User login
Pharmacovigilance and PML in the oncology setting
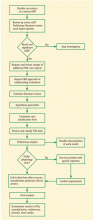
Rare and serious drug-related events are often not detected until after clinical trials have been completed and a drug becomes widely used. Methods traditionally used by pharmaceutical companies and the US Food and Drug Administration (FDA) are not the most effective ways to promptly identify a treatment-related adverse event and quickly notify the medical community. In 1998, an academically based surveillance group was created to identify and disseminate information on unrecognized adverse drug reactions.1 In 2010, with funding from the state of South Carolina, this program became the Southern Network on Adverse Reactions (SONAR), the only state-funded pharmacovigilance initiative in the nation. SONAR and its earlier incarnation have identified potentially fatal and previously unreported side effects associated with 43 drugs—with the majority of these drugs involving the hematology and oncology discisplines.
While the earlier incarnation of drug safety monitoring relied on data mining, or detecting specific signals from large amounts of data, investigations of possible adverse drug event occurrences have a much broader scope. SONAR, an enhanced surveillance program, was created to address this issue. Based jointly at the South Carolina College of Pharmacy and a National Cancer Institute–designated cancer center, the Hollings Cancer Center at the Medical University of South Carolina, SONAR more accurately reflects the nature of our adverse effects investigations: identification of small numbers of important cases from a variety of unique data sources, including case reports, the medical literature, FDA MedWatch, and pharmaceutical manufacturers.
This article reviews methods that underlie the successful investigations of the SONAR initiative, and it examines our SONAR investigation of the association between the immune modulatory monoclonal antibody rituximab and progressive multifocal leukoencephalopathy (PML).
DETECTING, INVESTIGATING, AND DISSEMINATING FINDINGS
Surveillance programs are needed because important rare side effects are seldom discovered in a clinical trial. The Safety and Efficacy of Natalizumab in Combination with Interferon Beta-1a in Patients with Relapsing Remitting Multiple Sclerosis (SENTINEL) trial was unusual in that it detected two cases of PML associated with the use of natalizumab.2 Most rare side effects are undetected at the time of FDA approval, and usually many years elapse from the time a potential problem is detected until it is identified as a rare side effect of the drug. The average time for a “black box” warning to appear on a package insert following FDA approval is 7 to 10 years.3
Timely and thorough data collection
SONAR investigators perform extensive literature reviews, may request more data from authors, and request and review additional FDA case reports. Unfortunately, obtaining data from the FDA can be difficult and time-consuming. Data can be requested through the Freedom of Information Act (FOIA), but receiving it may take more than a year, and the information in the public record may be redacted. SONAR obtains laboratory tests and imaging records and works with scientists to better understand the pathophysiology of potential treatment-related rare adverse events, investigate epidemiologic estimates of the side effect rate, and evaluate risk factors for development of toxicity.
Adverse events are usually identified by SONAR within 2 years post–drug approval—a 5-year improvement over the FDA on this important metric. Once an adverse event is positively identified, the information is disseminated throughout the worldwide medical community via journal articles and presentations at medical conferences. Funding is grant-based from sources such as the National Institutes of Health (NIH), the state of South Carolina, and the University of South Carolina.
FDA, manufacturer reports may be incomplete and delayed
In contrast with SONAR, the FDA relies heavily on MedWatch to detect cases of adverse events. The safety record compiled by MedWatch is often incomplete because the program relies on voluntary submissions of adverse events; further, the inordinate amount of followup required of physicians discourages many from participating. The time to identify an adverse event can be several years, and the FDA disseminates adverse event reports via package inserts. The network that evaluates the safety information and identifies initial safety signals is mainly internal to FDA employees, as is the funding.
Pharmaceutical manufacturers frequently compile data from their own proprietary databases. Although they attempt to follow up on reports of rare adverse events, it is often difficult or impossible for the company to obtain followup information from busy clinicians. Identification of an adverse event typically takes 7 to 12 years for most pharmaceutical manufacturers—reflecting the barriers experienced in obtaining detailed information from clinicians about potential new serious adverse drug reactions. Findings are frequently disseminated through “Dear Doctor” letters. Manufacturers’ investigative networks, like those of the FDA, are largely internal and the amount of funding of they allocate to drug safety investigations is unknown.
RARE EVENTS MAY INVOLVE FEW CASES
Of our major publications,5–14 many findings are based on a small number of cases—for example, only 13 cases for clopidogrel-associated thrombotic thrombocytopenic purpura (TTP)13 and 9 for pure red cell aplasia caused by epoetin alfa.12 Important findings also come from meta-analyses,8,10 although this avenue in our pharmacovigilance approach is less typical.
The 2008 study6 on mortality and venous thromboembolism associated with erythropoiesis-stimulating agents highlights the importance of basic scientific investigation in identifying rare events. Administration of epoetin alfa to raise hemoglobin levels had been approved by the FDA in 1989 for use in patients undergoing dialysis and in 1993 for supportive use in patients with some types of cancers. We discovered that epoetin alfa promoted cancer growth based on analysis of published data and reports in conjunction with basic scientific studies of erythropoietin and erythropoietin receptors in solid cancers.
RITUXIMAB AND VIRAL REACTIVATION
In the case of viral reactivation associated with the use of rituximab, a warning about hepatitis B reactivation was added to the package insert in 2004.15 In 2006, a warning about other viral infections was added to the package insert.16 In late 2006, a letter was sent to health care professionals from the manufacturer and the FDA with the warning that PML had been observed in two patients with systemic lupus erythematosus (SLE) who were treated with rituximab (an off-label use), both of whom were negative for human immunodeficiency virus (HIV).16 A few months later, a black box warning to this effect was added to the package insert.16
After we identified PML as an adverse event from rituximab in HIV-negative patients,14 we obtained case reports from clinicians at 12 cancer centers or academic hospitals (22 cases). We also reviewed FDA reports (11 cases), the manufacturer’s database (30 cases), and publications (18 cases) using the search terms “leukoencephalopathy,” “rituximab,” “immunosuppressed,” “lymphoma,” and “leukemia.” The unique data sources included clinical observations, the medical literature, FDA MedWatch, and the manufacturer.17
Of rituximab-treated patients who developed PML, the mean age was 61 years (range, 30 to 89 years), 56% of patients were women, and the mean number of rituximab doses was six (range, 1 to 28). Six patients had undergone stem cell transplants (four autologous), and 26 were also taking a purine analogue.17
Among 57 patients, a median of 16 months elapsed between first taking rituximab to development of PML (range, 1.0 to 90.0 months), and 5.5 months from the last dose of rituximab to development of PML (range, 0.3 to 66.0 months). The median time from diagnosis of PML to death was only 2.0 months (range, 0.4 to 122 months). Reported survival rates for patients with rituximab-associated PML who did not undergo stem cell transplantation was less than 10%.17
The symptoms of PML are easily confused with those that might be expected in an older patient with lymphoma, making early detection especially difficult. More than one-half (54.4%) had confusion or disorientation, and many had focal motor weakness (33.3%), loss of coordination (24.6%), difficulty speaking (21.2%), and vision changes (17.5%).
Effects on T and B cells and role of JC virus
At the time of PML diagnosis, 90% of patients had either a severely low CD4+ count or a low CD4+:CD8+ ratio. Based on clinical trial data, cytotoxic chemotherapy and not rituximab appears responsible for the abnormal CD4+ count and the low CD4+:CD8+ ratio in rituximab-treated patients.
Little is known about how T cells function after rituximab administration. In idiopathic thrombocytopenic purpura, the response to B-cell depletion induced by rituximab is associated with significant changes in the T-cell compartment.18 In a study of patients with either SLE or Evans syndrome, rituximab therapy was found to modify T-cell phenotype and cytokine profiles.19 The rapid effect of rituximab in multiple sclerosis suggests that it targets a process thought to be T-cell mediated.20
Our early hypothesis was that rituximab contributes to viral reactivation and PML through inhibiting T-and B-lymphocyte interactions. We now believe that the bone marrow plays an important role, which may explain the process by which natalizumab can cause PML. Five of five bone marrow samples from patients with lymphoma and PML tested positive for JC virus (JCV) compared with only two of 86 bone marrow samples from patients without PML. The JCV is latent in CD34+ hematopoietic cells and probably in early B lymphocytes. Chemotherapy mobilizes the stem cells from bone marrow and causes quantitative T-cell depletion. Rituximab reduces the qualitative T-cell response, and B-cell depletion results in expansion of progenitor cells containing the latent JCV. The hypothesis is limited in that it is based on a retrospective case series and is not verified in a laboratory model.
Of the 57 cases of PML identified in 2009, two patients were given rituximab for hematologic disorders and had no chemotherapy other than steroids. These data suggest that rituximab confers risk on its own.17
Quantifying risk of developing PML from rituximab
Calculating the odds of developing PML from rituximab therapy is difficult. The background rate of PML is an important consideration. One population-based study estimated the incidence of PML in patients with hematologic malignancies at 0.07%. This estimate was based on three cases of PML observed in patients with hematologic malignancies over a period of 11 years in a single Canadian province.21 Another study found a higher incidence of 0.52% in patients with chronic lymphocytic leukemia, although all of these patients were also treated with fludarabine.22 Accurately calculating the risk of PML attributable to the underlying malignancy as opposed to immune suppression from treatment is complicated by the rarity of the disease. Fludarabine is the chemotherapeutic agent most closely associated with PML. However, its well known side effects of T-lymphocyte depletion and complicating opportunistic infections similar to those seen in acquired immunodeficiency syndrome (AIDS) make such an association intuitive.23
Kavenaugh and Matteson reported that about 8,000 SLE patients had received rituximab treatment and two of these patients had developed PML.24 PML has been reported previously among 30 SLE patients who had not received rituximab, suggesting that SLE is a predisposing disorder.25,26
In the setting of hematologic malignancy, rituximab-associated PML incidence estimates are complicated by a low basal risk of PML seen among persons with the disease state prompting rituximab therapy and an inability to determine risk attributable to rituximab. A recent study demonstrated an association between rituximab and PML in patients with non-Hodgkin lymphoma (NHL). The retrospective, monocentric cohort study assessed data from 976 NHL patients diagnosed in Italy from 1994 to 2008, including 517 patients who received at least one dose of rituximab. Inclusion of rituximab into standard chemotherapy regimens for NHL caused a significantly higher incidence of PML cases (rate difference, 2.2 every 1,000 patient-years; 95% confidence interval, 0.1–4.3).27 More such studies of viral reactivation syndromes are obviously needed.
Ideally, randomized clinical trials of the use of rituximab in patients with lymphoma would serve as guidance, but because the drug, as the standard of care for treatment of lymphoma, is so widely used, randomization would be impractical.
Future planned studies include a case-control study of T-cell markers after chemotherapy administration with or without exposure to rituximab, a case-control study of bone marrow specimens from disease-matched and treatment-matched controls, and a cohort study using a large electronic medical records database or a government database.
CONCLUSION
The methods developed in the SONAR project will permit exploration of important hypotheses regarding the detection and prevention of rare adverse events in oncology, forming a basis for subsequent investigations. Based on our recent findings, rituximab may be associated with multiple viral reactivation syndromes; screening and early detection can potentially be helpful in preventing these complications.
DISCUSSION
Dr. Calabrese: Your approach to identifying rare adverse events is novel and aggressive, but the seeming limitations in a disease such as lymphoma are (1) rituximab is now a standard of care so everybody with lymphoma gets it, and (2) going back to the earliest descriptions of PML, lymphoma has always been represented as a predisposing factor. Moving ahead, how then can you calculate an effect size for a drug like rituximab?
Dr. Bennett: There’s no way to do it; we’re sort of stuck. Of the 57 cases with PML that we reported in Blood,17 two patients received rituximab for hematologic disorders and received no chemotherapy besides steroids. In those two patients, we could not blame the development of PML on lymphoma. Those types of patients suggest that rituximab may be implicated, but examining this question with a case-control or even a cohort study is an expensive proposition.
Dr. Simpson: My experience in terms of collaborating with the FDA has been distinctly unrewarding. Some years ago I had been looking into an adverse effect related to the nucleoside analog reverse transcriptase inhibitor d4T, in which there was a rapidly progressive neuromuscular weakness syndrome that looked like Guillain-Barré and lactic acidosis. The FDA itself reported 12 cases at an international AIDS conference and did not have any answers. I was charged by the AIDS Clinical Trials Group and other branches at the NIH to try to figure it out. When I requested access to FDA data, I ran into an unbelievable bureaucratic morass. Ultimately, we had to go through the FOIA to get them to release anything.
Dr. Bennett: The FOIA is the only way to get anything from the FDA. It takes about a year and a half and much information is redacted.
Dr. Berger: As we roll out these newer compounds, we need a mechanism to look for both foreseen and unforeseen consequences, perhaps with close collaboration between pharmaceutical companies and governmental agencies.
Dr. Bennett: The Risk Evaluation and Mitigation Strategies program authorizes the FDA to require post-marketing surveillance of all adverse events from manufacturers. We published 11 cases of TTP in association with clopidogrel, obtained from surveillance of directors of plasmapheresis centers in the United States. Not one of them had been reported to the FDA directly. However, we had an article 6 weeks after clopidogrel received FDA approval. Now, 10 years later, there are about 120 clopidogrel-associated TTP cases in the FDA database. Its estimated incidence is still one in a million, although we hear about the side effect every night on TV during commercials for the drug on the evening news.
Dr. Major: The FDA is more open now than in the past to trying to get a handle on what’s going on with biologic therapies. We need to do a little more homework up front on biologic agents in order to anticipate some adverse events. For example, the migratory nature of CD34+ cells through the circulation following natalizumab therapy was not appreciated, even though data in the literature already supported this phenomenon when integrin receptors are blocked.
- Hampton T. Postmarket “pharmacovigilance” program on alert for adverse events from drugs. JAMA 2007; 298:851–852.
- Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006; 354:911–923.
- Lasser KE, Allen PD, Woolhandler SJ, Himmelstein Du, Wolfe SM, Bor DH. Timing of new black box warnings and withdrawals for prescription medications. JAMA 2002; 287:2215–2220.
- Bennett CL, Nebeker JR, Lyons EA, et al. The Research on Adverse Drug Events and Reports (RADAR) project. JAMA 2005; 293:2131–2140.
- Edwards BJ, Gounder M, McKoy JM, et al. Pharmacovigilance and reporting oversight in US FDA fast-track process: bisphosphonates and osteonecrosis of the jaw. Lancet Oncol 2008; 9:1166–1172.
- Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 2008; 299:914–924.
- Beohar N, Davidson CJ, Kip KE, et al. Outcomes and complications associated with off-label and untested use of drug-eluting stents. JAMA 2007; 297:1992–2000.
- Hershman D, Neugut AI, Jacobson JS, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst 2007; 99:196–205.
- Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 2006; 98:708–714.
- Nebeker JR, Virmani R, Bennett CL, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol 2006; 47:175–181.
- Bennett CL, Angelotta C, Yarnold PR, et al. Thalidomide- and lenalidomide-associated thromboembolism among patients with cancer. JAMA 2006; 296:2558–2560.
- Bennett CL, Luminari S, Nissenson AR, et al. Pure red-cell aplasia and epoetin therapy. N Engl J Med 2004; 351:1403–1408.
- Bennett CL, Connors JM, Carwile JM, et al. Thrombotic thrombocytopenic purpura associated with clopidogrel. N Engl J Med 2000; 342:1773–1777.
- Bennett CL, Kiss JE, Weinberg PD, et al. Thrombotic thrombocytopenic purpura after stenting and ticlopidine. Lancet 1998; 352:1036–1037.
- Rituxan (rituximab) Oct 2004. U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm166521.htm. Updated June 19, 2009. Accessed September 13, 2011.
- Rituxan (rituximab). U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm150747.htm. Updated June 19, 2009. Accessed September 13, 2011.
- Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 2009; 113:4834–4840.
- Stasi R, Del Poeta G, Stipa E, et al. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood 2007; 110:2924–2930.
- Tamimoto Y, Horiuchi T, Tsukamoto H, et al. A dose-escalation study of rituximab for treatment of systemic lupus erythematosus and Evans’ syndrome: immunological analysis of B cells, T cells and cytokines. Rheumatology (Oxford) 2008; 47:821–827.
- McFarland HF. The B cell—old player, new position on the team. N Engl J Med 2008; 358:664–665.
- Power C, Gladden JG, Halliday W, et al. AIDS- and non-AIDS-related PML association with distinct p53 polymorphism. Neurology 2000; 54:743–746.
- Gonzalez H, Bolgert F, Camporo P, Leblond V. Progressive multi focal leukoencephalitis (PML) in three patients treated with standard-dose fludarabine (FAMP). Hematol Cell Ther 1999; 41:183–186.
- Garcia-Suárez J, de Miguel D, Krsnik I, Bañas H, Arribas I, Burgaleta C. Changes in the natural history of progressive multifocal leukoencephalopathy in HIV-negative lymphoproliferative disorders: impact of novel therapies. Am J Hematol 2005; 80:271–281.
- Kavanaugh A, Matteson E. Hotline: rituximab and progressive multifocal leukoencephalopathy. American College of Rheumatology Web site. http://www.rheumatology.org/publications/hotline/0107leuko.asp. Updated January 2007. Accessed September 13, 2011.
- Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy in patients with rheumatic diseases: are patients with systemic lupus erythematosus at particular risk? Autoimmun Rev 2008; 8:144–146.
- Calabrese LH, Molloy ES. Progressive multifocal leucoencephalopathy in the rheumatic diseases: assessing the risks of biological immunosuppressive therapies. Ann Rheum Dis 2008; 67 (suppl 3):iii64–iii65.
- Tuccori M, Focosi D, Blandizzi C, et al. Inclusion of rituximab in treatment protocols for non-Hodgkin’s lymphomas and risk for progressive multifocal leukoencephalopathy. Oncologist 2010; 15:1214–1219. Epub 2010 Nov 1.
Rare and serious drug-related events are often not detected until after clinical trials have been completed and a drug becomes widely used. Methods traditionally used by pharmaceutical companies and the US Food and Drug Administration (FDA) are not the most effective ways to promptly identify a treatment-related adverse event and quickly notify the medical community. In 1998, an academically based surveillance group was created to identify and disseminate information on unrecognized adverse drug reactions.1 In 2010, with funding from the state of South Carolina, this program became the Southern Network on Adverse Reactions (SONAR), the only state-funded pharmacovigilance initiative in the nation. SONAR and its earlier incarnation have identified potentially fatal and previously unreported side effects associated with 43 drugs—with the majority of these drugs involving the hematology and oncology discisplines.
While the earlier incarnation of drug safety monitoring relied on data mining, or detecting specific signals from large amounts of data, investigations of possible adverse drug event occurrences have a much broader scope. SONAR, an enhanced surveillance program, was created to address this issue. Based jointly at the South Carolina College of Pharmacy and a National Cancer Institute–designated cancer center, the Hollings Cancer Center at the Medical University of South Carolina, SONAR more accurately reflects the nature of our adverse effects investigations: identification of small numbers of important cases from a variety of unique data sources, including case reports, the medical literature, FDA MedWatch, and pharmaceutical manufacturers.
This article reviews methods that underlie the successful investigations of the SONAR initiative, and it examines our SONAR investigation of the association between the immune modulatory monoclonal antibody rituximab and progressive multifocal leukoencephalopathy (PML).
DETECTING, INVESTIGATING, AND DISSEMINATING FINDINGS
Surveillance programs are needed because important rare side effects are seldom discovered in a clinical trial. The Safety and Efficacy of Natalizumab in Combination with Interferon Beta-1a in Patients with Relapsing Remitting Multiple Sclerosis (SENTINEL) trial was unusual in that it detected two cases of PML associated with the use of natalizumab.2 Most rare side effects are undetected at the time of FDA approval, and usually many years elapse from the time a potential problem is detected until it is identified as a rare side effect of the drug. The average time for a “black box” warning to appear on a package insert following FDA approval is 7 to 10 years.3
Timely and thorough data collection
SONAR investigators perform extensive literature reviews, may request more data from authors, and request and review additional FDA case reports. Unfortunately, obtaining data from the FDA can be difficult and time-consuming. Data can be requested through the Freedom of Information Act (FOIA), but receiving it may take more than a year, and the information in the public record may be redacted. SONAR obtains laboratory tests and imaging records and works with scientists to better understand the pathophysiology of potential treatment-related rare adverse events, investigate epidemiologic estimates of the side effect rate, and evaluate risk factors for development of toxicity.
Adverse events are usually identified by SONAR within 2 years post–drug approval—a 5-year improvement over the FDA on this important metric. Once an adverse event is positively identified, the information is disseminated throughout the worldwide medical community via journal articles and presentations at medical conferences. Funding is grant-based from sources such as the National Institutes of Health (NIH), the state of South Carolina, and the University of South Carolina.
FDA, manufacturer reports may be incomplete and delayed
In contrast with SONAR, the FDA relies heavily on MedWatch to detect cases of adverse events. The safety record compiled by MedWatch is often incomplete because the program relies on voluntary submissions of adverse events; further, the inordinate amount of followup required of physicians discourages many from participating. The time to identify an adverse event can be several years, and the FDA disseminates adverse event reports via package inserts. The network that evaluates the safety information and identifies initial safety signals is mainly internal to FDA employees, as is the funding.
Pharmaceutical manufacturers frequently compile data from their own proprietary databases. Although they attempt to follow up on reports of rare adverse events, it is often difficult or impossible for the company to obtain followup information from busy clinicians. Identification of an adverse event typically takes 7 to 12 years for most pharmaceutical manufacturers—reflecting the barriers experienced in obtaining detailed information from clinicians about potential new serious adverse drug reactions. Findings are frequently disseminated through “Dear Doctor” letters. Manufacturers’ investigative networks, like those of the FDA, are largely internal and the amount of funding of they allocate to drug safety investigations is unknown.
RARE EVENTS MAY INVOLVE FEW CASES
Of our major publications,5–14 many findings are based on a small number of cases—for example, only 13 cases for clopidogrel-associated thrombotic thrombocytopenic purpura (TTP)13 and 9 for pure red cell aplasia caused by epoetin alfa.12 Important findings also come from meta-analyses,8,10 although this avenue in our pharmacovigilance approach is less typical.
The 2008 study6 on mortality and venous thromboembolism associated with erythropoiesis-stimulating agents highlights the importance of basic scientific investigation in identifying rare events. Administration of epoetin alfa to raise hemoglobin levels had been approved by the FDA in 1989 for use in patients undergoing dialysis and in 1993 for supportive use in patients with some types of cancers. We discovered that epoetin alfa promoted cancer growth based on analysis of published data and reports in conjunction with basic scientific studies of erythropoietin and erythropoietin receptors in solid cancers.
RITUXIMAB AND VIRAL REACTIVATION
In the case of viral reactivation associated with the use of rituximab, a warning about hepatitis B reactivation was added to the package insert in 2004.15 In 2006, a warning about other viral infections was added to the package insert.16 In late 2006, a letter was sent to health care professionals from the manufacturer and the FDA with the warning that PML had been observed in two patients with systemic lupus erythematosus (SLE) who were treated with rituximab (an off-label use), both of whom were negative for human immunodeficiency virus (HIV).16 A few months later, a black box warning to this effect was added to the package insert.16
After we identified PML as an adverse event from rituximab in HIV-negative patients,14 we obtained case reports from clinicians at 12 cancer centers or academic hospitals (22 cases). We also reviewed FDA reports (11 cases), the manufacturer’s database (30 cases), and publications (18 cases) using the search terms “leukoencephalopathy,” “rituximab,” “immunosuppressed,” “lymphoma,” and “leukemia.” The unique data sources included clinical observations, the medical literature, FDA MedWatch, and the manufacturer.17
Of rituximab-treated patients who developed PML, the mean age was 61 years (range, 30 to 89 years), 56% of patients were women, and the mean number of rituximab doses was six (range, 1 to 28). Six patients had undergone stem cell transplants (four autologous), and 26 were also taking a purine analogue.17
Among 57 patients, a median of 16 months elapsed between first taking rituximab to development of PML (range, 1.0 to 90.0 months), and 5.5 months from the last dose of rituximab to development of PML (range, 0.3 to 66.0 months). The median time from diagnosis of PML to death was only 2.0 months (range, 0.4 to 122 months). Reported survival rates for patients with rituximab-associated PML who did not undergo stem cell transplantation was less than 10%.17
The symptoms of PML are easily confused with those that might be expected in an older patient with lymphoma, making early detection especially difficult. More than one-half (54.4%) had confusion or disorientation, and many had focal motor weakness (33.3%), loss of coordination (24.6%), difficulty speaking (21.2%), and vision changes (17.5%).
Effects on T and B cells and role of JC virus
At the time of PML diagnosis, 90% of patients had either a severely low CD4+ count or a low CD4+:CD8+ ratio. Based on clinical trial data, cytotoxic chemotherapy and not rituximab appears responsible for the abnormal CD4+ count and the low CD4+:CD8+ ratio in rituximab-treated patients.
Little is known about how T cells function after rituximab administration. In idiopathic thrombocytopenic purpura, the response to B-cell depletion induced by rituximab is associated with significant changes in the T-cell compartment.18 In a study of patients with either SLE or Evans syndrome, rituximab therapy was found to modify T-cell phenotype and cytokine profiles.19 The rapid effect of rituximab in multiple sclerosis suggests that it targets a process thought to be T-cell mediated.20
Our early hypothesis was that rituximab contributes to viral reactivation and PML through inhibiting T-and B-lymphocyte interactions. We now believe that the bone marrow plays an important role, which may explain the process by which natalizumab can cause PML. Five of five bone marrow samples from patients with lymphoma and PML tested positive for JC virus (JCV) compared with only two of 86 bone marrow samples from patients without PML. The JCV is latent in CD34+ hematopoietic cells and probably in early B lymphocytes. Chemotherapy mobilizes the stem cells from bone marrow and causes quantitative T-cell depletion. Rituximab reduces the qualitative T-cell response, and B-cell depletion results in expansion of progenitor cells containing the latent JCV. The hypothesis is limited in that it is based on a retrospective case series and is not verified in a laboratory model.
Of the 57 cases of PML identified in 2009, two patients were given rituximab for hematologic disorders and had no chemotherapy other than steroids. These data suggest that rituximab confers risk on its own.17
Quantifying risk of developing PML from rituximab
Calculating the odds of developing PML from rituximab therapy is difficult. The background rate of PML is an important consideration. One population-based study estimated the incidence of PML in patients with hematologic malignancies at 0.07%. This estimate was based on three cases of PML observed in patients with hematologic malignancies over a period of 11 years in a single Canadian province.21 Another study found a higher incidence of 0.52% in patients with chronic lymphocytic leukemia, although all of these patients were also treated with fludarabine.22 Accurately calculating the risk of PML attributable to the underlying malignancy as opposed to immune suppression from treatment is complicated by the rarity of the disease. Fludarabine is the chemotherapeutic agent most closely associated with PML. However, its well known side effects of T-lymphocyte depletion and complicating opportunistic infections similar to those seen in acquired immunodeficiency syndrome (AIDS) make such an association intuitive.23
Kavenaugh and Matteson reported that about 8,000 SLE patients had received rituximab treatment and two of these patients had developed PML.24 PML has been reported previously among 30 SLE patients who had not received rituximab, suggesting that SLE is a predisposing disorder.25,26
In the setting of hematologic malignancy, rituximab-associated PML incidence estimates are complicated by a low basal risk of PML seen among persons with the disease state prompting rituximab therapy and an inability to determine risk attributable to rituximab. A recent study demonstrated an association between rituximab and PML in patients with non-Hodgkin lymphoma (NHL). The retrospective, monocentric cohort study assessed data from 976 NHL patients diagnosed in Italy from 1994 to 2008, including 517 patients who received at least one dose of rituximab. Inclusion of rituximab into standard chemotherapy regimens for NHL caused a significantly higher incidence of PML cases (rate difference, 2.2 every 1,000 patient-years; 95% confidence interval, 0.1–4.3).27 More such studies of viral reactivation syndromes are obviously needed.
Ideally, randomized clinical trials of the use of rituximab in patients with lymphoma would serve as guidance, but because the drug, as the standard of care for treatment of lymphoma, is so widely used, randomization would be impractical.
Future planned studies include a case-control study of T-cell markers after chemotherapy administration with or without exposure to rituximab, a case-control study of bone marrow specimens from disease-matched and treatment-matched controls, and a cohort study using a large electronic medical records database or a government database.
CONCLUSION
The methods developed in the SONAR project will permit exploration of important hypotheses regarding the detection and prevention of rare adverse events in oncology, forming a basis for subsequent investigations. Based on our recent findings, rituximab may be associated with multiple viral reactivation syndromes; screening and early detection can potentially be helpful in preventing these complications.
DISCUSSION
Dr. Calabrese: Your approach to identifying rare adverse events is novel and aggressive, but the seeming limitations in a disease such as lymphoma are (1) rituximab is now a standard of care so everybody with lymphoma gets it, and (2) going back to the earliest descriptions of PML, lymphoma has always been represented as a predisposing factor. Moving ahead, how then can you calculate an effect size for a drug like rituximab?
Dr. Bennett: There’s no way to do it; we’re sort of stuck. Of the 57 cases with PML that we reported in Blood,17 two patients received rituximab for hematologic disorders and received no chemotherapy besides steroids. In those two patients, we could not blame the development of PML on lymphoma. Those types of patients suggest that rituximab may be implicated, but examining this question with a case-control or even a cohort study is an expensive proposition.
Dr. Simpson: My experience in terms of collaborating with the FDA has been distinctly unrewarding. Some years ago I had been looking into an adverse effect related to the nucleoside analog reverse transcriptase inhibitor d4T, in which there was a rapidly progressive neuromuscular weakness syndrome that looked like Guillain-Barré and lactic acidosis. The FDA itself reported 12 cases at an international AIDS conference and did not have any answers. I was charged by the AIDS Clinical Trials Group and other branches at the NIH to try to figure it out. When I requested access to FDA data, I ran into an unbelievable bureaucratic morass. Ultimately, we had to go through the FOIA to get them to release anything.
Dr. Bennett: The FOIA is the only way to get anything from the FDA. It takes about a year and a half and much information is redacted.
Dr. Berger: As we roll out these newer compounds, we need a mechanism to look for both foreseen and unforeseen consequences, perhaps with close collaboration between pharmaceutical companies and governmental agencies.
Dr. Bennett: The Risk Evaluation and Mitigation Strategies program authorizes the FDA to require post-marketing surveillance of all adverse events from manufacturers. We published 11 cases of TTP in association with clopidogrel, obtained from surveillance of directors of plasmapheresis centers in the United States. Not one of them had been reported to the FDA directly. However, we had an article 6 weeks after clopidogrel received FDA approval. Now, 10 years later, there are about 120 clopidogrel-associated TTP cases in the FDA database. Its estimated incidence is still one in a million, although we hear about the side effect every night on TV during commercials for the drug on the evening news.
Dr. Major: The FDA is more open now than in the past to trying to get a handle on what’s going on with biologic therapies. We need to do a little more homework up front on biologic agents in order to anticipate some adverse events. For example, the migratory nature of CD34+ cells through the circulation following natalizumab therapy was not appreciated, even though data in the literature already supported this phenomenon when integrin receptors are blocked.
Rare and serious drug-related events are often not detected until after clinical trials have been completed and a drug becomes widely used. Methods traditionally used by pharmaceutical companies and the US Food and Drug Administration (FDA) are not the most effective ways to promptly identify a treatment-related adverse event and quickly notify the medical community. In 1998, an academically based surveillance group was created to identify and disseminate information on unrecognized adverse drug reactions.1 In 2010, with funding from the state of South Carolina, this program became the Southern Network on Adverse Reactions (SONAR), the only state-funded pharmacovigilance initiative in the nation. SONAR and its earlier incarnation have identified potentially fatal and previously unreported side effects associated with 43 drugs—with the majority of these drugs involving the hematology and oncology discisplines.
While the earlier incarnation of drug safety monitoring relied on data mining, or detecting specific signals from large amounts of data, investigations of possible adverse drug event occurrences have a much broader scope. SONAR, an enhanced surveillance program, was created to address this issue. Based jointly at the South Carolina College of Pharmacy and a National Cancer Institute–designated cancer center, the Hollings Cancer Center at the Medical University of South Carolina, SONAR more accurately reflects the nature of our adverse effects investigations: identification of small numbers of important cases from a variety of unique data sources, including case reports, the medical literature, FDA MedWatch, and pharmaceutical manufacturers.
This article reviews methods that underlie the successful investigations of the SONAR initiative, and it examines our SONAR investigation of the association between the immune modulatory monoclonal antibody rituximab and progressive multifocal leukoencephalopathy (PML).
DETECTING, INVESTIGATING, AND DISSEMINATING FINDINGS
Surveillance programs are needed because important rare side effects are seldom discovered in a clinical trial. The Safety and Efficacy of Natalizumab in Combination with Interferon Beta-1a in Patients with Relapsing Remitting Multiple Sclerosis (SENTINEL) trial was unusual in that it detected two cases of PML associated with the use of natalizumab.2 Most rare side effects are undetected at the time of FDA approval, and usually many years elapse from the time a potential problem is detected until it is identified as a rare side effect of the drug. The average time for a “black box” warning to appear on a package insert following FDA approval is 7 to 10 years.3
Timely and thorough data collection
SONAR investigators perform extensive literature reviews, may request more data from authors, and request and review additional FDA case reports. Unfortunately, obtaining data from the FDA can be difficult and time-consuming. Data can be requested through the Freedom of Information Act (FOIA), but receiving it may take more than a year, and the information in the public record may be redacted. SONAR obtains laboratory tests and imaging records and works with scientists to better understand the pathophysiology of potential treatment-related rare adverse events, investigate epidemiologic estimates of the side effect rate, and evaluate risk factors for development of toxicity.
Adverse events are usually identified by SONAR within 2 years post–drug approval—a 5-year improvement over the FDA on this important metric. Once an adverse event is positively identified, the information is disseminated throughout the worldwide medical community via journal articles and presentations at medical conferences. Funding is grant-based from sources such as the National Institutes of Health (NIH), the state of South Carolina, and the University of South Carolina.
FDA, manufacturer reports may be incomplete and delayed
In contrast with SONAR, the FDA relies heavily on MedWatch to detect cases of adverse events. The safety record compiled by MedWatch is often incomplete because the program relies on voluntary submissions of adverse events; further, the inordinate amount of followup required of physicians discourages many from participating. The time to identify an adverse event can be several years, and the FDA disseminates adverse event reports via package inserts. The network that evaluates the safety information and identifies initial safety signals is mainly internal to FDA employees, as is the funding.
Pharmaceutical manufacturers frequently compile data from their own proprietary databases. Although they attempt to follow up on reports of rare adverse events, it is often difficult or impossible for the company to obtain followup information from busy clinicians. Identification of an adverse event typically takes 7 to 12 years for most pharmaceutical manufacturers—reflecting the barriers experienced in obtaining detailed information from clinicians about potential new serious adverse drug reactions. Findings are frequently disseminated through “Dear Doctor” letters. Manufacturers’ investigative networks, like those of the FDA, are largely internal and the amount of funding of they allocate to drug safety investigations is unknown.
RARE EVENTS MAY INVOLVE FEW CASES
Of our major publications,5–14 many findings are based on a small number of cases—for example, only 13 cases for clopidogrel-associated thrombotic thrombocytopenic purpura (TTP)13 and 9 for pure red cell aplasia caused by epoetin alfa.12 Important findings also come from meta-analyses,8,10 although this avenue in our pharmacovigilance approach is less typical.
The 2008 study6 on mortality and venous thromboembolism associated with erythropoiesis-stimulating agents highlights the importance of basic scientific investigation in identifying rare events. Administration of epoetin alfa to raise hemoglobin levels had been approved by the FDA in 1989 for use in patients undergoing dialysis and in 1993 for supportive use in patients with some types of cancers. We discovered that epoetin alfa promoted cancer growth based on analysis of published data and reports in conjunction with basic scientific studies of erythropoietin and erythropoietin receptors in solid cancers.
RITUXIMAB AND VIRAL REACTIVATION
In the case of viral reactivation associated with the use of rituximab, a warning about hepatitis B reactivation was added to the package insert in 2004.15 In 2006, a warning about other viral infections was added to the package insert.16 In late 2006, a letter was sent to health care professionals from the manufacturer and the FDA with the warning that PML had been observed in two patients with systemic lupus erythematosus (SLE) who were treated with rituximab (an off-label use), both of whom were negative for human immunodeficiency virus (HIV).16 A few months later, a black box warning to this effect was added to the package insert.16
After we identified PML as an adverse event from rituximab in HIV-negative patients,14 we obtained case reports from clinicians at 12 cancer centers or academic hospitals (22 cases). We also reviewed FDA reports (11 cases), the manufacturer’s database (30 cases), and publications (18 cases) using the search terms “leukoencephalopathy,” “rituximab,” “immunosuppressed,” “lymphoma,” and “leukemia.” The unique data sources included clinical observations, the medical literature, FDA MedWatch, and the manufacturer.17
Of rituximab-treated patients who developed PML, the mean age was 61 years (range, 30 to 89 years), 56% of patients were women, and the mean number of rituximab doses was six (range, 1 to 28). Six patients had undergone stem cell transplants (four autologous), and 26 were also taking a purine analogue.17
Among 57 patients, a median of 16 months elapsed between first taking rituximab to development of PML (range, 1.0 to 90.0 months), and 5.5 months from the last dose of rituximab to development of PML (range, 0.3 to 66.0 months). The median time from diagnosis of PML to death was only 2.0 months (range, 0.4 to 122 months). Reported survival rates for patients with rituximab-associated PML who did not undergo stem cell transplantation was less than 10%.17
The symptoms of PML are easily confused with those that might be expected in an older patient with lymphoma, making early detection especially difficult. More than one-half (54.4%) had confusion or disorientation, and many had focal motor weakness (33.3%), loss of coordination (24.6%), difficulty speaking (21.2%), and vision changes (17.5%).
Effects on T and B cells and role of JC virus
At the time of PML diagnosis, 90% of patients had either a severely low CD4+ count or a low CD4+:CD8+ ratio. Based on clinical trial data, cytotoxic chemotherapy and not rituximab appears responsible for the abnormal CD4+ count and the low CD4+:CD8+ ratio in rituximab-treated patients.
Little is known about how T cells function after rituximab administration. In idiopathic thrombocytopenic purpura, the response to B-cell depletion induced by rituximab is associated with significant changes in the T-cell compartment.18 In a study of patients with either SLE or Evans syndrome, rituximab therapy was found to modify T-cell phenotype and cytokine profiles.19 The rapid effect of rituximab in multiple sclerosis suggests that it targets a process thought to be T-cell mediated.20
Our early hypothesis was that rituximab contributes to viral reactivation and PML through inhibiting T-and B-lymphocyte interactions. We now believe that the bone marrow plays an important role, which may explain the process by which natalizumab can cause PML. Five of five bone marrow samples from patients with lymphoma and PML tested positive for JC virus (JCV) compared with only two of 86 bone marrow samples from patients without PML. The JCV is latent in CD34+ hematopoietic cells and probably in early B lymphocytes. Chemotherapy mobilizes the stem cells from bone marrow and causes quantitative T-cell depletion. Rituximab reduces the qualitative T-cell response, and B-cell depletion results in expansion of progenitor cells containing the latent JCV. The hypothesis is limited in that it is based on a retrospective case series and is not verified in a laboratory model.
Of the 57 cases of PML identified in 2009, two patients were given rituximab for hematologic disorders and had no chemotherapy other than steroids. These data suggest that rituximab confers risk on its own.17
Quantifying risk of developing PML from rituximab
Calculating the odds of developing PML from rituximab therapy is difficult. The background rate of PML is an important consideration. One population-based study estimated the incidence of PML in patients with hematologic malignancies at 0.07%. This estimate was based on three cases of PML observed in patients with hematologic malignancies over a period of 11 years in a single Canadian province.21 Another study found a higher incidence of 0.52% in patients with chronic lymphocytic leukemia, although all of these patients were also treated with fludarabine.22 Accurately calculating the risk of PML attributable to the underlying malignancy as opposed to immune suppression from treatment is complicated by the rarity of the disease. Fludarabine is the chemotherapeutic agent most closely associated with PML. However, its well known side effects of T-lymphocyte depletion and complicating opportunistic infections similar to those seen in acquired immunodeficiency syndrome (AIDS) make such an association intuitive.23
Kavenaugh and Matteson reported that about 8,000 SLE patients had received rituximab treatment and two of these patients had developed PML.24 PML has been reported previously among 30 SLE patients who had not received rituximab, suggesting that SLE is a predisposing disorder.25,26
In the setting of hematologic malignancy, rituximab-associated PML incidence estimates are complicated by a low basal risk of PML seen among persons with the disease state prompting rituximab therapy and an inability to determine risk attributable to rituximab. A recent study demonstrated an association between rituximab and PML in patients with non-Hodgkin lymphoma (NHL). The retrospective, monocentric cohort study assessed data from 976 NHL patients diagnosed in Italy from 1994 to 2008, including 517 patients who received at least one dose of rituximab. Inclusion of rituximab into standard chemotherapy regimens for NHL caused a significantly higher incidence of PML cases (rate difference, 2.2 every 1,000 patient-years; 95% confidence interval, 0.1–4.3).27 More such studies of viral reactivation syndromes are obviously needed.
Ideally, randomized clinical trials of the use of rituximab in patients with lymphoma would serve as guidance, but because the drug, as the standard of care for treatment of lymphoma, is so widely used, randomization would be impractical.
Future planned studies include a case-control study of T-cell markers after chemotherapy administration with or without exposure to rituximab, a case-control study of bone marrow specimens from disease-matched and treatment-matched controls, and a cohort study using a large electronic medical records database or a government database.
CONCLUSION
The methods developed in the SONAR project will permit exploration of important hypotheses regarding the detection and prevention of rare adverse events in oncology, forming a basis for subsequent investigations. Based on our recent findings, rituximab may be associated with multiple viral reactivation syndromes; screening and early detection can potentially be helpful in preventing these complications.
DISCUSSION
Dr. Calabrese: Your approach to identifying rare adverse events is novel and aggressive, but the seeming limitations in a disease such as lymphoma are (1) rituximab is now a standard of care so everybody with lymphoma gets it, and (2) going back to the earliest descriptions of PML, lymphoma has always been represented as a predisposing factor. Moving ahead, how then can you calculate an effect size for a drug like rituximab?
Dr. Bennett: There’s no way to do it; we’re sort of stuck. Of the 57 cases with PML that we reported in Blood,17 two patients received rituximab for hematologic disorders and received no chemotherapy besides steroids. In those two patients, we could not blame the development of PML on lymphoma. Those types of patients suggest that rituximab may be implicated, but examining this question with a case-control or even a cohort study is an expensive proposition.
Dr. Simpson: My experience in terms of collaborating with the FDA has been distinctly unrewarding. Some years ago I had been looking into an adverse effect related to the nucleoside analog reverse transcriptase inhibitor d4T, in which there was a rapidly progressive neuromuscular weakness syndrome that looked like Guillain-Barré and lactic acidosis. The FDA itself reported 12 cases at an international AIDS conference and did not have any answers. I was charged by the AIDS Clinical Trials Group and other branches at the NIH to try to figure it out. When I requested access to FDA data, I ran into an unbelievable bureaucratic morass. Ultimately, we had to go through the FOIA to get them to release anything.
Dr. Bennett: The FOIA is the only way to get anything from the FDA. It takes about a year and a half and much information is redacted.
Dr. Berger: As we roll out these newer compounds, we need a mechanism to look for both foreseen and unforeseen consequences, perhaps with close collaboration between pharmaceutical companies and governmental agencies.
Dr. Bennett: The Risk Evaluation and Mitigation Strategies program authorizes the FDA to require post-marketing surveillance of all adverse events from manufacturers. We published 11 cases of TTP in association with clopidogrel, obtained from surveillance of directors of plasmapheresis centers in the United States. Not one of them had been reported to the FDA directly. However, we had an article 6 weeks after clopidogrel received FDA approval. Now, 10 years later, there are about 120 clopidogrel-associated TTP cases in the FDA database. Its estimated incidence is still one in a million, although we hear about the side effect every night on TV during commercials for the drug on the evening news.
Dr. Major: The FDA is more open now than in the past to trying to get a handle on what’s going on with biologic therapies. We need to do a little more homework up front on biologic agents in order to anticipate some adverse events. For example, the migratory nature of CD34+ cells through the circulation following natalizumab therapy was not appreciated, even though data in the literature already supported this phenomenon when integrin receptors are blocked.
- Hampton T. Postmarket “pharmacovigilance” program on alert for adverse events from drugs. JAMA 2007; 298:851–852.
- Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006; 354:911–923.
- Lasser KE, Allen PD, Woolhandler SJ, Himmelstein Du, Wolfe SM, Bor DH. Timing of new black box warnings and withdrawals for prescription medications. JAMA 2002; 287:2215–2220.
- Bennett CL, Nebeker JR, Lyons EA, et al. The Research on Adverse Drug Events and Reports (RADAR) project. JAMA 2005; 293:2131–2140.
- Edwards BJ, Gounder M, McKoy JM, et al. Pharmacovigilance and reporting oversight in US FDA fast-track process: bisphosphonates and osteonecrosis of the jaw. Lancet Oncol 2008; 9:1166–1172.
- Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 2008; 299:914–924.
- Beohar N, Davidson CJ, Kip KE, et al. Outcomes and complications associated with off-label and untested use of drug-eluting stents. JAMA 2007; 297:1992–2000.
- Hershman D, Neugut AI, Jacobson JS, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst 2007; 99:196–205.
- Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 2006; 98:708–714.
- Nebeker JR, Virmani R, Bennett CL, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol 2006; 47:175–181.
- Bennett CL, Angelotta C, Yarnold PR, et al. Thalidomide- and lenalidomide-associated thromboembolism among patients with cancer. JAMA 2006; 296:2558–2560.
- Bennett CL, Luminari S, Nissenson AR, et al. Pure red-cell aplasia and epoetin therapy. N Engl J Med 2004; 351:1403–1408.
- Bennett CL, Connors JM, Carwile JM, et al. Thrombotic thrombocytopenic purpura associated with clopidogrel. N Engl J Med 2000; 342:1773–1777.
- Bennett CL, Kiss JE, Weinberg PD, et al. Thrombotic thrombocytopenic purpura after stenting and ticlopidine. Lancet 1998; 352:1036–1037.
- Rituxan (rituximab) Oct 2004. U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm166521.htm. Updated June 19, 2009. Accessed September 13, 2011.
- Rituxan (rituximab). U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm150747.htm. Updated June 19, 2009. Accessed September 13, 2011.
- Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 2009; 113:4834–4840.
- Stasi R, Del Poeta G, Stipa E, et al. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood 2007; 110:2924–2930.
- Tamimoto Y, Horiuchi T, Tsukamoto H, et al. A dose-escalation study of rituximab for treatment of systemic lupus erythematosus and Evans’ syndrome: immunological analysis of B cells, T cells and cytokines. Rheumatology (Oxford) 2008; 47:821–827.
- McFarland HF. The B cell—old player, new position on the team. N Engl J Med 2008; 358:664–665.
- Power C, Gladden JG, Halliday W, et al. AIDS- and non-AIDS-related PML association with distinct p53 polymorphism. Neurology 2000; 54:743–746.
- Gonzalez H, Bolgert F, Camporo P, Leblond V. Progressive multi focal leukoencephalitis (PML) in three patients treated with standard-dose fludarabine (FAMP). Hematol Cell Ther 1999; 41:183–186.
- Garcia-Suárez J, de Miguel D, Krsnik I, Bañas H, Arribas I, Burgaleta C. Changes in the natural history of progressive multifocal leukoencephalopathy in HIV-negative lymphoproliferative disorders: impact of novel therapies. Am J Hematol 2005; 80:271–281.
- Kavanaugh A, Matteson E. Hotline: rituximab and progressive multifocal leukoencephalopathy. American College of Rheumatology Web site. http://www.rheumatology.org/publications/hotline/0107leuko.asp. Updated January 2007. Accessed September 13, 2011.
- Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy in patients with rheumatic diseases: are patients with systemic lupus erythematosus at particular risk? Autoimmun Rev 2008; 8:144–146.
- Calabrese LH, Molloy ES. Progressive multifocal leucoencephalopathy in the rheumatic diseases: assessing the risks of biological immunosuppressive therapies. Ann Rheum Dis 2008; 67 (suppl 3):iii64–iii65.
- Tuccori M, Focosi D, Blandizzi C, et al. Inclusion of rituximab in treatment protocols for non-Hodgkin’s lymphomas and risk for progressive multifocal leukoencephalopathy. Oncologist 2010; 15:1214–1219. Epub 2010 Nov 1.
- Hampton T. Postmarket “pharmacovigilance” program on alert for adverse events from drugs. JAMA 2007; 298:851–852.
- Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006; 354:911–923.
- Lasser KE, Allen PD, Woolhandler SJ, Himmelstein Du, Wolfe SM, Bor DH. Timing of new black box warnings and withdrawals for prescription medications. JAMA 2002; 287:2215–2220.
- Bennett CL, Nebeker JR, Lyons EA, et al. The Research on Adverse Drug Events and Reports (RADAR) project. JAMA 2005; 293:2131–2140.
- Edwards BJ, Gounder M, McKoy JM, et al. Pharmacovigilance and reporting oversight in US FDA fast-track process: bisphosphonates and osteonecrosis of the jaw. Lancet Oncol 2008; 9:1166–1172.
- Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 2008; 299:914–924.
- Beohar N, Davidson CJ, Kip KE, et al. Outcomes and complications associated with off-label and untested use of drug-eluting stents. JAMA 2007; 297:1992–2000.
- Hershman D, Neugut AI, Jacobson JS, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst 2007; 99:196–205.
- Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 2006; 98:708–714.
- Nebeker JR, Virmani R, Bennett CL, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol 2006; 47:175–181.
- Bennett CL, Angelotta C, Yarnold PR, et al. Thalidomide- and lenalidomide-associated thromboembolism among patients with cancer. JAMA 2006; 296:2558–2560.
- Bennett CL, Luminari S, Nissenson AR, et al. Pure red-cell aplasia and epoetin therapy. N Engl J Med 2004; 351:1403–1408.
- Bennett CL, Connors JM, Carwile JM, et al. Thrombotic thrombocytopenic purpura associated with clopidogrel. N Engl J Med 2000; 342:1773–1777.
- Bennett CL, Kiss JE, Weinberg PD, et al. Thrombotic thrombocytopenic purpura after stenting and ticlopidine. Lancet 1998; 352:1036–1037.
- Rituxan (rituximab) Oct 2004. U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm166521.htm. Updated June 19, 2009. Accessed September 13, 2011.
- Rituxan (rituximab). U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm150747.htm. Updated June 19, 2009. Accessed September 13, 2011.
- Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 2009; 113:4834–4840.
- Stasi R, Del Poeta G, Stipa E, et al. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood 2007; 110:2924–2930.
- Tamimoto Y, Horiuchi T, Tsukamoto H, et al. A dose-escalation study of rituximab for treatment of systemic lupus erythematosus and Evans’ syndrome: immunological analysis of B cells, T cells and cytokines. Rheumatology (Oxford) 2008; 47:821–827.
- McFarland HF. The B cell—old player, new position on the team. N Engl J Med 2008; 358:664–665.
- Power C, Gladden JG, Halliday W, et al. AIDS- and non-AIDS-related PML association with distinct p53 polymorphism. Neurology 2000; 54:743–746.
- Gonzalez H, Bolgert F, Camporo P, Leblond V. Progressive multi focal leukoencephalitis (PML) in three patients treated with standard-dose fludarabine (FAMP). Hematol Cell Ther 1999; 41:183–186.
- Garcia-Suárez J, de Miguel D, Krsnik I, Bañas H, Arribas I, Burgaleta C. Changes in the natural history of progressive multifocal leukoencephalopathy in HIV-negative lymphoproliferative disorders: impact of novel therapies. Am J Hematol 2005; 80:271–281.
- Kavanaugh A, Matteson E. Hotline: rituximab and progressive multifocal leukoencephalopathy. American College of Rheumatology Web site. http://www.rheumatology.org/publications/hotline/0107leuko.asp. Updated January 2007. Accessed September 13, 2011.
- Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy in patients with rheumatic diseases: are patients with systemic lupus erythematosus at particular risk? Autoimmun Rev 2008; 8:144–146.
- Calabrese LH, Molloy ES. Progressive multifocal leucoencephalopathy in the rheumatic diseases: assessing the risks of biological immunosuppressive therapies. Ann Rheum Dis 2008; 67 (suppl 3):iii64–iii65.
- Tuccori M, Focosi D, Blandizzi C, et al. Inclusion of rituximab in treatment protocols for non-Hodgkin’s lymphomas and risk for progressive multifocal leukoencephalopathy. Oncologist 2010; 15:1214–1219. Epub 2010 Nov 1.