User login
HM14 Special Report: When Cellulitis Isn't: Identifying Cellulitis Mimics
Presenter: Daniela Kroshinsky, MD, MPH
Summation: Cellulitis accounts for up to 10% of infectious disease hospitalizations and for about 3 billion/year in healthcare costs for both inpatient and outpatient treatment. Dr. Kroshinsky pointed out that dependent on patient factors, inadequately treated or recurrent cellulitis can lead to significant complications with chronic stasis changes and ulcerations. The diagnosis of cellulitis is typically made on physical exam. Cellulitis may have unusual presentations and at times the diagnosis can be difficult.
Hospitalists need to be aware that cellulitis has multiple mimics, and between 28% to 33% of patients are misdiagnosed as having cellulitis.
Dr. Kroshinsky listed a number of differential diagnoses. Frequent alternative diagnoses are dermatitis due to venous stasis or caused by lymphedema. Other skin conditions that need to be considered include erysipeloid, erythema migrans, atypical zoster, tinea and other fungal infections as well as skin changes caused by underlying malignancies.
Key Takeaways
- The diagnosis of cellulitis has a high error rate
- It is important to treat cellulitis adequately to prevent chronic skin changes and ulcers
- If cellulitis does not respond to appropriate antibacterial treatment, consider alternative diagnoses
- Be aware that many skin conditions can mimic cellulitis in immunocompromised patients
Klaus Suehler is a hospitalist at Mercy Hospital at Allina Health in Coon Rapids, Minn., and a member of Team Hospitalist.
Presenter: Daniela Kroshinsky, MD, MPH
Summation: Cellulitis accounts for up to 10% of infectious disease hospitalizations and for about 3 billion/year in healthcare costs for both inpatient and outpatient treatment. Dr. Kroshinsky pointed out that dependent on patient factors, inadequately treated or recurrent cellulitis can lead to significant complications with chronic stasis changes and ulcerations. The diagnosis of cellulitis is typically made on physical exam. Cellulitis may have unusual presentations and at times the diagnosis can be difficult.
Hospitalists need to be aware that cellulitis has multiple mimics, and between 28% to 33% of patients are misdiagnosed as having cellulitis.
Dr. Kroshinsky listed a number of differential diagnoses. Frequent alternative diagnoses are dermatitis due to venous stasis or caused by lymphedema. Other skin conditions that need to be considered include erysipeloid, erythema migrans, atypical zoster, tinea and other fungal infections as well as skin changes caused by underlying malignancies.
Key Takeaways
- The diagnosis of cellulitis has a high error rate
- It is important to treat cellulitis adequately to prevent chronic skin changes and ulcers
- If cellulitis does not respond to appropriate antibacterial treatment, consider alternative diagnoses
- Be aware that many skin conditions can mimic cellulitis in immunocompromised patients
Klaus Suehler is a hospitalist at Mercy Hospital at Allina Health in Coon Rapids, Minn., and a member of Team Hospitalist.
Presenter: Daniela Kroshinsky, MD, MPH
Summation: Cellulitis accounts for up to 10% of infectious disease hospitalizations and for about 3 billion/year in healthcare costs for both inpatient and outpatient treatment. Dr. Kroshinsky pointed out that dependent on patient factors, inadequately treated or recurrent cellulitis can lead to significant complications with chronic stasis changes and ulcerations. The diagnosis of cellulitis is typically made on physical exam. Cellulitis may have unusual presentations and at times the diagnosis can be difficult.
Hospitalists need to be aware that cellulitis has multiple mimics, and between 28% to 33% of patients are misdiagnosed as having cellulitis.
Dr. Kroshinsky listed a number of differential diagnoses. Frequent alternative diagnoses are dermatitis due to venous stasis or caused by lymphedema. Other skin conditions that need to be considered include erysipeloid, erythema migrans, atypical zoster, tinea and other fungal infections as well as skin changes caused by underlying malignancies.
Key Takeaways
- The diagnosis of cellulitis has a high error rate
- It is important to treat cellulitis adequately to prevent chronic skin changes and ulcers
- If cellulitis does not respond to appropriate antibacterial treatment, consider alternative diagnoses
- Be aware that many skin conditions can mimic cellulitis in immunocompromised patients
Klaus Suehler is a hospitalist at Mercy Hospital at Allina Health in Coon Rapids, Minn., and a member of Team Hospitalist.
HM14 Special Report: The Future of the Healthcare Marketplace
A Scot who describes himself as a “professional futurist,” Ian Morrison, MD, helped HM14 get off to a start with laughter. Describing the background of the changes leading to the Affordable Care Act (ACA), Dr. Morrison repeated his key point several times: “We’ve got to change the delivery system.”
This overview is very timely as the deadline for enrollment to comply with the Affordable Care Act is March 31, 2014. Dr. Morrison cited that as of March 11, more than five million Americans have enrolled in exchanges and over 80% of them have paid premiums. Up to 14 million Americans are eligible for Medicaid expansion programs. It is unknown how many of these people were previously uninsured.
Dr. Morrison described several key current issues, including:
1. There are “two Americas”—those states expanding Medicaid and those states declining any expansion;
2. The ACA is the “the Law of the Land.” The difficult task is now implementing this large change in the industry;
3. Accountable Care is a megatrend, but accountable care organizations may not continue in the form they are now;
4. Pressure on costs and delivering value is intensifying;
5. Medicare is still a major part of the healthcare system. “Learning to live in Medicare” means taking out 10% to 20% of costs; and
6. There is a renewed focus on primary care.
Dr. Morrison shared a vision of the future as these trends continue. There will continue to be “massive consolidation” in which there may be only 100-200 large regional healthcare systems in the U.S. Related to this linked care, clinical protocols will be more widely used. Care coordination of transitions will be at a premium. The transition of moving away from hospital admissions to more home care will be economically and culturally challenging.
As a transition to the future of healthcare, Dr. Morrison reviewed the concept of “the second curve” in business. Most hospitals have mastered the first curve of volume-based care, which is daily business and operations. The second curve, which is more value-based care, is a new way of doing business. Individual hospitals and healthcare systems must plan for, and succeed with, the second curve to survive. Dr. Morrison said this pressure on the healthcare system and the second curve is real, stating, “We turned the corner and we ain’t going back.”
Public purchasers will continue to play a growing role in the future. Dr. Morrison explained why Medicare Advantage is so resilient. Public employers have huge retiree health benefit problems. Dr. Morrison predicts that public payers will be more dominant by 2020 and public exchanges will grow after a rocky start.
Even with a disruptive start to the healthcare exchanges, Dr. Morrison encouraged the audience to think of the long-term benefits of the healthcare system changes.
He envisions four scenarios for the exchanges:
1. Managed competition nirvana. In this system, both public and private exchanges can grow;
2. Minor miracle. This is where the system is now at the start of exchanges;
3. Single-payer system. This would enable public exchanges to continue to grow and succeed; and
4. Meltdown, caused by patient- and system-risk or politics.
The work of the future is the transformation of the delivery system. This difficult work includes the centrality of clinical integration, information technology, “learning to live on Medicare”, managing a business model migration (from curve 1 to 2), and finally, building a culture of quality and accountability.
Dr. Morrison ended this enlightening session with several ACA implications and roles for hospitalists:
1. Take the long view. This is an area where hospitalists can continue to be leaders;
2. Redesign acute care, with hospitalists taking the lead;
3. Reach out beyond the walls. It will be very important for hospitalists to work even more closely with primary care providers;
4. Bring your clinical colleagues along to pursue the “triple aim” (better health, better healthcare, and lower per capita costs); and
5. Benefit patients, payers and providers through these changes.
Key points:
• “We’ve got to change the delivery system;”
• The changes in the healthcare system are areas in which hospitalists can continue to be leaders;
• ACA changes can be better for the patient, payer and provider; and
• HM14 is off to a strong start with a clear, overarching goal of hospitalists leading the changing world of medicine.
Dr. Hale is a pediatric hospitalist at the Floating Hospital for Children at Tufts Medical Center in Boston.
A Scot who describes himself as a “professional futurist,” Ian Morrison, MD, helped HM14 get off to a start with laughter. Describing the background of the changes leading to the Affordable Care Act (ACA), Dr. Morrison repeated his key point several times: “We’ve got to change the delivery system.”
This overview is very timely as the deadline for enrollment to comply with the Affordable Care Act is March 31, 2014. Dr. Morrison cited that as of March 11, more than five million Americans have enrolled in exchanges and over 80% of them have paid premiums. Up to 14 million Americans are eligible for Medicaid expansion programs. It is unknown how many of these people were previously uninsured.
Dr. Morrison described several key current issues, including:
1. There are “two Americas”—those states expanding Medicaid and those states declining any expansion;
2. The ACA is the “the Law of the Land.” The difficult task is now implementing this large change in the industry;
3. Accountable Care is a megatrend, but accountable care organizations may not continue in the form they are now;
4. Pressure on costs and delivering value is intensifying;
5. Medicare is still a major part of the healthcare system. “Learning to live in Medicare” means taking out 10% to 20% of costs; and
6. There is a renewed focus on primary care.
Dr. Morrison shared a vision of the future as these trends continue. There will continue to be “massive consolidation” in which there may be only 100-200 large regional healthcare systems in the U.S. Related to this linked care, clinical protocols will be more widely used. Care coordination of transitions will be at a premium. The transition of moving away from hospital admissions to more home care will be economically and culturally challenging.
As a transition to the future of healthcare, Dr. Morrison reviewed the concept of “the second curve” in business. Most hospitals have mastered the first curve of volume-based care, which is daily business and operations. The second curve, which is more value-based care, is a new way of doing business. Individual hospitals and healthcare systems must plan for, and succeed with, the second curve to survive. Dr. Morrison said this pressure on the healthcare system and the second curve is real, stating, “We turned the corner and we ain’t going back.”
Public purchasers will continue to play a growing role in the future. Dr. Morrison explained why Medicare Advantage is so resilient. Public employers have huge retiree health benefit problems. Dr. Morrison predicts that public payers will be more dominant by 2020 and public exchanges will grow after a rocky start.
Even with a disruptive start to the healthcare exchanges, Dr. Morrison encouraged the audience to think of the long-term benefits of the healthcare system changes.
He envisions four scenarios for the exchanges:
1. Managed competition nirvana. In this system, both public and private exchanges can grow;
2. Minor miracle. This is where the system is now at the start of exchanges;
3. Single-payer system. This would enable public exchanges to continue to grow and succeed; and
4. Meltdown, caused by patient- and system-risk or politics.
The work of the future is the transformation of the delivery system. This difficult work includes the centrality of clinical integration, information technology, “learning to live on Medicare”, managing a business model migration (from curve 1 to 2), and finally, building a culture of quality and accountability.
Dr. Morrison ended this enlightening session with several ACA implications and roles for hospitalists:
1. Take the long view. This is an area where hospitalists can continue to be leaders;
2. Redesign acute care, with hospitalists taking the lead;
3. Reach out beyond the walls. It will be very important for hospitalists to work even more closely with primary care providers;
4. Bring your clinical colleagues along to pursue the “triple aim” (better health, better healthcare, and lower per capita costs); and
5. Benefit patients, payers and providers through these changes.
Key points:
• “We’ve got to change the delivery system;”
• The changes in the healthcare system are areas in which hospitalists can continue to be leaders;
• ACA changes can be better for the patient, payer and provider; and
• HM14 is off to a strong start with a clear, overarching goal of hospitalists leading the changing world of medicine.
Dr. Hale is a pediatric hospitalist at the Floating Hospital for Children at Tufts Medical Center in Boston.
A Scot who describes himself as a “professional futurist,” Ian Morrison, MD, helped HM14 get off to a start with laughter. Describing the background of the changes leading to the Affordable Care Act (ACA), Dr. Morrison repeated his key point several times: “We’ve got to change the delivery system.”
This overview is very timely as the deadline for enrollment to comply with the Affordable Care Act is March 31, 2014. Dr. Morrison cited that as of March 11, more than five million Americans have enrolled in exchanges and over 80% of them have paid premiums. Up to 14 million Americans are eligible for Medicaid expansion programs. It is unknown how many of these people were previously uninsured.
Dr. Morrison described several key current issues, including:
1. There are “two Americas”—those states expanding Medicaid and those states declining any expansion;
2. The ACA is the “the Law of the Land.” The difficult task is now implementing this large change in the industry;
3. Accountable Care is a megatrend, but accountable care organizations may not continue in the form they are now;
4. Pressure on costs and delivering value is intensifying;
5. Medicare is still a major part of the healthcare system. “Learning to live in Medicare” means taking out 10% to 20% of costs; and
6. There is a renewed focus on primary care.
Dr. Morrison shared a vision of the future as these trends continue. There will continue to be “massive consolidation” in which there may be only 100-200 large regional healthcare systems in the U.S. Related to this linked care, clinical protocols will be more widely used. Care coordination of transitions will be at a premium. The transition of moving away from hospital admissions to more home care will be economically and culturally challenging.
As a transition to the future of healthcare, Dr. Morrison reviewed the concept of “the second curve” in business. Most hospitals have mastered the first curve of volume-based care, which is daily business and operations. The second curve, which is more value-based care, is a new way of doing business. Individual hospitals and healthcare systems must plan for, and succeed with, the second curve to survive. Dr. Morrison said this pressure on the healthcare system and the second curve is real, stating, “We turned the corner and we ain’t going back.”
Public purchasers will continue to play a growing role in the future. Dr. Morrison explained why Medicare Advantage is so resilient. Public employers have huge retiree health benefit problems. Dr. Morrison predicts that public payers will be more dominant by 2020 and public exchanges will grow after a rocky start.
Even with a disruptive start to the healthcare exchanges, Dr. Morrison encouraged the audience to think of the long-term benefits of the healthcare system changes.
He envisions four scenarios for the exchanges:
1. Managed competition nirvana. In this system, both public and private exchanges can grow;
2. Minor miracle. This is where the system is now at the start of exchanges;
3. Single-payer system. This would enable public exchanges to continue to grow and succeed; and
4. Meltdown, caused by patient- and system-risk or politics.
The work of the future is the transformation of the delivery system. This difficult work includes the centrality of clinical integration, information technology, “learning to live on Medicare”, managing a business model migration (from curve 1 to 2), and finally, building a culture of quality and accountability.
Dr. Morrison ended this enlightening session with several ACA implications and roles for hospitalists:
1. Take the long view. This is an area where hospitalists can continue to be leaders;
2. Redesign acute care, with hospitalists taking the lead;
3. Reach out beyond the walls. It will be very important for hospitalists to work even more closely with primary care providers;
4. Bring your clinical colleagues along to pursue the “triple aim” (better health, better healthcare, and lower per capita costs); and
5. Benefit patients, payers and providers through these changes.
Key points:
• “We’ve got to change the delivery system;”
• The changes in the healthcare system are areas in which hospitalists can continue to be leaders;
• ACA changes can be better for the patient, payer and provider; and
• HM14 is off to a strong start with a clear, overarching goal of hospitalists leading the changing world of medicine.
Dr. Hale is a pediatric hospitalist at the Floating Hospital for Children at Tufts Medical Center in Boston.
HM14 Report: Review of New Guidelines for Pediatric UTI
Presenter: Maria Finnell, MD
Summation: Dr. Finnell reviewed in detail the recommendations and controversies surrounding the revised 2011 guidelines. The thrust was on more refined diagnostic criteria and rigorous review of diagnostic options (ultrasound, VCUG) and therapeutic options (length of treatment, IV vs oral antibiotics, and prophylactic therapy).
Key Takeaways
- The diagnosis of a UTI is based on an abnormal urinalysis and a positive urine culture, now defined as >50,000 CFU/ml. A bag-colleted urine is not very effective in truly diagnosing a UTI (due to excessive false positives).
- Oral treatment is as effective as IV therapy.
- Duration of 7-14 days is recommended. There is not definitive evidence to support a more specific length at this time.
- A VCUG is not recommended after a 1st febrile UTI for children 2 months- 2 years of age.
- Antibiotic prophylaxis does increase antibiotic resistance and is not clearly helpful for reflux grades 1-2. For reflux grades 3-5, it may still be effective.
- Educating parents of children who have had a 1st febrile UTI to arrange for early evaluation of a possible secondary febrile UTIs is key in catching UTIs early.
Dr. Harlan is a pediatric hospitalist, medical director with IPC The Hospitalist Company, and member of Team Hospitalist.
Presenter: Maria Finnell, MD
Summation: Dr. Finnell reviewed in detail the recommendations and controversies surrounding the revised 2011 guidelines. The thrust was on more refined diagnostic criteria and rigorous review of diagnostic options (ultrasound, VCUG) and therapeutic options (length of treatment, IV vs oral antibiotics, and prophylactic therapy).
Key Takeaways
- The diagnosis of a UTI is based on an abnormal urinalysis and a positive urine culture, now defined as >50,000 CFU/ml. A bag-colleted urine is not very effective in truly diagnosing a UTI (due to excessive false positives).
- Oral treatment is as effective as IV therapy.
- Duration of 7-14 days is recommended. There is not definitive evidence to support a more specific length at this time.
- A VCUG is not recommended after a 1st febrile UTI for children 2 months- 2 years of age.
- Antibiotic prophylaxis does increase antibiotic resistance and is not clearly helpful for reflux grades 1-2. For reflux grades 3-5, it may still be effective.
- Educating parents of children who have had a 1st febrile UTI to arrange for early evaluation of a possible secondary febrile UTIs is key in catching UTIs early.
Dr. Harlan is a pediatric hospitalist, medical director with IPC The Hospitalist Company, and member of Team Hospitalist.
Presenter: Maria Finnell, MD
Summation: Dr. Finnell reviewed in detail the recommendations and controversies surrounding the revised 2011 guidelines. The thrust was on more refined diagnostic criteria and rigorous review of diagnostic options (ultrasound, VCUG) and therapeutic options (length of treatment, IV vs oral antibiotics, and prophylactic therapy).
Key Takeaways
- The diagnosis of a UTI is based on an abnormal urinalysis and a positive urine culture, now defined as >50,000 CFU/ml. A bag-colleted urine is not very effective in truly diagnosing a UTI (due to excessive false positives).
- Oral treatment is as effective as IV therapy.
- Duration of 7-14 days is recommended. There is not definitive evidence to support a more specific length at this time.
- A VCUG is not recommended after a 1st febrile UTI for children 2 months- 2 years of age.
- Antibiotic prophylaxis does increase antibiotic resistance and is not clearly helpful for reflux grades 1-2. For reflux grades 3-5, it may still be effective.
- Educating parents of children who have had a 1st febrile UTI to arrange for early evaluation of a possible secondary febrile UTIs is key in catching UTIs early.
Dr. Harlan is a pediatric hospitalist, medical director with IPC The Hospitalist Company, and member of Team Hospitalist.
HM14 Special Report: Rationale and Review of the New Guidelines for First Febrile UTI
Presenter: Maria Finnell, M.D., a leading member of the American Academy of Pediatrics Subcommittee on Urinary Tract Infection
Summary: Dr. Finnell summarized the recent changes in diagnosis and management of pediatric urinary tract infections (UTIs). The 2011 publication of “Urinary Tract Infection: Clinical Practice Guideline for the Diagnosis and Management of the Initial UTI in Febrile Infants and Children 2 to 24 Months” was an update of the 1999 technical report of UTI management. Dr. Finnell reviewed the difference between evidence based and eminence based recommendations. She stated the term “recommendations” was changed to “key action statements” in a new explicit reporting format. Aggregate quality of the evidence is presented in the report in an effort to keep statements transparent.
The process of updating the new guideline was based on the U.S. Preventive Services Task Force approach using a stepwise process. For the revised UTI recommendations the steps were narrowed to:
- Risk of having infection
- Making a diagnosis
- Treatment of UTI
- Identification and Evaluation for high risk conditions
Patient population for this guideline includes initial UTI in child age 2 months to 2 years of age. Patients with neurological conditions or recurrent UTI or renal damage are excluded. Dr. Finnell reviewed action statements for the revised guidelines. A summary of some of these statements:
- If antibiotics are going to be administered, a urine specimen should be collected by catheterization or suprapubic aspiration (SPA).
- Assessment of UTI risk should be performed in a febrile child with no source of infection. The guideline cites specific data for risk. If the likelihood is low then it is reasonable to follow the child clinically without a urine specimen. If the likelihood of a UTI is high then a urine specimen should be obtained.
- To establish the diagnosis of UTI, clinicians should require both urinalysis results that suggest infection and the presence of at least 50,000 colony-forming units (CFUs) per mL of a uropathogen cultured from a urine specimen obtained through catheterization or SPA.
- Oral and parenteral routes are equally efficacious.
- The clinician should choose 7-14 days as duration of treatment.
- Febrile infants with UTIs should undergo renal and bladder ultrasonography.
- VCUG should not be routinely performed after first UTI if ultrasound is normal.
Dr. Finnell also discussed controversy of not performing a VCUG after a first febrile UTI, as was recommended in the 1999 technical report. She summarized that about 100 children would need to undergo one UTI in the first year. She also reviewed limitations of any guidelines. New studies will assist in monitoring population changes with the revised guideline.
Key Takeaways:
- Understand the evidence and limitations used for all clinical guidelines that you use in practice.
- The updated 2011 guideline for evaluation and management of first febrile UTIs uses risk stratification as an initial approach.
- A major change in the updated 2011 guideline for evaluation and management of first febrile UTIs is that a VCUG is not required for initial evaluation.
Dr. Hale is a pediatric hospitalist at the Floating Hospital for Children at Tufts Medical Center in Boston.
Reference:
Urinary Tract Infection: Clinical Practice Guideline for the Diagnosis and Management of the Initial UTI in Febrile Infants and Children 2 to 24 Months. Pediatrics. 2011;128(3).
Presenter: Maria Finnell, M.D., a leading member of the American Academy of Pediatrics Subcommittee on Urinary Tract Infection
Summary: Dr. Finnell summarized the recent changes in diagnosis and management of pediatric urinary tract infections (UTIs). The 2011 publication of “Urinary Tract Infection: Clinical Practice Guideline for the Diagnosis and Management of the Initial UTI in Febrile Infants and Children 2 to 24 Months” was an update of the 1999 technical report of UTI management. Dr. Finnell reviewed the difference between evidence based and eminence based recommendations. She stated the term “recommendations” was changed to “key action statements” in a new explicit reporting format. Aggregate quality of the evidence is presented in the report in an effort to keep statements transparent.
The process of updating the new guideline was based on the U.S. Preventive Services Task Force approach using a stepwise process. For the revised UTI recommendations the steps were narrowed to:
- Risk of having infection
- Making a diagnosis
- Treatment of UTI
- Identification and Evaluation for high risk conditions
Patient population for this guideline includes initial UTI in child age 2 months to 2 years of age. Patients with neurological conditions or recurrent UTI or renal damage are excluded. Dr. Finnell reviewed action statements for the revised guidelines. A summary of some of these statements:
- If antibiotics are going to be administered, a urine specimen should be collected by catheterization or suprapubic aspiration (SPA).
- Assessment of UTI risk should be performed in a febrile child with no source of infection. The guideline cites specific data for risk. If the likelihood is low then it is reasonable to follow the child clinically without a urine specimen. If the likelihood of a UTI is high then a urine specimen should be obtained.
- To establish the diagnosis of UTI, clinicians should require both urinalysis results that suggest infection and the presence of at least 50,000 colony-forming units (CFUs) per mL of a uropathogen cultured from a urine specimen obtained through catheterization or SPA.
- Oral and parenteral routes are equally efficacious.
- The clinician should choose 7-14 days as duration of treatment.
- Febrile infants with UTIs should undergo renal and bladder ultrasonography.
- VCUG should not be routinely performed after first UTI if ultrasound is normal.
Dr. Finnell also discussed controversy of not performing a VCUG after a first febrile UTI, as was recommended in the 1999 technical report. She summarized that about 100 children would need to undergo one UTI in the first year. She also reviewed limitations of any guidelines. New studies will assist in monitoring population changes with the revised guideline.
Key Takeaways:
- Understand the evidence and limitations used for all clinical guidelines that you use in practice.
- The updated 2011 guideline for evaluation and management of first febrile UTIs uses risk stratification as an initial approach.
- A major change in the updated 2011 guideline for evaluation and management of first febrile UTIs is that a VCUG is not required for initial evaluation.
Dr. Hale is a pediatric hospitalist at the Floating Hospital for Children at Tufts Medical Center in Boston.
Reference:
Urinary Tract Infection: Clinical Practice Guideline for the Diagnosis and Management of the Initial UTI in Febrile Infants and Children 2 to 24 Months. Pediatrics. 2011;128(3).
Presenter: Maria Finnell, M.D., a leading member of the American Academy of Pediatrics Subcommittee on Urinary Tract Infection
Summary: Dr. Finnell summarized the recent changes in diagnosis and management of pediatric urinary tract infections (UTIs). The 2011 publication of “Urinary Tract Infection: Clinical Practice Guideline for the Diagnosis and Management of the Initial UTI in Febrile Infants and Children 2 to 24 Months” was an update of the 1999 technical report of UTI management. Dr. Finnell reviewed the difference between evidence based and eminence based recommendations. She stated the term “recommendations” was changed to “key action statements” in a new explicit reporting format. Aggregate quality of the evidence is presented in the report in an effort to keep statements transparent.
The process of updating the new guideline was based on the U.S. Preventive Services Task Force approach using a stepwise process. For the revised UTI recommendations the steps were narrowed to:
- Risk of having infection
- Making a diagnosis
- Treatment of UTI
- Identification and Evaluation for high risk conditions
Patient population for this guideline includes initial UTI in child age 2 months to 2 years of age. Patients with neurological conditions or recurrent UTI or renal damage are excluded. Dr. Finnell reviewed action statements for the revised guidelines. A summary of some of these statements:
- If antibiotics are going to be administered, a urine specimen should be collected by catheterization or suprapubic aspiration (SPA).
- Assessment of UTI risk should be performed in a febrile child with no source of infection. The guideline cites specific data for risk. If the likelihood is low then it is reasonable to follow the child clinically without a urine specimen. If the likelihood of a UTI is high then a urine specimen should be obtained.
- To establish the diagnosis of UTI, clinicians should require both urinalysis results that suggest infection and the presence of at least 50,000 colony-forming units (CFUs) per mL of a uropathogen cultured from a urine specimen obtained through catheterization or SPA.
- Oral and parenteral routes are equally efficacious.
- The clinician should choose 7-14 days as duration of treatment.
- Febrile infants with UTIs should undergo renal and bladder ultrasonography.
- VCUG should not be routinely performed after first UTI if ultrasound is normal.
Dr. Finnell also discussed controversy of not performing a VCUG after a first febrile UTI, as was recommended in the 1999 technical report. She summarized that about 100 children would need to undergo one UTI in the first year. She also reviewed limitations of any guidelines. New studies will assist in monitoring population changes with the revised guideline.
Key Takeaways:
- Understand the evidence and limitations used for all clinical guidelines that you use in practice.
- The updated 2011 guideline for evaluation and management of first febrile UTIs uses risk stratification as an initial approach.
- A major change in the updated 2011 guideline for evaluation and management of first febrile UTIs is that a VCUG is not required for initial evaluation.
Dr. Hale is a pediatric hospitalist at the Floating Hospital for Children at Tufts Medical Center in Boston.
Reference:
Urinary Tract Infection: Clinical Practice Guideline for the Diagnosis and Management of the Initial UTI in Febrile Infants and Children 2 to 24 Months. Pediatrics. 2011;128(3).
HM14 Special Report: HFNC in Bronchiolitis
Session: HFNC in Bronchiolitis: Best Thing Since Sliced Bread?
Presenter: Shawn Ralston, MD
Summation: Shawn Ralston, known as “Dr. Bronchiolitis” for her work on the AAP Bronchiolitis Practice Guideline, described the limited data on the use of High Flow Nasal Cannula (HFNC) in bronchiolitis. For HFNC to be effective it must have a flow above the patient’s minute ventilation, or at least 2 LPM for the infant. Physiologic studies show that HFNC improve work of breathing by washing out dead space and providing “mini-CPAP.” Increasing flow increases the CPAP effect up to about 6 LPM with less effect at higher rates. HFNC can achieve equivalent CPAP levels of about 3-4, with higher flows increasing pneumothorax risk. Studies of HFNC in bronchiolitis have been observational and retrospective showing trends towards decrease risk of intubation and that HFNC can be safely used outside an ICU setting. There are no clear data or guidelines to indicate which patients with bronchiolitis will benefit from HFNC.
Key Points:
- Need cannula at least 50% diameter of nares;
- Mouth should be closed—can use pacifier;
- Need flow rate above estimated minute ventilation;
- Higher flow, increase pneumothorax risk;
- Need high quality studies to better understand the role of HFNC in bronchiolitis.
David Pressel is a Pediatric Hospitalist and Inpatient Medical Director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, DE and a member of Team Hospitalist.
Session: HFNC in Bronchiolitis: Best Thing Since Sliced Bread?
Presenter: Shawn Ralston, MD
Summation: Shawn Ralston, known as “Dr. Bronchiolitis” for her work on the AAP Bronchiolitis Practice Guideline, described the limited data on the use of High Flow Nasal Cannula (HFNC) in bronchiolitis. For HFNC to be effective it must have a flow above the patient’s minute ventilation, or at least 2 LPM for the infant. Physiologic studies show that HFNC improve work of breathing by washing out dead space and providing “mini-CPAP.” Increasing flow increases the CPAP effect up to about 6 LPM with less effect at higher rates. HFNC can achieve equivalent CPAP levels of about 3-4, with higher flows increasing pneumothorax risk. Studies of HFNC in bronchiolitis have been observational and retrospective showing trends towards decrease risk of intubation and that HFNC can be safely used outside an ICU setting. There are no clear data or guidelines to indicate which patients with bronchiolitis will benefit from HFNC.
Key Points:
- Need cannula at least 50% diameter of nares;
- Mouth should be closed—can use pacifier;
- Need flow rate above estimated minute ventilation;
- Higher flow, increase pneumothorax risk;
- Need high quality studies to better understand the role of HFNC in bronchiolitis.
David Pressel is a Pediatric Hospitalist and Inpatient Medical Director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, DE and a member of Team Hospitalist.
Session: HFNC in Bronchiolitis: Best Thing Since Sliced Bread?
Presenter: Shawn Ralston, MD
Summation: Shawn Ralston, known as “Dr. Bronchiolitis” for her work on the AAP Bronchiolitis Practice Guideline, described the limited data on the use of High Flow Nasal Cannula (HFNC) in bronchiolitis. For HFNC to be effective it must have a flow above the patient’s minute ventilation, or at least 2 LPM for the infant. Physiologic studies show that HFNC improve work of breathing by washing out dead space and providing “mini-CPAP.” Increasing flow increases the CPAP effect up to about 6 LPM with less effect at higher rates. HFNC can achieve equivalent CPAP levels of about 3-4, with higher flows increasing pneumothorax risk. Studies of HFNC in bronchiolitis have been observational and retrospective showing trends towards decrease risk of intubation and that HFNC can be safely used outside an ICU setting. There are no clear data or guidelines to indicate which patients with bronchiolitis will benefit from HFNC.
Key Points:
- Need cannula at least 50% diameter of nares;
- Mouth should be closed—can use pacifier;
- Need flow rate above estimated minute ventilation;
- Higher flow, increase pneumothorax risk;
- Need high quality studies to better understand the role of HFNC in bronchiolitis.
David Pressel is a Pediatric Hospitalist and Inpatient Medical Director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, DE and a member of Team Hospitalist.
Deaf and self-signing
CASE Self Signing
Mrs. H, a 47-year-old, deaf, African American woman, is brought into the emergency room because she is becoming increasingly withdrawn and is signing to herself. She was hospitalized more than 10 years ago after developing psychotic symptoms and received a diagnosis of psychotic disorder, not otherwise specified. She was treated with olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d, but she has not seen a psychiatrist or taken any psychotropics in 8 years. Upon admission to the inpatient psychiatric unit, Mrs. H reports, through an American Sign Language (ASL) interpreter, that she has had “problems with her parents” and with “being fair” and that she is 18 months pregnant. Urine pregnancy test is negative. Mrs. H also reports that her mother is pregnant. She indicates that it is difficult for her to describe what she is trying to say and that it is difficult to be deaf.
She endorses “very strong” racing thoughts, which she first states have been present for 15 years, then reports it has been 20 months. She endorses high-energy levels, feeling like there is “work to do,” and poor sleep. However, when asked, she indicates that she sleeps for 15 hours a day.
Which is critical when conducting a psychiatric assessment for a deaf patient?
a) rely only on the ASL interpreter
b) inquire about the patient’s communication preferences
c) use written language to communicate instead of speech
d) use a family member as interpreter
The authors’ observations
Mental health assessment of a deaf a patient involves a unique set of challenges and requires a specialized skill set for mental health practitioners—a skill set that is not routinely covered in psychiatric training programs.
a We use the term “deaf” to describe patients who have severe hearing loss. Other terms, such as “hearing impaired,” might be considered pejorative in the Deaf community. The term “Deaf” (capitalized) refers to Deaf culture and community, which deaf patients may or may not identify with.
Deafness history
It is important to assess the cause of deafness,1,2 if known, and its age of onset (Table 1). A person is considered to be prelingually deaf if hearing loss was diagnosed before age 3.2 Clinicians should establish the patient’s communication preferences (use of assistive devices or interpreters or preference for lip reading), home communication dynamic,2 and language fluency level.1-3 Ask the patient if she attended a specialized school for the deaf and, if so, if there was an emphasis on oral communication or signing.2
HISTORY Conflicting reports
Mrs. H reports that she has been deaf since age 9, and that she learned sign language in India, where she became the “star king.” Mrs. H states that she then moved to the United States where she went to a school for the deaf. When asked if her family is able to communicate with her in sign language, she nods and indicates that they speak to her in “African and Indian.”
Mrs. H’s husband, who is hearing, says that Mrs. H is congenitally deaf, and was raised in the Midwestern United States where she attended a specialized school for the deaf. Mr. H and his 2 adult sons are hearing but communicate with Mrs. H in basic ASL. He states that Mrs. H sometimes uses signs that he and his sons cannot interpret. In addition to increased self-preoccupation and self-signing, Mrs. H has become more impulsive.
What are limitations of the mental status examination when evaluating a deaf patient?
a) facial expressions have a specific linguistic function in ASL
b) there is no differentiation in the mental status exam of deaf patients from that of hearing patients
c) the Mini-Mental State Examination (MMSE) is a validated tool to assess cognition in deaf patients
d) the clinician should not rely on the interpreter to assist with the mental status examination
The authors’ observation
Performing a mental status examination of a deaf patient without recognizing some of the challenges inherent to this task can lead to misleading findings. For example, signing and gesturing can give the clinician an impression of psychomotor agitation.2 What appears to be socially withdrawn behavior might be a reaction to the patient’s inability to communicate with others.2,3 Social skills may be affected by language deprivation, if present.3 In ASL, facial expressions have specific linguistic functions in addition to representing emotions,2 and can affect the meaning of the sign used. An exaggerated or intense facial expression with the sign “quiet,” for example, usually means “very quiet.”4 In assessing cognition, the MMSE is not available in ASL and has not been validated in deaf patients.5 Also, deaf people have reduced access to information, and a lack of knowledge does not necessarily correlate with low IQ.2
The interpreter’s role
An ASL interpreter can aid in assessing a deaf patient’s communication skills. The interpreter can help with a thorough language evaluation1,6 and provide information about socio-cultural norms in the Deaf community.7 Using an ASL interpreter with special training in mental health1,3,6,7 is important to accurately diagnose thought disorders in deaf patients.1
EVALUATION Mental status exam
Mrs. H is poorly groomed and is wearing a pink housecoat, with her hair in disarray. She seems to be distracted by something next to the interpreter, because her eyes keep roving in this direction. She has moderate psychomotor agitation, based on the rapidity of her signing and gesturing. Mrs. H makes indecipherable vocalizations while signing, often loud and with an urgent quality. Her affect is elevated and expansive. She is not oriented to place or time and when asked where she is, signs, “many times, every day, 6-9-9, 2-5, more trouble…”
The ASL interpreter notes that Mrs. H signs so quickly that only about one-half of her signs are interpretable. Mrs. H’s grammar is not always correct and that her syntax is, at times, inappropriate. Mrs. H’s letters are difficult to interpret because she often starts and concludes a word with a clear sign, but the intervening letters are rapid and uninterpretable. She also uses several non-alphabet signs that cannot be interpreted (approximately 10% to 15% of signs) and repeats signs without clear context, such as “nothing off.” Mrs. H can pause to clarify for the interpreter at the beginning of the interview but is not able to do so by the end of the interview.
How does assessment of psychosis differ when evaluating deaf patients?
a) language dysfluency must be carefully differentiated from a thought disorder
b) signing to oneself does not necessarily indicate a response to internal stimuli
c) norms in Deaf culture might be misconstrued as delusions
d) all of the above
The authors’ observations
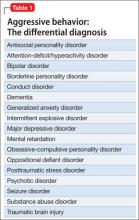
The prevalence of psychotic disorders among deaf patients is unknown.8 Although older studies have reported an increased prevalence of psychotic disorders among deaf patients, these studies suffer from methodological problems.1 Other studies are at odds with each other, variably reporting a greater,9 equivalent,10 and lesser incidence of psychotic disorders in deaf psychiatric inpatients.11 Deaf patients with psychotic disorders experience delusions, hallucinations, and thought disorders,1,3 and assessing for these symptoms in deaf patients can present a diagnostic challenge (Table 2).
Delusions are thought to present similarly in deaf patients with psychotic disorders compared with hearing patients.1,3 Paranoia may be increased in patients who are postlingually deaf, but has not been associated with prelingual deafness. Deficits in theory of mind related to hearing impairment have been thought to contribute to delusions in deaf patients.1,12
Many deaf patients distrust health care systems and providers,2,3,13 which may be misinterpreted as paranoia. Poor communication between deaf patients and clinicians and poor health literacy among deaf patients contribute to feelings of mistrust. Deaf patients often report experiencing prejudice within the health care system, and think that providers lack sufficient knowledge of deafness.13 Care must be taken to ensure that Deaf cultural norms are not misinterpreted as delusions.
Hallucinations. How deaf patients experience hallucinations, especially in prelingual deafness, likely is different from hallucinatory experiences of hearing patients.1,14 Deaf people with psychosis have described ”ideas coming into one’s head” and an almost “telepathic” process of “knowing.”14 Deaf patients with schizophrenia are more likely to report visual elements to their hallucinations; however, these may be subvisual precepts rather than true visual hallucinations.1,15 For example, hallucination might include the perception of being signed to.1
Deaf patients’ experience of auditory hallucinations is thought to be closely related to past auditory experiences. It is unlikely that prelingually deaf patients experience true auditory hallucinations.1,14 An endorsement of hearing a “voice” in ASL does not necessarily translate to an audiological experience.15 If profoundly prelingually deaf patients endorse hearing voices, generally they cannot assign acoustic properties (pitch, tone, volume, accent, etc.).1,14,15 It may not be necessary to fully comprehend the precise modality of how hallucinations are experienced by deaf patients to provide therapy.14
Self-signing, or signing to oneself, does not necessarily indicate that a deaf person is responding to a hallucinatory experience. Non-verbal patients may gesture to themselves without clear evidence of psychosis. When considering whether a patient is experiencing hallucinations, it is important to look for other evidence of psychosis.3
Possible approaches to evaluating hallucinations in deaf patients include asking,, “is someone signing in your head?” or “Is someone who is not in the room trying to communicate with you?”
Thought disorders in deaf psychiatric inpatients are difficult to diagnose, in part because of a high rate of language dysfluency in deaf patients; in samples of psychiatric inpatients, 75% are not fluent in ASL, 66% are not fluent in any language).1,3,11 Commonly, language dysfluency is related to language deprivation because of late or inadequate exposure to ASL, although it may be related to neurologic damage or aphasia.1,3,6,16 Deaf patients can have additional disabilities, including learning disabilities, that might contribute to language dysfluency.2 Language dysfluency can be misattributed to a psychotic process1-3,7 (Table 3).1
Language dysfluency and thought disorders can be difficult to differentiate and may be comorbid. Loose associations and flight of ideas can be hard to assess in patients with language dysfluency. In general, increasing looseness of association between concepts corresponds to an increasing likelihood that a patient has true loose associations rather than language dysfluency alone.3 Deaf patients with schizophrenia can be identified by the presence of associated symptoms of psychosis, especially if delusions are present.1,3
EVALUATION Psychotic symptoms
Mrs. H’s thought process appears disorganized and illogical, with flight of ideas. She might have an underlying language dysfluency. It is likely that Mrs. H is using neologisms to communicate because of her family’s lack of familiarity with some of her signs. She also demonstrates perseveration, with use of certain signs repeatedly without clear context (ie, “nothing off”).
Her thought content includes racial themes—she mentions Russia, Germany, and Vietnam without clear context—and delusions of being the “star king” and of being pregnant. She endorses paranoid feelings that people on the inpatient unit are trying to hurt her, although it isn’t clear whether this represents a true paranoid delusion because of the hectic climate of the unit, and she did not show unnecessarily defensive or guarded behaviors.
She is seen signing to herself in the dayroom and endorses feeling as though someone who is not in the room—described as an Indian teacher (and sometimes as a boss or principal) known as “Mr. Smith” or “Mr. Donald”—is trying to communicate with her. She describes this person as being male and female. She mentions that sometimes she sees an Indian man and another man fighting. It is likely that Mrs. H is experiencing hallucinations from decompensated psychosis, because of the constellation and trajectory of her symptoms. Her nonverbal behavior—her eyes rove around the room during interviews—also supports this conclusion.
Because of evidence of mood and psychotic symptoms, and with a collateral history that suggests significant baseline disorganization, Mrs. H receives a diagnosis of schizoaffective disorder, bipolar type. She is restarted on olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d.
Mrs. H’s psychomotor acceleration and affective elevation gradually improve with pharmacotherapy. After a 2-week hospitalization, despite ongoing disorganization and self-signing, Mrs. H’s husband says that he feels she is improved enough to return home, with plans to continue to take her medications and to reestablish outpatient follow-up.
Bottom Line
Psychiatric assessment of deaf patients presents distinctive challenges related to cultural and language barriers—making it important to engage an ASL interpreter with training in mental health during assessment of a deaf patient. Clinicians must become familiar with these challenges to provide effective care for mentally ill deaf patients.
Related Resources
• Landsberger SA, Diaz DR. Communicating with deaf patients: 10 tips to deliver appropriate care. Current Psychiatry. 2010;9(6):36-37.
• Deaf Wellness Center. University of Rochester School of Medicine. www.urmc.rochester.edu/deaf-wellness-center.
• Gallaudet University Mental Health Center. www.gallaudet.edu/
mental_health_center.html.
Drug Brand Names
Olanzapine • Zyprexa
Valproic acid • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Landsberger SA, Diaz DR. Identifying and assessing psychosis in deaf psychiatric patients. Curr Psychiatry Rep. 2011;13(3):198-202.
2. Fellinger J, Holzinger D, Pollard R. Mental health of deaf people. Lancet. 2012;379(9820):1037-1044.
3. Glickman N. Do you hear voices? Problems in assessment of mental status in deaf persons with severe language deprivation. J Deaf Stud Deaf Educ. 2007;12(2):127-147.
4. Vicars W. ASL University. Facial expressions. http://www.lifeprint.com/asl101/pages-layout/facialexpressions.htm. Accessed April 2, 2013.
5. Dean PM, Feldman DM, Morere D, et al. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753-760.
6. Crump C, Glickman N. Mental health interpreting with language dysfluent deaf clients. Journal of Interpretation. 2011;21(1):21-36.
7. Leigh IW, Pollard RQ Jr. Mental health and deaf adults. In: Marschark M, Spencer PE, eds. Oxford handbook of deaf studies, language, and education. Vol 1. New York, NY: Oxford University Press. 2011:214-226.
8. Øhre B, von Tezchner S, Falkum E. Deaf adults and mental health: A review of recent research on the prevalence and distribution of psychiatric symptoms and disorders in the prelingually deaf adult population. International Journal on Mental Health and Deafness. 2011;1(1):3-22.
9. Appleford J. Clinical activity within a specialist mental health service for deaf people: comparison with a general psychiatric service. Psychiatric Bulletin. 2003;27(10): 375-377.
10. Landsberger SA, Diaz DR. Inpatient psychiatric treatment of deaf adults: demographic and diagnostic comparisons with hearing inpatients. Psychiatr Serv. 2010;61(2):196-199.
11. Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006; 11(3):303-321.
12. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005; 76(1):99-103.
13. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility. Experiences and perceptions of deaf people. J Gen Inter Med. 2006;21(3):260-266.
14. Paijmans R, Cromwell J, Austen S. Do profoundly prelingually deaf patients with psychosis really hear voices? Am Ann Deaf. 2006;151(1):42-48.
15. Atkinson JR. The perceptual characteristics of voice-hallucinations in deaf people: insights into the nature of subvocal thought and sensory feedback loops. Schizophr Bull. 2006;32(4):701-708.
16. Trumbetta SL, Bonvillian JD, Siedlecki T, et al. Language-related symptoms in persons with schizophrenia and how deaf persons may manifest these symptoms. Sign Language Studies. 2001;1(3):228-253.
CASE Self Signing
Mrs. H, a 47-year-old, deaf, African American woman, is brought into the emergency room because she is becoming increasingly withdrawn and is signing to herself. She was hospitalized more than 10 years ago after developing psychotic symptoms and received a diagnosis of psychotic disorder, not otherwise specified. She was treated with olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d, but she has not seen a psychiatrist or taken any psychotropics in 8 years. Upon admission to the inpatient psychiatric unit, Mrs. H reports, through an American Sign Language (ASL) interpreter, that she has had “problems with her parents” and with “being fair” and that she is 18 months pregnant. Urine pregnancy test is negative. Mrs. H also reports that her mother is pregnant. She indicates that it is difficult for her to describe what she is trying to say and that it is difficult to be deaf.
She endorses “very strong” racing thoughts, which she first states have been present for 15 years, then reports it has been 20 months. She endorses high-energy levels, feeling like there is “work to do,” and poor sleep. However, when asked, she indicates that she sleeps for 15 hours a day.
Which is critical when conducting a psychiatric assessment for a deaf patient?
a) rely only on the ASL interpreter
b) inquire about the patient’s communication preferences
c) use written language to communicate instead of speech
d) use a family member as interpreter
The authors’ observations
Mental health assessment of a deaf a patient involves a unique set of challenges and requires a specialized skill set for mental health practitioners—a skill set that is not routinely covered in psychiatric training programs.
a We use the term “deaf” to describe patients who have severe hearing loss. Other terms, such as “hearing impaired,” might be considered pejorative in the Deaf community. The term “Deaf” (capitalized) refers to Deaf culture and community, which deaf patients may or may not identify with.
Deafness history
It is important to assess the cause of deafness,1,2 if known, and its age of onset (Table 1). A person is considered to be prelingually deaf if hearing loss was diagnosed before age 3.2 Clinicians should establish the patient’s communication preferences (use of assistive devices or interpreters or preference for lip reading), home communication dynamic,2 and language fluency level.1-3 Ask the patient if she attended a specialized school for the deaf and, if so, if there was an emphasis on oral communication or signing.2
HISTORY Conflicting reports
Mrs. H reports that she has been deaf since age 9, and that she learned sign language in India, where she became the “star king.” Mrs. H states that she then moved to the United States where she went to a school for the deaf. When asked if her family is able to communicate with her in sign language, she nods and indicates that they speak to her in “African and Indian.”
Mrs. H’s husband, who is hearing, says that Mrs. H is congenitally deaf, and was raised in the Midwestern United States where she attended a specialized school for the deaf. Mr. H and his 2 adult sons are hearing but communicate with Mrs. H in basic ASL. He states that Mrs. H sometimes uses signs that he and his sons cannot interpret. In addition to increased self-preoccupation and self-signing, Mrs. H has become more impulsive.
What are limitations of the mental status examination when evaluating a deaf patient?
a) facial expressions have a specific linguistic function in ASL
b) there is no differentiation in the mental status exam of deaf patients from that of hearing patients
c) the Mini-Mental State Examination (MMSE) is a validated tool to assess cognition in deaf patients
d) the clinician should not rely on the interpreter to assist with the mental status examination
The authors’ observation
Performing a mental status examination of a deaf patient without recognizing some of the challenges inherent to this task can lead to misleading findings. For example, signing and gesturing can give the clinician an impression of psychomotor agitation.2 What appears to be socially withdrawn behavior might be a reaction to the patient’s inability to communicate with others.2,3 Social skills may be affected by language deprivation, if present.3 In ASL, facial expressions have specific linguistic functions in addition to representing emotions,2 and can affect the meaning of the sign used. An exaggerated or intense facial expression with the sign “quiet,” for example, usually means “very quiet.”4 In assessing cognition, the MMSE is not available in ASL and has not been validated in deaf patients.5 Also, deaf people have reduced access to information, and a lack of knowledge does not necessarily correlate with low IQ.2
The interpreter’s role
An ASL interpreter can aid in assessing a deaf patient’s communication skills. The interpreter can help with a thorough language evaluation1,6 and provide information about socio-cultural norms in the Deaf community.7 Using an ASL interpreter with special training in mental health1,3,6,7 is important to accurately diagnose thought disorders in deaf patients.1
EVALUATION Mental status exam
Mrs. H is poorly groomed and is wearing a pink housecoat, with her hair in disarray. She seems to be distracted by something next to the interpreter, because her eyes keep roving in this direction. She has moderate psychomotor agitation, based on the rapidity of her signing and gesturing. Mrs. H makes indecipherable vocalizations while signing, often loud and with an urgent quality. Her affect is elevated and expansive. She is not oriented to place or time and when asked where she is, signs, “many times, every day, 6-9-9, 2-5, more trouble…”
The ASL interpreter notes that Mrs. H signs so quickly that only about one-half of her signs are interpretable. Mrs. H’s grammar is not always correct and that her syntax is, at times, inappropriate. Mrs. H’s letters are difficult to interpret because she often starts and concludes a word with a clear sign, but the intervening letters are rapid and uninterpretable. She also uses several non-alphabet signs that cannot be interpreted (approximately 10% to 15% of signs) and repeats signs without clear context, such as “nothing off.” Mrs. H can pause to clarify for the interpreter at the beginning of the interview but is not able to do so by the end of the interview.
How does assessment of psychosis differ when evaluating deaf patients?
a) language dysfluency must be carefully differentiated from a thought disorder
b) signing to oneself does not necessarily indicate a response to internal stimuli
c) norms in Deaf culture might be misconstrued as delusions
d) all of the above
The authors’ observations
The prevalence of psychotic disorders among deaf patients is unknown.8 Although older studies have reported an increased prevalence of psychotic disorders among deaf patients, these studies suffer from methodological problems.1 Other studies are at odds with each other, variably reporting a greater,9 equivalent,10 and lesser incidence of psychotic disorders in deaf psychiatric inpatients.11 Deaf patients with psychotic disorders experience delusions, hallucinations, and thought disorders,1,3 and assessing for these symptoms in deaf patients can present a diagnostic challenge (Table 2).
Delusions are thought to present similarly in deaf patients with psychotic disorders compared with hearing patients.1,3 Paranoia may be increased in patients who are postlingually deaf, but has not been associated with prelingual deafness. Deficits in theory of mind related to hearing impairment have been thought to contribute to delusions in deaf patients.1,12
Many deaf patients distrust health care systems and providers,2,3,13 which may be misinterpreted as paranoia. Poor communication between deaf patients and clinicians and poor health literacy among deaf patients contribute to feelings of mistrust. Deaf patients often report experiencing prejudice within the health care system, and think that providers lack sufficient knowledge of deafness.13 Care must be taken to ensure that Deaf cultural norms are not misinterpreted as delusions.
Hallucinations. How deaf patients experience hallucinations, especially in prelingual deafness, likely is different from hallucinatory experiences of hearing patients.1,14 Deaf people with psychosis have described ”ideas coming into one’s head” and an almost “telepathic” process of “knowing.”14 Deaf patients with schizophrenia are more likely to report visual elements to their hallucinations; however, these may be subvisual precepts rather than true visual hallucinations.1,15 For example, hallucination might include the perception of being signed to.1
Deaf patients’ experience of auditory hallucinations is thought to be closely related to past auditory experiences. It is unlikely that prelingually deaf patients experience true auditory hallucinations.1,14 An endorsement of hearing a “voice” in ASL does not necessarily translate to an audiological experience.15 If profoundly prelingually deaf patients endorse hearing voices, generally they cannot assign acoustic properties (pitch, tone, volume, accent, etc.).1,14,15 It may not be necessary to fully comprehend the precise modality of how hallucinations are experienced by deaf patients to provide therapy.14
Self-signing, or signing to oneself, does not necessarily indicate that a deaf person is responding to a hallucinatory experience. Non-verbal patients may gesture to themselves without clear evidence of psychosis. When considering whether a patient is experiencing hallucinations, it is important to look for other evidence of psychosis.3
Possible approaches to evaluating hallucinations in deaf patients include asking,, “is someone signing in your head?” or “Is someone who is not in the room trying to communicate with you?”
Thought disorders in deaf psychiatric inpatients are difficult to diagnose, in part because of a high rate of language dysfluency in deaf patients; in samples of psychiatric inpatients, 75% are not fluent in ASL, 66% are not fluent in any language).1,3,11 Commonly, language dysfluency is related to language deprivation because of late or inadequate exposure to ASL, although it may be related to neurologic damage or aphasia.1,3,6,16 Deaf patients can have additional disabilities, including learning disabilities, that might contribute to language dysfluency.2 Language dysfluency can be misattributed to a psychotic process1-3,7 (Table 3).1
Language dysfluency and thought disorders can be difficult to differentiate and may be comorbid. Loose associations and flight of ideas can be hard to assess in patients with language dysfluency. In general, increasing looseness of association between concepts corresponds to an increasing likelihood that a patient has true loose associations rather than language dysfluency alone.3 Deaf patients with schizophrenia can be identified by the presence of associated symptoms of psychosis, especially if delusions are present.1,3
EVALUATION Psychotic symptoms
Mrs. H’s thought process appears disorganized and illogical, with flight of ideas. She might have an underlying language dysfluency. It is likely that Mrs. H is using neologisms to communicate because of her family’s lack of familiarity with some of her signs. She also demonstrates perseveration, with use of certain signs repeatedly without clear context (ie, “nothing off”).
Her thought content includes racial themes—she mentions Russia, Germany, and Vietnam without clear context—and delusions of being the “star king” and of being pregnant. She endorses paranoid feelings that people on the inpatient unit are trying to hurt her, although it isn’t clear whether this represents a true paranoid delusion because of the hectic climate of the unit, and she did not show unnecessarily defensive or guarded behaviors.
She is seen signing to herself in the dayroom and endorses feeling as though someone who is not in the room—described as an Indian teacher (and sometimes as a boss or principal) known as “Mr. Smith” or “Mr. Donald”—is trying to communicate with her. She describes this person as being male and female. She mentions that sometimes she sees an Indian man and another man fighting. It is likely that Mrs. H is experiencing hallucinations from decompensated psychosis, because of the constellation and trajectory of her symptoms. Her nonverbal behavior—her eyes rove around the room during interviews—also supports this conclusion.
Because of evidence of mood and psychotic symptoms, and with a collateral history that suggests significant baseline disorganization, Mrs. H receives a diagnosis of schizoaffective disorder, bipolar type. She is restarted on olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d.
Mrs. H’s psychomotor acceleration and affective elevation gradually improve with pharmacotherapy. After a 2-week hospitalization, despite ongoing disorganization and self-signing, Mrs. H’s husband says that he feels she is improved enough to return home, with plans to continue to take her medications and to reestablish outpatient follow-up.
Bottom Line
Psychiatric assessment of deaf patients presents distinctive challenges related to cultural and language barriers—making it important to engage an ASL interpreter with training in mental health during assessment of a deaf patient. Clinicians must become familiar with these challenges to provide effective care for mentally ill deaf patients.
Related Resources
• Landsberger SA, Diaz DR. Communicating with deaf patients: 10 tips to deliver appropriate care. Current Psychiatry. 2010;9(6):36-37.
• Deaf Wellness Center. University of Rochester School of Medicine. www.urmc.rochester.edu/deaf-wellness-center.
• Gallaudet University Mental Health Center. www.gallaudet.edu/
mental_health_center.html.
Drug Brand Names
Olanzapine • Zyprexa
Valproic acid • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Self Signing
Mrs. H, a 47-year-old, deaf, African American woman, is brought into the emergency room because she is becoming increasingly withdrawn and is signing to herself. She was hospitalized more than 10 years ago after developing psychotic symptoms and received a diagnosis of psychotic disorder, not otherwise specified. She was treated with olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d, but she has not seen a psychiatrist or taken any psychotropics in 8 years. Upon admission to the inpatient psychiatric unit, Mrs. H reports, through an American Sign Language (ASL) interpreter, that she has had “problems with her parents” and with “being fair” and that she is 18 months pregnant. Urine pregnancy test is negative. Mrs. H also reports that her mother is pregnant. She indicates that it is difficult for her to describe what she is trying to say and that it is difficult to be deaf.
She endorses “very strong” racing thoughts, which she first states have been present for 15 years, then reports it has been 20 months. She endorses high-energy levels, feeling like there is “work to do,” and poor sleep. However, when asked, she indicates that she sleeps for 15 hours a day.
Which is critical when conducting a psychiatric assessment for a deaf patient?
a) rely only on the ASL interpreter
b) inquire about the patient’s communication preferences
c) use written language to communicate instead of speech
d) use a family member as interpreter
The authors’ observations
Mental health assessment of a deaf a patient involves a unique set of challenges and requires a specialized skill set for mental health practitioners—a skill set that is not routinely covered in psychiatric training programs.
a We use the term “deaf” to describe patients who have severe hearing loss. Other terms, such as “hearing impaired,” might be considered pejorative in the Deaf community. The term “Deaf” (capitalized) refers to Deaf culture and community, which deaf patients may or may not identify with.
Deafness history
It is important to assess the cause of deafness,1,2 if known, and its age of onset (Table 1). A person is considered to be prelingually deaf if hearing loss was diagnosed before age 3.2 Clinicians should establish the patient’s communication preferences (use of assistive devices or interpreters or preference for lip reading), home communication dynamic,2 and language fluency level.1-3 Ask the patient if she attended a specialized school for the deaf and, if so, if there was an emphasis on oral communication or signing.2
HISTORY Conflicting reports
Mrs. H reports that she has been deaf since age 9, and that she learned sign language in India, where she became the “star king.” Mrs. H states that she then moved to the United States where she went to a school for the deaf. When asked if her family is able to communicate with her in sign language, she nods and indicates that they speak to her in “African and Indian.”
Mrs. H’s husband, who is hearing, says that Mrs. H is congenitally deaf, and was raised in the Midwestern United States where she attended a specialized school for the deaf. Mr. H and his 2 adult sons are hearing but communicate with Mrs. H in basic ASL. He states that Mrs. H sometimes uses signs that he and his sons cannot interpret. In addition to increased self-preoccupation and self-signing, Mrs. H has become more impulsive.
What are limitations of the mental status examination when evaluating a deaf patient?
a) facial expressions have a specific linguistic function in ASL
b) there is no differentiation in the mental status exam of deaf patients from that of hearing patients
c) the Mini-Mental State Examination (MMSE) is a validated tool to assess cognition in deaf patients
d) the clinician should not rely on the interpreter to assist with the mental status examination
The authors’ observation
Performing a mental status examination of a deaf patient without recognizing some of the challenges inherent to this task can lead to misleading findings. For example, signing and gesturing can give the clinician an impression of psychomotor agitation.2 What appears to be socially withdrawn behavior might be a reaction to the patient’s inability to communicate with others.2,3 Social skills may be affected by language deprivation, if present.3 In ASL, facial expressions have specific linguistic functions in addition to representing emotions,2 and can affect the meaning of the sign used. An exaggerated or intense facial expression with the sign “quiet,” for example, usually means “very quiet.”4 In assessing cognition, the MMSE is not available in ASL and has not been validated in deaf patients.5 Also, deaf people have reduced access to information, and a lack of knowledge does not necessarily correlate with low IQ.2
The interpreter’s role
An ASL interpreter can aid in assessing a deaf patient’s communication skills. The interpreter can help with a thorough language evaluation1,6 and provide information about socio-cultural norms in the Deaf community.7 Using an ASL interpreter with special training in mental health1,3,6,7 is important to accurately diagnose thought disorders in deaf patients.1
EVALUATION Mental status exam
Mrs. H is poorly groomed and is wearing a pink housecoat, with her hair in disarray. She seems to be distracted by something next to the interpreter, because her eyes keep roving in this direction. She has moderate psychomotor agitation, based on the rapidity of her signing and gesturing. Mrs. H makes indecipherable vocalizations while signing, often loud and with an urgent quality. Her affect is elevated and expansive. She is not oriented to place or time and when asked where she is, signs, “many times, every day, 6-9-9, 2-5, more trouble…”
The ASL interpreter notes that Mrs. H signs so quickly that only about one-half of her signs are interpretable. Mrs. H’s grammar is not always correct and that her syntax is, at times, inappropriate. Mrs. H’s letters are difficult to interpret because she often starts and concludes a word with a clear sign, but the intervening letters are rapid and uninterpretable. She also uses several non-alphabet signs that cannot be interpreted (approximately 10% to 15% of signs) and repeats signs without clear context, such as “nothing off.” Mrs. H can pause to clarify for the interpreter at the beginning of the interview but is not able to do so by the end of the interview.
How does assessment of psychosis differ when evaluating deaf patients?
a) language dysfluency must be carefully differentiated from a thought disorder
b) signing to oneself does not necessarily indicate a response to internal stimuli
c) norms in Deaf culture might be misconstrued as delusions
d) all of the above
The authors’ observations
The prevalence of psychotic disorders among deaf patients is unknown.8 Although older studies have reported an increased prevalence of psychotic disorders among deaf patients, these studies suffer from methodological problems.1 Other studies are at odds with each other, variably reporting a greater,9 equivalent,10 and lesser incidence of psychotic disorders in deaf psychiatric inpatients.11 Deaf patients with psychotic disorders experience delusions, hallucinations, and thought disorders,1,3 and assessing for these symptoms in deaf patients can present a diagnostic challenge (Table 2).
Delusions are thought to present similarly in deaf patients with psychotic disorders compared with hearing patients.1,3 Paranoia may be increased in patients who are postlingually deaf, but has not been associated with prelingual deafness. Deficits in theory of mind related to hearing impairment have been thought to contribute to delusions in deaf patients.1,12
Many deaf patients distrust health care systems and providers,2,3,13 which may be misinterpreted as paranoia. Poor communication between deaf patients and clinicians and poor health literacy among deaf patients contribute to feelings of mistrust. Deaf patients often report experiencing prejudice within the health care system, and think that providers lack sufficient knowledge of deafness.13 Care must be taken to ensure that Deaf cultural norms are not misinterpreted as delusions.
Hallucinations. How deaf patients experience hallucinations, especially in prelingual deafness, likely is different from hallucinatory experiences of hearing patients.1,14 Deaf people with psychosis have described ”ideas coming into one’s head” and an almost “telepathic” process of “knowing.”14 Deaf patients with schizophrenia are more likely to report visual elements to their hallucinations; however, these may be subvisual precepts rather than true visual hallucinations.1,15 For example, hallucination might include the perception of being signed to.1
Deaf patients’ experience of auditory hallucinations is thought to be closely related to past auditory experiences. It is unlikely that prelingually deaf patients experience true auditory hallucinations.1,14 An endorsement of hearing a “voice” in ASL does not necessarily translate to an audiological experience.15 If profoundly prelingually deaf patients endorse hearing voices, generally they cannot assign acoustic properties (pitch, tone, volume, accent, etc.).1,14,15 It may not be necessary to fully comprehend the precise modality of how hallucinations are experienced by deaf patients to provide therapy.14
Self-signing, or signing to oneself, does not necessarily indicate that a deaf person is responding to a hallucinatory experience. Non-verbal patients may gesture to themselves without clear evidence of psychosis. When considering whether a patient is experiencing hallucinations, it is important to look for other evidence of psychosis.3
Possible approaches to evaluating hallucinations in deaf patients include asking,, “is someone signing in your head?” or “Is someone who is not in the room trying to communicate with you?”
Thought disorders in deaf psychiatric inpatients are difficult to diagnose, in part because of a high rate of language dysfluency in deaf patients; in samples of psychiatric inpatients, 75% are not fluent in ASL, 66% are not fluent in any language).1,3,11 Commonly, language dysfluency is related to language deprivation because of late or inadequate exposure to ASL, although it may be related to neurologic damage or aphasia.1,3,6,16 Deaf patients can have additional disabilities, including learning disabilities, that might contribute to language dysfluency.2 Language dysfluency can be misattributed to a psychotic process1-3,7 (Table 3).1
Language dysfluency and thought disorders can be difficult to differentiate and may be comorbid. Loose associations and flight of ideas can be hard to assess in patients with language dysfluency. In general, increasing looseness of association between concepts corresponds to an increasing likelihood that a patient has true loose associations rather than language dysfluency alone.3 Deaf patients with schizophrenia can be identified by the presence of associated symptoms of psychosis, especially if delusions are present.1,3
EVALUATION Psychotic symptoms
Mrs. H’s thought process appears disorganized and illogical, with flight of ideas. She might have an underlying language dysfluency. It is likely that Mrs. H is using neologisms to communicate because of her family’s lack of familiarity with some of her signs. She also demonstrates perseveration, with use of certain signs repeatedly without clear context (ie, “nothing off”).
Her thought content includes racial themes—she mentions Russia, Germany, and Vietnam without clear context—and delusions of being the “star king” and of being pregnant. She endorses paranoid feelings that people on the inpatient unit are trying to hurt her, although it isn’t clear whether this represents a true paranoid delusion because of the hectic climate of the unit, and she did not show unnecessarily defensive or guarded behaviors.
She is seen signing to herself in the dayroom and endorses feeling as though someone who is not in the room—described as an Indian teacher (and sometimes as a boss or principal) known as “Mr. Smith” or “Mr. Donald”—is trying to communicate with her. She describes this person as being male and female. She mentions that sometimes she sees an Indian man and another man fighting. It is likely that Mrs. H is experiencing hallucinations from decompensated psychosis, because of the constellation and trajectory of her symptoms. Her nonverbal behavior—her eyes rove around the room during interviews—also supports this conclusion.
Because of evidence of mood and psychotic symptoms, and with a collateral history that suggests significant baseline disorganization, Mrs. H receives a diagnosis of schizoaffective disorder, bipolar type. She is restarted on olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d.
Mrs. H’s psychomotor acceleration and affective elevation gradually improve with pharmacotherapy. After a 2-week hospitalization, despite ongoing disorganization and self-signing, Mrs. H’s husband says that he feels she is improved enough to return home, with plans to continue to take her medications and to reestablish outpatient follow-up.
Bottom Line
Psychiatric assessment of deaf patients presents distinctive challenges related to cultural and language barriers—making it important to engage an ASL interpreter with training in mental health during assessment of a deaf patient. Clinicians must become familiar with these challenges to provide effective care for mentally ill deaf patients.
Related Resources
• Landsberger SA, Diaz DR. Communicating with deaf patients: 10 tips to deliver appropriate care. Current Psychiatry. 2010;9(6):36-37.
• Deaf Wellness Center. University of Rochester School of Medicine. www.urmc.rochester.edu/deaf-wellness-center.
• Gallaudet University Mental Health Center. www.gallaudet.edu/
mental_health_center.html.
Drug Brand Names
Olanzapine • Zyprexa
Valproic acid • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Landsberger SA, Diaz DR. Identifying and assessing psychosis in deaf psychiatric patients. Curr Psychiatry Rep. 2011;13(3):198-202.
2. Fellinger J, Holzinger D, Pollard R. Mental health of deaf people. Lancet. 2012;379(9820):1037-1044.
3. Glickman N. Do you hear voices? Problems in assessment of mental status in deaf persons with severe language deprivation. J Deaf Stud Deaf Educ. 2007;12(2):127-147.
4. Vicars W. ASL University. Facial expressions. http://www.lifeprint.com/asl101/pages-layout/facialexpressions.htm. Accessed April 2, 2013.
5. Dean PM, Feldman DM, Morere D, et al. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753-760.
6. Crump C, Glickman N. Mental health interpreting with language dysfluent deaf clients. Journal of Interpretation. 2011;21(1):21-36.
7. Leigh IW, Pollard RQ Jr. Mental health and deaf adults. In: Marschark M, Spencer PE, eds. Oxford handbook of deaf studies, language, and education. Vol 1. New York, NY: Oxford University Press. 2011:214-226.
8. Øhre B, von Tezchner S, Falkum E. Deaf adults and mental health: A review of recent research on the prevalence and distribution of psychiatric symptoms and disorders in the prelingually deaf adult population. International Journal on Mental Health and Deafness. 2011;1(1):3-22.
9. Appleford J. Clinical activity within a specialist mental health service for deaf people: comparison with a general psychiatric service. Psychiatric Bulletin. 2003;27(10): 375-377.
10. Landsberger SA, Diaz DR. Inpatient psychiatric treatment of deaf adults: demographic and diagnostic comparisons with hearing inpatients. Psychiatr Serv. 2010;61(2):196-199.
11. Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006; 11(3):303-321.
12. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005; 76(1):99-103.
13. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility. Experiences and perceptions of deaf people. J Gen Inter Med. 2006;21(3):260-266.
14. Paijmans R, Cromwell J, Austen S. Do profoundly prelingually deaf patients with psychosis really hear voices? Am Ann Deaf. 2006;151(1):42-48.
15. Atkinson JR. The perceptual characteristics of voice-hallucinations in deaf people: insights into the nature of subvocal thought and sensory feedback loops. Schizophr Bull. 2006;32(4):701-708.
16. Trumbetta SL, Bonvillian JD, Siedlecki T, et al. Language-related symptoms in persons with schizophrenia and how deaf persons may manifest these symptoms. Sign Language Studies. 2001;1(3):228-253.
1. Landsberger SA, Diaz DR. Identifying and assessing psychosis in deaf psychiatric patients. Curr Psychiatry Rep. 2011;13(3):198-202.
2. Fellinger J, Holzinger D, Pollard R. Mental health of deaf people. Lancet. 2012;379(9820):1037-1044.
3. Glickman N. Do you hear voices? Problems in assessment of mental status in deaf persons with severe language deprivation. J Deaf Stud Deaf Educ. 2007;12(2):127-147.
4. Vicars W. ASL University. Facial expressions. http://www.lifeprint.com/asl101/pages-layout/facialexpressions.htm. Accessed April 2, 2013.
5. Dean PM, Feldman DM, Morere D, et al. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753-760.
6. Crump C, Glickman N. Mental health interpreting with language dysfluent deaf clients. Journal of Interpretation. 2011;21(1):21-36.
7. Leigh IW, Pollard RQ Jr. Mental health and deaf adults. In: Marschark M, Spencer PE, eds. Oxford handbook of deaf studies, language, and education. Vol 1. New York, NY: Oxford University Press. 2011:214-226.
8. Øhre B, von Tezchner S, Falkum E. Deaf adults and mental health: A review of recent research on the prevalence and distribution of psychiatric symptoms and disorders in the prelingually deaf adult population. International Journal on Mental Health and Deafness. 2011;1(1):3-22.
9. Appleford J. Clinical activity within a specialist mental health service for deaf people: comparison with a general psychiatric service. Psychiatric Bulletin. 2003;27(10): 375-377.
10. Landsberger SA, Diaz DR. Inpatient psychiatric treatment of deaf adults: demographic and diagnostic comparisons with hearing inpatients. Psychiatr Serv. 2010;61(2):196-199.
11. Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006; 11(3):303-321.
12. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005; 76(1):99-103.
13. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility. Experiences and perceptions of deaf people. J Gen Inter Med. 2006;21(3):260-266.
14. Paijmans R, Cromwell J, Austen S. Do profoundly prelingually deaf patients with psychosis really hear voices? Am Ann Deaf. 2006;151(1):42-48.
15. Atkinson JR. The perceptual characteristics of voice-hallucinations in deaf people: insights into the nature of subvocal thought and sensory feedback loops. Schizophr Bull. 2006;32(4):701-708.
16. Trumbetta SL, Bonvillian JD, Siedlecki T, et al. Language-related symptoms in persons with schizophrenia and how deaf persons may manifest these symptoms. Sign Language Studies. 2001;1(3):228-253.
Sinus surgery: new rigor in research
KEYSTONE, COLO. – Research-minded otolaryngologists have gotten serious about conducting high-quality, patient-centered outcomes studies of endoscopic sinus surgery for chronic rhinosinusitis, which more than 250,000 Americans undergo each year. And the results are eye opening.
Mounting evidence documents that endoscopic sinus surgery (ESS) in properly selected patients with chronic rhinosinusitis (CRS) results in markedly improved quality of life, functional status, and reduced use of medications, compared with medical management, Dr. Todd T. Kingdom said at a meeting on allergy and respiratory diseases sponsored by National Jewish Health.
These studies utilize validated measures of patient-centered quality of life and symptoms. They are nothing like the lightweight, less-than-persuasive ESS research published in the 1990s, which reported glowing ‘success’ rates of 80%-97% in single-institution retrospective studies using variable inclusion criteria and often-sketchy definitions of success.
"Those data are not acceptable, but that’s what we had. This was before evidence-based medicine with an emphasis on rigorously designed studies took hold," explained Dr. Kingdom, professor and vice chairman of the department of otolaryngology – head and neck surgery, at the University of Colorado, Denver, and immediate past president of the American Rhinologic Society.
Current research emphasizes the use of modern, validated patient-centered quality of life tools and symptom scores because CRS is a symptom-based diagnosis and it is symptom severity that drives patients to seek treatment. Also, objective measures, such as the Lund-Mackay CT staging system, fail to capture the full experience of disease burden. Nor do objective measures necessarily correlate with patient symptoms, according to the otolaryngologist.
A low point in the field of sinus surgery, in Dr. Kingdom’s view, was the 2006 Cochrane systematic review which concluded ESS "has not been demonstrated to confer additional benefit to that obtained by medical therapy" (Cochrane Database Syst. Rev. 2006:CD004458).
"This review was a disservice," he asserted.
The review was based entirely on three older randomized trials, which not only did not use current treatment paradigms but also did not study the key research question, which Dr. Kingdom believes is this: What’s the comparative effectiveness of ESS vs. continued medical therapy in patients who’ve failed initial medical therapy?
He offered as an example of the contemporary approach to comparative outcomes research in the field of ESS a recent multicenter prospective study led by otolaryngologists at Oregon Health and Science University, Portland. It involved 1 year of prospective follow-up of patients with CRS who had failed initial medical therapy, at which point they elected to undergo ESS or further medical management.
The 65 patients who opted for ESS and the 50 whose chose more medical management were comparable in terms of baseline CRS severity and comorbidities. Both groups showed durable improvement at 12 months, compared with baseline. But ESS was the clear winner, with a mean 71% improvement in the validated Chronic Sinusitis Survey total score, compared with a 46% improvement in the medically managed group. Moreover, during the year of follow-up 17 patients switched over from medical management to ESS and they, too, showed significantly greater improvement than those who remained on medical management (Int. Forum Allergy Rhinol. 2013;3:236-41).
An earlier interim report featuring 6 months of followup showed the surgical group experienced roughly twofold greater improvement, compared with the medical cohort in endpoints including number of days on oral antibiotics or oral corticosteroids and missed days of work or school (Int. Forum Allergy Rhinol. 2011;1:235-41).
Cost-effectiveness studies by various research groups are in the pipeline. The early indication is that the data will show an economic advantage for ESS over medical therapy in patients with recalcitrant disease, according to Dr. Kingdom.
The next research frontier in surgical outcomes in CRS is identification of cellular and molecular markers of disease activity and their genetic underpinnings, which it’s hoped can be used to select the best candidates for ESS, he added.
Dr. Kingdom reported having no financial conflicts of interest.
KEYSTONE, COLO. – Research-minded otolaryngologists have gotten serious about conducting high-quality, patient-centered outcomes studies of endoscopic sinus surgery for chronic rhinosinusitis, which more than 250,000 Americans undergo each year. And the results are eye opening.
Mounting evidence documents that endoscopic sinus surgery (ESS) in properly selected patients with chronic rhinosinusitis (CRS) results in markedly improved quality of life, functional status, and reduced use of medications, compared with medical management, Dr. Todd T. Kingdom said at a meeting on allergy and respiratory diseases sponsored by National Jewish Health.
These studies utilize validated measures of patient-centered quality of life and symptoms. They are nothing like the lightweight, less-than-persuasive ESS research published in the 1990s, which reported glowing ‘success’ rates of 80%-97% in single-institution retrospective studies using variable inclusion criteria and often-sketchy definitions of success.
"Those data are not acceptable, but that’s what we had. This was before evidence-based medicine with an emphasis on rigorously designed studies took hold," explained Dr. Kingdom, professor and vice chairman of the department of otolaryngology – head and neck surgery, at the University of Colorado, Denver, and immediate past president of the American Rhinologic Society.
Current research emphasizes the use of modern, validated patient-centered quality of life tools and symptom scores because CRS is a symptom-based diagnosis and it is symptom severity that drives patients to seek treatment. Also, objective measures, such as the Lund-Mackay CT staging system, fail to capture the full experience of disease burden. Nor do objective measures necessarily correlate with patient symptoms, according to the otolaryngologist.
A low point in the field of sinus surgery, in Dr. Kingdom’s view, was the 2006 Cochrane systematic review which concluded ESS "has not been demonstrated to confer additional benefit to that obtained by medical therapy" (Cochrane Database Syst. Rev. 2006:CD004458).
"This review was a disservice," he asserted.
The review was based entirely on three older randomized trials, which not only did not use current treatment paradigms but also did not study the key research question, which Dr. Kingdom believes is this: What’s the comparative effectiveness of ESS vs. continued medical therapy in patients who’ve failed initial medical therapy?
He offered as an example of the contemporary approach to comparative outcomes research in the field of ESS a recent multicenter prospective study led by otolaryngologists at Oregon Health and Science University, Portland. It involved 1 year of prospective follow-up of patients with CRS who had failed initial medical therapy, at which point they elected to undergo ESS or further medical management.
The 65 patients who opted for ESS and the 50 whose chose more medical management were comparable in terms of baseline CRS severity and comorbidities. Both groups showed durable improvement at 12 months, compared with baseline. But ESS was the clear winner, with a mean 71% improvement in the validated Chronic Sinusitis Survey total score, compared with a 46% improvement in the medically managed group. Moreover, during the year of follow-up 17 patients switched over from medical management to ESS and they, too, showed significantly greater improvement than those who remained on medical management (Int. Forum Allergy Rhinol. 2013;3:236-41).
An earlier interim report featuring 6 months of followup showed the surgical group experienced roughly twofold greater improvement, compared with the medical cohort in endpoints including number of days on oral antibiotics or oral corticosteroids and missed days of work or school (Int. Forum Allergy Rhinol. 2011;1:235-41).
Cost-effectiveness studies by various research groups are in the pipeline. The early indication is that the data will show an economic advantage for ESS over medical therapy in patients with recalcitrant disease, according to Dr. Kingdom.
The next research frontier in surgical outcomes in CRS is identification of cellular and molecular markers of disease activity and their genetic underpinnings, which it’s hoped can be used to select the best candidates for ESS, he added.
Dr. Kingdom reported having no financial conflicts of interest.
KEYSTONE, COLO. – Research-minded otolaryngologists have gotten serious about conducting high-quality, patient-centered outcomes studies of endoscopic sinus surgery for chronic rhinosinusitis, which more than 250,000 Americans undergo each year. And the results are eye opening.
Mounting evidence documents that endoscopic sinus surgery (ESS) in properly selected patients with chronic rhinosinusitis (CRS) results in markedly improved quality of life, functional status, and reduced use of medications, compared with medical management, Dr. Todd T. Kingdom said at a meeting on allergy and respiratory diseases sponsored by National Jewish Health.
These studies utilize validated measures of patient-centered quality of life and symptoms. They are nothing like the lightweight, less-than-persuasive ESS research published in the 1990s, which reported glowing ‘success’ rates of 80%-97% in single-institution retrospective studies using variable inclusion criteria and often-sketchy definitions of success.
"Those data are not acceptable, but that’s what we had. This was before evidence-based medicine with an emphasis on rigorously designed studies took hold," explained Dr. Kingdom, professor and vice chairman of the department of otolaryngology – head and neck surgery, at the University of Colorado, Denver, and immediate past president of the American Rhinologic Society.
Current research emphasizes the use of modern, validated patient-centered quality of life tools and symptom scores because CRS is a symptom-based diagnosis and it is symptom severity that drives patients to seek treatment. Also, objective measures, such as the Lund-Mackay CT staging system, fail to capture the full experience of disease burden. Nor do objective measures necessarily correlate with patient symptoms, according to the otolaryngologist.
A low point in the field of sinus surgery, in Dr. Kingdom’s view, was the 2006 Cochrane systematic review which concluded ESS "has not been demonstrated to confer additional benefit to that obtained by medical therapy" (Cochrane Database Syst. Rev. 2006:CD004458).
"This review was a disservice," he asserted.
The review was based entirely on three older randomized trials, which not only did not use current treatment paradigms but also did not study the key research question, which Dr. Kingdom believes is this: What’s the comparative effectiveness of ESS vs. continued medical therapy in patients who’ve failed initial medical therapy?
He offered as an example of the contemporary approach to comparative outcomes research in the field of ESS a recent multicenter prospective study led by otolaryngologists at Oregon Health and Science University, Portland. It involved 1 year of prospective follow-up of patients with CRS who had failed initial medical therapy, at which point they elected to undergo ESS or further medical management.
The 65 patients who opted for ESS and the 50 whose chose more medical management were comparable in terms of baseline CRS severity and comorbidities. Both groups showed durable improvement at 12 months, compared with baseline. But ESS was the clear winner, with a mean 71% improvement in the validated Chronic Sinusitis Survey total score, compared with a 46% improvement in the medically managed group. Moreover, during the year of follow-up 17 patients switched over from medical management to ESS and they, too, showed significantly greater improvement than those who remained on medical management (Int. Forum Allergy Rhinol. 2013;3:236-41).
An earlier interim report featuring 6 months of followup showed the surgical group experienced roughly twofold greater improvement, compared with the medical cohort in endpoints including number of days on oral antibiotics or oral corticosteroids and missed days of work or school (Int. Forum Allergy Rhinol. 2011;1:235-41).
Cost-effectiveness studies by various research groups are in the pipeline. The early indication is that the data will show an economic advantage for ESS over medical therapy in patients with recalcitrant disease, according to Dr. Kingdom.
The next research frontier in surgical outcomes in CRS is identification of cellular and molecular markers of disease activity and their genetic underpinnings, which it’s hoped can be used to select the best candidates for ESS, he added.
Dr. Kingdom reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM THE PULMONARY AND ALLERGY UPDATE
A new jail could prove transformative
In the jail cell I had appropriated as an office, the toilet was broken. The metal toilet bowl was wrapped carelessly in heavy plastic and the plastic was anchored in place by gray duct tape. The window opened hopefully to about 4 inches, just enough to let in the August humidity. Outside, the temperature reached 90 F. Inside the cell, the temperature was easily over 100° F. I was wilting, my patients were wilting, and I left the building at the end of the morning soaked in sweat carrying stacks of damp paper. For the first time since I was an intern, I donned a pair of surgical scrubs because the building was too intolerable for wearing street clothes. This was the legacy of my jail. The facility was opened in 1859, well before the invention of modern heating and air conditioning.
Thanks to the intervention of the Department of Justice, some improvements were made. Certain tiers were given air-conditioning units and designated for the housing of inmates vulnerable to heat exhaustion. Intake procedures were modified to identify and stratify new detainees according to their medical needs and heat tolerance. Inmates were given education on the symptoms of heat exhaustion and how to prevent it. Eventually summer passed, and I was able to lose the surgical scrubs and slip back into street clothes.
Yet the remaining need for a new jail was obvious to anyone working in the facility or incarcerated there. A recent legislative inquiry pointed out the security risks posed by the antiquated tier structures and other hazards. In the report published later, the cost of a new jail was estimated to be $553 million.
The protest started almost immediately. People wrote to the local newspaper to object to the idea of spending money on incarceration rather than crime prevention. Advocacy groups carved out territorial niches to promote diversion of juveniles, pregnant women, and the mentally ill, apparently leaving any prisoner who didn’t fit into one of those categories to fend for himself. In the end, the jail – which opened at a time when there were only 33 states in the union, the year that "A Tale of Two Cities" and "On the Origin of Species" were published, and when the works of Johannes Brahms were being heard for the first time – remained untouched.
These memories returned quickly after reading a recent New York Times story about a homeless veteran with mental illness who died in his jail cell, presumably because of excessive heat. The man had been incarcerated for a misdemeanor and held only because he couldn’t make bail. The fact that this happened in winter tells me a lot about the nature of the facility’s heating and ventilation system.
The first thing that struck me about this story was the need to invoke certain adjectives, as though readers would not care about the story if the prisoner who died was not mentally ill or was not a veteran. There may be some truth to that concern. When it comes to prison reform, I’ve seen the tendency within psychiatry to limit discussions only to conditions as they affect the mentally ill. This was not always the case. Dr. Benjamin Rush, founder of Pennsylvania Hospital, also founded one of the first prison reformation societies. He advocated humane care for everyone in the facility, not just a certain faction.
Unless someone figures out how to "cure" crime, we will always need jails and prisons. Building a new facility is neither a failure nor a waste of resources. It may be the beginning of a long-term commitment to rehabilitation.
Dr. Hanson is a forensic psychiatrist and coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work." The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson’s employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
In the jail cell I had appropriated as an office, the toilet was broken. The metal toilet bowl was wrapped carelessly in heavy plastic and the plastic was anchored in place by gray duct tape. The window opened hopefully to about 4 inches, just enough to let in the August humidity. Outside, the temperature reached 90 F. Inside the cell, the temperature was easily over 100° F. I was wilting, my patients were wilting, and I left the building at the end of the morning soaked in sweat carrying stacks of damp paper. For the first time since I was an intern, I donned a pair of surgical scrubs because the building was too intolerable for wearing street clothes. This was the legacy of my jail. The facility was opened in 1859, well before the invention of modern heating and air conditioning.
Thanks to the intervention of the Department of Justice, some improvements were made. Certain tiers were given air-conditioning units and designated for the housing of inmates vulnerable to heat exhaustion. Intake procedures were modified to identify and stratify new detainees according to their medical needs and heat tolerance. Inmates were given education on the symptoms of heat exhaustion and how to prevent it. Eventually summer passed, and I was able to lose the surgical scrubs and slip back into street clothes.
Yet the remaining need for a new jail was obvious to anyone working in the facility or incarcerated there. A recent legislative inquiry pointed out the security risks posed by the antiquated tier structures and other hazards. In the report published later, the cost of a new jail was estimated to be $553 million.
The protest started almost immediately. People wrote to the local newspaper to object to the idea of spending money on incarceration rather than crime prevention. Advocacy groups carved out territorial niches to promote diversion of juveniles, pregnant women, and the mentally ill, apparently leaving any prisoner who didn’t fit into one of those categories to fend for himself. In the end, the jail – which opened at a time when there were only 33 states in the union, the year that "A Tale of Two Cities" and "On the Origin of Species" were published, and when the works of Johannes Brahms were being heard for the first time – remained untouched.
These memories returned quickly after reading a recent New York Times story about a homeless veteran with mental illness who died in his jail cell, presumably because of excessive heat. The man had been incarcerated for a misdemeanor and held only because he couldn’t make bail. The fact that this happened in winter tells me a lot about the nature of the facility’s heating and ventilation system.
The first thing that struck me about this story was the need to invoke certain adjectives, as though readers would not care about the story if the prisoner who died was not mentally ill or was not a veteran. There may be some truth to that concern. When it comes to prison reform, I’ve seen the tendency within psychiatry to limit discussions only to conditions as they affect the mentally ill. This was not always the case. Dr. Benjamin Rush, founder of Pennsylvania Hospital, also founded one of the first prison reformation societies. He advocated humane care for everyone in the facility, not just a certain faction.
Unless someone figures out how to "cure" crime, we will always need jails and prisons. Building a new facility is neither a failure nor a waste of resources. It may be the beginning of a long-term commitment to rehabilitation.
Dr. Hanson is a forensic psychiatrist and coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work." The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson’s employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
In the jail cell I had appropriated as an office, the toilet was broken. The metal toilet bowl was wrapped carelessly in heavy plastic and the plastic was anchored in place by gray duct tape. The window opened hopefully to about 4 inches, just enough to let in the August humidity. Outside, the temperature reached 90 F. Inside the cell, the temperature was easily over 100° F. I was wilting, my patients were wilting, and I left the building at the end of the morning soaked in sweat carrying stacks of damp paper. For the first time since I was an intern, I donned a pair of surgical scrubs because the building was too intolerable for wearing street clothes. This was the legacy of my jail. The facility was opened in 1859, well before the invention of modern heating and air conditioning.
Thanks to the intervention of the Department of Justice, some improvements were made. Certain tiers were given air-conditioning units and designated for the housing of inmates vulnerable to heat exhaustion. Intake procedures were modified to identify and stratify new detainees according to their medical needs and heat tolerance. Inmates were given education on the symptoms of heat exhaustion and how to prevent it. Eventually summer passed, and I was able to lose the surgical scrubs and slip back into street clothes.
Yet the remaining need for a new jail was obvious to anyone working in the facility or incarcerated there. A recent legislative inquiry pointed out the security risks posed by the antiquated tier structures and other hazards. In the report published later, the cost of a new jail was estimated to be $553 million.
The protest started almost immediately. People wrote to the local newspaper to object to the idea of spending money on incarceration rather than crime prevention. Advocacy groups carved out territorial niches to promote diversion of juveniles, pregnant women, and the mentally ill, apparently leaving any prisoner who didn’t fit into one of those categories to fend for himself. In the end, the jail – which opened at a time when there were only 33 states in the union, the year that "A Tale of Two Cities" and "On the Origin of Species" were published, and when the works of Johannes Brahms were being heard for the first time – remained untouched.
These memories returned quickly after reading a recent New York Times story about a homeless veteran with mental illness who died in his jail cell, presumably because of excessive heat. The man had been incarcerated for a misdemeanor and held only because he couldn’t make bail. The fact that this happened in winter tells me a lot about the nature of the facility’s heating and ventilation system.
The first thing that struck me about this story was the need to invoke certain adjectives, as though readers would not care about the story if the prisoner who died was not mentally ill or was not a veteran. There may be some truth to that concern. When it comes to prison reform, I’ve seen the tendency within psychiatry to limit discussions only to conditions as they affect the mentally ill. This was not always the case. Dr. Benjamin Rush, founder of Pennsylvania Hospital, also founded one of the first prison reformation societies. He advocated humane care for everyone in the facility, not just a certain faction.
Unless someone figures out how to "cure" crime, we will always need jails and prisons. Building a new facility is neither a failure nor a waste of resources. It may be the beginning of a long-term commitment to rehabilitation.
Dr. Hanson is a forensic psychiatrist and coauthor of "Shrink Rap: Three Psychiatrists Explain Their Work." The opinions expressed are those of the author only, and do not represent those of any of Dr. Hanson’s employers or consultees, including the Maryland Department of Health and Mental Hygiene or the Maryland Division of Correction.
Should you use an anticonvulsant to treat impulsivity and aggression?
Mr. V, age 29, is a US Army veteran who presents to the psychiatric emergency department because of increasing aggression. He recently returned from deployment overseas and lives with his parents. Mr. V’s mother reports that he has been increasingly “unstable” and describes an incident during which he punched a hole in his bedroom window after a temporary slow-down in the home’s Internet connection.
The workup and review of the history rules out substance abuse, posttraumatic stress disorder, bipolar disorder, seizure disorder, and personality disorders. He is currently taking only omeprazole, 40 mg/d, for acid reflux. The psychiatrist considers prescribing an antiepileptic medication to treat the agitation. Why this choice of agent?
According to DSM-5, patients who have repeated episodes of aggression can be given a diagnosis of intermittent explosive disorder, but such behavior can occur secondary to other psychiatric diagnoses (Table 1). No medications are FDA approved for aggression.1
Aggression and associated verbal and physical acts fall into 2 subtypes: impulsive type and premeditated (predatory) type. Impulsive aggression generally is described as an emotionally charged aggressive response characterized by a loss of behavioral control.
Premeditated aggression
Pharmacotherapy is directed primarily at treating impulsive aggression because this subtype is thought to be caused by neurologic deficits that can affect a person’s ability to process, and react appropriately to, external stimuli. Agitation can result from neuronal hyperactivity.2 Agents such as antiepileptic drugs (AEDs) have the potential to reduce the intensity and frequency of such behaviors.2
In this article, we focus on the use of AEDs for treating impulsive aggression in adults.
Reviewing the evidence for AEDs
The neurobiology of aggression involves multiple neurotransmitters, intracellular pathways, and ion channels.3 AEDs have several mechanisms of action, however; primary mechanisms include action on sodium and calcium channels and modulation of γ-aminobutyric acid (GABA), glutamate, and carbonic anhydrase.2,3 Agent-specific mechanisms of actions are listed in Table 2.
Phenytoin. Several double-blind, placebo-controlled trials have found a statistically significant difference between phenytoin and placebo for treating impulsive aggression, as measured by the Overt Aggression Scale (OAS)a or a modified version (MOAS/ OAS-M).1,2,4 Researchers found that phenytoin, 300 mg/d, but not 100 mg/d, decreased impulsive aggression.4
a Studies generally used the OAS, or one of its modifications, to evaluate aggressive behavior.2,4
Valproate. Trials of valproate for decreasing aggressive behaviors have produced mixed results with regard to primary outcome when used at standard dosages and within the therapeutic range measured by serum concentration.2,3 In a pooled analysis of studies that met stringent criteria (randomized, controlled trial, aggressive behavior as primary outcome, patients free of organic illness or neurologic illness), Jones and colleagues1 reported that valproate/divalproex did not produce statistically significant results compared with placebo for treating impulsive aggression.
Carbamazepine and oxcarbazepine. Double-blind, placebo-controlled trials and case studies of carbamazepine have shown mixed results. In contrast, oxcarbazepine has been found to significantly decrease aggressive behavior, measured by OAS/MOAS/ OAS-M scores.2,3 Total daily dosages of oxcarbazepine ranged from 1,500 to 2,400 mg.2-4 It has been speculated that oxcarbazepine might be a useful option for treating impulsive aggression because of its therapeutic value in temporal lobe seizures—a subtype of seizure disorder that involves the limbic system, which also modulates aggressiveness.5
Additionally, when compared with carbamazepine, oxcarbazepine has a lower risk of cardiotoxicity, neurotoxicity, and blood dyscrasia. Oxcarbazepine has fewer drug-drug interactions because of a lower degree of hepatic enzyme induction.
Topiramate. Several studies have confirmed the efficacy of topiramate for aggressive behavior.2,3 However, there have been reports that topiramate can induce or exacerbate aggression in some patients, an effect that might be dose-related. Aggression might respond better to a higher, short-term dosage (eg, 400 mg/d) than to lower (100 to 300 mg/d) dosages, which might exacerbate aggression.3
Gabapentin. Research on using gabapentin for aggression is limited. Speculation is that the combined activity of gabapentin on GABA and glutamate give the drug its antiaggressive effect.3 No randomized, double-blind, placebo-controlled trials are underway comparing gabapentin and placebo or other active medication for impulsive aggression.
Some case reports and small-scale, open-label studies report a decrease in aggression with gabapentin. As is the case with topiramate, a lower dosage (200 mg to 400 mg) has been reported to result in increased aggression—whereas a higher dosages (800 mg) decreases aggressive behavior.2,3
Lamotrigine. The results of several studies, including double-blind, placebo-controlled trials, support the use of lamotrigine for aggressive behavior. A number of these studies, however, used scales other than OAS (or its modifications) to determine this outcome. One trial showed increased aggression in several patients on lower-dosage lamotrigine (100 mg/d) that resolved when the dosage was increased.2,3
Treatment recommendations
Although all AEDs have some documented efficacy against aggression, choosing the appropriate agent depends on patient-specific variables. Avoiding divalproex in patients with liver dysfunction, for example, or carbamazepine in those with a preexisting cardiac conduction abnormality will improve outcomes by avoiding complications.
It is important to rule out all other causes of aggression before selecting a treatment. The presence of one or more of the diagnoses listed in Table 1 could lead to selection of an alternate class of medication. Nondrug therapies, such as cognitive-behavioral therapy, also should be considered.
Related Resources
• Coccaro EF. Aggression. Psychiatric assessment and treatment. Chicago, IL: Marcel Dekker, Inc.; 2003.
• Citrome LL. Aggression. http://emedicine.medscape.com/article/288689-overview. Updated June 18, 2012. Accessed February 28, 2014.
Drug Brand Names
Carbamazepine • Tegretol Phenytoin • Dilantin
Gabapentin • Neurontin Topiramate • Topamax
Lamotrigine • Lamictal Valproate/Divalproex
Omeprazole • Prilosec • Depakote
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jones RM, Arlidge J, Gilham R, et al. Efficacy of mood stabilizers in the treatment of impulsive or repetitive aggression: systemic review and meta-analysis. Br J Psychiatry. 2011;198(2):93-98.
2. Stanford MS, Anderson NE, Lake SL, et al. Pharmacologic treatment of impulsive aggression with antiepileptic drugs. Curr Treat Options Neurol. 2009;11(5):383-390.
3. Comai S, Tau M, Pavlovic Z, et al. The psychopharmacology of aggressive behavior: a translational approach: part 2: clinical studies using atypical antipsychotics, anticonvulsants, and lithium. J Clin Psychopharmacol. 2012;32(2):237-260.
4. Huband N, Ferriter M, Nathan R, et al. Antiepileptics for aggression and associated impulsivity. Cochrane Database Sys Rev. 2010;2:CD003499.
5. Mattes JA. Medications for aggressiveness in prison: focus on oxcarbazepine. J Am Acad Psychiatry Law. 2012;40(2):234-238.
Mr. V, age 29, is a US Army veteran who presents to the psychiatric emergency department because of increasing aggression. He recently returned from deployment overseas and lives with his parents. Mr. V’s mother reports that he has been increasingly “unstable” and describes an incident during which he punched a hole in his bedroom window after a temporary slow-down in the home’s Internet connection.
The workup and review of the history rules out substance abuse, posttraumatic stress disorder, bipolar disorder, seizure disorder, and personality disorders. He is currently taking only omeprazole, 40 mg/d, for acid reflux. The psychiatrist considers prescribing an antiepileptic medication to treat the agitation. Why this choice of agent?
According to DSM-5, patients who have repeated episodes of aggression can be given a diagnosis of intermittent explosive disorder, but such behavior can occur secondary to other psychiatric diagnoses (Table 1). No medications are FDA approved for aggression.1
Aggression and associated verbal and physical acts fall into 2 subtypes: impulsive type and premeditated (predatory) type. Impulsive aggression generally is described as an emotionally charged aggressive response characterized by a loss of behavioral control.
Premeditated aggression
Pharmacotherapy is directed primarily at treating impulsive aggression because this subtype is thought to be caused by neurologic deficits that can affect a person’s ability to process, and react appropriately to, external stimuli. Agitation can result from neuronal hyperactivity.2 Agents such as antiepileptic drugs (AEDs) have the potential to reduce the intensity and frequency of such behaviors.2
In this article, we focus on the use of AEDs for treating impulsive aggression in adults.
Reviewing the evidence for AEDs
The neurobiology of aggression involves multiple neurotransmitters, intracellular pathways, and ion channels.3 AEDs have several mechanisms of action, however; primary mechanisms include action on sodium and calcium channels and modulation of γ-aminobutyric acid (GABA), glutamate, and carbonic anhydrase.2,3 Agent-specific mechanisms of actions are listed in Table 2.
Phenytoin. Several double-blind, placebo-controlled trials have found a statistically significant difference between phenytoin and placebo for treating impulsive aggression, as measured by the Overt Aggression Scale (OAS)a or a modified version (MOAS/ OAS-M).1,2,4 Researchers found that phenytoin, 300 mg/d, but not 100 mg/d, decreased impulsive aggression.4
a Studies generally used the OAS, or one of its modifications, to evaluate aggressive behavior.2,4
Valproate. Trials of valproate for decreasing aggressive behaviors have produced mixed results with regard to primary outcome when used at standard dosages and within the therapeutic range measured by serum concentration.2,3 In a pooled analysis of studies that met stringent criteria (randomized, controlled trial, aggressive behavior as primary outcome, patients free of organic illness or neurologic illness), Jones and colleagues1 reported that valproate/divalproex did not produce statistically significant results compared with placebo for treating impulsive aggression.
Carbamazepine and oxcarbazepine. Double-blind, placebo-controlled trials and case studies of carbamazepine have shown mixed results. In contrast, oxcarbazepine has been found to significantly decrease aggressive behavior, measured by OAS/MOAS/ OAS-M scores.2,3 Total daily dosages of oxcarbazepine ranged from 1,500 to 2,400 mg.2-4 It has been speculated that oxcarbazepine might be a useful option for treating impulsive aggression because of its therapeutic value in temporal lobe seizures—a subtype of seizure disorder that involves the limbic system, which also modulates aggressiveness.5
Additionally, when compared with carbamazepine, oxcarbazepine has a lower risk of cardiotoxicity, neurotoxicity, and blood dyscrasia. Oxcarbazepine has fewer drug-drug interactions because of a lower degree of hepatic enzyme induction.
Topiramate. Several studies have confirmed the efficacy of topiramate for aggressive behavior.2,3 However, there have been reports that topiramate can induce or exacerbate aggression in some patients, an effect that might be dose-related. Aggression might respond better to a higher, short-term dosage (eg, 400 mg/d) than to lower (100 to 300 mg/d) dosages, which might exacerbate aggression.3
Gabapentin. Research on using gabapentin for aggression is limited. Speculation is that the combined activity of gabapentin on GABA and glutamate give the drug its antiaggressive effect.3 No randomized, double-blind, placebo-controlled trials are underway comparing gabapentin and placebo or other active medication for impulsive aggression.
Some case reports and small-scale, open-label studies report a decrease in aggression with gabapentin. As is the case with topiramate, a lower dosage (200 mg to 400 mg) has been reported to result in increased aggression—whereas a higher dosages (800 mg) decreases aggressive behavior.2,3
Lamotrigine. The results of several studies, including double-blind, placebo-controlled trials, support the use of lamotrigine for aggressive behavior. A number of these studies, however, used scales other than OAS (or its modifications) to determine this outcome. One trial showed increased aggression in several patients on lower-dosage lamotrigine (100 mg/d) that resolved when the dosage was increased.2,3
Treatment recommendations
Although all AEDs have some documented efficacy against aggression, choosing the appropriate agent depends on patient-specific variables. Avoiding divalproex in patients with liver dysfunction, for example, or carbamazepine in those with a preexisting cardiac conduction abnormality will improve outcomes by avoiding complications.
It is important to rule out all other causes of aggression before selecting a treatment. The presence of one or more of the diagnoses listed in Table 1 could lead to selection of an alternate class of medication. Nondrug therapies, such as cognitive-behavioral therapy, also should be considered.
Related Resources
• Coccaro EF. Aggression. Psychiatric assessment and treatment. Chicago, IL: Marcel Dekker, Inc.; 2003.
• Citrome LL. Aggression. http://emedicine.medscape.com/article/288689-overview. Updated June 18, 2012. Accessed February 28, 2014.
Drug Brand Names
Carbamazepine • Tegretol Phenytoin • Dilantin
Gabapentin • Neurontin Topiramate • Topamax
Lamotrigine • Lamictal Valproate/Divalproex
Omeprazole • Prilosec • Depakote
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. V, age 29, is a US Army veteran who presents to the psychiatric emergency department because of increasing aggression. He recently returned from deployment overseas and lives with his parents. Mr. V’s mother reports that he has been increasingly “unstable” and describes an incident during which he punched a hole in his bedroom window after a temporary slow-down in the home’s Internet connection.
The workup and review of the history rules out substance abuse, posttraumatic stress disorder, bipolar disorder, seizure disorder, and personality disorders. He is currently taking only omeprazole, 40 mg/d, for acid reflux. The psychiatrist considers prescribing an antiepileptic medication to treat the agitation. Why this choice of agent?
According to DSM-5, patients who have repeated episodes of aggression can be given a diagnosis of intermittent explosive disorder, but such behavior can occur secondary to other psychiatric diagnoses (Table 1). No medications are FDA approved for aggression.1
Aggression and associated verbal and physical acts fall into 2 subtypes: impulsive type and premeditated (predatory) type. Impulsive aggression generally is described as an emotionally charged aggressive response characterized by a loss of behavioral control.
Premeditated aggression
Pharmacotherapy is directed primarily at treating impulsive aggression because this subtype is thought to be caused by neurologic deficits that can affect a person’s ability to process, and react appropriately to, external stimuli. Agitation can result from neuronal hyperactivity.2 Agents such as antiepileptic drugs (AEDs) have the potential to reduce the intensity and frequency of such behaviors.2
In this article, we focus on the use of AEDs for treating impulsive aggression in adults.
Reviewing the evidence for AEDs
The neurobiology of aggression involves multiple neurotransmitters, intracellular pathways, and ion channels.3 AEDs have several mechanisms of action, however; primary mechanisms include action on sodium and calcium channels and modulation of γ-aminobutyric acid (GABA), glutamate, and carbonic anhydrase.2,3 Agent-specific mechanisms of actions are listed in Table 2.
Phenytoin. Several double-blind, placebo-controlled trials have found a statistically significant difference between phenytoin and placebo for treating impulsive aggression, as measured by the Overt Aggression Scale (OAS)a or a modified version (MOAS/ OAS-M).1,2,4 Researchers found that phenytoin, 300 mg/d, but not 100 mg/d, decreased impulsive aggression.4
a Studies generally used the OAS, or one of its modifications, to evaluate aggressive behavior.2,4
Valproate. Trials of valproate for decreasing aggressive behaviors have produced mixed results with regard to primary outcome when used at standard dosages and within the therapeutic range measured by serum concentration.2,3 In a pooled analysis of studies that met stringent criteria (randomized, controlled trial, aggressive behavior as primary outcome, patients free of organic illness or neurologic illness), Jones and colleagues1 reported that valproate/divalproex did not produce statistically significant results compared with placebo for treating impulsive aggression.
Carbamazepine and oxcarbazepine. Double-blind, placebo-controlled trials and case studies of carbamazepine have shown mixed results. In contrast, oxcarbazepine has been found to significantly decrease aggressive behavior, measured by OAS/MOAS/ OAS-M scores.2,3 Total daily dosages of oxcarbazepine ranged from 1,500 to 2,400 mg.2-4 It has been speculated that oxcarbazepine might be a useful option for treating impulsive aggression because of its therapeutic value in temporal lobe seizures—a subtype of seizure disorder that involves the limbic system, which also modulates aggressiveness.5
Additionally, when compared with carbamazepine, oxcarbazepine has a lower risk of cardiotoxicity, neurotoxicity, and blood dyscrasia. Oxcarbazepine has fewer drug-drug interactions because of a lower degree of hepatic enzyme induction.
Topiramate. Several studies have confirmed the efficacy of topiramate for aggressive behavior.2,3 However, there have been reports that topiramate can induce or exacerbate aggression in some patients, an effect that might be dose-related. Aggression might respond better to a higher, short-term dosage (eg, 400 mg/d) than to lower (100 to 300 mg/d) dosages, which might exacerbate aggression.3
Gabapentin. Research on using gabapentin for aggression is limited. Speculation is that the combined activity of gabapentin on GABA and glutamate give the drug its antiaggressive effect.3 No randomized, double-blind, placebo-controlled trials are underway comparing gabapentin and placebo or other active medication for impulsive aggression.
Some case reports and small-scale, open-label studies report a decrease in aggression with gabapentin. As is the case with topiramate, a lower dosage (200 mg to 400 mg) has been reported to result in increased aggression—whereas a higher dosages (800 mg) decreases aggressive behavior.2,3
Lamotrigine. The results of several studies, including double-blind, placebo-controlled trials, support the use of lamotrigine for aggressive behavior. A number of these studies, however, used scales other than OAS (or its modifications) to determine this outcome. One trial showed increased aggression in several patients on lower-dosage lamotrigine (100 mg/d) that resolved when the dosage was increased.2,3
Treatment recommendations
Although all AEDs have some documented efficacy against aggression, choosing the appropriate agent depends on patient-specific variables. Avoiding divalproex in patients with liver dysfunction, for example, or carbamazepine in those with a preexisting cardiac conduction abnormality will improve outcomes by avoiding complications.
It is important to rule out all other causes of aggression before selecting a treatment. The presence of one or more of the diagnoses listed in Table 1 could lead to selection of an alternate class of medication. Nondrug therapies, such as cognitive-behavioral therapy, also should be considered.
Related Resources
• Coccaro EF. Aggression. Psychiatric assessment and treatment. Chicago, IL: Marcel Dekker, Inc.; 2003.
• Citrome LL. Aggression. http://emedicine.medscape.com/article/288689-overview. Updated June 18, 2012. Accessed February 28, 2014.
Drug Brand Names
Carbamazepine • Tegretol Phenytoin • Dilantin
Gabapentin • Neurontin Topiramate • Topamax
Lamotrigine • Lamictal Valproate/Divalproex
Omeprazole • Prilosec • Depakote
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jones RM, Arlidge J, Gilham R, et al. Efficacy of mood stabilizers in the treatment of impulsive or repetitive aggression: systemic review and meta-analysis. Br J Psychiatry. 2011;198(2):93-98.
2. Stanford MS, Anderson NE, Lake SL, et al. Pharmacologic treatment of impulsive aggression with antiepileptic drugs. Curr Treat Options Neurol. 2009;11(5):383-390.
3. Comai S, Tau M, Pavlovic Z, et al. The psychopharmacology of aggressive behavior: a translational approach: part 2: clinical studies using atypical antipsychotics, anticonvulsants, and lithium. J Clin Psychopharmacol. 2012;32(2):237-260.
4. Huband N, Ferriter M, Nathan R, et al. Antiepileptics for aggression and associated impulsivity. Cochrane Database Sys Rev. 2010;2:CD003499.
5. Mattes JA. Medications for aggressiveness in prison: focus on oxcarbazepine. J Am Acad Psychiatry Law. 2012;40(2):234-238.
1. Jones RM, Arlidge J, Gilham R, et al. Efficacy of mood stabilizers in the treatment of impulsive or repetitive aggression: systemic review and meta-analysis. Br J Psychiatry. 2011;198(2):93-98.
2. Stanford MS, Anderson NE, Lake SL, et al. Pharmacologic treatment of impulsive aggression with antiepileptic drugs. Curr Treat Options Neurol. 2009;11(5):383-390.
3. Comai S, Tau M, Pavlovic Z, et al. The psychopharmacology of aggressive behavior: a translational approach: part 2: clinical studies using atypical antipsychotics, anticonvulsants, and lithium. J Clin Psychopharmacol. 2012;32(2):237-260.
4. Huband N, Ferriter M, Nathan R, et al. Antiepileptics for aggression and associated impulsivity. Cochrane Database Sys Rev. 2010;2:CD003499.
5. Mattes JA. Medications for aggressiveness in prison: focus on oxcarbazepine. J Am Acad Psychiatry Law. 2012;40(2):234-238.
Evidence needed on obesity definition, treatment, AACE declares
WASHINGTON – Obesity requires a medical definition that goes beyond gauging a person’s body mass index if cost-effective care is to be delivered in an integrated fashion, according to a consensus statement issued by the American Association of Clinical Endocrinologists and the American College of Endocrinology.
"The definition of obesity as a disease is not perfect," Dr. W. Timothy Garvey, who chaired the AACE/ACE Obesity Consensus Conference, said in a media briefing. "We rely upon an [anthropometric] measure of body mass index, which is a measure of height versus weight, and there was consensus that this was ... divorced from the impact of weight gain on the health of the individual. This imprecision in our diagnosis of obesity was constraining us."
In 2013, the American Medical Association officially recognized obesity as a disease. Better codification of what actually constitutes "obesity, the disease," will allow a more integrated and effective approach to treating it, said Dr. Garvey, professor of medicine and chair of the department of nutrition sciences at the University of Alabama at Birmingham. To do so, the AACE/ACE held an intensive, 2-day session that largely featured spontaneous discussions between panelists and audience members representing four specific obesity "pillars": biomedical, government and regulation, health industry and economics, and research and education sectors.
A constant theme across the sectors was the need for a definition of obesity that accounts for cultural differences, ethnicity, and the presence or absence of cardiometabolic markers of disease in persons who are overweight or obese.
The conference’s multidisciplined approach informed the consensus statement that obesity is a chronic disease that should be treated with the established AACE/ACE obesity algorithm and met with lifestyle interventions. The consensus statement also addressed our current "obesogenic" environment, which many participants said was created in part by the abundance of nonnutritious foods.
In an interview, Dr. Susan Kansagra, deputy commissioner of the New York City Department of Health and Mental Hygiene, said that by working with local vendors and their suppliers, among other actions, her agency is focused on increasing access to more nutritious foods in neighborhoods across the city as a way to shape the food environment. "It’s not people who’ve changed over the past 30 years; it’s the environment," Dr. Kansagra said at the conference.
Also addressed by the consensus statement was the need for preventive care, particularly at the pediatric level, and more cohesive public awareness campaigns that could affect how private payers develop their reimbursement strategies. Audience member Dr. Robert Silverman, medical director of CIGNA Healthcare, said that payers would respond to the need for obesity care, but that what currently is missing is "a tie between the evidence and the complications [of obesity]."
"We learned that different stakeholders require different levels of evidence," AACE President Jeffrey I. Mechanick said in the media briefing. "So, we’re going to be able come up with a more efficient way to make recommendations about research so that private insurance carriers, the Centers for Medicare & Medicaid Services, or regulatory agencies have the type of data they require to facilitate the action [they need]."
These differences were brought to light during the conference as various audience members representing the Centers for Disease Control and Prevention, the Food and Drug Administration, the CMS, the National Institutes of Health, and others involved in research and policy making, addressed the panel to either explain or defend why their agency operates as it does.
In the case of the CMS, a statutory organization, it can apply coverage only according to what the agency is mandated to do, said Dr. Elizabeth Koller of the CMS. The level of evidence the agency looks for, she said, includes "hard endpoints of clinical relevance, like reductions in sleep apnea and degenerative joint disease." The CMS is also concerned about the lack of long-term data on interventions, the durability of interventions, and which characteristics are common in people who relapse in their disease, said Dr. Koller, who addressed the group as an audience member.
"Hearing from the CMS was incredibly helpful. We learned so much," said Dr. Mechanick, director of metabolic support at the Mt. Sinai School of Medicine in New York, in an interview.
Dr. Mechanick also said this was the first of three meetings, the next to be held in about a year, where the ultimate goal would be to use the evidence base they will have created to develop recommendations for all involved in delivering obesity care.
The talk was "polite," Dr. John Morton, chief of bariatric surgery at Stanford (Calif.) University and president of the American Society of Bariatric and Metabolic Surgery, said in an interview, but he said he thinks there is bias against people with obesity. "We wouldn’t be having this discussion if it were about cancer," he said in the interview. "Sometimes we think the consequences of obesity are the result of a personal decision, and that may skew people in a direction where they don’t necessarily want to provide help."
Regardless, Dr. Garvey said at the briefing, "the ‘old world’ thinking that obesity is a lifestyle choice has failed us."
Dr. Mechanick is a consultant for Abbott Nutrition. Dr. Garvey has multiple industry relationships, including with Merck, Vivus, and Eisai.
WASHINGTON – Obesity requires a medical definition that goes beyond gauging a person’s body mass index if cost-effective care is to be delivered in an integrated fashion, according to a consensus statement issued by the American Association of Clinical Endocrinologists and the American College of Endocrinology.
"The definition of obesity as a disease is not perfect," Dr. W. Timothy Garvey, who chaired the AACE/ACE Obesity Consensus Conference, said in a media briefing. "We rely upon an [anthropometric] measure of body mass index, which is a measure of height versus weight, and there was consensus that this was ... divorced from the impact of weight gain on the health of the individual. This imprecision in our diagnosis of obesity was constraining us."
In 2013, the American Medical Association officially recognized obesity as a disease. Better codification of what actually constitutes "obesity, the disease," will allow a more integrated and effective approach to treating it, said Dr. Garvey, professor of medicine and chair of the department of nutrition sciences at the University of Alabama at Birmingham. To do so, the AACE/ACE held an intensive, 2-day session that largely featured spontaneous discussions between panelists and audience members representing four specific obesity "pillars": biomedical, government and regulation, health industry and economics, and research and education sectors.
A constant theme across the sectors was the need for a definition of obesity that accounts for cultural differences, ethnicity, and the presence or absence of cardiometabolic markers of disease in persons who are overweight or obese.
The conference’s multidisciplined approach informed the consensus statement that obesity is a chronic disease that should be treated with the established AACE/ACE obesity algorithm and met with lifestyle interventions. The consensus statement also addressed our current "obesogenic" environment, which many participants said was created in part by the abundance of nonnutritious foods.
In an interview, Dr. Susan Kansagra, deputy commissioner of the New York City Department of Health and Mental Hygiene, said that by working with local vendors and their suppliers, among other actions, her agency is focused on increasing access to more nutritious foods in neighborhoods across the city as a way to shape the food environment. "It’s not people who’ve changed over the past 30 years; it’s the environment," Dr. Kansagra said at the conference.
Also addressed by the consensus statement was the need for preventive care, particularly at the pediatric level, and more cohesive public awareness campaigns that could affect how private payers develop their reimbursement strategies. Audience member Dr. Robert Silverman, medical director of CIGNA Healthcare, said that payers would respond to the need for obesity care, but that what currently is missing is "a tie between the evidence and the complications [of obesity]."
"We learned that different stakeholders require different levels of evidence," AACE President Jeffrey I. Mechanick said in the media briefing. "So, we’re going to be able come up with a more efficient way to make recommendations about research so that private insurance carriers, the Centers for Medicare & Medicaid Services, or regulatory agencies have the type of data they require to facilitate the action [they need]."
These differences were brought to light during the conference as various audience members representing the Centers for Disease Control and Prevention, the Food and Drug Administration, the CMS, the National Institutes of Health, and others involved in research and policy making, addressed the panel to either explain or defend why their agency operates as it does.
In the case of the CMS, a statutory organization, it can apply coverage only according to what the agency is mandated to do, said Dr. Elizabeth Koller of the CMS. The level of evidence the agency looks for, she said, includes "hard endpoints of clinical relevance, like reductions in sleep apnea and degenerative joint disease." The CMS is also concerned about the lack of long-term data on interventions, the durability of interventions, and which characteristics are common in people who relapse in their disease, said Dr. Koller, who addressed the group as an audience member.
"Hearing from the CMS was incredibly helpful. We learned so much," said Dr. Mechanick, director of metabolic support at the Mt. Sinai School of Medicine in New York, in an interview.
Dr. Mechanick also said this was the first of three meetings, the next to be held in about a year, where the ultimate goal would be to use the evidence base they will have created to develop recommendations for all involved in delivering obesity care.
The talk was "polite," Dr. John Morton, chief of bariatric surgery at Stanford (Calif.) University and president of the American Society of Bariatric and Metabolic Surgery, said in an interview, but he said he thinks there is bias against people with obesity. "We wouldn’t be having this discussion if it were about cancer," he said in the interview. "Sometimes we think the consequences of obesity are the result of a personal decision, and that may skew people in a direction where they don’t necessarily want to provide help."
Regardless, Dr. Garvey said at the briefing, "the ‘old world’ thinking that obesity is a lifestyle choice has failed us."
Dr. Mechanick is a consultant for Abbott Nutrition. Dr. Garvey has multiple industry relationships, including with Merck, Vivus, and Eisai.
WASHINGTON – Obesity requires a medical definition that goes beyond gauging a person’s body mass index if cost-effective care is to be delivered in an integrated fashion, according to a consensus statement issued by the American Association of Clinical Endocrinologists and the American College of Endocrinology.
"The definition of obesity as a disease is not perfect," Dr. W. Timothy Garvey, who chaired the AACE/ACE Obesity Consensus Conference, said in a media briefing. "We rely upon an [anthropometric] measure of body mass index, which is a measure of height versus weight, and there was consensus that this was ... divorced from the impact of weight gain on the health of the individual. This imprecision in our diagnosis of obesity was constraining us."
In 2013, the American Medical Association officially recognized obesity as a disease. Better codification of what actually constitutes "obesity, the disease," will allow a more integrated and effective approach to treating it, said Dr. Garvey, professor of medicine and chair of the department of nutrition sciences at the University of Alabama at Birmingham. To do so, the AACE/ACE held an intensive, 2-day session that largely featured spontaneous discussions between panelists and audience members representing four specific obesity "pillars": biomedical, government and regulation, health industry and economics, and research and education sectors.
A constant theme across the sectors was the need for a definition of obesity that accounts for cultural differences, ethnicity, and the presence or absence of cardiometabolic markers of disease in persons who are overweight or obese.
The conference’s multidisciplined approach informed the consensus statement that obesity is a chronic disease that should be treated with the established AACE/ACE obesity algorithm and met with lifestyle interventions. The consensus statement also addressed our current "obesogenic" environment, which many participants said was created in part by the abundance of nonnutritious foods.
In an interview, Dr. Susan Kansagra, deputy commissioner of the New York City Department of Health and Mental Hygiene, said that by working with local vendors and their suppliers, among other actions, her agency is focused on increasing access to more nutritious foods in neighborhoods across the city as a way to shape the food environment. "It’s not people who’ve changed over the past 30 years; it’s the environment," Dr. Kansagra said at the conference.
Also addressed by the consensus statement was the need for preventive care, particularly at the pediatric level, and more cohesive public awareness campaigns that could affect how private payers develop their reimbursement strategies. Audience member Dr. Robert Silverman, medical director of CIGNA Healthcare, said that payers would respond to the need for obesity care, but that what currently is missing is "a tie between the evidence and the complications [of obesity]."
"We learned that different stakeholders require different levels of evidence," AACE President Jeffrey I. Mechanick said in the media briefing. "So, we’re going to be able come up with a more efficient way to make recommendations about research so that private insurance carriers, the Centers for Medicare & Medicaid Services, or regulatory agencies have the type of data they require to facilitate the action [they need]."
These differences were brought to light during the conference as various audience members representing the Centers for Disease Control and Prevention, the Food and Drug Administration, the CMS, the National Institutes of Health, and others involved in research and policy making, addressed the panel to either explain or defend why their agency operates as it does.
In the case of the CMS, a statutory organization, it can apply coverage only according to what the agency is mandated to do, said Dr. Elizabeth Koller of the CMS. The level of evidence the agency looks for, she said, includes "hard endpoints of clinical relevance, like reductions in sleep apnea and degenerative joint disease." The CMS is also concerned about the lack of long-term data on interventions, the durability of interventions, and which characteristics are common in people who relapse in their disease, said Dr. Koller, who addressed the group as an audience member.
"Hearing from the CMS was incredibly helpful. We learned so much," said Dr. Mechanick, director of metabolic support at the Mt. Sinai School of Medicine in New York, in an interview.
Dr. Mechanick also said this was the first of three meetings, the next to be held in about a year, where the ultimate goal would be to use the evidence base they will have created to develop recommendations for all involved in delivering obesity care.
The talk was "polite," Dr. John Morton, chief of bariatric surgery at Stanford (Calif.) University and president of the American Society of Bariatric and Metabolic Surgery, said in an interview, but he said he thinks there is bias against people with obesity. "We wouldn’t be having this discussion if it were about cancer," he said in the interview. "Sometimes we think the consequences of obesity are the result of a personal decision, and that may skew people in a direction where they don’t necessarily want to provide help."
Regardless, Dr. Garvey said at the briefing, "the ‘old world’ thinking that obesity is a lifestyle choice has failed us."
Dr. Mechanick is a consultant for Abbott Nutrition. Dr. Garvey has multiple industry relationships, including with Merck, Vivus, and Eisai.
AT THE AACE/ACE CONSENSUS CONFERENCE ON OBESITY