User login
Mild cognitive impairment: Hope for stability, plan for progression
As our population ages, people are thinking more about preserving their quality of life, especially with regard to maintaining their cognitive and functional abilities. Older patients and caregivers often raise concerns about cognitive issues to their primary care providers: many patients have memory complaints, are worried about whether these are merely part of normal aging or symptoms of early dementia, and want strategies to forestall the progression of cognitive impairment.
Mild cognitive impairment (MCI) is a heterogeneous syndrome that in some cases represents a transition between normal aging and dementia. However, this condition is not yet well understood. Although some patients progress to dementia, others remain stable, or even improve. This article will review the current definitions and the underlying physiology of MCI, as well as diagnostic and management strategies.
COGNITIVE CHANGES OCCUR WITH NORMAL AGING
Cognition is defined as a means of acquiring and processing information about ourselves and our world. It includes memory as well as other domains such as attention, visuospatial skills, mental processing speed, language, and executive function. Cognitive abilities typically peak between ages 30 and 40, plateau in our 50s and 60s, and decline in our late 70s.
With age come detectable changes in the brain: brain weight declines by 10% by age 80, blood flow diminishes, neurons are lost throughout life, and nerve conduction slows. Despite these changes, the brain has a great deal of functional reserve capacity.
Table 1 compares the signs of normal aging, MCI, and dementia. Normally, cognitive abilities decline gradually with age without affecting overall function or activities of daily living. Even in normal aging, the processing of new information (new learning) is reduced. Mental processing becomes less efficient and slower. Visuospatial skills gradually decline, recall slows, and ultimately, the speed of performance slows as well. Additionally, distractibility increases. On the other hand, normal aging does not affect recognition, intelligence, or long-term memory.1
The line between the normal effects of aging on cognition and true pathologic cognitive decline is blurry. In a busy clinical practice, it is often difficult to determine whether problems with memory and cognition that elderly patients and their family members describe represent true pathologic decline. In general, the clinical presentation of MCI is more profound than that of age-associated cognitive impairment: whereas normal aging may involve forgetting names and words and misplacing things, MCI frequently involves forgetting conversations, information that one would ordinarily remember, appointments, and planned events.
BETWEEN NORMAL AGING AND DEMENTIA
MCI is a transitional state between normal cognition and dementia. But the course is not inevitably downward: on follow-up, patients with MCI may be better, stable, or worse (see PROGNOSIS VARIES, below).
On autopsy studies, the brains of people with MCI appear intermediate between normal brains and brains of people with Alzheimer-type dementia, which have neurofibrillary tangles, amyloid senile plaques, and neuronal degeneration.
Definitions of MCI vary
True cognitive decline that is more profound than normal aging was named and defined differently in different studies, making comparisons difficult. The concept of MCI arose from the term “benign senescent forgetfulness,” used by Kral in 1962.2 Other early terms include “cognitive impairment no dementia,” “memory impairment,” “mild cognitive disorder,” and “mild neurocognitive disorder.”3,4
MCI was first defined as a precursor to Alzheimer dementia. The term later described a sometimes reversible but abnormal state. It is a heterogeneous syndrome in terms of etiology, incidence, prevalence, presentation, and overall prognosis.
Most recently, MCI has been defined as5,6:
- Subjective memory complaints, preferably qualified by another person
- Memory impairment, with consideration for age and education
- Preserved general cognitive function
- Intact activities of daily living
- Absence of overt dementia.
MCI may arise from vascular, neurodegenerative, traumatic, metabolic, psychiatric, and other underlying medical disorders.7–9
The prevalence of MCI is difficult to determine because of the various definitions, populations studied (eg, clinic-based vs community-dwelling), and evaluation techniques. Published rates vary from 2% to 4% in all patients to 10% to 20% in the elderly. Incidence rates in the elderly vary from 14 to 75 per 1,000 patient-years.10–14
EARLY RECOGNITION ALLOWS PROMPT EVALUATION AND PLANNING
Pathologic cognitive decline is best detected early, for many reasons. Early recognition and intervention may help delay further decline. Establishing a diagnosis can also lessen family and caregiver stress and misunderstanding. Education of caregivers is important so that they can prepare for likely behavioral changes and plan for future care. Advance care planning, including advance directives, power of attorney, and designation of proxy for decision-making, is extremely important and is best considered before cognitive impairment becomes severe.
The diagnosis of MCI also provides the opportunity to assess safety concerns related to driving, working, medication compliance, the home environment, and firearms. Because patients with MCI are still highly functional, these issues need not be fully evaluated and should be handled on a case-by-case basis, depending on concerns raised. For example, if depression is an active concern, firearms safety should be addressed.
MEMORY LOSS MAY NOT BE THE PRIMARY CONCERN
MCI is categorized into two types based on whether memory loss is the primary cognitive deficit.
The amnestic type predominantly involves memory problems and is more common. Generally, several years elapse between initial memory concerns and a clinical diagnosis of MCI. Patients with amnestic MCI that progresses to dementia are more likely to develop Alzheimer disease.2,15
Nonamnestic types involve domains of cognition other than memory, such as executive function, attention, visuospatial ability, and language. Nonamnestic MCI can be subcategorized through extensive neuropsychological evaluation as involving single or multiple impaired domains.16,17 Such categorization is particularly important in determining prognosis, as patients with involvement of multiple domains are at higher risk of progressing to dementia.
Patients with nonamnestic MCI who progress to dementia are more likely to have non-Alzheimer types of dementia, such as Lewy body dementia and frontotemporal dementias.10
HISTORY SHOULD FOCUS ON FUNCTION, MEDICATIONS, AND DEPRESSION
Cognitive impairment should be clinically evaluated within the context of cognition, function, and behavior. Clinicians should focus on the time course of cognitive concerns, the specifics of the concerns, and their impact on day-to-day living and functioning. In assessing functional capacity, it is important to determine the level of assistance the patient needs to perform specific activities of daily living and instrumental activities of daily living (ie, the more advanced skills needed to live independently) (Table 2).
A thorough history includes consideration of baseline education, intellect, and previous learning disabilities; sensory impairments with emphasis on sight and hearing impairments; uncontrolled pain; head trauma; sleep disorders; concurrent medical and psychosocial illnesses such as depression and anxiety; substance abuse; and polypharmacy.
Depression, delirium, and the use of anticholinergic drugs are particularly important to evaluate, as these can result in cognitive deficits associated with MCI. The cognitive deficits may resolve with treatment or with stopping the drug.
Behavioral concerns such as wandering, agitation, and anger and sleep concerns, eating habits, and social etiquette are also important to evaluate.
PHYSICAL EVALUATION: RULE OUT REVERSIBLE CONDITIONS
The differential diagnosis of MCI includes delirium, depression, dementia, possibly reversible conditions affecting cognition (vitamin B12 deficiency, hypothyroidism, effects of anticholinergic drugs), and uncommonly, central nervous system conditions (normal pressure hydrocephalus, subdural hematoma, tumor, stroke), and others (Table 3).18
A thorough physical examination should include neurologic, cardiovascular, hearing, and vision examinations, as well as an evaluation of functional status.
Laboratory studies. Although evidence is lacking to support a laboratory diagnostic workup for MCI, a selective evaluation including a comprehensive metabolic profile, complete blood count, thyroid studies, and a vitamin B12 level can be useful. Occasionally, a treatable cause of impaired cognition such as vitamin B12 deficiency or thyroid disease can be identified and resolved. A further comprehensive laboratory evaluation should be obtained if a patient progresses to dementia.
Imaging can be used in conjunction with other supportive evidence but should not be used solely to establish a diagnosis of MCI. Magnetic resonance imaging (MRI) can detect metastatic disease, normal pressure hydrocephalus, and subdural hematoma, in addition to traumatic, inflammatory, infectious, and vascular causes of cognitive impairment. MRI can also determine focal areas of atrophy; temporal lobe atrophy is a risk factor for progression to dementia.
Other studies. Structural MRI using techniques to evaluate the hippocampus, functional imaging, genetic testing for ApoE4 alleles, and biomarkers in cerebrospinal fluid are currently under evaluation to identify those at risk of progression to dementia. Recently published guidelines by the Alzheimer’s Association and the National Institute on Aging indicate that pathophysiologic findings in MCI that may predict future Alzheimer disease are meant to guide research and are not part of clinical practice at this time.19
COGNITIVE AND NEUROLOGIC TESTING IDENTIFIES DEFICITS
A number of global measures of cognition can be used in the office in clinical practice to help in evaluating significant cognitive concerns and to determine areas and severity of deficits at presentation. These include the Mini-Mental State Examination, the Montreal Cognitive Assessment, the Saint Louis University Mental Status, and many others (Table 4).20
Caveats about interpreting the results: each of these tests has different sensitivities and specificities for detecting MCI. Also, we need to take into account the patient’s level of education, as highly educated people tend to do better on these tests.21–23 It is important to note that some patients with MCI have normal results or only minimally abnormal results on these tests.
Neuropsychological testing is reserved for patients needing further evaluation, eg, those with atypical or complex cases, and those in whom the specific domains of cognition involved need to be identified. It can also provide additional insight into the contribution of depression to cognitive deficits. Neuropsychological testing is usually very time-intensive and requires patients to be able to perform complicated cognitive tasks. Not all patients are good candidates for this testing; sensory and motor impairments must be considered to determine if patients can adequately participate in testing. The cost of neuropsychological testing for MCI may not be covered by insurance and should be discussed with patients before referral. Specific concerns about cognitive problems that need further evaluation should be stated in the referral.
No one test should be used to make a diagnosis of MCI or dementia; clinical judgment is also necessary. The need for referral to a neurologist, geriatrician, or psychiatrist depends on the nature of the cognitive and behavioral concerns, the complexity of making a diagnosis, the need for further assessment of functional ability, and the need for evaluation of risk of progression to dementia.
MEDICATIONS HAVE LITTLE ROLE IN MANAGEMENT
No drug has yet been approved by the US Food and Drug Administration for treating MCI.
The acetylcholinesterase inhibitors donepezil (Aricept), galantamine (Razadyne), and rivastigmine (Exelon) have undergone clinical trials for treatment of MCI but have not been definitely shown to significantly reduce the risk of progression to dementia.24
On the other hand, Diniz et al25 performed a meta-analysis of the use of cholinesterase inhibitors in patients with MCI as a means of delaying the progression to Alzheimer disease.25 They calculated that 15.4% of patients who received these drugs progressed to dementia, compared with 20.4% of those who received placebo, for a relative risk of 0.75 (95% confidence interval 0.66–0.87, P < .001). They concluded that the use of these drugs in patients with MCI “may attenuate the risk of progression” to Alzheimer disease and dementia.
In addition to not being approved for this indication and showing mixed evidence of efficacy, these drugs have well-known side effects such as diarrhea, nausea, vomiting, anorexia, and rhinitis, as well as significant but lesser-known side effects such as syncope, bradycardia, gastrointestinal bleeding, and vivid dreams.26
Nevertheless, some patients with MCI, particularly those at high risk with amnestic MCI, may still want to try these medications. In these cases, the risks and possible benefits (or lack of them) should be reviewed thoroughly with the patient and family, and the discussion should be documented before starting therapy. The lowest starting dose of acetylcholinesterase inhibitor should be used to determine tolerability; generally, the dose is increased after 4 weeks to a maintenance dosage, with particular consideration of side effects.
Other agents have also been evaluated for MCI but have shown no evidence of benefit. Nonsteroidal anti-inflammatory drugs have not been found to either improve symptoms or delay progression to dementia. Ginkgo biloba has shown unclear benefit in achieving important treatment goals for MCI,27 and it increases the risk of bleeding in the elderly. Vitamin E was evaluated in one study and did not slow progression to dementia.28
STAYING HEALTHY AND ACTIVE MAY HELP
We recommend optimizing vascular risk factors such as diabetes, blood pressure, smoking, and lipid levels in managing MCI, given that uncontrolled vascular risk factors may lead to progression to dementia. However, we can point to no research to support this recommendation.
Cognitive rehabilitation involves training in deficient domains and developing strategies to compensate for deficits. Different interventions are used, including computerized simulation exercises, memory aids, organizational techniques, personal digital assistants, crossword puzzles, mind games, and other mentally engaging activities.29
Increasing physical activity is another aspect of treatment. Some studies have shown that it improves cognitive performance in MCI, at least in the short term.30,31
Optimizing mood and emotions is also important. If present, depression should be identified and optimally treated. Social activity can be useful and leads to less emotional stress and to better coping mechanisms.
A multidisciplinary approach may help patients and may also help relieve the burden on the caregiver. Periodic reassessment of cognitive and functional symptoms may be warranted.
Maintaining disease-specific registries of patients who have MCI may be useful to longitudinally follow patients and ensure that they get the care they need.
PROGNOSIS VARIES
MCI is a heterogeneous condition that often does not predictably progress to dementia. Patients and families should be told that having MCI does not mean that the patient will necessarily get dementia.
Several studies have shown that the annual risk of progression to dementia for patients with MCI is 5% to 10% in community-dwelling populations and up to 15% in specialty-clinic patients.24,32 In comparison, the incidence of dementia in the general elderly population is 1% to 3% per year.
On the other hand, a number of studies show that MCI improves significantly in up to 15% to 40% of patients and sometimes reverts to a normal cognitive state.33,34 But prospective studies of patients with clinically diagnosed MCI usually find a low rate of reversion to a normal state.35,36 Many are short-term follow-up studies of different populations, making generalizations difficult.14
Patients with impairment in instrumental activities of daily living may be more likely to have nonreversible MCI and may be at higher risk of progressing to dementia.37
PATIENT AND FAMILY EDUCATION AND FOLLOW-UP CONSIDERATIONS
Caregiver education and stress management are important components of managing patients with MCI. Formally assessing caregiver stress is useful. Steps to prevent caregiver burnout include making use of respite care, counseling, education, and community resources such as adult day care and those offered by the Alzheimer’s Association.
Clinicians should follow patients with MCI closely to evaluate progression, address specific concerns, minimize risks, emphasize healthy habits, manage concurrent illnesses, and evaluate management.
Functional status, as demonstrated by activities of daily living, is the most important determinant of progression of MCI to dementia and should be evaluated at each visit. Repeat cognitive testing should be done on patients who have significant loss of functional status. Changes in work habits also warrant further attention.
Patients diagnosed with MCI or those who have persistent cognitive concerns should be considered for neuropsychological evaluation after 1 year to assess specific deficits and progression of cognitive impairment.
Finally, consideration should be given to current clinical research, and referrals should be made to research centers that focus on MCI management and treatment.
- Keefover RW. Aging and cognition. Neurol Clin 1998; 16:635–648.
- Kral VA. Senescent forgetfulness: benign and malignant. Can Med Assoc J 1962; 86:257–260.
- Bischkopf J, Busse A, Angermeyer MC. Mild cognitive impairment—a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand 2002; 106:403–414.
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1133–1142.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308.
- Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol 2009; 66:1447–1455.
- Bennett DA, Schneider JA, Bienias JL, et al. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology 2005; 64:834–841.
- Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 2006; 63:665–672.
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol 2003; 60:729–736.
- Molano J, Boeve B, Ferman T, et al. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain 2010; 133:540–556.
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol 2003; 60:1385–1389.
- Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology 2010; 75:889–897.
- Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol 2008; 63:494–506.
- Luck T, Luppa M, Briel S, et al. Mild cognitive impairment: incidence and risk factors: results of the Leipzig Longitudinal Study of the Aged. J Am Geriatr Soc 2010; 58:1903–1910.
- Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008; 30:58–69.
- Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol 2001; 58:411–416.
- DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol 2003; 2:15–21.
- Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet 1997; 349:1793–1796.
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:270–279.
- Tariq SH, Tumosa N, Chibnall JT, Perry MH, Morley JE. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry 2006; 14:900–910.
- Tang-Wai DF, Knopman DS, Geda YE, et al. Comparison of the short test of mental status and the Mini-Mental State Examination in mild cognitive impairment. Arch Neurol 2003; 60:1777–1781.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699.
- Banks WA, Morley JE. Memories are made of this: recent advances in understanding cognitive impairments and dementia. J Gerontol A Biol Sci Med Sci 2003; 58:314–321.
- Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med 2011; 364:2227–2234.
- Diniz BS, Pinto JA, Gonzaga MLC, Guimaraes FM, Gattaz WF, Forlenza OV. To treat or not to treat? A meta-analysis of the use of cholinesterase inhibitors in mild cognitive impairment for delaying progression to Alzheimer’s disease. Eur Arch Psychiatry Neurosci 2009; 259:248–256.
- Patel BB, Holland NW. Adverse effects of acetylcholinesterase inhibitors. Clin Geriatr 2011; 19:27–30.
- Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev 2009; 1:CD003120.
- Petersen RC, Thomas RG, Grundman M, et al; Alzheimer’s Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005; 352:2379–2388.
- Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry 2010; 18:281–296.
- Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008; 300:1027–1037.
- van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br J Sports Med 2008; 42:344–351.
- Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic-vs community-based cohorts. Arch Neurol 2009; 66:1151–1157.
- Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology 2001; 56:37–42.
- Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002; 59:1594–1599.
- Busse A, Hensel A, Gühne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology 2006; 67:2176–2185.
- Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology 2007; 68:288–291.
- Pérès K, Chrysostome V, Fabrigoule C, Orgogozo JM, Dartigues JF, Barberger-Gateau P. Restriction in complex activities of daily living in MCI: impact on outcome. Neurology 2006; 67:461–466.
As our population ages, people are thinking more about preserving their quality of life, especially with regard to maintaining their cognitive and functional abilities. Older patients and caregivers often raise concerns about cognitive issues to their primary care providers: many patients have memory complaints, are worried about whether these are merely part of normal aging or symptoms of early dementia, and want strategies to forestall the progression of cognitive impairment.
Mild cognitive impairment (MCI) is a heterogeneous syndrome that in some cases represents a transition between normal aging and dementia. However, this condition is not yet well understood. Although some patients progress to dementia, others remain stable, or even improve. This article will review the current definitions and the underlying physiology of MCI, as well as diagnostic and management strategies.
COGNITIVE CHANGES OCCUR WITH NORMAL AGING
Cognition is defined as a means of acquiring and processing information about ourselves and our world. It includes memory as well as other domains such as attention, visuospatial skills, mental processing speed, language, and executive function. Cognitive abilities typically peak between ages 30 and 40, plateau in our 50s and 60s, and decline in our late 70s.
With age come detectable changes in the brain: brain weight declines by 10% by age 80, blood flow diminishes, neurons are lost throughout life, and nerve conduction slows. Despite these changes, the brain has a great deal of functional reserve capacity.
Table 1 compares the signs of normal aging, MCI, and dementia. Normally, cognitive abilities decline gradually with age without affecting overall function or activities of daily living. Even in normal aging, the processing of new information (new learning) is reduced. Mental processing becomes less efficient and slower. Visuospatial skills gradually decline, recall slows, and ultimately, the speed of performance slows as well. Additionally, distractibility increases. On the other hand, normal aging does not affect recognition, intelligence, or long-term memory.1
The line between the normal effects of aging on cognition and true pathologic cognitive decline is blurry. In a busy clinical practice, it is often difficult to determine whether problems with memory and cognition that elderly patients and their family members describe represent true pathologic decline. In general, the clinical presentation of MCI is more profound than that of age-associated cognitive impairment: whereas normal aging may involve forgetting names and words and misplacing things, MCI frequently involves forgetting conversations, information that one would ordinarily remember, appointments, and planned events.
BETWEEN NORMAL AGING AND DEMENTIA
MCI is a transitional state between normal cognition and dementia. But the course is not inevitably downward: on follow-up, patients with MCI may be better, stable, or worse (see PROGNOSIS VARIES, below).
On autopsy studies, the brains of people with MCI appear intermediate between normal brains and brains of people with Alzheimer-type dementia, which have neurofibrillary tangles, amyloid senile plaques, and neuronal degeneration.
Definitions of MCI vary
True cognitive decline that is more profound than normal aging was named and defined differently in different studies, making comparisons difficult. The concept of MCI arose from the term “benign senescent forgetfulness,” used by Kral in 1962.2 Other early terms include “cognitive impairment no dementia,” “memory impairment,” “mild cognitive disorder,” and “mild neurocognitive disorder.”3,4
MCI was first defined as a precursor to Alzheimer dementia. The term later described a sometimes reversible but abnormal state. It is a heterogeneous syndrome in terms of etiology, incidence, prevalence, presentation, and overall prognosis.
Most recently, MCI has been defined as5,6:
- Subjective memory complaints, preferably qualified by another person
- Memory impairment, with consideration for age and education
- Preserved general cognitive function
- Intact activities of daily living
- Absence of overt dementia.
MCI may arise from vascular, neurodegenerative, traumatic, metabolic, psychiatric, and other underlying medical disorders.7–9
The prevalence of MCI is difficult to determine because of the various definitions, populations studied (eg, clinic-based vs community-dwelling), and evaluation techniques. Published rates vary from 2% to 4% in all patients to 10% to 20% in the elderly. Incidence rates in the elderly vary from 14 to 75 per 1,000 patient-years.10–14
EARLY RECOGNITION ALLOWS PROMPT EVALUATION AND PLANNING
Pathologic cognitive decline is best detected early, for many reasons. Early recognition and intervention may help delay further decline. Establishing a diagnosis can also lessen family and caregiver stress and misunderstanding. Education of caregivers is important so that they can prepare for likely behavioral changes and plan for future care. Advance care planning, including advance directives, power of attorney, and designation of proxy for decision-making, is extremely important and is best considered before cognitive impairment becomes severe.
The diagnosis of MCI also provides the opportunity to assess safety concerns related to driving, working, medication compliance, the home environment, and firearms. Because patients with MCI are still highly functional, these issues need not be fully evaluated and should be handled on a case-by-case basis, depending on concerns raised. For example, if depression is an active concern, firearms safety should be addressed.
MEMORY LOSS MAY NOT BE THE PRIMARY CONCERN
MCI is categorized into two types based on whether memory loss is the primary cognitive deficit.
The amnestic type predominantly involves memory problems and is more common. Generally, several years elapse between initial memory concerns and a clinical diagnosis of MCI. Patients with amnestic MCI that progresses to dementia are more likely to develop Alzheimer disease.2,15
Nonamnestic types involve domains of cognition other than memory, such as executive function, attention, visuospatial ability, and language. Nonamnestic MCI can be subcategorized through extensive neuropsychological evaluation as involving single or multiple impaired domains.16,17 Such categorization is particularly important in determining prognosis, as patients with involvement of multiple domains are at higher risk of progressing to dementia.
Patients with nonamnestic MCI who progress to dementia are more likely to have non-Alzheimer types of dementia, such as Lewy body dementia and frontotemporal dementias.10
HISTORY SHOULD FOCUS ON FUNCTION, MEDICATIONS, AND DEPRESSION
Cognitive impairment should be clinically evaluated within the context of cognition, function, and behavior. Clinicians should focus on the time course of cognitive concerns, the specifics of the concerns, and their impact on day-to-day living and functioning. In assessing functional capacity, it is important to determine the level of assistance the patient needs to perform specific activities of daily living and instrumental activities of daily living (ie, the more advanced skills needed to live independently) (Table 2).
A thorough history includes consideration of baseline education, intellect, and previous learning disabilities; sensory impairments with emphasis on sight and hearing impairments; uncontrolled pain; head trauma; sleep disorders; concurrent medical and psychosocial illnesses such as depression and anxiety; substance abuse; and polypharmacy.
Depression, delirium, and the use of anticholinergic drugs are particularly important to evaluate, as these can result in cognitive deficits associated with MCI. The cognitive deficits may resolve with treatment or with stopping the drug.
Behavioral concerns such as wandering, agitation, and anger and sleep concerns, eating habits, and social etiquette are also important to evaluate.
PHYSICAL EVALUATION: RULE OUT REVERSIBLE CONDITIONS
The differential diagnosis of MCI includes delirium, depression, dementia, possibly reversible conditions affecting cognition (vitamin B12 deficiency, hypothyroidism, effects of anticholinergic drugs), and uncommonly, central nervous system conditions (normal pressure hydrocephalus, subdural hematoma, tumor, stroke), and others (Table 3).18
A thorough physical examination should include neurologic, cardiovascular, hearing, and vision examinations, as well as an evaluation of functional status.
Laboratory studies. Although evidence is lacking to support a laboratory diagnostic workup for MCI, a selective evaluation including a comprehensive metabolic profile, complete blood count, thyroid studies, and a vitamin B12 level can be useful. Occasionally, a treatable cause of impaired cognition such as vitamin B12 deficiency or thyroid disease can be identified and resolved. A further comprehensive laboratory evaluation should be obtained if a patient progresses to dementia.
Imaging can be used in conjunction with other supportive evidence but should not be used solely to establish a diagnosis of MCI. Magnetic resonance imaging (MRI) can detect metastatic disease, normal pressure hydrocephalus, and subdural hematoma, in addition to traumatic, inflammatory, infectious, and vascular causes of cognitive impairment. MRI can also determine focal areas of atrophy; temporal lobe atrophy is a risk factor for progression to dementia.
Other studies. Structural MRI using techniques to evaluate the hippocampus, functional imaging, genetic testing for ApoE4 alleles, and biomarkers in cerebrospinal fluid are currently under evaluation to identify those at risk of progression to dementia. Recently published guidelines by the Alzheimer’s Association and the National Institute on Aging indicate that pathophysiologic findings in MCI that may predict future Alzheimer disease are meant to guide research and are not part of clinical practice at this time.19
COGNITIVE AND NEUROLOGIC TESTING IDENTIFIES DEFICITS
A number of global measures of cognition can be used in the office in clinical practice to help in evaluating significant cognitive concerns and to determine areas and severity of deficits at presentation. These include the Mini-Mental State Examination, the Montreal Cognitive Assessment, the Saint Louis University Mental Status, and many others (Table 4).20
Caveats about interpreting the results: each of these tests has different sensitivities and specificities for detecting MCI. Also, we need to take into account the patient’s level of education, as highly educated people tend to do better on these tests.21–23 It is important to note that some patients with MCI have normal results or only minimally abnormal results on these tests.
Neuropsychological testing is reserved for patients needing further evaluation, eg, those with atypical or complex cases, and those in whom the specific domains of cognition involved need to be identified. It can also provide additional insight into the contribution of depression to cognitive deficits. Neuropsychological testing is usually very time-intensive and requires patients to be able to perform complicated cognitive tasks. Not all patients are good candidates for this testing; sensory and motor impairments must be considered to determine if patients can adequately participate in testing. The cost of neuropsychological testing for MCI may not be covered by insurance and should be discussed with patients before referral. Specific concerns about cognitive problems that need further evaluation should be stated in the referral.
No one test should be used to make a diagnosis of MCI or dementia; clinical judgment is also necessary. The need for referral to a neurologist, geriatrician, or psychiatrist depends on the nature of the cognitive and behavioral concerns, the complexity of making a diagnosis, the need for further assessment of functional ability, and the need for evaluation of risk of progression to dementia.
MEDICATIONS HAVE LITTLE ROLE IN MANAGEMENT
No drug has yet been approved by the US Food and Drug Administration for treating MCI.
The acetylcholinesterase inhibitors donepezil (Aricept), galantamine (Razadyne), and rivastigmine (Exelon) have undergone clinical trials for treatment of MCI but have not been definitely shown to significantly reduce the risk of progression to dementia.24
On the other hand, Diniz et al25 performed a meta-analysis of the use of cholinesterase inhibitors in patients with MCI as a means of delaying the progression to Alzheimer disease.25 They calculated that 15.4% of patients who received these drugs progressed to dementia, compared with 20.4% of those who received placebo, for a relative risk of 0.75 (95% confidence interval 0.66–0.87, P < .001). They concluded that the use of these drugs in patients with MCI “may attenuate the risk of progression” to Alzheimer disease and dementia.
In addition to not being approved for this indication and showing mixed evidence of efficacy, these drugs have well-known side effects such as diarrhea, nausea, vomiting, anorexia, and rhinitis, as well as significant but lesser-known side effects such as syncope, bradycardia, gastrointestinal bleeding, and vivid dreams.26
Nevertheless, some patients with MCI, particularly those at high risk with amnestic MCI, may still want to try these medications. In these cases, the risks and possible benefits (or lack of them) should be reviewed thoroughly with the patient and family, and the discussion should be documented before starting therapy. The lowest starting dose of acetylcholinesterase inhibitor should be used to determine tolerability; generally, the dose is increased after 4 weeks to a maintenance dosage, with particular consideration of side effects.
Other agents have also been evaluated for MCI but have shown no evidence of benefit. Nonsteroidal anti-inflammatory drugs have not been found to either improve symptoms or delay progression to dementia. Ginkgo biloba has shown unclear benefit in achieving important treatment goals for MCI,27 and it increases the risk of bleeding in the elderly. Vitamin E was evaluated in one study and did not slow progression to dementia.28
STAYING HEALTHY AND ACTIVE MAY HELP
We recommend optimizing vascular risk factors such as diabetes, blood pressure, smoking, and lipid levels in managing MCI, given that uncontrolled vascular risk factors may lead to progression to dementia. However, we can point to no research to support this recommendation.
Cognitive rehabilitation involves training in deficient domains and developing strategies to compensate for deficits. Different interventions are used, including computerized simulation exercises, memory aids, organizational techniques, personal digital assistants, crossword puzzles, mind games, and other mentally engaging activities.29
Increasing physical activity is another aspect of treatment. Some studies have shown that it improves cognitive performance in MCI, at least in the short term.30,31
Optimizing mood and emotions is also important. If present, depression should be identified and optimally treated. Social activity can be useful and leads to less emotional stress and to better coping mechanisms.
A multidisciplinary approach may help patients and may also help relieve the burden on the caregiver. Periodic reassessment of cognitive and functional symptoms may be warranted.
Maintaining disease-specific registries of patients who have MCI may be useful to longitudinally follow patients and ensure that they get the care they need.
PROGNOSIS VARIES
MCI is a heterogeneous condition that often does not predictably progress to dementia. Patients and families should be told that having MCI does not mean that the patient will necessarily get dementia.
Several studies have shown that the annual risk of progression to dementia for patients with MCI is 5% to 10% in community-dwelling populations and up to 15% in specialty-clinic patients.24,32 In comparison, the incidence of dementia in the general elderly population is 1% to 3% per year.
On the other hand, a number of studies show that MCI improves significantly in up to 15% to 40% of patients and sometimes reverts to a normal cognitive state.33,34 But prospective studies of patients with clinically diagnosed MCI usually find a low rate of reversion to a normal state.35,36 Many are short-term follow-up studies of different populations, making generalizations difficult.14
Patients with impairment in instrumental activities of daily living may be more likely to have nonreversible MCI and may be at higher risk of progressing to dementia.37
PATIENT AND FAMILY EDUCATION AND FOLLOW-UP CONSIDERATIONS
Caregiver education and stress management are important components of managing patients with MCI. Formally assessing caregiver stress is useful. Steps to prevent caregiver burnout include making use of respite care, counseling, education, and community resources such as adult day care and those offered by the Alzheimer’s Association.
Clinicians should follow patients with MCI closely to evaluate progression, address specific concerns, minimize risks, emphasize healthy habits, manage concurrent illnesses, and evaluate management.
Functional status, as demonstrated by activities of daily living, is the most important determinant of progression of MCI to dementia and should be evaluated at each visit. Repeat cognitive testing should be done on patients who have significant loss of functional status. Changes in work habits also warrant further attention.
Patients diagnosed with MCI or those who have persistent cognitive concerns should be considered for neuropsychological evaluation after 1 year to assess specific deficits and progression of cognitive impairment.
Finally, consideration should be given to current clinical research, and referrals should be made to research centers that focus on MCI management and treatment.
As our population ages, people are thinking more about preserving their quality of life, especially with regard to maintaining their cognitive and functional abilities. Older patients and caregivers often raise concerns about cognitive issues to their primary care providers: many patients have memory complaints, are worried about whether these are merely part of normal aging or symptoms of early dementia, and want strategies to forestall the progression of cognitive impairment.
Mild cognitive impairment (MCI) is a heterogeneous syndrome that in some cases represents a transition between normal aging and dementia. However, this condition is not yet well understood. Although some patients progress to dementia, others remain stable, or even improve. This article will review the current definitions and the underlying physiology of MCI, as well as diagnostic and management strategies.
COGNITIVE CHANGES OCCUR WITH NORMAL AGING
Cognition is defined as a means of acquiring and processing information about ourselves and our world. It includes memory as well as other domains such as attention, visuospatial skills, mental processing speed, language, and executive function. Cognitive abilities typically peak between ages 30 and 40, plateau in our 50s and 60s, and decline in our late 70s.
With age come detectable changes in the brain: brain weight declines by 10% by age 80, blood flow diminishes, neurons are lost throughout life, and nerve conduction slows. Despite these changes, the brain has a great deal of functional reserve capacity.
Table 1 compares the signs of normal aging, MCI, and dementia. Normally, cognitive abilities decline gradually with age without affecting overall function or activities of daily living. Even in normal aging, the processing of new information (new learning) is reduced. Mental processing becomes less efficient and slower. Visuospatial skills gradually decline, recall slows, and ultimately, the speed of performance slows as well. Additionally, distractibility increases. On the other hand, normal aging does not affect recognition, intelligence, or long-term memory.1
The line between the normal effects of aging on cognition and true pathologic cognitive decline is blurry. In a busy clinical practice, it is often difficult to determine whether problems with memory and cognition that elderly patients and their family members describe represent true pathologic decline. In general, the clinical presentation of MCI is more profound than that of age-associated cognitive impairment: whereas normal aging may involve forgetting names and words and misplacing things, MCI frequently involves forgetting conversations, information that one would ordinarily remember, appointments, and planned events.
BETWEEN NORMAL AGING AND DEMENTIA
MCI is a transitional state between normal cognition and dementia. But the course is not inevitably downward: on follow-up, patients with MCI may be better, stable, or worse (see PROGNOSIS VARIES, below).
On autopsy studies, the brains of people with MCI appear intermediate between normal brains and brains of people with Alzheimer-type dementia, which have neurofibrillary tangles, amyloid senile plaques, and neuronal degeneration.
Definitions of MCI vary
True cognitive decline that is more profound than normal aging was named and defined differently in different studies, making comparisons difficult. The concept of MCI arose from the term “benign senescent forgetfulness,” used by Kral in 1962.2 Other early terms include “cognitive impairment no dementia,” “memory impairment,” “mild cognitive disorder,” and “mild neurocognitive disorder.”3,4
MCI was first defined as a precursor to Alzheimer dementia. The term later described a sometimes reversible but abnormal state. It is a heterogeneous syndrome in terms of etiology, incidence, prevalence, presentation, and overall prognosis.
Most recently, MCI has been defined as5,6:
- Subjective memory complaints, preferably qualified by another person
- Memory impairment, with consideration for age and education
- Preserved general cognitive function
- Intact activities of daily living
- Absence of overt dementia.
MCI may arise from vascular, neurodegenerative, traumatic, metabolic, psychiatric, and other underlying medical disorders.7–9
The prevalence of MCI is difficult to determine because of the various definitions, populations studied (eg, clinic-based vs community-dwelling), and evaluation techniques. Published rates vary from 2% to 4% in all patients to 10% to 20% in the elderly. Incidence rates in the elderly vary from 14 to 75 per 1,000 patient-years.10–14
EARLY RECOGNITION ALLOWS PROMPT EVALUATION AND PLANNING
Pathologic cognitive decline is best detected early, for many reasons. Early recognition and intervention may help delay further decline. Establishing a diagnosis can also lessen family and caregiver stress and misunderstanding. Education of caregivers is important so that they can prepare for likely behavioral changes and plan for future care. Advance care planning, including advance directives, power of attorney, and designation of proxy for decision-making, is extremely important and is best considered before cognitive impairment becomes severe.
The diagnosis of MCI also provides the opportunity to assess safety concerns related to driving, working, medication compliance, the home environment, and firearms. Because patients with MCI are still highly functional, these issues need not be fully evaluated and should be handled on a case-by-case basis, depending on concerns raised. For example, if depression is an active concern, firearms safety should be addressed.
MEMORY LOSS MAY NOT BE THE PRIMARY CONCERN
MCI is categorized into two types based on whether memory loss is the primary cognitive deficit.
The amnestic type predominantly involves memory problems and is more common. Generally, several years elapse between initial memory concerns and a clinical diagnosis of MCI. Patients with amnestic MCI that progresses to dementia are more likely to develop Alzheimer disease.2,15
Nonamnestic types involve domains of cognition other than memory, such as executive function, attention, visuospatial ability, and language. Nonamnestic MCI can be subcategorized through extensive neuropsychological evaluation as involving single or multiple impaired domains.16,17 Such categorization is particularly important in determining prognosis, as patients with involvement of multiple domains are at higher risk of progressing to dementia.
Patients with nonamnestic MCI who progress to dementia are more likely to have non-Alzheimer types of dementia, such as Lewy body dementia and frontotemporal dementias.10
HISTORY SHOULD FOCUS ON FUNCTION, MEDICATIONS, AND DEPRESSION
Cognitive impairment should be clinically evaluated within the context of cognition, function, and behavior. Clinicians should focus on the time course of cognitive concerns, the specifics of the concerns, and their impact on day-to-day living and functioning. In assessing functional capacity, it is important to determine the level of assistance the patient needs to perform specific activities of daily living and instrumental activities of daily living (ie, the more advanced skills needed to live independently) (Table 2).
A thorough history includes consideration of baseline education, intellect, and previous learning disabilities; sensory impairments with emphasis on sight and hearing impairments; uncontrolled pain; head trauma; sleep disorders; concurrent medical and psychosocial illnesses such as depression and anxiety; substance abuse; and polypharmacy.
Depression, delirium, and the use of anticholinergic drugs are particularly important to evaluate, as these can result in cognitive deficits associated with MCI. The cognitive deficits may resolve with treatment or with stopping the drug.
Behavioral concerns such as wandering, agitation, and anger and sleep concerns, eating habits, and social etiquette are also important to evaluate.
PHYSICAL EVALUATION: RULE OUT REVERSIBLE CONDITIONS
The differential diagnosis of MCI includes delirium, depression, dementia, possibly reversible conditions affecting cognition (vitamin B12 deficiency, hypothyroidism, effects of anticholinergic drugs), and uncommonly, central nervous system conditions (normal pressure hydrocephalus, subdural hematoma, tumor, stroke), and others (Table 3).18
A thorough physical examination should include neurologic, cardiovascular, hearing, and vision examinations, as well as an evaluation of functional status.
Laboratory studies. Although evidence is lacking to support a laboratory diagnostic workup for MCI, a selective evaluation including a comprehensive metabolic profile, complete blood count, thyroid studies, and a vitamin B12 level can be useful. Occasionally, a treatable cause of impaired cognition such as vitamin B12 deficiency or thyroid disease can be identified and resolved. A further comprehensive laboratory evaluation should be obtained if a patient progresses to dementia.
Imaging can be used in conjunction with other supportive evidence but should not be used solely to establish a diagnosis of MCI. Magnetic resonance imaging (MRI) can detect metastatic disease, normal pressure hydrocephalus, and subdural hematoma, in addition to traumatic, inflammatory, infectious, and vascular causes of cognitive impairment. MRI can also determine focal areas of atrophy; temporal lobe atrophy is a risk factor for progression to dementia.
Other studies. Structural MRI using techniques to evaluate the hippocampus, functional imaging, genetic testing for ApoE4 alleles, and biomarkers in cerebrospinal fluid are currently under evaluation to identify those at risk of progression to dementia. Recently published guidelines by the Alzheimer’s Association and the National Institute on Aging indicate that pathophysiologic findings in MCI that may predict future Alzheimer disease are meant to guide research and are not part of clinical practice at this time.19
COGNITIVE AND NEUROLOGIC TESTING IDENTIFIES DEFICITS
A number of global measures of cognition can be used in the office in clinical practice to help in evaluating significant cognitive concerns and to determine areas and severity of deficits at presentation. These include the Mini-Mental State Examination, the Montreal Cognitive Assessment, the Saint Louis University Mental Status, and many others (Table 4).20
Caveats about interpreting the results: each of these tests has different sensitivities and specificities for detecting MCI. Also, we need to take into account the patient’s level of education, as highly educated people tend to do better on these tests.21–23 It is important to note that some patients with MCI have normal results or only minimally abnormal results on these tests.
Neuropsychological testing is reserved for patients needing further evaluation, eg, those with atypical or complex cases, and those in whom the specific domains of cognition involved need to be identified. It can also provide additional insight into the contribution of depression to cognitive deficits. Neuropsychological testing is usually very time-intensive and requires patients to be able to perform complicated cognitive tasks. Not all patients are good candidates for this testing; sensory and motor impairments must be considered to determine if patients can adequately participate in testing. The cost of neuropsychological testing for MCI may not be covered by insurance and should be discussed with patients before referral. Specific concerns about cognitive problems that need further evaluation should be stated in the referral.
No one test should be used to make a diagnosis of MCI or dementia; clinical judgment is also necessary. The need for referral to a neurologist, geriatrician, or psychiatrist depends on the nature of the cognitive and behavioral concerns, the complexity of making a diagnosis, the need for further assessment of functional ability, and the need for evaluation of risk of progression to dementia.
MEDICATIONS HAVE LITTLE ROLE IN MANAGEMENT
No drug has yet been approved by the US Food and Drug Administration for treating MCI.
The acetylcholinesterase inhibitors donepezil (Aricept), galantamine (Razadyne), and rivastigmine (Exelon) have undergone clinical trials for treatment of MCI but have not been definitely shown to significantly reduce the risk of progression to dementia.24
On the other hand, Diniz et al25 performed a meta-analysis of the use of cholinesterase inhibitors in patients with MCI as a means of delaying the progression to Alzheimer disease.25 They calculated that 15.4% of patients who received these drugs progressed to dementia, compared with 20.4% of those who received placebo, for a relative risk of 0.75 (95% confidence interval 0.66–0.87, P < .001). They concluded that the use of these drugs in patients with MCI “may attenuate the risk of progression” to Alzheimer disease and dementia.
In addition to not being approved for this indication and showing mixed evidence of efficacy, these drugs have well-known side effects such as diarrhea, nausea, vomiting, anorexia, and rhinitis, as well as significant but lesser-known side effects such as syncope, bradycardia, gastrointestinal bleeding, and vivid dreams.26
Nevertheless, some patients with MCI, particularly those at high risk with amnestic MCI, may still want to try these medications. In these cases, the risks and possible benefits (or lack of them) should be reviewed thoroughly with the patient and family, and the discussion should be documented before starting therapy. The lowest starting dose of acetylcholinesterase inhibitor should be used to determine tolerability; generally, the dose is increased after 4 weeks to a maintenance dosage, with particular consideration of side effects.
Other agents have also been evaluated for MCI but have shown no evidence of benefit. Nonsteroidal anti-inflammatory drugs have not been found to either improve symptoms or delay progression to dementia. Ginkgo biloba has shown unclear benefit in achieving important treatment goals for MCI,27 and it increases the risk of bleeding in the elderly. Vitamin E was evaluated in one study and did not slow progression to dementia.28
STAYING HEALTHY AND ACTIVE MAY HELP
We recommend optimizing vascular risk factors such as diabetes, blood pressure, smoking, and lipid levels in managing MCI, given that uncontrolled vascular risk factors may lead to progression to dementia. However, we can point to no research to support this recommendation.
Cognitive rehabilitation involves training in deficient domains and developing strategies to compensate for deficits. Different interventions are used, including computerized simulation exercises, memory aids, organizational techniques, personal digital assistants, crossword puzzles, mind games, and other mentally engaging activities.29
Increasing physical activity is another aspect of treatment. Some studies have shown that it improves cognitive performance in MCI, at least in the short term.30,31
Optimizing mood and emotions is also important. If present, depression should be identified and optimally treated. Social activity can be useful and leads to less emotional stress and to better coping mechanisms.
A multidisciplinary approach may help patients and may also help relieve the burden on the caregiver. Periodic reassessment of cognitive and functional symptoms may be warranted.
Maintaining disease-specific registries of patients who have MCI may be useful to longitudinally follow patients and ensure that they get the care they need.
PROGNOSIS VARIES
MCI is a heterogeneous condition that often does not predictably progress to dementia. Patients and families should be told that having MCI does not mean that the patient will necessarily get dementia.
Several studies have shown that the annual risk of progression to dementia for patients with MCI is 5% to 10% in community-dwelling populations and up to 15% in specialty-clinic patients.24,32 In comparison, the incidence of dementia in the general elderly population is 1% to 3% per year.
On the other hand, a number of studies show that MCI improves significantly in up to 15% to 40% of patients and sometimes reverts to a normal cognitive state.33,34 But prospective studies of patients with clinically diagnosed MCI usually find a low rate of reversion to a normal state.35,36 Many are short-term follow-up studies of different populations, making generalizations difficult.14
Patients with impairment in instrumental activities of daily living may be more likely to have nonreversible MCI and may be at higher risk of progressing to dementia.37
PATIENT AND FAMILY EDUCATION AND FOLLOW-UP CONSIDERATIONS
Caregiver education and stress management are important components of managing patients with MCI. Formally assessing caregiver stress is useful. Steps to prevent caregiver burnout include making use of respite care, counseling, education, and community resources such as adult day care and those offered by the Alzheimer’s Association.
Clinicians should follow patients with MCI closely to evaluate progression, address specific concerns, minimize risks, emphasize healthy habits, manage concurrent illnesses, and evaluate management.
Functional status, as demonstrated by activities of daily living, is the most important determinant of progression of MCI to dementia and should be evaluated at each visit. Repeat cognitive testing should be done on patients who have significant loss of functional status. Changes in work habits also warrant further attention.
Patients diagnosed with MCI or those who have persistent cognitive concerns should be considered for neuropsychological evaluation after 1 year to assess specific deficits and progression of cognitive impairment.
Finally, consideration should be given to current clinical research, and referrals should be made to research centers that focus on MCI management and treatment.
- Keefover RW. Aging and cognition. Neurol Clin 1998; 16:635–648.
- Kral VA. Senescent forgetfulness: benign and malignant. Can Med Assoc J 1962; 86:257–260.
- Bischkopf J, Busse A, Angermeyer MC. Mild cognitive impairment—a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand 2002; 106:403–414.
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1133–1142.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308.
- Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol 2009; 66:1447–1455.
- Bennett DA, Schneider JA, Bienias JL, et al. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology 2005; 64:834–841.
- Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 2006; 63:665–672.
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol 2003; 60:729–736.
- Molano J, Boeve B, Ferman T, et al. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain 2010; 133:540–556.
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol 2003; 60:1385–1389.
- Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology 2010; 75:889–897.
- Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol 2008; 63:494–506.
- Luck T, Luppa M, Briel S, et al. Mild cognitive impairment: incidence and risk factors: results of the Leipzig Longitudinal Study of the Aged. J Am Geriatr Soc 2010; 58:1903–1910.
- Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008; 30:58–69.
- Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol 2001; 58:411–416.
- DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol 2003; 2:15–21.
- Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet 1997; 349:1793–1796.
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:270–279.
- Tariq SH, Tumosa N, Chibnall JT, Perry MH, Morley JE. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry 2006; 14:900–910.
- Tang-Wai DF, Knopman DS, Geda YE, et al. Comparison of the short test of mental status and the Mini-Mental State Examination in mild cognitive impairment. Arch Neurol 2003; 60:1777–1781.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699.
- Banks WA, Morley JE. Memories are made of this: recent advances in understanding cognitive impairments and dementia. J Gerontol A Biol Sci Med Sci 2003; 58:314–321.
- Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med 2011; 364:2227–2234.
- Diniz BS, Pinto JA, Gonzaga MLC, Guimaraes FM, Gattaz WF, Forlenza OV. To treat or not to treat? A meta-analysis of the use of cholinesterase inhibitors in mild cognitive impairment for delaying progression to Alzheimer’s disease. Eur Arch Psychiatry Neurosci 2009; 259:248–256.
- Patel BB, Holland NW. Adverse effects of acetylcholinesterase inhibitors. Clin Geriatr 2011; 19:27–30.
- Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev 2009; 1:CD003120.
- Petersen RC, Thomas RG, Grundman M, et al; Alzheimer’s Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005; 352:2379–2388.
- Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry 2010; 18:281–296.
- Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008; 300:1027–1037.
- van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br J Sports Med 2008; 42:344–351.
- Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic-vs community-based cohorts. Arch Neurol 2009; 66:1151–1157.
- Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology 2001; 56:37–42.
- Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002; 59:1594–1599.
- Busse A, Hensel A, Gühne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology 2006; 67:2176–2185.
- Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology 2007; 68:288–291.
- Pérès K, Chrysostome V, Fabrigoule C, Orgogozo JM, Dartigues JF, Barberger-Gateau P. Restriction in complex activities of daily living in MCI: impact on outcome. Neurology 2006; 67:461–466.
- Keefover RW. Aging and cognition. Neurol Clin 1998; 16:635–648.
- Kral VA. Senescent forgetfulness: benign and malignant. Can Med Assoc J 1962; 86:257–260.
- Bischkopf J, Busse A, Angermeyer MC. Mild cognitive impairment—a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand 2002; 106:403–414.
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1133–1142.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308.
- Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol 2009; 66:1447–1455.
- Bennett DA, Schneider JA, Bienias JL, et al. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology 2005; 64:834–841.
- Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 2006; 63:665–672.
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol 2003; 60:729–736.
- Molano J, Boeve B, Ferman T, et al. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain 2010; 133:540–556.
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol 2003; 60:1385–1389.
- Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology 2010; 75:889–897.
- Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol 2008; 63:494–506.
- Luck T, Luppa M, Briel S, et al. Mild cognitive impairment: incidence and risk factors: results of the Leipzig Longitudinal Study of the Aged. J Am Geriatr Soc 2010; 58:1903–1910.
- Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008; 30:58–69.
- Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol 2001; 58:411–416.
- DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol 2003; 2:15–21.
- Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet 1997; 349:1793–1796.
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:270–279.
- Tariq SH, Tumosa N, Chibnall JT, Perry MH, Morley JE. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry 2006; 14:900–910.
- Tang-Wai DF, Knopman DS, Geda YE, et al. Comparison of the short test of mental status and the Mini-Mental State Examination in mild cognitive impairment. Arch Neurol 2003; 60:1777–1781.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699.
- Banks WA, Morley JE. Memories are made of this: recent advances in understanding cognitive impairments and dementia. J Gerontol A Biol Sci Med Sci 2003; 58:314–321.
- Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med 2011; 364:2227–2234.
- Diniz BS, Pinto JA, Gonzaga MLC, Guimaraes FM, Gattaz WF, Forlenza OV. To treat or not to treat? A meta-analysis of the use of cholinesterase inhibitors in mild cognitive impairment for delaying progression to Alzheimer’s disease. Eur Arch Psychiatry Neurosci 2009; 259:248–256.
- Patel BB, Holland NW. Adverse effects of acetylcholinesterase inhibitors. Clin Geriatr 2011; 19:27–30.
- Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev 2009; 1:CD003120.
- Petersen RC, Thomas RG, Grundman M, et al; Alzheimer’s Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005; 352:2379–2388.
- Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry 2010; 18:281–296.
- Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008; 300:1027–1037.
- van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br J Sports Med 2008; 42:344–351.
- Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic-vs community-based cohorts. Arch Neurol 2009; 66:1151–1157.
- Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology 2001; 56:37–42.
- Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002; 59:1594–1599.
- Busse A, Hensel A, Gühne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology 2006; 67:2176–2185.
- Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology 2007; 68:288–291.
- Pérès K, Chrysostome V, Fabrigoule C, Orgogozo JM, Dartigues JF, Barberger-Gateau P. Restriction in complex activities of daily living in MCI: impact on outcome. Neurology 2006; 67:461–466.
KEY POINTS
- MCI that primarily involves memory or multiple domains has a higher risk of progressing to dementia.
- Depression and the effects of anticholinergic medication can mimic MCI, and these should be looked for in patients presenting with cognitive loss.
- Impaired functional status as reflected in activities of daily living is an important sign of progression from MCI to dementia.
- Acetylcholinesterase inhibitors are not approved for treating MCI, have shown little efficacy in altering progression to dementia, and have multiple side effects.
- Enhancing physical and mental health and developing strategies to compensate for deficits are key management approaches.
Bilateral adrenal masses
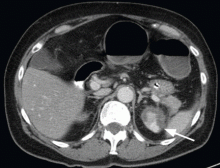
A 68-year-old woman presented to the emergency department with constipation and abdominal pain 13 days after left hip arthroplasty. Abdominal computed tomography (CT) revealed possible bowel obstruction, left renal infarction, and a thrombus in the abdominal aorta near the left renal artery (Figure 1).
Because of the aortic thrombus, anticoagulation with intravenous heparin and with warfarin (Coumadin) was started. Three days later, her platelet count decreased to 54 × 109/L (reference range 150–400), her serum creatinine rose to 2.38 mg/dL (0.70–1.40), sodium was stable at 131 mmol/L (132–148), and potassium was 4.1 mmol/L (3.5–5.0).
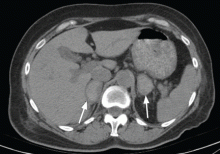
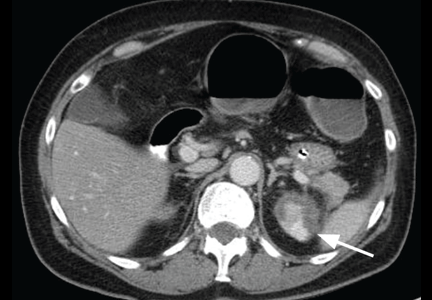
Physical examination revealed a temperature of 100.2°F (37.9°C), blood pressure 110/59 mm Hg, pulse 100 bpm, and no abdominal pain on palpation. Renal ultrasonography revealed a mass (2.4 × 2.1 × 3.6 cm) above the right kidney. Abdominal CT without contrast showed bilateral high-density adrenal masses (Figure 2).
Q: Which is the most likely diagnosis?
- Metastasis to the adrenal glands
- Adrenal adenoma
- Pheochromocytoma
- Adrenal cortical carcinoma
- Adrenal gland hemorrhage
A: The correct diagnosis is adrenal gland hemorrhage.
Acute or subacute hemorrhage typically results in an oval hyperdense mass with an attenuation of 50 to 90 Hounsfield units (H) on noncontrast CT (Figure 2),1,2 and this attenuation does not increase with the use of contrast.2
In contrast, adrenal cortical carcinoma typically appears as a large heterogeneous mass, with some lesions demonstrating central necrosis or calcification. Pheochromocytoma is well defined, with intense enhancement after contrast is given. Adenoma is usually homogenous, with well-defined margins. Many adenomas have increased intracytoplasmic lipid content and, therefore, will have an attenuation of less than 10 H or will demonstrate rapid washout of contrast. Metastasis to the adrenal gland may not have a characteristic radiographic appearance but typically has a slower contrast washout rate than adenoma.1
This patient’s initial abdominal image showed normal-appearing adrenal glands, thus making adenoma, adrenal metastasis, pheochromocytoma, and adrenal cortical carcinoma unlikely.
The patient’s baseline cortisol level, a random afternoon reading, was 0.4 μg/dL (reference range 3.4–26.9), and a 1-hour cortrosyn-stimulated cortisol was 0 μg/dL, which is diagnostic of primary adrenal insufficiency in the context of this clinical setting. She received hydrocortisone and fludrocortisone, and 8 am cortisol measurements 3 weeks and 5 months later, after the patient was off hydrocortisone for 24 hours, remained undetectable.
The diagnosis of adrenal hemorrhage can be difficult because the symptoms can be nonspecific and attributable to other clinical factors. In a review of 141 patients with adrenal hemorrhage,3 only 19% of patients with bilateral adrenal hemorrhage developed hypotension with a systolic blood pressure less than 90 mm Hg, only 15% developed hyponatremia (sodium < 130 mmol/L), and only 24% developed hyperkalemia (potassium > 5 mmol/L).3
If unrecognized, adrenal insufficiency from adrenal hemorrhage is fatal. Abdominal CT or magnetic resonance imaging can diagnose adrenal hemorrhage. Adrenal function may recover (although it did not in this patient), and a morning cortisol level should be obtained to reevaluate adrenal function.3
Risk factors for adrenal hemorrhage include anticoagulation therapy, sepsis, surgery, hypotension, and coagulopathy as seen in heparininduced thrombocytopenia and disseminated intravascular coagulation. This patient had coagulopathy, as evidenced by her abdominal aortic thrombus, for which she was placed on anticoagulation. Patients without these risk factors for adrenal gland hemorrhage should be investigated for an underlying adrenal neoplasm or cyst.2
- Dunnick NR, Korobkin M. Imaging of adrenal incidentalomas: current status. AJR Am J Roentgenol 2002; 179:559–568.
- Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics 1999; 19:949–963.
- Vella A, Nippoldt TB, Morris JC. Adrenal hemorrhage: a 25-year experience at the Mayo Clinic. Mayo Clin Proc 2001; 76:161–168.
A 68-year-old woman presented to the emergency department with constipation and abdominal pain 13 days after left hip arthroplasty. Abdominal computed tomography (CT) revealed possible bowel obstruction, left renal infarction, and a thrombus in the abdominal aorta near the left renal artery (Figure 1).
Because of the aortic thrombus, anticoagulation with intravenous heparin and with warfarin (Coumadin) was started. Three days later, her platelet count decreased to 54 × 109/L (reference range 150–400), her serum creatinine rose to 2.38 mg/dL (0.70–1.40), sodium was stable at 131 mmol/L (132–148), and potassium was 4.1 mmol/L (3.5–5.0).
Physical examination revealed a temperature of 100.2°F (37.9°C), blood pressure 110/59 mm Hg, pulse 100 bpm, and no abdominal pain on palpation. Renal ultrasonography revealed a mass (2.4 × 2.1 × 3.6 cm) above the right kidney. Abdominal CT without contrast showed bilateral high-density adrenal masses (Figure 2).
Q: Which is the most likely diagnosis?
- Metastasis to the adrenal glands
- Adrenal adenoma
- Pheochromocytoma
- Adrenal cortical carcinoma
- Adrenal gland hemorrhage
A: The correct diagnosis is adrenal gland hemorrhage.
Acute or subacute hemorrhage typically results in an oval hyperdense mass with an attenuation of 50 to 90 Hounsfield units (H) on noncontrast CT (Figure 2),1,2 and this attenuation does not increase with the use of contrast.2
In contrast, adrenal cortical carcinoma typically appears as a large heterogeneous mass, with some lesions demonstrating central necrosis or calcification. Pheochromocytoma is well defined, with intense enhancement after contrast is given. Adenoma is usually homogenous, with well-defined margins. Many adenomas have increased intracytoplasmic lipid content and, therefore, will have an attenuation of less than 10 H or will demonstrate rapid washout of contrast. Metastasis to the adrenal gland may not have a characteristic radiographic appearance but typically has a slower contrast washout rate than adenoma.1
This patient’s initial abdominal image showed normal-appearing adrenal glands, thus making adenoma, adrenal metastasis, pheochromocytoma, and adrenal cortical carcinoma unlikely.
The patient’s baseline cortisol level, a random afternoon reading, was 0.4 μg/dL (reference range 3.4–26.9), and a 1-hour cortrosyn-stimulated cortisol was 0 μg/dL, which is diagnostic of primary adrenal insufficiency in the context of this clinical setting. She received hydrocortisone and fludrocortisone, and 8 am cortisol measurements 3 weeks and 5 months later, after the patient was off hydrocortisone for 24 hours, remained undetectable.
The diagnosis of adrenal hemorrhage can be difficult because the symptoms can be nonspecific and attributable to other clinical factors. In a review of 141 patients with adrenal hemorrhage,3 only 19% of patients with bilateral adrenal hemorrhage developed hypotension with a systolic blood pressure less than 90 mm Hg, only 15% developed hyponatremia (sodium < 130 mmol/L), and only 24% developed hyperkalemia (potassium > 5 mmol/L).3
If unrecognized, adrenal insufficiency from adrenal hemorrhage is fatal. Abdominal CT or magnetic resonance imaging can diagnose adrenal hemorrhage. Adrenal function may recover (although it did not in this patient), and a morning cortisol level should be obtained to reevaluate adrenal function.3
Risk factors for adrenal hemorrhage include anticoagulation therapy, sepsis, surgery, hypotension, and coagulopathy as seen in heparininduced thrombocytopenia and disseminated intravascular coagulation. This patient had coagulopathy, as evidenced by her abdominal aortic thrombus, for which she was placed on anticoagulation. Patients without these risk factors for adrenal gland hemorrhage should be investigated for an underlying adrenal neoplasm or cyst.2
A 68-year-old woman presented to the emergency department with constipation and abdominal pain 13 days after left hip arthroplasty. Abdominal computed tomography (CT) revealed possible bowel obstruction, left renal infarction, and a thrombus in the abdominal aorta near the left renal artery (Figure 1).
Because of the aortic thrombus, anticoagulation with intravenous heparin and with warfarin (Coumadin) was started. Three days later, her platelet count decreased to 54 × 109/L (reference range 150–400), her serum creatinine rose to 2.38 mg/dL (0.70–1.40), sodium was stable at 131 mmol/L (132–148), and potassium was 4.1 mmol/L (3.5–5.0).
Physical examination revealed a temperature of 100.2°F (37.9°C), blood pressure 110/59 mm Hg, pulse 100 bpm, and no abdominal pain on palpation. Renal ultrasonography revealed a mass (2.4 × 2.1 × 3.6 cm) above the right kidney. Abdominal CT without contrast showed bilateral high-density adrenal masses (Figure 2).
Q: Which is the most likely diagnosis?
- Metastasis to the adrenal glands
- Adrenal adenoma
- Pheochromocytoma
- Adrenal cortical carcinoma
- Adrenal gland hemorrhage
A: The correct diagnosis is adrenal gland hemorrhage.
Acute or subacute hemorrhage typically results in an oval hyperdense mass with an attenuation of 50 to 90 Hounsfield units (H) on noncontrast CT (Figure 2),1,2 and this attenuation does not increase with the use of contrast.2
In contrast, adrenal cortical carcinoma typically appears as a large heterogeneous mass, with some lesions demonstrating central necrosis or calcification. Pheochromocytoma is well defined, with intense enhancement after contrast is given. Adenoma is usually homogenous, with well-defined margins. Many adenomas have increased intracytoplasmic lipid content and, therefore, will have an attenuation of less than 10 H or will demonstrate rapid washout of contrast. Metastasis to the adrenal gland may not have a characteristic radiographic appearance but typically has a slower contrast washout rate than adenoma.1
This patient’s initial abdominal image showed normal-appearing adrenal glands, thus making adenoma, adrenal metastasis, pheochromocytoma, and adrenal cortical carcinoma unlikely.
The patient’s baseline cortisol level, a random afternoon reading, was 0.4 μg/dL (reference range 3.4–26.9), and a 1-hour cortrosyn-stimulated cortisol was 0 μg/dL, which is diagnostic of primary adrenal insufficiency in the context of this clinical setting. She received hydrocortisone and fludrocortisone, and 8 am cortisol measurements 3 weeks and 5 months later, after the patient was off hydrocortisone for 24 hours, remained undetectable.
The diagnosis of adrenal hemorrhage can be difficult because the symptoms can be nonspecific and attributable to other clinical factors. In a review of 141 patients with adrenal hemorrhage,3 only 19% of patients with bilateral adrenal hemorrhage developed hypotension with a systolic blood pressure less than 90 mm Hg, only 15% developed hyponatremia (sodium < 130 mmol/L), and only 24% developed hyperkalemia (potassium > 5 mmol/L).3
If unrecognized, adrenal insufficiency from adrenal hemorrhage is fatal. Abdominal CT or magnetic resonance imaging can diagnose adrenal hemorrhage. Adrenal function may recover (although it did not in this patient), and a morning cortisol level should be obtained to reevaluate adrenal function.3
Risk factors for adrenal hemorrhage include anticoagulation therapy, sepsis, surgery, hypotension, and coagulopathy as seen in heparininduced thrombocytopenia and disseminated intravascular coagulation. This patient had coagulopathy, as evidenced by her abdominal aortic thrombus, for which she was placed on anticoagulation. Patients without these risk factors for adrenal gland hemorrhage should be investigated for an underlying adrenal neoplasm or cyst.2
- Dunnick NR, Korobkin M. Imaging of adrenal incidentalomas: current status. AJR Am J Roentgenol 2002; 179:559–568.
- Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics 1999; 19:949–963.
- Vella A, Nippoldt TB, Morris JC. Adrenal hemorrhage: a 25-year experience at the Mayo Clinic. Mayo Clin Proc 2001; 76:161–168.
- Dunnick NR, Korobkin M. Imaging of adrenal incidentalomas: current status. AJR Am J Roentgenol 2002; 179:559–568.
- Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics 1999; 19:949–963.
- Vella A, Nippoldt TB, Morris JC. Adrenal hemorrhage: a 25-year experience at the Mayo Clinic. Mayo Clin Proc 2001; 76:161–168.
Lupus in Hispanics: A matter of serious concern
Some diseases are either more serious or more frequent in US Hispanics, and systemic lupus erythematosus is one of them. This fact has not yet diffused to all providers, many of whom will be the ones dealing with these individuals when the disease first emerges.
In order to raise physicians’ awareness of this situation, we will briefly review here the salient features of lupus in US Hispanics and its short-term and long-term impact.
HISPANICS ARE THE LARGEST MINORITY IN THE UNITED STATES
Over the last 30 years, the Hispanic population in the United States has increased to the point that it is now the largest US minority group, and the fastest-growing. In the 2010 US census, Hispanics surpassed the 50 million mark.1 Physicians and health care providers are becoming familiar with this growing population and its ailments, but more needs to be done to familiarize them with specific conditions that are more frequent and more serious in US Hispanics.
No population-based study has yet defined the prevalence and incidence of lupus in US Hispanics. However, on the basis of hospital and outpatient visits in regions in which Hispanics make up a large part of the population, it has been inferred that this group has a higher frequency of lupus, probably as high as in African Americans.
Likewise, clinicians taking care of these patients have suspected that lupus is more severe in US Hispanics than in non-Hispanic Caucasians, but this was documented and brought to general attention only with the publication of reports from the Lupus in Minorities: Nature versus Nurture (LUMINA) study.2
LUMINA, a longitudinal study
LUMINA is a longitudinal study of 640 patients with lupus from four populations: Hispanic from Texas, Hispanic from Puerto Rico, African American, and Caucasian non-Hispanic (Table 1). At the time of recruitment, patients were at least 16 years old and had had lupus for 5 years or less. They come in for periodic visits to the University of Alabama at Birmingham, the University of Texas Health Science Center at Houston, and the University of Puerto Rico Medical Sciences Campus. Recruitment began in 1994 and finished in 2007. Follow-up ranges from 1 to 14 years, with a mean of 4.5 years.
LUMINA is supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institutes of Health General Clinical Research Centers program, the National Center for Research Resources Clinical Research Infrastructure Initiative, the Mary Kirkland Center for Lupus Research Scholars Program, and Rheuminations Inc (New York, NY).
The purpose of the study is to shed light on the interplay of genetics and environment in this disease and, in the process, to raise awareness about the problem of lupus in Hispanics. In fact, much of the information in the following sections is from the LUMINA study.
HISPANICS ARE NOT A HOMOGENEOUS GROUP
In the United States, the term Hispanic describes anyone whose origin goes back to a Spanish-speaking country. However, US Hispanics are not a homogeneous racial group: they differ in genetics, culture, and problems.
The largest US Hispanic subgroup and the one more likely to be seen by US physicians is Hispanics of Mexican origin, who account for 66% of all US Hispanics. This group has a higher percentage of Amerindian genes than those of Puerto Rican ancestry.3 LUMINA researchers analyzed the DNA of 492 patients and found the following mixtures of genes3:
- Hispanics in Texas (mostly of Mexican origin): 48% Amerindian, 18% African, 34% European
- Hispanics from Puerto Rico: 20% Amerindian, 45% African, 35% European
- African Americans: 0% Amerindian, 79% African, 21% European
- Non-Hispanic Caucasians: 10% Amerindian, 18% African, 72% European.
Latin Americans of mixed European and Amerindian ancestry (which includes Aztec, Mayan, Quechuan, Aymaran, and other Central and South American groups) are called mestizos. Not all people in Latin America are mestizos: some are of European, African, or Asian ancestry, but in the United States they are all called Hispanics.
LUPUS DIFFERS AMONG SUBGROUPS
LUMINA research has revealed that lupus is heterogeneous also among US Hispanic subgroups. When people from Puerto Rico get lupus, it is generally less serious and devastating than in those from Mexico or Central America. Since US Hispanics of Mexican or Central American origin possess more Amerindian genes, this observation supports the notion that these genes are important contributors to the occurrence and expression of the disease.
Amerindian genes contribute to a greater susceptibility to lupus,4,5 although there is an interplay between genetic and nongenetic factors in the etiology and expression.6 Lupus starts at a younger age in Hispanics of predominantly Amerindian ancestry than in non-Hispanic Caucasians, and the onset is more likely to be acute.7
Renal involvement in these patients8 and mestizos from Latin America is rather common, probably as common as it is in US African Americans, and it tends to develop earlier than in non-Hispanic Caucasians.9 Amerindian ancestral genes, like African genes, contribute to the occurrence of renal disease in lupus patients.4 Furthermore, once nephritis ensues, end-stage renal disease occurs more often in US Hispanic and African American than in non-Hispanic Caucasian children, as demonstrated by Hiraki et al10 using national databases, and the same is true in adults, as shown in the LUMINA cohort.11
Other potentially serious manifestations of the disease are also more common, including hematologic and central nervous system manifestations. Not surprisingly, then, these patients show a higher degree of disease activity, both early in the course of the disease12,13 and over time.14
Table 1 compares the demographic and clinical features of LUMINA patients according to ethnicity. By and large, Hispanics from Texas have lower levels of education and income (comparable with levels in African Americans), and this can adversely affect the disease course by limiting these patients’ access to adequate care.15
DISEASE ACTIVITY AND ORGAN DAMAGE ARE GREATER IN HISPANICS
Disease activity in lupus reflects the ongoing immune-mediated inflammatory process. In LUMINA patients, regardless of the time at which disease activity was ascertained, it was higher in Hispanics from Texas and in African Americans than in non-Hispanic Caucasians and in Hispanics from Puerto Rico.7,12,16–18 Similar findings were seen in the Grupo Latinoamericano de Estudio de Lupus (GLADEL) cohort,13 in which mestizos and Hispanics of mixed African and European ancestry had higher maximum disease activity scores than non-Hispanic Caucasians.13
In addition, organ damage in lupus—the irreversible changes that occur in organ systems as a consequence of the disease or its treatments (eg, glucocorticoids, immunosuppressive drugs)—is more severe and develops sooner in Hispanics from Texas than in other groups.6,18,19 Using multivariate analysis, LUMINA investigators19 estimated the hazard ratio for the time until organ damage appeared for various risk factors, with values of 1 or greater indicating a shorter time and lower values indicating a longer time. Being a Hispanic from Texas carried a hazard ratio of 2.11 (95% confidence interval 1.15–3.88).
Because organ damage is an important and independent predictor of further damage20 and death,21 physicians need to take this disease quite seriously and try to prevent damage early in people at risk. To achieve that, the need to control disease activity must be balanced against the risk of overtreatment, as the important contribution of glucocorticoids to organ damage is well recognized.22
HISPANICS HAVE MORE COMORBIDITIES
Obesity, hypertension, diabetes, and metabolic syndrome are more common in US Hispanics, particularly those of Amerindian ancestry, than in the majority population of non-Hispanic Caucasians.23,24 The potential deleterious effects of glucocorticoids in patients already predisposed to these conditions need to be considered, balancing adequate disease control against the potential adverse effects.22
QUALITY OF LIFE IS WORSE WITH LUPUS
Whether it is measured with a generic instrument such as the Short Form 36 (SF-36), as it was in LUMINA,25 or with a disease-specific tool such as the Lupus-Pro, quality of life is significantly worsened by lupus. Furthermore, Fernandez et al26 found that a low level of health-related quality of life, as measured by the SF-6D version of the SF-36, was predictive of poor outcomes in LUMINA patients.
POVERTY, NOT ETHNICITY, ACCOUNTS FOR HIGHER MORTALITY RATE
As yet, we have no population-based data comparing survival in US Hispanic patients with lupus vs that of other population groups.
At first inspection, data from LUMINA indicate that Hispanics of primarily Amerindian ancestry have a lower survival rate than patients in other ethnic groups (Figure 1).6 However, when all other factors are taken into consideration, poverty, not ethnicity, is the major contributing factor (Table 2).6,27
This finding illustrates the important interplay between genetic and nongenetic factors in the course and final outcome of lupus, as already alluded to, although the exact relationship between them is not clear. It remains to be determined whether poverty is only a proxy for other population characteristics such as illiteracy, limited access to specialized care, limited access to medications, or cultural beliefs that may interfere with proper care.
ANTIMALARIAL DRUGS INCREASE SURVIVAL
Using statistical analysis that adjusts for confounding by indication, we and others28–30 have shown that antimalarial drugs exert an independent and important protective effect on survival in lupus (Figure 2).
Important also is the protective effect of antimalarials on organ damage and the possibility of using them from disease outset in Hispanic patients at risk of early and rapid damage accrual,11 renal damage, and even lupus nephritis.31,32 This has very practical implications for the adequate and prompt management of these Hispanic patients.
PRACTICAL IMPLICATIONS
Lupus in US Hispanics is a serious disease with devastating consequences. Prompt diagnosis is paramount to prevent early organ damage and to prolong survival.
The disease may present in many different and unexpected ways, but joint pain, sun-sensitive rashes, renal involvement, cytopenias, and other manifestations should prompt the clinician to consider lupus in the differential diagnosis. Patients are often dismissed as having “arthritis” without being asked about other manifestations that may suggest a systemic connective tissue disease such as lupus. The same goes for skin rashes or unusual central nervous system manifestations.
The diagnosis of lupus is clinical, but some laboratory studies are essential to rule in or rule out renal or hematologic abnormalities and determine the level of disease activity. Tests usually ordered in patients suspected of having lupus include antinuclear antibody, complement levels, a complete blood cell count and differential, and a urinalysis. The need for additional tests depends on the results of the tests listed.
Once the disease is diagnosed, treatment should be tailored to the severity and type of clinical manifestations present. In general, glucocorticoids should be used at the smallest possible dose, antimalarials should be prescribed from the outset to all patients (following current guidelines in order to avoid ocular toxicity),33 and immunosuppressants and other treatments should be considered in certain instances. In parallel, consideration should be given to sun protection, adequate exercise, tobacco avoidance, osteoporosis and atherosclerosis prevention, planned conception, and compliance.
The goal in these people at risk is to control their lupus manifestations without causing undue damage, to preserve their quality of life, and to prevent an early demise.
- Humes KR, Jones NA, Ramirez RR. Overview of race and Hispanic origin: 2010. 2010 Census briefs; 2011. http://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf. Accessed October 20, 2012.
- Reveille JD, Moulds JM, Ahn C, et al; for the LUMINA study Group. Systemic lupus erythematosus in three ethnic groups. I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity and disease onset. Arthritis Rheum 1998; 41:1161–1172.
- Alarcón GS, Beasley TM, Roseman JM, et al; LUMINA Study Group. Ethnic disparities in health and disease: the need to account for ancestral admixture when estimating the genetic contribution to both (LUMINA XXVI) (Letter). Lupus 2005; 14:867–868.
- Alarcón GS, Bastian HM, Beasley TM, et al; LUMINA Study Group. Systemic lupus erythematosus in a multi-ethnic cohort (LUMINA) XXXII: [corrected] contributions of admixture and socioeconomic status to renal involvement. Lupus 2006; 15:26–31.
- Sanchez E, Webb RD, Rasmussen A, et al. Genetically determined Amerindian ancestry correlates with increased frequency of risk alleles for systemic lupus erythematosus. Arthritis Rheum 2010; 62:3722–3729.
- Fernández M, Alarcón GS, Calvo-Alén J, et al; LUMINA Study Group. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum 2007; 57:576–584.
- Alarcón GS, Friedman AW, Straaton KV, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs Nurture. Lupus 1999; 8:197–209.
- Bastian HM, Alarcón GS, Roseman JM, et al; LUMINA Study Group. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA) XL II: factors predictive of new or worsening proteinuria. Rheumatology (Oxford) 2007; 46:683–689.
- Burgos PI, McGwin G, Pons-Estel GJ, Reveille JD, Alarcón GS, Vilá LM. US patients of Hispanic and African ancestry develop lupus nephritis early in the disease course: data from LUMINA, a multiethnic US cohort (LUMINA LXXIV). Ann Rheum Dis 2011; 70:393–394.
- Hiraki LT, Lu B, Alexander SR, et al. End-stage renal disease due to lupus nephritis among children in the US, 1995–2006. Arthritis Rheum 2011; 63:1988–1997.
- Pons-Estel GJ, Alarcón GS, McGwin G, et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum 2009; 61:830–839.
- Alarcón GS, Roseman J, Bartolucci AA, et al. Systemic lupus erythematosus in three ethnic groups: II. Features predictive of disease activity early in its course. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum 1998; 41:1173–1180.
- Pons-Estel BA, Catoggio LJ, Cardiel MH, et al; Grupo Latinoamericano de Estudio del Lupus. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics.” Medicine (Baltimore) 2004; 83:1–17.
- Alarcón GS, Calvo-Alén J, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in a multiethnic cohort: LUMINA XXXV. Predictive factors of high disease activity over time. Ann Rheum Dis 2006; 65:1168–1174.
- Vilá LM, Alarcón GS, McGwin G, Bastian HM, Fessler BJ, Reveille JD; Lumina Study Group. Systemic lupus erythematosus in a multiethnic US cohort, XXXVII: association of lymphopenia with clinical manifestations, serologic abnormalities, disease activity, and damage accrual. Arthritis Rheum 2006; 55:799–806.
- Zhang J, González LA, Roseman JM, Vilá LM, Reveille JD, Alárcon GS. Predictors of the rate of change in disease activity over time in LUMINA, a multiethnic US cohort of patients with systemic lupus erythematosus: LUMINA LXX. Lupus 2010; 19:727–733.
- Vilá LM, Alarcón GS, McGwin G, et al; LUMINA Study Group. Early clinical manifestations, disease activity and damage of systemic lupus erythematosus among two distinct US Hispanic subpopulations. Rheumatology (Oxford) 2004; 43:358–363.
- Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996; 39:363–369.
- Toloza SM, Roseman JM, Alarcón GS, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA): XXII. Predictors of time to the occurrence of initial damage. Arthritis Rheum 2004; 50:3177–3186.
- Alarcón GS, Roseman JM, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups. XX. Damage as a predictor of further damage. Rheumatology (Oxford) 2004; 43:202–205.
- Alarcón GS, McGwin G, Bastian HM, et al. Systemic lupus erythematosus in three ethnic groups. VII [correction of VIII]. Predictors of early mortality in the LUMINA cohort. LUMINA Study Group. Arthritis Rheum 2001; 45:191–202.
- Ruiz-Irastorza G, Danza A, Khamashta M. Glucocorticoid use and abuse in SLE. Rheumatology (Oxford) 2012 E-pub ahead of print.
- Jordan HT, Tabaei BP, Nash D, Angell SY, Chamany S, Kerker B. Metabolic syndrome among adults in New York City, 2004 New York City Health and Nutrition Examination Survey. Prev Chronic Dis 2012; 9:E04.
- Matthews KA, Sowers MF, Derby CA, et al. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women’s Health Across the Nation (SWAN). Am Heart J 2005; 149:1066–1073.
- Alarcón GS, McGwin G, Uribe A, et al. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of selfreported health-related quality of life early in the disease course. Arthritis Rheum 2004; 51:465–474.
- Fernández M, Alarcón GS, McGwin G, et al; LUMINA Study Group. Using the Short Form 6D, as an overall measure of health, to predict damage accrual and mortality in patients with systemic lupus erythematosus: XLVII, results from a multiethnic US cohort. Arthritis Rheum 2007; 57:986–992.
- Durán S, Apte M, Alarcón GSLUMINA Study Group. Poverty, not ethnicity, accounts for the differential mortality rates among lupus patients of various ethnic groups. J Natl Med Assoc 2007; 99:1196–1198.
- Ruiz-Irastorza G, Egurbide MV, Pijoan JI, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus 2006; 15:577–583.
- Alarcón GS, McGwin G, Bertoli AM, et al; LUMINA Study Group. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 2007; 66:1168–1172.
- Shinjo SK, Bonfá E, Wojdyla D, et al; Grupo Latino Americano de Estudio del Lupus Eritematoso (Gladel). Antimalarial treatment may have a time-dependent effect on lupus survival: data from a multinational Latin American inception cohort. Arthritis Rheum 2010; 62:855–862.
- Fessler BJ, Alarcón GS, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum 2005; 52:1473–1480.
- Pons-Estel GJ, Alarcón GS, Hachuel L, et al. Antimalarials have a protective effect against the development of renal disease in Latin American SLE patients. The 9th International Congress on SLE June 24–27, 2010, Vancouver, Canada. Lupus 2010; 19(suppl 1):31–32.
- Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010; 69:20–28.
Some diseases are either more serious or more frequent in US Hispanics, and systemic lupus erythematosus is one of them. This fact has not yet diffused to all providers, many of whom will be the ones dealing with these individuals when the disease first emerges.
In order to raise physicians’ awareness of this situation, we will briefly review here the salient features of lupus in US Hispanics and its short-term and long-term impact.
HISPANICS ARE THE LARGEST MINORITY IN THE UNITED STATES
Over the last 30 years, the Hispanic population in the United States has increased to the point that it is now the largest US minority group, and the fastest-growing. In the 2010 US census, Hispanics surpassed the 50 million mark.1 Physicians and health care providers are becoming familiar with this growing population and its ailments, but more needs to be done to familiarize them with specific conditions that are more frequent and more serious in US Hispanics.
No population-based study has yet defined the prevalence and incidence of lupus in US Hispanics. However, on the basis of hospital and outpatient visits in regions in which Hispanics make up a large part of the population, it has been inferred that this group has a higher frequency of lupus, probably as high as in African Americans.
Likewise, clinicians taking care of these patients have suspected that lupus is more severe in US Hispanics than in non-Hispanic Caucasians, but this was documented and brought to general attention only with the publication of reports from the Lupus in Minorities: Nature versus Nurture (LUMINA) study.2
LUMINA, a longitudinal study
LUMINA is a longitudinal study of 640 patients with lupus from four populations: Hispanic from Texas, Hispanic from Puerto Rico, African American, and Caucasian non-Hispanic (Table 1). At the time of recruitment, patients were at least 16 years old and had had lupus for 5 years or less. They come in for periodic visits to the University of Alabama at Birmingham, the University of Texas Health Science Center at Houston, and the University of Puerto Rico Medical Sciences Campus. Recruitment began in 1994 and finished in 2007. Follow-up ranges from 1 to 14 years, with a mean of 4.5 years.
LUMINA is supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institutes of Health General Clinical Research Centers program, the National Center for Research Resources Clinical Research Infrastructure Initiative, the Mary Kirkland Center for Lupus Research Scholars Program, and Rheuminations Inc (New York, NY).
The purpose of the study is to shed light on the interplay of genetics and environment in this disease and, in the process, to raise awareness about the problem of lupus in Hispanics. In fact, much of the information in the following sections is from the LUMINA study.
HISPANICS ARE NOT A HOMOGENEOUS GROUP
In the United States, the term Hispanic describes anyone whose origin goes back to a Spanish-speaking country. However, US Hispanics are not a homogeneous racial group: they differ in genetics, culture, and problems.
The largest US Hispanic subgroup and the one more likely to be seen by US physicians is Hispanics of Mexican origin, who account for 66% of all US Hispanics. This group has a higher percentage of Amerindian genes than those of Puerto Rican ancestry.3 LUMINA researchers analyzed the DNA of 492 patients and found the following mixtures of genes3:
- Hispanics in Texas (mostly of Mexican origin): 48% Amerindian, 18% African, 34% European
- Hispanics from Puerto Rico: 20% Amerindian, 45% African, 35% European
- African Americans: 0% Amerindian, 79% African, 21% European
- Non-Hispanic Caucasians: 10% Amerindian, 18% African, 72% European.
Latin Americans of mixed European and Amerindian ancestry (which includes Aztec, Mayan, Quechuan, Aymaran, and other Central and South American groups) are called mestizos. Not all people in Latin America are mestizos: some are of European, African, or Asian ancestry, but in the United States they are all called Hispanics.
LUPUS DIFFERS AMONG SUBGROUPS
LUMINA research has revealed that lupus is heterogeneous also among US Hispanic subgroups. When people from Puerto Rico get lupus, it is generally less serious and devastating than in those from Mexico or Central America. Since US Hispanics of Mexican or Central American origin possess more Amerindian genes, this observation supports the notion that these genes are important contributors to the occurrence and expression of the disease.
Amerindian genes contribute to a greater susceptibility to lupus,4,5 although there is an interplay between genetic and nongenetic factors in the etiology and expression.6 Lupus starts at a younger age in Hispanics of predominantly Amerindian ancestry than in non-Hispanic Caucasians, and the onset is more likely to be acute.7
Renal involvement in these patients8 and mestizos from Latin America is rather common, probably as common as it is in US African Americans, and it tends to develop earlier than in non-Hispanic Caucasians.9 Amerindian ancestral genes, like African genes, contribute to the occurrence of renal disease in lupus patients.4 Furthermore, once nephritis ensues, end-stage renal disease occurs more often in US Hispanic and African American than in non-Hispanic Caucasian children, as demonstrated by Hiraki et al10 using national databases, and the same is true in adults, as shown in the LUMINA cohort.11
Other potentially serious manifestations of the disease are also more common, including hematologic and central nervous system manifestations. Not surprisingly, then, these patients show a higher degree of disease activity, both early in the course of the disease12,13 and over time.14
Table 1 compares the demographic and clinical features of LUMINA patients according to ethnicity. By and large, Hispanics from Texas have lower levels of education and income (comparable with levels in African Americans), and this can adversely affect the disease course by limiting these patients’ access to adequate care.15
DISEASE ACTIVITY AND ORGAN DAMAGE ARE GREATER IN HISPANICS
Disease activity in lupus reflects the ongoing immune-mediated inflammatory process. In LUMINA patients, regardless of the time at which disease activity was ascertained, it was higher in Hispanics from Texas and in African Americans than in non-Hispanic Caucasians and in Hispanics from Puerto Rico.7,12,16–18 Similar findings were seen in the Grupo Latinoamericano de Estudio de Lupus (GLADEL) cohort,13 in which mestizos and Hispanics of mixed African and European ancestry had higher maximum disease activity scores than non-Hispanic Caucasians.13
In addition, organ damage in lupus—the irreversible changes that occur in organ systems as a consequence of the disease or its treatments (eg, glucocorticoids, immunosuppressive drugs)—is more severe and develops sooner in Hispanics from Texas than in other groups.6,18,19 Using multivariate analysis, LUMINA investigators19 estimated the hazard ratio for the time until organ damage appeared for various risk factors, with values of 1 or greater indicating a shorter time and lower values indicating a longer time. Being a Hispanic from Texas carried a hazard ratio of 2.11 (95% confidence interval 1.15–3.88).
Because organ damage is an important and independent predictor of further damage20 and death,21 physicians need to take this disease quite seriously and try to prevent damage early in people at risk. To achieve that, the need to control disease activity must be balanced against the risk of overtreatment, as the important contribution of glucocorticoids to organ damage is well recognized.22
HISPANICS HAVE MORE COMORBIDITIES
Obesity, hypertension, diabetes, and metabolic syndrome are more common in US Hispanics, particularly those of Amerindian ancestry, than in the majority population of non-Hispanic Caucasians.23,24 The potential deleterious effects of glucocorticoids in patients already predisposed to these conditions need to be considered, balancing adequate disease control against the potential adverse effects.22
QUALITY OF LIFE IS WORSE WITH LUPUS
Whether it is measured with a generic instrument such as the Short Form 36 (SF-36), as it was in LUMINA,25 or with a disease-specific tool such as the Lupus-Pro, quality of life is significantly worsened by lupus. Furthermore, Fernandez et al26 found that a low level of health-related quality of life, as measured by the SF-6D version of the SF-36, was predictive of poor outcomes in LUMINA patients.
POVERTY, NOT ETHNICITY, ACCOUNTS FOR HIGHER MORTALITY RATE
As yet, we have no population-based data comparing survival in US Hispanic patients with lupus vs that of other population groups.
At first inspection, data from LUMINA indicate that Hispanics of primarily Amerindian ancestry have a lower survival rate than patients in other ethnic groups (Figure 1).6 However, when all other factors are taken into consideration, poverty, not ethnicity, is the major contributing factor (Table 2).6,27
This finding illustrates the important interplay between genetic and nongenetic factors in the course and final outcome of lupus, as already alluded to, although the exact relationship between them is not clear. It remains to be determined whether poverty is only a proxy for other population characteristics such as illiteracy, limited access to specialized care, limited access to medications, or cultural beliefs that may interfere with proper care.
ANTIMALARIAL DRUGS INCREASE SURVIVAL
Using statistical analysis that adjusts for confounding by indication, we and others28–30 have shown that antimalarial drugs exert an independent and important protective effect on survival in lupus (Figure 2).
Important also is the protective effect of antimalarials on organ damage and the possibility of using them from disease outset in Hispanic patients at risk of early and rapid damage accrual,11 renal damage, and even lupus nephritis.31,32 This has very practical implications for the adequate and prompt management of these Hispanic patients.
PRACTICAL IMPLICATIONS
Lupus in US Hispanics is a serious disease with devastating consequences. Prompt diagnosis is paramount to prevent early organ damage and to prolong survival.
The disease may present in many different and unexpected ways, but joint pain, sun-sensitive rashes, renal involvement, cytopenias, and other manifestations should prompt the clinician to consider lupus in the differential diagnosis. Patients are often dismissed as having “arthritis” without being asked about other manifestations that may suggest a systemic connective tissue disease such as lupus. The same goes for skin rashes or unusual central nervous system manifestations.
The diagnosis of lupus is clinical, but some laboratory studies are essential to rule in or rule out renal or hematologic abnormalities and determine the level of disease activity. Tests usually ordered in patients suspected of having lupus include antinuclear antibody, complement levels, a complete blood cell count and differential, and a urinalysis. The need for additional tests depends on the results of the tests listed.
Once the disease is diagnosed, treatment should be tailored to the severity and type of clinical manifestations present. In general, glucocorticoids should be used at the smallest possible dose, antimalarials should be prescribed from the outset to all patients (following current guidelines in order to avoid ocular toxicity),33 and immunosuppressants and other treatments should be considered in certain instances. In parallel, consideration should be given to sun protection, adequate exercise, tobacco avoidance, osteoporosis and atherosclerosis prevention, planned conception, and compliance.
The goal in these people at risk is to control their lupus manifestations without causing undue damage, to preserve their quality of life, and to prevent an early demise.
Some diseases are either more serious or more frequent in US Hispanics, and systemic lupus erythematosus is one of them. This fact has not yet diffused to all providers, many of whom will be the ones dealing with these individuals when the disease first emerges.
In order to raise physicians’ awareness of this situation, we will briefly review here the salient features of lupus in US Hispanics and its short-term and long-term impact.
HISPANICS ARE THE LARGEST MINORITY IN THE UNITED STATES
Over the last 30 years, the Hispanic population in the United States has increased to the point that it is now the largest US minority group, and the fastest-growing. In the 2010 US census, Hispanics surpassed the 50 million mark.1 Physicians and health care providers are becoming familiar with this growing population and its ailments, but more needs to be done to familiarize them with specific conditions that are more frequent and more serious in US Hispanics.
No population-based study has yet defined the prevalence and incidence of lupus in US Hispanics. However, on the basis of hospital and outpatient visits in regions in which Hispanics make up a large part of the population, it has been inferred that this group has a higher frequency of lupus, probably as high as in African Americans.
Likewise, clinicians taking care of these patients have suspected that lupus is more severe in US Hispanics than in non-Hispanic Caucasians, but this was documented and brought to general attention only with the publication of reports from the Lupus in Minorities: Nature versus Nurture (LUMINA) study.2
LUMINA, a longitudinal study
LUMINA is a longitudinal study of 640 patients with lupus from four populations: Hispanic from Texas, Hispanic from Puerto Rico, African American, and Caucasian non-Hispanic (Table 1). At the time of recruitment, patients were at least 16 years old and had had lupus for 5 years or less. They come in for periodic visits to the University of Alabama at Birmingham, the University of Texas Health Science Center at Houston, and the University of Puerto Rico Medical Sciences Campus. Recruitment began in 1994 and finished in 2007. Follow-up ranges from 1 to 14 years, with a mean of 4.5 years.
LUMINA is supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institutes of Health General Clinical Research Centers program, the National Center for Research Resources Clinical Research Infrastructure Initiative, the Mary Kirkland Center for Lupus Research Scholars Program, and Rheuminations Inc (New York, NY).
The purpose of the study is to shed light on the interplay of genetics and environment in this disease and, in the process, to raise awareness about the problem of lupus in Hispanics. In fact, much of the information in the following sections is from the LUMINA study.
HISPANICS ARE NOT A HOMOGENEOUS GROUP
In the United States, the term Hispanic describes anyone whose origin goes back to a Spanish-speaking country. However, US Hispanics are not a homogeneous racial group: they differ in genetics, culture, and problems.
The largest US Hispanic subgroup and the one more likely to be seen by US physicians is Hispanics of Mexican origin, who account for 66% of all US Hispanics. This group has a higher percentage of Amerindian genes than those of Puerto Rican ancestry.3 LUMINA researchers analyzed the DNA of 492 patients and found the following mixtures of genes3:
- Hispanics in Texas (mostly of Mexican origin): 48% Amerindian, 18% African, 34% European
- Hispanics from Puerto Rico: 20% Amerindian, 45% African, 35% European
- African Americans: 0% Amerindian, 79% African, 21% European
- Non-Hispanic Caucasians: 10% Amerindian, 18% African, 72% European.
Latin Americans of mixed European and Amerindian ancestry (which includes Aztec, Mayan, Quechuan, Aymaran, and other Central and South American groups) are called mestizos. Not all people in Latin America are mestizos: some are of European, African, or Asian ancestry, but in the United States they are all called Hispanics.
LUPUS DIFFERS AMONG SUBGROUPS
LUMINA research has revealed that lupus is heterogeneous also among US Hispanic subgroups. When people from Puerto Rico get lupus, it is generally less serious and devastating than in those from Mexico or Central America. Since US Hispanics of Mexican or Central American origin possess more Amerindian genes, this observation supports the notion that these genes are important contributors to the occurrence and expression of the disease.
Amerindian genes contribute to a greater susceptibility to lupus,4,5 although there is an interplay between genetic and nongenetic factors in the etiology and expression.6 Lupus starts at a younger age in Hispanics of predominantly Amerindian ancestry than in non-Hispanic Caucasians, and the onset is more likely to be acute.7
Renal involvement in these patients8 and mestizos from Latin America is rather common, probably as common as it is in US African Americans, and it tends to develop earlier than in non-Hispanic Caucasians.9 Amerindian ancestral genes, like African genes, contribute to the occurrence of renal disease in lupus patients.4 Furthermore, once nephritis ensues, end-stage renal disease occurs more often in US Hispanic and African American than in non-Hispanic Caucasian children, as demonstrated by Hiraki et al10 using national databases, and the same is true in adults, as shown in the LUMINA cohort.11
Other potentially serious manifestations of the disease are also more common, including hematologic and central nervous system manifestations. Not surprisingly, then, these patients show a higher degree of disease activity, both early in the course of the disease12,13 and over time.14
Table 1 compares the demographic and clinical features of LUMINA patients according to ethnicity. By and large, Hispanics from Texas have lower levels of education and income (comparable with levels in African Americans), and this can adversely affect the disease course by limiting these patients’ access to adequate care.15
DISEASE ACTIVITY AND ORGAN DAMAGE ARE GREATER IN HISPANICS
Disease activity in lupus reflects the ongoing immune-mediated inflammatory process. In LUMINA patients, regardless of the time at which disease activity was ascertained, it was higher in Hispanics from Texas and in African Americans than in non-Hispanic Caucasians and in Hispanics from Puerto Rico.7,12,16–18 Similar findings were seen in the Grupo Latinoamericano de Estudio de Lupus (GLADEL) cohort,13 in which mestizos and Hispanics of mixed African and European ancestry had higher maximum disease activity scores than non-Hispanic Caucasians.13
In addition, organ damage in lupus—the irreversible changes that occur in organ systems as a consequence of the disease or its treatments (eg, glucocorticoids, immunosuppressive drugs)—is more severe and develops sooner in Hispanics from Texas than in other groups.6,18,19 Using multivariate analysis, LUMINA investigators19 estimated the hazard ratio for the time until organ damage appeared for various risk factors, with values of 1 or greater indicating a shorter time and lower values indicating a longer time. Being a Hispanic from Texas carried a hazard ratio of 2.11 (95% confidence interval 1.15–3.88).
Because organ damage is an important and independent predictor of further damage20 and death,21 physicians need to take this disease quite seriously and try to prevent damage early in people at risk. To achieve that, the need to control disease activity must be balanced against the risk of overtreatment, as the important contribution of glucocorticoids to organ damage is well recognized.22
HISPANICS HAVE MORE COMORBIDITIES
Obesity, hypertension, diabetes, and metabolic syndrome are more common in US Hispanics, particularly those of Amerindian ancestry, than in the majority population of non-Hispanic Caucasians.23,24 The potential deleterious effects of glucocorticoids in patients already predisposed to these conditions need to be considered, balancing adequate disease control against the potential adverse effects.22
QUALITY OF LIFE IS WORSE WITH LUPUS
Whether it is measured with a generic instrument such as the Short Form 36 (SF-36), as it was in LUMINA,25 or with a disease-specific tool such as the Lupus-Pro, quality of life is significantly worsened by lupus. Furthermore, Fernandez et al26 found that a low level of health-related quality of life, as measured by the SF-6D version of the SF-36, was predictive of poor outcomes in LUMINA patients.
POVERTY, NOT ETHNICITY, ACCOUNTS FOR HIGHER MORTALITY RATE
As yet, we have no population-based data comparing survival in US Hispanic patients with lupus vs that of other population groups.
At first inspection, data from LUMINA indicate that Hispanics of primarily Amerindian ancestry have a lower survival rate than patients in other ethnic groups (Figure 1).6 However, when all other factors are taken into consideration, poverty, not ethnicity, is the major contributing factor (Table 2).6,27
This finding illustrates the important interplay between genetic and nongenetic factors in the course and final outcome of lupus, as already alluded to, although the exact relationship between them is not clear. It remains to be determined whether poverty is only a proxy for other population characteristics such as illiteracy, limited access to specialized care, limited access to medications, or cultural beliefs that may interfere with proper care.
ANTIMALARIAL DRUGS INCREASE SURVIVAL
Using statistical analysis that adjusts for confounding by indication, we and others28–30 have shown that antimalarial drugs exert an independent and important protective effect on survival in lupus (Figure 2).
Important also is the protective effect of antimalarials on organ damage and the possibility of using them from disease outset in Hispanic patients at risk of early and rapid damage accrual,11 renal damage, and even lupus nephritis.31,32 This has very practical implications for the adequate and prompt management of these Hispanic patients.
PRACTICAL IMPLICATIONS
Lupus in US Hispanics is a serious disease with devastating consequences. Prompt diagnosis is paramount to prevent early organ damage and to prolong survival.
The disease may present in many different and unexpected ways, but joint pain, sun-sensitive rashes, renal involvement, cytopenias, and other manifestations should prompt the clinician to consider lupus in the differential diagnosis. Patients are often dismissed as having “arthritis” without being asked about other manifestations that may suggest a systemic connective tissue disease such as lupus. The same goes for skin rashes or unusual central nervous system manifestations.
The diagnosis of lupus is clinical, but some laboratory studies are essential to rule in or rule out renal or hematologic abnormalities and determine the level of disease activity. Tests usually ordered in patients suspected of having lupus include antinuclear antibody, complement levels, a complete blood cell count and differential, and a urinalysis. The need for additional tests depends on the results of the tests listed.
Once the disease is diagnosed, treatment should be tailored to the severity and type of clinical manifestations present. In general, glucocorticoids should be used at the smallest possible dose, antimalarials should be prescribed from the outset to all patients (following current guidelines in order to avoid ocular toxicity),33 and immunosuppressants and other treatments should be considered in certain instances. In parallel, consideration should be given to sun protection, adequate exercise, tobacco avoidance, osteoporosis and atherosclerosis prevention, planned conception, and compliance.
The goal in these people at risk is to control their lupus manifestations without causing undue damage, to preserve their quality of life, and to prevent an early demise.
- Humes KR, Jones NA, Ramirez RR. Overview of race and Hispanic origin: 2010. 2010 Census briefs; 2011. http://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf. Accessed October 20, 2012.
- Reveille JD, Moulds JM, Ahn C, et al; for the LUMINA study Group. Systemic lupus erythematosus in three ethnic groups. I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity and disease onset. Arthritis Rheum 1998; 41:1161–1172.
- Alarcón GS, Beasley TM, Roseman JM, et al; LUMINA Study Group. Ethnic disparities in health and disease: the need to account for ancestral admixture when estimating the genetic contribution to both (LUMINA XXVI) (Letter). Lupus 2005; 14:867–868.
- Alarcón GS, Bastian HM, Beasley TM, et al; LUMINA Study Group. Systemic lupus erythematosus in a multi-ethnic cohort (LUMINA) XXXII: [corrected] contributions of admixture and socioeconomic status to renal involvement. Lupus 2006; 15:26–31.
- Sanchez E, Webb RD, Rasmussen A, et al. Genetically determined Amerindian ancestry correlates with increased frequency of risk alleles for systemic lupus erythematosus. Arthritis Rheum 2010; 62:3722–3729.
- Fernández M, Alarcón GS, Calvo-Alén J, et al; LUMINA Study Group. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum 2007; 57:576–584.
- Alarcón GS, Friedman AW, Straaton KV, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs Nurture. Lupus 1999; 8:197–209.
- Bastian HM, Alarcón GS, Roseman JM, et al; LUMINA Study Group. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA) XL II: factors predictive of new or worsening proteinuria. Rheumatology (Oxford) 2007; 46:683–689.
- Burgos PI, McGwin G, Pons-Estel GJ, Reveille JD, Alarcón GS, Vilá LM. US patients of Hispanic and African ancestry develop lupus nephritis early in the disease course: data from LUMINA, a multiethnic US cohort (LUMINA LXXIV). Ann Rheum Dis 2011; 70:393–394.
- Hiraki LT, Lu B, Alexander SR, et al. End-stage renal disease due to lupus nephritis among children in the US, 1995–2006. Arthritis Rheum 2011; 63:1988–1997.
- Pons-Estel GJ, Alarcón GS, McGwin G, et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum 2009; 61:830–839.
- Alarcón GS, Roseman J, Bartolucci AA, et al. Systemic lupus erythematosus in three ethnic groups: II. Features predictive of disease activity early in its course. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum 1998; 41:1173–1180.
- Pons-Estel BA, Catoggio LJ, Cardiel MH, et al; Grupo Latinoamericano de Estudio del Lupus. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics.” Medicine (Baltimore) 2004; 83:1–17.
- Alarcón GS, Calvo-Alén J, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in a multiethnic cohort: LUMINA XXXV. Predictive factors of high disease activity over time. Ann Rheum Dis 2006; 65:1168–1174.
- Vilá LM, Alarcón GS, McGwin G, Bastian HM, Fessler BJ, Reveille JD; Lumina Study Group. Systemic lupus erythematosus in a multiethnic US cohort, XXXVII: association of lymphopenia with clinical manifestations, serologic abnormalities, disease activity, and damage accrual. Arthritis Rheum 2006; 55:799–806.
- Zhang J, González LA, Roseman JM, Vilá LM, Reveille JD, Alárcon GS. Predictors of the rate of change in disease activity over time in LUMINA, a multiethnic US cohort of patients with systemic lupus erythematosus: LUMINA LXX. Lupus 2010; 19:727–733.
- Vilá LM, Alarcón GS, McGwin G, et al; LUMINA Study Group. Early clinical manifestations, disease activity and damage of systemic lupus erythematosus among two distinct US Hispanic subpopulations. Rheumatology (Oxford) 2004; 43:358–363.
- Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996; 39:363–369.
- Toloza SM, Roseman JM, Alarcón GS, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA): XXII. Predictors of time to the occurrence of initial damage. Arthritis Rheum 2004; 50:3177–3186.
- Alarcón GS, Roseman JM, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups. XX. Damage as a predictor of further damage. Rheumatology (Oxford) 2004; 43:202–205.
- Alarcón GS, McGwin G, Bastian HM, et al. Systemic lupus erythematosus in three ethnic groups. VII [correction of VIII]. Predictors of early mortality in the LUMINA cohort. LUMINA Study Group. Arthritis Rheum 2001; 45:191–202.
- Ruiz-Irastorza G, Danza A, Khamashta M. Glucocorticoid use and abuse in SLE. Rheumatology (Oxford) 2012 E-pub ahead of print.
- Jordan HT, Tabaei BP, Nash D, Angell SY, Chamany S, Kerker B. Metabolic syndrome among adults in New York City, 2004 New York City Health and Nutrition Examination Survey. Prev Chronic Dis 2012; 9:E04.
- Matthews KA, Sowers MF, Derby CA, et al. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women’s Health Across the Nation (SWAN). Am Heart J 2005; 149:1066–1073.
- Alarcón GS, McGwin G, Uribe A, et al. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of selfreported health-related quality of life early in the disease course. Arthritis Rheum 2004; 51:465–474.
- Fernández M, Alarcón GS, McGwin G, et al; LUMINA Study Group. Using the Short Form 6D, as an overall measure of health, to predict damage accrual and mortality in patients with systemic lupus erythematosus: XLVII, results from a multiethnic US cohort. Arthritis Rheum 2007; 57:986–992.
- Durán S, Apte M, Alarcón GSLUMINA Study Group. Poverty, not ethnicity, accounts for the differential mortality rates among lupus patients of various ethnic groups. J Natl Med Assoc 2007; 99:1196–1198.
- Ruiz-Irastorza G, Egurbide MV, Pijoan JI, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus 2006; 15:577–583.
- Alarcón GS, McGwin G, Bertoli AM, et al; LUMINA Study Group. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 2007; 66:1168–1172.
- Shinjo SK, Bonfá E, Wojdyla D, et al; Grupo Latino Americano de Estudio del Lupus Eritematoso (Gladel). Antimalarial treatment may have a time-dependent effect on lupus survival: data from a multinational Latin American inception cohort. Arthritis Rheum 2010; 62:855–862.
- Fessler BJ, Alarcón GS, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum 2005; 52:1473–1480.
- Pons-Estel GJ, Alarcón GS, Hachuel L, et al. Antimalarials have a protective effect against the development of renal disease in Latin American SLE patients. The 9th International Congress on SLE June 24–27, 2010, Vancouver, Canada. Lupus 2010; 19(suppl 1):31–32.
- Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010; 69:20–28.
- Humes KR, Jones NA, Ramirez RR. Overview of race and Hispanic origin: 2010. 2010 Census briefs; 2011. http://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf. Accessed October 20, 2012.
- Reveille JD, Moulds JM, Ahn C, et al; for the LUMINA study Group. Systemic lupus erythematosus in three ethnic groups. I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity and disease onset. Arthritis Rheum 1998; 41:1161–1172.
- Alarcón GS, Beasley TM, Roseman JM, et al; LUMINA Study Group. Ethnic disparities in health and disease: the need to account for ancestral admixture when estimating the genetic contribution to both (LUMINA XXVI) (Letter). Lupus 2005; 14:867–868.
- Alarcón GS, Bastian HM, Beasley TM, et al; LUMINA Study Group. Systemic lupus erythematosus in a multi-ethnic cohort (LUMINA) XXXII: [corrected] contributions of admixture and socioeconomic status to renal involvement. Lupus 2006; 15:26–31.
- Sanchez E, Webb RD, Rasmussen A, et al. Genetically determined Amerindian ancestry correlates with increased frequency of risk alleles for systemic lupus erythematosus. Arthritis Rheum 2010; 62:3722–3729.
- Fernández M, Alarcón GS, Calvo-Alén J, et al; LUMINA Study Group. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum 2007; 57:576–584.
- Alarcón GS, Friedman AW, Straaton KV, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs Nurture. Lupus 1999; 8:197–209.
- Bastian HM, Alarcón GS, Roseman JM, et al; LUMINA Study Group. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA) XL II: factors predictive of new or worsening proteinuria. Rheumatology (Oxford) 2007; 46:683–689.
- Burgos PI, McGwin G, Pons-Estel GJ, Reveille JD, Alarcón GS, Vilá LM. US patients of Hispanic and African ancestry develop lupus nephritis early in the disease course: data from LUMINA, a multiethnic US cohort (LUMINA LXXIV). Ann Rheum Dis 2011; 70:393–394.
- Hiraki LT, Lu B, Alexander SR, et al. End-stage renal disease due to lupus nephritis among children in the US, 1995–2006. Arthritis Rheum 2011; 63:1988–1997.
- Pons-Estel GJ, Alarcón GS, McGwin G, et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum 2009; 61:830–839.
- Alarcón GS, Roseman J, Bartolucci AA, et al. Systemic lupus erythematosus in three ethnic groups: II. Features predictive of disease activity early in its course. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum 1998; 41:1173–1180.
- Pons-Estel BA, Catoggio LJ, Cardiel MH, et al; Grupo Latinoamericano de Estudio del Lupus. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics.” Medicine (Baltimore) 2004; 83:1–17.
- Alarcón GS, Calvo-Alén J, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in a multiethnic cohort: LUMINA XXXV. Predictive factors of high disease activity over time. Ann Rheum Dis 2006; 65:1168–1174.
- Vilá LM, Alarcón GS, McGwin G, Bastian HM, Fessler BJ, Reveille JD; Lumina Study Group. Systemic lupus erythematosus in a multiethnic US cohort, XXXVII: association of lymphopenia with clinical manifestations, serologic abnormalities, disease activity, and damage accrual. Arthritis Rheum 2006; 55:799–806.
- Zhang J, González LA, Roseman JM, Vilá LM, Reveille JD, Alárcon GS. Predictors of the rate of change in disease activity over time in LUMINA, a multiethnic US cohort of patients with systemic lupus erythematosus: LUMINA LXX. Lupus 2010; 19:727–733.
- Vilá LM, Alarcón GS, McGwin G, et al; LUMINA Study Group. Early clinical manifestations, disease activity and damage of systemic lupus erythematosus among two distinct US Hispanic subpopulations. Rheumatology (Oxford) 2004; 43:358–363.
- Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996; 39:363–369.
- Toloza SM, Roseman JM, Alarcón GS, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA): XXII. Predictors of time to the occurrence of initial damage. Arthritis Rheum 2004; 50:3177–3186.
- Alarcón GS, Roseman JM, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups. XX. Damage as a predictor of further damage. Rheumatology (Oxford) 2004; 43:202–205.
- Alarcón GS, McGwin G, Bastian HM, et al. Systemic lupus erythematosus in three ethnic groups. VII [correction of VIII]. Predictors of early mortality in the LUMINA cohort. LUMINA Study Group. Arthritis Rheum 2001; 45:191–202.
- Ruiz-Irastorza G, Danza A, Khamashta M. Glucocorticoid use and abuse in SLE. Rheumatology (Oxford) 2012 E-pub ahead of print.
- Jordan HT, Tabaei BP, Nash D, Angell SY, Chamany S, Kerker B. Metabolic syndrome among adults in New York City, 2004 New York City Health and Nutrition Examination Survey. Prev Chronic Dis 2012; 9:E04.
- Matthews KA, Sowers MF, Derby CA, et al. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women’s Health Across the Nation (SWAN). Am Heart J 2005; 149:1066–1073.
- Alarcón GS, McGwin G, Uribe A, et al. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of selfreported health-related quality of life early in the disease course. Arthritis Rheum 2004; 51:465–474.
- Fernández M, Alarcón GS, McGwin G, et al; LUMINA Study Group. Using the Short Form 6D, as an overall measure of health, to predict damage accrual and mortality in patients with systemic lupus erythematosus: XLVII, results from a multiethnic US cohort. Arthritis Rheum 2007; 57:986–992.
- Durán S, Apte M, Alarcón GSLUMINA Study Group. Poverty, not ethnicity, accounts for the differential mortality rates among lupus patients of various ethnic groups. J Natl Med Assoc 2007; 99:1196–1198.
- Ruiz-Irastorza G, Egurbide MV, Pijoan JI, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus 2006; 15:577–583.
- Alarcón GS, McGwin G, Bertoli AM, et al; LUMINA Study Group. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 2007; 66:1168–1172.
- Shinjo SK, Bonfá E, Wojdyla D, et al; Grupo Latino Americano de Estudio del Lupus Eritematoso (Gladel). Antimalarial treatment may have a time-dependent effect on lupus survival: data from a multinational Latin American inception cohort. Arthritis Rheum 2010; 62:855–862.
- Fessler BJ, Alarcón GS, McGwin G, et al; LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum 2005; 52:1473–1480.
- Pons-Estel GJ, Alarcón GS, Hachuel L, et al. Antimalarials have a protective effect against the development of renal disease in Latin American SLE patients. The 9th International Congress on SLE June 24–27, 2010, Vancouver, Canada. Lupus 2010; 19(suppl 1):31–32.
- Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010; 69:20–28.
KEY POINTS
- Amerindian genes contribute to a greater susceptibility to lupus, although there is an interplay between genetic and nongenetic factors in its etiology and expression.
- In large studies, disease activity and organ damage were greater in African Americans and in Hispanics from Texas than in Caucasians and Hispanics from Puerto Rico.
- Hispanics of primarily Amerindian ancestry (which includes Aztec, Mayan, Quechuan, Aymaran, and other Central and South American groups) have a lower survival rate than patients in other ethnic groups, but poverty is the responsible factor.
- The need to control disease activity with corticosteroids must be balanced against the risk of overtreatment and organ damage.
- Antimalarial drugs such as chloroquine and hydroxychloroquine should be prescribed from the outset to all patients with lupus, according to current guidelines designed to avoid ocular toxicity.
Autism in the office
The numbers are striking: about 1% of 8-year-old children will receive a diagnosis of an autism spectrum disorder. We still have much to learn about autism, and many factors make study difficult. Reliable older data are scarce, and the diagnostic criteria change—for instance, the currently distinct diagnosis of Asperger syndrome will soon be redefined.
To many, the image of autism is of a cute sandy-haired boy, perhaps staring thoughtfully into space, perhaps reciting the batting averages of individual New York Yankees over the past 10 years—a kid stuck behind a wax-paper wall that blocks the full development of emotional connectivity and complex communication.
The autism spectrum is wide. Those diagnosed carry various features of social impairment, such as a limited ability to recognize and respond to social cues, language and communication challenges, and tendencies to get stuck on the literal. Some show severe social withdrawal and heightened sensitivity to sensory stimuli. Others perseverate on concepts, numbers, ritual behaviors, and repetitive movements.
Special schools and programs can offer a haven. They can buffer children from the unkindness of other children and from the unrealistic expectations of well-meaning but unaware adults; they can protect the more severely affected from self-destructive behaviors, and perhaps they can even decrease some distracting behaviors while promoting communication skills and reducing anxiety. But schools can’t provide forever-care.
Eight-year-olds have a way of growing into adults; nearly half a million autistic children will enter adulthood over the next 10 years. Many will need lifelong comprehensive care and support, others can function well in the workplace but are challenged in social interactions. Perhaps 25% of children diagnosed with an autism spectrum disorder will be high-functioning—with traits displayed graphically (but a little over-the-top) by Dustin Hoffman in the film Rain Man and by Christian Clemenson as the hopping, popping, brilliant attorney Jerry Espenson in the television series Boston Legal. But these are caricatures, and although they heighten our awareness they are limited in perspective.
The patients we see with Asperger syndrome or high-functioning autism do not always wear their diagnosis on their sleeve. Our office staff may recognize them as being a bit quirky. Most first come to our attention for common, unrelated diseases such as diabetes, abdominal pain, and cancer, needing extensive patient education as part of their disease management, but with whom we struggle to make our message clear. Our skills in recognizing these patients need to be refined in order to understand and respond to their unique needs.
At times, we are all challenged in communicating with some patients, even those not perceiving the emotional world through that wall of wax paper. In this issue of the Journal, Prayson and Franco and Shane offer practical advice in interacting with patients with Asperger syndrome. We need to pay attention. In fact, we would do well to follow many of their suggestions with all of our patients.
The numbers are striking: about 1% of 8-year-old children will receive a diagnosis of an autism spectrum disorder. We still have much to learn about autism, and many factors make study difficult. Reliable older data are scarce, and the diagnostic criteria change—for instance, the currently distinct diagnosis of Asperger syndrome will soon be redefined.
To many, the image of autism is of a cute sandy-haired boy, perhaps staring thoughtfully into space, perhaps reciting the batting averages of individual New York Yankees over the past 10 years—a kid stuck behind a wax-paper wall that blocks the full development of emotional connectivity and complex communication.
The autism spectrum is wide. Those diagnosed carry various features of social impairment, such as a limited ability to recognize and respond to social cues, language and communication challenges, and tendencies to get stuck on the literal. Some show severe social withdrawal and heightened sensitivity to sensory stimuli. Others perseverate on concepts, numbers, ritual behaviors, and repetitive movements.
Special schools and programs can offer a haven. They can buffer children from the unkindness of other children and from the unrealistic expectations of well-meaning but unaware adults; they can protect the more severely affected from self-destructive behaviors, and perhaps they can even decrease some distracting behaviors while promoting communication skills and reducing anxiety. But schools can’t provide forever-care.
Eight-year-olds have a way of growing into adults; nearly half a million autistic children will enter adulthood over the next 10 years. Many will need lifelong comprehensive care and support, others can function well in the workplace but are challenged in social interactions. Perhaps 25% of children diagnosed with an autism spectrum disorder will be high-functioning—with traits displayed graphically (but a little over-the-top) by Dustin Hoffman in the film Rain Man and by Christian Clemenson as the hopping, popping, brilliant attorney Jerry Espenson in the television series Boston Legal. But these are caricatures, and although they heighten our awareness they are limited in perspective.
The patients we see with Asperger syndrome or high-functioning autism do not always wear their diagnosis on their sleeve. Our office staff may recognize them as being a bit quirky. Most first come to our attention for common, unrelated diseases such as diabetes, abdominal pain, and cancer, needing extensive patient education as part of their disease management, but with whom we struggle to make our message clear. Our skills in recognizing these patients need to be refined in order to understand and respond to their unique needs.
At times, we are all challenged in communicating with some patients, even those not perceiving the emotional world through that wall of wax paper. In this issue of the Journal, Prayson and Franco and Shane offer practical advice in interacting with patients with Asperger syndrome. We need to pay attention. In fact, we would do well to follow many of their suggestions with all of our patients.
The numbers are striking: about 1% of 8-year-old children will receive a diagnosis of an autism spectrum disorder. We still have much to learn about autism, and many factors make study difficult. Reliable older data are scarce, and the diagnostic criteria change—for instance, the currently distinct diagnosis of Asperger syndrome will soon be redefined.
To many, the image of autism is of a cute sandy-haired boy, perhaps staring thoughtfully into space, perhaps reciting the batting averages of individual New York Yankees over the past 10 years—a kid stuck behind a wax-paper wall that blocks the full development of emotional connectivity and complex communication.
The autism spectrum is wide. Those diagnosed carry various features of social impairment, such as a limited ability to recognize and respond to social cues, language and communication challenges, and tendencies to get stuck on the literal. Some show severe social withdrawal and heightened sensitivity to sensory stimuli. Others perseverate on concepts, numbers, ritual behaviors, and repetitive movements.
Special schools and programs can offer a haven. They can buffer children from the unkindness of other children and from the unrealistic expectations of well-meaning but unaware adults; they can protect the more severely affected from self-destructive behaviors, and perhaps they can even decrease some distracting behaviors while promoting communication skills and reducing anxiety. But schools can’t provide forever-care.
Eight-year-olds have a way of growing into adults; nearly half a million autistic children will enter adulthood over the next 10 years. Many will need lifelong comprehensive care and support, others can function well in the workplace but are challenged in social interactions. Perhaps 25% of children diagnosed with an autism spectrum disorder will be high-functioning—with traits displayed graphically (but a little over-the-top) by Dustin Hoffman in the film Rain Man and by Christian Clemenson as the hopping, popping, brilliant attorney Jerry Espenson in the television series Boston Legal. But these are caricatures, and although they heighten our awareness they are limited in perspective.
The patients we see with Asperger syndrome or high-functioning autism do not always wear their diagnosis on their sleeve. Our office staff may recognize them as being a bit quirky. Most first come to our attention for common, unrelated diseases such as diabetes, abdominal pain, and cancer, needing extensive patient education as part of their disease management, but with whom we struggle to make our message clear. Our skills in recognizing these patients need to be refined in order to understand and respond to their unique needs.
At times, we are all challenged in communicating with some patients, even those not perceiving the emotional world through that wall of wax paper. In this issue of the Journal, Prayson and Franco and Shane offer practical advice in interacting with patients with Asperger syndrome. We need to pay attention. In fact, we would do well to follow many of their suggestions with all of our patients.
Appreciating Asperger syndrome: Implications for better care and outcomes
In this issue of the Cleveland Clinic Journal of Medicine, Prayson and Franco paint a comprehensive picture of the key medical and therapeutic issues faced by patients with Asperger syndrome.1 They offer a refreshing optimism about contemporary treatments aimed at enhancing independence and quality of life, while being realistic about the challenges for these patients, such as making the transition from pediatric care to adult care. Importantly, their overview offers practical suggestions for improving medical care through a greater understanding of the syndrome, along with strategies for how to relate to patients who have a difficult interpersonal style.
In this editorial, I focus on lessons learned in our practice that help identify the problems that people with Asperger syndrome have, and I build on the advice of Prayson and Franco on how to improve patient experiences in the adult medical setting, particularly by diminishing confusion and uncertainty in doctor-patient interactions and by supporting ongoing functioning.
PEOPLE WITH ASPERGER SYNDROME HAVE ALWAYS LIVED AMONG US
Asperger syndrome is being diagnosed more frequently, using criteria recognized by a greater number of professionals. This diagnostic distinction offers a clearer understanding of a group of people who have always lived among us—often standing out because of their appearance, behavior, and communication style, even before a common label existed for their condition.
In less-informed communities, they might be described by neighbors or peers as eccentric or odd, even when they present no obvious dysmorphic or other distinguishing physical features. In fact, some may stand out more because of their accomplishments. The behaviors reported for some innovative scientists (Einstein), inventors (Ford, Edison), musicians (Beethoven), and others might lead to a diagnosis of Asperger syndrome today, while an obsessive nature also characteristic of Asperger syndrome might well have enabled them to think and create in astonishing ways.
As we have come to understand this syndrome better, we have recognized that it is a spectrum. Some patients are highly functioning, for example, and different patients have different needs.
Steve Silberman,2 writing for Wired magazine, coined the term “geek syndrome” and suggested that geeks marrying geeks may help account for the comparatively high prevalence of autism and Asperger syndrome in “techheavy” communities such as Silicon Valley in California and Route 128 in Massachusetts. “At clinics and schools in the Valley, the observation that most parents of autistic kids are engineers and programmers who themselves display autistic behavior is not news.”2 Temple Grandin, arguably the best-known person with an autism spectrum condition, has characterized the NASA Space Center in Houston, TX, as a similar community.
Given this correlation, it follows that colleges and universities offering engineering, computer science, and other technical programs or degrees should have a relatively high prevalence of students with Asperger syndrome. The Massachusetts Institute of Technology, where such a pattern is often observed, offers a course entitled “Charm School,” and its online course description is suggestive of the unique needs of this population3:
“How do I ask for a date? Which bread plate is mine? At what point in a job interview can I ask about salary? Should I use a cell phone while on the T or the elevator? How can a student network to find the perfect position? Join us for MIT's 19th Annual Charm School to find out these answers and more.”
COMMUNICATION DISTURBANCES
The challenges a person with Asperger syndrome may be experiencing are often very difficult to understand. While these people may look normal and demonstrate average to above-average intellectual functioning, their sometimes-peculiar behaviors and deficits in social skills are often difficult for peers to interpret— and to forgive. People with Asperger syndrome want to get along with peers, develop relationships, and succeed in the workplace, and they feel perplexed that others sometimes seem put off by their behavior.
At the core of this discomfort are a range of communication disorders that negatively affect interactions with others. One practical indication of a communication disorder is whether more attention is paid to how something is said than what is being said. This may present to the physician in different ways.
Language
Difficulty with introspection and description may render a patient incapable of describing symptoms and related historical information. In addition, the idiomatic and figurative nature of English may lead Asperger syndrome patients to misunderstand what the physician is saying—even common nonliteral expressions such as “Hop up on the table,” “You’re as fit as a fiddle,” “Are you feeling under the weather?” and “I’m all ears.”
Speech and voice
For the person with Asperger syndrome, speech is often marked by prosodic disturbances, including problems with varying and atypical intonation and stress and, less commonly, unusual fluency patterns and residual articulation issues (l, r, and s sounds). These characteristics can be addressed in therapy.
Conversational style
When people with Asperger syndrome engage in conversation, it is usually brief, or they tend to monopolize it with topics of high interest to themselves or topics of a perseverative or obsessive nature. The patient also tends to have limited perspective and experiences difficulty with higher-order language (including inference and reasoning).
Nonverbal language
A host of nonverbal communication problems include the use of unacceptable social distance and the unintentional messages conveyed nonverbally by unusual clothing choices and poor grooming and hygiene.
WHAT CAN BE DONE IN THE OFFICE VISIT
The key to a successful visit with such patients is to help them anticipate and make sense of their experience. In the visit, predictability should be emphasized and “chaos” avoided. Try to schedule the patient with Asperger syndrome during less-busy days and times, and avoid surprises during medical examinations or procedures, as the unexpected often triggers an extreme reaction. Examinations and procedures should be conducted in a deliberate and slow manner, as rushing through the examination raises the risk of complicating the outcome. Care should also be taken to simplify communications to accommodate the language constraints of the patient.
ONGOING TREATMENT: THE PROMISE OF TECHNOLOGY
Access to support services is critical—especially as people with Asperger syndrome move into adulthood—while the apparent rise in the prevalence of Asperger syndrome and other forms of autism spectrum disorder call for an expansion of current service models. Typically eager to address areas of social deficit, people with Asperger syndrome could benefit from ongoing social-skills support.
Mobile devices such as tablets and smart phones are a transformative technology that shows great promise in supporting treatment innovation. I believe they will have the greatest impact on quality of life for patients with Asperger syndrome by enhancing the potential to live completely independently or semi-independently. These devices can function as personal assistants for those who experience difficulty with time management, human connectivity, way-finding, and other tasks. We have observed, for example, that visual connectivity with caregivers (and others) through a cell phone, messaging, or video chatting, or the provision of electronic reminders for medications or appointments, can reduce the anxiety of a child with Asperger syndrome living outside the parental home. It can also help the physician better ensure that treatment regimens are being followed. Finally, an endless supply of entertainment “apps” along with robust search engines to suit every interest is afforded by feature-rich mobile devices.
Armed with these gadgets, therapists now tailor support to meet the patient’s individual needs, which can range from basic social-skills development and social-cue reminders to higher-level conversational and organizational supports. New tools and techniques, along with better understanding of the condition, portend far more innovative and improved treatments for the future.
- Prayson B, Franco K. Is an adult with Asperger syndrome sitting in your waiting room? Cleve Clin J Med 2012; 79:875–882.
- Silberman S. The Geek syndrome. Autism—and its milder cousin Asperger’s syndrome—is surging among the children of Silicon Valley. Are math-and-tech genes to blame? Wired. http://www.wired.com/wired/archive/9.12/aspergers_pr.html. Accessed October 11, 2012.
- MIT Student Activities Office. The MIT Student Activities Office presents Charm School. http://studentlife.mit.edu/sao/charm. Accessed October 11, 2012.
In this issue of the Cleveland Clinic Journal of Medicine, Prayson and Franco paint a comprehensive picture of the key medical and therapeutic issues faced by patients with Asperger syndrome.1 They offer a refreshing optimism about contemporary treatments aimed at enhancing independence and quality of life, while being realistic about the challenges for these patients, such as making the transition from pediatric care to adult care. Importantly, their overview offers practical suggestions for improving medical care through a greater understanding of the syndrome, along with strategies for how to relate to patients who have a difficult interpersonal style.
In this editorial, I focus on lessons learned in our practice that help identify the problems that people with Asperger syndrome have, and I build on the advice of Prayson and Franco on how to improve patient experiences in the adult medical setting, particularly by diminishing confusion and uncertainty in doctor-patient interactions and by supporting ongoing functioning.
PEOPLE WITH ASPERGER SYNDROME HAVE ALWAYS LIVED AMONG US
Asperger syndrome is being diagnosed more frequently, using criteria recognized by a greater number of professionals. This diagnostic distinction offers a clearer understanding of a group of people who have always lived among us—often standing out because of their appearance, behavior, and communication style, even before a common label existed for their condition.
In less-informed communities, they might be described by neighbors or peers as eccentric or odd, even when they present no obvious dysmorphic or other distinguishing physical features. In fact, some may stand out more because of their accomplishments. The behaviors reported for some innovative scientists (Einstein), inventors (Ford, Edison), musicians (Beethoven), and others might lead to a diagnosis of Asperger syndrome today, while an obsessive nature also characteristic of Asperger syndrome might well have enabled them to think and create in astonishing ways.
As we have come to understand this syndrome better, we have recognized that it is a spectrum. Some patients are highly functioning, for example, and different patients have different needs.
Steve Silberman,2 writing for Wired magazine, coined the term “geek syndrome” and suggested that geeks marrying geeks may help account for the comparatively high prevalence of autism and Asperger syndrome in “techheavy” communities such as Silicon Valley in California and Route 128 in Massachusetts. “At clinics and schools in the Valley, the observation that most parents of autistic kids are engineers and programmers who themselves display autistic behavior is not news.”2 Temple Grandin, arguably the best-known person with an autism spectrum condition, has characterized the NASA Space Center in Houston, TX, as a similar community.
Given this correlation, it follows that colleges and universities offering engineering, computer science, and other technical programs or degrees should have a relatively high prevalence of students with Asperger syndrome. The Massachusetts Institute of Technology, where such a pattern is often observed, offers a course entitled “Charm School,” and its online course description is suggestive of the unique needs of this population3:
“How do I ask for a date? Which bread plate is mine? At what point in a job interview can I ask about salary? Should I use a cell phone while on the T or the elevator? How can a student network to find the perfect position? Join us for MIT's 19th Annual Charm School to find out these answers and more.”
COMMUNICATION DISTURBANCES
The challenges a person with Asperger syndrome may be experiencing are often very difficult to understand. While these people may look normal and demonstrate average to above-average intellectual functioning, their sometimes-peculiar behaviors and deficits in social skills are often difficult for peers to interpret— and to forgive. People with Asperger syndrome want to get along with peers, develop relationships, and succeed in the workplace, and they feel perplexed that others sometimes seem put off by their behavior.
At the core of this discomfort are a range of communication disorders that negatively affect interactions with others. One practical indication of a communication disorder is whether more attention is paid to how something is said than what is being said. This may present to the physician in different ways.
Language
Difficulty with introspection and description may render a patient incapable of describing symptoms and related historical information. In addition, the idiomatic and figurative nature of English may lead Asperger syndrome patients to misunderstand what the physician is saying—even common nonliteral expressions such as “Hop up on the table,” “You’re as fit as a fiddle,” “Are you feeling under the weather?” and “I’m all ears.”
Speech and voice
For the person with Asperger syndrome, speech is often marked by prosodic disturbances, including problems with varying and atypical intonation and stress and, less commonly, unusual fluency patterns and residual articulation issues (l, r, and s sounds). These characteristics can be addressed in therapy.
Conversational style
When people with Asperger syndrome engage in conversation, it is usually brief, or they tend to monopolize it with topics of high interest to themselves or topics of a perseverative or obsessive nature. The patient also tends to have limited perspective and experiences difficulty with higher-order language (including inference and reasoning).
Nonverbal language
A host of nonverbal communication problems include the use of unacceptable social distance and the unintentional messages conveyed nonverbally by unusual clothing choices and poor grooming and hygiene.
WHAT CAN BE DONE IN THE OFFICE VISIT
The key to a successful visit with such patients is to help them anticipate and make sense of their experience. In the visit, predictability should be emphasized and “chaos” avoided. Try to schedule the patient with Asperger syndrome during less-busy days and times, and avoid surprises during medical examinations or procedures, as the unexpected often triggers an extreme reaction. Examinations and procedures should be conducted in a deliberate and slow manner, as rushing through the examination raises the risk of complicating the outcome. Care should also be taken to simplify communications to accommodate the language constraints of the patient.
ONGOING TREATMENT: THE PROMISE OF TECHNOLOGY
Access to support services is critical—especially as people with Asperger syndrome move into adulthood—while the apparent rise in the prevalence of Asperger syndrome and other forms of autism spectrum disorder call for an expansion of current service models. Typically eager to address areas of social deficit, people with Asperger syndrome could benefit from ongoing social-skills support.
Mobile devices such as tablets and smart phones are a transformative technology that shows great promise in supporting treatment innovation. I believe they will have the greatest impact on quality of life for patients with Asperger syndrome by enhancing the potential to live completely independently or semi-independently. These devices can function as personal assistants for those who experience difficulty with time management, human connectivity, way-finding, and other tasks. We have observed, for example, that visual connectivity with caregivers (and others) through a cell phone, messaging, or video chatting, or the provision of electronic reminders for medications or appointments, can reduce the anxiety of a child with Asperger syndrome living outside the parental home. It can also help the physician better ensure that treatment regimens are being followed. Finally, an endless supply of entertainment “apps” along with robust search engines to suit every interest is afforded by feature-rich mobile devices.
Armed with these gadgets, therapists now tailor support to meet the patient’s individual needs, which can range from basic social-skills development and social-cue reminders to higher-level conversational and organizational supports. New tools and techniques, along with better understanding of the condition, portend far more innovative and improved treatments for the future.
In this issue of the Cleveland Clinic Journal of Medicine, Prayson and Franco paint a comprehensive picture of the key medical and therapeutic issues faced by patients with Asperger syndrome.1 They offer a refreshing optimism about contemporary treatments aimed at enhancing independence and quality of life, while being realistic about the challenges for these patients, such as making the transition from pediatric care to adult care. Importantly, their overview offers practical suggestions for improving medical care through a greater understanding of the syndrome, along with strategies for how to relate to patients who have a difficult interpersonal style.
In this editorial, I focus on lessons learned in our practice that help identify the problems that people with Asperger syndrome have, and I build on the advice of Prayson and Franco on how to improve patient experiences in the adult medical setting, particularly by diminishing confusion and uncertainty in doctor-patient interactions and by supporting ongoing functioning.
PEOPLE WITH ASPERGER SYNDROME HAVE ALWAYS LIVED AMONG US
Asperger syndrome is being diagnosed more frequently, using criteria recognized by a greater number of professionals. This diagnostic distinction offers a clearer understanding of a group of people who have always lived among us—often standing out because of their appearance, behavior, and communication style, even before a common label existed for their condition.
In less-informed communities, they might be described by neighbors or peers as eccentric or odd, even when they present no obvious dysmorphic or other distinguishing physical features. In fact, some may stand out more because of their accomplishments. The behaviors reported for some innovative scientists (Einstein), inventors (Ford, Edison), musicians (Beethoven), and others might lead to a diagnosis of Asperger syndrome today, while an obsessive nature also characteristic of Asperger syndrome might well have enabled them to think and create in astonishing ways.
As we have come to understand this syndrome better, we have recognized that it is a spectrum. Some patients are highly functioning, for example, and different patients have different needs.
Steve Silberman,2 writing for Wired magazine, coined the term “geek syndrome” and suggested that geeks marrying geeks may help account for the comparatively high prevalence of autism and Asperger syndrome in “techheavy” communities such as Silicon Valley in California and Route 128 in Massachusetts. “At clinics and schools in the Valley, the observation that most parents of autistic kids are engineers and programmers who themselves display autistic behavior is not news.”2 Temple Grandin, arguably the best-known person with an autism spectrum condition, has characterized the NASA Space Center in Houston, TX, as a similar community.
Given this correlation, it follows that colleges and universities offering engineering, computer science, and other technical programs or degrees should have a relatively high prevalence of students with Asperger syndrome. The Massachusetts Institute of Technology, where such a pattern is often observed, offers a course entitled “Charm School,” and its online course description is suggestive of the unique needs of this population3:
“How do I ask for a date? Which bread plate is mine? At what point in a job interview can I ask about salary? Should I use a cell phone while on the T or the elevator? How can a student network to find the perfect position? Join us for MIT's 19th Annual Charm School to find out these answers and more.”
COMMUNICATION DISTURBANCES
The challenges a person with Asperger syndrome may be experiencing are often very difficult to understand. While these people may look normal and demonstrate average to above-average intellectual functioning, their sometimes-peculiar behaviors and deficits in social skills are often difficult for peers to interpret— and to forgive. People with Asperger syndrome want to get along with peers, develop relationships, and succeed in the workplace, and they feel perplexed that others sometimes seem put off by their behavior.
At the core of this discomfort are a range of communication disorders that negatively affect interactions with others. One practical indication of a communication disorder is whether more attention is paid to how something is said than what is being said. This may present to the physician in different ways.
Language
Difficulty with introspection and description may render a patient incapable of describing symptoms and related historical information. In addition, the idiomatic and figurative nature of English may lead Asperger syndrome patients to misunderstand what the physician is saying—even common nonliteral expressions such as “Hop up on the table,” “You’re as fit as a fiddle,” “Are you feeling under the weather?” and “I’m all ears.”
Speech and voice
For the person with Asperger syndrome, speech is often marked by prosodic disturbances, including problems with varying and atypical intonation and stress and, less commonly, unusual fluency patterns and residual articulation issues (l, r, and s sounds). These characteristics can be addressed in therapy.
Conversational style
When people with Asperger syndrome engage in conversation, it is usually brief, or they tend to monopolize it with topics of high interest to themselves or topics of a perseverative or obsessive nature. The patient also tends to have limited perspective and experiences difficulty with higher-order language (including inference and reasoning).
Nonverbal language
A host of nonverbal communication problems include the use of unacceptable social distance and the unintentional messages conveyed nonverbally by unusual clothing choices and poor grooming and hygiene.
WHAT CAN BE DONE IN THE OFFICE VISIT
The key to a successful visit with such patients is to help them anticipate and make sense of their experience. In the visit, predictability should be emphasized and “chaos” avoided. Try to schedule the patient with Asperger syndrome during less-busy days and times, and avoid surprises during medical examinations or procedures, as the unexpected often triggers an extreme reaction. Examinations and procedures should be conducted in a deliberate and slow manner, as rushing through the examination raises the risk of complicating the outcome. Care should also be taken to simplify communications to accommodate the language constraints of the patient.
ONGOING TREATMENT: THE PROMISE OF TECHNOLOGY
Access to support services is critical—especially as people with Asperger syndrome move into adulthood—while the apparent rise in the prevalence of Asperger syndrome and other forms of autism spectrum disorder call for an expansion of current service models. Typically eager to address areas of social deficit, people with Asperger syndrome could benefit from ongoing social-skills support.
Mobile devices such as tablets and smart phones are a transformative technology that shows great promise in supporting treatment innovation. I believe they will have the greatest impact on quality of life for patients with Asperger syndrome by enhancing the potential to live completely independently or semi-independently. These devices can function as personal assistants for those who experience difficulty with time management, human connectivity, way-finding, and other tasks. We have observed, for example, that visual connectivity with caregivers (and others) through a cell phone, messaging, or video chatting, or the provision of electronic reminders for medications or appointments, can reduce the anxiety of a child with Asperger syndrome living outside the parental home. It can also help the physician better ensure that treatment regimens are being followed. Finally, an endless supply of entertainment “apps” along with robust search engines to suit every interest is afforded by feature-rich mobile devices.
Armed with these gadgets, therapists now tailor support to meet the patient’s individual needs, which can range from basic social-skills development and social-cue reminders to higher-level conversational and organizational supports. New tools and techniques, along with better understanding of the condition, portend far more innovative and improved treatments for the future.
- Prayson B, Franco K. Is an adult with Asperger syndrome sitting in your waiting room? Cleve Clin J Med 2012; 79:875–882.
- Silberman S. The Geek syndrome. Autism—and its milder cousin Asperger’s syndrome—is surging among the children of Silicon Valley. Are math-and-tech genes to blame? Wired. http://www.wired.com/wired/archive/9.12/aspergers_pr.html. Accessed October 11, 2012.
- MIT Student Activities Office. The MIT Student Activities Office presents Charm School. http://studentlife.mit.edu/sao/charm. Accessed October 11, 2012.
- Prayson B, Franco K. Is an adult with Asperger syndrome sitting in your waiting room? Cleve Clin J Med 2012; 79:875–882.
- Silberman S. The Geek syndrome. Autism—and its milder cousin Asperger’s syndrome—is surging among the children of Silicon Valley. Are math-and-tech genes to blame? Wired. http://www.wired.com/wired/archive/9.12/aspergers_pr.html. Accessed October 11, 2012.
- MIT Student Activities Office. The MIT Student Activities Office presents Charm School. http://studentlife.mit.edu/sao/charm. Accessed October 11, 2012.
Is an adult with Asperger syndrome sitting in your waiting room?
In 1944, Hans Asperger described a subset of children who exhibited “a lack of empathy, little ability to form friendships, one-sided conversation, intense absorption in a special interest, and clumsy movements.”1
In recent years, Asperger syndrome has become increasingly recognized in the medical community and by the general public. It has been popularized in the media in John Elder Robison’s bestselling book, Look Me in the Eye; with the television character Sheldon Cooper in The Big Bang Theory; and in the 2009 film, Adam, a romantic comedy with the title character accurately portraying a young man with Asperger syndrome.
In this article, we discuss the causes and characteristics of Asperger syndrome, with special focus on adults: how it presents, how to treat it, and how to enhance the delivery of care.
PREVALENCE SEEMS TO BE INCREASING
One in 88 children is diagnosed with an autism spectrum disorder, and the rates of Asperger syndrome and other autism spectrum disorders appear to be increasing.2 Whether this increase is the result of more thorough assessment and identification or of environmental changes is hotly debated.3 The rise began before the proposed changes to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) to combine autism, Asperger syndrome, and pervasive developmental disorder not otherwise specified to simplify diagnosis.4 Asperger syndrome affects males three to four times more often than females.5 For most patients, the effects persist throughout life.
BEHAVIORAL IMPAIRMENTS CHARACTERIZE THE SYNDROME
Poor social skills are a hallmark
People with Asperger syndrome struggle with social interaction and face challenges in forming and maintaining relationships. They tend to have less eye contact (often the first indicator), smiling, animated speech, and physical communication such as hand gestures. They tend not to solicit another’s attention to something they themselves find interesting. They often lack social and emotional reciprocity and have difficulty understanding another person’s thoughts or feelings,6 and they have marked difficulty reading social cues. Some adults may appear rigid, selfish, or narrow-minded.
Sometimes behavior is in the normal range but is out of context for a particular situation.7 For example, a preprofessional student with Asperger syndrome might walk into a psychiatric evaluation to assess fitness for duty and take a seat cross-legged on the floor and have a snack. Poor grooming inappropriate for the occasion may also be observed, such as showing up for a formal photo with unkempt hair and in a stained shirt that is half tucked in.
Many adults with autism spectrum disorders are oblivious to their social reputation.8 They are often unaware that their behavior is out of place and only learn that it is not normal when they are told. Others recognize that they have trouble empathizing with or understanding the perspectives of others, but they are at a loss as to how to improve. The syndrome has a tremendous impact on broader aspects of life, such as employment, functional independence, relationships, and social networks.
Other odd behaviors are common
Repetitive behaviors. Many patients with Asperger syndrome have repetitive behaviors, which can manifest as repeating phrases or expressions, attempting to imitate others, and rocking. They tend to follow routines, do not enjoy spontaneity, and are more inflexible and uncomfortable when their planned regimen is altered.
Gait or balance issues may be observed on physical examination.9 Uncoordinated motion and clumsiness are common,10 and some patients may have a bouncy, stilted gait or may walk on their toes, although the latter is more common in children than adults. Many patients have illegible handwriting.11
Fixations. Many Asperger patients have unusual and intense obsessions with subjects like numbers, dates, or aerodynamics of planes. Children with such fascinations are described as “little professors” or as having “geek syndrome.”12 Certain obsessions often continue into adulthood, although one area of interest may fade and another may take over. Such “expertise” in adults may gain them respect, even though they may seem very odd in other ways.
Lack of boundaries. Patients with Asperger syndrome tend to have poor spatial awareness and to be unaware of physical boundaries, standing too close for others’ comfort or unusually far away. Lack of boundaries may extend beyond the physical, as patients may inappropriately help themselves to food or use an item belonging to another without invitation, being unaware that the behavior may be intrusive or inappropriate.
BEHAVIORAL ASSESSMENTS HELP MAKE DIAGNOSIS
Asperger syndrome is most often diagnosed in early childhood, although it may remain undiagnosed into adulthood. Coexisting depression, attention deficit hyperactivity disorder, or anxiety disorders are also often present.
Establishing the diagnosis is aided by information from family members and others who interact with the patient, from the observations of trained professionals, and from self-reported data. However, self-reported assessments are not always reliable, because the syndrome can affect insight.
The most common assessment tool for autism spectrum disorders is the Autism Diagnostic Interview-Revised (ADI-R),13 a battery of tests given in a structured interview to identify and quantify symptoms, determine where a patient falls on the autism spectrum,14 and point toward interventions. The ADI-R also organizes critical developmental history to evaluate if something else is present, such as prodromal schizophrenia. Although the ADI-R can be very useful in the diagnostic process, it is based on parental reporting, which is neither always available nor fully reliable.
A specific diagnostic tool for adults is the Adult Asperger Assessment.15 Patients are asked to complete two screening questionnaires that gauge cognitive function and gather information about thinking, processing, and behavior.
Table 1 lists the criteria for Asperger syndrome from the DSM Fourth Edition, Text Revision (DSM-IV-TR).16 Asperger syndrome differs from general autism in that it is not associated with language delay. In addition, patients with Asperger syndrome usually have average or above-average IQ scores.17 Still, determining whether a patient has Asperger syndrome or high-functioning autism is sometimes challenging.6
In DSM-V, Asperger syndrome will be subsumed under autism spectrum disorder
In 2013, the DSM-V will replace the DSMIV-TR and will combine autism, Asperger syndrome, and pervasive developmental disorder not otherwise classified into a single diagnosis: autism spectrum disorder. The new system uses two instead of the previous three clusters of core symptoms, centered on “social reciprocity and communication” in one arm and “restricted interests and repetitive behavior” in the other.18 There will be less emphasis on play and imagination than in the past. Some authors suggest adding sensory criteria, particularly reduced pain and increased hearing sensitivity.19
The proposed system is sensitive and specific for autism spectrum disorders, allows early diagnosis, and indicates degree of severity.20 It is hoped that the new system, which accounts for the range and severity of symptoms, should help physicians refer patients to the correct level of treatment.
On the other hand, it may be difficult to think of the three disorders as a single diagnosis. Asperger syndrome manifests in distinct ways, and clear behavioral criteria for diagnosis can be invaluable in helping people with the syndrome. Also, the public may continue to refer to it as Asperger syndrome, and parents and patients may feel uncomfortable having it considered to be the same diagnosis as autism.
BEHAVIORAL THERAPY CAN HELP ACHIEVE INDEPENDENCE
Although there is no cure for Asperger syndrome, various interventions can dramatically improve quality of life and independence. The health care team may include a primary care physician, psychologist, psychiatrist, neurologist, and speech therapist.
Behavioral therapies can help patients with Asperger syndrome learn skills to reduce their symptoms. Occupational and physical therapy can improve dexterity, fluidity, and coordination. Desensitization training may help patients adapt to uncomfortable sights, sounds, or smells that may arise. This can be critical in a job situation. For example, while an average person exposed to a foul odor in public is likely to react tactfully, a person with Asperger syndrome may scream loudly, make inappropriate comments, or run from the room. Social training, especially targeted to the workplace, can provide strategies for promoting typical behaviors and be key to maximizing functional independence.
Speech therapists can teach patients how to sound more relaxed and help them master the natural give-and-take of conversational exchange. Psychotherapy can provide a safe place to work on anxiety, express emotions, and manage restricted interests or repeated behaviors. Group therapy or social training can be a venue for learning improved interactions.
Living independently can be very challenging, and patients with Asperger syndrome may need functional independence training to help with a variety of skills, from handling finances to organizing the home.
Improving quality of life includes determining the best learning environments from childhood into college years and beyond.21–23 Socialization can be enhanced with additional social support at home or on campus, through family interactions and collaborative learning, and by teaching empathy.24 Vocational training can be extremely useful.
DRUG THERAPY MAY HAVE A ROLE
Medications are not usually prescribed unless depression or anxiety is also present, but they may also help manage irritability, anger, stereotypical mannerisms, and disturbing movements. Fluoxetine (Prozac) helps reduce repetitive behaviors in adults with Asperger syndrome. Propranolol (Inderal), a well-known antihypertensive, is also used for performance anxiety and improves word fluency, understanding of verbal communication, and verbal problem-solving in patients with an autism spectrum disorder.25
Giving oxytocin (Pitocin) intranasally in a spray formulation is currently being tested to enhance social skills. Patients with an autism spectrum disorder were more able to perceive emotions of others and to respond more appropriately.26 Oxytocin has long been associated with bonding and is believed to enhance mothering skills. It is naturally present in both sexes, but levels are higher in women, which may in part explain the lower rate of autism spectrum disorders in females.27
HEALTH CARE REQUIRES SPECIAL CONSIDERATIONS
Medical care for patients with Asperger syndrome is enhanced by understanding the patient’s experience. Adults, in particular, may have learned to suppress symptoms of Asperger syndrome to better function in society but still experience stress in situations in which others would not. Patients with Asperger syndrome may struggle with social interactions during medical examinations or procedures, and clinicians may find interaction with the patient challenging.
It is important for health care providers to be calm and patient and to understand that anxiety may prevent people with Asperger syndrome from making eye contact. The clinician should confirm that a patient is engaged but should avoid seeming pushy or invasive.
When anxious, patients may employ strange gestures that they find soothing, such as flapping the hands, rocking, or cracking the knuckles. It is usually easier to allow them to continue unless the activity hinders the examination or treatment.
Patients are likely to respond better to direct requests than to subtle questions: eg, “Open your mouth, please” instead of “Could you open your mouth?” Using clear, specific language and avoiding metaphors, irony, and nonverbal communication are best. It is important to explicitly ask for everything needed, as patients may not volunteer information and may have trouble articulating what they are thinking or feeling. While educating patients about their health needs, physicians may need to reiterate guidance several times or approach the same topic from different angles in order for the patient to accept a concern.
All actions, especially touching the patient, should be explained clearly beforehand. If possible, the doctor should demonstrate using visuals or on his or her own body if appropriate. For invasive procedures, anesthetizing the local area is recommended.
People with Asperger syndrome often rely heavily on a regular routine to maintain a sense of organization. By interrupting this routine, a doctor’s visit can induce anxiety. Waiting also increases anxiety, so scheduling patients with Asperger syndrome either first or last in the day may help.
Hypersensitivity poses challenges
Many people with Asperger syndrome have abnormal sensitivity to stimuli, with differences in pain sensation and hearing perhaps most prominent. Loud noises, such as beeping equipment, whirring fans, or buzzing lights may be distressful and should be reduced if possible. Patients may also be strongly affected by bright lights or scents such as perfumes.
Patients may also have an altered sense of taste, with consequences that go beyond simple “picky eating.” Patients should be asked about unusual eating patterns, diets, or food aversions. People with autism spectrum disorders often do not consume adequate vitamin C because of an aversion to fruits and vegetables. Vitamin deficiency may have originated in infancy but may not be identified or treated until adulthood.28
The sense of touch may be intensified, causing patients to be extremely ticklish; they may actually prefer to be touched more firmly. When it is necessary to make physical contact with patients, it will make the process easier if the physician determines their comfort level and finds ways to help them endure the experience with the least amount of discomfort.
Some patients with impaired sensory expression may have a high tolerance for extreme temperatures and pain, leading to delay in seeking aid.29 Patients may downgrade pain levels, masking the severity of an illness or injury.
Transition from pediatric to adult care
Pediatrics is often a warm environment in which children develop a trusting relationship with their care providers. The transition to adult care can be daunting for patients with Asperger syndrome and their families, and many postpone the change for as long as possible.
Although time-consuming, a collaborative effort between the pediatric and adult care teams can dramatically smooth the transition. It can help to have a familiar person from the pediatric team, such as a nurse, be present at the initial interaction with the new adult care team. Both teams should be familiar with the other’s clinical practices and be aware of the patient’s stressors and ways to ameliorate them.30
THE SEARCH FOR A CAUSE CONTINUES
Numerous studies are attempting to understand the anatomic and physiologic causes of autism spectrum disorders, and to find effective treatments and improve the quality of life.
Prenatal factors implicated
Several recent studies have focused on environmental factors during pregnancy as risk factors for autism spectrum disorder. Selective serotonin reuptake inhibitors were found to increase the risk,31 but the severity of the mother’s depressive illness must be considered before counseling against using these drugs. Older maternal or paternal age was also found to increase the risk of an autism spectrum disorder.32 Recent research indicates that older fathers are in particular more likely to have children with disorders such as autism because of an increase in random mutations associated with advanced age.33
Maternal illness during pregnancy is also associated. Preliminary studies found an increased risk of autism if the mother had had a prenatal viral infection.34 A more recent study found that untreated fever during pregnancy rather than a specific viral infection is more strongly linked.35
Maternal antibodies have been implicated as well. One review found that psoriasis is the only maternal autoimmune condition significantly associated with the development of an autism spectrum disorder.36 Elevated levels of antibodies against the fetal brain have been found in mothers with autistic children.37 One study found that autistic children and their siblings have elevated antibrain antibodies in distinct brain regions, including the caudate nucleus, putamen, prefrontal cortex, cerebellum, and cingulate gyrus (why the siblings are spared from having the disorder is unclear).38 Some have questioned whether a child’s own immune system might even be involved.39
Functional magnetic resonance imaging reveals multiple differences
Functional magnetic resonance imaging (fMRI) has been used to investigate impaired social interaction, specific deficits of facial perception and recognition, sensory processing, working memory, and “theory of mind.” Hypoactivation, hyperactivation, and decreased functional connectivity have been observed depending on the mental processes evaluated.40
When undergoing facial perception tasks, subjects with autism spectrum disorders exhibit hypoactivation in the lateral aspect of the middle region of the fusiform gyrus, responsible for face identification. But they have significant activation of the limbic system, specifically the amygdala, during facial recognition. Hypoactivity in the fusiform gyrus is observed when trying to identify faces or read facial expressions.41,42 This cluster of findings helps explain misinterpretations, misidentification, and heightened affect.
A hallmark characteristic of autism is the difficulty patients have in determining intentions and interpreting others’ behavior, thoughts, or emotions. Studies of people with autism spectrum disorders show that areas often responsible for “sensitivity to others” are hypoactive.43 There is also diminished activation in the medial cingulate cortex, normally activated when these people are asked to think about themselves and who they are.44
The resting state in the brains of people with autism spectrum disorders is abnormally activated.45 They are often particularly good at attention to detail but challenged in integrating information needed for general executive functioning. Impaired sensory processing makes it difficult for them to simultaneously interpret multiple sources of sensory input.46
Perhaps some of the most exciting fMRI news comes from infant studies. Radical and axial diffusivity and fractional anisotropy techniques demonstrate differences in the brains of infants 6 to 24 months old, before symptoms of autism spectrum disorders are observed. It is hoped that early intervention could come into play before the syndrome develops fully.47
The synthesis of input of social and emotional cues is sometimes referred to in the literature as “theory of mind.” It is impaired in Asperger syndrome,48 as manifested by a lack of empathy and by challenges in perceiving others’ thoughts and feelings. The basis of impairment may be related to abnormalities in the amygdala.49 Normal awareness involves the integration of multiple neural networks in the anterior paracingulate cortex, the superior temporal sulci, and the temporal poles bilaterally, but different regions appear to be used in patients with Asperger syndrome.50 A small series of five case studies using positron emission tomography indicated that the left prefrontal cortex was the primary location for theory of mind in Asperger syndrome.51
Epilepsy, gastrointestinal problems, and sleep disturbances are associated
About 25% of people with autism spectrum disorders have epilepsy vs 2% to 3% in the general population. Asperger syndrome is associated with a much lower but still elevated risk of 4% to 6%.47,52
Gastrointestinal complaints, most often constipation or chronic diarrhea, are much more common in children with autism spectrum disorders than in the general population. Preliminary data showed that children with an autism spectrum disorder have a 42% rate of gastrointestinal problems vs 12% in unaffected siblings. There is also a correlation between the severity of gastrointestinal problems and severity of autistic symptoms.53
Research is ongoing to determine the prevalence of insomnia or interrupted sleep in those with autism spectrum disorders.54–56 Changes in sleep architecture can explain nighttime activity.
NONTRADITIONAL CONSIDERATIONS
Dietary treatment: Mixed findings
A popular hypothesis is that adherence to a gluten-free or casein-free diet can reduce symptoms of autism spectrum disorders. Preliminary reports identified several cases of children showing improvement.57 However, this has not been replicated, and more studies refute benefits of these diets.58
Essential nutritional needs should be met with any diet, whether it is designed to reduce symptoms or not. Patients with autism spectrum disorders may have strong food aversions, and dietary supplements of vitamins and minerals may be required.
Vaccines do not cause autism
Despite popular concern, recent research indicates that vaccines do not cause autism. Thimerosal, a mercury-based preservative used in childhood vaccines, was at one time implicated as a risk factor for autism spectrum disorders. The US Centers for Disease Control and Prevention (CDC) issued a precaution against using thimerosal-containing vaccines while testing was done to determine the effects on neuropsychological development.59 The CDC study as well as newer studies did not demonstrate that exposure to mercury causes these neuropsychological concerns, but researchers have continued to study the subject.60–62 The original study implicating thimerosal was disproven as scientifically unsound and fraught with conflict of interest and legal concerns. It has since been retracted, and its findings have been completely discredited.63
Other areas of research
Current research is exploring the higher prevalence of autism spectrum disorders in particular families.64–66 Autism and autism spectrum disorders may be caused by hundreds of simultaneous gene alterations or may develop as a result of reduced gene expression in two areas of the cerebral cortex where higher-order processing occurs, in the frontal and temporal lobes.67
Although genetic theories of autism predominate, a 2011 project suggests that environment is also important. A study of twins found that genetics accounted for 40% or less of cases of autism spectrum disorder, with at least 55% of cases being attributable to environmental factors.68
- Frith U, editor. Autism and Asperger Syndrome. New York: Cambridge University Press, 1991:37–92.
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ 2012; 61( 3):1–19.
- Rutter M. Incidence of autism spectrum disorders: changes over time and their meaning. Acta Paediatr 2005; 94:2–15.
- Happé F. Criteria, categories, and continua: autism and related disorders in DSM-5. J Am Acad Child Adolesc Psychiatry 2011; 50:540–542.
- National Institute of Neurological Disorders and Stroke. Asperger syndrome fact sheet. http://www.ninds.nih.gov/disorders/asperger/detail_asperger.htm. Accessed October 11, 2012.
- Toth K, King BH. Asperger’s syndrome: diagnosis and treatment. Am J Psychiatry 2008; 165:958–963.
- Vermeulen P. Autism: from mind blindness to context blindness. Asperger’s Digest November/December 2011. http://autismdigest.com/autism-from-mind-blindness-to-context-blindness/. Accessed October 11, 2012.
- Izuma K, Matsumoto K, Camerer CF, Adolphs R. Insensitivity to social reputation in autism. Proc Natl Acad Sci U S A. 2011; 108:17302–17307.
- Weimer AK, Schatz AM, Lincoln A, Ballantyne AO, Trauner DA. “Motor” impairment in Asperger’s syndrome: evidence for a deficit in proprioception. J Dev Behav Pediatr 2001; 22:92–101.
- Siaperas P, Ring HA, McAllister CJ, et al. Atypical movement performance and sensory integration in Asperger’s syndrome. J Autism Dev Disord 2012; 42:718–725.
- Kushki A, Chau T, Anagnostou E. Handwriting difficulties in children with autism spectrum disorders: a scoping review. J Autism Dev Disord 2011; 41:1706–1716.
- Nash JM, Bonesteel A. The geek syndrome. Time Magazine U.S. 2002. http://www.time.com/time/magazine/article/0,9171,1002365-1,00.html. Accessed October 11, 2012.
- Le Couteur A, Rutter M, Lord C, et al. Autism diagnostic interview: a standardized investigatorbased instrument. J Autism Dev Disord 1989; 19:363–387.
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised WPS Edition Manual. Los Angeles, CA. Western Psychological Services; 2003.
- Baron-Cohen S, Wheelwright S, Robinson J, Woodbury-Smith M. The Adult Asperger’s Assessment (AAA): a diagnostic method. J Autism Developmental Disord 2005; 35:807–819.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Text Revision DSM IV–TR 4th Ed. 2000; Washington, DC: American Psychiatric Association; 2000:80–84.
- Centers for Disease Control. Asperger syndrome fact sheet. http://www.cdc.gov/ncbddd/actearly/pdf/parents_pdfs/Asperger_Syndrome.pdf. Accessed October 11, 2012.
- Peckham C. The current state in autism—still tough to treat but encouraging progress. An expert interview with Fred R. Volkmar, MD. Medscape Pediatrics 2010. http://www.medscape.com/viewarticle/720802?src=mp&spon=17. Accessed October 31, 2012.
- Muscari ME. How should I evaluate an adult for possible Asperger’s syndrome? Medscape News Today 2006.
- Hollander E. Can we treat core symptoms of autism spectrum disorders in adults? December 21, 2011; 1( 18). http://www.medscape.com/viewarticle/531750. Accessed October 1, 2012.
- Müller E, Schuler A, Yates GB. Social challenges and supports from the perspective of individuals with Asperger’s syndrome and other autism spectrum disabilities. Autism 2008; 12:173–190.
- Helman T, Berger O. Parents of children with Asperger’s syndrome or with learning disabilities: family environment and social support. Res Dev Disabil 2008; 29;289–300.
- Taylor CM. Campus commons. When pigs fly: a new perspective on learning. About Campus 2011; 16:30–32.
- Cheng Y, Chiang H, Ye J, Cheng L. Enhancing empathy instruction using a collaborative virtual learning environment for children with autistic spectrum conditions. Comput Edu 2010; 55:1449–1458.
- Beversdorf DQ, Saklayen S, Higgins KF, Bodner KE, Kanne SM, Christ SE. Effect of propranolol on word fluency in autism. Cogn Behav Neurol 2011; 24:11–17.
- Kuehn BM. Scientists probe oxytocin therapy for social deficits in autism, schizophrenia. JAMA 2011; 305:659–661.
- Pfaff DW, Rapin I, Goldman S. Male dominance in autism: neuroendocrine influences on arousal and social anxiety. Autism Res 2011; 4:163–176.
- Brauser D. Children with autism routinely exhibit feeding difficulties in infancy. Medscape Medical News 2010. http://www.medscape.org/viewarticle/726060. Accessed October 31, 2012.
- Baron MG, Groden J, Groden G, Lipsitt L. Stress and coping in autism. New York: Oxford University Press; 2006:355.
- Camfield P, Camfield C. Transition to adult care for children with chronic neurological disorders. Ann Neurol 2001; 69:437–444.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011; 68:1104–1112.
- Croen LA, Najjar DV, Fireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med 2007; 161:334–340.
- Kong A, Frigge ML, Masson G, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 2012; 488:471–475.
- Atlandóttir HO, Thorsen P, Østergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 2010; 40:1423–1430.
- Zerbo O, Iosif A-M, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord 2012; 10.1007/s10803-012-1540-x.
- Crown LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med 2005; 159:151–157.
- Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neruoimmunol 2008; 194:165–172.
- Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol 2006; 178:149–155.
- Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmunity Rev 2004; 3:557–562.
- South M, Diehl JJ. Functional magnetic resonance imaging. In:Hollander E, Kolevzon A, Coyle J, editors. Textbook of Autism Spectrum Disorders. Washington, DC: American Psychiatric Publishing; 2011:409–414.
- Shultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry 2000; 57:331–340.
- Wang AT, Dapretto M, Hariri AR, et al. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2004; 43:481–490.
- Mason RA, Williams DL, Kana RK, et al. Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia 2008; 46:269–280.
- Chiu PH, Kayali MA, Kishida KT, et al. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron 2008; 57:463–437.
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A 2006; 103:8275–8280.
- Bölte S, Hubl D, Dierks T, et al. An fMRI-study of locally oriented perception in autism: altered early visual processing of the block design test. J Neural Transm 2008; 115:545–552.
- Maski KP, Jeste SS, Spencer SJ. Common neurological co-morbidities in autism spectrum disorders. Curr Opin Pediatr 2011; 23:609–615.
- Kleinman J, Marciano P, Ault RL. Advanced theory of mind in high functioning adults with autism. J Autism Dev Disord 2011; 31:29–36.
- Fine C, Lumsden J, Blair RJ. Dissociation between ‘theory of mind and executive functions in a patient with early left amygdala damage. Brain 2001; 124:287–298.
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind.’ Trends Cogn Sci 2003; 7:77–83.
- Happé F, Ehlers S, Pletcher P, et al. ‘Theory of mind’ in the brain. Evidence from a PET scan study of Asperger’s syndrome. Neuroreport 1996; 8:197–207.
- Kugimiya S. Clinical features and possible correlations between autism and epilepsy. Neurology Asia 2010; 15(suppl 1):44–45.
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32:351–360.
- Bruni O, Ferri R, Vittori E, et al. Sleep architecture and NREM alterations in children and adolescents with Asperger syndrome. Sleep 2007; 30:1577–1585.
- Richdale AL, Schreck KA. Sleep problems in autism spectrum disorders: prevalence, nature, & possible biopsychosocial aetiologies. Sleep Med Rev 2009; 13:403–411.
- Paavonen EJ, Vehkalahti K, Vanhala R, von Wendt L, Nieminen-von Wendt T, Aronen ET. Sleep in children with Asperger’s syndrome. J Autism Dev Disord 2007; 38:41–51.
- Elder JH, Shankar M, Shuster J, Theriaque D, Burns S, Sherrill L. The gluten-free, casein-free diet in autism: results of a preliminary double blind clinical trial. J Autism Dev Disord 2006; 36:413–420.
- Keller DM. Diet free of gluten and casein has no effect on autism symptoms. Medscape News May 24, 2010. http://www.medscape.com/viewarticle/722283.
- Centers for Disease Control and Prevention (CDC). Recommendations regarding the use of vaccines that contain thimerosal as a preservative. MMWR Morb Mortal Wkly Rep 1999; 48:996–998.
- Thompson WW, Price C, Goodson B, et al; Vaccine Safety Datalink Team. Early thimerosal exposure and neuropsychological outcomes at 7 to 10 years. N Engl J Med 2007; 357:1281–1292.
- Price CS, Thompson WW, Goodson B, et al. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics 2010; 126:656–664.
- Centers for Disease Control and Prevention (CDC). CDC study on “Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism.” www.cdc.gov/vaccinesafety/Concerns/Thimerosal/QA_Pediatrics-thimerosal-autism.html. Accessed November 5, 2012.
- Deer B. How the case against the MMR vaccine was fixed. BMJ 2011; 342:c5347.
- Losh M, Sullivan PF, Trembath D, Piven J. Current developments in the genetics of autism: from phenome to genome. J Neuropathol Exp Neurol 2008; 67:829–837.
- Curran S, Bolton P. Genetics of autism. In:Kim Y-K, editor. Handbook of Behavior Genetics, Part IV. New York, NY: Springer; 2009:397–410.
- State MW. The genetics of child psychiatric disorders: focus on autism and Tourette syndrome. Neuron 2010; 68:254–269.
- Voineagu I, Wang X, Johnston P, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011; 474:380–384.
- Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011; 68:1095–1102.
In 1944, Hans Asperger described a subset of children who exhibited “a lack of empathy, little ability to form friendships, one-sided conversation, intense absorption in a special interest, and clumsy movements.”1
In recent years, Asperger syndrome has become increasingly recognized in the medical community and by the general public. It has been popularized in the media in John Elder Robison’s bestselling book, Look Me in the Eye; with the television character Sheldon Cooper in The Big Bang Theory; and in the 2009 film, Adam, a romantic comedy with the title character accurately portraying a young man with Asperger syndrome.
In this article, we discuss the causes and characteristics of Asperger syndrome, with special focus on adults: how it presents, how to treat it, and how to enhance the delivery of care.
PREVALENCE SEEMS TO BE INCREASING
One in 88 children is diagnosed with an autism spectrum disorder, and the rates of Asperger syndrome and other autism spectrum disorders appear to be increasing.2 Whether this increase is the result of more thorough assessment and identification or of environmental changes is hotly debated.3 The rise began before the proposed changes to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) to combine autism, Asperger syndrome, and pervasive developmental disorder not otherwise specified to simplify diagnosis.4 Asperger syndrome affects males three to four times more often than females.5 For most patients, the effects persist throughout life.
BEHAVIORAL IMPAIRMENTS CHARACTERIZE THE SYNDROME
Poor social skills are a hallmark
People with Asperger syndrome struggle with social interaction and face challenges in forming and maintaining relationships. They tend to have less eye contact (often the first indicator), smiling, animated speech, and physical communication such as hand gestures. They tend not to solicit another’s attention to something they themselves find interesting. They often lack social and emotional reciprocity and have difficulty understanding another person’s thoughts or feelings,6 and they have marked difficulty reading social cues. Some adults may appear rigid, selfish, or narrow-minded.
Sometimes behavior is in the normal range but is out of context for a particular situation.7 For example, a preprofessional student with Asperger syndrome might walk into a psychiatric evaluation to assess fitness for duty and take a seat cross-legged on the floor and have a snack. Poor grooming inappropriate for the occasion may also be observed, such as showing up for a formal photo with unkempt hair and in a stained shirt that is half tucked in.
Many adults with autism spectrum disorders are oblivious to their social reputation.8 They are often unaware that their behavior is out of place and only learn that it is not normal when they are told. Others recognize that they have trouble empathizing with or understanding the perspectives of others, but they are at a loss as to how to improve. The syndrome has a tremendous impact on broader aspects of life, such as employment, functional independence, relationships, and social networks.
Other odd behaviors are common
Repetitive behaviors. Many patients with Asperger syndrome have repetitive behaviors, which can manifest as repeating phrases or expressions, attempting to imitate others, and rocking. They tend to follow routines, do not enjoy spontaneity, and are more inflexible and uncomfortable when their planned regimen is altered.
Gait or balance issues may be observed on physical examination.9 Uncoordinated motion and clumsiness are common,10 and some patients may have a bouncy, stilted gait or may walk on their toes, although the latter is more common in children than adults. Many patients have illegible handwriting.11
Fixations. Many Asperger patients have unusual and intense obsessions with subjects like numbers, dates, or aerodynamics of planes. Children with such fascinations are described as “little professors” or as having “geek syndrome.”12 Certain obsessions often continue into adulthood, although one area of interest may fade and another may take over. Such “expertise” in adults may gain them respect, even though they may seem very odd in other ways.
Lack of boundaries. Patients with Asperger syndrome tend to have poor spatial awareness and to be unaware of physical boundaries, standing too close for others’ comfort or unusually far away. Lack of boundaries may extend beyond the physical, as patients may inappropriately help themselves to food or use an item belonging to another without invitation, being unaware that the behavior may be intrusive or inappropriate.
BEHAVIORAL ASSESSMENTS HELP MAKE DIAGNOSIS
Asperger syndrome is most often diagnosed in early childhood, although it may remain undiagnosed into adulthood. Coexisting depression, attention deficit hyperactivity disorder, or anxiety disorders are also often present.
Establishing the diagnosis is aided by information from family members and others who interact with the patient, from the observations of trained professionals, and from self-reported data. However, self-reported assessments are not always reliable, because the syndrome can affect insight.
The most common assessment tool for autism spectrum disorders is the Autism Diagnostic Interview-Revised (ADI-R),13 a battery of tests given in a structured interview to identify and quantify symptoms, determine where a patient falls on the autism spectrum,14 and point toward interventions. The ADI-R also organizes critical developmental history to evaluate if something else is present, such as prodromal schizophrenia. Although the ADI-R can be very useful in the diagnostic process, it is based on parental reporting, which is neither always available nor fully reliable.
A specific diagnostic tool for adults is the Adult Asperger Assessment.15 Patients are asked to complete two screening questionnaires that gauge cognitive function and gather information about thinking, processing, and behavior.
Table 1 lists the criteria for Asperger syndrome from the DSM Fourth Edition, Text Revision (DSM-IV-TR).16 Asperger syndrome differs from general autism in that it is not associated with language delay. In addition, patients with Asperger syndrome usually have average or above-average IQ scores.17 Still, determining whether a patient has Asperger syndrome or high-functioning autism is sometimes challenging.6
In DSM-V, Asperger syndrome will be subsumed under autism spectrum disorder
In 2013, the DSM-V will replace the DSMIV-TR and will combine autism, Asperger syndrome, and pervasive developmental disorder not otherwise classified into a single diagnosis: autism spectrum disorder. The new system uses two instead of the previous three clusters of core symptoms, centered on “social reciprocity and communication” in one arm and “restricted interests and repetitive behavior” in the other.18 There will be less emphasis on play and imagination than in the past. Some authors suggest adding sensory criteria, particularly reduced pain and increased hearing sensitivity.19
The proposed system is sensitive and specific for autism spectrum disorders, allows early diagnosis, and indicates degree of severity.20 It is hoped that the new system, which accounts for the range and severity of symptoms, should help physicians refer patients to the correct level of treatment.
On the other hand, it may be difficult to think of the three disorders as a single diagnosis. Asperger syndrome manifests in distinct ways, and clear behavioral criteria for diagnosis can be invaluable in helping people with the syndrome. Also, the public may continue to refer to it as Asperger syndrome, and parents and patients may feel uncomfortable having it considered to be the same diagnosis as autism.
BEHAVIORAL THERAPY CAN HELP ACHIEVE INDEPENDENCE
Although there is no cure for Asperger syndrome, various interventions can dramatically improve quality of life and independence. The health care team may include a primary care physician, psychologist, psychiatrist, neurologist, and speech therapist.
Behavioral therapies can help patients with Asperger syndrome learn skills to reduce their symptoms. Occupational and physical therapy can improve dexterity, fluidity, and coordination. Desensitization training may help patients adapt to uncomfortable sights, sounds, or smells that may arise. This can be critical in a job situation. For example, while an average person exposed to a foul odor in public is likely to react tactfully, a person with Asperger syndrome may scream loudly, make inappropriate comments, or run from the room. Social training, especially targeted to the workplace, can provide strategies for promoting typical behaviors and be key to maximizing functional independence.
Speech therapists can teach patients how to sound more relaxed and help them master the natural give-and-take of conversational exchange. Psychotherapy can provide a safe place to work on anxiety, express emotions, and manage restricted interests or repeated behaviors. Group therapy or social training can be a venue for learning improved interactions.
Living independently can be very challenging, and patients with Asperger syndrome may need functional independence training to help with a variety of skills, from handling finances to organizing the home.
Improving quality of life includes determining the best learning environments from childhood into college years and beyond.21–23 Socialization can be enhanced with additional social support at home or on campus, through family interactions and collaborative learning, and by teaching empathy.24 Vocational training can be extremely useful.
DRUG THERAPY MAY HAVE A ROLE
Medications are not usually prescribed unless depression or anxiety is also present, but they may also help manage irritability, anger, stereotypical mannerisms, and disturbing movements. Fluoxetine (Prozac) helps reduce repetitive behaviors in adults with Asperger syndrome. Propranolol (Inderal), a well-known antihypertensive, is also used for performance anxiety and improves word fluency, understanding of verbal communication, and verbal problem-solving in patients with an autism spectrum disorder.25
Giving oxytocin (Pitocin) intranasally in a spray formulation is currently being tested to enhance social skills. Patients with an autism spectrum disorder were more able to perceive emotions of others and to respond more appropriately.26 Oxytocin has long been associated with bonding and is believed to enhance mothering skills. It is naturally present in both sexes, but levels are higher in women, which may in part explain the lower rate of autism spectrum disorders in females.27
HEALTH CARE REQUIRES SPECIAL CONSIDERATIONS
Medical care for patients with Asperger syndrome is enhanced by understanding the patient’s experience. Adults, in particular, may have learned to suppress symptoms of Asperger syndrome to better function in society but still experience stress in situations in which others would not. Patients with Asperger syndrome may struggle with social interactions during medical examinations or procedures, and clinicians may find interaction with the patient challenging.
It is important for health care providers to be calm and patient and to understand that anxiety may prevent people with Asperger syndrome from making eye contact. The clinician should confirm that a patient is engaged but should avoid seeming pushy or invasive.
When anxious, patients may employ strange gestures that they find soothing, such as flapping the hands, rocking, or cracking the knuckles. It is usually easier to allow them to continue unless the activity hinders the examination or treatment.
Patients are likely to respond better to direct requests than to subtle questions: eg, “Open your mouth, please” instead of “Could you open your mouth?” Using clear, specific language and avoiding metaphors, irony, and nonverbal communication are best. It is important to explicitly ask for everything needed, as patients may not volunteer information and may have trouble articulating what they are thinking or feeling. While educating patients about their health needs, physicians may need to reiterate guidance several times or approach the same topic from different angles in order for the patient to accept a concern.
All actions, especially touching the patient, should be explained clearly beforehand. If possible, the doctor should demonstrate using visuals or on his or her own body if appropriate. For invasive procedures, anesthetizing the local area is recommended.
People with Asperger syndrome often rely heavily on a regular routine to maintain a sense of organization. By interrupting this routine, a doctor’s visit can induce anxiety. Waiting also increases anxiety, so scheduling patients with Asperger syndrome either first or last in the day may help.
Hypersensitivity poses challenges
Many people with Asperger syndrome have abnormal sensitivity to stimuli, with differences in pain sensation and hearing perhaps most prominent. Loud noises, such as beeping equipment, whirring fans, or buzzing lights may be distressful and should be reduced if possible. Patients may also be strongly affected by bright lights or scents such as perfumes.
Patients may also have an altered sense of taste, with consequences that go beyond simple “picky eating.” Patients should be asked about unusual eating patterns, diets, or food aversions. People with autism spectrum disorders often do not consume adequate vitamin C because of an aversion to fruits and vegetables. Vitamin deficiency may have originated in infancy but may not be identified or treated until adulthood.28
The sense of touch may be intensified, causing patients to be extremely ticklish; they may actually prefer to be touched more firmly. When it is necessary to make physical contact with patients, it will make the process easier if the physician determines their comfort level and finds ways to help them endure the experience with the least amount of discomfort.
Some patients with impaired sensory expression may have a high tolerance for extreme temperatures and pain, leading to delay in seeking aid.29 Patients may downgrade pain levels, masking the severity of an illness or injury.
Transition from pediatric to adult care
Pediatrics is often a warm environment in which children develop a trusting relationship with their care providers. The transition to adult care can be daunting for patients with Asperger syndrome and their families, and many postpone the change for as long as possible.
Although time-consuming, a collaborative effort between the pediatric and adult care teams can dramatically smooth the transition. It can help to have a familiar person from the pediatric team, such as a nurse, be present at the initial interaction with the new adult care team. Both teams should be familiar with the other’s clinical practices and be aware of the patient’s stressors and ways to ameliorate them.30
THE SEARCH FOR A CAUSE CONTINUES
Numerous studies are attempting to understand the anatomic and physiologic causes of autism spectrum disorders, and to find effective treatments and improve the quality of life.
Prenatal factors implicated
Several recent studies have focused on environmental factors during pregnancy as risk factors for autism spectrum disorder. Selective serotonin reuptake inhibitors were found to increase the risk,31 but the severity of the mother’s depressive illness must be considered before counseling against using these drugs. Older maternal or paternal age was also found to increase the risk of an autism spectrum disorder.32 Recent research indicates that older fathers are in particular more likely to have children with disorders such as autism because of an increase in random mutations associated with advanced age.33
Maternal illness during pregnancy is also associated. Preliminary studies found an increased risk of autism if the mother had had a prenatal viral infection.34 A more recent study found that untreated fever during pregnancy rather than a specific viral infection is more strongly linked.35
Maternal antibodies have been implicated as well. One review found that psoriasis is the only maternal autoimmune condition significantly associated with the development of an autism spectrum disorder.36 Elevated levels of antibodies against the fetal brain have been found in mothers with autistic children.37 One study found that autistic children and their siblings have elevated antibrain antibodies in distinct brain regions, including the caudate nucleus, putamen, prefrontal cortex, cerebellum, and cingulate gyrus (why the siblings are spared from having the disorder is unclear).38 Some have questioned whether a child’s own immune system might even be involved.39
Functional magnetic resonance imaging reveals multiple differences
Functional magnetic resonance imaging (fMRI) has been used to investigate impaired social interaction, specific deficits of facial perception and recognition, sensory processing, working memory, and “theory of mind.” Hypoactivation, hyperactivation, and decreased functional connectivity have been observed depending on the mental processes evaluated.40
When undergoing facial perception tasks, subjects with autism spectrum disorders exhibit hypoactivation in the lateral aspect of the middle region of the fusiform gyrus, responsible for face identification. But they have significant activation of the limbic system, specifically the amygdala, during facial recognition. Hypoactivity in the fusiform gyrus is observed when trying to identify faces or read facial expressions.41,42 This cluster of findings helps explain misinterpretations, misidentification, and heightened affect.
A hallmark characteristic of autism is the difficulty patients have in determining intentions and interpreting others’ behavior, thoughts, or emotions. Studies of people with autism spectrum disorders show that areas often responsible for “sensitivity to others” are hypoactive.43 There is also diminished activation in the medial cingulate cortex, normally activated when these people are asked to think about themselves and who they are.44
The resting state in the brains of people with autism spectrum disorders is abnormally activated.45 They are often particularly good at attention to detail but challenged in integrating information needed for general executive functioning. Impaired sensory processing makes it difficult for them to simultaneously interpret multiple sources of sensory input.46
Perhaps some of the most exciting fMRI news comes from infant studies. Radical and axial diffusivity and fractional anisotropy techniques demonstrate differences in the brains of infants 6 to 24 months old, before symptoms of autism spectrum disorders are observed. It is hoped that early intervention could come into play before the syndrome develops fully.47
The synthesis of input of social and emotional cues is sometimes referred to in the literature as “theory of mind.” It is impaired in Asperger syndrome,48 as manifested by a lack of empathy and by challenges in perceiving others’ thoughts and feelings. The basis of impairment may be related to abnormalities in the amygdala.49 Normal awareness involves the integration of multiple neural networks in the anterior paracingulate cortex, the superior temporal sulci, and the temporal poles bilaterally, but different regions appear to be used in patients with Asperger syndrome.50 A small series of five case studies using positron emission tomography indicated that the left prefrontal cortex was the primary location for theory of mind in Asperger syndrome.51
Epilepsy, gastrointestinal problems, and sleep disturbances are associated
About 25% of people with autism spectrum disorders have epilepsy vs 2% to 3% in the general population. Asperger syndrome is associated with a much lower but still elevated risk of 4% to 6%.47,52
Gastrointestinal complaints, most often constipation or chronic diarrhea, are much more common in children with autism spectrum disorders than in the general population. Preliminary data showed that children with an autism spectrum disorder have a 42% rate of gastrointestinal problems vs 12% in unaffected siblings. There is also a correlation between the severity of gastrointestinal problems and severity of autistic symptoms.53
Research is ongoing to determine the prevalence of insomnia or interrupted sleep in those with autism spectrum disorders.54–56 Changes in sleep architecture can explain nighttime activity.
NONTRADITIONAL CONSIDERATIONS
Dietary treatment: Mixed findings
A popular hypothesis is that adherence to a gluten-free or casein-free diet can reduce symptoms of autism spectrum disorders. Preliminary reports identified several cases of children showing improvement.57 However, this has not been replicated, and more studies refute benefits of these diets.58
Essential nutritional needs should be met with any diet, whether it is designed to reduce symptoms or not. Patients with autism spectrum disorders may have strong food aversions, and dietary supplements of vitamins and minerals may be required.
Vaccines do not cause autism
Despite popular concern, recent research indicates that vaccines do not cause autism. Thimerosal, a mercury-based preservative used in childhood vaccines, was at one time implicated as a risk factor for autism spectrum disorders. The US Centers for Disease Control and Prevention (CDC) issued a precaution against using thimerosal-containing vaccines while testing was done to determine the effects on neuropsychological development.59 The CDC study as well as newer studies did not demonstrate that exposure to mercury causes these neuropsychological concerns, but researchers have continued to study the subject.60–62 The original study implicating thimerosal was disproven as scientifically unsound and fraught with conflict of interest and legal concerns. It has since been retracted, and its findings have been completely discredited.63
Other areas of research
Current research is exploring the higher prevalence of autism spectrum disorders in particular families.64–66 Autism and autism spectrum disorders may be caused by hundreds of simultaneous gene alterations or may develop as a result of reduced gene expression in two areas of the cerebral cortex where higher-order processing occurs, in the frontal and temporal lobes.67
Although genetic theories of autism predominate, a 2011 project suggests that environment is also important. A study of twins found that genetics accounted for 40% or less of cases of autism spectrum disorder, with at least 55% of cases being attributable to environmental factors.68
In 1944, Hans Asperger described a subset of children who exhibited “a lack of empathy, little ability to form friendships, one-sided conversation, intense absorption in a special interest, and clumsy movements.”1
In recent years, Asperger syndrome has become increasingly recognized in the medical community and by the general public. It has been popularized in the media in John Elder Robison’s bestselling book, Look Me in the Eye; with the television character Sheldon Cooper in The Big Bang Theory; and in the 2009 film, Adam, a romantic comedy with the title character accurately portraying a young man with Asperger syndrome.
In this article, we discuss the causes and characteristics of Asperger syndrome, with special focus on adults: how it presents, how to treat it, and how to enhance the delivery of care.
PREVALENCE SEEMS TO BE INCREASING
One in 88 children is diagnosed with an autism spectrum disorder, and the rates of Asperger syndrome and other autism spectrum disorders appear to be increasing.2 Whether this increase is the result of more thorough assessment and identification or of environmental changes is hotly debated.3 The rise began before the proposed changes to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) to combine autism, Asperger syndrome, and pervasive developmental disorder not otherwise specified to simplify diagnosis.4 Asperger syndrome affects males three to four times more often than females.5 For most patients, the effects persist throughout life.
BEHAVIORAL IMPAIRMENTS CHARACTERIZE THE SYNDROME
Poor social skills are a hallmark
People with Asperger syndrome struggle with social interaction and face challenges in forming and maintaining relationships. They tend to have less eye contact (often the first indicator), smiling, animated speech, and physical communication such as hand gestures. They tend not to solicit another’s attention to something they themselves find interesting. They often lack social and emotional reciprocity and have difficulty understanding another person’s thoughts or feelings,6 and they have marked difficulty reading social cues. Some adults may appear rigid, selfish, or narrow-minded.
Sometimes behavior is in the normal range but is out of context for a particular situation.7 For example, a preprofessional student with Asperger syndrome might walk into a psychiatric evaluation to assess fitness for duty and take a seat cross-legged on the floor and have a snack. Poor grooming inappropriate for the occasion may also be observed, such as showing up for a formal photo with unkempt hair and in a stained shirt that is half tucked in.
Many adults with autism spectrum disorders are oblivious to their social reputation.8 They are often unaware that their behavior is out of place and only learn that it is not normal when they are told. Others recognize that they have trouble empathizing with or understanding the perspectives of others, but they are at a loss as to how to improve. The syndrome has a tremendous impact on broader aspects of life, such as employment, functional independence, relationships, and social networks.
Other odd behaviors are common
Repetitive behaviors. Many patients with Asperger syndrome have repetitive behaviors, which can manifest as repeating phrases or expressions, attempting to imitate others, and rocking. They tend to follow routines, do not enjoy spontaneity, and are more inflexible and uncomfortable when their planned regimen is altered.
Gait or balance issues may be observed on physical examination.9 Uncoordinated motion and clumsiness are common,10 and some patients may have a bouncy, stilted gait or may walk on their toes, although the latter is more common in children than adults. Many patients have illegible handwriting.11
Fixations. Many Asperger patients have unusual and intense obsessions with subjects like numbers, dates, or aerodynamics of planes. Children with such fascinations are described as “little professors” or as having “geek syndrome.”12 Certain obsessions often continue into adulthood, although one area of interest may fade and another may take over. Such “expertise” in adults may gain them respect, even though they may seem very odd in other ways.
Lack of boundaries. Patients with Asperger syndrome tend to have poor spatial awareness and to be unaware of physical boundaries, standing too close for others’ comfort or unusually far away. Lack of boundaries may extend beyond the physical, as patients may inappropriately help themselves to food or use an item belonging to another without invitation, being unaware that the behavior may be intrusive or inappropriate.
BEHAVIORAL ASSESSMENTS HELP MAKE DIAGNOSIS
Asperger syndrome is most often diagnosed in early childhood, although it may remain undiagnosed into adulthood. Coexisting depression, attention deficit hyperactivity disorder, or anxiety disorders are also often present.
Establishing the diagnosis is aided by information from family members and others who interact with the patient, from the observations of trained professionals, and from self-reported data. However, self-reported assessments are not always reliable, because the syndrome can affect insight.
The most common assessment tool for autism spectrum disorders is the Autism Diagnostic Interview-Revised (ADI-R),13 a battery of tests given in a structured interview to identify and quantify symptoms, determine where a patient falls on the autism spectrum,14 and point toward interventions. The ADI-R also organizes critical developmental history to evaluate if something else is present, such as prodromal schizophrenia. Although the ADI-R can be very useful in the diagnostic process, it is based on parental reporting, which is neither always available nor fully reliable.
A specific diagnostic tool for adults is the Adult Asperger Assessment.15 Patients are asked to complete two screening questionnaires that gauge cognitive function and gather information about thinking, processing, and behavior.
Table 1 lists the criteria for Asperger syndrome from the DSM Fourth Edition, Text Revision (DSM-IV-TR).16 Asperger syndrome differs from general autism in that it is not associated with language delay. In addition, patients with Asperger syndrome usually have average or above-average IQ scores.17 Still, determining whether a patient has Asperger syndrome or high-functioning autism is sometimes challenging.6
In DSM-V, Asperger syndrome will be subsumed under autism spectrum disorder
In 2013, the DSM-V will replace the DSMIV-TR and will combine autism, Asperger syndrome, and pervasive developmental disorder not otherwise classified into a single diagnosis: autism spectrum disorder. The new system uses two instead of the previous three clusters of core symptoms, centered on “social reciprocity and communication” in one arm and “restricted interests and repetitive behavior” in the other.18 There will be less emphasis on play and imagination than in the past. Some authors suggest adding sensory criteria, particularly reduced pain and increased hearing sensitivity.19
The proposed system is sensitive and specific for autism spectrum disorders, allows early diagnosis, and indicates degree of severity.20 It is hoped that the new system, which accounts for the range and severity of symptoms, should help physicians refer patients to the correct level of treatment.
On the other hand, it may be difficult to think of the three disorders as a single diagnosis. Asperger syndrome manifests in distinct ways, and clear behavioral criteria for diagnosis can be invaluable in helping people with the syndrome. Also, the public may continue to refer to it as Asperger syndrome, and parents and patients may feel uncomfortable having it considered to be the same diagnosis as autism.
BEHAVIORAL THERAPY CAN HELP ACHIEVE INDEPENDENCE
Although there is no cure for Asperger syndrome, various interventions can dramatically improve quality of life and independence. The health care team may include a primary care physician, psychologist, psychiatrist, neurologist, and speech therapist.
Behavioral therapies can help patients with Asperger syndrome learn skills to reduce their symptoms. Occupational and physical therapy can improve dexterity, fluidity, and coordination. Desensitization training may help patients adapt to uncomfortable sights, sounds, or smells that may arise. This can be critical in a job situation. For example, while an average person exposed to a foul odor in public is likely to react tactfully, a person with Asperger syndrome may scream loudly, make inappropriate comments, or run from the room. Social training, especially targeted to the workplace, can provide strategies for promoting typical behaviors and be key to maximizing functional independence.
Speech therapists can teach patients how to sound more relaxed and help them master the natural give-and-take of conversational exchange. Psychotherapy can provide a safe place to work on anxiety, express emotions, and manage restricted interests or repeated behaviors. Group therapy or social training can be a venue for learning improved interactions.
Living independently can be very challenging, and patients with Asperger syndrome may need functional independence training to help with a variety of skills, from handling finances to organizing the home.
Improving quality of life includes determining the best learning environments from childhood into college years and beyond.21–23 Socialization can be enhanced with additional social support at home or on campus, through family interactions and collaborative learning, and by teaching empathy.24 Vocational training can be extremely useful.
DRUG THERAPY MAY HAVE A ROLE
Medications are not usually prescribed unless depression or anxiety is also present, but they may also help manage irritability, anger, stereotypical mannerisms, and disturbing movements. Fluoxetine (Prozac) helps reduce repetitive behaviors in adults with Asperger syndrome. Propranolol (Inderal), a well-known antihypertensive, is also used for performance anxiety and improves word fluency, understanding of verbal communication, and verbal problem-solving in patients with an autism spectrum disorder.25
Giving oxytocin (Pitocin) intranasally in a spray formulation is currently being tested to enhance social skills. Patients with an autism spectrum disorder were more able to perceive emotions of others and to respond more appropriately.26 Oxytocin has long been associated with bonding and is believed to enhance mothering skills. It is naturally present in both sexes, but levels are higher in women, which may in part explain the lower rate of autism spectrum disorders in females.27
HEALTH CARE REQUIRES SPECIAL CONSIDERATIONS
Medical care for patients with Asperger syndrome is enhanced by understanding the patient’s experience. Adults, in particular, may have learned to suppress symptoms of Asperger syndrome to better function in society but still experience stress in situations in which others would not. Patients with Asperger syndrome may struggle with social interactions during medical examinations or procedures, and clinicians may find interaction with the patient challenging.
It is important for health care providers to be calm and patient and to understand that anxiety may prevent people with Asperger syndrome from making eye contact. The clinician should confirm that a patient is engaged but should avoid seeming pushy or invasive.
When anxious, patients may employ strange gestures that they find soothing, such as flapping the hands, rocking, or cracking the knuckles. It is usually easier to allow them to continue unless the activity hinders the examination or treatment.
Patients are likely to respond better to direct requests than to subtle questions: eg, “Open your mouth, please” instead of “Could you open your mouth?” Using clear, specific language and avoiding metaphors, irony, and nonverbal communication are best. It is important to explicitly ask for everything needed, as patients may not volunteer information and may have trouble articulating what they are thinking or feeling. While educating patients about their health needs, physicians may need to reiterate guidance several times or approach the same topic from different angles in order for the patient to accept a concern.
All actions, especially touching the patient, should be explained clearly beforehand. If possible, the doctor should demonstrate using visuals or on his or her own body if appropriate. For invasive procedures, anesthetizing the local area is recommended.
People with Asperger syndrome often rely heavily on a regular routine to maintain a sense of organization. By interrupting this routine, a doctor’s visit can induce anxiety. Waiting also increases anxiety, so scheduling patients with Asperger syndrome either first or last in the day may help.
Hypersensitivity poses challenges
Many people with Asperger syndrome have abnormal sensitivity to stimuli, with differences in pain sensation and hearing perhaps most prominent. Loud noises, such as beeping equipment, whirring fans, or buzzing lights may be distressful and should be reduced if possible. Patients may also be strongly affected by bright lights or scents such as perfumes.
Patients may also have an altered sense of taste, with consequences that go beyond simple “picky eating.” Patients should be asked about unusual eating patterns, diets, or food aversions. People with autism spectrum disorders often do not consume adequate vitamin C because of an aversion to fruits and vegetables. Vitamin deficiency may have originated in infancy but may not be identified or treated until adulthood.28
The sense of touch may be intensified, causing patients to be extremely ticklish; they may actually prefer to be touched more firmly. When it is necessary to make physical contact with patients, it will make the process easier if the physician determines their comfort level and finds ways to help them endure the experience with the least amount of discomfort.
Some patients with impaired sensory expression may have a high tolerance for extreme temperatures and pain, leading to delay in seeking aid.29 Patients may downgrade pain levels, masking the severity of an illness or injury.
Transition from pediatric to adult care
Pediatrics is often a warm environment in which children develop a trusting relationship with their care providers. The transition to adult care can be daunting for patients with Asperger syndrome and their families, and many postpone the change for as long as possible.
Although time-consuming, a collaborative effort between the pediatric and adult care teams can dramatically smooth the transition. It can help to have a familiar person from the pediatric team, such as a nurse, be present at the initial interaction with the new adult care team. Both teams should be familiar with the other’s clinical practices and be aware of the patient’s stressors and ways to ameliorate them.30
THE SEARCH FOR A CAUSE CONTINUES
Numerous studies are attempting to understand the anatomic and physiologic causes of autism spectrum disorders, and to find effective treatments and improve the quality of life.
Prenatal factors implicated
Several recent studies have focused on environmental factors during pregnancy as risk factors for autism spectrum disorder. Selective serotonin reuptake inhibitors were found to increase the risk,31 but the severity of the mother’s depressive illness must be considered before counseling against using these drugs. Older maternal or paternal age was also found to increase the risk of an autism spectrum disorder.32 Recent research indicates that older fathers are in particular more likely to have children with disorders such as autism because of an increase in random mutations associated with advanced age.33
Maternal illness during pregnancy is also associated. Preliminary studies found an increased risk of autism if the mother had had a prenatal viral infection.34 A more recent study found that untreated fever during pregnancy rather than a specific viral infection is more strongly linked.35
Maternal antibodies have been implicated as well. One review found that psoriasis is the only maternal autoimmune condition significantly associated with the development of an autism spectrum disorder.36 Elevated levels of antibodies against the fetal brain have been found in mothers with autistic children.37 One study found that autistic children and their siblings have elevated antibrain antibodies in distinct brain regions, including the caudate nucleus, putamen, prefrontal cortex, cerebellum, and cingulate gyrus (why the siblings are spared from having the disorder is unclear).38 Some have questioned whether a child’s own immune system might even be involved.39
Functional magnetic resonance imaging reveals multiple differences
Functional magnetic resonance imaging (fMRI) has been used to investigate impaired social interaction, specific deficits of facial perception and recognition, sensory processing, working memory, and “theory of mind.” Hypoactivation, hyperactivation, and decreased functional connectivity have been observed depending on the mental processes evaluated.40
When undergoing facial perception tasks, subjects with autism spectrum disorders exhibit hypoactivation in the lateral aspect of the middle region of the fusiform gyrus, responsible for face identification. But they have significant activation of the limbic system, specifically the amygdala, during facial recognition. Hypoactivity in the fusiform gyrus is observed when trying to identify faces or read facial expressions.41,42 This cluster of findings helps explain misinterpretations, misidentification, and heightened affect.
A hallmark characteristic of autism is the difficulty patients have in determining intentions and interpreting others’ behavior, thoughts, or emotions. Studies of people with autism spectrum disorders show that areas often responsible for “sensitivity to others” are hypoactive.43 There is also diminished activation in the medial cingulate cortex, normally activated when these people are asked to think about themselves and who they are.44
The resting state in the brains of people with autism spectrum disorders is abnormally activated.45 They are often particularly good at attention to detail but challenged in integrating information needed for general executive functioning. Impaired sensory processing makes it difficult for them to simultaneously interpret multiple sources of sensory input.46
Perhaps some of the most exciting fMRI news comes from infant studies. Radical and axial diffusivity and fractional anisotropy techniques demonstrate differences in the brains of infants 6 to 24 months old, before symptoms of autism spectrum disorders are observed. It is hoped that early intervention could come into play before the syndrome develops fully.47
The synthesis of input of social and emotional cues is sometimes referred to in the literature as “theory of mind.” It is impaired in Asperger syndrome,48 as manifested by a lack of empathy and by challenges in perceiving others’ thoughts and feelings. The basis of impairment may be related to abnormalities in the amygdala.49 Normal awareness involves the integration of multiple neural networks in the anterior paracingulate cortex, the superior temporal sulci, and the temporal poles bilaterally, but different regions appear to be used in patients with Asperger syndrome.50 A small series of five case studies using positron emission tomography indicated that the left prefrontal cortex was the primary location for theory of mind in Asperger syndrome.51
Epilepsy, gastrointestinal problems, and sleep disturbances are associated
About 25% of people with autism spectrum disorders have epilepsy vs 2% to 3% in the general population. Asperger syndrome is associated with a much lower but still elevated risk of 4% to 6%.47,52
Gastrointestinal complaints, most often constipation or chronic diarrhea, are much more common in children with autism spectrum disorders than in the general population. Preliminary data showed that children with an autism spectrum disorder have a 42% rate of gastrointestinal problems vs 12% in unaffected siblings. There is also a correlation between the severity of gastrointestinal problems and severity of autistic symptoms.53
Research is ongoing to determine the prevalence of insomnia or interrupted sleep in those with autism spectrum disorders.54–56 Changes in sleep architecture can explain nighttime activity.
NONTRADITIONAL CONSIDERATIONS
Dietary treatment: Mixed findings
A popular hypothesis is that adherence to a gluten-free or casein-free diet can reduce symptoms of autism spectrum disorders. Preliminary reports identified several cases of children showing improvement.57 However, this has not been replicated, and more studies refute benefits of these diets.58
Essential nutritional needs should be met with any diet, whether it is designed to reduce symptoms or not. Patients with autism spectrum disorders may have strong food aversions, and dietary supplements of vitamins and minerals may be required.
Vaccines do not cause autism
Despite popular concern, recent research indicates that vaccines do not cause autism. Thimerosal, a mercury-based preservative used in childhood vaccines, was at one time implicated as a risk factor for autism spectrum disorders. The US Centers for Disease Control and Prevention (CDC) issued a precaution against using thimerosal-containing vaccines while testing was done to determine the effects on neuropsychological development.59 The CDC study as well as newer studies did not demonstrate that exposure to mercury causes these neuropsychological concerns, but researchers have continued to study the subject.60–62 The original study implicating thimerosal was disproven as scientifically unsound and fraught with conflict of interest and legal concerns. It has since been retracted, and its findings have been completely discredited.63
Other areas of research
Current research is exploring the higher prevalence of autism spectrum disorders in particular families.64–66 Autism and autism spectrum disorders may be caused by hundreds of simultaneous gene alterations or may develop as a result of reduced gene expression in two areas of the cerebral cortex where higher-order processing occurs, in the frontal and temporal lobes.67
Although genetic theories of autism predominate, a 2011 project suggests that environment is also important. A study of twins found that genetics accounted for 40% or less of cases of autism spectrum disorder, with at least 55% of cases being attributable to environmental factors.68
- Frith U, editor. Autism and Asperger Syndrome. New York: Cambridge University Press, 1991:37–92.
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ 2012; 61( 3):1–19.
- Rutter M. Incidence of autism spectrum disorders: changes over time and their meaning. Acta Paediatr 2005; 94:2–15.
- Happé F. Criteria, categories, and continua: autism and related disorders in DSM-5. J Am Acad Child Adolesc Psychiatry 2011; 50:540–542.
- National Institute of Neurological Disorders and Stroke. Asperger syndrome fact sheet. http://www.ninds.nih.gov/disorders/asperger/detail_asperger.htm. Accessed October 11, 2012.
- Toth K, King BH. Asperger’s syndrome: diagnosis and treatment. Am J Psychiatry 2008; 165:958–963.
- Vermeulen P. Autism: from mind blindness to context blindness. Asperger’s Digest November/December 2011. http://autismdigest.com/autism-from-mind-blindness-to-context-blindness/. Accessed October 11, 2012.
- Izuma K, Matsumoto K, Camerer CF, Adolphs R. Insensitivity to social reputation in autism. Proc Natl Acad Sci U S A. 2011; 108:17302–17307.
- Weimer AK, Schatz AM, Lincoln A, Ballantyne AO, Trauner DA. “Motor” impairment in Asperger’s syndrome: evidence for a deficit in proprioception. J Dev Behav Pediatr 2001; 22:92–101.
- Siaperas P, Ring HA, McAllister CJ, et al. Atypical movement performance and sensory integration in Asperger’s syndrome. J Autism Dev Disord 2012; 42:718–725.
- Kushki A, Chau T, Anagnostou E. Handwriting difficulties in children with autism spectrum disorders: a scoping review. J Autism Dev Disord 2011; 41:1706–1716.
- Nash JM, Bonesteel A. The geek syndrome. Time Magazine U.S. 2002. http://www.time.com/time/magazine/article/0,9171,1002365-1,00.html. Accessed October 11, 2012.
- Le Couteur A, Rutter M, Lord C, et al. Autism diagnostic interview: a standardized investigatorbased instrument. J Autism Dev Disord 1989; 19:363–387.
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised WPS Edition Manual. Los Angeles, CA. Western Psychological Services; 2003.
- Baron-Cohen S, Wheelwright S, Robinson J, Woodbury-Smith M. The Adult Asperger’s Assessment (AAA): a diagnostic method. J Autism Developmental Disord 2005; 35:807–819.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Text Revision DSM IV–TR 4th Ed. 2000; Washington, DC: American Psychiatric Association; 2000:80–84.
- Centers for Disease Control. Asperger syndrome fact sheet. http://www.cdc.gov/ncbddd/actearly/pdf/parents_pdfs/Asperger_Syndrome.pdf. Accessed October 11, 2012.
- Peckham C. The current state in autism—still tough to treat but encouraging progress. An expert interview with Fred R. Volkmar, MD. Medscape Pediatrics 2010. http://www.medscape.com/viewarticle/720802?src=mp&spon=17. Accessed October 31, 2012.
- Muscari ME. How should I evaluate an adult for possible Asperger’s syndrome? Medscape News Today 2006.
- Hollander E. Can we treat core symptoms of autism spectrum disorders in adults? December 21, 2011; 1( 18). http://www.medscape.com/viewarticle/531750. Accessed October 1, 2012.
- Müller E, Schuler A, Yates GB. Social challenges and supports from the perspective of individuals with Asperger’s syndrome and other autism spectrum disabilities. Autism 2008; 12:173–190.
- Helman T, Berger O. Parents of children with Asperger’s syndrome or with learning disabilities: family environment and social support. Res Dev Disabil 2008; 29;289–300.
- Taylor CM. Campus commons. When pigs fly: a new perspective on learning. About Campus 2011; 16:30–32.
- Cheng Y, Chiang H, Ye J, Cheng L. Enhancing empathy instruction using a collaborative virtual learning environment for children with autistic spectrum conditions. Comput Edu 2010; 55:1449–1458.
- Beversdorf DQ, Saklayen S, Higgins KF, Bodner KE, Kanne SM, Christ SE. Effect of propranolol on word fluency in autism. Cogn Behav Neurol 2011; 24:11–17.
- Kuehn BM. Scientists probe oxytocin therapy for social deficits in autism, schizophrenia. JAMA 2011; 305:659–661.
- Pfaff DW, Rapin I, Goldman S. Male dominance in autism: neuroendocrine influences on arousal and social anxiety. Autism Res 2011; 4:163–176.
- Brauser D. Children with autism routinely exhibit feeding difficulties in infancy. Medscape Medical News 2010. http://www.medscape.org/viewarticle/726060. Accessed October 31, 2012.
- Baron MG, Groden J, Groden G, Lipsitt L. Stress and coping in autism. New York: Oxford University Press; 2006:355.
- Camfield P, Camfield C. Transition to adult care for children with chronic neurological disorders. Ann Neurol 2001; 69:437–444.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011; 68:1104–1112.
- Croen LA, Najjar DV, Fireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med 2007; 161:334–340.
- Kong A, Frigge ML, Masson G, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 2012; 488:471–475.
- Atlandóttir HO, Thorsen P, Østergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 2010; 40:1423–1430.
- Zerbo O, Iosif A-M, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord 2012; 10.1007/s10803-012-1540-x.
- Crown LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med 2005; 159:151–157.
- Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neruoimmunol 2008; 194:165–172.
- Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol 2006; 178:149–155.
- Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmunity Rev 2004; 3:557–562.
- South M, Diehl JJ. Functional magnetic resonance imaging. In:Hollander E, Kolevzon A, Coyle J, editors. Textbook of Autism Spectrum Disorders. Washington, DC: American Psychiatric Publishing; 2011:409–414.
- Shultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry 2000; 57:331–340.
- Wang AT, Dapretto M, Hariri AR, et al. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2004; 43:481–490.
- Mason RA, Williams DL, Kana RK, et al. Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia 2008; 46:269–280.
- Chiu PH, Kayali MA, Kishida KT, et al. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron 2008; 57:463–437.
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A 2006; 103:8275–8280.
- Bölte S, Hubl D, Dierks T, et al. An fMRI-study of locally oriented perception in autism: altered early visual processing of the block design test. J Neural Transm 2008; 115:545–552.
- Maski KP, Jeste SS, Spencer SJ. Common neurological co-morbidities in autism spectrum disorders. Curr Opin Pediatr 2011; 23:609–615.
- Kleinman J, Marciano P, Ault RL. Advanced theory of mind in high functioning adults with autism. J Autism Dev Disord 2011; 31:29–36.
- Fine C, Lumsden J, Blair RJ. Dissociation between ‘theory of mind and executive functions in a patient with early left amygdala damage. Brain 2001; 124:287–298.
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind.’ Trends Cogn Sci 2003; 7:77–83.
- Happé F, Ehlers S, Pletcher P, et al. ‘Theory of mind’ in the brain. Evidence from a PET scan study of Asperger’s syndrome. Neuroreport 1996; 8:197–207.
- Kugimiya S. Clinical features and possible correlations between autism and epilepsy. Neurology Asia 2010; 15(suppl 1):44–45.
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32:351–360.
- Bruni O, Ferri R, Vittori E, et al. Sleep architecture and NREM alterations in children and adolescents with Asperger syndrome. Sleep 2007; 30:1577–1585.
- Richdale AL, Schreck KA. Sleep problems in autism spectrum disorders: prevalence, nature, & possible biopsychosocial aetiologies. Sleep Med Rev 2009; 13:403–411.
- Paavonen EJ, Vehkalahti K, Vanhala R, von Wendt L, Nieminen-von Wendt T, Aronen ET. Sleep in children with Asperger’s syndrome. J Autism Dev Disord 2007; 38:41–51.
- Elder JH, Shankar M, Shuster J, Theriaque D, Burns S, Sherrill L. The gluten-free, casein-free diet in autism: results of a preliminary double blind clinical trial. J Autism Dev Disord 2006; 36:413–420.
- Keller DM. Diet free of gluten and casein has no effect on autism symptoms. Medscape News May 24, 2010. http://www.medscape.com/viewarticle/722283.
- Centers for Disease Control and Prevention (CDC). Recommendations regarding the use of vaccines that contain thimerosal as a preservative. MMWR Morb Mortal Wkly Rep 1999; 48:996–998.
- Thompson WW, Price C, Goodson B, et al; Vaccine Safety Datalink Team. Early thimerosal exposure and neuropsychological outcomes at 7 to 10 years. N Engl J Med 2007; 357:1281–1292.
- Price CS, Thompson WW, Goodson B, et al. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics 2010; 126:656–664.
- Centers for Disease Control and Prevention (CDC). CDC study on “Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism.” www.cdc.gov/vaccinesafety/Concerns/Thimerosal/QA_Pediatrics-thimerosal-autism.html. Accessed November 5, 2012.
- Deer B. How the case against the MMR vaccine was fixed. BMJ 2011; 342:c5347.
- Losh M, Sullivan PF, Trembath D, Piven J. Current developments in the genetics of autism: from phenome to genome. J Neuropathol Exp Neurol 2008; 67:829–837.
- Curran S, Bolton P. Genetics of autism. In:Kim Y-K, editor. Handbook of Behavior Genetics, Part IV. New York, NY: Springer; 2009:397–410.
- State MW. The genetics of child psychiatric disorders: focus on autism and Tourette syndrome. Neuron 2010; 68:254–269.
- Voineagu I, Wang X, Johnston P, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011; 474:380–384.
- Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011; 68:1095–1102.
- Frith U, editor. Autism and Asperger Syndrome. New York: Cambridge University Press, 1991:37–92.
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ 2012; 61( 3):1–19.
- Rutter M. Incidence of autism spectrum disorders: changes over time and their meaning. Acta Paediatr 2005; 94:2–15.
- Happé F. Criteria, categories, and continua: autism and related disorders in DSM-5. J Am Acad Child Adolesc Psychiatry 2011; 50:540–542.
- National Institute of Neurological Disorders and Stroke. Asperger syndrome fact sheet. http://www.ninds.nih.gov/disorders/asperger/detail_asperger.htm. Accessed October 11, 2012.
- Toth K, King BH. Asperger’s syndrome: diagnosis and treatment. Am J Psychiatry 2008; 165:958–963.
- Vermeulen P. Autism: from mind blindness to context blindness. Asperger’s Digest November/December 2011. http://autismdigest.com/autism-from-mind-blindness-to-context-blindness/. Accessed October 11, 2012.
- Izuma K, Matsumoto K, Camerer CF, Adolphs R. Insensitivity to social reputation in autism. Proc Natl Acad Sci U S A. 2011; 108:17302–17307.
- Weimer AK, Schatz AM, Lincoln A, Ballantyne AO, Trauner DA. “Motor” impairment in Asperger’s syndrome: evidence for a deficit in proprioception. J Dev Behav Pediatr 2001; 22:92–101.
- Siaperas P, Ring HA, McAllister CJ, et al. Atypical movement performance and sensory integration in Asperger’s syndrome. J Autism Dev Disord 2012; 42:718–725.
- Kushki A, Chau T, Anagnostou E. Handwriting difficulties in children with autism spectrum disorders: a scoping review. J Autism Dev Disord 2011; 41:1706–1716.
- Nash JM, Bonesteel A. The geek syndrome. Time Magazine U.S. 2002. http://www.time.com/time/magazine/article/0,9171,1002365-1,00.html. Accessed October 11, 2012.
- Le Couteur A, Rutter M, Lord C, et al. Autism diagnostic interview: a standardized investigatorbased instrument. J Autism Dev Disord 1989; 19:363–387.
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised WPS Edition Manual. Los Angeles, CA. Western Psychological Services; 2003.
- Baron-Cohen S, Wheelwright S, Robinson J, Woodbury-Smith M. The Adult Asperger’s Assessment (AAA): a diagnostic method. J Autism Developmental Disord 2005; 35:807–819.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Text Revision DSM IV–TR 4th Ed. 2000; Washington, DC: American Psychiatric Association; 2000:80–84.
- Centers for Disease Control. Asperger syndrome fact sheet. http://www.cdc.gov/ncbddd/actearly/pdf/parents_pdfs/Asperger_Syndrome.pdf. Accessed October 11, 2012.
- Peckham C. The current state in autism—still tough to treat but encouraging progress. An expert interview with Fred R. Volkmar, MD. Medscape Pediatrics 2010. http://www.medscape.com/viewarticle/720802?src=mp&spon=17. Accessed October 31, 2012.
- Muscari ME. How should I evaluate an adult for possible Asperger’s syndrome? Medscape News Today 2006.
- Hollander E. Can we treat core symptoms of autism spectrum disorders in adults? December 21, 2011; 1( 18). http://www.medscape.com/viewarticle/531750. Accessed October 1, 2012.
- Müller E, Schuler A, Yates GB. Social challenges and supports from the perspective of individuals with Asperger’s syndrome and other autism spectrum disabilities. Autism 2008; 12:173–190.
- Helman T, Berger O. Parents of children with Asperger’s syndrome or with learning disabilities: family environment and social support. Res Dev Disabil 2008; 29;289–300.
- Taylor CM. Campus commons. When pigs fly: a new perspective on learning. About Campus 2011; 16:30–32.
- Cheng Y, Chiang H, Ye J, Cheng L. Enhancing empathy instruction using a collaborative virtual learning environment for children with autistic spectrum conditions. Comput Edu 2010; 55:1449–1458.
- Beversdorf DQ, Saklayen S, Higgins KF, Bodner KE, Kanne SM, Christ SE. Effect of propranolol on word fluency in autism. Cogn Behav Neurol 2011; 24:11–17.
- Kuehn BM. Scientists probe oxytocin therapy for social deficits in autism, schizophrenia. JAMA 2011; 305:659–661.
- Pfaff DW, Rapin I, Goldman S. Male dominance in autism: neuroendocrine influences on arousal and social anxiety. Autism Res 2011; 4:163–176.
- Brauser D. Children with autism routinely exhibit feeding difficulties in infancy. Medscape Medical News 2010. http://www.medscape.org/viewarticle/726060. Accessed October 31, 2012.
- Baron MG, Groden J, Groden G, Lipsitt L. Stress and coping in autism. New York: Oxford University Press; 2006:355.
- Camfield P, Camfield C. Transition to adult care for children with chronic neurological disorders. Ann Neurol 2001; 69:437–444.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011; 68:1104–1112.
- Croen LA, Najjar DV, Fireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med 2007; 161:334–340.
- Kong A, Frigge ML, Masson G, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 2012; 488:471–475.
- Atlandóttir HO, Thorsen P, Østergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 2010; 40:1423–1430.
- Zerbo O, Iosif A-M, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord 2012; 10.1007/s10803-012-1540-x.
- Crown LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med 2005; 159:151–157.
- Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neruoimmunol 2008; 194:165–172.
- Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol 2006; 178:149–155.
- Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmunity Rev 2004; 3:557–562.
- South M, Diehl JJ. Functional magnetic resonance imaging. In:Hollander E, Kolevzon A, Coyle J, editors. Textbook of Autism Spectrum Disorders. Washington, DC: American Psychiatric Publishing; 2011:409–414.
- Shultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry 2000; 57:331–340.
- Wang AT, Dapretto M, Hariri AR, et al. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2004; 43:481–490.
- Mason RA, Williams DL, Kana RK, et al. Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia 2008; 46:269–280.
- Chiu PH, Kayali MA, Kishida KT, et al. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron 2008; 57:463–437.
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A 2006; 103:8275–8280.
- Bölte S, Hubl D, Dierks T, et al. An fMRI-study of locally oriented perception in autism: altered early visual processing of the block design test. J Neural Transm 2008; 115:545–552.
- Maski KP, Jeste SS, Spencer SJ. Common neurological co-morbidities in autism spectrum disorders. Curr Opin Pediatr 2011; 23:609–615.
- Kleinman J, Marciano P, Ault RL. Advanced theory of mind in high functioning adults with autism. J Autism Dev Disord 2011; 31:29–36.
- Fine C, Lumsden J, Blair RJ. Dissociation between ‘theory of mind and executive functions in a patient with early left amygdala damage. Brain 2001; 124:287–298.
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind.’ Trends Cogn Sci 2003; 7:77–83.
- Happé F, Ehlers S, Pletcher P, et al. ‘Theory of mind’ in the brain. Evidence from a PET scan study of Asperger’s syndrome. Neuroreport 1996; 8:197–207.
- Kugimiya S. Clinical features and possible correlations between autism and epilepsy. Neurology Asia 2010; 15(suppl 1):44–45.
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32:351–360.
- Bruni O, Ferri R, Vittori E, et al. Sleep architecture and NREM alterations in children and adolescents with Asperger syndrome. Sleep 2007; 30:1577–1585.
- Richdale AL, Schreck KA. Sleep problems in autism spectrum disorders: prevalence, nature, & possible biopsychosocial aetiologies. Sleep Med Rev 2009; 13:403–411.
- Paavonen EJ, Vehkalahti K, Vanhala R, von Wendt L, Nieminen-von Wendt T, Aronen ET. Sleep in children with Asperger’s syndrome. J Autism Dev Disord 2007; 38:41–51.
- Elder JH, Shankar M, Shuster J, Theriaque D, Burns S, Sherrill L. The gluten-free, casein-free diet in autism: results of a preliminary double blind clinical trial. J Autism Dev Disord 2006; 36:413–420.
- Keller DM. Diet free of gluten and casein has no effect on autism symptoms. Medscape News May 24, 2010. http://www.medscape.com/viewarticle/722283.
- Centers for Disease Control and Prevention (CDC). Recommendations regarding the use of vaccines that contain thimerosal as a preservative. MMWR Morb Mortal Wkly Rep 1999; 48:996–998.
- Thompson WW, Price C, Goodson B, et al; Vaccine Safety Datalink Team. Early thimerosal exposure and neuropsychological outcomes at 7 to 10 years. N Engl J Med 2007; 357:1281–1292.
- Price CS, Thompson WW, Goodson B, et al. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics 2010; 126:656–664.
- Centers for Disease Control and Prevention (CDC). CDC study on “Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism.” www.cdc.gov/vaccinesafety/Concerns/Thimerosal/QA_Pediatrics-thimerosal-autism.html. Accessed November 5, 2012.
- Deer B. How the case against the MMR vaccine was fixed. BMJ 2011; 342:c5347.
- Losh M, Sullivan PF, Trembath D, Piven J. Current developments in the genetics of autism: from phenome to genome. J Neuropathol Exp Neurol 2008; 67:829–837.
- Curran S, Bolton P. Genetics of autism. In:Kim Y-K, editor. Handbook of Behavior Genetics, Part IV. New York, NY: Springer; 2009:397–410.
- State MW. The genetics of child psychiatric disorders: focus on autism and Tourette syndrome. Neuron 2010; 68:254–269.
- Voineagu I, Wang X, Johnston P, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011; 474:380–384.
- Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011; 68:1095–1102.
KEY POINTS
- Indicators of Asperger syndrome include lack of eye contact, inappropriate comments, odd posture, high anxiety, and intensely focused interests.
- Asperger syndrome is evident in childhood, but it also presents undiagnosed in adulthood.
- Physicians should be aware of patients’ social differences and increased sensitivities in order to improve health care delivery.
- Episodic cognitive behavioral therapy addressing interpersonal skills can dramatically improve quality of life and independence.
- Proposed diagnostic changes scheduled to take effect in 2013 involve including Asperger syndrome as an autism spectrum disorder.
New and Noteworthy Information—December
A majority of patients with mild traumatic brain injury (mTBI) have evidence of chronic traumatic encephalopathy (CTE), according to research published in the online December 2 Brain. Of 85 participants with a history of mTBI, 68 (80%) showed postmortem evidence of the degenerative brain disease. The persons with CTE were all males ages 17 to 98, most of whom were athletes and military veterans. The researchers used a four-stage system to classify CTE, and symptoms ranged from headache and concentration difficulties in stage one to dementia, aggression, and difficulty with words in stage four. Among American football players, stage of CTE correlated with increased duration of football play, survival after football, and age of death. “There is an ordered and predictable progression of hyperphosphorylated tau abnormalities through the nervous system in CTE that occurs in conjunction with widespread axonal disruption and loss,” the researchers said.
Persons who experience traumatic brain injury (TBI) and have also been exposed to the pesticide paraquat have triple the risk of developing Parkinson’s disease, according to research published in the November 13 Neurology. From 2001 to 2011, investigators examined 357 persons with idiopathic Parkinson’s disease and 754 population controls. A validated geographic information system based on records of pesticide application was used to assess paraquat exposure, while TBI was assessed through self-report of all head injuries that involved loss of consciousness for greater than five minutes. According to the researchers, exposure to paraquat and TBI each moderately increase the risk of Parkinson’s disease. However, the risk of developing Parkinson’s disease was threefold higher in study participants with both TBI and exposure to paraquat than in participants exposed to neither risk factor, the investigators said.
Preterm-born children have a significantly reduced capacity for cortical neuroplasticity, which affects learning and memory, researchers reported in the November 14 Journal of Neuroscience. The investigators used a noninvasive magnetic brain stimulation technique to induce long-term depressionlike neuroplasticity in groups of adolescents born after early preterm, late preterm, and term gestations. “Compared with term-born adolescents, both early and late preterm adolescents had reduced long-term depressionlike neuroplasticity in response to brain stimulation that was also associated with low salivary cortisol levels,” said the study authors, adding that these findings may show a potential mechanistic link between the brain physiology of preterm birth and behavioral deficits in learning and memory. Altered hypothalamic-pituitary-adrenal axis function may modulate the altered neuroplasticity and may offer options for therapeutic interventions, the researchers concluded.
MRI scans show that patients with mild traumatic brain injury (mTBI) have abnormal default-mode network connectivity patterns, researchers reported in the December Radiology. The study used resting-state functional MRI to characterize the default-mode network and included 18 healthy controls and 23 patients with mTBI who had post-traumatic symptoms less than two months after injury. Compared with controls, patients with mTBI showed significantly reduced connectivity in the posterior cingulate complex and parietal regions, which correlated positively with neurocognitive dysfunction. Patients with mTBI also showed increased frontal connectivity around the medial prefrontal cortex, which correlated with post-traumatic symptoms such as depression, anxiety, fatigue, and postconcussion syndrome. According to the researchers, the results may “provide insight into how neuronal communication and information integration are disrupted among default-mode network key structures after mild head injury.”
Increased concentration of phosphorylated neurofilament heavy subunit (pNF-H) in the plasma, serum, and CSF of patients with amyotrophic lateral sclerosis (ALS) may be associated with faster disease progression, according to research published in the online October 31 Journal of Neurology, Neurosurgery & Psychiatry. Investigators measured pNF-H concentration in the plasma and CSF of patients with ALS from the Mayo Clinic Florida and Emory University, as well as plasma from an earlier pilot study of 20 patients with ALS. Analysis showed that higher levels of pNF-H in plasma, serum, and CSF were linked with greater decline for ALS patients. The researchers also noted that patients with bulbar onset might have higher pNF-H concentration in plasma than those with spinal onset, though the results require confirmation. “These data support further study of pNF-H in CSF, serum, and plasma as a potential ALS biomarker,” the study authors said.
Paralyzed dogs who received intraspinal transplantation of cells derived from olfactory mucosal cultures regained some movement, researchers reported in the November Brain. The investigators conducted a randomized, double-blind clinical trial in which dogs with severe chronic thoracolumbar spinal injuries received an injection of either intraspinal autologous cells derived from olfactory mucosal cultures or cell transport medium alone. Dogs who received the olfactory mucosal transplants showed significantly better fore–hind coordination than those who received only the cell transport medium. “We conclude that intraspinal olfactory mucosal cell transplantation improves communication across the damaged region of the injured spinal cord, even in chronically injured individuals. However, we find no evidence for concomitant improvement in long tract function,” the researchers said.
Suvorexant, an orexin receptor antagonist, may offer a novel approach to treating insomnia, researchers reported in the December Neurology. In a randomized, double-blind, placebo-controlled study that took place during two periods of four weeks, patients received 10 mg, 20 mg, 40 mg, or 80 mg of suvorexant in one period and placebo in the other period. Coprimary end points were sleep efficiency on night one and at the end of week four. Patients receiving suvorexant showed significant dose-related improvements on both of the primary end points compared with those receiving placebo. “Dose-related effects were also observed for sleep induction (latency to persistent sleep) and maintenance (wake after sleep onset). Suvorexant was generally well tolerated,” the researchers said.
Persons born in April have significantly more risk of developing multiple sclerosis (MS) than those born in October and November, according to research published in the online November 14 Journal of Neurology, Neurosurgery & Psychiatry. The study was a meta-analysis of previously published data on month of birth of 151,978 patients with MS. According to the researchers, the month of birth effect is “likely to be due to ultraviolet light exposure and maternal vitamin D levels.” In a separate study published in the November 20 Neurology, investigators observed an association between high levels of vitamin D in the years prior to disease onset and a decreased risk of MS. However, there was no association between gestational levels of vitamin D and MS risk in the offspring. Decreasing levels of vitamin D in the population might help explain the increase in MS cases suggested from epidemiologic studies, the study authors said.
Mutations of the immune system gene TREM2 may be linked to an increased risk of Alzheimer’s disease, researchers reported in two studies in the online November 14 New England Journal of Medicine. In one study, investigators analyzed the genetic variability in TREM2 and performed a meta-analysis on imputed data for the TREM2 variant rs75932628, which is predicted to cause a R47H substitution. The R47H mutation was highly significantly associated with Alzheimer’s disease, said the authors. In the second study, researchers examined genome sequences of 2,261 Icelanders and found that the TREM2 mutation rs75932628-T conferred a significant risk of Alzheimer’s disease (odds ratio, 2.92). “Given the reported anti-inflammatory role of TREM2 in the brain, the R47H substitution may lead to an increased predisposition to Alzheimer’s disease through impaired containment of inflammatory processes,” the investigators concluded.
Exposure to traffic-related air pollution during pregnancy and the first year of life is associated with autism, according to a study published in the online November Archives of General Psychiatry. Researchers conducted a population-based case-control study that included data from 279 children with autism and 245 control children with typical development. Compared with controls, those with autism were more likely to live in areas with the highest quartile of exposure to traffic-related air pollution during gestation and during the first year of life. Exposure to particulate matter and nitrogen dioxide were also linked with autism. “Further epidemiological and toxicological examinations of likely biological pathways will help determine whether these associations are causal,” the researchers concluded.
Consumption of fish and long-chain omega 3 fatty acids may moderately reduce cerebrovascular risk, but fish oil supplements may not have the same beneficial effect, according to research published in the October 30 BMJ. The systemic review and meta-analysis examined 26 prospective cohort studies and 12 randomized controlled trials with aggregate data on 794,000 participants and 34,817 cerebrovascular outcomes. Results showed that persons who ate two to four servings of fish per week and those who ate five or more servings a week had a lower risk of cerebrovascular disease, compared with persons who ate one serving a week. However, no association was observed between risk for cerebrovascular disease and long-chain omega 3 fatty acids measured as circulating biomarkers in observational studies or supplements in primary and secondary prevention trials.
Depression is the most important factor affecting the health status of patients with Parkinson’s disease, according to early findings released as part of the Parkinson’s Outcome Project, a longitudinal study examining which treatments produce the best outcomes. The study, which began in 2009, includes data from more than 5,500 patients with Parkinson’s disease. Based on this research, the National Parkinson Foundation recommends screening patients for depression at least once a year and encouraging patients to discuss any mood change with a health care professional, particularly the physician treating them for Parkinson’s disease. Patients may also benefit from bringing a family member to doctor’s appointments and asking the family member to share any changes in the patient’s mood.
—Lauren LeBano
A majority of patients with mild traumatic brain injury (mTBI) have evidence of chronic traumatic encephalopathy (CTE), according to research published in the online December 2 Brain. Of 85 participants with a history of mTBI, 68 (80%) showed postmortem evidence of the degenerative brain disease. The persons with CTE were all males ages 17 to 98, most of whom were athletes and military veterans. The researchers used a four-stage system to classify CTE, and symptoms ranged from headache and concentration difficulties in stage one to dementia, aggression, and difficulty with words in stage four. Among American football players, stage of CTE correlated with increased duration of football play, survival after football, and age of death. “There is an ordered and predictable progression of hyperphosphorylated tau abnormalities through the nervous system in CTE that occurs in conjunction with widespread axonal disruption and loss,” the researchers said.
Persons who experience traumatic brain injury (TBI) and have also been exposed to the pesticide paraquat have triple the risk of developing Parkinson’s disease, according to research published in the November 13 Neurology. From 2001 to 2011, investigators examined 357 persons with idiopathic Parkinson’s disease and 754 population controls. A validated geographic information system based on records of pesticide application was used to assess paraquat exposure, while TBI was assessed through self-report of all head injuries that involved loss of consciousness for greater than five minutes. According to the researchers, exposure to paraquat and TBI each moderately increase the risk of Parkinson’s disease. However, the risk of developing Parkinson’s disease was threefold higher in study participants with both TBI and exposure to paraquat than in participants exposed to neither risk factor, the investigators said.
Preterm-born children have a significantly reduced capacity for cortical neuroplasticity, which affects learning and memory, researchers reported in the November 14 Journal of Neuroscience. The investigators used a noninvasive magnetic brain stimulation technique to induce long-term depressionlike neuroplasticity in groups of adolescents born after early preterm, late preterm, and term gestations. “Compared with term-born adolescents, both early and late preterm adolescents had reduced long-term depressionlike neuroplasticity in response to brain stimulation that was also associated with low salivary cortisol levels,” said the study authors, adding that these findings may show a potential mechanistic link between the brain physiology of preterm birth and behavioral deficits in learning and memory. Altered hypothalamic-pituitary-adrenal axis function may modulate the altered neuroplasticity and may offer options for therapeutic interventions, the researchers concluded.
MRI scans show that patients with mild traumatic brain injury (mTBI) have abnormal default-mode network connectivity patterns, researchers reported in the December Radiology. The study used resting-state functional MRI to characterize the default-mode network and included 18 healthy controls and 23 patients with mTBI who had post-traumatic symptoms less than two months after injury. Compared with controls, patients with mTBI showed significantly reduced connectivity in the posterior cingulate complex and parietal regions, which correlated positively with neurocognitive dysfunction. Patients with mTBI also showed increased frontal connectivity around the medial prefrontal cortex, which correlated with post-traumatic symptoms such as depression, anxiety, fatigue, and postconcussion syndrome. According to the researchers, the results may “provide insight into how neuronal communication and information integration are disrupted among default-mode network key structures after mild head injury.”
Increased concentration of phosphorylated neurofilament heavy subunit (pNF-H) in the plasma, serum, and CSF of patients with amyotrophic lateral sclerosis (ALS) may be associated with faster disease progression, according to research published in the online October 31 Journal of Neurology, Neurosurgery & Psychiatry. Investigators measured pNF-H concentration in the plasma and CSF of patients with ALS from the Mayo Clinic Florida and Emory University, as well as plasma from an earlier pilot study of 20 patients with ALS. Analysis showed that higher levels of pNF-H in plasma, serum, and CSF were linked with greater decline for ALS patients. The researchers also noted that patients with bulbar onset might have higher pNF-H concentration in plasma than those with spinal onset, though the results require confirmation. “These data support further study of pNF-H in CSF, serum, and plasma as a potential ALS biomarker,” the study authors said.
Paralyzed dogs who received intraspinal transplantation of cells derived from olfactory mucosal cultures regained some movement, researchers reported in the November Brain. The investigators conducted a randomized, double-blind clinical trial in which dogs with severe chronic thoracolumbar spinal injuries received an injection of either intraspinal autologous cells derived from olfactory mucosal cultures or cell transport medium alone. Dogs who received the olfactory mucosal transplants showed significantly better fore–hind coordination than those who received only the cell transport medium. “We conclude that intraspinal olfactory mucosal cell transplantation improves communication across the damaged region of the injured spinal cord, even in chronically injured individuals. However, we find no evidence for concomitant improvement in long tract function,” the researchers said.
Suvorexant, an orexin receptor antagonist, may offer a novel approach to treating insomnia, researchers reported in the December Neurology. In a randomized, double-blind, placebo-controlled study that took place during two periods of four weeks, patients received 10 mg, 20 mg, 40 mg, or 80 mg of suvorexant in one period and placebo in the other period. Coprimary end points were sleep efficiency on night one and at the end of week four. Patients receiving suvorexant showed significant dose-related improvements on both of the primary end points compared with those receiving placebo. “Dose-related effects were also observed for sleep induction (latency to persistent sleep) and maintenance (wake after sleep onset). Suvorexant was generally well tolerated,” the researchers said.
Persons born in April have significantly more risk of developing multiple sclerosis (MS) than those born in October and November, according to research published in the online November 14 Journal of Neurology, Neurosurgery & Psychiatry. The study was a meta-analysis of previously published data on month of birth of 151,978 patients with MS. According to the researchers, the month of birth effect is “likely to be due to ultraviolet light exposure and maternal vitamin D levels.” In a separate study published in the November 20 Neurology, investigators observed an association between high levels of vitamin D in the years prior to disease onset and a decreased risk of MS. However, there was no association between gestational levels of vitamin D and MS risk in the offspring. Decreasing levels of vitamin D in the population might help explain the increase in MS cases suggested from epidemiologic studies, the study authors said.
Mutations of the immune system gene TREM2 may be linked to an increased risk of Alzheimer’s disease, researchers reported in two studies in the online November 14 New England Journal of Medicine. In one study, investigators analyzed the genetic variability in TREM2 and performed a meta-analysis on imputed data for the TREM2 variant rs75932628, which is predicted to cause a R47H substitution. The R47H mutation was highly significantly associated with Alzheimer’s disease, said the authors. In the second study, researchers examined genome sequences of 2,261 Icelanders and found that the TREM2 mutation rs75932628-T conferred a significant risk of Alzheimer’s disease (odds ratio, 2.92). “Given the reported anti-inflammatory role of TREM2 in the brain, the R47H substitution may lead to an increased predisposition to Alzheimer’s disease through impaired containment of inflammatory processes,” the investigators concluded.
Exposure to traffic-related air pollution during pregnancy and the first year of life is associated with autism, according to a study published in the online November Archives of General Psychiatry. Researchers conducted a population-based case-control study that included data from 279 children with autism and 245 control children with typical development. Compared with controls, those with autism were more likely to live in areas with the highest quartile of exposure to traffic-related air pollution during gestation and during the first year of life. Exposure to particulate matter and nitrogen dioxide were also linked with autism. “Further epidemiological and toxicological examinations of likely biological pathways will help determine whether these associations are causal,” the researchers concluded.
Consumption of fish and long-chain omega 3 fatty acids may moderately reduce cerebrovascular risk, but fish oil supplements may not have the same beneficial effect, according to research published in the October 30 BMJ. The systemic review and meta-analysis examined 26 prospective cohort studies and 12 randomized controlled trials with aggregate data on 794,000 participants and 34,817 cerebrovascular outcomes. Results showed that persons who ate two to four servings of fish per week and those who ate five or more servings a week had a lower risk of cerebrovascular disease, compared with persons who ate one serving a week. However, no association was observed between risk for cerebrovascular disease and long-chain omega 3 fatty acids measured as circulating biomarkers in observational studies or supplements in primary and secondary prevention trials.
Depression is the most important factor affecting the health status of patients with Parkinson’s disease, according to early findings released as part of the Parkinson’s Outcome Project, a longitudinal study examining which treatments produce the best outcomes. The study, which began in 2009, includes data from more than 5,500 patients with Parkinson’s disease. Based on this research, the National Parkinson Foundation recommends screening patients for depression at least once a year and encouraging patients to discuss any mood change with a health care professional, particularly the physician treating them for Parkinson’s disease. Patients may also benefit from bringing a family member to doctor’s appointments and asking the family member to share any changes in the patient’s mood.
—Lauren LeBano
A majority of patients with mild traumatic brain injury (mTBI) have evidence of chronic traumatic encephalopathy (CTE), according to research published in the online December 2 Brain. Of 85 participants with a history of mTBI, 68 (80%) showed postmortem evidence of the degenerative brain disease. The persons with CTE were all males ages 17 to 98, most of whom were athletes and military veterans. The researchers used a four-stage system to classify CTE, and symptoms ranged from headache and concentration difficulties in stage one to dementia, aggression, and difficulty with words in stage four. Among American football players, stage of CTE correlated with increased duration of football play, survival after football, and age of death. “There is an ordered and predictable progression of hyperphosphorylated tau abnormalities through the nervous system in CTE that occurs in conjunction with widespread axonal disruption and loss,” the researchers said.
Persons who experience traumatic brain injury (TBI) and have also been exposed to the pesticide paraquat have triple the risk of developing Parkinson’s disease, according to research published in the November 13 Neurology. From 2001 to 2011, investigators examined 357 persons with idiopathic Parkinson’s disease and 754 population controls. A validated geographic information system based on records of pesticide application was used to assess paraquat exposure, while TBI was assessed through self-report of all head injuries that involved loss of consciousness for greater than five minutes. According to the researchers, exposure to paraquat and TBI each moderately increase the risk of Parkinson’s disease. However, the risk of developing Parkinson’s disease was threefold higher in study participants with both TBI and exposure to paraquat than in participants exposed to neither risk factor, the investigators said.
Preterm-born children have a significantly reduced capacity for cortical neuroplasticity, which affects learning and memory, researchers reported in the November 14 Journal of Neuroscience. The investigators used a noninvasive magnetic brain stimulation technique to induce long-term depressionlike neuroplasticity in groups of adolescents born after early preterm, late preterm, and term gestations. “Compared with term-born adolescents, both early and late preterm adolescents had reduced long-term depressionlike neuroplasticity in response to brain stimulation that was also associated with low salivary cortisol levels,” said the study authors, adding that these findings may show a potential mechanistic link between the brain physiology of preterm birth and behavioral deficits in learning and memory. Altered hypothalamic-pituitary-adrenal axis function may modulate the altered neuroplasticity and may offer options for therapeutic interventions, the researchers concluded.
MRI scans show that patients with mild traumatic brain injury (mTBI) have abnormal default-mode network connectivity patterns, researchers reported in the December Radiology. The study used resting-state functional MRI to characterize the default-mode network and included 18 healthy controls and 23 patients with mTBI who had post-traumatic symptoms less than two months after injury. Compared with controls, patients with mTBI showed significantly reduced connectivity in the posterior cingulate complex and parietal regions, which correlated positively with neurocognitive dysfunction. Patients with mTBI also showed increased frontal connectivity around the medial prefrontal cortex, which correlated with post-traumatic symptoms such as depression, anxiety, fatigue, and postconcussion syndrome. According to the researchers, the results may “provide insight into how neuronal communication and information integration are disrupted among default-mode network key structures after mild head injury.”
Increased concentration of phosphorylated neurofilament heavy subunit (pNF-H) in the plasma, serum, and CSF of patients with amyotrophic lateral sclerosis (ALS) may be associated with faster disease progression, according to research published in the online October 31 Journal of Neurology, Neurosurgery & Psychiatry. Investigators measured pNF-H concentration in the plasma and CSF of patients with ALS from the Mayo Clinic Florida and Emory University, as well as plasma from an earlier pilot study of 20 patients with ALS. Analysis showed that higher levels of pNF-H in plasma, serum, and CSF were linked with greater decline for ALS patients. The researchers also noted that patients with bulbar onset might have higher pNF-H concentration in plasma than those with spinal onset, though the results require confirmation. “These data support further study of pNF-H in CSF, serum, and plasma as a potential ALS biomarker,” the study authors said.
Paralyzed dogs who received intraspinal transplantation of cells derived from olfactory mucosal cultures regained some movement, researchers reported in the November Brain. The investigators conducted a randomized, double-blind clinical trial in which dogs with severe chronic thoracolumbar spinal injuries received an injection of either intraspinal autologous cells derived from olfactory mucosal cultures or cell transport medium alone. Dogs who received the olfactory mucosal transplants showed significantly better fore–hind coordination than those who received only the cell transport medium. “We conclude that intraspinal olfactory mucosal cell transplantation improves communication across the damaged region of the injured spinal cord, even in chronically injured individuals. However, we find no evidence for concomitant improvement in long tract function,” the researchers said.
Suvorexant, an orexin receptor antagonist, may offer a novel approach to treating insomnia, researchers reported in the December Neurology. In a randomized, double-blind, placebo-controlled study that took place during two periods of four weeks, patients received 10 mg, 20 mg, 40 mg, or 80 mg of suvorexant in one period and placebo in the other period. Coprimary end points were sleep efficiency on night one and at the end of week four. Patients receiving suvorexant showed significant dose-related improvements on both of the primary end points compared with those receiving placebo. “Dose-related effects were also observed for sleep induction (latency to persistent sleep) and maintenance (wake after sleep onset). Suvorexant was generally well tolerated,” the researchers said.
Persons born in April have significantly more risk of developing multiple sclerosis (MS) than those born in October and November, according to research published in the online November 14 Journal of Neurology, Neurosurgery & Psychiatry. The study was a meta-analysis of previously published data on month of birth of 151,978 patients with MS. According to the researchers, the month of birth effect is “likely to be due to ultraviolet light exposure and maternal vitamin D levels.” In a separate study published in the November 20 Neurology, investigators observed an association between high levels of vitamin D in the years prior to disease onset and a decreased risk of MS. However, there was no association between gestational levels of vitamin D and MS risk in the offspring. Decreasing levels of vitamin D in the population might help explain the increase in MS cases suggested from epidemiologic studies, the study authors said.
Mutations of the immune system gene TREM2 may be linked to an increased risk of Alzheimer’s disease, researchers reported in two studies in the online November 14 New England Journal of Medicine. In one study, investigators analyzed the genetic variability in TREM2 and performed a meta-analysis on imputed data for the TREM2 variant rs75932628, which is predicted to cause a R47H substitution. The R47H mutation was highly significantly associated with Alzheimer’s disease, said the authors. In the second study, researchers examined genome sequences of 2,261 Icelanders and found that the TREM2 mutation rs75932628-T conferred a significant risk of Alzheimer’s disease (odds ratio, 2.92). “Given the reported anti-inflammatory role of TREM2 in the brain, the R47H substitution may lead to an increased predisposition to Alzheimer’s disease through impaired containment of inflammatory processes,” the investigators concluded.
Exposure to traffic-related air pollution during pregnancy and the first year of life is associated with autism, according to a study published in the online November Archives of General Psychiatry. Researchers conducted a population-based case-control study that included data from 279 children with autism and 245 control children with typical development. Compared with controls, those with autism were more likely to live in areas with the highest quartile of exposure to traffic-related air pollution during gestation and during the first year of life. Exposure to particulate matter and nitrogen dioxide were also linked with autism. “Further epidemiological and toxicological examinations of likely biological pathways will help determine whether these associations are causal,” the researchers concluded.
Consumption of fish and long-chain omega 3 fatty acids may moderately reduce cerebrovascular risk, but fish oil supplements may not have the same beneficial effect, according to research published in the October 30 BMJ. The systemic review and meta-analysis examined 26 prospective cohort studies and 12 randomized controlled trials with aggregate data on 794,000 participants and 34,817 cerebrovascular outcomes. Results showed that persons who ate two to four servings of fish per week and those who ate five or more servings a week had a lower risk of cerebrovascular disease, compared with persons who ate one serving a week. However, no association was observed between risk for cerebrovascular disease and long-chain omega 3 fatty acids measured as circulating biomarkers in observational studies or supplements in primary and secondary prevention trials.
Depression is the most important factor affecting the health status of patients with Parkinson’s disease, according to early findings released as part of the Parkinson’s Outcome Project, a longitudinal study examining which treatments produce the best outcomes. The study, which began in 2009, includes data from more than 5,500 patients with Parkinson’s disease. Based on this research, the National Parkinson Foundation recommends screening patients for depression at least once a year and encouraging patients to discuss any mood change with a health care professional, particularly the physician treating them for Parkinson’s disease. Patients may also benefit from bringing a family member to doctor’s appointments and asking the family member to share any changes in the patient’s mood.
—Lauren LeBano
Grand Rounds: Woman, 38, With Pulseless Electrical Activity
On an autumn day, a 38-year-old woman with a history of asthma presented to the emergency department (ED) with the chief complaint of shortness of breath (SOB). The patient described her SOB as sudden in onset and not relieved by use of her albuterol inhaler; hence the ED visit.
She denied any chest pain, palpitations, dizziness, orthopnea, upper respiratory tract infection, cough, wheezing, fever or chills, headache, vision changes, body aches, sick contacts, or pets at home. She said she uses her albuterol inhaler as needed, and that she had used it that day for the first time in “a few months.” She denied any history of intubation or steroid use. Additionally, she had not been seen by a primary care provider in years.
The woman, a native of Ghana, had been living in the United States for many years. She denied any recent travel or exposure to toxic chemicals; any use of tobacco, alcohol, or illicit drugs; or any history of sexually transmitted disease.
The patient was afebrile (temperature, 98.6°F), with a respiratory rate of 20 breaths/min; blood pressure, 144/69 mm Hg; and ventricular rate, 125 beats/min. On physical examination, her extraocular movements were intact; pupils were equal, round, reactive to light and accommodation; and sclera were nonicteric. The patient’s head was normocephalic and atraumatic, and the neck was supple with normal range of motion and no jugular venous distension or lymphadenopathy. Her mucous membranes were moist with no pharyngeal erythema or exudates. Cardiovascular examination, including ECG, revealed tachycardia but no murmurs or gallops.
While being evaluated in the ED, the patient became tachypneic and began to experience respiratory distress. She was intubated for airway protection, at which time she developed pulseless electrical activity (PEA), with 30 beats/min. She responded to atropine and epinephrine injections. A repeat ECG showed sinus tachycardia and right atrial enlargement with right-axis deviation. Chest x-ray (see Figure 1) showed no consolidation, pleural effusion, or pneumothorax.
Results from the patient’s lab work are shown in the table, above. Negative results were reported for a urine pregnancy test.
Since there was no clear etiology for the patient’s PEA, she underwent pan-culturing, with the following tests ordered: HIV antibody testing, immunovirology for influenza A and B viruses, and urine toxicology. Doppler ultrasound of the bilateral lower extremities was also ordered, in addition to chest CT and transthoracic and transesophageal echocardiography (TTE and TEE, respectively). The patient was intubated and transferred to the medical ICU for further management.
The differential diagnosis included cardiac tamponade, acute MI, acute pulmonary embolus (PE), tension pneumothorax, hypovolemia, and asthma exacerbated by viral or bacterial infection.1,2 Although the case patient presented with PEA, she did not have the presenting signs of cardiac tamponade known as Beck’s triad: hypotension, jugular venous distension, and muffled heart sounds.3 TTE showed an ejection fraction of 65% and grade 2 diastolic dysfunction but no pericardial effusions (which accumulate rapidly in the patient with cardiac tamponade, resulting from fluid buildup in the pericardial layers),4 and TEE showed no atrial thrombi (which can masquerade as cardiac tamponade5). The patient had no signs of trauma and denied any history of malignancy (both potential causes of cardiac tamponade). Chest x-ray showed normal heart size and no pneumothorax, consolidations, or pleural effusions.4,6-8 Thus, the diagnosis of cardiac tamponade was ruled out.
Common presenting symptoms of acute MI include sudden-onset chest pain, SOB, palpitations, dizziness, nausea, and/or vomiting. Women may experience less dramatic symptoms—often little more than SOB and fatigue.9 According to a 2000 consensus document from a joint European Society of Cardiology/American College of Cardiology committee10 in which MI was redefined, the diagnosis of MI relies on a rise in cardiac troponin levels, typical MI symptoms, and changes in ECG showing pathological Q waves or ST elevation or depression. The case patient’s troponin I level was less than 0.02 ng/mL, and ECG did not reveal Q waves or ST-T wave changes; additionally, since the patient had no chest pain, palpitations, diaphoresis, nausea, or vomiting, acute MI was ruled out.
Blood clots capable of blocking the pulmonary artery usually originate in the deep veins of the lower extremities.11 Three main factors, called Virchow’s triad, are known to contribute to these deep vein thromboses (DVTs): venous stasis, endothelial injury, and a hypercoagulability state.12,13 The patient had denied any trauma, recent travel, history of malignancy, or use of tobacco or oral contraceptives, and the result of her urine pregnancy test was negative. Even though the patient presented with tachypnea and acute SOB, with ECG showing right-axis deviation and tachycardia (common presenting signs and symptoms for PE), her chest CT showed no evidence of PE (see Figure 2); additionally, Doppler ultrasound of the bilateral lower extremities revealed no DVTs. Thus, PE was also excluded.
Tension pneumothorax was also ruled out, as chest x-ray showed neither mediastinal shift nor tracheal deviation, and the patient had denied any trauma. Laboratory analyses did not indicate hyponatremia, and the patient’s hemoglobin and hematocrit were satisfactory. She was tachycardic on admission, but her blood pressure was stable. As the patient denied any use of vasodilators or diuretics, hypovolemia was ruled out.
Patients experiencing asthma exacerbation can present with acute SOB, which usually resolves following use of IV steroids, nebulizer therapy, and inhaler treatments. Despite being administered IV methylprednisolone and magnesium sulfate in the ED, the patient experienced PEA and respiratory distress and required intubation for airway protection.
The HIV test was nonreactive, and blood and urine cultures did not show any growth. Results of tests for Legionella urinary antigen and Streptococcus pneumoniae antigen were negative. Sputum culture showed normal flora. Immunovirology testing, however, was positive for both influenza A and B antigens.
Chest X-ray showed no acute pulmonary pathology, nor did chest CT show any central, interlobar, or segmental embolism or mediastinal lymphadenopathy. It was determined that the patient’s acute SOB might represent asthma exacerbation secondary to influenza viral infection. Her PEA was attributed to possible acute pericarditis secondary to concomitant influenza A and B viral infection.
DISCUSSION
Currently, the CDC recognizes three types of influenza virus: A, B, and C.14 Only influenza A viruses are further classified into subtypes, based on the presence of surface proteins called hemagglutinin (HA) or neuraminidase (NA) glycoproteins. Humans can be infected by influenza A subtypes H1N1 and H3N2.14 Influenza B viruses, found mostly in humans, are associated with significant morbidity and mortality.
Influenza A and B viruses are further classified into strains that change with each flu season—thus, the need to update vaccinations against influenza A and B each year. No vaccination exists against influenza C virus, which is known to cause only mild illness in humans.15
In patients with asthma (as in the case patient), chronic bronchitis, or emphysema, infection with the influenza virus can manifest with SOB, in addition to the more common symptoms of fever, sore throat, headache, rhinorrhea, chills, muscle aches, and general discomfort.16 Patients with coronary artery disease, congestive heart failure (CHF), and/or a history of smoking may experience more severe symptoms and increased risk for influenza-associated mortality than do other patients.17,18
Rare cardiac complications of influenza infections are myocarditis and benign acute pericarditis; myocarditis can progress to CHF and death.19,20 A case of acute myopericarditis was reported by Proby et al21 in a patient with acute influenza A infection who developed pericardial effusions, myositis, tamponade, and pleurisy. That patient recovered after pericardiocentesis and administration of inotropic drugs.
In the literature, a few cases of acute pericarditis have been reported in association with administration of the influenza vaccination.22,23
In the case patient, the diagnosis of influenza A and B was made following testing of nasal and nasopharyngeal swabs with an immunochromatographic assay that uses highly sensitive monoclonal antibodies to detect influenza A and B nucleoprotein antigens.24,25
According to reports in the literature, two-thirds of cases of acute pericarditis are caused by infection, most commonly viral infection (including influenza virus, adenovirus, enterovirus, cytomegalovirus, hepatitis B virus, and herpes simplex virus).26,27 Other etiologies for acute pericarditis are autoimmune (accounting for less than 10% of cases) and neoplastic conditions (5% to 7% of cases).26
PATIENT OUTCOME
Consultation with an infectious disease specialist was obtained. The patient was placed under droplet isolation precautions and was started on a nebulizer, IV steroid treatments, and oseltamivir 75 mg by mouth every 12 hours. She was transferred to a medical floor, where she completed a five-day course of oseltamivir.
As a result of timely intervention, the patient was discharged in stable condition on a therapeutic regimen that included albuterol, fluticasone, and salmeterol inhalation, in addition to tapered-dose steroids. She was advised to follow up with her primary care provider and at the pulmonary clinic.
CONCLUSION
To our knowledge, this is the first reported case of acute pericarditis in a patient with concomitant acute infections with influenza A and B. According to conclusions reached in recent literature, further research is needed to explain the pathophysiology of influenza viral infections, associated cardiovascular morbidity and mortality, and the degree to which these can be prevented by influenza vaccination.1,28 Also to be pursued through research is a better understanding of the morbidity and mortality associated with influenza viruses, especially in children and in adults affected by asthma, cardiac disease, and/or obesity.
REFERENCES
1. Finelli L, Chaves SS. Influenza and acute myocardial infarction. J Infect Dis. 2011;203(12):
1701-1704.
2. Steiger HV, Rimbach K, Müller E, Breitkreutz R. Focused emergency echocardiography: lifesaving tool for a 14-year-old girl suffering out-of-hospital pulseless electrical activity arrest because of cardiac tamponade. Eur J Emerg Med. 2009;16(2): 103-105.
3. Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade.
J Emerg Trauma Shock. 2012;5(1):72-75.
4. Restrepo CS, Lemos DF, Lemos JA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007;27(6):1595-1610.
5. Papanagnou D, Stone MB. Massive right atrial thrombus masquerading as cardiac tamponade. Acad Emerg Med. 2010;17(2):E11.
6. Saito Y, Donohue A, Attai S, et al. The syndrome of cardiac tamponade with “small” pericardial effusion. Echocardiography. 2008;25(3): 321-327.
7. Lin E, Boire A, Hemmige V, et al. Cardiac tamponade mimicking tuberculous pericarditis as the initial presentation of chronic lymphocytic leukemia in a 58-year-old woman: a case report. J Med Case Rep. 2010;4:246.
8. Meniconi A, Attenhofer Jost CH, Jenni R. How to survive myocardial rupture after myocardial infarction. Heart. 2000;84(5):552.
9. Kosuge M, Kimura K, Ishikawa T, et al. Differences between men and women in terms of clinical features of ST-segment elevation acute myocardial infarction. Circ J. 2006;70(3):222-226.
10. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959-969.
11. Goldhaber SZ. Deep venous thrombosis and pulmonary thromboembolism. In: Fauci AS, Braunwald E, Kasper DL, et al. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Medical; 2008:1651–1657.
12. Brooks EG, Trotman W, Wadsworth MP, et al. Valves of the deep venous system: an overlooked risk factor. Blood. 2009;114(6):1276-1279.
13. Kyrle PA, Eichinger S. Is Virchow’s triad complete? Blood. 2009;114(6):1138-1139.
14. CDC. Seasonal influenza (flu): types of influenza viruses (2012). www.cdc.gov/flu/about/viruses/types.htm. Accessed October 24, 2012.
15. CDC. Seasonal influenza (flu)(2012). www.cdc .gov/flu. Accessed October 24, 2012.
16. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
17. Angelo SJ, Marshall PS, Chrissoheris MP, Chaves AM. Clinical characteristics associated with poor outcome in patients acutely infected with Influenza A. Conn Med. 2004;68(4):199-205.
18. Murin S, Bilello K. Respiratory tract infections: another reason not to smoke. Cleve Clin J Med. 2005;72(10):916-920.
19. Ray CG, Icenogle TB, Minnich LL, et al. The use of intravenous ribavirin to treat influenza virus–associated acute myocarditis. J Infect Dis. 1989; 159(5):829-836.
20. Fairley CK, Ryan M, Wall PG, Weinberg J. The organism reported to cause infective myocarditis and pericarditis in England and Wales. J Infect. 1996;32(3):223-225.
21. Proby CM, Hackett D, Gupta S, Cox TM. Acute myopericarditis in influenza A infection. Q J Med. 1986;60(233):887-892.
22. Streifler JJ, Dux S, Garty M, Rosenfeld JB. Recurrent pericarditis: a rare complication of influenza vaccination. Br Med J (Clin Res Ed). 1981; 283(6290):526-527.
23. Desson JF, Leprévost M, Vabret F, Davy A. Acute benign pericarditis after anti-influenza vaccination [in French]. Presse Med. 1997;26 (9):415.
24. BinaxNOW® Influenza A&B Test Kit (product instructions). www.diagnosticsdirect2u.com/images/PDF/Binax%20Now%20416-022%20PPI .pdf. Accessed October 24, 2012.
25. 510(k) Substantial Equivalence Determination Decision Summary [BinaxNow® Influenza A & B Test] (2009). www.accessdata.fda.gov/cdrh_docs/reviews/K062109.pdf. Accessed October 24, 2012.
26. Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121(7):916-928.
27. Maisch B, Seferovic PM, Ristic AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases: executive summary. Eur Heart J. 2004;25(7):587-610.
28. McCullers JA, Hayden FG. Fatal influenza B infections: time to reexamine influenza research priorities. J Infect Dis. 2012;205(6):870-872.
On an autumn day, a 38-year-old woman with a history of asthma presented to the emergency department (ED) with the chief complaint of shortness of breath (SOB). The patient described her SOB as sudden in onset and not relieved by use of her albuterol inhaler; hence the ED visit.
She denied any chest pain, palpitations, dizziness, orthopnea, upper respiratory tract infection, cough, wheezing, fever or chills, headache, vision changes, body aches, sick contacts, or pets at home. She said she uses her albuterol inhaler as needed, and that she had used it that day for the first time in “a few months.” She denied any history of intubation or steroid use. Additionally, she had not been seen by a primary care provider in years.
The woman, a native of Ghana, had been living in the United States for many years. She denied any recent travel or exposure to toxic chemicals; any use of tobacco, alcohol, or illicit drugs; or any history of sexually transmitted disease.
The patient was afebrile (temperature, 98.6°F), with a respiratory rate of 20 breaths/min; blood pressure, 144/69 mm Hg; and ventricular rate, 125 beats/min. On physical examination, her extraocular movements were intact; pupils were equal, round, reactive to light and accommodation; and sclera were nonicteric. The patient’s head was normocephalic and atraumatic, and the neck was supple with normal range of motion and no jugular venous distension or lymphadenopathy. Her mucous membranes were moist with no pharyngeal erythema or exudates. Cardiovascular examination, including ECG, revealed tachycardia but no murmurs or gallops.
While being evaluated in the ED, the patient became tachypneic and began to experience respiratory distress. She was intubated for airway protection, at which time she developed pulseless electrical activity (PEA), with 30 beats/min. She responded to atropine and epinephrine injections. A repeat ECG showed sinus tachycardia and right atrial enlargement with right-axis deviation. Chest x-ray (see Figure 1) showed no consolidation, pleural effusion, or pneumothorax.
Results from the patient’s lab work are shown in the table, above. Negative results were reported for a urine pregnancy test.
Since there was no clear etiology for the patient’s PEA, she underwent pan-culturing, with the following tests ordered: HIV antibody testing, immunovirology for influenza A and B viruses, and urine toxicology. Doppler ultrasound of the bilateral lower extremities was also ordered, in addition to chest CT and transthoracic and transesophageal echocardiography (TTE and TEE, respectively). The patient was intubated and transferred to the medical ICU for further management.
The differential diagnosis included cardiac tamponade, acute MI, acute pulmonary embolus (PE), tension pneumothorax, hypovolemia, and asthma exacerbated by viral or bacterial infection.1,2 Although the case patient presented with PEA, she did not have the presenting signs of cardiac tamponade known as Beck’s triad: hypotension, jugular venous distension, and muffled heart sounds.3 TTE showed an ejection fraction of 65% and grade 2 diastolic dysfunction but no pericardial effusions (which accumulate rapidly in the patient with cardiac tamponade, resulting from fluid buildup in the pericardial layers),4 and TEE showed no atrial thrombi (which can masquerade as cardiac tamponade5). The patient had no signs of trauma and denied any history of malignancy (both potential causes of cardiac tamponade). Chest x-ray showed normal heart size and no pneumothorax, consolidations, or pleural effusions.4,6-8 Thus, the diagnosis of cardiac tamponade was ruled out.
Common presenting symptoms of acute MI include sudden-onset chest pain, SOB, palpitations, dizziness, nausea, and/or vomiting. Women may experience less dramatic symptoms—often little more than SOB and fatigue.9 According to a 2000 consensus document from a joint European Society of Cardiology/American College of Cardiology committee10 in which MI was redefined, the diagnosis of MI relies on a rise in cardiac troponin levels, typical MI symptoms, and changes in ECG showing pathological Q waves or ST elevation or depression. The case patient’s troponin I level was less than 0.02 ng/mL, and ECG did not reveal Q waves or ST-T wave changes; additionally, since the patient had no chest pain, palpitations, diaphoresis, nausea, or vomiting, acute MI was ruled out.
Blood clots capable of blocking the pulmonary artery usually originate in the deep veins of the lower extremities.11 Three main factors, called Virchow’s triad, are known to contribute to these deep vein thromboses (DVTs): venous stasis, endothelial injury, and a hypercoagulability state.12,13 The patient had denied any trauma, recent travel, history of malignancy, or use of tobacco or oral contraceptives, and the result of her urine pregnancy test was negative. Even though the patient presented with tachypnea and acute SOB, with ECG showing right-axis deviation and tachycardia (common presenting signs and symptoms for PE), her chest CT showed no evidence of PE (see Figure 2); additionally, Doppler ultrasound of the bilateral lower extremities revealed no DVTs. Thus, PE was also excluded.
Tension pneumothorax was also ruled out, as chest x-ray showed neither mediastinal shift nor tracheal deviation, and the patient had denied any trauma. Laboratory analyses did not indicate hyponatremia, and the patient’s hemoglobin and hematocrit were satisfactory. She was tachycardic on admission, but her blood pressure was stable. As the patient denied any use of vasodilators or diuretics, hypovolemia was ruled out.
Patients experiencing asthma exacerbation can present with acute SOB, which usually resolves following use of IV steroids, nebulizer therapy, and inhaler treatments. Despite being administered IV methylprednisolone and magnesium sulfate in the ED, the patient experienced PEA and respiratory distress and required intubation for airway protection.
The HIV test was nonreactive, and blood and urine cultures did not show any growth. Results of tests for Legionella urinary antigen and Streptococcus pneumoniae antigen were negative. Sputum culture showed normal flora. Immunovirology testing, however, was positive for both influenza A and B antigens.
Chest X-ray showed no acute pulmonary pathology, nor did chest CT show any central, interlobar, or segmental embolism or mediastinal lymphadenopathy. It was determined that the patient’s acute SOB might represent asthma exacerbation secondary to influenza viral infection. Her PEA was attributed to possible acute pericarditis secondary to concomitant influenza A and B viral infection.
DISCUSSION
Currently, the CDC recognizes three types of influenza virus: A, B, and C.14 Only influenza A viruses are further classified into subtypes, based on the presence of surface proteins called hemagglutinin (HA) or neuraminidase (NA) glycoproteins. Humans can be infected by influenza A subtypes H1N1 and H3N2.14 Influenza B viruses, found mostly in humans, are associated with significant morbidity and mortality.
Influenza A and B viruses are further classified into strains that change with each flu season—thus, the need to update vaccinations against influenza A and B each year. No vaccination exists against influenza C virus, which is known to cause only mild illness in humans.15
In patients with asthma (as in the case patient), chronic bronchitis, or emphysema, infection with the influenza virus can manifest with SOB, in addition to the more common symptoms of fever, sore throat, headache, rhinorrhea, chills, muscle aches, and general discomfort.16 Patients with coronary artery disease, congestive heart failure (CHF), and/or a history of smoking may experience more severe symptoms and increased risk for influenza-associated mortality than do other patients.17,18
Rare cardiac complications of influenza infections are myocarditis and benign acute pericarditis; myocarditis can progress to CHF and death.19,20 A case of acute myopericarditis was reported by Proby et al21 in a patient with acute influenza A infection who developed pericardial effusions, myositis, tamponade, and pleurisy. That patient recovered after pericardiocentesis and administration of inotropic drugs.
In the literature, a few cases of acute pericarditis have been reported in association with administration of the influenza vaccination.22,23
In the case patient, the diagnosis of influenza A and B was made following testing of nasal and nasopharyngeal swabs with an immunochromatographic assay that uses highly sensitive monoclonal antibodies to detect influenza A and B nucleoprotein antigens.24,25
According to reports in the literature, two-thirds of cases of acute pericarditis are caused by infection, most commonly viral infection (including influenza virus, adenovirus, enterovirus, cytomegalovirus, hepatitis B virus, and herpes simplex virus).26,27 Other etiologies for acute pericarditis are autoimmune (accounting for less than 10% of cases) and neoplastic conditions (5% to 7% of cases).26
PATIENT OUTCOME
Consultation with an infectious disease specialist was obtained. The patient was placed under droplet isolation precautions and was started on a nebulizer, IV steroid treatments, and oseltamivir 75 mg by mouth every 12 hours. She was transferred to a medical floor, where she completed a five-day course of oseltamivir.
As a result of timely intervention, the patient was discharged in stable condition on a therapeutic regimen that included albuterol, fluticasone, and salmeterol inhalation, in addition to tapered-dose steroids. She was advised to follow up with her primary care provider and at the pulmonary clinic.
CONCLUSION
To our knowledge, this is the first reported case of acute pericarditis in a patient with concomitant acute infections with influenza A and B. According to conclusions reached in recent literature, further research is needed to explain the pathophysiology of influenza viral infections, associated cardiovascular morbidity and mortality, and the degree to which these can be prevented by influenza vaccination.1,28 Also to be pursued through research is a better understanding of the morbidity and mortality associated with influenza viruses, especially in children and in adults affected by asthma, cardiac disease, and/or obesity.
REFERENCES
1. Finelli L, Chaves SS. Influenza and acute myocardial infarction. J Infect Dis. 2011;203(12):
1701-1704.
2. Steiger HV, Rimbach K, Müller E, Breitkreutz R. Focused emergency echocardiography: lifesaving tool for a 14-year-old girl suffering out-of-hospital pulseless electrical activity arrest because of cardiac tamponade. Eur J Emerg Med. 2009;16(2): 103-105.
3. Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade.
J Emerg Trauma Shock. 2012;5(1):72-75.
4. Restrepo CS, Lemos DF, Lemos JA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007;27(6):1595-1610.
5. Papanagnou D, Stone MB. Massive right atrial thrombus masquerading as cardiac tamponade. Acad Emerg Med. 2010;17(2):E11.
6. Saito Y, Donohue A, Attai S, et al. The syndrome of cardiac tamponade with “small” pericardial effusion. Echocardiography. 2008;25(3): 321-327.
7. Lin E, Boire A, Hemmige V, et al. Cardiac tamponade mimicking tuberculous pericarditis as the initial presentation of chronic lymphocytic leukemia in a 58-year-old woman: a case report. J Med Case Rep. 2010;4:246.
8. Meniconi A, Attenhofer Jost CH, Jenni R. How to survive myocardial rupture after myocardial infarction. Heart. 2000;84(5):552.
9. Kosuge M, Kimura K, Ishikawa T, et al. Differences between men and women in terms of clinical features of ST-segment elevation acute myocardial infarction. Circ J. 2006;70(3):222-226.
10. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959-969.
11. Goldhaber SZ. Deep venous thrombosis and pulmonary thromboembolism. In: Fauci AS, Braunwald E, Kasper DL, et al. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Medical; 2008:1651–1657.
12. Brooks EG, Trotman W, Wadsworth MP, et al. Valves of the deep venous system: an overlooked risk factor. Blood. 2009;114(6):1276-1279.
13. Kyrle PA, Eichinger S. Is Virchow’s triad complete? Blood. 2009;114(6):1138-1139.
14. CDC. Seasonal influenza (flu): types of influenza viruses (2012). www.cdc.gov/flu/about/viruses/types.htm. Accessed October 24, 2012.
15. CDC. Seasonal influenza (flu)(2012). www.cdc .gov/flu. Accessed October 24, 2012.
16. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
17. Angelo SJ, Marshall PS, Chrissoheris MP, Chaves AM. Clinical characteristics associated with poor outcome in patients acutely infected with Influenza A. Conn Med. 2004;68(4):199-205.
18. Murin S, Bilello K. Respiratory tract infections: another reason not to smoke. Cleve Clin J Med. 2005;72(10):916-920.
19. Ray CG, Icenogle TB, Minnich LL, et al. The use of intravenous ribavirin to treat influenza virus–associated acute myocarditis. J Infect Dis. 1989; 159(5):829-836.
20. Fairley CK, Ryan M, Wall PG, Weinberg J. The organism reported to cause infective myocarditis and pericarditis in England and Wales. J Infect. 1996;32(3):223-225.
21. Proby CM, Hackett D, Gupta S, Cox TM. Acute myopericarditis in influenza A infection. Q J Med. 1986;60(233):887-892.
22. Streifler JJ, Dux S, Garty M, Rosenfeld JB. Recurrent pericarditis: a rare complication of influenza vaccination. Br Med J (Clin Res Ed). 1981; 283(6290):526-527.
23. Desson JF, Leprévost M, Vabret F, Davy A. Acute benign pericarditis after anti-influenza vaccination [in French]. Presse Med. 1997;26 (9):415.
24. BinaxNOW® Influenza A&B Test Kit (product instructions). www.diagnosticsdirect2u.com/images/PDF/Binax%20Now%20416-022%20PPI .pdf. Accessed October 24, 2012.
25. 510(k) Substantial Equivalence Determination Decision Summary [BinaxNow® Influenza A & B Test] (2009). www.accessdata.fda.gov/cdrh_docs/reviews/K062109.pdf. Accessed October 24, 2012.
26. Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121(7):916-928.
27. Maisch B, Seferovic PM, Ristic AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases: executive summary. Eur Heart J. 2004;25(7):587-610.
28. McCullers JA, Hayden FG. Fatal influenza B infections: time to reexamine influenza research priorities. J Infect Dis. 2012;205(6):870-872.
On an autumn day, a 38-year-old woman with a history of asthma presented to the emergency department (ED) with the chief complaint of shortness of breath (SOB). The patient described her SOB as sudden in onset and not relieved by use of her albuterol inhaler; hence the ED visit.
She denied any chest pain, palpitations, dizziness, orthopnea, upper respiratory tract infection, cough, wheezing, fever or chills, headache, vision changes, body aches, sick contacts, or pets at home. She said she uses her albuterol inhaler as needed, and that she had used it that day for the first time in “a few months.” She denied any history of intubation or steroid use. Additionally, she had not been seen by a primary care provider in years.
The woman, a native of Ghana, had been living in the United States for many years. She denied any recent travel or exposure to toxic chemicals; any use of tobacco, alcohol, or illicit drugs; or any history of sexually transmitted disease.
The patient was afebrile (temperature, 98.6°F), with a respiratory rate of 20 breaths/min; blood pressure, 144/69 mm Hg; and ventricular rate, 125 beats/min. On physical examination, her extraocular movements were intact; pupils were equal, round, reactive to light and accommodation; and sclera were nonicteric. The patient’s head was normocephalic and atraumatic, and the neck was supple with normal range of motion and no jugular venous distension or lymphadenopathy. Her mucous membranes were moist with no pharyngeal erythema or exudates. Cardiovascular examination, including ECG, revealed tachycardia but no murmurs or gallops.
While being evaluated in the ED, the patient became tachypneic and began to experience respiratory distress. She was intubated for airway protection, at which time she developed pulseless electrical activity (PEA), with 30 beats/min. She responded to atropine and epinephrine injections. A repeat ECG showed sinus tachycardia and right atrial enlargement with right-axis deviation. Chest x-ray (see Figure 1) showed no consolidation, pleural effusion, or pneumothorax.
Results from the patient’s lab work are shown in the table, above. Negative results were reported for a urine pregnancy test.
Since there was no clear etiology for the patient’s PEA, she underwent pan-culturing, with the following tests ordered: HIV antibody testing, immunovirology for influenza A and B viruses, and urine toxicology. Doppler ultrasound of the bilateral lower extremities was also ordered, in addition to chest CT and transthoracic and transesophageal echocardiography (TTE and TEE, respectively). The patient was intubated and transferred to the medical ICU for further management.
The differential diagnosis included cardiac tamponade, acute MI, acute pulmonary embolus (PE), tension pneumothorax, hypovolemia, and asthma exacerbated by viral or bacterial infection.1,2 Although the case patient presented with PEA, she did not have the presenting signs of cardiac tamponade known as Beck’s triad: hypotension, jugular venous distension, and muffled heart sounds.3 TTE showed an ejection fraction of 65% and grade 2 diastolic dysfunction but no pericardial effusions (which accumulate rapidly in the patient with cardiac tamponade, resulting from fluid buildup in the pericardial layers),4 and TEE showed no atrial thrombi (which can masquerade as cardiac tamponade5). The patient had no signs of trauma and denied any history of malignancy (both potential causes of cardiac tamponade). Chest x-ray showed normal heart size and no pneumothorax, consolidations, or pleural effusions.4,6-8 Thus, the diagnosis of cardiac tamponade was ruled out.
Common presenting symptoms of acute MI include sudden-onset chest pain, SOB, palpitations, dizziness, nausea, and/or vomiting. Women may experience less dramatic symptoms—often little more than SOB and fatigue.9 According to a 2000 consensus document from a joint European Society of Cardiology/American College of Cardiology committee10 in which MI was redefined, the diagnosis of MI relies on a rise in cardiac troponin levels, typical MI symptoms, and changes in ECG showing pathological Q waves or ST elevation or depression. The case patient’s troponin I level was less than 0.02 ng/mL, and ECG did not reveal Q waves or ST-T wave changes; additionally, since the patient had no chest pain, palpitations, diaphoresis, nausea, or vomiting, acute MI was ruled out.
Blood clots capable of blocking the pulmonary artery usually originate in the deep veins of the lower extremities.11 Three main factors, called Virchow’s triad, are known to contribute to these deep vein thromboses (DVTs): venous stasis, endothelial injury, and a hypercoagulability state.12,13 The patient had denied any trauma, recent travel, history of malignancy, or use of tobacco or oral contraceptives, and the result of her urine pregnancy test was negative. Even though the patient presented with tachypnea and acute SOB, with ECG showing right-axis deviation and tachycardia (common presenting signs and symptoms for PE), her chest CT showed no evidence of PE (see Figure 2); additionally, Doppler ultrasound of the bilateral lower extremities revealed no DVTs. Thus, PE was also excluded.
Tension pneumothorax was also ruled out, as chest x-ray showed neither mediastinal shift nor tracheal deviation, and the patient had denied any trauma. Laboratory analyses did not indicate hyponatremia, and the patient’s hemoglobin and hematocrit were satisfactory. She was tachycardic on admission, but her blood pressure was stable. As the patient denied any use of vasodilators or diuretics, hypovolemia was ruled out.
Patients experiencing asthma exacerbation can present with acute SOB, which usually resolves following use of IV steroids, nebulizer therapy, and inhaler treatments. Despite being administered IV methylprednisolone and magnesium sulfate in the ED, the patient experienced PEA and respiratory distress and required intubation for airway protection.
The HIV test was nonreactive, and blood and urine cultures did not show any growth. Results of tests for Legionella urinary antigen and Streptococcus pneumoniae antigen were negative. Sputum culture showed normal flora. Immunovirology testing, however, was positive for both influenza A and B antigens.
Chest X-ray showed no acute pulmonary pathology, nor did chest CT show any central, interlobar, or segmental embolism or mediastinal lymphadenopathy. It was determined that the patient’s acute SOB might represent asthma exacerbation secondary to influenza viral infection. Her PEA was attributed to possible acute pericarditis secondary to concomitant influenza A and B viral infection.
DISCUSSION
Currently, the CDC recognizes three types of influenza virus: A, B, and C.14 Only influenza A viruses are further classified into subtypes, based on the presence of surface proteins called hemagglutinin (HA) or neuraminidase (NA) glycoproteins. Humans can be infected by influenza A subtypes H1N1 and H3N2.14 Influenza B viruses, found mostly in humans, are associated with significant morbidity and mortality.
Influenza A and B viruses are further classified into strains that change with each flu season—thus, the need to update vaccinations against influenza A and B each year. No vaccination exists against influenza C virus, which is known to cause only mild illness in humans.15
In patients with asthma (as in the case patient), chronic bronchitis, or emphysema, infection with the influenza virus can manifest with SOB, in addition to the more common symptoms of fever, sore throat, headache, rhinorrhea, chills, muscle aches, and general discomfort.16 Patients with coronary artery disease, congestive heart failure (CHF), and/or a history of smoking may experience more severe symptoms and increased risk for influenza-associated mortality than do other patients.17,18
Rare cardiac complications of influenza infections are myocarditis and benign acute pericarditis; myocarditis can progress to CHF and death.19,20 A case of acute myopericarditis was reported by Proby et al21 in a patient with acute influenza A infection who developed pericardial effusions, myositis, tamponade, and pleurisy. That patient recovered after pericardiocentesis and administration of inotropic drugs.
In the literature, a few cases of acute pericarditis have been reported in association with administration of the influenza vaccination.22,23
In the case patient, the diagnosis of influenza A and B was made following testing of nasal and nasopharyngeal swabs with an immunochromatographic assay that uses highly sensitive monoclonal antibodies to detect influenza A and B nucleoprotein antigens.24,25
According to reports in the literature, two-thirds of cases of acute pericarditis are caused by infection, most commonly viral infection (including influenza virus, adenovirus, enterovirus, cytomegalovirus, hepatitis B virus, and herpes simplex virus).26,27 Other etiologies for acute pericarditis are autoimmune (accounting for less than 10% of cases) and neoplastic conditions (5% to 7% of cases).26
PATIENT OUTCOME
Consultation with an infectious disease specialist was obtained. The patient was placed under droplet isolation precautions and was started on a nebulizer, IV steroid treatments, and oseltamivir 75 mg by mouth every 12 hours. She was transferred to a medical floor, where she completed a five-day course of oseltamivir.
As a result of timely intervention, the patient was discharged in stable condition on a therapeutic regimen that included albuterol, fluticasone, and salmeterol inhalation, in addition to tapered-dose steroids. She was advised to follow up with her primary care provider and at the pulmonary clinic.
CONCLUSION
To our knowledge, this is the first reported case of acute pericarditis in a patient with concomitant acute infections with influenza A and B. According to conclusions reached in recent literature, further research is needed to explain the pathophysiology of influenza viral infections, associated cardiovascular morbidity and mortality, and the degree to which these can be prevented by influenza vaccination.1,28 Also to be pursued through research is a better understanding of the morbidity and mortality associated with influenza viruses, especially in children and in adults affected by asthma, cardiac disease, and/or obesity.
REFERENCES
1. Finelli L, Chaves SS. Influenza and acute myocardial infarction. J Infect Dis. 2011;203(12):
1701-1704.
2. Steiger HV, Rimbach K, Müller E, Breitkreutz R. Focused emergency echocardiography: lifesaving tool for a 14-year-old girl suffering out-of-hospital pulseless electrical activity arrest because of cardiac tamponade. Eur J Emerg Med. 2009;16(2): 103-105.
3. Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade.
J Emerg Trauma Shock. 2012;5(1):72-75.
4. Restrepo CS, Lemos DF, Lemos JA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007;27(6):1595-1610.
5. Papanagnou D, Stone MB. Massive right atrial thrombus masquerading as cardiac tamponade. Acad Emerg Med. 2010;17(2):E11.
6. Saito Y, Donohue A, Attai S, et al. The syndrome of cardiac tamponade with “small” pericardial effusion. Echocardiography. 2008;25(3): 321-327.
7. Lin E, Boire A, Hemmige V, et al. Cardiac tamponade mimicking tuberculous pericarditis as the initial presentation of chronic lymphocytic leukemia in a 58-year-old woman: a case report. J Med Case Rep. 2010;4:246.
8. Meniconi A, Attenhofer Jost CH, Jenni R. How to survive myocardial rupture after myocardial infarction. Heart. 2000;84(5):552.
9. Kosuge M, Kimura K, Ishikawa T, et al. Differences between men and women in terms of clinical features of ST-segment elevation acute myocardial infarction. Circ J. 2006;70(3):222-226.
10. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959-969.
11. Goldhaber SZ. Deep venous thrombosis and pulmonary thromboembolism. In: Fauci AS, Braunwald E, Kasper DL, et al. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Medical; 2008:1651–1657.
12. Brooks EG, Trotman W, Wadsworth MP, et al. Valves of the deep venous system: an overlooked risk factor. Blood. 2009;114(6):1276-1279.
13. Kyrle PA, Eichinger S. Is Virchow’s triad complete? Blood. 2009;114(6):1138-1139.
14. CDC. Seasonal influenza (flu): types of influenza viruses (2012). www.cdc.gov/flu/about/viruses/types.htm. Accessed October 24, 2012.
15. CDC. Seasonal influenza (flu)(2012). www.cdc .gov/flu. Accessed October 24, 2012.
16. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
17. Angelo SJ, Marshall PS, Chrissoheris MP, Chaves AM. Clinical characteristics associated with poor outcome in patients acutely infected with Influenza A. Conn Med. 2004;68(4):199-205.
18. Murin S, Bilello K. Respiratory tract infections: another reason not to smoke. Cleve Clin J Med. 2005;72(10):916-920.
19. Ray CG, Icenogle TB, Minnich LL, et al. The use of intravenous ribavirin to treat influenza virus–associated acute myocarditis. J Infect Dis. 1989; 159(5):829-836.
20. Fairley CK, Ryan M, Wall PG, Weinberg J. The organism reported to cause infective myocarditis and pericarditis in England and Wales. J Infect. 1996;32(3):223-225.
21. Proby CM, Hackett D, Gupta S, Cox TM. Acute myopericarditis in influenza A infection. Q J Med. 1986;60(233):887-892.
22. Streifler JJ, Dux S, Garty M, Rosenfeld JB. Recurrent pericarditis: a rare complication of influenza vaccination. Br Med J (Clin Res Ed). 1981; 283(6290):526-527.
23. Desson JF, Leprévost M, Vabret F, Davy A. Acute benign pericarditis after anti-influenza vaccination [in French]. Presse Med. 1997;26 (9):415.
24. BinaxNOW® Influenza A&B Test Kit (product instructions). www.diagnosticsdirect2u.com/images/PDF/Binax%20Now%20416-022%20PPI .pdf. Accessed October 24, 2012.
25. 510(k) Substantial Equivalence Determination Decision Summary [BinaxNow® Influenza A & B Test] (2009). www.accessdata.fda.gov/cdrh_docs/reviews/K062109.pdf. Accessed October 24, 2012.
26. Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121(7):916-928.
27. Maisch B, Seferovic PM, Ristic AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases: executive summary. Eur Heart J. 2004;25(7):587-610.
28. McCullers JA, Hayden FG. Fatal influenza B infections: time to reexamine influenza research priorities. J Infect Dis. 2012;205(6):870-872.