and managing deterioration in children.Paediatr Nurs.2005;17(1):32–35.
- ,,.Promoting care for acutely ill children—development and evaluation of a Paediatric Early Warning Tool.Intensive Crit Care Nurs.2006;22(2):73–81.
- ,,,,.Prospective evaluation of a pediatric inpatient early warning scoring system.J Spec Pediatr Nurs.2009;14(2):79–85.
- ,,,.Prospective cohort study to test the predictability of the Cardiff and Vale paediatric early warning system.Arch Dis Child.2009;94(8):602–606.
- ,,,,,.Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services.Pediatrics.2001;107(6):e99.
- ,,,,.Early prediction of neurological sequelae or death after bacterial meningitis.Acta Paediatr.2002;91(4):391–398.
- ,,,,.Retrospective review of emergency response activations during a 13‐year period at a tertiary care children's hospital.J Hosp Med.2011;6(3):131–135.
- ,,,.Clinical profile of hospitalized children provided with urgent assistance from a medical emergency team.Pediatrics.2008;121(6):e1577–e1584.
- ,,, et al.Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit.Pediatr Crit Care Med.2007;8(3):236–246.
- ,,, et al.Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a children's hospital.JAMA.2007;298(19):2267–2274.
- ,,, et al.Transition from a traditional code team to a medical emergency team and categorization of cardiopulmonary arrests in a children's center.Arch Pediatr Adolesc Med.2008;162(2):117–122.
- ,.Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team.Pediatr Crit Care Med.2009;10(3):306–312.
Thousands of hospitals have implemented rapid response systems in recent years in attempts to reduce mortality outside the intensive care unit (ICU).1 These systems have 2 components, a response arm and an identification arm. The response arm is usually comprised of a multidisciplinary critical care team that responds to calls for urgent assistance outside the ICU; this team is often called a rapid response team or a medical emergency team. The identification arm comes in 2 forms, predictive and detective. Predictive tools estimate a patient's risk of deterioration over time based on factors that are not rapidly changing, such as elements of the patient's history. In contrast, detective tools include highly time‐varying signs of active deterioration, such as vital sign abnormalities.2 To date, most pediatric studies have focused on developing detective tools, including several early warning scores.38
In this study, we sought to identify the characteristics that increase the probability that a hospitalized child will deteriorate, and combine these characteristics into a predictive score. Tools like this may be helpful in identifying and triaging the subset of high‐risk children who should be intensively monitored for early signs of deterioration at the time of admission, as well as in identifying very low‐risk children who, in the absence of other clinical concerns, may be monitored less intensively.
METHODS
Detailed methods, including the inclusion/exclusion criteria, the matching procedures, and a full description of the statistical analysis are provided as an appendix (see Supporting Online Appendix: Supplement to Methods Section in the online version of this article). An abbreviated version follows.
Design
We performed a case‐control study among children, younger than 18 years old, hospitalized for >24 hours between January 1, 2005 and December 31, 2008. The case group consisted of children who experienced clinical deterioration, a composite outcome defined as cardiopulmonary arrest (CPA), acute respiratory compromise (ARC), or urgent ICU transfer, while on a non‐ICU unit. ICU transfers were considered urgent if they included at least one of the following outcomes in the 12 hours after transfer: death, CPA, intubation, initiation of noninvasive ventilation, or administration of a vasoactive medication infusion used for the treatment of shock. The control group consisted of a random sample of patients matched 3:1 to cases if they met the criteria of being on a non‐ICU unit at the same time as their matched case.
Variables and Measurements
We collected data on demographics, complex chronic conditions (CCCs), other patient characteristics, and laboratory studies. CCCs were specific diagnoses divided into the following 9 categories according to an established framework: neuromuscular, cardiovascular, respiratory, renal, gastrointestinal, hematologic/emmmunologic, metabolic, malignancy, and genetic/congenital defects.9 Other patient characteristics evaluated included age, weight‐for‐age, gestational age, history of transplant, time from hospital admission to event, recent ICU stays, administration of total parenteral nutrition, use of a patient‐controlled analgesia pump, and presence of medical devices including central venous lines and enteral tubes (naso‐gastric, gastrostomy, or jejunostomy).
Laboratory studies evaluated included hemoglobin value, white blood cell count, and blood culture drawn in the preceding 72 hours. We included these laboratory studies in this predictive score because we hypothesized that they represented factors that increased a child's risk of deterioration over time, as opposed to signs of acute deterioration that would be more appropriate for a detective score.
Statistical Analysis
We used conditional logistic regression for the bivariable and multivariable analyses to account for the matching. We derived the predictive score using an established method10 in which the regression coefficients for each covariate were divided by the smallest coefficient, and then rounded to the nearest integer, to establish each variable's sub‐score. We grouped the total scores into very low, low, intermediate, and high‐risk groups, calculated overall stratum‐specific likelihood ratios (SSLRs), and estimated stratum‐specific probabilities of deterioration for each group.
RESULTS
Patient Characteristics
We identified 12 CPAs, 41 ARCs, and 699 urgent ICU transfers during the study period. A total of 141 cases met our strict criteria for inclusion (see Figure in Supporting Online Appendix: Supplement to Methods Section in the online version of this article) among approximately 96,000 admissions during the study period, making the baseline incidence of events (pre‐test probability) approximately 0.15%. The case and control groups were similar in age, sex, and family‐reported race/ethnicity. Cases had been hospitalized longer than controls at the time of their event, were less likely to have been on a surgical service, and were less likely to survive to hospital discharge (Table 1). There was a high prevalence of CCCs among both cases and controls; 78% of cases and 52% of controls had at least 1 CCC.
| Cases (n = 141) | Controls (n = 423) | ||
|---|---|---|---|
| n (%) | n (%) | P Value | |
| |||
| Type of event | |||
| Cardiopulmonary arrest | 4 (3) | 0 | NA |
| Acute respiratory compromise | 29 (20) | 0 | NA |
| Urgent ICU transfer | 108 (77) | 0 | NA |
| Demographics | |||
| Age | 0.34 | ||
| 0‐6 mo | 17 (12) | 62 (15) | |
| 6‐12 mo | 22 (16) | 41 (10) | |
| 1‐4 yr | 34 (24) | 97 (23) | |
| 4‐10 yr | 26 (18) | 78 (18) | |
| 10‐18 yr | 42 (30) | 145 (34) | |
| Sex | 0.70 | ||
| Female | 60 (43) | 188 (44) | |
| Male | 81 (57) | 235 (56) | |
| Race | 0.40 | ||
| White | 69 (49) | 189 (45) | |
| Black/African‐American | 49 (35) | 163 (38) | |
| Asian/Pacific Islander | 0 (0) | 7 (2) | |
| Other | 23 (16) | 62 (15) | |
| Not reported | 0 (0) | 2 (1) | |
| Ethnicity | 0.53 | ||
| Non‐Hispanic | 127 (90) | 388 (92) | |
| Hispanic | 14 (10) | 33 (8) | |
| Unknown/not reported | 0 (0) | 2 (1) | |
| Hospitalization | |||
| Length of stay in days, median (interquartile range) | 7.8 (2.6‐18.2) | 3.9 (1.9‐11.2) | 0.001 |
| Surgical service | 4 (3) | 67 (16) | 0.001 |
| Survived to hospital discharge | 107 (76) | 421 (99.5) | 0.001 |
Unadjusted (Bivariable) Analysis
Results of bivariable analysis are shown in Table 2.
| Variable | Cases n (%) | Controls n (%) | OR* | 95% CI | P Value |
|---|---|---|---|---|---|
| |||||
| Complex chronic conditions categories | |||||
| Congenital/genetic | 19 (13) | 21 (5) | 3.0 | 1.6‐5.8 | 0.001 |
| Neuromuscular | 31 (22) | 48 (11) | 2.2 | 1.3‐3.7 | 0.002 |
| Respiratory | 18 (13) | 27 (6) | 2.0 | 1.1‐3.7 | 0.02 |
| Cardiovascular | 15 (10) | 24 (6) | 2.0 | 1.0‐3.9 | 0.05 |
| Metabolic | 5 (3) | 6 (1) | 2.5 | 0.8‐8.2 | 0.13 |
| Gastrointestinal | 10 (7) | 24 (6) | 1.3 | 0.6‐2.7 | 0.54 |
| Renal | 3 (2) | 8 (2) | 1.1 | 0.3‐4.2 | 0.86 |
| Hematology/emmmunodeficiency | 6 (4) | 19 (4) | 0.9 | 0.4‐2.4 | 0.91 |
| Specific conditions | |||||
| Mental retardation | 21 (15) | 25 (6) | 2.7 | 1.5‐4.9 | 0.001 |
| Malignancy | 49 (35) | 90 (21) | 1.9 | 1.3‐2.8 | 0.002 |
| Epilepsy | 22 (15) | 30 (7) | 2.4 | 1.3‐4.3 | 0.004 |
| Cardiac malformations | 14 (10) | 19 (4) | 2.2 | 1.1‐4.4 | 0.02 |
| Chronic respiratory disease arising in the perinatal period | 11 (8) | 15 (4) | 2.2 | 1.0‐4.8 | 0.05 |
| Cerebral palsy | 7 (5) | 13 (3) | 1.7 | 0.6‐4.2 | 0.30 |
| Cystic fibrosis | 1 (1) | 9 (2) | 0.3 | 0.1‐2.6 | 0.30 |
| Other patient characteristics | |||||
| Time from hospital admission to event 7 days | 74 (52) | 146 (35) | 2.1 | 1.4‐3.1 | 0.001 |
| History of any transplant | 27 (19) | 17 (4) | 5.7 | 2.9‐11.1 | 0.001 |
| Enteral tube | 65 (46) | 102 (24) | 2.6 | 1.8‐3.9 | 0.001 |
| Hospitalized in an intensive care unit during the same admission | 43 (31) | 77 (18) | 2.0 | 1.3‐3.1 | 0.002 |
| Administration of TPN in preceding 24 hr | 26 (18) | 36 (9) | 2.3 | 1.4‐3.9 | 0.002 |
| Administration of an opioid via a patient‐controlled analgesia pump in the preceding 24 hr | 14 (9) | 14 (3) | 3.6 | 1.6‐8.3 | 0.002 |
| Weight‐for‐age 5th percentile | 49 (35) | 94 (22) | 1.9 | 1.2‐2.9 | 0.003 |
| Central venous line | 55 (39) | 113 (27) | 1.8 | 1.2‐2.7 | 0.005 |
| Age 1 yr | 39 (28) | 103 (24) | 1.2 | 0.8‐1.9 | 0.42 |
| Gestational age 37 wk or documentation of prematurity | 21 (15) | 60 (14) | 1.1 | 0.6‐1.8 | 0.84 |
| Laboratory studies | |||||
| Hemoglobin in preceding 72 hr | |||||
| Not tested | 28 (20) | 190 (45) | 1.0 | [reference] | |
| 10 g/dL | 42 (30) | 144 (34) | 2.0 | 1.2‐3.5 | 0.01 |
| 10 g/dL | 71 (50) | 89 (21) | 5.6 | 3.3‐9.5 | 0.001 |
| White blood cell count in preceding 72 hr | |||||
| Not tested | 28 (20) | 190 (45) | 1.0 | [reference] | |
| 5000 to 15,000/l | 45 (32) | 131 (31) | 2.4 | 1.4‐4.1 | 0.001 |
| 15,000/l | 19 (13) | 25 (6) | 5.7 | 2.7‐12.0 | 0.001 |
| 5000/l | 49 (35) | 77 (18) | 4.5 | 2.6‐7.8 | 0.001 |
| Blood culture drawn in preceding 72 hr | 78 (55) | 85 (20) | 5.2 | 3.3‐8.1 | 0.001 |
Adjusted (Multivariable) Analysis
The multivariable conditional logistic regression model included 7 independent risk factors for deterioration (Table 3): age 1 year, epilepsy, congenital/genetic defects, history of transplant, enteral tubes, hemoglobin 10 g/dL, and blood culture drawn in the preceding 72 hours.
| Predictor | Adjusted OR (95% CI) | P Value | Regression Coefficient (95% CI) | Score* |
|---|---|---|---|---|
| ||||
| Age 1 yr | 1.9 (1.0‐3.4) | 0.038 | 0.6 (0.1‐1.2) | 1 |
| Epilepsy | 4.4 (1.9‐9.8) | 0.001 | 1.5 (0.7‐2.3) | 2 |
| Congenital/genetic defects | 2.1 (0.9‐4.9) | 0.075 | 0.8 (0.1‐1.6) | 1 |
| History of any transplant | 3.0 (1.3‐6.9) | 0.010 | 1.1 (0.3‐1.9) | 2 |
| Enteral tube | 2.1 (1.3‐3.6) | 0.003 | 0.8 (0.3‐1.3) | 1 |
| Hemoglobin 10 g/dL in preceding 72 hr | 3.0 (1.8‐5.1) | 0.001 | 1.1 (0.6‐1.6) | 2 |
| Blood culture drawn in preceding 72 hr | 5.8 (3.3‐10.3) | 0.001 | 1.8 (1.2‐2.3) | 3 |
Predictive Score
The range of the resulting predictive score was 0 to 12. The median score among cases was 4, and the median score among controls was 1 (P 0.001). The area under the receiver operating characteristic curve was 0.78 (95% confidence interval 0.74‐0.83).
We grouped the scores by SSLRs into 4 risk strata and calculated each group's estimated post‐test probability of deterioration based on the pre‐test probability of deterioration of 0.15% (Table 4). The very low‐risk group had a probability of deterioration of 0.06%, less than one‐half the pre‐test probability. The low‐risk group had a probability of deterioration of 0.18%, similar to the pre‐test probability. The intermediate‐risk group had a probability of deterioration of 0.39%, 2.6 times higher than the pre‐test probability. The high‐risk group had a probability of deterioration of 12.60%, 84 times higher than the pre‐test probability.
| Risk stratum | Score range | Cases in stratumn (%) | Controls in stratumn (%) | SSLR (95% CI) | Probability of deterioration (%)* |
|---|---|---|---|---|---|
| |||||
| Very low | 0‐2 | 37 (26) | 288 (68) | 0.4 (0.3‐0.5) | 0.06 |
| Low | 3‐4 | 37 (26) | 94 (22) | 1.2 (0.9‐1.6) | 0.2 |
| Intermediate | 5‐6 | 35 (25) | 40 (9) | 2.6 (1.7‐4.0) | 0.4 |
| High | 7‐12 | 32 (23) | 1 (1) | 96.0 (13.2‐696.2) | 12.6 |
DISCUSSION
Despite the widespread adoption of rapid response systems, we know little about the optimal methods to identify patients whose clinical characteristics alone put them at increased risk of deterioration, and triage the care they receive based on this risk. Pediatric case series have suggested that younger children and those with chronic illnesses are more likely to require assistance from a medical emergency team,1112 but this is the first study to measure their association with this outcome in children.
Most studies with the objective of identifying patients at risk have focused on tools designed to detect symptoms of deterioration that have already begun, using single‐parameter medical emergency team calling criteria1316 or multi‐parameter early warning scores.38 Rather than create a tool to detect deterioration that has already begun, we developed a predictive score that incorporates patient characteristics independently associated with deterioration in hospitalized children, including age 1 year, epilepsy, congenital/genetic defects, history of transplant, enteral tube, hemoglobin 10 g/dL, and blood culture drawn in the preceding 72 hours. The score has the potential to help clinicians identify the children at highest risk of deterioration who might benefit most from the use of vital sign‐based methods to detect deterioration, as well as the children at lowest risk for whom monitoring may be unnecessary. For example, this score could be performed at the time of admission, and those at very low risk of deterioration and without other clinically concerning findings might be considered for a low‐intensity schedule of vital signs and monitoring (such as vital signs every 8 hours, no continuous cardiorespiratory monitoring or pulse oximetry, and early warning score calculation daily), while patients in the intermediate and high‐risk groups might be considered for a more intensive schedule of vital signs and monitoring (such as vital signs every 4 hours, continuous cardiorespiratory monitoring and pulse oximetry, and early warning score calculation every 4 hours). It should be noted, however, that 37 cases (26%) fell into the very low‐risk category, raising the importance of external validation at the point of admission from the emergency department, before the score can be implemented for the potential clinical use described above. If the score performs well in validation studies, then its use in tailoring monitoring parameters has the potential to reduce the amount of time nurses spend responding to false monitor alarms and calculating early warning scores on patients at very low risk of deterioration.
Of note, we excluded children hospitalized for fewer than 24 hours, resulting in the exclusion of 31% of the potentially eligible events. We also excluded 40% of the potentially eligible ICU transfers because they did not meet urgent criteria. These may be limitations because: (1) the first 24 hours of hospitalization may be a high‐risk period; and (2) patients who were on trajectories toward severe deterioration and received interventions that prevented further deterioration, but did not meet urgent criteria, were excluded. It may be that the children we included as cases were at increased risk of deterioration that is either more difficult to recognize early, or more difficult to treat effectively without ICU interventions. In addition, the population of patients meeting urgent criteria may vary across hospitals, limiting generalizability of this score.
In summary, we developed a predictive score and risk stratification tool that may be useful in triaging the intensity of monitoring and surveillance for deterioration that children receive when hospitalized on non‐ICU units. External validation using the setting and frequency of score measurement that would be most valuable clinically (for example, in the emergency department at the time of admission) is needed before clinical use can be recommended.
Acknowledgements
The authors thank Annie Chung, BA, Emily Huang, and Susan Lipsett, MD, for their assistance with data collection.
Thousands of hospitals have implemented rapid response systems in recent years in attempts to reduce mortality outside the intensive care unit (ICU).1 These systems have 2 components, a response arm and an identification arm. The response arm is usually comprised of a multidisciplinary critical care team that responds to calls for urgent assistance outside the ICU; this team is often called a rapid response team or a medical emergency team. The identification arm comes in 2 forms, predictive and detective. Predictive tools estimate a patient's risk of deterioration over time based on factors that are not rapidly changing, such as elements of the patient's history. In contrast, detective tools include highly time‐varying signs of active deterioration, such as vital sign abnormalities.2 To date, most pediatric studies have focused on developing detective tools, including several early warning scores.38
In this study, we sought to identify the characteristics that increase the probability that a hospitalized child will deteriorate, and combine these characteristics into a predictive score. Tools like this may be helpful in identifying and triaging the subset of high‐risk children who should be intensively monitored for early signs of deterioration at the time of admission, as well as in identifying very low‐risk children who, in the absence of other clinical concerns, may be monitored less intensively.
METHODS
Detailed methods, including the inclusion/exclusion criteria, the matching procedures, and a full description of the statistical analysis are provided as an appendix (see Supporting Online Appendix: Supplement to Methods Section in the online version of this article). An abbreviated version follows.
Design
We performed a case‐control study among children, younger than 18 years old, hospitalized for >24 hours between January 1, 2005 and December 31, 2008. The case group consisted of children who experienced clinical deterioration, a composite outcome defined as cardiopulmonary arrest (CPA), acute respiratory compromise (ARC), or urgent ICU transfer, while on a non‐ICU unit. ICU transfers were considered urgent if they included at least one of the following outcomes in the 12 hours after transfer: death, CPA, intubation, initiation of noninvasive ventilation, or administration of a vasoactive medication infusion used for the treatment of shock. The control group consisted of a random sample of patients matched 3:1 to cases if they met the criteria of being on a non‐ICU unit at the same time as their matched case.
Variables and Measurements
We collected data on demographics, complex chronic conditions (CCCs), other patient characteristics, and laboratory studies. CCCs were specific diagnoses divided into the following 9 categories according to an established framework: neuromuscular, cardiovascular, respiratory, renal, gastrointestinal, hematologic/emmmunologic, metabolic, malignancy, and genetic/congenital defects.9 Other patient characteristics evaluated included age, weight‐for‐age, gestational age, history of transplant, time from hospital admission to event, recent ICU stays, administration of total parenteral nutrition, use of a patient‐controlled analgesia pump, and presence of medical devices including central venous lines and enteral tubes (naso‐gastric, gastrostomy, or jejunostomy).
Laboratory studies evaluated included hemoglobin value, white blood cell count, and blood culture drawn in the preceding 72 hours. We included these laboratory studies in this predictive score because we hypothesized that they represented factors that increased a child's risk of deterioration over time, as opposed to signs of acute deterioration that would be more appropriate for a detective score.
Statistical Analysis
We used conditional logistic regression for the bivariable and multivariable analyses to account for the matching. We derived the predictive score using an established method10 in which the regression coefficients for each covariate were divided by the smallest coefficient, and then rounded to the nearest integer, to establish each variable's sub‐score. We grouped the total scores into very low, low, intermediate, and high‐risk groups, calculated overall stratum‐specific likelihood ratios (SSLRs), and estimated stratum‐specific probabilities of deterioration for each group.
RESULTS
Patient Characteristics
We identified 12 CPAs, 41 ARCs, and 699 urgent ICU transfers during the study period. A total of 141 cases met our strict criteria for inclusion (see Figure in Supporting Online Appendix: Supplement to Methods Section in the online version of this article) among approximately 96,000 admissions during the study period, making the baseline incidence of events (pre‐test probability) approximately 0.15%. The case and control groups were similar in age, sex, and family‐reported race/ethnicity. Cases had been hospitalized longer than controls at the time of their event, were less likely to have been on a surgical service, and were less likely to survive to hospital discharge (Table 1). There was a high prevalence of CCCs among both cases and controls; 78% of cases and 52% of controls had at least 1 CCC.
| Cases (n = 141) | Controls (n = 423) | ||
|---|---|---|---|
| n (%) | n (%) | P Value | |
| |||
| Type of event | |||
| Cardiopulmonary arrest | 4 (3) | 0 | NA |
| Acute respiratory compromise | 29 (20) | 0 | NA |
| Urgent ICU transfer | 108 (77) | 0 | NA |
| Demographics | |||
| Age | 0.34 | ||
| 0‐6 mo | 17 (12) | 62 (15) | |
| 6‐12 mo | 22 (16) | 41 (10) | |
| 1‐4 yr | 34 (24) | 97 (23) | |
| 4‐10 yr | 26 (18) | 78 (18) | |
| 10‐18 yr | 42 (30) | 145 (34) | |
| Sex | 0.70 | ||
| Female | 60 (43) | 188 (44) | |
| Male | 81 (57) | 235 (56) | |
| Race | 0.40 | ||
| White | 69 (49) | 189 (45) | |
| Black/African‐American | 49 (35) | 163 (38) | |
| Asian/Pacific Islander | 0 (0) | 7 (2) | |
| Other | 23 (16) | 62 (15) | |
| Not reported | 0 (0) | 2 (1) | |
| Ethnicity | 0.53 | ||
| Non‐Hispanic | 127 (90) | 388 (92) | |
| Hispanic | 14 (10) | 33 (8) | |
| Unknown/not reported | 0 (0) | 2 (1) | |
| Hospitalization | |||
| Length of stay in days, median (interquartile range) | 7.8 (2.6‐18.2) | 3.9 (1.9‐11.2) | 0.001 |
| Surgical service | 4 (3) | 67 (16) | 0.001 |
| Survived to hospital discharge | 107 (76) | 421 (99.5) | 0.001 |
Unadjusted (Bivariable) Analysis
Results of bivariable analysis are shown in Table 2.
| Variable | Cases n (%) | Controls n (%) | OR* | 95% CI | P Value |
|---|---|---|---|---|---|
| |||||
| Complex chronic conditions categories | |||||
| Congenital/genetic | 19 (13) | 21 (5) | 3.0 | 1.6‐5.8 | 0.001 |
| Neuromuscular | 31 (22) | 48 (11) | 2.2 | 1.3‐3.7 | 0.002 |
| Respiratory | 18 (13) | 27 (6) | 2.0 | 1.1‐3.7 | 0.02 |
| Cardiovascular | 15 (10) | 24 (6) | 2.0 | 1.0‐3.9 | 0.05 |
| Metabolic | 5 (3) | 6 (1) | 2.5 | 0.8‐8.2 | 0.13 |
| Gastrointestinal | 10 (7) | 24 (6) | 1.3 | 0.6‐2.7 | 0.54 |
| Renal | 3 (2) | 8 (2) | 1.1 | 0.3‐4.2 | 0.86 |
| Hematology/emmmunodeficiency | 6 (4) | 19 (4) | 0.9 | 0.4‐2.4 | 0.91 |
| Specific conditions | |||||
| Mental retardation | 21 (15) | 25 (6) | 2.7 | 1.5‐4.9 | 0.001 |
| Malignancy | 49 (35) | 90 (21) | 1.9 | 1.3‐2.8 | 0.002 |
| Epilepsy | 22 (15) | 30 (7) | 2.4 | 1.3‐4.3 | 0.004 |
| Cardiac malformations | 14 (10) | 19 (4) | 2.2 | 1.1‐4.4 | 0.02 |
| Chronic respiratory disease arising in the perinatal period | 11 (8) | 15 (4) | 2.2 | 1.0‐4.8 | 0.05 |
| Cerebral palsy | 7 (5) | 13 (3) | 1.7 | 0.6‐4.2 | 0.30 |
| Cystic fibrosis | 1 (1) | 9 (2) | 0.3 | 0.1‐2.6 | 0.30 |
| Other patient characteristics | |||||
| Time from hospital admission to event 7 days | 74 (52) | 146 (35) | 2.1 | 1.4‐3.1 | 0.001 |
| History of any transplant | 27 (19) | 17 (4) | 5.7 | 2.9‐11.1 | 0.001 |
| Enteral tube | 65 (46) | 102 (24) | 2.6 | 1.8‐3.9 | 0.001 |
| Hospitalized in an intensive care unit during the same admission | 43 (31) | 77 (18) | 2.0 | 1.3‐3.1 | 0.002 |
| Administration of TPN in preceding 24 hr | 26 (18) | 36 (9) | 2.3 | 1.4‐3.9 | 0.002 |
| Administration of an opioid via a patient‐controlled analgesia pump in the preceding 24 hr | 14 (9) | 14 (3) | 3.6 | 1.6‐8.3 | 0.002 |
| Weight‐for‐age 5th percentile | 49 (35) | 94 (22) | 1.9 | 1.2‐2.9 | 0.003 |
| Central venous line | 55 (39) | 113 (27) | 1.8 | 1.2‐2.7 | 0.005 |
| Age 1 yr | 39 (28) | 103 (24) | 1.2 | 0.8‐1.9 | 0.42 |
| Gestational age 37 wk or documentation of prematurity | 21 (15) | 60 (14) | 1.1 | 0.6‐1.8 | 0.84 |
| Laboratory studies | |||||
| Hemoglobin in preceding 72 hr | |||||
| Not tested | 28 (20) | 190 (45) | 1.0 | [reference] | |
| 10 g/dL | 42 (30) | 144 (34) | 2.0 | 1.2‐3.5 | 0.01 |
| 10 g/dL | 71 (50) | 89 (21) | 5.6 | 3.3‐9.5 | 0.001 |
| White blood cell count in preceding 72 hr | |||||
| Not tested | 28 (20) | 190 (45) | 1.0 | [reference] | |
| 5000 to 15,000/l | 45 (32) | 131 (31) | 2.4 | 1.4‐4.1 | 0.001 |
| 15,000/l | 19 (13) | 25 (6) | 5.7 | 2.7‐12.0 | 0.001 |
| 5000/l | 49 (35) | 77 (18) | 4.5 | 2.6‐7.8 | 0.001 |
| Blood culture drawn in preceding 72 hr | 78 (55) | 85 (20) | 5.2 | 3.3‐8.1 | 0.001 |
Adjusted (Multivariable) Analysis
The multivariable conditional logistic regression model included 7 independent risk factors for deterioration (Table 3): age 1 year, epilepsy, congenital/genetic defects, history of transplant, enteral tubes, hemoglobin 10 g/dL, and blood culture drawn in the preceding 72 hours.
| Predictor | Adjusted OR (95% CI) | P Value | Regression Coefficient (95% CI) | Score* |
|---|---|---|---|---|
| ||||
| Age 1 yr | 1.9 (1.0‐3.4) | 0.038 | 0.6 (0.1‐1.2) | 1 |
| Epilepsy | 4.4 (1.9‐9.8) | 0.001 | 1.5 (0.7‐2.3) | 2 |
| Congenital/genetic defects | 2.1 (0.9‐4.9) | 0.075 | 0.8 (0.1‐1.6) | 1 |
| History of any transplant | 3.0 (1.3‐6.9) | 0.010 | 1.1 (0.3‐1.9) | 2 |
| Enteral tube | 2.1 (1.3‐3.6) | 0.003 | 0.8 (0.3‐1.3) | 1 |
| Hemoglobin 10 g/dL in preceding 72 hr | 3.0 (1.8‐5.1) | 0.001 | 1.1 (0.6‐1.6) | 2 |
| Blood culture drawn in preceding 72 hr | 5.8 (3.3‐10.3) | 0.001 | 1.8 (1.2‐2.3) | 3 |
Predictive Score
The range of the resulting predictive score was 0 to 12. The median score among cases was 4, and the median score among controls was 1 (P 0.001). The area under the receiver operating characteristic curve was 0.78 (95% confidence interval 0.74‐0.83).
We grouped the scores by SSLRs into 4 risk strata and calculated each group's estimated post‐test probability of deterioration based on the pre‐test probability of deterioration of 0.15% (Table 4). The very low‐risk group had a probability of deterioration of 0.06%, less than one‐half the pre‐test probability. The low‐risk group had a probability of deterioration of 0.18%, similar to the pre‐test probability. The intermediate‐risk group had a probability of deterioration of 0.39%, 2.6 times higher than the pre‐test probability. The high‐risk group had a probability of deterioration of 12.60%, 84 times higher than the pre‐test probability.
| Risk stratum | Score range | Cases in stratumn (%) | Controls in stratumn (%) | SSLR (95% CI) | Probability of deterioration (%)* |
|---|---|---|---|---|---|
| |||||
| Very low | 0‐2 | 37 (26) | 288 (68) | 0.4 (0.3‐0.5) | 0.06 |
| Low | 3‐4 | 37 (26) | 94 (22) | 1.2 (0.9‐1.6) | 0.2 |
| Intermediate | 5‐6 | 35 (25) | 40 (9) | 2.6 (1.7‐4.0) | 0.4 |
| High | 7‐12 | 32 (23) | 1 (1) | 96.0 (13.2‐696.2) | 12.6 |
DISCUSSION
Despite the widespread adoption of rapid response systems, we know little about the optimal methods to identify patients whose clinical characteristics alone put them at increased risk of deterioration, and triage the care they receive based on this risk. Pediatric case series have suggested that younger children and those with chronic illnesses are more likely to require assistance from a medical emergency team,1112 but this is the first study to measure their association with this outcome in children.
Most studies with the objective of identifying patients at risk have focused on tools designed to detect symptoms of deterioration that have already begun, using single‐parameter medical emergency team calling criteria1316 or multi‐parameter early warning scores.38 Rather than create a tool to detect deterioration that has already begun, we developed a predictive score that incorporates patient characteristics independently associated with deterioration in hospitalized children, including age 1 year, epilepsy, congenital/genetic defects, history of transplant, enteral tube, hemoglobin 10 g/dL, and blood culture drawn in the preceding 72 hours. The score has the potential to help clinicians identify the children at highest risk of deterioration who might benefit most from the use of vital sign‐based methods to detect deterioration, as well as the children at lowest risk for whom monitoring may be unnecessary. For example, this score could be performed at the time of admission, and those at very low risk of deterioration and without other clinically concerning findings might be considered for a low‐intensity schedule of vital signs and monitoring (such as vital signs every 8 hours, no continuous cardiorespiratory monitoring or pulse oximetry, and early warning score calculation daily), while patients in the intermediate and high‐risk groups might be considered for a more intensive schedule of vital signs and monitoring (such as vital signs every 4 hours, continuous cardiorespiratory monitoring and pulse oximetry, and early warning score calculation every 4 hours). It should be noted, however, that 37 cases (26%) fell into the very low‐risk category, raising the importance of external validation at the point of admission from the emergency department, before the score can be implemented for the potential clinical use described above. If the score performs well in validation studies, then its use in tailoring monitoring parameters has the potential to reduce the amount of time nurses spend responding to false monitor alarms and calculating early warning scores on patients at very low risk of deterioration.
Of note, we excluded children hospitalized for fewer than 24 hours, resulting in the exclusion of 31% of the potentially eligible events. We also excluded 40% of the potentially eligible ICU transfers because they did not meet urgent criteria. These may be limitations because: (1) the first 24 hours of hospitalization may be a high‐risk period; and (2) patients who were on trajectories toward severe deterioration and received interventions that prevented further deterioration, but did not meet urgent criteria, were excluded. It may be that the children we included as cases were at increased risk of deterioration that is either more difficult to recognize early, or more difficult to treat effectively without ICU interventions. In addition, the population of patients meeting urgent criteria may vary across hospitals, limiting generalizability of this score.
In summary, we developed a predictive score and risk stratification tool that may be useful in triaging the intensity of monitoring and surveillance for deterioration that children receive when hospitalized on non‐ICU units. External validation using the setting and frequency of score measurement that would be most valuable clinically (for example, in the emergency department at the time of admission) is needed before clinical use can be recommended.
Acknowledgements
The authors thank Annie Chung, BA, Emily Huang, and Susan Lipsett, MD, for their assistance with data collection.
- Institute for Healthcare Improvement. About IHI. Available at: http://www.ihi.org/ihi/about. Accessed July 18,2010.
- ,,, et al.“Identifying the hospitalised patient in crisis”—a consensus conference on the afferent limb of Rapid Response Systems.Resuscitation.2010;81(4):375–382.
- ,,.The Pediatric Early Warning System score: a severity of illness score to predict urgent medical need in hospitalized children.J Crit Care.2006;21(3):271–278.
- ,,.Development and initial validation of the Bedside Paediatric Early Warning System score.Crit Care.2009;13(4):R135.
- .Detecting and managing deterioration in children.Paediatr Nurs.2005;17(1):32–35.
- ,,.Promoting care for acutely ill children—development and evaluation of a Paediatric Early Warning Tool.Intensive Crit Care Nurs.2006;22(2):73–81.
- ,,,,.Prospective evaluation of a pediatric inpatient early warning scoring system.J Spec Pediatr Nurs.2009;14(2):79–85.
- ,,,.Prospective cohort study to test the predictability of the Cardiff and Vale paediatric early warning system.Arch Dis Child.2009;94(8):602–606.
- ,,,,,.Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services.Pediatrics.2001;107(6):e99.
- ,,,,.Early prediction of neurological sequelae or death after bacterial meningitis.Acta Paediatr.2002;91(4):391–398.
- ,,,,.Retrospective review of emergency response activations during a 13‐year period at a tertiary care children's hospital.J Hosp Med.2011;6(3):131–135.
- ,,,.Clinical profile of hospitalized children provided with urgent assistance from a medical emergency team.Pediatrics.2008;121(6):e1577–e1584.
- ,,, et al.Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit.Pediatr Crit Care Med.2007;8(3):236–246.
- ,,, et al.Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a children's hospital.JAMA.2007;298(19):2267–2274.
- ,,, et al.Transition from a traditional code team to a medical emergency team and categorization of cardiopulmonary arrests in a children's center.Arch Pediatr Adolesc Med.2008;162(2):117–122.
- ,.Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team.Pediatr Crit Care Med.2009;10(3):306–312.
- Institute for Healthcare Improvement. About IHI. Available at: http://www.ihi.org/ihi/about. Accessed July 18,2010.
- ,,, et al.“Identifying the hospitalised patient in crisis”—a consensus conference on the afferent limb of Rapid Response Systems.Resuscitation.2010;81(4):375–382.
- ,,.The Pediatric Early Warning System score: a severity of illness score to predict urgent medical need in hospitalized children.J Crit Care.2006;21(3):271–278.
- ,,.Development and initial validation of the Bedside Paediatric Early Warning System score.Crit Care.2009;13(4):R135.
- .Detecting and managing deterioration in children.Paediatr Nurs.2005;17(1):32–35.
- ,,.Promoting care for acutely ill children—development and evaluation of a Paediatric Early Warning Tool.Intensive Crit Care Nurs.2006;22(2):73–81.
- ,,,,.Prospective evaluation of a pediatric inpatient early warning scoring system.J Spec Pediatr Nurs.2009;14(2):79–85.
- ,,,.Prospective cohort study to test the predictability of the Cardiff and Vale paediatric early warning system.Arch Dis Child.2009;94(8):602–606.
- ,,,,,.Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services.Pediatrics.2001;107(6):e99.
- ,,,,.Early prediction of neurological sequelae or death after bacterial meningitis.Acta Paediatr.2002;91(4):391–398.
- ,,,,.Retrospective review of emergency response activations during a 13‐year period at a tertiary care children's hospital.J Hosp Med.2011;6(3):131–135.
- ,,,.Clinical profile of hospitalized children provided with urgent assistance from a medical emergency team.Pediatrics.2008;121(6):e1577–e1584.
- ,,, et al.Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit.Pediatr Crit Care Med.2007;8(3):236–246.
- ,,, et al.Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a children's hospital.JAMA.2007;298(19):2267–2274.
- ,,, et al.Transition from a traditional code team to a medical emergency team and categorization of cardiopulmonary arrests in a children's center.Arch Pediatr Adolesc Med.2008;162(2):117–122.
- ,.Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team.Pediatr Crit Care Med.2009;10(3):306–312.
In the Literature: Research You Need to Know
Clinical question: Which clinical decision rule—Wells rule, simplified Wells rule, revised Geneva score, or simplified revised Geneva score—is the best for evaluating a patient with a possible acute pulmonary embolism?
Background: The use of standardized clinical decision rules to determine the probability of an acute pulmonary embolism (PE) has significantly improved the diagnostic evaluation of patients with suspected PE. Several clinical decision rules are available and widely used, but they have not been previously directly compared.
Study design: Prospective cohort.
Setting: Seven hospitals in the Netherlands.
Synopsis: A total of 807 patients with suspected first episode of acute PE had a sequential workup with clinical probability assessment and D-dimer testing. When PE was considered unlikely according to all four clinical decision rules and a normal D-dimer result, PE was excluded. In the remaining patients, a CT scan was used to confirm or exclude the diagnosis.
The prevalence of PE was 23%. Combined with a normal D-dimer, the decision rules excluded PE in 22% to 24% of patients. Thirty percent of patients had discordant decision rule outcomes, but PE was not detected by CT in any of these patients when combined with a normal D-dimer.
This study has practical limitations because management was based on a combination of four decision rules and D-dimer testing rather than only one rule and D-dimer testing, which is the more realistic clinical approach.
Bottom line: When used correctly and in conjunction with a D-dimer result, the Wells rule, simplified Wells rule, revised Geneva score, and simplified revised Geneva score all perform similarly in the exclusion of acute PE.
Citation: Douma RA, Mos IC, Erkens PM, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism: a prospective cohort study. Ann Intern Med. 2011;154:709-718.
For more of physician reviews of HM-related literature, check out this month's"In the Literature".
Clinical question: Which clinical decision rule—Wells rule, simplified Wells rule, revised Geneva score, or simplified revised Geneva score—is the best for evaluating a patient with a possible acute pulmonary embolism?
Background: The use of standardized clinical decision rules to determine the probability of an acute pulmonary embolism (PE) has significantly improved the diagnostic evaluation of patients with suspected PE. Several clinical decision rules are available and widely used, but they have not been previously directly compared.
Study design: Prospective cohort.
Setting: Seven hospitals in the Netherlands.
Synopsis: A total of 807 patients with suspected first episode of acute PE had a sequential workup with clinical probability assessment and D-dimer testing. When PE was considered unlikely according to all four clinical decision rules and a normal D-dimer result, PE was excluded. In the remaining patients, a CT scan was used to confirm or exclude the diagnosis.
The prevalence of PE was 23%. Combined with a normal D-dimer, the decision rules excluded PE in 22% to 24% of patients. Thirty percent of patients had discordant decision rule outcomes, but PE was not detected by CT in any of these patients when combined with a normal D-dimer.
This study has practical limitations because management was based on a combination of four decision rules and D-dimer testing rather than only one rule and D-dimer testing, which is the more realistic clinical approach.
Bottom line: When used correctly and in conjunction with a D-dimer result, the Wells rule, simplified Wells rule, revised Geneva score, and simplified revised Geneva score all perform similarly in the exclusion of acute PE.
Citation: Douma RA, Mos IC, Erkens PM, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism: a prospective cohort study. Ann Intern Med. 2011;154:709-718.
For more of physician reviews of HM-related literature, check out this month's"In the Literature".
Clinical question: Which clinical decision rule—Wells rule, simplified Wells rule, revised Geneva score, or simplified revised Geneva score—is the best for evaluating a patient with a possible acute pulmonary embolism?
Background: The use of standardized clinical decision rules to determine the probability of an acute pulmonary embolism (PE) has significantly improved the diagnostic evaluation of patients with suspected PE. Several clinical decision rules are available and widely used, but they have not been previously directly compared.
Study design: Prospective cohort.
Setting: Seven hospitals in the Netherlands.
Synopsis: A total of 807 patients with suspected first episode of acute PE had a sequential workup with clinical probability assessment and D-dimer testing. When PE was considered unlikely according to all four clinical decision rules and a normal D-dimer result, PE was excluded. In the remaining patients, a CT scan was used to confirm or exclude the diagnosis.
The prevalence of PE was 23%. Combined with a normal D-dimer, the decision rules excluded PE in 22% to 24% of patients. Thirty percent of patients had discordant decision rule outcomes, but PE was not detected by CT in any of these patients when combined with a normal D-dimer.
This study has practical limitations because management was based on a combination of four decision rules and D-dimer testing rather than only one rule and D-dimer testing, which is the more realistic clinical approach.
Bottom line: When used correctly and in conjunction with a D-dimer result, the Wells rule, simplified Wells rule, revised Geneva score, and simplified revised Geneva score all perform similarly in the exclusion of acute PE.
Citation: Douma RA, Mos IC, Erkens PM, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism: a prospective cohort study. Ann Intern Med. 2011;154:709-718.
For more of physician reviews of HM-related literature, check out this month's"In the Literature".
Remote Weight-Loss Program Effective Long-Term
ORLANDO – Obese patients coached solely over the phone and Internet lost as much weight as did those counseled in person, according to the findings of a prospective, randomized controlled trial.
Moreover, both groups maintained their weight loss during the 2-year follow-up period of the POWER (Practice-Based Opportunities for Weight Reduction) trial.
At 24 months, 38% of patients in the remote group had lost at least 5% of their initial body weight vs. 41% of the in-person group and just 19% in a control group whose weight-loss was self-directed, Dr. Lawrence J. Appel said at the annual scientific sessions of the American Heart Association.
At 24 months, the mean weight loss was –4.6 kg or 10.1 pounds in the remote group vs. –5.1 kg or 11.2 pounds in the in-person group (P = .63), and –0.8 kg or 1.7 pounds in the control group.
At no point in the study did the weight loss in the two active treatment arms differ, said Dr. Appel, professor of medicine and director of the Welch Center for Prevention, Epidemiology and Clinical Research at Johns Hopkins University in Baltimore.
The sustained weight loss observed in POWER is unprecedented. "It could be considered something of a breakthrough in weight loss," Dr. Frank Sacks, professor of cardiovascular disease prevention at Harvard School of Public Health in Boston, observed at a press briefing. Dr. Sacks was an invited discussant for the paper.
Session moderator Dr. Donald Lloyd-Jones, chair of preventive medicine at Northwestern University, Chicago, called POWER an incredibly important trial given that obesity is by far the No. 1 public health problem in the United States and will drive cardiovascular disease in the coming decades.
"This is absolutely a game changer," he said in an interview. "To see a scalable, very inexpensive therapy, that can be done at arm’s length without too much [intensity] from the provider side, and yet fully engage patients in the process of their weight loss is a very exciting development."
The trial enrolled 415 obese patients with at least one cardiovascular risk factor and a mean weight of 103 kg and mean body mass index of 37 kg/m2.
Patients assigned to the remote group had to enter their weight on the study website before being guided to other educational modules on physical exercise and calorie counting. They could also view their weight-loss goal and progress, Dr. Appel explained.
Physicians reviewed the weight progress reports and played a supportive role through tailored e-mails. Counseling was provided by telephone by employees of Healthways, a disease management promotion company, with no face-to-face contact.
The website and physician’s roles were similar in the in-person group, but these patients received counseling in group meetings, individual meetings, and via telephone from employees of Johns Hopkins.
All patients were encouraged to reduce caloric intake, follow the DASH diet, exercise at least 180 min/week and to log in to the study website at least weekly.
During the first 6 months, the remote group took part in a median of 14 of the 15 recommended phone contacts with their coach and a median of 16 of 18 recommended phone calls over the next 18 months.
The experience in the in-person group was remarkably different, Dr. Appel said. Patients took part in just 6.5 of the 12 recommended group sessions in the first 6 months and only 1 of 18 sessions over the next 18 months, with attendance at individual sessions following a similar pattern. Ultimately, the program "morphed" into a phone intervention, likely because of the convenience and flexibility the format offers, he said. The in-person group maintained 3 of 4 recommended phone contacts with their coaches in the first 6 months and 11 of 12 contacts over the next 18 months.
The study website engaged the patients, with the remote group making 23 of the 26 recommended log ins during the first six months and the in-person group making 20.5 of the 26 log-ins, Dr. Appel said. Over the next 18 months both groups logged in to the website 35 of the 72 recommended times, and visited their primary care provider just once.
During a panel discussion, Dr. Darwin Labarthe, a professor of preventive medicine at Northwestern, said the results are probably the strongest evidence to date on the ability of adults to reduce weight on a sustained basis, but suggested that further follow-up is needed postintervention. He also asked whether the degree of weight loss observed in POWER had an impact on cardiovascular risk factors.
Dr. Appel said the trial was not set up to look at these outcomes, however evidence from other studies suggests that in a prediabetic population, a 5% loss in body weight will reduce the incidence of diabetes by 40%-50%. A reduction in systolic blood pressure also can be expected. Although patients were on medications for this, there was a relationship between systolic blood pressure reduction and weight loss across the entire study population, he said.
At baseline, 76% of patients had hypertension, 68% hypercholesterolemia, 23% diabetes, and 33% metabolic syndrome. Their mean age was 54 years, 64% were women, 56% were white and 41% were black.
The cost of such a remote program depends on how it is rolled out, but that the coaching staff was the biggest driver of expenses at about $600-$800 for the 2 years, Dr. Appel noted. Johns Hopkins is working on implementing the remote intervention and Healthways is expected to make the program commercially available, he said in an interview.
The remote intervention, consisting of phone counseling, an interactive website, and physician support, "has the potential for widespread implementation and should be applicable to the management of other chronic conditions," he told the attendees.
The trial was funded by the National Heart, Lung, and Blood Institute, with data analytic support provided by the National Institute of Diabetes and Digestive and Kidney Disease. Dr. Appel reported no conflicts of interest.
ORLANDO – Obese patients coached solely over the phone and Internet lost as much weight as did those counseled in person, according to the findings of a prospective, randomized controlled trial.
Moreover, both groups maintained their weight loss during the 2-year follow-up period of the POWER (Practice-Based Opportunities for Weight Reduction) trial.
At 24 months, 38% of patients in the remote group had lost at least 5% of their initial body weight vs. 41% of the in-person group and just 19% in a control group whose weight-loss was self-directed, Dr. Lawrence J. Appel said at the annual scientific sessions of the American Heart Association.
At 24 months, the mean weight loss was –4.6 kg or 10.1 pounds in the remote group vs. –5.1 kg or 11.2 pounds in the in-person group (P = .63), and –0.8 kg or 1.7 pounds in the control group.
At no point in the study did the weight loss in the two active treatment arms differ, said Dr. Appel, professor of medicine and director of the Welch Center for Prevention, Epidemiology and Clinical Research at Johns Hopkins University in Baltimore.
The sustained weight loss observed in POWER is unprecedented. "It could be considered something of a breakthrough in weight loss," Dr. Frank Sacks, professor of cardiovascular disease prevention at Harvard School of Public Health in Boston, observed at a press briefing. Dr. Sacks was an invited discussant for the paper.
Session moderator Dr. Donald Lloyd-Jones, chair of preventive medicine at Northwestern University, Chicago, called POWER an incredibly important trial given that obesity is by far the No. 1 public health problem in the United States and will drive cardiovascular disease in the coming decades.
"This is absolutely a game changer," he said in an interview. "To see a scalable, very inexpensive therapy, that can be done at arm’s length without too much [intensity] from the provider side, and yet fully engage patients in the process of their weight loss is a very exciting development."
The trial enrolled 415 obese patients with at least one cardiovascular risk factor and a mean weight of 103 kg and mean body mass index of 37 kg/m2.
Patients assigned to the remote group had to enter their weight on the study website before being guided to other educational modules on physical exercise and calorie counting. They could also view their weight-loss goal and progress, Dr. Appel explained.
Physicians reviewed the weight progress reports and played a supportive role through tailored e-mails. Counseling was provided by telephone by employees of Healthways, a disease management promotion company, with no face-to-face contact.
The website and physician’s roles were similar in the in-person group, but these patients received counseling in group meetings, individual meetings, and via telephone from employees of Johns Hopkins.
All patients were encouraged to reduce caloric intake, follow the DASH diet, exercise at least 180 min/week and to log in to the study website at least weekly.
During the first 6 months, the remote group took part in a median of 14 of the 15 recommended phone contacts with their coach and a median of 16 of 18 recommended phone calls over the next 18 months.
The experience in the in-person group was remarkably different, Dr. Appel said. Patients took part in just 6.5 of the 12 recommended group sessions in the first 6 months and only 1 of 18 sessions over the next 18 months, with attendance at individual sessions following a similar pattern. Ultimately, the program "morphed" into a phone intervention, likely because of the convenience and flexibility the format offers, he said. The in-person group maintained 3 of 4 recommended phone contacts with their coaches in the first 6 months and 11 of 12 contacts over the next 18 months.
The study website engaged the patients, with the remote group making 23 of the 26 recommended log ins during the first six months and the in-person group making 20.5 of the 26 log-ins, Dr. Appel said. Over the next 18 months both groups logged in to the website 35 of the 72 recommended times, and visited their primary care provider just once.
During a panel discussion, Dr. Darwin Labarthe, a professor of preventive medicine at Northwestern, said the results are probably the strongest evidence to date on the ability of adults to reduce weight on a sustained basis, but suggested that further follow-up is needed postintervention. He also asked whether the degree of weight loss observed in POWER had an impact on cardiovascular risk factors.
Dr. Appel said the trial was not set up to look at these outcomes, however evidence from other studies suggests that in a prediabetic population, a 5% loss in body weight will reduce the incidence of diabetes by 40%-50%. A reduction in systolic blood pressure also can be expected. Although patients were on medications for this, there was a relationship between systolic blood pressure reduction and weight loss across the entire study population, he said.
At baseline, 76% of patients had hypertension, 68% hypercholesterolemia, 23% diabetes, and 33% metabolic syndrome. Their mean age was 54 years, 64% were women, 56% were white and 41% were black.
The cost of such a remote program depends on how it is rolled out, but that the coaching staff was the biggest driver of expenses at about $600-$800 for the 2 years, Dr. Appel noted. Johns Hopkins is working on implementing the remote intervention and Healthways is expected to make the program commercially available, he said in an interview.
The remote intervention, consisting of phone counseling, an interactive website, and physician support, "has the potential for widespread implementation and should be applicable to the management of other chronic conditions," he told the attendees.
The trial was funded by the National Heart, Lung, and Blood Institute, with data analytic support provided by the National Institute of Diabetes and Digestive and Kidney Disease. Dr. Appel reported no conflicts of interest.
ORLANDO – Obese patients coached solely over the phone and Internet lost as much weight as did those counseled in person, according to the findings of a prospective, randomized controlled trial.
Moreover, both groups maintained their weight loss during the 2-year follow-up period of the POWER (Practice-Based Opportunities for Weight Reduction) trial.
At 24 months, 38% of patients in the remote group had lost at least 5% of their initial body weight vs. 41% of the in-person group and just 19% in a control group whose weight-loss was self-directed, Dr. Lawrence J. Appel said at the annual scientific sessions of the American Heart Association.
At 24 months, the mean weight loss was –4.6 kg or 10.1 pounds in the remote group vs. –5.1 kg or 11.2 pounds in the in-person group (P = .63), and –0.8 kg or 1.7 pounds in the control group.
At no point in the study did the weight loss in the two active treatment arms differ, said Dr. Appel, professor of medicine and director of the Welch Center for Prevention, Epidemiology and Clinical Research at Johns Hopkins University in Baltimore.
The sustained weight loss observed in POWER is unprecedented. "It could be considered something of a breakthrough in weight loss," Dr. Frank Sacks, professor of cardiovascular disease prevention at Harvard School of Public Health in Boston, observed at a press briefing. Dr. Sacks was an invited discussant for the paper.
Session moderator Dr. Donald Lloyd-Jones, chair of preventive medicine at Northwestern University, Chicago, called POWER an incredibly important trial given that obesity is by far the No. 1 public health problem in the United States and will drive cardiovascular disease in the coming decades.
"This is absolutely a game changer," he said in an interview. "To see a scalable, very inexpensive therapy, that can be done at arm’s length without too much [intensity] from the provider side, and yet fully engage patients in the process of their weight loss is a very exciting development."
The trial enrolled 415 obese patients with at least one cardiovascular risk factor and a mean weight of 103 kg and mean body mass index of 37 kg/m2.
Patients assigned to the remote group had to enter their weight on the study website before being guided to other educational modules on physical exercise and calorie counting. They could also view their weight-loss goal and progress, Dr. Appel explained.
Physicians reviewed the weight progress reports and played a supportive role through tailored e-mails. Counseling was provided by telephone by employees of Healthways, a disease management promotion company, with no face-to-face contact.
The website and physician’s roles were similar in the in-person group, but these patients received counseling in group meetings, individual meetings, and via telephone from employees of Johns Hopkins.
All patients were encouraged to reduce caloric intake, follow the DASH diet, exercise at least 180 min/week and to log in to the study website at least weekly.
During the first 6 months, the remote group took part in a median of 14 of the 15 recommended phone contacts with their coach and a median of 16 of 18 recommended phone calls over the next 18 months.
The experience in the in-person group was remarkably different, Dr. Appel said. Patients took part in just 6.5 of the 12 recommended group sessions in the first 6 months and only 1 of 18 sessions over the next 18 months, with attendance at individual sessions following a similar pattern. Ultimately, the program "morphed" into a phone intervention, likely because of the convenience and flexibility the format offers, he said. The in-person group maintained 3 of 4 recommended phone contacts with their coaches in the first 6 months and 11 of 12 contacts over the next 18 months.
The study website engaged the patients, with the remote group making 23 of the 26 recommended log ins during the first six months and the in-person group making 20.5 of the 26 log-ins, Dr. Appel said. Over the next 18 months both groups logged in to the website 35 of the 72 recommended times, and visited their primary care provider just once.
During a panel discussion, Dr. Darwin Labarthe, a professor of preventive medicine at Northwestern, said the results are probably the strongest evidence to date on the ability of adults to reduce weight on a sustained basis, but suggested that further follow-up is needed postintervention. He also asked whether the degree of weight loss observed in POWER had an impact on cardiovascular risk factors.
Dr. Appel said the trial was not set up to look at these outcomes, however evidence from other studies suggests that in a prediabetic population, a 5% loss in body weight will reduce the incidence of diabetes by 40%-50%. A reduction in systolic blood pressure also can be expected. Although patients were on medications for this, there was a relationship between systolic blood pressure reduction and weight loss across the entire study population, he said.
At baseline, 76% of patients had hypertension, 68% hypercholesterolemia, 23% diabetes, and 33% metabolic syndrome. Their mean age was 54 years, 64% were women, 56% were white and 41% were black.
The cost of such a remote program depends on how it is rolled out, but that the coaching staff was the biggest driver of expenses at about $600-$800 for the 2 years, Dr. Appel noted. Johns Hopkins is working on implementing the remote intervention and Healthways is expected to make the program commercially available, he said in an interview.
The remote intervention, consisting of phone counseling, an interactive website, and physician support, "has the potential for widespread implementation and should be applicable to the management of other chronic conditions," he told the attendees.
The trial was funded by the National Heart, Lung, and Blood Institute, with data analytic support provided by the National Institute of Diabetes and Digestive and Kidney Disease. Dr. Appel reported no conflicts of interest.
FROM THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN HEART ASSOCIATION
Major Finding: At 24 months, the mean weight loss was –4.6 kg or 10.1 pounds in the remote group vs. –5.1 kg or 11.2 pounds in the in-person group (P = .63), and –0.8 kg or 1.7 pounds in the control group.
Data Source: A 2-year prospective, randomized controlled trial.
Disclosures: The trial was funded by the National Heart, Lung, and Blood Institute, with data analytic support also provided by the National Institute of Diabetes and Digestive and Kidney Disease. Dr. Appel reported no conflicts of interest.
and Outcomes
New health information technologies hold enormous potential for improving the quality and efficiency of healthcare. One commonly used health information technology is computerized clinical knowledge management (CKM) systems, which provide clinicians with access to relevant and continually updated clinical information about major medical topics at the point of care. Studies indicate that clinicians often have questions about patient care, which go largely unanswered during patient encounters.13 The availability of answers to critical clinical questions can have a large impact on clinical decision‐making and practice.4
UpToDate is one of the most widely used computerized clinical knowledge management systems in the nation.1, 2, 5, 6 Previous studies of UpToDate and similar systems demonstrated that these systems improve acquisition of knowledge, increase the number of answered clinical questions, and change management decisions.7, 8 However, whether these changes lead to real improvements in clinical outcomes is unknown. Given the urgent need to improve both the quality and efficiency of healthcare, understanding whether UpToDate has the potential to improve outcomes is critical.
Therefore, we examined whether the use of UpToDate was associated with lower risk‐adjusted mortality rates, shorter lengths of stay, and better performance on standard quality process metrics. Further, we sought to determine whether the impact of UpToDate was particularly potent in certain subsets of hospitals. Finally, we examined whether the duration of use of UpToDate was associated with better outcomes.
METHODS
Overview
Our overall approach was to examine the relationship between UpToDate and 3 main outcomes: risk‐adjusted length of stay, risk‐adjusted mortality, and performance on standard quality process metrics in the period from 2004 to 2006. We took 4 approaches to try to reduce potential confounding (adopters of the UpToDate are likely different than non‐adopters). First, we used a longitudinal modeling approach where hospitals were allowed to serve as their own controls over time. For example, if a hospital was an adopter of UpToDate in the fourth quarter of 2005, all data for that hospital prior to that quarter of 2005 would be counted as part of the control hospitals. Second, we used multivariable models to adjust for observable differences between adopters and non‐adopters. Third, we tested for interactions to see if the effect of UpToDate was particularly concentrated in a subset of hospitals. Finally, we examined whether the duration of use, which reflects a potential dose‐response relationship, was related to the outcomes of interest.
UpToDate
The UpToDate system provides a compendium of regularly revised, evidence‐based monographs on topics in adult internal medicine, pediatrics, and obstetrics and gynecology.9 The system is available through multiple medias (ie, the Internet, handheld devices). Providers at subscribing hospitals can usually access it through any computer terminal within the facility, and often through remote access.
Data Sources and Linkage
We used 5 primary sources of data: UpToDate usage data, which was provided directly by UpToDate and includes a list of all hospitals in the United States who use the UpToDate software and the start date of usage; the American Hospital Association (AHA) Annual Survey, which contains individual hospital structural characteristics (such as size, location, teaching status); the 2007 Medicare Inpatient Impact files, which includes hospital characteristics not available in the AHA data; the 2004‐2006 Medicare Provider Analysis and Review (MEDPAR) databases, which have patient‐level discharge information about all Medicare fee‐for‐service patients hospitalized in a given year; and the 2004‐2007 Hospital Quality Alliance (HQA) database, which includes publicly available data for inpatient quality measures. We linked these 4 datasets with a database of hospitals that use UpToDate.
Outcomes
We chose, a priori, to examine 3 primary outcomes: risk‐adjusted length of stay (LOS), which is considered a measure of efficiency; risk‐adjusted mortality, a commonly used marker of quality of care; and performance outcomes on HQA quality metrics.
Risk‐Adjusted LOS and Risk‐Adjusted Mortality Rates
We examined risk‐adjusted LOS and risk‐adjusted mortality rates among all hospitalized patients and among 6 common medical and surgical conditions: acute myocardial infarction (AMI), congestive heart failure (CHF), pneumonia, gastrointestinal hemorrhage, stroke, and hip fracture. We selected these 6 conditions because they are used by the Agency for Healthcare Research and Quality (AHRQ) to measure hospital quality. We used International Classification of Diseases, Ninth Revision (ICD‐9) codes used by the AHRQ Inpatient Quality Indicators to identify patients admitted for these 6 conditions.10 We performed risk‐adjustment using the Elixhauser comorbidity adjustment scheme, which was developed by AHRQ and is commonly used to adjust for differences in severity using administrative data.
Quality Processes of Care
To examine hospital quality performance, we used the HQA process measures for 4 conditions from 2004 to 2007: AMI, CHF, pneumonia, and surgical infection prevention (SIP). We examined all HQA indicators publically available in 2004 (8 measures for AMI, 4 measures for CHF, 6 measures for pneumonia, and 2 measures for SIP). (The specific indicators are listed in Supporting Appendix Table 1 in the online version of this article.) We created summary scores for each condition, and an overall hospital summary score for the performance on all indicators, using methodology previously described by the Joint Commission.11 Each summary score represents the number of times a hospital performed the appropriate action across all measures for that condition divided by the number of opportunities the hospital had to provide appropriate care for that condition. Composite scores were only calculated if a hospital had at least 30 patients for at least one of the measures of each condition.
AnalysisCKM Users Versus Non‐Users
We chose, a priori, to perform several sets of analyses to understand the relationship between the use of UpToDate and clinical outcomes. Our primary approach was a longitudinal model where each hospital was allowed to serve as its own control. In sensitivity analyses, we used a differences‐in‐differences model where we examined whether the changes for hospitals that adopted UpToDate differed compared to changes in outcomes for non‐adopters, adjusting for temporal trends by using time as a covariate in the model.
In the first analysis using longitudinal data, we examined whether being admitted to a hospital with UpToDate was associated with shorter length of stay, lower risk‐adjusted 30‐day mortality or higher process quality. This model allowed each hospital to serve as its own control and tested to see how the outcomes changed after adoption of UpToDate, controlling for secular trends by including non‐UpToDate hospitals. The models were adjusted for hospital characteristics including size, region, location (urban vs rural), ownership (for‐profit, not‐for‐profit private, not‐for‐profit public), teaching status (member of the Council of Teaching Hospital vs not), the proportion of patients that had Medicaid, the Disproportionate Share Hospital (DSH) Index, and the presence or absence of a medical intensive care unit (ICU). We used a repeated‐measures generalized estimating equations (GEE) to account for both clustering at the hospital level and for the repeated measures nature of our analysis. We used the Elixhauser comorbidity adjustment scheme to account for patient‐level factors.12
In our first set of models, we included all patients. We subsequently built 6 condition‐specific models for each of the 6 common medical conditions: AMI, CHF, pneumonia, gastrointestinal hemorrhage, stroke, and hip fracture.
Identifying Subsets of Hospitals
Next, we postulated a priori that certain subsets of hospitalssmaller institutions and non‐teaching institutionsmight have less access to high‐quality clinical information and, thus, be mode likely to benefit from UpToDate. To determine if the potential impact of UpToDate on the outcomes varied based on these hospital characteristics, we repeated our analyses using multivariable models but tested interaction terms. We found significant interactions for 1 outcome (HQA quality performance scores), and present data with stratified analyses for this outcome.
Impact of Duration of Use
Finally, we calculated duration of UpToDate use for each hospital. For each hospital using UpToDate, we identified the date it started using the system. Based on the start date, we calculated each hospital's duration of use for each quarter. We used the midpoint of that quarter to calculate the number of days a hospital used UpToDate. For example, if a hospital started using UpToDate on January 1, 2002, we assigned 775 days for its duration of use for the first quarter of 2004 (365 days per year 2 years + 45 days for the first quarter of 2004) and 865 days for its duration of use for the second quarter of 2004. We excluded hospitals that did not use UpToDate.
Our primary analysis used a dataset that was restricted to just those hospitals using UpToDate. We ran the model for all discharges and for each of the 6 conditions for both LOS and for risk‐adjusted mortality rate, as well as for the 4 conditions encompassed in the HQA quality reporting program. In all analyses, we considered a 2‐sided P value as statistically significant.
Assessing the Overall Impact of UpToDate
To better assess the clinical significance of any change in mortality rates, we calculated the number of deaths prevented if all hospitals saw the same gain in mortality if they adopted UpToDate. To calculate this impact number, we identified the overall reduction in risk‐adjusted mortality associated with UpToDate adoption and multiplied this number by the number of elderly Medicare patients admitted to non‐UpToDate hospitals. We calculated a similar number for changes in length of stay.
RESULTS
We found that between 2004 through 2006, 1017 hospitals used UpToDate for at least 1 quarter. Users of UpToDate were more likely to be large, urban, teaching hospitals located in the Northeast, and either public or nonprofit (private) hospitals (Table 1).
| Characteristics | Using UpToDate (N = 1017) | Not Using UpToDate (N = 2305) | P Value |
|---|---|---|---|
| % | % | ||
| Hospital size | <0.001 | ||
| Small (6‐99) | 13 | 36 | |
| Medium (100‐399) | 64 | 55 | |
| Large (400+) | 23 | 8 | |
| Hospital region | <0.001 | ||
| Northeast | 27 | 12 | |
| Midwest | 26 | 23 | |
| South | 27 | 47 | |
| West | 20 | 18 | |
| Profit status | <0.001 | ||
| Profit hospitals | 10 | 21 | |
| Nongovernment nonprofit | 75 | 61 | |
| Government nonprofit | 15 | 18 | |
| Teaching hospitals | 19 | 4 | <0.001 |
| Urban location | 96 | 84 | <0.001 |
Over the 3 years, patients admitted to hospitals with UpToDate had generally shorter lengths of stay for all hospitalizations than patients admitted to hospitals without this specific system (5.6 days vs 5.7 days, P < 0.001; Table 2), and shorter lengths of stay for each of the 6 conditions examined (0.1 to 0.3 days shorter LOS, P < 0.001; Table 2).
| Conditions | Using UpToDate (Days) | Not Using UpToDate (Days) | Difference (CI) (Days) | P Value |
|---|---|---|---|---|
| ||||
| Total | 5.6 | 5.7 | 0.1 (0.2 to 0.0) | 0.001 |
| AMI | 5.3 | 5.5 | 0.2 (0.3 to 0.2) | <0.001 |
| CHF | 5.6 | 5.7 | 0.2 (0.2 to 0.1) | <0.001 |
| PN | 6.3 | 6.5 | 0.2 (0.2 to 0.1) | <0.001 |
| Stroke | 5.9 | 6.0 | 0.1 (0.2 to 0.1) | <0.001 |
| GIH | 5.3 | 5.4 | 0.2 (0.3 to 0.2) | <0.001 |
| Hip fracture | 6.7 | 6.8 | 0.1 (0.2 to 0.1) | <0.001 |
Similarly, we found that hospitals with UpToDate had lower risk‐adjusted 30‐day mortality rates, although the effects here were less consistent (Table 3). When we examined individual conditions, we found that patients admitted to UpToDate hospitals had lower risk‐adjusted 30‐day mortality rate for 4 of the 6 conditions, although only 3 of those 4 differences were statistically significant (Table 3). For example, patients in UpToDate hospitals had a lower likelihood of mortality for AMI (18.4% vs 18.9%, P = 0.03).
| Conditions | Using UpToDate (%) | Not Using UpToDate (%) | % Difference (CI) | P Value |
|---|---|---|---|---|
| ||||
| Total | 9.0 | 9.1 | 0.1 (0.2 to 0.0) | 0.04 |
| AMI | 18.4 | 19.0 | 0.7 (1.2 to 0.2) | 0.03 |
| CHF | 11.1 | 11.3 | 0.2 (0.4 to 0.1) | 0.21 |
| PN | 12.1 | 12.6 | 0.5 (0.7 to 0.2) | <0.001 |
| Stroke | 19.9 | 19.9 | 0.02 (0.5 to 0.5) | 0.91 |
| GIH | 6.9 | 7.3 | 0.4 (0.7 to 0.2) | 0.001 |
| Hip fracture | 8.8 | 8.6 | 0.2 (0.2 to 0.5) | 0.41 |
We found a more consistent association between the adoption of UpToDate and quality performance. Hospitals that had UpToDate had higher performance for each of the 4 conditions examined. For example, hospitals with UpToDate had higher quality performance for AMI compared to hospitals that did not adopt UpToDate (93.2% vs 90.4%, P < 0.001; Table 4). The results for CHF, pneumonia, and surgical complication prevention were qualitatively similar, and each reached statistical significance (Table 4).
| Conditions | Using UpToDate (%) | Not Using UpToDate (%) | % Difference (CI) | P Value |
|---|---|---|---|---|
| ||||
| AMI summary score | 93.4 | 90.2 | 3.2 (2.6, 3.6) | < 0.001 |
| CHF summary score | 81.0 | 75.1 | 5.9 (5.0, 6.8) | < 0.001 |
| PN summary score | 83.7 | 83.1 | 0.6 (0.3, 0.9) | 0.003 |
| SIP summary score | 80.0 | 78.1 | 1.9 (1.0, 2.9) | 0.002 |
In analyses that test for interaction, we found that the relationship between UpToDate use and quality performance was modified by hospital size and teaching status. Specifically, much of the benefit of UpToDate seemed limited to small and medium‐sized hospitals, as well as non‐teaching hospitals. For example, among small hospitals, those with UpToDate had, on average, 3‐7 point greater performance on the HQA scores, but almost no effect was found among large hospitals. Similarly, we found that non‐teaching hospitals were likely to have better performance in each of these areas if they had UpToDate, but this relationship was not consistent among major teaching hospitals (Table 5).
| Using UpToDate (%) | Not Using UpToDate (%) | % Difference (CI) | P Value | ||
|---|---|---|---|---|---|
| |||||
| Hospital size | Small (6‐99 beds) | ||||
| AMI summary score | 90.3 | 87.9 | 2.35 (0.91, 3.78) | <0.001 | |
| CHF summary score | 75.7 | 69.6 | 6.11 (4.04, 8.18) | <0.001 | |
| PN summary score | 84.9 | 83.2 | 1.67 (0.83, 2.51) | <0.001 | |
| Medium (100‐399 beds) | |||||
| AMI summary score | 93.0 | 90.5 | 2.52 (2.04, 3.00) | <0.001 | |
| CHF summary score | 81.1 | 78.9 | 2.22 (1.31, 3.12) | <0.001 | |
| PN summary score | 84.0 | 83.2 | 0.84 (0.31, 1.36) | <0.001 | |
| Large (>399 beds) | |||||
| AMI summary score | 94.7 | 93.8 | 0.82 (0.19, 1.46) | <0.001 | |
| CHF summary score | 82.9 | 82.7 | 0.21 (1.27, 1.69) | 0.83 | |
| PN summary score | 82.4 | 82.7 | 0.34 (1.49, 0.81) | 0.39 | |
| Teaching status | Major teaching | ||||
| AMI summary score | 94.9 | 94.8 | 0.15 (0.62, 0.92) | 0.60 | |
| CHF summary score | 83.3 | 83.0 | 0.26 (1.73, 2.25) | 0.83 | |
| PN summary score | 81.7 | 81.7 | 0.00 (1.67, 1.67) | 0.95 | |
| Not major teaching | |||||
| AMI summary score | 92.7 | 90.1 | 2.59 (2.15, 3.02) | <0.001 | |
| CHF summary score | 80.1 | 75.0 | 5.04 (4.18, 5.90) | <0.001 | |
| PN summary score | 84.3 | 83.2 | 1.05 (0.64, 1.47) | <0.001 | |
In our analyses of duration of use of UpToDate, we found a consistent relationship with shorter lengths of stay (see Supporting Appendix Table 2 in the online version of this article). Each 1000 days of UpToDate use was associated with a 0.08 day shorter length of stay (P < 0.001). A similar relationship was present and statistically significant in each of the 6 conditions examined. When we examined the impact of duration of use of UpToDate on risk‐adjusted mortality rate, we found a comparable relationship: Duration was associated with a lower mortality rate overall and for 5 of the 6 conditions examined, although only significant for 3 of the conditions (see Supporting Appendix Table 2 in the online version of this article). Similarly, greater duration was associated with better quality performance for each of the 4 conditions in the HQA program (data not shown).
When we quantified the overall impact of UpToDate, we found that if non‐adopters had a similar benefit in mortality (0.1%) seen in hospitals that adopted this system, it would lead, overall, to approximately 5550 fewer deaths annually (95% confidence interval 2601 fewer deaths to 7529 fewer deaths) and the 0.1 days shorter length of stay would lead to approximately 523,000 fewer patient days (95% confidence interval 160,000 to 799,000 fewer days) out of the approximately 30 million patient‐days that occurred in 2006 among non‐UpToDate hospitals.
DISCUSSION/CONCLUSION
We found that use of a commonly used computerized clinical knowledge management system (UpToDate) was associated with consistent, although small, reductions in lengths of stay, lower risk‐adjusted mortality rates, and higher quality performance. Much of the quality performance benefit seemed to be limited to small and medium‐sized, non‐teaching hospitals, while larger teaching hospitals realized little benefit. We found a stronger relationship between duration of use and better outcomes among UpToDate hospitals. Our findings suggest that hospitals using UpToDate had modestly better care that was also somewhat more efficient.
Prior studies have demonstrated that clinical decision support tools (ie, drug‐drug alerts and electronic reminders) can improve processes of care and enhance quality.13 Computerized clinical knowledge management systems, such as UpToDate, have unique advantages over other computerized clinical decision support tools. For example, computerized clinical knowledge management systems generally do not require electronic health records and can provide guidance to clinicians over a broader spectrum of diseases and clinical scenarios. UpToDate has previously shown to help providers answer questions rapidly, which can lead to changes in decision‐making that can improve management and efficiency.1, 7, 14
Ours is the first national study, to our knowledge, that has directly examined the relationship between UpToDate and key outcome metrics. Bonis et al. have previously examined the use of UpToDate and its relationship to risk‐adjusted LOS and mortality in a limited set of hospitals using a proprietary risk‐adjustment scheme.15 They found similar results among the Thompson 100 hospitals that were clinical knowledge management system users (compared to non‐users), including modestly shorter lengths of stay and a trend towards lower mortality. Our findings build on this work, but use publicly available data, a national sample, a well‐validated risk‐adjustment approach, and a much longer time period. The consistency of the findings across the 2 studies, despite differences in the approaches, help lend confidence that the results are unlikely to be due to chance alone.
There are likely to be important considerations surrounding the costs of UpToDate systems and whether those who purchase the system are wealthier than hospitals that chose not to. The typical annual subscription costs for a 100‐bed hospital between 2006 and 2010 was $10,578, which likely represents less than 0.01% of the annual operating costs for a 100‐bed institution (and is approximately the amount Medicare reimbursed for a single case of pneumonia without complications). Whether this cost would prohibit the adoption of UpToDate for most hospitals is unclear, and it is possible that a hospital that spent nearly $10,600 a year in such a system might therefore forego other quality improvement efforts. We suspect that these effects likely vary from hospital to hospital.
The primary limitation of the study is our inability to address whether or not the associations we found between UpToDate and outcomes are causally related. This is a fundamental limitation of all nonrandomized data. Four factors should lend some confidence to the interpretation that these findings may not be due to confounding alone. First, the effects were consistent across a series of measures (mortality, efficiency, and processes) that are not, themselves, highly correlated with each other1618. Our findings, that hospitals with UpToDate were somewhat better across all measures examined, point to the potential benefit of having high‐quality clinical information readily available for clinicians. Second, we found that the benefits persisted even after controlling for other hospital characteristics that were associated with adoption, including measures of hospital financial health (as measured by proportion of Medicaid patients and the DSH Index). Of course, this does not negate the possibility that other factors, such as the presence of medical libraries or a culture of quality and continuous learning, may be associated both with the use of UpToDate and with the outcomes. Third, the effects, at least for quality performance, were prominent among smaller, non‐teaching hospitals (which, one would surmise, a priori, to be most likely to benefit from UpToDate). Finally, the dose‐response relationship of duration of use, which was an analysis limited to only those hospitals with UpToDate, provides more evidence that the system itself may have some impact.
Another limitation of our work is that we used administrative data for risk‐adjustment, which has inherent challenges.1925 Given that users of UpToDate, such as teaching hospitals and larger institutions, generally have a much sicker patient population, inadequate risk‐adjustment may have lead us to underestimate the true effect. It is also possible that many of the hospitals designated as non‐users had other clinical knowledge management systems of which we were unaware. However, this would likely have made it harder to find an effect, biasing our study towards a null finding. We conducted a series of analyses and, yet, did not adjust for multiple testing. We had chosen these analyses a priori and, although the association we found may have been due to selection bias, it is unlikely that all of the associations in our analyses were due to random chance. Next, although we examined the potential impact of UpToDate, we suspect that any high‐quality clinical knowledge management system should allow clinicians to deliver higher quality, more efficient care. Finally, we are unsure whether or not the magnitude of effect we found is clinically significant. While the added advantage of having UpToDate appeared to be a reduction in mortality of only 0.1% (over all conditions), such a difference would be associated with approximately 5550 fewer deaths each year among Medicare fee‐for‐service beneficiaries. Whether such a benefit would be worth the cost of implementing systems like UpToDate needs to be further explored.
In conclusion, we found consistent association between use of a widely deployed computerized clinical knowledge management system, UpToDate, and reduced length of stay, lower risk‐adjusted mortality rates, and higher quality performance. Whether use of UpToDate led to better care is not definitive, but our findings suggest that these types of management software may play an important role as our nation endeavors to improve the quality and efficiency of the healthcare system.
- ,,,,.Answering physicians' clinical questions: obstacles and potential solutions.J Am Med Inform Assoc.2005;12(2):217–224.
- ,,, et al.Obstacles to answering doctors' questions about patient care with evidence: qualitative study.BMJ.2002;324(7339):710.
- ,.Why do residents fail to answer their clinical questions? A qualitative study of barriers to practicing evidence‐based medicine.Acad Med.2005;80(2):176–182.
- ,,.A clinical informaticist to support primary care decision making.Qual Health Care.2001;10(4):245–249.
- ,,, et al.Searching for medical information online: a survey of Canadian nephrologists.J Nephrol. Feb 23, 2011. doi: 10.5301/JN.2011.6373.
- ,,,.Answers to questions posed during daily patient care are more likely to be answered by UpToDate than PubMed.J Med Internet Res2008;10(4):e29.
- ,,, et al.The impact of evidence on physicians' inpatient treatment decisions.J Gen Intern Med.2004;19(5 pt 1):402–409.
- ,,.Can an electronic database help busy physicians answer clinical questions? [abstract].J Gen Intern Med.2002;17(suppl 1):220.
- ,.UpToDate: a comprehensive clinical database.J Fam Pract.2003;52(9):706–710.
- AHRQ Quality Indicators. Guide to Inpatient Quality Indicators: Quality of Care in Hospitals ‐ Volume, Mortality, and Utilization [Version 3.1]. Rockville, MD: AHRQ; Mar 12, 2007.
- ,,,,.Snapshot of hospital quality reporting and pay‐for‐performance under Medicare.Health Aff (Millwood).2006;25(1):149–162.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36(1):8–27.
- ,,, et al.Systematic review: impact of health information technology on quality, efficiency, and costs of medical care.Ann Intern Med.2006;144(10):742–752.
- ,,,.Answering clinical questions in the ED.Am J Emerg Med.2008;26(2):144–147.
- ,,,.Association of a clinical knowledge support system with improved patient safety, reduced complications and shorter length of stay among Medicare beneficiaries in acute care hospitals in the United States.Int J Med Informatics.2008;77(11):745–753.
- ,,,,.Measuring efficiency: the association of hospital costs and quality of care.Health Aff (Millwood).2009;28(3):897–906.
- ,,, et al.Hospital quality for acute myocardial infarction: correlates among process measures and relationship to short‐term mortality.JAMA.2006;295.
- ,,,.Hospital quality and intensity of spending: is there an association?Health Aff (Millwood).2009;28(4):w566–w572.
- ,,,,,.Discordance of databases designed for claims payment versus clinical information systems. Implications for outcomes research.Ann Intern Med.1993;119(8):844–850.
- ,,,.Accuracy of Medicare claims data for rheumatologic diagnoses in total hip replacement recipients.J Clin Epidemiol.2003;56(6):515–519.
- ,,,.The sensitivity of Medicare billing claims data for monitoring mammography use by elderly women.Med Care Res Rev.2004;61(1):116–127.
- ,,,,,.A comparison of ambulatory Medicaid claims to medical records: a reliability assessment.Am J Med Qual.1998;13(2):63–69.
- ,,,.Methodological issues in the use of administrative claims data to study surveillance after cancer treatment.Med Care.2002;40(8 suppl):IV‐69–74.
- ,,,,,.Using claims data for epidemiologic research. The concordance of claims‐based criteria with the medical record and patient survey for identifying a hypertensive population.Med Care.1993;31(6):498–507.
- .The Potential of Claims Data to Support the Measurement of Healthcare Quality.Santa Monica, CA:Rand Corporation;2003.
New health information technologies hold enormous potential for improving the quality and efficiency of healthcare. One commonly used health information technology is computerized clinical knowledge management (CKM) systems, which provide clinicians with access to relevant and continually updated clinical information about major medical topics at the point of care. Studies indicate that clinicians often have questions about patient care, which go largely unanswered during patient encounters.13 The availability of answers to critical clinical questions can have a large impact on clinical decision‐making and practice.4
UpToDate is one of the most widely used computerized clinical knowledge management systems in the nation.1, 2, 5, 6 Previous studies of UpToDate and similar systems demonstrated that these systems improve acquisition of knowledge, increase the number of answered clinical questions, and change management decisions.7, 8 However, whether these changes lead to real improvements in clinical outcomes is unknown. Given the urgent need to improve both the quality and efficiency of healthcare, understanding whether UpToDate has the potential to improve outcomes is critical.
Therefore, we examined whether the use of UpToDate was associated with lower risk‐adjusted mortality rates, shorter lengths of stay, and better performance on standard quality process metrics. Further, we sought to determine whether the impact of UpToDate was particularly potent in certain subsets of hospitals. Finally, we examined whether the duration of use of UpToDate was associated with better outcomes.
METHODS
Overview
Our overall approach was to examine the relationship between UpToDate and 3 main outcomes: risk‐adjusted length of stay, risk‐adjusted mortality, and performance on standard quality process metrics in the period from 2004 to 2006. We took 4 approaches to try to reduce potential confounding (adopters of the UpToDate are likely different than non‐adopters). First, we used a longitudinal modeling approach where hospitals were allowed to serve as their own controls over time. For example, if a hospital was an adopter of UpToDate in the fourth quarter of 2005, all data for that hospital prior to that quarter of 2005 would be counted as part of the control hospitals. Second, we used multivariable models to adjust for observable differences between adopters and non‐adopters. Third, we tested for interactions to see if the effect of UpToDate was particularly concentrated in a subset of hospitals. Finally, we examined whether the duration of use, which reflects a potential dose‐response relationship, was related to the outcomes of interest.
UpToDate
The UpToDate system provides a compendium of regularly revised, evidence‐based monographs on topics in adult internal medicine, pediatrics, and obstetrics and gynecology.9 The system is available through multiple medias (ie, the Internet, handheld devices). Providers at subscribing hospitals can usually access it through any computer terminal within the facility, and often through remote access.
Data Sources and Linkage
We used 5 primary sources of data: UpToDate usage data, which was provided directly by UpToDate and includes a list of all hospitals in the United States who use the UpToDate software and the start date of usage; the American Hospital Association (AHA) Annual Survey, which contains individual hospital structural characteristics (such as size, location, teaching status); the 2007 Medicare Inpatient Impact files, which includes hospital characteristics not available in the AHA data; the 2004‐2006 Medicare Provider Analysis and Review (MEDPAR) databases, which have patient‐level discharge information about all Medicare fee‐for‐service patients hospitalized in a given year; and the 2004‐2007 Hospital Quality Alliance (HQA) database, which includes publicly available data for inpatient quality measures. We linked these 4 datasets with a database of hospitals that use UpToDate.
Outcomes
We chose, a priori, to examine 3 primary outcomes: risk‐adjusted length of stay (LOS), which is considered a measure of efficiency; risk‐adjusted mortality, a commonly used marker of quality of care; and performance outcomes on HQA quality metrics.
Risk‐Adjusted LOS and Risk‐Adjusted Mortality Rates
We examined risk‐adjusted LOS and risk‐adjusted mortality rates among all hospitalized patients and among 6 common medical and surgical conditions: acute myocardial infarction (AMI), congestive heart failure (CHF), pneumonia, gastrointestinal hemorrhage, stroke, and hip fracture. We selected these 6 conditions because they are used by the Agency for Healthcare Research and Quality (AHRQ) to measure hospital quality. We used International Classification of Diseases, Ninth Revision (ICD‐9) codes used by the AHRQ Inpatient Quality Indicators to identify patients admitted for these 6 conditions.10 We performed risk‐adjustment using the Elixhauser comorbidity adjustment scheme, which was developed by AHRQ and is commonly used to adjust for differences in severity using administrative data.
Quality Processes of Care
To examine hospital quality performance, we used the HQA process measures for 4 conditions from 2004 to 2007: AMI, CHF, pneumonia, and surgical infection prevention (SIP). We examined all HQA indicators publically available in 2004 (8 measures for AMI, 4 measures for CHF, 6 measures for pneumonia, and 2 measures for SIP). (The specific indicators are listed in Supporting Appendix Table 1 in the online version of this article.) We created summary scores for each condition, and an overall hospital summary score for the performance on all indicators, using methodology previously described by the Joint Commission.11 Each summary score represents the number of times a hospital performed the appropriate action across all measures for that condition divided by the number of opportunities the hospital had to provide appropriate care for that condition. Composite scores were only calculated if a hospital had at least 30 patients for at least one of the measures of each condition.
AnalysisCKM Users Versus Non‐Users
We chose, a priori, to perform several sets of analyses to understand the relationship between the use of UpToDate and clinical outcomes. Our primary approach was a longitudinal model where each hospital was allowed to serve as its own control. In sensitivity analyses, we used a differences‐in‐differences model where we examined whether the changes for hospitals that adopted UpToDate differed compared to changes in outcomes for non‐adopters, adjusting for temporal trends by using time as a covariate in the model.
In the first analysis using longitudinal data, we examined whether being admitted to a hospital with UpToDate was associated with shorter length of stay, lower risk‐adjusted 30‐day mortality or higher process quality. This model allowed each hospital to serve as its own control and tested to see how the outcomes changed after adoption of UpToDate, controlling for secular trends by including non‐UpToDate hospitals. The models were adjusted for hospital characteristics including size, region, location (urban vs rural), ownership (for‐profit, not‐for‐profit private, not‐for‐profit public), teaching status (member of the Council of Teaching Hospital vs not), the proportion of patients that had Medicaid, the Disproportionate Share Hospital (DSH) Index, and the presence or absence of a medical intensive care unit (ICU). We used a repeated‐measures generalized estimating equations (GEE) to account for both clustering at the hospital level and for the repeated measures nature of our analysis. We used the Elixhauser comorbidity adjustment scheme to account for patient‐level factors.12
In our first set of models, we included all patients. We subsequently built 6 condition‐specific models for each of the 6 common medical conditions: AMI, CHF, pneumonia, gastrointestinal hemorrhage, stroke, and hip fracture.
Identifying Subsets of Hospitals
Next, we postulated a priori that certain subsets of hospitalssmaller institutions and non‐teaching institutionsmight have less access to high‐quality clinical information and, thus, be mode likely to benefit from UpToDate. To determine if the potential impact of UpToDate on the outcomes varied based on these hospital characteristics, we repeated our analyses using multivariable models but tested interaction terms. We found significant interactions for 1 outcome (HQA quality performance scores), and present data with stratified analyses for this outcome.
Impact of Duration of Use
Finally, we calculated duration of UpToDate use for each hospital. For each hospital using UpToDate, we identified the date it started using the system. Based on the start date, we calculated each hospital's duration of use for each quarter. We used the midpoint of that quarter to calculate the number of days a hospital used UpToDate. For example, if a hospital started using UpToDate on January 1, 2002, we assigned 775 days for its duration of use for the first quarter of 2004 (365 days per year 2 years + 45 days for the first quarter of 2004) and 865 days for its duration of use for the second quarter of 2004. We excluded hospitals that did not use UpToDate.
Our primary analysis used a dataset that was restricted to just those hospitals using UpToDate. We ran the model for all discharges and for each of the 6 conditions for both LOS and for risk‐adjusted mortality rate, as well as for the 4 conditions encompassed in the HQA quality reporting program. In all analyses, we considered a 2‐sided P value as statistically significant.
Assessing the Overall Impact of UpToDate
To better assess the clinical significance of any change in mortality rates, we calculated the number of deaths prevented if all hospitals saw the same gain in mortality if they adopted UpToDate. To calculate this impact number, we identified the overall reduction in risk‐adjusted mortality associated with UpToDate adoption and multiplied this number by the number of elderly Medicare patients admitted to non‐UpToDate hospitals. We calculated a similar number for changes in length of stay.
RESULTS
We found that between 2004 through 2006, 1017 hospitals used UpToDate for at least 1 quarter. Users of UpToDate were more likely to be large, urban, teaching hospitals located in the Northeast, and either public or nonprofit (private) hospitals (Table 1).
| Characteristics | Using UpToDate (N = 1017) | Not Using UpToDate (N = 2305) | P Value |
|---|---|---|---|
| % | % | ||
| Hospital size | <0.001 | ||
| Small (6‐99) | 13 | 36 | |
| Medium (100‐399) | 64 | 55 | |
| Large (400+) | 23 | 8 | |
| Hospital region | <0.001 | ||
| Northeast | 27 | 12 | |
| Midwest | 26 | 23 | |
| South | 27 | 47 | |
| West | 20 | 18 | |
| Profit status | <0.001 | ||
| Profit hospitals | 10 | 21 | |
| Nongovernment nonprofit | 75 | 61 | |
| Government nonprofit | 15 | 18 | |
| Teaching hospitals | 19 | 4 | <0.001 |
| Urban location | 96 | 84 | <0.001 |
Over the 3 years, patients admitted to hospitals with UpToDate had generally shorter lengths of stay for all hospitalizations than patients admitted to hospitals without this specific system (5.6 days vs 5.7 days, P < 0.001; Table 2), and shorter lengths of stay for each of the 6 conditions examined (0.1 to 0.3 days shorter LOS, P < 0.001; Table 2).
| Conditions | Using UpToDate (Days) | Not Using UpToDate (Days) | Difference (CI) (Days) | P Value |
|---|---|---|---|---|
| ||||
| Total | 5.6 | 5.7 | 0.1 (0.2 to 0.0) | 0.001 |
| AMI | 5.3 | 5.5 | 0.2 (0.3 to 0.2) | <0.001 |
| CHF | 5.6 | 5.7 | 0.2 (0.2 to 0.1) | <0.001 |
| PN | 6.3 | 6.5 | 0.2 (0.2 to 0.1) | <0.001 |
| Stroke | 5.9 | 6.0 | 0.1 (0.2 to 0.1) | <0.001 |
| GIH | 5.3 | 5.4 | 0.2 (0.3 to 0.2) | <0.001 |
| Hip fracture | 6.7 | 6.8 | 0.1 (0.2 to 0.1) | <0.001 |
Similarly, we found that hospitals with UpToDate had lower risk‐adjusted 30‐day mortality rates, although the effects here were less consistent (Table 3). When we examined individual conditions, we found that patients admitted to UpToDate hospitals had lower risk‐adjusted 30‐day mortality rate for 4 of the 6 conditions, although only 3 of those 4 differences were statistically significant (Table 3). For example, patients in UpToDate hospitals had a lower likelihood of mortality for AMI (18.4% vs 18.9%, P = 0.03).
| Conditions | Using UpToDate (%) | Not Using UpToDate (%) | % Difference (CI) | P Value |
|---|---|---|---|---|
| ||||
| Total | 9.0 | 9.1 | 0.1 (0.2 to 0.0) | 0.04 |
| AMI | 18.4 | 19.0 | 0.7 (1.2 to 0.2) | 0.03 |
| CHF | 11.1 | 11.3 | 0.2 (0.4 to 0.1) | 0.21 |
| PN | 12.1 | 12.6 | 0.5 (0.7 to 0.2) | <0.001 |
| Stroke | 19.9 | 19.9 | 0.02 (0.5 to 0.5) | 0.91 |
| GIH | 6.9 | 7.3 | 0.4 (0.7 to 0.2) | 0.001 |
| Hip fracture | 8.8 | 8.6 | 0.2 (0.2 to 0.5) | 0.41 |
We found a more consistent association between the adoption of UpToDate and quality performance. Hospitals that had UpToDate had higher performance for each of the 4 conditions examined. For example, hospitals with UpToDate had higher quality performance for AMI compared to hospitals that did not adopt UpToDate (93.2% vs 90.4%, P < 0.001; Table 4). The results for CHF, pneumonia, and surgical complication prevention were qualitatively similar, and each reached statistical significance (Table 4).
| Conditions | Using UpToDate (%) | Not Using UpToDate (%) | % Difference (CI) | P Value |
|---|---|---|---|---|
| ||||
| AMI summary score | 93.4 | 90.2 | 3.2 (2.6, 3.6) | < 0.001 |
| CHF summary score | 81.0 | 75.1 | 5.9 (5.0, 6.8) | < 0.001 |
| PN summary score | 83.7 | 83.1 | 0.6 (0.3, 0.9) | 0.003 |
| SIP summary score | 80.0 | 78.1 | 1.9 (1.0, 2.9) | 0.002 |
In analyses that test for interaction, we found that the relationship between UpToDate use and quality performance was modified by hospital size and teaching status. Specifically, much of the benefit of UpToDate seemed limited to small and medium‐sized hospitals, as well as non‐teaching hospitals. For example, among small hospitals, those with UpToDate had, on average, 3‐7 point greater performance on the HQA scores, but almost no effect was found among large hospitals. Similarly, we found that non‐teaching hospitals were likely to have better performance in each of these areas if they had UpToDate, but this relationship was not consistent among major teaching hospitals (Table 5).
| Using UpToDate (%) | Not Using UpToDate (%) | % Difference (CI) | P Value | ||
|---|---|---|---|---|---|
| |||||
| Hospital size | Small (6‐99 beds) | ||||
| AMI summary score | 90.3 | 87.9 | 2.35 (0.91, 3.78) | <0.001 | |
| CHF summary score | 75.7 | 69.6 | 6.11 (4.04, 8.18) | <0.001 | |
| PN summary score | 84.9 | 83.2 | 1.67 (0.83, 2.51) | <0.001 | |
| Medium (100‐399 beds) | |||||
| AMI summary score | 93.0 | 90.5 | 2.52 (2.04, 3.00) | <0.001 | |
| CHF summary score | 81.1 | 78.9 | 2.22 (1.31, 3.12) | <0.001 | |
| PN summary score | 84.0 | 83.2 | 0.84 (0.31, 1.36) | <0.001 | |
| Large (>399 beds) | |||||
| AMI summary score | 94.7 | 93.8 | 0.82 (0.19, 1.46) | <0.001 | |
| CHF summary score | 82.9 | 82.7 | 0.21 (1.27, 1.69) | 0.83 | |
| PN summary score | 82.4 | 82.7 | 0.34 (1.49, 0.81) | 0.39 | |
| Teaching status | Major teaching | ||||
| AMI summary score | 94.9 | 94.8 | 0.15 (0.62, 0.92) | 0.60 | |
| CHF summary score | 83.3 | 83.0 | 0.26 (1.73, 2.25) | 0.83 | |
| PN summary score | 81.7 | 81.7 | 0.00 (1.67, 1.67) | 0.95 | |
| Not major teaching | |||||
| AMI summary score | 92.7 | 90.1 | 2.59 (2.15, 3.02) | <0.001 | |
| CHF summary score | 80.1 | 75.0 | 5.04 (4.18, 5.90) | <0.001 | |
| PN summary score | 84.3 | 83.2 | 1.05 (0.64, 1.47) | <0.001 | |
In our analyses of duration of use of UpToDate, we found a consistent relationship with shorter lengths of stay (see Supporting Appendix Table 2 in the online version of this article). Each 1000 days of UpToDate use was associated with a 0.08 day shorter length of stay (P < 0.001). A similar relationship was present and statistically significant in each of the 6 conditions examined. When we examined the impact of duration of use of UpToDate on risk‐adjusted mortality rate, we found a comparable relationship: Duration was associated with a lower mortality rate overall and for 5 of the 6 conditions examined, although only significant for 3 of the conditions (see Supporting Appendix Table 2 in the online version of this article). Similarly, greater duration was associated with better quality performance for each of the 4 conditions in the HQA program (data not shown).
When we quantified the overall impact of UpToDate, we found that if non‐adopters had a similar benefit in mortality (0.1%) seen in hospitals that adopted this system, it would lead, overall, to approximately 5550 fewer deaths annually (95% confidence interval 2601 fewer deaths to 7529 fewer deaths) and the 0.1 days shorter length of stay would lead to approximately 523,000 fewer patient days (95% confidence interval 160,000 to 799,000 fewer days) out of the approximately 30 million patient‐days that occurred in 2006 among non‐UpToDate hospitals.
DISCUSSION/CONCLUSION
We found that use of a commonly used computerized clinical knowledge management system (UpToDate) was associated with consistent, although small, reductions in lengths of stay, lower risk‐adjusted mortality rates, and higher quality performance. Much of the quality performance benefit seemed to be limited to small and medium‐sized, non‐teaching hospitals, while larger teaching hospitals realized little benefit. We found a stronger relationship between duration of use and better outcomes among UpToDate hospitals. Our findings suggest that hospitals using UpToDate had modestly better care that was also somewhat more efficient.
Prior studies have demonstrated that clinical decision support tools (ie, drug‐drug alerts and electronic reminders) can improve processes of care and enhance quality.13 Computerized clinical knowledge management systems, such as UpToDate, have unique advantages over other computerized clinical decision support tools. For example, computerized clinical knowledge management systems generally do not require electronic health records and can provide guidance to clinicians over a broader spectrum of diseases and clinical scenarios. UpToDate has previously shown to help providers answer questions rapidly, which can lead to changes in decision‐making that can improve management and efficiency.1, 7, 14
Ours is the first national study, to our knowledge, that has directly examined the relationship between UpToDate and key outcome metrics. Bonis et al. have previously examined the use of UpToDate and its relationship to risk‐adjusted LOS and mortality in a limited set of hospitals using a proprietary risk‐adjustment scheme.15 They found similar results among the Thompson 100 hospitals that were clinical knowledge management system users (compared to non‐users), including modestly shorter lengths of stay and a trend towards lower mortality. Our findings build on this work, but use publicly available data, a national sample, a well‐validated risk‐adjustment approach, and a much longer time period. The consistency of the findings across the 2 studies, despite differences in the approaches, help lend confidence that the results are unlikely to be due to chance alone.
There are likely to be important considerations surrounding the costs of UpToDate systems and whether those who purchase the system are wealthier than hospitals that chose not to. The typical annual subscription costs for a 100‐bed hospital between 2006 and 2010 was $10,578, which likely represents less than 0.01% of the annual operating costs for a 100‐bed institution (and is approximately the amount Medicare reimbursed for a single case of pneumonia without complications). Whether this cost would prohibit the adoption of UpToDate for most hospitals is unclear, and it is possible that a hospital that spent nearly $10,600 a year in such a system might therefore forego other quality improvement efforts. We suspect that these effects likely vary from hospital to hospital.
The primary limitation of the study is our inability to address whether or not the associations we found between UpToDate and outcomes are causally related. This is a fundamental limitation of all nonrandomized data. Four factors should lend some confidence to the interpretation that these findings may not be due to confounding alone. First, the effects were consistent across a series of measures (mortality, efficiency, and processes) that are not, themselves, highly correlated with each other1618. Our findings, that hospitals with UpToDate were somewhat better across all measures examined, point to the potential benefit of having high‐quality clinical information readily available for clinicians. Second, we found that the benefits persisted even after controlling for other hospital characteristics that were associated with adoption, including measures of hospital financial health (as measured by proportion of Medicaid patients and the DSH Index). Of course, this does not negate the possibility that other factors, such as the presence of medical libraries or a culture of quality and continuous learning, may be associated both with the use of UpToDate and with the outcomes. Third, the effects, at least for quality performance, were prominent among smaller, non‐teaching hospitals (which, one would surmise, a priori, to be most likely to benefit from UpToDate). Finally, the dose‐response relationship of duration of use, which was an analysis limited to only those hospitals with UpToDate, provides more evidence that the system itself may have some impact.
Another limitation of our work is that we used administrative data for risk‐adjustment, which has inherent challenges.1925 Given that users of UpToDate, such as teaching hospitals and larger institutions, generally have a much sicker patient population, inadequate risk‐adjustment may have lead us to underestimate the true effect. It is also possible that many of the hospitals designated as non‐users had other clinical knowledge management systems of which we were unaware. However, this would likely have made it harder to find an effect, biasing our study towards a null finding. We conducted a series of analyses and, yet, did not adjust for multiple testing. We had chosen these analyses a priori and, although the association we found may have been due to selection bias, it is unlikely that all of the associations in our analyses were due to random chance. Next, although we examined the potential impact of UpToDate, we suspect that any high‐quality clinical knowledge management system should allow clinicians to deliver higher quality, more efficient care. Finally, we are unsure whether or not the magnitude of effect we found is clinically significant. While the added advantage of having UpToDate appeared to be a reduction in mortality of only 0.1% (over all conditions), such a difference would be associated with approximately 5550 fewer deaths each year among Medicare fee‐for‐service beneficiaries. Whether such a benefit would be worth the cost of implementing systems like UpToDate needs to be further explored.
In conclusion, we found consistent association between use of a widely deployed computerized clinical knowledge management system, UpToDate, and reduced length of stay, lower risk‐adjusted mortality rates, and higher quality performance. Whether use of UpToDate led to better care is not definitive, but our findings suggest that these types of management software may play an important role as our nation endeavors to improve the quality and efficiency of the healthcare system.
New health information technologies hold enormous potential for improving the quality and efficiency of healthcare. One commonly used health information technology is computerized clinical knowledge management (CKM) systems, which provide clinicians with access to relevant and continually updated clinical information about major medical topics at the point of care. Studies indicate that clinicians often have questions about patient care, which go largely unanswered during patient encounters.13 The availability of answers to critical clinical questions can have a large impact on clinical decision‐making and practice.4
UpToDate is one of the most widely used computerized clinical knowledge management systems in the nation.1, 2, 5, 6 Previous studies of UpToDate and similar systems demonstrated that these systems improve acquisition of knowledge, increase the number of answered clinical questions, and change management decisions.7, 8 However, whether these changes lead to real improvements in clinical outcomes is unknown. Given the urgent need to improve both the quality and efficiency of healthcare, understanding whether UpToDate has the potential to improve outcomes is critical.
Therefore, we examined whether the use of UpToDate was associated with lower risk‐adjusted mortality rates, shorter lengths of stay, and better performance on standard quality process metrics. Further, we sought to determine whether the impact of UpToDate was particularly potent in certain subsets of hospitals. Finally, we examined whether the duration of use of UpToDate was associated with better outcomes.
METHODS
Overview
Our overall approach was to examine the relationship between UpToDate and 3 main outcomes: risk‐adjusted length of stay, risk‐adjusted mortality, and performance on standard quality process metrics in the period from 2004 to 2006. We took 4 approaches to try to reduce potential confounding (adopters of the UpToDate are likely different than non‐adopters). First, we used a longitudinal modeling approach where hospitals were allowed to serve as their own controls over time. For example, if a hospital was an adopter of UpToDate in the fourth quarter of 2005, all data for that hospital prior to that quarter of 2005 would be counted as part of the control hospitals. Second, we used multivariable models to adjust for observable differences between adopters and non‐adopters. Third, we tested for interactions to see if the effect of UpToDate was particularly concentrated in a subset of hospitals. Finally, we examined whether the duration of use, which reflects a potential dose‐response relationship, was related to the outcomes of interest.
UpToDate
The UpToDate system provides a compendium of regularly revised, evidence‐based monographs on topics in adult internal medicine, pediatrics, and obstetrics and gynecology.9 The system is available through multiple medias (ie, the Internet, handheld devices). Providers at subscribing hospitals can usually access it through any computer terminal within the facility, and often through remote access.
Data Sources and Linkage
We used 5 primary sources of data: UpToDate usage data, which was provided directly by UpToDate and includes a list of all hospitals in the United States who use the UpToDate software and the start date of usage; the American Hospital Association (AHA) Annual Survey, which contains individual hospital structural characteristics (such as size, location, teaching status); the 2007 Medicare Inpatient Impact files, which includes hospital characteristics not available in the AHA data; the 2004‐2006 Medicare Provider Analysis and Review (MEDPAR) databases, which have patient‐level discharge information about all Medicare fee‐for‐service patients hospitalized in a given year; and the 2004‐2007 Hospital Quality Alliance (HQA) database, which includes publicly available data for inpatient quality measures. We linked these 4 datasets with a database of hospitals that use UpToDate.
Outcomes
We chose, a priori, to examine 3 primary outcomes: risk‐adjusted length of stay (LOS), which is considered a measure of efficiency; risk‐adjusted mortality, a commonly used marker of quality of care; and performance outcomes on HQA quality metrics.
Risk‐Adjusted LOS and Risk‐Adjusted Mortality Rates
We examined risk‐adjusted LOS and risk‐adjusted mortality rates among all hospitalized patients and among 6 common medical and surgical conditions: acute myocardial infarction (AMI), congestive heart failure (CHF), pneumonia, gastrointestinal hemorrhage, stroke, and hip fracture. We selected these 6 conditions because they are used by the Agency for Healthcare Research and Quality (AHRQ) to measure hospital quality. We used International Classification of Diseases, Ninth Revision (ICD‐9) codes used by the AHRQ Inpatient Quality Indicators to identify patients admitted for these 6 conditions.10 We performed risk‐adjustment using the Elixhauser comorbidity adjustment scheme, which was developed by AHRQ and is commonly used to adjust for differences in severity using administrative data.
Quality Processes of Care
To examine hospital quality performance, we used the HQA process measures for 4 conditions from 2004 to 2007: AMI, CHF, pneumonia, and surgical infection prevention (SIP). We examined all HQA indicators publically available in 2004 (8 measures for AMI, 4 measures for CHF, 6 measures for pneumonia, and 2 measures for SIP). (The specific indicators are listed in Supporting Appendix Table 1 in the online version of this article.) We created summary scores for each condition, and an overall hospital summary score for the performance on all indicators, using methodology previously described by the Joint Commission.11 Each summary score represents the number of times a hospital performed the appropriate action across all measures for that condition divided by the number of opportunities the hospital had to provide appropriate care for that condition. Composite scores were only calculated if a hospital had at least 30 patients for at least one of the measures of each condition.
AnalysisCKM Users Versus Non‐Users
We chose, a priori, to perform several sets of analyses to understand the relationship between the use of UpToDate and clinical outcomes. Our primary approach was a longitudinal model where each hospital was allowed to serve as its own control. In sensitivity analyses, we used a differences‐in‐differences model where we examined whether the changes for hospitals that adopted UpToDate differed compared to changes in outcomes for non‐adopters, adjusting for temporal trends by using time as a covariate in the model.
In the first analysis using longitudinal data, we examined whether being admitted to a hospital with UpToDate was associated with shorter length of stay, lower risk‐adjusted 30‐day mortality or higher process quality. This model allowed each hospital to serve as its own control and tested to see how the outcomes changed after adoption of UpToDate, controlling for secular trends by including non‐UpToDate hospitals. The models were adjusted for hospital characteristics including size, region, location (urban vs rural), ownership (for‐profit, not‐for‐profit private, not‐for‐profit public), teaching status (member of the Council of Teaching Hospital vs not), the proportion of patients that had Medicaid, the Disproportionate Share Hospital (DSH) Index, and the presence or absence of a medical intensive care unit (ICU). We used a repeated‐measures generalized estimating equations (GEE) to account for both clustering at the hospital level and for the repeated measures nature of our analysis. We used the Elixhauser comorbidity adjustment scheme to account for patient‐level factors.12
In our first set of models, we included all patients. We subsequently built 6 condition‐specific models for each of the 6 common medical conditions: AMI, CHF, pneumonia, gastrointestinal hemorrhage, stroke, and hip fracture.
Identifying Subsets of Hospitals
Next, we postulated a priori that certain subsets of hospitalssmaller institutions and non‐teaching institutionsmight have less access to high‐quality clinical information and, thus, be mode likely to benefit from UpToDate. To determine if the potential impact of UpToDate on the outcomes varied based on these hospital characteristics, we repeated our analyses using multivariable models but tested interaction terms. We found significant interactions for 1 outcome (HQA quality performance scores), and present data with stratified analyses for this outcome.
Impact of Duration of Use
Finally, we calculated duration of UpToDate use for each hospital. For each hospital using UpToDate, we identified the date it started using the system. Based on the start date, we calculated each hospital's duration of use for each quarter. We used the midpoint of that quarter to calculate the number of days a hospital used UpToDate. For example, if a hospital started using UpToDate on January 1, 2002, we assigned 775 days for its duration of use for the first quarter of 2004 (365 days per year 2 years + 45 days for the first quarter of 2004) and 865 days for its duration of use for the second quarter of 2004. We excluded hospitals that did not use UpToDate.
Our primary analysis used a dataset that was restricted to just those hospitals using UpToDate. We ran the model for all discharges and for each of the 6 conditions for both LOS and for risk‐adjusted mortality rate, as well as for the 4 conditions encompassed in the HQA quality reporting program. In all analyses, we considered a 2‐sided P value as statistically significant.
Assessing the Overall Impact of UpToDate
To better assess the clinical significance of any change in mortality rates, we calculated the number of deaths prevented if all hospitals saw the same gain in mortality if they adopted UpToDate. To calculate this impact number, we identified the overall reduction in risk‐adjusted mortality associated with UpToDate adoption and multiplied this number by the number of elderly Medicare patients admitted to non‐UpToDate hospitals. We calculated a similar number for changes in length of stay.
RESULTS
We found that between 2004 through 2006, 1017 hospitals used UpToDate for at least 1 quarter. Users of UpToDate were more likely to be large, urban, teaching hospitals located in the Northeast, and either public or nonprofit (private) hospitals (Table 1).
| Characteristics | Using UpToDate (N = 1017) | Not Using UpToDate (N = 2305) | P Value |
|---|---|---|---|
| % | % | ||
| Hospital size | <0.001 | ||
| Small (6‐99) | 13 | 36 | |
| Medium (100‐399) | 64 | 55 | |
| Large (400+) | 23 | 8 | |
| Hospital region | <0.001 | ||
| Northeast | 27 | 12 | |
| Midwest | 26 | 23 | |
| South | 27 | 47 | |
| West | 20 | 18 | |
| Profit status | <0.001 | ||
| Profit hospitals | 10 | 21 | |
| Nongovernment nonprofit | 75 | 61 | |
| Government nonprofit | 15 | 18 | |
| Teaching hospitals | 19 | 4 | <0.001 |
| Urban location | 96 | 84 | <0.001 |
Over the 3 years, patients admitted to hospitals with UpToDate had generally shorter lengths of stay for all hospitalizations than patients admitted to hospitals without this specific system (5.6 days vs 5.7 days, P < 0.001; Table 2), and shorter lengths of stay for each of the 6 conditions examined (0.1 to 0.3 days shorter LOS, P < 0.001; Table 2).
| Conditions | Using UpToDate (Days) | Not Using UpToDate (Days) | Difference (CI) (Days) | P Value |
|---|---|---|---|---|
| ||||
| Total | 5.6 | 5.7 | 0.1 (0.2 to 0.0) | 0.001 |
| AMI | 5.3 | 5.5 | 0.2 (0.3 to 0.2) | <0.001 |
| CHF | 5.6 | 5.7 | 0.2 (0.2 to 0.1) | <0.001 |
| PN | 6.3 | 6.5 | 0.2 (0.2 to 0.1) | <0.001 |
| Stroke | 5.9 | 6.0 | 0.1 (0.2 to 0.1) | <0.001 |
| GIH | 5.3 | 5.4 | 0.2 (0.3 to 0.2) | <0.001 |
| Hip fracture | 6.7 | 6.8 | 0.1 (0.2 to 0.1) | <0.001 |
Similarly, we found that hospitals with UpToDate had lower risk‐adjusted 30‐day mortality rates, although the effects here were less consistent (Table 3). When we examined individual conditions, we found that patients admitted to UpToDate hospitals had lower risk‐adjusted 30‐day mortality rate for 4 of the 6 conditions, although only 3 of those 4 differences were statistically significant (Table 3). For example, patients in UpToDate hospitals had a lower likelihood of mortality for AMI (18.4% vs 18.9%, P = 0.03).
| Conditions | Using UpToDate (%) | Not Using UpToDate (%) | % Difference (CI) | P Value |
|---|---|---|---|---|
| ||||
| Total | 9.0 | 9.1 | 0.1 (0.2 to 0.0) | 0.04 |
| AMI | 18.4 | 19.0 | 0.7 (1.2 to 0.2) | 0.03 |
| CHF | 11.1 | 11.3 | 0.2 (0.4 to 0.1) | 0.21 |
| PN | 12.1 | 12.6 | 0.5 (0.7 to 0.2) | <0.001 |
| Stroke | 19.9 | 19.9 | 0.02 (0.5 to 0.5) | 0.91 |
| GIH | 6.9 | 7.3 | 0.4 (0.7 to 0.2) | 0.001 |
| Hip fracture | 8.8 | 8.6 | 0.2 (0.2 to 0.5) | 0.41 |
We found a more consistent association between the adoption of UpToDate and quality performance. Hospitals that had UpToDate had higher performance for each of the 4 conditions examined. For example, hospitals with UpToDate had higher quality performance for AMI compared to hospitals that did not adopt UpToDate (93.2% vs 90.4%, P < 0.001; Table 4). The results for CHF, pneumonia, and surgical complication prevention were qualitatively similar, and each reached statistical significance (Table 4).
| Conditions | Using UpToDate (%) | Not Using UpToDate (%) | % Difference (CI) | P Value |
|---|---|---|---|---|
| ||||
| AMI summary score | 93.4 | 90.2 | 3.2 (2.6, 3.6) | < 0.001 |
| CHF summary score | 81.0 | 75.1 | 5.9 (5.0, 6.8) | < 0.001 |
| PN summary score | 83.7 | 83.1 | 0.6 (0.3, 0.9) | 0.003 |
| SIP summary score | 80.0 | 78.1 | 1.9 (1.0, 2.9) | 0.002 |
In analyses that test for interaction, we found that the relationship between UpToDate use and quality performance was modified by hospital size and teaching status. Specifically, much of the benefit of UpToDate seemed limited to small and medium‐sized hospitals, as well as non‐teaching hospitals. For example, among small hospitals, those with UpToDate had, on average, 3‐7 point greater performance on the HQA scores, but almost no effect was found among large hospitals. Similarly, we found that non‐teaching hospitals were likely to have better performance in each of these areas if they had UpToDate, but this relationship was not consistent among major teaching hospitals (Table 5).
| Using UpToDate (%) | Not Using UpToDate (%) | % Difference (CI) | P Value | ||
|---|---|---|---|---|---|
| |||||
| Hospital size | Small (6‐99 beds) | ||||
| AMI summary score | 90.3 | 87.9 | 2.35 (0.91, 3.78) | <0.001 | |
| CHF summary score | 75.7 | 69.6 | 6.11 (4.04, 8.18) | <0.001 | |
| PN summary score | 84.9 | 83.2 | 1.67 (0.83, 2.51) | <0.001 | |
| Medium (100‐399 beds) | |||||
| AMI summary score | 93.0 | 90.5 | 2.52 (2.04, 3.00) | <0.001 | |
| CHF summary score | 81.1 | 78.9 | 2.22 (1.31, 3.12) | <0.001 | |
| PN summary score | 84.0 | 83.2 | 0.84 (0.31, 1.36) | <0.001 | |
| Large (>399 beds) | |||||
| AMI summary score | 94.7 | 93.8 | 0.82 (0.19, 1.46) | <0.001 | |
| CHF summary score | 82.9 | 82.7 | 0.21 (1.27, 1.69) | 0.83 | |
| PN summary score | 82.4 | 82.7 | 0.34 (1.49, 0.81) | 0.39 | |
| Teaching status | Major teaching | ||||
| AMI summary score | 94.9 | 94.8 | 0.15 (0.62, 0.92) | 0.60 | |
| CHF summary score | 83.3 | 83.0 | 0.26 (1.73, 2.25) | 0.83 | |
| PN summary score | 81.7 | 81.7 | 0.00 (1.67, 1.67) | 0.95 | |
| Not major teaching | |||||
| AMI summary score | 92.7 | 90.1 | 2.59 (2.15, 3.02) | <0.001 | |
| CHF summary score | 80.1 | 75.0 | 5.04 (4.18, 5.90) | <0.001 | |
| PN summary score | 84.3 | 83.2 | 1.05 (0.64, 1.47) | <0.001 | |
In our analyses of duration of use of UpToDate, we found a consistent relationship with shorter lengths of stay (see Supporting Appendix Table 2 in the online version of this article). Each 1000 days of UpToDate use was associated with a 0.08 day shorter length of stay (P < 0.001). A similar relationship was present and statistically significant in each of the 6 conditions examined. When we examined the impact of duration of use of UpToDate on risk‐adjusted mortality rate, we found a comparable relationship: Duration was associated with a lower mortality rate overall and for 5 of the 6 conditions examined, although only significant for 3 of the conditions (see Supporting Appendix Table 2 in the online version of this article). Similarly, greater duration was associated with better quality performance for each of the 4 conditions in the HQA program (data not shown).
When we quantified the overall impact of UpToDate, we found that if non‐adopters had a similar benefit in mortality (0.1%) seen in hospitals that adopted this system, it would lead, overall, to approximately 5550 fewer deaths annually (95% confidence interval 2601 fewer deaths to 7529 fewer deaths) and the 0.1 days shorter length of stay would lead to approximately 523,000 fewer patient days (95% confidence interval 160,000 to 799,000 fewer days) out of the approximately 30 million patient‐days that occurred in 2006 among non‐UpToDate hospitals.
DISCUSSION/CONCLUSION
We found that use of a commonly used computerized clinical knowledge management system (UpToDate) was associated with consistent, although small, reductions in lengths of stay, lower risk‐adjusted mortality rates, and higher quality performance. Much of the quality performance benefit seemed to be limited to small and medium‐sized, non‐teaching hospitals, while larger teaching hospitals realized little benefit. We found a stronger relationship between duration of use and better outcomes among UpToDate hospitals. Our findings suggest that hospitals using UpToDate had modestly better care that was also somewhat more efficient.
Prior studies have demonstrated that clinical decision support tools (ie, drug‐drug alerts and electronic reminders) can improve processes of care and enhance quality.13 Computerized clinical knowledge management systems, such as UpToDate, have unique advantages over other computerized clinical decision support tools. For example, computerized clinical knowledge management systems generally do not require electronic health records and can provide guidance to clinicians over a broader spectrum of diseases and clinical scenarios. UpToDate has previously shown to help providers answer questions rapidly, which can lead to changes in decision‐making that can improve management and efficiency.1, 7, 14
Ours is the first national study, to our knowledge, that has directly examined the relationship between UpToDate and key outcome metrics. Bonis et al. have previously examined the use of UpToDate and its relationship to risk‐adjusted LOS and mortality in a limited set of hospitals using a proprietary risk‐adjustment scheme.15 They found similar results among the Thompson 100 hospitals that were clinical knowledge management system users (compared to non‐users), including modestly shorter lengths of stay and a trend towards lower mortality. Our findings build on this work, but use publicly available data, a national sample, a well‐validated risk‐adjustment approach, and a much longer time period. The consistency of the findings across the 2 studies, despite differences in the approaches, help lend confidence that the results are unlikely to be due to chance alone.
There are likely to be important considerations surrounding the costs of UpToDate systems and whether those who purchase the system are wealthier than hospitals that chose not to. The typical annual subscription costs for a 100‐bed hospital between 2006 and 2010 was $10,578, which likely represents less than 0.01% of the annual operating costs for a 100‐bed institution (and is approximately the amount Medicare reimbursed for a single case of pneumonia without complications). Whether this cost would prohibit the adoption of UpToDate for most hospitals is unclear, and it is possible that a hospital that spent nearly $10,600 a year in such a system might therefore forego other quality improvement efforts. We suspect that these effects likely vary from hospital to hospital.
The primary limitation of the study is our inability to address whether or not the associations we found between UpToDate and outcomes are causally related. This is a fundamental limitation of all nonrandomized data. Four factors should lend some confidence to the interpretation that these findings may not be due to confounding alone. First, the effects were consistent across a series of measures (mortality, efficiency, and processes) that are not, themselves, highly correlated with each other1618. Our findings, that hospitals with UpToDate were somewhat better across all measures examined, point to the potential benefit of having high‐quality clinical information readily available for clinicians. Second, we found that the benefits persisted even after controlling for other hospital characteristics that were associated with adoption, including measures of hospital financial health (as measured by proportion of Medicaid patients and the DSH Index). Of course, this does not negate the possibility that other factors, such as the presence of medical libraries or a culture of quality and continuous learning, may be associated both with the use of UpToDate and with the outcomes. Third, the effects, at least for quality performance, were prominent among smaller, non‐teaching hospitals (which, one would surmise, a priori, to be most likely to benefit from UpToDate). Finally, the dose‐response relationship of duration of use, which was an analysis limited to only those hospitals with UpToDate, provides more evidence that the system itself may have some impact.
Another limitation of our work is that we used administrative data for risk‐adjustment, which has inherent challenges.1925 Given that users of UpToDate, such as teaching hospitals and larger institutions, generally have a much sicker patient population, inadequate risk‐adjustment may have lead us to underestimate the true effect. It is also possible that many of the hospitals designated as non‐users had other clinical knowledge management systems of which we were unaware. However, this would likely have made it harder to find an effect, biasing our study towards a null finding. We conducted a series of analyses and, yet, did not adjust for multiple testing. We had chosen these analyses a priori and, although the association we found may have been due to selection bias, it is unlikely that all of the associations in our analyses were due to random chance. Next, although we examined the potential impact of UpToDate, we suspect that any high‐quality clinical knowledge management system should allow clinicians to deliver higher quality, more efficient care. Finally, we are unsure whether or not the magnitude of effect we found is clinically significant. While the added advantage of having UpToDate appeared to be a reduction in mortality of only 0.1% (over all conditions), such a difference would be associated with approximately 5550 fewer deaths each year among Medicare fee‐for‐service beneficiaries. Whether such a benefit would be worth the cost of implementing systems like UpToDate needs to be further explored.
In conclusion, we found consistent association between use of a widely deployed computerized clinical knowledge management system, UpToDate, and reduced length of stay, lower risk‐adjusted mortality rates, and higher quality performance. Whether use of UpToDate led to better care is not definitive, but our findings suggest that these types of management software may play an important role as our nation endeavors to improve the quality and efficiency of the healthcare system.
- ,,,,.Answering physicians' clinical questions: obstacles and potential solutions.J Am Med Inform Assoc.2005;12(2):217–224.
- ,,, et al.Obstacles to answering doctors' questions about patient care with evidence: qualitative study.BMJ.2002;324(7339):710.
- ,.Why do residents fail to answer their clinical questions? A qualitative study of barriers to practicing evidence‐based medicine.Acad Med.2005;80(2):176–182.
- ,,.A clinical informaticist to support primary care decision making.Qual Health Care.2001;10(4):245–249.
- ,,, et al.Searching for medical information online: a survey of Canadian nephrologists.J Nephrol. Feb 23, 2011. doi: 10.5301/JN.2011.6373.
- ,,,.Answers to questions posed during daily patient care are more likely to be answered by UpToDate than PubMed.J Med Internet Res2008;10(4):e29.
- ,,, et al.The impact of evidence on physicians' inpatient treatment decisions.J Gen Intern Med.2004;19(5 pt 1):402–409.
- ,,.Can an electronic database help busy physicians answer clinical questions? [abstract].J Gen Intern Med.2002;17(suppl 1):220.
- ,.UpToDate: a comprehensive clinical database.J Fam Pract.2003;52(9):706–710.
- AHRQ Quality Indicators. Guide to Inpatient Quality Indicators: Quality of Care in Hospitals ‐ Volume, Mortality, and Utilization [Version 3.1]. Rockville, MD: AHRQ; Mar 12, 2007.
- ,,,,.Snapshot of hospital quality reporting and pay‐for‐performance under Medicare.Health Aff (Millwood).2006;25(1):149–162.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36(1):8–27.
- ,,, et al.Systematic review: impact of health information technology on quality, efficiency, and costs of medical care.Ann Intern Med.2006;144(10):742–752.
- ,,,.Answering clinical questions in the ED.Am J Emerg Med.2008;26(2):144–147.
- ,,,.Association of a clinical knowledge support system with improved patient safety, reduced complications and shorter length of stay among Medicare beneficiaries in acute care hospitals in the United States.Int J Med Informatics.2008;77(11):745–753.
- ,,,,.Measuring efficiency: the association of hospital costs and quality of care.Health Aff (Millwood).2009;28(3):897–906.
- ,,, et al.Hospital quality for acute myocardial infarction: correlates among process measures and relationship to short‐term mortality.JAMA.2006;295.
- ,,,.Hospital quality and intensity of spending: is there an association?Health Aff (Millwood).2009;28(4):w566–w572.
- ,,,,,.Discordance of databases designed for claims payment versus clinical information systems. Implications for outcomes research.Ann Intern Med.1993;119(8):844–850.
- ,,,.Accuracy of Medicare claims data for rheumatologic diagnoses in total hip replacement recipients.J Clin Epidemiol.2003;56(6):515–519.
- ,,,.The sensitivity of Medicare billing claims data for monitoring mammography use by elderly women.Med Care Res Rev.2004;61(1):116–127.
- ,,,,,.A comparison of ambulatory Medicaid claims to medical records: a reliability assessment.Am J Med Qual.1998;13(2):63–69.
- ,,,.Methodological issues in the use of administrative claims data to study surveillance after cancer treatment.Med Care.2002;40(8 suppl):IV‐69–74.
- ,,,,,.Using claims data for epidemiologic research. The concordance of claims‐based criteria with the medical record and patient survey for identifying a hypertensive population.Med Care.1993;31(6):498–507.
- .The Potential of Claims Data to Support the Measurement of Healthcare Quality.Santa Monica, CA:Rand Corporation;2003.
- ,,,,.Answering physicians' clinical questions: obstacles and potential solutions.J Am Med Inform Assoc.2005;12(2):217–224.
- ,,, et al.Obstacles to answering doctors' questions about patient care with evidence: qualitative study.BMJ.2002;324(7339):710.
- ,.Why do residents fail to answer their clinical questions? A qualitative study of barriers to practicing evidence‐based medicine.Acad Med.2005;80(2):176–182.
- ,,.A clinical informaticist to support primary care decision making.Qual Health Care.2001;10(4):245–249.
- ,,, et al.Searching for medical information online: a survey of Canadian nephrologists.J Nephrol. Feb 23, 2011. doi: 10.5301/JN.2011.6373.
- ,,,.Answers to questions posed during daily patient care are more likely to be answered by UpToDate than PubMed.J Med Internet Res2008;10(4):e29.
- ,,, et al.The impact of evidence on physicians' inpatient treatment decisions.J Gen Intern Med.2004;19(5 pt 1):402–409.
- ,,.Can an electronic database help busy physicians answer clinical questions? [abstract].J Gen Intern Med.2002;17(suppl 1):220.
- ,.UpToDate: a comprehensive clinical database.J Fam Pract.2003;52(9):706–710.
- AHRQ Quality Indicators. Guide to Inpatient Quality Indicators: Quality of Care in Hospitals ‐ Volume, Mortality, and Utilization [Version 3.1]. Rockville, MD: AHRQ; Mar 12, 2007.
- ,,,,.Snapshot of hospital quality reporting and pay‐for‐performance under Medicare.Health Aff (Millwood).2006;25(1):149–162.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36(1):8–27.
- ,,, et al.Systematic review: impact of health information technology on quality, efficiency, and costs of medical care.Ann Intern Med.2006;144(10):742–752.
- ,,,.Answering clinical questions in the ED.Am J Emerg Med.2008;26(2):144–147.
- ,,,.Association of a clinical knowledge support system with improved patient safety, reduced complications and shorter length of stay among Medicare beneficiaries in acute care hospitals in the United States.Int J Med Informatics.2008;77(11):745–753.
- ,,,,.Measuring efficiency: the association of hospital costs and quality of care.Health Aff (Millwood).2009;28(3):897–906.
- ,,, et al.Hospital quality for acute myocardial infarction: correlates among process measures and relationship to short‐term mortality.JAMA.2006;295.
- ,,,.Hospital quality and intensity of spending: is there an association?Health Aff (Millwood).2009;28(4):w566–w572.
- ,,,,,.Discordance of databases designed for claims payment versus clinical information systems. Implications for outcomes research.Ann Intern Med.1993;119(8):844–850.
- ,,,.Accuracy of Medicare claims data for rheumatologic diagnoses in total hip replacement recipients.J Clin Epidemiol.2003;56(6):515–519.
- ,,,.The sensitivity of Medicare billing claims data for monitoring mammography use by elderly women.Med Care Res Rev.2004;61(1):116–127.
- ,,,,,.A comparison of ambulatory Medicaid claims to medical records: a reliability assessment.Am J Med Qual.1998;13(2):63–69.
- ,,,.Methodological issues in the use of administrative claims data to study surveillance after cancer treatment.Med Care.2002;40(8 suppl):IV‐69–74.
- ,,,,,.Using claims data for epidemiologic research. The concordance of claims‐based criteria with the medical record and patient survey for identifying a hypertensive population.Med Care.1993;31(6):498–507.
- .The Potential of Claims Data to Support the Measurement of Healthcare Quality.Santa Monica, CA:Rand Corporation;2003.
Copyright © 2011 Society of Hospital Medicine
Doctors Advised to Test Cholesterol in All Children Aged 9-11 Years
Perhaps the best news about the cholesterol testing now recommended for all children aged 9-11 years by an expert panel convened by the National Heart, Lung, and Blood Institute is that children don’t have to fast before getting their blood drawn. The guidelines were published online on Nov. 13 and appear in the December issue of Pediatrics.
Dr. Stephen R. Daniels, chair of the expert panel that reviewed the guidelines, emphasized that the new approach to cholesterol screening can be accomplished with a blood test that does not require fasting, so it should be relatively easy to include in a busy practice. This strategy "ensures that children with elevated LDL (or bad) cholesterol will be identified."
Data from studies of the previous cholesterol-screening approach suggest that children with high cholesterol have often been missed, said Dr. Daniels, pediatrician-in-chief at the University of Colorado at Denver, Aurora.
Data from previous studies have shown that atherosclerosis begins in youth, and that heart attacks, strokes, and other cardiovascular problems in adulthood are often the end result of cardiovascular risk factors that went unrecognized throughout childhood, according to the report (Pediatrics 2011 Nov. 13 [doi:10.1542/peds.2009-2107C]).
The current guidelines represent the latest update since the 1990s, said Dr. Daniels.
"These guidelines are different in that they are based on a comprehensive and systematic review of the literature, they are integrated across all risk factors (hypertension, dyslipidemia, obesity, diabetes, and cigarette smoking) and lifestyle factors (diet and physical activity), and they address issues across the pediatric age range," he said in an interview.
Data from studies of the previous cholesterol-screening approach suggest that children with high cholesterol have often been missed, said Dr. Stephen R. Daniels.
The "Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents Summary Report" provides details for how to reduce risk factors and help prevent cardiovascular problems in children from birth to 21 years of age, starting with a recommendation for exclusive breastfeeding of children for the first 6 months of life.
However, the most notable new element in the guidelines is universal cholesterol-screening recommendation for preadolescents.
According to the guidelines, doctors should obtain a universal lipid screen with nonfasting non-HDL cholesterol (that is, total cholesterol minus HDL cholesterol) or a fasting lipid profile (FLP) for all children at least once between the ages of 9 and 11 years, and "manage per lipid algorithms as needed." Diet and exercise are recommended as first-line treatment, but statins may be considered in children whose high cholesterol persists despite diet and lifestyle interventions.
The guidelines recommend obtaining an FLP at age 12-17 years if a child’s family history is newly positive, if a parent has dyslipidemia, or if the child has any other risk factors or high-risk conditions, and then managing per lipid algorithms as needed.
For all patients aged 18-21 years, the guidelines recommend measuring one nonfasting non-HDL or FLP, and then reviewing the results with patients and managing them with lipid algorithms per Adult Treatment Panel III as needed.
Preventive steps to reduce risk and prevent cardiovascular disease in all ages include regular physical activity, with vigorous activity 3 days a week, according to the "Physical Activity Guidelines Advisory Committee Report 2008" from the Department of Health and Human Services.
Other preventive measures include a diet low in saturated fat for all children starting at 1 year of age, as well as both practice- and school-based interventions to keep children from smoking and to help them quit.
The guidelines also recommend annual blood pressure measurement for all children starting at 3 years of age, and interpreted for age, sex, and height. The report has a chart with an algorithm and flow diagram to assist clinicians in diagnosing hypertension in children.
"The rationale for these guidelines is that there is more and more evidence for the concept that atherosclerosis begins in childhood and depends on the same risk factors we are concerned about in adults," Dr. Daniels said. "This means that we should try to prevent these risk factors from developing in the first place (primordial prevention) and identify children at higher risk, so we can work on improving their lifestyle," he added.
"Cardiovascular disease is the most common cause of death for both men and women. So, this places great importance on these issues for primary care pediatricians. The new approach to screening may actually be easier to implement than the old strategy, which requires constant updating of the family history," noted Dr. Daniels, who is also chairman and professor of pediatrics at the university.
"This test should be done once for every child in the 9- to 11-year age range. The universal approach to screening will identify children with a genetic cause for their high cholesterol (1 in 500 children) and children with cholesterol abnormalities based more on lifestyle," said Dr. Daniels. "Both groups will benefit from lifestyle intervention, which can be useful in lowering their lifetime risk of cardiovascular disease."
Dr. Daniels has served as a consultant for Abbott Laboratories, Merck, and Schering-Plough, and has received funding/grant support for research from the National Institutes of Health. Other members of the committee that reviewed the guidelines disclosed research support from various agencies and pharmaceutical companies.
Perhaps the best news about the cholesterol testing now recommended for all children aged 9-11 years by an expert panel convened by the National Heart, Lung, and Blood Institute is that children don’t have to fast before getting their blood drawn. The guidelines were published online on Nov. 13 and appear in the December issue of Pediatrics.
Dr. Stephen R. Daniels, chair of the expert panel that reviewed the guidelines, emphasized that the new approach to cholesterol screening can be accomplished with a blood test that does not require fasting, so it should be relatively easy to include in a busy practice. This strategy "ensures that children with elevated LDL (or bad) cholesterol will be identified."
Data from studies of the previous cholesterol-screening approach suggest that children with high cholesterol have often been missed, said Dr. Daniels, pediatrician-in-chief at the University of Colorado at Denver, Aurora.
Data from previous studies have shown that atherosclerosis begins in youth, and that heart attacks, strokes, and other cardiovascular problems in adulthood are often the end result of cardiovascular risk factors that went unrecognized throughout childhood, according to the report (Pediatrics 2011 Nov. 13 [doi:10.1542/peds.2009-2107C]).
The current guidelines represent the latest update since the 1990s, said Dr. Daniels.
"These guidelines are different in that they are based on a comprehensive and systematic review of the literature, they are integrated across all risk factors (hypertension, dyslipidemia, obesity, diabetes, and cigarette smoking) and lifestyle factors (diet and physical activity), and they address issues across the pediatric age range," he said in an interview.
Data from studies of the previous cholesterol-screening approach suggest that children with high cholesterol have often been missed, said Dr. Stephen R. Daniels.
The "Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents Summary Report" provides details for how to reduce risk factors and help prevent cardiovascular problems in children from birth to 21 years of age, starting with a recommendation for exclusive breastfeeding of children for the first 6 months of life.
However, the most notable new element in the guidelines is universal cholesterol-screening recommendation for preadolescents.
According to the guidelines, doctors should obtain a universal lipid screen with nonfasting non-HDL cholesterol (that is, total cholesterol minus HDL cholesterol) or a fasting lipid profile (FLP) for all children at least once between the ages of 9 and 11 years, and "manage per lipid algorithms as needed." Diet and exercise are recommended as first-line treatment, but statins may be considered in children whose high cholesterol persists despite diet and lifestyle interventions.
The guidelines recommend obtaining an FLP at age 12-17 years if a child’s family history is newly positive, if a parent has dyslipidemia, or if the child has any other risk factors or high-risk conditions, and then managing per lipid algorithms as needed.
For all patients aged 18-21 years, the guidelines recommend measuring one nonfasting non-HDL or FLP, and then reviewing the results with patients and managing them with lipid algorithms per Adult Treatment Panel III as needed.
Preventive steps to reduce risk and prevent cardiovascular disease in all ages include regular physical activity, with vigorous activity 3 days a week, according to the "Physical Activity Guidelines Advisory Committee Report 2008" from the Department of Health and Human Services.
Other preventive measures include a diet low in saturated fat for all children starting at 1 year of age, as well as both practice- and school-based interventions to keep children from smoking and to help them quit.
The guidelines also recommend annual blood pressure measurement for all children starting at 3 years of age, and interpreted for age, sex, and height. The report has a chart with an algorithm and flow diagram to assist clinicians in diagnosing hypertension in children.
"The rationale for these guidelines is that there is more and more evidence for the concept that atherosclerosis begins in childhood and depends on the same risk factors we are concerned about in adults," Dr. Daniels said. "This means that we should try to prevent these risk factors from developing in the first place (primordial prevention) and identify children at higher risk, so we can work on improving their lifestyle," he added.
"Cardiovascular disease is the most common cause of death for both men and women. So, this places great importance on these issues for primary care pediatricians. The new approach to screening may actually be easier to implement than the old strategy, which requires constant updating of the family history," noted Dr. Daniels, who is also chairman and professor of pediatrics at the university.
"This test should be done once for every child in the 9- to 11-year age range. The universal approach to screening will identify children with a genetic cause for their high cholesterol (1 in 500 children) and children with cholesterol abnormalities based more on lifestyle," said Dr. Daniels. "Both groups will benefit from lifestyle intervention, which can be useful in lowering their lifetime risk of cardiovascular disease."
Dr. Daniels has served as a consultant for Abbott Laboratories, Merck, and Schering-Plough, and has received funding/grant support for research from the National Institutes of Health. Other members of the committee that reviewed the guidelines disclosed research support from various agencies and pharmaceutical companies.
Perhaps the best news about the cholesterol testing now recommended for all children aged 9-11 years by an expert panel convened by the National Heart, Lung, and Blood Institute is that children don’t have to fast before getting their blood drawn. The guidelines were published online on Nov. 13 and appear in the December issue of Pediatrics.
Dr. Stephen R. Daniels, chair of the expert panel that reviewed the guidelines, emphasized that the new approach to cholesterol screening can be accomplished with a blood test that does not require fasting, so it should be relatively easy to include in a busy practice. This strategy "ensures that children with elevated LDL (or bad) cholesterol will be identified."
Data from studies of the previous cholesterol-screening approach suggest that children with high cholesterol have often been missed, said Dr. Daniels, pediatrician-in-chief at the University of Colorado at Denver, Aurora.
Data from previous studies have shown that atherosclerosis begins in youth, and that heart attacks, strokes, and other cardiovascular problems in adulthood are often the end result of cardiovascular risk factors that went unrecognized throughout childhood, according to the report (Pediatrics 2011 Nov. 13 [doi:10.1542/peds.2009-2107C]).
The current guidelines represent the latest update since the 1990s, said Dr. Daniels.
"These guidelines are different in that they are based on a comprehensive and systematic review of the literature, they are integrated across all risk factors (hypertension, dyslipidemia, obesity, diabetes, and cigarette smoking) and lifestyle factors (diet and physical activity), and they address issues across the pediatric age range," he said in an interview.
Data from studies of the previous cholesterol-screening approach suggest that children with high cholesterol have often been missed, said Dr. Stephen R. Daniels.
The "Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents Summary Report" provides details for how to reduce risk factors and help prevent cardiovascular problems in children from birth to 21 years of age, starting with a recommendation for exclusive breastfeeding of children for the first 6 months of life.
However, the most notable new element in the guidelines is universal cholesterol-screening recommendation for preadolescents.
According to the guidelines, doctors should obtain a universal lipid screen with nonfasting non-HDL cholesterol (that is, total cholesterol minus HDL cholesterol) or a fasting lipid profile (FLP) for all children at least once between the ages of 9 and 11 years, and "manage per lipid algorithms as needed." Diet and exercise are recommended as first-line treatment, but statins may be considered in children whose high cholesterol persists despite diet and lifestyle interventions.
The guidelines recommend obtaining an FLP at age 12-17 years if a child’s family history is newly positive, if a parent has dyslipidemia, or if the child has any other risk factors or high-risk conditions, and then managing per lipid algorithms as needed.
For all patients aged 18-21 years, the guidelines recommend measuring one nonfasting non-HDL or FLP, and then reviewing the results with patients and managing them with lipid algorithms per Adult Treatment Panel III as needed.
Preventive steps to reduce risk and prevent cardiovascular disease in all ages include regular physical activity, with vigorous activity 3 days a week, according to the "Physical Activity Guidelines Advisory Committee Report 2008" from the Department of Health and Human Services.
Other preventive measures include a diet low in saturated fat for all children starting at 1 year of age, as well as both practice- and school-based interventions to keep children from smoking and to help them quit.
The guidelines also recommend annual blood pressure measurement for all children starting at 3 years of age, and interpreted for age, sex, and height. The report has a chart with an algorithm and flow diagram to assist clinicians in diagnosing hypertension in children.
"The rationale for these guidelines is that there is more and more evidence for the concept that atherosclerosis begins in childhood and depends on the same risk factors we are concerned about in adults," Dr. Daniels said. "This means that we should try to prevent these risk factors from developing in the first place (primordial prevention) and identify children at higher risk, so we can work on improving their lifestyle," he added.
"Cardiovascular disease is the most common cause of death for both men and women. So, this places great importance on these issues for primary care pediatricians. The new approach to screening may actually be easier to implement than the old strategy, which requires constant updating of the family history," noted Dr. Daniels, who is also chairman and professor of pediatrics at the university.
"This test should be done once for every child in the 9- to 11-year age range. The universal approach to screening will identify children with a genetic cause for their high cholesterol (1 in 500 children) and children with cholesterol abnormalities based more on lifestyle," said Dr. Daniels. "Both groups will benefit from lifestyle intervention, which can be useful in lowering their lifetime risk of cardiovascular disease."
Dr. Daniels has served as a consultant for Abbott Laboratories, Merck, and Schering-Plough, and has received funding/grant support for research from the National Institutes of Health. Other members of the committee that reviewed the guidelines disclosed research support from various agencies and pharmaceutical companies.
FROM PEDIATRICS
Residents Improving Quality
To Err Is Human revealed the underappreciated tension between the enormous benefits of medical care and the potential for harm.1 Following this report, there has been an explosion of research and commentary detailing quality improvement (QI) opportunities. One area of growing emphasis has been resident physician training.2, 3 If medical care is dangerous, then a substantial contributor to the hazard must be the apprentice‐style process of physician training and the novice skill set of the trainees.4, 5 Many resident training programs have devised efforts to decrease the errors committed by physicians‐in‐training,6 change the culture of residency training,7 engage residents in quality improvement,8, 9 and improve resident training in quality improvement.10
Many of the programs devised to teach QI in the residency setting require substantial funding, a large pool of QI experts, or redesign of resident training programs.410 While effective, these programs are not feasible for many resource‐constrained residency programs. A less intense program, using resident‐led, hospitalist‐facilitated, limited root cause analysis (RCA), has been adopted at the Internal Medicine Residency Program at the Mount Sinai Hospital (MSH). We describe our 2‐year experience using this technique, including cases discussed, improvement strategies suggested, projects implemented, and resident perceptions.
METHODS
Setting
Departmental QI leaders developed this initiative in the Internal Medicine Residency Program at the MSH in New York City, New York. This residency program trains over 140 residents annually in categorical, preliminary, and research track positions, as well as an affiliated medicine/pediatrics program. The program's residents rotate at 3 clinic sites: a tertiary care hospital, a public safety‐net hospital, and a Veterans Affairs hospital. The QI program was only implemented at the MSH. Over 90% of the program's graduates go on to complete a subspecialty fellowship.
Intervention Description
The QI program was designed around a noon‐time quality improvement conference (QIC) occurring once every 4 weeks. In the weeks prior to the session, chief residents and a hospitalist mentor selected a case related to an inpatient care issue. Potential cases were solicited, and/or offered, from a range of sources including attending physicians, nurse managers, residents, and quality officers. Only cases from the teaching services were chosen. To ensure that participants on the case were able to recall relevant details, preference was given to more recent cases. A third‐year resident on an elective or outpatient block was chosen to investigate the case. To maximize the objectivity of the investigation, every effort was made to select a resident who was not involved in the care of the patient.
The resident was instructed to complete a limited RCA (fewer fact‐finding interviews and only 1 group meeting) and was directed to online resources.11 Each resident presenter worked closely with the chief residents and hospitalist mentor to identify appropriate strategies for collecting data and interviewing involved parties. If necessary, either due to volume of work or sensitivity of the case, the chief resident or hospitalist would assist with the data gathering. The resident contacted multiple parties involved in the patient care issue including, nurses, residents, attendings, pharmacists, social workers, and, if appropriate, the patient. The resident constructed a timeline for each case, and identified specific points in the patient care experience, where errors, near misses, or misunderstandings occurred. During the QIC, these findings were presented to Internal Medicine residents, chief residents, representatives from the Chief Medical Officer's office, attending physicians overseeing the residents on inpatient rotations, and representatives from any group (social work nursing, housekeeping, pharmacy, etc) that may have impacted patient care for the particular case being investigated. On average, 50 healthcare providers attended the QIC. Lunch was provided.
After the findings were presented, a chief resident and a lead hospitalist facilitated a group discussion on the circumstances surrounding the case. Discussions were focused on identifying system‐wide failures and proposing systems‐based solutions. Great efforts were made to remind all participants to refrain from individual blame. At the end of each QIC, participants summarized and prioritized suggestions to reduce the discussed error. Interested residents were invited to form improvement committees for cases with viable solutions. Each committee attempted to implement improvements discussed during the QIC. Committees, led by a representative from the Division of Hospital Medicine, included 2 to 4 residents as well as healthcare workers from other disciplines if appropriate. For all improvement efforts, the focus was on the interventions which appeared high yield with low cost.
Intervention Evaluation
The program was exempt from Institutional Review Board review as a part of the Department of Medicine's quality improvement and assurance portfolio.
The results of the QICs were tracked. After each case, a QI team consisting of chief residents and representatives from the Division of Hospital Medicine recorded the cases presented, and interventions suggested for each case, in an online database. After implementation, the success of each intervention was recorded. To evaluate the types of interventions suggested by residents, the 3 physician‐authors, who regularly attend these conferences and have a focused career interest in QI, grouped all suggestions into 4 broad categories: Educational, Reminder Systems, Design Changes (protocol‐based), and Design Changes (Information Technology [IT]‐based). Design change interventions (IT‐based) consisted of an adjustment to electronic systems, such as displaying specific lab results on a medication ordering system. Design changes (protocol‐based) consisted of changes made to standing protocols such as nursing protocols for reporting abnormal lab values. Reminder system interventions were endeavors such as a checklist for discharge planning. Educational interventions focused on providing additional training sessions or conferences.
The 3 physician‐authors independently reviewed each suggested intervention to determine its success. They first evaluated whether the change was attempted or not. For all attempted interventions, the reviewer then assessed if there was either objective permanent system‐wide change, subjective behavior change, or no change. To meet the objective change threshold, the intervention either had to have permanently changed provider workflow or have data demonstrating behavior change or improved outcome. Interventions with anecdotal evidence that behavior was improved or modified, but lacking systematic data, were qualified as subjective behavior change. For each assessment, 2 of the 3 reviewers needed to agree for an intervention to be recorded as a success.
Resident views on the monthly conferences were solicited via an anonymous and voluntary questionnaire. A first survey was designed to assess whether residents felt that the conferences provided them with the ability to recognize and improve systems errors which compromise patient care. This survey was administered at the conclusion of the first year of the program to residents who attended the final 2 QICs. A second survey assessed whether the tone of the conferences was constructive and blame‐free. This survey was administered at the conclusion of the second year of the program to residents who attended the year's final 2 QICs.
RESULTS
Over the first 22 months of the program, 20 conferences were held (Table 1). The topics covered ranged considerably and included: deficits in supervision, medication errors, patient satisfaction, staff safety, and 30‐day readmissions. Forty‐six distinct interventions were suggested during these conferences. Of those, an attempt was made to initiate 25 (54%) of these suggestions (Table 2). Of the 25 interventions that were initiated, 18 (72%) were determined to be successful. Eight resulted in objective permanent system‐wide change and 10 resulted in subjective behavior change among residents.
| QIC Topic | Interventions Suggested by Residents | Suggestion Results (Attempted/Not Attempted, Successful/Unsuccessful) |
|---|---|---|
| ||
| Central venous catheter guide wire lost during code placement | Improved supervision and training for line placement | Attempted, but unsuccessful |
| Avoid unnecessary line placement during codes | Attempted, but unsuccessful | |
| Inappropriate administration of warfarin | Decision support providing real‐time coagulation profile | Attempted and successful |
| Central line bloodstream infection | Clarified and encouraged use of line service | Attempted and successful |
| Daily documentation of catheter placement date | Not attempted | |
| Delayed administration of pain medication | Training nurses to use text paging communication system | Attempted and successful |
| Patient discharged on wrong medication dose | Do not use abbreviations | Not attempted |
| Electronic medication reconciliation | Attempted and successful | |
| Confusion over code status | Clarification of various forms used for DNR | Not attempted |
| Better communication of code status during signout | Not attempted | |
| Patient received hydromorphone IV instead of PO during verbal order at end‐of‐life | Verbal orders should have talk back verification | Attempted, but unsuccessful |
| Encourage informing patients of medical errors | Attempted, but unsuccessful | |
| Premature closure of diagnosis during transfer from MICU | Improve comfort level disagreeing with supervisors | Attempted, but unsuccessful |
| Reassessment of patient prior to late‐day MICU transfers | Not attempted | |
| Patient erroneously received clopidogrel bisulfate (Plavix) for years due to poor medication reconciliation | Improved discharge summary interface | Attempted and successful |
| Encourage physicians to call PCP on discharge | Attempted and successful | |
| Modified barium swallow ordered incorrectly, resulting in patient aspiration | Simplify electronic order entry system to clearly identify tests | Not attempted |
| Change radiology requisition form to facilitate communication | Not attempted | |
| Fingersticks leading to blood exposure | Train PGY1s on the needles used at all 3 hospitals | Not attempted |
| Improve mask with face shields and gown availability | Attempted and successful | |
| Patient discharged with central venous catheter still in place | Check list for lines and Foleys | Not attempted |
| Improved discharge documentation | Not attempted | |
| 30‐Day readmission | Mandatory discharge summary completion prior to discharge | Attempted and successful |
| Discharge summary training during intern year | Attempted and successful | |
| DKA developed in house when insulin not administered | Improve communication between floor and dialysis RNs | Not attempted |
| Better PA supervision by residents regarding order writing | Attempted and successful | |
| Compromised patient satisfaction | Patient handouts with name and role of each care team member | Attempted, but unsuccessful |
| Patient satisfaction coaching | Attempted and successful | |
| Elevated PTT and poor documentation | Improved feedback to residents regarding daily notes | Not attempted |
| Nurses must call physicians with alert values | Not attempted | |
| Hospital‐acquired MRSA | Improve availability of contact precaution gowns | Attempted, but unsuccessful |
| Direct observation of hand washing on morning rounds | Attempted and successful | |
| Staff safety with deranged family member | Education of staff regarding safety protocols | Attempted, but unsuccessful |
| Transfer of unstable patient from outside hospital ICU to general medicine floor | Standardization of OSH transfer guidelines | Not attempted |
| Improved documentation of transferring MD contract data | Attempted and successful | |
| Consult called, patient not seen by attending | Education of faculty on existing institutional consult policy | Attempted, but unsuccessful |
| Clarification of violations reporting process for hospital consults | Attempted, but unsuccessful | |
| Type of Intervention | No. of Interventions Suggested | No. of Interventions Implemented (%) | Of Implemented Interventions, No. Which Were Successful (%) | No. of Attempted Interventions With Objective Change (%) | No. of Attempted Interventions With Subjective Change (%) |
|---|---|---|---|---|---|
| Design changes: information technology‐based | 5 | 2 (40) | 2 (100%) | 2 (100) | 0 (0) |
| Design changes: protocol‐based | 17 | 10 (59) | 8 (80%) | 5 (50) | 3 (30) |
| Educational | 20 | 11 (55) | 7 (64) | 1 (9) | 6 (55) |
| Reminder systems | 4 | 2 (50) | 1 (50) | 0 (0) | 1 (50) |
| Total | 46 | 25 (54) | 18 (72) | 8 (32) | 10 (40) |
Two IT‐based system design changes were implemented; both resulted in objective system‐wide change. Eight protocol‐based design changes were implemented successfully, 5 objectively, and 3 subjectively. Seven educational interventions and 1 reminder system intervention were initiated.
The most successful intervention to come from these conferences was the implementation of an electronic medication reconciliation program. The reconciliation program was suggested following a conference on a patient who was discharged home on the wrong dose of a medication. The institution's paper‐based medication reconciliation process, particularly for heart‐failure patients, had long been known to be deficient. The QIC brought this issue to life by highlighting a cases that may have been ameliorated with a more robust medication reconciliation process. Enthusiastic residents were invited to build a case for medication reconciliation to the Chief Medical Officer, and this helped garner resources for the hospital‐wide project. Another successful IT‐based intervention was initiated after a case of inappropriate administration of warfarin to a patient with an already elevated international normalized ratio (INR). The computerized order entry system was changed so that, at the point of ordering warfarin, the most recent coagulation profile and platelet values appear before an order can be finalized.
An example of a protocol‐based intervention came from a conference that focused on poor communication at the time of discharge, which resulted in a 30‐day readmission. As a result, resident work flow was changed so that discharge summaries are expected to be completed at the time of discharge. Along with this protocol change was an educational initiative to improve the quality of discharge summaries by including essential data for the transition of care.
Overall, residents reviewed the conferences very positively (Table 3). The response rate for the first year survey was 40% (56/140) and the second year survey was 18% (26/143). The vast majority of participants felt that the conferences were of high quality (96%) and that the exercise could lead to improvement in quality (98%). Residents felt that the conference focused more on system issues than individual shortcomings (92%). A majority felt comfortable expressing their opinions during the conferences (77%).
| Overall Conference Quality | ||
|---|---|---|
| Question | Mean Score (n = 53) | Rating Question a 4 or 5 |
| Conference Tone | ||
| Question | Mean Score (n = 26) | Rating Question With a 4 or 5 |
| ||
| Please rate the overall quality of the QIC conferences. | 4.49* | 98% |
| The case highlighted an issue that is highly relevant to the quality of patient care. | 4.81 | 100% |
| Solutions discussed at this conference could lead to improved patient care and/or patient satisfaction. | 4.65 | 96% |
| My knowledge of issues related to hospital quality and patient safety has been enhanced by this conference. | 4.61 | 96% |
| The QIC focused on individuals, individual actions, or omissions, which compromised high quality care. | 3.35 | 50% |
| The QIC focused on system failures that compromised high quality care. | 4.35 | 92% |
| I felt comfortable sharing my honest opinions about the medical events presented during the conferences. | 4.15 | 77% |
| I avoided expressing my opinions about the medical events presented during the conferences because I did not want to criticize my peers. | 2.5 | 19% |
DISCUSSION
The first 20 sessions from this resident‐led, hospitalist‐facilitated QI program provided evidence that residents can contribute to patient safety within a large tertiary care center. The role of residents in actively addressing errors and unsatisfactory outcomes in the hospital has not been a traditional QI focus.12 Involvement has typically been a passive process for physician trainees, while more senior clinical staff members decide on and prioritize QI activities. We have observed that empowering residents to take a more active role in performance improvement yields significant change and does more than simply educate about basic QI methodology.
One reason for the success of these conferences was leveraging insights of residents as key front line providers. Residents spend more time than perhaps any other category of hospital employee working within clinical care systems. They are deeply aware of the quality struggles inherent to large healthcare organizations, and this insight can lead to high impact suggestions for improvement. Often, suggestions were simple proposals that were overlooked or unappreciated by other administrative leaders. An example of this type of contribution was when residents brought the lack of infection control equipment, on certain units, to the attention of the infection control staff and facility engineers. At a separate conference, residents informed the transfer office staff that valuable contact information for physicians accepting outside hospital transfers was not being collected. Both of these observations led to quick change, with better infection control gown availability and improved documentation by transfer office staff.
Our program also demonstrated that including residents in QI provides momentum for either a training program or an institution to pursue solutions that might have otherwise been resisted. The improvement suggestion to complete discharge summaries prior to the patient leaving the hospital had long been a goal for the residency program leadership, but there was hesitation to force this work flow change on the residents. After a QI conference, when a number of the residents themselves made the suggestion, implementing the change was much easier. Similarly, after several cases of clear errors relating to a suboptimal process of medication reconciliation, the institution dedicated scarce IT personnel to work with providers to develop a robust, user‐friendly medication reconciliation application to decrease transition of care errors.
Through this program, residents also demonstrated their ability to deconstruct patient care problems. For each case, resident session leaders interviewed physician providers, physician extenders, nurses, nurse managers, pharmacists, security staff, engineering staff, and administrative staff. They gathered crucial information regarding the patient care event and the gaps or errors that led to a poor outcome. After many of the conferences, the resident presenters commented on how the investigative exercise left them more appreciative of the complexity of the medical system and interested in fixing the problems uncovered.
The feedback from the resident surveys demonstrated that residents valued the QI program. The data collected also shows that such programs can be executed in a manner which highlights system flaws. Our data do, however, suggest that there is room to improve the tone of the conference to further decrease the sense from residents that quality discussions focus on individuals. Residents often struggle to master the myriad new expectations inherent in the transition from student to physician.13 A quality process which discourages already overworked and uncertain trainees, by creating a process which assigns blame for unintentional quality shortcomings, would be counterproductive.
Lessons Learned
While this QI program has had success uncovering clinical care issues, and creating a climate and process for resident participation in improvement, there has been a number of limitations and lessons learned. Most importantly, including busy residents in any process that requires regular participation and follow‐through is difficult. A number of suggested improvements which created substantial interest and early momentum were ultimately left unfinished, as residents and even faculty facilitators became overwhelmed by clinical responsibilities. In fact, the majority of suggestions have not been successfully implemented and even fewer have created lasting change. This must be carefully monitored, as experiencing multiple failures can undermine the empowerment that such QI programs are created to foster.
Regular reflection on the successful and unsuccessful projects yielded several important insights that resulted in changes over the course of the program. Suggestions were more likely to move from idea generation to execution if the QIC was attended by administrators with decision‐making authority. Several of the suggestionsimproved medication reconciliation, better transfer documentation, and improved availability of infection control productswere able to be acted upon because conference attendees were administrators with purview over these issues. Many times, these leaders were more than willing to implement helpful suggestions, but simply needed them to be brought to their attention. As a result, we have been more attentive to inviting as many stakeholders as possible to the QICs.
It was also clear that suggestions would not be realized without a physician leader and were more successful when resident interest was substantial. After each QIC, residents who had made promising suggestions were approached to continue to participate. If the residents agreed, the projects were pursued and a faculty or chief resident leader was assigned. Lastly, we have also made use of one of the department's QI data analysts to assist with project completion. This individual has been made available to provide administrative support (organizing meetings, paperwork, etc) but also to provide data for projects, should the need arise.
Another important finding is that the tone of the QI program must be constantly monitored. Despite reminding residents at each session that the exercise was for the purpose of identifying systems barriers to delivering high quality care, there were times when residents felt targeted or blamed. At one point, a number of residents voiced their concerns that the conferences had spent too much time highlighting quality failures without recognizing the many positive performances on the teaching service. As a result, subsequent conferences often began by highlighting quality improvements made. Additionally, a part of 1 session each year had been dedicated to reading letters and e‐mails sent by patients or families which highlight memorably positive performances by the residents. Finally, care was taken to make sure invited guests to the sessions were reminded of the session's blame‐free ground rules.
Care must be taken when investigating clinical cases. On several occasions, attending physicians expressed discomfort with having residents scrutinize a clinical event. Although this process was protected under the QI umbrella and faculty names were never shared at the conferences, some faculty believed that this process was the purview of departmental or hospital QI staff, not untrained residents. Given the support provided for this program by the department chair and program director, as well as the professional nature with which the residents conducted their inquiries, there was little difficulty rejecting this line of objection. This feedback did lead supervisors to be more involved with the resident presenters, coaching them regarding data gathering and interviewing. If a case appears that it will be particularly sensitive, the hospitalist mentor or chief resident will reach out to involved residents and faculty to notify them that the case will be reviewed.
A final development secured, in part, as a result of this quality program has been more protected faculty time. At the start of this program, all faculty time was donated time on top of other administrative and patient care responsibilities. After the first 18 months of the QIC program, the residency program named an assistant program director for quality. At the time of writing this manuscript, the program further invested in quality by naming both an assistant and associate program director for quality. These positions combined amount to at least 0.4 full‐time equivalents (FTE). Of that, roughly 0.1 FTE is spent working on the QICs and subsequent project implementation.
Limitations
The evaluation of the success of the interventions potentially biased our findings. The qualitative method of using multiple reviewers, all of whom were invested in the program's outcomes, to gauge the success of initiated interventions may have resulted in an overestimate of the project's effectiveness. Furthermore, the category of subjective change lacks measurable criteria, making replication of the findings difficult.
The results presented here are from a single institution, conceived of and executed by a group of dedicated faculty. Moreover, both the chair of the department and the program director were very supportive of this endeavor. Possibly, because of these aspects, the findings presented here would not be readily replicated at another institution.
The percentage of residents who completed the feedback surveys was low. This may result in an overestimate of quality, value, and tone of the conferences, as well as potentially missing an opportunity for improving the program. We will address this issue through more rigorous quantitative and qualitative feedback at the end of the third year of the program.
CONCLUSIONS
Residents are willing and effective participants in a QI program. As front line providers, their experiences are valuable and their willingness to share insights can be an impetus for change. Finally, a process which includes modest investigation by third year residents, has faculty support and oversight, and provides minimal administrative support can overcome the difficulty of involving overworked residents in quality efforts.
Acknowledgements
The authors acknowledge Michael Pourdehnad for his role in developing the quality program.
- ,,.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- ,,,,.Redesigning residency education in internal medicine: a position paper from the Association of Program Directors in Internal Medicine.Ann Intern Med.2006;144:920–926.
- Accreditation Council for Graduate Medical Education. Program directors guide to the common program requirements. Available at: http://www.acgme.org/acWebsite/navPages/commonpr_documents/ CompleteGuide_v2%20.pdf. Accessed May 5,2010.
- ,,,.Medical errors involving trainees: a study of closed malpractice claims from 5 insurers.Arch Intern Med.2007;167:2030–2036.
- ,,,,,.Residents report on adverse events and their causes.Arch Intern Med.2005;165:2607–2613.
- ,.A system of analyzing medical errors to improve GMA curricula and programs.Acad Med.2001;76:125–133.
- ,,, et al.Changing conversations: teaching safety and quality in residency training.Acad Med.2008;83(11):1080–1087.
- ,,.Practice‐based learning and improvement: a curriculum in continuous quality improvement for surgery residents.Arch Surg.2007;142:479–483.
- .Involving residents in quality improvement: contrasting “top‐down” and “bottom‐up” approaches. Accreditation Council for Graduate Medical Education and Institute for Healthcare Improvement‐day project.ACGME Bulletin. August2008.
- ,,,,.Creating a quality improvement elective for medical house officers.Gen Intern Med.2004;19(8):861–867.
- National Center for Patient Safety. United States Department for Veteran Affairs. Root cause analysis tools. Available at: http://www.patientsafety.gov/CogAids/RCA/. Accessed August 17,2010.
- ,,, et al.Residents' engagement in quality improvement: a systematic review of the literature.Acad Med.2009;84:1757–1764.
- ,,.Graduates from a traditional medical curriculum evaluate the effectiveness of their medical curriculum through interviews.BMC Med Educ.2009;9:64.
To Err Is Human revealed the underappreciated tension between the enormous benefits of medical care and the potential for harm.1 Following this report, there has been an explosion of research and commentary detailing quality improvement (QI) opportunities. One area of growing emphasis has been resident physician training.2, 3 If medical care is dangerous, then a substantial contributor to the hazard must be the apprentice‐style process of physician training and the novice skill set of the trainees.4, 5 Many resident training programs have devised efforts to decrease the errors committed by physicians‐in‐training,6 change the culture of residency training,7 engage residents in quality improvement,8, 9 and improve resident training in quality improvement.10
Many of the programs devised to teach QI in the residency setting require substantial funding, a large pool of QI experts, or redesign of resident training programs.410 While effective, these programs are not feasible for many resource‐constrained residency programs. A less intense program, using resident‐led, hospitalist‐facilitated, limited root cause analysis (RCA), has been adopted at the Internal Medicine Residency Program at the Mount Sinai Hospital (MSH). We describe our 2‐year experience using this technique, including cases discussed, improvement strategies suggested, projects implemented, and resident perceptions.
METHODS
Setting
Departmental QI leaders developed this initiative in the Internal Medicine Residency Program at the MSH in New York City, New York. This residency program trains over 140 residents annually in categorical, preliminary, and research track positions, as well as an affiliated medicine/pediatrics program. The program's residents rotate at 3 clinic sites: a tertiary care hospital, a public safety‐net hospital, and a Veterans Affairs hospital. The QI program was only implemented at the MSH. Over 90% of the program's graduates go on to complete a subspecialty fellowship.
Intervention Description
The QI program was designed around a noon‐time quality improvement conference (QIC) occurring once every 4 weeks. In the weeks prior to the session, chief residents and a hospitalist mentor selected a case related to an inpatient care issue. Potential cases were solicited, and/or offered, from a range of sources including attending physicians, nurse managers, residents, and quality officers. Only cases from the teaching services were chosen. To ensure that participants on the case were able to recall relevant details, preference was given to more recent cases. A third‐year resident on an elective or outpatient block was chosen to investigate the case. To maximize the objectivity of the investigation, every effort was made to select a resident who was not involved in the care of the patient.
The resident was instructed to complete a limited RCA (fewer fact‐finding interviews and only 1 group meeting) and was directed to online resources.11 Each resident presenter worked closely with the chief residents and hospitalist mentor to identify appropriate strategies for collecting data and interviewing involved parties. If necessary, either due to volume of work or sensitivity of the case, the chief resident or hospitalist would assist with the data gathering. The resident contacted multiple parties involved in the patient care issue including, nurses, residents, attendings, pharmacists, social workers, and, if appropriate, the patient. The resident constructed a timeline for each case, and identified specific points in the patient care experience, where errors, near misses, or misunderstandings occurred. During the QIC, these findings were presented to Internal Medicine residents, chief residents, representatives from the Chief Medical Officer's office, attending physicians overseeing the residents on inpatient rotations, and representatives from any group (social work nursing, housekeeping, pharmacy, etc) that may have impacted patient care for the particular case being investigated. On average, 50 healthcare providers attended the QIC. Lunch was provided.
After the findings were presented, a chief resident and a lead hospitalist facilitated a group discussion on the circumstances surrounding the case. Discussions were focused on identifying system‐wide failures and proposing systems‐based solutions. Great efforts were made to remind all participants to refrain from individual blame. At the end of each QIC, participants summarized and prioritized suggestions to reduce the discussed error. Interested residents were invited to form improvement committees for cases with viable solutions. Each committee attempted to implement improvements discussed during the QIC. Committees, led by a representative from the Division of Hospital Medicine, included 2 to 4 residents as well as healthcare workers from other disciplines if appropriate. For all improvement efforts, the focus was on the interventions which appeared high yield with low cost.
Intervention Evaluation
The program was exempt from Institutional Review Board review as a part of the Department of Medicine's quality improvement and assurance portfolio.
The results of the QICs were tracked. After each case, a QI team consisting of chief residents and representatives from the Division of Hospital Medicine recorded the cases presented, and interventions suggested for each case, in an online database. After implementation, the success of each intervention was recorded. To evaluate the types of interventions suggested by residents, the 3 physician‐authors, who regularly attend these conferences and have a focused career interest in QI, grouped all suggestions into 4 broad categories: Educational, Reminder Systems, Design Changes (protocol‐based), and Design Changes (Information Technology [IT]‐based). Design change interventions (IT‐based) consisted of an adjustment to electronic systems, such as displaying specific lab results on a medication ordering system. Design changes (protocol‐based) consisted of changes made to standing protocols such as nursing protocols for reporting abnormal lab values. Reminder system interventions were endeavors such as a checklist for discharge planning. Educational interventions focused on providing additional training sessions or conferences.
The 3 physician‐authors independently reviewed each suggested intervention to determine its success. They first evaluated whether the change was attempted or not. For all attempted interventions, the reviewer then assessed if there was either objective permanent system‐wide change, subjective behavior change, or no change. To meet the objective change threshold, the intervention either had to have permanently changed provider workflow or have data demonstrating behavior change or improved outcome. Interventions with anecdotal evidence that behavior was improved or modified, but lacking systematic data, were qualified as subjective behavior change. For each assessment, 2 of the 3 reviewers needed to agree for an intervention to be recorded as a success.
Resident views on the monthly conferences were solicited via an anonymous and voluntary questionnaire. A first survey was designed to assess whether residents felt that the conferences provided them with the ability to recognize and improve systems errors which compromise patient care. This survey was administered at the conclusion of the first year of the program to residents who attended the final 2 QICs. A second survey assessed whether the tone of the conferences was constructive and blame‐free. This survey was administered at the conclusion of the second year of the program to residents who attended the year's final 2 QICs.
RESULTS
Over the first 22 months of the program, 20 conferences were held (Table 1). The topics covered ranged considerably and included: deficits in supervision, medication errors, patient satisfaction, staff safety, and 30‐day readmissions. Forty‐six distinct interventions were suggested during these conferences. Of those, an attempt was made to initiate 25 (54%) of these suggestions (Table 2). Of the 25 interventions that were initiated, 18 (72%) were determined to be successful. Eight resulted in objective permanent system‐wide change and 10 resulted in subjective behavior change among residents.
| QIC Topic | Interventions Suggested by Residents | Suggestion Results (Attempted/Not Attempted, Successful/Unsuccessful) |
|---|---|---|
| ||
| Central venous catheter guide wire lost during code placement | Improved supervision and training for line placement | Attempted, but unsuccessful |
| Avoid unnecessary line placement during codes | Attempted, but unsuccessful | |
| Inappropriate administration of warfarin | Decision support providing real‐time coagulation profile | Attempted and successful |
| Central line bloodstream infection | Clarified and encouraged use of line service | Attempted and successful |
| Daily documentation of catheter placement date | Not attempted | |
| Delayed administration of pain medication | Training nurses to use text paging communication system | Attempted and successful |
| Patient discharged on wrong medication dose | Do not use abbreviations | Not attempted |
| Electronic medication reconciliation | Attempted and successful | |
| Confusion over code status | Clarification of various forms used for DNR | Not attempted |
| Better communication of code status during signout | Not attempted | |
| Patient received hydromorphone IV instead of PO during verbal order at end‐of‐life | Verbal orders should have talk back verification | Attempted, but unsuccessful |
| Encourage informing patients of medical errors | Attempted, but unsuccessful | |
| Premature closure of diagnosis during transfer from MICU | Improve comfort level disagreeing with supervisors | Attempted, but unsuccessful |
| Reassessment of patient prior to late‐day MICU transfers | Not attempted | |
| Patient erroneously received clopidogrel bisulfate (Plavix) for years due to poor medication reconciliation | Improved discharge summary interface | Attempted and successful |
| Encourage physicians to call PCP on discharge | Attempted and successful | |
| Modified barium swallow ordered incorrectly, resulting in patient aspiration | Simplify electronic order entry system to clearly identify tests | Not attempted |
| Change radiology requisition form to facilitate communication | Not attempted | |
| Fingersticks leading to blood exposure | Train PGY1s on the needles used at all 3 hospitals | Not attempted |
| Improve mask with face shields and gown availability | Attempted and successful | |
| Patient discharged with central venous catheter still in place | Check list for lines and Foleys | Not attempted |
| Improved discharge documentation | Not attempted | |
| 30‐Day readmission | Mandatory discharge summary completion prior to discharge | Attempted and successful |
| Discharge summary training during intern year | Attempted and successful | |
| DKA developed in house when insulin not administered | Improve communication between floor and dialysis RNs | Not attempted |
| Better PA supervision by residents regarding order writing | Attempted and successful | |
| Compromised patient satisfaction | Patient handouts with name and role of each care team member | Attempted, but unsuccessful |
| Patient satisfaction coaching | Attempted and successful | |
| Elevated PTT and poor documentation | Improved feedback to residents regarding daily notes | Not attempted |
| Nurses must call physicians with alert values | Not attempted | |
| Hospital‐acquired MRSA | Improve availability of contact precaution gowns | Attempted, but unsuccessful |
| Direct observation of hand washing on morning rounds | Attempted and successful | |
| Staff safety with deranged family member | Education of staff regarding safety protocols | Attempted, but unsuccessful |
| Transfer of unstable patient from outside hospital ICU to general medicine floor | Standardization of OSH transfer guidelines | Not attempted |
| Improved documentation of transferring MD contract data | Attempted and successful | |
| Consult called, patient not seen by attending | Education of faculty on existing institutional consult policy | Attempted, but unsuccessful |
| Clarification of violations reporting process for hospital consults | Attempted, but unsuccessful | |
| Type of Intervention | No. of Interventions Suggested | No. of Interventions Implemented (%) | Of Implemented Interventions, No. Which Were Successful (%) | No. of Attempted Interventions With Objective Change (%) | No. of Attempted Interventions With Subjective Change (%) |
|---|---|---|---|---|---|
| Design changes: information technology‐based | 5 | 2 (40) | 2 (100%) | 2 (100) | 0 (0) |
| Design changes: protocol‐based | 17 | 10 (59) | 8 (80%) | 5 (50) | 3 (30) |
| Educational | 20 | 11 (55) | 7 (64) | 1 (9) | 6 (55) |
| Reminder systems | 4 | 2 (50) | 1 (50) | 0 (0) | 1 (50) |
| Total | 46 | 25 (54) | 18 (72) | 8 (32) | 10 (40) |
Two IT‐based system design changes were implemented; both resulted in objective system‐wide change. Eight protocol‐based design changes were implemented successfully, 5 objectively, and 3 subjectively. Seven educational interventions and 1 reminder system intervention were initiated.
The most successful intervention to come from these conferences was the implementation of an electronic medication reconciliation program. The reconciliation program was suggested following a conference on a patient who was discharged home on the wrong dose of a medication. The institution's paper‐based medication reconciliation process, particularly for heart‐failure patients, had long been known to be deficient. The QIC brought this issue to life by highlighting a cases that may have been ameliorated with a more robust medication reconciliation process. Enthusiastic residents were invited to build a case for medication reconciliation to the Chief Medical Officer, and this helped garner resources for the hospital‐wide project. Another successful IT‐based intervention was initiated after a case of inappropriate administration of warfarin to a patient with an already elevated international normalized ratio (INR). The computerized order entry system was changed so that, at the point of ordering warfarin, the most recent coagulation profile and platelet values appear before an order can be finalized.
An example of a protocol‐based intervention came from a conference that focused on poor communication at the time of discharge, which resulted in a 30‐day readmission. As a result, resident work flow was changed so that discharge summaries are expected to be completed at the time of discharge. Along with this protocol change was an educational initiative to improve the quality of discharge summaries by including essential data for the transition of care.
Overall, residents reviewed the conferences very positively (Table 3). The response rate for the first year survey was 40% (56/140) and the second year survey was 18% (26/143). The vast majority of participants felt that the conferences were of high quality (96%) and that the exercise could lead to improvement in quality (98%). Residents felt that the conference focused more on system issues than individual shortcomings (92%). A majority felt comfortable expressing their opinions during the conferences (77%).
| Overall Conference Quality | ||
|---|---|---|
| Question | Mean Score (n = 53) | Rating Question a 4 or 5 |
| Conference Tone | ||
| Question | Mean Score (n = 26) | Rating Question With a 4 or 5 |
| ||
| Please rate the overall quality of the QIC conferences. | 4.49* | 98% |
| The case highlighted an issue that is highly relevant to the quality of patient care. | 4.81 | 100% |
| Solutions discussed at this conference could lead to improved patient care and/or patient satisfaction. | 4.65 | 96% |
| My knowledge of issues related to hospital quality and patient safety has been enhanced by this conference. | 4.61 | 96% |
| The QIC focused on individuals, individual actions, or omissions, which compromised high quality care. | 3.35 | 50% |
| The QIC focused on system failures that compromised high quality care. | 4.35 | 92% |
| I felt comfortable sharing my honest opinions about the medical events presented during the conferences. | 4.15 | 77% |
| I avoided expressing my opinions about the medical events presented during the conferences because I did not want to criticize my peers. | 2.5 | 19% |
DISCUSSION
The first 20 sessions from this resident‐led, hospitalist‐facilitated QI program provided evidence that residents can contribute to patient safety within a large tertiary care center. The role of residents in actively addressing errors and unsatisfactory outcomes in the hospital has not been a traditional QI focus.12 Involvement has typically been a passive process for physician trainees, while more senior clinical staff members decide on and prioritize QI activities. We have observed that empowering residents to take a more active role in performance improvement yields significant change and does more than simply educate about basic QI methodology.
One reason for the success of these conferences was leveraging insights of residents as key front line providers. Residents spend more time than perhaps any other category of hospital employee working within clinical care systems. They are deeply aware of the quality struggles inherent to large healthcare organizations, and this insight can lead to high impact suggestions for improvement. Often, suggestions were simple proposals that were overlooked or unappreciated by other administrative leaders. An example of this type of contribution was when residents brought the lack of infection control equipment, on certain units, to the attention of the infection control staff and facility engineers. At a separate conference, residents informed the transfer office staff that valuable contact information for physicians accepting outside hospital transfers was not being collected. Both of these observations led to quick change, with better infection control gown availability and improved documentation by transfer office staff.
Our program also demonstrated that including residents in QI provides momentum for either a training program or an institution to pursue solutions that might have otherwise been resisted. The improvement suggestion to complete discharge summaries prior to the patient leaving the hospital had long been a goal for the residency program leadership, but there was hesitation to force this work flow change on the residents. After a QI conference, when a number of the residents themselves made the suggestion, implementing the change was much easier. Similarly, after several cases of clear errors relating to a suboptimal process of medication reconciliation, the institution dedicated scarce IT personnel to work with providers to develop a robust, user‐friendly medication reconciliation application to decrease transition of care errors.
Through this program, residents also demonstrated their ability to deconstruct patient care problems. For each case, resident session leaders interviewed physician providers, physician extenders, nurses, nurse managers, pharmacists, security staff, engineering staff, and administrative staff. They gathered crucial information regarding the patient care event and the gaps or errors that led to a poor outcome. After many of the conferences, the resident presenters commented on how the investigative exercise left them more appreciative of the complexity of the medical system and interested in fixing the problems uncovered.
The feedback from the resident surveys demonstrated that residents valued the QI program. The data collected also shows that such programs can be executed in a manner which highlights system flaws. Our data do, however, suggest that there is room to improve the tone of the conference to further decrease the sense from residents that quality discussions focus on individuals. Residents often struggle to master the myriad new expectations inherent in the transition from student to physician.13 A quality process which discourages already overworked and uncertain trainees, by creating a process which assigns blame for unintentional quality shortcomings, would be counterproductive.
Lessons Learned
While this QI program has had success uncovering clinical care issues, and creating a climate and process for resident participation in improvement, there has been a number of limitations and lessons learned. Most importantly, including busy residents in any process that requires regular participation and follow‐through is difficult. A number of suggested improvements which created substantial interest and early momentum were ultimately left unfinished, as residents and even faculty facilitators became overwhelmed by clinical responsibilities. In fact, the majority of suggestions have not been successfully implemented and even fewer have created lasting change. This must be carefully monitored, as experiencing multiple failures can undermine the empowerment that such QI programs are created to foster.
Regular reflection on the successful and unsuccessful projects yielded several important insights that resulted in changes over the course of the program. Suggestions were more likely to move from idea generation to execution if the QIC was attended by administrators with decision‐making authority. Several of the suggestionsimproved medication reconciliation, better transfer documentation, and improved availability of infection control productswere able to be acted upon because conference attendees were administrators with purview over these issues. Many times, these leaders were more than willing to implement helpful suggestions, but simply needed them to be brought to their attention. As a result, we have been more attentive to inviting as many stakeholders as possible to the QICs.
It was also clear that suggestions would not be realized without a physician leader and were more successful when resident interest was substantial. After each QIC, residents who had made promising suggestions were approached to continue to participate. If the residents agreed, the projects were pursued and a faculty or chief resident leader was assigned. Lastly, we have also made use of one of the department's QI data analysts to assist with project completion. This individual has been made available to provide administrative support (organizing meetings, paperwork, etc) but also to provide data for projects, should the need arise.
Another important finding is that the tone of the QI program must be constantly monitored. Despite reminding residents at each session that the exercise was for the purpose of identifying systems barriers to delivering high quality care, there were times when residents felt targeted or blamed. At one point, a number of residents voiced their concerns that the conferences had spent too much time highlighting quality failures without recognizing the many positive performances on the teaching service. As a result, subsequent conferences often began by highlighting quality improvements made. Additionally, a part of 1 session each year had been dedicated to reading letters and e‐mails sent by patients or families which highlight memorably positive performances by the residents. Finally, care was taken to make sure invited guests to the sessions were reminded of the session's blame‐free ground rules.
Care must be taken when investigating clinical cases. On several occasions, attending physicians expressed discomfort with having residents scrutinize a clinical event. Although this process was protected under the QI umbrella and faculty names were never shared at the conferences, some faculty believed that this process was the purview of departmental or hospital QI staff, not untrained residents. Given the support provided for this program by the department chair and program director, as well as the professional nature with which the residents conducted their inquiries, there was little difficulty rejecting this line of objection. This feedback did lead supervisors to be more involved with the resident presenters, coaching them regarding data gathering and interviewing. If a case appears that it will be particularly sensitive, the hospitalist mentor or chief resident will reach out to involved residents and faculty to notify them that the case will be reviewed.
A final development secured, in part, as a result of this quality program has been more protected faculty time. At the start of this program, all faculty time was donated time on top of other administrative and patient care responsibilities. After the first 18 months of the QIC program, the residency program named an assistant program director for quality. At the time of writing this manuscript, the program further invested in quality by naming both an assistant and associate program director for quality. These positions combined amount to at least 0.4 full‐time equivalents (FTE). Of that, roughly 0.1 FTE is spent working on the QICs and subsequent project implementation.
Limitations
The evaluation of the success of the interventions potentially biased our findings. The qualitative method of using multiple reviewers, all of whom were invested in the program's outcomes, to gauge the success of initiated interventions may have resulted in an overestimate of the project's effectiveness. Furthermore, the category of subjective change lacks measurable criteria, making replication of the findings difficult.
The results presented here are from a single institution, conceived of and executed by a group of dedicated faculty. Moreover, both the chair of the department and the program director were very supportive of this endeavor. Possibly, because of these aspects, the findings presented here would not be readily replicated at another institution.
The percentage of residents who completed the feedback surveys was low. This may result in an overestimate of quality, value, and tone of the conferences, as well as potentially missing an opportunity for improving the program. We will address this issue through more rigorous quantitative and qualitative feedback at the end of the third year of the program.
CONCLUSIONS
Residents are willing and effective participants in a QI program. As front line providers, their experiences are valuable and their willingness to share insights can be an impetus for change. Finally, a process which includes modest investigation by third year residents, has faculty support and oversight, and provides minimal administrative support can overcome the difficulty of involving overworked residents in quality efforts.
Acknowledgements
The authors acknowledge Michael Pourdehnad for his role in developing the quality program.
To Err Is Human revealed the underappreciated tension between the enormous benefits of medical care and the potential for harm.1 Following this report, there has been an explosion of research and commentary detailing quality improvement (QI) opportunities. One area of growing emphasis has been resident physician training.2, 3 If medical care is dangerous, then a substantial contributor to the hazard must be the apprentice‐style process of physician training and the novice skill set of the trainees.4, 5 Many resident training programs have devised efforts to decrease the errors committed by physicians‐in‐training,6 change the culture of residency training,7 engage residents in quality improvement,8, 9 and improve resident training in quality improvement.10
Many of the programs devised to teach QI in the residency setting require substantial funding, a large pool of QI experts, or redesign of resident training programs.410 While effective, these programs are not feasible for many resource‐constrained residency programs. A less intense program, using resident‐led, hospitalist‐facilitated, limited root cause analysis (RCA), has been adopted at the Internal Medicine Residency Program at the Mount Sinai Hospital (MSH). We describe our 2‐year experience using this technique, including cases discussed, improvement strategies suggested, projects implemented, and resident perceptions.
METHODS
Setting
Departmental QI leaders developed this initiative in the Internal Medicine Residency Program at the MSH in New York City, New York. This residency program trains over 140 residents annually in categorical, preliminary, and research track positions, as well as an affiliated medicine/pediatrics program. The program's residents rotate at 3 clinic sites: a tertiary care hospital, a public safety‐net hospital, and a Veterans Affairs hospital. The QI program was only implemented at the MSH. Over 90% of the program's graduates go on to complete a subspecialty fellowship.
Intervention Description
The QI program was designed around a noon‐time quality improvement conference (QIC) occurring once every 4 weeks. In the weeks prior to the session, chief residents and a hospitalist mentor selected a case related to an inpatient care issue. Potential cases were solicited, and/or offered, from a range of sources including attending physicians, nurse managers, residents, and quality officers. Only cases from the teaching services were chosen. To ensure that participants on the case were able to recall relevant details, preference was given to more recent cases. A third‐year resident on an elective or outpatient block was chosen to investigate the case. To maximize the objectivity of the investigation, every effort was made to select a resident who was not involved in the care of the patient.
The resident was instructed to complete a limited RCA (fewer fact‐finding interviews and only 1 group meeting) and was directed to online resources.11 Each resident presenter worked closely with the chief residents and hospitalist mentor to identify appropriate strategies for collecting data and interviewing involved parties. If necessary, either due to volume of work or sensitivity of the case, the chief resident or hospitalist would assist with the data gathering. The resident contacted multiple parties involved in the patient care issue including, nurses, residents, attendings, pharmacists, social workers, and, if appropriate, the patient. The resident constructed a timeline for each case, and identified specific points in the patient care experience, where errors, near misses, or misunderstandings occurred. During the QIC, these findings were presented to Internal Medicine residents, chief residents, representatives from the Chief Medical Officer's office, attending physicians overseeing the residents on inpatient rotations, and representatives from any group (social work nursing, housekeeping, pharmacy, etc) that may have impacted patient care for the particular case being investigated. On average, 50 healthcare providers attended the QIC. Lunch was provided.
After the findings were presented, a chief resident and a lead hospitalist facilitated a group discussion on the circumstances surrounding the case. Discussions were focused on identifying system‐wide failures and proposing systems‐based solutions. Great efforts were made to remind all participants to refrain from individual blame. At the end of each QIC, participants summarized and prioritized suggestions to reduce the discussed error. Interested residents were invited to form improvement committees for cases with viable solutions. Each committee attempted to implement improvements discussed during the QIC. Committees, led by a representative from the Division of Hospital Medicine, included 2 to 4 residents as well as healthcare workers from other disciplines if appropriate. For all improvement efforts, the focus was on the interventions which appeared high yield with low cost.
Intervention Evaluation
The program was exempt from Institutional Review Board review as a part of the Department of Medicine's quality improvement and assurance portfolio.
The results of the QICs were tracked. After each case, a QI team consisting of chief residents and representatives from the Division of Hospital Medicine recorded the cases presented, and interventions suggested for each case, in an online database. After implementation, the success of each intervention was recorded. To evaluate the types of interventions suggested by residents, the 3 physician‐authors, who regularly attend these conferences and have a focused career interest in QI, grouped all suggestions into 4 broad categories: Educational, Reminder Systems, Design Changes (protocol‐based), and Design Changes (Information Technology [IT]‐based). Design change interventions (IT‐based) consisted of an adjustment to electronic systems, such as displaying specific lab results on a medication ordering system. Design changes (protocol‐based) consisted of changes made to standing protocols such as nursing protocols for reporting abnormal lab values. Reminder system interventions were endeavors such as a checklist for discharge planning. Educational interventions focused on providing additional training sessions or conferences.
The 3 physician‐authors independently reviewed each suggested intervention to determine its success. They first evaluated whether the change was attempted or not. For all attempted interventions, the reviewer then assessed if there was either objective permanent system‐wide change, subjective behavior change, or no change. To meet the objective change threshold, the intervention either had to have permanently changed provider workflow or have data demonstrating behavior change or improved outcome. Interventions with anecdotal evidence that behavior was improved or modified, but lacking systematic data, were qualified as subjective behavior change. For each assessment, 2 of the 3 reviewers needed to agree for an intervention to be recorded as a success.
Resident views on the monthly conferences were solicited via an anonymous and voluntary questionnaire. A first survey was designed to assess whether residents felt that the conferences provided them with the ability to recognize and improve systems errors which compromise patient care. This survey was administered at the conclusion of the first year of the program to residents who attended the final 2 QICs. A second survey assessed whether the tone of the conferences was constructive and blame‐free. This survey was administered at the conclusion of the second year of the program to residents who attended the year's final 2 QICs.
RESULTS
Over the first 22 months of the program, 20 conferences were held (Table 1). The topics covered ranged considerably and included: deficits in supervision, medication errors, patient satisfaction, staff safety, and 30‐day readmissions. Forty‐six distinct interventions were suggested during these conferences. Of those, an attempt was made to initiate 25 (54%) of these suggestions (Table 2). Of the 25 interventions that were initiated, 18 (72%) were determined to be successful. Eight resulted in objective permanent system‐wide change and 10 resulted in subjective behavior change among residents.
| QIC Topic | Interventions Suggested by Residents | Suggestion Results (Attempted/Not Attempted, Successful/Unsuccessful) |
|---|---|---|
| ||
| Central venous catheter guide wire lost during code placement | Improved supervision and training for line placement | Attempted, but unsuccessful |
| Avoid unnecessary line placement during codes | Attempted, but unsuccessful | |
| Inappropriate administration of warfarin | Decision support providing real‐time coagulation profile | Attempted and successful |
| Central line bloodstream infection | Clarified and encouraged use of line service | Attempted and successful |
| Daily documentation of catheter placement date | Not attempted | |
| Delayed administration of pain medication | Training nurses to use text paging communication system | Attempted and successful |
| Patient discharged on wrong medication dose | Do not use abbreviations | Not attempted |
| Electronic medication reconciliation | Attempted and successful | |
| Confusion over code status | Clarification of various forms used for DNR | Not attempted |
| Better communication of code status during signout | Not attempted | |
| Patient received hydromorphone IV instead of PO during verbal order at end‐of‐life | Verbal orders should have talk back verification | Attempted, but unsuccessful |
| Encourage informing patients of medical errors | Attempted, but unsuccessful | |
| Premature closure of diagnosis during transfer from MICU | Improve comfort level disagreeing with supervisors | Attempted, but unsuccessful |
| Reassessment of patient prior to late‐day MICU transfers | Not attempted | |
| Patient erroneously received clopidogrel bisulfate (Plavix) for years due to poor medication reconciliation | Improved discharge summary interface | Attempted and successful |
| Encourage physicians to call PCP on discharge | Attempted and successful | |
| Modified barium swallow ordered incorrectly, resulting in patient aspiration | Simplify electronic order entry system to clearly identify tests | Not attempted |
| Change radiology requisition form to facilitate communication | Not attempted | |
| Fingersticks leading to blood exposure | Train PGY1s on the needles used at all 3 hospitals | Not attempted |
| Improve mask with face shields and gown availability | Attempted and successful | |
| Patient discharged with central venous catheter still in place | Check list for lines and Foleys | Not attempted |
| Improved discharge documentation | Not attempted | |
| 30‐Day readmission | Mandatory discharge summary completion prior to discharge | Attempted and successful |
| Discharge summary training during intern year | Attempted and successful | |
| DKA developed in house when insulin not administered | Improve communication between floor and dialysis RNs | Not attempted |
| Better PA supervision by residents regarding order writing | Attempted and successful | |
| Compromised patient satisfaction | Patient handouts with name and role of each care team member | Attempted, but unsuccessful |
| Patient satisfaction coaching | Attempted and successful | |
| Elevated PTT and poor documentation | Improved feedback to residents regarding daily notes | Not attempted |
| Nurses must call physicians with alert values | Not attempted | |
| Hospital‐acquired MRSA | Improve availability of contact precaution gowns | Attempted, but unsuccessful |
| Direct observation of hand washing on morning rounds | Attempted and successful | |
| Staff safety with deranged family member | Education of staff regarding safety protocols | Attempted, but unsuccessful |
| Transfer of unstable patient from outside hospital ICU to general medicine floor | Standardization of OSH transfer guidelines | Not attempted |
| Improved documentation of transferring MD contract data | Attempted and successful | |
| Consult called, patient not seen by attending | Education of faculty on existing institutional consult policy | Attempted, but unsuccessful |
| Clarification of violations reporting process for hospital consults | Attempted, but unsuccessful | |
| Type of Intervention | No. of Interventions Suggested | No. of Interventions Implemented (%) | Of Implemented Interventions, No. Which Were Successful (%) | No. of Attempted Interventions With Objective Change (%) | No. of Attempted Interventions With Subjective Change (%) |
|---|---|---|---|---|---|
| Design changes: information technology‐based | 5 | 2 (40) | 2 (100%) | 2 (100) | 0 (0) |
| Design changes: protocol‐based | 17 | 10 (59) | 8 (80%) | 5 (50) | 3 (30) |
| Educational | 20 | 11 (55) | 7 (64) | 1 (9) | 6 (55) |
| Reminder systems | 4 | 2 (50) | 1 (50) | 0 (0) | 1 (50) |
| Total | 46 | 25 (54) | 18 (72) | 8 (32) | 10 (40) |
Two IT‐based system design changes were implemented; both resulted in objective system‐wide change. Eight protocol‐based design changes were implemented successfully, 5 objectively, and 3 subjectively. Seven educational interventions and 1 reminder system intervention were initiated.
The most successful intervention to come from these conferences was the implementation of an electronic medication reconciliation program. The reconciliation program was suggested following a conference on a patient who was discharged home on the wrong dose of a medication. The institution's paper‐based medication reconciliation process, particularly for heart‐failure patients, had long been known to be deficient. The QIC brought this issue to life by highlighting a cases that may have been ameliorated with a more robust medication reconciliation process. Enthusiastic residents were invited to build a case for medication reconciliation to the Chief Medical Officer, and this helped garner resources for the hospital‐wide project. Another successful IT‐based intervention was initiated after a case of inappropriate administration of warfarin to a patient with an already elevated international normalized ratio (INR). The computerized order entry system was changed so that, at the point of ordering warfarin, the most recent coagulation profile and platelet values appear before an order can be finalized.
An example of a protocol‐based intervention came from a conference that focused on poor communication at the time of discharge, which resulted in a 30‐day readmission. As a result, resident work flow was changed so that discharge summaries are expected to be completed at the time of discharge. Along with this protocol change was an educational initiative to improve the quality of discharge summaries by including essential data for the transition of care.
Overall, residents reviewed the conferences very positively (Table 3). The response rate for the first year survey was 40% (56/140) and the second year survey was 18% (26/143). The vast majority of participants felt that the conferences were of high quality (96%) and that the exercise could lead to improvement in quality (98%). Residents felt that the conference focused more on system issues than individual shortcomings (92%). A majority felt comfortable expressing their opinions during the conferences (77%).
| Overall Conference Quality | ||
|---|---|---|
| Question | Mean Score (n = 53) | Rating Question a 4 or 5 |
| Conference Tone | ||
| Question | Mean Score (n = 26) | Rating Question With a 4 or 5 |
| ||
| Please rate the overall quality of the QIC conferences. | 4.49* | 98% |
| The case highlighted an issue that is highly relevant to the quality of patient care. | 4.81 | 100% |
| Solutions discussed at this conference could lead to improved patient care and/or patient satisfaction. | 4.65 | 96% |
| My knowledge of issues related to hospital quality and patient safety has been enhanced by this conference. | 4.61 | 96% |
| The QIC focused on individuals, individual actions, or omissions, which compromised high quality care. | 3.35 | 50% |
| The QIC focused on system failures that compromised high quality care. | 4.35 | 92% |
| I felt comfortable sharing my honest opinions about the medical events presented during the conferences. | 4.15 | 77% |
| I avoided expressing my opinions about the medical events presented during the conferences because I did not want to criticize my peers. | 2.5 | 19% |
DISCUSSION
The first 20 sessions from this resident‐led, hospitalist‐facilitated QI program provided evidence that residents can contribute to patient safety within a large tertiary care center. The role of residents in actively addressing errors and unsatisfactory outcomes in the hospital has not been a traditional QI focus.12 Involvement has typically been a passive process for physician trainees, while more senior clinical staff members decide on and prioritize QI activities. We have observed that empowering residents to take a more active role in performance improvement yields significant change and does more than simply educate about basic QI methodology.
One reason for the success of these conferences was leveraging insights of residents as key front line providers. Residents spend more time than perhaps any other category of hospital employee working within clinical care systems. They are deeply aware of the quality struggles inherent to large healthcare organizations, and this insight can lead to high impact suggestions for improvement. Often, suggestions were simple proposals that were overlooked or unappreciated by other administrative leaders. An example of this type of contribution was when residents brought the lack of infection control equipment, on certain units, to the attention of the infection control staff and facility engineers. At a separate conference, residents informed the transfer office staff that valuable contact information for physicians accepting outside hospital transfers was not being collected. Both of these observations led to quick change, with better infection control gown availability and improved documentation by transfer office staff.
Our program also demonstrated that including residents in QI provides momentum for either a training program or an institution to pursue solutions that might have otherwise been resisted. The improvement suggestion to complete discharge summaries prior to the patient leaving the hospital had long been a goal for the residency program leadership, but there was hesitation to force this work flow change on the residents. After a QI conference, when a number of the residents themselves made the suggestion, implementing the change was much easier. Similarly, after several cases of clear errors relating to a suboptimal process of medication reconciliation, the institution dedicated scarce IT personnel to work with providers to develop a robust, user‐friendly medication reconciliation application to decrease transition of care errors.
Through this program, residents also demonstrated their ability to deconstruct patient care problems. For each case, resident session leaders interviewed physician providers, physician extenders, nurses, nurse managers, pharmacists, security staff, engineering staff, and administrative staff. They gathered crucial information regarding the patient care event and the gaps or errors that led to a poor outcome. After many of the conferences, the resident presenters commented on how the investigative exercise left them more appreciative of the complexity of the medical system and interested in fixing the problems uncovered.
The feedback from the resident surveys demonstrated that residents valued the QI program. The data collected also shows that such programs can be executed in a manner which highlights system flaws. Our data do, however, suggest that there is room to improve the tone of the conference to further decrease the sense from residents that quality discussions focus on individuals. Residents often struggle to master the myriad new expectations inherent in the transition from student to physician.13 A quality process which discourages already overworked and uncertain trainees, by creating a process which assigns blame for unintentional quality shortcomings, would be counterproductive.
Lessons Learned
While this QI program has had success uncovering clinical care issues, and creating a climate and process for resident participation in improvement, there has been a number of limitations and lessons learned. Most importantly, including busy residents in any process that requires regular participation and follow‐through is difficult. A number of suggested improvements which created substantial interest and early momentum were ultimately left unfinished, as residents and even faculty facilitators became overwhelmed by clinical responsibilities. In fact, the majority of suggestions have not been successfully implemented and even fewer have created lasting change. This must be carefully monitored, as experiencing multiple failures can undermine the empowerment that such QI programs are created to foster.
Regular reflection on the successful and unsuccessful projects yielded several important insights that resulted in changes over the course of the program. Suggestions were more likely to move from idea generation to execution if the QIC was attended by administrators with decision‐making authority. Several of the suggestionsimproved medication reconciliation, better transfer documentation, and improved availability of infection control productswere able to be acted upon because conference attendees were administrators with purview over these issues. Many times, these leaders were more than willing to implement helpful suggestions, but simply needed them to be brought to their attention. As a result, we have been more attentive to inviting as many stakeholders as possible to the QICs.
It was also clear that suggestions would not be realized without a physician leader and were more successful when resident interest was substantial. After each QIC, residents who had made promising suggestions were approached to continue to participate. If the residents agreed, the projects were pursued and a faculty or chief resident leader was assigned. Lastly, we have also made use of one of the department's QI data analysts to assist with project completion. This individual has been made available to provide administrative support (organizing meetings, paperwork, etc) but also to provide data for projects, should the need arise.
Another important finding is that the tone of the QI program must be constantly monitored. Despite reminding residents at each session that the exercise was for the purpose of identifying systems barriers to delivering high quality care, there were times when residents felt targeted or blamed. At one point, a number of residents voiced their concerns that the conferences had spent too much time highlighting quality failures without recognizing the many positive performances on the teaching service. As a result, subsequent conferences often began by highlighting quality improvements made. Additionally, a part of 1 session each year had been dedicated to reading letters and e‐mails sent by patients or families which highlight memorably positive performances by the residents. Finally, care was taken to make sure invited guests to the sessions were reminded of the session's blame‐free ground rules.
Care must be taken when investigating clinical cases. On several occasions, attending physicians expressed discomfort with having residents scrutinize a clinical event. Although this process was protected under the QI umbrella and faculty names were never shared at the conferences, some faculty believed that this process was the purview of departmental or hospital QI staff, not untrained residents. Given the support provided for this program by the department chair and program director, as well as the professional nature with which the residents conducted their inquiries, there was little difficulty rejecting this line of objection. This feedback did lead supervisors to be more involved with the resident presenters, coaching them regarding data gathering and interviewing. If a case appears that it will be particularly sensitive, the hospitalist mentor or chief resident will reach out to involved residents and faculty to notify them that the case will be reviewed.
A final development secured, in part, as a result of this quality program has been more protected faculty time. At the start of this program, all faculty time was donated time on top of other administrative and patient care responsibilities. After the first 18 months of the QIC program, the residency program named an assistant program director for quality. At the time of writing this manuscript, the program further invested in quality by naming both an assistant and associate program director for quality. These positions combined amount to at least 0.4 full‐time equivalents (FTE). Of that, roughly 0.1 FTE is spent working on the QICs and subsequent project implementation.
Limitations
The evaluation of the success of the interventions potentially biased our findings. The qualitative method of using multiple reviewers, all of whom were invested in the program's outcomes, to gauge the success of initiated interventions may have resulted in an overestimate of the project's effectiveness. Furthermore, the category of subjective change lacks measurable criteria, making replication of the findings difficult.
The results presented here are from a single institution, conceived of and executed by a group of dedicated faculty. Moreover, both the chair of the department and the program director were very supportive of this endeavor. Possibly, because of these aspects, the findings presented here would not be readily replicated at another institution.
The percentage of residents who completed the feedback surveys was low. This may result in an overestimate of quality, value, and tone of the conferences, as well as potentially missing an opportunity for improving the program. We will address this issue through more rigorous quantitative and qualitative feedback at the end of the third year of the program.
CONCLUSIONS
Residents are willing and effective participants in a QI program. As front line providers, their experiences are valuable and their willingness to share insights can be an impetus for change. Finally, a process which includes modest investigation by third year residents, has faculty support and oversight, and provides minimal administrative support can overcome the difficulty of involving overworked residents in quality efforts.
Acknowledgements
The authors acknowledge Michael Pourdehnad for his role in developing the quality program.
- ,,.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- ,,,,.Redesigning residency education in internal medicine: a position paper from the Association of Program Directors in Internal Medicine.Ann Intern Med.2006;144:920–926.
- Accreditation Council for Graduate Medical Education. Program directors guide to the common program requirements. Available at: http://www.acgme.org/acWebsite/navPages/commonpr_documents/ CompleteGuide_v2%20.pdf. Accessed May 5,2010.
- ,,,.Medical errors involving trainees: a study of closed malpractice claims from 5 insurers.Arch Intern Med.2007;167:2030–2036.
- ,,,,,.Residents report on adverse events and their causes.Arch Intern Med.2005;165:2607–2613.
- ,.A system of analyzing medical errors to improve GMA curricula and programs.Acad Med.2001;76:125–133.
- ,,, et al.Changing conversations: teaching safety and quality in residency training.Acad Med.2008;83(11):1080–1087.
- ,,.Practice‐based learning and improvement: a curriculum in continuous quality improvement for surgery residents.Arch Surg.2007;142:479–483.
- .Involving residents in quality improvement: contrasting “top‐down” and “bottom‐up” approaches. Accreditation Council for Graduate Medical Education and Institute for Healthcare Improvement‐day project.ACGME Bulletin. August2008.
- ,,,,.Creating a quality improvement elective for medical house officers.Gen Intern Med.2004;19(8):861–867.
- National Center for Patient Safety. United States Department for Veteran Affairs. Root cause analysis tools. Available at: http://www.patientsafety.gov/CogAids/RCA/. Accessed August 17,2010.
- ,,, et al.Residents' engagement in quality improvement: a systematic review of the literature.Acad Med.2009;84:1757–1764.
- ,,.Graduates from a traditional medical curriculum evaluate the effectiveness of their medical curriculum through interviews.BMC Med Educ.2009;9:64.
- ,,.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- ,,,,.Redesigning residency education in internal medicine: a position paper from the Association of Program Directors in Internal Medicine.Ann Intern Med.2006;144:920–926.
- Accreditation Council for Graduate Medical Education. Program directors guide to the common program requirements. Available at: http://www.acgme.org/acWebsite/navPages/commonpr_documents/ CompleteGuide_v2%20.pdf. Accessed May 5,2010.
- ,,,.Medical errors involving trainees: a study of closed malpractice claims from 5 insurers.Arch Intern Med.2007;167:2030–2036.
- ,,,,,.Residents report on adverse events and their causes.Arch Intern Med.2005;165:2607–2613.
- ,.A system of analyzing medical errors to improve GMA curricula and programs.Acad Med.2001;76:125–133.
- ,,, et al.Changing conversations: teaching safety and quality in residency training.Acad Med.2008;83(11):1080–1087.
- ,,.Practice‐based learning and improvement: a curriculum in continuous quality improvement for surgery residents.Arch Surg.2007;142:479–483.
- .Involving residents in quality improvement: contrasting “top‐down” and “bottom‐up” approaches. Accreditation Council for Graduate Medical Education and Institute for Healthcare Improvement‐day project.ACGME Bulletin. August2008.
- ,,,,.Creating a quality improvement elective for medical house officers.Gen Intern Med.2004;19(8):861–867.
- National Center for Patient Safety. United States Department for Veteran Affairs. Root cause analysis tools. Available at: http://www.patientsafety.gov/CogAids/RCA/. Accessed August 17,2010.
- ,,, et al.Residents' engagement in quality improvement: a systematic review of the literature.Acad Med.2009;84:1757–1764.
- ,,.Graduates from a traditional medical curriculum evaluate the effectiveness of their medical curriculum through interviews.BMC Med Educ.2009;9:64.
Complications of Bariatric Surgery
Obesity is a growing epidemic in the United States and worldwide. Over one‐third of Americans (33.8%) are considered obese (body mass index [BMI] 30).1 Nonsurgical interventions have failed to achieve the long‐lasting effects of weight loss surgery and the associated reduction in obesity‐related comorbidities such as type 2 diabetes mellitus, hyperlipidemia, hypertension, obstructive sleep apnea, cancer, coronary artery disease, osteoarthritis, and gastroesophageal reflux disease (GERD).27 The American Society for Metabolic and Bariatric Surgery estimates that 220,000 people underwent bariatric surgery in 2009 with over 1.5 million procedures performed since 1992.
Centers of excellence criteria include follow‐up with the bariatric surgeon for 5 years; however, the patient may be admitted to a hospital without immediate availability of the bariatric surgeon. Since hospitalists are often first responders to the majority of newly hospitalized patients, this growing number of post‐bariatric surgery patients necessitates hospitalists have a full understanding of their unique postoperative anatomical and physiological consequences. During the first hours of an acute inpatient presentation, post‐bariatric surgical patients can be divided into the following categories: surgical complications, surgical complications masquerading as acute medical conditions, and medical complications. Additionally, hospitalists should be aware of the nuances of radiographic imaging and appropriate endoscopic procedures in these patients. This article will discuss the common current bariatric surgical procedures; post‐bariatric surgery radiographic imaging pearls; and a review of the signs, symptoms, and treatment of common medical and surgical complications.
Descriptions of Contemporary Procedures
Contemporary weight loss procedures can be divided into 2 categories based on how they produce weight loss: restrictive only or combination malabsorptive with restriction. Most are performed laparoscopically to reduce postoperative pain, speed recovery, and decrease wound complications.
Restrictive Procedures (Laparoscopic Adjustable Gastric Band and Sleeve Gastrectomy)
These procedures produce weight loss by reducing the size of the stomach or creating an obstruction in the proximal stomach, limiting the consumption of large quantities at one time. They produce early satiety, but patients may still consume a large volume of calorie‐dense liquids compromising weight loss.
Laparoscopic Adjustable Gastric Band
Laparoscopic adjustable gastric band (LAGB; Figure 1A) is the primary form of restrictive procedures with 2 Food and Drug Administration‐approved bands (Lap Band [Allergan, Inc; Irvine, CA] and REALIZE band [Ethicon Endo‐Surgery, Inc; Cincinnati, OH]). A cuff is inflated around the proximal stomach creating a gastric pouch approximately 15‐30 mL in size. A subcutaneous reservoir is attached to the cuff allowing adjustment to the degree of restriction.8 LAGB has replaced the vertical banded gastroplasty (VBG). It is less invasive, adjustable, and reversible (0.1% operative mortality rate). Weight loss is maintained with this procedure but is generally less, with a higher failure rate compared to the more common gastric bypass procedure (Table 1).3, 9 Complications may include band dysfunction (ie, slippage, erosion, infections), esophageal dilatation, balloon failure, and port malposition, with rates approaching 3%‐5% per year requiring removal or repair.10 Patients may also experience GERD symptoms, especially if the condition was present preoperatively. Progressive GERD symptoms should be investigated with an upper gastrointestinal (GI) series to ensure there is no band slippage, esophageal dilation, or dysfunction.
| LAGB | Roux‐en‐Y Gastric Bypass | Biliopancreatic Diversion With and Without Duodenal Switch | |
|---|---|---|---|
| Excess weight loss | 48% | 62% | 70% |
| Resolution of diabetes | 48% | 84% | 98% |
Sleeve Gastrectomy
With the sleeve gastrectomy (Figure 1B) procedure, a small gastric tube is created by resecting the majority of the stomach. Early postoperative complications are comparable to those after Roux‐en‐Y gastric bypass (RYGB) operations. Leaks from the long gastric staple line are the most concerning. Recent report of a leak rate of 4.9% is much higher than contemporary reports of leaks after laparoscopic RYGB operations.11 Gastric tube stenosis is unique to the operation but comparable to gastrojejunal anastomotic stricture rates after RYGB. Weight loss is less than RYGB. Long‐term results from larger cohorts are needed to determine if the high incidence of esophageal complaints (GERD 26%, vomiting 21%), and weight regain issues are consistently experienced.
Combination Procedures (Roux‐en‐Y Gastric Bypass and Biliopancreatic Diversion With and Without Duodenal Switch)
These procedures produce weight loss by decreasing caloric intake and altering digestion and absorption.
Roux‐en‐Y Gastric Bypass
Roux‐en‐Y Gastric Bypass (RYGB) (Figure 1C) is the most common bariatric procedure performed in the United States. As the gold standard, it provides long‐term successful weight loss and a defined risk profile.9 This procedure involves the creation of a small (15‐30 mL) gastric pouch by transecting the stomach and then draining the pouch via a Roux limb. The Roux (aka alimentary) limb is the segment of bowel between the small gastric pouch and the jejunojejunostomy. Variations on this procedure include different length Roux limbs (75‐150 cm) and the use of a silastic ring at the gastrojejunal anastomosis. The latter is not commonly used because of the high incidence of band erosion. Weight loss seems to be independent of these variations. Postoperatively, food bypasses the biliopancreatic limb (ie, the stomach, duodenum, and part of the jejunum) resulting in selective malabsorption in the common channel (the segment distal to the jejunojejunostomy). Hormone secretions are altered, affecting satiety signaling and glucose metabolism.10, 12
Biliopancreatic Diversion With Duodenal Switch
In biliopancreatic diversion (BPD) with duodenal switch (DS) (Figure 1D), a sleeve gastrectomy is performed. The ileum is transected about 250 cm proximal to the ileocecal valve and is then attached to the transected duodenum just distal to the pylorus, forming the path for the food. The excluded duodenum, jejunum, and proximal ileum drain the biliary and pancreatic secretions and are reconnected to the distal ileum about 50‐100 cm proximal to the ileocecal valve. Food and digestive juices mix, allowing for absorption of nutrients over this short common channel. Greater malabsorption of calories, vitamins, and trace elements occurs, providing more reliable weight loss and significantly more nutritional problems.8, 9
Radiographic and Endoscopic Considerations
When evaluating abdominal complaints with radiographic imaging, the postoperative anatomic variations can challenge routine interpretation. An experienced radiologist and involvement of a bariatric surgeon, who is familiar with the post‐gastric bypass anatomical changes, are essential for accurate interpretation.
Computed tomography (CT) scans with oral contrast are the imaging modality of choice, particularly in the acute setting, to rule out small bowel obstruction. CT scans are helpful in delineating postoperative anatomy, detecting anastomotic leaks, obstructions and other intra‐abdominal problems.1315 Routine upper GI series (UGI) after gastric bypass is controversial, with some performing it routinely and others only for cause. Regardless, when UGI is performed, likewise for CT, small volumes of water‐soluble contrast should be used, followed by small volumes of dilute barium solution. A UGI may be complementary and more sensitive in the case of a small leak when done under fluoroscopy, but CT and UGI may not show the leak in as many as 30% of patients; CT scans may provide additional information to help guide the clinical decision making. A negative study should not preclude surgical exploration if a high suspicion for leak exists.16 Internal hernias (loop of bowel passing through a mesenteric defect created by the original surgery), a common cause of bowel obstructions, are frequently missed, therefore a high level of suspicion is necessary.1719 Several studies have identified 8 radiographic CT findings in bowel obstructions caused by internal hernias including swirl sign, mushroom sign, hurricane eye, small bowel obstruction, clustered loops, small bowel behind superior mesenteric artery, right‐side anastomosis, and engorged nodes.18, 19 The clinical picture should guide medical versus surgical management in those exceeding CT scanner weight limits (commonly 350 lb).
Imaging modalities such as UGI, endoscopy, or double balloon enteroscopy (DBE) should be used for patients with more chronic abdominal complaints. UGI may miss leaks and obstructions in the remnant stomach and bypassed intestine. If pathology, such as ulcers, retained sutures, and strictures are suspected in the bypassed stomach/emntestine, DBE can be used to diagnose and therapeutically intervene, but may not be available at all centers and referral may be considered. Endoscopy allows for direct visualization of subtle or mucosal pathology in the small bowel, but is unable to visualize the excluded stomach and duodenum.20
Early Medical and Surgical Complications
Early postoperative complications (within 30 days) occur in the minority of patients after weight loss operations. Clinical findings, even in life‐threatening conditions, may be subtle. Readmissions most often occur for dehydration secondary to inadequate oral intake. Pneumonias, and wound and urinary tract infections are not unique to the bariatric surgery patient, but there is a higher than average risk of pulmonary embolism and bleeding. Bleeding most frequently occurs into the GI tract from staple lines resulting in rapid catharsis or emesis, but can also be intraperitoneal and elusive. Most GI bleeding stops spontaneously, but some require transfusion and re‐exploration in extreme cases.21 Leaks may occur at any of the staple lines or anastomoses. The most common sites of leak are the g‐j anastomosis, gastric pouch, and remnant stomach. Again, remnant stomach and j‐j anastomosis leaks may escape detection by UGI and CT. Re‐exploration of a sick patient in the early postoperative period may be required despite normal imaging studies. Early consultation with, or transfer to, a bariatric surgery center should always be considered for patients readmitted after bariatric surgery.
Late Medical Complications
Gastrointestinal complaints, excessive weight loss, and vitamin/mineral deficiencies resulting in neurological problems and metabolic bone disease are post‐bariatric medical complications that may prompt hospital admission. If not the primary reason for admission, special attention to these issues may prevent readmission, another focus of hospital care.
Gastrointestinal Complaints
One of the most common causes of hospital admission any time postoperatively is abdominal pain. A differential diagnosis of abdominal pain, nausea, and/or vomiting in the post‐bariatric surgery patient should include small bowel obstruction, hernias (internal or incisional), band complications, food intolerance, dietary noncompliance, ileus, mesenteric venous thrombosis, strictures (such as outlet obstruction or anastomotic stenosis), ulcers, esophagitis, cholelithiasis, dumping syndrome, and Roux stasis syndrome.20
A thorough history targeted at the relationship between symptoms and food intake, attention to the character and location of the pain, and a thorough physical exam (specifically the presence or absence of palpable tenderness, guarding, or rebound) is essential. The physical exam may be misleading in obese patients and, if radiographic studies cannot be performed secondary to patient size, surgical exploration may be needed soon after presentation. Therefore, even lacking an obvious surgical need, the bariatric surgeon should be notified of admission.
Improper food choice, and failure to slowly and adequately chew food, can result in emesis and digestive difficulty. Physical incompatibility with the small gastric pouch and gastric outlet obstructions can be caused by nondigestible foods (ie, breads, steak, raw vegetables). This highlights the importance of ordering the appropriate hospital diet.8 Specific gastric bypass hospital diets for all consistencies should reflect the mechanical limitations and carbohydrate/protein requirements of these patients.
Increased gallstone formation is observed in patients with rapid weight loss (1.5 kg/wk), especially following RYGB and less often after LAGB procedures (40% vs 20% over 3 years). Routine use of ursodiol during rapid weight loss (6 months after RYGB) reduces this complication to 5%.8
Stenosis or ulceration at the anastomotic site for RYGB can cause abdominal pain and vomiting. The incidence of stomal stenosis has been reported at 5%‐19% and typically occurs within the first 3 postoperative months.22 This problem is often amenable to endoscopic dilatation, unless a ring was used to reinforce the anastomosis. Ulceration has been reported in 1%‐16% of patients and is usually secondary to tobacco or non‐steroidal anti‐inflammatory drug (NSAID) use, H. pylori, fistula‐induced acid exposure, reaction to foreign material, or ischemia from tension and poor tissue perfusion.23, 24 Endoscopy can diagnose the presence of ulcers, with biopsies to rule out H. pylori infection. Cessation of NSAIDs and tobacco are critical. Medical management including proton pump inhibitors and/or sucralfate is sufficient for up to 95% of patients. Surgical revision is reserved for persistent ulcers associated with obstruction, pain, and/or bleeding.25
Dumping syndrome is a complex of post‐prandial symptoms occurring most commonly in the RYGB patients. As many as 44% of RYGB patients may experience this syndrome characterized by flushing, dizziness, abdominal distension, pain, nausea, vomiting, and/or diarrhea.26 Symptoms may result from the ingestion of large amounts of sugars which empty from the altered gastric pouch at an unregulated rate. This large osmotic load causes fluid shifts and surges in peptide hormone levels, resulting in symptoms which may reinforce adherence to the prescribed postoperative diet. It occurs shortly after a meal and resolves over hours. Dietary modifications, such as increased protein and fiber intake with decreased consumption of simple sugars, will ameliorate symptoms in many patients, with most seeing resolution after the first year.8, 27 Some patients experience hyperglycemia secondary to ingestion of simple carbohydrates, with hypoglycemia approximately 2 hours later (late dumping). In our experience, limiting carbohydrate intake to 30 grams at any meal usually alleviates post‐prandial hypoglycemia.
If the patient reports an absence of bowel movements and flatus, an ileus from chronic narcotic use or a mechanical small bowel obstruction secondary to internal hernias or adhesions (see Late Surgical Complications) must be investigated. Severe or prolonged pain, lasting longer than a few hours, is cause for alarm and should prompt aggressive evaluation and possibly exploratory surgery.
Excessive Weight Loss
In diagnosing postoperative excessive weight loss, it is important to understand average anticipated weight loss parameters. Compared to the values expected for RYGB, LAGB produces less weight loss and BPD with and without DS produces more (Table 227). Patients experiencing more rapid or prolonged weight loss should be investigated for bacterial overgrowth syndrome, short bowel syndrome, or other anatomic abnormalities.
| Postoperative Time Period | Average Weight Loss (RYGB) | |
|---|---|---|
| Daily | By Time Period | |
| ||
| 0‐3 mo | 0.22‐0.45 kg/day | 15‐20 kg by 3 mo |
| 3‐9 mo | 0.11‐0.22 kg/day | 25‐35 kg by 6 mo |
| 9‐12 mo | 0.11 kg/day | 40‐60 kg in first year |
Known risk factors for bacterial overgrowth, which are prominent in this population, include decreased gastric acidity and slowed intestinal transit time (ie, narcotic use). Patients may be asymptomatic or experience weight loss, abdominal bloating and/or pain, nausea, vomiting, and diarrhea. The diagnosis can be made with a hydrogen breath test or by obtaining quantitative cultures of jejunal secretions during endoscopy. Questions remain on how the normalized values of these tests are affected by the postoperative environment, and on how this syndrome may present or be treated if it affects the excluded intestine. Bacterial overgrowth may be an incidental finding and not the cause of the gastrointestinal complaints. Although data is limited, treatment typically consists of a 7‐10 day course of rifaximin 1200 mg/day (divided doses) and/or a trial of dietary modifications.20, 2831 These may include avoiding lactose and eating a high fat, low carbohydrate, low fiber diet, so nutrients are readily absorbed and not left for bacterial consumption.32
Short bowel syndrome (100‐200 cm of intestinal tissue remaining and subsequent malabsorption) can occur after any extensive colonic resection or bypass of the intestine.33 This condition rarely results after an initial bariatric procedure; however, subsequent procedures for small bowel obstructions or intestinal ischemia may result in short bowel syndrome. Typical presentations include diarrhea, weight loss, and symptoms of vitamin and mineral deficiencies. Short bowel can also predispose patients to the development of bacterial overgrowth, further complicating weight loss. Management consists of nutritional supplementation, occasionally parenteral nutrition, and rarely reoperation to increase the length of the common channel.34 Avoidance of further bowel resection is crucial in preventing short bowel syndrome.33, 34 In the setting of carbohydrate malabsorption with concomitant bacterial overgrowth syndrome, production of d‐lactic acid causing a metabolic acidosis with encephalopathy has been reported.35
Once medical complications have been ruled out, it is prudent to evaluate for a psychological component such as anorexia nervosa. It is helpful to involve a qualified psychologist who is familiar with this population. Addictions to alcohol, gambling, and pain medications have been reported in the post‐bariatric surgery population as a substitute for food addiction.
Neurological Complications and Vitamin Deficiencies
Neurological complications develop months to years postoperatively, secondary to vitamin, mineral, and nutrient deficiencies that result from malabsorption or inadequate intake. An inpatient provider should be aware of the potential role these conditions may play in a hospitalized patient.
Peripheral neuropathy can develop secondary to several deficiencies, including vitamin B12, thiamine, vitamin E, and copper. Their sources, deficiencies, and replacement regimens are presented in Table 3.3642 Thiamine deficiency, manifesting as Wernicke's encephalopathy, is particularly important in the postoperative patient with excessive vomiting. For prevention, we recommend all patients readmitted with vomiting and dehydration receive a banana bag or rally pack (thiamine 100 mg, folic acid 1 mg, multivitamin with iron and magnesium 3 g in one liter of D5 normal saline) over 4‐8 hours. Additional deficiencies after gastric bypass include folate, selenium, zinc, vitamin B6, and riboflavin. A multivitamin with minerals will meet the needs of most patients. Multiple fat‐soluble vitamin deficiencies can occur with small bowel bacterial overgrowth or BPD.
| B12 | B1 (Thiamine) | Vitamin E | Copper | |
|---|---|---|---|---|
| Dietary sources | Meat and dairy | Fortified grains, cereals, nuts, and pork | Vegetable oil, nuts, leafy vegetables39 | Shellfish, organ meats (liver, kidney), chocolate, nuts, dried legumes/fruits41 |
| Location of absorption | Terminal ileum after combining with intrinsic factor | Proximal small intestine | Upper small intestine41 | Stomach and duodenum38 |
| Mechanism of deficiency | Inadequate intake intrinsic factor deficiency37, 39 | Bypass of primary absorption site Inadequate intake Excessive emesis | Fat malabsorption39 Inadequate intake | Defective intestinal mucosal transport40 Decreased absorptive surface area40 Inadequate intake Coadministered zinc which competes with copper for absorption38 |
| Time to develop deficiency | Years | 18 days37, 39 | 6‐12 mo39 | 3‐12 mo42 |
| Postoperative supplementation recommendation | Optimal prophylactic dose unknown Minimum 1‐2 mg/day | 1‐1.5 mg/day37 | Males: 10 mg/day Females: 8 mg/day | Multivitamin (900 g/day) |
| Pathology of deficiency | Macrocytic anemia Paresthesias Ataxia Subacute combined degeneration of the spinal cord | Dry beriberi Wernicke's encephalopathy Korsakoff's syndrome | Myopathy/neuropathy Ataxia | Demyelinating neuropathy with ataxia Anemia |
| Labs to document deficiency | Serum B12 | Erythrocyte transketolase activity Thiamine diphosphate effect37 | Serum alphatocopherol37, 39 Check for deficiencies of other fat soluble vitamins (A, D, K) | Serum copper level40 |
| Correcting deficiency | Intramuscular B12 (1000 mcg) Sublingual supplementation36 | 50‐100 mg/day (parenteral or oral)37 | 400 mg PO BID37 | 2‐4 mg/day38 |
Anemia
Iron deficiency affects 6%‐33% of patients after 1 year.43 Iron is preferentially absorbed in the duodenum and proximal jejunum which are bypassed postoperatively. The absence of gastric acid prevents conversion of ferric (Fe2+) to the absorbable ferrous (Fe3+) iron, further decreasing absorption.44 Ferritin reflects iron stores but is also an acute phase reactant and, therefore, may mask an underlying deficiency in an acutely ill hospitalized patient. A multivitamin with iron is recommended for all patients, but additional supplementation may be required for menstruating women.43 Parenteral administration may be necessary if oral supplements are not tolerated or are inadequately absorbed.44
Fractures and Osteomalacia
Calcium and vitamin D deficiencies are a significant problem in the bariatric surgery population, with resultant osteoporosis or osteomalacia and associated fractures.38, 43 Calcium is preferentially absorbed in the duodenum and proximal jejunum. Vitamin D is absorbed in the ileum or produced in the skin in response to ultraviolet B (UVB) radiation.45 Deficiency of vitamin D exacerbates calcium malabsorption, thereby causing secondary hyperparathyroidism, increased bone turnover, and osteomalacia. Dramatic weight loss can lead to bone loss, increasing the risk for osteoporosis and fractures.38 Hypocalcemia or osteomalacia may cause generalized bone pain, muscular weakness, tetany, and chronic musculoskeletal pain.45
Fat‐soluble vitamin deficiencies are more common in those undergoing malabsorptive versus restrictive procedures and, in the case of BPD, may be related to the length of the common channel.43 It is important to ensure that calcium and vitamin D levels are sufficient prior to surgery, and prior to starting any osteoporotic treatment such as bisphosphonates.45 We recommend at least 1200 mg of calcium citrate and 1000‐2000 IU of Vitamin D daily. Up to 50,000 IU weekly or daily may be required to correct deficiency and maintain sufficiency in this population.46, 47 Vitamin D2 (ergocalciferol) or D3 (cholecalciferol) can be used for supplementation. Cholecalciferol is preferred if given through a feeding tube because it is less prone to clogging the tube.46 With severe malabsorption, phototherapy may be necessary, as intravenous doses are often inadequate and intramuscular preparations require special compounding.46 Calcium carbonate requires acid for proper absorption, therefore calcium citrate may be preferred due to achlorhydria from gastric exclusion.
Late Surgical Complications
Hospitalists are increasingly responsible for managing and comanaging surgical patients. The post‐bariatric surgery patient may present with unique signs and symptoms of surgical conditions masquerading as medical conditions. Common conditions that present in uncommon ways include strictures (ie, outlet obstruction and stomal stenosis), hernias with strangulation (incisional and internal), and small bowel obstructions.
Small bowel obstruction (SBO) occurs in 0%‐5% of RYGB patients (less with LAGB, similar with BPD), which is similar to other abdominal surgery rates, and may occur months to years after the original surgery. The differential diagnosis of an SBO includes internal hernias, adhesions, ventral hernia (incisional and umbilical), postoperative ileus, and jejunojejunal anastomotic stricture. Typical symptoms are often present, but may be less obvious than with a non‐gastric bypass patient. Pain can range from acute to a chronic or intermittent pattern. Pain is the most common presenting symptom of obstruction. Pain relieved by emesis may indicate an obstruction in the Roux limb. Nausea, bloating, tachycardia, and hiccups with shoulder/back pain can occur when obstruction in the biliopancreatic limb causes gastric distension.48
Vomiting is seen in fewer than half of patients with SBOs due to the altered anatomy.49 Any post‐RYGB patient that vomits bile needs emergent surgical evaluation for a common channel obstruction. Radiographic imaging may be misleading as to the cause of the obstruction. SBO is crucial to consider since delayed diagnosis can result in bowel ischemia and death.18 For the hospitalist who is caring for a post‐bariatric patient with a bowel obstruction, early surgical consultation is mandatory, preferably with a bariatric surgeon. Traditional medical management such as nasogastric (NG) tube placement will not decompress the excluded stomach, therefore patients rarely benefit from nasogastric decompression. If necessary, an NG tube should only be placed by experienced hands or fluoroscopic guidance, due to the altered anatomy.
Conclusion
Weight loss surgery, developed to address the growing obesity problem, has been beneficial to hundreds of thousands of people by decreasing their excess weight and comorbidities. For some, the postoperative course is complicated by medical and surgical problems requiring hospitalization. It is critically important that, as this relatively new field of postoperative medicine evolves, the hospitalist stay informed on relevant presentations, complications, and treatment to better address this growing population. Early consultation with, and transfer to, a bariatric surgery center should be encouraged. The importance of arranging proper hospital follow‐up, including community‐based support groups, nutritional consults, psychological support, and close follow‐up with the bariatric surgeon, bariatrician, and/or primary care physician, should not be underestimated.
- ,,,.Prevalence and trends in obesity among US adults, 1999‐2008.JAMA.2010;303(3):235–241.
- ,,, et al.Long‐term mortality after gastric bypass surgery.N Engl J Med.2007;357(8):753–761.
- ,,, et al.Bariatric surgery: a systematic review and meta‐analysis.JAMA.2004;292(14):1724–1737.
- ,,.Results of laparoscopic sleeve gastrectomy (LSG) at 1 year in morbidly obese Korean patients.Obes Surg.2005;15(10):1469–1475.
- ,,, et al.Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery.N Engl J Med.2004;351(26):2683–2693.
- ,,, et al.Long‐term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II.Ann Intern Med.2001;134(1):1–11.
- ,,,.Narrative review: effect of bariatric surgery on type 2 diabetes mellitus.Ann Intern Med.2009;150(2):94–103.
- ,,.Metabolic consequences of bariatric surgery.J Clin Gastroenterol.2006;40(8):659–668.
- ,.Surgical approaches to obesity.Mayo Clin Proc.2006;81(10 suppl):S18–S24.
- ,,, et al.American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic 4(5 suppl):S109–S184.
- ,,.Long‐term results of laparoscopic sleeve gastrectomy for obesity.Ann Surg.2010;252(2):319–324.
- ,,,,.Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures.Am J Med.2008;121(10):885–893.
- ,.Gastrointestinal complications of laparoscopic Roux‐en‐Y gastric bypass surgery in patients who are morbidly obese: findings on radiography and CT.AJR Am J Roentgenol.2002;179(6):1437–1442.
- ,,,,.Gastrointestinal complications of laparoscopic Roux‐en‐Y gastric bypass surgery: clinical and imaging findings.Radiology.2002;223(3):625–632.
- ,,.Use of computed tomography in diagnosis of major postoperative gastrointestinal complications of laparoscopic Roux‐en‐Y gastric bypass surgery.Am Surg.2004;70(11):964–966.
- ,,, et al.Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity.J Am Coll Surg.2007;204(1):47–55.
- ,,,,.Small‐bowel obstruction after laparoscopic Roux‐en‐Y gastric bypass surgery.J Comput Assist Tomogr.2009;33(3):369–375.
- ,,,,,.Sensitivity and specificity of eight CT signs in the preoperative diagnosis of internal mesenteric hernia following Roux‐en‐Y gastric bypass surgery.Clin Radiol.2009;64(4):373–380.
- ,,, et al.Internal hernia after gastric bypass: sensitivity and specificity of seven CT signs with surgical correlation and controls.AJR Am J Roentgenol.2007;188(3):745–750.
- ,,,,.Nausea, bloating and abdominal pain in the Roux‐en‐Y gastric bypass patient: more questions than answers.Obes Surg.2007;17(11):1529–1533.
- ,,, et al.Management of acute bleeding after laparoscopic Roux‐en‐Y gastric bypass.Obes Surg.2003;13(6):842–847.
- ,,,,.Endoscopic balloon dilation of gastroenteric anastomotic stricture after laparoscopic gastric bypass.Endoscopy.2003;35(9):725–728.
- ,.Ulcer disease after gastric bypass surgery.Surg Obes Relat Dis.2006;2(4):455–459.
- ,,,.Marginal ulcer after gastric bypass: a prospective 3‐year study of 173 patients.Obes Surg.1998;8(5):505–516.
- ,,,,.Stomal complications of gastric bypass: incidence and outcome of therapy.Am J Gastroenterol.1992;87(9):1165–1169.
- ,,,,.[Analysis of the dumping syndrome on morbid obese patients submitted to Roux en Y gastric bypass].Rev Col Bras Cir.2009;36(5):413–419.
- ,,, et al.Clinical management after bariatric surgery: value of a multidisciplinary approach.Mayo Clin Proc.2006;81(10 suppl):S34–S45.
- ,,,,,.Antibiotic efficacy in small intestinal bacterial overgrowth‐related chronic diarrhea: a crossover, randomized trial.Gastroenterology.1999;117(4):794–797.
- ,,,,.Absorbable vs. non‐absorbable antibiotics in the treatment of small intestine bacterial overgrowth in patients with blind‐loop syndrome.Aliment Pharmacol Ther.2005;21(8):985–992.
- ,,, et al.Small intestinal bacterial overgrowth: diagnosis and treatment.Dig Dis.2007;25(3):237–240.
- ,,, et al.Antibiotic therapy in small intestinal bacterial overgrowth: rifaximin versus metronidazole.Eur Rev Med Pharmacol Sci.2009;13(2):111–116.
- ,,,.Treatment strategies for small bowel bacterial overgrowth in short bowel syndrome.J Pediatr Gastroenterol Nutr.1998;27(2):155–160.
- ,,,.Short bowel syndrome following bariatric surgical procedures.Am J Surg.2006;192(6):828–832.
- ,,,,.Postoperative short bowel syndrome.J Am Coll Surg.2005;201(1):85–89.
- ,,.D‐lactic acidosis. A review of clinical presentation, biochemical features, and pathophysiologic mechanisms.Medicine.1998;77(2):73–82.
- ,,, et al.Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials.Fam Pract.2006;23(3):279–285.
- ,,.Neuromuscular diseases and disorders of the alimentary system.Muscle Nerve.2002;25(6):768–784.
- .Nutritional deficiencies following bariatric surgery.General Surgery News: Obesity Care Special Edition.2007;65–72. Available at: http://www.generalsurgerynews.com/download/gsnse07issueWM.pdf.
- ,,,.Neurologic complications after surgery for obesity.Muscle Nerve.2006;33(2):166–176.
- ,,.Acquired hypocupremia after gastric surgery.Clin Gastroenterol Hepatol.2004;2(12):1074–1079.
- ,.Krause's Food 2008.
- ,.Nutritional consequences of bariatric surgery.Curr Opin Clin Nutr Metab Care.2006;9(4):489–496.
- ,,,,.Nutritional deficiencies following bariatric surgery: what have we learned?Obes Surg.2005;15(2):145–154.
- .Managing micronutrient deficiencies in the bariatric surgical patient.Obesity Management.2005;1(5):203–206. Available at: http://www.liebertonline.com/doi/pdf/10.1089/obe.2005.1.203.
- ,,, et al.Abnormalities of vitamin D and calcium metabolism after surgical treatment of morbid obesity: a study of 136 patients.Endocr Pract.2007;13(2):131–136.
- ,,.Vitamin D deficiency in adults: when to test and how to treat.Mayo Clinic Proc.85(8):752–757; quiz757–758.
- ,,, et al.Endocrine and nutritional management of the post‐bariatric surgery patient: an Endocrine Society Clinical Practice Guideline.J Clin Endocrinol Metab.2010;95(11):4823–4843.
- ,,.Small bowel obstruction after laparoscopic Roux‐en‐Y gastric bypass: a review of 9,527 patients.J Am Coll Surg.2008;206(3):571–584.
- ,,,,.Small‐bowel obstruction after laparoscopic Roux‐en‐Y gastric bypass: etiology, diagnosis, and management.Arch Surg.2007;142(10):988–993.
Obesity is a growing epidemic in the United States and worldwide. Over one‐third of Americans (33.8%) are considered obese (body mass index [BMI] 30).1 Nonsurgical interventions have failed to achieve the long‐lasting effects of weight loss surgery and the associated reduction in obesity‐related comorbidities such as type 2 diabetes mellitus, hyperlipidemia, hypertension, obstructive sleep apnea, cancer, coronary artery disease, osteoarthritis, and gastroesophageal reflux disease (GERD).27 The American Society for Metabolic and Bariatric Surgery estimates that 220,000 people underwent bariatric surgery in 2009 with over 1.5 million procedures performed since 1992.
Centers of excellence criteria include follow‐up with the bariatric surgeon for 5 years; however, the patient may be admitted to a hospital without immediate availability of the bariatric surgeon. Since hospitalists are often first responders to the majority of newly hospitalized patients, this growing number of post‐bariatric surgery patients necessitates hospitalists have a full understanding of their unique postoperative anatomical and physiological consequences. During the first hours of an acute inpatient presentation, post‐bariatric surgical patients can be divided into the following categories: surgical complications, surgical complications masquerading as acute medical conditions, and medical complications. Additionally, hospitalists should be aware of the nuances of radiographic imaging and appropriate endoscopic procedures in these patients. This article will discuss the common current bariatric surgical procedures; post‐bariatric surgery radiographic imaging pearls; and a review of the signs, symptoms, and treatment of common medical and surgical complications.
Descriptions of Contemporary Procedures
Contemporary weight loss procedures can be divided into 2 categories based on how they produce weight loss: restrictive only or combination malabsorptive with restriction. Most are performed laparoscopically to reduce postoperative pain, speed recovery, and decrease wound complications.
Restrictive Procedures (Laparoscopic Adjustable Gastric Band and Sleeve Gastrectomy)
These procedures produce weight loss by reducing the size of the stomach or creating an obstruction in the proximal stomach, limiting the consumption of large quantities at one time. They produce early satiety, but patients may still consume a large volume of calorie‐dense liquids compromising weight loss.
Laparoscopic Adjustable Gastric Band
Laparoscopic adjustable gastric band (LAGB; Figure 1A) is the primary form of restrictive procedures with 2 Food and Drug Administration‐approved bands (Lap Band [Allergan, Inc; Irvine, CA] and REALIZE band [Ethicon Endo‐Surgery, Inc; Cincinnati, OH]). A cuff is inflated around the proximal stomach creating a gastric pouch approximately 15‐30 mL in size. A subcutaneous reservoir is attached to the cuff allowing adjustment to the degree of restriction.8 LAGB has replaced the vertical banded gastroplasty (VBG). It is less invasive, adjustable, and reversible (0.1% operative mortality rate). Weight loss is maintained with this procedure but is generally less, with a higher failure rate compared to the more common gastric bypass procedure (Table 1).3, 9 Complications may include band dysfunction (ie, slippage, erosion, infections), esophageal dilatation, balloon failure, and port malposition, with rates approaching 3%‐5% per year requiring removal or repair.10 Patients may also experience GERD symptoms, especially if the condition was present preoperatively. Progressive GERD symptoms should be investigated with an upper gastrointestinal (GI) series to ensure there is no band slippage, esophageal dilation, or dysfunction.

| LAGB | Roux‐en‐Y Gastric Bypass | Biliopancreatic Diversion With and Without Duodenal Switch | |
|---|---|---|---|
| Excess weight loss | 48% | 62% | 70% |
| Resolution of diabetes | 48% | 84% | 98% |
Sleeve Gastrectomy
With the sleeve gastrectomy (Figure 1B) procedure, a small gastric tube is created by resecting the majority of the stomach. Early postoperative complications are comparable to those after Roux‐en‐Y gastric bypass (RYGB) operations. Leaks from the long gastric staple line are the most concerning. Recent report of a leak rate of 4.9% is much higher than contemporary reports of leaks after laparoscopic RYGB operations.11 Gastric tube stenosis is unique to the operation but comparable to gastrojejunal anastomotic stricture rates after RYGB. Weight loss is less than RYGB. Long‐term results from larger cohorts are needed to determine if the high incidence of esophageal complaints (GERD 26%, vomiting 21%), and weight regain issues are consistently experienced.
Combination Procedures (Roux‐en‐Y Gastric Bypass and Biliopancreatic Diversion With and Without Duodenal Switch)
These procedures produce weight loss by decreasing caloric intake and altering digestion and absorption.
Roux‐en‐Y Gastric Bypass
Roux‐en‐Y Gastric Bypass (RYGB) (Figure 1C) is the most common bariatric procedure performed in the United States. As the gold standard, it provides long‐term successful weight loss and a defined risk profile.9 This procedure involves the creation of a small (15‐30 mL) gastric pouch by transecting the stomach and then draining the pouch via a Roux limb. The Roux (aka alimentary) limb is the segment of bowel between the small gastric pouch and the jejunojejunostomy. Variations on this procedure include different length Roux limbs (75‐150 cm) and the use of a silastic ring at the gastrojejunal anastomosis. The latter is not commonly used because of the high incidence of band erosion. Weight loss seems to be independent of these variations. Postoperatively, food bypasses the biliopancreatic limb (ie, the stomach, duodenum, and part of the jejunum) resulting in selective malabsorption in the common channel (the segment distal to the jejunojejunostomy). Hormone secretions are altered, affecting satiety signaling and glucose metabolism.10, 12
Biliopancreatic Diversion With Duodenal Switch
In biliopancreatic diversion (BPD) with duodenal switch (DS) (Figure 1D), a sleeve gastrectomy is performed. The ileum is transected about 250 cm proximal to the ileocecal valve and is then attached to the transected duodenum just distal to the pylorus, forming the path for the food. The excluded duodenum, jejunum, and proximal ileum drain the biliary and pancreatic secretions and are reconnected to the distal ileum about 50‐100 cm proximal to the ileocecal valve. Food and digestive juices mix, allowing for absorption of nutrients over this short common channel. Greater malabsorption of calories, vitamins, and trace elements occurs, providing more reliable weight loss and significantly more nutritional problems.8, 9
Radiographic and Endoscopic Considerations
When evaluating abdominal complaints with radiographic imaging, the postoperative anatomic variations can challenge routine interpretation. An experienced radiologist and involvement of a bariatric surgeon, who is familiar with the post‐gastric bypass anatomical changes, are essential for accurate interpretation.
Computed tomography (CT) scans with oral contrast are the imaging modality of choice, particularly in the acute setting, to rule out small bowel obstruction. CT scans are helpful in delineating postoperative anatomy, detecting anastomotic leaks, obstructions and other intra‐abdominal problems.1315 Routine upper GI series (UGI) after gastric bypass is controversial, with some performing it routinely and others only for cause. Regardless, when UGI is performed, likewise for CT, small volumes of water‐soluble contrast should be used, followed by small volumes of dilute barium solution. A UGI may be complementary and more sensitive in the case of a small leak when done under fluoroscopy, but CT and UGI may not show the leak in as many as 30% of patients; CT scans may provide additional information to help guide the clinical decision making. A negative study should not preclude surgical exploration if a high suspicion for leak exists.16 Internal hernias (loop of bowel passing through a mesenteric defect created by the original surgery), a common cause of bowel obstructions, are frequently missed, therefore a high level of suspicion is necessary.1719 Several studies have identified 8 radiographic CT findings in bowel obstructions caused by internal hernias including swirl sign, mushroom sign, hurricane eye, small bowel obstruction, clustered loops, small bowel behind superior mesenteric artery, right‐side anastomosis, and engorged nodes.18, 19 The clinical picture should guide medical versus surgical management in those exceeding CT scanner weight limits (commonly 350 lb).
Imaging modalities such as UGI, endoscopy, or double balloon enteroscopy (DBE) should be used for patients with more chronic abdominal complaints. UGI may miss leaks and obstructions in the remnant stomach and bypassed intestine. If pathology, such as ulcers, retained sutures, and strictures are suspected in the bypassed stomach/emntestine, DBE can be used to diagnose and therapeutically intervene, but may not be available at all centers and referral may be considered. Endoscopy allows for direct visualization of subtle or mucosal pathology in the small bowel, but is unable to visualize the excluded stomach and duodenum.20
Early Medical and Surgical Complications
Early postoperative complications (within 30 days) occur in the minority of patients after weight loss operations. Clinical findings, even in life‐threatening conditions, may be subtle. Readmissions most often occur for dehydration secondary to inadequate oral intake. Pneumonias, and wound and urinary tract infections are not unique to the bariatric surgery patient, but there is a higher than average risk of pulmonary embolism and bleeding. Bleeding most frequently occurs into the GI tract from staple lines resulting in rapid catharsis or emesis, but can also be intraperitoneal and elusive. Most GI bleeding stops spontaneously, but some require transfusion and re‐exploration in extreme cases.21 Leaks may occur at any of the staple lines or anastomoses. The most common sites of leak are the g‐j anastomosis, gastric pouch, and remnant stomach. Again, remnant stomach and j‐j anastomosis leaks may escape detection by UGI and CT. Re‐exploration of a sick patient in the early postoperative period may be required despite normal imaging studies. Early consultation with, or transfer to, a bariatric surgery center should always be considered for patients readmitted after bariatric surgery.
Late Medical Complications
Gastrointestinal complaints, excessive weight loss, and vitamin/mineral deficiencies resulting in neurological problems and metabolic bone disease are post‐bariatric medical complications that may prompt hospital admission. If not the primary reason for admission, special attention to these issues may prevent readmission, another focus of hospital care.
Gastrointestinal Complaints
One of the most common causes of hospital admission any time postoperatively is abdominal pain. A differential diagnosis of abdominal pain, nausea, and/or vomiting in the post‐bariatric surgery patient should include small bowel obstruction, hernias (internal or incisional), band complications, food intolerance, dietary noncompliance, ileus, mesenteric venous thrombosis, strictures (such as outlet obstruction or anastomotic stenosis), ulcers, esophagitis, cholelithiasis, dumping syndrome, and Roux stasis syndrome.20
A thorough history targeted at the relationship between symptoms and food intake, attention to the character and location of the pain, and a thorough physical exam (specifically the presence or absence of palpable tenderness, guarding, or rebound) is essential. The physical exam may be misleading in obese patients and, if radiographic studies cannot be performed secondary to patient size, surgical exploration may be needed soon after presentation. Therefore, even lacking an obvious surgical need, the bariatric surgeon should be notified of admission.
Improper food choice, and failure to slowly and adequately chew food, can result in emesis and digestive difficulty. Physical incompatibility with the small gastric pouch and gastric outlet obstructions can be caused by nondigestible foods (ie, breads, steak, raw vegetables). This highlights the importance of ordering the appropriate hospital diet.8 Specific gastric bypass hospital diets for all consistencies should reflect the mechanical limitations and carbohydrate/protein requirements of these patients.
Increased gallstone formation is observed in patients with rapid weight loss (1.5 kg/wk), especially following RYGB and less often after LAGB procedures (40% vs 20% over 3 years). Routine use of ursodiol during rapid weight loss (6 months after RYGB) reduces this complication to 5%.8
Stenosis or ulceration at the anastomotic site for RYGB can cause abdominal pain and vomiting. The incidence of stomal stenosis has been reported at 5%‐19% and typically occurs within the first 3 postoperative months.22 This problem is often amenable to endoscopic dilatation, unless a ring was used to reinforce the anastomosis. Ulceration has been reported in 1%‐16% of patients and is usually secondary to tobacco or non‐steroidal anti‐inflammatory drug (NSAID) use, H. pylori, fistula‐induced acid exposure, reaction to foreign material, or ischemia from tension and poor tissue perfusion.23, 24 Endoscopy can diagnose the presence of ulcers, with biopsies to rule out H. pylori infection. Cessation of NSAIDs and tobacco are critical. Medical management including proton pump inhibitors and/or sucralfate is sufficient for up to 95% of patients. Surgical revision is reserved for persistent ulcers associated with obstruction, pain, and/or bleeding.25
Dumping syndrome is a complex of post‐prandial symptoms occurring most commonly in the RYGB patients. As many as 44% of RYGB patients may experience this syndrome characterized by flushing, dizziness, abdominal distension, pain, nausea, vomiting, and/or diarrhea.26 Symptoms may result from the ingestion of large amounts of sugars which empty from the altered gastric pouch at an unregulated rate. This large osmotic load causes fluid shifts and surges in peptide hormone levels, resulting in symptoms which may reinforce adherence to the prescribed postoperative diet. It occurs shortly after a meal and resolves over hours. Dietary modifications, such as increased protein and fiber intake with decreased consumption of simple sugars, will ameliorate symptoms in many patients, with most seeing resolution after the first year.8, 27 Some patients experience hyperglycemia secondary to ingestion of simple carbohydrates, with hypoglycemia approximately 2 hours later (late dumping). In our experience, limiting carbohydrate intake to 30 grams at any meal usually alleviates post‐prandial hypoglycemia.
If the patient reports an absence of bowel movements and flatus, an ileus from chronic narcotic use or a mechanical small bowel obstruction secondary to internal hernias or adhesions (see Late Surgical Complications) must be investigated. Severe or prolonged pain, lasting longer than a few hours, is cause for alarm and should prompt aggressive evaluation and possibly exploratory surgery.
Excessive Weight Loss
In diagnosing postoperative excessive weight loss, it is important to understand average anticipated weight loss parameters. Compared to the values expected for RYGB, LAGB produces less weight loss and BPD with and without DS produces more (Table 227). Patients experiencing more rapid or prolonged weight loss should be investigated for bacterial overgrowth syndrome, short bowel syndrome, or other anatomic abnormalities.
| Postoperative Time Period | Average Weight Loss (RYGB) | |
|---|---|---|
| Daily | By Time Period | |
| ||
| 0‐3 mo | 0.22‐0.45 kg/day | 15‐20 kg by 3 mo |
| 3‐9 mo | 0.11‐0.22 kg/day | 25‐35 kg by 6 mo |
| 9‐12 mo | 0.11 kg/day | 40‐60 kg in first year |
Known risk factors for bacterial overgrowth, which are prominent in this population, include decreased gastric acidity and slowed intestinal transit time (ie, narcotic use). Patients may be asymptomatic or experience weight loss, abdominal bloating and/or pain, nausea, vomiting, and diarrhea. The diagnosis can be made with a hydrogen breath test or by obtaining quantitative cultures of jejunal secretions during endoscopy. Questions remain on how the normalized values of these tests are affected by the postoperative environment, and on how this syndrome may present or be treated if it affects the excluded intestine. Bacterial overgrowth may be an incidental finding and not the cause of the gastrointestinal complaints. Although data is limited, treatment typically consists of a 7‐10 day course of rifaximin 1200 mg/day (divided doses) and/or a trial of dietary modifications.20, 2831 These may include avoiding lactose and eating a high fat, low carbohydrate, low fiber diet, so nutrients are readily absorbed and not left for bacterial consumption.32
Short bowel syndrome (100‐200 cm of intestinal tissue remaining and subsequent malabsorption) can occur after any extensive colonic resection or bypass of the intestine.33 This condition rarely results after an initial bariatric procedure; however, subsequent procedures for small bowel obstructions or intestinal ischemia may result in short bowel syndrome. Typical presentations include diarrhea, weight loss, and symptoms of vitamin and mineral deficiencies. Short bowel can also predispose patients to the development of bacterial overgrowth, further complicating weight loss. Management consists of nutritional supplementation, occasionally parenteral nutrition, and rarely reoperation to increase the length of the common channel.34 Avoidance of further bowel resection is crucial in preventing short bowel syndrome.33, 34 In the setting of carbohydrate malabsorption with concomitant bacterial overgrowth syndrome, production of d‐lactic acid causing a metabolic acidosis with encephalopathy has been reported.35
Once medical complications have been ruled out, it is prudent to evaluate for a psychological component such as anorexia nervosa. It is helpful to involve a qualified psychologist who is familiar with this population. Addictions to alcohol, gambling, and pain medications have been reported in the post‐bariatric surgery population as a substitute for food addiction.
Neurological Complications and Vitamin Deficiencies
Neurological complications develop months to years postoperatively, secondary to vitamin, mineral, and nutrient deficiencies that result from malabsorption or inadequate intake. An inpatient provider should be aware of the potential role these conditions may play in a hospitalized patient.
Peripheral neuropathy can develop secondary to several deficiencies, including vitamin B12, thiamine, vitamin E, and copper. Their sources, deficiencies, and replacement regimens are presented in Table 3.3642 Thiamine deficiency, manifesting as Wernicke's encephalopathy, is particularly important in the postoperative patient with excessive vomiting. For prevention, we recommend all patients readmitted with vomiting and dehydration receive a banana bag or rally pack (thiamine 100 mg, folic acid 1 mg, multivitamin with iron and magnesium 3 g in one liter of D5 normal saline) over 4‐8 hours. Additional deficiencies after gastric bypass include folate, selenium, zinc, vitamin B6, and riboflavin. A multivitamin with minerals will meet the needs of most patients. Multiple fat‐soluble vitamin deficiencies can occur with small bowel bacterial overgrowth or BPD.
| B12 | B1 (Thiamine) | Vitamin E | Copper | |
|---|---|---|---|---|
| Dietary sources | Meat and dairy | Fortified grains, cereals, nuts, and pork | Vegetable oil, nuts, leafy vegetables39 | Shellfish, organ meats (liver, kidney), chocolate, nuts, dried legumes/fruits41 |
| Location of absorption | Terminal ileum after combining with intrinsic factor | Proximal small intestine | Upper small intestine41 | Stomach and duodenum38 |
| Mechanism of deficiency | Inadequate intake intrinsic factor deficiency37, 39 | Bypass of primary absorption site Inadequate intake Excessive emesis | Fat malabsorption39 Inadequate intake | Defective intestinal mucosal transport40 Decreased absorptive surface area40 Inadequate intake Coadministered zinc which competes with copper for absorption38 |
| Time to develop deficiency | Years | 18 days37, 39 | 6‐12 mo39 | 3‐12 mo42 |
| Postoperative supplementation recommendation | Optimal prophylactic dose unknown Minimum 1‐2 mg/day | 1‐1.5 mg/day37 | Males: 10 mg/day Females: 8 mg/day | Multivitamin (900 g/day) |
| Pathology of deficiency | Macrocytic anemia Paresthesias Ataxia Subacute combined degeneration of the spinal cord | Dry beriberi Wernicke's encephalopathy Korsakoff's syndrome | Myopathy/neuropathy Ataxia | Demyelinating neuropathy with ataxia Anemia |
| Labs to document deficiency | Serum B12 | Erythrocyte transketolase activity Thiamine diphosphate effect37 | Serum alphatocopherol37, 39 Check for deficiencies of other fat soluble vitamins (A, D, K) | Serum copper level40 |
| Correcting deficiency | Intramuscular B12 (1000 mcg) Sublingual supplementation36 | 50‐100 mg/day (parenteral or oral)37 | 400 mg PO BID37 | 2‐4 mg/day38 |
Anemia
Iron deficiency affects 6%‐33% of patients after 1 year.43 Iron is preferentially absorbed in the duodenum and proximal jejunum which are bypassed postoperatively. The absence of gastric acid prevents conversion of ferric (Fe2+) to the absorbable ferrous (Fe3+) iron, further decreasing absorption.44 Ferritin reflects iron stores but is also an acute phase reactant and, therefore, may mask an underlying deficiency in an acutely ill hospitalized patient. A multivitamin with iron is recommended for all patients, but additional supplementation may be required for menstruating women.43 Parenteral administration may be necessary if oral supplements are not tolerated or are inadequately absorbed.44
Fractures and Osteomalacia
Calcium and vitamin D deficiencies are a significant problem in the bariatric surgery population, with resultant osteoporosis or osteomalacia and associated fractures.38, 43 Calcium is preferentially absorbed in the duodenum and proximal jejunum. Vitamin D is absorbed in the ileum or produced in the skin in response to ultraviolet B (UVB) radiation.45 Deficiency of vitamin D exacerbates calcium malabsorption, thereby causing secondary hyperparathyroidism, increased bone turnover, and osteomalacia. Dramatic weight loss can lead to bone loss, increasing the risk for osteoporosis and fractures.38 Hypocalcemia or osteomalacia may cause generalized bone pain, muscular weakness, tetany, and chronic musculoskeletal pain.45
Fat‐soluble vitamin deficiencies are more common in those undergoing malabsorptive versus restrictive procedures and, in the case of BPD, may be related to the length of the common channel.43 It is important to ensure that calcium and vitamin D levels are sufficient prior to surgery, and prior to starting any osteoporotic treatment such as bisphosphonates.45 We recommend at least 1200 mg of calcium citrate and 1000‐2000 IU of Vitamin D daily. Up to 50,000 IU weekly or daily may be required to correct deficiency and maintain sufficiency in this population.46, 47 Vitamin D2 (ergocalciferol) or D3 (cholecalciferol) can be used for supplementation. Cholecalciferol is preferred if given through a feeding tube because it is less prone to clogging the tube.46 With severe malabsorption, phototherapy may be necessary, as intravenous doses are often inadequate and intramuscular preparations require special compounding.46 Calcium carbonate requires acid for proper absorption, therefore calcium citrate may be preferred due to achlorhydria from gastric exclusion.
Late Surgical Complications
Hospitalists are increasingly responsible for managing and comanaging surgical patients. The post‐bariatric surgery patient may present with unique signs and symptoms of surgical conditions masquerading as medical conditions. Common conditions that present in uncommon ways include strictures (ie, outlet obstruction and stomal stenosis), hernias with strangulation (incisional and internal), and small bowel obstructions.
Small bowel obstruction (SBO) occurs in 0%‐5% of RYGB patients (less with LAGB, similar with BPD), which is similar to other abdominal surgery rates, and may occur months to years after the original surgery. The differential diagnosis of an SBO includes internal hernias, adhesions, ventral hernia (incisional and umbilical), postoperative ileus, and jejunojejunal anastomotic stricture. Typical symptoms are often present, but may be less obvious than with a non‐gastric bypass patient. Pain can range from acute to a chronic or intermittent pattern. Pain is the most common presenting symptom of obstruction. Pain relieved by emesis may indicate an obstruction in the Roux limb. Nausea, bloating, tachycardia, and hiccups with shoulder/back pain can occur when obstruction in the biliopancreatic limb causes gastric distension.48
Vomiting is seen in fewer than half of patients with SBOs due to the altered anatomy.49 Any post‐RYGB patient that vomits bile needs emergent surgical evaluation for a common channel obstruction. Radiographic imaging may be misleading as to the cause of the obstruction. SBO is crucial to consider since delayed diagnosis can result in bowel ischemia and death.18 For the hospitalist who is caring for a post‐bariatric patient with a bowel obstruction, early surgical consultation is mandatory, preferably with a bariatric surgeon. Traditional medical management such as nasogastric (NG) tube placement will not decompress the excluded stomach, therefore patients rarely benefit from nasogastric decompression. If necessary, an NG tube should only be placed by experienced hands or fluoroscopic guidance, due to the altered anatomy.
Conclusion
Weight loss surgery, developed to address the growing obesity problem, has been beneficial to hundreds of thousands of people by decreasing their excess weight and comorbidities. For some, the postoperative course is complicated by medical and surgical problems requiring hospitalization. It is critically important that, as this relatively new field of postoperative medicine evolves, the hospitalist stay informed on relevant presentations, complications, and treatment to better address this growing population. Early consultation with, and transfer to, a bariatric surgery center should be encouraged. The importance of arranging proper hospital follow‐up, including community‐based support groups, nutritional consults, psychological support, and close follow‐up with the bariatric surgeon, bariatrician, and/or primary care physician, should not be underestimated.
Obesity is a growing epidemic in the United States and worldwide. Over one‐third of Americans (33.8%) are considered obese (body mass index [BMI] 30).1 Nonsurgical interventions have failed to achieve the long‐lasting effects of weight loss surgery and the associated reduction in obesity‐related comorbidities such as type 2 diabetes mellitus, hyperlipidemia, hypertension, obstructive sleep apnea, cancer, coronary artery disease, osteoarthritis, and gastroesophageal reflux disease (GERD).27 The American Society for Metabolic and Bariatric Surgery estimates that 220,000 people underwent bariatric surgery in 2009 with over 1.5 million procedures performed since 1992.
Centers of excellence criteria include follow‐up with the bariatric surgeon for 5 years; however, the patient may be admitted to a hospital without immediate availability of the bariatric surgeon. Since hospitalists are often first responders to the majority of newly hospitalized patients, this growing number of post‐bariatric surgery patients necessitates hospitalists have a full understanding of their unique postoperative anatomical and physiological consequences. During the first hours of an acute inpatient presentation, post‐bariatric surgical patients can be divided into the following categories: surgical complications, surgical complications masquerading as acute medical conditions, and medical complications. Additionally, hospitalists should be aware of the nuances of radiographic imaging and appropriate endoscopic procedures in these patients. This article will discuss the common current bariatric surgical procedures; post‐bariatric surgery radiographic imaging pearls; and a review of the signs, symptoms, and treatment of common medical and surgical complications.
Descriptions of Contemporary Procedures
Contemporary weight loss procedures can be divided into 2 categories based on how they produce weight loss: restrictive only or combination malabsorptive with restriction. Most are performed laparoscopically to reduce postoperative pain, speed recovery, and decrease wound complications.
Restrictive Procedures (Laparoscopic Adjustable Gastric Band and Sleeve Gastrectomy)
These procedures produce weight loss by reducing the size of the stomach or creating an obstruction in the proximal stomach, limiting the consumption of large quantities at one time. They produce early satiety, but patients may still consume a large volume of calorie‐dense liquids compromising weight loss.
Laparoscopic Adjustable Gastric Band
Laparoscopic adjustable gastric band (LAGB; Figure 1A) is the primary form of restrictive procedures with 2 Food and Drug Administration‐approved bands (Lap Band [Allergan, Inc; Irvine, CA] and REALIZE band [Ethicon Endo‐Surgery, Inc; Cincinnati, OH]). A cuff is inflated around the proximal stomach creating a gastric pouch approximately 15‐30 mL in size. A subcutaneous reservoir is attached to the cuff allowing adjustment to the degree of restriction.8 LAGB has replaced the vertical banded gastroplasty (VBG). It is less invasive, adjustable, and reversible (0.1% operative mortality rate). Weight loss is maintained with this procedure but is generally less, with a higher failure rate compared to the more common gastric bypass procedure (Table 1).3, 9 Complications may include band dysfunction (ie, slippage, erosion, infections), esophageal dilatation, balloon failure, and port malposition, with rates approaching 3%‐5% per year requiring removal or repair.10 Patients may also experience GERD symptoms, especially if the condition was present preoperatively. Progressive GERD symptoms should be investigated with an upper gastrointestinal (GI) series to ensure there is no band slippage, esophageal dilation, or dysfunction.

| LAGB | Roux‐en‐Y Gastric Bypass | Biliopancreatic Diversion With and Without Duodenal Switch | |
|---|---|---|---|
| Excess weight loss | 48% | 62% | 70% |
| Resolution of diabetes | 48% | 84% | 98% |
Sleeve Gastrectomy
With the sleeve gastrectomy (Figure 1B) procedure, a small gastric tube is created by resecting the majority of the stomach. Early postoperative complications are comparable to those after Roux‐en‐Y gastric bypass (RYGB) operations. Leaks from the long gastric staple line are the most concerning. Recent report of a leak rate of 4.9% is much higher than contemporary reports of leaks after laparoscopic RYGB operations.11 Gastric tube stenosis is unique to the operation but comparable to gastrojejunal anastomotic stricture rates after RYGB. Weight loss is less than RYGB. Long‐term results from larger cohorts are needed to determine if the high incidence of esophageal complaints (GERD 26%, vomiting 21%), and weight regain issues are consistently experienced.
Combination Procedures (Roux‐en‐Y Gastric Bypass and Biliopancreatic Diversion With and Without Duodenal Switch)
These procedures produce weight loss by decreasing caloric intake and altering digestion and absorption.
Roux‐en‐Y Gastric Bypass
Roux‐en‐Y Gastric Bypass (RYGB) (Figure 1C) is the most common bariatric procedure performed in the United States. As the gold standard, it provides long‐term successful weight loss and a defined risk profile.9 This procedure involves the creation of a small (15‐30 mL) gastric pouch by transecting the stomach and then draining the pouch via a Roux limb. The Roux (aka alimentary) limb is the segment of bowel between the small gastric pouch and the jejunojejunostomy. Variations on this procedure include different length Roux limbs (75‐150 cm) and the use of a silastic ring at the gastrojejunal anastomosis. The latter is not commonly used because of the high incidence of band erosion. Weight loss seems to be independent of these variations. Postoperatively, food bypasses the biliopancreatic limb (ie, the stomach, duodenum, and part of the jejunum) resulting in selective malabsorption in the common channel (the segment distal to the jejunojejunostomy). Hormone secretions are altered, affecting satiety signaling and glucose metabolism.10, 12
Biliopancreatic Diversion With Duodenal Switch
In biliopancreatic diversion (BPD) with duodenal switch (DS) (Figure 1D), a sleeve gastrectomy is performed. The ileum is transected about 250 cm proximal to the ileocecal valve and is then attached to the transected duodenum just distal to the pylorus, forming the path for the food. The excluded duodenum, jejunum, and proximal ileum drain the biliary and pancreatic secretions and are reconnected to the distal ileum about 50‐100 cm proximal to the ileocecal valve. Food and digestive juices mix, allowing for absorption of nutrients over this short common channel. Greater malabsorption of calories, vitamins, and trace elements occurs, providing more reliable weight loss and significantly more nutritional problems.8, 9
Radiographic and Endoscopic Considerations
When evaluating abdominal complaints with radiographic imaging, the postoperative anatomic variations can challenge routine interpretation. An experienced radiologist and involvement of a bariatric surgeon, who is familiar with the post‐gastric bypass anatomical changes, are essential for accurate interpretation.
Computed tomography (CT) scans with oral contrast are the imaging modality of choice, particularly in the acute setting, to rule out small bowel obstruction. CT scans are helpful in delineating postoperative anatomy, detecting anastomotic leaks, obstructions and other intra‐abdominal problems.1315 Routine upper GI series (UGI) after gastric bypass is controversial, with some performing it routinely and others only for cause. Regardless, when UGI is performed, likewise for CT, small volumes of water‐soluble contrast should be used, followed by small volumes of dilute barium solution. A UGI may be complementary and more sensitive in the case of a small leak when done under fluoroscopy, but CT and UGI may not show the leak in as many as 30% of patients; CT scans may provide additional information to help guide the clinical decision making. A negative study should not preclude surgical exploration if a high suspicion for leak exists.16 Internal hernias (loop of bowel passing through a mesenteric defect created by the original surgery), a common cause of bowel obstructions, are frequently missed, therefore a high level of suspicion is necessary.1719 Several studies have identified 8 radiographic CT findings in bowel obstructions caused by internal hernias including swirl sign, mushroom sign, hurricane eye, small bowel obstruction, clustered loops, small bowel behind superior mesenteric artery, right‐side anastomosis, and engorged nodes.18, 19 The clinical picture should guide medical versus surgical management in those exceeding CT scanner weight limits (commonly 350 lb).
Imaging modalities such as UGI, endoscopy, or double balloon enteroscopy (DBE) should be used for patients with more chronic abdominal complaints. UGI may miss leaks and obstructions in the remnant stomach and bypassed intestine. If pathology, such as ulcers, retained sutures, and strictures are suspected in the bypassed stomach/emntestine, DBE can be used to diagnose and therapeutically intervene, but may not be available at all centers and referral may be considered. Endoscopy allows for direct visualization of subtle or mucosal pathology in the small bowel, but is unable to visualize the excluded stomach and duodenum.20
Early Medical and Surgical Complications
Early postoperative complications (within 30 days) occur in the minority of patients after weight loss operations. Clinical findings, even in life‐threatening conditions, may be subtle. Readmissions most often occur for dehydration secondary to inadequate oral intake. Pneumonias, and wound and urinary tract infections are not unique to the bariatric surgery patient, but there is a higher than average risk of pulmonary embolism and bleeding. Bleeding most frequently occurs into the GI tract from staple lines resulting in rapid catharsis or emesis, but can also be intraperitoneal and elusive. Most GI bleeding stops spontaneously, but some require transfusion and re‐exploration in extreme cases.21 Leaks may occur at any of the staple lines or anastomoses. The most common sites of leak are the g‐j anastomosis, gastric pouch, and remnant stomach. Again, remnant stomach and j‐j anastomosis leaks may escape detection by UGI and CT. Re‐exploration of a sick patient in the early postoperative period may be required despite normal imaging studies. Early consultation with, or transfer to, a bariatric surgery center should always be considered for patients readmitted after bariatric surgery.
Late Medical Complications
Gastrointestinal complaints, excessive weight loss, and vitamin/mineral deficiencies resulting in neurological problems and metabolic bone disease are post‐bariatric medical complications that may prompt hospital admission. If not the primary reason for admission, special attention to these issues may prevent readmission, another focus of hospital care.
Gastrointestinal Complaints
One of the most common causes of hospital admission any time postoperatively is abdominal pain. A differential diagnosis of abdominal pain, nausea, and/or vomiting in the post‐bariatric surgery patient should include small bowel obstruction, hernias (internal or incisional), band complications, food intolerance, dietary noncompliance, ileus, mesenteric venous thrombosis, strictures (such as outlet obstruction or anastomotic stenosis), ulcers, esophagitis, cholelithiasis, dumping syndrome, and Roux stasis syndrome.20
A thorough history targeted at the relationship between symptoms and food intake, attention to the character and location of the pain, and a thorough physical exam (specifically the presence or absence of palpable tenderness, guarding, or rebound) is essential. The physical exam may be misleading in obese patients and, if radiographic studies cannot be performed secondary to patient size, surgical exploration may be needed soon after presentation. Therefore, even lacking an obvious surgical need, the bariatric surgeon should be notified of admission.
Improper food choice, and failure to slowly and adequately chew food, can result in emesis and digestive difficulty. Physical incompatibility with the small gastric pouch and gastric outlet obstructions can be caused by nondigestible foods (ie, breads, steak, raw vegetables). This highlights the importance of ordering the appropriate hospital diet.8 Specific gastric bypass hospital diets for all consistencies should reflect the mechanical limitations and carbohydrate/protein requirements of these patients.
Increased gallstone formation is observed in patients with rapid weight loss (1.5 kg/wk), especially following RYGB and less often after LAGB procedures (40% vs 20% over 3 years). Routine use of ursodiol during rapid weight loss (6 months after RYGB) reduces this complication to 5%.8
Stenosis or ulceration at the anastomotic site for RYGB can cause abdominal pain and vomiting. The incidence of stomal stenosis has been reported at 5%‐19% and typically occurs within the first 3 postoperative months.22 This problem is often amenable to endoscopic dilatation, unless a ring was used to reinforce the anastomosis. Ulceration has been reported in 1%‐16% of patients and is usually secondary to tobacco or non‐steroidal anti‐inflammatory drug (NSAID) use, H. pylori, fistula‐induced acid exposure, reaction to foreign material, or ischemia from tension and poor tissue perfusion.23, 24 Endoscopy can diagnose the presence of ulcers, with biopsies to rule out H. pylori infection. Cessation of NSAIDs and tobacco are critical. Medical management including proton pump inhibitors and/or sucralfate is sufficient for up to 95% of patients. Surgical revision is reserved for persistent ulcers associated with obstruction, pain, and/or bleeding.25
Dumping syndrome is a complex of post‐prandial symptoms occurring most commonly in the RYGB patients. As many as 44% of RYGB patients may experience this syndrome characterized by flushing, dizziness, abdominal distension, pain, nausea, vomiting, and/or diarrhea.26 Symptoms may result from the ingestion of large amounts of sugars which empty from the altered gastric pouch at an unregulated rate. This large osmotic load causes fluid shifts and surges in peptide hormone levels, resulting in symptoms which may reinforce adherence to the prescribed postoperative diet. It occurs shortly after a meal and resolves over hours. Dietary modifications, such as increased protein and fiber intake with decreased consumption of simple sugars, will ameliorate symptoms in many patients, with most seeing resolution after the first year.8, 27 Some patients experience hyperglycemia secondary to ingestion of simple carbohydrates, with hypoglycemia approximately 2 hours later (late dumping). In our experience, limiting carbohydrate intake to 30 grams at any meal usually alleviates post‐prandial hypoglycemia.
If the patient reports an absence of bowel movements and flatus, an ileus from chronic narcotic use or a mechanical small bowel obstruction secondary to internal hernias or adhesions (see Late Surgical Complications) must be investigated. Severe or prolonged pain, lasting longer than a few hours, is cause for alarm and should prompt aggressive evaluation and possibly exploratory surgery.
Excessive Weight Loss
In diagnosing postoperative excessive weight loss, it is important to understand average anticipated weight loss parameters. Compared to the values expected for RYGB, LAGB produces less weight loss and BPD with and without DS produces more (Table 227). Patients experiencing more rapid or prolonged weight loss should be investigated for bacterial overgrowth syndrome, short bowel syndrome, or other anatomic abnormalities.
| Postoperative Time Period | Average Weight Loss (RYGB) | |
|---|---|---|
| Daily | By Time Period | |
| ||
| 0‐3 mo | 0.22‐0.45 kg/day | 15‐20 kg by 3 mo |
| 3‐9 mo | 0.11‐0.22 kg/day | 25‐35 kg by 6 mo |
| 9‐12 mo | 0.11 kg/day | 40‐60 kg in first year |
Known risk factors for bacterial overgrowth, which are prominent in this population, include decreased gastric acidity and slowed intestinal transit time (ie, narcotic use). Patients may be asymptomatic or experience weight loss, abdominal bloating and/or pain, nausea, vomiting, and diarrhea. The diagnosis can be made with a hydrogen breath test or by obtaining quantitative cultures of jejunal secretions during endoscopy. Questions remain on how the normalized values of these tests are affected by the postoperative environment, and on how this syndrome may present or be treated if it affects the excluded intestine. Bacterial overgrowth may be an incidental finding and not the cause of the gastrointestinal complaints. Although data is limited, treatment typically consists of a 7‐10 day course of rifaximin 1200 mg/day (divided doses) and/or a trial of dietary modifications.20, 2831 These may include avoiding lactose and eating a high fat, low carbohydrate, low fiber diet, so nutrients are readily absorbed and not left for bacterial consumption.32
Short bowel syndrome (100‐200 cm of intestinal tissue remaining and subsequent malabsorption) can occur after any extensive colonic resection or bypass of the intestine.33 This condition rarely results after an initial bariatric procedure; however, subsequent procedures for small bowel obstructions or intestinal ischemia may result in short bowel syndrome. Typical presentations include diarrhea, weight loss, and symptoms of vitamin and mineral deficiencies. Short bowel can also predispose patients to the development of bacterial overgrowth, further complicating weight loss. Management consists of nutritional supplementation, occasionally parenteral nutrition, and rarely reoperation to increase the length of the common channel.34 Avoidance of further bowel resection is crucial in preventing short bowel syndrome.33, 34 In the setting of carbohydrate malabsorption with concomitant bacterial overgrowth syndrome, production of d‐lactic acid causing a metabolic acidosis with encephalopathy has been reported.35
Once medical complications have been ruled out, it is prudent to evaluate for a psychological component such as anorexia nervosa. It is helpful to involve a qualified psychologist who is familiar with this population. Addictions to alcohol, gambling, and pain medications have been reported in the post‐bariatric surgery population as a substitute for food addiction.
Neurological Complications and Vitamin Deficiencies
Neurological complications develop months to years postoperatively, secondary to vitamin, mineral, and nutrient deficiencies that result from malabsorption or inadequate intake. An inpatient provider should be aware of the potential role these conditions may play in a hospitalized patient.
Peripheral neuropathy can develop secondary to several deficiencies, including vitamin B12, thiamine, vitamin E, and copper. Their sources, deficiencies, and replacement regimens are presented in Table 3.3642 Thiamine deficiency, manifesting as Wernicke's encephalopathy, is particularly important in the postoperative patient with excessive vomiting. For prevention, we recommend all patients readmitted with vomiting and dehydration receive a banana bag or rally pack (thiamine 100 mg, folic acid 1 mg, multivitamin with iron and magnesium 3 g in one liter of D5 normal saline) over 4‐8 hours. Additional deficiencies after gastric bypass include folate, selenium, zinc, vitamin B6, and riboflavin. A multivitamin with minerals will meet the needs of most patients. Multiple fat‐soluble vitamin deficiencies can occur with small bowel bacterial overgrowth or BPD.
| B12 | B1 (Thiamine) | Vitamin E | Copper | |
|---|---|---|---|---|
| Dietary sources | Meat and dairy | Fortified grains, cereals, nuts, and pork | Vegetable oil, nuts, leafy vegetables39 | Shellfish, organ meats (liver, kidney), chocolate, nuts, dried legumes/fruits41 |
| Location of absorption | Terminal ileum after combining with intrinsic factor | Proximal small intestine | Upper small intestine41 | Stomach and duodenum38 |
| Mechanism of deficiency | Inadequate intake intrinsic factor deficiency37, 39 | Bypass of primary absorption site Inadequate intake Excessive emesis | Fat malabsorption39 Inadequate intake | Defective intestinal mucosal transport40 Decreased absorptive surface area40 Inadequate intake Coadministered zinc which competes with copper for absorption38 |
| Time to develop deficiency | Years | 18 days37, 39 | 6‐12 mo39 | 3‐12 mo42 |
| Postoperative supplementation recommendation | Optimal prophylactic dose unknown Minimum 1‐2 mg/day | 1‐1.5 mg/day37 | Males: 10 mg/day Females: 8 mg/day | Multivitamin (900 g/day) |
| Pathology of deficiency | Macrocytic anemia Paresthesias Ataxia Subacute combined degeneration of the spinal cord | Dry beriberi Wernicke's encephalopathy Korsakoff's syndrome | Myopathy/neuropathy Ataxia | Demyelinating neuropathy with ataxia Anemia |
| Labs to document deficiency | Serum B12 | Erythrocyte transketolase activity Thiamine diphosphate effect37 | Serum alphatocopherol37, 39 Check for deficiencies of other fat soluble vitamins (A, D, K) | Serum copper level40 |
| Correcting deficiency | Intramuscular B12 (1000 mcg) Sublingual supplementation36 | 50‐100 mg/day (parenteral or oral)37 | 400 mg PO BID37 | 2‐4 mg/day38 |
Anemia
Iron deficiency affects 6%‐33% of patients after 1 year.43 Iron is preferentially absorbed in the duodenum and proximal jejunum which are bypassed postoperatively. The absence of gastric acid prevents conversion of ferric (Fe2+) to the absorbable ferrous (Fe3+) iron, further decreasing absorption.44 Ferritin reflects iron stores but is also an acute phase reactant and, therefore, may mask an underlying deficiency in an acutely ill hospitalized patient. A multivitamin with iron is recommended for all patients, but additional supplementation may be required for menstruating women.43 Parenteral administration may be necessary if oral supplements are not tolerated or are inadequately absorbed.44
Fractures and Osteomalacia
Calcium and vitamin D deficiencies are a significant problem in the bariatric surgery population, with resultant osteoporosis or osteomalacia and associated fractures.38, 43 Calcium is preferentially absorbed in the duodenum and proximal jejunum. Vitamin D is absorbed in the ileum or produced in the skin in response to ultraviolet B (UVB) radiation.45 Deficiency of vitamin D exacerbates calcium malabsorption, thereby causing secondary hyperparathyroidism, increased bone turnover, and osteomalacia. Dramatic weight loss can lead to bone loss, increasing the risk for osteoporosis and fractures.38 Hypocalcemia or osteomalacia may cause generalized bone pain, muscular weakness, tetany, and chronic musculoskeletal pain.45
Fat‐soluble vitamin deficiencies are more common in those undergoing malabsorptive versus restrictive procedures and, in the case of BPD, may be related to the length of the common channel.43 It is important to ensure that calcium and vitamin D levels are sufficient prior to surgery, and prior to starting any osteoporotic treatment such as bisphosphonates.45 We recommend at least 1200 mg of calcium citrate and 1000‐2000 IU of Vitamin D daily. Up to 50,000 IU weekly or daily may be required to correct deficiency and maintain sufficiency in this population.46, 47 Vitamin D2 (ergocalciferol) or D3 (cholecalciferol) can be used for supplementation. Cholecalciferol is preferred if given through a feeding tube because it is less prone to clogging the tube.46 With severe malabsorption, phototherapy may be necessary, as intravenous doses are often inadequate and intramuscular preparations require special compounding.46 Calcium carbonate requires acid for proper absorption, therefore calcium citrate may be preferred due to achlorhydria from gastric exclusion.
Late Surgical Complications
Hospitalists are increasingly responsible for managing and comanaging surgical patients. The post‐bariatric surgery patient may present with unique signs and symptoms of surgical conditions masquerading as medical conditions. Common conditions that present in uncommon ways include strictures (ie, outlet obstruction and stomal stenosis), hernias with strangulation (incisional and internal), and small bowel obstructions.
Small bowel obstruction (SBO) occurs in 0%‐5% of RYGB patients (less with LAGB, similar with BPD), which is similar to other abdominal surgery rates, and may occur months to years after the original surgery. The differential diagnosis of an SBO includes internal hernias, adhesions, ventral hernia (incisional and umbilical), postoperative ileus, and jejunojejunal anastomotic stricture. Typical symptoms are often present, but may be less obvious than with a non‐gastric bypass patient. Pain can range from acute to a chronic or intermittent pattern. Pain is the most common presenting symptom of obstruction. Pain relieved by emesis may indicate an obstruction in the Roux limb. Nausea, bloating, tachycardia, and hiccups with shoulder/back pain can occur when obstruction in the biliopancreatic limb causes gastric distension.48
Vomiting is seen in fewer than half of patients with SBOs due to the altered anatomy.49 Any post‐RYGB patient that vomits bile needs emergent surgical evaluation for a common channel obstruction. Radiographic imaging may be misleading as to the cause of the obstruction. SBO is crucial to consider since delayed diagnosis can result in bowel ischemia and death.18 For the hospitalist who is caring for a post‐bariatric patient with a bowel obstruction, early surgical consultation is mandatory, preferably with a bariatric surgeon. Traditional medical management such as nasogastric (NG) tube placement will not decompress the excluded stomach, therefore patients rarely benefit from nasogastric decompression. If necessary, an NG tube should only be placed by experienced hands or fluoroscopic guidance, due to the altered anatomy.
Conclusion
Weight loss surgery, developed to address the growing obesity problem, has been beneficial to hundreds of thousands of people by decreasing their excess weight and comorbidities. For some, the postoperative course is complicated by medical and surgical problems requiring hospitalization. It is critically important that, as this relatively new field of postoperative medicine evolves, the hospitalist stay informed on relevant presentations, complications, and treatment to better address this growing population. Early consultation with, and transfer to, a bariatric surgery center should be encouraged. The importance of arranging proper hospital follow‐up, including community‐based support groups, nutritional consults, psychological support, and close follow‐up with the bariatric surgeon, bariatrician, and/or primary care physician, should not be underestimated.
- ,,,.Prevalence and trends in obesity among US adults, 1999‐2008.JAMA.2010;303(3):235–241.
- ,,, et al.Long‐term mortality after gastric bypass surgery.N Engl J Med.2007;357(8):753–761.
- ,,, et al.Bariatric surgery: a systematic review and meta‐analysis.JAMA.2004;292(14):1724–1737.
- ,,.Results of laparoscopic sleeve gastrectomy (LSG) at 1 year in morbidly obese Korean patients.Obes Surg.2005;15(10):1469–1475.
- ,,, et al.Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery.N Engl J Med.2004;351(26):2683–2693.
- ,,, et al.Long‐term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II.Ann Intern Med.2001;134(1):1–11.
- ,,,.Narrative review: effect of bariatric surgery on type 2 diabetes mellitus.Ann Intern Med.2009;150(2):94–103.
- ,,.Metabolic consequences of bariatric surgery.J Clin Gastroenterol.2006;40(8):659–668.
- ,.Surgical approaches to obesity.Mayo Clin Proc.2006;81(10 suppl):S18–S24.
- ,,, et al.American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic 4(5 suppl):S109–S184.
- ,,.Long‐term results of laparoscopic sleeve gastrectomy for obesity.Ann Surg.2010;252(2):319–324.
- ,,,,.Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures.Am J Med.2008;121(10):885–893.
- ,.Gastrointestinal complications of laparoscopic Roux‐en‐Y gastric bypass surgery in patients who are morbidly obese: findings on radiography and CT.AJR Am J Roentgenol.2002;179(6):1437–1442.
- ,,,,.Gastrointestinal complications of laparoscopic Roux‐en‐Y gastric bypass surgery: clinical and imaging findings.Radiology.2002;223(3):625–632.
- ,,.Use of computed tomography in diagnosis of major postoperative gastrointestinal complications of laparoscopic Roux‐en‐Y gastric bypass surgery.Am Surg.2004;70(11):964–966.
- ,,, et al.Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity.J Am Coll Surg.2007;204(1):47–55.
- ,,,,.Small‐bowel obstruction after laparoscopic Roux‐en‐Y gastric bypass surgery.J Comput Assist Tomogr.2009;33(3):369–375.
- ,,,,,.Sensitivity and specificity of eight CT signs in the preoperative diagnosis of internal mesenteric hernia following Roux‐en‐Y gastric bypass surgery.Clin Radiol.2009;64(4):373–380.
- ,,, et al.Internal hernia after gastric bypass: sensitivity and specificity of seven CT signs with surgical correlation and controls.AJR Am J Roentgenol.2007;188(3):745–750.
- ,,,,.Nausea, bloating and abdominal pain in the Roux‐en‐Y gastric bypass patient: more questions than answers.Obes Surg.2007;17(11):1529–1533.
- ,,, et al.Management of acute bleeding after laparoscopic Roux‐en‐Y gastric bypass.Obes Surg.2003;13(6):842–847.
- ,,,,.Endoscopic balloon dilation of gastroenteric anastomotic stricture after laparoscopic gastric bypass.Endoscopy.2003;35(9):725–728.
- ,.Ulcer disease after gastric bypass surgery.Surg Obes Relat Dis.2006;2(4):455–459.
- ,,,.Marginal ulcer after gastric bypass: a prospective 3‐year study of 173 patients.Obes Surg.1998;8(5):505–516.
- ,,,,.Stomal complications of gastric bypass: incidence and outcome of therapy.Am J Gastroenterol.1992;87(9):1165–1169.
- ,,,,.[Analysis of the dumping syndrome on morbid obese patients submitted to Roux en Y gastric bypass].Rev Col Bras Cir.2009;36(5):413–419.
- ,,, et al.Clinical management after bariatric surgery: value of a multidisciplinary approach.Mayo Clin Proc.2006;81(10 suppl):S34–S45.
- ,,,,,.Antibiotic efficacy in small intestinal bacterial overgrowth‐related chronic diarrhea: a crossover, randomized trial.Gastroenterology.1999;117(4):794–797.
- ,,,,.Absorbable vs. non‐absorbable antibiotics in the treatment of small intestine bacterial overgrowth in patients with blind‐loop syndrome.Aliment Pharmacol Ther.2005;21(8):985–992.
- ,,, et al.Small intestinal bacterial overgrowth: diagnosis and treatment.Dig Dis.2007;25(3):237–240.
- ,,, et al.Antibiotic therapy in small intestinal bacterial overgrowth: rifaximin versus metronidazole.Eur Rev Med Pharmacol Sci.2009;13(2):111–116.
- ,,,.Treatment strategies for small bowel bacterial overgrowth in short bowel syndrome.J Pediatr Gastroenterol Nutr.1998;27(2):155–160.
- ,,,.Short bowel syndrome following bariatric surgical procedures.Am J Surg.2006;192(6):828–832.
- ,,,,.Postoperative short bowel syndrome.J Am Coll Surg.2005;201(1):85–89.
- ,,.D‐lactic acidosis. A review of clinical presentation, biochemical features, and pathophysiologic mechanisms.Medicine.1998;77(2):73–82.
- ,,, et al.Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials.Fam Pract.2006;23(3):279–285.
- ,,.Neuromuscular diseases and disorders of the alimentary system.Muscle Nerve.2002;25(6):768–784.
- .Nutritional deficiencies following bariatric surgery.General Surgery News: Obesity Care Special Edition.2007;65–72. Available at: http://www.generalsurgerynews.com/download/gsnse07issueWM.pdf.
- ,,,.Neurologic complications after surgery for obesity.Muscle Nerve.2006;33(2):166–176.
- ,,.Acquired hypocupremia after gastric surgery.Clin Gastroenterol Hepatol.2004;2(12):1074–1079.
- ,.Krause's Food 2008.
- ,.Nutritional consequences of bariatric surgery.Curr Opin Clin Nutr Metab Care.2006;9(4):489–496.
- ,,,,.Nutritional deficiencies following bariatric surgery: what have we learned?Obes Surg.2005;15(2):145–154.
- .Managing micronutrient deficiencies in the bariatric surgical patient.Obesity Management.2005;1(5):203–206. Available at: http://www.liebertonline.com/doi/pdf/10.1089/obe.2005.1.203.
- ,,, et al.Abnormalities of vitamin D and calcium metabolism after surgical treatment of morbid obesity: a study of 136 patients.Endocr Pract.2007;13(2):131–136.
- ,,.Vitamin D deficiency in adults: when to test and how to treat.Mayo Clinic Proc.85(8):752–757; quiz757–758.
- ,,, et al.Endocrine and nutritional management of the post‐bariatric surgery patient: an Endocrine Society Clinical Practice Guideline.J Clin Endocrinol Metab.2010;95(11):4823–4843.
- ,,.Small bowel obstruction after laparoscopic Roux‐en‐Y gastric bypass: a review of 9,527 patients.J Am Coll Surg.2008;206(3):571–584.
- ,,,,.Small‐bowel obstruction after laparoscopic Roux‐en‐Y gastric bypass: etiology, diagnosis, and management.Arch Surg.2007;142(10):988–993.
- ,,,.Prevalence and trends in obesity among US adults, 1999‐2008.JAMA.2010;303(3):235–241.
- ,,, et al.Long‐term mortality after gastric bypass surgery.N Engl J Med.2007;357(8):753–761.
- ,,, et al.Bariatric surgery: a systematic review and meta‐analysis.JAMA.2004;292(14):1724–1737.
- ,,.Results of laparoscopic sleeve gastrectomy (LSG) at 1 year in morbidly obese Korean patients.Obes Surg.2005;15(10):1469–1475.
- ,,, et al.Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery.N Engl J Med.2004;351(26):2683–2693.
- ,,, et al.Long‐term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II.Ann Intern Med.2001;134(1):1–11.
- ,,,.Narrative review: effect of bariatric surgery on type 2 diabetes mellitus.Ann Intern Med.2009;150(2):94–103.
- ,,.Metabolic consequences of bariatric surgery.J Clin Gastroenterol.2006;40(8):659–668.
- ,.Surgical approaches to obesity.Mayo Clin Proc.2006;81(10 suppl):S18–S24.
- ,,, et al.American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic 4(5 suppl):S109–S184.
- ,,.Long‐term results of laparoscopic sleeve gastrectomy for obesity.Ann Surg.2010;252(2):319–324.
- ,,,,.Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures.Am J Med.2008;121(10):885–893.
- ,.Gastrointestinal complications of laparoscopic Roux‐en‐Y gastric bypass surgery in patients who are morbidly obese: findings on radiography and CT.AJR Am J Roentgenol.2002;179(6):1437–1442.
- ,,,,.Gastrointestinal complications of laparoscopic Roux‐en‐Y gastric bypass surgery: clinical and imaging findings.Radiology.2002;223(3):625–632.
- ,,.Use of computed tomography in diagnosis of major postoperative gastrointestinal complications of laparoscopic Roux‐en‐Y gastric bypass surgery.Am Surg.2004;70(11):964–966.
- ,,, et al.Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity.J Am Coll Surg.2007;204(1):47–55.
- ,,,,.Small‐bowel obstruction after laparoscopic Roux‐en‐Y gastric bypass surgery.J Comput Assist Tomogr.2009;33(3):369–375.
- ,,,,,.Sensitivity and specificity of eight CT signs in the preoperative diagnosis of internal mesenteric hernia following Roux‐en‐Y gastric bypass surgery.Clin Radiol.2009;64(4):373–380.
- ,,, et al.Internal hernia after gastric bypass: sensitivity and specificity of seven CT signs with surgical correlation and controls.AJR Am J Roentgenol.2007;188(3):745–750.
- ,,,,.Nausea, bloating and abdominal pain in the Roux‐en‐Y gastric bypass patient: more questions than answers.Obes Surg.2007;17(11):1529–1533.
- ,,, et al.Management of acute bleeding after laparoscopic Roux‐en‐Y gastric bypass.Obes Surg.2003;13(6):842–847.
- ,,,,.Endoscopic balloon dilation of gastroenteric anastomotic stricture after laparoscopic gastric bypass.Endoscopy.2003;35(9):725–728.
- ,.Ulcer disease after gastric bypass surgery.Surg Obes Relat Dis.2006;2(4):455–459.
- ,,,.Marginal ulcer after gastric bypass: a prospective 3‐year study of 173 patients.Obes Surg.1998;8(5):505–516.
- ,,,,.Stomal complications of gastric bypass: incidence and outcome of therapy.Am J Gastroenterol.1992;87(9):1165–1169.
- ,,,,.[Analysis of the dumping syndrome on morbid obese patients submitted to Roux en Y gastric bypass].Rev Col Bras Cir.2009;36(5):413–419.
- ,,, et al.Clinical management after bariatric surgery: value of a multidisciplinary approach.Mayo Clin Proc.2006;81(10 suppl):S34–S45.
- ,,,,,.Antibiotic efficacy in small intestinal bacterial overgrowth‐related chronic diarrhea: a crossover, randomized trial.Gastroenterology.1999;117(4):794–797.
- ,,,,.Absorbable vs. non‐absorbable antibiotics in the treatment of small intestine bacterial overgrowth in patients with blind‐loop syndrome.Aliment Pharmacol Ther.2005;21(8):985–992.
- ,,, et al.Small intestinal bacterial overgrowth: diagnosis and treatment.Dig Dis.2007;25(3):237–240.
- ,,, et al.Antibiotic therapy in small intestinal bacterial overgrowth: rifaximin versus metronidazole.Eur Rev Med Pharmacol Sci.2009;13(2):111–116.
- ,,,.Treatment strategies for small bowel bacterial overgrowth in short bowel syndrome.J Pediatr Gastroenterol Nutr.1998;27(2):155–160.
- ,,,.Short bowel syndrome following bariatric surgical procedures.Am J Surg.2006;192(6):828–832.
- ,,,,.Postoperative short bowel syndrome.J Am Coll Surg.2005;201(1):85–89.
- ,,.D‐lactic acidosis. A review of clinical presentation, biochemical features, and pathophysiologic mechanisms.Medicine.1998;77(2):73–82.
- ,,, et al.Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials.Fam Pract.2006;23(3):279–285.
- ,,.Neuromuscular diseases and disorders of the alimentary system.Muscle Nerve.2002;25(6):768–784.
- .Nutritional deficiencies following bariatric surgery.General Surgery News: Obesity Care Special Edition.2007;65–72. Available at: http://www.generalsurgerynews.com/download/gsnse07issueWM.pdf.
- ,,,.Neurologic complications after surgery for obesity.Muscle Nerve.2006;33(2):166–176.
- ,,.Acquired hypocupremia after gastric surgery.Clin Gastroenterol Hepatol.2004;2(12):1074–1079.
- ,.Krause's Food 2008.
- ,.Nutritional consequences of bariatric surgery.Curr Opin Clin Nutr Metab Care.2006;9(4):489–496.
- ,,,,.Nutritional deficiencies following bariatric surgery: what have we learned?Obes Surg.2005;15(2):145–154.
- .Managing micronutrient deficiencies in the bariatric surgical patient.Obesity Management.2005;1(5):203–206. Available at: http://www.liebertonline.com/doi/pdf/10.1089/obe.2005.1.203.
- ,,, et al.Abnormalities of vitamin D and calcium metabolism after surgical treatment of morbid obesity: a study of 136 patients.Endocr Pract.2007;13(2):131–136.
- ,,.Vitamin D deficiency in adults: when to test and how to treat.Mayo Clinic Proc.85(8):752–757; quiz757–758.
- ,,, et al.Endocrine and nutritional management of the post‐bariatric surgery patient: an Endocrine Society Clinical Practice Guideline.J Clin Endocrinol Metab.2010;95(11):4823–4843.
- ,,.Small bowel obstruction after laparoscopic Roux‐en‐Y gastric bypass: a review of 9,527 patients.J Am Coll Surg.2008;206(3):571–584.
- ,,,,.Small‐bowel obstruction after laparoscopic Roux‐en‐Y gastric bypass: etiology, diagnosis, and management.Arch Surg.2007;142(10):988–993.