User login
History and current concepts in the pathogenesis of PML
The neuropathology of progressive multifocal leukoencephalopathy (PML) was first reported in 1958 following examination of brain tissue from two cases of chronic lymphocytic leukemia and one case of Hodgkin lymphoma.1 The classic triad of symptoms of PML—cognitive impairment, visual deficits, and motor dysfunction—had been observed previously but had not been formally described.2
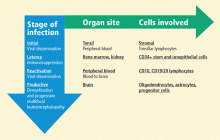
Until PML was discovered in patients with autoimmune diseases treated with biologic therapies that do not directly suppress immunity, PML had been considered a very rare, virus-induced demyelinating disease of the white matter that occurred in immune-compromised patients. The incidence of PML rose sharply in the mid-1980s with the pandemic of human immunodeficiency virus (HIV)-1 infection and continues as an acquired immunodeficiency syndrome–defining illness at a rate of approximately 1% to 3% of HIV-1 seropositive individuals; more recently, it has been seen in approximately 1 in 850 natalizumab-treated individuals who have multiple sclerosis (MS). The incidence of PML in natalizumab-treated MS patients increases with dosing; among those who receive 24 or more doses, the incidence is 1 in 400.
The cause of PML was unknown until 1971, when viral particles were observed by electron microscopy in PML brain lesions and subsequently isolated at the University of Wisconsin, Madison, in cultures of human fetal brain tissue.3 The designation of JC virus (JCV) was derived from the initials of the patient whose brain tissue was used for culture and isolation. Variants in the noncoding region of the genome were then serially identified as Mad 1, Mad 2, and so on, representing the geographic location, Madison, Wisconsin, where the virus was identified.
The JCV, a polyomavirus, is a nonenveloped DNA virus with icosahedral structure containing double-stranded DNA genomes. The circular genome of JCV contains early and late transcription units, the latter of which encodes three virion structural proteins—VPl, VP2, and VP3. Humans generate antibodies directed against the amino terminal end of VP1 and perhaps VP2 and VP3.
JC VIRUS PATHOGENESIS
JCV pathogenesis is studied in cell cultures derived from human fetal brain tissue. In vitro, JCV robustly infects astrocytes, making it important to identify the culture’s cellular phenotypes. A cell line was developed that allows multiplication of JCV and, more recently, human multipotential progenitor cells were isolated and are being grown from the human developing brain at various gestational stages. The lineage pathways of these cells can be differentiated into astrocytes, oligodendrocytes, and neurons. Initiating infection in progenitor cells with JC virions made it possible to determine which cells were susceptible to infection. JCV susceptibility is evident in progenitor-derived astrocytes and glial cells, which reflects the pathologic process in PML brain tissue. Neuronal cells, by contrast, are not susceptible to infection.4
JC VIRUS CHARACTERISTICS: GLOBAL DISTRIBUTION, TRIAD OF SYMPTOMS
Subcortical multifocal white matter lesions are the classic feature of PML on neuroimaging. Seroepidemiology of JCV has revealed ubiquitous distribution, with 50% to 60% of adults aged 20 to 50 years demonstrating antibody to JCV.5 The percentage of the population with antibody increases with age, but may vary among geographic regions. Prevalence is lower among remote populations.
Although the initial site of JCV infection is not well characterized, we know that the primary infection is not in the brain. The JCV has a selective tropism for replication in glial cells in the human brain, but the absence of an animal model for PML has hindered our understanding of the JCV migration to the brain and the initiation and development of central nervous system infection.
Although humans carry JCV-specific antibodies, the clinical significance of these antibodies is unknown. Antibody levels rise during active infection, at times to very high titers, but offer no protection. T-cell–mediated immune responses directed to structural and nonstructural proteins are important in controlling infection.
A high index of suspicion for PML is warranted in individuals who demonstrate the classic triad of symptoms (cognitive impairment, visual deficits, and motor dysfunction) and in whom magnetic resonance imaging shows evidence of demyelinated plaque lesions; however, evidence of the presence of JCV DNA in pathologic tissue is necessary to confirm a diagnosis of PML.
The development of an in situ DNA hybridization assay using a biotinylated probe has facilitated identification of JCV DNA in the infected nuclei of the pathologic tissue. The presence of JCV DNA in cerebrospinal fluid (CSF) samples can be detected using a quantitative polymerase chain reaction assay, targeting the viral genome in the amino terminal end of the viral T protein.6 This T protein coding region was targeted because it does not crossreact, even with other human polyomaviruses, and it is intolerant of mutations. This assay is certified by the Clinical Laboratory Improvement Amendments, licensed by the National Institutes of Health; it is the most sensitive (to levels of 10 copies/mL sample) assay available.
JC VIRUS SUSCEPTIBILITY FACTORS
Despite the high prevalence of JCV infection, PML is rare, suggesting important barriers to its development. Although the receptor for JCV has been identified as alpha 2,6-linked sialic acid, the host range for productive infection is controlled by factors within the cell nucleus that bind to the viral promoter; this process initiates transcription of mRNA for the coordinated synthesis of viral proteins. Only certain cells have the necessary DNA binding proteins in high enough concentrations to allow lytic infection to take place, spreading by cell-to-cell contact. These cells include oligodendrocytes, the primary target for JCV, whose destruction leads to PML; astrocytes; and the CD34+ and CD19+ cells of the immune system. JCV can also be found in urine, at times in very high concentrations. It is present in the uroepithelial cells and multiplies without apparent pathologic consequences. Virus isolated from the urine has not been grown in cell culture systems in the laboratory setting.
Bone marrow CD34+ hematopoietic progenitor cells represent a potential pathway of JCV pathogenesis: in six people with PML, latent JCV DNA was demonstrated in pathologic tissue from lymph, spleen, or bone marrow biopsies taken months to years before the patient developed neurologic disease.7
Upon immunosuppression, reactivation of the virus occurs, with evidence of the virus found in CD10 and CD19/20 lymphocytes in the peripheral blood of some individuals. Blood-to-brain viral dissemination results in infection of oligodendrocytes, astrocytes, and progenitor cells.
Susceptibility is related to nucleotide sequences
Susceptibility to PML is associated with promoter/enhancer nucleotide sequences. The tandem repeat nucleotide structure has been found in the peripheral blood leukocytes and the CSF of patients with PML. Although the arrangement of nucleotide sequences in the viral regulatory region is highly variable among patients with PML, there are no alterations in the sequence within the origin of DNA replication. These highly conserved sequences contain regions for DNA-binding proteins that drive transcription, initiating the life cycle of the virus.
The nuclear transcription factor NF-1 is a cell-specific regulator of JCV promoter/enhancer activity. In humans, the NF-1 family of DNA-binding proteins is encoded by four discrete genes, one of which is NF-1 class X (NF-1X), a critical transcription factor that affects JCV cells. The human brain makes NF-1X in concentrations greater than the concentrations of other NF-1 transcription family members of DNA-binding proteins. NF-1X is located adjacent to and interacts with another family of transcription factors, activator protein-1, which has also been associated with JC viral activity.
Spi-B expression a factor in natalizumab-treated patients
Another transcription factor, Spi-B, binds to sequences present in the JCV promoter/enhancer. Spi-B is a regulator of JCV gene expression in susceptible cells and appears to play an important role in JCV activity. The expression of Spi-B is upregulated in patients with MS who are treated with the monoclonal antibody natalizumab, a population of patients in whom PML has been recently described.11–15
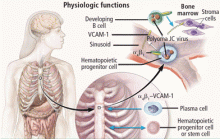
Natalizumab binds to the alpha-4 integrin molecule, preventing hematopoietic stem cells and developing B cells from attaching to a vascular-cell adhesion molecule and forcing them to migrate from the bone marrow (Figure 2).16 An ideal environment is created for JCV when the natalizumab-induced increase in CD34+ cells in the circulation is combined with upregulation of gene cells involved in B-cell maturation. JCV can reside in the bone marrow in a latent state and can use B cells and their DNA-binding proteins to initiate viral multiplication, eventually gaining entry into the brain to cause PML.
In addition to natalizumab, PML has been described in patients treated with efalizumab, another biologic agent that binds alpha-4 integrin molecules on the surface of T and B cells, preventing their entry into the brain, gut, and skin, and forcing migration of bone marrow CD34+ into peripheral circulation for long periods.9,17,18 Rituximab, another monoclonal antibody, binds the CD20 surface molecule on B cells, causing their depletion from the peripheral circulation through complement-mediated cytolysis.7
Risk factors for development of PML
Measurable risk factors for PML include:
- Rising antibody titers
- Evidence of viremia, especially persistent viremia associated with repeat sequences in the regulatory region of the viral genome
- Ineffective T-cell (CD4 and CD8) responses
- Molecular host factors (ie, Spi-B expression in B cells) that support JCV infection in potentially susceptible cells.
The presence of more than one of these risk factors is necessary for development of PML.
VIRAL LATENCY IN B LYMPHOCYTES IN BONE MARROW
A strong link between JCV infection in cells of the immune system and those of the nervous system points to the importance of the tissue origin of JCV latency. Bone marrow harbors CD34+ cells that migrate into the peripheral circulation and undergo differentiation to pre-B and mature B cells, augmenting JCV growth. The emergence of PML in patients treated with natalizumab, rituximab, efalizumab, and other immune-altering drugs underscores this observation.
As noted, the incidence of PML in natalizumab-treated patients with MS and Crohn disease rises as the number of doses increases. Analysis of blood samples collected from patients treated with natalizumab at baseline and again during treatment at months 1 to 12 and beyond 24 months demonstrates that the frequency of CD34+ cells in the peripheral circulation increases with the duration of therapy, adding credence to the theory that CD34+ cells act as a reservoir for latent virus. A higher frequency of CD34+ cells is associated with viremia.
The role of Spi-B in JC virus latency
Understanding the role of Spi-B during JCV latency and reactivation is increasingly important as the number of patients treated with immunomodulatory agents that can develop PML continues to rise. Spi-B is highly represented in the B cell and CD34+ cell fractions. Spi-B expression in B cells correlates with reactivation of JCV in immune cells in natalizumab-treated patients. In a sample of four patients with MS treated with natalizumab who developed PML, T-cell responses have been ineffective (absent or aberrant). Two patients had no detectable T-cell response to JCV; the other two demonstrated response, but their CD4 T-cell responses were dominated by interleukin-10–producing cells.
Longitudinal examination of CSF samples from 13 MS patients who were treated with natalizumab and subsequently developed PML revealed persistence of viral load even though all patients experienced immune reconstitution inflammatory syndrome and most had high levels of anti-JCV antibodies.19
SUMMARY
Despite the prevalence of JCV in the population, the development of PML is rare. Levels of JCV antibody rise during the course of active JCV infection, but they do not protect against infection. T-cell responses directed to structural and nonstructural proteins play a role in controlling infection. Latency of JCV is associated with specific cells of the immune system, and its reactivation can follow alteration of normal immune cell function—either immunosuppression or immunomodulation. Risk factors for the development of PML include rising antibody titers and ineffective T-cell (CD4 and CD8) responses.
DISCUSSION
Dr. Berger: Does natalizumab upregulate Spi-B in glial cells?
Dr. Major: We never tested this directly. From human brain cultures, we know that Spi-B is made in glial cells, not in neurons. We are considering the idea that wherever JCV binds, it takes advantage of certain types of DNA-binding proteins in the molecular regulation. If the binding takes place in an immune system cell, for example, Spi-B plays an important role.
Dr. Berger: Koralnik et al demonstrated JCV excretion in urine in MS patients after 12 months of treatment with natalizumab, and at 18 months, viremia in 60% of the patients.20 Yet, repeated studies of patients taking natalizumab have failed to demonstrate viremia or conversion of virus in the archetype. How do these findings correlate with your thoughts on the action of natalizumab in the pathogenesis of PML?
Dr. Major: We certainly know that natalizumab forces migration of hematopoietic stem cells and pre-B cells out of the marrow, but our findings have differed somewhat from those of Koralnik’s laboratory. For example, in the several hundred nucleotide sequences we have looked at in PML brain tissue, we have found the Mad 1 genotype once. We consider Mad 1 to be a potential laboratory contamination, so if we find Mad 1 we resequence the sample. We never clone because cloning can introduce alterations; we sequence directly from the clinical tissue. We can identify Mad 1 because our assay is very sensitive. In normal individuals, CD34+ cells compose approximately 0.01% of the peripheral circulation; in individuals treated with natalizumab, however, their composition is 0.1% to 0.3%. So if there is a potential for latent infection, we have an opportunity to find it in those cells. Its presence does not necessarily mean that the individual is going to develop PML, however; there are other controlling factors.
Dr. Rudick: Have you found the virus in B cells in healthy people?
Dr. Major: Yes we have, in about one-third. It is higher than what we would expect to see in the normal population.
Dr. Rudick: How can that finding be turned into something that’s clinically useful?
Dr. Major: If you’re trying to identify persons who are more susceptible to PML given underlying risk factors—treatment with natalizumab or rituximab, presence of HIV infection, or some other immune-altering condition—looking at one parameter isn’t going to help. Based on the available data, rising antibody titers signals an active infection, and viremia of any kind means probable latent infection. Because this is a small event in very few cells, you will not have the numbers of cells needed to identify susceptibility in a normal population. For now, we monitor patients at risk and, if we find viremia, we assess the cell population to determine whether a molecular factor like Spi-B is upregulated. We hope to develop an assay in which we can obtain one test tube of blood and report T-cell responses, molecular factors, antibody titer, and presence or absence of viremia. Such an assay would provide the data necessary to make a clinical decision.
- Astrom KE, Mancall EL, Richardson EP. Progressive multifocal leukoencephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin’s disease. Brain 1958; 81:93–111.
- Hallervorden J. Eigennartige und nicht rubriziebare Prozesse. In:Bumke O, ed. Handbuch der Geiteskranheiten. Vol. 2. Die Anatomie der Psychosen. Berlin: Springer; 1930:1063–1107.
- Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH. Cultivation of a papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 1971; 1:1257–1260.
- Major EO, Amemiya K, Tornatore CS, Houff SA, Berger JR. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev 1992; 5:49–73.
- Walker D, Padgett B. The epidemiology of human polyomaviruses. In:Sever J, Madden D, eds. Polyomaviruses and Human Neurological Disease. New York, NY: Alan R. Liss, Inc.; 1983:99–106.
- Ryschkewitsch C, Jensen P, Hou J, Fahle G, Fischer S, Major EO. Comparison of PCR-southern hybridization and quantitative realtime PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J Virol Methods 2004; 121:217–221.
- Monaco MC, Jensen PN, Hou J, Durham LC, Major EO. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol 1998; 72:9918–9923.
- Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med 2010; 61:35–47.
- Imperiale M, Major E. Polyomavirus. In:Knipe D, Howley P, eds. Field Virology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:2263–2298.
- Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 2005; 353:375–381.
- Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med 2005; 353:362–368.
- Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med 2005; 353:369–374.
- Bozic C, Belcher G, Kooijmans-Coutinho M, et al. Natalizumab utilization and safety in patients with relapsing multiple sclerosis: updated results from TOUCH™ and TYGRIS. Paper presented at: 60th Annual Meeting of the American Academy of Neurology; April 15, 2008; Chicago, IL.
- Kappos L, Bates D, Hartung HP, et al. Natalizumab treatment for multiple sclerosis: recommendations for patient selection and monitoring. Lancet Neurol 2007; 6:431–441.
- Major EO. Reemergence of PML in natalizumab-treated patients—new cases, same concerns. N Engl J Med 2009; 361:1041–1043.
- Vugmeyster Y, Kikuchi T, Lowes MA, et al. Efalizumab (anti-CD11a)-induced increase in peripheral blood leukocytes in psoriasis patients is preferentially mediated by altered trafficking of memory CD8+ T cells into lesional skin. Clin Immunol 2004; 113:38–46.
- Guttman-Yassky E, Vugmeyster Y, Lowes MA, et al. Blockade of CD11a by efalizumab in psoriasis patients induces a unique state of T-cell hyporesponsiveness. J Invest Dermatol 2008; 128:1182–1191.
- Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 2009; 113:4834–4840.
- Ryschkewitsch CF, Jensen PN, Monaco MC, Major EO. JC virus persistence following progressive multifocal leukoencephalopathy in multiple sclerosis patients treated with natalizumab. Ann Neurol 2010; 68:384–391.
- Koralnik IJ, Du Pasquier RA, Kuroda MJ, et al. Association of prolonged survival in HLA-A2+ progressive multifocal leuko encephalopathy patients with a CTL response specific for a commonly recognized JC virus epitope. J Immunol 2002; 168:499–504.
The neuropathology of progressive multifocal leukoencephalopathy (PML) was first reported in 1958 following examination of brain tissue from two cases of chronic lymphocytic leukemia and one case of Hodgkin lymphoma.1 The classic triad of symptoms of PML—cognitive impairment, visual deficits, and motor dysfunction—had been observed previously but had not been formally described.2
Until PML was discovered in patients with autoimmune diseases treated with biologic therapies that do not directly suppress immunity, PML had been considered a very rare, virus-induced demyelinating disease of the white matter that occurred in immune-compromised patients. The incidence of PML rose sharply in the mid-1980s with the pandemic of human immunodeficiency virus (HIV)-1 infection and continues as an acquired immunodeficiency syndrome–defining illness at a rate of approximately 1% to 3% of HIV-1 seropositive individuals; more recently, it has been seen in approximately 1 in 850 natalizumab-treated individuals who have multiple sclerosis (MS). The incidence of PML in natalizumab-treated MS patients increases with dosing; among those who receive 24 or more doses, the incidence is 1 in 400.
The cause of PML was unknown until 1971, when viral particles were observed by electron microscopy in PML brain lesions and subsequently isolated at the University of Wisconsin, Madison, in cultures of human fetal brain tissue.3 The designation of JC virus (JCV) was derived from the initials of the patient whose brain tissue was used for culture and isolation. Variants in the noncoding region of the genome were then serially identified as Mad 1, Mad 2, and so on, representing the geographic location, Madison, Wisconsin, where the virus was identified.
The JCV, a polyomavirus, is a nonenveloped DNA virus with icosahedral structure containing double-stranded DNA genomes. The circular genome of JCV contains early and late transcription units, the latter of which encodes three virion structural proteins—VPl, VP2, and VP3. Humans generate antibodies directed against the amino terminal end of VP1 and perhaps VP2 and VP3.
JC VIRUS PATHOGENESIS
JCV pathogenesis is studied in cell cultures derived from human fetal brain tissue. In vitro, JCV robustly infects astrocytes, making it important to identify the culture’s cellular phenotypes. A cell line was developed that allows multiplication of JCV and, more recently, human multipotential progenitor cells were isolated and are being grown from the human developing brain at various gestational stages. The lineage pathways of these cells can be differentiated into astrocytes, oligodendrocytes, and neurons. Initiating infection in progenitor cells with JC virions made it possible to determine which cells were susceptible to infection. JCV susceptibility is evident in progenitor-derived astrocytes and glial cells, which reflects the pathologic process in PML brain tissue. Neuronal cells, by contrast, are not susceptible to infection.4
JC VIRUS CHARACTERISTICS: GLOBAL DISTRIBUTION, TRIAD OF SYMPTOMS
Subcortical multifocal white matter lesions are the classic feature of PML on neuroimaging. Seroepidemiology of JCV has revealed ubiquitous distribution, with 50% to 60% of adults aged 20 to 50 years demonstrating antibody to JCV.5 The percentage of the population with antibody increases with age, but may vary among geographic regions. Prevalence is lower among remote populations.
Although the initial site of JCV infection is not well characterized, we know that the primary infection is not in the brain. The JCV has a selective tropism for replication in glial cells in the human brain, but the absence of an animal model for PML has hindered our understanding of the JCV migration to the brain and the initiation and development of central nervous system infection.
Although humans carry JCV-specific antibodies, the clinical significance of these antibodies is unknown. Antibody levels rise during active infection, at times to very high titers, but offer no protection. T-cell–mediated immune responses directed to structural and nonstructural proteins are important in controlling infection.
A high index of suspicion for PML is warranted in individuals who demonstrate the classic triad of symptoms (cognitive impairment, visual deficits, and motor dysfunction) and in whom magnetic resonance imaging shows evidence of demyelinated plaque lesions; however, evidence of the presence of JCV DNA in pathologic tissue is necessary to confirm a diagnosis of PML.
The development of an in situ DNA hybridization assay using a biotinylated probe has facilitated identification of JCV DNA in the infected nuclei of the pathologic tissue. The presence of JCV DNA in cerebrospinal fluid (CSF) samples can be detected using a quantitative polymerase chain reaction assay, targeting the viral genome in the amino terminal end of the viral T protein.6 This T protein coding region was targeted because it does not crossreact, even with other human polyomaviruses, and it is intolerant of mutations. This assay is certified by the Clinical Laboratory Improvement Amendments, licensed by the National Institutes of Health; it is the most sensitive (to levels of 10 copies/mL sample) assay available.
JC VIRUS SUSCEPTIBILITY FACTORS
Despite the high prevalence of JCV infection, PML is rare, suggesting important barriers to its development. Although the receptor for JCV has been identified as alpha 2,6-linked sialic acid, the host range for productive infection is controlled by factors within the cell nucleus that bind to the viral promoter; this process initiates transcription of mRNA for the coordinated synthesis of viral proteins. Only certain cells have the necessary DNA binding proteins in high enough concentrations to allow lytic infection to take place, spreading by cell-to-cell contact. These cells include oligodendrocytes, the primary target for JCV, whose destruction leads to PML; astrocytes; and the CD34+ and CD19+ cells of the immune system. JCV can also be found in urine, at times in very high concentrations. It is present in the uroepithelial cells and multiplies without apparent pathologic consequences. Virus isolated from the urine has not been grown in cell culture systems in the laboratory setting.
Bone marrow CD34+ hematopoietic progenitor cells represent a potential pathway of JCV pathogenesis: in six people with PML, latent JCV DNA was demonstrated in pathologic tissue from lymph, spleen, or bone marrow biopsies taken months to years before the patient developed neurologic disease.7
Upon immunosuppression, reactivation of the virus occurs, with evidence of the virus found in CD10 and CD19/20 lymphocytes in the peripheral blood of some individuals. Blood-to-brain viral dissemination results in infection of oligodendrocytes, astrocytes, and progenitor cells.
Susceptibility is related to nucleotide sequences
Susceptibility to PML is associated with promoter/enhancer nucleotide sequences. The tandem repeat nucleotide structure has been found in the peripheral blood leukocytes and the CSF of patients with PML. Although the arrangement of nucleotide sequences in the viral regulatory region is highly variable among patients with PML, there are no alterations in the sequence within the origin of DNA replication. These highly conserved sequences contain regions for DNA-binding proteins that drive transcription, initiating the life cycle of the virus.
The nuclear transcription factor NF-1 is a cell-specific regulator of JCV promoter/enhancer activity. In humans, the NF-1 family of DNA-binding proteins is encoded by four discrete genes, one of which is NF-1 class X (NF-1X), a critical transcription factor that affects JCV cells. The human brain makes NF-1X in concentrations greater than the concentrations of other NF-1 transcription family members of DNA-binding proteins. NF-1X is located adjacent to and interacts with another family of transcription factors, activator protein-1, which has also been associated with JC viral activity.
Spi-B expression a factor in natalizumab-treated patients
Another transcription factor, Spi-B, binds to sequences present in the JCV promoter/enhancer. Spi-B is a regulator of JCV gene expression in susceptible cells and appears to play an important role in JCV activity. The expression of Spi-B is upregulated in patients with MS who are treated with the monoclonal antibody natalizumab, a population of patients in whom PML has been recently described.11–15
Natalizumab binds to the alpha-4 integrin molecule, preventing hematopoietic stem cells and developing B cells from attaching to a vascular-cell adhesion molecule and forcing them to migrate from the bone marrow (Figure 2).16 An ideal environment is created for JCV when the natalizumab-induced increase in CD34+ cells in the circulation is combined with upregulation of gene cells involved in B-cell maturation. JCV can reside in the bone marrow in a latent state and can use B cells and their DNA-binding proteins to initiate viral multiplication, eventually gaining entry into the brain to cause PML.
In addition to natalizumab, PML has been described in patients treated with efalizumab, another biologic agent that binds alpha-4 integrin molecules on the surface of T and B cells, preventing their entry into the brain, gut, and skin, and forcing migration of bone marrow CD34+ into peripheral circulation for long periods.9,17,18 Rituximab, another monoclonal antibody, binds the CD20 surface molecule on B cells, causing their depletion from the peripheral circulation through complement-mediated cytolysis.7
Risk factors for development of PML
Measurable risk factors for PML include:
- Rising antibody titers
- Evidence of viremia, especially persistent viremia associated with repeat sequences in the regulatory region of the viral genome
- Ineffective T-cell (CD4 and CD8) responses
- Molecular host factors (ie, Spi-B expression in B cells) that support JCV infection in potentially susceptible cells.
The presence of more than one of these risk factors is necessary for development of PML.
VIRAL LATENCY IN B LYMPHOCYTES IN BONE MARROW
A strong link between JCV infection in cells of the immune system and those of the nervous system points to the importance of the tissue origin of JCV latency. Bone marrow harbors CD34+ cells that migrate into the peripheral circulation and undergo differentiation to pre-B and mature B cells, augmenting JCV growth. The emergence of PML in patients treated with natalizumab, rituximab, efalizumab, and other immune-altering drugs underscores this observation.
As noted, the incidence of PML in natalizumab-treated patients with MS and Crohn disease rises as the number of doses increases. Analysis of blood samples collected from patients treated with natalizumab at baseline and again during treatment at months 1 to 12 and beyond 24 months demonstrates that the frequency of CD34+ cells in the peripheral circulation increases with the duration of therapy, adding credence to the theory that CD34+ cells act as a reservoir for latent virus. A higher frequency of CD34+ cells is associated with viremia.
The role of Spi-B in JC virus latency
Understanding the role of Spi-B during JCV latency and reactivation is increasingly important as the number of patients treated with immunomodulatory agents that can develop PML continues to rise. Spi-B is highly represented in the B cell and CD34+ cell fractions. Spi-B expression in B cells correlates with reactivation of JCV in immune cells in natalizumab-treated patients. In a sample of four patients with MS treated with natalizumab who developed PML, T-cell responses have been ineffective (absent or aberrant). Two patients had no detectable T-cell response to JCV; the other two demonstrated response, but their CD4 T-cell responses were dominated by interleukin-10–producing cells.
Longitudinal examination of CSF samples from 13 MS patients who were treated with natalizumab and subsequently developed PML revealed persistence of viral load even though all patients experienced immune reconstitution inflammatory syndrome and most had high levels of anti-JCV antibodies.19
SUMMARY
Despite the prevalence of JCV in the population, the development of PML is rare. Levels of JCV antibody rise during the course of active JCV infection, but they do not protect against infection. T-cell responses directed to structural and nonstructural proteins play a role in controlling infection. Latency of JCV is associated with specific cells of the immune system, and its reactivation can follow alteration of normal immune cell function—either immunosuppression or immunomodulation. Risk factors for the development of PML include rising antibody titers and ineffective T-cell (CD4 and CD8) responses.
DISCUSSION
Dr. Berger: Does natalizumab upregulate Spi-B in glial cells?
Dr. Major: We never tested this directly. From human brain cultures, we know that Spi-B is made in glial cells, not in neurons. We are considering the idea that wherever JCV binds, it takes advantage of certain types of DNA-binding proteins in the molecular regulation. If the binding takes place in an immune system cell, for example, Spi-B plays an important role.
Dr. Berger: Koralnik et al demonstrated JCV excretion in urine in MS patients after 12 months of treatment with natalizumab, and at 18 months, viremia in 60% of the patients.20 Yet, repeated studies of patients taking natalizumab have failed to demonstrate viremia or conversion of virus in the archetype. How do these findings correlate with your thoughts on the action of natalizumab in the pathogenesis of PML?
Dr. Major: We certainly know that natalizumab forces migration of hematopoietic stem cells and pre-B cells out of the marrow, but our findings have differed somewhat from those of Koralnik’s laboratory. For example, in the several hundred nucleotide sequences we have looked at in PML brain tissue, we have found the Mad 1 genotype once. We consider Mad 1 to be a potential laboratory contamination, so if we find Mad 1 we resequence the sample. We never clone because cloning can introduce alterations; we sequence directly from the clinical tissue. We can identify Mad 1 because our assay is very sensitive. In normal individuals, CD34+ cells compose approximately 0.01% of the peripheral circulation; in individuals treated with natalizumab, however, their composition is 0.1% to 0.3%. So if there is a potential for latent infection, we have an opportunity to find it in those cells. Its presence does not necessarily mean that the individual is going to develop PML, however; there are other controlling factors.
Dr. Rudick: Have you found the virus in B cells in healthy people?
Dr. Major: Yes we have, in about one-third. It is higher than what we would expect to see in the normal population.
Dr. Rudick: How can that finding be turned into something that’s clinically useful?
Dr. Major: If you’re trying to identify persons who are more susceptible to PML given underlying risk factors—treatment with natalizumab or rituximab, presence of HIV infection, or some other immune-altering condition—looking at one parameter isn’t going to help. Based on the available data, rising antibody titers signals an active infection, and viremia of any kind means probable latent infection. Because this is a small event in very few cells, you will not have the numbers of cells needed to identify susceptibility in a normal population. For now, we monitor patients at risk and, if we find viremia, we assess the cell population to determine whether a molecular factor like Spi-B is upregulated. We hope to develop an assay in which we can obtain one test tube of blood and report T-cell responses, molecular factors, antibody titer, and presence or absence of viremia. Such an assay would provide the data necessary to make a clinical decision.
The neuropathology of progressive multifocal leukoencephalopathy (PML) was first reported in 1958 following examination of brain tissue from two cases of chronic lymphocytic leukemia and one case of Hodgkin lymphoma.1 The classic triad of symptoms of PML—cognitive impairment, visual deficits, and motor dysfunction—had been observed previously but had not been formally described.2
Until PML was discovered in patients with autoimmune diseases treated with biologic therapies that do not directly suppress immunity, PML had been considered a very rare, virus-induced demyelinating disease of the white matter that occurred in immune-compromised patients. The incidence of PML rose sharply in the mid-1980s with the pandemic of human immunodeficiency virus (HIV)-1 infection and continues as an acquired immunodeficiency syndrome–defining illness at a rate of approximately 1% to 3% of HIV-1 seropositive individuals; more recently, it has been seen in approximately 1 in 850 natalizumab-treated individuals who have multiple sclerosis (MS). The incidence of PML in natalizumab-treated MS patients increases with dosing; among those who receive 24 or more doses, the incidence is 1 in 400.
The cause of PML was unknown until 1971, when viral particles were observed by electron microscopy in PML brain lesions and subsequently isolated at the University of Wisconsin, Madison, in cultures of human fetal brain tissue.3 The designation of JC virus (JCV) was derived from the initials of the patient whose brain tissue was used for culture and isolation. Variants in the noncoding region of the genome were then serially identified as Mad 1, Mad 2, and so on, representing the geographic location, Madison, Wisconsin, where the virus was identified.
The JCV, a polyomavirus, is a nonenveloped DNA virus with icosahedral structure containing double-stranded DNA genomes. The circular genome of JCV contains early and late transcription units, the latter of which encodes three virion structural proteins—VPl, VP2, and VP3. Humans generate antibodies directed against the amino terminal end of VP1 and perhaps VP2 and VP3.
JC VIRUS PATHOGENESIS
JCV pathogenesis is studied in cell cultures derived from human fetal brain tissue. In vitro, JCV robustly infects astrocytes, making it important to identify the culture’s cellular phenotypes. A cell line was developed that allows multiplication of JCV and, more recently, human multipotential progenitor cells were isolated and are being grown from the human developing brain at various gestational stages. The lineage pathways of these cells can be differentiated into astrocytes, oligodendrocytes, and neurons. Initiating infection in progenitor cells with JC virions made it possible to determine which cells were susceptible to infection. JCV susceptibility is evident in progenitor-derived astrocytes and glial cells, which reflects the pathologic process in PML brain tissue. Neuronal cells, by contrast, are not susceptible to infection.4
JC VIRUS CHARACTERISTICS: GLOBAL DISTRIBUTION, TRIAD OF SYMPTOMS
Subcortical multifocal white matter lesions are the classic feature of PML on neuroimaging. Seroepidemiology of JCV has revealed ubiquitous distribution, with 50% to 60% of adults aged 20 to 50 years demonstrating antibody to JCV.5 The percentage of the population with antibody increases with age, but may vary among geographic regions. Prevalence is lower among remote populations.
Although the initial site of JCV infection is not well characterized, we know that the primary infection is not in the brain. The JCV has a selective tropism for replication in glial cells in the human brain, but the absence of an animal model for PML has hindered our understanding of the JCV migration to the brain and the initiation and development of central nervous system infection.
Although humans carry JCV-specific antibodies, the clinical significance of these antibodies is unknown. Antibody levels rise during active infection, at times to very high titers, but offer no protection. T-cell–mediated immune responses directed to structural and nonstructural proteins are important in controlling infection.
A high index of suspicion for PML is warranted in individuals who demonstrate the classic triad of symptoms (cognitive impairment, visual deficits, and motor dysfunction) and in whom magnetic resonance imaging shows evidence of demyelinated plaque lesions; however, evidence of the presence of JCV DNA in pathologic tissue is necessary to confirm a diagnosis of PML.
The development of an in situ DNA hybridization assay using a biotinylated probe has facilitated identification of JCV DNA in the infected nuclei of the pathologic tissue. The presence of JCV DNA in cerebrospinal fluid (CSF) samples can be detected using a quantitative polymerase chain reaction assay, targeting the viral genome in the amino terminal end of the viral T protein.6 This T protein coding region was targeted because it does not crossreact, even with other human polyomaviruses, and it is intolerant of mutations. This assay is certified by the Clinical Laboratory Improvement Amendments, licensed by the National Institutes of Health; it is the most sensitive (to levels of 10 copies/mL sample) assay available.
JC VIRUS SUSCEPTIBILITY FACTORS
Despite the high prevalence of JCV infection, PML is rare, suggesting important barriers to its development. Although the receptor for JCV has been identified as alpha 2,6-linked sialic acid, the host range for productive infection is controlled by factors within the cell nucleus that bind to the viral promoter; this process initiates transcription of mRNA for the coordinated synthesis of viral proteins. Only certain cells have the necessary DNA binding proteins in high enough concentrations to allow lytic infection to take place, spreading by cell-to-cell contact. These cells include oligodendrocytes, the primary target for JCV, whose destruction leads to PML; astrocytes; and the CD34+ and CD19+ cells of the immune system. JCV can also be found in urine, at times in very high concentrations. It is present in the uroepithelial cells and multiplies without apparent pathologic consequences. Virus isolated from the urine has not been grown in cell culture systems in the laboratory setting.
Bone marrow CD34+ hematopoietic progenitor cells represent a potential pathway of JCV pathogenesis: in six people with PML, latent JCV DNA was demonstrated in pathologic tissue from lymph, spleen, or bone marrow biopsies taken months to years before the patient developed neurologic disease.7
Upon immunosuppression, reactivation of the virus occurs, with evidence of the virus found in CD10 and CD19/20 lymphocytes in the peripheral blood of some individuals. Blood-to-brain viral dissemination results in infection of oligodendrocytes, astrocytes, and progenitor cells.
Susceptibility is related to nucleotide sequences
Susceptibility to PML is associated with promoter/enhancer nucleotide sequences. The tandem repeat nucleotide structure has been found in the peripheral blood leukocytes and the CSF of patients with PML. Although the arrangement of nucleotide sequences in the viral regulatory region is highly variable among patients with PML, there are no alterations in the sequence within the origin of DNA replication. These highly conserved sequences contain regions for DNA-binding proteins that drive transcription, initiating the life cycle of the virus.
The nuclear transcription factor NF-1 is a cell-specific regulator of JCV promoter/enhancer activity. In humans, the NF-1 family of DNA-binding proteins is encoded by four discrete genes, one of which is NF-1 class X (NF-1X), a critical transcription factor that affects JCV cells. The human brain makes NF-1X in concentrations greater than the concentrations of other NF-1 transcription family members of DNA-binding proteins. NF-1X is located adjacent to and interacts with another family of transcription factors, activator protein-1, which has also been associated with JC viral activity.
Spi-B expression a factor in natalizumab-treated patients
Another transcription factor, Spi-B, binds to sequences present in the JCV promoter/enhancer. Spi-B is a regulator of JCV gene expression in susceptible cells and appears to play an important role in JCV activity. The expression of Spi-B is upregulated in patients with MS who are treated with the monoclonal antibody natalizumab, a population of patients in whom PML has been recently described.11–15
Natalizumab binds to the alpha-4 integrin molecule, preventing hematopoietic stem cells and developing B cells from attaching to a vascular-cell adhesion molecule and forcing them to migrate from the bone marrow (Figure 2).16 An ideal environment is created for JCV when the natalizumab-induced increase in CD34+ cells in the circulation is combined with upregulation of gene cells involved in B-cell maturation. JCV can reside in the bone marrow in a latent state and can use B cells and their DNA-binding proteins to initiate viral multiplication, eventually gaining entry into the brain to cause PML.
In addition to natalizumab, PML has been described in patients treated with efalizumab, another biologic agent that binds alpha-4 integrin molecules on the surface of T and B cells, preventing their entry into the brain, gut, and skin, and forcing migration of bone marrow CD34+ into peripheral circulation for long periods.9,17,18 Rituximab, another monoclonal antibody, binds the CD20 surface molecule on B cells, causing their depletion from the peripheral circulation through complement-mediated cytolysis.7
Risk factors for development of PML
Measurable risk factors for PML include:
- Rising antibody titers
- Evidence of viremia, especially persistent viremia associated with repeat sequences in the regulatory region of the viral genome
- Ineffective T-cell (CD4 and CD8) responses
- Molecular host factors (ie, Spi-B expression in B cells) that support JCV infection in potentially susceptible cells.
The presence of more than one of these risk factors is necessary for development of PML.
VIRAL LATENCY IN B LYMPHOCYTES IN BONE MARROW
A strong link between JCV infection in cells of the immune system and those of the nervous system points to the importance of the tissue origin of JCV latency. Bone marrow harbors CD34+ cells that migrate into the peripheral circulation and undergo differentiation to pre-B and mature B cells, augmenting JCV growth. The emergence of PML in patients treated with natalizumab, rituximab, efalizumab, and other immune-altering drugs underscores this observation.
As noted, the incidence of PML in natalizumab-treated patients with MS and Crohn disease rises as the number of doses increases. Analysis of blood samples collected from patients treated with natalizumab at baseline and again during treatment at months 1 to 12 and beyond 24 months demonstrates that the frequency of CD34+ cells in the peripheral circulation increases with the duration of therapy, adding credence to the theory that CD34+ cells act as a reservoir for latent virus. A higher frequency of CD34+ cells is associated with viremia.
The role of Spi-B in JC virus latency
Understanding the role of Spi-B during JCV latency and reactivation is increasingly important as the number of patients treated with immunomodulatory agents that can develop PML continues to rise. Spi-B is highly represented in the B cell and CD34+ cell fractions. Spi-B expression in B cells correlates with reactivation of JCV in immune cells in natalizumab-treated patients. In a sample of four patients with MS treated with natalizumab who developed PML, T-cell responses have been ineffective (absent or aberrant). Two patients had no detectable T-cell response to JCV; the other two demonstrated response, but their CD4 T-cell responses were dominated by interleukin-10–producing cells.
Longitudinal examination of CSF samples from 13 MS patients who were treated with natalizumab and subsequently developed PML revealed persistence of viral load even though all patients experienced immune reconstitution inflammatory syndrome and most had high levels of anti-JCV antibodies.19
SUMMARY
Despite the prevalence of JCV in the population, the development of PML is rare. Levels of JCV antibody rise during the course of active JCV infection, but they do not protect against infection. T-cell responses directed to structural and nonstructural proteins play a role in controlling infection. Latency of JCV is associated with specific cells of the immune system, and its reactivation can follow alteration of normal immune cell function—either immunosuppression or immunomodulation. Risk factors for the development of PML include rising antibody titers and ineffective T-cell (CD4 and CD8) responses.
DISCUSSION
Dr. Berger: Does natalizumab upregulate Spi-B in glial cells?
Dr. Major: We never tested this directly. From human brain cultures, we know that Spi-B is made in glial cells, not in neurons. We are considering the idea that wherever JCV binds, it takes advantage of certain types of DNA-binding proteins in the molecular regulation. If the binding takes place in an immune system cell, for example, Spi-B plays an important role.
Dr. Berger: Koralnik et al demonstrated JCV excretion in urine in MS patients after 12 months of treatment with natalizumab, and at 18 months, viremia in 60% of the patients.20 Yet, repeated studies of patients taking natalizumab have failed to demonstrate viremia or conversion of virus in the archetype. How do these findings correlate with your thoughts on the action of natalizumab in the pathogenesis of PML?
Dr. Major: We certainly know that natalizumab forces migration of hematopoietic stem cells and pre-B cells out of the marrow, but our findings have differed somewhat from those of Koralnik’s laboratory. For example, in the several hundred nucleotide sequences we have looked at in PML brain tissue, we have found the Mad 1 genotype once. We consider Mad 1 to be a potential laboratory contamination, so if we find Mad 1 we resequence the sample. We never clone because cloning can introduce alterations; we sequence directly from the clinical tissue. We can identify Mad 1 because our assay is very sensitive. In normal individuals, CD34+ cells compose approximately 0.01% of the peripheral circulation; in individuals treated with natalizumab, however, their composition is 0.1% to 0.3%. So if there is a potential for latent infection, we have an opportunity to find it in those cells. Its presence does not necessarily mean that the individual is going to develop PML, however; there are other controlling factors.
Dr. Rudick: Have you found the virus in B cells in healthy people?
Dr. Major: Yes we have, in about one-third. It is higher than what we would expect to see in the normal population.
Dr. Rudick: How can that finding be turned into something that’s clinically useful?
Dr. Major: If you’re trying to identify persons who are more susceptible to PML given underlying risk factors—treatment with natalizumab or rituximab, presence of HIV infection, or some other immune-altering condition—looking at one parameter isn’t going to help. Based on the available data, rising antibody titers signals an active infection, and viremia of any kind means probable latent infection. Because this is a small event in very few cells, you will not have the numbers of cells needed to identify susceptibility in a normal population. For now, we monitor patients at risk and, if we find viremia, we assess the cell population to determine whether a molecular factor like Spi-B is upregulated. We hope to develop an assay in which we can obtain one test tube of blood and report T-cell responses, molecular factors, antibody titer, and presence or absence of viremia. Such an assay would provide the data necessary to make a clinical decision.
- Astrom KE, Mancall EL, Richardson EP. Progressive multifocal leukoencephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin’s disease. Brain 1958; 81:93–111.
- Hallervorden J. Eigennartige und nicht rubriziebare Prozesse. In:Bumke O, ed. Handbuch der Geiteskranheiten. Vol. 2. Die Anatomie der Psychosen. Berlin: Springer; 1930:1063–1107.
- Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH. Cultivation of a papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 1971; 1:1257–1260.
- Major EO, Amemiya K, Tornatore CS, Houff SA, Berger JR. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev 1992; 5:49–73.
- Walker D, Padgett B. The epidemiology of human polyomaviruses. In:Sever J, Madden D, eds. Polyomaviruses and Human Neurological Disease. New York, NY: Alan R. Liss, Inc.; 1983:99–106.
- Ryschkewitsch C, Jensen P, Hou J, Fahle G, Fischer S, Major EO. Comparison of PCR-southern hybridization and quantitative realtime PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J Virol Methods 2004; 121:217–221.
- Monaco MC, Jensen PN, Hou J, Durham LC, Major EO. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol 1998; 72:9918–9923.
- Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med 2010; 61:35–47.
- Imperiale M, Major E. Polyomavirus. In:Knipe D, Howley P, eds. Field Virology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:2263–2298.
- Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 2005; 353:375–381.
- Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med 2005; 353:362–368.
- Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med 2005; 353:369–374.
- Bozic C, Belcher G, Kooijmans-Coutinho M, et al. Natalizumab utilization and safety in patients with relapsing multiple sclerosis: updated results from TOUCH™ and TYGRIS. Paper presented at: 60th Annual Meeting of the American Academy of Neurology; April 15, 2008; Chicago, IL.
- Kappos L, Bates D, Hartung HP, et al. Natalizumab treatment for multiple sclerosis: recommendations for patient selection and monitoring. Lancet Neurol 2007; 6:431–441.
- Major EO. Reemergence of PML in natalizumab-treated patients—new cases, same concerns. N Engl J Med 2009; 361:1041–1043.
- Vugmeyster Y, Kikuchi T, Lowes MA, et al. Efalizumab (anti-CD11a)-induced increase in peripheral blood leukocytes in psoriasis patients is preferentially mediated by altered trafficking of memory CD8+ T cells into lesional skin. Clin Immunol 2004; 113:38–46.
- Guttman-Yassky E, Vugmeyster Y, Lowes MA, et al. Blockade of CD11a by efalizumab in psoriasis patients induces a unique state of T-cell hyporesponsiveness. J Invest Dermatol 2008; 128:1182–1191.
- Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 2009; 113:4834–4840.
- Ryschkewitsch CF, Jensen PN, Monaco MC, Major EO. JC virus persistence following progressive multifocal leukoencephalopathy in multiple sclerosis patients treated with natalizumab. Ann Neurol 2010; 68:384–391.
- Koralnik IJ, Du Pasquier RA, Kuroda MJ, et al. Association of prolonged survival in HLA-A2+ progressive multifocal leuko encephalopathy patients with a CTL response specific for a commonly recognized JC virus epitope. J Immunol 2002; 168:499–504.
- Astrom KE, Mancall EL, Richardson EP. Progressive multifocal leukoencephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin’s disease. Brain 1958; 81:93–111.
- Hallervorden J. Eigennartige und nicht rubriziebare Prozesse. In:Bumke O, ed. Handbuch der Geiteskranheiten. Vol. 2. Die Anatomie der Psychosen. Berlin: Springer; 1930:1063–1107.
- Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH. Cultivation of a papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 1971; 1:1257–1260.
- Major EO, Amemiya K, Tornatore CS, Houff SA, Berger JR. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev 1992; 5:49–73.
- Walker D, Padgett B. The epidemiology of human polyomaviruses. In:Sever J, Madden D, eds. Polyomaviruses and Human Neurological Disease. New York, NY: Alan R. Liss, Inc.; 1983:99–106.
- Ryschkewitsch C, Jensen P, Hou J, Fahle G, Fischer S, Major EO. Comparison of PCR-southern hybridization and quantitative realtime PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J Virol Methods 2004; 121:217–221.
- Monaco MC, Jensen PN, Hou J, Durham LC, Major EO. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol 1998; 72:9918–9923.
- Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med 2010; 61:35–47.
- Imperiale M, Major E. Polyomavirus. In:Knipe D, Howley P, eds. Field Virology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:2263–2298.
- Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 2005; 353:375–381.
- Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med 2005; 353:362–368.
- Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med 2005; 353:369–374.
- Bozic C, Belcher G, Kooijmans-Coutinho M, et al. Natalizumab utilization and safety in patients with relapsing multiple sclerosis: updated results from TOUCH™ and TYGRIS. Paper presented at: 60th Annual Meeting of the American Academy of Neurology; April 15, 2008; Chicago, IL.
- Kappos L, Bates D, Hartung HP, et al. Natalizumab treatment for multiple sclerosis: recommendations for patient selection and monitoring. Lancet Neurol 2007; 6:431–441.
- Major EO. Reemergence of PML in natalizumab-treated patients—new cases, same concerns. N Engl J Med 2009; 361:1041–1043.
- Vugmeyster Y, Kikuchi T, Lowes MA, et al. Efalizumab (anti-CD11a)-induced increase in peripheral blood leukocytes in psoriasis patients is preferentially mediated by altered trafficking of memory CD8+ T cells into lesional skin. Clin Immunol 2004; 113:38–46.
- Guttman-Yassky E, Vugmeyster Y, Lowes MA, et al. Blockade of CD11a by efalizumab in psoriasis patients induces a unique state of T-cell hyporesponsiveness. J Invest Dermatol 2008; 128:1182–1191.
- Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 2009; 113:4834–4840.
- Ryschkewitsch CF, Jensen PN, Monaco MC, Major EO. JC virus persistence following progressive multifocal leukoencephalopathy in multiple sclerosis patients treated with natalizumab. Ann Neurol 2010; 68:384–391.
- Koralnik IJ, Du Pasquier RA, Kuroda MJ, et al. Association of prolonged survival in HLA-A2+ progressive multifocal leuko encephalopathy patients with a CTL response specific for a commonly recognized JC virus epitope. J Immunol 2002; 168:499–504.