User login
Economic Impact of Enoxaparin in Stroke
Venous thromboembolism (VTE), which encompasses both deep‐vein thrombosis (DVT) and pulmonary embolism (PE), is a major health problem in the United States and worldwide. It represents one of the most significant causes of morbidity and mortality with an estimated 300,000 VTE‐related deaths,1 and 300,000‐600,000 hospitalizations in the United States annually.2 Hospitalization for medical illness is associated with a similar proportion of VTE cases as hospitalization for surgery.3 Several groups of medical patients have been shown to be at an increased risk of VTE, including those with cancer, severe respiratory disease, acute infectious illness, heart failure, myocardial infarction, and acute ischemic stroke.47 Ischemic stroke patients represent approximately 4.6% of medical patients at high risk of VTE in US hospitals.8 The incidence of DVT in such patients has been reported to be as high as 75%9 and PE has been reported to be responsible for up to 25% of early deaths after stroke.10
Several studies have demonstrated the efficacy of unfractionated heparin (UFH) or a low‐molecular‐weight heparin (LMWH) in the prevention of VTE in stroke patients, and have demonstrated that LMWHs are at least as effective as UFH.1114 The open‐label, randomized Prevention of VTE after acute ischemic stroke with LMWH and UFH (PREVAIL) trial demonstrated that in patients with acute ischemic stroke, prophylaxis for 10 days with the LMWH enoxaparin reduces the risk of VTE by 43% compared with UFH (10.2% vs 18.1%, respectively; relative risk = 0.57; 95% confidence interval [CI] = 0.44‐0.76; P = 0.0001) without increasing the incidence of overall bleeding events (7.9% vs 8.1%, respectively; P = 0.83), or the composite of symptomatic intracranial and major extracranial hemorrhage (1% in each group; P = 0.23). There was, however, a slight but significant increase in major extracranial hemorrhage alone with enoxaparin (1% vs 0%; P = 0.015).14 Evidence‐based guidelines from the American College of Chest Physicians (ACCP) provide recommendations for appropriate thromboprophylaxis regimens for patients at risk of VTE.15 Thromboprophylaxis with UFH, LMWH, and, more recently, fondaparinux is recommended for medical patients admitted to hospital with congestive heart failure or severe respiratory disease, or those who are confined to bed and have one or more additional risk factors, including active cancer, previous VTE, or acute neurologic disease.15 Similarly, in the Eighth ACCP Clinical Practice Guidelines, low‐dose UFH or LMWH are recommended for VTE prevention in patients with ischemic stroke who have restricted mobility.16
VTE is also associated with a substantial economic burden on the healthcare system, costing an estimated $1.5 billion annually in the United States.17 Thromboprophylaxis has been shown to be a cost‐effective strategy in hospitalized medical patients. Prophylaxis with a LMWH has been shown to be more cost‐effective than UFH in these patients.1821
However, despite the clinical and economic benefits, prophylaxis is still commonly underused in medical patients.22, 23 In surgical patients, the Surgical Care Improvement Project (SCIP) focuses on reducing surgical complications, and has endorsed 2 measures: VTE‐1, relating to the proportion of patients for whom VTE prophylaxis is ordered; and VTE‐2, relating to those who receive the recommended regimen (
The objective of the current study was to determine the economic impact, in terms of hospital costs, of enoxaparin compared with UFH for VTE prophylaxis after acute ischemic stroke. A decision‐analytic model was constructed using data from the PREVAIL study and historical inpatient data from a multi‐hospital database.
METHODS
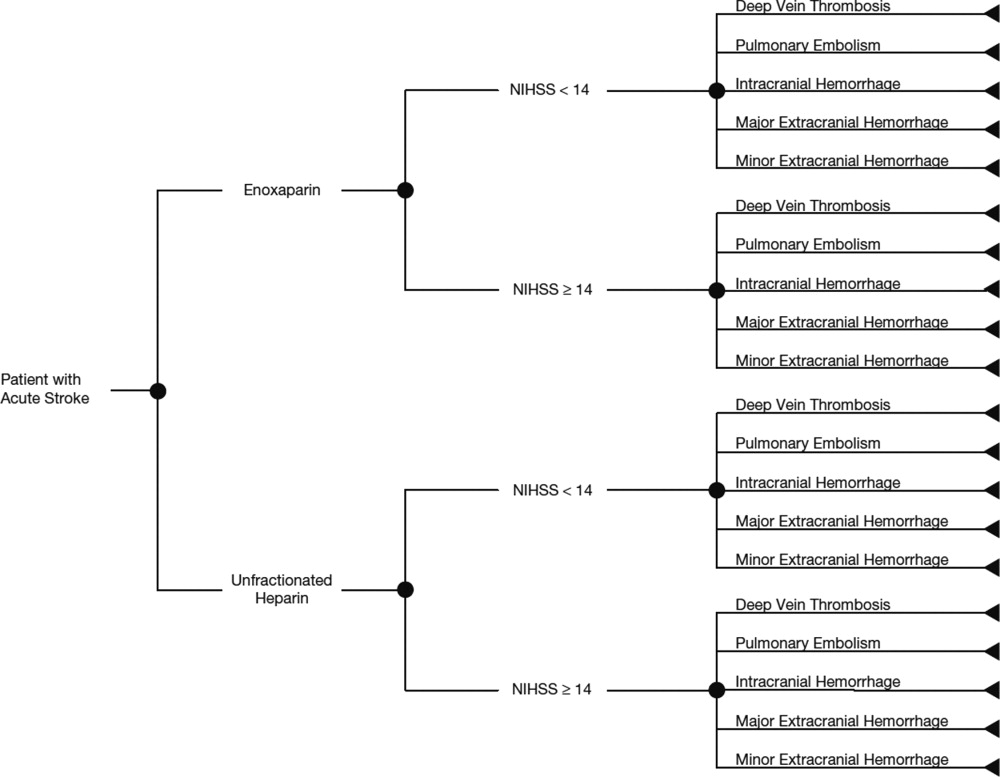
In this study, the cost implications, from the hospital perspective, of VTE prophylaxis with enoxaparin or UFH in patients with acute ischemic stroke, were determined using a decision‐analytic model in TreeAge Pro Suite (TreeAge Software, Inc., Williamstown, MA, USA). The decision‐tree was based on 3 stages: (a) whether patients received enoxaparin or UFH; (b) how patients were classified according to their National Institutes of Health Stroke Scale (NIHSS) classification scores (<14 or 14); and (c) which clinical event each patient experienced, as defined per the PREVAIL trial (DVT, PE, intracranial hemorrhage, major extracranial hemorrhage, and minor extracranial hemorrhage) (Figure 1). The time horizon for the model was established at 90 days to mirror the length of follow‐up in the PREVAIL trial.
Total hospital costs were calculated based on clinical event rates (from the PREVAIL trial) and the costs of each clinical event, which were calculated separately according to the descriptions below, and then inserted into the decision‐analytic model. The clinical event rates were calculated from the efficacy and safety endpoints collected in the PREVAIL trial, and included VTE events (DVT and PE) and bleeding events (intracranial hemorrhage, major extracranial hemorrhage, and minor extracranial hemorrhage). Details of the patient population, eligibility criteria, and treatment regimen have previously been published in full elsewhere.14, 27
The costs of clinical events during hospitalization were estimated using a multivariate cost‐evaluation model, based on mean hospital costs for the events in the (Premier Inc., Charlotte, NC, USA) multi‐hospital database, one of the largest US hospital clinical and economic databases. The data are received from over 600 hospitals, representing all geographical areas of the United States, a broad range of bed sizes, teaching and non‐teaching, and urban and rural facilities. This database contains detailed US inpatient care records of principal and secondary diagnoses, inpatient procedures, administered laboratory tests, dispensed drugs, and demographic information. The evaluation of hospital cost for each type of clinical event was conducted by i3 Innovus (Ingenix, Inc., Eden Prairie, MN, USA). Total hospital costs were cumulative from all events, so if patients experienced multiple clinical events, the costs of the events were additive. The cost for stroke treatment and management was not included because it is an inclusion criterion of the PREVAIL trial and, thus, all patients in the trial have such costs.
Default drug costs were taken from the 2008 US wholesalers' acquisition cost data. The default dosing schedule is based on information extracted from the PREVAIL trial: enoxaparin 40 mg (once‐daily) and UFH 5000 U (twice‐daily) for 10 days each ($25.97 and $2.97, respectively). A drug‐administration fee was added for each dose of either enoxaparin or UFH ($10 for each).19
The estimated hospital cost of clinical events, along with drug costs, were inserted into the decision‐analytic model in TreeAge Pro Suite to estimate the cost per discharge from the hospital perspective in patients with ischemic stroke receiving VTE prophylaxis with enoxaparin or UFH. An additional analysis was performed to investigate the costs and cost differences in patients with less severe stroke (NIHSS scores <14) and more severe stroke (NIHSS scores 14).
Sensitivity analyses were performed to examine the impact of varying the cost inputs on the total hospital cost of each treatment arm by 5%, 10%, 15%, 20%, 30%, and 40%, and the robustness in the difference in costs between the enoxaparin and UFH groups. Univariate (via tornado diagram in TreeAge Pro Suite) and multivariate (via Monte Carlo simulation in TreeAge Pro Suite) analyses were performed. For the univariate analysis, each clinical event cost was adjusted individually, increasing or decreasing by 5%, 10%, 15%, 20%, 30% and 40% while other parameters remained unchanged. For the Monte Carlo simulation (TreeAge Pro Suite), all the parameters were simultaneously varied in a random fashion, within a range of 5%, 10%, 15%, 20%, 30%, and 40% over 10,000 trials. The simulation adopted a gamma distribution assumption for input sampling for cost parameters and a beta distribution for the event probability parameters. The confidence intervals for the probability parameters were obtained from the PREVAIL trial. The differences between the enoxaparin and UFH treatment groups were plotted in a graph against the variation in costs of each clinical event.
RESULTS
The clinical VTE and bleeding event rates as collected from the PREVAIL trial are shown in Table 1. The hospital costs per clinical event are shown in Table 2. The most costly clinical event from the hospital perspective was intracranial hemorrhage at $4001, followed by major extracranial hemorrhage at $3534. The costs of DVT and PE were $3003 and $2143, respectively.
| Event Rate | 95% CI | |
|---|---|---|
| ||
| Enoxaparin (NIHSS <14) | ||
| Deep‐vein thrombosis | 0.081 | 0.05730.1048 |
| Pulmonary embolism | 0.002 | 0.000050.011 |
| Intracranial hemorrhage | 0.0031 | 00.0074 |
| Major extracranial hemorrhage | 0.0047 | 00.0099 |
| Minor hemorrhage | 0.0372 | 0.0240.0549 |
| Enoxaparin (NIHSS 14) | ||
| Deep‐vein thrombosis | 0.1625 | 0.10530.2197 |
| Pulmonary embolism | 0 | 00 |
| Intracranial hemorrhage | 0.0086 | 00.0205 |
| Major extracranial hemorrhage | 0.0172 | 0.04660.1198 |
| Minor hemorrhage | 0.0776 | 0.04660.1198 |
| UFH (NIHSS <14) | ||
| Deep‐vein thrombosis | 0.1356 | 0.10540.1658 |
| Pulmonary embolism | 0.004 | 0.00050.0145 |
| Intracranial hemorrhage | 0.0032 | 00.0077 |
| Major extracranial hemorrhage | 0 | 00 |
| Minor hemorrhage | 0.0514 | 0.03550.0719 |
| UFH (NIHSS 14) | ||
| Deep‐vein thrombosis | 0.2914 | 0.22410.3588 |
| Pulmonary embolism | 0.0229 | 0.00630.0575 |
| Intracranial hemorrhage | 0.016 | 0.00040.0316 |
| Major extracranial hemorrhage | 0 | 00 |
| Minor hemorrhage | 0.064 | 0.0370.1019 |
| Event | Cost per Event ($)* | ||
|---|---|---|---|
| Likeliest | Minimum | Maximum | |
| |||
| Deep‐vein thrombosis | 3,003 | 2,402 | 3,604 |
| Pulmonary embolism | 2,143 | 1,714 | 2,572 |
| Intracranial hemorrhage | 4,001 | 3,201 | 4,801 |
| Major extracranial hemorrhage | 3,534 | 2,827 | 4,241 |
| Minor hemorrhage | 1,322 | 1,058 | 1,586 |
| Enoxaparin cost per dose | 26 | 21 | 31 |
| Unfractionated heparin cost per dose | 3 | 2 | 4 |
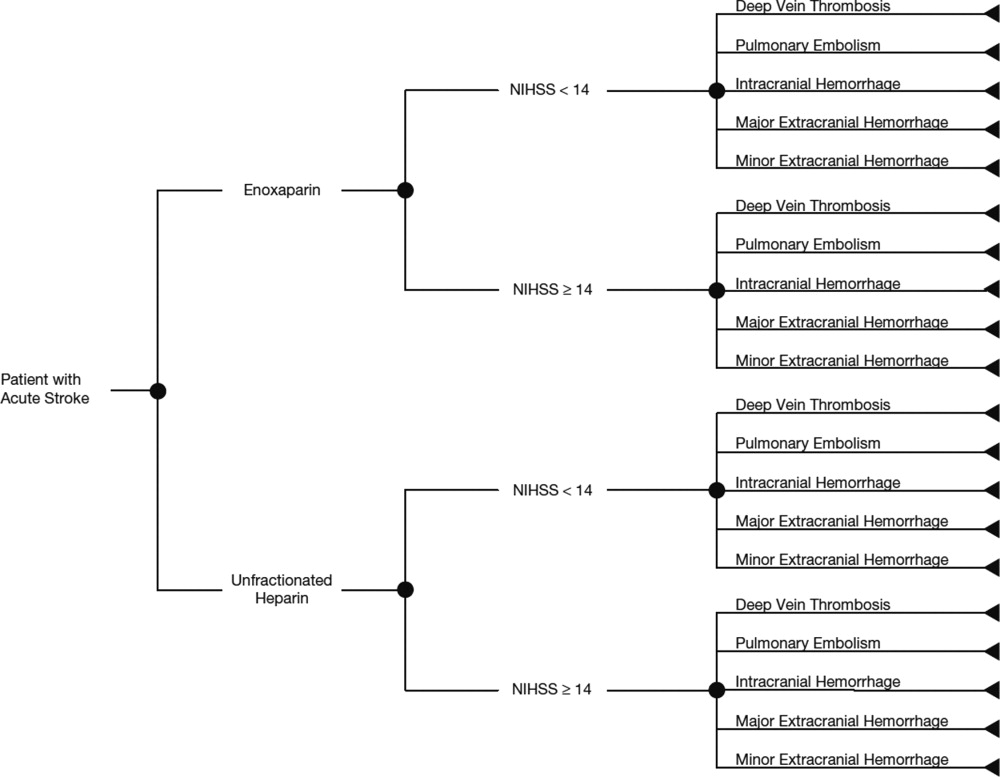
The average hospital cost with enoxaparin, when taking into account the costs of VTE and bleeding, was lower than with UFH ($422 vs $662, respectively), with a net savings of $240 per patient if enoxaparin was used. The average drug costs, including drug‐administration costs, were higher in the enoxaparin group ($360) compared with the UFH group ($259; difference $101). Nevertheless, the total hospital cost when clinical events and drug costs were considered together, was lower with enoxaparin than UFH. The total hospital costs per patient were $782 in patients receiving prophylaxis with enoxaparin and $922 in patients receiving UFH. Thus, enoxaparin was associated with a total cost‐savings of $140 per patient (Figure 2).
The cost estimates according to the stroke severity score (NIHSS scores <14 vs 14) are described in Table 3. The drug costs were consistent, regardless of stroke severity, for enoxaparin ($360) and for UFH ($259). However, in both treatment groups, the event costs were higher in patients with more severe stroke, compared with less severe stroke. For example, in the enoxaparin group, the event costs were $686 in patients with NIHSS scores 14 and $326 in patients with NIHSS scores <14. Nevertheless, the overall costs (event costs plus drug costs) were lower with enoxaparin compared with UFH, both in patients with less severe and more severe stroke. In fact, the total hospital cost‐savings were greater when enoxaparin was used instead of UFH in patients with more severe stroke (cost‐saving $287 if NIHSS score 14 vs $71 if NIHSS score <14) (Table 3).
| Enoxaparin ($) | UFH ($) | Difference ($ [UFHEnoxaparin]) | |
|---|---|---|---|
| |||
| NIHSS score <14 | |||
| Mean event costs per patient | 326 | 497 | 171 |
| Mean drug costs per patient* | 360 | 259 | 101 |
| Total costs | 685 | 756 | 71 |
| NIHSS score 14 | |||
| Mean event costs per patient | 686 | 1,073 | 387 |
| Mean drug costs per patient* | 360 | 259 | 101 |
| Total costs | 1,046 | 1,332 | 287 |
Multiple sensitivity analyses were performed. In the base case univariate sensitivity analysis, individual costs were adjusted by 20% (Table 4). If the cost of DVT increased by 20% (from $3003 to $3604) the difference between the enoxaparin and UFH groups was $187. When the cost of DVT was decreased by 20% to $2402, enoxaparin was still cost‐saving, with a difference of $94. For each of the individual cost parameters that were varied (DVT, PE, intracranial hemorrhage, major extracranial hemorrhage, and minor hemorrhage), enoxaparin was always less costly than UFH. Subsequent sensitivity analyses were performed (not shown) where cost parameters were varied by 5%, 10%, 15%, 30%, and 40%. Enoxaparin remained less costly than UFH in all cases.
| Event | Baseline Cost Input ($) | +20% Cost Input ($) | +20% Difference ($ [UFH Enoxaparin]) (% Change) | 20% Cost Input ($) | 20% Difference ($ [UFH Enoxaparin]) (% Change) |
|---|---|---|---|---|---|
| |||||
| Deep‐vein thrombosis | 3,003 | 3,604 | 187 (33) | 2,402 | 94 (33) |
| Pulmonary embolism | 2,143 | 2,572 | 144 (2.5) | 1,714 | 137 (2.5) |
| Intracranial hemorrhage | 4,001 | 4,801 | 142 (1.3) | 3,201 | 138 (1.3) |
| Major extracranial hemorrhage | 3,534 | 4,241 | 134 (4.0) | 2,827 | 146 (4.0) |
| Minor hemorrhage | 1,322 | 1,586 | 142 (1.3) | 1,058 | 138 (1.3) |
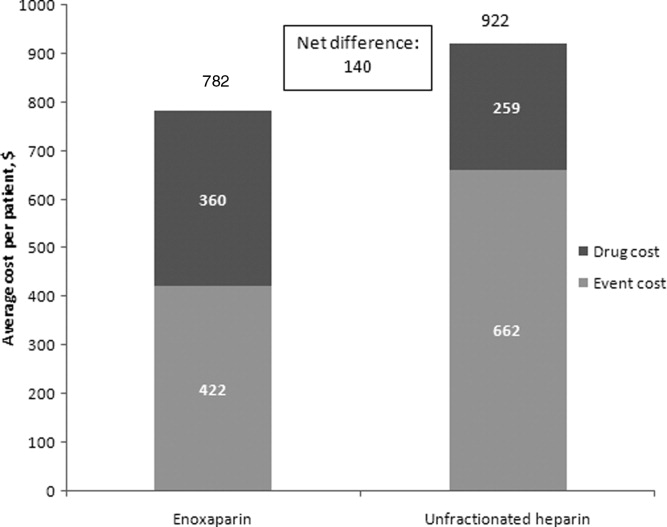
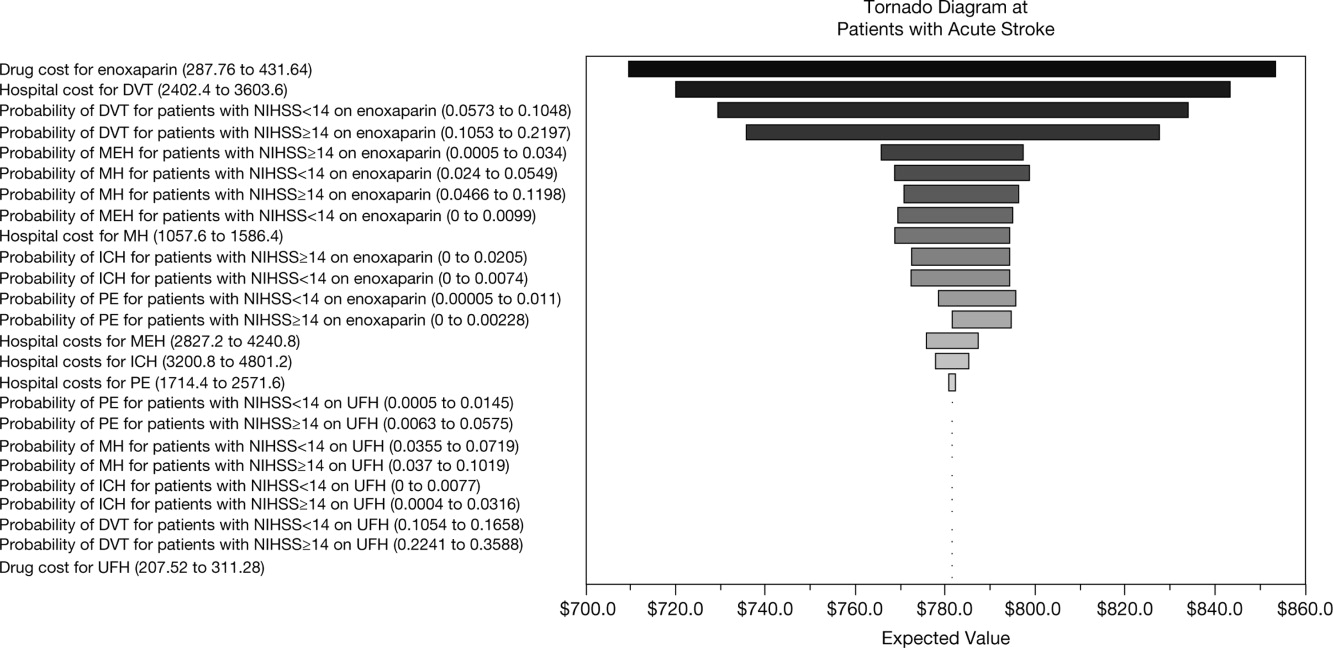
A multivariate analysis was performed using a Monte Carlo simulation in TreeAge Pro (Figure 3). When all parameters were varied simultaneously (by 5%, 10%, 15%, 20%, 30%, and 40%) and the differences in costs between the enoxaparin and UFH groups were measured and plotted, the mean (standard deviation) difference between enoxaparin and UFH prophylaxis was $140 ($79) (Figure 3). Figure 4 shows a graphical presentation of the sensitivity analysis results for event probabilities and costs. Differences in enoxaparin drug costs, hospital costs for DVT, and probability of DVT for patients on enoxaparin are the factors that have the greatest effect on the overall cost.
Finally, an additional scenario was performed using a published ratio of asymptomatic DVT to symptomatic VTE, due to the fact that not all VTE events in the real‐world present with symptoms prompting treatment. Quinlan et al. determined a ratio of asymptomatic DVT to symptomatic VTE of 5 for total hip replacement patients and of 21 for total knee replacement patients.28 Although derived from different patient populations who received different anticoagulants, we utilized the symptomatic event rates from the pooled studies to recalculate cost differences between enoxaparin and UFH in acute ischemic stroke. Using only symptomatic event rates, based on the 21:1 ratio in patients undergoing total knee replacement, the total cost for enoxaparin was $485 compared to $386 for UFH. Similar results were found based on the 5:1 ratio in patients with total hip replacement (enoxaparin $532 vs $472 for UFH). This was the only scenario where the higher drug cost of prophylaxis with enoxaparin was not completely offset by the reduction in events compared to UFH, likely due to the smaller difference in event rates once examining only symptomatic VTE.
DISCUSSION
This analysis demonstrates that, from the hospital perspective, enoxaparin 40 mg subcutaneously once‐daily is associated with lower total hospital costs and is more cost‐effective than twice‐daily UFH 5000 U subcutaneously for the prevention of VTE in patients with acute ischemic stroke. Despite higher drug‐acquisition costs, enoxaparin was associated with total cost‐savings of $140 per patient. This is due to the lower event rates with enoxaparin compared with UFH.
Previous studies, using hospital or payer information, have shown that VTE prophylaxis is more cost‐effective compared with no prophylaxis. In terms of the different VTE prophylaxis regimens, enoxaparin represents a more cost‐effective option in comparison with UFH19, 21, 2932 and also when compared with fondaparinux.21, 33 When comparing the results between different trials, it should be noted that previous analyses were mainly modeled on the Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) study, which was performed in general medical patients and reported a VTE rate of 5.5%.6 However, patients with acute ischemic stroke are at a higher risk of VTE, with a 10% incidence of VTE reported in the PREVAIL study.14 Furthermore, twice‐daily rather than three‐timesdaily administration of UFH was used in the PREVAIL study, based on the current practice patterns seen during the PREVAIL trial design.
A recent retrospective analysis of transactional billing records demonstrated that, despite higher mean costs of anticoagulation therapy, the mean, total, adjusted direct hospital costs were lower with LMWH thromboprophylaxis compared with UFH ($7358 vs $8680, respectively; difference $1322; P < 0.001).21 A previous study by Burleigh and colleagues based on hospital discharge information extracted from both medical and surgical patients, has a sub‐analysis in patients with stroke. In these patients also, the total costs were lower for enoxaparin compared with UFH ($8608 vs $8911, respectively; difference $303).29 In the Burleigh study, drug costs and total discharge costs (eg, room and board, laboratory, and diagnostic imaging) were derived from drug charges and total charges, and were converted to estimated costs using cost‐to‐charge methods, so the absolute figures are not directly comparable with the current analysis.
This study adds to current literature by using data from a prospective study to analyze the hospital costs of VTE prophylaxis in stroke patients. The current study also provides a valuable cost‐analysis regarding a specific subgroup of medical patients at particularly high risk of VTE, and provides an economic comparison among stroke patients with NIHSS scores of <14 versus 14. In the PREVAIL study, despite a 2‐fold higher incidence of VTE in patients with more severe stroke (16.3% vs 8.3%), a similar reduction in VTE risk was observed with enoxaparin versus UFH in patients with NIHSS scores of 14 (odds ratio = 0.56; 95% CI = 0.37‐0.84; P = 0.0036) and <14 (odds ratio = 0.46; 95% CI = 0.27‐0.78; P = 0.0043).14 Enoxaparin was shown to be cost‐saving relative to UFH in both patient groups and, in particular, in patients with more severe stroke.
Potential limitations of the current analysis include the applicability of the figures obtained from the highly selected clinical trial population to real‐world clinical practice, and the fact that it is difficult to match cost estimates to trial data definitions. For example, this analysis was conducted with a comparator of twice‐daily UFH (as opposed to three‐timesdaily) which may be used in the real‐world setting and may have resulted in the increased number of events in the UFH group seen in the PREVAIL study. Due to a variety of differences between real‐world practice patterns and the PREVAIL clinical trial, we can only speculate as to the true cost‐consequences of utilizing enoxaparin versus UFH.
Furthermore, the original model did not include a sub‐analysis regarding the rates and, therefore, costs of proximal/symptomatic VTE. In the primary study of PREVAIL, the rates of symptomatic DVT were 1 in 666 patients (<1%) for enoxaparin and 4 in 669 patients (1%) for UFH, whereas the rates of proximal DVT were 30 in 666 patients (5%) and 64 in 669 patients (10%), respectively. Sensitivity analyses were performed to investigate the impact of lower rates of both DVT and PE (up to 40%), and the differences between groups were found to be robust. However, it is important to note that overall costs for both groups may have been increased through the inclusion of asymptomatic costs, with a more distinct separation of these costs making for a good follow‐up study. In a similar cost‐analysis we performed based on the PREVAIL study, which assessed the cost to the payer, we included an analysis of costs according to 3 different VTE definitions: the PREVAIL VTE definition (as in the current study); a definition of major VTE (PE, symptomatic DVT, and asymptomatic proximal DVT); and primary endpoints recommended by the European Medicines Agency Committee for Medicinal Products for Human Use for studies on VTE (proximal DVT, nonfatal PE, and all‐cause mortality). We found similar results irrespective of clinical event definitions.34 In an additional model scenario using a published ratio of asymptomatic DVT to symptomatic VTE,28 the higher drug cost of prophylaxis with enoxaparin was not completely offset by the reduction in events compared to UFH. This was likely due to the smaller difference in event rates once examining only symptomatic VTE. This scenario was limited by the fact that the ratio was derived from different patient populations receiving different anticoagulants than stroke patients.
In conclusion, data from this analysis adds to the evidence that, from the hospital perspective, the higher drug cost of enoxaparin is offset by the economic consequences of the events avoided as compared with UFH for the prevention of VTE following acute ischemic stroke, particularly in patients with severe stroke.
Acknowledgements
The authors thank Aylin Lee from I3 Innovus for her contribution to this study. The authors also acknowledge Min Chen for her assistance in statistical analysis, and Essy Mozaffari for his contribution to this study.
- .Venous thromboembolism: disease burden, outcomes and risk factors.J Thromb Haemost.2005;3:1611–1617.
- .The epidemiology of venous thromboembolism in the community: implications for prevention and management.J Thromb Thrombolysis.2006;21:23–29.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study.Arch Intern Med.2002;162:1245–1248.
- ,,,,,.Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study.Arch Intern Med.2000;160:809–815.
- ,.A safety analysis of thromboprophylaxis in acute medical illness.Thromb Haemost.2003;89:590–591.
- ,,,,.Quantification of risk factors for venous thromboembolism: a preliminary study for the development of a risk assessment tool.Haematologica.2003;88:1410–1421.
- ,.Prevalence of venous thromboembolism in acute hemorrhagic and thromboembolic stroke.Am J Phys Med Rehabil.2003;82:364–369.
- ,,,.Thromboprophylaxis rates in US medical centers: success or failure?J Thromb Haemost.2007;5:1610–1616.
- ,,,.Complications after acute stroke.Stroke.1996;27:415–420.
- ,,,.Venous thromboembolism after acute stroke.Stroke.2001;32:262–267.
- ,,,,,.Enoxaparin vs heparin for prevention of deep‐vein thrombosis in acute ischaemic stroke: a randomized, double‐blind study.Acta Neurol Scand.2002;106:84–92.
- ,,.Low‐molecular‐weight heparins or heparinoids versus standard unfractionated heparin for acute ischaemic stroke.Cochrane Database Syst Rev.2008;(3):CD000119.
- ,,, et al;for the PROTECT Trial Group.Prophylaxis of thrombotic and embolic events in acute ischemic stroke with the low‐molecular‐weight heparin certoparin: results of the PROTECT Trial.Stroke.2006;37:139–144.
- ,,, et al;for the PREVAIL Investigators.The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL study): an open‐label randomised comparison.Lancet.2007;369:1347–1355.
- ,,, et al;for the American College of Chest Physicians.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th ed).Chest.2008;133(6 suppl):381S–453S.
- ,,,,;for the American College of Chest Physicians.Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th ed).Chest.2008;133(6 suppl):630S–669S.
- ,,,.Management of acute proximal deep vein thrombosis: pharmacoeconomic evaluation of outpatient treatment with enoxaparin vs inpatient treatment with unfractionated heparin.Chest.2002;122:108–114.
- ,.Economic evaluation of enoxaparin as prophylaxis against venous thromboembolism in seriously ill medical patients: a US perspective.Am J Manag Care.2002;8:1082–1088.
- ,,,.Cost effectiveness of thromboprophylaxis with a low‐molecular‐weight heparin versus unfractionated heparin in acutely ill medical inpatients.Am J Manag Care.2004;10:632–642.
- ,,, et al.Cost effectiveness of enoxaparin as prophylaxis against venous thromboembolic complications in acutely ill medical inpatients: modelling study from the hospital perspective in Germany.Pharmacoeconomics.2006;24:571–591.
- ,,,,.Hospital‐based costs associated with venous thromboembolism prophylaxis regimens.J Thromb Thrombolysis.2010;29:449–458.
- ,,, et al;for the ENDORSE Investigators.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371:387–394.
- ,,, et al;for the IMPROVE Investigators.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism.Chest.2007;132:936–945.
- United States Department of Health August 2008. Available at: http://www.ahrq.gov/qual/vtguide/. Accessed August 18,2010.
- ,,,.Enoxaparin versus unfractionated heparin in the prevention of venous thromboembolism after acute ischemic stroke: rationale, design, and methods of an open‐label, randomized, parallel‐group multicenter trial.J Stroke Cerebrovasc Dis.2005;14:95–100.
- ,,,,,.Association between asymptomatic deep vein thrombosis detected by venography and symptomatic venous thromboembolism in patients undergoing elective hip or knee surgery.J Thromb Haemost.2007;5:1438–1443.
- ,,, et al.Thromboprophylaxis in medically ill patients at risk for venous thromboembolism.Am J Health Syst Pharm.2006;63(20 suppl 6):S23–S29.
- ,,,.Comparison of the two‐year outcomes and costs of prophylaxis in medical patients at risk of venous thromboembolism.Thromb Haemost.2008;100:810–820.
- ,,,,,.Cost for inpatient care of venous thrombosis: a trial of enoxaparin vs standard heparin.Arch Intern Med.2000;160:3160–3165.
- ,,,.Economic evaluation of enoxaparin for the prevention of venous thromboembolism in acutely ill medical patients.Pharm World Sci.2004;26:214–220.
- ,,,,.Total hospital‐based costs of enoxaparin or fondaparinux prophylaxis in patients at risk of venous thromboembolism [abstract]. Presented at the Chest 2008 Annual Meeting; October 25–30,2008; Philadelphia, PA.
- ,,,,.Economic impact of enoxaparin after acute ischemic stroke based on PREVAIL.Clin Appl Thromb Hemost.2011;17:150–157.
Venous thromboembolism (VTE), which encompasses both deep‐vein thrombosis (DVT) and pulmonary embolism (PE), is a major health problem in the United States and worldwide. It represents one of the most significant causes of morbidity and mortality with an estimated 300,000 VTE‐related deaths,1 and 300,000‐600,000 hospitalizations in the United States annually.2 Hospitalization for medical illness is associated with a similar proportion of VTE cases as hospitalization for surgery.3 Several groups of medical patients have been shown to be at an increased risk of VTE, including those with cancer, severe respiratory disease, acute infectious illness, heart failure, myocardial infarction, and acute ischemic stroke.47 Ischemic stroke patients represent approximately 4.6% of medical patients at high risk of VTE in US hospitals.8 The incidence of DVT in such patients has been reported to be as high as 75%9 and PE has been reported to be responsible for up to 25% of early deaths after stroke.10
Several studies have demonstrated the efficacy of unfractionated heparin (UFH) or a low‐molecular‐weight heparin (LMWH) in the prevention of VTE in stroke patients, and have demonstrated that LMWHs are at least as effective as UFH.1114 The open‐label, randomized Prevention of VTE after acute ischemic stroke with LMWH and UFH (PREVAIL) trial demonstrated that in patients with acute ischemic stroke, prophylaxis for 10 days with the LMWH enoxaparin reduces the risk of VTE by 43% compared with UFH (10.2% vs 18.1%, respectively; relative risk = 0.57; 95% confidence interval [CI] = 0.44‐0.76; P = 0.0001) without increasing the incidence of overall bleeding events (7.9% vs 8.1%, respectively; P = 0.83), or the composite of symptomatic intracranial and major extracranial hemorrhage (1% in each group; P = 0.23). There was, however, a slight but significant increase in major extracranial hemorrhage alone with enoxaparin (1% vs 0%; P = 0.015).14 Evidence‐based guidelines from the American College of Chest Physicians (ACCP) provide recommendations for appropriate thromboprophylaxis regimens for patients at risk of VTE.15 Thromboprophylaxis with UFH, LMWH, and, more recently, fondaparinux is recommended for medical patients admitted to hospital with congestive heart failure or severe respiratory disease, or those who are confined to bed and have one or more additional risk factors, including active cancer, previous VTE, or acute neurologic disease.15 Similarly, in the Eighth ACCP Clinical Practice Guidelines, low‐dose UFH or LMWH are recommended for VTE prevention in patients with ischemic stroke who have restricted mobility.16
VTE is also associated with a substantial economic burden on the healthcare system, costing an estimated $1.5 billion annually in the United States.17 Thromboprophylaxis has been shown to be a cost‐effective strategy in hospitalized medical patients. Prophylaxis with a LMWH has been shown to be more cost‐effective than UFH in these patients.1821
However, despite the clinical and economic benefits, prophylaxis is still commonly underused in medical patients.22, 23 In surgical patients, the Surgical Care Improvement Project (SCIP) focuses on reducing surgical complications, and has endorsed 2 measures: VTE‐1, relating to the proportion of patients for whom VTE prophylaxis is ordered; and VTE‐2, relating to those who receive the recommended regimen (
The objective of the current study was to determine the economic impact, in terms of hospital costs, of enoxaparin compared with UFH for VTE prophylaxis after acute ischemic stroke. A decision‐analytic model was constructed using data from the PREVAIL study and historical inpatient data from a multi‐hospital database.
METHODS
In this study, the cost implications, from the hospital perspective, of VTE prophylaxis with enoxaparin or UFH in patients with acute ischemic stroke, were determined using a decision‐analytic model in TreeAge Pro Suite (TreeAge Software, Inc., Williamstown, MA, USA). The decision‐tree was based on 3 stages: (a) whether patients received enoxaparin or UFH; (b) how patients were classified according to their National Institutes of Health Stroke Scale (NIHSS) classification scores (<14 or 14); and (c) which clinical event each patient experienced, as defined per the PREVAIL trial (DVT, PE, intracranial hemorrhage, major extracranial hemorrhage, and minor extracranial hemorrhage) (Figure 1). The time horizon for the model was established at 90 days to mirror the length of follow‐up in the PREVAIL trial.

Total hospital costs were calculated based on clinical event rates (from the PREVAIL trial) and the costs of each clinical event, which were calculated separately according to the descriptions below, and then inserted into the decision‐analytic model. The clinical event rates were calculated from the efficacy and safety endpoints collected in the PREVAIL trial, and included VTE events (DVT and PE) and bleeding events (intracranial hemorrhage, major extracranial hemorrhage, and minor extracranial hemorrhage). Details of the patient population, eligibility criteria, and treatment regimen have previously been published in full elsewhere.14, 27
The costs of clinical events during hospitalization were estimated using a multivariate cost‐evaluation model, based on mean hospital costs for the events in the (Premier Inc., Charlotte, NC, USA) multi‐hospital database, one of the largest US hospital clinical and economic databases. The data are received from over 600 hospitals, representing all geographical areas of the United States, a broad range of bed sizes, teaching and non‐teaching, and urban and rural facilities. This database contains detailed US inpatient care records of principal and secondary diagnoses, inpatient procedures, administered laboratory tests, dispensed drugs, and demographic information. The evaluation of hospital cost for each type of clinical event was conducted by i3 Innovus (Ingenix, Inc., Eden Prairie, MN, USA). Total hospital costs were cumulative from all events, so if patients experienced multiple clinical events, the costs of the events were additive. The cost for stroke treatment and management was not included because it is an inclusion criterion of the PREVAIL trial and, thus, all patients in the trial have such costs.
Default drug costs were taken from the 2008 US wholesalers' acquisition cost data. The default dosing schedule is based on information extracted from the PREVAIL trial: enoxaparin 40 mg (once‐daily) and UFH 5000 U (twice‐daily) for 10 days each ($25.97 and $2.97, respectively). A drug‐administration fee was added for each dose of either enoxaparin or UFH ($10 for each).19
The estimated hospital cost of clinical events, along with drug costs, were inserted into the decision‐analytic model in TreeAge Pro Suite to estimate the cost per discharge from the hospital perspective in patients with ischemic stroke receiving VTE prophylaxis with enoxaparin or UFH. An additional analysis was performed to investigate the costs and cost differences in patients with less severe stroke (NIHSS scores <14) and more severe stroke (NIHSS scores 14).
Sensitivity analyses were performed to examine the impact of varying the cost inputs on the total hospital cost of each treatment arm by 5%, 10%, 15%, 20%, 30%, and 40%, and the robustness in the difference in costs between the enoxaparin and UFH groups. Univariate (via tornado diagram in TreeAge Pro Suite) and multivariate (via Monte Carlo simulation in TreeAge Pro Suite) analyses were performed. For the univariate analysis, each clinical event cost was adjusted individually, increasing or decreasing by 5%, 10%, 15%, 20%, 30% and 40% while other parameters remained unchanged. For the Monte Carlo simulation (TreeAge Pro Suite), all the parameters were simultaneously varied in a random fashion, within a range of 5%, 10%, 15%, 20%, 30%, and 40% over 10,000 trials. The simulation adopted a gamma distribution assumption for input sampling for cost parameters and a beta distribution for the event probability parameters. The confidence intervals for the probability parameters were obtained from the PREVAIL trial. The differences between the enoxaparin and UFH treatment groups were plotted in a graph against the variation in costs of each clinical event.
RESULTS
The clinical VTE and bleeding event rates as collected from the PREVAIL trial are shown in Table 1. The hospital costs per clinical event are shown in Table 2. The most costly clinical event from the hospital perspective was intracranial hemorrhage at $4001, followed by major extracranial hemorrhage at $3534. The costs of DVT and PE were $3003 and $2143, respectively.
| Event Rate | 95% CI | |
|---|---|---|
| ||
| Enoxaparin (NIHSS <14) | ||
| Deep‐vein thrombosis | 0.081 | 0.05730.1048 |
| Pulmonary embolism | 0.002 | 0.000050.011 |
| Intracranial hemorrhage | 0.0031 | 00.0074 |
| Major extracranial hemorrhage | 0.0047 | 00.0099 |
| Minor hemorrhage | 0.0372 | 0.0240.0549 |
| Enoxaparin (NIHSS 14) | ||
| Deep‐vein thrombosis | 0.1625 | 0.10530.2197 |
| Pulmonary embolism | 0 | 00 |
| Intracranial hemorrhage | 0.0086 | 00.0205 |
| Major extracranial hemorrhage | 0.0172 | 0.04660.1198 |
| Minor hemorrhage | 0.0776 | 0.04660.1198 |
| UFH (NIHSS <14) | ||
| Deep‐vein thrombosis | 0.1356 | 0.10540.1658 |
| Pulmonary embolism | 0.004 | 0.00050.0145 |
| Intracranial hemorrhage | 0.0032 | 00.0077 |
| Major extracranial hemorrhage | 0 | 00 |
| Minor hemorrhage | 0.0514 | 0.03550.0719 |
| UFH (NIHSS 14) | ||
| Deep‐vein thrombosis | 0.2914 | 0.22410.3588 |
| Pulmonary embolism | 0.0229 | 0.00630.0575 |
| Intracranial hemorrhage | 0.016 | 0.00040.0316 |
| Major extracranial hemorrhage | 0 | 00 |
| Minor hemorrhage | 0.064 | 0.0370.1019 |
| Event | Cost per Event ($)* | ||
|---|---|---|---|
| Likeliest | Minimum | Maximum | |
| |||
| Deep‐vein thrombosis | 3,003 | 2,402 | 3,604 |
| Pulmonary embolism | 2,143 | 1,714 | 2,572 |
| Intracranial hemorrhage | 4,001 | 3,201 | 4,801 |
| Major extracranial hemorrhage | 3,534 | 2,827 | 4,241 |
| Minor hemorrhage | 1,322 | 1,058 | 1,586 |
| Enoxaparin cost per dose | 26 | 21 | 31 |
| Unfractionated heparin cost per dose | 3 | 2 | 4 |
The average hospital cost with enoxaparin, when taking into account the costs of VTE and bleeding, was lower than with UFH ($422 vs $662, respectively), with a net savings of $240 per patient if enoxaparin was used. The average drug costs, including drug‐administration costs, were higher in the enoxaparin group ($360) compared with the UFH group ($259; difference $101). Nevertheless, the total hospital cost when clinical events and drug costs were considered together, was lower with enoxaparin than UFH. The total hospital costs per patient were $782 in patients receiving prophylaxis with enoxaparin and $922 in patients receiving UFH. Thus, enoxaparin was associated with a total cost‐savings of $140 per patient (Figure 2).

The cost estimates according to the stroke severity score (NIHSS scores <14 vs 14) are described in Table 3. The drug costs were consistent, regardless of stroke severity, for enoxaparin ($360) and for UFH ($259). However, in both treatment groups, the event costs were higher in patients with more severe stroke, compared with less severe stroke. For example, in the enoxaparin group, the event costs were $686 in patients with NIHSS scores 14 and $326 in patients with NIHSS scores <14. Nevertheless, the overall costs (event costs plus drug costs) were lower with enoxaparin compared with UFH, both in patients with less severe and more severe stroke. In fact, the total hospital cost‐savings were greater when enoxaparin was used instead of UFH in patients with more severe stroke (cost‐saving $287 if NIHSS score 14 vs $71 if NIHSS score <14) (Table 3).
| Enoxaparin ($) | UFH ($) | Difference ($ [UFHEnoxaparin]) | |
|---|---|---|---|
| |||
| NIHSS score <14 | |||
| Mean event costs per patient | 326 | 497 | 171 |
| Mean drug costs per patient* | 360 | 259 | 101 |
| Total costs | 685 | 756 | 71 |
| NIHSS score 14 | |||
| Mean event costs per patient | 686 | 1,073 | 387 |
| Mean drug costs per patient* | 360 | 259 | 101 |
| Total costs | 1,046 | 1,332 | 287 |
Multiple sensitivity analyses were performed. In the base case univariate sensitivity analysis, individual costs were adjusted by 20% (Table 4). If the cost of DVT increased by 20% (from $3003 to $3604) the difference between the enoxaparin and UFH groups was $187. When the cost of DVT was decreased by 20% to $2402, enoxaparin was still cost‐saving, with a difference of $94. For each of the individual cost parameters that were varied (DVT, PE, intracranial hemorrhage, major extracranial hemorrhage, and minor hemorrhage), enoxaparin was always less costly than UFH. Subsequent sensitivity analyses were performed (not shown) where cost parameters were varied by 5%, 10%, 15%, 30%, and 40%. Enoxaparin remained less costly than UFH in all cases.
| Event | Baseline Cost Input ($) | +20% Cost Input ($) | +20% Difference ($ [UFH Enoxaparin]) (% Change) | 20% Cost Input ($) | 20% Difference ($ [UFH Enoxaparin]) (% Change) |
|---|---|---|---|---|---|
| |||||
| Deep‐vein thrombosis | 3,003 | 3,604 | 187 (33) | 2,402 | 94 (33) |
| Pulmonary embolism | 2,143 | 2,572 | 144 (2.5) | 1,714 | 137 (2.5) |
| Intracranial hemorrhage | 4,001 | 4,801 | 142 (1.3) | 3,201 | 138 (1.3) |
| Major extracranial hemorrhage | 3,534 | 4,241 | 134 (4.0) | 2,827 | 146 (4.0) |
| Minor hemorrhage | 1,322 | 1,586 | 142 (1.3) | 1,058 | 138 (1.3) |
A multivariate analysis was performed using a Monte Carlo simulation in TreeAge Pro (Figure 3). When all parameters were varied simultaneously (by 5%, 10%, 15%, 20%, 30%, and 40%) and the differences in costs between the enoxaparin and UFH groups were measured and plotted, the mean (standard deviation) difference between enoxaparin and UFH prophylaxis was $140 ($79) (Figure 3). Figure 4 shows a graphical presentation of the sensitivity analysis results for event probabilities and costs. Differences in enoxaparin drug costs, hospital costs for DVT, and probability of DVT for patients on enoxaparin are the factors that have the greatest effect on the overall cost.


Finally, an additional scenario was performed using a published ratio of asymptomatic DVT to symptomatic VTE, due to the fact that not all VTE events in the real‐world present with symptoms prompting treatment. Quinlan et al. determined a ratio of asymptomatic DVT to symptomatic VTE of 5 for total hip replacement patients and of 21 for total knee replacement patients.28 Although derived from different patient populations who received different anticoagulants, we utilized the symptomatic event rates from the pooled studies to recalculate cost differences between enoxaparin and UFH in acute ischemic stroke. Using only symptomatic event rates, based on the 21:1 ratio in patients undergoing total knee replacement, the total cost for enoxaparin was $485 compared to $386 for UFH. Similar results were found based on the 5:1 ratio in patients with total hip replacement (enoxaparin $532 vs $472 for UFH). This was the only scenario where the higher drug cost of prophylaxis with enoxaparin was not completely offset by the reduction in events compared to UFH, likely due to the smaller difference in event rates once examining only symptomatic VTE.
DISCUSSION
This analysis demonstrates that, from the hospital perspective, enoxaparin 40 mg subcutaneously once‐daily is associated with lower total hospital costs and is more cost‐effective than twice‐daily UFH 5000 U subcutaneously for the prevention of VTE in patients with acute ischemic stroke. Despite higher drug‐acquisition costs, enoxaparin was associated with total cost‐savings of $140 per patient. This is due to the lower event rates with enoxaparin compared with UFH.
Previous studies, using hospital or payer information, have shown that VTE prophylaxis is more cost‐effective compared with no prophylaxis. In terms of the different VTE prophylaxis regimens, enoxaparin represents a more cost‐effective option in comparison with UFH19, 21, 2932 and also when compared with fondaparinux.21, 33 When comparing the results between different trials, it should be noted that previous analyses were mainly modeled on the Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) study, which was performed in general medical patients and reported a VTE rate of 5.5%.6 However, patients with acute ischemic stroke are at a higher risk of VTE, with a 10% incidence of VTE reported in the PREVAIL study.14 Furthermore, twice‐daily rather than three‐timesdaily administration of UFH was used in the PREVAIL study, based on the current practice patterns seen during the PREVAIL trial design.
A recent retrospective analysis of transactional billing records demonstrated that, despite higher mean costs of anticoagulation therapy, the mean, total, adjusted direct hospital costs were lower with LMWH thromboprophylaxis compared with UFH ($7358 vs $8680, respectively; difference $1322; P < 0.001).21 A previous study by Burleigh and colleagues based on hospital discharge information extracted from both medical and surgical patients, has a sub‐analysis in patients with stroke. In these patients also, the total costs were lower for enoxaparin compared with UFH ($8608 vs $8911, respectively; difference $303).29 In the Burleigh study, drug costs and total discharge costs (eg, room and board, laboratory, and diagnostic imaging) were derived from drug charges and total charges, and were converted to estimated costs using cost‐to‐charge methods, so the absolute figures are not directly comparable with the current analysis.
This study adds to current literature by using data from a prospective study to analyze the hospital costs of VTE prophylaxis in stroke patients. The current study also provides a valuable cost‐analysis regarding a specific subgroup of medical patients at particularly high risk of VTE, and provides an economic comparison among stroke patients with NIHSS scores of <14 versus 14. In the PREVAIL study, despite a 2‐fold higher incidence of VTE in patients with more severe stroke (16.3% vs 8.3%), a similar reduction in VTE risk was observed with enoxaparin versus UFH in patients with NIHSS scores of 14 (odds ratio = 0.56; 95% CI = 0.37‐0.84; P = 0.0036) and <14 (odds ratio = 0.46; 95% CI = 0.27‐0.78; P = 0.0043).14 Enoxaparin was shown to be cost‐saving relative to UFH in both patient groups and, in particular, in patients with more severe stroke.
Potential limitations of the current analysis include the applicability of the figures obtained from the highly selected clinical trial population to real‐world clinical practice, and the fact that it is difficult to match cost estimates to trial data definitions. For example, this analysis was conducted with a comparator of twice‐daily UFH (as opposed to three‐timesdaily) which may be used in the real‐world setting and may have resulted in the increased number of events in the UFH group seen in the PREVAIL study. Due to a variety of differences between real‐world practice patterns and the PREVAIL clinical trial, we can only speculate as to the true cost‐consequences of utilizing enoxaparin versus UFH.
Furthermore, the original model did not include a sub‐analysis regarding the rates and, therefore, costs of proximal/symptomatic VTE. In the primary study of PREVAIL, the rates of symptomatic DVT were 1 in 666 patients (<1%) for enoxaparin and 4 in 669 patients (1%) for UFH, whereas the rates of proximal DVT were 30 in 666 patients (5%) and 64 in 669 patients (10%), respectively. Sensitivity analyses were performed to investigate the impact of lower rates of both DVT and PE (up to 40%), and the differences between groups were found to be robust. However, it is important to note that overall costs for both groups may have been increased through the inclusion of asymptomatic costs, with a more distinct separation of these costs making for a good follow‐up study. In a similar cost‐analysis we performed based on the PREVAIL study, which assessed the cost to the payer, we included an analysis of costs according to 3 different VTE definitions: the PREVAIL VTE definition (as in the current study); a definition of major VTE (PE, symptomatic DVT, and asymptomatic proximal DVT); and primary endpoints recommended by the European Medicines Agency Committee for Medicinal Products for Human Use for studies on VTE (proximal DVT, nonfatal PE, and all‐cause mortality). We found similar results irrespective of clinical event definitions.34 In an additional model scenario using a published ratio of asymptomatic DVT to symptomatic VTE,28 the higher drug cost of prophylaxis with enoxaparin was not completely offset by the reduction in events compared to UFH. This was likely due to the smaller difference in event rates once examining only symptomatic VTE. This scenario was limited by the fact that the ratio was derived from different patient populations receiving different anticoagulants than stroke patients.
In conclusion, data from this analysis adds to the evidence that, from the hospital perspective, the higher drug cost of enoxaparin is offset by the economic consequences of the events avoided as compared with UFH for the prevention of VTE following acute ischemic stroke, particularly in patients with severe stroke.
Acknowledgements
The authors thank Aylin Lee from I3 Innovus for her contribution to this study. The authors also acknowledge Min Chen for her assistance in statistical analysis, and Essy Mozaffari for his contribution to this study.
Venous thromboembolism (VTE), which encompasses both deep‐vein thrombosis (DVT) and pulmonary embolism (PE), is a major health problem in the United States and worldwide. It represents one of the most significant causes of morbidity and mortality with an estimated 300,000 VTE‐related deaths,1 and 300,000‐600,000 hospitalizations in the United States annually.2 Hospitalization for medical illness is associated with a similar proportion of VTE cases as hospitalization for surgery.3 Several groups of medical patients have been shown to be at an increased risk of VTE, including those with cancer, severe respiratory disease, acute infectious illness, heart failure, myocardial infarction, and acute ischemic stroke.47 Ischemic stroke patients represent approximately 4.6% of medical patients at high risk of VTE in US hospitals.8 The incidence of DVT in such patients has been reported to be as high as 75%9 and PE has been reported to be responsible for up to 25% of early deaths after stroke.10
Several studies have demonstrated the efficacy of unfractionated heparin (UFH) or a low‐molecular‐weight heparin (LMWH) in the prevention of VTE in stroke patients, and have demonstrated that LMWHs are at least as effective as UFH.1114 The open‐label, randomized Prevention of VTE after acute ischemic stroke with LMWH and UFH (PREVAIL) trial demonstrated that in patients with acute ischemic stroke, prophylaxis for 10 days with the LMWH enoxaparin reduces the risk of VTE by 43% compared with UFH (10.2% vs 18.1%, respectively; relative risk = 0.57; 95% confidence interval [CI] = 0.44‐0.76; P = 0.0001) without increasing the incidence of overall bleeding events (7.9% vs 8.1%, respectively; P = 0.83), or the composite of symptomatic intracranial and major extracranial hemorrhage (1% in each group; P = 0.23). There was, however, a slight but significant increase in major extracranial hemorrhage alone with enoxaparin (1% vs 0%; P = 0.015).14 Evidence‐based guidelines from the American College of Chest Physicians (ACCP) provide recommendations for appropriate thromboprophylaxis regimens for patients at risk of VTE.15 Thromboprophylaxis with UFH, LMWH, and, more recently, fondaparinux is recommended for medical patients admitted to hospital with congestive heart failure or severe respiratory disease, or those who are confined to bed and have one or more additional risk factors, including active cancer, previous VTE, or acute neurologic disease.15 Similarly, in the Eighth ACCP Clinical Practice Guidelines, low‐dose UFH or LMWH are recommended for VTE prevention in patients with ischemic stroke who have restricted mobility.16
VTE is also associated with a substantial economic burden on the healthcare system, costing an estimated $1.5 billion annually in the United States.17 Thromboprophylaxis has been shown to be a cost‐effective strategy in hospitalized medical patients. Prophylaxis with a LMWH has been shown to be more cost‐effective than UFH in these patients.1821
However, despite the clinical and economic benefits, prophylaxis is still commonly underused in medical patients.22, 23 In surgical patients, the Surgical Care Improvement Project (SCIP) focuses on reducing surgical complications, and has endorsed 2 measures: VTE‐1, relating to the proportion of patients for whom VTE prophylaxis is ordered; and VTE‐2, relating to those who receive the recommended regimen (
The objective of the current study was to determine the economic impact, in terms of hospital costs, of enoxaparin compared with UFH for VTE prophylaxis after acute ischemic stroke. A decision‐analytic model was constructed using data from the PREVAIL study and historical inpatient data from a multi‐hospital database.
METHODS
In this study, the cost implications, from the hospital perspective, of VTE prophylaxis with enoxaparin or UFH in patients with acute ischemic stroke, were determined using a decision‐analytic model in TreeAge Pro Suite (TreeAge Software, Inc., Williamstown, MA, USA). The decision‐tree was based on 3 stages: (a) whether patients received enoxaparin or UFH; (b) how patients were classified according to their National Institutes of Health Stroke Scale (NIHSS) classification scores (<14 or 14); and (c) which clinical event each patient experienced, as defined per the PREVAIL trial (DVT, PE, intracranial hemorrhage, major extracranial hemorrhage, and minor extracranial hemorrhage) (Figure 1). The time horizon for the model was established at 90 days to mirror the length of follow‐up in the PREVAIL trial.

Total hospital costs were calculated based on clinical event rates (from the PREVAIL trial) and the costs of each clinical event, which were calculated separately according to the descriptions below, and then inserted into the decision‐analytic model. The clinical event rates were calculated from the efficacy and safety endpoints collected in the PREVAIL trial, and included VTE events (DVT and PE) and bleeding events (intracranial hemorrhage, major extracranial hemorrhage, and minor extracranial hemorrhage). Details of the patient population, eligibility criteria, and treatment regimen have previously been published in full elsewhere.14, 27
The costs of clinical events during hospitalization were estimated using a multivariate cost‐evaluation model, based on mean hospital costs for the events in the (Premier Inc., Charlotte, NC, USA) multi‐hospital database, one of the largest US hospital clinical and economic databases. The data are received from over 600 hospitals, representing all geographical areas of the United States, a broad range of bed sizes, teaching and non‐teaching, and urban and rural facilities. This database contains detailed US inpatient care records of principal and secondary diagnoses, inpatient procedures, administered laboratory tests, dispensed drugs, and demographic information. The evaluation of hospital cost for each type of clinical event was conducted by i3 Innovus (Ingenix, Inc., Eden Prairie, MN, USA). Total hospital costs were cumulative from all events, so if patients experienced multiple clinical events, the costs of the events were additive. The cost for stroke treatment and management was not included because it is an inclusion criterion of the PREVAIL trial and, thus, all patients in the trial have such costs.
Default drug costs were taken from the 2008 US wholesalers' acquisition cost data. The default dosing schedule is based on information extracted from the PREVAIL trial: enoxaparin 40 mg (once‐daily) and UFH 5000 U (twice‐daily) for 10 days each ($25.97 and $2.97, respectively). A drug‐administration fee was added for each dose of either enoxaparin or UFH ($10 for each).19
The estimated hospital cost of clinical events, along with drug costs, were inserted into the decision‐analytic model in TreeAge Pro Suite to estimate the cost per discharge from the hospital perspective in patients with ischemic stroke receiving VTE prophylaxis with enoxaparin or UFH. An additional analysis was performed to investigate the costs and cost differences in patients with less severe stroke (NIHSS scores <14) and more severe stroke (NIHSS scores 14).
Sensitivity analyses were performed to examine the impact of varying the cost inputs on the total hospital cost of each treatment arm by 5%, 10%, 15%, 20%, 30%, and 40%, and the robustness in the difference in costs between the enoxaparin and UFH groups. Univariate (via tornado diagram in TreeAge Pro Suite) and multivariate (via Monte Carlo simulation in TreeAge Pro Suite) analyses were performed. For the univariate analysis, each clinical event cost was adjusted individually, increasing or decreasing by 5%, 10%, 15%, 20%, 30% and 40% while other parameters remained unchanged. For the Monte Carlo simulation (TreeAge Pro Suite), all the parameters were simultaneously varied in a random fashion, within a range of 5%, 10%, 15%, 20%, 30%, and 40% over 10,000 trials. The simulation adopted a gamma distribution assumption for input sampling for cost parameters and a beta distribution for the event probability parameters. The confidence intervals for the probability parameters were obtained from the PREVAIL trial. The differences between the enoxaparin and UFH treatment groups were plotted in a graph against the variation in costs of each clinical event.
RESULTS
The clinical VTE and bleeding event rates as collected from the PREVAIL trial are shown in Table 1. The hospital costs per clinical event are shown in Table 2. The most costly clinical event from the hospital perspective was intracranial hemorrhage at $4001, followed by major extracranial hemorrhage at $3534. The costs of DVT and PE were $3003 and $2143, respectively.
| Event Rate | 95% CI | |
|---|---|---|
| ||
| Enoxaparin (NIHSS <14) | ||
| Deep‐vein thrombosis | 0.081 | 0.05730.1048 |
| Pulmonary embolism | 0.002 | 0.000050.011 |
| Intracranial hemorrhage | 0.0031 | 00.0074 |
| Major extracranial hemorrhage | 0.0047 | 00.0099 |
| Minor hemorrhage | 0.0372 | 0.0240.0549 |
| Enoxaparin (NIHSS 14) | ||
| Deep‐vein thrombosis | 0.1625 | 0.10530.2197 |
| Pulmonary embolism | 0 | 00 |
| Intracranial hemorrhage | 0.0086 | 00.0205 |
| Major extracranial hemorrhage | 0.0172 | 0.04660.1198 |
| Minor hemorrhage | 0.0776 | 0.04660.1198 |
| UFH (NIHSS <14) | ||
| Deep‐vein thrombosis | 0.1356 | 0.10540.1658 |
| Pulmonary embolism | 0.004 | 0.00050.0145 |
| Intracranial hemorrhage | 0.0032 | 00.0077 |
| Major extracranial hemorrhage | 0 | 00 |
| Minor hemorrhage | 0.0514 | 0.03550.0719 |
| UFH (NIHSS 14) | ||
| Deep‐vein thrombosis | 0.2914 | 0.22410.3588 |
| Pulmonary embolism | 0.0229 | 0.00630.0575 |
| Intracranial hemorrhage | 0.016 | 0.00040.0316 |
| Major extracranial hemorrhage | 0 | 00 |
| Minor hemorrhage | 0.064 | 0.0370.1019 |
| Event | Cost per Event ($)* | ||
|---|---|---|---|
| Likeliest | Minimum | Maximum | |
| |||
| Deep‐vein thrombosis | 3,003 | 2,402 | 3,604 |
| Pulmonary embolism | 2,143 | 1,714 | 2,572 |
| Intracranial hemorrhage | 4,001 | 3,201 | 4,801 |
| Major extracranial hemorrhage | 3,534 | 2,827 | 4,241 |
| Minor hemorrhage | 1,322 | 1,058 | 1,586 |
| Enoxaparin cost per dose | 26 | 21 | 31 |
| Unfractionated heparin cost per dose | 3 | 2 | 4 |
The average hospital cost with enoxaparin, when taking into account the costs of VTE and bleeding, was lower than with UFH ($422 vs $662, respectively), with a net savings of $240 per patient if enoxaparin was used. The average drug costs, including drug‐administration costs, were higher in the enoxaparin group ($360) compared with the UFH group ($259; difference $101). Nevertheless, the total hospital cost when clinical events and drug costs were considered together, was lower with enoxaparin than UFH. The total hospital costs per patient were $782 in patients receiving prophylaxis with enoxaparin and $922 in patients receiving UFH. Thus, enoxaparin was associated with a total cost‐savings of $140 per patient (Figure 2).

The cost estimates according to the stroke severity score (NIHSS scores <14 vs 14) are described in Table 3. The drug costs were consistent, regardless of stroke severity, for enoxaparin ($360) and for UFH ($259). However, in both treatment groups, the event costs were higher in patients with more severe stroke, compared with less severe stroke. For example, in the enoxaparin group, the event costs were $686 in patients with NIHSS scores 14 and $326 in patients with NIHSS scores <14. Nevertheless, the overall costs (event costs plus drug costs) were lower with enoxaparin compared with UFH, both in patients with less severe and more severe stroke. In fact, the total hospital cost‐savings were greater when enoxaparin was used instead of UFH in patients with more severe stroke (cost‐saving $287 if NIHSS score 14 vs $71 if NIHSS score <14) (Table 3).
| Enoxaparin ($) | UFH ($) | Difference ($ [UFHEnoxaparin]) | |
|---|---|---|---|
| |||
| NIHSS score <14 | |||
| Mean event costs per patient | 326 | 497 | 171 |
| Mean drug costs per patient* | 360 | 259 | 101 |
| Total costs | 685 | 756 | 71 |
| NIHSS score 14 | |||
| Mean event costs per patient | 686 | 1,073 | 387 |
| Mean drug costs per patient* | 360 | 259 | 101 |
| Total costs | 1,046 | 1,332 | 287 |
Multiple sensitivity analyses were performed. In the base case univariate sensitivity analysis, individual costs were adjusted by 20% (Table 4). If the cost of DVT increased by 20% (from $3003 to $3604) the difference between the enoxaparin and UFH groups was $187. When the cost of DVT was decreased by 20% to $2402, enoxaparin was still cost‐saving, with a difference of $94. For each of the individual cost parameters that were varied (DVT, PE, intracranial hemorrhage, major extracranial hemorrhage, and minor hemorrhage), enoxaparin was always less costly than UFH. Subsequent sensitivity analyses were performed (not shown) where cost parameters were varied by 5%, 10%, 15%, 30%, and 40%. Enoxaparin remained less costly than UFH in all cases.
| Event | Baseline Cost Input ($) | +20% Cost Input ($) | +20% Difference ($ [UFH Enoxaparin]) (% Change) | 20% Cost Input ($) | 20% Difference ($ [UFH Enoxaparin]) (% Change) |
|---|---|---|---|---|---|
| |||||
| Deep‐vein thrombosis | 3,003 | 3,604 | 187 (33) | 2,402 | 94 (33) |
| Pulmonary embolism | 2,143 | 2,572 | 144 (2.5) | 1,714 | 137 (2.5) |
| Intracranial hemorrhage | 4,001 | 4,801 | 142 (1.3) | 3,201 | 138 (1.3) |
| Major extracranial hemorrhage | 3,534 | 4,241 | 134 (4.0) | 2,827 | 146 (4.0) |
| Minor hemorrhage | 1,322 | 1,586 | 142 (1.3) | 1,058 | 138 (1.3) |
A multivariate analysis was performed using a Monte Carlo simulation in TreeAge Pro (Figure 3). When all parameters were varied simultaneously (by 5%, 10%, 15%, 20%, 30%, and 40%) and the differences in costs between the enoxaparin and UFH groups were measured and plotted, the mean (standard deviation) difference between enoxaparin and UFH prophylaxis was $140 ($79) (Figure 3). Figure 4 shows a graphical presentation of the sensitivity analysis results for event probabilities and costs. Differences in enoxaparin drug costs, hospital costs for DVT, and probability of DVT for patients on enoxaparin are the factors that have the greatest effect on the overall cost.


Finally, an additional scenario was performed using a published ratio of asymptomatic DVT to symptomatic VTE, due to the fact that not all VTE events in the real‐world present with symptoms prompting treatment. Quinlan et al. determined a ratio of asymptomatic DVT to symptomatic VTE of 5 for total hip replacement patients and of 21 for total knee replacement patients.28 Although derived from different patient populations who received different anticoagulants, we utilized the symptomatic event rates from the pooled studies to recalculate cost differences between enoxaparin and UFH in acute ischemic stroke. Using only symptomatic event rates, based on the 21:1 ratio in patients undergoing total knee replacement, the total cost for enoxaparin was $485 compared to $386 for UFH. Similar results were found based on the 5:1 ratio in patients with total hip replacement (enoxaparin $532 vs $472 for UFH). This was the only scenario where the higher drug cost of prophylaxis with enoxaparin was not completely offset by the reduction in events compared to UFH, likely due to the smaller difference in event rates once examining only symptomatic VTE.
DISCUSSION
This analysis demonstrates that, from the hospital perspective, enoxaparin 40 mg subcutaneously once‐daily is associated with lower total hospital costs and is more cost‐effective than twice‐daily UFH 5000 U subcutaneously for the prevention of VTE in patients with acute ischemic stroke. Despite higher drug‐acquisition costs, enoxaparin was associated with total cost‐savings of $140 per patient. This is due to the lower event rates with enoxaparin compared with UFH.
Previous studies, using hospital or payer information, have shown that VTE prophylaxis is more cost‐effective compared with no prophylaxis. In terms of the different VTE prophylaxis regimens, enoxaparin represents a more cost‐effective option in comparison with UFH19, 21, 2932 and also when compared with fondaparinux.21, 33 When comparing the results between different trials, it should be noted that previous analyses were mainly modeled on the Prophylaxis in Medical Patients with Enoxaparin (MEDENOX) study, which was performed in general medical patients and reported a VTE rate of 5.5%.6 However, patients with acute ischemic stroke are at a higher risk of VTE, with a 10% incidence of VTE reported in the PREVAIL study.14 Furthermore, twice‐daily rather than three‐timesdaily administration of UFH was used in the PREVAIL study, based on the current practice patterns seen during the PREVAIL trial design.
A recent retrospective analysis of transactional billing records demonstrated that, despite higher mean costs of anticoagulation therapy, the mean, total, adjusted direct hospital costs were lower with LMWH thromboprophylaxis compared with UFH ($7358 vs $8680, respectively; difference $1322; P < 0.001).21 A previous study by Burleigh and colleagues based on hospital discharge information extracted from both medical and surgical patients, has a sub‐analysis in patients with stroke. In these patients also, the total costs were lower for enoxaparin compared with UFH ($8608 vs $8911, respectively; difference $303).29 In the Burleigh study, drug costs and total discharge costs (eg, room and board, laboratory, and diagnostic imaging) were derived from drug charges and total charges, and were converted to estimated costs using cost‐to‐charge methods, so the absolute figures are not directly comparable with the current analysis.
This study adds to current literature by using data from a prospective study to analyze the hospital costs of VTE prophylaxis in stroke patients. The current study also provides a valuable cost‐analysis regarding a specific subgroup of medical patients at particularly high risk of VTE, and provides an economic comparison among stroke patients with NIHSS scores of <14 versus 14. In the PREVAIL study, despite a 2‐fold higher incidence of VTE in patients with more severe stroke (16.3% vs 8.3%), a similar reduction in VTE risk was observed with enoxaparin versus UFH in patients with NIHSS scores of 14 (odds ratio = 0.56; 95% CI = 0.37‐0.84; P = 0.0036) and <14 (odds ratio = 0.46; 95% CI = 0.27‐0.78; P = 0.0043).14 Enoxaparin was shown to be cost‐saving relative to UFH in both patient groups and, in particular, in patients with more severe stroke.
Potential limitations of the current analysis include the applicability of the figures obtained from the highly selected clinical trial population to real‐world clinical practice, and the fact that it is difficult to match cost estimates to trial data definitions. For example, this analysis was conducted with a comparator of twice‐daily UFH (as opposed to three‐timesdaily) which may be used in the real‐world setting and may have resulted in the increased number of events in the UFH group seen in the PREVAIL study. Due to a variety of differences between real‐world practice patterns and the PREVAIL clinical trial, we can only speculate as to the true cost‐consequences of utilizing enoxaparin versus UFH.
Furthermore, the original model did not include a sub‐analysis regarding the rates and, therefore, costs of proximal/symptomatic VTE. In the primary study of PREVAIL, the rates of symptomatic DVT were 1 in 666 patients (<1%) for enoxaparin and 4 in 669 patients (1%) for UFH, whereas the rates of proximal DVT were 30 in 666 patients (5%) and 64 in 669 patients (10%), respectively. Sensitivity analyses were performed to investigate the impact of lower rates of both DVT and PE (up to 40%), and the differences between groups were found to be robust. However, it is important to note that overall costs for both groups may have been increased through the inclusion of asymptomatic costs, with a more distinct separation of these costs making for a good follow‐up study. In a similar cost‐analysis we performed based on the PREVAIL study, which assessed the cost to the payer, we included an analysis of costs according to 3 different VTE definitions: the PREVAIL VTE definition (as in the current study); a definition of major VTE (PE, symptomatic DVT, and asymptomatic proximal DVT); and primary endpoints recommended by the European Medicines Agency Committee for Medicinal Products for Human Use for studies on VTE (proximal DVT, nonfatal PE, and all‐cause mortality). We found similar results irrespective of clinical event definitions.34 In an additional model scenario using a published ratio of asymptomatic DVT to symptomatic VTE,28 the higher drug cost of prophylaxis with enoxaparin was not completely offset by the reduction in events compared to UFH. This was likely due to the smaller difference in event rates once examining only symptomatic VTE. This scenario was limited by the fact that the ratio was derived from different patient populations receiving different anticoagulants than stroke patients.
In conclusion, data from this analysis adds to the evidence that, from the hospital perspective, the higher drug cost of enoxaparin is offset by the economic consequences of the events avoided as compared with UFH for the prevention of VTE following acute ischemic stroke, particularly in patients with severe stroke.
Acknowledgements
The authors thank Aylin Lee from I3 Innovus for her contribution to this study. The authors also acknowledge Min Chen for her assistance in statistical analysis, and Essy Mozaffari for his contribution to this study.
- .Venous thromboembolism: disease burden, outcomes and risk factors.J Thromb Haemost.2005;3:1611–1617.
- .The epidemiology of venous thromboembolism in the community: implications for prevention and management.J Thromb Thrombolysis.2006;21:23–29.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study.Arch Intern Med.2002;162:1245–1248.
- ,,,,,.Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study.Arch Intern Med.2000;160:809–815.
- ,.A safety analysis of thromboprophylaxis in acute medical illness.Thromb Haemost.2003;89:590–591.
- ,,,,.Quantification of risk factors for venous thromboembolism: a preliminary study for the development of a risk assessment tool.Haematologica.2003;88:1410–1421.
- ,.Prevalence of venous thromboembolism in acute hemorrhagic and thromboembolic stroke.Am J Phys Med Rehabil.2003;82:364–369.
- ,,,.Thromboprophylaxis rates in US medical centers: success or failure?J Thromb Haemost.2007;5:1610–1616.
- ,,,.Complications after acute stroke.Stroke.1996;27:415–420.
- ,,,.Venous thromboembolism after acute stroke.Stroke.2001;32:262–267.
- ,,,,,.Enoxaparin vs heparin for prevention of deep‐vein thrombosis in acute ischaemic stroke: a randomized, double‐blind study.Acta Neurol Scand.2002;106:84–92.
- ,,.Low‐molecular‐weight heparins or heparinoids versus standard unfractionated heparin for acute ischaemic stroke.Cochrane Database Syst Rev.2008;(3):CD000119.
- ,,, et al;for the PROTECT Trial Group.Prophylaxis of thrombotic and embolic events in acute ischemic stroke with the low‐molecular‐weight heparin certoparin: results of the PROTECT Trial.Stroke.2006;37:139–144.
- ,,, et al;for the PREVAIL Investigators.The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL study): an open‐label randomised comparison.Lancet.2007;369:1347–1355.
- ,,, et al;for the American College of Chest Physicians.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th ed).Chest.2008;133(6 suppl):381S–453S.
- ,,,,;for the American College of Chest Physicians.Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th ed).Chest.2008;133(6 suppl):630S–669S.
- ,,,.Management of acute proximal deep vein thrombosis: pharmacoeconomic evaluation of outpatient treatment with enoxaparin vs inpatient treatment with unfractionated heparin.Chest.2002;122:108–114.
- ,.Economic evaluation of enoxaparin as prophylaxis against venous thromboembolism in seriously ill medical patients: a US perspective.Am J Manag Care.2002;8:1082–1088.
- ,,,.Cost effectiveness of thromboprophylaxis with a low‐molecular‐weight heparin versus unfractionated heparin in acutely ill medical inpatients.Am J Manag Care.2004;10:632–642.
- ,,, et al.Cost effectiveness of enoxaparin as prophylaxis against venous thromboembolic complications in acutely ill medical inpatients: modelling study from the hospital perspective in Germany.Pharmacoeconomics.2006;24:571–591.
- ,,,,.Hospital‐based costs associated with venous thromboembolism prophylaxis regimens.J Thromb Thrombolysis.2010;29:449–458.
- ,,, et al;for the ENDORSE Investigators.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371:387–394.
- ,,, et al;for the IMPROVE Investigators.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism.Chest.2007;132:936–945.
- United States Department of Health August 2008. Available at: http://www.ahrq.gov/qual/vtguide/. Accessed August 18,2010.
- ,,,.Enoxaparin versus unfractionated heparin in the prevention of venous thromboembolism after acute ischemic stroke: rationale, design, and methods of an open‐label, randomized, parallel‐group multicenter trial.J Stroke Cerebrovasc Dis.2005;14:95–100.
- ,,,,,.Association between asymptomatic deep vein thrombosis detected by venography and symptomatic venous thromboembolism in patients undergoing elective hip or knee surgery.J Thromb Haemost.2007;5:1438–1443.
- ,,, et al.Thromboprophylaxis in medically ill patients at risk for venous thromboembolism.Am J Health Syst Pharm.2006;63(20 suppl 6):S23–S29.
- ,,,.Comparison of the two‐year outcomes and costs of prophylaxis in medical patients at risk of venous thromboembolism.Thromb Haemost.2008;100:810–820.
- ,,,,,.Cost for inpatient care of venous thrombosis: a trial of enoxaparin vs standard heparin.Arch Intern Med.2000;160:3160–3165.
- ,,,.Economic evaluation of enoxaparin for the prevention of venous thromboembolism in acutely ill medical patients.Pharm World Sci.2004;26:214–220.
- ,,,,.Total hospital‐based costs of enoxaparin or fondaparinux prophylaxis in patients at risk of venous thromboembolism [abstract]. Presented at the Chest 2008 Annual Meeting; October 25–30,2008; Philadelphia, PA.
- ,,,,.Economic impact of enoxaparin after acute ischemic stroke based on PREVAIL.Clin Appl Thromb Hemost.2011;17:150–157.
- .Venous thromboembolism: disease burden, outcomes and risk factors.J Thromb Haemost.2005;3:1611–1617.
- .The epidemiology of venous thromboembolism in the community: implications for prevention and management.J Thromb Thrombolysis.2006;21:23–29.
- ,,, et al.Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study.Arch Intern Med.2002;162:1245–1248.
- ,,,,,.Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study.Arch Intern Med.2000;160:809–815.
- ,.A safety analysis of thromboprophylaxis in acute medical illness.Thromb Haemost.2003;89:590–591.
- ,,,,.Quantification of risk factors for venous thromboembolism: a preliminary study for the development of a risk assessment tool.Haematologica.2003;88:1410–1421.
- ,.Prevalence of venous thromboembolism in acute hemorrhagic and thromboembolic stroke.Am J Phys Med Rehabil.2003;82:364–369.
- ,,,.Thromboprophylaxis rates in US medical centers: success or failure?J Thromb Haemost.2007;5:1610–1616.
- ,,,.Complications after acute stroke.Stroke.1996;27:415–420.
- ,,,.Venous thromboembolism after acute stroke.Stroke.2001;32:262–267.
- ,,,,,.Enoxaparin vs heparin for prevention of deep‐vein thrombosis in acute ischaemic stroke: a randomized, double‐blind study.Acta Neurol Scand.2002;106:84–92.
- ,,.Low‐molecular‐weight heparins or heparinoids versus standard unfractionated heparin for acute ischaemic stroke.Cochrane Database Syst Rev.2008;(3):CD000119.
- ,,, et al;for the PROTECT Trial Group.Prophylaxis of thrombotic and embolic events in acute ischemic stroke with the low‐molecular‐weight heparin certoparin: results of the PROTECT Trial.Stroke.2006;37:139–144.
- ,,, et al;for the PREVAIL Investigators.The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL study): an open‐label randomised comparison.Lancet.2007;369:1347–1355.
- ,,, et al;for the American College of Chest Physicians.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th ed).Chest.2008;133(6 suppl):381S–453S.
- ,,,,;for the American College of Chest Physicians.Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th ed).Chest.2008;133(6 suppl):630S–669S.
- ,,,.Management of acute proximal deep vein thrombosis: pharmacoeconomic evaluation of outpatient treatment with enoxaparin vs inpatient treatment with unfractionated heparin.Chest.2002;122:108–114.
- ,.Economic evaluation of enoxaparin as prophylaxis against venous thromboembolism in seriously ill medical patients: a US perspective.Am J Manag Care.2002;8:1082–1088.
- ,,,.Cost effectiveness of thromboprophylaxis with a low‐molecular‐weight heparin versus unfractionated heparin in acutely ill medical inpatients.Am J Manag Care.2004;10:632–642.
- ,,, et al.Cost effectiveness of enoxaparin as prophylaxis against venous thromboembolic complications in acutely ill medical inpatients: modelling study from the hospital perspective in Germany.Pharmacoeconomics.2006;24:571–591.
- ,,,,.Hospital‐based costs associated with venous thromboembolism prophylaxis regimens.J Thromb Thrombolysis.2010;29:449–458.
- ,,, et al;for the ENDORSE Investigators.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371:387–394.
- ,,, et al;for the IMPROVE Investigators.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism.Chest.2007;132:936–945.
- United States Department of Health August 2008. Available at: http://www.ahrq.gov/qual/vtguide/. Accessed August 18,2010.
- ,,,.Enoxaparin versus unfractionated heparin in the prevention of venous thromboembolism after acute ischemic stroke: rationale, design, and methods of an open‐label, randomized, parallel‐group multicenter trial.J Stroke Cerebrovasc Dis.2005;14:95–100.
- ,,,,,.Association between asymptomatic deep vein thrombosis detected by venography and symptomatic venous thromboembolism in patients undergoing elective hip or knee surgery.J Thromb Haemost.2007;5:1438–1443.
- ,,, et al.Thromboprophylaxis in medically ill patients at risk for venous thromboembolism.Am J Health Syst Pharm.2006;63(20 suppl 6):S23–S29.
- ,,,.Comparison of the two‐year outcomes and costs of prophylaxis in medical patients at risk of venous thromboembolism.Thromb Haemost.2008;100:810–820.
- ,,,,,.Cost for inpatient care of venous thrombosis: a trial of enoxaparin vs standard heparin.Arch Intern Med.2000;160:3160–3165.
- ,,,.Economic evaluation of enoxaparin for the prevention of venous thromboembolism in acutely ill medical patients.Pharm World Sci.2004;26:214–220.
- ,,,,.Total hospital‐based costs of enoxaparin or fondaparinux prophylaxis in patients at risk of venous thromboembolism [abstract]. Presented at the Chest 2008 Annual Meeting; October 25–30,2008; Philadelphia, PA.
- ,,,,.Economic impact of enoxaparin after acute ischemic stroke based on PREVAIL.Clin Appl Thromb Hemost.2011;17:150–157.
Copyright © 2011 Society of Hospital Medicine
Chief Resident Year
Academic hospitalists have multiple duties including direct patient care, hospital management, teaching, and scholarly productivity. We are frequently pulled in divergent directions, while attending to these responsibilities. Without a framework to manage these assorted tasks, we are at risk of subpar performance and career dissatisfaction. Alternatively, we often thrive in our roles as utility players when armed with a developed skill set. Our profession could benefit greatly from encouraging future academic hospitalists to obtain further training prior to starting as an attending. Although hospital medicine fellowship training exists, there are still relatively few programs available. A well‐crafted year spent as a chief medical resident (CMR) may be a viable alternative.
My year as CMR allowed me to develop the skills necessary for success as an academic hospitalist in a supportive setting, and has given me an advantage in accelerating my career. This experience provided me with important tools that my initial 3 years of internal medicine residency did not. Even during my final year of residency, I was focused mostly on obtaining medical knowledge, learning its clinical applications, and honing team leadership skills. My mind rarely trespassed into the broader concerns of quality improvement initiatives, educational enhancements, or hospital management issues. Although the American College of Graduate Medical Education (ACGME) core competencies are helping to better focus residents' attention to these more diverse aspects of healthcare, residents still spend the majority of their time providing direct patient care.1 Now in my fourth year as an attending academic hospitalist, I continue to appreciate how my chief experience provided the foundation for much of the work I perform today.
My motivations for becoming a CMR included a desire to spend more time teaching and learning medicine, and an interest in helping to improve the residency program itself. I did not appreciate how much of the job would be spent managing people, and evaluating systems of care within the hospital, while working closely with nurses and hospital administrators. However, the skills I learned while addressing those unexpected tasks are what continue to help me in my position as a multifaceted hospitalist today. CMRs have been described as middle managers, being pushed and pulled by the demands and requirements of the groups above, below and around them.2 Academic hospitalists who frequently wear administrative and educational hats are not dissimilar. Retrospectively, I realize how fortunate I was to be exposed to those aspects as a CMR, with many of the same responsibilities but without the full expectations of a more seasoned attending.
The most memorable interaction during my first day as junior ward attending was with a revered internist, himself a former CMR, who dryly commented, So, you're pretending now. It took me a moment to catch his play on words until he clarified, you are now a pre‐attending. The true meaning of this was elucidated over the next several weeks as I was expected to perform many of the same duties of a seasoned attending, but often had the sense that I was only pretending to be an attending and still had much to learn.
CMR positions vary in terms of clinical, educational, and administrative responsibilities. Moreover, many institutions mix inpatient and outpatient roles. My position was focused almost entirely on inpatient duties at a single hospital, which gave me an in‐depth and longitudinal view of how a hospital is managed. Like many other CMRs, much of my time was spent on educational activities, such as running morning report, preparing for chief of medicine rounds, coordinating noon teaching conferences, and spending time with the medical students. Administrative tasks included various institutional‐based meetings for student grading, educational review committees, and program scheduling. In addition, I spent 1 month as junior attending on a ward team. Many other programs' CMRs spend more time as junior attending; however, by offloading some of this ward service requirement, I feel fortunate to have had that time to use for my own scholarly activity and teaching/administrative opportunities. Perhaps unique to my CMR position, I also was involved with the daily running of the hospital by working with administrators to evaluate patient transfer requests and addressing provider work flow issues. These additional tasks provided an invaluable learning experience.
Organizing morning report, running physical diagnosis rounds, and preparing cases and speakers for Chair's Rounds allowed me to hone and expand my teaching skills in ways that 3 years of residency did not. Moreover, it put me in direct contact with an energetic, inspiring group of learners that challenged me to solidify my own medical knowledge. (Try explaining the delta‐delta equation to figure out if there are 2 metabolic processes going on, in front of a group of 20 residents, and you'll discover what I mean.) I quickly learned that the one doing the talking is the one doing the learning and changed my teaching style to better facilitate student learning. My bedside learning was further augmented by attending Masters' Rounds to which I owe my ongoing interests in physical diagnosis. Masters Rounds were given once a week by 2 master clinicians. It was only for chief residents and was directed at teaching us how to teach others the art of bedside physical diagnosis. The majority of physical exam teaching points I focus on today come from those sessions.
As chief, I felt like I had the pulse of the hospital at all times. Most of my mornings were spent on the wards floating between teams. I owe thanks to my predecessor who told me that the true sign of a good CMR was to never sit long enough in your chair to let it get warm. My office was located on the wards, between a team room and a double patient room. Aside from times when I was having confidential conversations with residents, the door was open. Nurses looking to vent, phlebotomists wanting to sit down, and attendings needing a break from their teams to get work done were common visitors. Administrative personnel were also frequent visitors, usually requesting me to disseminate new policies to the residents. Because of this, I learned to understand and better interact with the diverse group of people responsible for making a teaching hospital function. These are the same constituencies that I now sit down with on various committees to attempt to make my present hospital operate more smoothly.
Despite running morning report, attending rounds with teams, and developing plans for better patient flow, I had time for scholarly work and found easy mentorship. I was able to revive 2 projects I had started as a resident and bring them close to conclusion under the continued mentorship of my coinvestigators. Offers for career skill development were also abound, and I benefited greatly from one associate director's tutorials on preparing effective PowerPoint presentations. Another attending mentored me in student feedback skills, which have allowed me to become a much more effective educator. I was also able to model that mentorship and begin to build my own mentor relationships with my students. In fact, this mentorship has become one of the most fulfilling aspects of my job. I was fortunate to have that mentorship early on in my career, as similar mentorship becomes difficult to obtain once in an attending hospitalist position.3
In conclusion, although current internal medicine residency training provides intensive direct patient care experiences, it only allows glimpses into the other aspects of an academic hospitalist's job. Unfortunately, it does not adequately prepare one to begin this type of position with a full complement of skills. Only a minority of hospitalists pursue additional structured training directly after residency; the majority jump into hospitalist positions and opt for on‐the‐job training. While there is an early economic advantage to starting an attending position without delay, I believe that the skills learned during an additional year of dedicated training allow for a more meaningful work experience and, ultimately, a faster rise within the track of an academic hospitalist.
The tasks that residency programs and hospitals may give to CMRs provide fertile material for developing the skills necessary to become a productive academic hospitalist. I thrived on the multifaceted work of caring for a diverse group of patients, teaching different levels of learners, helping to manage various hospital systems, and better understanding the hospital as the sum of its parts. As noted above, this pre‐attending league gave me the exposure to more fully develop my academic hospitalist game in a supportive environment. A CMR year may be beneficial for residents entering any career in internal medicine; however, I believe it is most aptly suited as a stepping stone for future academic hospitalists. I strongly recommend that current residents interested in academic hospital medicine consider a CMR position, and encourage program directors to consider molding their inpatient CMR experiences to facilitate this. Moreover, unless fellowship training in hospital medicine becomes the norm, I propose that current academic hospitalists do more to closely court and usher these pretenders into our ranks.
- ,,,,.A systems approach for implementing practice‐based learning and improvement and systems‐based practice in graduate medical education.Acad Med.2009;84(3):335–339.
- ,.Middle manager role of the chief medical resident: an organizational psychologist's perspective.J Gen Intern Med.2007;22(12):1771–1774.
- ,,,.Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups.J Hosp Med.2011;6:5–9.
Academic hospitalists have multiple duties including direct patient care, hospital management, teaching, and scholarly productivity. We are frequently pulled in divergent directions, while attending to these responsibilities. Without a framework to manage these assorted tasks, we are at risk of subpar performance and career dissatisfaction. Alternatively, we often thrive in our roles as utility players when armed with a developed skill set. Our profession could benefit greatly from encouraging future academic hospitalists to obtain further training prior to starting as an attending. Although hospital medicine fellowship training exists, there are still relatively few programs available. A well‐crafted year spent as a chief medical resident (CMR) may be a viable alternative.
My year as CMR allowed me to develop the skills necessary for success as an academic hospitalist in a supportive setting, and has given me an advantage in accelerating my career. This experience provided me with important tools that my initial 3 years of internal medicine residency did not. Even during my final year of residency, I was focused mostly on obtaining medical knowledge, learning its clinical applications, and honing team leadership skills. My mind rarely trespassed into the broader concerns of quality improvement initiatives, educational enhancements, or hospital management issues. Although the American College of Graduate Medical Education (ACGME) core competencies are helping to better focus residents' attention to these more diverse aspects of healthcare, residents still spend the majority of their time providing direct patient care.1 Now in my fourth year as an attending academic hospitalist, I continue to appreciate how my chief experience provided the foundation for much of the work I perform today.
My motivations for becoming a CMR included a desire to spend more time teaching and learning medicine, and an interest in helping to improve the residency program itself. I did not appreciate how much of the job would be spent managing people, and evaluating systems of care within the hospital, while working closely with nurses and hospital administrators. However, the skills I learned while addressing those unexpected tasks are what continue to help me in my position as a multifaceted hospitalist today. CMRs have been described as middle managers, being pushed and pulled by the demands and requirements of the groups above, below and around them.2 Academic hospitalists who frequently wear administrative and educational hats are not dissimilar. Retrospectively, I realize how fortunate I was to be exposed to those aspects as a CMR, with many of the same responsibilities but without the full expectations of a more seasoned attending.
The most memorable interaction during my first day as junior ward attending was with a revered internist, himself a former CMR, who dryly commented, So, you're pretending now. It took me a moment to catch his play on words until he clarified, you are now a pre‐attending. The true meaning of this was elucidated over the next several weeks as I was expected to perform many of the same duties of a seasoned attending, but often had the sense that I was only pretending to be an attending and still had much to learn.
CMR positions vary in terms of clinical, educational, and administrative responsibilities. Moreover, many institutions mix inpatient and outpatient roles. My position was focused almost entirely on inpatient duties at a single hospital, which gave me an in‐depth and longitudinal view of how a hospital is managed. Like many other CMRs, much of my time was spent on educational activities, such as running morning report, preparing for chief of medicine rounds, coordinating noon teaching conferences, and spending time with the medical students. Administrative tasks included various institutional‐based meetings for student grading, educational review committees, and program scheduling. In addition, I spent 1 month as junior attending on a ward team. Many other programs' CMRs spend more time as junior attending; however, by offloading some of this ward service requirement, I feel fortunate to have had that time to use for my own scholarly activity and teaching/administrative opportunities. Perhaps unique to my CMR position, I also was involved with the daily running of the hospital by working with administrators to evaluate patient transfer requests and addressing provider work flow issues. These additional tasks provided an invaluable learning experience.
Organizing morning report, running physical diagnosis rounds, and preparing cases and speakers for Chair's Rounds allowed me to hone and expand my teaching skills in ways that 3 years of residency did not. Moreover, it put me in direct contact with an energetic, inspiring group of learners that challenged me to solidify my own medical knowledge. (Try explaining the delta‐delta equation to figure out if there are 2 metabolic processes going on, in front of a group of 20 residents, and you'll discover what I mean.) I quickly learned that the one doing the talking is the one doing the learning and changed my teaching style to better facilitate student learning. My bedside learning was further augmented by attending Masters' Rounds to which I owe my ongoing interests in physical diagnosis. Masters Rounds were given once a week by 2 master clinicians. It was only for chief residents and was directed at teaching us how to teach others the art of bedside physical diagnosis. The majority of physical exam teaching points I focus on today come from those sessions.
As chief, I felt like I had the pulse of the hospital at all times. Most of my mornings were spent on the wards floating between teams. I owe thanks to my predecessor who told me that the true sign of a good CMR was to never sit long enough in your chair to let it get warm. My office was located on the wards, between a team room and a double patient room. Aside from times when I was having confidential conversations with residents, the door was open. Nurses looking to vent, phlebotomists wanting to sit down, and attendings needing a break from their teams to get work done were common visitors. Administrative personnel were also frequent visitors, usually requesting me to disseminate new policies to the residents. Because of this, I learned to understand and better interact with the diverse group of people responsible for making a teaching hospital function. These are the same constituencies that I now sit down with on various committees to attempt to make my present hospital operate more smoothly.
Despite running morning report, attending rounds with teams, and developing plans for better patient flow, I had time for scholarly work and found easy mentorship. I was able to revive 2 projects I had started as a resident and bring them close to conclusion under the continued mentorship of my coinvestigators. Offers for career skill development were also abound, and I benefited greatly from one associate director's tutorials on preparing effective PowerPoint presentations. Another attending mentored me in student feedback skills, which have allowed me to become a much more effective educator. I was also able to model that mentorship and begin to build my own mentor relationships with my students. In fact, this mentorship has become one of the most fulfilling aspects of my job. I was fortunate to have that mentorship early on in my career, as similar mentorship becomes difficult to obtain once in an attending hospitalist position.3
In conclusion, although current internal medicine residency training provides intensive direct patient care experiences, it only allows glimpses into the other aspects of an academic hospitalist's job. Unfortunately, it does not adequately prepare one to begin this type of position with a full complement of skills. Only a minority of hospitalists pursue additional structured training directly after residency; the majority jump into hospitalist positions and opt for on‐the‐job training. While there is an early economic advantage to starting an attending position without delay, I believe that the skills learned during an additional year of dedicated training allow for a more meaningful work experience and, ultimately, a faster rise within the track of an academic hospitalist.
The tasks that residency programs and hospitals may give to CMRs provide fertile material for developing the skills necessary to become a productive academic hospitalist. I thrived on the multifaceted work of caring for a diverse group of patients, teaching different levels of learners, helping to manage various hospital systems, and better understanding the hospital as the sum of its parts. As noted above, this pre‐attending league gave me the exposure to more fully develop my academic hospitalist game in a supportive environment. A CMR year may be beneficial for residents entering any career in internal medicine; however, I believe it is most aptly suited as a stepping stone for future academic hospitalists. I strongly recommend that current residents interested in academic hospital medicine consider a CMR position, and encourage program directors to consider molding their inpatient CMR experiences to facilitate this. Moreover, unless fellowship training in hospital medicine becomes the norm, I propose that current academic hospitalists do more to closely court and usher these pretenders into our ranks.
Academic hospitalists have multiple duties including direct patient care, hospital management, teaching, and scholarly productivity. We are frequently pulled in divergent directions, while attending to these responsibilities. Without a framework to manage these assorted tasks, we are at risk of subpar performance and career dissatisfaction. Alternatively, we often thrive in our roles as utility players when armed with a developed skill set. Our profession could benefit greatly from encouraging future academic hospitalists to obtain further training prior to starting as an attending. Although hospital medicine fellowship training exists, there are still relatively few programs available. A well‐crafted year spent as a chief medical resident (CMR) may be a viable alternative.
My year as CMR allowed me to develop the skills necessary for success as an academic hospitalist in a supportive setting, and has given me an advantage in accelerating my career. This experience provided me with important tools that my initial 3 years of internal medicine residency did not. Even during my final year of residency, I was focused mostly on obtaining medical knowledge, learning its clinical applications, and honing team leadership skills. My mind rarely trespassed into the broader concerns of quality improvement initiatives, educational enhancements, or hospital management issues. Although the American College of Graduate Medical Education (ACGME) core competencies are helping to better focus residents' attention to these more diverse aspects of healthcare, residents still spend the majority of their time providing direct patient care.1 Now in my fourth year as an attending academic hospitalist, I continue to appreciate how my chief experience provided the foundation for much of the work I perform today.
My motivations for becoming a CMR included a desire to spend more time teaching and learning medicine, and an interest in helping to improve the residency program itself. I did not appreciate how much of the job would be spent managing people, and evaluating systems of care within the hospital, while working closely with nurses and hospital administrators. However, the skills I learned while addressing those unexpected tasks are what continue to help me in my position as a multifaceted hospitalist today. CMRs have been described as middle managers, being pushed and pulled by the demands and requirements of the groups above, below and around them.2 Academic hospitalists who frequently wear administrative and educational hats are not dissimilar. Retrospectively, I realize how fortunate I was to be exposed to those aspects as a CMR, with many of the same responsibilities but without the full expectations of a more seasoned attending.
The most memorable interaction during my first day as junior ward attending was with a revered internist, himself a former CMR, who dryly commented, So, you're pretending now. It took me a moment to catch his play on words until he clarified, you are now a pre‐attending. The true meaning of this was elucidated over the next several weeks as I was expected to perform many of the same duties of a seasoned attending, but often had the sense that I was only pretending to be an attending and still had much to learn.
CMR positions vary in terms of clinical, educational, and administrative responsibilities. Moreover, many institutions mix inpatient and outpatient roles. My position was focused almost entirely on inpatient duties at a single hospital, which gave me an in‐depth and longitudinal view of how a hospital is managed. Like many other CMRs, much of my time was spent on educational activities, such as running morning report, preparing for chief of medicine rounds, coordinating noon teaching conferences, and spending time with the medical students. Administrative tasks included various institutional‐based meetings for student grading, educational review committees, and program scheduling. In addition, I spent 1 month as junior attending on a ward team. Many other programs' CMRs spend more time as junior attending; however, by offloading some of this ward service requirement, I feel fortunate to have had that time to use for my own scholarly activity and teaching/administrative opportunities. Perhaps unique to my CMR position, I also was involved with the daily running of the hospital by working with administrators to evaluate patient transfer requests and addressing provider work flow issues. These additional tasks provided an invaluable learning experience.
Organizing morning report, running physical diagnosis rounds, and preparing cases and speakers for Chair's Rounds allowed me to hone and expand my teaching skills in ways that 3 years of residency did not. Moreover, it put me in direct contact with an energetic, inspiring group of learners that challenged me to solidify my own medical knowledge. (Try explaining the delta‐delta equation to figure out if there are 2 metabolic processes going on, in front of a group of 20 residents, and you'll discover what I mean.) I quickly learned that the one doing the talking is the one doing the learning and changed my teaching style to better facilitate student learning. My bedside learning was further augmented by attending Masters' Rounds to which I owe my ongoing interests in physical diagnosis. Masters Rounds were given once a week by 2 master clinicians. It was only for chief residents and was directed at teaching us how to teach others the art of bedside physical diagnosis. The majority of physical exam teaching points I focus on today come from those sessions.
As chief, I felt like I had the pulse of the hospital at all times. Most of my mornings were spent on the wards floating between teams. I owe thanks to my predecessor who told me that the true sign of a good CMR was to never sit long enough in your chair to let it get warm. My office was located on the wards, between a team room and a double patient room. Aside from times when I was having confidential conversations with residents, the door was open. Nurses looking to vent, phlebotomists wanting to sit down, and attendings needing a break from their teams to get work done were common visitors. Administrative personnel were also frequent visitors, usually requesting me to disseminate new policies to the residents. Because of this, I learned to understand and better interact with the diverse group of people responsible for making a teaching hospital function. These are the same constituencies that I now sit down with on various committees to attempt to make my present hospital operate more smoothly.
Despite running morning report, attending rounds with teams, and developing plans for better patient flow, I had time for scholarly work and found easy mentorship. I was able to revive 2 projects I had started as a resident and bring them close to conclusion under the continued mentorship of my coinvestigators. Offers for career skill development were also abound, and I benefited greatly from one associate director's tutorials on preparing effective PowerPoint presentations. Another attending mentored me in student feedback skills, which have allowed me to become a much more effective educator. I was also able to model that mentorship and begin to build my own mentor relationships with my students. In fact, this mentorship has become one of the most fulfilling aspects of my job. I was fortunate to have that mentorship early on in my career, as similar mentorship becomes difficult to obtain once in an attending hospitalist position.3
In conclusion, although current internal medicine residency training provides intensive direct patient care experiences, it only allows glimpses into the other aspects of an academic hospitalist's job. Unfortunately, it does not adequately prepare one to begin this type of position with a full complement of skills. Only a minority of hospitalists pursue additional structured training directly after residency; the majority jump into hospitalist positions and opt for on‐the‐job training. While there is an early economic advantage to starting an attending position without delay, I believe that the skills learned during an additional year of dedicated training allow for a more meaningful work experience and, ultimately, a faster rise within the track of an academic hospitalist.
The tasks that residency programs and hospitals may give to CMRs provide fertile material for developing the skills necessary to become a productive academic hospitalist. I thrived on the multifaceted work of caring for a diverse group of patients, teaching different levels of learners, helping to manage various hospital systems, and better understanding the hospital as the sum of its parts. As noted above, this pre‐attending league gave me the exposure to more fully develop my academic hospitalist game in a supportive environment. A CMR year may be beneficial for residents entering any career in internal medicine; however, I believe it is most aptly suited as a stepping stone for future academic hospitalists. I strongly recommend that current residents interested in academic hospital medicine consider a CMR position, and encourage program directors to consider molding their inpatient CMR experiences to facilitate this. Moreover, unless fellowship training in hospital medicine becomes the norm, I propose that current academic hospitalists do more to closely court and usher these pretenders into our ranks.
- ,,,,.A systems approach for implementing practice‐based learning and improvement and systems‐based practice in graduate medical education.Acad Med.2009;84(3):335–339.
- ,.Middle manager role of the chief medical resident: an organizational psychologist's perspective.J Gen Intern Med.2007;22(12):1771–1774.
- ,,,.Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups.J Hosp Med.2011;6:5–9.
- ,,,,.A systems approach for implementing practice‐based learning and improvement and systems‐based practice in graduate medical education.Acad Med.2009;84(3):335–339.
- ,.Middle manager role of the chief medical resident: an organizational psychologist's perspective.J Gen Intern Med.2007;22(12):1771–1774.
- ,,,.Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups.J Hosp Med.2011;6:5–9.
New Jersey Hospital Funds Care-Transitions “Coach”
Robert Wood Johnson University Hospital in Hamilton, N.J., has partnered with Jewish Family and Children’s Services of Greater Mercer County to support care transitions for 350 chronically ill older patients. Patients will receive a transitions coach following hospital discharge for education, support, and encouragement to keep appointments with their physicians. This “coach” will develop a plan of care for the patient, making one hospital visit, one home visit, and three phone calls, says Joyce Schwarz, the hospital’s vice president of quality and the project’s director.
The hospital received a $300,000 grant under the New Jersey Health Initiative from the Robert Wood Johnson Foundation to use an evidence-based intervention to improve care transitions and reduce readmissions, acting as a bridge between hospital personnel and community physicians.
Robert Wood Johnson University Hospital in Hamilton, N.J., has partnered with Jewish Family and Children’s Services of Greater Mercer County to support care transitions for 350 chronically ill older patients. Patients will receive a transitions coach following hospital discharge for education, support, and encouragement to keep appointments with their physicians. This “coach” will develop a plan of care for the patient, making one hospital visit, one home visit, and three phone calls, says Joyce Schwarz, the hospital’s vice president of quality and the project’s director.
The hospital received a $300,000 grant under the New Jersey Health Initiative from the Robert Wood Johnson Foundation to use an evidence-based intervention to improve care transitions and reduce readmissions, acting as a bridge between hospital personnel and community physicians.
Robert Wood Johnson University Hospital in Hamilton, N.J., has partnered with Jewish Family and Children’s Services of Greater Mercer County to support care transitions for 350 chronically ill older patients. Patients will receive a transitions coach following hospital discharge for education, support, and encouragement to keep appointments with their physicians. This “coach” will develop a plan of care for the patient, making one hospital visit, one home visit, and three phone calls, says Joyce Schwarz, the hospital’s vice president of quality and the project’s director.
The hospital received a $300,000 grant under the New Jersey Health Initiative from the Robert Wood Johnson Foundation to use an evidence-based intervention to improve care transitions and reduce readmissions, acting as a bridge between hospital personnel and community physicians.
‘Smoothing’ Strategies in Children’s Hospitals Reduce Overcrowding
A report published online May 24 in the Journal of Hospital Medicine found that smoothing inpatient occupancy and scheduled admissions in 39 children’s hospitals helped reduce midweek overcrowding. Evan S. Fieldston, MD, MBA, MSHP, of the University of Pennsylvania School of Medicine in Philadelphia and colleagues previously demonstrated occupancy variability and midweek crowding weekends (J Hosp Med. 2011;6:81-87). Strategies the team studied included controlling admissions when possible to achieve more level occupancy, with a mean of 2.6% of admissions moved to a different day of the week.
A report published online May 24 in the Journal of Hospital Medicine found that smoothing inpatient occupancy and scheduled admissions in 39 children’s hospitals helped reduce midweek overcrowding. Evan S. Fieldston, MD, MBA, MSHP, of the University of Pennsylvania School of Medicine in Philadelphia and colleagues previously demonstrated occupancy variability and midweek crowding weekends (J Hosp Med. 2011;6:81-87). Strategies the team studied included controlling admissions when possible to achieve more level occupancy, with a mean of 2.6% of admissions moved to a different day of the week.
A report published online May 24 in the Journal of Hospital Medicine found that smoothing inpatient occupancy and scheduled admissions in 39 children’s hospitals helped reduce midweek overcrowding. Evan S. Fieldston, MD, MBA, MSHP, of the University of Pennsylvania School of Medicine in Philadelphia and colleagues previously demonstrated occupancy variability and midweek crowding weekends (J Hosp Med. 2011;6:81-87). Strategies the team studied included controlling admissions when possible to achieve more level occupancy, with a mean of 2.6% of admissions moved to a different day of the week.
By the numbers - 0.5%
The reduction President Obama has proposed to a formula used by the Independent Payment Advisory Board (IPAB), created last year by the Affordable Care Act to cut Medicare costs without affecting quality. The formula IPAB currently uses as a baseline for growth estimates is GDP per capita, plus 1%. The president has proposed to reduce that figure to GDP plus 0.5%. The lower threshold means IPAB will have deeper cuts to make. Accordingly, hospitalists are watching the proposal, as it could lower federal reimbursements as IPAB looks for ways to cut Medicare spending
The reduction President Obama has proposed to a formula used by the Independent Payment Advisory Board (IPAB), created last year by the Affordable Care Act to cut Medicare costs without affecting quality. The formula IPAB currently uses as a baseline for growth estimates is GDP per capita, plus 1%. The president has proposed to reduce that figure to GDP plus 0.5%. The lower threshold means IPAB will have deeper cuts to make. Accordingly, hospitalists are watching the proposal, as it could lower federal reimbursements as IPAB looks for ways to cut Medicare spending
The reduction President Obama has proposed to a formula used by the Independent Payment Advisory Board (IPAB), created last year by the Affordable Care Act to cut Medicare costs without affecting quality. The formula IPAB currently uses as a baseline for growth estimates is GDP per capita, plus 1%. The president has proposed to reduce that figure to GDP plus 0.5%. The lower threshold means IPAB will have deeper cuts to make. Accordingly, hospitalists are watching the proposal, as it could lower federal reimbursements as IPAB looks for ways to cut Medicare spending
“Teachback” Reduces Readmissions for CHF Patients
A sking “teachback” questions to hospitalized chronic heart failure (CHF) patients at Lehigh Valley Health Network in Allentown, Pa., helps them better understand their condition, treatment, and post-discharge care—thereby impacting readmissions. In an abstract presented at HM11 in Dallas in May, CHF patients who received teachback had a 7.3% readmission rate in the first three months of 2011, compared with 9.7% for those who did not.
Teachback, according to hospitalist and lead author Michael Pistoria, DO, FACP, SFHM, represents “humble inquiry—the simple need and ability to ask patients: ‘Can you tell me what I said to you?’” The provider then needs to listen to the reply and confirm the understanding, he adds.
Lehigh Valley convened a multidisciplinary quality team to study transitions of care, with a subgroup focused on patient-caregiver education, Dr. Pistoria explains. “The first thing the patient-family caregiver education team looked at was how to identify the key learner,” he says. “We had assumed it was the patient, but that’s not always the person who needs to learn about managing the patient’s condition.”
The subgroup then developed a curriculum of questions to be asked sequentially over three days to test patients and their caregivers’ understanding of heart failure and need for reinforcement. These questions, drawing upon educational resources already used within the system, assess the key learner’s knowledge, attitudes about healthy behaviors, and how to incorporate those behaviors into effective self-care.
The teachback system was tested on a few patients, then disseminated to 1,400 nurses through Lehigh Valley’s professional e-learning network using a brief training video. “We learned that doing a good job of staff teaching is not enough, unless we go back and periodically revisit the issues and audit their performance,” Dr. Pistoria says. “In our system, starting with our nurses was the right approach. It’s important for everybody to take ownership of the initiative. It’s also important, from unit to unit, to ask the questions the same way.”
Subsequent analysis shows continued reductions in readmissions, Dr. Pistoria says. Lehigh Valley’s next targets for teachback are community-acquired pneumonia, myocardial infarction, hypoglycemia, COPD, and anti-coagulant treatment.
A sking “teachback” questions to hospitalized chronic heart failure (CHF) patients at Lehigh Valley Health Network in Allentown, Pa., helps them better understand their condition, treatment, and post-discharge care—thereby impacting readmissions. In an abstract presented at HM11 in Dallas in May, CHF patients who received teachback had a 7.3% readmission rate in the first three months of 2011, compared with 9.7% for those who did not.
Teachback, according to hospitalist and lead author Michael Pistoria, DO, FACP, SFHM, represents “humble inquiry—the simple need and ability to ask patients: ‘Can you tell me what I said to you?’” The provider then needs to listen to the reply and confirm the understanding, he adds.
Lehigh Valley convened a multidisciplinary quality team to study transitions of care, with a subgroup focused on patient-caregiver education, Dr. Pistoria explains. “The first thing the patient-family caregiver education team looked at was how to identify the key learner,” he says. “We had assumed it was the patient, but that’s not always the person who needs to learn about managing the patient’s condition.”
The subgroup then developed a curriculum of questions to be asked sequentially over three days to test patients and their caregivers’ understanding of heart failure and need for reinforcement. These questions, drawing upon educational resources already used within the system, assess the key learner’s knowledge, attitudes about healthy behaviors, and how to incorporate those behaviors into effective self-care.
The teachback system was tested on a few patients, then disseminated to 1,400 nurses through Lehigh Valley’s professional e-learning network using a brief training video. “We learned that doing a good job of staff teaching is not enough, unless we go back and periodically revisit the issues and audit their performance,” Dr. Pistoria says. “In our system, starting with our nurses was the right approach. It’s important for everybody to take ownership of the initiative. It’s also important, from unit to unit, to ask the questions the same way.”
Subsequent analysis shows continued reductions in readmissions, Dr. Pistoria says. Lehigh Valley’s next targets for teachback are community-acquired pneumonia, myocardial infarction, hypoglycemia, COPD, and anti-coagulant treatment.
A sking “teachback” questions to hospitalized chronic heart failure (CHF) patients at Lehigh Valley Health Network in Allentown, Pa., helps them better understand their condition, treatment, and post-discharge care—thereby impacting readmissions. In an abstract presented at HM11 in Dallas in May, CHF patients who received teachback had a 7.3% readmission rate in the first three months of 2011, compared with 9.7% for those who did not.
Teachback, according to hospitalist and lead author Michael Pistoria, DO, FACP, SFHM, represents “humble inquiry—the simple need and ability to ask patients: ‘Can you tell me what I said to you?’” The provider then needs to listen to the reply and confirm the understanding, he adds.
Lehigh Valley convened a multidisciplinary quality team to study transitions of care, with a subgroup focused on patient-caregiver education, Dr. Pistoria explains. “The first thing the patient-family caregiver education team looked at was how to identify the key learner,” he says. “We had assumed it was the patient, but that’s not always the person who needs to learn about managing the patient’s condition.”
The subgroup then developed a curriculum of questions to be asked sequentially over three days to test patients and their caregivers’ understanding of heart failure and need for reinforcement. These questions, drawing upon educational resources already used within the system, assess the key learner’s knowledge, attitudes about healthy behaviors, and how to incorporate those behaviors into effective self-care.
The teachback system was tested on a few patients, then disseminated to 1,400 nurses through Lehigh Valley’s professional e-learning network using a brief training video. “We learned that doing a good job of staff teaching is not enough, unless we go back and periodically revisit the issues and audit their performance,” Dr. Pistoria says. “In our system, starting with our nurses was the right approach. It’s important for everybody to take ownership of the initiative. It’s also important, from unit to unit, to ask the questions the same way.”
Subsequent analysis shows continued reductions in readmissions, Dr. Pistoria says. Lehigh Valley’s next targets for teachback are community-acquired pneumonia, myocardial infarction, hypoglycemia, COPD, and anti-coagulant treatment.
Documenting the Symptom Experience of Cancer Patients
Volume 9, Issue 6, November-December 2011, Pages 216-223
| doi:10.1016/j.suponc.2011.06.003 | How to Cite or Link Using DOI |
| Permissions & Reprints |
Original research
Teresa L. Deshields PhD ![]()
![]()
Received 11 January 2011; Accepted 9 June 2011. Available online 3 November 2011.
Abstract
Background
Cancer patients experience symptoms associated with their disease, treatment, and comorbidities. Symptom experience is complicated, reflecting symptom prevalence, frequency, and severity. Symptom burden is associated with treatment tolerance as well as patients' quality of life (QOL).
Objectives
The purpose of this study was to document the symptom experience and QOL of patients with commonly diagnosed cancers. The relationship between symptoms and QOL was also explored.
Methods
A convenience sample of patients with the five most common cancers at a comprehensive cancer center completed surveys assessing symptom experience (Memorial Symptom Assessment Survey) and QOL (Functional Assessment of Cancer Therapy). Patients completed surveys at baseline and at 3, 6, 9, and 12 months thereafter. This article describes the study's baseline findings.
Results
Surveys were completed by 558 cancer patients with breast, colorectal, gynecologic, lung, or prostate cancer. Patients reported an average of 9.1 symptoms, with symptom experience varying by cancer type. The mean overall QOL for the total sample was 85.1, with results differing by cancer type. Prostate cancer patients reported the lowest symptom burden and the highest QOL.
Limitations
The sample was limited in terms of racial diversity. Because of the method of recruitment, baseline data were collected 6–8 months after diagnosis, meaning that participants were at various stages of treatment.
Conclusions
The symptom experience of cancer patients varies widely depending on cancer type. Nevertheless, most patients report symptoms, regardless of whether or not they are currently receiving treatment. Patients' QOL is inversely related to their symptom burden.
Volume 9, Issue 6, November-December 2011, Pages 216-223
Volume 9, Issue 6, November-December 2011, Pages 216-223
| doi:10.1016/j.suponc.2011.06.003 | How to Cite or Link Using DOI |
| Permissions & Reprints |
Original research
Teresa L. Deshields PhD ![]()
![]()
Received 11 January 2011; Accepted 9 June 2011. Available online 3 November 2011.
Abstract
Background
Cancer patients experience symptoms associated with their disease, treatment, and comorbidities. Symptom experience is complicated, reflecting symptom prevalence, frequency, and severity. Symptom burden is associated with treatment tolerance as well as patients' quality of life (QOL).
Objectives
The purpose of this study was to document the symptom experience and QOL of patients with commonly diagnosed cancers. The relationship between symptoms and QOL was also explored.
Methods
A convenience sample of patients with the five most common cancers at a comprehensive cancer center completed surveys assessing symptom experience (Memorial Symptom Assessment Survey) and QOL (Functional Assessment of Cancer Therapy). Patients completed surveys at baseline and at 3, 6, 9, and 12 months thereafter. This article describes the study's baseline findings.
Results
Surveys were completed by 558 cancer patients with breast, colorectal, gynecologic, lung, or prostate cancer. Patients reported an average of 9.1 symptoms, with symptom experience varying by cancer type. The mean overall QOL for the total sample was 85.1, with results differing by cancer type. Prostate cancer patients reported the lowest symptom burden and the highest QOL.
Limitations
The sample was limited in terms of racial diversity. Because of the method of recruitment, baseline data were collected 6–8 months after diagnosis, meaning that participants were at various stages of treatment.
Conclusions
The symptom experience of cancer patients varies widely depending on cancer type. Nevertheless, most patients report symptoms, regardless of whether or not they are currently receiving treatment. Patients' QOL is inversely related to their symptom burden.
Volume 9, Issue 6, November-December 2011, Pages 216-223
Volume 9, Issue 6, November-December 2011, Pages 216-223
| doi:10.1016/j.suponc.2011.06.003 | How to Cite or Link Using DOI |
| Permissions & Reprints |
Original research
Teresa L. Deshields PhD ![]()
![]()
Received 11 January 2011; Accepted 9 June 2011. Available online 3 November 2011.
Abstract
Background
Cancer patients experience symptoms associated with their disease, treatment, and comorbidities. Symptom experience is complicated, reflecting symptom prevalence, frequency, and severity. Symptom burden is associated with treatment tolerance as well as patients' quality of life (QOL).
Objectives
The purpose of this study was to document the symptom experience and QOL of patients with commonly diagnosed cancers. The relationship between symptoms and QOL was also explored.
Methods
A convenience sample of patients with the five most common cancers at a comprehensive cancer center completed surveys assessing symptom experience (Memorial Symptom Assessment Survey) and QOL (Functional Assessment of Cancer Therapy). Patients completed surveys at baseline and at 3, 6, 9, and 12 months thereafter. This article describes the study's baseline findings.
Results
Surveys were completed by 558 cancer patients with breast, colorectal, gynecologic, lung, or prostate cancer. Patients reported an average of 9.1 symptoms, with symptom experience varying by cancer type. The mean overall QOL for the total sample was 85.1, with results differing by cancer type. Prostate cancer patients reported the lowest symptom burden and the highest QOL.
Limitations
The sample was limited in terms of racial diversity. Because of the method of recruitment, baseline data were collected 6–8 months after diagnosis, meaning that participants were at various stages of treatment.
Conclusions
The symptom experience of cancer patients varies widely depending on cancer type. Nevertheless, most patients report symptoms, regardless of whether or not they are currently receiving treatment. Patients' QOL is inversely related to their symptom burden.
Volume 9, Issue 6, November-December 2011, Pages 216-223
Efficacy and Safety of Fentanyl Pectin Nasal Spray Compared with Immediate-Release Morphine Sulfate Tablets in the Treatment of Breakthrough Cancer Pain: A Multicenter, Randomized, Controlled, Double-Blind, Double-Dummy Multiple-Crossover Study
Volume 9, Issue 6, November-December 2011, Pages 224-231
| doi:10.1016/j.suponc.2011.07.004 | How to Cite or Link Using DOI |
| Permissions & Reprints |
Original research
Marie Fallon MB, ChB, MD, FRCP ![]()
![]()
Received 10 February 2011; Accepted 18 July 2011. Available online 3 November 2011.
Background
Immediate-release morphine sulfate (IRMS) remains the standard treatment for breakthrough cancer pain (BTCP), but its onset of effect does not match the rapid onset and short duration of most BTCP episodes.
Objective
This study will evaluate the efficacy/tolerability of fentanyl pectin nasal spray (FPNS) compared with IRMS for BTCP.
Methods
Patients (n = 110) experiencing one to four BTCP episodes/day while taking ≥60 mg/day oral morphine (or equivalent) for background cancer pain entered a double-blind, double-dummy (DB/DD), multiple-crossover study. Patients completing a titration phase (n = 84) continued to a DB/DD phase: 10 episodes of BTCP were randomly treated with FPNS and oral capsule placebo (five episodes) or IRMS and nasal spray placebo (5 episodes). The primary end point was pain intensity (P < .05 FPNS vs. IRMS) difference from baseline at 15 minutes (PID15). Secondary end points were onset of pain intensity (PI) decrease (≥1-point) and time to clinically meaningful pain relief (CMPR, ≥2-point PI decrease). Safety and tolerability were evaluated by adverse events (AEs) and nasal assessments. By-patient and by-episode analyses were completed.
Results
Compared with IRMS, FPNS significantly improved mean PID15 scores. 57.5% of FPNS-treated episodes significantly demonstrated onset of PI improvement by 5 minutes and 95.7% by 30 minutes. CMPR (≥2-point PI decrease) was seen in 52.4% of episodes by 10 minutes. Only 4.7% of patients withdrew from titration (2.4% in DB/DD phase) because of AEs; no significant nasal effects were reported.
Conclusion
FPNS was efficacious and well tolerated in the treatment of BTCP and provided faster onset of analgesia and attainment of CMPR than IRMS.
The authors acknowledge i3Research, which conducted the study; the technical and editorial support provided by Anita Chadha-Patel at ApotheCom; and the Fentanyl Nasal Spray Study 044 Investigators. This study was sponsored by Archimedes Development, Ltd.
Volume 9, Issue 6, November-December 2011, Pages 224-231
| doi:10.1016/j.suponc.2011.07.004 | How to Cite or Link Using DOI |
| Permissions & Reprints |
Original research
Marie Fallon MB, ChB, MD, FRCP ![]()
![]()
Received 10 February 2011; Accepted 18 July 2011. Available online 3 November 2011.
Background
Immediate-release morphine sulfate (IRMS) remains the standard treatment for breakthrough cancer pain (BTCP), but its onset of effect does not match the rapid onset and short duration of most BTCP episodes.
Objective
This study will evaluate the efficacy/tolerability of fentanyl pectin nasal spray (FPNS) compared with IRMS for BTCP.
Methods
Patients (n = 110) experiencing one to four BTCP episodes/day while taking ≥60 mg/day oral morphine (or equivalent) for background cancer pain entered a double-blind, double-dummy (DB/DD), multiple-crossover study. Patients completing a titration phase (n = 84) continued to a DB/DD phase: 10 episodes of BTCP were randomly treated with FPNS and oral capsule placebo (five episodes) or IRMS and nasal spray placebo (5 episodes). The primary end point was pain intensity (P < .05 FPNS vs. IRMS) difference from baseline at 15 minutes (PID15). Secondary end points were onset of pain intensity (PI) decrease (≥1-point) and time to clinically meaningful pain relief (CMPR, ≥2-point PI decrease). Safety and tolerability were evaluated by adverse events (AEs) and nasal assessments. By-patient and by-episode analyses were completed.
Results
Compared with IRMS, FPNS significantly improved mean PID15 scores. 57.5% of FPNS-treated episodes significantly demonstrated onset of PI improvement by 5 minutes and 95.7% by 30 minutes. CMPR (≥2-point PI decrease) was seen in 52.4% of episodes by 10 minutes. Only 4.7% of patients withdrew from titration (2.4% in DB/DD phase) because of AEs; no significant nasal effects were reported.
Conclusion
FPNS was efficacious and well tolerated in the treatment of BTCP and provided faster onset of analgesia and attainment of CMPR than IRMS.
The authors acknowledge i3Research, which conducted the study; the technical and editorial support provided by Anita Chadha-Patel at ApotheCom; and the Fentanyl Nasal Spray Study 044 Investigators. This study was sponsored by Archimedes Development, Ltd.
Volume 9, Issue 6, November-December 2011, Pages 224-231
| doi:10.1016/j.suponc.2011.07.004 | How to Cite or Link Using DOI |
| Permissions & Reprints |
Original research
Marie Fallon MB, ChB, MD, FRCP ![]()
![]()
Received 10 February 2011; Accepted 18 July 2011. Available online 3 November 2011.
Background
Immediate-release morphine sulfate (IRMS) remains the standard treatment for breakthrough cancer pain (BTCP), but its onset of effect does not match the rapid onset and short duration of most BTCP episodes.
Objective
This study will evaluate the efficacy/tolerability of fentanyl pectin nasal spray (FPNS) compared with IRMS for BTCP.
Methods
Patients (n = 110) experiencing one to four BTCP episodes/day while taking ≥60 mg/day oral morphine (or equivalent) for background cancer pain entered a double-blind, double-dummy (DB/DD), multiple-crossover study. Patients completing a titration phase (n = 84) continued to a DB/DD phase: 10 episodes of BTCP were randomly treated with FPNS and oral capsule placebo (five episodes) or IRMS and nasal spray placebo (5 episodes). The primary end point was pain intensity (P < .05 FPNS vs. IRMS) difference from baseline at 15 minutes (PID15). Secondary end points were onset of pain intensity (PI) decrease (≥1-point) and time to clinically meaningful pain relief (CMPR, ≥2-point PI decrease). Safety and tolerability were evaluated by adverse events (AEs) and nasal assessments. By-patient and by-episode analyses were completed.
Results
Compared with IRMS, FPNS significantly improved mean PID15 scores. 57.5% of FPNS-treated episodes significantly demonstrated onset of PI improvement by 5 minutes and 95.7% by 30 minutes. CMPR (≥2-point PI decrease) was seen in 52.4% of episodes by 10 minutes. Only 4.7% of patients withdrew from titration (2.4% in DB/DD phase) because of AEs; no significant nasal effects were reported.
Conclusion
FPNS was efficacious and well tolerated in the treatment of BTCP and provided faster onset of analgesia and attainment of CMPR than IRMS.
The authors acknowledge i3Research, which conducted the study; the technical and editorial support provided by Anita Chadha-Patel at ApotheCom; and the Fentanyl Nasal Spray Study 044 Investigators. This study was sponsored by Archimedes Development, Ltd.
Coordination of Care in Breast Cancer Survivors: An Overview
Volume 9, Issue 6, November-December 2011, Pages 210-215
| doi:10.1016/j.suponc.2011.06.008 | How to Cite or Link Using DOI |
| Permissions & Reprints |
How We Do It

TO READ THE ENTIRE ARTICLE, CLICK ON THE ADJACENT LINK TO THE PDF FILE
Abstract
The number of breast cancer survivors in the United States is increasing. With longer survival, there has been an increase in the complexity and duration of posttreatment care. Multidisciplinary care teams are needed to participate across the broad spectrum of issues that breast cancer survivors face. In this setting, the need for well-established patterns of communication between care providers is increasingly apparent. We have created a multidisciplinary approach to the management of breast cancer survivors to improve communication and education between providers and patients. This approach could be extended to the care and management of survivors of other types of cancer.
Case
Vitae
Dr. Peairs is from the Johns Hopkins School of Medicine, Baltimore, Maryland. |
Dr. Wolff is from the Johns Hopkins School of Medicine, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland. |
Dr. Olsenis from the Johns Hopkins School of Nursing, Baltimore, Maryland. |
Dr. Bantugis from the Johns Hopkins School of Medicine, Baltimore, Maryland. |
Dr. Shockney is from the Johns Hopkins School of Medicine, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland. |
Dr. Kantsiper is from the Johns Hopkins School of Medicine, Baltimore, Maryland. |
Dr. Carrino-Tamasi is from the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland. |
Dr. Snyder is from the Johns Hopkins School of Medicine, Baltimore, Maryland. |
Volume 9, Issue 6, November-December 2011, Pages 210-215
Volume 9, Issue 6, November-December 2011, Pages 210-215
| doi:10.1016/j.suponc.2011.06.008 | How to Cite or Link Using DOI |
| Permissions & Reprints |
How We Do It

TO READ THE ENTIRE ARTICLE, CLICK ON THE ADJACENT LINK TO THE PDF FILE
Abstract
The number of breast cancer survivors in the United States is increasing. With longer survival, there has been an increase in the complexity and duration of posttreatment care. Multidisciplinary care teams are needed to participate across the broad spectrum of issues that breast cancer survivors face. In this setting, the need for well-established patterns of communication between care providers is increasingly apparent. We have created a multidisciplinary approach to the management of breast cancer survivors to improve communication and education between providers and patients. This approach could be extended to the care and management of survivors of other types of cancer.
Case
Vitae
Dr. Peairs is from the Johns Hopkins School of Medicine, Baltimore, Maryland. |
Dr. Wolff is from the Johns Hopkins School of Medicine, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland. |
Dr. Olsenis from the Johns Hopkins School of Nursing, Baltimore, Maryland. |
Dr. Bantugis from the Johns Hopkins School of Medicine, Baltimore, Maryland. |
Dr. Shockney is from the Johns Hopkins School of Medicine, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland. |
Dr. Kantsiper is from the Johns Hopkins School of Medicine, Baltimore, Maryland. |
Dr. Carrino-Tamasi is from the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland. |
Dr. Snyder is from the Johns Hopkins School of Medicine, Baltimore, Maryland. |
Volume 9, Issue 6, November-December 2011, Pages 210-215
Volume 9, Issue 6, November-December 2011, Pages 210-215
| doi:10.1016/j.suponc.2011.06.008 | How to Cite or Link Using DOI |
| Permissions & Reprints |
How We Do It

TO READ THE ENTIRE ARTICLE, CLICK ON THE ADJACENT LINK TO THE PDF FILE
Abstract
The number of breast cancer survivors in the United States is increasing. With longer survival, there has been an increase in the complexity and duration of posttreatment care. Multidisciplinary care teams are needed to participate across the broad spectrum of issues that breast cancer survivors face. In this setting, the need for well-established patterns of communication between care providers is increasingly apparent. We have created a multidisciplinary approach to the management of breast cancer survivors to improve communication and education between providers and patients. This approach could be extended to the care and management of survivors of other types of cancer.
Case
Vitae
Dr. Peairs is from the Johns Hopkins School of Medicine, Baltimore, Maryland. |
Dr. Wolff is from the Johns Hopkins School of Medicine, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland. |
Dr. Olsenis from the Johns Hopkins School of Nursing, Baltimore, Maryland. |
Dr. Bantugis from the Johns Hopkins School of Medicine, Baltimore, Maryland. |
Dr. Shockney is from the Johns Hopkins School of Medicine, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland. |
Dr. Kantsiper is from the Johns Hopkins School of Medicine, Baltimore, Maryland. |
Dr. Carrino-Tamasi is from the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland. |
Dr. Snyder is from the Johns Hopkins School of Medicine, Baltimore, Maryland. |
Volume 9, Issue 6, November-December 2011, Pages 210-215
UPDATED: Vytorin Gets FDA Panel Nod for Some CKD Patients
[UPDATED]SILVER SPRING, MD. – Vytorin should be approved for preventing major vascular events in patients who have chronic kidney disease but are not on dialysis, according to a unanimous vote by a Food and Drug Administration advisory panel.
The panel also voted 10-6, however, that the safety and effectiveness data did not support approval of the combination of 10 mg of ezetimibe with 20 mg of simvastatin for the same indication in patients with end-stage renal disease who are on dialysis.
Their votes at the Nov. 2 meeting were based on the results of the Study of Heart and Renal Protection (SHARP), which evaluated the effects of reducing LDL cholesterol on the risk of coronary vascular disease in patients with chronic kidney disease who are at an increased risk of cardiovascular morbidity and mortality. About two-thirds of the patients in the trial were not on dialysis at baseline, and in these patients, there was a 23% reduction in the primary end point – the risk of a major vascular event (nonfatal MI or cardiac death, stroke, or a revascularization procedure that excluded dialysis access-related procedures) – compared with those on placebo over a mean of 5 years (Lancet 2011;377:2181-92).
But in the patients who were on dialysis at baseline, the risk was reduced by about 6% over placebo. Panelists who did not support approval in this group cited the lower degree of effectiveness, and the fact that end stage renal disease patients on dialysis are very different from predialysis patients – and that patients with ESRD in the United States are different than those elsewhere. They noted that only 4% of the patients in SHARP were in the United States, and management and outcomes of patients with ESRD are different in the United States than in other parts of the world.
Dr. Lamont Weide, chief of diabetes and endocrinology, at the University of Missouri, Kansas City, backed approval for predialysis patients, but not for those on dialysis. Like several other panelists, he also considered the results of two previous large studies of about 4,000 CKD patients on dialysis, which found no significant beneficial effects of treatment with other statins on cardiovascular outcomes. While these were three different studies with different agents and different entry criteria, he said that the results go in the same direction as SHARP, in a large group of patients "without any clear indication of benefit." Those studies were the 4D study (N. Engl. J. Med. 2005;353:238-48) and the AURORA study (N. Engl J. Med. 2009;360:1395-407).
Also splitting her votes, Dr. Julia Lewis, professor of medicine in the department of nephrology, Vanderbilt University, Nashville, referred to those two trials and added that she considers her dialysis patients considerably different than her clinic patients who are not on dialysis. She commended the SHARP study and investigators for "providing what I think is going to be a wonderful addition to the care of CKD patients. It is going to change care of CKD patients and prevent the bad [cardiovascular] outcomes that affect them," she said.
In the study overall, the risk of major vascular events was reduced by 16% among those treated with the combination as compared to those on placebo, which was primarily driven by a reduction in the revascularization component. Cancers and all-cause mortality were similar in treated patients and those on placebo, and the panel agreed there were no new safety concerns at the dose studied. (About one-quarter of those enrolled died during the study.)
Merck, the manufacturer of ezetimibe (Zetia) and Vytorin, filed for approval of the claim that 10 mg of ezetimibe plus 20 mg of simvastatin (in the fixed-dose combination pill or taken separately) reduces the risk of major cardiovascular events in patients with chronic kidney disease on the basis of the SHARP results. SHARP was funded by Merck and Schering-Plough, but was independently conducted by the Oxford (England) University Clinical Trials Service Unit. Ezetimibe was used to make it possible to use a lower dose of simvastatin in the CKD patients, who are at a greater risk of myopathy and other adverse effects of statins.
Panelists emphasized that the 10 mg of ezetimibe with the 20-mg dose of simvastatin was the dose combination studied and was shown to be safe, and that doses should not be increased in this population of patients.
The two drugs are only approved for lipid-lowering indications: Ezetimibe, a selective inhibitor of the absorption of intestinal cholesterol and related phytosterol marketed as Zetia, was approved by the FDA in 2002; Vytorin, a combination of ezetimibe and the HMG-CoA reductase inhibitor simvastatin was approved in 2004; simvastatin was approved in 1991.
The mean age of the patients in the SHARP trial was 61 years; they did not have a history of MI or coronary revascularization.
The six panelists who voted in favor of approval for patient not yet on dialysis and those on dialysis included Dr. William Hiatt, professor of medicine, division of cardiology, at the University of Colorado, Denver, voted in favor of approval for both populations, based on the overall trial results. The differences between the two populations could be explained in the Vytorin label, and "the consistency between the vascular and atherosclerotic events led me to believe that the overall trial had integrity and the various components of the primary outcome were relatively consistent," he said. "To deny an indication to extend to patients who are on chronic hemodialysis ... would not respect the totality of the data," he added.
Dr. Lawrence Hunsicker, professor of medicine in the nephrology division, and emeritus medical director of organ transplantation, University of Iowa, Iowa City, also voted in favor of approval for both groups, but added that the FDA should ensure that advertising and detailing of the product should reflect that "the data are far more clear for patients not on dialysis."
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of disclosures, although in some cases, they are given a waiver-but not at this meeting.
[UPDATED]SILVER SPRING, MD. – Vytorin should be approved for preventing major vascular events in patients who have chronic kidney disease but are not on dialysis, according to a unanimous vote by a Food and Drug Administration advisory panel.
The panel also voted 10-6, however, that the safety and effectiveness data did not support approval of the combination of 10 mg of ezetimibe with 20 mg of simvastatin for the same indication in patients with end-stage renal disease who are on dialysis.
Their votes at the Nov. 2 meeting were based on the results of the Study of Heart and Renal Protection (SHARP), which evaluated the effects of reducing LDL cholesterol on the risk of coronary vascular disease in patients with chronic kidney disease who are at an increased risk of cardiovascular morbidity and mortality. About two-thirds of the patients in the trial were not on dialysis at baseline, and in these patients, there was a 23% reduction in the primary end point – the risk of a major vascular event (nonfatal MI or cardiac death, stroke, or a revascularization procedure that excluded dialysis access-related procedures) – compared with those on placebo over a mean of 5 years (Lancet 2011;377:2181-92).
But in the patients who were on dialysis at baseline, the risk was reduced by about 6% over placebo. Panelists who did not support approval in this group cited the lower degree of effectiveness, and the fact that end stage renal disease patients on dialysis are very different from predialysis patients – and that patients with ESRD in the United States are different than those elsewhere. They noted that only 4% of the patients in SHARP were in the United States, and management and outcomes of patients with ESRD are different in the United States than in other parts of the world.
Dr. Lamont Weide, chief of diabetes and endocrinology, at the University of Missouri, Kansas City, backed approval for predialysis patients, but not for those on dialysis. Like several other panelists, he also considered the results of two previous large studies of about 4,000 CKD patients on dialysis, which found no significant beneficial effects of treatment with other statins on cardiovascular outcomes. While these were three different studies with different agents and different entry criteria, he said that the results go in the same direction as SHARP, in a large group of patients "without any clear indication of benefit." Those studies were the 4D study (N. Engl. J. Med. 2005;353:238-48) and the AURORA study (N. Engl J. Med. 2009;360:1395-407).
Also splitting her votes, Dr. Julia Lewis, professor of medicine in the department of nephrology, Vanderbilt University, Nashville, referred to those two trials and added that she considers her dialysis patients considerably different than her clinic patients who are not on dialysis. She commended the SHARP study and investigators for "providing what I think is going to be a wonderful addition to the care of CKD patients. It is going to change care of CKD patients and prevent the bad [cardiovascular] outcomes that affect them," she said.
In the study overall, the risk of major vascular events was reduced by 16% among those treated with the combination as compared to those on placebo, which was primarily driven by a reduction in the revascularization component. Cancers and all-cause mortality were similar in treated patients and those on placebo, and the panel agreed there were no new safety concerns at the dose studied. (About one-quarter of those enrolled died during the study.)
Merck, the manufacturer of ezetimibe (Zetia) and Vytorin, filed for approval of the claim that 10 mg of ezetimibe plus 20 mg of simvastatin (in the fixed-dose combination pill or taken separately) reduces the risk of major cardiovascular events in patients with chronic kidney disease on the basis of the SHARP results. SHARP was funded by Merck and Schering-Plough, but was independently conducted by the Oxford (England) University Clinical Trials Service Unit. Ezetimibe was used to make it possible to use a lower dose of simvastatin in the CKD patients, who are at a greater risk of myopathy and other adverse effects of statins.
Panelists emphasized that the 10 mg of ezetimibe with the 20-mg dose of simvastatin was the dose combination studied and was shown to be safe, and that doses should not be increased in this population of patients.
The two drugs are only approved for lipid-lowering indications: Ezetimibe, a selective inhibitor of the absorption of intestinal cholesterol and related phytosterol marketed as Zetia, was approved by the FDA in 2002; Vytorin, a combination of ezetimibe and the HMG-CoA reductase inhibitor simvastatin was approved in 2004; simvastatin was approved in 1991.
The mean age of the patients in the SHARP trial was 61 years; they did not have a history of MI or coronary revascularization.
The six panelists who voted in favor of approval for patient not yet on dialysis and those on dialysis included Dr. William Hiatt, professor of medicine, division of cardiology, at the University of Colorado, Denver, voted in favor of approval for both populations, based on the overall trial results. The differences between the two populations could be explained in the Vytorin label, and "the consistency between the vascular and atherosclerotic events led me to believe that the overall trial had integrity and the various components of the primary outcome were relatively consistent," he said. "To deny an indication to extend to patients who are on chronic hemodialysis ... would not respect the totality of the data," he added.
Dr. Lawrence Hunsicker, professor of medicine in the nephrology division, and emeritus medical director of organ transplantation, University of Iowa, Iowa City, also voted in favor of approval for both groups, but added that the FDA should ensure that advertising and detailing of the product should reflect that "the data are far more clear for patients not on dialysis."
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of disclosures, although in some cases, they are given a waiver-but not at this meeting.
[UPDATED]SILVER SPRING, MD. – Vytorin should be approved for preventing major vascular events in patients who have chronic kidney disease but are not on dialysis, according to a unanimous vote by a Food and Drug Administration advisory panel.
The panel also voted 10-6, however, that the safety and effectiveness data did not support approval of the combination of 10 mg of ezetimibe with 20 mg of simvastatin for the same indication in patients with end-stage renal disease who are on dialysis.
Their votes at the Nov. 2 meeting were based on the results of the Study of Heart and Renal Protection (SHARP), which evaluated the effects of reducing LDL cholesterol on the risk of coronary vascular disease in patients with chronic kidney disease who are at an increased risk of cardiovascular morbidity and mortality. About two-thirds of the patients in the trial were not on dialysis at baseline, and in these patients, there was a 23% reduction in the primary end point – the risk of a major vascular event (nonfatal MI or cardiac death, stroke, or a revascularization procedure that excluded dialysis access-related procedures) – compared with those on placebo over a mean of 5 years (Lancet 2011;377:2181-92).
But in the patients who were on dialysis at baseline, the risk was reduced by about 6% over placebo. Panelists who did not support approval in this group cited the lower degree of effectiveness, and the fact that end stage renal disease patients on dialysis are very different from predialysis patients – and that patients with ESRD in the United States are different than those elsewhere. They noted that only 4% of the patients in SHARP were in the United States, and management and outcomes of patients with ESRD are different in the United States than in other parts of the world.
Dr. Lamont Weide, chief of diabetes and endocrinology, at the University of Missouri, Kansas City, backed approval for predialysis patients, but not for those on dialysis. Like several other panelists, he also considered the results of two previous large studies of about 4,000 CKD patients on dialysis, which found no significant beneficial effects of treatment with other statins on cardiovascular outcomes. While these were three different studies with different agents and different entry criteria, he said that the results go in the same direction as SHARP, in a large group of patients "without any clear indication of benefit." Those studies were the 4D study (N. Engl. J. Med. 2005;353:238-48) and the AURORA study (N. Engl J. Med. 2009;360:1395-407).
Also splitting her votes, Dr. Julia Lewis, professor of medicine in the department of nephrology, Vanderbilt University, Nashville, referred to those two trials and added that she considers her dialysis patients considerably different than her clinic patients who are not on dialysis. She commended the SHARP study and investigators for "providing what I think is going to be a wonderful addition to the care of CKD patients. It is going to change care of CKD patients and prevent the bad [cardiovascular] outcomes that affect them," she said.
In the study overall, the risk of major vascular events was reduced by 16% among those treated with the combination as compared to those on placebo, which was primarily driven by a reduction in the revascularization component. Cancers and all-cause mortality were similar in treated patients and those on placebo, and the panel agreed there were no new safety concerns at the dose studied. (About one-quarter of those enrolled died during the study.)
Merck, the manufacturer of ezetimibe (Zetia) and Vytorin, filed for approval of the claim that 10 mg of ezetimibe plus 20 mg of simvastatin (in the fixed-dose combination pill or taken separately) reduces the risk of major cardiovascular events in patients with chronic kidney disease on the basis of the SHARP results. SHARP was funded by Merck and Schering-Plough, but was independently conducted by the Oxford (England) University Clinical Trials Service Unit. Ezetimibe was used to make it possible to use a lower dose of simvastatin in the CKD patients, who are at a greater risk of myopathy and other adverse effects of statins.
Panelists emphasized that the 10 mg of ezetimibe with the 20-mg dose of simvastatin was the dose combination studied and was shown to be safe, and that doses should not be increased in this population of patients.
The two drugs are only approved for lipid-lowering indications: Ezetimibe, a selective inhibitor of the absorption of intestinal cholesterol and related phytosterol marketed as Zetia, was approved by the FDA in 2002; Vytorin, a combination of ezetimibe and the HMG-CoA reductase inhibitor simvastatin was approved in 2004; simvastatin was approved in 1991.
The mean age of the patients in the SHARP trial was 61 years; they did not have a history of MI or coronary revascularization.
The six panelists who voted in favor of approval for patient not yet on dialysis and those on dialysis included Dr. William Hiatt, professor of medicine, division of cardiology, at the University of Colorado, Denver, voted in favor of approval for both populations, based on the overall trial results. The differences between the two populations could be explained in the Vytorin label, and "the consistency between the vascular and atherosclerotic events led me to believe that the overall trial had integrity and the various components of the primary outcome were relatively consistent," he said. "To deny an indication to extend to patients who are on chronic hemodialysis ... would not respect the totality of the data," he added.
Dr. Lawrence Hunsicker, professor of medicine in the nephrology division, and emeritus medical director of organ transplantation, University of Iowa, Iowa City, also voted in favor of approval for both groups, but added that the FDA should ensure that advertising and detailing of the product should reflect that "the data are far more clear for patients not on dialysis."
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of disclosures, although in some cases, they are given a waiver-but not at this meeting.
FROM A MEETING OF THE FDA’S ENDOCRINOLOGIC AND METABOLIC DRUGS ADVISORY PANEL